User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
Powered by CHEST Physician, Clinician Reviews, MDedge Family Medicine, Internal Medicine News, and The Journal of Clinical Outcomes Management.
Asthma ruled out in 33% of diagnosed adults
Asthma was ruled out in 33% of adults in the general Canadian population who had been diagnosed by a physician during the preceding 5 years, according to a report published online Jan. 17 in JAMA.
In a prospective multicenter cohort study involving 613 asthma patients, 203 had no evidence of current asthma when they underwent serial assessments of respiratory symptoms, lung function, and bronchial provocation testing while not taking asthma medications. More than 90% of these 203 participants safely refrained from using the medications for an additional 1-year follow-up period, said Shawn D. Aaron, MD, of Ottawa (Ont.) Hospital Research Institute, and his associates in the Canadian Respiratory Research Network.
Some of these patients were likely misdiagnosed initially and some likely experienced remission since their initial diagnosis. Either way, reassessing asthma diagnoses may be warranted in many patients, the investigators said (JAMA 2017;317[3]:269-79).
To assess whether some patients could safely discontinue asthma medications because they no longer had the disease, the researchers performed a random sampling of the general adult population (approximately 17,000 people) living in urban, suburban, or rural areas in and around the 10 largest cities in Canada during a 3-year period. Those who reported that a member of the household had been diagnosed as having asthma within the previous 5 years were invited to participate in the study.
A total of 613 men and women (mean age, 51 years) completed the study, undergoing spirometry to assess airflow obstruction, methacholine challenges to assess airway hyperresponsiveness, clinical examination by a pulmonologist, and, if indicated, tapering and discontinuation of asthma medications. Those in whom asthma was ruled out were closely followed for 1 year, undergoing repeat bronchial challenge testing and reporting any worsening of asthma signs and symptoms.
At baseline, 87% of the participants said that they had recently used asthma medications and 45% said they used such medications daily. The remainder had already stopped using asthma medications, an indication that many patients can tell when their asthma has remitted (or was never present) and may adjust their medication use with or without a physician’s guidance, Dr. Aaron and his associates said.
Current asthma was confirmed in 62.3% of the study participants. The primary study outcome – the proportion of patients in whom a current asthma diagnosis was ruled out – was 33.1%, or 203 patients. Only 44% of these participants who did not have current asthma had undergone objective testing before their initial diagnosis, compared with 56% of patients in whom asthma was confirmed. This indicates that “whenever possible, physicians should order objective tests, such as prebronchodilator and postbronchodilator spirometry, serial peak flow measurements, or bronchial challenge tests, to confirm asthma at the time of initial diagnosis,” the investigators said.
A total of 35% of the participants in whom asthma was ruled out had been using daily asthma medications. “Use of asthma medications in these patients presumably provided only risks for medication adverse effects and cost, with little opportunity for therapeutic benefit,” the researchers noted. Twelve patients – 2% of the study population – were found to have serious cardiorespiratory conditions that had been misdiagnosed as asthma: four people with ischemic heart disease (two requiring percutaneous coronary intervention), two with subglottic stenosis (both requiring airway dilation procedures), two with bronchiectasis, and one each with interstitial lung disease, pulmonary hypertension, sarcoidosis, and tracheobronchomalacia.
During the additional year of follow-up, 22 of the 203 patients in whom asthma had been ruled out had a positive bronchial challenge test result at 6 or 12 months. Six resumed using asthma medications, one was treated with a brief course of oral corticosteroid, and the others did not require asthma medications.
The Canadian Institutes of Health Research supported the study. Methapharm provided provocholine and Trudell Medical International provided the peak flow meters used in the study. Dr. Aaron reported having no relevant financial disclosures; his associates reported ties to numerous industry sources.
“The study by Aaron [et al.] is an important reminder that in addition to reviewing asthma symptoms and treatment, trying to understand if the diagnosis is still appropriate is an important part of clinical care.”
The study gives clinicians two insights: First, adults diagnosed as having asthma may not continue to have the disease years later, or at least may not require treatment indefinitely. And second, physiological testing is an essential component of diagnosis and will help avoid unnecessary treatment and missed alternative causes for signs and symptoms.
Helen M. Hollingsworth, MD, and George T. O’Connor, MD, are at the Pulmonary Center at Boston University. Dr. O’Connor is an associate editor of JAMA. He reported serving as a consultant for AstraZeneca and receiving grants from Janssen Pharmaceuticals. Dr. Hollingsworth and Dr. O’Connor made these remarks in an editorial accompanying Dr. Aaron’s report (JAMA 2017;317[3]:262-3).
“The study by Aaron [et al.] is an important reminder that in addition to reviewing asthma symptoms and treatment, trying to understand if the diagnosis is still appropriate is an important part of clinical care.”
The study gives clinicians two insights: First, adults diagnosed as having asthma may not continue to have the disease years later, or at least may not require treatment indefinitely. And second, physiological testing is an essential component of diagnosis and will help avoid unnecessary treatment and missed alternative causes for signs and symptoms.
Helen M. Hollingsworth, MD, and George T. O’Connor, MD, are at the Pulmonary Center at Boston University. Dr. O’Connor is an associate editor of JAMA. He reported serving as a consultant for AstraZeneca and receiving grants from Janssen Pharmaceuticals. Dr. Hollingsworth and Dr. O’Connor made these remarks in an editorial accompanying Dr. Aaron’s report (JAMA 2017;317[3]:262-3).
“The study by Aaron [et al.] is an important reminder that in addition to reviewing asthma symptoms and treatment, trying to understand if the diagnosis is still appropriate is an important part of clinical care.”
The study gives clinicians two insights: First, adults diagnosed as having asthma may not continue to have the disease years later, or at least may not require treatment indefinitely. And second, physiological testing is an essential component of diagnosis and will help avoid unnecessary treatment and missed alternative causes for signs and symptoms.
Helen M. Hollingsworth, MD, and George T. O’Connor, MD, are at the Pulmonary Center at Boston University. Dr. O’Connor is an associate editor of JAMA. He reported serving as a consultant for AstraZeneca and receiving grants from Janssen Pharmaceuticals. Dr. Hollingsworth and Dr. O’Connor made these remarks in an editorial accompanying Dr. Aaron’s report (JAMA 2017;317[3]:262-3).
Asthma was ruled out in 33% of adults in the general Canadian population who had been diagnosed by a physician during the preceding 5 years, according to a report published online Jan. 17 in JAMA.
In a prospective multicenter cohort study involving 613 asthma patients, 203 had no evidence of current asthma when they underwent serial assessments of respiratory symptoms, lung function, and bronchial provocation testing while not taking asthma medications. More than 90% of these 203 participants safely refrained from using the medications for an additional 1-year follow-up period, said Shawn D. Aaron, MD, of Ottawa (Ont.) Hospital Research Institute, and his associates in the Canadian Respiratory Research Network.
Some of these patients were likely misdiagnosed initially and some likely experienced remission since their initial diagnosis. Either way, reassessing asthma diagnoses may be warranted in many patients, the investigators said (JAMA 2017;317[3]:269-79).
To assess whether some patients could safely discontinue asthma medications because they no longer had the disease, the researchers performed a random sampling of the general adult population (approximately 17,000 people) living in urban, suburban, or rural areas in and around the 10 largest cities in Canada during a 3-year period. Those who reported that a member of the household had been diagnosed as having asthma within the previous 5 years were invited to participate in the study.
A total of 613 men and women (mean age, 51 years) completed the study, undergoing spirometry to assess airflow obstruction, methacholine challenges to assess airway hyperresponsiveness, clinical examination by a pulmonologist, and, if indicated, tapering and discontinuation of asthma medications. Those in whom asthma was ruled out were closely followed for 1 year, undergoing repeat bronchial challenge testing and reporting any worsening of asthma signs and symptoms.
At baseline, 87% of the participants said that they had recently used asthma medications and 45% said they used such medications daily. The remainder had already stopped using asthma medications, an indication that many patients can tell when their asthma has remitted (or was never present) and may adjust their medication use with or without a physician’s guidance, Dr. Aaron and his associates said.
Current asthma was confirmed in 62.3% of the study participants. The primary study outcome – the proportion of patients in whom a current asthma diagnosis was ruled out – was 33.1%, or 203 patients. Only 44% of these participants who did not have current asthma had undergone objective testing before their initial diagnosis, compared with 56% of patients in whom asthma was confirmed. This indicates that “whenever possible, physicians should order objective tests, such as prebronchodilator and postbronchodilator spirometry, serial peak flow measurements, or bronchial challenge tests, to confirm asthma at the time of initial diagnosis,” the investigators said.
A total of 35% of the participants in whom asthma was ruled out had been using daily asthma medications. “Use of asthma medications in these patients presumably provided only risks for medication adverse effects and cost, with little opportunity for therapeutic benefit,” the researchers noted. Twelve patients – 2% of the study population – were found to have serious cardiorespiratory conditions that had been misdiagnosed as asthma: four people with ischemic heart disease (two requiring percutaneous coronary intervention), two with subglottic stenosis (both requiring airway dilation procedures), two with bronchiectasis, and one each with interstitial lung disease, pulmonary hypertension, sarcoidosis, and tracheobronchomalacia.
During the additional year of follow-up, 22 of the 203 patients in whom asthma had been ruled out had a positive bronchial challenge test result at 6 or 12 months. Six resumed using asthma medications, one was treated with a brief course of oral corticosteroid, and the others did not require asthma medications.
The Canadian Institutes of Health Research supported the study. Methapharm provided provocholine and Trudell Medical International provided the peak flow meters used in the study. Dr. Aaron reported having no relevant financial disclosures; his associates reported ties to numerous industry sources.
Asthma was ruled out in 33% of adults in the general Canadian population who had been diagnosed by a physician during the preceding 5 years, according to a report published online Jan. 17 in JAMA.
In a prospective multicenter cohort study involving 613 asthma patients, 203 had no evidence of current asthma when they underwent serial assessments of respiratory symptoms, lung function, and bronchial provocation testing while not taking asthma medications. More than 90% of these 203 participants safely refrained from using the medications for an additional 1-year follow-up period, said Shawn D. Aaron, MD, of Ottawa (Ont.) Hospital Research Institute, and his associates in the Canadian Respiratory Research Network.
Some of these patients were likely misdiagnosed initially and some likely experienced remission since their initial diagnosis. Either way, reassessing asthma diagnoses may be warranted in many patients, the investigators said (JAMA 2017;317[3]:269-79).
To assess whether some patients could safely discontinue asthma medications because they no longer had the disease, the researchers performed a random sampling of the general adult population (approximately 17,000 people) living in urban, suburban, or rural areas in and around the 10 largest cities in Canada during a 3-year period. Those who reported that a member of the household had been diagnosed as having asthma within the previous 5 years were invited to participate in the study.
A total of 613 men and women (mean age, 51 years) completed the study, undergoing spirometry to assess airflow obstruction, methacholine challenges to assess airway hyperresponsiveness, clinical examination by a pulmonologist, and, if indicated, tapering and discontinuation of asthma medications. Those in whom asthma was ruled out were closely followed for 1 year, undergoing repeat bronchial challenge testing and reporting any worsening of asthma signs and symptoms.
At baseline, 87% of the participants said that they had recently used asthma medications and 45% said they used such medications daily. The remainder had already stopped using asthma medications, an indication that many patients can tell when their asthma has remitted (or was never present) and may adjust their medication use with or without a physician’s guidance, Dr. Aaron and his associates said.
Current asthma was confirmed in 62.3% of the study participants. The primary study outcome – the proportion of patients in whom a current asthma diagnosis was ruled out – was 33.1%, or 203 patients. Only 44% of these participants who did not have current asthma had undergone objective testing before their initial diagnosis, compared with 56% of patients in whom asthma was confirmed. This indicates that “whenever possible, physicians should order objective tests, such as prebronchodilator and postbronchodilator spirometry, serial peak flow measurements, or bronchial challenge tests, to confirm asthma at the time of initial diagnosis,” the investigators said.
A total of 35% of the participants in whom asthma was ruled out had been using daily asthma medications. “Use of asthma medications in these patients presumably provided only risks for medication adverse effects and cost, with little opportunity for therapeutic benefit,” the researchers noted. Twelve patients – 2% of the study population – were found to have serious cardiorespiratory conditions that had been misdiagnosed as asthma: four people with ischemic heart disease (two requiring percutaneous coronary intervention), two with subglottic stenosis (both requiring airway dilation procedures), two with bronchiectasis, and one each with interstitial lung disease, pulmonary hypertension, sarcoidosis, and tracheobronchomalacia.
During the additional year of follow-up, 22 of the 203 patients in whom asthma had been ruled out had a positive bronchial challenge test result at 6 or 12 months. Six resumed using asthma medications, one was treated with a brief course of oral corticosteroid, and the others did not require asthma medications.
The Canadian Institutes of Health Research supported the study. Methapharm provided provocholine and Trudell Medical International provided the peak flow meters used in the study. Dr. Aaron reported having no relevant financial disclosures; his associates reported ties to numerous industry sources.
FROM JAMA
Key clinical point: Asthma was ruled out in 33% of adults in the general Canadian population who had been diagnosed by a physician.
Major finding: Only 44% of the participants in whom asthma was ruled out had undergone objective testing before their initial diagnosis, compared with 56% of patients in whom asthma was confirmed.
Data source: A prospective multicenter cohort study involving 613 adults who had been diagnosed as having asthma during the preceding 5 years.
Disclosures: The Canadian Institutes of Health Research supported the study. Methapharm provided provocholine and Trudell Medical International provided the peak flow meters used in the study. Dr. Aaron reported having no relevant financial disclosures; his associates reported ties to numerous industry sources.
Assay testing accurate in distinguishing bacterial from viral respiratory tract infections
An assay designed to distinguish between bacterial and viral infections of the lower respiratory tract appears effective and shows promise for helping hospital physicians reduce overprescribing of antibiotics to children, a study showed.
“It is often not possible to differentiate between bacterial and nonbacterial disease on the basis of clinical judgment alone, [so] antibiotics are prescribed almost twice as often as required in children with acute respiratory tract infections in the USA,” wrote Chantal B. van Houten of the University Medical Centre Utrecht (the Netherlands) and associates in a study published in the Lancet Infectious Diseases.
The assay in question is called ImmunoXpert, which uses three biomarkers – tumor necrosis factor–related apoptosis-inducing ligand (TRAIL), interferon-gamma–induced protein-10 (IP-10), and C-reactive protein (CRP) – to determine if a lower respiratory tract infection has a viral or bacterial origin. A total of 777 subjects, aged 2-60 months, were recruited from four hospitals in the Netherlands and two hospitals in Israel between October 16, 2013, and March 1, 2015 (Lancet Inf Dis. 2016 Dec. doi: 10.1016/S1473-3099(16)30519-9).
The patients all had fevers with unidentified sources when they presented, and had a follow-up assessment carried out 28 days after baseline. Blood samples and nasal swabs were collected within 24 hours of presentation for assay analysis. Additionally, every subject was diagnosed as “bacterial” or “viral” by a three-member panel of pediatricians, whose diagnoses were based on the data available from the follow-up assessment and from clinical and laboratory data. The panel diagnosis for each subject was used as the reference standard.
Of the 777 subjects initially recruited, 200 were excluded from the final analysis for various reasons. Of the 577 who remained, the panel diagnosed 435 as having a viral infection and 71 as having a bacterial infection; 71 were deemed “inconclusive.” The panel was unanimous in 354 of these cases, and a majority of the panel (two of the three experts) agreed in 443 of these cases. In unanimous cases, the sensitivity of distinguishing between viral and bacterial cases correctly was 87.8%, with a specificity of 93.0%. The panel’s positive and negative predictive value were 62.1% and 98.3%, respectively.
The assay’s sensitivity rate in distinguishing between viral and bacterial infections was very close: 86.7%, with a specificity of 91.1%, which the authors noted was “promising diagnostic accuracy.” The positive predictive value of the assay was 60.5%, while the negative predictive value was found to be 97.8%.
Regarding the 71 cases that were deemed “inconclusive,” Dr. van Houten and coauthors acknowledged that “such inconclusive cases are inherent to studies without a gold standard, and this was taken into account when calculating the sample size.” Additionally, they noted that follow-up studies should take into consideration the costs of utilizing assay testing like ImmunoXpert, in order to better assess the financial implications that adopting the technology would have on a health care facility.
Nevertheless, the investigators concluded, “our findings [support] the need for implementation research to examine the added clinical utility of ImmunoXpert to diagnose bacterial infection in clinical care for children with lower respiratory tract infection and fever without source presenting at the hospital.”
Funding for this study was provided by MeMed Diagnostics. Dr. van Houten and coauthors did not report any relevant financial disclosures.
“A study by Chantal van Houten and colleagues in this issue of the Lancet Infectious Diseases tested the combined measurement of CRP, TRAIL, and IP-10, and found that this test distinguished bacterial from viral infections with a sensitivity of 86.7% and a specificity of 91.1%. This assay is significantly more effective than procalcitonin determinations in identifying the cause of infection because it improves the diagnostic classification of bacterial infections by 6.3% and of viral infections by 5.4%. However, by comparison with CRP, the CRP, TRAIL, and IP-10 combined assay is as effective at classifying bacterial cases, although it does improve the identification of patients with viral infections by 8.6%. Furthermore, it still has some limitations that currently preclude its routine use in clinical practice.
“First, the test requires advanced laboratory techniques and cannot be used outside hospitals. Second, the collected data came from a relatively small number of children, none of whom had an underlying disease that might modify host response to infection. Third – as in the case of all of the studies that have tried to differentiate bacterial and viral infection – the definition of cause of infection used in these studies varies. Finally, respiratory infections are frequently classified on the basis of clinical and radiological findings, and the results of a microbiological assessment of nasopharyngeal swabs.
“However, it is well known that the investigation into upper respiratory secretions in children can be confounding and lead to the erroneous classification of a lower respiratory disease, and that bacteria and viruses can simply be carried and could have no association with the cause of a disease. This means that future studies should confirm the results of host protein-based assays in larger study populations with various characteristics, and consider their cost to benefit ratios in relation to their real effect on reducing antibiotic use.”
Susanna Esposito, MD, and Nicola Principi, MD, are with the University of Milan. Their opinions are excerpted from a commentary on the article by Dr. van Houten et al. (Lancet Inf Dis. 2016 Dec. doi: 10.1016/S1473-3099(16)30536-9). They had no relevant financial disclosures.
“A study by Chantal van Houten and colleagues in this issue of the Lancet Infectious Diseases tested the combined measurement of CRP, TRAIL, and IP-10, and found that this test distinguished bacterial from viral infections with a sensitivity of 86.7% and a specificity of 91.1%. This assay is significantly more effective than procalcitonin determinations in identifying the cause of infection because it improves the diagnostic classification of bacterial infections by 6.3% and of viral infections by 5.4%. However, by comparison with CRP, the CRP, TRAIL, and IP-10 combined assay is as effective at classifying bacterial cases, although it does improve the identification of patients with viral infections by 8.6%. Furthermore, it still has some limitations that currently preclude its routine use in clinical practice.
“First, the test requires advanced laboratory techniques and cannot be used outside hospitals. Second, the collected data came from a relatively small number of children, none of whom had an underlying disease that might modify host response to infection. Third – as in the case of all of the studies that have tried to differentiate bacterial and viral infection – the definition of cause of infection used in these studies varies. Finally, respiratory infections are frequently classified on the basis of clinical and radiological findings, and the results of a microbiological assessment of nasopharyngeal swabs.
“However, it is well known that the investigation into upper respiratory secretions in children can be confounding and lead to the erroneous classification of a lower respiratory disease, and that bacteria and viruses can simply be carried and could have no association with the cause of a disease. This means that future studies should confirm the results of host protein-based assays in larger study populations with various characteristics, and consider their cost to benefit ratios in relation to their real effect on reducing antibiotic use.”
Susanna Esposito, MD, and Nicola Principi, MD, are with the University of Milan. Their opinions are excerpted from a commentary on the article by Dr. van Houten et al. (Lancet Inf Dis. 2016 Dec. doi: 10.1016/S1473-3099(16)30536-9). They had no relevant financial disclosures.
“A study by Chantal van Houten and colleagues in this issue of the Lancet Infectious Diseases tested the combined measurement of CRP, TRAIL, and IP-10, and found that this test distinguished bacterial from viral infections with a sensitivity of 86.7% and a specificity of 91.1%. This assay is significantly more effective than procalcitonin determinations in identifying the cause of infection because it improves the diagnostic classification of bacterial infections by 6.3% and of viral infections by 5.4%. However, by comparison with CRP, the CRP, TRAIL, and IP-10 combined assay is as effective at classifying bacterial cases, although it does improve the identification of patients with viral infections by 8.6%. Furthermore, it still has some limitations that currently preclude its routine use in clinical practice.
“First, the test requires advanced laboratory techniques and cannot be used outside hospitals. Second, the collected data came from a relatively small number of children, none of whom had an underlying disease that might modify host response to infection. Third – as in the case of all of the studies that have tried to differentiate bacterial and viral infection – the definition of cause of infection used in these studies varies. Finally, respiratory infections are frequently classified on the basis of clinical and radiological findings, and the results of a microbiological assessment of nasopharyngeal swabs.
“However, it is well known that the investigation into upper respiratory secretions in children can be confounding and lead to the erroneous classification of a lower respiratory disease, and that bacteria and viruses can simply be carried and could have no association with the cause of a disease. This means that future studies should confirm the results of host protein-based assays in larger study populations with various characteristics, and consider their cost to benefit ratios in relation to their real effect on reducing antibiotic use.”
Susanna Esposito, MD, and Nicola Principi, MD, are with the University of Milan. Their opinions are excerpted from a commentary on the article by Dr. van Houten et al. (Lancet Inf Dis. 2016 Dec. doi: 10.1016/S1473-3099(16)30536-9). They had no relevant financial disclosures.
An assay designed to distinguish between bacterial and viral infections of the lower respiratory tract appears effective and shows promise for helping hospital physicians reduce overprescribing of antibiotics to children, a study showed.
“It is often not possible to differentiate between bacterial and nonbacterial disease on the basis of clinical judgment alone, [so] antibiotics are prescribed almost twice as often as required in children with acute respiratory tract infections in the USA,” wrote Chantal B. van Houten of the University Medical Centre Utrecht (the Netherlands) and associates in a study published in the Lancet Infectious Diseases.
The assay in question is called ImmunoXpert, which uses three biomarkers – tumor necrosis factor–related apoptosis-inducing ligand (TRAIL), interferon-gamma–induced protein-10 (IP-10), and C-reactive protein (CRP) – to determine if a lower respiratory tract infection has a viral or bacterial origin. A total of 777 subjects, aged 2-60 months, were recruited from four hospitals in the Netherlands and two hospitals in Israel between October 16, 2013, and March 1, 2015 (Lancet Inf Dis. 2016 Dec. doi: 10.1016/S1473-3099(16)30519-9).
The patients all had fevers with unidentified sources when they presented, and had a follow-up assessment carried out 28 days after baseline. Blood samples and nasal swabs were collected within 24 hours of presentation for assay analysis. Additionally, every subject was diagnosed as “bacterial” or “viral” by a three-member panel of pediatricians, whose diagnoses were based on the data available from the follow-up assessment and from clinical and laboratory data. The panel diagnosis for each subject was used as the reference standard.
Of the 777 subjects initially recruited, 200 were excluded from the final analysis for various reasons. Of the 577 who remained, the panel diagnosed 435 as having a viral infection and 71 as having a bacterial infection; 71 were deemed “inconclusive.” The panel was unanimous in 354 of these cases, and a majority of the panel (two of the three experts) agreed in 443 of these cases. In unanimous cases, the sensitivity of distinguishing between viral and bacterial cases correctly was 87.8%, with a specificity of 93.0%. The panel’s positive and negative predictive value were 62.1% and 98.3%, respectively.
The assay’s sensitivity rate in distinguishing between viral and bacterial infections was very close: 86.7%, with a specificity of 91.1%, which the authors noted was “promising diagnostic accuracy.” The positive predictive value of the assay was 60.5%, while the negative predictive value was found to be 97.8%.
Regarding the 71 cases that were deemed “inconclusive,” Dr. van Houten and coauthors acknowledged that “such inconclusive cases are inherent to studies without a gold standard, and this was taken into account when calculating the sample size.” Additionally, they noted that follow-up studies should take into consideration the costs of utilizing assay testing like ImmunoXpert, in order to better assess the financial implications that adopting the technology would have on a health care facility.
Nevertheless, the investigators concluded, “our findings [support] the need for implementation research to examine the added clinical utility of ImmunoXpert to diagnose bacterial infection in clinical care for children with lower respiratory tract infection and fever without source presenting at the hospital.”
Funding for this study was provided by MeMed Diagnostics. Dr. van Houten and coauthors did not report any relevant financial disclosures.
An assay designed to distinguish between bacterial and viral infections of the lower respiratory tract appears effective and shows promise for helping hospital physicians reduce overprescribing of antibiotics to children, a study showed.
“It is often not possible to differentiate between bacterial and nonbacterial disease on the basis of clinical judgment alone, [so] antibiotics are prescribed almost twice as often as required in children with acute respiratory tract infections in the USA,” wrote Chantal B. van Houten of the University Medical Centre Utrecht (the Netherlands) and associates in a study published in the Lancet Infectious Diseases.
The assay in question is called ImmunoXpert, which uses three biomarkers – tumor necrosis factor–related apoptosis-inducing ligand (TRAIL), interferon-gamma–induced protein-10 (IP-10), and C-reactive protein (CRP) – to determine if a lower respiratory tract infection has a viral or bacterial origin. A total of 777 subjects, aged 2-60 months, were recruited from four hospitals in the Netherlands and two hospitals in Israel between October 16, 2013, and March 1, 2015 (Lancet Inf Dis. 2016 Dec. doi: 10.1016/S1473-3099(16)30519-9).
The patients all had fevers with unidentified sources when they presented, and had a follow-up assessment carried out 28 days after baseline. Blood samples and nasal swabs were collected within 24 hours of presentation for assay analysis. Additionally, every subject was diagnosed as “bacterial” or “viral” by a three-member panel of pediatricians, whose diagnoses were based on the data available from the follow-up assessment and from clinical and laboratory data. The panel diagnosis for each subject was used as the reference standard.
Of the 777 subjects initially recruited, 200 were excluded from the final analysis for various reasons. Of the 577 who remained, the panel diagnosed 435 as having a viral infection and 71 as having a bacterial infection; 71 were deemed “inconclusive.” The panel was unanimous in 354 of these cases, and a majority of the panel (two of the three experts) agreed in 443 of these cases. In unanimous cases, the sensitivity of distinguishing between viral and bacterial cases correctly was 87.8%, with a specificity of 93.0%. The panel’s positive and negative predictive value were 62.1% and 98.3%, respectively.
The assay’s sensitivity rate in distinguishing between viral and bacterial infections was very close: 86.7%, with a specificity of 91.1%, which the authors noted was “promising diagnostic accuracy.” The positive predictive value of the assay was 60.5%, while the negative predictive value was found to be 97.8%.
Regarding the 71 cases that were deemed “inconclusive,” Dr. van Houten and coauthors acknowledged that “such inconclusive cases are inherent to studies without a gold standard, and this was taken into account when calculating the sample size.” Additionally, they noted that follow-up studies should take into consideration the costs of utilizing assay testing like ImmunoXpert, in order to better assess the financial implications that adopting the technology would have on a health care facility.
Nevertheless, the investigators concluded, “our findings [support] the need for implementation research to examine the added clinical utility of ImmunoXpert to diagnose bacterial infection in clinical care for children with lower respiratory tract infection and fever without source presenting at the hospital.”
Funding for this study was provided by MeMed Diagnostics. Dr. van Houten and coauthors did not report any relevant financial disclosures.
FROM THE LANCET INFECTIOUS DISEASES
Key clinical point:
Major finding: The assay distinguished between bacterial and viral infections with 86.7% sensitivity, compared with unanimous panel diagnosis, which did so with 87.8% sensitivity.
Data source: A double-blind, multicenter, validation study of 577 children aged 2-60 months from October 2013 through March 2015.
Disclosures: The study was funded by MeMed Diagnostics. The authors reported no relevant financial disclosures.
Pulmonary rehabilitation helps wide range of COPD patients
Participation in at least 20 days of pulmonary rehabilitation by patients with chronic obstructive pulmonary disease (COPD) resulted in statistically significant improvements in quality of life, perception of health status, functional capacity, dyspnea, and depression, in a retrospective analysis.
Furthermore, improvements were seen regardless of the degree of exercise function, dyspnea, or lung function at baseline, reported lead investigator Praful Schroff.
The analysis focused on 229 COPD patients enrolled in the pulmonary rehabilitation program at UAB during 1996-2013, all of whom completed questionnaires both at enrollment and after completion of their respective exercise programs. The mean forced expiratory volume in 1 second (FEV1) percent predicted for the cohort was 46.3%.The researchers used pulmonary function data from tests performed within 2 years of enrollment. Change in quality of life and perception of health status were measured using the 36-item Short Form Health Survey (SF-36), dyspnea was assessed using the San Diego Shortness of Breath Questionnaire (SOBQ), depression was assessed using the Beck Depression Inventory (BDI)-II, and functional capacity was assessed using the 6-minute-walk-distance (6MWD) test. On average, the patients reported clinically significant improvements in most components of the SF-36, including an 11.5-unit, a 16.4-U, and a 12.4-U increase in physical function, social function, and vitality, respectively (P less than .001 for all three). The patients also experienced clinically important improvements in the 6MWD test (increase of 52.4 m; P less than .001) and dyspnea (decrease of 9.1 U in the SOBQ; P less than .001). On average, patients’ depression decreased by 3 U, using the BDI II (P less than .001).
When patients in this study were divided into groups based on various aspects of their health at baseline, the levels of improvements seen by each group in most components of the SF-36, the 6MWD, the SOBQ, and the BDI-II were almost always similar.
“We add to the literature by comparing outcomes across quartiles of baseline exercise capacity and showing that patients benefit independent of underlying functional capacity,” said Mr. Schroff and his colleagues.
A few differences in the outcomes following pulmonary rehabilitation were observed between these baseline characteristics–based groups. When patients were grouped by exercise capacity, for example, those with the lowest baseline exercise capacity (as measured by the 6MWD) experienced the largest incremental improvement in the 6MWD. Additionally, when patients were grouped by dyspnea score, those with the worst baseline dyspnea experienced the greatest reduction in dyspnea. However, those with the lowest lung function made the smallest improvement in the 6MWD. Another of these differences was observed between the patients with the lowest baseline lung function and the patients in the other lung function-based quartiles. Those with the worst lung function made the smallest improvement in the 6MWD, which was 39.0 m, on average. All of these subgroups achieved clinically significant improvements in the 6MWD and SOBQ (Ann Am Thorac Soc. 2017 Jan 1;14[1]:26-32).
Each exercise session included aerobic exercises, resistance training, and breathing techniques. Cardiovascular exercise was prescribed starting at 20-30 minutes of continuous or interval bouts and was gradually increasing until 30-45 minutes of exercise was achieved. Patients also received education sessions lasting 40-60 minutes on understanding their disease, smoking cessation counseling, appropriate use of inhalers, diet and nutrition, and stress management. Subjects with other concurrent chronic respiratory diseases were excluded from the analysis.
The researchers noted that their findings may not be generalizable to patients with disease burdens causing them to drop out of the study. “Although we have previously shown that baseline levels of dyspnea, FEV1, and exercise capacity did not influence dropout rates, this could be a source of bias,” they noted.
“Patients with COPD experienced meaningful improvements in quality of life, dyspnea, exercise capacity, and depression, regardless of baseline lung function, dyspnea, and exercise capacity. Current guidelines should be amended to recommend pulmonary rehabilitation to all patients with COPD, regardless of their baseline level of disease burden,” the analysis’ authors said.
Three of the study’s authors reported receiving grants and other fees or a single grant from various sources. The other authors, including Mr. Schroff, said they had nothing to disclose.
Participation in at least 20 days of pulmonary rehabilitation by patients with chronic obstructive pulmonary disease (COPD) resulted in statistically significant improvements in quality of life, perception of health status, functional capacity, dyspnea, and depression, in a retrospective analysis.
Furthermore, improvements were seen regardless of the degree of exercise function, dyspnea, or lung function at baseline, reported lead investigator Praful Schroff.
The analysis focused on 229 COPD patients enrolled in the pulmonary rehabilitation program at UAB during 1996-2013, all of whom completed questionnaires both at enrollment and after completion of their respective exercise programs. The mean forced expiratory volume in 1 second (FEV1) percent predicted for the cohort was 46.3%.The researchers used pulmonary function data from tests performed within 2 years of enrollment. Change in quality of life and perception of health status were measured using the 36-item Short Form Health Survey (SF-36), dyspnea was assessed using the San Diego Shortness of Breath Questionnaire (SOBQ), depression was assessed using the Beck Depression Inventory (BDI)-II, and functional capacity was assessed using the 6-minute-walk-distance (6MWD) test. On average, the patients reported clinically significant improvements in most components of the SF-36, including an 11.5-unit, a 16.4-U, and a 12.4-U increase in physical function, social function, and vitality, respectively (P less than .001 for all three). The patients also experienced clinically important improvements in the 6MWD test (increase of 52.4 m; P less than .001) and dyspnea (decrease of 9.1 U in the SOBQ; P less than .001). On average, patients’ depression decreased by 3 U, using the BDI II (P less than .001).
When patients in this study were divided into groups based on various aspects of their health at baseline, the levels of improvements seen by each group in most components of the SF-36, the 6MWD, the SOBQ, and the BDI-II were almost always similar.
“We add to the literature by comparing outcomes across quartiles of baseline exercise capacity and showing that patients benefit independent of underlying functional capacity,” said Mr. Schroff and his colleagues.
A few differences in the outcomes following pulmonary rehabilitation were observed between these baseline characteristics–based groups. When patients were grouped by exercise capacity, for example, those with the lowest baseline exercise capacity (as measured by the 6MWD) experienced the largest incremental improvement in the 6MWD. Additionally, when patients were grouped by dyspnea score, those with the worst baseline dyspnea experienced the greatest reduction in dyspnea. However, those with the lowest lung function made the smallest improvement in the 6MWD. Another of these differences was observed between the patients with the lowest baseline lung function and the patients in the other lung function-based quartiles. Those with the worst lung function made the smallest improvement in the 6MWD, which was 39.0 m, on average. All of these subgroups achieved clinically significant improvements in the 6MWD and SOBQ (Ann Am Thorac Soc. 2017 Jan 1;14[1]:26-32).
Each exercise session included aerobic exercises, resistance training, and breathing techniques. Cardiovascular exercise was prescribed starting at 20-30 minutes of continuous or interval bouts and was gradually increasing until 30-45 minutes of exercise was achieved. Patients also received education sessions lasting 40-60 minutes on understanding their disease, smoking cessation counseling, appropriate use of inhalers, diet and nutrition, and stress management. Subjects with other concurrent chronic respiratory diseases were excluded from the analysis.
The researchers noted that their findings may not be generalizable to patients with disease burdens causing them to drop out of the study. “Although we have previously shown that baseline levels of dyspnea, FEV1, and exercise capacity did not influence dropout rates, this could be a source of bias,” they noted.
“Patients with COPD experienced meaningful improvements in quality of life, dyspnea, exercise capacity, and depression, regardless of baseline lung function, dyspnea, and exercise capacity. Current guidelines should be amended to recommend pulmonary rehabilitation to all patients with COPD, regardless of their baseline level of disease burden,” the analysis’ authors said.
Three of the study’s authors reported receiving grants and other fees or a single grant from various sources. The other authors, including Mr. Schroff, said they had nothing to disclose.
Participation in at least 20 days of pulmonary rehabilitation by patients with chronic obstructive pulmonary disease (COPD) resulted in statistically significant improvements in quality of life, perception of health status, functional capacity, dyspnea, and depression, in a retrospective analysis.
Furthermore, improvements were seen regardless of the degree of exercise function, dyspnea, or lung function at baseline, reported lead investigator Praful Schroff.
The analysis focused on 229 COPD patients enrolled in the pulmonary rehabilitation program at UAB during 1996-2013, all of whom completed questionnaires both at enrollment and after completion of their respective exercise programs. The mean forced expiratory volume in 1 second (FEV1) percent predicted for the cohort was 46.3%.The researchers used pulmonary function data from tests performed within 2 years of enrollment. Change in quality of life and perception of health status were measured using the 36-item Short Form Health Survey (SF-36), dyspnea was assessed using the San Diego Shortness of Breath Questionnaire (SOBQ), depression was assessed using the Beck Depression Inventory (BDI)-II, and functional capacity was assessed using the 6-minute-walk-distance (6MWD) test. On average, the patients reported clinically significant improvements in most components of the SF-36, including an 11.5-unit, a 16.4-U, and a 12.4-U increase in physical function, social function, and vitality, respectively (P less than .001 for all three). The patients also experienced clinically important improvements in the 6MWD test (increase of 52.4 m; P less than .001) and dyspnea (decrease of 9.1 U in the SOBQ; P less than .001). On average, patients’ depression decreased by 3 U, using the BDI II (P less than .001).
When patients in this study were divided into groups based on various aspects of their health at baseline, the levels of improvements seen by each group in most components of the SF-36, the 6MWD, the SOBQ, and the BDI-II were almost always similar.
“We add to the literature by comparing outcomes across quartiles of baseline exercise capacity and showing that patients benefit independent of underlying functional capacity,” said Mr. Schroff and his colleagues.
A few differences in the outcomes following pulmonary rehabilitation were observed between these baseline characteristics–based groups. When patients were grouped by exercise capacity, for example, those with the lowest baseline exercise capacity (as measured by the 6MWD) experienced the largest incremental improvement in the 6MWD. Additionally, when patients were grouped by dyspnea score, those with the worst baseline dyspnea experienced the greatest reduction in dyspnea. However, those with the lowest lung function made the smallest improvement in the 6MWD. Another of these differences was observed between the patients with the lowest baseline lung function and the patients in the other lung function-based quartiles. Those with the worst lung function made the smallest improvement in the 6MWD, which was 39.0 m, on average. All of these subgroups achieved clinically significant improvements in the 6MWD and SOBQ (Ann Am Thorac Soc. 2017 Jan 1;14[1]:26-32).
Each exercise session included aerobic exercises, resistance training, and breathing techniques. Cardiovascular exercise was prescribed starting at 20-30 minutes of continuous or interval bouts and was gradually increasing until 30-45 minutes of exercise was achieved. Patients also received education sessions lasting 40-60 minutes on understanding their disease, smoking cessation counseling, appropriate use of inhalers, diet and nutrition, and stress management. Subjects with other concurrent chronic respiratory diseases were excluded from the analysis.
The researchers noted that their findings may not be generalizable to patients with disease burdens causing them to drop out of the study. “Although we have previously shown that baseline levels of dyspnea, FEV1, and exercise capacity did not influence dropout rates, this could be a source of bias,” they noted.
“Patients with COPD experienced meaningful improvements in quality of life, dyspnea, exercise capacity, and depression, regardless of baseline lung function, dyspnea, and exercise capacity. Current guidelines should be amended to recommend pulmonary rehabilitation to all patients with COPD, regardless of their baseline level of disease burden,” the analysis’ authors said.
Three of the study’s authors reported receiving grants and other fees or a single grant from various sources. The other authors, including Mr. Schroff, said they had nothing to disclose.
Key clinical point: COPD patients with a variety of baseline health characteristics experienced clinically significant improvements in quality of life, functional capacity, and dyspnea following at least 20 days of pulmonary rehabilitation.
Major finding: The patients experienced clinically important improvements in the 6-minute-walk-distance test (an average increase of 52.4 m; P less than .001) and dyspnea (an average decrease of 9.1 U in the San Diego Shortness of Breath Questionnaire, P less than .001).
Data source: A retrospective analysis of 229 COPD patients from a prospectively maintained database.
Disclosures: Three of the study’s authors reported receiving grants and other fees or a single grant from various sources. The other authors, including Mr. Schroff, said they had nothing to disclose.
Rhinovirus most often caused HA-VRIs in two hospitals
Health care–associated viral respiratory infections (HA-VRIs) were common in two pediatric hospitals, with rhinovirus the most frequent cause of the infections in a 3-year analysis.
The incidence rate of laboratory-confirmed HA-VRIs was 1.29/1,000 patient-days in an examination of the hospitals’ patient data. Forty-eight percent of all 323 HA-VRI cases were caused by rhinovirus, with an overall incidence rate of 0.72/1,000 patient-days. Additionally, rhinovirus was the most frequently identified virus in cases of HA-VRI in all units of both hospitals, followed by parainfluenza virus and respiratory syncytial virus. An exception was the medical/surgical ward of Steven and Alexandra Cohen Children’s Medical Center (CCMC) of New York; in this unit of the CCMC, the incidence rate of parainfluenza virus was higher than that of rhinovirus (0.21/1,000 patient-days vs. 0.15/1,000 patient-days) (J Ped Inf Dis. 2016. doi: 10.1093/jpids/piw072).
The researchers used infection prevention and control surveillance databases from Montreal Children’s Hospital (MCH) in Quebec and the CCMC to identify HA-VRIs that occurred between April 1, 2010, and March 31, 2013, In both hospitals, HAIs were attributed to the unit to which the patient was admitted at the time of transmission. Both hospitals used a multiplex nucleic acid amplification test for respiratory virus detection on nasopharyngeal swabs or aspirates.
“An HA-VRI with an onset of symptoms after hospital discharge would be detected and included only for patients who presented to the emergency department or were readmitted for VRI and tested,” according to Caroline Quach, MD, of the Montreal Children’s Hospital, McGill University Health Centre, Quebec, and her colleagues.
The HA-VRI rate was 1.91/1,000 patient-days at Montreal Children’s Hospital, compared with 0.80/1,000 patient-days at the CCMC (P less than .0001). At the CCMC, the HA-VRI incidence rate was lowest in the neonatal ICU, but at Montgomery Children’s Hospital, the hematology/oncology ward had the lowest rate of HA-VRI.
Having less than 50% single rooms in a given unit was associated with a statistically significantly higher rate of HA-VRI, after the investigators adjusted for unit type and took the correlation of HA-VRI rates within a hospital into consideration. The study authors’ model predicted that units with less than 50% single rooms have 1.33 times higher HA-VRI rates than units with at least 50% single rooms, regardless of unit type.
Dr. Quach has received funding from GlaxoSmithKline, Pfizer, Sage, and AbbVie for an unrelated research project, while the other authors disclosed no financial relationships.
Health care–associated viral respiratory infections (HA-VRIs) were common in two pediatric hospitals, with rhinovirus the most frequent cause of the infections in a 3-year analysis.
The incidence rate of laboratory-confirmed HA-VRIs was 1.29/1,000 patient-days in an examination of the hospitals’ patient data. Forty-eight percent of all 323 HA-VRI cases were caused by rhinovirus, with an overall incidence rate of 0.72/1,000 patient-days. Additionally, rhinovirus was the most frequently identified virus in cases of HA-VRI in all units of both hospitals, followed by parainfluenza virus and respiratory syncytial virus. An exception was the medical/surgical ward of Steven and Alexandra Cohen Children’s Medical Center (CCMC) of New York; in this unit of the CCMC, the incidence rate of parainfluenza virus was higher than that of rhinovirus (0.21/1,000 patient-days vs. 0.15/1,000 patient-days) (J Ped Inf Dis. 2016. doi: 10.1093/jpids/piw072).
The researchers used infection prevention and control surveillance databases from Montreal Children’s Hospital (MCH) in Quebec and the CCMC to identify HA-VRIs that occurred between April 1, 2010, and March 31, 2013, In both hospitals, HAIs were attributed to the unit to which the patient was admitted at the time of transmission. Both hospitals used a multiplex nucleic acid amplification test for respiratory virus detection on nasopharyngeal swabs or aspirates.
“An HA-VRI with an onset of symptoms after hospital discharge would be detected and included only for patients who presented to the emergency department or were readmitted for VRI and tested,” according to Caroline Quach, MD, of the Montreal Children’s Hospital, McGill University Health Centre, Quebec, and her colleagues.
The HA-VRI rate was 1.91/1,000 patient-days at Montreal Children’s Hospital, compared with 0.80/1,000 patient-days at the CCMC (P less than .0001). At the CCMC, the HA-VRI incidence rate was lowest in the neonatal ICU, but at Montgomery Children’s Hospital, the hematology/oncology ward had the lowest rate of HA-VRI.
Having less than 50% single rooms in a given unit was associated with a statistically significantly higher rate of HA-VRI, after the investigators adjusted for unit type and took the correlation of HA-VRI rates within a hospital into consideration. The study authors’ model predicted that units with less than 50% single rooms have 1.33 times higher HA-VRI rates than units with at least 50% single rooms, regardless of unit type.
Dr. Quach has received funding from GlaxoSmithKline, Pfizer, Sage, and AbbVie for an unrelated research project, while the other authors disclosed no financial relationships.
Health care–associated viral respiratory infections (HA-VRIs) were common in two pediatric hospitals, with rhinovirus the most frequent cause of the infections in a 3-year analysis.
The incidence rate of laboratory-confirmed HA-VRIs was 1.29/1,000 patient-days in an examination of the hospitals’ patient data. Forty-eight percent of all 323 HA-VRI cases were caused by rhinovirus, with an overall incidence rate of 0.72/1,000 patient-days. Additionally, rhinovirus was the most frequently identified virus in cases of HA-VRI in all units of both hospitals, followed by parainfluenza virus and respiratory syncytial virus. An exception was the medical/surgical ward of Steven and Alexandra Cohen Children’s Medical Center (CCMC) of New York; in this unit of the CCMC, the incidence rate of parainfluenza virus was higher than that of rhinovirus (0.21/1,000 patient-days vs. 0.15/1,000 patient-days) (J Ped Inf Dis. 2016. doi: 10.1093/jpids/piw072).
The researchers used infection prevention and control surveillance databases from Montreal Children’s Hospital (MCH) in Quebec and the CCMC to identify HA-VRIs that occurred between April 1, 2010, and March 31, 2013, In both hospitals, HAIs were attributed to the unit to which the patient was admitted at the time of transmission. Both hospitals used a multiplex nucleic acid amplification test for respiratory virus detection on nasopharyngeal swabs or aspirates.
“An HA-VRI with an onset of symptoms after hospital discharge would be detected and included only for patients who presented to the emergency department or were readmitted for VRI and tested,” according to Caroline Quach, MD, of the Montreal Children’s Hospital, McGill University Health Centre, Quebec, and her colleagues.
The HA-VRI rate was 1.91/1,000 patient-days at Montreal Children’s Hospital, compared with 0.80/1,000 patient-days at the CCMC (P less than .0001). At the CCMC, the HA-VRI incidence rate was lowest in the neonatal ICU, but at Montgomery Children’s Hospital, the hematology/oncology ward had the lowest rate of HA-VRI.
Having less than 50% single rooms in a given unit was associated with a statistically significantly higher rate of HA-VRI, after the investigators adjusted for unit type and took the correlation of HA-VRI rates within a hospital into consideration. The study authors’ model predicted that units with less than 50% single rooms have 1.33 times higher HA-VRI rates than units with at least 50% single rooms, regardless of unit type.
Dr. Quach has received funding from GlaxoSmithKline, Pfizer, Sage, and AbbVie for an unrelated research project, while the other authors disclosed no financial relationships.
FROM THE JOURNAL OF THE PEDIATRIC INFECTIOUS DISEASES SOCIETY
Key clinical point:
Major finding: The incidence rate of HA-VRIs was 1.29/1,000 patient-days in an examination of two pediatric hospitals’ patient data between April 1, 2010, and March 31, 2013.
Data source: A retrospective comparison of two hospitals’ 3 years of infection prevention and control surveillance data.
Disclosures: Dr. Quach has received funding from GlaxoSmithKline, Pfizer, Sage, and AbbVie for an unrelated research project, while the other authors disclosed no relevant financial relationships.
Macitentan boosts quality of life in PAH patients
Macitentan, a recent addition to the drugs that treat pulmonary arterial hypertension (PAH), improves and stabilizes quality of life for patients with the condition, according to an industry-funded study.
Macitentan (Opsumit) remains tremendously expensive, costing as much as $100,000 per year in the United States, and the study provides little in the way of direct comparison to other drugs in its class. Still, the drug’s effects on quality of life are dramatic, said study lead author Sanjay Mehta, MD, FRCPC, FCCP, professor of medicine at the University of Western Ontario and director of the Southwest Ontario Pulmonary Hypertension Clinic at the London Health Sciences Center in London, Ont.
Researchers found that those who took the 10-mg dose, versus placebo, reported significant improvement in seven of eight quality-of-life domains, and in physical and mental components scores, as measured by the 36-item Short Form Health Survey (SF-36). In addition, the study linked 10-mg doses, versus placebo, to a lower risk of a decline of three points or more in the physical component score (hazard ratio [HR], 0.60; 95% CI, 0.47-0.76; P less than .0001] and the mental component scores (HR, 0.76; 95% CI, 0.61-0.95; P = .0173) until end of treatment.
“The drug has shown stability in patients’ quality of life over 6 months and 12 months,” Dr. Mehta said in an interview. “I can’t cure anybody, and they’ll get worse at some point, but I can improve them. They physically feel better, they’re less short of breath with less body pain, and they feel better psychologically.”
Macitentan, an endothelin receptor antagonist, received Food and Drug Administration approval in 2013 following a study that year (N Engl J Med. 2013 Aug 29;369[9]:809-18) that linked 10-mg doses to a significantly lower risk of death and various complications, compared with placebo and the 3-mg dose. The new study (Chest. 2017 Jan;151[1]:106-18), is an analysis of data from the 2013 study.
The PAH patients were randomly assigned to one of three groups: macitentan 10 mg once daily (234), macitentan 3 mg (237), and placebo (239). The study examined responses from 710 patients (76.9% were female, 55.2% were white, mean age was 45.5) to the SF-36 at baseline, 6 months, 12 months, and end of treatment.
Dr. Mehta noted that macitentan has not been clinically compared to the other drugs. The study, however, notes that it is the first PAH treatment to show improvement in seven of eight domains in the quality-of-life survey.
The new study was funded by Actelion Pharmaceuticals, maker of macitentan. Dr. Mehta has received consulting and speaking fees and institutional support for clinical trials from Actelion, among other drug companies. The other authors report various disclosures, including relationships with Actelion.
Macitentan, a recent addition to the drugs that treat pulmonary arterial hypertension (PAH), improves and stabilizes quality of life for patients with the condition, according to an industry-funded study.
Macitentan (Opsumit) remains tremendously expensive, costing as much as $100,000 per year in the United States, and the study provides little in the way of direct comparison to other drugs in its class. Still, the drug’s effects on quality of life are dramatic, said study lead author Sanjay Mehta, MD, FRCPC, FCCP, professor of medicine at the University of Western Ontario and director of the Southwest Ontario Pulmonary Hypertension Clinic at the London Health Sciences Center in London, Ont.
Researchers found that those who took the 10-mg dose, versus placebo, reported significant improvement in seven of eight quality-of-life domains, and in physical and mental components scores, as measured by the 36-item Short Form Health Survey (SF-36). In addition, the study linked 10-mg doses, versus placebo, to a lower risk of a decline of three points or more in the physical component score (hazard ratio [HR], 0.60; 95% CI, 0.47-0.76; P less than .0001] and the mental component scores (HR, 0.76; 95% CI, 0.61-0.95; P = .0173) until end of treatment.
“The drug has shown stability in patients’ quality of life over 6 months and 12 months,” Dr. Mehta said in an interview. “I can’t cure anybody, and they’ll get worse at some point, but I can improve them. They physically feel better, they’re less short of breath with less body pain, and they feel better psychologically.”
Macitentan, an endothelin receptor antagonist, received Food and Drug Administration approval in 2013 following a study that year (N Engl J Med. 2013 Aug 29;369[9]:809-18) that linked 10-mg doses to a significantly lower risk of death and various complications, compared with placebo and the 3-mg dose. The new study (Chest. 2017 Jan;151[1]:106-18), is an analysis of data from the 2013 study.
The PAH patients were randomly assigned to one of three groups: macitentan 10 mg once daily (234), macitentan 3 mg (237), and placebo (239). The study examined responses from 710 patients (76.9% were female, 55.2% were white, mean age was 45.5) to the SF-36 at baseline, 6 months, 12 months, and end of treatment.
Dr. Mehta noted that macitentan has not been clinically compared to the other drugs. The study, however, notes that it is the first PAH treatment to show improvement in seven of eight domains in the quality-of-life survey.
The new study was funded by Actelion Pharmaceuticals, maker of macitentan. Dr. Mehta has received consulting and speaking fees and institutional support for clinical trials from Actelion, among other drug companies. The other authors report various disclosures, including relationships with Actelion.
Macitentan, a recent addition to the drugs that treat pulmonary arterial hypertension (PAH), improves and stabilizes quality of life for patients with the condition, according to an industry-funded study.
Macitentan (Opsumit) remains tremendously expensive, costing as much as $100,000 per year in the United States, and the study provides little in the way of direct comparison to other drugs in its class. Still, the drug’s effects on quality of life are dramatic, said study lead author Sanjay Mehta, MD, FRCPC, FCCP, professor of medicine at the University of Western Ontario and director of the Southwest Ontario Pulmonary Hypertension Clinic at the London Health Sciences Center in London, Ont.
Researchers found that those who took the 10-mg dose, versus placebo, reported significant improvement in seven of eight quality-of-life domains, and in physical and mental components scores, as measured by the 36-item Short Form Health Survey (SF-36). In addition, the study linked 10-mg doses, versus placebo, to a lower risk of a decline of three points or more in the physical component score (hazard ratio [HR], 0.60; 95% CI, 0.47-0.76; P less than .0001] and the mental component scores (HR, 0.76; 95% CI, 0.61-0.95; P = .0173) until end of treatment.
“The drug has shown stability in patients’ quality of life over 6 months and 12 months,” Dr. Mehta said in an interview. “I can’t cure anybody, and they’ll get worse at some point, but I can improve them. They physically feel better, they’re less short of breath with less body pain, and they feel better psychologically.”
Macitentan, an endothelin receptor antagonist, received Food and Drug Administration approval in 2013 following a study that year (N Engl J Med. 2013 Aug 29;369[9]:809-18) that linked 10-mg doses to a significantly lower risk of death and various complications, compared with placebo and the 3-mg dose. The new study (Chest. 2017 Jan;151[1]:106-18), is an analysis of data from the 2013 study.
The PAH patients were randomly assigned to one of three groups: macitentan 10 mg once daily (234), macitentan 3 mg (237), and placebo (239). The study examined responses from 710 patients (76.9% were female, 55.2% were white, mean age was 45.5) to the SF-36 at baseline, 6 months, 12 months, and end of treatment.
Dr. Mehta noted that macitentan has not been clinically compared to the other drugs. The study, however, notes that it is the first PAH treatment to show improvement in seven of eight domains in the quality-of-life survey.
The new study was funded by Actelion Pharmaceuticals, maker of macitentan. Dr. Mehta has received consulting and speaking fees and institutional support for clinical trials from Actelion, among other drug companies. The other authors report various disclosures, including relationships with Actelion.
FROM CHEST
Key clinical point: Macitentan improves and stabilizes quality of life in patients with pulmonary arterial hypertension.
Major finding: Patients who took 10 mg daily macitentan improved in seven of eight quality-of-life domains and in combined physical and mental health measures.
Data source: Multicenter, double-blind, placebo-controlled, randomized phase III study of 710 patients (76.9% female, 55.2% white, mean age 45.5) assigned to placebo, macitentan 3 mg, or macitentan 10 mg once daily.
Disclosures: Actelion Pharmaceuticals, maker of macitentan, funded the study. The authors disclosed ties with Actelion.
CVS selling low-cost generic epinephrine autoinjector
CVS Pharmacy is currently selling a generic epinephrine autoinjector for a price of $109.99 per two-pack, which is about one-sixth the cost of Mylan’s EpiPen two-pack.
The product, an authorized generic for Adrenaclick, is manufactured by Lineage Therapeutics, which is a wholly owned subsidiary of Fort Washington, Pa.–based Impax Laboratories. CVS Pharmacy characterized the product as having “the lowest cash price in the market” and said in a Jan. 12 statement that the move was undertaken to address the “urgent need for a less-expensive epinephrine autoinjector.”
Data from a Kaiser Family Foundation analysis found that the average total Part D Medicare spending per EpiPen prescription increased nearly fivefold, from an average of $71 in 2007 to $344 in 2014. This trend continued, and in September 2016, Mylan’s CEO Heather Bresch faced questioning on Capitol Hill about the price hikes from members of the House Oversight Committee.
“We’re encouraged to see national efforts to make epinephrine autoinjectors more affordable and more available to Americans across the country,” Cary Sennett, MD, PhD, president and CEO of the Landover, Md.–based Asthma and Allergy Foundation of America, said in the CVS statement. “Partnerships that increase access to vital medications are key in helping those suffering from life-threatening allergies.”
CVS Pharmacy is currently selling a generic epinephrine autoinjector for a price of $109.99 per two-pack, which is about one-sixth the cost of Mylan’s EpiPen two-pack.
The product, an authorized generic for Adrenaclick, is manufactured by Lineage Therapeutics, which is a wholly owned subsidiary of Fort Washington, Pa.–based Impax Laboratories. CVS Pharmacy characterized the product as having “the lowest cash price in the market” and said in a Jan. 12 statement that the move was undertaken to address the “urgent need for a less-expensive epinephrine autoinjector.”
Data from a Kaiser Family Foundation analysis found that the average total Part D Medicare spending per EpiPen prescription increased nearly fivefold, from an average of $71 in 2007 to $344 in 2014. This trend continued, and in September 2016, Mylan’s CEO Heather Bresch faced questioning on Capitol Hill about the price hikes from members of the House Oversight Committee.
“We’re encouraged to see national efforts to make epinephrine autoinjectors more affordable and more available to Americans across the country,” Cary Sennett, MD, PhD, president and CEO of the Landover, Md.–based Asthma and Allergy Foundation of America, said in the CVS statement. “Partnerships that increase access to vital medications are key in helping those suffering from life-threatening allergies.”
CVS Pharmacy is currently selling a generic epinephrine autoinjector for a price of $109.99 per two-pack, which is about one-sixth the cost of Mylan’s EpiPen two-pack.
The product, an authorized generic for Adrenaclick, is manufactured by Lineage Therapeutics, which is a wholly owned subsidiary of Fort Washington, Pa.–based Impax Laboratories. CVS Pharmacy characterized the product as having “the lowest cash price in the market” and said in a Jan. 12 statement that the move was undertaken to address the “urgent need for a less-expensive epinephrine autoinjector.”
Data from a Kaiser Family Foundation analysis found that the average total Part D Medicare spending per EpiPen prescription increased nearly fivefold, from an average of $71 in 2007 to $344 in 2014. This trend continued, and in September 2016, Mylan’s CEO Heather Bresch faced questioning on Capitol Hill about the price hikes from members of the House Oversight Committee.
“We’re encouraged to see national efforts to make epinephrine autoinjectors more affordable and more available to Americans across the country,” Cary Sennett, MD, PhD, president and CEO of the Landover, Md.–based Asthma and Allergy Foundation of America, said in the CVS statement. “Partnerships that increase access to vital medications are key in helping those suffering from life-threatening allergies.”
Smoking-cessation interest and success vary by race, ethnicity
Just over 55% of adult cigarette smokers made an attempt to quit in the past year, and 7.4% said that they recently quit, according to investigators from the Centers or Disease Control and Prevention.
Data from the 2015 National Health Interview Survey (NHIS) show that 68% of cigarette smokers were interested in quitting, with considerable variation seen according to race and ethnicity (MMWR. 2017;65[52]:1457-64).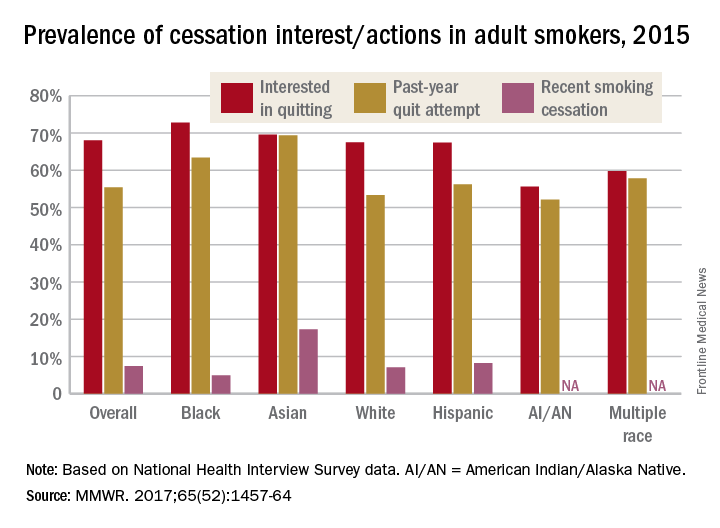
American Indian/Alaska Native smokers were the least likely to be interested in quitting (55.6%) and to have attempted to quit (52.1%), but the sample size was too small to report a reliable quit rate. The amount of survey participants of multiple races was also too small to report a reliable quit rate. Among that group, 59.8% were interested in quitting and 57.8% had attempted to quit in the past year, the NHIS data showed.
The sizes of surveyed populations for individual races and ethnicities were not reported, but the total sample size for the 2015 NHIS was 33,672.
Just over 55% of adult cigarette smokers made an attempt to quit in the past year, and 7.4% said that they recently quit, according to investigators from the Centers or Disease Control and Prevention.
Data from the 2015 National Health Interview Survey (NHIS) show that 68% of cigarette smokers were interested in quitting, with considerable variation seen according to race and ethnicity (MMWR. 2017;65[52]:1457-64).
American Indian/Alaska Native smokers were the least likely to be interested in quitting (55.6%) and to have attempted to quit (52.1%), but the sample size was too small to report a reliable quit rate. The amount of survey participants of multiple races was also too small to report a reliable quit rate. Among that group, 59.8% were interested in quitting and 57.8% had attempted to quit in the past year, the NHIS data showed.
The sizes of surveyed populations for individual races and ethnicities were not reported, but the total sample size for the 2015 NHIS was 33,672.
Just over 55% of adult cigarette smokers made an attempt to quit in the past year, and 7.4% said that they recently quit, according to investigators from the Centers or Disease Control and Prevention.
Data from the 2015 National Health Interview Survey (NHIS) show that 68% of cigarette smokers were interested in quitting, with considerable variation seen according to race and ethnicity (MMWR. 2017;65[52]:1457-64).
American Indian/Alaska Native smokers were the least likely to be interested in quitting (55.6%) and to have attempted to quit (52.1%), but the sample size was too small to report a reliable quit rate. The amount of survey participants of multiple races was also too small to report a reliable quit rate. Among that group, 59.8% were interested in quitting and 57.8% had attempted to quit in the past year, the NHIS data showed.
The sizes of surveyed populations for individual races and ethnicities were not reported, but the total sample size for the 2015 NHIS was 33,672.
Most cigarette smokers attempt to quit without evidence-based techniques
More than half of cigarette smokers have received advice to quit from a health care professional, but less than a third used medication or counseling in their cessation attempt, according to investigators from the Centers for Disease Control and Prevention.
In 2015, just over 57% of adult smokers said that a health care professional had advised them to quit in the past year. Of those who tried to quit, 29% used medication such as nicotine patches or gum, varenicline, or bupropion; 7% used counseling (including a stop-smoking clinic, class, or support group and a telephone help line); and 31% used counseling and/or medication, the investigators reported (MMWR 2017;65[52]:1457-64).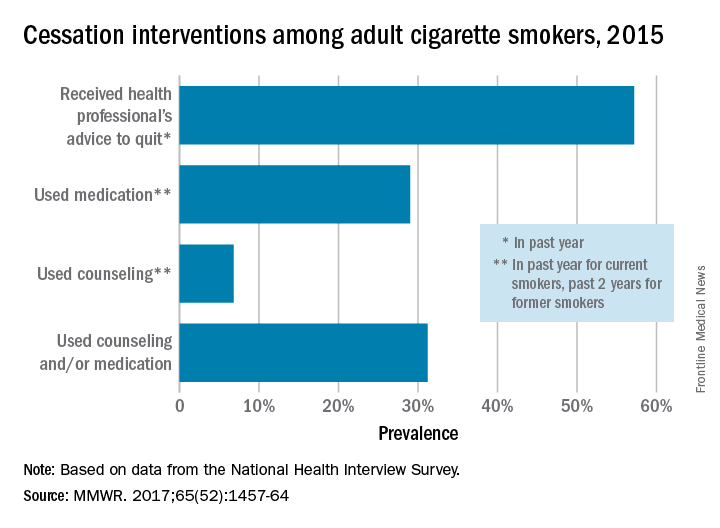
With the overall cessation rate at less than 10%, “it is critical for health care providers to consistently identify smokers, advise them to quit, and offer evidence-based cessation treatments, and for insurers to cover and promote the use of these treatments and remove barriers to accessing them,” the investigators wrote.
More than half of cigarette smokers have received advice to quit from a health care professional, but less than a third used medication or counseling in their cessation attempt, according to investigators from the Centers for Disease Control and Prevention.
In 2015, just over 57% of adult smokers said that a health care professional had advised them to quit in the past year. Of those who tried to quit, 29% used medication such as nicotine patches or gum, varenicline, or bupropion; 7% used counseling (including a stop-smoking clinic, class, or support group and a telephone help line); and 31% used counseling and/or medication, the investigators reported (MMWR 2017;65[52]:1457-64).
With the overall cessation rate at less than 10%, “it is critical for health care providers to consistently identify smokers, advise them to quit, and offer evidence-based cessation treatments, and for insurers to cover and promote the use of these treatments and remove barriers to accessing them,” the investigators wrote.
More than half of cigarette smokers have received advice to quit from a health care professional, but less than a third used medication or counseling in their cessation attempt, according to investigators from the Centers for Disease Control and Prevention.
In 2015, just over 57% of adult smokers said that a health care professional had advised them to quit in the past year. Of those who tried to quit, 29% used medication such as nicotine patches or gum, varenicline, or bupropion; 7% used counseling (including a stop-smoking clinic, class, or support group and a telephone help line); and 31% used counseling and/or medication, the investigators reported (MMWR 2017;65[52]:1457-64).
With the overall cessation rate at less than 10%, “it is critical for health care providers to consistently identify smokers, advise them to quit, and offer evidence-based cessation treatments, and for insurers to cover and promote the use of these treatments and remove barriers to accessing them,” the investigators wrote.
FROM MMWR
Streptococcal pneumonia’s resistance to macrolides increasing
The incidence of resistance of Streptococcus pneumoniae to the macrolide azithromycin – one of the most commonly prescribed antibiotics for treating pneumonia – was almost 50% in 2014, according to a report by Kara Keedy, PhD, executive director of microbiology at Cempra Pharmaceuticals, and her colleagues.
The researchers prospectively collected and investigated 4,567 nonreplicative community-acquired bacterial pneumonia (CABP) S. pneumoniae isolates between 2008 and 2014 in the United States, according to the report presented as a poster at IDWeek 2016. The isolates were tested for susceptibility by broth microdilution methods, according to Clinical and Laboratory Standards Institute breakpoint criteria. Macrolide resistance rates were based on azithromycin and/or clarithromycin minimal inhibitory concentrations as available, with only data on azithromycin having been collected in 2014.
The overall resistance of S. pneumoniae to azithromycin exceeded 30% in all of the nine geographical divisions of the Centers for Disease Control and Prevention (CDC), with the high-level resistance of this bacterial cause of CABP to azithromycin having been greater than 25% in eight of the CDC divisions.
The co-resistance of S. pneumoniae to azithromycin and penicillin was highest in the CDC’s East South Central division in 2014. The regions with the largest percentages of isolates with high-level macrolide resistance were the East South Central (43.2%), the West South Central (38.1%), and the Mid-Atlantic (35.0%). The regions with the largest percentages of overall macrolide resistance were the West South Central (62.9%), the East South Central (56.8%), and the South Atlantic (53.2%).
The analysis also determined that the 2014 overall rate of macrolide resistance in S. pneumoniae in the United States of 48.4% is higher than it was for any of the four earlier years examined. In 2008, 2009, 2010, and 2011, those macrolide resistance rates were 39.7%, 40.2%, 37.1%, and 44.3%, respectively.
The researchers concluded that S. pneumoniae is the most common bacterial cause of CABP and that antibiotic resistance to it is “a significant clinical challenge as highlighted by” the CDC having listed it as a threatening pathogen in the urgent category. Dr. Keedy and her associates noted that in the United States, macrolides, amoxicillin/clavulanate, and respiratory fluoroquinolones are the most frequent agents prescribed to treat almost all community-acquired respiratory infections.
“Macrolide resistance in S. pneumoniae is continuing to increase in the U.S.,” the researchers reported in the poster. “Both low- and high-level macrolide resistance have been reported to cause clinical failures and other negative outcomes including longer hospital stays and higher costs.”
The study also examined the abilities of several other drugs, including the fourth-generation macrolide solithromycin, to inhibit S. pneumoniae isolates. Solithromycin does not yet have approved Clinical and Laboratory Standards Institute breakpoints, so only minimum inhibitory concentrations (MICs) were presented.
According to the study, more than 50% of S. pneumoniae isolates were inhibited by 0.008 mcg/mL solithromycin. Additionally, solithromycin had one of the lowest MICs against S. pneumoniae of all of the drugs tested in the study. The higher end of the MICs against S. pneumoniae for solithromycin and moxifloxacin was 0.25, which was lower than the higher end of the MICs for any of the other drugs tested against S. pneumoniae isolates.
Solithromycin is the first fluoroketolide in Phase III clinical development. It “shows activity against all macrolide-resistant strains of S. pneumoniae isolates, irrespective of the location in the U.S.,” according to the poster.
The data included in the poster was extracted from a global study by JMI Laboratories. Cempra funded this study. Dr. Keedy and the other authors of the poster are employees of Cempra.
How many of us have heard that line? How many of us have done that ourselves? Did you do that today? Dr. Keedy and her colleagues report that in all geographic areas in the US, resistance to azithromycin for S pneumoniae now exceeds 30%. On average, 48.4% of S pneumoniae isolates display resistance in the US. Without antibiotic stewardship by all of us, azithromycin, along with other antibiotics, will become an expensive placebo.
How many of us have heard that line? How many of us have done that ourselves? Did you do that today? Dr. Keedy and her colleagues report that in all geographic areas in the US, resistance to azithromycin for S pneumoniae now exceeds 30%. On average, 48.4% of S pneumoniae isolates display resistance in the US. Without antibiotic stewardship by all of us, azithromycin, along with other antibiotics, will become an expensive placebo.
How many of us have heard that line? How many of us have done that ourselves? Did you do that today? Dr. Keedy and her colleagues report that in all geographic areas in the US, resistance to azithromycin for S pneumoniae now exceeds 30%. On average, 48.4% of S pneumoniae isolates display resistance in the US. Without antibiotic stewardship by all of us, azithromycin, along with other antibiotics, will become an expensive placebo.
The incidence of resistance of Streptococcus pneumoniae to the macrolide azithromycin – one of the most commonly prescribed antibiotics for treating pneumonia – was almost 50% in 2014, according to a report by Kara Keedy, PhD, executive director of microbiology at Cempra Pharmaceuticals, and her colleagues.
The researchers prospectively collected and investigated 4,567 nonreplicative community-acquired bacterial pneumonia (CABP) S. pneumoniae isolates between 2008 and 2014 in the United States, according to the report presented as a poster at IDWeek 2016. The isolates were tested for susceptibility by broth microdilution methods, according to Clinical and Laboratory Standards Institute breakpoint criteria. Macrolide resistance rates were based on azithromycin and/or clarithromycin minimal inhibitory concentrations as available, with only data on azithromycin having been collected in 2014.
The overall resistance of S. pneumoniae to azithromycin exceeded 30% in all of the nine geographical divisions of the Centers for Disease Control and Prevention (CDC), with the high-level resistance of this bacterial cause of CABP to azithromycin having been greater than 25% in eight of the CDC divisions.
The co-resistance of S. pneumoniae to azithromycin and penicillin was highest in the CDC’s East South Central division in 2014. The regions with the largest percentages of isolates with high-level macrolide resistance were the East South Central (43.2%), the West South Central (38.1%), and the Mid-Atlantic (35.0%). The regions with the largest percentages of overall macrolide resistance were the West South Central (62.9%), the East South Central (56.8%), and the South Atlantic (53.2%).
The analysis also determined that the 2014 overall rate of macrolide resistance in S. pneumoniae in the United States of 48.4% is higher than it was for any of the four earlier years examined. In 2008, 2009, 2010, and 2011, those macrolide resistance rates were 39.7%, 40.2%, 37.1%, and 44.3%, respectively.
The researchers concluded that S. pneumoniae is the most common bacterial cause of CABP and that antibiotic resistance to it is “a significant clinical challenge as highlighted by” the CDC having listed it as a threatening pathogen in the urgent category. Dr. Keedy and her associates noted that in the United States, macrolides, amoxicillin/clavulanate, and respiratory fluoroquinolones are the most frequent agents prescribed to treat almost all community-acquired respiratory infections.
“Macrolide resistance in S. pneumoniae is continuing to increase in the U.S.,” the researchers reported in the poster. “Both low- and high-level macrolide resistance have been reported to cause clinical failures and other negative outcomes including longer hospital stays and higher costs.”
The study also examined the abilities of several other drugs, including the fourth-generation macrolide solithromycin, to inhibit S. pneumoniae isolates. Solithromycin does not yet have approved Clinical and Laboratory Standards Institute breakpoints, so only minimum inhibitory concentrations (MICs) were presented.
According to the study, more than 50% of S. pneumoniae isolates were inhibited by 0.008 mcg/mL solithromycin. Additionally, solithromycin had one of the lowest MICs against S. pneumoniae of all of the drugs tested in the study. The higher end of the MICs against S. pneumoniae for solithromycin and moxifloxacin was 0.25, which was lower than the higher end of the MICs for any of the other drugs tested against S. pneumoniae isolates.
Solithromycin is the first fluoroketolide in Phase III clinical development. It “shows activity against all macrolide-resistant strains of S. pneumoniae isolates, irrespective of the location in the U.S.,” according to the poster.
The data included in the poster was extracted from a global study by JMI Laboratories. Cempra funded this study. Dr. Keedy and the other authors of the poster are employees of Cempra.
The incidence of resistance of Streptococcus pneumoniae to the macrolide azithromycin – one of the most commonly prescribed antibiotics for treating pneumonia – was almost 50% in 2014, according to a report by Kara Keedy, PhD, executive director of microbiology at Cempra Pharmaceuticals, and her colleagues.
The researchers prospectively collected and investigated 4,567 nonreplicative community-acquired bacterial pneumonia (CABP) S. pneumoniae isolates between 2008 and 2014 in the United States, according to the report presented as a poster at IDWeek 2016. The isolates were tested for susceptibility by broth microdilution methods, according to Clinical and Laboratory Standards Institute breakpoint criteria. Macrolide resistance rates were based on azithromycin and/or clarithromycin minimal inhibitory concentrations as available, with only data on azithromycin having been collected in 2014.
The overall resistance of S. pneumoniae to azithromycin exceeded 30% in all of the nine geographical divisions of the Centers for Disease Control and Prevention (CDC), with the high-level resistance of this bacterial cause of CABP to azithromycin having been greater than 25% in eight of the CDC divisions.
The co-resistance of S. pneumoniae to azithromycin and penicillin was highest in the CDC’s East South Central division in 2014. The regions with the largest percentages of isolates with high-level macrolide resistance were the East South Central (43.2%), the West South Central (38.1%), and the Mid-Atlantic (35.0%). The regions with the largest percentages of overall macrolide resistance were the West South Central (62.9%), the East South Central (56.8%), and the South Atlantic (53.2%).
The analysis also determined that the 2014 overall rate of macrolide resistance in S. pneumoniae in the United States of 48.4% is higher than it was for any of the four earlier years examined. In 2008, 2009, 2010, and 2011, those macrolide resistance rates were 39.7%, 40.2%, 37.1%, and 44.3%, respectively.
The researchers concluded that S. pneumoniae is the most common bacterial cause of CABP and that antibiotic resistance to it is “a significant clinical challenge as highlighted by” the CDC having listed it as a threatening pathogen in the urgent category. Dr. Keedy and her associates noted that in the United States, macrolides, amoxicillin/clavulanate, and respiratory fluoroquinolones are the most frequent agents prescribed to treat almost all community-acquired respiratory infections.
“Macrolide resistance in S. pneumoniae is continuing to increase in the U.S.,” the researchers reported in the poster. “Both low- and high-level macrolide resistance have been reported to cause clinical failures and other negative outcomes including longer hospital stays and higher costs.”
The study also examined the abilities of several other drugs, including the fourth-generation macrolide solithromycin, to inhibit S. pneumoniae isolates. Solithromycin does not yet have approved Clinical and Laboratory Standards Institute breakpoints, so only minimum inhibitory concentrations (MICs) were presented.
According to the study, more than 50% of S. pneumoniae isolates were inhibited by 0.008 mcg/mL solithromycin. Additionally, solithromycin had one of the lowest MICs against S. pneumoniae of all of the drugs tested in the study. The higher end of the MICs against S. pneumoniae for solithromycin and moxifloxacin was 0.25, which was lower than the higher end of the MICs for any of the other drugs tested against S. pneumoniae isolates.
Solithromycin is the first fluoroketolide in Phase III clinical development. It “shows activity against all macrolide-resistant strains of S. pneumoniae isolates, irrespective of the location in the U.S.,” according to the poster.
The data included in the poster was extracted from a global study by JMI Laboratories. Cempra funded this study. Dr. Keedy and the other authors of the poster are employees of Cempra.
FROM IDWEEK 2016
Key clinical point:
Major finding: S. pneumoniae isolates’ average resistance to the macrolide azithromycin was 48.4% in 2014.
Data source: A prospective collection and investigation of 4,567 non-replicative community-acquired bacterial pneumonia isolates.
Disclosures: The data included in the poster was extracted from a global study by JMI Laboratories. Cempra funded this study. Dr. Keedy and the other authors of the poster are employees of Cempra.
Giant cell arteritis independently raises risk for venous thromboembolism
The risk of venous thromboembolism increases markedly shortly before the diagnosis of giant cell arteritis regardless of glucocorticoid exposure, peaks at the time of diagnosis, and then progressively declines, according to a matched cohort review involving more than 6,000 arteritis patients.
It’s not been clear until now if the recently recognized risk of venous thromboembolism (VTE) in giant cell arteritis (GCA) was due to the disease itself, or the glucocorticoids used to treat it. “Because inflammation in GCA spares the venous circulation, our finding that patients are at greatest risk of VTE in the period surrounding GCA diagnosis (when inflammation is at its highest level), and the demonstration that this risk is not associated with the use of glucocorticoids, suggest that immunothrombosis could play a pathogenic role,” said investigators led by Sebastian Unizony, MD, of Massachusetts General Hospital, Boston (Arthritis Rheumatol. 2017 Jan;69[1]:176-84).
The report was short on advice about what to do to prevent VTE in GCA, but the investigators did recommend “adequate monitoring ... for early recognition of this potentially serious complication.”
The team used a British medical record database covering 1990-2013 to compare 6,441 patients with new-onset GCA to 63,985 controls without GCA matched for age, sex, and date of study entry. VTE was defined as pulmonary embolism and/or deep vein thrombosis.
The incidence of VTE shortly before diagnosis was 4.2 cases per 1,000 person-years in the GCA group, but 2.3 cases per 1,000 person-years among controls. It was about the same when the analysis was limited to GCA patients not exposed to oral glucocorticoids before diagnosis: 4.0 cases versus 2.2 cases in the control group per 1,000 person-years. The finding was key to the conclusion that GCA is an independent VTE risk factor.
During the 12, 9, 6, and 3 months leading up to GCA diagnosis, the relative risks for VTE among patients not treated with glucocorticoids – versus controls – were 1.8, 2.2, 2.4, and 3.6. In the first 3, 6, 12, 24, 48, and 96 months after GCA diagnosis, when virtually all patients were on glucocorticoids at least for the first 6 months, the relative risks for VTE were 9.9, 7.7, 5.9, 4.4, 3.3, 2.4; the last risk score of 2.4 indicated that GCA patients were still slightly more likely than controls to have a VTE even 8 years after diagnosis.
The mean age of patients in the study was 73 years, and 70% of the subjects were women. GCA patients were more likely than were controls to be smokers and to have cardiovascular disease. Also, a greater proportion of GCA patients used aspirin and had recent surgery and hospitalizations. There was no difference in body mass index (mean in both groups 27 kg/m2) or the prevalence of fracture, trauma, or cancer between the groups.
The National Institutes of Health funded the work. There was no disclosure information in the report.
Whether these findings have implications for treatment is unclear. Should a patient with GCA who sustains a VTE early in the course of disease receive anticoagulation short term, with the thought that the VTE was provoked by a risk factor that has been neutralized? Or should treatment be long term, out of concern that the risk factor is still present? Does this finding bear on the controversial question of whether a patient with GCA should receive aspirin?
[A] robust finding of the analysis is that the risk of a first VTE declines steadily over at least the first 2 years after diagnosis of GCA. However, the problem of distinguishing the effects of disease from the effects of treatment has returned. All patients with GCA are now receiving corticosteroids, at least during the period of very high risk in the first 6 months after diagnosis, and one can expect that the average severity of inflammation and average dose of prednisone/prednisolone will decline in parallel. The steadily declining risk of VTE for at least 1 year after diagnosis suggests that both GCA and corticosteroids increase the risk of VTE. It remains impossible to prove or disprove that hypothesis or to estimate the independent risks conferred by the disease and its treatment.
Having GCA probably increases the risk of VTE at least for the first 24 months after diagnosis and the beginning of treatment, but after 24 months, it is unclear. The clinician will still need to make a guess regarding duration of anticoagulation.
Paul Monach, MD, PhD, is a vasculitis specialist at Boston University. He made his comments in an accompanying editorial (Arthritis Rheumatol. 2017 Jan;69[1]:3-5).
Whether these findings have implications for treatment is unclear. Should a patient with GCA who sustains a VTE early in the course of disease receive anticoagulation short term, with the thought that the VTE was provoked by a risk factor that has been neutralized? Or should treatment be long term, out of concern that the risk factor is still present? Does this finding bear on the controversial question of whether a patient with GCA should receive aspirin?
[A] robust finding of the analysis is that the risk of a first VTE declines steadily over at least the first 2 years after diagnosis of GCA. However, the problem of distinguishing the effects of disease from the effects of treatment has returned. All patients with GCA are now receiving corticosteroids, at least during the period of very high risk in the first 6 months after diagnosis, and one can expect that the average severity of inflammation and average dose of prednisone/prednisolone will decline in parallel. The steadily declining risk of VTE for at least 1 year after diagnosis suggests that both GCA and corticosteroids increase the risk of VTE. It remains impossible to prove or disprove that hypothesis or to estimate the independent risks conferred by the disease and its treatment.
Having GCA probably increases the risk of VTE at least for the first 24 months after diagnosis and the beginning of treatment, but after 24 months, it is unclear. The clinician will still need to make a guess regarding duration of anticoagulation.
Paul Monach, MD, PhD, is a vasculitis specialist at Boston University. He made his comments in an accompanying editorial (Arthritis Rheumatol. 2017 Jan;69[1]:3-5).
Whether these findings have implications for treatment is unclear. Should a patient with GCA who sustains a VTE early in the course of disease receive anticoagulation short term, with the thought that the VTE was provoked by a risk factor that has been neutralized? Or should treatment be long term, out of concern that the risk factor is still present? Does this finding bear on the controversial question of whether a patient with GCA should receive aspirin?
[A] robust finding of the analysis is that the risk of a first VTE declines steadily over at least the first 2 years after diagnosis of GCA. However, the problem of distinguishing the effects of disease from the effects of treatment has returned. All patients with GCA are now receiving corticosteroids, at least during the period of very high risk in the first 6 months after diagnosis, and one can expect that the average severity of inflammation and average dose of prednisone/prednisolone will decline in parallel. The steadily declining risk of VTE for at least 1 year after diagnosis suggests that both GCA and corticosteroids increase the risk of VTE. It remains impossible to prove or disprove that hypothesis or to estimate the independent risks conferred by the disease and its treatment.
Having GCA probably increases the risk of VTE at least for the first 24 months after diagnosis and the beginning of treatment, but after 24 months, it is unclear. The clinician will still need to make a guess regarding duration of anticoagulation.
Paul Monach, MD, PhD, is a vasculitis specialist at Boston University. He made his comments in an accompanying editorial (Arthritis Rheumatol. 2017 Jan;69[1]:3-5).
The risk of venous thromboembolism increases markedly shortly before the diagnosis of giant cell arteritis regardless of glucocorticoid exposure, peaks at the time of diagnosis, and then progressively declines, according to a matched cohort review involving more than 6,000 arteritis patients.
It’s not been clear until now if the recently recognized risk of venous thromboembolism (VTE) in giant cell arteritis (GCA) was due to the disease itself, or the glucocorticoids used to treat it. “Because inflammation in GCA spares the venous circulation, our finding that patients are at greatest risk of VTE in the period surrounding GCA diagnosis (when inflammation is at its highest level), and the demonstration that this risk is not associated with the use of glucocorticoids, suggest that immunothrombosis could play a pathogenic role,” said investigators led by Sebastian Unizony, MD, of Massachusetts General Hospital, Boston (Arthritis Rheumatol. 2017 Jan;69[1]:176-84).
The report was short on advice about what to do to prevent VTE in GCA, but the investigators did recommend “adequate monitoring ... for early recognition of this potentially serious complication.”
The team used a British medical record database covering 1990-2013 to compare 6,441 patients with new-onset GCA to 63,985 controls without GCA matched for age, sex, and date of study entry. VTE was defined as pulmonary embolism and/or deep vein thrombosis.
The incidence of VTE shortly before diagnosis was 4.2 cases per 1,000 person-years in the GCA group, but 2.3 cases per 1,000 person-years among controls. It was about the same when the analysis was limited to GCA patients not exposed to oral glucocorticoids before diagnosis: 4.0 cases versus 2.2 cases in the control group per 1,000 person-years. The finding was key to the conclusion that GCA is an independent VTE risk factor.
During the 12, 9, 6, and 3 months leading up to GCA diagnosis, the relative risks for VTE among patients not treated with glucocorticoids – versus controls – were 1.8, 2.2, 2.4, and 3.6. In the first 3, 6, 12, 24, 48, and 96 months after GCA diagnosis, when virtually all patients were on glucocorticoids at least for the first 6 months, the relative risks for VTE were 9.9, 7.7, 5.9, 4.4, 3.3, 2.4; the last risk score of 2.4 indicated that GCA patients were still slightly more likely than controls to have a VTE even 8 years after diagnosis.
The mean age of patients in the study was 73 years, and 70% of the subjects were women. GCA patients were more likely than were controls to be smokers and to have cardiovascular disease. Also, a greater proportion of GCA patients used aspirin and had recent surgery and hospitalizations. There was no difference in body mass index (mean in both groups 27 kg/m2) or the prevalence of fracture, trauma, or cancer between the groups.
The National Institutes of Health funded the work. There was no disclosure information in the report.
The risk of venous thromboembolism increases markedly shortly before the diagnosis of giant cell arteritis regardless of glucocorticoid exposure, peaks at the time of diagnosis, and then progressively declines, according to a matched cohort review involving more than 6,000 arteritis patients.
It’s not been clear until now if the recently recognized risk of venous thromboembolism (VTE) in giant cell arteritis (GCA) was due to the disease itself, or the glucocorticoids used to treat it. “Because inflammation in GCA spares the venous circulation, our finding that patients are at greatest risk of VTE in the period surrounding GCA diagnosis (when inflammation is at its highest level), and the demonstration that this risk is not associated with the use of glucocorticoids, suggest that immunothrombosis could play a pathogenic role,” said investigators led by Sebastian Unizony, MD, of Massachusetts General Hospital, Boston (Arthritis Rheumatol. 2017 Jan;69[1]:176-84).
The report was short on advice about what to do to prevent VTE in GCA, but the investigators did recommend “adequate monitoring ... for early recognition of this potentially serious complication.”
The team used a British medical record database covering 1990-2013 to compare 6,441 patients with new-onset GCA to 63,985 controls without GCA matched for age, sex, and date of study entry. VTE was defined as pulmonary embolism and/or deep vein thrombosis.
The incidence of VTE shortly before diagnosis was 4.2 cases per 1,000 person-years in the GCA group, but 2.3 cases per 1,000 person-years among controls. It was about the same when the analysis was limited to GCA patients not exposed to oral glucocorticoids before diagnosis: 4.0 cases versus 2.2 cases in the control group per 1,000 person-years. The finding was key to the conclusion that GCA is an independent VTE risk factor.
During the 12, 9, 6, and 3 months leading up to GCA diagnosis, the relative risks for VTE among patients not treated with glucocorticoids – versus controls – were 1.8, 2.2, 2.4, and 3.6. In the first 3, 6, 12, 24, 48, and 96 months after GCA diagnosis, when virtually all patients were on glucocorticoids at least for the first 6 months, the relative risks for VTE were 9.9, 7.7, 5.9, 4.4, 3.3, 2.4; the last risk score of 2.4 indicated that GCA patients were still slightly more likely than controls to have a VTE even 8 years after diagnosis.
The mean age of patients in the study was 73 years, and 70% of the subjects were women. GCA patients were more likely than were controls to be smokers and to have cardiovascular disease. Also, a greater proportion of GCA patients used aspirin and had recent surgery and hospitalizations. There was no difference in body mass index (mean in both groups 27 kg/m2) or the prevalence of fracture, trauma, or cancer between the groups.
The National Institutes of Health funded the work. There was no disclosure information in the report.
FROM ARTHRITIS & RHEUMATOLOGY
Key clinical point:
Major finding: In the 12, 9, 6, and 3 months before GCA diagnosis, the relative risks for VTE among patients not treated with glucocorticoids – versus controls without GCA – were 1.8, 2.2, 2.4, and 3.6.
Data source: Matched cohort review involving more than 6,000 arteritis patients.
Disclosures: The National Institutes of Health funded the work. There was no disclosure information in the report.