User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
Powered by CHEST Physician, Clinician Reviews, MDedge Family Medicine, Internal Medicine News, and The Journal of Clinical Outcomes Management.
Osteopenia risk up in men with sarcopenia and COPD
Men experiencing sarcopenia who also have been diagnosed with chronic obstructive pulmonary disease (COPD) are at a significantly higher risk of developing osteopenia and osteoporosis than are men who do not suffer from COPD, according to a new study published in Chest.
“Muscle depletion has been considered a risk factor for low [bone mineral density (BMD)] in the healthy general population [but] data on the association between sarcopenia and osteopenia/osteoporosis in COPD patients are lacking,” wrote the investigators of the study, coauthored by Moo Suk Park, MD, of Yonsei University in Seoul, South Korea (Chest. 2017 Jan. doi: 10.1016/j.chest.2016.12.006).
“Although previous studies showed that loss of fat-free mass (FFM) was related to BMD loss in COPD patients, it is difficult to know the genuine relationship between skeletal muscle mass and BMD because whole body FFM contains a large proportion of water-retaining organs and nonmuscle soft tissue,” the authors continued.
The investigators examined data from the Korean National Health and Nutritional Examination Survey (KNHANES), looking for men at least 20 years of age with COPD who had both pulmonary function test and the dual-energy x-ray absorptiometry (DXA) performed on them during the years 2008-2011. A total of 864 men were deemed eligible for inclusion, and were scored for sarcopenia and osteopenia/osteoporosis; the former was assessed via the appendicular skeletal mass index (ASMI), with the latter done via T-score.
“Sarcopenia and presarcopenia were defined according to the presence of ASMI values that were less than two standard deviations (SDs) and between 2SDs and 1SD, respectively, below the mean value of a young male reference group aged 20-39 years,” according to the investigators. “Osteoporosis, osteopenia, and normal BMD were identified according to the lowest T-score of the three measured locations and were defined according to the World Health Organization criteria.”
“This study affirms the systemic nature of COPD, as it is not merely a disease that manifests as breathlessness and other respiratory complaints, but affects many aspects of a patient’s functionality and overall health,” explained Eric J. Gartman, MD, of Brown University, Providence, Rhode Island. “In clinical practice, this study reminds us that we need to consider these other issues in a COPD patient’s care, since the outcomes from these problems (e.g. hip fractures) can be devastating.”
A critical limitation of this study, however, is the sample population, according to Dr. Gartman. “It is solely made up of Korean men, thus somewhat limiting the generalizability to a larger population [and] especially to women, given that there are several other considerations surrounding effects on BMD.”
No funding sources were disclosed. The authors reported no conflicts of interest.
*This article was updated on 1/20/17 at 1:30 p.m. It misstated the affiliation for Vera Palo, MD, FCCP.
Men experiencing sarcopenia who also have been diagnosed with chronic obstructive pulmonary disease (COPD) are at a significantly higher risk of developing osteopenia and osteoporosis than are men who do not suffer from COPD, according to a new study published in Chest.
“Muscle depletion has been considered a risk factor for low [bone mineral density (BMD)] in the healthy general population [but] data on the association between sarcopenia and osteopenia/osteoporosis in COPD patients are lacking,” wrote the investigators of the study, coauthored by Moo Suk Park, MD, of Yonsei University in Seoul, South Korea (Chest. 2017 Jan. doi: 10.1016/j.chest.2016.12.006).
“Although previous studies showed that loss of fat-free mass (FFM) was related to BMD loss in COPD patients, it is difficult to know the genuine relationship between skeletal muscle mass and BMD because whole body FFM contains a large proportion of water-retaining organs and nonmuscle soft tissue,” the authors continued.
The investigators examined data from the Korean National Health and Nutritional Examination Survey (KNHANES), looking for men at least 20 years of age with COPD who had both pulmonary function test and the dual-energy x-ray absorptiometry (DXA) performed on them during the years 2008-2011. A total of 864 men were deemed eligible for inclusion, and were scored for sarcopenia and osteopenia/osteoporosis; the former was assessed via the appendicular skeletal mass index (ASMI), with the latter done via T-score.
“Sarcopenia and presarcopenia were defined according to the presence of ASMI values that were less than two standard deviations (SDs) and between 2SDs and 1SD, respectively, below the mean value of a young male reference group aged 20-39 years,” according to the investigators. “Osteoporosis, osteopenia, and normal BMD were identified according to the lowest T-score of the three measured locations and were defined according to the World Health Organization criteria.”
“This study affirms the systemic nature of COPD, as it is not merely a disease that manifests as breathlessness and other respiratory complaints, but affects many aspects of a patient’s functionality and overall health,” explained Eric J. Gartman, MD, of Brown University, Providence, Rhode Island. “In clinical practice, this study reminds us that we need to consider these other issues in a COPD patient’s care, since the outcomes from these problems (e.g. hip fractures) can be devastating.”
A critical limitation of this study, however, is the sample population, according to Dr. Gartman. “It is solely made up of Korean men, thus somewhat limiting the generalizability to a larger population [and] especially to women, given that there are several other considerations surrounding effects on BMD.”
No funding sources were disclosed. The authors reported no conflicts of interest.
*This article was updated on 1/20/17 at 1:30 p.m. It misstated the affiliation for Vera Palo, MD, FCCP.
Men experiencing sarcopenia who also have been diagnosed with chronic obstructive pulmonary disease (COPD) are at a significantly higher risk of developing osteopenia and osteoporosis than are men who do not suffer from COPD, according to a new study published in Chest.
“Muscle depletion has been considered a risk factor for low [bone mineral density (BMD)] in the healthy general population [but] data on the association between sarcopenia and osteopenia/osteoporosis in COPD patients are lacking,” wrote the investigators of the study, coauthored by Moo Suk Park, MD, of Yonsei University in Seoul, South Korea (Chest. 2017 Jan. doi: 10.1016/j.chest.2016.12.006).
“Although previous studies showed that loss of fat-free mass (FFM) was related to BMD loss in COPD patients, it is difficult to know the genuine relationship between skeletal muscle mass and BMD because whole body FFM contains a large proportion of water-retaining organs and nonmuscle soft tissue,” the authors continued.
The investigators examined data from the Korean National Health and Nutritional Examination Survey (KNHANES), looking for men at least 20 years of age with COPD who had both pulmonary function test and the dual-energy x-ray absorptiometry (DXA) performed on them during the years 2008-2011. A total of 864 men were deemed eligible for inclusion, and were scored for sarcopenia and osteopenia/osteoporosis; the former was assessed via the appendicular skeletal mass index (ASMI), with the latter done via T-score.
“Sarcopenia and presarcopenia were defined according to the presence of ASMI values that were less than two standard deviations (SDs) and between 2SDs and 1SD, respectively, below the mean value of a young male reference group aged 20-39 years,” according to the investigators. “Osteoporosis, osteopenia, and normal BMD were identified according to the lowest T-score of the three measured locations and were defined according to the World Health Organization criteria.”
“This study affirms the systemic nature of COPD, as it is not merely a disease that manifests as breathlessness and other respiratory complaints, but affects many aspects of a patient’s functionality and overall health,” explained Eric J. Gartman, MD, of Brown University, Providence, Rhode Island. “In clinical practice, this study reminds us that we need to consider these other issues in a COPD patient’s care, since the outcomes from these problems (e.g. hip fractures) can be devastating.”
A critical limitation of this study, however, is the sample population, according to Dr. Gartman. “It is solely made up of Korean men, thus somewhat limiting the generalizability to a larger population [and] especially to women, given that there are several other considerations surrounding effects on BMD.”
No funding sources were disclosed. The authors reported no conflicts of interest.
*This article was updated on 1/20/17 at 1:30 p.m. It misstated the affiliation for Vera Palo, MD, FCCP.
FROM CHEST
Key clinical point:
Major finding: Sarcopenia in men with COPD carried a significantly higher risk of bone mineral density loss: OR = 2.31 (95% CI 1.53–3.46) (P less than .001).
Data source: Retrospective cross-sectional study of data on 777 men with COPD during 2008-2011.
Disclosures: No funding sources were disclosed. The authors reported no conflicts of interest.
NIAID panel: Introduce peanut foods early to cut allergy risk
Introducing peanut foods to children who are at different levels of risk for peanut allergies may prevent or mitigate the risk, and the strategies for clinicians are explained in new addendum guidelines issued by an expert panel sponsored by the National Institute of Allergy and Infectious Diseases.
The guidelines were published online Jan. 5 in the Journal of Allergy and Clinical Immunology (J Allergy Clin Immunol. 2017. doi: 10.1016/j.jaci.2016.10.010).
“In the majority of patients, peanut allergy begins early in life and persists as a lifelong problem,” wrote lead author Alkis Togias, MD, of NIAID in Bethesda, Md., and colleagues. Previous guidelines published in 2010 did not provide specific treatment strategies for peanut allergies because of a lack of research, but the significant results of the Learning Early About Peanut Allergy (LEAP) study suggested that early exposure to peanut-containing foods reduces the risk of developing allergies.
The NIAID’s Guidelines Coordinating Committee conducted a literature review covering research from January 2010 to June 2016 and developed addendum guidelines, as follows:
For infants with severe eczema, egg allergies, or both, peanut-containing foods should be introduced at 4-6 months of age at the earliest, after the introduction of other solid foods to confirm developmental readiness. If the infant is developmentally ready for solids, clinicians should “strongly consider” evaluation by peanut-specific IgE (peanut sIgE) measurement and/or skin prick test before introducing peanut products to determine the potential sensitivity and need for supervised feeding vs. feeding at home.
If dietary peanut will be introduced based on the recommendations, “the total amount of peanut protein to be regularly consumed per week should be approximately 6 to 7 g over 3 or more feedings,” the authors wrote.
However, children already identified as allergic to peanut should practice strict peanut avoidance, they added. In addition, they recommend that clinicians review risks and benefits for high-risk children who may have family members with established peanut allergies.
For infants with mild to moderate eczema, the recommendation is to introduce peanut-containing foods at approximately 6 months of age, “in accordance with family preferences and cultural practices,” after the introduction of other solid foods, to help reduce the risk of peanut allergies. The expert panel recommends that infants in this moderate-risk category may receive peanut foods at home without an office visit, although caregivers or clinicians may choose an office visit for supervised feeding, evaluation, or both. Although the LEAP trial did not target infants with mild or moderate eczema, the panel has no reason to believe that the protective mechanisms are different in these children.
For infants with no eczema or any food allergies, the guidelines recommend introducing peanut-containing foods at any age, as appropriate and in keeping with a family’s preferences and cultural practices.
“The early introduction of dietary peanut in children without risk factors for peanut allergy is generally anticipated to be safe and to contribute modestly to an overall reduction in the prevalence of peanut allergy,” the researchers said.
The findings of the LEAP and accompanying LEAP-On trials were so compelling (approximately 80% relative reduction in peanut allergy at 5 years of age for peanut-exposed children, compared with standard of care) that the NIAID and expert panel “felt it was necessary to review and revise the previous recommendations from the 2010 guidelines on the diagnosis and management of food allergy,” Hugh Sampson, MD, director of the Jaffe Food Allergy Institute at the Icahn School of Medicine at Mount Sinai, New York, and a member of the panel, said in an interview.
“It is critical that pediatricians and family practitioners identify infants at high risk for developing peanut allergy (severe atopic dermatitis or egg allergy) between 4 and 6 months of age, evaluate them, or refer them to a food allergy specialist when necessary,” Dr. Sampson said.
“Have parents introduce peanut into the infant’s diet on a regular basis. It is important for parents to notify their pediatrician or family physician if they suspect their infant is at high risk for developing peanut allergy,” he added. “Also, once early peanut introduction is started, it is important that parents continue to provide peanut on a regular basis for several years.”
Next steps for research include pursuing other allergens, said Dr. Sampson. “Similar studies need to be done to determine if early introduction of other foods, such as milk, egg, [or] tree nuts will prevent these common food allergies in high-risk infants.”
Also, it will be important to study whether infants at mild to moderate risk for developing peanut or other food allergies as evidenced by mild to moderate eczema will experience the same benefits in allergy risk reduction seen in the highest-risk children, he added.
The panelists had no relevant financial conflicts to disclose.
Introducing peanut foods to children who are at different levels of risk for peanut allergies may prevent or mitigate the risk, and the strategies for clinicians are explained in new addendum guidelines issued by an expert panel sponsored by the National Institute of Allergy and Infectious Diseases.
The guidelines were published online Jan. 5 in the Journal of Allergy and Clinical Immunology (J Allergy Clin Immunol. 2017. doi: 10.1016/j.jaci.2016.10.010).
“In the majority of patients, peanut allergy begins early in life and persists as a lifelong problem,” wrote lead author Alkis Togias, MD, of NIAID in Bethesda, Md., and colleagues. Previous guidelines published in 2010 did not provide specific treatment strategies for peanut allergies because of a lack of research, but the significant results of the Learning Early About Peanut Allergy (LEAP) study suggested that early exposure to peanut-containing foods reduces the risk of developing allergies.
The NIAID’s Guidelines Coordinating Committee conducted a literature review covering research from January 2010 to June 2016 and developed addendum guidelines, as follows:
For infants with severe eczema, egg allergies, or both, peanut-containing foods should be introduced at 4-6 months of age at the earliest, after the introduction of other solid foods to confirm developmental readiness. If the infant is developmentally ready for solids, clinicians should “strongly consider” evaluation by peanut-specific IgE (peanut sIgE) measurement and/or skin prick test before introducing peanut products to determine the potential sensitivity and need for supervised feeding vs. feeding at home.
If dietary peanut will be introduced based on the recommendations, “the total amount of peanut protein to be regularly consumed per week should be approximately 6 to 7 g over 3 or more feedings,” the authors wrote.
However, children already identified as allergic to peanut should practice strict peanut avoidance, they added. In addition, they recommend that clinicians review risks and benefits for high-risk children who may have family members with established peanut allergies.
For infants with mild to moderate eczema, the recommendation is to introduce peanut-containing foods at approximately 6 months of age, “in accordance with family preferences and cultural practices,” after the introduction of other solid foods, to help reduce the risk of peanut allergies. The expert panel recommends that infants in this moderate-risk category may receive peanut foods at home without an office visit, although caregivers or clinicians may choose an office visit for supervised feeding, evaluation, or both. Although the LEAP trial did not target infants with mild or moderate eczema, the panel has no reason to believe that the protective mechanisms are different in these children.
For infants with no eczema or any food allergies, the guidelines recommend introducing peanut-containing foods at any age, as appropriate and in keeping with a family’s preferences and cultural practices.
“The early introduction of dietary peanut in children without risk factors for peanut allergy is generally anticipated to be safe and to contribute modestly to an overall reduction in the prevalence of peanut allergy,” the researchers said.
The findings of the LEAP and accompanying LEAP-On trials were so compelling (approximately 80% relative reduction in peanut allergy at 5 years of age for peanut-exposed children, compared with standard of care) that the NIAID and expert panel “felt it was necessary to review and revise the previous recommendations from the 2010 guidelines on the diagnosis and management of food allergy,” Hugh Sampson, MD, director of the Jaffe Food Allergy Institute at the Icahn School of Medicine at Mount Sinai, New York, and a member of the panel, said in an interview.
“It is critical that pediatricians and family practitioners identify infants at high risk for developing peanut allergy (severe atopic dermatitis or egg allergy) between 4 and 6 months of age, evaluate them, or refer them to a food allergy specialist when necessary,” Dr. Sampson said.
“Have parents introduce peanut into the infant’s diet on a regular basis. It is important for parents to notify their pediatrician or family physician if they suspect their infant is at high risk for developing peanut allergy,” he added. “Also, once early peanut introduction is started, it is important that parents continue to provide peanut on a regular basis for several years.”
Next steps for research include pursuing other allergens, said Dr. Sampson. “Similar studies need to be done to determine if early introduction of other foods, such as milk, egg, [or] tree nuts will prevent these common food allergies in high-risk infants.”
Also, it will be important to study whether infants at mild to moderate risk for developing peanut or other food allergies as evidenced by mild to moderate eczema will experience the same benefits in allergy risk reduction seen in the highest-risk children, he added.
The panelists had no relevant financial conflicts to disclose.
Introducing peanut foods to children who are at different levels of risk for peanut allergies may prevent or mitigate the risk, and the strategies for clinicians are explained in new addendum guidelines issued by an expert panel sponsored by the National Institute of Allergy and Infectious Diseases.
The guidelines were published online Jan. 5 in the Journal of Allergy and Clinical Immunology (J Allergy Clin Immunol. 2017. doi: 10.1016/j.jaci.2016.10.010).
“In the majority of patients, peanut allergy begins early in life and persists as a lifelong problem,” wrote lead author Alkis Togias, MD, of NIAID in Bethesda, Md., and colleagues. Previous guidelines published in 2010 did not provide specific treatment strategies for peanut allergies because of a lack of research, but the significant results of the Learning Early About Peanut Allergy (LEAP) study suggested that early exposure to peanut-containing foods reduces the risk of developing allergies.
The NIAID’s Guidelines Coordinating Committee conducted a literature review covering research from January 2010 to June 2016 and developed addendum guidelines, as follows:
For infants with severe eczema, egg allergies, or both, peanut-containing foods should be introduced at 4-6 months of age at the earliest, after the introduction of other solid foods to confirm developmental readiness. If the infant is developmentally ready for solids, clinicians should “strongly consider” evaluation by peanut-specific IgE (peanut sIgE) measurement and/or skin prick test before introducing peanut products to determine the potential sensitivity and need for supervised feeding vs. feeding at home.
If dietary peanut will be introduced based on the recommendations, “the total amount of peanut protein to be regularly consumed per week should be approximately 6 to 7 g over 3 or more feedings,” the authors wrote.
However, children already identified as allergic to peanut should practice strict peanut avoidance, they added. In addition, they recommend that clinicians review risks and benefits for high-risk children who may have family members with established peanut allergies.
For infants with mild to moderate eczema, the recommendation is to introduce peanut-containing foods at approximately 6 months of age, “in accordance with family preferences and cultural practices,” after the introduction of other solid foods, to help reduce the risk of peanut allergies. The expert panel recommends that infants in this moderate-risk category may receive peanut foods at home without an office visit, although caregivers or clinicians may choose an office visit for supervised feeding, evaluation, or both. Although the LEAP trial did not target infants with mild or moderate eczema, the panel has no reason to believe that the protective mechanisms are different in these children.
For infants with no eczema or any food allergies, the guidelines recommend introducing peanut-containing foods at any age, as appropriate and in keeping with a family’s preferences and cultural practices.
“The early introduction of dietary peanut in children without risk factors for peanut allergy is generally anticipated to be safe and to contribute modestly to an overall reduction in the prevalence of peanut allergy,” the researchers said.
The findings of the LEAP and accompanying LEAP-On trials were so compelling (approximately 80% relative reduction in peanut allergy at 5 years of age for peanut-exposed children, compared with standard of care) that the NIAID and expert panel “felt it was necessary to review and revise the previous recommendations from the 2010 guidelines on the diagnosis and management of food allergy,” Hugh Sampson, MD, director of the Jaffe Food Allergy Institute at the Icahn School of Medicine at Mount Sinai, New York, and a member of the panel, said in an interview.
“It is critical that pediatricians and family practitioners identify infants at high risk for developing peanut allergy (severe atopic dermatitis or egg allergy) between 4 and 6 months of age, evaluate them, or refer them to a food allergy specialist when necessary,” Dr. Sampson said.
“Have parents introduce peanut into the infant’s diet on a regular basis. It is important for parents to notify their pediatrician or family physician if they suspect their infant is at high risk for developing peanut allergy,” he added. “Also, once early peanut introduction is started, it is important that parents continue to provide peanut on a regular basis for several years.”
Next steps for research include pursuing other allergens, said Dr. Sampson. “Similar studies need to be done to determine if early introduction of other foods, such as milk, egg, [or] tree nuts will prevent these common food allergies in high-risk infants.”
Also, it will be important to study whether infants at mild to moderate risk for developing peanut or other food allergies as evidenced by mild to moderate eczema will experience the same benefits in allergy risk reduction seen in the highest-risk children, he added.
The panelists had no relevant financial conflicts to disclose.
FROM THE JOURNAL OF ALLERGY AND CLINICAL IMMUNOLOGY
Survey shines new light on weighty comorbidity burden in adult atopic dermatitis
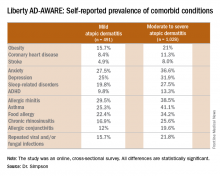
VIENNA – Newly enhanced appreciation of the profound burden of comorbidities associated with adult atopic dermatitis (AD) is provided by the Liberty AD-AWARE study, investigators said at a joint program of the International Eczema Council and the International Psoriasis Council held in conjunction with the annual congress of the European Academy of Dermatology and Venereology.
“I think the only reason we thought psoriasis is a systemic disease and atopic dermatitis is not is because people were researching it much more in psoriasis. I think atopic dermatitis will emerge as potentially more systemic than psoriasis, including the comorbidities. It’s just a matter of time before the evidence is put forth for atopic dermatitis,” predicted Emma Guttman-Yassky, MD, PhD, professor and vice chair of the department of dermatology at Mount Sinai School of Medicine in New York.
Dr. Guttman-Yassky noted that 85% of cases of AD begin before 5 years of age. Many cases resolve later in childhood, but for others it becomes a chronic lifelong condition. And while the burden of AD has been well characterized in the pediatric population, that’s not so in affected adults. This was the impetus for the Liberty AD-AWARE (Adults With Atopic Dermatitis Reporting on their Experience) study, an Internet-based cross-sectional survey of more than 1,500 adults with AD receiving their care from dermatologists at eight major U.S. academic medical centers.
Eric L. Simpson, MD, a coinvestigator with Dr. Guttman-Yassky in Liberty AD-AWARE, observed that the study documented self-reported high rates of a range of psychiatric, cardiovascular, allergic, respiratory, and infectious diseases in participants. And while a cross-sectional study can’t establish causality, it’s important to appreciate that rates of these comorbidities were across the board significantly higher in the 1,028 patients with moderate to severe AD over the prior 12 months than in the 491 classified as having mild AD.
These associations between AD and mental health problems have been confirmed in other studies. For example, a recent analysis of data on more than 354,000 children and nearly 35,000 adults in the United States demonstrated that AD was independently associated with a 14% increased likelihood of attention-deficit/hyperactivity disorder in children and a 61% increased risk in adults. Those risks of ADHD rose far higher in individuals with severe AD and sleep disruption (Br J Dermatol. 2016 Nov;175[5]:920-9).
A number of theories have been put forth to explain these associations, including altered brain development stemming from early exposure to inflammatory cytokines or perhaps shared genetic predisposition, but Dr. Simpson proposed a simpler explanation which carries more optimistic implications.
“I suspect the mental health problems associated with adult atopic dermatitis are probably nonspecific sequelae of any chronic skin disorder involving severe itch and sleep disturbances,” said Dr. Simpson, professor of dermatology at Oregon Health & Science University, Portland.
Moreover, there is good reason to believe that novel therapies targeting inflammation more effectively than what’s been available to date may help improve mental health outcomes, as well as asthma in affected adults with AD, he added. He cited a phase IIb, randomized, double-blind, placebo-controlled study for which he was lead investigator. In this trial, 16 weeks of treatment with dupilumab, a first-in-class investigational blocker of the interleukin-4/interleukin-13 signaling pathway, not only resulted in significant reductions in itch and sleep problems, it also decreased anxiety and depression symptoms and improved multiple validated measures of health-related quality of life (J Am Acad Dermatol. 2016 Sep;75[3]:506-15).
Liberty AD-AWARE provides hints of the profound cumulative negative impact moderate to severe AD can have on a patient’s life course. Among the group with moderate to severe disease, 7.5% said AD had a large negative effect on their pursuit of an education, 10.7% said their disease had influenced their career choice “a lot/very much,” 13.3% were unemployed for reasons other than being retired or a student, and 17.1% reported an annual family income of less than $25,000. All these rates were multifold higher than in patients with mild AD in the study, which didn’t include a non-AD control group.
Dr. Guttman-Yassky observed that 42% of the moderate to severe AD group in Liberty AD-AWARE reported their current treatments were ineffective at controlling their disease, even though study participants were presumably receiving high-quality care at academic medical centers. Twenty-eight percent of patients with inadequately controlled AD had used phototherapy or an immunomodulatory drug within the past 7 days, underscoring the limitations of those forms of therapy in patients with more severe AD as well as the need for new and better treatments.
Dr. Guttman-Yassky has played a key role in the paradigm shift regarding understanding of the pathogenesis of AD as involving not just disordered skin barrier function but also immunologic impairment. She was senior author of a study that showed the nonlesional skin of patients with AD is characterized by high-level expression of inflammatory cytokines, whereas the nonlesional skin of psoriasis patients is not, an observation that serves to highlight the need for proactive treatments for AD (J Allergy Clin Immunol. 2011 Apr;127[4]:954-64.e1-4). Later, she and her coworkers demonstrated that AD is characterized by greater levels of T-cell activation among central and effector CD4+ and CD8+CLA+ and CD8+CLA– memory cell subsets (J Allergy Clin Immunol. 2015 Jul;136[1]:208-11).
More recently, she was also senior author of a landmark study that provides a mechanism to account for the reason AD patients would potentially have more comorbid illnesses than psoriasis patients. The investigators demonstrated that AD is accompanied by systemic expansion of transitional and chronically activated memory B cells, plasmablasts, and IgE-expressing memory B cells in both skin and blood. In other words, AD is characterized by a greater level of systemic immune activation, compared with psoriasis, where activated T cells are largely confined to the skin, and activated central memory B cells don’t figure prominently (J Allergy Clin Immunol. 2016 Jan;137[1]:118-29.e5).
The Liberty AD-AWARE study was sponsored by Sanofi and Regeneron. Dr. Simpson and Dr. Guttman-Yassky reported receiving research grants from and serving as consultants to those and other pharmaceutical companies.
VIENNA – Newly enhanced appreciation of the profound burden of comorbidities associated with adult atopic dermatitis (AD) is provided by the Liberty AD-AWARE study, investigators said at a joint program of the International Eczema Council and the International Psoriasis Council held in conjunction with the annual congress of the European Academy of Dermatology and Venereology.
“I think the only reason we thought psoriasis is a systemic disease and atopic dermatitis is not is because people were researching it much more in psoriasis. I think atopic dermatitis will emerge as potentially more systemic than psoriasis, including the comorbidities. It’s just a matter of time before the evidence is put forth for atopic dermatitis,” predicted Emma Guttman-Yassky, MD, PhD, professor and vice chair of the department of dermatology at Mount Sinai School of Medicine in New York.
Dr. Guttman-Yassky noted that 85% of cases of AD begin before 5 years of age. Many cases resolve later in childhood, but for others it becomes a chronic lifelong condition. And while the burden of AD has been well characterized in the pediatric population, that’s not so in affected adults. This was the impetus for the Liberty AD-AWARE (Adults With Atopic Dermatitis Reporting on their Experience) study, an Internet-based cross-sectional survey of more than 1,500 adults with AD receiving their care from dermatologists at eight major U.S. academic medical centers.
Eric L. Simpson, MD, a coinvestigator with Dr. Guttman-Yassky in Liberty AD-AWARE, observed that the study documented self-reported high rates of a range of psychiatric, cardiovascular, allergic, respiratory, and infectious diseases in participants. And while a cross-sectional study can’t establish causality, it’s important to appreciate that rates of these comorbidities were across the board significantly higher in the 1,028 patients with moderate to severe AD over the prior 12 months than in the 491 classified as having mild AD.
These associations between AD and mental health problems have been confirmed in other studies. For example, a recent analysis of data on more than 354,000 children and nearly 35,000 adults in the United States demonstrated that AD was independently associated with a 14% increased likelihood of attention-deficit/hyperactivity disorder in children and a 61% increased risk in adults. Those risks of ADHD rose far higher in individuals with severe AD and sleep disruption (Br J Dermatol. 2016 Nov;175[5]:920-9).
A number of theories have been put forth to explain these associations, including altered brain development stemming from early exposure to inflammatory cytokines or perhaps shared genetic predisposition, but Dr. Simpson proposed a simpler explanation which carries more optimistic implications.
“I suspect the mental health problems associated with adult atopic dermatitis are probably nonspecific sequelae of any chronic skin disorder involving severe itch and sleep disturbances,” said Dr. Simpson, professor of dermatology at Oregon Health & Science University, Portland.
Moreover, there is good reason to believe that novel therapies targeting inflammation more effectively than what’s been available to date may help improve mental health outcomes, as well as asthma in affected adults with AD, he added. He cited a phase IIb, randomized, double-blind, placebo-controlled study for which he was lead investigator. In this trial, 16 weeks of treatment with dupilumab, a first-in-class investigational blocker of the interleukin-4/interleukin-13 signaling pathway, not only resulted in significant reductions in itch and sleep problems, it also decreased anxiety and depression symptoms and improved multiple validated measures of health-related quality of life (J Am Acad Dermatol. 2016 Sep;75[3]:506-15).
Liberty AD-AWARE provides hints of the profound cumulative negative impact moderate to severe AD can have on a patient’s life course. Among the group with moderate to severe disease, 7.5% said AD had a large negative effect on their pursuit of an education, 10.7% said their disease had influenced their career choice “a lot/very much,” 13.3% were unemployed for reasons other than being retired or a student, and 17.1% reported an annual family income of less than $25,000. All these rates were multifold higher than in patients with mild AD in the study, which didn’t include a non-AD control group.
Dr. Guttman-Yassky observed that 42% of the moderate to severe AD group in Liberty AD-AWARE reported their current treatments were ineffective at controlling their disease, even though study participants were presumably receiving high-quality care at academic medical centers. Twenty-eight percent of patients with inadequately controlled AD had used phototherapy or an immunomodulatory drug within the past 7 days, underscoring the limitations of those forms of therapy in patients with more severe AD as well as the need for new and better treatments.
Dr. Guttman-Yassky has played a key role in the paradigm shift regarding understanding of the pathogenesis of AD as involving not just disordered skin barrier function but also immunologic impairment. She was senior author of a study that showed the nonlesional skin of patients with AD is characterized by high-level expression of inflammatory cytokines, whereas the nonlesional skin of psoriasis patients is not, an observation that serves to highlight the need for proactive treatments for AD (J Allergy Clin Immunol. 2011 Apr;127[4]:954-64.e1-4). Later, she and her coworkers demonstrated that AD is characterized by greater levels of T-cell activation among central and effector CD4+ and CD8+CLA+ and CD8+CLA– memory cell subsets (J Allergy Clin Immunol. 2015 Jul;136[1]:208-11).
More recently, she was also senior author of a landmark study that provides a mechanism to account for the reason AD patients would potentially have more comorbid illnesses than psoriasis patients. The investigators demonstrated that AD is accompanied by systemic expansion of transitional and chronically activated memory B cells, plasmablasts, and IgE-expressing memory B cells in both skin and blood. In other words, AD is characterized by a greater level of systemic immune activation, compared with psoriasis, where activated T cells are largely confined to the skin, and activated central memory B cells don’t figure prominently (J Allergy Clin Immunol. 2016 Jan;137[1]:118-29.e5).
The Liberty AD-AWARE study was sponsored by Sanofi and Regeneron. Dr. Simpson and Dr. Guttman-Yassky reported receiving research grants from and serving as consultants to those and other pharmaceutical companies.
VIENNA – Newly enhanced appreciation of the profound burden of comorbidities associated with adult atopic dermatitis (AD) is provided by the Liberty AD-AWARE study, investigators said at a joint program of the International Eczema Council and the International Psoriasis Council held in conjunction with the annual congress of the European Academy of Dermatology and Venereology.
“I think the only reason we thought psoriasis is a systemic disease and atopic dermatitis is not is because people were researching it much more in psoriasis. I think atopic dermatitis will emerge as potentially more systemic than psoriasis, including the comorbidities. It’s just a matter of time before the evidence is put forth for atopic dermatitis,” predicted Emma Guttman-Yassky, MD, PhD, professor and vice chair of the department of dermatology at Mount Sinai School of Medicine in New York.
Dr. Guttman-Yassky noted that 85% of cases of AD begin before 5 years of age. Many cases resolve later in childhood, but for others it becomes a chronic lifelong condition. And while the burden of AD has been well characterized in the pediatric population, that’s not so in affected adults. This was the impetus for the Liberty AD-AWARE (Adults With Atopic Dermatitis Reporting on their Experience) study, an Internet-based cross-sectional survey of more than 1,500 adults with AD receiving their care from dermatologists at eight major U.S. academic medical centers.
Eric L. Simpson, MD, a coinvestigator with Dr. Guttman-Yassky in Liberty AD-AWARE, observed that the study documented self-reported high rates of a range of psychiatric, cardiovascular, allergic, respiratory, and infectious diseases in participants. And while a cross-sectional study can’t establish causality, it’s important to appreciate that rates of these comorbidities were across the board significantly higher in the 1,028 patients with moderate to severe AD over the prior 12 months than in the 491 classified as having mild AD.
These associations between AD and mental health problems have been confirmed in other studies. For example, a recent analysis of data on more than 354,000 children and nearly 35,000 adults in the United States demonstrated that AD was independently associated with a 14% increased likelihood of attention-deficit/hyperactivity disorder in children and a 61% increased risk in adults. Those risks of ADHD rose far higher in individuals with severe AD and sleep disruption (Br J Dermatol. 2016 Nov;175[5]:920-9).
A number of theories have been put forth to explain these associations, including altered brain development stemming from early exposure to inflammatory cytokines or perhaps shared genetic predisposition, but Dr. Simpson proposed a simpler explanation which carries more optimistic implications.
“I suspect the mental health problems associated with adult atopic dermatitis are probably nonspecific sequelae of any chronic skin disorder involving severe itch and sleep disturbances,” said Dr. Simpson, professor of dermatology at Oregon Health & Science University, Portland.
Moreover, there is good reason to believe that novel therapies targeting inflammation more effectively than what’s been available to date may help improve mental health outcomes, as well as asthma in affected adults with AD, he added. He cited a phase IIb, randomized, double-blind, placebo-controlled study for which he was lead investigator. In this trial, 16 weeks of treatment with dupilumab, a first-in-class investigational blocker of the interleukin-4/interleukin-13 signaling pathway, not only resulted in significant reductions in itch and sleep problems, it also decreased anxiety and depression symptoms and improved multiple validated measures of health-related quality of life (J Am Acad Dermatol. 2016 Sep;75[3]:506-15).
Liberty AD-AWARE provides hints of the profound cumulative negative impact moderate to severe AD can have on a patient’s life course. Among the group with moderate to severe disease, 7.5% said AD had a large negative effect on their pursuit of an education, 10.7% said their disease had influenced their career choice “a lot/very much,” 13.3% were unemployed for reasons other than being retired or a student, and 17.1% reported an annual family income of less than $25,000. All these rates were multifold higher than in patients with mild AD in the study, which didn’t include a non-AD control group.
Dr. Guttman-Yassky observed that 42% of the moderate to severe AD group in Liberty AD-AWARE reported their current treatments were ineffective at controlling their disease, even though study participants were presumably receiving high-quality care at academic medical centers. Twenty-eight percent of patients with inadequately controlled AD had used phototherapy or an immunomodulatory drug within the past 7 days, underscoring the limitations of those forms of therapy in patients with more severe AD as well as the need for new and better treatments.
Dr. Guttman-Yassky has played a key role in the paradigm shift regarding understanding of the pathogenesis of AD as involving not just disordered skin barrier function but also immunologic impairment. She was senior author of a study that showed the nonlesional skin of patients with AD is characterized by high-level expression of inflammatory cytokines, whereas the nonlesional skin of psoriasis patients is not, an observation that serves to highlight the need for proactive treatments for AD (J Allergy Clin Immunol. 2011 Apr;127[4]:954-64.e1-4). Later, she and her coworkers demonstrated that AD is characterized by greater levels of T-cell activation among central and effector CD4+ and CD8+CLA+ and CD8+CLA– memory cell subsets (J Allergy Clin Immunol. 2015 Jul;136[1]:208-11).
More recently, she was also senior author of a landmark study that provides a mechanism to account for the reason AD patients would potentially have more comorbid illnesses than psoriasis patients. The investigators demonstrated that AD is accompanied by systemic expansion of transitional and chronically activated memory B cells, plasmablasts, and IgE-expressing memory B cells in both skin and blood. In other words, AD is characterized by a greater level of systemic immune activation, compared with psoriasis, where activated T cells are largely confined to the skin, and activated central memory B cells don’t figure prominently (J Allergy Clin Immunol. 2016 Jan;137[1]:118-29.e5).
The Liberty AD-AWARE study was sponsored by Sanofi and Regeneron. Dr. Simpson and Dr. Guttman-Yassky reported receiving research grants from and serving as consultants to those and other pharmaceutical companies.
EXPERT ANALYSIS FROM THE EADV CONGRESS
FDA eases mental health warnings in smoking cessation drugs’ labels
Labels on two smoking cessation treatments will offer less severe warnings for mental health risk potentials in people with no history of psychiatric disorders, the Food and Drug Administration has announced.
Varenicline (Chantix) will no longer include a boxed warning for serious mental health side effects. The label for bupropion (Zyban) will still include a boxed warning, but language describing the potential for serious psychiatric adverse events will no longer appear within it. Updates will also be made to both labels to describe side effects on mood, behavior, or thinking.
In addition, varenicline’s label will reflect trial data showing its superior efficacy, compared with oral bupropion or nicotine patch. Although a patient medication guide will still be included with each prescription, the risk evaluation and mitigation strategy that prompted the guide will no longer be in place.
Earlier this year, two FDA advisory committees voted in favor of updating varenicline’s label, based on data from a randomized, controlled trial of more than 8,000 smokers, half of whom had a history of psychiatric disorders.
The trial showed no clinically significant difference in risk of adverse events across the smoking cessation treatments varenicline, bupropion, nicotine patch, or placebo study arms, although the risk was higher in the psychiatric cohorts in each.
Overall, 2% of those without a history of mental illness experienced neuropsychiatric adverse events, compared with between 5% and 7% of those with such a history.
The trial was cosponsored by Pfizer, maker of Chantix, and GlaxoSmithKline, maker of Zyban.
The FDA approved varenicline for smoking cessation in 2006 and approved bupropion, which also is indicated to treat depression and seasonal affective disorder, in 1997. After numerous postmarketing reports of increased incidents of psychiatric disorders occurring in smokers who used either drug, the agency added the boxed warning to each in 2009.
FDA officials advised clinicians to guard against changes in mental health status in smokers using these therapies. However, “the results of the trial confirm that the benefits of stopping smoking outweigh the risks of these medicines,” they noted.
On Twitter @whitneymcknight
Labels on two smoking cessation treatments will offer less severe warnings for mental health risk potentials in people with no history of psychiatric disorders, the Food and Drug Administration has announced.
Varenicline (Chantix) will no longer include a boxed warning for serious mental health side effects. The label for bupropion (Zyban) will still include a boxed warning, but language describing the potential for serious psychiatric adverse events will no longer appear within it. Updates will also be made to both labels to describe side effects on mood, behavior, or thinking.
In addition, varenicline’s label will reflect trial data showing its superior efficacy, compared with oral bupropion or nicotine patch. Although a patient medication guide will still be included with each prescription, the risk evaluation and mitigation strategy that prompted the guide will no longer be in place.
Earlier this year, two FDA advisory committees voted in favor of updating varenicline’s label, based on data from a randomized, controlled trial of more than 8,000 smokers, half of whom had a history of psychiatric disorders.
The trial showed no clinically significant difference in risk of adverse events across the smoking cessation treatments varenicline, bupropion, nicotine patch, or placebo study arms, although the risk was higher in the psychiatric cohorts in each.
Overall, 2% of those without a history of mental illness experienced neuropsychiatric adverse events, compared with between 5% and 7% of those with such a history.
The trial was cosponsored by Pfizer, maker of Chantix, and GlaxoSmithKline, maker of Zyban.
The FDA approved varenicline for smoking cessation in 2006 and approved bupropion, which also is indicated to treat depression and seasonal affective disorder, in 1997. After numerous postmarketing reports of increased incidents of psychiatric disorders occurring in smokers who used either drug, the agency added the boxed warning to each in 2009.
FDA officials advised clinicians to guard against changes in mental health status in smokers using these therapies. However, “the results of the trial confirm that the benefits of stopping smoking outweigh the risks of these medicines,” they noted.
On Twitter @whitneymcknight
Labels on two smoking cessation treatments will offer less severe warnings for mental health risk potentials in people with no history of psychiatric disorders, the Food and Drug Administration has announced.
Varenicline (Chantix) will no longer include a boxed warning for serious mental health side effects. The label for bupropion (Zyban) will still include a boxed warning, but language describing the potential for serious psychiatric adverse events will no longer appear within it. Updates will also be made to both labels to describe side effects on mood, behavior, or thinking.
In addition, varenicline’s label will reflect trial data showing its superior efficacy, compared with oral bupropion or nicotine patch. Although a patient medication guide will still be included with each prescription, the risk evaluation and mitigation strategy that prompted the guide will no longer be in place.
Earlier this year, two FDA advisory committees voted in favor of updating varenicline’s label, based on data from a randomized, controlled trial of more than 8,000 smokers, half of whom had a history of psychiatric disorders.
The trial showed no clinically significant difference in risk of adverse events across the smoking cessation treatments varenicline, bupropion, nicotine patch, or placebo study arms, although the risk was higher in the psychiatric cohorts in each.
Overall, 2% of those without a history of mental illness experienced neuropsychiatric adverse events, compared with between 5% and 7% of those with such a history.
The trial was cosponsored by Pfizer, maker of Chantix, and GlaxoSmithKline, maker of Zyban.
The FDA approved varenicline for smoking cessation in 2006 and approved bupropion, which also is indicated to treat depression and seasonal affective disorder, in 1997. After numerous postmarketing reports of increased incidents of psychiatric disorders occurring in smokers who used either drug, the agency added the boxed warning to each in 2009.
FDA officials advised clinicians to guard against changes in mental health status in smokers using these therapies. However, “the results of the trial confirm that the benefits of stopping smoking outweigh the risks of these medicines,” they noted.
On Twitter @whitneymcknight
How to Categorize Pediatric Asthma
Mass spectrometry of gastric aspirates can predict RDS in premature infants
A mass spectrometry test was able to rapidly measure lung maturity in premature infants at risk for respiratory distress syndrome (RDS), according to Henrik Verder, MD, of Holbaek (Denmark) University Hospital, and his associates.
Samples of gastric aspirates were taken from 136 infants with gestation periods of 24-31 weeks, and analyzed with mass spectrometry. Of this group, 61 developed RDS, and 7 died before the end of the study period. With a lecithin/sphingomyelin (L/S) cut-off ratio of 2.2, sensitivity of the mass spectrometry test was 92%, specificity was 73%, positive predictive value was 74%, and negative predictive was value of 92%. Sensitivity was high for all gestational age groups, the investigators noted.
Oropharyngeal secretions were sampled from an additional group of 59 infants and analyzed using spectrometry, with an L/S cut-off value of 3.7; however sensitivity and specificity were lower than for gastric aspirates.
“This test could help identify which infants will benefit from very early surfactant treatment, with the potential to significantly improve clinical outcomes resulting in less severe RDS, less need of mechanical ventilation and oxygen and less severe bronchopulmonary dysplasia,” the investigators concluded.
Find the study in Acta Paediatrica (2016. doi: 10.1111/apa.13683)
A mass spectrometry test was able to rapidly measure lung maturity in premature infants at risk for respiratory distress syndrome (RDS), according to Henrik Verder, MD, of Holbaek (Denmark) University Hospital, and his associates.
Samples of gastric aspirates were taken from 136 infants with gestation periods of 24-31 weeks, and analyzed with mass spectrometry. Of this group, 61 developed RDS, and 7 died before the end of the study period. With a lecithin/sphingomyelin (L/S) cut-off ratio of 2.2, sensitivity of the mass spectrometry test was 92%, specificity was 73%, positive predictive value was 74%, and negative predictive was value of 92%. Sensitivity was high for all gestational age groups, the investigators noted.
Oropharyngeal secretions were sampled from an additional group of 59 infants and analyzed using spectrometry, with an L/S cut-off value of 3.7; however sensitivity and specificity were lower than for gastric aspirates.
“This test could help identify which infants will benefit from very early surfactant treatment, with the potential to significantly improve clinical outcomes resulting in less severe RDS, less need of mechanical ventilation and oxygen and less severe bronchopulmonary dysplasia,” the investigators concluded.
Find the study in Acta Paediatrica (2016. doi: 10.1111/apa.13683)
A mass spectrometry test was able to rapidly measure lung maturity in premature infants at risk for respiratory distress syndrome (RDS), according to Henrik Verder, MD, of Holbaek (Denmark) University Hospital, and his associates.
Samples of gastric aspirates were taken from 136 infants with gestation periods of 24-31 weeks, and analyzed with mass spectrometry. Of this group, 61 developed RDS, and 7 died before the end of the study period. With a lecithin/sphingomyelin (L/S) cut-off ratio of 2.2, sensitivity of the mass spectrometry test was 92%, specificity was 73%, positive predictive value was 74%, and negative predictive was value of 92%. Sensitivity was high for all gestational age groups, the investigators noted.
Oropharyngeal secretions were sampled from an additional group of 59 infants and analyzed using spectrometry, with an L/S cut-off value of 3.7; however sensitivity and specificity were lower than for gastric aspirates.
“This test could help identify which infants will benefit from very early surfactant treatment, with the potential to significantly improve clinical outcomes resulting in less severe RDS, less need of mechanical ventilation and oxygen and less severe bronchopulmonary dysplasia,” the investigators concluded.
Find the study in Acta Paediatrica (2016. doi: 10.1111/apa.13683)
Partnerships with pediatric tertiary care centers improve community ED asthma treatment
Partnerships between community emergency departments and pediatric tertiary care centers are feasible and improve care of pediatric asthma, according to Theresa A. Walls, MD, of the Children’s National Health Systems, Washington, D.C., and her associates.
A total of 724 asthma patients aged 2-17 years were included in the study. Of this group, 289 (40%) were treated at the community ED before the pediatric tertiary care center intervention and 435 (60%) were treated after the intervention. Treatment with steroids was significantly increased post intervention, with 76% of patients receiving steroids, compared with 60% of patients before the intervention.
“Because the overwhelming majority of pediatric emergency visits occur in community EDs, partnerships with these EDs can broaden the impact of quality improvement activities and should be part of future quality improvement efforts,” the investigators concluded.
Find the full study in Pediatrics (2016. doi: 10.1542/peds.2016-0088).
Dr. Walls and her group developed a quality improvement (QI) initiative with a community emergency department. One important part of the study was the use of an asthma score, which helped determine steps for ED therapy.
Dr. Walls and her group developed a quality improvement (QI) initiative with a community emergency department. One important part of the study was the use of an asthma score, which helped determine steps for ED therapy.
Dr. Walls and her group developed a quality improvement (QI) initiative with a community emergency department. One important part of the study was the use of an asthma score, which helped determine steps for ED therapy.
Partnerships between community emergency departments and pediatric tertiary care centers are feasible and improve care of pediatric asthma, according to Theresa A. Walls, MD, of the Children’s National Health Systems, Washington, D.C., and her associates.
A total of 724 asthma patients aged 2-17 years were included in the study. Of this group, 289 (40%) were treated at the community ED before the pediatric tertiary care center intervention and 435 (60%) were treated after the intervention. Treatment with steroids was significantly increased post intervention, with 76% of patients receiving steroids, compared with 60% of patients before the intervention.
“Because the overwhelming majority of pediatric emergency visits occur in community EDs, partnerships with these EDs can broaden the impact of quality improvement activities and should be part of future quality improvement efforts,” the investigators concluded.
Find the full study in Pediatrics (2016. doi: 10.1542/peds.2016-0088).
Partnerships between community emergency departments and pediatric tertiary care centers are feasible and improve care of pediatric asthma, according to Theresa A. Walls, MD, of the Children’s National Health Systems, Washington, D.C., and her associates.
A total of 724 asthma patients aged 2-17 years were included in the study. Of this group, 289 (40%) were treated at the community ED before the pediatric tertiary care center intervention and 435 (60%) were treated after the intervention. Treatment with steroids was significantly increased post intervention, with 76% of patients receiving steroids, compared with 60% of patients before the intervention.
“Because the overwhelming majority of pediatric emergency visits occur in community EDs, partnerships with these EDs can broaden the impact of quality improvement activities and should be part of future quality improvement efforts,” the investigators concluded.
Find the full study in Pediatrics (2016. doi: 10.1542/peds.2016-0088).
FROM PEDIATRICS
VIDEO: Pivotal results nail osimertinib’s role in NSCLC
VIENNA – Osimertinib, a third-generation epidermal growth factor receptor tyrosine kinase inhibitor that received U.S. marketing approval in November 2015 as second-line treatment for selected patients with non–small-cell lung cancer based on phase II trial data, now has the pivotal-trial results that completely justify that action.
During a median follow-up of just over 8 months, patients who had progressed on their first-line EGFR tyrosine kinase inhibitor (TKI) and carried the T790M mutation and switched to osimertinib (Tagrisso) had “overwhelmingly” better response rates and progression-free survival, compared with patients put on standard-of-care chemotherapy in a multicenter randomized trial involving 419 patients, Vassiliki A. Papadimitrakopoulou, MD, reported at the World Conference on Lung Cancer.
The AZD9291 Versus Platinum-Based Doublet-Chemotherapy in Locally Advanced or Metastatic Non-Small Cell Lung Cancer (AURA3) trial enrolled 419 patients at 126 international sites during 2014 and 2015. The trial’s primary endpoint was investigator-assessed progression-free survival, which occurred after a median of 10.1 months with osimertinib and 4.4 months with standard chemotherapy, a statistically significant 70% relative risk reduction (P less than .001) in the hazard for death or progressive disease. The drug was as effective for patients with central nervous system metastases as it was for the other patients, which Dr. Papadimitrakopoulou attributed to osimertinib’s good penetration across the blood-brain barrier. The drug’s overall performance in AURA3 was completely consistent with the results of earlier studies that led to its U.S. approval.
Despite that approval, routine testing for T790M mutations and routine prescribing of osimertinib to positive patients “has not fully penetrated U.S. practice,” Dr. Papadimitrakopoulou said, but she hoped that these new confirmatory data will now firmly establish it as standard of care for the tested population.
Osimertinib is now under testing as first-line TKI treatment for EGFR-positive NSCLC regardless of the tumor’s T790M status. It’s going head to head with two first-generation EGFR TKIs, gefitinib and erlotinib, in the FLAURA trial, which should have reportable results in 2017. “We are very encouraged by the AURA3 data that osimertinib could beat the first-generation TKIs” for first-line treatment, she said.
AURA3 was sponsored by AstraZeneca, which markets osimertinib (Tagrisso). Dr. Papadimitrakopoulou is a consultant to, and has received research support from, AstraZeneca and several other companies.
[email protected]
On Twitter @mitchelzoler
Osimertinib overcomes the T790M mutation, which is the cause of about half of the non–small-cell lung cancers that are EGFR positive and develop resistance to a first- or-second generation tyrosine kinase inhibitor. The AURA3 results officially make osimertinib the standard of care for these patients.
Osimertinib’s performance in AURA3 was consistent with what it did in the earlier studies, producing an overall response rate of about 60%-70% and extending progression-free survival out to a median of 10-11 months.
Tetsuya Mitsudomi, MD, is professor of thoracic surgery at Kindai University in Osaka-Sayama, Japan. He has been a consultant to, and has received honoraria and research support from, AstraZeneca and several other companies. He was principal investigator for AURA2, one of the phase II studies of osimertinib. He made these comments as designated discussant for AURA3.
Osimertinib overcomes the T790M mutation, which is the cause of about half of the non–small-cell lung cancers that are EGFR positive and develop resistance to a first- or-second generation tyrosine kinase inhibitor. The AURA3 results officially make osimertinib the standard of care for these patients.
Osimertinib’s performance in AURA3 was consistent with what it did in the earlier studies, producing an overall response rate of about 60%-70% and extending progression-free survival out to a median of 10-11 months.
Tetsuya Mitsudomi, MD, is professor of thoracic surgery at Kindai University in Osaka-Sayama, Japan. He has been a consultant to, and has received honoraria and research support from, AstraZeneca and several other companies. He was principal investigator for AURA2, one of the phase II studies of osimertinib. He made these comments as designated discussant for AURA3.
Osimertinib overcomes the T790M mutation, which is the cause of about half of the non–small-cell lung cancers that are EGFR positive and develop resistance to a first- or-second generation tyrosine kinase inhibitor. The AURA3 results officially make osimertinib the standard of care for these patients.
Osimertinib’s performance in AURA3 was consistent with what it did in the earlier studies, producing an overall response rate of about 60%-70% and extending progression-free survival out to a median of 10-11 months.
Tetsuya Mitsudomi, MD, is professor of thoracic surgery at Kindai University in Osaka-Sayama, Japan. He has been a consultant to, and has received honoraria and research support from, AstraZeneca and several other companies. He was principal investigator for AURA2, one of the phase II studies of osimertinib. He made these comments as designated discussant for AURA3.
VIENNA – Osimertinib, a third-generation epidermal growth factor receptor tyrosine kinase inhibitor that received U.S. marketing approval in November 2015 as second-line treatment for selected patients with non–small-cell lung cancer based on phase II trial data, now has the pivotal-trial results that completely justify that action.
During a median follow-up of just over 8 months, patients who had progressed on their first-line EGFR tyrosine kinase inhibitor (TKI) and carried the T790M mutation and switched to osimertinib (Tagrisso) had “overwhelmingly” better response rates and progression-free survival, compared with patients put on standard-of-care chemotherapy in a multicenter randomized trial involving 419 patients, Vassiliki A. Papadimitrakopoulou, MD, reported at the World Conference on Lung Cancer.
The AZD9291 Versus Platinum-Based Doublet-Chemotherapy in Locally Advanced or Metastatic Non-Small Cell Lung Cancer (AURA3) trial enrolled 419 patients at 126 international sites during 2014 and 2015. The trial’s primary endpoint was investigator-assessed progression-free survival, which occurred after a median of 10.1 months with osimertinib and 4.4 months with standard chemotherapy, a statistically significant 70% relative risk reduction (P less than .001) in the hazard for death or progressive disease. The drug was as effective for patients with central nervous system metastases as it was for the other patients, which Dr. Papadimitrakopoulou attributed to osimertinib’s good penetration across the blood-brain barrier. The drug’s overall performance in AURA3 was completely consistent with the results of earlier studies that led to its U.S. approval.
Despite that approval, routine testing for T790M mutations and routine prescribing of osimertinib to positive patients “has not fully penetrated U.S. practice,” Dr. Papadimitrakopoulou said, but she hoped that these new confirmatory data will now firmly establish it as standard of care for the tested population.
Osimertinib is now under testing as first-line TKI treatment for EGFR-positive NSCLC regardless of the tumor’s T790M status. It’s going head to head with two first-generation EGFR TKIs, gefitinib and erlotinib, in the FLAURA trial, which should have reportable results in 2017. “We are very encouraged by the AURA3 data that osimertinib could beat the first-generation TKIs” for first-line treatment, she said.
AURA3 was sponsored by AstraZeneca, which markets osimertinib (Tagrisso). Dr. Papadimitrakopoulou is a consultant to, and has received research support from, AstraZeneca and several other companies.
[email protected]
On Twitter @mitchelzoler
VIENNA – Osimertinib, a third-generation epidermal growth factor receptor tyrosine kinase inhibitor that received U.S. marketing approval in November 2015 as second-line treatment for selected patients with non–small-cell lung cancer based on phase II trial data, now has the pivotal-trial results that completely justify that action.
During a median follow-up of just over 8 months, patients who had progressed on their first-line EGFR tyrosine kinase inhibitor (TKI) and carried the T790M mutation and switched to osimertinib (Tagrisso) had “overwhelmingly” better response rates and progression-free survival, compared with patients put on standard-of-care chemotherapy in a multicenter randomized trial involving 419 patients, Vassiliki A. Papadimitrakopoulou, MD, reported at the World Conference on Lung Cancer.
The AZD9291 Versus Platinum-Based Doublet-Chemotherapy in Locally Advanced or Metastatic Non-Small Cell Lung Cancer (AURA3) trial enrolled 419 patients at 126 international sites during 2014 and 2015. The trial’s primary endpoint was investigator-assessed progression-free survival, which occurred after a median of 10.1 months with osimertinib and 4.4 months with standard chemotherapy, a statistically significant 70% relative risk reduction (P less than .001) in the hazard for death or progressive disease. The drug was as effective for patients with central nervous system metastases as it was for the other patients, which Dr. Papadimitrakopoulou attributed to osimertinib’s good penetration across the blood-brain barrier. The drug’s overall performance in AURA3 was completely consistent with the results of earlier studies that led to its U.S. approval.
Despite that approval, routine testing for T790M mutations and routine prescribing of osimertinib to positive patients “has not fully penetrated U.S. practice,” Dr. Papadimitrakopoulou said, but she hoped that these new confirmatory data will now firmly establish it as standard of care for the tested population.
Osimertinib is now under testing as first-line TKI treatment for EGFR-positive NSCLC regardless of the tumor’s T790M status. It’s going head to head with two first-generation EGFR TKIs, gefitinib and erlotinib, in the FLAURA trial, which should have reportable results in 2017. “We are very encouraged by the AURA3 data that osimertinib could beat the first-generation TKIs” for first-line treatment, she said.
AURA3 was sponsored by AstraZeneca, which markets osimertinib (Tagrisso). Dr. Papadimitrakopoulou is a consultant to, and has received research support from, AstraZeneca and several other companies.
[email protected]
On Twitter @mitchelzoler
Key clinical point:
Major finding: Progression-free survival averaged 10.1 months with osimertinib and 4.4 months in patients on standard chemotherapy.
Data source: AURA3, which randomized 419 patients at 126 international centers.
Disclosures: AURA3 was sponsored by AstraZeneca, which markets osimertinib (Tagrisso). Dr. Papadimitrakopoulou is a consultant to, and has received research support from, AstraZeneca and several other companies.
‘Vanishing’ role forecast for whole-brain irradiation for NSCLC metastases
VIENNA – Icotinib proved significantly more effective and less toxic than standard therapy with whole-brain irradiation and chemotherapy in patients with multiple brain metastases from epidermal growth factor receptor (EGFR)–mutated non–small cell lung cancer in the phase III BRAIN trial.
“With favorable objective response and disease control rates, icotinib was superior to whole-brain irradiation with chemotherapy, and therefore icotinib should be used as first-line therapy for advanced EGFR-mutant non–small cell lung cancers with brain metastases,” Yi-long Wu, MD, said in presenting the BRAIN results at the World Congress on Lung Cancer.
Patients with brain metastases are often excluded from participation in clinical trials because their prognosis is so poor. BRAIN is the first phase III trial to report results comparing an EGFR tyrosine kinase inhibitor (TKI) – icotinib – to whole-brain irradiation (WBI) plus chemotherapy, regarded in National Comprehensive Cancer Network guidelines as standard therapy in the setting of brain metastases from NSCLC, noted Dr. Wu of the Guangdong Lung Cancer Institute in Guangzhou, China.
BRAIN was a multicenter Chinese trial in which investigators randomized 158 patients with three or more brain metastases from EGFR-mutated NSCLC to oral icotinib at 125 mg t.i.d. or to WBI with four to six cycles of concurrent or sequential platinum-based chemotherapy.
Icotinib outperformed standard therapy with WBI plus chemotherapy on multiple efficacy endpoints. Median intracranial progression-free survival was 10 months with icotinib, compared with only 4.8 months in patients on WBI with chemotherapy. At 6 months, 72% of patients assigned to icotinib remained free of intracranial disease progression, compared with just 48% of controls on WBI and chemotherapy. Six-month overall PFS, intracranial as well as extracranial, was 6.8 months with icotinib and 3.4 months in the WBI group. The intracranial and overall objective response rates were 67% and 55%, respectively, with icotinib, compared with 41% and 11% with WBI.
In addition, the EGFR TKI had a significantly better safety profile: grade 3 or worse toxicities occurred in just 8% of the icotinib group, compared with 26% of controls on standard therapy, Dr. Wu reported at the meeting sponsored by the International Association for the Study of Lung Cancer.
There was, however, no significant difference between the two study arms in overall survival: 18 months with icotinib, 20.5 months with standard therapy, noted Dr. Wu, who is president of the Chinese Society of Clinical Oncology.
Discussant Jacek Jassem, MD, said “This is potentially, and likely, a practice-changing study.” He added that he’s looking forward to planned future presentation of neurotoxicity and quality of life data from BRAIN. Those important endpoints are also incorporated in the ongoing phase III trials of gefitinib and erlotinib, EGFR TKIs which are far more readily accessible at present to physicians outside the Far East.
“Whole-brain radiotherapy for brain metastases from EGFR-mutated non–small cell lung cancer is a vanishing approach. The remaining role in this setting is as salvage in cases of symptomatic primary or secondary resistance to EGFR TKIs,” said Dr. Jassem, head of the department of oncology and radiotherapy at the Medical University of Gdansk, Poland.
By way of background, he noted that 10%-15% of patients already have brain metastases at the time of diagnosis of NSCLC, and 40% develop them eventually. EGFR-mutated primary tumors are particularly likely to metastasize to the brain.
Remaining questions in the wake of the BRAIN trial include the efficacy of gefitinib and erlotinib versus WBI plus chemotherapy, as well as the broader question of the efficacy of EGFR TKIs in non-Asian patients with brain metastases.
“Most of the studies of EGFR TKIs have been in East Asian populations. These agents are particularly active in East Asians. There’s a question as to whether the BRAIN results can be applied to other populations,” Dr. Jassem said.
The BRAIN study was sponsored by the Guangdong Association of Clinical Trials. Dr. Wu reported serving as a consultant to AstraZeneca, Roche, Eli Lilly, Pfizer, and Sanofi. Dr. Jassem reported having no financial conflicts of interest.
VIENNA – Icotinib proved significantly more effective and less toxic than standard therapy with whole-brain irradiation and chemotherapy in patients with multiple brain metastases from epidermal growth factor receptor (EGFR)–mutated non–small cell lung cancer in the phase III BRAIN trial.
“With favorable objective response and disease control rates, icotinib was superior to whole-brain irradiation with chemotherapy, and therefore icotinib should be used as first-line therapy for advanced EGFR-mutant non–small cell lung cancers with brain metastases,” Yi-long Wu, MD, said in presenting the BRAIN results at the World Congress on Lung Cancer.
Patients with brain metastases are often excluded from participation in clinical trials because their prognosis is so poor. BRAIN is the first phase III trial to report results comparing an EGFR tyrosine kinase inhibitor (TKI) – icotinib – to whole-brain irradiation (WBI) plus chemotherapy, regarded in National Comprehensive Cancer Network guidelines as standard therapy in the setting of brain metastases from NSCLC, noted Dr. Wu of the Guangdong Lung Cancer Institute in Guangzhou, China.
BRAIN was a multicenter Chinese trial in which investigators randomized 158 patients with three or more brain metastases from EGFR-mutated NSCLC to oral icotinib at 125 mg t.i.d. or to WBI with four to six cycles of concurrent or sequential platinum-based chemotherapy.
Icotinib outperformed standard therapy with WBI plus chemotherapy on multiple efficacy endpoints. Median intracranial progression-free survival was 10 months with icotinib, compared with only 4.8 months in patients on WBI with chemotherapy. At 6 months, 72% of patients assigned to icotinib remained free of intracranial disease progression, compared with just 48% of controls on WBI and chemotherapy. Six-month overall PFS, intracranial as well as extracranial, was 6.8 months with icotinib and 3.4 months in the WBI group. The intracranial and overall objective response rates were 67% and 55%, respectively, with icotinib, compared with 41% and 11% with WBI.
In addition, the EGFR TKI had a significantly better safety profile: grade 3 or worse toxicities occurred in just 8% of the icotinib group, compared with 26% of controls on standard therapy, Dr. Wu reported at the meeting sponsored by the International Association for the Study of Lung Cancer.
There was, however, no significant difference between the two study arms in overall survival: 18 months with icotinib, 20.5 months with standard therapy, noted Dr. Wu, who is president of the Chinese Society of Clinical Oncology.
Discussant Jacek Jassem, MD, said “This is potentially, and likely, a practice-changing study.” He added that he’s looking forward to planned future presentation of neurotoxicity and quality of life data from BRAIN. Those important endpoints are also incorporated in the ongoing phase III trials of gefitinib and erlotinib, EGFR TKIs which are far more readily accessible at present to physicians outside the Far East.
“Whole-brain radiotherapy for brain metastases from EGFR-mutated non–small cell lung cancer is a vanishing approach. The remaining role in this setting is as salvage in cases of symptomatic primary or secondary resistance to EGFR TKIs,” said Dr. Jassem, head of the department of oncology and radiotherapy at the Medical University of Gdansk, Poland.
By way of background, he noted that 10%-15% of patients already have brain metastases at the time of diagnosis of NSCLC, and 40% develop them eventually. EGFR-mutated primary tumors are particularly likely to metastasize to the brain.
Remaining questions in the wake of the BRAIN trial include the efficacy of gefitinib and erlotinib versus WBI plus chemotherapy, as well as the broader question of the efficacy of EGFR TKIs in non-Asian patients with brain metastases.
“Most of the studies of EGFR TKIs have been in East Asian populations. These agents are particularly active in East Asians. There’s a question as to whether the BRAIN results can be applied to other populations,” Dr. Jassem said.
The BRAIN study was sponsored by the Guangdong Association of Clinical Trials. Dr. Wu reported serving as a consultant to AstraZeneca, Roche, Eli Lilly, Pfizer, and Sanofi. Dr. Jassem reported having no financial conflicts of interest.
VIENNA – Icotinib proved significantly more effective and less toxic than standard therapy with whole-brain irradiation and chemotherapy in patients with multiple brain metastases from epidermal growth factor receptor (EGFR)–mutated non–small cell lung cancer in the phase III BRAIN trial.
“With favorable objective response and disease control rates, icotinib was superior to whole-brain irradiation with chemotherapy, and therefore icotinib should be used as first-line therapy for advanced EGFR-mutant non–small cell lung cancers with brain metastases,” Yi-long Wu, MD, said in presenting the BRAIN results at the World Congress on Lung Cancer.
Patients with brain metastases are often excluded from participation in clinical trials because their prognosis is so poor. BRAIN is the first phase III trial to report results comparing an EGFR tyrosine kinase inhibitor (TKI) – icotinib – to whole-brain irradiation (WBI) plus chemotherapy, regarded in National Comprehensive Cancer Network guidelines as standard therapy in the setting of brain metastases from NSCLC, noted Dr. Wu of the Guangdong Lung Cancer Institute in Guangzhou, China.
BRAIN was a multicenter Chinese trial in which investigators randomized 158 patients with three or more brain metastases from EGFR-mutated NSCLC to oral icotinib at 125 mg t.i.d. or to WBI with four to six cycles of concurrent or sequential platinum-based chemotherapy.
Icotinib outperformed standard therapy with WBI plus chemotherapy on multiple efficacy endpoints. Median intracranial progression-free survival was 10 months with icotinib, compared with only 4.8 months in patients on WBI with chemotherapy. At 6 months, 72% of patients assigned to icotinib remained free of intracranial disease progression, compared with just 48% of controls on WBI and chemotherapy. Six-month overall PFS, intracranial as well as extracranial, was 6.8 months with icotinib and 3.4 months in the WBI group. The intracranial and overall objective response rates were 67% and 55%, respectively, with icotinib, compared with 41% and 11% with WBI.
In addition, the EGFR TKI had a significantly better safety profile: grade 3 or worse toxicities occurred in just 8% of the icotinib group, compared with 26% of controls on standard therapy, Dr. Wu reported at the meeting sponsored by the International Association for the Study of Lung Cancer.
There was, however, no significant difference between the two study arms in overall survival: 18 months with icotinib, 20.5 months with standard therapy, noted Dr. Wu, who is president of the Chinese Society of Clinical Oncology.
Discussant Jacek Jassem, MD, said “This is potentially, and likely, a practice-changing study.” He added that he’s looking forward to planned future presentation of neurotoxicity and quality of life data from BRAIN. Those important endpoints are also incorporated in the ongoing phase III trials of gefitinib and erlotinib, EGFR TKIs which are far more readily accessible at present to physicians outside the Far East.
“Whole-brain radiotherapy for brain metastases from EGFR-mutated non–small cell lung cancer is a vanishing approach. The remaining role in this setting is as salvage in cases of symptomatic primary or secondary resistance to EGFR TKIs,” said Dr. Jassem, head of the department of oncology and radiotherapy at the Medical University of Gdansk, Poland.
By way of background, he noted that 10%-15% of patients already have brain metastases at the time of diagnosis of NSCLC, and 40% develop them eventually. EGFR-mutated primary tumors are particularly likely to metastasize to the brain.
Remaining questions in the wake of the BRAIN trial include the efficacy of gefitinib and erlotinib versus WBI plus chemotherapy, as well as the broader question of the efficacy of EGFR TKIs in non-Asian patients with brain metastases.
“Most of the studies of EGFR TKIs have been in East Asian populations. These agents are particularly active in East Asians. There’s a question as to whether the BRAIN results can be applied to other populations,” Dr. Jassem said.
The BRAIN study was sponsored by the Guangdong Association of Clinical Trials. Dr. Wu reported serving as a consultant to AstraZeneca, Roche, Eli Lilly, Pfizer, and Sanofi. Dr. Jassem reported having no financial conflicts of interest.
AT WCLC 2016
Key clinical point:
Major finding: Median intracranial progression-free survival in patients with multiple brain metastases from EGFR-mutated NSCLC was 10 months in those randomized to icotinib, compared with 4.8 months with standard-of-care whole-brain irradiation plus chemotherapy.
Data source: This phase III multicenter Chinese trial included 158 patients with multiple brain metastases from EGFR-mutated NSCLC.
Disclosures: The BRAIN study was sponsored by the Guangdong Association of Clinical Trials. The presenter reported serving as a consultant to AstraZeneca, Roche, Eli Lilly, Pfizer, and Sanofi.
Ganetespib fails to hit mark in phase III NSCLC trial
VIENNA – The addition of ganetespib to docetaxel for the treatment of advanced non–small cell lung cancer (NSCLC) did not live up to its earlier promise of improved efficacy over docetaxel alone in a phase III study.
Interim results of the GALAXY-2 study, which led to the trial’s termination for futility, showed that there was no difference in the primary endpoint of overall survival (OS). The median OS was a respective 10.9 months and 10.5 months for the combination of ganetespib plus docetaxel versus docetaxel alone (hazard ratio, 1.111, 95% confidence interval, 0.899-1.372, P = .03293).
The combination had looked promising in the phase II GALAXY-1 study (Ann Oncol. 2015;26:1741-8), with a trend towards improved PFS and OS, with patients diagnosed with advanced disease perhaps gaining the most benefit.
“The addition of ganetespib to docetaxel did not result in improved efficacy for the salvage therapy of patients with advanced-stage lung adenocarcinoma,” Rathi Pillai, MD, said at the World Conference on Lung Cancer.
“Based on these findings, further development of Hsp [heat shock protein] 90 inhibitors in lung cancer should be limited to patients with driver mutations with relevant client proteins,” added Dr. Pillai of the Winship Cancer Institute at Emory University in Atlanta.
In GALAXY-2, 672 patients were randomized, 1:1, to receive docetaxel (75 mg/m2 on day 1) with ganetespib (150 mg/m2 on days 1 and 15) or a placebo every 3 weeks. The median number of cycles received was five in the combination arm and four in the docetaxel-only arm. All patients had advanced non–small cell lung adenocarcinoma diagnosed at least 6 months prior to enrollment and had received only one prior treatment regimen.
Response rates were similar between the two groups, with 13.7% and 16% of ganetespib/docetaxel– and docetaxel/placebo–treated patients achieving a partial response (P = .448) and 56.1% and 50.1%, respectively, having stable disease (P = .123).
Almost two-thirds (65%) of patients given ganetespib/docetaxel reported any grade 3-4 adverse event versus 54% of patients given docetaxel/placebo. The most frequent treatment-emergent grade 3-4 adverse event was neutropenia, occurring in 34.6% ad 29.5% of patients in each arm (P = .1807), with only diarrhea (46.2% vs. 14.6%; P less than .0001) and weight loss (11.3% vs. 5.2%; P = .0044) occurring more frequently in the combination than the docetaxel-only arm.
Ganetespib is a second-generation Hsp90 inhibitor and the rationale for using Hsp90 inhibitors as cancer therapy remains sound, Dr. Gandara maintained. One of the issues is finding a biomarker to predict the benefit for these drugs in a clinical setting, he said. In the phase II GALAXY-1 trial it was noted that patients diagnosed with advanced disease more than 6 months prior to study entry fared better than those with more recent diagnosis if they were treated with ganetespib/docetaxel than docetaxel alone. Patients with elevated levels of lactate dehydrogenase (eLDH) also seemed to do better than those with normal LDH levels if they were treated with the combination.
These two subpopulations were specifically looked at the in the phase III study, but again no differences in OS or PFS were observed, nor were differences observed in any of the other subgroups analyzed, which included EGFR- and ALK-negative patients, by response rate or progression because of new metastatic lesions.
“Were these the right surrogates to use as predictive biomarkers for heat shock protein inhibition? Of course, in retrospect, it is easy to say that they were not,” Dr. Gandara said.
“Is there still an opportunity for this class of drugs to make a meaningful impact in NSCLC? In my own opinion I think the answer is yes, but not in this broad page approach and not with these potential surrogates as were used in GALAXY-2,” he said at the meeting sponsored by the International Association for the Study of Lung Cancer.
The study was sponsored by Synta Pharmaceuticals, which became part of Madrigal Pharmaceuticals in June 2016. Dr. Pillai reported having no financial disclosures. Dr. Gandara disclosed that he had received research grants from several pharmaceutical companies.
VIENNA – The addition of ganetespib to docetaxel for the treatment of advanced non–small cell lung cancer (NSCLC) did not live up to its earlier promise of improved efficacy over docetaxel alone in a phase III study.
Interim results of the GALAXY-2 study, which led to the trial’s termination for futility, showed that there was no difference in the primary endpoint of overall survival (OS). The median OS was a respective 10.9 months and 10.5 months for the combination of ganetespib plus docetaxel versus docetaxel alone (hazard ratio, 1.111, 95% confidence interval, 0.899-1.372, P = .03293).
The combination had looked promising in the phase II GALAXY-1 study (Ann Oncol. 2015;26:1741-8), with a trend towards improved PFS and OS, with patients diagnosed with advanced disease perhaps gaining the most benefit.
“The addition of ganetespib to docetaxel did not result in improved efficacy for the salvage therapy of patients with advanced-stage lung adenocarcinoma,” Rathi Pillai, MD, said at the World Conference on Lung Cancer.
“Based on these findings, further development of Hsp [heat shock protein] 90 inhibitors in lung cancer should be limited to patients with driver mutations with relevant client proteins,” added Dr. Pillai of the Winship Cancer Institute at Emory University in Atlanta.
In GALAXY-2, 672 patients were randomized, 1:1, to receive docetaxel (75 mg/m2 on day 1) with ganetespib (150 mg/m2 on days 1 and 15) or a placebo every 3 weeks. The median number of cycles received was five in the combination arm and four in the docetaxel-only arm. All patients had advanced non–small cell lung adenocarcinoma diagnosed at least 6 months prior to enrollment and had received only one prior treatment regimen.
Response rates were similar between the two groups, with 13.7% and 16% of ganetespib/docetaxel– and docetaxel/placebo–treated patients achieving a partial response (P = .448) and 56.1% and 50.1%, respectively, having stable disease (P = .123).
Almost two-thirds (65%) of patients given ganetespib/docetaxel reported any grade 3-4 adverse event versus 54% of patients given docetaxel/placebo. The most frequent treatment-emergent grade 3-4 adverse event was neutropenia, occurring in 34.6% ad 29.5% of patients in each arm (P = .1807), with only diarrhea (46.2% vs. 14.6%; P less than .0001) and weight loss (11.3% vs. 5.2%; P = .0044) occurring more frequently in the combination than the docetaxel-only arm.
Ganetespib is a second-generation Hsp90 inhibitor and the rationale for using Hsp90 inhibitors as cancer therapy remains sound, Dr. Gandara maintained. One of the issues is finding a biomarker to predict the benefit for these drugs in a clinical setting, he said. In the phase II GALAXY-1 trial it was noted that patients diagnosed with advanced disease more than 6 months prior to study entry fared better than those with more recent diagnosis if they were treated with ganetespib/docetaxel than docetaxel alone. Patients with elevated levels of lactate dehydrogenase (eLDH) also seemed to do better than those with normal LDH levels if they were treated with the combination.
These two subpopulations were specifically looked at the in the phase III study, but again no differences in OS or PFS were observed, nor were differences observed in any of the other subgroups analyzed, which included EGFR- and ALK-negative patients, by response rate or progression because of new metastatic lesions.
“Were these the right surrogates to use as predictive biomarkers for heat shock protein inhibition? Of course, in retrospect, it is easy to say that they were not,” Dr. Gandara said.
“Is there still an opportunity for this class of drugs to make a meaningful impact in NSCLC? In my own opinion I think the answer is yes, but not in this broad page approach and not with these potential surrogates as were used in GALAXY-2,” he said at the meeting sponsored by the International Association for the Study of Lung Cancer.
The study was sponsored by Synta Pharmaceuticals, which became part of Madrigal Pharmaceuticals in June 2016. Dr. Pillai reported having no financial disclosures. Dr. Gandara disclosed that he had received research grants from several pharmaceutical companies.
VIENNA – The addition of ganetespib to docetaxel for the treatment of advanced non–small cell lung cancer (NSCLC) did not live up to its earlier promise of improved efficacy over docetaxel alone in a phase III study.
Interim results of the GALAXY-2 study, which led to the trial’s termination for futility, showed that there was no difference in the primary endpoint of overall survival (OS). The median OS was a respective 10.9 months and 10.5 months for the combination of ganetespib plus docetaxel versus docetaxel alone (hazard ratio, 1.111, 95% confidence interval, 0.899-1.372, P = .03293).
The combination had looked promising in the phase II GALAXY-1 study (Ann Oncol. 2015;26:1741-8), with a trend towards improved PFS and OS, with patients diagnosed with advanced disease perhaps gaining the most benefit.
“The addition of ganetespib to docetaxel did not result in improved efficacy for the salvage therapy of patients with advanced-stage lung adenocarcinoma,” Rathi Pillai, MD, said at the World Conference on Lung Cancer.
“Based on these findings, further development of Hsp [heat shock protein] 90 inhibitors in lung cancer should be limited to patients with driver mutations with relevant client proteins,” added Dr. Pillai of the Winship Cancer Institute at Emory University in Atlanta.
In GALAXY-2, 672 patients were randomized, 1:1, to receive docetaxel (75 mg/m2 on day 1) with ganetespib (150 mg/m2 on days 1 and 15) or a placebo every 3 weeks. The median number of cycles received was five in the combination arm and four in the docetaxel-only arm. All patients had advanced non–small cell lung adenocarcinoma diagnosed at least 6 months prior to enrollment and had received only one prior treatment regimen.
Response rates were similar between the two groups, with 13.7% and 16% of ganetespib/docetaxel– and docetaxel/placebo–treated patients achieving a partial response (P = .448) and 56.1% and 50.1%, respectively, having stable disease (P = .123).
Almost two-thirds (65%) of patients given ganetespib/docetaxel reported any grade 3-4 adverse event versus 54% of patients given docetaxel/placebo. The most frequent treatment-emergent grade 3-4 adverse event was neutropenia, occurring in 34.6% ad 29.5% of patients in each arm (P = .1807), with only diarrhea (46.2% vs. 14.6%; P less than .0001) and weight loss (11.3% vs. 5.2%; P = .0044) occurring more frequently in the combination than the docetaxel-only arm.
Ganetespib is a second-generation Hsp90 inhibitor and the rationale for using Hsp90 inhibitors as cancer therapy remains sound, Dr. Gandara maintained. One of the issues is finding a biomarker to predict the benefit for these drugs in a clinical setting, he said. In the phase II GALAXY-1 trial it was noted that patients diagnosed with advanced disease more than 6 months prior to study entry fared better than those with more recent diagnosis if they were treated with ganetespib/docetaxel than docetaxel alone. Patients with elevated levels of lactate dehydrogenase (eLDH) also seemed to do better than those with normal LDH levels if they were treated with the combination.
These two subpopulations were specifically looked at the in the phase III study, but again no differences in OS or PFS were observed, nor were differences observed in any of the other subgroups analyzed, which included EGFR- and ALK-negative patients, by response rate or progression because of new metastatic lesions.
“Were these the right surrogates to use as predictive biomarkers for heat shock protein inhibition? Of course, in retrospect, it is easy to say that they were not,” Dr. Gandara said.
“Is there still an opportunity for this class of drugs to make a meaningful impact in NSCLC? In my own opinion I think the answer is yes, but not in this broad page approach and not with these potential surrogates as were used in GALAXY-2,” he said at the meeting sponsored by the International Association for the Study of Lung Cancer.
The study was sponsored by Synta Pharmaceuticals, which became part of Madrigal Pharmaceuticals in June 2016. Dr. Pillai reported having no financial disclosures. Dr. Gandara disclosed that he had received research grants from several pharmaceutical companies.
AT WCLC 2016
Key clinical point: Adding ganetespib to docetaxel did not improve the survival of the patients studied versus docetaxel alone.
Major finding: Median overall survival was 10.9 months for ganetespib/docetaxel and 10.5 months for docetaxel alone (HR, 1.111; 95% CI, 0.899-1.372; P = .03293).
Data source: International, randomized, phase III, open-label GALAXY-2 study of docetaxel with or without ganetespib in 672 patients with advanced non–small cell lung adenocarcinoma.
Disclosures: The study was sponsored by Synta Pharmaceuticals (Madrigal Pharmaceuticals since June 2016). Dr. Pillai reported having no financial disclosures. Dr. Gandara disclosed that he had received research grants from several pharmaceutical companies.