User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'main-prefix')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
Mixed results for intensive home care for psychiatric crises
Intensive home treatment may offer an alternative to inpatient care for patients in acute psychiatric crisis – but the intervention is no outright substitute, new research suggests.
However, there was no difference between treatment groups in improvement in quality of life or patient satisfaction; and a reduction in symptom severity noted after 6 weeks of home treatment faded within 6 months.
“We found no differences in admission rates either, which suggests that intensive home treatment is not a substitute for inpatient care but a different treatment opportunity for psychiatric patients in crisis,” Jurgen Cornelis, MD, Arkin Institute for Mental Health, Amsterdam, and colleagues write.
The findings were published online in The Lancet Psychiatry.
Increasingly popular
“Intensive home treatment is increasingly popular as an alternative to hospitalization. It was developed to prevent or reduce levels of inpatient care and facilitate the transition between inpatient care and low-intensity outpatient care,” the investigators write.
However, there have previously been only two randomized controlled trials published that assessed this type of care, resulting in “somewhat conflicting findings,” they add.
For the current study, participants presented to psychiatric emergency wards at two medical centers in the Netherlands. They were included only if they were able to offer informed consent within 14 days.
The intensive home treatment group (n = 183) worked with a multidisciplinary team that designed a care plan tailored to their specific crisis. Treatment components included pharmacotherapy, up to three home visits each day, psychoeducation, brief supportive and cognitive behavioral therapy, social care, and support and empowerment of the patient’s informal care system.
The usual care group (n = 63) commonly received a combination of highly intensive inpatient treatment in the first phase and outpatient treatment up to two times a week in the second phase. Treatment included similar components as those in intensive home treatment.
The most common primary clinical diagnosis in both groups was mood disorder, followed by psychotic disorders, personality disorders, or anxiety disorders.
The home treatment group had a significantly higher total mean item score on the Brief Psychiatric Rating Scale (BPRS) at baseline (2.23 vs. 2.04, P = .04).
Mixed results
Results at 6 weeks showed the number of hospital days was 25.3% lower in the home treatment group, compared with those who received usual care.
That trend continued at 1 year, with the intensive home treatment group recording 36.6% fewer hospital days than the usual care group (mean, 42.5 days vs. 67 days, respectively; P = .03).
However, the number of patients who were admitted in the first 6 weeks and at 1 year stayed the same, as did the mean number of admissions per patient over 12 months.
The home treatment group reported significantly fewer symptoms on the BPRS depression and anxiety scale at 6 weeks, compared with the usual treatment group (P = .025), but that difference was not maintained after 6 months.
The number of adverse events, including suicide attempts, was similar between the groups. Three patients in the home treatment group and two in the usual care group died by suicide.
“Future research should focus on which components of intensive home treatment or hospitalization can be used when, for whom, and meet which goals, so that both hospital care and intensive home treatment can be used proportionally and efficiently for patients in psychiatric crisis,” the investigators write.
Not generalizable?
In an accompanying editorial, Claire Henderson, PhD, Institute of Psychiatry, Psychology, and Neuroscience at King’s College London, noted that generalizing the study’s results to other countries could be problematic, especially to regions such as North America, which have shorter lengths of stay for psychiatric hospitalization.
“Future trials looking at intensive home treatment would be most informative if done in countries with relatively short lengths of stay, and without separate crisis services for people receiving assertive community treatment,” Dr. Henderson writes.
The study was funded by De Stichting tot Steun Vereniging voor Christelijke Verzorging van Geestes-en Zenuwzieken. The investigators and Dr. Henderson have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Intensive home treatment may offer an alternative to inpatient care for patients in acute psychiatric crisis – but the intervention is no outright substitute, new research suggests.
However, there was no difference between treatment groups in improvement in quality of life or patient satisfaction; and a reduction in symptom severity noted after 6 weeks of home treatment faded within 6 months.
“We found no differences in admission rates either, which suggests that intensive home treatment is not a substitute for inpatient care but a different treatment opportunity for psychiatric patients in crisis,” Jurgen Cornelis, MD, Arkin Institute for Mental Health, Amsterdam, and colleagues write.
The findings were published online in The Lancet Psychiatry.
Increasingly popular
“Intensive home treatment is increasingly popular as an alternative to hospitalization. It was developed to prevent or reduce levels of inpatient care and facilitate the transition between inpatient care and low-intensity outpatient care,” the investigators write.
However, there have previously been only two randomized controlled trials published that assessed this type of care, resulting in “somewhat conflicting findings,” they add.
For the current study, participants presented to psychiatric emergency wards at two medical centers in the Netherlands. They were included only if they were able to offer informed consent within 14 days.
The intensive home treatment group (n = 183) worked with a multidisciplinary team that designed a care plan tailored to their specific crisis. Treatment components included pharmacotherapy, up to three home visits each day, psychoeducation, brief supportive and cognitive behavioral therapy, social care, and support and empowerment of the patient’s informal care system.
The usual care group (n = 63) commonly received a combination of highly intensive inpatient treatment in the first phase and outpatient treatment up to two times a week in the second phase. Treatment included similar components as those in intensive home treatment.
The most common primary clinical diagnosis in both groups was mood disorder, followed by psychotic disorders, personality disorders, or anxiety disorders.
The home treatment group had a significantly higher total mean item score on the Brief Psychiatric Rating Scale (BPRS) at baseline (2.23 vs. 2.04, P = .04).
Mixed results
Results at 6 weeks showed the number of hospital days was 25.3% lower in the home treatment group, compared with those who received usual care.
That trend continued at 1 year, with the intensive home treatment group recording 36.6% fewer hospital days than the usual care group (mean, 42.5 days vs. 67 days, respectively; P = .03).
However, the number of patients who were admitted in the first 6 weeks and at 1 year stayed the same, as did the mean number of admissions per patient over 12 months.
The home treatment group reported significantly fewer symptoms on the BPRS depression and anxiety scale at 6 weeks, compared with the usual treatment group (P = .025), but that difference was not maintained after 6 months.
The number of adverse events, including suicide attempts, was similar between the groups. Three patients in the home treatment group and two in the usual care group died by suicide.
“Future research should focus on which components of intensive home treatment or hospitalization can be used when, for whom, and meet which goals, so that both hospital care and intensive home treatment can be used proportionally and efficiently for patients in psychiatric crisis,” the investigators write.
Not generalizable?
In an accompanying editorial, Claire Henderson, PhD, Institute of Psychiatry, Psychology, and Neuroscience at King’s College London, noted that generalizing the study’s results to other countries could be problematic, especially to regions such as North America, which have shorter lengths of stay for psychiatric hospitalization.
“Future trials looking at intensive home treatment would be most informative if done in countries with relatively short lengths of stay, and without separate crisis services for people receiving assertive community treatment,” Dr. Henderson writes.
The study was funded by De Stichting tot Steun Vereniging voor Christelijke Verzorging van Geestes-en Zenuwzieken. The investigators and Dr. Henderson have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Intensive home treatment may offer an alternative to inpatient care for patients in acute psychiatric crisis – but the intervention is no outright substitute, new research suggests.
However, there was no difference between treatment groups in improvement in quality of life or patient satisfaction; and a reduction in symptom severity noted after 6 weeks of home treatment faded within 6 months.
“We found no differences in admission rates either, which suggests that intensive home treatment is not a substitute for inpatient care but a different treatment opportunity for psychiatric patients in crisis,” Jurgen Cornelis, MD, Arkin Institute for Mental Health, Amsterdam, and colleagues write.
The findings were published online in The Lancet Psychiatry.
Increasingly popular
“Intensive home treatment is increasingly popular as an alternative to hospitalization. It was developed to prevent or reduce levels of inpatient care and facilitate the transition between inpatient care and low-intensity outpatient care,” the investigators write.
However, there have previously been only two randomized controlled trials published that assessed this type of care, resulting in “somewhat conflicting findings,” they add.
For the current study, participants presented to psychiatric emergency wards at two medical centers in the Netherlands. They were included only if they were able to offer informed consent within 14 days.
The intensive home treatment group (n = 183) worked with a multidisciplinary team that designed a care plan tailored to their specific crisis. Treatment components included pharmacotherapy, up to three home visits each day, psychoeducation, brief supportive and cognitive behavioral therapy, social care, and support and empowerment of the patient’s informal care system.
The usual care group (n = 63) commonly received a combination of highly intensive inpatient treatment in the first phase and outpatient treatment up to two times a week in the second phase. Treatment included similar components as those in intensive home treatment.
The most common primary clinical diagnosis in both groups was mood disorder, followed by psychotic disorders, personality disorders, or anxiety disorders.
The home treatment group had a significantly higher total mean item score on the Brief Psychiatric Rating Scale (BPRS) at baseline (2.23 vs. 2.04, P = .04).
Mixed results
Results at 6 weeks showed the number of hospital days was 25.3% lower in the home treatment group, compared with those who received usual care.
That trend continued at 1 year, with the intensive home treatment group recording 36.6% fewer hospital days than the usual care group (mean, 42.5 days vs. 67 days, respectively; P = .03).
However, the number of patients who were admitted in the first 6 weeks and at 1 year stayed the same, as did the mean number of admissions per patient over 12 months.
The home treatment group reported significantly fewer symptoms on the BPRS depression and anxiety scale at 6 weeks, compared with the usual treatment group (P = .025), but that difference was not maintained after 6 months.
The number of adverse events, including suicide attempts, was similar between the groups. Three patients in the home treatment group and two in the usual care group died by suicide.
“Future research should focus on which components of intensive home treatment or hospitalization can be used when, for whom, and meet which goals, so that both hospital care and intensive home treatment can be used proportionally and efficiently for patients in psychiatric crisis,” the investigators write.
Not generalizable?
In an accompanying editorial, Claire Henderson, PhD, Institute of Psychiatry, Psychology, and Neuroscience at King’s College London, noted that generalizing the study’s results to other countries could be problematic, especially to regions such as North America, which have shorter lengths of stay for psychiatric hospitalization.
“Future trials looking at intensive home treatment would be most informative if done in countries with relatively short lengths of stay, and without separate crisis services for people receiving assertive community treatment,” Dr. Henderson writes.
The study was funded by De Stichting tot Steun Vereniging voor Christelijke Verzorging van Geestes-en Zenuwzieken. The investigators and Dr. Henderson have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE LANCET PSYCHIATRY
University to train ‘trip facilitators’ for psychedelic therapy
The UC Berkeley Center for the Science of Psychedelics (BCSP) training program aims to create a cadre of facilitators who will be ready to help if, and when, substances such as psilocybin, MDMA, and LSD are approved in the United States, Tina Trujillo, PhD, an associate professor at UC Berkeley’s School of Education, told reporters at a press briefing.
Hallucinogenic drugs are on the Drug Enforcement Administration’s (DEA) Schedule I list because they are considered to have no currently accepted medical use and high abuse potential. But there has been an explosion of research into psychedelics – combined with therapy – as treatment for severe depression, posttraumatic stress disorder, substance-use disorder, and other mental health conditions. Some 100 clinical trials are underway.
“The estimates are that we’re going to need 100,000 trained psychedelic facilitators once psilocybin and MDMA are approved by the [U.S. Food and Drug Administration] FDA, which is expected to happen within the next 5 years or so,” said Michael Pollan, co-founder of the BCSP. He is author of “How to Change Your Mind,” a 2018 book about psychedelics, which has been adapted into a four-part docuseries currently streaming on Netflix.
Nine-month program
The first 24 trainees – a mix of physicians, nurses, psychotherapists, and social workers – will undergo 9 months of education and preparation in “the technical, the cultural, the mystical, and the ethical dimensions of psychedelic facilitation,” said Dr. Trujillo.
The BCSP’s Certificate Program in Psychedelic Facilitation will have “an emphasis on both western science and spiritual care traditions,” she said.
Trainees will receive 150 instructional hours and a 25-hour practicum and will take part in a final 5-day retreat. The program will initially focus only on psilocybin, in part because the BCSP is involved in several FDA-approved trials testing the drug.
In one study – which aims to enroll participants in the fall – researchers will use functional MRI to examine the neural correlates of the psychedelic experience in individuals receiving low-dose psilocybin.
Eligible trainees will have an opportunity to participate in the Berkeley psilocybin trials and “increase their first-hand knowledge,” Dr. Trujillo said.
At the conclusion of the training, students will receive a certificate, “not a license or sanction to go off and practice,” she said. She noted that eventually, when facilitation is legal, certificate holders will be able to practice in clinical research settings or in health care settings.
Growing acceptance in psychiatry
Mr. Pollan said there has been a radical change in acceptance of psychedelics as potential therapies.
“The shift from destroyer of young minds in the ‘60s to effective medicine in the 2020s is as sudden as it is confusing for many people,” he said. He noted that the Berkeley center hopes to provide evidence-based information for journalists, the public, and clinicians.
He said that after his book was released, he expected pushback from “mainstream psychiatry.” Instead, he was invited to give grand rounds talks. Psychiatrists are “very open to the potential of psychedelics,” Mr. Pollan said.
“The reason for that, quite frankly, is because they are desperate,” he said. “The tools of conventional psychiatry to deal with things like depression and anxiety and addiction are not very good, and some of them are failing,” he said.
Mr. Pollan cited some other indicators of acceptance. In Oregon, beginning in 2023, psilocybin will be available to anyone older than 21 years but only for use in licensed facilities with licensed facilitators, and the substance must be produced by a licensed manufacturer.
In November, Colorado will ask voters whether they want to follow the Oregon model and legalize psilocybin. If approved, another Colorado ballot initiative would decriminalize possession.
Mr. Pollan noted that Cory Booker, the Democratic Senator from New Jersey, and Rand Paul, a conservative Republican Senator from Kentucky, have found a common cause, introducing legislation to let select terminally ill patients have access to psychedelics and other Schedule I drugs.
Some 400 companies are conducting research on psychedelics. Researchers must have a license from the DEA to obtain and study the substances, Andrea Gomez, assistant professor of neurobiology at UC Berkeley, told reporters.
She said growing interest in the potential of these drugs might lead more researchers to “jump through the hoops” to get the licenses. The floodgates would truly open if the National Institutes of Health started funding studies, she said.
A version of this article first appeared on Medscape.com.
The UC Berkeley Center for the Science of Psychedelics (BCSP) training program aims to create a cadre of facilitators who will be ready to help if, and when, substances such as psilocybin, MDMA, and LSD are approved in the United States, Tina Trujillo, PhD, an associate professor at UC Berkeley’s School of Education, told reporters at a press briefing.
Hallucinogenic drugs are on the Drug Enforcement Administration’s (DEA) Schedule I list because they are considered to have no currently accepted medical use and high abuse potential. But there has been an explosion of research into psychedelics – combined with therapy – as treatment for severe depression, posttraumatic stress disorder, substance-use disorder, and other mental health conditions. Some 100 clinical trials are underway.
“The estimates are that we’re going to need 100,000 trained psychedelic facilitators once psilocybin and MDMA are approved by the [U.S. Food and Drug Administration] FDA, which is expected to happen within the next 5 years or so,” said Michael Pollan, co-founder of the BCSP. He is author of “How to Change Your Mind,” a 2018 book about psychedelics, which has been adapted into a four-part docuseries currently streaming on Netflix.
Nine-month program
The first 24 trainees – a mix of physicians, nurses, psychotherapists, and social workers – will undergo 9 months of education and preparation in “the technical, the cultural, the mystical, and the ethical dimensions of psychedelic facilitation,” said Dr. Trujillo.
The BCSP’s Certificate Program in Psychedelic Facilitation will have “an emphasis on both western science and spiritual care traditions,” she said.
Trainees will receive 150 instructional hours and a 25-hour practicum and will take part in a final 5-day retreat. The program will initially focus only on psilocybin, in part because the BCSP is involved in several FDA-approved trials testing the drug.
In one study – which aims to enroll participants in the fall – researchers will use functional MRI to examine the neural correlates of the psychedelic experience in individuals receiving low-dose psilocybin.
Eligible trainees will have an opportunity to participate in the Berkeley psilocybin trials and “increase their first-hand knowledge,” Dr. Trujillo said.
At the conclusion of the training, students will receive a certificate, “not a license or sanction to go off and practice,” she said. She noted that eventually, when facilitation is legal, certificate holders will be able to practice in clinical research settings or in health care settings.
Growing acceptance in psychiatry
Mr. Pollan said there has been a radical change in acceptance of psychedelics as potential therapies.
“The shift from destroyer of young minds in the ‘60s to effective medicine in the 2020s is as sudden as it is confusing for many people,” he said. He noted that the Berkeley center hopes to provide evidence-based information for journalists, the public, and clinicians.
He said that after his book was released, he expected pushback from “mainstream psychiatry.” Instead, he was invited to give grand rounds talks. Psychiatrists are “very open to the potential of psychedelics,” Mr. Pollan said.
“The reason for that, quite frankly, is because they are desperate,” he said. “The tools of conventional psychiatry to deal with things like depression and anxiety and addiction are not very good, and some of them are failing,” he said.
Mr. Pollan cited some other indicators of acceptance. In Oregon, beginning in 2023, psilocybin will be available to anyone older than 21 years but only for use in licensed facilities with licensed facilitators, and the substance must be produced by a licensed manufacturer.
In November, Colorado will ask voters whether they want to follow the Oregon model and legalize psilocybin. If approved, another Colorado ballot initiative would decriminalize possession.
Mr. Pollan noted that Cory Booker, the Democratic Senator from New Jersey, and Rand Paul, a conservative Republican Senator from Kentucky, have found a common cause, introducing legislation to let select terminally ill patients have access to psychedelics and other Schedule I drugs.
Some 400 companies are conducting research on psychedelics. Researchers must have a license from the DEA to obtain and study the substances, Andrea Gomez, assistant professor of neurobiology at UC Berkeley, told reporters.
She said growing interest in the potential of these drugs might lead more researchers to “jump through the hoops” to get the licenses. The floodgates would truly open if the National Institutes of Health started funding studies, she said.
A version of this article first appeared on Medscape.com.
The UC Berkeley Center for the Science of Psychedelics (BCSP) training program aims to create a cadre of facilitators who will be ready to help if, and when, substances such as psilocybin, MDMA, and LSD are approved in the United States, Tina Trujillo, PhD, an associate professor at UC Berkeley’s School of Education, told reporters at a press briefing.
Hallucinogenic drugs are on the Drug Enforcement Administration’s (DEA) Schedule I list because they are considered to have no currently accepted medical use and high abuse potential. But there has been an explosion of research into psychedelics – combined with therapy – as treatment for severe depression, posttraumatic stress disorder, substance-use disorder, and other mental health conditions. Some 100 clinical trials are underway.
“The estimates are that we’re going to need 100,000 trained psychedelic facilitators once psilocybin and MDMA are approved by the [U.S. Food and Drug Administration] FDA, which is expected to happen within the next 5 years or so,” said Michael Pollan, co-founder of the BCSP. He is author of “How to Change Your Mind,” a 2018 book about psychedelics, which has been adapted into a four-part docuseries currently streaming on Netflix.
Nine-month program
The first 24 trainees – a mix of physicians, nurses, psychotherapists, and social workers – will undergo 9 months of education and preparation in “the technical, the cultural, the mystical, and the ethical dimensions of psychedelic facilitation,” said Dr. Trujillo.
The BCSP’s Certificate Program in Psychedelic Facilitation will have “an emphasis on both western science and spiritual care traditions,” she said.
Trainees will receive 150 instructional hours and a 25-hour practicum and will take part in a final 5-day retreat. The program will initially focus only on psilocybin, in part because the BCSP is involved in several FDA-approved trials testing the drug.
In one study – which aims to enroll participants in the fall – researchers will use functional MRI to examine the neural correlates of the psychedelic experience in individuals receiving low-dose psilocybin.
Eligible trainees will have an opportunity to participate in the Berkeley psilocybin trials and “increase their first-hand knowledge,” Dr. Trujillo said.
At the conclusion of the training, students will receive a certificate, “not a license or sanction to go off and practice,” she said. She noted that eventually, when facilitation is legal, certificate holders will be able to practice in clinical research settings or in health care settings.
Growing acceptance in psychiatry
Mr. Pollan said there has been a radical change in acceptance of psychedelics as potential therapies.
“The shift from destroyer of young minds in the ‘60s to effective medicine in the 2020s is as sudden as it is confusing for many people,” he said. He noted that the Berkeley center hopes to provide evidence-based information for journalists, the public, and clinicians.
He said that after his book was released, he expected pushback from “mainstream psychiatry.” Instead, he was invited to give grand rounds talks. Psychiatrists are “very open to the potential of psychedelics,” Mr. Pollan said.
“The reason for that, quite frankly, is because they are desperate,” he said. “The tools of conventional psychiatry to deal with things like depression and anxiety and addiction are not very good, and some of them are failing,” he said.
Mr. Pollan cited some other indicators of acceptance. In Oregon, beginning in 2023, psilocybin will be available to anyone older than 21 years but only for use in licensed facilities with licensed facilitators, and the substance must be produced by a licensed manufacturer.
In November, Colorado will ask voters whether they want to follow the Oregon model and legalize psilocybin. If approved, another Colorado ballot initiative would decriminalize possession.
Mr. Pollan noted that Cory Booker, the Democratic Senator from New Jersey, and Rand Paul, a conservative Republican Senator from Kentucky, have found a common cause, introducing legislation to let select terminally ill patients have access to psychedelics and other Schedule I drugs.
Some 400 companies are conducting research on psychedelics. Researchers must have a license from the DEA to obtain and study the substances, Andrea Gomez, assistant professor of neurobiology at UC Berkeley, told reporters.
She said growing interest in the potential of these drugs might lead more researchers to “jump through the hoops” to get the licenses. The floodgates would truly open if the National Institutes of Health started funding studies, she said.
A version of this article first appeared on Medscape.com.
Sexual assault flagged as a possible psychosis trigger
A new study sheds light on some of the risk factors for the development of psychosis, including the potentially causative role of sexual assault.
Investigators conducted an exposome-wide association analysis on more than 155,000 individuals. Of more than 140 correlates of psychotic experiences that they identified, they narrowed it down to 36 variables, which they further explored using Mendelian randomization analysis.
On the other hand, having experienced a physical violent crime, cannabis use, and prolonged worry after embarrassment showed a pleiotropic association and appeared to be an aftereffect of psychotic experience.
“From a public health perspective, we need more investment in comprehensive strategies to prevent traumatic experiences at the population level to decrease the burden of psychosis,” senior author Sinan Gülöksüz, MD, PhD, associate professor in the department of psychiatry and neuropsychiatry, Maastricht University Medical Center, the Netherlands, said in an interview.
“From a clinical perspective, clinicians should be aware of the harmful influence of traumatic experiences on mental health and address this through interventions such as trauma-informed care,” he said.
The study was published online in JAMA Psychiatry.
‘Disentangling’ cause and effect
“Previous research has shown associations between psychosis and a few environmental factors, such as substance use, urbanicity, pregnancy complications, and traumatic experiences, but research has so far investigated only a few specific environmental factors by singling them out in individual studies,” Dr. Gülöksüz said.
“Yet, environment is a much more complex and interactive network that includes many factors shaping our health – where we live, what we eat, our lifestyle preferences and habits such as exercise and smoking, and our social surrounding,” he continued. “Rarely has it been possible to understand whether these environmental factors have causal roles in developing psychosis.”
To investigate the question, the researchers turned to the UK Biobank, one of the largest population-based datasets in the world. The current study focused on individuals with completed data on mental questionnaires that assessed psychotic experiences (n = 155,247; mean [SD] age, 55.94 [7.74] years; 57% female).
They began by conducting an exposome-wide association study, using logistic regression analyses with psychotic experiences as the outcome and adjusting all analyses for age and sex.
“Initially, we identified many associations between environmental factors and psychotic experiences in this large cohort,” Dr. Gülöksüz reported.
In the final multivariable model, variables associated with psychotic experiences were further analyzed using “genetically informed approaches to probe potential associations.”
The researchers utilized Mendelian randomization (MR) methodology “to disentangle cause and effect in this observational study,” Dr. Gülöksüz said. “This method reduces confounding and reverse causation in observational studies by using genetic variants that have been passed on from generation to generation randomly as instruments.”
MR analysis “has allowed us to assess whether these associations reflect potentially causal influences of environmental factors on psychotic experiences,” he added.
Well-studied and unexplored risk factors
The researchers identified 162 variables associated with psychotic experiences in the discovery dataset and were able to replicate 148. When these 148 variables were subjected to multivariable analyses, 36 were found to be statistically significantly associated with psychotic experiences. Of these variables, 28 had “significant genetic overlap” with psychotic experiences.
When the researchers conducted one-sample MR analyses, they found forward associations with three variables and reverse associations with three variables.
Forward associations were found with ever having experienced sexual assault (odds ratio [OR], 1.32; 95% confidence interval [CI], 1.14-1.52; P = 2.67), and forward associations (with pleiotropy) were found with ever having experienced a physically violent crime and risk-taking behavior (OR, 1.25, 95% CI, 1.11-1.41; P = 3.28 and OR, 1.21, 95% CI, 1.08-1.35; P = 1.34, respectively).
“The allele scores for these 3 variables explained 0.03% to 0.23% variance of the corresponding variable” and the F statistics “ranged from 21.53 to 181.84, indicating that the results did not suffer from a weak-instrument bias,” the authors reported.
The researchers calculated an instrument based on increasing psychotic experiences risk allele scores and found that these scores explained 0.14% variance of psychotic experiences (F statistic, 19.26).
Using that calculation, they found a reverse association with having experienced a physically violent crime (OR, 1.08; 95% CI, 1.04-1.13; P = 3.92 × 10-4), cannabis use (OR, 1.11; 95% CI, 1.06-1.15; P = 2.64 × 10-6), and worrying too long after embarrassment (OR, 1.06; 95% CI, 1.03-1.10; P = 3.96 × 10-4). They then validated these associations.
The presence of all five correlates was associated with tenfold increased odds of psychotic experiences (OR, 10.63; 95% CI, 8.27-13.65, P = 1.2 × 10-114).
“Associations with psychotic experiences were found with both well-studied and unexplored multiple correlated variables,” the authors stated.
Era of ‘big data’
In a comment, Chirag Patel, PhD, associate professor of biomedical informatics at Harvard Medical School, Boston, who was not involved with the study, said he thought the study was “a nice example of a data-driven and comprehensive study of the environment coupled with attempts to triangulate evidence from genetics, made possible by biobank data.
“To guide public health policies and implementation of prevention strategies for psychosis, we need more systematic analyses and triangulate evidence with genetically informed methods to identify potentially modifiable risk factors in the era of ‘big data,’ ” he said.
“For instance, traumatic experiences contribute to poor mental and physical health, including psychosis,” Dr. Gülöksüz added.
The Kootstra Talent Fellowship, the Ophelia Research Project, and the Vidi Award from the Netherlands Scientific Organization provided funding to individual investigators. Dr. Gülöksüz and coauthors declared no relevant financial conflicts. Dr. Patel served as a reviewer on the study.
A version of this article first appeared on Medscape.com.
A new study sheds light on some of the risk factors for the development of psychosis, including the potentially causative role of sexual assault.
Investigators conducted an exposome-wide association analysis on more than 155,000 individuals. Of more than 140 correlates of psychotic experiences that they identified, they narrowed it down to 36 variables, which they further explored using Mendelian randomization analysis.
On the other hand, having experienced a physical violent crime, cannabis use, and prolonged worry after embarrassment showed a pleiotropic association and appeared to be an aftereffect of psychotic experience.
“From a public health perspective, we need more investment in comprehensive strategies to prevent traumatic experiences at the population level to decrease the burden of psychosis,” senior author Sinan Gülöksüz, MD, PhD, associate professor in the department of psychiatry and neuropsychiatry, Maastricht University Medical Center, the Netherlands, said in an interview.
“From a clinical perspective, clinicians should be aware of the harmful influence of traumatic experiences on mental health and address this through interventions such as trauma-informed care,” he said.
The study was published online in JAMA Psychiatry.
‘Disentangling’ cause and effect
“Previous research has shown associations between psychosis and a few environmental factors, such as substance use, urbanicity, pregnancy complications, and traumatic experiences, but research has so far investigated only a few specific environmental factors by singling them out in individual studies,” Dr. Gülöksüz said.
“Yet, environment is a much more complex and interactive network that includes many factors shaping our health – where we live, what we eat, our lifestyle preferences and habits such as exercise and smoking, and our social surrounding,” he continued. “Rarely has it been possible to understand whether these environmental factors have causal roles in developing psychosis.”
To investigate the question, the researchers turned to the UK Biobank, one of the largest population-based datasets in the world. The current study focused on individuals with completed data on mental questionnaires that assessed psychotic experiences (n = 155,247; mean [SD] age, 55.94 [7.74] years; 57% female).
They began by conducting an exposome-wide association study, using logistic regression analyses with psychotic experiences as the outcome and adjusting all analyses for age and sex.
“Initially, we identified many associations between environmental factors and psychotic experiences in this large cohort,” Dr. Gülöksüz reported.
In the final multivariable model, variables associated with psychotic experiences were further analyzed using “genetically informed approaches to probe potential associations.”
The researchers utilized Mendelian randomization (MR) methodology “to disentangle cause and effect in this observational study,” Dr. Gülöksüz said. “This method reduces confounding and reverse causation in observational studies by using genetic variants that have been passed on from generation to generation randomly as instruments.”
MR analysis “has allowed us to assess whether these associations reflect potentially causal influences of environmental factors on psychotic experiences,” he added.
Well-studied and unexplored risk factors
The researchers identified 162 variables associated with psychotic experiences in the discovery dataset and were able to replicate 148. When these 148 variables were subjected to multivariable analyses, 36 were found to be statistically significantly associated with psychotic experiences. Of these variables, 28 had “significant genetic overlap” with psychotic experiences.
When the researchers conducted one-sample MR analyses, they found forward associations with three variables and reverse associations with three variables.
Forward associations were found with ever having experienced sexual assault (odds ratio [OR], 1.32; 95% confidence interval [CI], 1.14-1.52; P = 2.67), and forward associations (with pleiotropy) were found with ever having experienced a physically violent crime and risk-taking behavior (OR, 1.25, 95% CI, 1.11-1.41; P = 3.28 and OR, 1.21, 95% CI, 1.08-1.35; P = 1.34, respectively).
“The allele scores for these 3 variables explained 0.03% to 0.23% variance of the corresponding variable” and the F statistics “ranged from 21.53 to 181.84, indicating that the results did not suffer from a weak-instrument bias,” the authors reported.
The researchers calculated an instrument based on increasing psychotic experiences risk allele scores and found that these scores explained 0.14% variance of psychotic experiences (F statistic, 19.26).
Using that calculation, they found a reverse association with having experienced a physically violent crime (OR, 1.08; 95% CI, 1.04-1.13; P = 3.92 × 10-4), cannabis use (OR, 1.11; 95% CI, 1.06-1.15; P = 2.64 × 10-6), and worrying too long after embarrassment (OR, 1.06; 95% CI, 1.03-1.10; P = 3.96 × 10-4). They then validated these associations.
The presence of all five correlates was associated with tenfold increased odds of psychotic experiences (OR, 10.63; 95% CI, 8.27-13.65, P = 1.2 × 10-114).
“Associations with psychotic experiences were found with both well-studied and unexplored multiple correlated variables,” the authors stated.
Era of ‘big data’
In a comment, Chirag Patel, PhD, associate professor of biomedical informatics at Harvard Medical School, Boston, who was not involved with the study, said he thought the study was “a nice example of a data-driven and comprehensive study of the environment coupled with attempts to triangulate evidence from genetics, made possible by biobank data.
“To guide public health policies and implementation of prevention strategies for psychosis, we need more systematic analyses and triangulate evidence with genetically informed methods to identify potentially modifiable risk factors in the era of ‘big data,’ ” he said.
“For instance, traumatic experiences contribute to poor mental and physical health, including psychosis,” Dr. Gülöksüz added.
The Kootstra Talent Fellowship, the Ophelia Research Project, and the Vidi Award from the Netherlands Scientific Organization provided funding to individual investigators. Dr. Gülöksüz and coauthors declared no relevant financial conflicts. Dr. Patel served as a reviewer on the study.
A version of this article first appeared on Medscape.com.
A new study sheds light on some of the risk factors for the development of psychosis, including the potentially causative role of sexual assault.
Investigators conducted an exposome-wide association analysis on more than 155,000 individuals. Of more than 140 correlates of psychotic experiences that they identified, they narrowed it down to 36 variables, which they further explored using Mendelian randomization analysis.
On the other hand, having experienced a physical violent crime, cannabis use, and prolonged worry after embarrassment showed a pleiotropic association and appeared to be an aftereffect of psychotic experience.
“From a public health perspective, we need more investment in comprehensive strategies to prevent traumatic experiences at the population level to decrease the burden of psychosis,” senior author Sinan Gülöksüz, MD, PhD, associate professor in the department of psychiatry and neuropsychiatry, Maastricht University Medical Center, the Netherlands, said in an interview.
“From a clinical perspective, clinicians should be aware of the harmful influence of traumatic experiences on mental health and address this through interventions such as trauma-informed care,” he said.
The study was published online in JAMA Psychiatry.
‘Disentangling’ cause and effect
“Previous research has shown associations between psychosis and a few environmental factors, such as substance use, urbanicity, pregnancy complications, and traumatic experiences, but research has so far investigated only a few specific environmental factors by singling them out in individual studies,” Dr. Gülöksüz said.
“Yet, environment is a much more complex and interactive network that includes many factors shaping our health – where we live, what we eat, our lifestyle preferences and habits such as exercise and smoking, and our social surrounding,” he continued. “Rarely has it been possible to understand whether these environmental factors have causal roles in developing psychosis.”
To investigate the question, the researchers turned to the UK Biobank, one of the largest population-based datasets in the world. The current study focused on individuals with completed data on mental questionnaires that assessed psychotic experiences (n = 155,247; mean [SD] age, 55.94 [7.74] years; 57% female).
They began by conducting an exposome-wide association study, using logistic regression analyses with psychotic experiences as the outcome and adjusting all analyses for age and sex.
“Initially, we identified many associations between environmental factors and psychotic experiences in this large cohort,” Dr. Gülöksüz reported.
In the final multivariable model, variables associated with psychotic experiences were further analyzed using “genetically informed approaches to probe potential associations.”
The researchers utilized Mendelian randomization (MR) methodology “to disentangle cause and effect in this observational study,” Dr. Gülöksüz said. “This method reduces confounding and reverse causation in observational studies by using genetic variants that have been passed on from generation to generation randomly as instruments.”
MR analysis “has allowed us to assess whether these associations reflect potentially causal influences of environmental factors on psychotic experiences,” he added.
Well-studied and unexplored risk factors
The researchers identified 162 variables associated with psychotic experiences in the discovery dataset and were able to replicate 148. When these 148 variables were subjected to multivariable analyses, 36 were found to be statistically significantly associated with psychotic experiences. Of these variables, 28 had “significant genetic overlap” with psychotic experiences.
When the researchers conducted one-sample MR analyses, they found forward associations with three variables and reverse associations with three variables.
Forward associations were found with ever having experienced sexual assault (odds ratio [OR], 1.32; 95% confidence interval [CI], 1.14-1.52; P = 2.67), and forward associations (with pleiotropy) were found with ever having experienced a physically violent crime and risk-taking behavior (OR, 1.25, 95% CI, 1.11-1.41; P = 3.28 and OR, 1.21, 95% CI, 1.08-1.35; P = 1.34, respectively).
“The allele scores for these 3 variables explained 0.03% to 0.23% variance of the corresponding variable” and the F statistics “ranged from 21.53 to 181.84, indicating that the results did not suffer from a weak-instrument bias,” the authors reported.
The researchers calculated an instrument based on increasing psychotic experiences risk allele scores and found that these scores explained 0.14% variance of psychotic experiences (F statistic, 19.26).
Using that calculation, they found a reverse association with having experienced a physically violent crime (OR, 1.08; 95% CI, 1.04-1.13; P = 3.92 × 10-4), cannabis use (OR, 1.11; 95% CI, 1.06-1.15; P = 2.64 × 10-6), and worrying too long after embarrassment (OR, 1.06; 95% CI, 1.03-1.10; P = 3.96 × 10-4). They then validated these associations.
The presence of all five correlates was associated with tenfold increased odds of psychotic experiences (OR, 10.63; 95% CI, 8.27-13.65, P = 1.2 × 10-114).
“Associations with psychotic experiences were found with both well-studied and unexplored multiple correlated variables,” the authors stated.
Era of ‘big data’
In a comment, Chirag Patel, PhD, associate professor of biomedical informatics at Harvard Medical School, Boston, who was not involved with the study, said he thought the study was “a nice example of a data-driven and comprehensive study of the environment coupled with attempts to triangulate evidence from genetics, made possible by biobank data.
“To guide public health policies and implementation of prevention strategies for psychosis, we need more systematic analyses and triangulate evidence with genetically informed methods to identify potentially modifiable risk factors in the era of ‘big data,’ ” he said.
“For instance, traumatic experiences contribute to poor mental and physical health, including psychosis,” Dr. Gülöksüz added.
The Kootstra Talent Fellowship, the Ophelia Research Project, and the Vidi Award from the Netherlands Scientific Organization provided funding to individual investigators. Dr. Gülöksüz and coauthors declared no relevant financial conflicts. Dr. Patel served as a reviewer on the study.
A version of this article first appeared on Medscape.com.
FROM JAMA PSYCHIATRY
Skin-picking, hair-pulling disorders: Diagnostic criteria, prevalence, and treatment
INDIANAPOLIS –
And while both body-focused repetitive behavior disorders affect a greater proportion of females than males, “we have no current information that is useful about what hormonal influences may or may not play in terms of picking and pulling behaviors,” Jon E. Grant, MD, JD, MPH, professor of psychiatry and behavioral neuroscience at the University of Chicago, said at the annual meeting of the Society for Pediatric Dermatology. “On a cognitive level, affected children and adolescents often have impaired inhibitory control but they are often 1-2 standard deviations above average IQ. They have Type A personalities [and are] very driven young kids. They also do not tolerate any down time or boredom. They need to be doing something all the time.”
According to the DSM-5, the diagnostic criteria for skin picking includes recurrent skin picking that results in skin lesions and is not attributable to another medical condition or substance. It also involves repeated attempts to decrease or stop the behavior and causes clinically significant distress or impairment.
“The other medical condition that we are interested in is the misuse of or dependence upon amphetamines or other prescription-based or illicit stimulants,” Dr. Grant said. “I saw a young man who was using about 600 mg of Ritalin a day, and he was picking all over the place. He did not have a primary skin disorder.”
The lifetime prevalence of skin picking disorder ranges between 1.4% and 5.4% of the general population. However, about 63% of people in a community sample endorsed some form of skin picking, and in a study of 105 college students, almost 40% said they picked their skin and had noticeable tissue damage as a result.
“Skin picking is not the same as self-injury,” Dr. Grant said. “It is also not simply an anxiety disorder. Anxiety will make people who pick worse, so people will say that they pick when they’re under stress. I can give them benzodiazepines and they’re still going to pick.”
Animal and human studies demonstrate that skin picking and hair pulling primarily affect females. “You will encounter young boys that pick and pull, but it largely affects females, and it tends to start around puberty,” he said. “Picking can have an onset after the age of 30, which is quite uncommon.”
From a cognitive standpoint, pathological skin pickers demonstrate impaired inhibitory control, impaired stop signal reaction time, increased rates of negative urgency (a tendency to act impulsively in response to negative emotions), and increased rates of positive urgency (a tendency to act impulsively in response to exciting or pleasurable emotions).
Trichotillomania
The lifetime prevalence of trichotillomania ranges between 0.6% and 3.9%. The onset is typically from ages 10-13 years, and the mean duration of illness is 22 years.
The DSM-5 criteria for trichotillomania are similar to that of skin-picking disorder, “although we don’t really worry about the substance use issue with people who pull their hair,” Dr. Grant said. “It doesn’t seem to have a correlation.” In addition, sometimes, children “will worsen pulling or picking when they have co-occurring ADHD and they’ve been started on a stimulant, even at a typical dose. For kids who have those issues, we prefer to try nonstimulant options for their ADHD such as bupropion or atomoxetine.”
Individuals with trichotillomania also tend to have low self-esteem and increased social anxiety, he added, and about one-third report low or very low quality of life. “When you notice alopecia, particularly in young girls who often have longer hair, up to 20% will eat their hair,” Dr. Grant said. “We don’t know why. It’s not related to vitamin deficiencies; it’s not a pica type of iron deficiency. There seems to be a shame piece about eating one’s own hair, but it’s important to assess that. Ask about constipation or overflow incontinence because they can get a bezoar, which can rupture” and can be fatal.
Skin-picking disorder and trichotillomania co-occur in up to 20% of cases. “When they do it tends to be a more difficult problem,” he said. These patients often come for mental health care because of depression, and most, he added, say “I don’t think I would be depressed if I wasn’t covered with excoriations or missing most of my hair.”
Treatment for both conditions
According to Dr. Grant, the treatment of choice for skin-picking disorder and trichotillomania is a specific psychotherapy known as “habit reversal therapy,” which involves helping the patient gain better self-control. The drawback is that it’s difficult to find someone trained in habit reversal therapy, “who know anything about skin picking and hair pulling,” he said. “That has been a huge challenge in the field.”
In his experience, the medical treatment of choice for skin-picking disorder and trichotillomania is N-acetylcysteine, an over-the-counter amino acid and antioxidant, which has been shown to be helpful at a dose of 2,400 mg per day. “Patients report to me that some of the excoriations clear up a little quicker as they’re taking it,” Dr. Grant said.
There may also be a role for antipsychotic therapy, he said, “but because of the associated weight gain with most antipsychotics we prefer not to use them.”
The opioid antagonist naltrexone has been shown to be effective in the subset of patients with skin-picking or hair-pulling disorders whose parents have a substance use disorder, Dr. Grant said. “The thought is that there’s something addictive about this behavior in some kids. These kids will look forward to picking and find it rewarding and exciting.”
Dr. Grant reported having no relevant financial disclosures.
INDIANAPOLIS –
And while both body-focused repetitive behavior disorders affect a greater proportion of females than males, “we have no current information that is useful about what hormonal influences may or may not play in terms of picking and pulling behaviors,” Jon E. Grant, MD, JD, MPH, professor of psychiatry and behavioral neuroscience at the University of Chicago, said at the annual meeting of the Society for Pediatric Dermatology. “On a cognitive level, affected children and adolescents often have impaired inhibitory control but they are often 1-2 standard deviations above average IQ. They have Type A personalities [and are] very driven young kids. They also do not tolerate any down time or boredom. They need to be doing something all the time.”
According to the DSM-5, the diagnostic criteria for skin picking includes recurrent skin picking that results in skin lesions and is not attributable to another medical condition or substance. It also involves repeated attempts to decrease or stop the behavior and causes clinically significant distress or impairment.
“The other medical condition that we are interested in is the misuse of or dependence upon amphetamines or other prescription-based or illicit stimulants,” Dr. Grant said. “I saw a young man who was using about 600 mg of Ritalin a day, and he was picking all over the place. He did not have a primary skin disorder.”
The lifetime prevalence of skin picking disorder ranges between 1.4% and 5.4% of the general population. However, about 63% of people in a community sample endorsed some form of skin picking, and in a study of 105 college students, almost 40% said they picked their skin and had noticeable tissue damage as a result.
“Skin picking is not the same as self-injury,” Dr. Grant said. “It is also not simply an anxiety disorder. Anxiety will make people who pick worse, so people will say that they pick when they’re under stress. I can give them benzodiazepines and they’re still going to pick.”
Animal and human studies demonstrate that skin picking and hair pulling primarily affect females. “You will encounter young boys that pick and pull, but it largely affects females, and it tends to start around puberty,” he said. “Picking can have an onset after the age of 30, which is quite uncommon.”
From a cognitive standpoint, pathological skin pickers demonstrate impaired inhibitory control, impaired stop signal reaction time, increased rates of negative urgency (a tendency to act impulsively in response to negative emotions), and increased rates of positive urgency (a tendency to act impulsively in response to exciting or pleasurable emotions).
Trichotillomania
The lifetime prevalence of trichotillomania ranges between 0.6% and 3.9%. The onset is typically from ages 10-13 years, and the mean duration of illness is 22 years.
The DSM-5 criteria for trichotillomania are similar to that of skin-picking disorder, “although we don’t really worry about the substance use issue with people who pull their hair,” Dr. Grant said. “It doesn’t seem to have a correlation.” In addition, sometimes, children “will worsen pulling or picking when they have co-occurring ADHD and they’ve been started on a stimulant, even at a typical dose. For kids who have those issues, we prefer to try nonstimulant options for their ADHD such as bupropion or atomoxetine.”
Individuals with trichotillomania also tend to have low self-esteem and increased social anxiety, he added, and about one-third report low or very low quality of life. “When you notice alopecia, particularly in young girls who often have longer hair, up to 20% will eat their hair,” Dr. Grant said. “We don’t know why. It’s not related to vitamin deficiencies; it’s not a pica type of iron deficiency. There seems to be a shame piece about eating one’s own hair, but it’s important to assess that. Ask about constipation or overflow incontinence because they can get a bezoar, which can rupture” and can be fatal.
Skin-picking disorder and trichotillomania co-occur in up to 20% of cases. “When they do it tends to be a more difficult problem,” he said. These patients often come for mental health care because of depression, and most, he added, say “I don’t think I would be depressed if I wasn’t covered with excoriations or missing most of my hair.”
Treatment for both conditions
According to Dr. Grant, the treatment of choice for skin-picking disorder and trichotillomania is a specific psychotherapy known as “habit reversal therapy,” which involves helping the patient gain better self-control. The drawback is that it’s difficult to find someone trained in habit reversal therapy, “who know anything about skin picking and hair pulling,” he said. “That has been a huge challenge in the field.”
In his experience, the medical treatment of choice for skin-picking disorder and trichotillomania is N-acetylcysteine, an over-the-counter amino acid and antioxidant, which has been shown to be helpful at a dose of 2,400 mg per day. “Patients report to me that some of the excoriations clear up a little quicker as they’re taking it,” Dr. Grant said.
There may also be a role for antipsychotic therapy, he said, “but because of the associated weight gain with most antipsychotics we prefer not to use them.”
The opioid antagonist naltrexone has been shown to be effective in the subset of patients with skin-picking or hair-pulling disorders whose parents have a substance use disorder, Dr. Grant said. “The thought is that there’s something addictive about this behavior in some kids. These kids will look forward to picking and find it rewarding and exciting.”
Dr. Grant reported having no relevant financial disclosures.
INDIANAPOLIS –
And while both body-focused repetitive behavior disorders affect a greater proportion of females than males, “we have no current information that is useful about what hormonal influences may or may not play in terms of picking and pulling behaviors,” Jon E. Grant, MD, JD, MPH, professor of psychiatry and behavioral neuroscience at the University of Chicago, said at the annual meeting of the Society for Pediatric Dermatology. “On a cognitive level, affected children and adolescents often have impaired inhibitory control but they are often 1-2 standard deviations above average IQ. They have Type A personalities [and are] very driven young kids. They also do not tolerate any down time or boredom. They need to be doing something all the time.”
According to the DSM-5, the diagnostic criteria for skin picking includes recurrent skin picking that results in skin lesions and is not attributable to another medical condition or substance. It also involves repeated attempts to decrease or stop the behavior and causes clinically significant distress or impairment.
“The other medical condition that we are interested in is the misuse of or dependence upon amphetamines or other prescription-based or illicit stimulants,” Dr. Grant said. “I saw a young man who was using about 600 mg of Ritalin a day, and he was picking all over the place. He did not have a primary skin disorder.”
The lifetime prevalence of skin picking disorder ranges between 1.4% and 5.4% of the general population. However, about 63% of people in a community sample endorsed some form of skin picking, and in a study of 105 college students, almost 40% said they picked their skin and had noticeable tissue damage as a result.
“Skin picking is not the same as self-injury,” Dr. Grant said. “It is also not simply an anxiety disorder. Anxiety will make people who pick worse, so people will say that they pick when they’re under stress. I can give them benzodiazepines and they’re still going to pick.”
Animal and human studies demonstrate that skin picking and hair pulling primarily affect females. “You will encounter young boys that pick and pull, but it largely affects females, and it tends to start around puberty,” he said. “Picking can have an onset after the age of 30, which is quite uncommon.”
From a cognitive standpoint, pathological skin pickers demonstrate impaired inhibitory control, impaired stop signal reaction time, increased rates of negative urgency (a tendency to act impulsively in response to negative emotions), and increased rates of positive urgency (a tendency to act impulsively in response to exciting or pleasurable emotions).
Trichotillomania
The lifetime prevalence of trichotillomania ranges between 0.6% and 3.9%. The onset is typically from ages 10-13 years, and the mean duration of illness is 22 years.
The DSM-5 criteria for trichotillomania are similar to that of skin-picking disorder, “although we don’t really worry about the substance use issue with people who pull their hair,” Dr. Grant said. “It doesn’t seem to have a correlation.” In addition, sometimes, children “will worsen pulling or picking when they have co-occurring ADHD and they’ve been started on a stimulant, even at a typical dose. For kids who have those issues, we prefer to try nonstimulant options for their ADHD such as bupropion or atomoxetine.”
Individuals with trichotillomania also tend to have low self-esteem and increased social anxiety, he added, and about one-third report low or very low quality of life. “When you notice alopecia, particularly in young girls who often have longer hair, up to 20% will eat their hair,” Dr. Grant said. “We don’t know why. It’s not related to vitamin deficiencies; it’s not a pica type of iron deficiency. There seems to be a shame piece about eating one’s own hair, but it’s important to assess that. Ask about constipation or overflow incontinence because they can get a bezoar, which can rupture” and can be fatal.
Skin-picking disorder and trichotillomania co-occur in up to 20% of cases. “When they do it tends to be a more difficult problem,” he said. These patients often come for mental health care because of depression, and most, he added, say “I don’t think I would be depressed if I wasn’t covered with excoriations or missing most of my hair.”
Treatment for both conditions
According to Dr. Grant, the treatment of choice for skin-picking disorder and trichotillomania is a specific psychotherapy known as “habit reversal therapy,” which involves helping the patient gain better self-control. The drawback is that it’s difficult to find someone trained in habit reversal therapy, “who know anything about skin picking and hair pulling,” he said. “That has been a huge challenge in the field.”
In his experience, the medical treatment of choice for skin-picking disorder and trichotillomania is N-acetylcysteine, an over-the-counter amino acid and antioxidant, which has been shown to be helpful at a dose of 2,400 mg per day. “Patients report to me that some of the excoriations clear up a little quicker as they’re taking it,” Dr. Grant said.
There may also be a role for antipsychotic therapy, he said, “but because of the associated weight gain with most antipsychotics we prefer not to use them.”
The opioid antagonist naltrexone has been shown to be effective in the subset of patients with skin-picking or hair-pulling disorders whose parents have a substance use disorder, Dr. Grant said. “The thought is that there’s something addictive about this behavior in some kids. These kids will look forward to picking and find it rewarding and exciting.”
Dr. Grant reported having no relevant financial disclosures.
AT SPD 2022
B6 a new approach for depression, anxiety?
Investigators compared supplementation with a 1-month course of vitamin B6 or B12 to supplementation with placebo in almost 500 adults. Results showed that vitamin B6 supplementation was associated with reductions in self-reported anxiety and a trend toward decreased depressive symptoms.
In addition, the vitamin B6 group showed increased levels of gamma-aminobutyric acid (GABA), as indicated by results on a visual test that was administered at the end of the trial. The test results demonstrated subtle changes in participants’ visual performance. The researchers considered this to be consistent with controlled levels of GABA-related brain activity.
However, “before practicing clinicians would recommend taking high doses of vitamin B6, a full-scale clinical trial would have to be carried out to verify the findings, assess any side effects, and find out which types of patients do or don’t benefit,” study investigator David Field, PhD, associate professor, School of Psychological and Clinical Language Sciences, University of Reading (England), told this news organization.
“My relatively small study can only be considered as an initial proof of concept,” Dr. Field said.
The findings were published online in the Journal of Human Psychopharmacology: Clinical and Experimental.
Eat Marmite?
“Recent research has connected mood disorders and some other neuropsychiatric conditions with disturbance in this balance, often in the direction of raised levels of brain activity,” Dr. Field noted.
Vitamin B6 is a coenzyme in the synthesis of GABA, an inhibitory neurotransmitter, from glutamate. Some previous research has suggested that vitamins B6 and B12 have a role in improving mood-related outcomes.
Dr. Field had reviewed a 2017 study of the effects on visual processing of eating Marmite, a type of food spread rich in vitamin B, every day for a few weeks.
“Remarkably, the results of that study suggested that eating Marmite had increased the level of the inhibitory neurotransmitter GABA in the visual part of the brain, damping down the level of neural activity slightly,” he said.
However, Marmite contains other B vitamins and other ingredients that might potentially account for this result, “plus, a lot of people don’t like the taste of Marmite,” Dr. Field noted.
Therefore, he wanted to “find out which individual ingredients were driving the effect, and B6 and B12 were the most plausible candidates.”
He decided to test these vitamins individually and to compare them to placebo. “I added the measures of anxiety and depression that were not in the Marmite study because I reasoned that if GABA levels were altered, this could improve those disorders, because we know that decreased levels of GABA in the brain occur in both of those conditions,” Dr. Field added.
Over the course of 5 years, investigators recruited 478 participants aged 18-58 years (mean age, 23 years; 381 women). Of these, 265 reported having anxiety, and 146 reported having depression.
The study participants were randomly assigned to receive either vitamin B6 (100 mg pyroxidine hydrochloride), vitamin B12 (1,000 mg methylcobalmin), or placebo tablets once daily for a month.
They also completed the Screen for Adult Anxiety Related Disorders (SCAARED) and the Mood and Feelings Questionnaire (MFQ) long version at baseline and following supplementation (“post test”), and they underwent three sensory tests that acted as assays of inhibitory function at post test.
In addition, 307 participants completed the Visual Contrast Sensitivity and Surround Suppression, which “measures the minimum percentage contrast between the lighter and darker regions of a striped pattern that can be detected (called the contrast threshold),” the investigators note.
The contrast threshold was measured with and without a suppressive surround mask that increases the threshold – an effect mediated by GABAergic connections in the visual cortex.
Participants (n = 172) also completed the Binocular Rivalry test and the Tactile Test Battery (n = 180). Both tests are designed to measure responses requiring GABAergic inhibitory activity.
‘Subtle changes’
ANOVA analyses revealed a “highly significant” reduction in anxiety at post test (F[1,173] = 10.03; P = .002; np 2 = .055), driven primarily by reduced anxiety in the B6 group (t[88] = 3.51; P < .001; d = .37). The placebo group also showed some reduction in anxiety, but it was not deemed significant, and the overall interaction itself did not reach significance.
A comparison of the B12 group with the group that received placebo revealed a significant reduction in anxiety at post test (F[1,175] = 4.08; P = .045; np 2 = .023), similarly driven by reduced anxiety in the B12 group (t[89] = 1.84; P = .069; d = .19) – but the interaction was not significant.
Among the B6 group, there was a highly significant reduction in scores on the generalized anxiety disorder and social anxiety subscales of the SCAARED, and there was a trend toward reductions on the other subscales. Among the B12 group, there was a significant reduction only on scores on the separation anxiety subscale. No significant changes were found in the placebo group.
The ANOVA test analysis of the B6 and placebo group data showed “no uniform direction of change” in depression at post test. The researchers found a “tendency” for depression scores to decrease between baseline and post test in the B6 group but to increase in the placebo group – an interaction that “approached” significance (F[1,96] = 3.08; P = .083; np 2 = .031), they report.
The ANOVA analysis of the B12 and placebo group data revealed no significant or trending effects, and the t-test comparing baseline and post-test scores in the B12 group was similarly nonsignificant.
B6 supplementation did change visual contrast thresholds, but only when a suppressive surround was present. There were “no clear effects” of B6 supplementation on other outcome measures, including binocular rivalry reversal rate and the tactile test battery, the investigators note.
“We found that supplementation with B6 produced subtle changes in tests of visual processing in a way that suggested an increase in the level of the inhibitory neurotransmitter GABA,” Dr. Field reported.
Vitamin B6 is a “cofactor for a metabolic pathway in the brain that converts the excitatory neurotransmitter glutamate into the inhibitory/calming GABA,” he said.
“By increasing the quantity of the cofactor, we slightly speed up the rate of this metabolic process, and so you end up with a bit more of the GABA neurotransmitter and a bit less glutamate. The net effect of this is to slightly reduce the amount of activity in the brain,” Dr. Field added.
Most common nutrient deficiency
Carol Johnston, PhD, professor and associate dean for faculty success, College of Health Solutions, Arizona State University, Phoenix, said vitamin B6 is “the most common nutrient deficiency in the United States;” 16% of men and 32% of women are reportedly B6 deficient.
“Young women on birth control are at higher risk for B6 deficiency due to effects of oral contraceptives on B6 metabolism,” whereas vitamin B12 deficiency is more common in older adults, said Dr. Johnston, who was not involved with the study.
The current study’s population mainly consisted of young women, and the interpretation of the data is “limited” because the researchers did not measure blood status for B6 and B12, Dr. Johnston noted. It is possible the sample was low in B6 and that the supplements “improved cognitive measures.”
Because the population was young – no one was older than 60 years – B12 status was likely “adequate in the sample, and supplementation did not have an impact,” she said.
Overall, Dr. Johnston cautioned that it is important to “alert clinicians and the general public about the concerns of overdosing B6.” For example, supplementation at high amounts can cause potentially irreversible sensory neuropathy, she noted.
“The safe upper limit defined by experts is 100 mg per day – the dosage used in this trial. Daily supplementation should not exceed this level,” Dr. Johnston said.
The vitamin tablets used in the study were supplied by Innopure. The investigators and Dr. Johnston have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Investigators compared supplementation with a 1-month course of vitamin B6 or B12 to supplementation with placebo in almost 500 adults. Results showed that vitamin B6 supplementation was associated with reductions in self-reported anxiety and a trend toward decreased depressive symptoms.
In addition, the vitamin B6 group showed increased levels of gamma-aminobutyric acid (GABA), as indicated by results on a visual test that was administered at the end of the trial. The test results demonstrated subtle changes in participants’ visual performance. The researchers considered this to be consistent with controlled levels of GABA-related brain activity.
However, “before practicing clinicians would recommend taking high doses of vitamin B6, a full-scale clinical trial would have to be carried out to verify the findings, assess any side effects, and find out which types of patients do or don’t benefit,” study investigator David Field, PhD, associate professor, School of Psychological and Clinical Language Sciences, University of Reading (England), told this news organization.
“My relatively small study can only be considered as an initial proof of concept,” Dr. Field said.
The findings were published online in the Journal of Human Psychopharmacology: Clinical and Experimental.
Eat Marmite?
“Recent research has connected mood disorders and some other neuropsychiatric conditions with disturbance in this balance, often in the direction of raised levels of brain activity,” Dr. Field noted.
Vitamin B6 is a coenzyme in the synthesis of GABA, an inhibitory neurotransmitter, from glutamate. Some previous research has suggested that vitamins B6 and B12 have a role in improving mood-related outcomes.
Dr. Field had reviewed a 2017 study of the effects on visual processing of eating Marmite, a type of food spread rich in vitamin B, every day for a few weeks.
“Remarkably, the results of that study suggested that eating Marmite had increased the level of the inhibitory neurotransmitter GABA in the visual part of the brain, damping down the level of neural activity slightly,” he said.
However, Marmite contains other B vitamins and other ingredients that might potentially account for this result, “plus, a lot of people don’t like the taste of Marmite,” Dr. Field noted.
Therefore, he wanted to “find out which individual ingredients were driving the effect, and B6 and B12 were the most plausible candidates.”
He decided to test these vitamins individually and to compare them to placebo. “I added the measures of anxiety and depression that were not in the Marmite study because I reasoned that if GABA levels were altered, this could improve those disorders, because we know that decreased levels of GABA in the brain occur in both of those conditions,” Dr. Field added.
Over the course of 5 years, investigators recruited 478 participants aged 18-58 years (mean age, 23 years; 381 women). Of these, 265 reported having anxiety, and 146 reported having depression.
The study participants were randomly assigned to receive either vitamin B6 (100 mg pyroxidine hydrochloride), vitamin B12 (1,000 mg methylcobalmin), or placebo tablets once daily for a month.
They also completed the Screen for Adult Anxiety Related Disorders (SCAARED) and the Mood and Feelings Questionnaire (MFQ) long version at baseline and following supplementation (“post test”), and they underwent three sensory tests that acted as assays of inhibitory function at post test.
In addition, 307 participants completed the Visual Contrast Sensitivity and Surround Suppression, which “measures the minimum percentage contrast between the lighter and darker regions of a striped pattern that can be detected (called the contrast threshold),” the investigators note.
The contrast threshold was measured with and without a suppressive surround mask that increases the threshold – an effect mediated by GABAergic connections in the visual cortex.
Participants (n = 172) also completed the Binocular Rivalry test and the Tactile Test Battery (n = 180). Both tests are designed to measure responses requiring GABAergic inhibitory activity.
‘Subtle changes’
ANOVA analyses revealed a “highly significant” reduction in anxiety at post test (F[1,173] = 10.03; P = .002; np 2 = .055), driven primarily by reduced anxiety in the B6 group (t[88] = 3.51; P < .001; d = .37). The placebo group also showed some reduction in anxiety, but it was not deemed significant, and the overall interaction itself did not reach significance.
A comparison of the B12 group with the group that received placebo revealed a significant reduction in anxiety at post test (F[1,175] = 4.08; P = .045; np 2 = .023), similarly driven by reduced anxiety in the B12 group (t[89] = 1.84; P = .069; d = .19) – but the interaction was not significant.
Among the B6 group, there was a highly significant reduction in scores on the generalized anxiety disorder and social anxiety subscales of the SCAARED, and there was a trend toward reductions on the other subscales. Among the B12 group, there was a significant reduction only on scores on the separation anxiety subscale. No significant changes were found in the placebo group.
The ANOVA test analysis of the B6 and placebo group data showed “no uniform direction of change” in depression at post test. The researchers found a “tendency” for depression scores to decrease between baseline and post test in the B6 group but to increase in the placebo group – an interaction that “approached” significance (F[1,96] = 3.08; P = .083; np 2 = .031), they report.
The ANOVA analysis of the B12 and placebo group data revealed no significant or trending effects, and the t-test comparing baseline and post-test scores in the B12 group was similarly nonsignificant.
B6 supplementation did change visual contrast thresholds, but only when a suppressive surround was present. There were “no clear effects” of B6 supplementation on other outcome measures, including binocular rivalry reversal rate and the tactile test battery, the investigators note.
“We found that supplementation with B6 produced subtle changes in tests of visual processing in a way that suggested an increase in the level of the inhibitory neurotransmitter GABA,” Dr. Field reported.
Vitamin B6 is a “cofactor for a metabolic pathway in the brain that converts the excitatory neurotransmitter glutamate into the inhibitory/calming GABA,” he said.
“By increasing the quantity of the cofactor, we slightly speed up the rate of this metabolic process, and so you end up with a bit more of the GABA neurotransmitter and a bit less glutamate. The net effect of this is to slightly reduce the amount of activity in the brain,” Dr. Field added.
Most common nutrient deficiency
Carol Johnston, PhD, professor and associate dean for faculty success, College of Health Solutions, Arizona State University, Phoenix, said vitamin B6 is “the most common nutrient deficiency in the United States;” 16% of men and 32% of women are reportedly B6 deficient.
“Young women on birth control are at higher risk for B6 deficiency due to effects of oral contraceptives on B6 metabolism,” whereas vitamin B12 deficiency is more common in older adults, said Dr. Johnston, who was not involved with the study.
The current study’s population mainly consisted of young women, and the interpretation of the data is “limited” because the researchers did not measure blood status for B6 and B12, Dr. Johnston noted. It is possible the sample was low in B6 and that the supplements “improved cognitive measures.”
Because the population was young – no one was older than 60 years – B12 status was likely “adequate in the sample, and supplementation did not have an impact,” she said.
Overall, Dr. Johnston cautioned that it is important to “alert clinicians and the general public about the concerns of overdosing B6.” For example, supplementation at high amounts can cause potentially irreversible sensory neuropathy, she noted.
“The safe upper limit defined by experts is 100 mg per day – the dosage used in this trial. Daily supplementation should not exceed this level,” Dr. Johnston said.
The vitamin tablets used in the study were supplied by Innopure. The investigators and Dr. Johnston have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Investigators compared supplementation with a 1-month course of vitamin B6 or B12 to supplementation with placebo in almost 500 adults. Results showed that vitamin B6 supplementation was associated with reductions in self-reported anxiety and a trend toward decreased depressive symptoms.
In addition, the vitamin B6 group showed increased levels of gamma-aminobutyric acid (GABA), as indicated by results on a visual test that was administered at the end of the trial. The test results demonstrated subtle changes in participants’ visual performance. The researchers considered this to be consistent with controlled levels of GABA-related brain activity.
However, “before practicing clinicians would recommend taking high doses of vitamin B6, a full-scale clinical trial would have to be carried out to verify the findings, assess any side effects, and find out which types of patients do or don’t benefit,” study investigator David Field, PhD, associate professor, School of Psychological and Clinical Language Sciences, University of Reading (England), told this news organization.
“My relatively small study can only be considered as an initial proof of concept,” Dr. Field said.
The findings were published online in the Journal of Human Psychopharmacology: Clinical and Experimental.
Eat Marmite?
“Recent research has connected mood disorders and some other neuropsychiatric conditions with disturbance in this balance, often in the direction of raised levels of brain activity,” Dr. Field noted.
Vitamin B6 is a coenzyme in the synthesis of GABA, an inhibitory neurotransmitter, from glutamate. Some previous research has suggested that vitamins B6 and B12 have a role in improving mood-related outcomes.
Dr. Field had reviewed a 2017 study of the effects on visual processing of eating Marmite, a type of food spread rich in vitamin B, every day for a few weeks.
“Remarkably, the results of that study suggested that eating Marmite had increased the level of the inhibitory neurotransmitter GABA in the visual part of the brain, damping down the level of neural activity slightly,” he said.
However, Marmite contains other B vitamins and other ingredients that might potentially account for this result, “plus, a lot of people don’t like the taste of Marmite,” Dr. Field noted.
Therefore, he wanted to “find out which individual ingredients were driving the effect, and B6 and B12 were the most plausible candidates.”
He decided to test these vitamins individually and to compare them to placebo. “I added the measures of anxiety and depression that were not in the Marmite study because I reasoned that if GABA levels were altered, this could improve those disorders, because we know that decreased levels of GABA in the brain occur in both of those conditions,” Dr. Field added.
Over the course of 5 years, investigators recruited 478 participants aged 18-58 years (mean age, 23 years; 381 women). Of these, 265 reported having anxiety, and 146 reported having depression.
The study participants were randomly assigned to receive either vitamin B6 (100 mg pyroxidine hydrochloride), vitamin B12 (1,000 mg methylcobalmin), or placebo tablets once daily for a month.
They also completed the Screen for Adult Anxiety Related Disorders (SCAARED) and the Mood and Feelings Questionnaire (MFQ) long version at baseline and following supplementation (“post test”), and they underwent three sensory tests that acted as assays of inhibitory function at post test.
In addition, 307 participants completed the Visual Contrast Sensitivity and Surround Suppression, which “measures the minimum percentage contrast between the lighter and darker regions of a striped pattern that can be detected (called the contrast threshold),” the investigators note.
The contrast threshold was measured with and without a suppressive surround mask that increases the threshold – an effect mediated by GABAergic connections in the visual cortex.
Participants (n = 172) also completed the Binocular Rivalry test and the Tactile Test Battery (n = 180). Both tests are designed to measure responses requiring GABAergic inhibitory activity.
‘Subtle changes’
ANOVA analyses revealed a “highly significant” reduction in anxiety at post test (F[1,173] = 10.03; P = .002; np 2 = .055), driven primarily by reduced anxiety in the B6 group (t[88] = 3.51; P < .001; d = .37). The placebo group also showed some reduction in anxiety, but it was not deemed significant, and the overall interaction itself did not reach significance.
A comparison of the B12 group with the group that received placebo revealed a significant reduction in anxiety at post test (F[1,175] = 4.08; P = .045; np 2 = .023), similarly driven by reduced anxiety in the B12 group (t[89] = 1.84; P = .069; d = .19) – but the interaction was not significant.
Among the B6 group, there was a highly significant reduction in scores on the generalized anxiety disorder and social anxiety subscales of the SCAARED, and there was a trend toward reductions on the other subscales. Among the B12 group, there was a significant reduction only on scores on the separation anxiety subscale. No significant changes were found in the placebo group.
The ANOVA test analysis of the B6 and placebo group data showed “no uniform direction of change” in depression at post test. The researchers found a “tendency” for depression scores to decrease between baseline and post test in the B6 group but to increase in the placebo group – an interaction that “approached” significance (F[1,96] = 3.08; P = .083; np 2 = .031), they report.
The ANOVA analysis of the B12 and placebo group data revealed no significant or trending effects, and the t-test comparing baseline and post-test scores in the B12 group was similarly nonsignificant.
B6 supplementation did change visual contrast thresholds, but only when a suppressive surround was present. There were “no clear effects” of B6 supplementation on other outcome measures, including binocular rivalry reversal rate and the tactile test battery, the investigators note.
“We found that supplementation with B6 produced subtle changes in tests of visual processing in a way that suggested an increase in the level of the inhibitory neurotransmitter GABA,” Dr. Field reported.
Vitamin B6 is a “cofactor for a metabolic pathway in the brain that converts the excitatory neurotransmitter glutamate into the inhibitory/calming GABA,” he said.
“By increasing the quantity of the cofactor, we slightly speed up the rate of this metabolic process, and so you end up with a bit more of the GABA neurotransmitter and a bit less glutamate. The net effect of this is to slightly reduce the amount of activity in the brain,” Dr. Field added.
Most common nutrient deficiency
Carol Johnston, PhD, professor and associate dean for faculty success, College of Health Solutions, Arizona State University, Phoenix, said vitamin B6 is “the most common nutrient deficiency in the United States;” 16% of men and 32% of women are reportedly B6 deficient.
“Young women on birth control are at higher risk for B6 deficiency due to effects of oral contraceptives on B6 metabolism,” whereas vitamin B12 deficiency is more common in older adults, said Dr. Johnston, who was not involved with the study.
The current study’s population mainly consisted of young women, and the interpretation of the data is “limited” because the researchers did not measure blood status for B6 and B12, Dr. Johnston noted. It is possible the sample was low in B6 and that the supplements “improved cognitive measures.”
Because the population was young – no one was older than 60 years – B12 status was likely “adequate in the sample, and supplementation did not have an impact,” she said.
Overall, Dr. Johnston cautioned that it is important to “alert clinicians and the general public about the concerns of overdosing B6.” For example, supplementation at high amounts can cause potentially irreversible sensory neuropathy, she noted.
“The safe upper limit defined by experts is 100 mg per day – the dosage used in this trial. Daily supplementation should not exceed this level,” Dr. Johnston said.
The vitamin tablets used in the study were supplied by Innopure. The investigators and Dr. Johnston have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Pharmacogenomic testing may curb drug interactions in severe depression
Pharmacogenetic testing, which is used to classify how patients with major depressive disorder (MDD) metabolize medications, reduces adverse drug-gene interactions, new research shows.
In addition, among the intervention group, the rate of remission over 24 weeks was significantly greater.
“These tests can be helpful in rethinking choices of antidepressants, but clinicians should not expect them to be helpful for every patient,” study investigator David W. Oslin, MD, Corporal Michael J. Crescenz VA Medical Center and professor of psychiatry at Perelman School of Medicine, University of Pennsylvania, Philadelphia, said in an interview.
The findings were published online in JAMA.
Less trial and error
Pharmacogenomic testing can provide information to inform drug selection or dosing for patients with a genetic variation that alters pharmacokinetics or pharmacodynamics. Such testing may be particularly useful for patients with MDD, as fewer than 40% of these patients achieve clinical remission after an initial treatment with an antidepressant, the investigators note.
“To get to a treatment that works for an individual, it’s not unusual to have to try two or three or four antidepressants,” said Dr. Oslin. “If we could reduce that variance a little bit with a test like this, that would be huge from a public health perspective.”
The study included 676 physicians and 1,944 adults with MDD (mean age, 48 years; 24% women) who were receiving care at 22 Department of Veterans Affairs medical centers. Eligible patients were set to start a new antidepressant monotherapy, and all underwent a pharmacogenomic test using a cheek swab.
Investigators randomly assigned patients to receive test results when available (pharmacogenomic-guided group) or 24 weeks later (usual-care group). For the former group, clinicians were asked to initiate treatment when test results were available, typically within 2-3 days. For the latter group, they were asked to initiate treatment on a day of randomization.
Assessments included the 9-item Patient Health questionnaire (PHQ-9), scores for which range from 0-27 points, with higher scores indicating worse symptoms.
Of the total patient population, 79% completed the 24-week assessment.
Researchers characterized antidepressant medications on the basis of drug-gene interaction categories: no known interactions, moderate interactions, and substantial interactions.
The co-primary outcomes were treatment initiation within 30 days, determined on the basis of drug-gene interaction categories, and remission from depression symptoms, defined as a PHQ-9 score of less than or equal to 5.
Raters who were blinded to clinical care and study randomization assessed outcomes at 4, 8, 12, 18, and 24 weeks.
Significant impact?
Results showed that the pharmacogenomic-guided group was more likely to receive an antidepressant that had no potential drug-gene interaction, as opposed to one with a moderate/substantial interaction (odds ratio, 4.32; 95% confidence interval, 3.47-5.39; P < .001).
The usual-care group was more likely to receive a drug with mild potential drug-gene interaction (no/moderate interaction vs. substantial interaction: OR, 2.08; 95% CI, 1.52-2.84; P = .005).
For the intervention group, the estimated rates of receiving an antidepressant with no, moderate, and substantial drug-gene interactions were 59.3%, 30.0%, and 10.7%, respectively. For the usual-care group, the estimates were 25.7%, 54.6%, and 19.7%.
The finding that 1 in 5 patients who received usual care were initially given a medication for which there were significant drug-gene interactions means it is “not a rare event,” said Dr. Oslin. “If we can make an impact on 20% of the people we prescribe to, that’s actually pretty big.”
Rates of remission were greater in the pharmacogenomic-guided group over 24 weeks (OR, 1.28; 95% CI, 1.05-1.57; P = .02; absolute risk difference, 2.8%; 95% CI, 0.6%-5.1%).
The secondary outcomes of response to treatment, defined as at least a 50% decrease in PHQ-9 score, also favored the pharmacogenomic-guided group. This was also the case for the secondary outcome of reduction in symptom severity on the PHQ-9 score.
Some physicians have expressed skepticism about pharmacogenomic testing, but the study provides additional evidence of its usefulness, Dr. Oslin noted.
“While I don’t think testing should be standard of practice, I also don’t think we should put barriers into the testing until we can better understand how to target the testing” to those who will benefit the most, he added.
The tests are available at a commercial cost of about $1,000 – which may not be that expensive if testing has a significant impact on a patient’s life, said Dr. Oslin.
Important research, but with several limitations
In an accompanying editorial, Dan V. Iosifescu, MD, associate professor of psychiatry at New York University School of Medicine and director of clinical research at the Nathan Kline Institute for Psychiatric Research, called the study an important addition to the literature on pharmacogenomic testing for patients with MDD.
The study was significantly larger and had broader inclusion criteria and longer follow-up than previous clinical trials and is one of the few investigations not funded by a manufacturer of pharmacogenomic tests, writes Dr. Iosifescu, who was not involved with the research.
However, he notes that an antidepressant was not initiated for 30 days after randomization in 25% of the intervention group and in 31% of the usual-care group, which was “puzzling.” “Because these rates were comparable in the 2 groups, it cannot be explained primarily by the delay of the pharmacogenomic test results in the intervention group,” he writes.
In addition, in the co-primary outcome of symptom remission rate, the difference in clinical improvement in favor of the pharmacogenomic-guided treatment was only “modest” – the gain was of less than 2% in the proportion of patients achieving remission, Dr. Iosifescu adds.
He adds this is “likely not very meaningful clinically despite this difference achieving statistical significance in this large study sample.”
Other potential study limitations he cites include the lack of patient blinding to treatment assignment and the absence of clarity about why rates of MDD response and remission over time were relatively low in both treatment groups.
A possible approach to optimize antidepressant choices could involve integration of pharmacogenomic data into larger predictive models that include clinical and demographic variables, Dr. Iosifescu notes.
“The development of such complex models is challenging, but it is now possible given the recent substantial advances in the proficiency of computational tools,” he writes.
The study was funded by the U.S. Department of Veterans Affairs (VA), Health Services Research and Development Service, and the Mental Illness Research, Education, and Clinical Center at the Corporal Michael J. Crescenz VA Medical Center. Dr. Oslin reports having received grants from the VA Office of Research and Development and Janssen Pharmaceuticals and nonfinancial support from Myriad Genetics during the conduct of the study. Dr. Iosifescu report having received personal fees from Alkermes, Allergan, Axsome, Biogen, the Centers for Psychiatric Excellence, Jazz, Lundbeck, Precision Neuroscience, Sage, and Sunovion and grants from Alkermes, AstraZeneca, Brainsway, Litecure, Neosync, Otsuka, Roche, and Shire.
A version of this article first appeared on Medscape.com.
Pharmacogenetic testing, which is used to classify how patients with major depressive disorder (MDD) metabolize medications, reduces adverse drug-gene interactions, new research shows.
In addition, among the intervention group, the rate of remission over 24 weeks was significantly greater.
“These tests can be helpful in rethinking choices of antidepressants, but clinicians should not expect them to be helpful for every patient,” study investigator David W. Oslin, MD, Corporal Michael J. Crescenz VA Medical Center and professor of psychiatry at Perelman School of Medicine, University of Pennsylvania, Philadelphia, said in an interview.
The findings were published online in JAMA.
Less trial and error
Pharmacogenomic testing can provide information to inform drug selection or dosing for patients with a genetic variation that alters pharmacokinetics or pharmacodynamics. Such testing may be particularly useful for patients with MDD, as fewer than 40% of these patients achieve clinical remission after an initial treatment with an antidepressant, the investigators note.
“To get to a treatment that works for an individual, it’s not unusual to have to try two or three or four antidepressants,” said Dr. Oslin. “If we could reduce that variance a little bit with a test like this, that would be huge from a public health perspective.”
The study included 676 physicians and 1,944 adults with MDD (mean age, 48 years; 24% women) who were receiving care at 22 Department of Veterans Affairs medical centers. Eligible patients were set to start a new antidepressant monotherapy, and all underwent a pharmacogenomic test using a cheek swab.
Investigators randomly assigned patients to receive test results when available (pharmacogenomic-guided group) or 24 weeks later (usual-care group). For the former group, clinicians were asked to initiate treatment when test results were available, typically within 2-3 days. For the latter group, they were asked to initiate treatment on a day of randomization.
Assessments included the 9-item Patient Health questionnaire (PHQ-9), scores for which range from 0-27 points, with higher scores indicating worse symptoms.
Of the total patient population, 79% completed the 24-week assessment.
Researchers characterized antidepressant medications on the basis of drug-gene interaction categories: no known interactions, moderate interactions, and substantial interactions.
The co-primary outcomes were treatment initiation within 30 days, determined on the basis of drug-gene interaction categories, and remission from depression symptoms, defined as a PHQ-9 score of less than or equal to 5.
Raters who were blinded to clinical care and study randomization assessed outcomes at 4, 8, 12, 18, and 24 weeks.
Significant impact?
Results showed that the pharmacogenomic-guided group was more likely to receive an antidepressant that had no potential drug-gene interaction, as opposed to one with a moderate/substantial interaction (odds ratio, 4.32; 95% confidence interval, 3.47-5.39; P < .001).
The usual-care group was more likely to receive a drug with mild potential drug-gene interaction (no/moderate interaction vs. substantial interaction: OR, 2.08; 95% CI, 1.52-2.84; P = .005).
For the intervention group, the estimated rates of receiving an antidepressant with no, moderate, and substantial drug-gene interactions were 59.3%, 30.0%, and 10.7%, respectively. For the usual-care group, the estimates were 25.7%, 54.6%, and 19.7%.
The finding that 1 in 5 patients who received usual care were initially given a medication for which there were significant drug-gene interactions means it is “not a rare event,” said Dr. Oslin. “If we can make an impact on 20% of the people we prescribe to, that’s actually pretty big.”
Rates of remission were greater in the pharmacogenomic-guided group over 24 weeks (OR, 1.28; 95% CI, 1.05-1.57; P = .02; absolute risk difference, 2.8%; 95% CI, 0.6%-5.1%).
The secondary outcomes of response to treatment, defined as at least a 50% decrease in PHQ-9 score, also favored the pharmacogenomic-guided group. This was also the case for the secondary outcome of reduction in symptom severity on the PHQ-9 score.
Some physicians have expressed skepticism about pharmacogenomic testing, but the study provides additional evidence of its usefulness, Dr. Oslin noted.
“While I don’t think testing should be standard of practice, I also don’t think we should put barriers into the testing until we can better understand how to target the testing” to those who will benefit the most, he added.
The tests are available at a commercial cost of about $1,000 – which may not be that expensive if testing has a significant impact on a patient’s life, said Dr. Oslin.
Important research, but with several limitations
In an accompanying editorial, Dan V. Iosifescu, MD, associate professor of psychiatry at New York University School of Medicine and director of clinical research at the Nathan Kline Institute for Psychiatric Research, called the study an important addition to the literature on pharmacogenomic testing for patients with MDD.
The study was significantly larger and had broader inclusion criteria and longer follow-up than previous clinical trials and is one of the few investigations not funded by a manufacturer of pharmacogenomic tests, writes Dr. Iosifescu, who was not involved with the research.
However, he notes that an antidepressant was not initiated for 30 days after randomization in 25% of the intervention group and in 31% of the usual-care group, which was “puzzling.” “Because these rates were comparable in the 2 groups, it cannot be explained primarily by the delay of the pharmacogenomic test results in the intervention group,” he writes.
In addition, in the co-primary outcome of symptom remission rate, the difference in clinical improvement in favor of the pharmacogenomic-guided treatment was only “modest” – the gain was of less than 2% in the proportion of patients achieving remission, Dr. Iosifescu adds.
He adds this is “likely not very meaningful clinically despite this difference achieving statistical significance in this large study sample.”
Other potential study limitations he cites include the lack of patient blinding to treatment assignment and the absence of clarity about why rates of MDD response and remission over time were relatively low in both treatment groups.
A possible approach to optimize antidepressant choices could involve integration of pharmacogenomic data into larger predictive models that include clinical and demographic variables, Dr. Iosifescu notes.
“The development of such complex models is challenging, but it is now possible given the recent substantial advances in the proficiency of computational tools,” he writes.
The study was funded by the U.S. Department of Veterans Affairs (VA), Health Services Research and Development Service, and the Mental Illness Research, Education, and Clinical Center at the Corporal Michael J. Crescenz VA Medical Center. Dr. Oslin reports having received grants from the VA Office of Research and Development and Janssen Pharmaceuticals and nonfinancial support from Myriad Genetics during the conduct of the study. Dr. Iosifescu report having received personal fees from Alkermes, Allergan, Axsome, Biogen, the Centers for Psychiatric Excellence, Jazz, Lundbeck, Precision Neuroscience, Sage, and Sunovion and grants from Alkermes, AstraZeneca, Brainsway, Litecure, Neosync, Otsuka, Roche, and Shire.
A version of this article first appeared on Medscape.com.
Pharmacogenetic testing, which is used to classify how patients with major depressive disorder (MDD) metabolize medications, reduces adverse drug-gene interactions, new research shows.
In addition, among the intervention group, the rate of remission over 24 weeks was significantly greater.
“These tests can be helpful in rethinking choices of antidepressants, but clinicians should not expect them to be helpful for every patient,” study investigator David W. Oslin, MD, Corporal Michael J. Crescenz VA Medical Center and professor of psychiatry at Perelman School of Medicine, University of Pennsylvania, Philadelphia, said in an interview.
The findings were published online in JAMA.
Less trial and error
Pharmacogenomic testing can provide information to inform drug selection or dosing for patients with a genetic variation that alters pharmacokinetics or pharmacodynamics. Such testing may be particularly useful for patients with MDD, as fewer than 40% of these patients achieve clinical remission after an initial treatment with an antidepressant, the investigators note.
“To get to a treatment that works for an individual, it’s not unusual to have to try two or three or four antidepressants,” said Dr. Oslin. “If we could reduce that variance a little bit with a test like this, that would be huge from a public health perspective.”
The study included 676 physicians and 1,944 adults with MDD (mean age, 48 years; 24% women) who were receiving care at 22 Department of Veterans Affairs medical centers. Eligible patients were set to start a new antidepressant monotherapy, and all underwent a pharmacogenomic test using a cheek swab.
Investigators randomly assigned patients to receive test results when available (pharmacogenomic-guided group) or 24 weeks later (usual-care group). For the former group, clinicians were asked to initiate treatment when test results were available, typically within 2-3 days. For the latter group, they were asked to initiate treatment on a day of randomization.
Assessments included the 9-item Patient Health questionnaire (PHQ-9), scores for which range from 0-27 points, with higher scores indicating worse symptoms.
Of the total patient population, 79% completed the 24-week assessment.
Researchers characterized antidepressant medications on the basis of drug-gene interaction categories: no known interactions, moderate interactions, and substantial interactions.
The co-primary outcomes were treatment initiation within 30 days, determined on the basis of drug-gene interaction categories, and remission from depression symptoms, defined as a PHQ-9 score of less than or equal to 5.
Raters who were blinded to clinical care and study randomization assessed outcomes at 4, 8, 12, 18, and 24 weeks.
Significant impact?
Results showed that the pharmacogenomic-guided group was more likely to receive an antidepressant that had no potential drug-gene interaction, as opposed to one with a moderate/substantial interaction (odds ratio, 4.32; 95% confidence interval, 3.47-5.39; P < .001).
The usual-care group was more likely to receive a drug with mild potential drug-gene interaction (no/moderate interaction vs. substantial interaction: OR, 2.08; 95% CI, 1.52-2.84; P = .005).
For the intervention group, the estimated rates of receiving an antidepressant with no, moderate, and substantial drug-gene interactions were 59.3%, 30.0%, and 10.7%, respectively. For the usual-care group, the estimates were 25.7%, 54.6%, and 19.7%.
The finding that 1 in 5 patients who received usual care were initially given a medication for which there were significant drug-gene interactions means it is “not a rare event,” said Dr. Oslin. “If we can make an impact on 20% of the people we prescribe to, that’s actually pretty big.”
Rates of remission were greater in the pharmacogenomic-guided group over 24 weeks (OR, 1.28; 95% CI, 1.05-1.57; P = .02; absolute risk difference, 2.8%; 95% CI, 0.6%-5.1%).
The secondary outcomes of response to treatment, defined as at least a 50% decrease in PHQ-9 score, also favored the pharmacogenomic-guided group. This was also the case for the secondary outcome of reduction in symptom severity on the PHQ-9 score.
Some physicians have expressed skepticism about pharmacogenomic testing, but the study provides additional evidence of its usefulness, Dr. Oslin noted.
“While I don’t think testing should be standard of practice, I also don’t think we should put barriers into the testing until we can better understand how to target the testing” to those who will benefit the most, he added.
The tests are available at a commercial cost of about $1,000 – which may not be that expensive if testing has a significant impact on a patient’s life, said Dr. Oslin.
Important research, but with several limitations
In an accompanying editorial, Dan V. Iosifescu, MD, associate professor of psychiatry at New York University School of Medicine and director of clinical research at the Nathan Kline Institute for Psychiatric Research, called the study an important addition to the literature on pharmacogenomic testing for patients with MDD.
The study was significantly larger and had broader inclusion criteria and longer follow-up than previous clinical trials and is one of the few investigations not funded by a manufacturer of pharmacogenomic tests, writes Dr. Iosifescu, who was not involved with the research.
However, he notes that an antidepressant was not initiated for 30 days after randomization in 25% of the intervention group and in 31% of the usual-care group, which was “puzzling.” “Because these rates were comparable in the 2 groups, it cannot be explained primarily by the delay of the pharmacogenomic test results in the intervention group,” he writes.
In addition, in the co-primary outcome of symptom remission rate, the difference in clinical improvement in favor of the pharmacogenomic-guided treatment was only “modest” – the gain was of less than 2% in the proportion of patients achieving remission, Dr. Iosifescu adds.
He adds this is “likely not very meaningful clinically despite this difference achieving statistical significance in this large study sample.”
Other potential study limitations he cites include the lack of patient blinding to treatment assignment and the absence of clarity about why rates of MDD response and remission over time were relatively low in both treatment groups.
A possible approach to optimize antidepressant choices could involve integration of pharmacogenomic data into larger predictive models that include clinical and demographic variables, Dr. Iosifescu notes.
“The development of such complex models is challenging, but it is now possible given the recent substantial advances in the proficiency of computational tools,” he writes.
The study was funded by the U.S. Department of Veterans Affairs (VA), Health Services Research and Development Service, and the Mental Illness Research, Education, and Clinical Center at the Corporal Michael J. Crescenz VA Medical Center. Dr. Oslin reports having received grants from the VA Office of Research and Development and Janssen Pharmaceuticals and nonfinancial support from Myriad Genetics during the conduct of the study. Dr. Iosifescu report having received personal fees from Alkermes, Allergan, Axsome, Biogen, the Centers for Psychiatric Excellence, Jazz, Lundbeck, Precision Neuroscience, Sage, and Sunovion and grants from Alkermes, AstraZeneca, Brainsway, Litecure, Neosync, Otsuka, Roche, and Shire.
A version of this article first appeared on Medscape.com.
FROM JAMA
Novel guidance informs plasma biomarker use for Alzheimer’s disease
SAN DIEGO – The organization has previously published recommendations for use of amyloid positron emission tomography (PET) and cerebrospinal fluid (CSF) biomarkers for Alzheimer’s disease.
The recommendations were the subject of a presentation at the 2022 Alzheimer’s Association International Conference and were published online in Alzheimer’s & Dementia.
During his presentation, Oskar Hansson, MD, PhD, stressed that the document describes recommendations, not criteria, for use of blood-based biomarkers. He suggested that the recommendations will need to be updated within 9-12 months, and that criteria for blood-based biomarkers use could come within 2 years.
The new recommendations reflect the recent acceleration of progress in the field, according to Wiesje M. van der Flier, PhD, who moderated the session. “It’s just growing so quickly. I think within 5 years the whole field will have transformed. By starting to use them in specialized memory clinics first, but then also local memory clinics, and then finally, I think that they may also transform primary care,” said Dr. van der Flier, who is a professor of neurology at Amsterdam University Medical Center.
Guidance for clinical trials and memory clinics
The guidelines were created in part because blood-based biomarkers for Alzheimer’s disease have become increasingly available, and there has been a call from the community for guidance, according to Dr. Hansson. There is also a hazard that widespread adoption could interfere with the field itself, especially if physicians don’t understand how to interpret the results. That’s a particularly acute problem since Alzheimer’s disease pathology can precede symptoms. “It’s important to have some guidance about regulating their use so we don’t get the problem that they are misused and get a bad reputation,” said Dr. Hansson in an interview.
The current recommendations are for use in clinical trials to identify patients likely to have Alzheimer’s disease, as well as in memory clinics, though “we’re still a bit cautious. We still need to confirm it with other biomarkers. The reason for that is we still don’t know how these will perform in the clinical reality. So it’s a bit trying it out. You can start using these blood biomarkers to some degree,” said Dr. Hansson.
However, he offered the caveat that plasma-based biomarkers should only be used while confirming that the blood-based biomarkers agree with CSF tests, ideally more than 90% of the time. “If suddenly only 60% of the plasma biomarkers agree with CSF, you have a problem and you need to stop,” said Dr. Hansson.
The authors recommend that blood-based biomarkers be used in clinical trials to help select patients and identify healthy controls. Dr. Hansson said that there is not enough evidence that blood-based biomarkers have sufficient positive predictive value to be used as the sole criteria for clinical trial admission. However, they could also be used to inform decision-making in adaptive clinical trials.
Specifically, plasma Abeta42/Abeta40 and P-tau assays using established thresholds can be used in clinical studies first-screening step for clinical trials, though they should be confirmed by PET or CSF in those with abnormal blood biomarker levels. The biomarkers could also be used in non–Alzheimer’s disease clinical trials to exclude patients with probable Alzheimer’s disease copathology.
In memory clinics, the authors recommend that BBMs be used only in patients who are symptomatic and, when possible, should be confirmed by PET or CSF.
More work to be done
Dr. Hansson noted that 50%-70% of patients with Alzheimer’s disease are misdiagnosed in primary care, showing a clear need for biomarkers that could improve diagnosis. However, he stressed that blood-based biomarkers are not yet ready for use in that setting.
Still, they could eventually become a boon. “The majority of patients now do not get any biomarker support to diagnosis. They do not have access to amyloid PET or [CSF] biomarkers, but when the blood-based biomarkers are good enough, that means that biomarker support for an Alzheimer’s diagnosis [will be] available to many patients … across the globe,” said Dr. van der Flier.
There are numerous research efforts underway to validate blood-based biomarkers in more diverse groups of patients. That’s because the retrospective studies typically used to identify and validate biomarkers tend to recruit carefully selected patients, with clearly defined cases and good CSF characterization, according to Charlotte Teunissen, PhD, who is also a coauthor of the guidelines and professor of neuropsychiatry at Amsterdam University Medical Center. “Now we want to go one step further to go real-life practice, and there are several initiatives,” she said.
Dr. Hansson, Dr. Tenuissen, and Dr. van der Flier have no relevant financial disclosures.
SAN DIEGO – The organization has previously published recommendations for use of amyloid positron emission tomography (PET) and cerebrospinal fluid (CSF) biomarkers for Alzheimer’s disease.
The recommendations were the subject of a presentation at the 2022 Alzheimer’s Association International Conference and were published online in Alzheimer’s & Dementia.
During his presentation, Oskar Hansson, MD, PhD, stressed that the document describes recommendations, not criteria, for use of blood-based biomarkers. He suggested that the recommendations will need to be updated within 9-12 months, and that criteria for blood-based biomarkers use could come within 2 years.
The new recommendations reflect the recent acceleration of progress in the field, according to Wiesje M. van der Flier, PhD, who moderated the session. “It’s just growing so quickly. I think within 5 years the whole field will have transformed. By starting to use them in specialized memory clinics first, but then also local memory clinics, and then finally, I think that they may also transform primary care,” said Dr. van der Flier, who is a professor of neurology at Amsterdam University Medical Center.
Guidance for clinical trials and memory clinics
The guidelines were created in part because blood-based biomarkers for Alzheimer’s disease have become increasingly available, and there has been a call from the community for guidance, according to Dr. Hansson. There is also a hazard that widespread adoption could interfere with the field itself, especially if physicians don’t understand how to interpret the results. That’s a particularly acute problem since Alzheimer’s disease pathology can precede symptoms. “It’s important to have some guidance about regulating their use so we don’t get the problem that they are misused and get a bad reputation,” said Dr. Hansson in an interview.
The current recommendations are for use in clinical trials to identify patients likely to have Alzheimer’s disease, as well as in memory clinics, though “we’re still a bit cautious. We still need to confirm it with other biomarkers. The reason for that is we still don’t know how these will perform in the clinical reality. So it’s a bit trying it out. You can start using these blood biomarkers to some degree,” said Dr. Hansson.
However, he offered the caveat that plasma-based biomarkers should only be used while confirming that the blood-based biomarkers agree with CSF tests, ideally more than 90% of the time. “If suddenly only 60% of the plasma biomarkers agree with CSF, you have a problem and you need to stop,” said Dr. Hansson.
The authors recommend that blood-based biomarkers be used in clinical trials to help select patients and identify healthy controls. Dr. Hansson said that there is not enough evidence that blood-based biomarkers have sufficient positive predictive value to be used as the sole criteria for clinical trial admission. However, they could also be used to inform decision-making in adaptive clinical trials.
Specifically, plasma Abeta42/Abeta40 and P-tau assays using established thresholds can be used in clinical studies first-screening step for clinical trials, though they should be confirmed by PET or CSF in those with abnormal blood biomarker levels. The biomarkers could also be used in non–Alzheimer’s disease clinical trials to exclude patients with probable Alzheimer’s disease copathology.
In memory clinics, the authors recommend that BBMs be used only in patients who are symptomatic and, when possible, should be confirmed by PET or CSF.
More work to be done
Dr. Hansson noted that 50%-70% of patients with Alzheimer’s disease are misdiagnosed in primary care, showing a clear need for biomarkers that could improve diagnosis. However, he stressed that blood-based biomarkers are not yet ready for use in that setting.
Still, they could eventually become a boon. “The majority of patients now do not get any biomarker support to diagnosis. They do not have access to amyloid PET or [CSF] biomarkers, but when the blood-based biomarkers are good enough, that means that biomarker support for an Alzheimer’s diagnosis [will be] available to many patients … across the globe,” said Dr. van der Flier.
There are numerous research efforts underway to validate blood-based biomarkers in more diverse groups of patients. That’s because the retrospective studies typically used to identify and validate biomarkers tend to recruit carefully selected patients, with clearly defined cases and good CSF characterization, according to Charlotte Teunissen, PhD, who is also a coauthor of the guidelines and professor of neuropsychiatry at Amsterdam University Medical Center. “Now we want to go one step further to go real-life practice, and there are several initiatives,” she said.
Dr. Hansson, Dr. Tenuissen, and Dr. van der Flier have no relevant financial disclosures.
SAN DIEGO – The organization has previously published recommendations for use of amyloid positron emission tomography (PET) and cerebrospinal fluid (CSF) biomarkers for Alzheimer’s disease.
The recommendations were the subject of a presentation at the 2022 Alzheimer’s Association International Conference and were published online in Alzheimer’s & Dementia.
During his presentation, Oskar Hansson, MD, PhD, stressed that the document describes recommendations, not criteria, for use of blood-based biomarkers. He suggested that the recommendations will need to be updated within 9-12 months, and that criteria for blood-based biomarkers use could come within 2 years.
The new recommendations reflect the recent acceleration of progress in the field, according to Wiesje M. van der Flier, PhD, who moderated the session. “It’s just growing so quickly. I think within 5 years the whole field will have transformed. By starting to use them in specialized memory clinics first, but then also local memory clinics, and then finally, I think that they may also transform primary care,” said Dr. van der Flier, who is a professor of neurology at Amsterdam University Medical Center.
Guidance for clinical trials and memory clinics
The guidelines were created in part because blood-based biomarkers for Alzheimer’s disease have become increasingly available, and there has been a call from the community for guidance, according to Dr. Hansson. There is also a hazard that widespread adoption could interfere with the field itself, especially if physicians don’t understand how to interpret the results. That’s a particularly acute problem since Alzheimer’s disease pathology can precede symptoms. “It’s important to have some guidance about regulating their use so we don’t get the problem that they are misused and get a bad reputation,” said Dr. Hansson in an interview.
The current recommendations are for use in clinical trials to identify patients likely to have Alzheimer’s disease, as well as in memory clinics, though “we’re still a bit cautious. We still need to confirm it with other biomarkers. The reason for that is we still don’t know how these will perform in the clinical reality. So it’s a bit trying it out. You can start using these blood biomarkers to some degree,” said Dr. Hansson.
However, he offered the caveat that plasma-based biomarkers should only be used while confirming that the blood-based biomarkers agree with CSF tests, ideally more than 90% of the time. “If suddenly only 60% of the plasma biomarkers agree with CSF, you have a problem and you need to stop,” said Dr. Hansson.
The authors recommend that blood-based biomarkers be used in clinical trials to help select patients and identify healthy controls. Dr. Hansson said that there is not enough evidence that blood-based biomarkers have sufficient positive predictive value to be used as the sole criteria for clinical trial admission. However, they could also be used to inform decision-making in adaptive clinical trials.
Specifically, plasma Abeta42/Abeta40 and P-tau assays using established thresholds can be used in clinical studies first-screening step for clinical trials, though they should be confirmed by PET or CSF in those with abnormal blood biomarker levels. The biomarkers could also be used in non–Alzheimer’s disease clinical trials to exclude patients with probable Alzheimer’s disease copathology.
In memory clinics, the authors recommend that BBMs be used only in patients who are symptomatic and, when possible, should be confirmed by PET or CSF.
More work to be done
Dr. Hansson noted that 50%-70% of patients with Alzheimer’s disease are misdiagnosed in primary care, showing a clear need for biomarkers that could improve diagnosis. However, he stressed that blood-based biomarkers are not yet ready for use in that setting.
Still, they could eventually become a boon. “The majority of patients now do not get any biomarker support to diagnosis. They do not have access to amyloid PET or [CSF] biomarkers, but when the blood-based biomarkers are good enough, that means that biomarker support for an Alzheimer’s diagnosis [will be] available to many patients … across the globe,” said Dr. van der Flier.
There are numerous research efforts underway to validate blood-based biomarkers in more diverse groups of patients. That’s because the retrospective studies typically used to identify and validate biomarkers tend to recruit carefully selected patients, with clearly defined cases and good CSF characterization, according to Charlotte Teunissen, PhD, who is also a coauthor of the guidelines and professor of neuropsychiatry at Amsterdam University Medical Center. “Now we want to go one step further to go real-life practice, and there are several initiatives,” she said.
Dr. Hansson, Dr. Tenuissen, and Dr. van der Flier have no relevant financial disclosures.
FROM AAIC 2022
ICU stays linked to a doubling of dementia risk
compared with older adults who have never stayed in the ICU, new research suggests.
“ICU hospitalization may be an underrecognized risk factor for dementia in older adults,” Bryan D. James, PhD, epidemiologist with Rush Alzheimer’s Disease Center, Chicago, said in an interview.
“Health care providers caring for older patients who have experienced a hospitalization for critical illness should be prepared to assess and monitor their patients’ cognitive status as part of their long-term care plan,” Dr. James added.
The findings were presented at the Alzheimer’s Association International Conference.
Hidden risk factor?
ICU hospitalization as a result of critical illness has been linked to subsequent cognitive impairment in older patients. However, how ICU hospitalization relates to the long-term risk of developing Alzheimer’s and other age-related dementias is unknown.
“Given the high rate of ICU hospitalization in older persons, especially during the COVID-19 pandemic, it is critical to explore this relationship, Dr. James said.
The Rush team assessed the impact of an ICU stay on dementia risk in 3,822 older adults (mean age, 77 years) without known dementia at baseline participating in five diverse epidemiologic cohorts.
Participants were checked annually for development of Alzheimer’s and all-type dementia using standardized cognitive assessments.
Over an average of 7.8 years, 1,991 (52%) adults had at least one ICU stay; 1,031 (27%) had an ICU stay before study enrollment; and 961 (25%) had an ICU stay during the study period.
In models adjusted for age, sex, education, and race, ICU hospitalization was associated with 63% higher risk of Alzheimer’s dementia (hazard ratio, 1.63; 95% confidence interval, 1.41-1.88) and 71% higher risk of all-type dementia (HR, 1.71; 95% CI, 1.48-1.97).
In models further adjusted for other health factors such as vascular risk factors and disease, other chronic medical conditions and functional disabilities, the association was even stronger: ICU hospitalization was associated with roughly double the risk of Alzheimer’s dementia (HR 2.10; 95% CI, 1.66-2.65) and all-type dementia (HR, 2.20; 95% CI, 1.75-2.77).
Dr. James said in an interview that it remains unclear why an ICU stay may raise the dementia risk.
“This study was not designed to assess the causes of the higher risk of dementia in persons who had ICU hospitalizations. However, researchers have looked into a number of factors that could account for this increased risk,” he explained.
One is critical illness itself that leads to hospitalization, which could result in damage to the brain; for example, severe COVID-19 has been shown to directly harm the brain, Dr. James said.
He also noted that specific events experienced during ICU stay have been shown to increase risk for cognitive impairment, including infection and severe sepsis, acute dialysis, neurologic dysfunction and delirium, and sedation.
Important work
Commenting on the study, Heather Snyder, PhD, vice president of medical & scientific relations at the Alzheimer’s Association, said what’s interesting about the study is that it looks at individuals in the ICU, regardless of the cause.
“The study shows that having some type of health issue that results in some type of ICU stay is associated with an increased risk of declining cognition,” Dr. Snyder said.
“That’s really important,” she said, “especially given the increase in individuals, particularly those 60 and older, who did experience an ICU stay over the last couple of years and understanding how that might impact their long-term risk related to Alzheimer’s and other changes in memory.”
“If an individual has been in the ICU, that should be part of the conversation with their physician or health care provider,” Dr. Snyder advised.
The study was funded by the National Institute on Aging. Dr. James and Dr. Snyder disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
compared with older adults who have never stayed in the ICU, new research suggests.
“ICU hospitalization may be an underrecognized risk factor for dementia in older adults,” Bryan D. James, PhD, epidemiologist with Rush Alzheimer’s Disease Center, Chicago, said in an interview.
“Health care providers caring for older patients who have experienced a hospitalization for critical illness should be prepared to assess and monitor their patients’ cognitive status as part of their long-term care plan,” Dr. James added.
The findings were presented at the Alzheimer’s Association International Conference.
Hidden risk factor?
ICU hospitalization as a result of critical illness has been linked to subsequent cognitive impairment in older patients. However, how ICU hospitalization relates to the long-term risk of developing Alzheimer’s and other age-related dementias is unknown.
“Given the high rate of ICU hospitalization in older persons, especially during the COVID-19 pandemic, it is critical to explore this relationship, Dr. James said.
The Rush team assessed the impact of an ICU stay on dementia risk in 3,822 older adults (mean age, 77 years) without known dementia at baseline participating in five diverse epidemiologic cohorts.
Participants were checked annually for development of Alzheimer’s and all-type dementia using standardized cognitive assessments.
Over an average of 7.8 years, 1,991 (52%) adults had at least one ICU stay; 1,031 (27%) had an ICU stay before study enrollment; and 961 (25%) had an ICU stay during the study period.
In models adjusted for age, sex, education, and race, ICU hospitalization was associated with 63% higher risk of Alzheimer’s dementia (hazard ratio, 1.63; 95% confidence interval, 1.41-1.88) and 71% higher risk of all-type dementia (HR, 1.71; 95% CI, 1.48-1.97).
In models further adjusted for other health factors such as vascular risk factors and disease, other chronic medical conditions and functional disabilities, the association was even stronger: ICU hospitalization was associated with roughly double the risk of Alzheimer’s dementia (HR 2.10; 95% CI, 1.66-2.65) and all-type dementia (HR, 2.20; 95% CI, 1.75-2.77).
Dr. James said in an interview that it remains unclear why an ICU stay may raise the dementia risk.
“This study was not designed to assess the causes of the higher risk of dementia in persons who had ICU hospitalizations. However, researchers have looked into a number of factors that could account for this increased risk,” he explained.
One is critical illness itself that leads to hospitalization, which could result in damage to the brain; for example, severe COVID-19 has been shown to directly harm the brain, Dr. James said.
He also noted that specific events experienced during ICU stay have been shown to increase risk for cognitive impairment, including infection and severe sepsis, acute dialysis, neurologic dysfunction and delirium, and sedation.
Important work
Commenting on the study, Heather Snyder, PhD, vice president of medical & scientific relations at the Alzheimer’s Association, said what’s interesting about the study is that it looks at individuals in the ICU, regardless of the cause.
“The study shows that having some type of health issue that results in some type of ICU stay is associated with an increased risk of declining cognition,” Dr. Snyder said.
“That’s really important,” she said, “especially given the increase in individuals, particularly those 60 and older, who did experience an ICU stay over the last couple of years and understanding how that might impact their long-term risk related to Alzheimer’s and other changes in memory.”
“If an individual has been in the ICU, that should be part of the conversation with their physician or health care provider,” Dr. Snyder advised.
The study was funded by the National Institute on Aging. Dr. James and Dr. Snyder disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
compared with older adults who have never stayed in the ICU, new research suggests.
“ICU hospitalization may be an underrecognized risk factor for dementia in older adults,” Bryan D. James, PhD, epidemiologist with Rush Alzheimer’s Disease Center, Chicago, said in an interview.
“Health care providers caring for older patients who have experienced a hospitalization for critical illness should be prepared to assess and monitor their patients’ cognitive status as part of their long-term care plan,” Dr. James added.
The findings were presented at the Alzheimer’s Association International Conference.
Hidden risk factor?
ICU hospitalization as a result of critical illness has been linked to subsequent cognitive impairment in older patients. However, how ICU hospitalization relates to the long-term risk of developing Alzheimer’s and other age-related dementias is unknown.
“Given the high rate of ICU hospitalization in older persons, especially during the COVID-19 pandemic, it is critical to explore this relationship, Dr. James said.
The Rush team assessed the impact of an ICU stay on dementia risk in 3,822 older adults (mean age, 77 years) without known dementia at baseline participating in five diverse epidemiologic cohorts.
Participants were checked annually for development of Alzheimer’s and all-type dementia using standardized cognitive assessments.
Over an average of 7.8 years, 1,991 (52%) adults had at least one ICU stay; 1,031 (27%) had an ICU stay before study enrollment; and 961 (25%) had an ICU stay during the study period.
In models adjusted for age, sex, education, and race, ICU hospitalization was associated with 63% higher risk of Alzheimer’s dementia (hazard ratio, 1.63; 95% confidence interval, 1.41-1.88) and 71% higher risk of all-type dementia (HR, 1.71; 95% CI, 1.48-1.97).
In models further adjusted for other health factors such as vascular risk factors and disease, other chronic medical conditions and functional disabilities, the association was even stronger: ICU hospitalization was associated with roughly double the risk of Alzheimer’s dementia (HR 2.10; 95% CI, 1.66-2.65) and all-type dementia (HR, 2.20; 95% CI, 1.75-2.77).
Dr. James said in an interview that it remains unclear why an ICU stay may raise the dementia risk.
“This study was not designed to assess the causes of the higher risk of dementia in persons who had ICU hospitalizations. However, researchers have looked into a number of factors that could account for this increased risk,” he explained.
One is critical illness itself that leads to hospitalization, which could result in damage to the brain; for example, severe COVID-19 has been shown to directly harm the brain, Dr. James said.
He also noted that specific events experienced during ICU stay have been shown to increase risk for cognitive impairment, including infection and severe sepsis, acute dialysis, neurologic dysfunction and delirium, and sedation.
Important work
Commenting on the study, Heather Snyder, PhD, vice president of medical & scientific relations at the Alzheimer’s Association, said what’s interesting about the study is that it looks at individuals in the ICU, regardless of the cause.
“The study shows that having some type of health issue that results in some type of ICU stay is associated with an increased risk of declining cognition,” Dr. Snyder said.
“That’s really important,” she said, “especially given the increase in individuals, particularly those 60 and older, who did experience an ICU stay over the last couple of years and understanding how that might impact their long-term risk related to Alzheimer’s and other changes in memory.”
“If an individual has been in the ICU, that should be part of the conversation with their physician or health care provider,” Dr. Snyder advised.
The study was funded by the National Institute on Aging. Dr. James and Dr. Snyder disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM AAIC 2022
‘Med check’ appointments: How to minimize your malpractice risk
Medical malpractice claims can arise in any type of health care setting. The purpose of this article is to discuss the risk of medical malpractice suits in the context of brief “med checks,” which are 15- to 20-minute follow-up appointments for psychiatric outpatient medication management. Similar issues arise in brief new patient and transfer visits.
Malpractice hinges on ‘reasonableness’
Malpractice is an allegation of professional negligence.1 More specifically, it is an allegation that a clinician violated an existing duty by deviating from the standard of care, and that deviation caused damages.2 Medical malpractice claims involve questions about whether there was a deviation from the standard of care (whether the clinician failed to exercise a reasonable degree of skill and care given the context of the situation) and whether there was causation (whether a deviation caused a patient’s damages).3 These are fact-based determinations. Thus, the legal resolution of a malpractice claim is based on the facts of each specific case.
The advisability of 15-minute med checks and the associated limitation on a clinician’s ability to provide talk therapy are beyond the scope of this article. What is clear, however, is that not all brief med check appointments are created equal. Their safety and efficacy are dictated by the milieu in which they exist.
Practically speaking, although many factors need to be considered, the standard of care in a medical malpractice lawsuit is based on reasonableness.4-6 One strategy to proactively manage your malpractice risk is to consider—either for your existing job or before accepting a new position—whether your agency’s setup for brief med checks will allow you to practice reasonably. This article provides information to help you answer this question and describes a hypothetical case vignette to illustrate how certain factors might help lower the chances of facing a malpractice suit.
Established patients
In med check appointments for established patients, consider the patient population, the availability of pre- and postvisit support services, and contingency plans (Table).

Different patient populations require different levels of treatment. Consider, for example, a patient with anxiety and trauma who is actively engaged with a therapist who works at the same agency as their psychiatrist, where the medication management appointments are solely for selective serotonin reuptake inhibitor refills. Compare that to a dual-diagnosis patient—with a psychotic and substance use disorder—who has had poor medication compliance and frequent rehospitalizations. The first patient is more likely to be reasonably managed in a 15-minute med check. The second patient would need significantly more pre- and postvisit support services. This consideration is relevant from a clinical perspective, and if a bad outcome occurs, from a malpractice perspective. Patient populations are not homogeneous; the reasonableness of a clinician’s actions during a brief med check visit depends on the specific patient.
Pre- and postvisit support services vary greatly from clinic to clinic. They range from clerical support (eg, calling a pharmacy to ensure that a patient’s medication is available for same-day pickup) to nursing support (eg, an injection nurse who is on site and can immediately provide a patient with a missed injection) to case manager support (eg, a case manager to facilitate coordination of care, such as by having a patient fill out record releases and then working to ensure that relevant hospital records are received prior to the next visit). The real-world availability of these services can determine the feasibility of safely conducting a 15-minute med check visit.
Continue to: Regardless of the patient population...
Regardless of the patient population, unexpected situations will arise. It could be a patient with posttraumatic stress disorder who was recently retraumatized and is in the midst of disclosing this new trauma at the end of a 15-minute visit. Or it could be a patient with dual diagnoses who comes to the agency intoxicated and manic, describing a plan to kill his neighbor with a shotgun. A clinician’s ability to meet the standard of care, and act reasonably within the confines of a brief med check structure, can thus depend on whether there are means of adequately managing such emergent situations.
Some clinics have fairly high no-show rates. Leaving no-show slots open for administrative time can provide a means of managing emergent situations. If, however, they are automatically rebooked with walk-ins, brief visits become more challenging. Thus, when assessing contingency plan logistics, consider the no-show rate, what happens when there are no-shows, how many other clinicians are available on a given day, and whether staff is available to provide support (eg, sitting with a patient while waiting for an ambulance).
New and transfer patients
Brief visits for new or transfer patients require the same assessment described above. However, there are additional considerations regarding previsit support services. Some clinics use clinical social workers to perform intake evaluations before a new patient sees the psychiatrist. A high-quality intake evaluation can allow a psychiatrist to focus, in a shorter amount of time, on a patient’s medication needs. An additional time saver is having support staff who will obtain relevant medical records before a patient’s first psychiatric visit. Such actions can greatly increase the efficacy of a new patient appointment for the prescribing clinician.
The reliability of and level of detail assessed in prior evaluations can be particularly relevant when considering a job providing coverage as locum tenens, when all patients will be new to you. Unfortunately, if you are not employed at a clinic, it can be hard to assess this ahead of time. If you know colleagues in the area where you are considering taking a locum position, ask for their opinions about the quality of work at the agency.
Case vignette
Mr. J is a 30-year-old man with schizoaffective disorder. For several years, he has been followed once every 4 weeks at the local clinic. During the first year of treatment, he had numerous hospitalizations due to medication noncompliance, psychotic episodes, and threats of violence against his mother. For the past year, he had been stable on the same dose of an oral antipsychotic medication (risperidone 2 mg twice a day). Then he stopped taking his medication, became increasingly psychotic, and, while holding a butcher knife, threatened to kill his mother. His mother called 911 and Mr. J was hospitalized.
Continue to: While in the hospital...
While in the hospital, Mr. J was restarted on risperidone 2 mg twice a day, and lithium 600 mg twice a day was added. As part of discharge planning, the hospital social worker set up an outpatient appointment with Dr. R, Mr. J’s treating psychiatrist at the clinic. That appointment was scheduled as a 15-minute med check. At the visit, Dr. R did not have or try to obtain a copy of the hospital discharge summary. Mr. J told Dr. R that he had been hospitalized because he had run out of his oral antipsychotic, and that it had been restarted during the hospitalization. Dr. R—who did not know about the recent incident involving a butcher knife or the subsequent medication changes—continued Mr. J’s risperidone, but did not continue his lithium because she did not know it had been added.
Dr. R scheduled a 4-week follow-up visit for Mr. J. Then she went on maternity leave. Because the agency was short-staffed, they hired Dr. C—a locum tenens—to see all of Dr. R’s established patients in 15-minute time slots.
At their first visit, Mr. J told Dr. C that he was gaining too much weight from his antipsychotic and wanted to know if it would be OK to decrease the dose. Dr. C reviewed Dr. R’s last office note but, due to limited time, did not review any other notes. Although Dr. C had 2 no-shows that day, the clinic had a policy that required Dr. C to see walk-ins whenever there was a no-show.
Dr. C did not know of Mr. J’s threats of violence or the medication changes associated with his recent hospitalization (they were not referenced in Dr. R’s last note). Dr. C decreased the dose of Mr. J’s risperidone from 2 mg twice a day to 0.5 mg twice a day. He did not do a violence risk assessment. Two weeks after the visit with Dr. C, Mr. J, who had become increasingly depressed and psychotic, killed his mother and died by suicide.
The estates of Mr. J and his mother filed a medical malpractice lawsuit against Dr. R and Dr. C. Both psychiatrists had a duty to Mr. J. Whether there was a duty to Mr. J’s mother would depend in part on the state’s duty to protect laws. Either way, the malpractice case would hinge on whether the psychiatrists’ conduct fell below the standard of care.
Continue to: In this case...
In this case, the critical issues were Dr. R’s failure to obtain and review the recent hospital records and Dr. C’s decision to decrease the antipsychotic dose. Of particular concern is Dr. C’s decision to decrease the antipsychotic dose without reviewing more information from past records, and the resultant failure to perform a violence risk assessment. These deviations cannot be blamed entirely on the brevity of the med check appointment. They could happen in a clinic that allotted longer time periods for follow-up visits, but they are, however, more likely to occur in a short med check appointment due to time constraints.
The likelihood of these errors could have been reduced by additional support services, as well as more time for Dr. C to see each patient who was new to him. For example, if there had been a support person available to obtain hospital records prior to the postdischarge appointment, Dr. R and Dr. C would have been more likely to be aware of the violent threat associated with Mr. J’s hospitalization. Additionally, if the busy clinicians had contingency plans to assess complicated patients, such as the ability to use no-show time to deal with difficult situations, Dr. C could have taken more time to review past records.
Bottom Line
When working in a setting that involves brief med check appointments, assess the agency structure, and whether it will allow you to practice reasonably. This will be relevant clinically and may reduce the risk of malpractice lawsuits. Reasonableness of a clinician’s actions is a fact-specific question and is influenced by multiple factors, including the patient population, the availability and quality of an agency’s support services, and contingency plans.
Related Resources
- Mossman D. Successfully navigating the 15-minute ‘med check.’ Current Psychiatry. 2010;9(6):40-43.
- Olfson M, Marcus SC. National trends in outpatient psychotherapy. Am J Psychiatry. 2010;167(12):1456-1463.
Drug Brand Names
Lithium • Eskalith, Lithobid
Risperidone • Risperdal
1. Malpractice. In: Garner BA, ed. Black’s Law Dictionary. 11th ed. Thomson West; 2019:1148.
2. Frierson RL, Joshi KG. Malpractice law and psychiatry: an overview. Focus. 2019;17:332-336. doi:10.1176/appi.focus.20190017
3. Negligence Based Claims. In: Boumil MM, Hattis PA, eds. Medical Liability in a Nutshell. 4th ed. West Academic Publishing; 2017:43-88
4. Peters PG. The quiet demise of deference to custom: malpractice law at the millennium. Washington and Lee Law Review. 2000;57(1):163-205. Accessed July 8, 2022. https://scholarlycommons.law.wlu.edu/wlulr/vol57/iss1/5
5. Simon RI. Standard-of-care testimony: best practices or reasonable care? J Am Acad Psychiatry Law. 2005;33(1):8-11. Accessed July 8, 2022. http://jaapl.org/content/33/1/8
6. Behrens SA. Call in Houdini: the time has come to be released from the geographic straightjacket known as the locality rule. Drake Law Review. 2008; 56(3):753-790. Accessed June 20, 2022. https://lawreviewdrake.files.wordpress.com/2015/06/lrvol56-3_behrens.pdf
Medical malpractice claims can arise in any type of health care setting. The purpose of this article is to discuss the risk of medical malpractice suits in the context of brief “med checks,” which are 15- to 20-minute follow-up appointments for psychiatric outpatient medication management. Similar issues arise in brief new patient and transfer visits.
Malpractice hinges on ‘reasonableness’
Malpractice is an allegation of professional negligence.1 More specifically, it is an allegation that a clinician violated an existing duty by deviating from the standard of care, and that deviation caused damages.2 Medical malpractice claims involve questions about whether there was a deviation from the standard of care (whether the clinician failed to exercise a reasonable degree of skill and care given the context of the situation) and whether there was causation (whether a deviation caused a patient’s damages).3 These are fact-based determinations. Thus, the legal resolution of a malpractice claim is based on the facts of each specific case.
The advisability of 15-minute med checks and the associated limitation on a clinician’s ability to provide talk therapy are beyond the scope of this article. What is clear, however, is that not all brief med check appointments are created equal. Their safety and efficacy are dictated by the milieu in which they exist.
Practically speaking, although many factors need to be considered, the standard of care in a medical malpractice lawsuit is based on reasonableness.4-6 One strategy to proactively manage your malpractice risk is to consider—either for your existing job or before accepting a new position—whether your agency’s setup for brief med checks will allow you to practice reasonably. This article provides information to help you answer this question and describes a hypothetical case vignette to illustrate how certain factors might help lower the chances of facing a malpractice suit.
Established patients
In med check appointments for established patients, consider the patient population, the availability of pre- and postvisit support services, and contingency plans (Table).

Different patient populations require different levels of treatment. Consider, for example, a patient with anxiety and trauma who is actively engaged with a therapist who works at the same agency as their psychiatrist, where the medication management appointments are solely for selective serotonin reuptake inhibitor refills. Compare that to a dual-diagnosis patient—with a psychotic and substance use disorder—who has had poor medication compliance and frequent rehospitalizations. The first patient is more likely to be reasonably managed in a 15-minute med check. The second patient would need significantly more pre- and postvisit support services. This consideration is relevant from a clinical perspective, and if a bad outcome occurs, from a malpractice perspective. Patient populations are not homogeneous; the reasonableness of a clinician’s actions during a brief med check visit depends on the specific patient.
Pre- and postvisit support services vary greatly from clinic to clinic. They range from clerical support (eg, calling a pharmacy to ensure that a patient’s medication is available for same-day pickup) to nursing support (eg, an injection nurse who is on site and can immediately provide a patient with a missed injection) to case manager support (eg, a case manager to facilitate coordination of care, such as by having a patient fill out record releases and then working to ensure that relevant hospital records are received prior to the next visit). The real-world availability of these services can determine the feasibility of safely conducting a 15-minute med check visit.
Continue to: Regardless of the patient population...
Regardless of the patient population, unexpected situations will arise. It could be a patient with posttraumatic stress disorder who was recently retraumatized and is in the midst of disclosing this new trauma at the end of a 15-minute visit. Or it could be a patient with dual diagnoses who comes to the agency intoxicated and manic, describing a plan to kill his neighbor with a shotgun. A clinician’s ability to meet the standard of care, and act reasonably within the confines of a brief med check structure, can thus depend on whether there are means of adequately managing such emergent situations.
Some clinics have fairly high no-show rates. Leaving no-show slots open for administrative time can provide a means of managing emergent situations. If, however, they are automatically rebooked with walk-ins, brief visits become more challenging. Thus, when assessing contingency plan logistics, consider the no-show rate, what happens when there are no-shows, how many other clinicians are available on a given day, and whether staff is available to provide support (eg, sitting with a patient while waiting for an ambulance).
New and transfer patients
Brief visits for new or transfer patients require the same assessment described above. However, there are additional considerations regarding previsit support services. Some clinics use clinical social workers to perform intake evaluations before a new patient sees the psychiatrist. A high-quality intake evaluation can allow a psychiatrist to focus, in a shorter amount of time, on a patient’s medication needs. An additional time saver is having support staff who will obtain relevant medical records before a patient’s first psychiatric visit. Such actions can greatly increase the efficacy of a new patient appointment for the prescribing clinician.
The reliability of and level of detail assessed in prior evaluations can be particularly relevant when considering a job providing coverage as locum tenens, when all patients will be new to you. Unfortunately, if you are not employed at a clinic, it can be hard to assess this ahead of time. If you know colleagues in the area where you are considering taking a locum position, ask for their opinions about the quality of work at the agency.
Case vignette
Mr. J is a 30-year-old man with schizoaffective disorder. For several years, he has been followed once every 4 weeks at the local clinic. During the first year of treatment, he had numerous hospitalizations due to medication noncompliance, psychotic episodes, and threats of violence against his mother. For the past year, he had been stable on the same dose of an oral antipsychotic medication (risperidone 2 mg twice a day). Then he stopped taking his medication, became increasingly psychotic, and, while holding a butcher knife, threatened to kill his mother. His mother called 911 and Mr. J was hospitalized.
Continue to: While in the hospital...
While in the hospital, Mr. J was restarted on risperidone 2 mg twice a day, and lithium 600 mg twice a day was added. As part of discharge planning, the hospital social worker set up an outpatient appointment with Dr. R, Mr. J’s treating psychiatrist at the clinic. That appointment was scheduled as a 15-minute med check. At the visit, Dr. R did not have or try to obtain a copy of the hospital discharge summary. Mr. J told Dr. R that he had been hospitalized because he had run out of his oral antipsychotic, and that it had been restarted during the hospitalization. Dr. R—who did not know about the recent incident involving a butcher knife or the subsequent medication changes—continued Mr. J’s risperidone, but did not continue his lithium because she did not know it had been added.
Dr. R scheduled a 4-week follow-up visit for Mr. J. Then she went on maternity leave. Because the agency was short-staffed, they hired Dr. C—a locum tenens—to see all of Dr. R’s established patients in 15-minute time slots.
At their first visit, Mr. J told Dr. C that he was gaining too much weight from his antipsychotic and wanted to know if it would be OK to decrease the dose. Dr. C reviewed Dr. R’s last office note but, due to limited time, did not review any other notes. Although Dr. C had 2 no-shows that day, the clinic had a policy that required Dr. C to see walk-ins whenever there was a no-show.
Dr. C did not know of Mr. J’s threats of violence or the medication changes associated with his recent hospitalization (they were not referenced in Dr. R’s last note). Dr. C decreased the dose of Mr. J’s risperidone from 2 mg twice a day to 0.5 mg twice a day. He did not do a violence risk assessment. Two weeks after the visit with Dr. C, Mr. J, who had become increasingly depressed and psychotic, killed his mother and died by suicide.
The estates of Mr. J and his mother filed a medical malpractice lawsuit against Dr. R and Dr. C. Both psychiatrists had a duty to Mr. J. Whether there was a duty to Mr. J’s mother would depend in part on the state’s duty to protect laws. Either way, the malpractice case would hinge on whether the psychiatrists’ conduct fell below the standard of care.
Continue to: In this case...
In this case, the critical issues were Dr. R’s failure to obtain and review the recent hospital records and Dr. C’s decision to decrease the antipsychotic dose. Of particular concern is Dr. C’s decision to decrease the antipsychotic dose without reviewing more information from past records, and the resultant failure to perform a violence risk assessment. These deviations cannot be blamed entirely on the brevity of the med check appointment. They could happen in a clinic that allotted longer time periods for follow-up visits, but they are, however, more likely to occur in a short med check appointment due to time constraints.
The likelihood of these errors could have been reduced by additional support services, as well as more time for Dr. C to see each patient who was new to him. For example, if there had been a support person available to obtain hospital records prior to the postdischarge appointment, Dr. R and Dr. C would have been more likely to be aware of the violent threat associated with Mr. J’s hospitalization. Additionally, if the busy clinicians had contingency plans to assess complicated patients, such as the ability to use no-show time to deal with difficult situations, Dr. C could have taken more time to review past records.
Bottom Line
When working in a setting that involves brief med check appointments, assess the agency structure, and whether it will allow you to practice reasonably. This will be relevant clinically and may reduce the risk of malpractice lawsuits. Reasonableness of a clinician’s actions is a fact-specific question and is influenced by multiple factors, including the patient population, the availability and quality of an agency’s support services, and contingency plans.
Related Resources
- Mossman D. Successfully navigating the 15-minute ‘med check.’ Current Psychiatry. 2010;9(6):40-43.
- Olfson M, Marcus SC. National trends in outpatient psychotherapy. Am J Psychiatry. 2010;167(12):1456-1463.
Drug Brand Names
Lithium • Eskalith, Lithobid
Risperidone • Risperdal
Medical malpractice claims can arise in any type of health care setting. The purpose of this article is to discuss the risk of medical malpractice suits in the context of brief “med checks,” which are 15- to 20-minute follow-up appointments for psychiatric outpatient medication management. Similar issues arise in brief new patient and transfer visits.
Malpractice hinges on ‘reasonableness’
Malpractice is an allegation of professional negligence.1 More specifically, it is an allegation that a clinician violated an existing duty by deviating from the standard of care, and that deviation caused damages.2 Medical malpractice claims involve questions about whether there was a deviation from the standard of care (whether the clinician failed to exercise a reasonable degree of skill and care given the context of the situation) and whether there was causation (whether a deviation caused a patient’s damages).3 These are fact-based determinations. Thus, the legal resolution of a malpractice claim is based on the facts of each specific case.
The advisability of 15-minute med checks and the associated limitation on a clinician’s ability to provide talk therapy are beyond the scope of this article. What is clear, however, is that not all brief med check appointments are created equal. Their safety and efficacy are dictated by the milieu in which they exist.
Practically speaking, although many factors need to be considered, the standard of care in a medical malpractice lawsuit is based on reasonableness.4-6 One strategy to proactively manage your malpractice risk is to consider—either for your existing job or before accepting a new position—whether your agency’s setup for brief med checks will allow you to practice reasonably. This article provides information to help you answer this question and describes a hypothetical case vignette to illustrate how certain factors might help lower the chances of facing a malpractice suit.
Established patients
In med check appointments for established patients, consider the patient population, the availability of pre- and postvisit support services, and contingency plans (Table).

Different patient populations require different levels of treatment. Consider, for example, a patient with anxiety and trauma who is actively engaged with a therapist who works at the same agency as their psychiatrist, where the medication management appointments are solely for selective serotonin reuptake inhibitor refills. Compare that to a dual-diagnosis patient—with a psychotic and substance use disorder—who has had poor medication compliance and frequent rehospitalizations. The first patient is more likely to be reasonably managed in a 15-minute med check. The second patient would need significantly more pre- and postvisit support services. This consideration is relevant from a clinical perspective, and if a bad outcome occurs, from a malpractice perspective. Patient populations are not homogeneous; the reasonableness of a clinician’s actions during a brief med check visit depends on the specific patient.
Pre- and postvisit support services vary greatly from clinic to clinic. They range from clerical support (eg, calling a pharmacy to ensure that a patient’s medication is available for same-day pickup) to nursing support (eg, an injection nurse who is on site and can immediately provide a patient with a missed injection) to case manager support (eg, a case manager to facilitate coordination of care, such as by having a patient fill out record releases and then working to ensure that relevant hospital records are received prior to the next visit). The real-world availability of these services can determine the feasibility of safely conducting a 15-minute med check visit.
Continue to: Regardless of the patient population...
Regardless of the patient population, unexpected situations will arise. It could be a patient with posttraumatic stress disorder who was recently retraumatized and is in the midst of disclosing this new trauma at the end of a 15-minute visit. Or it could be a patient with dual diagnoses who comes to the agency intoxicated and manic, describing a plan to kill his neighbor with a shotgun. A clinician’s ability to meet the standard of care, and act reasonably within the confines of a brief med check structure, can thus depend on whether there are means of adequately managing such emergent situations.
Some clinics have fairly high no-show rates. Leaving no-show slots open for administrative time can provide a means of managing emergent situations. If, however, they are automatically rebooked with walk-ins, brief visits become more challenging. Thus, when assessing contingency plan logistics, consider the no-show rate, what happens when there are no-shows, how many other clinicians are available on a given day, and whether staff is available to provide support (eg, sitting with a patient while waiting for an ambulance).
New and transfer patients
Brief visits for new or transfer patients require the same assessment described above. However, there are additional considerations regarding previsit support services. Some clinics use clinical social workers to perform intake evaluations before a new patient sees the psychiatrist. A high-quality intake evaluation can allow a psychiatrist to focus, in a shorter amount of time, on a patient’s medication needs. An additional time saver is having support staff who will obtain relevant medical records before a patient’s first psychiatric visit. Such actions can greatly increase the efficacy of a new patient appointment for the prescribing clinician.
The reliability of and level of detail assessed in prior evaluations can be particularly relevant when considering a job providing coverage as locum tenens, when all patients will be new to you. Unfortunately, if you are not employed at a clinic, it can be hard to assess this ahead of time. If you know colleagues in the area where you are considering taking a locum position, ask for their opinions about the quality of work at the agency.
Case vignette
Mr. J is a 30-year-old man with schizoaffective disorder. For several years, he has been followed once every 4 weeks at the local clinic. During the first year of treatment, he had numerous hospitalizations due to medication noncompliance, psychotic episodes, and threats of violence against his mother. For the past year, he had been stable on the same dose of an oral antipsychotic medication (risperidone 2 mg twice a day). Then he stopped taking his medication, became increasingly psychotic, and, while holding a butcher knife, threatened to kill his mother. His mother called 911 and Mr. J was hospitalized.
Continue to: While in the hospital...
While in the hospital, Mr. J was restarted on risperidone 2 mg twice a day, and lithium 600 mg twice a day was added. As part of discharge planning, the hospital social worker set up an outpatient appointment with Dr. R, Mr. J’s treating psychiatrist at the clinic. That appointment was scheduled as a 15-minute med check. At the visit, Dr. R did not have or try to obtain a copy of the hospital discharge summary. Mr. J told Dr. R that he had been hospitalized because he had run out of his oral antipsychotic, and that it had been restarted during the hospitalization. Dr. R—who did not know about the recent incident involving a butcher knife or the subsequent medication changes—continued Mr. J’s risperidone, but did not continue his lithium because she did not know it had been added.
Dr. R scheduled a 4-week follow-up visit for Mr. J. Then she went on maternity leave. Because the agency was short-staffed, they hired Dr. C—a locum tenens—to see all of Dr. R’s established patients in 15-minute time slots.
At their first visit, Mr. J told Dr. C that he was gaining too much weight from his antipsychotic and wanted to know if it would be OK to decrease the dose. Dr. C reviewed Dr. R’s last office note but, due to limited time, did not review any other notes. Although Dr. C had 2 no-shows that day, the clinic had a policy that required Dr. C to see walk-ins whenever there was a no-show.
Dr. C did not know of Mr. J’s threats of violence or the medication changes associated with his recent hospitalization (they were not referenced in Dr. R’s last note). Dr. C decreased the dose of Mr. J’s risperidone from 2 mg twice a day to 0.5 mg twice a day. He did not do a violence risk assessment. Two weeks after the visit with Dr. C, Mr. J, who had become increasingly depressed and psychotic, killed his mother and died by suicide.
The estates of Mr. J and his mother filed a medical malpractice lawsuit against Dr. R and Dr. C. Both psychiatrists had a duty to Mr. J. Whether there was a duty to Mr. J’s mother would depend in part on the state’s duty to protect laws. Either way, the malpractice case would hinge on whether the psychiatrists’ conduct fell below the standard of care.
Continue to: In this case...
In this case, the critical issues were Dr. R’s failure to obtain and review the recent hospital records and Dr. C’s decision to decrease the antipsychotic dose. Of particular concern is Dr. C’s decision to decrease the antipsychotic dose without reviewing more information from past records, and the resultant failure to perform a violence risk assessment. These deviations cannot be blamed entirely on the brevity of the med check appointment. They could happen in a clinic that allotted longer time periods for follow-up visits, but they are, however, more likely to occur in a short med check appointment due to time constraints.
The likelihood of these errors could have been reduced by additional support services, as well as more time for Dr. C to see each patient who was new to him. For example, if there had been a support person available to obtain hospital records prior to the postdischarge appointment, Dr. R and Dr. C would have been more likely to be aware of the violent threat associated with Mr. J’s hospitalization. Additionally, if the busy clinicians had contingency plans to assess complicated patients, such as the ability to use no-show time to deal with difficult situations, Dr. C could have taken more time to review past records.
Bottom Line
When working in a setting that involves brief med check appointments, assess the agency structure, and whether it will allow you to practice reasonably. This will be relevant clinically and may reduce the risk of malpractice lawsuits. Reasonableness of a clinician’s actions is a fact-specific question and is influenced by multiple factors, including the patient population, the availability and quality of an agency’s support services, and contingency plans.
Related Resources
- Mossman D. Successfully navigating the 15-minute ‘med check.’ Current Psychiatry. 2010;9(6):40-43.
- Olfson M, Marcus SC. National trends in outpatient psychotherapy. Am J Psychiatry. 2010;167(12):1456-1463.
Drug Brand Names
Lithium • Eskalith, Lithobid
Risperidone • Risperdal
1. Malpractice. In: Garner BA, ed. Black’s Law Dictionary. 11th ed. Thomson West; 2019:1148.
2. Frierson RL, Joshi KG. Malpractice law and psychiatry: an overview. Focus. 2019;17:332-336. doi:10.1176/appi.focus.20190017
3. Negligence Based Claims. In: Boumil MM, Hattis PA, eds. Medical Liability in a Nutshell. 4th ed. West Academic Publishing; 2017:43-88
4. Peters PG. The quiet demise of deference to custom: malpractice law at the millennium. Washington and Lee Law Review. 2000;57(1):163-205. Accessed July 8, 2022. https://scholarlycommons.law.wlu.edu/wlulr/vol57/iss1/5
5. Simon RI. Standard-of-care testimony: best practices or reasonable care? J Am Acad Psychiatry Law. 2005;33(1):8-11. Accessed July 8, 2022. http://jaapl.org/content/33/1/8
6. Behrens SA. Call in Houdini: the time has come to be released from the geographic straightjacket known as the locality rule. Drake Law Review. 2008; 56(3):753-790. Accessed June 20, 2022. https://lawreviewdrake.files.wordpress.com/2015/06/lrvol56-3_behrens.pdf
1. Malpractice. In: Garner BA, ed. Black’s Law Dictionary. 11th ed. Thomson West; 2019:1148.
2. Frierson RL, Joshi KG. Malpractice law and psychiatry: an overview. Focus. 2019;17:332-336. doi:10.1176/appi.focus.20190017
3. Negligence Based Claims. In: Boumil MM, Hattis PA, eds. Medical Liability in a Nutshell. 4th ed. West Academic Publishing; 2017:43-88
4. Peters PG. The quiet demise of deference to custom: malpractice law at the millennium. Washington and Lee Law Review. 2000;57(1):163-205. Accessed July 8, 2022. https://scholarlycommons.law.wlu.edu/wlulr/vol57/iss1/5
5. Simon RI. Standard-of-care testimony: best practices or reasonable care? J Am Acad Psychiatry Law. 2005;33(1):8-11. Accessed July 8, 2022. http://jaapl.org/content/33/1/8
6. Behrens SA. Call in Houdini: the time has come to be released from the geographic straightjacket known as the locality rule. Drake Law Review. 2008; 56(3):753-790. Accessed June 20, 2022. https://lawreviewdrake.files.wordpress.com/2015/06/lrvol56-3_behrens.pdf
Risk factors for nonsuicidal self-injury: A review of the evidence
Nonsuicidal self-injury (NSSI) is the direct and deliberate destruction of body tissue without intent to die.1 Common forms of NSSI include cutting, burning, scraping/scratching skin, biting, hitting, and interfering with wound healing.2 Functional theories suggest that NSSI temporarily alleviates overwhelming negative emotions and can produce feelings of relief, resulting in a reinforcing effect.3
NSSI has been shown to be a risk factor for future suicide attempts.4 A 2018 study found that NSSI is associated with an increased risk of subsequent suicidal ideation (odds ratio [OR] 2.8), suicide plan (OR 3.0), and suicide attempt (OR 5.5).5 NSSI is also associated with individuals who had suicidal ideation and formed a suicide plan, and individuals who had a suicide plan and attempted suicide (ORs 1.7 to 2.1).5 Another study found that 70% of adolescents who engage in NSSI have attempted suicide during their lifetime, and 55% have multiple attempts.6
Given the overlap between suicide attempts and NSSI, performing a thorough suicide risk assessment (which is beyond the scope of this article) is crucial. This article describes the static and dynamic risk factors for NSSI in adolescents and adults, which can help us perform a suicide risk assessment and allow us to formulate an appropriate treatment plan that includes safety-based interventions.
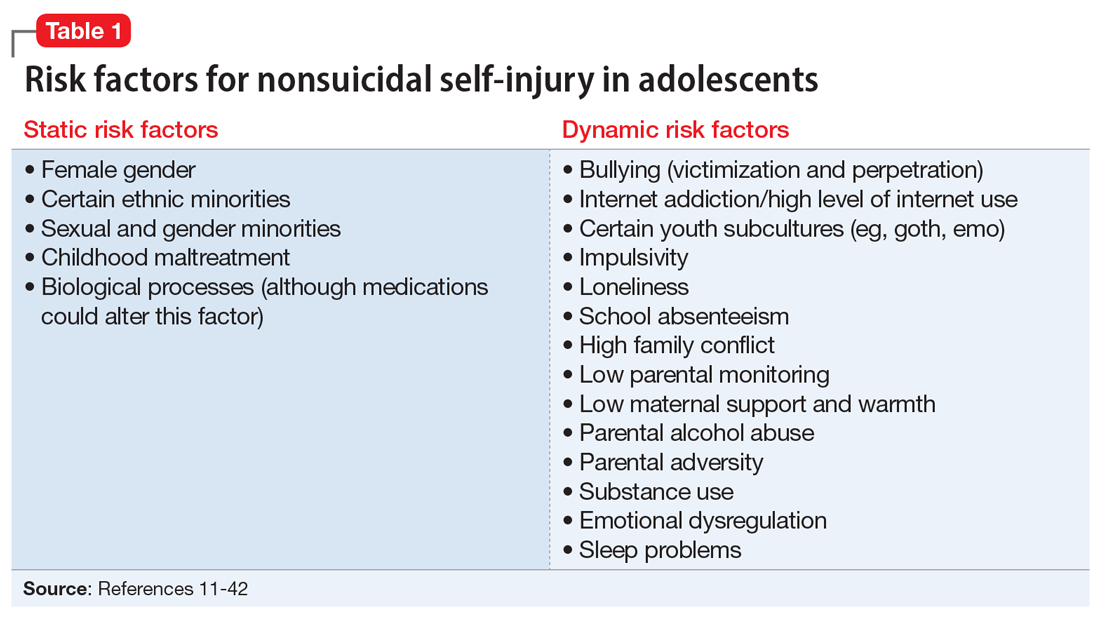
NSSI risk factors for adolescents
From developing sexual identity and undergoing puberty to achieving increased independence from their parents and developing a sense of autonomy, adolescents undergo many biological, psychological, and social changes before reaching adulthood.7 Data suggest that NSSI often begins in adolescence, with a typical onset at age 13 or 14.3 Community studies show that one-third to one-half of adolescents in the United States have engaged in NSSI.8,9 Previously, NSSI during adolescence was associated with 3 major diagnostic categories: eating disorders, developmental disabilities, and borderline personality disorder (BPD).10 However, recent data suggest that NSSI is also common outside of these categories. Here we describe static and dynamic risk factors for NSSI in adolescents (Table 111-42). Table 211-42 summarizes the studies of NSSI in adolescents that we reviewed.
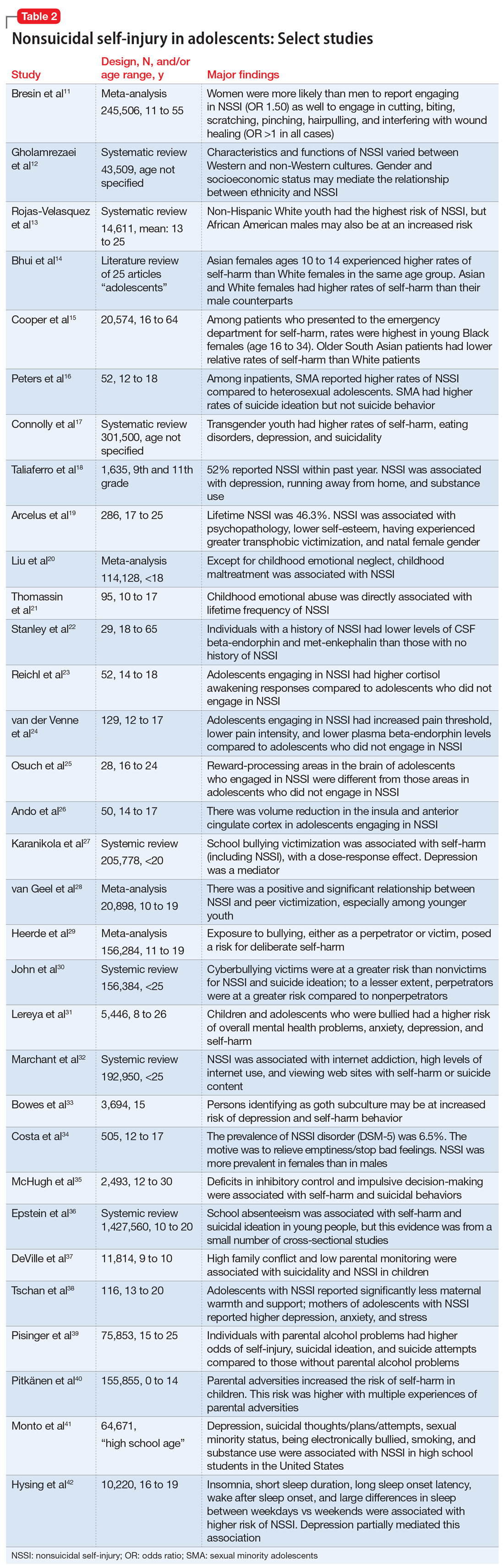
Static risk factors
Female adolescents and adults engage in NSSI at higher rates than males. The difference is larger in clinical populations compared to the general population.11
A large portion of research about NSSI has been conducted in studies in which the majority of participants were White.12 Most studies report a higher prevalence of NSSI among non-Hispanic White youth,13 but some suggest other ethnic groups may also experience high rates of self-harm and NSSI.13-15 Several studies have demonstrated high rates of self-harm among South Asian adult females compared with White adult females, but this difference may be less pronounced in adolescents.14 One study in the United Kingdom found that White females age 10 to 14 had higher rates of self-harm compared to South Asian females,14 while another found that risk and rates of self-harm in young South Asian people varied by city and country of origin.15 Young Black females15 and young Black males13 also may be at an increased risk of self-harm. One review found that Black females were more likely to self-harm than Asian or White groups.15
Several studies suggest that sexual minority adolescents (SMA) (eg, lesbian, gay, bisexual, transgender, queer) are at greater risk for NSSI than heterosexual adolescents.16 SMA have been shown to engage in a significantly greater frequency of NSSI and more types of NSSI than heterosexual adolescents.16 Furthermore, on the Inventory of Statements about Self-Injury, SMA self-reported using NSSI for intrapersonal functions (eg, for affect regulation, antisuicide, self-punishment) significantly greater than their heterosexual peers; however, there were no significant differences between the 2 groups on interpersonal functions (eg, autonomy, interpersonal boundaries, peer bonding, sensation-seeking).16
Continue to: Transgender and gender nonconfirming...
Transgender and gender nonconfirming (GNC) youth are at a particularly high risk for NSSI; 30% to 45.5% of transgender adolescents report self-injury.17 Factors shown to distinguish transgender/GNC youth who engage in NSSI from those who do not include having a mental health problem, depression, running away from home, substance use, lower self-esteem/greater self-criticism, experiencing transphobia victimization, and having more interpersonal problems.18,19 Among transgender/GNC youth, those whose biological sex is female are more likely to report NSSI than those whose biological sex is male (ie, transgendered adolescent males are more likely to report NSSI than transgendered adolescent females).18,19
Most forms of childhood maltreatment have been associated with NSSI. In a recently published review, Liu et al20 found that childhood maltreatment (including sexual abuse, physical abuse, emotional abuse, and physical neglect) was associated with an increased risk for NSSI. However, conflicting evidence suggests that when confounders are removed, only childhood emotional abuse was directly associated with NSSI.21 Current evidence is modest for childhood emotional neglect as a risk factor for NSSI.20
Increasing research is investigating the biological processes that may be implicated in NSSI. Some studies suggest that endogenous opioids,22 monoamine neurotransmitters,22 and the hypothalamic-pituitary-adrenal (HPA) axis23 may play a role in NSSI. Compared to healthy controls, adolescents engaging in NSSI have been shown to have lower pain intensity (P = .036), higher pain thresholds (P = .040), and lower beta-endorphins (endogenous opioid hormones involved in mediating stress and pain) (P = .002).24 There may be alterations in the HPA axis among adolescents who engage in NSSI, more specifically stronger cortisol awakening responses.23 Both functional and standard MRI have been used to study the neurobiology of NSSI. One study demonstrated differences in functional connectivity between brain areas linked to neuroregulation of emotions in adolescents who engage in NSSI,25 while another found volume reduction in the insula of these adolescents, which suggests a possible neurobiological reason for impulsivity and the increased risk of suicidal behavior.26
Dynamic risk factors
Research has repeatedly shown bullying is a risk factor for NSSI.27 One study found that younger children who were victimized reported significantly more NSSI than older children.28 New data suggest that perpetrators of bullying are also at risk for deliberate self-harm behavior (SHB), which this study defined as a behavior that is intended to cause self-harm but without suicidal intent and having a nonfatal outcome.29 Victims of cyberbullying also are at a greater risk for self-harm, suicidal behaviors, and suicide attempt.30 To a lesser extent, cyberbullying perpetrators are at greater risk for suicidal behaviors and suicidal ideation.30 Bullying is a risk factor for NSSI not only in adolescence, but also in adulthood. Lereya et al31 found that victims of bullying in childhood and early adolescence were more likely to have mental health problems (including anxiety and depression) and more likely to engage in SHB—which this study defined as hurting oneself on purpose in any way—as adults.
The effects of internet use on adolescents’ mental health also has been investigated. A recent review that explored the relationship between all types of internet use (general use, internet addiction, social media, self-harm websites, forums, etc) and SHB/suicidal behavior found that young people with internet addiction, high levels of internet use, and a tendency to view websites with self-harm or suicidal content were at higher risk of engaging in SHB/suicidal behavior.32 This study did not use a specific definition for SHB or suicidal behavior.32
Continue to: Membership in certain youth...
Membership in certain youth subcultures (eg, emo or goth) has been evaluated as potential risk factors for depression and deliberate self-harm. Bowes et al33 found that for each unit increase in goth affiliation (not at all, not very much, somewhat, more than somewhat, very much), youth were 1.52 times more likely to engage in SHB; these researchers also reported a dose-response association between goth identification and future SHB. This study asked participants if they have ever tried to harm or hurt themselves in any manner, but did not distinguish between individuals who had harmed themselves with and without suicidal intent.33
Personality traits such as impulsiveness and loneliness have been linked to NSSI among adolescents.34,35 A recent study found that adolescents who met the proposed DSM-5 diagnostic criteria for NSSI scored higher on the Barratt Impulsiveness Scale, specifically in measures of:
- motor impulsiveness (ie, acting without thinking)
- attentional impulsiveness (ie, making decisions quickly)
- impulsiveness due to lack of planning (ie, failure to plan for the future).34
This study also found that adolescents who identified as being lonely based on scores on the Brazilian Loneliness Scale were at a higher risk for NSSI.34
A recent systematic review (32 studies) and meta-analysis (9 studies) found that school absenteeism was associated with a risk of self-harm (pooled aOR 1.37, P = .01) and suicidal ideation (pooled aOR 1.20, P = .03).36 This study suggested that school absenteeism, an important marker of social exclusion, was associated with both SHB and suicidal ideation in young people.36 It defined SHB as any act of self-injury or self-poisoning, regardless of intent.36
Finally, family-related factors have been associated with an increased risk of NSSI. One study of 11,814 children age 9 and 10 revealed that high family conflict (OR 1.09; 95% CI, 1.05 to 1.14) and low parental monitoring (OR 0.95; 95% CI, 0.93 to 0.98) were associated with NSSI.37 A smaller, community-based study found that adolescents with NSSI reported significantly less maternal support and warmth than nonclinical controls, but a cause-and-effect relationship has not yet been determined.38 Parental history alone may influence adolescents’ risk of NSSI. A study that included nearly 76,000 youth found that adolescents with perceived parental alcohol problems had higher odds of self-injury, suicidal ideation, and suicide attempts.39 Adolescents exposed to maternal or paternal adversities were also at a higher risk of self-harm (hazard ratio 1.5 to 5.4 among males, 1.7 to 3.9 among females).40
Continue to: NSSI risk factors for adults
NSSI risk factors for adults
Although data regarding the prevalence of NSSI in adults are lacking, available studies report a 12-month prevalence of 0.9%2 and a lifetime prevalence of 5.5% to 5.9%.43 There is a significant overlap in risk factors for NSSI in adolescent and adult populations, but there are also many important differences. The static and dynamic risk factors for NSSI in adults are described in Table 3.44-66 Table 444-66 summarizes the studies of NSSI in adults that we reviewed.
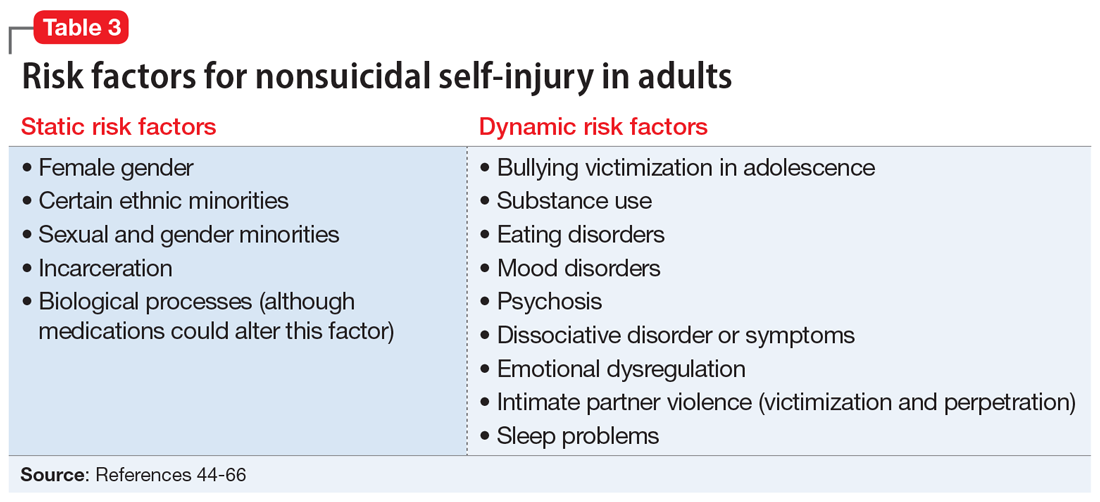
Static risk factors
Research findings regarding the prevalence of NSSI based on gender are varied. For years, it has been believed that women are more likely to engage in NSSI than men. Recent meta-analyses that have examined this relationship closely found that the gender difference is larger for clinical samples compared to community samples and more pronounced in younger individuals.11
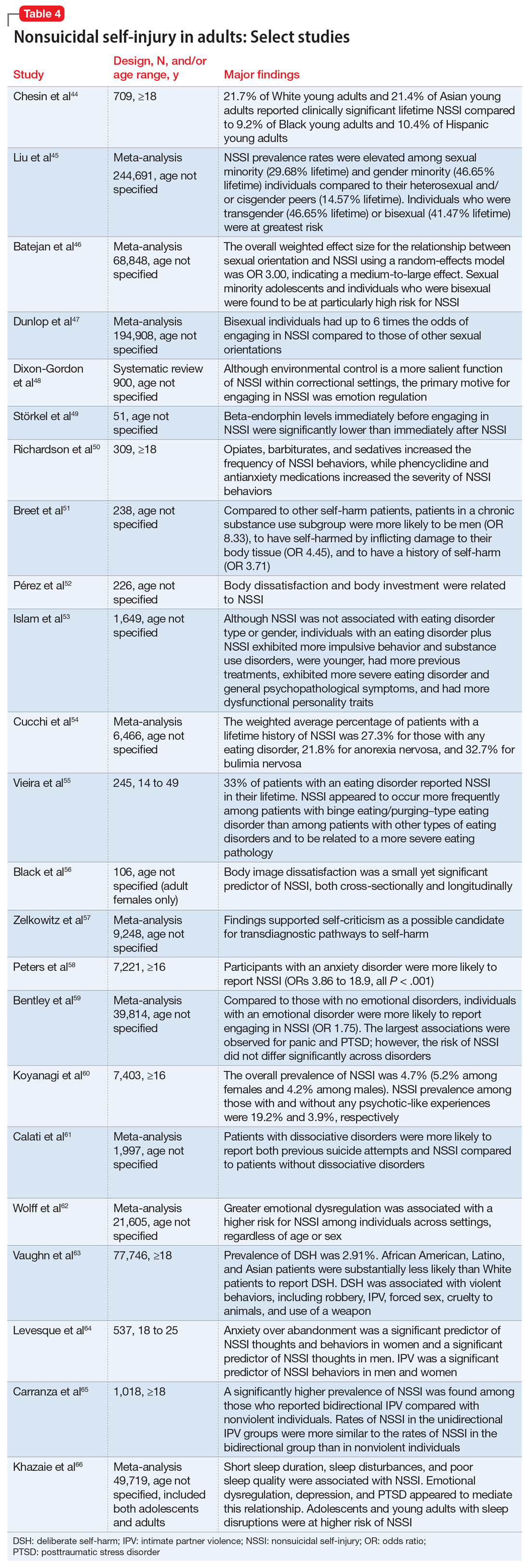
As is the case with adolescents, there may be ethnic variations in rates of self-harm and NSSI among adults. A 2013 study by Chesin et al44 found that Asian and White young adults experience higher rates of NSSI than their Hispanic and Black counterparts. Evidence suggests that relative rates of self-harm for older South Asian adults are lower than in older White adults.15
Compared to heterosexual or cisgender individuals, members of sexual and gender minorities have a higher past-year and lifetime prevalence of NSSI.45 One study found that the weighted effect size between sexual orientation and NSSI had an OR of 3 (95% CI, 2.46 to 3.66), indicating a medium-to-large effect.46 Bisexual and transgender individuals appear to be at the highest risk for NSSI when compared to members of other sexual and gender minority groups.45 One review that included mostly cross-sectional studies found that individuals identifying as bisexual had up to 6 times the odds of engaging in NSSI when compared to those of other sexual orientations.47
Incarceration is a risk factor for NSSI. The rates of NSSI in criminal justice settings are higher (up to 61%) than in the general adult population (approximately 4%).48 Recent research found that NSSI serves similar functions in correctional and non-correctional settings, primarily to regulate emotions.48 However, there is also evidence of higher rates of NSSI being motivated by an attempt to influence the environment (ie, engaging in NSSI in order to be transferred to another prison unit) compared to NSSI in community settings.48
Continue to: Though less robust than data...
Though less robust than data published regarding adolescents, the role of biological processes in adults engaging in NSSI has also been studied. A 2021 study by Störkel et al49 found that levels of salivary beta-endorphins were significantly lower in adults immediately before engaging in NSSI compared to after NSSI. Furthermore, adults who engage in NSSI have lower levels of met-enkephalin (P < .01), an opioid growth factor, compared to adults who have never engaged in NSSI.22
Dynamic risk factors
Individuals who engage in NSSI often report substance use, but there is little data on whether substance use is an independent risk factor for NSSI. Although limited, recent evidence suggests illicit substance use in both adolescents41 and adults50 increases risk for NSSI. Richardson et al50 found that the use of barbiturates, opiates, and sedatives significantly increased the frequency of NSSI, whereas use of marijuana, phencyclidine, and medications used to treat anxiety significantly increased the severity of NSSI. A smaller study conducted in South Africa found that individuals who engage in substance use and NSSI were more likely to be male (P < .001).51
Eating disorders and NSSI are highly comorbid.52 The lifetime prevalence of NSSI among individuals with eating disorders ranges from 20.6%to 37.1%.52,53 Results are inconsistent regarding which eating disorders (if any) are greater risk factors for NSSI. One study found that the prevalence of NSSI in patients with bulimia nervosa was 32.7% (95% CI, 26.9% to 39.1%) vs 21.8% in patients with anorexia nervosa (95% CI, 18.5% to 25.6%).54 Another study found that individuals with binge eating/purging–type eating disorders reported engaging in NSSI more frequently than those with other types of eating disorders.55 Among patients with eating disorders who reported NSSI, risk factors included younger age of onset, more negative self-evaluation, more impulsive behavior, concomitant substance use, history of suicide attempts, childhood abuse, and peer aggression.53,55 Body image dissatisfaction and self-criticism, even in individuals not formally diagnosed with an eating disorder, are small but significant predictors of NSSI.56,57
Mood disorders have also been linked to NSSI.58,59 Anxiety disorders (including generalized anxiety disorder, social phobia, panic disorder, and agoraphobia) as well as anxiety-related disorders such as obsessive-compulsive disorder have been significantly associated with NSSI (P < .001), but this relationship decreased in strength when mood instability was removed as a confounder.58 Among patients with anxiety and anxiety-related disorders, panic disorder and posttraumatic stress disorder (PTSD) have shown the strongest association with NSSI, with pooled aORs of 2.67 and 2.06, respectively.59
Recent studies have examined the association of other mental health disorders and symptoms with NSSI, including psychosis60 and dissociative symptoms.61 One study found that paranoia, thought control, and auditory hallucinations were significantly associated with NSSI60; however, after controlling for concomitant BPD, only paranoia was significantly associated with NSSI.60 Individuals diagnosed with dissociative disorders were more likely than patients without such disorders to endorse NSSI and suicide attempts.61
Continue to: Emotional dysregulation...
Emotional dysregulation (EDR)—defined as difficulty understanding, recognizing, and managing one’s emotions—has been researched extensively in relation to NSSI.62 A recent review that included studies of both adolescents and adults reported a significant association between EDR and NSSI, with an OR of 2.40 (95% CI, 2.01 to 2.86).62 A larger effect size was observed between EDR and lifetime NSSI (OR 3.21; 95% CI, 2.63 to 3.91) compared to past-year NSSI (OR 2.32; 95% CI, 1.84 to 2.92).62 Patient age, sex, and sample type (clinical vs community) were not significant moderators of strength between the reported associations.62
Studies examining intimate partner violence (IPV) and NSSI have found that young adults who engage in IPV (both as victims and as perpetrators) are more likely to report NSSI.63-65 Researchers have proposed that anxiety over abandonment may explain this relationship.64 A recent study found that individuals with bidirectional IPV (ie, both victimization and perpetration) engaged in NSSI at a higher prevalence than those engaging in unidirectional IPV or no IPV.65 This suggests that relationship violence in general (rather than just being a victim of IPV) may be a risk factor for NSSI.65
Finally, studies suggest that adolescents and adults who have sleep problems (insomnia, short sleep duration, long sleep onset latency, waking after sleep onset, and poor quality sleep) are more likely to report self-harm or NSSI than those without sleep problems.42,66 In adults, this relationship is partially mediated by depressive symptoms, EDR, and PTSD.66 In adolescents, depressive symptoms are a mediator for this relationship.42
Bottom Line
Nonsuicidal self-injury (NSSI) is a significant health concern due to its association with suicide attempts. Although there are similarities in NSSI risk factors between adolescents and adults, there are also important differences. Understanding these differences is necessary to develop appropriate treatment plans.
Related Resources
- American Foundation for Suicide Prevention. https://afsp.org/
- Cipriano A, Cella S, Cotrufo P. Nonsuicidal self-injury: a systematic review. Front Psych. 2017;8:1946. doi:10.3389/ fpsyg.2017.01946
- Gold LH, Frierson RL, eds. Textbook of Suicide Risk Assessment and Management. 3rd ed. American Psychiatric Association Publishing; 2020.
1. Nock MK. Self-injury. Annu Rev Clin Psychol. 2010;6:339-363.
2. Klonsky ED. Non-suicidal self-injury in United States adults: prevalence, sociodemographics, topography and functions. Psychol Med. 2011;41(9):1981-1986.
3. Klonsky ED. Nonsuicidal self-injury: what we know, and what we need to know. Can J Psychiatry. 2014;59(11):565-568.
4. Wilkinson P, Kelvin R, Roberts C, et al. Clinical and psychosocial predictors of suicide attempts and nonsuicidal self-injury in the Adolescent Depression Antidepressants and Psychotherapy Trial (ADAPT). Am J Psychiatry. 2011;168(5):495-501.
5. Kiekens G, Hasking P, Boyes M, et al. The associations between non-suicidal self-injury and first onset suicidal thoughts and behaviors. J Affect Disord. 2018;239:171-179.
6. Nock MK, Joiner TE, Gordon KH, et al. Non-suicidal self-injury among adolescents: diagnostic correlates and relation to suicide attempts. Psychiatry Res. 2006;144(1):65-72.
7. Christie D, Viner R. Adolescent development. BMJ. 2005;330(7486):301-304.
8. Yates TM, Tracy AJ, Luthar SS. Nonsuicidal self-injury among “privileged” youths: longitudinal and cross-sectional approaches to developmental process. J Consult Clin Psychol. 2008;76(1):52-62.
9. Lloyd-Richardson EE, Perrine N, Dierker L, et al. Characteristics and functions of non-suicidal self-injury in a community sample of adolescents. Psychol Med. 2007;37(8):1183-1192.
10. Peterson J, Freedenthal S, Sheldon C, et al. Nonsuicidal self injury in adolescents. Psychiatry(Edgmont). 2008;5(11):20-26.
11. Bresin K, Schoenleber M. Gender differences in the prevalence of nonsuicidal self-injury: a meta-analysis. Clin Psychol Rev. 2015;38:55-64.
12. Gholamrezaei M, Stefano JD, Heath NL. Nonsuicidal self-injury across cultures and ethnic and racial minorities: a review. Int J Psychol. 2015;52(4):316-326.
13. Rojas-Velasquez DA, Pluhar EI, Burns PA, et al. Nonsuicidal self-injury among African American and Hispanic adolescents and young adults: a systematic review. Prev Sci. 2021;22:367-377.
14. Bhui K, McKenzie K, Rasul F. Rates, risk factors & methods of self harm among minority ethnic groups in the UK: a systematic review. BMC Public Health. 2007;7:336.
15. Cooper J, Murphy E, Webb R, et al. Ethnic differences in self-harm, rates, characteristics and service provision: three-city cohort study. Br J Psychiatry. 2010;197(3):212-218.
16. Peters JR, Mereish EH, Krek MA, et al. Sexual orientation differences in non-suicidal self-injury, suicidality, and psychosocial factors among an inpatient psychiatric sample of adolescents. Psychiatry Res. 2020;284:112664.
17. Connolly MD, Zervos MJ, Barone 2nd CJ, et al. The mental health of transgender youth: advances in understanding. J Adolesc Health. 2016;59(5):489-495.
18. Taliaferro LA, McMorris BJ, Rider GN, et al. Risk and protective factors for self-harm in a population-based sample of transgender youth. Archives Suicide Res. 2019;23(2):203-221.
19. Arcelus J, Claes L, Witcomb GL, et al. Risk factors for non-suicidal self-injury among trans youth. J Sex Med. 2016;13(3):402-412.
20. Liu RT, Scopelliti KM, Pittman SK, et al. Childhood maltreatment and non-suicidal self-injury: a systematic review and meta-analysis. Lancet Psychiatry. 2018;5(1):51-64.
21. Thomassin K, Shaffer A, Madden A, et al. Specificity of childhood maltreatment and emotion deficit in nonsuicidal self-injury in an inpatient sample of youth. Psychiatry Res. 2016;244:103-108.
22. Stanley B, Sher L, Wilson S, et al. Non-suicidal self-injurious behavior, endogenous opioids and monoamine neurotransmitters. J Affect Disord. 2010;124(1-2):134-140.
23. Reichl C, Heyer A, Brunner R, et al. Hypothalamic-pituitary-adrenal axis, childhood adversity and adolescent nonsuicidal self-injury. Psychoneuroendocrinology. 2016;74:203-211.
24. van der Venne P, Balint A, Drews E, et al. Pain sensitivity and plasma beta-endorphin in adolescent non-suicidal self-injury. J Affect Disord. 2021;278:199-209.
25. Osuch E, Ford K, Wrath A, et al. Functional MRI of pain application in youth who engaged in repetitive non-suicidal self-injury vs. psychiatric controls. Psychiatry Res. 2014;223(2):104-112.
26. Ando A, Reichl C, Scheu F, et al. Regional grey matter volume reduction in adolescents engaging in non-suicidal self-injury. Psychiatry Res Neuroimaging. 2018;280:48-55.
27. Karanikola MNK, Lyberg A, Holm A-L, et al. The association between deliberate self-harm and school bullying victimization and the mediating effect of depressive symptoms and self-stigma: a systematic review. BioMed Res Int. 2018;4745791. doi: 10.1155/2018/4745791
28. van Geel M, Goemans A, Vedder P. A meta-analysis on the relation between peer victimization and adolescent non-suicidal self-injury. Psychiatry Res. 2015;230(2):364-368.
29. Heerde JA, Hemphill SA. Are bullying perpetration and victimization associated with adolescent deliberate self-harm? A meta-analysis. Arch Suicide Res. 2019;23(3):353-381.
30. John A, Glendenning AC, Marchant A, et al. Self-harm, suicidal behaviours, and cyberbullying in children and young people: systematic review. J Med Internet Res. 2018;20(4):e129. doi: 10.2196/jmir.9044
31. Lereya ST, Copeland WE, Costello EJ, et al. Adult mental health consequences of peer bullying and maltreatment in childhood: two cohorts in two countries. Lancet Psychiatry. 2015;2(6):524-531.
32. Marchant A, Hawton K, Stewart A, et al. A systematic review of the relationship between internet use, self-harm and suicidal behaviour in young people: the good, the bad and the unknown. PLoS One. 2017;12(8):e0181722. doi: 10.1371/journal.pone.0181722
33. Bowes L, Carnegie R, Pearson R, et al. Risk of depression and self-harm in teenagers identifying with goth subculture: a longitudinal cohort study. Lancet Psychiatry. 2015;2(9):793-800.
34. Costa RPO, Peixoto ALRP, Lucas CCA, et al. Profile of non-suicidal self-injury in adolescents: interface with impulsiveness and loneliness. J Pediatr (Rio J). 2021;97(2):184-190.
35. McHugh CM, Lee RSC, Hermens DF, et al. Impulsivity in the self-harm and suicidal behavior of young people: a systematic review and meta-analysis. J Psychiatr Res. 2019;116:51-60.
36. Epstein S, Roberts E, Sedgwick R, et al. School absenteeism as a risk factor for self-harm and suicidal ideation in children and adolescents: a systematic review and meta-analysis. Eur Child Adolesc Psychiatry. 2020;29(9):1175-1194.
37. DeVille DC, Whalen D, Breslin FJ, et al. Prevalence and family-related factors associated with suicidal ideation, suicide attempts, and self-injury in children aged 9 to 10 years. JAMA Netw Open. 2020;3(2):e1920956. doi: 10.1001/jamanetworkopen.2019.20956
38. Tschan T, Schmid M, In-Albon T. Parenting behavior in families of female adolescents with nonsuicidal self-injury in comparison to a clinical and a nonclinical control group. Child Adolesc Psychiatry Ment Health. 2015;9:17.
39. Pisinger V, Hawton K, Tolstrup JS. Self-injury and suicide behavior among young people with perceived parental alcohol problems in Denmark: a school-based survey. Eur Child Adolesc Psychiatry. 2018;27(2):201-208.
40. Pitkänen J, Remes H, Aaltonen M, et al. Experience of maternal and paternal adversities in childhood as determinants of self-harm in adolescence and young adulthood. J Epidemiol Community Health. 2019;73(11):1040-1046.
41. Monto MA, McRee N, Deryck FS. Nonsuicidal self-injury among a representative sample of US adolescents, 2015. Am J Public Health. 2018;108(8):1042-1048.
42. Hysing M, Sivertsen B, Stormark KM, et al. Sleep problems and self-harm in adolescence. Br J Psychiatry. 2015;207(4):306-312.
43. Swannell SV, Martin GE, Page A, et al. Prevalence of nonsuicidal self-injury in nonclinical samples: systematic review, meta-analysis and meta-regression. Suicide Life Threat Behav. 2014;44(3):273-303.
44. Chesin M, Moster A, Jeglic E. Non-suicidal self-injury among ethnically and racially diverse emerging adults: do factors unique to the minority experience matter? Current Psychology. 2013;32:318-328.
45. Liu RT, Sheehan AE, Walsh RFL, et al. Prevalence and correlates of non-suicidal self-injury among lesbian, gay, bisexual, and transgender individuals: a systematic review and meta-analysis. Clin Psychol Rev. 2019;74:101-783. doi:10.1016/j.cpr.2019.101783
46. Batejan KL, Jarvi SM, Swenson LP. Sexual orientation and non-suicidal self-injury: a meta-analytic review. Arch Suicide Res. 2015;19(2):131-150.
47. Dunlop BJ, Hartley S, Oladokun O, et al. Bisexuality and non-suicidal self-injury (NSSI): a narrative synthesis of associated variables and a meta-analysis of risk. J Affect Disord. 2020;276:1159-1172.
48. Dixon-Gordon K, Harrison N, Roesch R. Non-suicidal self-injury within offender populations: a systematic review. Int J Forensic Ment Health. 2012;11(1):33-50.
49. Störkel LM, Karabatsiakis A, Hepp K, et al. Salivary beta-endorphin in nonsuicidal self-injury: an ambulatory assessment study. Neuropsychopharmacology. 2021;46(7):1357-1363.
50. Richardson E, DePue MK, Therriault DJ, et al. The influence of substance use on engagement in non-suicidal self-injury (NSI) in adults. Subst Use Misuse. 2020;55(1):89-94.
51. Breet E, Bantjes J, Lewis I. Chronic substance use and self-harm in a primary health care setting. Afr J Prim Health Care Fam Med. 2018;10(1):e1-e9. doi: 10.4102/phcfm.v10i1.1544
52. Pérez S, Marco JH, Cañabate M. Non-suicidal self-injury in patients with eating disorders: prevalence, forms, functions, and body image correlates. Compr Psychiatry. 2018;84:32-38.
53. Islam MA, Steiger H, Jimenez-Murcia S, et al. Non-suicidal self-injury in different eating disorder types: relevance of personality traits and gender. Eur Eat Disord Rev. 2015;23(6):553-560.
54. Cucchi A, Ryan D, Konstantakopoulos G, et al. Lifetime prevalence of non-suicidal self-injury in patients with eating disorders: a systematic review and meta-analysis. Psychol Med. 2016;46(7):1345-1358.
55. Vieira AI, Machado BC, Machado PPP, et al. Putative risk factors for non-suicidal self-injury in eating disorders. Eur Eat Disord Rev. 2017;25(6):544-550.
56. Black EB, Garratt M, Beccaria G, et al. Body image as a predictor of nonsuicidal self-injury in women: a longitudinal study. Compr Psychiatry. 2019;88:83-89.
57. Zelkowitz RL, Cole DA. Self-criticism as a transdiagnostic process in nonsuicidal self-injury and disordered eating: systematic review and meta-analysis. Suicide Life Threat Behav. 2019;49(1):310-327.
58. Peters EM, Bowen R, Balbuena L. Mood instability contributes to impulsivity, non-suicidal self-injury, and binge eating/purging in people with anxiety disorders. Psychol Psychother. 2019;92(3):422-438.
59. Bentley KH, Cassiello-Robbins CF, Vittorio L, et al. The association between nonsuicidal self-injury and the emotional disorders: a meta-analytic review. Clin Psychol Rev. 2015;37:72-88.
60. Koyanagi A, Stickley A, Haro JM. Psychotic-like experiences and nonsuicidal self-injury in England: results from a national survey [corrected]. PLoS One. 2015;10(12):e0145533. doi: 10.1371/journal.pone.0145533
61. Calati R, Bensassi I, Courtet P. The link between dissociation and both suicide attempts and non-suicidal self-injury: meta-analyses. Psychiatry Res. 2017;251:103-114.
62. Wolff JC, Thompson E, Thomas SA, et al. Emotion dysregulation and non-suicidal self-injury: a systematic review and meta-analysis. Eur Psychiatry. 2019;59:25-36.
63. Vaughn MG, Salas-Wright CP, DeLisi M, et al. Deliberate self-harm and the nexus of violence, victimization, and mental health problems in the United States. Psychiatry Res. 2015;225(3):588-595.
64. Levesque C, Lafontaine M-F, Bureau J-F, et al. The influence of romantic attachment and intimate partner violence on nonsuicidal self-injury in young adults. J Youth Adolesc. 2010;39(5):474-483.
65. Carranza AB, Wallis CRD, Jonnson MR, et al. Nonsuicidal self-injury and intimate partner violence: directionality of violence and motives for self-injury. J Interpers Violence. 2020;886260520922372. doi: 10.1177/0886260520922372
66. Khazaie H, Zakiei A, McCall WV, et al. Relationship between sleep problems and self-injury: a systematic review. Behav Sleep Med. 2020;1-16. doi: 10.1080/15402002.2020.1822360
Nonsuicidal self-injury (NSSI) is the direct and deliberate destruction of body tissue without intent to die.1 Common forms of NSSI include cutting, burning, scraping/scratching skin, biting, hitting, and interfering with wound healing.2 Functional theories suggest that NSSI temporarily alleviates overwhelming negative emotions and can produce feelings of relief, resulting in a reinforcing effect.3
NSSI has been shown to be a risk factor for future suicide attempts.4 A 2018 study found that NSSI is associated with an increased risk of subsequent suicidal ideation (odds ratio [OR] 2.8), suicide plan (OR 3.0), and suicide attempt (OR 5.5).5 NSSI is also associated with individuals who had suicidal ideation and formed a suicide plan, and individuals who had a suicide plan and attempted suicide (ORs 1.7 to 2.1).5 Another study found that 70% of adolescents who engage in NSSI have attempted suicide during their lifetime, and 55% have multiple attempts.6
Given the overlap between suicide attempts and NSSI, performing a thorough suicide risk assessment (which is beyond the scope of this article) is crucial. This article describes the static and dynamic risk factors for NSSI in adolescents and adults, which can help us perform a suicide risk assessment and allow us to formulate an appropriate treatment plan that includes safety-based interventions.

NSSI risk factors for adolescents
From developing sexual identity and undergoing puberty to achieving increased independence from their parents and developing a sense of autonomy, adolescents undergo many biological, psychological, and social changes before reaching adulthood.7 Data suggest that NSSI often begins in adolescence, with a typical onset at age 13 or 14.3 Community studies show that one-third to one-half of adolescents in the United States have engaged in NSSI.8,9 Previously, NSSI during adolescence was associated with 3 major diagnostic categories: eating disorders, developmental disabilities, and borderline personality disorder (BPD).10 However, recent data suggest that NSSI is also common outside of these categories. Here we describe static and dynamic risk factors for NSSI in adolescents (Table 111-42). Table 211-42 summarizes the studies of NSSI in adolescents that we reviewed.

Static risk factors
Female adolescents and adults engage in NSSI at higher rates than males. The difference is larger in clinical populations compared to the general population.11
A large portion of research about NSSI has been conducted in studies in which the majority of participants were White.12 Most studies report a higher prevalence of NSSI among non-Hispanic White youth,13 but some suggest other ethnic groups may also experience high rates of self-harm and NSSI.13-15 Several studies have demonstrated high rates of self-harm among South Asian adult females compared with White adult females, but this difference may be less pronounced in adolescents.14 One study in the United Kingdom found that White females age 10 to 14 had higher rates of self-harm compared to South Asian females,14 while another found that risk and rates of self-harm in young South Asian people varied by city and country of origin.15 Young Black females15 and young Black males13 also may be at an increased risk of self-harm. One review found that Black females were more likely to self-harm than Asian or White groups.15
Several studies suggest that sexual minority adolescents (SMA) (eg, lesbian, gay, bisexual, transgender, queer) are at greater risk for NSSI than heterosexual adolescents.16 SMA have been shown to engage in a significantly greater frequency of NSSI and more types of NSSI than heterosexual adolescents.16 Furthermore, on the Inventory of Statements about Self-Injury, SMA self-reported using NSSI for intrapersonal functions (eg, for affect regulation, antisuicide, self-punishment) significantly greater than their heterosexual peers; however, there were no significant differences between the 2 groups on interpersonal functions (eg, autonomy, interpersonal boundaries, peer bonding, sensation-seeking).16
Continue to: Transgender and gender nonconfirming...
Transgender and gender nonconfirming (GNC) youth are at a particularly high risk for NSSI; 30% to 45.5% of transgender adolescents report self-injury.17 Factors shown to distinguish transgender/GNC youth who engage in NSSI from those who do not include having a mental health problem, depression, running away from home, substance use, lower self-esteem/greater self-criticism, experiencing transphobia victimization, and having more interpersonal problems.18,19 Among transgender/GNC youth, those whose biological sex is female are more likely to report NSSI than those whose biological sex is male (ie, transgendered adolescent males are more likely to report NSSI than transgendered adolescent females).18,19
Most forms of childhood maltreatment have been associated with NSSI. In a recently published review, Liu et al20 found that childhood maltreatment (including sexual abuse, physical abuse, emotional abuse, and physical neglect) was associated with an increased risk for NSSI. However, conflicting evidence suggests that when confounders are removed, only childhood emotional abuse was directly associated with NSSI.21 Current evidence is modest for childhood emotional neglect as a risk factor for NSSI.20
Increasing research is investigating the biological processes that may be implicated in NSSI. Some studies suggest that endogenous opioids,22 monoamine neurotransmitters,22 and the hypothalamic-pituitary-adrenal (HPA) axis23 may play a role in NSSI. Compared to healthy controls, adolescents engaging in NSSI have been shown to have lower pain intensity (P = .036), higher pain thresholds (P = .040), and lower beta-endorphins (endogenous opioid hormones involved in mediating stress and pain) (P = .002).24 There may be alterations in the HPA axis among adolescents who engage in NSSI, more specifically stronger cortisol awakening responses.23 Both functional and standard MRI have been used to study the neurobiology of NSSI. One study demonstrated differences in functional connectivity between brain areas linked to neuroregulation of emotions in adolescents who engage in NSSI,25 while another found volume reduction in the insula of these adolescents, which suggests a possible neurobiological reason for impulsivity and the increased risk of suicidal behavior.26
Dynamic risk factors
Research has repeatedly shown bullying is a risk factor for NSSI.27 One study found that younger children who were victimized reported significantly more NSSI than older children.28 New data suggest that perpetrators of bullying are also at risk for deliberate self-harm behavior (SHB), which this study defined as a behavior that is intended to cause self-harm but without suicidal intent and having a nonfatal outcome.29 Victims of cyberbullying also are at a greater risk for self-harm, suicidal behaviors, and suicide attempt.30 To a lesser extent, cyberbullying perpetrators are at greater risk for suicidal behaviors and suicidal ideation.30 Bullying is a risk factor for NSSI not only in adolescence, but also in adulthood. Lereya et al31 found that victims of bullying in childhood and early adolescence were more likely to have mental health problems (including anxiety and depression) and more likely to engage in SHB—which this study defined as hurting oneself on purpose in any way—as adults.
The effects of internet use on adolescents’ mental health also has been investigated. A recent review that explored the relationship between all types of internet use (general use, internet addiction, social media, self-harm websites, forums, etc) and SHB/suicidal behavior found that young people with internet addiction, high levels of internet use, and a tendency to view websites with self-harm or suicidal content were at higher risk of engaging in SHB/suicidal behavior.32 This study did not use a specific definition for SHB or suicidal behavior.32
Continue to: Membership in certain youth...
Membership in certain youth subcultures (eg, emo or goth) has been evaluated as potential risk factors for depression and deliberate self-harm. Bowes et al33 found that for each unit increase in goth affiliation (not at all, not very much, somewhat, more than somewhat, very much), youth were 1.52 times more likely to engage in SHB; these researchers also reported a dose-response association between goth identification and future SHB. This study asked participants if they have ever tried to harm or hurt themselves in any manner, but did not distinguish between individuals who had harmed themselves with and without suicidal intent.33
Personality traits such as impulsiveness and loneliness have been linked to NSSI among adolescents.34,35 A recent study found that adolescents who met the proposed DSM-5 diagnostic criteria for NSSI scored higher on the Barratt Impulsiveness Scale, specifically in measures of:
- motor impulsiveness (ie, acting without thinking)
- attentional impulsiveness (ie, making decisions quickly)
- impulsiveness due to lack of planning (ie, failure to plan for the future).34
This study also found that adolescents who identified as being lonely based on scores on the Brazilian Loneliness Scale were at a higher risk for NSSI.34
A recent systematic review (32 studies) and meta-analysis (9 studies) found that school absenteeism was associated with a risk of self-harm (pooled aOR 1.37, P = .01) and suicidal ideation (pooled aOR 1.20, P = .03).36 This study suggested that school absenteeism, an important marker of social exclusion, was associated with both SHB and suicidal ideation in young people.36 It defined SHB as any act of self-injury or self-poisoning, regardless of intent.36
Finally, family-related factors have been associated with an increased risk of NSSI. One study of 11,814 children age 9 and 10 revealed that high family conflict (OR 1.09; 95% CI, 1.05 to 1.14) and low parental monitoring (OR 0.95; 95% CI, 0.93 to 0.98) were associated with NSSI.37 A smaller, community-based study found that adolescents with NSSI reported significantly less maternal support and warmth than nonclinical controls, but a cause-and-effect relationship has not yet been determined.38 Parental history alone may influence adolescents’ risk of NSSI. A study that included nearly 76,000 youth found that adolescents with perceived parental alcohol problems had higher odds of self-injury, suicidal ideation, and suicide attempts.39 Adolescents exposed to maternal or paternal adversities were also at a higher risk of self-harm (hazard ratio 1.5 to 5.4 among males, 1.7 to 3.9 among females).40
Continue to: NSSI risk factors for adults
NSSI risk factors for adults
Although data regarding the prevalence of NSSI in adults are lacking, available studies report a 12-month prevalence of 0.9%2 and a lifetime prevalence of 5.5% to 5.9%.43 There is a significant overlap in risk factors for NSSI in adolescent and adult populations, but there are also many important differences. The static and dynamic risk factors for NSSI in adults are described in Table 3.44-66 Table 444-66 summarizes the studies of NSSI in adults that we reviewed.

Static risk factors
Research findings regarding the prevalence of NSSI based on gender are varied. For years, it has been believed that women are more likely to engage in NSSI than men. Recent meta-analyses that have examined this relationship closely found that the gender difference is larger for clinical samples compared to community samples and more pronounced in younger individuals.11

As is the case with adolescents, there may be ethnic variations in rates of self-harm and NSSI among adults. A 2013 study by Chesin et al44 found that Asian and White young adults experience higher rates of NSSI than their Hispanic and Black counterparts. Evidence suggests that relative rates of self-harm for older South Asian adults are lower than in older White adults.15
Compared to heterosexual or cisgender individuals, members of sexual and gender minorities have a higher past-year and lifetime prevalence of NSSI.45 One study found that the weighted effect size between sexual orientation and NSSI had an OR of 3 (95% CI, 2.46 to 3.66), indicating a medium-to-large effect.46 Bisexual and transgender individuals appear to be at the highest risk for NSSI when compared to members of other sexual and gender minority groups.45 One review that included mostly cross-sectional studies found that individuals identifying as bisexual had up to 6 times the odds of engaging in NSSI when compared to those of other sexual orientations.47
Incarceration is a risk factor for NSSI. The rates of NSSI in criminal justice settings are higher (up to 61%) than in the general adult population (approximately 4%).48 Recent research found that NSSI serves similar functions in correctional and non-correctional settings, primarily to regulate emotions.48 However, there is also evidence of higher rates of NSSI being motivated by an attempt to influence the environment (ie, engaging in NSSI in order to be transferred to another prison unit) compared to NSSI in community settings.48
Continue to: Though less robust than data...
Though less robust than data published regarding adolescents, the role of biological processes in adults engaging in NSSI has also been studied. A 2021 study by Störkel et al49 found that levels of salivary beta-endorphins were significantly lower in adults immediately before engaging in NSSI compared to after NSSI. Furthermore, adults who engage in NSSI have lower levels of met-enkephalin (P < .01), an opioid growth factor, compared to adults who have never engaged in NSSI.22
Dynamic risk factors
Individuals who engage in NSSI often report substance use, but there is little data on whether substance use is an independent risk factor for NSSI. Although limited, recent evidence suggests illicit substance use in both adolescents41 and adults50 increases risk for NSSI. Richardson et al50 found that the use of barbiturates, opiates, and sedatives significantly increased the frequency of NSSI, whereas use of marijuana, phencyclidine, and medications used to treat anxiety significantly increased the severity of NSSI. A smaller study conducted in South Africa found that individuals who engage in substance use and NSSI were more likely to be male (P < .001).51
Eating disorders and NSSI are highly comorbid.52 The lifetime prevalence of NSSI among individuals with eating disorders ranges from 20.6%to 37.1%.52,53 Results are inconsistent regarding which eating disorders (if any) are greater risk factors for NSSI. One study found that the prevalence of NSSI in patients with bulimia nervosa was 32.7% (95% CI, 26.9% to 39.1%) vs 21.8% in patients with anorexia nervosa (95% CI, 18.5% to 25.6%).54 Another study found that individuals with binge eating/purging–type eating disorders reported engaging in NSSI more frequently than those with other types of eating disorders.55 Among patients with eating disorders who reported NSSI, risk factors included younger age of onset, more negative self-evaluation, more impulsive behavior, concomitant substance use, history of suicide attempts, childhood abuse, and peer aggression.53,55 Body image dissatisfaction and self-criticism, even in individuals not formally diagnosed with an eating disorder, are small but significant predictors of NSSI.56,57
Mood disorders have also been linked to NSSI.58,59 Anxiety disorders (including generalized anxiety disorder, social phobia, panic disorder, and agoraphobia) as well as anxiety-related disorders such as obsessive-compulsive disorder have been significantly associated with NSSI (P < .001), but this relationship decreased in strength when mood instability was removed as a confounder.58 Among patients with anxiety and anxiety-related disorders, panic disorder and posttraumatic stress disorder (PTSD) have shown the strongest association with NSSI, with pooled aORs of 2.67 and 2.06, respectively.59
Recent studies have examined the association of other mental health disorders and symptoms with NSSI, including psychosis60 and dissociative symptoms.61 One study found that paranoia, thought control, and auditory hallucinations were significantly associated with NSSI60; however, after controlling for concomitant BPD, only paranoia was significantly associated with NSSI.60 Individuals diagnosed with dissociative disorders were more likely than patients without such disorders to endorse NSSI and suicide attempts.61
Continue to: Emotional dysregulation...
Emotional dysregulation (EDR)—defined as difficulty understanding, recognizing, and managing one’s emotions—has been researched extensively in relation to NSSI.62 A recent review that included studies of both adolescents and adults reported a significant association between EDR and NSSI, with an OR of 2.40 (95% CI, 2.01 to 2.86).62 A larger effect size was observed between EDR and lifetime NSSI (OR 3.21; 95% CI, 2.63 to 3.91) compared to past-year NSSI (OR 2.32; 95% CI, 1.84 to 2.92).62 Patient age, sex, and sample type (clinical vs community) were not significant moderators of strength between the reported associations.62
Studies examining intimate partner violence (IPV) and NSSI have found that young adults who engage in IPV (both as victims and as perpetrators) are more likely to report NSSI.63-65 Researchers have proposed that anxiety over abandonment may explain this relationship.64 A recent study found that individuals with bidirectional IPV (ie, both victimization and perpetration) engaged in NSSI at a higher prevalence than those engaging in unidirectional IPV or no IPV.65 This suggests that relationship violence in general (rather than just being a victim of IPV) may be a risk factor for NSSI.65
Finally, studies suggest that adolescents and adults who have sleep problems (insomnia, short sleep duration, long sleep onset latency, waking after sleep onset, and poor quality sleep) are more likely to report self-harm or NSSI than those without sleep problems.42,66 In adults, this relationship is partially mediated by depressive symptoms, EDR, and PTSD.66 In adolescents, depressive symptoms are a mediator for this relationship.42
Bottom Line
Nonsuicidal self-injury (NSSI) is a significant health concern due to its association with suicide attempts. Although there are similarities in NSSI risk factors between adolescents and adults, there are also important differences. Understanding these differences is necessary to develop appropriate treatment plans.
Related Resources
- American Foundation for Suicide Prevention. https://afsp.org/
- Cipriano A, Cella S, Cotrufo P. Nonsuicidal self-injury: a systematic review. Front Psych. 2017;8:1946. doi:10.3389/ fpsyg.2017.01946
- Gold LH, Frierson RL, eds. Textbook of Suicide Risk Assessment and Management. 3rd ed. American Psychiatric Association Publishing; 2020.
Nonsuicidal self-injury (NSSI) is the direct and deliberate destruction of body tissue without intent to die.1 Common forms of NSSI include cutting, burning, scraping/scratching skin, biting, hitting, and interfering with wound healing.2 Functional theories suggest that NSSI temporarily alleviates overwhelming negative emotions and can produce feelings of relief, resulting in a reinforcing effect.3
NSSI has been shown to be a risk factor for future suicide attempts.4 A 2018 study found that NSSI is associated with an increased risk of subsequent suicidal ideation (odds ratio [OR] 2.8), suicide plan (OR 3.0), and suicide attempt (OR 5.5).5 NSSI is also associated with individuals who had suicidal ideation and formed a suicide plan, and individuals who had a suicide plan and attempted suicide (ORs 1.7 to 2.1).5 Another study found that 70% of adolescents who engage in NSSI have attempted suicide during their lifetime, and 55% have multiple attempts.6
Given the overlap between suicide attempts and NSSI, performing a thorough suicide risk assessment (which is beyond the scope of this article) is crucial. This article describes the static and dynamic risk factors for NSSI in adolescents and adults, which can help us perform a suicide risk assessment and allow us to formulate an appropriate treatment plan that includes safety-based interventions.

NSSI risk factors for adolescents
From developing sexual identity and undergoing puberty to achieving increased independence from their parents and developing a sense of autonomy, adolescents undergo many biological, psychological, and social changes before reaching adulthood.7 Data suggest that NSSI often begins in adolescence, with a typical onset at age 13 or 14.3 Community studies show that one-third to one-half of adolescents in the United States have engaged in NSSI.8,9 Previously, NSSI during adolescence was associated with 3 major diagnostic categories: eating disorders, developmental disabilities, and borderline personality disorder (BPD).10 However, recent data suggest that NSSI is also common outside of these categories. Here we describe static and dynamic risk factors for NSSI in adolescents (Table 111-42). Table 211-42 summarizes the studies of NSSI in adolescents that we reviewed.

Static risk factors
Female adolescents and adults engage in NSSI at higher rates than males. The difference is larger in clinical populations compared to the general population.11
A large portion of research about NSSI has been conducted in studies in which the majority of participants were White.12 Most studies report a higher prevalence of NSSI among non-Hispanic White youth,13 but some suggest other ethnic groups may also experience high rates of self-harm and NSSI.13-15 Several studies have demonstrated high rates of self-harm among South Asian adult females compared with White adult females, but this difference may be less pronounced in adolescents.14 One study in the United Kingdom found that White females age 10 to 14 had higher rates of self-harm compared to South Asian females,14 while another found that risk and rates of self-harm in young South Asian people varied by city and country of origin.15 Young Black females15 and young Black males13 also may be at an increased risk of self-harm. One review found that Black females were more likely to self-harm than Asian or White groups.15
Several studies suggest that sexual minority adolescents (SMA) (eg, lesbian, gay, bisexual, transgender, queer) are at greater risk for NSSI than heterosexual adolescents.16 SMA have been shown to engage in a significantly greater frequency of NSSI and more types of NSSI than heterosexual adolescents.16 Furthermore, on the Inventory of Statements about Self-Injury, SMA self-reported using NSSI for intrapersonal functions (eg, for affect regulation, antisuicide, self-punishment) significantly greater than their heterosexual peers; however, there were no significant differences between the 2 groups on interpersonal functions (eg, autonomy, interpersonal boundaries, peer bonding, sensation-seeking).16
Continue to: Transgender and gender nonconfirming...
Transgender and gender nonconfirming (GNC) youth are at a particularly high risk for NSSI; 30% to 45.5% of transgender adolescents report self-injury.17 Factors shown to distinguish transgender/GNC youth who engage in NSSI from those who do not include having a mental health problem, depression, running away from home, substance use, lower self-esteem/greater self-criticism, experiencing transphobia victimization, and having more interpersonal problems.18,19 Among transgender/GNC youth, those whose biological sex is female are more likely to report NSSI than those whose biological sex is male (ie, transgendered adolescent males are more likely to report NSSI than transgendered adolescent females).18,19
Most forms of childhood maltreatment have been associated with NSSI. In a recently published review, Liu et al20 found that childhood maltreatment (including sexual abuse, physical abuse, emotional abuse, and physical neglect) was associated with an increased risk for NSSI. However, conflicting evidence suggests that when confounders are removed, only childhood emotional abuse was directly associated with NSSI.21 Current evidence is modest for childhood emotional neglect as a risk factor for NSSI.20
Increasing research is investigating the biological processes that may be implicated in NSSI. Some studies suggest that endogenous opioids,22 monoamine neurotransmitters,22 and the hypothalamic-pituitary-adrenal (HPA) axis23 may play a role in NSSI. Compared to healthy controls, adolescents engaging in NSSI have been shown to have lower pain intensity (P = .036), higher pain thresholds (P = .040), and lower beta-endorphins (endogenous opioid hormones involved in mediating stress and pain) (P = .002).24 There may be alterations in the HPA axis among adolescents who engage in NSSI, more specifically stronger cortisol awakening responses.23 Both functional and standard MRI have been used to study the neurobiology of NSSI. One study demonstrated differences in functional connectivity between brain areas linked to neuroregulation of emotions in adolescents who engage in NSSI,25 while another found volume reduction in the insula of these adolescents, which suggests a possible neurobiological reason for impulsivity and the increased risk of suicidal behavior.26
Dynamic risk factors
Research has repeatedly shown bullying is a risk factor for NSSI.27 One study found that younger children who were victimized reported significantly more NSSI than older children.28 New data suggest that perpetrators of bullying are also at risk for deliberate self-harm behavior (SHB), which this study defined as a behavior that is intended to cause self-harm but without suicidal intent and having a nonfatal outcome.29 Victims of cyberbullying also are at a greater risk for self-harm, suicidal behaviors, and suicide attempt.30 To a lesser extent, cyberbullying perpetrators are at greater risk for suicidal behaviors and suicidal ideation.30 Bullying is a risk factor for NSSI not only in adolescence, but also in adulthood. Lereya et al31 found that victims of bullying in childhood and early adolescence were more likely to have mental health problems (including anxiety and depression) and more likely to engage in SHB—which this study defined as hurting oneself on purpose in any way—as adults.
The effects of internet use on adolescents’ mental health also has been investigated. A recent review that explored the relationship between all types of internet use (general use, internet addiction, social media, self-harm websites, forums, etc) and SHB/suicidal behavior found that young people with internet addiction, high levels of internet use, and a tendency to view websites with self-harm or suicidal content were at higher risk of engaging in SHB/suicidal behavior.32 This study did not use a specific definition for SHB or suicidal behavior.32
Continue to: Membership in certain youth...
Membership in certain youth subcultures (eg, emo or goth) has been evaluated as potential risk factors for depression and deliberate self-harm. Bowes et al33 found that for each unit increase in goth affiliation (not at all, not very much, somewhat, more than somewhat, very much), youth were 1.52 times more likely to engage in SHB; these researchers also reported a dose-response association between goth identification and future SHB. This study asked participants if they have ever tried to harm or hurt themselves in any manner, but did not distinguish between individuals who had harmed themselves with and without suicidal intent.33
Personality traits such as impulsiveness and loneliness have been linked to NSSI among adolescents.34,35 A recent study found that adolescents who met the proposed DSM-5 diagnostic criteria for NSSI scored higher on the Barratt Impulsiveness Scale, specifically in measures of:
- motor impulsiveness (ie, acting without thinking)
- attentional impulsiveness (ie, making decisions quickly)
- impulsiveness due to lack of planning (ie, failure to plan for the future).34
This study also found that adolescents who identified as being lonely based on scores on the Brazilian Loneliness Scale were at a higher risk for NSSI.34
A recent systematic review (32 studies) and meta-analysis (9 studies) found that school absenteeism was associated with a risk of self-harm (pooled aOR 1.37, P = .01) and suicidal ideation (pooled aOR 1.20, P = .03).36 This study suggested that school absenteeism, an important marker of social exclusion, was associated with both SHB and suicidal ideation in young people.36 It defined SHB as any act of self-injury or self-poisoning, regardless of intent.36
Finally, family-related factors have been associated with an increased risk of NSSI. One study of 11,814 children age 9 and 10 revealed that high family conflict (OR 1.09; 95% CI, 1.05 to 1.14) and low parental monitoring (OR 0.95; 95% CI, 0.93 to 0.98) were associated with NSSI.37 A smaller, community-based study found that adolescents with NSSI reported significantly less maternal support and warmth than nonclinical controls, but a cause-and-effect relationship has not yet been determined.38 Parental history alone may influence adolescents’ risk of NSSI. A study that included nearly 76,000 youth found that adolescents with perceived parental alcohol problems had higher odds of self-injury, suicidal ideation, and suicide attempts.39 Adolescents exposed to maternal or paternal adversities were also at a higher risk of self-harm (hazard ratio 1.5 to 5.4 among males, 1.7 to 3.9 among females).40
Continue to: NSSI risk factors for adults
NSSI risk factors for adults
Although data regarding the prevalence of NSSI in adults are lacking, available studies report a 12-month prevalence of 0.9%2 and a lifetime prevalence of 5.5% to 5.9%.43 There is a significant overlap in risk factors for NSSI in adolescent and adult populations, but there are also many important differences. The static and dynamic risk factors for NSSI in adults are described in Table 3.44-66 Table 444-66 summarizes the studies of NSSI in adults that we reviewed.

Static risk factors
Research findings regarding the prevalence of NSSI based on gender are varied. For years, it has been believed that women are more likely to engage in NSSI than men. Recent meta-analyses that have examined this relationship closely found that the gender difference is larger for clinical samples compared to community samples and more pronounced in younger individuals.11

As is the case with adolescents, there may be ethnic variations in rates of self-harm and NSSI among adults. A 2013 study by Chesin et al44 found that Asian and White young adults experience higher rates of NSSI than their Hispanic and Black counterparts. Evidence suggests that relative rates of self-harm for older South Asian adults are lower than in older White adults.15
Compared to heterosexual or cisgender individuals, members of sexual and gender minorities have a higher past-year and lifetime prevalence of NSSI.45 One study found that the weighted effect size between sexual orientation and NSSI had an OR of 3 (95% CI, 2.46 to 3.66), indicating a medium-to-large effect.46 Bisexual and transgender individuals appear to be at the highest risk for NSSI when compared to members of other sexual and gender minority groups.45 One review that included mostly cross-sectional studies found that individuals identifying as bisexual had up to 6 times the odds of engaging in NSSI when compared to those of other sexual orientations.47
Incarceration is a risk factor for NSSI. The rates of NSSI in criminal justice settings are higher (up to 61%) than in the general adult population (approximately 4%).48 Recent research found that NSSI serves similar functions in correctional and non-correctional settings, primarily to regulate emotions.48 However, there is also evidence of higher rates of NSSI being motivated by an attempt to influence the environment (ie, engaging in NSSI in order to be transferred to another prison unit) compared to NSSI in community settings.48
Continue to: Though less robust than data...
Though less robust than data published regarding adolescents, the role of biological processes in adults engaging in NSSI has also been studied. A 2021 study by Störkel et al49 found that levels of salivary beta-endorphins were significantly lower in adults immediately before engaging in NSSI compared to after NSSI. Furthermore, adults who engage in NSSI have lower levels of met-enkephalin (P < .01), an opioid growth factor, compared to adults who have never engaged in NSSI.22
Dynamic risk factors
Individuals who engage in NSSI often report substance use, but there is little data on whether substance use is an independent risk factor for NSSI. Although limited, recent evidence suggests illicit substance use in both adolescents41 and adults50 increases risk for NSSI. Richardson et al50 found that the use of barbiturates, opiates, and sedatives significantly increased the frequency of NSSI, whereas use of marijuana, phencyclidine, and medications used to treat anxiety significantly increased the severity of NSSI. A smaller study conducted in South Africa found that individuals who engage in substance use and NSSI were more likely to be male (P < .001).51
Eating disorders and NSSI are highly comorbid.52 The lifetime prevalence of NSSI among individuals with eating disorders ranges from 20.6%to 37.1%.52,53 Results are inconsistent regarding which eating disorders (if any) are greater risk factors for NSSI. One study found that the prevalence of NSSI in patients with bulimia nervosa was 32.7% (95% CI, 26.9% to 39.1%) vs 21.8% in patients with anorexia nervosa (95% CI, 18.5% to 25.6%).54 Another study found that individuals with binge eating/purging–type eating disorders reported engaging in NSSI more frequently than those with other types of eating disorders.55 Among patients with eating disorders who reported NSSI, risk factors included younger age of onset, more negative self-evaluation, more impulsive behavior, concomitant substance use, history of suicide attempts, childhood abuse, and peer aggression.53,55 Body image dissatisfaction and self-criticism, even in individuals not formally diagnosed with an eating disorder, are small but significant predictors of NSSI.56,57
Mood disorders have also been linked to NSSI.58,59 Anxiety disorders (including generalized anxiety disorder, social phobia, panic disorder, and agoraphobia) as well as anxiety-related disorders such as obsessive-compulsive disorder have been significantly associated with NSSI (P < .001), but this relationship decreased in strength when mood instability was removed as a confounder.58 Among patients with anxiety and anxiety-related disorders, panic disorder and posttraumatic stress disorder (PTSD) have shown the strongest association with NSSI, with pooled aORs of 2.67 and 2.06, respectively.59
Recent studies have examined the association of other mental health disorders and symptoms with NSSI, including psychosis60 and dissociative symptoms.61 One study found that paranoia, thought control, and auditory hallucinations were significantly associated with NSSI60; however, after controlling for concomitant BPD, only paranoia was significantly associated with NSSI.60 Individuals diagnosed with dissociative disorders were more likely than patients without such disorders to endorse NSSI and suicide attempts.61
Continue to: Emotional dysregulation...
Emotional dysregulation (EDR)—defined as difficulty understanding, recognizing, and managing one’s emotions—has been researched extensively in relation to NSSI.62 A recent review that included studies of both adolescents and adults reported a significant association between EDR and NSSI, with an OR of 2.40 (95% CI, 2.01 to 2.86).62 A larger effect size was observed between EDR and lifetime NSSI (OR 3.21; 95% CI, 2.63 to 3.91) compared to past-year NSSI (OR 2.32; 95% CI, 1.84 to 2.92).62 Patient age, sex, and sample type (clinical vs community) were not significant moderators of strength between the reported associations.62
Studies examining intimate partner violence (IPV) and NSSI have found that young adults who engage in IPV (both as victims and as perpetrators) are more likely to report NSSI.63-65 Researchers have proposed that anxiety over abandonment may explain this relationship.64 A recent study found that individuals with bidirectional IPV (ie, both victimization and perpetration) engaged in NSSI at a higher prevalence than those engaging in unidirectional IPV or no IPV.65 This suggests that relationship violence in general (rather than just being a victim of IPV) may be a risk factor for NSSI.65
Finally, studies suggest that adolescents and adults who have sleep problems (insomnia, short sleep duration, long sleep onset latency, waking after sleep onset, and poor quality sleep) are more likely to report self-harm or NSSI than those without sleep problems.42,66 In adults, this relationship is partially mediated by depressive symptoms, EDR, and PTSD.66 In adolescents, depressive symptoms are a mediator for this relationship.42
Bottom Line
Nonsuicidal self-injury (NSSI) is a significant health concern due to its association with suicide attempts. Although there are similarities in NSSI risk factors between adolescents and adults, there are also important differences. Understanding these differences is necessary to develop appropriate treatment plans.
Related Resources
- American Foundation for Suicide Prevention. https://afsp.org/
- Cipriano A, Cella S, Cotrufo P. Nonsuicidal self-injury: a systematic review. Front Psych. 2017;8:1946. doi:10.3389/ fpsyg.2017.01946
- Gold LH, Frierson RL, eds. Textbook of Suicide Risk Assessment and Management. 3rd ed. American Psychiatric Association Publishing; 2020.
1. Nock MK. Self-injury. Annu Rev Clin Psychol. 2010;6:339-363.
2. Klonsky ED. Non-suicidal self-injury in United States adults: prevalence, sociodemographics, topography and functions. Psychol Med. 2011;41(9):1981-1986.
3. Klonsky ED. Nonsuicidal self-injury: what we know, and what we need to know. Can J Psychiatry. 2014;59(11):565-568.
4. Wilkinson P, Kelvin R, Roberts C, et al. Clinical and psychosocial predictors of suicide attempts and nonsuicidal self-injury in the Adolescent Depression Antidepressants and Psychotherapy Trial (ADAPT). Am J Psychiatry. 2011;168(5):495-501.
5. Kiekens G, Hasking P, Boyes M, et al. The associations between non-suicidal self-injury and first onset suicidal thoughts and behaviors. J Affect Disord. 2018;239:171-179.
6. Nock MK, Joiner TE, Gordon KH, et al. Non-suicidal self-injury among adolescents: diagnostic correlates and relation to suicide attempts. Psychiatry Res. 2006;144(1):65-72.
7. Christie D, Viner R. Adolescent development. BMJ. 2005;330(7486):301-304.
8. Yates TM, Tracy AJ, Luthar SS. Nonsuicidal self-injury among “privileged” youths: longitudinal and cross-sectional approaches to developmental process. J Consult Clin Psychol. 2008;76(1):52-62.
9. Lloyd-Richardson EE, Perrine N, Dierker L, et al. Characteristics and functions of non-suicidal self-injury in a community sample of adolescents. Psychol Med. 2007;37(8):1183-1192.
10. Peterson J, Freedenthal S, Sheldon C, et al. Nonsuicidal self injury in adolescents. Psychiatry(Edgmont). 2008;5(11):20-26.
11. Bresin K, Schoenleber M. Gender differences in the prevalence of nonsuicidal self-injury: a meta-analysis. Clin Psychol Rev. 2015;38:55-64.
12. Gholamrezaei M, Stefano JD, Heath NL. Nonsuicidal self-injury across cultures and ethnic and racial minorities: a review. Int J Psychol. 2015;52(4):316-326.
13. Rojas-Velasquez DA, Pluhar EI, Burns PA, et al. Nonsuicidal self-injury among African American and Hispanic adolescents and young adults: a systematic review. Prev Sci. 2021;22:367-377.
14. Bhui K, McKenzie K, Rasul F. Rates, risk factors & methods of self harm among minority ethnic groups in the UK: a systematic review. BMC Public Health. 2007;7:336.
15. Cooper J, Murphy E, Webb R, et al. Ethnic differences in self-harm, rates, characteristics and service provision: three-city cohort study. Br J Psychiatry. 2010;197(3):212-218.
16. Peters JR, Mereish EH, Krek MA, et al. Sexual orientation differences in non-suicidal self-injury, suicidality, and psychosocial factors among an inpatient psychiatric sample of adolescents. Psychiatry Res. 2020;284:112664.
17. Connolly MD, Zervos MJ, Barone 2nd CJ, et al. The mental health of transgender youth: advances in understanding. J Adolesc Health. 2016;59(5):489-495.
18. Taliaferro LA, McMorris BJ, Rider GN, et al. Risk and protective factors for self-harm in a population-based sample of transgender youth. Archives Suicide Res. 2019;23(2):203-221.
19. Arcelus J, Claes L, Witcomb GL, et al. Risk factors for non-suicidal self-injury among trans youth. J Sex Med. 2016;13(3):402-412.
20. Liu RT, Scopelliti KM, Pittman SK, et al. Childhood maltreatment and non-suicidal self-injury: a systematic review and meta-analysis. Lancet Psychiatry. 2018;5(1):51-64.
21. Thomassin K, Shaffer A, Madden A, et al. Specificity of childhood maltreatment and emotion deficit in nonsuicidal self-injury in an inpatient sample of youth. Psychiatry Res. 2016;244:103-108.
22. Stanley B, Sher L, Wilson S, et al. Non-suicidal self-injurious behavior, endogenous opioids and monoamine neurotransmitters. J Affect Disord. 2010;124(1-2):134-140.
23. Reichl C, Heyer A, Brunner R, et al. Hypothalamic-pituitary-adrenal axis, childhood adversity and adolescent nonsuicidal self-injury. Psychoneuroendocrinology. 2016;74:203-211.
24. van der Venne P, Balint A, Drews E, et al. Pain sensitivity and plasma beta-endorphin in adolescent non-suicidal self-injury. J Affect Disord. 2021;278:199-209.
25. Osuch E, Ford K, Wrath A, et al. Functional MRI of pain application in youth who engaged in repetitive non-suicidal self-injury vs. psychiatric controls. Psychiatry Res. 2014;223(2):104-112.
26. Ando A, Reichl C, Scheu F, et al. Regional grey matter volume reduction in adolescents engaging in non-suicidal self-injury. Psychiatry Res Neuroimaging. 2018;280:48-55.
27. Karanikola MNK, Lyberg A, Holm A-L, et al. The association between deliberate self-harm and school bullying victimization and the mediating effect of depressive symptoms and self-stigma: a systematic review. BioMed Res Int. 2018;4745791. doi: 10.1155/2018/4745791
28. van Geel M, Goemans A, Vedder P. A meta-analysis on the relation between peer victimization and adolescent non-suicidal self-injury. Psychiatry Res. 2015;230(2):364-368.
29. Heerde JA, Hemphill SA. Are bullying perpetration and victimization associated with adolescent deliberate self-harm? A meta-analysis. Arch Suicide Res. 2019;23(3):353-381.
30. John A, Glendenning AC, Marchant A, et al. Self-harm, suicidal behaviours, and cyberbullying in children and young people: systematic review. J Med Internet Res. 2018;20(4):e129. doi: 10.2196/jmir.9044
31. Lereya ST, Copeland WE, Costello EJ, et al. Adult mental health consequences of peer bullying and maltreatment in childhood: two cohorts in two countries. Lancet Psychiatry. 2015;2(6):524-531.
32. Marchant A, Hawton K, Stewart A, et al. A systematic review of the relationship between internet use, self-harm and suicidal behaviour in young people: the good, the bad and the unknown. PLoS One. 2017;12(8):e0181722. doi: 10.1371/journal.pone.0181722
33. Bowes L, Carnegie R, Pearson R, et al. Risk of depression and self-harm in teenagers identifying with goth subculture: a longitudinal cohort study. Lancet Psychiatry. 2015;2(9):793-800.
34. Costa RPO, Peixoto ALRP, Lucas CCA, et al. Profile of non-suicidal self-injury in adolescents: interface with impulsiveness and loneliness. J Pediatr (Rio J). 2021;97(2):184-190.
35. McHugh CM, Lee RSC, Hermens DF, et al. Impulsivity in the self-harm and suicidal behavior of young people: a systematic review and meta-analysis. J Psychiatr Res. 2019;116:51-60.
36. Epstein S, Roberts E, Sedgwick R, et al. School absenteeism as a risk factor for self-harm and suicidal ideation in children and adolescents: a systematic review and meta-analysis. Eur Child Adolesc Psychiatry. 2020;29(9):1175-1194.
37. DeVille DC, Whalen D, Breslin FJ, et al. Prevalence and family-related factors associated with suicidal ideation, suicide attempts, and self-injury in children aged 9 to 10 years. JAMA Netw Open. 2020;3(2):e1920956. doi: 10.1001/jamanetworkopen.2019.20956
38. Tschan T, Schmid M, In-Albon T. Parenting behavior in families of female adolescents with nonsuicidal self-injury in comparison to a clinical and a nonclinical control group. Child Adolesc Psychiatry Ment Health. 2015;9:17.
39. Pisinger V, Hawton K, Tolstrup JS. Self-injury and suicide behavior among young people with perceived parental alcohol problems in Denmark: a school-based survey. Eur Child Adolesc Psychiatry. 2018;27(2):201-208.
40. Pitkänen J, Remes H, Aaltonen M, et al. Experience of maternal and paternal adversities in childhood as determinants of self-harm in adolescence and young adulthood. J Epidemiol Community Health. 2019;73(11):1040-1046.
41. Monto MA, McRee N, Deryck FS. Nonsuicidal self-injury among a representative sample of US adolescents, 2015. Am J Public Health. 2018;108(8):1042-1048.
42. Hysing M, Sivertsen B, Stormark KM, et al. Sleep problems and self-harm in adolescence. Br J Psychiatry. 2015;207(4):306-312.
43. Swannell SV, Martin GE, Page A, et al. Prevalence of nonsuicidal self-injury in nonclinical samples: systematic review, meta-analysis and meta-regression. Suicide Life Threat Behav. 2014;44(3):273-303.
44. Chesin M, Moster A, Jeglic E. Non-suicidal self-injury among ethnically and racially diverse emerging adults: do factors unique to the minority experience matter? Current Psychology. 2013;32:318-328.
45. Liu RT, Sheehan AE, Walsh RFL, et al. Prevalence and correlates of non-suicidal self-injury among lesbian, gay, bisexual, and transgender individuals: a systematic review and meta-analysis. Clin Psychol Rev. 2019;74:101-783. doi:10.1016/j.cpr.2019.101783
46. Batejan KL, Jarvi SM, Swenson LP. Sexual orientation and non-suicidal self-injury: a meta-analytic review. Arch Suicide Res. 2015;19(2):131-150.
47. Dunlop BJ, Hartley S, Oladokun O, et al. Bisexuality and non-suicidal self-injury (NSSI): a narrative synthesis of associated variables and a meta-analysis of risk. J Affect Disord. 2020;276:1159-1172.
48. Dixon-Gordon K, Harrison N, Roesch R. Non-suicidal self-injury within offender populations: a systematic review. Int J Forensic Ment Health. 2012;11(1):33-50.
49. Störkel LM, Karabatsiakis A, Hepp K, et al. Salivary beta-endorphin in nonsuicidal self-injury: an ambulatory assessment study. Neuropsychopharmacology. 2021;46(7):1357-1363.
50. Richardson E, DePue MK, Therriault DJ, et al. The influence of substance use on engagement in non-suicidal self-injury (NSI) in adults. Subst Use Misuse. 2020;55(1):89-94.
51. Breet E, Bantjes J, Lewis I. Chronic substance use and self-harm in a primary health care setting. Afr J Prim Health Care Fam Med. 2018;10(1):e1-e9. doi: 10.4102/phcfm.v10i1.1544
52. Pérez S, Marco JH, Cañabate M. Non-suicidal self-injury in patients with eating disorders: prevalence, forms, functions, and body image correlates. Compr Psychiatry. 2018;84:32-38.
53. Islam MA, Steiger H, Jimenez-Murcia S, et al. Non-suicidal self-injury in different eating disorder types: relevance of personality traits and gender. Eur Eat Disord Rev. 2015;23(6):553-560.
54. Cucchi A, Ryan D, Konstantakopoulos G, et al. Lifetime prevalence of non-suicidal self-injury in patients with eating disorders: a systematic review and meta-analysis. Psychol Med. 2016;46(7):1345-1358.
55. Vieira AI, Machado BC, Machado PPP, et al. Putative risk factors for non-suicidal self-injury in eating disorders. Eur Eat Disord Rev. 2017;25(6):544-550.
56. Black EB, Garratt M, Beccaria G, et al. Body image as a predictor of nonsuicidal self-injury in women: a longitudinal study. Compr Psychiatry. 2019;88:83-89.
57. Zelkowitz RL, Cole DA. Self-criticism as a transdiagnostic process in nonsuicidal self-injury and disordered eating: systematic review and meta-analysis. Suicide Life Threat Behav. 2019;49(1):310-327.
58. Peters EM, Bowen R, Balbuena L. Mood instability contributes to impulsivity, non-suicidal self-injury, and binge eating/purging in people with anxiety disorders. Psychol Psychother. 2019;92(3):422-438.
59. Bentley KH, Cassiello-Robbins CF, Vittorio L, et al. The association between nonsuicidal self-injury and the emotional disorders: a meta-analytic review. Clin Psychol Rev. 2015;37:72-88.
60. Koyanagi A, Stickley A, Haro JM. Psychotic-like experiences and nonsuicidal self-injury in England: results from a national survey [corrected]. PLoS One. 2015;10(12):e0145533. doi: 10.1371/journal.pone.0145533
61. Calati R, Bensassi I, Courtet P. The link between dissociation and both suicide attempts and non-suicidal self-injury: meta-analyses. Psychiatry Res. 2017;251:103-114.
62. Wolff JC, Thompson E, Thomas SA, et al. Emotion dysregulation and non-suicidal self-injury: a systematic review and meta-analysis. Eur Psychiatry. 2019;59:25-36.
63. Vaughn MG, Salas-Wright CP, DeLisi M, et al. Deliberate self-harm and the nexus of violence, victimization, and mental health problems in the United States. Psychiatry Res. 2015;225(3):588-595.
64. Levesque C, Lafontaine M-F, Bureau J-F, et al. The influence of romantic attachment and intimate partner violence on nonsuicidal self-injury in young adults. J Youth Adolesc. 2010;39(5):474-483.
65. Carranza AB, Wallis CRD, Jonnson MR, et al. Nonsuicidal self-injury and intimate partner violence: directionality of violence and motives for self-injury. J Interpers Violence. 2020;886260520922372. doi: 10.1177/0886260520922372
66. Khazaie H, Zakiei A, McCall WV, et al. Relationship between sleep problems and self-injury: a systematic review. Behav Sleep Med. 2020;1-16. doi: 10.1080/15402002.2020.1822360
1. Nock MK. Self-injury. Annu Rev Clin Psychol. 2010;6:339-363.
2. Klonsky ED. Non-suicidal self-injury in United States adults: prevalence, sociodemographics, topography and functions. Psychol Med. 2011;41(9):1981-1986.
3. Klonsky ED. Nonsuicidal self-injury: what we know, and what we need to know. Can J Psychiatry. 2014;59(11):565-568.
4. Wilkinson P, Kelvin R, Roberts C, et al. Clinical and psychosocial predictors of suicide attempts and nonsuicidal self-injury in the Adolescent Depression Antidepressants and Psychotherapy Trial (ADAPT). Am J Psychiatry. 2011;168(5):495-501.
5. Kiekens G, Hasking P, Boyes M, et al. The associations between non-suicidal self-injury and first onset suicidal thoughts and behaviors. J Affect Disord. 2018;239:171-179.
6. Nock MK, Joiner TE, Gordon KH, et al. Non-suicidal self-injury among adolescents: diagnostic correlates and relation to suicide attempts. Psychiatry Res. 2006;144(1):65-72.
7. Christie D, Viner R. Adolescent development. BMJ. 2005;330(7486):301-304.
8. Yates TM, Tracy AJ, Luthar SS. Nonsuicidal self-injury among “privileged” youths: longitudinal and cross-sectional approaches to developmental process. J Consult Clin Psychol. 2008;76(1):52-62.
9. Lloyd-Richardson EE, Perrine N, Dierker L, et al. Characteristics and functions of non-suicidal self-injury in a community sample of adolescents. Psychol Med. 2007;37(8):1183-1192.
10. Peterson J, Freedenthal S, Sheldon C, et al. Nonsuicidal self injury in adolescents. Psychiatry(Edgmont). 2008;5(11):20-26.
11. Bresin K, Schoenleber M. Gender differences in the prevalence of nonsuicidal self-injury: a meta-analysis. Clin Psychol Rev. 2015;38:55-64.
12. Gholamrezaei M, Stefano JD, Heath NL. Nonsuicidal self-injury across cultures and ethnic and racial minorities: a review. Int J Psychol. 2015;52(4):316-326.
13. Rojas-Velasquez DA, Pluhar EI, Burns PA, et al. Nonsuicidal self-injury among African American and Hispanic adolescents and young adults: a systematic review. Prev Sci. 2021;22:367-377.
14. Bhui K, McKenzie K, Rasul F. Rates, risk factors & methods of self harm among minority ethnic groups in the UK: a systematic review. BMC Public Health. 2007;7:336.
15. Cooper J, Murphy E, Webb R, et al. Ethnic differences in self-harm, rates, characteristics and service provision: three-city cohort study. Br J Psychiatry. 2010;197(3):212-218.
16. Peters JR, Mereish EH, Krek MA, et al. Sexual orientation differences in non-suicidal self-injury, suicidality, and psychosocial factors among an inpatient psychiatric sample of adolescents. Psychiatry Res. 2020;284:112664.
17. Connolly MD, Zervos MJ, Barone 2nd CJ, et al. The mental health of transgender youth: advances in understanding. J Adolesc Health. 2016;59(5):489-495.
18. Taliaferro LA, McMorris BJ, Rider GN, et al. Risk and protective factors for self-harm in a population-based sample of transgender youth. Archives Suicide Res. 2019;23(2):203-221.
19. Arcelus J, Claes L, Witcomb GL, et al. Risk factors for non-suicidal self-injury among trans youth. J Sex Med. 2016;13(3):402-412.
20. Liu RT, Scopelliti KM, Pittman SK, et al. Childhood maltreatment and non-suicidal self-injury: a systematic review and meta-analysis. Lancet Psychiatry. 2018;5(1):51-64.
21. Thomassin K, Shaffer A, Madden A, et al. Specificity of childhood maltreatment and emotion deficit in nonsuicidal self-injury in an inpatient sample of youth. Psychiatry Res. 2016;244:103-108.
22. Stanley B, Sher L, Wilson S, et al. Non-suicidal self-injurious behavior, endogenous opioids and monoamine neurotransmitters. J Affect Disord. 2010;124(1-2):134-140.
23. Reichl C, Heyer A, Brunner R, et al. Hypothalamic-pituitary-adrenal axis, childhood adversity and adolescent nonsuicidal self-injury. Psychoneuroendocrinology. 2016;74:203-211.
24. van der Venne P, Balint A, Drews E, et al. Pain sensitivity and plasma beta-endorphin in adolescent non-suicidal self-injury. J Affect Disord. 2021;278:199-209.
25. Osuch E, Ford K, Wrath A, et al. Functional MRI of pain application in youth who engaged in repetitive non-suicidal self-injury vs. psychiatric controls. Psychiatry Res. 2014;223(2):104-112.
26. Ando A, Reichl C, Scheu F, et al. Regional grey matter volume reduction in adolescents engaging in non-suicidal self-injury. Psychiatry Res Neuroimaging. 2018;280:48-55.
27. Karanikola MNK, Lyberg A, Holm A-L, et al. The association between deliberate self-harm and school bullying victimization and the mediating effect of depressive symptoms and self-stigma: a systematic review. BioMed Res Int. 2018;4745791. doi: 10.1155/2018/4745791
28. van Geel M, Goemans A, Vedder P. A meta-analysis on the relation between peer victimization and adolescent non-suicidal self-injury. Psychiatry Res. 2015;230(2):364-368.
29. Heerde JA, Hemphill SA. Are bullying perpetration and victimization associated with adolescent deliberate self-harm? A meta-analysis. Arch Suicide Res. 2019;23(3):353-381.
30. John A, Glendenning AC, Marchant A, et al. Self-harm, suicidal behaviours, and cyberbullying in children and young people: systematic review. J Med Internet Res. 2018;20(4):e129. doi: 10.2196/jmir.9044
31. Lereya ST, Copeland WE, Costello EJ, et al. Adult mental health consequences of peer bullying and maltreatment in childhood: two cohorts in two countries. Lancet Psychiatry. 2015;2(6):524-531.
32. Marchant A, Hawton K, Stewart A, et al. A systematic review of the relationship between internet use, self-harm and suicidal behaviour in young people: the good, the bad and the unknown. PLoS One. 2017;12(8):e0181722. doi: 10.1371/journal.pone.0181722
33. Bowes L, Carnegie R, Pearson R, et al. Risk of depression and self-harm in teenagers identifying with goth subculture: a longitudinal cohort study. Lancet Psychiatry. 2015;2(9):793-800.
34. Costa RPO, Peixoto ALRP, Lucas CCA, et al. Profile of non-suicidal self-injury in adolescents: interface with impulsiveness and loneliness. J Pediatr (Rio J). 2021;97(2):184-190.
35. McHugh CM, Lee RSC, Hermens DF, et al. Impulsivity in the self-harm and suicidal behavior of young people: a systematic review and meta-analysis. J Psychiatr Res. 2019;116:51-60.
36. Epstein S, Roberts E, Sedgwick R, et al. School absenteeism as a risk factor for self-harm and suicidal ideation in children and adolescents: a systematic review and meta-analysis. Eur Child Adolesc Psychiatry. 2020;29(9):1175-1194.
37. DeVille DC, Whalen D, Breslin FJ, et al. Prevalence and family-related factors associated with suicidal ideation, suicide attempts, and self-injury in children aged 9 to 10 years. JAMA Netw Open. 2020;3(2):e1920956. doi: 10.1001/jamanetworkopen.2019.20956
38. Tschan T, Schmid M, In-Albon T. Parenting behavior in families of female adolescents with nonsuicidal self-injury in comparison to a clinical and a nonclinical control group. Child Adolesc Psychiatry Ment Health. 2015;9:17.
39. Pisinger V, Hawton K, Tolstrup JS. Self-injury and suicide behavior among young people with perceived parental alcohol problems in Denmark: a school-based survey. Eur Child Adolesc Psychiatry. 2018;27(2):201-208.
40. Pitkänen J, Remes H, Aaltonen M, et al. Experience of maternal and paternal adversities in childhood as determinants of self-harm in adolescence and young adulthood. J Epidemiol Community Health. 2019;73(11):1040-1046.
41. Monto MA, McRee N, Deryck FS. Nonsuicidal self-injury among a representative sample of US adolescents, 2015. Am J Public Health. 2018;108(8):1042-1048.
42. Hysing M, Sivertsen B, Stormark KM, et al. Sleep problems and self-harm in adolescence. Br J Psychiatry. 2015;207(4):306-312.
43. Swannell SV, Martin GE, Page A, et al. Prevalence of nonsuicidal self-injury in nonclinical samples: systematic review, meta-analysis and meta-regression. Suicide Life Threat Behav. 2014;44(3):273-303.
44. Chesin M, Moster A, Jeglic E. Non-suicidal self-injury among ethnically and racially diverse emerging adults: do factors unique to the minority experience matter? Current Psychology. 2013;32:318-328.
45. Liu RT, Sheehan AE, Walsh RFL, et al. Prevalence and correlates of non-suicidal self-injury among lesbian, gay, bisexual, and transgender individuals: a systematic review and meta-analysis. Clin Psychol Rev. 2019;74:101-783. doi:10.1016/j.cpr.2019.101783
46. Batejan KL, Jarvi SM, Swenson LP. Sexual orientation and non-suicidal self-injury: a meta-analytic review. Arch Suicide Res. 2015;19(2):131-150.
47. Dunlop BJ, Hartley S, Oladokun O, et al. Bisexuality and non-suicidal self-injury (NSSI): a narrative synthesis of associated variables and a meta-analysis of risk. J Affect Disord. 2020;276:1159-1172.
48. Dixon-Gordon K, Harrison N, Roesch R. Non-suicidal self-injury within offender populations: a systematic review. Int J Forensic Ment Health. 2012;11(1):33-50.
49. Störkel LM, Karabatsiakis A, Hepp K, et al. Salivary beta-endorphin in nonsuicidal self-injury: an ambulatory assessment study. Neuropsychopharmacology. 2021;46(7):1357-1363.
50. Richardson E, DePue MK, Therriault DJ, et al. The influence of substance use on engagement in non-suicidal self-injury (NSI) in adults. Subst Use Misuse. 2020;55(1):89-94.
51. Breet E, Bantjes J, Lewis I. Chronic substance use and self-harm in a primary health care setting. Afr J Prim Health Care Fam Med. 2018;10(1):e1-e9. doi: 10.4102/phcfm.v10i1.1544
52. Pérez S, Marco JH, Cañabate M. Non-suicidal self-injury in patients with eating disorders: prevalence, forms, functions, and body image correlates. Compr Psychiatry. 2018;84:32-38.
53. Islam MA, Steiger H, Jimenez-Murcia S, et al. Non-suicidal self-injury in different eating disorder types: relevance of personality traits and gender. Eur Eat Disord Rev. 2015;23(6):553-560.
54. Cucchi A, Ryan D, Konstantakopoulos G, et al. Lifetime prevalence of non-suicidal self-injury in patients with eating disorders: a systematic review and meta-analysis. Psychol Med. 2016;46(7):1345-1358.
55. Vieira AI, Machado BC, Machado PPP, et al. Putative risk factors for non-suicidal self-injury in eating disorders. Eur Eat Disord Rev. 2017;25(6):544-550.
56. Black EB, Garratt M, Beccaria G, et al. Body image as a predictor of nonsuicidal self-injury in women: a longitudinal study. Compr Psychiatry. 2019;88:83-89.
57. Zelkowitz RL, Cole DA. Self-criticism as a transdiagnostic process in nonsuicidal self-injury and disordered eating: systematic review and meta-analysis. Suicide Life Threat Behav. 2019;49(1):310-327.
58. Peters EM, Bowen R, Balbuena L. Mood instability contributes to impulsivity, non-suicidal self-injury, and binge eating/purging in people with anxiety disorders. Psychol Psychother. 2019;92(3):422-438.
59. Bentley KH, Cassiello-Robbins CF, Vittorio L, et al. The association between nonsuicidal self-injury and the emotional disorders: a meta-analytic review. Clin Psychol Rev. 2015;37:72-88.
60. Koyanagi A, Stickley A, Haro JM. Psychotic-like experiences and nonsuicidal self-injury in England: results from a national survey [corrected]. PLoS One. 2015;10(12):e0145533. doi: 10.1371/journal.pone.0145533
61. Calati R, Bensassi I, Courtet P. The link between dissociation and both suicide attempts and non-suicidal self-injury: meta-analyses. Psychiatry Res. 2017;251:103-114.
62. Wolff JC, Thompson E, Thomas SA, et al. Emotion dysregulation and non-suicidal self-injury: a systematic review and meta-analysis. Eur Psychiatry. 2019;59:25-36.
63. Vaughn MG, Salas-Wright CP, DeLisi M, et al. Deliberate self-harm and the nexus of violence, victimization, and mental health problems in the United States. Psychiatry Res. 2015;225(3):588-595.
64. Levesque C, Lafontaine M-F, Bureau J-F, et al. The influence of romantic attachment and intimate partner violence on nonsuicidal self-injury in young adults. J Youth Adolesc. 2010;39(5):474-483.
65. Carranza AB, Wallis CRD, Jonnson MR, et al. Nonsuicidal self-injury and intimate partner violence: directionality of violence and motives for self-injury. J Interpers Violence. 2020;886260520922372. doi: 10.1177/0886260520922372
66. Khazaie H, Zakiei A, McCall WV, et al. Relationship between sleep problems and self-injury: a systematic review. Behav Sleep Med. 2020;1-16. doi: 10.1080/15402002.2020.1822360













