User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'main-prefix')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
Is it psychosis, or an autoimmune encephalitis?
Hidden within routine presentations of first-episode psychosis is a rare subpopulation whose symptoms are mediated by an autoimmune process for which proper treatment differs significantly from standard care for typical psychotic illness. In this article, we present a hypothetical case and describe how to assess if a patient has an elevated probability of autoimmune encephalitis, determine what diagnostics or medication-induced effects to consider, and identify unresolved questions about best practices.
CASE REPORT
Bizarre behavior and isolation
Ms. L, age 21, is brought to the emergency department (ED) by her college roommate after exhibiting out-of-character behavior and gradual self-isolation over the last 2 months. Her roommate noticed that she had been spending more time isolated in her dorm room and remaining in bed into the early afternoon, though she does not appear to be asleep. Ms. L’s mother is concerned about her daughter’s uncharacteristic refusal to travel home for a family event. Ms. L expresses concern about the intentions of her research preceptor, and recalls messages from the association of colleges telling her to “change her future.” Ms. L hears voices telling her who she can and cannot trust. In the ED, she says she has a headache, experiences mild dizziness while standing, and reports having a brief upper respiratory illness at the end of last semester. Otherwise, a medical review of systems is negative.
Although the etiology of first-episode psychosis can be numerous or unknown, many psychiatrists feel comfortable with the initial diagnostic for this type of clinical presentation. However, for some clinicians, it may be challenging to feel confident in making a diagnosis of autoimmune encephalitis.
Autoimmune encephalitis is a family of syndromes caused by autoantibodies targeting either intracellular or extracellular neuronal antigens. Anti-N-methyl-
In this article, we focus on anti-NMDA receptor encephalitis and use the term interchangeably with autoimmune encephalitis for 2 reasons. First, anti-NMDA receptor encephalitis can present with psychotic symptoms as the only symptoms (prior to cognitive or neurologic manifestations) or can present with psychotic symptoms as the main indicator (with other symptoms that are more subtle and possibly missed). Second, anti-NMDA receptor encephalitis often occurs in young adults, which is when it is common to see the onset of a primary psychotic illness. These 2 factors make it likely that these cases will come into the evaluative sphere of psychiatrists. We give special attention to features of cases of anti-NMDA receptor encephalitis confirmed with antineuronal antibodies in the CSF, as it has emerged that antibodies in the serum can be nonspecific and nonpathogenic.2,3
What does anti-NMDA receptor encephalitis look like?
Symptoms of anti-NMDA receptor encephalitis resemble those of a primary psychotic disorder, which can make it challenging to differentiate between the 2 conditions, and might cause the correct diagnosis to be missed. Pollak et al4 proposed that psychiatrically confusing presentations that don’t clearly match an identifiable psychotic disorder should raise a red flag for an autoimmune etiology. However, studies often fail to describe the specific psychiatric features of anti-NMDA receptor encephalitis, and thus provide little practical evidence to guide diagnosis. In some of the largest studies of patients with anti-NMDA receptor encephalitis, psychiatric clinical findings are often combined into nonspecific headings such as “abnormal behavior” or “behavioral and cognitive” symptoms.5 Such groupings make this the most common clinical finding (95%)5 but make it difficult to discern particular clinical characteristics. Where available, specific symptoms identified across studies include agitation, aggression, changes in mood and/or irritability, insomnia, delusions, hallucinations, and occasionally catatonic features.6,7 Attempts to identify specific psychiatric phenotypes distinct from primary psychotic illnesses have fallen short due to contradictory findings and lack of clinical practicality.8 One exception is the presence of catatonic features, which have been found in CSF-confirmed studies.2 In contrast to the typical teaching that the hallucination modality (eg, visual or tactile) can be helpful in estimating the likelihood of a secondary psychosis (ie, drug-induced, neurodegenerative, or autoimmune), there does not appear to be a difference in hallucination modality between encephalitis and primary psychotic disorders.9
History and review of systems
Another red flag to consider is the rapidity of symptom presentation. Symptoms that progress within 3 months increase the likelihood that the patient has autoimmune encephalitis.10 Cases where collateral information indicates the psychotic episode was preceded by a long, subtle decline in school performance, social withdrawal, and attenuated psychotic symptoms typical of a schizophrenia prodrome are less likely to be an autoimmune psychosis.11 A more delayed presentation does not entirely exclude autoimmune encephalitis; however, a viral-like prodrome before the onset of psychosis increases the likelihood of autoimmune encephalitis. Such a prodrome may include fever, headache, nausea, vomiting, and diarrhea.7
Continue to: Another indication is the presence...
Another indication is the presence of new seizures within 1 year of presenting with psychotic symptoms.10 The possibility of undiagnosed seizures should be considered in a patient with psychosis who has episodes of unresponsiveness, dissociative episodes, or seizure-like activity that is thought to be psychogenic but has not been fully evaluated. Seizures in autoimmune encephalitis involve deep structures in the brain and can be present without overt epileptiform activity on EEG, but rather causing only bilateral slowing that is often described as nonspecific.12
In a young patient presenting with first-episode psychosis, a recent diagnosis of cancer or abnormal finding in the ovaries increases the likelihood of autoimmune encephalitis.4 Historically, however, this type of medical history has been irrelevant to psychosis. Although rare, any person presenting with first-episode psychosis and a history of herpes simplex virus (HSV) encephalitis should be evaluated for autoimmune encephalitis because anti-NMDA receptor antibodies have been reported to be present in approximately one-third of these patients.13 Finally, the report of focal neurologic symptoms, including neck stiffness or neck pain, should raise concern, although sensory, working memory, and cognitive deficits may be difficult to fully distinguish from common somatic and cognitive symptoms in a primary psychiatric presentation.
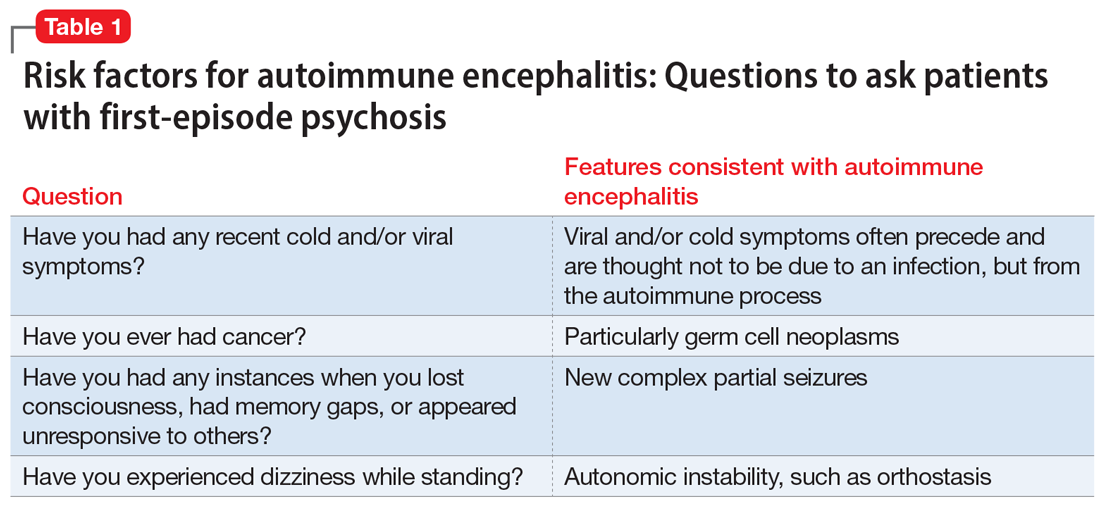
Table 1 lists 4 questions to ask patients who present with first-episode psychosis that may not usually be part of a typical evaluation.
CASE CONTINUED
Uncooperative with examination
In the ED, Ms. L’s heart rate is 101 beats per minute and her blood pressure is 102/72 mm Hg. Her body mass index (BMI) is 22, which suggests an approximate 8-pound weight loss since her BMI was last assessed. Ms. L responds to questions with 1- to 6-word sentences, without clear verbigeration. Though her speech is not pressured, it is of increased rate. Her gaze scans the room, occasionally becoming fixed for 5 to 10 seconds but is aborted by the interviewer’s comment on this behavior. Ms. L efficiently and accurately spells WORLD backwards, then asks “Why?” and refuses to engage in further cognitive testing, stating “Not doing that.” When the interviewer asks “Why not?” she responds “Not doing that.” Her cranial nerves are intact, and she refuses cerebellar testing or requests to assess tone. There are no observed stereotypies, posturing, or echopraxia.
While not necessary for a diagnosis of autoimmune encephalitis, short-term memory loss is a common cognitive finding across studies.5-7 A common clinical finding from a mental status exam is speech disorders, including (but not limited to) increased rates of speech or decreased verbal output.7 Autonomic instability—including tachycardia, markedly labile blood pressures, and orthostasis—all increase the likelihood of autoimmune encephalitis.14 Interpreting a patient’s vital sign changes can be confounded if they are agitated or anxious, or if they are taking an antipsychotic that produces adverse anticholinergic effects. However, vital sign abnormalities that precede medication administration or do not correlate with fluctuations in mental status increase suspicion for an autoimmune encephalitis.
Continue to: In the absence of the adverse effect...
In the absence of the adverse effect of a medication, orthostasis is uncommon in a well-hydrated young person. Some guidelines4 suggest that symptoms of catatonia should be considered a red flag for autoimmune encephalitis. According to the Bush-Francis Catatonia Rating Scale, commonly identified features include immobility, staring, mutism, posturing, withdrawal, rigidity, and gegenhalten.15 Catatonia is common among patients with anti-NDMA receptor encephalitis, though it may not be initially present and could emerge later.2 However, there are documented cases of autoimmune encephalitis where the patient had only isolated features of catatonia, such as echolalia or mutism.2
CASE CONTINUED
History helps narrow the diagnosis
Ms. L’s parents say their daughter has not had prior contact with a therapist or psychiatrist, previous psychiatric diagnoses, hospitalizations, suicide attempts, self-injury, or binging or purging behaviors. Ms. L’s paternal grandfather was diagnosed with schizophrenia, but he is currently employed, lives alone, and has not taken medication for many years. Her mother has hypothyroidism. Ms. L was born at full term via vaginal delivery without cardiac defects or a neonatal intensive care unit stay. Her mother said she did not have postpartum depression or anxiety, a complicated pregnancy, or exposure to tobacco, alcohol, or illicit drug use. Ms. L has no history of childhood seizures or head injury with loss of consciousness. She is an only child, born and raised in a house in a metropolitan area, walked at 13 months, did not require early intervention or speech therapy, and met normal language milestones.
She attended kindergarten at age 6 and progressed throughout public school without regressions in reading, writing, or behavioral manifestations, and did not require a 504 Plan or individualized education program. Ms. L graduated high school in the top 30% of her class, was socially active, and attended a local college. In college, she achieved honor roll, enrolled in a sorority, and was a part of a research lab. Her only medication is oral contraception. She consumes alcohol socially, and reports no cannabis, cigarette, or vaping use. Ms. L says she does not use hallucinogens, stimulants, opiates, or cocaine, and her roommate and family confirm this. She denies recent travel and is sexually active. Ms. L’s urinary and serum toxicology are unremarkable, human chorionic gonadotropin is undetectable, and her sodium level is 133 mEq/L. A measure of serum neutrophils is 6.8 x 109/L and serum lymphocytes is 1.7 x 109/L. Her parents adamantly request a Neurology consultation and further workup, including a lumbar puncture (LP), EEG, and brain imaging (MRI).
This information is useful in ruling out other potential causes of psychosis, such as substance-induced psychosis and neurodevelopmental disorders that can present with psychosis. Additionally, neurodevelopmental abnormalities and psychiatric prodromal symptoms are known precedents in individuals who develop a primary psychotic disorder such as schizophrenia.16 A family history that includes a psychotic illness may increase the likelihood of a primary psychotic disorder in offspring; however, clinicians must also consider the accuracy of diagnosis in the family, as this can often be inaccurate or influenced by historical cultural bias. We recommend further elucidating the likelihood of a genetic predisposition to a primary psychotic disorder by clarifying familial medication history and functionality.
For example, the fact that Ms. L’s grandfather has not taken medication for many years and has a high degree of functioning and/or absence of cognitive deficits would lower our suspicion for an accurate diagnosis of schizophrenia (given the typical cognitive decline with untreated illness). Another piece of family history relevant to autoimmune encephalitis includes the propensity for autoimmune disorders, but expert opinion on this matter is mixed.17 Ms. L’s mother has hypothyroidism, which is commonly caused by a prior episode of Hashimoto’s autoimmune thyroiditis. Some physicians advocate for measuring antithyroid antibodies and erythrocyte sedimentation rate or C-reactive protein to gauge the level of autoimmunity, but the usefulness of these measures for detecting autoimmune encephalitis is unclear. These serum markers can be useful in detecting additional important etiologies such as systemic infection or systemic inflammation, and there are conditions such as steroid-responsive encephalopathy with associated thyroiditis, which, as the name suggests, responds to steroids rather than other psychotropic medications. Other risk factors for autoimmune encephalitis include being female, being young, having viral infections (eg, HSV), prior tumor burden, and being in the postpartum period.18 Some experts also suggest the presence of neurologic symptoms 4 weeks after the first psychiatric or cognitive symptom presentation increases the likelihood of anti-NMDA receptor encephalitis, and a lack of neurologic symptoms would make this diagnosis less likely.6,19
Continue to: Another item of interest...
Another item of interest in Ms. L’s case is her parents’ request for a Neurology consultation and further workup, as there is an association between caregiver request for workup and eventual diagnosis.6 While the etiology of this phenomenon is unclear, the literature suggests individuals with autoimmune encephalitis who initially present to Psychiatry experience longer delays to the appropriate treatment with immunomodulatory therapy than those who first present to Neurology.20
Laboratory and diagnostic testing
Guasp et al2 recommend EEG, MRI, and serum autoimmune antibodies (ie, screening for anti-NMDA receptor antibodies) for patients who present with first-episode psychosis, even in the absence of some of the red flags previously discussed. A recent economic analysis suggested screening all patients with first-episode psychosis for serum antibodies may be cost-effective.21
For patients whose presentations include features concerning for anti-NMDA receptor encephalitis, an EEG and MRI are reasonable. In a review of EEG abnormalities in anti-NMDA receptor encephalitis, Gillinder et al23 noted that while 30% did not have initial findings, 83.6% of those with confirmed anti-NMDA receptor encephalitis demonstrated EEG abnormalities; the most common were generalized slowing, delta slowing, and focal abnormalities. Discovering an extreme delta-brush activity on EEG is specific for anti-NMDA receptor encephalitis, but its absence is not fully informative. Practically, slowing can be a nonspecific manifestation of encephalopathy or a medication effect, and many people who present with first-episode psychosis will have recently received antipsychotics, which alter EEG frequency. In a study of EEG changes with antipsychotics, Centorrino et al24 found that generalized background slowing into the theta range across all antipsychotics was not significantly different from control participants, while theta to delta range slowing occurred in 8.2% of those receiving antipsychotics vs 3.3% of controls. Clozapine and olanzapine may be associated with greater EEG abnormalities, while haloperidol and quetiapine contribute a lower risk.25 For young patients with first-episode psychosis without a clear alternative explanation, we advocate for further autoimmune encephalitis workup among all individuals with generalized theta or delta wave slowing.
Because these medication effects are most likely to decrease specificity but not sensitivity of EEG for autoimmune encephalitis, a normal EEG without slowing can be reassuring.26 Moreover, for patients who receive neuroimaging, an MRI may detect inflammation that is not visible on CT. The concerning findings for anti-NMDA receptor encephalitis are temporal or multifocal T2 hyperintensities, though the MRI is normal in most cases and thus should not be reassuring if other concerning features are present.27
The role of lumbar puncture
Another area of active debate surrounds the usefulness and timing of LP. Guasp et al2 proposed that all individuals with first-episode psychosis and focal neurologic findings should receive LP and CSF antineuronal antibody testing. They recommend that patients with first-episode psychosis without focal neurologic findings also should receive LP and CSF testing if ≥1 of the following is present:
- slowing on EEG
- temporal or multifocal T2 hyperintensities on MRI
- positive anti-NMDA receptor antibody in the serum.2
Continue to: Evidence suggests that basic CSF parameters...
Evidence suggests that basic CSF parameters, such as elevated protein and white blood cell counts, are some of the most sensitive and specific tests for autoimmune encephalitis.2 Thus, if the patient is amenable and logistical factors are in place, it may be reasonable to pursue LP earlier in some cases without waiting for serum antibody assays to return (these results can take several weeks). CSF inflammatory changes without neuronal antibodies should lead to other diagnostic considerations (eg, systemic inflammatory disease, psychosis attributed to systemic lupus erythematosus).7 While nonspecific, serum laboratory values that may increase suspicion of anti-NMDA receptor encephalitis include hyponatremia6 and an elevated neutrophil-to-lymphocyte ratio (NLR).28 An NLR >4 in conjunction with CSF albumin-to- serum albumin ratio >7 is associated with impaired blood brain barrier integrity and a worse prognosis for those with anti-NMDA receptor encephalitis.28
Additional clinical features that may sway decisions in favor of obtaining LP despite negative findings on EEG, MRI, and serum antibodies include increased adverse reactions to antipsychotics (eg, neuroleptic malignant syndrome), prodromal infectious symptoms, known tumor, or new-onset neurologic symptoms after initial evaluation.2,8
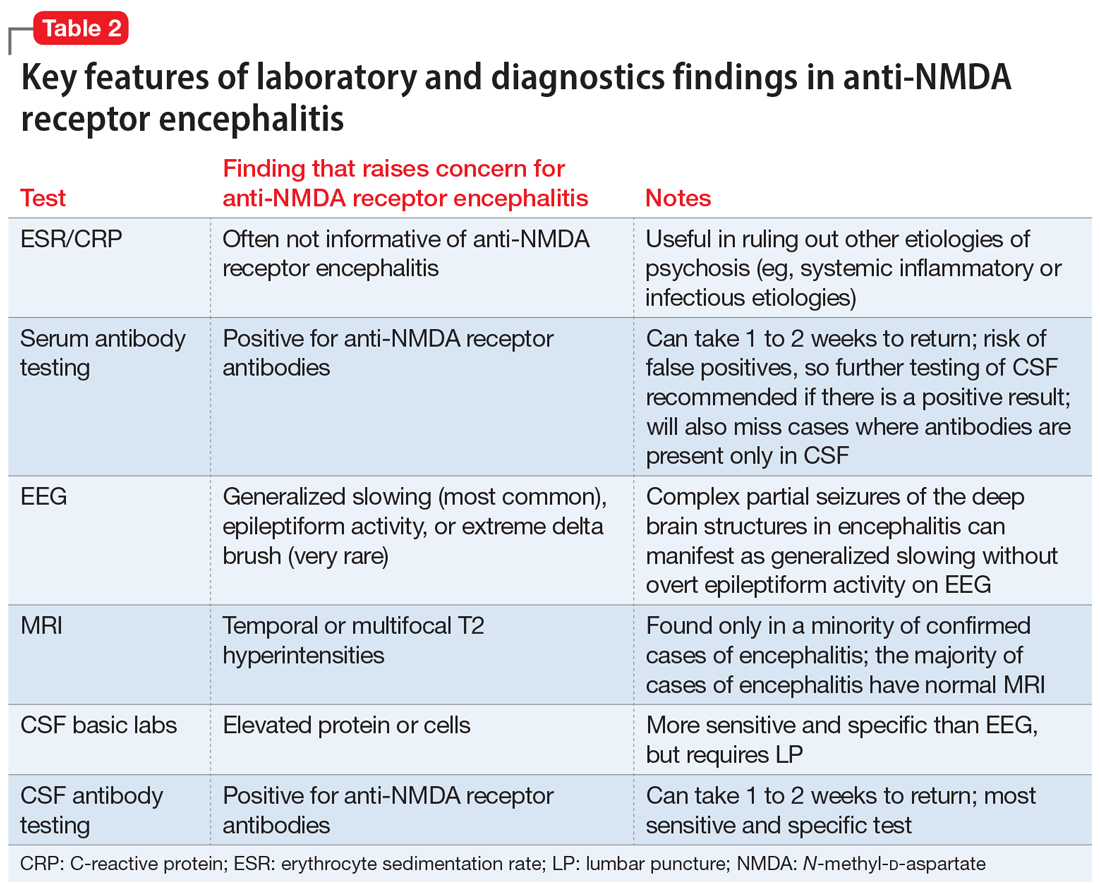
Table 2 summarizes key features of laboratory and diagnostic findings in anti-NMDA receptor encephalitis.
When should you pursue a more extensive workup?
There are some practical tools and rating scales to help clinicians conceptualize risk for autoimmune encephalitis. For psychiatric purposes, however, many of these scales assume that LP, MRI, and EEG have already been completed, and thus it is challenging to incorporate them into psychiatric practice. One such tool is the Antibody Prevalence in Epilepsy and Encephalopathy scale; a score ≥4 is 98% sensitive and 78% to 84% specific for predicting antineural autoantibody positivity.10 Table 3 describes warning signs that may be useful in helping clinicians decide how urgently to pursue a more extensive workup in the possibility of autoimmune encephalitis.
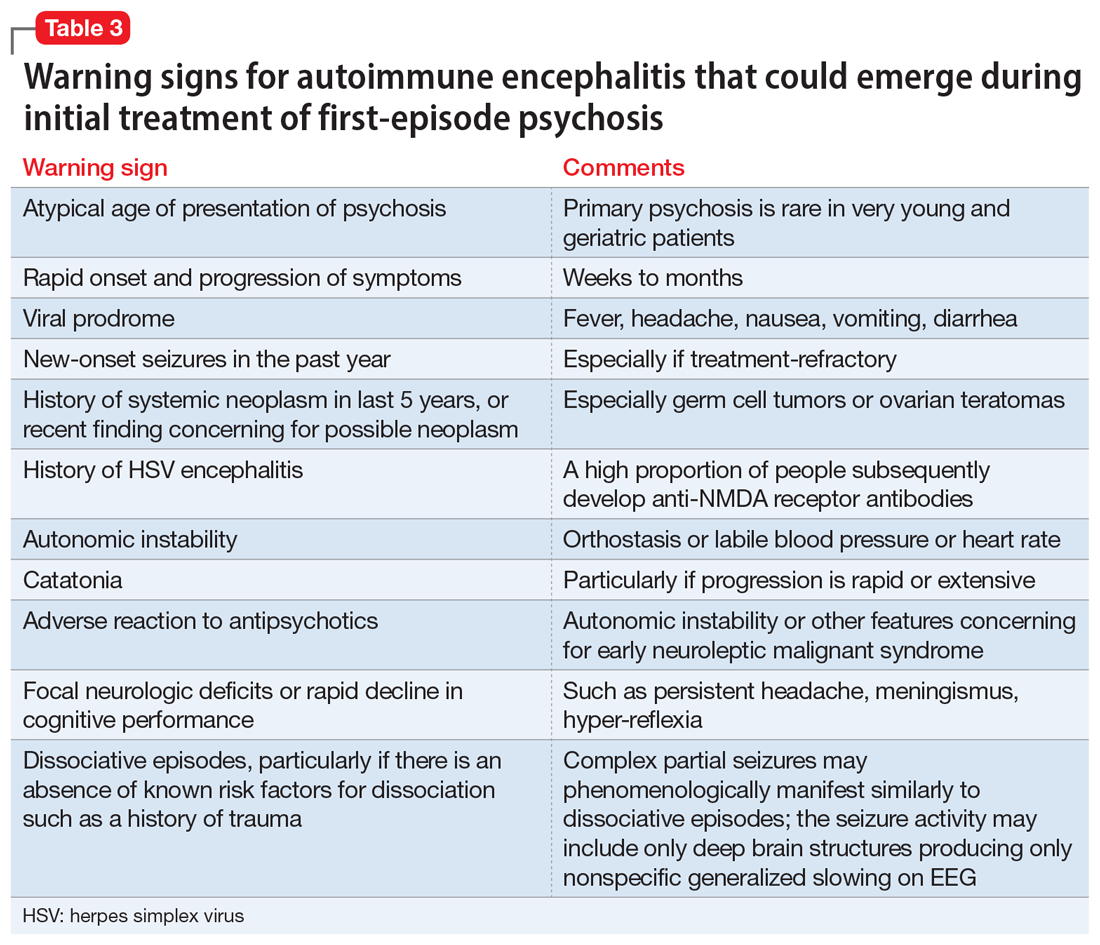
The importance of catching anti-NMDA receptor encephalitis is underscored by the fact that appropriate treatment is very different than for primary psychosis, and outcomes worsen with delay to appropriate treatment.20 Without treatment, severe cases may progress to autonomic instability, altered consciousness, and respiratory compromise warranting admission to an intensive care unit. While the details are beyond the scope of this review, the recommended treatment for confirmed cases of anti-NMDA receptor encephalitis includes tumor removal (if indicated), reducing inflammation (steroids), removing antibodies via IV immunoglobulins, or plasma exchange.8,29 Progression of the disease may warrant consideration of rituximab or cyclophosphamide. In nonresponsive cases, third-line treatments include proteasome inhibitors or interleukin-6 receptor antagonists.8 For patients with severe catatonia, some studies have investigated the utility of electroconvulsive therapy.30 Conceptually, clinicians may consider the utility of antipsychotics as similar to recommendations for hyperactive delirium for the management of psychotic symptoms, agitation, or insomnia. However, given the risk for antipsychotic intolerance, using the lowest effective dose and vigilant screening for the emergence of extrapyramidal symptoms, fever, and autonomic instability is recommended.
CASE CONTINUED
Finally, something objective
Ms. L receives haloperidol 2 mg and undergoes an MRI without contrast. Findings are unremarkable. A spot EEG notes diffuse background slowing in the theta range, prompting lumbar puncture. Findings note 0.40 g/L, 0.2 g/L, and 3.5 for the total protein, albumin, and albumin/CSF-serum quotient (QAlb), respectively; all values are within normal limits. A mild lymphocytic pleocytosis is present as evidenced by a cell count of 35 cells/µL. The CSF is sent for qualitative examination of immunoglobulin G and electrophoresis of proteins in the CSF and serum, of which an increased concentration of restricted bands (oligoclonal bands) in the CSF but not the serum would indicate findings of oligoclonal bands. CSF is sent for detection of anti-NMDA receptor antibodies by indirect immunofluorescence, with a plan to involve an interdisciplinary team for treatment if the antibodies return positive and to manage the case symptomatically in the interim.
Bottom Line
A small subpopulation of patients who present with apparent first-episode psychosis will have symptoms caused by autoimmune encephalitis (specifically, anti-NMDA receptor encephalitis). We provide 4 screening questions to determine when to pursue a workup for an autoimmune encephalitis, and describe relevant clinical symptoms and warning signs to help differentiate the 2 conditions.
Related Resources
- Askandaryan AS, Naqvi A, Varughese A, et al. Anti-N-methyl-D-aspartate receptor encephalitis: neuropsychiatric and multidisciplinary approach to a patient not responding to first-line treatment. Cureus. 2022;14(6):e25751.
- Kayser MS, Titulaer MJ, Gresa-Arribas N, et al. Frequency and characteristics of isolated psychiatric episodes in anti-NMDA receptor encephalitis. JAMA Neurol. 2013;70(9):1133-1139.
Drug Brand Names
Clozapine • Clozaril
Haloperidol • Haldol
Olanzapine • Zyprexa
Quetiapine • Seroquel
Rituximab • Rituxan
1. Granerod J, Ambrose HE, Davies NW, et al; UK Health Protection Agency (HPA) Aetiology of Encephalitis Study Group. Causes of encephalitis and differences in their clinical presentations in England: a multicentre, population-based prospective study. Lancet Infect Dis. 2010;10(12):835-44. doi:10.1016/S1473-3099(10)70222-X
2. Guasp M, Giné-Servén E, Maudes E, et al. Clinical, neuroimmunologic, and CSF investigations in first episode psychosis. Neurology. 2021;97(1):e61-e75.
3. From the American Association of Neurological Surgeons (AANS), American Society of Neuroradiology (ASNR), Cardiovascular and Interventional Radiology Society of Europe (CIRSE), Canadian Interventional Radiology Association (CIRA), Congress of Neurological Surgeons (CNS), European Society of Minimally Invasive Neurological Therapy (ESMINT), European Society of Neuroradiology (ESNR), European Stroke Organization (ESO), Society for Cardiovascular Angiography and Interventions (SCAI), Society of Interventional Radiology (SIR), Society of NeuroInterventional Surgery (SNIS), and World Stroke Organization (WSO), Sacks D, Baxter B, Campbell BCV, et al. Multisociety consensus quality improvement revised consensus statement for endovascular therapy of acute ischemic stroke. Int J Stroke. 2018;13(6):612-632. doi:10.1177/1747493018778713
4. Pollak TA, Lennox BR, Muller S, et al. Autoimmune psychosis: an international consensus on an approach to the diagnosis and management of psychosis of suspected autoimmune origin. Lancet Psychiatry. 2020;7(1):93-108.
5. Guasp M, Módena Y, Armangue T, et al. Clinical features of seronegative, but CSF antibody-positive, anti-NMDA receptor encephalitis. Neurol Neuroimmunol Neuroinflamm. 2020;7(2):e659.
6. Herken J, Prüss H. Red flags: clinical signs for identifying autoimmune encephalitis in psychiatric patients. Front Psychiatry. 2017;8:25. doi:10.3389/fpsyt.2017.00025
7. Graus F, Titulaer MJ, Balu R, et al. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol. 2016;15(4):391-404.
8. Dalmau J, Armangue T, Planaguma J, et al. An update on anti-NMDA receptor encephalitis for neurologists and psychiatrists: mechanisms and models. Lancet Neurol. 2019;18(11):1045-1057.
9. Rattay TW, Martin P, Vittore D, et al. Cerebrospinal fluid findings in patients with psychotic symptoms—a retrospective analysis. Sci Rep. 2021;11(1):7169.
10. Dubey D, Pittock SJ, McKeon A. Antibody prevalence in epilepsy and encephalopathy score: increased specificity and applicability. Epilepsia. 2019;60(2):367-369.
11. Maj M, van Os J, De Hert M, et al. The clinical characterization of the patient with primary psychosis aimed at personalization of management. World Psychiatry. 2021;20(1):4-33. doi:10.1002/wps.20809
12. Caplan JP, Binius T, Lennon VA, et al. Pseudopseudoseizures: conditions that may mimic psychogenic non-epileptic seizures. Psychosomatics. 2011;52(6):501-506.
13. Armangue T, Spatola M, Vlagea A, et al. Frequency, symptoms, risk factors, and outcomes of autoimmune encephalitis after herpes simplex encephalitis: a prospective observational study and retrospective analysis. Lancet Neurol. 2018;17(9):760-772.
14. Takamatsu K, Nakane S. Autonomic manifestations in autoimmune encephalitis. Neurol Clin Neurosci. 2022;10:130-136. doi:10.1111/ncn3.12557
15. Espinola-Nadurille M, Flores-Rivera J, Rivas-Alonso V, et al. Catatonia in patients with anti-NMDA receptor encephalitis. Psychiatry Clin Neurosci. 2019;73(9):574-580.
16. Keshavan M, Montrose DM, Rajarethinam R, et al. Psychopathology among offspring of parents with schizophrenia: relationship to premorbid impairments. Schizophr Res. 2008;103(1-3):114-120.
17. Jeppesen R, Benros ME. Autoimmune diseases and psychotic disorders. Front Psychiatry. 2019;10:131.
18. Bergink V, Armangue T, Titulaer MJ, et al. Autoimmune encephalitis in postpartum psychosis. Am J Psychiatry. 2015;172(9):901-908.
19. Dalmau J, Gleichman AJ, Hughes EG, et al. Anti-NMDA-receptor encephalitis: case series and analysis of the effects of antibodies. Lancet Neurol. 2008;7(12):1091-8. doi: 10.1016/S1474-4422(08)70224-2
20. Titulaer MJ, McCracken L, Gabilondo I, et al. Treatment and prognostic factors for long-term outcome in patients with anti-NMDA receptor encephalitis: an observational cohort study. Lancet Neurol. 2013;12(2):157-165.
21. Ross EL, Becker JE, Linnoila JJ, et al. Cost-effectiveness of routine screening for autoimmune encephalitis in patients with first-episode psychosis in the United States. J Clin Psychiatry. 2020;82(1):19m13168.
22. Sonderen AV, Arends S, Tavy DLJ, et al. Predictive value of electroencephalography in anti-NMDA receptor encephalitis. J Neurol Neurosurg Psychiatry. 2018;89(10):1101-1106.
23. Gillinder L, Warren N, Hartel G, et al. EEG findings in NMDA encephalitis--a systematic review. Seizure. 2019;65:20-24.
24. Centorrino F, Price BH, Tuttle M, et al. EEG abnormalities during treatment with typical and atypical antipsychotics. Am J Psychiatry. 2002;159(1):109-115.
25. Raymond N, Lizano P, Kelly S, et al. What can clozapine’s effect on neural oscillations tell us about its therapeutic effects? A scoping review and synthesis. Biomarkers in Neuropsychiatry. 2022;6:100048.
26. Kaufman DM, Geyer H, Milstein MJ. Kaufman’s Clinical Neurology for Psychiatrists. 8th ed. Elsevier Inc; 2016.
27. Kelley BP, Patel SC, Marin HL, et al. Autoimmune encephalitis: pathophysiology and imaging review of an overlooked diagnosis. AJNR Am J Neuroradiol. 2017;38(6):1070-1078.
28. Yu Y, Wu Y, Cao X, et al. The clinical features and prognosis of anti-NMDAR encephalitis depends on blood brain barrier integrity. Mult Scler Relat Disord. 2021;47:102604.
29. Dalmau J, Graus F. Antibody-mediated neuropsychiatric disorders. J Allergy Clin Immunol. 2022;149(1):37-40.
30. Warren N, Grote V, O’Gorman C, et al. Electroconvulsive therapy for anti-N-methyl-daspartate (NMDA) receptor encephalitis: a systematic review of cases. Brain Stimul. 2019;12(2):329-334.
Hidden within routine presentations of first-episode psychosis is a rare subpopulation whose symptoms are mediated by an autoimmune process for which proper treatment differs significantly from standard care for typical psychotic illness. In this article, we present a hypothetical case and describe how to assess if a patient has an elevated probability of autoimmune encephalitis, determine what diagnostics or medication-induced effects to consider, and identify unresolved questions about best practices.
CASE REPORT
Bizarre behavior and isolation
Ms. L, age 21, is brought to the emergency department (ED) by her college roommate after exhibiting out-of-character behavior and gradual self-isolation over the last 2 months. Her roommate noticed that she had been spending more time isolated in her dorm room and remaining in bed into the early afternoon, though she does not appear to be asleep. Ms. L’s mother is concerned about her daughter’s uncharacteristic refusal to travel home for a family event. Ms. L expresses concern about the intentions of her research preceptor, and recalls messages from the association of colleges telling her to “change her future.” Ms. L hears voices telling her who she can and cannot trust. In the ED, she says she has a headache, experiences mild dizziness while standing, and reports having a brief upper respiratory illness at the end of last semester. Otherwise, a medical review of systems is negative.
Although the etiology of first-episode psychosis can be numerous or unknown, many psychiatrists feel comfortable with the initial diagnostic for this type of clinical presentation. However, for some clinicians, it may be challenging to feel confident in making a diagnosis of autoimmune encephalitis.
Autoimmune encephalitis is a family of syndromes caused by autoantibodies targeting either intracellular or extracellular neuronal antigens. Anti-N-methyl-
In this article, we focus on anti-NMDA receptor encephalitis and use the term interchangeably with autoimmune encephalitis for 2 reasons. First, anti-NMDA receptor encephalitis can present with psychotic symptoms as the only symptoms (prior to cognitive or neurologic manifestations) or can present with psychotic symptoms as the main indicator (with other symptoms that are more subtle and possibly missed). Second, anti-NMDA receptor encephalitis often occurs in young adults, which is when it is common to see the onset of a primary psychotic illness. These 2 factors make it likely that these cases will come into the evaluative sphere of psychiatrists. We give special attention to features of cases of anti-NMDA receptor encephalitis confirmed with antineuronal antibodies in the CSF, as it has emerged that antibodies in the serum can be nonspecific and nonpathogenic.2,3
What does anti-NMDA receptor encephalitis look like?
Symptoms of anti-NMDA receptor encephalitis resemble those of a primary psychotic disorder, which can make it challenging to differentiate between the 2 conditions, and might cause the correct diagnosis to be missed. Pollak et al4 proposed that psychiatrically confusing presentations that don’t clearly match an identifiable psychotic disorder should raise a red flag for an autoimmune etiology. However, studies often fail to describe the specific psychiatric features of anti-NMDA receptor encephalitis, and thus provide little practical evidence to guide diagnosis. In some of the largest studies of patients with anti-NMDA receptor encephalitis, psychiatric clinical findings are often combined into nonspecific headings such as “abnormal behavior” or “behavioral and cognitive” symptoms.5 Such groupings make this the most common clinical finding (95%)5 but make it difficult to discern particular clinical characteristics. Where available, specific symptoms identified across studies include agitation, aggression, changes in mood and/or irritability, insomnia, delusions, hallucinations, and occasionally catatonic features.6,7 Attempts to identify specific psychiatric phenotypes distinct from primary psychotic illnesses have fallen short due to contradictory findings and lack of clinical practicality.8 One exception is the presence of catatonic features, which have been found in CSF-confirmed studies.2 In contrast to the typical teaching that the hallucination modality (eg, visual or tactile) can be helpful in estimating the likelihood of a secondary psychosis (ie, drug-induced, neurodegenerative, or autoimmune), there does not appear to be a difference in hallucination modality between encephalitis and primary psychotic disorders.9
History and review of systems
Another red flag to consider is the rapidity of symptom presentation. Symptoms that progress within 3 months increase the likelihood that the patient has autoimmune encephalitis.10 Cases where collateral information indicates the psychotic episode was preceded by a long, subtle decline in school performance, social withdrawal, and attenuated psychotic symptoms typical of a schizophrenia prodrome are less likely to be an autoimmune psychosis.11 A more delayed presentation does not entirely exclude autoimmune encephalitis; however, a viral-like prodrome before the onset of psychosis increases the likelihood of autoimmune encephalitis. Such a prodrome may include fever, headache, nausea, vomiting, and diarrhea.7
Continue to: Another indication is the presence...
Another indication is the presence of new seizures within 1 year of presenting with psychotic symptoms.10 The possibility of undiagnosed seizures should be considered in a patient with psychosis who has episodes of unresponsiveness, dissociative episodes, or seizure-like activity that is thought to be psychogenic but has not been fully evaluated. Seizures in autoimmune encephalitis involve deep structures in the brain and can be present without overt epileptiform activity on EEG, but rather causing only bilateral slowing that is often described as nonspecific.12
In a young patient presenting with first-episode psychosis, a recent diagnosis of cancer or abnormal finding in the ovaries increases the likelihood of autoimmune encephalitis.4 Historically, however, this type of medical history has been irrelevant to psychosis. Although rare, any person presenting with first-episode psychosis and a history of herpes simplex virus (HSV) encephalitis should be evaluated for autoimmune encephalitis because anti-NMDA receptor antibodies have been reported to be present in approximately one-third of these patients.13 Finally, the report of focal neurologic symptoms, including neck stiffness or neck pain, should raise concern, although sensory, working memory, and cognitive deficits may be difficult to fully distinguish from common somatic and cognitive symptoms in a primary psychiatric presentation.
Table 1 lists 4 questions to ask patients who present with first-episode psychosis that may not usually be part of a typical evaluation.

CASE CONTINUED
Uncooperative with examination
In the ED, Ms. L’s heart rate is 101 beats per minute and her blood pressure is 102/72 mm Hg. Her body mass index (BMI) is 22, which suggests an approximate 8-pound weight loss since her BMI was last assessed. Ms. L responds to questions with 1- to 6-word sentences, without clear verbigeration. Though her speech is not pressured, it is of increased rate. Her gaze scans the room, occasionally becoming fixed for 5 to 10 seconds but is aborted by the interviewer’s comment on this behavior. Ms. L efficiently and accurately spells WORLD backwards, then asks “Why?” and refuses to engage in further cognitive testing, stating “Not doing that.” When the interviewer asks “Why not?” she responds “Not doing that.” Her cranial nerves are intact, and she refuses cerebellar testing or requests to assess tone. There are no observed stereotypies, posturing, or echopraxia.
While not necessary for a diagnosis of autoimmune encephalitis, short-term memory loss is a common cognitive finding across studies.5-7 A common clinical finding from a mental status exam is speech disorders, including (but not limited to) increased rates of speech or decreased verbal output.7 Autonomic instability—including tachycardia, markedly labile blood pressures, and orthostasis—all increase the likelihood of autoimmune encephalitis.14 Interpreting a patient’s vital sign changes can be confounded if they are agitated or anxious, or if they are taking an antipsychotic that produces adverse anticholinergic effects. However, vital sign abnormalities that precede medication administration or do not correlate with fluctuations in mental status increase suspicion for an autoimmune encephalitis.
Continue to: In the absence of the adverse effect...
In the absence of the adverse effect of a medication, orthostasis is uncommon in a well-hydrated young person. Some guidelines4 suggest that symptoms of catatonia should be considered a red flag for autoimmune encephalitis. According to the Bush-Francis Catatonia Rating Scale, commonly identified features include immobility, staring, mutism, posturing, withdrawal, rigidity, and gegenhalten.15 Catatonia is common among patients with anti-NDMA receptor encephalitis, though it may not be initially present and could emerge later.2 However, there are documented cases of autoimmune encephalitis where the patient had only isolated features of catatonia, such as echolalia or mutism.2
CASE CONTINUED
History helps narrow the diagnosis
Ms. L’s parents say their daughter has not had prior contact with a therapist or psychiatrist, previous psychiatric diagnoses, hospitalizations, suicide attempts, self-injury, or binging or purging behaviors. Ms. L’s paternal grandfather was diagnosed with schizophrenia, but he is currently employed, lives alone, and has not taken medication for many years. Her mother has hypothyroidism. Ms. L was born at full term via vaginal delivery without cardiac defects or a neonatal intensive care unit stay. Her mother said she did not have postpartum depression or anxiety, a complicated pregnancy, or exposure to tobacco, alcohol, or illicit drug use. Ms. L has no history of childhood seizures or head injury with loss of consciousness. She is an only child, born and raised in a house in a metropolitan area, walked at 13 months, did not require early intervention or speech therapy, and met normal language milestones.
She attended kindergarten at age 6 and progressed throughout public school without regressions in reading, writing, or behavioral manifestations, and did not require a 504 Plan or individualized education program. Ms. L graduated high school in the top 30% of her class, was socially active, and attended a local college. In college, she achieved honor roll, enrolled in a sorority, and was a part of a research lab. Her only medication is oral contraception. She consumes alcohol socially, and reports no cannabis, cigarette, or vaping use. Ms. L says she does not use hallucinogens, stimulants, opiates, or cocaine, and her roommate and family confirm this. She denies recent travel and is sexually active. Ms. L’s urinary and serum toxicology are unremarkable, human chorionic gonadotropin is undetectable, and her sodium level is 133 mEq/L. A measure of serum neutrophils is 6.8 x 109/L and serum lymphocytes is 1.7 x 109/L. Her parents adamantly request a Neurology consultation and further workup, including a lumbar puncture (LP), EEG, and brain imaging (MRI).
This information is useful in ruling out other potential causes of psychosis, such as substance-induced psychosis and neurodevelopmental disorders that can present with psychosis. Additionally, neurodevelopmental abnormalities and psychiatric prodromal symptoms are known precedents in individuals who develop a primary psychotic disorder such as schizophrenia.16 A family history that includes a psychotic illness may increase the likelihood of a primary psychotic disorder in offspring; however, clinicians must also consider the accuracy of diagnosis in the family, as this can often be inaccurate or influenced by historical cultural bias. We recommend further elucidating the likelihood of a genetic predisposition to a primary psychotic disorder by clarifying familial medication history and functionality.
For example, the fact that Ms. L’s grandfather has not taken medication for many years and has a high degree of functioning and/or absence of cognitive deficits would lower our suspicion for an accurate diagnosis of schizophrenia (given the typical cognitive decline with untreated illness). Another piece of family history relevant to autoimmune encephalitis includes the propensity for autoimmune disorders, but expert opinion on this matter is mixed.17 Ms. L’s mother has hypothyroidism, which is commonly caused by a prior episode of Hashimoto’s autoimmune thyroiditis. Some physicians advocate for measuring antithyroid antibodies and erythrocyte sedimentation rate or C-reactive protein to gauge the level of autoimmunity, but the usefulness of these measures for detecting autoimmune encephalitis is unclear. These serum markers can be useful in detecting additional important etiologies such as systemic infection or systemic inflammation, and there are conditions such as steroid-responsive encephalopathy with associated thyroiditis, which, as the name suggests, responds to steroids rather than other psychotropic medications. Other risk factors for autoimmune encephalitis include being female, being young, having viral infections (eg, HSV), prior tumor burden, and being in the postpartum period.18 Some experts also suggest the presence of neurologic symptoms 4 weeks after the first psychiatric or cognitive symptom presentation increases the likelihood of anti-NMDA receptor encephalitis, and a lack of neurologic symptoms would make this diagnosis less likely.6,19
Continue to: Another item of interest...
Another item of interest in Ms. L’s case is her parents’ request for a Neurology consultation and further workup, as there is an association between caregiver request for workup and eventual diagnosis.6 While the etiology of this phenomenon is unclear, the literature suggests individuals with autoimmune encephalitis who initially present to Psychiatry experience longer delays to the appropriate treatment with immunomodulatory therapy than those who first present to Neurology.20
Laboratory and diagnostic testing
Guasp et al2 recommend EEG, MRI, and serum autoimmune antibodies (ie, screening for anti-NMDA receptor antibodies) for patients who present with first-episode psychosis, even in the absence of some of the red flags previously discussed. A recent economic analysis suggested screening all patients with first-episode psychosis for serum antibodies may be cost-effective.21
For patients whose presentations include features concerning for anti-NMDA receptor encephalitis, an EEG and MRI are reasonable. In a review of EEG abnormalities in anti-NMDA receptor encephalitis, Gillinder et al23 noted that while 30% did not have initial findings, 83.6% of those with confirmed anti-NMDA receptor encephalitis demonstrated EEG abnormalities; the most common were generalized slowing, delta slowing, and focal abnormalities. Discovering an extreme delta-brush activity on EEG is specific for anti-NMDA receptor encephalitis, but its absence is not fully informative. Practically, slowing can be a nonspecific manifestation of encephalopathy or a medication effect, and many people who present with first-episode psychosis will have recently received antipsychotics, which alter EEG frequency. In a study of EEG changes with antipsychotics, Centorrino et al24 found that generalized background slowing into the theta range across all antipsychotics was not significantly different from control participants, while theta to delta range slowing occurred in 8.2% of those receiving antipsychotics vs 3.3% of controls. Clozapine and olanzapine may be associated with greater EEG abnormalities, while haloperidol and quetiapine contribute a lower risk.25 For young patients with first-episode psychosis without a clear alternative explanation, we advocate for further autoimmune encephalitis workup among all individuals with generalized theta or delta wave slowing.
Because these medication effects are most likely to decrease specificity but not sensitivity of EEG for autoimmune encephalitis, a normal EEG without slowing can be reassuring.26 Moreover, for patients who receive neuroimaging, an MRI may detect inflammation that is not visible on CT. The concerning findings for anti-NMDA receptor encephalitis are temporal or multifocal T2 hyperintensities, though the MRI is normal in most cases and thus should not be reassuring if other concerning features are present.27
The role of lumbar puncture
Another area of active debate surrounds the usefulness and timing of LP. Guasp et al2 proposed that all individuals with first-episode psychosis and focal neurologic findings should receive LP and CSF antineuronal antibody testing. They recommend that patients with first-episode psychosis without focal neurologic findings also should receive LP and CSF testing if ≥1 of the following is present:
- slowing on EEG
- temporal or multifocal T2 hyperintensities on MRI
- positive anti-NMDA receptor antibody in the serum.2
Continue to: Evidence suggests that basic CSF parameters...
Evidence suggests that basic CSF parameters, such as elevated protein and white blood cell counts, are some of the most sensitive and specific tests for autoimmune encephalitis.2 Thus, if the patient is amenable and logistical factors are in place, it may be reasonable to pursue LP earlier in some cases without waiting for serum antibody assays to return (these results can take several weeks). CSF inflammatory changes without neuronal antibodies should lead to other diagnostic considerations (eg, systemic inflammatory disease, psychosis attributed to systemic lupus erythematosus).7 While nonspecific, serum laboratory values that may increase suspicion of anti-NMDA receptor encephalitis include hyponatremia6 and an elevated neutrophil-to-lymphocyte ratio (NLR).28 An NLR >4 in conjunction with CSF albumin-to- serum albumin ratio >7 is associated with impaired blood brain barrier integrity and a worse prognosis for those with anti-NMDA receptor encephalitis.28
Additional clinical features that may sway decisions in favor of obtaining LP despite negative findings on EEG, MRI, and serum antibodies include increased adverse reactions to antipsychotics (eg, neuroleptic malignant syndrome), prodromal infectious symptoms, known tumor, or new-onset neurologic symptoms after initial evaluation.2,8
Table 2 summarizes key features of laboratory and diagnostic findings in anti-NMDA receptor encephalitis.

When should you pursue a more extensive workup?
There are some practical tools and rating scales to help clinicians conceptualize risk for autoimmune encephalitis. For psychiatric purposes, however, many of these scales assume that LP, MRI, and EEG have already been completed, and thus it is challenging to incorporate them into psychiatric practice. One such tool is the Antibody Prevalence in Epilepsy and Encephalopathy scale; a score ≥4 is 98% sensitive and 78% to 84% specific for predicting antineural autoantibody positivity.10 Table 3 describes warning signs that may be useful in helping clinicians decide how urgently to pursue a more extensive workup in the possibility of autoimmune encephalitis.

The importance of catching anti-NMDA receptor encephalitis is underscored by the fact that appropriate treatment is very different than for primary psychosis, and outcomes worsen with delay to appropriate treatment.20 Without treatment, severe cases may progress to autonomic instability, altered consciousness, and respiratory compromise warranting admission to an intensive care unit. While the details are beyond the scope of this review, the recommended treatment for confirmed cases of anti-NMDA receptor encephalitis includes tumor removal (if indicated), reducing inflammation (steroids), removing antibodies via IV immunoglobulins, or plasma exchange.8,29 Progression of the disease may warrant consideration of rituximab or cyclophosphamide. In nonresponsive cases, third-line treatments include proteasome inhibitors or interleukin-6 receptor antagonists.8 For patients with severe catatonia, some studies have investigated the utility of electroconvulsive therapy.30 Conceptually, clinicians may consider the utility of antipsychotics as similar to recommendations for hyperactive delirium for the management of psychotic symptoms, agitation, or insomnia. However, given the risk for antipsychotic intolerance, using the lowest effective dose and vigilant screening for the emergence of extrapyramidal symptoms, fever, and autonomic instability is recommended.
CASE CONTINUED
Finally, something objective
Ms. L receives haloperidol 2 mg and undergoes an MRI without contrast. Findings are unremarkable. A spot EEG notes diffuse background slowing in the theta range, prompting lumbar puncture. Findings note 0.40 g/L, 0.2 g/L, and 3.5 for the total protein, albumin, and albumin/CSF-serum quotient (QAlb), respectively; all values are within normal limits. A mild lymphocytic pleocytosis is present as evidenced by a cell count of 35 cells/µL. The CSF is sent for qualitative examination of immunoglobulin G and electrophoresis of proteins in the CSF and serum, of which an increased concentration of restricted bands (oligoclonal bands) in the CSF but not the serum would indicate findings of oligoclonal bands. CSF is sent for detection of anti-NMDA receptor antibodies by indirect immunofluorescence, with a plan to involve an interdisciplinary team for treatment if the antibodies return positive and to manage the case symptomatically in the interim.
Bottom Line
A small subpopulation of patients who present with apparent first-episode psychosis will have symptoms caused by autoimmune encephalitis (specifically, anti-NMDA receptor encephalitis). We provide 4 screening questions to determine when to pursue a workup for an autoimmune encephalitis, and describe relevant clinical symptoms and warning signs to help differentiate the 2 conditions.
Related Resources
- Askandaryan AS, Naqvi A, Varughese A, et al. Anti-N-methyl-D-aspartate receptor encephalitis: neuropsychiatric and multidisciplinary approach to a patient not responding to first-line treatment. Cureus. 2022;14(6):e25751.
- Kayser MS, Titulaer MJ, Gresa-Arribas N, et al. Frequency and characteristics of isolated psychiatric episodes in anti-NMDA receptor encephalitis. JAMA Neurol. 2013;70(9):1133-1139.
Drug Brand Names
Clozapine • Clozaril
Haloperidol • Haldol
Olanzapine • Zyprexa
Quetiapine • Seroquel
Rituximab • Rituxan
Hidden within routine presentations of first-episode psychosis is a rare subpopulation whose symptoms are mediated by an autoimmune process for which proper treatment differs significantly from standard care for typical psychotic illness. In this article, we present a hypothetical case and describe how to assess if a patient has an elevated probability of autoimmune encephalitis, determine what diagnostics or medication-induced effects to consider, and identify unresolved questions about best practices.
CASE REPORT
Bizarre behavior and isolation
Ms. L, age 21, is brought to the emergency department (ED) by her college roommate after exhibiting out-of-character behavior and gradual self-isolation over the last 2 months. Her roommate noticed that she had been spending more time isolated in her dorm room and remaining in bed into the early afternoon, though she does not appear to be asleep. Ms. L’s mother is concerned about her daughter’s uncharacteristic refusal to travel home for a family event. Ms. L expresses concern about the intentions of her research preceptor, and recalls messages from the association of colleges telling her to “change her future.” Ms. L hears voices telling her who she can and cannot trust. In the ED, she says she has a headache, experiences mild dizziness while standing, and reports having a brief upper respiratory illness at the end of last semester. Otherwise, a medical review of systems is negative.
Although the etiology of first-episode psychosis can be numerous or unknown, many psychiatrists feel comfortable with the initial diagnostic for this type of clinical presentation. However, for some clinicians, it may be challenging to feel confident in making a diagnosis of autoimmune encephalitis.
Autoimmune encephalitis is a family of syndromes caused by autoantibodies targeting either intracellular or extracellular neuronal antigens. Anti-N-methyl-
In this article, we focus on anti-NMDA receptor encephalitis and use the term interchangeably with autoimmune encephalitis for 2 reasons. First, anti-NMDA receptor encephalitis can present with psychotic symptoms as the only symptoms (prior to cognitive or neurologic manifestations) or can present with psychotic symptoms as the main indicator (with other symptoms that are more subtle and possibly missed). Second, anti-NMDA receptor encephalitis often occurs in young adults, which is when it is common to see the onset of a primary psychotic illness. These 2 factors make it likely that these cases will come into the evaluative sphere of psychiatrists. We give special attention to features of cases of anti-NMDA receptor encephalitis confirmed with antineuronal antibodies in the CSF, as it has emerged that antibodies in the serum can be nonspecific and nonpathogenic.2,3
What does anti-NMDA receptor encephalitis look like?
Symptoms of anti-NMDA receptor encephalitis resemble those of a primary psychotic disorder, which can make it challenging to differentiate between the 2 conditions, and might cause the correct diagnosis to be missed. Pollak et al4 proposed that psychiatrically confusing presentations that don’t clearly match an identifiable psychotic disorder should raise a red flag for an autoimmune etiology. However, studies often fail to describe the specific psychiatric features of anti-NMDA receptor encephalitis, and thus provide little practical evidence to guide diagnosis. In some of the largest studies of patients with anti-NMDA receptor encephalitis, psychiatric clinical findings are often combined into nonspecific headings such as “abnormal behavior” or “behavioral and cognitive” symptoms.5 Such groupings make this the most common clinical finding (95%)5 but make it difficult to discern particular clinical characteristics. Where available, specific symptoms identified across studies include agitation, aggression, changes in mood and/or irritability, insomnia, delusions, hallucinations, and occasionally catatonic features.6,7 Attempts to identify specific psychiatric phenotypes distinct from primary psychotic illnesses have fallen short due to contradictory findings and lack of clinical practicality.8 One exception is the presence of catatonic features, which have been found in CSF-confirmed studies.2 In contrast to the typical teaching that the hallucination modality (eg, visual or tactile) can be helpful in estimating the likelihood of a secondary psychosis (ie, drug-induced, neurodegenerative, or autoimmune), there does not appear to be a difference in hallucination modality between encephalitis and primary psychotic disorders.9
History and review of systems
Another red flag to consider is the rapidity of symptom presentation. Symptoms that progress within 3 months increase the likelihood that the patient has autoimmune encephalitis.10 Cases where collateral information indicates the psychotic episode was preceded by a long, subtle decline in school performance, social withdrawal, and attenuated psychotic symptoms typical of a schizophrenia prodrome are less likely to be an autoimmune psychosis.11 A more delayed presentation does not entirely exclude autoimmune encephalitis; however, a viral-like prodrome before the onset of psychosis increases the likelihood of autoimmune encephalitis. Such a prodrome may include fever, headache, nausea, vomiting, and diarrhea.7
Continue to: Another indication is the presence...
Another indication is the presence of new seizures within 1 year of presenting with psychotic symptoms.10 The possibility of undiagnosed seizures should be considered in a patient with psychosis who has episodes of unresponsiveness, dissociative episodes, or seizure-like activity that is thought to be psychogenic but has not been fully evaluated. Seizures in autoimmune encephalitis involve deep structures in the brain and can be present without overt epileptiform activity on EEG, but rather causing only bilateral slowing that is often described as nonspecific.12
In a young patient presenting with first-episode psychosis, a recent diagnosis of cancer or abnormal finding in the ovaries increases the likelihood of autoimmune encephalitis.4 Historically, however, this type of medical history has been irrelevant to psychosis. Although rare, any person presenting with first-episode psychosis and a history of herpes simplex virus (HSV) encephalitis should be evaluated for autoimmune encephalitis because anti-NMDA receptor antibodies have been reported to be present in approximately one-third of these patients.13 Finally, the report of focal neurologic symptoms, including neck stiffness or neck pain, should raise concern, although sensory, working memory, and cognitive deficits may be difficult to fully distinguish from common somatic and cognitive symptoms in a primary psychiatric presentation.
Table 1 lists 4 questions to ask patients who present with first-episode psychosis that may not usually be part of a typical evaluation.

CASE CONTINUED
Uncooperative with examination
In the ED, Ms. L’s heart rate is 101 beats per minute and her blood pressure is 102/72 mm Hg. Her body mass index (BMI) is 22, which suggests an approximate 8-pound weight loss since her BMI was last assessed. Ms. L responds to questions with 1- to 6-word sentences, without clear verbigeration. Though her speech is not pressured, it is of increased rate. Her gaze scans the room, occasionally becoming fixed for 5 to 10 seconds but is aborted by the interviewer’s comment on this behavior. Ms. L efficiently and accurately spells WORLD backwards, then asks “Why?” and refuses to engage in further cognitive testing, stating “Not doing that.” When the interviewer asks “Why not?” she responds “Not doing that.” Her cranial nerves are intact, and she refuses cerebellar testing or requests to assess tone. There are no observed stereotypies, posturing, or echopraxia.
While not necessary for a diagnosis of autoimmune encephalitis, short-term memory loss is a common cognitive finding across studies.5-7 A common clinical finding from a mental status exam is speech disorders, including (but not limited to) increased rates of speech or decreased verbal output.7 Autonomic instability—including tachycardia, markedly labile blood pressures, and orthostasis—all increase the likelihood of autoimmune encephalitis.14 Interpreting a patient’s vital sign changes can be confounded if they are agitated or anxious, or if they are taking an antipsychotic that produces adverse anticholinergic effects. However, vital sign abnormalities that precede medication administration or do not correlate with fluctuations in mental status increase suspicion for an autoimmune encephalitis.
Continue to: In the absence of the adverse effect...
In the absence of the adverse effect of a medication, orthostasis is uncommon in a well-hydrated young person. Some guidelines4 suggest that symptoms of catatonia should be considered a red flag for autoimmune encephalitis. According to the Bush-Francis Catatonia Rating Scale, commonly identified features include immobility, staring, mutism, posturing, withdrawal, rigidity, and gegenhalten.15 Catatonia is common among patients with anti-NDMA receptor encephalitis, though it may not be initially present and could emerge later.2 However, there are documented cases of autoimmune encephalitis where the patient had only isolated features of catatonia, such as echolalia or mutism.2
CASE CONTINUED
History helps narrow the diagnosis
Ms. L’s parents say their daughter has not had prior contact with a therapist or psychiatrist, previous psychiatric diagnoses, hospitalizations, suicide attempts, self-injury, or binging or purging behaviors. Ms. L’s paternal grandfather was diagnosed with schizophrenia, but he is currently employed, lives alone, and has not taken medication for many years. Her mother has hypothyroidism. Ms. L was born at full term via vaginal delivery without cardiac defects or a neonatal intensive care unit stay. Her mother said she did not have postpartum depression or anxiety, a complicated pregnancy, or exposure to tobacco, alcohol, or illicit drug use. Ms. L has no history of childhood seizures or head injury with loss of consciousness. She is an only child, born and raised in a house in a metropolitan area, walked at 13 months, did not require early intervention or speech therapy, and met normal language milestones.
She attended kindergarten at age 6 and progressed throughout public school without regressions in reading, writing, or behavioral manifestations, and did not require a 504 Plan or individualized education program. Ms. L graduated high school in the top 30% of her class, was socially active, and attended a local college. In college, she achieved honor roll, enrolled in a sorority, and was a part of a research lab. Her only medication is oral contraception. She consumes alcohol socially, and reports no cannabis, cigarette, or vaping use. Ms. L says she does not use hallucinogens, stimulants, opiates, or cocaine, and her roommate and family confirm this. She denies recent travel and is sexually active. Ms. L’s urinary and serum toxicology are unremarkable, human chorionic gonadotropin is undetectable, and her sodium level is 133 mEq/L. A measure of serum neutrophils is 6.8 x 109/L and serum lymphocytes is 1.7 x 109/L. Her parents adamantly request a Neurology consultation and further workup, including a lumbar puncture (LP), EEG, and brain imaging (MRI).
This information is useful in ruling out other potential causes of psychosis, such as substance-induced psychosis and neurodevelopmental disorders that can present with psychosis. Additionally, neurodevelopmental abnormalities and psychiatric prodromal symptoms are known precedents in individuals who develop a primary psychotic disorder such as schizophrenia.16 A family history that includes a psychotic illness may increase the likelihood of a primary psychotic disorder in offspring; however, clinicians must also consider the accuracy of diagnosis in the family, as this can often be inaccurate or influenced by historical cultural bias. We recommend further elucidating the likelihood of a genetic predisposition to a primary psychotic disorder by clarifying familial medication history and functionality.
For example, the fact that Ms. L’s grandfather has not taken medication for many years and has a high degree of functioning and/or absence of cognitive deficits would lower our suspicion for an accurate diagnosis of schizophrenia (given the typical cognitive decline with untreated illness). Another piece of family history relevant to autoimmune encephalitis includes the propensity for autoimmune disorders, but expert opinion on this matter is mixed.17 Ms. L’s mother has hypothyroidism, which is commonly caused by a prior episode of Hashimoto’s autoimmune thyroiditis. Some physicians advocate for measuring antithyroid antibodies and erythrocyte sedimentation rate or C-reactive protein to gauge the level of autoimmunity, but the usefulness of these measures for detecting autoimmune encephalitis is unclear. These serum markers can be useful in detecting additional important etiologies such as systemic infection or systemic inflammation, and there are conditions such as steroid-responsive encephalopathy with associated thyroiditis, which, as the name suggests, responds to steroids rather than other psychotropic medications. Other risk factors for autoimmune encephalitis include being female, being young, having viral infections (eg, HSV), prior tumor burden, and being in the postpartum period.18 Some experts also suggest the presence of neurologic symptoms 4 weeks after the first psychiatric or cognitive symptom presentation increases the likelihood of anti-NMDA receptor encephalitis, and a lack of neurologic symptoms would make this diagnosis less likely.6,19
Continue to: Another item of interest...
Another item of interest in Ms. L’s case is her parents’ request for a Neurology consultation and further workup, as there is an association between caregiver request for workup and eventual diagnosis.6 While the etiology of this phenomenon is unclear, the literature suggests individuals with autoimmune encephalitis who initially present to Psychiatry experience longer delays to the appropriate treatment with immunomodulatory therapy than those who first present to Neurology.20
Laboratory and diagnostic testing
Guasp et al2 recommend EEG, MRI, and serum autoimmune antibodies (ie, screening for anti-NMDA receptor antibodies) for patients who present with first-episode psychosis, even in the absence of some of the red flags previously discussed. A recent economic analysis suggested screening all patients with first-episode psychosis for serum antibodies may be cost-effective.21
For patients whose presentations include features concerning for anti-NMDA receptor encephalitis, an EEG and MRI are reasonable. In a review of EEG abnormalities in anti-NMDA receptor encephalitis, Gillinder et al23 noted that while 30% did not have initial findings, 83.6% of those with confirmed anti-NMDA receptor encephalitis demonstrated EEG abnormalities; the most common were generalized slowing, delta slowing, and focal abnormalities. Discovering an extreme delta-brush activity on EEG is specific for anti-NMDA receptor encephalitis, but its absence is not fully informative. Practically, slowing can be a nonspecific manifestation of encephalopathy or a medication effect, and many people who present with first-episode psychosis will have recently received antipsychotics, which alter EEG frequency. In a study of EEG changes with antipsychotics, Centorrino et al24 found that generalized background slowing into the theta range across all antipsychotics was not significantly different from control participants, while theta to delta range slowing occurred in 8.2% of those receiving antipsychotics vs 3.3% of controls. Clozapine and olanzapine may be associated with greater EEG abnormalities, while haloperidol and quetiapine contribute a lower risk.25 For young patients with first-episode psychosis without a clear alternative explanation, we advocate for further autoimmune encephalitis workup among all individuals with generalized theta or delta wave slowing.
Because these medication effects are most likely to decrease specificity but not sensitivity of EEG for autoimmune encephalitis, a normal EEG without slowing can be reassuring.26 Moreover, for patients who receive neuroimaging, an MRI may detect inflammation that is not visible on CT. The concerning findings for anti-NMDA receptor encephalitis are temporal or multifocal T2 hyperintensities, though the MRI is normal in most cases and thus should not be reassuring if other concerning features are present.27
The role of lumbar puncture
Another area of active debate surrounds the usefulness and timing of LP. Guasp et al2 proposed that all individuals with first-episode psychosis and focal neurologic findings should receive LP and CSF antineuronal antibody testing. They recommend that patients with first-episode psychosis without focal neurologic findings also should receive LP and CSF testing if ≥1 of the following is present:
- slowing on EEG
- temporal or multifocal T2 hyperintensities on MRI
- positive anti-NMDA receptor antibody in the serum.2
Continue to: Evidence suggests that basic CSF parameters...
Evidence suggests that basic CSF parameters, such as elevated protein and white blood cell counts, are some of the most sensitive and specific tests for autoimmune encephalitis.2 Thus, if the patient is amenable and logistical factors are in place, it may be reasonable to pursue LP earlier in some cases without waiting for serum antibody assays to return (these results can take several weeks). CSF inflammatory changes without neuronal antibodies should lead to other diagnostic considerations (eg, systemic inflammatory disease, psychosis attributed to systemic lupus erythematosus).7 While nonspecific, serum laboratory values that may increase suspicion of anti-NMDA receptor encephalitis include hyponatremia6 and an elevated neutrophil-to-lymphocyte ratio (NLR).28 An NLR >4 in conjunction with CSF albumin-to- serum albumin ratio >7 is associated with impaired blood brain barrier integrity and a worse prognosis for those with anti-NMDA receptor encephalitis.28
Additional clinical features that may sway decisions in favor of obtaining LP despite negative findings on EEG, MRI, and serum antibodies include increased adverse reactions to antipsychotics (eg, neuroleptic malignant syndrome), prodromal infectious symptoms, known tumor, or new-onset neurologic symptoms after initial evaluation.2,8
Table 2 summarizes key features of laboratory and diagnostic findings in anti-NMDA receptor encephalitis.

When should you pursue a more extensive workup?
There are some practical tools and rating scales to help clinicians conceptualize risk for autoimmune encephalitis. For psychiatric purposes, however, many of these scales assume that LP, MRI, and EEG have already been completed, and thus it is challenging to incorporate them into psychiatric practice. One such tool is the Antibody Prevalence in Epilepsy and Encephalopathy scale; a score ≥4 is 98% sensitive and 78% to 84% specific for predicting antineural autoantibody positivity.10 Table 3 describes warning signs that may be useful in helping clinicians decide how urgently to pursue a more extensive workup in the possibility of autoimmune encephalitis.

The importance of catching anti-NMDA receptor encephalitis is underscored by the fact that appropriate treatment is very different than for primary psychosis, and outcomes worsen with delay to appropriate treatment.20 Without treatment, severe cases may progress to autonomic instability, altered consciousness, and respiratory compromise warranting admission to an intensive care unit. While the details are beyond the scope of this review, the recommended treatment for confirmed cases of anti-NMDA receptor encephalitis includes tumor removal (if indicated), reducing inflammation (steroids), removing antibodies via IV immunoglobulins, or plasma exchange.8,29 Progression of the disease may warrant consideration of rituximab or cyclophosphamide. In nonresponsive cases, third-line treatments include proteasome inhibitors or interleukin-6 receptor antagonists.8 For patients with severe catatonia, some studies have investigated the utility of electroconvulsive therapy.30 Conceptually, clinicians may consider the utility of antipsychotics as similar to recommendations for hyperactive delirium for the management of psychotic symptoms, agitation, or insomnia. However, given the risk for antipsychotic intolerance, using the lowest effective dose and vigilant screening for the emergence of extrapyramidal symptoms, fever, and autonomic instability is recommended.
CASE CONTINUED
Finally, something objective
Ms. L receives haloperidol 2 mg and undergoes an MRI without contrast. Findings are unremarkable. A spot EEG notes diffuse background slowing in the theta range, prompting lumbar puncture. Findings note 0.40 g/L, 0.2 g/L, and 3.5 for the total protein, albumin, and albumin/CSF-serum quotient (QAlb), respectively; all values are within normal limits. A mild lymphocytic pleocytosis is present as evidenced by a cell count of 35 cells/µL. The CSF is sent for qualitative examination of immunoglobulin G and electrophoresis of proteins in the CSF and serum, of which an increased concentration of restricted bands (oligoclonal bands) in the CSF but not the serum would indicate findings of oligoclonal bands. CSF is sent for detection of anti-NMDA receptor antibodies by indirect immunofluorescence, with a plan to involve an interdisciplinary team for treatment if the antibodies return positive and to manage the case symptomatically in the interim.
Bottom Line
A small subpopulation of patients who present with apparent first-episode psychosis will have symptoms caused by autoimmune encephalitis (specifically, anti-NMDA receptor encephalitis). We provide 4 screening questions to determine when to pursue a workup for an autoimmune encephalitis, and describe relevant clinical symptoms and warning signs to help differentiate the 2 conditions.
Related Resources
- Askandaryan AS, Naqvi A, Varughese A, et al. Anti-N-methyl-D-aspartate receptor encephalitis: neuropsychiatric and multidisciplinary approach to a patient not responding to first-line treatment. Cureus. 2022;14(6):e25751.
- Kayser MS, Titulaer MJ, Gresa-Arribas N, et al. Frequency and characteristics of isolated psychiatric episodes in anti-NMDA receptor encephalitis. JAMA Neurol. 2013;70(9):1133-1139.
Drug Brand Names
Clozapine • Clozaril
Haloperidol • Haldol
Olanzapine • Zyprexa
Quetiapine • Seroquel
Rituximab • Rituxan
1. Granerod J, Ambrose HE, Davies NW, et al; UK Health Protection Agency (HPA) Aetiology of Encephalitis Study Group. Causes of encephalitis and differences in their clinical presentations in England: a multicentre, population-based prospective study. Lancet Infect Dis. 2010;10(12):835-44. doi:10.1016/S1473-3099(10)70222-X
2. Guasp M, Giné-Servén E, Maudes E, et al. Clinical, neuroimmunologic, and CSF investigations in first episode psychosis. Neurology. 2021;97(1):e61-e75.
3. From the American Association of Neurological Surgeons (AANS), American Society of Neuroradiology (ASNR), Cardiovascular and Interventional Radiology Society of Europe (CIRSE), Canadian Interventional Radiology Association (CIRA), Congress of Neurological Surgeons (CNS), European Society of Minimally Invasive Neurological Therapy (ESMINT), European Society of Neuroradiology (ESNR), European Stroke Organization (ESO), Society for Cardiovascular Angiography and Interventions (SCAI), Society of Interventional Radiology (SIR), Society of NeuroInterventional Surgery (SNIS), and World Stroke Organization (WSO), Sacks D, Baxter B, Campbell BCV, et al. Multisociety consensus quality improvement revised consensus statement for endovascular therapy of acute ischemic stroke. Int J Stroke. 2018;13(6):612-632. doi:10.1177/1747493018778713
4. Pollak TA, Lennox BR, Muller S, et al. Autoimmune psychosis: an international consensus on an approach to the diagnosis and management of psychosis of suspected autoimmune origin. Lancet Psychiatry. 2020;7(1):93-108.
5. Guasp M, Módena Y, Armangue T, et al. Clinical features of seronegative, but CSF antibody-positive, anti-NMDA receptor encephalitis. Neurol Neuroimmunol Neuroinflamm. 2020;7(2):e659.
6. Herken J, Prüss H. Red flags: clinical signs for identifying autoimmune encephalitis in psychiatric patients. Front Psychiatry. 2017;8:25. doi:10.3389/fpsyt.2017.00025
7. Graus F, Titulaer MJ, Balu R, et al. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol. 2016;15(4):391-404.
8. Dalmau J, Armangue T, Planaguma J, et al. An update on anti-NMDA receptor encephalitis for neurologists and psychiatrists: mechanisms and models. Lancet Neurol. 2019;18(11):1045-1057.
9. Rattay TW, Martin P, Vittore D, et al. Cerebrospinal fluid findings in patients with psychotic symptoms—a retrospective analysis. Sci Rep. 2021;11(1):7169.
10. Dubey D, Pittock SJ, McKeon A. Antibody prevalence in epilepsy and encephalopathy score: increased specificity and applicability. Epilepsia. 2019;60(2):367-369.
11. Maj M, van Os J, De Hert M, et al. The clinical characterization of the patient with primary psychosis aimed at personalization of management. World Psychiatry. 2021;20(1):4-33. doi:10.1002/wps.20809
12. Caplan JP, Binius T, Lennon VA, et al. Pseudopseudoseizures: conditions that may mimic psychogenic non-epileptic seizures. Psychosomatics. 2011;52(6):501-506.
13. Armangue T, Spatola M, Vlagea A, et al. Frequency, symptoms, risk factors, and outcomes of autoimmune encephalitis after herpes simplex encephalitis: a prospective observational study and retrospective analysis. Lancet Neurol. 2018;17(9):760-772.
14. Takamatsu K, Nakane S. Autonomic manifestations in autoimmune encephalitis. Neurol Clin Neurosci. 2022;10:130-136. doi:10.1111/ncn3.12557
15. Espinola-Nadurille M, Flores-Rivera J, Rivas-Alonso V, et al. Catatonia in patients with anti-NMDA receptor encephalitis. Psychiatry Clin Neurosci. 2019;73(9):574-580.
16. Keshavan M, Montrose DM, Rajarethinam R, et al. Psychopathology among offspring of parents with schizophrenia: relationship to premorbid impairments. Schizophr Res. 2008;103(1-3):114-120.
17. Jeppesen R, Benros ME. Autoimmune diseases and psychotic disorders. Front Psychiatry. 2019;10:131.
18. Bergink V, Armangue T, Titulaer MJ, et al. Autoimmune encephalitis in postpartum psychosis. Am J Psychiatry. 2015;172(9):901-908.
19. Dalmau J, Gleichman AJ, Hughes EG, et al. Anti-NMDA-receptor encephalitis: case series and analysis of the effects of antibodies. Lancet Neurol. 2008;7(12):1091-8. doi: 10.1016/S1474-4422(08)70224-2
20. Titulaer MJ, McCracken L, Gabilondo I, et al. Treatment and prognostic factors for long-term outcome in patients with anti-NMDA receptor encephalitis: an observational cohort study. Lancet Neurol. 2013;12(2):157-165.
21. Ross EL, Becker JE, Linnoila JJ, et al. Cost-effectiveness of routine screening for autoimmune encephalitis in patients with first-episode psychosis in the United States. J Clin Psychiatry. 2020;82(1):19m13168.
22. Sonderen AV, Arends S, Tavy DLJ, et al. Predictive value of electroencephalography in anti-NMDA receptor encephalitis. J Neurol Neurosurg Psychiatry. 2018;89(10):1101-1106.
23. Gillinder L, Warren N, Hartel G, et al. EEG findings in NMDA encephalitis--a systematic review. Seizure. 2019;65:20-24.
24. Centorrino F, Price BH, Tuttle M, et al. EEG abnormalities during treatment with typical and atypical antipsychotics. Am J Psychiatry. 2002;159(1):109-115.
25. Raymond N, Lizano P, Kelly S, et al. What can clozapine’s effect on neural oscillations tell us about its therapeutic effects? A scoping review and synthesis. Biomarkers in Neuropsychiatry. 2022;6:100048.
26. Kaufman DM, Geyer H, Milstein MJ. Kaufman’s Clinical Neurology for Psychiatrists. 8th ed. Elsevier Inc; 2016.
27. Kelley BP, Patel SC, Marin HL, et al. Autoimmune encephalitis: pathophysiology and imaging review of an overlooked diagnosis. AJNR Am J Neuroradiol. 2017;38(6):1070-1078.
28. Yu Y, Wu Y, Cao X, et al. The clinical features and prognosis of anti-NMDAR encephalitis depends on blood brain barrier integrity. Mult Scler Relat Disord. 2021;47:102604.
29. Dalmau J, Graus F. Antibody-mediated neuropsychiatric disorders. J Allergy Clin Immunol. 2022;149(1):37-40.
30. Warren N, Grote V, O’Gorman C, et al. Electroconvulsive therapy for anti-N-methyl-daspartate (NMDA) receptor encephalitis: a systematic review of cases. Brain Stimul. 2019;12(2):329-334.
1. Granerod J, Ambrose HE, Davies NW, et al; UK Health Protection Agency (HPA) Aetiology of Encephalitis Study Group. Causes of encephalitis and differences in their clinical presentations in England: a multicentre, population-based prospective study. Lancet Infect Dis. 2010;10(12):835-44. doi:10.1016/S1473-3099(10)70222-X
2. Guasp M, Giné-Servén E, Maudes E, et al. Clinical, neuroimmunologic, and CSF investigations in first episode psychosis. Neurology. 2021;97(1):e61-e75.
3. From the American Association of Neurological Surgeons (AANS), American Society of Neuroradiology (ASNR), Cardiovascular and Interventional Radiology Society of Europe (CIRSE), Canadian Interventional Radiology Association (CIRA), Congress of Neurological Surgeons (CNS), European Society of Minimally Invasive Neurological Therapy (ESMINT), European Society of Neuroradiology (ESNR), European Stroke Organization (ESO), Society for Cardiovascular Angiography and Interventions (SCAI), Society of Interventional Radiology (SIR), Society of NeuroInterventional Surgery (SNIS), and World Stroke Organization (WSO), Sacks D, Baxter B, Campbell BCV, et al. Multisociety consensus quality improvement revised consensus statement for endovascular therapy of acute ischemic stroke. Int J Stroke. 2018;13(6):612-632. doi:10.1177/1747493018778713
4. Pollak TA, Lennox BR, Muller S, et al. Autoimmune psychosis: an international consensus on an approach to the diagnosis and management of psychosis of suspected autoimmune origin. Lancet Psychiatry. 2020;7(1):93-108.
5. Guasp M, Módena Y, Armangue T, et al. Clinical features of seronegative, but CSF antibody-positive, anti-NMDA receptor encephalitis. Neurol Neuroimmunol Neuroinflamm. 2020;7(2):e659.
6. Herken J, Prüss H. Red flags: clinical signs for identifying autoimmune encephalitis in psychiatric patients. Front Psychiatry. 2017;8:25. doi:10.3389/fpsyt.2017.00025
7. Graus F, Titulaer MJ, Balu R, et al. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol. 2016;15(4):391-404.
8. Dalmau J, Armangue T, Planaguma J, et al. An update on anti-NMDA receptor encephalitis for neurologists and psychiatrists: mechanisms and models. Lancet Neurol. 2019;18(11):1045-1057.
9. Rattay TW, Martin P, Vittore D, et al. Cerebrospinal fluid findings in patients with psychotic symptoms—a retrospective analysis. Sci Rep. 2021;11(1):7169.
10. Dubey D, Pittock SJ, McKeon A. Antibody prevalence in epilepsy and encephalopathy score: increased specificity and applicability. Epilepsia. 2019;60(2):367-369.
11. Maj M, van Os J, De Hert M, et al. The clinical characterization of the patient with primary psychosis aimed at personalization of management. World Psychiatry. 2021;20(1):4-33. doi:10.1002/wps.20809
12. Caplan JP, Binius T, Lennon VA, et al. Pseudopseudoseizures: conditions that may mimic psychogenic non-epileptic seizures. Psychosomatics. 2011;52(6):501-506.
13. Armangue T, Spatola M, Vlagea A, et al. Frequency, symptoms, risk factors, and outcomes of autoimmune encephalitis after herpes simplex encephalitis: a prospective observational study and retrospective analysis. Lancet Neurol. 2018;17(9):760-772.
14. Takamatsu K, Nakane S. Autonomic manifestations in autoimmune encephalitis. Neurol Clin Neurosci. 2022;10:130-136. doi:10.1111/ncn3.12557
15. Espinola-Nadurille M, Flores-Rivera J, Rivas-Alonso V, et al. Catatonia in patients with anti-NMDA receptor encephalitis. Psychiatry Clin Neurosci. 2019;73(9):574-580.
16. Keshavan M, Montrose DM, Rajarethinam R, et al. Psychopathology among offspring of parents with schizophrenia: relationship to premorbid impairments. Schizophr Res. 2008;103(1-3):114-120.
17. Jeppesen R, Benros ME. Autoimmune diseases and psychotic disorders. Front Psychiatry. 2019;10:131.
18. Bergink V, Armangue T, Titulaer MJ, et al. Autoimmune encephalitis in postpartum psychosis. Am J Psychiatry. 2015;172(9):901-908.
19. Dalmau J, Gleichman AJ, Hughes EG, et al. Anti-NMDA-receptor encephalitis: case series and analysis of the effects of antibodies. Lancet Neurol. 2008;7(12):1091-8. doi: 10.1016/S1474-4422(08)70224-2
20. Titulaer MJ, McCracken L, Gabilondo I, et al. Treatment and prognostic factors for long-term outcome in patients with anti-NMDA receptor encephalitis: an observational cohort study. Lancet Neurol. 2013;12(2):157-165.
21. Ross EL, Becker JE, Linnoila JJ, et al. Cost-effectiveness of routine screening for autoimmune encephalitis in patients with first-episode psychosis in the United States. J Clin Psychiatry. 2020;82(1):19m13168.
22. Sonderen AV, Arends S, Tavy DLJ, et al. Predictive value of electroencephalography in anti-NMDA receptor encephalitis. J Neurol Neurosurg Psychiatry. 2018;89(10):1101-1106.
23. Gillinder L, Warren N, Hartel G, et al. EEG findings in NMDA encephalitis--a systematic review. Seizure. 2019;65:20-24.
24. Centorrino F, Price BH, Tuttle M, et al. EEG abnormalities during treatment with typical and atypical antipsychotics. Am J Psychiatry. 2002;159(1):109-115.
25. Raymond N, Lizano P, Kelly S, et al. What can clozapine’s effect on neural oscillations tell us about its therapeutic effects? A scoping review and synthesis. Biomarkers in Neuropsychiatry. 2022;6:100048.
26. Kaufman DM, Geyer H, Milstein MJ. Kaufman’s Clinical Neurology for Psychiatrists. 8th ed. Elsevier Inc; 2016.
27. Kelley BP, Patel SC, Marin HL, et al. Autoimmune encephalitis: pathophysiology and imaging review of an overlooked diagnosis. AJNR Am J Neuroradiol. 2017;38(6):1070-1078.
28. Yu Y, Wu Y, Cao X, et al. The clinical features and prognosis of anti-NMDAR encephalitis depends on blood brain barrier integrity. Mult Scler Relat Disord. 2021;47:102604.
29. Dalmau J, Graus F. Antibody-mediated neuropsychiatric disorders. J Allergy Clin Immunol. 2022;149(1):37-40.
30. Warren N, Grote V, O’Gorman C, et al. Electroconvulsive therapy for anti-N-methyl-daspartate (NMDA) receptor encephalitis: a systematic review of cases. Brain Stimul. 2019;12(2):329-334.
Reversing depression: A plethora of therapeutic strategies and mechanisms
Despite much progress, major depressive disorder (MDD) continues to be a challenging and life-threatening neuropsychiatric disorder. It is highly prevalent and afflicts tens of millions of Americans.
It is also ranked as the No. 1 disabling medical (not just psychiatric) condition by the World Health Organization.1 A significant proportion of patients with MDD do not respond adequately to several rounds of antidepressant medications,2 and many are labeled as having “treatment-resistant depression” (TRD).
In a previous article, I provocatively proposed that TRD is a myth.3 What I meant is that in a heterogeneous syndrome such as depression, failure to respond to 1, 2, or even 3 antidepressants should not imply TRD, because there is a “right treatment” that has not yet been identified for a given depressed patient. Most of those labeled as TRD have simply not yet received the pharmacotherapy or somatic therapy with the requisite mechanism of action for their variant of depression within a heterogeneous syndrome. IV ketamine, which, astonishingly, often reverses severe TRD of chronic duration within a few hours, is a prime example of why the term TRD is often used prematurely. Ketamine’s mechanism of action (immediate neuroplasticity via glutamate N-methyl-
Some clinicians may not be aware of the abundance of mechanisms of action currently available for the treatment of MDD as well as bipolar depression. Many practitioners, in both psychiatry and primary care, usually start the treatment of depression with a selective serotonin reuptake inhibitor, and if that does not produce a response or remission, they might switch to a serotonin-norepinephrine reuptake inhibitor. If that does not control the patient’s depressive symptoms, they start entertaining the notion that the patient may have TRD, not realizing that they have barely scratched the surface of the many therapeutic options and mechanisms of action, one of which could be the “best match” for a given patient.4
There will come a day when “precision psychiatry” finally arrives, and specific biomarkers will be developed to identify the “right” treatment for each patient within the heterogenous syndrome of depression.5 Until that day arrives, the treatment of depression will continue to be a process of trial and error, and hit or miss. But research will eventually discover genetic, neurochemical, neurophysiological, neuroimaging, or neuroimmune biomarkers that will rapidly guide clinicians to the correct treatment. This is critical to avoid inordinate delays in achieving remission and avert the ever-present risk of suicidal behavior.
The Table6 provides an overview of the numerous treatments currently available to manage depression. All increase brain-derived neurotrophic factor and restore healthy neuroplasticity and neurogenesis, which are impaired in MDD and currently believed to be a final common pathway for all depression treatments.7
These 41 therapeutic approaches to treating MDD or bipolar depression reflect the heterogeneity of mechanisms of action to address an equally heterogeneous syndrome. This implies that clinicians have a wide array of on-label options to manage patients with depression, aiming for remission, not just a good response, which typically is defined as a ≥50% reduction in total score on one of the validated rating scales used to quantify depression severity, such as the Montgomery-Åsberg Depression Rating Scale, Hamilton Depression Rating Scale, or Calgary Depression Scale for Schizophrenia.
Continue to: When several FDA-approved pharmacotherapies...
When several FDA-approved pharmacotherapies fall short and produce a suboptimal response, clinicians can resort to other treatment options known to have a higher efficacy than oral antidepressants. These include electroconvulsive therapy, repetitive transcranial magnetic stimulation, and vagus nerve stimulation. Other on-label options include adjunctive therapy with one of the approved second-generation antipsychotic agents or with adjunctive esketamine.
But if the patient still does not improve, one of many emerging off-label treatment options may work. One of the exciting new discoveries is the hallucinogen psilocybin, whose mechanism of action is truly unique. Unlike standard antidepressant medications, which modulate neurotransmitters, psilocybin increases the brain’s network flexibility, decreases the modularity of several key brain networks (especially the default-brain network, or DMN), and alters the dark and distorted mental perspective of depression to a much healthier and optimistic outlook about the self and the world.8 Such novel breakthroughs in the treatment of severe depression will shed some unprecedented insights into the core neurobiology of depression, and may lead to early intervention and prevention.
As the saying goes, all roads lead to Rome. Psychiatric clinicians should rejoice that there are abundant approaches and therapeutic mechanisms to relieve their severely melancholic (and often suicidal) patients from the grips of this disabling and life-altering brain syndrome.
1. World Health Organization. Depression: let’s talk says WHO, as depression tops list of causes of ill health. March 30, 2017. Accessed July 5, 2022. www.who.int/news/item/30-03-2017--depression-let-s-talk-says-who-as-depression-tops-list-of-causes-of-ill-health
2. Trivedi MH, Fava M, Wisniewski SR, et al. Medication augmentation after the failure of SSRIs for depression. N Eng J Med. 2006;354(12)1243-1252.
3. Nasrallah HA. Treatment resistance is a myth! Current Psychiatry. 2021;20(3):14-16,28.
4. Nasrallah HA. 10 Recent paradigm shifts in the neurobiology and treatment of depression. Current Psychiatry. 2015;14(2):10-13.
5. Nasrallah HA. Biomarkers in neuropsychiatric disorders: translating research to clinical applications. Biomarkers in Neuropsychiatry. 2019;1:100001. doi:10.1016/j.bionps.2019.100001
6. Procyshyn RM, Bezchlibnyk-Butler KZ, Jeffries JJ. Clinical Handbook of Psychotropic Drugs. 23rd ed. Hogrefe; 2019.
7. Tartt AN, Mariani, MB, Hen R, et al. Dysregulation of adult hippocampal neuroplasticity in major depression: pathogenesis and therapeutic implications. Mol Psychiatry. 2022;27(6):2689-2699.
8. Lowe H, Toyang N, Steele B, et al. The therapeutic potential of psilocybin. Molecules. 2021;26(10):2948. doi: 10.3390/molecules26102948
Despite much progress, major depressive disorder (MDD) continues to be a challenging and life-threatening neuropsychiatric disorder. It is highly prevalent and afflicts tens of millions of Americans.
It is also ranked as the No. 1 disabling medical (not just psychiatric) condition by the World Health Organization.1 A significant proportion of patients with MDD do not respond adequately to several rounds of antidepressant medications,2 and many are labeled as having “treatment-resistant depression” (TRD).
In a previous article, I provocatively proposed that TRD is a myth.3 What I meant is that in a heterogeneous syndrome such as depression, failure to respond to 1, 2, or even 3 antidepressants should not imply TRD, because there is a “right treatment” that has not yet been identified for a given depressed patient. Most of those labeled as TRD have simply not yet received the pharmacotherapy or somatic therapy with the requisite mechanism of action for their variant of depression within a heterogeneous syndrome. IV ketamine, which, astonishingly, often reverses severe TRD of chronic duration within a few hours, is a prime example of why the term TRD is often used prematurely. Ketamine’s mechanism of action (immediate neuroplasticity via glutamate N-methyl-
Some clinicians may not be aware of the abundance of mechanisms of action currently available for the treatment of MDD as well as bipolar depression. Many practitioners, in both psychiatry and primary care, usually start the treatment of depression with a selective serotonin reuptake inhibitor, and if that does not produce a response or remission, they might switch to a serotonin-norepinephrine reuptake inhibitor. If that does not control the patient’s depressive symptoms, they start entertaining the notion that the patient may have TRD, not realizing that they have barely scratched the surface of the many therapeutic options and mechanisms of action, one of which could be the “best match” for a given patient.4
There will come a day when “precision psychiatry” finally arrives, and specific biomarkers will be developed to identify the “right” treatment for each patient within the heterogenous syndrome of depression.5 Until that day arrives, the treatment of depression will continue to be a process of trial and error, and hit or miss. But research will eventually discover genetic, neurochemical, neurophysiological, neuroimaging, or neuroimmune biomarkers that will rapidly guide clinicians to the correct treatment. This is critical to avoid inordinate delays in achieving remission and avert the ever-present risk of suicidal behavior.
The Table6 provides an overview of the numerous treatments currently available to manage depression. All increase brain-derived neurotrophic factor and restore healthy neuroplasticity and neurogenesis, which are impaired in MDD and currently believed to be a final common pathway for all depression treatments.7

These 41 therapeutic approaches to treating MDD or bipolar depression reflect the heterogeneity of mechanisms of action to address an equally heterogeneous syndrome. This implies that clinicians have a wide array of on-label options to manage patients with depression, aiming for remission, not just a good response, which typically is defined as a ≥50% reduction in total score on one of the validated rating scales used to quantify depression severity, such as the Montgomery-Åsberg Depression Rating Scale, Hamilton Depression Rating Scale, or Calgary Depression Scale for Schizophrenia.
Continue to: When several FDA-approved pharmacotherapies...
When several FDA-approved pharmacotherapies fall short and produce a suboptimal response, clinicians can resort to other treatment options known to have a higher efficacy than oral antidepressants. These include electroconvulsive therapy, repetitive transcranial magnetic stimulation, and vagus nerve stimulation. Other on-label options include adjunctive therapy with one of the approved second-generation antipsychotic agents or with adjunctive esketamine.
But if the patient still does not improve, one of many emerging off-label treatment options may work. One of the exciting new discoveries is the hallucinogen psilocybin, whose mechanism of action is truly unique. Unlike standard antidepressant medications, which modulate neurotransmitters, psilocybin increases the brain’s network flexibility, decreases the modularity of several key brain networks (especially the default-brain network, or DMN), and alters the dark and distorted mental perspective of depression to a much healthier and optimistic outlook about the self and the world.8 Such novel breakthroughs in the treatment of severe depression will shed some unprecedented insights into the core neurobiology of depression, and may lead to early intervention and prevention.
As the saying goes, all roads lead to Rome. Psychiatric clinicians should rejoice that there are abundant approaches and therapeutic mechanisms to relieve their severely melancholic (and often suicidal) patients from the grips of this disabling and life-altering brain syndrome.
Despite much progress, major depressive disorder (MDD) continues to be a challenging and life-threatening neuropsychiatric disorder. It is highly prevalent and afflicts tens of millions of Americans.
It is also ranked as the No. 1 disabling medical (not just psychiatric) condition by the World Health Organization.1 A significant proportion of patients with MDD do not respond adequately to several rounds of antidepressant medications,2 and many are labeled as having “treatment-resistant depression” (TRD).
In a previous article, I provocatively proposed that TRD is a myth.3 What I meant is that in a heterogeneous syndrome such as depression, failure to respond to 1, 2, or even 3 antidepressants should not imply TRD, because there is a “right treatment” that has not yet been identified for a given depressed patient. Most of those labeled as TRD have simply not yet received the pharmacotherapy or somatic therapy with the requisite mechanism of action for their variant of depression within a heterogeneous syndrome. IV ketamine, which, astonishingly, often reverses severe TRD of chronic duration within a few hours, is a prime example of why the term TRD is often used prematurely. Ketamine’s mechanism of action (immediate neuroplasticity via glutamate N-methyl-
Some clinicians may not be aware of the abundance of mechanisms of action currently available for the treatment of MDD as well as bipolar depression. Many practitioners, in both psychiatry and primary care, usually start the treatment of depression with a selective serotonin reuptake inhibitor, and if that does not produce a response or remission, they might switch to a serotonin-norepinephrine reuptake inhibitor. If that does not control the patient’s depressive symptoms, they start entertaining the notion that the patient may have TRD, not realizing that they have barely scratched the surface of the many therapeutic options and mechanisms of action, one of which could be the “best match” for a given patient.4
There will come a day when “precision psychiatry” finally arrives, and specific biomarkers will be developed to identify the “right” treatment for each patient within the heterogenous syndrome of depression.5 Until that day arrives, the treatment of depression will continue to be a process of trial and error, and hit or miss. But research will eventually discover genetic, neurochemical, neurophysiological, neuroimaging, or neuroimmune biomarkers that will rapidly guide clinicians to the correct treatment. This is critical to avoid inordinate delays in achieving remission and avert the ever-present risk of suicidal behavior.
The Table6 provides an overview of the numerous treatments currently available to manage depression. All increase brain-derived neurotrophic factor and restore healthy neuroplasticity and neurogenesis, which are impaired in MDD and currently believed to be a final common pathway for all depression treatments.7

These 41 therapeutic approaches to treating MDD or bipolar depression reflect the heterogeneity of mechanisms of action to address an equally heterogeneous syndrome. This implies that clinicians have a wide array of on-label options to manage patients with depression, aiming for remission, not just a good response, which typically is defined as a ≥50% reduction in total score on one of the validated rating scales used to quantify depression severity, such as the Montgomery-Åsberg Depression Rating Scale, Hamilton Depression Rating Scale, or Calgary Depression Scale for Schizophrenia.
Continue to: When several FDA-approved pharmacotherapies...
When several FDA-approved pharmacotherapies fall short and produce a suboptimal response, clinicians can resort to other treatment options known to have a higher efficacy than oral antidepressants. These include electroconvulsive therapy, repetitive transcranial magnetic stimulation, and vagus nerve stimulation. Other on-label options include adjunctive therapy with one of the approved second-generation antipsychotic agents or with adjunctive esketamine.
But if the patient still does not improve, one of many emerging off-label treatment options may work. One of the exciting new discoveries is the hallucinogen psilocybin, whose mechanism of action is truly unique. Unlike standard antidepressant medications, which modulate neurotransmitters, psilocybin increases the brain’s network flexibility, decreases the modularity of several key brain networks (especially the default-brain network, or DMN), and alters the dark and distorted mental perspective of depression to a much healthier and optimistic outlook about the self and the world.8 Such novel breakthroughs in the treatment of severe depression will shed some unprecedented insights into the core neurobiology of depression, and may lead to early intervention and prevention.
As the saying goes, all roads lead to Rome. Psychiatric clinicians should rejoice that there are abundant approaches and therapeutic mechanisms to relieve their severely melancholic (and often suicidal) patients from the grips of this disabling and life-altering brain syndrome.
1. World Health Organization. Depression: let’s talk says WHO, as depression tops list of causes of ill health. March 30, 2017. Accessed July 5, 2022. www.who.int/news/item/30-03-2017--depression-let-s-talk-says-who-as-depression-tops-list-of-causes-of-ill-health
2. Trivedi MH, Fava M, Wisniewski SR, et al. Medication augmentation after the failure of SSRIs for depression. N Eng J Med. 2006;354(12)1243-1252.
3. Nasrallah HA. Treatment resistance is a myth! Current Psychiatry. 2021;20(3):14-16,28.
4. Nasrallah HA. 10 Recent paradigm shifts in the neurobiology and treatment of depression. Current Psychiatry. 2015;14(2):10-13.
5. Nasrallah HA. Biomarkers in neuropsychiatric disorders: translating research to clinical applications. Biomarkers in Neuropsychiatry. 2019;1:100001. doi:10.1016/j.bionps.2019.100001
6. Procyshyn RM, Bezchlibnyk-Butler KZ, Jeffries JJ. Clinical Handbook of Psychotropic Drugs. 23rd ed. Hogrefe; 2019.
7. Tartt AN, Mariani, MB, Hen R, et al. Dysregulation of adult hippocampal neuroplasticity in major depression: pathogenesis and therapeutic implications. Mol Psychiatry. 2022;27(6):2689-2699.
8. Lowe H, Toyang N, Steele B, et al. The therapeutic potential of psilocybin. Molecules. 2021;26(10):2948. doi: 10.3390/molecules26102948
1. World Health Organization. Depression: let’s talk says WHO, as depression tops list of causes of ill health. March 30, 2017. Accessed July 5, 2022. www.who.int/news/item/30-03-2017--depression-let-s-talk-says-who-as-depression-tops-list-of-causes-of-ill-health
2. Trivedi MH, Fava M, Wisniewski SR, et al. Medication augmentation after the failure of SSRIs for depression. N Eng J Med. 2006;354(12)1243-1252.
3. Nasrallah HA. Treatment resistance is a myth! Current Psychiatry. 2021;20(3):14-16,28.
4. Nasrallah HA. 10 Recent paradigm shifts in the neurobiology and treatment of depression. Current Psychiatry. 2015;14(2):10-13.
5. Nasrallah HA. Biomarkers in neuropsychiatric disorders: translating research to clinical applications. Biomarkers in Neuropsychiatry. 2019;1:100001. doi:10.1016/j.bionps.2019.100001
6. Procyshyn RM, Bezchlibnyk-Butler KZ, Jeffries JJ. Clinical Handbook of Psychotropic Drugs. 23rd ed. Hogrefe; 2019.
7. Tartt AN, Mariani, MB, Hen R, et al. Dysregulation of adult hippocampal neuroplasticity in major depression: pathogenesis and therapeutic implications. Mol Psychiatry. 2022;27(6):2689-2699.
8. Lowe H, Toyang N, Steele B, et al. The therapeutic potential of psilocybin. Molecules. 2021;26(10):2948. doi: 10.3390/molecules26102948
How bariatric surgery affects psychotropic drug absorption
Ms. B, age 60, presents to the clinic with high blood pressure, hyperlipidemia, type 2 diabetes mellitus, depression, and anxiety. Her blood pressure is 138/82 mm Hg and pulse is 70 beats per minute. Her body mass index (BMI) is 41, which indicates she is obese. She has always struggled with her weight and has tried diet and lifestyle modifications, as well as medications, for the past 5 years with no success. Her current medication regimen includes lisinopril 40 mg daily, amlodipine 5 mg daily, atorvastatin 40 mg daily, metformin 500 mg twice daily, dulaglutide 0.75 mg weekly, lithium 600 mg daily, venlafaxine extended-release (XR) 150 mg daily, and alprazolam 0.5 mg as needed up to twice daily. Due to Ms. B’s BMI and because she has ≥1 comorbid health condition, her primary care physician refers her to a gastroenterologist to discuss gastric bypass surgery options.
Ms. B is scheduled for Roux-en-Y gastric bypass surgery. You need to determine if any changes should be made to her psychotropic medications after she undergoes this surgery.
There are multiple types of bariatric surgeries, including Roux-en-Y gastric bypass, sleeve gastrectomy, laparoscopic adjustable gastric band, and biliopancreatic diversion with duodenal switch (BPD/DS) (Figure1-4). These procedures all restrict the stomach’s capacity to hold food. In most cases, they also bypass areas of absorption in the intestine and cause increased secretion of hormones in the gut, including (but not limited to) peptide-YY (PYY) and glucagon-like peptide 1 (GLP-1). These hormonal changes impact several factors, including satiety, hunger, and blood sugar levels.5
Roux-en-Y is commonly referred to as the gold standard of weight loss surgery. It divides the top of the stomach into a smaller stomach pouch that connects directly to the small intestine to facilitate smaller meals and alters the release of gut hormones. Additionally, a segment of the small intestine that normally absorbs nutrients and medications is completely bypassed. In contrast, the sleeve gastrectomy removes approximately 80% of the stomach, consequently reducing the amount of food that can be consumed. The greatest impact of the sleeve gastrectomy procedure appears to result from changes in gut hormones. The adjustable gastric band procedure works by placing a band around the upper portion of the stomach to create a small pouch above the band to satisfy hunger with a smaller amount of food. Lastly, BPD/DS is a procedure that creates a tubular stomach pouch and bypasses a large portion of the small intestine. Like the gastric bypass and sleeve gastrectomy, BPD/DS affects gut hormones impacting hunger, satiety, and blood sugar control.
How bariatric surgery can affect drug absorption
As illustrated in the Table,6-19 each type of bariatric surgery may impact drug absorption differently depending on the mechanism by which the stomach is restricted.
Drug malabsorption is a concern for clinicians with patients who have undergone bariatric surgery. There is limited research measuring changes in psychotropic exposure and outcomes following bariatric surgery. A 2009 literature review by Padwal et al7 found that one-third of the 26 studies evaluated provided evidence of decreased absorption following bariatric surgery in patients taking medications that had intrinsic poor absorption, high lipophilicity, and/or undergo enterohepatic recirculation. In a review that included a small study of patients taking selective serotonin reuptake inhibitors or venlafaxine, Godini et al8 demonstrated that although there was a notable decrease in drug absorption closely following the surgery, drug absorption recovered for some patients 1 month after Roux-en-Y surgery. These reviews suggest patients who have undergone any form of bariatric surgery must be observed closely because drug absorption may vary based on the individual, the medication administered, and the amount of time postprocedure.
Until more research becomes available, current evidence supports recommendations to assist patients who have a decreased ability to absorb medications after gastric bypass surgery by switching from an extended-release formulation to an immediate-release or solution formulation. This allows patients to rely less on gastric mixing and unpredictable changes in drug release from extended- or controlled-release formulations.
Continue to: Aside from altered...
Aside from altered pharmacokinetics after bariatric surgery, many patients experience an increased risk of self-harm and suicide.20 Therefore, a continued emphasis on and reinforcement of proper antidepressant use and adjustment in these patients is important. This can be facilitated through frequent follow-up visits, either in-person or via telehealth.
Understanding the effect of bariatric surgery on drug absorption is critical to identifying a potential need to adjust a medication dose or formulation after the surgery. Available evidence and data suggest it is reasonable to switch from an extended- or sustained-release formulation to an immediate-release formulation, and to monitor patients more frequently immediately following the surgery.
CASE CONTINUED
Related Resources
- Colvin C, Tsia W, Silverman AL, et al. Nothing up his sleeve: decompensation after bariatric surgery. Current Psychiatry. 2021;20(4):15-19. doi:10.12788/cp.010
Drug Brand Names
Alprazolam • Xanax
Amlodipine • Norvasc
Atorvastatin • Lipitor
Dulaglutide • Trulicity
Lisinopril • Zestril, Prinivil
Lithium • Eskalith, Lithobid
Metformin • Glucophage
Olanzapine • Zyprexa
Venlafaxine • Effexor
1. Obesity Treatments: Gastric Bypass Surgery. UCLA Health. Accessed April 4, 2021. http://surgery.ucla.edu/bariatrics-gastric-bypass
2. Thomas L. Gastric bypass more likely to require further treatment than gastric sleeve. News Medical. January 15, 2020. Accessed April 4, 2021. https://www.news-medical.net/news/20200115/Gastric-bypass-more-likely-to-require-further-treatment-than-gastric-sleeve.aspx
3. Lap Adjustable Gastric Banding. Laser Stone Surgery & Endoscopy Centre. September 5, 2016. Accessed April 4, 2021. http://www.laserstonesurgery.org/project/lap-adjustable-gastric-banding/
4. BPD/DS Weight-Loss Surgery. Johns Hopkins Medicine. Accessed April 4, 2021. https://www.hopkinsmedicine.org/health/treatment-tests-and-therapies/bpdds-weightloss-surgery
5. Holst JJ, Madsbad S, Bojsen-Møller KN, et al. Mechanisms in bariatric surgery: gut hormones, diabetes resolution, and weight loss. Surg Obes Relat Dis. 2018;14(5):708-714. doi:10.1016/j.soard.2018.03.003
6. Public Education Committee. Bariatric Surgery Procedures. American Society for Metabolic and Bariatric Surgery. Updated May 2021. Accessed September 4, 2021. https://asmbs.org/patients/bariatric-surgery-procedures
7. Padwal R, Brocks D, Sharma AM. A systematic review of drug absorption following bariatric surgery and its theoretical implications. Obes Rev. 2010;11(1):41-50. doi:10.1111/j.1467-789x.2009.00614.x
8. Godini L, Castellini G, Facchiano E, et al. Mood disorders and bariatric surgery patients: pre- and post- surgery clinical course- an overview. J Obes Weight Loss Medicat. 2016;2(1). doi:10.23937/2572-4010.1510012
9. Smith A, Henriksen B, Cohen A. Pharmacokinetic considerations in Roux-en-Y gastric bypass patients. Am J Health Syst Pharm. 2011;68(23):2241-2247. doi:10.2146/ajhp100630
10. Brocks DR, Ben-Eltriki M, Gabr RQ, et al. The effects of gastric bypass surgery on drug absorption and pharmacokinetics. Expert Opin Drug Metab Toxicol. 2012;8(12):1505-1519. doi:10.1517/17425255.2012.722757
11. Hamad GG, Helsel JC, Perel JM, et al. The effect of gastric bypass on the pharmacokinetics of serotonin reuptake inhibitors. Am J Psychiatry. 2012;169(3):256-263. doi:10.1176/appi.ajp.2011.11050719
12. Angeles PC, Robertsen I, Seeberg LT, et al. The influence of bariatric surgery on oral drug bioavailability in patients with obesity: a systematic review. Obes Rev. 2019;20(9):1299-1311. doi:10.1111/obr.12869
13. Laparoscopic Sleeve Gastrectomy. University of California San Francisco Department of Surgery. Accessed April 1, 2021. https://surgery.ucsf.edu/conditions--procedures/laparoscopic-sleeve-gastrectomy.aspx
14. Brethauer S, Schauer P. Laparoscopic Sleeve Gastrectomy: A Newcomer to Bariatric Surgery. Obesity Action Coalition. 2007. Accessed May 15, 2021. https://www.obesityaction.org/community/article-library/laparoscopic-sleeve-gastrectomy-a-newcomer-to-bariatric-surgery/
15. Roerig JL, Steffen K. Psychopharmacology and bariatric surgery. Eur Eat Disord Rev. 2015;23(6):463-469. doi:10.1002/erv.2396
16. Bland CM, Quidley AM, Love BL, et al. Long-term pharmacotherapy considerations in the bariatric surgery patient. A J Health Syst Pharm. 2016;73(16):1230-1242. doi:10.2146/ajhp151062
17. Lin YH, Liu SW, Wu HL, et al. Lithium toxicity with prolonged neurologic sequelae following sleeve gastrectomy: a case report and review of literature. Medicine (Baltimore). 2020;99(28):e21122. doi:10.1097/MD.0000000000021122
18. Lorico S, Colton B. Medication management and pharmacokinetic changes after bariatric surgery. Can Fam Physician. 2020;66(6):409-416.
19. Homan J, Schijns W, Aarts EO, et al. Treatment of vitamin and mineral deficiencies after biliopancreatic diversion with or without duodenal switch: a major challenge. Obes Surg. 2018;28(1):234-241. doi:10.1007/s11695-017-2841-0
20. Neovius M, Bruze G, Jacobson P, et al. Risk of suicide and non-fatal self-harm after bariatric surgery: results from two matched cohort studies. Lancet Diabetes Endocrinol. 2018;6(3):197-207. doi:10.1016/S2213-8587(17)30437-0
Ms. B, age 60, presents to the clinic with high blood pressure, hyperlipidemia, type 2 diabetes mellitus, depression, and anxiety. Her blood pressure is 138/82 mm Hg and pulse is 70 beats per minute. Her body mass index (BMI) is 41, which indicates she is obese. She has always struggled with her weight and has tried diet and lifestyle modifications, as well as medications, for the past 5 years with no success. Her current medication regimen includes lisinopril 40 mg daily, amlodipine 5 mg daily, atorvastatin 40 mg daily, metformin 500 mg twice daily, dulaglutide 0.75 mg weekly, lithium 600 mg daily, venlafaxine extended-release (XR) 150 mg daily, and alprazolam 0.5 mg as needed up to twice daily. Due to Ms. B’s BMI and because she has ≥1 comorbid health condition, her primary care physician refers her to a gastroenterologist to discuss gastric bypass surgery options.
Ms. B is scheduled for Roux-en-Y gastric bypass surgery. You need to determine if any changes should be made to her psychotropic medications after she undergoes this surgery.
There are multiple types of bariatric surgeries, including Roux-en-Y gastric bypass, sleeve gastrectomy, laparoscopic adjustable gastric band, and biliopancreatic diversion with duodenal switch (BPD/DS) (Figure1-4). These procedures all restrict the stomach’s capacity to hold food. In most cases, they also bypass areas of absorption in the intestine and cause increased secretion of hormones in the gut, including (but not limited to) peptide-YY (PYY) and glucagon-like peptide 1 (GLP-1). These hormonal changes impact several factors, including satiety, hunger, and blood sugar levels.5

Roux-en-Y is commonly referred to as the gold standard of weight loss surgery. It divides the top of the stomach into a smaller stomach pouch that connects directly to the small intestine to facilitate smaller meals and alters the release of gut hormones. Additionally, a segment of the small intestine that normally absorbs nutrients and medications is completely bypassed. In contrast, the sleeve gastrectomy removes approximately 80% of the stomach, consequently reducing the amount of food that can be consumed. The greatest impact of the sleeve gastrectomy procedure appears to result from changes in gut hormones. The adjustable gastric band procedure works by placing a band around the upper portion of the stomach to create a small pouch above the band to satisfy hunger with a smaller amount of food. Lastly, BPD/DS is a procedure that creates a tubular stomach pouch and bypasses a large portion of the small intestine. Like the gastric bypass and sleeve gastrectomy, BPD/DS affects gut hormones impacting hunger, satiety, and blood sugar control.
How bariatric surgery can affect drug absorption
As illustrated in the Table,6-19 each type of bariatric surgery may impact drug absorption differently depending on the mechanism by which the stomach is restricted.

Drug malabsorption is a concern for clinicians with patients who have undergone bariatric surgery. There is limited research measuring changes in psychotropic exposure and outcomes following bariatric surgery. A 2009 literature review by Padwal et al7 found that one-third of the 26 studies evaluated provided evidence of decreased absorption following bariatric surgery in patients taking medications that had intrinsic poor absorption, high lipophilicity, and/or undergo enterohepatic recirculation. In a review that included a small study of patients taking selective serotonin reuptake inhibitors or venlafaxine, Godini et al8 demonstrated that although there was a notable decrease in drug absorption closely following the surgery, drug absorption recovered for some patients 1 month after Roux-en-Y surgery. These reviews suggest patients who have undergone any form of bariatric surgery must be observed closely because drug absorption may vary based on the individual, the medication administered, and the amount of time postprocedure.
Until more research becomes available, current evidence supports recommendations to assist patients who have a decreased ability to absorb medications after gastric bypass surgery by switching from an extended-release formulation to an immediate-release or solution formulation. This allows patients to rely less on gastric mixing and unpredictable changes in drug release from extended- or controlled-release formulations.
Continue to: Aside from altered...
Aside from altered pharmacokinetics after bariatric surgery, many patients experience an increased risk of self-harm and suicide.20 Therefore, a continued emphasis on and reinforcement of proper antidepressant use and adjustment in these patients is important. This can be facilitated through frequent follow-up visits, either in-person or via telehealth.
Understanding the effect of bariatric surgery on drug absorption is critical to identifying a potential need to adjust a medication dose or formulation after the surgery. Available evidence and data suggest it is reasonable to switch from an extended- or sustained-release formulation to an immediate-release formulation, and to monitor patients more frequently immediately following the surgery.
CASE CONTINUED
Related Resources
- Colvin C, Tsia W, Silverman AL, et al. Nothing up his sleeve: decompensation after bariatric surgery. Current Psychiatry. 2021;20(4):15-19. doi:10.12788/cp.010
Drug Brand Names
Alprazolam • Xanax
Amlodipine • Norvasc
Atorvastatin • Lipitor
Dulaglutide • Trulicity
Lisinopril • Zestril, Prinivil
Lithium • Eskalith, Lithobid
Metformin • Glucophage
Olanzapine • Zyprexa
Venlafaxine • Effexor
Ms. B, age 60, presents to the clinic with high blood pressure, hyperlipidemia, type 2 diabetes mellitus, depression, and anxiety. Her blood pressure is 138/82 mm Hg and pulse is 70 beats per minute. Her body mass index (BMI) is 41, which indicates she is obese. She has always struggled with her weight and has tried diet and lifestyle modifications, as well as medications, for the past 5 years with no success. Her current medication regimen includes lisinopril 40 mg daily, amlodipine 5 mg daily, atorvastatin 40 mg daily, metformin 500 mg twice daily, dulaglutide 0.75 mg weekly, lithium 600 mg daily, venlafaxine extended-release (XR) 150 mg daily, and alprazolam 0.5 mg as needed up to twice daily. Due to Ms. B’s BMI and because she has ≥1 comorbid health condition, her primary care physician refers her to a gastroenterologist to discuss gastric bypass surgery options.
Ms. B is scheduled for Roux-en-Y gastric bypass surgery. You need to determine if any changes should be made to her psychotropic medications after she undergoes this surgery.
There are multiple types of bariatric surgeries, including Roux-en-Y gastric bypass, sleeve gastrectomy, laparoscopic adjustable gastric band, and biliopancreatic diversion with duodenal switch (BPD/DS) (Figure1-4). These procedures all restrict the stomach’s capacity to hold food. In most cases, they also bypass areas of absorption in the intestine and cause increased secretion of hormones in the gut, including (but not limited to) peptide-YY (PYY) and glucagon-like peptide 1 (GLP-1). These hormonal changes impact several factors, including satiety, hunger, and blood sugar levels.5

Roux-en-Y is commonly referred to as the gold standard of weight loss surgery. It divides the top of the stomach into a smaller stomach pouch that connects directly to the small intestine to facilitate smaller meals and alters the release of gut hormones. Additionally, a segment of the small intestine that normally absorbs nutrients and medications is completely bypassed. In contrast, the sleeve gastrectomy removes approximately 80% of the stomach, consequently reducing the amount of food that can be consumed. The greatest impact of the sleeve gastrectomy procedure appears to result from changes in gut hormones. The adjustable gastric band procedure works by placing a band around the upper portion of the stomach to create a small pouch above the band to satisfy hunger with a smaller amount of food. Lastly, BPD/DS is a procedure that creates a tubular stomach pouch and bypasses a large portion of the small intestine. Like the gastric bypass and sleeve gastrectomy, BPD/DS affects gut hormones impacting hunger, satiety, and blood sugar control.
How bariatric surgery can affect drug absorption
As illustrated in the Table,6-19 each type of bariatric surgery may impact drug absorption differently depending on the mechanism by which the stomach is restricted.

Drug malabsorption is a concern for clinicians with patients who have undergone bariatric surgery. There is limited research measuring changes in psychotropic exposure and outcomes following bariatric surgery. A 2009 literature review by Padwal et al7 found that one-third of the 26 studies evaluated provided evidence of decreased absorption following bariatric surgery in patients taking medications that had intrinsic poor absorption, high lipophilicity, and/or undergo enterohepatic recirculation. In a review that included a small study of patients taking selective serotonin reuptake inhibitors or venlafaxine, Godini et al8 demonstrated that although there was a notable decrease in drug absorption closely following the surgery, drug absorption recovered for some patients 1 month after Roux-en-Y surgery. These reviews suggest patients who have undergone any form of bariatric surgery must be observed closely because drug absorption may vary based on the individual, the medication administered, and the amount of time postprocedure.
Until more research becomes available, current evidence supports recommendations to assist patients who have a decreased ability to absorb medications after gastric bypass surgery by switching from an extended-release formulation to an immediate-release or solution formulation. This allows patients to rely less on gastric mixing and unpredictable changes in drug release from extended- or controlled-release formulations.
Continue to: Aside from altered...
Aside from altered pharmacokinetics after bariatric surgery, many patients experience an increased risk of self-harm and suicide.20 Therefore, a continued emphasis on and reinforcement of proper antidepressant use and adjustment in these patients is important. This can be facilitated through frequent follow-up visits, either in-person or via telehealth.
Understanding the effect of bariatric surgery on drug absorption is critical to identifying a potential need to adjust a medication dose or formulation after the surgery. Available evidence and data suggest it is reasonable to switch from an extended- or sustained-release formulation to an immediate-release formulation, and to monitor patients more frequently immediately following the surgery.
CASE CONTINUED
Related Resources
- Colvin C, Tsia W, Silverman AL, et al. Nothing up his sleeve: decompensation after bariatric surgery. Current Psychiatry. 2021;20(4):15-19. doi:10.12788/cp.010
Drug Brand Names
Alprazolam • Xanax
Amlodipine • Norvasc
Atorvastatin • Lipitor
Dulaglutide • Trulicity
Lisinopril • Zestril, Prinivil
Lithium • Eskalith, Lithobid
Metformin • Glucophage
Olanzapine • Zyprexa
Venlafaxine • Effexor
1. Obesity Treatments: Gastric Bypass Surgery. UCLA Health. Accessed April 4, 2021. http://surgery.ucla.edu/bariatrics-gastric-bypass
2. Thomas L. Gastric bypass more likely to require further treatment than gastric sleeve. News Medical. January 15, 2020. Accessed April 4, 2021. https://www.news-medical.net/news/20200115/Gastric-bypass-more-likely-to-require-further-treatment-than-gastric-sleeve.aspx
3. Lap Adjustable Gastric Banding. Laser Stone Surgery & Endoscopy Centre. September 5, 2016. Accessed April 4, 2021. http://www.laserstonesurgery.org/project/lap-adjustable-gastric-banding/
4. BPD/DS Weight-Loss Surgery. Johns Hopkins Medicine. Accessed April 4, 2021. https://www.hopkinsmedicine.org/health/treatment-tests-and-therapies/bpdds-weightloss-surgery
5. Holst JJ, Madsbad S, Bojsen-Møller KN, et al. Mechanisms in bariatric surgery: gut hormones, diabetes resolution, and weight loss. Surg Obes Relat Dis. 2018;14(5):708-714. doi:10.1016/j.soard.2018.03.003
6. Public Education Committee. Bariatric Surgery Procedures. American Society for Metabolic and Bariatric Surgery. Updated May 2021. Accessed September 4, 2021. https://asmbs.org/patients/bariatric-surgery-procedures
7. Padwal R, Brocks D, Sharma AM. A systematic review of drug absorption following bariatric surgery and its theoretical implications. Obes Rev. 2010;11(1):41-50. doi:10.1111/j.1467-789x.2009.00614.x
8. Godini L, Castellini G, Facchiano E, et al. Mood disorders and bariatric surgery patients: pre- and post- surgery clinical course- an overview. J Obes Weight Loss Medicat. 2016;2(1). doi:10.23937/2572-4010.1510012
9. Smith A, Henriksen B, Cohen A. Pharmacokinetic considerations in Roux-en-Y gastric bypass patients. Am J Health Syst Pharm. 2011;68(23):2241-2247. doi:10.2146/ajhp100630
10. Brocks DR, Ben-Eltriki M, Gabr RQ, et al. The effects of gastric bypass surgery on drug absorption and pharmacokinetics. Expert Opin Drug Metab Toxicol. 2012;8(12):1505-1519. doi:10.1517/17425255.2012.722757
11. Hamad GG, Helsel JC, Perel JM, et al. The effect of gastric bypass on the pharmacokinetics of serotonin reuptake inhibitors. Am J Psychiatry. 2012;169(3):256-263. doi:10.1176/appi.ajp.2011.11050719
12. Angeles PC, Robertsen I, Seeberg LT, et al. The influence of bariatric surgery on oral drug bioavailability in patients with obesity: a systematic review. Obes Rev. 2019;20(9):1299-1311. doi:10.1111/obr.12869
13. Laparoscopic Sleeve Gastrectomy. University of California San Francisco Department of Surgery. Accessed April 1, 2021. https://surgery.ucsf.edu/conditions--procedures/laparoscopic-sleeve-gastrectomy.aspx
14. Brethauer S, Schauer P. Laparoscopic Sleeve Gastrectomy: A Newcomer to Bariatric Surgery. Obesity Action Coalition. 2007. Accessed May 15, 2021. https://www.obesityaction.org/community/article-library/laparoscopic-sleeve-gastrectomy-a-newcomer-to-bariatric-surgery/
15. Roerig JL, Steffen K. Psychopharmacology and bariatric surgery. Eur Eat Disord Rev. 2015;23(6):463-469. doi:10.1002/erv.2396
16. Bland CM, Quidley AM, Love BL, et al. Long-term pharmacotherapy considerations in the bariatric surgery patient. A J Health Syst Pharm. 2016;73(16):1230-1242. doi:10.2146/ajhp151062
17. Lin YH, Liu SW, Wu HL, et al. Lithium toxicity with prolonged neurologic sequelae following sleeve gastrectomy: a case report and review of literature. Medicine (Baltimore). 2020;99(28):e21122. doi:10.1097/MD.0000000000021122
18. Lorico S, Colton B. Medication management and pharmacokinetic changes after bariatric surgery. Can Fam Physician. 2020;66(6):409-416.
19. Homan J, Schijns W, Aarts EO, et al. Treatment of vitamin and mineral deficiencies after biliopancreatic diversion with or without duodenal switch: a major challenge. Obes Surg. 2018;28(1):234-241. doi:10.1007/s11695-017-2841-0
20. Neovius M, Bruze G, Jacobson P, et al. Risk of suicide and non-fatal self-harm after bariatric surgery: results from two matched cohort studies. Lancet Diabetes Endocrinol. 2018;6(3):197-207. doi:10.1016/S2213-8587(17)30437-0
1. Obesity Treatments: Gastric Bypass Surgery. UCLA Health. Accessed April 4, 2021. http://surgery.ucla.edu/bariatrics-gastric-bypass
2. Thomas L. Gastric bypass more likely to require further treatment than gastric sleeve. News Medical. January 15, 2020. Accessed April 4, 2021. https://www.news-medical.net/news/20200115/Gastric-bypass-more-likely-to-require-further-treatment-than-gastric-sleeve.aspx
3. Lap Adjustable Gastric Banding. Laser Stone Surgery & Endoscopy Centre. September 5, 2016. Accessed April 4, 2021. http://www.laserstonesurgery.org/project/lap-adjustable-gastric-banding/
4. BPD/DS Weight-Loss Surgery. Johns Hopkins Medicine. Accessed April 4, 2021. https://www.hopkinsmedicine.org/health/treatment-tests-and-therapies/bpdds-weightloss-surgery
5. Holst JJ, Madsbad S, Bojsen-Møller KN, et al. Mechanisms in bariatric surgery: gut hormones, diabetes resolution, and weight loss. Surg Obes Relat Dis. 2018;14(5):708-714. doi:10.1016/j.soard.2018.03.003
6. Public Education Committee. Bariatric Surgery Procedures. American Society for Metabolic and Bariatric Surgery. Updated May 2021. Accessed September 4, 2021. https://asmbs.org/patients/bariatric-surgery-procedures
7. Padwal R, Brocks D, Sharma AM. A systematic review of drug absorption following bariatric surgery and its theoretical implications. Obes Rev. 2010;11(1):41-50. doi:10.1111/j.1467-789x.2009.00614.x
8. Godini L, Castellini G, Facchiano E, et al. Mood disorders and bariatric surgery patients: pre- and post- surgery clinical course- an overview. J Obes Weight Loss Medicat. 2016;2(1). doi:10.23937/2572-4010.1510012
9. Smith A, Henriksen B, Cohen A. Pharmacokinetic considerations in Roux-en-Y gastric bypass patients. Am J Health Syst Pharm. 2011;68(23):2241-2247. doi:10.2146/ajhp100630
10. Brocks DR, Ben-Eltriki M, Gabr RQ, et al. The effects of gastric bypass surgery on drug absorption and pharmacokinetics. Expert Opin Drug Metab Toxicol. 2012;8(12):1505-1519. doi:10.1517/17425255.2012.722757
11. Hamad GG, Helsel JC, Perel JM, et al. The effect of gastric bypass on the pharmacokinetics of serotonin reuptake inhibitors. Am J Psychiatry. 2012;169(3):256-263. doi:10.1176/appi.ajp.2011.11050719
12. Angeles PC, Robertsen I, Seeberg LT, et al. The influence of bariatric surgery on oral drug bioavailability in patients with obesity: a systematic review. Obes Rev. 2019;20(9):1299-1311. doi:10.1111/obr.12869
13. Laparoscopic Sleeve Gastrectomy. University of California San Francisco Department of Surgery. Accessed April 1, 2021. https://surgery.ucsf.edu/conditions--procedures/laparoscopic-sleeve-gastrectomy.aspx
14. Brethauer S, Schauer P. Laparoscopic Sleeve Gastrectomy: A Newcomer to Bariatric Surgery. Obesity Action Coalition. 2007. Accessed May 15, 2021. https://www.obesityaction.org/community/article-library/laparoscopic-sleeve-gastrectomy-a-newcomer-to-bariatric-surgery/
15. Roerig JL, Steffen K. Psychopharmacology and bariatric surgery. Eur Eat Disord Rev. 2015;23(6):463-469. doi:10.1002/erv.2396
16. Bland CM, Quidley AM, Love BL, et al. Long-term pharmacotherapy considerations in the bariatric surgery patient. A J Health Syst Pharm. 2016;73(16):1230-1242. doi:10.2146/ajhp151062
17. Lin YH, Liu SW, Wu HL, et al. Lithium toxicity with prolonged neurologic sequelae following sleeve gastrectomy: a case report and review of literature. Medicine (Baltimore). 2020;99(28):e21122. doi:10.1097/MD.0000000000021122
18. Lorico S, Colton B. Medication management and pharmacokinetic changes after bariatric surgery. Can Fam Physician. 2020;66(6):409-416.
19. Homan J, Schijns W, Aarts EO, et al. Treatment of vitamin and mineral deficiencies after biliopancreatic diversion with or without duodenal switch: a major challenge. Obes Surg. 2018;28(1):234-241. doi:10.1007/s11695-017-2841-0
20. Neovius M, Bruze G, Jacobson P, et al. Risk of suicide and non-fatal self-harm after bariatric surgery: results from two matched cohort studies. Lancet Diabetes Endocrinol. 2018;6(3):197-207. doi:10.1016/S2213-8587(17)30437-0
Impaired cognition in a patient with schizophrenia and HIV
CASE Psychotic episode in a patient with HIV
Mr. F, age 32, has schizophrenia and HIV. He presents to the emergency department with auditory and visual hallucinations in addition to paranoia. The treatment team refers him to the state psychiatric facility on an involuntary hold. Mr. F has had multiple previous hospitalizations, none of which had resulted in successful treatment. According to his most recent records, Mr. F failed to improve while taking olanzapine. Upon examination, Mr. F reports he hears command auditory hallucinations to hurt others and endorses paranoia. He is agitated, with a constricted affect, and his thought content is paranoid, disorganized, and circumstantial. Mr. F provides vague and evasive answers upon admission. His physical examination is unremarkable. He has an eighth-grade education level and limited insight into his illnesses. His Positive and Negative Syndrome Scale (PANSS) score is 122, indicating severe symptoms. The PANSS score is formulated based on 30 items, each scored between 1 and 7. Higher scores indicate more severe symptoms.
[polldaddy:11167946]
The authors’ observations
Compared to other medically ill patients, those with AIDS are 7 times more likely to experience EPS associated with antipsychotics. This may be a result of HIV infiltration of the basal ganglia causing regional changes that predispose these patients to EPS.
[polldaddy:11167948]
TREATMENT Haloperidol and antiretroviral therapy
The treatment team decides to start Mr. F on haloperidol for his psychotic symptoms as well as bictegravir, emtricitabine, and tenofovir for HIV. One week after admission, the team starts Mr. F on haloperidol decanoate 150 mg IM, and continues oral haloperidol and antiretroviral therapy. Mr. F reports some improvement in his hallucinations and appears to have reduced paranoia. He attends psychotherapy treatment groups over the next several days and scores 80 on a retrospective PANSS assessment (Figure 1). Mr. F receives haloperidol decanoate 200 mg IM 28 days after his first dose, and his oral haloperidol dose is reduced.
During the following 2 weeks, Mr. F endorses continued improvement of his symptoms and insight and begins discharge planning by calling his sister to discuss living arrangements. However, his mental state begins to decline; he becomes paranoid, withdrawn, and irritable, and endorses increased hallucinations. His PANSS score is 87, and he scores 11 on the Montreal Cognitive Assessment (MoCA), indicating moderate cognitive impairment. MoCA scores range from 0 to 30, with scores <10 indicating severe impairment, 10 to 17 indicating moderate impairment, 18 to 25 indicating mild impairment, and 26 to 30 considered normal. Figure 2 shows a timeline of Mr. F’s MoCA scores during treatment.
The treatment team increases the dose of haloperidol, and Mr. F continues to receive haloperidol deaconate injections monthly. After an adequate trial of haloperidol, the patient exhibits only partial response to treatment—his symptoms wax and wane—and he continues to display limited insight into both his mental illness and HIV diagnosis. Another PANSS assessment yields an essentially unchanged score of 88.
After a discussion of risks and benefits, Mr. F consents to initiating clozapine. The treatment team starts clozapine 25 mg/d and increases the dosage to 400 mg in the evening with a concomitant clozapine level of 487 ng/mL. Mr. F’s absolute neutrophil count was within normal limits (2,500 to 6,000 µL) during this period for weekly complete blood cell count monitoring. Over the next few weeks, his MoCA score increases to 17 and PANSS score decreases to 52. Haloperidol decanoate 200 mg IM is discontinued 3 days after Mr. F received a dose of clozapine 400 mg at bedtime. After an additional 2 weeks of clozapine at the same dosage, Mr. F scores 20 on the MoCA, an increase of 9 points from his baseline score while receiving haloperidol. There is a washout period for haloperidol decanoate and oral haloperidol before he completes a third MoCA. Mr. F participates in a discussion regarding his HIV diagnosis and the importance of consistently continuing treatment for this chronic infection. After some education, he has a better understanding of his condition and is more insightful about wanting to remain compliant with clozapine and bictegravir, emtricitabine, and tenofovir for his HIV.
The authors’ observations
Many patients receive treatment for comorbid HIV and schizophrenia. Patients with schizophrenia and other psychoses are at increased risk of contracting HIV due to numerous psychosocial factors, including an increased frequency of illicit drug use as well as an increased propensity for high-risk sexual behaviors secondary to impaired neurocognitive functioning, delusions, and victimization.1 In addition to deficits in functioning related to psychiatric illness, patients with HIV also experience virus-related neurocognitive insults. After crossing the blood-brain barrier, HIV viral proteins circulate in the blood, inducing brain endothelial cells to release cytokines, causing neuroinflammation.2
Continue to: Recently, inflammation and inflammatory...
Recently, inflammation and inflammatory biomarkers have become an important topic of psychiatric research. A meta-analysis by Fraguas et al3 concluded that greater inflammation and oxidative stress might lead to poorer outcomes in patients with first-episode psychosis. Based on this evidence, inflammation associated with untreated HIV infection may compound the pre-existing neurocognitive decline seen in patients with schizophrenia and other psychoses, thereby contributing to poor outcomes and treatment-resistant pathology.
Clozapine has been the superior treatment for refractory and nonrefractory schizophrenia.4 Factor et al5 report there are limited basal ganglia reserves in patients with HIV, which make clozapine the preferred option due to its low potential for causing EPS.
In this case, starting Mr. F on clozapine and titrating to therapeutic blood levels was associated with improved MoCA scores. Low MoCA scores could be due to untreated HIV, as well as inadequately treated psychosis. For Mr. F, improved MoCA scores were associated with increased insight into his HIV. It is important to note that Mr. F’s improved MoCA score also coincided with discontinuing monthly haloperidol decanoate injections. Haloperidol and its metabolites are believed to cause some neurotoxicity at high doses, and can contribute to cognitive impairment. This may partially explain the increased MoCA score after Mr. F stopped receiving haloperidol decanoate monthly injections.6 For the first time, he felt the need to be on antiretroviral therapy for his HIV, and was able to understand the chronic nature of HIV infection.
The benefit of clozapine treatment for patients with schizophrenia and comorbid HIV extends beyond symptomatic control. Long-term and consistent treatment of schizophrenia can be a stepping stone for improving many psychosocial factors. Improved insight allows patients to better understand their illness, treatment regimen, and follow-up needs. Improved self-care contributes to increased adherence to treatment regimens and overall health.
It is likely that patients who are consistently treated for schizophrenia will also have an increased capacity to understand their HIV diagnosis. With gained understanding, patients may be more likely to adhere to highly active antiretroviral therapy (HAART) for HIV and attend follow-up appointments with infectious disease or primary care physicians. Furthermore, with adherence to HAART therapy, patients can enjoy improved quality and duration of life by raising CD4 counts and preventing progression to AIDS and AIDS-related infections.
Continue to: In the case of...
In the case of Mr. F, we noted significant improvement in MoCA scores following treatment with clozapine. This led to improved insight into understanding the chronicity of HIV, understanding the complications of not being treated, and adherence to HAART medication. Improved cognition, as evidenced by an increased MoCA score, can significantly improve patient insight and adherence with medication.7 Insight into illness is particularly important when managing a patient with a chronic infectious illness such as HIV, where consistency with the medication regimen can decrease mortality and improve quality of life.8 Furthermore, with close monitoring, clozapine was a safe treatment option for this patient with HIV and schizophrenia.
Bottom Line
Patients with schizophrenia are at an increased risk of contracting HIV, and untreated schizophrenia decreases the likelihood patients will adhere to highly active antiretroviral therapy (HAART). Clozapine treatment in comorbid HIV and schizophrenia can improve cognition and insight into HIV diagnosis, possibly increasing the likelihood patients will remain compliant with HAART.
Related Resources
- Diduch MN, Campbell RH, Borovicka M, et al. Treating psychosis in patients with HIV/AIDS. Current Psychiatry. 2018;17(5):35-36,41-44,46.
Drug Brand Names
Bictegravir, emtricitabine, and tenofovir • Biktarvy
Clozapine • Clozaril
Haloperidol • Haldol
Haloperidol decanoate • Haldol decanoate
Olanzapine • Zyprexa
Ziprasidone • Geodon
1. Bahorik AL, Newhill CE, Eack SM. Neurocognitive functioning of individuals with schizophrenia: using and not using drugs. Schizophrenia Bull. 2014;40(4):856-867. doi:10.1093/schbul/sbt099
2. Hong S, Banks WA. Role of the immune system in HIV-associated neuroinflammation and neurocognitive implications. Brain Behav Immun. 2015;45:1-12. doi:10.1016/j.bbi.2014.10.008
3. Fraguas D, Díaz-Caneja CM, Rodríguez-Quiroga A, et al. Oxidative stress and inflammation in early onset first episode psychosis: a systematic review and meta-analysis. Int J Neuropsychopharmacol. 2017;20(6):435-444. doi:10.1093/ijnp/pyx015
4. Wahlbeck K, Cheine M, Essali A, et al. Evidence of clozapine’s effectiveness in schizophrenia: a systematic review and meta-analysis of randomized trials. Am J Psychiatry. 1999;156(7):990-999.
5. Factor SA, Brown D, Molho ES, et al. Clozapine: a 2-year open trial in Parkinson’s disease patients with psychosis. Neurology. 1994;44(3 Pt 1):544-546.
6. Raudenska M, Gumulec J, Babula P, et al. Haloperidol cytotoxicity and its relation to oxidative stress. Mini Rev Med Chem. 2013;13(14):1993-1998. doi:10.2174/13895575113136660100
7. El Abdellati K, De Picker L, Morrens M. Antipsychotic treatment failure: a systematic review on risk factors and interventions for treatment adherence in psychosis. Front Neurosci. 2020;14:531763. doi:10.3389/fnins.2020.531763
8. Margalho R, Pereira M, Ouakinin S, et al. Adesão à HAART, qualidade de vida e sintomat ologia psicopat ológica em doentes infectados pelo VIH/SIDA [Adherence to HAART, quality of life and psychopathological symptoms among HIV/AIDS infected patients]. Acta Med Port. 2011;24 Suppl 2:539-548.
CASE Psychotic episode in a patient with HIV
Mr. F, age 32, has schizophrenia and HIV. He presents to the emergency department with auditory and visual hallucinations in addition to paranoia. The treatment team refers him to the state psychiatric facility on an involuntary hold. Mr. F has had multiple previous hospitalizations, none of which had resulted in successful treatment. According to his most recent records, Mr. F failed to improve while taking olanzapine. Upon examination, Mr. F reports he hears command auditory hallucinations to hurt others and endorses paranoia. He is agitated, with a constricted affect, and his thought content is paranoid, disorganized, and circumstantial. Mr. F provides vague and evasive answers upon admission. His physical examination is unremarkable. He has an eighth-grade education level and limited insight into his illnesses. His Positive and Negative Syndrome Scale (PANSS) score is 122, indicating severe symptoms. The PANSS score is formulated based on 30 items, each scored between 1 and 7. Higher scores indicate more severe symptoms.
[polldaddy:11167946]
The authors’ observations
Compared to other medically ill patients, those with AIDS are 7 times more likely to experience EPS associated with antipsychotics. This may be a result of HIV infiltration of the basal ganglia causing regional changes that predispose these patients to EPS.
[polldaddy:11167948]
TREATMENT Haloperidol and antiretroviral therapy
The treatment team decides to start Mr. F on haloperidol for his psychotic symptoms as well as bictegravir, emtricitabine, and tenofovir for HIV. One week after admission, the team starts Mr. F on haloperidol decanoate 150 mg IM, and continues oral haloperidol and antiretroviral therapy. Mr. F reports some improvement in his hallucinations and appears to have reduced paranoia. He attends psychotherapy treatment groups over the next several days and scores 80 on a retrospective PANSS assessment (Figure 1). Mr. F receives haloperidol decanoate 200 mg IM 28 days after his first dose, and his oral haloperidol dose is reduced.

During the following 2 weeks, Mr. F endorses continued improvement of his symptoms and insight and begins discharge planning by calling his sister to discuss living arrangements. However, his mental state begins to decline; he becomes paranoid, withdrawn, and irritable, and endorses increased hallucinations. His PANSS score is 87, and he scores 11 on the Montreal Cognitive Assessment (MoCA), indicating moderate cognitive impairment. MoCA scores range from 0 to 30, with scores <10 indicating severe impairment, 10 to 17 indicating moderate impairment, 18 to 25 indicating mild impairment, and 26 to 30 considered normal. Figure 2 shows a timeline of Mr. F’s MoCA scores during treatment.

The treatment team increases the dose of haloperidol, and Mr. F continues to receive haloperidol deaconate injections monthly. After an adequate trial of haloperidol, the patient exhibits only partial response to treatment—his symptoms wax and wane—and he continues to display limited insight into both his mental illness and HIV diagnosis. Another PANSS assessment yields an essentially unchanged score of 88.
After a discussion of risks and benefits, Mr. F consents to initiating clozapine. The treatment team starts clozapine 25 mg/d and increases the dosage to 400 mg in the evening with a concomitant clozapine level of 487 ng/mL. Mr. F’s absolute neutrophil count was within normal limits (2,500 to 6,000 µL) during this period for weekly complete blood cell count monitoring. Over the next few weeks, his MoCA score increases to 17 and PANSS score decreases to 52. Haloperidol decanoate 200 mg IM is discontinued 3 days after Mr. F received a dose of clozapine 400 mg at bedtime. After an additional 2 weeks of clozapine at the same dosage, Mr. F scores 20 on the MoCA, an increase of 9 points from his baseline score while receiving haloperidol. There is a washout period for haloperidol decanoate and oral haloperidol before he completes a third MoCA. Mr. F participates in a discussion regarding his HIV diagnosis and the importance of consistently continuing treatment for this chronic infection. After some education, he has a better understanding of his condition and is more insightful about wanting to remain compliant with clozapine and bictegravir, emtricitabine, and tenofovir for his HIV.
The authors’ observations
Many patients receive treatment for comorbid HIV and schizophrenia. Patients with schizophrenia and other psychoses are at increased risk of contracting HIV due to numerous psychosocial factors, including an increased frequency of illicit drug use as well as an increased propensity for high-risk sexual behaviors secondary to impaired neurocognitive functioning, delusions, and victimization.1 In addition to deficits in functioning related to psychiatric illness, patients with HIV also experience virus-related neurocognitive insults. After crossing the blood-brain barrier, HIV viral proteins circulate in the blood, inducing brain endothelial cells to release cytokines, causing neuroinflammation.2
Continue to: Recently, inflammation and inflammatory...
Recently, inflammation and inflammatory biomarkers have become an important topic of psychiatric research. A meta-analysis by Fraguas et al3 concluded that greater inflammation and oxidative stress might lead to poorer outcomes in patients with first-episode psychosis. Based on this evidence, inflammation associated with untreated HIV infection may compound the pre-existing neurocognitive decline seen in patients with schizophrenia and other psychoses, thereby contributing to poor outcomes and treatment-resistant pathology.
Clozapine has been the superior treatment for refractory and nonrefractory schizophrenia.4 Factor et al5 report there are limited basal ganglia reserves in patients with HIV, which make clozapine the preferred option due to its low potential for causing EPS.
In this case, starting Mr. F on clozapine and titrating to therapeutic blood levels was associated with improved MoCA scores. Low MoCA scores could be due to untreated HIV, as well as inadequately treated psychosis. For Mr. F, improved MoCA scores were associated with increased insight into his HIV. It is important to note that Mr. F’s improved MoCA score also coincided with discontinuing monthly haloperidol decanoate injections. Haloperidol and its metabolites are believed to cause some neurotoxicity at high doses, and can contribute to cognitive impairment. This may partially explain the increased MoCA score after Mr. F stopped receiving haloperidol decanoate monthly injections.6 For the first time, he felt the need to be on antiretroviral therapy for his HIV, and was able to understand the chronic nature of HIV infection.
The benefit of clozapine treatment for patients with schizophrenia and comorbid HIV extends beyond symptomatic control. Long-term and consistent treatment of schizophrenia can be a stepping stone for improving many psychosocial factors. Improved insight allows patients to better understand their illness, treatment regimen, and follow-up needs. Improved self-care contributes to increased adherence to treatment regimens and overall health.
It is likely that patients who are consistently treated for schizophrenia will also have an increased capacity to understand their HIV diagnosis. With gained understanding, patients may be more likely to adhere to highly active antiretroviral therapy (HAART) for HIV and attend follow-up appointments with infectious disease or primary care physicians. Furthermore, with adherence to HAART therapy, patients can enjoy improved quality and duration of life by raising CD4 counts and preventing progression to AIDS and AIDS-related infections.
Continue to: In the case of...
In the case of Mr. F, we noted significant improvement in MoCA scores following treatment with clozapine. This led to improved insight into understanding the chronicity of HIV, understanding the complications of not being treated, and adherence to HAART medication. Improved cognition, as evidenced by an increased MoCA score, can significantly improve patient insight and adherence with medication.7 Insight into illness is particularly important when managing a patient with a chronic infectious illness such as HIV, where consistency with the medication regimen can decrease mortality and improve quality of life.8 Furthermore, with close monitoring, clozapine was a safe treatment option for this patient with HIV and schizophrenia.
Bottom Line
Patients with schizophrenia are at an increased risk of contracting HIV, and untreated schizophrenia decreases the likelihood patients will adhere to highly active antiretroviral therapy (HAART). Clozapine treatment in comorbid HIV and schizophrenia can improve cognition and insight into HIV diagnosis, possibly increasing the likelihood patients will remain compliant with HAART.
Related Resources
- Diduch MN, Campbell RH, Borovicka M, et al. Treating psychosis in patients with HIV/AIDS. Current Psychiatry. 2018;17(5):35-36,41-44,46.
Drug Brand Names
Bictegravir, emtricitabine, and tenofovir • Biktarvy
Clozapine • Clozaril
Haloperidol • Haldol
Haloperidol decanoate • Haldol decanoate
Olanzapine • Zyprexa
Ziprasidone • Geodon
CASE Psychotic episode in a patient with HIV
Mr. F, age 32, has schizophrenia and HIV. He presents to the emergency department with auditory and visual hallucinations in addition to paranoia. The treatment team refers him to the state psychiatric facility on an involuntary hold. Mr. F has had multiple previous hospitalizations, none of which had resulted in successful treatment. According to his most recent records, Mr. F failed to improve while taking olanzapine. Upon examination, Mr. F reports he hears command auditory hallucinations to hurt others and endorses paranoia. He is agitated, with a constricted affect, and his thought content is paranoid, disorganized, and circumstantial. Mr. F provides vague and evasive answers upon admission. His physical examination is unremarkable. He has an eighth-grade education level and limited insight into his illnesses. His Positive and Negative Syndrome Scale (PANSS) score is 122, indicating severe symptoms. The PANSS score is formulated based on 30 items, each scored between 1 and 7. Higher scores indicate more severe symptoms.
[polldaddy:11167946]
The authors’ observations
Compared to other medically ill patients, those with AIDS are 7 times more likely to experience EPS associated with antipsychotics. This may be a result of HIV infiltration of the basal ganglia causing regional changes that predispose these patients to EPS.
[polldaddy:11167948]
TREATMENT Haloperidol and antiretroviral therapy
The treatment team decides to start Mr. F on haloperidol for his psychotic symptoms as well as bictegravir, emtricitabine, and tenofovir for HIV. One week after admission, the team starts Mr. F on haloperidol decanoate 150 mg IM, and continues oral haloperidol and antiretroviral therapy. Mr. F reports some improvement in his hallucinations and appears to have reduced paranoia. He attends psychotherapy treatment groups over the next several days and scores 80 on a retrospective PANSS assessment (Figure 1). Mr. F receives haloperidol decanoate 200 mg IM 28 days after his first dose, and his oral haloperidol dose is reduced.

During the following 2 weeks, Mr. F endorses continued improvement of his symptoms and insight and begins discharge planning by calling his sister to discuss living arrangements. However, his mental state begins to decline; he becomes paranoid, withdrawn, and irritable, and endorses increased hallucinations. His PANSS score is 87, and he scores 11 on the Montreal Cognitive Assessment (MoCA), indicating moderate cognitive impairment. MoCA scores range from 0 to 30, with scores <10 indicating severe impairment, 10 to 17 indicating moderate impairment, 18 to 25 indicating mild impairment, and 26 to 30 considered normal. Figure 2 shows a timeline of Mr. F’s MoCA scores during treatment.

The treatment team increases the dose of haloperidol, and Mr. F continues to receive haloperidol deaconate injections monthly. After an adequate trial of haloperidol, the patient exhibits only partial response to treatment—his symptoms wax and wane—and he continues to display limited insight into both his mental illness and HIV diagnosis. Another PANSS assessment yields an essentially unchanged score of 88.
After a discussion of risks and benefits, Mr. F consents to initiating clozapine. The treatment team starts clozapine 25 mg/d and increases the dosage to 400 mg in the evening with a concomitant clozapine level of 487 ng/mL. Mr. F’s absolute neutrophil count was within normal limits (2,500 to 6,000 µL) during this period for weekly complete blood cell count monitoring. Over the next few weeks, his MoCA score increases to 17 and PANSS score decreases to 52. Haloperidol decanoate 200 mg IM is discontinued 3 days after Mr. F received a dose of clozapine 400 mg at bedtime. After an additional 2 weeks of clozapine at the same dosage, Mr. F scores 20 on the MoCA, an increase of 9 points from his baseline score while receiving haloperidol. There is a washout period for haloperidol decanoate and oral haloperidol before he completes a third MoCA. Mr. F participates in a discussion regarding his HIV diagnosis and the importance of consistently continuing treatment for this chronic infection. After some education, he has a better understanding of his condition and is more insightful about wanting to remain compliant with clozapine and bictegravir, emtricitabine, and tenofovir for his HIV.
The authors’ observations
Many patients receive treatment for comorbid HIV and schizophrenia. Patients with schizophrenia and other psychoses are at increased risk of contracting HIV due to numerous psychosocial factors, including an increased frequency of illicit drug use as well as an increased propensity for high-risk sexual behaviors secondary to impaired neurocognitive functioning, delusions, and victimization.1 In addition to deficits in functioning related to psychiatric illness, patients with HIV also experience virus-related neurocognitive insults. After crossing the blood-brain barrier, HIV viral proteins circulate in the blood, inducing brain endothelial cells to release cytokines, causing neuroinflammation.2
Continue to: Recently, inflammation and inflammatory...
Recently, inflammation and inflammatory biomarkers have become an important topic of psychiatric research. A meta-analysis by Fraguas et al3 concluded that greater inflammation and oxidative stress might lead to poorer outcomes in patients with first-episode psychosis. Based on this evidence, inflammation associated with untreated HIV infection may compound the pre-existing neurocognitive decline seen in patients with schizophrenia and other psychoses, thereby contributing to poor outcomes and treatment-resistant pathology.
Clozapine has been the superior treatment for refractory and nonrefractory schizophrenia.4 Factor et al5 report there are limited basal ganglia reserves in patients with HIV, which make clozapine the preferred option due to its low potential for causing EPS.
In this case, starting Mr. F on clozapine and titrating to therapeutic blood levels was associated with improved MoCA scores. Low MoCA scores could be due to untreated HIV, as well as inadequately treated psychosis. For Mr. F, improved MoCA scores were associated with increased insight into his HIV. It is important to note that Mr. F’s improved MoCA score also coincided with discontinuing monthly haloperidol decanoate injections. Haloperidol and its metabolites are believed to cause some neurotoxicity at high doses, and can contribute to cognitive impairment. This may partially explain the increased MoCA score after Mr. F stopped receiving haloperidol decanoate monthly injections.6 For the first time, he felt the need to be on antiretroviral therapy for his HIV, and was able to understand the chronic nature of HIV infection.
The benefit of clozapine treatment for patients with schizophrenia and comorbid HIV extends beyond symptomatic control. Long-term and consistent treatment of schizophrenia can be a stepping stone for improving many psychosocial factors. Improved insight allows patients to better understand their illness, treatment regimen, and follow-up needs. Improved self-care contributes to increased adherence to treatment regimens and overall health.
It is likely that patients who are consistently treated for schizophrenia will also have an increased capacity to understand their HIV diagnosis. With gained understanding, patients may be more likely to adhere to highly active antiretroviral therapy (HAART) for HIV and attend follow-up appointments with infectious disease or primary care physicians. Furthermore, with adherence to HAART therapy, patients can enjoy improved quality and duration of life by raising CD4 counts and preventing progression to AIDS and AIDS-related infections.
Continue to: In the case of...
In the case of Mr. F, we noted significant improvement in MoCA scores following treatment with clozapine. This led to improved insight into understanding the chronicity of HIV, understanding the complications of not being treated, and adherence to HAART medication. Improved cognition, as evidenced by an increased MoCA score, can significantly improve patient insight and adherence with medication.7 Insight into illness is particularly important when managing a patient with a chronic infectious illness such as HIV, where consistency with the medication regimen can decrease mortality and improve quality of life.8 Furthermore, with close monitoring, clozapine was a safe treatment option for this patient with HIV and schizophrenia.
Bottom Line
Patients with schizophrenia are at an increased risk of contracting HIV, and untreated schizophrenia decreases the likelihood patients will adhere to highly active antiretroviral therapy (HAART). Clozapine treatment in comorbid HIV and schizophrenia can improve cognition and insight into HIV diagnosis, possibly increasing the likelihood patients will remain compliant with HAART.
Related Resources
- Diduch MN, Campbell RH, Borovicka M, et al. Treating psychosis in patients with HIV/AIDS. Current Psychiatry. 2018;17(5):35-36,41-44,46.
Drug Brand Names
Bictegravir, emtricitabine, and tenofovir • Biktarvy
Clozapine • Clozaril
Haloperidol • Haldol
Haloperidol decanoate • Haldol decanoate
Olanzapine • Zyprexa
Ziprasidone • Geodon
1. Bahorik AL, Newhill CE, Eack SM. Neurocognitive functioning of individuals with schizophrenia: using and not using drugs. Schizophrenia Bull. 2014;40(4):856-867. doi:10.1093/schbul/sbt099
2. Hong S, Banks WA. Role of the immune system in HIV-associated neuroinflammation and neurocognitive implications. Brain Behav Immun. 2015;45:1-12. doi:10.1016/j.bbi.2014.10.008
3. Fraguas D, Díaz-Caneja CM, Rodríguez-Quiroga A, et al. Oxidative stress and inflammation in early onset first episode psychosis: a systematic review and meta-analysis. Int J Neuropsychopharmacol. 2017;20(6):435-444. doi:10.1093/ijnp/pyx015
4. Wahlbeck K, Cheine M, Essali A, et al. Evidence of clozapine’s effectiveness in schizophrenia: a systematic review and meta-analysis of randomized trials. Am J Psychiatry. 1999;156(7):990-999.
5. Factor SA, Brown D, Molho ES, et al. Clozapine: a 2-year open trial in Parkinson’s disease patients with psychosis. Neurology. 1994;44(3 Pt 1):544-546.
6. Raudenska M, Gumulec J, Babula P, et al. Haloperidol cytotoxicity and its relation to oxidative stress. Mini Rev Med Chem. 2013;13(14):1993-1998. doi:10.2174/13895575113136660100
7. El Abdellati K, De Picker L, Morrens M. Antipsychotic treatment failure: a systematic review on risk factors and interventions for treatment adherence in psychosis. Front Neurosci. 2020;14:531763. doi:10.3389/fnins.2020.531763
8. Margalho R, Pereira M, Ouakinin S, et al. Adesão à HAART, qualidade de vida e sintomat ologia psicopat ológica em doentes infectados pelo VIH/SIDA [Adherence to HAART, quality of life and psychopathological symptoms among HIV/AIDS infected patients]. Acta Med Port. 2011;24 Suppl 2:539-548.
1. Bahorik AL, Newhill CE, Eack SM. Neurocognitive functioning of individuals with schizophrenia: using and not using drugs. Schizophrenia Bull. 2014;40(4):856-867. doi:10.1093/schbul/sbt099
2. Hong S, Banks WA. Role of the immune system in HIV-associated neuroinflammation and neurocognitive implications. Brain Behav Immun. 2015;45:1-12. doi:10.1016/j.bbi.2014.10.008
3. Fraguas D, Díaz-Caneja CM, Rodríguez-Quiroga A, et al. Oxidative stress and inflammation in early onset first episode psychosis: a systematic review and meta-analysis. Int J Neuropsychopharmacol. 2017;20(6):435-444. doi:10.1093/ijnp/pyx015
4. Wahlbeck K, Cheine M, Essali A, et al. Evidence of clozapine’s effectiveness in schizophrenia: a systematic review and meta-analysis of randomized trials. Am J Psychiatry. 1999;156(7):990-999.
5. Factor SA, Brown D, Molho ES, et al. Clozapine: a 2-year open trial in Parkinson’s disease patients with psychosis. Neurology. 1994;44(3 Pt 1):544-546.
6. Raudenska M, Gumulec J, Babula P, et al. Haloperidol cytotoxicity and its relation to oxidative stress. Mini Rev Med Chem. 2013;13(14):1993-1998. doi:10.2174/13895575113136660100
7. El Abdellati K, De Picker L, Morrens M. Antipsychotic treatment failure: a systematic review on risk factors and interventions for treatment adherence in psychosis. Front Neurosci. 2020;14:531763. doi:10.3389/fnins.2020.531763
8. Margalho R, Pereira M, Ouakinin S, et al. Adesão à HAART, qualidade de vida e sintomat ologia psicopat ológica em doentes infectados pelo VIH/SIDA [Adherence to HAART, quality of life and psychopathological symptoms among HIV/AIDS infected patients]. Acta Med Port. 2011;24 Suppl 2:539-548.
Hope, help, and humor when facing a life-threatening illness
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in
My father, Morty Sosland, MD, was a psychiatrist in a community health setting when he was diagnosed with amyotrophic lateral sclerosis (ALS; Lou Gehrig’s disease) in April 2020. He continued to work until February 2021 and credits his ongoing resilience to what he refers to as “the 3 Hs”: hope, help, and humor. Although he can no longer speak, I was able to interview him over the advanced technology that is text messaging.
Sarah: Hi, Dad.
Morty: It’s Doctor Dad to you.
Sarah: I guess we are starting with humor, then?
Humor
Research has demonstrated that humor can have serious health benefits, such as decreasing stress-making hormones and altering dopamine activity.1 For individuals facing a life-threatening illness, humor can help them gain a sense of perspective in a situation that would otherwise feel overwhelming.
Sarah: I feel like a lot of the humor you used with patients was to help them gain perspective.
Continue to: Morty
Morty: Yes. I’d have to know the client well enough, though—and timing is important. My patients would come to me with a long list of challenges they had faced in the week, and I would say, “But besides that, everything’s good?”
Sarah: And besides the ALS, everything’s good?
Morty: Exactly. I’d also use magic or math tricks to make kids like coming to therapy or to reinforce important concepts.
Sarah: How has humor helped you cope?
Morty: Thinking about things in humorous ways has always been helpful. I used to say my Olympic sport was walking to the dining room with my walker. Unfortunately, I can’t do that anymore, so now my Olympic sport is getting out of bed. It’s a team sport.
Sarah: And that’s a good segue to…
Help
Countless studies have shown the impact of social support on health. Good social support can increase resilience, protect against mental illness, and even increase life expectancy.2 Support becomes even more critical when you are physically dependent on others due to illness.
Continue to: Sarah
Sarah: Was it difficult for you to accept help at first?
Morty: I would say yes—but at the same time, I accepted it because the illness was so shocking. I learned early on this was a fight that my family would also fight alongside me.
Sarah: I remember you would quote Fred Rogers.
Morty: Actually, it was Fred Rogers’ mother. She would tell her son during hard times, “Look for the helpers. You will always find people who are helping.” Helpers can be family members, friends, doctors, and aides, as well as others who have the same illness.
Hope
In the face of all life’s challenges, hope is important, but in the face of a life-threatening illness, hope must be multifaceted.3 In addition to hope for a cure, patients may focus their hopes on deepening relationships, maintaining dignity, or living each day to its fullest.
Morty: Early on in this illness, I chose to set a positive tone when I told people. I would say I have the top doctors and there is more research now than ever. Years ago, I wrote a children’s book with the mantra, “I say I can, I make a plan, I get right to it and then I do it.”4 My plan is to be around for at least 30 more years.
Sarah: Do you think it’s possible to hold acceptance and hope at the same time?
Morty: Acceptance and hope are not easy, but possible. I get down about this illness. In my dreams, I walk and talk, and most mornings I wake up and see my wheelchair and I think this is absurd or a different choice word. But I focus on the things I still can do, and that gives me a feeling of hope. I can read the latest research, I can enjoy moments of laughter, and I can spend time with my family and close friends.
1. Yim J. Therapeutic benefits of laughter in mental health: a theoretical review. Tohoku J Exp Med. 2016;239(3):243-249. doi:10.1620/tjem.239.243
2. Ozbay F, Johnson DC, Dimoulas E, et al. Social support and resilience to stress: from neurobiology to clinical practice. Psychiatry (Edgmont). 2007;4(5):35-40.
3. Hill DL, Feudnter C. Hope in the midst of terminal illness. In: Gallagher MW, Lopez SJ, eds. The Oxford Handbook of Hope. Oxford University Press; 2018:191-206.
4. Sosland MD. The Can Do Duck: A Story About Believing in Yourself. Can Do Duck Publishing; 2019.
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in
My father, Morty Sosland, MD, was a psychiatrist in a community health setting when he was diagnosed with amyotrophic lateral sclerosis (ALS; Lou Gehrig’s disease) in April 2020. He continued to work until February 2021 and credits his ongoing resilience to what he refers to as “the 3 Hs”: hope, help, and humor. Although he can no longer speak, I was able to interview him over the advanced technology that is text messaging.
Sarah: Hi, Dad.
Morty: It’s Doctor Dad to you.
Sarah: I guess we are starting with humor, then?
Humor
Research has demonstrated that humor can have serious health benefits, such as decreasing stress-making hormones and altering dopamine activity.1 For individuals facing a life-threatening illness, humor can help them gain a sense of perspective in a situation that would otherwise feel overwhelming.
Sarah: I feel like a lot of the humor you used with patients was to help them gain perspective.
Continue to: Morty
Morty: Yes. I’d have to know the client well enough, though—and timing is important. My patients would come to me with a long list of challenges they had faced in the week, and I would say, “But besides that, everything’s good?”
Sarah: And besides the ALS, everything’s good?
Morty: Exactly. I’d also use magic or math tricks to make kids like coming to therapy or to reinforce important concepts.
Sarah: How has humor helped you cope?
Morty: Thinking about things in humorous ways has always been helpful. I used to say my Olympic sport was walking to the dining room with my walker. Unfortunately, I can’t do that anymore, so now my Olympic sport is getting out of bed. It’s a team sport.
Sarah: And that’s a good segue to…
Help
Countless studies have shown the impact of social support on health. Good social support can increase resilience, protect against mental illness, and even increase life expectancy.2 Support becomes even more critical when you are physically dependent on others due to illness.
Continue to: Sarah
Sarah: Was it difficult for you to accept help at first?
Morty: I would say yes—but at the same time, I accepted it because the illness was so shocking. I learned early on this was a fight that my family would also fight alongside me.
Sarah: I remember you would quote Fred Rogers.
Morty: Actually, it was Fred Rogers’ mother. She would tell her son during hard times, “Look for the helpers. You will always find people who are helping.” Helpers can be family members, friends, doctors, and aides, as well as others who have the same illness.
Hope
In the face of all life’s challenges, hope is important, but in the face of a life-threatening illness, hope must be multifaceted.3 In addition to hope for a cure, patients may focus their hopes on deepening relationships, maintaining dignity, or living each day to its fullest.
Morty: Early on in this illness, I chose to set a positive tone when I told people. I would say I have the top doctors and there is more research now than ever. Years ago, I wrote a children’s book with the mantra, “I say I can, I make a plan, I get right to it and then I do it.”4 My plan is to be around for at least 30 more years.
Sarah: Do you think it’s possible to hold acceptance and hope at the same time?
Morty: Acceptance and hope are not easy, but possible. I get down about this illness. In my dreams, I walk and talk, and most mornings I wake up and see my wheelchair and I think this is absurd or a different choice word. But I focus on the things I still can do, and that gives me a feeling of hope. I can read the latest research, I can enjoy moments of laughter, and I can spend time with my family and close friends.
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in
My father, Morty Sosland, MD, was a psychiatrist in a community health setting when he was diagnosed with amyotrophic lateral sclerosis (ALS; Lou Gehrig’s disease) in April 2020. He continued to work until February 2021 and credits his ongoing resilience to what he refers to as “the 3 Hs”: hope, help, and humor. Although he can no longer speak, I was able to interview him over the advanced technology that is text messaging.
Sarah: Hi, Dad.
Morty: It’s Doctor Dad to you.
Sarah: I guess we are starting with humor, then?
Humor
Research has demonstrated that humor can have serious health benefits, such as decreasing stress-making hormones and altering dopamine activity.1 For individuals facing a life-threatening illness, humor can help them gain a sense of perspective in a situation that would otherwise feel overwhelming.
Sarah: I feel like a lot of the humor you used with patients was to help them gain perspective.
Continue to: Morty
Morty: Yes. I’d have to know the client well enough, though—and timing is important. My patients would come to me with a long list of challenges they had faced in the week, and I would say, “But besides that, everything’s good?”
Sarah: And besides the ALS, everything’s good?
Morty: Exactly. I’d also use magic or math tricks to make kids like coming to therapy or to reinforce important concepts.
Sarah: How has humor helped you cope?
Morty: Thinking about things in humorous ways has always been helpful. I used to say my Olympic sport was walking to the dining room with my walker. Unfortunately, I can’t do that anymore, so now my Olympic sport is getting out of bed. It’s a team sport.
Sarah: And that’s a good segue to…
Help
Countless studies have shown the impact of social support on health. Good social support can increase resilience, protect against mental illness, and even increase life expectancy.2 Support becomes even more critical when you are physically dependent on others due to illness.
Continue to: Sarah
Sarah: Was it difficult for you to accept help at first?
Morty: I would say yes—but at the same time, I accepted it because the illness was so shocking. I learned early on this was a fight that my family would also fight alongside me.
Sarah: I remember you would quote Fred Rogers.
Morty: Actually, it was Fred Rogers’ mother. She would tell her son during hard times, “Look for the helpers. You will always find people who are helping.” Helpers can be family members, friends, doctors, and aides, as well as others who have the same illness.
Hope
In the face of all life’s challenges, hope is important, but in the face of a life-threatening illness, hope must be multifaceted.3 In addition to hope for a cure, patients may focus their hopes on deepening relationships, maintaining dignity, or living each day to its fullest.
Morty: Early on in this illness, I chose to set a positive tone when I told people. I would say I have the top doctors and there is more research now than ever. Years ago, I wrote a children’s book with the mantra, “I say I can, I make a plan, I get right to it and then I do it.”4 My plan is to be around for at least 30 more years.
Sarah: Do you think it’s possible to hold acceptance and hope at the same time?
Morty: Acceptance and hope are not easy, but possible. I get down about this illness. In my dreams, I walk and talk, and most mornings I wake up and see my wheelchair and I think this is absurd or a different choice word. But I focus on the things I still can do, and that gives me a feeling of hope. I can read the latest research, I can enjoy moments of laughter, and I can spend time with my family and close friends.
1. Yim J. Therapeutic benefits of laughter in mental health: a theoretical review. Tohoku J Exp Med. 2016;239(3):243-249. doi:10.1620/tjem.239.243
2. Ozbay F, Johnson DC, Dimoulas E, et al. Social support and resilience to stress: from neurobiology to clinical practice. Psychiatry (Edgmont). 2007;4(5):35-40.
3. Hill DL, Feudnter C. Hope in the midst of terminal illness. In: Gallagher MW, Lopez SJ, eds. The Oxford Handbook of Hope. Oxford University Press; 2018:191-206.
4. Sosland MD. The Can Do Duck: A Story About Believing in Yourself. Can Do Duck Publishing; 2019.
1. Yim J. Therapeutic benefits of laughter in mental health: a theoretical review. Tohoku J Exp Med. 2016;239(3):243-249. doi:10.1620/tjem.239.243
2. Ozbay F, Johnson DC, Dimoulas E, et al. Social support and resilience to stress: from neurobiology to clinical practice. Psychiatry (Edgmont). 2007;4(5):35-40.
3. Hill DL, Feudnter C. Hope in the midst of terminal illness. In: Gallagher MW, Lopez SJ, eds. The Oxford Handbook of Hope. Oxford University Press; 2018:191-206.
4. Sosland MD. The Can Do Duck: A Story About Believing in Yourself. Can Do Duck Publishing; 2019.
Pregnancy termination: What psychiatrists need to know
Approximately half of pregnancies in the United States are unplanned, and approximately one-fifth of pregnancies end in elective termination.1 Psychiatrists who treat women of childbearing potential should understand critical aspects of abortion that could affect their patients’ mental health.
Discuss the potential for pregnancy with your patients. Individuals with psychiatric illness are less likely to adhere to contraceptive methods and are more likely to have unplanned pregnancies and detect pregnancies late.2 Women receiving psychiatric care could be at risk of not detecting pregnancy early enough to meet state laws that restrict the time frames in which abortions are allowed.
Understand that patients face barriers to abortion. Almost immediately after the Supreme Court overturned Roe v Wade in June 2022, abortion became illegal in several states. Even if abortion remains legal and available in your jurisdiction, patients could face barriers, including strict limits on abortion timing, monetary and travel challenges, preabortion counseling mandates, and timely access to an abortion provider.
Know that most patients can provide informed consent. Most patients with psychiatric illness have capacity to make medical decisions, including whether to consent to an abortion. Pro forma assessment is not necessary. Assessing capacity to consent to abortion should be the same as any other capacity assessment. If a woman lacks medical decision-making capacity, a substitute decision-maker must be used.
Recognize that ambivalence is normal. Even when a woman is certain about her decision to terminate a pregnancy, she might experience ambivalence. Ambivalence about important life decisions is common and should be validated and explored.3
Be aware of bias. As psychiatrists, we must ensure that our personal opinions about abortion do not impact patient care. An impartial and nondirective approach is key, and any effort to persuade or manipulate a woman’s decision is unethical. Because women with mental illness might be vulnerable to coercion, it is important to ensure that the woman’s choice is voluntary.
Accurately communicate information about mental health and abortion to your patients. Abortion does not worsen mental health. Research on abortion and mental health is rife with poorly designed studies that contain methodological flaws, including failure to control for confounding effects, such as pre-existing mental illness, and inadequate control group comparisons.4 For example, the correct comparison group in which to consider mental health outcomes for women who are seeking an abortion is those who sought an abortion but were not able to have one—not women with planned and desired pregnancies. The best predictor of postabortion mental wellness is preabortion mental health.5 Well-designed studies, such as the Turnaway Study, have demonstrated that abortion does not cause a significant increase in mental illness.6 The Turnaway Study was a well-designed, prospective study of thousands of women who obtained a wanted abortion. It compared many outcomes, including mental health, among women who wanted an abortion vs women who could not obtain a wanted abortion.
Continue to: Know that patients might not receive accurate information about the risks and impact of abortion
Know that patients might not receive accurate information about the risks and impact of abortion. A number of states have requirements—known as “informed consent laws”—that mandate physicians to provide state-authored informational packets about the risks and alternatives to abortion to patients seeking abortions. Some of this information is scientifically inaccurate, which poses a significant ethical dilemma for doctors who must choose between legal requirements and an obligation to scientific integrity.7
Recognize that abortion being illegal could negatively impact mental health. The consequences of being forced to carry out an unwanted pregnancy are profound. Women unable to obtain an abortion are more likely to have adverse health and pregnancy outcomes, live in poverty, stay with an abusive partner, and have difficulty bonding with the child.6 Abortion is highly stigmatized in the United States, and belonging to a stigmatized group is a risk factor for adverse mental health sequalae, including anxiety, depression, substance use, and cognitive deficits.4-6
Stay up-to-date on your state’s abortion laws. The legal landscape regarding abortion is changing rapidly, and it is important to stay abreast of these changes.
Restrictions on abortion likely will significantly affect women with psychiatric illness. As psychiatrists, we must be aware of the impact of the country’s changing laws will have on our patients and their mental health.
1. Guttmacher Institute. Accessed July 21, 2022. https://www.guttmacher.org/
2. Miller LJ. Sexuality, reproduction, and family planning in women with schizophrenia. Schizophr Bull. 1997;23(4):623-635. doi:10.1093/schbul/23.4.623
3. Brody BD, Chaudhry SK, Penzner JB, et al. A woman with major depression with psychotic features requesting a termination of pregnancy. Am J Psychiatry. 2016;173(1):12-15. doi:10.1176/appi.ajp.2015.15030380
4. Major B, Appelbaum M, Beckman L, et al. Abortion and mental health: Evaluating the evidence. Am Psychol. 2009;64(9):863-890. doi:10.1037/a0017497
5. Steinberg JR, Tschann JM, Furgerson D, et al. Psychosocial factors and pre-abortion psychological health: the significance of stigma. Soc Sci Med. 2016;150:67-75. doi:10.1016/j.socscimed.2015.12.007
6. ANSIRH. The Turnaway Study. Accessed June 29, 2022. https://www.ansirh.org/research/ongoing/turnaway-study
7. Daniels CR, Ferguson J, Howard G, et al. Informed or misinformed consent? Abortion policy in the United States. J Health Polit Policy Law. 2016;41(2):181-209. doi:10.1215/03616878-3476105
Approximately half of pregnancies in the United States are unplanned, and approximately one-fifth of pregnancies end in elective termination.1 Psychiatrists who treat women of childbearing potential should understand critical aspects of abortion that could affect their patients’ mental health.
Discuss the potential for pregnancy with your patients. Individuals with psychiatric illness are less likely to adhere to contraceptive methods and are more likely to have unplanned pregnancies and detect pregnancies late.2 Women receiving psychiatric care could be at risk of not detecting pregnancy early enough to meet state laws that restrict the time frames in which abortions are allowed.
Understand that patients face barriers to abortion. Almost immediately after the Supreme Court overturned Roe v Wade in June 2022, abortion became illegal in several states. Even if abortion remains legal and available in your jurisdiction, patients could face barriers, including strict limits on abortion timing, monetary and travel challenges, preabortion counseling mandates, and timely access to an abortion provider.
Know that most patients can provide informed consent. Most patients with psychiatric illness have capacity to make medical decisions, including whether to consent to an abortion. Pro forma assessment is not necessary. Assessing capacity to consent to abortion should be the same as any other capacity assessment. If a woman lacks medical decision-making capacity, a substitute decision-maker must be used.
Recognize that ambivalence is normal. Even when a woman is certain about her decision to terminate a pregnancy, she might experience ambivalence. Ambivalence about important life decisions is common and should be validated and explored.3
Be aware of bias. As psychiatrists, we must ensure that our personal opinions about abortion do not impact patient care. An impartial and nondirective approach is key, and any effort to persuade or manipulate a woman’s decision is unethical. Because women with mental illness might be vulnerable to coercion, it is important to ensure that the woman’s choice is voluntary.
Accurately communicate information about mental health and abortion to your patients. Abortion does not worsen mental health. Research on abortion and mental health is rife with poorly designed studies that contain methodological flaws, including failure to control for confounding effects, such as pre-existing mental illness, and inadequate control group comparisons.4 For example, the correct comparison group in which to consider mental health outcomes for women who are seeking an abortion is those who sought an abortion but were not able to have one—not women with planned and desired pregnancies. The best predictor of postabortion mental wellness is preabortion mental health.5 Well-designed studies, such as the Turnaway Study, have demonstrated that abortion does not cause a significant increase in mental illness.6 The Turnaway Study was a well-designed, prospective study of thousands of women who obtained a wanted abortion. It compared many outcomes, including mental health, among women who wanted an abortion vs women who could not obtain a wanted abortion.
Continue to: Know that patients might not receive accurate information about the risks and impact of abortion
Know that patients might not receive accurate information about the risks and impact of abortion. A number of states have requirements—known as “informed consent laws”—that mandate physicians to provide state-authored informational packets about the risks and alternatives to abortion to patients seeking abortions. Some of this information is scientifically inaccurate, which poses a significant ethical dilemma for doctors who must choose between legal requirements and an obligation to scientific integrity.7
Recognize that abortion being illegal could negatively impact mental health. The consequences of being forced to carry out an unwanted pregnancy are profound. Women unable to obtain an abortion are more likely to have adverse health and pregnancy outcomes, live in poverty, stay with an abusive partner, and have difficulty bonding with the child.6 Abortion is highly stigmatized in the United States, and belonging to a stigmatized group is a risk factor for adverse mental health sequalae, including anxiety, depression, substance use, and cognitive deficits.4-6
Stay up-to-date on your state’s abortion laws. The legal landscape regarding abortion is changing rapidly, and it is important to stay abreast of these changes.
Restrictions on abortion likely will significantly affect women with psychiatric illness. As psychiatrists, we must be aware of the impact of the country’s changing laws will have on our patients and their mental health.
Approximately half of pregnancies in the United States are unplanned, and approximately one-fifth of pregnancies end in elective termination.1 Psychiatrists who treat women of childbearing potential should understand critical aspects of abortion that could affect their patients’ mental health.
Discuss the potential for pregnancy with your patients. Individuals with psychiatric illness are less likely to adhere to contraceptive methods and are more likely to have unplanned pregnancies and detect pregnancies late.2 Women receiving psychiatric care could be at risk of not detecting pregnancy early enough to meet state laws that restrict the time frames in which abortions are allowed.
Understand that patients face barriers to abortion. Almost immediately after the Supreme Court overturned Roe v Wade in June 2022, abortion became illegal in several states. Even if abortion remains legal and available in your jurisdiction, patients could face barriers, including strict limits on abortion timing, monetary and travel challenges, preabortion counseling mandates, and timely access to an abortion provider.
Know that most patients can provide informed consent. Most patients with psychiatric illness have capacity to make medical decisions, including whether to consent to an abortion. Pro forma assessment is not necessary. Assessing capacity to consent to abortion should be the same as any other capacity assessment. If a woman lacks medical decision-making capacity, a substitute decision-maker must be used.
Recognize that ambivalence is normal. Even when a woman is certain about her decision to terminate a pregnancy, she might experience ambivalence. Ambivalence about important life decisions is common and should be validated and explored.3
Be aware of bias. As psychiatrists, we must ensure that our personal opinions about abortion do not impact patient care. An impartial and nondirective approach is key, and any effort to persuade or manipulate a woman’s decision is unethical. Because women with mental illness might be vulnerable to coercion, it is important to ensure that the woman’s choice is voluntary.
Accurately communicate information about mental health and abortion to your patients. Abortion does not worsen mental health. Research on abortion and mental health is rife with poorly designed studies that contain methodological flaws, including failure to control for confounding effects, such as pre-existing mental illness, and inadequate control group comparisons.4 For example, the correct comparison group in which to consider mental health outcomes for women who are seeking an abortion is those who sought an abortion but were not able to have one—not women with planned and desired pregnancies. The best predictor of postabortion mental wellness is preabortion mental health.5 Well-designed studies, such as the Turnaway Study, have demonstrated that abortion does not cause a significant increase in mental illness.6 The Turnaway Study was a well-designed, prospective study of thousands of women who obtained a wanted abortion. It compared many outcomes, including mental health, among women who wanted an abortion vs women who could not obtain a wanted abortion.
Continue to: Know that patients might not receive accurate information about the risks and impact of abortion
Know that patients might not receive accurate information about the risks and impact of abortion. A number of states have requirements—known as “informed consent laws”—that mandate physicians to provide state-authored informational packets about the risks and alternatives to abortion to patients seeking abortions. Some of this information is scientifically inaccurate, which poses a significant ethical dilemma for doctors who must choose between legal requirements and an obligation to scientific integrity.7
Recognize that abortion being illegal could negatively impact mental health. The consequences of being forced to carry out an unwanted pregnancy are profound. Women unable to obtain an abortion are more likely to have adverse health and pregnancy outcomes, live in poverty, stay with an abusive partner, and have difficulty bonding with the child.6 Abortion is highly stigmatized in the United States, and belonging to a stigmatized group is a risk factor for adverse mental health sequalae, including anxiety, depression, substance use, and cognitive deficits.4-6
Stay up-to-date on your state’s abortion laws. The legal landscape regarding abortion is changing rapidly, and it is important to stay abreast of these changes.
Restrictions on abortion likely will significantly affect women with psychiatric illness. As psychiatrists, we must be aware of the impact of the country’s changing laws will have on our patients and their mental health.
1. Guttmacher Institute. Accessed July 21, 2022. https://www.guttmacher.org/
2. Miller LJ. Sexuality, reproduction, and family planning in women with schizophrenia. Schizophr Bull. 1997;23(4):623-635. doi:10.1093/schbul/23.4.623
3. Brody BD, Chaudhry SK, Penzner JB, et al. A woman with major depression with psychotic features requesting a termination of pregnancy. Am J Psychiatry. 2016;173(1):12-15. doi:10.1176/appi.ajp.2015.15030380
4. Major B, Appelbaum M, Beckman L, et al. Abortion and mental health: Evaluating the evidence. Am Psychol. 2009;64(9):863-890. doi:10.1037/a0017497
5. Steinberg JR, Tschann JM, Furgerson D, et al. Psychosocial factors and pre-abortion psychological health: the significance of stigma. Soc Sci Med. 2016;150:67-75. doi:10.1016/j.socscimed.2015.12.007
6. ANSIRH. The Turnaway Study. Accessed June 29, 2022. https://www.ansirh.org/research/ongoing/turnaway-study
7. Daniels CR, Ferguson J, Howard G, et al. Informed or misinformed consent? Abortion policy in the United States. J Health Polit Policy Law. 2016;41(2):181-209. doi:10.1215/03616878-3476105
1. Guttmacher Institute. Accessed July 21, 2022. https://www.guttmacher.org/
2. Miller LJ. Sexuality, reproduction, and family planning in women with schizophrenia. Schizophr Bull. 1997;23(4):623-635. doi:10.1093/schbul/23.4.623
3. Brody BD, Chaudhry SK, Penzner JB, et al. A woman with major depression with psychotic features requesting a termination of pregnancy. Am J Psychiatry. 2016;173(1):12-15. doi:10.1176/appi.ajp.2015.15030380
4. Major B, Appelbaum M, Beckman L, et al. Abortion and mental health: Evaluating the evidence. Am Psychol. 2009;64(9):863-890. doi:10.1037/a0017497
5. Steinberg JR, Tschann JM, Furgerson D, et al. Psychosocial factors and pre-abortion psychological health: the significance of stigma. Soc Sci Med. 2016;150:67-75. doi:10.1016/j.socscimed.2015.12.007
6. ANSIRH. The Turnaway Study. Accessed June 29, 2022. https://www.ansirh.org/research/ongoing/turnaway-study
7. Daniels CR, Ferguson J, Howard G, et al. Informed or misinformed consent? Abortion policy in the United States. J Health Polit Policy Law. 2016;41(2):181-209. doi:10.1215/03616878-3476105
The impact of COVID-19 on adolescents’ mental health
While the COVID-19 pandemic has impacted the mental health of a wide range of individuals, its adverse effects have been particularly detrimental to adolescents. In this article, I discuss evidence that shows the effects of the pandemic on adolescent patients, potential reasons for this increased distress, and what types of coping mechanisms adolescents have used to counter these effects.
Increases in multiple measures of psychopathology
Multiple online surveys and other studies have documented the pandemic’s impact on younger individuals. In the United States, visits to emergency departments by pediatric patients increased in the months after the first lockdown period.1 Several studies found increased rates of anxiety and depression among adolescents during the COVID-19 pandemic.2,3 In an online survey of 359 children and 3,254 adolescents in China, 22% of respondents reported that they experienced depressive symptoms.3 In an online survey of 1,054 Canadian adolescents, 43% said they were “very concerned” about the pandemic.4 In an online survey of 7,353 adolescents in the United States, 37% reported suicidal ideation during the pandemic compared to 17% in 2017.5 A Chinese study found that smartphone and internet addiction was significantly associated with increased levels of depressive symptoms during the pandemic.3 In a survey in the Philippines, 16.3% of adolescents reported moderate-to-severe psychological impairment during the pandemic; the rates of COVID-19–related anxiety were higher among girls vs boys.6 Alcohol and cannabis use increased among Canadian adolescents during the pandemic, according to an online survey.7 Adolescents with anorexia nervosa reported a 70% increase in poor eating habits and more thoughts associated with eating disorders during the pandemic.8 A Danish study found that children and adolescents newly diagnosed with obsessive-compulsive disorder (OCD) or who had completed treatment exhibited worsening OCD, anxiety, and depressive symptoms during the pandemic.9 An online survey of 6,196 Chinese adolescents found that those with a higher number of pre-pandemic adverse childhood experiences, such as abuse and neglect, had elevated posttraumatic stress symptoms and anxiety during the onset of the pandemic.10
Underlying causes of pandemic-induced distress
Limited social connectedness during the pandemic is a major reason for distress among adolescents. A review of 80 studies found that social isolation and loneliness as a result of social distancing and quarantining were associated with an increased risk of depression, anxiety, suicidal ideation, and self-harm.11 Parents’ stress about the risks of COVID-19 was correlated with worsening mental health in their adolescent children.12 A Chinese study found that the amount of time students spent on smartphones and social media doubled during the pandemic.13 In an online survey of 7,890 Chinese adolescents, greater social media, internet, and smartphone use was associated with increased anxiety and depression.14 This may be in part the result of adolescents spending time reading COVID-related news.
Coping mechanisms to increase well-being
Researchers have identified several positive coping mechanisms adolescents employed during the pandemic. Although some data suggest that increased internet use raises the risk of COVID-related distress, for certain adolescents, using social media to stay connected with friends and relatives was a buffer for feelings of loneliness and might have increased mental well-being.15 Other common coping mechanisms include relying on faith, volunteering, and starting new hobbies.16 During the pandemic, there were higher rates of playing outside and increased physical activity, which correlated with positive mental health outcomes.16 An online survey of 1,040 adolescents found that those who looked to the future optimistically and confidently had a higher health-related quality of life.17
Continuing an emphasis on adolescent well-being
Although data are limited, adolescents can continue to use these coping mechanisms to maintain their well-being, even if COVID-related restrictions are lifted or reimplemented. During these difficult times, it is imperative for adolescents to get the mental health services they need, and for psychiatric clinicians to continue to find avenues to promote resilience and mental wellness among young patients.
1. Leeb RT, Bitsko RH, Radhakrishnan L, et al. Mental health–related emergency department visits among children aged <18 years during the COVID-19 pandemic—United States, January 1-October 17, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(45):1675-1680. doi:10.15585/mmwr.mm6945a3
2. Oosterhoff B, Palmer CA, Wilson J, et al. Adolescents’ motivations to engage in social distancing during the COVID-19 pandemic: associations with mental and social health. J Adolesc Health. 2020;67(2):179-185. doi:10.1016/j.jadohealth.2020.05.004
3. Duan L, Shao X, Wang Y, et al. An investigation of mental health status of children and adolescents in China during the outbreak of COVID-19. J Affect Disord. 2020;275:112-118. doi:10.1016/j.jad.2020.06.029
4. Ellis WE, Dumas TM, Forbes LM. Physically isolated but socially connected: psychological adjustment and stress among adolescents during the initial COVID-19 crisis. Can J Behav Sci. 2020;52(3):177-187. doi:10.1037/cbs0000215
5. Murata S, Rezeppa T, Thoma B, et al. The psychiatric sequelae of the COVID-19 pandemic in adolescents, adults, and health care workers. Depress Anxiety. 2021;38(2):233-246. doi:10.1002/da.23120
6. Tee ML, Tee CA, Anlacan JP, et al. Psychological impact of COVID-19 pandemic in the Philippines. J Affect Disord. 2020;277:379-391. doi:10.1016/j.jad.2020.08.043
7. Dumas TM, Ellis W, Litt DM. What does adolescent substance use look like during the COVID-19 pandemic? Examining changes in frequency, social contexts, and pandemic-related predictors. J Adolesc Health. 2020;67(3):354-361. doi:10.1016/j.jadohealth.2020.06.018
8. Schlegl S, Maier J, Meule A, et al. Eating disorders in times of the COVID-19 pandemic—results from an online survey of patients with anorexia nervosa. Int J Eat Disord. 2020;53:1791-1800. doi:10.1002/eat.23374.
9. Nissen JB, Højgaard D, Thomsen PH. The immediate effect of COVID-19 pandemic on children and adolescents with obsessive compulsive disorder. BMC Psychiatry. 2020;20(1):511. doi:10.1186/s12888-020-02905-5
10. Guo J, Fu M, Liu D, et al. Is the psychological impact of exposure to COVID-19 stronger in adolescents with pre-pandemic maltreatment experiences? A survey of rural Chinese adolescents. Child Abuse Negl. 2020;110(Pt 2):104667. doi:10.1016/j.chiabu.2020.104667
11. Loades ME, Chatburn E, Higson-Sweeney N, et al. Rapid Systematic Review: The impact of social isolation and loneliness on the mental health of children and adolescents in the context of COVID-19. J Am Acad Child Adolesc Psychiatry. 2020;59(11):1218-1239.e3. doi:10.1016/j.jaac.2020.05.009
12. Spinelli M, Lionetti F, Setti A, et al. Parenting stress during the COVID-19 outbreak: socioeconomic and environmental risk factors and implications for children emotion regulation. Fam Process. 2021;60(2):639-653. doi:10.1111/famp.12601
13. Chen IH, Chen CY, Pakpour AH, et al. Internet-related behaviors and psychological distress among schoolchildren during COVID-19 school suspension. J Am Acad Child Adolesc Psychiatry. 2020;59(10):1099-1102.e1. doi:10.1016/j.jaac.2020.06.007
14. Li W, Zhang Y, Wang J, et al. Association of home quarantine and mental health among teenagers in Wuhan, China, during the COVID-19 pandemic. JAMA Pediatr. 2021;175(3):313-316. doi:10.1001/jamapediatrics.2020.5499
15. Janssen, LHC, Kullberg, MJ, Verkuil B, et al. Does the COVID-19 pandemic impact parents’ and adolescents’ well-being? An EMA-study on daily affect and parenting. PLoS One. 2020;15(10):e0240962. doi:10.1371/journal.pone.0240962
16. Banati P, Jones N, Youssef S. Intersecting vulnerabilities: the impacts of COVID-19 on the psycho-emotional lives of young people in low- and middle-income countries. Eur J Dev Res. 2020;32(5):1613-1638. doi:10.1057/s41287-020-00325-5
17. Ravens-Sieberer U, Kaman A, Otto C, et al. Mental health and quality of life in children and adolescents during the COVID-19 pandemic—results of the COPSY study. Dtsch Arztebl Int. 2020;117(48):828-829. doi:10.3238/arztebl.2020.0828
While the COVID-19 pandemic has impacted the mental health of a wide range of individuals, its adverse effects have been particularly detrimental to adolescents. In this article, I discuss evidence that shows the effects of the pandemic on adolescent patients, potential reasons for this increased distress, and what types of coping mechanisms adolescents have used to counter these effects.
Increases in multiple measures of psychopathology
Multiple online surveys and other studies have documented the pandemic’s impact on younger individuals. In the United States, visits to emergency departments by pediatric patients increased in the months after the first lockdown period.1 Several studies found increased rates of anxiety and depression among adolescents during the COVID-19 pandemic.2,3 In an online survey of 359 children and 3,254 adolescents in China, 22% of respondents reported that they experienced depressive symptoms.3 In an online survey of 1,054 Canadian adolescents, 43% said they were “very concerned” about the pandemic.4 In an online survey of 7,353 adolescents in the United States, 37% reported suicidal ideation during the pandemic compared to 17% in 2017.5 A Chinese study found that smartphone and internet addiction was significantly associated with increased levels of depressive symptoms during the pandemic.3 In a survey in the Philippines, 16.3% of adolescents reported moderate-to-severe psychological impairment during the pandemic; the rates of COVID-19–related anxiety were higher among girls vs boys.6 Alcohol and cannabis use increased among Canadian adolescents during the pandemic, according to an online survey.7 Adolescents with anorexia nervosa reported a 70% increase in poor eating habits and more thoughts associated with eating disorders during the pandemic.8 A Danish study found that children and adolescents newly diagnosed with obsessive-compulsive disorder (OCD) or who had completed treatment exhibited worsening OCD, anxiety, and depressive symptoms during the pandemic.9 An online survey of 6,196 Chinese adolescents found that those with a higher number of pre-pandemic adverse childhood experiences, such as abuse and neglect, had elevated posttraumatic stress symptoms and anxiety during the onset of the pandemic.10
Underlying causes of pandemic-induced distress
Limited social connectedness during the pandemic is a major reason for distress among adolescents. A review of 80 studies found that social isolation and loneliness as a result of social distancing and quarantining were associated with an increased risk of depression, anxiety, suicidal ideation, and self-harm.11 Parents’ stress about the risks of COVID-19 was correlated with worsening mental health in their adolescent children.12 A Chinese study found that the amount of time students spent on smartphones and social media doubled during the pandemic.13 In an online survey of 7,890 Chinese adolescents, greater social media, internet, and smartphone use was associated with increased anxiety and depression.14 This may be in part the result of adolescents spending time reading COVID-related news.
Coping mechanisms to increase well-being
Researchers have identified several positive coping mechanisms adolescents employed during the pandemic. Although some data suggest that increased internet use raises the risk of COVID-related distress, for certain adolescents, using social media to stay connected with friends and relatives was a buffer for feelings of loneliness and might have increased mental well-being.15 Other common coping mechanisms include relying on faith, volunteering, and starting new hobbies.16 During the pandemic, there were higher rates of playing outside and increased physical activity, which correlated with positive mental health outcomes.16 An online survey of 1,040 adolescents found that those who looked to the future optimistically and confidently had a higher health-related quality of life.17
Continuing an emphasis on adolescent well-being
Although data are limited, adolescents can continue to use these coping mechanisms to maintain their well-being, even if COVID-related restrictions are lifted or reimplemented. During these difficult times, it is imperative for adolescents to get the mental health services they need, and for psychiatric clinicians to continue to find avenues to promote resilience and mental wellness among young patients.
While the COVID-19 pandemic has impacted the mental health of a wide range of individuals, its adverse effects have been particularly detrimental to adolescents. In this article, I discuss evidence that shows the effects of the pandemic on adolescent patients, potential reasons for this increased distress, and what types of coping mechanisms adolescents have used to counter these effects.
Increases in multiple measures of psychopathology
Multiple online surveys and other studies have documented the pandemic’s impact on younger individuals. In the United States, visits to emergency departments by pediatric patients increased in the months after the first lockdown period.1 Several studies found increased rates of anxiety and depression among adolescents during the COVID-19 pandemic.2,3 In an online survey of 359 children and 3,254 adolescents in China, 22% of respondents reported that they experienced depressive symptoms.3 In an online survey of 1,054 Canadian adolescents, 43% said they were “very concerned” about the pandemic.4 In an online survey of 7,353 adolescents in the United States, 37% reported suicidal ideation during the pandemic compared to 17% in 2017.5 A Chinese study found that smartphone and internet addiction was significantly associated with increased levels of depressive symptoms during the pandemic.3 In a survey in the Philippines, 16.3% of adolescents reported moderate-to-severe psychological impairment during the pandemic; the rates of COVID-19–related anxiety were higher among girls vs boys.6 Alcohol and cannabis use increased among Canadian adolescents during the pandemic, according to an online survey.7 Adolescents with anorexia nervosa reported a 70% increase in poor eating habits and more thoughts associated with eating disorders during the pandemic.8 A Danish study found that children and adolescents newly diagnosed with obsessive-compulsive disorder (OCD) or who had completed treatment exhibited worsening OCD, anxiety, and depressive symptoms during the pandemic.9 An online survey of 6,196 Chinese adolescents found that those with a higher number of pre-pandemic adverse childhood experiences, such as abuse and neglect, had elevated posttraumatic stress symptoms and anxiety during the onset of the pandemic.10
Underlying causes of pandemic-induced distress
Limited social connectedness during the pandemic is a major reason for distress among adolescents. A review of 80 studies found that social isolation and loneliness as a result of social distancing and quarantining were associated with an increased risk of depression, anxiety, suicidal ideation, and self-harm.11 Parents’ stress about the risks of COVID-19 was correlated with worsening mental health in their adolescent children.12 A Chinese study found that the amount of time students spent on smartphones and social media doubled during the pandemic.13 In an online survey of 7,890 Chinese adolescents, greater social media, internet, and smartphone use was associated with increased anxiety and depression.14 This may be in part the result of adolescents spending time reading COVID-related news.
Coping mechanisms to increase well-being
Researchers have identified several positive coping mechanisms adolescents employed during the pandemic. Although some data suggest that increased internet use raises the risk of COVID-related distress, for certain adolescents, using social media to stay connected with friends and relatives was a buffer for feelings of loneliness and might have increased mental well-being.15 Other common coping mechanisms include relying on faith, volunteering, and starting new hobbies.16 During the pandemic, there were higher rates of playing outside and increased physical activity, which correlated with positive mental health outcomes.16 An online survey of 1,040 adolescents found that those who looked to the future optimistically and confidently had a higher health-related quality of life.17
Continuing an emphasis on adolescent well-being
Although data are limited, adolescents can continue to use these coping mechanisms to maintain their well-being, even if COVID-related restrictions are lifted or reimplemented. During these difficult times, it is imperative for adolescents to get the mental health services they need, and for psychiatric clinicians to continue to find avenues to promote resilience and mental wellness among young patients.
1. Leeb RT, Bitsko RH, Radhakrishnan L, et al. Mental health–related emergency department visits among children aged <18 years during the COVID-19 pandemic—United States, January 1-October 17, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(45):1675-1680. doi:10.15585/mmwr.mm6945a3
2. Oosterhoff B, Palmer CA, Wilson J, et al. Adolescents’ motivations to engage in social distancing during the COVID-19 pandemic: associations with mental and social health. J Adolesc Health. 2020;67(2):179-185. doi:10.1016/j.jadohealth.2020.05.004
3. Duan L, Shao X, Wang Y, et al. An investigation of mental health status of children and adolescents in China during the outbreak of COVID-19. J Affect Disord. 2020;275:112-118. doi:10.1016/j.jad.2020.06.029
4. Ellis WE, Dumas TM, Forbes LM. Physically isolated but socially connected: psychological adjustment and stress among adolescents during the initial COVID-19 crisis. Can J Behav Sci. 2020;52(3):177-187. doi:10.1037/cbs0000215
5. Murata S, Rezeppa T, Thoma B, et al. The psychiatric sequelae of the COVID-19 pandemic in adolescents, adults, and health care workers. Depress Anxiety. 2021;38(2):233-246. doi:10.1002/da.23120
6. Tee ML, Tee CA, Anlacan JP, et al. Psychological impact of COVID-19 pandemic in the Philippines. J Affect Disord. 2020;277:379-391. doi:10.1016/j.jad.2020.08.043
7. Dumas TM, Ellis W, Litt DM. What does adolescent substance use look like during the COVID-19 pandemic? Examining changes in frequency, social contexts, and pandemic-related predictors. J Adolesc Health. 2020;67(3):354-361. doi:10.1016/j.jadohealth.2020.06.018
8. Schlegl S, Maier J, Meule A, et al. Eating disorders in times of the COVID-19 pandemic—results from an online survey of patients with anorexia nervosa. Int J Eat Disord. 2020;53:1791-1800. doi:10.1002/eat.23374.
9. Nissen JB, Højgaard D, Thomsen PH. The immediate effect of COVID-19 pandemic on children and adolescents with obsessive compulsive disorder. BMC Psychiatry. 2020;20(1):511. doi:10.1186/s12888-020-02905-5
10. Guo J, Fu M, Liu D, et al. Is the psychological impact of exposure to COVID-19 stronger in adolescents with pre-pandemic maltreatment experiences? A survey of rural Chinese adolescents. Child Abuse Negl. 2020;110(Pt 2):104667. doi:10.1016/j.chiabu.2020.104667
11. Loades ME, Chatburn E, Higson-Sweeney N, et al. Rapid Systematic Review: The impact of social isolation and loneliness on the mental health of children and adolescents in the context of COVID-19. J Am Acad Child Adolesc Psychiatry. 2020;59(11):1218-1239.e3. doi:10.1016/j.jaac.2020.05.009
12. Spinelli M, Lionetti F, Setti A, et al. Parenting stress during the COVID-19 outbreak: socioeconomic and environmental risk factors and implications for children emotion regulation. Fam Process. 2021;60(2):639-653. doi:10.1111/famp.12601
13. Chen IH, Chen CY, Pakpour AH, et al. Internet-related behaviors and psychological distress among schoolchildren during COVID-19 school suspension. J Am Acad Child Adolesc Psychiatry. 2020;59(10):1099-1102.e1. doi:10.1016/j.jaac.2020.06.007
14. Li W, Zhang Y, Wang J, et al. Association of home quarantine and mental health among teenagers in Wuhan, China, during the COVID-19 pandemic. JAMA Pediatr. 2021;175(3):313-316. doi:10.1001/jamapediatrics.2020.5499
15. Janssen, LHC, Kullberg, MJ, Verkuil B, et al. Does the COVID-19 pandemic impact parents’ and adolescents’ well-being? An EMA-study on daily affect and parenting. PLoS One. 2020;15(10):e0240962. doi:10.1371/journal.pone.0240962
16. Banati P, Jones N, Youssef S. Intersecting vulnerabilities: the impacts of COVID-19 on the psycho-emotional lives of young people in low- and middle-income countries. Eur J Dev Res. 2020;32(5):1613-1638. doi:10.1057/s41287-020-00325-5
17. Ravens-Sieberer U, Kaman A, Otto C, et al. Mental health and quality of life in children and adolescents during the COVID-19 pandemic—results of the COPSY study. Dtsch Arztebl Int. 2020;117(48):828-829. doi:10.3238/arztebl.2020.0828
1. Leeb RT, Bitsko RH, Radhakrishnan L, et al. Mental health–related emergency department visits among children aged <18 years during the COVID-19 pandemic—United States, January 1-October 17, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(45):1675-1680. doi:10.15585/mmwr.mm6945a3
2. Oosterhoff B, Palmer CA, Wilson J, et al. Adolescents’ motivations to engage in social distancing during the COVID-19 pandemic: associations with mental and social health. J Adolesc Health. 2020;67(2):179-185. doi:10.1016/j.jadohealth.2020.05.004
3. Duan L, Shao X, Wang Y, et al. An investigation of mental health status of children and adolescents in China during the outbreak of COVID-19. J Affect Disord. 2020;275:112-118. doi:10.1016/j.jad.2020.06.029
4. Ellis WE, Dumas TM, Forbes LM. Physically isolated but socially connected: psychological adjustment and stress among adolescents during the initial COVID-19 crisis. Can J Behav Sci. 2020;52(3):177-187. doi:10.1037/cbs0000215
5. Murata S, Rezeppa T, Thoma B, et al. The psychiatric sequelae of the COVID-19 pandemic in adolescents, adults, and health care workers. Depress Anxiety. 2021;38(2):233-246. doi:10.1002/da.23120
6. Tee ML, Tee CA, Anlacan JP, et al. Psychological impact of COVID-19 pandemic in the Philippines. J Affect Disord. 2020;277:379-391. doi:10.1016/j.jad.2020.08.043
7. Dumas TM, Ellis W, Litt DM. What does adolescent substance use look like during the COVID-19 pandemic? Examining changes in frequency, social contexts, and pandemic-related predictors. J Adolesc Health. 2020;67(3):354-361. doi:10.1016/j.jadohealth.2020.06.018
8. Schlegl S, Maier J, Meule A, et al. Eating disorders in times of the COVID-19 pandemic—results from an online survey of patients with anorexia nervosa. Int J Eat Disord. 2020;53:1791-1800. doi:10.1002/eat.23374.
9. Nissen JB, Højgaard D, Thomsen PH. The immediate effect of COVID-19 pandemic on children and adolescents with obsessive compulsive disorder. BMC Psychiatry. 2020;20(1):511. doi:10.1186/s12888-020-02905-5
10. Guo J, Fu M, Liu D, et al. Is the psychological impact of exposure to COVID-19 stronger in adolescents with pre-pandemic maltreatment experiences? A survey of rural Chinese adolescents. Child Abuse Negl. 2020;110(Pt 2):104667. doi:10.1016/j.chiabu.2020.104667
11. Loades ME, Chatburn E, Higson-Sweeney N, et al. Rapid Systematic Review: The impact of social isolation and loneliness on the mental health of children and adolescents in the context of COVID-19. J Am Acad Child Adolesc Psychiatry. 2020;59(11):1218-1239.e3. doi:10.1016/j.jaac.2020.05.009
12. Spinelli M, Lionetti F, Setti A, et al. Parenting stress during the COVID-19 outbreak: socioeconomic and environmental risk factors and implications for children emotion regulation. Fam Process. 2021;60(2):639-653. doi:10.1111/famp.12601
13. Chen IH, Chen CY, Pakpour AH, et al. Internet-related behaviors and psychological distress among schoolchildren during COVID-19 school suspension. J Am Acad Child Adolesc Psychiatry. 2020;59(10):1099-1102.e1. doi:10.1016/j.jaac.2020.06.007
14. Li W, Zhang Y, Wang J, et al. Association of home quarantine and mental health among teenagers in Wuhan, China, during the COVID-19 pandemic. JAMA Pediatr. 2021;175(3):313-316. doi:10.1001/jamapediatrics.2020.5499
15. Janssen, LHC, Kullberg, MJ, Verkuil B, et al. Does the COVID-19 pandemic impact parents’ and adolescents’ well-being? An EMA-study on daily affect and parenting. PLoS One. 2020;15(10):e0240962. doi:10.1371/journal.pone.0240962
16. Banati P, Jones N, Youssef S. Intersecting vulnerabilities: the impacts of COVID-19 on the psycho-emotional lives of young people in low- and middle-income countries. Eur J Dev Res. 2020;32(5):1613-1638. doi:10.1057/s41287-020-00325-5
17. Ravens-Sieberer U, Kaman A, Otto C, et al. Mental health and quality of life in children and adolescents during the COVID-19 pandemic—results of the COPSY study. Dtsch Arztebl Int. 2020;117(48):828-829. doi:10.3238/arztebl.2020.0828
More on stigma
I just finished reading your editorial “A PSYCHIATRIC MANIFESTO: Stigma is hate speech and a hate crime” (
Our son went from an honor roll student before the pandemic to a child I barely recognized. Approximately 6 months into the pandemic, he was using drugs, vaping nicotine, destroying our property, and eloping at night. The journey of watching his decline and getting him help was agonizing. But the stigma around what was happening to him was an entirely separate animal.
Our society vilifies, ridicules, dismisses, and often makes fun of those with mental health issues. I experience it daily with my son and am on constant guard to shoot down any comments and to calmly teach those who say such cruel things. But the shame my son feels is the most devastating part. Although we keep reminding him that his condition is a medical condition like diabetes or heart disease, for a teenage boy, that makes no sense. He just wants to be “normal.” And living in a world that rarely represents mental illness this way, it’s almost a lost cause to get him to let go of this shame. All we can do is love him, be there for him, support him, and do what we can to educate those around us about the stigma of mental illness.
What a powerful and accurate article. Thank you for putting into words what I have been thinking and feeling, and for being as outraged as we are at how this vulnerable population is treated. My husband is a psychiatrist and we live in an affluent urban area, so we are not in the middle of nowhere with no knowledge of what is happening to our son. And despite that, we still suffer from the stigma.
Thank you, Dr. Nasrallah.
Name withheld
I need to take a moment to thank you for your editorial about stigma being hate speech and a hate crime. I really agree with you, and I think the way you formulated and articulated this message is very compelling.
I have focused on normalizing mental health differences among entrepreneurs as a destigmatization strategy (see https://www.sciencedirect.com/science/article/pii/S0883902622000027 and https://link.springer.com/article/10.1007/s11187-018-0059-8). Entrepreneurs clearly illustrate the fallacy of stigma. As a simple example, Elon Musk—the wealthiest person in the world—talks openly about being autistic, and possibly bipolar. These mental health differences help him create jobs and contribute to our shared prosperity. Nothing to be ashamed of there.
Thanks again for being such an effective advocate.
Michael A. Freeman, MD
Kentfield, California
Continue to: Thank you...
Thank you so much for your “Psychiatric Manifesto.” I will do my best to disseminate it amongst colleagues, patients, friends, family, and as many others as possible.
Daniel N. Pistone, MD
San Francisco, California
Once again, your words hit the pin on the head.
Robert W. Pollack, MD, ABPN, DLFAPA
Fort Myers, Florida
I just finished reading your editorial “A PSYCHIATRIC MANIFESTO: Stigma is hate speech and a hate crime” (
Our son went from an honor roll student before the pandemic to a child I barely recognized. Approximately 6 months into the pandemic, he was using drugs, vaping nicotine, destroying our property, and eloping at night. The journey of watching his decline and getting him help was agonizing. But the stigma around what was happening to him was an entirely separate animal.
Our society vilifies, ridicules, dismisses, and often makes fun of those with mental health issues. I experience it daily with my son and am on constant guard to shoot down any comments and to calmly teach those who say such cruel things. But the shame my son feels is the most devastating part. Although we keep reminding him that his condition is a medical condition like diabetes or heart disease, for a teenage boy, that makes no sense. He just wants to be “normal.” And living in a world that rarely represents mental illness this way, it’s almost a lost cause to get him to let go of this shame. All we can do is love him, be there for him, support him, and do what we can to educate those around us about the stigma of mental illness.
What a powerful and accurate article. Thank you for putting into words what I have been thinking and feeling, and for being as outraged as we are at how this vulnerable population is treated. My husband is a psychiatrist and we live in an affluent urban area, so we are not in the middle of nowhere with no knowledge of what is happening to our son. And despite that, we still suffer from the stigma.
Thank you, Dr. Nasrallah.
Name withheld
I need to take a moment to thank you for your editorial about stigma being hate speech and a hate crime. I really agree with you, and I think the way you formulated and articulated this message is very compelling.
I have focused on normalizing mental health differences among entrepreneurs as a destigmatization strategy (see https://www.sciencedirect.com/science/article/pii/S0883902622000027 and https://link.springer.com/article/10.1007/s11187-018-0059-8). Entrepreneurs clearly illustrate the fallacy of stigma. As a simple example, Elon Musk—the wealthiest person in the world—talks openly about being autistic, and possibly bipolar. These mental health differences help him create jobs and contribute to our shared prosperity. Nothing to be ashamed of there.
Thanks again for being such an effective advocate.
Michael A. Freeman, MD
Kentfield, California
Continue to: Thank you...
Thank you so much for your “Psychiatric Manifesto.” I will do my best to disseminate it amongst colleagues, patients, friends, family, and as many others as possible.
Daniel N. Pistone, MD
San Francisco, California
Once again, your words hit the pin on the head.
Robert W. Pollack, MD, ABPN, DLFAPA
Fort Myers, Florida
I just finished reading your editorial “A PSYCHIATRIC MANIFESTO: Stigma is hate speech and a hate crime” (
Our son went from an honor roll student before the pandemic to a child I barely recognized. Approximately 6 months into the pandemic, he was using drugs, vaping nicotine, destroying our property, and eloping at night. The journey of watching his decline and getting him help was agonizing. But the stigma around what was happening to him was an entirely separate animal.
Our society vilifies, ridicules, dismisses, and often makes fun of those with mental health issues. I experience it daily with my son and am on constant guard to shoot down any comments and to calmly teach those who say such cruel things. But the shame my son feels is the most devastating part. Although we keep reminding him that his condition is a medical condition like diabetes or heart disease, for a teenage boy, that makes no sense. He just wants to be “normal.” And living in a world that rarely represents mental illness this way, it’s almost a lost cause to get him to let go of this shame. All we can do is love him, be there for him, support him, and do what we can to educate those around us about the stigma of mental illness.
What a powerful and accurate article. Thank you for putting into words what I have been thinking and feeling, and for being as outraged as we are at how this vulnerable population is treated. My husband is a psychiatrist and we live in an affluent urban area, so we are not in the middle of nowhere with no knowledge of what is happening to our son. And despite that, we still suffer from the stigma.
Thank you, Dr. Nasrallah.
Name withheld
I need to take a moment to thank you for your editorial about stigma being hate speech and a hate crime. I really agree with you, and I think the way you formulated and articulated this message is very compelling.
I have focused on normalizing mental health differences among entrepreneurs as a destigmatization strategy (see https://www.sciencedirect.com/science/article/pii/S0883902622000027 and https://link.springer.com/article/10.1007/s11187-018-0059-8). Entrepreneurs clearly illustrate the fallacy of stigma. As a simple example, Elon Musk—the wealthiest person in the world—talks openly about being autistic, and possibly bipolar. These mental health differences help him create jobs and contribute to our shared prosperity. Nothing to be ashamed of there.
Thanks again for being such an effective advocate.
Michael A. Freeman, MD
Kentfield, California
Continue to: Thank you...
Thank you so much for your “Psychiatric Manifesto.” I will do my best to disseminate it amongst colleagues, patients, friends, family, and as many others as possible.
Daniel N. Pistone, MD
San Francisco, California
Once again, your words hit the pin on the head.
Robert W. Pollack, MD, ABPN, DLFAPA
Fort Myers, Florida
High rate of mental health problems in transgender children
Transgender children, even those as young as 9 or 10 years old, already show increased susceptibility to mental health problems compared with their cisgender peers, new research suggests.
Investigators assessed a sample of more than 7000 children aged 9-10 years in the general population and found those who reported being transgender scored considerably higher on all six subscales of the DSM-5-oriented Child Behavior Checklist (CBCL).
Transgender children had almost sixfold higher odds of suicidality and over twice the odds of depressive and anxiety problems, compared with cisgender children. Moreover, transgender children displayed higher levels of mental health problems compared with previous studies of transgender children recruited from specialist gender clinics.
“Our findings emphasize the vulnerability of transgender children, including those who may not yet have accessed specialist support,” senior author Kenneth C. Pang, MBBS, BMedSc, PhD, associate professor, Murdoch Children’s Research Institute, University of Melbourne, Royal Children’s Hospital, Australia, told this news organization.
“Clinicians providing general health care to transgender children should keep this vulnerability in mind and proactively address any mental health problems that exist,” he said.
The findings were published online as a research letter in JAMA Network Open.
Higher levels of support?
“We felt this study was important to conduct because previous studies regarding the mental health of transgender children have been drawn from children receiving specialist gender-related care,” Dr. Pang said.
“Transgender children receiving such care are likely to enjoy higher levels of support than those unable to access such services, and this might create differences in mental health,” he added.
To investigate this issue, the researchers turned to participants (n = 7,169; mean age, 10.3 years) in the Adolescent Brain Cognitive Development (ABCD) study.
“The ABCD study is a longitudinal study of over 11,000 children who were recruited to reflect the sociodemographic variation of the U.S. population,” lead author Douglas H. Russell, MSc, a PhD candidate at the University of Melbourne, told this news organization.
To be included in the current study, children had to understand and respond to the question “Are you transgender?”
The researchers compared mental health outcomes between transgender and cisgender children (n = 58 and n = 7,111, respectively) using the CBCL, which study participants had completed at baseline.
Key protective factor
The transgender children recorded higher mean T scores for all six subscales of the CBCL, although all children scored in the references range; and the standardized mean difference was “small.”
Suicidality was measured by summing the two suicide-related items in the parent-report CBCL assessing suicidal ideation and attempts.
“For the CBCL, T scores are calculated for measures that are scored on a continuous scale,” Dr. Pang noted. “Responses to the suicidality questions on the CBCL were assessed in a categorical manner (at risk of suicide vs. not), as previously described by others. So T scores were therefore not able to be calculated.”
When the investigators determined the proportion of cisgender and transgender children who scored in the “borderline” or “clinical” range (T score, 65), they found increased odds of transgender children scoring in that range in all six subscales, as well as suicidality.
The researchers note the results for attention-deficit/hyperactivity disorder and oppositional defiant problems were not statistically significant.
Previous studies that used clinical samples of young transgender children (aged 5 -11 years) reported lower rates of depression and anxiety than what was found in the current study.
“Transgender children in the general population displayed higher levels of mental health problems compared to previous studies of transgender children recruited from specialist gender clinics,” Mr. Russell said.
One reason for that may be children in specialist clinics “are likely to have support from their families (a key protective factor for the mental health of transgender young people); in comparison, many transgender children in the general population lack parental support for their gender,” the investigators wrote.
“Our findings suggest that by 9 to 10 years of age transgender children already show increased susceptibility to mental health problems compared with their cisgender peers, which has important public health implications,” they added.
The researchers noted that whether this susceptibility “is due to stigma, minority stress, discrimination, or gender dysphoria is unclear, but providing appropriate mental health supports to this vulnerable group is paramount.”
“Pathologizing and damaging”
Commenting for this news organiztion, Jack L. Turban, MD, incoming assistant professor of child and adolescent psychiatry, University of California, San Francisco, said that “sadly” the findings are “largely in line with past studies that have shown dramatic mental health disparities” for transgender and gender diverse youth.
“The dramatically elevated odds of suicidality warrants particular public health concern,” said Dr. Turban, who was not involved with the study.
He noted these results “come at a time when transgender youth are under legislative attack in many states throughout the country, and the national rhetoric around them has been pathologizing and damaging.”
Dr. Turban said that he worries “if our national discourse around trans youth doesn’t change soon, that these disparities will worsen.”
Funding was provided to individual investigators by the Hugh Williamson Foundation, the Royal Children’s Hospital foundation, the National Health and Medical Research Council, and the Australian Government Research Training Program Scholarship. Mr. Russell and Dr. Pang reported being members of the Australian Professional Association for Trans Health. Dr. Pang is a member of the World Professional Association for Transgender Health and a member of the editorial board of the journal Transgender Health. Dr. Turban reported textbook royalties from Springer Nature, being on the scientific advisory board of Panorama Global (UpSwing Fund), and payments as an expert witness for the American Civil Liberties Union, Lambda Legal, and Cooley LLP. He has received a pilot research award from AACAP and pharmaceutical partners (Arbor and Pfizer), a research fellowship from the Sorensen Foundation, and freelance payments from the New York Times, the Washington Post, and the Los Angeles Times.
A version of this article first appeared on Medscape.com.
Transgender children, even those as young as 9 or 10 years old, already show increased susceptibility to mental health problems compared with their cisgender peers, new research suggests.
Investigators assessed a sample of more than 7000 children aged 9-10 years in the general population and found those who reported being transgender scored considerably higher on all six subscales of the DSM-5-oriented Child Behavior Checklist (CBCL).
Transgender children had almost sixfold higher odds of suicidality and over twice the odds of depressive and anxiety problems, compared with cisgender children. Moreover, transgender children displayed higher levels of mental health problems compared with previous studies of transgender children recruited from specialist gender clinics.
“Our findings emphasize the vulnerability of transgender children, including those who may not yet have accessed specialist support,” senior author Kenneth C. Pang, MBBS, BMedSc, PhD, associate professor, Murdoch Children’s Research Institute, University of Melbourne, Royal Children’s Hospital, Australia, told this news organization.
“Clinicians providing general health care to transgender children should keep this vulnerability in mind and proactively address any mental health problems that exist,” he said.
The findings were published online as a research letter in JAMA Network Open.
Higher levels of support?
“We felt this study was important to conduct because previous studies regarding the mental health of transgender children have been drawn from children receiving specialist gender-related care,” Dr. Pang said.
“Transgender children receiving such care are likely to enjoy higher levels of support than those unable to access such services, and this might create differences in mental health,” he added.
To investigate this issue, the researchers turned to participants (n = 7,169; mean age, 10.3 years) in the Adolescent Brain Cognitive Development (ABCD) study.
“The ABCD study is a longitudinal study of over 11,000 children who were recruited to reflect the sociodemographic variation of the U.S. population,” lead author Douglas H. Russell, MSc, a PhD candidate at the University of Melbourne, told this news organization.
To be included in the current study, children had to understand and respond to the question “Are you transgender?”
The researchers compared mental health outcomes between transgender and cisgender children (n = 58 and n = 7,111, respectively) using the CBCL, which study participants had completed at baseline.
Key protective factor
The transgender children recorded higher mean T scores for all six subscales of the CBCL, although all children scored in the references range; and the standardized mean difference was “small.”
Suicidality was measured by summing the two suicide-related items in the parent-report CBCL assessing suicidal ideation and attempts.
“For the CBCL, T scores are calculated for measures that are scored on a continuous scale,” Dr. Pang noted. “Responses to the suicidality questions on the CBCL were assessed in a categorical manner (at risk of suicide vs. not), as previously described by others. So T scores were therefore not able to be calculated.”
When the investigators determined the proportion of cisgender and transgender children who scored in the “borderline” or “clinical” range (T score, 65), they found increased odds of transgender children scoring in that range in all six subscales, as well as suicidality.
The researchers note the results for attention-deficit/hyperactivity disorder and oppositional defiant problems were not statistically significant.
Previous studies that used clinical samples of young transgender children (aged 5 -11 years) reported lower rates of depression and anxiety than what was found in the current study.
“Transgender children in the general population displayed higher levels of mental health problems compared to previous studies of transgender children recruited from specialist gender clinics,” Mr. Russell said.
One reason for that may be children in specialist clinics “are likely to have support from their families (a key protective factor for the mental health of transgender young people); in comparison, many transgender children in the general population lack parental support for their gender,” the investigators wrote.
“Our findings suggest that by 9 to 10 years of age transgender children already show increased susceptibility to mental health problems compared with their cisgender peers, which has important public health implications,” they added.
The researchers noted that whether this susceptibility “is due to stigma, minority stress, discrimination, or gender dysphoria is unclear, but providing appropriate mental health supports to this vulnerable group is paramount.”
“Pathologizing and damaging”
Commenting for this news organiztion, Jack L. Turban, MD, incoming assistant professor of child and adolescent psychiatry, University of California, San Francisco, said that “sadly” the findings are “largely in line with past studies that have shown dramatic mental health disparities” for transgender and gender diverse youth.
“The dramatically elevated odds of suicidality warrants particular public health concern,” said Dr. Turban, who was not involved with the study.
He noted these results “come at a time when transgender youth are under legislative attack in many states throughout the country, and the national rhetoric around them has been pathologizing and damaging.”
Dr. Turban said that he worries “if our national discourse around trans youth doesn’t change soon, that these disparities will worsen.”
Funding was provided to individual investigators by the Hugh Williamson Foundation, the Royal Children’s Hospital foundation, the National Health and Medical Research Council, and the Australian Government Research Training Program Scholarship. Mr. Russell and Dr. Pang reported being members of the Australian Professional Association for Trans Health. Dr. Pang is a member of the World Professional Association for Transgender Health and a member of the editorial board of the journal Transgender Health. Dr. Turban reported textbook royalties from Springer Nature, being on the scientific advisory board of Panorama Global (UpSwing Fund), and payments as an expert witness for the American Civil Liberties Union, Lambda Legal, and Cooley LLP. He has received a pilot research award from AACAP and pharmaceutical partners (Arbor and Pfizer), a research fellowship from the Sorensen Foundation, and freelance payments from the New York Times, the Washington Post, and the Los Angeles Times.
A version of this article first appeared on Medscape.com.
Transgender children, even those as young as 9 or 10 years old, already show increased susceptibility to mental health problems compared with their cisgender peers, new research suggests.
Investigators assessed a sample of more than 7000 children aged 9-10 years in the general population and found those who reported being transgender scored considerably higher on all six subscales of the DSM-5-oriented Child Behavior Checklist (CBCL).
Transgender children had almost sixfold higher odds of suicidality and over twice the odds of depressive and anxiety problems, compared with cisgender children. Moreover, transgender children displayed higher levels of mental health problems compared with previous studies of transgender children recruited from specialist gender clinics.
“Our findings emphasize the vulnerability of transgender children, including those who may not yet have accessed specialist support,” senior author Kenneth C. Pang, MBBS, BMedSc, PhD, associate professor, Murdoch Children’s Research Institute, University of Melbourne, Royal Children’s Hospital, Australia, told this news organization.
“Clinicians providing general health care to transgender children should keep this vulnerability in mind and proactively address any mental health problems that exist,” he said.
The findings were published online as a research letter in JAMA Network Open.
Higher levels of support?
“We felt this study was important to conduct because previous studies regarding the mental health of transgender children have been drawn from children receiving specialist gender-related care,” Dr. Pang said.
“Transgender children receiving such care are likely to enjoy higher levels of support than those unable to access such services, and this might create differences in mental health,” he added.
To investigate this issue, the researchers turned to participants (n = 7,169; mean age, 10.3 years) in the Adolescent Brain Cognitive Development (ABCD) study.
“The ABCD study is a longitudinal study of over 11,000 children who were recruited to reflect the sociodemographic variation of the U.S. population,” lead author Douglas H. Russell, MSc, a PhD candidate at the University of Melbourne, told this news organization.
To be included in the current study, children had to understand and respond to the question “Are you transgender?”
The researchers compared mental health outcomes between transgender and cisgender children (n = 58 and n = 7,111, respectively) using the CBCL, which study participants had completed at baseline.
Key protective factor
The transgender children recorded higher mean T scores for all six subscales of the CBCL, although all children scored in the references range; and the standardized mean difference was “small.”
Suicidality was measured by summing the two suicide-related items in the parent-report CBCL assessing suicidal ideation and attempts.
“For the CBCL, T scores are calculated for measures that are scored on a continuous scale,” Dr. Pang noted. “Responses to the suicidality questions on the CBCL were assessed in a categorical manner (at risk of suicide vs. not), as previously described by others. So T scores were therefore not able to be calculated.”
When the investigators determined the proportion of cisgender and transgender children who scored in the “borderline” or “clinical” range (T score, 65), they found increased odds of transgender children scoring in that range in all six subscales, as well as suicidality.
The researchers note the results for attention-deficit/hyperactivity disorder and oppositional defiant problems were not statistically significant.
Previous studies that used clinical samples of young transgender children (aged 5 -11 years) reported lower rates of depression and anxiety than what was found in the current study.
“Transgender children in the general population displayed higher levels of mental health problems compared to previous studies of transgender children recruited from specialist gender clinics,” Mr. Russell said.
One reason for that may be children in specialist clinics “are likely to have support from their families (a key protective factor for the mental health of transgender young people); in comparison, many transgender children in the general population lack parental support for their gender,” the investigators wrote.
“Our findings suggest that by 9 to 10 years of age transgender children already show increased susceptibility to mental health problems compared with their cisgender peers, which has important public health implications,” they added.
The researchers noted that whether this susceptibility “is due to stigma, minority stress, discrimination, or gender dysphoria is unclear, but providing appropriate mental health supports to this vulnerable group is paramount.”
“Pathologizing and damaging”
Commenting for this news organiztion, Jack L. Turban, MD, incoming assistant professor of child and adolescent psychiatry, University of California, San Francisco, said that “sadly” the findings are “largely in line with past studies that have shown dramatic mental health disparities” for transgender and gender diverse youth.
“The dramatically elevated odds of suicidality warrants particular public health concern,” said Dr. Turban, who was not involved with the study.
He noted these results “come at a time when transgender youth are under legislative attack in many states throughout the country, and the national rhetoric around them has been pathologizing and damaging.”
Dr. Turban said that he worries “if our national discourse around trans youth doesn’t change soon, that these disparities will worsen.”
Funding was provided to individual investigators by the Hugh Williamson Foundation, the Royal Children’s Hospital foundation, the National Health and Medical Research Council, and the Australian Government Research Training Program Scholarship. Mr. Russell and Dr. Pang reported being members of the Australian Professional Association for Trans Health. Dr. Pang is a member of the World Professional Association for Transgender Health and a member of the editorial board of the journal Transgender Health. Dr. Turban reported textbook royalties from Springer Nature, being on the scientific advisory board of Panorama Global (UpSwing Fund), and payments as an expert witness for the American Civil Liberties Union, Lambda Legal, and Cooley LLP. He has received a pilot research award from AACAP and pharmaceutical partners (Arbor and Pfizer), a research fellowship from the Sorensen Foundation, and freelance payments from the New York Times, the Washington Post, and the Los Angeles Times.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN