User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'main-prefix')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
What ketamine and psilocybin can and cannot do in depression
Recent studies with hallucinogens have raised hopes for an effective drug-based therapy to treat chronic depression. At the German Congress of Psychosomatic Medicine and Psychotherapy, Torsten Passie, MD, PhD, professor of psychiatry and psychotherapy at the Hannover (Germay) Medical School, gave a presentation on the current state of psilocybin and ketamine/esketamine research.
Dr. Passie, who also is head physician of the specialist unit for addiction and addiction prevention at the Diakonisches Werk in Hannover, has been investigating hallucinogenic substances and their application in psychotherapy for decades.
New therapies sought
In depression, gloom extends beyond the patient’s mood. For some time there has been little cause for joy with regard to chronic depression therapy. Established drug therapies hardly perform any better than placebo in meta-analyses, as a study recently confirmed. The pharmaceutical industry pulled out of psycho-pharmaceutical development more than 10 years ago. What’s more, the number of cases is rising, especially among young people, and there are long waiting times for psychotherapy appointments.
It is no wonder that some are welcoming new drug-based approaches with lysergic acid diethylamide (LSD)–like hallucinogens. In 2016, a study on psilocybin was published in The Lancet Psychiatry, although the study was unblinded and included only 24 patients.
Evoking emotions
A range of substances can be classed as hallucinogens, including psilocybin, mescaline, LSD, 3,4-methylenedioxy-methamphetamine (MDMA, also known as ecstasy), and ketamine.
Taking hallucinogens can cause a release of serotonin and dopamine, an increase in activity levels in the brain, a shift in stimulus filtering, an increase in the production of internal stimuli (inner experiences), and a change in sensory integration (for example, synesthesia).
Besides falling into a dreamlike state, patients can achieve an expansion or narrowing of consciousness if they focus on an inner experience. Internal perception increases. Perceptual routines are broken apart. Thought processes become more image-based and are more associative than normal.
Patients therefore are more capable of making new and unusual connections between different biographical or current situations. Previously unconscious ideas can become conscious. At higher doses, ego loss can occur, which can be associated with a mystical feeling of connectedness.
Hallucinogens mainly evoke and heighten emotions. Those effects may be experienced strongly as internal visions or in physical manifestations (for example, crying or laughing). In contrast, conventional antidepressants work by suppressing emotions (that is, emotional blunting).
These different mechanisms result in two contrasting management strategies. For example, SSRI antidepressants cause a patient to perceive workplace bullying as less severe and to do nothing to change the situation; the patient remains passive.
In contrast, a therapeutically guided, emotionally activating experience on hallucinogens can help the patient to try more actively to change the stressful situation.
Ketamine has a special place among hallucinogens. Unlike other hallucinogens, ketamine causes a strong clouding of consciousness, a reduction in physical sensory perception, and significant disruption in thinking and memory. It is therefore only suitable as a short-term intervention and is therapeutically impractical over the long term.
Ketamine’s effects
Ketamine, a racemic mixture of the enantiomers S-ketamine and R-ketamine, was originally used only as an analgesic and anesthetic. Owing to its rapid antidepressant effect, it has since also been used as an emergency medication for severe depression, sometimes in combination with SSRIs or serotonin noradrenaline reuptake inhibitors.
Approximately 60% of patients respond to the treatment. Whereas with conventional antidepressants, onset of action requires 10-14 days, ketamine is effective within a few hours. However, relapse always occurs, usually very quickly. After 2-3 days, the effect is usually approximately that of a placebo. An administration interval of about 2 days is optimal. However, “resistance” to the effect often develops after some time: the drug’s antidepressant effect diminishes.
Ketamine also has some unpleasant side effects, such as depersonalization, dissociation, impaired thinking, nystagmus, and psychotomimetic effects. Nausea and vomiting also occur. Interestingly, the latter does not bother the patient much, owing to the drug’s psychological effects, and it does not lead to treatment discontinuation, said Dr. Passie, who described his clinical experiences with ketamine.
Since ketamine causes a considerable clouding of consciousness, sensory disorders, and significant memory problems, it is not suitable for psychedelic-assisted psychotherapy, unlike LSD or psilocybin, he emphasized.
Ketamine 2.0?
Esketamine, the pure S-enantiomer of ketamine, has been on the market since 2019 in the form of a nasal spray (Spravato). Esketamine has been approved in combination with oral antidepressant therapy for adults with a moderate to severe episode of major depression for acute treatment of a psychiatric emergency.
A meta-analysis from 2022 concluded that the original racemic ketamine is better than the new esketamine in reducing symptoms of depression.
In his own comprehensive study, Dr. Passie concluded that the mental impairments that occur during therapy did not differ significantly between substances. The patients even felt that the side effects from esketamine therapy were much more mentally unpleasant, said Dr. Passie. He concluded that the R-enantiomer may have a kind of protective effect against some of the psychopathological effects of the S-enantiomer (esketamine).
In addition, preclinical studies have indicated that the antidepressant effects of R-enantiomer, which is not contained in esketamine, are longer lasting and stronger.
Another problem is absorption, which can be inconsistent with a nasal spray. It may differ, for example, depending on the ambient humidity or whether the patient has recently had a cold. In addition, the spray is far more expensive than the ketamine injection, said Dr. Passie. Patients must also use the nasal spray under supervision at a medical practice (as with the intravenous application) and must receive follow-up care there. It therefore offers no advantage over the ketamine injection.
According to the Institute for Quality and Efficiency in Healthcare, no additional benefit has been proven for esketamine over standard therapies for adults who have experienced a moderate to severe depressive episode when used as short-term treatment for the rapid reduction of depressive symptoms in a psychiatric emergency. The German Medical Association agreed with this evaluation in October 2021.
In the United Kingdom, the medication was never approved, owing to the fact that it was too expensive and that no studies comparing it with psychotherapy were available.
Add-on psilocybin?
What was experienced under the influence of psilocybin can also be subsequently processed and used in psychotherapy.
The acute effect of psilocybin begins after approximately 40 minutes and lasts for 4-6 hours. The antidepressant effect, if it occurs at all, is of immediate onset. Unlike ketamine/esketamine, psilocybin hardly has any physical side effects.
The neurologic mechanism of action has been investigated recently using fMRI and PET techniques. According to the investigations, the substance causes individual networks of activity in the patient’s brain to interconnect more strongly, said Dr. Passie. The thalamus, the filter station for sensory information, as well as the limbic and paralimbic structures, which generate emotions, and the cortex are all activated more strongly.
Two therapeutic settings
Psilocybin, at least in the context of studies, is used in two settings: psycholytic therapy and psychedelic therapy. Both settings originated in the 1950s and were also used with LSD as the active substance.
Psycholytic therapy with psilocybin entails multiple administrations at low doses (for example, 10-18 mg), incorporated into a longer, mostly psychodynamic therapy of around 50-100 hours (often on an inpatient basis at the beginning). It results in what is described as an extended encounter with oneself. The focus is on psychodynamic experiences, such as memories and internal conflicts. In addition, novel experiences with oneself and self-recognition are important.
Psychedelic therapy generally entails one or two sessions with a high dose (for example, 25-35 mg psilocybin). The preparation and follow-up are limited to a few sessions. These methods refer to so-called transpersonal psychology, which addresses extraordinary states of consciousness in line with religious experiences. It often leads to an intense self-confrontation as well as to new evaluations of self and world. The central element to this therapy is the experience of a mystical ego loss and the concomitant feeling of connectedness, which should help to expand one’s perspective.
Euphoria and disillusionment
The first promising studies with a few patients suffering from depression were followed by others in which the euphoria was allowed to fade away somewhat. In the first direct comparison in a methodically high-grade double-blind study, psilocybin was inferior to the SSRI antidepressant escitalopram.
“There is a great variation in response from person to person,” said Dr. Passie. “The better the study is methodically controlled, the worse the results,” he hypothesized.
“Since the method is up to 50 times more expensive in practice, compared to SSRI therapy over 6-12 weeks, the question clearly must be asked as to whether it really has any great future.”
Outlook for psilocybin
Nevertheless, Dr. Passie still sees potential in psilocybin. He considers an approach in which psilocybin therapy is more firmly incorporated into psychotherapy, with between four and 10 therapy sessions before and after administration of a lower therapeutic dose of the substance, to be more promising.
“With this kind of intensive preparation and follow-up, as well as the repeated psilocybin sessions, the patient can benefit much more than is possible with one or two high-dose sessions,” said Dr. Passie, who also is chair of the International Society for Substance-Assisted Psychotherapy. “The constant ‘in-depth work on the ego’ required for drastic therapeutic changes can be more effective and lead to permanent improvements. I have no doubt about this.”
In Dr. Passie’s opinion, the best approach would involve a dignified inpatient setting with a longer period of follow-up care and consistent posttreatment care, including group therapy. The shape of future psilocybin therapy depends on whether the rather abrupt change seen with high-dose psychedelic therapy is permanent. The answer to this question will be decisive for the method and manner of its future clinical use.
Because of the somewhat negative study results, however, the initial investors are pulling out. Dr. Passie is therefore skeptical about whether the necessary larger studies will take place and whether psilocybin will make it onto the market.
In Switzerland, which is not subject to EU restrictions, more than 30 physicians have been authorized to use psilocybin, LSD, and MDMA in psychotherapy sessions. Still, in some respects this is a special case that cannot be transferred easily to other countries, said Dr. Passie.
Possible psilocybin improvement?
Various chemical derivatives of psychoactive substances have been researched, including a psilocybin variant with the label CYB003. With CYB003, the length of the acute psychedelic experience is reduced from around 6 hours (such as with psilocybin) to 1 hour. The plasma concentration of the substance is less variable between different patients. It is assumed that its effects will also differ less from person to person.
In July, researchers began a study of the use of CYB003 in the treatment of major depression. In the randomized, double-blind, placebo-controlled study with 40 patients, multiple doses of the substance will be administered.
When asked, Dr. Passie was rather skeptical about the study. He considers the approaches with psilocybin derivatives to be the consequences of a “gold-rush atmosphere” and expects there will be no real additional benefit, especially not a reduction in the period of action.
A version of this article first appeared on Medscape.com.
Recent studies with hallucinogens have raised hopes for an effective drug-based therapy to treat chronic depression. At the German Congress of Psychosomatic Medicine and Psychotherapy, Torsten Passie, MD, PhD, professor of psychiatry and psychotherapy at the Hannover (Germay) Medical School, gave a presentation on the current state of psilocybin and ketamine/esketamine research.
Dr. Passie, who also is head physician of the specialist unit for addiction and addiction prevention at the Diakonisches Werk in Hannover, has been investigating hallucinogenic substances and their application in psychotherapy for decades.
New therapies sought
In depression, gloom extends beyond the patient’s mood. For some time there has been little cause for joy with regard to chronic depression therapy. Established drug therapies hardly perform any better than placebo in meta-analyses, as a study recently confirmed. The pharmaceutical industry pulled out of psycho-pharmaceutical development more than 10 years ago. What’s more, the number of cases is rising, especially among young people, and there are long waiting times for psychotherapy appointments.
It is no wonder that some are welcoming new drug-based approaches with lysergic acid diethylamide (LSD)–like hallucinogens. In 2016, a study on psilocybin was published in The Lancet Psychiatry, although the study was unblinded and included only 24 patients.
Evoking emotions
A range of substances can be classed as hallucinogens, including psilocybin, mescaline, LSD, 3,4-methylenedioxy-methamphetamine (MDMA, also known as ecstasy), and ketamine.
Taking hallucinogens can cause a release of serotonin and dopamine, an increase in activity levels in the brain, a shift in stimulus filtering, an increase in the production of internal stimuli (inner experiences), and a change in sensory integration (for example, synesthesia).
Besides falling into a dreamlike state, patients can achieve an expansion or narrowing of consciousness if they focus on an inner experience. Internal perception increases. Perceptual routines are broken apart. Thought processes become more image-based and are more associative than normal.
Patients therefore are more capable of making new and unusual connections between different biographical or current situations. Previously unconscious ideas can become conscious. At higher doses, ego loss can occur, which can be associated with a mystical feeling of connectedness.
Hallucinogens mainly evoke and heighten emotions. Those effects may be experienced strongly as internal visions or in physical manifestations (for example, crying or laughing). In contrast, conventional antidepressants work by suppressing emotions (that is, emotional blunting).
These different mechanisms result in two contrasting management strategies. For example, SSRI antidepressants cause a patient to perceive workplace bullying as less severe and to do nothing to change the situation; the patient remains passive.
In contrast, a therapeutically guided, emotionally activating experience on hallucinogens can help the patient to try more actively to change the stressful situation.
Ketamine has a special place among hallucinogens. Unlike other hallucinogens, ketamine causes a strong clouding of consciousness, a reduction in physical sensory perception, and significant disruption in thinking and memory. It is therefore only suitable as a short-term intervention and is therapeutically impractical over the long term.
Ketamine’s effects
Ketamine, a racemic mixture of the enantiomers S-ketamine and R-ketamine, was originally used only as an analgesic and anesthetic. Owing to its rapid antidepressant effect, it has since also been used as an emergency medication for severe depression, sometimes in combination with SSRIs or serotonin noradrenaline reuptake inhibitors.
Approximately 60% of patients respond to the treatment. Whereas with conventional antidepressants, onset of action requires 10-14 days, ketamine is effective within a few hours. However, relapse always occurs, usually very quickly. After 2-3 days, the effect is usually approximately that of a placebo. An administration interval of about 2 days is optimal. However, “resistance” to the effect often develops after some time: the drug’s antidepressant effect diminishes.
Ketamine also has some unpleasant side effects, such as depersonalization, dissociation, impaired thinking, nystagmus, and psychotomimetic effects. Nausea and vomiting also occur. Interestingly, the latter does not bother the patient much, owing to the drug’s psychological effects, and it does not lead to treatment discontinuation, said Dr. Passie, who described his clinical experiences with ketamine.
Since ketamine causes a considerable clouding of consciousness, sensory disorders, and significant memory problems, it is not suitable for psychedelic-assisted psychotherapy, unlike LSD or psilocybin, he emphasized.
Ketamine 2.0?
Esketamine, the pure S-enantiomer of ketamine, has been on the market since 2019 in the form of a nasal spray (Spravato). Esketamine has been approved in combination with oral antidepressant therapy for adults with a moderate to severe episode of major depression for acute treatment of a psychiatric emergency.
A meta-analysis from 2022 concluded that the original racemic ketamine is better than the new esketamine in reducing symptoms of depression.
In his own comprehensive study, Dr. Passie concluded that the mental impairments that occur during therapy did not differ significantly between substances. The patients even felt that the side effects from esketamine therapy were much more mentally unpleasant, said Dr. Passie. He concluded that the R-enantiomer may have a kind of protective effect against some of the psychopathological effects of the S-enantiomer (esketamine).
In addition, preclinical studies have indicated that the antidepressant effects of R-enantiomer, which is not contained in esketamine, are longer lasting and stronger.
Another problem is absorption, which can be inconsistent with a nasal spray. It may differ, for example, depending on the ambient humidity or whether the patient has recently had a cold. In addition, the spray is far more expensive than the ketamine injection, said Dr. Passie. Patients must also use the nasal spray under supervision at a medical practice (as with the intravenous application) and must receive follow-up care there. It therefore offers no advantage over the ketamine injection.
According to the Institute for Quality and Efficiency in Healthcare, no additional benefit has been proven for esketamine over standard therapies for adults who have experienced a moderate to severe depressive episode when used as short-term treatment for the rapid reduction of depressive symptoms in a psychiatric emergency. The German Medical Association agreed with this evaluation in October 2021.
In the United Kingdom, the medication was never approved, owing to the fact that it was too expensive and that no studies comparing it with psychotherapy were available.
Add-on psilocybin?
What was experienced under the influence of psilocybin can also be subsequently processed and used in psychotherapy.
The acute effect of psilocybin begins after approximately 40 minutes and lasts for 4-6 hours. The antidepressant effect, if it occurs at all, is of immediate onset. Unlike ketamine/esketamine, psilocybin hardly has any physical side effects.
The neurologic mechanism of action has been investigated recently using fMRI and PET techniques. According to the investigations, the substance causes individual networks of activity in the patient’s brain to interconnect more strongly, said Dr. Passie. The thalamus, the filter station for sensory information, as well as the limbic and paralimbic structures, which generate emotions, and the cortex are all activated more strongly.
Two therapeutic settings
Psilocybin, at least in the context of studies, is used in two settings: psycholytic therapy and psychedelic therapy. Both settings originated in the 1950s and were also used with LSD as the active substance.
Psycholytic therapy with psilocybin entails multiple administrations at low doses (for example, 10-18 mg), incorporated into a longer, mostly psychodynamic therapy of around 50-100 hours (often on an inpatient basis at the beginning). It results in what is described as an extended encounter with oneself. The focus is on psychodynamic experiences, such as memories and internal conflicts. In addition, novel experiences with oneself and self-recognition are important.
Psychedelic therapy generally entails one or two sessions with a high dose (for example, 25-35 mg psilocybin). The preparation and follow-up are limited to a few sessions. These methods refer to so-called transpersonal psychology, which addresses extraordinary states of consciousness in line with religious experiences. It often leads to an intense self-confrontation as well as to new evaluations of self and world. The central element to this therapy is the experience of a mystical ego loss and the concomitant feeling of connectedness, which should help to expand one’s perspective.
Euphoria and disillusionment
The first promising studies with a few patients suffering from depression were followed by others in which the euphoria was allowed to fade away somewhat. In the first direct comparison in a methodically high-grade double-blind study, psilocybin was inferior to the SSRI antidepressant escitalopram.
“There is a great variation in response from person to person,” said Dr. Passie. “The better the study is methodically controlled, the worse the results,” he hypothesized.
“Since the method is up to 50 times more expensive in practice, compared to SSRI therapy over 6-12 weeks, the question clearly must be asked as to whether it really has any great future.”
Outlook for psilocybin
Nevertheless, Dr. Passie still sees potential in psilocybin. He considers an approach in which psilocybin therapy is more firmly incorporated into psychotherapy, with between four and 10 therapy sessions before and after administration of a lower therapeutic dose of the substance, to be more promising.
“With this kind of intensive preparation and follow-up, as well as the repeated psilocybin sessions, the patient can benefit much more than is possible with one or two high-dose sessions,” said Dr. Passie, who also is chair of the International Society for Substance-Assisted Psychotherapy. “The constant ‘in-depth work on the ego’ required for drastic therapeutic changes can be more effective and lead to permanent improvements. I have no doubt about this.”
In Dr. Passie’s opinion, the best approach would involve a dignified inpatient setting with a longer period of follow-up care and consistent posttreatment care, including group therapy. The shape of future psilocybin therapy depends on whether the rather abrupt change seen with high-dose psychedelic therapy is permanent. The answer to this question will be decisive for the method and manner of its future clinical use.
Because of the somewhat negative study results, however, the initial investors are pulling out. Dr. Passie is therefore skeptical about whether the necessary larger studies will take place and whether psilocybin will make it onto the market.
In Switzerland, which is not subject to EU restrictions, more than 30 physicians have been authorized to use psilocybin, LSD, and MDMA in psychotherapy sessions. Still, in some respects this is a special case that cannot be transferred easily to other countries, said Dr. Passie.
Possible psilocybin improvement?
Various chemical derivatives of psychoactive substances have been researched, including a psilocybin variant with the label CYB003. With CYB003, the length of the acute psychedelic experience is reduced from around 6 hours (such as with psilocybin) to 1 hour. The plasma concentration of the substance is less variable between different patients. It is assumed that its effects will also differ less from person to person.
In July, researchers began a study of the use of CYB003 in the treatment of major depression. In the randomized, double-blind, placebo-controlled study with 40 patients, multiple doses of the substance will be administered.
When asked, Dr. Passie was rather skeptical about the study. He considers the approaches with psilocybin derivatives to be the consequences of a “gold-rush atmosphere” and expects there will be no real additional benefit, especially not a reduction in the period of action.
A version of this article first appeared on Medscape.com.
Recent studies with hallucinogens have raised hopes for an effective drug-based therapy to treat chronic depression. At the German Congress of Psychosomatic Medicine and Psychotherapy, Torsten Passie, MD, PhD, professor of psychiatry and psychotherapy at the Hannover (Germay) Medical School, gave a presentation on the current state of psilocybin and ketamine/esketamine research.
Dr. Passie, who also is head physician of the specialist unit for addiction and addiction prevention at the Diakonisches Werk in Hannover, has been investigating hallucinogenic substances and their application in psychotherapy for decades.
New therapies sought
In depression, gloom extends beyond the patient’s mood. For some time there has been little cause for joy with regard to chronic depression therapy. Established drug therapies hardly perform any better than placebo in meta-analyses, as a study recently confirmed. The pharmaceutical industry pulled out of psycho-pharmaceutical development more than 10 years ago. What’s more, the number of cases is rising, especially among young people, and there are long waiting times for psychotherapy appointments.
It is no wonder that some are welcoming new drug-based approaches with lysergic acid diethylamide (LSD)–like hallucinogens. In 2016, a study on psilocybin was published in The Lancet Psychiatry, although the study was unblinded and included only 24 patients.
Evoking emotions
A range of substances can be classed as hallucinogens, including psilocybin, mescaline, LSD, 3,4-methylenedioxy-methamphetamine (MDMA, also known as ecstasy), and ketamine.
Taking hallucinogens can cause a release of serotonin and dopamine, an increase in activity levels in the brain, a shift in stimulus filtering, an increase in the production of internal stimuli (inner experiences), and a change in sensory integration (for example, synesthesia).
Besides falling into a dreamlike state, patients can achieve an expansion or narrowing of consciousness if they focus on an inner experience. Internal perception increases. Perceptual routines are broken apart. Thought processes become more image-based and are more associative than normal.
Patients therefore are more capable of making new and unusual connections between different biographical or current situations. Previously unconscious ideas can become conscious. At higher doses, ego loss can occur, which can be associated with a mystical feeling of connectedness.
Hallucinogens mainly evoke and heighten emotions. Those effects may be experienced strongly as internal visions or in physical manifestations (for example, crying or laughing). In contrast, conventional antidepressants work by suppressing emotions (that is, emotional blunting).
These different mechanisms result in two contrasting management strategies. For example, SSRI antidepressants cause a patient to perceive workplace bullying as less severe and to do nothing to change the situation; the patient remains passive.
In contrast, a therapeutically guided, emotionally activating experience on hallucinogens can help the patient to try more actively to change the stressful situation.
Ketamine has a special place among hallucinogens. Unlike other hallucinogens, ketamine causes a strong clouding of consciousness, a reduction in physical sensory perception, and significant disruption in thinking and memory. It is therefore only suitable as a short-term intervention and is therapeutically impractical over the long term.
Ketamine’s effects
Ketamine, a racemic mixture of the enantiomers S-ketamine and R-ketamine, was originally used only as an analgesic and anesthetic. Owing to its rapid antidepressant effect, it has since also been used as an emergency medication for severe depression, sometimes in combination with SSRIs or serotonin noradrenaline reuptake inhibitors.
Approximately 60% of patients respond to the treatment. Whereas with conventional antidepressants, onset of action requires 10-14 days, ketamine is effective within a few hours. However, relapse always occurs, usually very quickly. After 2-3 days, the effect is usually approximately that of a placebo. An administration interval of about 2 days is optimal. However, “resistance” to the effect often develops after some time: the drug’s antidepressant effect diminishes.
Ketamine also has some unpleasant side effects, such as depersonalization, dissociation, impaired thinking, nystagmus, and psychotomimetic effects. Nausea and vomiting also occur. Interestingly, the latter does not bother the patient much, owing to the drug’s psychological effects, and it does not lead to treatment discontinuation, said Dr. Passie, who described his clinical experiences with ketamine.
Since ketamine causes a considerable clouding of consciousness, sensory disorders, and significant memory problems, it is not suitable for psychedelic-assisted psychotherapy, unlike LSD or psilocybin, he emphasized.
Ketamine 2.0?
Esketamine, the pure S-enantiomer of ketamine, has been on the market since 2019 in the form of a nasal spray (Spravato). Esketamine has been approved in combination with oral antidepressant therapy for adults with a moderate to severe episode of major depression for acute treatment of a psychiatric emergency.
A meta-analysis from 2022 concluded that the original racemic ketamine is better than the new esketamine in reducing symptoms of depression.
In his own comprehensive study, Dr. Passie concluded that the mental impairments that occur during therapy did not differ significantly between substances. The patients even felt that the side effects from esketamine therapy were much more mentally unpleasant, said Dr. Passie. He concluded that the R-enantiomer may have a kind of protective effect against some of the psychopathological effects of the S-enantiomer (esketamine).
In addition, preclinical studies have indicated that the antidepressant effects of R-enantiomer, which is not contained in esketamine, are longer lasting and stronger.
Another problem is absorption, which can be inconsistent with a nasal spray. It may differ, for example, depending on the ambient humidity or whether the patient has recently had a cold. In addition, the spray is far more expensive than the ketamine injection, said Dr. Passie. Patients must also use the nasal spray under supervision at a medical practice (as with the intravenous application) and must receive follow-up care there. It therefore offers no advantage over the ketamine injection.
According to the Institute for Quality and Efficiency in Healthcare, no additional benefit has been proven for esketamine over standard therapies for adults who have experienced a moderate to severe depressive episode when used as short-term treatment for the rapid reduction of depressive symptoms in a psychiatric emergency. The German Medical Association agreed with this evaluation in October 2021.
In the United Kingdom, the medication was never approved, owing to the fact that it was too expensive and that no studies comparing it with psychotherapy were available.
Add-on psilocybin?
What was experienced under the influence of psilocybin can also be subsequently processed and used in psychotherapy.
The acute effect of psilocybin begins after approximately 40 minutes and lasts for 4-6 hours. The antidepressant effect, if it occurs at all, is of immediate onset. Unlike ketamine/esketamine, psilocybin hardly has any physical side effects.
The neurologic mechanism of action has been investigated recently using fMRI and PET techniques. According to the investigations, the substance causes individual networks of activity in the patient’s brain to interconnect more strongly, said Dr. Passie. The thalamus, the filter station for sensory information, as well as the limbic and paralimbic structures, which generate emotions, and the cortex are all activated more strongly.
Two therapeutic settings
Psilocybin, at least in the context of studies, is used in two settings: psycholytic therapy and psychedelic therapy. Both settings originated in the 1950s and were also used with LSD as the active substance.
Psycholytic therapy with psilocybin entails multiple administrations at low doses (for example, 10-18 mg), incorporated into a longer, mostly psychodynamic therapy of around 50-100 hours (often on an inpatient basis at the beginning). It results in what is described as an extended encounter with oneself. The focus is on psychodynamic experiences, such as memories and internal conflicts. In addition, novel experiences with oneself and self-recognition are important.
Psychedelic therapy generally entails one or two sessions with a high dose (for example, 25-35 mg psilocybin). The preparation and follow-up are limited to a few sessions. These methods refer to so-called transpersonal psychology, which addresses extraordinary states of consciousness in line with religious experiences. It often leads to an intense self-confrontation as well as to new evaluations of self and world. The central element to this therapy is the experience of a mystical ego loss and the concomitant feeling of connectedness, which should help to expand one’s perspective.
Euphoria and disillusionment
The first promising studies with a few patients suffering from depression were followed by others in which the euphoria was allowed to fade away somewhat. In the first direct comparison in a methodically high-grade double-blind study, psilocybin was inferior to the SSRI antidepressant escitalopram.
“There is a great variation in response from person to person,” said Dr. Passie. “The better the study is methodically controlled, the worse the results,” he hypothesized.
“Since the method is up to 50 times more expensive in practice, compared to SSRI therapy over 6-12 weeks, the question clearly must be asked as to whether it really has any great future.”
Outlook for psilocybin
Nevertheless, Dr. Passie still sees potential in psilocybin. He considers an approach in which psilocybin therapy is more firmly incorporated into psychotherapy, with between four and 10 therapy sessions before and after administration of a lower therapeutic dose of the substance, to be more promising.
“With this kind of intensive preparation and follow-up, as well as the repeated psilocybin sessions, the patient can benefit much more than is possible with one or two high-dose sessions,” said Dr. Passie, who also is chair of the International Society for Substance-Assisted Psychotherapy. “The constant ‘in-depth work on the ego’ required for drastic therapeutic changes can be more effective and lead to permanent improvements. I have no doubt about this.”
In Dr. Passie’s opinion, the best approach would involve a dignified inpatient setting with a longer period of follow-up care and consistent posttreatment care, including group therapy. The shape of future psilocybin therapy depends on whether the rather abrupt change seen with high-dose psychedelic therapy is permanent. The answer to this question will be decisive for the method and manner of its future clinical use.
Because of the somewhat negative study results, however, the initial investors are pulling out. Dr. Passie is therefore skeptical about whether the necessary larger studies will take place and whether psilocybin will make it onto the market.
In Switzerland, which is not subject to EU restrictions, more than 30 physicians have been authorized to use psilocybin, LSD, and MDMA in psychotherapy sessions. Still, in some respects this is a special case that cannot be transferred easily to other countries, said Dr. Passie.
Possible psilocybin improvement?
Various chemical derivatives of psychoactive substances have been researched, including a psilocybin variant with the label CYB003. With CYB003, the length of the acute psychedelic experience is reduced from around 6 hours (such as with psilocybin) to 1 hour. The plasma concentration of the substance is less variable between different patients. It is assumed that its effects will also differ less from person to person.
In July, researchers began a study of the use of CYB003 in the treatment of major depression. In the randomized, double-blind, placebo-controlled study with 40 patients, multiple doses of the substance will be administered.
When asked, Dr. Passie was rather skeptical about the study. He considers the approaches with psilocybin derivatives to be the consequences of a “gold-rush atmosphere” and expects there will be no real additional benefit, especially not a reduction in the period of action.
A version of this article first appeared on Medscape.com.
Doctors using fake positive reviews to boost business
Five years ago, Kay Dean relied upon Yelp! and Google reviews in her search for a doctor in her area. After finding a physician with fairly high reviews, Ms. Dean was shocked when her personal experience was significantly worse than patients on the review platforms.
Following her experience, Ms. Dean, a former federal government investigator, became skeptical and used her skills to investigate the practice on all review platforms. She uncovered that the practice had a review from an individual who was involved in a review trading group on Facebook, where organizations openly barter their services in exchange for positive reviews fraud.
“I discovered that the online review world was just saturated with fake reviews, much more so than I think most people are aware ... and law enforcement regulators aren’t doing anything to address the problem,” said Ms. Dean. “In this online space, it’s the Wild West; cheating is rewarded.”
Ms. Dean decided to take matters into her own hands. She created a YouTube channel called Fake Review Watch, where she exposes real businesses and their attempts to dupe potential consumers with fake positive reviews.
For example, one video analyzes an orthopedic surgeon in Manhattan with an abundance of five-star reviews. Through her detailed analysis, Ms. Dean created a spreadsheet of the 26 alleged patients of the orthopedic surgeon that had submitted glowing reviews. She looked into other businesses that the individuals had left reviews for and found a significant amount of overlap.
According to the video, 19 of the doctor’s reviewers had left high reviews for the same moving company in Las Vegas, and 18 of them reviewed the same locksmith in Texas. Overall, eight of the patients reviewed the same mover, locksmith, and hotel in New Zealand.
A matter of trust
Ms. Dean expressed the gravity of this phenomenon, especially in health care, as patients often head online first when searching for care options. Based on a survey by Software Advice, about 84% of patients use online reviews to assess a physician, and 77% use review sites as the first step in finding a doctor.
Patient trust has continued to diminish in recent years, particularly following the pandemic. In a 2021 global ranking of trust levels towards health care by country, the U.S. health care system ranked 19th, far below those of several developing countries.
Owing to the rise of fake patient reviews and their inscrutable nature, Ms. Dean advises staying away from online review platforms. Instead, she suggests sticking to the old-fashioned method of getting recommendations from friends and relatives, not virtual people.
Ms. Dean explained a few indicators that she looks for when trying to identify a fake review.
“The business has all five-star reviews, negative reviews are followed by five-star reviews, or the business has an abnormal number of positive reviews in a short period of time,” she noted. “Some businesses try to bury legitimate negative reviews by obtaining more recent, fake, positive ones. The recent reviews will contradict the specific criticisms in the negative review.”
She warned that consumers should not give credibility to reviews simply because the reviewer is dubbed “Elite” or a Google Local Guide, because she has seen plenty of these individuals posting fake reviews.
Unfortunately, review platforms haven’t been doing much self-policing. Google and Healthgrades have a series of policies against fake engagement, impersonation, misinformation, and misrepresentation, according to their websites. However, the only consequence of these violations is review removal.
Both Yelp! and Google say they have automated software that distinguishes real versus fake reviews. When Yelp! uncovers users engaging in compensation review activity, it removes their reviews, closes their account, and blocks those users from creating future Yelp! accounts.
Physicians’ basis
Moreover,
“I think there’s an erosion of business ethics because cheating is rewarded. You can’t compete in an environment where your competition is allowed to accumulate numerous fake reviews while you’re still trying to fill chairs in your business,” said Ms. Dean. “Your competition is then getting the business because the tech companies are allowing this fraud.”
Family physician and practice owner Mike Woo-Ming, MD, MPH, provides career coaching for physicians, including maintaining a good reputation – in-person and online. He has seen physicians bumping up their own five-star reviews personally as well as posting negative reviews for their competition.
“I’ve seen where they’re going to lose business, as many practices were affected through COVID,” he said. “Business owners can become desperate and may decide to start posting or buying reviews because they know people will choose certain services these days based upon reviews.”
Dr. Woo-Ming expressed his frustration with fellow physicians who give in to purchasing fake reviews, because the patients have no idea whether reviews are genuine or not.
To encourage genuine positive reviews, Dr. Woo-Ming’s practice uses a third-party app system that sends patients a follow-up email or text asking about their experience with a link to review sites.
“Honest reviews are a reflection of what I can do to improve my business. At the end of the day, if you’re truly providing great service and you’re helping people by providing great medical care, those are going to win out,” he said. “I would rather, as a responsible practice owner, improve the experience and outcome for the patient.”
A version of this article first appeared on Medscape.com.
Five years ago, Kay Dean relied upon Yelp! and Google reviews in her search for a doctor in her area. After finding a physician with fairly high reviews, Ms. Dean was shocked when her personal experience was significantly worse than patients on the review platforms.
Following her experience, Ms. Dean, a former federal government investigator, became skeptical and used her skills to investigate the practice on all review platforms. She uncovered that the practice had a review from an individual who was involved in a review trading group on Facebook, where organizations openly barter their services in exchange for positive reviews fraud.
“I discovered that the online review world was just saturated with fake reviews, much more so than I think most people are aware ... and law enforcement regulators aren’t doing anything to address the problem,” said Ms. Dean. “In this online space, it’s the Wild West; cheating is rewarded.”
Ms. Dean decided to take matters into her own hands. She created a YouTube channel called Fake Review Watch, where she exposes real businesses and their attempts to dupe potential consumers with fake positive reviews.
For example, one video analyzes an orthopedic surgeon in Manhattan with an abundance of five-star reviews. Through her detailed analysis, Ms. Dean created a spreadsheet of the 26 alleged patients of the orthopedic surgeon that had submitted glowing reviews. She looked into other businesses that the individuals had left reviews for and found a significant amount of overlap.
According to the video, 19 of the doctor’s reviewers had left high reviews for the same moving company in Las Vegas, and 18 of them reviewed the same locksmith in Texas. Overall, eight of the patients reviewed the same mover, locksmith, and hotel in New Zealand.
A matter of trust
Ms. Dean expressed the gravity of this phenomenon, especially in health care, as patients often head online first when searching for care options. Based on a survey by Software Advice, about 84% of patients use online reviews to assess a physician, and 77% use review sites as the first step in finding a doctor.
Patient trust has continued to diminish in recent years, particularly following the pandemic. In a 2021 global ranking of trust levels towards health care by country, the U.S. health care system ranked 19th, far below those of several developing countries.
Owing to the rise of fake patient reviews and their inscrutable nature, Ms. Dean advises staying away from online review platforms. Instead, she suggests sticking to the old-fashioned method of getting recommendations from friends and relatives, not virtual people.
Ms. Dean explained a few indicators that she looks for when trying to identify a fake review.
“The business has all five-star reviews, negative reviews are followed by five-star reviews, or the business has an abnormal number of positive reviews in a short period of time,” she noted. “Some businesses try to bury legitimate negative reviews by obtaining more recent, fake, positive ones. The recent reviews will contradict the specific criticisms in the negative review.”
She warned that consumers should not give credibility to reviews simply because the reviewer is dubbed “Elite” or a Google Local Guide, because she has seen plenty of these individuals posting fake reviews.
Unfortunately, review platforms haven’t been doing much self-policing. Google and Healthgrades have a series of policies against fake engagement, impersonation, misinformation, and misrepresentation, according to their websites. However, the only consequence of these violations is review removal.
Both Yelp! and Google say they have automated software that distinguishes real versus fake reviews. When Yelp! uncovers users engaging in compensation review activity, it removes their reviews, closes their account, and blocks those users from creating future Yelp! accounts.
Physicians’ basis
Moreover,
“I think there’s an erosion of business ethics because cheating is rewarded. You can’t compete in an environment where your competition is allowed to accumulate numerous fake reviews while you’re still trying to fill chairs in your business,” said Ms. Dean. “Your competition is then getting the business because the tech companies are allowing this fraud.”
Family physician and practice owner Mike Woo-Ming, MD, MPH, provides career coaching for physicians, including maintaining a good reputation – in-person and online. He has seen physicians bumping up their own five-star reviews personally as well as posting negative reviews for their competition.
“I’ve seen where they’re going to lose business, as many practices were affected through COVID,” he said. “Business owners can become desperate and may decide to start posting or buying reviews because they know people will choose certain services these days based upon reviews.”
Dr. Woo-Ming expressed his frustration with fellow physicians who give in to purchasing fake reviews, because the patients have no idea whether reviews are genuine or not.
To encourage genuine positive reviews, Dr. Woo-Ming’s practice uses a third-party app system that sends patients a follow-up email or text asking about their experience with a link to review sites.
“Honest reviews are a reflection of what I can do to improve my business. At the end of the day, if you’re truly providing great service and you’re helping people by providing great medical care, those are going to win out,” he said. “I would rather, as a responsible practice owner, improve the experience and outcome for the patient.”
A version of this article first appeared on Medscape.com.
Five years ago, Kay Dean relied upon Yelp! and Google reviews in her search for a doctor in her area. After finding a physician with fairly high reviews, Ms. Dean was shocked when her personal experience was significantly worse than patients on the review platforms.
Following her experience, Ms. Dean, a former federal government investigator, became skeptical and used her skills to investigate the practice on all review platforms. She uncovered that the practice had a review from an individual who was involved in a review trading group on Facebook, where organizations openly barter their services in exchange for positive reviews fraud.
“I discovered that the online review world was just saturated with fake reviews, much more so than I think most people are aware ... and law enforcement regulators aren’t doing anything to address the problem,” said Ms. Dean. “In this online space, it’s the Wild West; cheating is rewarded.”
Ms. Dean decided to take matters into her own hands. She created a YouTube channel called Fake Review Watch, where she exposes real businesses and their attempts to dupe potential consumers with fake positive reviews.
For example, one video analyzes an orthopedic surgeon in Manhattan with an abundance of five-star reviews. Through her detailed analysis, Ms. Dean created a spreadsheet of the 26 alleged patients of the orthopedic surgeon that had submitted glowing reviews. She looked into other businesses that the individuals had left reviews for and found a significant amount of overlap.
According to the video, 19 of the doctor’s reviewers had left high reviews for the same moving company in Las Vegas, and 18 of them reviewed the same locksmith in Texas. Overall, eight of the patients reviewed the same mover, locksmith, and hotel in New Zealand.
A matter of trust
Ms. Dean expressed the gravity of this phenomenon, especially in health care, as patients often head online first when searching for care options. Based on a survey by Software Advice, about 84% of patients use online reviews to assess a physician, and 77% use review sites as the first step in finding a doctor.
Patient trust has continued to diminish in recent years, particularly following the pandemic. In a 2021 global ranking of trust levels towards health care by country, the U.S. health care system ranked 19th, far below those of several developing countries.
Owing to the rise of fake patient reviews and their inscrutable nature, Ms. Dean advises staying away from online review platforms. Instead, she suggests sticking to the old-fashioned method of getting recommendations from friends and relatives, not virtual people.
Ms. Dean explained a few indicators that she looks for when trying to identify a fake review.
“The business has all five-star reviews, negative reviews are followed by five-star reviews, or the business has an abnormal number of positive reviews in a short period of time,” she noted. “Some businesses try to bury legitimate negative reviews by obtaining more recent, fake, positive ones. The recent reviews will contradict the specific criticisms in the negative review.”
She warned that consumers should not give credibility to reviews simply because the reviewer is dubbed “Elite” or a Google Local Guide, because she has seen plenty of these individuals posting fake reviews.
Unfortunately, review platforms haven’t been doing much self-policing. Google and Healthgrades have a series of policies against fake engagement, impersonation, misinformation, and misrepresentation, according to their websites. However, the only consequence of these violations is review removal.
Both Yelp! and Google say they have automated software that distinguishes real versus fake reviews. When Yelp! uncovers users engaging in compensation review activity, it removes their reviews, closes their account, and blocks those users from creating future Yelp! accounts.
Physicians’ basis
Moreover,
“I think there’s an erosion of business ethics because cheating is rewarded. You can’t compete in an environment where your competition is allowed to accumulate numerous fake reviews while you’re still trying to fill chairs in your business,” said Ms. Dean. “Your competition is then getting the business because the tech companies are allowing this fraud.”
Family physician and practice owner Mike Woo-Ming, MD, MPH, provides career coaching for physicians, including maintaining a good reputation – in-person and online. He has seen physicians bumping up their own five-star reviews personally as well as posting negative reviews for their competition.
“I’ve seen where they’re going to lose business, as many practices were affected through COVID,” he said. “Business owners can become desperate and may decide to start posting or buying reviews because they know people will choose certain services these days based upon reviews.”
Dr. Woo-Ming expressed his frustration with fellow physicians who give in to purchasing fake reviews, because the patients have no idea whether reviews are genuine or not.
To encourage genuine positive reviews, Dr. Woo-Ming’s practice uses a third-party app system that sends patients a follow-up email or text asking about their experience with a link to review sites.
“Honest reviews are a reflection of what I can do to improve my business. At the end of the day, if you’re truly providing great service and you’re helping people by providing great medical care, those are going to win out,” he said. “I would rather, as a responsible practice owner, improve the experience and outcome for the patient.”
A version of this article first appeared on Medscape.com.
Why our brains wear out at the end of the day
The transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr. F. Perry Wilson of the Yale School of Medicine.
Once again, we’re doing an informal journal club to talk about a really interesting study, “A Neuro-metabolic Account of Why Daylong Cognitive Work Alters the Control of Economic Decisions,” that just came out. It tries to answer the question of why our brains wear out. I’m going to put myself in the corner here. Let’s walk through this study, which appears in Current Biology, by lead author Antonius Wiehler from Paris.
The big question is what’s going on with cognitive fatigue. If you look at chess players who are exerting a lot of cognitive effort, it’s well documented that over hours of play, they get worse and make more mistakes. It takes them longer to make decisions. The question is, why?
Why does your brain get tired?
To date, it’s been a little bit hard to tease that out. Now, there is some suggestion of what is responsible for this. The cognitive control center of the brain is probably somewhere in the left lateral prefrontal cortex (LLPC).
The prefrontal cortex is responsible for higher-level thinking. It’s what causes you to be inhibited. It gets shut off by alcohol and leads to impulsive behaviors. The LLPC, according to functional MRI studies, has reduced activity as people become more and more cognitively fatigued. The LLPC helps you think through choices. As you become more fatigued, this area of the brain isn’t working as well. But why would it not work as well? What is going on in that particular part of the brain? It doesn’t seem to be something simple, like glucose levels; that’s been investigated and glucose levels are pretty constant throughout the brain, regardless of cognitive task. This paper seeks to tease out what is actually going on in the LLPC when you are becoming cognitively tired.
They did an experiment where they induced cognitive fatigue, and it sounds like a painful experiment. For more than 6 hours, volunteers completed sessions during which they had to perform cognitive switching tasks. Investigators showed participants a letter, in either red or green, and the participant would respond with whether it was a vowel or a consonant or whether it was a capital or lowercase letter, based on the color. If it’s red, say whether it’s a consonant or vowel. If it’s green, say whether it’s upper- or lowercase.
It’s hard, and doing it for 6 hours is likely to induce a lot of cognitive fatigue. They had a control group as well, which is really important here. The control group also did a task like this for 6 hours, but for them, investigators didn’t change the color as often – perhaps only once per session. For the study group, they were switching colors back and forth quite a lot. They also incorporated a memory challenge that worked in a similar way.
So, what are the readouts of this study? They had a group who went through the hard cognitive challenge and a group who went through the easy cognitive challenge. They looked at a variety of metrics. I’ll describe a few.
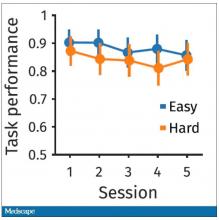
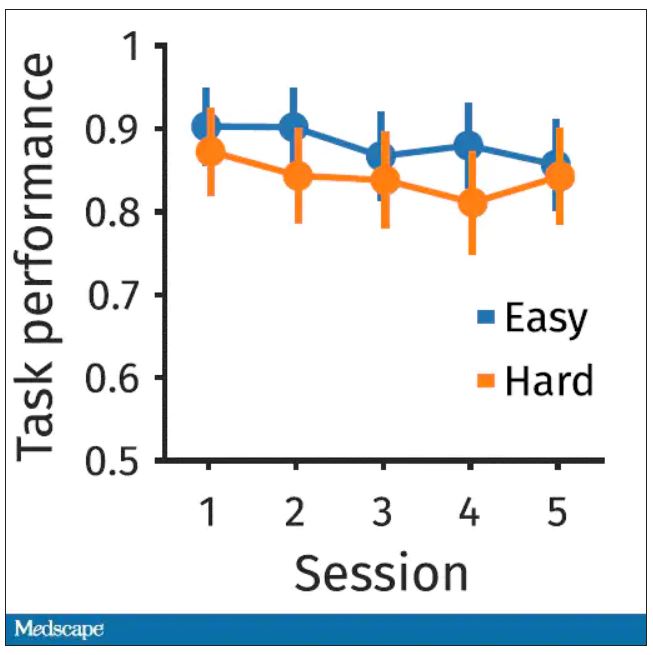
The first is performance decrement. Did they get it wrong? What percentage of the time did the participant say “consonant” when they should have said “lowercase?”
You can see here that the hard group did a little bit worse overall. It was harder, so they don’t do as well. That makes sense. But both groups kind of waned over time a little bit. It’s not as though the hard group declines much more. The slopes of those lines are pretty similar. So, not very robust findings there.
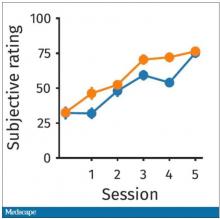
What about subjective fatigue? They asked the participants how exhausted they were from doing the tasks.
Both groups were worn out. It was a long day. There was a suggestion that the hard group became worn out a little bit sooner, but I don’t think this achieves statistical significance. Everyone was getting tired by hour 6 here.
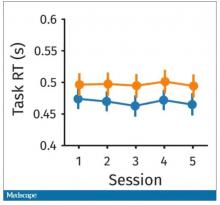
What about response time? How quickly could the participant say “consonant,” “vowel,” “lowercase,” or “uppercase?”
The hard group took longer to respond because it was a harder task. But over time, the response times were pretty flat.
So far there isn’t a robust readout that would make us say, oh, yeah, that is a good marker of cognitive fatigue. That’s how you measure cognitive fatigue. It’s not what people say. It’s not how quick they are. It’s not even how accurate they are.
But then the investigators got a little bit clever. Participants were asked to play a “would you rather” game, a reward game. Here are two examples.
Would you rather:
- Have a 25% chance of earning $50 OR a 95% chance of earning $17.30?
- Earn $50, but your next task session will be hard or earn $40 and your next task session will be easy?
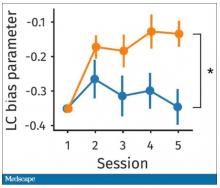
Participants had to figure out the better odds – what should they be choosing here? They had to tease out whether they preferred lower cost lower-risk choices – when they are cognitively fatigued, which has been shown in prior studies.
This showed a pretty dramatic difference between the groups in terms of the low-cost bias – how much more likely they were to pick the low-cost, easier choice as they became more and more cognitively fatigued. The hard group participants were more likely to pick the easy thing rather than the potentially more lucrative thing, which is really interesting when we think about how our own cognitive fatigue happens at the end of a difficult workday, how you may just be likely to go with the flow and do something easy because you just don’t have that much decision-making power left.
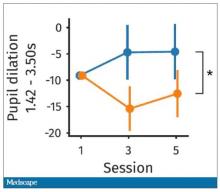
It would be nice to have some objective physiologic measurements for this, and they do. This is pupil dilation.
When you’re paying attention to something, your pupils dilate a little bit. They were able to show that as the hard group became more and more fatigued, pupil dilation sort of went away. In fact, if anything, their pupils constricted a little bit. But basically there was a significant difference here. The easy group’s pupils were still fine; they were still dilating. The hard group’s pupils got more sluggish. This is a physiologic correlate of what’s going on.
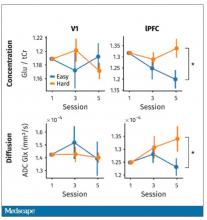
But again, these are all downstream of whatever is happening in the LLPC. So the real meat of this study is a functional MRI analysis, and the way they did this is pretty clever. They were looking for metabolites in the various parts of the brain using a labeled hydrogen MRI, which is even fancier than a functional MRI. It’s like MRI spectroscopy, and it can measure the levels of certain chemicals in the brain. They hypothesized that if there is a chemical that builds up when you are tired, it should build up preferentially in the LLPC.
Whereas in the rest of the brain, there shouldn’t be that much difference because we know the action is happening in the LLPC. The control part of the brain is a section called V1. They looked at a variety of metabolites, but the only one that behaved the way they expected was glutamate and glutamic acid (glutamate metabolites). In the hard group, the glutamate is building up over time, so there is a higher concentration of glutamate in the LLPC but not the rest of the brain. There is also a greater diffusion of glutamate from the intracellular to the extracellular space, which suggests that it’s kind of leaking out of the cells.
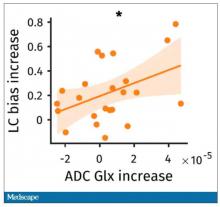
So the signal here is that the thing that’s impacting that part of the brain is this buildup of glutamate. To tie this together, they showed in the scatterplot the relationship between the increase in glutamate and the low-cost bias from the decision fatigue example.
It’s not the strongest correlation, but it is statistically significant that the more glutamate in your LLPC, the more likely you are to just take the easy decision as opposed to really thinking things through. That is pretty powerful. It’s telling us that your brain making you fatigued, and making you less likely to continue to use your LLPC, may be a self-defense mechanism against a buildup of glutamate, which may be neurotoxic. And that’s a fascinating bit of homeostasis.
Of course, it makes you wonder how we might adjust glutamate levels in the brain, although maybe we should let the brain be tired if the brain wants to be tired. It reminds me of that old Far Side cartoon where the guy is raising his hand and asking: “Can I be excused? My brain is full.” That is essentially what’s happening. This part of your brain is becoming taxed and building up glutamate. There’s some kind of negative feedback loop. The authors don’t know what the receptor pathway is that down-regulates that part of the brain based on the glutamate buildup, but some kind of negative feedback loop is saying, okay, give this part of the brain a rest. Things have gone on too far here.
It’s a fascinating study, although it’s not clear what we can do with this information. It’s not clear whether we can manipulate glutamate levels in this particular part of the brain or not. But it’s nice to see some biologic correlates of a psychological phenomenon that is incredibly well described – the phenomenon of decision fatigue. I think we all feel it at the end of a hard workday. If you’ve been doing a lot of cognitively intensive tasks, you just don’t have it in you anymore. And maybe the act of a good night’s sleep is clearing out some of that glutamate in the LLPC, which lets you start over and make some good decisions again. So I hope you all make some good decisions and keep your glutamate levels low. And I’ll see you next time.
For Medscape, I’m Perry Wilson.
Dr. Wilson is an associate professor of medicine and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
The transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr. F. Perry Wilson of the Yale School of Medicine.
Once again, we’re doing an informal journal club to talk about a really interesting study, “A Neuro-metabolic Account of Why Daylong Cognitive Work Alters the Control of Economic Decisions,” that just came out. It tries to answer the question of why our brains wear out. I’m going to put myself in the corner here. Let’s walk through this study, which appears in Current Biology, by lead author Antonius Wiehler from Paris.
The big question is what’s going on with cognitive fatigue. If you look at chess players who are exerting a lot of cognitive effort, it’s well documented that over hours of play, they get worse and make more mistakes. It takes them longer to make decisions. The question is, why?
Why does your brain get tired?
To date, it’s been a little bit hard to tease that out. Now, there is some suggestion of what is responsible for this. The cognitive control center of the brain is probably somewhere in the left lateral prefrontal cortex (LLPC).
The prefrontal cortex is responsible for higher-level thinking. It’s what causes you to be inhibited. It gets shut off by alcohol and leads to impulsive behaviors. The LLPC, according to functional MRI studies, has reduced activity as people become more and more cognitively fatigued. The LLPC helps you think through choices. As you become more fatigued, this area of the brain isn’t working as well. But why would it not work as well? What is going on in that particular part of the brain? It doesn’t seem to be something simple, like glucose levels; that’s been investigated and glucose levels are pretty constant throughout the brain, regardless of cognitive task. This paper seeks to tease out what is actually going on in the LLPC when you are becoming cognitively tired.
They did an experiment where they induced cognitive fatigue, and it sounds like a painful experiment. For more than 6 hours, volunteers completed sessions during which they had to perform cognitive switching tasks. Investigators showed participants a letter, in either red or green, and the participant would respond with whether it was a vowel or a consonant or whether it was a capital or lowercase letter, based on the color. If it’s red, say whether it’s a consonant or vowel. If it’s green, say whether it’s upper- or lowercase.
It’s hard, and doing it for 6 hours is likely to induce a lot of cognitive fatigue. They had a control group as well, which is really important here. The control group also did a task like this for 6 hours, but for them, investigators didn’t change the color as often – perhaps only once per session. For the study group, they were switching colors back and forth quite a lot. They also incorporated a memory challenge that worked in a similar way.
So, what are the readouts of this study? They had a group who went through the hard cognitive challenge and a group who went through the easy cognitive challenge. They looked at a variety of metrics. I’ll describe a few.
The first is performance decrement. Did they get it wrong? What percentage of the time did the participant say “consonant” when they should have said “lowercase?”
You can see here that the hard group did a little bit worse overall. It was harder, so they don’t do as well. That makes sense. But both groups kind of waned over time a little bit. It’s not as though the hard group declines much more. The slopes of those lines are pretty similar. So, not very robust findings there.
What about subjective fatigue? They asked the participants how exhausted they were from doing the tasks.
Both groups were worn out. It was a long day. There was a suggestion that the hard group became worn out a little bit sooner, but I don’t think this achieves statistical significance. Everyone was getting tired by hour 6 here.
What about response time? How quickly could the participant say “consonant,” “vowel,” “lowercase,” or “uppercase?”
The hard group took longer to respond because it was a harder task. But over time, the response times were pretty flat.
So far there isn’t a robust readout that would make us say, oh, yeah, that is a good marker of cognitive fatigue. That’s how you measure cognitive fatigue. It’s not what people say. It’s not how quick they are. It’s not even how accurate they are.
But then the investigators got a little bit clever. Participants were asked to play a “would you rather” game, a reward game. Here are two examples.
Would you rather:
- Have a 25% chance of earning $50 OR a 95% chance of earning $17.30?
- Earn $50, but your next task session will be hard or earn $40 and your next task session will be easy?
Participants had to figure out the better odds – what should they be choosing here? They had to tease out whether they preferred lower cost lower-risk choices – when they are cognitively fatigued, which has been shown in prior studies.
This showed a pretty dramatic difference between the groups in terms of the low-cost bias – how much more likely they were to pick the low-cost, easier choice as they became more and more cognitively fatigued. The hard group participants were more likely to pick the easy thing rather than the potentially more lucrative thing, which is really interesting when we think about how our own cognitive fatigue happens at the end of a difficult workday, how you may just be likely to go with the flow and do something easy because you just don’t have that much decision-making power left.
It would be nice to have some objective physiologic measurements for this, and they do. This is pupil dilation.
When you’re paying attention to something, your pupils dilate a little bit. They were able to show that as the hard group became more and more fatigued, pupil dilation sort of went away. In fact, if anything, their pupils constricted a little bit. But basically there was a significant difference here. The easy group’s pupils were still fine; they were still dilating. The hard group’s pupils got more sluggish. This is a physiologic correlate of what’s going on.
But again, these are all downstream of whatever is happening in the LLPC. So the real meat of this study is a functional MRI analysis, and the way they did this is pretty clever. They were looking for metabolites in the various parts of the brain using a labeled hydrogen MRI, which is even fancier than a functional MRI. It’s like MRI spectroscopy, and it can measure the levels of certain chemicals in the brain. They hypothesized that if there is a chemical that builds up when you are tired, it should build up preferentially in the LLPC.
Whereas in the rest of the brain, there shouldn’t be that much difference because we know the action is happening in the LLPC. The control part of the brain is a section called V1. They looked at a variety of metabolites, but the only one that behaved the way they expected was glutamate and glutamic acid (glutamate metabolites). In the hard group, the glutamate is building up over time, so there is a higher concentration of glutamate in the LLPC but not the rest of the brain. There is also a greater diffusion of glutamate from the intracellular to the extracellular space, which suggests that it’s kind of leaking out of the cells.
So the signal here is that the thing that’s impacting that part of the brain is this buildup of glutamate. To tie this together, they showed in the scatterplot the relationship between the increase in glutamate and the low-cost bias from the decision fatigue example.
It’s not the strongest correlation, but it is statistically significant that the more glutamate in your LLPC, the more likely you are to just take the easy decision as opposed to really thinking things through. That is pretty powerful. It’s telling us that your brain making you fatigued, and making you less likely to continue to use your LLPC, may be a self-defense mechanism against a buildup of glutamate, which may be neurotoxic. And that’s a fascinating bit of homeostasis.
Of course, it makes you wonder how we might adjust glutamate levels in the brain, although maybe we should let the brain be tired if the brain wants to be tired. It reminds me of that old Far Side cartoon where the guy is raising his hand and asking: “Can I be excused? My brain is full.” That is essentially what’s happening. This part of your brain is becoming taxed and building up glutamate. There’s some kind of negative feedback loop. The authors don’t know what the receptor pathway is that down-regulates that part of the brain based on the glutamate buildup, but some kind of negative feedback loop is saying, okay, give this part of the brain a rest. Things have gone on too far here.
It’s a fascinating study, although it’s not clear what we can do with this information. It’s not clear whether we can manipulate glutamate levels in this particular part of the brain or not. But it’s nice to see some biologic correlates of a psychological phenomenon that is incredibly well described – the phenomenon of decision fatigue. I think we all feel it at the end of a hard workday. If you’ve been doing a lot of cognitively intensive tasks, you just don’t have it in you anymore. And maybe the act of a good night’s sleep is clearing out some of that glutamate in the LLPC, which lets you start over and make some good decisions again. So I hope you all make some good decisions and keep your glutamate levels low. And I’ll see you next time.
For Medscape, I’m Perry Wilson.
Dr. Wilson is an associate professor of medicine and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
The transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr. F. Perry Wilson of the Yale School of Medicine.
Once again, we’re doing an informal journal club to talk about a really interesting study, “A Neuro-metabolic Account of Why Daylong Cognitive Work Alters the Control of Economic Decisions,” that just came out. It tries to answer the question of why our brains wear out. I’m going to put myself in the corner here. Let’s walk through this study, which appears in Current Biology, by lead author Antonius Wiehler from Paris.
The big question is what’s going on with cognitive fatigue. If you look at chess players who are exerting a lot of cognitive effort, it’s well documented that over hours of play, they get worse and make more mistakes. It takes them longer to make decisions. The question is, why?
Why does your brain get tired?
To date, it’s been a little bit hard to tease that out. Now, there is some suggestion of what is responsible for this. The cognitive control center of the brain is probably somewhere in the left lateral prefrontal cortex (LLPC).
The prefrontal cortex is responsible for higher-level thinking. It’s what causes you to be inhibited. It gets shut off by alcohol and leads to impulsive behaviors. The LLPC, according to functional MRI studies, has reduced activity as people become more and more cognitively fatigued. The LLPC helps you think through choices. As you become more fatigued, this area of the brain isn’t working as well. But why would it not work as well? What is going on in that particular part of the brain? It doesn’t seem to be something simple, like glucose levels; that’s been investigated and glucose levels are pretty constant throughout the brain, regardless of cognitive task. This paper seeks to tease out what is actually going on in the LLPC when you are becoming cognitively tired.
They did an experiment where they induced cognitive fatigue, and it sounds like a painful experiment. For more than 6 hours, volunteers completed sessions during which they had to perform cognitive switching tasks. Investigators showed participants a letter, in either red or green, and the participant would respond with whether it was a vowel or a consonant or whether it was a capital or lowercase letter, based on the color. If it’s red, say whether it’s a consonant or vowel. If it’s green, say whether it’s upper- or lowercase.
It’s hard, and doing it for 6 hours is likely to induce a lot of cognitive fatigue. They had a control group as well, which is really important here. The control group also did a task like this for 6 hours, but for them, investigators didn’t change the color as often – perhaps only once per session. For the study group, they were switching colors back and forth quite a lot. They also incorporated a memory challenge that worked in a similar way.
So, what are the readouts of this study? They had a group who went through the hard cognitive challenge and a group who went through the easy cognitive challenge. They looked at a variety of metrics. I’ll describe a few.
The first is performance decrement. Did they get it wrong? What percentage of the time did the participant say “consonant” when they should have said “lowercase?”
You can see here that the hard group did a little bit worse overall. It was harder, so they don’t do as well. That makes sense. But both groups kind of waned over time a little bit. It’s not as though the hard group declines much more. The slopes of those lines are pretty similar. So, not very robust findings there.
What about subjective fatigue? They asked the participants how exhausted they were from doing the tasks.
Both groups were worn out. It was a long day. There was a suggestion that the hard group became worn out a little bit sooner, but I don’t think this achieves statistical significance. Everyone was getting tired by hour 6 here.
What about response time? How quickly could the participant say “consonant,” “vowel,” “lowercase,” or “uppercase?”
The hard group took longer to respond because it was a harder task. But over time, the response times were pretty flat.
So far there isn’t a robust readout that would make us say, oh, yeah, that is a good marker of cognitive fatigue. That’s how you measure cognitive fatigue. It’s not what people say. It’s not how quick they are. It’s not even how accurate they are.
But then the investigators got a little bit clever. Participants were asked to play a “would you rather” game, a reward game. Here are two examples.
Would you rather:
- Have a 25% chance of earning $50 OR a 95% chance of earning $17.30?
- Earn $50, but your next task session will be hard or earn $40 and your next task session will be easy?
Participants had to figure out the better odds – what should they be choosing here? They had to tease out whether they preferred lower cost lower-risk choices – when they are cognitively fatigued, which has been shown in prior studies.
This showed a pretty dramatic difference between the groups in terms of the low-cost bias – how much more likely they were to pick the low-cost, easier choice as they became more and more cognitively fatigued. The hard group participants were more likely to pick the easy thing rather than the potentially more lucrative thing, which is really interesting when we think about how our own cognitive fatigue happens at the end of a difficult workday, how you may just be likely to go with the flow and do something easy because you just don’t have that much decision-making power left.
It would be nice to have some objective physiologic measurements for this, and they do. This is pupil dilation.
When you’re paying attention to something, your pupils dilate a little bit. They were able to show that as the hard group became more and more fatigued, pupil dilation sort of went away. In fact, if anything, their pupils constricted a little bit. But basically there was a significant difference here. The easy group’s pupils were still fine; they were still dilating. The hard group’s pupils got more sluggish. This is a physiologic correlate of what’s going on.
But again, these are all downstream of whatever is happening in the LLPC. So the real meat of this study is a functional MRI analysis, and the way they did this is pretty clever. They were looking for metabolites in the various parts of the brain using a labeled hydrogen MRI, which is even fancier than a functional MRI. It’s like MRI spectroscopy, and it can measure the levels of certain chemicals in the brain. They hypothesized that if there is a chemical that builds up when you are tired, it should build up preferentially in the LLPC.
Whereas in the rest of the brain, there shouldn’t be that much difference because we know the action is happening in the LLPC. The control part of the brain is a section called V1. They looked at a variety of metabolites, but the only one that behaved the way they expected was glutamate and glutamic acid (glutamate metabolites). In the hard group, the glutamate is building up over time, so there is a higher concentration of glutamate in the LLPC but not the rest of the brain. There is also a greater diffusion of glutamate from the intracellular to the extracellular space, which suggests that it’s kind of leaking out of the cells.
So the signal here is that the thing that’s impacting that part of the brain is this buildup of glutamate. To tie this together, they showed in the scatterplot the relationship between the increase in glutamate and the low-cost bias from the decision fatigue example.
It’s not the strongest correlation, but it is statistically significant that the more glutamate in your LLPC, the more likely you are to just take the easy decision as opposed to really thinking things through. That is pretty powerful. It’s telling us that your brain making you fatigued, and making you less likely to continue to use your LLPC, may be a self-defense mechanism against a buildup of glutamate, which may be neurotoxic. And that’s a fascinating bit of homeostasis.
Of course, it makes you wonder how we might adjust glutamate levels in the brain, although maybe we should let the brain be tired if the brain wants to be tired. It reminds me of that old Far Side cartoon where the guy is raising his hand and asking: “Can I be excused? My brain is full.” That is essentially what’s happening. This part of your brain is becoming taxed and building up glutamate. There’s some kind of negative feedback loop. The authors don’t know what the receptor pathway is that down-regulates that part of the brain based on the glutamate buildup, but some kind of negative feedback loop is saying, okay, give this part of the brain a rest. Things have gone on too far here.
It’s a fascinating study, although it’s not clear what we can do with this information. It’s not clear whether we can manipulate glutamate levels in this particular part of the brain or not. But it’s nice to see some biologic correlates of a psychological phenomenon that is incredibly well described – the phenomenon of decision fatigue. I think we all feel it at the end of a hard workday. If you’ve been doing a lot of cognitively intensive tasks, you just don’t have it in you anymore. And maybe the act of a good night’s sleep is clearing out some of that glutamate in the LLPC, which lets you start over and make some good decisions again. So I hope you all make some good decisions and keep your glutamate levels low. And I’ll see you next time.
For Medscape, I’m Perry Wilson.
Dr. Wilson is an associate professor of medicine and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Diagnostic criterion may hide borderline personality disorder
Investigators compared characteristics of almost 400 psychiatric outpatients diagnosed with BPD. About half of the participants met the suicidality/self-injury diagnostic criterion for the disorder, while the other half did not.
Results showed no differences between the two groups in degree of impairment in occupational or social functioning, comorbid psychiatric disorders, history of childhood trauma, or severity of depression, anxiety, or anger.
“Just because a person doesn’t engage in self-harm or suicidal behavior doesn’t mean that the person is free of borderline personality disorder,” lead author Mark Zimmerman, MD, professor of psychiatry and human behavior, Brown University, Providence, R.I., told this news organization.
“Clinicians need to screen for borderline personality disorder in patients with other suggestive symptoms, even if those patients don’t self-harm, just as they would for similar patients who do self-harm,” said Dr. Zimmerman, who is also the director of the Outpatient Division at the Partial Hospital Program, Rhode Island Hospital.
The findings were published online in Psychological Medicine.
A ‘polythetic diagnosis’
Dr. Zimmerman noted the impetus for conducting the study originated with a patient he saw who had all of the features of BPD except for self-harm and suicidality. However, because she didn’t have those two features, she was told by her therapist she could not have BPD.
“This sparked the idea that perhaps there are other individuals whose BPD may not be recognized because they don’t engage in self-harm or suicidal behavior,” Dr. Zimmerman said.
“Most individuals with BPD don’t present for treatment saying, ‘I’m here because I don’t have a sense of myself’ or ‘I feel empty inside’ – but they do say, ‘I’m here because I’m cutting myself’ or ‘I’m suicidal,’ ” he added.
The investigators wondered if there were other “hidden” cases of BPD that were being missed by therapists.
They had previously analyzed each diagnostic criterion for BPD to ascertain its sensitivity. “We had been interested in wanting to see whether there was a criterion so frequent in BPD that every patient with BPD has it,” Dr. Zimmerman said.
BPD is a “polythetic diagnosis,” he added. It is “based on a list of features, with a certain minimum number of those features necessary to make the diagnosis rather than one specific criterion.”
His group’s previous research showed affective instability criterion to be present in more than 90% of individuals with BPD. “It had a very high negative predictive value, meaning that if you didn’t have affective instability, you didn’t have the disorder,” he said.
“Given the clinical and public health significance of suicidal and self-harm behavior in patients with BPD, an important question is whether the absence of this criterion, which might attenuate the likelihood of recognizing and diagnosing the disorder, identifies a subgroup of patients with BPD who are ‘less borderline’ than patients with BPD who do not manifest this criterion,” the investigators write.
The researchers wanted to see if a similar finding applied to self-injury and suicidal behavior and turned to the Rhode Island Methods to Improve Diagnostic Assessment and Services (MIDAS) project to compare the demographic and clinical characteristics of patients with BPD who do and do not engage in repeated suicidal and self-harm behavior.
MIDAS project
The study population was derived from 3,800 psychiatric outpatients who had been evaluated in the MIDAS project with semi-structured diagnostic interviews.
Of these, 390 patients were diagnosed with BPD. Since the suicidality/self-harm item was not rated in one patient, the analyzed sample consisted of 389 individuals with BPD (28.3% male; mean age, 32.6 years; 86.3% White). A little more than half the participants (54%) met the BPD suicidality/ self-harm criterion.
Only one-fifth (20.5%) of patients with BPD presented with a chief complaint that was related to a feature of BPD and had received BPD as their principal diagnosis.
Patients who met the suicidality/self-injury criterion were almost twice as likely to be diagnosed with BPD as the principal diagnosis, compared with those who did not have that criterion (24.8% vs. 14.5%, respectively; P < .01).
On the other hand, there was no difference in the mean number of BPD criteria that were met, other than suicidality/self-harm, between those who did and did not present with suicidality/self-harm (5.5 ± 1.2 vs. 5.7 ± 0.8, t = 1.44). The investigators note that this finding was “not significant.”
There also was no difference between patients who did and did not meet the criterion in number of psychiatric diagnoses at time of evaluation (3.4 ± 1.9 vs. 3.5 ± 1.8, t = 0.56).
Hidden BPD
Similarly, there was no difference in any specific Axis I or personality disorder – except for generalized anxiety disorder (GAD) and histrionic personality disorder. Both were more frequent in the patients who did not meet the suicidality/self-injury criterion. However, after controlling for age, the group difference in GAD was no longer deemed significant (F = 3.45, P = .064).
By contrast, histrionic personality disorder remained significant with age as the covariate (F = 6.03, P = .015).
“The patients who met the suicidality/self-injury criterion were significantly more likely to have been hospitalized and reported more suicidal ideation at the time of the evaluation,” the researchers write. Both variables remained significant even after including age as a covariate.
There were no between-group differences on severity of depression, anxiety, or anger at initial evaluation nor were there differences in social functioning, adolescent social functioning, likelihood of persistent unemployment or receiving disability payments, childhood trauma, or neglect.
“I suspect that there are a number of individuals whose BPD is not recognized because they don’t have the more overt feature of self-injury or suicidal behavior,” said Dr. Zimmerman, noting that these patients might be considered as having “hidden” BPD.
“Repeated self-injurious and suicidal behavior is not synonymous with BPD, and clinicians should be aware that the absence of these behaviors does not rule out a diagnosis of BPD,” he added.
Stigmatizing diagnosis?
Monica Carsky, PhD, clinical assistant professor of psychology in psychiatry and senior fellow, Personality Disorders Institute, Weill Cornell Medical College, New York, said the study “will be particularly useful in the education of clinicians about the characteristics of individuals with BPD.”
Dr. Carsky, who is also an adjunct assistant professor in the NYU Postdoctoral Program in Psychoanalysis and Psychotherapy, was not involved with the study. She noted that other factors “can contribute to misdiagnosis of the borderline patients who do not have suicidality/self-harm.”
Clinicians and patients “may see BPD as a stigmatizing diagnosis so that clinicians become reluctant to make, share, and explain this personality disorder diagnosis,” she said.
Dr. Carsky suggested that increasing use of the Alternate Model for Personality Disorders in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-V), which first rates the severity level of personality by assessing identity and relationship problems and then notes traits of specific personality disorders, “will help clinicians who dread telling patients they are ‘borderline.’ ”
No source of study funding has been reported. The investigators and Dr. Carsky reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Investigators compared characteristics of almost 400 psychiatric outpatients diagnosed with BPD. About half of the participants met the suicidality/self-injury diagnostic criterion for the disorder, while the other half did not.
Results showed no differences between the two groups in degree of impairment in occupational or social functioning, comorbid psychiatric disorders, history of childhood trauma, or severity of depression, anxiety, or anger.
“Just because a person doesn’t engage in self-harm or suicidal behavior doesn’t mean that the person is free of borderline personality disorder,” lead author Mark Zimmerman, MD, professor of psychiatry and human behavior, Brown University, Providence, R.I., told this news organization.
“Clinicians need to screen for borderline personality disorder in patients with other suggestive symptoms, even if those patients don’t self-harm, just as they would for similar patients who do self-harm,” said Dr. Zimmerman, who is also the director of the Outpatient Division at the Partial Hospital Program, Rhode Island Hospital.
The findings were published online in Psychological Medicine.
A ‘polythetic diagnosis’
Dr. Zimmerman noted the impetus for conducting the study originated with a patient he saw who had all of the features of BPD except for self-harm and suicidality. However, because she didn’t have those two features, she was told by her therapist she could not have BPD.
“This sparked the idea that perhaps there are other individuals whose BPD may not be recognized because they don’t engage in self-harm or suicidal behavior,” Dr. Zimmerman said.
“Most individuals with BPD don’t present for treatment saying, ‘I’m here because I don’t have a sense of myself’ or ‘I feel empty inside’ – but they do say, ‘I’m here because I’m cutting myself’ or ‘I’m suicidal,’ ” he added.
The investigators wondered if there were other “hidden” cases of BPD that were being missed by therapists.
They had previously analyzed each diagnostic criterion for BPD to ascertain its sensitivity. “We had been interested in wanting to see whether there was a criterion so frequent in BPD that every patient with BPD has it,” Dr. Zimmerman said.
BPD is a “polythetic diagnosis,” he added. It is “based on a list of features, with a certain minimum number of those features necessary to make the diagnosis rather than one specific criterion.”
His group’s previous research showed affective instability criterion to be present in more than 90% of individuals with BPD. “It had a very high negative predictive value, meaning that if you didn’t have affective instability, you didn’t have the disorder,” he said.
“Given the clinical and public health significance of suicidal and self-harm behavior in patients with BPD, an important question is whether the absence of this criterion, which might attenuate the likelihood of recognizing and diagnosing the disorder, identifies a subgroup of patients with BPD who are ‘less borderline’ than patients with BPD who do not manifest this criterion,” the investigators write.
The researchers wanted to see if a similar finding applied to self-injury and suicidal behavior and turned to the Rhode Island Methods to Improve Diagnostic Assessment and Services (MIDAS) project to compare the demographic and clinical characteristics of patients with BPD who do and do not engage in repeated suicidal and self-harm behavior.
MIDAS project
The study population was derived from 3,800 psychiatric outpatients who had been evaluated in the MIDAS project with semi-structured diagnostic interviews.
Of these, 390 patients were diagnosed with BPD. Since the suicidality/self-harm item was not rated in one patient, the analyzed sample consisted of 389 individuals with BPD (28.3% male; mean age, 32.6 years; 86.3% White). A little more than half the participants (54%) met the BPD suicidality/ self-harm criterion.
Only one-fifth (20.5%) of patients with BPD presented with a chief complaint that was related to a feature of BPD and had received BPD as their principal diagnosis.
Patients who met the suicidality/self-injury criterion were almost twice as likely to be diagnosed with BPD as the principal diagnosis, compared with those who did not have that criterion (24.8% vs. 14.5%, respectively; P < .01).
On the other hand, there was no difference in the mean number of BPD criteria that were met, other than suicidality/self-harm, between those who did and did not present with suicidality/self-harm (5.5 ± 1.2 vs. 5.7 ± 0.8, t = 1.44). The investigators note that this finding was “not significant.”
There also was no difference between patients who did and did not meet the criterion in number of psychiatric diagnoses at time of evaluation (3.4 ± 1.9 vs. 3.5 ± 1.8, t = 0.56).
Hidden BPD
Similarly, there was no difference in any specific Axis I or personality disorder – except for generalized anxiety disorder (GAD) and histrionic personality disorder. Both were more frequent in the patients who did not meet the suicidality/self-injury criterion. However, after controlling for age, the group difference in GAD was no longer deemed significant (F = 3.45, P = .064).
By contrast, histrionic personality disorder remained significant with age as the covariate (F = 6.03, P = .015).
“The patients who met the suicidality/self-injury criterion were significantly more likely to have been hospitalized and reported more suicidal ideation at the time of the evaluation,” the researchers write. Both variables remained significant even after including age as a covariate.
There were no between-group differences on severity of depression, anxiety, or anger at initial evaluation nor were there differences in social functioning, adolescent social functioning, likelihood of persistent unemployment or receiving disability payments, childhood trauma, or neglect.
“I suspect that there are a number of individuals whose BPD is not recognized because they don’t have the more overt feature of self-injury or suicidal behavior,” said Dr. Zimmerman, noting that these patients might be considered as having “hidden” BPD.
“Repeated self-injurious and suicidal behavior is not synonymous with BPD, and clinicians should be aware that the absence of these behaviors does not rule out a diagnosis of BPD,” he added.
Stigmatizing diagnosis?
Monica Carsky, PhD, clinical assistant professor of psychology in psychiatry and senior fellow, Personality Disorders Institute, Weill Cornell Medical College, New York, said the study “will be particularly useful in the education of clinicians about the characteristics of individuals with BPD.”
Dr. Carsky, who is also an adjunct assistant professor in the NYU Postdoctoral Program in Psychoanalysis and Psychotherapy, was not involved with the study. She noted that other factors “can contribute to misdiagnosis of the borderline patients who do not have suicidality/self-harm.”
Clinicians and patients “may see BPD as a stigmatizing diagnosis so that clinicians become reluctant to make, share, and explain this personality disorder diagnosis,” she said.
Dr. Carsky suggested that increasing use of the Alternate Model for Personality Disorders in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-V), which first rates the severity level of personality by assessing identity and relationship problems and then notes traits of specific personality disorders, “will help clinicians who dread telling patients they are ‘borderline.’ ”
No source of study funding has been reported. The investigators and Dr. Carsky reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Investigators compared characteristics of almost 400 psychiatric outpatients diagnosed with BPD. About half of the participants met the suicidality/self-injury diagnostic criterion for the disorder, while the other half did not.
Results showed no differences between the two groups in degree of impairment in occupational or social functioning, comorbid psychiatric disorders, history of childhood trauma, or severity of depression, anxiety, or anger.
“Just because a person doesn’t engage in self-harm or suicidal behavior doesn’t mean that the person is free of borderline personality disorder,” lead author Mark Zimmerman, MD, professor of psychiatry and human behavior, Brown University, Providence, R.I., told this news organization.
“Clinicians need to screen for borderline personality disorder in patients with other suggestive symptoms, even if those patients don’t self-harm, just as they would for similar patients who do self-harm,” said Dr. Zimmerman, who is also the director of the Outpatient Division at the Partial Hospital Program, Rhode Island Hospital.
The findings were published online in Psychological Medicine.
A ‘polythetic diagnosis’
Dr. Zimmerman noted the impetus for conducting the study originated with a patient he saw who had all of the features of BPD except for self-harm and suicidality. However, because she didn’t have those two features, she was told by her therapist she could not have BPD.
“This sparked the idea that perhaps there are other individuals whose BPD may not be recognized because they don’t engage in self-harm or suicidal behavior,” Dr. Zimmerman said.
“Most individuals with BPD don’t present for treatment saying, ‘I’m here because I don’t have a sense of myself’ or ‘I feel empty inside’ – but they do say, ‘I’m here because I’m cutting myself’ or ‘I’m suicidal,’ ” he added.
The investigators wondered if there were other “hidden” cases of BPD that were being missed by therapists.
They had previously analyzed each diagnostic criterion for BPD to ascertain its sensitivity. “We had been interested in wanting to see whether there was a criterion so frequent in BPD that every patient with BPD has it,” Dr. Zimmerman said.
BPD is a “polythetic diagnosis,” he added. It is “based on a list of features, with a certain minimum number of those features necessary to make the diagnosis rather than one specific criterion.”
His group’s previous research showed affective instability criterion to be present in more than 90% of individuals with BPD. “It had a very high negative predictive value, meaning that if you didn’t have affective instability, you didn’t have the disorder,” he said.
“Given the clinical and public health significance of suicidal and self-harm behavior in patients with BPD, an important question is whether the absence of this criterion, which might attenuate the likelihood of recognizing and diagnosing the disorder, identifies a subgroup of patients with BPD who are ‘less borderline’ than patients with BPD who do not manifest this criterion,” the investigators write.
The researchers wanted to see if a similar finding applied to self-injury and suicidal behavior and turned to the Rhode Island Methods to Improve Diagnostic Assessment and Services (MIDAS) project to compare the demographic and clinical characteristics of patients with BPD who do and do not engage in repeated suicidal and self-harm behavior.
MIDAS project
The study population was derived from 3,800 psychiatric outpatients who had been evaluated in the MIDAS project with semi-structured diagnostic interviews.
Of these, 390 patients were diagnosed with BPD. Since the suicidality/self-harm item was not rated in one patient, the analyzed sample consisted of 389 individuals with BPD (28.3% male; mean age, 32.6 years; 86.3% White). A little more than half the participants (54%) met the BPD suicidality/ self-harm criterion.
Only one-fifth (20.5%) of patients with BPD presented with a chief complaint that was related to a feature of BPD and had received BPD as their principal diagnosis.
Patients who met the suicidality/self-injury criterion were almost twice as likely to be diagnosed with BPD as the principal diagnosis, compared with those who did not have that criterion (24.8% vs. 14.5%, respectively; P < .01).
On the other hand, there was no difference in the mean number of BPD criteria that were met, other than suicidality/self-harm, between those who did and did not present with suicidality/self-harm (5.5 ± 1.2 vs. 5.7 ± 0.8, t = 1.44). The investigators note that this finding was “not significant.”
There also was no difference between patients who did and did not meet the criterion in number of psychiatric diagnoses at time of evaluation (3.4 ± 1.9 vs. 3.5 ± 1.8, t = 0.56).
Hidden BPD
Similarly, there was no difference in any specific Axis I or personality disorder – except for generalized anxiety disorder (GAD) and histrionic personality disorder. Both were more frequent in the patients who did not meet the suicidality/self-injury criterion. However, after controlling for age, the group difference in GAD was no longer deemed significant (F = 3.45, P = .064).
By contrast, histrionic personality disorder remained significant with age as the covariate (F = 6.03, P = .015).
“The patients who met the suicidality/self-injury criterion were significantly more likely to have been hospitalized and reported more suicidal ideation at the time of the evaluation,” the researchers write. Both variables remained significant even after including age as a covariate.
There were no between-group differences on severity of depression, anxiety, or anger at initial evaluation nor were there differences in social functioning, adolescent social functioning, likelihood of persistent unemployment or receiving disability payments, childhood trauma, or neglect.
“I suspect that there are a number of individuals whose BPD is not recognized because they don’t have the more overt feature of self-injury or suicidal behavior,” said Dr. Zimmerman, noting that these patients might be considered as having “hidden” BPD.
“Repeated self-injurious and suicidal behavior is not synonymous with BPD, and clinicians should be aware that the absence of these behaviors does not rule out a diagnosis of BPD,” he added.
Stigmatizing diagnosis?
Monica Carsky, PhD, clinical assistant professor of psychology in psychiatry and senior fellow, Personality Disorders Institute, Weill Cornell Medical College, New York, said the study “will be particularly useful in the education of clinicians about the characteristics of individuals with BPD.”
Dr. Carsky, who is also an adjunct assistant professor in the NYU Postdoctoral Program in Psychoanalysis and Psychotherapy, was not involved with the study. She noted that other factors “can contribute to misdiagnosis of the borderline patients who do not have suicidality/self-harm.”
Clinicians and patients “may see BPD as a stigmatizing diagnosis so that clinicians become reluctant to make, share, and explain this personality disorder diagnosis,” she said.
Dr. Carsky suggested that increasing use of the Alternate Model for Personality Disorders in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-V), which first rates the severity level of personality by assessing identity and relationship problems and then notes traits of specific personality disorders, “will help clinicians who dread telling patients they are ‘borderline.’ ”
No source of study funding has been reported. The investigators and Dr. Carsky reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Psychiatrists’ income, net worth rise as COVID wanes
Last year brought welcome relief to psychiatrists, with incomes generally rising as practices reopened after COVID-19 restrictions lifted and patients ventured out of their homes.
Psychiatrists’ average annual income rose to $287,000, according to the Medscape Psychiatrist Wealth and Debt Report 2022. That is up about 4% from $275,000, which was listed in last year’s report.
However, psychiatrists still rank in the bottom third of all specialties when it comes to physicians’ income.
According to the overall Medscape Physician Wealth and Debt Report 2022, the highest-paying speciality is plastic surgery ($576,000), followed by orthopedics ($557,000) and cardiology ($490,000).
The lowest-paying areas of medicine are family medicine ($255,000), pediatrics ($244,000), and public health and preventive medicine ($243,000).
The report is based on responses from more than 13,000 physicians in 29 specialties. All were surveyed between Oct. 5, 2021 and Jan. 19, 2022.
Money-conscious?
Similar to last year’s report, three-quarters of psychiatrists have not done anything to reduce major expenses. Those who have taken cost-cutting measures cited deferring or refinancing loans, moving to a different home, or changing cars as ways to do so.
Most psychiatrists (80%) reported having avoided major financial losses, which is up slightly from last year (76%). Only 6% of psychiatrists (9% last year) reported monetary losses because of issues at their medical practice.
One-quarter reported having a stock or corporate investment go south, which is about the same as last year. In addition, 42% said that they have yet to make a particular investment mistake and 19% said that they have not made any investments.
This year, a somewhat smaller percentage of psychiatrists reported keeping their rates of saving in after-tax accounts level or at increased rates, compared with last year (47% vs. 52%).
About 28% of psychiatrists do not regularly put money into after-tax savings accounts, compared with 25% of physicians overall.
The vast majority said that they kept up with bills amid COVID, as they also did last year.
The percentage of psychiatrists who paid mortgages or other bills late during the pandemic is about the same this year as last year (3% and 5%, respectively).
That is in contrast to one 2021 industry survey, which showed that 46% of Americans missed one or more rent or mortgage payments because of COVID.
Other key findings from Medscape’s latest psychiatrist wealth and debt report include that:
- 61% live in a home of 3,000 square feet or less, which is greater that the current average size of a U.S. house (2,261 square feet)
- 22% have one or two credit cards and 42% have five or more credit cards, while the average American has four cards
- 63% differ in opinion, at least sporadically, with their significant other about spending. A Northwestern Mutual study showed that across the country, around 1 in 4 couples argue about money at least once a month.
In addition, 70% of psychiatrists said that they typically tip at least the recommended 20% for decent service, which is somewhat more generous than the average physician (64%).
A version of this article first appeared on Medscape.com.
Last year brought welcome relief to psychiatrists, with incomes generally rising as practices reopened after COVID-19 restrictions lifted and patients ventured out of their homes.
Psychiatrists’ average annual income rose to $287,000, according to the Medscape Psychiatrist Wealth and Debt Report 2022. That is up about 4% from $275,000, which was listed in last year’s report.
However, psychiatrists still rank in the bottom third of all specialties when it comes to physicians’ income.
According to the overall Medscape Physician Wealth and Debt Report 2022, the highest-paying speciality is plastic surgery ($576,000), followed by orthopedics ($557,000) and cardiology ($490,000).
The lowest-paying areas of medicine are family medicine ($255,000), pediatrics ($244,000), and public health and preventive medicine ($243,000).
The report is based on responses from more than 13,000 physicians in 29 specialties. All were surveyed between Oct. 5, 2021 and Jan. 19, 2022.
Money-conscious?
Similar to last year’s report, three-quarters of psychiatrists have not done anything to reduce major expenses. Those who have taken cost-cutting measures cited deferring or refinancing loans, moving to a different home, or changing cars as ways to do so.
Most psychiatrists (80%) reported having avoided major financial losses, which is up slightly from last year (76%). Only 6% of psychiatrists (9% last year) reported monetary losses because of issues at their medical practice.
One-quarter reported having a stock or corporate investment go south, which is about the same as last year. In addition, 42% said that they have yet to make a particular investment mistake and 19% said that they have not made any investments.
This year, a somewhat smaller percentage of psychiatrists reported keeping their rates of saving in after-tax accounts level or at increased rates, compared with last year (47% vs. 52%).
About 28% of psychiatrists do not regularly put money into after-tax savings accounts, compared with 25% of physicians overall.
The vast majority said that they kept up with bills amid COVID, as they also did last year.
The percentage of psychiatrists who paid mortgages or other bills late during the pandemic is about the same this year as last year (3% and 5%, respectively).
That is in contrast to one 2021 industry survey, which showed that 46% of Americans missed one or more rent or mortgage payments because of COVID.
Other key findings from Medscape’s latest psychiatrist wealth and debt report include that:
- 61% live in a home of 3,000 square feet or less, which is greater that the current average size of a U.S. house (2,261 square feet)
- 22% have one or two credit cards and 42% have five or more credit cards, while the average American has four cards
- 63% differ in opinion, at least sporadically, with their significant other about spending. A Northwestern Mutual study showed that across the country, around 1 in 4 couples argue about money at least once a month.
In addition, 70% of psychiatrists said that they typically tip at least the recommended 20% for decent service, which is somewhat more generous than the average physician (64%).
A version of this article first appeared on Medscape.com.
Last year brought welcome relief to psychiatrists, with incomes generally rising as practices reopened after COVID-19 restrictions lifted and patients ventured out of their homes.
Psychiatrists’ average annual income rose to $287,000, according to the Medscape Psychiatrist Wealth and Debt Report 2022. That is up about 4% from $275,000, which was listed in last year’s report.
However, psychiatrists still rank in the bottom third of all specialties when it comes to physicians’ income.
According to the overall Medscape Physician Wealth and Debt Report 2022, the highest-paying speciality is plastic surgery ($576,000), followed by orthopedics ($557,000) and cardiology ($490,000).
The lowest-paying areas of medicine are family medicine ($255,000), pediatrics ($244,000), and public health and preventive medicine ($243,000).
The report is based on responses from more than 13,000 physicians in 29 specialties. All were surveyed between Oct. 5, 2021 and Jan. 19, 2022.
Money-conscious?
Similar to last year’s report, three-quarters of psychiatrists have not done anything to reduce major expenses. Those who have taken cost-cutting measures cited deferring or refinancing loans, moving to a different home, or changing cars as ways to do so.
Most psychiatrists (80%) reported having avoided major financial losses, which is up slightly from last year (76%). Only 6% of psychiatrists (9% last year) reported monetary losses because of issues at their medical practice.
One-quarter reported having a stock or corporate investment go south, which is about the same as last year. In addition, 42% said that they have yet to make a particular investment mistake and 19% said that they have not made any investments.
This year, a somewhat smaller percentage of psychiatrists reported keeping their rates of saving in after-tax accounts level or at increased rates, compared with last year (47% vs. 52%).
About 28% of psychiatrists do not regularly put money into after-tax savings accounts, compared with 25% of physicians overall.
The vast majority said that they kept up with bills amid COVID, as they also did last year.
The percentage of psychiatrists who paid mortgages or other bills late during the pandemic is about the same this year as last year (3% and 5%, respectively).
That is in contrast to one 2021 industry survey, which showed that 46% of Americans missed one or more rent or mortgage payments because of COVID.
Other key findings from Medscape’s latest psychiatrist wealth and debt report include that:
- 61% live in a home of 3,000 square feet or less, which is greater that the current average size of a U.S. house (2,261 square feet)
- 22% have one or two credit cards and 42% have five or more credit cards, while the average American has four cards
- 63% differ in opinion, at least sporadically, with their significant other about spending. A Northwestern Mutual study showed that across the country, around 1 in 4 couples argue about money at least once a month.
In addition, 70% of psychiatrists said that they typically tip at least the recommended 20% for decent service, which is somewhat more generous than the average physician (64%).
A version of this article first appeared on Medscape.com.
Postpartum psychosis: Does longitudinal course inform treatment?
The last 15 years have brought increased effort to screen for postpartum psychiatric illness. That’s exceedingly welcome given the morbidity and potential mortality associated with postpartum psychiatric disorders across the country.
From small community hospitals to major academic centers, screening for postpartum depression is part of the clinical fabric of routine obstetrical care. There is a growing appreciation for the complexity of perinatal psychiatric illness, particularly with respect to the commingling of both mood and anxiety disorders during the postpartum period. However, willingness to treat and appreciation of the urgency to treat with both pharmacologic and nonpharmacologic interventions can vary. For women who suffer from postpartum depression and their families, there are real-world implications of both treating and failing to treat this illness, and there is an urgent need to really help these women “climb out of the darkness” that is and defines postpartum depression.
Less common but of great clinical importance is postpartum psychosis, which occurs in approximately 1 in 1,000-2,000 women based on estimates from several studies. As noted in previous columns, the presentation is a dramatic one, with the typical onset of psychotic symptoms in the first days to weeks post partum. The disorder typically has a mood component and is not an exacerbation of underlying chronic psychotic illness. While there have been few systematic treatment studies, the clinical consensus is treatment usually includes hospitalization to ensure the safety of both the patient and infant. Use of medications, including mood stabilizers, antipsychotics, and benzodiazepines may be appropriate when expeditious treatment is needed.
Appropriate treatment by informed clinical staff is essential, as untreated or incompletely treated postpartum psychosis with its attendant morbidity and potential mortality is a very real concern. As I speak with women across the country with histories of postpartum psychosis, I’m often told of the difficult exchanges that women and their partners have at EDs in various clinical settings where diagnosis was delayed, or treatment was incomplete because of staff without expertise in postpartum psychosis management.
Another dilemma that patients and clinicians face after acute treatment is treatment duration, which is derived from how we conceptualize the illness. Even for experts in the area, there is not a consensus on whether postpartum psychosis should be considered as bipolar disorder or whether it is a circumscribed diagnostic entity. This issue has been hotly debated for many years and is one of the reasons why the illness is not included in the DSM classification system.
At Massachusetts General Hospital, we are systematically studying a large cohort of women with histories of postpartum psychosis as part of the MGH Postpartum Psychosis Project to better understand the phenomenology of postpartum psychosis, and also to understand the possible genomic underpinning of the illness. Most recently, we are conducting a neuroimaging study of women with histories of postpartum psychosis, compared with women in a healthy control group. We hope the results of this novel investigation will help to answer whether there is a neural signature identifiable with neuroimaging techniques such as functional MRI, if those findings are similar to other findings of neural circuitry we see in other forms of psychotic illness, or if the illness has a more distinct neural signature.
A question patients and colleagues often ask is what is the long-term nature of postpartum psychosis. If one considers it clearly to be bipolar disorder, the most intuitive approach would be long-term treatment with mood stabilizers. We now have a growing amount of data on the longitudinal course of postpartum psychosis. In one meta-analysis, 64% of women who had an episode of postpartum psychosis developed episodes of recurrent psychiatric disorder mostly consistent with bipolar illness. However, 36% of women appear to have more circumscribed illness without recurrence. In those women with recurrent disease, the presumption was those patients who had bipolar disorder and their presentation postpartum was simply their index episode of bipolar illness. However, there were other women who looked as if they had developed subsequent illness over the 11-26 years of follow-up, and those women did not receive long-term treatment.
A more recent prospective study of 106 women with postpartum psychosis who had their medication tapered and discontinued showed that 32% of women went on to have recurrent disease with a median time to illness of 20.3 months, and those patients presented primarily with illness that looked like bipolar disorder.
These accumulating data support the impression we’ve had for years that there’s a very strong relationship between bipolar disorder and postpartum psychiatric illness. Regardless of what side of the debate you fall on, the acute treatment is really the same. The real question for the clinician is what to do over the long term. Frequently, patients feel very strongly about a taper and discontinuation of medicine, and even the data show between 30% and 45% of women seem to have relatively circumscribed disease. There may be an issue in terms of prophylaxis if a patient gets pregnant and delivers another child, but that’s a separate issue. The issue is really whether there is a way to “thread the clinical needle” and meet patients where they are who do not want to continue long-term treatment.
I think we are at a point where we could argue the clinical treatment algorithm for patients who present with a new-onset manic-like psychosis postpartum is clear: initial treatment to stabilize, and then treatment with mood stabilizers for at least 12 months to follow is indicated. However, it may also be reasonable to taper treatment at 12-18 months, particularly for patients who have discussed this option with their clinician and who have been totally well for a year. (Women with previously documented bipolar disorder who have episodes of postpartum psychosis should obviously be treated with longer-term treatment aimed at maintenance of euthymia, as discontinuation of mood stabilizer is well known to be associated with risk for relapse.)
It should be noted that the longitudinal course and the treatment implications for women with postpartum psychosis are not etched in stone absent a clear evidence base driving care guidelines. Treatment must still be individualized. Women with underlying mood diatheses will typically declare themselves over time, and others may do well if they discontinue treatment, particularly if they are followed closely and instructed to present to a clinician at the earliest symptoms of mood dysregulation. The good news is we’ve seen an evolution of both interest and expertise in acute management of postpartum psychosis and a richer appreciation of the potential heterogeneity of this sample of women. There may be some variability in terms of long-term course requiring personalized treatment and obviously close follow-up of these women.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications. Email Dr. Cohen at [email protected].
The last 15 years have brought increased effort to screen for postpartum psychiatric illness. That’s exceedingly welcome given the morbidity and potential mortality associated with postpartum psychiatric disorders across the country.
From small community hospitals to major academic centers, screening for postpartum depression is part of the clinical fabric of routine obstetrical care. There is a growing appreciation for the complexity of perinatal psychiatric illness, particularly with respect to the commingling of both mood and anxiety disorders during the postpartum period. However, willingness to treat and appreciation of the urgency to treat with both pharmacologic and nonpharmacologic interventions can vary. For women who suffer from postpartum depression and their families, there are real-world implications of both treating and failing to treat this illness, and there is an urgent need to really help these women “climb out of the darkness” that is and defines postpartum depression.
Less common but of great clinical importance is postpartum psychosis, which occurs in approximately 1 in 1,000-2,000 women based on estimates from several studies. As noted in previous columns, the presentation is a dramatic one, with the typical onset of psychotic symptoms in the first days to weeks post partum. The disorder typically has a mood component and is not an exacerbation of underlying chronic psychotic illness. While there have been few systematic treatment studies, the clinical consensus is treatment usually includes hospitalization to ensure the safety of both the patient and infant. Use of medications, including mood stabilizers, antipsychotics, and benzodiazepines may be appropriate when expeditious treatment is needed.
Appropriate treatment by informed clinical staff is essential, as untreated or incompletely treated postpartum psychosis with its attendant morbidity and potential mortality is a very real concern. As I speak with women across the country with histories of postpartum psychosis, I’m often told of the difficult exchanges that women and their partners have at EDs in various clinical settings where diagnosis was delayed, or treatment was incomplete because of staff without expertise in postpartum psychosis management.
Another dilemma that patients and clinicians face after acute treatment is treatment duration, which is derived from how we conceptualize the illness. Even for experts in the area, there is not a consensus on whether postpartum psychosis should be considered as bipolar disorder or whether it is a circumscribed diagnostic entity. This issue has been hotly debated for many years and is one of the reasons why the illness is not included in the DSM classification system.
At Massachusetts General Hospital, we are systematically studying a large cohort of women with histories of postpartum psychosis as part of the MGH Postpartum Psychosis Project to better understand the phenomenology of postpartum psychosis, and also to understand the possible genomic underpinning of the illness. Most recently, we are conducting a neuroimaging study of women with histories of postpartum psychosis, compared with women in a healthy control group. We hope the results of this novel investigation will help to answer whether there is a neural signature identifiable with neuroimaging techniques such as functional MRI, if those findings are similar to other findings of neural circuitry we see in other forms of psychotic illness, or if the illness has a more distinct neural signature.
A question patients and colleagues often ask is what is the long-term nature of postpartum psychosis. If one considers it clearly to be bipolar disorder, the most intuitive approach would be long-term treatment with mood stabilizers. We now have a growing amount of data on the longitudinal course of postpartum psychosis. In one meta-analysis, 64% of women who had an episode of postpartum psychosis developed episodes of recurrent psychiatric disorder mostly consistent with bipolar illness. However, 36% of women appear to have more circumscribed illness without recurrence. In those women with recurrent disease, the presumption was those patients who had bipolar disorder and their presentation postpartum was simply their index episode of bipolar illness. However, there were other women who looked as if they had developed subsequent illness over the 11-26 years of follow-up, and those women did not receive long-term treatment.
A more recent prospective study of 106 women with postpartum psychosis who had their medication tapered and discontinued showed that 32% of women went on to have recurrent disease with a median time to illness of 20.3 months, and those patients presented primarily with illness that looked like bipolar disorder.
These accumulating data support the impression we’ve had for years that there’s a very strong relationship between bipolar disorder and postpartum psychiatric illness. Regardless of what side of the debate you fall on, the acute treatment is really the same. The real question for the clinician is what to do over the long term. Frequently, patients feel very strongly about a taper and discontinuation of medicine, and even the data show between 30% and 45% of women seem to have relatively circumscribed disease. There may be an issue in terms of prophylaxis if a patient gets pregnant and delivers another child, but that’s a separate issue. The issue is really whether there is a way to “thread the clinical needle” and meet patients where they are who do not want to continue long-term treatment.
I think we are at a point where we could argue the clinical treatment algorithm for patients who present with a new-onset manic-like psychosis postpartum is clear: initial treatment to stabilize, and then treatment with mood stabilizers for at least 12 months to follow is indicated. However, it may also be reasonable to taper treatment at 12-18 months, particularly for patients who have discussed this option with their clinician and who have been totally well for a year. (Women with previously documented bipolar disorder who have episodes of postpartum psychosis should obviously be treated with longer-term treatment aimed at maintenance of euthymia, as discontinuation of mood stabilizer is well known to be associated with risk for relapse.)
It should be noted that the longitudinal course and the treatment implications for women with postpartum psychosis are not etched in stone absent a clear evidence base driving care guidelines. Treatment must still be individualized. Women with underlying mood diatheses will typically declare themselves over time, and others may do well if they discontinue treatment, particularly if they are followed closely and instructed to present to a clinician at the earliest symptoms of mood dysregulation. The good news is we’ve seen an evolution of both interest and expertise in acute management of postpartum psychosis and a richer appreciation of the potential heterogeneity of this sample of women. There may be some variability in terms of long-term course requiring personalized treatment and obviously close follow-up of these women.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications. Email Dr. Cohen at [email protected].
The last 15 years have brought increased effort to screen for postpartum psychiatric illness. That’s exceedingly welcome given the morbidity and potential mortality associated with postpartum psychiatric disorders across the country.
From small community hospitals to major academic centers, screening for postpartum depression is part of the clinical fabric of routine obstetrical care. There is a growing appreciation for the complexity of perinatal psychiatric illness, particularly with respect to the commingling of both mood and anxiety disorders during the postpartum period. However, willingness to treat and appreciation of the urgency to treat with both pharmacologic and nonpharmacologic interventions can vary. For women who suffer from postpartum depression and their families, there are real-world implications of both treating and failing to treat this illness, and there is an urgent need to really help these women “climb out of the darkness” that is and defines postpartum depression.
Less common but of great clinical importance is postpartum psychosis, which occurs in approximately 1 in 1,000-2,000 women based on estimates from several studies. As noted in previous columns, the presentation is a dramatic one, with the typical onset of psychotic symptoms in the first days to weeks post partum. The disorder typically has a mood component and is not an exacerbation of underlying chronic psychotic illness. While there have been few systematic treatment studies, the clinical consensus is treatment usually includes hospitalization to ensure the safety of both the patient and infant. Use of medications, including mood stabilizers, antipsychotics, and benzodiazepines may be appropriate when expeditious treatment is needed.
Appropriate treatment by informed clinical staff is essential, as untreated or incompletely treated postpartum psychosis with its attendant morbidity and potential mortality is a very real concern. As I speak with women across the country with histories of postpartum psychosis, I’m often told of the difficult exchanges that women and their partners have at EDs in various clinical settings where diagnosis was delayed, or treatment was incomplete because of staff without expertise in postpartum psychosis management.
Another dilemma that patients and clinicians face after acute treatment is treatment duration, which is derived from how we conceptualize the illness. Even for experts in the area, there is not a consensus on whether postpartum psychosis should be considered as bipolar disorder or whether it is a circumscribed diagnostic entity. This issue has been hotly debated for many years and is one of the reasons why the illness is not included in the DSM classification system.
At Massachusetts General Hospital, we are systematically studying a large cohort of women with histories of postpartum psychosis as part of the MGH Postpartum Psychosis Project to better understand the phenomenology of postpartum psychosis, and also to understand the possible genomic underpinning of the illness. Most recently, we are conducting a neuroimaging study of women with histories of postpartum psychosis, compared with women in a healthy control group. We hope the results of this novel investigation will help to answer whether there is a neural signature identifiable with neuroimaging techniques such as functional MRI, if those findings are similar to other findings of neural circuitry we see in other forms of psychotic illness, or if the illness has a more distinct neural signature.
A question patients and colleagues often ask is what is the long-term nature of postpartum psychosis. If one considers it clearly to be bipolar disorder, the most intuitive approach would be long-term treatment with mood stabilizers. We now have a growing amount of data on the longitudinal course of postpartum psychosis. In one meta-analysis, 64% of women who had an episode of postpartum psychosis developed episodes of recurrent psychiatric disorder mostly consistent with bipolar illness. However, 36% of women appear to have more circumscribed illness without recurrence. In those women with recurrent disease, the presumption was those patients who had bipolar disorder and their presentation postpartum was simply their index episode of bipolar illness. However, there were other women who looked as if they had developed subsequent illness over the 11-26 years of follow-up, and those women did not receive long-term treatment.
A more recent prospective study of 106 women with postpartum psychosis who had their medication tapered and discontinued showed that 32% of women went on to have recurrent disease with a median time to illness of 20.3 months, and those patients presented primarily with illness that looked like bipolar disorder.
These accumulating data support the impression we’ve had for years that there’s a very strong relationship between bipolar disorder and postpartum psychiatric illness. Regardless of what side of the debate you fall on, the acute treatment is really the same. The real question for the clinician is what to do over the long term. Frequently, patients feel very strongly about a taper and discontinuation of medicine, and even the data show between 30% and 45% of women seem to have relatively circumscribed disease. There may be an issue in terms of prophylaxis if a patient gets pregnant and delivers another child, but that’s a separate issue. The issue is really whether there is a way to “thread the clinical needle” and meet patients where they are who do not want to continue long-term treatment.
I think we are at a point where we could argue the clinical treatment algorithm for patients who present with a new-onset manic-like psychosis postpartum is clear: initial treatment to stabilize, and then treatment with mood stabilizers for at least 12 months to follow is indicated. However, it may also be reasonable to taper treatment at 12-18 months, particularly for patients who have discussed this option with their clinician and who have been totally well for a year. (Women with previously documented bipolar disorder who have episodes of postpartum psychosis should obviously be treated with longer-term treatment aimed at maintenance of euthymia, as discontinuation of mood stabilizer is well known to be associated with risk for relapse.)
It should be noted that the longitudinal course and the treatment implications for women with postpartum psychosis are not etched in stone absent a clear evidence base driving care guidelines. Treatment must still be individualized. Women with underlying mood diatheses will typically declare themselves over time, and others may do well if they discontinue treatment, particularly if they are followed closely and instructed to present to a clinician at the earliest symptoms of mood dysregulation. The good news is we’ve seen an evolution of both interest and expertise in acute management of postpartum psychosis and a richer appreciation of the potential heterogeneity of this sample of women. There may be some variability in terms of long-term course requiring personalized treatment and obviously close follow-up of these women.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications. Email Dr. Cohen at [email protected].
Mindfulness ‘changes the biology’ of pain
In a randomized trial, more than 100 healthy individuals were assigned to an 8-week mindfulness-based stress reduction (MBSR) program, a health improvement program (HEP) of the same length, or a waiting list.
Scanning participants’ brains during a heat-based stimulus pain task showed those who completed the MBSR had a reduction in a brain signature linked to the sensory intensity of pain.
“Our finding supports the idea that for new practitioners, mindfulness training directly affects how sensory signals from the body are converted into a brain response,” lead investigator Joseph Wielgosz, PhD, of the Center for Healthy Minds, University of Wisconsin–Madison, said in a release.
Further analysis in long-term meditation practitioners showed the total time spent on intensive retreats was associated with neural changes associated with the perceived stress of pain.
“Just like an experienced athlete plays a sport differently than a first-timer, experienced mindfulness practitioners seem to use their mental ‘muscles’ differently in response to pain than first-time meditators,” Dr. Wielgosz noted.
The findings were published online in the American Journal of Psychiatry.
A complex condition
Dr. Wielgosz told this news organization that pain is “complex,” with multiple stages and several phases between the time signals are sent from pain receptors and the experience of pain.
“The way that mindfulness affects pain processing has more to do with the way the brain interprets pain signals.”
The investigators note that understanding the neurocognitive mechanisms underlying the efficacy of nonpharmacologic pain interventions is a “high-priority objective for improving pain treatment.”
Evidence from brief laboratory interventions and cross-sectional studies suggests that mindfulness training is associated with alterations in both sensory processing and cognitive-emotional regulatory networks, the investigators note.
“However, no such study has yet been conducted on a standardized, full-length, and widely used clinical intervention, such as MBSR,” they add.
Thermal pain task
The randomized, active-control trial included 115 healthy, meditation-naive individuals (61.7% women; average age, 48.3 years). Just over half (58%) had a graduate degree and their mean score on the Hollingshead index was 58.3, indicting a higher socioeconomic status.
All were randomly assigned to an 8-week MBSR course, an 8-week HEP course as an active control group, or a waiting-list control group with no intervention.
The MBSR involved instruction and practice in continuous focused attention on the breath, bodily sensations, and mental content while in seated postures, walking, and doing yoga.
The HEP matched the MBSR in terms of its length, structure, and nonspecific therapeutic elements, which included a supportive group atmosphere, expert instruction, and positive expectancy for benefit.
To examine the interventions’ effect on the pain experience, participants underwent a pain task in which they had 20 thermal stimuli applied to the inside of the left wrist for 12 seconds, including 8 seconds at peak temperature.
The stimuli were separated by a distractor task and intervals for cued anticipation, recovery, and subjective ratings of intensity and unpleasantness on a scale of 0-20.
During the task, participants underwent MRI to assess the neurologic pain signature (NPS) and the stimulus intensity independent pain signature-1 (SIIPS-1) within the brain.
The NPS is activated by various types of pain stimuli, while responding minimally or not at all to “emotionally evocative stimuli” relating to pain or to placebo treatment, the researchers note.
In contrast, the SIIPS-1 is activated in response to aspects of pain unrelated to the stimulus itself. It incorporates a “broader range of cognitive and emotional modulatory circuits,” including those related to expectancy and cognitive processes to modulate the pain experience.
Neural signatures
Results showed that in all groups, age was significantly negatively associated with both NPS (P = .001) and SIIPS-1 response (P < .001), although not subjective pain reports, and was subsequently included in all analyses of neural signatures.
Persons in the MBSR group had a significant decrease in the NPS, compared with those in the HEP group (P = .05), and from pre- to postintervention assessments (P = .023).
Those in the MBSR group also had “marginal” decreases in the NPS vs. the waiting list group (P = .096), and in the SIIPS-1 relative to both the HEP (P = .089) and waiting list groups (P = .087).
In subjective pain ratings, the MBSR group showed a marginal decrease, compared with the waiting list group (P = .078), and from the pre- to postintervention assessments (P = .028).
The HEP group also had marginal decreases in pain unpleasantness vs. the waiting list group (P = .043), and from the pre- to postintervention assessments for pain intensity (P = .046) and unpleasantness (P = .007).
The researchers also assessed 30 long-term meditators who had undertaken at least 3 years of formal experience with meditation, including participating in multiple intensive retreats and ongoing daily practice, and compared them with meditation-naive individuals.
Long-term meditators reported significantly less pain intensity and unpleasantness than those who had not undergone the training (P < .001).
In addition, a higher number of practice hours during a retreat was linked to a greater reduction in pain ratings. This association remained even after adjustment for gender and respiration rate.
However, the number of daily practice hours was not significantly associated with pain ratings among long-term meditators.
Although there were no average differences in neural signature responses between long-term meditators and individuals who were naive to the technique, there was an inverse relationship between hours on retreat and SIIPS-1 response (P = .027).
‘We’re seeing the biology change’
Commenting for this news organization, Fadel Zeidan, PhD, associate professor of anesthesiology, University of California, San Diego, said that in attenuating the experience of pain, mindfulness engages “very novel” mechanisms.
However, the “most remarkable thing about this study” is that the pain effect occurred when the participants were not meditating, “which gives rise to the notion that mental training is just like physical training,” said Dr. Zeidan, who was not involved with the research.
He noted that the notion was not appreciated previously, “because we weren’t able to see the changes,” as they were based on self-report alone.
However, combining those reports with brain imaging and other objective methods means that “we’re actually seeing the biology change,” Dr. Zeidan said.
He added that mindfulness is different from other techniques for modulating the pain experience, because it is self-facilitated.
“People can learn this technique, ideally, for free online. They can learn the recipe, and it’s one of the only techniques out there that can be used immediately to assuage one’s own pain,” he said.
“There’s nothing else out there on this planet that could immediately reduce one’s own pain. You have to wait 45 minutes for Tylenol, distraction can only work for so long, and you can’t really placebo yourself,” Dr. Zeidan added.
The study was funded by a National Center for Complementary and Alternative Medicine grant, National Institute of Mental Health grants, a Fetzer Institute grant, and a John Templeton Foundation grant, as well as a core grant to the Waisman Center from the National Institute of Child Health and Human Development to Albee Messing. Dr. Wielgosz and Dr. Zeidan have reported no relevant financial relationships. Disclosures for the coinvestigators are listed in the original article.
A version of this article first appeared on Medscape.com.
In a randomized trial, more than 100 healthy individuals were assigned to an 8-week mindfulness-based stress reduction (MBSR) program, a health improvement program (HEP) of the same length, or a waiting list.
Scanning participants’ brains during a heat-based stimulus pain task showed those who completed the MBSR had a reduction in a brain signature linked to the sensory intensity of pain.
“Our finding supports the idea that for new practitioners, mindfulness training directly affects how sensory signals from the body are converted into a brain response,” lead investigator Joseph Wielgosz, PhD, of the Center for Healthy Minds, University of Wisconsin–Madison, said in a release.
Further analysis in long-term meditation practitioners showed the total time spent on intensive retreats was associated with neural changes associated with the perceived stress of pain.
“Just like an experienced athlete plays a sport differently than a first-timer, experienced mindfulness practitioners seem to use their mental ‘muscles’ differently in response to pain than first-time meditators,” Dr. Wielgosz noted.
The findings were published online in the American Journal of Psychiatry.
A complex condition
Dr. Wielgosz told this news organization that pain is “complex,” with multiple stages and several phases between the time signals are sent from pain receptors and the experience of pain.
“The way that mindfulness affects pain processing has more to do with the way the brain interprets pain signals.”
The investigators note that understanding the neurocognitive mechanisms underlying the efficacy of nonpharmacologic pain interventions is a “high-priority objective for improving pain treatment.”
Evidence from brief laboratory interventions and cross-sectional studies suggests that mindfulness training is associated with alterations in both sensory processing and cognitive-emotional regulatory networks, the investigators note.
“However, no such study has yet been conducted on a standardized, full-length, and widely used clinical intervention, such as MBSR,” they add.
Thermal pain task
The randomized, active-control trial included 115 healthy, meditation-naive individuals (61.7% women; average age, 48.3 years). Just over half (58%) had a graduate degree and their mean score on the Hollingshead index was 58.3, indicting a higher socioeconomic status.
All were randomly assigned to an 8-week MBSR course, an 8-week HEP course as an active control group, or a waiting-list control group with no intervention.
The MBSR involved instruction and practice in continuous focused attention on the breath, bodily sensations, and mental content while in seated postures, walking, and doing yoga.
The HEP matched the MBSR in terms of its length, structure, and nonspecific therapeutic elements, which included a supportive group atmosphere, expert instruction, and positive expectancy for benefit.
To examine the interventions’ effect on the pain experience, participants underwent a pain task in which they had 20 thermal stimuli applied to the inside of the left wrist for 12 seconds, including 8 seconds at peak temperature.
The stimuli were separated by a distractor task and intervals for cued anticipation, recovery, and subjective ratings of intensity and unpleasantness on a scale of 0-20.
During the task, participants underwent MRI to assess the neurologic pain signature (NPS) and the stimulus intensity independent pain signature-1 (SIIPS-1) within the brain.
The NPS is activated by various types of pain stimuli, while responding minimally or not at all to “emotionally evocative stimuli” relating to pain or to placebo treatment, the researchers note.
In contrast, the SIIPS-1 is activated in response to aspects of pain unrelated to the stimulus itself. It incorporates a “broader range of cognitive and emotional modulatory circuits,” including those related to expectancy and cognitive processes to modulate the pain experience.
Neural signatures
Results showed that in all groups, age was significantly negatively associated with both NPS (P = .001) and SIIPS-1 response (P < .001), although not subjective pain reports, and was subsequently included in all analyses of neural signatures.
Persons in the MBSR group had a significant decrease in the NPS, compared with those in the HEP group (P = .05), and from pre- to postintervention assessments (P = .023).
Those in the MBSR group also had “marginal” decreases in the NPS vs. the waiting list group (P = .096), and in the SIIPS-1 relative to both the HEP (P = .089) and waiting list groups (P = .087).
In subjective pain ratings, the MBSR group showed a marginal decrease, compared with the waiting list group (P = .078), and from the pre- to postintervention assessments (P = .028).
The HEP group also had marginal decreases in pain unpleasantness vs. the waiting list group (P = .043), and from the pre- to postintervention assessments for pain intensity (P = .046) and unpleasantness (P = .007).
The researchers also assessed 30 long-term meditators who had undertaken at least 3 years of formal experience with meditation, including participating in multiple intensive retreats and ongoing daily practice, and compared them with meditation-naive individuals.
Long-term meditators reported significantly less pain intensity and unpleasantness than those who had not undergone the training (P < .001).
In addition, a higher number of practice hours during a retreat was linked to a greater reduction in pain ratings. This association remained even after adjustment for gender and respiration rate.
However, the number of daily practice hours was not significantly associated with pain ratings among long-term meditators.
Although there were no average differences in neural signature responses between long-term meditators and individuals who were naive to the technique, there was an inverse relationship between hours on retreat and SIIPS-1 response (P = .027).
‘We’re seeing the biology change’
Commenting for this news organization, Fadel Zeidan, PhD, associate professor of anesthesiology, University of California, San Diego, said that in attenuating the experience of pain, mindfulness engages “very novel” mechanisms.
However, the “most remarkable thing about this study” is that the pain effect occurred when the participants were not meditating, “which gives rise to the notion that mental training is just like physical training,” said Dr. Zeidan, who was not involved with the research.
He noted that the notion was not appreciated previously, “because we weren’t able to see the changes,” as they were based on self-report alone.
However, combining those reports with brain imaging and other objective methods means that “we’re actually seeing the biology change,” Dr. Zeidan said.
He added that mindfulness is different from other techniques for modulating the pain experience, because it is self-facilitated.
“People can learn this technique, ideally, for free online. They can learn the recipe, and it’s one of the only techniques out there that can be used immediately to assuage one’s own pain,” he said.
“There’s nothing else out there on this planet that could immediately reduce one’s own pain. You have to wait 45 minutes for Tylenol, distraction can only work for so long, and you can’t really placebo yourself,” Dr. Zeidan added.
The study was funded by a National Center for Complementary and Alternative Medicine grant, National Institute of Mental Health grants, a Fetzer Institute grant, and a John Templeton Foundation grant, as well as a core grant to the Waisman Center from the National Institute of Child Health and Human Development to Albee Messing. Dr. Wielgosz and Dr. Zeidan have reported no relevant financial relationships. Disclosures for the coinvestigators are listed in the original article.
A version of this article first appeared on Medscape.com.
In a randomized trial, more than 100 healthy individuals were assigned to an 8-week mindfulness-based stress reduction (MBSR) program, a health improvement program (HEP) of the same length, or a waiting list.
Scanning participants’ brains during a heat-based stimulus pain task showed those who completed the MBSR had a reduction in a brain signature linked to the sensory intensity of pain.
“Our finding supports the idea that for new practitioners, mindfulness training directly affects how sensory signals from the body are converted into a brain response,” lead investigator Joseph Wielgosz, PhD, of the Center for Healthy Minds, University of Wisconsin–Madison, said in a release.
Further analysis in long-term meditation practitioners showed the total time spent on intensive retreats was associated with neural changes associated with the perceived stress of pain.
“Just like an experienced athlete plays a sport differently than a first-timer, experienced mindfulness practitioners seem to use their mental ‘muscles’ differently in response to pain than first-time meditators,” Dr. Wielgosz noted.
The findings were published online in the American Journal of Psychiatry.
A complex condition
Dr. Wielgosz told this news organization that pain is “complex,” with multiple stages and several phases between the time signals are sent from pain receptors and the experience of pain.
“The way that mindfulness affects pain processing has more to do with the way the brain interprets pain signals.”
The investigators note that understanding the neurocognitive mechanisms underlying the efficacy of nonpharmacologic pain interventions is a “high-priority objective for improving pain treatment.”
Evidence from brief laboratory interventions and cross-sectional studies suggests that mindfulness training is associated with alterations in both sensory processing and cognitive-emotional regulatory networks, the investigators note.
“However, no such study has yet been conducted on a standardized, full-length, and widely used clinical intervention, such as MBSR,” they add.
Thermal pain task
The randomized, active-control trial included 115 healthy, meditation-naive individuals (61.7% women; average age, 48.3 years). Just over half (58%) had a graduate degree and their mean score on the Hollingshead index was 58.3, indicting a higher socioeconomic status.
All were randomly assigned to an 8-week MBSR course, an 8-week HEP course as an active control group, or a waiting-list control group with no intervention.
The MBSR involved instruction and practice in continuous focused attention on the breath, bodily sensations, and mental content while in seated postures, walking, and doing yoga.
The HEP matched the MBSR in terms of its length, structure, and nonspecific therapeutic elements, which included a supportive group atmosphere, expert instruction, and positive expectancy for benefit.
To examine the interventions’ effect on the pain experience, participants underwent a pain task in which they had 20 thermal stimuli applied to the inside of the left wrist for 12 seconds, including 8 seconds at peak temperature.
The stimuli were separated by a distractor task and intervals for cued anticipation, recovery, and subjective ratings of intensity and unpleasantness on a scale of 0-20.
During the task, participants underwent MRI to assess the neurologic pain signature (NPS) and the stimulus intensity independent pain signature-1 (SIIPS-1) within the brain.
The NPS is activated by various types of pain stimuli, while responding minimally or not at all to “emotionally evocative stimuli” relating to pain or to placebo treatment, the researchers note.
In contrast, the SIIPS-1 is activated in response to aspects of pain unrelated to the stimulus itself. It incorporates a “broader range of cognitive and emotional modulatory circuits,” including those related to expectancy and cognitive processes to modulate the pain experience.
Neural signatures
Results showed that in all groups, age was significantly negatively associated with both NPS (P = .001) and SIIPS-1 response (P < .001), although not subjective pain reports, and was subsequently included in all analyses of neural signatures.
Persons in the MBSR group had a significant decrease in the NPS, compared with those in the HEP group (P = .05), and from pre- to postintervention assessments (P = .023).
Those in the MBSR group also had “marginal” decreases in the NPS vs. the waiting list group (P = .096), and in the SIIPS-1 relative to both the HEP (P = .089) and waiting list groups (P = .087).
In subjective pain ratings, the MBSR group showed a marginal decrease, compared with the waiting list group (P = .078), and from the pre- to postintervention assessments (P = .028).
The HEP group also had marginal decreases in pain unpleasantness vs. the waiting list group (P = .043), and from the pre- to postintervention assessments for pain intensity (P = .046) and unpleasantness (P = .007).
The researchers also assessed 30 long-term meditators who had undertaken at least 3 years of formal experience with meditation, including participating in multiple intensive retreats and ongoing daily practice, and compared them with meditation-naive individuals.
Long-term meditators reported significantly less pain intensity and unpleasantness than those who had not undergone the training (P < .001).
In addition, a higher number of practice hours during a retreat was linked to a greater reduction in pain ratings. This association remained even after adjustment for gender and respiration rate.
However, the number of daily practice hours was not significantly associated with pain ratings among long-term meditators.
Although there were no average differences in neural signature responses between long-term meditators and individuals who were naive to the technique, there was an inverse relationship between hours on retreat and SIIPS-1 response (P = .027).
‘We’re seeing the biology change’
Commenting for this news organization, Fadel Zeidan, PhD, associate professor of anesthesiology, University of California, San Diego, said that in attenuating the experience of pain, mindfulness engages “very novel” mechanisms.
However, the “most remarkable thing about this study” is that the pain effect occurred when the participants were not meditating, “which gives rise to the notion that mental training is just like physical training,” said Dr. Zeidan, who was not involved with the research.
He noted that the notion was not appreciated previously, “because we weren’t able to see the changes,” as they were based on self-report alone.
However, combining those reports with brain imaging and other objective methods means that “we’re actually seeing the biology change,” Dr. Zeidan said.
He added that mindfulness is different from other techniques for modulating the pain experience, because it is self-facilitated.
“People can learn this technique, ideally, for free online. They can learn the recipe, and it’s one of the only techniques out there that can be used immediately to assuage one’s own pain,” he said.
“There’s nothing else out there on this planet that could immediately reduce one’s own pain. You have to wait 45 minutes for Tylenol, distraction can only work for so long, and you can’t really placebo yourself,” Dr. Zeidan added.
The study was funded by a National Center for Complementary and Alternative Medicine grant, National Institute of Mental Health grants, a Fetzer Institute grant, and a John Templeton Foundation grant, as well as a core grant to the Waisman Center from the National Institute of Child Health and Human Development to Albee Messing. Dr. Wielgosz and Dr. Zeidan have reported no relevant financial relationships. Disclosures for the coinvestigators are listed in the original article.
A version of this article first appeared on Medscape.com.
FROM AMERICAN JOURNAL OF PSYCHIATRY
Patients who won’t pay: What’s your recourse?
Owing to the pandemic, job loss, and the possible loss of health insurance, patients have had more difficulty managing copays, coinsurance, and deductibles, not to mention other out-of-pocket health care charges.
“Many of our patients have lost their jobs or have had their hours cut back, and as a result, they are struggling to make ends meet,” said Ahmad Chaudhry, MD, a cardiothoracic surgeon in Lexington, Ky. “However, we cannot continue to provide care if our patients do not pay their bills.”
This news organization asked physicians what they do when their patients don’t pay. About 43% said that they continue to treat them and develop a payment plan; 13% send their bill to collections; 12% continue their care and write off their balance, and 25% choose other actions. Only 8% of physicians drop patients if they don’t pay.
Because you need to pay your own bills, what can you do about nonpaying patients?
Start with price transparency
In the past, patients never knew what their lab work or a chest EKG would cost because it wasn’t listed anywhere, and it was usually more than expected. Because of new legislation concerning health care price transparency, hospitals, health plans, and insurers must pony up with the actual fees, making them transparent to patients. Physician practices should follow suit and keep prices transparent too. Patients are more likely to pay their bills when prepared for the expense.
Patients with insurance often don’t know what they’ll be paying for their visit or their tests because they don’t know how much insurance will cover and what will be left for them to pay. Also, they may not know if they’ve met their deductible yet so they’re unsure whether insurance will even kick in. And patients without insurance still need to know what their costs will be upfront.
According to 10 insights from the Primary Care Consumer Choice Survey, 74% of health care consumers were willing to pay a $50 out-of-pocket charge to know the cost of their primary care visit.
Provide payment plans
Many patients have always needed payment plans. It’s one thing to post a sign at check-in telling patients that all monies are due at the time of service, but it’s another reality for a patient who can’t fork over the $250 charge they just unexpectedly spent in your office.
Discover Financial Services recently ran a survey, with results presented in the press release Americans are Delaying Non-Emergency Medical Care in Higher Numbers than Last Year, and found that many Americans with medical debt are delaying nonemergency medical care. For example, they put off seeing a specialist (52%), seeing a doctor for sickness (41%), and undergoing treatment plans recommended by their doctor (31%).
Turning an account over to collections should be a last resort. In addition, agencies typically charge 30%-40% of the total collected off the top.
Though collecting that amount is better than nothing, using a collection agency may have unexpected consequences. For instance, you’re trusting the agency you hire to collect to represent you and act on your practice’s behalf. If they’re rude or their tactics are harsh in the eyes of the patient or their relatives, it’s your reputation that is on the line.
Rather than use a collection agency, you could collect the payments yourself. When a patient fails to pay within about 3 months, begin mailing statements from the office, followed by firm but generous phone calls trying to collect. Industry estimates put the average cost of sending an invoice, including staff labor, printing, and postage, at about $35 per mailer. Some practices combat the added costs by offering a 20% prompt-pay discount. Offering payment plans is another option that helps garner eventual payment. Plus, practices should direct patients to third-party lenders such as CareCredit for larger bills.
On occasion, some small practices may allow a swap, such as allowing a patient to provide a service such as plumbing, electrical, or painting in exchange for working off the bill. Though it’s not ideal when it comes to finances, you may find it can work in a pinch for a cash-strapped patient. Make sure to keep records of what bills the patient’s work goes toward.
It often helps to incentivize your billing staff to follow up regularly, with various suggestions and tactics, to get patients to pay their bills. The incentive amount you offer will probably be less than if you had to use a collection agency.
Have a payment policy
Because your practice’s primary job is caring for patients’ physical and emotional needs, payment collection without coming off as insensitive can be tricky. “We understand these are difficult times for everyone, and we are doing our best to work with our patients,” said Dr. Chaudhry. Having a written payment policy can help build the bridge. A policy lets patients know what they can expect and can help prevent surprises over what occurs in the event of nonpayment. Your written policy should include:
- When payment is due.
- How the practice handles copays and deductibles.
- What forms of payment are accepted.
- Your policy regarding nonpayment.
Why patients don’t pay
A 2021 Healthcare Consumer Experience Study from Cedar found that medical bills are a source of anxiety and frustration for most patients, affecting their financial experience. More than half of the respondents said that paying a medical bill is stressful. Complicating matters, many health care practices rely on outdated payment systems, which may not provide patients with a clear view of what they owe and how to pay it.
The study found that 53% of respondents find understanding their plan’s coverage and benefits stressful, and 37% of patients won’t pay their bill if they can’t understand it.
People may think the patient is trying to get out of paying, which, of course, is sometimes true, but most of the time they want to pay, concluded the study. Most patients need a better explanation, communication, and accurate accounting of their out-of-pocket costs.
What can doctors do?
If you’re a physician who regularly sees patients who have problems paying their bills, you can take a few steps to minimize the financial impact on your practice:
- Bill the patient’s insurance directly to ensure you receive at least partial payment.
- Keep adequate records of services in case you need to pursue legal action.
- “Be understanding and flexible when it comes to payment arrangements, as this can often be the difference between getting paid and not getting paid at all,” said Dr. Chaudhry.
Distance yourself
When discussing payment policies, physicians should try to distance themselves from the actual collection process as much as possible. Well-meaning physicians often tell patients things like they can “figure something out “ financially or “work them in” during a scheduling conflict, but that often undermines the authority and credibility of the practice’s office staff. Plus, it teaches patients they can get their way if they work on the doctor’s soft spot – something you don’t want to encourage.
By following some of these measures, you can help ensure that your practice continues to thrive despite the challenges posed by nonpaying patients.
A version of this article first appeared on Medscape.com.
Owing to the pandemic, job loss, and the possible loss of health insurance, patients have had more difficulty managing copays, coinsurance, and deductibles, not to mention other out-of-pocket health care charges.
“Many of our patients have lost their jobs or have had their hours cut back, and as a result, they are struggling to make ends meet,” said Ahmad Chaudhry, MD, a cardiothoracic surgeon in Lexington, Ky. “However, we cannot continue to provide care if our patients do not pay their bills.”
This news organization asked physicians what they do when their patients don’t pay. About 43% said that they continue to treat them and develop a payment plan; 13% send their bill to collections; 12% continue their care and write off their balance, and 25% choose other actions. Only 8% of physicians drop patients if they don’t pay.
Because you need to pay your own bills, what can you do about nonpaying patients?
Start with price transparency
In the past, patients never knew what their lab work or a chest EKG would cost because it wasn’t listed anywhere, and it was usually more than expected. Because of new legislation concerning health care price transparency, hospitals, health plans, and insurers must pony up with the actual fees, making them transparent to patients. Physician practices should follow suit and keep prices transparent too. Patients are more likely to pay their bills when prepared for the expense.
Patients with insurance often don’t know what they’ll be paying for their visit or their tests because they don’t know how much insurance will cover and what will be left for them to pay. Also, they may not know if they’ve met their deductible yet so they’re unsure whether insurance will even kick in. And patients without insurance still need to know what their costs will be upfront.
According to 10 insights from the Primary Care Consumer Choice Survey, 74% of health care consumers were willing to pay a $50 out-of-pocket charge to know the cost of their primary care visit.
Provide payment plans
Many patients have always needed payment plans. It’s one thing to post a sign at check-in telling patients that all monies are due at the time of service, but it’s another reality for a patient who can’t fork over the $250 charge they just unexpectedly spent in your office.
Discover Financial Services recently ran a survey, with results presented in the press release Americans are Delaying Non-Emergency Medical Care in Higher Numbers than Last Year, and found that many Americans with medical debt are delaying nonemergency medical care. For example, they put off seeing a specialist (52%), seeing a doctor for sickness (41%), and undergoing treatment plans recommended by their doctor (31%).
Turning an account over to collections should be a last resort. In addition, agencies typically charge 30%-40% of the total collected off the top.
Though collecting that amount is better than nothing, using a collection agency may have unexpected consequences. For instance, you’re trusting the agency you hire to collect to represent you and act on your practice’s behalf. If they’re rude or their tactics are harsh in the eyes of the patient or their relatives, it’s your reputation that is on the line.
Rather than use a collection agency, you could collect the payments yourself. When a patient fails to pay within about 3 months, begin mailing statements from the office, followed by firm but generous phone calls trying to collect. Industry estimates put the average cost of sending an invoice, including staff labor, printing, and postage, at about $35 per mailer. Some practices combat the added costs by offering a 20% prompt-pay discount. Offering payment plans is another option that helps garner eventual payment. Plus, practices should direct patients to third-party lenders such as CareCredit for larger bills.
On occasion, some small practices may allow a swap, such as allowing a patient to provide a service such as plumbing, electrical, or painting in exchange for working off the bill. Though it’s not ideal when it comes to finances, you may find it can work in a pinch for a cash-strapped patient. Make sure to keep records of what bills the patient’s work goes toward.
It often helps to incentivize your billing staff to follow up regularly, with various suggestions and tactics, to get patients to pay their bills. The incentive amount you offer will probably be less than if you had to use a collection agency.
Have a payment policy
Because your practice’s primary job is caring for patients’ physical and emotional needs, payment collection without coming off as insensitive can be tricky. “We understand these are difficult times for everyone, and we are doing our best to work with our patients,” said Dr. Chaudhry. Having a written payment policy can help build the bridge. A policy lets patients know what they can expect and can help prevent surprises over what occurs in the event of nonpayment. Your written policy should include:
- When payment is due.
- How the practice handles copays and deductibles.
- What forms of payment are accepted.
- Your policy regarding nonpayment.
Why patients don’t pay
A 2021 Healthcare Consumer Experience Study from Cedar found that medical bills are a source of anxiety and frustration for most patients, affecting their financial experience. More than half of the respondents said that paying a medical bill is stressful. Complicating matters, many health care practices rely on outdated payment systems, which may not provide patients with a clear view of what they owe and how to pay it.
The study found that 53% of respondents find understanding their plan’s coverage and benefits stressful, and 37% of patients won’t pay their bill if they can’t understand it.
People may think the patient is trying to get out of paying, which, of course, is sometimes true, but most of the time they want to pay, concluded the study. Most patients need a better explanation, communication, and accurate accounting of their out-of-pocket costs.
What can doctors do?
If you’re a physician who regularly sees patients who have problems paying their bills, you can take a few steps to minimize the financial impact on your practice:
- Bill the patient’s insurance directly to ensure you receive at least partial payment.
- Keep adequate records of services in case you need to pursue legal action.
- “Be understanding and flexible when it comes to payment arrangements, as this can often be the difference between getting paid and not getting paid at all,” said Dr. Chaudhry.
Distance yourself
When discussing payment policies, physicians should try to distance themselves from the actual collection process as much as possible. Well-meaning physicians often tell patients things like they can “figure something out “ financially or “work them in” during a scheduling conflict, but that often undermines the authority and credibility of the practice’s office staff. Plus, it teaches patients they can get their way if they work on the doctor’s soft spot – something you don’t want to encourage.
By following some of these measures, you can help ensure that your practice continues to thrive despite the challenges posed by nonpaying patients.
A version of this article first appeared on Medscape.com.
Owing to the pandemic, job loss, and the possible loss of health insurance, patients have had more difficulty managing copays, coinsurance, and deductibles, not to mention other out-of-pocket health care charges.
“Many of our patients have lost their jobs or have had their hours cut back, and as a result, they are struggling to make ends meet,” said Ahmad Chaudhry, MD, a cardiothoracic surgeon in Lexington, Ky. “However, we cannot continue to provide care if our patients do not pay their bills.”
This news organization asked physicians what they do when their patients don’t pay. About 43% said that they continue to treat them and develop a payment plan; 13% send their bill to collections; 12% continue their care and write off their balance, and 25% choose other actions. Only 8% of physicians drop patients if they don’t pay.
Because you need to pay your own bills, what can you do about nonpaying patients?
Start with price transparency
In the past, patients never knew what their lab work or a chest EKG would cost because it wasn’t listed anywhere, and it was usually more than expected. Because of new legislation concerning health care price transparency, hospitals, health plans, and insurers must pony up with the actual fees, making them transparent to patients. Physician practices should follow suit and keep prices transparent too. Patients are more likely to pay their bills when prepared for the expense.
Patients with insurance often don’t know what they’ll be paying for their visit or their tests because they don’t know how much insurance will cover and what will be left for them to pay. Also, they may not know if they’ve met their deductible yet so they’re unsure whether insurance will even kick in. And patients without insurance still need to know what their costs will be upfront.
According to 10 insights from the Primary Care Consumer Choice Survey, 74% of health care consumers were willing to pay a $50 out-of-pocket charge to know the cost of their primary care visit.
Provide payment plans
Many patients have always needed payment plans. It’s one thing to post a sign at check-in telling patients that all monies are due at the time of service, but it’s another reality for a patient who can’t fork over the $250 charge they just unexpectedly spent in your office.
Discover Financial Services recently ran a survey, with results presented in the press release Americans are Delaying Non-Emergency Medical Care in Higher Numbers than Last Year, and found that many Americans with medical debt are delaying nonemergency medical care. For example, they put off seeing a specialist (52%), seeing a doctor for sickness (41%), and undergoing treatment plans recommended by their doctor (31%).
Turning an account over to collections should be a last resort. In addition, agencies typically charge 30%-40% of the total collected off the top.
Though collecting that amount is better than nothing, using a collection agency may have unexpected consequences. For instance, you’re trusting the agency you hire to collect to represent you and act on your practice’s behalf. If they’re rude or their tactics are harsh in the eyes of the patient or their relatives, it’s your reputation that is on the line.
Rather than use a collection agency, you could collect the payments yourself. When a patient fails to pay within about 3 months, begin mailing statements from the office, followed by firm but generous phone calls trying to collect. Industry estimates put the average cost of sending an invoice, including staff labor, printing, and postage, at about $35 per mailer. Some practices combat the added costs by offering a 20% prompt-pay discount. Offering payment plans is another option that helps garner eventual payment. Plus, practices should direct patients to third-party lenders such as CareCredit for larger bills.
On occasion, some small practices may allow a swap, such as allowing a patient to provide a service such as plumbing, electrical, or painting in exchange for working off the bill. Though it’s not ideal when it comes to finances, you may find it can work in a pinch for a cash-strapped patient. Make sure to keep records of what bills the patient’s work goes toward.
It often helps to incentivize your billing staff to follow up regularly, with various suggestions and tactics, to get patients to pay their bills. The incentive amount you offer will probably be less than if you had to use a collection agency.
Have a payment policy
Because your practice’s primary job is caring for patients’ physical and emotional needs, payment collection without coming off as insensitive can be tricky. “We understand these are difficult times for everyone, and we are doing our best to work with our patients,” said Dr. Chaudhry. Having a written payment policy can help build the bridge. A policy lets patients know what they can expect and can help prevent surprises over what occurs in the event of nonpayment. Your written policy should include:
- When payment is due.
- How the practice handles copays and deductibles.
- What forms of payment are accepted.
- Your policy regarding nonpayment.
Why patients don’t pay
A 2021 Healthcare Consumer Experience Study from Cedar found that medical bills are a source of anxiety and frustration for most patients, affecting their financial experience. More than half of the respondents said that paying a medical bill is stressful. Complicating matters, many health care practices rely on outdated payment systems, which may not provide patients with a clear view of what they owe and how to pay it.
The study found that 53% of respondents find understanding their plan’s coverage and benefits stressful, and 37% of patients won’t pay their bill if they can’t understand it.
People may think the patient is trying to get out of paying, which, of course, is sometimes true, but most of the time they want to pay, concluded the study. Most patients need a better explanation, communication, and accurate accounting of their out-of-pocket costs.
What can doctors do?
If you’re a physician who regularly sees patients who have problems paying their bills, you can take a few steps to minimize the financial impact on your practice:
- Bill the patient’s insurance directly to ensure you receive at least partial payment.
- Keep adequate records of services in case you need to pursue legal action.
- “Be understanding and flexible when it comes to payment arrangements, as this can often be the difference between getting paid and not getting paid at all,” said Dr. Chaudhry.
Distance yourself
When discussing payment policies, physicians should try to distance themselves from the actual collection process as much as possible. Well-meaning physicians often tell patients things like they can “figure something out “ financially or “work them in” during a scheduling conflict, but that often undermines the authority and credibility of the practice’s office staff. Plus, it teaches patients they can get their way if they work on the doctor’s soft spot – something you don’t want to encourage.
By following some of these measures, you can help ensure that your practice continues to thrive despite the challenges posed by nonpaying patients.
A version of this article first appeared on Medscape.com.
Active shooter drills may be harming children, but doctors offer help
The drills can range from staging lockdowns and sheltering in place to quasi dramas with mock shooters roaming the halls. Although the goals of these training exercises are important, equally important are the potential negative effects of drills on students’ mental health, according to doctors with expertise in pediatrics and mental health.
“Dramatic simulation of an active shooter event at school would be expected to provoke the same stress response as the real thing,” said Peter L. Loper Jr., MD, a pediatrician and psychiatrist, in an interview. “While ensuring their physical safety is very important, we must be intentional about making sure that we are not doing so at the expense of their psychosocial or emotional safety.”
“Children may not be able to differentiate a dramatic drill from a real event,” emphasized Dr. Loper, of the neuropsychiatry and behavioral science departments at the University of South Carolina, Columbia. “The parts of the brain responsible for our flight-fight-or-freeze response would interpret both simulated and real events identically and produce the same neurohormonal stress-response.”
Indeed, a study published in the journal Humanities & Social Sciences Communications suggested children experienced mental health problems related to participating in active shooter drills. In the large study, a team of statisticians from the Georgia Institute of Technology found that students reported a 42% increase in stress and anxiety and a 38.7% increase in depression during the 90 days following active shooter drills, compared with the 90 days before the drills.
The authors of this study, including Mai ElSherief, PhD, drew these conclusions after analyzing 54 million social media posts before and after drills in 114 schools across 33 states. The researchers analyzed the language of the social media posts by teachers, parents, and students and found increased use of the words hope, love, home, school, kids, community, support, and help after the drills. The researchers considered posting with these terms in the aftermath of the drills to be indicative of having high anxiety.
They included examples of how high stress, anxiety, and depression manifested in specific posts from parents in their report. The following is an example of a poster expressing high anxiety and stress: “are we really gonna normalize school shooter drills?! holy sh* there has to be a real way to avoid these tragedies. sh*t like this cannot be normalized. teachers injured after being shot with plastic pellets ‘execution style’ in active shooter drill.”
The authors also shared this post to serve as an example of a person who seems depressed: “and now we are revisiting the trauma on our kids, forcing them to act out school drills monthly. i don’t get why gen x parents buy into this concept wholeheartedly. things need to change.”
The published material did not include posts from students, but the researchers’ analysis of the content of posts overall showed increased concerns for health and increased concerns about death during the period after drills, compared with before drills.
The authors also conducted focus groups in communities in which drills occurred, and many teachers and parents reported anecdotal evidence of children who were nervous long after the drills were over, with some showing extreme reactions such as panic over a standard fire alarm at school. Overall, the results show that school shooter drills can negatively affect school communities over prolonged periods of time, they concluded.
According to a statement from the American Academy of Pediatrics, “there is a need to be cautious about the potential psychological risks and other unintended consequences of directly involving children in live exercises and drills.”
“These risks and consequences are especially a concern when children are deceived and led to believe there is an actual attack and not a drill,” wrote David Schonfeld, MD, the lead author of the statement on Participation of Children and Adolescents in Live Crisis Drills and Exercises, and colleagues.
Managing the fallout
Physicians can help students experiencing mental health problems from these drills, according to doctors interviewed for this piece.
It’s important for providers to know that stress will show up differently in children than in adults, said Chelsea Younghans, MD, a psychiatrist and military officer in Bethesda, Md., in an interview.
“They may see children with headaches, stomach aches, or nonspecific complaints. They may also see children who have not had difficulty with sleep present with nightmares or bed wetting,” she added.
For teens and preteens, validated tools such as the Child PTSD Symptom Scale (CPSS-5) and Child and Adolescent Trauma Screen (CATS) to assess PTSD in youth, may help serve as a starting point for a conversation between providers and their older child population, she noted.
Children who exhibit avoidance or withdrawal behaviors including consistent school refusal, an increase in reassurance-seeking behaviors, or somatic symptoms like vague abdominal pain or headaches that prevent school attendance after participating in a drill, may need more robust mental health services, Dr. Loper noted.
Dr. Schonfeld, who is also director of the National Center for School Crisis and Bereavement at Children’s Hospital Los Angeles, called for health care providers to be available to help children process traumatic reactions to these exercises.
Agreeing with Dr. Schonfeld, Dr. Younghans said: “It is vital to debrief with students and staff after drills, making sure that students have a safe space and ample time to speak with trusted staff. As children will undoubtedly have questions and concerns, creating open lines of communication will help alleviate any traumatic effect these drills may have.”
Communicating with various stakeholders
Experts also gave recommendations for how clinicians communicate with leaders in their area’s school districts and other members of their communities about these training exercises.
“For primary care providers, it is important to establish meaningful relationships within your community and patient population as much as possible,” Dr. Younghans said. “Having a good relationship with the local schools and being part of the conversation can help increase school and community awareness on the impact these drills can have on students and staff,” she added.
For those pediatricians or other health care providers who serve as consultants to schools, Dr. Schonfeld advised they ask about policies related to exercises and drills, such as what are the limits to what children might be exposed to in a drill, and what requirements there might be at the local and state level in terms of frequency and what the drills will and will not involve.
He also noted that clinicians should encourage school leaders to consider the fact that kids may have personal histories of trauma that are completely unknown to the school when they design these exercises.
School staff and health care providers should explain the nature and reasons for drills, invite family members to express concerns, and make accommodations if necessary for some children to participate in drills in a more limited way, noted Dr. Schonfeld, who is also clinical professor of pediatrics at the University of Southern California, Los Angeles.
“I think health care providers should work with legislators, so that if they require a drill, it must be done in a way that is physically and emotionally safe,” he added.
Executing better drills for students’ mental health
Experts also advised on ways to execute these drills that will be least damaging to students.
The AAP statement on Participation of Children and Adolescents in Live Crisis Drills and Exercises, for example, advocates eliminating high-intensity drills, prohibiting deception in drills, and providing accommodations based on children’s vulnerabilities.
Dr. Schonfeld also emphasized, in an interview, that training for an attack need not be extremely realistic to be effective.
“When you are preparing for a crisis, the drills and exercises are for children to practice and develop mastery over something they don’t know how to do fully yet,” said Dr. Schonfeld.
Citing a suggestion from a 2020 report conducted by Everytown for Gun Safety on keeping schools safe from gun violence, Dr. Younghans said, “Schools should be in clear communication with communities and families regarding when drills will be happening,” and advised ensuring that the explanation of drills is developmentally appropriate to the age of the children participating.
The report also recommends conducting drills that do not simulate an actual incident, combining drills with trauma-informed approaches to address students’ well-being during and for a sustained period after the drills, and tracking data on the efficacy and effects of drills.
Dr. Loper suggested ways that clinicians and parents can help navigate the tricky territory of school safety drills.
In his view, they should not be random or unexpected, and anticipatory guidance should be given regarding any visual or auditory stimuli, such as flashing lights or sirens, alarms, or announcements.
“A preventive approach should be utilized to ensure that any child who is experiencing extreme drill-distress be excused from any future disaster drills to prevent retraumatization,” Dr. Loper said.
Physicians interviewed for this piece also provided tips on how to talk about these events with children in a way that is beneficial to their mental health.
“What we want to do is [have a] calm discussion [with kids] about what we are doing and why we are doing it” and guide them through the movements, Dr. Schonfeld said.
When teaching children how to respond to an emergency, some elements of uncertainty need to be discussed. Children need to anticipate “what you might do if you are not in the classroom if something occurs, such as being in the bathroom, or out at recess,” he continued.
Dr. Younghans recommended that parents and staff schedule time to prepare children for the drill and practice in advance, and that behavioral health providers, counselors, and/or primary care providers should be involved in the planning and execution of the drill.
The Georgia Tech study was supported through a grant from Everytown for Gun Safety.
The study authors and experts interviewed for this piece had no financial conflicts to disclose.
The drills can range from staging lockdowns and sheltering in place to quasi dramas with mock shooters roaming the halls. Although the goals of these training exercises are important, equally important are the potential negative effects of drills on students’ mental health, according to doctors with expertise in pediatrics and mental health.
“Dramatic simulation of an active shooter event at school would be expected to provoke the same stress response as the real thing,” said Peter L. Loper Jr., MD, a pediatrician and psychiatrist, in an interview. “While ensuring their physical safety is very important, we must be intentional about making sure that we are not doing so at the expense of their psychosocial or emotional safety.”
“Children may not be able to differentiate a dramatic drill from a real event,” emphasized Dr. Loper, of the neuropsychiatry and behavioral science departments at the University of South Carolina, Columbia. “The parts of the brain responsible for our flight-fight-or-freeze response would interpret both simulated and real events identically and produce the same neurohormonal stress-response.”
Indeed, a study published in the journal Humanities & Social Sciences Communications suggested children experienced mental health problems related to participating in active shooter drills. In the large study, a team of statisticians from the Georgia Institute of Technology found that students reported a 42% increase in stress and anxiety and a 38.7% increase in depression during the 90 days following active shooter drills, compared with the 90 days before the drills.
The authors of this study, including Mai ElSherief, PhD, drew these conclusions after analyzing 54 million social media posts before and after drills in 114 schools across 33 states. The researchers analyzed the language of the social media posts by teachers, parents, and students and found increased use of the words hope, love, home, school, kids, community, support, and help after the drills. The researchers considered posting with these terms in the aftermath of the drills to be indicative of having high anxiety.
They included examples of how high stress, anxiety, and depression manifested in specific posts from parents in their report. The following is an example of a poster expressing high anxiety and stress: “are we really gonna normalize school shooter drills?! holy sh* there has to be a real way to avoid these tragedies. sh*t like this cannot be normalized. teachers injured after being shot with plastic pellets ‘execution style’ in active shooter drill.”
The authors also shared this post to serve as an example of a person who seems depressed: “and now we are revisiting the trauma on our kids, forcing them to act out school drills monthly. i don’t get why gen x parents buy into this concept wholeheartedly. things need to change.”
The published material did not include posts from students, but the researchers’ analysis of the content of posts overall showed increased concerns for health and increased concerns about death during the period after drills, compared with before drills.
The authors also conducted focus groups in communities in which drills occurred, and many teachers and parents reported anecdotal evidence of children who were nervous long after the drills were over, with some showing extreme reactions such as panic over a standard fire alarm at school. Overall, the results show that school shooter drills can negatively affect school communities over prolonged periods of time, they concluded.
According to a statement from the American Academy of Pediatrics, “there is a need to be cautious about the potential psychological risks and other unintended consequences of directly involving children in live exercises and drills.”
“These risks and consequences are especially a concern when children are deceived and led to believe there is an actual attack and not a drill,” wrote David Schonfeld, MD, the lead author of the statement on Participation of Children and Adolescents in Live Crisis Drills and Exercises, and colleagues.
Managing the fallout
Physicians can help students experiencing mental health problems from these drills, according to doctors interviewed for this piece.
It’s important for providers to know that stress will show up differently in children than in adults, said Chelsea Younghans, MD, a psychiatrist and military officer in Bethesda, Md., in an interview.
“They may see children with headaches, stomach aches, or nonspecific complaints. They may also see children who have not had difficulty with sleep present with nightmares or bed wetting,” she added.
For teens and preteens, validated tools such as the Child PTSD Symptom Scale (CPSS-5) and Child and Adolescent Trauma Screen (CATS) to assess PTSD in youth, may help serve as a starting point for a conversation between providers and their older child population, she noted.
Children who exhibit avoidance or withdrawal behaviors including consistent school refusal, an increase in reassurance-seeking behaviors, or somatic symptoms like vague abdominal pain or headaches that prevent school attendance after participating in a drill, may need more robust mental health services, Dr. Loper noted.
Dr. Schonfeld, who is also director of the National Center for School Crisis and Bereavement at Children’s Hospital Los Angeles, called for health care providers to be available to help children process traumatic reactions to these exercises.
Agreeing with Dr. Schonfeld, Dr. Younghans said: “It is vital to debrief with students and staff after drills, making sure that students have a safe space and ample time to speak with trusted staff. As children will undoubtedly have questions and concerns, creating open lines of communication will help alleviate any traumatic effect these drills may have.”
Communicating with various stakeholders
Experts also gave recommendations for how clinicians communicate with leaders in their area’s school districts and other members of their communities about these training exercises.
“For primary care providers, it is important to establish meaningful relationships within your community and patient population as much as possible,” Dr. Younghans said. “Having a good relationship with the local schools and being part of the conversation can help increase school and community awareness on the impact these drills can have on students and staff,” she added.
For those pediatricians or other health care providers who serve as consultants to schools, Dr. Schonfeld advised they ask about policies related to exercises and drills, such as what are the limits to what children might be exposed to in a drill, and what requirements there might be at the local and state level in terms of frequency and what the drills will and will not involve.
He also noted that clinicians should encourage school leaders to consider the fact that kids may have personal histories of trauma that are completely unknown to the school when they design these exercises.
School staff and health care providers should explain the nature and reasons for drills, invite family members to express concerns, and make accommodations if necessary for some children to participate in drills in a more limited way, noted Dr. Schonfeld, who is also clinical professor of pediatrics at the University of Southern California, Los Angeles.
“I think health care providers should work with legislators, so that if they require a drill, it must be done in a way that is physically and emotionally safe,” he added.
Executing better drills for students’ mental health
Experts also advised on ways to execute these drills that will be least damaging to students.
The AAP statement on Participation of Children and Adolescents in Live Crisis Drills and Exercises, for example, advocates eliminating high-intensity drills, prohibiting deception in drills, and providing accommodations based on children’s vulnerabilities.
Dr. Schonfeld also emphasized, in an interview, that training for an attack need not be extremely realistic to be effective.
“When you are preparing for a crisis, the drills and exercises are for children to practice and develop mastery over something they don’t know how to do fully yet,” said Dr. Schonfeld.
Citing a suggestion from a 2020 report conducted by Everytown for Gun Safety on keeping schools safe from gun violence, Dr. Younghans said, “Schools should be in clear communication with communities and families regarding when drills will be happening,” and advised ensuring that the explanation of drills is developmentally appropriate to the age of the children participating.
The report also recommends conducting drills that do not simulate an actual incident, combining drills with trauma-informed approaches to address students’ well-being during and for a sustained period after the drills, and tracking data on the efficacy and effects of drills.
Dr. Loper suggested ways that clinicians and parents can help navigate the tricky territory of school safety drills.
In his view, they should not be random or unexpected, and anticipatory guidance should be given regarding any visual or auditory stimuli, such as flashing lights or sirens, alarms, or announcements.
“A preventive approach should be utilized to ensure that any child who is experiencing extreme drill-distress be excused from any future disaster drills to prevent retraumatization,” Dr. Loper said.
Physicians interviewed for this piece also provided tips on how to talk about these events with children in a way that is beneficial to their mental health.
“What we want to do is [have a] calm discussion [with kids] about what we are doing and why we are doing it” and guide them through the movements, Dr. Schonfeld said.
When teaching children how to respond to an emergency, some elements of uncertainty need to be discussed. Children need to anticipate “what you might do if you are not in the classroom if something occurs, such as being in the bathroom, or out at recess,” he continued.
Dr. Younghans recommended that parents and staff schedule time to prepare children for the drill and practice in advance, and that behavioral health providers, counselors, and/or primary care providers should be involved in the planning and execution of the drill.
The Georgia Tech study was supported through a grant from Everytown for Gun Safety.
The study authors and experts interviewed for this piece had no financial conflicts to disclose.
The drills can range from staging lockdowns and sheltering in place to quasi dramas with mock shooters roaming the halls. Although the goals of these training exercises are important, equally important are the potential negative effects of drills on students’ mental health, according to doctors with expertise in pediatrics and mental health.
“Dramatic simulation of an active shooter event at school would be expected to provoke the same stress response as the real thing,” said Peter L. Loper Jr., MD, a pediatrician and psychiatrist, in an interview. “While ensuring their physical safety is very important, we must be intentional about making sure that we are not doing so at the expense of their psychosocial or emotional safety.”
“Children may not be able to differentiate a dramatic drill from a real event,” emphasized Dr. Loper, of the neuropsychiatry and behavioral science departments at the University of South Carolina, Columbia. “The parts of the brain responsible for our flight-fight-or-freeze response would interpret both simulated and real events identically and produce the same neurohormonal stress-response.”
Indeed, a study published in the journal Humanities & Social Sciences Communications suggested children experienced mental health problems related to participating in active shooter drills. In the large study, a team of statisticians from the Georgia Institute of Technology found that students reported a 42% increase in stress and anxiety and a 38.7% increase in depression during the 90 days following active shooter drills, compared with the 90 days before the drills.
The authors of this study, including Mai ElSherief, PhD, drew these conclusions after analyzing 54 million social media posts before and after drills in 114 schools across 33 states. The researchers analyzed the language of the social media posts by teachers, parents, and students and found increased use of the words hope, love, home, school, kids, community, support, and help after the drills. The researchers considered posting with these terms in the aftermath of the drills to be indicative of having high anxiety.
They included examples of how high stress, anxiety, and depression manifested in specific posts from parents in their report. The following is an example of a poster expressing high anxiety and stress: “are we really gonna normalize school shooter drills?! holy sh* there has to be a real way to avoid these tragedies. sh*t like this cannot be normalized. teachers injured after being shot with plastic pellets ‘execution style’ in active shooter drill.”
The authors also shared this post to serve as an example of a person who seems depressed: “and now we are revisiting the trauma on our kids, forcing them to act out school drills monthly. i don’t get why gen x parents buy into this concept wholeheartedly. things need to change.”
The published material did not include posts from students, but the researchers’ analysis of the content of posts overall showed increased concerns for health and increased concerns about death during the period after drills, compared with before drills.
The authors also conducted focus groups in communities in which drills occurred, and many teachers and parents reported anecdotal evidence of children who were nervous long after the drills were over, with some showing extreme reactions such as panic over a standard fire alarm at school. Overall, the results show that school shooter drills can negatively affect school communities over prolonged periods of time, they concluded.
According to a statement from the American Academy of Pediatrics, “there is a need to be cautious about the potential psychological risks and other unintended consequences of directly involving children in live exercises and drills.”
“These risks and consequences are especially a concern when children are deceived and led to believe there is an actual attack and not a drill,” wrote David Schonfeld, MD, the lead author of the statement on Participation of Children and Adolescents in Live Crisis Drills and Exercises, and colleagues.
Managing the fallout
Physicians can help students experiencing mental health problems from these drills, according to doctors interviewed for this piece.
It’s important for providers to know that stress will show up differently in children than in adults, said Chelsea Younghans, MD, a psychiatrist and military officer in Bethesda, Md., in an interview.
“They may see children with headaches, stomach aches, or nonspecific complaints. They may also see children who have not had difficulty with sleep present with nightmares or bed wetting,” she added.
For teens and preteens, validated tools such as the Child PTSD Symptom Scale (CPSS-5) and Child and Adolescent Trauma Screen (CATS) to assess PTSD in youth, may help serve as a starting point for a conversation between providers and their older child population, she noted.
Children who exhibit avoidance or withdrawal behaviors including consistent school refusal, an increase in reassurance-seeking behaviors, or somatic symptoms like vague abdominal pain or headaches that prevent school attendance after participating in a drill, may need more robust mental health services, Dr. Loper noted.
Dr. Schonfeld, who is also director of the National Center for School Crisis and Bereavement at Children’s Hospital Los Angeles, called for health care providers to be available to help children process traumatic reactions to these exercises.
Agreeing with Dr. Schonfeld, Dr. Younghans said: “It is vital to debrief with students and staff after drills, making sure that students have a safe space and ample time to speak with trusted staff. As children will undoubtedly have questions and concerns, creating open lines of communication will help alleviate any traumatic effect these drills may have.”
Communicating with various stakeholders
Experts also gave recommendations for how clinicians communicate with leaders in their area’s school districts and other members of their communities about these training exercises.
“For primary care providers, it is important to establish meaningful relationships within your community and patient population as much as possible,” Dr. Younghans said. “Having a good relationship with the local schools and being part of the conversation can help increase school and community awareness on the impact these drills can have on students and staff,” she added.
For those pediatricians or other health care providers who serve as consultants to schools, Dr. Schonfeld advised they ask about policies related to exercises and drills, such as what are the limits to what children might be exposed to in a drill, and what requirements there might be at the local and state level in terms of frequency and what the drills will and will not involve.
He also noted that clinicians should encourage school leaders to consider the fact that kids may have personal histories of trauma that are completely unknown to the school when they design these exercises.
School staff and health care providers should explain the nature and reasons for drills, invite family members to express concerns, and make accommodations if necessary for some children to participate in drills in a more limited way, noted Dr. Schonfeld, who is also clinical professor of pediatrics at the University of Southern California, Los Angeles.
“I think health care providers should work with legislators, so that if they require a drill, it must be done in a way that is physically and emotionally safe,” he added.
Executing better drills for students’ mental health
Experts also advised on ways to execute these drills that will be least damaging to students.
The AAP statement on Participation of Children and Adolescents in Live Crisis Drills and Exercises, for example, advocates eliminating high-intensity drills, prohibiting deception in drills, and providing accommodations based on children’s vulnerabilities.
Dr. Schonfeld also emphasized, in an interview, that training for an attack need not be extremely realistic to be effective.
“When you are preparing for a crisis, the drills and exercises are for children to practice and develop mastery over something they don’t know how to do fully yet,” said Dr. Schonfeld.
Citing a suggestion from a 2020 report conducted by Everytown for Gun Safety on keeping schools safe from gun violence, Dr. Younghans said, “Schools should be in clear communication with communities and families regarding when drills will be happening,” and advised ensuring that the explanation of drills is developmentally appropriate to the age of the children participating.
The report also recommends conducting drills that do not simulate an actual incident, combining drills with trauma-informed approaches to address students’ well-being during and for a sustained period after the drills, and tracking data on the efficacy and effects of drills.
Dr. Loper suggested ways that clinicians and parents can help navigate the tricky territory of school safety drills.
In his view, they should not be random or unexpected, and anticipatory guidance should be given regarding any visual or auditory stimuli, such as flashing lights or sirens, alarms, or announcements.
“A preventive approach should be utilized to ensure that any child who is experiencing extreme drill-distress be excused from any future disaster drills to prevent retraumatization,” Dr. Loper said.
Physicians interviewed for this piece also provided tips on how to talk about these events with children in a way that is beneficial to their mental health.
“What we want to do is [have a] calm discussion [with kids] about what we are doing and why we are doing it” and guide them through the movements, Dr. Schonfeld said.
When teaching children how to respond to an emergency, some elements of uncertainty need to be discussed. Children need to anticipate “what you might do if you are not in the classroom if something occurs, such as being in the bathroom, or out at recess,” he continued.
Dr. Younghans recommended that parents and staff schedule time to prepare children for the drill and practice in advance, and that behavioral health providers, counselors, and/or primary care providers should be involved in the planning and execution of the drill.
The Georgia Tech study was supported through a grant from Everytown for Gun Safety.
The study authors and experts interviewed for this piece had no financial conflicts to disclose.
LAIAs safer, more effective than oral antipsychotics for schizophrenia
– and they carry no increased risk for adverse events, new research shows.
Investigators analyzed data for more than 70,000 patients with schizophrenia and found that, compared with OAs, LAIAs were associated with lower risk for hospitalizations for any cause, hospitalizations for psychiatric disorders, hospitalizations for schizophrenia, and incident suicide attempts.
Among patients treated fully with LAIAs, there were fewer hospitalizations for somatic disorders and cardiovascular diseases and less extrapyramidal symptoms (EPS), compared with those treated fully with OAs. In addition, among those for whom treatment with LAIAs was initiated early, the reduction in these outcome events was greater in comparison with patients for whom treatment was initiated later.
The results “reinforced that LAIAs were associated with a lower risk of hospitalizations, disease relapses, and suicide attempts than OAs, and this association remained during subsequent treatment periods,” wrote the investigators, led by Esther Chan, PhD, Center for Safe Medication Practice and Research, department of pharmacology and pharmacy, University of Hong Kong.
The findings were published online in JAMA Network Open.
Misclassification common
“Current clinical guidelines on the use of LAIAs are derived mainly from randomized clinical trials in which strict inclusion criteria limit generalizability,” the investigators noted. In addition, most of these trials were of “relatively short duration,” so long-term observational studies are “important to establish the safety and effectiveness of LAIAs.”
Moreover, most studies have been based on Western populations, and the findings may not be generalizable to Asian populations, which have been less studied.
Previous studies were also often subject to “misclassification of exposure,” since patients treated with LAIAs alone and those treated concurrently with LAIAs and OAs were categorized together as “LAIA” users and were compared with users of OAs alone, the researchers noted.
To investigate the long-term safety and efficacy of LAIAs, they assessed data from the Clinical Data Analysis and Reporting System, an electronic health records database of the Hong Kong Hospital Authority. They identified 70,396 patients with schizophrenia (52.8% women; mean age, 44.2 years).
The investigators used a self-controlled case series design – “within-individual comparison, based on a case-only approach” – to analyze the data. This model uses incidence rate ratios derived by “comparing the rate of outcomes between exposed and unexposed or reference periods for the same individual.” In this approach, “only people with both the exposure and the outcome are eligible.”
To be included, a patient had to have received at least one OA and LAIA and had to have had at least one outcome event during the observation period of January 2004 or the date of first schizophrenia diagnosis (whichever came later) through December 2019 or death (whichever came first).
The observation period was further subdivided into four periods: a nontreatment period, a period in which OAs were used alone, a period in which LAIAs were used alone, and a period in which a combination of OAs and LAIAs were used together.
Primary outcomes included health care use and disease relapses, such as hospitalizations for psychiatric disorders, hospitalizations for schizophrenia, and incident suicide attempt. Secondary outcomes included hospitalizations for somatic disorders, hospitalizations for cardiovascular diseases, and EPS.
LAIAs superior
Of the total study population, 23,719 patients (33.7%) were prescribed both OAs and LAIAs (mean age, 41.7 years). Of these participants, 15.4% died during the observation period.
The mean duration of follow-up was 12.5 years. The mean duration of exposure to OAs alone was 5 years; for LAIA exposure alone, 1.4 years; and for OA plus LAIA exposure, 4.4 years.
During the observation period, almost all individuals (92.8%) had one or more ED visits, and most (88.4%) had one or more hospitalizations for any psychiatric disorder. Over three-quarters (77.5%) were hospitalized for schizophrenia, and a small percentage (6.1%) had an incident suicide attempt.
Almost all patients experienced EPS (93.5%); over half (64.9%) were hospitalized for somatic disorders; and 15.6% were hospitalized for cardiovascular diseases.
After adjustment, compared with OAs, use of LAIAs was associated with a significantly lower risk for most outcomes.
There were no differences between LAIAs and OAs regarding ED visits.
The reduction in EPS suggests that LAIAs “were not associated with a higher risk of those adverse events than OAs,” the investigators write.
When the patients were stratified by initiation time of LAIAs, early initiators had 76% fewer hospitalizations for schizophrenia during LAIA vs OA treatment (IRR, 0.24; 95% confidence interval, 0.21-0 .27), while late initiators of LAIAs had 55% fewer hospitalizations for schizophrenia (IRR, 0.45; 95% CI, 0.40-0.49), “suggesting that early LAIA initiators could have greater reduction in disease relapse,” the researchers noted.
Participants with comorbid substance use had a significantly lower risk for hospitalizations for any cause, hospitalizations for psychiatric disorders, hospitalizations for schizophrenia, hospitalizations for somatic disorders, incident suicide attempts, and EPS during the time they were treated with LAIAs versus the time they were treated with OAs.
Older adults (> 65 years) treated with LAIAs had a lower risk for ED visits, hospitalizations for any cause, hospitalizations for psychiatric disorders, and hospitalizations for schizophrenia. They were not at increased risk for hospitalizations for somatic disorders or cardiovascular diseases.
There was, however, a higher risk for EPS during initial treatment with LAIAs, so “caution should be exercised when initiating LAIAs” in older individuals, the investigators wrote.
Cited study limitations include the fact that pooled estimates were used for all LAIAs, rather than for individual antipsychotics. Additionally, the dose of these antipsychotics “was not accounted for because the disease information was recorded in a different way for LAIAs and OAs.”
Proactive approach
Commenting on the study, Brittany Gouse, MD, assistant professor at Boston University School of Medicine and a psychiatrist in the wellness and recovery after psychosis program, Boston Medical Center, said the study “adds to the growing body of evidence supporting the longitudinal benefits” of LAIAs for patients with schizophrenia spectrum disorders.
“In particular, there may be a unique benefit to transitioning to long-acting injectable antipsychotics within the first 2 years of illness,” said Dr. Gouse, who coauthored an accompanying editorial and was not involved with the research.
She also called the study “important,” because it suggests that early initiation of LAIAs “may serve as an important tool to reduce morbidity and bridge the premature mortality gap in schizophrenia.”
Dr. Gouse emphasized the importance of prioritizing “tertiary prevention” right from the onset of psychotic illness.
“Clinicians must take a proactive approach and include long-acting antipsychotics in shared decision-making conversations with patients within the first few years of illness,” she said.
The study was funded by the Excellent Young Scientists Fund of the National Natural Science Foundation of China. Dr. Chan received grants from the National Natural Science Foundation of China during the conduct of the study; nonfinancial support from the Wellcome Trust; grants from the Research Grants Council, the Research Fund Secretariat of the Food and Health Bureau, the National Health and Medical Research Council (Australia), the narcotics division of the Security Bureau of Hong Kong SAR, Amgen, AstraZeneca, Bayer, Bristol-Myers Squibb, Janssen, Pfizer, Takeda, and Novartis; and personal fees from Pfizer, Novartis, and the Hong Kong SAR Hospital Authority outside the submitted work. The editorialists reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
– and they carry no increased risk for adverse events, new research shows.
Investigators analyzed data for more than 70,000 patients with schizophrenia and found that, compared with OAs, LAIAs were associated with lower risk for hospitalizations for any cause, hospitalizations for psychiatric disorders, hospitalizations for schizophrenia, and incident suicide attempts.
Among patients treated fully with LAIAs, there were fewer hospitalizations for somatic disorders and cardiovascular diseases and less extrapyramidal symptoms (EPS), compared with those treated fully with OAs. In addition, among those for whom treatment with LAIAs was initiated early, the reduction in these outcome events was greater in comparison with patients for whom treatment was initiated later.
The results “reinforced that LAIAs were associated with a lower risk of hospitalizations, disease relapses, and suicide attempts than OAs, and this association remained during subsequent treatment periods,” wrote the investigators, led by Esther Chan, PhD, Center for Safe Medication Practice and Research, department of pharmacology and pharmacy, University of Hong Kong.
The findings were published online in JAMA Network Open.
Misclassification common
“Current clinical guidelines on the use of LAIAs are derived mainly from randomized clinical trials in which strict inclusion criteria limit generalizability,” the investigators noted. In addition, most of these trials were of “relatively short duration,” so long-term observational studies are “important to establish the safety and effectiveness of LAIAs.”
Moreover, most studies have been based on Western populations, and the findings may not be generalizable to Asian populations, which have been less studied.
Previous studies were also often subject to “misclassification of exposure,” since patients treated with LAIAs alone and those treated concurrently with LAIAs and OAs were categorized together as “LAIA” users and were compared with users of OAs alone, the researchers noted.
To investigate the long-term safety and efficacy of LAIAs, they assessed data from the Clinical Data Analysis and Reporting System, an electronic health records database of the Hong Kong Hospital Authority. They identified 70,396 patients with schizophrenia (52.8% women; mean age, 44.2 years).
The investigators used a self-controlled case series design – “within-individual comparison, based on a case-only approach” – to analyze the data. This model uses incidence rate ratios derived by “comparing the rate of outcomes between exposed and unexposed or reference periods for the same individual.” In this approach, “only people with both the exposure and the outcome are eligible.”
To be included, a patient had to have received at least one OA and LAIA and had to have had at least one outcome event during the observation period of January 2004 or the date of first schizophrenia diagnosis (whichever came later) through December 2019 or death (whichever came first).
The observation period was further subdivided into four periods: a nontreatment period, a period in which OAs were used alone, a period in which LAIAs were used alone, and a period in which a combination of OAs and LAIAs were used together.
Primary outcomes included health care use and disease relapses, such as hospitalizations for psychiatric disorders, hospitalizations for schizophrenia, and incident suicide attempt. Secondary outcomes included hospitalizations for somatic disorders, hospitalizations for cardiovascular diseases, and EPS.
LAIAs superior
Of the total study population, 23,719 patients (33.7%) were prescribed both OAs and LAIAs (mean age, 41.7 years). Of these participants, 15.4% died during the observation period.
The mean duration of follow-up was 12.5 years. The mean duration of exposure to OAs alone was 5 years; for LAIA exposure alone, 1.4 years; and for OA plus LAIA exposure, 4.4 years.
During the observation period, almost all individuals (92.8%) had one or more ED visits, and most (88.4%) had one or more hospitalizations for any psychiatric disorder. Over three-quarters (77.5%) were hospitalized for schizophrenia, and a small percentage (6.1%) had an incident suicide attempt.
Almost all patients experienced EPS (93.5%); over half (64.9%) were hospitalized for somatic disorders; and 15.6% were hospitalized for cardiovascular diseases.
After adjustment, compared with OAs, use of LAIAs was associated with a significantly lower risk for most outcomes.
There were no differences between LAIAs and OAs regarding ED visits.
The reduction in EPS suggests that LAIAs “were not associated with a higher risk of those adverse events than OAs,” the investigators write.
When the patients were stratified by initiation time of LAIAs, early initiators had 76% fewer hospitalizations for schizophrenia during LAIA vs OA treatment (IRR, 0.24; 95% confidence interval, 0.21-0 .27), while late initiators of LAIAs had 55% fewer hospitalizations for schizophrenia (IRR, 0.45; 95% CI, 0.40-0.49), “suggesting that early LAIA initiators could have greater reduction in disease relapse,” the researchers noted.
Participants with comorbid substance use had a significantly lower risk for hospitalizations for any cause, hospitalizations for psychiatric disorders, hospitalizations for schizophrenia, hospitalizations for somatic disorders, incident suicide attempts, and EPS during the time they were treated with LAIAs versus the time they were treated with OAs.
Older adults (> 65 years) treated with LAIAs had a lower risk for ED visits, hospitalizations for any cause, hospitalizations for psychiatric disorders, and hospitalizations for schizophrenia. They were not at increased risk for hospitalizations for somatic disorders or cardiovascular diseases.
There was, however, a higher risk for EPS during initial treatment with LAIAs, so “caution should be exercised when initiating LAIAs” in older individuals, the investigators wrote.
Cited study limitations include the fact that pooled estimates were used for all LAIAs, rather than for individual antipsychotics. Additionally, the dose of these antipsychotics “was not accounted for because the disease information was recorded in a different way for LAIAs and OAs.”
Proactive approach
Commenting on the study, Brittany Gouse, MD, assistant professor at Boston University School of Medicine and a psychiatrist in the wellness and recovery after psychosis program, Boston Medical Center, said the study “adds to the growing body of evidence supporting the longitudinal benefits” of LAIAs for patients with schizophrenia spectrum disorders.
“In particular, there may be a unique benefit to transitioning to long-acting injectable antipsychotics within the first 2 years of illness,” said Dr. Gouse, who coauthored an accompanying editorial and was not involved with the research.
She also called the study “important,” because it suggests that early initiation of LAIAs “may serve as an important tool to reduce morbidity and bridge the premature mortality gap in schizophrenia.”
Dr. Gouse emphasized the importance of prioritizing “tertiary prevention” right from the onset of psychotic illness.
“Clinicians must take a proactive approach and include long-acting antipsychotics in shared decision-making conversations with patients within the first few years of illness,” she said.
The study was funded by the Excellent Young Scientists Fund of the National Natural Science Foundation of China. Dr. Chan received grants from the National Natural Science Foundation of China during the conduct of the study; nonfinancial support from the Wellcome Trust; grants from the Research Grants Council, the Research Fund Secretariat of the Food and Health Bureau, the National Health and Medical Research Council (Australia), the narcotics division of the Security Bureau of Hong Kong SAR, Amgen, AstraZeneca, Bayer, Bristol-Myers Squibb, Janssen, Pfizer, Takeda, and Novartis; and personal fees from Pfizer, Novartis, and the Hong Kong SAR Hospital Authority outside the submitted work. The editorialists reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
– and they carry no increased risk for adverse events, new research shows.
Investigators analyzed data for more than 70,000 patients with schizophrenia and found that, compared with OAs, LAIAs were associated with lower risk for hospitalizations for any cause, hospitalizations for psychiatric disorders, hospitalizations for schizophrenia, and incident suicide attempts.
Among patients treated fully with LAIAs, there were fewer hospitalizations for somatic disorders and cardiovascular diseases and less extrapyramidal symptoms (EPS), compared with those treated fully with OAs. In addition, among those for whom treatment with LAIAs was initiated early, the reduction in these outcome events was greater in comparison with patients for whom treatment was initiated later.
The results “reinforced that LAIAs were associated with a lower risk of hospitalizations, disease relapses, and suicide attempts than OAs, and this association remained during subsequent treatment periods,” wrote the investigators, led by Esther Chan, PhD, Center for Safe Medication Practice and Research, department of pharmacology and pharmacy, University of Hong Kong.
The findings were published online in JAMA Network Open.
Misclassification common
“Current clinical guidelines on the use of LAIAs are derived mainly from randomized clinical trials in which strict inclusion criteria limit generalizability,” the investigators noted. In addition, most of these trials were of “relatively short duration,” so long-term observational studies are “important to establish the safety and effectiveness of LAIAs.”
Moreover, most studies have been based on Western populations, and the findings may not be generalizable to Asian populations, which have been less studied.
Previous studies were also often subject to “misclassification of exposure,” since patients treated with LAIAs alone and those treated concurrently with LAIAs and OAs were categorized together as “LAIA” users and were compared with users of OAs alone, the researchers noted.
To investigate the long-term safety and efficacy of LAIAs, they assessed data from the Clinical Data Analysis and Reporting System, an electronic health records database of the Hong Kong Hospital Authority. They identified 70,396 patients with schizophrenia (52.8% women; mean age, 44.2 years).
The investigators used a self-controlled case series design – “within-individual comparison, based on a case-only approach” – to analyze the data. This model uses incidence rate ratios derived by “comparing the rate of outcomes between exposed and unexposed or reference periods for the same individual.” In this approach, “only people with both the exposure and the outcome are eligible.”
To be included, a patient had to have received at least one OA and LAIA and had to have had at least one outcome event during the observation period of January 2004 or the date of first schizophrenia diagnosis (whichever came later) through December 2019 or death (whichever came first).
The observation period was further subdivided into four periods: a nontreatment period, a period in which OAs were used alone, a period in which LAIAs were used alone, and a period in which a combination of OAs and LAIAs were used together.
Primary outcomes included health care use and disease relapses, such as hospitalizations for psychiatric disorders, hospitalizations for schizophrenia, and incident suicide attempt. Secondary outcomes included hospitalizations for somatic disorders, hospitalizations for cardiovascular diseases, and EPS.
LAIAs superior
Of the total study population, 23,719 patients (33.7%) were prescribed both OAs and LAIAs (mean age, 41.7 years). Of these participants, 15.4% died during the observation period.
The mean duration of follow-up was 12.5 years. The mean duration of exposure to OAs alone was 5 years; for LAIA exposure alone, 1.4 years; and for OA plus LAIA exposure, 4.4 years.
During the observation period, almost all individuals (92.8%) had one or more ED visits, and most (88.4%) had one or more hospitalizations for any psychiatric disorder. Over three-quarters (77.5%) were hospitalized for schizophrenia, and a small percentage (6.1%) had an incident suicide attempt.
Almost all patients experienced EPS (93.5%); over half (64.9%) were hospitalized for somatic disorders; and 15.6% were hospitalized for cardiovascular diseases.
After adjustment, compared with OAs, use of LAIAs was associated with a significantly lower risk for most outcomes.
There were no differences between LAIAs and OAs regarding ED visits.
The reduction in EPS suggests that LAIAs “were not associated with a higher risk of those adverse events than OAs,” the investigators write.
When the patients were stratified by initiation time of LAIAs, early initiators had 76% fewer hospitalizations for schizophrenia during LAIA vs OA treatment (IRR, 0.24; 95% confidence interval, 0.21-0 .27), while late initiators of LAIAs had 55% fewer hospitalizations for schizophrenia (IRR, 0.45; 95% CI, 0.40-0.49), “suggesting that early LAIA initiators could have greater reduction in disease relapse,” the researchers noted.
Participants with comorbid substance use had a significantly lower risk for hospitalizations for any cause, hospitalizations for psychiatric disorders, hospitalizations for schizophrenia, hospitalizations for somatic disorders, incident suicide attempts, and EPS during the time they were treated with LAIAs versus the time they were treated with OAs.
Older adults (> 65 years) treated with LAIAs had a lower risk for ED visits, hospitalizations for any cause, hospitalizations for psychiatric disorders, and hospitalizations for schizophrenia. They were not at increased risk for hospitalizations for somatic disorders or cardiovascular diseases.
There was, however, a higher risk for EPS during initial treatment with LAIAs, so “caution should be exercised when initiating LAIAs” in older individuals, the investigators wrote.
Cited study limitations include the fact that pooled estimates were used for all LAIAs, rather than for individual antipsychotics. Additionally, the dose of these antipsychotics “was not accounted for because the disease information was recorded in a different way for LAIAs and OAs.”
Proactive approach
Commenting on the study, Brittany Gouse, MD, assistant professor at Boston University School of Medicine and a psychiatrist in the wellness and recovery after psychosis program, Boston Medical Center, said the study “adds to the growing body of evidence supporting the longitudinal benefits” of LAIAs for patients with schizophrenia spectrum disorders.
“In particular, there may be a unique benefit to transitioning to long-acting injectable antipsychotics within the first 2 years of illness,” said Dr. Gouse, who coauthored an accompanying editorial and was not involved with the research.
She also called the study “important,” because it suggests that early initiation of LAIAs “may serve as an important tool to reduce morbidity and bridge the premature mortality gap in schizophrenia.”
Dr. Gouse emphasized the importance of prioritizing “tertiary prevention” right from the onset of psychotic illness.
“Clinicians must take a proactive approach and include long-acting antipsychotics in shared decision-making conversations with patients within the first few years of illness,” she said.
The study was funded by the Excellent Young Scientists Fund of the National Natural Science Foundation of China. Dr. Chan received grants from the National Natural Science Foundation of China during the conduct of the study; nonfinancial support from the Wellcome Trust; grants from the Research Grants Council, the Research Fund Secretariat of the Food and Health Bureau, the National Health and Medical Research Council (Australia), the narcotics division of the Security Bureau of Hong Kong SAR, Amgen, AstraZeneca, Bayer, Bristol-Myers Squibb, Janssen, Pfizer, Takeda, and Novartis; and personal fees from Pfizer, Novartis, and the Hong Kong SAR Hospital Authority outside the submitted work. The editorialists reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN