User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'main-prefix')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
Watching TV, using computer have opposite ties to dementia risk
The relationship to dementia with these activities remained strong no matter how much physical activity a person did, the authors wrote in Proceedings of the National Academy of Sciences.
Both watching TV and using a computer have been linked to increased risk of chronic disease and mortality, while exercise and physical activity (PA) have shown benefit in reducing cognitive decline, structural brain atrophy, and dementia risk in older adults, the authors wrote.
The authors said they wanted to try to understand the effects of watching TV and using computers on dementia risk, because people in the United States and Europe have been engaging in both of these activities more often.
They concluded that it’s not the sitting part of sedentary behavior (SB) that potentially has the effect on dementia but what people are doing while sitting.
Some of the results were surprising, lead author David Raichlen, PhD, professor of Human and Evolutionary Biology at University of Southern California, Los Angeles, said in an interview.
Previous literature on sedentary behaviors have documented their negative effects on a wide range of health outcomes, rather than finding positive associations, he explained.
More than 140,000 included in study
The researchers conducted their prospective cohort study using data from the United Kingdom Biobank. After excluding people younger than 60, those with prevalent dementia at the start of follow-up, and those without complete data, 146,651 participants were included.
The participants were followed from their baseline visit until they received a dementia diagnosis, died, were lost to follow-up, or were last admitted to the hospital.
TV-watching time was linked with an increased risk of incident dementia (HR [95% confidence interval] = 1.31 [1.23-1.40]), and computer use was linked with a reduced risk of incident dementia HR [95% CI] = 0.80 [0.76-0.85]).
TV’s link with higher dementia risk increased in those who had the highest use, compared with those who had the lowest use (HR [95% CI] = 1.28 [1.18-1.39].
Similarly, the link with risk reduction for dementia with computer use increased with more use.
Both medium and high computer time were associated with reduced risk of incident dementia (HR [95% CI] = 0.70 [0.64-0.76] and HR [95% CI] = 0.76 [0.70-0.83] respectively).
Dr. Raichlen pointed out that the high use of TV in this study was 4 or more hours a day and computer use – which included leisure use, not work use – had benefits on dementia risk after just half an hour.
These results remained significant after researchers adjusted for demographic, health, and lifestyle variables, including time spent on physical activity, sleeping, obesity, alcohol consumption, smoking status, diet scores, education level, body mass index, and employment type.
Physical is still better than sedentary activity
One potential reason for the different effects on dementia risk in the two activities studied, the authors write, is that sitting down to watch TV is associated with “uniquely low levels of muscle activity and energy expenditure, compared with sitting to use a computer.”
Andrew Budson, MD, chief of Cognitive & Behavioral Neurology and Associate Chief of Staff for Education for the VA Boston Healthcare System, Mass., who was not part of the study, said he thinks a more likely explanation for the study findings lies in the active versus passive tasks required in the two kinds of viewing that the authors reference.
“When we’re doing cognitive activity involving using the computer, we’re using large parts of our cortex to carry out that activity, whereas when we’re watching TV, there are probably relatively small amounts of our brain that are actually active,” Dr. Budson, author of Seven Steps to Managing Your Memory, explained in an interview.
“This is one of the first times I’ve been convinced that even when the computer activity isn’t completely new and novel, it may be beneficial,” Dr. Budson said.
It would be much better to do physical activity, but if the choice is sedentary activity, active cognitive activities, such as computer use, are better than TV watching, he continued.
The results of the current study are consistent with previous work showing that the type of sedentary behavior matters, according to the authors.
“Several studies have shown that TV time is associated with mortality and poor cardiometabolic biomarkers, whereas computer time is not,” they wrote.
A limitation of the study is that sedentary behaviors were self-reported via questionnaires, and there may be errors in recall.
“The use of objective methods for measuring both SB and PA are needed in future studies,” they write.
The authors receive support from the National Institutes of Health, the State of Arizona, the Arizona Department of Health Services, and the McKnight Brain Research Foundation. Neither the authors nor Dr. Budson declared relevant financial relationships.
The relationship to dementia with these activities remained strong no matter how much physical activity a person did, the authors wrote in Proceedings of the National Academy of Sciences.
Both watching TV and using a computer have been linked to increased risk of chronic disease and mortality, while exercise and physical activity (PA) have shown benefit in reducing cognitive decline, structural brain atrophy, and dementia risk in older adults, the authors wrote.
The authors said they wanted to try to understand the effects of watching TV and using computers on dementia risk, because people in the United States and Europe have been engaging in both of these activities more often.
They concluded that it’s not the sitting part of sedentary behavior (SB) that potentially has the effect on dementia but what people are doing while sitting.
Some of the results were surprising, lead author David Raichlen, PhD, professor of Human and Evolutionary Biology at University of Southern California, Los Angeles, said in an interview.
Previous literature on sedentary behaviors have documented their negative effects on a wide range of health outcomes, rather than finding positive associations, he explained.
More than 140,000 included in study
The researchers conducted their prospective cohort study using data from the United Kingdom Biobank. After excluding people younger than 60, those with prevalent dementia at the start of follow-up, and those without complete data, 146,651 participants were included.
The participants were followed from their baseline visit until they received a dementia diagnosis, died, were lost to follow-up, or were last admitted to the hospital.
TV-watching time was linked with an increased risk of incident dementia (HR [95% confidence interval] = 1.31 [1.23-1.40]), and computer use was linked with a reduced risk of incident dementia HR [95% CI] = 0.80 [0.76-0.85]).
TV’s link with higher dementia risk increased in those who had the highest use, compared with those who had the lowest use (HR [95% CI] = 1.28 [1.18-1.39].
Similarly, the link with risk reduction for dementia with computer use increased with more use.
Both medium and high computer time were associated with reduced risk of incident dementia (HR [95% CI] = 0.70 [0.64-0.76] and HR [95% CI] = 0.76 [0.70-0.83] respectively).
Dr. Raichlen pointed out that the high use of TV in this study was 4 or more hours a day and computer use – which included leisure use, not work use – had benefits on dementia risk after just half an hour.
These results remained significant after researchers adjusted for demographic, health, and lifestyle variables, including time spent on physical activity, sleeping, obesity, alcohol consumption, smoking status, diet scores, education level, body mass index, and employment type.
Physical is still better than sedentary activity
One potential reason for the different effects on dementia risk in the two activities studied, the authors write, is that sitting down to watch TV is associated with “uniquely low levels of muscle activity and energy expenditure, compared with sitting to use a computer.”
Andrew Budson, MD, chief of Cognitive & Behavioral Neurology and Associate Chief of Staff for Education for the VA Boston Healthcare System, Mass., who was not part of the study, said he thinks a more likely explanation for the study findings lies in the active versus passive tasks required in the two kinds of viewing that the authors reference.
“When we’re doing cognitive activity involving using the computer, we’re using large parts of our cortex to carry out that activity, whereas when we’re watching TV, there are probably relatively small amounts of our brain that are actually active,” Dr. Budson, author of Seven Steps to Managing Your Memory, explained in an interview.
“This is one of the first times I’ve been convinced that even when the computer activity isn’t completely new and novel, it may be beneficial,” Dr. Budson said.
It would be much better to do physical activity, but if the choice is sedentary activity, active cognitive activities, such as computer use, are better than TV watching, he continued.
The results of the current study are consistent with previous work showing that the type of sedentary behavior matters, according to the authors.
“Several studies have shown that TV time is associated with mortality and poor cardiometabolic biomarkers, whereas computer time is not,” they wrote.
A limitation of the study is that sedentary behaviors were self-reported via questionnaires, and there may be errors in recall.
“The use of objective methods for measuring both SB and PA are needed in future studies,” they write.
The authors receive support from the National Institutes of Health, the State of Arizona, the Arizona Department of Health Services, and the McKnight Brain Research Foundation. Neither the authors nor Dr. Budson declared relevant financial relationships.
The relationship to dementia with these activities remained strong no matter how much physical activity a person did, the authors wrote in Proceedings of the National Academy of Sciences.
Both watching TV and using a computer have been linked to increased risk of chronic disease and mortality, while exercise and physical activity (PA) have shown benefit in reducing cognitive decline, structural brain atrophy, and dementia risk in older adults, the authors wrote.
The authors said they wanted to try to understand the effects of watching TV and using computers on dementia risk, because people in the United States and Europe have been engaging in both of these activities more often.
They concluded that it’s not the sitting part of sedentary behavior (SB) that potentially has the effect on dementia but what people are doing while sitting.
Some of the results were surprising, lead author David Raichlen, PhD, professor of Human and Evolutionary Biology at University of Southern California, Los Angeles, said in an interview.
Previous literature on sedentary behaviors have documented their negative effects on a wide range of health outcomes, rather than finding positive associations, he explained.
More than 140,000 included in study
The researchers conducted their prospective cohort study using data from the United Kingdom Biobank. After excluding people younger than 60, those with prevalent dementia at the start of follow-up, and those without complete data, 146,651 participants were included.
The participants were followed from their baseline visit until they received a dementia diagnosis, died, were lost to follow-up, or were last admitted to the hospital.
TV-watching time was linked with an increased risk of incident dementia (HR [95% confidence interval] = 1.31 [1.23-1.40]), and computer use was linked with a reduced risk of incident dementia HR [95% CI] = 0.80 [0.76-0.85]).
TV’s link with higher dementia risk increased in those who had the highest use, compared with those who had the lowest use (HR [95% CI] = 1.28 [1.18-1.39].
Similarly, the link with risk reduction for dementia with computer use increased with more use.
Both medium and high computer time were associated with reduced risk of incident dementia (HR [95% CI] = 0.70 [0.64-0.76] and HR [95% CI] = 0.76 [0.70-0.83] respectively).
Dr. Raichlen pointed out that the high use of TV in this study was 4 or more hours a day and computer use – which included leisure use, not work use – had benefits on dementia risk after just half an hour.
These results remained significant after researchers adjusted for demographic, health, and lifestyle variables, including time spent on physical activity, sleeping, obesity, alcohol consumption, smoking status, diet scores, education level, body mass index, and employment type.
Physical is still better than sedentary activity
One potential reason for the different effects on dementia risk in the two activities studied, the authors write, is that sitting down to watch TV is associated with “uniquely low levels of muscle activity and energy expenditure, compared with sitting to use a computer.”
Andrew Budson, MD, chief of Cognitive & Behavioral Neurology and Associate Chief of Staff for Education for the VA Boston Healthcare System, Mass., who was not part of the study, said he thinks a more likely explanation for the study findings lies in the active versus passive tasks required in the two kinds of viewing that the authors reference.
“When we’re doing cognitive activity involving using the computer, we’re using large parts of our cortex to carry out that activity, whereas when we’re watching TV, there are probably relatively small amounts of our brain that are actually active,” Dr. Budson, author of Seven Steps to Managing Your Memory, explained in an interview.
“This is one of the first times I’ve been convinced that even when the computer activity isn’t completely new and novel, it may be beneficial,” Dr. Budson said.
It would be much better to do physical activity, but if the choice is sedentary activity, active cognitive activities, such as computer use, are better than TV watching, he continued.
The results of the current study are consistent with previous work showing that the type of sedentary behavior matters, according to the authors.
“Several studies have shown that TV time is associated with mortality and poor cardiometabolic biomarkers, whereas computer time is not,” they wrote.
A limitation of the study is that sedentary behaviors were self-reported via questionnaires, and there may be errors in recall.
“The use of objective methods for measuring both SB and PA are needed in future studies,” they write.
The authors receive support from the National Institutes of Health, the State of Arizona, the Arizona Department of Health Services, and the McKnight Brain Research Foundation. Neither the authors nor Dr. Budson declared relevant financial relationships.
FROM PROCEEDINGS OF THE NATIONAL ACADEMY OF SCIENCES
Is it COVID or long COVID? Your organs may know
There’s little doubt long COVID is real. The federal government recognizes long COVID as a condition and said in two reports issued in August that one in five adult COVID-19 survivors have a health condition related to their illness.
COVID-19 can damage multiple organs in the body. Sometimes this damage leads to long COVID; sometimes other reasons are at play. Doctors are beginning to sort it out.
“COVID itself can actually cause prolonged illness, and we don’t really call that long COVID,” said Nisha Viswanathan, MD, a doctor at UCLA Health in Los Angeles. But if symptoms extend beyond 12 weeks, that puts patients in the realm of long COVID.
Symptoms can range from mild to severe and can keep people from resuming their normal lives and jobs. Sometimes they last for months, according to the U.S. Department of Health & Human Services.
Multiorgan damage
Lung scarring and other lung problems are common after COVID, said Leora Horwitz, MD, an internal medicine specialist at New York University. Even after a mild case, people can have breathing issues for months, a team at Johns Hopkins Medicine, Baltimore, said in an online briefing. One study published in the journal Radiology found damage in people a full year after a COVID-19 diagnosis.
Some people have persistent heart, kidney, liver, and nervous system problems after COVID-19. A study published in 2020 in JAMA Cardiology found 60% of people who had COVID-19 had ongoing signs of heart inflammation. Nearly a third of people hospitalized for COVID-19 get kidney damage that can become chronic, and some end up needing dialysis or a transplant, said C. John Sperati, MD, a kidney specialist at Johns Hopkins Medicine.
This might be, in part, because SARS-CoV-2, the virus that causes COVID-19, directly infects the cells in many organs.
Nicole Bhave, MD, a cardiologist at University of Michigan Health, Ann Arbor is concerned that COVID-19 appears to increase the risk of heart problems in some people.
“Some of the uptick may just be recognition bias, in that people with symptoms are seeking care,” she said. “But there’s definitely a biological basis by which COVID could tip people over into a new diagnosis of heart failure.”
Inflammation
Inflammation is probably a key part of the long-term effects of COVID-19.
Some people have a serious immune reaction to COVID-19 called a cytokine storm, said Nitra Aggarwal Gilotra, MD, a cardiologist at Johns Hopkins Medicine. This release of inflammation-causing molecules called cytokines is meant to attack the invading virus. But it can be so severe that it wreaks havoc on healthy tissues and organs and causes lasting damage – if patients even survive it.
In some people, inflammation can affect the heart, causing myocarditis. Myocarditis symptoms include chest pain, breathlessness, and heart palpitations. Though rare, it can be serious and can raise the risk of other heart problems, including heart failure, down the line.
Long COVID may also trigger an autoimmune condition, said Eline Luning Prak, MD, PhD, a pathologist at the Hospital of the University of Pennsylvania, Philadelphia. Long COVID can share many hallmark symptoms with autoimmune diseases, including fatigue, widespread pain, memory problems, and mood disorders.
Blood clots
Studies have shown the overcharged inflammatory response to COVID-19 can cause blood clots. This sometimes overwhelming clotting was an early hallmark of COVID-19 infection, and when clots restrict blood flow in the brain, lungs, kidneys, or limbs, they can cause long-term damage. Some can be deadly. Researchers in Sweden found patients were at risk of deep vein thrombosis – a blood clot usually in the leg – up to 3 months after infection and at higher risk of a blood clot in the lung, called pulmonary embolism, for as long as 3 months.
Viral reservoirs
The virus itself may also linger in a patient’s body, causing continued symptoms and, potentially, new flare-ups. Zoe Swank, PhD, of Harvard Medical School, Boston, and colleagues reported in a preprint study that they found pieces of the SARS-CoV-2 virus in the blood of most patients with long COVID symptoms they tested – some as long as a year after infection. The study has not yet been peer reviewed.
Another team found evidence of the virus in stool up to 7 months later, which suggests the virus hides out in the gut. Other early studies have found bits of viral RNA in the appendix, breast tissue, heart, eyes, and brain.
Diabetes
Diabetes is a risk factor for getting severe COVID-19, and multiple studies have shown people can get diabetes both while battling infection and afterward. One study of veterans, published in The Lancet Diabetes and Endocrinology, found COVID-19 survivors were about 40% more likely to get diabetes over the next year.
There are a few ways this might happen. Insulin-producing cells in the pancreas have SARS-CoV-2 receptors – a type of molecular doorway the coronavirus can attach to. Damage to these cells could make the body less able to produce insulin, which in turn can lead to diabetes. The virus could also disrupt the balance in the body or cause inflammation that leads to insulin resistance, which can develop into diabetes, Ziad Al-Aly, MD, of the Veterans Affairs St. Louis Health Care System, and colleagues wrote.
Nervous system issues
People who get COVID-19 are also more vulnerable to postural orthostatic tachycardia syndrome (POTS). This affects what’s known as the autonomic nervous system, which regulates blood circulation, and includes those things that happen in your body without your having to think about them, like breathing, heartbeat, and digestion. POTS can cause common long COVID neurologic symptoms, including headaches, fatigue, brain fog, insomnia, and problems thinking and concentrating. “This was a known condition prior to COVID, but it was incredibly rare,” said Dr. Viswanathan. “After COVID, I’ve seen it with increasing frequency.”
Long-term outlook
Lasting issues after COVID-19 are much more likely after a moderate or severe infection. Still, plenty of people are battling them even after a mild illness. “As for why, that’s the billion-dollar question,” said Dr. Horwitz. “It’s well known that viral infections can cause long-term dysregulation. Why that is, we really just don’t know.”
Whether it’s virus hiding out in the body, long-term organ damage, or an autoimmune reaction likely differs from person to person. “I’m believing, increasingly, that it’s a combination of all of these, just based on how different patients are responding to different medications,” said Dr. Viswanathan. “One patient will respond to something beautifully, and another patient won’t at all.”
But it’s clear a significant number of people are facing long-term health struggles because of COVID-19, which has infected at least 580 million people globally and 92 million – likely many more – in the United States, according to Johns Hopkins University.
Even a small increased risk of conditions like heart disease or diabetes translates to a huge number of people, Dr. Horwitz said. “If even 1% of people getting COVID have long-term symptoms, that’s a major public health crisis, because that’s 1% of pretty much everybody in the country.”
A version of this article first appeared on WebMD.com.
There’s little doubt long COVID is real. The federal government recognizes long COVID as a condition and said in two reports issued in August that one in five adult COVID-19 survivors have a health condition related to their illness.
COVID-19 can damage multiple organs in the body. Sometimes this damage leads to long COVID; sometimes other reasons are at play. Doctors are beginning to sort it out.
“COVID itself can actually cause prolonged illness, and we don’t really call that long COVID,” said Nisha Viswanathan, MD, a doctor at UCLA Health in Los Angeles. But if symptoms extend beyond 12 weeks, that puts patients in the realm of long COVID.
Symptoms can range from mild to severe and can keep people from resuming their normal lives and jobs. Sometimes they last for months, according to the U.S. Department of Health & Human Services.
Multiorgan damage
Lung scarring and other lung problems are common after COVID, said Leora Horwitz, MD, an internal medicine specialist at New York University. Even after a mild case, people can have breathing issues for months, a team at Johns Hopkins Medicine, Baltimore, said in an online briefing. One study published in the journal Radiology found damage in people a full year after a COVID-19 diagnosis.
Some people have persistent heart, kidney, liver, and nervous system problems after COVID-19. A study published in 2020 in JAMA Cardiology found 60% of people who had COVID-19 had ongoing signs of heart inflammation. Nearly a third of people hospitalized for COVID-19 get kidney damage that can become chronic, and some end up needing dialysis or a transplant, said C. John Sperati, MD, a kidney specialist at Johns Hopkins Medicine.
This might be, in part, because SARS-CoV-2, the virus that causes COVID-19, directly infects the cells in many organs.
Nicole Bhave, MD, a cardiologist at University of Michigan Health, Ann Arbor is concerned that COVID-19 appears to increase the risk of heart problems in some people.
“Some of the uptick may just be recognition bias, in that people with symptoms are seeking care,” she said. “But there’s definitely a biological basis by which COVID could tip people over into a new diagnosis of heart failure.”
Inflammation
Inflammation is probably a key part of the long-term effects of COVID-19.
Some people have a serious immune reaction to COVID-19 called a cytokine storm, said Nitra Aggarwal Gilotra, MD, a cardiologist at Johns Hopkins Medicine. This release of inflammation-causing molecules called cytokines is meant to attack the invading virus. But it can be so severe that it wreaks havoc on healthy tissues and organs and causes lasting damage – if patients even survive it.
In some people, inflammation can affect the heart, causing myocarditis. Myocarditis symptoms include chest pain, breathlessness, and heart palpitations. Though rare, it can be serious and can raise the risk of other heart problems, including heart failure, down the line.
Long COVID may also trigger an autoimmune condition, said Eline Luning Prak, MD, PhD, a pathologist at the Hospital of the University of Pennsylvania, Philadelphia. Long COVID can share many hallmark symptoms with autoimmune diseases, including fatigue, widespread pain, memory problems, and mood disorders.
Blood clots
Studies have shown the overcharged inflammatory response to COVID-19 can cause blood clots. This sometimes overwhelming clotting was an early hallmark of COVID-19 infection, and when clots restrict blood flow in the brain, lungs, kidneys, or limbs, they can cause long-term damage. Some can be deadly. Researchers in Sweden found patients were at risk of deep vein thrombosis – a blood clot usually in the leg – up to 3 months after infection and at higher risk of a blood clot in the lung, called pulmonary embolism, for as long as 3 months.
Viral reservoirs
The virus itself may also linger in a patient’s body, causing continued symptoms and, potentially, new flare-ups. Zoe Swank, PhD, of Harvard Medical School, Boston, and colleagues reported in a preprint study that they found pieces of the SARS-CoV-2 virus in the blood of most patients with long COVID symptoms they tested – some as long as a year after infection. The study has not yet been peer reviewed.
Another team found evidence of the virus in stool up to 7 months later, which suggests the virus hides out in the gut. Other early studies have found bits of viral RNA in the appendix, breast tissue, heart, eyes, and brain.
Diabetes
Diabetes is a risk factor for getting severe COVID-19, and multiple studies have shown people can get diabetes both while battling infection and afterward. One study of veterans, published in The Lancet Diabetes and Endocrinology, found COVID-19 survivors were about 40% more likely to get diabetes over the next year.
There are a few ways this might happen. Insulin-producing cells in the pancreas have SARS-CoV-2 receptors – a type of molecular doorway the coronavirus can attach to. Damage to these cells could make the body less able to produce insulin, which in turn can lead to diabetes. The virus could also disrupt the balance in the body or cause inflammation that leads to insulin resistance, which can develop into diabetes, Ziad Al-Aly, MD, of the Veterans Affairs St. Louis Health Care System, and colleagues wrote.
Nervous system issues
People who get COVID-19 are also more vulnerable to postural orthostatic tachycardia syndrome (POTS). This affects what’s known as the autonomic nervous system, which regulates blood circulation, and includes those things that happen in your body without your having to think about them, like breathing, heartbeat, and digestion. POTS can cause common long COVID neurologic symptoms, including headaches, fatigue, brain fog, insomnia, and problems thinking and concentrating. “This was a known condition prior to COVID, but it was incredibly rare,” said Dr. Viswanathan. “After COVID, I’ve seen it with increasing frequency.”
Long-term outlook
Lasting issues after COVID-19 are much more likely after a moderate or severe infection. Still, plenty of people are battling them even after a mild illness. “As for why, that’s the billion-dollar question,” said Dr. Horwitz. “It’s well known that viral infections can cause long-term dysregulation. Why that is, we really just don’t know.”
Whether it’s virus hiding out in the body, long-term organ damage, or an autoimmune reaction likely differs from person to person. “I’m believing, increasingly, that it’s a combination of all of these, just based on how different patients are responding to different medications,” said Dr. Viswanathan. “One patient will respond to something beautifully, and another patient won’t at all.”
But it’s clear a significant number of people are facing long-term health struggles because of COVID-19, which has infected at least 580 million people globally and 92 million – likely many more – in the United States, according to Johns Hopkins University.
Even a small increased risk of conditions like heart disease or diabetes translates to a huge number of people, Dr. Horwitz said. “If even 1% of people getting COVID have long-term symptoms, that’s a major public health crisis, because that’s 1% of pretty much everybody in the country.”
A version of this article first appeared on WebMD.com.
There’s little doubt long COVID is real. The federal government recognizes long COVID as a condition and said in two reports issued in August that one in five adult COVID-19 survivors have a health condition related to their illness.
COVID-19 can damage multiple organs in the body. Sometimes this damage leads to long COVID; sometimes other reasons are at play. Doctors are beginning to sort it out.
“COVID itself can actually cause prolonged illness, and we don’t really call that long COVID,” said Nisha Viswanathan, MD, a doctor at UCLA Health in Los Angeles. But if symptoms extend beyond 12 weeks, that puts patients in the realm of long COVID.
Symptoms can range from mild to severe and can keep people from resuming their normal lives and jobs. Sometimes they last for months, according to the U.S. Department of Health & Human Services.
Multiorgan damage
Lung scarring and other lung problems are common after COVID, said Leora Horwitz, MD, an internal medicine specialist at New York University. Even after a mild case, people can have breathing issues for months, a team at Johns Hopkins Medicine, Baltimore, said in an online briefing. One study published in the journal Radiology found damage in people a full year after a COVID-19 diagnosis.
Some people have persistent heart, kidney, liver, and nervous system problems after COVID-19. A study published in 2020 in JAMA Cardiology found 60% of people who had COVID-19 had ongoing signs of heart inflammation. Nearly a third of people hospitalized for COVID-19 get kidney damage that can become chronic, and some end up needing dialysis or a transplant, said C. John Sperati, MD, a kidney specialist at Johns Hopkins Medicine.
This might be, in part, because SARS-CoV-2, the virus that causes COVID-19, directly infects the cells in many organs.
Nicole Bhave, MD, a cardiologist at University of Michigan Health, Ann Arbor is concerned that COVID-19 appears to increase the risk of heart problems in some people.
“Some of the uptick may just be recognition bias, in that people with symptoms are seeking care,” she said. “But there’s definitely a biological basis by which COVID could tip people over into a new diagnosis of heart failure.”
Inflammation
Inflammation is probably a key part of the long-term effects of COVID-19.
Some people have a serious immune reaction to COVID-19 called a cytokine storm, said Nitra Aggarwal Gilotra, MD, a cardiologist at Johns Hopkins Medicine. This release of inflammation-causing molecules called cytokines is meant to attack the invading virus. But it can be so severe that it wreaks havoc on healthy tissues and organs and causes lasting damage – if patients even survive it.
In some people, inflammation can affect the heart, causing myocarditis. Myocarditis symptoms include chest pain, breathlessness, and heart palpitations. Though rare, it can be serious and can raise the risk of other heart problems, including heart failure, down the line.
Long COVID may also trigger an autoimmune condition, said Eline Luning Prak, MD, PhD, a pathologist at the Hospital of the University of Pennsylvania, Philadelphia. Long COVID can share many hallmark symptoms with autoimmune diseases, including fatigue, widespread pain, memory problems, and mood disorders.
Blood clots
Studies have shown the overcharged inflammatory response to COVID-19 can cause blood clots. This sometimes overwhelming clotting was an early hallmark of COVID-19 infection, and when clots restrict blood flow in the brain, lungs, kidneys, or limbs, they can cause long-term damage. Some can be deadly. Researchers in Sweden found patients were at risk of deep vein thrombosis – a blood clot usually in the leg – up to 3 months after infection and at higher risk of a blood clot in the lung, called pulmonary embolism, for as long as 3 months.
Viral reservoirs
The virus itself may also linger in a patient’s body, causing continued symptoms and, potentially, new flare-ups. Zoe Swank, PhD, of Harvard Medical School, Boston, and colleagues reported in a preprint study that they found pieces of the SARS-CoV-2 virus in the blood of most patients with long COVID symptoms they tested – some as long as a year after infection. The study has not yet been peer reviewed.
Another team found evidence of the virus in stool up to 7 months later, which suggests the virus hides out in the gut. Other early studies have found bits of viral RNA in the appendix, breast tissue, heart, eyes, and brain.
Diabetes
Diabetes is a risk factor for getting severe COVID-19, and multiple studies have shown people can get diabetes both while battling infection and afterward. One study of veterans, published in The Lancet Diabetes and Endocrinology, found COVID-19 survivors were about 40% more likely to get diabetes over the next year.
There are a few ways this might happen. Insulin-producing cells in the pancreas have SARS-CoV-2 receptors – a type of molecular doorway the coronavirus can attach to. Damage to these cells could make the body less able to produce insulin, which in turn can lead to diabetes. The virus could also disrupt the balance in the body or cause inflammation that leads to insulin resistance, which can develop into diabetes, Ziad Al-Aly, MD, of the Veterans Affairs St. Louis Health Care System, and colleagues wrote.
Nervous system issues
People who get COVID-19 are also more vulnerable to postural orthostatic tachycardia syndrome (POTS). This affects what’s known as the autonomic nervous system, which regulates blood circulation, and includes those things that happen in your body without your having to think about them, like breathing, heartbeat, and digestion. POTS can cause common long COVID neurologic symptoms, including headaches, fatigue, brain fog, insomnia, and problems thinking and concentrating. “This was a known condition prior to COVID, but it was incredibly rare,” said Dr. Viswanathan. “After COVID, I’ve seen it with increasing frequency.”
Long-term outlook
Lasting issues after COVID-19 are much more likely after a moderate or severe infection. Still, plenty of people are battling them even after a mild illness. “As for why, that’s the billion-dollar question,” said Dr. Horwitz. “It’s well known that viral infections can cause long-term dysregulation. Why that is, we really just don’t know.”
Whether it’s virus hiding out in the body, long-term organ damage, or an autoimmune reaction likely differs from person to person. “I’m believing, increasingly, that it’s a combination of all of these, just based on how different patients are responding to different medications,” said Dr. Viswanathan. “One patient will respond to something beautifully, and another patient won’t at all.”
But it’s clear a significant number of people are facing long-term health struggles because of COVID-19, which has infected at least 580 million people globally and 92 million – likely many more – in the United States, according to Johns Hopkins University.
Even a small increased risk of conditions like heart disease or diabetes translates to a huge number of people, Dr. Horwitz said. “If even 1% of people getting COVID have long-term symptoms, that’s a major public health crisis, because that’s 1% of pretty much everybody in the country.”
A version of this article first appeared on WebMD.com.
APA task force highlights U.S. psychiatric bed crisis
The model, introduced in a recent report from the organization, can predict how changes in any component of mental health care in a community, including mobile trauma teams and assertive community treatment, will affect other components and the overall capacity to care for patients with mental illness.
Leaders of the APA task force that drafted the report noted that communities can use the model to confront the ongoing mental health crisis brought about by a lack of inpatient beds, a shortage of mental health professionals, shorter inpatient stays, and a rising number of individuals with mental illness.
The report was first released at the APA’s annual meeting in May 2022 and was discussed in further detail at a press briefing in mid-August.
“Part of the wisdom of the APA leadership of releasing this report in this format now is to keep attention and awareness on the issue and acknowledge that there is a terrible shortage of beds,” Anita Everett, MD, past president of the APA and chair of the report’s task force, told briefing attendees.
“We need to have ongoing conversations about how we can solve this problem,” said Dr. Everett, who is also director of the Center for Mental Health Services at the Substance Abuse and Mental Health Services Administration.
A virtual world
The report describes both historic and current psychiatric bed use and discusses how the availability of community resources affects the need for inpatient care. It includes analyses of inpatient medical care spending and describes barriers to accessing inpatient psychiatric care.
Historically, the number of state-operated psychiatric hospital beds in the United States was 337 per 100,000 people in the mid-1950s. Today, that figure is about 11.7 state psychiatric hospital beds per 100,000 people, the report says.
The average length of an inpatient stay has also decreased significantly both for adults and children. Pediatric length of stay declined from 12.2 days to 4.4 days between 1990 and 2000.
Launched in 2020, the APA Presidential Task Force on the Assessment of Psychiatric Bed Needs in the United States includes more than 30 mental health professionals and members of the APA administration.
The group was charged with drafting a report that explains and defines the current mental health crisis. They were also charged with developing a method for calculating the number of psychiatric beds needed in any given community.
Task force leaders said the model considers how individuals enter the mental health care system and are routed to appropriate services, how long they remain in the system, and the capacity of the system to respond to demand.
The model is based on a “virtual world” that has a number of care components. These include mobile crisis teams, intensive team-based outpatient care, community-based crisis beds, psychiatric hospital beds, and residential and step-down programs.
The model factors in the magnitude of the need for beds in many service areas. Factors include population size, estimates of the rate of acute mental health crises per 100,000 population, adequacy of the community mental health system, the intersection between the mental health and criminal justice systems, and outpatient and inpatient capacities.
The model computes the estimated number of patients waiting in the emergency department, crisis receiving centers, and jail, as well as average wait times. It also calculates the percentage of use of the various services.
The model will be continually updated and can be modified to better reflect the current situation in any given community.
Real-world testing
A team led by the University of Michigan, Ann Arbor, and two area hospitals is testing the APA model by using it to calculate the number of beds needed in their community.
“Because the model is focused on the continuum of care services, it allows communities to try to focus on what is the right mix of services needed to try to reduce the need for in-patient hospitalization and measure the impact of development of resources across the continuum, including inpatient beds, to try to achieve the right mix,” Gregory Dalack, MD, chair of the department of psychiatry at the University of Michigan Health System, told this news organization.
Ultimately, Dr. Dalack expects that the model will tell the team something they already know: that additional psychiatric beds are needed in their community.
However, meeting the needs of patients and families is not just about beds, he noted. The model will help provide a fuller picture of psychiatric care and will take into account existing services from many aspects of the care field.
“If we put all the focus just on hospital beds, we are only addressing one part of the challenge,” Dr. Dalack said.
The challenge is also about “identifying what resources/services are already in the continuum of care, where expansion of those or development of new programs might be needed, and what the impact is on the system, particularly with folks who arrive in the emergency room who might need inpatient admission,” he added.
Dr. Everett said the APA leadership team is now actively recruiting others to test the model in their communities, which will help to calibrate the system.
A version of this article first appeared on Medscape.com.
The model, introduced in a recent report from the organization, can predict how changes in any component of mental health care in a community, including mobile trauma teams and assertive community treatment, will affect other components and the overall capacity to care for patients with mental illness.
Leaders of the APA task force that drafted the report noted that communities can use the model to confront the ongoing mental health crisis brought about by a lack of inpatient beds, a shortage of mental health professionals, shorter inpatient stays, and a rising number of individuals with mental illness.
The report was first released at the APA’s annual meeting in May 2022 and was discussed in further detail at a press briefing in mid-August.
“Part of the wisdom of the APA leadership of releasing this report in this format now is to keep attention and awareness on the issue and acknowledge that there is a terrible shortage of beds,” Anita Everett, MD, past president of the APA and chair of the report’s task force, told briefing attendees.
“We need to have ongoing conversations about how we can solve this problem,” said Dr. Everett, who is also director of the Center for Mental Health Services at the Substance Abuse and Mental Health Services Administration.
A virtual world
The report describes both historic and current psychiatric bed use and discusses how the availability of community resources affects the need for inpatient care. It includes analyses of inpatient medical care spending and describes barriers to accessing inpatient psychiatric care.
Historically, the number of state-operated psychiatric hospital beds in the United States was 337 per 100,000 people in the mid-1950s. Today, that figure is about 11.7 state psychiatric hospital beds per 100,000 people, the report says.
The average length of an inpatient stay has also decreased significantly both for adults and children. Pediatric length of stay declined from 12.2 days to 4.4 days between 1990 and 2000.
Launched in 2020, the APA Presidential Task Force on the Assessment of Psychiatric Bed Needs in the United States includes more than 30 mental health professionals and members of the APA administration.
The group was charged with drafting a report that explains and defines the current mental health crisis. They were also charged with developing a method for calculating the number of psychiatric beds needed in any given community.
Task force leaders said the model considers how individuals enter the mental health care system and are routed to appropriate services, how long they remain in the system, and the capacity of the system to respond to demand.
The model is based on a “virtual world” that has a number of care components. These include mobile crisis teams, intensive team-based outpatient care, community-based crisis beds, psychiatric hospital beds, and residential and step-down programs.
The model factors in the magnitude of the need for beds in many service areas. Factors include population size, estimates of the rate of acute mental health crises per 100,000 population, adequacy of the community mental health system, the intersection between the mental health and criminal justice systems, and outpatient and inpatient capacities.
The model computes the estimated number of patients waiting in the emergency department, crisis receiving centers, and jail, as well as average wait times. It also calculates the percentage of use of the various services.
The model will be continually updated and can be modified to better reflect the current situation in any given community.
Real-world testing
A team led by the University of Michigan, Ann Arbor, and two area hospitals is testing the APA model by using it to calculate the number of beds needed in their community.
“Because the model is focused on the continuum of care services, it allows communities to try to focus on what is the right mix of services needed to try to reduce the need for in-patient hospitalization and measure the impact of development of resources across the continuum, including inpatient beds, to try to achieve the right mix,” Gregory Dalack, MD, chair of the department of psychiatry at the University of Michigan Health System, told this news organization.
Ultimately, Dr. Dalack expects that the model will tell the team something they already know: that additional psychiatric beds are needed in their community.
However, meeting the needs of patients and families is not just about beds, he noted. The model will help provide a fuller picture of psychiatric care and will take into account existing services from many aspects of the care field.
“If we put all the focus just on hospital beds, we are only addressing one part of the challenge,” Dr. Dalack said.
The challenge is also about “identifying what resources/services are already in the continuum of care, where expansion of those or development of new programs might be needed, and what the impact is on the system, particularly with folks who arrive in the emergency room who might need inpatient admission,” he added.
Dr. Everett said the APA leadership team is now actively recruiting others to test the model in their communities, which will help to calibrate the system.
A version of this article first appeared on Medscape.com.
The model, introduced in a recent report from the organization, can predict how changes in any component of mental health care in a community, including mobile trauma teams and assertive community treatment, will affect other components and the overall capacity to care for patients with mental illness.
Leaders of the APA task force that drafted the report noted that communities can use the model to confront the ongoing mental health crisis brought about by a lack of inpatient beds, a shortage of mental health professionals, shorter inpatient stays, and a rising number of individuals with mental illness.
The report was first released at the APA’s annual meeting in May 2022 and was discussed in further detail at a press briefing in mid-August.
“Part of the wisdom of the APA leadership of releasing this report in this format now is to keep attention and awareness on the issue and acknowledge that there is a terrible shortage of beds,” Anita Everett, MD, past president of the APA and chair of the report’s task force, told briefing attendees.
“We need to have ongoing conversations about how we can solve this problem,” said Dr. Everett, who is also director of the Center for Mental Health Services at the Substance Abuse and Mental Health Services Administration.
A virtual world
The report describes both historic and current psychiatric bed use and discusses how the availability of community resources affects the need for inpatient care. It includes analyses of inpatient medical care spending and describes barriers to accessing inpatient psychiatric care.
Historically, the number of state-operated psychiatric hospital beds in the United States was 337 per 100,000 people in the mid-1950s. Today, that figure is about 11.7 state psychiatric hospital beds per 100,000 people, the report says.
The average length of an inpatient stay has also decreased significantly both for adults and children. Pediatric length of stay declined from 12.2 days to 4.4 days between 1990 and 2000.
Launched in 2020, the APA Presidential Task Force on the Assessment of Psychiatric Bed Needs in the United States includes more than 30 mental health professionals and members of the APA administration.
The group was charged with drafting a report that explains and defines the current mental health crisis. They were also charged with developing a method for calculating the number of psychiatric beds needed in any given community.
Task force leaders said the model considers how individuals enter the mental health care system and are routed to appropriate services, how long they remain in the system, and the capacity of the system to respond to demand.
The model is based on a “virtual world” that has a number of care components. These include mobile crisis teams, intensive team-based outpatient care, community-based crisis beds, psychiatric hospital beds, and residential and step-down programs.
The model factors in the magnitude of the need for beds in many service areas. Factors include population size, estimates of the rate of acute mental health crises per 100,000 population, adequacy of the community mental health system, the intersection between the mental health and criminal justice systems, and outpatient and inpatient capacities.
The model computes the estimated number of patients waiting in the emergency department, crisis receiving centers, and jail, as well as average wait times. It also calculates the percentage of use of the various services.
The model will be continually updated and can be modified to better reflect the current situation in any given community.
Real-world testing
A team led by the University of Michigan, Ann Arbor, and two area hospitals is testing the APA model by using it to calculate the number of beds needed in their community.
“Because the model is focused on the continuum of care services, it allows communities to try to focus on what is the right mix of services needed to try to reduce the need for in-patient hospitalization and measure the impact of development of resources across the continuum, including inpatient beds, to try to achieve the right mix,” Gregory Dalack, MD, chair of the department of psychiatry at the University of Michigan Health System, told this news organization.
Ultimately, Dr. Dalack expects that the model will tell the team something they already know: that additional psychiatric beds are needed in their community.
However, meeting the needs of patients and families is not just about beds, he noted. The model will help provide a fuller picture of psychiatric care and will take into account existing services from many aspects of the care field.
“If we put all the focus just on hospital beds, we are only addressing one part of the challenge,” Dr. Dalack said.
The challenge is also about “identifying what resources/services are already in the continuum of care, where expansion of those or development of new programs might be needed, and what the impact is on the system, particularly with folks who arrive in the emergency room who might need inpatient admission,” he added.
Dr. Everett said the APA leadership team is now actively recruiting others to test the model in their communities, which will help to calibrate the system.
A version of this article first appeared on Medscape.com.
New panic disorder model flags risk for recurrence, persistence
Investigators based in France and the United States analyzed data for almost 800 patients with DSM-IV–diagnosed PD.
Results showed that having a “general psychopathology factor,” defined as the shared effects of all comorbid conditions, or PD liability, significantly and independently predicted 3-year recurrence or persistence of PD symptoms.
Having a lower physical health-related quality of life (QOL), a greater number of stressful life events, and not seeking treatment at baseline were also significant and independent predictors.
“This integrative model could help clinicians to identify individuals at high risk of recurrence or persistence of panic disorder and provide content for future research,” Valentin Scheer, MD, MPH, a resident in psychiatry at AP-HP, Assistance Publique, Hôpitaux de Paris, and colleagues wrote.
The findings were published online in the Journal of Clinical Psychiatry.
Integration needed
PD is a disabling disorder with a “chronic course” – and a recurrence rate ranging from 25% to 50%, the investigators noted.
“Because of the heterogeneous course of PD, there is a need to develop a comprehensive predictive model of recurrence or persistence,” they wrote. This could “help practitioners adapt therapeutic strategies and develop prevention strategies in high-risk individuals.”
Most previous studies that have investigated risk factors for PD recurrence and persistence have relied on clinical samples, often with limited sample sizes.
Moreover, each risk factor, when considered individually, accounts for only a “small proportion” of the variance in risk, the researchers noted. The co-occurrence of these risk factors “suggests the need to combine them into a broad multivariable model.”
However, currently proposed integrative models do not identify independent predictors or mitigate the influence of confounding variables. To fill this gap, the investigators conducted a study using structural equation modeling “to take into account multiple correlations across predictors.”
They drew on data from 775 participants (mean age, 40 years) in the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC). For the current analysis, they examined two waves of NESARC (2001-2002 and 2004-2005) to “build a comprehensive model” of the 3-year recurrence or persistence of PD.
The researchers used a “latent variable approach” that simultaneously examined the effect of the following five groups of potential predictors of recurrence or persistence: PD severity, severity of comorbidity, family history of psychiatric disorders, sociodemographic characteristics, and treatment-seeking behavior.
They also distinguished between risk factors responsible for recurrence and those responsible for persistence.
Psychiatric diagnoses were determined on the basis of the Alcohol Use Disorder and Associated Disabilities Interview Schedule-IV. Participants also completed Version 2 of the Short Form 12-Item Health Survey, which assesses both mental and physical QOL over the previous 4 weeks.
Early treatment needed
Among participants with a 12-month diagnosis of PD at wave 1, 13% had persistent PD and 27.6% had recurrent PD during the 3-year period. The mean duration of illness was 9.5 years.
A greater number of lifetime panic attacks, the presence of any Axis I or II comorbid disorder, and any Axis I disorder, especially social anxiety disorder, were significantly associated with 3-year risk for recurrence and for persistence.
Sweating, choking, paresthesias, the comorbid disorders of mania/hypomania and general anxiety disorder, nicotine dependence, lower mental and physical QOL scores, and exposure to a greater number of stressful life events in the previous year were all significantly associated with 3-year risk for recurrence.
Only variables shown with a P value were statistically significant, “with the a priori fixed at .05,” the researchers noted.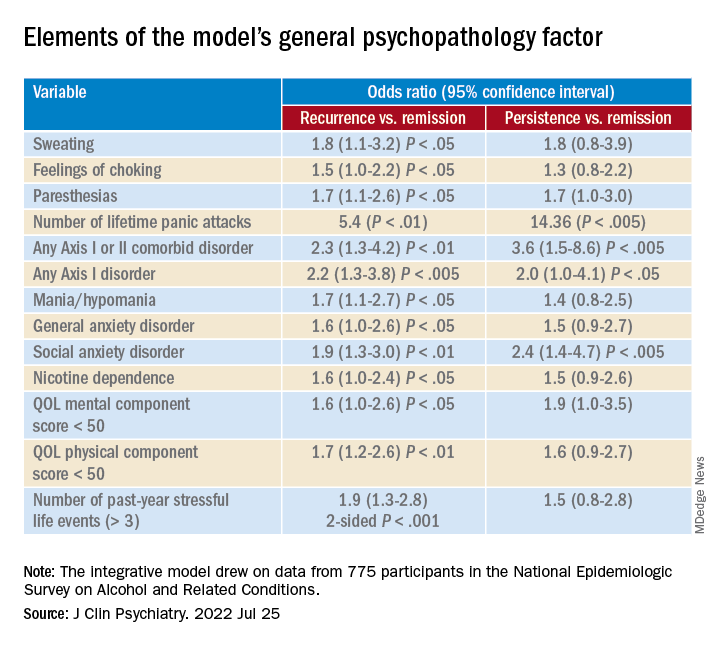
A combination of psychopathology factors, such as the shared effect of all comorbid psychiatric conditions, PD liability, lower physical health-related QOL, more life stressors during the past year, and not seeking treatment at baseline “significantly and independently” predicted recurrence or persistence of symptoms between the two waves (all Ps < .05), the investigators reported.
One study limitation cited was that several psychiatric disorders known to be associated with PD recurrence or persistence, such as borderline personality disorder, were not examined. Additionally, the study used a 3-year follow-up period – and the results might have differed for other follow-up time frames, the researchers noted.
Nevertheless, the findings constitute a “comprehensive model” to predict recurrence and persistence of PD, they wrote. Moreover, early treatment-seeking behavior “should be promoted, as it may reduce the risk of recurrence.”
Not much new?
Commenting on the study, Peter Roy-Byrne, MD, professor of psychiatry, University of Washington, Seattle, noted, “there is not much that is new here.”
Dr. Roy-Byrne, who was not involved with the study, said that a “general theme for years has been that more severe illness, whether you measure it by greater number of other Axis I disorders or symptom severity or a general psychopathology factor, usually predicts worse outcome – here codified as persistence and recurrence.”
Greater stress and reluctance to seek treatment may also predict worse outcomes, he noted.
In addition, the study “did not examine another very important factor: the degree of social connection/social support that someone has,” Dr. Roy-Byrne said. However, “perhaps some of this was contained in specific life events.”
A version of this article first appeared on Medscape.com.
Investigators based in France and the United States analyzed data for almost 800 patients with DSM-IV–diagnosed PD.
Results showed that having a “general psychopathology factor,” defined as the shared effects of all comorbid conditions, or PD liability, significantly and independently predicted 3-year recurrence or persistence of PD symptoms.
Having a lower physical health-related quality of life (QOL), a greater number of stressful life events, and not seeking treatment at baseline were also significant and independent predictors.
“This integrative model could help clinicians to identify individuals at high risk of recurrence or persistence of panic disorder and provide content for future research,” Valentin Scheer, MD, MPH, a resident in psychiatry at AP-HP, Assistance Publique, Hôpitaux de Paris, and colleagues wrote.
The findings were published online in the Journal of Clinical Psychiatry.
Integration needed
PD is a disabling disorder with a “chronic course” – and a recurrence rate ranging from 25% to 50%, the investigators noted.
“Because of the heterogeneous course of PD, there is a need to develop a comprehensive predictive model of recurrence or persistence,” they wrote. This could “help practitioners adapt therapeutic strategies and develop prevention strategies in high-risk individuals.”
Most previous studies that have investigated risk factors for PD recurrence and persistence have relied on clinical samples, often with limited sample sizes.
Moreover, each risk factor, when considered individually, accounts for only a “small proportion” of the variance in risk, the researchers noted. The co-occurrence of these risk factors “suggests the need to combine them into a broad multivariable model.”
However, currently proposed integrative models do not identify independent predictors or mitigate the influence of confounding variables. To fill this gap, the investigators conducted a study using structural equation modeling “to take into account multiple correlations across predictors.”
They drew on data from 775 participants (mean age, 40 years) in the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC). For the current analysis, they examined two waves of NESARC (2001-2002 and 2004-2005) to “build a comprehensive model” of the 3-year recurrence or persistence of PD.
The researchers used a “latent variable approach” that simultaneously examined the effect of the following five groups of potential predictors of recurrence or persistence: PD severity, severity of comorbidity, family history of psychiatric disorders, sociodemographic characteristics, and treatment-seeking behavior.
They also distinguished between risk factors responsible for recurrence and those responsible for persistence.
Psychiatric diagnoses were determined on the basis of the Alcohol Use Disorder and Associated Disabilities Interview Schedule-IV. Participants also completed Version 2 of the Short Form 12-Item Health Survey, which assesses both mental and physical QOL over the previous 4 weeks.
Early treatment needed
Among participants with a 12-month diagnosis of PD at wave 1, 13% had persistent PD and 27.6% had recurrent PD during the 3-year period. The mean duration of illness was 9.5 years.
A greater number of lifetime panic attacks, the presence of any Axis I or II comorbid disorder, and any Axis I disorder, especially social anxiety disorder, were significantly associated with 3-year risk for recurrence and for persistence.
Sweating, choking, paresthesias, the comorbid disorders of mania/hypomania and general anxiety disorder, nicotine dependence, lower mental and physical QOL scores, and exposure to a greater number of stressful life events in the previous year were all significantly associated with 3-year risk for recurrence.
Only variables shown with a P value were statistically significant, “with the a priori fixed at .05,” the researchers noted.
A combination of psychopathology factors, such as the shared effect of all comorbid psychiatric conditions, PD liability, lower physical health-related QOL, more life stressors during the past year, and not seeking treatment at baseline “significantly and independently” predicted recurrence or persistence of symptoms between the two waves (all Ps < .05), the investigators reported.
One study limitation cited was that several psychiatric disorders known to be associated with PD recurrence or persistence, such as borderline personality disorder, were not examined. Additionally, the study used a 3-year follow-up period – and the results might have differed for other follow-up time frames, the researchers noted.
Nevertheless, the findings constitute a “comprehensive model” to predict recurrence and persistence of PD, they wrote. Moreover, early treatment-seeking behavior “should be promoted, as it may reduce the risk of recurrence.”
Not much new?
Commenting on the study, Peter Roy-Byrne, MD, professor of psychiatry, University of Washington, Seattle, noted, “there is not much that is new here.”
Dr. Roy-Byrne, who was not involved with the study, said that a “general theme for years has been that more severe illness, whether you measure it by greater number of other Axis I disorders or symptom severity or a general psychopathology factor, usually predicts worse outcome – here codified as persistence and recurrence.”
Greater stress and reluctance to seek treatment may also predict worse outcomes, he noted.
In addition, the study “did not examine another very important factor: the degree of social connection/social support that someone has,” Dr. Roy-Byrne said. However, “perhaps some of this was contained in specific life events.”
A version of this article first appeared on Medscape.com.
Investigators based in France and the United States analyzed data for almost 800 patients with DSM-IV–diagnosed PD.
Results showed that having a “general psychopathology factor,” defined as the shared effects of all comorbid conditions, or PD liability, significantly and independently predicted 3-year recurrence or persistence of PD symptoms.
Having a lower physical health-related quality of life (QOL), a greater number of stressful life events, and not seeking treatment at baseline were also significant and independent predictors.
“This integrative model could help clinicians to identify individuals at high risk of recurrence or persistence of panic disorder and provide content for future research,” Valentin Scheer, MD, MPH, a resident in psychiatry at AP-HP, Assistance Publique, Hôpitaux de Paris, and colleagues wrote.
The findings were published online in the Journal of Clinical Psychiatry.
Integration needed
PD is a disabling disorder with a “chronic course” – and a recurrence rate ranging from 25% to 50%, the investigators noted.
“Because of the heterogeneous course of PD, there is a need to develop a comprehensive predictive model of recurrence or persistence,” they wrote. This could “help practitioners adapt therapeutic strategies and develop prevention strategies in high-risk individuals.”
Most previous studies that have investigated risk factors for PD recurrence and persistence have relied on clinical samples, often with limited sample sizes.
Moreover, each risk factor, when considered individually, accounts for only a “small proportion” of the variance in risk, the researchers noted. The co-occurrence of these risk factors “suggests the need to combine them into a broad multivariable model.”
However, currently proposed integrative models do not identify independent predictors or mitigate the influence of confounding variables. To fill this gap, the investigators conducted a study using structural equation modeling “to take into account multiple correlations across predictors.”
They drew on data from 775 participants (mean age, 40 years) in the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC). For the current analysis, they examined two waves of NESARC (2001-2002 and 2004-2005) to “build a comprehensive model” of the 3-year recurrence or persistence of PD.
The researchers used a “latent variable approach” that simultaneously examined the effect of the following five groups of potential predictors of recurrence or persistence: PD severity, severity of comorbidity, family history of psychiatric disorders, sociodemographic characteristics, and treatment-seeking behavior.
They also distinguished between risk factors responsible for recurrence and those responsible for persistence.
Psychiatric diagnoses were determined on the basis of the Alcohol Use Disorder and Associated Disabilities Interview Schedule-IV. Participants also completed Version 2 of the Short Form 12-Item Health Survey, which assesses both mental and physical QOL over the previous 4 weeks.
Early treatment needed
Among participants with a 12-month diagnosis of PD at wave 1, 13% had persistent PD and 27.6% had recurrent PD during the 3-year period. The mean duration of illness was 9.5 years.
A greater number of lifetime panic attacks, the presence of any Axis I or II comorbid disorder, and any Axis I disorder, especially social anxiety disorder, were significantly associated with 3-year risk for recurrence and for persistence.
Sweating, choking, paresthesias, the comorbid disorders of mania/hypomania and general anxiety disorder, nicotine dependence, lower mental and physical QOL scores, and exposure to a greater number of stressful life events in the previous year were all significantly associated with 3-year risk for recurrence.
Only variables shown with a P value were statistically significant, “with the a priori fixed at .05,” the researchers noted.
A combination of psychopathology factors, such as the shared effect of all comorbid psychiatric conditions, PD liability, lower physical health-related QOL, more life stressors during the past year, and not seeking treatment at baseline “significantly and independently” predicted recurrence or persistence of symptoms between the two waves (all Ps < .05), the investigators reported.
One study limitation cited was that several psychiatric disorders known to be associated with PD recurrence or persistence, such as borderline personality disorder, were not examined. Additionally, the study used a 3-year follow-up period – and the results might have differed for other follow-up time frames, the researchers noted.
Nevertheless, the findings constitute a “comprehensive model” to predict recurrence and persistence of PD, they wrote. Moreover, early treatment-seeking behavior “should be promoted, as it may reduce the risk of recurrence.”
Not much new?
Commenting on the study, Peter Roy-Byrne, MD, professor of psychiatry, University of Washington, Seattle, noted, “there is not much that is new here.”
Dr. Roy-Byrne, who was not involved with the study, said that a “general theme for years has been that more severe illness, whether you measure it by greater number of other Axis I disorders or symptom severity or a general psychopathology factor, usually predicts worse outcome – here codified as persistence and recurrence.”
Greater stress and reluctance to seek treatment may also predict worse outcomes, he noted.
In addition, the study “did not examine another very important factor: the degree of social connection/social support that someone has,” Dr. Roy-Byrne said. However, “perhaps some of this was contained in specific life events.”
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF CLINICAL PSYCHIATRY
Siblings of children with chronic health conditions may have increased mental health risks
Siblings of children with chronic health conditions could be at an increased risk for depression, according to a new report.
In a systematic review of 34 studies, siblings of children with chronic health conditions had significantly higher scores on depressive rating scales than individuals without a sibling with a chronic health condition (standardized mean difference = 0.53; P < .001). Findings related to other clinical health outcomes, such as physical health conditions or mortality, were inconsistent.
“We’ve known for a long time that siblings of kids with chronic conditions undergo stress, and there have been conflicting data on how that stress is manifested in terms of their own health,” senior study author Eyal Cohen, MD, program head for child health evaluative sciences at the Hospital for Sick Children, Toronto, told this news organization.
“For some siblings, having the experience of being raised with a child with a chronic condition may be an asset and build resiliency, while other siblings may feel strong negative emotions, such as sadness, anger, and fear,” he said. “Although we know that this experience is stressful for many siblings, it is important to know whether it changes their health outcomes, so that appropriate support can be put in place for those who need it.”
The study was published online in the Journal of Pediatrics.
Risk for psychological challenges
About a quarter of children in the United States have a mental, emotional, developmental, or behavioral condition, and more than a third have at least one current or lifelong health condition, the study authors write. A childhood chronic health condition can affect family members through worse mental health outcomes, increased stress, and poorer health-related quality of life.
Dr. Cohen and colleagues conducted a systematic review and meta-analysis to assess the clinical mental and physical health outcomes of siblings of children with chronic health conditions in comparison with siblings of healthy children or normative data.
The research team included English-language studies that reported on clinically diagnosable mental or physical health outcomes among siblings of persons younger than 18 years who had a chronic health condition. They included a comparison group and used an experimental or observational design for their study. The researchers analyzed 34 studies, including 28 that reported on mental health, 3 that reported on physical health, and 3 that reported on mortality.
Overall, siblings of children with chronic health conditions had significantly higher scores on depression rating scales than their comparison groups. Siblings’ anxiety scores weren’t substantially higher, however (standard mean difference = 0.21; P = .07).
The effects for confirmed psychiatric diagnoses, physical health outcomes, and mortality could not be included in the meta-analysis, owing to the limited number of studies and the high level of heterogeneity among the studies.
Dr. Cohen noted that although the researchers weren’t surprised that siblings may be at increased risk of mental health challenges, they were surprised by the limited data regarding physical health.
“At a minimum, our findings support the importance of asking open-ended questions about how a family is doing during clinical encounters,” he said. “These siblings may also benefit from programs such as support groups or summer camps, which have been shown to improve mental health and behavioral outcomes in siblings of children with chronic health conditions, such as cancer and neurodevelopmental disabilities.”
Future studies should assess the specific risk factors for mental health problems in siblings of children with chronic health conditions, Dr. Cohen said. Additional research could also investigate the design and effectiveness of interventions that address these concerns.
Message of inclusiveness
“The message that resonates with me is about the interventions and resources needed to support siblings,” Linda Nguyen, a doctoral student in rehabilitation science and researcher with the CanChild Center for Childhood Disability Research at McMaster University in Hamilton, Ont., told this news organization.
Ms. Nguyen, who wasn’t involved with this study, has researched the resources available to siblings in Canada and has found a lack of support options, particularly when it comes to specific health care management roles.
“Consistently throughout my research, I’ve seen the need for resources that go beyond a focus on siblings’ well-being and instead support them in their different roles,” she said. “Some want to be friends, mentors, supporters, and caregivers for their siblings in the future.”
Siblings often adopt different roles as they form their own identity, Ms. Nguyen noted, which becomes a larger part of the health care conversation as children with chronic conditions make the transition from pediatric to adult health care. Siblings want to be asked how they’d like to be involved, she said. Some would like to be involved with health care appointments, the chronic condition community, research, and policy making.
“At the societal level and public level, there’s also a message of inclusiveness and making sure that we’re welcoming youth with disabilities and chronic conditions,” Jan Willem Gorter, MD, PhD, a professor of pediatrics and scientist for CanChild at McMaster University, told this news organization.
Dr. Gorter, who also was not involved with this study, noted that children with chronic conditions often feel left behind, which can influence the involvement of their siblings as well.
“There are a lot of places in the world where children with disabilities go to special schools, and they spend a lot of time in a different world, with different experiences than their siblings,” he said. “At the public health level, we want to advocate for an inclusive society and support the whole family, which benefits everybody.”
The study was funded by the Canadian Institutes of Health Research and the CHILD-BRIGHT Network summer studentship, which is supported by the Canadian Institute for Health Research Strategy for Patient-Oriented Research. Dr. Cohen, Ms. Nguyen, and Dr. Gorter have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Siblings of children with chronic health conditions could be at an increased risk for depression, according to a new report.
In a systematic review of 34 studies, siblings of children with chronic health conditions had significantly higher scores on depressive rating scales than individuals without a sibling with a chronic health condition (standardized mean difference = 0.53; P < .001). Findings related to other clinical health outcomes, such as physical health conditions or mortality, were inconsistent.
“We’ve known for a long time that siblings of kids with chronic conditions undergo stress, and there have been conflicting data on how that stress is manifested in terms of their own health,” senior study author Eyal Cohen, MD, program head for child health evaluative sciences at the Hospital for Sick Children, Toronto, told this news organization.
“For some siblings, having the experience of being raised with a child with a chronic condition may be an asset and build resiliency, while other siblings may feel strong negative emotions, such as sadness, anger, and fear,” he said. “Although we know that this experience is stressful for many siblings, it is important to know whether it changes their health outcomes, so that appropriate support can be put in place for those who need it.”
The study was published online in the Journal of Pediatrics.
Risk for psychological challenges
About a quarter of children in the United States have a mental, emotional, developmental, or behavioral condition, and more than a third have at least one current or lifelong health condition, the study authors write. A childhood chronic health condition can affect family members through worse mental health outcomes, increased stress, and poorer health-related quality of life.
Dr. Cohen and colleagues conducted a systematic review and meta-analysis to assess the clinical mental and physical health outcomes of siblings of children with chronic health conditions in comparison with siblings of healthy children or normative data.
The research team included English-language studies that reported on clinically diagnosable mental or physical health outcomes among siblings of persons younger than 18 years who had a chronic health condition. They included a comparison group and used an experimental or observational design for their study. The researchers analyzed 34 studies, including 28 that reported on mental health, 3 that reported on physical health, and 3 that reported on mortality.
Overall, siblings of children with chronic health conditions had significantly higher scores on depression rating scales than their comparison groups. Siblings’ anxiety scores weren’t substantially higher, however (standard mean difference = 0.21; P = .07).
The effects for confirmed psychiatric diagnoses, physical health outcomes, and mortality could not be included in the meta-analysis, owing to the limited number of studies and the high level of heterogeneity among the studies.
Dr. Cohen noted that although the researchers weren’t surprised that siblings may be at increased risk of mental health challenges, they were surprised by the limited data regarding physical health.
“At a minimum, our findings support the importance of asking open-ended questions about how a family is doing during clinical encounters,” he said. “These siblings may also benefit from programs such as support groups or summer camps, which have been shown to improve mental health and behavioral outcomes in siblings of children with chronic health conditions, such as cancer and neurodevelopmental disabilities.”
Future studies should assess the specific risk factors for mental health problems in siblings of children with chronic health conditions, Dr. Cohen said. Additional research could also investigate the design and effectiveness of interventions that address these concerns.
Message of inclusiveness
“The message that resonates with me is about the interventions and resources needed to support siblings,” Linda Nguyen, a doctoral student in rehabilitation science and researcher with the CanChild Center for Childhood Disability Research at McMaster University in Hamilton, Ont., told this news organization.
Ms. Nguyen, who wasn’t involved with this study, has researched the resources available to siblings in Canada and has found a lack of support options, particularly when it comes to specific health care management roles.
“Consistently throughout my research, I’ve seen the need for resources that go beyond a focus on siblings’ well-being and instead support them in their different roles,” she said. “Some want to be friends, mentors, supporters, and caregivers for their siblings in the future.”
Siblings often adopt different roles as they form their own identity, Ms. Nguyen noted, which becomes a larger part of the health care conversation as children with chronic conditions make the transition from pediatric to adult health care. Siblings want to be asked how they’d like to be involved, she said. Some would like to be involved with health care appointments, the chronic condition community, research, and policy making.
“At the societal level and public level, there’s also a message of inclusiveness and making sure that we’re welcoming youth with disabilities and chronic conditions,” Jan Willem Gorter, MD, PhD, a professor of pediatrics and scientist for CanChild at McMaster University, told this news organization.
Dr. Gorter, who also was not involved with this study, noted that children with chronic conditions often feel left behind, which can influence the involvement of their siblings as well.
“There are a lot of places in the world where children with disabilities go to special schools, and they spend a lot of time in a different world, with different experiences than their siblings,” he said. “At the public health level, we want to advocate for an inclusive society and support the whole family, which benefits everybody.”
The study was funded by the Canadian Institutes of Health Research and the CHILD-BRIGHT Network summer studentship, which is supported by the Canadian Institute for Health Research Strategy for Patient-Oriented Research. Dr. Cohen, Ms. Nguyen, and Dr. Gorter have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Siblings of children with chronic health conditions could be at an increased risk for depression, according to a new report.
In a systematic review of 34 studies, siblings of children with chronic health conditions had significantly higher scores on depressive rating scales than individuals without a sibling with a chronic health condition (standardized mean difference = 0.53; P < .001). Findings related to other clinical health outcomes, such as physical health conditions or mortality, were inconsistent.
“We’ve known for a long time that siblings of kids with chronic conditions undergo stress, and there have been conflicting data on how that stress is manifested in terms of their own health,” senior study author Eyal Cohen, MD, program head for child health evaluative sciences at the Hospital for Sick Children, Toronto, told this news organization.
“For some siblings, having the experience of being raised with a child with a chronic condition may be an asset and build resiliency, while other siblings may feel strong negative emotions, such as sadness, anger, and fear,” he said. “Although we know that this experience is stressful for many siblings, it is important to know whether it changes their health outcomes, so that appropriate support can be put in place for those who need it.”
The study was published online in the Journal of Pediatrics.
Risk for psychological challenges
About a quarter of children in the United States have a mental, emotional, developmental, or behavioral condition, and more than a third have at least one current or lifelong health condition, the study authors write. A childhood chronic health condition can affect family members through worse mental health outcomes, increased stress, and poorer health-related quality of life.
Dr. Cohen and colleagues conducted a systematic review and meta-analysis to assess the clinical mental and physical health outcomes of siblings of children with chronic health conditions in comparison with siblings of healthy children or normative data.
The research team included English-language studies that reported on clinically diagnosable mental or physical health outcomes among siblings of persons younger than 18 years who had a chronic health condition. They included a comparison group and used an experimental or observational design for their study. The researchers analyzed 34 studies, including 28 that reported on mental health, 3 that reported on physical health, and 3 that reported on mortality.
Overall, siblings of children with chronic health conditions had significantly higher scores on depression rating scales than their comparison groups. Siblings’ anxiety scores weren’t substantially higher, however (standard mean difference = 0.21; P = .07).
The effects for confirmed psychiatric diagnoses, physical health outcomes, and mortality could not be included in the meta-analysis, owing to the limited number of studies and the high level of heterogeneity among the studies.
Dr. Cohen noted that although the researchers weren’t surprised that siblings may be at increased risk of mental health challenges, they were surprised by the limited data regarding physical health.
“At a minimum, our findings support the importance of asking open-ended questions about how a family is doing during clinical encounters,” he said. “These siblings may also benefit from programs such as support groups or summer camps, which have been shown to improve mental health and behavioral outcomes in siblings of children with chronic health conditions, such as cancer and neurodevelopmental disabilities.”
Future studies should assess the specific risk factors for mental health problems in siblings of children with chronic health conditions, Dr. Cohen said. Additional research could also investigate the design and effectiveness of interventions that address these concerns.
Message of inclusiveness
“The message that resonates with me is about the interventions and resources needed to support siblings,” Linda Nguyen, a doctoral student in rehabilitation science and researcher with the CanChild Center for Childhood Disability Research at McMaster University in Hamilton, Ont., told this news organization.
Ms. Nguyen, who wasn’t involved with this study, has researched the resources available to siblings in Canada and has found a lack of support options, particularly when it comes to specific health care management roles.
“Consistently throughout my research, I’ve seen the need for resources that go beyond a focus on siblings’ well-being and instead support them in their different roles,” she said. “Some want to be friends, mentors, supporters, and caregivers for their siblings in the future.”
Siblings often adopt different roles as they form their own identity, Ms. Nguyen noted, which becomes a larger part of the health care conversation as children with chronic conditions make the transition from pediatric to adult health care. Siblings want to be asked how they’d like to be involved, she said. Some would like to be involved with health care appointments, the chronic condition community, research, and policy making.
“At the societal level and public level, there’s also a message of inclusiveness and making sure that we’re welcoming youth with disabilities and chronic conditions,” Jan Willem Gorter, MD, PhD, a professor of pediatrics and scientist for CanChild at McMaster University, told this news organization.
Dr. Gorter, who also was not involved with this study, noted that children with chronic conditions often feel left behind, which can influence the involvement of their siblings as well.
“There are a lot of places in the world where children with disabilities go to special schools, and they spend a lot of time in a different world, with different experiences than their siblings,” he said. “At the public health level, we want to advocate for an inclusive society and support the whole family, which benefits everybody.”
The study was funded by the Canadian Institutes of Health Research and the CHILD-BRIGHT Network summer studentship, which is supported by the Canadian Institute for Health Research Strategy for Patient-Oriented Research. Dr. Cohen, Ms. Nguyen, and Dr. Gorter have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JOURNAL OF PEDIATRICS
Postpartum depression risk higher with family psych history
Mothers who have a family history of any psychiatric disorder have almost two times the risk of postpartum depression as do mothers without such history, according to a new study.
Mette-Marie Zacher Kjeldsen, MSc, with the National Centre for Register-based Research at Aarhus (Denmark) University, led the study, a meta-analysis that included 26 studies with information on 100,877 women.
Findings were published online in JAMA Psychiatry.
When mothers had a family history of psychiatric disorders, the odds ratio for PPD was 2.08 (95% confidence interval, 1.67-2.59). That corresponds to a risk ratio of 1.79 (95% CI, 1.52-2.09), assuming a 15% postpartum depression prevalence in the general population.
Not doomed to develop PPD
Polina Teslyar, MD, a perinatal psychiatrist at Brigham and Women’s Hospital in Boston told this news organization it’s important to point out that though the risk is higher, women with a family psychiatric history should not feel as though they are destined to develop PPD.
“You are still more likely to not have postpartum depression, but it is important to be aware of personal risk factors so that if a person is experiencing that, they ask for help quickly rather than suffering and not knowing something is amiss,” she emphasized. Dr. Teslyar says she does see the higher risk for PPD, which is preventable and treatable, in her own practice when women have had a family history of psychiatric disorders.
The association makes sense, but literature on why that is has been varied, she said, and likely involves both genetics and socioeconomic factors. It’s difficult to tease apart how big a part each plays.
In her perinatal practice she sees women even before they are pregnant to discuss risk factors for PPD so she does ask about family history of psychiatric disorders, specifically about history of PPD and anxiety.
The researchers suggest routine perinatal care should include an easy low-cost, two-part question about both personal and family history of psychiatric disorders.
“As the assessment is possible even prior to conception, this would leave time for planning preventive efforts, such as psychosocial and psychological interventions targeting these at-risk women,” the authors write.
Asking about family history a challenge
Dr. Teslyar noted though that one of the challenges in asking about family history is that families may not have openly shared psychiatric history details with offspring. Family members may also report conditions they suspect a family member had rather than having a documented diagnosis.
In places where there is universal health care, she noted, finding documented diagnoses is easier, but otherwise “you’re really taking a subjective interpretation.”
The researchers found that subgroup, sensitivity, and meta–regression analyses aligned with the primary findings. The overall certainty of evidence was graded as moderate.
This study was not able to make clear how the specific diagnoses of family members affect the risk of developing PPD because much of the data from the studies came from self-report and questions were not consistent across the studies.
For instance, only 7 studies asked specifically about first-degree family members and 10 asked about specific diagnoses. Diagnoses ranged from mild affective disorders to more intrusive disorders, such as schizophrenia.
And while this study doesn’t seek to determine why the family history and risk of PPD appear to be connected, the authors offer some possible explanations.
“Growing up in an environment with parents struggling with mental health problems potentially influences the social support received from these parents when going into motherhood,” the authors write. “This particular explanation is supported by umbrella reviews concluding that lack of social support is a significant PPD risk factor.”
Screening, extraction, and assessment of studies included was done independently by two reviewers, increasing validity, the authors note.
The authors state that approximately 10%-15% of new mothers experience PPD, but Dr. Teslyar points out the numbers in the United States are typically quoted at up to 20%-30%. PPD ranges from mild to severe episodes and includes symptoms like those for major depression outside the postpartum period.
Study authors received funding from The Lundbeck Foundation and the European Union’s Horizon 2020 Research and Innovation Programme. A coauthor, Vibe G. Frokjaer, MD, PhD, has served as consultant and lecturer for H. Lundbeck and Sage Therapeutics. No other disclosures were reported. Dr. Teslyar reports no relevant financial relationships.
Mothers who have a family history of any psychiatric disorder have almost two times the risk of postpartum depression as do mothers without such history, according to a new study.
Mette-Marie Zacher Kjeldsen, MSc, with the National Centre for Register-based Research at Aarhus (Denmark) University, led the study, a meta-analysis that included 26 studies with information on 100,877 women.
Findings were published online in JAMA Psychiatry.
When mothers had a family history of psychiatric disorders, the odds ratio for PPD was 2.08 (95% confidence interval, 1.67-2.59). That corresponds to a risk ratio of 1.79 (95% CI, 1.52-2.09), assuming a 15% postpartum depression prevalence in the general population.
Not doomed to develop PPD
Polina Teslyar, MD, a perinatal psychiatrist at Brigham and Women’s Hospital in Boston told this news organization it’s important to point out that though the risk is higher, women with a family psychiatric history should not feel as though they are destined to develop PPD.
“You are still more likely to not have postpartum depression, but it is important to be aware of personal risk factors so that if a person is experiencing that, they ask for help quickly rather than suffering and not knowing something is amiss,” she emphasized. Dr. Teslyar says she does see the higher risk for PPD, which is preventable and treatable, in her own practice when women have had a family history of psychiatric disorders.
The association makes sense, but literature on why that is has been varied, she said, and likely involves both genetics and socioeconomic factors. It’s difficult to tease apart how big a part each plays.
In her perinatal practice she sees women even before they are pregnant to discuss risk factors for PPD so she does ask about family history of psychiatric disorders, specifically about history of PPD and anxiety.
The researchers suggest routine perinatal care should include an easy low-cost, two-part question about both personal and family history of psychiatric disorders.
“As the assessment is possible even prior to conception, this would leave time for planning preventive efforts, such as psychosocial and psychological interventions targeting these at-risk women,” the authors write.
Asking about family history a challenge
Dr. Teslyar noted though that one of the challenges in asking about family history is that families may not have openly shared psychiatric history details with offspring. Family members may also report conditions they suspect a family member had rather than having a documented diagnosis.
In places where there is universal health care, she noted, finding documented diagnoses is easier, but otherwise “you’re really taking a subjective interpretation.”
The researchers found that subgroup, sensitivity, and meta–regression analyses aligned with the primary findings. The overall certainty of evidence was graded as moderate.
This study was not able to make clear how the specific diagnoses of family members affect the risk of developing PPD because much of the data from the studies came from self-report and questions were not consistent across the studies.
For instance, only 7 studies asked specifically about first-degree family members and 10 asked about specific diagnoses. Diagnoses ranged from mild affective disorders to more intrusive disorders, such as schizophrenia.
And while this study doesn’t seek to determine why the family history and risk of PPD appear to be connected, the authors offer some possible explanations.
“Growing up in an environment with parents struggling with mental health problems potentially influences the social support received from these parents when going into motherhood,” the authors write. “This particular explanation is supported by umbrella reviews concluding that lack of social support is a significant PPD risk factor.”
Screening, extraction, and assessment of studies included was done independently by two reviewers, increasing validity, the authors note.
The authors state that approximately 10%-15% of new mothers experience PPD, but Dr. Teslyar points out the numbers in the United States are typically quoted at up to 20%-30%. PPD ranges from mild to severe episodes and includes symptoms like those for major depression outside the postpartum period.
Study authors received funding from The Lundbeck Foundation and the European Union’s Horizon 2020 Research and Innovation Programme. A coauthor, Vibe G. Frokjaer, MD, PhD, has served as consultant and lecturer for H. Lundbeck and Sage Therapeutics. No other disclosures were reported. Dr. Teslyar reports no relevant financial relationships.
Mothers who have a family history of any psychiatric disorder have almost two times the risk of postpartum depression as do mothers without such history, according to a new study.
Mette-Marie Zacher Kjeldsen, MSc, with the National Centre for Register-based Research at Aarhus (Denmark) University, led the study, a meta-analysis that included 26 studies with information on 100,877 women.
Findings were published online in JAMA Psychiatry.
When mothers had a family history of psychiatric disorders, the odds ratio for PPD was 2.08 (95% confidence interval, 1.67-2.59). That corresponds to a risk ratio of 1.79 (95% CI, 1.52-2.09), assuming a 15% postpartum depression prevalence in the general population.
Not doomed to develop PPD
Polina Teslyar, MD, a perinatal psychiatrist at Brigham and Women’s Hospital in Boston told this news organization it’s important to point out that though the risk is higher, women with a family psychiatric history should not feel as though they are destined to develop PPD.
“You are still more likely to not have postpartum depression, but it is important to be aware of personal risk factors so that if a person is experiencing that, they ask for help quickly rather than suffering and not knowing something is amiss,” she emphasized. Dr. Teslyar says she does see the higher risk for PPD, which is preventable and treatable, in her own practice when women have had a family history of psychiatric disorders.
The association makes sense, but literature on why that is has been varied, she said, and likely involves both genetics and socioeconomic factors. It’s difficult to tease apart how big a part each plays.
In her perinatal practice she sees women even before they are pregnant to discuss risk factors for PPD so she does ask about family history of psychiatric disorders, specifically about history of PPD and anxiety.
The researchers suggest routine perinatal care should include an easy low-cost, two-part question about both personal and family history of psychiatric disorders.
“As the assessment is possible even prior to conception, this would leave time for planning preventive efforts, such as psychosocial and psychological interventions targeting these at-risk women,” the authors write.
Asking about family history a challenge
Dr. Teslyar noted though that one of the challenges in asking about family history is that families may not have openly shared psychiatric history details with offspring. Family members may also report conditions they suspect a family member had rather than having a documented diagnosis.
In places where there is universal health care, she noted, finding documented diagnoses is easier, but otherwise “you’re really taking a subjective interpretation.”
The researchers found that subgroup, sensitivity, and meta–regression analyses aligned with the primary findings. The overall certainty of evidence was graded as moderate.
This study was not able to make clear how the specific diagnoses of family members affect the risk of developing PPD because much of the data from the studies came from self-report and questions were not consistent across the studies.
For instance, only 7 studies asked specifically about first-degree family members and 10 asked about specific diagnoses. Diagnoses ranged from mild affective disorders to more intrusive disorders, such as schizophrenia.
And while this study doesn’t seek to determine why the family history and risk of PPD appear to be connected, the authors offer some possible explanations.
“Growing up in an environment with parents struggling with mental health problems potentially influences the social support received from these parents when going into motherhood,” the authors write. “This particular explanation is supported by umbrella reviews concluding that lack of social support is a significant PPD risk factor.”
Screening, extraction, and assessment of studies included was done independently by two reviewers, increasing validity, the authors note.
The authors state that approximately 10%-15% of new mothers experience PPD, but Dr. Teslyar points out the numbers in the United States are typically quoted at up to 20%-30%. PPD ranges from mild to severe episodes and includes symptoms like those for major depression outside the postpartum period.
Study authors received funding from The Lundbeck Foundation and the European Union’s Horizon 2020 Research and Innovation Programme. A coauthor, Vibe G. Frokjaer, MD, PhD, has served as consultant and lecturer for H. Lundbeck and Sage Therapeutics. No other disclosures were reported. Dr. Teslyar reports no relevant financial relationships.
FROM JAMA PSYCHIATRY
COVID-19 may trigger irritable bowel syndrome
Gastrointestinal symptoms are common with long COVID, also known as post-acute COVID-19 syndrome, according to Walter Chan, MD, MPH, and Madhusudan Grover, MBBS.
Dr. Chan, an assistant professor at Harvard Medical School, Boston, and Dr. Grover, an associate professor of medicine and physiology at Mayo Clinic, Rochester, Minn., conducted a review of the literature on COVID-19’s long-term gastrointestinal effects. Their review was published in Clinical Gastroenterology and Hepatology.
Estimates of the prevalence of gastrointestinal symptoms with COVID-19 have ranged as high as 60%, Dr. Chan and Dr. Grover report, and the symptoms may be present in patients with long COVID, a syndrome that continues 4 weeks or longer.
In one survey of 749 COVID-19 survivors, 29% reported at least one new chronic gastrointestinal symptom. The most common were heartburn, constipation, diarrhea, and abdominal pain. Of those with abdominal pain, 39% had symptoms that met Rome IV criteria for irritable bowel syndrome.
People who have gastrointestinal symptoms after their initial SARS-CoV-2 infection are more likely to have them with long COVID. Psychiatric diagnoses, hospitalization, and the loss of smell and taste are predictors of gastrointestinal symptoms.
Infectious gastroenteritis can increase the risk for disorders of gut-brain interaction, especially postinfection IBS, Dr. Chan and Dr. Grover write.
COVID-19 likely causes gastrointestinal symptoms through multiple mechanisms. It may suppress angiotensin-converting enzyme 2, which protects intestinal cells. It can alter the microbiome. It can cause or worsen weight gain and diabetes. It may disrupt the immune system and trigger an autoimmune reaction. It can cause depression and anxiety, and it can alter dietary habits.
No specific treatments for gastrointestinal symptoms associated with long COVID have emerged, so clinicians should make use of established therapies for disorders of gut-brain interaction, Dr. Chan and Dr. Grover recommend.
Beyond adequate sleep and exercise, these may include high-fiber, low FODMAP (fermentable oligosaccharides, disaccharides, monosaccharides, and polyols), gluten-free, low-carbohydrate, or elimination diets.
For diarrhea, they list loperamide, ondansetron, alosetron, eluxadoline, antispasmodics, rifaximin, and bile acid sequestrants.
For constipation, they mention fiber supplements, polyethylene glycol, linaclotide, plecanatide, lubiprostone, tenapanor, tegaserod, and prucalopride.
For modulating intestinal permeability, they recommend glutamine.
Neuromodulation may be achieved with tricyclic antidepressants, selective serotonin reuptake inhibitors, serotonin norepinephrine reuptake inhibitors, azaperones, and delta ligands, they write.
For psychological therapy, they recommend cognitive-behavioral therapy and gut-directed hypnotherapy.
A handful of studies have suggested benefits from Lactiplantibacillus plantarum and Pediococcus acidilactici as probiotic therapies. Additionally, one study showed positive results with a high-fiber formula, perhaps by nourishing short-chain fatty acid-producing bacteria, Dr. Chan and Dr. Grover write.
Dr. Chan reported financial relationships with Ironwood, Takeda, and Phathom Pharmaceuticals. Dr. Grover reported financial relationships with Takeda, Donga, Alexza Pharmaceuticals, and Alfasigma.
A version of this article first appeared on Medscape.com.
Gastrointestinal symptoms are common with long COVID, also known as post-acute COVID-19 syndrome, according to Walter Chan, MD, MPH, and Madhusudan Grover, MBBS.
Dr. Chan, an assistant professor at Harvard Medical School, Boston, and Dr. Grover, an associate professor of medicine and physiology at Mayo Clinic, Rochester, Minn., conducted a review of the literature on COVID-19’s long-term gastrointestinal effects. Their review was published in Clinical Gastroenterology and Hepatology.
Estimates of the prevalence of gastrointestinal symptoms with COVID-19 have ranged as high as 60%, Dr. Chan and Dr. Grover report, and the symptoms may be present in patients with long COVID, a syndrome that continues 4 weeks or longer.
In one survey of 749 COVID-19 survivors, 29% reported at least one new chronic gastrointestinal symptom. The most common were heartburn, constipation, diarrhea, and abdominal pain. Of those with abdominal pain, 39% had symptoms that met Rome IV criteria for irritable bowel syndrome.
People who have gastrointestinal symptoms after their initial SARS-CoV-2 infection are more likely to have them with long COVID. Psychiatric diagnoses, hospitalization, and the loss of smell and taste are predictors of gastrointestinal symptoms.
Infectious gastroenteritis can increase the risk for disorders of gut-brain interaction, especially postinfection IBS, Dr. Chan and Dr. Grover write.
COVID-19 likely causes gastrointestinal symptoms through multiple mechanisms. It may suppress angiotensin-converting enzyme 2, which protects intestinal cells. It can alter the microbiome. It can cause or worsen weight gain and diabetes. It may disrupt the immune system and trigger an autoimmune reaction. It can cause depression and anxiety, and it can alter dietary habits.
No specific treatments for gastrointestinal symptoms associated with long COVID have emerged, so clinicians should make use of established therapies for disorders of gut-brain interaction, Dr. Chan and Dr. Grover recommend.
Beyond adequate sleep and exercise, these may include high-fiber, low FODMAP (fermentable oligosaccharides, disaccharides, monosaccharides, and polyols), gluten-free, low-carbohydrate, or elimination diets.
For diarrhea, they list loperamide, ondansetron, alosetron, eluxadoline, antispasmodics, rifaximin, and bile acid sequestrants.
For constipation, they mention fiber supplements, polyethylene glycol, linaclotide, plecanatide, lubiprostone, tenapanor, tegaserod, and prucalopride.
For modulating intestinal permeability, they recommend glutamine.
Neuromodulation may be achieved with tricyclic antidepressants, selective serotonin reuptake inhibitors, serotonin norepinephrine reuptake inhibitors, azaperones, and delta ligands, they write.
For psychological therapy, they recommend cognitive-behavioral therapy and gut-directed hypnotherapy.
A handful of studies have suggested benefits from Lactiplantibacillus plantarum and Pediococcus acidilactici as probiotic therapies. Additionally, one study showed positive results with a high-fiber formula, perhaps by nourishing short-chain fatty acid-producing bacteria, Dr. Chan and Dr. Grover write.
Dr. Chan reported financial relationships with Ironwood, Takeda, and Phathom Pharmaceuticals. Dr. Grover reported financial relationships with Takeda, Donga, Alexza Pharmaceuticals, and Alfasigma.
A version of this article first appeared on Medscape.com.
Gastrointestinal symptoms are common with long COVID, also known as post-acute COVID-19 syndrome, according to Walter Chan, MD, MPH, and Madhusudan Grover, MBBS.
Dr. Chan, an assistant professor at Harvard Medical School, Boston, and Dr. Grover, an associate professor of medicine and physiology at Mayo Clinic, Rochester, Minn., conducted a review of the literature on COVID-19’s long-term gastrointestinal effects. Their review was published in Clinical Gastroenterology and Hepatology.
Estimates of the prevalence of gastrointestinal symptoms with COVID-19 have ranged as high as 60%, Dr. Chan and Dr. Grover report, and the symptoms may be present in patients with long COVID, a syndrome that continues 4 weeks or longer.
In one survey of 749 COVID-19 survivors, 29% reported at least one new chronic gastrointestinal symptom. The most common were heartburn, constipation, diarrhea, and abdominal pain. Of those with abdominal pain, 39% had symptoms that met Rome IV criteria for irritable bowel syndrome.
People who have gastrointestinal symptoms after their initial SARS-CoV-2 infection are more likely to have them with long COVID. Psychiatric diagnoses, hospitalization, and the loss of smell and taste are predictors of gastrointestinal symptoms.
Infectious gastroenteritis can increase the risk for disorders of gut-brain interaction, especially postinfection IBS, Dr. Chan and Dr. Grover write.
COVID-19 likely causes gastrointestinal symptoms through multiple mechanisms. It may suppress angiotensin-converting enzyme 2, which protects intestinal cells. It can alter the microbiome. It can cause or worsen weight gain and diabetes. It may disrupt the immune system and trigger an autoimmune reaction. It can cause depression and anxiety, and it can alter dietary habits.
No specific treatments for gastrointestinal symptoms associated with long COVID have emerged, so clinicians should make use of established therapies for disorders of gut-brain interaction, Dr. Chan and Dr. Grover recommend.
Beyond adequate sleep and exercise, these may include high-fiber, low FODMAP (fermentable oligosaccharides, disaccharides, monosaccharides, and polyols), gluten-free, low-carbohydrate, or elimination diets.
For diarrhea, they list loperamide, ondansetron, alosetron, eluxadoline, antispasmodics, rifaximin, and bile acid sequestrants.
For constipation, they mention fiber supplements, polyethylene glycol, linaclotide, plecanatide, lubiprostone, tenapanor, tegaserod, and prucalopride.
For modulating intestinal permeability, they recommend glutamine.
Neuromodulation may be achieved with tricyclic antidepressants, selective serotonin reuptake inhibitors, serotonin norepinephrine reuptake inhibitors, azaperones, and delta ligands, they write.
For psychological therapy, they recommend cognitive-behavioral therapy and gut-directed hypnotherapy.
A handful of studies have suggested benefits from Lactiplantibacillus plantarum and Pediococcus acidilactici as probiotic therapies. Additionally, one study showed positive results with a high-fiber formula, perhaps by nourishing short-chain fatty acid-producing bacteria, Dr. Chan and Dr. Grover write.
Dr. Chan reported financial relationships with Ironwood, Takeda, and Phathom Pharmaceuticals. Dr. Grover reported financial relationships with Takeda, Donga, Alexza Pharmaceuticals, and Alfasigma.
A version of this article first appeared on Medscape.com.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Early dementia but no specialists: Reinforcements needed?
Patients in rural areas are also less likely to see psychologists and undergo neuropsychological testing, according to the study, published in JAMA Network Open.
Patients who forgo such specialist visits and testing may be missing information about their condition that could help them prepare for changes in job responsibilities and future care decisions, said Wendy Yi Xu, PhD, of The Ohio State University, Columbus, who led the research.
“A lot of them are still in the workforce,” Dr. Xu said. Patients in the study were an average age of 56 years, well before the conventional age of retirement.
Location, location, location
To examine rural versus urban differences in the use of diagnostic tests and health care visits for early onset Alzheimer’s disease and related dementias, Dr. Xu and colleagues analyzed commercial claims data from 2012-2018. They identified more than 71,000 patients aged 40-64 years with those conditions and focused on health care use by 7,311 patients in urban areas and 1,119 in rural areas within 90 days of a new dementia diagnosis.
The proportion who received neuropsychological testing was 19% among urban patients and 16% among rural patients. Psychological assessments, which are less specialized and detailed than neuropsychological testing, and brain imaging occurred at similar rates in both groups. Similar proportions of rural and urban patients visited neurologists (17.7% and 17.96%, respectively) and psychiatrists (6.02% and 6.47%).
But more urban patients than rural patients visited a psychologist, at 19% versus 15%, according to the researchers.
Approximately 18% of patients in rural areas saw a primary care provider without visiting other specialists, compared with 13% in urban areas.
The researchers found that rural patients were significantly less likely to undergo neuropsychological testing (odds ratio, 0.83; 95% confidence interval, 0.70-0.98) or see a psychologist (OR, 0.72; 95% CI, 0.60-0.85).
Similarly, rural patients had significantly higher odds of having only primary care providers involved in the diagnosis of dementia and symptom management (OR, 1.40; 95% CI, 1.19-1.66).
Addressing workforce deficiencies
More primary care training in dementia care and collaboration with specialist colleagues could help address differences in care, Dr. Xu’s group writes. Such efforts are already underway.
In 2018, the Alzheimer’s Association launched telementoring programs focused on dementia care using the Project ECHO (Extension for Community Healthcare Outcomes) model. Researchers originally developed Project ECHO at the University of New Mexico in 2003 to teach primary care clinicians in remote settings how to treat patients infected with the hepatitis C virus.
With the Alzheimer’s and Dementia Care ECHO Program for Clinicians, primary care clinicians can participate in interactive case-based video conferencing sessions to better understand dementia and how to provide high-quality care in community settings, according to the association.
The program covers guidelines for diagnosis, disclosure, and follow-up; the initiation of care planning; managing disease-related challenges; and resources for patients and caregivers.
Since 2018, nearly 100 primary care practices in the United States have completed training in dementia care using Project ECHO, said Morgan Daven, vice president of health systems for the Alzheimer’s Association. Many cases featured in the program are challenging, he added.
“With primary care being on the front lines, it is really important that primary care physicians are equipped to do what they can to detect or diagnose and know when to refer,” Mr. Daven said.
The association has compiled other resources for clinicians as well.
A 2020 report from the association examined the role that primary care physicians play in dementia care. One survey found that 82% of primary care physicians consider themselves on the front lines of providing care for patients with dementia.
Meanwhile, about half say medical professionals are not prepared to meet rising demands associated with Alzheimer’s disease and dementia care.
Mr. Daven said the geographic disparities Dr. Xu and colleagues found are unsurprising. More than half of primary care physicians who care for people with Alzheimer’s disease say dementia specialists in their communities cannot meet demand. The problem is more urgent in rural areas. Roughly half of nonmetropolitan counties in the United States lack a practicing psychologist, according to a 2018 study published in the American Journal of Preventive Medicine.
“We really need to approach this on both sides – build the capacity in primary care, but we also need to address the dementia care specialty shortages,” Mr. Daven said.
The lack of obvious differences in access to neurologists in the new study “was surprising, given the more than fourfold difference between urban and rural areas in the supply of neurologists,” the researchers note. Health plans may maintain more access to neurologists than psychologists because of relatively higher reimbursement for neurologists, they observed.
One of the study coauthors disclosed ties to Aveanna Healthcare, a company that delivers home health and hospice care.
A version of this article first appeared on Medscape.com.
Patients in rural areas are also less likely to see psychologists and undergo neuropsychological testing, according to the study, published in JAMA Network Open.
Patients who forgo such specialist visits and testing may be missing information about their condition that could help them prepare for changes in job responsibilities and future care decisions, said Wendy Yi Xu, PhD, of The Ohio State University, Columbus, who led the research.
“A lot of them are still in the workforce,” Dr. Xu said. Patients in the study were an average age of 56 years, well before the conventional age of retirement.
Location, location, location
To examine rural versus urban differences in the use of diagnostic tests and health care visits for early onset Alzheimer’s disease and related dementias, Dr. Xu and colleagues analyzed commercial claims data from 2012-2018. They identified more than 71,000 patients aged 40-64 years with those conditions and focused on health care use by 7,311 patients in urban areas and 1,119 in rural areas within 90 days of a new dementia diagnosis.
The proportion who received neuropsychological testing was 19% among urban patients and 16% among rural patients. Psychological assessments, which are less specialized and detailed than neuropsychological testing, and brain imaging occurred at similar rates in both groups. Similar proportions of rural and urban patients visited neurologists (17.7% and 17.96%, respectively) and psychiatrists (6.02% and 6.47%).
But more urban patients than rural patients visited a psychologist, at 19% versus 15%, according to the researchers.
Approximately 18% of patients in rural areas saw a primary care provider without visiting other specialists, compared with 13% in urban areas.
The researchers found that rural patients were significantly less likely to undergo neuropsychological testing (odds ratio, 0.83; 95% confidence interval, 0.70-0.98) or see a psychologist (OR, 0.72; 95% CI, 0.60-0.85).
Similarly, rural patients had significantly higher odds of having only primary care providers involved in the diagnosis of dementia and symptom management (OR, 1.40; 95% CI, 1.19-1.66).
Addressing workforce deficiencies
More primary care training in dementia care and collaboration with specialist colleagues could help address differences in care, Dr. Xu’s group writes. Such efforts are already underway.
In 2018, the Alzheimer’s Association launched telementoring programs focused on dementia care using the Project ECHO (Extension for Community Healthcare Outcomes) model. Researchers originally developed Project ECHO at the University of New Mexico in 2003 to teach primary care clinicians in remote settings how to treat patients infected with the hepatitis C virus.
With the Alzheimer’s and Dementia Care ECHO Program for Clinicians, primary care clinicians can participate in interactive case-based video conferencing sessions to better understand dementia and how to provide high-quality care in community settings, according to the association.
The program covers guidelines for diagnosis, disclosure, and follow-up; the initiation of care planning; managing disease-related challenges; and resources for patients and caregivers.
Since 2018, nearly 100 primary care practices in the United States have completed training in dementia care using Project ECHO, said Morgan Daven, vice president of health systems for the Alzheimer’s Association. Many cases featured in the program are challenging, he added.
“With primary care being on the front lines, it is really important that primary care physicians are equipped to do what they can to detect or diagnose and know when to refer,” Mr. Daven said.
The association has compiled other resources for clinicians as well.
A 2020 report from the association examined the role that primary care physicians play in dementia care. One survey found that 82% of primary care physicians consider themselves on the front lines of providing care for patients with dementia.
Meanwhile, about half say medical professionals are not prepared to meet rising demands associated with Alzheimer’s disease and dementia care.
Mr. Daven said the geographic disparities Dr. Xu and colleagues found are unsurprising. More than half of primary care physicians who care for people with Alzheimer’s disease say dementia specialists in their communities cannot meet demand. The problem is more urgent in rural areas. Roughly half of nonmetropolitan counties in the United States lack a practicing psychologist, according to a 2018 study published in the American Journal of Preventive Medicine.
“We really need to approach this on both sides – build the capacity in primary care, but we also need to address the dementia care specialty shortages,” Mr. Daven said.
The lack of obvious differences in access to neurologists in the new study “was surprising, given the more than fourfold difference between urban and rural areas in the supply of neurologists,” the researchers note. Health plans may maintain more access to neurologists than psychologists because of relatively higher reimbursement for neurologists, they observed.
One of the study coauthors disclosed ties to Aveanna Healthcare, a company that delivers home health and hospice care.
A version of this article first appeared on Medscape.com.
Patients in rural areas are also less likely to see psychologists and undergo neuropsychological testing, according to the study, published in JAMA Network Open.
Patients who forgo such specialist visits and testing may be missing information about their condition that could help them prepare for changes in job responsibilities and future care decisions, said Wendy Yi Xu, PhD, of The Ohio State University, Columbus, who led the research.
“A lot of them are still in the workforce,” Dr. Xu said. Patients in the study were an average age of 56 years, well before the conventional age of retirement.
Location, location, location
To examine rural versus urban differences in the use of diagnostic tests and health care visits for early onset Alzheimer’s disease and related dementias, Dr. Xu and colleagues analyzed commercial claims data from 2012-2018. They identified more than 71,000 patients aged 40-64 years with those conditions and focused on health care use by 7,311 patients in urban areas and 1,119 in rural areas within 90 days of a new dementia diagnosis.
The proportion who received neuropsychological testing was 19% among urban patients and 16% among rural patients. Psychological assessments, which are less specialized and detailed than neuropsychological testing, and brain imaging occurred at similar rates in both groups. Similar proportions of rural and urban patients visited neurologists (17.7% and 17.96%, respectively) and psychiatrists (6.02% and 6.47%).
But more urban patients than rural patients visited a psychologist, at 19% versus 15%, according to the researchers.
Approximately 18% of patients in rural areas saw a primary care provider without visiting other specialists, compared with 13% in urban areas.
The researchers found that rural patients were significantly less likely to undergo neuropsychological testing (odds ratio, 0.83; 95% confidence interval, 0.70-0.98) or see a psychologist (OR, 0.72; 95% CI, 0.60-0.85).
Similarly, rural patients had significantly higher odds of having only primary care providers involved in the diagnosis of dementia and symptom management (OR, 1.40; 95% CI, 1.19-1.66).
Addressing workforce deficiencies
More primary care training in dementia care and collaboration with specialist colleagues could help address differences in care, Dr. Xu’s group writes. Such efforts are already underway.
In 2018, the Alzheimer’s Association launched telementoring programs focused on dementia care using the Project ECHO (Extension for Community Healthcare Outcomes) model. Researchers originally developed Project ECHO at the University of New Mexico in 2003 to teach primary care clinicians in remote settings how to treat patients infected with the hepatitis C virus.
With the Alzheimer’s and Dementia Care ECHO Program for Clinicians, primary care clinicians can participate in interactive case-based video conferencing sessions to better understand dementia and how to provide high-quality care in community settings, according to the association.
The program covers guidelines for diagnosis, disclosure, and follow-up; the initiation of care planning; managing disease-related challenges; and resources for patients and caregivers.
Since 2018, nearly 100 primary care practices in the United States have completed training in dementia care using Project ECHO, said Morgan Daven, vice president of health systems for the Alzheimer’s Association. Many cases featured in the program are challenging, he added.
“With primary care being on the front lines, it is really important that primary care physicians are equipped to do what they can to detect or diagnose and know when to refer,” Mr. Daven said.
The association has compiled other resources for clinicians as well.
A 2020 report from the association examined the role that primary care physicians play in dementia care. One survey found that 82% of primary care physicians consider themselves on the front lines of providing care for patients with dementia.
Meanwhile, about half say medical professionals are not prepared to meet rising demands associated with Alzheimer’s disease and dementia care.
Mr. Daven said the geographic disparities Dr. Xu and colleagues found are unsurprising. More than half of primary care physicians who care for people with Alzheimer’s disease say dementia specialists in their communities cannot meet demand. The problem is more urgent in rural areas. Roughly half of nonmetropolitan counties in the United States lack a practicing psychologist, according to a 2018 study published in the American Journal of Preventive Medicine.
“We really need to approach this on both sides – build the capacity in primary care, but we also need to address the dementia care specialty shortages,” Mr. Daven said.
The lack of obvious differences in access to neurologists in the new study “was surprising, given the more than fourfold difference between urban and rural areas in the supply of neurologists,” the researchers note. Health plans may maintain more access to neurologists than psychologists because of relatively higher reimbursement for neurologists, they observed.
One of the study coauthors disclosed ties to Aveanna Healthcare, a company that delivers home health and hospice care.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
Understanding the relationship between life satisfaction and cognitive decline
Every day, we depend on our working memory, spatial cognition, and processing speed abilities to optimize productivity, interpersonal interactions, and psychological wellbeing. These cognitive functioning indices relate closely with academic and work performance, managing emotions, physical fitness, and a sense of fulfillment in personal and work relationships. They are linked intimately to complex cognitive skills (van Dijk et al., 2020). It is thus imperative to identify modifiable predictors of cognitive functioning in the brain to protect against aging-related cognitive decline and maximize the quality of life.
A decline in life satisfaction can worsen cognitive functioning over long periods via lifestyle factors (e.g., suboptimal diet and nutrition, lack of exercise) (Ratigan et al., 2016). Inadequate engagement in these health-enhancing pursuits could build up inflammation in EF-linked brain areas, thus negatively impacting cognitive functioning in adulthood (Grant et al., 2009). Possible pathways include long-term wear and tear of the hypothalamic-pituitary axis and brain regions linked to executive functioning (Zainal and Newman, 2022a). These processes may deteriorate working memory, spatial cognition, and processing speed across time.
Similarly, it is plausible that a reduction in cognitive functioning may lead to a long-term decrease in life satisfaction. Working memory, processing speed, spatial cognition, and related capacities are essential to meaningful activities and feelings of gratification in personal and professional relationships and other spheres of health throughout life (Baumeister et al., 2007). These cognitive functioning markers safeguard against reduced life satisfaction by facilitating effective problem-solving, and choices (Swanson and Fung, 2016). For example, stronger working memory, processing speed, and related domains coincided with better tolerance for stress and trading off immediate rewards for long-term values and life goals (Hofmann et al., 2012). Therefore, reduction in cognitive functioning abilities could precede a future decline in life satisfaction.
Nonetheless, the literature on this topic has several limitations. Most of the studies have been cross-sectional (i.e., across a single time-point) and thus do not permit inferences between cause and effect (e.g., Toh et al., 2020). Also, most studies used statistical methods that did not differentiate between between-person (trait-like individual differences) and within-person (state-like) relations. Distinguishing within- and between-person relations is necessary because they may vary in magnitude and direction. The preceding theories emphasize change-to-future change relations within persons rather than between persons (Wright and Woods, 2020).
Clinical implications
Our recent work (Zainal and Newman, 2022b) added to the literature by using an advanced statistical method to determine the relations between change in life satisfaction and future change in cognitive functioning domains within persons. The choice of an advanced statistical technique minimizes biases due to the passage of time and assessment unreliability. It also adjusts for between-person effects (Klopack and Wickrama, 2020). Improving understanding of the within-person factors leading to the deterioration of cognitive functioning and life satisfaction is crucial given the rising rates of psychiatric and neurocognitive illnesses (Cui et al., 2020). Identifying these changeable risk factors can optimize prevention, early detection, and treatment approaches.
Specifically, we analyzed the publicly available Swedish Adoption/Twin Study of Aging (SATSA) dataset (Petkus et al., 2017). Their dataset comprised 520 middle- to older-aged twin adults without dementia. Participants provided data across 23 years with five time points. Each time lag ranged from 3 to 11 years. The analyses demonstrated that greater decreases in life satisfaction predicted larger future declines in processing speed, verbal working memory, and spatial cognition. Moreover, declines in verbal working memory and processing speed predicted a reduction in life satisfaction. However, change in spatial awareness did not predict change in life satisfaction.
Our study offers multiple theoretical perspectives. Scar theories propose that decreased life satisfaction and related mental health problems can compromise working memory, processing speed, and spatial cognition in the long term. This scarring process occurs through the buildup of allostatic load, such as increased biomarkers of chronic stress (e.g., cortisol) and inflammation (e.g., interleukin-6, C-reactive protein) (Fancourt and Steptoe, 2020; Zainal and Newman, 2021a). Also, findings suggest the importance of executive functioning domains to attain desired milestones and aspirations to enhance a sense of fulfillment (Baddeley, 2013; Toh and Yang, 2020). Reductions in these cognitive functioning capacities could, over time, adversely affect the ability to engage in daily living activities and manage negative moods.
Limitations of our study include the lack of a multiple-assessment approach to measuring diverse cognitive functioning domains. Also, the absence of cognitive self-reports is a shortcoming since perceived cognitive difficulties might not align with performance on cognitive tests. Relatedly, future studies should administer cognitive tests that parallel and transfer to everyday tasks. However, our study’s strengths include the robust findings across different intervals between study waves, advanced statistics, and the large sample size.
If future studies replicate a similar pattern of results, the clinical applications of this study merit attention. Mindfulness-based interventions can promote working memory, sustained awareness, and spatial cognition or protect against cognitive decline (Jha et al., 2019; Zainal and Newman, 2021b). Further, clinical science can profit from exploring cognitive-behavioral therapies to improve adults’ cognitive function or life satisfaction (Sok et al., 2021).
Dr. Zainal recently accepted a 2-year postdoctoral research associate position at Harvard Medical School, Boston, starting in summer 2022. She received her Ph.D. from Pennsylvania State University, University Park, and completed a predoctoral clinical fellowship at the HMS-affiliated Massachusetts General Hospital – Cognitive Behavioral Scientist Track. Her research interests focus on how executive functioning, social cognition, and cognitive-behavioral strategies link to the etiology, maintenance, and treatment of anxiety and depressive disorders. Dr. Newman is a professor of psychology and psychiatry, and the director of the Center for the Treatment of Anxiety and Depression, at Pennsylvania State University. She has conducted basic and applied research on anxiety disorders and depression and has published over 200 papers on these topics.
Sources
Baddeley A. Working memory and emotion: Ruminations on a theory of depression. Rev Gen Psychol. 2013;17(1):20-7. doi: 10.1037/a0030029.
Baumeister RF et al. “Self-regulation and the executive function: The self as controlling agent,” in Social Psychology: Handbook of Basic Principles, 2nd ed. (pp. 516-39). The Guilford Press: New York, 2007.
Cui L et al. Prevalence of alzheimer’s disease and parkinson’s disease in China: An updated systematical analysis. Front Aging Neurosci. 2020 Dec 21;12:603854. doi: 10.3389/fnagi.2020.603854.
Fancourt D and Steptoe A. The longitudinal relationship between changes in wellbeing and inflammatory markers: Are associations independent of depression? Brain Behav Immun. 2020 Jan;83:146-52. doi: 10.1016/j.bbi.2019.10.004.
Grant N et al. The relationship between life satisfaction and health behavior: A cross-cultural analysis of young adults. Int J Behav Med. 2009;16(3):259-68. doi: 10.1007/s12529-009-9032-x.
Hofmann W et al. Executive functions and self-regulation. Trends Cogn Sci. 2012 Mar;16(3):174-80. doi: 10.1016/j.tics.2012.01.006.
Jha AP et al. Bolstering cognitive resilience via train-the-trainer delivery of mindfulness training in applied high-demand settings. Mindfulness. 2019;11(3):683-97. doi: 10.1007/s12671-019-01284-7.
Klopack ET and Wickrama K. Modeling latent change score analysis and extensions in Mplus: A practical guide for researchers. Struct Equ Modeling. 2020;27(1):97-110. doi: 10.1080/10705511.2018.1562929.
Petkus AJ et al. Temporal dynamics of cognitive performance and anxiety across older adulthood. Psychol Aging. 2017 May;32(3):278-92. doi: 10.1037/pag0000164.
Ratigan A et al. Sex differences in the association of physical function and cognitive function with life satisfaction in older age: The Rancho Bernardo Study. Maturitas. 2016 Jul;89:29-35. doi: 10.1016/j.maturitas.2016.04.007.
Sok S et al. Effects of cognitive/exercise dual-task program on the cognitive function, health status, depression, and life satisfaction of the elderly living in the community. Int J Environ Res Public Health. 2021 Jul 24;18(15):7848. doi: 10.3390/ijerph18157848.
Swanson HL and Fung W. Working memory components and problem-solving accuracy: Are there multiple pathways? J Educ Psychol. 2016;108(8):1153-77. doi: 10.1037/edu0000116.
Toh WX and Yang H. Executive function moderates the effect of reappraisal on life satisfaction: A latent variable analysis. Emotion. 2020;22(3):554-71. doi: 10.1037/emo0000907.
Toh WX et al. Executive function and subjective wellbeing in middle and late adulthood. J Gerontol B Psychol Sci Soc Sci. 2020 Jun 2;75(6):e69-e77. doi: 10.1093/geronb/gbz006.
van Dijk DM, et al. Cognitive functioning, sleep quality, and work performance in non-clinical burnout: The role of working memory. PLoS One. 2020 Apr 23;15(4):e0231906. doi: 10.1371/journal.pone.0231906.
Wright AGC and Woods WC. Personalized models of psychopathology. Annu Rev Clin Psychol. 2020 May 7;16:49-74. doi: 10.1146/annurev-clinpsy-102419-125032.
Zainal NH and Newman MG. (2021a). Depression and worry symptoms predict future executive functioning impairment via inflammation. Psychol Med. 2021 Mar 3;1-11. doi: 10.1017/S0033291721000398.
Zainal NH and Newman MG. (2021b). Mindfulness enhances cognitive functioning: A meta-analysis of 111 randomized controlled trials. PsyArXiv Preprints. 2021 May 11. doi: 10.31234/osf.io/vzxw7.
Zainal NH and Newman MG. (2022a). Inflammation mediates depression and generalized anxiety symptoms predicting executive function impairment after 18 years. J Affect Disord. 2022 Jan 1;296:465-75. doi: 10.1016/j.jad.2021.08.077.
Zainal NH and Newman MG. (2022b). Life satisfaction prevents decline in working memory, spatial cognition, and processing speed: Latent change score analyses across 23 years. Eur Psychiatry. 2022 Apr 19;65(1):1-55. doi: 10.1192/j.eurpsy.2022.19.
Every day, we depend on our working memory, spatial cognition, and processing speed abilities to optimize productivity, interpersonal interactions, and psychological wellbeing. These cognitive functioning indices relate closely with academic and work performance, managing emotions, physical fitness, and a sense of fulfillment in personal and work relationships. They are linked intimately to complex cognitive skills (van Dijk et al., 2020). It is thus imperative to identify modifiable predictors of cognitive functioning in the brain to protect against aging-related cognitive decline and maximize the quality of life.
A decline in life satisfaction can worsen cognitive functioning over long periods via lifestyle factors (e.g., suboptimal diet and nutrition, lack of exercise) (Ratigan et al., 2016). Inadequate engagement in these health-enhancing pursuits could build up inflammation in EF-linked brain areas, thus negatively impacting cognitive functioning in adulthood (Grant et al., 2009). Possible pathways include long-term wear and tear of the hypothalamic-pituitary axis and brain regions linked to executive functioning (Zainal and Newman, 2022a). These processes may deteriorate working memory, spatial cognition, and processing speed across time.
Similarly, it is plausible that a reduction in cognitive functioning may lead to a long-term decrease in life satisfaction. Working memory, processing speed, spatial cognition, and related capacities are essential to meaningful activities and feelings of gratification in personal and professional relationships and other spheres of health throughout life (Baumeister et al., 2007). These cognitive functioning markers safeguard against reduced life satisfaction by facilitating effective problem-solving, and choices (Swanson and Fung, 2016). For example, stronger working memory, processing speed, and related domains coincided with better tolerance for stress and trading off immediate rewards for long-term values and life goals (Hofmann et al., 2012). Therefore, reduction in cognitive functioning abilities could precede a future decline in life satisfaction.
Nonetheless, the literature on this topic has several limitations. Most of the studies have been cross-sectional (i.e., across a single time-point) and thus do not permit inferences between cause and effect (e.g., Toh et al., 2020). Also, most studies used statistical methods that did not differentiate between between-person (trait-like individual differences) and within-person (state-like) relations. Distinguishing within- and between-person relations is necessary because they may vary in magnitude and direction. The preceding theories emphasize change-to-future change relations within persons rather than between persons (Wright and Woods, 2020).
Clinical implications
Our recent work (Zainal and Newman, 2022b) added to the literature by using an advanced statistical method to determine the relations between change in life satisfaction and future change in cognitive functioning domains within persons. The choice of an advanced statistical technique minimizes biases due to the passage of time and assessment unreliability. It also adjusts for between-person effects (Klopack and Wickrama, 2020). Improving understanding of the within-person factors leading to the deterioration of cognitive functioning and life satisfaction is crucial given the rising rates of psychiatric and neurocognitive illnesses (Cui et al., 2020). Identifying these changeable risk factors can optimize prevention, early detection, and treatment approaches.
Specifically, we analyzed the publicly available Swedish Adoption/Twin Study of Aging (SATSA) dataset (Petkus et al., 2017). Their dataset comprised 520 middle- to older-aged twin adults without dementia. Participants provided data across 23 years with five time points. Each time lag ranged from 3 to 11 years. The analyses demonstrated that greater decreases in life satisfaction predicted larger future declines in processing speed, verbal working memory, and spatial cognition. Moreover, declines in verbal working memory and processing speed predicted a reduction in life satisfaction. However, change in spatial awareness did not predict change in life satisfaction.
Our study offers multiple theoretical perspectives. Scar theories propose that decreased life satisfaction and related mental health problems can compromise working memory, processing speed, and spatial cognition in the long term. This scarring process occurs through the buildup of allostatic load, such as increased biomarkers of chronic stress (e.g., cortisol) and inflammation (e.g., interleukin-6, C-reactive protein) (Fancourt and Steptoe, 2020; Zainal and Newman, 2021a). Also, findings suggest the importance of executive functioning domains to attain desired milestones and aspirations to enhance a sense of fulfillment (Baddeley, 2013; Toh and Yang, 2020). Reductions in these cognitive functioning capacities could, over time, adversely affect the ability to engage in daily living activities and manage negative moods.
Limitations of our study include the lack of a multiple-assessment approach to measuring diverse cognitive functioning domains. Also, the absence of cognitive self-reports is a shortcoming since perceived cognitive difficulties might not align with performance on cognitive tests. Relatedly, future studies should administer cognitive tests that parallel and transfer to everyday tasks. However, our study’s strengths include the robust findings across different intervals between study waves, advanced statistics, and the large sample size.
If future studies replicate a similar pattern of results, the clinical applications of this study merit attention. Mindfulness-based interventions can promote working memory, sustained awareness, and spatial cognition or protect against cognitive decline (Jha et al., 2019; Zainal and Newman, 2021b). Further, clinical science can profit from exploring cognitive-behavioral therapies to improve adults’ cognitive function or life satisfaction (Sok et al., 2021).
Dr. Zainal recently accepted a 2-year postdoctoral research associate position at Harvard Medical School, Boston, starting in summer 2022. She received her Ph.D. from Pennsylvania State University, University Park, and completed a predoctoral clinical fellowship at the HMS-affiliated Massachusetts General Hospital – Cognitive Behavioral Scientist Track. Her research interests focus on how executive functioning, social cognition, and cognitive-behavioral strategies link to the etiology, maintenance, and treatment of anxiety and depressive disorders. Dr. Newman is a professor of psychology and psychiatry, and the director of the Center for the Treatment of Anxiety and Depression, at Pennsylvania State University. She has conducted basic and applied research on anxiety disorders and depression and has published over 200 papers on these topics.
Sources
Baddeley A. Working memory and emotion: Ruminations on a theory of depression. Rev Gen Psychol. 2013;17(1):20-7. doi: 10.1037/a0030029.
Baumeister RF et al. “Self-regulation and the executive function: The self as controlling agent,” in Social Psychology: Handbook of Basic Principles, 2nd ed. (pp. 516-39). The Guilford Press: New York, 2007.
Cui L et al. Prevalence of alzheimer’s disease and parkinson’s disease in China: An updated systematical analysis. Front Aging Neurosci. 2020 Dec 21;12:603854. doi: 10.3389/fnagi.2020.603854.
Fancourt D and Steptoe A. The longitudinal relationship between changes in wellbeing and inflammatory markers: Are associations independent of depression? Brain Behav Immun. 2020 Jan;83:146-52. doi: 10.1016/j.bbi.2019.10.004.
Grant N et al. The relationship between life satisfaction and health behavior: A cross-cultural analysis of young adults. Int J Behav Med. 2009;16(3):259-68. doi: 10.1007/s12529-009-9032-x.
Hofmann W et al. Executive functions and self-regulation. Trends Cogn Sci. 2012 Mar;16(3):174-80. doi: 10.1016/j.tics.2012.01.006.
Jha AP et al. Bolstering cognitive resilience via train-the-trainer delivery of mindfulness training in applied high-demand settings. Mindfulness. 2019;11(3):683-97. doi: 10.1007/s12671-019-01284-7.
Klopack ET and Wickrama K. Modeling latent change score analysis and extensions in Mplus: A practical guide for researchers. Struct Equ Modeling. 2020;27(1):97-110. doi: 10.1080/10705511.2018.1562929.
Petkus AJ et al. Temporal dynamics of cognitive performance and anxiety across older adulthood. Psychol Aging. 2017 May;32(3):278-92. doi: 10.1037/pag0000164.
Ratigan A et al. Sex differences in the association of physical function and cognitive function with life satisfaction in older age: The Rancho Bernardo Study. Maturitas. 2016 Jul;89:29-35. doi: 10.1016/j.maturitas.2016.04.007.
Sok S et al. Effects of cognitive/exercise dual-task program on the cognitive function, health status, depression, and life satisfaction of the elderly living in the community. Int J Environ Res Public Health. 2021 Jul 24;18(15):7848. doi: 10.3390/ijerph18157848.
Swanson HL and Fung W. Working memory components and problem-solving accuracy: Are there multiple pathways? J Educ Psychol. 2016;108(8):1153-77. doi: 10.1037/edu0000116.
Toh WX and Yang H. Executive function moderates the effect of reappraisal on life satisfaction: A latent variable analysis. Emotion. 2020;22(3):554-71. doi: 10.1037/emo0000907.
Toh WX et al. Executive function and subjective wellbeing in middle and late adulthood. J Gerontol B Psychol Sci Soc Sci. 2020 Jun 2;75(6):e69-e77. doi: 10.1093/geronb/gbz006.
van Dijk DM, et al. Cognitive functioning, sleep quality, and work performance in non-clinical burnout: The role of working memory. PLoS One. 2020 Apr 23;15(4):e0231906. doi: 10.1371/journal.pone.0231906.
Wright AGC and Woods WC. Personalized models of psychopathology. Annu Rev Clin Psychol. 2020 May 7;16:49-74. doi: 10.1146/annurev-clinpsy-102419-125032.
Zainal NH and Newman MG. (2021a). Depression and worry symptoms predict future executive functioning impairment via inflammation. Psychol Med. 2021 Mar 3;1-11. doi: 10.1017/S0033291721000398.
Zainal NH and Newman MG. (2021b). Mindfulness enhances cognitive functioning: A meta-analysis of 111 randomized controlled trials. PsyArXiv Preprints. 2021 May 11. doi: 10.31234/osf.io/vzxw7.
Zainal NH and Newman MG. (2022a). Inflammation mediates depression and generalized anxiety symptoms predicting executive function impairment after 18 years. J Affect Disord. 2022 Jan 1;296:465-75. doi: 10.1016/j.jad.2021.08.077.
Zainal NH and Newman MG. (2022b). Life satisfaction prevents decline in working memory, spatial cognition, and processing speed: Latent change score analyses across 23 years. Eur Psychiatry. 2022 Apr 19;65(1):1-55. doi: 10.1192/j.eurpsy.2022.19.
Every day, we depend on our working memory, spatial cognition, and processing speed abilities to optimize productivity, interpersonal interactions, and psychological wellbeing. These cognitive functioning indices relate closely with academic and work performance, managing emotions, physical fitness, and a sense of fulfillment in personal and work relationships. They are linked intimately to complex cognitive skills (van Dijk et al., 2020). It is thus imperative to identify modifiable predictors of cognitive functioning in the brain to protect against aging-related cognitive decline and maximize the quality of life.
A decline in life satisfaction can worsen cognitive functioning over long periods via lifestyle factors (e.g., suboptimal diet and nutrition, lack of exercise) (Ratigan et al., 2016). Inadequate engagement in these health-enhancing pursuits could build up inflammation in EF-linked brain areas, thus negatively impacting cognitive functioning in adulthood (Grant et al., 2009). Possible pathways include long-term wear and tear of the hypothalamic-pituitary axis and brain regions linked to executive functioning (Zainal and Newman, 2022a). These processes may deteriorate working memory, spatial cognition, and processing speed across time.
Similarly, it is plausible that a reduction in cognitive functioning may lead to a long-term decrease in life satisfaction. Working memory, processing speed, spatial cognition, and related capacities are essential to meaningful activities and feelings of gratification in personal and professional relationships and other spheres of health throughout life (Baumeister et al., 2007). These cognitive functioning markers safeguard against reduced life satisfaction by facilitating effective problem-solving, and choices (Swanson and Fung, 2016). For example, stronger working memory, processing speed, and related domains coincided with better tolerance for stress and trading off immediate rewards for long-term values and life goals (Hofmann et al., 2012). Therefore, reduction in cognitive functioning abilities could precede a future decline in life satisfaction.
Nonetheless, the literature on this topic has several limitations. Most of the studies have been cross-sectional (i.e., across a single time-point) and thus do not permit inferences between cause and effect (e.g., Toh et al., 2020). Also, most studies used statistical methods that did not differentiate between between-person (trait-like individual differences) and within-person (state-like) relations. Distinguishing within- and between-person relations is necessary because they may vary in magnitude and direction. The preceding theories emphasize change-to-future change relations within persons rather than between persons (Wright and Woods, 2020).
Clinical implications
Our recent work (Zainal and Newman, 2022b) added to the literature by using an advanced statistical method to determine the relations between change in life satisfaction and future change in cognitive functioning domains within persons. The choice of an advanced statistical technique minimizes biases due to the passage of time and assessment unreliability. It also adjusts for between-person effects (Klopack and Wickrama, 2020). Improving understanding of the within-person factors leading to the deterioration of cognitive functioning and life satisfaction is crucial given the rising rates of psychiatric and neurocognitive illnesses (Cui et al., 2020). Identifying these changeable risk factors can optimize prevention, early detection, and treatment approaches.
Specifically, we analyzed the publicly available Swedish Adoption/Twin Study of Aging (SATSA) dataset (Petkus et al., 2017). Their dataset comprised 520 middle- to older-aged twin adults without dementia. Participants provided data across 23 years with five time points. Each time lag ranged from 3 to 11 years. The analyses demonstrated that greater decreases in life satisfaction predicted larger future declines in processing speed, verbal working memory, and spatial cognition. Moreover, declines in verbal working memory and processing speed predicted a reduction in life satisfaction. However, change in spatial awareness did not predict change in life satisfaction.
Our study offers multiple theoretical perspectives. Scar theories propose that decreased life satisfaction and related mental health problems can compromise working memory, processing speed, and spatial cognition in the long term. This scarring process occurs through the buildup of allostatic load, such as increased biomarkers of chronic stress (e.g., cortisol) and inflammation (e.g., interleukin-6, C-reactive protein) (Fancourt and Steptoe, 2020; Zainal and Newman, 2021a). Also, findings suggest the importance of executive functioning domains to attain desired milestones and aspirations to enhance a sense of fulfillment (Baddeley, 2013; Toh and Yang, 2020). Reductions in these cognitive functioning capacities could, over time, adversely affect the ability to engage in daily living activities and manage negative moods.
Limitations of our study include the lack of a multiple-assessment approach to measuring diverse cognitive functioning domains. Also, the absence of cognitive self-reports is a shortcoming since perceived cognitive difficulties might not align with performance on cognitive tests. Relatedly, future studies should administer cognitive tests that parallel and transfer to everyday tasks. However, our study’s strengths include the robust findings across different intervals between study waves, advanced statistics, and the large sample size.
If future studies replicate a similar pattern of results, the clinical applications of this study merit attention. Mindfulness-based interventions can promote working memory, sustained awareness, and spatial cognition or protect against cognitive decline (Jha et al., 2019; Zainal and Newman, 2021b). Further, clinical science can profit from exploring cognitive-behavioral therapies to improve adults’ cognitive function or life satisfaction (Sok et al., 2021).
Dr. Zainal recently accepted a 2-year postdoctoral research associate position at Harvard Medical School, Boston, starting in summer 2022. She received her Ph.D. from Pennsylvania State University, University Park, and completed a predoctoral clinical fellowship at the HMS-affiliated Massachusetts General Hospital – Cognitive Behavioral Scientist Track. Her research interests focus on how executive functioning, social cognition, and cognitive-behavioral strategies link to the etiology, maintenance, and treatment of anxiety and depressive disorders. Dr. Newman is a professor of psychology and psychiatry, and the director of the Center for the Treatment of Anxiety and Depression, at Pennsylvania State University. She has conducted basic and applied research on anxiety disorders and depression and has published over 200 papers on these topics.
Sources
Baddeley A. Working memory and emotion: Ruminations on a theory of depression. Rev Gen Psychol. 2013;17(1):20-7. doi: 10.1037/a0030029.
Baumeister RF et al. “Self-regulation and the executive function: The self as controlling agent,” in Social Psychology: Handbook of Basic Principles, 2nd ed. (pp. 516-39). The Guilford Press: New York, 2007.
Cui L et al. Prevalence of alzheimer’s disease and parkinson’s disease in China: An updated systematical analysis. Front Aging Neurosci. 2020 Dec 21;12:603854. doi: 10.3389/fnagi.2020.603854.
Fancourt D and Steptoe A. The longitudinal relationship between changes in wellbeing and inflammatory markers: Are associations independent of depression? Brain Behav Immun. 2020 Jan;83:146-52. doi: 10.1016/j.bbi.2019.10.004.
Grant N et al. The relationship between life satisfaction and health behavior: A cross-cultural analysis of young adults. Int J Behav Med. 2009;16(3):259-68. doi: 10.1007/s12529-009-9032-x.
Hofmann W et al. Executive functions and self-regulation. Trends Cogn Sci. 2012 Mar;16(3):174-80. doi: 10.1016/j.tics.2012.01.006.
Jha AP et al. Bolstering cognitive resilience via train-the-trainer delivery of mindfulness training in applied high-demand settings. Mindfulness. 2019;11(3):683-97. doi: 10.1007/s12671-019-01284-7.
Klopack ET and Wickrama K. Modeling latent change score analysis and extensions in Mplus: A practical guide for researchers. Struct Equ Modeling. 2020;27(1):97-110. doi: 10.1080/10705511.2018.1562929.
Petkus AJ et al. Temporal dynamics of cognitive performance and anxiety across older adulthood. Psychol Aging. 2017 May;32(3):278-92. doi: 10.1037/pag0000164.
Ratigan A et al. Sex differences in the association of physical function and cognitive function with life satisfaction in older age: The Rancho Bernardo Study. Maturitas. 2016 Jul;89:29-35. doi: 10.1016/j.maturitas.2016.04.007.
Sok S et al. Effects of cognitive/exercise dual-task program on the cognitive function, health status, depression, and life satisfaction of the elderly living in the community. Int J Environ Res Public Health. 2021 Jul 24;18(15):7848. doi: 10.3390/ijerph18157848.
Swanson HL and Fung W. Working memory components and problem-solving accuracy: Are there multiple pathways? J Educ Psychol. 2016;108(8):1153-77. doi: 10.1037/edu0000116.
Toh WX and Yang H. Executive function moderates the effect of reappraisal on life satisfaction: A latent variable analysis. Emotion. 2020;22(3):554-71. doi: 10.1037/emo0000907.
Toh WX et al. Executive function and subjective wellbeing in middle and late adulthood. J Gerontol B Psychol Sci Soc Sci. 2020 Jun 2;75(6):e69-e77. doi: 10.1093/geronb/gbz006.
van Dijk DM, et al. Cognitive functioning, sleep quality, and work performance in non-clinical burnout: The role of working memory. PLoS One. 2020 Apr 23;15(4):e0231906. doi: 10.1371/journal.pone.0231906.
Wright AGC and Woods WC. Personalized models of psychopathology. Annu Rev Clin Psychol. 2020 May 7;16:49-74. doi: 10.1146/annurev-clinpsy-102419-125032.
Zainal NH and Newman MG. (2021a). Depression and worry symptoms predict future executive functioning impairment via inflammation. Psychol Med. 2021 Mar 3;1-11. doi: 10.1017/S0033291721000398.
Zainal NH and Newman MG. (2021b). Mindfulness enhances cognitive functioning: A meta-analysis of 111 randomized controlled trials. PsyArXiv Preprints. 2021 May 11. doi: 10.31234/osf.io/vzxw7.
Zainal NH and Newman MG. (2022a). Inflammation mediates depression and generalized anxiety symptoms predicting executive function impairment after 18 years. J Affect Disord. 2022 Jan 1;296:465-75. doi: 10.1016/j.jad.2021.08.077.
Zainal NH and Newman MG. (2022b). Life satisfaction prevents decline in working memory, spatial cognition, and processing speed: Latent change score analyses across 23 years. Eur Psychiatry. 2022 Apr 19;65(1):1-55. doi: 10.1192/j.eurpsy.2022.19.
Primary care now offering physicians the 26.7-hour day
Taking ‘not enough hours in the day’ to new heights
It’s no secret that there’s a big doctor shortage in the United States. Going through medical school is long, expensive, and stressful, and it’s not like those long, stressful hours stop once you finally do get that degree. There is, however, an excellent reason to take that dive into doctorhood: You’ll gain mastery over time itself.
A study from the University of Chicago, Johns Hopkins University, and Imperial College London has revealed the truth. By using data pulled from the National Health and Nutrition Examination Survey, the researchers found that primary care physicians who see an average number of patients and follow all the current national guidelines for preventive care, chronic disease care, and acute care – plus administrative tasks – must work 26.7 hours a day. That works out to 14.1 hours of preventive care, 7.2 hours of chronic disease care, 2.2 hours of acute care, and 3.2 hours of documentation and inbox management.
Astute readers may note that this is a bit more than the traditional 8-hour workday. It is, in fact, more hours than there actually are in a day. As it turns out, Doctor Strange is more of a documentary than …
Hang on, we’re receiving word that doctors are not in fact wizards who can bend time and space to their will, nor are they sitting on a stash of Time-Turners they saved from the Ministry of Magic before Voldemort destroyed them all. They are, according to the study, overworked and overburdened with too many things and too little time. This is why outcomes haven’t improved despite technological advances and why burnout is so common. We’d be burned out too, having to work temporally impossible hours.
The study authors suggested a team-based approach to medicine that would spread the workload out to nurses, physician assistants, dietitians, etc., estimating that about two-thirds of what a primary care physician does can be handled by someone else. A team-based approach would reduce the physician’s required hours down to 9.3 hours a day, which is at least physically possible. It’s either that or we make the day longer, which sounds like the plot of an episode of Futurama. Swap overwork for global warming and a longer day for a longer year and it is actually the plot of an episode of Futurama.
After a hard day of thinking, brains need their rest
Do you ever feel like you have no more capacity to think or make any more decisions after a long day at work? Do you need a few extra cups of coffee to even make it through the day, even though you’re mostly just sitting around talking and typing? Have we got the research for you: Mental exhaustion is an actual thing. Imagine that double whammy of having a job that’s physically and mentally demanding.
A recent study in Current Biology explained why we feel so exhausted after doing something mentally demanding for several hours. Over that time, glutamate builds up in synapses of the prefrontal cortex, which affects our decision making and leads to cognitive lethargy. Your brain eventually becomes more interested in tasks that are less mentally fatiguing, and that’s probably why you’re reading this LOTME right now instead of getting back to work.
“Our findings show that cognitive work results in a true functional alteration – accumulation of noxious substances – so fatigue would indeed be a signal that makes us stop working but for a different purpose: to preserve the integrity of brain functioning,” senior author Mathias Pessiglione of Pitié-Salpêtrière University, Paris, said in a written statement.
The group of researchers conducted studies by using magnetic resonance spectroscopy to look at two groups of people over the course of a workday: One group had mentally tasking jobs and one didn’t. Those who had to think harder for their jobs had more signs of fatigue, such as reduced pupil dilation and glutamate in synapses of the prefrontal cortex. They also looked for more rewards that required less thinking.
For those whose mentally exhausting jobs probably won’t get better or change, the researchers suggest getting as much rest as possible. Those who don’t have that option will have to continue drinking those 7 cups of coffee a day. ... and reading LOTME.
Hmm, might be a new tagline for us in there somewhere. LOTME: Tired brains love us? When you’re too tired to think, think of LOTME? You can’t spell mental exhaustion without L-O-T-M-E?
Testosterone shows its warm and fuzzy side
Stereotypically, men are loud, knuckle-dragging Neanderthals. The hair coming out of our faces is kind of a dead giveaway, right? We grunt, we scratch, we start wars, we watch sports on TV. But why? It’s the testosterone. Everyone knows that. Testosterone makes men aggressive … or does it?
Since this sort of research generally isn’t done with actual men, investigators at Emory University used Mongolian gerbils. The advantage being that males exhibit cuddling behavior after females become pregnant and they don’t watch a lot of sports on TV. They introduced a male and female gerbil, who then formed a pair bond and the female became pregnant. When the male started displaying cuddling behaviors, the researchers injected him with testosterone, expecting to see his antisocial side.
“Instead, we were surprised that a male gerbil became even more cuddly and prosocial with his partner. He became like ‘super partner,’ ” lead author Aubrey Kelly, PhD, said in a written statement from the university.
For the next experiment, the female was removed and another male was introduced to a male who had already received a testosterone injection. That male was surprisingly unaggressive toward the intruder, at least initially. Then he received a second injection of testosterone. “It was like they suddenly woke up and realized they weren’t supposed to be friendly in that context,” Dr. Kelly said.
The testosterone seemed to influence the activity of oxytocin, the so-called “love hormone,” the investigators suggested. “It’s surprising because normally we think of testosterone as increasing sexual behaviors and aggression. But we’ve shown that it can have more nuanced effects, depending on the social context.”
The researchers were not as surprised when their use of the phrase “super partner” led to a bidding war between DC and Marvel. Then came the contact from the Department of Defense, wondering about weaponized testosterone: Would it be possible for some sort of bomb to turn Vlad “the Impaler” Putin into Vlad “the Cuddler” Putin?
Are instruments spreading the sounds of COVID?
COVID restrictions are practically a thing of the past now. With more people laxed on being in close proximity to each other and the CDC not even recommending social distancing anymore, live concerts and events are back in full swing. But with new variants on the rise and people being a little more cautious, should we be worried about musical instruments spreading COVID?
Yes and no.
A study published in Physics of Fluids looked at wind instruments specifically and how much aerosol is produced and dispersed when playing them. For the study, the investigators measured fog particles with a laser and aerosol concentration with a particle counter to see how fast these particles decay in the air from the distance of the instrument.
Musicians in an orchestra typically would sit close together to produce the best sound, but with COVID that became an issue, senior author Paulo Arratia of the University of Pennsylvania, Philadelphia, noted in a separate written statement. By looking at the distance traveled by the particles coming from a single instrument and how quickly they decayed, they could determine if sitting in close proximity is an actual threat.
Well, the threat was no greater than talking to someone face to face. Particle exit speeds were lower than for a cough or a sneeze, and the maximum decay length was 2 meters from the instrument’s opening.
But that’s just one instrument: What kind of impact does a whole orchestra have on a space? The researchers are looking into that too, but for now they suggest that musicians continue to stay 6 feet away from each other.
So, yeah, there is a threat, but it’s probably safer for you to see that orchestra than have someone sneeze on you.
Music to our ears.
Taking ‘not enough hours in the day’ to new heights
It’s no secret that there’s a big doctor shortage in the United States. Going through medical school is long, expensive, and stressful, and it’s not like those long, stressful hours stop once you finally do get that degree. There is, however, an excellent reason to take that dive into doctorhood: You’ll gain mastery over time itself.
A study from the University of Chicago, Johns Hopkins University, and Imperial College London has revealed the truth. By using data pulled from the National Health and Nutrition Examination Survey, the researchers found that primary care physicians who see an average number of patients and follow all the current national guidelines for preventive care, chronic disease care, and acute care – plus administrative tasks – must work 26.7 hours a day. That works out to 14.1 hours of preventive care, 7.2 hours of chronic disease care, 2.2 hours of acute care, and 3.2 hours of documentation and inbox management.
Astute readers may note that this is a bit more than the traditional 8-hour workday. It is, in fact, more hours than there actually are in a day. As it turns out, Doctor Strange is more of a documentary than …
Hang on, we’re receiving word that doctors are not in fact wizards who can bend time and space to their will, nor are they sitting on a stash of Time-Turners they saved from the Ministry of Magic before Voldemort destroyed them all. They are, according to the study, overworked and overburdened with too many things and too little time. This is why outcomes haven’t improved despite technological advances and why burnout is so common. We’d be burned out too, having to work temporally impossible hours.
The study authors suggested a team-based approach to medicine that would spread the workload out to nurses, physician assistants, dietitians, etc., estimating that about two-thirds of what a primary care physician does can be handled by someone else. A team-based approach would reduce the physician’s required hours down to 9.3 hours a day, which is at least physically possible. It’s either that or we make the day longer, which sounds like the plot of an episode of Futurama. Swap overwork for global warming and a longer day for a longer year and it is actually the plot of an episode of Futurama.
After a hard day of thinking, brains need their rest
Do you ever feel like you have no more capacity to think or make any more decisions after a long day at work? Do you need a few extra cups of coffee to even make it through the day, even though you’re mostly just sitting around talking and typing? Have we got the research for you: Mental exhaustion is an actual thing. Imagine that double whammy of having a job that’s physically and mentally demanding.
A recent study in Current Biology explained why we feel so exhausted after doing something mentally demanding for several hours. Over that time, glutamate builds up in synapses of the prefrontal cortex, which affects our decision making and leads to cognitive lethargy. Your brain eventually becomes more interested in tasks that are less mentally fatiguing, and that’s probably why you’re reading this LOTME right now instead of getting back to work.
“Our findings show that cognitive work results in a true functional alteration – accumulation of noxious substances – so fatigue would indeed be a signal that makes us stop working but for a different purpose: to preserve the integrity of brain functioning,” senior author Mathias Pessiglione of Pitié-Salpêtrière University, Paris, said in a written statement.
The group of researchers conducted studies by using magnetic resonance spectroscopy to look at two groups of people over the course of a workday: One group had mentally tasking jobs and one didn’t. Those who had to think harder for their jobs had more signs of fatigue, such as reduced pupil dilation and glutamate in synapses of the prefrontal cortex. They also looked for more rewards that required less thinking.
For those whose mentally exhausting jobs probably won’t get better or change, the researchers suggest getting as much rest as possible. Those who don’t have that option will have to continue drinking those 7 cups of coffee a day. ... and reading LOTME.
Hmm, might be a new tagline for us in there somewhere. LOTME: Tired brains love us? When you’re too tired to think, think of LOTME? You can’t spell mental exhaustion without L-O-T-M-E?
Testosterone shows its warm and fuzzy side
Stereotypically, men are loud, knuckle-dragging Neanderthals. The hair coming out of our faces is kind of a dead giveaway, right? We grunt, we scratch, we start wars, we watch sports on TV. But why? It’s the testosterone. Everyone knows that. Testosterone makes men aggressive … or does it?
Since this sort of research generally isn’t done with actual men, investigators at Emory University used Mongolian gerbils. The advantage being that males exhibit cuddling behavior after females become pregnant and they don’t watch a lot of sports on TV. They introduced a male and female gerbil, who then formed a pair bond and the female became pregnant. When the male started displaying cuddling behaviors, the researchers injected him with testosterone, expecting to see his antisocial side.
“Instead, we were surprised that a male gerbil became even more cuddly and prosocial with his partner. He became like ‘super partner,’ ” lead author Aubrey Kelly, PhD, said in a written statement from the university.
For the next experiment, the female was removed and another male was introduced to a male who had already received a testosterone injection. That male was surprisingly unaggressive toward the intruder, at least initially. Then he received a second injection of testosterone. “It was like they suddenly woke up and realized they weren’t supposed to be friendly in that context,” Dr. Kelly said.
The testosterone seemed to influence the activity of oxytocin, the so-called “love hormone,” the investigators suggested. “It’s surprising because normally we think of testosterone as increasing sexual behaviors and aggression. But we’ve shown that it can have more nuanced effects, depending on the social context.”
The researchers were not as surprised when their use of the phrase “super partner” led to a bidding war between DC and Marvel. Then came the contact from the Department of Defense, wondering about weaponized testosterone: Would it be possible for some sort of bomb to turn Vlad “the Impaler” Putin into Vlad “the Cuddler” Putin?
Are instruments spreading the sounds of COVID?
COVID restrictions are practically a thing of the past now. With more people laxed on being in close proximity to each other and the CDC not even recommending social distancing anymore, live concerts and events are back in full swing. But with new variants on the rise and people being a little more cautious, should we be worried about musical instruments spreading COVID?
Yes and no.
A study published in Physics of Fluids looked at wind instruments specifically and how much aerosol is produced and dispersed when playing them. For the study, the investigators measured fog particles with a laser and aerosol concentration with a particle counter to see how fast these particles decay in the air from the distance of the instrument.
Musicians in an orchestra typically would sit close together to produce the best sound, but with COVID that became an issue, senior author Paulo Arratia of the University of Pennsylvania, Philadelphia, noted in a separate written statement. By looking at the distance traveled by the particles coming from a single instrument and how quickly they decayed, they could determine if sitting in close proximity is an actual threat.
Well, the threat was no greater than talking to someone face to face. Particle exit speeds were lower than for a cough or a sneeze, and the maximum decay length was 2 meters from the instrument’s opening.
But that’s just one instrument: What kind of impact does a whole orchestra have on a space? The researchers are looking into that too, but for now they suggest that musicians continue to stay 6 feet away from each other.
So, yeah, there is a threat, but it’s probably safer for you to see that orchestra than have someone sneeze on you.
Music to our ears.
Taking ‘not enough hours in the day’ to new heights
It’s no secret that there’s a big doctor shortage in the United States. Going through medical school is long, expensive, and stressful, and it’s not like those long, stressful hours stop once you finally do get that degree. There is, however, an excellent reason to take that dive into doctorhood: You’ll gain mastery over time itself.
A study from the University of Chicago, Johns Hopkins University, and Imperial College London has revealed the truth. By using data pulled from the National Health and Nutrition Examination Survey, the researchers found that primary care physicians who see an average number of patients and follow all the current national guidelines for preventive care, chronic disease care, and acute care – plus administrative tasks – must work 26.7 hours a day. That works out to 14.1 hours of preventive care, 7.2 hours of chronic disease care, 2.2 hours of acute care, and 3.2 hours of documentation and inbox management.
Astute readers may note that this is a bit more than the traditional 8-hour workday. It is, in fact, more hours than there actually are in a day. As it turns out, Doctor Strange is more of a documentary than …
Hang on, we’re receiving word that doctors are not in fact wizards who can bend time and space to their will, nor are they sitting on a stash of Time-Turners they saved from the Ministry of Magic before Voldemort destroyed them all. They are, according to the study, overworked and overburdened with too many things and too little time. This is why outcomes haven’t improved despite technological advances and why burnout is so common. We’d be burned out too, having to work temporally impossible hours.
The study authors suggested a team-based approach to medicine that would spread the workload out to nurses, physician assistants, dietitians, etc., estimating that about two-thirds of what a primary care physician does can be handled by someone else. A team-based approach would reduce the physician’s required hours down to 9.3 hours a day, which is at least physically possible. It’s either that or we make the day longer, which sounds like the plot of an episode of Futurama. Swap overwork for global warming and a longer day for a longer year and it is actually the plot of an episode of Futurama.
After a hard day of thinking, brains need their rest
Do you ever feel like you have no more capacity to think or make any more decisions after a long day at work? Do you need a few extra cups of coffee to even make it through the day, even though you’re mostly just sitting around talking and typing? Have we got the research for you: Mental exhaustion is an actual thing. Imagine that double whammy of having a job that’s physically and mentally demanding.
A recent study in Current Biology explained why we feel so exhausted after doing something mentally demanding for several hours. Over that time, glutamate builds up in synapses of the prefrontal cortex, which affects our decision making and leads to cognitive lethargy. Your brain eventually becomes more interested in tasks that are less mentally fatiguing, and that’s probably why you’re reading this LOTME right now instead of getting back to work.
“Our findings show that cognitive work results in a true functional alteration – accumulation of noxious substances – so fatigue would indeed be a signal that makes us stop working but for a different purpose: to preserve the integrity of brain functioning,” senior author Mathias Pessiglione of Pitié-Salpêtrière University, Paris, said in a written statement.
The group of researchers conducted studies by using magnetic resonance spectroscopy to look at two groups of people over the course of a workday: One group had mentally tasking jobs and one didn’t. Those who had to think harder for their jobs had more signs of fatigue, such as reduced pupil dilation and glutamate in synapses of the prefrontal cortex. They also looked for more rewards that required less thinking.
For those whose mentally exhausting jobs probably won’t get better or change, the researchers suggest getting as much rest as possible. Those who don’t have that option will have to continue drinking those 7 cups of coffee a day. ... and reading LOTME.
Hmm, might be a new tagline for us in there somewhere. LOTME: Tired brains love us? When you’re too tired to think, think of LOTME? You can’t spell mental exhaustion without L-O-T-M-E?
Testosterone shows its warm and fuzzy side
Stereotypically, men are loud, knuckle-dragging Neanderthals. The hair coming out of our faces is kind of a dead giveaway, right? We grunt, we scratch, we start wars, we watch sports on TV. But why? It’s the testosterone. Everyone knows that. Testosterone makes men aggressive … or does it?
Since this sort of research generally isn’t done with actual men, investigators at Emory University used Mongolian gerbils. The advantage being that males exhibit cuddling behavior after females become pregnant and they don’t watch a lot of sports on TV. They introduced a male and female gerbil, who then formed a pair bond and the female became pregnant. When the male started displaying cuddling behaviors, the researchers injected him with testosterone, expecting to see his antisocial side.
“Instead, we were surprised that a male gerbil became even more cuddly and prosocial with his partner. He became like ‘super partner,’ ” lead author Aubrey Kelly, PhD, said in a written statement from the university.
For the next experiment, the female was removed and another male was introduced to a male who had already received a testosterone injection. That male was surprisingly unaggressive toward the intruder, at least initially. Then he received a second injection of testosterone. “It was like they suddenly woke up and realized they weren’t supposed to be friendly in that context,” Dr. Kelly said.
The testosterone seemed to influence the activity of oxytocin, the so-called “love hormone,” the investigators suggested. “It’s surprising because normally we think of testosterone as increasing sexual behaviors and aggression. But we’ve shown that it can have more nuanced effects, depending on the social context.”
The researchers were not as surprised when their use of the phrase “super partner” led to a bidding war between DC and Marvel. Then came the contact from the Department of Defense, wondering about weaponized testosterone: Would it be possible for some sort of bomb to turn Vlad “the Impaler” Putin into Vlad “the Cuddler” Putin?
Are instruments spreading the sounds of COVID?
COVID restrictions are practically a thing of the past now. With more people laxed on being in close proximity to each other and the CDC not even recommending social distancing anymore, live concerts and events are back in full swing. But with new variants on the rise and people being a little more cautious, should we be worried about musical instruments spreading COVID?
Yes and no.
A study published in Physics of Fluids looked at wind instruments specifically and how much aerosol is produced and dispersed when playing them. For the study, the investigators measured fog particles with a laser and aerosol concentration with a particle counter to see how fast these particles decay in the air from the distance of the instrument.
Musicians in an orchestra typically would sit close together to produce the best sound, but with COVID that became an issue, senior author Paulo Arratia of the University of Pennsylvania, Philadelphia, noted in a separate written statement. By looking at the distance traveled by the particles coming from a single instrument and how quickly they decayed, they could determine if sitting in close proximity is an actual threat.
Well, the threat was no greater than talking to someone face to face. Particle exit speeds were lower than for a cough or a sneeze, and the maximum decay length was 2 meters from the instrument’s opening.
But that’s just one instrument: What kind of impact does a whole orchestra have on a space? The researchers are looking into that too, but for now they suggest that musicians continue to stay 6 feet away from each other.
So, yeah, there is a threat, but it’s probably safer for you to see that orchestra than have someone sneeze on you.
Music to our ears.















