User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'main-prefix')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
Pediatricians, specialists largely agree on ASD diagnoses
General pediatricians and a multidisciplinary team of specialists agreed most of the time on which children should be diagnosed with autism spectrum disorder (ASD), data from a new study suggest.
But when it came to ruling out ASD, the agreement rate was much lower.
The study by Melanie Penner, MSc, MD, with the Autism Research Centre at Bloorview Research Institute, Toronto, and colleagues found that 89% of the time when a physician determined a child had ASD, the multidisciplinary team agreed. But when a pediatrician thought a child did not have ASD, the multidisciplinary team agreed only 60% of the time. The study was published in JAMA Network Open.
Multidisciplinary team model can’t keep up with demand
The findings are important as many guidelines recommend multidisciplinary teams (MDTs) for all ASD diagnostic assessment. However, the resources for this model can’t meet the demand of children needing a diagnosis and can lead to long waits for ASD therapies.
In Canada, the researchers note, the average wait time from referral to receipt of ASD diagnosis has been reported as 7 months and “has likely lengthened since the COVID-19 pandemic.”
Jennifer Gerdts, PhD, an attending psychologist at the Seattle Children’s Autism Center, said in an interview that the wait there for diagnosis in children older than 4 is “multiple years,” a length of time that’s common across the United States. Meanwhile, in many states families can’t access services without a diagnosis.
Expanding capacity with diagnoses by general pediatricians may improve access, but the diagnostic accuracy is critical.
Dr. Gerdts, who was not part of the study, said this research is “hugely important in the work that is under way to build community capacity for diagnostic evaluation.”
She said this study shows that not all diagnoses need the resources of a multiple-disciplinary team and that “pediatricians can do it, too, and they can do it pretty accurately.” Dr. Gerdts evaluates children for autism and helps train pediatricians to make diagnoses.
Pediatricians, specialist team completed blinded assessments
The 17 pediatricians in the study and the specialist team independently completed blinded assessment and each recorded a decision on whether the child had ASD. The prospective diagnostic study was conducted in a specialist assessment center in Toronto and in general pediatrician practices in Ontario from June 2016 to March 2020.
Children were younger than 5.5 years, did not have an ASD diagnosis and were referred because there was a development concern. The pediatricians referred 106 children (75% boys; average age, 3.5 years). More than half (57%) of the participating children were from minority racial and ethnic groups.
The children were randomly assigned to two groups: One included children who had their MDT visits before their pediatrician assessment and the other group included those who had their MDT visits after their pediatrician assessment.
The MDT diagnosed more than two-thirds of the children (68%) with ASD.
Sensitivity and specificity of the pediatrician assessments, compared with that of the specialist team, were 0.75 (95% confidence interval, 0.67-0.83) and 0.79 (95% CI, 0.62-0.91), respectively.
A look at pediatricians’ accuracy
Pediatricians reported the decisions they would have made had the child not been in the study.
- In 69% of the true-positive cases, pediatricians would have given an ASD diagnosis.
- In 44% of true-negative cases, they would have told the family the child did not have autism; in 30% of those case, they would give alternative diagnoses (most commonly ADHD and language delay).
- The pediatrician would have diagnosed ASD in only one of the seven false-positive cases and would refer those patients to a subspecialist 71% of the time.
- In false-negative cases, the pediatrician would incorrectly tell the family the child does not have autism 44% of the time.
Regarding the false-negative cases, the authors wrote, “more caution is needed for pediatricians when definitively ruling out ASD, which might result in diagnostic delays.”
Confidence is key
Physician confidence was also correlated with accuracy.
The authors wrote: “Among true-positive cases (MDT and pediatrician agree the child has ASD), the pediatrician was certain or very certain 80% of the time (43 cases) and the MDT was certain or very certain 96% of the time (52 cases). As such, if pediatricians conferred ASD diagnoses when feeling certain or very certain, they would make 46 correct diagnoses and 2 incorrect diagnoses.”
The high accuracy of diagnosis when physicians are confident suggests “listening to that sense of certainty is important,” Dr. Gerdts said. Conversely, these numbers show when a physician is uncertain about diagnosing ASD, they should listen to that instinct, too, and refer.
The results of the study support having general pediatricians diagnose and move forward with their patients when the signs of ASD are more definitive, saving the less-certain cases for the more resource-intensive teams to diagnose. Many states are moving toward that “tiered” system, Dr. Gerdts said.
“For many, and in fact most children, general pediatricians are pretty accurate when making an autism diagnosis,” she said.
“Let’s get [general pediatricians] confident in recognizing when this is outside their skill and ability level,” she said. “If you’re not sure, it is better to refer them on than to misdiagnose them.”
The important missing piece she said is how to support them “so they don’t feel pressure to make that call,” Dr. Gerdts said.
This project was funded by a grant from the Bloorview Research Institute, a grant from the Canadian Institutes of Health Research and a grant from the Canadian Institutes of Health. Three coauthors consult for and receive grants from several pharmaceutical companies and other organizations. Dr. Gerdts declared no relevant financial relationships.
General pediatricians and a multidisciplinary team of specialists agreed most of the time on which children should be diagnosed with autism spectrum disorder (ASD), data from a new study suggest.
But when it came to ruling out ASD, the agreement rate was much lower.
The study by Melanie Penner, MSc, MD, with the Autism Research Centre at Bloorview Research Institute, Toronto, and colleagues found that 89% of the time when a physician determined a child had ASD, the multidisciplinary team agreed. But when a pediatrician thought a child did not have ASD, the multidisciplinary team agreed only 60% of the time. The study was published in JAMA Network Open.
Multidisciplinary team model can’t keep up with demand
The findings are important as many guidelines recommend multidisciplinary teams (MDTs) for all ASD diagnostic assessment. However, the resources for this model can’t meet the demand of children needing a diagnosis and can lead to long waits for ASD therapies.
In Canada, the researchers note, the average wait time from referral to receipt of ASD diagnosis has been reported as 7 months and “has likely lengthened since the COVID-19 pandemic.”
Jennifer Gerdts, PhD, an attending psychologist at the Seattle Children’s Autism Center, said in an interview that the wait there for diagnosis in children older than 4 is “multiple years,” a length of time that’s common across the United States. Meanwhile, in many states families can’t access services without a diagnosis.
Expanding capacity with diagnoses by general pediatricians may improve access, but the diagnostic accuracy is critical.
Dr. Gerdts, who was not part of the study, said this research is “hugely important in the work that is under way to build community capacity for diagnostic evaluation.”
She said this study shows that not all diagnoses need the resources of a multiple-disciplinary team and that “pediatricians can do it, too, and they can do it pretty accurately.” Dr. Gerdts evaluates children for autism and helps train pediatricians to make diagnoses.
Pediatricians, specialist team completed blinded assessments
The 17 pediatricians in the study and the specialist team independently completed blinded assessment and each recorded a decision on whether the child had ASD. The prospective diagnostic study was conducted in a specialist assessment center in Toronto and in general pediatrician practices in Ontario from June 2016 to March 2020.
Children were younger than 5.5 years, did not have an ASD diagnosis and were referred because there was a development concern. The pediatricians referred 106 children (75% boys; average age, 3.5 years). More than half (57%) of the participating children were from minority racial and ethnic groups.
The children were randomly assigned to two groups: One included children who had their MDT visits before their pediatrician assessment and the other group included those who had their MDT visits after their pediatrician assessment.
The MDT diagnosed more than two-thirds of the children (68%) with ASD.
Sensitivity and specificity of the pediatrician assessments, compared with that of the specialist team, were 0.75 (95% confidence interval, 0.67-0.83) and 0.79 (95% CI, 0.62-0.91), respectively.
A look at pediatricians’ accuracy
Pediatricians reported the decisions they would have made had the child not been in the study.
- In 69% of the true-positive cases, pediatricians would have given an ASD diagnosis.
- In 44% of true-negative cases, they would have told the family the child did not have autism; in 30% of those case, they would give alternative diagnoses (most commonly ADHD and language delay).
- The pediatrician would have diagnosed ASD in only one of the seven false-positive cases and would refer those patients to a subspecialist 71% of the time.
- In false-negative cases, the pediatrician would incorrectly tell the family the child does not have autism 44% of the time.
Regarding the false-negative cases, the authors wrote, “more caution is needed for pediatricians when definitively ruling out ASD, which might result in diagnostic delays.”
Confidence is key
Physician confidence was also correlated with accuracy.
The authors wrote: “Among true-positive cases (MDT and pediatrician agree the child has ASD), the pediatrician was certain or very certain 80% of the time (43 cases) and the MDT was certain or very certain 96% of the time (52 cases). As such, if pediatricians conferred ASD diagnoses when feeling certain or very certain, they would make 46 correct diagnoses and 2 incorrect diagnoses.”
The high accuracy of diagnosis when physicians are confident suggests “listening to that sense of certainty is important,” Dr. Gerdts said. Conversely, these numbers show when a physician is uncertain about diagnosing ASD, they should listen to that instinct, too, and refer.
The results of the study support having general pediatricians diagnose and move forward with their patients when the signs of ASD are more definitive, saving the less-certain cases for the more resource-intensive teams to diagnose. Many states are moving toward that “tiered” system, Dr. Gerdts said.
“For many, and in fact most children, general pediatricians are pretty accurate when making an autism diagnosis,” she said.
“Let’s get [general pediatricians] confident in recognizing when this is outside their skill and ability level,” she said. “If you’re not sure, it is better to refer them on than to misdiagnose them.”
The important missing piece she said is how to support them “so they don’t feel pressure to make that call,” Dr. Gerdts said.
This project was funded by a grant from the Bloorview Research Institute, a grant from the Canadian Institutes of Health Research and a grant from the Canadian Institutes of Health. Three coauthors consult for and receive grants from several pharmaceutical companies and other organizations. Dr. Gerdts declared no relevant financial relationships.
General pediatricians and a multidisciplinary team of specialists agreed most of the time on which children should be diagnosed with autism spectrum disorder (ASD), data from a new study suggest.
But when it came to ruling out ASD, the agreement rate was much lower.
The study by Melanie Penner, MSc, MD, with the Autism Research Centre at Bloorview Research Institute, Toronto, and colleagues found that 89% of the time when a physician determined a child had ASD, the multidisciplinary team agreed. But when a pediatrician thought a child did not have ASD, the multidisciplinary team agreed only 60% of the time. The study was published in JAMA Network Open.
Multidisciplinary team model can’t keep up with demand
The findings are important as many guidelines recommend multidisciplinary teams (MDTs) for all ASD diagnostic assessment. However, the resources for this model can’t meet the demand of children needing a diagnosis and can lead to long waits for ASD therapies.
In Canada, the researchers note, the average wait time from referral to receipt of ASD diagnosis has been reported as 7 months and “has likely lengthened since the COVID-19 pandemic.”
Jennifer Gerdts, PhD, an attending psychologist at the Seattle Children’s Autism Center, said in an interview that the wait there for diagnosis in children older than 4 is “multiple years,” a length of time that’s common across the United States. Meanwhile, in many states families can’t access services without a diagnosis.
Expanding capacity with diagnoses by general pediatricians may improve access, but the diagnostic accuracy is critical.
Dr. Gerdts, who was not part of the study, said this research is “hugely important in the work that is under way to build community capacity for diagnostic evaluation.”
She said this study shows that not all diagnoses need the resources of a multiple-disciplinary team and that “pediatricians can do it, too, and they can do it pretty accurately.” Dr. Gerdts evaluates children for autism and helps train pediatricians to make diagnoses.
Pediatricians, specialist team completed blinded assessments
The 17 pediatricians in the study and the specialist team independently completed blinded assessment and each recorded a decision on whether the child had ASD. The prospective diagnostic study was conducted in a specialist assessment center in Toronto and in general pediatrician practices in Ontario from June 2016 to March 2020.
Children were younger than 5.5 years, did not have an ASD diagnosis and were referred because there was a development concern. The pediatricians referred 106 children (75% boys; average age, 3.5 years). More than half (57%) of the participating children were from minority racial and ethnic groups.
The children were randomly assigned to two groups: One included children who had their MDT visits before their pediatrician assessment and the other group included those who had their MDT visits after their pediatrician assessment.
The MDT diagnosed more than two-thirds of the children (68%) with ASD.
Sensitivity and specificity of the pediatrician assessments, compared with that of the specialist team, were 0.75 (95% confidence interval, 0.67-0.83) and 0.79 (95% CI, 0.62-0.91), respectively.
A look at pediatricians’ accuracy
Pediatricians reported the decisions they would have made had the child not been in the study.
- In 69% of the true-positive cases, pediatricians would have given an ASD diagnosis.
- In 44% of true-negative cases, they would have told the family the child did not have autism; in 30% of those case, they would give alternative diagnoses (most commonly ADHD and language delay).
- The pediatrician would have diagnosed ASD in only one of the seven false-positive cases and would refer those patients to a subspecialist 71% of the time.
- In false-negative cases, the pediatrician would incorrectly tell the family the child does not have autism 44% of the time.
Regarding the false-negative cases, the authors wrote, “more caution is needed for pediatricians when definitively ruling out ASD, which might result in diagnostic delays.”
Confidence is key
Physician confidence was also correlated with accuracy.
The authors wrote: “Among true-positive cases (MDT and pediatrician agree the child has ASD), the pediatrician was certain or very certain 80% of the time (43 cases) and the MDT was certain or very certain 96% of the time (52 cases). As such, if pediatricians conferred ASD diagnoses when feeling certain or very certain, they would make 46 correct diagnoses and 2 incorrect diagnoses.”
The high accuracy of diagnosis when physicians are confident suggests “listening to that sense of certainty is important,” Dr. Gerdts said. Conversely, these numbers show when a physician is uncertain about diagnosing ASD, they should listen to that instinct, too, and refer.
The results of the study support having general pediatricians diagnose and move forward with their patients when the signs of ASD are more definitive, saving the less-certain cases for the more resource-intensive teams to diagnose. Many states are moving toward that “tiered” system, Dr. Gerdts said.
“For many, and in fact most children, general pediatricians are pretty accurate when making an autism diagnosis,” she said.
“Let’s get [general pediatricians] confident in recognizing when this is outside their skill and ability level,” she said. “If you’re not sure, it is better to refer them on than to misdiagnose them.”
The important missing piece she said is how to support them “so they don’t feel pressure to make that call,” Dr. Gerdts said.
This project was funded by a grant from the Bloorview Research Institute, a grant from the Canadian Institutes of Health Research and a grant from the Canadian Institutes of Health. Three coauthors consult for and receive grants from several pharmaceutical companies and other organizations. Dr. Gerdts declared no relevant financial relationships.
FROM JAMA NETWORK OPEN
Nine more minutes a day of vigorous exercise tied to better cognition
such as running and cycling, plays in brain health.
“Even minor differences in daily behavior appeared meaningful for cognition in this study,” researcher John J. Mitchell, MSci and PhD candidate, Medical Research Council, London, told this news organization.
The findings were published online in the Journal of Epidemiology and Community Health.
Research gap
Previous research has linked physical activity (PA) with increased cognitive reserve, which delays the onset of cognitive decline in later life. But disentangling the most important components of PA for cognition – such as intensity and volume – has not been well researched.
Previous studies didn’t capture sleep time, which typically takes up the largest component of the day. Sleep is “acutely relevant” when examining cognition, the investigators noted.
In addition, studies in this area often focus on just one or two activity components of the day, which “neglects the growing awareness” that movements “are all tightly interlinked,” said Mr. Mitchell.
The new study included 4,481 participants in the British Cohort Study who were born in 1970 across England, Scotland, and Wales. The participants were followed throughout childhood and adulthood.
The median age of the participants was 47 years, and they were predominantly White, female (52%), married (66%), and well educated. Most were occasional or nonrisky alcohol consumers, and half had never smoked.
The researchers collected biometric measurements and health, demographic, and lifestyle information. Participants wore a thigh-mounted accelerometer at least 7 consecutive hours a day for up to 7 days to track PA, sedentary behavior (SB), and sleep time.
The device used in the study could detect subtle movements as well as speed of accelerations, said Mr. Mitchell. “From this, we can distinguish MVPA from slow walking, standing, and sitting. It’s the current best practice for detecting the more subtle movements we make, such as brisk walking and stair climbing, beyond just ‘exercise,’ “ he added.
Light intensity PA (LIPA) describes movement such as walking and moving around the house or office, while MVPA includes activities such as brisk walking and running that accelerate the heart rate. SB, defined as time spent sitting or lying, is distinguished from standing by the thigh inclination.
On an average day, the cohort spent 51 minutes in MVPA; 5 hours, 42 minutes in LIPA; 9 hours, 16 minutes in SB; and 8 hours, 11 minutes sleeping.
Researchers calculated an overall global score for verbal memory and executive function.
The study used “compositional data analysis,” a statistical method that can examine the associations of cognition and PA in the context of all components of daily movement.
The analysis revealed a positive association between MVPA and cognition relative to all other behaviors, after adjustment for sociodemographic factors that included sex, age, education, and marital status. But the relationship lessened after further adjustment for health status – for example, cardiovascular disease or disability – and lifestyle factors, such as alcohol consumption and smoking status.
SB relative to all other movements remained positively associated with cognition after full adjustment. This, the authors speculated, may reflect engagement in cognitively stimulating activities such as reading.
To better understand the associations, the researchers used a statistical method to reallocate time in the cohort’s average day from one activity component to another.
“We held two of the components static but moved time between the other two and monitored the theoretical ramifications of that change for cognition,” said Mr. Mitchell.
Real cognitive change
There was a 1.31% improvement in cognition ranking compared to the sample average after replacing 9 minutes of sedentary activity with MVPA (1.31; 95% confidence interval [CI], 0.09-2.50). There was a 1.27% improvement after replacing 7 minutes of LIPA with MVPA, and a 1.2% improvement after replacing 7 minutes of sleep with MVPA.
Individuals might move up from about the 50th percentile to the 51st or 52nd percentile after just 9 minutes of more moderate to vigorous movement in place of sitting, said Mr. Mitchell. “This highlights how even very modest differences in people’s daily movement – less than 10 minutes – is linked to quite real changes in our cognitive health.”
The impact of physical activity appeared greatest on working memory and mental processes, such as planning and organization.
On the other hand, cognition declined by 1%-2% after replacing MVPA with 8 minutes of SB, 6 minutes of LIPA, or 7 minutes of sleep.
The activity tracking device couldn’t determine how well participants slept, which is “a clear limitation” of the study, said Mr. Mitchell. “We have to be cautious when trying to interpret our findings surrounding sleep.”
Another limitation is that despite a large sample size, people of color were underrepresented, limiting the generalizability of the findings. As well, other healthy pursuits – for example, reading – might have contributed to improved cognition.
Important findings
In a comment, Jennifer J. Heisz, PhD, associate professor and Canada research chair in brain health and aging, department of kinesiology, McMaster University, Hamilton, Ont., said the findings from the study are important.
“Through the statistical modelling, the authors demonstrate that swapping just 9 minutes of sedentary behavior with moderate to vigorous physical activity, such as a brisk walk or bike ride, was associated with an increase in cognition.”
She added that this seemed to be especially true for people who sit while at work.
The findings “confer with the growing consensus” that some exercise is better than none when it comes to brain health, said Dr. Heisz.
“Clinicians should encourage their patients to add a brisk, 10-minute walk to their daily routine and break up prolonged sitting with short movement breaks.”
She noted the study was cross-sectional, “so it is not possible to infer causation.”
The study received funding from the Medical Research Council and the British Heart Foundation. Mr. Mitchell and Dr. Heisz have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
such as running and cycling, plays in brain health.
“Even minor differences in daily behavior appeared meaningful for cognition in this study,” researcher John J. Mitchell, MSci and PhD candidate, Medical Research Council, London, told this news organization.
The findings were published online in the Journal of Epidemiology and Community Health.
Research gap
Previous research has linked physical activity (PA) with increased cognitive reserve, which delays the onset of cognitive decline in later life. But disentangling the most important components of PA for cognition – such as intensity and volume – has not been well researched.
Previous studies didn’t capture sleep time, which typically takes up the largest component of the day. Sleep is “acutely relevant” when examining cognition, the investigators noted.
In addition, studies in this area often focus on just one or two activity components of the day, which “neglects the growing awareness” that movements “are all tightly interlinked,” said Mr. Mitchell.
The new study included 4,481 participants in the British Cohort Study who were born in 1970 across England, Scotland, and Wales. The participants were followed throughout childhood and adulthood.
The median age of the participants was 47 years, and they were predominantly White, female (52%), married (66%), and well educated. Most were occasional or nonrisky alcohol consumers, and half had never smoked.
The researchers collected biometric measurements and health, demographic, and lifestyle information. Participants wore a thigh-mounted accelerometer at least 7 consecutive hours a day for up to 7 days to track PA, sedentary behavior (SB), and sleep time.
The device used in the study could detect subtle movements as well as speed of accelerations, said Mr. Mitchell. “From this, we can distinguish MVPA from slow walking, standing, and sitting. It’s the current best practice for detecting the more subtle movements we make, such as brisk walking and stair climbing, beyond just ‘exercise,’ “ he added.
Light intensity PA (LIPA) describes movement such as walking and moving around the house or office, while MVPA includes activities such as brisk walking and running that accelerate the heart rate. SB, defined as time spent sitting or lying, is distinguished from standing by the thigh inclination.
On an average day, the cohort spent 51 minutes in MVPA; 5 hours, 42 minutes in LIPA; 9 hours, 16 minutes in SB; and 8 hours, 11 minutes sleeping.
Researchers calculated an overall global score for verbal memory and executive function.
The study used “compositional data analysis,” a statistical method that can examine the associations of cognition and PA in the context of all components of daily movement.
The analysis revealed a positive association between MVPA and cognition relative to all other behaviors, after adjustment for sociodemographic factors that included sex, age, education, and marital status. But the relationship lessened after further adjustment for health status – for example, cardiovascular disease or disability – and lifestyle factors, such as alcohol consumption and smoking status.
SB relative to all other movements remained positively associated with cognition after full adjustment. This, the authors speculated, may reflect engagement in cognitively stimulating activities such as reading.
To better understand the associations, the researchers used a statistical method to reallocate time in the cohort’s average day from one activity component to another.
“We held two of the components static but moved time between the other two and monitored the theoretical ramifications of that change for cognition,” said Mr. Mitchell.
Real cognitive change
There was a 1.31% improvement in cognition ranking compared to the sample average after replacing 9 minutes of sedentary activity with MVPA (1.31; 95% confidence interval [CI], 0.09-2.50). There was a 1.27% improvement after replacing 7 minutes of LIPA with MVPA, and a 1.2% improvement after replacing 7 minutes of sleep with MVPA.
Individuals might move up from about the 50th percentile to the 51st or 52nd percentile after just 9 minutes of more moderate to vigorous movement in place of sitting, said Mr. Mitchell. “This highlights how even very modest differences in people’s daily movement – less than 10 minutes – is linked to quite real changes in our cognitive health.”
The impact of physical activity appeared greatest on working memory and mental processes, such as planning and organization.
On the other hand, cognition declined by 1%-2% after replacing MVPA with 8 minutes of SB, 6 minutes of LIPA, or 7 minutes of sleep.
The activity tracking device couldn’t determine how well participants slept, which is “a clear limitation” of the study, said Mr. Mitchell. “We have to be cautious when trying to interpret our findings surrounding sleep.”
Another limitation is that despite a large sample size, people of color were underrepresented, limiting the generalizability of the findings. As well, other healthy pursuits – for example, reading – might have contributed to improved cognition.
Important findings
In a comment, Jennifer J. Heisz, PhD, associate professor and Canada research chair in brain health and aging, department of kinesiology, McMaster University, Hamilton, Ont., said the findings from the study are important.
“Through the statistical modelling, the authors demonstrate that swapping just 9 minutes of sedentary behavior with moderate to vigorous physical activity, such as a brisk walk or bike ride, was associated with an increase in cognition.”
She added that this seemed to be especially true for people who sit while at work.
The findings “confer with the growing consensus” that some exercise is better than none when it comes to brain health, said Dr. Heisz.
“Clinicians should encourage their patients to add a brisk, 10-minute walk to their daily routine and break up prolonged sitting with short movement breaks.”
She noted the study was cross-sectional, “so it is not possible to infer causation.”
The study received funding from the Medical Research Council and the British Heart Foundation. Mr. Mitchell and Dr. Heisz have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
such as running and cycling, plays in brain health.
“Even minor differences in daily behavior appeared meaningful for cognition in this study,” researcher John J. Mitchell, MSci and PhD candidate, Medical Research Council, London, told this news organization.
The findings were published online in the Journal of Epidemiology and Community Health.
Research gap
Previous research has linked physical activity (PA) with increased cognitive reserve, which delays the onset of cognitive decline in later life. But disentangling the most important components of PA for cognition – such as intensity and volume – has not been well researched.
Previous studies didn’t capture sleep time, which typically takes up the largest component of the day. Sleep is “acutely relevant” when examining cognition, the investigators noted.
In addition, studies in this area often focus on just one or two activity components of the day, which “neglects the growing awareness” that movements “are all tightly interlinked,” said Mr. Mitchell.
The new study included 4,481 participants in the British Cohort Study who were born in 1970 across England, Scotland, and Wales. The participants were followed throughout childhood and adulthood.
The median age of the participants was 47 years, and they were predominantly White, female (52%), married (66%), and well educated. Most were occasional or nonrisky alcohol consumers, and half had never smoked.
The researchers collected biometric measurements and health, demographic, and lifestyle information. Participants wore a thigh-mounted accelerometer at least 7 consecutive hours a day for up to 7 days to track PA, sedentary behavior (SB), and sleep time.
The device used in the study could detect subtle movements as well as speed of accelerations, said Mr. Mitchell. “From this, we can distinguish MVPA from slow walking, standing, and sitting. It’s the current best practice for detecting the more subtle movements we make, such as brisk walking and stair climbing, beyond just ‘exercise,’ “ he added.
Light intensity PA (LIPA) describes movement such as walking and moving around the house or office, while MVPA includes activities such as brisk walking and running that accelerate the heart rate. SB, defined as time spent sitting or lying, is distinguished from standing by the thigh inclination.
On an average day, the cohort spent 51 minutes in MVPA; 5 hours, 42 minutes in LIPA; 9 hours, 16 minutes in SB; and 8 hours, 11 minutes sleeping.
Researchers calculated an overall global score for verbal memory and executive function.
The study used “compositional data analysis,” a statistical method that can examine the associations of cognition and PA in the context of all components of daily movement.
The analysis revealed a positive association between MVPA and cognition relative to all other behaviors, after adjustment for sociodemographic factors that included sex, age, education, and marital status. But the relationship lessened after further adjustment for health status – for example, cardiovascular disease or disability – and lifestyle factors, such as alcohol consumption and smoking status.
SB relative to all other movements remained positively associated with cognition after full adjustment. This, the authors speculated, may reflect engagement in cognitively stimulating activities such as reading.
To better understand the associations, the researchers used a statistical method to reallocate time in the cohort’s average day from one activity component to another.
“We held two of the components static but moved time between the other two and monitored the theoretical ramifications of that change for cognition,” said Mr. Mitchell.
Real cognitive change
There was a 1.31% improvement in cognition ranking compared to the sample average after replacing 9 minutes of sedentary activity with MVPA (1.31; 95% confidence interval [CI], 0.09-2.50). There was a 1.27% improvement after replacing 7 minutes of LIPA with MVPA, and a 1.2% improvement after replacing 7 minutes of sleep with MVPA.
Individuals might move up from about the 50th percentile to the 51st or 52nd percentile after just 9 minutes of more moderate to vigorous movement in place of sitting, said Mr. Mitchell. “This highlights how even very modest differences in people’s daily movement – less than 10 minutes – is linked to quite real changes in our cognitive health.”
The impact of physical activity appeared greatest on working memory and mental processes, such as planning and organization.
On the other hand, cognition declined by 1%-2% after replacing MVPA with 8 minutes of SB, 6 minutes of LIPA, or 7 minutes of sleep.
The activity tracking device couldn’t determine how well participants slept, which is “a clear limitation” of the study, said Mr. Mitchell. “We have to be cautious when trying to interpret our findings surrounding sleep.”
Another limitation is that despite a large sample size, people of color were underrepresented, limiting the generalizability of the findings. As well, other healthy pursuits – for example, reading – might have contributed to improved cognition.
Important findings
In a comment, Jennifer J. Heisz, PhD, associate professor and Canada research chair in brain health and aging, department of kinesiology, McMaster University, Hamilton, Ont., said the findings from the study are important.
“Through the statistical modelling, the authors demonstrate that swapping just 9 minutes of sedentary behavior with moderate to vigorous physical activity, such as a brisk walk or bike ride, was associated with an increase in cognition.”
She added that this seemed to be especially true for people who sit while at work.
The findings “confer with the growing consensus” that some exercise is better than none when it comes to brain health, said Dr. Heisz.
“Clinicians should encourage their patients to add a brisk, 10-minute walk to their daily routine and break up prolonged sitting with short movement breaks.”
She noted the study was cross-sectional, “so it is not possible to infer causation.”
The study received funding from the Medical Research Council and the British Heart Foundation. Mr. Mitchell and Dr. Heisz have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF EPIDEMIOLOGY AND COMMUNITY HEALTH
The longevity gene: Healthy mutant reverses heart aging
Everybody wants a younger heart
As more people live well past 90, scientists have been taking a closer look at how they’ve been doing it. Mostly it boiled down to genetics. You either had it or you didn’t. Well, a recent study suggests that doesn’t have to be true anymore, at least for the heart.
Scientists from the United Kingdom and Italy found an antiaging gene in some centenarians that has shown possible antiaging effects in mice and in human heart cells. A single administration of the mutant antiaging gene, they found, stopped heart function decay in middle-aged mice and even reversed the biological clock by the human equivalent of 10 years in elderly mice.
When the researchers applied the antiaging gene to samples of human heart cells from elderly people with heart problems, the cells “resumed functioning properly, proving to be more efficient in building new blood vessels,” they said in a written statement. It all kind of sounds like something out of Dr. Frankenstein’s lab.
I want to believe … in better sleep
The “X-Files” theme song plays. Mulder and Scully are sitting in a diner, breakfast laid out around them. The diner is quiet, with only a few people inside.
Mulder: I’m telling you, Scully, there’s something spooky going on here.
Scully: You mean other than the fact that this town in Georgia looks suspiciously like Vancouver?
Mulder: Not one person we spoke to yesterday has gotten a full night’s sleep since the UFO sighting last month. I’m telling you, they’re here, they’re experimenting.
Scully: Do you really want me to do this to you again?
Mulder: Do what again?
Scully: There’s nothing going on here that can’t be explained by the current research. Why, in January 2023 a study was published revealing a link between poor sleep and belief in paranormal phenomena like UFOS, demons, or ghosts. Which probably explains why you’re on your third cup of coffee for the morning.
Mulder: Scully, you’ve literally been abducted by aliens. Do we have to play this game every time?
Scully: Look, it’s simple. In a sample of nearly 9,000 people, nearly two-thirds of those who reported experiencing sleep paralysis or exploding head syndrome reported believing in UFOs and aliens walking amongst humanity, despite making up just 3% of the overall sample.
Furthermore, about 60% of those reporting sleep paralysis also reported believing near-death experiences prove the soul lingers on after death, and those with stronger insomnia symptoms were more likely to believe in the devil.
Mulder: Aha!
Scully: Aha what?
Mulder: You’re a devout Christian. You believe in the devil and the soul.
Scully: Yes, but I don’t let it interfere with a good night’s sleep, Mulder. These people saw something strange, convinced themselves it was a UFO, and now they can’t sleep. It’s a vicious cycle. The study authors even said that people experiencing strange nighttime phenomena could interpret this as evidence of aliens or other paranormal beings, thus making them even more susceptible to further sleep disruption and deepening beliefs. Look who I’m talking to.
Mulder: Always with the facts, eh?
Scully: I am a doctor, after all. And if you want more research into how paranormal belief and poor sleep quality are linked, I’d be happy to dig out the literature, because the truth is out there, Mulder.
Mulder: I hate you sometimes.
It’s ChatGPT’s world. We’re just living in it
Have you heard about ChatGPT? The artificial intelligence chatbot was just launched in November and it’s already more important to the Internet than either Vladimir Putin or “Rick and Morty.”
What’s that? You’re wondering why you should care? Well, excuuuuuse us, but we thought you might want to know that ChatGPT is in the process of taking over the world. Let’s take a quick look at what it’s been up to.
“ChatGPT bot passes law school exam”
“ChatGPT passes MBA exam given by a Wharton professor”
“A freelance writer says ChatGPT wrote a $600 article in just 30 seconds”
And here’s one that might be of interest to those of the health care persuasion: “ChatGPT can pass part of the U.S. Medical Licensing Exam.” See? It’s coming for you, too.
The artificial intelligence known as ChatGPT “performed at >50% accuracy across [the three USMLE] examinations, exceeding 60% in most analyses,” a group of researchers wrote on the preprint server medRxiv, noting that 60% is usually the pass threshold for humans taking the exam in any given year.
ChatGPT was not given any special medical training before the exam, but the investigators pointed out that another AI, PubMedGPT, which is trained exclusively on biomedical domain literature, was only 50.8% accurate on the USMLE. Its reliance on “ongoing academic discourse that tends to be inconclusive, contradictory, or highly conservative or noncommittal in its language” was its undoing, the team suggested.
To top it off, ChatGPT is listed as one of the authors at the top of the medRxiv report, with an acknowledgment at the end saying that “ChatGPT contributed to the writing of several sections of this manuscript.”
We’ve said it before, and no doubt we’ll say it again: We’re doomed.
Everybody wants a younger heart
As more people live well past 90, scientists have been taking a closer look at how they’ve been doing it. Mostly it boiled down to genetics. You either had it or you didn’t. Well, a recent study suggests that doesn’t have to be true anymore, at least for the heart.
Scientists from the United Kingdom and Italy found an antiaging gene in some centenarians that has shown possible antiaging effects in mice and in human heart cells. A single administration of the mutant antiaging gene, they found, stopped heart function decay in middle-aged mice and even reversed the biological clock by the human equivalent of 10 years in elderly mice.
When the researchers applied the antiaging gene to samples of human heart cells from elderly people with heart problems, the cells “resumed functioning properly, proving to be more efficient in building new blood vessels,” they said in a written statement. It all kind of sounds like something out of Dr. Frankenstein’s lab.
I want to believe … in better sleep
The “X-Files” theme song plays. Mulder and Scully are sitting in a diner, breakfast laid out around them. The diner is quiet, with only a few people inside.
Mulder: I’m telling you, Scully, there’s something spooky going on here.
Scully: You mean other than the fact that this town in Georgia looks suspiciously like Vancouver?
Mulder: Not one person we spoke to yesterday has gotten a full night’s sleep since the UFO sighting last month. I’m telling you, they’re here, they’re experimenting.
Scully: Do you really want me to do this to you again?
Mulder: Do what again?
Scully: There’s nothing going on here that can’t be explained by the current research. Why, in January 2023 a study was published revealing a link between poor sleep and belief in paranormal phenomena like UFOS, demons, or ghosts. Which probably explains why you’re on your third cup of coffee for the morning.
Mulder: Scully, you’ve literally been abducted by aliens. Do we have to play this game every time?
Scully: Look, it’s simple. In a sample of nearly 9,000 people, nearly two-thirds of those who reported experiencing sleep paralysis or exploding head syndrome reported believing in UFOs and aliens walking amongst humanity, despite making up just 3% of the overall sample.
Furthermore, about 60% of those reporting sleep paralysis also reported believing near-death experiences prove the soul lingers on after death, and those with stronger insomnia symptoms were more likely to believe in the devil.
Mulder: Aha!
Scully: Aha what?
Mulder: You’re a devout Christian. You believe in the devil and the soul.
Scully: Yes, but I don’t let it interfere with a good night’s sleep, Mulder. These people saw something strange, convinced themselves it was a UFO, and now they can’t sleep. It’s a vicious cycle. The study authors even said that people experiencing strange nighttime phenomena could interpret this as evidence of aliens or other paranormal beings, thus making them even more susceptible to further sleep disruption and deepening beliefs. Look who I’m talking to.
Mulder: Always with the facts, eh?
Scully: I am a doctor, after all. And if you want more research into how paranormal belief and poor sleep quality are linked, I’d be happy to dig out the literature, because the truth is out there, Mulder.
Mulder: I hate you sometimes.
It’s ChatGPT’s world. We’re just living in it
Have you heard about ChatGPT? The artificial intelligence chatbot was just launched in November and it’s already more important to the Internet than either Vladimir Putin or “Rick and Morty.”
What’s that? You’re wondering why you should care? Well, excuuuuuse us, but we thought you might want to know that ChatGPT is in the process of taking over the world. Let’s take a quick look at what it’s been up to.
“ChatGPT bot passes law school exam”
“ChatGPT passes MBA exam given by a Wharton professor”
“A freelance writer says ChatGPT wrote a $600 article in just 30 seconds”
And here’s one that might be of interest to those of the health care persuasion: “ChatGPT can pass part of the U.S. Medical Licensing Exam.” See? It’s coming for you, too.
The artificial intelligence known as ChatGPT “performed at >50% accuracy across [the three USMLE] examinations, exceeding 60% in most analyses,” a group of researchers wrote on the preprint server medRxiv, noting that 60% is usually the pass threshold for humans taking the exam in any given year.
ChatGPT was not given any special medical training before the exam, but the investigators pointed out that another AI, PubMedGPT, which is trained exclusively on biomedical domain literature, was only 50.8% accurate on the USMLE. Its reliance on “ongoing academic discourse that tends to be inconclusive, contradictory, or highly conservative or noncommittal in its language” was its undoing, the team suggested.
To top it off, ChatGPT is listed as one of the authors at the top of the medRxiv report, with an acknowledgment at the end saying that “ChatGPT contributed to the writing of several sections of this manuscript.”
We’ve said it before, and no doubt we’ll say it again: We’re doomed.
Everybody wants a younger heart
As more people live well past 90, scientists have been taking a closer look at how they’ve been doing it. Mostly it boiled down to genetics. You either had it or you didn’t. Well, a recent study suggests that doesn’t have to be true anymore, at least for the heart.
Scientists from the United Kingdom and Italy found an antiaging gene in some centenarians that has shown possible antiaging effects in mice and in human heart cells. A single administration of the mutant antiaging gene, they found, stopped heart function decay in middle-aged mice and even reversed the biological clock by the human equivalent of 10 years in elderly mice.
When the researchers applied the antiaging gene to samples of human heart cells from elderly people with heart problems, the cells “resumed functioning properly, proving to be more efficient in building new blood vessels,” they said in a written statement. It all kind of sounds like something out of Dr. Frankenstein’s lab.
I want to believe … in better sleep
The “X-Files” theme song plays. Mulder and Scully are sitting in a diner, breakfast laid out around them. The diner is quiet, with only a few people inside.
Mulder: I’m telling you, Scully, there’s something spooky going on here.
Scully: You mean other than the fact that this town in Georgia looks suspiciously like Vancouver?
Mulder: Not one person we spoke to yesterday has gotten a full night’s sleep since the UFO sighting last month. I’m telling you, they’re here, they’re experimenting.
Scully: Do you really want me to do this to you again?
Mulder: Do what again?
Scully: There’s nothing going on here that can’t be explained by the current research. Why, in January 2023 a study was published revealing a link between poor sleep and belief in paranormal phenomena like UFOS, demons, or ghosts. Which probably explains why you’re on your third cup of coffee for the morning.
Mulder: Scully, you’ve literally been abducted by aliens. Do we have to play this game every time?
Scully: Look, it’s simple. In a sample of nearly 9,000 people, nearly two-thirds of those who reported experiencing sleep paralysis or exploding head syndrome reported believing in UFOs and aliens walking amongst humanity, despite making up just 3% of the overall sample.
Furthermore, about 60% of those reporting sleep paralysis also reported believing near-death experiences prove the soul lingers on after death, and those with stronger insomnia symptoms were more likely to believe in the devil.
Mulder: Aha!
Scully: Aha what?
Mulder: You’re a devout Christian. You believe in the devil and the soul.
Scully: Yes, but I don’t let it interfere with a good night’s sleep, Mulder. These people saw something strange, convinced themselves it was a UFO, and now they can’t sleep. It’s a vicious cycle. The study authors even said that people experiencing strange nighttime phenomena could interpret this as evidence of aliens or other paranormal beings, thus making them even more susceptible to further sleep disruption and deepening beliefs. Look who I’m talking to.
Mulder: Always with the facts, eh?
Scully: I am a doctor, after all. And if you want more research into how paranormal belief and poor sleep quality are linked, I’d be happy to dig out the literature, because the truth is out there, Mulder.
Mulder: I hate you sometimes.
It’s ChatGPT’s world. We’re just living in it
Have you heard about ChatGPT? The artificial intelligence chatbot was just launched in November and it’s already more important to the Internet than either Vladimir Putin or “Rick and Morty.”
What’s that? You’re wondering why you should care? Well, excuuuuuse us, but we thought you might want to know that ChatGPT is in the process of taking over the world. Let’s take a quick look at what it’s been up to.
“ChatGPT bot passes law school exam”
“ChatGPT passes MBA exam given by a Wharton professor”
“A freelance writer says ChatGPT wrote a $600 article in just 30 seconds”
And here’s one that might be of interest to those of the health care persuasion: “ChatGPT can pass part of the U.S. Medical Licensing Exam.” See? It’s coming for you, too.
The artificial intelligence known as ChatGPT “performed at >50% accuracy across [the three USMLE] examinations, exceeding 60% in most analyses,” a group of researchers wrote on the preprint server medRxiv, noting that 60% is usually the pass threshold for humans taking the exam in any given year.
ChatGPT was not given any special medical training before the exam, but the investigators pointed out that another AI, PubMedGPT, which is trained exclusively on biomedical domain literature, was only 50.8% accurate on the USMLE. Its reliance on “ongoing academic discourse that tends to be inconclusive, contradictory, or highly conservative or noncommittal in its language” was its undoing, the team suggested.
To top it off, ChatGPT is listed as one of the authors at the top of the medRxiv report, with an acknowledgment at the end saying that “ChatGPT contributed to the writing of several sections of this manuscript.”
We’ve said it before, and no doubt we’ll say it again: We’re doomed.
Canadian guidance recommends reducing alcohol consumption
“Drinking less is better,” says the guidance, which replaces Canada’s 2011 Low-Risk Drinking Guidelines (LRDGs).
Developed in consultation with an executive committee from federal, provincial, and territorial governments; national organizations; three scientific expert panels; and an internal evidence review working group, the guidance presents the following findings:
- Consuming no drinks per week has benefits, such as better health and better sleep, and it’s the only safe option during pregnancy.
- Consuming one or two standard drinks weekly will likely not have alcohol-related consequences.
- Three to six drinks raise the risk of developing breast, colon, and other cancers.
- Seven or more increase the risk of heart disease or stroke.
- Each additional drink “radically increases” the risk of these health consequences.
“Alcohol is more harmful than was previously thought and is a key component of the health of your patients,” Adam Sherk, PhD, a scientist at the Canadian Institute for Substance Use Research at the University of Victoria (B.C.), and a member of the scientific expert panel that contributed to the guidance, said in an interview. “Display and discuss the new guidance with your patients with the main message that drinking less is better.”
Peter Butt, MD, a clinical associate professor at the University of Saskatchewan, Saskatoon, and cochair of the guidance project, said in an interview: “The World Health Organization has identified over 200 ICD-coded conditions associated with alcohol use. This creates many opportunities to inquire into quantity and frequency of alcohol use, relate it to the patient’s health and well-being, and provide advice on reduction.”
“Canada’s Guidance on Alcohol and Health: Final Report” and a related infographic were published online Jan. 17.
Continuum of risk
The impetus for the new guidance came from the fact that “our 2011 LRDGs were no longer current, and there was emerging evidence that people drinking within those levels were coming to harm,” said Dr. Butt.
That evidence indicates that alcohol causes at least seven types of cancer, mostly of the breast or colon; is a risk factor for most types of heart disease; and is a main cause of liver disease. Evidence also indicates that avoiding drinking to the point of intoxication will reduce people’s risk of perpetrating alcohol-related violence.
Responding to the need to accurately quantify the risk, the guidance defines a “standard” drink as 12 oz of beer, cooler, or cider (5% alcohol); 5 oz of wine (12% alcohol); and 1.5 oz of spirits such as whiskey, vodka, or gin (40% alcohol).
Using different mortality risk thresholds, the project’s experts developed the following continuum of risk:
- Low for individuals who consume two standard drinks or fewer per week
- Moderate for those who consume from three to six standard drinks per week
- Increasingly high for those who consume seven standard drinks or more per week
The guidance makes the following observations:
- Consuming more than two standard drinks per drinking occasion is associated with an increased risk of harms to self and others, including injuries and violence.
- When pregnant or trying to get pregnant, no amount of alcohol is safe.
- When breastfeeding, not drinking is safest.
- Above the upper limit of the moderate risk zone, health risks increase more steeply for females than males.
- Far more injuries, violence, and deaths result from men’s alcohol use, especially for per occasion drinking, than from women’s alcohol use.
- Young people should delay alcohol use for as long as possible.
- Individuals should not start to use alcohol or increase their alcohol use for health benefits.
- Any reduction in alcohol use is beneficial.
Other national guidelines
“Countries that haven’t updated their alcohol use guidelines recently should do so, as the evidence regarding alcohol and health has advanced considerably in the past 10 years,” said Dr. Sherk. He acknowledged that “any time health guidance changes substantially, it’s reasonable to expect a period of readjustment.”
“Some will be resistant,” Dr. Butt agreed. “Some professionals will need more education than others on the health effects of alcohol. Some patients will also be more invested in drinking than others. The harm-reduction, risk-zone approach should assist in the process of engaging patients and helping them reduce over time.
“Just as we benefited from the updates done in the United Kingdom, France, and especially Australia, so also researchers elsewhere will critique our work and our approach and make their own decisions on how best to communicate with their public,” Dr. Butt said. He noted that Canada’s contributions regarding the association between alcohol and violence, as well as their sex/gender approach to the evidence, “may influence the next country’s review.”
Commenting on whether the United States should consider changing its guidance, Timothy Brennan, MD, MPH, chief of clinical services for the Addiction Institute of Mount Sinai Health System in New York, said in an interview, “A lot of people will be surprised at the recommended limits on alcohol. Most think that they can have one or two glasses of alcohol per day and not have any increased risk to their health. I think the Canadians deserve credit for putting themselves out there.”
Dr. Brennan said there will “certainly be pushback by the drinking lobby, which is very strong both in the U.S. and in Canada.” In fact, the national trade group Beer Canada was recently quoted as stating that it still supports the 2011 guidelines and that the updating process lacked full transparency and expert technical peer review.
Nevertheless, Dr. Brennan said, “it’s overwhelmingly clear that alcohol affects a ton of different parts of our body, so limiting the amount of alcohol we take in is always going to be a good thing. The Canadian graphic is great because it color-codes the risk. I recommend that clinicians put it up in their offices and begin quantifying the units of alcohol that are going into a patient’s body each day.”
A version of this article originally appeared on Medscape.com.
“Drinking less is better,” says the guidance, which replaces Canada’s 2011 Low-Risk Drinking Guidelines (LRDGs).
Developed in consultation with an executive committee from federal, provincial, and territorial governments; national organizations; three scientific expert panels; and an internal evidence review working group, the guidance presents the following findings:
- Consuming no drinks per week has benefits, such as better health and better sleep, and it’s the only safe option during pregnancy.
- Consuming one or two standard drinks weekly will likely not have alcohol-related consequences.
- Three to six drinks raise the risk of developing breast, colon, and other cancers.
- Seven or more increase the risk of heart disease or stroke.
- Each additional drink “radically increases” the risk of these health consequences.
“Alcohol is more harmful than was previously thought and is a key component of the health of your patients,” Adam Sherk, PhD, a scientist at the Canadian Institute for Substance Use Research at the University of Victoria (B.C.), and a member of the scientific expert panel that contributed to the guidance, said in an interview. “Display and discuss the new guidance with your patients with the main message that drinking less is better.”
Peter Butt, MD, a clinical associate professor at the University of Saskatchewan, Saskatoon, and cochair of the guidance project, said in an interview: “The World Health Organization has identified over 200 ICD-coded conditions associated with alcohol use. This creates many opportunities to inquire into quantity and frequency of alcohol use, relate it to the patient’s health and well-being, and provide advice on reduction.”
“Canada’s Guidance on Alcohol and Health: Final Report” and a related infographic were published online Jan. 17.
Continuum of risk
The impetus for the new guidance came from the fact that “our 2011 LRDGs were no longer current, and there was emerging evidence that people drinking within those levels were coming to harm,” said Dr. Butt.
That evidence indicates that alcohol causes at least seven types of cancer, mostly of the breast or colon; is a risk factor for most types of heart disease; and is a main cause of liver disease. Evidence also indicates that avoiding drinking to the point of intoxication will reduce people’s risk of perpetrating alcohol-related violence.
Responding to the need to accurately quantify the risk, the guidance defines a “standard” drink as 12 oz of beer, cooler, or cider (5% alcohol); 5 oz of wine (12% alcohol); and 1.5 oz of spirits such as whiskey, vodka, or gin (40% alcohol).
Using different mortality risk thresholds, the project’s experts developed the following continuum of risk:
- Low for individuals who consume two standard drinks or fewer per week
- Moderate for those who consume from three to six standard drinks per week
- Increasingly high for those who consume seven standard drinks or more per week
The guidance makes the following observations:
- Consuming more than two standard drinks per drinking occasion is associated with an increased risk of harms to self and others, including injuries and violence.
- When pregnant or trying to get pregnant, no amount of alcohol is safe.
- When breastfeeding, not drinking is safest.
- Above the upper limit of the moderate risk zone, health risks increase more steeply for females than males.
- Far more injuries, violence, and deaths result from men’s alcohol use, especially for per occasion drinking, than from women’s alcohol use.
- Young people should delay alcohol use for as long as possible.
- Individuals should not start to use alcohol or increase their alcohol use for health benefits.
- Any reduction in alcohol use is beneficial.
Other national guidelines
“Countries that haven’t updated their alcohol use guidelines recently should do so, as the evidence regarding alcohol and health has advanced considerably in the past 10 years,” said Dr. Sherk. He acknowledged that “any time health guidance changes substantially, it’s reasonable to expect a period of readjustment.”
“Some will be resistant,” Dr. Butt agreed. “Some professionals will need more education than others on the health effects of alcohol. Some patients will also be more invested in drinking than others. The harm-reduction, risk-zone approach should assist in the process of engaging patients and helping them reduce over time.
“Just as we benefited from the updates done in the United Kingdom, France, and especially Australia, so also researchers elsewhere will critique our work and our approach and make their own decisions on how best to communicate with their public,” Dr. Butt said. He noted that Canada’s contributions regarding the association between alcohol and violence, as well as their sex/gender approach to the evidence, “may influence the next country’s review.”
Commenting on whether the United States should consider changing its guidance, Timothy Brennan, MD, MPH, chief of clinical services for the Addiction Institute of Mount Sinai Health System in New York, said in an interview, “A lot of people will be surprised at the recommended limits on alcohol. Most think that they can have one or two glasses of alcohol per day and not have any increased risk to their health. I think the Canadians deserve credit for putting themselves out there.”
Dr. Brennan said there will “certainly be pushback by the drinking lobby, which is very strong both in the U.S. and in Canada.” In fact, the national trade group Beer Canada was recently quoted as stating that it still supports the 2011 guidelines and that the updating process lacked full transparency and expert technical peer review.
Nevertheless, Dr. Brennan said, “it’s overwhelmingly clear that alcohol affects a ton of different parts of our body, so limiting the amount of alcohol we take in is always going to be a good thing. The Canadian graphic is great because it color-codes the risk. I recommend that clinicians put it up in their offices and begin quantifying the units of alcohol that are going into a patient’s body each day.”
A version of this article originally appeared on Medscape.com.
“Drinking less is better,” says the guidance, which replaces Canada’s 2011 Low-Risk Drinking Guidelines (LRDGs).
Developed in consultation with an executive committee from federal, provincial, and territorial governments; national organizations; three scientific expert panels; and an internal evidence review working group, the guidance presents the following findings:
- Consuming no drinks per week has benefits, such as better health and better sleep, and it’s the only safe option during pregnancy.
- Consuming one or two standard drinks weekly will likely not have alcohol-related consequences.
- Three to six drinks raise the risk of developing breast, colon, and other cancers.
- Seven or more increase the risk of heart disease or stroke.
- Each additional drink “radically increases” the risk of these health consequences.
“Alcohol is more harmful than was previously thought and is a key component of the health of your patients,” Adam Sherk, PhD, a scientist at the Canadian Institute for Substance Use Research at the University of Victoria (B.C.), and a member of the scientific expert panel that contributed to the guidance, said in an interview. “Display and discuss the new guidance with your patients with the main message that drinking less is better.”
Peter Butt, MD, a clinical associate professor at the University of Saskatchewan, Saskatoon, and cochair of the guidance project, said in an interview: “The World Health Organization has identified over 200 ICD-coded conditions associated with alcohol use. This creates many opportunities to inquire into quantity and frequency of alcohol use, relate it to the patient’s health and well-being, and provide advice on reduction.”
“Canada’s Guidance on Alcohol and Health: Final Report” and a related infographic were published online Jan. 17.
Continuum of risk
The impetus for the new guidance came from the fact that “our 2011 LRDGs were no longer current, and there was emerging evidence that people drinking within those levels were coming to harm,” said Dr. Butt.
That evidence indicates that alcohol causes at least seven types of cancer, mostly of the breast or colon; is a risk factor for most types of heart disease; and is a main cause of liver disease. Evidence also indicates that avoiding drinking to the point of intoxication will reduce people’s risk of perpetrating alcohol-related violence.
Responding to the need to accurately quantify the risk, the guidance defines a “standard” drink as 12 oz of beer, cooler, or cider (5% alcohol); 5 oz of wine (12% alcohol); and 1.5 oz of spirits such as whiskey, vodka, or gin (40% alcohol).
Using different mortality risk thresholds, the project’s experts developed the following continuum of risk:
- Low for individuals who consume two standard drinks or fewer per week
- Moderate for those who consume from three to six standard drinks per week
- Increasingly high for those who consume seven standard drinks or more per week
The guidance makes the following observations:
- Consuming more than two standard drinks per drinking occasion is associated with an increased risk of harms to self and others, including injuries and violence.
- When pregnant or trying to get pregnant, no amount of alcohol is safe.
- When breastfeeding, not drinking is safest.
- Above the upper limit of the moderate risk zone, health risks increase more steeply for females than males.
- Far more injuries, violence, and deaths result from men’s alcohol use, especially for per occasion drinking, than from women’s alcohol use.
- Young people should delay alcohol use for as long as possible.
- Individuals should not start to use alcohol or increase their alcohol use for health benefits.
- Any reduction in alcohol use is beneficial.
Other national guidelines
“Countries that haven’t updated their alcohol use guidelines recently should do so, as the evidence regarding alcohol and health has advanced considerably in the past 10 years,” said Dr. Sherk. He acknowledged that “any time health guidance changes substantially, it’s reasonable to expect a period of readjustment.”
“Some will be resistant,” Dr. Butt agreed. “Some professionals will need more education than others on the health effects of alcohol. Some patients will also be more invested in drinking than others. The harm-reduction, risk-zone approach should assist in the process of engaging patients and helping them reduce over time.
“Just as we benefited from the updates done in the United Kingdom, France, and especially Australia, so also researchers elsewhere will critique our work and our approach and make their own decisions on how best to communicate with their public,” Dr. Butt said. He noted that Canada’s contributions regarding the association between alcohol and violence, as well as their sex/gender approach to the evidence, “may influence the next country’s review.”
Commenting on whether the United States should consider changing its guidance, Timothy Brennan, MD, MPH, chief of clinical services for the Addiction Institute of Mount Sinai Health System in New York, said in an interview, “A lot of people will be surprised at the recommended limits on alcohol. Most think that they can have one or two glasses of alcohol per day and not have any increased risk to their health. I think the Canadians deserve credit for putting themselves out there.”
Dr. Brennan said there will “certainly be pushback by the drinking lobby, which is very strong both in the U.S. and in Canada.” In fact, the national trade group Beer Canada was recently quoted as stating that it still supports the 2011 guidelines and that the updating process lacked full transparency and expert technical peer review.
Nevertheless, Dr. Brennan said, “it’s overwhelmingly clear that alcohol affects a ton of different parts of our body, so limiting the amount of alcohol we take in is always going to be a good thing. The Canadian graphic is great because it color-codes the risk. I recommend that clinicians put it up in their offices and begin quantifying the units of alcohol that are going into a patient’s body each day.”
A version of this article originally appeared on Medscape.com.
Concern grows over ‘medical assistance in dying for mental illness’ law
Canada already has the largest number of deaths by MAID of any nation, with 10,064 in 2021, a 32% increase from 2020. With the addition of serious mental illness (SMI) as an eligible category, the country is on track to have the most liberal assisted-death policy in the world.
Concerns about the additional number of patients who could become eligible for MAID, and a lack of evidence-backed standards from disability rights groups, mental health advocates, First Nations leaders, psychiatrists, and other mental health providers, seems to have led the Canadian government to give the proposed law some sober second thought.
“Listening to experts and Canadians, we believe this date needs to be temporarily delayed,” said David Lametti, Canada’s minister of Justice and attorney general of Canada; Jean-Yves Duclos, minister of Health; and Carolyn Bennett, minister of Mental Health and Addictions, in a Dec. 15, 2022, joint statement.
Canada’s Parliament – which approved the expansion – will now have to vote on whether to okay a pause on the legislation.
However, the Canadian Psychiatric Association has not been calling for a delay in the proposed legislation. In a November 2021 statement, the CPA said it “does not take a position on the legality or morality of MAID,” but added that to deny MAID to people with mental illness was discriminatory, and that, as it was the law, it must be followed.
“CPA has not taken a position about MAID,” the association’s president Gary Chaimowitz, MBChB, told this news organization. “We know this is coming and our organization is trying to get its members ready for what will be most likely the ability of people with mental conditions to be able to request MAID,” said Dr. Chaimowitz, who is also head of forensic psychiatry at St. Joseph’s Healthcare and a professor of psychiatry at McMaster University, both in Hamilton, Ont.
Dr. Chaimowitz acknowledges that “a majority of psychiatrists do not want to be involved in anything to do with MAID.”
“The idea, certainly in psychiatry, is to get people well and we’ve been taught that people dying from a major mental disorder is something that we’re trained to prevent,” he added.
A ‘clinical option’
Assisted medical death is especially fraught in psychiatry, said Rebecca Brendel, MD, president of the American Psychiatric Association. She noted a 25-year life expectancy gap between people with SMI and those who do not have such conditions.
“As a profession we have very serious obligations to advance treatment so that a person with serious mental illness can live [a] full, productive, and healthy [life],” Dr. Brendel, associate director of the Center for Bioethics at Harvard Medical School in Boston, said in an interview.
Under the Canadian proposal, psychiatrists would be allowed to suggest MAID as a “clinical option.”
Harold Braswell, PhD, a fellow with The Hastings Center, a bioethics research institute, calls that problematic.
“It’s not neutral to suggest to someone that it would be theoretically reasonable to end their lives,” Dr. Braswell, associate professor at the Albert Gnaegi Center for Health Care Ethics at Saint Louis University, told this news organization.
It also creates a double standard in the treatment of suicidal ideation, in which suicide prevention is absolute for some, but encouraging it as a possibility for others, he added.
“To have that come from an authority figure is something that’s very harsh and, in my opinion, very potentially destructive,” especially for vulnerable groups, like First Nations people, who already have elevated rates of suicide, said Dr. Braswell.
Fierce debate
Since 2016, Canada has allowed MAID for medical conditions and diseases that will not improve and in cases where the evidence shows that medical providers can accurately predict the condition will not improve.
However, in 2019, a Quebec court ruled that the law unconstitutionally barred euthanasia in people who were not terminally ill. In March 2021, Canada’s criminal code was amended to allow MAID for people whose natural death was not “reasonably foreseeable,” but it excluded SMI for a period of 2 years, ending in March 2023.
The 2-year stay was intended to allow for study and to give mental health providers and MAID assessors time to develop standards.
The federal government charged a 12-member expert panel with determining how to safely allow MAID for SMI. In its final report released in May 2022 it recommended that standards be developed.
The panel acknowledged that for many conditions it may be impossible to make predictions about whether an individual might improve. However, it did not mention SMI.
In those cases, when MAID is requested, “establishing incurability and irreversibility on the basis of the evolution and response to past interventions is necessary,” the panel noted, adding that these are the criteria used by psychiatrists assessing euthanasia requests in the Netherlands and Belgium.
But the notion that mental illness can be irremediable has been fiercely debated.
Soon after the expert report was released, the Center for Addiction and Mental Health in Toronto noted on its website that there are currently “no agreed upon standards for psychiatrists or other health care practitioners to use to determine if a person’s mental illness is ‘grievous and irremediable’ for the purposes of MAID.”
Dr. Chaimowitz acknowledged that “there’s no agreed-upon definition of incurability” in mental illness. Some psychiatrists “will argue that there’s always another treatment that can be attempted,” he said, adding that there has been a lack of consensus on irremediability among CPA members.
Protecting vulnerable populations
Matt Wynia, MD, MPH, FACP, director of the Center for Bioethics and Humanities at the University of Colorado at Denver, Aurora, said the question of irremediability is crucial. “Most people with mental illness do get better, especially if they’re in treatment,” Dr. Wynia said.
For MAID assessors it may be difficult to know when someone has tried all possible treatments, especially given the wide array of options, including psychedelics, said Dr. Wynia.
Dr. Braswell said there is not enough evidence that mental illness is incurable. With SMI, “there’s a lot more potential for the causes of the individual’s suffering to be ameliorated. By offering MAID, you’re going to kill people who might have been able to get out of this through other nonlethal means.”
Currently, MAID is provided for an irremediable medical condition, “in other words, a condition that will not improve and that we can predict will not improve,” said Karandeep S. Gaind, MD, chief of psychiatry at Toronto’s Humber River Hospital and physician chair of the hospital’s MAID team.
“If that’s the premise, then I think we cannot provide MAID for sole mental illness,” Dr. Gaind said. “Because we can’t honestly make those predictions” with mental illness, he added.
Dr. Gaind does not support MAID for mental illness and believes that it will put the vulnerable – including those living in poverty – at particular risk.
With the proposed expansion, MAID is “now becoming something which is being sought as a way to escape a painful life rather than to avoid a painful death,” said Dr. Gaind, who is also a past president of the CPA.
One member of the federal government’s expert panel – Ellen Cohen, who had a psychiatric condition – wrote in The Globe and Mail that she quit early on when it became apparent that the panel was not seriously considering her own experiences or the possibility that poverty and lack of access to care or social supports could strongly influence a request for MAID.
Social determinants of suffering
People with mental illness often are without homes, have substance use disorders, have been stigmatized and discriminated against, and have poor social supports, said Dr. Wynia. “You worry that it’s all of those things that are making them want to end their lives,” he said.
The Daily Mail ran a story in December 2022 about a 65-year-old Canadian who said he’d applied for MAID solely because of fears that his disability benefits for various chronic health conditions were being cut off and that he didn’t want to live in poverty.
A 51-year-old Ontario woman with multiple chemical sensitivities was granted MAID after she said she could not find housing that could keep her safe, according to an August report by CTV News.
Tarek Rajji, MD, chief of the Adult Neurodevelopment and Geriatric Psychiatry Division at CAMH, said social determinants of health need to be considered in standards created to guide MAID for mental illness.
“We’re very mindful of the fact that the suffering, that is, the grievousness that the person is living with, in the context of mental illness, many times is due to the social determinants of their illness and the social determinants of their suffering,” Dr. Rajji said.
Many are also concerned that it will be difficult to separate out suicidality from sheer hopelessness.
The CPA has advised a group that’s working on developing guidelines for MAID in SMI and is also developing a curriculum for mental health providers, Dr. Chaimowitz said. As part of that, there will be a process to ensure that someone who is actively suicidal is not granted MAID.
“I do not believe that it’s contemplated that MAID is going to accelerate or facilitate suicidal ideation,” he said. Someone who is suicidal will be referred to treatment, said Dr. Chaimowitz.
“People with depression often feel hopeless,” and may refuse treatments that have worked in the past, countered Dr. Gaind. Some of his patients “are absolutely convinced that nothing will help,” he said.
Troublesome cases
The expert panel said in its final report that “it is not possible to provide fixed rules for how many attempts at interventions, how many types of interventions, and over how much time,” are necessary to establish “irreversibility” of mental illness.
Dr. Chaimowitz said MAID will not be offered to anyone “refusing treatment for their condition without any good reason.” They will be “unlikely to meet criteria for incurable,” as they will have needed to avail themselves of the array of treatments available, he said.
That would be similar to rules in Belgium and the Netherlands, which allow euthanasia for psychiatric conditions.
An estimated 100-300 psychiatric patients receive euthanasia each year in those countries, according to a 2021 commentary in Psychiatric Times (Jun 7;38[6]) by Mark S. Komrad, MD, a Towson, Maryland-based psychiatrist.
There are still troublesome cases.
As previously reported by this news organization, many in Belgium were distressed recently at the news that a 23-year-old woman who had survived a terrorist attack, Shanti De Corte, requested and was granted euthanasia.
As the deadline for implementation of MAID grew closer, calls for delay grew louder, especially given the lack of concrete standards for providers.
During the waning months of 2022, Dr. Gaind – who said he was suspended from CPA for “unprofessional interactions” and allegedly misrepresenting CPA’s processes and governance matters – announced the launch of a new organization, the Society of Canadian Psychiatry, in November calling for a delay in MAID of at least 1 year so that evidence-based safeguards could be implemented. The petition has been signed by more than 200 psychiatrists, along with several dozen physicians, MAID assessors, and individuals with mental illness and family members.
The Association of Chairs of Psychiatry in Canada, the Canadian Association for Suicide Prevention, the Council of Canadians with Disabilities, a group of indigenous leaders, and the Ontario Association for ACT and FACT, psychiatrists who provide care to individuals with severe mental illness, among other groups, joined the call for a delay.
In its December announcement, the Canadian federal ministers said a factor in seeking a delay was that standards guiding clinicians would not be delivered until at least February – too close to when applications would be opened.
Upon hearing about the federal government’s intentions, the chair of the expert panel, Mona Gupta, MD, told The Canadian Press that she did not think it was necessary to put off implementation because necessary safeguards were already in place.
Dr. Chaimowitz awaits the standards but is optimistic that for mental illness, “the process will be tightly controlled, closely monitored, and open to scrutiny,” he said.
Dr. Braswell is not convinced. The concern is that adding people with mental illness is “going to overload the capacity of the government to monitor this practice,” he said.
Is the United States next?
Although Canada and the United States share a border, it’s unlikely that U.S. states will allow aid in dying for nonterminal illness, much less for psychiatric conditions any time soon, said Dr. Braswell and others.
Ten states – California, Colorado, Hawaii, Maine, Montana, New Jersey, New Mexico, Oregon, Vermont, and Washington – have laws allowing assistance in dying, but for terminal illness only.
In 2016, the APA adopted the American Medical Association policy on medical euthanasia, stating, “that a psychiatrist should not prescribe or administer any intervention to a nonterminally ill person for the purpose of causing death.”
Dr. Brendel said the field is acutely aware that people with mental illness do suffer, but that more work needs to be done – and is being done – on “distinguishing wishes to hasten death or end one’s life from these historical or traditional notions that any premature death is a suicide.”
There is also increasing discussion within the medical community, not just psychiatry, about a physician’s duty to relieve suffering, said Dr. Wynia. “There’s debate basically about whether we stand for preserving life essentially at all costs and never being involved in the taking of life, or whether we stand for reduction of suffering and being the advocate for the patients that we serve,” he said.
“Those are both legitimate,” said Dr. Wynia, adding, “there are good reasons to want both of those to be true.”
“I suspect that 20 years from now we will still be having conversations about how physicians, how psychiatrists ought to participate in preserving life and in shepherding death,” said Dr. Brendel.
But to Dr. Gaind, the debate is not just esoteric, it’s a soon-to-be reality in Canada. “When we’re providing death to people who aren’t dying, to me that’s like providing what amounts to a wrongful death,” he said.
A version of this article originally appeared on Medscape.com.
Canada already has the largest number of deaths by MAID of any nation, with 10,064 in 2021, a 32% increase from 2020. With the addition of serious mental illness (SMI) as an eligible category, the country is on track to have the most liberal assisted-death policy in the world.
Concerns about the additional number of patients who could become eligible for MAID, and a lack of evidence-backed standards from disability rights groups, mental health advocates, First Nations leaders, psychiatrists, and other mental health providers, seems to have led the Canadian government to give the proposed law some sober second thought.
“Listening to experts and Canadians, we believe this date needs to be temporarily delayed,” said David Lametti, Canada’s minister of Justice and attorney general of Canada; Jean-Yves Duclos, minister of Health; and Carolyn Bennett, minister of Mental Health and Addictions, in a Dec. 15, 2022, joint statement.
Canada’s Parliament – which approved the expansion – will now have to vote on whether to okay a pause on the legislation.
However, the Canadian Psychiatric Association has not been calling for a delay in the proposed legislation. In a November 2021 statement, the CPA said it “does not take a position on the legality or morality of MAID,” but added that to deny MAID to people with mental illness was discriminatory, and that, as it was the law, it must be followed.
“CPA has not taken a position about MAID,” the association’s president Gary Chaimowitz, MBChB, told this news organization. “We know this is coming and our organization is trying to get its members ready for what will be most likely the ability of people with mental conditions to be able to request MAID,” said Dr. Chaimowitz, who is also head of forensic psychiatry at St. Joseph’s Healthcare and a professor of psychiatry at McMaster University, both in Hamilton, Ont.
Dr. Chaimowitz acknowledges that “a majority of psychiatrists do not want to be involved in anything to do with MAID.”
“The idea, certainly in psychiatry, is to get people well and we’ve been taught that people dying from a major mental disorder is something that we’re trained to prevent,” he added.
A ‘clinical option’
Assisted medical death is especially fraught in psychiatry, said Rebecca Brendel, MD, president of the American Psychiatric Association. She noted a 25-year life expectancy gap between people with SMI and those who do not have such conditions.
“As a profession we have very serious obligations to advance treatment so that a person with serious mental illness can live [a] full, productive, and healthy [life],” Dr. Brendel, associate director of the Center for Bioethics at Harvard Medical School in Boston, said in an interview.
Under the Canadian proposal, psychiatrists would be allowed to suggest MAID as a “clinical option.”
Harold Braswell, PhD, a fellow with The Hastings Center, a bioethics research institute, calls that problematic.
“It’s not neutral to suggest to someone that it would be theoretically reasonable to end their lives,” Dr. Braswell, associate professor at the Albert Gnaegi Center for Health Care Ethics at Saint Louis University, told this news organization.
It also creates a double standard in the treatment of suicidal ideation, in which suicide prevention is absolute for some, but encouraging it as a possibility for others, he added.
“To have that come from an authority figure is something that’s very harsh and, in my opinion, very potentially destructive,” especially for vulnerable groups, like First Nations people, who already have elevated rates of suicide, said Dr. Braswell.
Fierce debate
Since 2016, Canada has allowed MAID for medical conditions and diseases that will not improve and in cases where the evidence shows that medical providers can accurately predict the condition will not improve.
However, in 2019, a Quebec court ruled that the law unconstitutionally barred euthanasia in people who were not terminally ill. In March 2021, Canada’s criminal code was amended to allow MAID for people whose natural death was not “reasonably foreseeable,” but it excluded SMI for a period of 2 years, ending in March 2023.
The 2-year stay was intended to allow for study and to give mental health providers and MAID assessors time to develop standards.
The federal government charged a 12-member expert panel with determining how to safely allow MAID for SMI. In its final report released in May 2022 it recommended that standards be developed.
The panel acknowledged that for many conditions it may be impossible to make predictions about whether an individual might improve. However, it did not mention SMI.
In those cases, when MAID is requested, “establishing incurability and irreversibility on the basis of the evolution and response to past interventions is necessary,” the panel noted, adding that these are the criteria used by psychiatrists assessing euthanasia requests in the Netherlands and Belgium.
But the notion that mental illness can be irremediable has been fiercely debated.
Soon after the expert report was released, the Center for Addiction and Mental Health in Toronto noted on its website that there are currently “no agreed upon standards for psychiatrists or other health care practitioners to use to determine if a person’s mental illness is ‘grievous and irremediable’ for the purposes of MAID.”
Dr. Chaimowitz acknowledged that “there’s no agreed-upon definition of incurability” in mental illness. Some psychiatrists “will argue that there’s always another treatment that can be attempted,” he said, adding that there has been a lack of consensus on irremediability among CPA members.
Protecting vulnerable populations
Matt Wynia, MD, MPH, FACP, director of the Center for Bioethics and Humanities at the University of Colorado at Denver, Aurora, said the question of irremediability is crucial. “Most people with mental illness do get better, especially if they’re in treatment,” Dr. Wynia said.
For MAID assessors it may be difficult to know when someone has tried all possible treatments, especially given the wide array of options, including psychedelics, said Dr. Wynia.
Dr. Braswell said there is not enough evidence that mental illness is incurable. With SMI, “there’s a lot more potential for the causes of the individual’s suffering to be ameliorated. By offering MAID, you’re going to kill people who might have been able to get out of this through other nonlethal means.”
Currently, MAID is provided for an irremediable medical condition, “in other words, a condition that will not improve and that we can predict will not improve,” said Karandeep S. Gaind, MD, chief of psychiatry at Toronto’s Humber River Hospital and physician chair of the hospital’s MAID team.
“If that’s the premise, then I think we cannot provide MAID for sole mental illness,” Dr. Gaind said. “Because we can’t honestly make those predictions” with mental illness, he added.
Dr. Gaind does not support MAID for mental illness and believes that it will put the vulnerable – including those living in poverty – at particular risk.
With the proposed expansion, MAID is “now becoming something which is being sought as a way to escape a painful life rather than to avoid a painful death,” said Dr. Gaind, who is also a past president of the CPA.
One member of the federal government’s expert panel – Ellen Cohen, who had a psychiatric condition – wrote in The Globe and Mail that she quit early on when it became apparent that the panel was not seriously considering her own experiences or the possibility that poverty and lack of access to care or social supports could strongly influence a request for MAID.
Social determinants of suffering
People with mental illness often are without homes, have substance use disorders, have been stigmatized and discriminated against, and have poor social supports, said Dr. Wynia. “You worry that it’s all of those things that are making them want to end their lives,” he said.
The Daily Mail ran a story in December 2022 about a 65-year-old Canadian who said he’d applied for MAID solely because of fears that his disability benefits for various chronic health conditions were being cut off and that he didn’t want to live in poverty.
A 51-year-old Ontario woman with multiple chemical sensitivities was granted MAID after she said she could not find housing that could keep her safe, according to an August report by CTV News.
Tarek Rajji, MD, chief of the Adult Neurodevelopment and Geriatric Psychiatry Division at CAMH, said social determinants of health need to be considered in standards created to guide MAID for mental illness.
“We’re very mindful of the fact that the suffering, that is, the grievousness that the person is living with, in the context of mental illness, many times is due to the social determinants of their illness and the social determinants of their suffering,” Dr. Rajji said.
Many are also concerned that it will be difficult to separate out suicidality from sheer hopelessness.
The CPA has advised a group that’s working on developing guidelines for MAID in SMI and is also developing a curriculum for mental health providers, Dr. Chaimowitz said. As part of that, there will be a process to ensure that someone who is actively suicidal is not granted MAID.
“I do not believe that it’s contemplated that MAID is going to accelerate or facilitate suicidal ideation,” he said. Someone who is suicidal will be referred to treatment, said Dr. Chaimowitz.
“People with depression often feel hopeless,” and may refuse treatments that have worked in the past, countered Dr. Gaind. Some of his patients “are absolutely convinced that nothing will help,” he said.
Troublesome cases
The expert panel said in its final report that “it is not possible to provide fixed rules for how many attempts at interventions, how many types of interventions, and over how much time,” are necessary to establish “irreversibility” of mental illness.
Dr. Chaimowitz said MAID will not be offered to anyone “refusing treatment for their condition without any good reason.” They will be “unlikely to meet criteria for incurable,” as they will have needed to avail themselves of the array of treatments available, he said.
That would be similar to rules in Belgium and the Netherlands, which allow euthanasia for psychiatric conditions.
An estimated 100-300 psychiatric patients receive euthanasia each year in those countries, according to a 2021 commentary in Psychiatric Times (Jun 7;38[6]) by Mark S. Komrad, MD, a Towson, Maryland-based psychiatrist.
There are still troublesome cases.
As previously reported by this news organization, many in Belgium were distressed recently at the news that a 23-year-old woman who had survived a terrorist attack, Shanti De Corte, requested and was granted euthanasia.
As the deadline for implementation of MAID grew closer, calls for delay grew louder, especially given the lack of concrete standards for providers.
During the waning months of 2022, Dr. Gaind – who said he was suspended from CPA for “unprofessional interactions” and allegedly misrepresenting CPA’s processes and governance matters – announced the launch of a new organization, the Society of Canadian Psychiatry, in November calling for a delay in MAID of at least 1 year so that evidence-based safeguards could be implemented. The petition has been signed by more than 200 psychiatrists, along with several dozen physicians, MAID assessors, and individuals with mental illness and family members.
The Association of Chairs of Psychiatry in Canada, the Canadian Association for Suicide Prevention, the Council of Canadians with Disabilities, a group of indigenous leaders, and the Ontario Association for ACT and FACT, psychiatrists who provide care to individuals with severe mental illness, among other groups, joined the call for a delay.
In its December announcement, the Canadian federal ministers said a factor in seeking a delay was that standards guiding clinicians would not be delivered until at least February – too close to when applications would be opened.
Upon hearing about the federal government’s intentions, the chair of the expert panel, Mona Gupta, MD, told The Canadian Press that she did not think it was necessary to put off implementation because necessary safeguards were already in place.
Dr. Chaimowitz awaits the standards but is optimistic that for mental illness, “the process will be tightly controlled, closely monitored, and open to scrutiny,” he said.
Dr. Braswell is not convinced. The concern is that adding people with mental illness is “going to overload the capacity of the government to monitor this practice,” he said.
Is the United States next?
Although Canada and the United States share a border, it’s unlikely that U.S. states will allow aid in dying for nonterminal illness, much less for psychiatric conditions any time soon, said Dr. Braswell and others.
Ten states – California, Colorado, Hawaii, Maine, Montana, New Jersey, New Mexico, Oregon, Vermont, and Washington – have laws allowing assistance in dying, but for terminal illness only.
In 2016, the APA adopted the American Medical Association policy on medical euthanasia, stating, “that a psychiatrist should not prescribe or administer any intervention to a nonterminally ill person for the purpose of causing death.”
Dr. Brendel said the field is acutely aware that people with mental illness do suffer, but that more work needs to be done – and is being done – on “distinguishing wishes to hasten death or end one’s life from these historical or traditional notions that any premature death is a suicide.”
There is also increasing discussion within the medical community, not just psychiatry, about a physician’s duty to relieve suffering, said Dr. Wynia. “There’s debate basically about whether we stand for preserving life essentially at all costs and never being involved in the taking of life, or whether we stand for reduction of suffering and being the advocate for the patients that we serve,” he said.
“Those are both legitimate,” said Dr. Wynia, adding, “there are good reasons to want both of those to be true.”
“I suspect that 20 years from now we will still be having conversations about how physicians, how psychiatrists ought to participate in preserving life and in shepherding death,” said Dr. Brendel.
But to Dr. Gaind, the debate is not just esoteric, it’s a soon-to-be reality in Canada. “When we’re providing death to people who aren’t dying, to me that’s like providing what amounts to a wrongful death,” he said.
A version of this article originally appeared on Medscape.com.
Canada already has the largest number of deaths by MAID of any nation, with 10,064 in 2021, a 32% increase from 2020. With the addition of serious mental illness (SMI) as an eligible category, the country is on track to have the most liberal assisted-death policy in the world.
Concerns about the additional number of patients who could become eligible for MAID, and a lack of evidence-backed standards from disability rights groups, mental health advocates, First Nations leaders, psychiatrists, and other mental health providers, seems to have led the Canadian government to give the proposed law some sober second thought.
“Listening to experts and Canadians, we believe this date needs to be temporarily delayed,” said David Lametti, Canada’s minister of Justice and attorney general of Canada; Jean-Yves Duclos, minister of Health; and Carolyn Bennett, minister of Mental Health and Addictions, in a Dec. 15, 2022, joint statement.
Canada’s Parliament – which approved the expansion – will now have to vote on whether to okay a pause on the legislation.
However, the Canadian Psychiatric Association has not been calling for a delay in the proposed legislation. In a November 2021 statement, the CPA said it “does not take a position on the legality or morality of MAID,” but added that to deny MAID to people with mental illness was discriminatory, and that, as it was the law, it must be followed.
“CPA has not taken a position about MAID,” the association’s president Gary Chaimowitz, MBChB, told this news organization. “We know this is coming and our organization is trying to get its members ready for what will be most likely the ability of people with mental conditions to be able to request MAID,” said Dr. Chaimowitz, who is also head of forensic psychiatry at St. Joseph’s Healthcare and a professor of psychiatry at McMaster University, both in Hamilton, Ont.
Dr. Chaimowitz acknowledges that “a majority of psychiatrists do not want to be involved in anything to do with MAID.”
“The idea, certainly in psychiatry, is to get people well and we’ve been taught that people dying from a major mental disorder is something that we’re trained to prevent,” he added.
A ‘clinical option’
Assisted medical death is especially fraught in psychiatry, said Rebecca Brendel, MD, president of the American Psychiatric Association. She noted a 25-year life expectancy gap between people with SMI and those who do not have such conditions.
“As a profession we have very serious obligations to advance treatment so that a person with serious mental illness can live [a] full, productive, and healthy [life],” Dr. Brendel, associate director of the Center for Bioethics at Harvard Medical School in Boston, said in an interview.
Under the Canadian proposal, psychiatrists would be allowed to suggest MAID as a “clinical option.”
Harold Braswell, PhD, a fellow with The Hastings Center, a bioethics research institute, calls that problematic.
“It’s not neutral to suggest to someone that it would be theoretically reasonable to end their lives,” Dr. Braswell, associate professor at the Albert Gnaegi Center for Health Care Ethics at Saint Louis University, told this news organization.
It also creates a double standard in the treatment of suicidal ideation, in which suicide prevention is absolute for some, but encouraging it as a possibility for others, he added.
“To have that come from an authority figure is something that’s very harsh and, in my opinion, very potentially destructive,” especially for vulnerable groups, like First Nations people, who already have elevated rates of suicide, said Dr. Braswell.
Fierce debate
Since 2016, Canada has allowed MAID for medical conditions and diseases that will not improve and in cases where the evidence shows that medical providers can accurately predict the condition will not improve.
However, in 2019, a Quebec court ruled that the law unconstitutionally barred euthanasia in people who were not terminally ill. In March 2021, Canada’s criminal code was amended to allow MAID for people whose natural death was not “reasonably foreseeable,” but it excluded SMI for a period of 2 years, ending in March 2023.
The 2-year stay was intended to allow for study and to give mental health providers and MAID assessors time to develop standards.
The federal government charged a 12-member expert panel with determining how to safely allow MAID for SMI. In its final report released in May 2022 it recommended that standards be developed.
The panel acknowledged that for many conditions it may be impossible to make predictions about whether an individual might improve. However, it did not mention SMI.
In those cases, when MAID is requested, “establishing incurability and irreversibility on the basis of the evolution and response to past interventions is necessary,” the panel noted, adding that these are the criteria used by psychiatrists assessing euthanasia requests in the Netherlands and Belgium.
But the notion that mental illness can be irremediable has been fiercely debated.
Soon after the expert report was released, the Center for Addiction and Mental Health in Toronto noted on its website that there are currently “no agreed upon standards for psychiatrists or other health care practitioners to use to determine if a person’s mental illness is ‘grievous and irremediable’ for the purposes of MAID.”
Dr. Chaimowitz acknowledged that “there’s no agreed-upon definition of incurability” in mental illness. Some psychiatrists “will argue that there’s always another treatment that can be attempted,” he said, adding that there has been a lack of consensus on irremediability among CPA members.
Protecting vulnerable populations
Matt Wynia, MD, MPH, FACP, director of the Center for Bioethics and Humanities at the University of Colorado at Denver, Aurora, said the question of irremediability is crucial. “Most people with mental illness do get better, especially if they’re in treatment,” Dr. Wynia said.
For MAID assessors it may be difficult to know when someone has tried all possible treatments, especially given the wide array of options, including psychedelics, said Dr. Wynia.
Dr. Braswell said there is not enough evidence that mental illness is incurable. With SMI, “there’s a lot more potential for the causes of the individual’s suffering to be ameliorated. By offering MAID, you’re going to kill people who might have been able to get out of this through other nonlethal means.”
Currently, MAID is provided for an irremediable medical condition, “in other words, a condition that will not improve and that we can predict will not improve,” said Karandeep S. Gaind, MD, chief of psychiatry at Toronto’s Humber River Hospital and physician chair of the hospital’s MAID team.
“If that’s the premise, then I think we cannot provide MAID for sole mental illness,” Dr. Gaind said. “Because we can’t honestly make those predictions” with mental illness, he added.
Dr. Gaind does not support MAID for mental illness and believes that it will put the vulnerable – including those living in poverty – at particular risk.
With the proposed expansion, MAID is “now becoming something which is being sought as a way to escape a painful life rather than to avoid a painful death,” said Dr. Gaind, who is also a past president of the CPA.
One member of the federal government’s expert panel – Ellen Cohen, who had a psychiatric condition – wrote in The Globe and Mail that she quit early on when it became apparent that the panel was not seriously considering her own experiences or the possibility that poverty and lack of access to care or social supports could strongly influence a request for MAID.
Social determinants of suffering
People with mental illness often are without homes, have substance use disorders, have been stigmatized and discriminated against, and have poor social supports, said Dr. Wynia. “You worry that it’s all of those things that are making them want to end their lives,” he said.
The Daily Mail ran a story in December 2022 about a 65-year-old Canadian who said he’d applied for MAID solely because of fears that his disability benefits for various chronic health conditions were being cut off and that he didn’t want to live in poverty.
A 51-year-old Ontario woman with multiple chemical sensitivities was granted MAID after she said she could not find housing that could keep her safe, according to an August report by CTV News.
Tarek Rajji, MD, chief of the Adult Neurodevelopment and Geriatric Psychiatry Division at CAMH, said social determinants of health need to be considered in standards created to guide MAID for mental illness.
“We’re very mindful of the fact that the suffering, that is, the grievousness that the person is living with, in the context of mental illness, many times is due to the social determinants of their illness and the social determinants of their suffering,” Dr. Rajji said.
Many are also concerned that it will be difficult to separate out suicidality from sheer hopelessness.
The CPA has advised a group that’s working on developing guidelines for MAID in SMI and is also developing a curriculum for mental health providers, Dr. Chaimowitz said. As part of that, there will be a process to ensure that someone who is actively suicidal is not granted MAID.
“I do not believe that it’s contemplated that MAID is going to accelerate or facilitate suicidal ideation,” he said. Someone who is suicidal will be referred to treatment, said Dr. Chaimowitz.
“People with depression often feel hopeless,” and may refuse treatments that have worked in the past, countered Dr. Gaind. Some of his patients “are absolutely convinced that nothing will help,” he said.
Troublesome cases
The expert panel said in its final report that “it is not possible to provide fixed rules for how many attempts at interventions, how many types of interventions, and over how much time,” are necessary to establish “irreversibility” of mental illness.
Dr. Chaimowitz said MAID will not be offered to anyone “refusing treatment for their condition without any good reason.” They will be “unlikely to meet criteria for incurable,” as they will have needed to avail themselves of the array of treatments available, he said.
That would be similar to rules in Belgium and the Netherlands, which allow euthanasia for psychiatric conditions.
An estimated 100-300 psychiatric patients receive euthanasia each year in those countries, according to a 2021 commentary in Psychiatric Times (Jun 7;38[6]) by Mark S. Komrad, MD, a Towson, Maryland-based psychiatrist.
There are still troublesome cases.
As previously reported by this news organization, many in Belgium were distressed recently at the news that a 23-year-old woman who had survived a terrorist attack, Shanti De Corte, requested and was granted euthanasia.
As the deadline for implementation of MAID grew closer, calls for delay grew louder, especially given the lack of concrete standards for providers.
During the waning months of 2022, Dr. Gaind – who said he was suspended from CPA for “unprofessional interactions” and allegedly misrepresenting CPA’s processes and governance matters – announced the launch of a new organization, the Society of Canadian Psychiatry, in November calling for a delay in MAID of at least 1 year so that evidence-based safeguards could be implemented. The petition has been signed by more than 200 psychiatrists, along with several dozen physicians, MAID assessors, and individuals with mental illness and family members.
The Association of Chairs of Psychiatry in Canada, the Canadian Association for Suicide Prevention, the Council of Canadians with Disabilities, a group of indigenous leaders, and the Ontario Association for ACT and FACT, psychiatrists who provide care to individuals with severe mental illness, among other groups, joined the call for a delay.
In its December announcement, the Canadian federal ministers said a factor in seeking a delay was that standards guiding clinicians would not be delivered until at least February – too close to when applications would be opened.
Upon hearing about the federal government’s intentions, the chair of the expert panel, Mona Gupta, MD, told The Canadian Press that she did not think it was necessary to put off implementation because necessary safeguards were already in place.
Dr. Chaimowitz awaits the standards but is optimistic that for mental illness, “the process will be tightly controlled, closely monitored, and open to scrutiny,” he said.
Dr. Braswell is not convinced. The concern is that adding people with mental illness is “going to overload the capacity of the government to monitor this practice,” he said.
Is the United States next?
Although Canada and the United States share a border, it’s unlikely that U.S. states will allow aid in dying for nonterminal illness, much less for psychiatric conditions any time soon, said Dr. Braswell and others.
Ten states – California, Colorado, Hawaii, Maine, Montana, New Jersey, New Mexico, Oregon, Vermont, and Washington – have laws allowing assistance in dying, but for terminal illness only.
In 2016, the APA adopted the American Medical Association policy on medical euthanasia, stating, “that a psychiatrist should not prescribe or administer any intervention to a nonterminally ill person for the purpose of causing death.”
Dr. Brendel said the field is acutely aware that people with mental illness do suffer, but that more work needs to be done – and is being done – on “distinguishing wishes to hasten death or end one’s life from these historical or traditional notions that any premature death is a suicide.”
There is also increasing discussion within the medical community, not just psychiatry, about a physician’s duty to relieve suffering, said Dr. Wynia. “There’s debate basically about whether we stand for preserving life essentially at all costs and never being involved in the taking of life, or whether we stand for reduction of suffering and being the advocate for the patients that we serve,” he said.
“Those are both legitimate,” said Dr. Wynia, adding, “there are good reasons to want both of those to be true.”
“I suspect that 20 years from now we will still be having conversations about how physicians, how psychiatrists ought to participate in preserving life and in shepherding death,” said Dr. Brendel.
But to Dr. Gaind, the debate is not just esoteric, it’s a soon-to-be reality in Canada. “When we’re providing death to people who aren’t dying, to me that’s like providing what amounts to a wrongful death,” he said.
A version of this article originally appeared on Medscape.com.
Managing patients with comorbid opioid and alcohol use disorders
When left untreated, opioid use disorder (OUD) is a debilitating and potentially lethal illness. Despite the availability of safe and effective medications for OUD, the prevalence of opioid use and overdose deaths has been increasing every year.1 An additional challenge in OUD treatment is the high prevalence of comorbid alcohol use disorder (AUD).2-6 A Clinical Trials Network survey from the National Institute on Drug Abuse found 38% of persons seeking treatment for OUD also had AUD.7 Other analyses have found alcohol was involved in approximately one-fifth of opioid-related deaths.8 Research also reveals that comorbid OUD and AUD contributes to poor treatment outcomes, more medical comorbidities, and a high risk of death (including overdose death).4,9 There is no standard of care for this particular patient population.3 This article reviews the evidence and summarizes practical considerations regarding the clinical management of patients with comorbid OUD and AUD.
To illustrate the various decision points, we will follow 2 hypothetical patients through various stages of treatment (Figure), from their presentation in the emergency department (ED) or outpatient clinic, through their hospital admission (if needed), and into their outpatient follow-up treatment.
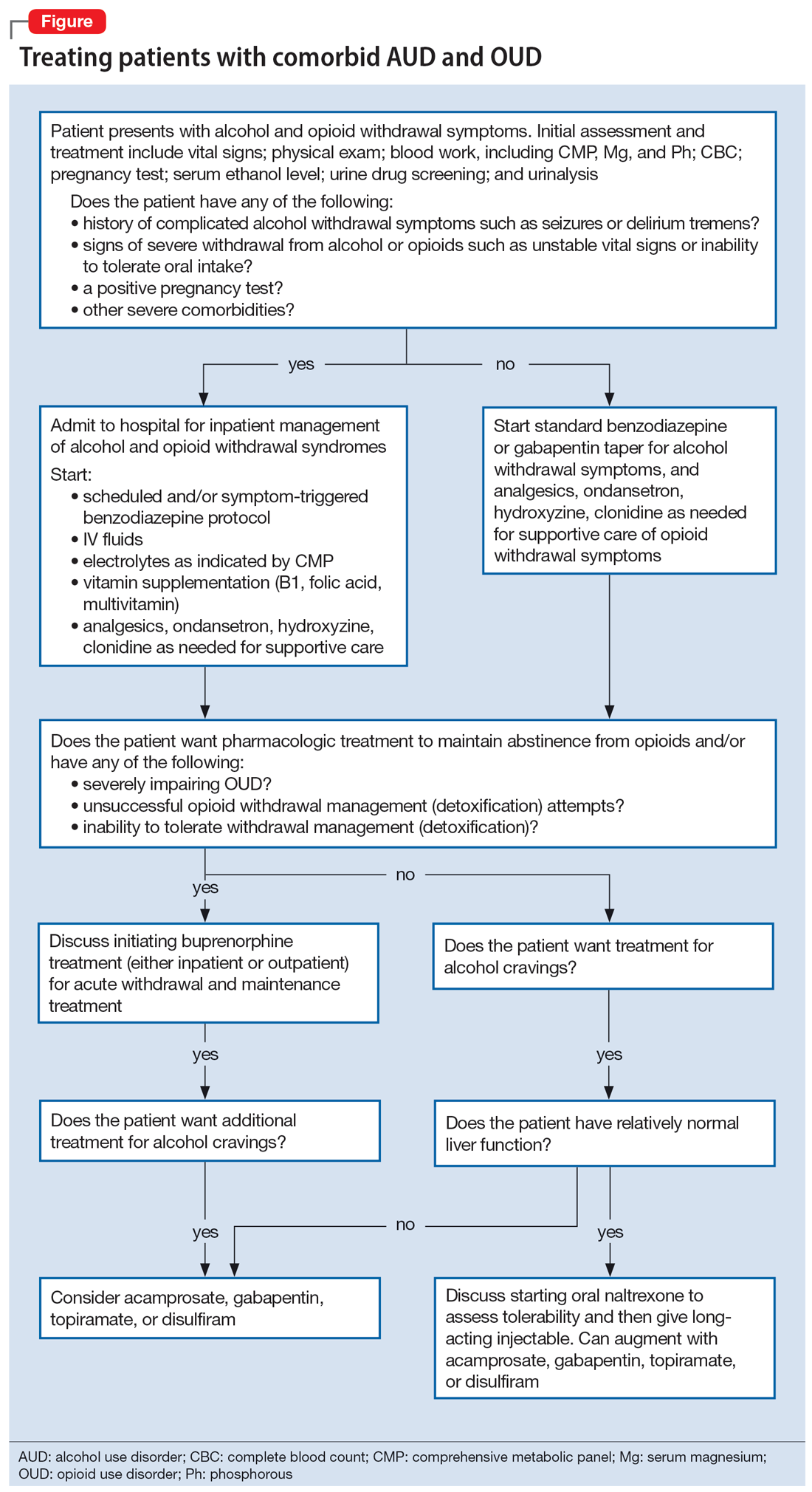
CASE REPORTS
Ms. A and Ms. B present to the ED for evaluation of nausea, vomiting, sweating, anxiety, and tremor. Both patients describe their most recent use of both alcohol and opioids approximately 12 hours ago, and each has been attempting to stop using both substances at home.
Decision-making in the emergency setting
In the ED, a few important decisions need to be made regarding treatment:
- Are the presenting symptoms primarily due to alcohol withdrawal syndrome (AWS), opioid withdrawal syndrome (OWS), or both?
- Does the patient require inpatient medical withdrawal management (detoxification) based on the history and severity of the withdrawal symptoms?
- What are the patient’s treatment goals for their AUD and OUD?
- Is maintenance medication for OUD indicated? If so, which medication is most appropriate?
In the ED, the presentation of individuals affected by both OUD and AUD can be challenging because OWS shares overlapping features with AWS, including nausea, vomiting, diarrhea, sweating, anxiety, and tremor. However, although acute OWS is typically very uncomfortable, it is rarely lethal. On the other hand, severe AWS may result in delirium, seizures, and death,10 which makes it essential to recognize and treat appropriately.
Both Ms. A and Ms. B should be medically evaluated and treated by an emergency medicine physician in conjunction with psychiatric (or addiction medicine) consultation. The ED assessment of a patient presenting with both AUD and OUD should include vital signs monitoring; physical examination; blood work including comprehensive metabolic panel, serum magnesium, and phosphorus; complete blood count; pregnancy test for women of reproductive age; urine drug screen (UDS); urinalysis; and serum ethanol level. Of note, sympathetic hyperactivity is found in both alcohol and opioid withdrawal, and patients with alcohol withdrawal may also have hypokalemia, a condition associated with an increased risk of arrhythmia. Furthermore, a prolonged QTc would affect clinical decision-making about medications for OUD (ie, methadone) and withdrawal management (ie, ondansetron, trazodone, and hydroxyzine). Therefore, an electrocardiogram should be conducted, where appropriate.
Initial treatment of AWS includes vitamin supplementation (thiamine, folic acid, and multivitamins) and benzodiazepine administration (symptom-triggered and/or scheduled taper). It may also include IV fluid resuscitation, analgesics for pain, ondansetron for nausea and vomiting, and other electrolyte repletion as indicated by the laboratory results.11 Additional measures for patients in opioid withdrawal should include alpha-2 agonists such as clonidine or lofexidine for adrenergic symptoms, antiemetics, antidiarrheals, muscle relaxants, anxiolytics such as hydroxyzine, and sleep medications such as trazodone.12
Continue to: The next decision...
The next decision is whether the patient needs to be admitted for inpatient treatment. This decision is based primarily on the risk assessment and severity of AWS, including a compelling history of complicated AWS such as seizures or delirium tremens as well as consideration of the complexity and severity of any comorbid medical or psychiatric conditions. Other indications for medical withdrawal management include a history of unsuccessful ambulatory withdrawal management and pregnancy. For severe AWS, a scheduled benzodiazepine taper in addition to the symptom-triggered protocol should be considered.13-15 A psychiatric evaluation may be obtained in the ED, as long as the patient is sober enough to meaningfully participate in the psychiatric interview. Wherever possible, psychiatric interviews should be supplemented by collateral information.
CASE REPORTS CONTINUED
Ms. A admits to a 5-year history of alcohol and opioid use that meets the criteria for severe AUD and severe OUD. She has previously required inpatient treatment for seizures related to AWS. Laboratory results are notable for a serum ethanol level of 380 mg/dL, UDS positive for opioids, and a negative pregnancy test.
Disposition of patients in alcohol and opioid withdrawal
Given Ms. A’s history of seizures while withdrawing from alcohol, she is appropriate for hospital admission for medically managed withdrawal observation. As previously mentioned, there is clinical overlap between AWS and OWS, and differentiating between the 2 syndromes is essential and may be lifesaving. Whereas anxiety, agitation, diaphoresis, tachycardia, hypertension, and insomnia can be seen in both opioid and alcohol withdrawal, OWS-specific symptoms include mydriasis, lacrimation, rhinorrhea, bone or joint aches, yawning, and piloerection. AWS may present with visual or tactile hallucinations, delirium, and grand mal seizures.15
The details of inpatient management are beyond the scope of this article; however, both patients should be started on thiamine, folic acid, and a multivitamin. For patients in alcohol withdrawal with a history of poor diet who appear malnourished or have a history of malabsorption (such as gastric bypass surgery), thiamine 100 mg/d IV should be given for 3 to 5 days to prevent Wernicke encephalopathy.16 Where there is any concern the patient may be exhibiting signs of Wernicke-Korsakoff Syndrome (impaired cognition, evident malnourishment, ataxia, or eye movement abnormalities), high-dose thiamine IV should be given presumptively as follows: 500 mg IV 3 times a day for 3 days, 250 mg/d IV for 5 days, and then oral supplementation 100 mg/d for at least 30 days.17
In summary, on presentation to the ED, both patients should be medically stabilized and started on benzodiazepines for alcohol withdrawal. The risk assessment and the severity of the AWS often determines the level of care.
CASE REPORTS CONTINUED
On hospital Day 2, Ms. A tells the consulting psychiatrist she would like to start medications to treat her substance use disorders. She has a long history of failed attempts to achieve abstinence from opioids, so she and the psychiatrist agree to initiate a trial of buprenorphine/naloxone for her OUD, 4 mg/1 mg to 8 mg/2 mg for Day 1. Although buprenorphine/naloxone seems to help her alcohol cravings somewhat, she requests additional help. She experiences migraine headaches, which is in part why she began using opioid medications. Via joint decision making with her psychiatrist, she agrees to a trial of topiramate, with a slow titration schedule starting at 25 mg/d.
Continue to: Management decisions
Management decisions: Buprenorphine for OUD
The next issue is to determine the appropriate treatment for the patient’s OUD. Although treating OWS is important in improving the patient’s health, decreasing their discomfort, and facilitating their participation in a psychosocial treatment program,18 current evidence suggests that opioid withdrawal management alone without medication for OUD rarely leads to long-term recovery.19,20 Some research suggests that the risk of accidental opioid overdose immediately following acute withdrawal management may actually be increased due to decreased tolerance in these patients.12,21,22
Three medications have the most evidence for OUD treatment: buprenorphine, methadone, and naltrexone.15 The decision to use buprenorphine, methadone, or naltrexone depends on a variety of factors, including the severity of the OUD, patient history of prior treatment successes and failures, comorbid medical and psychiatric conditions, and patient preference.4 Treatment with buprenorphine or methadone is preferred over naltrexone for patients who do not want to or cannot tolerate the physical and emotional discomfort of the opioid withdrawal process, who experience moderate to severe OUD, who have a history of failed abstinence-based treatment, or who have more severe physiological tolerance/dependence.12 Buprenorphine is a mu opioid receptor partial agonist that has been shown to reduce opioid cravings,23 provide moderate pain relief,24 and ameliorate OWS.12 It does not typically result in significant respiratory depression, which is the biggest safety concern for opioid use.12 Buprenorphine may also treat comorbid AUD at higher doses; however, the data are inconclusive.25,26 Buprenorphine should be prescribed with caution to patients with comorbid, uncontrolled AUD, due to the risk of respiratory depression when combined with alcohol. Patients who continue to drink alcohol but are able to abstain from opioids may consider starting an AUD-specific medication. Pharmacologic options are discussed in more detail in the next section.
For patients who have higher physiological dependence or more severe OUD, methadone may be a reasonable alternative to buprenorphine. Methadone, a mu-opioid receptor agonist, ameliorates OWS, reduces opioid cravings, and reduces the euphoric effects of opioid ingestion if the patient relapses. However, methadone can only be dispensed for the treatment of OUD by a federally-certified treatment program governed by restrictive and federally mandated guidelines. Compared to buprenorphine, methadone is more dangerous in overdose, has more drug interactions, and is more commonly diverted for recreational use.27 Furthermore, methadone should be prescribed with caution to patients with comorbid, uncontrolled AUD, because both alcohol and methadone can result in respiratory depression.
By contrast, the first-line treatment for individuals experiencing moderateto severe AUD is typically naltrexone.28 Naltrexone is contraindicated in Ms. A because she has a severe OUD and is unlikely to tolerate the opioid withdrawal process. Research suggests that the use of naltrexone for OUD should be limited to patients who have a mild disorder or who show low physiological dependence.29 Alternatively, acamprosate, disulfiram, topiramate, or gabapentin should be considered for Ms. A.4,28,30 Because each of these medications have specific strengths and weaknesses, medication selection should be based on individual patient factors such as comorbid psychiatric and medical conditions and/or patient preference.28
Management decisions: AUD augmentation strategies
Naltrexone is contraindicated for patients who are receiving opioids, including opioid agonist therapy for OUD. Therefore, clinicians need to consider other options for these individuals. There are several medications with good evidence, including acamprosate, disulfiram, topiramate, and gabapentin. Acamprosate and disulfiram are FDA-approved for AUD; the latter 2 have been used off-label.
Continue to: Acamprosate is a glutamate receptor modulator...
Acamprosate is a glutamate receptor modulator that reduces alcohol cravings and is recommended for patients who have achieved and wish to maintain abstinence. It can be used in patients with liver disease, because it is not hepatically metabolized.30 Topiramate is also used to reduce alcohol cravings. It antagonizes glutamate at alpha-amino-3-hydroxy-5-methyl-4-isoxazole-propionate (AMPA) and kainite receptors, facilitates gamma-aminobutyric acid (GABA) function, and reduces the extracellular release of dopamine in the mesocorticolimbic regions of the brain.30 Topiramate is a reasonable option for patients with a seizure disorder, a history of migraine headaches,30 or who are overweight or obese and wish to lose weight.31 In a nonrandomized study, topiramate reduced alcohol intake and cravings more than naltrexone.32
Disulfiram is another second-line therapy for AUD. It is best used under close supervision because it does not reduce alcohol cravings but makes ingesting alcohol extremely aversive by preventing the breakdown of the alcohol metabolite acetaldehyde, and in doing so causes a cluster of unpleasant symptoms, including sweating, palpitations, flushing, nausea/vomiting, and increased sympathetic tone.28 Disulfiram only works if it is taken daily, and it requires a high degree of motivation and/or daily supervision at home or in the clinic.33 It is not recommended to be used as a first-line treatment based on its potential toxicity, adverse effects, and mixed findings on its efficacy. In addition, it should not be given to medically vulnerable/fragile individuals.
Lastly, gabapentin, a voltage-gated calcium channel modulator, may also be used as a second-line agent for AUD. Patients who have started alcohol withdrawal management with gabapentin may wish to continue treatment to assist with craving suppression.30 It is also a good choice for patients who have comorbid diabetic neuropathy or other neuropathic pain conditions, anxiety, or insomnia.30,34 Of note, there have been reports of gabapentin misuse.
CASE REPORTS CONTINUED
Ms. B presents to the ED with a 5-year history of moderate AUD and a 2-year history of mild OUD. She denies a history of severe or complicated AWS. Her laboratory results are significant for a serum ethanol level of 250 mg/dL, UDS positive for opioids, and a negative pregnancy test.
Management decisions: Naltrexone for OUD
In contrast to Ms. A, Ms. B is likely able to complete the opioid withdrawal management process. It is reasonable to treat her uncomplicated, moderate alcohol withdrawal as an outpatient with gabapentin or a benzodiazepine taper. Had her AUD been as severe as Ms. A’s, or if she were unsuccessful with ambulatory withdrawal treatment attempts, Ms. B would also be a candidate for inpatient medical treatment for alcohol withdrawal regardless of the severity of her OUD. Ongoing pharmacotherapy for her AUD after withdrawal management is the same as previously outlined. After Ms. B completes the taper (typically 1 week after the ED visit), she should follow up for initiation of pharmacotherapy for AUD. Ms. B is an ideal candidate for naltrexone, which targets both AUD and OUD.
Continue to: Naltrexone is a semi-synthetic...
Naltrexone is a semi-synthetic competitive antagonist at mu-opioid receptors and a partial agonist at kappa receptors; it has little to no activity at delta receptors. Naltrexone has been shown to reduce alcohol cravings and diminish the euphoric effects of alcohol by reducing endogenous opioid release and receptor activation.35 Thus, even when patients do use alcohol while taking naltrexone, the amount of alcohol they use is typically substantially reduced.36 In fact, at a standard dose of 50 mg/d, 95% of mu-opioid receptors are occupied and are shown to yield approximately 40% alcohol abstinence rates at 1 year.36
Once Ms. B has completed withdrawal management from both alcohol and opioids, she should have a trial period of oral naltrexone to prove tolerability, and then transition to the long-acting injectable (LAI) formulation. Patients able to complete withdrawal management from opioids and transition to LAI naltrexone have been shown to have equivalent rates of successful abstinence from opioids compared to buprenorphine.37 Though Ms. B could opt to try buprenorphine to treat her mild OUD, naltrexone would be the preferred option because it has 3 advantages:
- it blocks the mu-opioid receptor, which prevents euphoria if an illicit substance is used
- it does not cause physiologic dependence or withdrawal syndrome if/when stopped
- if it is not effective, it is easy to switch to buprenorphine.
Lastly, all patients with OUD should be prescribed a rescue naloxone kit, in accordance with harm-reduction guidelines. Naloxone, a potent opioid receptor antagonist, is used to prevent or reverse respiratory depression in opioid overdose. Naloxone rescue kits include intranasal naloxone, which makes it easy for nonclinician bystanders to administer while waiting for emergency transport.38 Most states allow naloxone kits to be prescribed to individuals who have a concern for overdose among friends, family, or others in the community. The wide distribution and easy availability of naloxone rescue kits have been essential in decreasing overdose deaths among patients who misuse opioids.39
Take-home points
Patients with both OUD and AUD are relatively common and often pose significant management challenges when they present to the clinic or the ED in withdrawal. Because severe AWS can be life-threatening, hospitalization should be considered. OWS is often accompanied by intense cravings that can lead to relapse and the risk of accidental opioid overdose/death. As soon as patients are able to engage in a discussion about their treatment options, clinicians need to clarify the patient’s goals and priorities. In medications for OUD, the decision of whether to use buprenorphine, naltrexone, or methadone is guided by the severity of the OUD, the patient’s past treatment experience (illicit as well as prescribed), and patient preference. If the OUD is mild or if the patient prefers to avoid opioid agonist medications and can tolerate the opioid withdrawal process, both the AUD and OUD can be treated with naltrexone, preferably with the LAI formulation. Other AUD medications and outpatient psychotherapy may be used to augment treatment outcomes. For patients with a moderate to severe OUD, buprenorphine (preferably with immediate initiation) or methadone therapy should be offered. Patients with comorbid OUD and AUD who are treated with opioid agonists should be offered medication for AUD other than naltrexone, as outlined above. All patients with substance use disorders would benefit from psychosocial interventions, including group and individual therapy as well as community sober support groups.
Bottom Line
Patients with comorbid opioid use disorder (OUD) and alcohol use disorder (AUD) often pose significant management challenges when they present in withdrawal. This article reviews the evidence and summarizes practical considerations regarding the clinical management of patients with comorbid OUD and AUD.
Related Resources
- Chaney L, Mathia C, Cole T. Transitioning patients with opioid use disorder from methadone to buprenorphine. Current Psychiatry. 2022;21(12):23-24,28. doi:10.12788/ cp.0305
- Eatmon CV, Trent K. Pharmacotherapy for alcohol use disorder in patients with hepatic impairment. Current Psychiatry. 2021;20(12):25-28. doi:10.12788/cp.0068
Drug Brand Names
Acamprosate • Campral
Buprenorphine/naloxone • Suboxone, Zubsolv
Clonidine • Catapres
Disulfiram • Antabuse
Gabapentin • Neurontin
Hydroxyzine • Vistaril
Lofexidine • Lucemyra
Methadone • Methadose, Dolophine
Naloxone • Narcan
Naltrexone • ReVia, Vivitrol
Ondansetron • Zofran
Topiramate • Topamax
Trazodone • Desyrel, Oleptro
1. Mattson CL, Tanz LJ, Quinn K, et al. Trends and geographic patterns in drug and synthetic opioid overdose deaths - United States, 2013-2019. MMWR Morb Mortal Wkly Rep. 2021;70(6):202-207.
2. Hartzler B, Donovan DM, Huang Z. Comparison of opiate-primary treatment seekers with and without alcohol use disorder. J Subst Abuse Treat. 2010;39(2):114-123.
3. Nolan S, Klimas J, Wood E. Alcohol use in opioid agonist treatment. Addict Sci Clin Pract. 2016;11(1):17.
4. Hood LE, Leyrer-Hackson JM, Olive MF. Pharmacotherapeutic management of co-morbid alcohol and opioid use. Expert Opin Pharmacother. 2020;21(7):823-839.
5. Pikovsky M, Peacock A, Larney S, et al. Alcohol use disorder and associated physical health complications and treatment amongst individuals with and without opioid dependence: a case-control study. Drug Alcohol Depend. 2018;188:304-310.
6. Jones CM, McCance-Katz EF. Co-occurring substance use and mental disorders among adults with opioid use disorder. Drug Alcohol Depend. 2019;197:78-82.
7. Hartzler B, Donovan DM, Huang Z. Comparison of opiate-primary treatment seekers with and without alcohol use disorder. J Subst Abuse Treat. 2010;39(2):114-123.
8. Jones CM, Paulozzi LJ, Mack KA; Centers for Disease Control and Prevention (CDC). Alcohol involvement in opioid pain reliever and benzodiazepine drug abuse-related emergency department visits and drug-related deaths - United States, 2010. MMWR Morb Mortal Wkly Rep. 2014;63(40):881-885.
9. Stapleton RD, Comiskey CM. Alcohol usage and associated treatment outcomes for opiate users entering treatment in Ireland. Drug Alcohol Depend. 2010;107(1):56-61.
10. Turner RC, Lichstein PR, Peden JG Jr, et al. Alcohol withdrawal syndromes: a review of pathophysiology, clinical presentation, and treatment. J Gen Intern Med. 1989;4(5):432-444.
11. Boba A. Management of acute alcohol intoxication. Am J Emerg Med. 1999;17(4):431.
12. The ASAM national practice guideline for the treatment of opioid use disorder: 2020 focused update. J Addict Med. 2020;14(2S Suppl1):1-91.
13. Shaw JM, Kolesar GS, Sellers EM, et al. Development of optimal treatment tactics for alcohol withdrawal. I. Assessment and effectiveness of supportive care. J Clin Psychopharmacol. 1981;1(6):382-389.
14. Naranjo CA, Sellers EM. Clinical assessment and pharmacotherapy of the alcohol withdrawal syndrome. Recent Dev Alcohol. 1986;4:265-281.
15. Kampman K, Jarvis M. American Society of Addiction Medicine (ASAM) national practice guideline for the use of medications in the treatment of addiction involving opioid use. J Addict Med. 2015;9(5):358-367.
16. The ASAM clinical practice guideline on alcohol withdrawal management. J Addict Med. 2020;14(3S Suppl 1):1-72.
17. Isenberg-Grzeda E, Kutner HE, Nicolson SE. Wernicke-Korsakoff-syndrome: under-recognized and under-treated. Psychosomatics. 2012;53(6):507-516.
18. Schuckit MA. Treatment of opioid-use disorders. N Engl J Med. 2016;375(4):357-368.
19. Tang Y-L, Hao W. Improving drug addiction treatment in China. Addiction. 2007;102(7):1057-1063.
20. Wakeman SE, Larochelle MR, Ameli O, et al. Comparative effectiveness of different treatment pathways for opioid use disorder. JAMA Netw Open. 2020;3(2):e1920622.
21. Wines JD Jr, Saitz R, Horton NJ, et al. Overdose after detoxification: a prospective study. Drug Alcohol Depend. 2007;89(2-3):161-169.
22. Maughan BC, Becker EA. Drug-related mortality after discharge from treatment: a record-linkage study of substance abuse clients in Texas, 2006-2012. Drug Alcohol Depend. 2019;204:107473.
23. Gowing L, Ali R, White J. Buprenorphine for the management of opioid withdrawal. Cochrane Database Syst Rev. 2002;(2):CD002025.
24. Malinoff HL, Barkin RL, Wilson G. Sublingual buprenorphine is effective in the treatment of chronic pain syndrome. Am J Ther. 2005;12(5):379-384.
25. Nava F, Manzato E, Leonardi C, et al. Opioid maintenance therapy suppresses alcohol intake in heroin addicts with alcohol dependence: preliminary results of an open randomized study. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(8):1867-1872.
26. Srivastava A, Kahan M, Ross S. The effect of methadone maintenance treatment on alcohol consumption: a systematic review. J Subst Abuse Treat. 2008;34(2):215-223.
27. Davids E, Gastpar M. Buprenorphine in the treatment of opioid dependence. Eur Neuropsychopharmacol. 2004;14(3):209-216.
28. American Psychiatric Association. Practice Guideline for the Pharmacological Treatment of Patients With Alcohol Use Disorder. American Psychiatric Association; 2018.
29. Hassanian-Moghaddam H, Afzali S, Pooya A. Withdrawal syndrome caused by naltrexone in opioid abusers. Hum Exp Toxicol. 2014;33(6):561-567.
30. Fairbanks J, Umbreit A, Kolla BP, et al. Evidence-based pharmacotherapies for alcohol use disorder: clinical pearls. Mayo Clin Proc. 2020;95(9):1964-1977.
31. Verrotti A, Scaparrotta A, Agostinelli S, et al. Topiramate-induced weight loss: a review. Epilepsy Res. 2011;95(3):189-199.
32. Flórez G, García-Portilla P, Alvarez S, et al. Using topiramate or naltrexone for the treatment of alcohol-dependent patients. Alcohol Clin Exp Res. 2008;32(7):1251-1259.
33. Jørgensen CH, Pedersen B, Tønnesen H. The efficacy of disulfiram for the treatment of alcohol use disorder. Alcohol Clin Exp Res. 2011;35(10):1749-1758.
34. Mason BJ, Quello S, Shadan F. Gabapentin for the treatment of alcohol use disorder. Expert Opin Investig Drugs. 2018;27(1):113-124.
35. Sudakin D. Naltrexone: not just for opioids anymore. J Med Toxicol. 2016;12(1):71-75.
36. Rubio G, Jiménez-Arrieri MA, Ponce G, et al. Naltrexone versus acamprosate: one year follow-up of alcohol dependence treatment. Alcohol Alcohol. 2001;36(5):419-425.
37. Lee JD, Nunes EV Jr, Novo P, et al. Comparative effectiveness of extended-release naltrexone versus buprenorphine-naloxone for opioid relapse prevention (X:BOT): a multicentre, open-label, randomised controlled trial. Lancet. 2018;391(10118):309-318.
38. Clark AK, Wilder CM, Winstanley EL. A systematic review of community opioid overdose prevention and naloxone distribution programs. J Addict Med. 2014;8(3):153-163.
39. Dunne RB. Prescribing naloxone for opioid overdose intervention. Pain Manag. 2018;8(3):197-208.
When left untreated, opioid use disorder (OUD) is a debilitating and potentially lethal illness. Despite the availability of safe and effective medications for OUD, the prevalence of opioid use and overdose deaths has been increasing every year.1 An additional challenge in OUD treatment is the high prevalence of comorbid alcohol use disorder (AUD).2-6 A Clinical Trials Network survey from the National Institute on Drug Abuse found 38% of persons seeking treatment for OUD also had AUD.7 Other analyses have found alcohol was involved in approximately one-fifth of opioid-related deaths.8 Research also reveals that comorbid OUD and AUD contributes to poor treatment outcomes, more medical comorbidities, and a high risk of death (including overdose death).4,9 There is no standard of care for this particular patient population.3 This article reviews the evidence and summarizes practical considerations regarding the clinical management of patients with comorbid OUD and AUD.
To illustrate the various decision points, we will follow 2 hypothetical patients through various stages of treatment (Figure), from their presentation in the emergency department (ED) or outpatient clinic, through their hospital admission (if needed), and into their outpatient follow-up treatment.

CASE REPORTS
Ms. A and Ms. B present to the ED for evaluation of nausea, vomiting, sweating, anxiety, and tremor. Both patients describe their most recent use of both alcohol and opioids approximately 12 hours ago, and each has been attempting to stop using both substances at home.
Decision-making in the emergency setting
In the ED, a few important decisions need to be made regarding treatment:
- Are the presenting symptoms primarily due to alcohol withdrawal syndrome (AWS), opioid withdrawal syndrome (OWS), or both?
- Does the patient require inpatient medical withdrawal management (detoxification) based on the history and severity of the withdrawal symptoms?
- What are the patient’s treatment goals for their AUD and OUD?
- Is maintenance medication for OUD indicated? If so, which medication is most appropriate?
In the ED, the presentation of individuals affected by both OUD and AUD can be challenging because OWS shares overlapping features with AWS, including nausea, vomiting, diarrhea, sweating, anxiety, and tremor. However, although acute OWS is typically very uncomfortable, it is rarely lethal. On the other hand, severe AWS may result in delirium, seizures, and death,10 which makes it essential to recognize and treat appropriately.
Both Ms. A and Ms. B should be medically evaluated and treated by an emergency medicine physician in conjunction with psychiatric (or addiction medicine) consultation. The ED assessment of a patient presenting with both AUD and OUD should include vital signs monitoring; physical examination; blood work including comprehensive metabolic panel, serum magnesium, and phosphorus; complete blood count; pregnancy test for women of reproductive age; urine drug screen (UDS); urinalysis; and serum ethanol level. Of note, sympathetic hyperactivity is found in both alcohol and opioid withdrawal, and patients with alcohol withdrawal may also have hypokalemia, a condition associated with an increased risk of arrhythmia. Furthermore, a prolonged QTc would affect clinical decision-making about medications for OUD (ie, methadone) and withdrawal management (ie, ondansetron, trazodone, and hydroxyzine). Therefore, an electrocardiogram should be conducted, where appropriate.
Initial treatment of AWS includes vitamin supplementation (thiamine, folic acid, and multivitamins) and benzodiazepine administration (symptom-triggered and/or scheduled taper). It may also include IV fluid resuscitation, analgesics for pain, ondansetron for nausea and vomiting, and other electrolyte repletion as indicated by the laboratory results.11 Additional measures for patients in opioid withdrawal should include alpha-2 agonists such as clonidine or lofexidine for adrenergic symptoms, antiemetics, antidiarrheals, muscle relaxants, anxiolytics such as hydroxyzine, and sleep medications such as trazodone.12
Continue to: The next decision...
The next decision is whether the patient needs to be admitted for inpatient treatment. This decision is based primarily on the risk assessment and severity of AWS, including a compelling history of complicated AWS such as seizures or delirium tremens as well as consideration of the complexity and severity of any comorbid medical or psychiatric conditions. Other indications for medical withdrawal management include a history of unsuccessful ambulatory withdrawal management and pregnancy. For severe AWS, a scheduled benzodiazepine taper in addition to the symptom-triggered protocol should be considered.13-15 A psychiatric evaluation may be obtained in the ED, as long as the patient is sober enough to meaningfully participate in the psychiatric interview. Wherever possible, psychiatric interviews should be supplemented by collateral information.
CASE REPORTS CONTINUED
Ms. A admits to a 5-year history of alcohol and opioid use that meets the criteria for severe AUD and severe OUD. She has previously required inpatient treatment for seizures related to AWS. Laboratory results are notable for a serum ethanol level of 380 mg/dL, UDS positive for opioids, and a negative pregnancy test.
Disposition of patients in alcohol and opioid withdrawal
Given Ms. A’s history of seizures while withdrawing from alcohol, she is appropriate for hospital admission for medically managed withdrawal observation. As previously mentioned, there is clinical overlap between AWS and OWS, and differentiating between the 2 syndromes is essential and may be lifesaving. Whereas anxiety, agitation, diaphoresis, tachycardia, hypertension, and insomnia can be seen in both opioid and alcohol withdrawal, OWS-specific symptoms include mydriasis, lacrimation, rhinorrhea, bone or joint aches, yawning, and piloerection. AWS may present with visual or tactile hallucinations, delirium, and grand mal seizures.15
The details of inpatient management are beyond the scope of this article; however, both patients should be started on thiamine, folic acid, and a multivitamin. For patients in alcohol withdrawal with a history of poor diet who appear malnourished or have a history of malabsorption (such as gastric bypass surgery), thiamine 100 mg/d IV should be given for 3 to 5 days to prevent Wernicke encephalopathy.16 Where there is any concern the patient may be exhibiting signs of Wernicke-Korsakoff Syndrome (impaired cognition, evident malnourishment, ataxia, or eye movement abnormalities), high-dose thiamine IV should be given presumptively as follows: 500 mg IV 3 times a day for 3 days, 250 mg/d IV for 5 days, and then oral supplementation 100 mg/d for at least 30 days.17
In summary, on presentation to the ED, both patients should be medically stabilized and started on benzodiazepines for alcohol withdrawal. The risk assessment and the severity of the AWS often determines the level of care.
CASE REPORTS CONTINUED
On hospital Day 2, Ms. A tells the consulting psychiatrist she would like to start medications to treat her substance use disorders. She has a long history of failed attempts to achieve abstinence from opioids, so she and the psychiatrist agree to initiate a trial of buprenorphine/naloxone for her OUD, 4 mg/1 mg to 8 mg/2 mg for Day 1. Although buprenorphine/naloxone seems to help her alcohol cravings somewhat, she requests additional help. She experiences migraine headaches, which is in part why she began using opioid medications. Via joint decision making with her psychiatrist, she agrees to a trial of topiramate, with a slow titration schedule starting at 25 mg/d.
Continue to: Management decisions
Management decisions: Buprenorphine for OUD
The next issue is to determine the appropriate treatment for the patient’s OUD. Although treating OWS is important in improving the patient’s health, decreasing their discomfort, and facilitating their participation in a psychosocial treatment program,18 current evidence suggests that opioid withdrawal management alone without medication for OUD rarely leads to long-term recovery.19,20 Some research suggests that the risk of accidental opioid overdose immediately following acute withdrawal management may actually be increased due to decreased tolerance in these patients.12,21,22
Three medications have the most evidence for OUD treatment: buprenorphine, methadone, and naltrexone.15 The decision to use buprenorphine, methadone, or naltrexone depends on a variety of factors, including the severity of the OUD, patient history of prior treatment successes and failures, comorbid medical and psychiatric conditions, and patient preference.4 Treatment with buprenorphine or methadone is preferred over naltrexone for patients who do not want to or cannot tolerate the physical and emotional discomfort of the opioid withdrawal process, who experience moderate to severe OUD, who have a history of failed abstinence-based treatment, or who have more severe physiological tolerance/dependence.12 Buprenorphine is a mu opioid receptor partial agonist that has been shown to reduce opioid cravings,23 provide moderate pain relief,24 and ameliorate OWS.12 It does not typically result in significant respiratory depression, which is the biggest safety concern for opioid use.12 Buprenorphine may also treat comorbid AUD at higher doses; however, the data are inconclusive.25,26 Buprenorphine should be prescribed with caution to patients with comorbid, uncontrolled AUD, due to the risk of respiratory depression when combined with alcohol. Patients who continue to drink alcohol but are able to abstain from opioids may consider starting an AUD-specific medication. Pharmacologic options are discussed in more detail in the next section.
For patients who have higher physiological dependence or more severe OUD, methadone may be a reasonable alternative to buprenorphine. Methadone, a mu-opioid receptor agonist, ameliorates OWS, reduces opioid cravings, and reduces the euphoric effects of opioid ingestion if the patient relapses. However, methadone can only be dispensed for the treatment of OUD by a federally-certified treatment program governed by restrictive and federally mandated guidelines. Compared to buprenorphine, methadone is more dangerous in overdose, has more drug interactions, and is more commonly diverted for recreational use.27 Furthermore, methadone should be prescribed with caution to patients with comorbid, uncontrolled AUD, because both alcohol and methadone can result in respiratory depression.
By contrast, the first-line treatment for individuals experiencing moderateto severe AUD is typically naltrexone.28 Naltrexone is contraindicated in Ms. A because she has a severe OUD and is unlikely to tolerate the opioid withdrawal process. Research suggests that the use of naltrexone for OUD should be limited to patients who have a mild disorder or who show low physiological dependence.29 Alternatively, acamprosate, disulfiram, topiramate, or gabapentin should be considered for Ms. A.4,28,30 Because each of these medications have specific strengths and weaknesses, medication selection should be based on individual patient factors such as comorbid psychiatric and medical conditions and/or patient preference.28
Management decisions: AUD augmentation strategies
Naltrexone is contraindicated for patients who are receiving opioids, including opioid agonist therapy for OUD. Therefore, clinicians need to consider other options for these individuals. There are several medications with good evidence, including acamprosate, disulfiram, topiramate, and gabapentin. Acamprosate and disulfiram are FDA-approved for AUD; the latter 2 have been used off-label.
Continue to: Acamprosate is a glutamate receptor modulator...
Acamprosate is a glutamate receptor modulator that reduces alcohol cravings and is recommended for patients who have achieved and wish to maintain abstinence. It can be used in patients with liver disease, because it is not hepatically metabolized.30 Topiramate is also used to reduce alcohol cravings. It antagonizes glutamate at alpha-amino-3-hydroxy-5-methyl-4-isoxazole-propionate (AMPA) and kainite receptors, facilitates gamma-aminobutyric acid (GABA) function, and reduces the extracellular release of dopamine in the mesocorticolimbic regions of the brain.30 Topiramate is a reasonable option for patients with a seizure disorder, a history of migraine headaches,30 or who are overweight or obese and wish to lose weight.31 In a nonrandomized study, topiramate reduced alcohol intake and cravings more than naltrexone.32
Disulfiram is another second-line therapy for AUD. It is best used under close supervision because it does not reduce alcohol cravings but makes ingesting alcohol extremely aversive by preventing the breakdown of the alcohol metabolite acetaldehyde, and in doing so causes a cluster of unpleasant symptoms, including sweating, palpitations, flushing, nausea/vomiting, and increased sympathetic tone.28 Disulfiram only works if it is taken daily, and it requires a high degree of motivation and/or daily supervision at home or in the clinic.33 It is not recommended to be used as a first-line treatment based on its potential toxicity, adverse effects, and mixed findings on its efficacy. In addition, it should not be given to medically vulnerable/fragile individuals.
Lastly, gabapentin, a voltage-gated calcium channel modulator, may also be used as a second-line agent for AUD. Patients who have started alcohol withdrawal management with gabapentin may wish to continue treatment to assist with craving suppression.30 It is also a good choice for patients who have comorbid diabetic neuropathy or other neuropathic pain conditions, anxiety, or insomnia.30,34 Of note, there have been reports of gabapentin misuse.
CASE REPORTS CONTINUED
Ms. B presents to the ED with a 5-year history of moderate AUD and a 2-year history of mild OUD. She denies a history of severe or complicated AWS. Her laboratory results are significant for a serum ethanol level of 250 mg/dL, UDS positive for opioids, and a negative pregnancy test.
Management decisions: Naltrexone for OUD
In contrast to Ms. A, Ms. B is likely able to complete the opioid withdrawal management process. It is reasonable to treat her uncomplicated, moderate alcohol withdrawal as an outpatient with gabapentin or a benzodiazepine taper. Had her AUD been as severe as Ms. A’s, or if she were unsuccessful with ambulatory withdrawal treatment attempts, Ms. B would also be a candidate for inpatient medical treatment for alcohol withdrawal regardless of the severity of her OUD. Ongoing pharmacotherapy for her AUD after withdrawal management is the same as previously outlined. After Ms. B completes the taper (typically 1 week after the ED visit), she should follow up for initiation of pharmacotherapy for AUD. Ms. B is an ideal candidate for naltrexone, which targets both AUD and OUD.
Continue to: Naltrexone is a semi-synthetic...
Naltrexone is a semi-synthetic competitive antagonist at mu-opioid receptors and a partial agonist at kappa receptors; it has little to no activity at delta receptors. Naltrexone has been shown to reduce alcohol cravings and diminish the euphoric effects of alcohol by reducing endogenous opioid release and receptor activation.35 Thus, even when patients do use alcohol while taking naltrexone, the amount of alcohol they use is typically substantially reduced.36 In fact, at a standard dose of 50 mg/d, 95% of mu-opioid receptors are occupied and are shown to yield approximately 40% alcohol abstinence rates at 1 year.36
Once Ms. B has completed withdrawal management from both alcohol and opioids, she should have a trial period of oral naltrexone to prove tolerability, and then transition to the long-acting injectable (LAI) formulation. Patients able to complete withdrawal management from opioids and transition to LAI naltrexone have been shown to have equivalent rates of successful abstinence from opioids compared to buprenorphine.37 Though Ms. B could opt to try buprenorphine to treat her mild OUD, naltrexone would be the preferred option because it has 3 advantages:
- it blocks the mu-opioid receptor, which prevents euphoria if an illicit substance is used
- it does not cause physiologic dependence or withdrawal syndrome if/when stopped
- if it is not effective, it is easy to switch to buprenorphine.
Lastly, all patients with OUD should be prescribed a rescue naloxone kit, in accordance with harm-reduction guidelines. Naloxone, a potent opioid receptor antagonist, is used to prevent or reverse respiratory depression in opioid overdose. Naloxone rescue kits include intranasal naloxone, which makes it easy for nonclinician bystanders to administer while waiting for emergency transport.38 Most states allow naloxone kits to be prescribed to individuals who have a concern for overdose among friends, family, or others in the community. The wide distribution and easy availability of naloxone rescue kits have been essential in decreasing overdose deaths among patients who misuse opioids.39
Take-home points
Patients with both OUD and AUD are relatively common and often pose significant management challenges when they present to the clinic or the ED in withdrawal. Because severe AWS can be life-threatening, hospitalization should be considered. OWS is often accompanied by intense cravings that can lead to relapse and the risk of accidental opioid overdose/death. As soon as patients are able to engage in a discussion about their treatment options, clinicians need to clarify the patient’s goals and priorities. In medications for OUD, the decision of whether to use buprenorphine, naltrexone, or methadone is guided by the severity of the OUD, the patient’s past treatment experience (illicit as well as prescribed), and patient preference. If the OUD is mild or if the patient prefers to avoid opioid agonist medications and can tolerate the opioid withdrawal process, both the AUD and OUD can be treated with naltrexone, preferably with the LAI formulation. Other AUD medications and outpatient psychotherapy may be used to augment treatment outcomes. For patients with a moderate to severe OUD, buprenorphine (preferably with immediate initiation) or methadone therapy should be offered. Patients with comorbid OUD and AUD who are treated with opioid agonists should be offered medication for AUD other than naltrexone, as outlined above. All patients with substance use disorders would benefit from psychosocial interventions, including group and individual therapy as well as community sober support groups.
Bottom Line
Patients with comorbid opioid use disorder (OUD) and alcohol use disorder (AUD) often pose significant management challenges when they present in withdrawal. This article reviews the evidence and summarizes practical considerations regarding the clinical management of patients with comorbid OUD and AUD.
Related Resources
- Chaney L, Mathia C, Cole T. Transitioning patients with opioid use disorder from methadone to buprenorphine. Current Psychiatry. 2022;21(12):23-24,28. doi:10.12788/ cp.0305
- Eatmon CV, Trent K. Pharmacotherapy for alcohol use disorder in patients with hepatic impairment. Current Psychiatry. 2021;20(12):25-28. doi:10.12788/cp.0068
Drug Brand Names
Acamprosate • Campral
Buprenorphine/naloxone • Suboxone, Zubsolv
Clonidine • Catapres
Disulfiram • Antabuse
Gabapentin • Neurontin
Hydroxyzine • Vistaril
Lofexidine • Lucemyra
Methadone • Methadose, Dolophine
Naloxone • Narcan
Naltrexone • ReVia, Vivitrol
Ondansetron • Zofran
Topiramate • Topamax
Trazodone • Desyrel, Oleptro
When left untreated, opioid use disorder (OUD) is a debilitating and potentially lethal illness. Despite the availability of safe and effective medications for OUD, the prevalence of opioid use and overdose deaths has been increasing every year.1 An additional challenge in OUD treatment is the high prevalence of comorbid alcohol use disorder (AUD).2-6 A Clinical Trials Network survey from the National Institute on Drug Abuse found 38% of persons seeking treatment for OUD also had AUD.7 Other analyses have found alcohol was involved in approximately one-fifth of opioid-related deaths.8 Research also reveals that comorbid OUD and AUD contributes to poor treatment outcomes, more medical comorbidities, and a high risk of death (including overdose death).4,9 There is no standard of care for this particular patient population.3 This article reviews the evidence and summarizes practical considerations regarding the clinical management of patients with comorbid OUD and AUD.
To illustrate the various decision points, we will follow 2 hypothetical patients through various stages of treatment (Figure), from their presentation in the emergency department (ED) or outpatient clinic, through their hospital admission (if needed), and into their outpatient follow-up treatment.

CASE REPORTS
Ms. A and Ms. B present to the ED for evaluation of nausea, vomiting, sweating, anxiety, and tremor. Both patients describe their most recent use of both alcohol and opioids approximately 12 hours ago, and each has been attempting to stop using both substances at home.
Decision-making in the emergency setting
In the ED, a few important decisions need to be made regarding treatment:
- Are the presenting symptoms primarily due to alcohol withdrawal syndrome (AWS), opioid withdrawal syndrome (OWS), or both?
- Does the patient require inpatient medical withdrawal management (detoxification) based on the history and severity of the withdrawal symptoms?
- What are the patient’s treatment goals for their AUD and OUD?
- Is maintenance medication for OUD indicated? If so, which medication is most appropriate?
In the ED, the presentation of individuals affected by both OUD and AUD can be challenging because OWS shares overlapping features with AWS, including nausea, vomiting, diarrhea, sweating, anxiety, and tremor. However, although acute OWS is typically very uncomfortable, it is rarely lethal. On the other hand, severe AWS may result in delirium, seizures, and death,10 which makes it essential to recognize and treat appropriately.
Both Ms. A and Ms. B should be medically evaluated and treated by an emergency medicine physician in conjunction with psychiatric (or addiction medicine) consultation. The ED assessment of a patient presenting with both AUD and OUD should include vital signs monitoring; physical examination; blood work including comprehensive metabolic panel, serum magnesium, and phosphorus; complete blood count; pregnancy test for women of reproductive age; urine drug screen (UDS); urinalysis; and serum ethanol level. Of note, sympathetic hyperactivity is found in both alcohol and opioid withdrawal, and patients with alcohol withdrawal may also have hypokalemia, a condition associated with an increased risk of arrhythmia. Furthermore, a prolonged QTc would affect clinical decision-making about medications for OUD (ie, methadone) and withdrawal management (ie, ondansetron, trazodone, and hydroxyzine). Therefore, an electrocardiogram should be conducted, where appropriate.
Initial treatment of AWS includes vitamin supplementation (thiamine, folic acid, and multivitamins) and benzodiazepine administration (symptom-triggered and/or scheduled taper). It may also include IV fluid resuscitation, analgesics for pain, ondansetron for nausea and vomiting, and other electrolyte repletion as indicated by the laboratory results.11 Additional measures for patients in opioid withdrawal should include alpha-2 agonists such as clonidine or lofexidine for adrenergic symptoms, antiemetics, antidiarrheals, muscle relaxants, anxiolytics such as hydroxyzine, and sleep medications such as trazodone.12
Continue to: The next decision...
The next decision is whether the patient needs to be admitted for inpatient treatment. This decision is based primarily on the risk assessment and severity of AWS, including a compelling history of complicated AWS such as seizures or delirium tremens as well as consideration of the complexity and severity of any comorbid medical or psychiatric conditions. Other indications for medical withdrawal management include a history of unsuccessful ambulatory withdrawal management and pregnancy. For severe AWS, a scheduled benzodiazepine taper in addition to the symptom-triggered protocol should be considered.13-15 A psychiatric evaluation may be obtained in the ED, as long as the patient is sober enough to meaningfully participate in the psychiatric interview. Wherever possible, psychiatric interviews should be supplemented by collateral information.
CASE REPORTS CONTINUED
Ms. A admits to a 5-year history of alcohol and opioid use that meets the criteria for severe AUD and severe OUD. She has previously required inpatient treatment for seizures related to AWS. Laboratory results are notable for a serum ethanol level of 380 mg/dL, UDS positive for opioids, and a negative pregnancy test.
Disposition of patients in alcohol and opioid withdrawal
Given Ms. A’s history of seizures while withdrawing from alcohol, she is appropriate for hospital admission for medically managed withdrawal observation. As previously mentioned, there is clinical overlap between AWS and OWS, and differentiating between the 2 syndromes is essential and may be lifesaving. Whereas anxiety, agitation, diaphoresis, tachycardia, hypertension, and insomnia can be seen in both opioid and alcohol withdrawal, OWS-specific symptoms include mydriasis, lacrimation, rhinorrhea, bone or joint aches, yawning, and piloerection. AWS may present with visual or tactile hallucinations, delirium, and grand mal seizures.15
The details of inpatient management are beyond the scope of this article; however, both patients should be started on thiamine, folic acid, and a multivitamin. For patients in alcohol withdrawal with a history of poor diet who appear malnourished or have a history of malabsorption (such as gastric bypass surgery), thiamine 100 mg/d IV should be given for 3 to 5 days to prevent Wernicke encephalopathy.16 Where there is any concern the patient may be exhibiting signs of Wernicke-Korsakoff Syndrome (impaired cognition, evident malnourishment, ataxia, or eye movement abnormalities), high-dose thiamine IV should be given presumptively as follows: 500 mg IV 3 times a day for 3 days, 250 mg/d IV for 5 days, and then oral supplementation 100 mg/d for at least 30 days.17
In summary, on presentation to the ED, both patients should be medically stabilized and started on benzodiazepines for alcohol withdrawal. The risk assessment and the severity of the AWS often determines the level of care.
CASE REPORTS CONTINUED
On hospital Day 2, Ms. A tells the consulting psychiatrist she would like to start medications to treat her substance use disorders. She has a long history of failed attempts to achieve abstinence from opioids, so she and the psychiatrist agree to initiate a trial of buprenorphine/naloxone for her OUD, 4 mg/1 mg to 8 mg/2 mg for Day 1. Although buprenorphine/naloxone seems to help her alcohol cravings somewhat, she requests additional help. She experiences migraine headaches, which is in part why she began using opioid medications. Via joint decision making with her psychiatrist, she agrees to a trial of topiramate, with a slow titration schedule starting at 25 mg/d.
Continue to: Management decisions
Management decisions: Buprenorphine for OUD
The next issue is to determine the appropriate treatment for the patient’s OUD. Although treating OWS is important in improving the patient’s health, decreasing their discomfort, and facilitating their participation in a psychosocial treatment program,18 current evidence suggests that opioid withdrawal management alone without medication for OUD rarely leads to long-term recovery.19,20 Some research suggests that the risk of accidental opioid overdose immediately following acute withdrawal management may actually be increased due to decreased tolerance in these patients.12,21,22
Three medications have the most evidence for OUD treatment: buprenorphine, methadone, and naltrexone.15 The decision to use buprenorphine, methadone, or naltrexone depends on a variety of factors, including the severity of the OUD, patient history of prior treatment successes and failures, comorbid medical and psychiatric conditions, and patient preference.4 Treatment with buprenorphine or methadone is preferred over naltrexone for patients who do not want to or cannot tolerate the physical and emotional discomfort of the opioid withdrawal process, who experience moderate to severe OUD, who have a history of failed abstinence-based treatment, or who have more severe physiological tolerance/dependence.12 Buprenorphine is a mu opioid receptor partial agonist that has been shown to reduce opioid cravings,23 provide moderate pain relief,24 and ameliorate OWS.12 It does not typically result in significant respiratory depression, which is the biggest safety concern for opioid use.12 Buprenorphine may also treat comorbid AUD at higher doses; however, the data are inconclusive.25,26 Buprenorphine should be prescribed with caution to patients with comorbid, uncontrolled AUD, due to the risk of respiratory depression when combined with alcohol. Patients who continue to drink alcohol but are able to abstain from opioids may consider starting an AUD-specific medication. Pharmacologic options are discussed in more detail in the next section.
For patients who have higher physiological dependence or more severe OUD, methadone may be a reasonable alternative to buprenorphine. Methadone, a mu-opioid receptor agonist, ameliorates OWS, reduces opioid cravings, and reduces the euphoric effects of opioid ingestion if the patient relapses. However, methadone can only be dispensed for the treatment of OUD by a federally-certified treatment program governed by restrictive and federally mandated guidelines. Compared to buprenorphine, methadone is more dangerous in overdose, has more drug interactions, and is more commonly diverted for recreational use.27 Furthermore, methadone should be prescribed with caution to patients with comorbid, uncontrolled AUD, because both alcohol and methadone can result in respiratory depression.
By contrast, the first-line treatment for individuals experiencing moderateto severe AUD is typically naltrexone.28 Naltrexone is contraindicated in Ms. A because she has a severe OUD and is unlikely to tolerate the opioid withdrawal process. Research suggests that the use of naltrexone for OUD should be limited to patients who have a mild disorder or who show low physiological dependence.29 Alternatively, acamprosate, disulfiram, topiramate, or gabapentin should be considered for Ms. A.4,28,30 Because each of these medications have specific strengths and weaknesses, medication selection should be based on individual patient factors such as comorbid psychiatric and medical conditions and/or patient preference.28
Management decisions: AUD augmentation strategies
Naltrexone is contraindicated for patients who are receiving opioids, including opioid agonist therapy for OUD. Therefore, clinicians need to consider other options for these individuals. There are several medications with good evidence, including acamprosate, disulfiram, topiramate, and gabapentin. Acamprosate and disulfiram are FDA-approved for AUD; the latter 2 have been used off-label.
Continue to: Acamprosate is a glutamate receptor modulator...
Acamprosate is a glutamate receptor modulator that reduces alcohol cravings and is recommended for patients who have achieved and wish to maintain abstinence. It can be used in patients with liver disease, because it is not hepatically metabolized.30 Topiramate is also used to reduce alcohol cravings. It antagonizes glutamate at alpha-amino-3-hydroxy-5-methyl-4-isoxazole-propionate (AMPA) and kainite receptors, facilitates gamma-aminobutyric acid (GABA) function, and reduces the extracellular release of dopamine in the mesocorticolimbic regions of the brain.30 Topiramate is a reasonable option for patients with a seizure disorder, a history of migraine headaches,30 or who are overweight or obese and wish to lose weight.31 In a nonrandomized study, topiramate reduced alcohol intake and cravings more than naltrexone.32
Disulfiram is another second-line therapy for AUD. It is best used under close supervision because it does not reduce alcohol cravings but makes ingesting alcohol extremely aversive by preventing the breakdown of the alcohol metabolite acetaldehyde, and in doing so causes a cluster of unpleasant symptoms, including sweating, palpitations, flushing, nausea/vomiting, and increased sympathetic tone.28 Disulfiram only works if it is taken daily, and it requires a high degree of motivation and/or daily supervision at home or in the clinic.33 It is not recommended to be used as a first-line treatment based on its potential toxicity, adverse effects, and mixed findings on its efficacy. In addition, it should not be given to medically vulnerable/fragile individuals.
Lastly, gabapentin, a voltage-gated calcium channel modulator, may also be used as a second-line agent for AUD. Patients who have started alcohol withdrawal management with gabapentin may wish to continue treatment to assist with craving suppression.30 It is also a good choice for patients who have comorbid diabetic neuropathy or other neuropathic pain conditions, anxiety, or insomnia.30,34 Of note, there have been reports of gabapentin misuse.
CASE REPORTS CONTINUED
Ms. B presents to the ED with a 5-year history of moderate AUD and a 2-year history of mild OUD. She denies a history of severe or complicated AWS. Her laboratory results are significant for a serum ethanol level of 250 mg/dL, UDS positive for opioids, and a negative pregnancy test.
Management decisions: Naltrexone for OUD
In contrast to Ms. A, Ms. B is likely able to complete the opioid withdrawal management process. It is reasonable to treat her uncomplicated, moderate alcohol withdrawal as an outpatient with gabapentin or a benzodiazepine taper. Had her AUD been as severe as Ms. A’s, or if she were unsuccessful with ambulatory withdrawal treatment attempts, Ms. B would also be a candidate for inpatient medical treatment for alcohol withdrawal regardless of the severity of her OUD. Ongoing pharmacotherapy for her AUD after withdrawal management is the same as previously outlined. After Ms. B completes the taper (typically 1 week after the ED visit), she should follow up for initiation of pharmacotherapy for AUD. Ms. B is an ideal candidate for naltrexone, which targets both AUD and OUD.
Continue to: Naltrexone is a semi-synthetic...
Naltrexone is a semi-synthetic competitive antagonist at mu-opioid receptors and a partial agonist at kappa receptors; it has little to no activity at delta receptors. Naltrexone has been shown to reduce alcohol cravings and diminish the euphoric effects of alcohol by reducing endogenous opioid release and receptor activation.35 Thus, even when patients do use alcohol while taking naltrexone, the amount of alcohol they use is typically substantially reduced.36 In fact, at a standard dose of 50 mg/d, 95% of mu-opioid receptors are occupied and are shown to yield approximately 40% alcohol abstinence rates at 1 year.36
Once Ms. B has completed withdrawal management from both alcohol and opioids, she should have a trial period of oral naltrexone to prove tolerability, and then transition to the long-acting injectable (LAI) formulation. Patients able to complete withdrawal management from opioids and transition to LAI naltrexone have been shown to have equivalent rates of successful abstinence from opioids compared to buprenorphine.37 Though Ms. B could opt to try buprenorphine to treat her mild OUD, naltrexone would be the preferred option because it has 3 advantages:
- it blocks the mu-opioid receptor, which prevents euphoria if an illicit substance is used
- it does not cause physiologic dependence or withdrawal syndrome if/when stopped
- if it is not effective, it is easy to switch to buprenorphine.
Lastly, all patients with OUD should be prescribed a rescue naloxone kit, in accordance with harm-reduction guidelines. Naloxone, a potent opioid receptor antagonist, is used to prevent or reverse respiratory depression in opioid overdose. Naloxone rescue kits include intranasal naloxone, which makes it easy for nonclinician bystanders to administer while waiting for emergency transport.38 Most states allow naloxone kits to be prescribed to individuals who have a concern for overdose among friends, family, or others in the community. The wide distribution and easy availability of naloxone rescue kits have been essential in decreasing overdose deaths among patients who misuse opioids.39
Take-home points
Patients with both OUD and AUD are relatively common and often pose significant management challenges when they present to the clinic or the ED in withdrawal. Because severe AWS can be life-threatening, hospitalization should be considered. OWS is often accompanied by intense cravings that can lead to relapse and the risk of accidental opioid overdose/death. As soon as patients are able to engage in a discussion about their treatment options, clinicians need to clarify the patient’s goals and priorities. In medications for OUD, the decision of whether to use buprenorphine, naltrexone, or methadone is guided by the severity of the OUD, the patient’s past treatment experience (illicit as well as prescribed), and patient preference. If the OUD is mild or if the patient prefers to avoid opioid agonist medications and can tolerate the opioid withdrawal process, both the AUD and OUD can be treated with naltrexone, preferably with the LAI formulation. Other AUD medications and outpatient psychotherapy may be used to augment treatment outcomes. For patients with a moderate to severe OUD, buprenorphine (preferably with immediate initiation) or methadone therapy should be offered. Patients with comorbid OUD and AUD who are treated with opioid agonists should be offered medication for AUD other than naltrexone, as outlined above. All patients with substance use disorders would benefit from psychosocial interventions, including group and individual therapy as well as community sober support groups.
Bottom Line
Patients with comorbid opioid use disorder (OUD) and alcohol use disorder (AUD) often pose significant management challenges when they present in withdrawal. This article reviews the evidence and summarizes practical considerations regarding the clinical management of patients with comorbid OUD and AUD.
Related Resources
- Chaney L, Mathia C, Cole T. Transitioning patients with opioid use disorder from methadone to buprenorphine. Current Psychiatry. 2022;21(12):23-24,28. doi:10.12788/ cp.0305
- Eatmon CV, Trent K. Pharmacotherapy for alcohol use disorder in patients with hepatic impairment. Current Psychiatry. 2021;20(12):25-28. doi:10.12788/cp.0068
Drug Brand Names
Acamprosate • Campral
Buprenorphine/naloxone • Suboxone, Zubsolv
Clonidine • Catapres
Disulfiram • Antabuse
Gabapentin • Neurontin
Hydroxyzine • Vistaril
Lofexidine • Lucemyra
Methadone • Methadose, Dolophine
Naloxone • Narcan
Naltrexone • ReVia, Vivitrol
Ondansetron • Zofran
Topiramate • Topamax
Trazodone • Desyrel, Oleptro
1. Mattson CL, Tanz LJ, Quinn K, et al. Trends and geographic patterns in drug and synthetic opioid overdose deaths - United States, 2013-2019. MMWR Morb Mortal Wkly Rep. 2021;70(6):202-207.
2. Hartzler B, Donovan DM, Huang Z. Comparison of opiate-primary treatment seekers with and without alcohol use disorder. J Subst Abuse Treat. 2010;39(2):114-123.
3. Nolan S, Klimas J, Wood E. Alcohol use in opioid agonist treatment. Addict Sci Clin Pract. 2016;11(1):17.
4. Hood LE, Leyrer-Hackson JM, Olive MF. Pharmacotherapeutic management of co-morbid alcohol and opioid use. Expert Opin Pharmacother. 2020;21(7):823-839.
5. Pikovsky M, Peacock A, Larney S, et al. Alcohol use disorder and associated physical health complications and treatment amongst individuals with and without opioid dependence: a case-control study. Drug Alcohol Depend. 2018;188:304-310.
6. Jones CM, McCance-Katz EF. Co-occurring substance use and mental disorders among adults with opioid use disorder. Drug Alcohol Depend. 2019;197:78-82.
7. Hartzler B, Donovan DM, Huang Z. Comparison of opiate-primary treatment seekers with and without alcohol use disorder. J Subst Abuse Treat. 2010;39(2):114-123.
8. Jones CM, Paulozzi LJ, Mack KA; Centers for Disease Control and Prevention (CDC). Alcohol involvement in opioid pain reliever and benzodiazepine drug abuse-related emergency department visits and drug-related deaths - United States, 2010. MMWR Morb Mortal Wkly Rep. 2014;63(40):881-885.
9. Stapleton RD, Comiskey CM. Alcohol usage and associated treatment outcomes for opiate users entering treatment in Ireland. Drug Alcohol Depend. 2010;107(1):56-61.
10. Turner RC, Lichstein PR, Peden JG Jr, et al. Alcohol withdrawal syndromes: a review of pathophysiology, clinical presentation, and treatment. J Gen Intern Med. 1989;4(5):432-444.
11. Boba A. Management of acute alcohol intoxication. Am J Emerg Med. 1999;17(4):431.
12. The ASAM national practice guideline for the treatment of opioid use disorder: 2020 focused update. J Addict Med. 2020;14(2S Suppl1):1-91.
13. Shaw JM, Kolesar GS, Sellers EM, et al. Development of optimal treatment tactics for alcohol withdrawal. I. Assessment and effectiveness of supportive care. J Clin Psychopharmacol. 1981;1(6):382-389.
14. Naranjo CA, Sellers EM. Clinical assessment and pharmacotherapy of the alcohol withdrawal syndrome. Recent Dev Alcohol. 1986;4:265-281.
15. Kampman K, Jarvis M. American Society of Addiction Medicine (ASAM) national practice guideline for the use of medications in the treatment of addiction involving opioid use. J Addict Med. 2015;9(5):358-367.
16. The ASAM clinical practice guideline on alcohol withdrawal management. J Addict Med. 2020;14(3S Suppl 1):1-72.
17. Isenberg-Grzeda E, Kutner HE, Nicolson SE. Wernicke-Korsakoff-syndrome: under-recognized and under-treated. Psychosomatics. 2012;53(6):507-516.
18. Schuckit MA. Treatment of opioid-use disorders. N Engl J Med. 2016;375(4):357-368.
19. Tang Y-L, Hao W. Improving drug addiction treatment in China. Addiction. 2007;102(7):1057-1063.
20. Wakeman SE, Larochelle MR, Ameli O, et al. Comparative effectiveness of different treatment pathways for opioid use disorder. JAMA Netw Open. 2020;3(2):e1920622.
21. Wines JD Jr, Saitz R, Horton NJ, et al. Overdose after detoxification: a prospective study. Drug Alcohol Depend. 2007;89(2-3):161-169.
22. Maughan BC, Becker EA. Drug-related mortality after discharge from treatment: a record-linkage study of substance abuse clients in Texas, 2006-2012. Drug Alcohol Depend. 2019;204:107473.
23. Gowing L, Ali R, White J. Buprenorphine for the management of opioid withdrawal. Cochrane Database Syst Rev. 2002;(2):CD002025.
24. Malinoff HL, Barkin RL, Wilson G. Sublingual buprenorphine is effective in the treatment of chronic pain syndrome. Am J Ther. 2005;12(5):379-384.
25. Nava F, Manzato E, Leonardi C, et al. Opioid maintenance therapy suppresses alcohol intake in heroin addicts with alcohol dependence: preliminary results of an open randomized study. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(8):1867-1872.
26. Srivastava A, Kahan M, Ross S. The effect of methadone maintenance treatment on alcohol consumption: a systematic review. J Subst Abuse Treat. 2008;34(2):215-223.
27. Davids E, Gastpar M. Buprenorphine in the treatment of opioid dependence. Eur Neuropsychopharmacol. 2004;14(3):209-216.
28. American Psychiatric Association. Practice Guideline for the Pharmacological Treatment of Patients With Alcohol Use Disorder. American Psychiatric Association; 2018.
29. Hassanian-Moghaddam H, Afzali S, Pooya A. Withdrawal syndrome caused by naltrexone in opioid abusers. Hum Exp Toxicol. 2014;33(6):561-567.
30. Fairbanks J, Umbreit A, Kolla BP, et al. Evidence-based pharmacotherapies for alcohol use disorder: clinical pearls. Mayo Clin Proc. 2020;95(9):1964-1977.
31. Verrotti A, Scaparrotta A, Agostinelli S, et al. Topiramate-induced weight loss: a review. Epilepsy Res. 2011;95(3):189-199.
32. Flórez G, García-Portilla P, Alvarez S, et al. Using topiramate or naltrexone for the treatment of alcohol-dependent patients. Alcohol Clin Exp Res. 2008;32(7):1251-1259.
33. Jørgensen CH, Pedersen B, Tønnesen H. The efficacy of disulfiram for the treatment of alcohol use disorder. Alcohol Clin Exp Res. 2011;35(10):1749-1758.
34. Mason BJ, Quello S, Shadan F. Gabapentin for the treatment of alcohol use disorder. Expert Opin Investig Drugs. 2018;27(1):113-124.
35. Sudakin D. Naltrexone: not just for opioids anymore. J Med Toxicol. 2016;12(1):71-75.
36. Rubio G, Jiménez-Arrieri MA, Ponce G, et al. Naltrexone versus acamprosate: one year follow-up of alcohol dependence treatment. Alcohol Alcohol. 2001;36(5):419-425.
37. Lee JD, Nunes EV Jr, Novo P, et al. Comparative effectiveness of extended-release naltrexone versus buprenorphine-naloxone for opioid relapse prevention (X:BOT): a multicentre, open-label, randomised controlled trial. Lancet. 2018;391(10118):309-318.
38. Clark AK, Wilder CM, Winstanley EL. A systematic review of community opioid overdose prevention and naloxone distribution programs. J Addict Med. 2014;8(3):153-163.
39. Dunne RB. Prescribing naloxone for opioid overdose intervention. Pain Manag. 2018;8(3):197-208.
1. Mattson CL, Tanz LJ, Quinn K, et al. Trends and geographic patterns in drug and synthetic opioid overdose deaths - United States, 2013-2019. MMWR Morb Mortal Wkly Rep. 2021;70(6):202-207.
2. Hartzler B, Donovan DM, Huang Z. Comparison of opiate-primary treatment seekers with and without alcohol use disorder. J Subst Abuse Treat. 2010;39(2):114-123.
3. Nolan S, Klimas J, Wood E. Alcohol use in opioid agonist treatment. Addict Sci Clin Pract. 2016;11(1):17.
4. Hood LE, Leyrer-Hackson JM, Olive MF. Pharmacotherapeutic management of co-morbid alcohol and opioid use. Expert Opin Pharmacother. 2020;21(7):823-839.
5. Pikovsky M, Peacock A, Larney S, et al. Alcohol use disorder and associated physical health complications and treatment amongst individuals with and without opioid dependence: a case-control study. Drug Alcohol Depend. 2018;188:304-310.
6. Jones CM, McCance-Katz EF. Co-occurring substance use and mental disorders among adults with opioid use disorder. Drug Alcohol Depend. 2019;197:78-82.
7. Hartzler B, Donovan DM, Huang Z. Comparison of opiate-primary treatment seekers with and without alcohol use disorder. J Subst Abuse Treat. 2010;39(2):114-123.
8. Jones CM, Paulozzi LJ, Mack KA; Centers for Disease Control and Prevention (CDC). Alcohol involvement in opioid pain reliever and benzodiazepine drug abuse-related emergency department visits and drug-related deaths - United States, 2010. MMWR Morb Mortal Wkly Rep. 2014;63(40):881-885.
9. Stapleton RD, Comiskey CM. Alcohol usage and associated treatment outcomes for opiate users entering treatment in Ireland. Drug Alcohol Depend. 2010;107(1):56-61.
10. Turner RC, Lichstein PR, Peden JG Jr, et al. Alcohol withdrawal syndromes: a review of pathophysiology, clinical presentation, and treatment. J Gen Intern Med. 1989;4(5):432-444.
11. Boba A. Management of acute alcohol intoxication. Am J Emerg Med. 1999;17(4):431.
12. The ASAM national practice guideline for the treatment of opioid use disorder: 2020 focused update. J Addict Med. 2020;14(2S Suppl1):1-91.
13. Shaw JM, Kolesar GS, Sellers EM, et al. Development of optimal treatment tactics for alcohol withdrawal. I. Assessment and effectiveness of supportive care. J Clin Psychopharmacol. 1981;1(6):382-389.
14. Naranjo CA, Sellers EM. Clinical assessment and pharmacotherapy of the alcohol withdrawal syndrome. Recent Dev Alcohol. 1986;4:265-281.
15. Kampman K, Jarvis M. American Society of Addiction Medicine (ASAM) national practice guideline for the use of medications in the treatment of addiction involving opioid use. J Addict Med. 2015;9(5):358-367.
16. The ASAM clinical practice guideline on alcohol withdrawal management. J Addict Med. 2020;14(3S Suppl 1):1-72.
17. Isenberg-Grzeda E, Kutner HE, Nicolson SE. Wernicke-Korsakoff-syndrome: under-recognized and under-treated. Psychosomatics. 2012;53(6):507-516.
18. Schuckit MA. Treatment of opioid-use disorders. N Engl J Med. 2016;375(4):357-368.
19. Tang Y-L, Hao W. Improving drug addiction treatment in China. Addiction. 2007;102(7):1057-1063.
20. Wakeman SE, Larochelle MR, Ameli O, et al. Comparative effectiveness of different treatment pathways for opioid use disorder. JAMA Netw Open. 2020;3(2):e1920622.
21. Wines JD Jr, Saitz R, Horton NJ, et al. Overdose after detoxification: a prospective study. Drug Alcohol Depend. 2007;89(2-3):161-169.
22. Maughan BC, Becker EA. Drug-related mortality after discharge from treatment: a record-linkage study of substance abuse clients in Texas, 2006-2012. Drug Alcohol Depend. 2019;204:107473.
23. Gowing L, Ali R, White J. Buprenorphine for the management of opioid withdrawal. Cochrane Database Syst Rev. 2002;(2):CD002025.
24. Malinoff HL, Barkin RL, Wilson G. Sublingual buprenorphine is effective in the treatment of chronic pain syndrome. Am J Ther. 2005;12(5):379-384.
25. Nava F, Manzato E, Leonardi C, et al. Opioid maintenance therapy suppresses alcohol intake in heroin addicts with alcohol dependence: preliminary results of an open randomized study. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(8):1867-1872.
26. Srivastava A, Kahan M, Ross S. The effect of methadone maintenance treatment on alcohol consumption: a systematic review. J Subst Abuse Treat. 2008;34(2):215-223.
27. Davids E, Gastpar M. Buprenorphine in the treatment of opioid dependence. Eur Neuropsychopharmacol. 2004;14(3):209-216.
28. American Psychiatric Association. Practice Guideline for the Pharmacological Treatment of Patients With Alcohol Use Disorder. American Psychiatric Association; 2018.
29. Hassanian-Moghaddam H, Afzali S, Pooya A. Withdrawal syndrome caused by naltrexone in opioid abusers. Hum Exp Toxicol. 2014;33(6):561-567.
30. Fairbanks J, Umbreit A, Kolla BP, et al. Evidence-based pharmacotherapies for alcohol use disorder: clinical pearls. Mayo Clin Proc. 2020;95(9):1964-1977.
31. Verrotti A, Scaparrotta A, Agostinelli S, et al. Topiramate-induced weight loss: a review. Epilepsy Res. 2011;95(3):189-199.
32. Flórez G, García-Portilla P, Alvarez S, et al. Using topiramate or naltrexone for the treatment of alcohol-dependent patients. Alcohol Clin Exp Res. 2008;32(7):1251-1259.
33. Jørgensen CH, Pedersen B, Tønnesen H. The efficacy of disulfiram for the treatment of alcohol use disorder. Alcohol Clin Exp Res. 2011;35(10):1749-1758.
34. Mason BJ, Quello S, Shadan F. Gabapentin for the treatment of alcohol use disorder. Expert Opin Investig Drugs. 2018;27(1):113-124.
35. Sudakin D. Naltrexone: not just for opioids anymore. J Med Toxicol. 2016;12(1):71-75.
36. Rubio G, Jiménez-Arrieri MA, Ponce G, et al. Naltrexone versus acamprosate: one year follow-up of alcohol dependence treatment. Alcohol Alcohol. 2001;36(5):419-425.
37. Lee JD, Nunes EV Jr, Novo P, et al. Comparative effectiveness of extended-release naltrexone versus buprenorphine-naloxone for opioid relapse prevention (X:BOT): a multicentre, open-label, randomised controlled trial. Lancet. 2018;391(10118):309-318.
38. Clark AK, Wilder CM, Winstanley EL. A systematic review of community opioid overdose prevention and naloxone distribution programs. J Addict Med. 2014;8(3):153-163.
39. Dunne RB. Prescribing naloxone for opioid overdose intervention. Pain Manag. 2018;8(3):197-208.
Evaluation after a suicide attempt: What to ask
In 2021, suicide was the 11th leading cause of death in the United States.1 Suicide resulted in 49,000 US deaths during 2021; it was the second most common cause of death in individuals age 10 to 34, and the fifth leading cause among children.1,2 Women are 3 to 4 times more likely than men to attempt suicide, but men are 4 times more likely to die by suicide.2
The evaluation of patients with suicidal ideation who have not made an attempt generally involves assessing 4 factors: the specific plan, access to lethal means, any recent social stressors, and the presence of a psychiatric disorder.3 The clinician should also assess which potential deterrents, such as religious beliefs or dependent children, might be present.
Mental health clinicians are often called upon to evaluate a patient after a suicide attempt to assess intent for continued self-harm and to determine appropriate disposition. Such an evaluation must consider multiple factors, including the method used, premeditation, consequences of the attempt, the presence of severe depression and/or psychosis, and the role of substance use. Assessment after a suicide attempt differs from the examination of individuals who harbor suicidal thoughts but have not made an attempt; the latter group may be more likely to respond to interventions such as intensive outpatient care, mobilization of family support, and religious proscriptions against suicide. However, for patients who make an attempt to end their life, whatever potential safeguards or deterrents to suicide that were in place obviously did not prevent the self-harm act. The consequences of the attempt, such as disabling injuries or medical complications, and possible involuntary commitment, need to be considered. Assessment of the patient’s feelings about having survived the attempt is important because the psychological impact of the attempt on family members may serve to intensify the patient’s depression and make a subsequent attempt more likely.
Many individuals who think of suicide have communicated self-harm thoughts or intentions, but such comments are often minimized or ignored. There is a common but erroneous belief that if patients are encouraged to discuss thoughts of self-harm, they will be more likely to act upon them. Because the opposite is true,4 clinicians should ask vulnerable patients about suicidal ideation or intent. Importantly, noncompliance with life-saving medical care, risk-taking behaviors, and substance use may also signal a desire for self-harm. Passive thoughts of death, typified by comments such as “I don’t care whether I wake up or not,” should also be elicited. Many patients who think of suicide speak of being in a “bad place” where reason and logic give way to an intense desire to end their misery.

The evaluation of a patient who has attempted suicide is an important component of providing psychiatric care. This article reflects our 45 years of evaluating such patients. As such, it reflects our clinical experience and is not evidence-based. We offer a checklist of 14 questions that we have found helpful when determining if it would be best for a patient to receive inpatient psychiatric hospitalization or a discharge referral for outpatient care (Table). Questions 1 through 6 are specific for patients who have made a suicide attempt, while questions 7 through 14 are helpful for assessing global risk factors for suicide.
1. Was the attempt premeditated?
Determining premeditation vs impulsivity is an essential element of the assessment following a suicide attempt. Many such acts may occur without forethought in response to an unexpected stressor, such as an altercation between partners or family conflicts. Impulsive attempts can occur when an individual is involved in a distressing event and/or while intoxicated. Conversely, premeditation involves forethought and planning, which may increase the risk of suicide in the near future.
Examples of premeditated behavior include:
- Contemplating the attempt days or weeks beforehand
- Researching the effects of a medication or combination of medications in terms of potential lethality
- Engaging in behavior that would decrease the likelihood of their body being discovered after the attempt
- Obtaining weapons and/or stockpiling pills
- Canvassing potential sites such as bridges or tall buildings
- Engaging in a suicide attempt “practice run”
- Leaving a suicide note or message on social media
- Making funeral arrangements, such as choosing burial clothing
- Writing a will and arranging for the custody of dependent children
- Purchasing life insurance that does not deny payment of benefits in cases of death by suicide.
Continue to: Patients with a premeditated...
Patients with a premeditated suicide attempt generally do not expect to survive and are often surprised or upset that the act was not fatal. The presence of indicators that the attempt was premeditated should direct the disposition more toward hospitalization than discharge. In assessing the impact of premeditation, it is important to gauge not just the examples listed above, but also the patient’s perception of these issues (such as potential loss of child custody). Consider how much the patient is emotionally affected by such thinking.
2. What were the consequences of the attempt?
Assessing the reason for the attempt (if any) and determining whether the inciting circumstance has changed due to the suicide attempt are an important part of the evaluation. A suicide attempt may result in reconciliation with and/or renewed support from family members or partners, who might not have been aware of the patient’s emotional distress. Such unexpected support often results in the patient exhibiting improved mood and affect, and possibly temporary resolution of suicidal thoughts. This “flight into health” may be short-lived, but it also may be enough to engage the patient in a therapeutic alliance. That may permit a discharge with safe disposition to the outpatient clinic while in the custody of a family member, partner, or close friend.
Alternatively, some people experience a troubling worsening of precipitants following a suicide attempt. Preexisting medical conditions and financial, occupational, and/or social woes may be exacerbated. Child custody determinations may be affected, assuming the patient understands the possibility of this adverse consequence. Violent methods may result in disfigurement and body image issues. Individuals from small, close-knit communities may experience stigmatization and unwanted notoriety because of their suicide attempt. Such negative consequences may render some patients more likely to make another attempt to die by suicide. It is crucial to consider how a suicide attempt may have changed the original stress that led to the attempt.
3. Which method was used?
Most fatal suicides in the US are by firearms, and many individuals who survive such attempts do so because of unfamiliarity with the weapon, gun malfunction, faulty aim, or alcohol use.5-7 Some survivors report intending to shoot themselves in the heart, but instead suffered shoulder injuries. Unfortunately, for a patient who survives self-inflicted gunshot wounds, the sequelae of chronic pain, multiple surgical procedures, disability, and disfigurement may serve as constant negative reminders of the event. Some individuals with suicidal intent eschew the idea of using firearms because they hope to avoid having a family member be the first to discover them. Witnessing the aftermath of a fatal suicide by gunshot can induce symptoms of posttraumatic stress disorder in family members and/or partners.8
For a patient with self-inflicted gunshot wounds, always determine whether the weapon has been secured or if the patient still has access to it. Asking about weapon availability is essential during the evaluation of any patient with depression, major life crises, or other factors that may yield a desire to die; this is especially true for individuals with substance use disorders (SUDs). Whenever readily available to such individuals, weapons need to be safely removed.
Continue to: Other self-harm methods...
Other self-harm methods with a high degree of lethality include jumping from bridges or buildings, poisonings, self-immolation, cutting, and hangings. Individuals who choose these approaches generally do not intend to survive. Many of these methods also entail premeditation, as in the case of individuals who canvass bridges and note time when traffic is light so they are less likely to be interrupted. Between 1937 and 2012, there were >1,600 deaths by suicide from San Francisco’s Golden Gate Bridge.9 Patients who choose highly lethal methods are often irritated during the postattempt evaluation because their plans were not fatal. Usually, patients who choose such potentially lethal methods are hospitalized initially on medical and surgical floors, and receive most of their psychiatric care from consultation psychiatrists. Following discharge, these patients may be at high risk for subsequent suicide attempts.
In the US, the most common method of attempting suicide is by overdose.4 Lethality is determined by the agent or combination of substances ingested, the amount taken, the person’s health status, and the length of time before they are discovered. Many patients mistakenly assume that readily available agents such as acetaminophen and aspirin are less likely to be fatal than prescription medications. Evaluators may want to assess for suicidality in individuals with erratic, risk-taking behaviors, who are at especially high risk for death. Learning about the method the patient used can help the clinician determine the imminent risk of another suicide attempt. The more potentially fatal the patient’s method, the more serious their suicide intent, and the higher the risk they will make another suicide attempt, possibly using an even more lethal method.
4. What was the intent?
“What did you want to happen when you made this attempt?” Many patients will respond that they wanted to die, sleep, not wake up, or did not care what happened. Others say it was a gesture to evoke a certain response from another person. If this is the case, it is important to know whether the desired outcome was achieved. These so-called gestures often involve making sure the intended person is aware of the attempt, often by writing a letter, sending a text, or posting on social media. Such behaviors may be exhibited by patients with personality disorders. While such attempts often are impulsive, if the attempt fails to generate the anticipated effect, the patient may try to gain more attention by escalating their suicide actions.
Conversely, if a spouse or partner reconciles with the patient solely because of a suicide attempt, this may set a pattern for future self-harm events in which the patient hopes to achieve the same outcome. Nevertheless, it is better to err for safety because some of these patients will make another attempt, just to prove that they should have been taken more seriously. An exploration of such intent can help the evaluation because even supposed “gestures” can have dangerous consequences. Acts that do not result in the desired outcome should precipitate hospitalization rather than discharge.
5. What facilitated the patient’s rescue?
“Why is this patient still alive?” Determine if the patient did anything to save themself, such as calling an ambulance, inducing emesis, telling someone what they did, or coming to the hospital on their own. If yes, asking them what changed their mind may provide information about what exists in their lives to potentially prevent future attempts, or about wishes to stay alive. These issues can be used to guide outpatient therapy.
Continue to: How does the patient feel about having survived?
6. How does the patient feel about having survived?
When a patient is asked how they feel about having survived a suicide attempt, some will label their act “stupid” and profess embarrassment. Others exhibit future-oriented thought, which is a very good prognostic sign. More ominous is subsequent dysphoria or lamenting that “I could not even do this right.” Patients often express anger toward anyone who rescued them, especially those whose attempts were carefully planned or were discovered by accident. Some patients might also express ambivalence about having survived.
The patient’s response to this question may be shaped by their desire to avoid hospitalization, so beyond their verbal answers, be attentive to clinical cues that may suggest the patient is not being fully transparent. Anger or ambivalence about having survived, a lack of future-oriented thought, and a restricted affect despite verbalizing joy about still being alive are features that suggest psychiatric hospitalization may be warranted.
7. Has the patient made previous suicide attempts?
Compared to individuals with no previous suicide attempts, patients with a history of suicide attempts are 30 to 40 times more likely to die by suicide.2 Many patients who present after a suicide attempt have tried to kill themselves multiple times. Exploring the number of past attempts, how recent the attempts were, and what dispositions were made can be of benefit. Reviewing the potential lethality of past attempts (eg, was hospitalization required, was the patient placed in an intensive care unit, and/or was intubation needed) is recommended. If outpatient care was suggested or medication prescribed, was the patient adherent? Consider asking about passive suicidal behavior, such as not seeking care for medical issues, discontinuing life-saving medication, or engaging in reckless behavior. While such behaviors may not have been classified as a suicide attempt, it might indicate a feeling of indifference toward staying alive. A patient with a past attempt, especially if recent, merits consideration for inpatient care. Once again, referring previously nonadherent patients to outpatient treatment is less likely to be effective.
8. Does the patient have a support network?
Before discharging a patient who has made a suicide attempt, consider the quality of their support network. Gauging the response of the family and friends to the patient’s attempt can be beneficial. Indifference or resentment on the part of loved ones is a bad sign. Some patients have access to support networks they either did not know were available or chose not to utilize. In other instances, after realizing how depressed the patient has been, the family might provide a new safety net. Strong religious affiliations can also be valuable because devout spirituality can be a deterrent to suicide behaviors.10 For an individual whose attempt was motivated by loneliness or feeling unloved or underappreciated, a newly realized support network can be an additional protective deterrent.
9. Does the patient have a family history of suicide?
There may be a familial component to suicide. Knowing about any suicide history in the family contributes to future therapeutic planning. The clinician may want to explore the patient’s family suicide history in detail because such information can have substantial impact on the patient’s motivation for attempting suicide. The evaluator may want to determine if the anniversary of a family suicide is coming. Triggers for a suicide attempt could include the anniversary of a death, birthdays, family-oriented holidays, and similar events. It is productive to understand how the patient feels about family members who have died by suicide. Some will empathize with the deceased, commenting that they did the “right thing.” Others, upon realizing how their own attempt affected others, will be remorseful and determined not to inflict more pain on their family. Such patients may need to be reminded of the misery associated with their family being left without them. These understandings are helpful at setting a safe disposition. However, a history of death by suicide in the family should always be thoroughly evaluated, regardless of the patient’s attitude about that death.
Continue to: Was the attempt the result of depression?
10. Was the attempt the result of depression?
For a patient experiencing depressive symptoms, the prognosis is less positive; they are more likely to harbor serious intent, premeditation, hopelessness, and social isolation, and less likely to express future-oriented thought. They often exhibit a temporary “flight into health.” Such progress is often transitory and may not represent recovery. Because mood disorders may still be present despite a temporary improvement, inpatient and pharmacologic treatment may be needed. If a patient’s suicide attempt occurred as a result of severe depression, it is possible they will make another suicide attempt unless their depression is addressed in a safe and secure setting, such as inpatient hospitalization, or through close family observation while the patient is receiving intensive outpatient treatment.
11. Does the patient have a psychotic disorder?
Many patients with a psychotic illness die following their first attempt without ever having contact with a mental health professional.11 Features of psychosis might include malevolent auditory hallucinations that suggest self-destruction.11 Such “voices” can be intense and self-deprecating; many patients with this type of hallucination report having made a suicide attempt “just to make the voices stop.”
Symptoms of paranoia can make it less likely for individuals with psychosis to confide in family members, friends, or medical personnel. Religious elements are often of a delusional nature and can be dangerous. Psychosis is more difficult to hide than depression and the presence of psychoses concurrent with major depressive disorder (MDD) increases the probability of suicidality.11 Psychosis secondary to substance use may diminish inhibitions and heighten impulsivity, thereby exacerbating the likelihood of self-harm. Usually, the presence of psychotic features precipitating or following a suicide attempt leads to psychiatric hospitalization.
12. Is the patient in a high-risk demographic group?
When evaluating a patient who has attempted suicide, it helps to consider not just what they did, but who they are. Specifically, does the individual belong to a demographic group that traditionally has a high rate of suicide? For example, patients who are Native American or Alaska Natives warrant extra caution.2 Older White males, especially those who are divorced, widowed, retired, and/or have chronic health problems, are also at greater risk. Compared to the general population, individuals age >80 have a massively elevated chance for self-induced death.12 Some of the reasons include:
- medical comorbidities make surviving an attempt less likely
- access to large amounts of medications
- more irreversible issues, such as chronic pain, disability, or widowhood
- living alone, which may delay discovery.
Patients who are members of any of these demographic groups may deserve serious consideration for inpatient psychiatric admission, regardless of other factors.
Continue to: Were drugs or alcohol involved?
13. Were drugs or alcohol involved?
This factor is unique in that it is both a chronic risk factor (SUDs) and a warning sign for imminent suicide, as in the case of an individual who gets intoxicated to disinhibit their fear of death so they can attempt suicide. Alcohol use disorders are associated with depression and suicide. Overdoses by fentanyl and other opiates have become more frequent.13 In many cases, fatalities are unintentional because users overestimate their tolerance or ingest contaminated substances.14 Disinhibition by alcohol and/or other drugs is a risk factor for attempting suicide and can intensify the depth of MDD. Some patients will ingest substances before an attempt just to give them the courage to act; many think of suicide only when intoxicated. Toxicology screens are indicated as part of the evaluation after a suicide attempt.
Depressive and suicidal thoughts often occur in people “coming down” from cocaine or other stimulants. These circumstances require determining whether to refer the patient for treatment for an SUD or psychiatric hospitalization.
In summary, getting intoxicated solely to diminish anxiety about suicide is a dangerous feature, whereas attempting suicide due to intoxication is less concerning. The latter patient may not consider suicide unless they become intoxicated again. When available, dual diagnosis treatment facilities can be an appropriate referral for such patients. Emergency department holding beds can allow these individuals to detoxify prior to the evaluation.
14. Does the patient have future-oriented thoughts?
When evaluating a patient who has attempted suicide, the presence of future planning and anticipation can be reassuring, but these features should be carefully assessed.14-16
After-the-fact comments may be more reliable when a patient offers them spontaneously, as opposed to in response to direct questioning.
- the specificity of the future plans
- corroboration from the family and others about the patient’s previous investment in the upcoming event
- whether the patient mentions such plans spontaneously or only in response to direct questioning
- the patient’s emotional expression or affect when discussing their future
- whether such plans are reasonable, grandiose, and/or unrealistic.
Bottom Line
When assessing a patient after a suicide attempt, both the patient’s presentation and history and the clinician’s instincts are important. Careful consideration of the method, stated intent, premeditation vs impulsivity, feelings about having survived, presence of psychiatric illness, high-risk demographic, postattempt demeanor and affect, quality of support, presence of self-rescue behaviors, future-oriented thoughts, and other factors can help in making the appropriate disposition.
Related Resources
- Kim H, Kim Y, Shin MH, et al. Early psychiatric referral after attempted suicide helps prevent suicide reattempts: a longitudinal national cohort study in South Korea. Front Psychiatry. 2022;13:607892. doi:10.3389/fpsyt.2022.607892
- Michaud L, Berva S, Ostertag L, et al. When to discharge and when to voluntary or compulsory hospitalize? Factors associated with treatment decision after self-harm. Psychiatry Res. 2022;317:114810. doi:10.1016/j.psychres.2022.114810
1. Ten Leading Causes of Death, United States 2020. Centers for Disease Control and Prevention WISQARS. Accessed March 4, 2022. https://wisqars.cdc.gov/data/lcd/home
2. Norris D, Clark MS. Evaluation and treatment of suicidal patients. Am Fam Physician. 2012;15;85(6):602-605.
3. Gliatto MF, Rai AK. Evaluation and treatment patients with suicidal ideation. Am Fam Phys. 1999;59(6):1500-1506.
4. Dazzi T, Gribble R, Wessely S, et al. Does asking about suicide and related behaviors induce suicidal ideation? What is the evidence? Psychol Med. 2014;44(16):3361-3363.
5. Lewiecki EM, Miller SA. Suicide, guns and public policy. Am J Public Health. 2013;103(1):27-31.
6. Frierson RL. Women who shoot themselves. Hosp Community Psychiatry. 1989;40(8):841-843.
7. Frierson RL, Lippmann SB. Psychiatric consultation for patients with self-inflicted gunshot wounds. Psychosomatics. 1990;31(1):67-74.
8. Mitchell AM, Terhorst L. PTSD symptoms in survivors bereaved by the suicide of a significant other. J Am Psychiatr Nurses Assoc. 2017;23(1):61-65.
9. Bateson J. The Golden Gate Bridge’s fatal flaw. Los Angeles Times. May 25, 2012. Accessed March 2, 2022. https://www.latimes.com/opinion/la-xpm-2012-may-25-la-oe-adv-bateson-golden-gate-20120525-story.html
10. Dervic K, Oquendoma MA, Grunebaum MF, et al. Religious affiliation and suicide attempt. Am J Psychiatry. 2004;161(12):2303-2308.
11. Nordentoft H, Madsen T, Fedyszyn IF. Suicidal behavior and mortality in first episode psychosis. J Nerv Ment Dis. 2015;203(5):387-392.
12. Frierson R, Lippmann S. Suicide attempts by the old and the very old. Arch Intern Med. 1991;151(1):141-144.
13. Braden JB, Edlund MJ, Sullivan MD. Suicide deaths with opiate poisonings in the United States: 1999-2014. Am J Public Health. 2017;107(3):421-426.
14. Morin KA, Acharya S, Eibl JK, et al: Evidence of increased fentanyl use during the COVID-19 pandemic among opioid agonist treated patients in Ontario, Canada. Int J Drug Policy. 2021;90:103088.
15. Shobassy A, Abu-Mohammad AS. Assessing imminent suicide risk: what about future planning? Current Psychiatry. 2022;21(2):12-17.
16. MacLeod AK, Pankhania B, Lee M, et al. Parasuicide, depression and the anticipation of positive and negative future experiences. Psychol Med. 1997;27(4):973-977.
17. Macleod AK, Tata P, Tyrer P, et al. Hopelessness and positive and negative future thinking in parasuicide. Br J Clin Psychol. 2010;44(Pt 4):495-504.
In 2021, suicide was the 11th leading cause of death in the United States.1 Suicide resulted in 49,000 US deaths during 2021; it was the second most common cause of death in individuals age 10 to 34, and the fifth leading cause among children.1,2 Women are 3 to 4 times more likely than men to attempt suicide, but men are 4 times more likely to die by suicide.2
The evaluation of patients with suicidal ideation who have not made an attempt generally involves assessing 4 factors: the specific plan, access to lethal means, any recent social stressors, and the presence of a psychiatric disorder.3 The clinician should also assess which potential deterrents, such as religious beliefs or dependent children, might be present.
Mental health clinicians are often called upon to evaluate a patient after a suicide attempt to assess intent for continued self-harm and to determine appropriate disposition. Such an evaluation must consider multiple factors, including the method used, premeditation, consequences of the attempt, the presence of severe depression and/or psychosis, and the role of substance use. Assessment after a suicide attempt differs from the examination of individuals who harbor suicidal thoughts but have not made an attempt; the latter group may be more likely to respond to interventions such as intensive outpatient care, mobilization of family support, and religious proscriptions against suicide. However, for patients who make an attempt to end their life, whatever potential safeguards or deterrents to suicide that were in place obviously did not prevent the self-harm act. The consequences of the attempt, such as disabling injuries or medical complications, and possible involuntary commitment, need to be considered. Assessment of the patient’s feelings about having survived the attempt is important because the psychological impact of the attempt on family members may serve to intensify the patient’s depression and make a subsequent attempt more likely.
Many individuals who think of suicide have communicated self-harm thoughts or intentions, but such comments are often minimized or ignored. There is a common but erroneous belief that if patients are encouraged to discuss thoughts of self-harm, they will be more likely to act upon them. Because the opposite is true,4 clinicians should ask vulnerable patients about suicidal ideation or intent. Importantly, noncompliance with life-saving medical care, risk-taking behaviors, and substance use may also signal a desire for self-harm. Passive thoughts of death, typified by comments such as “I don’t care whether I wake up or not,” should also be elicited. Many patients who think of suicide speak of being in a “bad place” where reason and logic give way to an intense desire to end their misery.

The evaluation of a patient who has attempted suicide is an important component of providing psychiatric care. This article reflects our 45 years of evaluating such patients. As such, it reflects our clinical experience and is not evidence-based. We offer a checklist of 14 questions that we have found helpful when determining if it would be best for a patient to receive inpatient psychiatric hospitalization or a discharge referral for outpatient care (Table). Questions 1 through 6 are specific for patients who have made a suicide attempt, while questions 7 through 14 are helpful for assessing global risk factors for suicide.
1. Was the attempt premeditated?
Determining premeditation vs impulsivity is an essential element of the assessment following a suicide attempt. Many such acts may occur without forethought in response to an unexpected stressor, such as an altercation between partners or family conflicts. Impulsive attempts can occur when an individual is involved in a distressing event and/or while intoxicated. Conversely, premeditation involves forethought and planning, which may increase the risk of suicide in the near future.
Examples of premeditated behavior include:
- Contemplating the attempt days or weeks beforehand
- Researching the effects of a medication or combination of medications in terms of potential lethality
- Engaging in behavior that would decrease the likelihood of their body being discovered after the attempt
- Obtaining weapons and/or stockpiling pills
- Canvassing potential sites such as bridges or tall buildings
- Engaging in a suicide attempt “practice run”
- Leaving a suicide note or message on social media
- Making funeral arrangements, such as choosing burial clothing
- Writing a will and arranging for the custody of dependent children
- Purchasing life insurance that does not deny payment of benefits in cases of death by suicide.
Continue to: Patients with a premeditated...
Patients with a premeditated suicide attempt generally do not expect to survive and are often surprised or upset that the act was not fatal. The presence of indicators that the attempt was premeditated should direct the disposition more toward hospitalization than discharge. In assessing the impact of premeditation, it is important to gauge not just the examples listed above, but also the patient’s perception of these issues (such as potential loss of child custody). Consider how much the patient is emotionally affected by such thinking.
2. What were the consequences of the attempt?
Assessing the reason for the attempt (if any) and determining whether the inciting circumstance has changed due to the suicide attempt are an important part of the evaluation. A suicide attempt may result in reconciliation with and/or renewed support from family members or partners, who might not have been aware of the patient’s emotional distress. Such unexpected support often results in the patient exhibiting improved mood and affect, and possibly temporary resolution of suicidal thoughts. This “flight into health” may be short-lived, but it also may be enough to engage the patient in a therapeutic alliance. That may permit a discharge with safe disposition to the outpatient clinic while in the custody of a family member, partner, or close friend.
Alternatively, some people experience a troubling worsening of precipitants following a suicide attempt. Preexisting medical conditions and financial, occupational, and/or social woes may be exacerbated. Child custody determinations may be affected, assuming the patient understands the possibility of this adverse consequence. Violent methods may result in disfigurement and body image issues. Individuals from small, close-knit communities may experience stigmatization and unwanted notoriety because of their suicide attempt. Such negative consequences may render some patients more likely to make another attempt to die by suicide. It is crucial to consider how a suicide attempt may have changed the original stress that led to the attempt.
3. Which method was used?
Most fatal suicides in the US are by firearms, and many individuals who survive such attempts do so because of unfamiliarity with the weapon, gun malfunction, faulty aim, or alcohol use.5-7 Some survivors report intending to shoot themselves in the heart, but instead suffered shoulder injuries. Unfortunately, for a patient who survives self-inflicted gunshot wounds, the sequelae of chronic pain, multiple surgical procedures, disability, and disfigurement may serve as constant negative reminders of the event. Some individuals with suicidal intent eschew the idea of using firearms because they hope to avoid having a family member be the first to discover them. Witnessing the aftermath of a fatal suicide by gunshot can induce symptoms of posttraumatic stress disorder in family members and/or partners.8
For a patient with self-inflicted gunshot wounds, always determine whether the weapon has been secured or if the patient still has access to it. Asking about weapon availability is essential during the evaluation of any patient with depression, major life crises, or other factors that may yield a desire to die; this is especially true for individuals with substance use disorders (SUDs). Whenever readily available to such individuals, weapons need to be safely removed.
Continue to: Other self-harm methods...
Other self-harm methods with a high degree of lethality include jumping from bridges or buildings, poisonings, self-immolation, cutting, and hangings. Individuals who choose these approaches generally do not intend to survive. Many of these methods also entail premeditation, as in the case of individuals who canvass bridges and note time when traffic is light so they are less likely to be interrupted. Between 1937 and 2012, there were >1,600 deaths by suicide from San Francisco’s Golden Gate Bridge.9 Patients who choose highly lethal methods are often irritated during the postattempt evaluation because their plans were not fatal. Usually, patients who choose such potentially lethal methods are hospitalized initially on medical and surgical floors, and receive most of their psychiatric care from consultation psychiatrists. Following discharge, these patients may be at high risk for subsequent suicide attempts.
In the US, the most common method of attempting suicide is by overdose.4 Lethality is determined by the agent or combination of substances ingested, the amount taken, the person’s health status, and the length of time before they are discovered. Many patients mistakenly assume that readily available agents such as acetaminophen and aspirin are less likely to be fatal than prescription medications. Evaluators may want to assess for suicidality in individuals with erratic, risk-taking behaviors, who are at especially high risk for death. Learning about the method the patient used can help the clinician determine the imminent risk of another suicide attempt. The more potentially fatal the patient’s method, the more serious their suicide intent, and the higher the risk they will make another suicide attempt, possibly using an even more lethal method.
4. What was the intent?
“What did you want to happen when you made this attempt?” Many patients will respond that they wanted to die, sleep, not wake up, or did not care what happened. Others say it was a gesture to evoke a certain response from another person. If this is the case, it is important to know whether the desired outcome was achieved. These so-called gestures often involve making sure the intended person is aware of the attempt, often by writing a letter, sending a text, or posting on social media. Such behaviors may be exhibited by patients with personality disorders. While such attempts often are impulsive, if the attempt fails to generate the anticipated effect, the patient may try to gain more attention by escalating their suicide actions.
Conversely, if a spouse or partner reconciles with the patient solely because of a suicide attempt, this may set a pattern for future self-harm events in which the patient hopes to achieve the same outcome. Nevertheless, it is better to err for safety because some of these patients will make another attempt, just to prove that they should have been taken more seriously. An exploration of such intent can help the evaluation because even supposed “gestures” can have dangerous consequences. Acts that do not result in the desired outcome should precipitate hospitalization rather than discharge.
5. What facilitated the patient’s rescue?
“Why is this patient still alive?” Determine if the patient did anything to save themself, such as calling an ambulance, inducing emesis, telling someone what they did, or coming to the hospital on their own. If yes, asking them what changed their mind may provide information about what exists in their lives to potentially prevent future attempts, or about wishes to stay alive. These issues can be used to guide outpatient therapy.
Continue to: How does the patient feel about having survived?
6. How does the patient feel about having survived?
When a patient is asked how they feel about having survived a suicide attempt, some will label their act “stupid” and profess embarrassment. Others exhibit future-oriented thought, which is a very good prognostic sign. More ominous is subsequent dysphoria or lamenting that “I could not even do this right.” Patients often express anger toward anyone who rescued them, especially those whose attempts were carefully planned or were discovered by accident. Some patients might also express ambivalence about having survived.
The patient’s response to this question may be shaped by their desire to avoid hospitalization, so beyond their verbal answers, be attentive to clinical cues that may suggest the patient is not being fully transparent. Anger or ambivalence about having survived, a lack of future-oriented thought, and a restricted affect despite verbalizing joy about still being alive are features that suggest psychiatric hospitalization may be warranted.
7. Has the patient made previous suicide attempts?
Compared to individuals with no previous suicide attempts, patients with a history of suicide attempts are 30 to 40 times more likely to die by suicide.2 Many patients who present after a suicide attempt have tried to kill themselves multiple times. Exploring the number of past attempts, how recent the attempts were, and what dispositions were made can be of benefit. Reviewing the potential lethality of past attempts (eg, was hospitalization required, was the patient placed in an intensive care unit, and/or was intubation needed) is recommended. If outpatient care was suggested or medication prescribed, was the patient adherent? Consider asking about passive suicidal behavior, such as not seeking care for medical issues, discontinuing life-saving medication, or engaging in reckless behavior. While such behaviors may not have been classified as a suicide attempt, it might indicate a feeling of indifference toward staying alive. A patient with a past attempt, especially if recent, merits consideration for inpatient care. Once again, referring previously nonadherent patients to outpatient treatment is less likely to be effective.
8. Does the patient have a support network?
Before discharging a patient who has made a suicide attempt, consider the quality of their support network. Gauging the response of the family and friends to the patient’s attempt can be beneficial. Indifference or resentment on the part of loved ones is a bad sign. Some patients have access to support networks they either did not know were available or chose not to utilize. In other instances, after realizing how depressed the patient has been, the family might provide a new safety net. Strong religious affiliations can also be valuable because devout spirituality can be a deterrent to suicide behaviors.10 For an individual whose attempt was motivated by loneliness or feeling unloved or underappreciated, a newly realized support network can be an additional protective deterrent.
9. Does the patient have a family history of suicide?
There may be a familial component to suicide. Knowing about any suicide history in the family contributes to future therapeutic planning. The clinician may want to explore the patient’s family suicide history in detail because such information can have substantial impact on the patient’s motivation for attempting suicide. The evaluator may want to determine if the anniversary of a family suicide is coming. Triggers for a suicide attempt could include the anniversary of a death, birthdays, family-oriented holidays, and similar events. It is productive to understand how the patient feels about family members who have died by suicide. Some will empathize with the deceased, commenting that they did the “right thing.” Others, upon realizing how their own attempt affected others, will be remorseful and determined not to inflict more pain on their family. Such patients may need to be reminded of the misery associated with their family being left without them. These understandings are helpful at setting a safe disposition. However, a history of death by suicide in the family should always be thoroughly evaluated, regardless of the patient’s attitude about that death.
Continue to: Was the attempt the result of depression?
10. Was the attempt the result of depression?
For a patient experiencing depressive symptoms, the prognosis is less positive; they are more likely to harbor serious intent, premeditation, hopelessness, and social isolation, and less likely to express future-oriented thought. They often exhibit a temporary “flight into health.” Such progress is often transitory and may not represent recovery. Because mood disorders may still be present despite a temporary improvement, inpatient and pharmacologic treatment may be needed. If a patient’s suicide attempt occurred as a result of severe depression, it is possible they will make another suicide attempt unless their depression is addressed in a safe and secure setting, such as inpatient hospitalization, or through close family observation while the patient is receiving intensive outpatient treatment.
11. Does the patient have a psychotic disorder?
Many patients with a psychotic illness die following their first attempt without ever having contact with a mental health professional.11 Features of psychosis might include malevolent auditory hallucinations that suggest self-destruction.11 Such “voices” can be intense and self-deprecating; many patients with this type of hallucination report having made a suicide attempt “just to make the voices stop.”
Symptoms of paranoia can make it less likely for individuals with psychosis to confide in family members, friends, or medical personnel. Religious elements are often of a delusional nature and can be dangerous. Psychosis is more difficult to hide than depression and the presence of psychoses concurrent with major depressive disorder (MDD) increases the probability of suicidality.11 Psychosis secondary to substance use may diminish inhibitions and heighten impulsivity, thereby exacerbating the likelihood of self-harm. Usually, the presence of psychotic features precipitating or following a suicide attempt leads to psychiatric hospitalization.
12. Is the patient in a high-risk demographic group?
When evaluating a patient who has attempted suicide, it helps to consider not just what they did, but who they are. Specifically, does the individual belong to a demographic group that traditionally has a high rate of suicide? For example, patients who are Native American or Alaska Natives warrant extra caution.2 Older White males, especially those who are divorced, widowed, retired, and/or have chronic health problems, are also at greater risk. Compared to the general population, individuals age >80 have a massively elevated chance for self-induced death.12 Some of the reasons include:
- medical comorbidities make surviving an attempt less likely
- access to large amounts of medications
- more irreversible issues, such as chronic pain, disability, or widowhood
- living alone, which may delay discovery.
Patients who are members of any of these demographic groups may deserve serious consideration for inpatient psychiatric admission, regardless of other factors.
Continue to: Were drugs or alcohol involved?
13. Were drugs or alcohol involved?
This factor is unique in that it is both a chronic risk factor (SUDs) and a warning sign for imminent suicide, as in the case of an individual who gets intoxicated to disinhibit their fear of death so they can attempt suicide. Alcohol use disorders are associated with depression and suicide. Overdoses by fentanyl and other opiates have become more frequent.13 In many cases, fatalities are unintentional because users overestimate their tolerance or ingest contaminated substances.14 Disinhibition by alcohol and/or other drugs is a risk factor for attempting suicide and can intensify the depth of MDD. Some patients will ingest substances before an attempt just to give them the courage to act; many think of suicide only when intoxicated. Toxicology screens are indicated as part of the evaluation after a suicide attempt.
Depressive and suicidal thoughts often occur in people “coming down” from cocaine or other stimulants. These circumstances require determining whether to refer the patient for treatment for an SUD or psychiatric hospitalization.
In summary, getting intoxicated solely to diminish anxiety about suicide is a dangerous feature, whereas attempting suicide due to intoxication is less concerning. The latter patient may not consider suicide unless they become intoxicated again. When available, dual diagnosis treatment facilities can be an appropriate referral for such patients. Emergency department holding beds can allow these individuals to detoxify prior to the evaluation.
14. Does the patient have future-oriented thoughts?
When evaluating a patient who has attempted suicide, the presence of future planning and anticipation can be reassuring, but these features should be carefully assessed.14-16
After-the-fact comments may be more reliable when a patient offers them spontaneously, as opposed to in response to direct questioning.
- the specificity of the future plans
- corroboration from the family and others about the patient’s previous investment in the upcoming event
- whether the patient mentions such plans spontaneously or only in response to direct questioning
- the patient’s emotional expression or affect when discussing their future
- whether such plans are reasonable, grandiose, and/or unrealistic.
Bottom Line
When assessing a patient after a suicide attempt, both the patient’s presentation and history and the clinician’s instincts are important. Careful consideration of the method, stated intent, premeditation vs impulsivity, feelings about having survived, presence of psychiatric illness, high-risk demographic, postattempt demeanor and affect, quality of support, presence of self-rescue behaviors, future-oriented thoughts, and other factors can help in making the appropriate disposition.
Related Resources
- Kim H, Kim Y, Shin MH, et al. Early psychiatric referral after attempted suicide helps prevent suicide reattempts: a longitudinal national cohort study in South Korea. Front Psychiatry. 2022;13:607892. doi:10.3389/fpsyt.2022.607892
- Michaud L, Berva S, Ostertag L, et al. When to discharge and when to voluntary or compulsory hospitalize? Factors associated with treatment decision after self-harm. Psychiatry Res. 2022;317:114810. doi:10.1016/j.psychres.2022.114810
In 2021, suicide was the 11th leading cause of death in the United States.1 Suicide resulted in 49,000 US deaths during 2021; it was the second most common cause of death in individuals age 10 to 34, and the fifth leading cause among children.1,2 Women are 3 to 4 times more likely than men to attempt suicide, but men are 4 times more likely to die by suicide.2
The evaluation of patients with suicidal ideation who have not made an attempt generally involves assessing 4 factors: the specific plan, access to lethal means, any recent social stressors, and the presence of a psychiatric disorder.3 The clinician should also assess which potential deterrents, such as religious beliefs or dependent children, might be present.
Mental health clinicians are often called upon to evaluate a patient after a suicide attempt to assess intent for continued self-harm and to determine appropriate disposition. Such an evaluation must consider multiple factors, including the method used, premeditation, consequences of the attempt, the presence of severe depression and/or psychosis, and the role of substance use. Assessment after a suicide attempt differs from the examination of individuals who harbor suicidal thoughts but have not made an attempt; the latter group may be more likely to respond to interventions such as intensive outpatient care, mobilization of family support, and religious proscriptions against suicide. However, for patients who make an attempt to end their life, whatever potential safeguards or deterrents to suicide that were in place obviously did not prevent the self-harm act. The consequences of the attempt, such as disabling injuries or medical complications, and possible involuntary commitment, need to be considered. Assessment of the patient’s feelings about having survived the attempt is important because the psychological impact of the attempt on family members may serve to intensify the patient’s depression and make a subsequent attempt more likely.
Many individuals who think of suicide have communicated self-harm thoughts or intentions, but such comments are often minimized or ignored. There is a common but erroneous belief that if patients are encouraged to discuss thoughts of self-harm, they will be more likely to act upon them. Because the opposite is true,4 clinicians should ask vulnerable patients about suicidal ideation or intent. Importantly, noncompliance with life-saving medical care, risk-taking behaviors, and substance use may also signal a desire for self-harm. Passive thoughts of death, typified by comments such as “I don’t care whether I wake up or not,” should also be elicited. Many patients who think of suicide speak of being in a “bad place” where reason and logic give way to an intense desire to end their misery.

The evaluation of a patient who has attempted suicide is an important component of providing psychiatric care. This article reflects our 45 years of evaluating such patients. As such, it reflects our clinical experience and is not evidence-based. We offer a checklist of 14 questions that we have found helpful when determining if it would be best for a patient to receive inpatient psychiatric hospitalization or a discharge referral for outpatient care (Table). Questions 1 through 6 are specific for patients who have made a suicide attempt, while questions 7 through 14 are helpful for assessing global risk factors for suicide.
1. Was the attempt premeditated?
Determining premeditation vs impulsivity is an essential element of the assessment following a suicide attempt. Many such acts may occur without forethought in response to an unexpected stressor, such as an altercation between partners or family conflicts. Impulsive attempts can occur when an individual is involved in a distressing event and/or while intoxicated. Conversely, premeditation involves forethought and planning, which may increase the risk of suicide in the near future.
Examples of premeditated behavior include:
- Contemplating the attempt days or weeks beforehand
- Researching the effects of a medication or combination of medications in terms of potential lethality
- Engaging in behavior that would decrease the likelihood of their body being discovered after the attempt
- Obtaining weapons and/or stockpiling pills
- Canvassing potential sites such as bridges or tall buildings
- Engaging in a suicide attempt “practice run”
- Leaving a suicide note or message on social media
- Making funeral arrangements, such as choosing burial clothing
- Writing a will and arranging for the custody of dependent children
- Purchasing life insurance that does not deny payment of benefits in cases of death by suicide.
Continue to: Patients with a premeditated...
Patients with a premeditated suicide attempt generally do not expect to survive and are often surprised or upset that the act was not fatal. The presence of indicators that the attempt was premeditated should direct the disposition more toward hospitalization than discharge. In assessing the impact of premeditation, it is important to gauge not just the examples listed above, but also the patient’s perception of these issues (such as potential loss of child custody). Consider how much the patient is emotionally affected by such thinking.
2. What were the consequences of the attempt?
Assessing the reason for the attempt (if any) and determining whether the inciting circumstance has changed due to the suicide attempt are an important part of the evaluation. A suicide attempt may result in reconciliation with and/or renewed support from family members or partners, who might not have been aware of the patient’s emotional distress. Such unexpected support often results in the patient exhibiting improved mood and affect, and possibly temporary resolution of suicidal thoughts. This “flight into health” may be short-lived, but it also may be enough to engage the patient in a therapeutic alliance. That may permit a discharge with safe disposition to the outpatient clinic while in the custody of a family member, partner, or close friend.
Alternatively, some people experience a troubling worsening of precipitants following a suicide attempt. Preexisting medical conditions and financial, occupational, and/or social woes may be exacerbated. Child custody determinations may be affected, assuming the patient understands the possibility of this adverse consequence. Violent methods may result in disfigurement and body image issues. Individuals from small, close-knit communities may experience stigmatization and unwanted notoriety because of their suicide attempt. Such negative consequences may render some patients more likely to make another attempt to die by suicide. It is crucial to consider how a suicide attempt may have changed the original stress that led to the attempt.
3. Which method was used?
Most fatal suicides in the US are by firearms, and many individuals who survive such attempts do so because of unfamiliarity with the weapon, gun malfunction, faulty aim, or alcohol use.5-7 Some survivors report intending to shoot themselves in the heart, but instead suffered shoulder injuries. Unfortunately, for a patient who survives self-inflicted gunshot wounds, the sequelae of chronic pain, multiple surgical procedures, disability, and disfigurement may serve as constant negative reminders of the event. Some individuals with suicidal intent eschew the idea of using firearms because they hope to avoid having a family member be the first to discover them. Witnessing the aftermath of a fatal suicide by gunshot can induce symptoms of posttraumatic stress disorder in family members and/or partners.8
For a patient with self-inflicted gunshot wounds, always determine whether the weapon has been secured or if the patient still has access to it. Asking about weapon availability is essential during the evaluation of any patient with depression, major life crises, or other factors that may yield a desire to die; this is especially true for individuals with substance use disorders (SUDs). Whenever readily available to such individuals, weapons need to be safely removed.
Continue to: Other self-harm methods...
Other self-harm methods with a high degree of lethality include jumping from bridges or buildings, poisonings, self-immolation, cutting, and hangings. Individuals who choose these approaches generally do not intend to survive. Many of these methods also entail premeditation, as in the case of individuals who canvass bridges and note time when traffic is light so they are less likely to be interrupted. Between 1937 and 2012, there were >1,600 deaths by suicide from San Francisco’s Golden Gate Bridge.9 Patients who choose highly lethal methods are often irritated during the postattempt evaluation because their plans were not fatal. Usually, patients who choose such potentially lethal methods are hospitalized initially on medical and surgical floors, and receive most of their psychiatric care from consultation psychiatrists. Following discharge, these patients may be at high risk for subsequent suicide attempts.
In the US, the most common method of attempting suicide is by overdose.4 Lethality is determined by the agent or combination of substances ingested, the amount taken, the person’s health status, and the length of time before they are discovered. Many patients mistakenly assume that readily available agents such as acetaminophen and aspirin are less likely to be fatal than prescription medications. Evaluators may want to assess for suicidality in individuals with erratic, risk-taking behaviors, who are at especially high risk for death. Learning about the method the patient used can help the clinician determine the imminent risk of another suicide attempt. The more potentially fatal the patient’s method, the more serious their suicide intent, and the higher the risk they will make another suicide attempt, possibly using an even more lethal method.
4. What was the intent?
“What did you want to happen when you made this attempt?” Many patients will respond that they wanted to die, sleep, not wake up, or did not care what happened. Others say it was a gesture to evoke a certain response from another person. If this is the case, it is important to know whether the desired outcome was achieved. These so-called gestures often involve making sure the intended person is aware of the attempt, often by writing a letter, sending a text, or posting on social media. Such behaviors may be exhibited by patients with personality disorders. While such attempts often are impulsive, if the attempt fails to generate the anticipated effect, the patient may try to gain more attention by escalating their suicide actions.
Conversely, if a spouse or partner reconciles with the patient solely because of a suicide attempt, this may set a pattern for future self-harm events in which the patient hopes to achieve the same outcome. Nevertheless, it is better to err for safety because some of these patients will make another attempt, just to prove that they should have been taken more seriously. An exploration of such intent can help the evaluation because even supposed “gestures” can have dangerous consequences. Acts that do not result in the desired outcome should precipitate hospitalization rather than discharge.
5. What facilitated the patient’s rescue?
“Why is this patient still alive?” Determine if the patient did anything to save themself, such as calling an ambulance, inducing emesis, telling someone what they did, or coming to the hospital on their own. If yes, asking them what changed their mind may provide information about what exists in their lives to potentially prevent future attempts, or about wishes to stay alive. These issues can be used to guide outpatient therapy.
Continue to: How does the patient feel about having survived?
6. How does the patient feel about having survived?
When a patient is asked how they feel about having survived a suicide attempt, some will label their act “stupid” and profess embarrassment. Others exhibit future-oriented thought, which is a very good prognostic sign. More ominous is subsequent dysphoria or lamenting that “I could not even do this right.” Patients often express anger toward anyone who rescued them, especially those whose attempts were carefully planned or were discovered by accident. Some patients might also express ambivalence about having survived.
The patient’s response to this question may be shaped by their desire to avoid hospitalization, so beyond their verbal answers, be attentive to clinical cues that may suggest the patient is not being fully transparent. Anger or ambivalence about having survived, a lack of future-oriented thought, and a restricted affect despite verbalizing joy about still being alive are features that suggest psychiatric hospitalization may be warranted.
7. Has the patient made previous suicide attempts?
Compared to individuals with no previous suicide attempts, patients with a history of suicide attempts are 30 to 40 times more likely to die by suicide.2 Many patients who present after a suicide attempt have tried to kill themselves multiple times. Exploring the number of past attempts, how recent the attempts were, and what dispositions were made can be of benefit. Reviewing the potential lethality of past attempts (eg, was hospitalization required, was the patient placed in an intensive care unit, and/or was intubation needed) is recommended. If outpatient care was suggested or medication prescribed, was the patient adherent? Consider asking about passive suicidal behavior, such as not seeking care for medical issues, discontinuing life-saving medication, or engaging in reckless behavior. While such behaviors may not have been classified as a suicide attempt, it might indicate a feeling of indifference toward staying alive. A patient with a past attempt, especially if recent, merits consideration for inpatient care. Once again, referring previously nonadherent patients to outpatient treatment is less likely to be effective.
8. Does the patient have a support network?
Before discharging a patient who has made a suicide attempt, consider the quality of their support network. Gauging the response of the family and friends to the patient’s attempt can be beneficial. Indifference or resentment on the part of loved ones is a bad sign. Some patients have access to support networks they either did not know were available or chose not to utilize. In other instances, after realizing how depressed the patient has been, the family might provide a new safety net. Strong religious affiliations can also be valuable because devout spirituality can be a deterrent to suicide behaviors.10 For an individual whose attempt was motivated by loneliness or feeling unloved or underappreciated, a newly realized support network can be an additional protective deterrent.
9. Does the patient have a family history of suicide?
There may be a familial component to suicide. Knowing about any suicide history in the family contributes to future therapeutic planning. The clinician may want to explore the patient’s family suicide history in detail because such information can have substantial impact on the patient’s motivation for attempting suicide. The evaluator may want to determine if the anniversary of a family suicide is coming. Triggers for a suicide attempt could include the anniversary of a death, birthdays, family-oriented holidays, and similar events. It is productive to understand how the patient feels about family members who have died by suicide. Some will empathize with the deceased, commenting that they did the “right thing.” Others, upon realizing how their own attempt affected others, will be remorseful and determined not to inflict more pain on their family. Such patients may need to be reminded of the misery associated with their family being left without them. These understandings are helpful at setting a safe disposition. However, a history of death by suicide in the family should always be thoroughly evaluated, regardless of the patient’s attitude about that death.
Continue to: Was the attempt the result of depression?
10. Was the attempt the result of depression?
For a patient experiencing depressive symptoms, the prognosis is less positive; they are more likely to harbor serious intent, premeditation, hopelessness, and social isolation, and less likely to express future-oriented thought. They often exhibit a temporary “flight into health.” Such progress is often transitory and may not represent recovery. Because mood disorders may still be present despite a temporary improvement, inpatient and pharmacologic treatment may be needed. If a patient’s suicide attempt occurred as a result of severe depression, it is possible they will make another suicide attempt unless their depression is addressed in a safe and secure setting, such as inpatient hospitalization, or through close family observation while the patient is receiving intensive outpatient treatment.
11. Does the patient have a psychotic disorder?
Many patients with a psychotic illness die following their first attempt without ever having contact with a mental health professional.11 Features of psychosis might include malevolent auditory hallucinations that suggest self-destruction.11 Such “voices” can be intense and self-deprecating; many patients with this type of hallucination report having made a suicide attempt “just to make the voices stop.”
Symptoms of paranoia can make it less likely for individuals with psychosis to confide in family members, friends, or medical personnel. Religious elements are often of a delusional nature and can be dangerous. Psychosis is more difficult to hide than depression and the presence of psychoses concurrent with major depressive disorder (MDD) increases the probability of suicidality.11 Psychosis secondary to substance use may diminish inhibitions and heighten impulsivity, thereby exacerbating the likelihood of self-harm. Usually, the presence of psychotic features precipitating or following a suicide attempt leads to psychiatric hospitalization.
12. Is the patient in a high-risk demographic group?
When evaluating a patient who has attempted suicide, it helps to consider not just what they did, but who they are. Specifically, does the individual belong to a demographic group that traditionally has a high rate of suicide? For example, patients who are Native American or Alaska Natives warrant extra caution.2 Older White males, especially those who are divorced, widowed, retired, and/or have chronic health problems, are also at greater risk. Compared to the general population, individuals age >80 have a massively elevated chance for self-induced death.12 Some of the reasons include:
- medical comorbidities make surviving an attempt less likely
- access to large amounts of medications
- more irreversible issues, such as chronic pain, disability, or widowhood
- living alone, which may delay discovery.
Patients who are members of any of these demographic groups may deserve serious consideration for inpatient psychiatric admission, regardless of other factors.
Continue to: Were drugs or alcohol involved?
13. Were drugs or alcohol involved?
This factor is unique in that it is both a chronic risk factor (SUDs) and a warning sign for imminent suicide, as in the case of an individual who gets intoxicated to disinhibit their fear of death so they can attempt suicide. Alcohol use disorders are associated with depression and suicide. Overdoses by fentanyl and other opiates have become more frequent.13 In many cases, fatalities are unintentional because users overestimate their tolerance or ingest contaminated substances.14 Disinhibition by alcohol and/or other drugs is a risk factor for attempting suicide and can intensify the depth of MDD. Some patients will ingest substances before an attempt just to give them the courage to act; many think of suicide only when intoxicated. Toxicology screens are indicated as part of the evaluation after a suicide attempt.
Depressive and suicidal thoughts often occur in people “coming down” from cocaine or other stimulants. These circumstances require determining whether to refer the patient for treatment for an SUD or psychiatric hospitalization.
In summary, getting intoxicated solely to diminish anxiety about suicide is a dangerous feature, whereas attempting suicide due to intoxication is less concerning. The latter patient may not consider suicide unless they become intoxicated again. When available, dual diagnosis treatment facilities can be an appropriate referral for such patients. Emergency department holding beds can allow these individuals to detoxify prior to the evaluation.
14. Does the patient have future-oriented thoughts?
When evaluating a patient who has attempted suicide, the presence of future planning and anticipation can be reassuring, but these features should be carefully assessed.14-16
After-the-fact comments may be more reliable when a patient offers them spontaneously, as opposed to in response to direct questioning.
- the specificity of the future plans
- corroboration from the family and others about the patient’s previous investment in the upcoming event
- whether the patient mentions such plans spontaneously or only in response to direct questioning
- the patient’s emotional expression or affect when discussing their future
- whether such plans are reasonable, grandiose, and/or unrealistic.
Bottom Line
When assessing a patient after a suicide attempt, both the patient’s presentation and history and the clinician’s instincts are important. Careful consideration of the method, stated intent, premeditation vs impulsivity, feelings about having survived, presence of psychiatric illness, high-risk demographic, postattempt demeanor and affect, quality of support, presence of self-rescue behaviors, future-oriented thoughts, and other factors can help in making the appropriate disposition.
Related Resources
- Kim H, Kim Y, Shin MH, et al. Early psychiatric referral after attempted suicide helps prevent suicide reattempts: a longitudinal national cohort study in South Korea. Front Psychiatry. 2022;13:607892. doi:10.3389/fpsyt.2022.607892
- Michaud L, Berva S, Ostertag L, et al. When to discharge and when to voluntary or compulsory hospitalize? Factors associated with treatment decision after self-harm. Psychiatry Res. 2022;317:114810. doi:10.1016/j.psychres.2022.114810
1. Ten Leading Causes of Death, United States 2020. Centers for Disease Control and Prevention WISQARS. Accessed March 4, 2022. https://wisqars.cdc.gov/data/lcd/home
2. Norris D, Clark MS. Evaluation and treatment of suicidal patients. Am Fam Physician. 2012;15;85(6):602-605.
3. Gliatto MF, Rai AK. Evaluation and treatment patients with suicidal ideation. Am Fam Phys. 1999;59(6):1500-1506.
4. Dazzi T, Gribble R, Wessely S, et al. Does asking about suicide and related behaviors induce suicidal ideation? What is the evidence? Psychol Med. 2014;44(16):3361-3363.
5. Lewiecki EM, Miller SA. Suicide, guns and public policy. Am J Public Health. 2013;103(1):27-31.
6. Frierson RL. Women who shoot themselves. Hosp Community Psychiatry. 1989;40(8):841-843.
7. Frierson RL, Lippmann SB. Psychiatric consultation for patients with self-inflicted gunshot wounds. Psychosomatics. 1990;31(1):67-74.
8. Mitchell AM, Terhorst L. PTSD symptoms in survivors bereaved by the suicide of a significant other. J Am Psychiatr Nurses Assoc. 2017;23(1):61-65.
9. Bateson J. The Golden Gate Bridge’s fatal flaw. Los Angeles Times. May 25, 2012. Accessed March 2, 2022. https://www.latimes.com/opinion/la-xpm-2012-may-25-la-oe-adv-bateson-golden-gate-20120525-story.html
10. Dervic K, Oquendoma MA, Grunebaum MF, et al. Religious affiliation and suicide attempt. Am J Psychiatry. 2004;161(12):2303-2308.
11. Nordentoft H, Madsen T, Fedyszyn IF. Suicidal behavior and mortality in first episode psychosis. J Nerv Ment Dis. 2015;203(5):387-392.
12. Frierson R, Lippmann S. Suicide attempts by the old and the very old. Arch Intern Med. 1991;151(1):141-144.
13. Braden JB, Edlund MJ, Sullivan MD. Suicide deaths with opiate poisonings in the United States: 1999-2014. Am J Public Health. 2017;107(3):421-426.
14. Morin KA, Acharya S, Eibl JK, et al: Evidence of increased fentanyl use during the COVID-19 pandemic among opioid agonist treated patients in Ontario, Canada. Int J Drug Policy. 2021;90:103088.
15. Shobassy A, Abu-Mohammad AS. Assessing imminent suicide risk: what about future planning? Current Psychiatry. 2022;21(2):12-17.
16. MacLeod AK, Pankhania B, Lee M, et al. Parasuicide, depression and the anticipation of positive and negative future experiences. Psychol Med. 1997;27(4):973-977.
17. Macleod AK, Tata P, Tyrer P, et al. Hopelessness and positive and negative future thinking in parasuicide. Br J Clin Psychol. 2010;44(Pt 4):495-504.
1. Ten Leading Causes of Death, United States 2020. Centers for Disease Control and Prevention WISQARS. Accessed March 4, 2022. https://wisqars.cdc.gov/data/lcd/home
2. Norris D, Clark MS. Evaluation and treatment of suicidal patients. Am Fam Physician. 2012;15;85(6):602-605.
3. Gliatto MF, Rai AK. Evaluation and treatment patients with suicidal ideation. Am Fam Phys. 1999;59(6):1500-1506.
4. Dazzi T, Gribble R, Wessely S, et al. Does asking about suicide and related behaviors induce suicidal ideation? What is the evidence? Psychol Med. 2014;44(16):3361-3363.
5. Lewiecki EM, Miller SA. Suicide, guns and public policy. Am J Public Health. 2013;103(1):27-31.
6. Frierson RL. Women who shoot themselves. Hosp Community Psychiatry. 1989;40(8):841-843.
7. Frierson RL, Lippmann SB. Psychiatric consultation for patients with self-inflicted gunshot wounds. Psychosomatics. 1990;31(1):67-74.
8. Mitchell AM, Terhorst L. PTSD symptoms in survivors bereaved by the suicide of a significant other. J Am Psychiatr Nurses Assoc. 2017;23(1):61-65.
9. Bateson J. The Golden Gate Bridge’s fatal flaw. Los Angeles Times. May 25, 2012. Accessed March 2, 2022. https://www.latimes.com/opinion/la-xpm-2012-may-25-la-oe-adv-bateson-golden-gate-20120525-story.html
10. Dervic K, Oquendoma MA, Grunebaum MF, et al. Religious affiliation and suicide attempt. Am J Psychiatry. 2004;161(12):2303-2308.
11. Nordentoft H, Madsen T, Fedyszyn IF. Suicidal behavior and mortality in first episode psychosis. J Nerv Ment Dis. 2015;203(5):387-392.
12. Frierson R, Lippmann S. Suicide attempts by the old and the very old. Arch Intern Med. 1991;151(1):141-144.
13. Braden JB, Edlund MJ, Sullivan MD. Suicide deaths with opiate poisonings in the United States: 1999-2014. Am J Public Health. 2017;107(3):421-426.
14. Morin KA, Acharya S, Eibl JK, et al: Evidence of increased fentanyl use during the COVID-19 pandemic among opioid agonist treated patients in Ontario, Canada. Int J Drug Policy. 2021;90:103088.
15. Shobassy A, Abu-Mohammad AS. Assessing imminent suicide risk: what about future planning? Current Psychiatry. 2022;21(2):12-17.
16. MacLeod AK, Pankhania B, Lee M, et al. Parasuicide, depression and the anticipation of positive and negative future experiences. Psychol Med. 1997;27(4):973-977.
17. Macleod AK, Tata P, Tyrer P, et al. Hopelessness and positive and negative future thinking in parasuicide. Br J Clin Psychol. 2010;44(Pt 4):495-504.
Gut microbiota and symptoms of psychosis: Is there a link?
The human microbiota refers to the collection of bacteria, archaea, eukarya, and viruses that reside within the human body. The term gut microbiome indicates the composition of these microbes and genetic codes in the intestine.1 Harkening back to the ancient Greek physician Galen, who treated gastrointestinal (GI) symptoms to relieve mental disturbances such as psychosis, the gut has been a therapeutic target in schizophrenia long before antipsychotics and the DSM.2 In recent years, research into the gut microbiome has drastically increased, with genetic sequencing affording a more precise look into the specific bacteria that call the human intestines their home. This has led to the recognition that the gut microbiome may be severely disrupted in schizophrenia, a condition known as dysbiosis. Preliminary research suggests that gut bacteria are more helpful than many human genes in distinguishing individuals with schizophrenia from their healthy counterparts.3,4 In this article, we discuss the potential role of the gut microbiome in schizophrenia, including new research correlating clinical symptoms of psychosis with dysbiosis. We also provide recommendations for promoting a healthy gut microbiome.
The enteric brain across life
The composition of our bodies is far more microbiota than human. Strikingly, microbiota cells in the gut outnumber human cells, and the distal gut alone hosts bacteria with 100 times the genetic content of the entire genome.5 The intricate meshwork of nerves in the gut is often called the enteric brain because the gut consists of 100 million neurons and synthesizes many neuroactive chemicals implicated in mood disorders and psychosis, including serotonin, dopamine, gamma-aminobutyric acid (GABA), and acetylcholine.6 The variety of neuroimmunologic, hormonal, and metabolic paths by which the gut microbiome and the brain interact are collectively known as the gut-microbiota-brain axis.7
How do we acquire our gut microbiome, and how does it come to influence our brain and behavior? On the first day of life, as babies pass through the birth canal, they are bathed in their mother’s vaginal microbiota. In the following weeks, the microbiome expands and colonizes the gut as bacteria are introduced from environmental sources such as skin-to-skin contact and breastmilk.8 The microbiome continues to evolve throughout early life. As children expand their diets and navigate new aspects of the physical world, additional bacteria join the unseen ecosystem growing inside.9 The development of the microbiome coincides with the development of the brain. From preclinical studies, we know the gut microbiome mediates important aspects of neurodevelopment such as the formation of the blood-brain barrier (BBB), synaptic pruning, glial activation, and myelination.10 Interestingly, many of the risk factors for schizophrenia are associated with gut dysbiosis, including obstetric complications, infections treated with antibiotics, and urbanization.11-15
Throughout human life, the gut and brain remain in close communication. The gut microbiota continue to produce monoamines, along with other metabolites that are able to cross the BBB.6 The HPA axis, stimulation of the immune system, and the vagus nerve all provide highways of communication between the gut and the brain.7 The relationship between the enteric brain and cephalic brain continues through life, even up to a person’s final hour. One autopsy study that is often cited (but soberingly, cannot be found online) allegedly revealed that 92% of schizophrenia patients had developed colitis by the time of death.16,17
First-episode psychosis and antipsychotic treatment
For patients with schizophrenia, first-episode psychosis (FEP) represents a cocktail of mounting genetic and environmental factors. Typically, by the time a patient receives psychiatric care, they present with characteristic psychotic symptoms—hallucinations, delusions, bizarre behavior, and unusual thought process—along with a unique gut microbiome profile.
This disrupted microbiome coincides with a marked state of inflammation in the intestines. Inflammation triggers increased endothelial barrier permeability, similar to the way immune signals increase capillary permeability to allow immune cells into the periphery of the blood. Specific gut bacteria play specific roles in maintaining the gut barrier.18,19 Disruptions in the bacteria that maintain the gut barrier, combined with inflammation, contribute to a leaky gut. A leaky gut barrier allows bacterial and immune products to more easily enter the bloodstream and then the brain, which is a potential source of neuroinflammation in schizophrenia.20 This increase in gut permeability (leaky gut syndrome) is likely one of several reasons low-grade inflammation is common in schizophrenia—numerous studies show higher serum levels of proinflammatory cytokines along with antibacterial immunoglobulins in patients with FEP.21,22
Fortunately, antipsychotics, especially the second-generation agents, help restore a healthy gut microbiome and have substantial anti-inflammatory properties.23,24 These medications interact heavily with the gut microbiome: they have been found to have antibiotic properties, even in doses lower than would normally reach the gut microbiome.25 In humans, a randomized controlled trial of probiotic supplementation for schizophrenia patients taking antipsychotics showed a reduction in GI symptoms but no significant improvement in psychotic symptoms.26
Dysbiosis in schizophrenia: cause or effect?
There is no consensus on what constitutes a healthy gut microbiome because the gut microbiome is highly variable, even among healthy individuals, and can change quickly. Those who adopt new diets, for example, see drastic shifts in the gut microbiome within a few days.27 Despite this variation, the main separation between a healthy and dysbiotic gut comes from the diversity of bacteria present in the gut—a healthy gut microbiome is associated with increased diversity. Numerous disease states have been associated with decreased bacterial diversity, including Clostridium difficile infection, Parkinson disease, depression, Crohn disease, and schizophrenia spectrum disorders.28,29
Although there are ethical limitations to studying causality in humans directly, animal models have provided a great deal of insight into the gut microbiome’s role in the development of schizophrenia. A recent study used fecal transplant to provide the gut microbiome from patients with schizophrenia to a group of germ-free mice and compared these animals to a group of mice that received a fecal transplant from individuals with a healthy gut microbiome. The mice receiving the schizophrenia microbiome showed an increased startle response and hyperactivity.3 This was consistent with mouse models of schizophrenia, although with obvious limitations.30 In addition, the brains of these animals showed changes in glutamate, glutamine, and GABA in the hippocampus; these chemicals play a role in the neurophysiology of schizophrenia.3,31 This study has not yet been replicated, and considerable variation remains within the schizophrenia biosignature.
Continue to: Clinical symptoms of psychosis and the gut microbiome
Clinical symptoms of psychosis and the gut microbiome
Previous literature has grouped patients with schizophrenia spectrum disorders as 1 unified study group. But as is the case with many psychiatric conditions, there is a great deal of heterogeneity in neurobiology, genetics, and microbiome composition among individuals with schizophrenia.32
Researchers have begun to investigate ways in which the gut microbiome varies regarding the clinical symptoms of psychosis.33 The Table3,34-39 provides an overview of 7 human studies of gut microbiome changes relating to clinical features of schizophrenia. In these studies, researchers have found correlations between the gut microbiome and a tendency toward violence,37 cognitive deficits,34-36,39 depressive symptoms,35,39 and numerous other clinical features of psychosis. Most of these correlations have not yet been replicated by further studies. But among studies with similar clinical questions, 3 reported changes in gut microbiome correlated with overall symptom severity, and 4 studies correlated changes with negative symptom severity. In 2 studies,3,34 Lachnospiraceae was correlated with worsened symptom severity. However, this may have been the result of poor control for antipsychotic use, as 1 study in bipolar patients found that Lachnospiraceae was increased in those taking antipsychotics compared to those who were not treated with antipsychotics.40 The specific shifts in bacteria seen for overall symptom and negative symptom severity were not consistent across studies. This is not surprising because the gut microbiome varies with diet and geographic region,41 and patients in these studies were from a variety of regions. Multiple studies demonstrated gut microbiome alterations for patients with more severe negative symptoms. This is particularly interesting because negative symptoms are often difficult to treat and do not respond to antipsychotics.42 This research suggests the gut microbiome may be helpful in developing future treatments for patients with negative symptoms that do not respond to existing treatments.
Research of probiotic supplementation for ameliorating symptoms of schizophrenia has yielded mixed results.43 It is possible that studies of probiotic supplementation have failed to consider the variations in the gut microbiome among individuals with schizophrenia. A better understanding of the variations in gut microbiome may allow for the development of more personalized interventions.
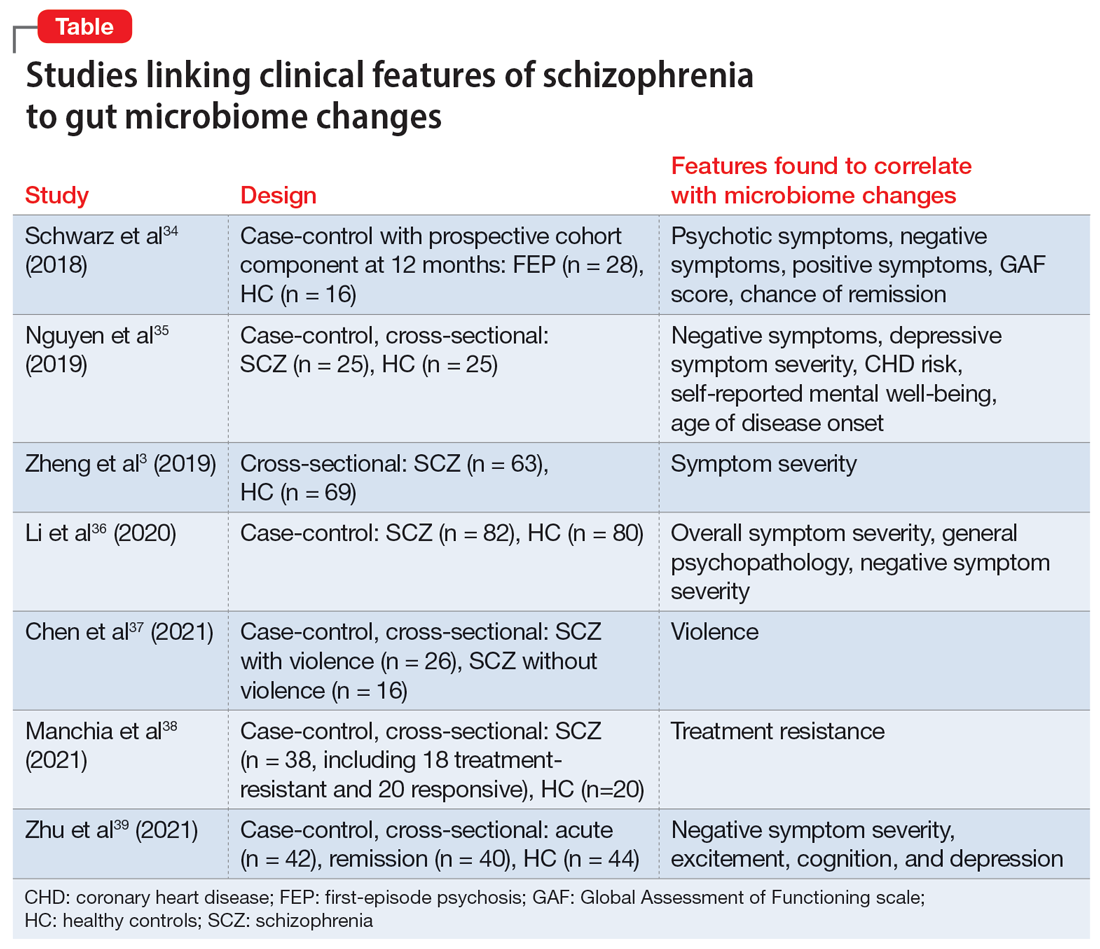
Recommendations for a healthy gut microbiome
In addition to antipsychotics, many other evidence-based interventions can be used to help restore a healthy gut microbiome in patients with schizophrenia. To improve the gut microbiome, we suggest discussing the following changes with patients:
- Quitting smoking. Smoking is common among patients with schizophrenia but decreases gut microbiome diversity.44
- Avoiding excessive alcohol use. Excessive alcohol use contributes to dysbiosis and increased intestinal permeability.45 Moderate alcohol consumption does not appear to have the same harmful effects on the microbiome.46
- Avoiding the use of recreational drugs, including marijuana, which impact the gut microbiome.47
- Consuming a diet rich in fiber.48 Presently, there is not enough evidence to recommend probiotic supplementation to reduce symptoms of schizophrenia.41 Similar to probiotics, fermented foods contain Lactobacillus, a bacterial species that produces lactic acid.49 Lactobacillus is enriched in the gut microbiome in some neurodegenerative diseases, and lactic acid can be neurotoxic at high levels.50-52 Therefore, clinicians should not explicitly recommend fermented foods under the assumption of improved brain health. A diet rich in soluble fiber has been consistently shown to promote anti-inflammatory bacteria and is much more likely to be beneficial.53,54 Soluble fiber is found in foods such as fruits, vegetables, beans, and oats.
- Exercising can increase microbiome diversity and provide anti-inflammatory effects in the gut.55,56 A recent review found that steady-state aerobic and high-intensity exercise interventions have positive effects on mood, cognition, and other negative symptoms in patients with schizophrenia.55
- Minimizing stress. Psychological stress and physiological stress from untreated medical conditions are toxic to healthy gut bacteria and weaken the gut barrier.57
- Mitigating exposure to pollution. Environmental pollution, including exposures to air pollution, heavy metals, and pesticides, disrupts the gut microbiome.58
The American Heart Association publishes lifestyle recommendations for individuals with heart disease and the National Institutes of Health publishes lifestyle recommendations for patients with chronic kidney disease. This leads us to question why the American Psychiatric Association has not published lifestyle recommendations for those with severe mental illness. The effects of lifestyle on both the gut microbiome and symptom mitigation is critical. With increasingly shortened appointments, standardized guidelines would benefit psychiatrists and patients alike.
Bottom Line
The gut microbiome is connected to the clinical symptoms of psychosis via a variety of hormonal, neuroimmune, and metabolic mechanisms active across the lifespan. Despite advances in research, there is still much to be understood regarding this relationship. Clinicians should discuss with patients ways to promote a healthy gut microbiome, including consuming a diet rich in fiber, avoiding use of recreational drugs, and exercising regularly.
Related Resources
- Nocera A, Nasrallah HA. The association of the gut microbiota with clinical features in schizophrenia. Behav Sci. 2022;12(4):89.
- Nasrallah HA. It takes guts to be mentally ill: microbiota and psychopathology. Current Psychiatry. 2018;17(9):4-6.
1. Bäckhed F, Ley RE, Sonnenburg JL, et al. Host-bacterial mutualism in the human intestine. Science. 2005;307(5717):1915-1920. doi:10.1126/science.1104816
2. Jackson SW. Galen—on mental disorders. J Hist Behav Sci. 1969;5(4):365-384. doi:10.1002/1520-6696(196910)5:4<365::AID-JHBS2300050408>3.0.CO;2-9
3. Zheng P, Zeng B, Liu M, et al. The gut microbiome from patients with schizophrenia modulates the glutamate-glutamine-GABA cycle and schizophrenia-relevant behaviors in mice. Sci Adv. 2019;5(2):eaau8317. doi:10.1126/sciadv.aau8317
4. Schizophrenia Working Group of the Psychiatric Genomics Consortium. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511(7510):421-427. doi:10.1038/nature13595
5. Gill SR, Pop M, DeBoy RT, et al. Metagenomic analysis of the human distal gut microbiome. Science. 2006;312(5778):1355-1359. doi:10.1126/science.1124234
6. Alam R, Abdolmaleky HM, Zhou JR. Microbiome, inflammation, epigenetic alterations, and mental diseases. Am J Med Genet B Neuropsychiatr Genet. 2017;174(6):651-660. doi:10.1002/ajmg.b.32567
7. Cryan JF, O’Riordan KJ, Cowan CSM, et al. The microbiota-gut-brain axis. Physiol Rev. 2019;99(4):1877-2013. doi:10.1152/physrev.00018.2018
8. Mueller NT, Bakacs E, Combellick J, et al. The infant microbiome development: mom matters. Trends Mol Med. 2015;21(2):109-117. doi:10.1016/j.molmed.2014.12.002
9. Fouhy F, Watkins C, Hill CJ, et al. Perinatal factors affect the gut microbiota up to four years after birth. Nat Commun. 2019;10(1):1517. doi:10.1038/s41467-019-09252-4
10. Sharon G, Sampson TR, Geschwind DH, et al. The central nervous system and the gut microbiome. Cell. 2016;167(4):915-932. doi:10.1016/j.cell.2016.10.027
11. Hill CJ, Lynch DB, Murphy K, et al. Evolution of gut microbiota composition from birth to 24 weeks in the INFANTMET Cohort. Microbiome. 2017;5:4. doi:10.1186/s40168-016-0213-y
12. Gareau MG, Wine E, Rodrigues DM, et al. Bacterial infection causes stress-induced memory dysfunction in mice. Gut. 2011;60(3):307-317. doi:10.1136/gut.2009.202515
13. Bokulich NA, Chung J, Battaglia T, et al. Antibiotics, birth mode, and diet shape microbiome maturation during early life. Sci Transl Med. 2016;8(343):343ra82. doi:10.1126/scitranslmed.aad7121
14. Mancabelli L, Milani C, Lugli GA, et al. Meta-analysis of the human gut microbiome from urbanized and pre-agricultural populations. Environ Microbiol. 2017;19(4):1379-1390. doi:10.1111/1462-2920.13692
15. Stilo SA, Murray RM. Non-genetic factors in schizophrenia. Curr Psychiatry Rep. 2019;21(10):100. doi:10.1007/s11920-019-1091-3
16. Buscaino VM. Patologia extraneurale della schizofrenia: fegato, tubo digerente, sistema reticolo-endoteliale. Acta Neurologica. 1953;VIII:1-60.
17. Hemmings G. Schizophrenia. Lancet. 2004;364(9442):1312-1313. doi:10.1016/S0140- 6736(04)17181-X
18. Hooper LV, Gordon JI. Commensal host-bacterial relationships in the gut. Science. 2001;292(5519):1115-1118. doi:10.1126/science.1058709
19. Ewaschuk JB, Diaz H, Meddings L, et al. Secreted bioactive factors from Bifidobacterium infantis enhance epithelial cell barrier function. Am J Physiol-Gastrointest Liver Physiol. 2008;295(5):G1025-G1034. doi:10.1152/ajpgi.90227.2008
20. Alhasson F, Das S, Seth R, et al. Altered gut microbiome in a mouse model of Gulf War Illness causes neuroinflammation and intestinal injury via leaky gut and TLR4 activation. PLoS One. 2017;12(3):e0172914. doi:10.1371/journal.pone.0172914
21. Fillman SG, Cloonan N, Catts VS, et al. Increased inflammatory markers identified in the dorsolateral prefrontal cortex of individuals with schizophrenia. Mol Psychiatry. 2013;18(2):206-214. doi:10.1038/mp.2012.110
22. Miller BJ, Buckley P, Seabolt W, et al. Meta-analysis of cytokine alterations in schizophrenia: clinical status and antipsychotic effects. Biol Psychiatry. 2011;70(7):663-671. doi:10.1016/j.biopsych.2011.04.013
23. Al-Amin M, Uddin MMN, Reza HM. Effects of antipsychotics on the inflammatory response system of patients with schizophrenia in peripheral blood mononuclear cell cultures. Clin Psychopharmacol Neurosci. 2013;11(3):144-151. doi:10.9758/cpn.2013.11.3.144
24. Yuan X, Zhang P, Wang Y, et al. Changes in metabolism and microbiota after 24-week risperidone treatment in drug naïve, normal weight patients with first episode schizophrenia. Schizophr Res. 2018;201:299-306. doi:10.1016/j.schres.2018.05.017
25. Maier L, Pruteanu M, Kuhn M, et al. Extensive impact of non-antibiotic drugs on human gut bacteria. Nature. 2018;555(7698):623-628. doi:10.1038/nature25979
26. Dickerson FB, Stallings C, Origoni A, et al. Effect of probiotic supplementation on schizophrenia symptoms and association with gastrointestinal functioning: a randomized, placebo-controlled trial. Prim Care Companion CNS Disord. 2014;15(1):PCC.13m01579. doi:10.4088/PCC.13m01579
27. David LA, Maurice CF, Carmody RN, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505(7484):559-563. doi:10.1038/nature12820
28. Bien J, Palagani V, Bozko P. The intestinal microbiota dysbiosis and Clostridium difficile infection: is there a relationship with inflammatory bowel disease? Ther Adv Gastroenterol. 2013;6(1):53-68. doi:10.1177/1756283X12454590
29. Cryan JF, O’Riordan KJ, Sandhu K, et al. The gut microbiome in neurological disorders. Lancet Neurol. 2020;19(2):179-194. doi:10.1016/S1474-4422(19)30356-4
30. Jones CA, Watson DJG, Fone KCF. Animal models of schizophrenia. Br J Pharmacol. 2011;164(4):1162-1194. doi:10.1111/j.1476-5381.2011.01386.x
31. Schmidt MJ, Mirnics K. Neurodevelopment, GABA system dysfunction, and schizophrenia. Neuropsychopharmacology. 2015;40(1):190-206. doi:10.1038/npp.2014.95
32. Nasrallah, HA. The daunting challenge of schizophrenia: hundreds of biotypes and dozens of theories. Curr. Psychiatry 2018;17(12):4-6,50.
33. Nocera A, Nasrallah HA. The association of the gut microbiota with clinical features in schizophrenia. Behav Sci (Basel). 2022;12(4):89. doi:10.3390/bs12040089
34. Schwarz E, Maukonen J, Hyytiäinen T, et al. Analysis of microbiota in first episode psychosis identifies preliminary associations with symptom severity and treatment response. Schizophr Res. 2018;192:398-403. doi:10.1016/j.schres.2017.04.017
35. Nguyen TT, Kosciolek T, Maldonado Y, et al. Differences in gut microbiome composition between persons with chronic schizophrenia and healthy comparison subjects. Schizophr Res. 2019;204:23-29. doi:10.1016/j.schres.2018.09.014
36. Li S, Zhuo M, Huang X, et al. Altered gut microbiota associated with symptom severity in schizophrenia. PeerJ. 2020;8:e9574. doi:10.7717/peerj.9574
37. Chen X, Xu J, Wang H, et al. Profiling the differences of gut microbial structure between schizophrenia patients with and without violent behaviors based on 16S rRNA gene sequencing. Int J Legal Med. 2021;135(1):131-141. doi:10.1007/s00414-020-02439-1
38. Manchia M, Fontana A, Panebianco C, et al. Involvement of gut microbiota in schizophrenia and treatment resistance to antipsychotics. Biomedicines. 2021;9(8):875. doi:10.3390/biomedicines9080875
39. Zhu C, Zheng M, Ali U, et al. Association between abundance of haemophilus in the gut microbiota and negative symptoms of schizophrenia. Front Psychiatry. 2021;12:685910. doi:10.3389/fpsyt.2021.685910
40. Flowers SA, Evans SJ, Ward KM, et al. Interaction between atypical antipsychotics and the gut microbiome in a bipolar disease cohort. Pharmacotherapy. 2017;37(3):261-267. doi:10.1002/phar.1890
41. Yatsunenko T, Rey FE, Manary MJ, et al. Human gut microbiome viewed across age and geography. Nature. 2012;486(7402):222-227. doi:10.1038/nature11053
42. Buchanan RW. Persistent negative symptoms in schizophrenia: an overview. Schizophr Bull. 2007;33(4):1013-1022. doi:10.1093/schbul/sb1057
43. Liu JCW, Gorbovskaya I, Hahn MK, et al. The gut microbiome in schizophrenia and the potential benefits of prebiotic and probiotic treatment. Nutrients. 2021;13(4):1152. doi:10.3390/nu13041152
44. Biedermann L, Zeitz J, Mwinyi J, et al. Smoking cessation induces profound changes in the composition of the intestinal microbiota in humans. PloS One. 2013;8(3):e59260. doi:10.1371/journal.pone.0059260
45. Leclercq S, Matamoros S, Cani PD, et al. Intestinal permeability, gut-bacterial dysbiosis, and behavioral markers of alcohol-dependence severity. Proc Natl Acad Sci. 2014;111(42):e4485-e4493. doi:10.1073/pnas.1415174111
46. Hernández-Quiroz F, Nirmalkar K, Villalobos-Flores LE, et al. Influence of moderate beer consumption on human gut microbiota and its impact on fasting glucose and ß-cell function. Alcohol. 2020;85:77-94. doi:10.1016/j.alcohol.2019.05.006
47. Panee J, Gerschenson M, Chang L. Associations between microbiota, mitochondrial function, and cognition in chronic marijuana users. J Neuroimmune Pharmacol. 2018;13(1):113-122. doi:10.1007/s11481-017-9767-0
48. Wu GD, Chen J, Hoffmann C, et al. Linking long-term dietary patterns with gut microbial enterotypes. Science. 2011;334(6052):105-108. doi:10.1126/science.1208344
49. Rezac S, Kok CR, Heermann M, et al. Fermented foods as a dietary source of live organisms. Front Microbiol. 2018;9:1785. doi:10.3389/fmicb.2018.01785
50. Chen X, Zhang Y, Wang H, et al. The regulatory effects of lactic acid on neuropsychiatric disorders. Discover Ment Health. 2022;2(1). doi:10.1007/s44192-022-00011-4
51. Karbownik MS, Mokros Ł, Dobielska M, et al. Association between consumption of fermented food and food-derived prebiotics with cognitive performance, depressive, and anxiety symptoms in psychiatrically healthy medical students under psychological stress: a prospective cohort study. Front Nutr. 2022;9:850249. doi:10.3389/fnut.2022.850249
52. Romano S, Savva GM, Bedarf JR, et al. Meta-analysis of the Parkinson’s disease gut microbiome suggests alterations linked to intestinal inflammation. NPJ Parkinsons Dis. 2021;7(1):27. doi:10.1038/s41531-021-00156-z
53. Bourassa MW, Alim I, Bultman SJ, et al. Butyrate, neuroepigenetics and the gut microbiome: can a high fiber diet improve brain health? Neurosci Lett. 2016;625:56-63. doi:10.1016/j.neulet.2016.02.009
54. Matt SM, Allen JM, Lawson MA, et al. Butyrate and dietary soluble fiber improve neuroinflammation associated with aging in mice. Front Immunol. 2018;9:1832. doi:10.3389/fimmu.2018.01832
55. Mittal VA, Vargas T, Osborne KJ, et al. Exercise treatments for psychosis: a review. Curr Treat Options Psychiatry. 2017;4(2):152-166. doi:10.1007/s40501-017-0112-2
56. Estaki M, Pither J, Baumeister P, et al. Cardiorespiratory fitness as a predictor of intestinal microbial diversity and distinct metagenomic functions. Microbiome. 2016;4(1):42. doi:10.1186/s40168-016-0189-7
57. Karl JP, Margolis LM, Madslien EH, et al. Changes in intestinal microbiota composition and metabolism coincide with increased intestinal permeability in young adults under prolonged physiological stress. Am J Physiol-Gastrointest Liver Physiol. 2017;312(6):G559-G571. doi:10.1152/ajpgi.00066.2017
58. Claus SP, Guillou H, Ellero-Simatos S. The gut microbiota: a major player in the toxicity of environmental pollutants? NPJ Biofilms Microbiomes. 2016;2:16003. doi:10.1038/npjbiofilms.2016.3
The human microbiota refers to the collection of bacteria, archaea, eukarya, and viruses that reside within the human body. The term gut microbiome indicates the composition of these microbes and genetic codes in the intestine.1 Harkening back to the ancient Greek physician Galen, who treated gastrointestinal (GI) symptoms to relieve mental disturbances such as psychosis, the gut has been a therapeutic target in schizophrenia long before antipsychotics and the DSM.2 In recent years, research into the gut microbiome has drastically increased, with genetic sequencing affording a more precise look into the specific bacteria that call the human intestines their home. This has led to the recognition that the gut microbiome may be severely disrupted in schizophrenia, a condition known as dysbiosis. Preliminary research suggests that gut bacteria are more helpful than many human genes in distinguishing individuals with schizophrenia from their healthy counterparts.3,4 In this article, we discuss the potential role of the gut microbiome in schizophrenia, including new research correlating clinical symptoms of psychosis with dysbiosis. We also provide recommendations for promoting a healthy gut microbiome.
The enteric brain across life
The composition of our bodies is far more microbiota than human. Strikingly, microbiota cells in the gut outnumber human cells, and the distal gut alone hosts bacteria with 100 times the genetic content of the entire genome.5 The intricate meshwork of nerves in the gut is often called the enteric brain because the gut consists of 100 million neurons and synthesizes many neuroactive chemicals implicated in mood disorders and psychosis, including serotonin, dopamine, gamma-aminobutyric acid (GABA), and acetylcholine.6 The variety of neuroimmunologic, hormonal, and metabolic paths by which the gut microbiome and the brain interact are collectively known as the gut-microbiota-brain axis.7
How do we acquire our gut microbiome, and how does it come to influence our brain and behavior? On the first day of life, as babies pass through the birth canal, they are bathed in their mother’s vaginal microbiota. In the following weeks, the microbiome expands and colonizes the gut as bacteria are introduced from environmental sources such as skin-to-skin contact and breastmilk.8 The microbiome continues to evolve throughout early life. As children expand their diets and navigate new aspects of the physical world, additional bacteria join the unseen ecosystem growing inside.9 The development of the microbiome coincides with the development of the brain. From preclinical studies, we know the gut microbiome mediates important aspects of neurodevelopment such as the formation of the blood-brain barrier (BBB), synaptic pruning, glial activation, and myelination.10 Interestingly, many of the risk factors for schizophrenia are associated with gut dysbiosis, including obstetric complications, infections treated with antibiotics, and urbanization.11-15
Throughout human life, the gut and brain remain in close communication. The gut microbiota continue to produce monoamines, along with other metabolites that are able to cross the BBB.6 The HPA axis, stimulation of the immune system, and the vagus nerve all provide highways of communication between the gut and the brain.7 The relationship between the enteric brain and cephalic brain continues through life, even up to a person’s final hour. One autopsy study that is often cited (but soberingly, cannot be found online) allegedly revealed that 92% of schizophrenia patients had developed colitis by the time of death.16,17
First-episode psychosis and antipsychotic treatment
For patients with schizophrenia, first-episode psychosis (FEP) represents a cocktail of mounting genetic and environmental factors. Typically, by the time a patient receives psychiatric care, they present with characteristic psychotic symptoms—hallucinations, delusions, bizarre behavior, and unusual thought process—along with a unique gut microbiome profile.
This disrupted microbiome coincides with a marked state of inflammation in the intestines. Inflammation triggers increased endothelial barrier permeability, similar to the way immune signals increase capillary permeability to allow immune cells into the periphery of the blood. Specific gut bacteria play specific roles in maintaining the gut barrier.18,19 Disruptions in the bacteria that maintain the gut barrier, combined with inflammation, contribute to a leaky gut. A leaky gut barrier allows bacterial and immune products to more easily enter the bloodstream and then the brain, which is a potential source of neuroinflammation in schizophrenia.20 This increase in gut permeability (leaky gut syndrome) is likely one of several reasons low-grade inflammation is common in schizophrenia—numerous studies show higher serum levels of proinflammatory cytokines along with antibacterial immunoglobulins in patients with FEP.21,22
Fortunately, antipsychotics, especially the second-generation agents, help restore a healthy gut microbiome and have substantial anti-inflammatory properties.23,24 These medications interact heavily with the gut microbiome: they have been found to have antibiotic properties, even in doses lower than would normally reach the gut microbiome.25 In humans, a randomized controlled trial of probiotic supplementation for schizophrenia patients taking antipsychotics showed a reduction in GI symptoms but no significant improvement in psychotic symptoms.26
Dysbiosis in schizophrenia: cause or effect?
There is no consensus on what constitutes a healthy gut microbiome because the gut microbiome is highly variable, even among healthy individuals, and can change quickly. Those who adopt new diets, for example, see drastic shifts in the gut microbiome within a few days.27 Despite this variation, the main separation between a healthy and dysbiotic gut comes from the diversity of bacteria present in the gut—a healthy gut microbiome is associated with increased diversity. Numerous disease states have been associated with decreased bacterial diversity, including Clostridium difficile infection, Parkinson disease, depression, Crohn disease, and schizophrenia spectrum disorders.28,29
Although there are ethical limitations to studying causality in humans directly, animal models have provided a great deal of insight into the gut microbiome’s role in the development of schizophrenia. A recent study used fecal transplant to provide the gut microbiome from patients with schizophrenia to a group of germ-free mice and compared these animals to a group of mice that received a fecal transplant from individuals with a healthy gut microbiome. The mice receiving the schizophrenia microbiome showed an increased startle response and hyperactivity.3 This was consistent with mouse models of schizophrenia, although with obvious limitations.30 In addition, the brains of these animals showed changes in glutamate, glutamine, and GABA in the hippocampus; these chemicals play a role in the neurophysiology of schizophrenia.3,31 This study has not yet been replicated, and considerable variation remains within the schizophrenia biosignature.
Continue to: Clinical symptoms of psychosis and the gut microbiome
Clinical symptoms of psychosis and the gut microbiome
Previous literature has grouped patients with schizophrenia spectrum disorders as 1 unified study group. But as is the case with many psychiatric conditions, there is a great deal of heterogeneity in neurobiology, genetics, and microbiome composition among individuals with schizophrenia.32
Researchers have begun to investigate ways in which the gut microbiome varies regarding the clinical symptoms of psychosis.33 The Table3,34-39 provides an overview of 7 human studies of gut microbiome changes relating to clinical features of schizophrenia. In these studies, researchers have found correlations between the gut microbiome and a tendency toward violence,37 cognitive deficits,34-36,39 depressive symptoms,35,39 and numerous other clinical features of psychosis. Most of these correlations have not yet been replicated by further studies. But among studies with similar clinical questions, 3 reported changes in gut microbiome correlated with overall symptom severity, and 4 studies correlated changes with negative symptom severity. In 2 studies,3,34 Lachnospiraceae was correlated with worsened symptom severity. However, this may have been the result of poor control for antipsychotic use, as 1 study in bipolar patients found that Lachnospiraceae was increased in those taking antipsychotics compared to those who were not treated with antipsychotics.40 The specific shifts in bacteria seen for overall symptom and negative symptom severity were not consistent across studies. This is not surprising because the gut microbiome varies with diet and geographic region,41 and patients in these studies were from a variety of regions. Multiple studies demonstrated gut microbiome alterations for patients with more severe negative symptoms. This is particularly interesting because negative symptoms are often difficult to treat and do not respond to antipsychotics.42 This research suggests the gut microbiome may be helpful in developing future treatments for patients with negative symptoms that do not respond to existing treatments.
Research of probiotic supplementation for ameliorating symptoms of schizophrenia has yielded mixed results.43 It is possible that studies of probiotic supplementation have failed to consider the variations in the gut microbiome among individuals with schizophrenia. A better understanding of the variations in gut microbiome may allow for the development of more personalized interventions.

Recommendations for a healthy gut microbiome
In addition to antipsychotics, many other evidence-based interventions can be used to help restore a healthy gut microbiome in patients with schizophrenia. To improve the gut microbiome, we suggest discussing the following changes with patients:
- Quitting smoking. Smoking is common among patients with schizophrenia but decreases gut microbiome diversity.44
- Avoiding excessive alcohol use. Excessive alcohol use contributes to dysbiosis and increased intestinal permeability.45 Moderate alcohol consumption does not appear to have the same harmful effects on the microbiome.46
- Avoiding the use of recreational drugs, including marijuana, which impact the gut microbiome.47
- Consuming a diet rich in fiber.48 Presently, there is not enough evidence to recommend probiotic supplementation to reduce symptoms of schizophrenia.41 Similar to probiotics, fermented foods contain Lactobacillus, a bacterial species that produces lactic acid.49 Lactobacillus is enriched in the gut microbiome in some neurodegenerative diseases, and lactic acid can be neurotoxic at high levels.50-52 Therefore, clinicians should not explicitly recommend fermented foods under the assumption of improved brain health. A diet rich in soluble fiber has been consistently shown to promote anti-inflammatory bacteria and is much more likely to be beneficial.53,54 Soluble fiber is found in foods such as fruits, vegetables, beans, and oats.
- Exercising can increase microbiome diversity and provide anti-inflammatory effects in the gut.55,56 A recent review found that steady-state aerobic and high-intensity exercise interventions have positive effects on mood, cognition, and other negative symptoms in patients with schizophrenia.55
- Minimizing stress. Psychological stress and physiological stress from untreated medical conditions are toxic to healthy gut bacteria and weaken the gut barrier.57
- Mitigating exposure to pollution. Environmental pollution, including exposures to air pollution, heavy metals, and pesticides, disrupts the gut microbiome.58
The American Heart Association publishes lifestyle recommendations for individuals with heart disease and the National Institutes of Health publishes lifestyle recommendations for patients with chronic kidney disease. This leads us to question why the American Psychiatric Association has not published lifestyle recommendations for those with severe mental illness. The effects of lifestyle on both the gut microbiome and symptom mitigation is critical. With increasingly shortened appointments, standardized guidelines would benefit psychiatrists and patients alike.
Bottom Line
The gut microbiome is connected to the clinical symptoms of psychosis via a variety of hormonal, neuroimmune, and metabolic mechanisms active across the lifespan. Despite advances in research, there is still much to be understood regarding this relationship. Clinicians should discuss with patients ways to promote a healthy gut microbiome, including consuming a diet rich in fiber, avoiding use of recreational drugs, and exercising regularly.
Related Resources
- Nocera A, Nasrallah HA. The association of the gut microbiota with clinical features in schizophrenia. Behav Sci. 2022;12(4):89.
- Nasrallah HA. It takes guts to be mentally ill: microbiota and psychopathology. Current Psychiatry. 2018;17(9):4-6.
The human microbiota refers to the collection of bacteria, archaea, eukarya, and viruses that reside within the human body. The term gut microbiome indicates the composition of these microbes and genetic codes in the intestine.1 Harkening back to the ancient Greek physician Galen, who treated gastrointestinal (GI) symptoms to relieve mental disturbances such as psychosis, the gut has been a therapeutic target in schizophrenia long before antipsychotics and the DSM.2 In recent years, research into the gut microbiome has drastically increased, with genetic sequencing affording a more precise look into the specific bacteria that call the human intestines their home. This has led to the recognition that the gut microbiome may be severely disrupted in schizophrenia, a condition known as dysbiosis. Preliminary research suggests that gut bacteria are more helpful than many human genes in distinguishing individuals with schizophrenia from their healthy counterparts.3,4 In this article, we discuss the potential role of the gut microbiome in schizophrenia, including new research correlating clinical symptoms of psychosis with dysbiosis. We also provide recommendations for promoting a healthy gut microbiome.
The enteric brain across life
The composition of our bodies is far more microbiota than human. Strikingly, microbiota cells in the gut outnumber human cells, and the distal gut alone hosts bacteria with 100 times the genetic content of the entire genome.5 The intricate meshwork of nerves in the gut is often called the enteric brain because the gut consists of 100 million neurons and synthesizes many neuroactive chemicals implicated in mood disorders and psychosis, including serotonin, dopamine, gamma-aminobutyric acid (GABA), and acetylcholine.6 The variety of neuroimmunologic, hormonal, and metabolic paths by which the gut microbiome and the brain interact are collectively known as the gut-microbiota-brain axis.7
How do we acquire our gut microbiome, and how does it come to influence our brain and behavior? On the first day of life, as babies pass through the birth canal, they are bathed in their mother’s vaginal microbiota. In the following weeks, the microbiome expands and colonizes the gut as bacteria are introduced from environmental sources such as skin-to-skin contact and breastmilk.8 The microbiome continues to evolve throughout early life. As children expand their diets and navigate new aspects of the physical world, additional bacteria join the unseen ecosystem growing inside.9 The development of the microbiome coincides with the development of the brain. From preclinical studies, we know the gut microbiome mediates important aspects of neurodevelopment such as the formation of the blood-brain barrier (BBB), synaptic pruning, glial activation, and myelination.10 Interestingly, many of the risk factors for schizophrenia are associated with gut dysbiosis, including obstetric complications, infections treated with antibiotics, and urbanization.11-15
Throughout human life, the gut and brain remain in close communication. The gut microbiota continue to produce monoamines, along with other metabolites that are able to cross the BBB.6 The HPA axis, stimulation of the immune system, and the vagus nerve all provide highways of communication between the gut and the brain.7 The relationship between the enteric brain and cephalic brain continues through life, even up to a person’s final hour. One autopsy study that is often cited (but soberingly, cannot be found online) allegedly revealed that 92% of schizophrenia patients had developed colitis by the time of death.16,17
First-episode psychosis and antipsychotic treatment
For patients with schizophrenia, first-episode psychosis (FEP) represents a cocktail of mounting genetic and environmental factors. Typically, by the time a patient receives psychiatric care, they present with characteristic psychotic symptoms—hallucinations, delusions, bizarre behavior, and unusual thought process—along with a unique gut microbiome profile.
This disrupted microbiome coincides with a marked state of inflammation in the intestines. Inflammation triggers increased endothelial barrier permeability, similar to the way immune signals increase capillary permeability to allow immune cells into the periphery of the blood. Specific gut bacteria play specific roles in maintaining the gut barrier.18,19 Disruptions in the bacteria that maintain the gut barrier, combined with inflammation, contribute to a leaky gut. A leaky gut barrier allows bacterial and immune products to more easily enter the bloodstream and then the brain, which is a potential source of neuroinflammation in schizophrenia.20 This increase in gut permeability (leaky gut syndrome) is likely one of several reasons low-grade inflammation is common in schizophrenia—numerous studies show higher serum levels of proinflammatory cytokines along with antibacterial immunoglobulins in patients with FEP.21,22
Fortunately, antipsychotics, especially the second-generation agents, help restore a healthy gut microbiome and have substantial anti-inflammatory properties.23,24 These medications interact heavily with the gut microbiome: they have been found to have antibiotic properties, even in doses lower than would normally reach the gut microbiome.25 In humans, a randomized controlled trial of probiotic supplementation for schizophrenia patients taking antipsychotics showed a reduction in GI symptoms but no significant improvement in psychotic symptoms.26
Dysbiosis in schizophrenia: cause or effect?
There is no consensus on what constitutes a healthy gut microbiome because the gut microbiome is highly variable, even among healthy individuals, and can change quickly. Those who adopt new diets, for example, see drastic shifts in the gut microbiome within a few days.27 Despite this variation, the main separation between a healthy and dysbiotic gut comes from the diversity of bacteria present in the gut—a healthy gut microbiome is associated with increased diversity. Numerous disease states have been associated with decreased bacterial diversity, including Clostridium difficile infection, Parkinson disease, depression, Crohn disease, and schizophrenia spectrum disorders.28,29
Although there are ethical limitations to studying causality in humans directly, animal models have provided a great deal of insight into the gut microbiome’s role in the development of schizophrenia. A recent study used fecal transplant to provide the gut microbiome from patients with schizophrenia to a group of germ-free mice and compared these animals to a group of mice that received a fecal transplant from individuals with a healthy gut microbiome. The mice receiving the schizophrenia microbiome showed an increased startle response and hyperactivity.3 This was consistent with mouse models of schizophrenia, although with obvious limitations.30 In addition, the brains of these animals showed changes in glutamate, glutamine, and GABA in the hippocampus; these chemicals play a role in the neurophysiology of schizophrenia.3,31 This study has not yet been replicated, and considerable variation remains within the schizophrenia biosignature.
Continue to: Clinical symptoms of psychosis and the gut microbiome
Clinical symptoms of psychosis and the gut microbiome
Previous literature has grouped patients with schizophrenia spectrum disorders as 1 unified study group. But as is the case with many psychiatric conditions, there is a great deal of heterogeneity in neurobiology, genetics, and microbiome composition among individuals with schizophrenia.32
Researchers have begun to investigate ways in which the gut microbiome varies regarding the clinical symptoms of psychosis.33 The Table3,34-39 provides an overview of 7 human studies of gut microbiome changes relating to clinical features of schizophrenia. In these studies, researchers have found correlations between the gut microbiome and a tendency toward violence,37 cognitive deficits,34-36,39 depressive symptoms,35,39 and numerous other clinical features of psychosis. Most of these correlations have not yet been replicated by further studies. But among studies with similar clinical questions, 3 reported changes in gut microbiome correlated with overall symptom severity, and 4 studies correlated changes with negative symptom severity. In 2 studies,3,34 Lachnospiraceae was correlated with worsened symptom severity. However, this may have been the result of poor control for antipsychotic use, as 1 study in bipolar patients found that Lachnospiraceae was increased in those taking antipsychotics compared to those who were not treated with antipsychotics.40 The specific shifts in bacteria seen for overall symptom and negative symptom severity were not consistent across studies. This is not surprising because the gut microbiome varies with diet and geographic region,41 and patients in these studies were from a variety of regions. Multiple studies demonstrated gut microbiome alterations for patients with more severe negative symptoms. This is particularly interesting because negative symptoms are often difficult to treat and do not respond to antipsychotics.42 This research suggests the gut microbiome may be helpful in developing future treatments for patients with negative symptoms that do not respond to existing treatments.
Research of probiotic supplementation for ameliorating symptoms of schizophrenia has yielded mixed results.43 It is possible that studies of probiotic supplementation have failed to consider the variations in the gut microbiome among individuals with schizophrenia. A better understanding of the variations in gut microbiome may allow for the development of more personalized interventions.

Recommendations for a healthy gut microbiome
In addition to antipsychotics, many other evidence-based interventions can be used to help restore a healthy gut microbiome in patients with schizophrenia. To improve the gut microbiome, we suggest discussing the following changes with patients:
- Quitting smoking. Smoking is common among patients with schizophrenia but decreases gut microbiome diversity.44
- Avoiding excessive alcohol use. Excessive alcohol use contributes to dysbiosis and increased intestinal permeability.45 Moderate alcohol consumption does not appear to have the same harmful effects on the microbiome.46
- Avoiding the use of recreational drugs, including marijuana, which impact the gut microbiome.47
- Consuming a diet rich in fiber.48 Presently, there is not enough evidence to recommend probiotic supplementation to reduce symptoms of schizophrenia.41 Similar to probiotics, fermented foods contain Lactobacillus, a bacterial species that produces lactic acid.49 Lactobacillus is enriched in the gut microbiome in some neurodegenerative diseases, and lactic acid can be neurotoxic at high levels.50-52 Therefore, clinicians should not explicitly recommend fermented foods under the assumption of improved brain health. A diet rich in soluble fiber has been consistently shown to promote anti-inflammatory bacteria and is much more likely to be beneficial.53,54 Soluble fiber is found in foods such as fruits, vegetables, beans, and oats.
- Exercising can increase microbiome diversity and provide anti-inflammatory effects in the gut.55,56 A recent review found that steady-state aerobic and high-intensity exercise interventions have positive effects on mood, cognition, and other negative symptoms in patients with schizophrenia.55
- Minimizing stress. Psychological stress and physiological stress from untreated medical conditions are toxic to healthy gut bacteria and weaken the gut barrier.57
- Mitigating exposure to pollution. Environmental pollution, including exposures to air pollution, heavy metals, and pesticides, disrupts the gut microbiome.58
The American Heart Association publishes lifestyle recommendations for individuals with heart disease and the National Institutes of Health publishes lifestyle recommendations for patients with chronic kidney disease. This leads us to question why the American Psychiatric Association has not published lifestyle recommendations for those with severe mental illness. The effects of lifestyle on both the gut microbiome and symptom mitigation is critical. With increasingly shortened appointments, standardized guidelines would benefit psychiatrists and patients alike.
Bottom Line
The gut microbiome is connected to the clinical symptoms of psychosis via a variety of hormonal, neuroimmune, and metabolic mechanisms active across the lifespan. Despite advances in research, there is still much to be understood regarding this relationship. Clinicians should discuss with patients ways to promote a healthy gut microbiome, including consuming a diet rich in fiber, avoiding use of recreational drugs, and exercising regularly.
Related Resources
- Nocera A, Nasrallah HA. The association of the gut microbiota with clinical features in schizophrenia. Behav Sci. 2022;12(4):89.
- Nasrallah HA. It takes guts to be mentally ill: microbiota and psychopathology. Current Psychiatry. 2018;17(9):4-6.
1. Bäckhed F, Ley RE, Sonnenburg JL, et al. Host-bacterial mutualism in the human intestine. Science. 2005;307(5717):1915-1920. doi:10.1126/science.1104816
2. Jackson SW. Galen—on mental disorders. J Hist Behav Sci. 1969;5(4):365-384. doi:10.1002/1520-6696(196910)5:4<365::AID-JHBS2300050408>3.0.CO;2-9
3. Zheng P, Zeng B, Liu M, et al. The gut microbiome from patients with schizophrenia modulates the glutamate-glutamine-GABA cycle and schizophrenia-relevant behaviors in mice. Sci Adv. 2019;5(2):eaau8317. doi:10.1126/sciadv.aau8317
4. Schizophrenia Working Group of the Psychiatric Genomics Consortium. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511(7510):421-427. doi:10.1038/nature13595
5. Gill SR, Pop M, DeBoy RT, et al. Metagenomic analysis of the human distal gut microbiome. Science. 2006;312(5778):1355-1359. doi:10.1126/science.1124234
6. Alam R, Abdolmaleky HM, Zhou JR. Microbiome, inflammation, epigenetic alterations, and mental diseases. Am J Med Genet B Neuropsychiatr Genet. 2017;174(6):651-660. doi:10.1002/ajmg.b.32567
7. Cryan JF, O’Riordan KJ, Cowan CSM, et al. The microbiota-gut-brain axis. Physiol Rev. 2019;99(4):1877-2013. doi:10.1152/physrev.00018.2018
8. Mueller NT, Bakacs E, Combellick J, et al. The infant microbiome development: mom matters. Trends Mol Med. 2015;21(2):109-117. doi:10.1016/j.molmed.2014.12.002
9. Fouhy F, Watkins C, Hill CJ, et al. Perinatal factors affect the gut microbiota up to four years after birth. Nat Commun. 2019;10(1):1517. doi:10.1038/s41467-019-09252-4
10. Sharon G, Sampson TR, Geschwind DH, et al. The central nervous system and the gut microbiome. Cell. 2016;167(4):915-932. doi:10.1016/j.cell.2016.10.027
11. Hill CJ, Lynch DB, Murphy K, et al. Evolution of gut microbiota composition from birth to 24 weeks in the INFANTMET Cohort. Microbiome. 2017;5:4. doi:10.1186/s40168-016-0213-y
12. Gareau MG, Wine E, Rodrigues DM, et al. Bacterial infection causes stress-induced memory dysfunction in mice. Gut. 2011;60(3):307-317. doi:10.1136/gut.2009.202515
13. Bokulich NA, Chung J, Battaglia T, et al. Antibiotics, birth mode, and diet shape microbiome maturation during early life. Sci Transl Med. 2016;8(343):343ra82. doi:10.1126/scitranslmed.aad7121
14. Mancabelli L, Milani C, Lugli GA, et al. Meta-analysis of the human gut microbiome from urbanized and pre-agricultural populations. Environ Microbiol. 2017;19(4):1379-1390. doi:10.1111/1462-2920.13692
15. Stilo SA, Murray RM. Non-genetic factors in schizophrenia. Curr Psychiatry Rep. 2019;21(10):100. doi:10.1007/s11920-019-1091-3
16. Buscaino VM. Patologia extraneurale della schizofrenia: fegato, tubo digerente, sistema reticolo-endoteliale. Acta Neurologica. 1953;VIII:1-60.
17. Hemmings G. Schizophrenia. Lancet. 2004;364(9442):1312-1313. doi:10.1016/S0140- 6736(04)17181-X
18. Hooper LV, Gordon JI. Commensal host-bacterial relationships in the gut. Science. 2001;292(5519):1115-1118. doi:10.1126/science.1058709
19. Ewaschuk JB, Diaz H, Meddings L, et al. Secreted bioactive factors from Bifidobacterium infantis enhance epithelial cell barrier function. Am J Physiol-Gastrointest Liver Physiol. 2008;295(5):G1025-G1034. doi:10.1152/ajpgi.90227.2008
20. Alhasson F, Das S, Seth R, et al. Altered gut microbiome in a mouse model of Gulf War Illness causes neuroinflammation and intestinal injury via leaky gut and TLR4 activation. PLoS One. 2017;12(3):e0172914. doi:10.1371/journal.pone.0172914
21. Fillman SG, Cloonan N, Catts VS, et al. Increased inflammatory markers identified in the dorsolateral prefrontal cortex of individuals with schizophrenia. Mol Psychiatry. 2013;18(2):206-214. doi:10.1038/mp.2012.110
22. Miller BJ, Buckley P, Seabolt W, et al. Meta-analysis of cytokine alterations in schizophrenia: clinical status and antipsychotic effects. Biol Psychiatry. 2011;70(7):663-671. doi:10.1016/j.biopsych.2011.04.013
23. Al-Amin M, Uddin MMN, Reza HM. Effects of antipsychotics on the inflammatory response system of patients with schizophrenia in peripheral blood mononuclear cell cultures. Clin Psychopharmacol Neurosci. 2013;11(3):144-151. doi:10.9758/cpn.2013.11.3.144
24. Yuan X, Zhang P, Wang Y, et al. Changes in metabolism and microbiota after 24-week risperidone treatment in drug naïve, normal weight patients with first episode schizophrenia. Schizophr Res. 2018;201:299-306. doi:10.1016/j.schres.2018.05.017
25. Maier L, Pruteanu M, Kuhn M, et al. Extensive impact of non-antibiotic drugs on human gut bacteria. Nature. 2018;555(7698):623-628. doi:10.1038/nature25979
26. Dickerson FB, Stallings C, Origoni A, et al. Effect of probiotic supplementation on schizophrenia symptoms and association with gastrointestinal functioning: a randomized, placebo-controlled trial. Prim Care Companion CNS Disord. 2014;15(1):PCC.13m01579. doi:10.4088/PCC.13m01579
27. David LA, Maurice CF, Carmody RN, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505(7484):559-563. doi:10.1038/nature12820
28. Bien J, Palagani V, Bozko P. The intestinal microbiota dysbiosis and Clostridium difficile infection: is there a relationship with inflammatory bowel disease? Ther Adv Gastroenterol. 2013;6(1):53-68. doi:10.1177/1756283X12454590
29. Cryan JF, O’Riordan KJ, Sandhu K, et al. The gut microbiome in neurological disorders. Lancet Neurol. 2020;19(2):179-194. doi:10.1016/S1474-4422(19)30356-4
30. Jones CA, Watson DJG, Fone KCF. Animal models of schizophrenia. Br J Pharmacol. 2011;164(4):1162-1194. doi:10.1111/j.1476-5381.2011.01386.x
31. Schmidt MJ, Mirnics K. Neurodevelopment, GABA system dysfunction, and schizophrenia. Neuropsychopharmacology. 2015;40(1):190-206. doi:10.1038/npp.2014.95
32. Nasrallah, HA. The daunting challenge of schizophrenia: hundreds of biotypes and dozens of theories. Curr. Psychiatry 2018;17(12):4-6,50.
33. Nocera A, Nasrallah HA. The association of the gut microbiota with clinical features in schizophrenia. Behav Sci (Basel). 2022;12(4):89. doi:10.3390/bs12040089
34. Schwarz E, Maukonen J, Hyytiäinen T, et al. Analysis of microbiota in first episode psychosis identifies preliminary associations with symptom severity and treatment response. Schizophr Res. 2018;192:398-403. doi:10.1016/j.schres.2017.04.017
35. Nguyen TT, Kosciolek T, Maldonado Y, et al. Differences in gut microbiome composition between persons with chronic schizophrenia and healthy comparison subjects. Schizophr Res. 2019;204:23-29. doi:10.1016/j.schres.2018.09.014
36. Li S, Zhuo M, Huang X, et al. Altered gut microbiota associated with symptom severity in schizophrenia. PeerJ. 2020;8:e9574. doi:10.7717/peerj.9574
37. Chen X, Xu J, Wang H, et al. Profiling the differences of gut microbial structure between schizophrenia patients with and without violent behaviors based on 16S rRNA gene sequencing. Int J Legal Med. 2021;135(1):131-141. doi:10.1007/s00414-020-02439-1
38. Manchia M, Fontana A, Panebianco C, et al. Involvement of gut microbiota in schizophrenia and treatment resistance to antipsychotics. Biomedicines. 2021;9(8):875. doi:10.3390/biomedicines9080875
39. Zhu C, Zheng M, Ali U, et al. Association between abundance of haemophilus in the gut microbiota and negative symptoms of schizophrenia. Front Psychiatry. 2021;12:685910. doi:10.3389/fpsyt.2021.685910
40. Flowers SA, Evans SJ, Ward KM, et al. Interaction between atypical antipsychotics and the gut microbiome in a bipolar disease cohort. Pharmacotherapy. 2017;37(3):261-267. doi:10.1002/phar.1890
41. Yatsunenko T, Rey FE, Manary MJ, et al. Human gut microbiome viewed across age and geography. Nature. 2012;486(7402):222-227. doi:10.1038/nature11053
42. Buchanan RW. Persistent negative symptoms in schizophrenia: an overview. Schizophr Bull. 2007;33(4):1013-1022. doi:10.1093/schbul/sb1057
43. Liu JCW, Gorbovskaya I, Hahn MK, et al. The gut microbiome in schizophrenia and the potential benefits of prebiotic and probiotic treatment. Nutrients. 2021;13(4):1152. doi:10.3390/nu13041152
44. Biedermann L, Zeitz J, Mwinyi J, et al. Smoking cessation induces profound changes in the composition of the intestinal microbiota in humans. PloS One. 2013;8(3):e59260. doi:10.1371/journal.pone.0059260
45. Leclercq S, Matamoros S, Cani PD, et al. Intestinal permeability, gut-bacterial dysbiosis, and behavioral markers of alcohol-dependence severity. Proc Natl Acad Sci. 2014;111(42):e4485-e4493. doi:10.1073/pnas.1415174111
46. Hernández-Quiroz F, Nirmalkar K, Villalobos-Flores LE, et al. Influence of moderate beer consumption on human gut microbiota and its impact on fasting glucose and ß-cell function. Alcohol. 2020;85:77-94. doi:10.1016/j.alcohol.2019.05.006
47. Panee J, Gerschenson M, Chang L. Associations between microbiota, mitochondrial function, and cognition in chronic marijuana users. J Neuroimmune Pharmacol. 2018;13(1):113-122. doi:10.1007/s11481-017-9767-0
48. Wu GD, Chen J, Hoffmann C, et al. Linking long-term dietary patterns with gut microbial enterotypes. Science. 2011;334(6052):105-108. doi:10.1126/science.1208344
49. Rezac S, Kok CR, Heermann M, et al. Fermented foods as a dietary source of live organisms. Front Microbiol. 2018;9:1785. doi:10.3389/fmicb.2018.01785
50. Chen X, Zhang Y, Wang H, et al. The regulatory effects of lactic acid on neuropsychiatric disorders. Discover Ment Health. 2022;2(1). doi:10.1007/s44192-022-00011-4
51. Karbownik MS, Mokros Ł, Dobielska M, et al. Association between consumption of fermented food and food-derived prebiotics with cognitive performance, depressive, and anxiety symptoms in psychiatrically healthy medical students under psychological stress: a prospective cohort study. Front Nutr. 2022;9:850249. doi:10.3389/fnut.2022.850249
52. Romano S, Savva GM, Bedarf JR, et al. Meta-analysis of the Parkinson’s disease gut microbiome suggests alterations linked to intestinal inflammation. NPJ Parkinsons Dis. 2021;7(1):27. doi:10.1038/s41531-021-00156-z
53. Bourassa MW, Alim I, Bultman SJ, et al. Butyrate, neuroepigenetics and the gut microbiome: can a high fiber diet improve brain health? Neurosci Lett. 2016;625:56-63. doi:10.1016/j.neulet.2016.02.009
54. Matt SM, Allen JM, Lawson MA, et al. Butyrate and dietary soluble fiber improve neuroinflammation associated with aging in mice. Front Immunol. 2018;9:1832. doi:10.3389/fimmu.2018.01832
55. Mittal VA, Vargas T, Osborne KJ, et al. Exercise treatments for psychosis: a review. Curr Treat Options Psychiatry. 2017;4(2):152-166. doi:10.1007/s40501-017-0112-2
56. Estaki M, Pither J, Baumeister P, et al. Cardiorespiratory fitness as a predictor of intestinal microbial diversity and distinct metagenomic functions. Microbiome. 2016;4(1):42. doi:10.1186/s40168-016-0189-7
57. Karl JP, Margolis LM, Madslien EH, et al. Changes in intestinal microbiota composition and metabolism coincide with increased intestinal permeability in young adults under prolonged physiological stress. Am J Physiol-Gastrointest Liver Physiol. 2017;312(6):G559-G571. doi:10.1152/ajpgi.00066.2017
58. Claus SP, Guillou H, Ellero-Simatos S. The gut microbiota: a major player in the toxicity of environmental pollutants? NPJ Biofilms Microbiomes. 2016;2:16003. doi:10.1038/npjbiofilms.2016.3
1. Bäckhed F, Ley RE, Sonnenburg JL, et al. Host-bacterial mutualism in the human intestine. Science. 2005;307(5717):1915-1920. doi:10.1126/science.1104816
2. Jackson SW. Galen—on mental disorders. J Hist Behav Sci. 1969;5(4):365-384. doi:10.1002/1520-6696(196910)5:4<365::AID-JHBS2300050408>3.0.CO;2-9
3. Zheng P, Zeng B, Liu M, et al. The gut microbiome from patients with schizophrenia modulates the glutamate-glutamine-GABA cycle and schizophrenia-relevant behaviors in mice. Sci Adv. 2019;5(2):eaau8317. doi:10.1126/sciadv.aau8317
4. Schizophrenia Working Group of the Psychiatric Genomics Consortium. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511(7510):421-427. doi:10.1038/nature13595
5. Gill SR, Pop M, DeBoy RT, et al. Metagenomic analysis of the human distal gut microbiome. Science. 2006;312(5778):1355-1359. doi:10.1126/science.1124234
6. Alam R, Abdolmaleky HM, Zhou JR. Microbiome, inflammation, epigenetic alterations, and mental diseases. Am J Med Genet B Neuropsychiatr Genet. 2017;174(6):651-660. doi:10.1002/ajmg.b.32567
7. Cryan JF, O’Riordan KJ, Cowan CSM, et al. The microbiota-gut-brain axis. Physiol Rev. 2019;99(4):1877-2013. doi:10.1152/physrev.00018.2018
8. Mueller NT, Bakacs E, Combellick J, et al. The infant microbiome development: mom matters. Trends Mol Med. 2015;21(2):109-117. doi:10.1016/j.molmed.2014.12.002
9. Fouhy F, Watkins C, Hill CJ, et al. Perinatal factors affect the gut microbiota up to four years after birth. Nat Commun. 2019;10(1):1517. doi:10.1038/s41467-019-09252-4
10. Sharon G, Sampson TR, Geschwind DH, et al. The central nervous system and the gut microbiome. Cell. 2016;167(4):915-932. doi:10.1016/j.cell.2016.10.027
11. Hill CJ, Lynch DB, Murphy K, et al. Evolution of gut microbiota composition from birth to 24 weeks in the INFANTMET Cohort. Microbiome. 2017;5:4. doi:10.1186/s40168-016-0213-y
12. Gareau MG, Wine E, Rodrigues DM, et al. Bacterial infection causes stress-induced memory dysfunction in mice. Gut. 2011;60(3):307-317. doi:10.1136/gut.2009.202515
13. Bokulich NA, Chung J, Battaglia T, et al. Antibiotics, birth mode, and diet shape microbiome maturation during early life. Sci Transl Med. 2016;8(343):343ra82. doi:10.1126/scitranslmed.aad7121
14. Mancabelli L, Milani C, Lugli GA, et al. Meta-analysis of the human gut microbiome from urbanized and pre-agricultural populations. Environ Microbiol. 2017;19(4):1379-1390. doi:10.1111/1462-2920.13692
15. Stilo SA, Murray RM. Non-genetic factors in schizophrenia. Curr Psychiatry Rep. 2019;21(10):100. doi:10.1007/s11920-019-1091-3
16. Buscaino VM. Patologia extraneurale della schizofrenia: fegato, tubo digerente, sistema reticolo-endoteliale. Acta Neurologica. 1953;VIII:1-60.
17. Hemmings G. Schizophrenia. Lancet. 2004;364(9442):1312-1313. doi:10.1016/S0140- 6736(04)17181-X
18. Hooper LV, Gordon JI. Commensal host-bacterial relationships in the gut. Science. 2001;292(5519):1115-1118. doi:10.1126/science.1058709
19. Ewaschuk JB, Diaz H, Meddings L, et al. Secreted bioactive factors from Bifidobacterium infantis enhance epithelial cell barrier function. Am J Physiol-Gastrointest Liver Physiol. 2008;295(5):G1025-G1034. doi:10.1152/ajpgi.90227.2008
20. Alhasson F, Das S, Seth R, et al. Altered gut microbiome in a mouse model of Gulf War Illness causes neuroinflammation and intestinal injury via leaky gut and TLR4 activation. PLoS One. 2017;12(3):e0172914. doi:10.1371/journal.pone.0172914
21. Fillman SG, Cloonan N, Catts VS, et al. Increased inflammatory markers identified in the dorsolateral prefrontal cortex of individuals with schizophrenia. Mol Psychiatry. 2013;18(2):206-214. doi:10.1038/mp.2012.110
22. Miller BJ, Buckley P, Seabolt W, et al. Meta-analysis of cytokine alterations in schizophrenia: clinical status and antipsychotic effects. Biol Psychiatry. 2011;70(7):663-671. doi:10.1016/j.biopsych.2011.04.013
23. Al-Amin M, Uddin MMN, Reza HM. Effects of antipsychotics on the inflammatory response system of patients with schizophrenia in peripheral blood mononuclear cell cultures. Clin Psychopharmacol Neurosci. 2013;11(3):144-151. doi:10.9758/cpn.2013.11.3.144
24. Yuan X, Zhang P, Wang Y, et al. Changes in metabolism and microbiota after 24-week risperidone treatment in drug naïve, normal weight patients with first episode schizophrenia. Schizophr Res. 2018;201:299-306. doi:10.1016/j.schres.2018.05.017
25. Maier L, Pruteanu M, Kuhn M, et al. Extensive impact of non-antibiotic drugs on human gut bacteria. Nature. 2018;555(7698):623-628. doi:10.1038/nature25979
26. Dickerson FB, Stallings C, Origoni A, et al. Effect of probiotic supplementation on schizophrenia symptoms and association with gastrointestinal functioning: a randomized, placebo-controlled trial. Prim Care Companion CNS Disord. 2014;15(1):PCC.13m01579. doi:10.4088/PCC.13m01579
27. David LA, Maurice CF, Carmody RN, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505(7484):559-563. doi:10.1038/nature12820
28. Bien J, Palagani V, Bozko P. The intestinal microbiota dysbiosis and Clostridium difficile infection: is there a relationship with inflammatory bowel disease? Ther Adv Gastroenterol. 2013;6(1):53-68. doi:10.1177/1756283X12454590
29. Cryan JF, O’Riordan KJ, Sandhu K, et al. The gut microbiome in neurological disorders. Lancet Neurol. 2020;19(2):179-194. doi:10.1016/S1474-4422(19)30356-4
30. Jones CA, Watson DJG, Fone KCF. Animal models of schizophrenia. Br J Pharmacol. 2011;164(4):1162-1194. doi:10.1111/j.1476-5381.2011.01386.x
31. Schmidt MJ, Mirnics K. Neurodevelopment, GABA system dysfunction, and schizophrenia. Neuropsychopharmacology. 2015;40(1):190-206. doi:10.1038/npp.2014.95
32. Nasrallah, HA. The daunting challenge of schizophrenia: hundreds of biotypes and dozens of theories. Curr. Psychiatry 2018;17(12):4-6,50.
33. Nocera A, Nasrallah HA. The association of the gut microbiota with clinical features in schizophrenia. Behav Sci (Basel). 2022;12(4):89. doi:10.3390/bs12040089
34. Schwarz E, Maukonen J, Hyytiäinen T, et al. Analysis of microbiota in first episode psychosis identifies preliminary associations with symptom severity and treatment response. Schizophr Res. 2018;192:398-403. doi:10.1016/j.schres.2017.04.017
35. Nguyen TT, Kosciolek T, Maldonado Y, et al. Differences in gut microbiome composition between persons with chronic schizophrenia and healthy comparison subjects. Schizophr Res. 2019;204:23-29. doi:10.1016/j.schres.2018.09.014
36. Li S, Zhuo M, Huang X, et al. Altered gut microbiota associated with symptom severity in schizophrenia. PeerJ. 2020;8:e9574. doi:10.7717/peerj.9574
37. Chen X, Xu J, Wang H, et al. Profiling the differences of gut microbial structure between schizophrenia patients with and without violent behaviors based on 16S rRNA gene sequencing. Int J Legal Med. 2021;135(1):131-141. doi:10.1007/s00414-020-02439-1
38. Manchia M, Fontana A, Panebianco C, et al. Involvement of gut microbiota in schizophrenia and treatment resistance to antipsychotics. Biomedicines. 2021;9(8):875. doi:10.3390/biomedicines9080875
39. Zhu C, Zheng M, Ali U, et al. Association between abundance of haemophilus in the gut microbiota and negative symptoms of schizophrenia. Front Psychiatry. 2021;12:685910. doi:10.3389/fpsyt.2021.685910
40. Flowers SA, Evans SJ, Ward KM, et al. Interaction between atypical antipsychotics and the gut microbiome in a bipolar disease cohort. Pharmacotherapy. 2017;37(3):261-267. doi:10.1002/phar.1890
41. Yatsunenko T, Rey FE, Manary MJ, et al. Human gut microbiome viewed across age and geography. Nature. 2012;486(7402):222-227. doi:10.1038/nature11053
42. Buchanan RW. Persistent negative symptoms in schizophrenia: an overview. Schizophr Bull. 2007;33(4):1013-1022. doi:10.1093/schbul/sb1057
43. Liu JCW, Gorbovskaya I, Hahn MK, et al. The gut microbiome in schizophrenia and the potential benefits of prebiotic and probiotic treatment. Nutrients. 2021;13(4):1152. doi:10.3390/nu13041152
44. Biedermann L, Zeitz J, Mwinyi J, et al. Smoking cessation induces profound changes in the composition of the intestinal microbiota in humans. PloS One. 2013;8(3):e59260. doi:10.1371/journal.pone.0059260
45. Leclercq S, Matamoros S, Cani PD, et al. Intestinal permeability, gut-bacterial dysbiosis, and behavioral markers of alcohol-dependence severity. Proc Natl Acad Sci. 2014;111(42):e4485-e4493. doi:10.1073/pnas.1415174111
46. Hernández-Quiroz F, Nirmalkar K, Villalobos-Flores LE, et al. Influence of moderate beer consumption on human gut microbiota and its impact on fasting glucose and ß-cell function. Alcohol. 2020;85:77-94. doi:10.1016/j.alcohol.2019.05.006
47. Panee J, Gerschenson M, Chang L. Associations between microbiota, mitochondrial function, and cognition in chronic marijuana users. J Neuroimmune Pharmacol. 2018;13(1):113-122. doi:10.1007/s11481-017-9767-0
48. Wu GD, Chen J, Hoffmann C, et al. Linking long-term dietary patterns with gut microbial enterotypes. Science. 2011;334(6052):105-108. doi:10.1126/science.1208344
49. Rezac S, Kok CR, Heermann M, et al. Fermented foods as a dietary source of live organisms. Front Microbiol. 2018;9:1785. doi:10.3389/fmicb.2018.01785
50. Chen X, Zhang Y, Wang H, et al. The regulatory effects of lactic acid on neuropsychiatric disorders. Discover Ment Health. 2022;2(1). doi:10.1007/s44192-022-00011-4
51. Karbownik MS, Mokros Ł, Dobielska M, et al. Association between consumption of fermented food and food-derived prebiotics with cognitive performance, depressive, and anxiety symptoms in psychiatrically healthy medical students under psychological stress: a prospective cohort study. Front Nutr. 2022;9:850249. doi:10.3389/fnut.2022.850249
52. Romano S, Savva GM, Bedarf JR, et al. Meta-analysis of the Parkinson’s disease gut microbiome suggests alterations linked to intestinal inflammation. NPJ Parkinsons Dis. 2021;7(1):27. doi:10.1038/s41531-021-00156-z
53. Bourassa MW, Alim I, Bultman SJ, et al. Butyrate, neuroepigenetics and the gut microbiome: can a high fiber diet improve brain health? Neurosci Lett. 2016;625:56-63. doi:10.1016/j.neulet.2016.02.009
54. Matt SM, Allen JM, Lawson MA, et al. Butyrate and dietary soluble fiber improve neuroinflammation associated with aging in mice. Front Immunol. 2018;9:1832. doi:10.3389/fimmu.2018.01832
55. Mittal VA, Vargas T, Osborne KJ, et al. Exercise treatments for psychosis: a review. Curr Treat Options Psychiatry. 2017;4(2):152-166. doi:10.1007/s40501-017-0112-2
56. Estaki M, Pither J, Baumeister P, et al. Cardiorespiratory fitness as a predictor of intestinal microbial diversity and distinct metagenomic functions. Microbiome. 2016;4(1):42. doi:10.1186/s40168-016-0189-7
57. Karl JP, Margolis LM, Madslien EH, et al. Changes in intestinal microbiota composition and metabolism coincide with increased intestinal permeability in young adults under prolonged physiological stress. Am J Physiol-Gastrointest Liver Physiol. 2017;312(6):G559-G571. doi:10.1152/ajpgi.00066.2017
58. Claus SP, Guillou H, Ellero-Simatos S. The gut microbiota: a major player in the toxicity of environmental pollutants? NPJ Biofilms Microbiomes. 2016;2:16003. doi:10.1038/npjbiofilms.2016.3
Depression guidelines fall short in characterizing withdrawal
Previous research suggests that approximately half of patients who discontinue or decrease dosage of antidepressants experience withdrawal symptoms, wrote Anders Sørensen, MD, of Copenhagen University Hospital, and colleagues. These symptoms are diverse and may include flulike symptoms, fatigue, anxiety, and sensations of electric shock, they noted. Most withdrawal effects last for a few weeks, but some persist for months or years, sometimes described as persistent postwithdrawal disorder, they added.
“Symptoms of withdrawal and depression overlap considerably but constitute two fundamentally different clinical conditions, which makes it important to distinguish between the two,” the researchers emphasized.
In a study published in the Journal of Affective Disorders, the researchers identified 21 clinical practice guidelines (CPGs) for depression published between 1998 and 2022. The guidelines were published in the United Kingdom, the United States, Canada, Australia, Singapore, Ireland, and New Zealand. They compared descriptions of withdrawal from antidepressants and calculated the proportion of CPGs with different information.
Overall, 15 of the 21 studies in the review (71%) noted that antidepressants are associated with withdrawal symptoms, but less than half (43%) used the term “withdrawal symptoms,” or similar. Of the nine guidelines that mentioned withdrawal symptoms, five used the term interchangeably with “discontinuation symptoms” and six used the term “discontinuation symptoms” only when discussing antidepressant withdrawal. In addition, six CPGs specifically stated that patients who stop antidepressants can experience withdrawal symptoms, and five stated that these symptoms also can occur in patients who are reducing or tapering their doses.
The type of withdrawal symptoms was mentioned in 10 CPGs, and the other 11 had no information on potential withdrawal symptoms, the researchers noted. Of the CPGs that mentioned symptoms specifically associated with withdrawal, the number of potential symptoms ranged from 4 to 39.
“None of the CPGs provided an exhaustive list of the potential withdrawal symptoms identified in the research literature,” the researchers wrote in their discussion.
Only four of the guidelines (19%) mentioned the overlap in symptoms between withdrawal from antidepressants and depression relapse, and only one provided guidance on distinguishing between the two conditions. Most of the symptoms of withdrawal, when described, were characterized as mild, brief, or self-limiting, the researchers noted.
“Being in withdrawal is a fundamentally different clinical situation than experiencing relapse, requiring two distinctly different treatment approaches,” the researchers emphasized. “Withdrawal reactions that are more severe and longer lasting than currently defined in the CPGs could risk getting misinterpreted as relapse, potentially leading to resumed unnecessary long-term antidepressant treatment in some patients,” they added.
The findings were limited by several factors including the inclusion only of guidelines from English-speaking countries, which may limit generalizability, the researchers noted. Other potential limitations include the subjective judgments involved in creating different guidelines, they said.
However, the results support the need for improved CPGs that help clinicians distinguish potential withdrawal reactions from depression relapse, and the need for more research on optimal dose reduction strategies for antidepressants, they concluded.
The study received no outside funding. The researchers had no financial conflicts to disclose.
Previous research suggests that approximately half of patients who discontinue or decrease dosage of antidepressants experience withdrawal symptoms, wrote Anders Sørensen, MD, of Copenhagen University Hospital, and colleagues. These symptoms are diverse and may include flulike symptoms, fatigue, anxiety, and sensations of electric shock, they noted. Most withdrawal effects last for a few weeks, but some persist for months or years, sometimes described as persistent postwithdrawal disorder, they added.
“Symptoms of withdrawal and depression overlap considerably but constitute two fundamentally different clinical conditions, which makes it important to distinguish between the two,” the researchers emphasized.
In a study published in the Journal of Affective Disorders, the researchers identified 21 clinical practice guidelines (CPGs) for depression published between 1998 and 2022. The guidelines were published in the United Kingdom, the United States, Canada, Australia, Singapore, Ireland, and New Zealand. They compared descriptions of withdrawal from antidepressants and calculated the proportion of CPGs with different information.
Overall, 15 of the 21 studies in the review (71%) noted that antidepressants are associated with withdrawal symptoms, but less than half (43%) used the term “withdrawal symptoms,” or similar. Of the nine guidelines that mentioned withdrawal symptoms, five used the term interchangeably with “discontinuation symptoms” and six used the term “discontinuation symptoms” only when discussing antidepressant withdrawal. In addition, six CPGs specifically stated that patients who stop antidepressants can experience withdrawal symptoms, and five stated that these symptoms also can occur in patients who are reducing or tapering their doses.
The type of withdrawal symptoms was mentioned in 10 CPGs, and the other 11 had no information on potential withdrawal symptoms, the researchers noted. Of the CPGs that mentioned symptoms specifically associated with withdrawal, the number of potential symptoms ranged from 4 to 39.
“None of the CPGs provided an exhaustive list of the potential withdrawal symptoms identified in the research literature,” the researchers wrote in their discussion.
Only four of the guidelines (19%) mentioned the overlap in symptoms between withdrawal from antidepressants and depression relapse, and only one provided guidance on distinguishing between the two conditions. Most of the symptoms of withdrawal, when described, were characterized as mild, brief, or self-limiting, the researchers noted.
“Being in withdrawal is a fundamentally different clinical situation than experiencing relapse, requiring two distinctly different treatment approaches,” the researchers emphasized. “Withdrawal reactions that are more severe and longer lasting than currently defined in the CPGs could risk getting misinterpreted as relapse, potentially leading to resumed unnecessary long-term antidepressant treatment in some patients,” they added.
The findings were limited by several factors including the inclusion only of guidelines from English-speaking countries, which may limit generalizability, the researchers noted. Other potential limitations include the subjective judgments involved in creating different guidelines, they said.
However, the results support the need for improved CPGs that help clinicians distinguish potential withdrawal reactions from depression relapse, and the need for more research on optimal dose reduction strategies for antidepressants, they concluded.
The study received no outside funding. The researchers had no financial conflicts to disclose.
Previous research suggests that approximately half of patients who discontinue or decrease dosage of antidepressants experience withdrawal symptoms, wrote Anders Sørensen, MD, of Copenhagen University Hospital, and colleagues. These symptoms are diverse and may include flulike symptoms, fatigue, anxiety, and sensations of electric shock, they noted. Most withdrawal effects last for a few weeks, but some persist for months or years, sometimes described as persistent postwithdrawal disorder, they added.
“Symptoms of withdrawal and depression overlap considerably but constitute two fundamentally different clinical conditions, which makes it important to distinguish between the two,” the researchers emphasized.
In a study published in the Journal of Affective Disorders, the researchers identified 21 clinical practice guidelines (CPGs) for depression published between 1998 and 2022. The guidelines were published in the United Kingdom, the United States, Canada, Australia, Singapore, Ireland, and New Zealand. They compared descriptions of withdrawal from antidepressants and calculated the proportion of CPGs with different information.
Overall, 15 of the 21 studies in the review (71%) noted that antidepressants are associated with withdrawal symptoms, but less than half (43%) used the term “withdrawal symptoms,” or similar. Of the nine guidelines that mentioned withdrawal symptoms, five used the term interchangeably with “discontinuation symptoms” and six used the term “discontinuation symptoms” only when discussing antidepressant withdrawal. In addition, six CPGs specifically stated that patients who stop antidepressants can experience withdrawal symptoms, and five stated that these symptoms also can occur in patients who are reducing or tapering their doses.
The type of withdrawal symptoms was mentioned in 10 CPGs, and the other 11 had no information on potential withdrawal symptoms, the researchers noted. Of the CPGs that mentioned symptoms specifically associated with withdrawal, the number of potential symptoms ranged from 4 to 39.
“None of the CPGs provided an exhaustive list of the potential withdrawal symptoms identified in the research literature,” the researchers wrote in their discussion.
Only four of the guidelines (19%) mentioned the overlap in symptoms between withdrawal from antidepressants and depression relapse, and only one provided guidance on distinguishing between the two conditions. Most of the symptoms of withdrawal, when described, were characterized as mild, brief, or self-limiting, the researchers noted.
“Being in withdrawal is a fundamentally different clinical situation than experiencing relapse, requiring two distinctly different treatment approaches,” the researchers emphasized. “Withdrawal reactions that are more severe and longer lasting than currently defined in the CPGs could risk getting misinterpreted as relapse, potentially leading to resumed unnecessary long-term antidepressant treatment in some patients,” they added.
The findings were limited by several factors including the inclusion only of guidelines from English-speaking countries, which may limit generalizability, the researchers noted. Other potential limitations include the subjective judgments involved in creating different guidelines, they said.
However, the results support the need for improved CPGs that help clinicians distinguish potential withdrawal reactions from depression relapse, and the need for more research on optimal dose reduction strategies for antidepressants, they concluded.
The study received no outside funding. The researchers had no financial conflicts to disclose.
FROM THE JOURNAL OF AFFECTIVE DISORDERS
Depression and schizophrenia: Many biological and clinical similarities
Clinicians generally regard major depressive disorder (MDD) and schizophrenia as 2 separate and distinct psychiatric brain disorders. However, despite some differences, those 2 psychiatric syndromes have numerous similarities across clinical features and neurobiologic parameters.
Biological similarities
Both disorders share the following variables:
- Highly genetic in etiology but with environmental influences and epigenetics
- Associated with childhood maltreatment, abuse, or neglect
- Disrupted neuroplasticity, especially shrinkage in hippocampal volume
- Significant drop in brain-derived neurotrophic factor resulting in decreased neurogenesis
- Extensive white matter pathology across interhemispheric and intrahemispheric bundles
- Increased levels of serum cortisol, a stress hormone and inflammatory biomarker
- Hypofrontal cerebral blood flow during acute episodes of both MDD and schizophrenia
- Reduced dendritic spines (in number and size) and impaired experiential neuroplasticity
- Neuroinflammation (eg, cytokines, tumor necrosis factor-alpha, C-reactive protein) during acute episodes
- Elevated oxidative stress biomarkers, indicating an increase in free radicals
- Overactive default mode network associated with ruminations in MDD and “daydreaming” in schizophrenia
- Decrease in gamma-aminobutyric acid (GABA) and its inhibitory activity, translating into dysregulation of glutamatergic pathways and other neurotransmitters
- Immune dysregulation and comorbid autoimmune disorders
Clinical similarities
- Psychotic symptoms, especially delusional thinking such as paranoia in schizophrenia and severe self-deprecation in MDD
- Significantly elevated lifetime suicide risk
- Cognitive impairment (more severe in schizophrenia across several cognitive functions)
- Similarity of depressive and negative symptoms (especially anhedonia, apathy, restricted facial expression, social withdrawal)
- Antidepressant medications im-prove depressive and negative symptoms (though not completely in the case of negative symptoms of schizophrenia)
- Both have treatment-resistant subtypes that fail to respond to standard therapies
- Both are associated with comorbid generalized anxiety disorder
- Both are associated with comorbid obsessive-compulsive disorder
- Both are associated with serious alcohol and drug use
- Early mortality from general medical conditions, especially cardiovascular risks due to obesity, diabetes, hypertension, dyslipidemia
- Elevated risk of dementia with aging compared to the unaffected general population
- Opioids improve MDD and psychosis (buprenorphine in MDD and morphine in schizophrenia)
- Several second-generation antipsychotic medications are approved for both MDD and schizophrenia
- Electroconvulsive therapy is effective when pharmacotherapy fails in both MDD and schizophrenia
Biological differences
- Glutamate N-methyl-D-aspartate receptor antagonists (eg, ketamine) improve MDD but worsen schizophrenia
- Muscarinic agonists improve psychosis but worsen depression
- High pain threshold in schizophrenia (pain insensitivity) and low threshold in MDD (in which pain is a common comorbidity)
- Cortical thinning more severe in schizophrenia
- Hippocampal atrophy is reversible with successful treatment in MDD but not in schizophrenia
- Hypofrontality is reversible with remission in MDD but not in schizophrenia
Clinical differences
- Auditory and visual hallucinations are more common in schizophrenia than in MDD
- Anosognosia is common in schizophrenia but not in MDD
- Implausible delusions are more common in schizophrenia than in MDD
- Mood-congruent delusions are more common in MDD than in schizophrenia
- Sadness, crying, pessimism, and self-deprecation are common in MDD but not in schizophrenia
- Achieving full remission is more common in MDD than in schizophrenia
- Long-acting injectable medications are available for schizophrenia but not for MDD
- Evidence-based psychotherapy, without pharmacotherapy, is more likely to be effective in MDD than in schizophrenia
A transdiagnostic model of psychopathology
The significant overlap between MDD and schizophrenia should not be surprising. They are both generated by the same organ, the human brain, with disrupted neurochemical and physiological circuits in the brain.
The overlap is also consistent with the emerging transdiagnostic model of psychopathology.1-9 This model proposes that there is a “core” genetic risk for psychopathology with different iterations. The transdiagnostic model is in stark contrast to the prevailing DSM-5, which categorizes psychiatric disorders in “silos,” as if they are completely independent from each other despite many shared features. This is highly debatable according to the substantial evidence that multiple psychiatric disorders share many genes that influence brain development in utero and predispose individuals to a variety of clinical symptoms in adolescence and young adulthood.
The origin of mental illness is being disentangled by emerging research, which is identifying the common links among the various disorders currently listed in DSM-5.10 However, the evolution of psychiatric diagnosis has come full circle from a single entity before DSM, to multiple entities with DSM, and now back to a unified transdiagnostic model that is rapidly emerging.11 This has implications for the FDA’s persistent dogma that clinical trials for new drugs must be targeted for 1 of the DSM-5 categories, a flawed and narrow assumption. Given the accelerating body of evidence for a unified, transdiagnostic model, it makes much more sense for the FDA to approve medications that target a psychiatric symptom that is shared by multiple psychiatric conditions within a transdiagnostic clinical system. When medications are approved for a symptom regardless of a DSM diagnosis, the term “off-label” and its “stigma” will then fade into history, along with the malignant preauthorization racket that was invented by greedy insurance companies that exploit the off-label use of medications (even when an FDA-approved medication for the patient’s condition does not yet exist) simply to deny coverage, lower their expenses, and fatten their profits.
1. Goodkind M, Eickhoff SB, Oathes DJ, et al. Identification of a common neurobiological substrate for mental illness. JAMA Psychiatry. 2015;72(4):305-315.
2. Caspi A, Moffitt TE. All for one and one for all: mental disorders in one dimension. Am J Psychiatry. 2018;175(9):831-844.
3. Krueger RF, Easton NR. Transdiagnostic factors in mental disorders. World Psychiatry. 2015;14(1):27-29.
4. Hyman SE. New evidence for shared risk architecture for mental disorders. JAMA Psychiatry. 2019;76(3):235-236.
5. Selzam S, Coleman JRI, Caspi A, et al. A polygenic p factor for major psychiatric disorders. Translational Psychiatry. 2018;8(1):205.
6. Barch DM. What it means to be transdiagnostic and how do we know? Am J Psychiatry. 2020;177(5):370-372.
7. Nasrallah HA. Is there only 1 neurobiologic psychiatric disorder with different clinical expressions? Current Psychiatry. 2015;14(7):10-12.
8. Nasrallah HA. Pleiotropy of psychiatric disorders will reinvent DSM. Current Psychiatry. 2013;12(4):6-7.
9. Nasrallah HA. Beyond DSM-5: clinical and biological features shared by major psychiatric syndromes. Current Psychiatry. 2017;16(10):4-7.
10. Marshall M. Roots of mental illness: researchers are beginning to untangle the common biology that links supposedly distinct psychiatric conditions. Nature. 2020;581:19-21.
11. Kendler KS. From many to one to many--the search for causes of psychiatric illness. JAMA Psychiatry. 2019;76(10):1085-1091.
Clinicians generally regard major depressive disorder (MDD) and schizophrenia as 2 separate and distinct psychiatric brain disorders. However, despite some differences, those 2 psychiatric syndromes have numerous similarities across clinical features and neurobiologic parameters.
Biological similarities
Both disorders share the following variables:
- Highly genetic in etiology but with environmental influences and epigenetics
- Associated with childhood maltreatment, abuse, or neglect
- Disrupted neuroplasticity, especially shrinkage in hippocampal volume
- Significant drop in brain-derived neurotrophic factor resulting in decreased neurogenesis
- Extensive white matter pathology across interhemispheric and intrahemispheric bundles
- Increased levels of serum cortisol, a stress hormone and inflammatory biomarker
- Hypofrontal cerebral blood flow during acute episodes of both MDD and schizophrenia
- Reduced dendritic spines (in number and size) and impaired experiential neuroplasticity
- Neuroinflammation (eg, cytokines, tumor necrosis factor-alpha, C-reactive protein) during acute episodes
- Elevated oxidative stress biomarkers, indicating an increase in free radicals
- Overactive default mode network associated with ruminations in MDD and “daydreaming” in schizophrenia
- Decrease in gamma-aminobutyric acid (GABA) and its inhibitory activity, translating into dysregulation of glutamatergic pathways and other neurotransmitters
- Immune dysregulation and comorbid autoimmune disorders
Clinical similarities
- Psychotic symptoms, especially delusional thinking such as paranoia in schizophrenia and severe self-deprecation in MDD
- Significantly elevated lifetime suicide risk
- Cognitive impairment (more severe in schizophrenia across several cognitive functions)
- Similarity of depressive and negative symptoms (especially anhedonia, apathy, restricted facial expression, social withdrawal)
- Antidepressant medications im-prove depressive and negative symptoms (though not completely in the case of negative symptoms of schizophrenia)
- Both have treatment-resistant subtypes that fail to respond to standard therapies
- Both are associated with comorbid generalized anxiety disorder
- Both are associated with comorbid obsessive-compulsive disorder
- Both are associated with serious alcohol and drug use
- Early mortality from general medical conditions, especially cardiovascular risks due to obesity, diabetes, hypertension, dyslipidemia
- Elevated risk of dementia with aging compared to the unaffected general population
- Opioids improve MDD and psychosis (buprenorphine in MDD and morphine in schizophrenia)
- Several second-generation antipsychotic medications are approved for both MDD and schizophrenia
- Electroconvulsive therapy is effective when pharmacotherapy fails in both MDD and schizophrenia
Biological differences
- Glutamate N-methyl-D-aspartate receptor antagonists (eg, ketamine) improve MDD but worsen schizophrenia
- Muscarinic agonists improve psychosis but worsen depression
- High pain threshold in schizophrenia (pain insensitivity) and low threshold in MDD (in which pain is a common comorbidity)
- Cortical thinning more severe in schizophrenia
- Hippocampal atrophy is reversible with successful treatment in MDD but not in schizophrenia
- Hypofrontality is reversible with remission in MDD but not in schizophrenia
Clinical differences
- Auditory and visual hallucinations are more common in schizophrenia than in MDD
- Anosognosia is common in schizophrenia but not in MDD
- Implausible delusions are more common in schizophrenia than in MDD
- Mood-congruent delusions are more common in MDD than in schizophrenia
- Sadness, crying, pessimism, and self-deprecation are common in MDD but not in schizophrenia
- Achieving full remission is more common in MDD than in schizophrenia
- Long-acting injectable medications are available for schizophrenia but not for MDD
- Evidence-based psychotherapy, without pharmacotherapy, is more likely to be effective in MDD than in schizophrenia
A transdiagnostic model of psychopathology
The significant overlap between MDD and schizophrenia should not be surprising. They are both generated by the same organ, the human brain, with disrupted neurochemical and physiological circuits in the brain.
The overlap is also consistent with the emerging transdiagnostic model of psychopathology.1-9 This model proposes that there is a “core” genetic risk for psychopathology with different iterations. The transdiagnostic model is in stark contrast to the prevailing DSM-5, which categorizes psychiatric disorders in “silos,” as if they are completely independent from each other despite many shared features. This is highly debatable according to the substantial evidence that multiple psychiatric disorders share many genes that influence brain development in utero and predispose individuals to a variety of clinical symptoms in adolescence and young adulthood.
The origin of mental illness is being disentangled by emerging research, which is identifying the common links among the various disorders currently listed in DSM-5.10 However, the evolution of psychiatric diagnosis has come full circle from a single entity before DSM, to multiple entities with DSM, and now back to a unified transdiagnostic model that is rapidly emerging.11 This has implications for the FDA’s persistent dogma that clinical trials for new drugs must be targeted for 1 of the DSM-5 categories, a flawed and narrow assumption. Given the accelerating body of evidence for a unified, transdiagnostic model, it makes much more sense for the FDA to approve medications that target a psychiatric symptom that is shared by multiple psychiatric conditions within a transdiagnostic clinical system. When medications are approved for a symptom regardless of a DSM diagnosis, the term “off-label” and its “stigma” will then fade into history, along with the malignant preauthorization racket that was invented by greedy insurance companies that exploit the off-label use of medications (even when an FDA-approved medication for the patient’s condition does not yet exist) simply to deny coverage, lower their expenses, and fatten their profits.
Clinicians generally regard major depressive disorder (MDD) and schizophrenia as 2 separate and distinct psychiatric brain disorders. However, despite some differences, those 2 psychiatric syndromes have numerous similarities across clinical features and neurobiologic parameters.
Biological similarities
Both disorders share the following variables:
- Highly genetic in etiology but with environmental influences and epigenetics
- Associated with childhood maltreatment, abuse, or neglect
- Disrupted neuroplasticity, especially shrinkage in hippocampal volume
- Significant drop in brain-derived neurotrophic factor resulting in decreased neurogenesis
- Extensive white matter pathology across interhemispheric and intrahemispheric bundles
- Increased levels of serum cortisol, a stress hormone and inflammatory biomarker
- Hypofrontal cerebral blood flow during acute episodes of both MDD and schizophrenia
- Reduced dendritic spines (in number and size) and impaired experiential neuroplasticity
- Neuroinflammation (eg, cytokines, tumor necrosis factor-alpha, C-reactive protein) during acute episodes
- Elevated oxidative stress biomarkers, indicating an increase in free radicals
- Overactive default mode network associated with ruminations in MDD and “daydreaming” in schizophrenia
- Decrease in gamma-aminobutyric acid (GABA) and its inhibitory activity, translating into dysregulation of glutamatergic pathways and other neurotransmitters
- Immune dysregulation and comorbid autoimmune disorders
Clinical similarities
- Psychotic symptoms, especially delusional thinking such as paranoia in schizophrenia and severe self-deprecation in MDD
- Significantly elevated lifetime suicide risk
- Cognitive impairment (more severe in schizophrenia across several cognitive functions)
- Similarity of depressive and negative symptoms (especially anhedonia, apathy, restricted facial expression, social withdrawal)
- Antidepressant medications im-prove depressive and negative symptoms (though not completely in the case of negative symptoms of schizophrenia)
- Both have treatment-resistant subtypes that fail to respond to standard therapies
- Both are associated with comorbid generalized anxiety disorder
- Both are associated with comorbid obsessive-compulsive disorder
- Both are associated with serious alcohol and drug use
- Early mortality from general medical conditions, especially cardiovascular risks due to obesity, diabetes, hypertension, dyslipidemia
- Elevated risk of dementia with aging compared to the unaffected general population
- Opioids improve MDD and psychosis (buprenorphine in MDD and morphine in schizophrenia)
- Several second-generation antipsychotic medications are approved for both MDD and schizophrenia
- Electroconvulsive therapy is effective when pharmacotherapy fails in both MDD and schizophrenia
Biological differences
- Glutamate N-methyl-D-aspartate receptor antagonists (eg, ketamine) improve MDD but worsen schizophrenia
- Muscarinic agonists improve psychosis but worsen depression
- High pain threshold in schizophrenia (pain insensitivity) and low threshold in MDD (in which pain is a common comorbidity)
- Cortical thinning more severe in schizophrenia
- Hippocampal atrophy is reversible with successful treatment in MDD but not in schizophrenia
- Hypofrontality is reversible with remission in MDD but not in schizophrenia
Clinical differences
- Auditory and visual hallucinations are more common in schizophrenia than in MDD
- Anosognosia is common in schizophrenia but not in MDD
- Implausible delusions are more common in schizophrenia than in MDD
- Mood-congruent delusions are more common in MDD than in schizophrenia
- Sadness, crying, pessimism, and self-deprecation are common in MDD but not in schizophrenia
- Achieving full remission is more common in MDD than in schizophrenia
- Long-acting injectable medications are available for schizophrenia but not for MDD
- Evidence-based psychotherapy, without pharmacotherapy, is more likely to be effective in MDD than in schizophrenia
A transdiagnostic model of psychopathology
The significant overlap between MDD and schizophrenia should not be surprising. They are both generated by the same organ, the human brain, with disrupted neurochemical and physiological circuits in the brain.
The overlap is also consistent with the emerging transdiagnostic model of psychopathology.1-9 This model proposes that there is a “core” genetic risk for psychopathology with different iterations. The transdiagnostic model is in stark contrast to the prevailing DSM-5, which categorizes psychiatric disorders in “silos,” as if they are completely independent from each other despite many shared features. This is highly debatable according to the substantial evidence that multiple psychiatric disorders share many genes that influence brain development in utero and predispose individuals to a variety of clinical symptoms in adolescence and young adulthood.
The origin of mental illness is being disentangled by emerging research, which is identifying the common links among the various disorders currently listed in DSM-5.10 However, the evolution of psychiatric diagnosis has come full circle from a single entity before DSM, to multiple entities with DSM, and now back to a unified transdiagnostic model that is rapidly emerging.11 This has implications for the FDA’s persistent dogma that clinical trials for new drugs must be targeted for 1 of the DSM-5 categories, a flawed and narrow assumption. Given the accelerating body of evidence for a unified, transdiagnostic model, it makes much more sense for the FDA to approve medications that target a psychiatric symptom that is shared by multiple psychiatric conditions within a transdiagnostic clinical system. When medications are approved for a symptom regardless of a DSM diagnosis, the term “off-label” and its “stigma” will then fade into history, along with the malignant preauthorization racket that was invented by greedy insurance companies that exploit the off-label use of medications (even when an FDA-approved medication for the patient’s condition does not yet exist) simply to deny coverage, lower their expenses, and fatten their profits.
1. Goodkind M, Eickhoff SB, Oathes DJ, et al. Identification of a common neurobiological substrate for mental illness. JAMA Psychiatry. 2015;72(4):305-315.
2. Caspi A, Moffitt TE. All for one and one for all: mental disorders in one dimension. Am J Psychiatry. 2018;175(9):831-844.
3. Krueger RF, Easton NR. Transdiagnostic factors in mental disorders. World Psychiatry. 2015;14(1):27-29.
4. Hyman SE. New evidence for shared risk architecture for mental disorders. JAMA Psychiatry. 2019;76(3):235-236.
5. Selzam S, Coleman JRI, Caspi A, et al. A polygenic p factor for major psychiatric disorders. Translational Psychiatry. 2018;8(1):205.
6. Barch DM. What it means to be transdiagnostic and how do we know? Am J Psychiatry. 2020;177(5):370-372.
7. Nasrallah HA. Is there only 1 neurobiologic psychiatric disorder with different clinical expressions? Current Psychiatry. 2015;14(7):10-12.
8. Nasrallah HA. Pleiotropy of psychiatric disorders will reinvent DSM. Current Psychiatry. 2013;12(4):6-7.
9. Nasrallah HA. Beyond DSM-5: clinical and biological features shared by major psychiatric syndromes. Current Psychiatry. 2017;16(10):4-7.
10. Marshall M. Roots of mental illness: researchers are beginning to untangle the common biology that links supposedly distinct psychiatric conditions. Nature. 2020;581:19-21.
11. Kendler KS. From many to one to many--the search for causes of psychiatric illness. JAMA Psychiatry. 2019;76(10):1085-1091.
1. Goodkind M, Eickhoff SB, Oathes DJ, et al. Identification of a common neurobiological substrate for mental illness. JAMA Psychiatry. 2015;72(4):305-315.
2. Caspi A, Moffitt TE. All for one and one for all: mental disorders in one dimension. Am J Psychiatry. 2018;175(9):831-844.
3. Krueger RF, Easton NR. Transdiagnostic factors in mental disorders. World Psychiatry. 2015;14(1):27-29.
4. Hyman SE. New evidence for shared risk architecture for mental disorders. JAMA Psychiatry. 2019;76(3):235-236.
5. Selzam S, Coleman JRI, Caspi A, et al. A polygenic p factor for major psychiatric disorders. Translational Psychiatry. 2018;8(1):205.
6. Barch DM. What it means to be transdiagnostic and how do we know? Am J Psychiatry. 2020;177(5):370-372.
7. Nasrallah HA. Is there only 1 neurobiologic psychiatric disorder with different clinical expressions? Current Psychiatry. 2015;14(7):10-12.
8. Nasrallah HA. Pleiotropy of psychiatric disorders will reinvent DSM. Current Psychiatry. 2013;12(4):6-7.
9. Nasrallah HA. Beyond DSM-5: clinical and biological features shared by major psychiatric syndromes. Current Psychiatry. 2017;16(10):4-7.
10. Marshall M. Roots of mental illness: researchers are beginning to untangle the common biology that links supposedly distinct psychiatric conditions. Nature. 2020;581:19-21.
11. Kendler KS. From many to one to many--the search for causes of psychiatric illness. JAMA Psychiatry. 2019;76(10):1085-1091.