User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'main-prefix')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
Disability in medicine: My experience
What does a doctor look like? Throughout history, this concept has shifted due to societal norms and increased access to medical education. Today, the idea of a physician has expanded to incorporate a myriad of people; however, stigma still exists in medicine regarding mental illness and disability. I would like to share my personal journey through high school, college, medical school, and now residency, and how my identity and struggles have shaped me into the physician I am today. There are few conversations around disability—especially disability and mental health—in medicine, and through my own advocacy, I have met many students with disability who feel that medical school is unattainable. Additionally, I have met many medical students, residents, and pre-health advisors who are happy for the experience to learn more about a marginalized group in medicine. My hope in sharing my story is to offer a space for conversation about intersectionality within medical communities and how physicians and physicians in training can facilitate that change, regardless of their position or specialty. Additionally, I hope to shed light on the unique mental health needs of patients with disabilities and how mental health clinicians can address those needs.
Perceived weaknesses turned into strengths
“Why do you walk like that?” “What is that brace on your leg?” The early years of my childhood were marked by these questions and others like them. I was the kid with the limp, the kid with a brace on his leg, and the kid who disappeared multiple times a week for doctor’s appointments or physical therapy. I learned to deflect these questions or give nebulous answers about an accident or injury. The reality is that I was born with cerebral palsy (CP). My CP manifested as hemiparesis on the left side of my body. I was in aggressive physical therapy throughout childhood, received Botox injections for muscle spasticity, and underwent corrective surgery on my left leg to straighten my foot. In childhood, the diagnosis meant nothing more than 2 words that sounded like they belonged to superheroes in comic books. Even with supportive parents and family, I kept my disability a secret, much like the powers and abilities of my favorite superheroes.
However, like all great origin stories, what I once thought were weaknesses turned out to be strengths that pushed me through college, medical school, and now psychiatry residency. Living with a disability has shaped how I see the world and relate to my patients. My experience has helped me connect to my patients in ways others might not. These properties are important in any physician but vital in psychiatry, where many patients feel neglected or stigmatized; this is another reason there should be more doctors with disabilities in medicine. Unfortunately, systemic barriers are still in place that disincentivize those with a disability from pursuing careers in medicine. Stories like mine are important to inspire a reexamination of what a physician should be and how medicine, patients, and communities benefit from this change.
My experience through medical school
My path to psychiatry and residency was shaped by my early experience with the medical field and treatment. From the early days of my diagnosis at age 4, I was told that my brain was “wired differently” and that, because of this disruption in circuitry, I would have difficulty with physical activity. I grew to appreciate the intricacies of the brain and pathology to understand my body. With greater understanding came the existential realization that I would live with a disability for the rest of my life. Rather than dream of a future where I would be “normal,” I focused on adapting my life to my normal. An unfortunate reality of this normal was that no doctor would be able to relate to me, and my health care would focus on limitations rather than possibilities.
I focused on school as a distraction and slowly warmed to the idea of pursuing medicine as a career. The seed was planted years prior by the numerous doctors’ visits and procedures, and was cultivated by a desire to understand pathologies and offer treatment to patients from the perspective of a patient. When I applied to medical school, I did not know how to address my CP. Living as a person with CP was a core reason for my decision to pursue medicine, but I was afraid that a disclosure of disability would preclude any admission to medical school. Research into programs offered little guidance because most institutions only listed vague “physical expectations” of each student. There were times I doubted if I would be accepted anywhere. Many programs I reached out to about my situation seemed unenthusiastic about the prospect of a student with CP, and when I brought up my CP in interviews, the reaction was often of surprise and an admission that they had forgotten about “that part” of my application. Fortunately, I was accepted to medical school, but still struggled with the fear that one day I would be found out and not allowed to continue. No one in my class or school was like me, and a meeting with an Americans with Disabilities Act coordinator who asked me to reexamine the physical competencies of the school before advancing to clinical clerkships only further reinforced this fear. I decided to fly under the radar and not say anything about my disability to my attendings. I slowly worked my way through clerkships by making do with adapted ways to perform procedures and exams with additional practice and maneuvering at home. I found myself drawn to psychiatry because of the similarities I saw in the patients and myself. I empathized with how the patients struggled with chronic conditions that left them feeling separated from society and how they felt that their diagnosis was something they needed to hide. When medical school ended and I decided to pursue psychiatry, I wanted to share my story to inspire others with a disability to consider medicine as a career given their unique experiences. My experience thus far has been uplifting as my journey has echoed so many others.
A need for greater representation
Disability representation in medicine is needed more than ever. According to the CDC, >60 million adults in the United States (1 in 4) live with a disability.1 Although the physical health disparities are often discussed, there is less conversation surrounding mental health for individuals with disabilities. A 2018 study by Cree et al2 found that approximately 17.4 million adults with disabilities experienced frequent mental distress, defined as reporting ≥14 mentally unhealthy days in the past 30 days. Furthermore, compared to individuals without a disability, those with a disability are statistically more likely to have suicidal ideation, suicidal planning, and suicide attempts.3 One way to address this disparity is to recruit medical students with disabilities to become physicians with disabilities. Evidence suggests that physicians who are members of groups that are underrepresented in medicine are more likely to deliver care to underrepresented patients.4 However, medical schools and institutions have been slow to address the disparity. A 2019 survey found an estimated 4.6% of medical students responded “yes” when asked if they had a disability, with most students reporting a psychological or attention/hyperactive disorder.5 Existing barriers include restrictive language surrounding technical standards influenced by long-standing vestiges of what a physician should be.6
An opportunity to connect with patients
I now do not see myself as having a secret identity to hide. Although my CP does not give me any superpowers, it has given me the opportunity to connect with my patients and serve as an example of why medical school recruitment and admissions should expand. Psychiatrists have been on the forefront of change in medicine and can shift the perception of a physician. In doing so, we not only enrich our field but also the lives of our patients who may need it most.
1. Okoro CA, Hollis ND, Cyrus AC, et al. Prevalence of disabilities and health care access by disability status and type among adults—United States, 2016. MMWR Morb Mortal Wkly Rep. 2018;67(32):882-887.
2. Cree RA, Okoro CA, Zack MM, et al. Frequent mental distress among adults, by disability status, disability type, and selected characteristics—United States 2018. MMWR Morb Mortal Wkly Rep. 2020;69(36):1238-1243.
3. Marlow NM, Xie Z, Tanner R, et al. Association between disability and suicide-related outcomes among US adults. Am J Prev Med. 2021;61(6):852-862.
4. Thurmond VB, Kirch DG. Impact of minority physicians on health care. South Med J. 1998;91(11):1009-1013.
5. Meeks LM, Case B, Herzer K, et al. Change in prevalence of disabilities and accommodation practices among US medical schools, 2016 vs 2019. JAMA. 2019;322(20):2022-2024.
6. Stauffer C, Case B, Moreland CJ, et al. Technical standards from newly established medical schools: a review of disability inclusive practices. J Med Educ Curric Dev. 2022;9:23821205211072763.
What does a doctor look like? Throughout history, this concept has shifted due to societal norms and increased access to medical education. Today, the idea of a physician has expanded to incorporate a myriad of people; however, stigma still exists in medicine regarding mental illness and disability. I would like to share my personal journey through high school, college, medical school, and now residency, and how my identity and struggles have shaped me into the physician I am today. There are few conversations around disability—especially disability and mental health—in medicine, and through my own advocacy, I have met many students with disability who feel that medical school is unattainable. Additionally, I have met many medical students, residents, and pre-health advisors who are happy for the experience to learn more about a marginalized group in medicine. My hope in sharing my story is to offer a space for conversation about intersectionality within medical communities and how physicians and physicians in training can facilitate that change, regardless of their position or specialty. Additionally, I hope to shed light on the unique mental health needs of patients with disabilities and how mental health clinicians can address those needs.
Perceived weaknesses turned into strengths
“Why do you walk like that?” “What is that brace on your leg?” The early years of my childhood were marked by these questions and others like them. I was the kid with the limp, the kid with a brace on his leg, and the kid who disappeared multiple times a week for doctor’s appointments or physical therapy. I learned to deflect these questions or give nebulous answers about an accident or injury. The reality is that I was born with cerebral palsy (CP). My CP manifested as hemiparesis on the left side of my body. I was in aggressive physical therapy throughout childhood, received Botox injections for muscle spasticity, and underwent corrective surgery on my left leg to straighten my foot. In childhood, the diagnosis meant nothing more than 2 words that sounded like they belonged to superheroes in comic books. Even with supportive parents and family, I kept my disability a secret, much like the powers and abilities of my favorite superheroes.
However, like all great origin stories, what I once thought were weaknesses turned out to be strengths that pushed me through college, medical school, and now psychiatry residency. Living with a disability has shaped how I see the world and relate to my patients. My experience has helped me connect to my patients in ways others might not. These properties are important in any physician but vital in psychiatry, where many patients feel neglected or stigmatized; this is another reason there should be more doctors with disabilities in medicine. Unfortunately, systemic barriers are still in place that disincentivize those with a disability from pursuing careers in medicine. Stories like mine are important to inspire a reexamination of what a physician should be and how medicine, patients, and communities benefit from this change.
My experience through medical school
My path to psychiatry and residency was shaped by my early experience with the medical field and treatment. From the early days of my diagnosis at age 4, I was told that my brain was “wired differently” and that, because of this disruption in circuitry, I would have difficulty with physical activity. I grew to appreciate the intricacies of the brain and pathology to understand my body. With greater understanding came the existential realization that I would live with a disability for the rest of my life. Rather than dream of a future where I would be “normal,” I focused on adapting my life to my normal. An unfortunate reality of this normal was that no doctor would be able to relate to me, and my health care would focus on limitations rather than possibilities.
I focused on school as a distraction and slowly warmed to the idea of pursuing medicine as a career. The seed was planted years prior by the numerous doctors’ visits and procedures, and was cultivated by a desire to understand pathologies and offer treatment to patients from the perspective of a patient. When I applied to medical school, I did not know how to address my CP. Living as a person with CP was a core reason for my decision to pursue medicine, but I was afraid that a disclosure of disability would preclude any admission to medical school. Research into programs offered little guidance because most institutions only listed vague “physical expectations” of each student. There were times I doubted if I would be accepted anywhere. Many programs I reached out to about my situation seemed unenthusiastic about the prospect of a student with CP, and when I brought up my CP in interviews, the reaction was often of surprise and an admission that they had forgotten about “that part” of my application. Fortunately, I was accepted to medical school, but still struggled with the fear that one day I would be found out and not allowed to continue. No one in my class or school was like me, and a meeting with an Americans with Disabilities Act coordinator who asked me to reexamine the physical competencies of the school before advancing to clinical clerkships only further reinforced this fear. I decided to fly under the radar and not say anything about my disability to my attendings. I slowly worked my way through clerkships by making do with adapted ways to perform procedures and exams with additional practice and maneuvering at home. I found myself drawn to psychiatry because of the similarities I saw in the patients and myself. I empathized with how the patients struggled with chronic conditions that left them feeling separated from society and how they felt that their diagnosis was something they needed to hide. When medical school ended and I decided to pursue psychiatry, I wanted to share my story to inspire others with a disability to consider medicine as a career given their unique experiences. My experience thus far has been uplifting as my journey has echoed so many others.
A need for greater representation
Disability representation in medicine is needed more than ever. According to the CDC, >60 million adults in the United States (1 in 4) live with a disability.1 Although the physical health disparities are often discussed, there is less conversation surrounding mental health for individuals with disabilities. A 2018 study by Cree et al2 found that approximately 17.4 million adults with disabilities experienced frequent mental distress, defined as reporting ≥14 mentally unhealthy days in the past 30 days. Furthermore, compared to individuals without a disability, those with a disability are statistically more likely to have suicidal ideation, suicidal planning, and suicide attempts.3 One way to address this disparity is to recruit medical students with disabilities to become physicians with disabilities. Evidence suggests that physicians who are members of groups that are underrepresented in medicine are more likely to deliver care to underrepresented patients.4 However, medical schools and institutions have been slow to address the disparity. A 2019 survey found an estimated 4.6% of medical students responded “yes” when asked if they had a disability, with most students reporting a psychological or attention/hyperactive disorder.5 Existing barriers include restrictive language surrounding technical standards influenced by long-standing vestiges of what a physician should be.6
An opportunity to connect with patients
I now do not see myself as having a secret identity to hide. Although my CP does not give me any superpowers, it has given me the opportunity to connect with my patients and serve as an example of why medical school recruitment and admissions should expand. Psychiatrists have been on the forefront of change in medicine and can shift the perception of a physician. In doing so, we not only enrich our field but also the lives of our patients who may need it most.
What does a doctor look like? Throughout history, this concept has shifted due to societal norms and increased access to medical education. Today, the idea of a physician has expanded to incorporate a myriad of people; however, stigma still exists in medicine regarding mental illness and disability. I would like to share my personal journey through high school, college, medical school, and now residency, and how my identity and struggles have shaped me into the physician I am today. There are few conversations around disability—especially disability and mental health—in medicine, and through my own advocacy, I have met many students with disability who feel that medical school is unattainable. Additionally, I have met many medical students, residents, and pre-health advisors who are happy for the experience to learn more about a marginalized group in medicine. My hope in sharing my story is to offer a space for conversation about intersectionality within medical communities and how physicians and physicians in training can facilitate that change, regardless of their position or specialty. Additionally, I hope to shed light on the unique mental health needs of patients with disabilities and how mental health clinicians can address those needs.
Perceived weaknesses turned into strengths
“Why do you walk like that?” “What is that brace on your leg?” The early years of my childhood were marked by these questions and others like them. I was the kid with the limp, the kid with a brace on his leg, and the kid who disappeared multiple times a week for doctor’s appointments or physical therapy. I learned to deflect these questions or give nebulous answers about an accident or injury. The reality is that I was born with cerebral palsy (CP). My CP manifested as hemiparesis on the left side of my body. I was in aggressive physical therapy throughout childhood, received Botox injections for muscle spasticity, and underwent corrective surgery on my left leg to straighten my foot. In childhood, the diagnosis meant nothing more than 2 words that sounded like they belonged to superheroes in comic books. Even with supportive parents and family, I kept my disability a secret, much like the powers and abilities of my favorite superheroes.
However, like all great origin stories, what I once thought were weaknesses turned out to be strengths that pushed me through college, medical school, and now psychiatry residency. Living with a disability has shaped how I see the world and relate to my patients. My experience has helped me connect to my patients in ways others might not. These properties are important in any physician but vital in psychiatry, where many patients feel neglected or stigmatized; this is another reason there should be more doctors with disabilities in medicine. Unfortunately, systemic barriers are still in place that disincentivize those with a disability from pursuing careers in medicine. Stories like mine are important to inspire a reexamination of what a physician should be and how medicine, patients, and communities benefit from this change.
My experience through medical school
My path to psychiatry and residency was shaped by my early experience with the medical field and treatment. From the early days of my diagnosis at age 4, I was told that my brain was “wired differently” and that, because of this disruption in circuitry, I would have difficulty with physical activity. I grew to appreciate the intricacies of the brain and pathology to understand my body. With greater understanding came the existential realization that I would live with a disability for the rest of my life. Rather than dream of a future where I would be “normal,” I focused on adapting my life to my normal. An unfortunate reality of this normal was that no doctor would be able to relate to me, and my health care would focus on limitations rather than possibilities.
I focused on school as a distraction and slowly warmed to the idea of pursuing medicine as a career. The seed was planted years prior by the numerous doctors’ visits and procedures, and was cultivated by a desire to understand pathologies and offer treatment to patients from the perspective of a patient. When I applied to medical school, I did not know how to address my CP. Living as a person with CP was a core reason for my decision to pursue medicine, but I was afraid that a disclosure of disability would preclude any admission to medical school. Research into programs offered little guidance because most institutions only listed vague “physical expectations” of each student. There were times I doubted if I would be accepted anywhere. Many programs I reached out to about my situation seemed unenthusiastic about the prospect of a student with CP, and when I brought up my CP in interviews, the reaction was often of surprise and an admission that they had forgotten about “that part” of my application. Fortunately, I was accepted to medical school, but still struggled with the fear that one day I would be found out and not allowed to continue. No one in my class or school was like me, and a meeting with an Americans with Disabilities Act coordinator who asked me to reexamine the physical competencies of the school before advancing to clinical clerkships only further reinforced this fear. I decided to fly under the radar and not say anything about my disability to my attendings. I slowly worked my way through clerkships by making do with adapted ways to perform procedures and exams with additional practice and maneuvering at home. I found myself drawn to psychiatry because of the similarities I saw in the patients and myself. I empathized with how the patients struggled with chronic conditions that left them feeling separated from society and how they felt that their diagnosis was something they needed to hide. When medical school ended and I decided to pursue psychiatry, I wanted to share my story to inspire others with a disability to consider medicine as a career given their unique experiences. My experience thus far has been uplifting as my journey has echoed so many others.
A need for greater representation
Disability representation in medicine is needed more than ever. According to the CDC, >60 million adults in the United States (1 in 4) live with a disability.1 Although the physical health disparities are often discussed, there is less conversation surrounding mental health for individuals with disabilities. A 2018 study by Cree et al2 found that approximately 17.4 million adults with disabilities experienced frequent mental distress, defined as reporting ≥14 mentally unhealthy days in the past 30 days. Furthermore, compared to individuals without a disability, those with a disability are statistically more likely to have suicidal ideation, suicidal planning, and suicide attempts.3 One way to address this disparity is to recruit medical students with disabilities to become physicians with disabilities. Evidence suggests that physicians who are members of groups that are underrepresented in medicine are more likely to deliver care to underrepresented patients.4 However, medical schools and institutions have been slow to address the disparity. A 2019 survey found an estimated 4.6% of medical students responded “yes” when asked if they had a disability, with most students reporting a psychological or attention/hyperactive disorder.5 Existing barriers include restrictive language surrounding technical standards influenced by long-standing vestiges of what a physician should be.6
An opportunity to connect with patients
I now do not see myself as having a secret identity to hide. Although my CP does not give me any superpowers, it has given me the opportunity to connect with my patients and serve as an example of why medical school recruitment and admissions should expand. Psychiatrists have been on the forefront of change in medicine and can shift the perception of a physician. In doing so, we not only enrich our field but also the lives of our patients who may need it most.
1. Okoro CA, Hollis ND, Cyrus AC, et al. Prevalence of disabilities and health care access by disability status and type among adults—United States, 2016. MMWR Morb Mortal Wkly Rep. 2018;67(32):882-887.
2. Cree RA, Okoro CA, Zack MM, et al. Frequent mental distress among adults, by disability status, disability type, and selected characteristics—United States 2018. MMWR Morb Mortal Wkly Rep. 2020;69(36):1238-1243.
3. Marlow NM, Xie Z, Tanner R, et al. Association between disability and suicide-related outcomes among US adults. Am J Prev Med. 2021;61(6):852-862.
4. Thurmond VB, Kirch DG. Impact of minority physicians on health care. South Med J. 1998;91(11):1009-1013.
5. Meeks LM, Case B, Herzer K, et al. Change in prevalence of disabilities and accommodation practices among US medical schools, 2016 vs 2019. JAMA. 2019;322(20):2022-2024.
6. Stauffer C, Case B, Moreland CJ, et al. Technical standards from newly established medical schools: a review of disability inclusive practices. J Med Educ Curric Dev. 2022;9:23821205211072763.
1. Okoro CA, Hollis ND, Cyrus AC, et al. Prevalence of disabilities and health care access by disability status and type among adults—United States, 2016. MMWR Morb Mortal Wkly Rep. 2018;67(32):882-887.
2. Cree RA, Okoro CA, Zack MM, et al. Frequent mental distress among adults, by disability status, disability type, and selected characteristics—United States 2018. MMWR Morb Mortal Wkly Rep. 2020;69(36):1238-1243.
3. Marlow NM, Xie Z, Tanner R, et al. Association between disability and suicide-related outcomes among US adults. Am J Prev Med. 2021;61(6):852-862.
4. Thurmond VB, Kirch DG. Impact of minority physicians on health care. South Med J. 1998;91(11):1009-1013.
5. Meeks LM, Case B, Herzer K, et al. Change in prevalence of disabilities and accommodation practices among US medical schools, 2016 vs 2019. JAMA. 2019;322(20):2022-2024.
6. Stauffer C, Case B, Moreland CJ, et al. Technical standards from newly established medical schools: a review of disability inclusive practices. J Med Educ Curric Dev. 2022;9:23821205211072763.
Discontinuing a long-acting injectable antipsychotic: What to consider
Mr. R, age 29, was diagnosed with schizophrenia 6 years ago. To manage his disorder, he has been receiving paliperidone palmitate long-acting injectable (LAI) 156 mg once a month for 2 years. Prior to maintenance with paliperidone palmitate, Mr. R was stabilized on oral paliperidone 9 mg/d. Though he was originally initiated on paliperidone palmitate due to nonadherence concerns, Mr. R has been adherent with each injection for 1 year.
At a recent visit, Mr. R says he wants to discontinue the injection because he is not interested in receiving an ongoing injectable medication and is not able to continue monthly clinic visits. He wants to take a daily oral antipsychotic again, despite the availability of longer-acting products.
A paucity of evidence exists regarding the discontinuation of LAI antipsychotics and the next steps that follow in treatment. There is neither a consensus nor recognized guidelines advising how and when to discontinue an LAI and restart an oral antipsychotic. A recent systematic review and meta-analysis evaluated different maintenance treatment strategies; however, switching from an LAI antipsychotic to an oral medication was not a focus.1 In this article, we outline a possible approach to discontinuing an LAI antipsychotic and restarting an oral formulation. Before discontinuing an LAI antipsychotic, clinicians should review with the patient the risks and benefits of switching medications, including the risk of decompensation and potential adverse effects.
Switching to an oral antipsychotic
The first step in the discontinuation process is to determine whether the patient will continue the same oral medication as the LAI antipsychotic or if a different oral antipsychotic will be initiated. Next, determining when to initiate the oral medication requires several pieces of information, including the oral dose equivalent of the patient’s current LAI, the half-life of the LAI, and the release mechanism of the LAI (Table 1).2-5 To determine the appropriate time frame for restarting oral treatment, it is also vital to know the date of the last injection.
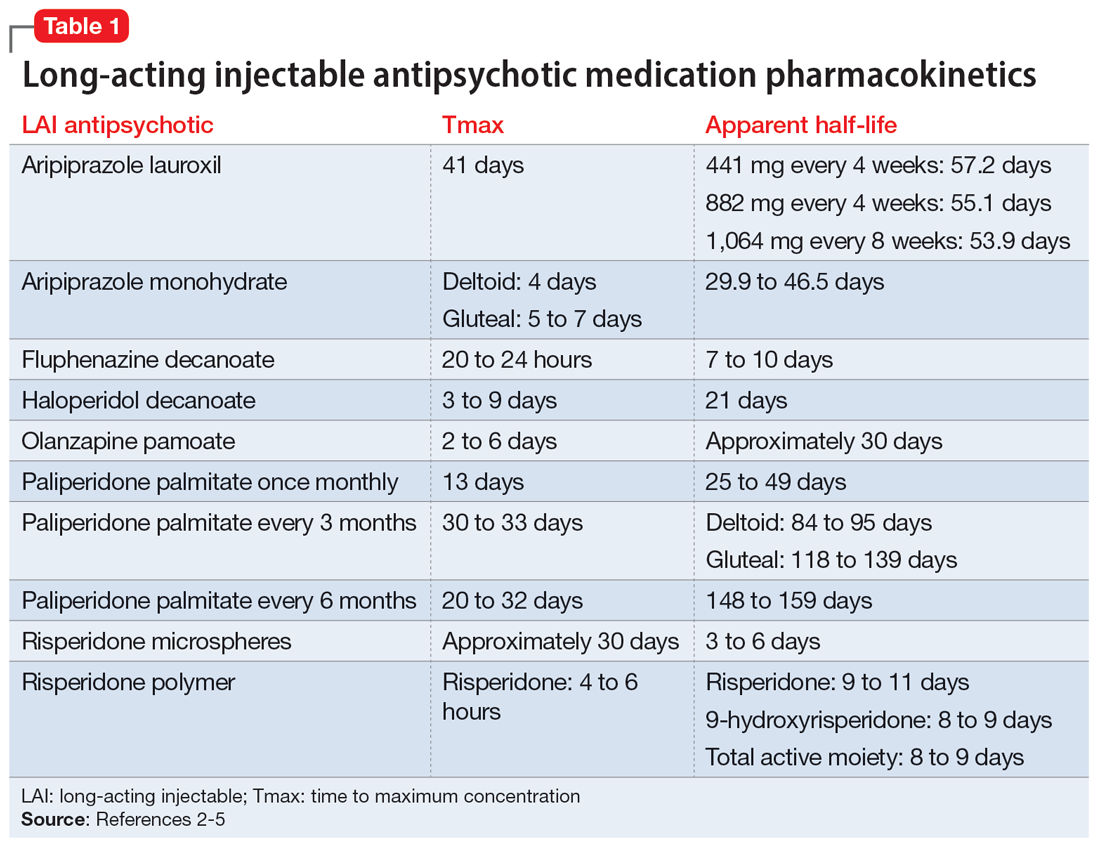
Based on the date of the next injection, the clinician will utilize the LAI’s half-life and its release mechanism to determine the appropriate time to start a new oral antipsychotic. Research demonstrates that in patients who have achieved steady state with a first-generation antipsychotic, plasma concentrations stay relatively consistent for 6 to 7 weeks after the last injection, which suggests oral medications may not need to be initiated until that time.6-9
For many second-generation LAI antipsychotics, oral medications may be initiated at the date of the next injection. Initiation of an oral antipsychotic may require more time between the last injection dose and the date of administration for oral medication due to the pharmacokinetic profile of risperidone microspheres. Once a patient is at steady state with risperidone microspheres, trough levels are not observed until 3 to 4 weeks after discontinuation.10
Previous pharmacokinetic model–based stimulations of active moiety plasma concentrations of risperidone microspheres demonstrate that 2 weeks after an injection of risperidone microspheres, the concentration of active moiety continued to approximate the steady-state concentration for 3 to 5 weeks.11 This is likely due to the product’s delay in release being 3 weeks from the time of injection to the last release phase. Of note, there was a rapid decline in the active moiety concentration; it reached nearly 0 by Week 5.11 The same pharmacokinetic model–based stimulation demonstrated a steady and slow decline of the concentration of active moiety of paliperidone palmitate after discontinuation of the LAI.11
Continue to: No guidance exists for...
No guidance exists for aripiprazole LAI medications; however, based on the pharmacokinetic data, administration of oral medications should be initiated at the date of next injection. Given the long half-life of aripiprazole, a cross-titration of the LAI with oral medication is reasonable.
Monitoring drug levels
In addition to utilizing the pharmacokinetic data from LAI antipsychotics, therapeutic drug levels can be instrumental in determining the dose of oral medication to use and when to begin titration (Table 2).12-14 Obtaining a drug level on the date of the next injection can provide the clinician with data regarding the release of the medication specific to the patient. Based on the level and the current symptomatology, the clinician could choose to start the oral medication at a lower dose and titrate back to the LAI equivalent oral dose, or initiate the oral dose at the LAI equivalent oral dose. Continued therapeutic drug levels can aid in this determination.
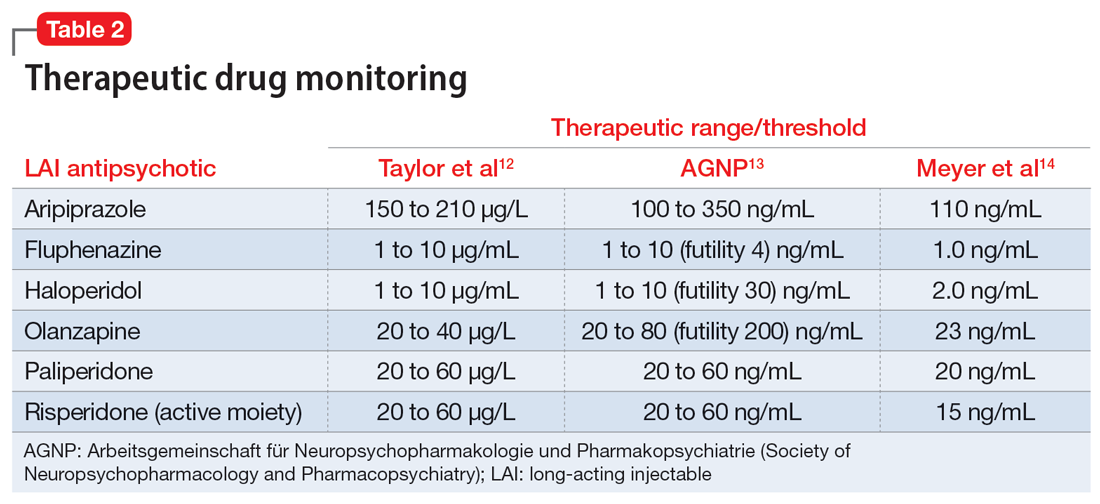
No guidance exists on the appropriate discontinuation of LAI antipsychotics. Utilizing a medication’s half-life and release mechanism, as well as the patient’s previous medication history, date of last injection, and therapeutic drug levels, should be considered when determining the schedule for restarting an oral antipsychotic.
CASE CONTINUED
Based on the current dosing of paliperidone palmitate of 156 mg once a month, Mr. R likely requires 9 mg/d of oral paliperidone upon discontinuation of the LAI. On the date of the next injection, the clinician could decide to initiate a lower dose of paliperidone, such as to 3 mg/d or 6 mg/d, and increase the dose as tolerated over the next 10 to 14 days as the paliperidone palmitate is further metabolized. Additionally, the clinician may consider obtaining a therapeutic drug level to determine the current paliperidone level prior to initiating the oral medication. Each treatment option offers individual risks and benefits. The decision on when and how to initiate the oral medication will be based on the individual patient’s situation and history, as well as the comfort and discretion of the clinician. The clinician should arrange appropriate monitoring for potential increased symptomatology during the transition, and adverse effects should be assessed regularly until steady state is achieved with the targeted oral dose of medication.
Related Resources
- Parmentier BL. Second-generation long-acting injectable antipsychotics: a practical guide. Current Psychiatry. 2020;19(3):24-32.
- Thippaiah SM, Fargason RE, Birur B. Switching antipsychotics: a guide to dose equivalents. Current Psychiatry. 2021;20(4):13-14. doi:10.12788/cp.0103
Drug Brand Names
Aripiprazole lauroxil • Aristada
Aripiprazole monohydrate • Maintena
Haloperidol injection • Haldol decanoate
Olanzapine pamoate • Zyprexa Relprevv
Paliperidone • Invega
Paliperidone palmitate once monthly • Invega Sustenna
Paliperidone palmitate every 3 months • Invega Trinza
Paliperidone palmitate every 6 months • Invega Hafyera
Risperidone microspheres • Risperdal Consta
Risperidone polymer • Perseris
1. Ostuzzi G, Vita G, Bertolini F, et al. Continuing, reducing, switching, or stopping antipsychotics in individuals with schizophrenia-spectrum disorders who are clinically stable: a systematic review and network meta-analysis. Lancet Psychiatry. 2022;9(8):614-624.
2. Correll CU, Kim E, Sliwa JK, et al. Pharmacokinetic characteristics of long-acting injectable antipsychotics for schizophrenia: an overview. CNS Drugs. 2021;35(1):39-59.
3. Spanarello S, La Ferla T. The pharmacokinetics of long-acting antipsychotic medications. Curr Clin Pharmacol. 2014;9(3):310-317.
4. Meyer JM. Understanding depot antipsychotics: an illustrated guide to kinetics. CNS Spectr. 2013;18(Suppl 1):58-68.
5. Invega Hafyera [package insert]. Janssen Pharmaceuticals, Inc; 2021.
6. Gitlin MJ, Midha KK, Fogelson D, et al. Persistence of fluphenazine in plasma after decanoate withdrawal. J Clin Psychopharmacol. 1988;8(1):53-56.
7. Wistedt B, Jørgensen A, Wiles D. A depot neuroleptic withdrawal study. Plasma concentration of fluphenazine and flupenthixol and relapse frequency. Psychopharmacology. 1982;78(4):301-304.
8. Chang WH, Lin SK, Juang DJ, et al. Prolonged haloperidol and reduced haloperidol plasma concentrations after decanoate withdrawal. Schizophr Res. 1993;9(1):35-40.
9. Eklund K, Forsman A. Minimal effective dose and relapse—double-blind trial: haloperidol decanoate vs. placebo. Clin Neuropharmacol. 1991;1(Suppl 2):S7-S15.
10. Wilson WH. A visual guide to expected blood levels of long-acting injectable risperidone in clinical practice. J Psychiatry Pract. 2004;10(6):393-401.
11. Samtani MN, Sheehan JJ, Fu DJ, et al. Management of antipsychotic treatment discontinuation and interruptions using model-based simulations. Clin Pharmacol. 2012;4:25-40.
12. Taylor D, Barnes TRE, Young AH. The Maudsley Prescribing Guidelines in Psychiatry. 13th ed. Wiley-Blackwell; 2018.
13. Hiemke C, Bergemann N, Clement HW, et al. Consensus guidelines for therapeutic drug monitoring in neuropsychopharmacology: update 2017. Pharmacopsychiatry. 2018;51(1-2):9-62.
14. Meyer JM, Stahl SM. The Clinical Use of Antipsychotic Plasma Levels. Cambridge University Press; 2021.
Mr. R, age 29, was diagnosed with schizophrenia 6 years ago. To manage his disorder, he has been receiving paliperidone palmitate long-acting injectable (LAI) 156 mg once a month for 2 years. Prior to maintenance with paliperidone palmitate, Mr. R was stabilized on oral paliperidone 9 mg/d. Though he was originally initiated on paliperidone palmitate due to nonadherence concerns, Mr. R has been adherent with each injection for 1 year.
At a recent visit, Mr. R says he wants to discontinue the injection because he is not interested in receiving an ongoing injectable medication and is not able to continue monthly clinic visits. He wants to take a daily oral antipsychotic again, despite the availability of longer-acting products.
A paucity of evidence exists regarding the discontinuation of LAI antipsychotics and the next steps that follow in treatment. There is neither a consensus nor recognized guidelines advising how and when to discontinue an LAI and restart an oral antipsychotic. A recent systematic review and meta-analysis evaluated different maintenance treatment strategies; however, switching from an LAI antipsychotic to an oral medication was not a focus.1 In this article, we outline a possible approach to discontinuing an LAI antipsychotic and restarting an oral formulation. Before discontinuing an LAI antipsychotic, clinicians should review with the patient the risks and benefits of switching medications, including the risk of decompensation and potential adverse effects.
Switching to an oral antipsychotic
The first step in the discontinuation process is to determine whether the patient will continue the same oral medication as the LAI antipsychotic or if a different oral antipsychotic will be initiated. Next, determining when to initiate the oral medication requires several pieces of information, including the oral dose equivalent of the patient’s current LAI, the half-life of the LAI, and the release mechanism of the LAI (Table 1).2-5 To determine the appropriate time frame for restarting oral treatment, it is also vital to know the date of the last injection.

Based on the date of the next injection, the clinician will utilize the LAI’s half-life and its release mechanism to determine the appropriate time to start a new oral antipsychotic. Research demonstrates that in patients who have achieved steady state with a first-generation antipsychotic, plasma concentrations stay relatively consistent for 6 to 7 weeks after the last injection, which suggests oral medications may not need to be initiated until that time.6-9
For many second-generation LAI antipsychotics, oral medications may be initiated at the date of the next injection. Initiation of an oral antipsychotic may require more time between the last injection dose and the date of administration for oral medication due to the pharmacokinetic profile of risperidone microspheres. Once a patient is at steady state with risperidone microspheres, trough levels are not observed until 3 to 4 weeks after discontinuation.10
Previous pharmacokinetic model–based stimulations of active moiety plasma concentrations of risperidone microspheres demonstrate that 2 weeks after an injection of risperidone microspheres, the concentration of active moiety continued to approximate the steady-state concentration for 3 to 5 weeks.11 This is likely due to the product’s delay in release being 3 weeks from the time of injection to the last release phase. Of note, there was a rapid decline in the active moiety concentration; it reached nearly 0 by Week 5.11 The same pharmacokinetic model–based stimulation demonstrated a steady and slow decline of the concentration of active moiety of paliperidone palmitate after discontinuation of the LAI.11
Continue to: No guidance exists for...
No guidance exists for aripiprazole LAI medications; however, based on the pharmacokinetic data, administration of oral medications should be initiated at the date of next injection. Given the long half-life of aripiprazole, a cross-titration of the LAI with oral medication is reasonable.
Monitoring drug levels
In addition to utilizing the pharmacokinetic data from LAI antipsychotics, therapeutic drug levels can be instrumental in determining the dose of oral medication to use and when to begin titration (Table 2).12-14 Obtaining a drug level on the date of the next injection can provide the clinician with data regarding the release of the medication specific to the patient. Based on the level and the current symptomatology, the clinician could choose to start the oral medication at a lower dose and titrate back to the LAI equivalent oral dose, or initiate the oral dose at the LAI equivalent oral dose. Continued therapeutic drug levels can aid in this determination.

No guidance exists on the appropriate discontinuation of LAI antipsychotics. Utilizing a medication’s half-life and release mechanism, as well as the patient’s previous medication history, date of last injection, and therapeutic drug levels, should be considered when determining the schedule for restarting an oral antipsychotic.
CASE CONTINUED
Based on the current dosing of paliperidone palmitate of 156 mg once a month, Mr. R likely requires 9 mg/d of oral paliperidone upon discontinuation of the LAI. On the date of the next injection, the clinician could decide to initiate a lower dose of paliperidone, such as to 3 mg/d or 6 mg/d, and increase the dose as tolerated over the next 10 to 14 days as the paliperidone palmitate is further metabolized. Additionally, the clinician may consider obtaining a therapeutic drug level to determine the current paliperidone level prior to initiating the oral medication. Each treatment option offers individual risks and benefits. The decision on when and how to initiate the oral medication will be based on the individual patient’s situation and history, as well as the comfort and discretion of the clinician. The clinician should arrange appropriate monitoring for potential increased symptomatology during the transition, and adverse effects should be assessed regularly until steady state is achieved with the targeted oral dose of medication.
Related Resources
- Parmentier BL. Second-generation long-acting injectable antipsychotics: a practical guide. Current Psychiatry. 2020;19(3):24-32.
- Thippaiah SM, Fargason RE, Birur B. Switching antipsychotics: a guide to dose equivalents. Current Psychiatry. 2021;20(4):13-14. doi:10.12788/cp.0103
Drug Brand Names
Aripiprazole lauroxil • Aristada
Aripiprazole monohydrate • Maintena
Haloperidol injection • Haldol decanoate
Olanzapine pamoate • Zyprexa Relprevv
Paliperidone • Invega
Paliperidone palmitate once monthly • Invega Sustenna
Paliperidone palmitate every 3 months • Invega Trinza
Paliperidone palmitate every 6 months • Invega Hafyera
Risperidone microspheres • Risperdal Consta
Risperidone polymer • Perseris
Mr. R, age 29, was diagnosed with schizophrenia 6 years ago. To manage his disorder, he has been receiving paliperidone palmitate long-acting injectable (LAI) 156 mg once a month for 2 years. Prior to maintenance with paliperidone palmitate, Mr. R was stabilized on oral paliperidone 9 mg/d. Though he was originally initiated on paliperidone palmitate due to nonadherence concerns, Mr. R has been adherent with each injection for 1 year.
At a recent visit, Mr. R says he wants to discontinue the injection because he is not interested in receiving an ongoing injectable medication and is not able to continue monthly clinic visits. He wants to take a daily oral antipsychotic again, despite the availability of longer-acting products.
A paucity of evidence exists regarding the discontinuation of LAI antipsychotics and the next steps that follow in treatment. There is neither a consensus nor recognized guidelines advising how and when to discontinue an LAI and restart an oral antipsychotic. A recent systematic review and meta-analysis evaluated different maintenance treatment strategies; however, switching from an LAI antipsychotic to an oral medication was not a focus.1 In this article, we outline a possible approach to discontinuing an LAI antipsychotic and restarting an oral formulation. Before discontinuing an LAI antipsychotic, clinicians should review with the patient the risks and benefits of switching medications, including the risk of decompensation and potential adverse effects.
Switching to an oral antipsychotic
The first step in the discontinuation process is to determine whether the patient will continue the same oral medication as the LAI antipsychotic or if a different oral antipsychotic will be initiated. Next, determining when to initiate the oral medication requires several pieces of information, including the oral dose equivalent of the patient’s current LAI, the half-life of the LAI, and the release mechanism of the LAI (Table 1).2-5 To determine the appropriate time frame for restarting oral treatment, it is also vital to know the date of the last injection.

Based on the date of the next injection, the clinician will utilize the LAI’s half-life and its release mechanism to determine the appropriate time to start a new oral antipsychotic. Research demonstrates that in patients who have achieved steady state with a first-generation antipsychotic, plasma concentrations stay relatively consistent for 6 to 7 weeks after the last injection, which suggests oral medications may not need to be initiated until that time.6-9
For many second-generation LAI antipsychotics, oral medications may be initiated at the date of the next injection. Initiation of an oral antipsychotic may require more time between the last injection dose and the date of administration for oral medication due to the pharmacokinetic profile of risperidone microspheres. Once a patient is at steady state with risperidone microspheres, trough levels are not observed until 3 to 4 weeks after discontinuation.10
Previous pharmacokinetic model–based stimulations of active moiety plasma concentrations of risperidone microspheres demonstrate that 2 weeks after an injection of risperidone microspheres, the concentration of active moiety continued to approximate the steady-state concentration for 3 to 5 weeks.11 This is likely due to the product’s delay in release being 3 weeks from the time of injection to the last release phase. Of note, there was a rapid decline in the active moiety concentration; it reached nearly 0 by Week 5.11 The same pharmacokinetic model–based stimulation demonstrated a steady and slow decline of the concentration of active moiety of paliperidone palmitate after discontinuation of the LAI.11
Continue to: No guidance exists for...
No guidance exists for aripiprazole LAI medications; however, based on the pharmacokinetic data, administration of oral medications should be initiated at the date of next injection. Given the long half-life of aripiprazole, a cross-titration of the LAI with oral medication is reasonable.
Monitoring drug levels
In addition to utilizing the pharmacokinetic data from LAI antipsychotics, therapeutic drug levels can be instrumental in determining the dose of oral medication to use and when to begin titration (Table 2).12-14 Obtaining a drug level on the date of the next injection can provide the clinician with data regarding the release of the medication specific to the patient. Based on the level and the current symptomatology, the clinician could choose to start the oral medication at a lower dose and titrate back to the LAI equivalent oral dose, or initiate the oral dose at the LAI equivalent oral dose. Continued therapeutic drug levels can aid in this determination.

No guidance exists on the appropriate discontinuation of LAI antipsychotics. Utilizing a medication’s half-life and release mechanism, as well as the patient’s previous medication history, date of last injection, and therapeutic drug levels, should be considered when determining the schedule for restarting an oral antipsychotic.
CASE CONTINUED
Based on the current dosing of paliperidone palmitate of 156 mg once a month, Mr. R likely requires 9 mg/d of oral paliperidone upon discontinuation of the LAI. On the date of the next injection, the clinician could decide to initiate a lower dose of paliperidone, such as to 3 mg/d or 6 mg/d, and increase the dose as tolerated over the next 10 to 14 days as the paliperidone palmitate is further metabolized. Additionally, the clinician may consider obtaining a therapeutic drug level to determine the current paliperidone level prior to initiating the oral medication. Each treatment option offers individual risks and benefits. The decision on when and how to initiate the oral medication will be based on the individual patient’s situation and history, as well as the comfort and discretion of the clinician. The clinician should arrange appropriate monitoring for potential increased symptomatology during the transition, and adverse effects should be assessed regularly until steady state is achieved with the targeted oral dose of medication.
Related Resources
- Parmentier BL. Second-generation long-acting injectable antipsychotics: a practical guide. Current Psychiatry. 2020;19(3):24-32.
- Thippaiah SM, Fargason RE, Birur B. Switching antipsychotics: a guide to dose equivalents. Current Psychiatry. 2021;20(4):13-14. doi:10.12788/cp.0103
Drug Brand Names
Aripiprazole lauroxil • Aristada
Aripiprazole monohydrate • Maintena
Haloperidol injection • Haldol decanoate
Olanzapine pamoate • Zyprexa Relprevv
Paliperidone • Invega
Paliperidone palmitate once monthly • Invega Sustenna
Paliperidone palmitate every 3 months • Invega Trinza
Paliperidone palmitate every 6 months • Invega Hafyera
Risperidone microspheres • Risperdal Consta
Risperidone polymer • Perseris
1. Ostuzzi G, Vita G, Bertolini F, et al. Continuing, reducing, switching, or stopping antipsychotics in individuals with schizophrenia-spectrum disorders who are clinically stable: a systematic review and network meta-analysis. Lancet Psychiatry. 2022;9(8):614-624.
2. Correll CU, Kim E, Sliwa JK, et al. Pharmacokinetic characteristics of long-acting injectable antipsychotics for schizophrenia: an overview. CNS Drugs. 2021;35(1):39-59.
3. Spanarello S, La Ferla T. The pharmacokinetics of long-acting antipsychotic medications. Curr Clin Pharmacol. 2014;9(3):310-317.
4. Meyer JM. Understanding depot antipsychotics: an illustrated guide to kinetics. CNS Spectr. 2013;18(Suppl 1):58-68.
5. Invega Hafyera [package insert]. Janssen Pharmaceuticals, Inc; 2021.
6. Gitlin MJ, Midha KK, Fogelson D, et al. Persistence of fluphenazine in plasma after decanoate withdrawal. J Clin Psychopharmacol. 1988;8(1):53-56.
7. Wistedt B, Jørgensen A, Wiles D. A depot neuroleptic withdrawal study. Plasma concentration of fluphenazine and flupenthixol and relapse frequency. Psychopharmacology. 1982;78(4):301-304.
8. Chang WH, Lin SK, Juang DJ, et al. Prolonged haloperidol and reduced haloperidol plasma concentrations after decanoate withdrawal. Schizophr Res. 1993;9(1):35-40.
9. Eklund K, Forsman A. Minimal effective dose and relapse—double-blind trial: haloperidol decanoate vs. placebo. Clin Neuropharmacol. 1991;1(Suppl 2):S7-S15.
10. Wilson WH. A visual guide to expected blood levels of long-acting injectable risperidone in clinical practice. J Psychiatry Pract. 2004;10(6):393-401.
11. Samtani MN, Sheehan JJ, Fu DJ, et al. Management of antipsychotic treatment discontinuation and interruptions using model-based simulations. Clin Pharmacol. 2012;4:25-40.
12. Taylor D, Barnes TRE, Young AH. The Maudsley Prescribing Guidelines in Psychiatry. 13th ed. Wiley-Blackwell; 2018.
13. Hiemke C, Bergemann N, Clement HW, et al. Consensus guidelines for therapeutic drug monitoring in neuropsychopharmacology: update 2017. Pharmacopsychiatry. 2018;51(1-2):9-62.
14. Meyer JM, Stahl SM. The Clinical Use of Antipsychotic Plasma Levels. Cambridge University Press; 2021.
1. Ostuzzi G, Vita G, Bertolini F, et al. Continuing, reducing, switching, or stopping antipsychotics in individuals with schizophrenia-spectrum disorders who are clinically stable: a systematic review and network meta-analysis. Lancet Psychiatry. 2022;9(8):614-624.
2. Correll CU, Kim E, Sliwa JK, et al. Pharmacokinetic characteristics of long-acting injectable antipsychotics for schizophrenia: an overview. CNS Drugs. 2021;35(1):39-59.
3. Spanarello S, La Ferla T. The pharmacokinetics of long-acting antipsychotic medications. Curr Clin Pharmacol. 2014;9(3):310-317.
4. Meyer JM. Understanding depot antipsychotics: an illustrated guide to kinetics. CNS Spectr. 2013;18(Suppl 1):58-68.
5. Invega Hafyera [package insert]. Janssen Pharmaceuticals, Inc; 2021.
6. Gitlin MJ, Midha KK, Fogelson D, et al. Persistence of fluphenazine in plasma after decanoate withdrawal. J Clin Psychopharmacol. 1988;8(1):53-56.
7. Wistedt B, Jørgensen A, Wiles D. A depot neuroleptic withdrawal study. Plasma concentration of fluphenazine and flupenthixol and relapse frequency. Psychopharmacology. 1982;78(4):301-304.
8. Chang WH, Lin SK, Juang DJ, et al. Prolonged haloperidol and reduced haloperidol plasma concentrations after decanoate withdrawal. Schizophr Res. 1993;9(1):35-40.
9. Eklund K, Forsman A. Minimal effective dose and relapse—double-blind trial: haloperidol decanoate vs. placebo. Clin Neuropharmacol. 1991;1(Suppl 2):S7-S15.
10. Wilson WH. A visual guide to expected blood levels of long-acting injectable risperidone in clinical practice. J Psychiatry Pract. 2004;10(6):393-401.
11. Samtani MN, Sheehan JJ, Fu DJ, et al. Management of antipsychotic treatment discontinuation and interruptions using model-based simulations. Clin Pharmacol. 2012;4:25-40.
12. Taylor D, Barnes TRE, Young AH. The Maudsley Prescribing Guidelines in Psychiatry. 13th ed. Wiley-Blackwell; 2018.
13. Hiemke C, Bergemann N, Clement HW, et al. Consensus guidelines for therapeutic drug monitoring in neuropsychopharmacology: update 2017. Pharmacopsychiatry. 2018;51(1-2):9-62.
14. Meyer JM, Stahl SM. The Clinical Use of Antipsychotic Plasma Levels. Cambridge University Press; 2021.
Medication-induced rhabdomyolysis
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in
Ms. A, age 32, has a history of anxiety, bipolar disorder, and borderline personality disorder. She is undergoing treatment with lamotrigine 200 mg/d at bedtime, aripiprazole 5 mg/d, trazodone 100 mg/d at bedtime, clonazepam 0.5 mg twice a day, and hydroxyzine 25 mg twice a day. She presents to the emergency department with myalgia, left upper and lower extremity numbness, and weakness. These symptoms started at approximately 3
Ms. A’s vital signs are hemodynamically stable, but her pulse is 113 bpm. On examination, she appears anxious and has decreased sensation in her upper and lower extremities, with 3/5 strength on the left side. Her laboratory results indicate mild leukocytosis, hyponatremia (129 mmol/L; reference range 136 to 145 mmol/L), and elevations in serum creatinine (3.7 mg/dL; reference range 0.6 to 1.2 mg/dL), aspartate aminotransferase (654 U/L; reference range 10 to 42 U/L), alanine transaminase (234 U/L; reference range 10 to 60 U/L), and troponin (2.11 ng/mL; reference range 0 to 0.04 ng/mL). A urinalysis reveals darkly colored urine with large red blood cells.
Neurology and Cardiology consultations are requested to rule out stroke and acute coronary syndromes. A computed tomography scan of the head shows no acute intracranial findings. Her creatinine kinase (CK) level is elevated (>42,670 U/L; reference range 22 to 232 U/L), which prompts a search for causes of rhabdomyolysis, a breakdown of muscle tissue that releases muscle fiber contents into the blood. Ms. A reports no history of recent trauma or strenuous exercise. Infectious, endocrine, and other workups are negative. After a consult to Psychiatry, the treating clinicians suspect that the most likely cause for rhabdomyolysis is aripiprazole.
Ms. A is treated with IV isotonic fluids. Aripiprazole is stopped and her CK levels are closely monitored. CK levels continue to trend down, and by Day 6 of hospitalization her CK level is 1,648 U/L. Her transaminase levels also improve; these elevations are considered likely secondary to rhabdomyolysis. Because there is notable improvement in CK and transaminase levels after stopping aripiprazole, Ms. A is discharged and instructed to follow up with a psychiatrist for further management.
Aripiprazole and rhabdomyolysis
According to the National Institute of Mental Health, an estimated 2.8% of the US population has bipolar disorder and 0.24% to 0.64% has schizophrenia.1,2 Antipsychotics are often used to treat these disorders. The prevalence of antipsychotic use in the general adult population is 1.6%.3 The use of second-generation antipsychotics (SGAs) has increased over recent years with the availability of a variety of formulations, such as immediate-release injectable, long-acting injectable, and orally disintegrating tablets in addition to the customary oral tablets. SGAs can cause several adverse effects, including weight gain, hyperlipidemia, diabetes, QTc prolongation, extrapyramidal side effects, myocarditis, agranulocytosis, cataracts, and sexual adverse effects.4
Antipsychotic use is more commonly associated with serotonin syndrome and neuroleptic malignant syndrome than it is with rhabdomyolysis. Rhabdomyolysis as an adverse effect of antipsychotic use has not been well understood or reported. One study found the prevalence of rhabdomyolysis was approximately 10% among patients who received an antipsychotic medication.5 There have been 4 case reports of clozapine use, 6 of olanzapine use, and 3 of aripiprazole use associated with rhabdomyolysis.6-8 Therefore, this would be the fourth case report to describe aripiprazole-associated rhabdomyolysis.
Aripiprazole is FDA-approved for the treatment of schizophrenia. In this case report, we found that aripiprazole could have led to rhabdomyolysis. Aripiprazole is a quinoline derivative that acts by binding to the 5-HT1A and 5-HT2A receptors.9,10 It acts as a partial agonist at 5-HT1A receptors, an antagonist at 5-HT2A receptors, and a partial agonist and stabilizer at the D2 receptor. By binding to the dopamine receptor in its G protein–coupled state, aripiprazole blocks the receptor in the presence of excessive dopamine.11-13 The mechanism of how aripiprazole could cause rhabdomyolysis is unclear. One proposed mechanism is that it can increase the permeability of skeletal muscle by 5-HT2A antagonism. This leads to a decrease in glucose reuptake in the cell and increases the permeability of the cell membrane, leading to elevations in CK levels.14 Another proposed mechanism is that dopamine blockade in the nigrostriatal pathway can result in muscle stiffness, rigidity, parkinsonian-like symptoms, and akathisia, which can result in elevated CK levels.15 There are only 3 other published cases of aripiprazole-induced rhabdomyolysis; we hope this case report will add value to the available literature. More evidence is needed to establish the safety profile of aripiprazole.
1. National Institute of Mental Health. Prevalence of bipolar disorder among adults. Accessed December 21, 2022. https://www.nimh.nih.gov/health/statistics/bipolar-disorder#part_2605
2. National Institute of Mental Health. Schizophrenia. Accessed December 21, 2022. https://www.nimh.nih.gov/health/statistics/schizophrenia#part_2543
3. Dennis JA, Gittner LS, Payne JD, et al. Characteristics of U.S. adults taking prescription antipsychotic medications, National Health and Nutrition Examination Survey 2013-2018. BMC Psychiatry. 2020;20(1):483. doi: 10.1186/s12888-020-02895-4
4. Willner K, Vasan S, Abdijadid S. Atypical antipsychotic agents. In: StatPearls [Internet]. StatPearls Publishing; 2022. Updated May 2, 2022. Accessed December 22, 2022. https://www.ncbi.nlm.nih.gov/books/NBK448156/
5. Packard K, Price P, Hanson A. Antipsychotic use and the risk of rhabdomyolysis. J Pharm Pract 2014;27(5):501-512. doi: 10.1177/0897190013516509
6. Wu YF, Chang KY. Aripiprazole-associated rhabdomyolysis in a patient with schizophrenia. J Neuropsychiatry Clin Neurosci. 2011;23(3):E51.
7. Marzetti E, Bocchino L, Teramo S, et al. Rhabdomyolysis in a patient on aripiprazole with traumatic hip prosthesis luxation. J Neuropsychiatry Clin Neurosci. 2012;24(4):E40-E41.
8. Zhu X, Hu J, Deng S, et al. Rhabdomyolysis and elevated liver enzymes after rapid correction of hyponatremia due to pneumonia and concurrent use of aripiprazole: a case report. Aust N Z J Psychiatry. 2018;52(2):206. doi:10.1177/0004867417743342
9. Stahl SM. Essential Psychopharmacology: Neuroscientific Basis and Practical Application. 2nd ed. Cambridge University Press; 2000.
10. Stahl SM. “Hit-and-run” actions at dopamine receptors, part 1: mechanism of action of atypical antipsychotics. J Clin Psychiatry. 2001;62(9):670-671.
11. Leysen JE, Janssen PM, Schotte A, et al. Interaction of antipsychotic drugs with neurotransmitter receptor sites in vitro and in vivo in relation to pharmacological and clinical effects: role of 5HT2 receptors. Psychopharmacology (Berl). 1993;112(1 Suppl):S40-S54.
12. Millan MJ. Improving the treatment of schizophrenia: focus on serotonin (5-HT)(1A) receptors. J Pharmacol Exp Ther. 2000;295(3):853-861.
13. Millan MJ. The neurobiology and control of anxious states. Prog Neurobiol. 2003;70(2):83-244.
14. Meltzer HY, Cola PA, Parsa M. Marked elevations of serum creatine kinase activity associated with antipsychotic drug treatment. Neuropsychopharmacology. 1996;15(4):395-405.
15. Devarajan S, Dursun SM. Antipsychotic drugs, serum creatine kinase (CPK) and possible mechanisms. Psychopharmacology (Berl). 2000;152(1):122.
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in
Ms. A, age 32, has a history of anxiety, bipolar disorder, and borderline personality disorder. She is undergoing treatment with lamotrigine 200 mg/d at bedtime, aripiprazole 5 mg/d, trazodone 100 mg/d at bedtime, clonazepam 0.5 mg twice a day, and hydroxyzine 25 mg twice a day. She presents to the emergency department with myalgia, left upper and lower extremity numbness, and weakness. These symptoms started at approximately 3
Ms. A’s vital signs are hemodynamically stable, but her pulse is 113 bpm. On examination, she appears anxious and has decreased sensation in her upper and lower extremities, with 3/5 strength on the left side. Her laboratory results indicate mild leukocytosis, hyponatremia (129 mmol/L; reference range 136 to 145 mmol/L), and elevations in serum creatinine (3.7 mg/dL; reference range 0.6 to 1.2 mg/dL), aspartate aminotransferase (654 U/L; reference range 10 to 42 U/L), alanine transaminase (234 U/L; reference range 10 to 60 U/L), and troponin (2.11 ng/mL; reference range 0 to 0.04 ng/mL). A urinalysis reveals darkly colored urine with large red blood cells.
Neurology and Cardiology consultations are requested to rule out stroke and acute coronary syndromes. A computed tomography scan of the head shows no acute intracranial findings. Her creatinine kinase (CK) level is elevated (>42,670 U/L; reference range 22 to 232 U/L), which prompts a search for causes of rhabdomyolysis, a breakdown of muscle tissue that releases muscle fiber contents into the blood. Ms. A reports no history of recent trauma or strenuous exercise. Infectious, endocrine, and other workups are negative. After a consult to Psychiatry, the treating clinicians suspect that the most likely cause for rhabdomyolysis is aripiprazole.
Ms. A is treated with IV isotonic fluids. Aripiprazole is stopped and her CK levels are closely monitored. CK levels continue to trend down, and by Day 6 of hospitalization her CK level is 1,648 U/L. Her transaminase levels also improve; these elevations are considered likely secondary to rhabdomyolysis. Because there is notable improvement in CK and transaminase levels after stopping aripiprazole, Ms. A is discharged and instructed to follow up with a psychiatrist for further management.
Aripiprazole and rhabdomyolysis
According to the National Institute of Mental Health, an estimated 2.8% of the US population has bipolar disorder and 0.24% to 0.64% has schizophrenia.1,2 Antipsychotics are often used to treat these disorders. The prevalence of antipsychotic use in the general adult population is 1.6%.3 The use of second-generation antipsychotics (SGAs) has increased over recent years with the availability of a variety of formulations, such as immediate-release injectable, long-acting injectable, and orally disintegrating tablets in addition to the customary oral tablets. SGAs can cause several adverse effects, including weight gain, hyperlipidemia, diabetes, QTc prolongation, extrapyramidal side effects, myocarditis, agranulocytosis, cataracts, and sexual adverse effects.4
Antipsychotic use is more commonly associated with serotonin syndrome and neuroleptic malignant syndrome than it is with rhabdomyolysis. Rhabdomyolysis as an adverse effect of antipsychotic use has not been well understood or reported. One study found the prevalence of rhabdomyolysis was approximately 10% among patients who received an antipsychotic medication.5 There have been 4 case reports of clozapine use, 6 of olanzapine use, and 3 of aripiprazole use associated with rhabdomyolysis.6-8 Therefore, this would be the fourth case report to describe aripiprazole-associated rhabdomyolysis.
Aripiprazole is FDA-approved for the treatment of schizophrenia. In this case report, we found that aripiprazole could have led to rhabdomyolysis. Aripiprazole is a quinoline derivative that acts by binding to the 5-HT1A and 5-HT2A receptors.9,10 It acts as a partial agonist at 5-HT1A receptors, an antagonist at 5-HT2A receptors, and a partial agonist and stabilizer at the D2 receptor. By binding to the dopamine receptor in its G protein–coupled state, aripiprazole blocks the receptor in the presence of excessive dopamine.11-13 The mechanism of how aripiprazole could cause rhabdomyolysis is unclear. One proposed mechanism is that it can increase the permeability of skeletal muscle by 5-HT2A antagonism. This leads to a decrease in glucose reuptake in the cell and increases the permeability of the cell membrane, leading to elevations in CK levels.14 Another proposed mechanism is that dopamine blockade in the nigrostriatal pathway can result in muscle stiffness, rigidity, parkinsonian-like symptoms, and akathisia, which can result in elevated CK levels.15 There are only 3 other published cases of aripiprazole-induced rhabdomyolysis; we hope this case report will add value to the available literature. More evidence is needed to establish the safety profile of aripiprazole.
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in
Ms. A, age 32, has a history of anxiety, bipolar disorder, and borderline personality disorder. She is undergoing treatment with lamotrigine 200 mg/d at bedtime, aripiprazole 5 mg/d, trazodone 100 mg/d at bedtime, clonazepam 0.5 mg twice a day, and hydroxyzine 25 mg twice a day. She presents to the emergency department with myalgia, left upper and lower extremity numbness, and weakness. These symptoms started at approximately 3
Ms. A’s vital signs are hemodynamically stable, but her pulse is 113 bpm. On examination, she appears anxious and has decreased sensation in her upper and lower extremities, with 3/5 strength on the left side. Her laboratory results indicate mild leukocytosis, hyponatremia (129 mmol/L; reference range 136 to 145 mmol/L), and elevations in serum creatinine (3.7 mg/dL; reference range 0.6 to 1.2 mg/dL), aspartate aminotransferase (654 U/L; reference range 10 to 42 U/L), alanine transaminase (234 U/L; reference range 10 to 60 U/L), and troponin (2.11 ng/mL; reference range 0 to 0.04 ng/mL). A urinalysis reveals darkly colored urine with large red blood cells.
Neurology and Cardiology consultations are requested to rule out stroke and acute coronary syndromes. A computed tomography scan of the head shows no acute intracranial findings. Her creatinine kinase (CK) level is elevated (>42,670 U/L; reference range 22 to 232 U/L), which prompts a search for causes of rhabdomyolysis, a breakdown of muscle tissue that releases muscle fiber contents into the blood. Ms. A reports no history of recent trauma or strenuous exercise. Infectious, endocrine, and other workups are negative. After a consult to Psychiatry, the treating clinicians suspect that the most likely cause for rhabdomyolysis is aripiprazole.
Ms. A is treated with IV isotonic fluids. Aripiprazole is stopped and her CK levels are closely monitored. CK levels continue to trend down, and by Day 6 of hospitalization her CK level is 1,648 U/L. Her transaminase levels also improve; these elevations are considered likely secondary to rhabdomyolysis. Because there is notable improvement in CK and transaminase levels after stopping aripiprazole, Ms. A is discharged and instructed to follow up with a psychiatrist for further management.
Aripiprazole and rhabdomyolysis
According to the National Institute of Mental Health, an estimated 2.8% of the US population has bipolar disorder and 0.24% to 0.64% has schizophrenia.1,2 Antipsychotics are often used to treat these disorders. The prevalence of antipsychotic use in the general adult population is 1.6%.3 The use of second-generation antipsychotics (SGAs) has increased over recent years with the availability of a variety of formulations, such as immediate-release injectable, long-acting injectable, and orally disintegrating tablets in addition to the customary oral tablets. SGAs can cause several adverse effects, including weight gain, hyperlipidemia, diabetes, QTc prolongation, extrapyramidal side effects, myocarditis, agranulocytosis, cataracts, and sexual adverse effects.4
Antipsychotic use is more commonly associated with serotonin syndrome and neuroleptic malignant syndrome than it is with rhabdomyolysis. Rhabdomyolysis as an adverse effect of antipsychotic use has not been well understood or reported. One study found the prevalence of rhabdomyolysis was approximately 10% among patients who received an antipsychotic medication.5 There have been 4 case reports of clozapine use, 6 of olanzapine use, and 3 of aripiprazole use associated with rhabdomyolysis.6-8 Therefore, this would be the fourth case report to describe aripiprazole-associated rhabdomyolysis.
Aripiprazole is FDA-approved for the treatment of schizophrenia. In this case report, we found that aripiprazole could have led to rhabdomyolysis. Aripiprazole is a quinoline derivative that acts by binding to the 5-HT1A and 5-HT2A receptors.9,10 It acts as a partial agonist at 5-HT1A receptors, an antagonist at 5-HT2A receptors, and a partial agonist and stabilizer at the D2 receptor. By binding to the dopamine receptor in its G protein–coupled state, aripiprazole blocks the receptor in the presence of excessive dopamine.11-13 The mechanism of how aripiprazole could cause rhabdomyolysis is unclear. One proposed mechanism is that it can increase the permeability of skeletal muscle by 5-HT2A antagonism. This leads to a decrease in glucose reuptake in the cell and increases the permeability of the cell membrane, leading to elevations in CK levels.14 Another proposed mechanism is that dopamine blockade in the nigrostriatal pathway can result in muscle stiffness, rigidity, parkinsonian-like symptoms, and akathisia, which can result in elevated CK levels.15 There are only 3 other published cases of aripiprazole-induced rhabdomyolysis; we hope this case report will add value to the available literature. More evidence is needed to establish the safety profile of aripiprazole.
1. National Institute of Mental Health. Prevalence of bipolar disorder among adults. Accessed December 21, 2022. https://www.nimh.nih.gov/health/statistics/bipolar-disorder#part_2605
2. National Institute of Mental Health. Schizophrenia. Accessed December 21, 2022. https://www.nimh.nih.gov/health/statistics/schizophrenia#part_2543
3. Dennis JA, Gittner LS, Payne JD, et al. Characteristics of U.S. adults taking prescription antipsychotic medications, National Health and Nutrition Examination Survey 2013-2018. BMC Psychiatry. 2020;20(1):483. doi: 10.1186/s12888-020-02895-4
4. Willner K, Vasan S, Abdijadid S. Atypical antipsychotic agents. In: StatPearls [Internet]. StatPearls Publishing; 2022. Updated May 2, 2022. Accessed December 22, 2022. https://www.ncbi.nlm.nih.gov/books/NBK448156/
5. Packard K, Price P, Hanson A. Antipsychotic use and the risk of rhabdomyolysis. J Pharm Pract 2014;27(5):501-512. doi: 10.1177/0897190013516509
6. Wu YF, Chang KY. Aripiprazole-associated rhabdomyolysis in a patient with schizophrenia. J Neuropsychiatry Clin Neurosci. 2011;23(3):E51.
7. Marzetti E, Bocchino L, Teramo S, et al. Rhabdomyolysis in a patient on aripiprazole with traumatic hip prosthesis luxation. J Neuropsychiatry Clin Neurosci. 2012;24(4):E40-E41.
8. Zhu X, Hu J, Deng S, et al. Rhabdomyolysis and elevated liver enzymes after rapid correction of hyponatremia due to pneumonia and concurrent use of aripiprazole: a case report. Aust N Z J Psychiatry. 2018;52(2):206. doi:10.1177/0004867417743342
9. Stahl SM. Essential Psychopharmacology: Neuroscientific Basis and Practical Application. 2nd ed. Cambridge University Press; 2000.
10. Stahl SM. “Hit-and-run” actions at dopamine receptors, part 1: mechanism of action of atypical antipsychotics. J Clin Psychiatry. 2001;62(9):670-671.
11. Leysen JE, Janssen PM, Schotte A, et al. Interaction of antipsychotic drugs with neurotransmitter receptor sites in vitro and in vivo in relation to pharmacological and clinical effects: role of 5HT2 receptors. Psychopharmacology (Berl). 1993;112(1 Suppl):S40-S54.
12. Millan MJ. Improving the treatment of schizophrenia: focus on serotonin (5-HT)(1A) receptors. J Pharmacol Exp Ther. 2000;295(3):853-861.
13. Millan MJ. The neurobiology and control of anxious states. Prog Neurobiol. 2003;70(2):83-244.
14. Meltzer HY, Cola PA, Parsa M. Marked elevations of serum creatine kinase activity associated with antipsychotic drug treatment. Neuropsychopharmacology. 1996;15(4):395-405.
15. Devarajan S, Dursun SM. Antipsychotic drugs, serum creatine kinase (CPK) and possible mechanisms. Psychopharmacology (Berl). 2000;152(1):122.
1. National Institute of Mental Health. Prevalence of bipolar disorder among adults. Accessed December 21, 2022. https://www.nimh.nih.gov/health/statistics/bipolar-disorder#part_2605
2. National Institute of Mental Health. Schizophrenia. Accessed December 21, 2022. https://www.nimh.nih.gov/health/statistics/schizophrenia#part_2543
3. Dennis JA, Gittner LS, Payne JD, et al. Characteristics of U.S. adults taking prescription antipsychotic medications, National Health and Nutrition Examination Survey 2013-2018. BMC Psychiatry. 2020;20(1):483. doi: 10.1186/s12888-020-02895-4
4. Willner K, Vasan S, Abdijadid S. Atypical antipsychotic agents. In: StatPearls [Internet]. StatPearls Publishing; 2022. Updated May 2, 2022. Accessed December 22, 2022. https://www.ncbi.nlm.nih.gov/books/NBK448156/
5. Packard K, Price P, Hanson A. Antipsychotic use and the risk of rhabdomyolysis. J Pharm Pract 2014;27(5):501-512. doi: 10.1177/0897190013516509
6. Wu YF, Chang KY. Aripiprazole-associated rhabdomyolysis in a patient with schizophrenia. J Neuropsychiatry Clin Neurosci. 2011;23(3):E51.
7. Marzetti E, Bocchino L, Teramo S, et al. Rhabdomyolysis in a patient on aripiprazole with traumatic hip prosthesis luxation. J Neuropsychiatry Clin Neurosci. 2012;24(4):E40-E41.
8. Zhu X, Hu J, Deng S, et al. Rhabdomyolysis and elevated liver enzymes after rapid correction of hyponatremia due to pneumonia and concurrent use of aripiprazole: a case report. Aust N Z J Psychiatry. 2018;52(2):206. doi:10.1177/0004867417743342
9. Stahl SM. Essential Psychopharmacology: Neuroscientific Basis and Practical Application. 2nd ed. Cambridge University Press; 2000.
10. Stahl SM. “Hit-and-run” actions at dopamine receptors, part 1: mechanism of action of atypical antipsychotics. J Clin Psychiatry. 2001;62(9):670-671.
11. Leysen JE, Janssen PM, Schotte A, et al. Interaction of antipsychotic drugs with neurotransmitter receptor sites in vitro and in vivo in relation to pharmacological and clinical effects: role of 5HT2 receptors. Psychopharmacology (Berl). 1993;112(1 Suppl):S40-S54.
12. Millan MJ. Improving the treatment of schizophrenia: focus on serotonin (5-HT)(1A) receptors. J Pharmacol Exp Ther. 2000;295(3):853-861.
13. Millan MJ. The neurobiology and control of anxious states. Prog Neurobiol. 2003;70(2):83-244.
14. Meltzer HY, Cola PA, Parsa M. Marked elevations of serum creatine kinase activity associated with antipsychotic drug treatment. Neuropsychopharmacology. 1996;15(4):395-405.
15. Devarajan S, Dursun SM. Antipsychotic drugs, serum creatine kinase (CPK) and possible mechanisms. Psychopharmacology (Berl). 2000;152(1):122.
Subtle cognitive decline in a patient with depression and anxiety
CASE Anxious and confused
Mr. M, age 53, a surgeon, presents to the emergency department (ED) following a panic attack and concerns from his staff that he appears confused. Specifically, staff members report that in the past 4 months, Mr. M was observed having problems completing some postoperative tasks related to chart documentation. Mr. M has a history of major depressive disorder (MDD), hypertension, hyperlipidemia, and type 2 diabetes.
HISTORY A long-standing diagnosis of depression
Mr. M reports that 30 years ago, he received care from a psychiatrist to address symptoms of MDD. He says that around the time he arrived at the ED, he had noticed subtle but gradual changes in his cognition, which led him to skip words and often struggle to find the correct words. These episodes left him confused. Mr. M started getting anxious about these cognitive issues because they disrupted his work and forced him to reduce his duties. He does not have any known family history of mental illness, is single, and lives alone.
EVALUATION After stroke is ruled out, a psychiatric workup
In the ED, a comprehensive exam rules out an acute cerebrovascular event. A neurologic evaluation notes some delay in processing information and observes Mr. M having difficulty following simple commands. Laboratory investigations, including a comprehensive metabolic panel, are unremarkable. An MRI of Mr. M’s brain, with and without contrast, notes no acute findings. He is discharged from the ED with a diagnosis of MDD.
Before he presented to the ED, Mr. M’s medication regimen included duloxetine 60 mg/d, buspirone 10 mg 3 times a day, and aripiprazole 5 mg/d for MDD and anxiety. After the ED visit, Mr. M’s physician refers him to an outpatient psychiatrist for management of worsening depression and panic attacks. During the psychiatrist’s evaluation, Mr. M reports a decreased interest in activities, decreased motivation, being easily fatigued, and having poor sleep. He denies having a depressed mood, difficulty concentrating, or having problems with his appetite. He also denies suicidal thoughts, both past and present.
Mr. M describes his mood as anxious, primarily surrounding his recent cognitive changes. He does not have a substance use disorder, psychotic illness, mania or hypomania, posttraumatic stress disorder, or obsessive-compulsive disorder. He reports adherence to his psychiatric medications. A mental status exam reveals Mr. M to be anxious. His attention is not well sustained, and he has difficulty describing details of his cognitive struggles, providing vague descriptions such as “skipping thought” and “skipping words.” Mr. M’s affect is congruent to his mood with some restriction and the psychiatrist notes that he is experiencing thought latency, poverty of content of thoughts, word-finding difficulties, and circumlocution. Mr. M denies any perceptual abnormalities, and there is no evidence of delusions.
[polldaddy:11320112]
The authors’ observations
Mr. M’s symptoms are significant for subacute cognitive decline that is subtle but gradual and can be easily missed, especially in the beginning. Though his ED evaluation—including brain imaging—ruled out acute or focal neurologic findings and his primary psychiatric presentation was anxiety, Mr. M’s medical history and mental status exam were suggestive of cognitive deficits.
Collateral information was obtained from his work colleagues, which confirmed both cognitive problems and comorbid anxiety. Additionally, given Mr. M’s high cognitive baseline as a surgeon, the new-onset cognitive changes over 4 months warranted further cognitive and neurologic evaluation. There are many causes of cognitive impairment (vascular, cancer, infection, autoimmune, medications, substances or toxins, neurodegenerative, psychiatric, vitamin deficiencies), all of which need to be considered in a patient with a nonspecific presentation such as Mr. M’s. The psychiatrist confirmed Mr. M’s current medication regimen, and discussed tapering aripiprazole while continuing duloxetine and buspirone.
Continue to: EVALUATION A closer look at cognitive deficits
EVALUATION A closer look at cognitive deficits
Mr. M scores 12/30 on the Montreal Cognitive Assessment (MoCA), indicating moderate cognitive impairment (Table 1). The psychiatrist refers Mr. M to Neurology. During his neurologic evaluation, Mr. M continues to report feeling anxious that “something is wrong” and skips his words. The neurologist confirms Mr. M’s symptoms may have started 2 to 3 months before he presented to the ED. Mr. M reports unusual eating habits, including yogurt and cookies for breakfast, Mexican food for lunch, and more cookies for dinner. He denies having a fever, gaining or losing weight, rashes, headaches, neck stiffness, tingling or weakness or stiffness of limbs, vertigo, visual changes, photophobia, unsteady gait, bowel or bladder incontinence, or tremors.
When the neurologist repeats the MoCA, Mr. M again scores 12. The neurologist notes that Mr. M answers questions a little slowly and pauses for thoughts when unable to find an answer. Mr. M has difficulty following some simple commands, such as “touch a finger to your nose.” Other in-office neurologic physical exams (cranial nerves, involuntary movements or tremors, sensation, muscle strength, reflexes, cerebellar signs) are unremarkable except for mildly decreased vibration sense of his toes. The neurologist concludes that Mr. M’s presentation is suggestive of subacute to chronic bradyphrenia and orders additional evaluation, including neuropsychological testing.
[polldaddy:11320114]
The authors’ observations
Physical and neurologic exams were not suggestive of any obvious causes of cognitive decline. Both the mental status exam and 2 serial MoCAs suggested deficits in executive function, language, and memory. Each of the differential diagnoses considered was ruled out with workup or exams (Table 2), which led to a most likely diagnosis of neurodegenerative disorder with PPA. Neuropsychological testing confirmed the diagnosis of nonfluent PPA.
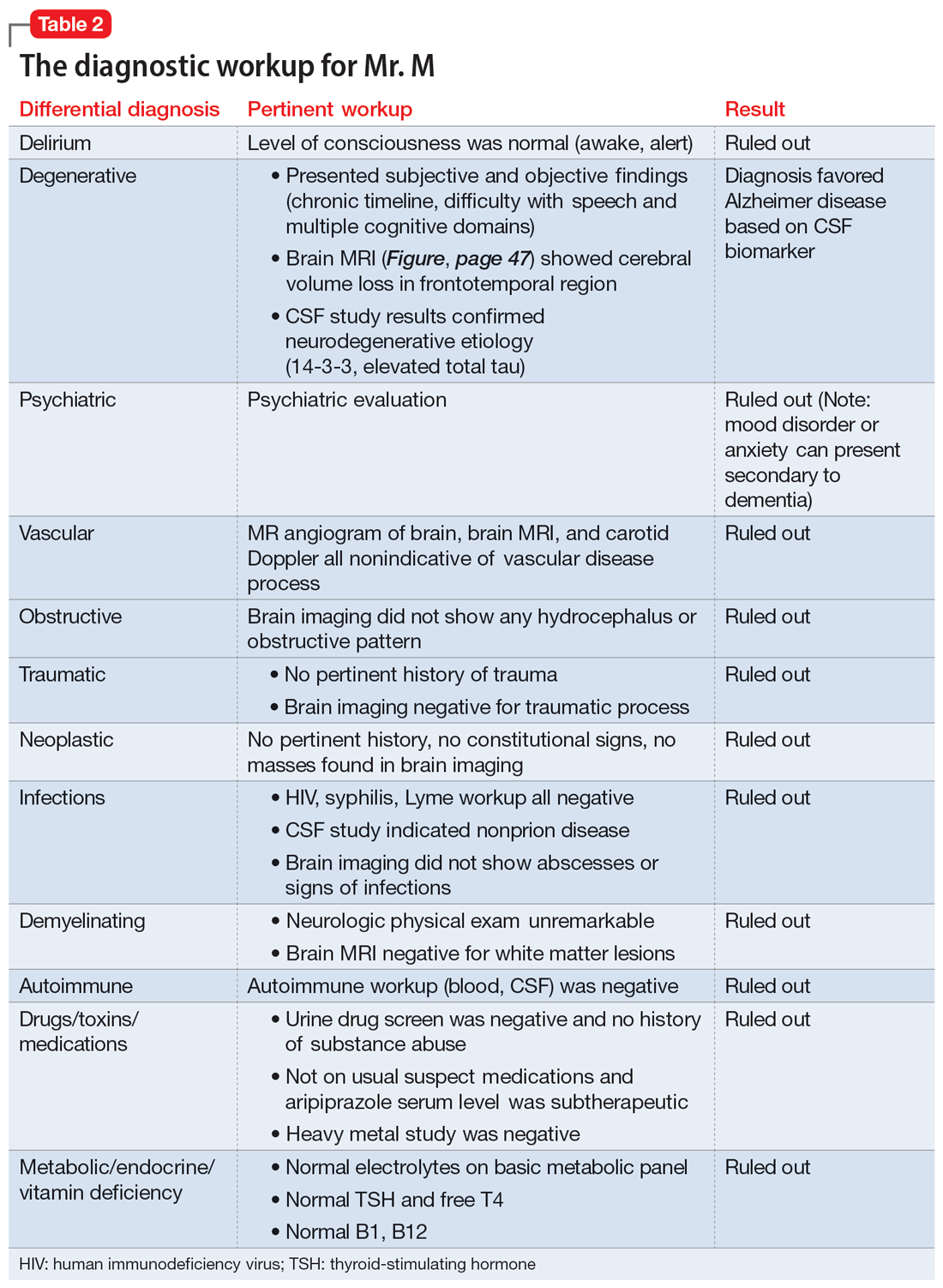
Primary progressive aphasia
PPA is an uncommon, heterogeneous group of disorders stemming from focal degeneration of language-governing centers of the brain.1,2 The estimated prevalence of PPA is 3 in 100,000 cases.2,3 There are 4 major variants of PPA (Table 34), and each presents with distinct language, cognitive, neuroanatomical, and neuropathological characteristics.4 PPA is usually diagnosed in late middle life; however, diagnosis is often delayed due to the relative obscurity of the disorder.4 In Mr. M’s case, it took approximately 4 months of evaluations by various specialists before a diagnosis was confirmed.
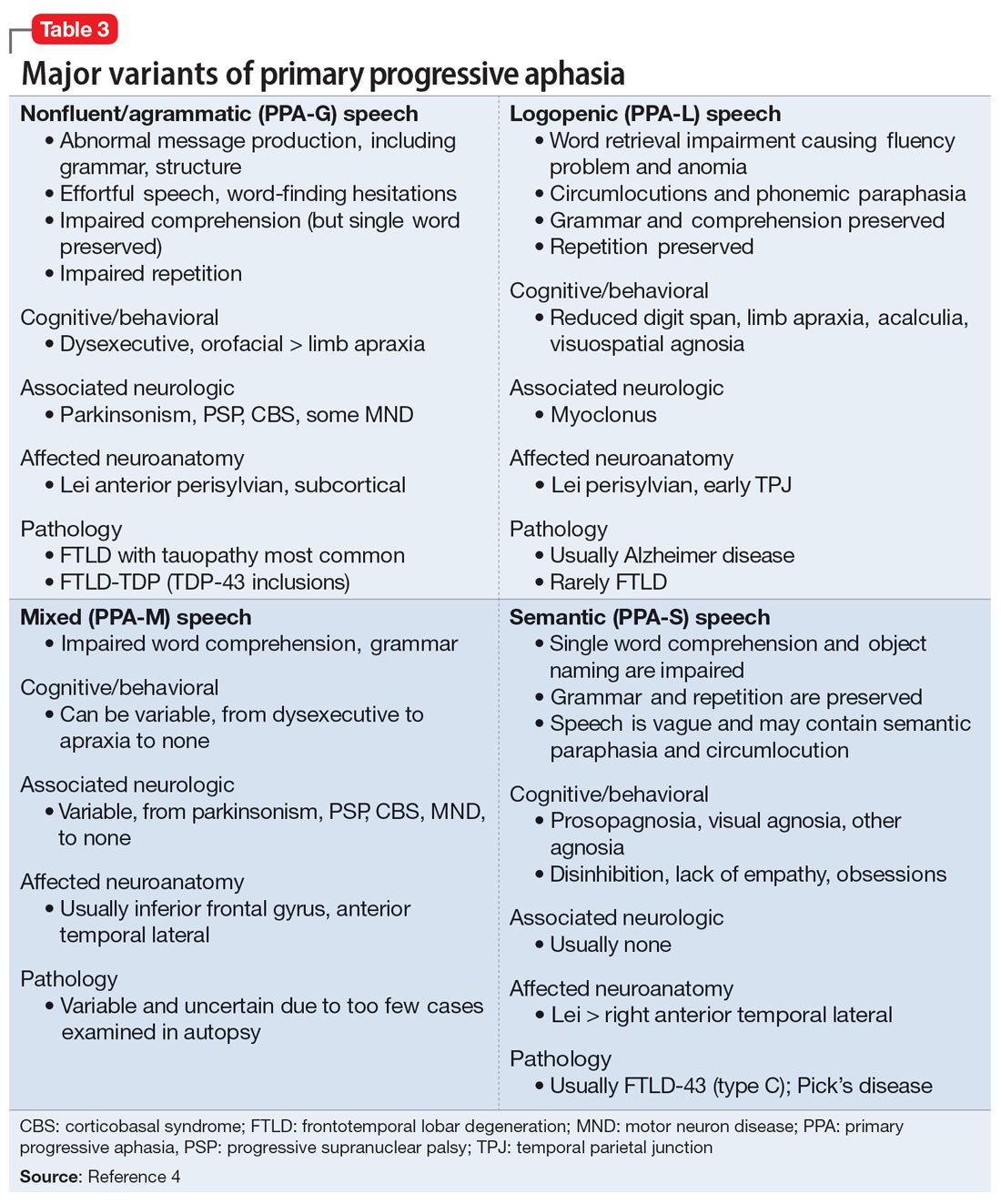
The initial phase of PPA can present as a diagnostic challenge because patients can have difficulty articulating their cognitive and language deficits. PPA can be commonly mistaken for a primary psychiatric disorder such as MDD or anxiety, which can further delay an accurate diagnosis and treatment. Special attention to the mental status exam, close observation of the patient’s language, and assessment of cognitive abilities using standardized screenings such as the MoCA or Mini-Mental State Examination can be helpful in clarifying the diagnosis. It is also important to rule out developmental problems (eg, dyslexia) and hearing difficulties, particularly in older patients.
Continue to: TREATMENT Adjusting the medication regimen
TREATMENT Adjusting the medication regimen
The neurologist completes additional examinations to rule out causes of rare neurodegenerative disorders, including CSF autoimmune disorders, Creutzfeldt-Jakob disease, and Alzheimer disease (AD) (Table 4). Mr. M continues to follow up with his outpatient psychiatrist and his medication regimen is adjusted. Aripiprazole and buspirone are discontinued, and duloxetine is titrated to 60 mg twice a day. During follow-up visits, Mr. M discusses his understanding of his neurologic condition. His concerns shift to his illness and prognosis. During these visits, he continues to deny suicidality.
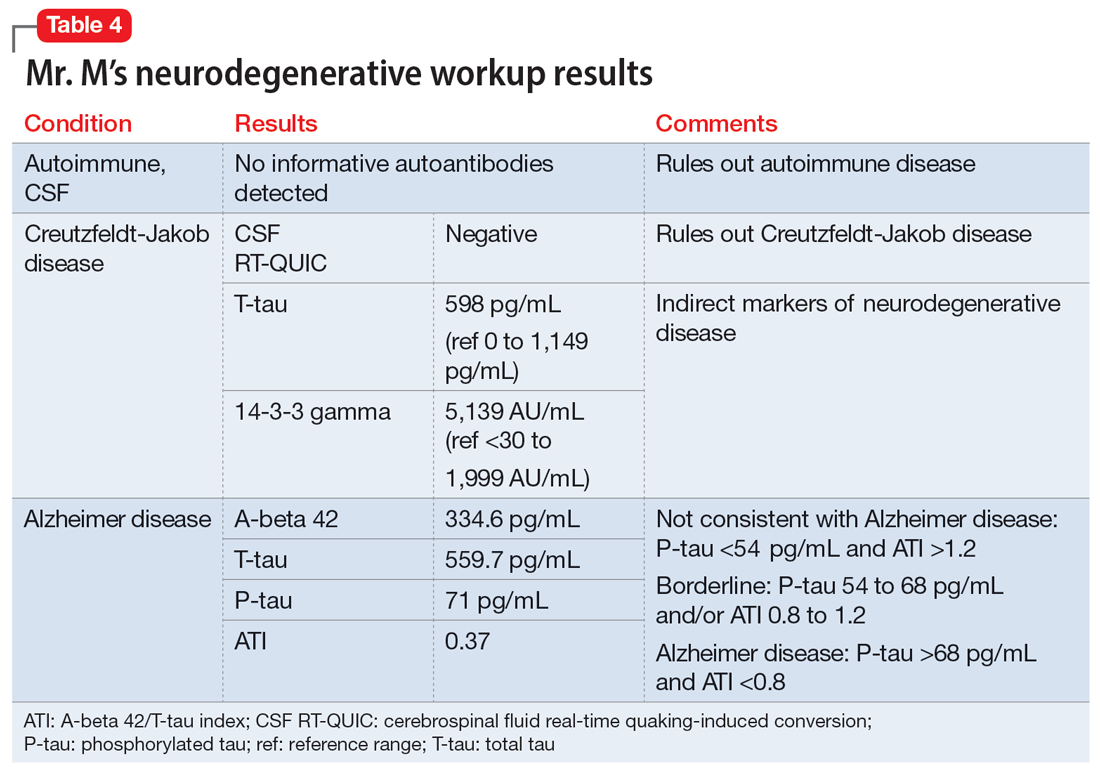
[polldaddy:11320115]
The authors’ observations
Mr. M’s neurodegenerative workup identified an intriguing diagnostic challenge. A repeat brain MRI (Figure) showed atrophy patterns suggestive of frontotemporal lobar degeneration (FTLD). On the other hand, his CSF ATI (A-beta 42/T-tau index, a value used to aid in the diagnosis of AD) was <1, suggesting early-onset AD.5,6 Although significant advances have been made to distinguish AD and FTLD following an autopsy, there are still no reliable or definitive biomarkers to distinguish AD from FTLD (particularly in the early stages of FTLD). This can often leave the confirmatory diagnosis as a question.7
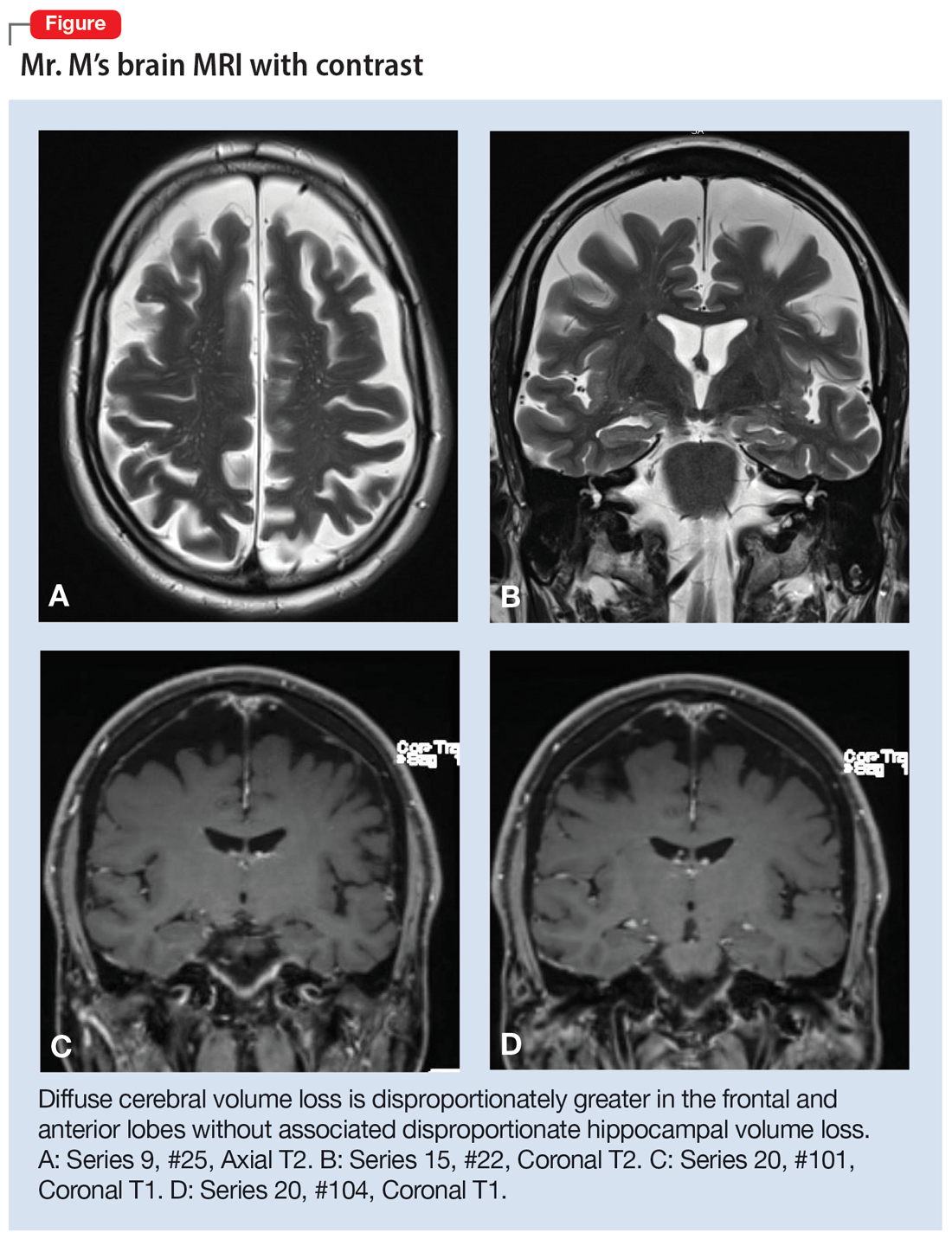
A PPA diagnosis (and other dementias) can have a significant impact on the patient and their family due to the uncertain nature of the progression of the disease and quality-of-life issues related to language and other cognitive deficits. Early identification and accurate diagnosis of PPA and its etiology (ie, AD vs FTLD) is important to avoid unnecessary exposure to medications or the use of polypharmacy to treat an inaccurate diagnosis of a primary psychiatric illness. For example, Mr. M was being treated with 3 psychiatric medications (aripiprazole, buspirone, and duloxetine) for depression and anxiety prior to the diagnosis of PPA.
Nonpharmacologic interventions can play an important role in the management of patients with PPA. These include educating the patient and their family about the diagnosis and discussions about future planning, including appropriate social support, employment, and finances.4 Pharmacologic interventions may be limited, as there are currently no disease-modifying treatments for PPA or FTLD. For patients with nonfluent PPA or AD, cholinesterase inhibitors such as donepezil or N-methyl
Psychiatrists should continue to treat patients with PPA for comorbid anxiety or depression, with appropriate medications and/or supportive therapy to guide the patient through the process of grief. Assessing for suicide risk is also important in patients diagnosed with dementia. A retrospective cohort study of patients age ≥60 with a diagnosis of dementia suggested that the majority of suicides occurred in those with a new dementia diagnosis.9 End-of-life decisions such as advanced directives should be made when the patient still has legal capacity, ideally as soon as possible after diagnosis.10
OUTCOME Remaining engaged in treatment
Mr. M continues to follow-up with the Neurology team. He has also been regularly seeing his psychiatric team for medication management and supportive therapy, and his psychiatric medications have been optimized to reduce polypharmacy. During his sessions, Mr. M discusses his grief and plans for the future. Despite his anxiety about the uncertainty of his prognosis, Mr. M continues to report that he is doing reasonably well and remains engaged in treatment.
Bottom Line
Patients with primary progressive aphasia and rare neurodegenerative disorders may present to an outpatient or emergency setting with symptoms of anxiety and confusion. They are frequently misdiagnosed with a primary psychiatric disorder due to the nature of cognitive and language deficits, particularly in the early stages of the disease. Paying close attention to language and conducting cognitive screening are critical in identifying the true cause of a patient’s symptoms.
Related Resources
- Primary progressive aphasia. National Center for Advancing Translational Sciences. Genetic and Rare Diseases Information Center. https://rarediseases.info.nih.gov/diseases/8541/primary-progressive-aphasia
- Moller MD, Parmenter BA, Lane DW. Neuropsychological testing: A useful but underutilized resource. Current Psychiatry. 2019;18(11):40-46,51.
Drug Brand Names
Aripiprazole • Abilify
Donepezil • Aricept
Duloxetine • Cymbalta
Memantine • Namenda
1. Grossman M. Primary progressive aphasia: clinicopathological correlations. Nat Rev Neurol. 2010;6(2):88-97. doi:10.1038/nrneurol.2009.216
2. Mesulam M-M, Rogalski EJ, Wieneke C, et al. Primary progressive aphasia and the evolving neurology of the language network. Nat Rev Neurol. 2014;10(10):554-569. doi:10.1038/nrneurol.2014.159
3. Coyle-Gilchrist ITS, Dick KM, Patterson K, et al. Prevalence, characteristics, and survival of frontotemporal lobar degeneration syndromes. Neurology. 2016;86(18):1736-1743. doi:10.1212/WNL.0000000000002638
4. Marshall CR, Hardy CJD, Volkmer A, et al. Primary progressive aphasia: a clinical approach. J Neurol. 2018;265(6):1474-1490. doi:10.1007/s00415-018-8762-6
5. Blennow K. Cerebrospinal fluid protein biomarkers for Alzheimer’s disease. NeuroRx. 2004;1(2):213-225. doi:10.1602/neurorx.1.2.213
6. Hulstaert F, Blennow K, Ivanoiu A, et al. Improved discrimination of AD patients using beta-amyloid(1-42) and tau levels in CSF. Neurology. 1999;52(8):1555-1562. doi:10.1212/wnl.52.8.1555
7. Thijssen EH, La Joie R, Wolf A, et al. Diagnostic value of plasma phosphorylated tau181 in Alzheimer’s disease and frontotemporal lobar degeneration. Nat Med. 2020;26(3):387-397. doi:10.1038/s41591-020-0762-2
8. Newhart M, Davis C, Kannan V, et al. Therapy for naming deficits in two variants of primary progressive aphasia. Aphasiology. 2009;23(7-8):823-834. doi:10.1080/02687030802661762
9. Seyfried LS, Kales HC, Ignacio RV, et al. Predictors of suicide in patients with dementia. Alzheimers Dement. 2011;7(6):567-573. doi:10.1016/j.jalz.2011.01.006
10. Porteri C. Advance directives as a tool to respect patients’ values and preferences: discussion on the case of Alzheimer’s disease. BMC Med Ethics. 2018;19(1):9. doi:10.1186/s12910-018-0249-6
CASE Anxious and confused
Mr. M, age 53, a surgeon, presents to the emergency department (ED) following a panic attack and concerns from his staff that he appears confused. Specifically, staff members report that in the past 4 months, Mr. M was observed having problems completing some postoperative tasks related to chart documentation. Mr. M has a history of major depressive disorder (MDD), hypertension, hyperlipidemia, and type 2 diabetes.
HISTORY A long-standing diagnosis of depression
Mr. M reports that 30 years ago, he received care from a psychiatrist to address symptoms of MDD. He says that around the time he arrived at the ED, he had noticed subtle but gradual changes in his cognition, which led him to skip words and often struggle to find the correct words. These episodes left him confused. Mr. M started getting anxious about these cognitive issues because they disrupted his work and forced him to reduce his duties. He does not have any known family history of mental illness, is single, and lives alone.
EVALUATION After stroke is ruled out, a psychiatric workup
In the ED, a comprehensive exam rules out an acute cerebrovascular event. A neurologic evaluation notes some delay in processing information and observes Mr. M having difficulty following simple commands. Laboratory investigations, including a comprehensive metabolic panel, are unremarkable. An MRI of Mr. M’s brain, with and without contrast, notes no acute findings. He is discharged from the ED with a diagnosis of MDD.
Before he presented to the ED, Mr. M’s medication regimen included duloxetine 60 mg/d, buspirone 10 mg 3 times a day, and aripiprazole 5 mg/d for MDD and anxiety. After the ED visit, Mr. M’s physician refers him to an outpatient psychiatrist for management of worsening depression and panic attacks. During the psychiatrist’s evaluation, Mr. M reports a decreased interest in activities, decreased motivation, being easily fatigued, and having poor sleep. He denies having a depressed mood, difficulty concentrating, or having problems with his appetite. He also denies suicidal thoughts, both past and present.
Mr. M describes his mood as anxious, primarily surrounding his recent cognitive changes. He does not have a substance use disorder, psychotic illness, mania or hypomania, posttraumatic stress disorder, or obsessive-compulsive disorder. He reports adherence to his psychiatric medications. A mental status exam reveals Mr. M to be anxious. His attention is not well sustained, and he has difficulty describing details of his cognitive struggles, providing vague descriptions such as “skipping thought” and “skipping words.” Mr. M’s affect is congruent to his mood with some restriction and the psychiatrist notes that he is experiencing thought latency, poverty of content of thoughts, word-finding difficulties, and circumlocution. Mr. M denies any perceptual abnormalities, and there is no evidence of delusions.
[polldaddy:11320112]
The authors’ observations
Mr. M’s symptoms are significant for subacute cognitive decline that is subtle but gradual and can be easily missed, especially in the beginning. Though his ED evaluation—including brain imaging—ruled out acute or focal neurologic findings and his primary psychiatric presentation was anxiety, Mr. M’s medical history and mental status exam were suggestive of cognitive deficits.
Collateral information was obtained from his work colleagues, which confirmed both cognitive problems and comorbid anxiety. Additionally, given Mr. M’s high cognitive baseline as a surgeon, the new-onset cognitive changes over 4 months warranted further cognitive and neurologic evaluation. There are many causes of cognitive impairment (vascular, cancer, infection, autoimmune, medications, substances or toxins, neurodegenerative, psychiatric, vitamin deficiencies), all of which need to be considered in a patient with a nonspecific presentation such as Mr. M’s. The psychiatrist confirmed Mr. M’s current medication regimen, and discussed tapering aripiprazole while continuing duloxetine and buspirone.
Continue to: EVALUATION A closer look at cognitive deficits
EVALUATION A closer look at cognitive deficits
Mr. M scores 12/30 on the Montreal Cognitive Assessment (MoCA), indicating moderate cognitive impairment (Table 1). The psychiatrist refers Mr. M to Neurology. During his neurologic evaluation, Mr. M continues to report feeling anxious that “something is wrong” and skips his words. The neurologist confirms Mr. M’s symptoms may have started 2 to 3 months before he presented to the ED. Mr. M reports unusual eating habits, including yogurt and cookies for breakfast, Mexican food for lunch, and more cookies for dinner. He denies having a fever, gaining or losing weight, rashes, headaches, neck stiffness, tingling or weakness or stiffness of limbs, vertigo, visual changes, photophobia, unsteady gait, bowel or bladder incontinence, or tremors.

When the neurologist repeats the MoCA, Mr. M again scores 12. The neurologist notes that Mr. M answers questions a little slowly and pauses for thoughts when unable to find an answer. Mr. M has difficulty following some simple commands, such as “touch a finger to your nose.” Other in-office neurologic physical exams (cranial nerves, involuntary movements or tremors, sensation, muscle strength, reflexes, cerebellar signs) are unremarkable except for mildly decreased vibration sense of his toes. The neurologist concludes that Mr. M’s presentation is suggestive of subacute to chronic bradyphrenia and orders additional evaluation, including neuropsychological testing.
[polldaddy:11320114]
The authors’ observations
Physical and neurologic exams were not suggestive of any obvious causes of cognitive decline. Both the mental status exam and 2 serial MoCAs suggested deficits in executive function, language, and memory. Each of the differential diagnoses considered was ruled out with workup or exams (Table 2), which led to a most likely diagnosis of neurodegenerative disorder with PPA. Neuropsychological testing confirmed the diagnosis of nonfluent PPA.

Primary progressive aphasia
PPA is an uncommon, heterogeneous group of disorders stemming from focal degeneration of language-governing centers of the brain.1,2 The estimated prevalence of PPA is 3 in 100,000 cases.2,3 There are 4 major variants of PPA (Table 34), and each presents with distinct language, cognitive, neuroanatomical, and neuropathological characteristics.4 PPA is usually diagnosed in late middle life; however, diagnosis is often delayed due to the relative obscurity of the disorder.4 In Mr. M’s case, it took approximately 4 months of evaluations by various specialists before a diagnosis was confirmed.

The initial phase of PPA can present as a diagnostic challenge because patients can have difficulty articulating their cognitive and language deficits. PPA can be commonly mistaken for a primary psychiatric disorder such as MDD or anxiety, which can further delay an accurate diagnosis and treatment. Special attention to the mental status exam, close observation of the patient’s language, and assessment of cognitive abilities using standardized screenings such as the MoCA or Mini-Mental State Examination can be helpful in clarifying the diagnosis. It is also important to rule out developmental problems (eg, dyslexia) and hearing difficulties, particularly in older patients.
Continue to: TREATMENT Adjusting the medication regimen
TREATMENT Adjusting the medication regimen
The neurologist completes additional examinations to rule out causes of rare neurodegenerative disorders, including CSF autoimmune disorders, Creutzfeldt-Jakob disease, and Alzheimer disease (AD) (Table 4). Mr. M continues to follow up with his outpatient psychiatrist and his medication regimen is adjusted. Aripiprazole and buspirone are discontinued, and duloxetine is titrated to 60 mg twice a day. During follow-up visits, Mr. M discusses his understanding of his neurologic condition. His concerns shift to his illness and prognosis. During these visits, he continues to deny suicidality.

[polldaddy:11320115]
The authors’ observations
Mr. M’s neurodegenerative workup identified an intriguing diagnostic challenge. A repeat brain MRI (Figure) showed atrophy patterns suggestive of frontotemporal lobar degeneration (FTLD). On the other hand, his CSF ATI (A-beta 42/T-tau index, a value used to aid in the diagnosis of AD) was <1, suggesting early-onset AD.5,6 Although significant advances have been made to distinguish AD and FTLD following an autopsy, there are still no reliable or definitive biomarkers to distinguish AD from FTLD (particularly in the early stages of FTLD). This can often leave the confirmatory diagnosis as a question.7

A PPA diagnosis (and other dementias) can have a significant impact on the patient and their family due to the uncertain nature of the progression of the disease and quality-of-life issues related to language and other cognitive deficits. Early identification and accurate diagnosis of PPA and its etiology (ie, AD vs FTLD) is important to avoid unnecessary exposure to medications or the use of polypharmacy to treat an inaccurate diagnosis of a primary psychiatric illness. For example, Mr. M was being treated with 3 psychiatric medications (aripiprazole, buspirone, and duloxetine) for depression and anxiety prior to the diagnosis of PPA.
Nonpharmacologic interventions can play an important role in the management of patients with PPA. These include educating the patient and their family about the diagnosis and discussions about future planning, including appropriate social support, employment, and finances.4 Pharmacologic interventions may be limited, as there are currently no disease-modifying treatments for PPA or FTLD. For patients with nonfluent PPA or AD, cholinesterase inhibitors such as donepezil or N-methyl
Psychiatrists should continue to treat patients with PPA for comorbid anxiety or depression, with appropriate medications and/or supportive therapy to guide the patient through the process of grief. Assessing for suicide risk is also important in patients diagnosed with dementia. A retrospective cohort study of patients age ≥60 with a diagnosis of dementia suggested that the majority of suicides occurred in those with a new dementia diagnosis.9 End-of-life decisions such as advanced directives should be made when the patient still has legal capacity, ideally as soon as possible after diagnosis.10
OUTCOME Remaining engaged in treatment
Mr. M continues to follow-up with the Neurology team. He has also been regularly seeing his psychiatric team for medication management and supportive therapy, and his psychiatric medications have been optimized to reduce polypharmacy. During his sessions, Mr. M discusses his grief and plans for the future. Despite his anxiety about the uncertainty of his prognosis, Mr. M continues to report that he is doing reasonably well and remains engaged in treatment.
Bottom Line
Patients with primary progressive aphasia and rare neurodegenerative disorders may present to an outpatient or emergency setting with symptoms of anxiety and confusion. They are frequently misdiagnosed with a primary psychiatric disorder due to the nature of cognitive and language deficits, particularly in the early stages of the disease. Paying close attention to language and conducting cognitive screening are critical in identifying the true cause of a patient’s symptoms.
Related Resources
- Primary progressive aphasia. National Center for Advancing Translational Sciences. Genetic and Rare Diseases Information Center. https://rarediseases.info.nih.gov/diseases/8541/primary-progressive-aphasia
- Moller MD, Parmenter BA, Lane DW. Neuropsychological testing: A useful but underutilized resource. Current Psychiatry. 2019;18(11):40-46,51.
Drug Brand Names
Aripiprazole • Abilify
Donepezil • Aricept
Duloxetine • Cymbalta
Memantine • Namenda
CASE Anxious and confused
Mr. M, age 53, a surgeon, presents to the emergency department (ED) following a panic attack and concerns from his staff that he appears confused. Specifically, staff members report that in the past 4 months, Mr. M was observed having problems completing some postoperative tasks related to chart documentation. Mr. M has a history of major depressive disorder (MDD), hypertension, hyperlipidemia, and type 2 diabetes.
HISTORY A long-standing diagnosis of depression
Mr. M reports that 30 years ago, he received care from a psychiatrist to address symptoms of MDD. He says that around the time he arrived at the ED, he had noticed subtle but gradual changes in his cognition, which led him to skip words and often struggle to find the correct words. These episodes left him confused. Mr. M started getting anxious about these cognitive issues because they disrupted his work and forced him to reduce his duties. He does not have any known family history of mental illness, is single, and lives alone.
EVALUATION After stroke is ruled out, a psychiatric workup
In the ED, a comprehensive exam rules out an acute cerebrovascular event. A neurologic evaluation notes some delay in processing information and observes Mr. M having difficulty following simple commands. Laboratory investigations, including a comprehensive metabolic panel, are unremarkable. An MRI of Mr. M’s brain, with and without contrast, notes no acute findings. He is discharged from the ED with a diagnosis of MDD.
Before he presented to the ED, Mr. M’s medication regimen included duloxetine 60 mg/d, buspirone 10 mg 3 times a day, and aripiprazole 5 mg/d for MDD and anxiety. After the ED visit, Mr. M’s physician refers him to an outpatient psychiatrist for management of worsening depression and panic attacks. During the psychiatrist’s evaluation, Mr. M reports a decreased interest in activities, decreased motivation, being easily fatigued, and having poor sleep. He denies having a depressed mood, difficulty concentrating, or having problems with his appetite. He also denies suicidal thoughts, both past and present.
Mr. M describes his mood as anxious, primarily surrounding his recent cognitive changes. He does not have a substance use disorder, psychotic illness, mania or hypomania, posttraumatic stress disorder, or obsessive-compulsive disorder. He reports adherence to his psychiatric medications. A mental status exam reveals Mr. M to be anxious. His attention is not well sustained, and he has difficulty describing details of his cognitive struggles, providing vague descriptions such as “skipping thought” and “skipping words.” Mr. M’s affect is congruent to his mood with some restriction and the psychiatrist notes that he is experiencing thought latency, poverty of content of thoughts, word-finding difficulties, and circumlocution. Mr. M denies any perceptual abnormalities, and there is no evidence of delusions.
[polldaddy:11320112]
The authors’ observations
Mr. M’s symptoms are significant for subacute cognitive decline that is subtle but gradual and can be easily missed, especially in the beginning. Though his ED evaluation—including brain imaging—ruled out acute or focal neurologic findings and his primary psychiatric presentation was anxiety, Mr. M’s medical history and mental status exam were suggestive of cognitive deficits.
Collateral information was obtained from his work colleagues, which confirmed both cognitive problems and comorbid anxiety. Additionally, given Mr. M’s high cognitive baseline as a surgeon, the new-onset cognitive changes over 4 months warranted further cognitive and neurologic evaluation. There are many causes of cognitive impairment (vascular, cancer, infection, autoimmune, medications, substances or toxins, neurodegenerative, psychiatric, vitamin deficiencies), all of which need to be considered in a patient with a nonspecific presentation such as Mr. M’s. The psychiatrist confirmed Mr. M’s current medication regimen, and discussed tapering aripiprazole while continuing duloxetine and buspirone.
Continue to: EVALUATION A closer look at cognitive deficits
EVALUATION A closer look at cognitive deficits
Mr. M scores 12/30 on the Montreal Cognitive Assessment (MoCA), indicating moderate cognitive impairment (Table 1). The psychiatrist refers Mr. M to Neurology. During his neurologic evaluation, Mr. M continues to report feeling anxious that “something is wrong” and skips his words. The neurologist confirms Mr. M’s symptoms may have started 2 to 3 months before he presented to the ED. Mr. M reports unusual eating habits, including yogurt and cookies for breakfast, Mexican food for lunch, and more cookies for dinner. He denies having a fever, gaining or losing weight, rashes, headaches, neck stiffness, tingling or weakness or stiffness of limbs, vertigo, visual changes, photophobia, unsteady gait, bowel or bladder incontinence, or tremors.

When the neurologist repeats the MoCA, Mr. M again scores 12. The neurologist notes that Mr. M answers questions a little slowly and pauses for thoughts when unable to find an answer. Mr. M has difficulty following some simple commands, such as “touch a finger to your nose.” Other in-office neurologic physical exams (cranial nerves, involuntary movements or tremors, sensation, muscle strength, reflexes, cerebellar signs) are unremarkable except for mildly decreased vibration sense of his toes. The neurologist concludes that Mr. M’s presentation is suggestive of subacute to chronic bradyphrenia and orders additional evaluation, including neuropsychological testing.
[polldaddy:11320114]
The authors’ observations
Physical and neurologic exams were not suggestive of any obvious causes of cognitive decline. Both the mental status exam and 2 serial MoCAs suggested deficits in executive function, language, and memory. Each of the differential diagnoses considered was ruled out with workup or exams (Table 2), which led to a most likely diagnosis of neurodegenerative disorder with PPA. Neuropsychological testing confirmed the diagnosis of nonfluent PPA.

Primary progressive aphasia
PPA is an uncommon, heterogeneous group of disorders stemming from focal degeneration of language-governing centers of the brain.1,2 The estimated prevalence of PPA is 3 in 100,000 cases.2,3 There are 4 major variants of PPA (Table 34), and each presents with distinct language, cognitive, neuroanatomical, and neuropathological characteristics.4 PPA is usually diagnosed in late middle life; however, diagnosis is often delayed due to the relative obscurity of the disorder.4 In Mr. M’s case, it took approximately 4 months of evaluations by various specialists before a diagnosis was confirmed.

The initial phase of PPA can present as a diagnostic challenge because patients can have difficulty articulating their cognitive and language deficits. PPA can be commonly mistaken for a primary psychiatric disorder such as MDD or anxiety, which can further delay an accurate diagnosis and treatment. Special attention to the mental status exam, close observation of the patient’s language, and assessment of cognitive abilities using standardized screenings such as the MoCA or Mini-Mental State Examination can be helpful in clarifying the diagnosis. It is also important to rule out developmental problems (eg, dyslexia) and hearing difficulties, particularly in older patients.
Continue to: TREATMENT Adjusting the medication regimen
TREATMENT Adjusting the medication regimen
The neurologist completes additional examinations to rule out causes of rare neurodegenerative disorders, including CSF autoimmune disorders, Creutzfeldt-Jakob disease, and Alzheimer disease (AD) (Table 4). Mr. M continues to follow up with his outpatient psychiatrist and his medication regimen is adjusted. Aripiprazole and buspirone are discontinued, and duloxetine is titrated to 60 mg twice a day. During follow-up visits, Mr. M discusses his understanding of his neurologic condition. His concerns shift to his illness and prognosis. During these visits, he continues to deny suicidality.

[polldaddy:11320115]
The authors’ observations
Mr. M’s neurodegenerative workup identified an intriguing diagnostic challenge. A repeat brain MRI (Figure) showed atrophy patterns suggestive of frontotemporal lobar degeneration (FTLD). On the other hand, his CSF ATI (A-beta 42/T-tau index, a value used to aid in the diagnosis of AD) was <1, suggesting early-onset AD.5,6 Although significant advances have been made to distinguish AD and FTLD following an autopsy, there are still no reliable or definitive biomarkers to distinguish AD from FTLD (particularly in the early stages of FTLD). This can often leave the confirmatory diagnosis as a question.7

A PPA diagnosis (and other dementias) can have a significant impact on the patient and their family due to the uncertain nature of the progression of the disease and quality-of-life issues related to language and other cognitive deficits. Early identification and accurate diagnosis of PPA and its etiology (ie, AD vs FTLD) is important to avoid unnecessary exposure to medications or the use of polypharmacy to treat an inaccurate diagnosis of a primary psychiatric illness. For example, Mr. M was being treated with 3 psychiatric medications (aripiprazole, buspirone, and duloxetine) for depression and anxiety prior to the diagnosis of PPA.
Nonpharmacologic interventions can play an important role in the management of patients with PPA. These include educating the patient and their family about the diagnosis and discussions about future planning, including appropriate social support, employment, and finances.4 Pharmacologic interventions may be limited, as there are currently no disease-modifying treatments for PPA or FTLD. For patients with nonfluent PPA or AD, cholinesterase inhibitors such as donepezil or N-methyl
Psychiatrists should continue to treat patients with PPA for comorbid anxiety or depression, with appropriate medications and/or supportive therapy to guide the patient through the process of grief. Assessing for suicide risk is also important in patients diagnosed with dementia. A retrospective cohort study of patients age ≥60 with a diagnosis of dementia suggested that the majority of suicides occurred in those with a new dementia diagnosis.9 End-of-life decisions such as advanced directives should be made when the patient still has legal capacity, ideally as soon as possible after diagnosis.10
OUTCOME Remaining engaged in treatment
Mr. M continues to follow-up with the Neurology team. He has also been regularly seeing his psychiatric team for medication management and supportive therapy, and his psychiatric medications have been optimized to reduce polypharmacy. During his sessions, Mr. M discusses his grief and plans for the future. Despite his anxiety about the uncertainty of his prognosis, Mr. M continues to report that he is doing reasonably well and remains engaged in treatment.
Bottom Line
Patients with primary progressive aphasia and rare neurodegenerative disorders may present to an outpatient or emergency setting with symptoms of anxiety and confusion. They are frequently misdiagnosed with a primary psychiatric disorder due to the nature of cognitive and language deficits, particularly in the early stages of the disease. Paying close attention to language and conducting cognitive screening are critical in identifying the true cause of a patient’s symptoms.
Related Resources
- Primary progressive aphasia. National Center for Advancing Translational Sciences. Genetic and Rare Diseases Information Center. https://rarediseases.info.nih.gov/diseases/8541/primary-progressive-aphasia
- Moller MD, Parmenter BA, Lane DW. Neuropsychological testing: A useful but underutilized resource. Current Psychiatry. 2019;18(11):40-46,51.
Drug Brand Names
Aripiprazole • Abilify
Donepezil • Aricept
Duloxetine • Cymbalta
Memantine • Namenda
1. Grossman M. Primary progressive aphasia: clinicopathological correlations. Nat Rev Neurol. 2010;6(2):88-97. doi:10.1038/nrneurol.2009.216
2. Mesulam M-M, Rogalski EJ, Wieneke C, et al. Primary progressive aphasia and the evolving neurology of the language network. Nat Rev Neurol. 2014;10(10):554-569. doi:10.1038/nrneurol.2014.159
3. Coyle-Gilchrist ITS, Dick KM, Patterson K, et al. Prevalence, characteristics, and survival of frontotemporal lobar degeneration syndromes. Neurology. 2016;86(18):1736-1743. doi:10.1212/WNL.0000000000002638
4. Marshall CR, Hardy CJD, Volkmer A, et al. Primary progressive aphasia: a clinical approach. J Neurol. 2018;265(6):1474-1490. doi:10.1007/s00415-018-8762-6
5. Blennow K. Cerebrospinal fluid protein biomarkers for Alzheimer’s disease. NeuroRx. 2004;1(2):213-225. doi:10.1602/neurorx.1.2.213
6. Hulstaert F, Blennow K, Ivanoiu A, et al. Improved discrimination of AD patients using beta-amyloid(1-42) and tau levels in CSF. Neurology. 1999;52(8):1555-1562. doi:10.1212/wnl.52.8.1555
7. Thijssen EH, La Joie R, Wolf A, et al. Diagnostic value of plasma phosphorylated tau181 in Alzheimer’s disease and frontotemporal lobar degeneration. Nat Med. 2020;26(3):387-397. doi:10.1038/s41591-020-0762-2
8. Newhart M, Davis C, Kannan V, et al. Therapy for naming deficits in two variants of primary progressive aphasia. Aphasiology. 2009;23(7-8):823-834. doi:10.1080/02687030802661762
9. Seyfried LS, Kales HC, Ignacio RV, et al. Predictors of suicide in patients with dementia. Alzheimers Dement. 2011;7(6):567-573. doi:10.1016/j.jalz.2011.01.006
10. Porteri C. Advance directives as a tool to respect patients’ values and preferences: discussion on the case of Alzheimer’s disease. BMC Med Ethics. 2018;19(1):9. doi:10.1186/s12910-018-0249-6
1. Grossman M. Primary progressive aphasia: clinicopathological correlations. Nat Rev Neurol. 2010;6(2):88-97. doi:10.1038/nrneurol.2009.216
2. Mesulam M-M, Rogalski EJ, Wieneke C, et al. Primary progressive aphasia and the evolving neurology of the language network. Nat Rev Neurol. 2014;10(10):554-569. doi:10.1038/nrneurol.2014.159
3. Coyle-Gilchrist ITS, Dick KM, Patterson K, et al. Prevalence, characteristics, and survival of frontotemporal lobar degeneration syndromes. Neurology. 2016;86(18):1736-1743. doi:10.1212/WNL.0000000000002638
4. Marshall CR, Hardy CJD, Volkmer A, et al. Primary progressive aphasia: a clinical approach. J Neurol. 2018;265(6):1474-1490. doi:10.1007/s00415-018-8762-6
5. Blennow K. Cerebrospinal fluid protein biomarkers for Alzheimer’s disease. NeuroRx. 2004;1(2):213-225. doi:10.1602/neurorx.1.2.213
6. Hulstaert F, Blennow K, Ivanoiu A, et al. Improved discrimination of AD patients using beta-amyloid(1-42) and tau levels in CSF. Neurology. 1999;52(8):1555-1562. doi:10.1212/wnl.52.8.1555
7. Thijssen EH, La Joie R, Wolf A, et al. Diagnostic value of plasma phosphorylated tau181 in Alzheimer’s disease and frontotemporal lobar degeneration. Nat Med. 2020;26(3):387-397. doi:10.1038/s41591-020-0762-2
8. Newhart M, Davis C, Kannan V, et al. Therapy for naming deficits in two variants of primary progressive aphasia. Aphasiology. 2009;23(7-8):823-834. doi:10.1080/02687030802661762
9. Seyfried LS, Kales HC, Ignacio RV, et al. Predictors of suicide in patients with dementia. Alzheimers Dement. 2011;7(6):567-573. doi:10.1016/j.jalz.2011.01.006
10. Porteri C. Advance directives as a tool to respect patients’ values and preferences: discussion on the case of Alzheimer’s disease. BMC Med Ethics. 2018;19(1):9. doi:10.1186/s12910-018-0249-6
More on psilocybin
I would like to remark on “Psychedelics for treating psychiatric disorders: Are they safe?” (
The Oregon Psilocybin Services that will begin in 2023 are not specific to therapeutic use; this is a common misconception. These are specifically referred to as “psilocybin services” in the Oregon Administrative Rules (OAR), and psilocybin facilitators are required to limit their scope such that they are not practicing psychotherapy or other interventions, even if they do have a medical or psychotherapy background. The intention of the Oregon Psilocybin Services rollout was that these services would not be of the medical model. In the spirit of this, services do not require a medical diagnosis or referral, and services are not a medical or clinical treatment (OAR 333-333-5040). Additionally, services cannot be provided in a health care facility (OAR 441). Facilitators receive robust training as defined by Oregon law, and licensed facilitators provide this information during preparation for services. When discussing this model on a large public scale, I have noticed substantial misconceptions; it is imperative that we refer to these services as they are defined so that individuals with mental health conditions who seek them are aware that such services are different from psilocybin-assisted psychotherapy. Instead, Oregon Psilocybin Services might be better categorized as supported psilocybin use.
I would like to remark on “Psychedelics for treating psychiatric disorders: Are they safe?” (
The Oregon Psilocybin Services that will begin in 2023 are not specific to therapeutic use; this is a common misconception. These are specifically referred to as “psilocybin services” in the Oregon Administrative Rules (OAR), and psilocybin facilitators are required to limit their scope such that they are not practicing psychotherapy or other interventions, even if they do have a medical or psychotherapy background. The intention of the Oregon Psilocybin Services rollout was that these services would not be of the medical model. In the spirit of this, services do not require a medical diagnosis or referral, and services are not a medical or clinical treatment (OAR 333-333-5040). Additionally, services cannot be provided in a health care facility (OAR 441). Facilitators receive robust training as defined by Oregon law, and licensed facilitators provide this information during preparation for services. When discussing this model on a large public scale, I have noticed substantial misconceptions; it is imperative that we refer to these services as they are defined so that individuals with mental health conditions who seek them are aware that such services are different from psilocybin-assisted psychotherapy. Instead, Oregon Psilocybin Services might be better categorized as supported psilocybin use.
I would like to remark on “Psychedelics for treating psychiatric disorders: Are they safe?” (
The Oregon Psilocybin Services that will begin in 2023 are not specific to therapeutic use; this is a common misconception. These are specifically referred to as “psilocybin services” in the Oregon Administrative Rules (OAR), and psilocybin facilitators are required to limit their scope such that they are not practicing psychotherapy or other interventions, even if they do have a medical or psychotherapy background. The intention of the Oregon Psilocybin Services rollout was that these services would not be of the medical model. In the spirit of this, services do not require a medical diagnosis or referral, and services are not a medical or clinical treatment (OAR 333-333-5040). Additionally, services cannot be provided in a health care facility (OAR 441). Facilitators receive robust training as defined by Oregon law, and licensed facilitators provide this information during preparation for services. When discussing this model on a large public scale, I have noticed substantial misconceptions; it is imperative that we refer to these services as they are defined so that individuals with mental health conditions who seek them are aware that such services are different from psilocybin-assisted psychotherapy. Instead, Oregon Psilocybin Services might be better categorized as supported psilocybin use.
Guidelines recommend CBT alone for mild acute depression, more options for more severe cases
The guidelines also state that patients with mild depression should start with CBT alone, and if a patient with moderate to severe depression prefers, they can use a combination of both CBT and an SGA.
These nuanced recommendations contrast sharply with the 2016 ACP guidelines for depression, which lumped all stages and severity levels together, and came with just one recommendation: Clinicians should choose between CBT and an SGA.
More data have come to light over the years, requiring the present update, reported lead author Amir Qaseem, MD, PhD, vice president of Clinical Policy and the Center for Evidence Reviews at the ACP, and adjunct faculty at Thomas Jefferson University, Philadelphia, and colleagues.
In addition to the focus on acute depression, Dr. Qaseem and colleagues highlighted the new guidelines' “consideration of patient values and preferences, and costs,” as well as responses to therapy.
Recommendations were derived from a network meta-analysis that included studies evaluating nonpharmacologic and pharmacologic therapies, the authors wrote in Annals of Internal Medicine. They compared effectiveness across a range of SGAs, “including selective serotonin reuptake inhibitors; serotonin-norepinephrine reuptake inhibitors; and others such as bupropion, mirtazapine, nefazodone, trazodone, vilazodone, and vortioxetine.”
This analysis yielded three pieces of clinical advice.
First, patients in the acute phase of mild depression should receive CBT alone as their initial treatment.
Dr. Qaseem and colleagues noted that many depression studies for pharmacologic therapies excluded these patients in favor of those with moderate to severe depression, leaving an evidence gap.
“Furthermore, the Clinical Guidelines Committee had concerns about adverse effects of SGAs in these patients and suggests that the use of SGAs as initial treatment of these patients should be based on additional considerations, such as limited access to or cost of CBT, history of moderate or severe major depressive disorder, or patient preferences,” they added.
The committee’s next recommendation, based on moderate-certainty evidence, suggested that CBT alone or an SGA alone should be considered for patients in the acute phase of moderate to severe depression. This call for monotherapy is balanced by a conditional recommendation based on low-certainty evidence that the same group may benefit from initial combination therapy with both CBT and an SGA.
“The informed decision on the options of monotherapy with CBT versus SGAs, or combination therapy, should be personalized and based on discussion of potential treatment benefits, harms, adverse effect profiles, cost, feasibility, patients’ specific symptoms (such as insomnia, hypersomnia, or fluctuation in appetite), comorbidities, concomitant medication use, and patient preferences,” the guidelines state.
The third and final recommendation offers an algorithm for patients who do not respond to initial therapy with an SGA. Multiple pathways are provided: Switch to CBT or augment with CBT; or switch to a different SGA or augment with a second pharmacologic therapy, such as mirtazapine, bupropion, or buspirone.
“These second-line treatment strategies show similar efficacy when compared with each other,” the guidelines committee noted.
Again, the guidelines suggest that second-line choices should be personalized based on the various factors previously discussed.
A timely update
“The new guideline is very different from the last guideline,” said Ryan Mire, MD, president of the ACP and practicing internal medicine physician in Nashville, Tenn. in a written comment. “ACP decided to update the depression guidelines with a focus on acute depression because approximately 70% of patients with major depressive disorder do not achieve remission and remain in the acute phase after the initial pharmacologic treatment attempt. In addition, there is new evidence on second-line treatments since the 2016 ACP guideline was published.”
Neil S. Skolnik, MD, of Thomas Jefferson University, Philadelphia, agreed that the guidelines offer a necessary and fresh perspective on caring for patients with depression.
“These guidelines are a helpful update, assuring us that we are using the latest, evidence-based therapies, and [they] are written in a practical, easy-to-implement manner,” Dr. Skolnik said in a written comment.
“First, the guidelines reaffirm that CBT is an effective first-line option, with or without the concurrent use of an SGA,” Dr. Skolnik said, noting that CBT alone may reduce likelihood of recurrence, compared with an SGA alone. “Many patients do not like the idea of medication, or the potential side effects of medications, and CBT is an evidenced-based approach that can be very helpful for patients.”
Dr. Skolnik also applauded the guidelines authors for offering a clear path forward for patients who do not have full remission after treatment – a common clinical scenario.
He went on to offer some more detailed steps forward.
“If someone chooses to be treated with an SGA alone and has not had much response at all to an initial SGA, usually a selective serotonin reuptake inhibitor, I’ll usually switch to a different SSRI or serotonin and norepinephrine reuptake inhibitor (SNRI) and/or add CBT,” Dr. Skolnik said. “If they have had a partial response, I’ll often encourage CBT and consider the addition of augmentation with an additional medication as discussed in the guidelines.”
Valuable despite the gaps
Other experts expressed mixed impressions of the update, noting both highs and lows.
“Although [this guideline] has some gaps, it is more valuable in several ways than other widely consulted practice guidelines for depression,” wrote Miriam Shuchman, MD and Elia Abi-Jaoude, MSc, MD, PhD, of the University of Toronto, in an accompanying editorial.
Specifically, they praised the publication’s focus on shared decision-making in the treatment planning process.
“This effort to respond to patient preferences is crucial and may even increase the chance that patients will improve with treatment,” they wrote.
They also applauded the ACP’s efforts to recuse any committee members who may have had conflicts of interest “that could affect their judgment about treatments for depression.”
After highlighting these attributes, Dr. Shuchman and Dr. Abi-Jaoude noted that the guidelines still contain “significant gaps.”
Foremost, they pointed out the guidelines' emphasis on CBT to the exclusion of other nonpharmacologic options.
“The guideline does patients a disservice by leaving out several nonmedication treatment options that clinicians can offer as first- or second-line therapies,” they wrote.
This oversight may increase risk that patients simply hop from one SGA to another, which is a common, and often ineffective, strategy, according to Dr. Shuchman and Dr. Abi-Jaoude.
“Patients often go from one drug to the next in the hopes of landing on one that ‘works,’ ” the editorialists wrote. “This narrow clinical approach of pursuing medication-based treatments ignores the ways difficulties in a person’s work or relationships may contribute to their struggles with depression. At a time when the COVID-19 pandemic has underscored the importance of the social context of mental health, clinicians may need to consider other forms of support and tailor prescribing to what is most relevant and accessible for a particular patient.”
Dr. Shuchman and Dr. Abi-Jaoude went on to suggest several nonpharmacologic options beyond CBT, including interpersonal therapy, psychodynamic therapy, problem solving, behavioral activation, and guided self-help.
The other key gap they pointed out relates to withdrawal.
Although the guideline does advise physicians to taper antidepressants to reduce risk of withdrawal, the editorialists suggested that this recommendation lacked sufficient emphasis, as it can be a particularly difficult period in the treatment process.
“Tapering of an antidepressant may need to be done over months or years, not weeks, and a patient may need to visit a compounding pharmacy to obtain doses of a second-generation antidepressant not marketed by drug manufacturers so that prescriptions can be tapered even more slowly,” they suggested.
Financial costs remain unclear
Beyond the above medical considerations, one other piece of the depression puzzle remains unsolved: cost.
In a simultaneously published rapid review, Andreea Dobrescu, MD, PhD, of Cochrane Austria, and colleagues evaluated the relative cost-effectiveness of first- and second-step treatment strategies.
For most comparisons, evidence was insufficient to reach a conclusion, although they suggested that CBT may be more cost effective at the 5-year mark.
“For most pharmacologic and nonpharmacologic interventions for major depressive disorder, evidence was missing or was insufficient to draw conclusions about the cost-effectiveness of first- or second-step treatments for MDD,” Dr. Dobrescu and colleagues wrote. “The strongest evidence (albeit still low certainty of evidence) was for the cost-effectiveness of CBT compared with SGA as a first-step treatment over a 5-year time horizon from the societal and health care sector perspectives. However, this evidence should also be interpreted cautiously considering it is based on a single study.”
When asked about the financial findings, Dr. Mire agreed that more data are needed, especially because CBT and SGA costs range widely. He suggested that cost, for each patient, should be considered in the personalized approach now highlighted by the new guidelines.
The guidelines and the Cochrane cost-effectiveness study were supported by the ACP. The guidelines' authors and other individuals quoted in this article reported no conflicts of interest.
The guidelines also state that patients with mild depression should start with CBT alone, and if a patient with moderate to severe depression prefers, they can use a combination of both CBT and an SGA.
These nuanced recommendations contrast sharply with the 2016 ACP guidelines for depression, which lumped all stages and severity levels together, and came with just one recommendation: Clinicians should choose between CBT and an SGA.
More data have come to light over the years, requiring the present update, reported lead author Amir Qaseem, MD, PhD, vice president of Clinical Policy and the Center for Evidence Reviews at the ACP, and adjunct faculty at Thomas Jefferson University, Philadelphia, and colleagues.
In addition to the focus on acute depression, Dr. Qaseem and colleagues highlighted the new guidelines' “consideration of patient values and preferences, and costs,” as well as responses to therapy.
Recommendations were derived from a network meta-analysis that included studies evaluating nonpharmacologic and pharmacologic therapies, the authors wrote in Annals of Internal Medicine. They compared effectiveness across a range of SGAs, “including selective serotonin reuptake inhibitors; serotonin-norepinephrine reuptake inhibitors; and others such as bupropion, mirtazapine, nefazodone, trazodone, vilazodone, and vortioxetine.”
This analysis yielded three pieces of clinical advice.
First, patients in the acute phase of mild depression should receive CBT alone as their initial treatment.
Dr. Qaseem and colleagues noted that many depression studies for pharmacologic therapies excluded these patients in favor of those with moderate to severe depression, leaving an evidence gap.
“Furthermore, the Clinical Guidelines Committee had concerns about adverse effects of SGAs in these patients and suggests that the use of SGAs as initial treatment of these patients should be based on additional considerations, such as limited access to or cost of CBT, history of moderate or severe major depressive disorder, or patient preferences,” they added.
The committee’s next recommendation, based on moderate-certainty evidence, suggested that CBT alone or an SGA alone should be considered for patients in the acute phase of moderate to severe depression. This call for monotherapy is balanced by a conditional recommendation based on low-certainty evidence that the same group may benefit from initial combination therapy with both CBT and an SGA.
“The informed decision on the options of monotherapy with CBT versus SGAs, or combination therapy, should be personalized and based on discussion of potential treatment benefits, harms, adverse effect profiles, cost, feasibility, patients’ specific symptoms (such as insomnia, hypersomnia, or fluctuation in appetite), comorbidities, concomitant medication use, and patient preferences,” the guidelines state.
The third and final recommendation offers an algorithm for patients who do not respond to initial therapy with an SGA. Multiple pathways are provided: Switch to CBT or augment with CBT; or switch to a different SGA or augment with a second pharmacologic therapy, such as mirtazapine, bupropion, or buspirone.
“These second-line treatment strategies show similar efficacy when compared with each other,” the guidelines committee noted.
Again, the guidelines suggest that second-line choices should be personalized based on the various factors previously discussed.
A timely update
“The new guideline is very different from the last guideline,” said Ryan Mire, MD, president of the ACP and practicing internal medicine physician in Nashville, Tenn. in a written comment. “ACP decided to update the depression guidelines with a focus on acute depression because approximately 70% of patients with major depressive disorder do not achieve remission and remain in the acute phase after the initial pharmacologic treatment attempt. In addition, there is new evidence on second-line treatments since the 2016 ACP guideline was published.”
Neil S. Skolnik, MD, of Thomas Jefferson University, Philadelphia, agreed that the guidelines offer a necessary and fresh perspective on caring for patients with depression.
“These guidelines are a helpful update, assuring us that we are using the latest, evidence-based therapies, and [they] are written in a practical, easy-to-implement manner,” Dr. Skolnik said in a written comment.
“First, the guidelines reaffirm that CBT is an effective first-line option, with or without the concurrent use of an SGA,” Dr. Skolnik said, noting that CBT alone may reduce likelihood of recurrence, compared with an SGA alone. “Many patients do not like the idea of medication, or the potential side effects of medications, and CBT is an evidenced-based approach that can be very helpful for patients.”
Dr. Skolnik also applauded the guidelines authors for offering a clear path forward for patients who do not have full remission after treatment – a common clinical scenario.
He went on to offer some more detailed steps forward.
“If someone chooses to be treated with an SGA alone and has not had much response at all to an initial SGA, usually a selective serotonin reuptake inhibitor, I’ll usually switch to a different SSRI or serotonin and norepinephrine reuptake inhibitor (SNRI) and/or add CBT,” Dr. Skolnik said. “If they have had a partial response, I’ll often encourage CBT and consider the addition of augmentation with an additional medication as discussed in the guidelines.”
Valuable despite the gaps
Other experts expressed mixed impressions of the update, noting both highs and lows.
“Although [this guideline] has some gaps, it is more valuable in several ways than other widely consulted practice guidelines for depression,” wrote Miriam Shuchman, MD and Elia Abi-Jaoude, MSc, MD, PhD, of the University of Toronto, in an accompanying editorial.
Specifically, they praised the publication’s focus on shared decision-making in the treatment planning process.
“This effort to respond to patient preferences is crucial and may even increase the chance that patients will improve with treatment,” they wrote.
They also applauded the ACP’s efforts to recuse any committee members who may have had conflicts of interest “that could affect their judgment about treatments for depression.”
After highlighting these attributes, Dr. Shuchman and Dr. Abi-Jaoude noted that the guidelines still contain “significant gaps.”
Foremost, they pointed out the guidelines' emphasis on CBT to the exclusion of other nonpharmacologic options.
“The guideline does patients a disservice by leaving out several nonmedication treatment options that clinicians can offer as first- or second-line therapies,” they wrote.
This oversight may increase risk that patients simply hop from one SGA to another, which is a common, and often ineffective, strategy, according to Dr. Shuchman and Dr. Abi-Jaoude.
“Patients often go from one drug to the next in the hopes of landing on one that ‘works,’ ” the editorialists wrote. “This narrow clinical approach of pursuing medication-based treatments ignores the ways difficulties in a person’s work or relationships may contribute to their struggles with depression. At a time when the COVID-19 pandemic has underscored the importance of the social context of mental health, clinicians may need to consider other forms of support and tailor prescribing to what is most relevant and accessible for a particular patient.”
Dr. Shuchman and Dr. Abi-Jaoude went on to suggest several nonpharmacologic options beyond CBT, including interpersonal therapy, psychodynamic therapy, problem solving, behavioral activation, and guided self-help.
The other key gap they pointed out relates to withdrawal.
Although the guideline does advise physicians to taper antidepressants to reduce risk of withdrawal, the editorialists suggested that this recommendation lacked sufficient emphasis, as it can be a particularly difficult period in the treatment process.
“Tapering of an antidepressant may need to be done over months or years, not weeks, and a patient may need to visit a compounding pharmacy to obtain doses of a second-generation antidepressant not marketed by drug manufacturers so that prescriptions can be tapered even more slowly,” they suggested.
Financial costs remain unclear
Beyond the above medical considerations, one other piece of the depression puzzle remains unsolved: cost.
In a simultaneously published rapid review, Andreea Dobrescu, MD, PhD, of Cochrane Austria, and colleagues evaluated the relative cost-effectiveness of first- and second-step treatment strategies.
For most comparisons, evidence was insufficient to reach a conclusion, although they suggested that CBT may be more cost effective at the 5-year mark.
“For most pharmacologic and nonpharmacologic interventions for major depressive disorder, evidence was missing or was insufficient to draw conclusions about the cost-effectiveness of first- or second-step treatments for MDD,” Dr. Dobrescu and colleagues wrote. “The strongest evidence (albeit still low certainty of evidence) was for the cost-effectiveness of CBT compared with SGA as a first-step treatment over a 5-year time horizon from the societal and health care sector perspectives. However, this evidence should also be interpreted cautiously considering it is based on a single study.”
When asked about the financial findings, Dr. Mire agreed that more data are needed, especially because CBT and SGA costs range widely. He suggested that cost, for each patient, should be considered in the personalized approach now highlighted by the new guidelines.
The guidelines and the Cochrane cost-effectiveness study were supported by the ACP. The guidelines' authors and other individuals quoted in this article reported no conflicts of interest.
The guidelines also state that patients with mild depression should start with CBT alone, and if a patient with moderate to severe depression prefers, they can use a combination of both CBT and an SGA.
These nuanced recommendations contrast sharply with the 2016 ACP guidelines for depression, which lumped all stages and severity levels together, and came with just one recommendation: Clinicians should choose between CBT and an SGA.
More data have come to light over the years, requiring the present update, reported lead author Amir Qaseem, MD, PhD, vice president of Clinical Policy and the Center for Evidence Reviews at the ACP, and adjunct faculty at Thomas Jefferson University, Philadelphia, and colleagues.
In addition to the focus on acute depression, Dr. Qaseem and colleagues highlighted the new guidelines' “consideration of patient values and preferences, and costs,” as well as responses to therapy.
Recommendations were derived from a network meta-analysis that included studies evaluating nonpharmacologic and pharmacologic therapies, the authors wrote in Annals of Internal Medicine. They compared effectiveness across a range of SGAs, “including selective serotonin reuptake inhibitors; serotonin-norepinephrine reuptake inhibitors; and others such as bupropion, mirtazapine, nefazodone, trazodone, vilazodone, and vortioxetine.”
This analysis yielded three pieces of clinical advice.
First, patients in the acute phase of mild depression should receive CBT alone as their initial treatment.
Dr. Qaseem and colleagues noted that many depression studies for pharmacologic therapies excluded these patients in favor of those with moderate to severe depression, leaving an evidence gap.
“Furthermore, the Clinical Guidelines Committee had concerns about adverse effects of SGAs in these patients and suggests that the use of SGAs as initial treatment of these patients should be based on additional considerations, such as limited access to or cost of CBT, history of moderate or severe major depressive disorder, or patient preferences,” they added.
The committee’s next recommendation, based on moderate-certainty evidence, suggested that CBT alone or an SGA alone should be considered for patients in the acute phase of moderate to severe depression. This call for monotherapy is balanced by a conditional recommendation based on low-certainty evidence that the same group may benefit from initial combination therapy with both CBT and an SGA.
“The informed decision on the options of monotherapy with CBT versus SGAs, or combination therapy, should be personalized and based on discussion of potential treatment benefits, harms, adverse effect profiles, cost, feasibility, patients’ specific symptoms (such as insomnia, hypersomnia, or fluctuation in appetite), comorbidities, concomitant medication use, and patient preferences,” the guidelines state.
The third and final recommendation offers an algorithm for patients who do not respond to initial therapy with an SGA. Multiple pathways are provided: Switch to CBT or augment with CBT; or switch to a different SGA or augment with a second pharmacologic therapy, such as mirtazapine, bupropion, or buspirone.
“These second-line treatment strategies show similar efficacy when compared with each other,” the guidelines committee noted.
Again, the guidelines suggest that second-line choices should be personalized based on the various factors previously discussed.
A timely update
“The new guideline is very different from the last guideline,” said Ryan Mire, MD, president of the ACP and practicing internal medicine physician in Nashville, Tenn. in a written comment. “ACP decided to update the depression guidelines with a focus on acute depression because approximately 70% of patients with major depressive disorder do not achieve remission and remain in the acute phase after the initial pharmacologic treatment attempt. In addition, there is new evidence on second-line treatments since the 2016 ACP guideline was published.”
Neil S. Skolnik, MD, of Thomas Jefferson University, Philadelphia, agreed that the guidelines offer a necessary and fresh perspective on caring for patients with depression.
“These guidelines are a helpful update, assuring us that we are using the latest, evidence-based therapies, and [they] are written in a practical, easy-to-implement manner,” Dr. Skolnik said in a written comment.
“First, the guidelines reaffirm that CBT is an effective first-line option, with or without the concurrent use of an SGA,” Dr. Skolnik said, noting that CBT alone may reduce likelihood of recurrence, compared with an SGA alone. “Many patients do not like the idea of medication, or the potential side effects of medications, and CBT is an evidenced-based approach that can be very helpful for patients.”
Dr. Skolnik also applauded the guidelines authors for offering a clear path forward for patients who do not have full remission after treatment – a common clinical scenario.
He went on to offer some more detailed steps forward.
“If someone chooses to be treated with an SGA alone and has not had much response at all to an initial SGA, usually a selective serotonin reuptake inhibitor, I’ll usually switch to a different SSRI or serotonin and norepinephrine reuptake inhibitor (SNRI) and/or add CBT,” Dr. Skolnik said. “If they have had a partial response, I’ll often encourage CBT and consider the addition of augmentation with an additional medication as discussed in the guidelines.”
Valuable despite the gaps
Other experts expressed mixed impressions of the update, noting both highs and lows.
“Although [this guideline] has some gaps, it is more valuable in several ways than other widely consulted practice guidelines for depression,” wrote Miriam Shuchman, MD and Elia Abi-Jaoude, MSc, MD, PhD, of the University of Toronto, in an accompanying editorial.
Specifically, they praised the publication’s focus on shared decision-making in the treatment planning process.
“This effort to respond to patient preferences is crucial and may even increase the chance that patients will improve with treatment,” they wrote.
They also applauded the ACP’s efforts to recuse any committee members who may have had conflicts of interest “that could affect their judgment about treatments for depression.”
After highlighting these attributes, Dr. Shuchman and Dr. Abi-Jaoude noted that the guidelines still contain “significant gaps.”
Foremost, they pointed out the guidelines' emphasis on CBT to the exclusion of other nonpharmacologic options.
“The guideline does patients a disservice by leaving out several nonmedication treatment options that clinicians can offer as first- or second-line therapies,” they wrote.
This oversight may increase risk that patients simply hop from one SGA to another, which is a common, and often ineffective, strategy, according to Dr. Shuchman and Dr. Abi-Jaoude.
“Patients often go from one drug to the next in the hopes of landing on one that ‘works,’ ” the editorialists wrote. “This narrow clinical approach of pursuing medication-based treatments ignores the ways difficulties in a person’s work or relationships may contribute to their struggles with depression. At a time when the COVID-19 pandemic has underscored the importance of the social context of mental health, clinicians may need to consider other forms of support and tailor prescribing to what is most relevant and accessible for a particular patient.”
Dr. Shuchman and Dr. Abi-Jaoude went on to suggest several nonpharmacologic options beyond CBT, including interpersonal therapy, psychodynamic therapy, problem solving, behavioral activation, and guided self-help.
The other key gap they pointed out relates to withdrawal.
Although the guideline does advise physicians to taper antidepressants to reduce risk of withdrawal, the editorialists suggested that this recommendation lacked sufficient emphasis, as it can be a particularly difficult period in the treatment process.
“Tapering of an antidepressant may need to be done over months or years, not weeks, and a patient may need to visit a compounding pharmacy to obtain doses of a second-generation antidepressant not marketed by drug manufacturers so that prescriptions can be tapered even more slowly,” they suggested.
Financial costs remain unclear
Beyond the above medical considerations, one other piece of the depression puzzle remains unsolved: cost.
In a simultaneously published rapid review, Andreea Dobrescu, MD, PhD, of Cochrane Austria, and colleagues evaluated the relative cost-effectiveness of first- and second-step treatment strategies.
For most comparisons, evidence was insufficient to reach a conclusion, although they suggested that CBT may be more cost effective at the 5-year mark.
“For most pharmacologic and nonpharmacologic interventions for major depressive disorder, evidence was missing or was insufficient to draw conclusions about the cost-effectiveness of first- or second-step treatments for MDD,” Dr. Dobrescu and colleagues wrote. “The strongest evidence (albeit still low certainty of evidence) was for the cost-effectiveness of CBT compared with SGA as a first-step treatment over a 5-year time horizon from the societal and health care sector perspectives. However, this evidence should also be interpreted cautiously considering it is based on a single study.”
When asked about the financial findings, Dr. Mire agreed that more data are needed, especially because CBT and SGA costs range widely. He suggested that cost, for each patient, should be considered in the personalized approach now highlighted by the new guidelines.
The guidelines and the Cochrane cost-effectiveness study were supported by the ACP. The guidelines' authors and other individuals quoted in this article reported no conflicts of interest.
FROM ANNALS OF INTERNAL MEDICINE
Doctors’ happiness has not rebounded as pandemic drags on
Physicians reported similar levels of unhappiness in 2022 too.
Fewer than half of physicians said they were currently somewhat or very happy at work, compared with 75% of physicians who said they were somewhat or very happy at work in a previous survey conducted before the pandemic, the new Medscape Physician Lifestyle & Happiness Report 2023 shows.*
“I am not surprised that we’re less happy now,” said Amaryllis Sánchez, MD, a board-certified family medicine physician and a certified physician coach.
“I speak to physicians around the country and I hear that their workplaces are understaffed, they’re overworked and they don’t feel safe. Although we’re in a different phase of the pandemic, doctors feel that the ground beneath them is still shaky,” said Dr. Sánchez, the author of “Recapturing Joy in Medicine.”
Most doctors are seeing more patients than they can handle and are expected to do that consistently. “When you no longer have the capacity to give of yourself, that becomes a nearly impossible task,” said Dr. Sánchez.
Also, physicians in understaffed workplaces often must take on additional work such as administrative or nursing duties, said Katie Cole, DO, a board-certified psychiatrist and a physician coach.
While health systems are aware that physicians need time to rest and recharge, staffing shortages prevent doctors from taking time off because they can’t find coverage, said Dr. Cole.
“While we know that it’s important for physicians to take vacations, more than one-third of doctors still take 2 weeks or less of vacation annually,” said Dr. Cole.
Physicians also tend to have less compassion for themselves and sacrifice self-care compared to other health care workers. “When a patient dies, nurses get together, debrief, and hug each other, whereas doctors have another patient to see. The culture of medicine doesn’t support self-compassion for physicians,” said Dr. Cole.
Physicians also felt less safe at work during the pandemic because of to shortages of personal protective equipment, said Dr. Sánchez. They have also witnessed or experienced an increase in abusive behavior, violence and threats of violence.
Physicians’ personal life suffers
Doctors maintain their mental health primarily by spending time with family members and friends, according to 2022’s Medscape Physician Lifestyle & Happiness Report. Yet half of doctors reported in a survey by the Physicians Foundation that they withdrew from family, friends or coworkers in 2022, said Dr. Sánchez.
“When you exceed your mental, emotional, and physical capacity at work, you have no reserve left for your personal life,” said Dr. Cole.
That may explain why only 58% of doctors reported feeling somewhat or very happy outside of work, compared with 84% who felt that way before the pandemic.
More women doctors said they deal with stronger feelings of conflict in trying to balance parenting responsibilities with a highly demanding job. Nearly one in two women physician-parents reported feeling very conflicted at work, compared with about one in four male physician-parents.
When physicians go home, they may be emotionally drained and tired mentally from making a lot of decisions at work, said Dr. Cole.
“As a woman, if you have children and a husband and you’re responsible for dinner, picking up the kids at daycare or helping them with homework, and making all these decisions when you get home, it’s overwhelming,” said Dr. Cole.
Prioritize your well-being
Doctors need to prioritize their own well-being, said Dr. Sánchez. “That’s not being selfish, that’s doing what’s necessary to stay well and be able to take care of patients. If doctors don’t take care of themselves, no one else will.”
Dr. Sánchez recommended that doctors regularly interact with relatives, friends, trusted colleagues, or clergy to help maintain their well-being, rather than waiting until a crisis to reach out.
A good coach, mentor, or counselor can help physicians gain enough self-awareness to handle their emotions and gain more clarity about what changes need to be made, she said.
Dr. Cole suggested that doctors figure out what makes them happy and fulfilled at work and try to spend more time on that activity. “Knowing what makes you happy and your strengths are foundational for creating a life you love.”
She urged doctors to “start thinking now about what you love about medicine and what is going right at home, and what areas you want to change. Then, start advocating for your needs.”
A version of this article originally appeared on Medscape.com.
Correction, 1/26/23: An earlier version of this article misstated the findings of the survey.
Physicians reported similar levels of unhappiness in 2022 too.
Fewer than half of physicians said they were currently somewhat or very happy at work, compared with 75% of physicians who said they were somewhat or very happy at work in a previous survey conducted before the pandemic, the new Medscape Physician Lifestyle & Happiness Report 2023 shows.*
“I am not surprised that we’re less happy now,” said Amaryllis Sánchez, MD, a board-certified family medicine physician and a certified physician coach.
“I speak to physicians around the country and I hear that their workplaces are understaffed, they’re overworked and they don’t feel safe. Although we’re in a different phase of the pandemic, doctors feel that the ground beneath them is still shaky,” said Dr. Sánchez, the author of “Recapturing Joy in Medicine.”
Most doctors are seeing more patients than they can handle and are expected to do that consistently. “When you no longer have the capacity to give of yourself, that becomes a nearly impossible task,” said Dr. Sánchez.
Also, physicians in understaffed workplaces often must take on additional work such as administrative or nursing duties, said Katie Cole, DO, a board-certified psychiatrist and a physician coach.
While health systems are aware that physicians need time to rest and recharge, staffing shortages prevent doctors from taking time off because they can’t find coverage, said Dr. Cole.
“While we know that it’s important for physicians to take vacations, more than one-third of doctors still take 2 weeks or less of vacation annually,” said Dr. Cole.
Physicians also tend to have less compassion for themselves and sacrifice self-care compared to other health care workers. “When a patient dies, nurses get together, debrief, and hug each other, whereas doctors have another patient to see. The culture of medicine doesn’t support self-compassion for physicians,” said Dr. Cole.
Physicians also felt less safe at work during the pandemic because of to shortages of personal protective equipment, said Dr. Sánchez. They have also witnessed or experienced an increase in abusive behavior, violence and threats of violence.
Physicians’ personal life suffers
Doctors maintain their mental health primarily by spending time with family members and friends, according to 2022’s Medscape Physician Lifestyle & Happiness Report. Yet half of doctors reported in a survey by the Physicians Foundation that they withdrew from family, friends or coworkers in 2022, said Dr. Sánchez.
“When you exceed your mental, emotional, and physical capacity at work, you have no reserve left for your personal life,” said Dr. Cole.
That may explain why only 58% of doctors reported feeling somewhat or very happy outside of work, compared with 84% who felt that way before the pandemic.
More women doctors said they deal with stronger feelings of conflict in trying to balance parenting responsibilities with a highly demanding job. Nearly one in two women physician-parents reported feeling very conflicted at work, compared with about one in four male physician-parents.
When physicians go home, they may be emotionally drained and tired mentally from making a lot of decisions at work, said Dr. Cole.
“As a woman, if you have children and a husband and you’re responsible for dinner, picking up the kids at daycare or helping them with homework, and making all these decisions when you get home, it’s overwhelming,” said Dr. Cole.
Prioritize your well-being
Doctors need to prioritize their own well-being, said Dr. Sánchez. “That’s not being selfish, that’s doing what’s necessary to stay well and be able to take care of patients. If doctors don’t take care of themselves, no one else will.”
Dr. Sánchez recommended that doctors regularly interact with relatives, friends, trusted colleagues, or clergy to help maintain their well-being, rather than waiting until a crisis to reach out.
A good coach, mentor, or counselor can help physicians gain enough self-awareness to handle their emotions and gain more clarity about what changes need to be made, she said.
Dr. Cole suggested that doctors figure out what makes them happy and fulfilled at work and try to spend more time on that activity. “Knowing what makes you happy and your strengths are foundational for creating a life you love.”
She urged doctors to “start thinking now about what you love about medicine and what is going right at home, and what areas you want to change. Then, start advocating for your needs.”
A version of this article originally appeared on Medscape.com.
Correction, 1/26/23: An earlier version of this article misstated the findings of the survey.
Physicians reported similar levels of unhappiness in 2022 too.
Fewer than half of physicians said they were currently somewhat or very happy at work, compared with 75% of physicians who said they were somewhat or very happy at work in a previous survey conducted before the pandemic, the new Medscape Physician Lifestyle & Happiness Report 2023 shows.*
“I am not surprised that we’re less happy now,” said Amaryllis Sánchez, MD, a board-certified family medicine physician and a certified physician coach.
“I speak to physicians around the country and I hear that their workplaces are understaffed, they’re overworked and they don’t feel safe. Although we’re in a different phase of the pandemic, doctors feel that the ground beneath them is still shaky,” said Dr. Sánchez, the author of “Recapturing Joy in Medicine.”
Most doctors are seeing more patients than they can handle and are expected to do that consistently. “When you no longer have the capacity to give of yourself, that becomes a nearly impossible task,” said Dr. Sánchez.
Also, physicians in understaffed workplaces often must take on additional work such as administrative or nursing duties, said Katie Cole, DO, a board-certified psychiatrist and a physician coach.
While health systems are aware that physicians need time to rest and recharge, staffing shortages prevent doctors from taking time off because they can’t find coverage, said Dr. Cole.
“While we know that it’s important for physicians to take vacations, more than one-third of doctors still take 2 weeks or less of vacation annually,” said Dr. Cole.
Physicians also tend to have less compassion for themselves and sacrifice self-care compared to other health care workers. “When a patient dies, nurses get together, debrief, and hug each other, whereas doctors have another patient to see. The culture of medicine doesn’t support self-compassion for physicians,” said Dr. Cole.
Physicians also felt less safe at work during the pandemic because of to shortages of personal protective equipment, said Dr. Sánchez. They have also witnessed or experienced an increase in abusive behavior, violence and threats of violence.
Physicians’ personal life suffers
Doctors maintain their mental health primarily by spending time with family members and friends, according to 2022’s Medscape Physician Lifestyle & Happiness Report. Yet half of doctors reported in a survey by the Physicians Foundation that they withdrew from family, friends or coworkers in 2022, said Dr. Sánchez.
“When you exceed your mental, emotional, and physical capacity at work, you have no reserve left for your personal life,” said Dr. Cole.
That may explain why only 58% of doctors reported feeling somewhat or very happy outside of work, compared with 84% who felt that way before the pandemic.
More women doctors said they deal with stronger feelings of conflict in trying to balance parenting responsibilities with a highly demanding job. Nearly one in two women physician-parents reported feeling very conflicted at work, compared with about one in four male physician-parents.
When physicians go home, they may be emotionally drained and tired mentally from making a lot of decisions at work, said Dr. Cole.
“As a woman, if you have children and a husband and you’re responsible for dinner, picking up the kids at daycare or helping them with homework, and making all these decisions when you get home, it’s overwhelming,” said Dr. Cole.
Prioritize your well-being
Doctors need to prioritize their own well-being, said Dr. Sánchez. “That’s not being selfish, that’s doing what’s necessary to stay well and be able to take care of patients. If doctors don’t take care of themselves, no one else will.”
Dr. Sánchez recommended that doctors regularly interact with relatives, friends, trusted colleagues, or clergy to help maintain their well-being, rather than waiting until a crisis to reach out.
A good coach, mentor, or counselor can help physicians gain enough self-awareness to handle their emotions and gain more clarity about what changes need to be made, she said.
Dr. Cole suggested that doctors figure out what makes them happy and fulfilled at work and try to spend more time on that activity. “Knowing what makes you happy and your strengths are foundational for creating a life you love.”
She urged doctors to “start thinking now about what you love about medicine and what is going right at home, and what areas you want to change. Then, start advocating for your needs.”
A version of this article originally appeared on Medscape.com.
Correction, 1/26/23: An earlier version of this article misstated the findings of the survey.
A patient named ‘Settle’ decides to sue instead
On Nov. 1, 2020, Dallas Settle went to Plateau Medical Center, Oak Hill, W.Va., complaining of pain that was later described in court documents as being “in his right mid-abdomen migrating to his right lower abdomen.” Following a CT scan, Mr. Settle was diagnosed with diverticulitis resulting in pneumoperitoneum, which is the presence of air or other gas in the abdominal cavity. The patient, it was decided, required surgery to correct the problem, but Plateau Medical Center didn’t have the staff to perform the procedure.
Mr. Settle was then transferred to another West Virginia hospital, Charleston Area Medical Center (CAMC). Here, he was evaluated by doctors in the facility’s General Division, who initiated treatment with IV fluids and opiate analgesics. He was then placed under the care of a trauma surgeon, who initially decided to treat the patient nonoperatively. If that approach failed, the surgeon believed, Mr. Settle would probably require a laparotomy, bowel resection, and ostomy.
Another surgical team performed an exploratory laparotomy the following day. The team determined that Mr. Settle was suffering from a ruptured appendicitis and allegedly performed an appendectomy. But Mr. Settle’s condition continued to deteriorate the following day.
Another CT scan followed. It revealed various problems – multiple fluid collections, an ileus, distended loops of the patient’s small bowel, a left renal cyst, subcentimeter mesenteric, and retroperitoneal adenopathy. Additional CT scans conducted 4 days later indicated other problems, including fluid collections in the patient’s right- and left-lower quadrants.
Over the next few days, doctors performed further exploratory laparotomies. Finally, on Nov. 22, Mr. Settle was transferred out of the intensive care unit in preparation for his discharge the following day.
His pain continued to worsen, however, and he was readmitted to CAMC a day later. At this point, an examination revealed that his surgical incisions had become infected.
Worse news was on the horizon. On Nov. 28, the trauma surgeon who had first agreed to treat Mr. Settle informed him that, despite claims to the contrary, his appendix hadn’t been removed.
Eventually, Mr. Settle was referred to the Cleveland Clinic, where at press time he was still being treated.
Mr. Settle has hired the firm Calwell Luce diTrapano to sue CAMC, accusing it of medical malpractice, medical negligence, and other lapses in the standard of care. In his complaint, he accused the hospital and its staff of breaching their duty of care “by negligently and improperly treating him” and by failing “to exercise the degree of care, skill, and learning required and expected of reasonable health care providers.”
His suit seeks not only compensatory damages and other relief but also punitive damages.
The content contained in this article is for informational purposes only and does not constitute legal advice. Reliance on any information provided in this article is solely at your own risk.
A version of this article originally appeared on Medscape.com.
On Nov. 1, 2020, Dallas Settle went to Plateau Medical Center, Oak Hill, W.Va., complaining of pain that was later described in court documents as being “in his right mid-abdomen migrating to his right lower abdomen.” Following a CT scan, Mr. Settle was diagnosed with diverticulitis resulting in pneumoperitoneum, which is the presence of air or other gas in the abdominal cavity. The patient, it was decided, required surgery to correct the problem, but Plateau Medical Center didn’t have the staff to perform the procedure.
Mr. Settle was then transferred to another West Virginia hospital, Charleston Area Medical Center (CAMC). Here, he was evaluated by doctors in the facility’s General Division, who initiated treatment with IV fluids and opiate analgesics. He was then placed under the care of a trauma surgeon, who initially decided to treat the patient nonoperatively. If that approach failed, the surgeon believed, Mr. Settle would probably require a laparotomy, bowel resection, and ostomy.
Another surgical team performed an exploratory laparotomy the following day. The team determined that Mr. Settle was suffering from a ruptured appendicitis and allegedly performed an appendectomy. But Mr. Settle’s condition continued to deteriorate the following day.
Another CT scan followed. It revealed various problems – multiple fluid collections, an ileus, distended loops of the patient’s small bowel, a left renal cyst, subcentimeter mesenteric, and retroperitoneal adenopathy. Additional CT scans conducted 4 days later indicated other problems, including fluid collections in the patient’s right- and left-lower quadrants.
Over the next few days, doctors performed further exploratory laparotomies. Finally, on Nov. 22, Mr. Settle was transferred out of the intensive care unit in preparation for his discharge the following day.
His pain continued to worsen, however, and he was readmitted to CAMC a day later. At this point, an examination revealed that his surgical incisions had become infected.
Worse news was on the horizon. On Nov. 28, the trauma surgeon who had first agreed to treat Mr. Settle informed him that, despite claims to the contrary, his appendix hadn’t been removed.
Eventually, Mr. Settle was referred to the Cleveland Clinic, where at press time he was still being treated.
Mr. Settle has hired the firm Calwell Luce diTrapano to sue CAMC, accusing it of medical malpractice, medical negligence, and other lapses in the standard of care. In his complaint, he accused the hospital and its staff of breaching their duty of care “by negligently and improperly treating him” and by failing “to exercise the degree of care, skill, and learning required and expected of reasonable health care providers.”
His suit seeks not only compensatory damages and other relief but also punitive damages.
The content contained in this article is for informational purposes only and does not constitute legal advice. Reliance on any information provided in this article is solely at your own risk.
A version of this article originally appeared on Medscape.com.
On Nov. 1, 2020, Dallas Settle went to Plateau Medical Center, Oak Hill, W.Va., complaining of pain that was later described in court documents as being “in his right mid-abdomen migrating to his right lower abdomen.” Following a CT scan, Mr. Settle was diagnosed with diverticulitis resulting in pneumoperitoneum, which is the presence of air or other gas in the abdominal cavity. The patient, it was decided, required surgery to correct the problem, but Plateau Medical Center didn’t have the staff to perform the procedure.
Mr. Settle was then transferred to another West Virginia hospital, Charleston Area Medical Center (CAMC). Here, he was evaluated by doctors in the facility’s General Division, who initiated treatment with IV fluids and opiate analgesics. He was then placed under the care of a trauma surgeon, who initially decided to treat the patient nonoperatively. If that approach failed, the surgeon believed, Mr. Settle would probably require a laparotomy, bowel resection, and ostomy.
Another surgical team performed an exploratory laparotomy the following day. The team determined that Mr. Settle was suffering from a ruptured appendicitis and allegedly performed an appendectomy. But Mr. Settle’s condition continued to deteriorate the following day.
Another CT scan followed. It revealed various problems – multiple fluid collections, an ileus, distended loops of the patient’s small bowel, a left renal cyst, subcentimeter mesenteric, and retroperitoneal adenopathy. Additional CT scans conducted 4 days later indicated other problems, including fluid collections in the patient’s right- and left-lower quadrants.
Over the next few days, doctors performed further exploratory laparotomies. Finally, on Nov. 22, Mr. Settle was transferred out of the intensive care unit in preparation for his discharge the following day.
His pain continued to worsen, however, and he was readmitted to CAMC a day later. At this point, an examination revealed that his surgical incisions had become infected.
Worse news was on the horizon. On Nov. 28, the trauma surgeon who had first agreed to treat Mr. Settle informed him that, despite claims to the contrary, his appendix hadn’t been removed.
Eventually, Mr. Settle was referred to the Cleveland Clinic, where at press time he was still being treated.
Mr. Settle has hired the firm Calwell Luce diTrapano to sue CAMC, accusing it of medical malpractice, medical negligence, and other lapses in the standard of care. In his complaint, he accused the hospital and its staff of breaching their duty of care “by negligently and improperly treating him” and by failing “to exercise the degree of care, skill, and learning required and expected of reasonable health care providers.”
His suit seeks not only compensatory damages and other relief but also punitive damages.
The content contained in this article is for informational purposes only and does not constitute legal advice. Reliance on any information provided in this article is solely at your own risk.
A version of this article originally appeared on Medscape.com.
Geriatrician advises on use of vitamin D supplementation, lecanemab, and texting for her patients
Vitamin D supplementation and incident fractures
Vitamin D supplementation is a commonly recommended intervention for bone health, but data to support its impact on reducing fracture risk has been variable.
A study in the New England Journal of Medicine by LeBoff and colleagues has garnered much attention since its publication in July 2022.1 In the ancillary study of the Vitamin D and Omega-3-Trial (VITAL), the authors examined the impact of vitamin D supplementation versus placebo on incident fractures. The study found that vitamin D supplementation, as compared with placebo, led to no significant difference in the incidence of total, nonvertebral, and hip fractures in midlife and older adults over the 5-year period of follow-up.
The generalizability of these findings has been raised as a concern as the study does not describe adults at higher risk for fracture. The authors of the study specified in their conclusion that vitamin D supplementation does not reduce fracture risk in “generally healthy midlife and older adults who were not selected for vitamin D deficiency, low bone mass or osteoporosis.”
With a mean participant age of 67 and exclusion of participants with a history of cardiovascular disease, stroke, cirrhosis and other serious illnesses, the study does not reflect the multimorbid older adult population that geriatricians typically care for. Furthermore, efficacy of vitamin D supplementation on fracture risk may be the most impactful in those with osteoporosis and with severe vitamin D deficiency (defined by vitamin D 25[OH]D level less than 12 ng/mL).
In post hoc analyses, there was no significant difference in fracture risk in these subgroups, however the authors acknowledged that the findings may be limited by the small percentage of participants with severe vitamin D deficiency (2.4%) and osteoporosis included in the study (5%).
Lecanemab for mild cognitive impairment and early Alzheimer’s dementia
On Jan. 6, 2023, the Food and Drug Administration approved lecanemab, the second-ever disease-modifying treatment for Alzheimer’s dementia following the approval of aducanumab in 2021. Lecanemab is a monoclonal antibody targeting larger amyloid-beta oligomers, which has been shown in vitro to have higher affinity for amyloid-beta, compared with aducanumab. FDA approval followed shortly after the publication of the CLARITY-AD trial, which investigated the effect of lecanemab versus placebo on cognitive decline and burden of amyloid in adults with mild cognitive impairment and mild Alzheimer’s dementia. Over an 18-month period, the study found that participants who received lecanemab, compared with placebo, had a significantly smaller decline in cognition and function, and reduction in amyloid burden on PET CT.2
The clinical significance of these findings, however, is unclear. As noted by an editorial published in the Lancet in 2022, the difference in Clinical Dementia Rating-Sum of Boxes (CDR-SB) scale between the treatment and placebo groups was 0.45. On an 18-point scale, prior research has noted that a minimal clinically significance difference of 0.98 is necessary in those with mild cognitive impairment and 1.63 in mild Alzheimer dementia.3
Additionally, the CLARITY-AD trial reported that lecanemab resulted in infusion reactions in 26.4% of participants and brain edema (an amyloid-related imaging abnormality referred to as ARIA-E) in 12.6% of participants. This finding highlights concerns for safety and the need for close monitoring, as well as ongoing implications of economic feasibility and equitable access for all those who qualify for treatment.2
Social isolation and dementia risk
There is growing awareness of the impact of social isolation on health outcomes, particularly among older adults. Prior research has reported that one in four older adults are considered socially isolated and that social isolation increases risk of premature death, dementia, depression, and cardiovascular disease.4
A study by Huang and colleagues is the first nationally representative cohort study examining the association between social isolation and incident dementia for older adults in community dwelling settings. A cohort of 5,022 older adults participating in the National Health and Aging Trends Study was followed from 2011 to 2020. When adjusting for demographic and health factors, including race, level of education, and number of chronic health conditions, socially isolated adults had a greater risk of developing dementia, compared with adults who were not socially isolated (hazard ratio, 1.27; 95% confidence interval, 1.08-1.49). Potential mechanisms to explain this association include the increased risk of cardiovascular disease and depression in older adults who are socially isolated, thereby increasing dementia risk.
Decreased cognitive activity/engagement and access to resources such as caregiving and health care may also be linked to the increased risk of dementia in socially isolated older adults.5
Another observational cohort study from the National Health and Aging Trends Study investigated whether access and use of technology can lower the risk of social isolation. The study found that older adults who used email or text messaging had a lower risk of social isolation than older adults who did not use technology (incidence rate ratio, 0.64; 95% CI, 0.51-0.80).6 These findings highlight the importance of addressing social isolation as an important modifiable health risk factor, and the need for providing equitable access to technology in vulnerable populations as health intervention.
Dr. Mengru “Ruru” Wang is a geriatrician and internist at the University of Washington, Seattle. She practices full-spectrum medicine, seeing patients in primary care, nursing homes, and acute care. Dr. Wang has no disclosures related to this piece.
References
1. LeBoff MS et al. Supplemental vitamin D and incident fractures in midlife and older adults. N Engl J Med. 2022;387(4):299-30.
2. van Dyck CH et al. Lecanemab in early Alzheimer’s disease. N Engl J Med. 2023;388(1):9-21.
3. The Lancet. Lecanemab for Alzheimer’s disease: tempering hype and hope. Lancet. 2022; 400:1899.
4. National Academies of Sciences, Engineering, and Medicine. Social Isolation and Loneliness in Older Adults: Opportunities for the Health Care System. Washington, DC: 2020, The National Academies Press.
5. Huang, AR et al. Social isolation and 9-year dementia risk in community dwelling Medicare beneficiaries in the United States. J Am Geriatr Soc. 2023 Jan 11. doi: 10.1111/jgs18140.
6. Umoh ME etal. Impact of technology on social isolation: Longitudinal analysis from the National Health Aging Trends Study. J Am Geriatr Soc. 2022 Dec 15. doi 10.1111/jgs.18179.
Vitamin D supplementation and incident fractures
Vitamin D supplementation is a commonly recommended intervention for bone health, but data to support its impact on reducing fracture risk has been variable.
A study in the New England Journal of Medicine by LeBoff and colleagues has garnered much attention since its publication in July 2022.1 In the ancillary study of the Vitamin D and Omega-3-Trial (VITAL), the authors examined the impact of vitamin D supplementation versus placebo on incident fractures. The study found that vitamin D supplementation, as compared with placebo, led to no significant difference in the incidence of total, nonvertebral, and hip fractures in midlife and older adults over the 5-year period of follow-up.
The generalizability of these findings has been raised as a concern as the study does not describe adults at higher risk for fracture. The authors of the study specified in their conclusion that vitamin D supplementation does not reduce fracture risk in “generally healthy midlife and older adults who were not selected for vitamin D deficiency, low bone mass or osteoporosis.”
With a mean participant age of 67 and exclusion of participants with a history of cardiovascular disease, stroke, cirrhosis and other serious illnesses, the study does not reflect the multimorbid older adult population that geriatricians typically care for. Furthermore, efficacy of vitamin D supplementation on fracture risk may be the most impactful in those with osteoporosis and with severe vitamin D deficiency (defined by vitamin D 25[OH]D level less than 12 ng/mL).
In post hoc analyses, there was no significant difference in fracture risk in these subgroups, however the authors acknowledged that the findings may be limited by the small percentage of participants with severe vitamin D deficiency (2.4%) and osteoporosis included in the study (5%).
Lecanemab for mild cognitive impairment and early Alzheimer’s dementia
On Jan. 6, 2023, the Food and Drug Administration approved lecanemab, the second-ever disease-modifying treatment for Alzheimer’s dementia following the approval of aducanumab in 2021. Lecanemab is a monoclonal antibody targeting larger amyloid-beta oligomers, which has been shown in vitro to have higher affinity for amyloid-beta, compared with aducanumab. FDA approval followed shortly after the publication of the CLARITY-AD trial, which investigated the effect of lecanemab versus placebo on cognitive decline and burden of amyloid in adults with mild cognitive impairment and mild Alzheimer’s dementia. Over an 18-month period, the study found that participants who received lecanemab, compared with placebo, had a significantly smaller decline in cognition and function, and reduction in amyloid burden on PET CT.2
The clinical significance of these findings, however, is unclear. As noted by an editorial published in the Lancet in 2022, the difference in Clinical Dementia Rating-Sum of Boxes (CDR-SB) scale between the treatment and placebo groups was 0.45. On an 18-point scale, prior research has noted that a minimal clinically significance difference of 0.98 is necessary in those with mild cognitive impairment and 1.63 in mild Alzheimer dementia.3
Additionally, the CLARITY-AD trial reported that lecanemab resulted in infusion reactions in 26.4% of participants and brain edema (an amyloid-related imaging abnormality referred to as ARIA-E) in 12.6% of participants. This finding highlights concerns for safety and the need for close monitoring, as well as ongoing implications of economic feasibility and equitable access for all those who qualify for treatment.2
Social isolation and dementia risk
There is growing awareness of the impact of social isolation on health outcomes, particularly among older adults. Prior research has reported that one in four older adults are considered socially isolated and that social isolation increases risk of premature death, dementia, depression, and cardiovascular disease.4
A study by Huang and colleagues is the first nationally representative cohort study examining the association between social isolation and incident dementia for older adults in community dwelling settings. A cohort of 5,022 older adults participating in the National Health and Aging Trends Study was followed from 2011 to 2020. When adjusting for demographic and health factors, including race, level of education, and number of chronic health conditions, socially isolated adults had a greater risk of developing dementia, compared with adults who were not socially isolated (hazard ratio, 1.27; 95% confidence interval, 1.08-1.49). Potential mechanisms to explain this association include the increased risk of cardiovascular disease and depression in older adults who are socially isolated, thereby increasing dementia risk.
Decreased cognitive activity/engagement and access to resources such as caregiving and health care may also be linked to the increased risk of dementia in socially isolated older adults.5
Another observational cohort study from the National Health and Aging Trends Study investigated whether access and use of technology can lower the risk of social isolation. The study found that older adults who used email or text messaging had a lower risk of social isolation than older adults who did not use technology (incidence rate ratio, 0.64; 95% CI, 0.51-0.80).6 These findings highlight the importance of addressing social isolation as an important modifiable health risk factor, and the need for providing equitable access to technology in vulnerable populations as health intervention.
Dr. Mengru “Ruru” Wang is a geriatrician and internist at the University of Washington, Seattle. She practices full-spectrum medicine, seeing patients in primary care, nursing homes, and acute care. Dr. Wang has no disclosures related to this piece.
References
1. LeBoff MS et al. Supplemental vitamin D and incident fractures in midlife and older adults. N Engl J Med. 2022;387(4):299-30.
2. van Dyck CH et al. Lecanemab in early Alzheimer’s disease. N Engl J Med. 2023;388(1):9-21.
3. The Lancet. Lecanemab for Alzheimer’s disease: tempering hype and hope. Lancet. 2022; 400:1899.
4. National Academies of Sciences, Engineering, and Medicine. Social Isolation and Loneliness in Older Adults: Opportunities for the Health Care System. Washington, DC: 2020, The National Academies Press.
5. Huang, AR et al. Social isolation and 9-year dementia risk in community dwelling Medicare beneficiaries in the United States. J Am Geriatr Soc. 2023 Jan 11. doi: 10.1111/jgs18140.
6. Umoh ME etal. Impact of technology on social isolation: Longitudinal analysis from the National Health Aging Trends Study. J Am Geriatr Soc. 2022 Dec 15. doi 10.1111/jgs.18179.
Vitamin D supplementation and incident fractures
Vitamin D supplementation is a commonly recommended intervention for bone health, but data to support its impact on reducing fracture risk has been variable.
A study in the New England Journal of Medicine by LeBoff and colleagues has garnered much attention since its publication in July 2022.1 In the ancillary study of the Vitamin D and Omega-3-Trial (VITAL), the authors examined the impact of vitamin D supplementation versus placebo on incident fractures. The study found that vitamin D supplementation, as compared with placebo, led to no significant difference in the incidence of total, nonvertebral, and hip fractures in midlife and older adults over the 5-year period of follow-up.
The generalizability of these findings has been raised as a concern as the study does not describe adults at higher risk for fracture. The authors of the study specified in their conclusion that vitamin D supplementation does not reduce fracture risk in “generally healthy midlife and older adults who were not selected for vitamin D deficiency, low bone mass or osteoporosis.”
With a mean participant age of 67 and exclusion of participants with a history of cardiovascular disease, stroke, cirrhosis and other serious illnesses, the study does not reflect the multimorbid older adult population that geriatricians typically care for. Furthermore, efficacy of vitamin D supplementation on fracture risk may be the most impactful in those with osteoporosis and with severe vitamin D deficiency (defined by vitamin D 25[OH]D level less than 12 ng/mL).
In post hoc analyses, there was no significant difference in fracture risk in these subgroups, however the authors acknowledged that the findings may be limited by the small percentage of participants with severe vitamin D deficiency (2.4%) and osteoporosis included in the study (5%).
Lecanemab for mild cognitive impairment and early Alzheimer’s dementia
On Jan. 6, 2023, the Food and Drug Administration approved lecanemab, the second-ever disease-modifying treatment for Alzheimer’s dementia following the approval of aducanumab in 2021. Lecanemab is a monoclonal antibody targeting larger amyloid-beta oligomers, which has been shown in vitro to have higher affinity for amyloid-beta, compared with aducanumab. FDA approval followed shortly after the publication of the CLARITY-AD trial, which investigated the effect of lecanemab versus placebo on cognitive decline and burden of amyloid in adults with mild cognitive impairment and mild Alzheimer’s dementia. Over an 18-month period, the study found that participants who received lecanemab, compared with placebo, had a significantly smaller decline in cognition and function, and reduction in amyloid burden on PET CT.2
The clinical significance of these findings, however, is unclear. As noted by an editorial published in the Lancet in 2022, the difference in Clinical Dementia Rating-Sum of Boxes (CDR-SB) scale between the treatment and placebo groups was 0.45. On an 18-point scale, prior research has noted that a minimal clinically significance difference of 0.98 is necessary in those with mild cognitive impairment and 1.63 in mild Alzheimer dementia.3
Additionally, the CLARITY-AD trial reported that lecanemab resulted in infusion reactions in 26.4% of participants and brain edema (an amyloid-related imaging abnormality referred to as ARIA-E) in 12.6% of participants. This finding highlights concerns for safety and the need for close monitoring, as well as ongoing implications of economic feasibility and equitable access for all those who qualify for treatment.2
Social isolation and dementia risk
There is growing awareness of the impact of social isolation on health outcomes, particularly among older adults. Prior research has reported that one in four older adults are considered socially isolated and that social isolation increases risk of premature death, dementia, depression, and cardiovascular disease.4
A study by Huang and colleagues is the first nationally representative cohort study examining the association between social isolation and incident dementia for older adults in community dwelling settings. A cohort of 5,022 older adults participating in the National Health and Aging Trends Study was followed from 2011 to 2020. When adjusting for demographic and health factors, including race, level of education, and number of chronic health conditions, socially isolated adults had a greater risk of developing dementia, compared with adults who were not socially isolated (hazard ratio, 1.27; 95% confidence interval, 1.08-1.49). Potential mechanisms to explain this association include the increased risk of cardiovascular disease and depression in older adults who are socially isolated, thereby increasing dementia risk.
Decreased cognitive activity/engagement and access to resources such as caregiving and health care may also be linked to the increased risk of dementia in socially isolated older adults.5
Another observational cohort study from the National Health and Aging Trends Study investigated whether access and use of technology can lower the risk of social isolation. The study found that older adults who used email or text messaging had a lower risk of social isolation than older adults who did not use technology (incidence rate ratio, 0.64; 95% CI, 0.51-0.80).6 These findings highlight the importance of addressing social isolation as an important modifiable health risk factor, and the need for providing equitable access to technology in vulnerable populations as health intervention.
Dr. Mengru “Ruru” Wang is a geriatrician and internist at the University of Washington, Seattle. She practices full-spectrum medicine, seeing patients in primary care, nursing homes, and acute care. Dr. Wang has no disclosures related to this piece.
References
1. LeBoff MS et al. Supplemental vitamin D and incident fractures in midlife and older adults. N Engl J Med. 2022;387(4):299-30.
2. van Dyck CH et al. Lecanemab in early Alzheimer’s disease. N Engl J Med. 2023;388(1):9-21.
3. The Lancet. Lecanemab for Alzheimer’s disease: tempering hype and hope. Lancet. 2022; 400:1899.
4. National Academies of Sciences, Engineering, and Medicine. Social Isolation and Loneliness in Older Adults: Opportunities for the Health Care System. Washington, DC: 2020, The National Academies Press.
5. Huang, AR et al. Social isolation and 9-year dementia risk in community dwelling Medicare beneficiaries in the United States. J Am Geriatr Soc. 2023 Jan 11. doi: 10.1111/jgs18140.
6. Umoh ME etal. Impact of technology on social isolation: Longitudinal analysis from the National Health Aging Trends Study. J Am Geriatr Soc. 2022 Dec 15. doi 10.1111/jgs.18179.
Not all white coats are doctors: Why titles are important at the doctor’s office
says Cyndy Flores, a physician assistant (PA) in the emergency department at Vituity, Emeryville, Calif. “Sometimes, I can go through a complete history and physical, explain a treatment plan, and perform a procedure, and [the patient] will say, ‘Thank you, doctor.’ ”
“I always come back and say, ‘You’re very welcome, but my name is Cyndy, and I’m the PA.’ ”
Ms. Flores is used to patients calling her “doctor” when she greets them. She typically offers a quick correction and moves on with the appointment.
With 355,000 nurse practitioners (NPs) and 149,000 certified PAs practicing in the United States, it’s more common than ever for health care providers who don’t go by the title “doctor” to diagnose and treat patients.
A recent report, Evolving Scope of Practice, found that more than 70% of physicians were “somewhat satisfied to very satisfied” with patient treatment by PAs and NPs.
But for patients, having a health care team that includes physicians, NPs, and PAs can be confusing. Additionally, it creates a need for education about their correct titles and roles in patient care.
“It’s really important for patients to understand who is taking care of them,” Ms. Flores says.
Education starts in your practice
Educating patients about the roles of different providers on their health care team starts long before patients enter the exam room, Ms. Flores explains.
Some patients may not understand the difference, some may just forget because they’re used to calling all providers doctors, and others may find it awkward to use a provider’s first name or not know the respectful way to address an NP or a PA.
Practices can help by listing the names and biographies of the health care team on the clinic website. In addition, when patients call for an appointment, Ms. Flores believes front desk staff can reinforce that information. When offering appointments with a physician, NP, or PA, clearly use the practitioner’s title and reiterate it throughout the conversation. For example, “Would you like to see our nurse practitioner, Alice Smith, next week?” or “So, our physician assistant Mrs. Jones will see you Friday at 3 PM.”
The report also found that 76% of patients expressed a preference to see a physician over a PA, and 71% expressed a preference to see a physician over an NP, but offering appointments with nonphysician providers is part of the education process.
“Some families are super savvy and know the differences between nurse practitioners, physician assistants, and doctors, and ... there are families who don’t understand those titles, [and] we need to explain what they do in our practice,” adds Nicole Aaronson, MD, MBA, attending surgeon at Nemours Children’s Health of Delaware. Dr. Aaronson believes there’s an opportunity for educating patients when speaking about all the available providers they may see.
Hanging posters or using brochures in the clinic or hospital is another effective way to reinforce the roles of various providers on the care team. Include biographies and educational information on practice materials and video programs running in the waiting room.
“Patients mean it [calling everyone doctor] as a way to respectfully address the nurse practitioner or physician assistant rather than meaning it as a denigration of the physician,” Dr. Aaronson says. “But everyone appreciates being called by the correct title.”
Helping patients understand the members of their care team and the correct titles to use for those health care professionals could also help patients feel more confident about their health care experience.
“Patients really like knowing that there are specialists in each of the areas taking care of them,” Ms. Flores says. “I think that conveys a feeling of trust in your provider.”
Not everyone is a doctor
Even when PAs and NPs remind patients of their roles and reinforce the use of their preferred names, there will still be patients who continue referring to their nonphysician provider as “doctor.”
“There’s a perception that anyone who walks into a room with a stethoscope is your doctor,” says Graig Straus, DNP, an NP and president and CEO of Rockland Urgent Care Family Health NP, P.C., West Haverstraw, N.Y. “You do get a little bit of burnout correcting people all the time.”
Dr. Straus, who earned his doctorate in nursing practice, notes that patients using the honorific with him aren’t incorrect, but he still educates them on his role within the health care team.
“NPs and PAs have a valuable role to play independently and in concert with the physician,” Dr. Aaronson says. This understanding is essential, as states consider expanding treatment abilities for NPs and PAs.
NPs have expanded treatment abilities or full practice authority in almost half the states, and 31% of the physicians surveyed agreed that NPs should have expanded treatment abilities.
An estimated 1 in 5 states characterizes the physician-PA relationship as collaborative, not supervisory, according to the American Academy of Physician Associates. At the same time, only 39% of physicians surveyed said they favored this trend.
“Patients need great quality care, and there are many different types of providers that can provide that care as part of the team,” Ms. Flores says. “When you have a team taking care of a patient, that patient [gets] the best care possible – and ... that’s why we went into medicine: to deliver high-quality, compassionate care to our patients, and we should all be in this together.”
When practices do their part explaining who is and isn’t a doctor and what each provider’s title and role is and what to call them, and everyone reinforces it, health care becomes not only more manageable for patients to traverse but easier to understand, leading to a better experience.
A version of this article first appeared on Medscape.com.
says Cyndy Flores, a physician assistant (PA) in the emergency department at Vituity, Emeryville, Calif. “Sometimes, I can go through a complete history and physical, explain a treatment plan, and perform a procedure, and [the patient] will say, ‘Thank you, doctor.’ ”
“I always come back and say, ‘You’re very welcome, but my name is Cyndy, and I’m the PA.’ ”
Ms. Flores is used to patients calling her “doctor” when she greets them. She typically offers a quick correction and moves on with the appointment.
With 355,000 nurse practitioners (NPs) and 149,000 certified PAs practicing in the United States, it’s more common than ever for health care providers who don’t go by the title “doctor” to diagnose and treat patients.
A recent report, Evolving Scope of Practice, found that more than 70% of physicians were “somewhat satisfied to very satisfied” with patient treatment by PAs and NPs.
But for patients, having a health care team that includes physicians, NPs, and PAs can be confusing. Additionally, it creates a need for education about their correct titles and roles in patient care.
“It’s really important for patients to understand who is taking care of them,” Ms. Flores says.
Education starts in your practice
Educating patients about the roles of different providers on their health care team starts long before patients enter the exam room, Ms. Flores explains.
Some patients may not understand the difference, some may just forget because they’re used to calling all providers doctors, and others may find it awkward to use a provider’s first name or not know the respectful way to address an NP or a PA.
Practices can help by listing the names and biographies of the health care team on the clinic website. In addition, when patients call for an appointment, Ms. Flores believes front desk staff can reinforce that information. When offering appointments with a physician, NP, or PA, clearly use the practitioner’s title and reiterate it throughout the conversation. For example, “Would you like to see our nurse practitioner, Alice Smith, next week?” or “So, our physician assistant Mrs. Jones will see you Friday at 3 PM.”
The report also found that 76% of patients expressed a preference to see a physician over a PA, and 71% expressed a preference to see a physician over an NP, but offering appointments with nonphysician providers is part of the education process.
“Some families are super savvy and know the differences between nurse practitioners, physician assistants, and doctors, and ... there are families who don’t understand those titles, [and] we need to explain what they do in our practice,” adds Nicole Aaronson, MD, MBA, attending surgeon at Nemours Children’s Health of Delaware. Dr. Aaronson believes there’s an opportunity for educating patients when speaking about all the available providers they may see.
Hanging posters or using brochures in the clinic or hospital is another effective way to reinforce the roles of various providers on the care team. Include biographies and educational information on practice materials and video programs running in the waiting room.
“Patients mean it [calling everyone doctor] as a way to respectfully address the nurse practitioner or physician assistant rather than meaning it as a denigration of the physician,” Dr. Aaronson says. “But everyone appreciates being called by the correct title.”
Helping patients understand the members of their care team and the correct titles to use for those health care professionals could also help patients feel more confident about their health care experience.
“Patients really like knowing that there are specialists in each of the areas taking care of them,” Ms. Flores says. “I think that conveys a feeling of trust in your provider.”
Not everyone is a doctor
Even when PAs and NPs remind patients of their roles and reinforce the use of their preferred names, there will still be patients who continue referring to their nonphysician provider as “doctor.”
“There’s a perception that anyone who walks into a room with a stethoscope is your doctor,” says Graig Straus, DNP, an NP and president and CEO of Rockland Urgent Care Family Health NP, P.C., West Haverstraw, N.Y. “You do get a little bit of burnout correcting people all the time.”
Dr. Straus, who earned his doctorate in nursing practice, notes that patients using the honorific with him aren’t incorrect, but he still educates them on his role within the health care team.
“NPs and PAs have a valuable role to play independently and in concert with the physician,” Dr. Aaronson says. This understanding is essential, as states consider expanding treatment abilities for NPs and PAs.
NPs have expanded treatment abilities or full practice authority in almost half the states, and 31% of the physicians surveyed agreed that NPs should have expanded treatment abilities.
An estimated 1 in 5 states characterizes the physician-PA relationship as collaborative, not supervisory, according to the American Academy of Physician Associates. At the same time, only 39% of physicians surveyed said they favored this trend.
“Patients need great quality care, and there are many different types of providers that can provide that care as part of the team,” Ms. Flores says. “When you have a team taking care of a patient, that patient [gets] the best care possible – and ... that’s why we went into medicine: to deliver high-quality, compassionate care to our patients, and we should all be in this together.”
When practices do their part explaining who is and isn’t a doctor and what each provider’s title and role is and what to call them, and everyone reinforces it, health care becomes not only more manageable for patients to traverse but easier to understand, leading to a better experience.
A version of this article first appeared on Medscape.com.
says Cyndy Flores, a physician assistant (PA) in the emergency department at Vituity, Emeryville, Calif. “Sometimes, I can go through a complete history and physical, explain a treatment plan, and perform a procedure, and [the patient] will say, ‘Thank you, doctor.’ ”
“I always come back and say, ‘You’re very welcome, but my name is Cyndy, and I’m the PA.’ ”
Ms. Flores is used to patients calling her “doctor” when she greets them. She typically offers a quick correction and moves on with the appointment.
With 355,000 nurse practitioners (NPs) and 149,000 certified PAs practicing in the United States, it’s more common than ever for health care providers who don’t go by the title “doctor” to diagnose and treat patients.
A recent report, Evolving Scope of Practice, found that more than 70% of physicians were “somewhat satisfied to very satisfied” with patient treatment by PAs and NPs.
But for patients, having a health care team that includes physicians, NPs, and PAs can be confusing. Additionally, it creates a need for education about their correct titles and roles in patient care.
“It’s really important for patients to understand who is taking care of them,” Ms. Flores says.
Education starts in your practice
Educating patients about the roles of different providers on their health care team starts long before patients enter the exam room, Ms. Flores explains.
Some patients may not understand the difference, some may just forget because they’re used to calling all providers doctors, and others may find it awkward to use a provider’s first name or not know the respectful way to address an NP or a PA.
Practices can help by listing the names and biographies of the health care team on the clinic website. In addition, when patients call for an appointment, Ms. Flores believes front desk staff can reinforce that information. When offering appointments with a physician, NP, or PA, clearly use the practitioner’s title and reiterate it throughout the conversation. For example, “Would you like to see our nurse practitioner, Alice Smith, next week?” or “So, our physician assistant Mrs. Jones will see you Friday at 3 PM.”
The report also found that 76% of patients expressed a preference to see a physician over a PA, and 71% expressed a preference to see a physician over an NP, but offering appointments with nonphysician providers is part of the education process.
“Some families are super savvy and know the differences between nurse practitioners, physician assistants, and doctors, and ... there are families who don’t understand those titles, [and] we need to explain what they do in our practice,” adds Nicole Aaronson, MD, MBA, attending surgeon at Nemours Children’s Health of Delaware. Dr. Aaronson believes there’s an opportunity for educating patients when speaking about all the available providers they may see.
Hanging posters or using brochures in the clinic or hospital is another effective way to reinforce the roles of various providers on the care team. Include biographies and educational information on practice materials and video programs running in the waiting room.
“Patients mean it [calling everyone doctor] as a way to respectfully address the nurse practitioner or physician assistant rather than meaning it as a denigration of the physician,” Dr. Aaronson says. “But everyone appreciates being called by the correct title.”
Helping patients understand the members of their care team and the correct titles to use for those health care professionals could also help patients feel more confident about their health care experience.
“Patients really like knowing that there are specialists in each of the areas taking care of them,” Ms. Flores says. “I think that conveys a feeling of trust in your provider.”
Not everyone is a doctor
Even when PAs and NPs remind patients of their roles and reinforce the use of their preferred names, there will still be patients who continue referring to their nonphysician provider as “doctor.”
“There’s a perception that anyone who walks into a room with a stethoscope is your doctor,” says Graig Straus, DNP, an NP and president and CEO of Rockland Urgent Care Family Health NP, P.C., West Haverstraw, N.Y. “You do get a little bit of burnout correcting people all the time.”
Dr. Straus, who earned his doctorate in nursing practice, notes that patients using the honorific with him aren’t incorrect, but he still educates them on his role within the health care team.
“NPs and PAs have a valuable role to play independently and in concert with the physician,” Dr. Aaronson says. This understanding is essential, as states consider expanding treatment abilities for NPs and PAs.
NPs have expanded treatment abilities or full practice authority in almost half the states, and 31% of the physicians surveyed agreed that NPs should have expanded treatment abilities.
An estimated 1 in 5 states characterizes the physician-PA relationship as collaborative, not supervisory, according to the American Academy of Physician Associates. At the same time, only 39% of physicians surveyed said they favored this trend.
“Patients need great quality care, and there are many different types of providers that can provide that care as part of the team,” Ms. Flores says. “When you have a team taking care of a patient, that patient [gets] the best care possible – and ... that’s why we went into medicine: to deliver high-quality, compassionate care to our patients, and we should all be in this together.”
When practices do their part explaining who is and isn’t a doctor and what each provider’s title and role is and what to call them, and everyone reinforces it, health care becomes not only more manageable for patients to traverse but easier to understand, leading to a better experience.
A version of this article first appeared on Medscape.com.