User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
COVID-19 vaccine distribution could start in 2 weeks, Pence says
Initial doses of a coronavirus vaccine could be sent out as early as mid-December, Vice President Mike Pence told governors during a call on Monday.
The distribution process could start during the week of Dec. 14, according to audio of a White House Coronavirus Task Force call obtained by CBS News. The call focused on the timeline of vaccine approval and distribution.
“With this morning’s news that Moderna is joining Pfizer in submitting an emergency-use authorization [to the Food and Drug Administration], we continue to be on pace,” Pence said.
The FDA is scheduled to make a decision about Pfizer’s emergency use authorization after an advisory panel meets on Dec. 10 to review the company’s application. FDA Commissioner Stephen Hahn, MD, didn’t commit to the Dec. 14 date, CBS News reported.
“We do all the number crunching ourselves,” Dr. Hahn said. “We look line by line by line on all the data, on all the patients and manufacturing. We do statistical analyses and we come to our own conclusions to support a decision of either thumbs-up or thumbs-down.”
According to a meeting agenda, Pfizer vaccine deliveries should start on Dec. 15, followed by the Moderna vaccine on Dec. 22, CBS News reported.
Between Dec. 13-19, Pfizer is slated to deliver 6.4 million doses, which is enough to immunize about 3 million people with two shots. An “undetermined number” are reserved for backup doses, the news outlet reported.
During the next week, Pfizer and Moderna are scheduled to produce enough doses to vaccinate an additional 10 million people. By the end of the month, about 30 million people should receive doses.
As vaccines begin to roll out, Mr. Pence said “we have a ways to go” in reassuring the public about immunization. He urged governors to use their “bully pulpit” to educate their states and “develop public confidence” in the vaccines.
During the call, Anthony Fauci, MD, director of the National Institute for Allergy and Infectious Diseases, supported the safety and efficacy of the vaccines. Although the vaccine development and approval process was accelerated this year, he said, it “does not at all compromise safety, nor does it compromise scientific integrity.”
“Any misrepresentation that the vaccines had government interference or company interference is patently untrue,” he said.
This article first appeared on Medscape.com.
Initial doses of a coronavirus vaccine could be sent out as early as mid-December, Vice President Mike Pence told governors during a call on Monday.
The distribution process could start during the week of Dec. 14, according to audio of a White House Coronavirus Task Force call obtained by CBS News. The call focused on the timeline of vaccine approval and distribution.
“With this morning’s news that Moderna is joining Pfizer in submitting an emergency-use authorization [to the Food and Drug Administration], we continue to be on pace,” Pence said.
The FDA is scheduled to make a decision about Pfizer’s emergency use authorization after an advisory panel meets on Dec. 10 to review the company’s application. FDA Commissioner Stephen Hahn, MD, didn’t commit to the Dec. 14 date, CBS News reported.
“We do all the number crunching ourselves,” Dr. Hahn said. “We look line by line by line on all the data, on all the patients and manufacturing. We do statistical analyses and we come to our own conclusions to support a decision of either thumbs-up or thumbs-down.”
According to a meeting agenda, Pfizer vaccine deliveries should start on Dec. 15, followed by the Moderna vaccine on Dec. 22, CBS News reported.
Between Dec. 13-19, Pfizer is slated to deliver 6.4 million doses, which is enough to immunize about 3 million people with two shots. An “undetermined number” are reserved for backup doses, the news outlet reported.
During the next week, Pfizer and Moderna are scheduled to produce enough doses to vaccinate an additional 10 million people. By the end of the month, about 30 million people should receive doses.
As vaccines begin to roll out, Mr. Pence said “we have a ways to go” in reassuring the public about immunization. He urged governors to use their “bully pulpit” to educate their states and “develop public confidence” in the vaccines.
During the call, Anthony Fauci, MD, director of the National Institute for Allergy and Infectious Diseases, supported the safety and efficacy of the vaccines. Although the vaccine development and approval process was accelerated this year, he said, it “does not at all compromise safety, nor does it compromise scientific integrity.”
“Any misrepresentation that the vaccines had government interference or company interference is patently untrue,” he said.
This article first appeared on Medscape.com.
Initial doses of a coronavirus vaccine could be sent out as early as mid-December, Vice President Mike Pence told governors during a call on Monday.
The distribution process could start during the week of Dec. 14, according to audio of a White House Coronavirus Task Force call obtained by CBS News. The call focused on the timeline of vaccine approval and distribution.
“With this morning’s news that Moderna is joining Pfizer in submitting an emergency-use authorization [to the Food and Drug Administration], we continue to be on pace,” Pence said.
The FDA is scheduled to make a decision about Pfizer’s emergency use authorization after an advisory panel meets on Dec. 10 to review the company’s application. FDA Commissioner Stephen Hahn, MD, didn’t commit to the Dec. 14 date, CBS News reported.
“We do all the number crunching ourselves,” Dr. Hahn said. “We look line by line by line on all the data, on all the patients and manufacturing. We do statistical analyses and we come to our own conclusions to support a decision of either thumbs-up or thumbs-down.”
According to a meeting agenda, Pfizer vaccine deliveries should start on Dec. 15, followed by the Moderna vaccine on Dec. 22, CBS News reported.
Between Dec. 13-19, Pfizer is slated to deliver 6.4 million doses, which is enough to immunize about 3 million people with two shots. An “undetermined number” are reserved for backup doses, the news outlet reported.
During the next week, Pfizer and Moderna are scheduled to produce enough doses to vaccinate an additional 10 million people. By the end of the month, about 30 million people should receive doses.
As vaccines begin to roll out, Mr. Pence said “we have a ways to go” in reassuring the public about immunization. He urged governors to use their “bully pulpit” to educate their states and “develop public confidence” in the vaccines.
During the call, Anthony Fauci, MD, director of the National Institute for Allergy and Infectious Diseases, supported the safety and efficacy of the vaccines. Although the vaccine development and approval process was accelerated this year, he said, it “does not at all compromise safety, nor does it compromise scientific integrity.”
“Any misrepresentation that the vaccines had government interference or company interference is patently untrue,” he said.
This article first appeared on Medscape.com.
Medicare finalizes 2021 physician pay rule with E/M changes
Medicare officials stuck with their plan to increase payments for office visits for primary care and several other specialties that focus on helping patients manage complex conditions such as diabetes. In doing so, Medicare also finalized cuts for other fields, triggering a new wave of protests.
The final version of the 2021 Medicare physician fee schedule was unveiled on the night of Dec. 1. The Centers for Medicare & Medicaid Services posted an unofficial copy of the rule, which will later be published in the Federal Register.
CMS said it completed work on this massive annual review of payments for clinicians later than it usually does because of the demands of the federal response to the COVID-19 pandemic. The 2021 physician fee rule will take effect within a 30-day period instead of the usual 60-day time frame.
The most contentious item proposed for 2021 was a reshuffling of payments among specialties as part of an overhaul of Medicare’s approach to valuing evaluation and management (E/M) services. There was broader support for other aspects of the E/M overhaul, which are intended to cut some of the administrative hassle clinicians face.
“This finalized policy marks the most significant updates to E/M codes in 30 years, reducing burden on doctors imposed by the coding system and rewarding time spent evaluating and managing their patients’ care,” CMS Administrator Seema Verma said in a statement. “In the past, the system has rewarded interventions and procedures over time spent with patients – time taken preventing disease and managing chronic illnesses.”
In the final rule, CMS summarized these results of the E/M changes in Table 106. CMS largely stuck with the approach outlined in a draft rule released in August, with minor changes in the amounts of cuts and increases.
Specialties in line for increases under the 2021 final physician fee schedule include allergy/immunology (9%), endocrinology (16%), family practice (13%), general practice (7%), geriatrics (3%), hematology/oncology (14%), internal medicine (4%), nephrology (6%), physician assistants (8%), psychiatry (7%), rheumatology (15%), and urology (8%).
In line for cuts would be anesthesiology (–8%), cardiac surgery (–8%), emergency medicine (–6%), general surgery (–6%), infectious disease (–4%), neurosurgery (–6%), physical/occupational therapy (–9%), plastic surgery (–7%), radiology (–10%), and thoracic surgery (–8%).
CMS had initially set these changes in 2021 pay in motion in the 2020 physician fee schedule. The agency subsequently faced significant opposition to its plans. Many physician groups sought to waive a “budget-neutral” approach to the E/M overhaul, which makes the offsetting of cuts necessary. They argued this would allow increased compensation for clinicians whose practices focus on office visits without requiring offsetting cuts from other fields of medicine.
The American Medical Association is among those urging Congress to prevent or postpone the payment reductions resulting from Medicare’s budget neutrality requirement as applied to the E/M overhaul.
In a Tuesday statement, AMA President Susan R. Bailey, MD, noted that many physicians are facing “substantial economic hardships due to COVID-19.”
By AMA’s calculations, CMS’ planned 2021 E/M overhaul could result in “a shocking reduction of 10.2% to Medicare payment rates,” according to Bailey’s statement. The AMA strongly supports other aspects of the E/M changes CMS finalized, which Bailey said will result in “simpler and more flexible” coding and documentation.
The Surgical Care Coalition, which represents about a dozen medical specialty associations, is asking members of Congress to block the full implementation of the E/M overhaul.
In a Dec. 1 statement, the coalition urged the passage of a bill (HR 8702) that has been introduced in the House by a bipartisan duo of physicians, Rep. Ami Bera, MD (D-Calif.), and Rep. Larry Bucshon, MD (R-Ind.). Their bill would effectively block the cuts from going into effect on January 1, 2021. It would provide an additional Medicare payment for certain services in 2021 and 2022 if the otherwise applicable payment is less than it would have been in 2020.
The Medicare E/M overhaul “was a dangerous policy even before the pandemic, and enacting it during the worst health care crisis in a century is unconscionable. If Congress fails to act, it will further strain a health care system that’s already been pushed to the brink due to the COVID-19 pandemic and undermine patient care,” said John A. Wilson, MD, president of the American Association of Neurological Surgeons, in a statement.
Also backing the Bera-Bucshon bill is the American College of Emergency Physicians. In a statement on Tuesday, ACEP President Mark Rosenberg, DO, MBA, urged Congress to act on this measure.
“Emergency physicians and other health care providers battling on the front lines of the ongoing pandemic are already under unprecedented financial strain as they continue to bear the brunt of COVID-19,” Dr. Rosenberg said. “These cuts would have a devastating impact for the future of emergency medicine and could seriously impede patients’ access to emergency care when they need it most.”
“Long overdue”
But there also are champions for the approach CMS took in the E/M overhaul. The influential Medicare Payment Advisory Commission (MedPAC) has argued strongly for keeping the budget-neutral approach to the E/M overhaul.
In an Oct. 2 comment to CMS about the draft 2021 physician fee schedule, MedPAC Chairman Michael E. Chernew, PhD, said this approach would “help rebalance the fee schedule from services that have become overvalued to services that have become undervalued.”
This budget-neutral approach also “will go further in reducing the large gap in compensation between primary care physicians (who had a median income of $243,000 in 2018) and specialists such as surgeons (whose median income was $426,000 in 2018),” Dr. Chernew wrote.
In a Tuesday tweet, Robert B. Doherty, senior vice president of governmental affairs and public policy for the American College of Physicians, said CMS had “finalized long overdue payment increases for primary and comprehensive care including an add-in for more complex visits.”
The American Academy of Family Physicians joined ACP in a November 30 letter to congressional leaders, urging them to allow Medicare “to increase investment in primary care, benefiting millions of Medicare patients and the program itself, and reject last minute efforts to prevent these essential and long-overdue changes from going fully into effect on January 1, 2021.”
In the letter, AAFP and ACP and their cosigners argued for a need to address “underinvestment” in primary care by finalizing the E/M overhaul.
“Given that six in ten American adults have a chronic disease and four in ten have two or more chronic conditions, why would we, as a country, accept such an inadequate investment in the very care model that stands to provide maximum value to these patients?” they wrote. “Since we know that individuals with a longitudinal relationship with a primary care physician have better health outcomes and use fewer health care resources, why would we continue to direct money to higher-cost, marginal value services?”
A version of this article originally appeared on Medscape.com.
Medicare officials stuck with their plan to increase payments for office visits for primary care and several other specialties that focus on helping patients manage complex conditions such as diabetes. In doing so, Medicare also finalized cuts for other fields, triggering a new wave of protests.
The final version of the 2021 Medicare physician fee schedule was unveiled on the night of Dec. 1. The Centers for Medicare & Medicaid Services posted an unofficial copy of the rule, which will later be published in the Federal Register.
CMS said it completed work on this massive annual review of payments for clinicians later than it usually does because of the demands of the federal response to the COVID-19 pandemic. The 2021 physician fee rule will take effect within a 30-day period instead of the usual 60-day time frame.
The most contentious item proposed for 2021 was a reshuffling of payments among specialties as part of an overhaul of Medicare’s approach to valuing evaluation and management (E/M) services. There was broader support for other aspects of the E/M overhaul, which are intended to cut some of the administrative hassle clinicians face.
“This finalized policy marks the most significant updates to E/M codes in 30 years, reducing burden on doctors imposed by the coding system and rewarding time spent evaluating and managing their patients’ care,” CMS Administrator Seema Verma said in a statement. “In the past, the system has rewarded interventions and procedures over time spent with patients – time taken preventing disease and managing chronic illnesses.”
In the final rule, CMS summarized these results of the E/M changes in Table 106. CMS largely stuck with the approach outlined in a draft rule released in August, with minor changes in the amounts of cuts and increases.
Specialties in line for increases under the 2021 final physician fee schedule include allergy/immunology (9%), endocrinology (16%), family practice (13%), general practice (7%), geriatrics (3%), hematology/oncology (14%), internal medicine (4%), nephrology (6%), physician assistants (8%), psychiatry (7%), rheumatology (15%), and urology (8%).
In line for cuts would be anesthesiology (–8%), cardiac surgery (–8%), emergency medicine (–6%), general surgery (–6%), infectious disease (–4%), neurosurgery (–6%), physical/occupational therapy (–9%), plastic surgery (–7%), radiology (–10%), and thoracic surgery (–8%).
CMS had initially set these changes in 2021 pay in motion in the 2020 physician fee schedule. The agency subsequently faced significant opposition to its plans. Many physician groups sought to waive a “budget-neutral” approach to the E/M overhaul, which makes the offsetting of cuts necessary. They argued this would allow increased compensation for clinicians whose practices focus on office visits without requiring offsetting cuts from other fields of medicine.
The American Medical Association is among those urging Congress to prevent or postpone the payment reductions resulting from Medicare’s budget neutrality requirement as applied to the E/M overhaul.
In a Tuesday statement, AMA President Susan R. Bailey, MD, noted that many physicians are facing “substantial economic hardships due to COVID-19.”
By AMA’s calculations, CMS’ planned 2021 E/M overhaul could result in “a shocking reduction of 10.2% to Medicare payment rates,” according to Bailey’s statement. The AMA strongly supports other aspects of the E/M changes CMS finalized, which Bailey said will result in “simpler and more flexible” coding and documentation.
The Surgical Care Coalition, which represents about a dozen medical specialty associations, is asking members of Congress to block the full implementation of the E/M overhaul.
In a Dec. 1 statement, the coalition urged the passage of a bill (HR 8702) that has been introduced in the House by a bipartisan duo of physicians, Rep. Ami Bera, MD (D-Calif.), and Rep. Larry Bucshon, MD (R-Ind.). Their bill would effectively block the cuts from going into effect on January 1, 2021. It would provide an additional Medicare payment for certain services in 2021 and 2022 if the otherwise applicable payment is less than it would have been in 2020.
The Medicare E/M overhaul “was a dangerous policy even before the pandemic, and enacting it during the worst health care crisis in a century is unconscionable. If Congress fails to act, it will further strain a health care system that’s already been pushed to the brink due to the COVID-19 pandemic and undermine patient care,” said John A. Wilson, MD, president of the American Association of Neurological Surgeons, in a statement.
Also backing the Bera-Bucshon bill is the American College of Emergency Physicians. In a statement on Tuesday, ACEP President Mark Rosenberg, DO, MBA, urged Congress to act on this measure.
“Emergency physicians and other health care providers battling on the front lines of the ongoing pandemic are already under unprecedented financial strain as they continue to bear the brunt of COVID-19,” Dr. Rosenberg said. “These cuts would have a devastating impact for the future of emergency medicine and could seriously impede patients’ access to emergency care when they need it most.”
“Long overdue”
But there also are champions for the approach CMS took in the E/M overhaul. The influential Medicare Payment Advisory Commission (MedPAC) has argued strongly for keeping the budget-neutral approach to the E/M overhaul.
In an Oct. 2 comment to CMS about the draft 2021 physician fee schedule, MedPAC Chairman Michael E. Chernew, PhD, said this approach would “help rebalance the fee schedule from services that have become overvalued to services that have become undervalued.”
This budget-neutral approach also “will go further in reducing the large gap in compensation between primary care physicians (who had a median income of $243,000 in 2018) and specialists such as surgeons (whose median income was $426,000 in 2018),” Dr. Chernew wrote.
In a Tuesday tweet, Robert B. Doherty, senior vice president of governmental affairs and public policy for the American College of Physicians, said CMS had “finalized long overdue payment increases for primary and comprehensive care including an add-in for more complex visits.”
The American Academy of Family Physicians joined ACP in a November 30 letter to congressional leaders, urging them to allow Medicare “to increase investment in primary care, benefiting millions of Medicare patients and the program itself, and reject last minute efforts to prevent these essential and long-overdue changes from going fully into effect on January 1, 2021.”
In the letter, AAFP and ACP and their cosigners argued for a need to address “underinvestment” in primary care by finalizing the E/M overhaul.
“Given that six in ten American adults have a chronic disease and four in ten have two or more chronic conditions, why would we, as a country, accept such an inadequate investment in the very care model that stands to provide maximum value to these patients?” they wrote. “Since we know that individuals with a longitudinal relationship with a primary care physician have better health outcomes and use fewer health care resources, why would we continue to direct money to higher-cost, marginal value services?”
A version of this article originally appeared on Medscape.com.
Medicare officials stuck with their plan to increase payments for office visits for primary care and several other specialties that focus on helping patients manage complex conditions such as diabetes. In doing so, Medicare also finalized cuts for other fields, triggering a new wave of protests.
The final version of the 2021 Medicare physician fee schedule was unveiled on the night of Dec. 1. The Centers for Medicare & Medicaid Services posted an unofficial copy of the rule, which will later be published in the Federal Register.
CMS said it completed work on this massive annual review of payments for clinicians later than it usually does because of the demands of the federal response to the COVID-19 pandemic. The 2021 physician fee rule will take effect within a 30-day period instead of the usual 60-day time frame.
The most contentious item proposed for 2021 was a reshuffling of payments among specialties as part of an overhaul of Medicare’s approach to valuing evaluation and management (E/M) services. There was broader support for other aspects of the E/M overhaul, which are intended to cut some of the administrative hassle clinicians face.
“This finalized policy marks the most significant updates to E/M codes in 30 years, reducing burden on doctors imposed by the coding system and rewarding time spent evaluating and managing their patients’ care,” CMS Administrator Seema Verma said in a statement. “In the past, the system has rewarded interventions and procedures over time spent with patients – time taken preventing disease and managing chronic illnesses.”
In the final rule, CMS summarized these results of the E/M changes in Table 106. CMS largely stuck with the approach outlined in a draft rule released in August, with minor changes in the amounts of cuts and increases.
Specialties in line for increases under the 2021 final physician fee schedule include allergy/immunology (9%), endocrinology (16%), family practice (13%), general practice (7%), geriatrics (3%), hematology/oncology (14%), internal medicine (4%), nephrology (6%), physician assistants (8%), psychiatry (7%), rheumatology (15%), and urology (8%).
In line for cuts would be anesthesiology (–8%), cardiac surgery (–8%), emergency medicine (–6%), general surgery (–6%), infectious disease (–4%), neurosurgery (–6%), physical/occupational therapy (–9%), plastic surgery (–7%), radiology (–10%), and thoracic surgery (–8%).
CMS had initially set these changes in 2021 pay in motion in the 2020 physician fee schedule. The agency subsequently faced significant opposition to its plans. Many physician groups sought to waive a “budget-neutral” approach to the E/M overhaul, which makes the offsetting of cuts necessary. They argued this would allow increased compensation for clinicians whose practices focus on office visits without requiring offsetting cuts from other fields of medicine.
The American Medical Association is among those urging Congress to prevent or postpone the payment reductions resulting from Medicare’s budget neutrality requirement as applied to the E/M overhaul.
In a Tuesday statement, AMA President Susan R. Bailey, MD, noted that many physicians are facing “substantial economic hardships due to COVID-19.”
By AMA’s calculations, CMS’ planned 2021 E/M overhaul could result in “a shocking reduction of 10.2% to Medicare payment rates,” according to Bailey’s statement. The AMA strongly supports other aspects of the E/M changes CMS finalized, which Bailey said will result in “simpler and more flexible” coding and documentation.
The Surgical Care Coalition, which represents about a dozen medical specialty associations, is asking members of Congress to block the full implementation of the E/M overhaul.
In a Dec. 1 statement, the coalition urged the passage of a bill (HR 8702) that has been introduced in the House by a bipartisan duo of physicians, Rep. Ami Bera, MD (D-Calif.), and Rep. Larry Bucshon, MD (R-Ind.). Their bill would effectively block the cuts from going into effect on January 1, 2021. It would provide an additional Medicare payment for certain services in 2021 and 2022 if the otherwise applicable payment is less than it would have been in 2020.
The Medicare E/M overhaul “was a dangerous policy even before the pandemic, and enacting it during the worst health care crisis in a century is unconscionable. If Congress fails to act, it will further strain a health care system that’s already been pushed to the brink due to the COVID-19 pandemic and undermine patient care,” said John A. Wilson, MD, president of the American Association of Neurological Surgeons, in a statement.
Also backing the Bera-Bucshon bill is the American College of Emergency Physicians. In a statement on Tuesday, ACEP President Mark Rosenberg, DO, MBA, urged Congress to act on this measure.
“Emergency physicians and other health care providers battling on the front lines of the ongoing pandemic are already under unprecedented financial strain as they continue to bear the brunt of COVID-19,” Dr. Rosenberg said. “These cuts would have a devastating impact for the future of emergency medicine and could seriously impede patients’ access to emergency care when they need it most.”
“Long overdue”
But there also are champions for the approach CMS took in the E/M overhaul. The influential Medicare Payment Advisory Commission (MedPAC) has argued strongly for keeping the budget-neutral approach to the E/M overhaul.
In an Oct. 2 comment to CMS about the draft 2021 physician fee schedule, MedPAC Chairman Michael E. Chernew, PhD, said this approach would “help rebalance the fee schedule from services that have become overvalued to services that have become undervalued.”
This budget-neutral approach also “will go further in reducing the large gap in compensation between primary care physicians (who had a median income of $243,000 in 2018) and specialists such as surgeons (whose median income was $426,000 in 2018),” Dr. Chernew wrote.
In a Tuesday tweet, Robert B. Doherty, senior vice president of governmental affairs and public policy for the American College of Physicians, said CMS had “finalized long overdue payment increases for primary and comprehensive care including an add-in for more complex visits.”
The American Academy of Family Physicians joined ACP in a November 30 letter to congressional leaders, urging them to allow Medicare “to increase investment in primary care, benefiting millions of Medicare patients and the program itself, and reject last minute efforts to prevent these essential and long-overdue changes from going fully into effect on January 1, 2021.”
In the letter, AAFP and ACP and their cosigners argued for a need to address “underinvestment” in primary care by finalizing the E/M overhaul.
“Given that six in ten American adults have a chronic disease and four in ten have two or more chronic conditions, why would we, as a country, accept such an inadequate investment in the very care model that stands to provide maximum value to these patients?” they wrote. “Since we know that individuals with a longitudinal relationship with a primary care physician have better health outcomes and use fewer health care resources, why would we continue to direct money to higher-cost, marginal value services?”
A version of this article originally appeared on Medscape.com.
CDC shortens COVID-19 quarantine time to 10 or 7 days, with conditions
Citing new evidence and an “acceptable risk” of transmission, the agency hopes reducing the 14-day quarantine will increase overall compliance and improve public health and economic constraints.
The agency also suggested people postpone travel during the upcoming winter holidays and stay home because of the pandemic.
These shorter quarantine options do not replace initial CDC guidance. “CDC continues to recommend quarantining for 14 days as the best way to reduce risk for spreading COVID-19,” said Henry Walke, MD, MPH, the CDC’s COVID-19 incident manager, during a media briefing on Wednesday.
However, “after reviewing and analyzing new research and data, CDC has identified two acceptable alternative quarantine periods.”
People can now quarantine for 10 days without a COVID-19 test if they have no symptoms. Alternatively, a quarantine can end after 7 days for someone with a negative test and no symptoms. The agency recommends a polymerase chain reaction test or an antigen assay within 48 hours before the end of a quarantine.
The agency also suggests people still monitor for symptoms for a full 14 days.
Reducing the length of quarantine “may make it easier for people to take this critical public health action, by reducing the economic hardship associated with a longer period, especially if they cannot work during that time,” Dr. Walke said. “In addition, a shorter quarantine period can lessen stress on the public health system and communities, especially when new infections are rapidly rising.”
The federal guidance leaves flexibility for local jurisdictions to make their own quarantine recommendations, as warranted, he added.
An ‘acceptable risk’ calculation
Modeling by the CDC and academic and public health partners led to the new quarantine recommendations, said John Brooks, MD, chief medical officer for the CDC’s COVID-19 response. Multiple studies “point in the same direction, which is that we can safely reduce the length of quarantine but accept there is a small residual risk that a person who is leaving quarantine early could transmit to someone else.”
The residual risk is approximately 1%, with an upper limit of 10%, when people quarantine for 10 days. A 7-day quarantine carries a residual risk of about 5% and an upper limit of 12%.
“Ten days is where the risk got into a sweet spot we like, at about 1%,” Dr. Brooks said. “That is a very acceptable risk, I think, for many people.”
Although it remains unknown what proportion of people spending 14 days in quarantine leave early, “we are hearing anecdotally from our partners in public health that many people are discontinuing quarantine ahead of time because there is pressure to go back to work, to get people back into school – and it imposes a burden on the individual,” Dr. Brooks said.
“One of our hopes is that ... if we reduce the amount of time they have to spend in quarantine, people will be more compliant,” he added.
A reporter asked why the CDC is shortening quarantines when the pandemic numbers are increasing nationwide. The timing has to do with capacity, Dr. Brooks said. “We are in situation where the number of cases is rising, the number of contacts is rising and the number of people who require quarantine is rising. That is a lot of burden, not just on the people who have to quarantine, but on public health.”
Home for the holidays
Similar to its pre-Thanksgiving advisory, the CDC also recommends people avoid travel during the upcoming winter holidays. “The best way to protect yourself and others is to postpone travel and stay home,” Dr. Walke said.
If people do decide to travel, the agency recommends COVID-19 testing 1-3 days prior to travel and again 3-5 days afterward, as well as reducing nonessential activities for a full 7 days after returning home. Furthermore, if someone does not have follow-up testing, the CDC recommends reducing nonessential activities for 10 days.
Testing does not eliminate all risk, Dr. Walke said, “but when combined with reducing nonessential activities, symptom screening and continuing with precautions like wearing masks, social distancing and hand washing, it can make travel safer.”
“We are trying to reduce the number of infections by postponing travel over the winter holiday,” Cindy Friedman, MD, chief of the CDC Travelers’ Health Branch, said during the media briefing.
“Travel volume was high during Thanksgiving,” she said, “and even if only a small percentage of those travelers were asymptomatically infected, this can translate into hundreds of thousands of additional infections moving from one community to another.”
This article first appeared on Medscape.com.
Citing new evidence and an “acceptable risk” of transmission, the agency hopes reducing the 14-day quarantine will increase overall compliance and improve public health and economic constraints.
The agency also suggested people postpone travel during the upcoming winter holidays and stay home because of the pandemic.
These shorter quarantine options do not replace initial CDC guidance. “CDC continues to recommend quarantining for 14 days as the best way to reduce risk for spreading COVID-19,” said Henry Walke, MD, MPH, the CDC’s COVID-19 incident manager, during a media briefing on Wednesday.
However, “after reviewing and analyzing new research and data, CDC has identified two acceptable alternative quarantine periods.”
People can now quarantine for 10 days without a COVID-19 test if they have no symptoms. Alternatively, a quarantine can end after 7 days for someone with a negative test and no symptoms. The agency recommends a polymerase chain reaction test or an antigen assay within 48 hours before the end of a quarantine.
The agency also suggests people still monitor for symptoms for a full 14 days.
Reducing the length of quarantine “may make it easier for people to take this critical public health action, by reducing the economic hardship associated with a longer period, especially if they cannot work during that time,” Dr. Walke said. “In addition, a shorter quarantine period can lessen stress on the public health system and communities, especially when new infections are rapidly rising.”
The federal guidance leaves flexibility for local jurisdictions to make their own quarantine recommendations, as warranted, he added.
An ‘acceptable risk’ calculation
Modeling by the CDC and academic and public health partners led to the new quarantine recommendations, said John Brooks, MD, chief medical officer for the CDC’s COVID-19 response. Multiple studies “point in the same direction, which is that we can safely reduce the length of quarantine but accept there is a small residual risk that a person who is leaving quarantine early could transmit to someone else.”
The residual risk is approximately 1%, with an upper limit of 10%, when people quarantine for 10 days. A 7-day quarantine carries a residual risk of about 5% and an upper limit of 12%.
“Ten days is where the risk got into a sweet spot we like, at about 1%,” Dr. Brooks said. “That is a very acceptable risk, I think, for many people.”
Although it remains unknown what proportion of people spending 14 days in quarantine leave early, “we are hearing anecdotally from our partners in public health that many people are discontinuing quarantine ahead of time because there is pressure to go back to work, to get people back into school – and it imposes a burden on the individual,” Dr. Brooks said.
“One of our hopes is that ... if we reduce the amount of time they have to spend in quarantine, people will be more compliant,” he added.
A reporter asked why the CDC is shortening quarantines when the pandemic numbers are increasing nationwide. The timing has to do with capacity, Dr. Brooks said. “We are in situation where the number of cases is rising, the number of contacts is rising and the number of people who require quarantine is rising. That is a lot of burden, not just on the people who have to quarantine, but on public health.”
Home for the holidays
Similar to its pre-Thanksgiving advisory, the CDC also recommends people avoid travel during the upcoming winter holidays. “The best way to protect yourself and others is to postpone travel and stay home,” Dr. Walke said.
If people do decide to travel, the agency recommends COVID-19 testing 1-3 days prior to travel and again 3-5 days afterward, as well as reducing nonessential activities for a full 7 days after returning home. Furthermore, if someone does not have follow-up testing, the CDC recommends reducing nonessential activities for 10 days.
Testing does not eliminate all risk, Dr. Walke said, “but when combined with reducing nonessential activities, symptom screening and continuing with precautions like wearing masks, social distancing and hand washing, it can make travel safer.”
“We are trying to reduce the number of infections by postponing travel over the winter holiday,” Cindy Friedman, MD, chief of the CDC Travelers’ Health Branch, said during the media briefing.
“Travel volume was high during Thanksgiving,” she said, “and even if only a small percentage of those travelers were asymptomatically infected, this can translate into hundreds of thousands of additional infections moving from one community to another.”
This article first appeared on Medscape.com.
Citing new evidence and an “acceptable risk” of transmission, the agency hopes reducing the 14-day quarantine will increase overall compliance and improve public health and economic constraints.
The agency also suggested people postpone travel during the upcoming winter holidays and stay home because of the pandemic.
These shorter quarantine options do not replace initial CDC guidance. “CDC continues to recommend quarantining for 14 days as the best way to reduce risk for spreading COVID-19,” said Henry Walke, MD, MPH, the CDC’s COVID-19 incident manager, during a media briefing on Wednesday.
However, “after reviewing and analyzing new research and data, CDC has identified two acceptable alternative quarantine periods.”
People can now quarantine for 10 days without a COVID-19 test if they have no symptoms. Alternatively, a quarantine can end after 7 days for someone with a negative test and no symptoms. The agency recommends a polymerase chain reaction test or an antigen assay within 48 hours before the end of a quarantine.
The agency also suggests people still monitor for symptoms for a full 14 days.
Reducing the length of quarantine “may make it easier for people to take this critical public health action, by reducing the economic hardship associated with a longer period, especially if they cannot work during that time,” Dr. Walke said. “In addition, a shorter quarantine period can lessen stress on the public health system and communities, especially when new infections are rapidly rising.”
The federal guidance leaves flexibility for local jurisdictions to make their own quarantine recommendations, as warranted, he added.
An ‘acceptable risk’ calculation
Modeling by the CDC and academic and public health partners led to the new quarantine recommendations, said John Brooks, MD, chief medical officer for the CDC’s COVID-19 response. Multiple studies “point in the same direction, which is that we can safely reduce the length of quarantine but accept there is a small residual risk that a person who is leaving quarantine early could transmit to someone else.”
The residual risk is approximately 1%, with an upper limit of 10%, when people quarantine for 10 days. A 7-day quarantine carries a residual risk of about 5% and an upper limit of 12%.
“Ten days is where the risk got into a sweet spot we like, at about 1%,” Dr. Brooks said. “That is a very acceptable risk, I think, for many people.”
Although it remains unknown what proportion of people spending 14 days in quarantine leave early, “we are hearing anecdotally from our partners in public health that many people are discontinuing quarantine ahead of time because there is pressure to go back to work, to get people back into school – and it imposes a burden on the individual,” Dr. Brooks said.
“One of our hopes is that ... if we reduce the amount of time they have to spend in quarantine, people will be more compliant,” he added.
A reporter asked why the CDC is shortening quarantines when the pandemic numbers are increasing nationwide. The timing has to do with capacity, Dr. Brooks said. “We are in situation where the number of cases is rising, the number of contacts is rising and the number of people who require quarantine is rising. That is a lot of burden, not just on the people who have to quarantine, but on public health.”
Home for the holidays
Similar to its pre-Thanksgiving advisory, the CDC also recommends people avoid travel during the upcoming winter holidays. “The best way to protect yourself and others is to postpone travel and stay home,” Dr. Walke said.
If people do decide to travel, the agency recommends COVID-19 testing 1-3 days prior to travel and again 3-5 days afterward, as well as reducing nonessential activities for a full 7 days after returning home. Furthermore, if someone does not have follow-up testing, the CDC recommends reducing nonessential activities for 10 days.
Testing does not eliminate all risk, Dr. Walke said, “but when combined with reducing nonessential activities, symptom screening and continuing with precautions like wearing masks, social distancing and hand washing, it can make travel safer.”
“We are trying to reduce the number of infections by postponing travel over the winter holiday,” Cindy Friedman, MD, chief of the CDC Travelers’ Health Branch, said during the media briefing.
“Travel volume was high during Thanksgiving,” she said, “and even if only a small percentage of those travelers were asymptomatically infected, this can translate into hundreds of thousands of additional infections moving from one community to another.”
This article first appeared on Medscape.com.
Acute-on-chronic itch is new frontier in atopic dermatitis
Brian S. Kim, MD, said at MedscapeLive’s annual Las Vegas Dermatology Seminar, held virtually this year.
Recent years have brought enormous progress in understanding how chronic itch in patients with atopic dermatitis (AD) is mediated by type 2 cytokines, including interleukin-13, IL-4, and IL-31, as well as by Janus kinase (JAK) signaling. This has led to development of potent therapies targeting these mediators, including dupilumab (Dupixent) and the investigational agents tralokinumab, lebrikizumab, abrocitinib, upadacitinib, baricitinib, and the IL-31 inhibitor nemolizumab.
“This is now one of the most active areas in the field of dermatology,” observed Dr. Kim, a dermatologist and codirector of the Center for the Study of Itch and Sensory Disorders at Washington University in St. Louis.
He has figured prominently in this effort. He and his coinvestigators conducted translational studies in mouse models which unraveled key mechanisms by which the immune system responsible for skin inflammation in AD communicates with the nervous system to trigger the neural sensation of itch. He also led a phase 2 randomized trial in 307 patients with AD, which demonstrated that the investigational JAK1/JAK2 inhibitor ruxolitinib cream markedly improved itch within 36 hours, well before subsequent improvement in skin inflammation – and the topical JAK inhibitor did so with minimal systemic absorption.
Compared with chronic itch, much less research attention has been devoted to the phenomenon of acute itch flares superimposed upon the chronic itch of AD. These acute-on-chronic itch flares are a common feature of the disease. In a soon-to-be-published study of 159 AD patients in the placebo arm of a clinical trial, Dr. Kim and coinvestigators found that 26% exhibited a pattern of acute itch flares during the course of a single month. During the next month, 3.1% of patients under study went from an acute-on-chronic itch pattern in month 1 to a nonflare pattern, 20% went from a nonflare pattern in month 1 to acute itch flares in month 2, and 23% of the overall study population retained their pattern of acute itch flares through both months.
“This does not seem to be just a static phenotype, but rather these patients can evolve over time. And we think that this can be driven by allergen-specific IgE,” according to Dr. Kim.
Indeed, the investigators found that patients with allergen-specific IgE in their serum were roughly twice as likely to exhibit the acute-on-chronic itch flare pattern than those without allergen-specific IgE.
The classical thinking has been that IgE binds to its receptors on mast cells, causing mast cell degranulation and release of histamine and other itch-inducing molecules. Yet antihistamines have proven notoriously ineffective for the treatment of AD.
Circulating basophils capable of working their way into inflamed skin also have IgE receptors. Dr. Kim and colleagues have shown that allergen-specific IgE in mice binds to those receptors, causing the basophils to degenerate, releasing itch-promoting chemicals. They have subsequently carried over this work into the clinical arena.
“We’ve found that patients with atopic dermatitis have significantly higher expression of receptors for IgE in their basophils than in the basophils of healthy controls, indicating perhaps that the basophils in patients with atopic dermatitis are much more prone to stimulation by allergen by way of IgE. This is a new concept that we’re exploring,” Dr. Kim said.
“We haven’t really known before what IgE does in atopic dermatitis, but it turns out that it may actually play a very important role in triggering acute flares of itch,” the dermatologist explained. “What’s been surprising is that the IgE activity is not mediated so much by mast cells, which are tissue-resident; the predominant means appears to be that IgE acts on basophils. That then creates release not of histamine, but of leukotriene C4, which is a very potent pruritogen. This may be responsible for those acute itch flares.”
Asked during an audience Q&A how allergen-specific IgE–mediated basophil activation might be targeted therapeutically in order to prevent acute-on-chronic itch flares in patients with AD, Dr. Kim mentioned two possibilities. One is treatment with potent anti-IgE agents, which to date have not been adequately tested for their antipruritic prowess in AD.
“Also, there’s another molecule that seems to be relatively basophil-selective and -specific that’s just been discovered by my colleague Xinzhong Dong at Johns Hopkins University [in Baltimore] – called MRGPRX2 – that may actually be a potentially viable way to go after basophils, maybe even by depleting them if you had an antibody against that,” Dr. Kim said. He was a coinvestigator in Dr. Dong’s recent study characterizing MRGPRX2, the mast-cell-expressed Mas-related G-protein–coupled receptor activator.
Dr. Kim reported receiving research funding from Cara Therapeutics and LEO Pharma, holding a patent for the use of JAK inhibitors in chronic itch, and serving as a consultant to numerous pharmaceutical companies.
MedscapeLive and this news organization are owned by the same parent company.
Brian S. Kim, MD, said at MedscapeLive’s annual Las Vegas Dermatology Seminar, held virtually this year.
Recent years have brought enormous progress in understanding how chronic itch in patients with atopic dermatitis (AD) is mediated by type 2 cytokines, including interleukin-13, IL-4, and IL-31, as well as by Janus kinase (JAK) signaling. This has led to development of potent therapies targeting these mediators, including dupilumab (Dupixent) and the investigational agents tralokinumab, lebrikizumab, abrocitinib, upadacitinib, baricitinib, and the IL-31 inhibitor nemolizumab.
“This is now one of the most active areas in the field of dermatology,” observed Dr. Kim, a dermatologist and codirector of the Center for the Study of Itch and Sensory Disorders at Washington University in St. Louis.
He has figured prominently in this effort. He and his coinvestigators conducted translational studies in mouse models which unraveled key mechanisms by which the immune system responsible for skin inflammation in AD communicates with the nervous system to trigger the neural sensation of itch. He also led a phase 2 randomized trial in 307 patients with AD, which demonstrated that the investigational JAK1/JAK2 inhibitor ruxolitinib cream markedly improved itch within 36 hours, well before subsequent improvement in skin inflammation – and the topical JAK inhibitor did so with minimal systemic absorption.
Compared with chronic itch, much less research attention has been devoted to the phenomenon of acute itch flares superimposed upon the chronic itch of AD. These acute-on-chronic itch flares are a common feature of the disease. In a soon-to-be-published study of 159 AD patients in the placebo arm of a clinical trial, Dr. Kim and coinvestigators found that 26% exhibited a pattern of acute itch flares during the course of a single month. During the next month, 3.1% of patients under study went from an acute-on-chronic itch pattern in month 1 to a nonflare pattern, 20% went from a nonflare pattern in month 1 to acute itch flares in month 2, and 23% of the overall study population retained their pattern of acute itch flares through both months.
“This does not seem to be just a static phenotype, but rather these patients can evolve over time. And we think that this can be driven by allergen-specific IgE,” according to Dr. Kim.
Indeed, the investigators found that patients with allergen-specific IgE in their serum were roughly twice as likely to exhibit the acute-on-chronic itch flare pattern than those without allergen-specific IgE.
The classical thinking has been that IgE binds to its receptors on mast cells, causing mast cell degranulation and release of histamine and other itch-inducing molecules. Yet antihistamines have proven notoriously ineffective for the treatment of AD.
Circulating basophils capable of working their way into inflamed skin also have IgE receptors. Dr. Kim and colleagues have shown that allergen-specific IgE in mice binds to those receptors, causing the basophils to degenerate, releasing itch-promoting chemicals. They have subsequently carried over this work into the clinical arena.
“We’ve found that patients with atopic dermatitis have significantly higher expression of receptors for IgE in their basophils than in the basophils of healthy controls, indicating perhaps that the basophils in patients with atopic dermatitis are much more prone to stimulation by allergen by way of IgE. This is a new concept that we’re exploring,” Dr. Kim said.
“We haven’t really known before what IgE does in atopic dermatitis, but it turns out that it may actually play a very important role in triggering acute flares of itch,” the dermatologist explained. “What’s been surprising is that the IgE activity is not mediated so much by mast cells, which are tissue-resident; the predominant means appears to be that IgE acts on basophils. That then creates release not of histamine, but of leukotriene C4, which is a very potent pruritogen. This may be responsible for those acute itch flares.”
Asked during an audience Q&A how allergen-specific IgE–mediated basophil activation might be targeted therapeutically in order to prevent acute-on-chronic itch flares in patients with AD, Dr. Kim mentioned two possibilities. One is treatment with potent anti-IgE agents, which to date have not been adequately tested for their antipruritic prowess in AD.
“Also, there’s another molecule that seems to be relatively basophil-selective and -specific that’s just been discovered by my colleague Xinzhong Dong at Johns Hopkins University [in Baltimore] – called MRGPRX2 – that may actually be a potentially viable way to go after basophils, maybe even by depleting them if you had an antibody against that,” Dr. Kim said. He was a coinvestigator in Dr. Dong’s recent study characterizing MRGPRX2, the mast-cell-expressed Mas-related G-protein–coupled receptor activator.
Dr. Kim reported receiving research funding from Cara Therapeutics and LEO Pharma, holding a patent for the use of JAK inhibitors in chronic itch, and serving as a consultant to numerous pharmaceutical companies.
MedscapeLive and this news organization are owned by the same parent company.
Brian S. Kim, MD, said at MedscapeLive’s annual Las Vegas Dermatology Seminar, held virtually this year.
Recent years have brought enormous progress in understanding how chronic itch in patients with atopic dermatitis (AD) is mediated by type 2 cytokines, including interleukin-13, IL-4, and IL-31, as well as by Janus kinase (JAK) signaling. This has led to development of potent therapies targeting these mediators, including dupilumab (Dupixent) and the investigational agents tralokinumab, lebrikizumab, abrocitinib, upadacitinib, baricitinib, and the IL-31 inhibitor nemolizumab.
“This is now one of the most active areas in the field of dermatology,” observed Dr. Kim, a dermatologist and codirector of the Center for the Study of Itch and Sensory Disorders at Washington University in St. Louis.
He has figured prominently in this effort. He and his coinvestigators conducted translational studies in mouse models which unraveled key mechanisms by which the immune system responsible for skin inflammation in AD communicates with the nervous system to trigger the neural sensation of itch. He also led a phase 2 randomized trial in 307 patients with AD, which demonstrated that the investigational JAK1/JAK2 inhibitor ruxolitinib cream markedly improved itch within 36 hours, well before subsequent improvement in skin inflammation – and the topical JAK inhibitor did so with minimal systemic absorption.
Compared with chronic itch, much less research attention has been devoted to the phenomenon of acute itch flares superimposed upon the chronic itch of AD. These acute-on-chronic itch flares are a common feature of the disease. In a soon-to-be-published study of 159 AD patients in the placebo arm of a clinical trial, Dr. Kim and coinvestigators found that 26% exhibited a pattern of acute itch flares during the course of a single month. During the next month, 3.1% of patients under study went from an acute-on-chronic itch pattern in month 1 to a nonflare pattern, 20% went from a nonflare pattern in month 1 to acute itch flares in month 2, and 23% of the overall study population retained their pattern of acute itch flares through both months.
“This does not seem to be just a static phenotype, but rather these patients can evolve over time. And we think that this can be driven by allergen-specific IgE,” according to Dr. Kim.
Indeed, the investigators found that patients with allergen-specific IgE in their serum were roughly twice as likely to exhibit the acute-on-chronic itch flare pattern than those without allergen-specific IgE.
The classical thinking has been that IgE binds to its receptors on mast cells, causing mast cell degranulation and release of histamine and other itch-inducing molecules. Yet antihistamines have proven notoriously ineffective for the treatment of AD.
Circulating basophils capable of working their way into inflamed skin also have IgE receptors. Dr. Kim and colleagues have shown that allergen-specific IgE in mice binds to those receptors, causing the basophils to degenerate, releasing itch-promoting chemicals. They have subsequently carried over this work into the clinical arena.
“We’ve found that patients with atopic dermatitis have significantly higher expression of receptors for IgE in their basophils than in the basophils of healthy controls, indicating perhaps that the basophils in patients with atopic dermatitis are much more prone to stimulation by allergen by way of IgE. This is a new concept that we’re exploring,” Dr. Kim said.
“We haven’t really known before what IgE does in atopic dermatitis, but it turns out that it may actually play a very important role in triggering acute flares of itch,” the dermatologist explained. “What’s been surprising is that the IgE activity is not mediated so much by mast cells, which are tissue-resident; the predominant means appears to be that IgE acts on basophils. That then creates release not of histamine, but of leukotriene C4, which is a very potent pruritogen. This may be responsible for those acute itch flares.”
Asked during an audience Q&A how allergen-specific IgE–mediated basophil activation might be targeted therapeutically in order to prevent acute-on-chronic itch flares in patients with AD, Dr. Kim mentioned two possibilities. One is treatment with potent anti-IgE agents, which to date have not been adequately tested for their antipruritic prowess in AD.
“Also, there’s another molecule that seems to be relatively basophil-selective and -specific that’s just been discovered by my colleague Xinzhong Dong at Johns Hopkins University [in Baltimore] – called MRGPRX2 – that may actually be a potentially viable way to go after basophils, maybe even by depleting them if you had an antibody against that,” Dr. Kim said. He was a coinvestigator in Dr. Dong’s recent study characterizing MRGPRX2, the mast-cell-expressed Mas-related G-protein–coupled receptor activator.
Dr. Kim reported receiving research funding from Cara Therapeutics and LEO Pharma, holding a patent for the use of JAK inhibitors in chronic itch, and serving as a consultant to numerous pharmaceutical companies.
MedscapeLive and this news organization are owned by the same parent company.
FROM MEDSCAPELIVE LAS VEGAS DERMATOLOGY SEMINAR
Daily sunscreen use will prevent more melanoma deaths than early detection
The dramatic advances in targeted therapies for late-stage melanoma capture the headlines, but a recent Australian study quietly concluded that according to Laura Korb Ferris, MD, PhD, a dermatologist and director of clinical trials in the department of dermatology at the University of Pittsburgh.
“I think it’s really important that we recognize the importance of preventing skin cancer, and not just early detection, not just treatment of late disease,” Dr. Ferris said at a virtual forum on cutaneous malignancies jointly presented by Postgraduate Institute for Medicine and Global Academy for Medical Education.
She highlighted the Australian cost-effectiveness analysis, which used Markov modeling of data from two published population-based, randomized controlled trials carried out in Queensland, Australia.
The cost-effectiveness study compared the estimated long-term impact of three different approaches to control of melanoma: a primary prevention strategy, which basically consisted of promoting daily sunscreen use and other forms of sun protection; early detection through annual whole-body skin examinations by physicians starting at age 50; and no intervention. The analysis provided estimates of the number of cases of melanoma, deaths caused by melanoma, nonmelanoma skin cancers, and quality of life outcomes over the course of 30 years starting in 50-year-old men and women.
Primary prevention through sun protection was the clear winner, as shown by the results:
- A 44% reduction in the incidence of melanoma, compared with early detection via annual physician skin examinations.
- A 39% reduction in projected melanoma deaths compared with early detection, which in turn achieved only a 2% reduction when compared with no intervention.
- 27% fewer keratinocyte cancers excised than with annual skin examinations.
- A 21.7% reduction in societal costs, compared with an early-detection program.
Daily sunscreen use for primary prevention was also associated with a modest 0.1% increase in quality-adjusted life-years. “Prevention is low cost, low risk, and effective,” Dr. Ferris observed.
The investigators noted that, while residents of the Australian state of Queensland are mainly fair-skinned and confront high UV radiation levels throughout the year, somewhat limiting the generalizability of the study findings, the relationships between the costs of interventional strategies and their outcomes should be proportional in other countries.
True enough, but a strategy of annual skin examinations starting at age 50 years as modeled in the Australian study is not the most productive way to conduct a melanoma early-detection program, Dr. Ferris said. She noted that data from the National Cancer Institute’s Surveillance, Epidemiology, and End Results Program show that the median age at diagnosis of melanoma in the United States is 65 years, while the median age at death caused by the malignancy is 71 years. That information is helpful in formulating strategies to improve early detection through more focused, higher-yield screening.
Case in point: European investigators have estimated that, by screening everyone age 50 years and older, 475 people need to be screened and an average of 19.6 lesions must be biopsied in order to detect one melanoma. But by reserving screening for those age 50 years and up who have any one of three risk factors – a personal history of melanoma, atypical nevi, or at least 40 common nevi – those numbers drop dramatically: 98 people need to be screened and 13.5 lesions biopsied to detect one melanoma. And by further narrowing the screened population to those age 65 years or older with any of the three risk factors, 63 seniors would need to be screened and 9.2 lesions excised to find one melanoma.
Total-body skin examinations are time-consuming for dermatologists. In a recent U.S. study, investigators determined that the additional face-to-face time required per skin cancer detected by doing a total-body skin exam in adults who present to a dermatologist for another reason is 4.5 hours. And that’s just the time involved in detecting any type of skin cancer.
“To get that number for melanoma, multiply by 15 to 20,” Dr. Ferris said.
The investigators also determined that, for each decade of advancing age and increment in lighter skin phototype, the number-needed-to-examine in order to identify one skin cancer of any type decreased.
“By focusing on patients who are older and have fair skin types we can get that time down to about 1 hour,” commented Dr. Ferris, who penned an editorial perspective on the study.
While many dermatologists recommend that people with a high common nevus count undergo frequent screening for melanoma because they are at particularly high risk for invasive disease, a couple of recent studies challenge that notion, she pointed out. One was a retrospective study of 326 consecutive new melanoma patients which found that patients with a higher nevus count had thinner melanomas and a greater likelihood of in situ melanoma. Patients who presented with invasive melanoma had a mean total nevus count of 31.5 lesions, while those with in situ melanoma averaged 57.2 nevi. Each additional nevus was associated with a 4% reduction in the likelihood of invasive melanoma, independent of age and sex.
The other study included 566 newly diagnosed melanoma patients in two U.S. centers. Among the 56% of patients who were younger than 60 years, those who had more than 50 total nevi were 68% less likely to have a thick melanoma in a logistic regression analysis that controlled for demographic factors, as well as anatomic location of the melanoma, histologic subtype, and skin cancer screening frequency. In contrast, younger patients with more than 5 atypical nevi were 2.43-fold more likely to have thicker melanomas than were those with no such lesions. The lesson, according to the investigators, is that total nevus count isn’t a reliable determinant of a patient’s risk status or the need for skin examinations.
Dr. Ferris reported no financial conflicts of interest regarding her presentation.
Global Academy for Medical Education and this news organization are owned by the same company.
The dramatic advances in targeted therapies for late-stage melanoma capture the headlines, but a recent Australian study quietly concluded that according to Laura Korb Ferris, MD, PhD, a dermatologist and director of clinical trials in the department of dermatology at the University of Pittsburgh.
“I think it’s really important that we recognize the importance of preventing skin cancer, and not just early detection, not just treatment of late disease,” Dr. Ferris said at a virtual forum on cutaneous malignancies jointly presented by Postgraduate Institute for Medicine and Global Academy for Medical Education.
She highlighted the Australian cost-effectiveness analysis, which used Markov modeling of data from two published population-based, randomized controlled trials carried out in Queensland, Australia.
The cost-effectiveness study compared the estimated long-term impact of three different approaches to control of melanoma: a primary prevention strategy, which basically consisted of promoting daily sunscreen use and other forms of sun protection; early detection through annual whole-body skin examinations by physicians starting at age 50; and no intervention. The analysis provided estimates of the number of cases of melanoma, deaths caused by melanoma, nonmelanoma skin cancers, and quality of life outcomes over the course of 30 years starting in 50-year-old men and women.
Primary prevention through sun protection was the clear winner, as shown by the results:
- A 44% reduction in the incidence of melanoma, compared with early detection via annual physician skin examinations.
- A 39% reduction in projected melanoma deaths compared with early detection, which in turn achieved only a 2% reduction when compared with no intervention.
- 27% fewer keratinocyte cancers excised than with annual skin examinations.
- A 21.7% reduction in societal costs, compared with an early-detection program.
Daily sunscreen use for primary prevention was also associated with a modest 0.1% increase in quality-adjusted life-years. “Prevention is low cost, low risk, and effective,” Dr. Ferris observed.
The investigators noted that, while residents of the Australian state of Queensland are mainly fair-skinned and confront high UV radiation levels throughout the year, somewhat limiting the generalizability of the study findings, the relationships between the costs of interventional strategies and their outcomes should be proportional in other countries.
True enough, but a strategy of annual skin examinations starting at age 50 years as modeled in the Australian study is not the most productive way to conduct a melanoma early-detection program, Dr. Ferris said. She noted that data from the National Cancer Institute’s Surveillance, Epidemiology, and End Results Program show that the median age at diagnosis of melanoma in the United States is 65 years, while the median age at death caused by the malignancy is 71 years. That information is helpful in formulating strategies to improve early detection through more focused, higher-yield screening.
Case in point: European investigators have estimated that, by screening everyone age 50 years and older, 475 people need to be screened and an average of 19.6 lesions must be biopsied in order to detect one melanoma. But by reserving screening for those age 50 years and up who have any one of three risk factors – a personal history of melanoma, atypical nevi, or at least 40 common nevi – those numbers drop dramatically: 98 people need to be screened and 13.5 lesions biopsied to detect one melanoma. And by further narrowing the screened population to those age 65 years or older with any of the three risk factors, 63 seniors would need to be screened and 9.2 lesions excised to find one melanoma.
Total-body skin examinations are time-consuming for dermatologists. In a recent U.S. study, investigators determined that the additional face-to-face time required per skin cancer detected by doing a total-body skin exam in adults who present to a dermatologist for another reason is 4.5 hours. And that’s just the time involved in detecting any type of skin cancer.
“To get that number for melanoma, multiply by 15 to 20,” Dr. Ferris said.
The investigators also determined that, for each decade of advancing age and increment in lighter skin phototype, the number-needed-to-examine in order to identify one skin cancer of any type decreased.
“By focusing on patients who are older and have fair skin types we can get that time down to about 1 hour,” commented Dr. Ferris, who penned an editorial perspective on the study.
While many dermatologists recommend that people with a high common nevus count undergo frequent screening for melanoma because they are at particularly high risk for invasive disease, a couple of recent studies challenge that notion, she pointed out. One was a retrospective study of 326 consecutive new melanoma patients which found that patients with a higher nevus count had thinner melanomas and a greater likelihood of in situ melanoma. Patients who presented with invasive melanoma had a mean total nevus count of 31.5 lesions, while those with in situ melanoma averaged 57.2 nevi. Each additional nevus was associated with a 4% reduction in the likelihood of invasive melanoma, independent of age and sex.
The other study included 566 newly diagnosed melanoma patients in two U.S. centers. Among the 56% of patients who were younger than 60 years, those who had more than 50 total nevi were 68% less likely to have a thick melanoma in a logistic regression analysis that controlled for demographic factors, as well as anatomic location of the melanoma, histologic subtype, and skin cancer screening frequency. In contrast, younger patients with more than 5 atypical nevi were 2.43-fold more likely to have thicker melanomas than were those with no such lesions. The lesson, according to the investigators, is that total nevus count isn’t a reliable determinant of a patient’s risk status or the need for skin examinations.
Dr. Ferris reported no financial conflicts of interest regarding her presentation.
Global Academy for Medical Education and this news organization are owned by the same company.
The dramatic advances in targeted therapies for late-stage melanoma capture the headlines, but a recent Australian study quietly concluded that according to Laura Korb Ferris, MD, PhD, a dermatologist and director of clinical trials in the department of dermatology at the University of Pittsburgh.
“I think it’s really important that we recognize the importance of preventing skin cancer, and not just early detection, not just treatment of late disease,” Dr. Ferris said at a virtual forum on cutaneous malignancies jointly presented by Postgraduate Institute for Medicine and Global Academy for Medical Education.
She highlighted the Australian cost-effectiveness analysis, which used Markov modeling of data from two published population-based, randomized controlled trials carried out in Queensland, Australia.
The cost-effectiveness study compared the estimated long-term impact of three different approaches to control of melanoma: a primary prevention strategy, which basically consisted of promoting daily sunscreen use and other forms of sun protection; early detection through annual whole-body skin examinations by physicians starting at age 50; and no intervention. The analysis provided estimates of the number of cases of melanoma, deaths caused by melanoma, nonmelanoma skin cancers, and quality of life outcomes over the course of 30 years starting in 50-year-old men and women.
Primary prevention through sun protection was the clear winner, as shown by the results:
- A 44% reduction in the incidence of melanoma, compared with early detection via annual physician skin examinations.
- A 39% reduction in projected melanoma deaths compared with early detection, which in turn achieved only a 2% reduction when compared with no intervention.
- 27% fewer keratinocyte cancers excised than with annual skin examinations.
- A 21.7% reduction in societal costs, compared with an early-detection program.
Daily sunscreen use for primary prevention was also associated with a modest 0.1% increase in quality-adjusted life-years. “Prevention is low cost, low risk, and effective,” Dr. Ferris observed.
The investigators noted that, while residents of the Australian state of Queensland are mainly fair-skinned and confront high UV radiation levels throughout the year, somewhat limiting the generalizability of the study findings, the relationships between the costs of interventional strategies and their outcomes should be proportional in other countries.
True enough, but a strategy of annual skin examinations starting at age 50 years as modeled in the Australian study is not the most productive way to conduct a melanoma early-detection program, Dr. Ferris said. She noted that data from the National Cancer Institute’s Surveillance, Epidemiology, and End Results Program show that the median age at diagnosis of melanoma in the United States is 65 years, while the median age at death caused by the malignancy is 71 years. That information is helpful in formulating strategies to improve early detection through more focused, higher-yield screening.
Case in point: European investigators have estimated that, by screening everyone age 50 years and older, 475 people need to be screened and an average of 19.6 lesions must be biopsied in order to detect one melanoma. But by reserving screening for those age 50 years and up who have any one of three risk factors – a personal history of melanoma, atypical nevi, or at least 40 common nevi – those numbers drop dramatically: 98 people need to be screened and 13.5 lesions biopsied to detect one melanoma. And by further narrowing the screened population to those age 65 years or older with any of the three risk factors, 63 seniors would need to be screened and 9.2 lesions excised to find one melanoma.
Total-body skin examinations are time-consuming for dermatologists. In a recent U.S. study, investigators determined that the additional face-to-face time required per skin cancer detected by doing a total-body skin exam in adults who present to a dermatologist for another reason is 4.5 hours. And that’s just the time involved in detecting any type of skin cancer.
“To get that number for melanoma, multiply by 15 to 20,” Dr. Ferris said.
The investigators also determined that, for each decade of advancing age and increment in lighter skin phototype, the number-needed-to-examine in order to identify one skin cancer of any type decreased.
“By focusing on patients who are older and have fair skin types we can get that time down to about 1 hour,” commented Dr. Ferris, who penned an editorial perspective on the study.
While many dermatologists recommend that people with a high common nevus count undergo frequent screening for melanoma because they are at particularly high risk for invasive disease, a couple of recent studies challenge that notion, she pointed out. One was a retrospective study of 326 consecutive new melanoma patients which found that patients with a higher nevus count had thinner melanomas and a greater likelihood of in situ melanoma. Patients who presented with invasive melanoma had a mean total nevus count of 31.5 lesions, while those with in situ melanoma averaged 57.2 nevi. Each additional nevus was associated with a 4% reduction in the likelihood of invasive melanoma, independent of age and sex.
The other study included 566 newly diagnosed melanoma patients in two U.S. centers. Among the 56% of patients who were younger than 60 years, those who had more than 50 total nevi were 68% less likely to have a thick melanoma in a logistic regression analysis that controlled for demographic factors, as well as anatomic location of the melanoma, histologic subtype, and skin cancer screening frequency. In contrast, younger patients with more than 5 atypical nevi were 2.43-fold more likely to have thicker melanomas than were those with no such lesions. The lesson, according to the investigators, is that total nevus count isn’t a reliable determinant of a patient’s risk status or the need for skin examinations.
Dr. Ferris reported no financial conflicts of interest regarding her presentation.
Global Academy for Medical Education and this news organization are owned by the same company.
FROM THE CUTANEOUS MALIGNANCIES FORUM
U.S. passes 1.3 million COVID-19 cases in children
The news on children and COVID-19 for Thanksgiving week does not provide a lot of room for thankfulness.
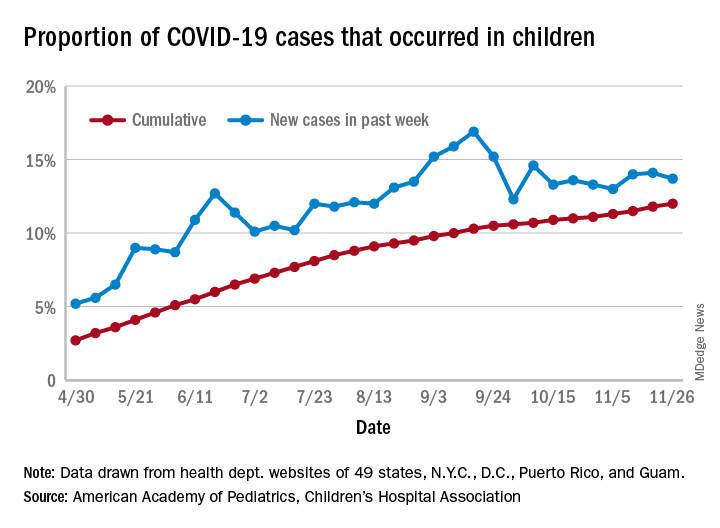
the American Academy of Pediatrics and the Children’s Hospital Association said in their latest weekly report.
For those not counting, the week ending Nov. 26 was the fifth in a row to show “the highest weekly increase since the pandemic began,” based on data the AAP and CHA have been collecting from 49 state health departments (New York does not report ages), as well as the District of Columbia, New York City, Puerto Rico, and Guam.
The 153,608 new cases bring the total number of COVID-19 cases in children to almost 1.34 million in those jurisdictions, which is 12% of the total number of cases (11.2 million) among all ages. For just the week ending Nov. 26, children represented 13.7% of all new cases in the United States, down from 14.1% the previous week, according to the AAP/CHA data.
Among the states reporting child cases, Florida has the lowest cumulative proportion of child cases, 6.4%, but the state is using an age range of 0-14 years (no other state goes lower than 17 years). New Jersey and Texas are next at 6.9%, although Texas “reported age for only 6% of total confirmed cases,” the AAP and CHA noted.
There are 35 states above the national number of 12.0%, the highest being Wyoming at 23.3%, followed by Tennessee at 18.3% and South Carolina at 18.2%. The two southern states are the only ones to use an age range of 0-20 years for child cases, the two groups said in this week’s report, which did not include the usual data on testing, hospitalization, and mortality because of the holiday.
The news on children and COVID-19 for Thanksgiving week does not provide a lot of room for thankfulness.

the American Academy of Pediatrics and the Children’s Hospital Association said in their latest weekly report.
For those not counting, the week ending Nov. 26 was the fifth in a row to show “the highest weekly increase since the pandemic began,” based on data the AAP and CHA have been collecting from 49 state health departments (New York does not report ages), as well as the District of Columbia, New York City, Puerto Rico, and Guam.
The 153,608 new cases bring the total number of COVID-19 cases in children to almost 1.34 million in those jurisdictions, which is 12% of the total number of cases (11.2 million) among all ages. For just the week ending Nov. 26, children represented 13.7% of all new cases in the United States, down from 14.1% the previous week, according to the AAP/CHA data.
Among the states reporting child cases, Florida has the lowest cumulative proportion of child cases, 6.4%, but the state is using an age range of 0-14 years (no other state goes lower than 17 years). New Jersey and Texas are next at 6.9%, although Texas “reported age for only 6% of total confirmed cases,” the AAP and CHA noted.
There are 35 states above the national number of 12.0%, the highest being Wyoming at 23.3%, followed by Tennessee at 18.3% and South Carolina at 18.2%. The two southern states are the only ones to use an age range of 0-20 years for child cases, the two groups said in this week’s report, which did not include the usual data on testing, hospitalization, and mortality because of the holiday.
The news on children and COVID-19 for Thanksgiving week does not provide a lot of room for thankfulness.

the American Academy of Pediatrics and the Children’s Hospital Association said in their latest weekly report.
For those not counting, the week ending Nov. 26 was the fifth in a row to show “the highest weekly increase since the pandemic began,” based on data the AAP and CHA have been collecting from 49 state health departments (New York does not report ages), as well as the District of Columbia, New York City, Puerto Rico, and Guam.
The 153,608 new cases bring the total number of COVID-19 cases in children to almost 1.34 million in those jurisdictions, which is 12% of the total number of cases (11.2 million) among all ages. For just the week ending Nov. 26, children represented 13.7% of all new cases in the United States, down from 14.1% the previous week, according to the AAP/CHA data.
Among the states reporting child cases, Florida has the lowest cumulative proportion of child cases, 6.4%, but the state is using an age range of 0-14 years (no other state goes lower than 17 years). New Jersey and Texas are next at 6.9%, although Texas “reported age for only 6% of total confirmed cases,” the AAP and CHA noted.
There are 35 states above the national number of 12.0%, the highest being Wyoming at 23.3%, followed by Tennessee at 18.3% and South Carolina at 18.2%. The two southern states are the only ones to use an age range of 0-20 years for child cases, the two groups said in this week’s report, which did not include the usual data on testing, hospitalization, and mortality because of the holiday.
ACIP: Health workers, long-term care residents first tier for COVID-19 vaccine
The Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices voted 13-1 that both groups be in the highest-priority group for vaccination. As such, ACIP recommends that both be included in phase 1a of the committee’s allocation plan.
The recommendation now goes to CDC director Robert Redfield, MD, for approval. State health departments are expected to rely on the recommendation, but ultimately can make their own decisions on how to allocate vaccine in their states.
“We hope that this vote gets us all one step closer to the day when we can all feel safe again and when this pandemic is over,” said Nancy Messonnier, MD, director of the CDC’s National Center for Immunization and Respiratory Diseases, at today’s meeting.
Health care workers are defined as paid and unpaid individuals serving in health care settings who have the potential for direct or indirect exposure to patients or infectious materials. Long-term care residents are defined as adults who reside in facilities that provide a variety of services, including medical and personal care. Phase 1a would not include children who live in such facilities.
“Our goal in phase 1a with regard to health care personnel is to preserve the workforce and health care capacity regardless of where exposure occurs,” said ACIP panelist Grace Lee, MD, MPH, professor of paediatrics at Stanford (Calif.) University. Thus vaccination would cover clinical support staff, such as nursing assistants, environmental services staff, and food support staff.
“It is crucial to maintain our health care capacity,” said ACIP member Sharon Frey, MD, clinical director at the Center for Vaccine Development at Saint Louis University. “But it’s also important to prevent severe disease and death in the group that is at highest risk of those complications and that includes those in long-term care facilities.”
CDC staff said that staff and residents in those facilities account for 6% of COVID-19 cases and 40% of deaths.
But Helen Keipp Talbot, MD, associate professor of medicine at Vanderbilt University, Nashville, Tenn., voted against putting long-term care residents into the 1a phase. “We have traditionally tried a vaccine in a young healthy population and then hope it works in our frail older adults. So we enter this realm of ‘we hope it works and that it’s safe,’ and that concerns me on many levels particularly for this vaccine,” she said, noting that the vaccines closest to FDA authorization have not been studied in elderly adults who live in nursing homes or assisted living facilities.
She added: “I have no reservations for health care workers taking this vaccine.”
Prioritization could change
The phase 1a allocation fits within the “four ethical principles” outlined by ACIP and CDC staff Nov. 23: to maximize benefits and minimize harms, promote justice, mitigate health inequities, and promote transparency.
“My vote reflects maximum benefit, minimum harm, promoting justice and mitigating the health inequalities that exist with regard to distribution of this vaccine,” said ACIP Chair Jose Romero, MD. Romero, chief medical officer of the Arkansas Department of Health, voted in favor of the phase 1a plan.
He and other panelists noted, however, that allocation priorities could change after the FDA reviews and authorizes a vaccine.
The FDA’s Vaccines and Related Biological Products Advisory Committee (VRBPAC) will meet December 10 to review the Pfizer/BioNTech’s messenger RNA-based vaccine (BNT162b2). The companies filed for emergency use on November 20.
A second vaccine, made by Moderna, is not far behind. The company reported on Nov. 30 that its messenger RNA vaccine was 94.1% effective and filed for emergency use the same day. The FDA’s VRBPAC will review the safety and efficacy data for the Moderna vaccine on Dec. 17.
“If individual vaccines receive emergency use authorization, we will have more data to consider, and that could lead to revision of our prioritization,” said ACIP member Robert Atmar, MD, John S. Dunn Research Foundation Clinical Professor in Infectious Diseases at Baylor College of Medicine, Houston.
ACIP will meet again after the Dec. 10 FDA advisory panel. But it won’t recommend a product until after the FDA has authorized it, said Amanda Cohn, MD, senior advisor for vaccines at the CDC’s National Center for Immunization and Respiratory Diseases.
Staggered immunization subprioritization urged
The CDC staff said that given the potential that not enough vaccine will be available immediately, it was recommending that health care organizations plan on creating a hierarchy of prioritization within institutions. And, they also urged staggering vaccination for personnel in similar units or positions, citing potential systemic or other reactions among health care workers.
“Consider planning for personnel to have time away from clinical care if health care personnel experience systemic symptoms post vaccination,” said Sarah Oliver, MD, MSPH, from the CDC.
The CDC will soon be issuing guidance on how to handle systemic symptoms with health care workers, Dr. Oliver noted.
Some 40 million doses of the Pfizer/BioNTech and Moderna vaccines are expected to be available by the end of December, with 5 million to 10 million a week coming online after that, Dr. Cohn said. That means not all health care workers will be vaccinated immediately. That may require “subprioritization, but for a limited period of time,” she said.
Dr. Messonnier said that, even with limited supplies, most of the states have told the CDC that they think they can vaccinate all of their health care workers within 3 weeks – some in less time.
The ACIP allocation plan is similar to but not exactly the same as that issued by the National Academy of Sciences, Engineering, and Medicine, which issued recommendations in October. That organization said that health care workers, first responders, older Americans living in congregate settings, and people with underlying health conditions should be the first to receive a vaccine.
ACIP has said that phase 1b would include essential workers, including police officers and firefighters, and those in education, transportation, and food and agriculture sectors. Phase 1c would include adults with high-risk medical conditions and those aged 65 years or older.
This article first appeared on Medscape.com.
The Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices voted 13-1 that both groups be in the highest-priority group for vaccination. As such, ACIP recommends that both be included in phase 1a of the committee’s allocation plan.
The recommendation now goes to CDC director Robert Redfield, MD, for approval. State health departments are expected to rely on the recommendation, but ultimately can make their own decisions on how to allocate vaccine in their states.
“We hope that this vote gets us all one step closer to the day when we can all feel safe again and when this pandemic is over,” said Nancy Messonnier, MD, director of the CDC’s National Center for Immunization and Respiratory Diseases, at today’s meeting.
Health care workers are defined as paid and unpaid individuals serving in health care settings who have the potential for direct or indirect exposure to patients or infectious materials. Long-term care residents are defined as adults who reside in facilities that provide a variety of services, including medical and personal care. Phase 1a would not include children who live in such facilities.
“Our goal in phase 1a with regard to health care personnel is to preserve the workforce and health care capacity regardless of where exposure occurs,” said ACIP panelist Grace Lee, MD, MPH, professor of paediatrics at Stanford (Calif.) University. Thus vaccination would cover clinical support staff, such as nursing assistants, environmental services staff, and food support staff.
“It is crucial to maintain our health care capacity,” said ACIP member Sharon Frey, MD, clinical director at the Center for Vaccine Development at Saint Louis University. “But it’s also important to prevent severe disease and death in the group that is at highest risk of those complications and that includes those in long-term care facilities.”
CDC staff said that staff and residents in those facilities account for 6% of COVID-19 cases and 40% of deaths.
But Helen Keipp Talbot, MD, associate professor of medicine at Vanderbilt University, Nashville, Tenn., voted against putting long-term care residents into the 1a phase. “We have traditionally tried a vaccine in a young healthy population and then hope it works in our frail older adults. So we enter this realm of ‘we hope it works and that it’s safe,’ and that concerns me on many levels particularly for this vaccine,” she said, noting that the vaccines closest to FDA authorization have not been studied in elderly adults who live in nursing homes or assisted living facilities.
She added: “I have no reservations for health care workers taking this vaccine.”
Prioritization could change
The phase 1a allocation fits within the “four ethical principles” outlined by ACIP and CDC staff Nov. 23: to maximize benefits and minimize harms, promote justice, mitigate health inequities, and promote transparency.
“My vote reflects maximum benefit, minimum harm, promoting justice and mitigating the health inequalities that exist with regard to distribution of this vaccine,” said ACIP Chair Jose Romero, MD. Romero, chief medical officer of the Arkansas Department of Health, voted in favor of the phase 1a plan.
He and other panelists noted, however, that allocation priorities could change after the FDA reviews and authorizes a vaccine.
The FDA’s Vaccines and Related Biological Products Advisory Committee (VRBPAC) will meet December 10 to review the Pfizer/BioNTech’s messenger RNA-based vaccine (BNT162b2). The companies filed for emergency use on November 20.
A second vaccine, made by Moderna, is not far behind. The company reported on Nov. 30 that its messenger RNA vaccine was 94.1% effective and filed for emergency use the same day. The FDA’s VRBPAC will review the safety and efficacy data for the Moderna vaccine on Dec. 17.
“If individual vaccines receive emergency use authorization, we will have more data to consider, and that could lead to revision of our prioritization,” said ACIP member Robert Atmar, MD, John S. Dunn Research Foundation Clinical Professor in Infectious Diseases at Baylor College of Medicine, Houston.
ACIP will meet again after the Dec. 10 FDA advisory panel. But it won’t recommend a product until after the FDA has authorized it, said Amanda Cohn, MD, senior advisor for vaccines at the CDC’s National Center for Immunization and Respiratory Diseases.
Staggered immunization subprioritization urged
The CDC staff said that given the potential that not enough vaccine will be available immediately, it was recommending that health care organizations plan on creating a hierarchy of prioritization within institutions. And, they also urged staggering vaccination for personnel in similar units or positions, citing potential systemic or other reactions among health care workers.
“Consider planning for personnel to have time away from clinical care if health care personnel experience systemic symptoms post vaccination,” said Sarah Oliver, MD, MSPH, from the CDC.
The CDC will soon be issuing guidance on how to handle systemic symptoms with health care workers, Dr. Oliver noted.
Some 40 million doses of the Pfizer/BioNTech and Moderna vaccines are expected to be available by the end of December, with 5 million to 10 million a week coming online after that, Dr. Cohn said. That means not all health care workers will be vaccinated immediately. That may require “subprioritization, but for a limited period of time,” she said.
Dr. Messonnier said that, even with limited supplies, most of the states have told the CDC that they think they can vaccinate all of their health care workers within 3 weeks – some in less time.
The ACIP allocation plan is similar to but not exactly the same as that issued by the National Academy of Sciences, Engineering, and Medicine, which issued recommendations in October. That organization said that health care workers, first responders, older Americans living in congregate settings, and people with underlying health conditions should be the first to receive a vaccine.
ACIP has said that phase 1b would include essential workers, including police officers and firefighters, and those in education, transportation, and food and agriculture sectors. Phase 1c would include adults with high-risk medical conditions and those aged 65 years or older.
This article first appeared on Medscape.com.
The Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices voted 13-1 that both groups be in the highest-priority group for vaccination. As such, ACIP recommends that both be included in phase 1a of the committee’s allocation plan.
The recommendation now goes to CDC director Robert Redfield, MD, for approval. State health departments are expected to rely on the recommendation, but ultimately can make their own decisions on how to allocate vaccine in their states.
“We hope that this vote gets us all one step closer to the day when we can all feel safe again and when this pandemic is over,” said Nancy Messonnier, MD, director of the CDC’s National Center for Immunization and Respiratory Diseases, at today’s meeting.
Health care workers are defined as paid and unpaid individuals serving in health care settings who have the potential for direct or indirect exposure to patients or infectious materials. Long-term care residents are defined as adults who reside in facilities that provide a variety of services, including medical and personal care. Phase 1a would not include children who live in such facilities.
“Our goal in phase 1a with regard to health care personnel is to preserve the workforce and health care capacity regardless of where exposure occurs,” said ACIP panelist Grace Lee, MD, MPH, professor of paediatrics at Stanford (Calif.) University. Thus vaccination would cover clinical support staff, such as nursing assistants, environmental services staff, and food support staff.
“It is crucial to maintain our health care capacity,” said ACIP member Sharon Frey, MD, clinical director at the Center for Vaccine Development at Saint Louis University. “But it’s also important to prevent severe disease and death in the group that is at highest risk of those complications and that includes those in long-term care facilities.”
CDC staff said that staff and residents in those facilities account for 6% of COVID-19 cases and 40% of deaths.
But Helen Keipp Talbot, MD, associate professor of medicine at Vanderbilt University, Nashville, Tenn., voted against putting long-term care residents into the 1a phase. “We have traditionally tried a vaccine in a young healthy population and then hope it works in our frail older adults. So we enter this realm of ‘we hope it works and that it’s safe,’ and that concerns me on many levels particularly for this vaccine,” she said, noting that the vaccines closest to FDA authorization have not been studied in elderly adults who live in nursing homes or assisted living facilities.
She added: “I have no reservations for health care workers taking this vaccine.”
Prioritization could change
The phase 1a allocation fits within the “four ethical principles” outlined by ACIP and CDC staff Nov. 23: to maximize benefits and minimize harms, promote justice, mitigate health inequities, and promote transparency.
“My vote reflects maximum benefit, minimum harm, promoting justice and mitigating the health inequalities that exist with regard to distribution of this vaccine,” said ACIP Chair Jose Romero, MD. Romero, chief medical officer of the Arkansas Department of Health, voted in favor of the phase 1a plan.
He and other panelists noted, however, that allocation priorities could change after the FDA reviews and authorizes a vaccine.
The FDA’s Vaccines and Related Biological Products Advisory Committee (VRBPAC) will meet December 10 to review the Pfizer/BioNTech’s messenger RNA-based vaccine (BNT162b2). The companies filed for emergency use on November 20.
A second vaccine, made by Moderna, is not far behind. The company reported on Nov. 30 that its messenger RNA vaccine was 94.1% effective and filed for emergency use the same day. The FDA’s VRBPAC will review the safety and efficacy data for the Moderna vaccine on Dec. 17.
“If individual vaccines receive emergency use authorization, we will have more data to consider, and that could lead to revision of our prioritization,” said ACIP member Robert Atmar, MD, John S. Dunn Research Foundation Clinical Professor in Infectious Diseases at Baylor College of Medicine, Houston.
ACIP will meet again after the Dec. 10 FDA advisory panel. But it won’t recommend a product until after the FDA has authorized it, said Amanda Cohn, MD, senior advisor for vaccines at the CDC’s National Center for Immunization and Respiratory Diseases.
Staggered immunization subprioritization urged
The CDC staff said that given the potential that not enough vaccine will be available immediately, it was recommending that health care organizations plan on creating a hierarchy of prioritization within institutions. And, they also urged staggering vaccination for personnel in similar units or positions, citing potential systemic or other reactions among health care workers.
“Consider planning for personnel to have time away from clinical care if health care personnel experience systemic symptoms post vaccination,” said Sarah Oliver, MD, MSPH, from the CDC.
The CDC will soon be issuing guidance on how to handle systemic symptoms with health care workers, Dr. Oliver noted.
Some 40 million doses of the Pfizer/BioNTech and Moderna vaccines are expected to be available by the end of December, with 5 million to 10 million a week coming online after that, Dr. Cohn said. That means not all health care workers will be vaccinated immediately. That may require “subprioritization, but for a limited period of time,” she said.
Dr. Messonnier said that, even with limited supplies, most of the states have told the CDC that they think they can vaccinate all of their health care workers within 3 weeks – some in less time.
The ACIP allocation plan is similar to but not exactly the same as that issued by the National Academy of Sciences, Engineering, and Medicine, which issued recommendations in October. That organization said that health care workers, first responders, older Americans living in congregate settings, and people with underlying health conditions should be the first to receive a vaccine.
ACIP has said that phase 1b would include essential workers, including police officers and firefighters, and those in education, transportation, and food and agriculture sectors. Phase 1c would include adults with high-risk medical conditions and those aged 65 years or older.
This article first appeared on Medscape.com.
CMS launches hospital-at-home program to free up hospital capacity
As an increasing number of health systems implement “hospital-at-home” (HaH) programs to increase their traditional hospital capacity, the Centers for Medicare & Medicaid Services has given the movement a boost by changing its regulations to allow acute care to be provided in a patient’s home under certain conditions.
The CMS announced Nov. 25 that it was launching its Acute Hospital Care at Home program “to increase the capacity of the American health care system” during the COVID-19 pandemic.
At the same time, the agency announced it was giving more flexibility to ambulatory surgery centers (ASCs) to provide hospital-level care.
The CMS said its new HaH program is an expansion of the Hospitals Without Walls initiative that was unveiled last March. Hospitals Without Walls is a set of “temporary new rules” that provide flexibility for hospitals to provide acute care outside of inpatient settings. Under those rules, hospitals are able to transfer patients to outside facilities, such as ASCs, inpatient rehabilitation hospitals, hotels, and dormitories, while still receiving Medicare hospital payments.
Under CMS’ new Acute Hospital Care at Home, which is not described as temporary, patients can be transferred from emergency departments or inpatient wards to hospital-level care at home. The CMS said the HaH program is designed for people with conditions such as the acute phases of asthma, heart failure, pneumonia, and chronic obstructive pulmonary disease. Altogether, the agency said, more than 60 acute conditions can be treated safely at home.
However, the agency didn’t say that facilities can’t admit COVID-19 patients to the hospital at home. Rami Karjian, MBA, cofounder and CEO of Medically Home, a firm that supplies health systems with technical services and software for HaH programs, said in an interview that several Medically Home clients plan to treat both COVID-19 and non-COVID-19 patients at home when they begin to participate in the CMS program in the near future.
The CMS said it consulted extensively with academic and private industry leaders in building its HaH program. Before rolling out the initiative, the agency noted, it conducted successful pilot programs in leading hospitals and health systems. The results of some of these pilots have been reported in academic journals.
Participating hospitals will be required to have specified screening protocols in place before beginning acute care at home, the CMS announced. An in-person physician evaluation will be required before starting care at home. A nurse will evaluate each patient once daily in person or remotely, and either nurses or paramedics will visit the patient in person twice a day.
In contrast, Medicare regulations require nursing staff to be available around the clock in traditional hospitals. So the CMS has to grant waivers to hospitals for HaH programs.
While not going into detail on the telemonitoring capabilities that will be required in the acute hospital care at home, the release said, “Today’s announcement builds upon the critical work by CMS to expand telehealth coverage to keep beneficiaries safe and prevent the spread of COVID-19.”
More flexibility for ASCs
The agency is also giving ASCs the flexibility to provide 24-hour nursing services only when one or more patients are receiving care on site. This flexibility will be available to any of the 5,700 ASCs that wish to participate, and will be immediately effective for the 85 ASCs currently participating in the Hospital Without Walls initiative, the CMS said.
The new ASC regulations, the CMS said, are aimed at allowing communities “to maintain surgical capacity and other life-saving non-COVID-19 [care], like cancer surgeries.” Patients who need such procedures will be able to receive them in ASCs without being exposed to known COVID-19 cases.
Similarly, the CMS said patients and families not diagnosed with COVID-19 may prefer to receive acute care at home if local hospitals are full of COVID-19 patients. In addition, the CMS said it anticipates patients may value the ability to be treated at home without the visitation restrictions of hospitals.
Early HaH participants
Six health systems with extensive experience in providing acute hospital care at home have been approved for the new HaH waivers from Medicare rules. They include Brigham and Women’s Hospital (Massachusetts); Huntsman Cancer Institute (Utah); Massachusetts General Hospital (Massachusetts); Mount Sinai Health System (New York City); Presbyterian Healthcare Services (New Mexico); and UnityPoint Health (Iowa).
The CMS said that it’s in discussions with other health care systems and expects new applications to be submitted soon.
To support these efforts, the CMS has launched an online portal to streamline the waiver request process. The agency said it will closely monitor the program to safeguard beneficiaries and will require participating hospitals to report quality and safety data on a regular basis.
Support from hospitals
The first health systems participating in the CMS HaH appear to be supportive of the program, with some hospital leaders submitting comments to the CMS about their view of the initiative.
“The CMS has taken an extraordinary step today, facilitating the rapid expansion of Hospitalization at Home, an innovative care model with proven results,” said Kenneth L. Davis, MD, president and CEO of the Mount Sinai Health System in New York City. “This important and timely move will enable hospitals across the country to use effective tools to safely care for patients during this pandemic.”
David Levine, MD, assistant professor of medicine and medical director of strategy and innovation for Brigham Health Home Hospital in Boston, was similarly laudatory: “Our research at Brigham Health Home has shown that we can deliver hospital-level care in our patients’ homes with lower readmission rates, more physical mobility, and a positive patient experience,” he said. “During these challenging times, a focus on the home is critical. We are so encouraged that CMS is taking this important step, which will allow hospitals across the country to increase their capacity while delivering the care all patients deserve.”
Scaling up quickly
If other hospitals and health systems recognize the value of HaH, how long might it take them to develop and implement these programs in the midst of a pandemic?
Atrium Health, a large health system in the Southeast, ramped up a hospital-at-home initiative last spring for its 10 hospitals in the Charlotte, N.C., area, in just 2 weeks. However, it had been working on the project for some time before the pandemic struck. Focusing mostly on COVID-19 patients, the initiative reduced the COVID-19 patient load by 20%-25% in Atrium’s hospitals.
Medically Home, the HaH infrastructure company, said in a news release that it “enables health systems to establish new hospital-at-home services in as little as 30 days.” Medically Home has partnered in this venture with Huron Consulting Group, which has about 200 HaH-trained consultants, and Cardinal Health, a large global medical supplies distributor.
Mr. Karjian said in an interview that he expects private insurers to follow CMS’ example, as they often do. “We think this decision will cause not only CMS but private insurers to cover hospital at home after the pandemic, if it becomes the standard of care, because patients have better outcomes when treated at home,” he said.
Asked for his view on why the CMS specified that patients could be admitted to an HaH only from emergency departments or inpatient settings, Mr. Karjian said that the CMS wants to make sure that patients have access to brick-and-mortar hospital care if that’s what they need. Also, he noted, this model is new to most hospitals, so the CMS wants to make sure it starts “with all the safety guardrails” in place.
Overall, Mr. Karjian said, “This is an exciting development for patients across the country. What CMS has done is terrific in terms of letting patients get the care they want, where they want it, and get the benefit of better outcomes while the nation is going through this capacity crunch for hospital beds.”
A version of this article originally appeared on Medscape.com.
As an increasing number of health systems implement “hospital-at-home” (HaH) programs to increase their traditional hospital capacity, the Centers for Medicare & Medicaid Services has given the movement a boost by changing its regulations to allow acute care to be provided in a patient’s home under certain conditions.
The CMS announced Nov. 25 that it was launching its Acute Hospital Care at Home program “to increase the capacity of the American health care system” during the COVID-19 pandemic.
At the same time, the agency announced it was giving more flexibility to ambulatory surgery centers (ASCs) to provide hospital-level care.
The CMS said its new HaH program is an expansion of the Hospitals Without Walls initiative that was unveiled last March. Hospitals Without Walls is a set of “temporary new rules” that provide flexibility for hospitals to provide acute care outside of inpatient settings. Under those rules, hospitals are able to transfer patients to outside facilities, such as ASCs, inpatient rehabilitation hospitals, hotels, and dormitories, while still receiving Medicare hospital payments.
Under CMS’ new Acute Hospital Care at Home, which is not described as temporary, patients can be transferred from emergency departments or inpatient wards to hospital-level care at home. The CMS said the HaH program is designed for people with conditions such as the acute phases of asthma, heart failure, pneumonia, and chronic obstructive pulmonary disease. Altogether, the agency said, more than 60 acute conditions can be treated safely at home.
However, the agency didn’t say that facilities can’t admit COVID-19 patients to the hospital at home. Rami Karjian, MBA, cofounder and CEO of Medically Home, a firm that supplies health systems with technical services and software for HaH programs, said in an interview that several Medically Home clients plan to treat both COVID-19 and non-COVID-19 patients at home when they begin to participate in the CMS program in the near future.
The CMS said it consulted extensively with academic and private industry leaders in building its HaH program. Before rolling out the initiative, the agency noted, it conducted successful pilot programs in leading hospitals and health systems. The results of some of these pilots have been reported in academic journals.
Participating hospitals will be required to have specified screening protocols in place before beginning acute care at home, the CMS announced. An in-person physician evaluation will be required before starting care at home. A nurse will evaluate each patient once daily in person or remotely, and either nurses or paramedics will visit the patient in person twice a day.
In contrast, Medicare regulations require nursing staff to be available around the clock in traditional hospitals. So the CMS has to grant waivers to hospitals for HaH programs.
While not going into detail on the telemonitoring capabilities that will be required in the acute hospital care at home, the release said, “Today’s announcement builds upon the critical work by CMS to expand telehealth coverage to keep beneficiaries safe and prevent the spread of COVID-19.”
More flexibility for ASCs
The agency is also giving ASCs the flexibility to provide 24-hour nursing services only when one or more patients are receiving care on site. This flexibility will be available to any of the 5,700 ASCs that wish to participate, and will be immediately effective for the 85 ASCs currently participating in the Hospital Without Walls initiative, the CMS said.
The new ASC regulations, the CMS said, are aimed at allowing communities “to maintain surgical capacity and other life-saving non-COVID-19 [care], like cancer surgeries.” Patients who need such procedures will be able to receive them in ASCs without being exposed to known COVID-19 cases.
Similarly, the CMS said patients and families not diagnosed with COVID-19 may prefer to receive acute care at home if local hospitals are full of COVID-19 patients. In addition, the CMS said it anticipates patients may value the ability to be treated at home without the visitation restrictions of hospitals.
Early HaH participants
Six health systems with extensive experience in providing acute hospital care at home have been approved for the new HaH waivers from Medicare rules. They include Brigham and Women’s Hospital (Massachusetts); Huntsman Cancer Institute (Utah); Massachusetts General Hospital (Massachusetts); Mount Sinai Health System (New York City); Presbyterian Healthcare Services (New Mexico); and UnityPoint Health (Iowa).
The CMS said that it’s in discussions with other health care systems and expects new applications to be submitted soon.
To support these efforts, the CMS has launched an online portal to streamline the waiver request process. The agency said it will closely monitor the program to safeguard beneficiaries and will require participating hospitals to report quality and safety data on a regular basis.
Support from hospitals
The first health systems participating in the CMS HaH appear to be supportive of the program, with some hospital leaders submitting comments to the CMS about their view of the initiative.
“The CMS has taken an extraordinary step today, facilitating the rapid expansion of Hospitalization at Home, an innovative care model with proven results,” said Kenneth L. Davis, MD, president and CEO of the Mount Sinai Health System in New York City. “This important and timely move will enable hospitals across the country to use effective tools to safely care for patients during this pandemic.”
David Levine, MD, assistant professor of medicine and medical director of strategy and innovation for Brigham Health Home Hospital in Boston, was similarly laudatory: “Our research at Brigham Health Home has shown that we can deliver hospital-level care in our patients’ homes with lower readmission rates, more physical mobility, and a positive patient experience,” he said. “During these challenging times, a focus on the home is critical. We are so encouraged that CMS is taking this important step, which will allow hospitals across the country to increase their capacity while delivering the care all patients deserve.”
Scaling up quickly
If other hospitals and health systems recognize the value of HaH, how long might it take them to develop and implement these programs in the midst of a pandemic?
Atrium Health, a large health system in the Southeast, ramped up a hospital-at-home initiative last spring for its 10 hospitals in the Charlotte, N.C., area, in just 2 weeks. However, it had been working on the project for some time before the pandemic struck. Focusing mostly on COVID-19 patients, the initiative reduced the COVID-19 patient load by 20%-25% in Atrium’s hospitals.
Medically Home, the HaH infrastructure company, said in a news release that it “enables health systems to establish new hospital-at-home services in as little as 30 days.” Medically Home has partnered in this venture with Huron Consulting Group, which has about 200 HaH-trained consultants, and Cardinal Health, a large global medical supplies distributor.
Mr. Karjian said in an interview that he expects private insurers to follow CMS’ example, as they often do. “We think this decision will cause not only CMS but private insurers to cover hospital at home after the pandemic, if it becomes the standard of care, because patients have better outcomes when treated at home,” he said.
Asked for his view on why the CMS specified that patients could be admitted to an HaH only from emergency departments or inpatient settings, Mr. Karjian said that the CMS wants to make sure that patients have access to brick-and-mortar hospital care if that’s what they need. Also, he noted, this model is new to most hospitals, so the CMS wants to make sure it starts “with all the safety guardrails” in place.
Overall, Mr. Karjian said, “This is an exciting development for patients across the country. What CMS has done is terrific in terms of letting patients get the care they want, where they want it, and get the benefit of better outcomes while the nation is going through this capacity crunch for hospital beds.”
A version of this article originally appeared on Medscape.com.
As an increasing number of health systems implement “hospital-at-home” (HaH) programs to increase their traditional hospital capacity, the Centers for Medicare & Medicaid Services has given the movement a boost by changing its regulations to allow acute care to be provided in a patient’s home under certain conditions.
The CMS announced Nov. 25 that it was launching its Acute Hospital Care at Home program “to increase the capacity of the American health care system” during the COVID-19 pandemic.
At the same time, the agency announced it was giving more flexibility to ambulatory surgery centers (ASCs) to provide hospital-level care.
The CMS said its new HaH program is an expansion of the Hospitals Without Walls initiative that was unveiled last March. Hospitals Without Walls is a set of “temporary new rules” that provide flexibility for hospitals to provide acute care outside of inpatient settings. Under those rules, hospitals are able to transfer patients to outside facilities, such as ASCs, inpatient rehabilitation hospitals, hotels, and dormitories, while still receiving Medicare hospital payments.
Under CMS’ new Acute Hospital Care at Home, which is not described as temporary, patients can be transferred from emergency departments or inpatient wards to hospital-level care at home. The CMS said the HaH program is designed for people with conditions such as the acute phases of asthma, heart failure, pneumonia, and chronic obstructive pulmonary disease. Altogether, the agency said, more than 60 acute conditions can be treated safely at home.
However, the agency didn’t say that facilities can’t admit COVID-19 patients to the hospital at home. Rami Karjian, MBA, cofounder and CEO of Medically Home, a firm that supplies health systems with technical services and software for HaH programs, said in an interview that several Medically Home clients plan to treat both COVID-19 and non-COVID-19 patients at home when they begin to participate in the CMS program in the near future.
The CMS said it consulted extensively with academic and private industry leaders in building its HaH program. Before rolling out the initiative, the agency noted, it conducted successful pilot programs in leading hospitals and health systems. The results of some of these pilots have been reported in academic journals.
Participating hospitals will be required to have specified screening protocols in place before beginning acute care at home, the CMS announced. An in-person physician evaluation will be required before starting care at home. A nurse will evaluate each patient once daily in person or remotely, and either nurses or paramedics will visit the patient in person twice a day.
In contrast, Medicare regulations require nursing staff to be available around the clock in traditional hospitals. So the CMS has to grant waivers to hospitals for HaH programs.
While not going into detail on the telemonitoring capabilities that will be required in the acute hospital care at home, the release said, “Today’s announcement builds upon the critical work by CMS to expand telehealth coverage to keep beneficiaries safe and prevent the spread of COVID-19.”
More flexibility for ASCs
The agency is also giving ASCs the flexibility to provide 24-hour nursing services only when one or more patients are receiving care on site. This flexibility will be available to any of the 5,700 ASCs that wish to participate, and will be immediately effective for the 85 ASCs currently participating in the Hospital Without Walls initiative, the CMS said.
The new ASC regulations, the CMS said, are aimed at allowing communities “to maintain surgical capacity and other life-saving non-COVID-19 [care], like cancer surgeries.” Patients who need such procedures will be able to receive them in ASCs without being exposed to known COVID-19 cases.
Similarly, the CMS said patients and families not diagnosed with COVID-19 may prefer to receive acute care at home if local hospitals are full of COVID-19 patients. In addition, the CMS said it anticipates patients may value the ability to be treated at home without the visitation restrictions of hospitals.
Early HaH participants
Six health systems with extensive experience in providing acute hospital care at home have been approved for the new HaH waivers from Medicare rules. They include Brigham and Women’s Hospital (Massachusetts); Huntsman Cancer Institute (Utah); Massachusetts General Hospital (Massachusetts); Mount Sinai Health System (New York City); Presbyterian Healthcare Services (New Mexico); and UnityPoint Health (Iowa).
The CMS said that it’s in discussions with other health care systems and expects new applications to be submitted soon.
To support these efforts, the CMS has launched an online portal to streamline the waiver request process. The agency said it will closely monitor the program to safeguard beneficiaries and will require participating hospitals to report quality and safety data on a regular basis.
Support from hospitals
The first health systems participating in the CMS HaH appear to be supportive of the program, with some hospital leaders submitting comments to the CMS about their view of the initiative.
“The CMS has taken an extraordinary step today, facilitating the rapid expansion of Hospitalization at Home, an innovative care model with proven results,” said Kenneth L. Davis, MD, president and CEO of the Mount Sinai Health System in New York City. “This important and timely move will enable hospitals across the country to use effective tools to safely care for patients during this pandemic.”
David Levine, MD, assistant professor of medicine and medical director of strategy and innovation for Brigham Health Home Hospital in Boston, was similarly laudatory: “Our research at Brigham Health Home has shown that we can deliver hospital-level care in our patients’ homes with lower readmission rates, more physical mobility, and a positive patient experience,” he said. “During these challenging times, a focus on the home is critical. We are so encouraged that CMS is taking this important step, which will allow hospitals across the country to increase their capacity while delivering the care all patients deserve.”
Scaling up quickly
If other hospitals and health systems recognize the value of HaH, how long might it take them to develop and implement these programs in the midst of a pandemic?
Atrium Health, a large health system in the Southeast, ramped up a hospital-at-home initiative last spring for its 10 hospitals in the Charlotte, N.C., area, in just 2 weeks. However, it had been working on the project for some time before the pandemic struck. Focusing mostly on COVID-19 patients, the initiative reduced the COVID-19 patient load by 20%-25% in Atrium’s hospitals.
Medically Home, the HaH infrastructure company, said in a news release that it “enables health systems to establish new hospital-at-home services in as little as 30 days.” Medically Home has partnered in this venture with Huron Consulting Group, which has about 200 HaH-trained consultants, and Cardinal Health, a large global medical supplies distributor.
Mr. Karjian said in an interview that he expects private insurers to follow CMS’ example, as they often do. “We think this decision will cause not only CMS but private insurers to cover hospital at home after the pandemic, if it becomes the standard of care, because patients have better outcomes when treated at home,” he said.
Asked for his view on why the CMS specified that patients could be admitted to an HaH only from emergency departments or inpatient settings, Mr. Karjian said that the CMS wants to make sure that patients have access to brick-and-mortar hospital care if that’s what they need. Also, he noted, this model is new to most hospitals, so the CMS wants to make sure it starts “with all the safety guardrails” in place.
Overall, Mr. Karjian said, “This is an exciting development for patients across the country. What CMS has done is terrific in terms of letting patients get the care they want, where they want it, and get the benefit of better outcomes while the nation is going through this capacity crunch for hospital beds.”
A version of this article originally appeared on Medscape.com.
Are more female physicians leaving medicine as pandemic surges?
For mid-career oncologist Tanya Wildes, MD, the pandemic was the last straw. In late September, she tweeted: “I have done the academically unfathomable: I am resigning my faculty position without another job lined up.”
She wasn’t burned out, she insisted. She loved her patients and her research. But she was also “100% confident” in her decision and “also 100% sad. This did not have to happen,” she lamented, asking not to disclose her workplace for fear of retribution.
Being a woman in medicine “is a hard life to start with,” Dr. Wildes said in an interview. “We all have that tenuous balance going on and the pandemic made everything just a little bit harder.”
She describes her prepandemic work-life balance as a “Jenga tower, with everything only just in place.” But she realized that the balance had tipped, when after a difficult clinic she felt emotionally wrung out. Her 11-year-old son had asked her to help him fly his model airplane. “I told him, ‘Honey, I can’t do it because if it crashes or gets stuck in a tree ... you’re going to be devastated and I have nothing left for you.’ “
This was a eureka moment, as “I realized, this is not who I want to be,” she said, holding back tears. “Seventy years from now my son is going to tell his grandchildren about the pandemic and I don’t want his memory of his mom to be that she couldn’t be there for him because she was too spent.”
When Dr. Wildes shared her story on Twitter, other female oncologists and physicians responded that they too have felt they’re under increased pressure this year, with the extra stress of the pandemic leading others to quit as well.
The trend of doctors leaving medicine has been noticeable. A July survey from the Physicians Foundation found that roughly 16,000 medical practices had already closed during the pandemic, with another 8,000 predicted to close within the next year.
“Similar patterns” were evident in another analysis by the Larry A. Green Center and the Primary Care Collaborative, as reported in The New York Times. In that survey, nearly one-fifth of primary care clinicians said “someone in their practice plans to retire early or has already retired because of COVID-19,” and 15% say “someone has left or plans to leave the practice.” About half said their mental exhaustion was at an all-time high, the survey found.
“COVID-19 is a burden, and that added burden has tipped people over the edge of many things,” said Monica Bertagnolli, MD, chief of the division of surgical oncology at Brigham and Women’s Hospital, Boston, and former president of the American Society of Clinical Oncology.
“It has illustrated that we do have a lot of people who are working kind of on the edge of not being able to handle everything,” she said.
While many in medicine are struggling, the pandemic seems to be pushing more women to leave, highlighting longtime gender disparities and increased caregiving burdens. And their absence may be felt for years to come.
Firm numbers are hard to come by, said Julie Silver, MD, associate professor, associate chair, and director of cancer rehabilitation in the department of physical medicine and rehabilitation at Harvard Medical School, Boston, and an expert in gender equity in medicine. But she sees some troubling trends.
“There are many indications that women are leaving medicine in disproportionately high numbers,” Dr. Silver said in an interview. “A lack of fair pay and promotion opportunities that were present before COVID-19 are now combined with a host of pandemic-related challenges.”
A survey of 1,809 women conducted in mid-April with the Physician Moms Facebook Group and accepted for online publication by the American Journal of Psychiatry found that 41% scored over the cutoff points for moderate or severe anxiety, with 46% meeting these criteria among front-line workers.
“It’s really important for society to recognize the extraordinary impact this pandemic is having on physician mothers, as there will be profound ripple effects on the ability of this key segment of the health care workforce to serve others if we do not address this problem urgently,” co-senior author Reshma Jagsi, MD, DPhil, a radiation oncologist at the University of Michigan, Ann Arbor, said in an interview.
Women weighed in on Twitter, in response to Dr. Silver’s tweet to #WomenInMedicine: “If you are thinking of leaving #medicine & need a reason to stay: we value you & need you.”
In reply, Emmy Betz, MD, MPH, associate professor of emergency medicine at the University of Colorado at Denver, Aurora, said via Twitter, “I’ve had lots of conversations with women considering leaving medicine.”
“I have thought about leaving many times. I love what I do, but medicine can be an unkind world at times,” responded Valerie Fitzhugh MD, associate professor and pathologist at New Jersey Medical School, Newark.
“Too late. Left at the end of July and it was the best decision ever,” wrote Michelle Gordon, DO, who was previously a board-certified general surgeon at Northern Westchester Surgical Associates in Putnam Valley, N.Y.
Prepandemic disparities accentuated
The pandemic “has merely accentuated – or made more apparent – some of the longstanding issues and struggles of women in oncology, women in medicine, women in academia,” said Sarah Holstein, MD, PhD, another mid-career oncologist and associate professor at the University of Nebraska Medical Center, Omaha.
“There are disparities in first-author/last-author publications, disparities in being asked to give speaking engagements, disparities in leadership,” Dr. Holstein said in an interview. “And then ... put on top of that the various surges with the pandemic where you are being asked to do clinical responsibilities you don’t normally do, perhaps some things you haven’t done since your training 10 or 20 years ago.”
This is backed up with data: There is already a “robust” body of prepandemic literature demonstrating pay gaps for female physicians and scientists, noted Dr. Silver, who founded the Her Time Is Now campaign for gender equity in medicine and runs a women’s leadership course at Harvard.
In addition, female physicians are more likely to be involved in “nonpromotable” work, group projects and educator roles that are often underappreciated and undercompensated, she said.
Writing recently in a blog post for the BMJ, Dr. Silver and colleagues predict that as a result of the pandemic, female physicians will “face disadvantages from unconscious bias in decisions about whose pay should be cut, whose operating schedules should take priority when resources are limited, and whose contributions merit retention ... The ground that women lose now will likely have a profound effect for many years to come, perhaps putting them at a disadvantage for the rest of their careers.”
There is already evidence of reduced publishing by female scientists during the pandemic, something that “could undermine the careers of an entire generation of women scholars,” noted Caitlyn Collins, PhD, assistant professor of sociology at Washington University in St. Louis.
“Science needs to address the culture of overwork,” Dr. Collins said in an interview. “Parents and other caregivers deserve support. The stress and ‘overwhelm’ they feel is not inevitable. A more fair, just, and humane approach to combining work and family is possible – what we need is the political will to pass better policies and a massive shift in our cultural understandings about how work should fit into family life, not the other way around.”
Lack of support for “vulnerable scientists,” particularly “junior scientists who are parents, women, or minorities” could lead to “severe attrition in cancer research in the coming years,” Cullen Taniguchi, MD, PhD, a radiation oncologist and associate professor at the University of Texas MD Anderson Cancer Center, Houston, and colleagues, warned in a recent letter to the journal Cancer Cell.
“The biggest worries of attrition will come from young faculty who started just before or after the pandemic,” Dr. Taniguchi said in an interview. “The first year in an academic setting is incredibly challenging but also important for establishing research efforts and building networks of colleagues to collaborate with. While completely necessary, the restrictions put in place during the pandemic made doing these things even more difficult.”
Another stressor: Caregiving at home
Another reason female physicians may be marginalized during the pandemic is that they are more often the primary caregivers at home.
“Anyone who is a caregiver, be it to kids, parents, or spouses, can relate to the challenges brought [on] by the pandemic,” said Ishwaria Subbiah, MD, a palliative care physician and medical oncologist at MD Anderson.
“Most of us work toward meeting our responsibilities by engaging a network of support, whether it’s home care workers, center-based or at-home child care, schools, or activities outside of school. The pandemic led to a level of disruption that brought most (if not all) of those responsibilities onto the caregivers themselves,” she said in an interview.
As the mother of an adult son with severe epilepsy, Dr. Bertagnolli has certainly experienced the challenges of parenting during the pandemic. “Our son is now 24 but he is handicapped, and lives with us. The care issues we have to deal with as professionals have been enormously magnified by COVID,” she said.
But she cautions against making gender distinctions when it comes to caregiving. “Has it fallen on the women? Well, this kind of stuff generally falls on the women, but I am certain it has fallen on an awful lot of men as well, because I think the world is changing that way, so it’s fallen on all of us.”
There is no question that female oncologists are bearing the brunt, both at work and at home, contended Dr. Taniguchi. “Absolutely. I have seen this first-hand,” he said.
“If it was difficult for women, underrepresented minorities, and junior faculty to find a voice in the room prepandemic, I think it can be harder in the times of virtual meetings when it is difficult to engage audiences,” he said.
Dr. Holstein said she is lucky to be well-supported at her institution, with both a female chief of hematology/oncology and a female chair of internal medicine, but still, she worries about the long-term consequences of the pandemic on the gender landscape of medicine.
“If you’re having to put aside research projects because you have extra responsibilities – again because women just tend to have a lot of other things going on – that might not be a big deal for 3 months, 6 months, but this is going to be a year or 2 years before ‘normal’ comes back,” she says. “One to two years of underpublishing or not getting the grants could be career killers for women in academic oncology.”
Cancer COVID-19 combo
As Dr. Wildes completed her final weeks of seeing cancer patients, she received an outpouring of support, which she says convinced her of the shared experience of all doctors, and especially female doctors, during the pandemic. But even more specifically, she feels that she has tapped into the unique burden shouldered by oncologists during this time.
“It’s intimidating being an oncologist; we are literally giving people poison for a living. Then throw into it a pandemic where early in March we had so little data. I was helping my patients make decisions about their cancer care based on a case series of four patients in China. The burden of those conversations is something I never want to have to live through again,” she said.
“Oncology is a particularly intense subspecialty within medicine,” agreed Dr. Subbiah. “The people we care for have received a life-altering and potentially life-limiting diagnosis. Coupled with that, the COVID-19 pandemic has brought an unprecedented cloud of uncertainty ... Whether the patients can see it overtly or not, oncologists carry the weight of this worry with them for not just one but all of their patients.”
Dr. Wildes said she plans to return to academic medicine and clinical care “in time,” but for now, the gap that she and others like her leave is troubling to those who have stayed on.
“We need these women in medicine,” said Dr. Holstein. “We have data suggesting that women take more time with their patients than men, that patient outcomes are better if they have a female physician. But also for the generations coming up, we need the mid-career and senior women to be in place to mentor and guide and make sure we continue to increase women in leadership.”
A version of this article originally appeared on Medscape.com.
For mid-career oncologist Tanya Wildes, MD, the pandemic was the last straw. In late September, she tweeted: “I have done the academically unfathomable: I am resigning my faculty position without another job lined up.”
She wasn’t burned out, she insisted. She loved her patients and her research. But she was also “100% confident” in her decision and “also 100% sad. This did not have to happen,” she lamented, asking not to disclose her workplace for fear of retribution.
Being a woman in medicine “is a hard life to start with,” Dr. Wildes said in an interview. “We all have that tenuous balance going on and the pandemic made everything just a little bit harder.”
She describes her prepandemic work-life balance as a “Jenga tower, with everything only just in place.” But she realized that the balance had tipped, when after a difficult clinic she felt emotionally wrung out. Her 11-year-old son had asked her to help him fly his model airplane. “I told him, ‘Honey, I can’t do it because if it crashes or gets stuck in a tree ... you’re going to be devastated and I have nothing left for you.’ “
This was a eureka moment, as “I realized, this is not who I want to be,” she said, holding back tears. “Seventy years from now my son is going to tell his grandchildren about the pandemic and I don’t want his memory of his mom to be that she couldn’t be there for him because she was too spent.”
When Dr. Wildes shared her story on Twitter, other female oncologists and physicians responded that they too have felt they’re under increased pressure this year, with the extra stress of the pandemic leading others to quit as well.
The trend of doctors leaving medicine has been noticeable. A July survey from the Physicians Foundation found that roughly 16,000 medical practices had already closed during the pandemic, with another 8,000 predicted to close within the next year.
“Similar patterns” were evident in another analysis by the Larry A. Green Center and the Primary Care Collaborative, as reported in The New York Times. In that survey, nearly one-fifth of primary care clinicians said “someone in their practice plans to retire early or has already retired because of COVID-19,” and 15% say “someone has left or plans to leave the practice.” About half said their mental exhaustion was at an all-time high, the survey found.
“COVID-19 is a burden, and that added burden has tipped people over the edge of many things,” said Monica Bertagnolli, MD, chief of the division of surgical oncology at Brigham and Women’s Hospital, Boston, and former president of the American Society of Clinical Oncology.
“It has illustrated that we do have a lot of people who are working kind of on the edge of not being able to handle everything,” she said.
While many in medicine are struggling, the pandemic seems to be pushing more women to leave, highlighting longtime gender disparities and increased caregiving burdens. And their absence may be felt for years to come.
Firm numbers are hard to come by, said Julie Silver, MD, associate professor, associate chair, and director of cancer rehabilitation in the department of physical medicine and rehabilitation at Harvard Medical School, Boston, and an expert in gender equity in medicine. But she sees some troubling trends.
“There are many indications that women are leaving medicine in disproportionately high numbers,” Dr. Silver said in an interview. “A lack of fair pay and promotion opportunities that were present before COVID-19 are now combined with a host of pandemic-related challenges.”
A survey of 1,809 women conducted in mid-April with the Physician Moms Facebook Group and accepted for online publication by the American Journal of Psychiatry found that 41% scored over the cutoff points for moderate or severe anxiety, with 46% meeting these criteria among front-line workers.
“It’s really important for society to recognize the extraordinary impact this pandemic is having on physician mothers, as there will be profound ripple effects on the ability of this key segment of the health care workforce to serve others if we do not address this problem urgently,” co-senior author Reshma Jagsi, MD, DPhil, a radiation oncologist at the University of Michigan, Ann Arbor, said in an interview.
Women weighed in on Twitter, in response to Dr. Silver’s tweet to #WomenInMedicine: “If you are thinking of leaving #medicine & need a reason to stay: we value you & need you.”
In reply, Emmy Betz, MD, MPH, associate professor of emergency medicine at the University of Colorado at Denver, Aurora, said via Twitter, “I’ve had lots of conversations with women considering leaving medicine.”
“I have thought about leaving many times. I love what I do, but medicine can be an unkind world at times,” responded Valerie Fitzhugh MD, associate professor and pathologist at New Jersey Medical School, Newark.
“Too late. Left at the end of July and it was the best decision ever,” wrote Michelle Gordon, DO, who was previously a board-certified general surgeon at Northern Westchester Surgical Associates in Putnam Valley, N.Y.
Prepandemic disparities accentuated
The pandemic “has merely accentuated – or made more apparent – some of the longstanding issues and struggles of women in oncology, women in medicine, women in academia,” said Sarah Holstein, MD, PhD, another mid-career oncologist and associate professor at the University of Nebraska Medical Center, Omaha.
“There are disparities in first-author/last-author publications, disparities in being asked to give speaking engagements, disparities in leadership,” Dr. Holstein said in an interview. “And then ... put on top of that the various surges with the pandemic where you are being asked to do clinical responsibilities you don’t normally do, perhaps some things you haven’t done since your training 10 or 20 years ago.”
This is backed up with data: There is already a “robust” body of prepandemic literature demonstrating pay gaps for female physicians and scientists, noted Dr. Silver, who founded the Her Time Is Now campaign for gender equity in medicine and runs a women’s leadership course at Harvard.
In addition, female physicians are more likely to be involved in “nonpromotable” work, group projects and educator roles that are often underappreciated and undercompensated, she said.
Writing recently in a blog post for the BMJ, Dr. Silver and colleagues predict that as a result of the pandemic, female physicians will “face disadvantages from unconscious bias in decisions about whose pay should be cut, whose operating schedules should take priority when resources are limited, and whose contributions merit retention ... The ground that women lose now will likely have a profound effect for many years to come, perhaps putting them at a disadvantage for the rest of their careers.”
There is already evidence of reduced publishing by female scientists during the pandemic, something that “could undermine the careers of an entire generation of women scholars,” noted Caitlyn Collins, PhD, assistant professor of sociology at Washington University in St. Louis.
“Science needs to address the culture of overwork,” Dr. Collins said in an interview. “Parents and other caregivers deserve support. The stress and ‘overwhelm’ they feel is not inevitable. A more fair, just, and humane approach to combining work and family is possible – what we need is the political will to pass better policies and a massive shift in our cultural understandings about how work should fit into family life, not the other way around.”
Lack of support for “vulnerable scientists,” particularly “junior scientists who are parents, women, or minorities” could lead to “severe attrition in cancer research in the coming years,” Cullen Taniguchi, MD, PhD, a radiation oncologist and associate professor at the University of Texas MD Anderson Cancer Center, Houston, and colleagues, warned in a recent letter to the journal Cancer Cell.
“The biggest worries of attrition will come from young faculty who started just before or after the pandemic,” Dr. Taniguchi said in an interview. “The first year in an academic setting is incredibly challenging but also important for establishing research efforts and building networks of colleagues to collaborate with. While completely necessary, the restrictions put in place during the pandemic made doing these things even more difficult.”
Another stressor: Caregiving at home
Another reason female physicians may be marginalized during the pandemic is that they are more often the primary caregivers at home.
“Anyone who is a caregiver, be it to kids, parents, or spouses, can relate to the challenges brought [on] by the pandemic,” said Ishwaria Subbiah, MD, a palliative care physician and medical oncologist at MD Anderson.
“Most of us work toward meeting our responsibilities by engaging a network of support, whether it’s home care workers, center-based or at-home child care, schools, or activities outside of school. The pandemic led to a level of disruption that brought most (if not all) of those responsibilities onto the caregivers themselves,” she said in an interview.
As the mother of an adult son with severe epilepsy, Dr. Bertagnolli has certainly experienced the challenges of parenting during the pandemic. “Our son is now 24 but he is handicapped, and lives with us. The care issues we have to deal with as professionals have been enormously magnified by COVID,” she said.
But she cautions against making gender distinctions when it comes to caregiving. “Has it fallen on the women? Well, this kind of stuff generally falls on the women, but I am certain it has fallen on an awful lot of men as well, because I think the world is changing that way, so it’s fallen on all of us.”
There is no question that female oncologists are bearing the brunt, both at work and at home, contended Dr. Taniguchi. “Absolutely. I have seen this first-hand,” he said.
“If it was difficult for women, underrepresented minorities, and junior faculty to find a voice in the room prepandemic, I think it can be harder in the times of virtual meetings when it is difficult to engage audiences,” he said.
Dr. Holstein said she is lucky to be well-supported at her institution, with both a female chief of hematology/oncology and a female chair of internal medicine, but still, she worries about the long-term consequences of the pandemic on the gender landscape of medicine.
“If you’re having to put aside research projects because you have extra responsibilities – again because women just tend to have a lot of other things going on – that might not be a big deal for 3 months, 6 months, but this is going to be a year or 2 years before ‘normal’ comes back,” she says. “One to two years of underpublishing or not getting the grants could be career killers for women in academic oncology.”
Cancer COVID-19 combo
As Dr. Wildes completed her final weeks of seeing cancer patients, she received an outpouring of support, which she says convinced her of the shared experience of all doctors, and especially female doctors, during the pandemic. But even more specifically, she feels that she has tapped into the unique burden shouldered by oncologists during this time.
“It’s intimidating being an oncologist; we are literally giving people poison for a living. Then throw into it a pandemic where early in March we had so little data. I was helping my patients make decisions about their cancer care based on a case series of four patients in China. The burden of those conversations is something I never want to have to live through again,” she said.
“Oncology is a particularly intense subspecialty within medicine,” agreed Dr. Subbiah. “The people we care for have received a life-altering and potentially life-limiting diagnosis. Coupled with that, the COVID-19 pandemic has brought an unprecedented cloud of uncertainty ... Whether the patients can see it overtly or not, oncologists carry the weight of this worry with them for not just one but all of their patients.”
Dr. Wildes said she plans to return to academic medicine and clinical care “in time,” but for now, the gap that she and others like her leave is troubling to those who have stayed on.
“We need these women in medicine,” said Dr. Holstein. “We have data suggesting that women take more time with their patients than men, that patient outcomes are better if they have a female physician. But also for the generations coming up, we need the mid-career and senior women to be in place to mentor and guide and make sure we continue to increase women in leadership.”
A version of this article originally appeared on Medscape.com.
For mid-career oncologist Tanya Wildes, MD, the pandemic was the last straw. In late September, she tweeted: “I have done the academically unfathomable: I am resigning my faculty position without another job lined up.”
She wasn’t burned out, she insisted. She loved her patients and her research. But she was also “100% confident” in her decision and “also 100% sad. This did not have to happen,” she lamented, asking not to disclose her workplace for fear of retribution.
Being a woman in medicine “is a hard life to start with,” Dr. Wildes said in an interview. “We all have that tenuous balance going on and the pandemic made everything just a little bit harder.”
She describes her prepandemic work-life balance as a “Jenga tower, with everything only just in place.” But she realized that the balance had tipped, when after a difficult clinic she felt emotionally wrung out. Her 11-year-old son had asked her to help him fly his model airplane. “I told him, ‘Honey, I can’t do it because if it crashes or gets stuck in a tree ... you’re going to be devastated and I have nothing left for you.’ “
This was a eureka moment, as “I realized, this is not who I want to be,” she said, holding back tears. “Seventy years from now my son is going to tell his grandchildren about the pandemic and I don’t want his memory of his mom to be that she couldn’t be there for him because she was too spent.”
When Dr. Wildes shared her story on Twitter, other female oncologists and physicians responded that they too have felt they’re under increased pressure this year, with the extra stress of the pandemic leading others to quit as well.
The trend of doctors leaving medicine has been noticeable. A July survey from the Physicians Foundation found that roughly 16,000 medical practices had already closed during the pandemic, with another 8,000 predicted to close within the next year.
“Similar patterns” were evident in another analysis by the Larry A. Green Center and the Primary Care Collaborative, as reported in The New York Times. In that survey, nearly one-fifth of primary care clinicians said “someone in their practice plans to retire early or has already retired because of COVID-19,” and 15% say “someone has left or plans to leave the practice.” About half said their mental exhaustion was at an all-time high, the survey found.
“COVID-19 is a burden, and that added burden has tipped people over the edge of many things,” said Monica Bertagnolli, MD, chief of the division of surgical oncology at Brigham and Women’s Hospital, Boston, and former president of the American Society of Clinical Oncology.
“It has illustrated that we do have a lot of people who are working kind of on the edge of not being able to handle everything,” she said.
While many in medicine are struggling, the pandemic seems to be pushing more women to leave, highlighting longtime gender disparities and increased caregiving burdens. And their absence may be felt for years to come.
Firm numbers are hard to come by, said Julie Silver, MD, associate professor, associate chair, and director of cancer rehabilitation in the department of physical medicine and rehabilitation at Harvard Medical School, Boston, and an expert in gender equity in medicine. But she sees some troubling trends.
“There are many indications that women are leaving medicine in disproportionately high numbers,” Dr. Silver said in an interview. “A lack of fair pay and promotion opportunities that were present before COVID-19 are now combined with a host of pandemic-related challenges.”
A survey of 1,809 women conducted in mid-April with the Physician Moms Facebook Group and accepted for online publication by the American Journal of Psychiatry found that 41% scored over the cutoff points for moderate or severe anxiety, with 46% meeting these criteria among front-line workers.
“It’s really important for society to recognize the extraordinary impact this pandemic is having on physician mothers, as there will be profound ripple effects on the ability of this key segment of the health care workforce to serve others if we do not address this problem urgently,” co-senior author Reshma Jagsi, MD, DPhil, a radiation oncologist at the University of Michigan, Ann Arbor, said in an interview.
Women weighed in on Twitter, in response to Dr. Silver’s tweet to #WomenInMedicine: “If you are thinking of leaving #medicine & need a reason to stay: we value you & need you.”
In reply, Emmy Betz, MD, MPH, associate professor of emergency medicine at the University of Colorado at Denver, Aurora, said via Twitter, “I’ve had lots of conversations with women considering leaving medicine.”
“I have thought about leaving many times. I love what I do, but medicine can be an unkind world at times,” responded Valerie Fitzhugh MD, associate professor and pathologist at New Jersey Medical School, Newark.
“Too late. Left at the end of July and it was the best decision ever,” wrote Michelle Gordon, DO, who was previously a board-certified general surgeon at Northern Westchester Surgical Associates in Putnam Valley, N.Y.
Prepandemic disparities accentuated
The pandemic “has merely accentuated – or made more apparent – some of the longstanding issues and struggles of women in oncology, women in medicine, women in academia,” said Sarah Holstein, MD, PhD, another mid-career oncologist and associate professor at the University of Nebraska Medical Center, Omaha.
“There are disparities in first-author/last-author publications, disparities in being asked to give speaking engagements, disparities in leadership,” Dr. Holstein said in an interview. “And then ... put on top of that the various surges with the pandemic where you are being asked to do clinical responsibilities you don’t normally do, perhaps some things you haven’t done since your training 10 or 20 years ago.”
This is backed up with data: There is already a “robust” body of prepandemic literature demonstrating pay gaps for female physicians and scientists, noted Dr. Silver, who founded the Her Time Is Now campaign for gender equity in medicine and runs a women’s leadership course at Harvard.
In addition, female physicians are more likely to be involved in “nonpromotable” work, group projects and educator roles that are often underappreciated and undercompensated, she said.
Writing recently in a blog post for the BMJ, Dr. Silver and colleagues predict that as a result of the pandemic, female physicians will “face disadvantages from unconscious bias in decisions about whose pay should be cut, whose operating schedules should take priority when resources are limited, and whose contributions merit retention ... The ground that women lose now will likely have a profound effect for many years to come, perhaps putting them at a disadvantage for the rest of their careers.”
There is already evidence of reduced publishing by female scientists during the pandemic, something that “could undermine the careers of an entire generation of women scholars,” noted Caitlyn Collins, PhD, assistant professor of sociology at Washington University in St. Louis.
“Science needs to address the culture of overwork,” Dr. Collins said in an interview. “Parents and other caregivers deserve support. The stress and ‘overwhelm’ they feel is not inevitable. A more fair, just, and humane approach to combining work and family is possible – what we need is the political will to pass better policies and a massive shift in our cultural understandings about how work should fit into family life, not the other way around.”
Lack of support for “vulnerable scientists,” particularly “junior scientists who are parents, women, or minorities” could lead to “severe attrition in cancer research in the coming years,” Cullen Taniguchi, MD, PhD, a radiation oncologist and associate professor at the University of Texas MD Anderson Cancer Center, Houston, and colleagues, warned in a recent letter to the journal Cancer Cell.
“The biggest worries of attrition will come from young faculty who started just before or after the pandemic,” Dr. Taniguchi said in an interview. “The first year in an academic setting is incredibly challenging but also important for establishing research efforts and building networks of colleagues to collaborate with. While completely necessary, the restrictions put in place during the pandemic made doing these things even more difficult.”
Another stressor: Caregiving at home
Another reason female physicians may be marginalized during the pandemic is that they are more often the primary caregivers at home.
“Anyone who is a caregiver, be it to kids, parents, or spouses, can relate to the challenges brought [on] by the pandemic,” said Ishwaria Subbiah, MD, a palliative care physician and medical oncologist at MD Anderson.
“Most of us work toward meeting our responsibilities by engaging a network of support, whether it’s home care workers, center-based or at-home child care, schools, or activities outside of school. The pandemic led to a level of disruption that brought most (if not all) of those responsibilities onto the caregivers themselves,” she said in an interview.
As the mother of an adult son with severe epilepsy, Dr. Bertagnolli has certainly experienced the challenges of parenting during the pandemic. “Our son is now 24 but he is handicapped, and lives with us. The care issues we have to deal with as professionals have been enormously magnified by COVID,” she said.
But she cautions against making gender distinctions when it comes to caregiving. “Has it fallen on the women? Well, this kind of stuff generally falls on the women, but I am certain it has fallen on an awful lot of men as well, because I think the world is changing that way, so it’s fallen on all of us.”
There is no question that female oncologists are bearing the brunt, both at work and at home, contended Dr. Taniguchi. “Absolutely. I have seen this first-hand,” he said.
“If it was difficult for women, underrepresented minorities, and junior faculty to find a voice in the room prepandemic, I think it can be harder in the times of virtual meetings when it is difficult to engage audiences,” he said.
Dr. Holstein said she is lucky to be well-supported at her institution, with both a female chief of hematology/oncology and a female chair of internal medicine, but still, she worries about the long-term consequences of the pandemic on the gender landscape of medicine.
“If you’re having to put aside research projects because you have extra responsibilities – again because women just tend to have a lot of other things going on – that might not be a big deal for 3 months, 6 months, but this is going to be a year or 2 years before ‘normal’ comes back,” she says. “One to two years of underpublishing or not getting the grants could be career killers for women in academic oncology.”
Cancer COVID-19 combo
As Dr. Wildes completed her final weeks of seeing cancer patients, she received an outpouring of support, which she says convinced her of the shared experience of all doctors, and especially female doctors, during the pandemic. But even more specifically, she feels that she has tapped into the unique burden shouldered by oncologists during this time.
“It’s intimidating being an oncologist; we are literally giving people poison for a living. Then throw into it a pandemic where early in March we had so little data. I was helping my patients make decisions about their cancer care based on a case series of four patients in China. The burden of those conversations is something I never want to have to live through again,” she said.
“Oncology is a particularly intense subspecialty within medicine,” agreed Dr. Subbiah. “The people we care for have received a life-altering and potentially life-limiting diagnosis. Coupled with that, the COVID-19 pandemic has brought an unprecedented cloud of uncertainty ... Whether the patients can see it overtly or not, oncologists carry the weight of this worry with them for not just one but all of their patients.”
Dr. Wildes said she plans to return to academic medicine and clinical care “in time,” but for now, the gap that she and others like her leave is troubling to those who have stayed on.
“We need these women in medicine,” said Dr. Holstein. “We have data suggesting that women take more time with their patients than men, that patient outcomes are better if they have a female physician. But also for the generations coming up, we need the mid-career and senior women to be in place to mentor and guide and make sure we continue to increase women in leadership.”
A version of this article originally appeared on Medscape.com.
Patient health suffers amid pandemic health care shortages
More than half (56%) of responding clinicians reported seeing a decline in patient health because of delayed or inaccessible care amid the pandemic, according to the results of the latest survey by the Larry A. Green Center and the Primary Care Collaborative. The survey was conducted in mid-October and the results were published online Nov. 17.
In addition, 37% of respondents said their patients with chronic conditions showed “noticeably worse health resulting from the pandemic.” And a resounding 85% said patient mental health had worsened.
“I think it’s worse than we thought,” said Rebecca Etz, PhD, codirector of the Larry Green Center. “It’s the outcome of not sufficiently sending resources to primary care either before or during the pandemic.” According to Dr. Etz, survey respondents noted substantial increases in patient weight gain as well as weight loss, anxiety and depression, sleep issues, domestic abuse, and poor oral and eye health, among others.
One clinician from Pennsylvania wrote: “Patients are becoming sicker during the pandemic. I’m seeing more uncontrolled [diabetes]and new [patients with diabetes]. They prefer telehealth yet [have] no access to glucose monitoring or a blood pressure cuff. I am concerned about patients’ isolation and mental health. People are delaying care.”
Now, with COVID numbers peaking across much of the country, many clinicians are trying to close the gap in care with telehealth – something they’re more prepared to do now than they were in March. Over two-thirds of practices are using telehealth for visits to keep up with patients who have stable chronic conditions, according to the survey.
Over 60% of physicians report using telehealth for mental health visits. But a much smaller number – only 16% of respondents – said their practice had added staff to help manage the rising number of behavioral and mental health cases. About one-third (35%) of practices say they’re not financially able to take on new staff.
“We’ve been looking for more ways for patients to do self-support. A big part of chronic disease is health behaviors,” Alex Krist, MD, MPH, a family doctor in Fairfax, Va., and chairperson of the U.S. Preventive Services Task Force, said in an interview. And unfortunately, on top of limited access to basic care, healthy habits that are essential to managing many chronic conditions have become more difficult and less consistent during the pandemic.
The survey – the 22nd iteration in a series of surveys the Green Center and the Primary Care Collaborative have conducted – received 580 respondents from 47 states and Guam. Over two-thirds of respondents were primary care physicians (MDs and DOs). Over half were owners, partners, or employees of a private practice, 66% of which were family medicine practices. And one fifth of respondents provided care in a rural area.
Funding and support for primary care has been wildly insufficient, Dr. Etz said in an interview. If that doesn’t change, patient health, clinic staffing, and public health strategies amid the pandemic will continue to suffer.
“When you think of the COVID vaccine, who do you think is going to be sending that out?” Dr. Etz asked. “If we don’t bolster primary care now how are they going to handle that.”
A version of this article originally appeared on Medscape.com.
More than half (56%) of responding clinicians reported seeing a decline in patient health because of delayed or inaccessible care amid the pandemic, according to the results of the latest survey by the Larry A. Green Center and the Primary Care Collaborative. The survey was conducted in mid-October and the results were published online Nov. 17.
In addition, 37% of respondents said their patients with chronic conditions showed “noticeably worse health resulting from the pandemic.” And a resounding 85% said patient mental health had worsened.
“I think it’s worse than we thought,” said Rebecca Etz, PhD, codirector of the Larry Green Center. “It’s the outcome of not sufficiently sending resources to primary care either before or during the pandemic.” According to Dr. Etz, survey respondents noted substantial increases in patient weight gain as well as weight loss, anxiety and depression, sleep issues, domestic abuse, and poor oral and eye health, among others.
One clinician from Pennsylvania wrote: “Patients are becoming sicker during the pandemic. I’m seeing more uncontrolled [diabetes]and new [patients with diabetes]. They prefer telehealth yet [have] no access to glucose monitoring or a blood pressure cuff. I am concerned about patients’ isolation and mental health. People are delaying care.”
Now, with COVID numbers peaking across much of the country, many clinicians are trying to close the gap in care with telehealth – something they’re more prepared to do now than they were in March. Over two-thirds of practices are using telehealth for visits to keep up with patients who have stable chronic conditions, according to the survey.
Over 60% of physicians report using telehealth for mental health visits. But a much smaller number – only 16% of respondents – said their practice had added staff to help manage the rising number of behavioral and mental health cases. About one-third (35%) of practices say they’re not financially able to take on new staff.
“We’ve been looking for more ways for patients to do self-support. A big part of chronic disease is health behaviors,” Alex Krist, MD, MPH, a family doctor in Fairfax, Va., and chairperson of the U.S. Preventive Services Task Force, said in an interview. And unfortunately, on top of limited access to basic care, healthy habits that are essential to managing many chronic conditions have become more difficult and less consistent during the pandemic.
The survey – the 22nd iteration in a series of surveys the Green Center and the Primary Care Collaborative have conducted – received 580 respondents from 47 states and Guam. Over two-thirds of respondents were primary care physicians (MDs and DOs). Over half were owners, partners, or employees of a private practice, 66% of which were family medicine practices. And one fifth of respondents provided care in a rural area.
Funding and support for primary care has been wildly insufficient, Dr. Etz said in an interview. If that doesn’t change, patient health, clinic staffing, and public health strategies amid the pandemic will continue to suffer.
“When you think of the COVID vaccine, who do you think is going to be sending that out?” Dr. Etz asked. “If we don’t bolster primary care now how are they going to handle that.”
A version of this article originally appeared on Medscape.com.
More than half (56%) of responding clinicians reported seeing a decline in patient health because of delayed or inaccessible care amid the pandemic, according to the results of the latest survey by the Larry A. Green Center and the Primary Care Collaborative. The survey was conducted in mid-October and the results were published online Nov. 17.
In addition, 37% of respondents said their patients with chronic conditions showed “noticeably worse health resulting from the pandemic.” And a resounding 85% said patient mental health had worsened.
“I think it’s worse than we thought,” said Rebecca Etz, PhD, codirector of the Larry Green Center. “It’s the outcome of not sufficiently sending resources to primary care either before or during the pandemic.” According to Dr. Etz, survey respondents noted substantial increases in patient weight gain as well as weight loss, anxiety and depression, sleep issues, domestic abuse, and poor oral and eye health, among others.
One clinician from Pennsylvania wrote: “Patients are becoming sicker during the pandemic. I’m seeing more uncontrolled [diabetes]and new [patients with diabetes]. They prefer telehealth yet [have] no access to glucose monitoring or a blood pressure cuff. I am concerned about patients’ isolation and mental health. People are delaying care.”
Now, with COVID numbers peaking across much of the country, many clinicians are trying to close the gap in care with telehealth – something they’re more prepared to do now than they were in March. Over two-thirds of practices are using telehealth for visits to keep up with patients who have stable chronic conditions, according to the survey.
Over 60% of physicians report using telehealth for mental health visits. But a much smaller number – only 16% of respondents – said their practice had added staff to help manage the rising number of behavioral and mental health cases. About one-third (35%) of practices say they’re not financially able to take on new staff.
“We’ve been looking for more ways for patients to do self-support. A big part of chronic disease is health behaviors,” Alex Krist, MD, MPH, a family doctor in Fairfax, Va., and chairperson of the U.S. Preventive Services Task Force, said in an interview. And unfortunately, on top of limited access to basic care, healthy habits that are essential to managing many chronic conditions have become more difficult and less consistent during the pandemic.
The survey – the 22nd iteration in a series of surveys the Green Center and the Primary Care Collaborative have conducted – received 580 respondents from 47 states and Guam. Over two-thirds of respondents were primary care physicians (MDs and DOs). Over half were owners, partners, or employees of a private practice, 66% of which were family medicine practices. And one fifth of respondents provided care in a rural area.
Funding and support for primary care has been wildly insufficient, Dr. Etz said in an interview. If that doesn’t change, patient health, clinic staffing, and public health strategies amid the pandemic will continue to suffer.
“When you think of the COVID vaccine, who do you think is going to be sending that out?” Dr. Etz asked. “If we don’t bolster primary care now how are they going to handle that.”
A version of this article originally appeared on Medscape.com.