User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Seeking new vaccines against whooping cough: The PERISCOPE project
Although there is an effective vaccine against Bordetella pertussis, whooping cough remains a leading cause of death. Cases are increasing, and scientists face challenges in developing new vaccines.
In a key research session at the start of the annual meeting of the European Society for Paediatric Infectious Diseases, held virtually this year, Dimitri Diavatopoulos, PhD, associate professor at the Radboud University Medical Centre Nijmegen, the Netherlands, summarized the pertussis vaccination problem and what the Pertussis Correlates of Protection Europe (PERISCOPE) project seeks to achieve. Dr. Diavatopoulos has a longstanding interest in pertussis and immunity and will soon take over as the scientific coordinator of PERISCOPE.
Pertussis is a highly contagious infectious disease that causes uncontrollable coughing. The disease begins with an atypical cough and rhinorrhea before entering a paroxysmal stage characterized by cyanosis, lymphocytosis, vomiting, and whoops. Generally, fever is absent and coughing increases at night. Finally, after weeks to months, the patient enters a convalescent stage. The World Health Organization estimates that there are 16 million pertussis cases annually and approximately 195,000 deaths in children. Most cases are caused by Bordetella pertussis and are preventable by vaccination.
In the United States, following the introduction of a national immunization program using a whole-cell vaccine in the 1950s, cases fell significantly. After a lag phase, the adoption of an acellular vaccine in the United States in 1997 and the Netherlands in 2005 – usually in combination with diphtheria and tetanus via DTaP – saw an increase in case numbers. Dr. Diavatopoulos stated that control is no longer as good, compared with other infectious diseases prevented by the MMR vaccine, such as mumps, measles, and rubella.
In the face of increasing numbers, how do we move to the next generation of vaccines to improve control? There are several barriers to licensure, including the following:
• Universal recommendation for pertussis prevention means that more than 90% of the population will have received DTaP (usually in combination with polio and Haemophilus influenzae B) and be protected for several years after vaccination.
• Because DTaP vaccines are only efficacious for a limited time, the problem is not immediately apparent.
• Pertussis epidemics are cyclical, occurring every 3-5 years. These peaks and troughs complicate the development of epidemiological studies.
What this means is that large-scale Phase III efficacy studies, in which disease is used as the endpoint, are not feasible. Also, formal correlates of protection have not been identified.
The PERISCOPE Project started in March 2016 and is designed to respond to some of these issues. Funding is made available by a public private consortium involving the Bill & Melinda Gates foundation, the European Union, and European Federation of Pharmaceutical Industries and Associations (EFPIA) partners, and in this case, GlaxoSmithKline and Sanofi Pasteur. In total, there are 22 partners in this project.
The strategic objectives of this partnership include the following:
• Foster expertise and increase capacity in Europe to evaluate new pertussis vaccines both in clinical and preclinical models.
• Identify early biomarkers of long-lasting protective immunity to pertussis in humans. (This step will accelerate and de-risk clinical development of next generation pertussis vaccines.)
• Investigate the impact of maternal vaccination on infant response to pertussis vaccination.
The problem is that there is no one single study design that addresses all questions about the pertussis vaccine. For example, in PERISCOPE, the results of preclinical studies using the baboon or mouse models and addressing disease and colonization endpoints or immunogenicity do not perfectly model human infection and disease.
By comparison, controlled human infection studies provide information on colonization but not disease endpoints. Such studies, however, do provide information on immunogenicity endpoints. Also available are booster vaccination studies and infant vaccination studies providing data on immunogenicity, as well as safety information.
Finally, there are patient studies, such as household contact studies where immunogenicity can be correlated to disease endpoints. From these studies, it will be seen that what is needed is integration of evidence from clinical and preclinical studies to support a new vaccine registration.
PERISCOPE addresses these issues by developing novel, functional antibody and cellular assays and employing cutting-edge methods to characterize innate immune responses and cell-mediated systemic and mucosal immunity. PERISCOPE combines two major industrial partners with public researchers from academic and public health institutes and small and medium-sized enterprises with expertise in clinical trials, vaccinology, immunology, molecular microbiology, challenge models, and bioinformatics.
Andrew Gorringe, PhD, from Public Health England and the Research and Development Institute at Porton Down, Wiltshire, England, said, “Vaccines have greatly reduced the incidence of pertussis, but it remains the most prevalent ‘vaccine preventable’ disease. This is an exciting period for pertussis vaccine research as we find new ways to understand the immunity that protects from both infection and disease. The PERISCOPE project provides a collaborative environment that combines expertise across Europe to provide a route to the development of new, more effective vaccines.”
GSK and Sanofi Pasteur have cofunded the PERISCOPE Project. Dr. Diavatopoulos made no other financial disclosures.
Although there is an effective vaccine against Bordetella pertussis, whooping cough remains a leading cause of death. Cases are increasing, and scientists face challenges in developing new vaccines.
In a key research session at the start of the annual meeting of the European Society for Paediatric Infectious Diseases, held virtually this year, Dimitri Diavatopoulos, PhD, associate professor at the Radboud University Medical Centre Nijmegen, the Netherlands, summarized the pertussis vaccination problem and what the Pertussis Correlates of Protection Europe (PERISCOPE) project seeks to achieve. Dr. Diavatopoulos has a longstanding interest in pertussis and immunity and will soon take over as the scientific coordinator of PERISCOPE.
Pertussis is a highly contagious infectious disease that causes uncontrollable coughing. The disease begins with an atypical cough and rhinorrhea before entering a paroxysmal stage characterized by cyanosis, lymphocytosis, vomiting, and whoops. Generally, fever is absent and coughing increases at night. Finally, after weeks to months, the patient enters a convalescent stage. The World Health Organization estimates that there are 16 million pertussis cases annually and approximately 195,000 deaths in children. Most cases are caused by Bordetella pertussis and are preventable by vaccination.
In the United States, following the introduction of a national immunization program using a whole-cell vaccine in the 1950s, cases fell significantly. After a lag phase, the adoption of an acellular vaccine in the United States in 1997 and the Netherlands in 2005 – usually in combination with diphtheria and tetanus via DTaP – saw an increase in case numbers. Dr. Diavatopoulos stated that control is no longer as good, compared with other infectious diseases prevented by the MMR vaccine, such as mumps, measles, and rubella.
In the face of increasing numbers, how do we move to the next generation of vaccines to improve control? There are several barriers to licensure, including the following:
• Universal recommendation for pertussis prevention means that more than 90% of the population will have received DTaP (usually in combination with polio and Haemophilus influenzae B) and be protected for several years after vaccination.
• Because DTaP vaccines are only efficacious for a limited time, the problem is not immediately apparent.
• Pertussis epidemics are cyclical, occurring every 3-5 years. These peaks and troughs complicate the development of epidemiological studies.
What this means is that large-scale Phase III efficacy studies, in which disease is used as the endpoint, are not feasible. Also, formal correlates of protection have not been identified.
The PERISCOPE Project started in March 2016 and is designed to respond to some of these issues. Funding is made available by a public private consortium involving the Bill & Melinda Gates foundation, the European Union, and European Federation of Pharmaceutical Industries and Associations (EFPIA) partners, and in this case, GlaxoSmithKline and Sanofi Pasteur. In total, there are 22 partners in this project.
The strategic objectives of this partnership include the following:
• Foster expertise and increase capacity in Europe to evaluate new pertussis vaccines both in clinical and preclinical models.
• Identify early biomarkers of long-lasting protective immunity to pertussis in humans. (This step will accelerate and de-risk clinical development of next generation pertussis vaccines.)
• Investigate the impact of maternal vaccination on infant response to pertussis vaccination.
The problem is that there is no one single study design that addresses all questions about the pertussis vaccine. For example, in PERISCOPE, the results of preclinical studies using the baboon or mouse models and addressing disease and colonization endpoints or immunogenicity do not perfectly model human infection and disease.
By comparison, controlled human infection studies provide information on colonization but not disease endpoints. Such studies, however, do provide information on immunogenicity endpoints. Also available are booster vaccination studies and infant vaccination studies providing data on immunogenicity, as well as safety information.
Finally, there are patient studies, such as household contact studies where immunogenicity can be correlated to disease endpoints. From these studies, it will be seen that what is needed is integration of evidence from clinical and preclinical studies to support a new vaccine registration.
PERISCOPE addresses these issues by developing novel, functional antibody and cellular assays and employing cutting-edge methods to characterize innate immune responses and cell-mediated systemic and mucosal immunity. PERISCOPE combines two major industrial partners with public researchers from academic and public health institutes and small and medium-sized enterprises with expertise in clinical trials, vaccinology, immunology, molecular microbiology, challenge models, and bioinformatics.
Andrew Gorringe, PhD, from Public Health England and the Research and Development Institute at Porton Down, Wiltshire, England, said, “Vaccines have greatly reduced the incidence of pertussis, but it remains the most prevalent ‘vaccine preventable’ disease. This is an exciting period for pertussis vaccine research as we find new ways to understand the immunity that protects from both infection and disease. The PERISCOPE project provides a collaborative environment that combines expertise across Europe to provide a route to the development of new, more effective vaccines.”
GSK and Sanofi Pasteur have cofunded the PERISCOPE Project. Dr. Diavatopoulos made no other financial disclosures.
Although there is an effective vaccine against Bordetella pertussis, whooping cough remains a leading cause of death. Cases are increasing, and scientists face challenges in developing new vaccines.
In a key research session at the start of the annual meeting of the European Society for Paediatric Infectious Diseases, held virtually this year, Dimitri Diavatopoulos, PhD, associate professor at the Radboud University Medical Centre Nijmegen, the Netherlands, summarized the pertussis vaccination problem and what the Pertussis Correlates of Protection Europe (PERISCOPE) project seeks to achieve. Dr. Diavatopoulos has a longstanding interest in pertussis and immunity and will soon take over as the scientific coordinator of PERISCOPE.
Pertussis is a highly contagious infectious disease that causes uncontrollable coughing. The disease begins with an atypical cough and rhinorrhea before entering a paroxysmal stage characterized by cyanosis, lymphocytosis, vomiting, and whoops. Generally, fever is absent and coughing increases at night. Finally, after weeks to months, the patient enters a convalescent stage. The World Health Organization estimates that there are 16 million pertussis cases annually and approximately 195,000 deaths in children. Most cases are caused by Bordetella pertussis and are preventable by vaccination.
In the United States, following the introduction of a national immunization program using a whole-cell vaccine in the 1950s, cases fell significantly. After a lag phase, the adoption of an acellular vaccine in the United States in 1997 and the Netherlands in 2005 – usually in combination with diphtheria and tetanus via DTaP – saw an increase in case numbers. Dr. Diavatopoulos stated that control is no longer as good, compared with other infectious diseases prevented by the MMR vaccine, such as mumps, measles, and rubella.
In the face of increasing numbers, how do we move to the next generation of vaccines to improve control? There are several barriers to licensure, including the following:
• Universal recommendation for pertussis prevention means that more than 90% of the population will have received DTaP (usually in combination with polio and Haemophilus influenzae B) and be protected for several years after vaccination.
• Because DTaP vaccines are only efficacious for a limited time, the problem is not immediately apparent.
• Pertussis epidemics are cyclical, occurring every 3-5 years. These peaks and troughs complicate the development of epidemiological studies.
What this means is that large-scale Phase III efficacy studies, in which disease is used as the endpoint, are not feasible. Also, formal correlates of protection have not been identified.
The PERISCOPE Project started in March 2016 and is designed to respond to some of these issues. Funding is made available by a public private consortium involving the Bill & Melinda Gates foundation, the European Union, and European Federation of Pharmaceutical Industries and Associations (EFPIA) partners, and in this case, GlaxoSmithKline and Sanofi Pasteur. In total, there are 22 partners in this project.
The strategic objectives of this partnership include the following:
• Foster expertise and increase capacity in Europe to evaluate new pertussis vaccines both in clinical and preclinical models.
• Identify early biomarkers of long-lasting protective immunity to pertussis in humans. (This step will accelerate and de-risk clinical development of next generation pertussis vaccines.)
• Investigate the impact of maternal vaccination on infant response to pertussis vaccination.
The problem is that there is no one single study design that addresses all questions about the pertussis vaccine. For example, in PERISCOPE, the results of preclinical studies using the baboon or mouse models and addressing disease and colonization endpoints or immunogenicity do not perfectly model human infection and disease.
By comparison, controlled human infection studies provide information on colonization but not disease endpoints. Such studies, however, do provide information on immunogenicity endpoints. Also available are booster vaccination studies and infant vaccination studies providing data on immunogenicity, as well as safety information.
Finally, there are patient studies, such as household contact studies where immunogenicity can be correlated to disease endpoints. From these studies, it will be seen that what is needed is integration of evidence from clinical and preclinical studies to support a new vaccine registration.
PERISCOPE addresses these issues by developing novel, functional antibody and cellular assays and employing cutting-edge methods to characterize innate immune responses and cell-mediated systemic and mucosal immunity. PERISCOPE combines two major industrial partners with public researchers from academic and public health institutes and small and medium-sized enterprises with expertise in clinical trials, vaccinology, immunology, molecular microbiology, challenge models, and bioinformatics.
Andrew Gorringe, PhD, from Public Health England and the Research and Development Institute at Porton Down, Wiltshire, England, said, “Vaccines have greatly reduced the incidence of pertussis, but it remains the most prevalent ‘vaccine preventable’ disease. This is an exciting period for pertussis vaccine research as we find new ways to understand the immunity that protects from both infection and disease. The PERISCOPE project provides a collaborative environment that combines expertise across Europe to provide a route to the development of new, more effective vaccines.”
GSK and Sanofi Pasteur have cofunded the PERISCOPE Project. Dr. Diavatopoulos made no other financial disclosures.
FROM ESPID 2020
Moderna’s COVID-19 vaccine deemed ‘highly effective,’ but further studies needed
The Food and Drug Administration’s Vaccines and Related Biological Products Advisory Committee (VRBPAC) evaluated
The panel acknowledged that further studies will be required post issuance of an Emergency Use Authorization (EUA) to collect additional data on the safety and effectiveness of the vaccine. A briefing document released by the FDA on Dec. 17, 2020, summarized interim results and included recommendations from VRBPAC on use of Moderna’s mRNA-1273 COVID-19 vaccine.
“On November 30, 2020, ModernaTX (the Sponsor) submitted an EUA request to FDA for an investigational COVID-19 vaccine (mRNA-1273) intended to prevent COVID-19,” the committee wrote.
The mRNA-1273 vaccine trial
Among 30,351 individuals aged 18 years and older, the efficacy, safety, and immunogenicity of the mRNA-1273 vaccine candidate was evaluated in a randomized, stratified, observer-blind, placebo-controlled phase 3 study. Participants were randomly assigned (1:1) to receive two injections of either 100 mcg of mRNA-1273 (n = 15,181) or saline placebo (n = 15,170) administered intramuscularly on day 1 and day 29.
The primary efficacy endpoint was efficacy of mRNA-1273 against PCR-confirmed COVID-19 with onset at least 14 days following the second dose. The primary safety endpoint was to characterize the safety of the vaccine following one or two doses.
Efficacy
Among 27,817 subjects included in the first interim analysis (data cutoff: Nov. 7, 2020), 5 cases of COVID-19 with onset at least 14 days after the second dose occurred among vaccine recipients and 90 case occurred among placebo recipients, corresponding to 94.5% vaccine efficacy (95% confidence interval, 86.5%-97.8%).
“Subgroup analyses of the primary efficacy endpoint showed similar efficacy point estimates across age groups, genders, racial and ethnic groups, and participants with medical comorbidities associated with high risk of severe COVID-19,” they reported.
Data from the final scheduled analysis of the primary efficacy endpoint (data cutoff: Nov. 21, 2020; median follow-up of >2 months after dose 2), demonstrated 94.1% vaccine efficacy (95% confidence interval, 89.3%-96.8%), corresponding to 11 cases of COVID-19 in the vaccine group and 185 cases in the placebo group.
When stratified by age, the vaccine efficacy was 95.6% (95% CI, 90.6%-97.9%) for individuals 18-64 years of age and 86.4% (95% CI, 61.4%-95.5%) for those 65 years of age or older.
In addition, results from secondary analyses indicated benefit for mRNA-1273 in preventing severe COVID-19 cases, COVID-19 in those with prior SARS-CoV-2 infection, and infection after the first dose, but these data were not conclusive.
Safety
Among 30,350 subjects included in the first interim analysis (data cutoff: Nov. 11, 2020; median follow-up of 7 weeks post second dose), no specific safety concerns were observed that would prevent issuance of an EUA.
Additional safety data (data cutoff: Nov. 25, 2020; median follow-up of 9 weeks post second dose) were provided on Dec. 7, 2020, but did not change the conclusions from the first interim analysis.
The most common vaccine-related adverse reactions were injection site pain (91.6%), fatigue (68.5%), headache (63.0%), muscle pain (59.6%), joint pain (44.8%), and chills (43.4%).
“The frequency of serious adverse events (SAEs) was low (1.0% in the mRNA-1273 arm and 1.0% in the placebo arm), without meaningful imbalances between study arms,” they reported.
Myocardial infarction (0.03%), nephrolithiasis (0.02%), and cholecystitis (0.02%) were the most common SAEs that were numerically greater in the vaccine arm than the placebo arm; however, the small number of cases does not infer a casual relationship.
“The 2-dose vaccination regimen was highly effective in preventing PCR-confirmed COVID-19 occurring at least 14 days after receipt of the second dose,” the committee wrote. “[However], it is critical to continue to gather data about the vaccine even after it is made available under EUA.”
The associated phase 3 study was sponsored by ModernaTX.
SOURCE: FDA Briefing Document: Moderna COVID-19 Vaccine. FDA Vaccines and Related Biological Products Advisory Committee. Published Dec. 17, 2020.
The Food and Drug Administration’s Vaccines and Related Biological Products Advisory Committee (VRBPAC) evaluated
The panel acknowledged that further studies will be required post issuance of an Emergency Use Authorization (EUA) to collect additional data on the safety and effectiveness of the vaccine. A briefing document released by the FDA on Dec. 17, 2020, summarized interim results and included recommendations from VRBPAC on use of Moderna’s mRNA-1273 COVID-19 vaccine.
“On November 30, 2020, ModernaTX (the Sponsor) submitted an EUA request to FDA for an investigational COVID-19 vaccine (mRNA-1273) intended to prevent COVID-19,” the committee wrote.
The mRNA-1273 vaccine trial
Among 30,351 individuals aged 18 years and older, the efficacy, safety, and immunogenicity of the mRNA-1273 vaccine candidate was evaluated in a randomized, stratified, observer-blind, placebo-controlled phase 3 study. Participants were randomly assigned (1:1) to receive two injections of either 100 mcg of mRNA-1273 (n = 15,181) or saline placebo (n = 15,170) administered intramuscularly on day 1 and day 29.
The primary efficacy endpoint was efficacy of mRNA-1273 against PCR-confirmed COVID-19 with onset at least 14 days following the second dose. The primary safety endpoint was to characterize the safety of the vaccine following one or two doses.
Efficacy
Among 27,817 subjects included in the first interim analysis (data cutoff: Nov. 7, 2020), 5 cases of COVID-19 with onset at least 14 days after the second dose occurred among vaccine recipients and 90 case occurred among placebo recipients, corresponding to 94.5% vaccine efficacy (95% confidence interval, 86.5%-97.8%).
“Subgroup analyses of the primary efficacy endpoint showed similar efficacy point estimates across age groups, genders, racial and ethnic groups, and participants with medical comorbidities associated with high risk of severe COVID-19,” they reported.
Data from the final scheduled analysis of the primary efficacy endpoint (data cutoff: Nov. 21, 2020; median follow-up of >2 months after dose 2), demonstrated 94.1% vaccine efficacy (95% confidence interval, 89.3%-96.8%), corresponding to 11 cases of COVID-19 in the vaccine group and 185 cases in the placebo group.
When stratified by age, the vaccine efficacy was 95.6% (95% CI, 90.6%-97.9%) for individuals 18-64 years of age and 86.4% (95% CI, 61.4%-95.5%) for those 65 years of age or older.
In addition, results from secondary analyses indicated benefit for mRNA-1273 in preventing severe COVID-19 cases, COVID-19 in those with prior SARS-CoV-2 infection, and infection after the first dose, but these data were not conclusive.
Safety
Among 30,350 subjects included in the first interim analysis (data cutoff: Nov. 11, 2020; median follow-up of 7 weeks post second dose), no specific safety concerns were observed that would prevent issuance of an EUA.
Additional safety data (data cutoff: Nov. 25, 2020; median follow-up of 9 weeks post second dose) were provided on Dec. 7, 2020, but did not change the conclusions from the first interim analysis.
The most common vaccine-related adverse reactions were injection site pain (91.6%), fatigue (68.5%), headache (63.0%), muscle pain (59.6%), joint pain (44.8%), and chills (43.4%).
“The frequency of serious adverse events (SAEs) was low (1.0% in the mRNA-1273 arm and 1.0% in the placebo arm), without meaningful imbalances between study arms,” they reported.
Myocardial infarction (0.03%), nephrolithiasis (0.02%), and cholecystitis (0.02%) were the most common SAEs that were numerically greater in the vaccine arm than the placebo arm; however, the small number of cases does not infer a casual relationship.
“The 2-dose vaccination regimen was highly effective in preventing PCR-confirmed COVID-19 occurring at least 14 days after receipt of the second dose,” the committee wrote. “[However], it is critical to continue to gather data about the vaccine even after it is made available under EUA.”
The associated phase 3 study was sponsored by ModernaTX.
SOURCE: FDA Briefing Document: Moderna COVID-19 Vaccine. FDA Vaccines and Related Biological Products Advisory Committee. Published Dec. 17, 2020.
The Food and Drug Administration’s Vaccines and Related Biological Products Advisory Committee (VRBPAC) evaluated
The panel acknowledged that further studies will be required post issuance of an Emergency Use Authorization (EUA) to collect additional data on the safety and effectiveness of the vaccine. A briefing document released by the FDA on Dec. 17, 2020, summarized interim results and included recommendations from VRBPAC on use of Moderna’s mRNA-1273 COVID-19 vaccine.
“On November 30, 2020, ModernaTX (the Sponsor) submitted an EUA request to FDA for an investigational COVID-19 vaccine (mRNA-1273) intended to prevent COVID-19,” the committee wrote.
The mRNA-1273 vaccine trial
Among 30,351 individuals aged 18 years and older, the efficacy, safety, and immunogenicity of the mRNA-1273 vaccine candidate was evaluated in a randomized, stratified, observer-blind, placebo-controlled phase 3 study. Participants were randomly assigned (1:1) to receive two injections of either 100 mcg of mRNA-1273 (n = 15,181) or saline placebo (n = 15,170) administered intramuscularly on day 1 and day 29.
The primary efficacy endpoint was efficacy of mRNA-1273 against PCR-confirmed COVID-19 with onset at least 14 days following the second dose. The primary safety endpoint was to characterize the safety of the vaccine following one or two doses.
Efficacy
Among 27,817 subjects included in the first interim analysis (data cutoff: Nov. 7, 2020), 5 cases of COVID-19 with onset at least 14 days after the second dose occurred among vaccine recipients and 90 case occurred among placebo recipients, corresponding to 94.5% vaccine efficacy (95% confidence interval, 86.5%-97.8%).
“Subgroup analyses of the primary efficacy endpoint showed similar efficacy point estimates across age groups, genders, racial and ethnic groups, and participants with medical comorbidities associated with high risk of severe COVID-19,” they reported.
Data from the final scheduled analysis of the primary efficacy endpoint (data cutoff: Nov. 21, 2020; median follow-up of >2 months after dose 2), demonstrated 94.1% vaccine efficacy (95% confidence interval, 89.3%-96.8%), corresponding to 11 cases of COVID-19 in the vaccine group and 185 cases in the placebo group.
When stratified by age, the vaccine efficacy was 95.6% (95% CI, 90.6%-97.9%) for individuals 18-64 years of age and 86.4% (95% CI, 61.4%-95.5%) for those 65 years of age or older.
In addition, results from secondary analyses indicated benefit for mRNA-1273 in preventing severe COVID-19 cases, COVID-19 in those with prior SARS-CoV-2 infection, and infection after the first dose, but these data were not conclusive.
Safety
Among 30,350 subjects included in the first interim analysis (data cutoff: Nov. 11, 2020; median follow-up of 7 weeks post second dose), no specific safety concerns were observed that would prevent issuance of an EUA.
Additional safety data (data cutoff: Nov. 25, 2020; median follow-up of 9 weeks post second dose) were provided on Dec. 7, 2020, but did not change the conclusions from the first interim analysis.
The most common vaccine-related adverse reactions were injection site pain (91.6%), fatigue (68.5%), headache (63.0%), muscle pain (59.6%), joint pain (44.8%), and chills (43.4%).
“The frequency of serious adverse events (SAEs) was low (1.0% in the mRNA-1273 arm and 1.0% in the placebo arm), without meaningful imbalances between study arms,” they reported.
Myocardial infarction (0.03%), nephrolithiasis (0.02%), and cholecystitis (0.02%) were the most common SAEs that were numerically greater in the vaccine arm than the placebo arm; however, the small number of cases does not infer a casual relationship.
“The 2-dose vaccination regimen was highly effective in preventing PCR-confirmed COVID-19 occurring at least 14 days after receipt of the second dose,” the committee wrote. “[However], it is critical to continue to gather data about the vaccine even after it is made available under EUA.”
The associated phase 3 study was sponsored by ModernaTX.
SOURCE: FDA Briefing Document: Moderna COVID-19 Vaccine. FDA Vaccines and Related Biological Products Advisory Committee. Published Dec. 17, 2020.
Key clinical point: The FDA’s Vaccines and Related Biological Products Advisory Committee regarded Moderna’s COVID-19 vaccine as highly effective with a favorable safety profile, based on interim phase 3 results.
Major finding: The two-dose vaccine regimen had a low frequency of serious adverse events (1.0% each in the mRNA-1273 and placebo arms, respectively) and demonstrated 94.1% (95% CI, 89.3%-96.8%) vaccine efficacy.
Study details: A briefing document summarized interim data and recommendations from the FDA’s VRBPAC on Moderna’s mRNA-1273 COVID-19 vaccine.
Disclosures: The associated phase 3 study was sponsored by ModernaTX.
Source: FDA Briefing Document: Moderna COVID-19 Vaccine. FDA Vaccines and Related Biological Products Advisory Committee. Published Dec. 17, 2020.
Letters from Maine: Role playing
It’s not unusual when I run into a former patient that I am addressed as “Doctor” Wilkoff. I guess that is to be expected because when I was in practice I seldom introduced myself as Will. However, I will admit now that I never quite felt comfortable with the “Doctor” label. Today, if you addressed me as “Doctor” I would correct you and refer to myself as the “ex-Doctor Wilkoff.”
The term doctor derived from the Latin word to teach and eventually morphed into an academic title. In common parlance it is sometimes used as verb meaning to treat, e.g., “he doctored the wound.” Regardless of what academic field we are talking about, the title “doctor” has become a term of respect for someone who has spent an unusually long time learning his or her subject or craft. The holder of a doctorate, particularly in medicine, receives a rank, earned or unearned, near the top of the social hierarchy.
When I look back at more than 50 years of doing pediatrics I’m not sure that “doctor” really captures what I was up to. I will grant you that it is nice that folks want to acknowledge all those years I spent in training. But I don’t think one could say that what I did as a primary care small town pediatrician fits in with the original definition “to teach.” I did spend a few hours teaching students every so often but my primary time was spent with patients and I don’t consider what I was doing with them as teaching. There just wasn’t enough time. I had to take as a given that families who came to see me already had a basic knowledge base either as the result of their schooling, family lore, or public service announcements from the American Academy of Pediatrics.
I certainly wasn’t doing much doctoring in the sense of treating or curing disease. If one removes administering immunizations and delivery room resuscitations, I saved very few lives.
So you may ask, if not as “doctor,” how would I prefer to be labeled? Good question, but easy for me to answer. The term “coach” quickly comes to mind. As someone who played a variety of team sports there is no term that I can think of that commands more respect than “Coach.” While the term doesn’t carry the burden of a particularly long educational journey it does imply the person is wise, observant, and experienced.
There is some teaching involved but primarily a coach is going to assess the players (or in this cases the families) he is presented with and then do the best he can to guide them toward good decisions they can make themselves given their specific situations. This requires spending most of one’s time getting to know each family and understanding their strengths and limitations. One can’t coach speed to an athlete who is slow footed. And, one isn’t going to get a family that is dominated by anxiety to become bold risk takers. The best you can do is to help them learn strategies to minimize their anxieties.
A good coach can help his players learn to set reasonable goals given their skill sets. And, a good pediatrician can coach his families how to adapt their strengths and weakness to the challenges of each of their children. For example, for a physician faced with a mother-infant dyad that is struggling with breastfeeding, once the education piece is in place, it is up to him or her to function as a coach and assist the team in setting a reasonable goal that can result in a win-win for the family.
A coach must be humble, putting his or her players’ needs first and flexible enough to adjust his or her goals to define success in terms for what is best for each individual team. “Coach” may not carry the ring of authority that can come with “Doctor” but it is a role equally as challenging and rewarding.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
It’s not unusual when I run into a former patient that I am addressed as “Doctor” Wilkoff. I guess that is to be expected because when I was in practice I seldom introduced myself as Will. However, I will admit now that I never quite felt comfortable with the “Doctor” label. Today, if you addressed me as “Doctor” I would correct you and refer to myself as the “ex-Doctor Wilkoff.”
The term doctor derived from the Latin word to teach and eventually morphed into an academic title. In common parlance it is sometimes used as verb meaning to treat, e.g., “he doctored the wound.” Regardless of what academic field we are talking about, the title “doctor” has become a term of respect for someone who has spent an unusually long time learning his or her subject or craft. The holder of a doctorate, particularly in medicine, receives a rank, earned or unearned, near the top of the social hierarchy.
When I look back at more than 50 years of doing pediatrics I’m not sure that “doctor” really captures what I was up to. I will grant you that it is nice that folks want to acknowledge all those years I spent in training. But I don’t think one could say that what I did as a primary care small town pediatrician fits in with the original definition “to teach.” I did spend a few hours teaching students every so often but my primary time was spent with patients and I don’t consider what I was doing with them as teaching. There just wasn’t enough time. I had to take as a given that families who came to see me already had a basic knowledge base either as the result of their schooling, family lore, or public service announcements from the American Academy of Pediatrics.
I certainly wasn’t doing much doctoring in the sense of treating or curing disease. If one removes administering immunizations and delivery room resuscitations, I saved very few lives.
So you may ask, if not as “doctor,” how would I prefer to be labeled? Good question, but easy for me to answer. The term “coach” quickly comes to mind. As someone who played a variety of team sports there is no term that I can think of that commands more respect than “Coach.” While the term doesn’t carry the burden of a particularly long educational journey it does imply the person is wise, observant, and experienced.
There is some teaching involved but primarily a coach is going to assess the players (or in this cases the families) he is presented with and then do the best he can to guide them toward good decisions they can make themselves given their specific situations. This requires spending most of one’s time getting to know each family and understanding their strengths and limitations. One can’t coach speed to an athlete who is slow footed. And, one isn’t going to get a family that is dominated by anxiety to become bold risk takers. The best you can do is to help them learn strategies to minimize their anxieties.
A good coach can help his players learn to set reasonable goals given their skill sets. And, a good pediatrician can coach his families how to adapt their strengths and weakness to the challenges of each of their children. For example, for a physician faced with a mother-infant dyad that is struggling with breastfeeding, once the education piece is in place, it is up to him or her to function as a coach and assist the team in setting a reasonable goal that can result in a win-win for the family.
A coach must be humble, putting his or her players’ needs first and flexible enough to adjust his or her goals to define success in terms for what is best for each individual team. “Coach” may not carry the ring of authority that can come with “Doctor” but it is a role equally as challenging and rewarding.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
It’s not unusual when I run into a former patient that I am addressed as “Doctor” Wilkoff. I guess that is to be expected because when I was in practice I seldom introduced myself as Will. However, I will admit now that I never quite felt comfortable with the “Doctor” label. Today, if you addressed me as “Doctor” I would correct you and refer to myself as the “ex-Doctor Wilkoff.”
The term doctor derived from the Latin word to teach and eventually morphed into an academic title. In common parlance it is sometimes used as verb meaning to treat, e.g., “he doctored the wound.” Regardless of what academic field we are talking about, the title “doctor” has become a term of respect for someone who has spent an unusually long time learning his or her subject or craft. The holder of a doctorate, particularly in medicine, receives a rank, earned or unearned, near the top of the social hierarchy.
When I look back at more than 50 years of doing pediatrics I’m not sure that “doctor” really captures what I was up to. I will grant you that it is nice that folks want to acknowledge all those years I spent in training. But I don’t think one could say that what I did as a primary care small town pediatrician fits in with the original definition “to teach.” I did spend a few hours teaching students every so often but my primary time was spent with patients and I don’t consider what I was doing with them as teaching. There just wasn’t enough time. I had to take as a given that families who came to see me already had a basic knowledge base either as the result of their schooling, family lore, or public service announcements from the American Academy of Pediatrics.
I certainly wasn’t doing much doctoring in the sense of treating or curing disease. If one removes administering immunizations and delivery room resuscitations, I saved very few lives.
So you may ask, if not as “doctor,” how would I prefer to be labeled? Good question, but easy for me to answer. The term “coach” quickly comes to mind. As someone who played a variety of team sports there is no term that I can think of that commands more respect than “Coach.” While the term doesn’t carry the burden of a particularly long educational journey it does imply the person is wise, observant, and experienced.
There is some teaching involved but primarily a coach is going to assess the players (or in this cases the families) he is presented with and then do the best he can to guide them toward good decisions they can make themselves given their specific situations. This requires spending most of one’s time getting to know each family and understanding their strengths and limitations. One can’t coach speed to an athlete who is slow footed. And, one isn’t going to get a family that is dominated by anxiety to become bold risk takers. The best you can do is to help them learn strategies to minimize their anxieties.
A good coach can help his players learn to set reasonable goals given their skill sets. And, a good pediatrician can coach his families how to adapt their strengths and weakness to the challenges of each of their children. For example, for a physician faced with a mother-infant dyad that is struggling with breastfeeding, once the education piece is in place, it is up to him or her to function as a coach and assist the team in setting a reasonable goal that can result in a win-win for the family.
A coach must be humble, putting his or her players’ needs first and flexible enough to adjust his or her goals to define success in terms for what is best for each individual team. “Coach” may not carry the ring of authority that can come with “Doctor” but it is a role equally as challenging and rewarding.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
Child abuse visits to EDs declined in 2020, but not admissions
but the visits in 2020 were significantly more likely to result in hospitalization, based on analysis of a national ED database.
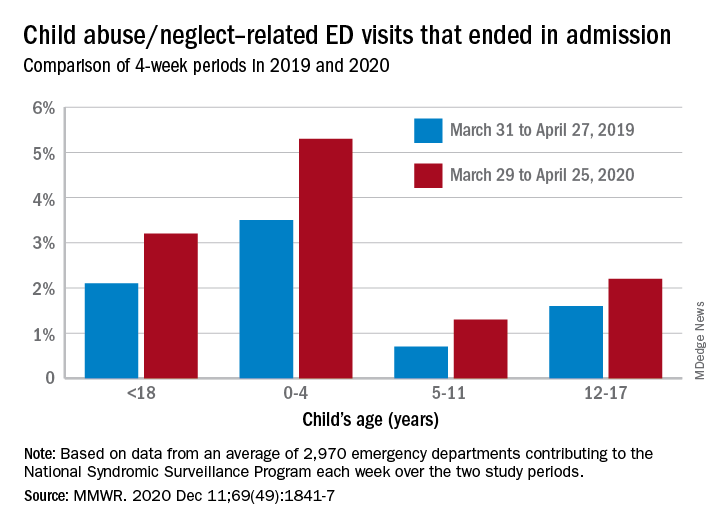
The number of ED visits involving child abuse and neglect was down by 53% during the 4-week period from March 29 to April 25, 2020, compared with the 4 weeks from March 31 to April 27, 2019. The proportion of those ED visits that ended in hospitalizations, however, increased from 2.1% in 2019 to 3.2% in 2020, Elizabeth Swedo, MD, and associates at the Centers for Disease Control and Prevention said in the Morbidity and Mortality Weekly Report.
“ED visits related to suspected or confirmed child abuse and neglect decreased beginning the week of March 15, 2020, coinciding with the declaration of a national emergency related to COVID-19 and implementation of community mitigation measures,” they wrote.
An earlier study involving the same database (the National Syndromic Surveillance Program) showed that, over the two same 4-week periods, the volume of all ED visits in 2020 was down 72% for children aged 10 years and younger and 71% for those aged 11-14 years.
In the current study, however, all age subgroups had significant increases in hospital admissions. The proportion of ED visits related to child abuse and neglect that resulted in hospitalization rose from 3.5% in 2019 to 5.3% in 2020 among ages 0-4 years, 0.7% to 1.3% for ages 5-11 years, and 1.6% to 2.2% for adolescents aged 12-17, Dr. Swedo and associates reported.
The absence of a corresponding drop in hospitalizations may be tied to risk factors related to the pandemic, “such as loss of income, increased stress related to parental child care and schooling responsibilities, and increased substance use and mental health conditions among adults,” the investigators added.
The National Syndromic Surveillance Program receives daily data from 3,310 EDs in 47 states, but the number of facilities meeting the investigators’ criteria averaged 2,970 a week for the 8 weeks of the study period.
SOURCE: Swedo E et al. MMWR. 2020 Dec. 11;69(49):1841-7.
but the visits in 2020 were significantly more likely to result in hospitalization, based on analysis of a national ED database.

The number of ED visits involving child abuse and neglect was down by 53% during the 4-week period from March 29 to April 25, 2020, compared with the 4 weeks from March 31 to April 27, 2019. The proportion of those ED visits that ended in hospitalizations, however, increased from 2.1% in 2019 to 3.2% in 2020, Elizabeth Swedo, MD, and associates at the Centers for Disease Control and Prevention said in the Morbidity and Mortality Weekly Report.
“ED visits related to suspected or confirmed child abuse and neglect decreased beginning the week of March 15, 2020, coinciding with the declaration of a national emergency related to COVID-19 and implementation of community mitigation measures,” they wrote.
An earlier study involving the same database (the National Syndromic Surveillance Program) showed that, over the two same 4-week periods, the volume of all ED visits in 2020 was down 72% for children aged 10 years and younger and 71% for those aged 11-14 years.
In the current study, however, all age subgroups had significant increases in hospital admissions. The proportion of ED visits related to child abuse and neglect that resulted in hospitalization rose from 3.5% in 2019 to 5.3% in 2020 among ages 0-4 years, 0.7% to 1.3% for ages 5-11 years, and 1.6% to 2.2% for adolescents aged 12-17, Dr. Swedo and associates reported.
The absence of a corresponding drop in hospitalizations may be tied to risk factors related to the pandemic, “such as loss of income, increased stress related to parental child care and schooling responsibilities, and increased substance use and mental health conditions among adults,” the investigators added.
The National Syndromic Surveillance Program receives daily data from 3,310 EDs in 47 states, but the number of facilities meeting the investigators’ criteria averaged 2,970 a week for the 8 weeks of the study period.
SOURCE: Swedo E et al. MMWR. 2020 Dec. 11;69(49):1841-7.
but the visits in 2020 were significantly more likely to result in hospitalization, based on analysis of a national ED database.

The number of ED visits involving child abuse and neglect was down by 53% during the 4-week period from March 29 to April 25, 2020, compared with the 4 weeks from March 31 to April 27, 2019. The proportion of those ED visits that ended in hospitalizations, however, increased from 2.1% in 2019 to 3.2% in 2020, Elizabeth Swedo, MD, and associates at the Centers for Disease Control and Prevention said in the Morbidity and Mortality Weekly Report.
“ED visits related to suspected or confirmed child abuse and neglect decreased beginning the week of March 15, 2020, coinciding with the declaration of a national emergency related to COVID-19 and implementation of community mitigation measures,” they wrote.
An earlier study involving the same database (the National Syndromic Surveillance Program) showed that, over the two same 4-week periods, the volume of all ED visits in 2020 was down 72% for children aged 10 years and younger and 71% for those aged 11-14 years.
In the current study, however, all age subgroups had significant increases in hospital admissions. The proportion of ED visits related to child abuse and neglect that resulted in hospitalization rose from 3.5% in 2019 to 5.3% in 2020 among ages 0-4 years, 0.7% to 1.3% for ages 5-11 years, and 1.6% to 2.2% for adolescents aged 12-17, Dr. Swedo and associates reported.
The absence of a corresponding drop in hospitalizations may be tied to risk factors related to the pandemic, “such as loss of income, increased stress related to parental child care and schooling responsibilities, and increased substance use and mental health conditions among adults,” the investigators added.
The National Syndromic Surveillance Program receives daily data from 3,310 EDs in 47 states, but the number of facilities meeting the investigators’ criteria averaged 2,970 a week for the 8 weeks of the study period.
SOURCE: Swedo E et al. MMWR. 2020 Dec. 11;69(49):1841-7.
FROM MMWR
Current PERISCOPE vaccine studies: Toward better pertussis prevention?
With increasing whooping cough numbers, developing an effective new vaccine against Bordetella pertussis is a priority. Results from the multifactorial PERISCOPE Project will help scientists and clinicians move forward.
Dominic Kelly, PhD, talked about vaccine-induced immunity and provided an overview of ongoing clinical trials in the PERISCOPE (Pertussis Correlates of Protection Europe) project in a key research session at the start of the annual meeting of the European Society for Paediatric Infectious Diseases, held virtually this year. Dr. Kelly, a pediatrician at the Children’s Hospital in Oxford and a member of the Oxford Vaccines Group, leads one of the studies in the project looking at infant vaccination.
Dr. Kelly began his presentation by showing a figure depicting where vaccine-induced immunity fits into the larger suite of clinical studies. These studies involve mouse models, human challenge models, and infection patients. A key theme is the use of a core group of immunoassays across all studies, with the hope that they will allow effective cross comparisons.
Dr. Kelly stated, “If we find a correlate of protection in the challenge model, we can then interpret the vaccine studies in the light of that because we are using standardized constant immunoassays.”
The assays being used depend in part on the specific study and the volume of blood available. They will generally include Bordetella-specific antibody and functional antibody assays, as well as interesting studies collecting mucosal samples from infants and adults to look at serological responses. Also under examination are a range of enzyme-linked immune absorbent spot, flow cytometry, and culture techniques looking at Memory B cells, T cells, and gene expression.
Complementing these assay studies, PERISCOPE includes a series of clinical investigations designed to throw light on three areas of interest, described below:
First, researchers hope to gain a better understanding regarding the effects of the original whole cell vaccine versus the current acellular variety. The former uses an inactivated version of the whole organism. Epidemiological studies, animal data, and experience in the field demonstrate that whole-cell vaccination results in a broad, long-lasting, and effective immune response.
By comparison, the acellular pertussis vaccine consists of between three and five protein components, which are purified from cultured Bordetella pertussis. While it is an effective vaccine, its effects are less durable; routine use in some countries is associated with cyclical outbreaks of increasing severity.
A second issue for researchers involved in the PERISCOPE project concerns the effects of maternal immunization. In the United Kingdom in 2012, for example, an increasing number of cases were noted 6-7 years after adoption of an acellular vaccine for routine vaccination in the 2nd-3rd trimester of pregnancy. Vaccination appears to effectively control neonatal disease, but whether this influences infant immune responses and long-term control of pertussis for a population is unknown.
Finally, the group is interested in the effects of an acellular booster across all age groups. While the effects may be short-lived, the booster is a potential strategy for controlling a population by repeated boosting of immunity. This is another area where using novel immunoassays may aid better understanding.
To find answers, the consortium has established four studies: the Gambia Pertussis study (GaPs) in Gambia and AWARE, the sister study to GaPs in the United Kingdom, addressing the acellular pertussis versus cellular pertussis question; the Pertussis Maternal Immunization Study in Finland (MIFI) addressing maternal immunization; and the Booster against Pertussis (BERT) study across three countries (U.K., the Netherlands, and Finland) looking at acellular booster across age groups.
Gambia pertussis study
GaPs is the largest single study in the project and is being run at the Medical Research Council–funded London School of Tropical Medicine center in Gambia. Beate Kampmann, MD, PhD, of Imperial College London, England, is the project lead. It is due to complete in 2022. GaPs seeks to enroll 600 mother/infant pairs and randomize the mothers to either an acellular pertussis booster in pregnancy or a tetanus toxoid control vaccine. Infants are subsequently randomized to an acellular or whole-cell pertussis schedule of primary immunization. The vaccine doses are being given at 2, 3, and 4 months. The primary endpoint is a serological finding being measured at 9 months of age, when the infant would usually receive yellow fever, measles, and rubella vaccination.
GaPs has a number of pathways. Within each of the four arms generated by the two randomizations, the maternal randomization and the infant randomization, there are five subgroups. They are designed to study time points in subgroups A and B after the first dose in more detail, looking at the innate immune responses using gene expression. It will enable researchers to study adaptive immune responses to T cells and B cells after the second dose of vaccine. By employing a range of subgroups, the team can explore the immune profile using the assays referred to above. Such information should provide new insights into the differences between acellular and whole-cell vaccines.
The AWARE study
AWARE is the sister study to GaPs and looks at the acellular/whole pertussis issue. Because many developed countries, such as the United Kingdom, have established maternal immunization programs, it is not possible to randomize mothers. Consequently, researchers have opted to recruit infants of mothers who have received an acellular vaccine in pregnancy and randomize them to either an acellular schedule of primary immunization or a whole-cell schedule.
The selected vaccine is ComVac5 from Bharat Biotech. This whole-cell vaccine differs from that used in Gambia. An early obstacle for AWARE has been seeking permission to import a non-conventional vaccine into Europe. It has delayed the anticipated end date to 2023. Participating infants will receive a two-dose schedule at 2 and 4 months of age per their randomization; then, both groups will go on to receive an acellular pertussis booster at 12 months. At all time points, the team will sample blood for cells and serum, as well as mucosal fluid from the nose. Because the mucosal surface is where the action is, this approach will likely generate new data around antibody responses.
The MIFI
The Pertussis Maternal Immunization Study in Finland is being run by Jussi Mertsola, of the University of Turku, Finland, and Qiushui He, of the National Public Health Institute, Turku. It is due to complete in late 2021. Where, in the United Kingdom, researchers are unable to randomize mothers because of the current guidelines, researchers in Finland do not have a maternal immunization program to consider. MIFI will randomize 80 mothers, 40 to immunization with acellular pertussis and 40 to a control group. Dr. Kelly stated that whole cell vaccines are not available for use in Finland. Participants will receive a two-dose schedule at 3 and 5 months. Blood samples will then be taken to compare the serological and cellular responses, which will help researchers understand the effects of maternal immunization. In addition, there will be sampling of mucosal fluid using a device that collects a standardized aliquot of fluid.
The BERT study
The final clinical element of PERISCOPE presented by Dr. Kelly was the Booster against Pertussis study. This study is near completion. It seeks to examine the use of an acellular booster across different age groups and three countries: the United Kingdom, the Netherlands, and Finland. The study is being coordinated by Guy Berbers, PhD, at the National Institute for Public Health and the Environment in the Netherlands.
BERT comprises four cohorts (A, B, C, D) of different ages: 7-10 years (36 participants), 11-15 years (36 participants), mid-adult (25 participants), and older age (25 participants). After receiving an acellular booster, participants will undergo intense sampling. Sampling will take place immediately after immunization at day 7 and look at adaptive effects, then again at day 28 and day 365.
Because some participants will have already received whole cell or acellular vaccination, this approach will allow researchers to look at the effects of priming (i.e., how long the B cell/T cell antibody responses last).
Involving different countries across Europe ensures wide applicability of results, but also allows researchers to compare the effects of very different immunization histories.
At the end of this ESPID session, Dimitri Diavatopoulos, PhD, assistant professor at the Radboud University Medical Centre Nijmegen, the Netherlands, commented that a future problem in studying pertussis vaccines and their potential clinical application is that most vaccination schedules now involve combination products. Obtaining a stand-alone vaccination may prove difficult, and there may be resistance if it complicates current vaccination programs.
Dr. Kelly acknowledged funding for the PERISCOPE project from GlaxoSmithKline and Pasteur Sanofi.
With increasing whooping cough numbers, developing an effective new vaccine against Bordetella pertussis is a priority. Results from the multifactorial PERISCOPE Project will help scientists and clinicians move forward.
Dominic Kelly, PhD, talked about vaccine-induced immunity and provided an overview of ongoing clinical trials in the PERISCOPE (Pertussis Correlates of Protection Europe) project in a key research session at the start of the annual meeting of the European Society for Paediatric Infectious Diseases, held virtually this year. Dr. Kelly, a pediatrician at the Children’s Hospital in Oxford and a member of the Oxford Vaccines Group, leads one of the studies in the project looking at infant vaccination.
Dr. Kelly began his presentation by showing a figure depicting where vaccine-induced immunity fits into the larger suite of clinical studies. These studies involve mouse models, human challenge models, and infection patients. A key theme is the use of a core group of immunoassays across all studies, with the hope that they will allow effective cross comparisons.
Dr. Kelly stated, “If we find a correlate of protection in the challenge model, we can then interpret the vaccine studies in the light of that because we are using standardized constant immunoassays.”
The assays being used depend in part on the specific study and the volume of blood available. They will generally include Bordetella-specific antibody and functional antibody assays, as well as interesting studies collecting mucosal samples from infants and adults to look at serological responses. Also under examination are a range of enzyme-linked immune absorbent spot, flow cytometry, and culture techniques looking at Memory B cells, T cells, and gene expression.
Complementing these assay studies, PERISCOPE includes a series of clinical investigations designed to throw light on three areas of interest, described below:
First, researchers hope to gain a better understanding regarding the effects of the original whole cell vaccine versus the current acellular variety. The former uses an inactivated version of the whole organism. Epidemiological studies, animal data, and experience in the field demonstrate that whole-cell vaccination results in a broad, long-lasting, and effective immune response.
By comparison, the acellular pertussis vaccine consists of between three and five protein components, which are purified from cultured Bordetella pertussis. While it is an effective vaccine, its effects are less durable; routine use in some countries is associated with cyclical outbreaks of increasing severity.
A second issue for researchers involved in the PERISCOPE project concerns the effects of maternal immunization. In the United Kingdom in 2012, for example, an increasing number of cases were noted 6-7 years after adoption of an acellular vaccine for routine vaccination in the 2nd-3rd trimester of pregnancy. Vaccination appears to effectively control neonatal disease, but whether this influences infant immune responses and long-term control of pertussis for a population is unknown.
Finally, the group is interested in the effects of an acellular booster across all age groups. While the effects may be short-lived, the booster is a potential strategy for controlling a population by repeated boosting of immunity. This is another area where using novel immunoassays may aid better understanding.
To find answers, the consortium has established four studies: the Gambia Pertussis study (GaPs) in Gambia and AWARE, the sister study to GaPs in the United Kingdom, addressing the acellular pertussis versus cellular pertussis question; the Pertussis Maternal Immunization Study in Finland (MIFI) addressing maternal immunization; and the Booster against Pertussis (BERT) study across three countries (U.K., the Netherlands, and Finland) looking at acellular booster across age groups.
Gambia pertussis study
GaPs is the largest single study in the project and is being run at the Medical Research Council–funded London School of Tropical Medicine center in Gambia. Beate Kampmann, MD, PhD, of Imperial College London, England, is the project lead. It is due to complete in 2022. GaPs seeks to enroll 600 mother/infant pairs and randomize the mothers to either an acellular pertussis booster in pregnancy or a tetanus toxoid control vaccine. Infants are subsequently randomized to an acellular or whole-cell pertussis schedule of primary immunization. The vaccine doses are being given at 2, 3, and 4 months. The primary endpoint is a serological finding being measured at 9 months of age, when the infant would usually receive yellow fever, measles, and rubella vaccination.
GaPs has a number of pathways. Within each of the four arms generated by the two randomizations, the maternal randomization and the infant randomization, there are five subgroups. They are designed to study time points in subgroups A and B after the first dose in more detail, looking at the innate immune responses using gene expression. It will enable researchers to study adaptive immune responses to T cells and B cells after the second dose of vaccine. By employing a range of subgroups, the team can explore the immune profile using the assays referred to above. Such information should provide new insights into the differences between acellular and whole-cell vaccines.
The AWARE study
AWARE is the sister study to GaPs and looks at the acellular/whole pertussis issue. Because many developed countries, such as the United Kingdom, have established maternal immunization programs, it is not possible to randomize mothers. Consequently, researchers have opted to recruit infants of mothers who have received an acellular vaccine in pregnancy and randomize them to either an acellular schedule of primary immunization or a whole-cell schedule.
The selected vaccine is ComVac5 from Bharat Biotech. This whole-cell vaccine differs from that used in Gambia. An early obstacle for AWARE has been seeking permission to import a non-conventional vaccine into Europe. It has delayed the anticipated end date to 2023. Participating infants will receive a two-dose schedule at 2 and 4 months of age per their randomization; then, both groups will go on to receive an acellular pertussis booster at 12 months. At all time points, the team will sample blood for cells and serum, as well as mucosal fluid from the nose. Because the mucosal surface is where the action is, this approach will likely generate new data around antibody responses.
The MIFI
The Pertussis Maternal Immunization Study in Finland is being run by Jussi Mertsola, of the University of Turku, Finland, and Qiushui He, of the National Public Health Institute, Turku. It is due to complete in late 2021. Where, in the United Kingdom, researchers are unable to randomize mothers because of the current guidelines, researchers in Finland do not have a maternal immunization program to consider. MIFI will randomize 80 mothers, 40 to immunization with acellular pertussis and 40 to a control group. Dr. Kelly stated that whole cell vaccines are not available for use in Finland. Participants will receive a two-dose schedule at 3 and 5 months. Blood samples will then be taken to compare the serological and cellular responses, which will help researchers understand the effects of maternal immunization. In addition, there will be sampling of mucosal fluid using a device that collects a standardized aliquot of fluid.
The BERT study
The final clinical element of PERISCOPE presented by Dr. Kelly was the Booster against Pertussis study. This study is near completion. It seeks to examine the use of an acellular booster across different age groups and three countries: the United Kingdom, the Netherlands, and Finland. The study is being coordinated by Guy Berbers, PhD, at the National Institute for Public Health and the Environment in the Netherlands.
BERT comprises four cohorts (A, B, C, D) of different ages: 7-10 years (36 participants), 11-15 years (36 participants), mid-adult (25 participants), and older age (25 participants). After receiving an acellular booster, participants will undergo intense sampling. Sampling will take place immediately after immunization at day 7 and look at adaptive effects, then again at day 28 and day 365.
Because some participants will have already received whole cell or acellular vaccination, this approach will allow researchers to look at the effects of priming (i.e., how long the B cell/T cell antibody responses last).
Involving different countries across Europe ensures wide applicability of results, but also allows researchers to compare the effects of very different immunization histories.
At the end of this ESPID session, Dimitri Diavatopoulos, PhD, assistant professor at the Radboud University Medical Centre Nijmegen, the Netherlands, commented that a future problem in studying pertussis vaccines and their potential clinical application is that most vaccination schedules now involve combination products. Obtaining a stand-alone vaccination may prove difficult, and there may be resistance if it complicates current vaccination programs.
Dr. Kelly acknowledged funding for the PERISCOPE project from GlaxoSmithKline and Pasteur Sanofi.
With increasing whooping cough numbers, developing an effective new vaccine against Bordetella pertussis is a priority. Results from the multifactorial PERISCOPE Project will help scientists and clinicians move forward.
Dominic Kelly, PhD, talked about vaccine-induced immunity and provided an overview of ongoing clinical trials in the PERISCOPE (Pertussis Correlates of Protection Europe) project in a key research session at the start of the annual meeting of the European Society for Paediatric Infectious Diseases, held virtually this year. Dr. Kelly, a pediatrician at the Children’s Hospital in Oxford and a member of the Oxford Vaccines Group, leads one of the studies in the project looking at infant vaccination.
Dr. Kelly began his presentation by showing a figure depicting where vaccine-induced immunity fits into the larger suite of clinical studies. These studies involve mouse models, human challenge models, and infection patients. A key theme is the use of a core group of immunoassays across all studies, with the hope that they will allow effective cross comparisons.
Dr. Kelly stated, “If we find a correlate of protection in the challenge model, we can then interpret the vaccine studies in the light of that because we are using standardized constant immunoassays.”
The assays being used depend in part on the specific study and the volume of blood available. They will generally include Bordetella-specific antibody and functional antibody assays, as well as interesting studies collecting mucosal samples from infants and adults to look at serological responses. Also under examination are a range of enzyme-linked immune absorbent spot, flow cytometry, and culture techniques looking at Memory B cells, T cells, and gene expression.
Complementing these assay studies, PERISCOPE includes a series of clinical investigations designed to throw light on three areas of interest, described below:
First, researchers hope to gain a better understanding regarding the effects of the original whole cell vaccine versus the current acellular variety. The former uses an inactivated version of the whole organism. Epidemiological studies, animal data, and experience in the field demonstrate that whole-cell vaccination results in a broad, long-lasting, and effective immune response.
By comparison, the acellular pertussis vaccine consists of between three and five protein components, which are purified from cultured Bordetella pertussis. While it is an effective vaccine, its effects are less durable; routine use in some countries is associated with cyclical outbreaks of increasing severity.
A second issue for researchers involved in the PERISCOPE project concerns the effects of maternal immunization. In the United Kingdom in 2012, for example, an increasing number of cases were noted 6-7 years after adoption of an acellular vaccine for routine vaccination in the 2nd-3rd trimester of pregnancy. Vaccination appears to effectively control neonatal disease, but whether this influences infant immune responses and long-term control of pertussis for a population is unknown.
Finally, the group is interested in the effects of an acellular booster across all age groups. While the effects may be short-lived, the booster is a potential strategy for controlling a population by repeated boosting of immunity. This is another area where using novel immunoassays may aid better understanding.
To find answers, the consortium has established four studies: the Gambia Pertussis study (GaPs) in Gambia and AWARE, the sister study to GaPs in the United Kingdom, addressing the acellular pertussis versus cellular pertussis question; the Pertussis Maternal Immunization Study in Finland (MIFI) addressing maternal immunization; and the Booster against Pertussis (BERT) study across three countries (U.K., the Netherlands, and Finland) looking at acellular booster across age groups.
Gambia pertussis study
GaPs is the largest single study in the project and is being run at the Medical Research Council–funded London School of Tropical Medicine center in Gambia. Beate Kampmann, MD, PhD, of Imperial College London, England, is the project lead. It is due to complete in 2022. GaPs seeks to enroll 600 mother/infant pairs and randomize the mothers to either an acellular pertussis booster in pregnancy or a tetanus toxoid control vaccine. Infants are subsequently randomized to an acellular or whole-cell pertussis schedule of primary immunization. The vaccine doses are being given at 2, 3, and 4 months. The primary endpoint is a serological finding being measured at 9 months of age, when the infant would usually receive yellow fever, measles, and rubella vaccination.
GaPs has a number of pathways. Within each of the four arms generated by the two randomizations, the maternal randomization and the infant randomization, there are five subgroups. They are designed to study time points in subgroups A and B after the first dose in more detail, looking at the innate immune responses using gene expression. It will enable researchers to study adaptive immune responses to T cells and B cells after the second dose of vaccine. By employing a range of subgroups, the team can explore the immune profile using the assays referred to above. Such information should provide new insights into the differences between acellular and whole-cell vaccines.
The AWARE study
AWARE is the sister study to GaPs and looks at the acellular/whole pertussis issue. Because many developed countries, such as the United Kingdom, have established maternal immunization programs, it is not possible to randomize mothers. Consequently, researchers have opted to recruit infants of mothers who have received an acellular vaccine in pregnancy and randomize them to either an acellular schedule of primary immunization or a whole-cell schedule.
The selected vaccine is ComVac5 from Bharat Biotech. This whole-cell vaccine differs from that used in Gambia. An early obstacle for AWARE has been seeking permission to import a non-conventional vaccine into Europe. It has delayed the anticipated end date to 2023. Participating infants will receive a two-dose schedule at 2 and 4 months of age per their randomization; then, both groups will go on to receive an acellular pertussis booster at 12 months. At all time points, the team will sample blood for cells and serum, as well as mucosal fluid from the nose. Because the mucosal surface is where the action is, this approach will likely generate new data around antibody responses.
The MIFI
The Pertussis Maternal Immunization Study in Finland is being run by Jussi Mertsola, of the University of Turku, Finland, and Qiushui He, of the National Public Health Institute, Turku. It is due to complete in late 2021. Where, in the United Kingdom, researchers are unable to randomize mothers because of the current guidelines, researchers in Finland do not have a maternal immunization program to consider. MIFI will randomize 80 mothers, 40 to immunization with acellular pertussis and 40 to a control group. Dr. Kelly stated that whole cell vaccines are not available for use in Finland. Participants will receive a two-dose schedule at 3 and 5 months. Blood samples will then be taken to compare the serological and cellular responses, which will help researchers understand the effects of maternal immunization. In addition, there will be sampling of mucosal fluid using a device that collects a standardized aliquot of fluid.
The BERT study
The final clinical element of PERISCOPE presented by Dr. Kelly was the Booster against Pertussis study. This study is near completion. It seeks to examine the use of an acellular booster across different age groups and three countries: the United Kingdom, the Netherlands, and Finland. The study is being coordinated by Guy Berbers, PhD, at the National Institute for Public Health and the Environment in the Netherlands.
BERT comprises four cohorts (A, B, C, D) of different ages: 7-10 years (36 participants), 11-15 years (36 participants), mid-adult (25 participants), and older age (25 participants). After receiving an acellular booster, participants will undergo intense sampling. Sampling will take place immediately after immunization at day 7 and look at adaptive effects, then again at day 28 and day 365.
Because some participants will have already received whole cell or acellular vaccination, this approach will allow researchers to look at the effects of priming (i.e., how long the B cell/T cell antibody responses last).
Involving different countries across Europe ensures wide applicability of results, but also allows researchers to compare the effects of very different immunization histories.
At the end of this ESPID session, Dimitri Diavatopoulos, PhD, assistant professor at the Radboud University Medical Centre Nijmegen, the Netherlands, commented that a future problem in studying pertussis vaccines and their potential clinical application is that most vaccination schedules now involve combination products. Obtaining a stand-alone vaccination may prove difficult, and there may be resistance if it complicates current vaccination programs.
Dr. Kelly acknowledged funding for the PERISCOPE project from GlaxoSmithKline and Pasteur Sanofi.
FROM ESPID 2020
Call to arms: vaccinating the health workforce of 21 million strong
As the first American health care workers rolled up their sleeves for a COVID-19 vaccine, the images were instantly frozen in history, marking the triumph of scientific know-how and ingenuity. Cameras captured the first trucks pulling out of a warehouse in Portage, Mich., to the applause of workers and area residents. A day later, Boston Medical Center employees – some dressed in scrubs and wearing masks, face shields, and protective gowns – literally danced on the sidewalk when doses arrived. Some have photographed themselves getting the vaccine and posted it on social media, tagging it #MyCOVIDVax.
But the real story of the debut of COVID-19 vaccination is more methodical than monumental, a celebration of teamwork rather than of conquest. As hospitals waited for their first allotment, they reviewed their carefully drafted plans. They relied on each other, reaching across the usual divisions of competition and working collaboratively to share the limited supply. Their priority lists for the first vaccinations included environmental services workers who clean patient rooms and the critical care physicians who work to save lives.
“Health care workers have pulled together throughout this pandemic,” said Melanie Swift, MD, cochair of the COVID-19 Vaccine Allocation and Distribution Work Group at Mayo Clinic in Rochester, Minn. “We’ve gone through the darkest of years relying so heavily on each other,” she said. “Now we’re pulling together to get out of it.”
Still, a rollout of this magnitude has hitches. Stanford issued an apology Dec. 18 after its medical residents protested a vaccine distribution plan that left out nearly all of its residents and fellows, many of whom regularly treat patients with COVID-19.
There have already been more than 287,000 COVID-19 cases and 953 deaths among health care workers, according to the Centers for Disease Control and Prevention. In its guidance, the agency pointed out that the “continued protection of them at work, at home, and in the community remains a national priority.” That means vaccinating a workforce of about 21 million people, often the largest group of employees in a community.
“It collectively takes all of us to vaccinate our teams to maintain that stability in our health care infrastructure across the metro Atlanta area,” Christy Norman, PharmD, vice president of pharmacy services at Emory Healthcare, told reporters in a briefing as the health system awaited its first delivery.
Don’t waste a dose
One overriding imperative prevails: Hospitals don’t want to waste any doses. The storage requirements of the Pfizer vaccine make that tricky.
Once vials are removed from the pizza-box-shaped containers in ultracold storage and placed in a refrigerator, they must be used within 5 days. Thawed five-dose vials must be brought to room temperature before they are diluted, and they can remain at room temperature for no more than 2 hours. Once they are diluted with 1.8 mL of a 0.9% sodium chloride injection, the vials must be used within 6 hours.
COVID-19 precautions require employees to stay physically distant while they wait their turn for vaccination, which means the process can’t mirror typical large-scale flu immunization programs.
To prioritize groups, the vaccination planners at Mayo conducted a thorough risk stratification, considering each employee’s duties. Do they work in a dedicated COVID-19 unit? Do they handle lab tests or collect swabs? Do they work in the ICU or emergency department?
“We have applied some principles to make sure that as we roll it out, we prioritize people who are at greatest risk of ongoing exposure and who are really critical to maintaining the COVID response and other essential health services,” said Dr. Swift, associate medical director of Mayo’s occupational health service.
Mayo employees who are eligible for the first doses can sign up for appointments through the medical record system. If it seems likely that some doses will be left over at the end of the vaccination period – perhaps because of missed appointments – supervisors in high-risk areas can refer other health care workers. Mayo gave its first vaccines on Dec. 18, but the vaccination program began in earnest the following week. With the pleasant surprise that each five-dose vial actually provides six doses, 474 vials will allow for the vaccination of 2,844 employees in the top-priority group. “It’s going to expand each week or few days as we get more and more vaccine,” Dr. Swift said.
Sharing vials with small rural hospitals
Minnesota is using a hub-and-spoke system to give small rural hospitals access to the Pfizer vaccine, even though they lack ultracold storage and can’t use a minimum order of 975 doses. Large hospitals, acting as hubs, are sharing their orders. (The minimum order for Moderna is 100 doses.)
In south-central Minnesota, for example, two hub hospitals each have six spoke hospitals. Five of the 14 hospitals are independent, and the rest are part of large hospital systems, but affiliation doesn’t matter, said Eric Weller, regional health care preparedness coordinator for the South Central Healthcare Coalition. “We are all working together. It doesn’t matter what system you’re from,” he said. “We’re working for the good of the community.”
Each hospital designed a process to provide vaccine education, prioritize groups, allocate appointments, register people for vaccination, obtain signed consent forms, administer vaccines in a COVID-safe way, and provide follow-up appointments for the second dose. “We’re using some of the lessons we learned during H1N1,” said Mr. Weller, referring to immunization during the 2009 influenza pandemic. “The difference is that during H1N1, you could have lines of people.”
Coordinating the appointments will be more important than ever. “One of the vaccination strategies is to get people in groups of five, so you use one vial on those five people and don’t waste it,” he said.
Logistics are somewhat different for the Moderna vaccine, which will come in 10-dose vials that can be refrigerated for up to 30 days.
Both vaccines may produce mild flulike symptoms, such as fatigue, headache, or muscle pain, particularly after the second dose. That’s a sign that the immune system is reacting to the vaccine, but it’s also another consideration in the vaccination plans, because health care workers might take a day or two off work. “We’re not going to vaccinate a whole department at one time. It will be staggered,” said Kevin Smith, MD, medical director of the occupational medicine program at ProMedica, a health care system based in Toledo, Ohio.
Dr. Smith said he plans to encourage employees to use V-Safe, an app created by the CDC to track adverse effects in people who receive the vaccine. He pointed out that a day or two of achiness will be better than coping with the symptoms of COVID-19. Some employees who recovered from the infection still feel fatigued or haven’t regained their sense of taste and smell. “We are still monitoring quite a few employees to make sure they get back to 100%,” he said.
Hope for ending the pandemic
Public health officials have worried about vaccine hesitancy, even among health care workers, but so far, that concern seems overshadowed by enthusiasm. Dr. Smith said his department has been fielding calls from employees who want to know when they will be able to get the vaccine. “I think everyone feels relief,” he said. “We’re at the beginning of the end.”
At Mayo, Dr. Swift is surveying staff to gauge the willingness to get the vaccine, but she already senses excitement among employees. “No doubt there are still people who are hesitant, but I’m feeling a shift,” she said. “I’m feeling this momentum building of health care workers coming on board and wanting to take this vaccine, which is good, because they will set an example for their patients.”
For Colleen Kelley, MD, an infectious disease physician at Emory University in Atlanta who was principal investigator for an Emory-affiliated Moderna clinical trial site, it has been an emotional time. “Things were looking very bleak and dark for a time, and then we started to get these efficacy results that were greater than anyone imagined,” she said.
Dr. Kelley spends time talking to journalists and educating physician colleagues and hospital employees about how the vaccine was developed so quickly and how it works. “Everyone asks me, ‘Should I get it? Are you going to get it?’ My answer is ‘yes’ and ‘yes,’ “ she said. “I am 1,000% confident that the benefits of widespread vaccination outweigh the risks of continued COVID and a continued pandemic.”
A version of this article first appeared on Medscape.com.
As the first American health care workers rolled up their sleeves for a COVID-19 vaccine, the images were instantly frozen in history, marking the triumph of scientific know-how and ingenuity. Cameras captured the first trucks pulling out of a warehouse in Portage, Mich., to the applause of workers and area residents. A day later, Boston Medical Center employees – some dressed in scrubs and wearing masks, face shields, and protective gowns – literally danced on the sidewalk when doses arrived. Some have photographed themselves getting the vaccine and posted it on social media, tagging it #MyCOVIDVax.
But the real story of the debut of COVID-19 vaccination is more methodical than monumental, a celebration of teamwork rather than of conquest. As hospitals waited for their first allotment, they reviewed their carefully drafted plans. They relied on each other, reaching across the usual divisions of competition and working collaboratively to share the limited supply. Their priority lists for the first vaccinations included environmental services workers who clean patient rooms and the critical care physicians who work to save lives.
“Health care workers have pulled together throughout this pandemic,” said Melanie Swift, MD, cochair of the COVID-19 Vaccine Allocation and Distribution Work Group at Mayo Clinic in Rochester, Minn. “We’ve gone through the darkest of years relying so heavily on each other,” she said. “Now we’re pulling together to get out of it.”
Still, a rollout of this magnitude has hitches. Stanford issued an apology Dec. 18 after its medical residents protested a vaccine distribution plan that left out nearly all of its residents and fellows, many of whom regularly treat patients with COVID-19.
There have already been more than 287,000 COVID-19 cases and 953 deaths among health care workers, according to the Centers for Disease Control and Prevention. In its guidance, the agency pointed out that the “continued protection of them at work, at home, and in the community remains a national priority.” That means vaccinating a workforce of about 21 million people, often the largest group of employees in a community.
“It collectively takes all of us to vaccinate our teams to maintain that stability in our health care infrastructure across the metro Atlanta area,” Christy Norman, PharmD, vice president of pharmacy services at Emory Healthcare, told reporters in a briefing as the health system awaited its first delivery.
Don’t waste a dose
One overriding imperative prevails: Hospitals don’t want to waste any doses. The storage requirements of the Pfizer vaccine make that tricky.
Once vials are removed from the pizza-box-shaped containers in ultracold storage and placed in a refrigerator, they must be used within 5 days. Thawed five-dose vials must be brought to room temperature before they are diluted, and they can remain at room temperature for no more than 2 hours. Once they are diluted with 1.8 mL of a 0.9% sodium chloride injection, the vials must be used within 6 hours.
COVID-19 precautions require employees to stay physically distant while they wait their turn for vaccination, which means the process can’t mirror typical large-scale flu immunization programs.
To prioritize groups, the vaccination planners at Mayo conducted a thorough risk stratification, considering each employee’s duties. Do they work in a dedicated COVID-19 unit? Do they handle lab tests or collect swabs? Do they work in the ICU or emergency department?
“We have applied some principles to make sure that as we roll it out, we prioritize people who are at greatest risk of ongoing exposure and who are really critical to maintaining the COVID response and other essential health services,” said Dr. Swift, associate medical director of Mayo’s occupational health service.
Mayo employees who are eligible for the first doses can sign up for appointments through the medical record system. If it seems likely that some doses will be left over at the end of the vaccination period – perhaps because of missed appointments – supervisors in high-risk areas can refer other health care workers. Mayo gave its first vaccines on Dec. 18, but the vaccination program began in earnest the following week. With the pleasant surprise that each five-dose vial actually provides six doses, 474 vials will allow for the vaccination of 2,844 employees in the top-priority group. “It’s going to expand each week or few days as we get more and more vaccine,” Dr. Swift said.
Sharing vials with small rural hospitals
Minnesota is using a hub-and-spoke system to give small rural hospitals access to the Pfizer vaccine, even though they lack ultracold storage and can’t use a minimum order of 975 doses. Large hospitals, acting as hubs, are sharing their orders. (The minimum order for Moderna is 100 doses.)
In south-central Minnesota, for example, two hub hospitals each have six spoke hospitals. Five of the 14 hospitals are independent, and the rest are part of large hospital systems, but affiliation doesn’t matter, said Eric Weller, regional health care preparedness coordinator for the South Central Healthcare Coalition. “We are all working together. It doesn’t matter what system you’re from,” he said. “We’re working for the good of the community.”
Each hospital designed a process to provide vaccine education, prioritize groups, allocate appointments, register people for vaccination, obtain signed consent forms, administer vaccines in a COVID-safe way, and provide follow-up appointments for the second dose. “We’re using some of the lessons we learned during H1N1,” said Mr. Weller, referring to immunization during the 2009 influenza pandemic. “The difference is that during H1N1, you could have lines of people.”
Coordinating the appointments will be more important than ever. “One of the vaccination strategies is to get people in groups of five, so you use one vial on those five people and don’t waste it,” he said.
Logistics are somewhat different for the Moderna vaccine, which will come in 10-dose vials that can be refrigerated for up to 30 days.
Both vaccines may produce mild flulike symptoms, such as fatigue, headache, or muscle pain, particularly after the second dose. That’s a sign that the immune system is reacting to the vaccine, but it’s also another consideration in the vaccination plans, because health care workers might take a day or two off work. “We’re not going to vaccinate a whole department at one time. It will be staggered,” said Kevin Smith, MD, medical director of the occupational medicine program at ProMedica, a health care system based in Toledo, Ohio.
Dr. Smith said he plans to encourage employees to use V-Safe, an app created by the CDC to track adverse effects in people who receive the vaccine. He pointed out that a day or two of achiness will be better than coping with the symptoms of COVID-19. Some employees who recovered from the infection still feel fatigued or haven’t regained their sense of taste and smell. “We are still monitoring quite a few employees to make sure they get back to 100%,” he said.
Hope for ending the pandemic
Public health officials have worried about vaccine hesitancy, even among health care workers, but so far, that concern seems overshadowed by enthusiasm. Dr. Smith said his department has been fielding calls from employees who want to know when they will be able to get the vaccine. “I think everyone feels relief,” he said. “We’re at the beginning of the end.”
At Mayo, Dr. Swift is surveying staff to gauge the willingness to get the vaccine, but she already senses excitement among employees. “No doubt there are still people who are hesitant, but I’m feeling a shift,” she said. “I’m feeling this momentum building of health care workers coming on board and wanting to take this vaccine, which is good, because they will set an example for their patients.”
For Colleen Kelley, MD, an infectious disease physician at Emory University in Atlanta who was principal investigator for an Emory-affiliated Moderna clinical trial site, it has been an emotional time. “Things were looking very bleak and dark for a time, and then we started to get these efficacy results that were greater than anyone imagined,” she said.
Dr. Kelley spends time talking to journalists and educating physician colleagues and hospital employees about how the vaccine was developed so quickly and how it works. “Everyone asks me, ‘Should I get it? Are you going to get it?’ My answer is ‘yes’ and ‘yes,’ “ she said. “I am 1,000% confident that the benefits of widespread vaccination outweigh the risks of continued COVID and a continued pandemic.”
A version of this article first appeared on Medscape.com.
As the first American health care workers rolled up their sleeves for a COVID-19 vaccine, the images were instantly frozen in history, marking the triumph of scientific know-how and ingenuity. Cameras captured the first trucks pulling out of a warehouse in Portage, Mich., to the applause of workers and area residents. A day later, Boston Medical Center employees – some dressed in scrubs and wearing masks, face shields, and protective gowns – literally danced on the sidewalk when doses arrived. Some have photographed themselves getting the vaccine and posted it on social media, tagging it #MyCOVIDVax.
But the real story of the debut of COVID-19 vaccination is more methodical than monumental, a celebration of teamwork rather than of conquest. As hospitals waited for their first allotment, they reviewed their carefully drafted plans. They relied on each other, reaching across the usual divisions of competition and working collaboratively to share the limited supply. Their priority lists for the first vaccinations included environmental services workers who clean patient rooms and the critical care physicians who work to save lives.
“Health care workers have pulled together throughout this pandemic,” said Melanie Swift, MD, cochair of the COVID-19 Vaccine Allocation and Distribution Work Group at Mayo Clinic in Rochester, Minn. “We’ve gone through the darkest of years relying so heavily on each other,” she said. “Now we’re pulling together to get out of it.”
Still, a rollout of this magnitude has hitches. Stanford issued an apology Dec. 18 after its medical residents protested a vaccine distribution plan that left out nearly all of its residents and fellows, many of whom regularly treat patients with COVID-19.
There have already been more than 287,000 COVID-19 cases and 953 deaths among health care workers, according to the Centers for Disease Control and Prevention. In its guidance, the agency pointed out that the “continued protection of them at work, at home, and in the community remains a national priority.” That means vaccinating a workforce of about 21 million people, often the largest group of employees in a community.
“It collectively takes all of us to vaccinate our teams to maintain that stability in our health care infrastructure across the metro Atlanta area,” Christy Norman, PharmD, vice president of pharmacy services at Emory Healthcare, told reporters in a briefing as the health system awaited its first delivery.
Don’t waste a dose
One overriding imperative prevails: Hospitals don’t want to waste any doses. The storage requirements of the Pfizer vaccine make that tricky.
Once vials are removed from the pizza-box-shaped containers in ultracold storage and placed in a refrigerator, they must be used within 5 days. Thawed five-dose vials must be brought to room temperature before they are diluted, and they can remain at room temperature for no more than 2 hours. Once they are diluted with 1.8 mL of a 0.9% sodium chloride injection, the vials must be used within 6 hours.
COVID-19 precautions require employees to stay physically distant while they wait their turn for vaccination, which means the process can’t mirror typical large-scale flu immunization programs.
To prioritize groups, the vaccination planners at Mayo conducted a thorough risk stratification, considering each employee’s duties. Do they work in a dedicated COVID-19 unit? Do they handle lab tests or collect swabs? Do they work in the ICU or emergency department?
“We have applied some principles to make sure that as we roll it out, we prioritize people who are at greatest risk of ongoing exposure and who are really critical to maintaining the COVID response and other essential health services,” said Dr. Swift, associate medical director of Mayo’s occupational health service.
Mayo employees who are eligible for the first doses can sign up for appointments through the medical record system. If it seems likely that some doses will be left over at the end of the vaccination period – perhaps because of missed appointments – supervisors in high-risk areas can refer other health care workers. Mayo gave its first vaccines on Dec. 18, but the vaccination program began in earnest the following week. With the pleasant surprise that each five-dose vial actually provides six doses, 474 vials will allow for the vaccination of 2,844 employees in the top-priority group. “It’s going to expand each week or few days as we get more and more vaccine,” Dr. Swift said.
Sharing vials with small rural hospitals
Minnesota is using a hub-and-spoke system to give small rural hospitals access to the Pfizer vaccine, even though they lack ultracold storage and can’t use a minimum order of 975 doses. Large hospitals, acting as hubs, are sharing their orders. (The minimum order for Moderna is 100 doses.)
In south-central Minnesota, for example, two hub hospitals each have six spoke hospitals. Five of the 14 hospitals are independent, and the rest are part of large hospital systems, but affiliation doesn’t matter, said Eric Weller, regional health care preparedness coordinator for the South Central Healthcare Coalition. “We are all working together. It doesn’t matter what system you’re from,” he said. “We’re working for the good of the community.”
Each hospital designed a process to provide vaccine education, prioritize groups, allocate appointments, register people for vaccination, obtain signed consent forms, administer vaccines in a COVID-safe way, and provide follow-up appointments for the second dose. “We’re using some of the lessons we learned during H1N1,” said Mr. Weller, referring to immunization during the 2009 influenza pandemic. “The difference is that during H1N1, you could have lines of people.”
Coordinating the appointments will be more important than ever. “One of the vaccination strategies is to get people in groups of five, so you use one vial on those five people and don’t waste it,” he said.
Logistics are somewhat different for the Moderna vaccine, which will come in 10-dose vials that can be refrigerated for up to 30 days.
Both vaccines may produce mild flulike symptoms, such as fatigue, headache, or muscle pain, particularly after the second dose. That’s a sign that the immune system is reacting to the vaccine, but it’s also another consideration in the vaccination plans, because health care workers might take a day or two off work. “We’re not going to vaccinate a whole department at one time. It will be staggered,” said Kevin Smith, MD, medical director of the occupational medicine program at ProMedica, a health care system based in Toledo, Ohio.
Dr. Smith said he plans to encourage employees to use V-Safe, an app created by the CDC to track adverse effects in people who receive the vaccine. He pointed out that a day or two of achiness will be better than coping with the symptoms of COVID-19. Some employees who recovered from the infection still feel fatigued or haven’t regained their sense of taste and smell. “We are still monitoring quite a few employees to make sure they get back to 100%,” he said.
Hope for ending the pandemic
Public health officials have worried about vaccine hesitancy, even among health care workers, but so far, that concern seems overshadowed by enthusiasm. Dr. Smith said his department has been fielding calls from employees who want to know when they will be able to get the vaccine. “I think everyone feels relief,” he said. “We’re at the beginning of the end.”
At Mayo, Dr. Swift is surveying staff to gauge the willingness to get the vaccine, but she already senses excitement among employees. “No doubt there are still people who are hesitant, but I’m feeling a shift,” she said. “I’m feeling this momentum building of health care workers coming on board and wanting to take this vaccine, which is good, because they will set an example for their patients.”
For Colleen Kelley, MD, an infectious disease physician at Emory University in Atlanta who was principal investigator for an Emory-affiliated Moderna clinical trial site, it has been an emotional time. “Things were looking very bleak and dark for a time, and then we started to get these efficacy results that were greater than anyone imagined,” she said.
Dr. Kelley spends time talking to journalists and educating physician colleagues and hospital employees about how the vaccine was developed so quickly and how it works. “Everyone asks me, ‘Should I get it? Are you going to get it?’ My answer is ‘yes’ and ‘yes,’ “ she said. “I am 1,000% confident that the benefits of widespread vaccination outweigh the risks of continued COVID and a continued pandemic.”
A version of this article first appeared on Medscape.com.
COVID-19 anticoagulation trials ‘paused’ for futility, safety
Parts of three linked studies investigating increased levels of anticoagulation in hospitalized COVID-19 patients have been “paused” because of futility and safety concerns, a statement from the U.S. National Heart, Lung, and Blood Institute (NHLBI) confirms.
The trials involved are the REMAP-CAP, ACTIV-4, and ATTACC studies.
The statement also says that a potential for harm in this subgroup could not be excluded, noting that increased bleeding is a known complication of full-dose anticoagulation. The trials are working urgently to undertake additional analyses, which will be made available as soon as possible.
The three clinical trial platforms are working together to test the effects of full therapeutic doses of anticoagulants vs. lower prophylactic doses in COVID-19 patients.
Informed by the deliberations of the data safety monitoring boards of these trials, all of the trial sites have paused enrollment of the most critically ill hospitalized patients with COVID-19.
Enrollment continues in the trials for moderately ill hospitalized COVID-19 patients, the statement notes.
“Whether the use of full-dose compared to low-dose anticoagulants leads to better outcomes in hospitalized patients with less COVID-19 severe disease remains a very important question,” the NHLBI statement says.
Patients who require full dose anticoagulants for another medical indication are not included in these trials.
The statement explains that COVID-19 is associated with significant inflammation and clinical and pathologic evidence of widespread blood clots. These trials were launched because clinicians have observed that many patients ill with COVID-19, including those who have died from the disease, formed blood clots throughout their bodies, even in their smallest blood vessels. This unusual clotting can cause multiple health complications, including lung failure, myocardial infarction, and stroke.
The three trials are the result of a collaboration between major international partners. The trials include: the Randomized, Embedded, Multi-factorial Adaptive Platform Trial for Community-Acquired Pneumonia (REMAP-CAP) Therapeutic Anticoagulation; Accelerating COVID-19 Therapeutic Interventions and Vaccines-4 (ACTIV-4) Antithrombotics Inpatient; and Antithrombotic Therapy to Ameliorate Complications of COVID-19 (ATTACC).
The trials, which span four continents, have the common goal of assessing the benefit of full doses of anticoagulants to treat moderately ill or critically ill adults hospitalized for COVID-19, compared with a lower dose often used to prevent blood clots in hospitalized patients.
In the United States, the ACTIV-4 trial is being led by a collaborative effort involving a number of universities, including the University of Pittsburgh and New York University.
The trials are supported by multiple international funding organizations including the National Institutes of Health, Canadian Institutes of Health Research, the National Institute for Health Research (UK), the National Health and Medical Research Council (Australia), and the PREPARE and RECOVER consortia (European Union).
A version of this story first appeared on Medscape.com.
Parts of three linked studies investigating increased levels of anticoagulation in hospitalized COVID-19 patients have been “paused” because of futility and safety concerns, a statement from the U.S. National Heart, Lung, and Blood Institute (NHLBI) confirms.
The trials involved are the REMAP-CAP, ACTIV-4, and ATTACC studies.
The statement also says that a potential for harm in this subgroup could not be excluded, noting that increased bleeding is a known complication of full-dose anticoagulation. The trials are working urgently to undertake additional analyses, which will be made available as soon as possible.
The three clinical trial platforms are working together to test the effects of full therapeutic doses of anticoagulants vs. lower prophylactic doses in COVID-19 patients.
Informed by the deliberations of the data safety monitoring boards of these trials, all of the trial sites have paused enrollment of the most critically ill hospitalized patients with COVID-19.
Enrollment continues in the trials for moderately ill hospitalized COVID-19 patients, the statement notes.
“Whether the use of full-dose compared to low-dose anticoagulants leads to better outcomes in hospitalized patients with less COVID-19 severe disease remains a very important question,” the NHLBI statement says.
Patients who require full dose anticoagulants for another medical indication are not included in these trials.
The statement explains that COVID-19 is associated with significant inflammation and clinical and pathologic evidence of widespread blood clots. These trials were launched because clinicians have observed that many patients ill with COVID-19, including those who have died from the disease, formed blood clots throughout their bodies, even in their smallest blood vessels. This unusual clotting can cause multiple health complications, including lung failure, myocardial infarction, and stroke.
The three trials are the result of a collaboration between major international partners. The trials include: the Randomized, Embedded, Multi-factorial Adaptive Platform Trial for Community-Acquired Pneumonia (REMAP-CAP) Therapeutic Anticoagulation; Accelerating COVID-19 Therapeutic Interventions and Vaccines-4 (ACTIV-4) Antithrombotics Inpatient; and Antithrombotic Therapy to Ameliorate Complications of COVID-19 (ATTACC).
The trials, which span four continents, have the common goal of assessing the benefit of full doses of anticoagulants to treat moderately ill or critically ill adults hospitalized for COVID-19, compared with a lower dose often used to prevent blood clots in hospitalized patients.
In the United States, the ACTIV-4 trial is being led by a collaborative effort involving a number of universities, including the University of Pittsburgh and New York University.
The trials are supported by multiple international funding organizations including the National Institutes of Health, Canadian Institutes of Health Research, the National Institute for Health Research (UK), the National Health and Medical Research Council (Australia), and the PREPARE and RECOVER consortia (European Union).
A version of this story first appeared on Medscape.com.
Parts of three linked studies investigating increased levels of anticoagulation in hospitalized COVID-19 patients have been “paused” because of futility and safety concerns, a statement from the U.S. National Heart, Lung, and Blood Institute (NHLBI) confirms.
The trials involved are the REMAP-CAP, ACTIV-4, and ATTACC studies.
The statement also says that a potential for harm in this subgroup could not be excluded, noting that increased bleeding is a known complication of full-dose anticoagulation. The trials are working urgently to undertake additional analyses, which will be made available as soon as possible.
The three clinical trial platforms are working together to test the effects of full therapeutic doses of anticoagulants vs. lower prophylactic doses in COVID-19 patients.
Informed by the deliberations of the data safety monitoring boards of these trials, all of the trial sites have paused enrollment of the most critically ill hospitalized patients with COVID-19.
Enrollment continues in the trials for moderately ill hospitalized COVID-19 patients, the statement notes.
“Whether the use of full-dose compared to low-dose anticoagulants leads to better outcomes in hospitalized patients with less COVID-19 severe disease remains a very important question,” the NHLBI statement says.
Patients who require full dose anticoagulants for another medical indication are not included in these trials.
The statement explains that COVID-19 is associated with significant inflammation and clinical and pathologic evidence of widespread blood clots. These trials were launched because clinicians have observed that many patients ill with COVID-19, including those who have died from the disease, formed blood clots throughout their bodies, even in their smallest blood vessels. This unusual clotting can cause multiple health complications, including lung failure, myocardial infarction, and stroke.
The three trials are the result of a collaboration between major international partners. The trials include: the Randomized, Embedded, Multi-factorial Adaptive Platform Trial for Community-Acquired Pneumonia (REMAP-CAP) Therapeutic Anticoagulation; Accelerating COVID-19 Therapeutic Interventions and Vaccines-4 (ACTIV-4) Antithrombotics Inpatient; and Antithrombotic Therapy to Ameliorate Complications of COVID-19 (ATTACC).
The trials, which span four continents, have the common goal of assessing the benefit of full doses of anticoagulants to treat moderately ill or critically ill adults hospitalized for COVID-19, compared with a lower dose often used to prevent blood clots in hospitalized patients.
In the United States, the ACTIV-4 trial is being led by a collaborative effort involving a number of universities, including the University of Pittsburgh and New York University.
The trials are supported by multiple international funding organizations including the National Institutes of Health, Canadian Institutes of Health Research, the National Institute for Health Research (UK), the National Health and Medical Research Council (Australia), and the PREPARE and RECOVER consortia (European Union).
A version of this story first appeared on Medscape.com.
COVID-19–induced drop in first measles vaccinations sparks resurgence concerns
Widespread use of the MMR vaccine is not only crucial for protecting the community against infectious outbreaks, but also serves as the overall pacesetter for preventive services, said Sara M. Bode, MD and colleagues at Nationwide Children’s Hospital in Columbus.
As part of a bivariate logistic regression analysis, Dr. Bode and colleagues sought to evaluate changes in measles vaccination rates across 12 clinic sites of the Nationwide Children’s Hospital pediatric primary care network in Columbus among 23,534 children aged 16 months. The study period targeted the time between April and May 2020, when clinic access and appointment attendance declined following the start of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic, until the June-to-August 2020 time period, when clinical care was allowed to return.
The need for the study was prompted by Centers for Disease Control and Prevention reporting on a state-specific precipitous decline in MMR vaccination rates shortly after the onset of COVID-19 in May 2020. Citing the results of one study, such reductions in vaccination have raised concerns over the possibility of a measles resurgence, noted Dr. Bode and associates.
MMR vaccination rates begin to drop with onset of COVID-19 pandemic.
From March 2017 to March 2020, the average rate of MMR vaccination in 16-month-olds was 72%. It subsequently decreased to 67% from April to May 2020, and then dropped further to 62% during the period June to August, 2020 (P = .001). Those without insurance were less likely to be vaccinated than were those carrying private insurance or Medicaid.
Among patients who had not attended a preventive care visit after 12 months of age, the proportion who received vaccines declined during the same time periods, from 10% before the pandemic to 6% at the start of the pandemic and 3% during the summer months of 2020.
“Given the baseline low vaccination rates even before the pandemic and the subsequent decline, we face a critical need to improve timely vaccination and provide catch-up opportunities” in areas with the highest incidence of COVID-19, observed Dr. Bode and colleagues.
Innovative approaches are needed to encourage families to seek preventive care.
In response, the researchers announced the implementation of new community-based vaccination approaches in Ohio, including pop-up vaccine clinics, mobile clinics, and school-based clinics to provide families, who are reluctant to visit health care facilities over COVID-19 related concerns, with safe alternatives. “We believe that it is critical to develop innovative approaches to have families return for preventive care,” they added.
In a separate interview, Herschel Lessin, MD, a private practice pediatrician in Poughkeepsie, N.Y., noted: “This study confirms the anecdotal experience of pediatricians around the country, and our greatest fear that the pandemic will interfere with herd immunity of children for vaccine-preventable illness. Although the study was of urban offices with a primarily Medicaid population, I believe the results to be very worrisome should they prove to be generalizable to the country, as a whole. The significant reduction of well-child visits due to COVID-19 (and fear of COVID-19) seriously impaired the vaccination status of a standard required vaccine in a large population. What is even more worrisome is that the rates continued to fall even after the initial closure of many offices and well into their reopening, despite concerted efforts to try to catch up these missed visits and immunizations.”
Measles is an intensely contagious illness that has not been eradicated, as evidenced by the enormous measles outbreak stemming from Disneyland in 2014-2015, and again with the possible exposure of hundreds to an infected Disneyland visitor last fall, where coverage rates were even higher than in this study, added Dr. Lessin. “This phenomenon, unless forcefully remedied, could easily result in large outbreaks of other vaccine-preventable illness besides COVID-19,” he cautioned.
Dr. Bode and colleagues as well as Dr. Lessin had no conflicts of interest and no relevant financial disclosures.
SOURCE: Bode SM et al. Pediatrics. 2021. doi: 10.1542/peds.2020-035576.
Widespread use of the MMR vaccine is not only crucial for protecting the community against infectious outbreaks, but also serves as the overall pacesetter for preventive services, said Sara M. Bode, MD and colleagues at Nationwide Children’s Hospital in Columbus.
As part of a bivariate logistic regression analysis, Dr. Bode and colleagues sought to evaluate changes in measles vaccination rates across 12 clinic sites of the Nationwide Children’s Hospital pediatric primary care network in Columbus among 23,534 children aged 16 months. The study period targeted the time between April and May 2020, when clinic access and appointment attendance declined following the start of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic, until the June-to-August 2020 time period, when clinical care was allowed to return.
The need for the study was prompted by Centers for Disease Control and Prevention reporting on a state-specific precipitous decline in MMR vaccination rates shortly after the onset of COVID-19 in May 2020. Citing the results of one study, such reductions in vaccination have raised concerns over the possibility of a measles resurgence, noted Dr. Bode and associates.
MMR vaccination rates begin to drop with onset of COVID-19 pandemic.
From March 2017 to March 2020, the average rate of MMR vaccination in 16-month-olds was 72%. It subsequently decreased to 67% from April to May 2020, and then dropped further to 62% during the period June to August, 2020 (P = .001). Those without insurance were less likely to be vaccinated than were those carrying private insurance or Medicaid.
Among patients who had not attended a preventive care visit after 12 months of age, the proportion who received vaccines declined during the same time periods, from 10% before the pandemic to 6% at the start of the pandemic and 3% during the summer months of 2020.
“Given the baseline low vaccination rates even before the pandemic and the subsequent decline, we face a critical need to improve timely vaccination and provide catch-up opportunities” in areas with the highest incidence of COVID-19, observed Dr. Bode and colleagues.
Innovative approaches are needed to encourage families to seek preventive care.
In response, the researchers announced the implementation of new community-based vaccination approaches in Ohio, including pop-up vaccine clinics, mobile clinics, and school-based clinics to provide families, who are reluctant to visit health care facilities over COVID-19 related concerns, with safe alternatives. “We believe that it is critical to develop innovative approaches to have families return for preventive care,” they added.
In a separate interview, Herschel Lessin, MD, a private practice pediatrician in Poughkeepsie, N.Y., noted: “This study confirms the anecdotal experience of pediatricians around the country, and our greatest fear that the pandemic will interfere with herd immunity of children for vaccine-preventable illness. Although the study was of urban offices with a primarily Medicaid population, I believe the results to be very worrisome should they prove to be generalizable to the country, as a whole. The significant reduction of well-child visits due to COVID-19 (and fear of COVID-19) seriously impaired the vaccination status of a standard required vaccine in a large population. What is even more worrisome is that the rates continued to fall even after the initial closure of many offices and well into their reopening, despite concerted efforts to try to catch up these missed visits and immunizations.”
Measles is an intensely contagious illness that has not been eradicated, as evidenced by the enormous measles outbreak stemming from Disneyland in 2014-2015, and again with the possible exposure of hundreds to an infected Disneyland visitor last fall, where coverage rates were even higher than in this study, added Dr. Lessin. “This phenomenon, unless forcefully remedied, could easily result in large outbreaks of other vaccine-preventable illness besides COVID-19,” he cautioned.
Dr. Bode and colleagues as well as Dr. Lessin had no conflicts of interest and no relevant financial disclosures.
SOURCE: Bode SM et al. Pediatrics. 2021. doi: 10.1542/peds.2020-035576.
Widespread use of the MMR vaccine is not only crucial for protecting the community against infectious outbreaks, but also serves as the overall pacesetter for preventive services, said Sara M. Bode, MD and colleagues at Nationwide Children’s Hospital in Columbus.
As part of a bivariate logistic regression analysis, Dr. Bode and colleagues sought to evaluate changes in measles vaccination rates across 12 clinic sites of the Nationwide Children’s Hospital pediatric primary care network in Columbus among 23,534 children aged 16 months. The study period targeted the time between April and May 2020, when clinic access and appointment attendance declined following the start of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic, until the June-to-August 2020 time period, when clinical care was allowed to return.
The need for the study was prompted by Centers for Disease Control and Prevention reporting on a state-specific precipitous decline in MMR vaccination rates shortly after the onset of COVID-19 in May 2020. Citing the results of one study, such reductions in vaccination have raised concerns over the possibility of a measles resurgence, noted Dr. Bode and associates.
MMR vaccination rates begin to drop with onset of COVID-19 pandemic.
From March 2017 to March 2020, the average rate of MMR vaccination in 16-month-olds was 72%. It subsequently decreased to 67% from April to May 2020, and then dropped further to 62% during the period June to August, 2020 (P = .001). Those without insurance were less likely to be vaccinated than were those carrying private insurance or Medicaid.
Among patients who had not attended a preventive care visit after 12 months of age, the proportion who received vaccines declined during the same time periods, from 10% before the pandemic to 6% at the start of the pandemic and 3% during the summer months of 2020.
“Given the baseline low vaccination rates even before the pandemic and the subsequent decline, we face a critical need to improve timely vaccination and provide catch-up opportunities” in areas with the highest incidence of COVID-19, observed Dr. Bode and colleagues.
Innovative approaches are needed to encourage families to seek preventive care.
In response, the researchers announced the implementation of new community-based vaccination approaches in Ohio, including pop-up vaccine clinics, mobile clinics, and school-based clinics to provide families, who are reluctant to visit health care facilities over COVID-19 related concerns, with safe alternatives. “We believe that it is critical to develop innovative approaches to have families return for preventive care,” they added.
In a separate interview, Herschel Lessin, MD, a private practice pediatrician in Poughkeepsie, N.Y., noted: “This study confirms the anecdotal experience of pediatricians around the country, and our greatest fear that the pandemic will interfere with herd immunity of children for vaccine-preventable illness. Although the study was of urban offices with a primarily Medicaid population, I believe the results to be very worrisome should they prove to be generalizable to the country, as a whole. The significant reduction of well-child visits due to COVID-19 (and fear of COVID-19) seriously impaired the vaccination status of a standard required vaccine in a large population. What is even more worrisome is that the rates continued to fall even after the initial closure of many offices and well into their reopening, despite concerted efforts to try to catch up these missed visits and immunizations.”
Measles is an intensely contagious illness that has not been eradicated, as evidenced by the enormous measles outbreak stemming from Disneyland in 2014-2015, and again with the possible exposure of hundreds to an infected Disneyland visitor last fall, where coverage rates were even higher than in this study, added Dr. Lessin. “This phenomenon, unless forcefully remedied, could easily result in large outbreaks of other vaccine-preventable illness besides COVID-19,” he cautioned.
Dr. Bode and colleagues as well as Dr. Lessin had no conflicts of interest and no relevant financial disclosures.
SOURCE: Bode SM et al. Pediatrics. 2021. doi: 10.1542/peds.2020-035576.
FROM PEDIATRICS
Latest rise in child COVID-19 cases is relatively small
For the seventh week out of the last eight, more new cases of COVID-19 in children were reported in the United States than any week before, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
There were just over 182,000 new cases of COVID-19 in children during the week ending Dec. 17, topping the previous high of almost 179,000 set the previous week. – a stretch of 11 weeks that has produced only one decline, based on data from the latest AAP/CHA weekly report.
As of Dec. 17, there had been over 1.8 million cases of COVID-19 in children, which represents 12.3% of all U.S. cases. For the week, 14% of all cases occurred in children, which was up slightly from 13.8% the week before (Dec. 10). The overall rate of coronavirus infection is now 2,420 cases per 100,000 children in the population, the AAP and CHA said.
A total of 30 states are above that national rate, with North Dakota the highest at 7,515 cases per 100,000 children, followed by South Dakota (5,618), Wyoming (5,157), Wisconsin (5,106), and Tennessee (4,994). Wyoming has the highest proportion of cases occurring in children at 20.8%, but that is down from 23.4% in mid-November, based on data collected by the AAP and CHA from the health department websites of 49 states (New York does not provide age distributions), the District of Columbia, New York City, Puerto Rico, and Guam.
In the last 2 weeks, however, the largest percent increases in new cases came in states with low-to-average rates of cumulative child infection. California, Connecticut, Delaware, Maine, Maryland, New Hampshire, and Vermont all saw increases of over 35% from Dec. 3 to Dec. 17, while the smallest increases occurred in Hawaii, North Dakota, and Wyoming, the AAP and CHA reported.
For the seventh week out of the last eight, more new cases of COVID-19 in children were reported in the United States than any week before, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

There were just over 182,000 new cases of COVID-19 in children during the week ending Dec. 17, topping the previous high of almost 179,000 set the previous week. – a stretch of 11 weeks that has produced only one decline, based on data from the latest AAP/CHA weekly report.
As of Dec. 17, there had been over 1.8 million cases of COVID-19 in children, which represents 12.3% of all U.S. cases. For the week, 14% of all cases occurred in children, which was up slightly from 13.8% the week before (Dec. 10). The overall rate of coronavirus infection is now 2,420 cases per 100,000 children in the population, the AAP and CHA said.
A total of 30 states are above that national rate, with North Dakota the highest at 7,515 cases per 100,000 children, followed by South Dakota (5,618), Wyoming (5,157), Wisconsin (5,106), and Tennessee (4,994). Wyoming has the highest proportion of cases occurring in children at 20.8%, but that is down from 23.4% in mid-November, based on data collected by the AAP and CHA from the health department websites of 49 states (New York does not provide age distributions), the District of Columbia, New York City, Puerto Rico, and Guam.
In the last 2 weeks, however, the largest percent increases in new cases came in states with low-to-average rates of cumulative child infection. California, Connecticut, Delaware, Maine, Maryland, New Hampshire, and Vermont all saw increases of over 35% from Dec. 3 to Dec. 17, while the smallest increases occurred in Hawaii, North Dakota, and Wyoming, the AAP and CHA reported.
For the seventh week out of the last eight, more new cases of COVID-19 in children were reported in the United States than any week before, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

There were just over 182,000 new cases of COVID-19 in children during the week ending Dec. 17, topping the previous high of almost 179,000 set the previous week. – a stretch of 11 weeks that has produced only one decline, based on data from the latest AAP/CHA weekly report.
As of Dec. 17, there had been over 1.8 million cases of COVID-19 in children, which represents 12.3% of all U.S. cases. For the week, 14% of all cases occurred in children, which was up slightly from 13.8% the week before (Dec. 10). The overall rate of coronavirus infection is now 2,420 cases per 100,000 children in the population, the AAP and CHA said.
A total of 30 states are above that national rate, with North Dakota the highest at 7,515 cases per 100,000 children, followed by South Dakota (5,618), Wyoming (5,157), Wisconsin (5,106), and Tennessee (4,994). Wyoming has the highest proportion of cases occurring in children at 20.8%, but that is down from 23.4% in mid-November, based on data collected by the AAP and CHA from the health department websites of 49 states (New York does not provide age distributions), the District of Columbia, New York City, Puerto Rico, and Guam.
In the last 2 weeks, however, the largest percent increases in new cases came in states with low-to-average rates of cumulative child infection. California, Connecticut, Delaware, Maine, Maryland, New Hampshire, and Vermont all saw increases of over 35% from Dec. 3 to Dec. 17, while the smallest increases occurred in Hawaii, North Dakota, and Wyoming, the AAP and CHA reported.
Shortcomings identified in study of acne videos on TikTok
, according to an analysis of the top 100 videos using a consumer health validation tool.
The popularity of TikTok among adolescents in particular has implications for the dissemination of acne information, as some teens become “skinfluencers” and receive sponsorship from skin care brands in exchange for social media promotion, wrote David X. Zheng, BA, of the department of dermatology, Case Western Reserve University, Cleveland, and colleagues.
“However, the quality of dermatologic information found on TikTok is largely unknown,” they said.
In a brief report published in Pediatric Dermatology, the researchers identified the top 100 videos on TikTok on May 1, 2020, that were tagged with “#acne.” The information on each video included date of upload, type and gender of the individual uploading the video, physician specialty if applicable, and video category. These top 100 videos had 13,470,501 likes and 64,775 comments over a 7.6-month time period.
The researchers used the DISCERN criteria, a validated 1-5 scale designed to assess consumer health information, to evaluate the video content, with 1 (having “serious” or “extensive shortcomings”) and 5 (having “minimal shortcomings.”)
Overall, the average quality rating of the TikTok acne videos was 2.03. A total of 9 videos were produced by board-certified physicians in the United States, with an average DISCERN score of 2.41.
“Analysis of the DISCERN criteria dimensions suggested that major shortcomings common to both physician and nonphysician uploaders included failure to cite information sources, discuss treatment risks, and provide support for shared decision-making,” the researchers said.
Approximately one-third (34%) of the videos fell into the treatment-product advertisement category, while 26% were personal anecdotes, 20% presented information related to acne, 13% featured home remedy treatments, and 7% were classified as “other.” The researchers also identified the top 200 “#acne” videos on TikTok once a week from May 8, 2020 to June 5, 2020, to determine the evolution of acne content on the app and found a turnover rate of 10.9% per week.
Based on the high turnover and low quality based on DISCERN ratings, the authors suggested that patients seeking acne information should “view acne-related TikTok videos with caution and consult evidence-based resources whenever possible.”
The study findings were limited by several factors including the small sample size of physicians uploading videos, lack of information about the number of nonphysician medical professionals who uploaded videos, and lack of information about the number of video views and country of origin, the researchers noted. However, the results highlight the need for dermatologists to be aware that patients, especially teens, may be using TikTok for acne information that may be of poor quality, they said.
“Conversely, we understand that social media can be a powerful tool for advancing health literacy,” the researchers noted. “Therefore, we also recommend that health care professionals engaging on TikTok create thorough and perhaps standardized educational videos regarding acne, as well as correct any acne-related misinformation that may be present,” they concluded.
The other authors of the study were from the departments of dermatology at Case Western Reserve, University Hospitals Cleveland, and Johns Hopkins University, Baltimore.
The study received no outside funding. The researchers had no financial conflicts to disclose.
SOURCE: Zheng DX et al. Pediatr Dermatol. 2020 Nov 28. doi: 10.1111/pde.14471.
, according to an analysis of the top 100 videos using a consumer health validation tool.
The popularity of TikTok among adolescents in particular has implications for the dissemination of acne information, as some teens become “skinfluencers” and receive sponsorship from skin care brands in exchange for social media promotion, wrote David X. Zheng, BA, of the department of dermatology, Case Western Reserve University, Cleveland, and colleagues.
“However, the quality of dermatologic information found on TikTok is largely unknown,” they said.
In a brief report published in Pediatric Dermatology, the researchers identified the top 100 videos on TikTok on May 1, 2020, that were tagged with “#acne.” The information on each video included date of upload, type and gender of the individual uploading the video, physician specialty if applicable, and video category. These top 100 videos had 13,470,501 likes and 64,775 comments over a 7.6-month time period.
The researchers used the DISCERN criteria, a validated 1-5 scale designed to assess consumer health information, to evaluate the video content, with 1 (having “serious” or “extensive shortcomings”) and 5 (having “minimal shortcomings.”)
Overall, the average quality rating of the TikTok acne videos was 2.03. A total of 9 videos were produced by board-certified physicians in the United States, with an average DISCERN score of 2.41.
“Analysis of the DISCERN criteria dimensions suggested that major shortcomings common to both physician and nonphysician uploaders included failure to cite information sources, discuss treatment risks, and provide support for shared decision-making,” the researchers said.
Approximately one-third (34%) of the videos fell into the treatment-product advertisement category, while 26% were personal anecdotes, 20% presented information related to acne, 13% featured home remedy treatments, and 7% were classified as “other.” The researchers also identified the top 200 “#acne” videos on TikTok once a week from May 8, 2020 to June 5, 2020, to determine the evolution of acne content on the app and found a turnover rate of 10.9% per week.
Based on the high turnover and low quality based on DISCERN ratings, the authors suggested that patients seeking acne information should “view acne-related TikTok videos with caution and consult evidence-based resources whenever possible.”
The study findings were limited by several factors including the small sample size of physicians uploading videos, lack of information about the number of nonphysician medical professionals who uploaded videos, and lack of information about the number of video views and country of origin, the researchers noted. However, the results highlight the need for dermatologists to be aware that patients, especially teens, may be using TikTok for acne information that may be of poor quality, they said.
“Conversely, we understand that social media can be a powerful tool for advancing health literacy,” the researchers noted. “Therefore, we also recommend that health care professionals engaging on TikTok create thorough and perhaps standardized educational videos regarding acne, as well as correct any acne-related misinformation that may be present,” they concluded.
The other authors of the study were from the departments of dermatology at Case Western Reserve, University Hospitals Cleveland, and Johns Hopkins University, Baltimore.
The study received no outside funding. The researchers had no financial conflicts to disclose.
SOURCE: Zheng DX et al. Pediatr Dermatol. 2020 Nov 28. doi: 10.1111/pde.14471.
, according to an analysis of the top 100 videos using a consumer health validation tool.
The popularity of TikTok among adolescents in particular has implications for the dissemination of acne information, as some teens become “skinfluencers” and receive sponsorship from skin care brands in exchange for social media promotion, wrote David X. Zheng, BA, of the department of dermatology, Case Western Reserve University, Cleveland, and colleagues.
“However, the quality of dermatologic information found on TikTok is largely unknown,” they said.
In a brief report published in Pediatric Dermatology, the researchers identified the top 100 videos on TikTok on May 1, 2020, that were tagged with “#acne.” The information on each video included date of upload, type and gender of the individual uploading the video, physician specialty if applicable, and video category. These top 100 videos had 13,470,501 likes and 64,775 comments over a 7.6-month time period.
The researchers used the DISCERN criteria, a validated 1-5 scale designed to assess consumer health information, to evaluate the video content, with 1 (having “serious” or “extensive shortcomings”) and 5 (having “minimal shortcomings.”)
Overall, the average quality rating of the TikTok acne videos was 2.03. A total of 9 videos were produced by board-certified physicians in the United States, with an average DISCERN score of 2.41.
“Analysis of the DISCERN criteria dimensions suggested that major shortcomings common to both physician and nonphysician uploaders included failure to cite information sources, discuss treatment risks, and provide support for shared decision-making,” the researchers said.
Approximately one-third (34%) of the videos fell into the treatment-product advertisement category, while 26% were personal anecdotes, 20% presented information related to acne, 13% featured home remedy treatments, and 7% were classified as “other.” The researchers also identified the top 200 “#acne” videos on TikTok once a week from May 8, 2020 to June 5, 2020, to determine the evolution of acne content on the app and found a turnover rate of 10.9% per week.
Based on the high turnover and low quality based on DISCERN ratings, the authors suggested that patients seeking acne information should “view acne-related TikTok videos with caution and consult evidence-based resources whenever possible.”
The study findings were limited by several factors including the small sample size of physicians uploading videos, lack of information about the number of nonphysician medical professionals who uploaded videos, and lack of information about the number of video views and country of origin, the researchers noted. However, the results highlight the need for dermatologists to be aware that patients, especially teens, may be using TikTok for acne information that may be of poor quality, they said.
“Conversely, we understand that social media can be a powerful tool for advancing health literacy,” the researchers noted. “Therefore, we also recommend that health care professionals engaging on TikTok create thorough and perhaps standardized educational videos regarding acne, as well as correct any acne-related misinformation that may be present,” they concluded.
The other authors of the study were from the departments of dermatology at Case Western Reserve, University Hospitals Cleveland, and Johns Hopkins University, Baltimore.
The study received no outside funding. The researchers had no financial conflicts to disclose.
SOURCE: Zheng DX et al. Pediatr Dermatol. 2020 Nov 28. doi: 10.1111/pde.14471.
FROM PEDIATRIC DERMATOLOGY