User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Family physicians can help achieve national goals on STIs
Among these are the U.S. Department of Health and Human Services’ first “Sexually Transmitted Infections (STIs) National Strategic Plan for the United States,” which has a strong encompassing vision.
“The United States will be a place where sexually transmitted infections are prevented and where every person has high-quality STI prevention care, and treatment while living free from stigma and discrimination. The vision includes all people, regardless of age, sex, gender identity, sexual orientation, race, ethnicity, religion, disability, geographic location, or socioeconomic circumstance,” the new HHS plan states.1
Family physicians can and should play important roles in helping our country meet this plan’s goals particularly by following two important updated clinical guidelines, one from the U.S. Preventive Services Task Force (USPSTF) and another from the Centers for Disease Control and Prevention (CDC).
This strategic plan includes the following five overarching goals with associated objectives:
- Prevent New STIs.
- Improve the health of people by reducing adverse outcomes of STIs.
- Accelerate progress in STI research, technology, and innovation.
- Reduce STI-related health disparities and health inequities.
- Achieve integrated, coordinated efforts that address the STI epidemic.1
In my opinion, family physicians have important roles to play in order for each of these goals to be achieved.Unfortunately, there are approximately 20 million new cases of STIs each year, and the U.S. has seen increases in the rates of STIs in the past decade.
“Sexually transmitted infections are frequently asymptomatic, which may delay diagnosis and treatment and lead persons to unknowingly transmit STIs to others,” according to a new recommendation statement from the USPSTF.2 STIs may lead to serious health consequences for patients, cause harms to a mother and infant during pregnancy, and lead to cases of cancer among other concerning outcomes. As such, following the HHS new national strategic plan is critical for us to address the needs of our communities.
Preventing new STIs
Family physicians can be vital in achieving the first goal of the plan by helping to prevent new STIs. In August 2020, the USPSTF updated its guideline on behavioral counseling interventions to prevent STIs. In my opinion, the USPSTF offers some practical improvements from the earlier version of this guideline.
The task force provides a grade B recommendation that all sexually active adolescents and adults at increased risk for STIs be provided with behavioral counseling to prevent STIs. The guideline indicates that behavioral counseling interventions reduce the likelihood of those at increased risk for acquiring STIs.2
The 2014 guideline had recommended intensive interventions with a minimum of 30 minutes of counseling. Many family physicians may have found this previous recommendation impractical to implement. These updated recommendations now include a variety of interventions, such as those that take less than 30 minutes.
Although interventions with more than 120 minutes of contact time had the most effect, those with less than 30 minutes still demonstrated statistically significant fewer acquisitions of STIs during follow-up. These options include in-person counseling, and providing written materials, websites, videos, and telephone and text support to patients. These interventions can be delivered directly by the family physician, or patients may be referred to other settings or the media interventions.
The task force’s updated recommendation statement refers to a variety of resources that can be used to identify these interventions. Many of the studies reviewed for this guideline were conducted in STI clinics, and the guideline authors recommended further studies in primary care as opportunities for more generalizability.
In addition to behavioral counseling for STI prevention, family physicians can help prevent STIs in their patients through HPV vaccination and HIV pre-exposure prophylaxis (PrEP provision) within their practices. As the first contact for health care for many patients, we have an opportunity to significantly impact this first goal of prevention.
Treating STIs
Within the second goal of the national strategic plan is treatment of STIs, which family physicians should include in their practices as well as the diagnosis of STIs.
In December 2020, an update to the CDC’s treatment guideline for gonococcal infection was released. Prior to the publishing of this updated recommendation, the CDC recommended combination therapy of 250 mg intramuscular (IM) dose of ceftriaxone and either doxycycline or azithromycin. This recommendation has been changed to a single 500-mg IM dose of ceftriaxone for uncomplicated urogenital, anorectal, and pharyngeal gonorrhea. If chlamydia cannot be excluded, then the addition of oral doxycycline 100 mg twice daily for 7 days is recommended for nonpregnant persons, and 1 g oral azithromycin for pregnant persons. The previous treatment was recommended based on a concern for gonococcal resistance.
This updated guideline reflects increasing concerns for antimicrobial stewardship and emerging azithromycin resistance. It does not recommend a test-of-cure for urogenital or rectal gonorrhea, though did recommend a test-of-cure 7-14 days after treatment of pharyngeal gonorrhea. The guideline also recommends testing for reinfection 3-12 months after treatment as the rate of reinfection ranges from 7% to 12% among those previously treated.3
For some offices, the provision of the IM injection may be challenging, though having this medication in stock with the possibility of provision can greatly improve access and ease of treatment for patients. Family physicians can incorporate these updated recommendations along with those for other STIs such as chlamydia and syphilis with standing orders for treatment and testing within their offices.
Accelerating progress in STI research
Family physicians can also support the national strategic plan by participating in studies looking at the impact of behavioral counseling in the primary care office as opposed to in STI clinics. In addition, by following the STI treatment and screening guidelines, family physicians will contribute to the body of knowledge of prevalence, treatment failure, and reinfection rates of STIs. We can also help advance the research by providing feedback on interventions that have success within our practices.
Reducing STI-related health disparities and inequities
Family physicians are also in important places to support the strategic plan’s fourth goal of reducing health disparities and health inequities.
If we continue to ask the questions to identify those at high risk and ensure that we are offering appropriate STI prevention, care, and treatment services within our clinics, we can expand access to all who need services and improve equity. By offering these services within the primary care office, we may be able to decrease the stigma some may feel going to an STI clinic for services.
By incorporating additional screening and counseling in our practices we may identify some patients who were not aware that they were at risk for an STI and offer them preventive services.
Achieving integrated and coordinated efforts
Finally, as many family physicians have integrated practices, we are uniquely poised to support the fifth goal of the strategic plan of achieving integrated and coordinated efforts addressing the STI epidemic. In our practices we can participate in, lead, and refer to programs for substance use disorders, viral hepatitis, STIs, and HIV as part of full scope primary care.
Family physicians and other primary care providers should work to support the entire strategic plan to ensure that we are fully caring for our patients and communities and stopping the past decade’s increase in STIs. We have an opportunity to use this strategy and make a large impact in our communities.
Dr. Wheat is a family physician at Erie Family Health Center in Chicago. She is program director of Northwestern’s McGaw Family Medicine residency program at Humboldt Park, Chicago. Dr. Wheat serves on the editorial advisory board of Family Practice News. You can contact her at [email protected].
References
1. U.S. Department of Health and Human Services. 2020. Sexually Transmitted Infections National Strategic Plan for the United States: 2021-2025. Washington.
2. U.S. Preventive Services Task Force. Behavioral counseling interventions to prevent sexually transmitted infections: U.S. Preventive Services Task Force Recommendation Statement. JAMA. 2020;324(7):674-81. doi: 10.1001/jama.2020.13095.
3. St. Cyr S et al. Update to CDC’s Treatment Guideline for Gonococcal Infection, 2020. MMWR Morb Mortal Wkly Rep 2020;69:1911-6. doi: 10.15585/mmwr.mm6950a6external_icon.
Among these are the U.S. Department of Health and Human Services’ first “Sexually Transmitted Infections (STIs) National Strategic Plan for the United States,” which has a strong encompassing vision.
“The United States will be a place where sexually transmitted infections are prevented and where every person has high-quality STI prevention care, and treatment while living free from stigma and discrimination. The vision includes all people, regardless of age, sex, gender identity, sexual orientation, race, ethnicity, religion, disability, geographic location, or socioeconomic circumstance,” the new HHS plan states.1
Family physicians can and should play important roles in helping our country meet this plan’s goals particularly by following two important updated clinical guidelines, one from the U.S. Preventive Services Task Force (USPSTF) and another from the Centers for Disease Control and Prevention (CDC).
This strategic plan includes the following five overarching goals with associated objectives:
- Prevent New STIs.
- Improve the health of people by reducing adverse outcomes of STIs.
- Accelerate progress in STI research, technology, and innovation.
- Reduce STI-related health disparities and health inequities.
- Achieve integrated, coordinated efforts that address the STI epidemic.1
In my opinion, family physicians have important roles to play in order for each of these goals to be achieved.Unfortunately, there are approximately 20 million new cases of STIs each year, and the U.S. has seen increases in the rates of STIs in the past decade.
“Sexually transmitted infections are frequently asymptomatic, which may delay diagnosis and treatment and lead persons to unknowingly transmit STIs to others,” according to a new recommendation statement from the USPSTF.2 STIs may lead to serious health consequences for patients, cause harms to a mother and infant during pregnancy, and lead to cases of cancer among other concerning outcomes. As such, following the HHS new national strategic plan is critical for us to address the needs of our communities.
Preventing new STIs
Family physicians can be vital in achieving the first goal of the plan by helping to prevent new STIs. In August 2020, the USPSTF updated its guideline on behavioral counseling interventions to prevent STIs. In my opinion, the USPSTF offers some practical improvements from the earlier version of this guideline.
The task force provides a grade B recommendation that all sexually active adolescents and adults at increased risk for STIs be provided with behavioral counseling to prevent STIs. The guideline indicates that behavioral counseling interventions reduce the likelihood of those at increased risk for acquiring STIs.2
The 2014 guideline had recommended intensive interventions with a minimum of 30 minutes of counseling. Many family physicians may have found this previous recommendation impractical to implement. These updated recommendations now include a variety of interventions, such as those that take less than 30 minutes.
Although interventions with more than 120 minutes of contact time had the most effect, those with less than 30 minutes still demonstrated statistically significant fewer acquisitions of STIs during follow-up. These options include in-person counseling, and providing written materials, websites, videos, and telephone and text support to patients. These interventions can be delivered directly by the family physician, or patients may be referred to other settings or the media interventions.
The task force’s updated recommendation statement refers to a variety of resources that can be used to identify these interventions. Many of the studies reviewed for this guideline were conducted in STI clinics, and the guideline authors recommended further studies in primary care as opportunities for more generalizability.
In addition to behavioral counseling for STI prevention, family physicians can help prevent STIs in their patients through HPV vaccination and HIV pre-exposure prophylaxis (PrEP provision) within their practices. As the first contact for health care for many patients, we have an opportunity to significantly impact this first goal of prevention.
Treating STIs
Within the second goal of the national strategic plan is treatment of STIs, which family physicians should include in their practices as well as the diagnosis of STIs.
In December 2020, an update to the CDC’s treatment guideline for gonococcal infection was released. Prior to the publishing of this updated recommendation, the CDC recommended combination therapy of 250 mg intramuscular (IM) dose of ceftriaxone and either doxycycline or azithromycin. This recommendation has been changed to a single 500-mg IM dose of ceftriaxone for uncomplicated urogenital, anorectal, and pharyngeal gonorrhea. If chlamydia cannot be excluded, then the addition of oral doxycycline 100 mg twice daily for 7 days is recommended for nonpregnant persons, and 1 g oral azithromycin for pregnant persons. The previous treatment was recommended based on a concern for gonococcal resistance.
This updated guideline reflects increasing concerns for antimicrobial stewardship and emerging azithromycin resistance. It does not recommend a test-of-cure for urogenital or rectal gonorrhea, though did recommend a test-of-cure 7-14 days after treatment of pharyngeal gonorrhea. The guideline also recommends testing for reinfection 3-12 months after treatment as the rate of reinfection ranges from 7% to 12% among those previously treated.3
For some offices, the provision of the IM injection may be challenging, though having this medication in stock with the possibility of provision can greatly improve access and ease of treatment for patients. Family physicians can incorporate these updated recommendations along with those for other STIs such as chlamydia and syphilis with standing orders for treatment and testing within their offices.
Accelerating progress in STI research
Family physicians can also support the national strategic plan by participating in studies looking at the impact of behavioral counseling in the primary care office as opposed to in STI clinics. In addition, by following the STI treatment and screening guidelines, family physicians will contribute to the body of knowledge of prevalence, treatment failure, and reinfection rates of STIs. We can also help advance the research by providing feedback on interventions that have success within our practices.
Reducing STI-related health disparities and inequities
Family physicians are also in important places to support the strategic plan’s fourth goal of reducing health disparities and health inequities.
If we continue to ask the questions to identify those at high risk and ensure that we are offering appropriate STI prevention, care, and treatment services within our clinics, we can expand access to all who need services and improve equity. By offering these services within the primary care office, we may be able to decrease the stigma some may feel going to an STI clinic for services.
By incorporating additional screening and counseling in our practices we may identify some patients who were not aware that they were at risk for an STI and offer them preventive services.
Achieving integrated and coordinated efforts
Finally, as many family physicians have integrated practices, we are uniquely poised to support the fifth goal of the strategic plan of achieving integrated and coordinated efforts addressing the STI epidemic. In our practices we can participate in, lead, and refer to programs for substance use disorders, viral hepatitis, STIs, and HIV as part of full scope primary care.
Family physicians and other primary care providers should work to support the entire strategic plan to ensure that we are fully caring for our patients and communities and stopping the past decade’s increase in STIs. We have an opportunity to use this strategy and make a large impact in our communities.
Dr. Wheat is a family physician at Erie Family Health Center in Chicago. She is program director of Northwestern’s McGaw Family Medicine residency program at Humboldt Park, Chicago. Dr. Wheat serves on the editorial advisory board of Family Practice News. You can contact her at [email protected].
References
1. U.S. Department of Health and Human Services. 2020. Sexually Transmitted Infections National Strategic Plan for the United States: 2021-2025. Washington.
2. U.S. Preventive Services Task Force. Behavioral counseling interventions to prevent sexually transmitted infections: U.S. Preventive Services Task Force Recommendation Statement. JAMA. 2020;324(7):674-81. doi: 10.1001/jama.2020.13095.
3. St. Cyr S et al. Update to CDC’s Treatment Guideline for Gonococcal Infection, 2020. MMWR Morb Mortal Wkly Rep 2020;69:1911-6. doi: 10.15585/mmwr.mm6950a6external_icon.
Among these are the U.S. Department of Health and Human Services’ first “Sexually Transmitted Infections (STIs) National Strategic Plan for the United States,” which has a strong encompassing vision.
“The United States will be a place where sexually transmitted infections are prevented and where every person has high-quality STI prevention care, and treatment while living free from stigma and discrimination. The vision includes all people, regardless of age, sex, gender identity, sexual orientation, race, ethnicity, religion, disability, geographic location, or socioeconomic circumstance,” the new HHS plan states.1
Family physicians can and should play important roles in helping our country meet this plan’s goals particularly by following two important updated clinical guidelines, one from the U.S. Preventive Services Task Force (USPSTF) and another from the Centers for Disease Control and Prevention (CDC).
This strategic plan includes the following five overarching goals with associated objectives:
- Prevent New STIs.
- Improve the health of people by reducing adverse outcomes of STIs.
- Accelerate progress in STI research, technology, and innovation.
- Reduce STI-related health disparities and health inequities.
- Achieve integrated, coordinated efforts that address the STI epidemic.1
In my opinion, family physicians have important roles to play in order for each of these goals to be achieved.Unfortunately, there are approximately 20 million new cases of STIs each year, and the U.S. has seen increases in the rates of STIs in the past decade.
“Sexually transmitted infections are frequently asymptomatic, which may delay diagnosis and treatment and lead persons to unknowingly transmit STIs to others,” according to a new recommendation statement from the USPSTF.2 STIs may lead to serious health consequences for patients, cause harms to a mother and infant during pregnancy, and lead to cases of cancer among other concerning outcomes. As such, following the HHS new national strategic plan is critical for us to address the needs of our communities.
Preventing new STIs
Family physicians can be vital in achieving the first goal of the plan by helping to prevent new STIs. In August 2020, the USPSTF updated its guideline on behavioral counseling interventions to prevent STIs. In my opinion, the USPSTF offers some practical improvements from the earlier version of this guideline.
The task force provides a grade B recommendation that all sexually active adolescents and adults at increased risk for STIs be provided with behavioral counseling to prevent STIs. The guideline indicates that behavioral counseling interventions reduce the likelihood of those at increased risk for acquiring STIs.2
The 2014 guideline had recommended intensive interventions with a minimum of 30 minutes of counseling. Many family physicians may have found this previous recommendation impractical to implement. These updated recommendations now include a variety of interventions, such as those that take less than 30 minutes.
Although interventions with more than 120 minutes of contact time had the most effect, those with less than 30 minutes still demonstrated statistically significant fewer acquisitions of STIs during follow-up. These options include in-person counseling, and providing written materials, websites, videos, and telephone and text support to patients. These interventions can be delivered directly by the family physician, or patients may be referred to other settings or the media interventions.
The task force’s updated recommendation statement refers to a variety of resources that can be used to identify these interventions. Many of the studies reviewed for this guideline were conducted in STI clinics, and the guideline authors recommended further studies in primary care as opportunities for more generalizability.
In addition to behavioral counseling for STI prevention, family physicians can help prevent STIs in their patients through HPV vaccination and HIV pre-exposure prophylaxis (PrEP provision) within their practices. As the first contact for health care for many patients, we have an opportunity to significantly impact this first goal of prevention.
Treating STIs
Within the second goal of the national strategic plan is treatment of STIs, which family physicians should include in their practices as well as the diagnosis of STIs.
In December 2020, an update to the CDC’s treatment guideline for gonococcal infection was released. Prior to the publishing of this updated recommendation, the CDC recommended combination therapy of 250 mg intramuscular (IM) dose of ceftriaxone and either doxycycline or azithromycin. This recommendation has been changed to a single 500-mg IM dose of ceftriaxone for uncomplicated urogenital, anorectal, and pharyngeal gonorrhea. If chlamydia cannot be excluded, then the addition of oral doxycycline 100 mg twice daily for 7 days is recommended for nonpregnant persons, and 1 g oral azithromycin for pregnant persons. The previous treatment was recommended based on a concern for gonococcal resistance.
This updated guideline reflects increasing concerns for antimicrobial stewardship and emerging azithromycin resistance. It does not recommend a test-of-cure for urogenital or rectal gonorrhea, though did recommend a test-of-cure 7-14 days after treatment of pharyngeal gonorrhea. The guideline also recommends testing for reinfection 3-12 months after treatment as the rate of reinfection ranges from 7% to 12% among those previously treated.3
For some offices, the provision of the IM injection may be challenging, though having this medication in stock with the possibility of provision can greatly improve access and ease of treatment for patients. Family physicians can incorporate these updated recommendations along with those for other STIs such as chlamydia and syphilis with standing orders for treatment and testing within their offices.
Accelerating progress in STI research
Family physicians can also support the national strategic plan by participating in studies looking at the impact of behavioral counseling in the primary care office as opposed to in STI clinics. In addition, by following the STI treatment and screening guidelines, family physicians will contribute to the body of knowledge of prevalence, treatment failure, and reinfection rates of STIs. We can also help advance the research by providing feedback on interventions that have success within our practices.
Reducing STI-related health disparities and inequities
Family physicians are also in important places to support the strategic plan’s fourth goal of reducing health disparities and health inequities.
If we continue to ask the questions to identify those at high risk and ensure that we are offering appropriate STI prevention, care, and treatment services within our clinics, we can expand access to all who need services and improve equity. By offering these services within the primary care office, we may be able to decrease the stigma some may feel going to an STI clinic for services.
By incorporating additional screening and counseling in our practices we may identify some patients who were not aware that they were at risk for an STI and offer them preventive services.
Achieving integrated and coordinated efforts
Finally, as many family physicians have integrated practices, we are uniquely poised to support the fifth goal of the strategic plan of achieving integrated and coordinated efforts addressing the STI epidemic. In our practices we can participate in, lead, and refer to programs for substance use disorders, viral hepatitis, STIs, and HIV as part of full scope primary care.
Family physicians and other primary care providers should work to support the entire strategic plan to ensure that we are fully caring for our patients and communities and stopping the past decade’s increase in STIs. We have an opportunity to use this strategy and make a large impact in our communities.
Dr. Wheat is a family physician at Erie Family Health Center in Chicago. She is program director of Northwestern’s McGaw Family Medicine residency program at Humboldt Park, Chicago. Dr. Wheat serves on the editorial advisory board of Family Practice News. You can contact her at [email protected].
References
1. U.S. Department of Health and Human Services. 2020. Sexually Transmitted Infections National Strategic Plan for the United States: 2021-2025. Washington.
2. U.S. Preventive Services Task Force. Behavioral counseling interventions to prevent sexually transmitted infections: U.S. Preventive Services Task Force Recommendation Statement. JAMA. 2020;324(7):674-81. doi: 10.1001/jama.2020.13095.
3. St. Cyr S et al. Update to CDC’s Treatment Guideline for Gonococcal Infection, 2020. MMWR Morb Mortal Wkly Rep 2020;69:1911-6. doi: 10.15585/mmwr.mm6950a6external_icon.
What's the diagnosis?
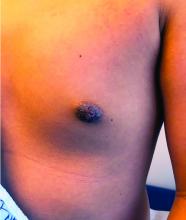
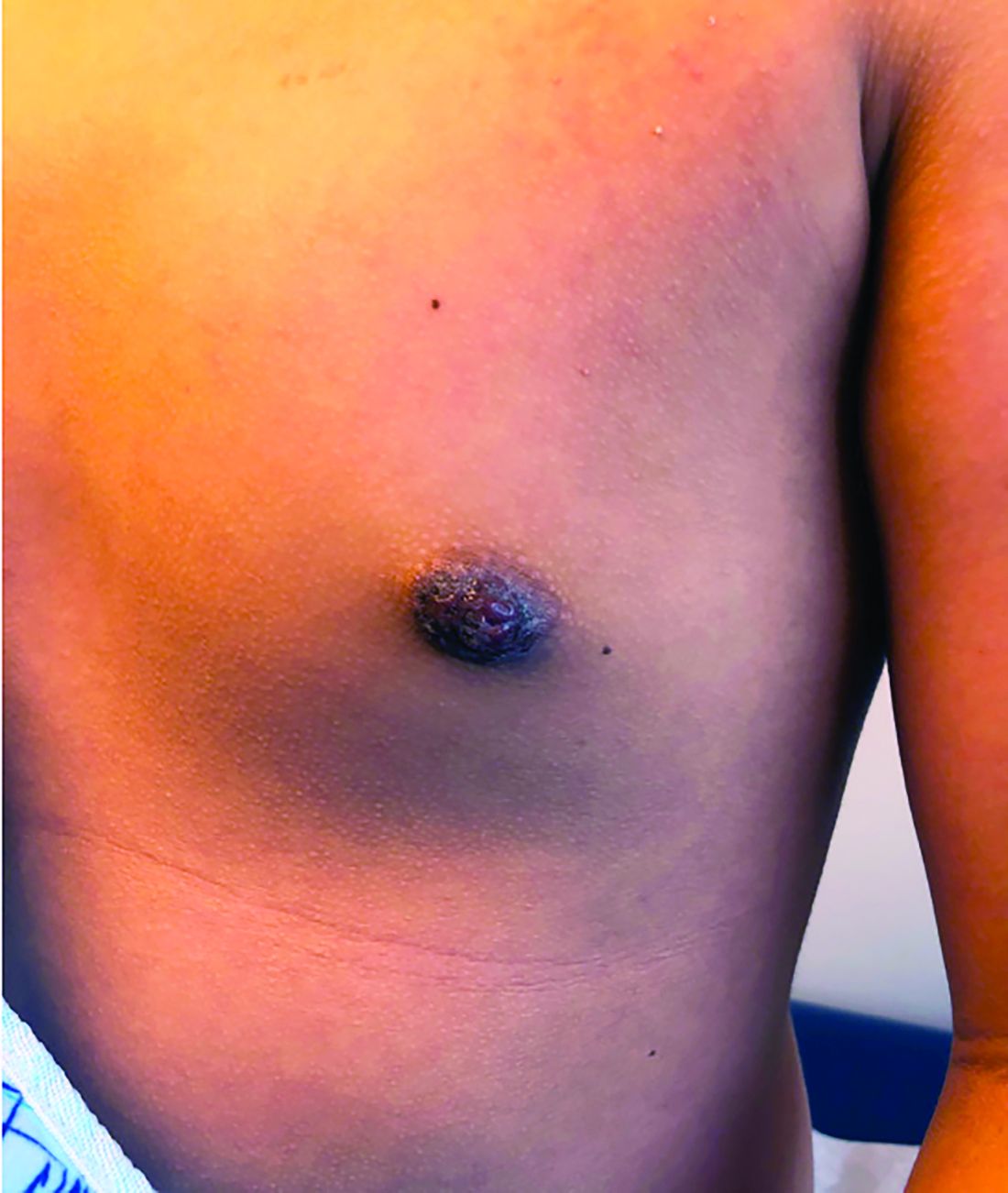
Nipple eczema is a dermatitis of the nipple and areola with clinical features such as erythema, fissures, scaling, pruritus, and crusting.1,2 It is classically associated with atopic dermatitis (AD), though it may occur as an isolated condition less commonly. While it may affect female adolescents, nipple eczema has also been reported in boys and breastfeeding women.3,4 The overall risk of incidence of nipple dermatitis has also been shown to increase with age.5 Nipple eczema is considered a cutaneous finding of AD, and is listed as a minor diagnostic criteria for AD in the Hanifin-Rajka criteria.6 The patient had not related his history of AD, which was elicited after finding typical antecubital eczematous dermatitis, and he had not been actively treating it.
Diagnosis and differential
Nipple eczema may be a challenging diagnosis for various reasons. For example, a unilateral presentation and the changes in the eczematous lesions overlying the nipple and areola’s varying textures and colors can make it difficult for clinicians to identify.3 Many children and adolescents, including our patient, are initially diagnosed as having impetigo and treated with antibiotics. The diagnosis of nipple eczema is made clinically, and management straightforward (see below). However, additional testing may be appropriate including patch testing for allergic contact dermatitis or bacterial cultures if bacterial infection or superinfection is considered.7,8 The differential diagnosis for nipple eczema includes impetigo, gynecomastia, scabies, and allergic contact dermatitis.
Impetigo typically presents with honey-colored crusts or pustules caused by infection with Staphylococcus aureus or Streptococcus. Patients with AD have higher rates of colonization with S. aureus and impetiginized eczema in common. Impetigo of the nipple and areola is more common in breastfeeding women as skin cracking from lactation can lead to exposure to bacteria from the infant’s mouth.9 Treatments involve topical or oral antibiotics.
Gynecomastia is the development of male breast tissue with most cases hypothesized to be caused by an imbalance between androgens and estrogens.10 Some other causes include direct skin contact with topical estrogen sprays and recreational use of marijuana and heroin.11 It is usually a benign exam finding in adolescent boys. However, clinical findings such as overlying skin changes, rapidly enlarging masses, and constitutional symptoms are concerning in the setting of gynecomastia and warrant further evaluation.
Scabies, which is caused by the infestation of scabies mites, is a common infectious skin disease. The classic presentation includes a rash that is intensely itchy, especially at night. Crusted scabies of the nipples may be difficult to distinguish from nipple eczema. Areas of frequent involvement of scabies include palms, between fingers, armpits, groin, between toes, and feet. Treatments include treating all household members with permethrin cream and washing all clothes and bedding in contact with a scabies-infected patient in high heat, or oral ivermectin in certain circumstances.12
Allergic contact dermatitis is a common cause of breast and nipple dermatitis and should be considered within the differential diagnosis of nipple eczema with atopic dermatitis, or as an exacerbator.7,9 Patients in particular who present with bilateral involvement extending to the periareolar skin, or unusual bilateral focal patterns suggestive for contact allergy should be considered for allergic contact dermatitis evaluation with patch tests. A common causative agent for allergic contact dermatitis of the breast and nipple includes Cl+Me-isothiazolinone, commonly found in detergents and fabric softeners.7 Primary treatment includes avoidance of the offending agents.
Treatment
Topical corticosteroids are first-line treatment for treating nipple eczema. Low-potency topical steroids can be used for maintenance and mild eczema while more potent steroids are useful for more severe cases. In addition to topical medication therapy, frequent emollient use to protect the skin barrier and the elimination of any irritants are essential to a successful treatment course. Unilateral nipple eczema can also be secondary to inadequate treatment of AD, demonstrating the importance of addressing the underlying AD with therapy.3
Our patient was diagnosed with nipple eczema based on clinical presentation of an eczematous left nipple in the setting of active atopic dermatitis and minimal improvement on topical antibiotic. He was started on a 3-week course of fluocinonide 0.05% topical ointment (a potent topical corticosteroid) twice daily for 2 weeks with plans to transition to triamcinolone 0.1% topical ointment several times a week.
Ms. Park is a pediatric dermatology research associate in the division of pediatric and adolescent dermatology, University of California, San Diego, and Rady Children’s Hospital, San Diego. Dr. Eichenfield is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego, and Rady Children’s Hospital. Neither Ms. Park nor Dr. Eichenfield have any relevant financial disclosures.
References
1. Pediatr Dermatol. 2005;22(1):64-6.
2. Am J Dermatopathol. 2015;37(4):284-8.
3. Pediatr Dermatol. 2015;32(5):718-22.
4. J Cutan Med Surg. 2004;8(2):126-30.
5. Pediatr Dermatol. 2012;29(5):580-3.
6. Dermatologica. 1988;177(6):360-4.
7. Ann Dermatol. 2014;26(3):413-4.
8. BMJ Case Rep. 2020;13(8).
9. J Am Acad Dermatol. 2019;80(6):1483-94.
10. Pediatr Endocrinol Rev. 2017;14(4):371-7.
11. JAMA. 2010;304(9):953.
12. JAMA. 2018;320(6):612.
Nipple eczema is a dermatitis of the nipple and areola with clinical features such as erythema, fissures, scaling, pruritus, and crusting.1,2 It is classically associated with atopic dermatitis (AD), though it may occur as an isolated condition less commonly. While it may affect female adolescents, nipple eczema has also been reported in boys and breastfeeding women.3,4 The overall risk of incidence of nipple dermatitis has also been shown to increase with age.5 Nipple eczema is considered a cutaneous finding of AD, and is listed as a minor diagnostic criteria for AD in the Hanifin-Rajka criteria.6 The patient had not related his history of AD, which was elicited after finding typical antecubital eczematous dermatitis, and he had not been actively treating it.
Diagnosis and differential
Nipple eczema may be a challenging diagnosis for various reasons. For example, a unilateral presentation and the changes in the eczematous lesions overlying the nipple and areola’s varying textures and colors can make it difficult for clinicians to identify.3 Many children and adolescents, including our patient, are initially diagnosed as having impetigo and treated with antibiotics. The diagnosis of nipple eczema is made clinically, and management straightforward (see below). However, additional testing may be appropriate including patch testing for allergic contact dermatitis or bacterial cultures if bacterial infection or superinfection is considered.7,8 The differential diagnosis for nipple eczema includes impetigo, gynecomastia, scabies, and allergic contact dermatitis.
Impetigo typically presents with honey-colored crusts or pustules caused by infection with Staphylococcus aureus or Streptococcus. Patients with AD have higher rates of colonization with S. aureus and impetiginized eczema in common. Impetigo of the nipple and areola is more common in breastfeeding women as skin cracking from lactation can lead to exposure to bacteria from the infant’s mouth.9 Treatments involve topical or oral antibiotics.
Gynecomastia is the development of male breast tissue with most cases hypothesized to be caused by an imbalance between androgens and estrogens.10 Some other causes include direct skin contact with topical estrogen sprays and recreational use of marijuana and heroin.11 It is usually a benign exam finding in adolescent boys. However, clinical findings such as overlying skin changes, rapidly enlarging masses, and constitutional symptoms are concerning in the setting of gynecomastia and warrant further evaluation.
Scabies, which is caused by the infestation of scabies mites, is a common infectious skin disease. The classic presentation includes a rash that is intensely itchy, especially at night. Crusted scabies of the nipples may be difficult to distinguish from nipple eczema. Areas of frequent involvement of scabies include palms, between fingers, armpits, groin, between toes, and feet. Treatments include treating all household members with permethrin cream and washing all clothes and bedding in contact with a scabies-infected patient in high heat, or oral ivermectin in certain circumstances.12
Allergic contact dermatitis is a common cause of breast and nipple dermatitis and should be considered within the differential diagnosis of nipple eczema with atopic dermatitis, or as an exacerbator.7,9 Patients in particular who present with bilateral involvement extending to the periareolar skin, or unusual bilateral focal patterns suggestive for contact allergy should be considered for allergic contact dermatitis evaluation with patch tests. A common causative agent for allergic contact dermatitis of the breast and nipple includes Cl+Me-isothiazolinone, commonly found in detergents and fabric softeners.7 Primary treatment includes avoidance of the offending agents.
Treatment
Topical corticosteroids are first-line treatment for treating nipple eczema. Low-potency topical steroids can be used for maintenance and mild eczema while more potent steroids are useful for more severe cases. In addition to topical medication therapy, frequent emollient use to protect the skin barrier and the elimination of any irritants are essential to a successful treatment course. Unilateral nipple eczema can also be secondary to inadequate treatment of AD, demonstrating the importance of addressing the underlying AD with therapy.3
Our patient was diagnosed with nipple eczema based on clinical presentation of an eczematous left nipple in the setting of active atopic dermatitis and minimal improvement on topical antibiotic. He was started on a 3-week course of fluocinonide 0.05% topical ointment (a potent topical corticosteroid) twice daily for 2 weeks with plans to transition to triamcinolone 0.1% topical ointment several times a week.
Ms. Park is a pediatric dermatology research associate in the division of pediatric and adolescent dermatology, University of California, San Diego, and Rady Children’s Hospital, San Diego. Dr. Eichenfield is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego, and Rady Children’s Hospital. Neither Ms. Park nor Dr. Eichenfield have any relevant financial disclosures.
References
1. Pediatr Dermatol. 2005;22(1):64-6.
2. Am J Dermatopathol. 2015;37(4):284-8.
3. Pediatr Dermatol. 2015;32(5):718-22.
4. J Cutan Med Surg. 2004;8(2):126-30.
5. Pediatr Dermatol. 2012;29(5):580-3.
6. Dermatologica. 1988;177(6):360-4.
7. Ann Dermatol. 2014;26(3):413-4.
8. BMJ Case Rep. 2020;13(8).
9. J Am Acad Dermatol. 2019;80(6):1483-94.
10. Pediatr Endocrinol Rev. 2017;14(4):371-7.
11. JAMA. 2010;304(9):953.
12. JAMA. 2018;320(6):612.
Nipple eczema is a dermatitis of the nipple and areola with clinical features such as erythema, fissures, scaling, pruritus, and crusting.1,2 It is classically associated with atopic dermatitis (AD), though it may occur as an isolated condition less commonly. While it may affect female adolescents, nipple eczema has also been reported in boys and breastfeeding women.3,4 The overall risk of incidence of nipple dermatitis has also been shown to increase with age.5 Nipple eczema is considered a cutaneous finding of AD, and is listed as a minor diagnostic criteria for AD in the Hanifin-Rajka criteria.6 The patient had not related his history of AD, which was elicited after finding typical antecubital eczematous dermatitis, and he had not been actively treating it.
Diagnosis and differential
Nipple eczema may be a challenging diagnosis for various reasons. For example, a unilateral presentation and the changes in the eczematous lesions overlying the nipple and areola’s varying textures and colors can make it difficult for clinicians to identify.3 Many children and adolescents, including our patient, are initially diagnosed as having impetigo and treated with antibiotics. The diagnosis of nipple eczema is made clinically, and management straightforward (see below). However, additional testing may be appropriate including patch testing for allergic contact dermatitis or bacterial cultures if bacterial infection or superinfection is considered.7,8 The differential diagnosis for nipple eczema includes impetigo, gynecomastia, scabies, and allergic contact dermatitis.
Impetigo typically presents with honey-colored crusts or pustules caused by infection with Staphylococcus aureus or Streptococcus. Patients with AD have higher rates of colonization with S. aureus and impetiginized eczema in common. Impetigo of the nipple and areola is more common in breastfeeding women as skin cracking from lactation can lead to exposure to bacteria from the infant’s mouth.9 Treatments involve topical or oral antibiotics.
Gynecomastia is the development of male breast tissue with most cases hypothesized to be caused by an imbalance between androgens and estrogens.10 Some other causes include direct skin contact with topical estrogen sprays and recreational use of marijuana and heroin.11 It is usually a benign exam finding in adolescent boys. However, clinical findings such as overlying skin changes, rapidly enlarging masses, and constitutional symptoms are concerning in the setting of gynecomastia and warrant further evaluation.
Scabies, which is caused by the infestation of scabies mites, is a common infectious skin disease. The classic presentation includes a rash that is intensely itchy, especially at night. Crusted scabies of the nipples may be difficult to distinguish from nipple eczema. Areas of frequent involvement of scabies include palms, between fingers, armpits, groin, between toes, and feet. Treatments include treating all household members with permethrin cream and washing all clothes and bedding in contact with a scabies-infected patient in high heat, or oral ivermectin in certain circumstances.12
Allergic contact dermatitis is a common cause of breast and nipple dermatitis and should be considered within the differential diagnosis of nipple eczema with atopic dermatitis, or as an exacerbator.7,9 Patients in particular who present with bilateral involvement extending to the periareolar skin, or unusual bilateral focal patterns suggestive for contact allergy should be considered for allergic contact dermatitis evaluation with patch tests. A common causative agent for allergic contact dermatitis of the breast and nipple includes Cl+Me-isothiazolinone, commonly found in detergents and fabric softeners.7 Primary treatment includes avoidance of the offending agents.
Treatment
Topical corticosteroids are first-line treatment for treating nipple eczema. Low-potency topical steroids can be used for maintenance and mild eczema while more potent steroids are useful for more severe cases. In addition to topical medication therapy, frequent emollient use to protect the skin barrier and the elimination of any irritants are essential to a successful treatment course. Unilateral nipple eczema can also be secondary to inadequate treatment of AD, demonstrating the importance of addressing the underlying AD with therapy.3
Our patient was diagnosed with nipple eczema based on clinical presentation of an eczematous left nipple in the setting of active atopic dermatitis and minimal improvement on topical antibiotic. He was started on a 3-week course of fluocinonide 0.05% topical ointment (a potent topical corticosteroid) twice daily for 2 weeks with plans to transition to triamcinolone 0.1% topical ointment several times a week.
Ms. Park is a pediatric dermatology research associate in the division of pediatric and adolescent dermatology, University of California, San Diego, and Rady Children’s Hospital, San Diego. Dr. Eichenfield is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego, and Rady Children’s Hospital. Neither Ms. Park nor Dr. Eichenfield have any relevant financial disclosures.
References
1. Pediatr Dermatol. 2005;22(1):64-6.
2. Am J Dermatopathol. 2015;37(4):284-8.
3. Pediatr Dermatol. 2015;32(5):718-22.
4. J Cutan Med Surg. 2004;8(2):126-30.
5. Pediatr Dermatol. 2012;29(5):580-3.
6. Dermatologica. 1988;177(6):360-4.
7. Ann Dermatol. 2014;26(3):413-4.
8. BMJ Case Rep. 2020;13(8).
9. J Am Acad Dermatol. 2019;80(6):1483-94.
10. Pediatr Endocrinol Rev. 2017;14(4):371-7.
11. JAMA. 2010;304(9):953.
12. JAMA. 2018;320(6):612.
A 12-year-old boy presents to the dermatology clinic with a 1-month history of crusting and watery sticky drainage from the left nipple. Given concern for a possible skin infection, the patient was initially treated with mupirocin ointment for several weeks but without improvement. The affected area is sometimes itchy but not painful. He reports no prior history of similar problems.
On physical exam, he is noted to have an eczematous left nipple with edema, xerosis, and scaling overlying the entire areola. There is no evidence of visible discharge, pustules, or honey-colored crusts in the area. The extensor surfaces of his arms bilaterally have skin-colored follicular papules, and his antecubital fossa display erythematous scaling plaques with mild lichenification and excoriations.
Pressure builds on CDC to prioritize both diabetes types for vaccine
The American Diabetes Association, along with 18 other organizations, has sent a letter to the U.S. Centers for Disease Control and Prevention urging them to rank people with type 1 diabetes as equally high risk for COVID-19 severity, and therefore vaccination, as those with type 2 diabetes.
On Jan. 12, the CDC recommended states vaccinate all Americans over age 65 and those with underlying health conditions that make them more vulnerable to COVID-19.
Currently, type 2 diabetes is listed among 12 conditions that place adults “at increased risk of severe illness from the virus that causes COVID-19,” with the latter defined as “hospitalization, admission to the intensive care unit, intubation or mechanical ventilation, or death.”
On the other hand, the autoimmune condition type 1 diabetes is among 11 conditions the CDC says “might be at increased risk” for COVID-19, but limited data were available at the time of the last update on Dec. 23, 2020.
“States are utilizing the CDC risk classification when designing their vaccine distribution plans. This raises an obvious concern as it could result in the approximately 1.6 million with type 1 diabetes receiving the vaccination later than others with the same risk,” states the ADA letter, sent to the CDC on Jan. 13.
Representatives from the Endocrine Society, American Association of Clinical Endocrinology, Pediatric Endocrine Society, Association of Diabetes Care & Education Specialists, and JDRF, among others, cosigned the letter.
Newer data show those with type 1 diabetes at equally high risk
While acknowledging that “early data did not provide as much clarity about the extent to which those with type 1 diabetes are at high risk,” the ADA says newer evidence has emerged, as previously reported by this news organization, that “convincingly demonstrates that COVID-19 severity is more than tripled in individuals with type 1 diabetes.”
The letter also cites another study showing that people with type 1 diabetes “have a 3.3-fold greater risk of severe illness, are 3.9 times more likely to be hospitalized with COVID-19, and have a 3-fold increase in mortality compared to those without type 1 diabetes.”
Those risks, they note, are comparable to the increased risk established for those with type 2 diabetes, as shown in a third study from Scotland, published last month.
Asked for comment, CDC representative Kirsten Nordlund said in an interview, “This list is a living document that will be periodically updated by CDC, and it could rapidly change as the science evolves.”
In addition, Ms. Nordlund said, “Decisions about transitioning to subsequent phases should depend on supply; demand; equitable vaccine distribution; and local, state, or territorial context.”
“Phased vaccine recommendations are meant to be fluid and not restrictive for jurisdictions. It is not necessary to vaccinate all individuals in one phase before initiating the next phase; phases may overlap,” she noted. More information is available here.
Tennessee gives type 1 and type 2 diabetes equal priority for vaccination
Meanwhile, at least one state, Tennessee, has updated its guidance to include both types of diabetes as being priority for COVID-19 vaccination.
Vanderbilt University pediatric endocrinologist Justin M. Gregory, MD, said in an interview: “I was thrilled when our state modified its guidance on December 30th to include both type 1 and type 2 diabetes in the ‘high-risk category.’ Other states have not modified that guidance though.”
It’s unclear how this might play out on the ground, noted Dr. Gregory, who led one of the three studies demonstrating increased COVID-19 risk for people with type 1 diabetes.
“To tell you the truth, I don’t really know how individual organizations dispensing the vaccination [will handle] people who come to their facility saying they have ‘diabetes.’ Individual states set the vaccine-dispensing guidance and individual county health departments and health care systems mirror that guidance,” he said.
Thus, he added, “Although it’s possible an individual nurse may take the ‘I’ll ask you no questions, and you’ll tell me no lies’ approach if someone with type 1 diabetes says they have ‘diabetes’, websites and health department–recorded telephone messages are going to tell people with type 1 diabetes they have to wait further back in line if that is what their state’s guidance directs.”
A version of this article first appeared on Medscape.com.
The American Diabetes Association, along with 18 other organizations, has sent a letter to the U.S. Centers for Disease Control and Prevention urging them to rank people with type 1 diabetes as equally high risk for COVID-19 severity, and therefore vaccination, as those with type 2 diabetes.
On Jan. 12, the CDC recommended states vaccinate all Americans over age 65 and those with underlying health conditions that make them more vulnerable to COVID-19.
Currently, type 2 diabetes is listed among 12 conditions that place adults “at increased risk of severe illness from the virus that causes COVID-19,” with the latter defined as “hospitalization, admission to the intensive care unit, intubation or mechanical ventilation, or death.”
On the other hand, the autoimmune condition type 1 diabetes is among 11 conditions the CDC says “might be at increased risk” for COVID-19, but limited data were available at the time of the last update on Dec. 23, 2020.
“States are utilizing the CDC risk classification when designing their vaccine distribution plans. This raises an obvious concern as it could result in the approximately 1.6 million with type 1 diabetes receiving the vaccination later than others with the same risk,” states the ADA letter, sent to the CDC on Jan. 13.
Representatives from the Endocrine Society, American Association of Clinical Endocrinology, Pediatric Endocrine Society, Association of Diabetes Care & Education Specialists, and JDRF, among others, cosigned the letter.
Newer data show those with type 1 diabetes at equally high risk
While acknowledging that “early data did not provide as much clarity about the extent to which those with type 1 diabetes are at high risk,” the ADA says newer evidence has emerged, as previously reported by this news organization, that “convincingly demonstrates that COVID-19 severity is more than tripled in individuals with type 1 diabetes.”
The letter also cites another study showing that people with type 1 diabetes “have a 3.3-fold greater risk of severe illness, are 3.9 times more likely to be hospitalized with COVID-19, and have a 3-fold increase in mortality compared to those without type 1 diabetes.”
Those risks, they note, are comparable to the increased risk established for those with type 2 diabetes, as shown in a third study from Scotland, published last month.
Asked for comment, CDC representative Kirsten Nordlund said in an interview, “This list is a living document that will be periodically updated by CDC, and it could rapidly change as the science evolves.”
In addition, Ms. Nordlund said, “Decisions about transitioning to subsequent phases should depend on supply; demand; equitable vaccine distribution; and local, state, or territorial context.”
“Phased vaccine recommendations are meant to be fluid and not restrictive for jurisdictions. It is not necessary to vaccinate all individuals in one phase before initiating the next phase; phases may overlap,” she noted. More information is available here.
Tennessee gives type 1 and type 2 diabetes equal priority for vaccination
Meanwhile, at least one state, Tennessee, has updated its guidance to include both types of diabetes as being priority for COVID-19 vaccination.
Vanderbilt University pediatric endocrinologist Justin M. Gregory, MD, said in an interview: “I was thrilled when our state modified its guidance on December 30th to include both type 1 and type 2 diabetes in the ‘high-risk category.’ Other states have not modified that guidance though.”
It’s unclear how this might play out on the ground, noted Dr. Gregory, who led one of the three studies demonstrating increased COVID-19 risk for people with type 1 diabetes.
“To tell you the truth, I don’t really know how individual organizations dispensing the vaccination [will handle] people who come to their facility saying they have ‘diabetes.’ Individual states set the vaccine-dispensing guidance and individual county health departments and health care systems mirror that guidance,” he said.
Thus, he added, “Although it’s possible an individual nurse may take the ‘I’ll ask you no questions, and you’ll tell me no lies’ approach if someone with type 1 diabetes says they have ‘diabetes’, websites and health department–recorded telephone messages are going to tell people with type 1 diabetes they have to wait further back in line if that is what their state’s guidance directs.”
A version of this article first appeared on Medscape.com.
The American Diabetes Association, along with 18 other organizations, has sent a letter to the U.S. Centers for Disease Control and Prevention urging them to rank people with type 1 diabetes as equally high risk for COVID-19 severity, and therefore vaccination, as those with type 2 diabetes.
On Jan. 12, the CDC recommended states vaccinate all Americans over age 65 and those with underlying health conditions that make them more vulnerable to COVID-19.
Currently, type 2 diabetes is listed among 12 conditions that place adults “at increased risk of severe illness from the virus that causes COVID-19,” with the latter defined as “hospitalization, admission to the intensive care unit, intubation or mechanical ventilation, or death.”
On the other hand, the autoimmune condition type 1 diabetes is among 11 conditions the CDC says “might be at increased risk” for COVID-19, but limited data were available at the time of the last update on Dec. 23, 2020.
“States are utilizing the CDC risk classification when designing their vaccine distribution plans. This raises an obvious concern as it could result in the approximately 1.6 million with type 1 diabetes receiving the vaccination later than others with the same risk,” states the ADA letter, sent to the CDC on Jan. 13.
Representatives from the Endocrine Society, American Association of Clinical Endocrinology, Pediatric Endocrine Society, Association of Diabetes Care & Education Specialists, and JDRF, among others, cosigned the letter.
Newer data show those with type 1 diabetes at equally high risk
While acknowledging that “early data did not provide as much clarity about the extent to which those with type 1 diabetes are at high risk,” the ADA says newer evidence has emerged, as previously reported by this news organization, that “convincingly demonstrates that COVID-19 severity is more than tripled in individuals with type 1 diabetes.”
The letter also cites another study showing that people with type 1 diabetes “have a 3.3-fold greater risk of severe illness, are 3.9 times more likely to be hospitalized with COVID-19, and have a 3-fold increase in mortality compared to those without type 1 diabetes.”
Those risks, they note, are comparable to the increased risk established for those with type 2 diabetes, as shown in a third study from Scotland, published last month.
Asked for comment, CDC representative Kirsten Nordlund said in an interview, “This list is a living document that will be periodically updated by CDC, and it could rapidly change as the science evolves.”
In addition, Ms. Nordlund said, “Decisions about transitioning to subsequent phases should depend on supply; demand; equitable vaccine distribution; and local, state, or territorial context.”
“Phased vaccine recommendations are meant to be fluid and not restrictive for jurisdictions. It is not necessary to vaccinate all individuals in one phase before initiating the next phase; phases may overlap,” she noted. More information is available here.
Tennessee gives type 1 and type 2 diabetes equal priority for vaccination
Meanwhile, at least one state, Tennessee, has updated its guidance to include both types of diabetes as being priority for COVID-19 vaccination.
Vanderbilt University pediatric endocrinologist Justin M. Gregory, MD, said in an interview: “I was thrilled when our state modified its guidance on December 30th to include both type 1 and type 2 diabetes in the ‘high-risk category.’ Other states have not modified that guidance though.”
It’s unclear how this might play out on the ground, noted Dr. Gregory, who led one of the three studies demonstrating increased COVID-19 risk for people with type 1 diabetes.
“To tell you the truth, I don’t really know how individual organizations dispensing the vaccination [will handle] people who come to their facility saying they have ‘diabetes.’ Individual states set the vaccine-dispensing guidance and individual county health departments and health care systems mirror that guidance,” he said.
Thus, he added, “Although it’s possible an individual nurse may take the ‘I’ll ask you no questions, and you’ll tell me no lies’ approach if someone with type 1 diabetes says they have ‘diabetes’, websites and health department–recorded telephone messages are going to tell people with type 1 diabetes they have to wait further back in line if that is what their state’s guidance directs.”
A version of this article first appeared on Medscape.com.
Coping with vaccine refusal
Do you accept new families into your practice who have already chosen to not have their children immunized? What about families who have been in your practice for several months or years? In 2016 the American Academy of Pediatrics published a clinical report in which it stated that, under some circumstances, dismissing families who refuse to vaccinate is permissible. Have you felt sufficiently supported by that statement and dismissed any families after multiple attempts at education on your part?
In a Pediatrics Perspective article in the December issue of Pediatrics, two philosophers and a physician make the argument that, while in some situations dismissing a family who refuses vaccines may be “an ethically acceptable option” refusing to accept a family with the same philosophy is not. It is an interesting paper and worth reading regardless of whether or not you already accept and continue to tolerate vaccine deniers in your practice.
The Pediatrics Perspective is certainly not the last word on the ethics of caring for families who deny their children care that we believe is critical to their health and the welfare of the community at large. There has been a lot of discussion about the issue but little has been written about how we as the physicians on the front line are coping emotionally with what the authors of the paper call the “burdens associated with treating” families who refuse to follow our guidance.
It is hard not to feel angry when a family you have invested valuable office time in discussing the benefits and safety of vaccines continues to disregard what you see as the facts. The time you have spent with them is not just income-generating time for your practice, it is time stolen from other families who are more willing to follow your recommendations. In how many visits will you continue to raise the issue? Unless I saw a glimmer of hope I would usually stop after two wasted encounters. But, the issue would still linger as the elephant in the examination room for as long as I continued to see the patient.
How have you expressed your anger? Have you been argumentative or rude? You may have been able maintain your composure and remain civil and appear caring, but I suspect the anger is still gnawing at you. And, there is still the frustration and feeling of impotence. You may have questioned your ability as an educator. You should get over that notion quickly. There is ample evidence that most vaccine deniers are not going to be convinced by even the most carefully presented information. I suggest you leave it to others to try their hands at education. Let them invest their time while you tend to the needs of your other patients. You can try being a fear monger and, while fear can be effective, you have better ways to spend your office day than telling horror stories.
If vaccine denial makes you feel powerless, you should get over that pretty quickly as well and accept the fact that you are simply an advisor. If you believe that most of the families in your practice are following your recommendations as though you had presented them on stone tablets, it is time for a wakeup call.
Finally, there is the most troubling emotion associated with vaccine refusal and that is fear, the fear of being sued. Establishing a relationship with a family is one that requires mutual trust and certainly vaccine refusal will put that trust in question, particularly if you have done a less than adequate job of hiding your anger and frustration with their unfortunate decision.
For now, vaccine refusal is just another one of those crosses that those of us in primary care must bear together wearing the best face we can put forward. That doesn’t mean we can’t share those emotions with our peers. Misery does love company.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
Do you accept new families into your practice who have already chosen to not have their children immunized? What about families who have been in your practice for several months or years? In 2016 the American Academy of Pediatrics published a clinical report in which it stated that, under some circumstances, dismissing families who refuse to vaccinate is permissible. Have you felt sufficiently supported by that statement and dismissed any families after multiple attempts at education on your part?
In a Pediatrics Perspective article in the December issue of Pediatrics, two philosophers and a physician make the argument that, while in some situations dismissing a family who refuses vaccines may be “an ethically acceptable option” refusing to accept a family with the same philosophy is not. It is an interesting paper and worth reading regardless of whether or not you already accept and continue to tolerate vaccine deniers in your practice.
The Pediatrics Perspective is certainly not the last word on the ethics of caring for families who deny their children care that we believe is critical to their health and the welfare of the community at large. There has been a lot of discussion about the issue but little has been written about how we as the physicians on the front line are coping emotionally with what the authors of the paper call the “burdens associated with treating” families who refuse to follow our guidance.
It is hard not to feel angry when a family you have invested valuable office time in discussing the benefits and safety of vaccines continues to disregard what you see as the facts. The time you have spent with them is not just income-generating time for your practice, it is time stolen from other families who are more willing to follow your recommendations. In how many visits will you continue to raise the issue? Unless I saw a glimmer of hope I would usually stop after two wasted encounters. But, the issue would still linger as the elephant in the examination room for as long as I continued to see the patient.
How have you expressed your anger? Have you been argumentative or rude? You may have been able maintain your composure and remain civil and appear caring, but I suspect the anger is still gnawing at you. And, there is still the frustration and feeling of impotence. You may have questioned your ability as an educator. You should get over that notion quickly. There is ample evidence that most vaccine deniers are not going to be convinced by even the most carefully presented information. I suggest you leave it to others to try their hands at education. Let them invest their time while you tend to the needs of your other patients. You can try being a fear monger and, while fear can be effective, you have better ways to spend your office day than telling horror stories.
If vaccine denial makes you feel powerless, you should get over that pretty quickly as well and accept the fact that you are simply an advisor. If you believe that most of the families in your practice are following your recommendations as though you had presented them on stone tablets, it is time for a wakeup call.
Finally, there is the most troubling emotion associated with vaccine refusal and that is fear, the fear of being sued. Establishing a relationship with a family is one that requires mutual trust and certainly vaccine refusal will put that trust in question, particularly if you have done a less than adequate job of hiding your anger and frustration with their unfortunate decision.
For now, vaccine refusal is just another one of those crosses that those of us in primary care must bear together wearing the best face we can put forward. That doesn’t mean we can’t share those emotions with our peers. Misery does love company.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
Do you accept new families into your practice who have already chosen to not have their children immunized? What about families who have been in your practice for several months or years? In 2016 the American Academy of Pediatrics published a clinical report in which it stated that, under some circumstances, dismissing families who refuse to vaccinate is permissible. Have you felt sufficiently supported by that statement and dismissed any families after multiple attempts at education on your part?
In a Pediatrics Perspective article in the December issue of Pediatrics, two philosophers and a physician make the argument that, while in some situations dismissing a family who refuses vaccines may be “an ethically acceptable option” refusing to accept a family with the same philosophy is not. It is an interesting paper and worth reading regardless of whether or not you already accept and continue to tolerate vaccine deniers in your practice.
The Pediatrics Perspective is certainly not the last word on the ethics of caring for families who deny their children care that we believe is critical to their health and the welfare of the community at large. There has been a lot of discussion about the issue but little has been written about how we as the physicians on the front line are coping emotionally with what the authors of the paper call the “burdens associated with treating” families who refuse to follow our guidance.
It is hard not to feel angry when a family you have invested valuable office time in discussing the benefits and safety of vaccines continues to disregard what you see as the facts. The time you have spent with them is not just income-generating time for your practice, it is time stolen from other families who are more willing to follow your recommendations. In how many visits will you continue to raise the issue? Unless I saw a glimmer of hope I would usually stop after two wasted encounters. But, the issue would still linger as the elephant in the examination room for as long as I continued to see the patient.
How have you expressed your anger? Have you been argumentative or rude? You may have been able maintain your composure and remain civil and appear caring, but I suspect the anger is still gnawing at you. And, there is still the frustration and feeling of impotence. You may have questioned your ability as an educator. You should get over that notion quickly. There is ample evidence that most vaccine deniers are not going to be convinced by even the most carefully presented information. I suggest you leave it to others to try their hands at education. Let them invest their time while you tend to the needs of your other patients. You can try being a fear monger and, while fear can be effective, you have better ways to spend your office day than telling horror stories.
If vaccine denial makes you feel powerless, you should get over that pretty quickly as well and accept the fact that you are simply an advisor. If you believe that most of the families in your practice are following your recommendations as though you had presented them on stone tablets, it is time for a wakeup call.
Finally, there is the most troubling emotion associated with vaccine refusal and that is fear, the fear of being sued. Establishing a relationship with a family is one that requires mutual trust and certainly vaccine refusal will put that trust in question, particularly if you have done a less than adequate job of hiding your anger and frustration with their unfortunate decision.
For now, vaccine refusal is just another one of those crosses that those of us in primary care must bear together wearing the best face we can put forward. That doesn’t mean we can’t share those emotions with our peers. Misery does love company.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
Invasive bacterial infections uncommon in afebrile infants with diagnosed AOM
Outpatient management of most afebrile infants with acute otitis media who haven’t been tested for invasive bacterial infection may be reasonable given the low occurrence of adverse events, said Son H. McLaren, MD, MS, of Columbia University, New York, and colleagues.
Dr. McLaren and associates conducted an international cross-sectional study at 33 emergency departments participating in the Pediatric Emergency Medicine Collaborative Research Committee of the American Academy of Pediatrics (AAP): 29 in the United States, 2 in Canada and 2 in Spain.
The researchers sought first to assess prevalence of invasive bacterial infections and adverse events tied to acute otitis media (AOM) in infants 90 days and younger. Those who were clinically diagnosed with AOM and presented without fever between January 2007 and December 2017 were included in the study. The presence of fever, they explained, “is a primary driver for more expanded testing and/or empirical treatment of invasive bacterial infection (IBI). Secondarily, they sought to characterize patterns of diagnostic testing and the factors associated with it specifically in this patient population.
Of 5,270 patients screened, 1,637 met study criteria. Included patients were a median age of 68 days. A total of 1,459 (89.1%) met AAP diagnostic criteria for AOM. The remaining 178 patients were examined and found to have more than one of these criteria: 113 had opacification of tympanic membrane, 57 had dull tympanic membrane, 25 had decreased visualization of middle ear structures, 9 had middle ear effusion, 8 had visible tympanic membrane perforation and 5 had decreased tympanic membrane mobility with insufflation. None of the 278 infants with blood cultures had bacteremia, nor were they diagnosed with bacterial meningitis. Two of 645 (0.3%) infants experienced adverse events, as evidenced with 30-day follow-up or history of hospitalization.
Dr. McLaren and colleagues observed that despite a low prevalence of IBI and AOM-associated adverse events, more than one-fifth of patients were prescribed diagnostic testing for IBI and subsequently hospitalized, a practice that appeared more common with younger patients.
Significant testing and hospitalizations persisted despite low prevalence of IBIs
Although diagnostic testing and hospitalizations differed by site, they were, in fact, “substantial in contrast to the low prevalence of IBIs and adverse events,” the researchers noted. “Our data may be used to help guide clinical management of afebrile infants with clinician-diagnosed AOM, who are not included in the current AAP AOM practice guideline,” the authors said. They speculated that this practice may be due, in part, to young-age risk of IBI and the concern for IBI in this population based on febrile infant population data and a general hesitance to begin antibiotics without first evaluating for IBI. They also cited a low prevalence ranging from 0.8% to 2.5% as evidence for low risk of IBI in afebrile infants with AOM.
Also of note, given that roughly three-fourths of infants included in the study were reported to have symptoms of upper respiratory infection that can lead to viral AOM, including these infants who could have a lower likelihood of IBI than those with known bacterial AOM, may have led the researchers to underestimate IBI prevalence. Because existing data do not allow for clear distinction of viral from bacterial AOM without tympanocentesis, and because more than 85% of older patients with clinically diagnosed AOM also have observed bacterial otopathogens, the authors clarify that “it is understandable why clinicians would manage infants with AOM conservatively, regardless of the presence of concurrent viral illnesses.” They also acknowledged that one major challenge in working with infants believed to have AOM is ensuring that it is actually present since it is so hard to diagnose.
Dr. McLaren and colleagues cited several study limitations: 1) completeness and accuracy of data couldn’t be ensured because of the retrospective study design; 2) because not all infants were tested for IBI, its prevalence may have been underestimated; 3) infants whose discharge codes did not include AOM may have been missed, although all infants with positive blood or cerebrospinal fluid cultures were screened for missed AOM diagnosis; and 4) it is important to consider that any issues associated with testing and hospitalization that were identified may have been the result of management decisions driven by factors that cannot be captured retrospectively or by a diagnosis of AOM.
The findings are not generalizable to infants aged younger than 28 days
Finally, the authors cautioned that because the number of infants younger than 28 days was quite small, and it is therefore infinitely more challenging to diagnose AOM for these patients, results of the study should be applied to infants older than 28 days and are not generalizable to febrile infants.
“This report will not resolve the significant challenge faced by clinicians in treating infants aged [younger than] 28 days who have the highest risk of occult bacteremia and systemic spread of a focal bacterial infection,” Joseph Ravera, MD, and M.W. Stevens, MD, of the University of Vermont, Burlington, noted in an accompanying editorial. Previous studies have identified this age group “to be at the highest risk for systemic bacterial involvement and the most difficult to risk stratify on the basis of physical examination findings and initial laboratory results,” they noted. That the subjects aged younger than 28 days in this study had nearly a 50% admission rate illustrates the clinical uncertainty pediatric emergency medicine providers are challenged with, they added. Just 100 (6%) of the 1,637 patients in the study sample were in this age category, which makes it difficult, given the lack of sufficient data, to generalize findings to the youngest infants.
“Despite a paucity of young infants and limitations inherent to the design, this study does contribute to the literature with a robust retrospective data set of afebrile infants between 1 and 3 months of age with an ED diagnosis of AOM ... It certainly provides a base of support for carefully designed prospective studies in which researchers aim to determine the best care for AOM in children under 6 months of age,” reflected Dr. Ravera and Dr. Stevens.
In a separate interview, Karalyn Kinsella, MD, private practice, Cheshire, Conn. noted, “What is confusing is the absence of documented symptoms for infants presenting to the emergency department, as the symptoms they presented with would influence our concern for IBI. Diagnosing AOM in infants under 90 days old is extremely uncommon as an outpatient pediatrician. Although the finding of AOM in an afebrile infant is very rare in the outpatient setting, this study assures us the risk of IBI is almost nonexistent. Therefore, further workup is unnecessary unless providers have clinical suspicions to the contrary.”
Dr. McLaren and colleagues as well as Dr. Ravera, Dr. Stevens, and Dr. Kinsella, had no conflicts of interest and no relevant financial disclosures.
Outpatient management of most afebrile infants with acute otitis media who haven’t been tested for invasive bacterial infection may be reasonable given the low occurrence of adverse events, said Son H. McLaren, MD, MS, of Columbia University, New York, and colleagues.
Dr. McLaren and associates conducted an international cross-sectional study at 33 emergency departments participating in the Pediatric Emergency Medicine Collaborative Research Committee of the American Academy of Pediatrics (AAP): 29 in the United States, 2 in Canada and 2 in Spain.
The researchers sought first to assess prevalence of invasive bacterial infections and adverse events tied to acute otitis media (AOM) in infants 90 days and younger. Those who were clinically diagnosed with AOM and presented without fever between January 2007 and December 2017 were included in the study. The presence of fever, they explained, “is a primary driver for more expanded testing and/or empirical treatment of invasive bacterial infection (IBI). Secondarily, they sought to characterize patterns of diagnostic testing and the factors associated with it specifically in this patient population.
Of 5,270 patients screened, 1,637 met study criteria. Included patients were a median age of 68 days. A total of 1,459 (89.1%) met AAP diagnostic criteria for AOM. The remaining 178 patients were examined and found to have more than one of these criteria: 113 had opacification of tympanic membrane, 57 had dull tympanic membrane, 25 had decreased visualization of middle ear structures, 9 had middle ear effusion, 8 had visible tympanic membrane perforation and 5 had decreased tympanic membrane mobility with insufflation. None of the 278 infants with blood cultures had bacteremia, nor were they diagnosed with bacterial meningitis. Two of 645 (0.3%) infants experienced adverse events, as evidenced with 30-day follow-up or history of hospitalization.
Dr. McLaren and colleagues observed that despite a low prevalence of IBI and AOM-associated adverse events, more than one-fifth of patients were prescribed diagnostic testing for IBI and subsequently hospitalized, a practice that appeared more common with younger patients.
Significant testing and hospitalizations persisted despite low prevalence of IBIs
Although diagnostic testing and hospitalizations differed by site, they were, in fact, “substantial in contrast to the low prevalence of IBIs and adverse events,” the researchers noted. “Our data may be used to help guide clinical management of afebrile infants with clinician-diagnosed AOM, who are not included in the current AAP AOM practice guideline,” the authors said. They speculated that this practice may be due, in part, to young-age risk of IBI and the concern for IBI in this population based on febrile infant population data and a general hesitance to begin antibiotics without first evaluating for IBI. They also cited a low prevalence ranging from 0.8% to 2.5% as evidence for low risk of IBI in afebrile infants with AOM.
Also of note, given that roughly three-fourths of infants included in the study were reported to have symptoms of upper respiratory infection that can lead to viral AOM, including these infants who could have a lower likelihood of IBI than those with known bacterial AOM, may have led the researchers to underestimate IBI prevalence. Because existing data do not allow for clear distinction of viral from bacterial AOM without tympanocentesis, and because more than 85% of older patients with clinically diagnosed AOM also have observed bacterial otopathogens, the authors clarify that “it is understandable why clinicians would manage infants with AOM conservatively, regardless of the presence of concurrent viral illnesses.” They also acknowledged that one major challenge in working with infants believed to have AOM is ensuring that it is actually present since it is so hard to diagnose.
Dr. McLaren and colleagues cited several study limitations: 1) completeness and accuracy of data couldn’t be ensured because of the retrospective study design; 2) because not all infants were tested for IBI, its prevalence may have been underestimated; 3) infants whose discharge codes did not include AOM may have been missed, although all infants with positive blood or cerebrospinal fluid cultures were screened for missed AOM diagnosis; and 4) it is important to consider that any issues associated with testing and hospitalization that were identified may have been the result of management decisions driven by factors that cannot be captured retrospectively or by a diagnosis of AOM.
The findings are not generalizable to infants aged younger than 28 days
Finally, the authors cautioned that because the number of infants younger than 28 days was quite small, and it is therefore infinitely more challenging to diagnose AOM for these patients, results of the study should be applied to infants older than 28 days and are not generalizable to febrile infants.
“This report will not resolve the significant challenge faced by clinicians in treating infants aged [younger than] 28 days who have the highest risk of occult bacteremia and systemic spread of a focal bacterial infection,” Joseph Ravera, MD, and M.W. Stevens, MD, of the University of Vermont, Burlington, noted in an accompanying editorial. Previous studies have identified this age group “to be at the highest risk for systemic bacterial involvement and the most difficult to risk stratify on the basis of physical examination findings and initial laboratory results,” they noted. That the subjects aged younger than 28 days in this study had nearly a 50% admission rate illustrates the clinical uncertainty pediatric emergency medicine providers are challenged with, they added. Just 100 (6%) of the 1,637 patients in the study sample were in this age category, which makes it difficult, given the lack of sufficient data, to generalize findings to the youngest infants.
“Despite a paucity of young infants and limitations inherent to the design, this study does contribute to the literature with a robust retrospective data set of afebrile infants between 1 and 3 months of age with an ED diagnosis of AOM ... It certainly provides a base of support for carefully designed prospective studies in which researchers aim to determine the best care for AOM in children under 6 months of age,” reflected Dr. Ravera and Dr. Stevens.
In a separate interview, Karalyn Kinsella, MD, private practice, Cheshire, Conn. noted, “What is confusing is the absence of documented symptoms for infants presenting to the emergency department, as the symptoms they presented with would influence our concern for IBI. Diagnosing AOM in infants under 90 days old is extremely uncommon as an outpatient pediatrician. Although the finding of AOM in an afebrile infant is very rare in the outpatient setting, this study assures us the risk of IBI is almost nonexistent. Therefore, further workup is unnecessary unless providers have clinical suspicions to the contrary.”
Dr. McLaren and colleagues as well as Dr. Ravera, Dr. Stevens, and Dr. Kinsella, had no conflicts of interest and no relevant financial disclosures.
Outpatient management of most afebrile infants with acute otitis media who haven’t been tested for invasive bacterial infection may be reasonable given the low occurrence of adverse events, said Son H. McLaren, MD, MS, of Columbia University, New York, and colleagues.
Dr. McLaren and associates conducted an international cross-sectional study at 33 emergency departments participating in the Pediatric Emergency Medicine Collaborative Research Committee of the American Academy of Pediatrics (AAP): 29 in the United States, 2 in Canada and 2 in Spain.
The researchers sought first to assess prevalence of invasive bacterial infections and adverse events tied to acute otitis media (AOM) in infants 90 days and younger. Those who were clinically diagnosed with AOM and presented without fever between January 2007 and December 2017 were included in the study. The presence of fever, they explained, “is a primary driver for more expanded testing and/or empirical treatment of invasive bacterial infection (IBI). Secondarily, they sought to characterize patterns of diagnostic testing and the factors associated with it specifically in this patient population.
Of 5,270 patients screened, 1,637 met study criteria. Included patients were a median age of 68 days. A total of 1,459 (89.1%) met AAP diagnostic criteria for AOM. The remaining 178 patients were examined and found to have more than one of these criteria: 113 had opacification of tympanic membrane, 57 had dull tympanic membrane, 25 had decreased visualization of middle ear structures, 9 had middle ear effusion, 8 had visible tympanic membrane perforation and 5 had decreased tympanic membrane mobility with insufflation. None of the 278 infants with blood cultures had bacteremia, nor were they diagnosed with bacterial meningitis. Two of 645 (0.3%) infants experienced adverse events, as evidenced with 30-day follow-up or history of hospitalization.
Dr. McLaren and colleagues observed that despite a low prevalence of IBI and AOM-associated adverse events, more than one-fifth of patients were prescribed diagnostic testing for IBI and subsequently hospitalized, a practice that appeared more common with younger patients.
Significant testing and hospitalizations persisted despite low prevalence of IBIs
Although diagnostic testing and hospitalizations differed by site, they were, in fact, “substantial in contrast to the low prevalence of IBIs and adverse events,” the researchers noted. “Our data may be used to help guide clinical management of afebrile infants with clinician-diagnosed AOM, who are not included in the current AAP AOM practice guideline,” the authors said. They speculated that this practice may be due, in part, to young-age risk of IBI and the concern for IBI in this population based on febrile infant population data and a general hesitance to begin antibiotics without first evaluating for IBI. They also cited a low prevalence ranging from 0.8% to 2.5% as evidence for low risk of IBI in afebrile infants with AOM.
Also of note, given that roughly three-fourths of infants included in the study were reported to have symptoms of upper respiratory infection that can lead to viral AOM, including these infants who could have a lower likelihood of IBI than those with known bacterial AOM, may have led the researchers to underestimate IBI prevalence. Because existing data do not allow for clear distinction of viral from bacterial AOM without tympanocentesis, and because more than 85% of older patients with clinically diagnosed AOM also have observed bacterial otopathogens, the authors clarify that “it is understandable why clinicians would manage infants with AOM conservatively, regardless of the presence of concurrent viral illnesses.” They also acknowledged that one major challenge in working with infants believed to have AOM is ensuring that it is actually present since it is so hard to diagnose.
Dr. McLaren and colleagues cited several study limitations: 1) completeness and accuracy of data couldn’t be ensured because of the retrospective study design; 2) because not all infants were tested for IBI, its prevalence may have been underestimated; 3) infants whose discharge codes did not include AOM may have been missed, although all infants with positive blood or cerebrospinal fluid cultures were screened for missed AOM diagnosis; and 4) it is important to consider that any issues associated with testing and hospitalization that were identified may have been the result of management decisions driven by factors that cannot be captured retrospectively or by a diagnosis of AOM.
The findings are not generalizable to infants aged younger than 28 days
Finally, the authors cautioned that because the number of infants younger than 28 days was quite small, and it is therefore infinitely more challenging to diagnose AOM for these patients, results of the study should be applied to infants older than 28 days and are not generalizable to febrile infants.
“This report will not resolve the significant challenge faced by clinicians in treating infants aged [younger than] 28 days who have the highest risk of occult bacteremia and systemic spread of a focal bacterial infection,” Joseph Ravera, MD, and M.W. Stevens, MD, of the University of Vermont, Burlington, noted in an accompanying editorial. Previous studies have identified this age group “to be at the highest risk for systemic bacterial involvement and the most difficult to risk stratify on the basis of physical examination findings and initial laboratory results,” they noted. That the subjects aged younger than 28 days in this study had nearly a 50% admission rate illustrates the clinical uncertainty pediatric emergency medicine providers are challenged with, they added. Just 100 (6%) of the 1,637 patients in the study sample were in this age category, which makes it difficult, given the lack of sufficient data, to generalize findings to the youngest infants.
“Despite a paucity of young infants and limitations inherent to the design, this study does contribute to the literature with a robust retrospective data set of afebrile infants between 1 and 3 months of age with an ED diagnosis of AOM ... It certainly provides a base of support for carefully designed prospective studies in which researchers aim to determine the best care for AOM in children under 6 months of age,” reflected Dr. Ravera and Dr. Stevens.
In a separate interview, Karalyn Kinsella, MD, private practice, Cheshire, Conn. noted, “What is confusing is the absence of documented symptoms for infants presenting to the emergency department, as the symptoms they presented with would influence our concern for IBI. Diagnosing AOM in infants under 90 days old is extremely uncommon as an outpatient pediatrician. Although the finding of AOM in an afebrile infant is very rare in the outpatient setting, this study assures us the risk of IBI is almost nonexistent. Therefore, further workup is unnecessary unless providers have clinical suspicions to the contrary.”
Dr. McLaren and colleagues as well as Dr. Ravera, Dr. Stevens, and Dr. Kinsella, had no conflicts of interest and no relevant financial disclosures.
FROM PEDIATRICS
Waiting for the COVID 19 vaccine, or not?
A shot of relief. A shot of hope. Those are the words used to describe COVID-19 vaccines on a television commercial running in prime time in Kentucky.
“We all can’t get the vaccine at once,” the announcer says solemnly, “but we’ll all get a turn.”
For some of us, that turn came quickly. In December, the Advisory Committee on Immunization Practices recommended that health care personnel (HCP) and long-term care facility residents be the first to be immunized with COVID-19 vaccines (see table). 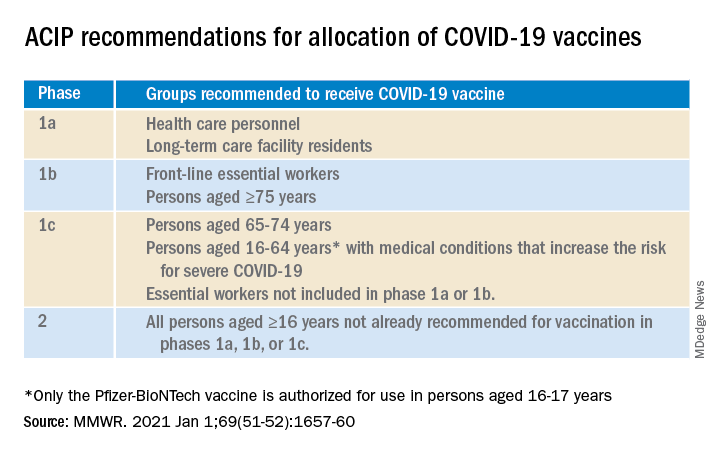
On Dec. 14, 2020, Sandra Lindsay, a nurse and director of patient care services in the intensive care unit at Long Island Jewish Medical Center, was the first person in the United States to receive a COVID-19 vaccine outside a clinical trial.
In subsequent days, social media sites were quickly flooded with photos of HCP rolling up their sleeves or flashing their immunization cards. There was jubilation ... and perhaps a little bit of jealousy. There were tears of joy and some tears of frustration.
There are more than 21 million HCP in the United States and to date, there have not been enough vaccines nor adequate infrastructure to immunize all of them. According to the Centers for Disease Control and Prevention Data Tracker, as of Jan. 7, 2021, 21,419,800 doses of vaccine had been distributed to states to immunize everyone identified in phase 1a, but only 5,919,418 people had received a first dose. Limited supply has necessitated prioritization of subgroups of HCP; those in the front of the line have varied by state, and even by hospital or health care systems within states. Both the American Academy of Pediatrics and the American Academy of Family Physicians have noted that primary care providers not employed by a hospital may have more difficulty accessing vaccine.
The mismatch between supply and demand has created an intense focus on improving supply and distribution. Soon though, we’re going to shift our attention to how we increase demand. We don’t have good data on those who being are offered COVID-19 vaccine and declining, but several studies that predate the Emergency Use Authorization for the Pfizer-BioNTech and Moderna vaccines suggest significant COVID-19 vaccine hesitancy among adults in the United States.
One large, longitudinal Internet-based study of U.S. adults found that the proportion who reported they were “somewhat or very likely” to receive COVID-19 vaccine declined from 74% in early April to 56% in early December.
In the Understanding America Study, self-reported likelihood of being vaccinated with COVID-19 vaccine was lower among Black compared to White respondents (38% vs. 59%; aRR, 0.7 [95% confidence interval, 0.6-0.8]), and lower among women compared to men (51% vs. 62%; aRR, 0.9 [95% CI, 0.8-0.9]). Those 65 years of age and older were more likely to report a willingness to be vaccinated than were those 18-49 years of age, as were those with at least a bachelor’s degree compared to those with a high school education or less.
A study conducted by the Pew Research Center in November – before any COVID-19 vaccines were available – found that only 60% of American adults said they would “definitely or probably get a vaccine for coronavirus” if one were available. That was an increase from 51% in September, but and overall decrease of 72% in May. Of the remaining 40%, just over half said they did not intend to get vaccinated and were “pretty certain” that more information would not change their minds.
Concern about acquiring a serious case of COVID-19 and trust in the vaccine development process were associated with an intent to receive vaccine, as was a personal history of receiving a flu shot annually. Willingness to be vaccinated varied by age, race, and family income, with Black respondents, women, and those with a lower family incomes less likely to accept a vaccine.
To date, few data are available about HCP and willingness to receive COVID-19 vaccine. A preprint posted at medrxiv.org reports on a cross-sectional study of more than 3,400 HCP surveyed between Oct. 7 and Nov. 9, 2020. In that study, only 36% of respondents voiced a willingness to be immunized as soon as vaccine is available. Vaccine acceptance increased with increasing age, income level, and education. As in other studies, self-reported willingness to accept vaccine was lower in women and Black individuals. While vaccine acceptance was higher in direct medical care providers than others, it was still only 49%.
So here’s the paradox: Even as limited supplies of vaccine are available and many are frustrated about lack of access, we need to promote the value of immunization to those who are hesitant. Pediatricians are trusted sources of vaccine information and we are in a good position to educate our colleagues, our staff, the parents of our patients and the community at-large.
A useful resource for those ready to take that step it is the CDC’s COVID-19 Vaccination Communication Toolkit. While this collection is designed to build vaccine confidence and promote immunization among health care providers, many of the strategies will be easily adapted for use with patients.
It’s not clear when we might have a COVID 19 vaccine for most children. The Pfizer-BioNTech vaccine emergency use authorization includes those as young as 16 years of age, and 16- and 17-year-olds with high risk medical conditions are included in phase 1c of vaccine allocation. Pfizer is currently enrolling children as young as 12 years of age in clinical trials, and Moderna and Janssen are poised to do the same. It is conceivable but far from certain that we could have a vaccine for children late this year. Are parents going to be ready to vaccinate their children?
Limited data about parental acceptance of vaccine for their children mirrors what was seen in the Understanding America Study and the Pew Research Study. In December 2020, the National Parents Union surveyed 1,008 parents of public school students enrolled in kindergarten through 12th grade. Sixty percent of parents said they would allow their children to receive a COVID-19 vaccine, while 25% would not and 15% were unsure. This suggests that now is the time to begin building vaccine confidence with parents. One conversation starter might be, “I am going to be vaccinated as soon as the vaccine is available.” Ideally, many of you will soon be able to say what I do: “I am excited to tell you that I have been immunized with the COVID-19 vaccine. I did this to protect myself, my family, and our community. I’m hopeful that vaccine will soon be available for all of us.”
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She said she had no relevant financial disclosures. Email her at [email protected].
A shot of relief. A shot of hope. Those are the words used to describe COVID-19 vaccines on a television commercial running in prime time in Kentucky.
“We all can’t get the vaccine at once,” the announcer says solemnly, “but we’ll all get a turn.”
For some of us, that turn came quickly. In December, the Advisory Committee on Immunization Practices recommended that health care personnel (HCP) and long-term care facility residents be the first to be immunized with COVID-19 vaccines (see table). 
On Dec. 14, 2020, Sandra Lindsay, a nurse and director of patient care services in the intensive care unit at Long Island Jewish Medical Center, was the first person in the United States to receive a COVID-19 vaccine outside a clinical trial.
In subsequent days, social media sites were quickly flooded with photos of HCP rolling up their sleeves or flashing their immunization cards. There was jubilation ... and perhaps a little bit of jealousy. There were tears of joy and some tears of frustration.
There are more than 21 million HCP in the United States and to date, there have not been enough vaccines nor adequate infrastructure to immunize all of them. According to the Centers for Disease Control and Prevention Data Tracker, as of Jan. 7, 2021, 21,419,800 doses of vaccine had been distributed to states to immunize everyone identified in phase 1a, but only 5,919,418 people had received a first dose. Limited supply has necessitated prioritization of subgroups of HCP; those in the front of the line have varied by state, and even by hospital or health care systems within states. Both the American Academy of Pediatrics and the American Academy of Family Physicians have noted that primary care providers not employed by a hospital may have more difficulty accessing vaccine.
The mismatch between supply and demand has created an intense focus on improving supply and distribution. Soon though, we’re going to shift our attention to how we increase demand. We don’t have good data on those who being are offered COVID-19 vaccine and declining, but several studies that predate the Emergency Use Authorization for the Pfizer-BioNTech and Moderna vaccines suggest significant COVID-19 vaccine hesitancy among adults in the United States.
One large, longitudinal Internet-based study of U.S. adults found that the proportion who reported they were “somewhat or very likely” to receive COVID-19 vaccine declined from 74% in early April to 56% in early December.
In the Understanding America Study, self-reported likelihood of being vaccinated with COVID-19 vaccine was lower among Black compared to White respondents (38% vs. 59%; aRR, 0.7 [95% confidence interval, 0.6-0.8]), and lower among women compared to men (51% vs. 62%; aRR, 0.9 [95% CI, 0.8-0.9]). Those 65 years of age and older were more likely to report a willingness to be vaccinated than were those 18-49 years of age, as were those with at least a bachelor’s degree compared to those with a high school education or less.
A study conducted by the Pew Research Center in November – before any COVID-19 vaccines were available – found that only 60% of American adults said they would “definitely or probably get a vaccine for coronavirus” if one were available. That was an increase from 51% in September, but and overall decrease of 72% in May. Of the remaining 40%, just over half said they did not intend to get vaccinated and were “pretty certain” that more information would not change their minds.
Concern about acquiring a serious case of COVID-19 and trust in the vaccine development process were associated with an intent to receive vaccine, as was a personal history of receiving a flu shot annually. Willingness to be vaccinated varied by age, race, and family income, with Black respondents, women, and those with a lower family incomes less likely to accept a vaccine.
To date, few data are available about HCP and willingness to receive COVID-19 vaccine. A preprint posted at medrxiv.org reports on a cross-sectional study of more than 3,400 HCP surveyed between Oct. 7 and Nov. 9, 2020. In that study, only 36% of respondents voiced a willingness to be immunized as soon as vaccine is available. Vaccine acceptance increased with increasing age, income level, and education. As in other studies, self-reported willingness to accept vaccine was lower in women and Black individuals. While vaccine acceptance was higher in direct medical care providers than others, it was still only 49%.
So here’s the paradox: Even as limited supplies of vaccine are available and many are frustrated about lack of access, we need to promote the value of immunization to those who are hesitant. Pediatricians are trusted sources of vaccine information and we are in a good position to educate our colleagues, our staff, the parents of our patients and the community at-large.
A useful resource for those ready to take that step it is the CDC’s COVID-19 Vaccination Communication Toolkit. While this collection is designed to build vaccine confidence and promote immunization among health care providers, many of the strategies will be easily adapted for use with patients.
It’s not clear when we might have a COVID 19 vaccine for most children. The Pfizer-BioNTech vaccine emergency use authorization includes those as young as 16 years of age, and 16- and 17-year-olds with high risk medical conditions are included in phase 1c of vaccine allocation. Pfizer is currently enrolling children as young as 12 years of age in clinical trials, and Moderna and Janssen are poised to do the same. It is conceivable but far from certain that we could have a vaccine for children late this year. Are parents going to be ready to vaccinate their children?
Limited data about parental acceptance of vaccine for their children mirrors what was seen in the Understanding America Study and the Pew Research Study. In December 2020, the National Parents Union surveyed 1,008 parents of public school students enrolled in kindergarten through 12th grade. Sixty percent of parents said they would allow their children to receive a COVID-19 vaccine, while 25% would not and 15% were unsure. This suggests that now is the time to begin building vaccine confidence with parents. One conversation starter might be, “I am going to be vaccinated as soon as the vaccine is available.” Ideally, many of you will soon be able to say what I do: “I am excited to tell you that I have been immunized with the COVID-19 vaccine. I did this to protect myself, my family, and our community. I’m hopeful that vaccine will soon be available for all of us.”
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She said she had no relevant financial disclosures. Email her at [email protected].
A shot of relief. A shot of hope. Those are the words used to describe COVID-19 vaccines on a television commercial running in prime time in Kentucky.
“We all can’t get the vaccine at once,” the announcer says solemnly, “but we’ll all get a turn.”
For some of us, that turn came quickly. In December, the Advisory Committee on Immunization Practices recommended that health care personnel (HCP) and long-term care facility residents be the first to be immunized with COVID-19 vaccines (see table). 
On Dec. 14, 2020, Sandra Lindsay, a nurse and director of patient care services in the intensive care unit at Long Island Jewish Medical Center, was the first person in the United States to receive a COVID-19 vaccine outside a clinical trial.
In subsequent days, social media sites were quickly flooded with photos of HCP rolling up their sleeves or flashing their immunization cards. There was jubilation ... and perhaps a little bit of jealousy. There were tears of joy and some tears of frustration.
There are more than 21 million HCP in the United States and to date, there have not been enough vaccines nor adequate infrastructure to immunize all of them. According to the Centers for Disease Control and Prevention Data Tracker, as of Jan. 7, 2021, 21,419,800 doses of vaccine had been distributed to states to immunize everyone identified in phase 1a, but only 5,919,418 people had received a first dose. Limited supply has necessitated prioritization of subgroups of HCP; those in the front of the line have varied by state, and even by hospital or health care systems within states. Both the American Academy of Pediatrics and the American Academy of Family Physicians have noted that primary care providers not employed by a hospital may have more difficulty accessing vaccine.
The mismatch between supply and demand has created an intense focus on improving supply and distribution. Soon though, we’re going to shift our attention to how we increase demand. We don’t have good data on those who being are offered COVID-19 vaccine and declining, but several studies that predate the Emergency Use Authorization for the Pfizer-BioNTech and Moderna vaccines suggest significant COVID-19 vaccine hesitancy among adults in the United States.
One large, longitudinal Internet-based study of U.S. adults found that the proportion who reported they were “somewhat or very likely” to receive COVID-19 vaccine declined from 74% in early April to 56% in early December.
In the Understanding America Study, self-reported likelihood of being vaccinated with COVID-19 vaccine was lower among Black compared to White respondents (38% vs. 59%; aRR, 0.7 [95% confidence interval, 0.6-0.8]), and lower among women compared to men (51% vs. 62%; aRR, 0.9 [95% CI, 0.8-0.9]). Those 65 years of age and older were more likely to report a willingness to be vaccinated than were those 18-49 years of age, as were those with at least a bachelor’s degree compared to those with a high school education or less.
A study conducted by the Pew Research Center in November – before any COVID-19 vaccines were available – found that only 60% of American adults said they would “definitely or probably get a vaccine for coronavirus” if one were available. That was an increase from 51% in September, but and overall decrease of 72% in May. Of the remaining 40%, just over half said they did not intend to get vaccinated and were “pretty certain” that more information would not change their minds.
Concern about acquiring a serious case of COVID-19 and trust in the vaccine development process were associated with an intent to receive vaccine, as was a personal history of receiving a flu shot annually. Willingness to be vaccinated varied by age, race, and family income, with Black respondents, women, and those with a lower family incomes less likely to accept a vaccine.
To date, few data are available about HCP and willingness to receive COVID-19 vaccine. A preprint posted at medrxiv.org reports on a cross-sectional study of more than 3,400 HCP surveyed between Oct. 7 and Nov. 9, 2020. In that study, only 36% of respondents voiced a willingness to be immunized as soon as vaccine is available. Vaccine acceptance increased with increasing age, income level, and education. As in other studies, self-reported willingness to accept vaccine was lower in women and Black individuals. While vaccine acceptance was higher in direct medical care providers than others, it was still only 49%.
So here’s the paradox: Even as limited supplies of vaccine are available and many are frustrated about lack of access, we need to promote the value of immunization to those who are hesitant. Pediatricians are trusted sources of vaccine information and we are in a good position to educate our colleagues, our staff, the parents of our patients and the community at-large.
A useful resource for those ready to take that step it is the CDC’s COVID-19 Vaccination Communication Toolkit. While this collection is designed to build vaccine confidence and promote immunization among health care providers, many of the strategies will be easily adapted for use with patients.
It’s not clear when we might have a COVID 19 vaccine for most children. The Pfizer-BioNTech vaccine emergency use authorization includes those as young as 16 years of age, and 16- and 17-year-olds with high risk medical conditions are included in phase 1c of vaccine allocation. Pfizer is currently enrolling children as young as 12 years of age in clinical trials, and Moderna and Janssen are poised to do the same. It is conceivable but far from certain that we could have a vaccine for children late this year. Are parents going to be ready to vaccinate their children?
Limited data about parental acceptance of vaccine for their children mirrors what was seen in the Understanding America Study and the Pew Research Study. In December 2020, the National Parents Union surveyed 1,008 parents of public school students enrolled in kindergarten through 12th grade. Sixty percent of parents said they would allow their children to receive a COVID-19 vaccine, while 25% would not and 15% were unsure. This suggests that now is the time to begin building vaccine confidence with parents. One conversation starter might be, “I am going to be vaccinated as soon as the vaccine is available.” Ideally, many of you will soon be able to say what I do: “I am excited to tell you that I have been immunized with the COVID-19 vaccine. I did this to protect myself, my family, and our community. I’m hopeful that vaccine will soon be available for all of us.”
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She said she had no relevant financial disclosures. Email her at [email protected].
CVD deaths rose, imaging declined during pandemic
While the direct toll of the COVID-19 pandemic is being tallied and shared on the nightly news, the indirect effects will undoubtedly take years to fully measure.
In two papers published online Jan. 11 in the Journal of the American College of Cardiology, researchers have started the process of quantifying the impact of the pandemic on the care of patients with cardiovascular disease (CVD).
In the first study, Rishi Wadhera, MD, MPP, MPhil, and colleagues from the Beth Israel Deaconess Medical Center and Harvard Medical School in Boston examined population-level data to determine how deaths from cardiovascular causes changed in the United States in the early months of the pandemic relative to the same periods in 2019.
In a second paper, Andrew J. Einstein, MD, PhD, from Columbia University Irving Medical Center/New York–Presbyterian Hospital and colleagues looked at the pandemic’s international impact on the diagnosis of heart disease.
Using data from the National Center for Health Statistics, Dr. Wadhera and colleagues compared death rates from cardiovascular causes in the United States from March 18, 2020, to June 2, 2020, (the first wave of the pandemic) and from Jan. 1, 2020, to March 17, 2020, (the period just before the pandemic started) and compared them to the same periods in 2019. ICD codes were used to identify underlying causes of death.
Relative to 2019, they found a significant increase in deaths from ischemic heart disease nationally (1.11; 95% confidence interval, 1.04-1.18), as well as an increase in deaths caused by hypertensive disease (1.17; 95% CI, 1.09-1.26). There was no apparent increase in deaths from heart failure, cerebrovascular disease, or other diseases of the circulatory system.
When they looked just at New York City, the area hit hardest during the early part of the pandemic, the relative increases in deaths from ischemic heart disease were more pronounced.
Deaths from ischemic heart disease or hypertensive diseases jumped 139% and 164%, respectively, between March 18, 2020, and June 2, 2020.
More modest increases in deaths were seen in the remainder of New York state, New Jersey, Michigan and Illinois, while Massachusetts and Louisiana did not see a change in cardiovascular deaths.
Several studies from different parts of the world have indicated a 40%-50% drop in hospitalization for myocardial infarction in the initial months of the pandemic, said Dr. Wadhera in an interview.
“We wanted to understand where did all the heart attacks go? And we worried that patients with urgent heart conditions were not seeking the medical care they needed. I think our data suggest that this may have been the case,” reported Dr. Wadhera.
“This very much reflects the reality of what we’re seeing on the ground,” he told this news organization. “After the initial surge ended, when hospital volumes began to return to normal, we saw patients come into the hospital who clearly had a heart attack during the surge months – and were now experiencing complications of that event – because they had initially not come into the hospital due to concerns about exposure to the virus.”
A limitation of their data, he stressed, is whether some deaths coded as CVD deaths were really deaths from undiagnosed COVID-19. “It’s possible that some portion of the increased deaths we observed really reflect the cardiovascular complications of undiagnosed COVID-19, because we know that testing was quite limited during the early first surge of cases.”
“I think that basically three factors – patients avoiding the health care system because of fear of getting COVID, health care systems being strained and overwhelmed leading to the deferral of cardiovascular care and semi-elective procedures, and the cardiovascular complications of COVID-19 itself – all probably collectively contributed to the rise in cardiovascular deaths that we observed,” said Dr. Wadhera.
In an accompanying editorial, Michael N. Young, MD, Geisel School of Medicine at Dartmouth, Lebanon, N.H., and colleagues write that these data, taken together with an earlier study showing an increase in out-of-hospital cardiac arrests at the pandemic peak in New York City, “support the notion of excess fatalities due to unattended comorbid illnesses.” That said, attribution of death in the COVID era “remains problematic.”
In the second article, Andrew Einstein, MD, PhD, and the INCAPS COVID Investigators Group took a broader approach and looked at the impact of COVID-19 on cardiac diagnostic procedures in over 100 countries.
The INCAPS (International Atomic Energy Agency Noninvasive Cardiology Protocols Study) group has for the past decade conducted numerous studies addressing the use of best practices and worldwide practice variation in CVD diagnosis.
For this effort, they sent a survey link to INCAPS participants worldwide, ultimately including 909 survey responses from 108 countries in the final analysis.
Compared with March 2019, overall procedure volume decreased 42% in March 2020 and 64% in April 2020.
The greatest decreases were seen in stress testing (78%) and transesophageal echocardiography (76%), both procedures, noted Dr. Einstein, associated with a greater risk of aerosolization.
“Whether as we reset after COVID we return to the same place in terms of the use of cardiovascular diagnostic testing remains to be seen, but it certainly poses an opportunity to improve our utilization of various modes of testing,” said Dr. Einstein.
Using regression analysis, Dr. Einstein and colleagues were able to see that sites located in low-income and lower-middle-income countries saw an additional 22% reduction in cardiac procedures and less availability of personal protective equipment (PPE) and telehealth.
Fifty-two percent of survey respondents reported significant shortages of N95 masks early in the pandemic, with fewer issues in supplies of gloves, gowns, and face shields. Lower-income countries were more likely to face significant PPE shortages and less likely to be able to implement telehealth strategies to make up for reduced in-person care. PPE shortage itself, however, was not related to lower procedural volume on multivariable regression.
“It all really begs the question of whether there is more that the world can do to help out the developing world in terms of managing the pandemic in all its facets,” said Dr. Einstein in an interview, adding he was “shocked” to learn how difficult it was for some lower-income countries to get sufficient PPE.
Did shutdowns go too far?
Calling this a “remarkable study,” an editorial written by Darryl P. Leong, MBBS, PhD, John W. Eikelboom, MBBS, and Salim Yusuf, MBBS, DPhil, all from McMaster University, Hamilton, Ont., suggests that perhaps health systems in some places went too far in closing down during the first wave of the pandemic, naming specifically Canada, Eastern Europe, and Saudi Arabia as examples.
“Although these measures were taken to prepare for the worst, overwhelming numbers of patients with COVID-19 did not materialize during the first wave of the pandemic in these countries. It is possible that delaying so-called nonessential services may have been unnecessary and potentially harmful, because it likely led to delays in providing care for the treatment of serious non–COVID-19 illnesses.”
Since then, more experience and more data have largely allowed hospital systems to “tackle the ebb and flow” of COVID-19 cases in ways that limit shutdowns of important health services, they said.
Given the more pronounced effect in low- and middle-income countries, they stressed the need to focus resources on ways to promote prevention and treatment that do not rely on diagnostic procedures.
“This calls for more emphasis on developing efficient systems of telehealth, especially in poorer countries or in remote settings in all countries,” Dr. Leong and colleagues conclude.
Dr. Wadhera has reported research support from the National Heart, Lung, and Blood Institute, along with fellow senior author Robert W. Yeh, MD, MBA, who has also received personal fees and grants from several companies not related to the submitted work. Dr. Einstein, Dr. Leong, Dr. Eikelboom, and Dr. Yusuf have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
While the direct toll of the COVID-19 pandemic is being tallied and shared on the nightly news, the indirect effects will undoubtedly take years to fully measure.
In two papers published online Jan. 11 in the Journal of the American College of Cardiology, researchers have started the process of quantifying the impact of the pandemic on the care of patients with cardiovascular disease (CVD).
In the first study, Rishi Wadhera, MD, MPP, MPhil, and colleagues from the Beth Israel Deaconess Medical Center and Harvard Medical School in Boston examined population-level data to determine how deaths from cardiovascular causes changed in the United States in the early months of the pandemic relative to the same periods in 2019.
In a second paper, Andrew J. Einstein, MD, PhD, from Columbia University Irving Medical Center/New York–Presbyterian Hospital and colleagues looked at the pandemic’s international impact on the diagnosis of heart disease.
Using data from the National Center for Health Statistics, Dr. Wadhera and colleagues compared death rates from cardiovascular causes in the United States from March 18, 2020, to June 2, 2020, (the first wave of the pandemic) and from Jan. 1, 2020, to March 17, 2020, (the period just before the pandemic started) and compared them to the same periods in 2019. ICD codes were used to identify underlying causes of death.
Relative to 2019, they found a significant increase in deaths from ischemic heart disease nationally (1.11; 95% confidence interval, 1.04-1.18), as well as an increase in deaths caused by hypertensive disease (1.17; 95% CI, 1.09-1.26). There was no apparent increase in deaths from heart failure, cerebrovascular disease, or other diseases of the circulatory system.
When they looked just at New York City, the area hit hardest during the early part of the pandemic, the relative increases in deaths from ischemic heart disease were more pronounced.
Deaths from ischemic heart disease or hypertensive diseases jumped 139% and 164%, respectively, between March 18, 2020, and June 2, 2020.
More modest increases in deaths were seen in the remainder of New York state, New Jersey, Michigan and Illinois, while Massachusetts and Louisiana did not see a change in cardiovascular deaths.
Several studies from different parts of the world have indicated a 40%-50% drop in hospitalization for myocardial infarction in the initial months of the pandemic, said Dr. Wadhera in an interview.
“We wanted to understand where did all the heart attacks go? And we worried that patients with urgent heart conditions were not seeking the medical care they needed. I think our data suggest that this may have been the case,” reported Dr. Wadhera.
“This very much reflects the reality of what we’re seeing on the ground,” he told this news organization. “After the initial surge ended, when hospital volumes began to return to normal, we saw patients come into the hospital who clearly had a heart attack during the surge months – and were now experiencing complications of that event – because they had initially not come into the hospital due to concerns about exposure to the virus.”
A limitation of their data, he stressed, is whether some deaths coded as CVD deaths were really deaths from undiagnosed COVID-19. “It’s possible that some portion of the increased deaths we observed really reflect the cardiovascular complications of undiagnosed COVID-19, because we know that testing was quite limited during the early first surge of cases.”
“I think that basically three factors – patients avoiding the health care system because of fear of getting COVID, health care systems being strained and overwhelmed leading to the deferral of cardiovascular care and semi-elective procedures, and the cardiovascular complications of COVID-19 itself – all probably collectively contributed to the rise in cardiovascular deaths that we observed,” said Dr. Wadhera.
In an accompanying editorial, Michael N. Young, MD, Geisel School of Medicine at Dartmouth, Lebanon, N.H., and colleagues write that these data, taken together with an earlier study showing an increase in out-of-hospital cardiac arrests at the pandemic peak in New York City, “support the notion of excess fatalities due to unattended comorbid illnesses.” That said, attribution of death in the COVID era “remains problematic.”
In the second article, Andrew Einstein, MD, PhD, and the INCAPS COVID Investigators Group took a broader approach and looked at the impact of COVID-19 on cardiac diagnostic procedures in over 100 countries.
The INCAPS (International Atomic Energy Agency Noninvasive Cardiology Protocols Study) group has for the past decade conducted numerous studies addressing the use of best practices and worldwide practice variation in CVD diagnosis.
For this effort, they sent a survey link to INCAPS participants worldwide, ultimately including 909 survey responses from 108 countries in the final analysis.
Compared with March 2019, overall procedure volume decreased 42% in March 2020 and 64% in April 2020.
The greatest decreases were seen in stress testing (78%) and transesophageal echocardiography (76%), both procedures, noted Dr. Einstein, associated with a greater risk of aerosolization.
“Whether as we reset after COVID we return to the same place in terms of the use of cardiovascular diagnostic testing remains to be seen, but it certainly poses an opportunity to improve our utilization of various modes of testing,” said Dr. Einstein.
Using regression analysis, Dr. Einstein and colleagues were able to see that sites located in low-income and lower-middle-income countries saw an additional 22% reduction in cardiac procedures and less availability of personal protective equipment (PPE) and telehealth.
Fifty-two percent of survey respondents reported significant shortages of N95 masks early in the pandemic, with fewer issues in supplies of gloves, gowns, and face shields. Lower-income countries were more likely to face significant PPE shortages and less likely to be able to implement telehealth strategies to make up for reduced in-person care. PPE shortage itself, however, was not related to lower procedural volume on multivariable regression.
“It all really begs the question of whether there is more that the world can do to help out the developing world in terms of managing the pandemic in all its facets,” said Dr. Einstein in an interview, adding he was “shocked” to learn how difficult it was for some lower-income countries to get sufficient PPE.
Did shutdowns go too far?
Calling this a “remarkable study,” an editorial written by Darryl P. Leong, MBBS, PhD, John W. Eikelboom, MBBS, and Salim Yusuf, MBBS, DPhil, all from McMaster University, Hamilton, Ont., suggests that perhaps health systems in some places went too far in closing down during the first wave of the pandemic, naming specifically Canada, Eastern Europe, and Saudi Arabia as examples.
“Although these measures were taken to prepare for the worst, overwhelming numbers of patients with COVID-19 did not materialize during the first wave of the pandemic in these countries. It is possible that delaying so-called nonessential services may have been unnecessary and potentially harmful, because it likely led to delays in providing care for the treatment of serious non–COVID-19 illnesses.”
Since then, more experience and more data have largely allowed hospital systems to “tackle the ebb and flow” of COVID-19 cases in ways that limit shutdowns of important health services, they said.
Given the more pronounced effect in low- and middle-income countries, they stressed the need to focus resources on ways to promote prevention and treatment that do not rely on diagnostic procedures.
“This calls for more emphasis on developing efficient systems of telehealth, especially in poorer countries or in remote settings in all countries,” Dr. Leong and colleagues conclude.
Dr. Wadhera has reported research support from the National Heart, Lung, and Blood Institute, along with fellow senior author Robert W. Yeh, MD, MBA, who has also received personal fees and grants from several companies not related to the submitted work. Dr. Einstein, Dr. Leong, Dr. Eikelboom, and Dr. Yusuf have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
While the direct toll of the COVID-19 pandemic is being tallied and shared on the nightly news, the indirect effects will undoubtedly take years to fully measure.
In two papers published online Jan. 11 in the Journal of the American College of Cardiology, researchers have started the process of quantifying the impact of the pandemic on the care of patients with cardiovascular disease (CVD).
In the first study, Rishi Wadhera, MD, MPP, MPhil, and colleagues from the Beth Israel Deaconess Medical Center and Harvard Medical School in Boston examined population-level data to determine how deaths from cardiovascular causes changed in the United States in the early months of the pandemic relative to the same periods in 2019.
In a second paper, Andrew J. Einstein, MD, PhD, from Columbia University Irving Medical Center/New York–Presbyterian Hospital and colleagues looked at the pandemic’s international impact on the diagnosis of heart disease.
Using data from the National Center for Health Statistics, Dr. Wadhera and colleagues compared death rates from cardiovascular causes in the United States from March 18, 2020, to June 2, 2020, (the first wave of the pandemic) and from Jan. 1, 2020, to March 17, 2020, (the period just before the pandemic started) and compared them to the same periods in 2019. ICD codes were used to identify underlying causes of death.
Relative to 2019, they found a significant increase in deaths from ischemic heart disease nationally (1.11; 95% confidence interval, 1.04-1.18), as well as an increase in deaths caused by hypertensive disease (1.17; 95% CI, 1.09-1.26). There was no apparent increase in deaths from heart failure, cerebrovascular disease, or other diseases of the circulatory system.
When they looked just at New York City, the area hit hardest during the early part of the pandemic, the relative increases in deaths from ischemic heart disease were more pronounced.
Deaths from ischemic heart disease or hypertensive diseases jumped 139% and 164%, respectively, between March 18, 2020, and June 2, 2020.
More modest increases in deaths were seen in the remainder of New York state, New Jersey, Michigan and Illinois, while Massachusetts and Louisiana did not see a change in cardiovascular deaths.
Several studies from different parts of the world have indicated a 40%-50% drop in hospitalization for myocardial infarction in the initial months of the pandemic, said Dr. Wadhera in an interview.
“We wanted to understand where did all the heart attacks go? And we worried that patients with urgent heart conditions were not seeking the medical care they needed. I think our data suggest that this may have been the case,” reported Dr. Wadhera.
“This very much reflects the reality of what we’re seeing on the ground,” he told this news organization. “After the initial surge ended, when hospital volumes began to return to normal, we saw patients come into the hospital who clearly had a heart attack during the surge months – and were now experiencing complications of that event – because they had initially not come into the hospital due to concerns about exposure to the virus.”
A limitation of their data, he stressed, is whether some deaths coded as CVD deaths were really deaths from undiagnosed COVID-19. “It’s possible that some portion of the increased deaths we observed really reflect the cardiovascular complications of undiagnosed COVID-19, because we know that testing was quite limited during the early first surge of cases.”
“I think that basically three factors – patients avoiding the health care system because of fear of getting COVID, health care systems being strained and overwhelmed leading to the deferral of cardiovascular care and semi-elective procedures, and the cardiovascular complications of COVID-19 itself – all probably collectively contributed to the rise in cardiovascular deaths that we observed,” said Dr. Wadhera.
In an accompanying editorial, Michael N. Young, MD, Geisel School of Medicine at Dartmouth, Lebanon, N.H., and colleagues write that these data, taken together with an earlier study showing an increase in out-of-hospital cardiac arrests at the pandemic peak in New York City, “support the notion of excess fatalities due to unattended comorbid illnesses.” That said, attribution of death in the COVID era “remains problematic.”
In the second article, Andrew Einstein, MD, PhD, and the INCAPS COVID Investigators Group took a broader approach and looked at the impact of COVID-19 on cardiac diagnostic procedures in over 100 countries.
The INCAPS (International Atomic Energy Agency Noninvasive Cardiology Protocols Study) group has for the past decade conducted numerous studies addressing the use of best practices and worldwide practice variation in CVD diagnosis.
For this effort, they sent a survey link to INCAPS participants worldwide, ultimately including 909 survey responses from 108 countries in the final analysis.
Compared with March 2019, overall procedure volume decreased 42% in March 2020 and 64% in April 2020.
The greatest decreases were seen in stress testing (78%) and transesophageal echocardiography (76%), both procedures, noted Dr. Einstein, associated with a greater risk of aerosolization.
“Whether as we reset after COVID we return to the same place in terms of the use of cardiovascular diagnostic testing remains to be seen, but it certainly poses an opportunity to improve our utilization of various modes of testing,” said Dr. Einstein.
Using regression analysis, Dr. Einstein and colleagues were able to see that sites located in low-income and lower-middle-income countries saw an additional 22% reduction in cardiac procedures and less availability of personal protective equipment (PPE) and telehealth.
Fifty-two percent of survey respondents reported significant shortages of N95 masks early in the pandemic, with fewer issues in supplies of gloves, gowns, and face shields. Lower-income countries were more likely to face significant PPE shortages and less likely to be able to implement telehealth strategies to make up for reduced in-person care. PPE shortage itself, however, was not related to lower procedural volume on multivariable regression.
“It all really begs the question of whether there is more that the world can do to help out the developing world in terms of managing the pandemic in all its facets,” said Dr. Einstein in an interview, adding he was “shocked” to learn how difficult it was for some lower-income countries to get sufficient PPE.
Did shutdowns go too far?
Calling this a “remarkable study,” an editorial written by Darryl P. Leong, MBBS, PhD, John W. Eikelboom, MBBS, and Salim Yusuf, MBBS, DPhil, all from McMaster University, Hamilton, Ont., suggests that perhaps health systems in some places went too far in closing down during the first wave of the pandemic, naming specifically Canada, Eastern Europe, and Saudi Arabia as examples.
“Although these measures were taken to prepare for the worst, overwhelming numbers of patients with COVID-19 did not materialize during the first wave of the pandemic in these countries. It is possible that delaying so-called nonessential services may have been unnecessary and potentially harmful, because it likely led to delays in providing care for the treatment of serious non–COVID-19 illnesses.”
Since then, more experience and more data have largely allowed hospital systems to “tackle the ebb and flow” of COVID-19 cases in ways that limit shutdowns of important health services, they said.
Given the more pronounced effect in low- and middle-income countries, they stressed the need to focus resources on ways to promote prevention and treatment that do not rely on diagnostic procedures.
“This calls for more emphasis on developing efficient systems of telehealth, especially in poorer countries or in remote settings in all countries,” Dr. Leong and colleagues conclude.
Dr. Wadhera has reported research support from the National Heart, Lung, and Blood Institute, along with fellow senior author Robert W. Yeh, MD, MBA, who has also received personal fees and grants from several companies not related to the submitted work. Dr. Einstein, Dr. Leong, Dr. Eikelboom, and Dr. Yusuf have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Natural immunity from COVID-19 ‘may last months’
Infection with the SARS-CoV-2 virus may provide some immunity for at least 5 months, interim results from a study has found.
The first report from the Sarscov2 Immunity & Reinfection Evaluation (SIREN) study suggested that antibodies from people who had recovered from COVID-19 gave at least 83% protection against reinfection compared with people who had not had the disease before.
However, Public Health England (PHE) researchers said some people with antibodies may still be able to carry and transmit the SARS-CoV-2 virus.
‘Strongly encouraged’
Susan Hopkins, PhD, senior medical advisor at PHE, who is leading the study, said the overall findings were good news. She told a briefing hosted by the Science Media Centre: “I am strongly encouraged that people have immunity that is lasting much more than the few months that was speculated before the summer.”
She added: “It allows people to feel that their prior infection will protect them from future infections but at the same time it is not complete protection, and therefore they still need to be careful when they are out and about.”
PHE scientists said they would continue to assess whether protection might last longer than 5 months.
Eleanor Riley, PhD, professor of immunology and infectious disease at the University of Edinburgh, said the report suggested that “natural infection provides short-term protection against COVID-19 that is very similar to that conferred by vaccination.”
Simon Clarke, PhD, associate professor in cellular microbiology at the University of Reading, said: “The concerning finding is that some people who have COVID antibodies appear to still be able to carry the coronavirus and could spread it to others. This means that the vast majority of the population will either need to have natural immunity or have been immunised for us to fully lift restrictions on our lives.”
The analysis took place before the new variant of SARS-CoV-2 became widespread in the UK. The PHE scientists said that further work was underway to establish whether and to what extent antibodies also provide protection from the VOC202012/01 variant.
Healthcare Workers
The SIREN preprint analysed data from 20,787 health care workers from 102 NHS trusts who had undergone antibody and PCR testing from June 18 to November 9, 2020.
Of those, 6614 tested positive for COVID-19 antibodies.
Of the 44 potential reinfections identified, two were designated ‘probable’ and 42 ‘possible’, based on available evidence.
Both of the two individuals classified as probable reinfections reported having experienced COVID-19 symptoms during the first wave of the pandemic but were not tested at the time. Both reported that their symptoms were less severe the second time.
None of the 44 potential reinfection cases were PCR tested during the first wave, but all tested positive for COVID-19 antibodies at the time they were recruited to the study.
Tom Wingfield, PhD, senior clinical lecturer at the Liverpool School of Tropical Medicine, said that given the high risk of SARS-CoV-2 infection for frontline NHS staff, it was “vital that we do all that we can to understand, predict, and prevent risk of SARS-CoV-2 amongst healthcare workers”.
The study will continue to follow participants for 12 months to explore how long any immunity may last, the effectiveness of vaccines, and to what extent people with immunity are able to carry and transmit the virus.
A version of this article first appeared on Medscape.com.
Infection with the SARS-CoV-2 virus may provide some immunity for at least 5 months, interim results from a study has found.
The first report from the Sarscov2 Immunity & Reinfection Evaluation (SIREN) study suggested that antibodies from people who had recovered from COVID-19 gave at least 83% protection against reinfection compared with people who had not had the disease before.
However, Public Health England (PHE) researchers said some people with antibodies may still be able to carry and transmit the SARS-CoV-2 virus.
‘Strongly encouraged’
Susan Hopkins, PhD, senior medical advisor at PHE, who is leading the study, said the overall findings were good news. She told a briefing hosted by the Science Media Centre: “I am strongly encouraged that people have immunity that is lasting much more than the few months that was speculated before the summer.”
She added: “It allows people to feel that their prior infection will protect them from future infections but at the same time it is not complete protection, and therefore they still need to be careful when they are out and about.”
PHE scientists said they would continue to assess whether protection might last longer than 5 months.
Eleanor Riley, PhD, professor of immunology and infectious disease at the University of Edinburgh, said the report suggested that “natural infection provides short-term protection against COVID-19 that is very similar to that conferred by vaccination.”
Simon Clarke, PhD, associate professor in cellular microbiology at the University of Reading, said: “The concerning finding is that some people who have COVID antibodies appear to still be able to carry the coronavirus and could spread it to others. This means that the vast majority of the population will either need to have natural immunity or have been immunised for us to fully lift restrictions on our lives.”
The analysis took place before the new variant of SARS-CoV-2 became widespread in the UK. The PHE scientists said that further work was underway to establish whether and to what extent antibodies also provide protection from the VOC202012/01 variant.
Healthcare Workers
The SIREN preprint analysed data from 20,787 health care workers from 102 NHS trusts who had undergone antibody and PCR testing from June 18 to November 9, 2020.
Of those, 6614 tested positive for COVID-19 antibodies.
Of the 44 potential reinfections identified, two were designated ‘probable’ and 42 ‘possible’, based on available evidence.
Both of the two individuals classified as probable reinfections reported having experienced COVID-19 symptoms during the first wave of the pandemic but were not tested at the time. Both reported that their symptoms were less severe the second time.
None of the 44 potential reinfection cases were PCR tested during the first wave, but all tested positive for COVID-19 antibodies at the time they were recruited to the study.
Tom Wingfield, PhD, senior clinical lecturer at the Liverpool School of Tropical Medicine, said that given the high risk of SARS-CoV-2 infection for frontline NHS staff, it was “vital that we do all that we can to understand, predict, and prevent risk of SARS-CoV-2 amongst healthcare workers”.
The study will continue to follow participants for 12 months to explore how long any immunity may last, the effectiveness of vaccines, and to what extent people with immunity are able to carry and transmit the virus.
A version of this article first appeared on Medscape.com.
Infection with the SARS-CoV-2 virus may provide some immunity for at least 5 months, interim results from a study has found.
The first report from the Sarscov2 Immunity & Reinfection Evaluation (SIREN) study suggested that antibodies from people who had recovered from COVID-19 gave at least 83% protection against reinfection compared with people who had not had the disease before.
However, Public Health England (PHE) researchers said some people with antibodies may still be able to carry and transmit the SARS-CoV-2 virus.
‘Strongly encouraged’
Susan Hopkins, PhD, senior medical advisor at PHE, who is leading the study, said the overall findings were good news. She told a briefing hosted by the Science Media Centre: “I am strongly encouraged that people have immunity that is lasting much more than the few months that was speculated before the summer.”
She added: “It allows people to feel that their prior infection will protect them from future infections but at the same time it is not complete protection, and therefore they still need to be careful when they are out and about.”
PHE scientists said they would continue to assess whether protection might last longer than 5 months.
Eleanor Riley, PhD, professor of immunology and infectious disease at the University of Edinburgh, said the report suggested that “natural infection provides short-term protection against COVID-19 that is very similar to that conferred by vaccination.”
Simon Clarke, PhD, associate professor in cellular microbiology at the University of Reading, said: “The concerning finding is that some people who have COVID antibodies appear to still be able to carry the coronavirus and could spread it to others. This means that the vast majority of the population will either need to have natural immunity or have been immunised for us to fully lift restrictions on our lives.”
The analysis took place before the new variant of SARS-CoV-2 became widespread in the UK. The PHE scientists said that further work was underway to establish whether and to what extent antibodies also provide protection from the VOC202012/01 variant.
Healthcare Workers
The SIREN preprint analysed data from 20,787 health care workers from 102 NHS trusts who had undergone antibody and PCR testing from June 18 to November 9, 2020.
Of those, 6614 tested positive for COVID-19 antibodies.
Of the 44 potential reinfections identified, two were designated ‘probable’ and 42 ‘possible’, based on available evidence.
Both of the two individuals classified as probable reinfections reported having experienced COVID-19 symptoms during the first wave of the pandemic but were not tested at the time. Both reported that their symptoms were less severe the second time.
None of the 44 potential reinfection cases were PCR tested during the first wave, but all tested positive for COVID-19 antibodies at the time they were recruited to the study.
Tom Wingfield, PhD, senior clinical lecturer at the Liverpool School of Tropical Medicine, said that given the high risk of SARS-CoV-2 infection for frontline NHS staff, it was “vital that we do all that we can to understand, predict, and prevent risk of SARS-CoV-2 amongst healthcare workers”.
The study will continue to follow participants for 12 months to explore how long any immunity may last, the effectiveness of vaccines, and to what extent people with immunity are able to carry and transmit the virus.
A version of this article first appeared on Medscape.com.
COVID protections suppressed flu season in U.S.
Last fall, health experts said it was possible the United States could experience an easy 2020-21 flu season because health measures to fight COVID-19 would also thwart the spread of influenza.
It looks like that happened – and then some. Numbers are strikingly low for cases of the flu and other common respiratory and gastrointestinal viruses, health experts told the Washington Post.
“It’s crazy,” Lynnette Brammer, MPH, who leads the domestic influenza surveillance team at the Centers for Disease Control and Prevention, told the Washington Post. “This is my 30th flu season. I never would have expected to see flu activity this low.”
Influenza A, influenza B, parainfluenza, norovirus, respiratory syncytial virus, human metapneumovirus, and the bacteria that cause whooping cough and pneumonia are circulating at near-record-low levels.
As an example, the Washington Post said in the third week of December 2019, the CDC’s network of clinical labs reported 16.2% of almost 30,000 samples tested positive for influenza A. During the same period in 2020, only 0.3% tested positive.
But there’s a possible downside to this suppression of viruses, because flu and other viruses may rebound once the coronavirus is brought under control.
“The best analogy is to a forest fire,” Bryan Grenfell, PhD, an epidemiologist and population biologist at Princeton (N.J.) University, told the Washington Post. “For the fire to spread, it needs to have unburned wood. For epidemics to spread, they require people who haven’t previously been infected. So if people don’t get infected this year by these viruses, they likely will at some point later on.”
American health experts like Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Disease, said last fall that they noticed Australia and other nations in the southern hemisphere had easy flu seasons, apparently because of COVID protection measures. The flu season there runs March through August.
COVID-19 now has a very low presence in Australia, but in recent months the flu has been making a comeback. Flu cases among children aged 5 and younger rose sixfold by December, when such cases are usually at their lowest, the Washington Post said.
“That’s an important cautionary tale for us,” said Kevin Messacar, MD, an infectious disease doctor at Children’s Hospital Colorado, Aurora. “Just because we get through the winter and don’t see much RSV or influenza doesn’t mean we’ll be out of the woods.”
A version of this article first appeared on WebMD.com.
Last fall, health experts said it was possible the United States could experience an easy 2020-21 flu season because health measures to fight COVID-19 would also thwart the spread of influenza.
It looks like that happened – and then some. Numbers are strikingly low for cases of the flu and other common respiratory and gastrointestinal viruses, health experts told the Washington Post.
“It’s crazy,” Lynnette Brammer, MPH, who leads the domestic influenza surveillance team at the Centers for Disease Control and Prevention, told the Washington Post. “This is my 30th flu season. I never would have expected to see flu activity this low.”
Influenza A, influenza B, parainfluenza, norovirus, respiratory syncytial virus, human metapneumovirus, and the bacteria that cause whooping cough and pneumonia are circulating at near-record-low levels.
As an example, the Washington Post said in the third week of December 2019, the CDC’s network of clinical labs reported 16.2% of almost 30,000 samples tested positive for influenza A. During the same period in 2020, only 0.3% tested positive.
But there’s a possible downside to this suppression of viruses, because flu and other viruses may rebound once the coronavirus is brought under control.
“The best analogy is to a forest fire,” Bryan Grenfell, PhD, an epidemiologist and population biologist at Princeton (N.J.) University, told the Washington Post. “For the fire to spread, it needs to have unburned wood. For epidemics to spread, they require people who haven’t previously been infected. So if people don’t get infected this year by these viruses, they likely will at some point later on.”
American health experts like Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Disease, said last fall that they noticed Australia and other nations in the southern hemisphere had easy flu seasons, apparently because of COVID protection measures. The flu season there runs March through August.
COVID-19 now has a very low presence in Australia, but in recent months the flu has been making a comeback. Flu cases among children aged 5 and younger rose sixfold by December, when such cases are usually at their lowest, the Washington Post said.
“That’s an important cautionary tale for us,” said Kevin Messacar, MD, an infectious disease doctor at Children’s Hospital Colorado, Aurora. “Just because we get through the winter and don’t see much RSV or influenza doesn’t mean we’ll be out of the woods.”
A version of this article first appeared on WebMD.com.
Last fall, health experts said it was possible the United States could experience an easy 2020-21 flu season because health measures to fight COVID-19 would also thwart the spread of influenza.
It looks like that happened – and then some. Numbers are strikingly low for cases of the flu and other common respiratory and gastrointestinal viruses, health experts told the Washington Post.
“It’s crazy,” Lynnette Brammer, MPH, who leads the domestic influenza surveillance team at the Centers for Disease Control and Prevention, told the Washington Post. “This is my 30th flu season. I never would have expected to see flu activity this low.”
Influenza A, influenza B, parainfluenza, norovirus, respiratory syncytial virus, human metapneumovirus, and the bacteria that cause whooping cough and pneumonia are circulating at near-record-low levels.
As an example, the Washington Post said in the third week of December 2019, the CDC’s network of clinical labs reported 16.2% of almost 30,000 samples tested positive for influenza A. During the same period in 2020, only 0.3% tested positive.
But there’s a possible downside to this suppression of viruses, because flu and other viruses may rebound once the coronavirus is brought under control.
“The best analogy is to a forest fire,” Bryan Grenfell, PhD, an epidemiologist and population biologist at Princeton (N.J.) University, told the Washington Post. “For the fire to spread, it needs to have unburned wood. For epidemics to spread, they require people who haven’t previously been infected. So if people don’t get infected this year by these viruses, they likely will at some point later on.”
American health experts like Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Disease, said last fall that they noticed Australia and other nations in the southern hemisphere had easy flu seasons, apparently because of COVID protection measures. The flu season there runs March through August.
COVID-19 now has a very low presence in Australia, but in recent months the flu has been making a comeback. Flu cases among children aged 5 and younger rose sixfold by December, when such cases are usually at their lowest, the Washington Post said.
“That’s an important cautionary tale for us,” said Kevin Messacar, MD, an infectious disease doctor at Children’s Hospital Colorado, Aurora. “Just because we get through the winter and don’t see much RSV or influenza doesn’t mean we’ll be out of the woods.”
A version of this article first appeared on WebMD.com.
Childhood smoking and depression contribute to young adult opioid use
Depression and tobacco use in childhood significantly increased the risk for opioid use in young adults, according to data from a prospective study of approximately 1,000 individuals.
Previous research, including the annual Monitoring the Future study, documents opioid use among adolescents in the United States, but childhood risk factors for opioid use in young adults have not been well studied, wrote Lilly Shanahan, PhD, of the University of Zürich, and colleagues.
In a prospective cohort study published in JAMA Pediatrics, the researchers identified 1,252 non-Hispanic White and American Indian opioid-naive individuals aged 9-16 years in rural North Carolina. They interviewed participants and parents up to 7 times between January 1993 and December 2000, and interviewed participants only at ages 19, 21, 25, and 30 years between January 1999 and December 2015.
Overall, 24.2% of study participants had used a nonheroin opioid by age 30 years, and both chronic depression and dysthymia were significantly associated with this use (odds ratios 5.43 and 7.13, respectively).
In addition, 155 participants (8.8%) reported weekly use of a nonheroin opioid, and 95 (6.6%) reported weekly heroin use by age 30 years. Chronic depression and dysthymia also were strongly associated with weekly nonheroin opioid use (OR 8.89 and 11.51, respectively).
In a multivariate analysis, depression, tobacco use, and cannabis use at ages 9-16 years were strongly associated with overall opioid use at ages 19-30 years.
“One possible reason childhood chronic depression increases the risk of later opioid use is self-medication, including the use of psychoactive substances, to alleviate depression,” the researchers noted. In addition, the mood-altering properties of opioids may increase their appeal to depressed youth as a way to relieve impaired reward system function, they said.
Potential mechanisms for the association between early tobacco use and later opioid use include the alterations to neurodevelopment caused by nicotine exposure in adolescence, as well as increased risk for depression, reduced pain thresholds, and use of nicotine as a gateway to harder drugs, the researchers added.
Several childhood risk factors were not associated with young adult opioid use in multivariate analysis in this study, including alcohol use, sociodemographic status, maltreatment, family dysfunction, and anxiety, the researchers wrote. “Previous studies typically measured these risk factors retrospectively or in late adolescence and young adulthood, and most did not consider depressive disorders, which may mediate associations between select childhood risk factors and later opioid use,” they said.
The study findings were limited by several factors, including the inability to distinguish between medical and nonmedical opioid use, the incomplete list of available opioids, and the exclusion of Black participants because of low sample size, the researchers noted. However, the results were strengthened by the longitudinal, community-representative design and the inclusion of up to 11 assessments of opioid use, they said.
“Our findings suggest strong opportunities for early prevention and intervention, including in primary care settings,” using known evidence-based strategies, they concluded.
More screening is needed
“Children in the United States are at high risk of serious adult health issues as a result of childhood factors such as ACEs (adverse childhood experiences),” said Suzanne C. Boulter, MD, of the Geisel School of Medicine at Dartmouth, Hanover, N.H. “This study looks prospectively at other factors in childhood over a long period of time leading to opioid usage, with its serious risks and health consequences including overdose death,” she said. “It is unclear what the effects of COVID-19 will be on the population of children growing up now and how opioid usage might change as a result,” she noted.
“Some of the links to adult usage are predictable, such as depression, tobacco use, and cannabis use in early adolescence,” said Dr. Boulter. “Surprising was the lack of correlation between anxiety, early alcohol use, child mistreatment, and sociodemographic factors with future opioid use,” she said.
The take-home message for clinicians is to screen children and adolescents for factors leading to opioid usage in young adults “with preventive strategies including avoidance of pain medication prescriptions and early referral and treatment for depression and use of cannabis and tobacco products using tools like SBIRT (Screening, Brief Intervention, and Referral to Treatment),” Dr. Boulter emphasized.
As for additional research, “It would be interesting to study e-cigarette usage and see if the correlation with future opioid usage is similar to older tobacco products,” she said. “Also helpful would be to delve deeper into connections between medical or dental diagnoses when opioids were first prescribed and later usage of those products,” Dr. Boulter noted.
The study was supported in part by the by the National Institute of Mental Health and the National Institute on Drug Abuse. The researchers had no financial conflicts to disclose. Dr. Boulter had no disclosures but serves on the Pediatric News Editorial Advisory Board.
Depression and tobacco use in childhood significantly increased the risk for opioid use in young adults, according to data from a prospective study of approximately 1,000 individuals.
Previous research, including the annual Monitoring the Future study, documents opioid use among adolescents in the United States, but childhood risk factors for opioid use in young adults have not been well studied, wrote Lilly Shanahan, PhD, of the University of Zürich, and colleagues.
In a prospective cohort study published in JAMA Pediatrics, the researchers identified 1,252 non-Hispanic White and American Indian opioid-naive individuals aged 9-16 years in rural North Carolina. They interviewed participants and parents up to 7 times between January 1993 and December 2000, and interviewed participants only at ages 19, 21, 25, and 30 years between January 1999 and December 2015.
Overall, 24.2% of study participants had used a nonheroin opioid by age 30 years, and both chronic depression and dysthymia were significantly associated with this use (odds ratios 5.43 and 7.13, respectively).
In addition, 155 participants (8.8%) reported weekly use of a nonheroin opioid, and 95 (6.6%) reported weekly heroin use by age 30 years. Chronic depression and dysthymia also were strongly associated with weekly nonheroin opioid use (OR 8.89 and 11.51, respectively).
In a multivariate analysis, depression, tobacco use, and cannabis use at ages 9-16 years were strongly associated with overall opioid use at ages 19-30 years.
“One possible reason childhood chronic depression increases the risk of later opioid use is self-medication, including the use of psychoactive substances, to alleviate depression,” the researchers noted. In addition, the mood-altering properties of opioids may increase their appeal to depressed youth as a way to relieve impaired reward system function, they said.
Potential mechanisms for the association between early tobacco use and later opioid use include the alterations to neurodevelopment caused by nicotine exposure in adolescence, as well as increased risk for depression, reduced pain thresholds, and use of nicotine as a gateway to harder drugs, the researchers added.
Several childhood risk factors were not associated with young adult opioid use in multivariate analysis in this study, including alcohol use, sociodemographic status, maltreatment, family dysfunction, and anxiety, the researchers wrote. “Previous studies typically measured these risk factors retrospectively or in late adolescence and young adulthood, and most did not consider depressive disorders, which may mediate associations between select childhood risk factors and later opioid use,” they said.
The study findings were limited by several factors, including the inability to distinguish between medical and nonmedical opioid use, the incomplete list of available opioids, and the exclusion of Black participants because of low sample size, the researchers noted. However, the results were strengthened by the longitudinal, community-representative design and the inclusion of up to 11 assessments of opioid use, they said.
“Our findings suggest strong opportunities for early prevention and intervention, including in primary care settings,” using known evidence-based strategies, they concluded.
More screening is needed
“Children in the United States are at high risk of serious adult health issues as a result of childhood factors such as ACEs (adverse childhood experiences),” said Suzanne C. Boulter, MD, of the Geisel School of Medicine at Dartmouth, Hanover, N.H. “This study looks prospectively at other factors in childhood over a long period of time leading to opioid usage, with its serious risks and health consequences including overdose death,” she said. “It is unclear what the effects of COVID-19 will be on the population of children growing up now and how opioid usage might change as a result,” she noted.
“Some of the links to adult usage are predictable, such as depression, tobacco use, and cannabis use in early adolescence,” said Dr. Boulter. “Surprising was the lack of correlation between anxiety, early alcohol use, child mistreatment, and sociodemographic factors with future opioid use,” she said.
The take-home message for clinicians is to screen children and adolescents for factors leading to opioid usage in young adults “with preventive strategies including avoidance of pain medication prescriptions and early referral and treatment for depression and use of cannabis and tobacco products using tools like SBIRT (Screening, Brief Intervention, and Referral to Treatment),” Dr. Boulter emphasized.
As for additional research, “It would be interesting to study e-cigarette usage and see if the correlation with future opioid usage is similar to older tobacco products,” she said. “Also helpful would be to delve deeper into connections between medical or dental diagnoses when opioids were first prescribed and later usage of those products,” Dr. Boulter noted.
The study was supported in part by the by the National Institute of Mental Health and the National Institute on Drug Abuse. The researchers had no financial conflicts to disclose. Dr. Boulter had no disclosures but serves on the Pediatric News Editorial Advisory Board.
Depression and tobacco use in childhood significantly increased the risk for opioid use in young adults, according to data from a prospective study of approximately 1,000 individuals.
Previous research, including the annual Monitoring the Future study, documents opioid use among adolescents in the United States, but childhood risk factors for opioid use in young adults have not been well studied, wrote Lilly Shanahan, PhD, of the University of Zürich, and colleagues.
In a prospective cohort study published in JAMA Pediatrics, the researchers identified 1,252 non-Hispanic White and American Indian opioid-naive individuals aged 9-16 years in rural North Carolina. They interviewed participants and parents up to 7 times between January 1993 and December 2000, and interviewed participants only at ages 19, 21, 25, and 30 years between January 1999 and December 2015.
Overall, 24.2% of study participants had used a nonheroin opioid by age 30 years, and both chronic depression and dysthymia were significantly associated with this use (odds ratios 5.43 and 7.13, respectively).
In addition, 155 participants (8.8%) reported weekly use of a nonheroin opioid, and 95 (6.6%) reported weekly heroin use by age 30 years. Chronic depression and dysthymia also were strongly associated with weekly nonheroin opioid use (OR 8.89 and 11.51, respectively).
In a multivariate analysis, depression, tobacco use, and cannabis use at ages 9-16 years were strongly associated with overall opioid use at ages 19-30 years.
“One possible reason childhood chronic depression increases the risk of later opioid use is self-medication, including the use of psychoactive substances, to alleviate depression,” the researchers noted. In addition, the mood-altering properties of opioids may increase their appeal to depressed youth as a way to relieve impaired reward system function, they said.
Potential mechanisms for the association between early tobacco use and later opioid use include the alterations to neurodevelopment caused by nicotine exposure in adolescence, as well as increased risk for depression, reduced pain thresholds, and use of nicotine as a gateway to harder drugs, the researchers added.
Several childhood risk factors were not associated with young adult opioid use in multivariate analysis in this study, including alcohol use, sociodemographic status, maltreatment, family dysfunction, and anxiety, the researchers wrote. “Previous studies typically measured these risk factors retrospectively or in late adolescence and young adulthood, and most did not consider depressive disorders, which may mediate associations between select childhood risk factors and later opioid use,” they said.
The study findings were limited by several factors, including the inability to distinguish between medical and nonmedical opioid use, the incomplete list of available opioids, and the exclusion of Black participants because of low sample size, the researchers noted. However, the results were strengthened by the longitudinal, community-representative design and the inclusion of up to 11 assessments of opioid use, they said.
“Our findings suggest strong opportunities for early prevention and intervention, including in primary care settings,” using known evidence-based strategies, they concluded.
More screening is needed
“Children in the United States are at high risk of serious adult health issues as a result of childhood factors such as ACEs (adverse childhood experiences),” said Suzanne C. Boulter, MD, of the Geisel School of Medicine at Dartmouth, Hanover, N.H. “This study looks prospectively at other factors in childhood over a long period of time leading to opioid usage, with its serious risks and health consequences including overdose death,” she said. “It is unclear what the effects of COVID-19 will be on the population of children growing up now and how opioid usage might change as a result,” she noted.
“Some of the links to adult usage are predictable, such as depression, tobacco use, and cannabis use in early adolescence,” said Dr. Boulter. “Surprising was the lack of correlation between anxiety, early alcohol use, child mistreatment, and sociodemographic factors with future opioid use,” she said.
The take-home message for clinicians is to screen children and adolescents for factors leading to opioid usage in young adults “with preventive strategies including avoidance of pain medication prescriptions and early referral and treatment for depression and use of cannabis and tobacco products using tools like SBIRT (Screening, Brief Intervention, and Referral to Treatment),” Dr. Boulter emphasized.
As for additional research, “It would be interesting to study e-cigarette usage and see if the correlation with future opioid usage is similar to older tobacco products,” she said. “Also helpful would be to delve deeper into connections between medical or dental diagnoses when opioids were first prescribed and later usage of those products,” Dr. Boulter noted.
The study was supported in part by the by the National Institute of Mental Health and the National Institute on Drug Abuse. The researchers had no financial conflicts to disclose. Dr. Boulter had no disclosures but serves on the Pediatric News Editorial Advisory Board.
FROM JAMA PEDIATRICS