User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
COVID-19: Peginterferon lambda may prevent clinical deterioration, shorten viral shedding
and shorten the duration of viral shedding, according to results of a double-blind, placebo-controlled trial (NCT04354259).
Reductions in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) RNA were greater with peginterferon lambda than with placebo from day 3 onward in the phase 2 study led by Jordan J. Feld, MD, of the Toronto Centre for Liver Disease. The findings were reported in The Lancet Respiratory Medicine.
Fewer side effects
To date in randomized clinical trials, efficacy in treatment of COVID-19 has been shown only for remdesivir and dexamethasone in hospitalized patients, and in an interim analysis of accelerated viral clearance for a monoclonal antibody infusion in outpatients.
Activity against respiratory pathogens has been demonstrated for interferon lambda-1, a type III interferon shown to be involved in innate antiviral responses. Interferons, Dr. Feld and coauthors stated, drive induction of genes with antiviral, antiproliferative and immunoregulatory properties, and early treatment with interferons might halt clinical progression and shorten the duration of viral shedding with reduced onward transmission. In addition, interferon lambdas (type III) use a distinct receptor complex with high expression levels limited to epithelial cells in the lung, liver, and intestine, leading to fewer side effects than other interferons, including avoiding risk of promoting cytokine storm syndrome.
The researchers investigated peginterferon lambda safety and efficacy in treatment of patients with laboratory-confirmed, mild to moderate COVID-19. Sixty patients (median age 46 years, about 60% female, about 50% White) were recruited from outpatient testing centers at six institutions in Toronto, and referred to a single ambulatory site. Patients were randomly assigned 1:1 to a single subcutaneous injection of peginterferon lambda 180 mcg or placebo within 7 days of symptom onset or, if asymptomatic, of their first positive swab. Mean time from symptom onset to injection was about 4.5 days, and about 18.5% were asymptomatic. The primary outcome was the proportion of patients negative for SARS-CoV-2 RNA on day 7 after the injection.
Greater benefit with higher baseline load
A higher baseline SARS-CoV-2 RNA concentration found in the peginterferon lambda group was found to be significantly associated with day 7 clearance (odds ratio [OR] 0.69 [95% confidence interval 0.51-0.87]; P = ·001). In the peginterferon lambda group, also, the mean decline in SARS-CoV-2 RNA was significantly larger than in the placebo group across all time points (days 3, 5, 7, and14). While viral load decline was 0.81 log greater in the treatment group (P = .14) by day 3, viral load decline increased to 1.67 log copies per mL by day 5 (P = .013) and 2.42 log copies per mL by day 7 (P = .0041). At day 14, the viral decline was 1.77 log copies per mL larger in the peginterferon lambda group (P = .048). The investigators pointed out that the difference in viral load decline between groups was greater in patients with high baseline viral load (at or above 106 copies per mL). In the peginterferon lambda high baseline viral load group, the reduction was 7.17 log copies per mL, versus 4.92 log copies per mL in the placebo group (P = .004).
More patients SARS-CoV-2 RNA negative
By day 7, 80% of patients in the peginterferon lambda group were negative for SARS-CoV-2 RNA, compared with 63% in the placebo group (P = .15). After baseline load adjustment, however, the peginterferon lambda treatment was significantly associated with day 7 clearance (OR 4·12 [95% CI 1·15-16·73]; P = .029).
Respiratory symptoms improved faster
Most symptoms in both groups were mild to moderate, without difference in frequency or severity. While symptom improvement was generally similar over time for both groups, respiratory symptoms improved faster with peginterferon lambda, with the effect more pronounced in the high baseline viral load group (OR 5·88 (0·81-42·46; P =. 079).
Laboratory adverse events, similar for both groups, were mild.
“Peginterferon lambda has potential to prevent clinical deterioration and shorten duration of viral shedding,” the investigators concluded.
“This clinical trial is important” because it suggests that a single intravenous dose of peginterferon lambda administered to outpatients with a positive SARS-CoV-2 nasopharyngeal swab speeds reduction of SARS-CoV-2 viral load, David L. Bowton, MD, FCCP, professor emeritus, Wake Forest Baptist Health, Winston-Salem, N.C., said in an interview. He observed that the smaller viral load difference observed at day 14 likely reflects host immune responses.
Dr. Bowton also noted that two placebo group baseline characteristics (five placebo group patients with anti-SARS-CoV-2 S protein IgG antibodies; two times more undetectable SARS-CoV-2 RNA at baseline assessment) would tend to reduce differences between the peginterferon lambda and placebo groups. He added that the study findings were concordant with another phase 2 trial of hospitalized COVID-19 patients receiving inhaled interferon beta-1a.
“Thus, interferons may find a place in the treatment of COVID-19 and perhaps other severe viral illnesses,” Dr. Bowton said.
The study was funded by the Toronto COVID-19 Action Initiative, University of Toronto, and the Ontario First COVID-19 Rapid Research Fund, Toronto General & Western Hospital Foundation.
Dr. Bowton had no disclosures. Disclosures for Dr. Feld and coauthors are listed on the Lancet Respiratory Medicine website.
and shorten the duration of viral shedding, according to results of a double-blind, placebo-controlled trial (NCT04354259).
Reductions in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) RNA were greater with peginterferon lambda than with placebo from day 3 onward in the phase 2 study led by Jordan J. Feld, MD, of the Toronto Centre for Liver Disease. The findings were reported in The Lancet Respiratory Medicine.
Fewer side effects
To date in randomized clinical trials, efficacy in treatment of COVID-19 has been shown only for remdesivir and dexamethasone in hospitalized patients, and in an interim analysis of accelerated viral clearance for a monoclonal antibody infusion in outpatients.
Activity against respiratory pathogens has been demonstrated for interferon lambda-1, a type III interferon shown to be involved in innate antiviral responses. Interferons, Dr. Feld and coauthors stated, drive induction of genes with antiviral, antiproliferative and immunoregulatory properties, and early treatment with interferons might halt clinical progression and shorten the duration of viral shedding with reduced onward transmission. In addition, interferon lambdas (type III) use a distinct receptor complex with high expression levels limited to epithelial cells in the lung, liver, and intestine, leading to fewer side effects than other interferons, including avoiding risk of promoting cytokine storm syndrome.
The researchers investigated peginterferon lambda safety and efficacy in treatment of patients with laboratory-confirmed, mild to moderate COVID-19. Sixty patients (median age 46 years, about 60% female, about 50% White) were recruited from outpatient testing centers at six institutions in Toronto, and referred to a single ambulatory site. Patients were randomly assigned 1:1 to a single subcutaneous injection of peginterferon lambda 180 mcg or placebo within 7 days of symptom onset or, if asymptomatic, of their first positive swab. Mean time from symptom onset to injection was about 4.5 days, and about 18.5% were asymptomatic. The primary outcome was the proportion of patients negative for SARS-CoV-2 RNA on day 7 after the injection.
Greater benefit with higher baseline load
A higher baseline SARS-CoV-2 RNA concentration found in the peginterferon lambda group was found to be significantly associated with day 7 clearance (odds ratio [OR] 0.69 [95% confidence interval 0.51-0.87]; P = ·001). In the peginterferon lambda group, also, the mean decline in SARS-CoV-2 RNA was significantly larger than in the placebo group across all time points (days 3, 5, 7, and14). While viral load decline was 0.81 log greater in the treatment group (P = .14) by day 3, viral load decline increased to 1.67 log copies per mL by day 5 (P = .013) and 2.42 log copies per mL by day 7 (P = .0041). At day 14, the viral decline was 1.77 log copies per mL larger in the peginterferon lambda group (P = .048). The investigators pointed out that the difference in viral load decline between groups was greater in patients with high baseline viral load (at or above 106 copies per mL). In the peginterferon lambda high baseline viral load group, the reduction was 7.17 log copies per mL, versus 4.92 log copies per mL in the placebo group (P = .004).
More patients SARS-CoV-2 RNA negative
By day 7, 80% of patients in the peginterferon lambda group were negative for SARS-CoV-2 RNA, compared with 63% in the placebo group (P = .15). After baseline load adjustment, however, the peginterferon lambda treatment was significantly associated with day 7 clearance (OR 4·12 [95% CI 1·15-16·73]; P = .029).
Respiratory symptoms improved faster
Most symptoms in both groups were mild to moderate, without difference in frequency or severity. While symptom improvement was generally similar over time for both groups, respiratory symptoms improved faster with peginterferon lambda, with the effect more pronounced in the high baseline viral load group (OR 5·88 (0·81-42·46; P =. 079).
Laboratory adverse events, similar for both groups, were mild.
“Peginterferon lambda has potential to prevent clinical deterioration and shorten duration of viral shedding,” the investigators concluded.
“This clinical trial is important” because it suggests that a single intravenous dose of peginterferon lambda administered to outpatients with a positive SARS-CoV-2 nasopharyngeal swab speeds reduction of SARS-CoV-2 viral load, David L. Bowton, MD, FCCP, professor emeritus, Wake Forest Baptist Health, Winston-Salem, N.C., said in an interview. He observed that the smaller viral load difference observed at day 14 likely reflects host immune responses.
Dr. Bowton also noted that two placebo group baseline characteristics (five placebo group patients with anti-SARS-CoV-2 S protein IgG antibodies; two times more undetectable SARS-CoV-2 RNA at baseline assessment) would tend to reduce differences between the peginterferon lambda and placebo groups. He added that the study findings were concordant with another phase 2 trial of hospitalized COVID-19 patients receiving inhaled interferon beta-1a.
“Thus, interferons may find a place in the treatment of COVID-19 and perhaps other severe viral illnesses,” Dr. Bowton said.
The study was funded by the Toronto COVID-19 Action Initiative, University of Toronto, and the Ontario First COVID-19 Rapid Research Fund, Toronto General & Western Hospital Foundation.
Dr. Bowton had no disclosures. Disclosures for Dr. Feld and coauthors are listed on the Lancet Respiratory Medicine website.
and shorten the duration of viral shedding, according to results of a double-blind, placebo-controlled trial (NCT04354259).
Reductions in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) RNA were greater with peginterferon lambda than with placebo from day 3 onward in the phase 2 study led by Jordan J. Feld, MD, of the Toronto Centre for Liver Disease. The findings were reported in The Lancet Respiratory Medicine.
Fewer side effects
To date in randomized clinical trials, efficacy in treatment of COVID-19 has been shown only for remdesivir and dexamethasone in hospitalized patients, and in an interim analysis of accelerated viral clearance for a monoclonal antibody infusion in outpatients.
Activity against respiratory pathogens has been demonstrated for interferon lambda-1, a type III interferon shown to be involved in innate antiviral responses. Interferons, Dr. Feld and coauthors stated, drive induction of genes with antiviral, antiproliferative and immunoregulatory properties, and early treatment with interferons might halt clinical progression and shorten the duration of viral shedding with reduced onward transmission. In addition, interferon lambdas (type III) use a distinct receptor complex with high expression levels limited to epithelial cells in the lung, liver, and intestine, leading to fewer side effects than other interferons, including avoiding risk of promoting cytokine storm syndrome.
The researchers investigated peginterferon lambda safety and efficacy in treatment of patients with laboratory-confirmed, mild to moderate COVID-19. Sixty patients (median age 46 years, about 60% female, about 50% White) were recruited from outpatient testing centers at six institutions in Toronto, and referred to a single ambulatory site. Patients were randomly assigned 1:1 to a single subcutaneous injection of peginterferon lambda 180 mcg or placebo within 7 days of symptom onset or, if asymptomatic, of their first positive swab. Mean time from symptom onset to injection was about 4.5 days, and about 18.5% were asymptomatic. The primary outcome was the proportion of patients negative for SARS-CoV-2 RNA on day 7 after the injection.
Greater benefit with higher baseline load
A higher baseline SARS-CoV-2 RNA concentration found in the peginterferon lambda group was found to be significantly associated with day 7 clearance (odds ratio [OR] 0.69 [95% confidence interval 0.51-0.87]; P = ·001). In the peginterferon lambda group, also, the mean decline in SARS-CoV-2 RNA was significantly larger than in the placebo group across all time points (days 3, 5, 7, and14). While viral load decline was 0.81 log greater in the treatment group (P = .14) by day 3, viral load decline increased to 1.67 log copies per mL by day 5 (P = .013) and 2.42 log copies per mL by day 7 (P = .0041). At day 14, the viral decline was 1.77 log copies per mL larger in the peginterferon lambda group (P = .048). The investigators pointed out that the difference in viral load decline between groups was greater in patients with high baseline viral load (at or above 106 copies per mL). In the peginterferon lambda high baseline viral load group, the reduction was 7.17 log copies per mL, versus 4.92 log copies per mL in the placebo group (P = .004).
More patients SARS-CoV-2 RNA negative
By day 7, 80% of patients in the peginterferon lambda group were negative for SARS-CoV-2 RNA, compared with 63% in the placebo group (P = .15). After baseline load adjustment, however, the peginterferon lambda treatment was significantly associated with day 7 clearance (OR 4·12 [95% CI 1·15-16·73]; P = .029).
Respiratory symptoms improved faster
Most symptoms in both groups were mild to moderate, without difference in frequency or severity. While symptom improvement was generally similar over time for both groups, respiratory symptoms improved faster with peginterferon lambda, with the effect more pronounced in the high baseline viral load group (OR 5·88 (0·81-42·46; P =. 079).
Laboratory adverse events, similar for both groups, were mild.
“Peginterferon lambda has potential to prevent clinical deterioration and shorten duration of viral shedding,” the investigators concluded.
“This clinical trial is important” because it suggests that a single intravenous dose of peginterferon lambda administered to outpatients with a positive SARS-CoV-2 nasopharyngeal swab speeds reduction of SARS-CoV-2 viral load, David L. Bowton, MD, FCCP, professor emeritus, Wake Forest Baptist Health, Winston-Salem, N.C., said in an interview. He observed that the smaller viral load difference observed at day 14 likely reflects host immune responses.
Dr. Bowton also noted that two placebo group baseline characteristics (five placebo group patients with anti-SARS-CoV-2 S protein IgG antibodies; two times more undetectable SARS-CoV-2 RNA at baseline assessment) would tend to reduce differences between the peginterferon lambda and placebo groups. He added that the study findings were concordant with another phase 2 trial of hospitalized COVID-19 patients receiving inhaled interferon beta-1a.
“Thus, interferons may find a place in the treatment of COVID-19 and perhaps other severe viral illnesses,” Dr. Bowton said.
The study was funded by the Toronto COVID-19 Action Initiative, University of Toronto, and the Ontario First COVID-19 Rapid Research Fund, Toronto General & Western Hospital Foundation.
Dr. Bowton had no disclosures. Disclosures for Dr. Feld and coauthors are listed on the Lancet Respiratory Medicine website.
FROM THE LANCET RESPIRATORY MEDICINE
Teenagers get in the queue for COVID-19 vaccines
The vaccinations can’t come soon enough for parents like Stacy Hillenburg, a developmental therapist in Aurora, Ill., whose 9-year-old son takes immunosuppressants because he had a heart transplant when he was 7 weeks old. Although school-age children aren’t yet included in clinical trials, if her 12- and 13-year-old daughters could get vaccinated, along with both parents, then the family could relax some of the protocols they currently follow to prevent infection.
Whenever they are around other people, even masked and socially distanced, they come home and immediately shower and change their clothes. So far, no one in the family has been infected with COVID, but the anxiety is ever-present. “I can’t wait for it to come out,” Ms. Hillenburg said of a pediatric COVID vaccine. “It will ease my mind so much.”
She isn’t alone in that anticipation. In the fall, the American Academy of Pediatrics and other pediatric vaccine experts urged faster action on pediatric vaccine trials and worried that children would be left behind as adults gained protection from COVID. But recent developments have eased those concerns.
“Over the next couple of months, we will be doing trials in an age-deescalation manner,” with studies moving gradually to younger children, Anthony S. Fauci, MD, chief medical adviser on COVID-19 to the president, said in a coronavirus response team briefing on Jan. 29. “So that hopefully, as we get to the late spring and summer, we will have children being able to be vaccinated.”
Pfizer completed enrollment of 2,259 teens aged 12-15 years in late January and expects to move forward with a separate pediatric trial of children aged 5-11 years by this spring, Keanna Ghazvini, senior associate for global media relations at Pfizer, said in an interview.
Enrollment in Moderna’s TeenCove study of adolescents ages 12-17 years began slowly in late December, but the pace has since picked up, said company spokesperson Colleen Hussey. “We continue to bring clinical trial sites online, and we are on track to provide updated data around mid-year 2021.” A trial extension in children 11 years and younger is expected to begin later in 2021.
Johnson & Johnson and AstraZeneca said they expect to begin adolescent trials in early 2021, according to data shared by the Advisory Committee on Immunization Practices. An interim analysis of J&J’s Janssen COVID-19 vaccine trial data, released on Jan. 29, showed it was 72% effective in US participants aged 18 years or older. AstraZeneca’s U.S. trial in adults is ongoing.
Easing the burden
Vaccination could lessen children’s risk of severe disease as well as the social and emotional burdens of the pandemic, says James Campbell, MD, a pediatric infectious disease specialist at the University of Maryland’s Center for Vaccine Development in Baltimore, which was involved in the Moderna and early-phase Pfizer trials. He coauthored a September 2020 article in Clinical Infectious Diseases titled: “Warp Speed for COVID-19 vaccines: Why are children stuck in neutral?”
The adolescent trials are a vital step to ensure timely vaccine access for teens and younger children. “It is reasonable, when you have limited vaccine, that your rollout goes to the highest priority and then moves to lower and lower priorities. In adults, we’re just saying: ‘Wait your turn,’ ” he said of the current vaccination effort. “If we didn’t have the [vaccine trial] data in children, we’d be saying: ‘You don’t have a turn.’ ”
As the pandemic has worn on, the burden on children has grown. As of Tuesday, 269 children had died of COVID-19. That is well above the highest annual death toll recorded during a regular flu season – 188 flu deaths among children and adolescents under 18 in the 2019-2020 and 2017-2018 flu seasons.
Children are less likely to transmit COVID-19 in their household than adults, according to a meta-analysis of 54 studies published in JAMA Network Open. But that does not necessarily mean children are less infectious, the authors said, noting that unmeasured factors could have affected the spread of infection among adults.
Moreover, children and adolescents need protection from COVID infection – and from the potential for severe disease or lingering effects – and, given that there are 74 million children and teens in the United States, their vaccination is an important part of stopping the pandemic, said Grace Lee, MD, professor of pediatrics at Stanford (Calif.) University, and cochair of ACIP’s COVID-19 Vaccine Safety Technical Subgroup.
“In order to interrupt transmission, I don’t see how we’re going to do that without vaccinating children and adolescents,” she said.
Dr. Lee said her 16-year-old daughter misses the normal teenage social life and is excited about getting the vaccine when she is eligible. (Adolescents without high-risk conditions are in the lowest vaccination tier, according to ACIP recommendations.) “There is truly individual protection to be gained,” Dr. Lee said.
She noted that researchers continue to assess the immune responses to the adult vaccines – even looking at immune characteristics of the small percentage of people who aren’t protected from infection – and that information helps in the evaluation of the pediatric immune responses. As the trials expand to younger children and infants, dosing will be a major focus. “How many doses do they need they need to receive the same immunity? Safety considerations will be critically important,” she said.
Teen trials underway
Pfizer/BioNTech extended its adult trial to 16- and 17-year-olds in October, which enabled older teens to be included in its emergency-use authorization. They and younger teens, ages 12-15, receive the same dose as adults.
The ongoing trials with Pfizer and Moderna vaccines are immunobridging trials, designed to study safety and immunogenicity. Investigators will compare the teens’ immune response with the findings from the larger adult trials. When the trials expand to school-age children (6-12 years), protocols call for testing the safety and immunogenicity of a half-dose vaccine as well as the full dose.
Children ages 2-5 years and infants and toddlers will be enrolled in future trials, studying safety and immunogenicity of full, half, or even quarter dosages. The Pediatric Research Equity Act of 2003 requires licensed vaccines to be tested for safety and efficacy in children, unless they are not appropriate for a pediatric population.
Demand for the teen trials has been strong. At Cincinnati Children’s Hospital Medical Center, 259 teenagers joined the Pfizer/BioNTech trial, but some teenagers were turned away when the trial’s national enrollment closed in late January.
“Many of the children are having no side effects, and if they are, they’re having the same [effects] as the young adults – local redness or pain, fatigue, and headaches,” said Robert Frenck, MD, director of the Cincinnati Children’s Gamble Program for Clinical Studies.
Parents may share some of the vaccine hesitancy that has affected adult vaccination. But that is balanced by the hope that vaccines will end the pandemic and usher in a new normal. “If it looks like [vaccines] will increase the likelihood of children returning to school safely, that may be a motivating factor,” Dr. Frenck said.
Cody Meissner, MD, chief of the pediatric infectious disease service at Tufts Medical Center, Boston, was initially cautious about the extension of vaccination to adolescents. A member of the Vaccine and Related Biological Products Advisory Committee, which evaluates data and makes recommendations to the Food and Drug Administration, Dr. Meissner initially abstained in the vote on the Pfizer/BioNTech emergency-use authorization for people 16 and older.
He noted that, at the time the committee reviewed the Pfizer vaccine, the company had data available for just 134 teenagers, half of whom received a placebo. But the vaccination of 34 million adults has provided robust data about the vaccine’s safety, and the trial expansion into adolescents is important.
“I’m comfortable with the way these trials are going now,” he said. “This is the way I was hoping they would go.”
Ms. Hillenburg is on the parent advisory board of Voices for Vaccines, an organization of parents supporting vaccination that is affiliated with the Task Force for Global Health, an Atlanta-based independent public health organization. Dr. Campbell’s institution has received funds to conduct clinical trials from the National Institutes of Health and several companies, including Merck, GlaxoSmithKline, Sanofi, Pfizer, and Moderna. He has served pro bono on many safety and data monitoring committees. Dr. Frenck’s institution is receiving funds to conduct the Pfizer trial. In the past 5 years, he has also participated in clinical trials for GlaxoSmithKline, Merck, and Meissa vaccines. Dr. Lee and Dr. Meissner disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The vaccinations can’t come soon enough for parents like Stacy Hillenburg, a developmental therapist in Aurora, Ill., whose 9-year-old son takes immunosuppressants because he had a heart transplant when he was 7 weeks old. Although school-age children aren’t yet included in clinical trials, if her 12- and 13-year-old daughters could get vaccinated, along with both parents, then the family could relax some of the protocols they currently follow to prevent infection.
Whenever they are around other people, even masked and socially distanced, they come home and immediately shower and change their clothes. So far, no one in the family has been infected with COVID, but the anxiety is ever-present. “I can’t wait for it to come out,” Ms. Hillenburg said of a pediatric COVID vaccine. “It will ease my mind so much.”
She isn’t alone in that anticipation. In the fall, the American Academy of Pediatrics and other pediatric vaccine experts urged faster action on pediatric vaccine trials and worried that children would be left behind as adults gained protection from COVID. But recent developments have eased those concerns.
“Over the next couple of months, we will be doing trials in an age-deescalation manner,” with studies moving gradually to younger children, Anthony S. Fauci, MD, chief medical adviser on COVID-19 to the president, said in a coronavirus response team briefing on Jan. 29. “So that hopefully, as we get to the late spring and summer, we will have children being able to be vaccinated.”
Pfizer completed enrollment of 2,259 teens aged 12-15 years in late January and expects to move forward with a separate pediatric trial of children aged 5-11 years by this spring, Keanna Ghazvini, senior associate for global media relations at Pfizer, said in an interview.
Enrollment in Moderna’s TeenCove study of adolescents ages 12-17 years began slowly in late December, but the pace has since picked up, said company spokesperson Colleen Hussey. “We continue to bring clinical trial sites online, and we are on track to provide updated data around mid-year 2021.” A trial extension in children 11 years and younger is expected to begin later in 2021.
Johnson & Johnson and AstraZeneca said they expect to begin adolescent trials in early 2021, according to data shared by the Advisory Committee on Immunization Practices. An interim analysis of J&J’s Janssen COVID-19 vaccine trial data, released on Jan. 29, showed it was 72% effective in US participants aged 18 years or older. AstraZeneca’s U.S. trial in adults is ongoing.
Easing the burden
Vaccination could lessen children’s risk of severe disease as well as the social and emotional burdens of the pandemic, says James Campbell, MD, a pediatric infectious disease specialist at the University of Maryland’s Center for Vaccine Development in Baltimore, which was involved in the Moderna and early-phase Pfizer trials. He coauthored a September 2020 article in Clinical Infectious Diseases titled: “Warp Speed for COVID-19 vaccines: Why are children stuck in neutral?”
The adolescent trials are a vital step to ensure timely vaccine access for teens and younger children. “It is reasonable, when you have limited vaccine, that your rollout goes to the highest priority and then moves to lower and lower priorities. In adults, we’re just saying: ‘Wait your turn,’ ” he said of the current vaccination effort. “If we didn’t have the [vaccine trial] data in children, we’d be saying: ‘You don’t have a turn.’ ”
As the pandemic has worn on, the burden on children has grown. As of Tuesday, 269 children had died of COVID-19. That is well above the highest annual death toll recorded during a regular flu season – 188 flu deaths among children and adolescents under 18 in the 2019-2020 and 2017-2018 flu seasons.
Children are less likely to transmit COVID-19 in their household than adults, according to a meta-analysis of 54 studies published in JAMA Network Open. But that does not necessarily mean children are less infectious, the authors said, noting that unmeasured factors could have affected the spread of infection among adults.
Moreover, children and adolescents need protection from COVID infection – and from the potential for severe disease or lingering effects – and, given that there are 74 million children and teens in the United States, their vaccination is an important part of stopping the pandemic, said Grace Lee, MD, professor of pediatrics at Stanford (Calif.) University, and cochair of ACIP’s COVID-19 Vaccine Safety Technical Subgroup.
“In order to interrupt transmission, I don’t see how we’re going to do that without vaccinating children and adolescents,” she said.
Dr. Lee said her 16-year-old daughter misses the normal teenage social life and is excited about getting the vaccine when she is eligible. (Adolescents without high-risk conditions are in the lowest vaccination tier, according to ACIP recommendations.) “There is truly individual protection to be gained,” Dr. Lee said.
She noted that researchers continue to assess the immune responses to the adult vaccines – even looking at immune characteristics of the small percentage of people who aren’t protected from infection – and that information helps in the evaluation of the pediatric immune responses. As the trials expand to younger children and infants, dosing will be a major focus. “How many doses do they need they need to receive the same immunity? Safety considerations will be critically important,” she said.
Teen trials underway
Pfizer/BioNTech extended its adult trial to 16- and 17-year-olds in October, which enabled older teens to be included in its emergency-use authorization. They and younger teens, ages 12-15, receive the same dose as adults.
The ongoing trials with Pfizer and Moderna vaccines are immunobridging trials, designed to study safety and immunogenicity. Investigators will compare the teens’ immune response with the findings from the larger adult trials. When the trials expand to school-age children (6-12 years), protocols call for testing the safety and immunogenicity of a half-dose vaccine as well as the full dose.
Children ages 2-5 years and infants and toddlers will be enrolled in future trials, studying safety and immunogenicity of full, half, or even quarter dosages. The Pediatric Research Equity Act of 2003 requires licensed vaccines to be tested for safety and efficacy in children, unless they are not appropriate for a pediatric population.
Demand for the teen trials has been strong. At Cincinnati Children’s Hospital Medical Center, 259 teenagers joined the Pfizer/BioNTech trial, but some teenagers were turned away when the trial’s national enrollment closed in late January.
“Many of the children are having no side effects, and if they are, they’re having the same [effects] as the young adults – local redness or pain, fatigue, and headaches,” said Robert Frenck, MD, director of the Cincinnati Children’s Gamble Program for Clinical Studies.
Parents may share some of the vaccine hesitancy that has affected adult vaccination. But that is balanced by the hope that vaccines will end the pandemic and usher in a new normal. “If it looks like [vaccines] will increase the likelihood of children returning to school safely, that may be a motivating factor,” Dr. Frenck said.
Cody Meissner, MD, chief of the pediatric infectious disease service at Tufts Medical Center, Boston, was initially cautious about the extension of vaccination to adolescents. A member of the Vaccine and Related Biological Products Advisory Committee, which evaluates data and makes recommendations to the Food and Drug Administration, Dr. Meissner initially abstained in the vote on the Pfizer/BioNTech emergency-use authorization for people 16 and older.
He noted that, at the time the committee reviewed the Pfizer vaccine, the company had data available for just 134 teenagers, half of whom received a placebo. But the vaccination of 34 million adults has provided robust data about the vaccine’s safety, and the trial expansion into adolescents is important.
“I’m comfortable with the way these trials are going now,” he said. “This is the way I was hoping they would go.”
Ms. Hillenburg is on the parent advisory board of Voices for Vaccines, an organization of parents supporting vaccination that is affiliated with the Task Force for Global Health, an Atlanta-based independent public health organization. Dr. Campbell’s institution has received funds to conduct clinical trials from the National Institutes of Health and several companies, including Merck, GlaxoSmithKline, Sanofi, Pfizer, and Moderna. He has served pro bono on many safety and data monitoring committees. Dr. Frenck’s institution is receiving funds to conduct the Pfizer trial. In the past 5 years, he has also participated in clinical trials for GlaxoSmithKline, Merck, and Meissa vaccines. Dr. Lee and Dr. Meissner disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The vaccinations can’t come soon enough for parents like Stacy Hillenburg, a developmental therapist in Aurora, Ill., whose 9-year-old son takes immunosuppressants because he had a heart transplant when he was 7 weeks old. Although school-age children aren’t yet included in clinical trials, if her 12- and 13-year-old daughters could get vaccinated, along with both parents, then the family could relax some of the protocols they currently follow to prevent infection.
Whenever they are around other people, even masked and socially distanced, they come home and immediately shower and change their clothes. So far, no one in the family has been infected with COVID, but the anxiety is ever-present. “I can’t wait for it to come out,” Ms. Hillenburg said of a pediatric COVID vaccine. “It will ease my mind so much.”
She isn’t alone in that anticipation. In the fall, the American Academy of Pediatrics and other pediatric vaccine experts urged faster action on pediatric vaccine trials and worried that children would be left behind as adults gained protection from COVID. But recent developments have eased those concerns.
“Over the next couple of months, we will be doing trials in an age-deescalation manner,” with studies moving gradually to younger children, Anthony S. Fauci, MD, chief medical adviser on COVID-19 to the president, said in a coronavirus response team briefing on Jan. 29. “So that hopefully, as we get to the late spring and summer, we will have children being able to be vaccinated.”
Pfizer completed enrollment of 2,259 teens aged 12-15 years in late January and expects to move forward with a separate pediatric trial of children aged 5-11 years by this spring, Keanna Ghazvini, senior associate for global media relations at Pfizer, said in an interview.
Enrollment in Moderna’s TeenCove study of adolescents ages 12-17 years began slowly in late December, but the pace has since picked up, said company spokesperson Colleen Hussey. “We continue to bring clinical trial sites online, and we are on track to provide updated data around mid-year 2021.” A trial extension in children 11 years and younger is expected to begin later in 2021.
Johnson & Johnson and AstraZeneca said they expect to begin adolescent trials in early 2021, according to data shared by the Advisory Committee on Immunization Practices. An interim analysis of J&J’s Janssen COVID-19 vaccine trial data, released on Jan. 29, showed it was 72% effective in US participants aged 18 years or older. AstraZeneca’s U.S. trial in adults is ongoing.
Easing the burden
Vaccination could lessen children’s risk of severe disease as well as the social and emotional burdens of the pandemic, says James Campbell, MD, a pediatric infectious disease specialist at the University of Maryland’s Center for Vaccine Development in Baltimore, which was involved in the Moderna and early-phase Pfizer trials. He coauthored a September 2020 article in Clinical Infectious Diseases titled: “Warp Speed for COVID-19 vaccines: Why are children stuck in neutral?”
The adolescent trials are a vital step to ensure timely vaccine access for teens and younger children. “It is reasonable, when you have limited vaccine, that your rollout goes to the highest priority and then moves to lower and lower priorities. In adults, we’re just saying: ‘Wait your turn,’ ” he said of the current vaccination effort. “If we didn’t have the [vaccine trial] data in children, we’d be saying: ‘You don’t have a turn.’ ”
As the pandemic has worn on, the burden on children has grown. As of Tuesday, 269 children had died of COVID-19. That is well above the highest annual death toll recorded during a regular flu season – 188 flu deaths among children and adolescents under 18 in the 2019-2020 and 2017-2018 flu seasons.
Children are less likely to transmit COVID-19 in their household than adults, according to a meta-analysis of 54 studies published in JAMA Network Open. But that does not necessarily mean children are less infectious, the authors said, noting that unmeasured factors could have affected the spread of infection among adults.
Moreover, children and adolescents need protection from COVID infection – and from the potential for severe disease or lingering effects – and, given that there are 74 million children and teens in the United States, their vaccination is an important part of stopping the pandemic, said Grace Lee, MD, professor of pediatrics at Stanford (Calif.) University, and cochair of ACIP’s COVID-19 Vaccine Safety Technical Subgroup.
“In order to interrupt transmission, I don’t see how we’re going to do that without vaccinating children and adolescents,” she said.
Dr. Lee said her 16-year-old daughter misses the normal teenage social life and is excited about getting the vaccine when she is eligible. (Adolescents without high-risk conditions are in the lowest vaccination tier, according to ACIP recommendations.) “There is truly individual protection to be gained,” Dr. Lee said.
She noted that researchers continue to assess the immune responses to the adult vaccines – even looking at immune characteristics of the small percentage of people who aren’t protected from infection – and that information helps in the evaluation of the pediatric immune responses. As the trials expand to younger children and infants, dosing will be a major focus. “How many doses do they need they need to receive the same immunity? Safety considerations will be critically important,” she said.
Teen trials underway
Pfizer/BioNTech extended its adult trial to 16- and 17-year-olds in October, which enabled older teens to be included in its emergency-use authorization. They and younger teens, ages 12-15, receive the same dose as adults.
The ongoing trials with Pfizer and Moderna vaccines are immunobridging trials, designed to study safety and immunogenicity. Investigators will compare the teens’ immune response with the findings from the larger adult trials. When the trials expand to school-age children (6-12 years), protocols call for testing the safety and immunogenicity of a half-dose vaccine as well as the full dose.
Children ages 2-5 years and infants and toddlers will be enrolled in future trials, studying safety and immunogenicity of full, half, or even quarter dosages. The Pediatric Research Equity Act of 2003 requires licensed vaccines to be tested for safety and efficacy in children, unless they are not appropriate for a pediatric population.
Demand for the teen trials has been strong. At Cincinnati Children’s Hospital Medical Center, 259 teenagers joined the Pfizer/BioNTech trial, but some teenagers were turned away when the trial’s national enrollment closed in late January.
“Many of the children are having no side effects, and if they are, they’re having the same [effects] as the young adults – local redness or pain, fatigue, and headaches,” said Robert Frenck, MD, director of the Cincinnati Children’s Gamble Program for Clinical Studies.
Parents may share some of the vaccine hesitancy that has affected adult vaccination. But that is balanced by the hope that vaccines will end the pandemic and usher in a new normal. “If it looks like [vaccines] will increase the likelihood of children returning to school safely, that may be a motivating factor,” Dr. Frenck said.
Cody Meissner, MD, chief of the pediatric infectious disease service at Tufts Medical Center, Boston, was initially cautious about the extension of vaccination to adolescents. A member of the Vaccine and Related Biological Products Advisory Committee, which evaluates data and makes recommendations to the Food and Drug Administration, Dr. Meissner initially abstained in the vote on the Pfizer/BioNTech emergency-use authorization for people 16 and older.
He noted that, at the time the committee reviewed the Pfizer vaccine, the company had data available for just 134 teenagers, half of whom received a placebo. But the vaccination of 34 million adults has provided robust data about the vaccine’s safety, and the trial expansion into adolescents is important.
“I’m comfortable with the way these trials are going now,” he said. “This is the way I was hoping they would go.”
Ms. Hillenburg is on the parent advisory board of Voices for Vaccines, an organization of parents supporting vaccination that is affiliated with the Task Force for Global Health, an Atlanta-based independent public health organization. Dr. Campbell’s institution has received funds to conduct clinical trials from the National Institutes of Health and several companies, including Merck, GlaxoSmithKline, Sanofi, Pfizer, and Moderna. He has served pro bono on many safety and data monitoring committees. Dr. Frenck’s institution is receiving funds to conduct the Pfizer trial. In the past 5 years, he has also participated in clinical trials for GlaxoSmithKline, Merck, and Meissa vaccines. Dr. Lee and Dr. Meissner disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
COVID-19 in children: New cases down for third straight week
New COVID-19 cases in children dropped for the third consecutive week, even as children continue to make up a larger share of all cases, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
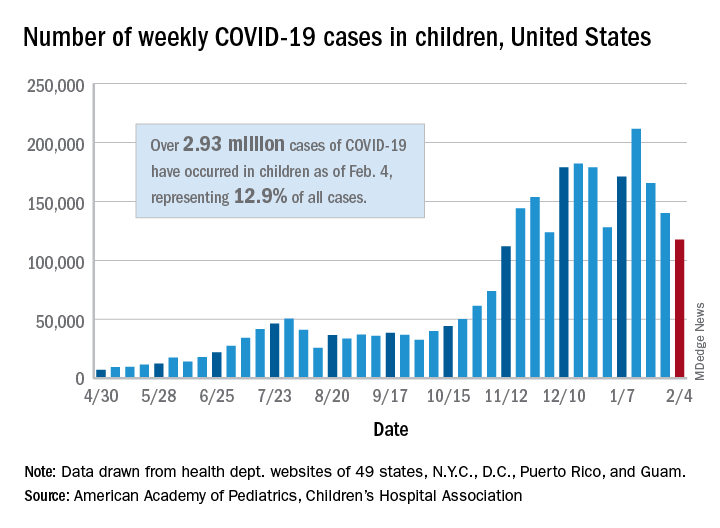
New child cases totaled almost 118,000 for the week of Jan. 29-Feb. 4, continuing the decline that began right after the United States topped 200,000 cases for the only time Jan. 8-14, the AAP and the CHA said in their weekly COVID-19 report.
For the latest week, however, children represented 16.0% of all new COVID-19 cases, continuing a 5-week increase that began in early December 2020, after the proportion had dropped to 12.6%, based on data collected from the health departments of 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam. During the week of Sept. 11-17, children made up 16.9% of all cases, the highest level seen during the pandemic.
The 2.93 million cases that have been reported in children make up 12.9% of all cases since the pandemic began, and the overall rate of pediatric coronavirus infection is 3,899 cases per 100,000 children in the population. Taking a step down from the national level, 30 states are above that rate and 18 are below it, along with D.C., New York City, Puerto Rico, and Guam (New York and Texas are excluded), the AAP and CHA reported.
There were 12 new COVID-19–related child deaths in the 43 states, along with New York City and Guam, that are reporting such data, bringing the total to 227. Nationally, 0.06% of all deaths have occurred in children, with rates ranging from 0.00% (11 states) to 0.26% (Nebraska) in the 45 jurisdictions, the AAP/CHA report shows.
Child hospitalizations rose to 1.9% of all hospitalizations after holding at 1.8% since mid-November in 25 reporting jurisdictions (24 states and New York City), but the hospitalization rate among children with COVID held at 0.8%, where it has been for the last 4 weeks. Hospitalization rates as high as 3.8% were recorded early in the pandemic, the AAP and CHA noted.
New COVID-19 cases in children dropped for the third consecutive week, even as children continue to make up a larger share of all cases, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

New child cases totaled almost 118,000 for the week of Jan. 29-Feb. 4, continuing the decline that began right after the United States topped 200,000 cases for the only time Jan. 8-14, the AAP and the CHA said in their weekly COVID-19 report.
For the latest week, however, children represented 16.0% of all new COVID-19 cases, continuing a 5-week increase that began in early December 2020, after the proportion had dropped to 12.6%, based on data collected from the health departments of 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam. During the week of Sept. 11-17, children made up 16.9% of all cases, the highest level seen during the pandemic.
The 2.93 million cases that have been reported in children make up 12.9% of all cases since the pandemic began, and the overall rate of pediatric coronavirus infection is 3,899 cases per 100,000 children in the population. Taking a step down from the national level, 30 states are above that rate and 18 are below it, along with D.C., New York City, Puerto Rico, and Guam (New York and Texas are excluded), the AAP and CHA reported.
There were 12 new COVID-19–related child deaths in the 43 states, along with New York City and Guam, that are reporting such data, bringing the total to 227. Nationally, 0.06% of all deaths have occurred in children, with rates ranging from 0.00% (11 states) to 0.26% (Nebraska) in the 45 jurisdictions, the AAP/CHA report shows.
Child hospitalizations rose to 1.9% of all hospitalizations after holding at 1.8% since mid-November in 25 reporting jurisdictions (24 states and New York City), but the hospitalization rate among children with COVID held at 0.8%, where it has been for the last 4 weeks. Hospitalization rates as high as 3.8% were recorded early in the pandemic, the AAP and CHA noted.
New COVID-19 cases in children dropped for the third consecutive week, even as children continue to make up a larger share of all cases, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

New child cases totaled almost 118,000 for the week of Jan. 29-Feb. 4, continuing the decline that began right after the United States topped 200,000 cases for the only time Jan. 8-14, the AAP and the CHA said in their weekly COVID-19 report.
For the latest week, however, children represented 16.0% of all new COVID-19 cases, continuing a 5-week increase that began in early December 2020, after the proportion had dropped to 12.6%, based on data collected from the health departments of 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam. During the week of Sept. 11-17, children made up 16.9% of all cases, the highest level seen during the pandemic.
The 2.93 million cases that have been reported in children make up 12.9% of all cases since the pandemic began, and the overall rate of pediatric coronavirus infection is 3,899 cases per 100,000 children in the population. Taking a step down from the national level, 30 states are above that rate and 18 are below it, along with D.C., New York City, Puerto Rico, and Guam (New York and Texas are excluded), the AAP and CHA reported.
There were 12 new COVID-19–related child deaths in the 43 states, along with New York City and Guam, that are reporting such data, bringing the total to 227. Nationally, 0.06% of all deaths have occurred in children, with rates ranging from 0.00% (11 states) to 0.26% (Nebraska) in the 45 jurisdictions, the AAP/CHA report shows.
Child hospitalizations rose to 1.9% of all hospitalizations after holding at 1.8% since mid-November in 25 reporting jurisdictions (24 states and New York City), but the hospitalization rate among children with COVID held at 0.8%, where it has been for the last 4 weeks. Hospitalization rates as high as 3.8% were recorded early in the pandemic, the AAP and CHA noted.
Study tests ways to increase autism screening and referrals
To improve autism screening rates, researchers in Utah tried a range of interventions.
They added automatic reminders to the electronic health record (EHR). They started using a shorter, more sensitive screening instrument. And they trained clinicians to perform autism-specific evaluations in a primary care clinic.
The researchers found that these interventions were associated with increased rates of autism screening and referrals.
At the same time, they looked at screening and referral rates at other community clinics in their health care system. These clinics incorporated EHR reminders but not all of the other changes.
“The community clinics had an increase in screening frequency with only automatic reminders,” the researchers reported. At the two intervention clinics, however, screening rates increased more than they did at the community clinics. Referrals did not significantly increase at the community clinics.
Kathleen Campbell, MD, MHSc, a pediatric resident at the University of Utah in Salt Lake City, and colleagues described their research in a study published in Pediatrics.
Three phases
They examined more than 12,000 well-child visits for children aged 16-30 months between July 2017 and June 2019.
In all, 4,155 visits occurred at the 2 intervention clinics, and 8,078 visits occurred at the 27 community clinics in the University of Utah health care system.
From baseline through the interventions, the proportion of visits with screening increased by 51% in the intervention clinics (from 58.6% to 88.8%), and by 21% in the community clinics (from 43.4% to 52.4%). The proportion of referrals increased 1.5-fold in intervention clinics, from 1.3% to 3.3%, the authors said.
The American Academy of Pediatrics (AAP) supports screening for autism in all children starting at age 18 months, but “only 44% of children with autism have had a comprehensive autism evaluation before age 36 months,” Dr. Campbell and colleagues wrote.
In their system, about half of the children were being screened for autism, and 0.5% had autism diagnosed.
In an effort to increase the proportion of visits with screening for autism and the proportion of visits with referrals for autism evaluation, Dr. Campbell and colleagues designed a quality improvement study.
Following a baseline period, they implemented interventions in three phases.
Initially, all clinics used the Modified Checklist for Autism in Toddlers, Revised (M-CHAT-R) for autism screening. For the first phase starting in July 2018, the researchers changed the screening instrument at the two intervention clinics to the Parent’s Observation of Social Interaction (POSI). This instrument “is embedded in a broadband developmental screen, is shorter than the M-CHAT-R, and includes questions about the consistency of the child’s behavior,” the authors said. “The POSI has greater sensitivity than the M-CHAT-R ... and similar, although somewhat lower, specificity.”
In intervention phase 2 starting in November 2018, the researchers “added an automatic reminder for autism screening to the EHR health maintenance screen.” Both the intervention clinics and the community clinics received the automatic reminders.
In intervention phase 3 starting in February 2019, they “added a referral option that clinicians could use for rapid access to autism-specific evaluation ... for children who had a POSI result suggestive of autism and for whom the clinician had sufficient concerns about autism that would indicate the need for referral for autism evaluation,” the researchers said.
“Using an online tutorial, we trained three clinicians in the intervention clinics to administer an observational assessment known as the Screening Tool for Autism in Toddlers (STAT),” which requires a 30-minute visit, they said. “Children who had a STAT result suggestive of autism were referred for expedited autism diagnostic evaluation, which was performed by a multidisciplinary team in our university-based developmental assessment clinic. Children who had a STAT result that did not suggest autism did not receive further autism evaluations unless the clinician felt they still needed further evaluation at the developmental clinic.”
After the switch to POSI, the percentage of visits with a positive screen result increased from 4.7% to 13.5% in the intervention clinics.
Furthermore, referrals were 3.4 times more frequent for visits during phase 3 in the intervention clinics, relative to the baseline period.
Potential to overwhelm
“The change to a more sensitive screening instrument increased the frequency of screening results suggestive of autism and informed our improvement team of the need to implement autism evaluation in primary care to avoid overwhelming our referral system,” Dr. Campbell and coauthors reported.
Future studies may assess whether increased screening and referrals speed the time to diagnosis and treatment and improve long-term functional abilities of children with autism. Some children in the study have received an autism diagnosis, while others have not yet been evaluated.
The use of STAT in primary care may be limited by “the barriers of training providers and purchasing materials,” the authors noted. “However, the time-based billing for lengthier appointments and billing for developmental testing help to cover cost.”
The intervention clinics and community clinics were staffed by pediatric providers, including residents and attendings, said Dr. Campbell.
“The staffing is similar at the community and intervention clinics, with mostly pediatricians and some nurse practitioners,” Dr. Campbell said. “One difference is that there are a few family medicine physicians in the community clinics, but we did not study whether that made a difference in screening. At the beginning of the study the approach to screening was the same.”
From the start, the community clinics were screening for autism and referring for further autism evaluation less often than the intervention clinics. “I don’t know why they were screening less, but they did improve with the automatic reminders,” said Dr. Campbell. “We didn’t examine type of provider or type of practice in this study, but the literature suggests that family physicians do not screen for autism as often as pediatricians.”
Payment and referral challenges
In theory, the approach in the study is a great idea, but it may not be feasible to implement for many private practices, said Herschel Lessin, MD. Dr. Lessin is a senior partner of the Children’s Medical Group in New York.
“We desperately need autism screening in a primary care setting,” Dr. Lessin said. “These authors found that wasn’t being done as recommended by the AAP Bright Futures, which is a problem.”
However, the researchers incorporated the interventions in a health care system with “far more resources than most people in practice would ever have” and substituted a less familiar screening tool.
In addition, the ability to use confirmatory STAT for primary care evaluations may be limited. “Unless you can find pediatricians willing to commit 30 to 45 minutes on one of these evaluations ... few are going to do that,” he said.
“The whole problem is that there are no referrals available or very few referrals available, and that insurance payments so underpay for developmental screening and evaluation that it does not justify the time doing it, so a lot of doctors are unable to do it,” said Dr. Lessin. When a referral is warranted, developmental pediatricians may have 6- to 12-month waiting lists, he said.
“For people in clinical practice, this is not news,” Dr. Lessin said. “We know we should screen for autism. The problem is it’s time consuming. Nobody pays for it. We have no place to send them even when we are suspicious.”
From screening to diagnosis to treatment
“Autism screen approaches vary but with educational efforts on the part of the AAP, CDC, and family organizations the rates for autism screening have dramatically improved,” said Susan L. Hyman, MD, professor of pediatrics at the University of Rochester in New York. “I do not know if screening rates have been impacted by COVID.”
Dr. Hyman and coauthors wrote an AAP clinical report on the identification, evaluation, and management of children with autism spectrum disorder. The report was published in the January 2020 issue of Pediatrics.
After screening and diagnostic testing, patients most importantly need to be able to access “timely and equitable evidence-based intervention,” which should be available, said Dr. Hyman.
Although researchers have proposed training primary care providers in autism diagnostics, “older, more complex patients with co-occurring behavioral health or other developmental disorders may need more specialized diagnostic assessment than could be accomplished in a primary care setting,” Dr. Hyman added.
“However, it is very important to identify children with therapeutic needs as early as possible and move them through the continuum from screening to diagnosis to treatment in a timely fashion. It would be wonderful if symptoms could be addressed without the need for diagnosis in the very youngest children,” Dr. Hyman said. “Early symptoms, even if not autism, are likely to be appropriate for intervention – whether it is speech therapy, attention to food selectivity, sleep problems – things that impact quality of life and potential future symptoms.”
The research was supported by the Utah Stimulating Access to Research in Residency Transition Scholar award, which is funded by the National Institutes of Health.
Dr. Campbell is an inventor on a patent related to screening for autism. The study authors otherwise had no disclosures. Dr. Lessin is on the editorial advisory board for Pediatric News and is on an advisory board for Cognoa, which is developing a medical device to diagnose autism and he is also the co-editor of the AAP's current ADHD Toolkit. Dr. Hyman had no relevant financial disclosures.
*This story was updated on Feb. 11, 2021.
To improve autism screening rates, researchers in Utah tried a range of interventions.
They added automatic reminders to the electronic health record (EHR). They started using a shorter, more sensitive screening instrument. And they trained clinicians to perform autism-specific evaluations in a primary care clinic.
The researchers found that these interventions were associated with increased rates of autism screening and referrals.
At the same time, they looked at screening and referral rates at other community clinics in their health care system. These clinics incorporated EHR reminders but not all of the other changes.
“The community clinics had an increase in screening frequency with only automatic reminders,” the researchers reported. At the two intervention clinics, however, screening rates increased more than they did at the community clinics. Referrals did not significantly increase at the community clinics.
Kathleen Campbell, MD, MHSc, a pediatric resident at the University of Utah in Salt Lake City, and colleagues described their research in a study published in Pediatrics.
Three phases
They examined more than 12,000 well-child visits for children aged 16-30 months between July 2017 and June 2019.
In all, 4,155 visits occurred at the 2 intervention clinics, and 8,078 visits occurred at the 27 community clinics in the University of Utah health care system.
From baseline through the interventions, the proportion of visits with screening increased by 51% in the intervention clinics (from 58.6% to 88.8%), and by 21% in the community clinics (from 43.4% to 52.4%). The proportion of referrals increased 1.5-fold in intervention clinics, from 1.3% to 3.3%, the authors said.
The American Academy of Pediatrics (AAP) supports screening for autism in all children starting at age 18 months, but “only 44% of children with autism have had a comprehensive autism evaluation before age 36 months,” Dr. Campbell and colleagues wrote.
In their system, about half of the children were being screened for autism, and 0.5% had autism diagnosed.
In an effort to increase the proportion of visits with screening for autism and the proportion of visits with referrals for autism evaluation, Dr. Campbell and colleagues designed a quality improvement study.
Following a baseline period, they implemented interventions in three phases.
Initially, all clinics used the Modified Checklist for Autism in Toddlers, Revised (M-CHAT-R) for autism screening. For the first phase starting in July 2018, the researchers changed the screening instrument at the two intervention clinics to the Parent’s Observation of Social Interaction (POSI). This instrument “is embedded in a broadband developmental screen, is shorter than the M-CHAT-R, and includes questions about the consistency of the child’s behavior,” the authors said. “The POSI has greater sensitivity than the M-CHAT-R ... and similar, although somewhat lower, specificity.”
In intervention phase 2 starting in November 2018, the researchers “added an automatic reminder for autism screening to the EHR health maintenance screen.” Both the intervention clinics and the community clinics received the automatic reminders.
In intervention phase 3 starting in February 2019, they “added a referral option that clinicians could use for rapid access to autism-specific evaluation ... for children who had a POSI result suggestive of autism and for whom the clinician had sufficient concerns about autism that would indicate the need for referral for autism evaluation,” the researchers said.
“Using an online tutorial, we trained three clinicians in the intervention clinics to administer an observational assessment known as the Screening Tool for Autism in Toddlers (STAT),” which requires a 30-minute visit, they said. “Children who had a STAT result suggestive of autism were referred for expedited autism diagnostic evaluation, which was performed by a multidisciplinary team in our university-based developmental assessment clinic. Children who had a STAT result that did not suggest autism did not receive further autism evaluations unless the clinician felt they still needed further evaluation at the developmental clinic.”
After the switch to POSI, the percentage of visits with a positive screen result increased from 4.7% to 13.5% in the intervention clinics.
Furthermore, referrals were 3.4 times more frequent for visits during phase 3 in the intervention clinics, relative to the baseline period.
Potential to overwhelm
“The change to a more sensitive screening instrument increased the frequency of screening results suggestive of autism and informed our improvement team of the need to implement autism evaluation in primary care to avoid overwhelming our referral system,” Dr. Campbell and coauthors reported.
Future studies may assess whether increased screening and referrals speed the time to diagnosis and treatment and improve long-term functional abilities of children with autism. Some children in the study have received an autism diagnosis, while others have not yet been evaluated.
The use of STAT in primary care may be limited by “the barriers of training providers and purchasing materials,” the authors noted. “However, the time-based billing for lengthier appointments and billing for developmental testing help to cover cost.”
The intervention clinics and community clinics were staffed by pediatric providers, including residents and attendings, said Dr. Campbell.
“The staffing is similar at the community and intervention clinics, with mostly pediatricians and some nurse practitioners,” Dr. Campbell said. “One difference is that there are a few family medicine physicians in the community clinics, but we did not study whether that made a difference in screening. At the beginning of the study the approach to screening was the same.”
From the start, the community clinics were screening for autism and referring for further autism evaluation less often than the intervention clinics. “I don’t know why they were screening less, but they did improve with the automatic reminders,” said Dr. Campbell. “We didn’t examine type of provider or type of practice in this study, but the literature suggests that family physicians do not screen for autism as often as pediatricians.”
Payment and referral challenges
In theory, the approach in the study is a great idea, but it may not be feasible to implement for many private practices, said Herschel Lessin, MD. Dr. Lessin is a senior partner of the Children’s Medical Group in New York.
“We desperately need autism screening in a primary care setting,” Dr. Lessin said. “These authors found that wasn’t being done as recommended by the AAP Bright Futures, which is a problem.”
However, the researchers incorporated the interventions in a health care system with “far more resources than most people in practice would ever have” and substituted a less familiar screening tool.
In addition, the ability to use confirmatory STAT for primary care evaluations may be limited. “Unless you can find pediatricians willing to commit 30 to 45 minutes on one of these evaluations ... few are going to do that,” he said.
“The whole problem is that there are no referrals available or very few referrals available, and that insurance payments so underpay for developmental screening and evaluation that it does not justify the time doing it, so a lot of doctors are unable to do it,” said Dr. Lessin. When a referral is warranted, developmental pediatricians may have 6- to 12-month waiting lists, he said.
“For people in clinical practice, this is not news,” Dr. Lessin said. “We know we should screen for autism. The problem is it’s time consuming. Nobody pays for it. We have no place to send them even when we are suspicious.”
From screening to diagnosis to treatment
“Autism screen approaches vary but with educational efforts on the part of the AAP, CDC, and family organizations the rates for autism screening have dramatically improved,” said Susan L. Hyman, MD, professor of pediatrics at the University of Rochester in New York. “I do not know if screening rates have been impacted by COVID.”
Dr. Hyman and coauthors wrote an AAP clinical report on the identification, evaluation, and management of children with autism spectrum disorder. The report was published in the January 2020 issue of Pediatrics.
After screening and diagnostic testing, patients most importantly need to be able to access “timely and equitable evidence-based intervention,” which should be available, said Dr. Hyman.
Although researchers have proposed training primary care providers in autism diagnostics, “older, more complex patients with co-occurring behavioral health or other developmental disorders may need more specialized diagnostic assessment than could be accomplished in a primary care setting,” Dr. Hyman added.
“However, it is very important to identify children with therapeutic needs as early as possible and move them through the continuum from screening to diagnosis to treatment in a timely fashion. It would be wonderful if symptoms could be addressed without the need for diagnosis in the very youngest children,” Dr. Hyman said. “Early symptoms, even if not autism, are likely to be appropriate for intervention – whether it is speech therapy, attention to food selectivity, sleep problems – things that impact quality of life and potential future symptoms.”
The research was supported by the Utah Stimulating Access to Research in Residency Transition Scholar award, which is funded by the National Institutes of Health.
Dr. Campbell is an inventor on a patent related to screening for autism. The study authors otherwise had no disclosures. Dr. Lessin is on the editorial advisory board for Pediatric News and is on an advisory board for Cognoa, which is developing a medical device to diagnose autism and he is also the co-editor of the AAP's current ADHD Toolkit. Dr. Hyman had no relevant financial disclosures.
*This story was updated on Feb. 11, 2021.
To improve autism screening rates, researchers in Utah tried a range of interventions.
They added automatic reminders to the electronic health record (EHR). They started using a shorter, more sensitive screening instrument. And they trained clinicians to perform autism-specific evaluations in a primary care clinic.
The researchers found that these interventions were associated with increased rates of autism screening and referrals.
At the same time, they looked at screening and referral rates at other community clinics in their health care system. These clinics incorporated EHR reminders but not all of the other changes.
“The community clinics had an increase in screening frequency with only automatic reminders,” the researchers reported. At the two intervention clinics, however, screening rates increased more than they did at the community clinics. Referrals did not significantly increase at the community clinics.
Kathleen Campbell, MD, MHSc, a pediatric resident at the University of Utah in Salt Lake City, and colleagues described their research in a study published in Pediatrics.
Three phases
They examined more than 12,000 well-child visits for children aged 16-30 months between July 2017 and June 2019.
In all, 4,155 visits occurred at the 2 intervention clinics, and 8,078 visits occurred at the 27 community clinics in the University of Utah health care system.
From baseline through the interventions, the proportion of visits with screening increased by 51% in the intervention clinics (from 58.6% to 88.8%), and by 21% in the community clinics (from 43.4% to 52.4%). The proportion of referrals increased 1.5-fold in intervention clinics, from 1.3% to 3.3%, the authors said.
The American Academy of Pediatrics (AAP) supports screening for autism in all children starting at age 18 months, but “only 44% of children with autism have had a comprehensive autism evaluation before age 36 months,” Dr. Campbell and colleagues wrote.
In their system, about half of the children were being screened for autism, and 0.5% had autism diagnosed.
In an effort to increase the proportion of visits with screening for autism and the proportion of visits with referrals for autism evaluation, Dr. Campbell and colleagues designed a quality improvement study.
Following a baseline period, they implemented interventions in three phases.
Initially, all clinics used the Modified Checklist for Autism in Toddlers, Revised (M-CHAT-R) for autism screening. For the first phase starting in July 2018, the researchers changed the screening instrument at the two intervention clinics to the Parent’s Observation of Social Interaction (POSI). This instrument “is embedded in a broadband developmental screen, is shorter than the M-CHAT-R, and includes questions about the consistency of the child’s behavior,” the authors said. “The POSI has greater sensitivity than the M-CHAT-R ... and similar, although somewhat lower, specificity.”
In intervention phase 2 starting in November 2018, the researchers “added an automatic reminder for autism screening to the EHR health maintenance screen.” Both the intervention clinics and the community clinics received the automatic reminders.
In intervention phase 3 starting in February 2019, they “added a referral option that clinicians could use for rapid access to autism-specific evaluation ... for children who had a POSI result suggestive of autism and for whom the clinician had sufficient concerns about autism that would indicate the need for referral for autism evaluation,” the researchers said.
“Using an online tutorial, we trained three clinicians in the intervention clinics to administer an observational assessment known as the Screening Tool for Autism in Toddlers (STAT),” which requires a 30-minute visit, they said. “Children who had a STAT result suggestive of autism were referred for expedited autism diagnostic evaluation, which was performed by a multidisciplinary team in our university-based developmental assessment clinic. Children who had a STAT result that did not suggest autism did not receive further autism evaluations unless the clinician felt they still needed further evaluation at the developmental clinic.”
After the switch to POSI, the percentage of visits with a positive screen result increased from 4.7% to 13.5% in the intervention clinics.
Furthermore, referrals were 3.4 times more frequent for visits during phase 3 in the intervention clinics, relative to the baseline period.
Potential to overwhelm
“The change to a more sensitive screening instrument increased the frequency of screening results suggestive of autism and informed our improvement team of the need to implement autism evaluation in primary care to avoid overwhelming our referral system,” Dr. Campbell and coauthors reported.
Future studies may assess whether increased screening and referrals speed the time to diagnosis and treatment and improve long-term functional abilities of children with autism. Some children in the study have received an autism diagnosis, while others have not yet been evaluated.
The use of STAT in primary care may be limited by “the barriers of training providers and purchasing materials,” the authors noted. “However, the time-based billing for lengthier appointments and billing for developmental testing help to cover cost.”
The intervention clinics and community clinics were staffed by pediatric providers, including residents and attendings, said Dr. Campbell.
“The staffing is similar at the community and intervention clinics, with mostly pediatricians and some nurse practitioners,” Dr. Campbell said. “One difference is that there are a few family medicine physicians in the community clinics, but we did not study whether that made a difference in screening. At the beginning of the study the approach to screening was the same.”
From the start, the community clinics were screening for autism and referring for further autism evaluation less often than the intervention clinics. “I don’t know why they were screening less, but they did improve with the automatic reminders,” said Dr. Campbell. “We didn’t examine type of provider or type of practice in this study, but the literature suggests that family physicians do not screen for autism as often as pediatricians.”
Payment and referral challenges
In theory, the approach in the study is a great idea, but it may not be feasible to implement for many private practices, said Herschel Lessin, MD. Dr. Lessin is a senior partner of the Children’s Medical Group in New York.
“We desperately need autism screening in a primary care setting,” Dr. Lessin said. “These authors found that wasn’t being done as recommended by the AAP Bright Futures, which is a problem.”
However, the researchers incorporated the interventions in a health care system with “far more resources than most people in practice would ever have” and substituted a less familiar screening tool.
In addition, the ability to use confirmatory STAT for primary care evaluations may be limited. “Unless you can find pediatricians willing to commit 30 to 45 minutes on one of these evaluations ... few are going to do that,” he said.
“The whole problem is that there are no referrals available or very few referrals available, and that insurance payments so underpay for developmental screening and evaluation that it does not justify the time doing it, so a lot of doctors are unable to do it,” said Dr. Lessin. When a referral is warranted, developmental pediatricians may have 6- to 12-month waiting lists, he said.
“For people in clinical practice, this is not news,” Dr. Lessin said. “We know we should screen for autism. The problem is it’s time consuming. Nobody pays for it. We have no place to send them even when we are suspicious.”
From screening to diagnosis to treatment
“Autism screen approaches vary but with educational efforts on the part of the AAP, CDC, and family organizations the rates for autism screening have dramatically improved,” said Susan L. Hyman, MD, professor of pediatrics at the University of Rochester in New York. “I do not know if screening rates have been impacted by COVID.”
Dr. Hyman and coauthors wrote an AAP clinical report on the identification, evaluation, and management of children with autism spectrum disorder. The report was published in the January 2020 issue of Pediatrics.
After screening and diagnostic testing, patients most importantly need to be able to access “timely and equitable evidence-based intervention,” which should be available, said Dr. Hyman.
Although researchers have proposed training primary care providers in autism diagnostics, “older, more complex patients with co-occurring behavioral health or other developmental disorders may need more specialized diagnostic assessment than could be accomplished in a primary care setting,” Dr. Hyman added.
“However, it is very important to identify children with therapeutic needs as early as possible and move them through the continuum from screening to diagnosis to treatment in a timely fashion. It would be wonderful if symptoms could be addressed without the need for diagnosis in the very youngest children,” Dr. Hyman said. “Early symptoms, even if not autism, are likely to be appropriate for intervention – whether it is speech therapy, attention to food selectivity, sleep problems – things that impact quality of life and potential future symptoms.”
The research was supported by the Utah Stimulating Access to Research in Residency Transition Scholar award, which is funded by the National Institutes of Health.
Dr. Campbell is an inventor on a patent related to screening for autism. The study authors otherwise had no disclosures. Dr. Lessin is on the editorial advisory board for Pediatric News and is on an advisory board for Cognoa, which is developing a medical device to diagnose autism and he is also the co-editor of the AAP's current ADHD Toolkit. Dr. Hyman had no relevant financial disclosures.
*This story was updated on Feb. 11, 2021.
FROM PEDIATRICS
U.K. COVID-19 variant doubling every 10 days in the U.S.: Study
The SARS-CoV-2 variant first detected in the United Kingdom is rapidly becoming the dominant strain in several countries and is doubling every 10 days in the United States, according to new data.
The findings by Nicole L. Washington, PhD, associate director of research at the genomics company Helix, and colleagues were posted Feb. 7, 2021, on the preprint server medRxiv. The paper has not been peer-reviewed in a scientific journal.
The researchers also found that the transmission rate in the United States of the variant, labeled B.1.1.7, is 30%-40% higher than that of more common lineages.
While clinical outcomes initially were thought to be similar to those of other SARS-CoV-2 variants, early reports suggest that infection with the B.1.1.7 variant may increase death risk by about 30%.
A coauthor of the current study, Kristian Andersen, PhD, told the New York Times , “Nothing in this paper is surprising, but people need to see it.”
Dr. Andersen, a virologist at the Scripps Research Institute in La Jolla, Calif., added that “we should probably prepare for this being the predominant lineage in most places in the United States by March.”
The study of the B.1.1.7 variant adds support for the Centers for Disease Control and Prevention prediction in January that it would dominate by March.
“Our study shows that the U.S. is on a similar trajectory as other countries where B.1.1.7 rapidly became the dominant SARS-CoV-2 variant, requiring immediate and decisive action to minimize COVID-19 morbidity and mortality,” the researchers wrote.
The authors pointed out that the B.1.1.7 variant became the dominant SARS-CoV-2 strain in the United Kingdom within a couple of months of its detection.
“Since then, the variant has been increasingly observed across many European countries, including Portugal and Ireland, which, like the U.K., observed devastating waves of COVID-19 after B.1.1.7 became dominant,” the authors wrote.
“Category 5” storm
The B.1.1.7 variant has likely been spreading between U.S. states since at least December, they wrote.
This news organization reported on Jan. 15 that, as of Jan. 13, the B.1.1.7 variant was seen in 76 cases across 12 U.S. states, according to an early release of the CDC’s Morbidity and Mortality Weekly Report.
As of Feb. 7, there were 690 cases of the B.1.1.7 variant in the US in 33 states, according to the CDC.
Dr. Washington and colleagues examined more than 500,000 coronavirus test samples from cases across the United States that were tested at San Mateo, Calif.–based Helix facilities since July.
In the study, they found inconsistent prevalence of the variant across states. By the last week in January, the researchers estimated the proportion of B.1.1.7 in the U.S. population to be about 2.1% of all COVID-19 cases, though they found it made up about 2% of all COVID-19 cases in California and about 4.5% of cases in Florida. The authors acknowledged that their data is less robust outside of those two states.
Though that seems a relatively low frequency, “our estimates show that its growth rate is at least 35%-45% increased and doubling every week and a half,” the authors wrote.
“Because laboratories in the U.S. are only sequencing a small subset of SARS-CoV-2 samples, the true sequence diversity of SARS-CoV-2 in this country is still unknown,” they noted.
Michael Osterholm, PhD, MPH, director of the Center for Infectious Disease Research and Policy at the University of Minnesota, Minneapolis, said last week that the United States is facing a “Category 5” storm with the spread of the B.1.1.7 variant as well as the variants first identified in South Africa and Brazil.
“We are going to see something like we have not seen yet in this country,” Dr. Osterholm said recently on NBC’s Meet the Press.
Lead author Nicole L. Washington and many of the coauthors are employees of Helix. Other coauthors are employees of Illumina. Three coauthors own stock in ILMN. The work was funded by Illumina, Helix, the Innovative Genomics Institute, and the New Frontiers in Research Fund provided by the Canadian Institutes of Health Research.
A version of this article first appeared on Medscape.com.
The SARS-CoV-2 variant first detected in the United Kingdom is rapidly becoming the dominant strain in several countries and is doubling every 10 days in the United States, according to new data.
The findings by Nicole L. Washington, PhD, associate director of research at the genomics company Helix, and colleagues were posted Feb. 7, 2021, on the preprint server medRxiv. The paper has not been peer-reviewed in a scientific journal.
The researchers also found that the transmission rate in the United States of the variant, labeled B.1.1.7, is 30%-40% higher than that of more common lineages.
While clinical outcomes initially were thought to be similar to those of other SARS-CoV-2 variants, early reports suggest that infection with the B.1.1.7 variant may increase death risk by about 30%.
A coauthor of the current study, Kristian Andersen, PhD, told the New York Times , “Nothing in this paper is surprising, but people need to see it.”
Dr. Andersen, a virologist at the Scripps Research Institute in La Jolla, Calif., added that “we should probably prepare for this being the predominant lineage in most places in the United States by March.”
The study of the B.1.1.7 variant adds support for the Centers for Disease Control and Prevention prediction in January that it would dominate by March.
“Our study shows that the U.S. is on a similar trajectory as other countries where B.1.1.7 rapidly became the dominant SARS-CoV-2 variant, requiring immediate and decisive action to minimize COVID-19 morbidity and mortality,” the researchers wrote.
The authors pointed out that the B.1.1.7 variant became the dominant SARS-CoV-2 strain in the United Kingdom within a couple of months of its detection.
“Since then, the variant has been increasingly observed across many European countries, including Portugal and Ireland, which, like the U.K., observed devastating waves of COVID-19 after B.1.1.7 became dominant,” the authors wrote.
“Category 5” storm
The B.1.1.7 variant has likely been spreading between U.S. states since at least December, they wrote.
This news organization reported on Jan. 15 that, as of Jan. 13, the B.1.1.7 variant was seen in 76 cases across 12 U.S. states, according to an early release of the CDC’s Morbidity and Mortality Weekly Report.
As of Feb. 7, there were 690 cases of the B.1.1.7 variant in the US in 33 states, according to the CDC.
Dr. Washington and colleagues examined more than 500,000 coronavirus test samples from cases across the United States that were tested at San Mateo, Calif.–based Helix facilities since July.
In the study, they found inconsistent prevalence of the variant across states. By the last week in January, the researchers estimated the proportion of B.1.1.7 in the U.S. population to be about 2.1% of all COVID-19 cases, though they found it made up about 2% of all COVID-19 cases in California and about 4.5% of cases in Florida. The authors acknowledged that their data is less robust outside of those two states.
Though that seems a relatively low frequency, “our estimates show that its growth rate is at least 35%-45% increased and doubling every week and a half,” the authors wrote.
“Because laboratories in the U.S. are only sequencing a small subset of SARS-CoV-2 samples, the true sequence diversity of SARS-CoV-2 in this country is still unknown,” they noted.
Michael Osterholm, PhD, MPH, director of the Center for Infectious Disease Research and Policy at the University of Minnesota, Minneapolis, said last week that the United States is facing a “Category 5” storm with the spread of the B.1.1.7 variant as well as the variants first identified in South Africa and Brazil.
“We are going to see something like we have not seen yet in this country,” Dr. Osterholm said recently on NBC’s Meet the Press.
Lead author Nicole L. Washington and many of the coauthors are employees of Helix. Other coauthors are employees of Illumina. Three coauthors own stock in ILMN. The work was funded by Illumina, Helix, the Innovative Genomics Institute, and the New Frontiers in Research Fund provided by the Canadian Institutes of Health Research.
A version of this article first appeared on Medscape.com.
The SARS-CoV-2 variant first detected in the United Kingdom is rapidly becoming the dominant strain in several countries and is doubling every 10 days in the United States, according to new data.
The findings by Nicole L. Washington, PhD, associate director of research at the genomics company Helix, and colleagues were posted Feb. 7, 2021, on the preprint server medRxiv. The paper has not been peer-reviewed in a scientific journal.
The researchers also found that the transmission rate in the United States of the variant, labeled B.1.1.7, is 30%-40% higher than that of more common lineages.
While clinical outcomes initially were thought to be similar to those of other SARS-CoV-2 variants, early reports suggest that infection with the B.1.1.7 variant may increase death risk by about 30%.
A coauthor of the current study, Kristian Andersen, PhD, told the New York Times , “Nothing in this paper is surprising, but people need to see it.”
Dr. Andersen, a virologist at the Scripps Research Institute in La Jolla, Calif., added that “we should probably prepare for this being the predominant lineage in most places in the United States by March.”
The study of the B.1.1.7 variant adds support for the Centers for Disease Control and Prevention prediction in January that it would dominate by March.
“Our study shows that the U.S. is on a similar trajectory as other countries where B.1.1.7 rapidly became the dominant SARS-CoV-2 variant, requiring immediate and decisive action to minimize COVID-19 morbidity and mortality,” the researchers wrote.
The authors pointed out that the B.1.1.7 variant became the dominant SARS-CoV-2 strain in the United Kingdom within a couple of months of its detection.
“Since then, the variant has been increasingly observed across many European countries, including Portugal and Ireland, which, like the U.K., observed devastating waves of COVID-19 after B.1.1.7 became dominant,” the authors wrote.
“Category 5” storm
The B.1.1.7 variant has likely been spreading between U.S. states since at least December, they wrote.
This news organization reported on Jan. 15 that, as of Jan. 13, the B.1.1.7 variant was seen in 76 cases across 12 U.S. states, according to an early release of the CDC’s Morbidity and Mortality Weekly Report.
As of Feb. 7, there were 690 cases of the B.1.1.7 variant in the US in 33 states, according to the CDC.
Dr. Washington and colleagues examined more than 500,000 coronavirus test samples from cases across the United States that were tested at San Mateo, Calif.–based Helix facilities since July.
In the study, they found inconsistent prevalence of the variant across states. By the last week in January, the researchers estimated the proportion of B.1.1.7 in the U.S. population to be about 2.1% of all COVID-19 cases, though they found it made up about 2% of all COVID-19 cases in California and about 4.5% of cases in Florida. The authors acknowledged that their data is less robust outside of those two states.
Though that seems a relatively low frequency, “our estimates show that its growth rate is at least 35%-45% increased and doubling every week and a half,” the authors wrote.
“Because laboratories in the U.S. are only sequencing a small subset of SARS-CoV-2 samples, the true sequence diversity of SARS-CoV-2 in this country is still unknown,” they noted.
Michael Osterholm, PhD, MPH, director of the Center for Infectious Disease Research and Policy at the University of Minnesota, Minneapolis, said last week that the United States is facing a “Category 5” storm with the spread of the B.1.1.7 variant as well as the variants first identified in South Africa and Brazil.
“We are going to see something like we have not seen yet in this country,” Dr. Osterholm said recently on NBC’s Meet the Press.
Lead author Nicole L. Washington and many of the coauthors are employees of Helix. Other coauthors are employees of Illumina. Three coauthors own stock in ILMN. The work was funded by Illumina, Helix, the Innovative Genomics Institute, and the New Frontiers in Research Fund provided by the Canadian Institutes of Health Research.
A version of this article first appeared on Medscape.com.
Are diagnosticians chasing COVID-linked zebras and missing horses?
The emergence of multiple inflammatory syndrome in children (MIS-C) in association with COVID-19 may be complicating the investigation and diagnosis of more common viral and bacterial infections, potentially delaying treatment and prolonging hospital stays.
Two recent articles published online in Hospital Pediatrics provide evidence of this phenomenon. The articles outlined case studies of children who underwent extensive investigation for MIS-C when in fact they had less severe and more common infections. MIS-C is a severe but rare syndrome that involves systemic hyperinflammation with fever and multisystem organ dysfunction similar to that of Kawasaki disease (KD).
In one of the articles, Matthew Molloy, MD, MPH, of the division of pediatric hospital medicine at Cincinnati Children’s Hospital Medical Center, and colleagues aptly asked: “What are we missing in our search for MIS-C?”
E. coli, not SARS-CoV-2
That question arose from a case involving a 3-year-old boy who had a 6-day history of fever and fatigue. Three days earlier, he had tested negative for strep antigen and COVID-19. He had a persistent, high fever, reduced appetite, and reduced urine output and was taken to the ED. On physical examination, there was no rash, skin peeling, redness of the eye or oral mucosa, congestion, rhinorrhea, cough, shortness of breath, chest pain, abdominal pain, nausea, vomiting, or diarrhea.
Urinalysis results and exam findings were suspicious for pyelonephritis. Other findings from an extensive laboratory workup raised the alarm that the boy was suffering from MIS-C as opposed to incomplete KD. After admission to hospital medicine, the cardiology, rheumatology, and infectious disease teams were called in to consult.
Repeat labs were planned for the following day before initiating therapy. On day 2, the child’s urine culture was positive for gram-negative rods, later identified as Escherichia coli. The boy was started on ceftriaxone. Left renal scarring was apparent on ultrasound. The patient’s condition resolved after 36 hours, and he was discharged home with antibiotics.
‘Diagnosis derailed’
Calling this a case of “diagnosis derailed,” the authors noted that, in the pre-COVID era, this child’s signs and symptoms would likely have triggered a more targeted and less costly evaluation for more common infectious and noninfectious causes, including pyelonephritis, absent any physical exam findings consistent with KD.
“However, the patient presented in the midst of the COVID-19 pandemic with growing awareness of a new clinical entity,” Dr. Molloy and colleagues wrote. “Anchored to the patient’s persistent fever, the medical team initiated an extensive, costly, and ultimately unnecessary workup to avoid missing the diagnosis of MIS-C; a not yet well-described diagnosis with potentially severe morbidity.”
Confirmation bias and diagnostic momentum likely contributed to the early focus on MIS-C rather than more common alternatives, the authors acknowledged. The addition of mildly abnormal laboratory data not typically obtained in the evaluation of fever led the team astray. “The diagnosis and definitive treatment may have been made earlier had the focus on concern for MIS-C not been present,” Dr. Molloy said in an interview.
Keeping value in care
The authors recognized that their initial approach to evaluating for MIS-C provided low-value care. “In our desire to not ‘miss’ MIS-C, we were performing costly evaluations that at times produced mildly abnormal, nonspecific results,” they wrote. That triggered a cascade of specialty consultations, follow-up testing, and an unwarranted diagnostic preoccupation with MIS-C.
Determining the extra price tag for the child’s workup would be complex and difficult because there is a difference in the cost to the hospital and the cost to the family, Dr. Molloy said. “However, there are potential cost savings that would be related to making a correct diagnosis in a timely manner in terms of preventing downstream effects from delayed diagnoses.”
Even as clinicians struggle with the challenging SARS-CoV-2 learning curve, Dr. Molloy and associates urged them to continue to strive for high-value care, with an unwavering focus on using only necessary resources, a stewardship the pandemic has shown to be critical.
“The COVID-19 pandemic has been an incredibly stressful time for physicians and for families,” Dr. Molloy said. “COVID-19 and related conditions like MIS-C are new, and we are learning more and more about them every week. These diagnoses are understandably on the minds of physicians and families when children present with fever.” Notwithstanding, the boy’s case underscores the need for clinicians to consider alternate diagnoses and the value of the care provided.
Impact of bias
Dr. Molloy’s group brings home the cognitive biases practitioners often suffer from, including anchoring and confirmation bias and diagnostic momentum, according to J. Howard Smart, MD, chief of pediatrics at Sharp Mary Birch Hospital for Women and Newborns, San Diego, and an assistant clinical professor of pediatrics at University of California, San Diego.
“But it is one thing to recognize these in retrospect and quite another to consider whether they may be happening to you yourself in real time,” he said in an interview. “It is almost as if we need to have a ‘time out,’ where we stop and ask ourselves whether there is something else that could be explaining our patient’s presentation, something that would be more common and more likely to be occurring.”
According to Dr. Smart, who was not involved in Dr. Molloy’s study, the team’s premature diagnostic focus on MIS-C was almost the inverse of what typically happens with KD. “It is usually the case that Kawasaki disease does not enter the differential diagnosis until late in the course of the fever, typically on day 5 or later, when it may have been better to think of it earlier,” he said.
In the second article, Andrea Dean, MD, of the department of pediatrics at Baylor College of Medicine and Texas Children’s Hospital, both in Houston, and colleagues outlined the cases of five patients aged 8-17 years who were hospitalized in May 2020 for suspected MIS-C. They exhibited inflammatory and other concerning indicators but were eventually discharged with a diagnosis of murine typhus.
This flea-borne infection, most commonly reported in the United States in the southeastern Gulf Coast region, Hawaii, and California, is often associated with a triad of fever, rash, and headache.
Cases have been rising in southern Texas, and Dr. Dean and colleagues postulated that school closures and social distancing may have increased exposure as a result of children spending more time outdoors or with pets. “Alternatively, parental concern for SARS-CoV-2 infection could mean children with symptoms are presenting to care and being referred or admitted to the hospital more frequently due to provider concern for MIS-C,” they wrote.
Cardiac involvement
The most concerning of the five cases in terms of possible MIS-C, Dr. Dean said in an interview, was that of a 12-year-old boy who had fever for 6 days in association with headache, eczematous rash, dry lips, and conjunctivitis. Laboratory tests showed a mildly elevated C-reactive protein level, hyponatremia, and thrombocytopenia, as well as sterile pyuria and mildly elevated prothrombin time. He was treated empirically with doxycycline, and his fever resolved over the next 24 hours.
An echocardiogram at initial evaluation, however, revealed mild dilation of the left anterior descending and right coronary arteries, which led to the administration of intravenous immunoglobulin and aspirin for atypical KD, in contrast to MIS-C. The authors postulated that mild cardiac involvement in disorders other than MIS-C and KD may be underrecognized.
The lesson from these cases, Dr. Dean and associates concluded, is that hospitalists must maintain a wide differential diagnosis when assessing a child with prolonged fever and evidence of systemic inflammation. The CDC stipulates that a diagnosis of MIS-C requires the absence of a plausible alternative diagnosis.
In addition to common viral, bacterial, and noninfectious disorders, a range of regional endemic rickettsial and parasitic infections must be considered as alternative diagnoses to MIS-C. “Many of these diseases cannot be reliably differentiated from MIS-C on presentation, and as community exposure to SARS-CoV-2 grows, hospitalists should be prepared to admit febrile children with evidence of systemic inflammation for brief observation periods to evaluate for MIS-C,” Dr. Dean’s group wrote. In this context, however, empiric treatment for common or even uncommon infectious diseases may avoid overdiagnosis and overtreatment of MIS-C as well as improve patient outcomes.
“We do have specific MIS-C guidelines at our institution,” Dr. Dean said, “but like all institutions, we are dealing with the broad definition of MIS-C according to the World Health Organization and the CDC, which is really the takeaway from this paper.”
More difficult differentiation
Both groups of authors pointed out that, as SARS-CoV-2 spreads throughout a community, a higher percentage of the population will have positive results on antibody testing, and such results will become less useful for differentiating between MIS-C and other conditions.
Despite these series’ cautionary lessons, other experts point to the critical importance of including MIS-C early on in the interest of efficient diagnosis and therapy. “In the cases cited, other pathologies were evaluated for and treated accordingly,” said Kara Gross Margolis, MD, AGAF, an associate professor of pediatrics in the division of pediatric gastroenterology, hepatology, and nutrition at Morgan Stanley Children’s Hospital,New York. “These papers stress the need for a balance that is important, and all potential diagnoses need to be considered, but MIS-C, due to its potential severe consequences, also needs to be on our differential now.”
In her view, as this new high-morbidity entity becomes more widespread during the pandemic, it will be increasingly important to keep this condition on the diagnostic radar.
Interestingly, in a converse example of diagnostic clouding, Dr. Gross Margolis’s group reported (Gastroenterology. 2020 Oct;159[4]:1571-4.e2) last year on a pediatric case series in which the presence of gastrointestinal symptoms in children with COVID-19–related MIS-C muddied the diagnosis by confusing this potentially severe syndrome with more common and less toxic gastrointestinal infections.
According to Dr. Smart, although the two reports don’t offer evidence for a particular diagnostic practice, they can inform the decision-making process. “It may be that we will have enough evidence shortly to say what the best practice is regarding diagnostic evaluation of possible MIS-C cases,” he said. “Until then, we must remember that common things occur commonly, even during a global pandemic.”
Neither of the two reports received any specific funding. The authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The emergence of multiple inflammatory syndrome in children (MIS-C) in association with COVID-19 may be complicating the investigation and diagnosis of more common viral and bacterial infections, potentially delaying treatment and prolonging hospital stays.
Two recent articles published online in Hospital Pediatrics provide evidence of this phenomenon. The articles outlined case studies of children who underwent extensive investigation for MIS-C when in fact they had less severe and more common infections. MIS-C is a severe but rare syndrome that involves systemic hyperinflammation with fever and multisystem organ dysfunction similar to that of Kawasaki disease (KD).
In one of the articles, Matthew Molloy, MD, MPH, of the division of pediatric hospital medicine at Cincinnati Children’s Hospital Medical Center, and colleagues aptly asked: “What are we missing in our search for MIS-C?”
E. coli, not SARS-CoV-2
That question arose from a case involving a 3-year-old boy who had a 6-day history of fever and fatigue. Three days earlier, he had tested negative for strep antigen and COVID-19. He had a persistent, high fever, reduced appetite, and reduced urine output and was taken to the ED. On physical examination, there was no rash, skin peeling, redness of the eye or oral mucosa, congestion, rhinorrhea, cough, shortness of breath, chest pain, abdominal pain, nausea, vomiting, or diarrhea.
Urinalysis results and exam findings were suspicious for pyelonephritis. Other findings from an extensive laboratory workup raised the alarm that the boy was suffering from MIS-C as opposed to incomplete KD. After admission to hospital medicine, the cardiology, rheumatology, and infectious disease teams were called in to consult.
Repeat labs were planned for the following day before initiating therapy. On day 2, the child’s urine culture was positive for gram-negative rods, later identified as Escherichia coli. The boy was started on ceftriaxone. Left renal scarring was apparent on ultrasound. The patient’s condition resolved after 36 hours, and he was discharged home with antibiotics.
‘Diagnosis derailed’
Calling this a case of “diagnosis derailed,” the authors noted that, in the pre-COVID era, this child’s signs and symptoms would likely have triggered a more targeted and less costly evaluation for more common infectious and noninfectious causes, including pyelonephritis, absent any physical exam findings consistent with KD.
“However, the patient presented in the midst of the COVID-19 pandemic with growing awareness of a new clinical entity,” Dr. Molloy and colleagues wrote. “Anchored to the patient’s persistent fever, the medical team initiated an extensive, costly, and ultimately unnecessary workup to avoid missing the diagnosis of MIS-C; a not yet well-described diagnosis with potentially severe morbidity.”
Confirmation bias and diagnostic momentum likely contributed to the early focus on MIS-C rather than more common alternatives, the authors acknowledged. The addition of mildly abnormal laboratory data not typically obtained in the evaluation of fever led the team astray. “The diagnosis and definitive treatment may have been made earlier had the focus on concern for MIS-C not been present,” Dr. Molloy said in an interview.
Keeping value in care
The authors recognized that their initial approach to evaluating for MIS-C provided low-value care. “In our desire to not ‘miss’ MIS-C, we were performing costly evaluations that at times produced mildly abnormal, nonspecific results,” they wrote. That triggered a cascade of specialty consultations, follow-up testing, and an unwarranted diagnostic preoccupation with MIS-C.
Determining the extra price tag for the child’s workup would be complex and difficult because there is a difference in the cost to the hospital and the cost to the family, Dr. Molloy said. “However, there are potential cost savings that would be related to making a correct diagnosis in a timely manner in terms of preventing downstream effects from delayed diagnoses.”
Even as clinicians struggle with the challenging SARS-CoV-2 learning curve, Dr. Molloy and associates urged them to continue to strive for high-value care, with an unwavering focus on using only necessary resources, a stewardship the pandemic has shown to be critical.
“The COVID-19 pandemic has been an incredibly stressful time for physicians and for families,” Dr. Molloy said. “COVID-19 and related conditions like MIS-C are new, and we are learning more and more about them every week. These diagnoses are understandably on the minds of physicians and families when children present with fever.” Notwithstanding, the boy’s case underscores the need for clinicians to consider alternate diagnoses and the value of the care provided.
Impact of bias
Dr. Molloy’s group brings home the cognitive biases practitioners often suffer from, including anchoring and confirmation bias and diagnostic momentum, according to J. Howard Smart, MD, chief of pediatrics at Sharp Mary Birch Hospital for Women and Newborns, San Diego, and an assistant clinical professor of pediatrics at University of California, San Diego.
“But it is one thing to recognize these in retrospect and quite another to consider whether they may be happening to you yourself in real time,” he said in an interview. “It is almost as if we need to have a ‘time out,’ where we stop and ask ourselves whether there is something else that could be explaining our patient’s presentation, something that would be more common and more likely to be occurring.”
According to Dr. Smart, who was not involved in Dr. Molloy’s study, the team’s premature diagnostic focus on MIS-C was almost the inverse of what typically happens with KD. “It is usually the case that Kawasaki disease does not enter the differential diagnosis until late in the course of the fever, typically on day 5 or later, when it may have been better to think of it earlier,” he said.
In the second article, Andrea Dean, MD, of the department of pediatrics at Baylor College of Medicine and Texas Children’s Hospital, both in Houston, and colleagues outlined the cases of five patients aged 8-17 years who were hospitalized in May 2020 for suspected MIS-C. They exhibited inflammatory and other concerning indicators but were eventually discharged with a diagnosis of murine typhus.
This flea-borne infection, most commonly reported in the United States in the southeastern Gulf Coast region, Hawaii, and California, is often associated with a triad of fever, rash, and headache.
Cases have been rising in southern Texas, and Dr. Dean and colleagues postulated that school closures and social distancing may have increased exposure as a result of children spending more time outdoors or with pets. “Alternatively, parental concern for SARS-CoV-2 infection could mean children with symptoms are presenting to care and being referred or admitted to the hospital more frequently due to provider concern for MIS-C,” they wrote.
Cardiac involvement
The most concerning of the five cases in terms of possible MIS-C, Dr. Dean said in an interview, was that of a 12-year-old boy who had fever for 6 days in association with headache, eczematous rash, dry lips, and conjunctivitis. Laboratory tests showed a mildly elevated C-reactive protein level, hyponatremia, and thrombocytopenia, as well as sterile pyuria and mildly elevated prothrombin time. He was treated empirically with doxycycline, and his fever resolved over the next 24 hours.
An echocardiogram at initial evaluation, however, revealed mild dilation of the left anterior descending and right coronary arteries, which led to the administration of intravenous immunoglobulin and aspirin for atypical KD, in contrast to MIS-C. The authors postulated that mild cardiac involvement in disorders other than MIS-C and KD may be underrecognized.
The lesson from these cases, Dr. Dean and associates concluded, is that hospitalists must maintain a wide differential diagnosis when assessing a child with prolonged fever and evidence of systemic inflammation. The CDC stipulates that a diagnosis of MIS-C requires the absence of a plausible alternative diagnosis.
In addition to common viral, bacterial, and noninfectious disorders, a range of regional endemic rickettsial and parasitic infections must be considered as alternative diagnoses to MIS-C. “Many of these diseases cannot be reliably differentiated from MIS-C on presentation, and as community exposure to SARS-CoV-2 grows, hospitalists should be prepared to admit febrile children with evidence of systemic inflammation for brief observation periods to evaluate for MIS-C,” Dr. Dean’s group wrote. In this context, however, empiric treatment for common or even uncommon infectious diseases may avoid overdiagnosis and overtreatment of MIS-C as well as improve patient outcomes.
“We do have specific MIS-C guidelines at our institution,” Dr. Dean said, “but like all institutions, we are dealing with the broad definition of MIS-C according to the World Health Organization and the CDC, which is really the takeaway from this paper.”
More difficult differentiation
Both groups of authors pointed out that, as SARS-CoV-2 spreads throughout a community, a higher percentage of the population will have positive results on antibody testing, and such results will become less useful for differentiating between MIS-C and other conditions.
Despite these series’ cautionary lessons, other experts point to the critical importance of including MIS-C early on in the interest of efficient diagnosis and therapy. “In the cases cited, other pathologies were evaluated for and treated accordingly,” said Kara Gross Margolis, MD, AGAF, an associate professor of pediatrics in the division of pediatric gastroenterology, hepatology, and nutrition at Morgan Stanley Children’s Hospital,New York. “These papers stress the need for a balance that is important, and all potential diagnoses need to be considered, but MIS-C, due to its potential severe consequences, also needs to be on our differential now.”
In her view, as this new high-morbidity entity becomes more widespread during the pandemic, it will be increasingly important to keep this condition on the diagnostic radar.
Interestingly, in a converse example of diagnostic clouding, Dr. Gross Margolis’s group reported (Gastroenterology. 2020 Oct;159[4]:1571-4.e2) last year on a pediatric case series in which the presence of gastrointestinal symptoms in children with COVID-19–related MIS-C muddied the diagnosis by confusing this potentially severe syndrome with more common and less toxic gastrointestinal infections.
According to Dr. Smart, although the two reports don’t offer evidence for a particular diagnostic practice, they can inform the decision-making process. “It may be that we will have enough evidence shortly to say what the best practice is regarding diagnostic evaluation of possible MIS-C cases,” he said. “Until then, we must remember that common things occur commonly, even during a global pandemic.”
Neither of the two reports received any specific funding. The authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The emergence of multiple inflammatory syndrome in children (MIS-C) in association with COVID-19 may be complicating the investigation and diagnosis of more common viral and bacterial infections, potentially delaying treatment and prolonging hospital stays.
Two recent articles published online in Hospital Pediatrics provide evidence of this phenomenon. The articles outlined case studies of children who underwent extensive investigation for MIS-C when in fact they had less severe and more common infections. MIS-C is a severe but rare syndrome that involves systemic hyperinflammation with fever and multisystem organ dysfunction similar to that of Kawasaki disease (KD).
In one of the articles, Matthew Molloy, MD, MPH, of the division of pediatric hospital medicine at Cincinnati Children’s Hospital Medical Center, and colleagues aptly asked: “What are we missing in our search for MIS-C?”
E. coli, not SARS-CoV-2
That question arose from a case involving a 3-year-old boy who had a 6-day history of fever and fatigue. Three days earlier, he had tested negative for strep antigen and COVID-19. He had a persistent, high fever, reduced appetite, and reduced urine output and was taken to the ED. On physical examination, there was no rash, skin peeling, redness of the eye or oral mucosa, congestion, rhinorrhea, cough, shortness of breath, chest pain, abdominal pain, nausea, vomiting, or diarrhea.
Urinalysis results and exam findings were suspicious for pyelonephritis. Other findings from an extensive laboratory workup raised the alarm that the boy was suffering from MIS-C as opposed to incomplete KD. After admission to hospital medicine, the cardiology, rheumatology, and infectious disease teams were called in to consult.
Repeat labs were planned for the following day before initiating therapy. On day 2, the child’s urine culture was positive for gram-negative rods, later identified as Escherichia coli. The boy was started on ceftriaxone. Left renal scarring was apparent on ultrasound. The patient’s condition resolved after 36 hours, and he was discharged home with antibiotics.
‘Diagnosis derailed’
Calling this a case of “diagnosis derailed,” the authors noted that, in the pre-COVID era, this child’s signs and symptoms would likely have triggered a more targeted and less costly evaluation for more common infectious and noninfectious causes, including pyelonephritis, absent any physical exam findings consistent with KD.
“However, the patient presented in the midst of the COVID-19 pandemic with growing awareness of a new clinical entity,” Dr. Molloy and colleagues wrote. “Anchored to the patient’s persistent fever, the medical team initiated an extensive, costly, and ultimately unnecessary workup to avoid missing the diagnosis of MIS-C; a not yet well-described diagnosis with potentially severe morbidity.”
Confirmation bias and diagnostic momentum likely contributed to the early focus on MIS-C rather than more common alternatives, the authors acknowledged. The addition of mildly abnormal laboratory data not typically obtained in the evaluation of fever led the team astray. “The diagnosis and definitive treatment may have been made earlier had the focus on concern for MIS-C not been present,” Dr. Molloy said in an interview.
Keeping value in care
The authors recognized that their initial approach to evaluating for MIS-C provided low-value care. “In our desire to not ‘miss’ MIS-C, we were performing costly evaluations that at times produced mildly abnormal, nonspecific results,” they wrote. That triggered a cascade of specialty consultations, follow-up testing, and an unwarranted diagnostic preoccupation with MIS-C.
Determining the extra price tag for the child’s workup would be complex and difficult because there is a difference in the cost to the hospital and the cost to the family, Dr. Molloy said. “However, there are potential cost savings that would be related to making a correct diagnosis in a timely manner in terms of preventing downstream effects from delayed diagnoses.”
Even as clinicians struggle with the challenging SARS-CoV-2 learning curve, Dr. Molloy and associates urged them to continue to strive for high-value care, with an unwavering focus on using only necessary resources, a stewardship the pandemic has shown to be critical.
“The COVID-19 pandemic has been an incredibly stressful time for physicians and for families,” Dr. Molloy said. “COVID-19 and related conditions like MIS-C are new, and we are learning more and more about them every week. These diagnoses are understandably on the minds of physicians and families when children present with fever.” Notwithstanding, the boy’s case underscores the need for clinicians to consider alternate diagnoses and the value of the care provided.
Impact of bias
Dr. Molloy’s group brings home the cognitive biases practitioners often suffer from, including anchoring and confirmation bias and diagnostic momentum, according to J. Howard Smart, MD, chief of pediatrics at Sharp Mary Birch Hospital for Women and Newborns, San Diego, and an assistant clinical professor of pediatrics at University of California, San Diego.
“But it is one thing to recognize these in retrospect and quite another to consider whether they may be happening to you yourself in real time,” he said in an interview. “It is almost as if we need to have a ‘time out,’ where we stop and ask ourselves whether there is something else that could be explaining our patient’s presentation, something that would be more common and more likely to be occurring.”
According to Dr. Smart, who was not involved in Dr. Molloy’s study, the team’s premature diagnostic focus on MIS-C was almost the inverse of what typically happens with KD. “It is usually the case that Kawasaki disease does not enter the differential diagnosis until late in the course of the fever, typically on day 5 or later, when it may have been better to think of it earlier,” he said.
In the second article, Andrea Dean, MD, of the department of pediatrics at Baylor College of Medicine and Texas Children’s Hospital, both in Houston, and colleagues outlined the cases of five patients aged 8-17 years who were hospitalized in May 2020 for suspected MIS-C. They exhibited inflammatory and other concerning indicators but were eventually discharged with a diagnosis of murine typhus.
This flea-borne infection, most commonly reported in the United States in the southeastern Gulf Coast region, Hawaii, and California, is often associated with a triad of fever, rash, and headache.
Cases have been rising in southern Texas, and Dr. Dean and colleagues postulated that school closures and social distancing may have increased exposure as a result of children spending more time outdoors or with pets. “Alternatively, parental concern for SARS-CoV-2 infection could mean children with symptoms are presenting to care and being referred or admitted to the hospital more frequently due to provider concern for MIS-C,” they wrote.
Cardiac involvement
The most concerning of the five cases in terms of possible MIS-C, Dr. Dean said in an interview, was that of a 12-year-old boy who had fever for 6 days in association with headache, eczematous rash, dry lips, and conjunctivitis. Laboratory tests showed a mildly elevated C-reactive protein level, hyponatremia, and thrombocytopenia, as well as sterile pyuria and mildly elevated prothrombin time. He was treated empirically with doxycycline, and his fever resolved over the next 24 hours.
An echocardiogram at initial evaluation, however, revealed mild dilation of the left anterior descending and right coronary arteries, which led to the administration of intravenous immunoglobulin and aspirin for atypical KD, in contrast to MIS-C. The authors postulated that mild cardiac involvement in disorders other than MIS-C and KD may be underrecognized.
The lesson from these cases, Dr. Dean and associates concluded, is that hospitalists must maintain a wide differential diagnosis when assessing a child with prolonged fever and evidence of systemic inflammation. The CDC stipulates that a diagnosis of MIS-C requires the absence of a plausible alternative diagnosis.
In addition to common viral, bacterial, and noninfectious disorders, a range of regional endemic rickettsial and parasitic infections must be considered as alternative diagnoses to MIS-C. “Many of these diseases cannot be reliably differentiated from MIS-C on presentation, and as community exposure to SARS-CoV-2 grows, hospitalists should be prepared to admit febrile children with evidence of systemic inflammation for brief observation periods to evaluate for MIS-C,” Dr. Dean’s group wrote. In this context, however, empiric treatment for common or even uncommon infectious diseases may avoid overdiagnosis and overtreatment of MIS-C as well as improve patient outcomes.
“We do have specific MIS-C guidelines at our institution,” Dr. Dean said, “but like all institutions, we are dealing with the broad definition of MIS-C according to the World Health Organization and the CDC, which is really the takeaway from this paper.”
More difficult differentiation
Both groups of authors pointed out that, as SARS-CoV-2 spreads throughout a community, a higher percentage of the population will have positive results on antibody testing, and such results will become less useful for differentiating between MIS-C and other conditions.
Despite these series’ cautionary lessons, other experts point to the critical importance of including MIS-C early on in the interest of efficient diagnosis and therapy. “In the cases cited, other pathologies were evaluated for and treated accordingly,” said Kara Gross Margolis, MD, AGAF, an associate professor of pediatrics in the division of pediatric gastroenterology, hepatology, and nutrition at Morgan Stanley Children’s Hospital,New York. “These papers stress the need for a balance that is important, and all potential diagnoses need to be considered, but MIS-C, due to its potential severe consequences, also needs to be on our differential now.”
In her view, as this new high-morbidity entity becomes more widespread during the pandemic, it will be increasingly important to keep this condition on the diagnostic radar.
Interestingly, in a converse example of diagnostic clouding, Dr. Gross Margolis’s group reported (Gastroenterology. 2020 Oct;159[4]:1571-4.e2) last year on a pediatric case series in which the presence of gastrointestinal symptoms in children with COVID-19–related MIS-C muddied the diagnosis by confusing this potentially severe syndrome with more common and less toxic gastrointestinal infections.
According to Dr. Smart, although the two reports don’t offer evidence for a particular diagnostic practice, they can inform the decision-making process. “It may be that we will have enough evidence shortly to say what the best practice is regarding diagnostic evaluation of possible MIS-C cases,” he said. “Until then, we must remember that common things occur commonly, even during a global pandemic.”
Neither of the two reports received any specific funding. The authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Mask mandates reduced COVID-19 hospitalizations
States that implemented mask mandates in 2020 saw a decline in the growth of COVID-19 hospitalizations between March and October 2020, according to a new study published Feb. 5 in the CDC’s Morbidity and Mortality Weekly Report.
Hospitalization growth rates declined by 5.5 percentage points for adults between ages 18-64 about 3 weeks after the mandates were implemented, compared with climbing growth rates in the 4 weeks before mandates.
CDC Director Rochelle Walensky said she was pleased to see the results, but that it’s “too early” to tell whether President Joe Biden’s recent mask orders have had an effect on cases and hospitalizations in 2021.
“We’re going to be watching the mask data very carefully,” she said during a news briefing with the White House COVID-19 Response Team on Feb. 5. “I think it’s probably still a bit too early to tell, but I’m encouraged with the decrease in case rates right now.”
In another study published Feb. 5 in the Morbidity and Mortality Weekly Report, trained observers tracked mask use at six universities with mask mandates between September and November 2020. Overall, observers reported that about 92% of people wore masks correctly indoors, which varied based on the type of mask.
About 97% of people used N95 masks correctly, compared with 92% who used cloth masks, and 79% who used bandanas, scarves, or neck gaiters. Cloth masks were most common, and bandanas and scarves were least common.
The Biden administration is considering whether to send masks directly to American households to encourage people to wear them, according to NBC News. The White House COVID-19 Response Team is debating the logistics of mailing out masks, including how many to send and what the mask material would be, the news outlet reported.
Wisconsin Gov. Tony Evers reissued a new statewide mask mandate on Feb. 4, just an hour after the Republican-controlled legislature voted to repeal his previous mandate, according to The Associated Press. Gov. Evers said his priority is to keep people safe and that wearing a mask is the easiest way to do so.
“If the legislature keeps playing politics and we don’t keep wearing masks, we’re going to see more preventable deaths,” he said. “It’s going to take even longer to get our state and our economy back on track.”
A version of this article first appeared on WebMD.com.
States that implemented mask mandates in 2020 saw a decline in the growth of COVID-19 hospitalizations between March and October 2020, according to a new study published Feb. 5 in the CDC’s Morbidity and Mortality Weekly Report.
Hospitalization growth rates declined by 5.5 percentage points for adults between ages 18-64 about 3 weeks after the mandates were implemented, compared with climbing growth rates in the 4 weeks before mandates.
CDC Director Rochelle Walensky said she was pleased to see the results, but that it’s “too early” to tell whether President Joe Biden’s recent mask orders have had an effect on cases and hospitalizations in 2021.
“We’re going to be watching the mask data very carefully,” she said during a news briefing with the White House COVID-19 Response Team on Feb. 5. “I think it’s probably still a bit too early to tell, but I’m encouraged with the decrease in case rates right now.”
In another study published Feb. 5 in the Morbidity and Mortality Weekly Report, trained observers tracked mask use at six universities with mask mandates between September and November 2020. Overall, observers reported that about 92% of people wore masks correctly indoors, which varied based on the type of mask.
About 97% of people used N95 masks correctly, compared with 92% who used cloth masks, and 79% who used bandanas, scarves, or neck gaiters. Cloth masks were most common, and bandanas and scarves were least common.
The Biden administration is considering whether to send masks directly to American households to encourage people to wear them, according to NBC News. The White House COVID-19 Response Team is debating the logistics of mailing out masks, including how many to send and what the mask material would be, the news outlet reported.
Wisconsin Gov. Tony Evers reissued a new statewide mask mandate on Feb. 4, just an hour after the Republican-controlled legislature voted to repeal his previous mandate, according to The Associated Press. Gov. Evers said his priority is to keep people safe and that wearing a mask is the easiest way to do so.
“If the legislature keeps playing politics and we don’t keep wearing masks, we’re going to see more preventable deaths,” he said. “It’s going to take even longer to get our state and our economy back on track.”
A version of this article first appeared on WebMD.com.
States that implemented mask mandates in 2020 saw a decline in the growth of COVID-19 hospitalizations between March and October 2020, according to a new study published Feb. 5 in the CDC’s Morbidity and Mortality Weekly Report.
Hospitalization growth rates declined by 5.5 percentage points for adults between ages 18-64 about 3 weeks after the mandates were implemented, compared with climbing growth rates in the 4 weeks before mandates.
CDC Director Rochelle Walensky said she was pleased to see the results, but that it’s “too early” to tell whether President Joe Biden’s recent mask orders have had an effect on cases and hospitalizations in 2021.
“We’re going to be watching the mask data very carefully,” she said during a news briefing with the White House COVID-19 Response Team on Feb. 5. “I think it’s probably still a bit too early to tell, but I’m encouraged with the decrease in case rates right now.”
In another study published Feb. 5 in the Morbidity and Mortality Weekly Report, trained observers tracked mask use at six universities with mask mandates between September and November 2020. Overall, observers reported that about 92% of people wore masks correctly indoors, which varied based on the type of mask.
About 97% of people used N95 masks correctly, compared with 92% who used cloth masks, and 79% who used bandanas, scarves, or neck gaiters. Cloth masks were most common, and bandanas and scarves were least common.
The Biden administration is considering whether to send masks directly to American households to encourage people to wear them, according to NBC News. The White House COVID-19 Response Team is debating the logistics of mailing out masks, including how many to send and what the mask material would be, the news outlet reported.
Wisconsin Gov. Tony Evers reissued a new statewide mask mandate on Feb. 4, just an hour after the Republican-controlled legislature voted to repeal his previous mandate, according to The Associated Press. Gov. Evers said his priority is to keep people safe and that wearing a mask is the easiest way to do so.
“If the legislature keeps playing politics and we don’t keep wearing masks, we’re going to see more preventable deaths,” he said. “It’s going to take even longer to get our state and our economy back on track.”
A version of this article first appeared on WebMD.com.
Children in ICU for COVID-19 likely to be older, Black, and asthmatic
Little has been known about children sick enough with COVID-19 to require intensive care because such patients are relatively few, but preliminary data analyzed from a nationwide registry indicate that they are more likely to be older, to be Black, and to have asthma.
Gastrointestinal distress is also more common in children with severe COVID-19, according to research by Sandeep Tripathi, MD. Dr. Tripathi, a pediatric intensivist and associate professor at the University of Illinois at Peoria, presented the findings on Feb. 3 at the Society for Critical Care Medicine (SCCM) 2021 Critical Care Congress.
Registry data gathered from 49 sites
Results from the SCCM’s VIRUS: COVID-19 Registry, which involved data from 49 sites, included 181 children admitted to an intensive care unit between February and July 2020. Those in the ICU were older than patients who did not receive care in the ICU (10 years vs. 3.67 years; P < .01) and were more likely to be Black (28.8% vs. 17.8%; P = .02).
More of the patients who required intensive care had preexisting conditions (58.2% vs. 44.3%; P = .01), the most common of which was asthma.
For both the ICU patients and the non-ICU group, the most common presenting symptom was fever.
Symptoms that were more common among children needing ICU care included nausea/vomiting (38.4% vs. 22.1%; P < .01), dyspnea (31.8% vs. 17.7%; P < .01), and abdominal pain (25.2% vs. 14.1%; P < .01).
Significantly higher proportions of ICU patients had multisystem inflammatory syndrome of childhood (MIS-C) (44.2% vs. 6.8%; P < .01) and acute kidney injury (9.34% vs. 1.7%; P < .01).
“The children who presented with MIS-C tended to be much sicker than children who present with just COVID,” Dr. Tripathi said in an interview.
In this analysis, among children in ICUs with COVID, the mortality rate was 4%, Dr. Tripathi said.
He said he hopes the information, which will be periodically published with updated data, will raise awareness of which children might be likely to experience progression to severe disease.
“The information may help physicians be more mindful of deterioration in those patients and be more aggressive in their management,” he said. When children are brought to the emergency department with the features this analysis highlights, he said, “physicians should have a low threshold for treating or admitting the patients.”
Another study that was presented on Feb. 3 in parallel with the registry study described patterns of illness among 68 children hospitalized with COVID-19 in a tertiary-care pediatric center.
In that analysis, Meghana Nadiger, MD, a critical care fellow with Nicklaus Children’s Hospital in Miami, found that all patients admitted to the pediatric ICU (n = 17) had either MIS-C or severe illness and COVID-19-related Kawasaki-like disease.
The investigators also found that the patients with serious illness were more commonly adolescents with elevated body mass index (73%). In this study, 83.8% of the hospitalized children were Hispanic. They also found that 88.8% of the children older than 2 years who had been hospitalized with COVID-19 were overweight or obese, with a BMI >25 kg/m2.
Jerry Zimmerman, MD, PhD, SCCM’s immediate past president, said in an interview that he found it interesting that in the Nadiger study, “All of the children with severe illness had MIS-C as compared to adults, who typically are critically ill with severe acute respiratory distress syndrome.” Dr. Zimmerman was not involved in either study.
He said that although the high percentage of Hispanic patients in the hospitalized population may reflect the high percentage of Hispanic children in the Miami area, it may also reflect challenges of controlling the disease in the Hispanic community. Such challenges might include shortages of personal protective equipment, poorer access to health care, and difficulty in social distancing.
Dr. Zimmerman pointed out that obesity is an important risk factor for COVID-19 and that according to the Centers for Disease Control and Prevention, childhood obesity is much more common among Hispanics (25.8%) and non-Hispanic Blacks persons (22.0%) compared with non-Hispanic White persons (14.1%).
The VIRUS registry is funded in part by the Gordon and Betty Moore Foundation and Janssen Research and Development. Dr. Tripathi, Dr. Nadiger, and Dr. Zimmerman have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Little has been known about children sick enough with COVID-19 to require intensive care because such patients are relatively few, but preliminary data analyzed from a nationwide registry indicate that they are more likely to be older, to be Black, and to have asthma.
Gastrointestinal distress is also more common in children with severe COVID-19, according to research by Sandeep Tripathi, MD. Dr. Tripathi, a pediatric intensivist and associate professor at the University of Illinois at Peoria, presented the findings on Feb. 3 at the Society for Critical Care Medicine (SCCM) 2021 Critical Care Congress.
Registry data gathered from 49 sites
Results from the SCCM’s VIRUS: COVID-19 Registry, which involved data from 49 sites, included 181 children admitted to an intensive care unit between February and July 2020. Those in the ICU were older than patients who did not receive care in the ICU (10 years vs. 3.67 years; P < .01) and were more likely to be Black (28.8% vs. 17.8%; P = .02).
More of the patients who required intensive care had preexisting conditions (58.2% vs. 44.3%; P = .01), the most common of which was asthma.
For both the ICU patients and the non-ICU group, the most common presenting symptom was fever.
Symptoms that were more common among children needing ICU care included nausea/vomiting (38.4% vs. 22.1%; P < .01), dyspnea (31.8% vs. 17.7%; P < .01), and abdominal pain (25.2% vs. 14.1%; P < .01).
Significantly higher proportions of ICU patients had multisystem inflammatory syndrome of childhood (MIS-C) (44.2% vs. 6.8%; P < .01) and acute kidney injury (9.34% vs. 1.7%; P < .01).
“The children who presented with MIS-C tended to be much sicker than children who present with just COVID,” Dr. Tripathi said in an interview.
In this analysis, among children in ICUs with COVID, the mortality rate was 4%, Dr. Tripathi said.
He said he hopes the information, which will be periodically published with updated data, will raise awareness of which children might be likely to experience progression to severe disease.
“The information may help physicians be more mindful of deterioration in those patients and be more aggressive in their management,” he said. When children are brought to the emergency department with the features this analysis highlights, he said, “physicians should have a low threshold for treating or admitting the patients.”
Another study that was presented on Feb. 3 in parallel with the registry study described patterns of illness among 68 children hospitalized with COVID-19 in a tertiary-care pediatric center.
In that analysis, Meghana Nadiger, MD, a critical care fellow with Nicklaus Children’s Hospital in Miami, found that all patients admitted to the pediatric ICU (n = 17) had either MIS-C or severe illness and COVID-19-related Kawasaki-like disease.
The investigators also found that the patients with serious illness were more commonly adolescents with elevated body mass index (73%). In this study, 83.8% of the hospitalized children were Hispanic. They also found that 88.8% of the children older than 2 years who had been hospitalized with COVID-19 were overweight or obese, with a BMI >25 kg/m2.
Jerry Zimmerman, MD, PhD, SCCM’s immediate past president, said in an interview that he found it interesting that in the Nadiger study, “All of the children with severe illness had MIS-C as compared to adults, who typically are critically ill with severe acute respiratory distress syndrome.” Dr. Zimmerman was not involved in either study.
He said that although the high percentage of Hispanic patients in the hospitalized population may reflect the high percentage of Hispanic children in the Miami area, it may also reflect challenges of controlling the disease in the Hispanic community. Such challenges might include shortages of personal protective equipment, poorer access to health care, and difficulty in social distancing.
Dr. Zimmerman pointed out that obesity is an important risk factor for COVID-19 and that according to the Centers for Disease Control and Prevention, childhood obesity is much more common among Hispanics (25.8%) and non-Hispanic Blacks persons (22.0%) compared with non-Hispanic White persons (14.1%).
The VIRUS registry is funded in part by the Gordon and Betty Moore Foundation and Janssen Research and Development. Dr. Tripathi, Dr. Nadiger, and Dr. Zimmerman have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Little has been known about children sick enough with COVID-19 to require intensive care because such patients are relatively few, but preliminary data analyzed from a nationwide registry indicate that they are more likely to be older, to be Black, and to have asthma.
Gastrointestinal distress is also more common in children with severe COVID-19, according to research by Sandeep Tripathi, MD. Dr. Tripathi, a pediatric intensivist and associate professor at the University of Illinois at Peoria, presented the findings on Feb. 3 at the Society for Critical Care Medicine (SCCM) 2021 Critical Care Congress.
Registry data gathered from 49 sites
Results from the SCCM’s VIRUS: COVID-19 Registry, which involved data from 49 sites, included 181 children admitted to an intensive care unit between February and July 2020. Those in the ICU were older than patients who did not receive care in the ICU (10 years vs. 3.67 years; P < .01) and were more likely to be Black (28.8% vs. 17.8%; P = .02).
More of the patients who required intensive care had preexisting conditions (58.2% vs. 44.3%; P = .01), the most common of which was asthma.
For both the ICU patients and the non-ICU group, the most common presenting symptom was fever.
Symptoms that were more common among children needing ICU care included nausea/vomiting (38.4% vs. 22.1%; P < .01), dyspnea (31.8% vs. 17.7%; P < .01), and abdominal pain (25.2% vs. 14.1%; P < .01).
Significantly higher proportions of ICU patients had multisystem inflammatory syndrome of childhood (MIS-C) (44.2% vs. 6.8%; P < .01) and acute kidney injury (9.34% vs. 1.7%; P < .01).
“The children who presented with MIS-C tended to be much sicker than children who present with just COVID,” Dr. Tripathi said in an interview.
In this analysis, among children in ICUs with COVID, the mortality rate was 4%, Dr. Tripathi said.
He said he hopes the information, which will be periodically published with updated data, will raise awareness of which children might be likely to experience progression to severe disease.
“The information may help physicians be more mindful of deterioration in those patients and be more aggressive in their management,” he said. When children are brought to the emergency department with the features this analysis highlights, he said, “physicians should have a low threshold for treating or admitting the patients.”
Another study that was presented on Feb. 3 in parallel with the registry study described patterns of illness among 68 children hospitalized with COVID-19 in a tertiary-care pediatric center.
In that analysis, Meghana Nadiger, MD, a critical care fellow with Nicklaus Children’s Hospital in Miami, found that all patients admitted to the pediatric ICU (n = 17) had either MIS-C or severe illness and COVID-19-related Kawasaki-like disease.
The investigators also found that the patients with serious illness were more commonly adolescents with elevated body mass index (73%). In this study, 83.8% of the hospitalized children were Hispanic. They also found that 88.8% of the children older than 2 years who had been hospitalized with COVID-19 were overweight or obese, with a BMI >25 kg/m2.
Jerry Zimmerman, MD, PhD, SCCM’s immediate past president, said in an interview that he found it interesting that in the Nadiger study, “All of the children with severe illness had MIS-C as compared to adults, who typically are critically ill with severe acute respiratory distress syndrome.” Dr. Zimmerman was not involved in either study.
He said that although the high percentage of Hispanic patients in the hospitalized population may reflect the high percentage of Hispanic children in the Miami area, it may also reflect challenges of controlling the disease in the Hispanic community. Such challenges might include shortages of personal protective equipment, poorer access to health care, and difficulty in social distancing.
Dr. Zimmerman pointed out that obesity is an important risk factor for COVID-19 and that according to the Centers for Disease Control and Prevention, childhood obesity is much more common among Hispanics (25.8%) and non-Hispanic Blacks persons (22.0%) compared with non-Hispanic White persons (14.1%).
The VIRUS registry is funded in part by the Gordon and Betty Moore Foundation and Janssen Research and Development. Dr. Tripathi, Dr. Nadiger, and Dr. Zimmerman have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Rollout of COVID-19 monoclonal antibodies lacked unified plan: expert panel
Monoclonal antibodies (mAbs) to treat COVID-19 are in ample supply, but scant evidence on their effectiveness, paltry reimbursement, and a lack of a planned infrastructure to administer them has led to major underutilization of these potentially useful therapies, according to a new report from The National Academies of Sciences, Engineering, and Medicine.
The 35-page report described missed opportunities to work with states and hospitals to establish trust with clinicians and patients and to set up an infusion infrastructure to funnel patients to sites. Though the therapies still need more study, they should be an option for the right patient at the right time, said the National Academies experts in their report, Rapid Expert Consultation on Allocating COVID-19 Monoclonal Antibody Therapies and Other Novel Therapeutics.
“No potentially eligible patient should be left uninformed, and no eligible patient should be denied access, if there are doses available and the patient and doctor agree it is a reasonable course,” they concluded. The report also noted that underuse, and in particular underuse by members of vulnerable and underserved communities “raises concerns about exacerbating already dramatic health disparities.”
The federal government has spent $375 million on Eli Lilly’s bamlanivimab and $450 million on Regeneron’s casirivimab plus imdevimab cocktail, and agreed last month to spend as much as $2.6 billion more on up to 1.25 million additional doses.
Some 785,000 doses of the two therapeutics have been produced and about a half million have been distributed to states. But about three quarters have gone unused. The U.S. Department of Health & Human Services has launched an online treatment locater to try to spur interest in the therapies.
But the federal government hasn’t addressed some of the basic barriers to use of the monoclonals, said the National Academies experts.
“Lack of awareness, interest, and confidence in COVID-19 mAb therapies among patients and providers are major issues,” they said in the report. Patients who have tested positive might not want to travel to an infusion site, while others might not have access to health care or only seek such treatments when it’s too late. Some who are eligible might not have the time, resources, or transportation to go to a site and sit through a 2-hour treatment.
In addition, “the supply and availability of infusion centers and personnel was identified as a greater constraint than the supply of COVID-19 mAbs,” said the report.
Cost a big impediment
While the federal government has covered the cost of the therapies, hospitals and patients inevitably incur related costs.
“The fragmented payment system in the United States has not provided adequate support to cover the spectrum of costs associated with COVID-19 mAb therapies,” said the report. That is compounded by chronic underfunding and restrictions on federally qualified health centers for community health, the report said.
Patients may have to pay for testing, office visits, follow-up appointments, transportation to and from the infusion site, and potentially a copay for the administration of the drug.
While Medicare pays hospitals $309 per infusion, that might not be enough, especially if a hospital or other site had to build out a new infusion center, the report shows. For clinicians, the administrative payment under Medicare Part B does “not cover the total practice cost to furnish infusion services, resulting in a substantial cost-reimbursement disparity,” the report states.
In addition, there are no specific codes for observing patients during the 2-hour procedure.
“The established Medicare payment rate for furnishing COVID-19 mAb therapies does not cover the cost associated with coordinating care for those patients, nor does it justify the risk and opportunity costs associated with investing in infrastructure modifications to safely integrate COVID-19 patients into existing facilities or building temporary infusion capacity,” the report concluded.
More data needed
The U.S. Food and Drug Administration issued emergency-use authorizations (EUAs) for the two monoclonal therapies based on phase 2 trial data, and that leaves a lot of uncertainty, noted the National Academies.
In trials, both therapies reduced COVID-19-related hospitalizations and emergency room visits within 28 days after treatment among patients at high risk of progression, compared with those who received placebo.
But clinicians aren’t certain about who should use the monoclonals, said the report. The underuse has in turn led to trouble collecting data – either through ongoing trials or in starting new trials.
The National Academies recommended allocating the monoclonal antibodies in a way that would give rise to better data collection to inform clinicians. Payers could support the development of a core data platform or registry, or Medicare could develop pilot trials, said the report.
Lilly and UnitedHealth Group are collaborating on a study in high-risk Medicare patients, according to Reuters. Patients who test positive will be given bamlanivimab at home.
“Building infusion capacity and developing the evidence base about the impact of COVID-19 mAbs on clinical outcomes other than hospitalization, including mortality, are the most promising strategies for increasing effective utilization moving forward,” stated the National Academies report.
A version of this article first appeared on Medscape.com.
Monoclonal antibodies (mAbs) to treat COVID-19 are in ample supply, but scant evidence on their effectiveness, paltry reimbursement, and a lack of a planned infrastructure to administer them has led to major underutilization of these potentially useful therapies, according to a new report from The National Academies of Sciences, Engineering, and Medicine.
The 35-page report described missed opportunities to work with states and hospitals to establish trust with clinicians and patients and to set up an infusion infrastructure to funnel patients to sites. Though the therapies still need more study, they should be an option for the right patient at the right time, said the National Academies experts in their report, Rapid Expert Consultation on Allocating COVID-19 Monoclonal Antibody Therapies and Other Novel Therapeutics.
“No potentially eligible patient should be left uninformed, and no eligible patient should be denied access, if there are doses available and the patient and doctor agree it is a reasonable course,” they concluded. The report also noted that underuse, and in particular underuse by members of vulnerable and underserved communities “raises concerns about exacerbating already dramatic health disparities.”
The federal government has spent $375 million on Eli Lilly’s bamlanivimab and $450 million on Regeneron’s casirivimab plus imdevimab cocktail, and agreed last month to spend as much as $2.6 billion more on up to 1.25 million additional doses.
Some 785,000 doses of the two therapeutics have been produced and about a half million have been distributed to states. But about three quarters have gone unused. The U.S. Department of Health & Human Services has launched an online treatment locater to try to spur interest in the therapies.
But the federal government hasn’t addressed some of the basic barriers to use of the monoclonals, said the National Academies experts.
“Lack of awareness, interest, and confidence in COVID-19 mAb therapies among patients and providers are major issues,” they said in the report. Patients who have tested positive might not want to travel to an infusion site, while others might not have access to health care or only seek such treatments when it’s too late. Some who are eligible might not have the time, resources, or transportation to go to a site and sit through a 2-hour treatment.
In addition, “the supply and availability of infusion centers and personnel was identified as a greater constraint than the supply of COVID-19 mAbs,” said the report.
Cost a big impediment
While the federal government has covered the cost of the therapies, hospitals and patients inevitably incur related costs.
“The fragmented payment system in the United States has not provided adequate support to cover the spectrum of costs associated with COVID-19 mAb therapies,” said the report. That is compounded by chronic underfunding and restrictions on federally qualified health centers for community health, the report said.
Patients may have to pay for testing, office visits, follow-up appointments, transportation to and from the infusion site, and potentially a copay for the administration of the drug.
While Medicare pays hospitals $309 per infusion, that might not be enough, especially if a hospital or other site had to build out a new infusion center, the report shows. For clinicians, the administrative payment under Medicare Part B does “not cover the total practice cost to furnish infusion services, resulting in a substantial cost-reimbursement disparity,” the report states.
In addition, there are no specific codes for observing patients during the 2-hour procedure.
“The established Medicare payment rate for furnishing COVID-19 mAb therapies does not cover the cost associated with coordinating care for those patients, nor does it justify the risk and opportunity costs associated with investing in infrastructure modifications to safely integrate COVID-19 patients into existing facilities or building temporary infusion capacity,” the report concluded.
More data needed
The U.S. Food and Drug Administration issued emergency-use authorizations (EUAs) for the two monoclonal therapies based on phase 2 trial data, and that leaves a lot of uncertainty, noted the National Academies.
In trials, both therapies reduced COVID-19-related hospitalizations and emergency room visits within 28 days after treatment among patients at high risk of progression, compared with those who received placebo.
But clinicians aren’t certain about who should use the monoclonals, said the report. The underuse has in turn led to trouble collecting data – either through ongoing trials or in starting new trials.
The National Academies recommended allocating the monoclonal antibodies in a way that would give rise to better data collection to inform clinicians. Payers could support the development of a core data platform or registry, or Medicare could develop pilot trials, said the report.
Lilly and UnitedHealth Group are collaborating on a study in high-risk Medicare patients, according to Reuters. Patients who test positive will be given bamlanivimab at home.
“Building infusion capacity and developing the evidence base about the impact of COVID-19 mAbs on clinical outcomes other than hospitalization, including mortality, are the most promising strategies for increasing effective utilization moving forward,” stated the National Academies report.
A version of this article first appeared on Medscape.com.
Monoclonal antibodies (mAbs) to treat COVID-19 are in ample supply, but scant evidence on their effectiveness, paltry reimbursement, and a lack of a planned infrastructure to administer them has led to major underutilization of these potentially useful therapies, according to a new report from The National Academies of Sciences, Engineering, and Medicine.
The 35-page report described missed opportunities to work with states and hospitals to establish trust with clinicians and patients and to set up an infusion infrastructure to funnel patients to sites. Though the therapies still need more study, they should be an option for the right patient at the right time, said the National Academies experts in their report, Rapid Expert Consultation on Allocating COVID-19 Monoclonal Antibody Therapies and Other Novel Therapeutics.
“No potentially eligible patient should be left uninformed, and no eligible patient should be denied access, if there are doses available and the patient and doctor agree it is a reasonable course,” they concluded. The report also noted that underuse, and in particular underuse by members of vulnerable and underserved communities “raises concerns about exacerbating already dramatic health disparities.”
The federal government has spent $375 million on Eli Lilly’s bamlanivimab and $450 million on Regeneron’s casirivimab plus imdevimab cocktail, and agreed last month to spend as much as $2.6 billion more on up to 1.25 million additional doses.
Some 785,000 doses of the two therapeutics have been produced and about a half million have been distributed to states. But about three quarters have gone unused. The U.S. Department of Health & Human Services has launched an online treatment locater to try to spur interest in the therapies.
But the federal government hasn’t addressed some of the basic barriers to use of the monoclonals, said the National Academies experts.
“Lack of awareness, interest, and confidence in COVID-19 mAb therapies among patients and providers are major issues,” they said in the report. Patients who have tested positive might not want to travel to an infusion site, while others might not have access to health care or only seek such treatments when it’s too late. Some who are eligible might not have the time, resources, or transportation to go to a site and sit through a 2-hour treatment.
In addition, “the supply and availability of infusion centers and personnel was identified as a greater constraint than the supply of COVID-19 mAbs,” said the report.
Cost a big impediment
While the federal government has covered the cost of the therapies, hospitals and patients inevitably incur related costs.
“The fragmented payment system in the United States has not provided adequate support to cover the spectrum of costs associated with COVID-19 mAb therapies,” said the report. That is compounded by chronic underfunding and restrictions on federally qualified health centers for community health, the report said.
Patients may have to pay for testing, office visits, follow-up appointments, transportation to and from the infusion site, and potentially a copay for the administration of the drug.
While Medicare pays hospitals $309 per infusion, that might not be enough, especially if a hospital or other site had to build out a new infusion center, the report shows. For clinicians, the administrative payment under Medicare Part B does “not cover the total practice cost to furnish infusion services, resulting in a substantial cost-reimbursement disparity,” the report states.
In addition, there are no specific codes for observing patients during the 2-hour procedure.
“The established Medicare payment rate for furnishing COVID-19 mAb therapies does not cover the cost associated with coordinating care for those patients, nor does it justify the risk and opportunity costs associated with investing in infrastructure modifications to safely integrate COVID-19 patients into existing facilities or building temporary infusion capacity,” the report concluded.
More data needed
The U.S. Food and Drug Administration issued emergency-use authorizations (EUAs) for the two monoclonal therapies based on phase 2 trial data, and that leaves a lot of uncertainty, noted the National Academies.
In trials, both therapies reduced COVID-19-related hospitalizations and emergency room visits within 28 days after treatment among patients at high risk of progression, compared with those who received placebo.
But clinicians aren’t certain about who should use the monoclonals, said the report. The underuse has in turn led to trouble collecting data – either through ongoing trials or in starting new trials.
The National Academies recommended allocating the monoclonal antibodies in a way that would give rise to better data collection to inform clinicians. Payers could support the development of a core data platform or registry, or Medicare could develop pilot trials, said the report.
Lilly and UnitedHealth Group are collaborating on a study in high-risk Medicare patients, according to Reuters. Patients who test positive will be given bamlanivimab at home.
“Building infusion capacity and developing the evidence base about the impact of COVID-19 mAbs on clinical outcomes other than hospitalization, including mortality, are the most promising strategies for increasing effective utilization moving forward,” stated the National Academies report.
A version of this article first appeared on Medscape.com.
Pediatric minority patients less likely to undergo ED imaging
Significant racial and ethnic differences in diagnostic imaging rates exist among children receiving care in pediatric EDs across the United States, Jennifer R. Marin, MD, of the University of Pittsburgh Medical Center Children’s Hospital of Pittsburgh, and associates reported.
Specifically, visits with non-Hispanic Black and Hispanic patients less frequently included radiography, CT, ultrasonography, and MRI than those of non-Hispanic White patients. The findings persisted across most diagnostic groups, even when stratified according to insurance type, Dr. Marin and colleagues reported in a multicenter cross-sectional study in JAMA Network Open.
The authors collected administrative data from the Pediatric Health Information System on 44 tertiary care children’s hospitals in 17 major metropolitan areas across the United States. They evaluated a total of 13,087,522 ED visits by 6,230,911 patients that occurred between Jan. 1, 2016, and Dec. 31, 2019. Of these, 28.2% included at least one imaging study. Altogether, 33.5% were performed on non-Hispanic White children, compared with just 24.1% of non-Hispanic Black children (adjusted odds ratio, 0.82) and 26.1% of Hispanic children (aOR, 0.87). After adjusting for relevant confounding factors, non-Hispanic Black and Hispanic children were less likely to have any imaging at all during their visits.
“Our findings suggest that a child’s race and ethnicity may be independently associated with the decision to perform imaging during ED visits,” Dr. Marin and associates said, adding that “the differential use of diagnostic imaging by race/ethnicity may reflect underuse of imaging in non-Hispanic Black and Hispanic children, or alternatively, overuse in non-Hispanic White children.”
Overuse vs. underuse: Racial bias or parental anxiety?
Overuse of imaging carries its own risks, but underuse can lead to misdiagnosis, the need for additional care, and possibly worse outcomes in the long run, Dr. Marin and colleagues explained. “Although we were unable to discern underuse from overuse using an administrative database, it is likely that much of the imaging in White children is unnecessary.”
Higher parental anxiety was just one of the explanations the authors offered for excessive imaging in White children. Especially in cases of diagnostic imaging for head trauma, one survey of adult ED patients showed that the peace of mind CT offers with its more definitive diagnosis was worth the additional possible risk of radiation.
Language barriers in non–English-speaking patients may also affect likelihood of testing as part of an ED visit.
Implicit physician racial bias, which can be amplified under the stress of working in an ED, can affect patient interactions, treatment decisions and adherence, and ultimately overall health outcomes, the authors noted. The goal in ensuring parity is to routinely follow clinical guidelines and use objective scoring tools that minimize subjectivity. At the institutional level, internal quality assurance evaluations go a long way toward understanding and limiting bias.
Historically, White patients are more likely than minority patients to have a medical home, which can influence whether ED physicians order imaging studies and whether imaging of White patients may have been triggered by a primary care physician referral, Dr. Marin and associates said.
In an accompanying editorial, Anupam B. Kharbanda, MD, said these findings are “consistent with decades of previous research documenting inequalities in health care delivery ... [and] must be examined in the context of inequities within the social framework of a community.”
Going back to the drawing board
“Physicians, researchers, and health care leaders must partner with the communities they serve to develop and implement interventions to address these substantial inequities in care,” said Dr. Kharbanda, pediatric emergency medicine physician at Children’s Minnesota Hospital, Minneapolis. As previous studies have demonstrated, implicit bias and antiracism training are needed to help physicians develop empathy so they are better equipped to help patients and families in a multicultural environment. Partnering with community-based organizations to ensure that care is more community centered, as has been done successfully within the Kaiser Permanente health system, for example, and employing a more diverse workforce that mirrors the populations cared for will go a long way.
Citing a 1966 speech of the late Dr. Martin Luther King, Jr., in which he said: “Of all the forms of inequality, injustice in health care is the most shocking and inhumane,” Dr. Kharbanda urged clinicians to not only hear but believe these words and act on them by working in partnerships with the communities they serve.
In a separate interview, Walter Palmer, MD, pediatric emergency medicine fellow at Children’s National Medical Center, Washington, noted: “This study’s findings are disappointing and yet not at all unexpected, as the authors convincingly identify yet another step at which patients of color are treated unequally in the U.S. health care system. It highlights a frightening truth: That we are all at risk of letting invisible implicit biases impact our clinical decision-making process. This is especially true in the busy emergency department environment, where pressure to make swift decisions regarding diagnostic workup and management invites the influence of imperceptible biases. It is now incumbent upon us as health care providers to monitor our personal and departmental patterns of practice for areas in which we can improve racial health equity and become advocates for the children and families who entrust us with their care.”
The authors reported multiple financial disclosures. Dr. Kharbanda and Dr. Palmer had no conflicts of interest and reported no disclosures.
Significant racial and ethnic differences in diagnostic imaging rates exist among children receiving care in pediatric EDs across the United States, Jennifer R. Marin, MD, of the University of Pittsburgh Medical Center Children’s Hospital of Pittsburgh, and associates reported.
Specifically, visits with non-Hispanic Black and Hispanic patients less frequently included radiography, CT, ultrasonography, and MRI than those of non-Hispanic White patients. The findings persisted across most diagnostic groups, even when stratified according to insurance type, Dr. Marin and colleagues reported in a multicenter cross-sectional study in JAMA Network Open.
The authors collected administrative data from the Pediatric Health Information System on 44 tertiary care children’s hospitals in 17 major metropolitan areas across the United States. They evaluated a total of 13,087,522 ED visits by 6,230,911 patients that occurred between Jan. 1, 2016, and Dec. 31, 2019. Of these, 28.2% included at least one imaging study. Altogether, 33.5% were performed on non-Hispanic White children, compared with just 24.1% of non-Hispanic Black children (adjusted odds ratio, 0.82) and 26.1% of Hispanic children (aOR, 0.87). After adjusting for relevant confounding factors, non-Hispanic Black and Hispanic children were less likely to have any imaging at all during their visits.
“Our findings suggest that a child’s race and ethnicity may be independently associated with the decision to perform imaging during ED visits,” Dr. Marin and associates said, adding that “the differential use of diagnostic imaging by race/ethnicity may reflect underuse of imaging in non-Hispanic Black and Hispanic children, or alternatively, overuse in non-Hispanic White children.”
Overuse vs. underuse: Racial bias or parental anxiety?
Overuse of imaging carries its own risks, but underuse can lead to misdiagnosis, the need for additional care, and possibly worse outcomes in the long run, Dr. Marin and colleagues explained. “Although we were unable to discern underuse from overuse using an administrative database, it is likely that much of the imaging in White children is unnecessary.”
Higher parental anxiety was just one of the explanations the authors offered for excessive imaging in White children. Especially in cases of diagnostic imaging for head trauma, one survey of adult ED patients showed that the peace of mind CT offers with its more definitive diagnosis was worth the additional possible risk of radiation.
Language barriers in non–English-speaking patients may also affect likelihood of testing as part of an ED visit.
Implicit physician racial bias, which can be amplified under the stress of working in an ED, can affect patient interactions, treatment decisions and adherence, and ultimately overall health outcomes, the authors noted. The goal in ensuring parity is to routinely follow clinical guidelines and use objective scoring tools that minimize subjectivity. At the institutional level, internal quality assurance evaluations go a long way toward understanding and limiting bias.
Historically, White patients are more likely than minority patients to have a medical home, which can influence whether ED physicians order imaging studies and whether imaging of White patients may have been triggered by a primary care physician referral, Dr. Marin and associates said.
In an accompanying editorial, Anupam B. Kharbanda, MD, said these findings are “consistent with decades of previous research documenting inequalities in health care delivery ... [and] must be examined in the context of inequities within the social framework of a community.”
Going back to the drawing board
“Physicians, researchers, and health care leaders must partner with the communities they serve to develop and implement interventions to address these substantial inequities in care,” said Dr. Kharbanda, pediatric emergency medicine physician at Children’s Minnesota Hospital, Minneapolis. As previous studies have demonstrated, implicit bias and antiracism training are needed to help physicians develop empathy so they are better equipped to help patients and families in a multicultural environment. Partnering with community-based organizations to ensure that care is more community centered, as has been done successfully within the Kaiser Permanente health system, for example, and employing a more diverse workforce that mirrors the populations cared for will go a long way.
Citing a 1966 speech of the late Dr. Martin Luther King, Jr., in which he said: “Of all the forms of inequality, injustice in health care is the most shocking and inhumane,” Dr. Kharbanda urged clinicians to not only hear but believe these words and act on them by working in partnerships with the communities they serve.
In a separate interview, Walter Palmer, MD, pediatric emergency medicine fellow at Children’s National Medical Center, Washington, noted: “This study’s findings are disappointing and yet not at all unexpected, as the authors convincingly identify yet another step at which patients of color are treated unequally in the U.S. health care system. It highlights a frightening truth: That we are all at risk of letting invisible implicit biases impact our clinical decision-making process. This is especially true in the busy emergency department environment, where pressure to make swift decisions regarding diagnostic workup and management invites the influence of imperceptible biases. It is now incumbent upon us as health care providers to monitor our personal and departmental patterns of practice for areas in which we can improve racial health equity and become advocates for the children and families who entrust us with their care.”
The authors reported multiple financial disclosures. Dr. Kharbanda and Dr. Palmer had no conflicts of interest and reported no disclosures.
Significant racial and ethnic differences in diagnostic imaging rates exist among children receiving care in pediatric EDs across the United States, Jennifer R. Marin, MD, of the University of Pittsburgh Medical Center Children’s Hospital of Pittsburgh, and associates reported.
Specifically, visits with non-Hispanic Black and Hispanic patients less frequently included radiography, CT, ultrasonography, and MRI than those of non-Hispanic White patients. The findings persisted across most diagnostic groups, even when stratified according to insurance type, Dr. Marin and colleagues reported in a multicenter cross-sectional study in JAMA Network Open.
The authors collected administrative data from the Pediatric Health Information System on 44 tertiary care children’s hospitals in 17 major metropolitan areas across the United States. They evaluated a total of 13,087,522 ED visits by 6,230,911 patients that occurred between Jan. 1, 2016, and Dec. 31, 2019. Of these, 28.2% included at least one imaging study. Altogether, 33.5% were performed on non-Hispanic White children, compared with just 24.1% of non-Hispanic Black children (adjusted odds ratio, 0.82) and 26.1% of Hispanic children (aOR, 0.87). After adjusting for relevant confounding factors, non-Hispanic Black and Hispanic children were less likely to have any imaging at all during their visits.
“Our findings suggest that a child’s race and ethnicity may be independently associated with the decision to perform imaging during ED visits,” Dr. Marin and associates said, adding that “the differential use of diagnostic imaging by race/ethnicity may reflect underuse of imaging in non-Hispanic Black and Hispanic children, or alternatively, overuse in non-Hispanic White children.”
Overuse vs. underuse: Racial bias or parental anxiety?
Overuse of imaging carries its own risks, but underuse can lead to misdiagnosis, the need for additional care, and possibly worse outcomes in the long run, Dr. Marin and colleagues explained. “Although we were unable to discern underuse from overuse using an administrative database, it is likely that much of the imaging in White children is unnecessary.”
Higher parental anxiety was just one of the explanations the authors offered for excessive imaging in White children. Especially in cases of diagnostic imaging for head trauma, one survey of adult ED patients showed that the peace of mind CT offers with its more definitive diagnosis was worth the additional possible risk of radiation.
Language barriers in non–English-speaking patients may also affect likelihood of testing as part of an ED visit.
Implicit physician racial bias, which can be amplified under the stress of working in an ED, can affect patient interactions, treatment decisions and adherence, and ultimately overall health outcomes, the authors noted. The goal in ensuring parity is to routinely follow clinical guidelines and use objective scoring tools that minimize subjectivity. At the institutional level, internal quality assurance evaluations go a long way toward understanding and limiting bias.
Historically, White patients are more likely than minority patients to have a medical home, which can influence whether ED physicians order imaging studies and whether imaging of White patients may have been triggered by a primary care physician referral, Dr. Marin and associates said.
In an accompanying editorial, Anupam B. Kharbanda, MD, said these findings are “consistent with decades of previous research documenting inequalities in health care delivery ... [and] must be examined in the context of inequities within the social framework of a community.”
Going back to the drawing board
“Physicians, researchers, and health care leaders must partner with the communities they serve to develop and implement interventions to address these substantial inequities in care,” said Dr. Kharbanda, pediatric emergency medicine physician at Children’s Minnesota Hospital, Minneapolis. As previous studies have demonstrated, implicit bias and antiracism training are needed to help physicians develop empathy so they are better equipped to help patients and families in a multicultural environment. Partnering with community-based organizations to ensure that care is more community centered, as has been done successfully within the Kaiser Permanente health system, for example, and employing a more diverse workforce that mirrors the populations cared for will go a long way.
Citing a 1966 speech of the late Dr. Martin Luther King, Jr., in which he said: “Of all the forms of inequality, injustice in health care is the most shocking and inhumane,” Dr. Kharbanda urged clinicians to not only hear but believe these words and act on them by working in partnerships with the communities they serve.
In a separate interview, Walter Palmer, MD, pediatric emergency medicine fellow at Children’s National Medical Center, Washington, noted: “This study’s findings are disappointing and yet not at all unexpected, as the authors convincingly identify yet another step at which patients of color are treated unequally in the U.S. health care system. It highlights a frightening truth: That we are all at risk of letting invisible implicit biases impact our clinical decision-making process. This is especially true in the busy emergency department environment, where pressure to make swift decisions regarding diagnostic workup and management invites the influence of imperceptible biases. It is now incumbent upon us as health care providers to monitor our personal and departmental patterns of practice for areas in which we can improve racial health equity and become advocates for the children and families who entrust us with their care.”
The authors reported multiple financial disclosures. Dr. Kharbanda and Dr. Palmer had no conflicts of interest and reported no disclosures.
FROM JAMA NETWORK OPEN




