User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
COVID-19–related inflammatory syndrome tied to neurologic symptoms in children
About half of children with pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2 (PIMS-TS) have new-onset neurologic symptoms, research shows.
These symptoms involve the central and peripheral nervous systems but do not always affect the respiratory system. In addition, neurologic symptoms appear to be more common in severe presentations of this syndrome.
“These new data consolidate the initial findings in our JAMA Neurology publication on the neurological problems that children with PIMS-TS can present with, even in the absence of respiratory systems,” study investigator Omar Abdel-Mannan, MD, clinical research fellow at University College London Institute of Neurology and senior resident at Great Ormond Street Hospital for children in London, said in an interview.
He added that the findings are in keeping with other recent research studies on PIMS-TS, which is known more commonly in the United States as multisystem inflammatory syndrome in children (MIS-C).
The findings will be presented April 18 at the American Academy of Neurology (AAN) 2021 Annual Meeting.
Neurologic manifestations common
Many children and adults with COVID-19 have developed neurologic manifestations. PIMS-TS is a severe, postinfectious, immune-mediated disorder characterized by persistent fever and extreme inflammation.
Patients may have acute diarrhea or vomiting, rash or bilateral conjunctivitis, and low blood pressure. They should be examined by a pediatric specialist, and most children with this disorder need intensive care.
To report the neurologic manifestations in children with PIMS-TS, the researchers retrospectively examined data for children and adolescents younger than 18 years who had the disorder and presented to a single center between April 4, 2020, and Sept. 1, 2020.
Forty-six patients (median age, 10.2 years) were included in the analysis. Thirty (65.2%) were male, and 37 (80.4%) were of non-White ethnicities.
Twenty-four (52.2%) patients had new-onset neurologic symptoms, which included headache (n = 24), encephalopathy (n = 14 patients), dysarthria/dysphonia (n = 6), hallucinations (n = 6), ataxia (n = 4), peripheral nerve involvement (n = 3), and seizures (n=1).
Laboratory and imaging results provided further information. One patient had 118 leukocytes in cerebrospinal fluid. Children with neurologic involvement had higher levels of peak inflammatory markers and were more likely to be ventilated and require inotropic support in the PICU (P < .05).
Four of 16 patients who underwent brain MRI had splenium signal changes. Of 15 patients who underwent electroencephalogram (EEG), 14 had an excess of slow activity. Four of 7 patients who underwent nerve conduction studies and electromyography (EMG) had myopathic and neuropathic changes.
Response to SARS-CoV-2
Central neurologic problems of the brain and peripheral nerve involvement rarely occur at the same time in children.
“This makes it highly possible that the syndrome is secondary to cytokine release in response to the SARS-CoV-2 virus, as there is significant clinical overlap with both genetic and acquired forms of another immune-mediated condition known as hemophagocytic lymphohistiocytosis,” said Dr. Abdel-Mannan.
The researchers found no demographic differences between children with neurologic involvement at presentation and those without.
“However, the numbers are small given the rarity of this condition, which makes it difficult to extrapolate associations and differences between the two groups, and will require future collaborative larger scale studies to look at what potentially makes some children more susceptible to neurologic involvement than others,” said Dr. Abdel-Mannan.
Excluding potential causes of the symptoms other than COVID-19 also is important, he added.
The preponderance of ethnic minorities in the current study population mirrors that in other PIMS-TS cohorts in other countries, said Dr. Abdel-Mannan. It reflects the higher incidence of COVID-19 in ethnic minority groups. However, presentation, investigations, and management did not differ between White and non-White children in the current study.
“Although PIMS-TS patients with neurologic involvement are initially sicker, our center’s preliminary follow-up data up to 6 months post discharge from hospital demonstrates that most of these children make an almost complete functional recovery, which is reassuring,” said Dr. Abdel-Mannan.
The data underscore how important it is that clinicians be aware that children with PIMS-TS can present with neurologic symptoms, even in the absence of respiratory involvement, he added.
The researchers will soon begin a multicenter research study that will involve longitudinal clinical and cognitive assessments and advanced neuroimaging. The objective will be to determine whether all children with PIMS-TS, or only those with neurologic symptoms, are at risk of chronic longer-term neurocognitive and psychiatric outcomes.
Unanswered questions
John B. Bodensteiner, MD, professor of neurology and pediatrics at Mayo Clinic, Rochester, Minn., said in an interview that the findings help flesh out the range of neurologic involvement that PIMS-TS entails.
“It’s not a surprise to us as neurologists, but it’s not been emphasized in the general literature and in the public health sector,” he said.
The study’s most important implication is that neurologic conditions are not uncommon among children with PIMS-TS, Dr. Bodensteiner added.
“We have no idea how long or what the long-term effects of that are,” he said. Not enough time has elapsed to enable a clear understanding of the syndrome’s lasting effects on cognition, he said, “but I think this certainly raises a flag that this is a real entity. This is nothing to sniff at.”
He noted that the study has the limitations of any retrospective case series. The researchers did not perform prospective and systematic evaluations of children with the syndrome
The findings also raise unanswered questions.
“They had 14 kids with encephalopathy, but not all of them got the same evaluation,” said Dr. Bodensteiner. Although the researchers mention peripheral nerve involvement in three children, they do not describe it. “They said that the EMG showed myopathic and neuropathic changes, but peripheral nerve involvement wouldn’t give you myopathic changes, so maybe there’s some direct involvement of the muscle in this inflammatory process.”
The study also focused on a select group of patients, said Dr. Bodensteiner. “These are all patients admitted to Great Ormond Street Hospital, and we don’t know what percentage of kids who get COVID are hospitalized, which is an important issue.”
It is necessary to know what proportion of children with COVID-19 develop encephalopathy and MRI changes, he added. The findings do confirm that this coronavirus-related inflammatory condition is real and may have long-term sequelae. “We should be careful about kids getting this disease,” said Dr. Bodensteiner
The study had no funding. Dr. Abdel-Mannan and Dr. Bodensteiner have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
About half of children with pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2 (PIMS-TS) have new-onset neurologic symptoms, research shows.
These symptoms involve the central and peripheral nervous systems but do not always affect the respiratory system. In addition, neurologic symptoms appear to be more common in severe presentations of this syndrome.
“These new data consolidate the initial findings in our JAMA Neurology publication on the neurological problems that children with PIMS-TS can present with, even in the absence of respiratory systems,” study investigator Omar Abdel-Mannan, MD, clinical research fellow at University College London Institute of Neurology and senior resident at Great Ormond Street Hospital for children in London, said in an interview.
He added that the findings are in keeping with other recent research studies on PIMS-TS, which is known more commonly in the United States as multisystem inflammatory syndrome in children (MIS-C).
The findings will be presented April 18 at the American Academy of Neurology (AAN) 2021 Annual Meeting.
Neurologic manifestations common
Many children and adults with COVID-19 have developed neurologic manifestations. PIMS-TS is a severe, postinfectious, immune-mediated disorder characterized by persistent fever and extreme inflammation.
Patients may have acute diarrhea or vomiting, rash or bilateral conjunctivitis, and low blood pressure. They should be examined by a pediatric specialist, and most children with this disorder need intensive care.
To report the neurologic manifestations in children with PIMS-TS, the researchers retrospectively examined data for children and adolescents younger than 18 years who had the disorder and presented to a single center between April 4, 2020, and Sept. 1, 2020.
Forty-six patients (median age, 10.2 years) were included in the analysis. Thirty (65.2%) were male, and 37 (80.4%) were of non-White ethnicities.
Twenty-four (52.2%) patients had new-onset neurologic symptoms, which included headache (n = 24), encephalopathy (n = 14 patients), dysarthria/dysphonia (n = 6), hallucinations (n = 6), ataxia (n = 4), peripheral nerve involvement (n = 3), and seizures (n=1).
Laboratory and imaging results provided further information. One patient had 118 leukocytes in cerebrospinal fluid. Children with neurologic involvement had higher levels of peak inflammatory markers and were more likely to be ventilated and require inotropic support in the PICU (P < .05).
Four of 16 patients who underwent brain MRI had splenium signal changes. Of 15 patients who underwent electroencephalogram (EEG), 14 had an excess of slow activity. Four of 7 patients who underwent nerve conduction studies and electromyography (EMG) had myopathic and neuropathic changes.
Response to SARS-CoV-2
Central neurologic problems of the brain and peripheral nerve involvement rarely occur at the same time in children.
“This makes it highly possible that the syndrome is secondary to cytokine release in response to the SARS-CoV-2 virus, as there is significant clinical overlap with both genetic and acquired forms of another immune-mediated condition known as hemophagocytic lymphohistiocytosis,” said Dr. Abdel-Mannan.
The researchers found no demographic differences between children with neurologic involvement at presentation and those without.
“However, the numbers are small given the rarity of this condition, which makes it difficult to extrapolate associations and differences between the two groups, and will require future collaborative larger scale studies to look at what potentially makes some children more susceptible to neurologic involvement than others,” said Dr. Abdel-Mannan.
Excluding potential causes of the symptoms other than COVID-19 also is important, he added.
The preponderance of ethnic minorities in the current study population mirrors that in other PIMS-TS cohorts in other countries, said Dr. Abdel-Mannan. It reflects the higher incidence of COVID-19 in ethnic minority groups. However, presentation, investigations, and management did not differ between White and non-White children in the current study.
“Although PIMS-TS patients with neurologic involvement are initially sicker, our center’s preliminary follow-up data up to 6 months post discharge from hospital demonstrates that most of these children make an almost complete functional recovery, which is reassuring,” said Dr. Abdel-Mannan.
The data underscore how important it is that clinicians be aware that children with PIMS-TS can present with neurologic symptoms, even in the absence of respiratory involvement, he added.
The researchers will soon begin a multicenter research study that will involve longitudinal clinical and cognitive assessments and advanced neuroimaging. The objective will be to determine whether all children with PIMS-TS, or only those with neurologic symptoms, are at risk of chronic longer-term neurocognitive and psychiatric outcomes.
Unanswered questions
John B. Bodensteiner, MD, professor of neurology and pediatrics at Mayo Clinic, Rochester, Minn., said in an interview that the findings help flesh out the range of neurologic involvement that PIMS-TS entails.
“It’s not a surprise to us as neurologists, but it’s not been emphasized in the general literature and in the public health sector,” he said.
The study’s most important implication is that neurologic conditions are not uncommon among children with PIMS-TS, Dr. Bodensteiner added.
“We have no idea how long or what the long-term effects of that are,” he said. Not enough time has elapsed to enable a clear understanding of the syndrome’s lasting effects on cognition, he said, “but I think this certainly raises a flag that this is a real entity. This is nothing to sniff at.”
He noted that the study has the limitations of any retrospective case series. The researchers did not perform prospective and systematic evaluations of children with the syndrome
The findings also raise unanswered questions.
“They had 14 kids with encephalopathy, but not all of them got the same evaluation,” said Dr. Bodensteiner. Although the researchers mention peripheral nerve involvement in three children, they do not describe it. “They said that the EMG showed myopathic and neuropathic changes, but peripheral nerve involvement wouldn’t give you myopathic changes, so maybe there’s some direct involvement of the muscle in this inflammatory process.”
The study also focused on a select group of patients, said Dr. Bodensteiner. “These are all patients admitted to Great Ormond Street Hospital, and we don’t know what percentage of kids who get COVID are hospitalized, which is an important issue.”
It is necessary to know what proportion of children with COVID-19 develop encephalopathy and MRI changes, he added. The findings do confirm that this coronavirus-related inflammatory condition is real and may have long-term sequelae. “We should be careful about kids getting this disease,” said Dr. Bodensteiner
The study had no funding. Dr. Abdel-Mannan and Dr. Bodensteiner have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
About half of children with pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2 (PIMS-TS) have new-onset neurologic symptoms, research shows.
These symptoms involve the central and peripheral nervous systems but do not always affect the respiratory system. In addition, neurologic symptoms appear to be more common in severe presentations of this syndrome.
“These new data consolidate the initial findings in our JAMA Neurology publication on the neurological problems that children with PIMS-TS can present with, even in the absence of respiratory systems,” study investigator Omar Abdel-Mannan, MD, clinical research fellow at University College London Institute of Neurology and senior resident at Great Ormond Street Hospital for children in London, said in an interview.
He added that the findings are in keeping with other recent research studies on PIMS-TS, which is known more commonly in the United States as multisystem inflammatory syndrome in children (MIS-C).
The findings will be presented April 18 at the American Academy of Neurology (AAN) 2021 Annual Meeting.
Neurologic manifestations common
Many children and adults with COVID-19 have developed neurologic manifestations. PIMS-TS is a severe, postinfectious, immune-mediated disorder characterized by persistent fever and extreme inflammation.
Patients may have acute diarrhea or vomiting, rash or bilateral conjunctivitis, and low blood pressure. They should be examined by a pediatric specialist, and most children with this disorder need intensive care.
To report the neurologic manifestations in children with PIMS-TS, the researchers retrospectively examined data for children and adolescents younger than 18 years who had the disorder and presented to a single center between April 4, 2020, and Sept. 1, 2020.
Forty-six patients (median age, 10.2 years) were included in the analysis. Thirty (65.2%) were male, and 37 (80.4%) were of non-White ethnicities.
Twenty-four (52.2%) patients had new-onset neurologic symptoms, which included headache (n = 24), encephalopathy (n = 14 patients), dysarthria/dysphonia (n = 6), hallucinations (n = 6), ataxia (n = 4), peripheral nerve involvement (n = 3), and seizures (n=1).
Laboratory and imaging results provided further information. One patient had 118 leukocytes in cerebrospinal fluid. Children with neurologic involvement had higher levels of peak inflammatory markers and were more likely to be ventilated and require inotropic support in the PICU (P < .05).
Four of 16 patients who underwent brain MRI had splenium signal changes. Of 15 patients who underwent electroencephalogram (EEG), 14 had an excess of slow activity. Four of 7 patients who underwent nerve conduction studies and electromyography (EMG) had myopathic and neuropathic changes.
Response to SARS-CoV-2
Central neurologic problems of the brain and peripheral nerve involvement rarely occur at the same time in children.
“This makes it highly possible that the syndrome is secondary to cytokine release in response to the SARS-CoV-2 virus, as there is significant clinical overlap with both genetic and acquired forms of another immune-mediated condition known as hemophagocytic lymphohistiocytosis,” said Dr. Abdel-Mannan.
The researchers found no demographic differences between children with neurologic involvement at presentation and those without.
“However, the numbers are small given the rarity of this condition, which makes it difficult to extrapolate associations and differences between the two groups, and will require future collaborative larger scale studies to look at what potentially makes some children more susceptible to neurologic involvement than others,” said Dr. Abdel-Mannan.
Excluding potential causes of the symptoms other than COVID-19 also is important, he added.
The preponderance of ethnic minorities in the current study population mirrors that in other PIMS-TS cohorts in other countries, said Dr. Abdel-Mannan. It reflects the higher incidence of COVID-19 in ethnic minority groups. However, presentation, investigations, and management did not differ between White and non-White children in the current study.
“Although PIMS-TS patients with neurologic involvement are initially sicker, our center’s preliminary follow-up data up to 6 months post discharge from hospital demonstrates that most of these children make an almost complete functional recovery, which is reassuring,” said Dr. Abdel-Mannan.
The data underscore how important it is that clinicians be aware that children with PIMS-TS can present with neurologic symptoms, even in the absence of respiratory involvement, he added.
The researchers will soon begin a multicenter research study that will involve longitudinal clinical and cognitive assessments and advanced neuroimaging. The objective will be to determine whether all children with PIMS-TS, or only those with neurologic symptoms, are at risk of chronic longer-term neurocognitive and psychiatric outcomes.
Unanswered questions
John B. Bodensteiner, MD, professor of neurology and pediatrics at Mayo Clinic, Rochester, Minn., said in an interview that the findings help flesh out the range of neurologic involvement that PIMS-TS entails.
“It’s not a surprise to us as neurologists, but it’s not been emphasized in the general literature and in the public health sector,” he said.
The study’s most important implication is that neurologic conditions are not uncommon among children with PIMS-TS, Dr. Bodensteiner added.
“We have no idea how long or what the long-term effects of that are,” he said. Not enough time has elapsed to enable a clear understanding of the syndrome’s lasting effects on cognition, he said, “but I think this certainly raises a flag that this is a real entity. This is nothing to sniff at.”
He noted that the study has the limitations of any retrospective case series. The researchers did not perform prospective and systematic evaluations of children with the syndrome
The findings also raise unanswered questions.
“They had 14 kids with encephalopathy, but not all of them got the same evaluation,” said Dr. Bodensteiner. Although the researchers mention peripheral nerve involvement in three children, they do not describe it. “They said that the EMG showed myopathic and neuropathic changes, but peripheral nerve involvement wouldn’t give you myopathic changes, so maybe there’s some direct involvement of the muscle in this inflammatory process.”
The study also focused on a select group of patients, said Dr. Bodensteiner. “These are all patients admitted to Great Ormond Street Hospital, and we don’t know what percentage of kids who get COVID are hospitalized, which is an important issue.”
It is necessary to know what proportion of children with COVID-19 develop encephalopathy and MRI changes, he added. The findings do confirm that this coronavirus-related inflammatory condition is real and may have long-term sequelae. “We should be careful about kids getting this disease,” said Dr. Bodensteiner
The study had no funding. Dr. Abdel-Mannan and Dr. Bodensteiner have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Milk is overtaking nuts as top food allergy threat
When Lesley Solomon’s son was 10 years old, he was standing in an unlucky spot on the playground when a schoolmate kicked over a cup of hot chocolate, sending droplets flying into the air. For the young boy with a severe milk allergy, the hot liquid splattering was less of a hazard for him than the dairy stirred into the drink.
Ms. Solomon’s son quickly washed the fluids off his clothes and skin, took some Benadryl, and called his parents. But on the car ride home, his throat began to close and his pulse raced. It was one of about a dozen times he has needed an epinephrine injection, which increases blood flow, reduces swelling, and reverses anaphylaxis.
“Until you see a child going through that anaphylaxis and not being able to breathe, or throwing up so much that they can’t breathe, you don’t understand” how serious food allergies can be, said Ms. Solomon, who is senior vice president and chief innovation officer of the Dana-Farber Cancer Institute in Boston and cofounder of the Food Allergy Science Initiative, an independent nonprofit that funds food allergy research.
The rate of children hospitalized for food-induced anaphylaxis rose by 25% from 2006 to 2012 – from 1.2 to 1.5 per 100,000 – according to a 2019 analysis of data from pediatric hospitals in the United States. And severe symptoms were more often linked to milk than to peanuts or tree nuts, the study showed.
Cow’s milk is the most common food allergy in children aged younger than 5 years, and accounts for about half of all food allergies in children younger than 1. Most children grow out of it, but when milk allergy persists into the teenage years and adulthood, it is more likely to cause severe reactions.
A dangerous allergy
“Cow’s milk allergy is the most distressing of the food allergies. Many people are unaware that it can cause anaphylaxis that is so severe,” said Carla Davis, MD, director of the food allergy program at the Texas Children’s Hospital in Houston. “People do not think about how much of this is in our food.”
And cow’s milk was shown to be the food allergy most likely to lead to death in school-aged children in the United Kingdom, according to an analysis of national data reported by this news organization.
Lack of awareness is what makes milk allergy so dangerous, said Paul Turner, BMBCh, PhD, a pediatric allergist and immunologist from Imperial College London, who was involved in the British analysis. “We need to get that information out to the public and businesses so they take the same level of care that they have with nuts, and when someone says they have milk allergy, they take it seriously.
In food allergy, the body treats certain proteins, such as the casein and whey in milk, as invaders, mounting an immune response. Antibodies known as IgE – which normally protect against bacteria, viruses, and parasites – trigger inflammation, the release of histamine, and can lead to symptoms, typically within minutes, ranging from rash and swelling to vomiting, difficulty swallowing, and difficulty breathing.
So, the very thing that makes milk a healthy choice for kids – its high protein content – can cause serious reactions in a small portion of children and adults. “You don’t need much milk to get a decent dose” of the allergen, Dr. Turner pointed out.
The mechanisms of milk allergy are complex, even compared with other food allergies. The IgE antibody can be detected with a skin-prick test or IgE blood test, but some people have positive results even though they are not allergic. To complicate things further, people can also have non–IgE-mediated milk allergy, which cannot be detected with testing and can lead to symptoms that emerge hours or even days after exposure.
More serious than lactose intolerance
Unfortunately, milk allergy is often confused with a milk-related digestive problem. Globally, about 70% of people lack the enzyme to break down the sugar in milk; the condition, known as lactose intolerance, can cause bloating, abdominal cramps, and diarrhea but is not life-threatening.
“Because lactose intolerance is so common, people don’t think of milk allergy as something that can be significant or severe,” said Ruchi Gupta, MD, MPH, director of the Center for Food Allergy and Asthma Research at the Northwestern University, Chicago.
In babies, colic, the regurgitation of milk-based formula, and rash are sometimes misinterpreted as a milk allergy, leading parents to buy expensive, specialized formula unnecessarily.
Frustrated by a lack of data about food allergies, Dr. Gupta and colleagues launched a nationally representative survey of 38,408 American parents in 2009, which was updated in 2015 and 2016.
On average, children with milk allergy had their first reaction before the age of 2, most commonly vomiting, diarrhea, hives, and eczema; this is a younger age of onset than for other food allergies. And children with milk allergy were twice as likely as children with other allergies to grow out of it.
Yet about one-third of milk-allergic children in the updated study were 11 years and older. And in a similar survey of adults who self-reported symptoms, milk allergy was as common as peanut allergy (1.9% vs 1.8%). “We don’t know why milk allergy is becoming more persistent,” Dr. Gupta said. And, she warned, only one in four children with a milk allergy had a current prescription for an epinephrine autoinjector, compared with about 70% of children with peanut allergy.
Food allergy can’t be caused by genetics alone, said Christine Olsen, MD, cofounder and CEO of the Food Allergy Science Initiative at the Broad Institute in Cambridge, Mass. “There may be a genetic predisposition, but there must be something environmental” that has influenced the development of food allergies.
One theory is that the body’s natural defense against noxious substances has been disrupted in the modern world by processed foods, chemical additives, and hygienic surroundings.
Dr. Olson’s own son vomited when he had his first small taste of hummus as a baby; he is severely allergic to sesame. The immediacy of his bodily reaction made Dr. Olsen think that the response involved neurons, not just a misguided immune system.
Researchers are currently looking for drug targets that could shut off the immune response as quickly as it starts. If you think of the fact that some kids outgrow their allergies and some adults get allergies, that suggests there’s some lever that you can turn on and off,” said Dr. Olsen, who is also a radiation oncologist.
Preventing allergy
The approach to food allergy prevention has already been transformed by the Learning Early About Peanut Allergy (LEAP) study conducted in the United Kingdom. LEAP investigators randomly assigned 640 infants to ingest regular amounts of peanut snacks or peanut butter or to avoid peanut products until they reached 5 years of age. The babies who had regular exposure to peanut from an early age were much less likely to develop a peanut allergy than those who avoided peanuts.
The National Institute of Allergy and Infectious Diseases revised its guidelines and now recommends that all babies be exposed to peanut-containing food at around 6 months of age; for high-risk babies, that can start as early as 4 months.
Allergy experts are planning to study that concept again with other foods, including cow’s milk. The 5-year iREACH study, launched by the Center for Food Allergy & Asthma Research at Northwestern and Lurie Children’s Hospital in Chicago, is currently enrolling 10,500 infants to test early exposure to peanuts, milk, egg, and cashew. A portion of the infants will have severe eczema, putting them at high risk for food allergies, and others will be low risk, said Dr. Gupta, who is the principal iREACH investigator.
“Hopefully in the next 5 years we will have data showing whether this prevention technique will work for other common food allergens, in addition to peanuts,” she said.
Introducing foods early “promotes tolerance rather than early sensitization,” explained Stephanie Leeds, MD, an allergist and immunologist at Yale University, New Haven, Conn. In the future, rather than just diagnosing and treating food allergies, allergists might work with pediatricians to help prevent them from ever happening.
A version of this article first appeared on Medscape.com.
When Lesley Solomon’s son was 10 years old, he was standing in an unlucky spot on the playground when a schoolmate kicked over a cup of hot chocolate, sending droplets flying into the air. For the young boy with a severe milk allergy, the hot liquid splattering was less of a hazard for him than the dairy stirred into the drink.
Ms. Solomon’s son quickly washed the fluids off his clothes and skin, took some Benadryl, and called his parents. But on the car ride home, his throat began to close and his pulse raced. It was one of about a dozen times he has needed an epinephrine injection, which increases blood flow, reduces swelling, and reverses anaphylaxis.
“Until you see a child going through that anaphylaxis and not being able to breathe, or throwing up so much that they can’t breathe, you don’t understand” how serious food allergies can be, said Ms. Solomon, who is senior vice president and chief innovation officer of the Dana-Farber Cancer Institute in Boston and cofounder of the Food Allergy Science Initiative, an independent nonprofit that funds food allergy research.
The rate of children hospitalized for food-induced anaphylaxis rose by 25% from 2006 to 2012 – from 1.2 to 1.5 per 100,000 – according to a 2019 analysis of data from pediatric hospitals in the United States. And severe symptoms were more often linked to milk than to peanuts or tree nuts, the study showed.
Cow’s milk is the most common food allergy in children aged younger than 5 years, and accounts for about half of all food allergies in children younger than 1. Most children grow out of it, but when milk allergy persists into the teenage years and adulthood, it is more likely to cause severe reactions.
A dangerous allergy
“Cow’s milk allergy is the most distressing of the food allergies. Many people are unaware that it can cause anaphylaxis that is so severe,” said Carla Davis, MD, director of the food allergy program at the Texas Children’s Hospital in Houston. “People do not think about how much of this is in our food.”
And cow’s milk was shown to be the food allergy most likely to lead to death in school-aged children in the United Kingdom, according to an analysis of national data reported by this news organization.
Lack of awareness is what makes milk allergy so dangerous, said Paul Turner, BMBCh, PhD, a pediatric allergist and immunologist from Imperial College London, who was involved in the British analysis. “We need to get that information out to the public and businesses so they take the same level of care that they have with nuts, and when someone says they have milk allergy, they take it seriously.
In food allergy, the body treats certain proteins, such as the casein and whey in milk, as invaders, mounting an immune response. Antibodies known as IgE – which normally protect against bacteria, viruses, and parasites – trigger inflammation, the release of histamine, and can lead to symptoms, typically within minutes, ranging from rash and swelling to vomiting, difficulty swallowing, and difficulty breathing.
So, the very thing that makes milk a healthy choice for kids – its high protein content – can cause serious reactions in a small portion of children and adults. “You don’t need much milk to get a decent dose” of the allergen, Dr. Turner pointed out.
The mechanisms of milk allergy are complex, even compared with other food allergies. The IgE antibody can be detected with a skin-prick test or IgE blood test, but some people have positive results even though they are not allergic. To complicate things further, people can also have non–IgE-mediated milk allergy, which cannot be detected with testing and can lead to symptoms that emerge hours or even days after exposure.
More serious than lactose intolerance
Unfortunately, milk allergy is often confused with a milk-related digestive problem. Globally, about 70% of people lack the enzyme to break down the sugar in milk; the condition, known as lactose intolerance, can cause bloating, abdominal cramps, and diarrhea but is not life-threatening.
“Because lactose intolerance is so common, people don’t think of milk allergy as something that can be significant or severe,” said Ruchi Gupta, MD, MPH, director of the Center for Food Allergy and Asthma Research at the Northwestern University, Chicago.
In babies, colic, the regurgitation of milk-based formula, and rash are sometimes misinterpreted as a milk allergy, leading parents to buy expensive, specialized formula unnecessarily.
Frustrated by a lack of data about food allergies, Dr. Gupta and colleagues launched a nationally representative survey of 38,408 American parents in 2009, which was updated in 2015 and 2016.
On average, children with milk allergy had their first reaction before the age of 2, most commonly vomiting, diarrhea, hives, and eczema; this is a younger age of onset than for other food allergies. And children with milk allergy were twice as likely as children with other allergies to grow out of it.
Yet about one-third of milk-allergic children in the updated study were 11 years and older. And in a similar survey of adults who self-reported symptoms, milk allergy was as common as peanut allergy (1.9% vs 1.8%). “We don’t know why milk allergy is becoming more persistent,” Dr. Gupta said. And, she warned, only one in four children with a milk allergy had a current prescription for an epinephrine autoinjector, compared with about 70% of children with peanut allergy.
Food allergy can’t be caused by genetics alone, said Christine Olsen, MD, cofounder and CEO of the Food Allergy Science Initiative at the Broad Institute in Cambridge, Mass. “There may be a genetic predisposition, but there must be something environmental” that has influenced the development of food allergies.
One theory is that the body’s natural defense against noxious substances has been disrupted in the modern world by processed foods, chemical additives, and hygienic surroundings.
Dr. Olson’s own son vomited when he had his first small taste of hummus as a baby; he is severely allergic to sesame. The immediacy of his bodily reaction made Dr. Olsen think that the response involved neurons, not just a misguided immune system.
Researchers are currently looking for drug targets that could shut off the immune response as quickly as it starts. If you think of the fact that some kids outgrow their allergies and some adults get allergies, that suggests there’s some lever that you can turn on and off,” said Dr. Olsen, who is also a radiation oncologist.
Preventing allergy
The approach to food allergy prevention has already been transformed by the Learning Early About Peanut Allergy (LEAP) study conducted in the United Kingdom. LEAP investigators randomly assigned 640 infants to ingest regular amounts of peanut snacks or peanut butter or to avoid peanut products until they reached 5 years of age. The babies who had regular exposure to peanut from an early age were much less likely to develop a peanut allergy than those who avoided peanuts.
The National Institute of Allergy and Infectious Diseases revised its guidelines and now recommends that all babies be exposed to peanut-containing food at around 6 months of age; for high-risk babies, that can start as early as 4 months.
Allergy experts are planning to study that concept again with other foods, including cow’s milk. The 5-year iREACH study, launched by the Center for Food Allergy & Asthma Research at Northwestern and Lurie Children’s Hospital in Chicago, is currently enrolling 10,500 infants to test early exposure to peanuts, milk, egg, and cashew. A portion of the infants will have severe eczema, putting them at high risk for food allergies, and others will be low risk, said Dr. Gupta, who is the principal iREACH investigator.
“Hopefully in the next 5 years we will have data showing whether this prevention technique will work for other common food allergens, in addition to peanuts,” she said.
Introducing foods early “promotes tolerance rather than early sensitization,” explained Stephanie Leeds, MD, an allergist and immunologist at Yale University, New Haven, Conn. In the future, rather than just diagnosing and treating food allergies, allergists might work with pediatricians to help prevent them from ever happening.
A version of this article first appeared on Medscape.com.
When Lesley Solomon’s son was 10 years old, he was standing in an unlucky spot on the playground when a schoolmate kicked over a cup of hot chocolate, sending droplets flying into the air. For the young boy with a severe milk allergy, the hot liquid splattering was less of a hazard for him than the dairy stirred into the drink.
Ms. Solomon’s son quickly washed the fluids off his clothes and skin, took some Benadryl, and called his parents. But on the car ride home, his throat began to close and his pulse raced. It was one of about a dozen times he has needed an epinephrine injection, which increases blood flow, reduces swelling, and reverses anaphylaxis.
“Until you see a child going through that anaphylaxis and not being able to breathe, or throwing up so much that they can’t breathe, you don’t understand” how serious food allergies can be, said Ms. Solomon, who is senior vice president and chief innovation officer of the Dana-Farber Cancer Institute in Boston and cofounder of the Food Allergy Science Initiative, an independent nonprofit that funds food allergy research.
The rate of children hospitalized for food-induced anaphylaxis rose by 25% from 2006 to 2012 – from 1.2 to 1.5 per 100,000 – according to a 2019 analysis of data from pediatric hospitals in the United States. And severe symptoms were more often linked to milk than to peanuts or tree nuts, the study showed.
Cow’s milk is the most common food allergy in children aged younger than 5 years, and accounts for about half of all food allergies in children younger than 1. Most children grow out of it, but when milk allergy persists into the teenage years and adulthood, it is more likely to cause severe reactions.
A dangerous allergy
“Cow’s milk allergy is the most distressing of the food allergies. Many people are unaware that it can cause anaphylaxis that is so severe,” said Carla Davis, MD, director of the food allergy program at the Texas Children’s Hospital in Houston. “People do not think about how much of this is in our food.”
And cow’s milk was shown to be the food allergy most likely to lead to death in school-aged children in the United Kingdom, according to an analysis of national data reported by this news organization.
Lack of awareness is what makes milk allergy so dangerous, said Paul Turner, BMBCh, PhD, a pediatric allergist and immunologist from Imperial College London, who was involved in the British analysis. “We need to get that information out to the public and businesses so they take the same level of care that they have with nuts, and when someone says they have milk allergy, they take it seriously.
In food allergy, the body treats certain proteins, such as the casein and whey in milk, as invaders, mounting an immune response. Antibodies known as IgE – which normally protect against bacteria, viruses, and parasites – trigger inflammation, the release of histamine, and can lead to symptoms, typically within minutes, ranging from rash and swelling to vomiting, difficulty swallowing, and difficulty breathing.
So, the very thing that makes milk a healthy choice for kids – its high protein content – can cause serious reactions in a small portion of children and adults. “You don’t need much milk to get a decent dose” of the allergen, Dr. Turner pointed out.
The mechanisms of milk allergy are complex, even compared with other food allergies. The IgE antibody can be detected with a skin-prick test or IgE blood test, but some people have positive results even though they are not allergic. To complicate things further, people can also have non–IgE-mediated milk allergy, which cannot be detected with testing and can lead to symptoms that emerge hours or even days after exposure.
More serious than lactose intolerance
Unfortunately, milk allergy is often confused with a milk-related digestive problem. Globally, about 70% of people lack the enzyme to break down the sugar in milk; the condition, known as lactose intolerance, can cause bloating, abdominal cramps, and diarrhea but is not life-threatening.
“Because lactose intolerance is so common, people don’t think of milk allergy as something that can be significant or severe,” said Ruchi Gupta, MD, MPH, director of the Center for Food Allergy and Asthma Research at the Northwestern University, Chicago.
In babies, colic, the regurgitation of milk-based formula, and rash are sometimes misinterpreted as a milk allergy, leading parents to buy expensive, specialized formula unnecessarily.
Frustrated by a lack of data about food allergies, Dr. Gupta and colleagues launched a nationally representative survey of 38,408 American parents in 2009, which was updated in 2015 and 2016.
On average, children with milk allergy had their first reaction before the age of 2, most commonly vomiting, diarrhea, hives, and eczema; this is a younger age of onset than for other food allergies. And children with milk allergy were twice as likely as children with other allergies to grow out of it.
Yet about one-third of milk-allergic children in the updated study were 11 years and older. And in a similar survey of adults who self-reported symptoms, milk allergy was as common as peanut allergy (1.9% vs 1.8%). “We don’t know why milk allergy is becoming more persistent,” Dr. Gupta said. And, she warned, only one in four children with a milk allergy had a current prescription for an epinephrine autoinjector, compared with about 70% of children with peanut allergy.
Food allergy can’t be caused by genetics alone, said Christine Olsen, MD, cofounder and CEO of the Food Allergy Science Initiative at the Broad Institute in Cambridge, Mass. “There may be a genetic predisposition, but there must be something environmental” that has influenced the development of food allergies.
One theory is that the body’s natural defense against noxious substances has been disrupted in the modern world by processed foods, chemical additives, and hygienic surroundings.
Dr. Olson’s own son vomited when he had his first small taste of hummus as a baby; he is severely allergic to sesame. The immediacy of his bodily reaction made Dr. Olsen think that the response involved neurons, not just a misguided immune system.
Researchers are currently looking for drug targets that could shut off the immune response as quickly as it starts. If you think of the fact that some kids outgrow their allergies and some adults get allergies, that suggests there’s some lever that you can turn on and off,” said Dr. Olsen, who is also a radiation oncologist.
Preventing allergy
The approach to food allergy prevention has already been transformed by the Learning Early About Peanut Allergy (LEAP) study conducted in the United Kingdom. LEAP investigators randomly assigned 640 infants to ingest regular amounts of peanut snacks or peanut butter or to avoid peanut products until they reached 5 years of age. The babies who had regular exposure to peanut from an early age were much less likely to develop a peanut allergy than those who avoided peanuts.
The National Institute of Allergy and Infectious Diseases revised its guidelines and now recommends that all babies be exposed to peanut-containing food at around 6 months of age; for high-risk babies, that can start as early as 4 months.
Allergy experts are planning to study that concept again with other foods, including cow’s milk. The 5-year iREACH study, launched by the Center for Food Allergy & Asthma Research at Northwestern and Lurie Children’s Hospital in Chicago, is currently enrolling 10,500 infants to test early exposure to peanuts, milk, egg, and cashew. A portion of the infants will have severe eczema, putting them at high risk for food allergies, and others will be low risk, said Dr. Gupta, who is the principal iREACH investigator.
“Hopefully in the next 5 years we will have data showing whether this prevention technique will work for other common food allergens, in addition to peanuts,” she said.
Introducing foods early “promotes tolerance rather than early sensitization,” explained Stephanie Leeds, MD, an allergist and immunologist at Yale University, New Haven, Conn. In the future, rather than just diagnosing and treating food allergies, allergists might work with pediatricians to help prevent them from ever happening.
A version of this article first appeared on Medscape.com.
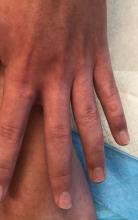
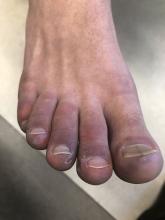
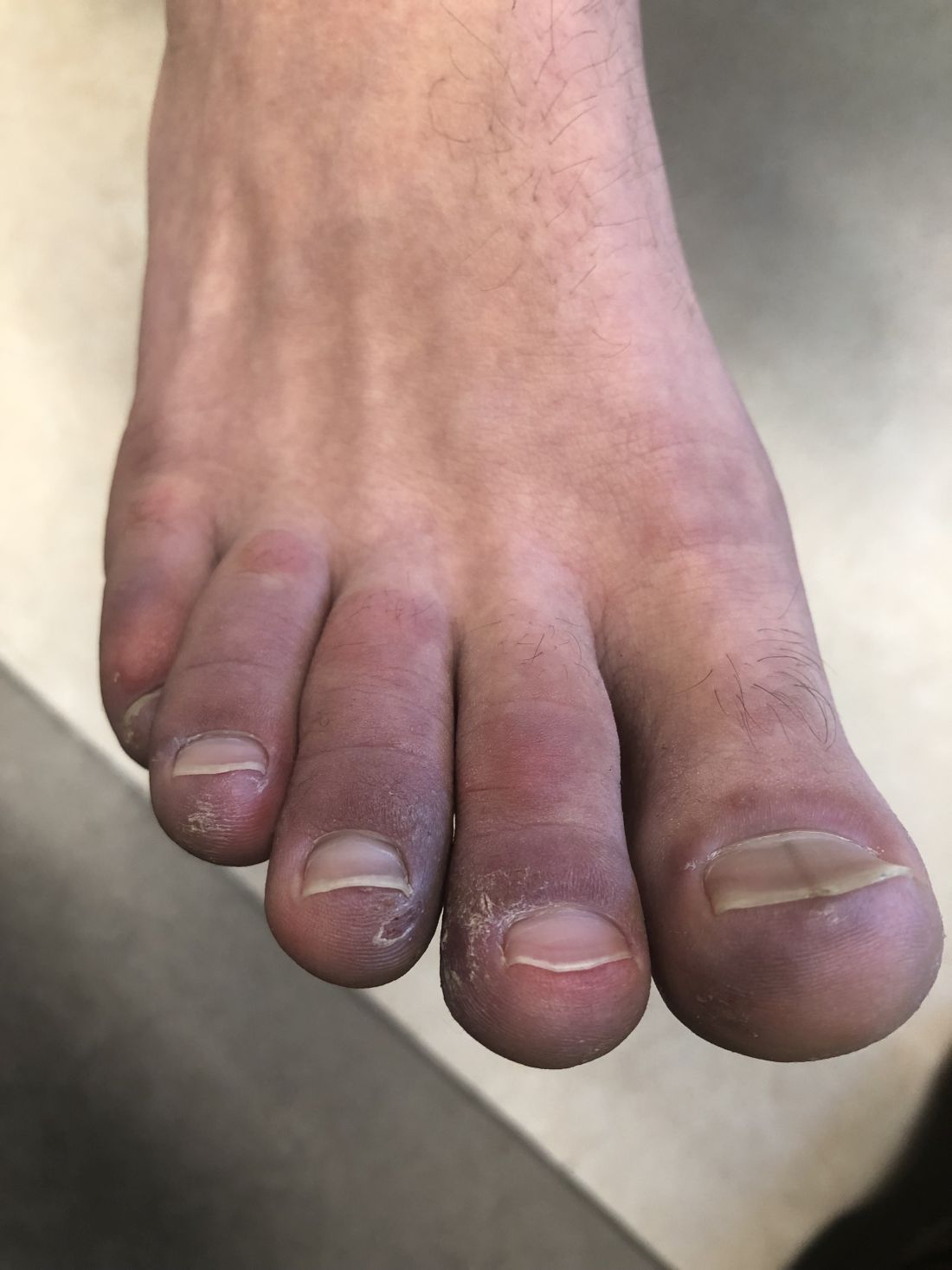
A 12-year-old male has persistent purple toes and new red lesions on his hands
A punch biopsy from one of the lesions on the feet showed subtle basal vacuolar interface inflammation on the epidermis and rare apoptotic keratinocytes. There was an underlying dermal lymphocytic inflammatory infiltrate around the vascular plexus. Dermal mucin appeared slightly increased. The histologic findings are consistent with pernio. He had a negative direct immunofluorescence study.
Laboratory work-up showed an elevated antinuclear antibody (ANA) of 1:620; positive anticardiolipin IgM was at 15.2. A complete blood count showed no anemia or lymphopenia, he had normal complement C3 and C4 levels, normal urinalysis, negative cryoglobulins and cold agglutinins, and a normal protein electrophoresis.
Given the chronicity of his lesions, the lack of improvement with weather changes, the histopathologic findings of a vacuolar interface dermatitis and the positive ANA titer he was diagnosed with chilblain lupus.
Chilblain lupus erythematosus (CLE) is an uncommon form of chronic cutaneous lupus erythematosus that presents with tender pink to violaceous macules, papules, and/or nodules that sometimes can ulcerate and are present on the fingers, toes, and sometimes the nose and ears. The lesions are usually triggered by cold exposure.1 These patients also have clinical and laboratory findings consistent with lupus erythematosus.
Even though more studies are needed to clarify the clinical and histopathologic features of chilblain lupus, compared with idiopathic pernio, some authors suggest several characteristics: CLE lesions tend to persist in summer months, as occurred in our patient, and histopathologic evaluation usually shows vacuolar and interface inflammation on the basal cell layer and may also have a positive lupus band on direct immunofluorescence.2 About 20% of patient with CLE may later develop systemic lupus erythematosus.3
There is also a familial form of CLE which is usually inherited as an autosomal-dominant trait. Mutations in TREX1, SAMHD1, and STING have been described in these patients.4 Affected children present with skin lesions at a young age and those with TREX1 mutations are at a higher risk to develop systemic lupus erythematosus.
The differential diagnosis of chilblain lupus includes idiopathic pernio or pernio secondary to other conditions. Other conditions that are thought to be associated with pernio, besides lupus erythematosus, include infectious causes (hepatitis B, COVID-19 infection),5 autoimmune conditions, malignancy and hematologic disorders (paraproteinemia).6 In histopathology, pernio lesions present with dermal edema and superficial and deep lymphocytic infiltrate.
The pathogenesis of pernio is not fully understood but is thought be related to vasospasm with secondary poor perfusion and ischemia and type I interferon (INF1) immune response. A recent review of the published studies trying to explain the causality between COVID 19 and pernio-like lesions, from January 2020 to December 2020, speculate several possible mechanisms: an increase in the vasoconstrictive, prothrombotic, and proinflammatory effects of the angiotensin II pathway through activation of the ACE2 by the virus; COVID-19 triggers a robust INF1 immune response in predisposed patients; pernio as a sign of mild disease, may be explained by genetic and hormonal differences in the patients affected.7
Another condition that can be confused with CLE is Raynaud phenomenon, were patients present with white to purple to red patches on the fingers and toes after exposure to cold, but in comparison with pernio, the lesions improve within minutes to hours after rewarming. Secondary Raynaud phenomenon can be seen in patients with systemic lupus erythematosus and in patients with other connective tissue disorders. The skin lesions in our patient were persistent and were not triggered by cold exposure, making Raynaud phenomenon less likely. Children with vasculitis can present with painful red, violaceous, or necrotic lesions on the extremities, which can mimic pernio. Vasculitis lesions tend to be more purpuric and angulated, compared with pernio lesions, though in severe cases of pernio with ulceration it may be difficult to distinguish between the two entities and a skin biopsy may be needed.
Sweet syndrome, also known as acute febrile neutrophilic dermatosis, is a rare skin condition in which children present with edematous tender nodules on the hands and with less frequency in other parts of the body with associated fever, malaise, conjunctivitis, or joint pain and it is usually associated with infection or malignancy. Our patient denied any systemic symptoms and had no conjunctivitis nor arthritis.
Most patients with idiopathic pernio do not require a biopsy or further laboratory evaluation unless the lesions are atypical, chronic, or there is a suspected associated condition. The workup for patients with prolonged or atypical pernio-like lesions include a skin biopsy with direct immunofluorescence, ANA, complete blood count, complement levels, antiphospholipid antibodies, cold agglutinins, and cryoglobulins.
Treatment of mild CLE is with moderate- to high-potency topical corticosteroids. In those patients not responding to topical measures and keeping the extremities warm, the use of hydroxychloroquine has been reported to be beneficial in some patients as well as the use of calcium-channel blockers.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego.
References
1. Su WP et al. Cutis. 1994 Dec;54(6):395-9.
2. Boada A et al. Am J Dermatopathol. 2010 Feb;32(1):19-23.
3. Patel et al. SBMJ Case Rep. 2013;2013:bcr2013201165.
4. Genes Yi et al. BMC. 2020 Apr 15;18(1):32.
5. Battesti G et al. J Am Acad Dermatol. 2020;83(4):1219-22.
6. Cappel JA et al. Mayo Clin Proc. 2014 Feb;89(2):207-15.
7. Cappel MA et al. Mayo Clin Proc. 2021;96(4):989-1005.
A punch biopsy from one of the lesions on the feet showed subtle basal vacuolar interface inflammation on the epidermis and rare apoptotic keratinocytes. There was an underlying dermal lymphocytic inflammatory infiltrate around the vascular plexus. Dermal mucin appeared slightly increased. The histologic findings are consistent with pernio. He had a negative direct immunofluorescence study.
Laboratory work-up showed an elevated antinuclear antibody (ANA) of 1:620; positive anticardiolipin IgM was at 15.2. A complete blood count showed no anemia or lymphopenia, he had normal complement C3 and C4 levels, normal urinalysis, negative cryoglobulins and cold agglutinins, and a normal protein electrophoresis.
Given the chronicity of his lesions, the lack of improvement with weather changes, the histopathologic findings of a vacuolar interface dermatitis and the positive ANA titer he was diagnosed with chilblain lupus.
Chilblain lupus erythematosus (CLE) is an uncommon form of chronic cutaneous lupus erythematosus that presents with tender pink to violaceous macules, papules, and/or nodules that sometimes can ulcerate and are present on the fingers, toes, and sometimes the nose and ears. The lesions are usually triggered by cold exposure.1 These patients also have clinical and laboratory findings consistent with lupus erythematosus.
Even though more studies are needed to clarify the clinical and histopathologic features of chilblain lupus, compared with idiopathic pernio, some authors suggest several characteristics: CLE lesions tend to persist in summer months, as occurred in our patient, and histopathologic evaluation usually shows vacuolar and interface inflammation on the basal cell layer and may also have a positive lupus band on direct immunofluorescence.2 About 20% of patient with CLE may later develop systemic lupus erythematosus.3
There is also a familial form of CLE which is usually inherited as an autosomal-dominant trait. Mutations in TREX1, SAMHD1, and STING have been described in these patients.4 Affected children present with skin lesions at a young age and those with TREX1 mutations are at a higher risk to develop systemic lupus erythematosus.
The differential diagnosis of chilblain lupus includes idiopathic pernio or pernio secondary to other conditions. Other conditions that are thought to be associated with pernio, besides lupus erythematosus, include infectious causes (hepatitis B, COVID-19 infection),5 autoimmune conditions, malignancy and hematologic disorders (paraproteinemia).6 In histopathology, pernio lesions present with dermal edema and superficial and deep lymphocytic infiltrate.
The pathogenesis of pernio is not fully understood but is thought be related to vasospasm with secondary poor perfusion and ischemia and type I interferon (INF1) immune response. A recent review of the published studies trying to explain the causality between COVID 19 and pernio-like lesions, from January 2020 to December 2020, speculate several possible mechanisms: an increase in the vasoconstrictive, prothrombotic, and proinflammatory effects of the angiotensin II pathway through activation of the ACE2 by the virus; COVID-19 triggers a robust INF1 immune response in predisposed patients; pernio as a sign of mild disease, may be explained by genetic and hormonal differences in the patients affected.7
Another condition that can be confused with CLE is Raynaud phenomenon, were patients present with white to purple to red patches on the fingers and toes after exposure to cold, but in comparison with pernio, the lesions improve within minutes to hours after rewarming. Secondary Raynaud phenomenon can be seen in patients with systemic lupus erythematosus and in patients with other connective tissue disorders. The skin lesions in our patient were persistent and were not triggered by cold exposure, making Raynaud phenomenon less likely. Children with vasculitis can present with painful red, violaceous, or necrotic lesions on the extremities, which can mimic pernio. Vasculitis lesions tend to be more purpuric and angulated, compared with pernio lesions, though in severe cases of pernio with ulceration it may be difficult to distinguish between the two entities and a skin biopsy may be needed.
Sweet syndrome, also known as acute febrile neutrophilic dermatosis, is a rare skin condition in which children present with edematous tender nodules on the hands and with less frequency in other parts of the body with associated fever, malaise, conjunctivitis, or joint pain and it is usually associated with infection or malignancy. Our patient denied any systemic symptoms and had no conjunctivitis nor arthritis.
Most patients with idiopathic pernio do not require a biopsy or further laboratory evaluation unless the lesions are atypical, chronic, or there is a suspected associated condition. The workup for patients with prolonged or atypical pernio-like lesions include a skin biopsy with direct immunofluorescence, ANA, complete blood count, complement levels, antiphospholipid antibodies, cold agglutinins, and cryoglobulins.
Treatment of mild CLE is with moderate- to high-potency topical corticosteroids. In those patients not responding to topical measures and keeping the extremities warm, the use of hydroxychloroquine has been reported to be beneficial in some patients as well as the use of calcium-channel blockers.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego.
References
1. Su WP et al. Cutis. 1994 Dec;54(6):395-9.
2. Boada A et al. Am J Dermatopathol. 2010 Feb;32(1):19-23.
3. Patel et al. SBMJ Case Rep. 2013;2013:bcr2013201165.
4. Genes Yi et al. BMC. 2020 Apr 15;18(1):32.
5. Battesti G et al. J Am Acad Dermatol. 2020;83(4):1219-22.
6. Cappel JA et al. Mayo Clin Proc. 2014 Feb;89(2):207-15.
7. Cappel MA et al. Mayo Clin Proc. 2021;96(4):989-1005.
A punch biopsy from one of the lesions on the feet showed subtle basal vacuolar interface inflammation on the epidermis and rare apoptotic keratinocytes. There was an underlying dermal lymphocytic inflammatory infiltrate around the vascular plexus. Dermal mucin appeared slightly increased. The histologic findings are consistent with pernio. He had a negative direct immunofluorescence study.
Laboratory work-up showed an elevated antinuclear antibody (ANA) of 1:620; positive anticardiolipin IgM was at 15.2. A complete blood count showed no anemia or lymphopenia, he had normal complement C3 and C4 levels, normal urinalysis, negative cryoglobulins and cold agglutinins, and a normal protein electrophoresis.
Given the chronicity of his lesions, the lack of improvement with weather changes, the histopathologic findings of a vacuolar interface dermatitis and the positive ANA titer he was diagnosed with chilblain lupus.
Chilblain lupus erythematosus (CLE) is an uncommon form of chronic cutaneous lupus erythematosus that presents with tender pink to violaceous macules, papules, and/or nodules that sometimes can ulcerate and are present on the fingers, toes, and sometimes the nose and ears. The lesions are usually triggered by cold exposure.1 These patients also have clinical and laboratory findings consistent with lupus erythematosus.
Even though more studies are needed to clarify the clinical and histopathologic features of chilblain lupus, compared with idiopathic pernio, some authors suggest several characteristics: CLE lesions tend to persist in summer months, as occurred in our patient, and histopathologic evaluation usually shows vacuolar and interface inflammation on the basal cell layer and may also have a positive lupus band on direct immunofluorescence.2 About 20% of patient with CLE may later develop systemic lupus erythematosus.3
There is also a familial form of CLE which is usually inherited as an autosomal-dominant trait. Mutations in TREX1, SAMHD1, and STING have been described in these patients.4 Affected children present with skin lesions at a young age and those with TREX1 mutations are at a higher risk to develop systemic lupus erythematosus.
The differential diagnosis of chilblain lupus includes idiopathic pernio or pernio secondary to other conditions. Other conditions that are thought to be associated with pernio, besides lupus erythematosus, include infectious causes (hepatitis B, COVID-19 infection),5 autoimmune conditions, malignancy and hematologic disorders (paraproteinemia).6 In histopathology, pernio lesions present with dermal edema and superficial and deep lymphocytic infiltrate.
The pathogenesis of pernio is not fully understood but is thought be related to vasospasm with secondary poor perfusion and ischemia and type I interferon (INF1) immune response. A recent review of the published studies trying to explain the causality between COVID 19 and pernio-like lesions, from January 2020 to December 2020, speculate several possible mechanisms: an increase in the vasoconstrictive, prothrombotic, and proinflammatory effects of the angiotensin II pathway through activation of the ACE2 by the virus; COVID-19 triggers a robust INF1 immune response in predisposed patients; pernio as a sign of mild disease, may be explained by genetic and hormonal differences in the patients affected.7
Another condition that can be confused with CLE is Raynaud phenomenon, were patients present with white to purple to red patches on the fingers and toes after exposure to cold, but in comparison with pernio, the lesions improve within minutes to hours after rewarming. Secondary Raynaud phenomenon can be seen in patients with systemic lupus erythematosus and in patients with other connective tissue disorders. The skin lesions in our patient were persistent and were not triggered by cold exposure, making Raynaud phenomenon less likely. Children with vasculitis can present with painful red, violaceous, or necrotic lesions on the extremities, which can mimic pernio. Vasculitis lesions tend to be more purpuric and angulated, compared with pernio lesions, though in severe cases of pernio with ulceration it may be difficult to distinguish between the two entities and a skin biopsy may be needed.
Sweet syndrome, also known as acute febrile neutrophilic dermatosis, is a rare skin condition in which children present with edematous tender nodules on the hands and with less frequency in other parts of the body with associated fever, malaise, conjunctivitis, or joint pain and it is usually associated with infection or malignancy. Our patient denied any systemic symptoms and had no conjunctivitis nor arthritis.
Most patients with idiopathic pernio do not require a biopsy or further laboratory evaluation unless the lesions are atypical, chronic, or there is a suspected associated condition. The workup for patients with prolonged or atypical pernio-like lesions include a skin biopsy with direct immunofluorescence, ANA, complete blood count, complement levels, antiphospholipid antibodies, cold agglutinins, and cryoglobulins.
Treatment of mild CLE is with moderate- to high-potency topical corticosteroids. In those patients not responding to topical measures and keeping the extremities warm, the use of hydroxychloroquine has been reported to be beneficial in some patients as well as the use of calcium-channel blockers.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego.
References
1. Su WP et al. Cutis. 1994 Dec;54(6):395-9.
2. Boada A et al. Am J Dermatopathol. 2010 Feb;32(1):19-23.
3. Patel et al. SBMJ Case Rep. 2013;2013:bcr2013201165.
4. Genes Yi et al. BMC. 2020 Apr 15;18(1):32.
5. Battesti G et al. J Am Acad Dermatol. 2020;83(4):1219-22.
6. Cappel JA et al. Mayo Clin Proc. 2014 Feb;89(2):207-15.
7. Cappel MA et al. Mayo Clin Proc. 2021;96(4):989-1005.
He denied any hair loss, mouth sores, sun sensitivity, headaches, gastrointestinal complaints, joint pain, or muscle weakness.
He is not taking any medications.
He has been at home doing virtual school and has not traveled. He likes to play the piano. There is no family history of similar lesions, connective tissue disorder, or autoimmunity.
On physical exam he has purple discoloration on the toes with some violaceous and pink papules. On the fingers he has pink to violaceous papules and macules.
There is no joint edema or pain.
Tick talk for families and pediatricians
Spring 2021 has arrived with summer quickly approaching. It is our second spring and summer during the pandemic. Travel restrictions have minimally eased for vaccinated adults. However, neither domestic nor international leisure travel is encouraged for anyone. Ironically, air travel is increasing. For many families, it is time to make decisions regarding summer activities. Outdoor activities have been encouraged throughout the pandemic, which makes it a good time to review tick-borne diseases. Depending on your location, your patients may only have to travel as far as their backyard to sustain a tick bite.
Ticks are a group of obligate, bloodsucking arthropods that feed on mammals, birds, and reptiles. There are three families of ticks. Two families, Ixodidae (hard-bodied ticks) and Argasidae (soft-bodied ticks) are responsible for transmitting the most diseases to humans in the United States. Once a tick is infected with a pathogen it usually survives and transmits it to its next host. Ticks efficiently transmit bacteria, spirochetes, protozoa, rickettsiae, nematodes, and toxins to humans during feeding when the site is exposed to infected salivary gland secretions or regurgitated midgut contents. Pathogen transmission can also occur when the feeding site is contaminated by feces or coxal fluid. Sometimes a tick can transmit multiple pathogens. Not all pathogens are infectious (e.g., tick paralysis, which occurs after exposure to a neurotoxin and red meat allergy because of alpha-gal). Ticks require a blood meal to transform to their next stage of development (larva to nymph to adult). Life cycles of hard and soft ticks differ with most hard ticks undergoing a 2-year life cycle and feeding slowly over many days. In contrast, soft ticks feed multiple times often for less than 1 hour and are capable of transmitting diseases in less than 1 minute.
Rocky Mountain spotted fever was the first recognized tick-borne disease (TBD) in humans. Since then, 18 additional pathogens transmitted by ticks have been identified with 40% being described since 1980. The increased discovery of tickborne pathogens has been attributed to physician awareness of TBD and improved diagnostics. The number of cases of TBD has risen yearly. Ticks are responsible for most vector-transmitted diseases in the United States with Lyme disease most frequently reported.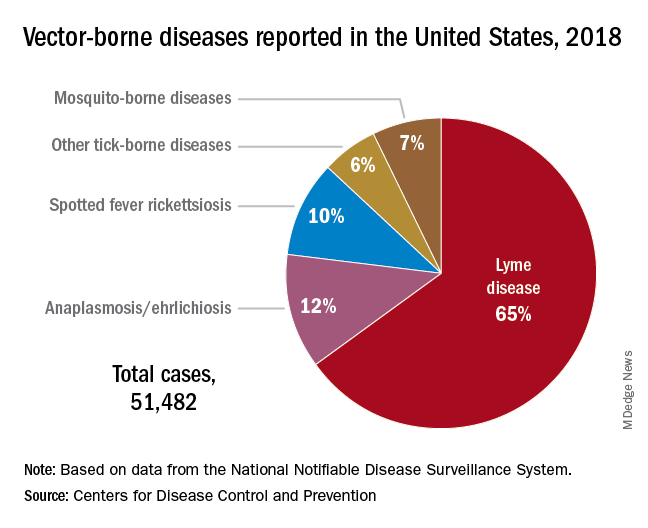
Mosquito transmission accounts for only 7% of vector-borne diseases. Three species of ticks are responsible for most human disease: Ixodes scapularis (Black-legged tick), Amblyomma americanum (Lone Star tick), and Dermacentor variabilis (American dog tick). Each is capable of transmitting agents that cause multiple diseases.
Risk for acquisition of a specific disease is dependent upon the type of tick, its geographic location, the season, and duration of the exposure.
Humans are usually incidental hosts. Tick exposure can occur year-round, but tick activity is greatest between April and September. Ticks are generally found near the ground, in brushy or wooded areas. They can climb tall grasses or shrubs and wait for a potential host to brush against them. When this occurs, they seek a site for attachment.
In the absence of a vaccine, prevention of TBD is totally dependent upon your patients/parents understanding of when and where they are at risk for exposure and for us as physicians to know which pathogens can potentially be transmitted by ticks. Data regarding potential exposure risks are based on where a TBD was diagnosed, not necessarily where it was acquired. National maps that illustrate the distribution of medically significant ticks and presence or prevalence of tick-borne pathogens in specific areas within a region previously may have been incomplete or outdated. The Centers for Disease Control and Prevention initiated a national tick surveillance program in 2017; five universities were established as regional centers of excellence to help prevent and rapidly respond to emerging vector-borne diseases across the United States. One goal is to standardize tick surveillance activities at the state level. For state-specific activity go to https://www.cdc.gov/ncezid/dvbd/vital-signs/index.html.
Prevention: Here are a few environmental interventions you can recommend to your patients
- Remove leaf litter, clear tall brush, and grass around the home and at edge of lawns. Mow the lawn frequently.
- Keep playground equipment, decks, and patios away from yard edges and trees.
- Live near a wooded area? Place a 3-ft.-wide barrier of gravel or wood chips between the areas.
- Put up a fence to keep unwanted animals out.
- Keep the yard free of potential hiding place for ticks (e.g., mattresses or furniture).
- Stack wood neatly and in a dry area.
- Use pesticides, but do not rely on them solely to prevent ticks exposure.
Personal interventions for patients when outdoors
- Use Environmental Protection Agency–registered insect repellents. Note: Oil of lemon-, eucalyptus-, and para-menthane-diol–containing products should not be used in children aged3 years or less.
- Treat clothing and gear with products containing 0.5% permethrin to repel mosquitoes and ticks.
- Check cloths for ticks. Drying clothes on high heat for 10 minutes will kill ticks. If washing is needed use hot water. Lower temperatures will not kill ticks.
- Do daily body checks for ticks after coming indoors.
- Check pets for ticks.
Tick removal
- Take tweezers, grasp the tick as close to the skin’s surface as possible.
- Pull upward. Do not twist or jerk the tick. Place in a container. Ideally submit for species identification.
- After removal, clean the bite area with alcohol or soap and water.
- Never crush a tick with your fingers.
When should you include TBD in your differential for a sick child?
Headache, fever, arthralgia, and rash are symptoms for several infectious diseases. Obtaining a history of recent activities, tick bite, or travel to areas where these diseases are more prevalent is important. You must have a high index of suspicion. Clinical and laboratory clues may help.
Delay in treatment is more detrimental. If you suspect rickettsia, ehrlichiosis, or anaplasmosis, doxycycline should be started promptly regardless of age. Consultation with an infectious disease specialist is recommended.
The United States recognizes it is not adequately prepared to address the continuing rise of vector-borne diseases. In response, on Jan. 20, 2021, the CDC’s division of vector-borne diseases with input from five federal departments and the EPA developed a joint National Public Health Framework for the Prevention and Control of Vector-Borne Diseases in Humans to tackle issues including risk, detection, diagnosis, treatment, prevention and control of TBD. Stay tuned.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures.
Spring 2021 has arrived with summer quickly approaching. It is our second spring and summer during the pandemic. Travel restrictions have minimally eased for vaccinated adults. However, neither domestic nor international leisure travel is encouraged for anyone. Ironically, air travel is increasing. For many families, it is time to make decisions regarding summer activities. Outdoor activities have been encouraged throughout the pandemic, which makes it a good time to review tick-borne diseases. Depending on your location, your patients may only have to travel as far as their backyard to sustain a tick bite.
Ticks are a group of obligate, bloodsucking arthropods that feed on mammals, birds, and reptiles. There are three families of ticks. Two families, Ixodidae (hard-bodied ticks) and Argasidae (soft-bodied ticks) are responsible for transmitting the most diseases to humans in the United States. Once a tick is infected with a pathogen it usually survives and transmits it to its next host. Ticks efficiently transmit bacteria, spirochetes, protozoa, rickettsiae, nematodes, and toxins to humans during feeding when the site is exposed to infected salivary gland secretions or regurgitated midgut contents. Pathogen transmission can also occur when the feeding site is contaminated by feces or coxal fluid. Sometimes a tick can transmit multiple pathogens. Not all pathogens are infectious (e.g., tick paralysis, which occurs after exposure to a neurotoxin and red meat allergy because of alpha-gal). Ticks require a blood meal to transform to their next stage of development (larva to nymph to adult). Life cycles of hard and soft ticks differ with most hard ticks undergoing a 2-year life cycle and feeding slowly over many days. In contrast, soft ticks feed multiple times often for less than 1 hour and are capable of transmitting diseases in less than 1 minute.
Rocky Mountain spotted fever was the first recognized tick-borne disease (TBD) in humans. Since then, 18 additional pathogens transmitted by ticks have been identified with 40% being described since 1980. The increased discovery of tickborne pathogens has been attributed to physician awareness of TBD and improved diagnostics. The number of cases of TBD has risen yearly. Ticks are responsible for most vector-transmitted diseases in the United States with Lyme disease most frequently reported.
Mosquito transmission accounts for only 7% of vector-borne diseases. Three species of ticks are responsible for most human disease: Ixodes scapularis (Black-legged tick), Amblyomma americanum (Lone Star tick), and Dermacentor variabilis (American dog tick). Each is capable of transmitting agents that cause multiple diseases.
Risk for acquisition of a specific disease is dependent upon the type of tick, its geographic location, the season, and duration of the exposure.
Humans are usually incidental hosts. Tick exposure can occur year-round, but tick activity is greatest between April and September. Ticks are generally found near the ground, in brushy or wooded areas. They can climb tall grasses or shrubs and wait for a potential host to brush against them. When this occurs, they seek a site for attachment.
In the absence of a vaccine, prevention of TBD is totally dependent upon your patients/parents understanding of when and where they are at risk for exposure and for us as physicians to know which pathogens can potentially be transmitted by ticks. Data regarding potential exposure risks are based on where a TBD was diagnosed, not necessarily where it was acquired. National maps that illustrate the distribution of medically significant ticks and presence or prevalence of tick-borne pathogens in specific areas within a region previously may have been incomplete or outdated. The Centers for Disease Control and Prevention initiated a national tick surveillance program in 2017; five universities were established as regional centers of excellence to help prevent and rapidly respond to emerging vector-borne diseases across the United States. One goal is to standardize tick surveillance activities at the state level. For state-specific activity go to https://www.cdc.gov/ncezid/dvbd/vital-signs/index.html.
Prevention: Here are a few environmental interventions you can recommend to your patients
- Remove leaf litter, clear tall brush, and grass around the home and at edge of lawns. Mow the lawn frequently.
- Keep playground equipment, decks, and patios away from yard edges and trees.
- Live near a wooded area? Place a 3-ft.-wide barrier of gravel or wood chips between the areas.
- Put up a fence to keep unwanted animals out.
- Keep the yard free of potential hiding place for ticks (e.g., mattresses or furniture).
- Stack wood neatly and in a dry area.
- Use pesticides, but do not rely on them solely to prevent ticks exposure.
Personal interventions for patients when outdoors
- Use Environmental Protection Agency–registered insect repellents. Note: Oil of lemon-, eucalyptus-, and para-menthane-diol–containing products should not be used in children aged3 years or less.
- Treat clothing and gear with products containing 0.5% permethrin to repel mosquitoes and ticks.
- Check cloths for ticks. Drying clothes on high heat for 10 minutes will kill ticks. If washing is needed use hot water. Lower temperatures will not kill ticks.
- Do daily body checks for ticks after coming indoors.
- Check pets for ticks.
Tick removal
- Take tweezers, grasp the tick as close to the skin’s surface as possible.
- Pull upward. Do not twist or jerk the tick. Place in a container. Ideally submit for species identification.
- After removal, clean the bite area with alcohol or soap and water.
- Never crush a tick with your fingers.
When should you include TBD in your differential for a sick child?
Headache, fever, arthralgia, and rash are symptoms for several infectious diseases. Obtaining a history of recent activities, tick bite, or travel to areas where these diseases are more prevalent is important. You must have a high index of suspicion. Clinical and laboratory clues may help.
Delay in treatment is more detrimental. If you suspect rickettsia, ehrlichiosis, or anaplasmosis, doxycycline should be started promptly regardless of age. Consultation with an infectious disease specialist is recommended.
The United States recognizes it is not adequately prepared to address the continuing rise of vector-borne diseases. In response, on Jan. 20, 2021, the CDC’s division of vector-borne diseases with input from five federal departments and the EPA developed a joint National Public Health Framework for the Prevention and Control of Vector-Borne Diseases in Humans to tackle issues including risk, detection, diagnosis, treatment, prevention and control of TBD. Stay tuned.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures.
Spring 2021 has arrived with summer quickly approaching. It is our second spring and summer during the pandemic. Travel restrictions have minimally eased for vaccinated adults. However, neither domestic nor international leisure travel is encouraged for anyone. Ironically, air travel is increasing. For many families, it is time to make decisions regarding summer activities. Outdoor activities have been encouraged throughout the pandemic, which makes it a good time to review tick-borne diseases. Depending on your location, your patients may only have to travel as far as their backyard to sustain a tick bite.
Ticks are a group of obligate, bloodsucking arthropods that feed on mammals, birds, and reptiles. There are three families of ticks. Two families, Ixodidae (hard-bodied ticks) and Argasidae (soft-bodied ticks) are responsible for transmitting the most diseases to humans in the United States. Once a tick is infected with a pathogen it usually survives and transmits it to its next host. Ticks efficiently transmit bacteria, spirochetes, protozoa, rickettsiae, nematodes, and toxins to humans during feeding when the site is exposed to infected salivary gland secretions or regurgitated midgut contents. Pathogen transmission can also occur when the feeding site is contaminated by feces or coxal fluid. Sometimes a tick can transmit multiple pathogens. Not all pathogens are infectious (e.g., tick paralysis, which occurs after exposure to a neurotoxin and red meat allergy because of alpha-gal). Ticks require a blood meal to transform to their next stage of development (larva to nymph to adult). Life cycles of hard and soft ticks differ with most hard ticks undergoing a 2-year life cycle and feeding slowly over many days. In contrast, soft ticks feed multiple times often for less than 1 hour and are capable of transmitting diseases in less than 1 minute.
Rocky Mountain spotted fever was the first recognized tick-borne disease (TBD) in humans. Since then, 18 additional pathogens transmitted by ticks have been identified with 40% being described since 1980. The increased discovery of tickborne pathogens has been attributed to physician awareness of TBD and improved diagnostics. The number of cases of TBD has risen yearly. Ticks are responsible for most vector-transmitted diseases in the United States with Lyme disease most frequently reported.
Mosquito transmission accounts for only 7% of vector-borne diseases. Three species of ticks are responsible for most human disease: Ixodes scapularis (Black-legged tick), Amblyomma americanum (Lone Star tick), and Dermacentor variabilis (American dog tick). Each is capable of transmitting agents that cause multiple diseases.
Risk for acquisition of a specific disease is dependent upon the type of tick, its geographic location, the season, and duration of the exposure.
Humans are usually incidental hosts. Tick exposure can occur year-round, but tick activity is greatest between April and September. Ticks are generally found near the ground, in brushy or wooded areas. They can climb tall grasses or shrubs and wait for a potential host to brush against them. When this occurs, they seek a site for attachment.
In the absence of a vaccine, prevention of TBD is totally dependent upon your patients/parents understanding of when and where they are at risk for exposure and for us as physicians to know which pathogens can potentially be transmitted by ticks. Data regarding potential exposure risks are based on where a TBD was diagnosed, not necessarily where it was acquired. National maps that illustrate the distribution of medically significant ticks and presence or prevalence of tick-borne pathogens in specific areas within a region previously may have been incomplete or outdated. The Centers for Disease Control and Prevention initiated a national tick surveillance program in 2017; five universities were established as regional centers of excellence to help prevent and rapidly respond to emerging vector-borne diseases across the United States. One goal is to standardize tick surveillance activities at the state level. For state-specific activity go to https://www.cdc.gov/ncezid/dvbd/vital-signs/index.html.
Prevention: Here are a few environmental interventions you can recommend to your patients
- Remove leaf litter, clear tall brush, and grass around the home and at edge of lawns. Mow the lawn frequently.
- Keep playground equipment, decks, and patios away from yard edges and trees.
- Live near a wooded area? Place a 3-ft.-wide barrier of gravel or wood chips between the areas.
- Put up a fence to keep unwanted animals out.
- Keep the yard free of potential hiding place for ticks (e.g., mattresses or furniture).
- Stack wood neatly and in a dry area.
- Use pesticides, but do not rely on them solely to prevent ticks exposure.
Personal interventions for patients when outdoors
- Use Environmental Protection Agency–registered insect repellents. Note: Oil of lemon-, eucalyptus-, and para-menthane-diol–containing products should not be used in children aged3 years or less.
- Treat clothing and gear with products containing 0.5% permethrin to repel mosquitoes and ticks.
- Check cloths for ticks. Drying clothes on high heat for 10 minutes will kill ticks. If washing is needed use hot water. Lower temperatures will not kill ticks.
- Do daily body checks for ticks after coming indoors.
- Check pets for ticks.
Tick removal
- Take tweezers, grasp the tick as close to the skin’s surface as possible.
- Pull upward. Do not twist or jerk the tick. Place in a container. Ideally submit for species identification.
- After removal, clean the bite area with alcohol or soap and water.
- Never crush a tick with your fingers.
When should you include TBD in your differential for a sick child?
Headache, fever, arthralgia, and rash are symptoms for several infectious diseases. Obtaining a history of recent activities, tick bite, or travel to areas where these diseases are more prevalent is important. You must have a high index of suspicion. Clinical and laboratory clues may help.
Delay in treatment is more detrimental. If you suspect rickettsia, ehrlichiosis, or anaplasmosis, doxycycline should be started promptly regardless of age. Consultation with an infectious disease specialist is recommended.
The United States recognizes it is not adequately prepared to address the continuing rise of vector-borne diseases. In response, on Jan. 20, 2021, the CDC’s division of vector-borne diseases with input from five federal departments and the EPA developed a joint National Public Health Framework for the Prevention and Control of Vector-Borne Diseases in Humans to tackle issues including risk, detection, diagnosis, treatment, prevention and control of TBD. Stay tuned.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures.
Adolescent substance use and the COVID-19 pandemic
During the past year, adolescents, families, educators, and health care providers have had to press forward through myriad challenges and stressors with flexibility and adaptability. With appropriate concern, we ask ourselves how children and youth are coping emotionally with the unprecedented changes of the past year.
Adolescent substance use represents an important area of concern. What has happened during the pandemic? Has youth substance use increased or decreased? Has access to substances increased or decreased, has monitoring and support for at-risk youth increased or decreased?
The answers to these questions are mixed. If anything, the pandemic has highlighted the heterogeneity of adolescent substance use. Now is a key time for assessment, support, and conversation with teens and families.
Monitoring the Future (MTF), a nationally representative annual survey, has provided a broad perspective on trends of adolescent substance use for decades.1 The MTF data is usually collected from February to May and was cut short in 2020 because of school closures associated with the pandemic. The sample size, though still nationally representative, was about a quarter of the typical volume. Some of the data are encouraging, including a flattening out of previous years’ stark increase in vaping of both nicotine and cannabis products (though overall numbers remain alarmingly high). Other data are more concerning including a continued increase in misuse of cough medicine, amphetamines, and inhalants among the youngest cohort surveyed (eighth graders). However, these data were largely representative of prepandemic circumstances.
The COVID-19 pandemic has significantly affected risk and protective factors for teen drug and alcohol use. Most notably, it has had a widely observed negative impact on adolescent mental health, across multiple disease categories.2 In addition, the cancellation of in-person academic and extracurricular activities such as arts and athletics markedly increased unstructured time, a known associated factor for higher-risk activities including substance use. This has also led to decreased contact with many supportive adults such as teachers and coaches. On the other hand, some adolescents now have more time with supportive parents and caregivers, more meals together, and more supervision, all of which are associated with decreased likelihood of substance use disorders.
The highly variable reasons for substance use affect highly variable pandemic-related changes in use. Understanding the impetus for use is a good place to start conversation and can help providers assess risk of escalation during the pandemic. Some teens primarily use for social enhancement while others use as a means of coping with stress or to mask or escape negative emotions. Still others continue use because of physiological dependence, craving, and other symptoms consistent with use disorders.
Highlighting the heterogeneity of this issue, one study assessing use early in the pandemic showed a decrease in the percentage of teens who use substances but an increase in frequency of use for those who are using.3 Though expected, an increase in frequency of use by oneself as compared with peers was also notable. Using substances alone is associated with more severe use disorders, carries greater risk of overdose, and can increase shame and secrecy, further fueling use disorders.
The pandemic has thus represented a protective pause for some experimental or socially motivated substance-using teens who have experienced a period of abstinence even if not fully by choice. For others, it has represented an acute amplification of risk factors and use has accelerated. This latter group includes those whose use represents an effort to cope with depression, anxiety, and loneliness or for whom isolation at home represents less monitoring, increased access, and greater exposure to substances.
Over the past year, in the treatment of adolescents struggling with substance use, many clinicians have observed a sifting effect during these unprecedented social changes. Many youth, who no longer have access to substances, have found they can “take it or leave it”. Other youth have been observed engaging in additional risk or going to greater lengths to access substances and continue their use. For both groups and everyone in between, this is an important time for screening, clinical assessment, and support.
While anticipating further research and data regarding broad substance use trends, including MTF data from 2021, recognizing that the impact of the COVID-19 pandemic is individual, with marked differences from adolescent to adolescent, will help us continue to act now to assess this important area of adolescent health. The first step for primary care providers is unchanged: to routinely screen for and discuss substance use in clinical settings.
Two brief, validated, easily accessible screening tools are available for primary care settings. They can both be self-administered and take less than 2 minutes to complete. Screening, Brief Intervention and Referral to Treatment and the Brief Screener for Tobacco, Alcohol and other Drugs can both be used for youth aged 12-17 years.4,5 Both screens are available online at drugabuse.gov.6
Routine screening will normalize conversations about substance use and healthy choices, provide opportunities for positive reinforcement, identify adolescents at risk, increase comfort and competence in providing brief intervention, and expedite referrals for additional support and treatment.
A false assumption that a particular adolescent isn’t using substances creates a missed opportunity to offer guidance and treatment. An oft-overlooked opportunity is that of providing positive reinforcement for an adolescent who isn’t using any substances or experimenting at all. Positive reinforcement is a strong component of reinforcing health maintenance.
Parent guidance and family assessment will also be critical tools. Parents and caregivers play a primary role in substance use treatment for teens and have a contributory impact on risk through both genes and environment. Of note, research suggests a moderate overall increase in adult substance use during the pandemic, particularly substances that are widely available such as alcohol. Adolescents may thus have greater access and exposure to substance use. A remarkably high percentage, 42%, of substance-using teens surveyed early in the pandemic indicated that they were using substances with their parents.3 Parents, who have equally been challenged by the pandemic, may need guidance in balancing compassion and support for struggling youth, while setting appropriate limits and maintaining expectations of healthy activities.
Unprecedented change and uncertainty provide an opportunity to reassess risks and openly discuss substance use with youth and families. Even with much on our minds during the COVID-19 pandemic, we can maintain focus on this significant risk to adolescent health and wellness. Our efforts now, from screening to treatment for adolescent substance use should be reinforced rather than delayed.
Dr. Jackson is assistant professor of psychiatry at the University of Vermont, Burlington.
References
1. Monitoringthefuture.org
2. Jones EAK et al. Int J Environ Res Public Health, 2021;18(5):2470.
3. Dumas TM et al. J Adolesc Health, 2020;67(3):354-61.
4. Levy S et al. JAMA Pediatr. 2014;168(9):822-8.
5. Kelly SM et al. Pediatrics. 2014;133(5):819-26.
6. National Institute on Drug Abuse. Adolescent Substance Use Screening Tools. 2016 Apr 27. https://www.drugabuse.gov/nidamed-medical-health-professionals/screening-tools-prevention/screening-tools-adolescent-substance-use/adolescent-substance-use-screening-tools
During the past year, adolescents, families, educators, and health care providers have had to press forward through myriad challenges and stressors with flexibility and adaptability. With appropriate concern, we ask ourselves how children and youth are coping emotionally with the unprecedented changes of the past year.
Adolescent substance use represents an important area of concern. What has happened during the pandemic? Has youth substance use increased or decreased? Has access to substances increased or decreased, has monitoring and support for at-risk youth increased or decreased?
The answers to these questions are mixed. If anything, the pandemic has highlighted the heterogeneity of adolescent substance use. Now is a key time for assessment, support, and conversation with teens and families.
Monitoring the Future (MTF), a nationally representative annual survey, has provided a broad perspective on trends of adolescent substance use for decades.1 The MTF data is usually collected from February to May and was cut short in 2020 because of school closures associated with the pandemic. The sample size, though still nationally representative, was about a quarter of the typical volume. Some of the data are encouraging, including a flattening out of previous years’ stark increase in vaping of both nicotine and cannabis products (though overall numbers remain alarmingly high). Other data are more concerning including a continued increase in misuse of cough medicine, amphetamines, and inhalants among the youngest cohort surveyed (eighth graders). However, these data were largely representative of prepandemic circumstances.
The COVID-19 pandemic has significantly affected risk and protective factors for teen drug and alcohol use. Most notably, it has had a widely observed negative impact on adolescent mental health, across multiple disease categories.2 In addition, the cancellation of in-person academic and extracurricular activities such as arts and athletics markedly increased unstructured time, a known associated factor for higher-risk activities including substance use. This has also led to decreased contact with many supportive adults such as teachers and coaches. On the other hand, some adolescents now have more time with supportive parents and caregivers, more meals together, and more supervision, all of which are associated with decreased likelihood of substance use disorders.
The highly variable reasons for substance use affect highly variable pandemic-related changes in use. Understanding the impetus for use is a good place to start conversation and can help providers assess risk of escalation during the pandemic. Some teens primarily use for social enhancement while others use as a means of coping with stress or to mask or escape negative emotions. Still others continue use because of physiological dependence, craving, and other symptoms consistent with use disorders.
Highlighting the heterogeneity of this issue, one study assessing use early in the pandemic showed a decrease in the percentage of teens who use substances but an increase in frequency of use for those who are using.3 Though expected, an increase in frequency of use by oneself as compared with peers was also notable. Using substances alone is associated with more severe use disorders, carries greater risk of overdose, and can increase shame and secrecy, further fueling use disorders.
The pandemic has thus represented a protective pause for some experimental or socially motivated substance-using teens who have experienced a period of abstinence even if not fully by choice. For others, it has represented an acute amplification of risk factors and use has accelerated. This latter group includes those whose use represents an effort to cope with depression, anxiety, and loneliness or for whom isolation at home represents less monitoring, increased access, and greater exposure to substances.
Over the past year, in the treatment of adolescents struggling with substance use, many clinicians have observed a sifting effect during these unprecedented social changes. Many youth, who no longer have access to substances, have found they can “take it or leave it”. Other youth have been observed engaging in additional risk or going to greater lengths to access substances and continue their use. For both groups and everyone in between, this is an important time for screening, clinical assessment, and support.
While anticipating further research and data regarding broad substance use trends, including MTF data from 2021, recognizing that the impact of the COVID-19 pandemic is individual, with marked differences from adolescent to adolescent, will help us continue to act now to assess this important area of adolescent health. The first step for primary care providers is unchanged: to routinely screen for and discuss substance use in clinical settings.
Two brief, validated, easily accessible screening tools are available for primary care settings. They can both be self-administered and take less than 2 minutes to complete. Screening, Brief Intervention and Referral to Treatment and the Brief Screener for Tobacco, Alcohol and other Drugs can both be used for youth aged 12-17 years.4,5 Both screens are available online at drugabuse.gov.6
Routine screening will normalize conversations about substance use and healthy choices, provide opportunities for positive reinforcement, identify adolescents at risk, increase comfort and competence in providing brief intervention, and expedite referrals for additional support and treatment.
A false assumption that a particular adolescent isn’t using substances creates a missed opportunity to offer guidance and treatment. An oft-overlooked opportunity is that of providing positive reinforcement for an adolescent who isn’t using any substances or experimenting at all. Positive reinforcement is a strong component of reinforcing health maintenance.
Parent guidance and family assessment will also be critical tools. Parents and caregivers play a primary role in substance use treatment for teens and have a contributory impact on risk through both genes and environment. Of note, research suggests a moderate overall increase in adult substance use during the pandemic, particularly substances that are widely available such as alcohol. Adolescents may thus have greater access and exposure to substance use. A remarkably high percentage, 42%, of substance-using teens surveyed early in the pandemic indicated that they were using substances with their parents.3 Parents, who have equally been challenged by the pandemic, may need guidance in balancing compassion and support for struggling youth, while setting appropriate limits and maintaining expectations of healthy activities.
Unprecedented change and uncertainty provide an opportunity to reassess risks and openly discuss substance use with youth and families. Even with much on our minds during the COVID-19 pandemic, we can maintain focus on this significant risk to adolescent health and wellness. Our efforts now, from screening to treatment for adolescent substance use should be reinforced rather than delayed.
Dr. Jackson is assistant professor of psychiatry at the University of Vermont, Burlington.
References
1. Monitoringthefuture.org
2. Jones EAK et al. Int J Environ Res Public Health, 2021;18(5):2470.
3. Dumas TM et al. J Adolesc Health, 2020;67(3):354-61.
4. Levy S et al. JAMA Pediatr. 2014;168(9):822-8.
5. Kelly SM et al. Pediatrics. 2014;133(5):819-26.
6. National Institute on Drug Abuse. Adolescent Substance Use Screening Tools. 2016 Apr 27. https://www.drugabuse.gov/nidamed-medical-health-professionals/screening-tools-prevention/screening-tools-adolescent-substance-use/adolescent-substance-use-screening-tools
During the past year, adolescents, families, educators, and health care providers have had to press forward through myriad challenges and stressors with flexibility and adaptability. With appropriate concern, we ask ourselves how children and youth are coping emotionally with the unprecedented changes of the past year.
Adolescent substance use represents an important area of concern. What has happened during the pandemic? Has youth substance use increased or decreased? Has access to substances increased or decreased, has monitoring and support for at-risk youth increased or decreased?
The answers to these questions are mixed. If anything, the pandemic has highlighted the heterogeneity of adolescent substance use. Now is a key time for assessment, support, and conversation with teens and families.
Monitoring the Future (MTF), a nationally representative annual survey, has provided a broad perspective on trends of adolescent substance use for decades.1 The MTF data is usually collected from February to May and was cut short in 2020 because of school closures associated with the pandemic. The sample size, though still nationally representative, was about a quarter of the typical volume. Some of the data are encouraging, including a flattening out of previous years’ stark increase in vaping of both nicotine and cannabis products (though overall numbers remain alarmingly high). Other data are more concerning including a continued increase in misuse of cough medicine, amphetamines, and inhalants among the youngest cohort surveyed (eighth graders). However, these data were largely representative of prepandemic circumstances.
The COVID-19 pandemic has significantly affected risk and protective factors for teen drug and alcohol use. Most notably, it has had a widely observed negative impact on adolescent mental health, across multiple disease categories.2 In addition, the cancellation of in-person academic and extracurricular activities such as arts and athletics markedly increased unstructured time, a known associated factor for higher-risk activities including substance use. This has also led to decreased contact with many supportive adults such as teachers and coaches. On the other hand, some adolescents now have more time with supportive parents and caregivers, more meals together, and more supervision, all of which are associated with decreased likelihood of substance use disorders.
The highly variable reasons for substance use affect highly variable pandemic-related changes in use. Understanding the impetus for use is a good place to start conversation and can help providers assess risk of escalation during the pandemic. Some teens primarily use for social enhancement while others use as a means of coping with stress or to mask or escape negative emotions. Still others continue use because of physiological dependence, craving, and other symptoms consistent with use disorders.
Highlighting the heterogeneity of this issue, one study assessing use early in the pandemic showed a decrease in the percentage of teens who use substances but an increase in frequency of use for those who are using.3 Though expected, an increase in frequency of use by oneself as compared with peers was also notable. Using substances alone is associated with more severe use disorders, carries greater risk of overdose, and can increase shame and secrecy, further fueling use disorders.
The pandemic has thus represented a protective pause for some experimental or socially motivated substance-using teens who have experienced a period of abstinence even if not fully by choice. For others, it has represented an acute amplification of risk factors and use has accelerated. This latter group includes those whose use represents an effort to cope with depression, anxiety, and loneliness or for whom isolation at home represents less monitoring, increased access, and greater exposure to substances.
Over the past year, in the treatment of adolescents struggling with substance use, many clinicians have observed a sifting effect during these unprecedented social changes. Many youth, who no longer have access to substances, have found they can “take it or leave it”. Other youth have been observed engaging in additional risk or going to greater lengths to access substances and continue their use. For both groups and everyone in between, this is an important time for screening, clinical assessment, and support.
While anticipating further research and data regarding broad substance use trends, including MTF data from 2021, recognizing that the impact of the COVID-19 pandemic is individual, with marked differences from adolescent to adolescent, will help us continue to act now to assess this important area of adolescent health. The first step for primary care providers is unchanged: to routinely screen for and discuss substance use in clinical settings.
Two brief, validated, easily accessible screening tools are available for primary care settings. They can both be self-administered and take less than 2 minutes to complete. Screening, Brief Intervention and Referral to Treatment and the Brief Screener for Tobacco, Alcohol and other Drugs can both be used for youth aged 12-17 years.4,5 Both screens are available online at drugabuse.gov.6
Routine screening will normalize conversations about substance use and healthy choices, provide opportunities for positive reinforcement, identify adolescents at risk, increase comfort and competence in providing brief intervention, and expedite referrals for additional support and treatment.
A false assumption that a particular adolescent isn’t using substances creates a missed opportunity to offer guidance and treatment. An oft-overlooked opportunity is that of providing positive reinforcement for an adolescent who isn’t using any substances or experimenting at all. Positive reinforcement is a strong component of reinforcing health maintenance.
Parent guidance and family assessment will also be critical tools. Parents and caregivers play a primary role in substance use treatment for teens and have a contributory impact on risk through both genes and environment. Of note, research suggests a moderate overall increase in adult substance use during the pandemic, particularly substances that are widely available such as alcohol. Adolescents may thus have greater access and exposure to substance use. A remarkably high percentage, 42%, of substance-using teens surveyed early in the pandemic indicated that they were using substances with their parents.3 Parents, who have equally been challenged by the pandemic, may need guidance in balancing compassion and support for struggling youth, while setting appropriate limits and maintaining expectations of healthy activities.
Unprecedented change and uncertainty provide an opportunity to reassess risks and openly discuss substance use with youth and families. Even with much on our minds during the COVID-19 pandemic, we can maintain focus on this significant risk to adolescent health and wellness. Our efforts now, from screening to treatment for adolescent substance use should be reinforced rather than delayed.
Dr. Jackson is assistant professor of psychiatry at the University of Vermont, Burlington.
References
1. Monitoringthefuture.org
2. Jones EAK et al. Int J Environ Res Public Health, 2021;18(5):2470.
3. Dumas TM et al. J Adolesc Health, 2020;67(3):354-61.
4. Levy S et al. JAMA Pediatr. 2014;168(9):822-8.
5. Kelly SM et al. Pediatrics. 2014;133(5):819-26.
6. National Institute on Drug Abuse. Adolescent Substance Use Screening Tools. 2016 Apr 27. https://www.drugabuse.gov/nidamed-medical-health-professionals/screening-tools-prevention/screening-tools-adolescent-substance-use/adolescent-substance-use-screening-tools
CDC panel: Pause of J&J COVID-19 vaccine to remain for now
The Advisory Committee on Immunization Practices decided there was not adequate information to change again recommend use of the Johnson & Johnson vaccine.
The committee’s decision comes the day after the CDC and Food and Drug Administration recommended that J&J injections be paused after reports of rare, but serious types of blood clots in six patients among the 6.8 million people who had received the J&J vaccine in the United States.
A member of the committee, Beth Bell, MD, said: “I do not want to be sending a message that there is some huge concern here on a different order of magnitude than any other vaccine safety signals that we evaluate. And I don’t want to send a message that there is something fundamentally wrong with the vaccine because that also I don’t agree with.”
At the end of the 4-hour meeting, ACIP members decided to call a meeting in 1 or 2 weeks and evaluate more safety data, specifically reports of people who have received the J&J vaccine in the past 2 weeks.
Some, however, pointed out that delaying a decision could have substantial consequences as well in terms of unused vaccine doses and public confidence.
Committee member Camiile Kotton, MD, described the pause as “devastating.”
“Putting this vaccine on pause for those of us that are frontline health care workers has really been devastating,” she said. “I agree in general that we don’t have enough data to make a decision at this time but we were planning on using this vaccine in the state of Massachusetts for people who were homebound and otherwise not able to get a vaccine. We were planning on using it for our vulnerable inpatient population often with many comorbidities and at high risk for disease but haven’t been able to get vaccinated otherwise.”
Pausing the one-and-done vaccine that doesn’t have the significant refrigeration requirements of the others “is a significant loss,” she said.
What is known, not known
Sara Oliver, MD, who leads the COVID-19 Vaccines ACIP Work Group, summarized what is known and unknown about the blood clots.
Among the six cases of cerebral venous sinus thrombosis reported to the Vaccine Adverse Event Reporting System after the J&J shot, all were women aged 18-48 years and all developed the clots 6-13 days after receiving the vaccine.
No cases of these clots have been reported from either the Pfizer or Moderna shots, she noted.
In the United States, the two mRNA vaccine alternatives – the Moderna and Pfizer vaccines – are available “and based on current projections supply of both vaccines are expected to be relatively stable in the near future,” she said.
She said 14 million doses of Pfizer and Moderna are expected each week in the United States and J&J vaccines makes up less than 5% of vaccines administered in the country.
Approximately 13 million J&J doses are available to order or are already at administration sites, she said.
But much more is unknown, she said.
“There may be more cases identified in the coming days to weeks,” Dr. Oliver said, referring back to the average time from vaccination to symptom onset.
Scott Ratzan, MD, editor-in-chief of the Journal of Health Communication: International Perspectives and executive director of Business Partners to CONVINCE (BP2C), a global network of employers that promotes COVID-19 vaccination among employees, suppliers, and customers, applauded ACIP’s delay on making a decision.
Dr. Ratzan, who watched the deliberations online, said in an interview the decision “shows an admirable abundance of caution in the distribution of COVID-19 vaccines.”
“Unfortunately,” he said, “the pause also worsens the existing and pervasive vaccine hesitancy issue.
“We need a rational strategy regarding who should or should not get the J&J/Janssen vaccine since these rare adverse events appear to affect a particular group of people, females aged 18-48. It is essential that we build vaccine confidence and retain the option of using this vaccine for people who are not in this risk group.”
He pointed out there are safety red flags with the Pfizer and Moderna COVID-19 vaccines.
“We should feel reassured about the process of ensuring vaccine safety as the FDA and CDC have quickly addressed risk and shared the data transparently of the J&J vaccine and taken appropriate action,” he said.
ACIP’s executive secretary, Amanda Cohn, MD, said the date for the next meeting would be set by April 16.
A version of this article first appeared on WebMD.com.
The Advisory Committee on Immunization Practices decided there was not adequate information to change again recommend use of the Johnson & Johnson vaccine.
The committee’s decision comes the day after the CDC and Food and Drug Administration recommended that J&J injections be paused after reports of rare, but serious types of blood clots in six patients among the 6.8 million people who had received the J&J vaccine in the United States.
A member of the committee, Beth Bell, MD, said: “I do not want to be sending a message that there is some huge concern here on a different order of magnitude than any other vaccine safety signals that we evaluate. And I don’t want to send a message that there is something fundamentally wrong with the vaccine because that also I don’t agree with.”
At the end of the 4-hour meeting, ACIP members decided to call a meeting in 1 or 2 weeks and evaluate more safety data, specifically reports of people who have received the J&J vaccine in the past 2 weeks.
Some, however, pointed out that delaying a decision could have substantial consequences as well in terms of unused vaccine doses and public confidence.
Committee member Camiile Kotton, MD, described the pause as “devastating.”
“Putting this vaccine on pause for those of us that are frontline health care workers has really been devastating,” she said. “I agree in general that we don’t have enough data to make a decision at this time but we were planning on using this vaccine in the state of Massachusetts for people who were homebound and otherwise not able to get a vaccine. We were planning on using it for our vulnerable inpatient population often with many comorbidities and at high risk for disease but haven’t been able to get vaccinated otherwise.”
Pausing the one-and-done vaccine that doesn’t have the significant refrigeration requirements of the others “is a significant loss,” she said.
What is known, not known
Sara Oliver, MD, who leads the COVID-19 Vaccines ACIP Work Group, summarized what is known and unknown about the blood clots.
Among the six cases of cerebral venous sinus thrombosis reported to the Vaccine Adverse Event Reporting System after the J&J shot, all were women aged 18-48 years and all developed the clots 6-13 days after receiving the vaccine.
No cases of these clots have been reported from either the Pfizer or Moderna shots, she noted.
In the United States, the two mRNA vaccine alternatives – the Moderna and Pfizer vaccines – are available “and based on current projections supply of both vaccines are expected to be relatively stable in the near future,” she said.
She said 14 million doses of Pfizer and Moderna are expected each week in the United States and J&J vaccines makes up less than 5% of vaccines administered in the country.
Approximately 13 million J&J doses are available to order or are already at administration sites, she said.
But much more is unknown, she said.
“There may be more cases identified in the coming days to weeks,” Dr. Oliver said, referring back to the average time from vaccination to symptom onset.
Scott Ratzan, MD, editor-in-chief of the Journal of Health Communication: International Perspectives and executive director of Business Partners to CONVINCE (BP2C), a global network of employers that promotes COVID-19 vaccination among employees, suppliers, and customers, applauded ACIP’s delay on making a decision.
Dr. Ratzan, who watched the deliberations online, said in an interview the decision “shows an admirable abundance of caution in the distribution of COVID-19 vaccines.”
“Unfortunately,” he said, “the pause also worsens the existing and pervasive vaccine hesitancy issue.
“We need a rational strategy regarding who should or should not get the J&J/Janssen vaccine since these rare adverse events appear to affect a particular group of people, females aged 18-48. It is essential that we build vaccine confidence and retain the option of using this vaccine for people who are not in this risk group.”
He pointed out there are safety red flags with the Pfizer and Moderna COVID-19 vaccines.
“We should feel reassured about the process of ensuring vaccine safety as the FDA and CDC have quickly addressed risk and shared the data transparently of the J&J vaccine and taken appropriate action,” he said.
ACIP’s executive secretary, Amanda Cohn, MD, said the date for the next meeting would be set by April 16.
A version of this article first appeared on WebMD.com.
The Advisory Committee on Immunization Practices decided there was not adequate information to change again recommend use of the Johnson & Johnson vaccine.
The committee’s decision comes the day after the CDC and Food and Drug Administration recommended that J&J injections be paused after reports of rare, but serious types of blood clots in six patients among the 6.8 million people who had received the J&J vaccine in the United States.
A member of the committee, Beth Bell, MD, said: “I do not want to be sending a message that there is some huge concern here on a different order of magnitude than any other vaccine safety signals that we evaluate. And I don’t want to send a message that there is something fundamentally wrong with the vaccine because that also I don’t agree with.”
At the end of the 4-hour meeting, ACIP members decided to call a meeting in 1 or 2 weeks and evaluate more safety data, specifically reports of people who have received the J&J vaccine in the past 2 weeks.
Some, however, pointed out that delaying a decision could have substantial consequences as well in terms of unused vaccine doses and public confidence.
Committee member Camiile Kotton, MD, described the pause as “devastating.”
“Putting this vaccine on pause for those of us that are frontline health care workers has really been devastating,” she said. “I agree in general that we don’t have enough data to make a decision at this time but we were planning on using this vaccine in the state of Massachusetts for people who were homebound and otherwise not able to get a vaccine. We were planning on using it for our vulnerable inpatient population often with many comorbidities and at high risk for disease but haven’t been able to get vaccinated otherwise.”
Pausing the one-and-done vaccine that doesn’t have the significant refrigeration requirements of the others “is a significant loss,” she said.
What is known, not known
Sara Oliver, MD, who leads the COVID-19 Vaccines ACIP Work Group, summarized what is known and unknown about the blood clots.
Among the six cases of cerebral venous sinus thrombosis reported to the Vaccine Adverse Event Reporting System after the J&J shot, all were women aged 18-48 years and all developed the clots 6-13 days after receiving the vaccine.
No cases of these clots have been reported from either the Pfizer or Moderna shots, she noted.
In the United States, the two mRNA vaccine alternatives – the Moderna and Pfizer vaccines – are available “and based on current projections supply of both vaccines are expected to be relatively stable in the near future,” she said.
She said 14 million doses of Pfizer and Moderna are expected each week in the United States and J&J vaccines makes up less than 5% of vaccines administered in the country.
Approximately 13 million J&J doses are available to order or are already at administration sites, she said.
But much more is unknown, she said.
“There may be more cases identified in the coming days to weeks,” Dr. Oliver said, referring back to the average time from vaccination to symptom onset.
Scott Ratzan, MD, editor-in-chief of the Journal of Health Communication: International Perspectives and executive director of Business Partners to CONVINCE (BP2C), a global network of employers that promotes COVID-19 vaccination among employees, suppliers, and customers, applauded ACIP’s delay on making a decision.
Dr. Ratzan, who watched the deliberations online, said in an interview the decision “shows an admirable abundance of caution in the distribution of COVID-19 vaccines.”
“Unfortunately,” he said, “the pause also worsens the existing and pervasive vaccine hesitancy issue.
“We need a rational strategy regarding who should or should not get the J&J/Janssen vaccine since these rare adverse events appear to affect a particular group of people, females aged 18-48. It is essential that we build vaccine confidence and retain the option of using this vaccine for people who are not in this risk group.”
He pointed out there are safety red flags with the Pfizer and Moderna COVID-19 vaccines.
“We should feel reassured about the process of ensuring vaccine safety as the FDA and CDC have quickly addressed risk and shared the data transparently of the J&J vaccine and taken appropriate action,” he said.
ACIP’s executive secretary, Amanda Cohn, MD, said the date for the next meeting would be set by April 16.
A version of this article first appeared on WebMD.com.
I sent my suicidal teen patient to the ED: Whew?
You read “thoughts of being better off dead” on your next patient’s PHQ-9 screen results and break into a sweat. After eliciting the teen’s realistic suicide plan and intent you send him to the ED with his parent for crisis mental health evaluation. When you call the family that evening to follow-up you hear that he was discharged with a “mental health counseling” appointment next week.
Have you done enough to prevent this child from dying at his own hand? I imagine that this haunts you as it does me. It is terrifying to know that, of youth with suicidal ideation, over one-third attempt suicide, most within 1-2 years, and 20%-40% do so without having had a plan.
We now know that certain kinds of psychotherapy have evidence for preventing subsequent suicide in teens at high risk due to suicidal ideation and past attempts. Cognitive behavioral therapy (CBT) has the best evidence including its subtypes for youth with relevant histories: for both suicide and substance use (integrated, or I-CBT), trauma focused (TF-CBT), traumatic grief (CTG-CBT), and CBT-I, for the potent risk factor of insomnia. The other treatment shown to reduce risk is dialectical behavioral therapy–adolescent (DBT-A) focused on strengthening skills in interpersonal effectiveness, mindfulness, distress tolerance, and emotion regulation adapted to youth by adding family therapy and multifamily skills training. Interpersonal psychotherapy (IPT) adapted for suicidal and self-harming adolescents (IPT-SA) also has evidence.
Some school programs have shown moderate efficacy, for example (IPT-A-IN) addresses the social and interpersonal context, and Youth Aware of Mental Health, a school curriculum to increase knowledge, help-seeking, and ways of coping with depression and suicidal behavior, that cut suicide attempts by half.
You may be able to recommend, refer to, or check to see if a youth can be provided one of the above therapies with best evidence but getting any counseling at all can be hard and some, especially minority families may decline formal interventions. Any therapy – CBT, DBT, or IPT – acceptable to the youth and family can be helpful. You can often determine if the key components are being provided by asking the teen what they are working on in therapy.
It is clear that checking in regularly with teens who have been through a suicide crisis is crucial to ensure that they continue in therapy long and consistently enough, that the family is involved in treatment, and that they are taught emotion regulation, distress tolerance, and safety planning. Warm, consistent parenting, good parent-child communication, and monitoring are protective factors but also skills that can be boosted to reduce future risk of suicide. When there is family dysfunction, conflict, or weak relationships, getting help for family relationships such as through attachment-based family therapy (ABFT) or family cognitive behavioral therapy is a priority. When bereavement or parental depression is contributing to youth suicidal thoughts, addressing these specifically can reduce suicide risk.
Sometimes family members, even with counseling, are not the best supporters for a teen in pain. When youths nominated their own support team to be informed about risk factors, diagnosis, and treatment plans and to stay in contact weekly there was a 6.6-fold lower risk of death than for nonsupported youth.
But how much of this evidence-based intervention can you ensure from your position in primary care? Refer if you can but regular supportive contacts alone reduce risk so you, trusted staff, school counselors, or even the now more available teletherapists may help. You can work with your patient to fill out a written commitment-to-safety plan (e.g. U. Colorado, CHADIS) of strategies they can use when having suicidal thoughts such as self-distractions, problem-solving, listing things they are looking forward to, things to do to get their mind off suicidal thoughts, and selecting support people to understand their situation with whom to be in regular contact. Any plan needs to take into account how understanding, supportive, and available the family is, factors you are most likely to be able to judge from your ongoing relationship, but that immediate risk may change. Contact within 48 hours, check-in within 1-2 weeks, and provision of crisis hotline information are essential actions.
Recommending home safety is part of routine anticipatory guidance but reduction of lethal means is essential in these cases. Guns are the most lethal method of suicide but discussing safe gun storage has been shown to be more effective than arguing in vain for gun removal. Medication overdose, a common means, can be reduced by not prescribing tricyclics (ineffective and more lethal), and advising parents to lock up all household medications.
You can ask about and coach teens on how to avoid the hazards of participating in online discussion groups, bullying, and cyberbullying (with risk for both perpetrator and victim), all risk factors for suicide. Managing insomnia can improve depression and is within your skills. While pediatricians can’t treat the suicide risk factors of family poverty, unemployment, or loss of culture/identity, we can refer affected families to community resources.
Repeated suicide screens can help but are imperfect, so listen to the child or parent for risk signs such as the youth having self-reported worthlessness, low self-esteem, speaking negatively about self, anhedonia, or poor emotion regulation. Children with impulsive aggression, often familial, are at special risk of suicide. This trait, while more common in ADHD, is not confined to that condition. You can help by optimizing medical management of impulsivity, when appropriate.
Most youth who attempt suicide have one or more mental health diagnoses, particularly major depressive disorder (MDD), eating disorder, ADHD, conduct, or intermittent explosive disorder. When MDD is comorbid with anxiety, suicides increase 9.5-fold. Children on the autism spectrum are more likely to have been bullied and eight times more likely to commit suicide. LGBTQ youth are five times more often bullied and are at high risk for suicide. The more common issues of school failure or substance use also confer risk. While we do our best caring for children with these conditions we may not be thinking about, screening, or monitoring for their suicide risk. It may be important for us to explain that, despite black-box warnings, rates of SSRI prescribing for depression are inversely related to suicides.
Child maltreatment is the highest risk factor for suicide (population attributed risk, or PAR, 9.6%-14.5%), particularly sexual misuse. All together, adverse childhood experiences have a PAR for suicide of 80%. Continuity allows you to monitor for developmental times when distress from past experiences often reemerges, e.g., puberty, dating onset, or divorce. Getting consent and sharing these highly sensitive but potentially triggering factors as well as prior diagnoses with a newly assigned therapist can be helpful to prioritize treatments to prevent a suicide attempt, because they may be difficult to elicit and timeliness is essential.
Dr. Howard is assistant professor of pediatrics at Johns Hopkins University, Baltimore, and creator of CHADIS. She had no other relevant disclosures. Dr. Howard’s contribution to this publication was as a paid expert to MDedge News. E-mail her at [email protected].
References
Brent DA. J Am Acad Child Adolesc Psychiatry. 2019;58(1):25-35.
Cha CB et al. J Child Psychol Psychiatry. 2018;59(4):460-82.
You read “thoughts of being better off dead” on your next patient’s PHQ-9 screen results and break into a sweat. After eliciting the teen’s realistic suicide plan and intent you send him to the ED with his parent for crisis mental health evaluation. When you call the family that evening to follow-up you hear that he was discharged with a “mental health counseling” appointment next week.
Have you done enough to prevent this child from dying at his own hand? I imagine that this haunts you as it does me. It is terrifying to know that, of youth with suicidal ideation, over one-third attempt suicide, most within 1-2 years, and 20%-40% do so without having had a plan.
We now know that certain kinds of psychotherapy have evidence for preventing subsequent suicide in teens at high risk due to suicidal ideation and past attempts. Cognitive behavioral therapy (CBT) has the best evidence including its subtypes for youth with relevant histories: for both suicide and substance use (integrated, or I-CBT), trauma focused (TF-CBT), traumatic grief (CTG-CBT), and CBT-I, for the potent risk factor of insomnia. The other treatment shown to reduce risk is dialectical behavioral therapy–adolescent (DBT-A) focused on strengthening skills in interpersonal effectiveness, mindfulness, distress tolerance, and emotion regulation adapted to youth by adding family therapy and multifamily skills training. Interpersonal psychotherapy (IPT) adapted for suicidal and self-harming adolescents (IPT-SA) also has evidence.
Some school programs have shown moderate efficacy, for example (IPT-A-IN) addresses the social and interpersonal context, and Youth Aware of Mental Health, a school curriculum to increase knowledge, help-seeking, and ways of coping with depression and suicidal behavior, that cut suicide attempts by half.
You may be able to recommend, refer to, or check to see if a youth can be provided one of the above therapies with best evidence but getting any counseling at all can be hard and some, especially minority families may decline formal interventions. Any therapy – CBT, DBT, or IPT – acceptable to the youth and family can be helpful. You can often determine if the key components are being provided by asking the teen what they are working on in therapy.
It is clear that checking in regularly with teens who have been through a suicide crisis is crucial to ensure that they continue in therapy long and consistently enough, that the family is involved in treatment, and that they are taught emotion regulation, distress tolerance, and safety planning. Warm, consistent parenting, good parent-child communication, and monitoring are protective factors but also skills that can be boosted to reduce future risk of suicide. When there is family dysfunction, conflict, or weak relationships, getting help for family relationships such as through attachment-based family therapy (ABFT) or family cognitive behavioral therapy is a priority. When bereavement or parental depression is contributing to youth suicidal thoughts, addressing these specifically can reduce suicide risk.
Sometimes family members, even with counseling, are not the best supporters for a teen in pain. When youths nominated their own support team to be informed about risk factors, diagnosis, and treatment plans and to stay in contact weekly there was a 6.6-fold lower risk of death than for nonsupported youth.
But how much of this evidence-based intervention can you ensure from your position in primary care? Refer if you can but regular supportive contacts alone reduce risk so you, trusted staff, school counselors, or even the now more available teletherapists may help. You can work with your patient to fill out a written commitment-to-safety plan (e.g. U. Colorado, CHADIS) of strategies they can use when having suicidal thoughts such as self-distractions, problem-solving, listing things they are looking forward to, things to do to get their mind off suicidal thoughts, and selecting support people to understand their situation with whom to be in regular contact. Any plan needs to take into account how understanding, supportive, and available the family is, factors you are most likely to be able to judge from your ongoing relationship, but that immediate risk may change. Contact within 48 hours, check-in within 1-2 weeks, and provision of crisis hotline information are essential actions.
Recommending home safety is part of routine anticipatory guidance but reduction of lethal means is essential in these cases. Guns are the most lethal method of suicide but discussing safe gun storage has been shown to be more effective than arguing in vain for gun removal. Medication overdose, a common means, can be reduced by not prescribing tricyclics (ineffective and more lethal), and advising parents to lock up all household medications.
You can ask about and coach teens on how to avoid the hazards of participating in online discussion groups, bullying, and cyberbullying (with risk for both perpetrator and victim), all risk factors for suicide. Managing insomnia can improve depression and is within your skills. While pediatricians can’t treat the suicide risk factors of family poverty, unemployment, or loss of culture/identity, we can refer affected families to community resources.
Repeated suicide screens can help but are imperfect, so listen to the child or parent for risk signs such as the youth having self-reported worthlessness, low self-esteem, speaking negatively about self, anhedonia, or poor emotion regulation. Children with impulsive aggression, often familial, are at special risk of suicide. This trait, while more common in ADHD, is not confined to that condition. You can help by optimizing medical management of impulsivity, when appropriate.
Most youth who attempt suicide have one or more mental health diagnoses, particularly major depressive disorder (MDD), eating disorder, ADHD, conduct, or intermittent explosive disorder. When MDD is comorbid with anxiety, suicides increase 9.5-fold. Children on the autism spectrum are more likely to have been bullied and eight times more likely to commit suicide. LGBTQ youth are five times more often bullied and are at high risk for suicide. The more common issues of school failure or substance use also confer risk. While we do our best caring for children with these conditions we may not be thinking about, screening, or monitoring for their suicide risk. It may be important for us to explain that, despite black-box warnings, rates of SSRI prescribing for depression are inversely related to suicides.
Child maltreatment is the highest risk factor for suicide (population attributed risk, or PAR, 9.6%-14.5%), particularly sexual misuse. All together, adverse childhood experiences have a PAR for suicide of 80%. Continuity allows you to monitor for developmental times when distress from past experiences often reemerges, e.g., puberty, dating onset, or divorce. Getting consent and sharing these highly sensitive but potentially triggering factors as well as prior diagnoses with a newly assigned therapist can be helpful to prioritize treatments to prevent a suicide attempt, because they may be difficult to elicit and timeliness is essential.
Dr. Howard is assistant professor of pediatrics at Johns Hopkins University, Baltimore, and creator of CHADIS. She had no other relevant disclosures. Dr. Howard’s contribution to this publication was as a paid expert to MDedge News. E-mail her at [email protected].
References
Brent DA. J Am Acad Child Adolesc Psychiatry. 2019;58(1):25-35.
Cha CB et al. J Child Psychol Psychiatry. 2018;59(4):460-82.
You read “thoughts of being better off dead” on your next patient’s PHQ-9 screen results and break into a sweat. After eliciting the teen’s realistic suicide plan and intent you send him to the ED with his parent for crisis mental health evaluation. When you call the family that evening to follow-up you hear that he was discharged with a “mental health counseling” appointment next week.
Have you done enough to prevent this child from dying at his own hand? I imagine that this haunts you as it does me. It is terrifying to know that, of youth with suicidal ideation, over one-third attempt suicide, most within 1-2 years, and 20%-40% do so without having had a plan.
We now know that certain kinds of psychotherapy have evidence for preventing subsequent suicide in teens at high risk due to suicidal ideation and past attempts. Cognitive behavioral therapy (CBT) has the best evidence including its subtypes for youth with relevant histories: for both suicide and substance use (integrated, or I-CBT), trauma focused (TF-CBT), traumatic grief (CTG-CBT), and CBT-I, for the potent risk factor of insomnia. The other treatment shown to reduce risk is dialectical behavioral therapy–adolescent (DBT-A) focused on strengthening skills in interpersonal effectiveness, mindfulness, distress tolerance, and emotion regulation adapted to youth by adding family therapy and multifamily skills training. Interpersonal psychotherapy (IPT) adapted for suicidal and self-harming adolescents (IPT-SA) also has evidence.
Some school programs have shown moderate efficacy, for example (IPT-A-IN) addresses the social and interpersonal context, and Youth Aware of Mental Health, a school curriculum to increase knowledge, help-seeking, and ways of coping with depression and suicidal behavior, that cut suicide attempts by half.
You may be able to recommend, refer to, or check to see if a youth can be provided one of the above therapies with best evidence but getting any counseling at all can be hard and some, especially minority families may decline formal interventions. Any therapy – CBT, DBT, or IPT – acceptable to the youth and family can be helpful. You can often determine if the key components are being provided by asking the teen what they are working on in therapy.
It is clear that checking in regularly with teens who have been through a suicide crisis is crucial to ensure that they continue in therapy long and consistently enough, that the family is involved in treatment, and that they are taught emotion regulation, distress tolerance, and safety planning. Warm, consistent parenting, good parent-child communication, and monitoring are protective factors but also skills that can be boosted to reduce future risk of suicide. When there is family dysfunction, conflict, or weak relationships, getting help for family relationships such as through attachment-based family therapy (ABFT) or family cognitive behavioral therapy is a priority. When bereavement or parental depression is contributing to youth suicidal thoughts, addressing these specifically can reduce suicide risk.
Sometimes family members, even with counseling, are not the best supporters for a teen in pain. When youths nominated their own support team to be informed about risk factors, diagnosis, and treatment plans and to stay in contact weekly there was a 6.6-fold lower risk of death than for nonsupported youth.
But how much of this evidence-based intervention can you ensure from your position in primary care? Refer if you can but regular supportive contacts alone reduce risk so you, trusted staff, school counselors, or even the now more available teletherapists may help. You can work with your patient to fill out a written commitment-to-safety plan (e.g. U. Colorado, CHADIS) of strategies they can use when having suicidal thoughts such as self-distractions, problem-solving, listing things they are looking forward to, things to do to get their mind off suicidal thoughts, and selecting support people to understand their situation with whom to be in regular contact. Any plan needs to take into account how understanding, supportive, and available the family is, factors you are most likely to be able to judge from your ongoing relationship, but that immediate risk may change. Contact within 48 hours, check-in within 1-2 weeks, and provision of crisis hotline information are essential actions.
Recommending home safety is part of routine anticipatory guidance but reduction of lethal means is essential in these cases. Guns are the most lethal method of suicide but discussing safe gun storage has been shown to be more effective than arguing in vain for gun removal. Medication overdose, a common means, can be reduced by not prescribing tricyclics (ineffective and more lethal), and advising parents to lock up all household medications.
You can ask about and coach teens on how to avoid the hazards of participating in online discussion groups, bullying, and cyberbullying (with risk for both perpetrator and victim), all risk factors for suicide. Managing insomnia can improve depression and is within your skills. While pediatricians can’t treat the suicide risk factors of family poverty, unemployment, or loss of culture/identity, we can refer affected families to community resources.
Repeated suicide screens can help but are imperfect, so listen to the child or parent for risk signs such as the youth having self-reported worthlessness, low self-esteem, speaking negatively about self, anhedonia, or poor emotion regulation. Children with impulsive aggression, often familial, are at special risk of suicide. This trait, while more common in ADHD, is not confined to that condition. You can help by optimizing medical management of impulsivity, when appropriate.
Most youth who attempt suicide have one or more mental health diagnoses, particularly major depressive disorder (MDD), eating disorder, ADHD, conduct, or intermittent explosive disorder. When MDD is comorbid with anxiety, suicides increase 9.5-fold. Children on the autism spectrum are more likely to have been bullied and eight times more likely to commit suicide. LGBTQ youth are five times more often bullied and are at high risk for suicide. The more common issues of school failure or substance use also confer risk. While we do our best caring for children with these conditions we may not be thinking about, screening, or monitoring for their suicide risk. It may be important for us to explain that, despite black-box warnings, rates of SSRI prescribing for depression are inversely related to suicides.
Child maltreatment is the highest risk factor for suicide (population attributed risk, or PAR, 9.6%-14.5%), particularly sexual misuse. All together, adverse childhood experiences have a PAR for suicide of 80%. Continuity allows you to monitor for developmental times when distress from past experiences often reemerges, e.g., puberty, dating onset, or divorce. Getting consent and sharing these highly sensitive but potentially triggering factors as well as prior diagnoses with a newly assigned therapist can be helpful to prioritize treatments to prevent a suicide attempt, because they may be difficult to elicit and timeliness is essential.
Dr. Howard is assistant professor of pediatrics at Johns Hopkins University, Baltimore, and creator of CHADIS. She had no other relevant disclosures. Dr. Howard’s contribution to this publication was as a paid expert to MDedge News. E-mail her at [email protected].
References
Brent DA. J Am Acad Child Adolesc Psychiatry. 2019;58(1):25-35.
Cha CB et al. J Child Psychol Psychiatry. 2018;59(4):460-82.
Seeing is bleeding, and smelling is perceiving
True Blood casting call!
If you’ve seen the show True Blood on HBO, you’re probably familiar with blood coming out instead of tears when any of the vampires start crying. Apparently, this interesting phenomenon isn’t unique to vampires on TV.
If you know about female anatomy, you know that the eyes aren’t usually involved in the menstrual cycle. However, a 25-year-old woman went to the ED when she experienced haemolacria, the term for blood tears, for the second time in 2 months during her cycle. She did not appear to have any injuries or illnesses that caused the eye bleeding, but physicians noted that both times she had eye bleeding, she also had her period.
Menstrual bleeding outside of the uterus, called vicarious menstruation, can occur, and it seems that the patient may have had that condition.
Since there are rumors of a True Blood remake circling, perhaps the show’s writers could blend in a little medical fact with vampire fiction.
What does skinny smell like? Lemons apparently
When you smell a lemon, what comes to your mind? How does it make you feel? Now think of the scent of vanilla. How does that one make you feel? Current research suggests certain smells may have an effect on how you perceive your body image.
Researchers from the University of Sussex (England) have found that certain olfactory stimuli (such as lemons and vanilla) and audio stimuli (light steps vs. heavy steps), have a moderate effect on self-image.
During their study, participants were put through a series of auditory and olfactory tests, from listening to stilettos and boots walking across the floor, to being exposed to certain essential oils with different sound pitches.
Exposure to lemon and higher-pitched sounds (like stilettos) made participants feel lighter and was associated with thin, spiky shapes. Exposure to vanilla and lower-pitched sounds was more associated with thicker, rounded shapes. This made researchers believe that multisensory stimuli, such as scents and sounds, can have a bigger role in treating eating disorders.
Our brain functions with multiple “mental models” of ourselves. Based on sensory stimuli from our day-to-day lives, those images and perceptions of ourselves change. Someone complimenting your snazzy new sweater provokes one self-perception, while someone letting you know that your fly is down provokes another.
Well, the researchers believe that, through a sense of smell, we can alter that perception of ourselves when paired with positive influence. Doing this through wearable “interactive clothes” could help boost the confidence and self-esteem of patients struggling with body image. Light smells equals light feelings. Of course, this won’t help the nearly 5% of the world who have some kind of smell disorder.
The researchers said that more research needs to be done, but you can do your own little experiment at home. Think about yourself and how you react to certain smells. How do they make you feel? If it makes you feel good, stop and smell more often.
Pregnancy with a side of pregnancy
It was a great day when Rebecca Roberts and her partner went to the obstetrician to confirm their positive pregnancy test. They’d been trying for more than a year without success, and now they would be having a baby. Note the usage of the singular there. That will become important in a moment.
When Ms. Roberts went back for her 12-week ultrasound appointment, there was an unexpected complication: Baby had become babies. The original fetus was there and doing fine, but there was now a second, less-developed fetus who’d invited herself in unannounced. While they were technically twins, the second fetus did not form at the same time, like normal fraternal twins, instead forming from an egg that was released weeks after the first egg was fertilized.
The phenomenon, called superfetation, is incredibly rare. Prior to 2008, there were fewer than 10 reported cases in the world, according to the European Journal of Obstetrics & Gynecology and Reproductive Biology. The odds of an egg being released during pregnancy, something that’s not supposed to happen, and then having that egg also become fertilized and successfully implanted in the uterus, is astronomically small.
It was not an easy pregnancy for Ms. Roberts, and at 33 weeks into the first pregnancy, the younger fetus’s umbilical cord began to malfunction, so delivery for both was induced in September 2020. Both infants spent time in the neonatal ICU, with the younger baby being in for 3 months, but after 6 months both are doing well and developing quickly. It’s always nice to have a happy ending to one of these weird medical phenomena, especially one with such an unpleasant-sounding name. If we didn’t know better, we’d think superfetation was something really, really smelly.
What’s a little misinformation among neighbors?
Vaccination will, hopefully, get the COVID-19 pandemic under control at some point, but the related misinformation floating around the Internet is another story. Already rampant in the United States, it’s now spreading … to Canada.
Investigators from that northern land took a look at the Twitter accounts of the platform’s 187,000 most active Canadian users and eventually ended up with a database of 147 million tweets, of which 154,000 contained terms associated with misinformation.
The Canadian social media users had more exposure to information from the United States than from Canada, and the exposure to U.S. outlets was more likely to involve misperceptions about COVID-19. “Most of the misinformation circulating on Twitter shared by Canadians was retweeted from U.S. sources,” the researchers said, and “Canadians who followed more American users were more likely to post misinformation.”
The study’s lead investigator, Aengus Bridgman of McGill University in Montreal, put it this way: “It’s hard for Canadian journalists, scientists, and public health experts to be heard by the average Canadian, given all the noise generated by American sources.”
People generally don’t take the time to read the fine print on contracts, and it looks like the Canadians have fallen into that trap. Not entirely their fault, of course, because most people coming from Canada to America don’t pass the Statue of Liberty, but she’s got some fine print of her own.
That poem written on the pedestal, the one that says, “Give me your tired, your poor, your huddled masses yearning to breathe free”? It’s actually a contract, and at the bottom, in very small print, it says, “In return for acceptance of the aforementioned ‘huddled masses,’ countries of origin agree to accept all of the social media noise generated by American sources.”
Sorry, Canada, but we gotcha.
True Blood casting call!
If you’ve seen the show True Blood on HBO, you’re probably familiar with blood coming out instead of tears when any of the vampires start crying. Apparently, this interesting phenomenon isn’t unique to vampires on TV.
If you know about female anatomy, you know that the eyes aren’t usually involved in the menstrual cycle. However, a 25-year-old woman went to the ED when she experienced haemolacria, the term for blood tears, for the second time in 2 months during her cycle. She did not appear to have any injuries or illnesses that caused the eye bleeding, but physicians noted that both times she had eye bleeding, she also had her period.
Menstrual bleeding outside of the uterus, called vicarious menstruation, can occur, and it seems that the patient may have had that condition.
Since there are rumors of a True Blood remake circling, perhaps the show’s writers could blend in a little medical fact with vampire fiction.
What does skinny smell like? Lemons apparently
When you smell a lemon, what comes to your mind? How does it make you feel? Now think of the scent of vanilla. How does that one make you feel? Current research suggests certain smells may have an effect on how you perceive your body image.
Researchers from the University of Sussex (England) have found that certain olfactory stimuli (such as lemons and vanilla) and audio stimuli (light steps vs. heavy steps), have a moderate effect on self-image.
During their study, participants were put through a series of auditory and olfactory tests, from listening to stilettos and boots walking across the floor, to being exposed to certain essential oils with different sound pitches.
Exposure to lemon and higher-pitched sounds (like stilettos) made participants feel lighter and was associated with thin, spiky shapes. Exposure to vanilla and lower-pitched sounds was more associated with thicker, rounded shapes. This made researchers believe that multisensory stimuli, such as scents and sounds, can have a bigger role in treating eating disorders.
Our brain functions with multiple “mental models” of ourselves. Based on sensory stimuli from our day-to-day lives, those images and perceptions of ourselves change. Someone complimenting your snazzy new sweater provokes one self-perception, while someone letting you know that your fly is down provokes another.
Well, the researchers believe that, through a sense of smell, we can alter that perception of ourselves when paired with positive influence. Doing this through wearable “interactive clothes” could help boost the confidence and self-esteem of patients struggling with body image. Light smells equals light feelings. Of course, this won’t help the nearly 5% of the world who have some kind of smell disorder.
The researchers said that more research needs to be done, but you can do your own little experiment at home. Think about yourself and how you react to certain smells. How do they make you feel? If it makes you feel good, stop and smell more often.
Pregnancy with a side of pregnancy
It was a great day when Rebecca Roberts and her partner went to the obstetrician to confirm their positive pregnancy test. They’d been trying for more than a year without success, and now they would be having a baby. Note the usage of the singular there. That will become important in a moment.
When Ms. Roberts went back for her 12-week ultrasound appointment, there was an unexpected complication: Baby had become babies. The original fetus was there and doing fine, but there was now a second, less-developed fetus who’d invited herself in unannounced. While they were technically twins, the second fetus did not form at the same time, like normal fraternal twins, instead forming from an egg that was released weeks after the first egg was fertilized.
The phenomenon, called superfetation, is incredibly rare. Prior to 2008, there were fewer than 10 reported cases in the world, according to the European Journal of Obstetrics & Gynecology and Reproductive Biology. The odds of an egg being released during pregnancy, something that’s not supposed to happen, and then having that egg also become fertilized and successfully implanted in the uterus, is astronomically small.
It was not an easy pregnancy for Ms. Roberts, and at 33 weeks into the first pregnancy, the younger fetus’s umbilical cord began to malfunction, so delivery for both was induced in September 2020. Both infants spent time in the neonatal ICU, with the younger baby being in for 3 months, but after 6 months both are doing well and developing quickly. It’s always nice to have a happy ending to one of these weird medical phenomena, especially one with such an unpleasant-sounding name. If we didn’t know better, we’d think superfetation was something really, really smelly.
What’s a little misinformation among neighbors?
Vaccination will, hopefully, get the COVID-19 pandemic under control at some point, but the related misinformation floating around the Internet is another story. Already rampant in the United States, it’s now spreading … to Canada.
Investigators from that northern land took a look at the Twitter accounts of the platform’s 187,000 most active Canadian users and eventually ended up with a database of 147 million tweets, of which 154,000 contained terms associated with misinformation.
The Canadian social media users had more exposure to information from the United States than from Canada, and the exposure to U.S. outlets was more likely to involve misperceptions about COVID-19. “Most of the misinformation circulating on Twitter shared by Canadians was retweeted from U.S. sources,” the researchers said, and “Canadians who followed more American users were more likely to post misinformation.”
The study’s lead investigator, Aengus Bridgman of McGill University in Montreal, put it this way: “It’s hard for Canadian journalists, scientists, and public health experts to be heard by the average Canadian, given all the noise generated by American sources.”
People generally don’t take the time to read the fine print on contracts, and it looks like the Canadians have fallen into that trap. Not entirely their fault, of course, because most people coming from Canada to America don’t pass the Statue of Liberty, but she’s got some fine print of her own.
That poem written on the pedestal, the one that says, “Give me your tired, your poor, your huddled masses yearning to breathe free”? It’s actually a contract, and at the bottom, in very small print, it says, “In return for acceptance of the aforementioned ‘huddled masses,’ countries of origin agree to accept all of the social media noise generated by American sources.”
Sorry, Canada, but we gotcha.
True Blood casting call!
If you’ve seen the show True Blood on HBO, you’re probably familiar with blood coming out instead of tears when any of the vampires start crying. Apparently, this interesting phenomenon isn’t unique to vampires on TV.
If you know about female anatomy, you know that the eyes aren’t usually involved in the menstrual cycle. However, a 25-year-old woman went to the ED when she experienced haemolacria, the term for blood tears, for the second time in 2 months during her cycle. She did not appear to have any injuries or illnesses that caused the eye bleeding, but physicians noted that both times she had eye bleeding, she also had her period.
Menstrual bleeding outside of the uterus, called vicarious menstruation, can occur, and it seems that the patient may have had that condition.
Since there are rumors of a True Blood remake circling, perhaps the show’s writers could blend in a little medical fact with vampire fiction.
What does skinny smell like? Lemons apparently
When you smell a lemon, what comes to your mind? How does it make you feel? Now think of the scent of vanilla. How does that one make you feel? Current research suggests certain smells may have an effect on how you perceive your body image.
Researchers from the University of Sussex (England) have found that certain olfactory stimuli (such as lemons and vanilla) and audio stimuli (light steps vs. heavy steps), have a moderate effect on self-image.
During their study, participants were put through a series of auditory and olfactory tests, from listening to stilettos and boots walking across the floor, to being exposed to certain essential oils with different sound pitches.
Exposure to lemon and higher-pitched sounds (like stilettos) made participants feel lighter and was associated with thin, spiky shapes. Exposure to vanilla and lower-pitched sounds was more associated with thicker, rounded shapes. This made researchers believe that multisensory stimuli, such as scents and sounds, can have a bigger role in treating eating disorders.
Our brain functions with multiple “mental models” of ourselves. Based on sensory stimuli from our day-to-day lives, those images and perceptions of ourselves change. Someone complimenting your snazzy new sweater provokes one self-perception, while someone letting you know that your fly is down provokes another.
Well, the researchers believe that, through a sense of smell, we can alter that perception of ourselves when paired with positive influence. Doing this through wearable “interactive clothes” could help boost the confidence and self-esteem of patients struggling with body image. Light smells equals light feelings. Of course, this won’t help the nearly 5% of the world who have some kind of smell disorder.
The researchers said that more research needs to be done, but you can do your own little experiment at home. Think about yourself and how you react to certain smells. How do they make you feel? If it makes you feel good, stop and smell more often.
Pregnancy with a side of pregnancy
It was a great day when Rebecca Roberts and her partner went to the obstetrician to confirm their positive pregnancy test. They’d been trying for more than a year without success, and now they would be having a baby. Note the usage of the singular there. That will become important in a moment.
When Ms. Roberts went back for her 12-week ultrasound appointment, there was an unexpected complication: Baby had become babies. The original fetus was there and doing fine, but there was now a second, less-developed fetus who’d invited herself in unannounced. While they were technically twins, the second fetus did not form at the same time, like normal fraternal twins, instead forming from an egg that was released weeks after the first egg was fertilized.
The phenomenon, called superfetation, is incredibly rare. Prior to 2008, there were fewer than 10 reported cases in the world, according to the European Journal of Obstetrics & Gynecology and Reproductive Biology. The odds of an egg being released during pregnancy, something that’s not supposed to happen, and then having that egg also become fertilized and successfully implanted in the uterus, is astronomically small.
It was not an easy pregnancy for Ms. Roberts, and at 33 weeks into the first pregnancy, the younger fetus’s umbilical cord began to malfunction, so delivery for both was induced in September 2020. Both infants spent time in the neonatal ICU, with the younger baby being in for 3 months, but after 6 months both are doing well and developing quickly. It’s always nice to have a happy ending to one of these weird medical phenomena, especially one with such an unpleasant-sounding name. If we didn’t know better, we’d think superfetation was something really, really smelly.
What’s a little misinformation among neighbors?
Vaccination will, hopefully, get the COVID-19 pandemic under control at some point, but the related misinformation floating around the Internet is another story. Already rampant in the United States, it’s now spreading … to Canada.
Investigators from that northern land took a look at the Twitter accounts of the platform’s 187,000 most active Canadian users and eventually ended up with a database of 147 million tweets, of which 154,000 contained terms associated with misinformation.
The Canadian social media users had more exposure to information from the United States than from Canada, and the exposure to U.S. outlets was more likely to involve misperceptions about COVID-19. “Most of the misinformation circulating on Twitter shared by Canadians was retweeted from U.S. sources,” the researchers said, and “Canadians who followed more American users were more likely to post misinformation.”
The study’s lead investigator, Aengus Bridgman of McGill University in Montreal, put it this way: “It’s hard for Canadian journalists, scientists, and public health experts to be heard by the average Canadian, given all the noise generated by American sources.”
People generally don’t take the time to read the fine print on contracts, and it looks like the Canadians have fallen into that trap. Not entirely their fault, of course, because most people coming from Canada to America don’t pass the Statue of Liberty, but she’s got some fine print of her own.
That poem written on the pedestal, the one that says, “Give me your tired, your poor, your huddled masses yearning to breathe free”? It’s actually a contract, and at the bottom, in very small print, it says, “In return for acceptance of the aforementioned ‘huddled masses,’ countries of origin agree to accept all of the social media noise generated by American sources.”
Sorry, Canada, but we gotcha.
How some COVID-19 vaccines could cause rare blood clots
on April 14, 2021, after the CDC and Food and Drug Administration recommended that states hold off on using it pending a detailed review of six cases of the same kind of rare but serious event – a blood clot in the vessels that drain blood from the brain combined with a large drop in platelets, which increases the risk for bleeding.
This combination can lead to severe strokes that can lead to brain damage or death. Among the six cases reported, which came to light over the past 3 weeks, one person died, according to the CDC. All six were women and ranged in age from 18 to 48 years.
According to a report from the Vaccine Adverse Event Reporting System (VAERS), which is maintained by the Department of Health & Human Services, the woman who died was 45. She developed a gradually worsening headache about a week after receiving the Johnson & Johnson vaccine.
On March 17, the day she came to the hospital, she was dry heaving. Her headache had suddenly gotten much worse, and the left side of her body was weak, which are signs of a stroke. A CT scan revealed both bleeding in her brain and a clot in her cortical vein. She died the following day.
In addition to VAERS, which accepts reports from anyone, the CDC and FDA are monitoring at least eight other safety systems maintained by hospitals, research centers, long-term care facilities, and insurance companies for signs of trouble with the vaccines. VAERS data is searchable and open to the public. Most of these systems are not publicly available to protect patient privacy. It’s unclear which systems detected the six cases cited by federal regulators.
“These are very serious and potentially fatal problems occurring in a healthy young adult. It’s serious and we need to get to the bottom of it,” said Ed Belongia, MD, director of the Center for Clinical Epidemiology and Population Health at the Marshfield (Wis.) Clinic Research Institute. Dr. Belongia leads a research team that helps the CDC monitor vaccine safety and effectiveness.
“Safety is always the highest priority, and I think what we’ve seen here in the past 24 hours is our vaccine safety monitoring system is working,” he said.
Others agree. “I think what CDC and FDA have detected is a rare, but likely real adverse event associated with this vaccine,” said Paul Offit, MD, director of vaccine education at Children’s Hospital of Philadelphia.
Although much is still unknown about these events, they follow a similar pattern of blood clots reported with the AstraZeneca vaccine in Europe. That vaccine is now sold under the brand name Vaxzevria.
This has experts questioning whether all vaccines of this type may cause these rare clots.
“I think it’s likely a class effect,” said Dr. Offit, who was a member of the FDA advisory committee that reviewed clinical trial data on the J&J vaccine before it was authorized for use.
Adenovirus vaccines scrutinized
Both the Johnson & Johnson and Vaxzevria vaccines use an adenovirus to ferry genetic instructions for making the coronaviruses spike protein into our cells.
Adenoviruses are common, relatively simple viruses that normally cause mild cold or flu symptoms. The ones used in the vaccine are disabled so they can’t make us sick. They’re more like Trojan horses.
Once inside our cells, they release the DNA instructions they carry to make the spike protein of the new coronavirus. Those cells then crank out copies of the spike protein, which then get displayed on the outer surface of the cell membrane where they are recognized by the immune system.
The immune system then makes antibodies and other defenses against the spike so that, when the real coronavirus comes along, our bodies are ready to fight the infection.
There’s no question the vaccine works. In clinical trials, the Johnson & Johnson vaccine was 66% percent effective at preventing against moderate to severe COVID-19 infection, and none of the patients who got COVID-19 after vaccination had to be admitted to the hospital or died.
The idea behind using adenoviruses in vaccines isn’t a new one. In a kind of fight-fire-with-fire approach, the idea is to use a virus, which is good at infecting us, to fight a different kind of virus.
Researchers have been working on the concept for about 10 years, but the COVID-19 vaccines that use this technology are some of the first adenovirus-vector vaccines deployed in humans.
Only one other adenovirus vaccine, for Ebola, has been approved for use in humans. It was approved in Europe last year. Before the Johnson & Johnson vaccine, no other adenovirus vector has been available for use in humans in the United States.
There are six adenovirus-vector vaccines for COVID-19. In addition to AstraZeneca and Johnson & Johnson, there’s the Russian-developed vaccine Sputnik V, along with CanSino from China, and the Covishield vaccine in India.
Adenovirus vaccines are more stable than the mRNA vaccines. That makes them easier to store and transport.
But they have a significant downside, too. Because adenoviruses infect humans out in the world, we already make antibodies against them. So there’s always a danger that our immune systems might recognize and react to the vaccine, rendering it ineffective. For that reason, scientists try to carefully select the adenovirus vectors, or carriers, they use.
The two vaccines under investigation for blood clots are slightly different. The Johnson & Johnson vaccine uses the vector AD26, because most of the population lacks preexisting immunity to it. Vaxzevria uses an adenovirus that infects chimpanzees, called ChAdOx1.
Vaxzevria has been widely used in Europe but has not yet been authorized in the United States.
On April 7, the European Medicines Agency, Europe’s counterpart to the FDA, ruled that unusual blood clots with low blood platelets should be listed as rare side effects on the Vaxzevria vaccine.
The decision came after reviewing 62 cases of cerebral venous sinus thrombosis (CVST) linked to the vaccine and 25 cases of another rare type of clot, called a splanchnic vein thrombosis. Splanchnic veins drain blood from the major organs in the digestive system, including the stomach, liver, and intestines; 18 of those events were fatal.
The reports were culled from reporting in Europe and the United Kingdom, where around 25 million people have received the Vaxzevria vaccine, making these clots exceptionally rare, but serious.
So far, six cases of CVST have been reported in the United States, after more than 7 million doses of the Johnson & Johnson vaccines have been administered.
A key question for U.S. regulators will be the background rate for these types of rare combinations of clots and deplenished platelets. The background rate is the number of events that would be expected to occur naturally in a population of unvaccinated people. On a press call on April 13, Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, was asked about the frequency of this dangerous combination. He said the combination of low platelets and clots was so rare that it was hard to pinpoint, but might be somewhere between 2 and 14 cases per million people over the course of a year.
The first Johnson & Johnson doses were given in early March. That means the six cases came to light within the first few weeks of use of the vaccine in the United States, a very short amount of time.
“These were six cases per million people for 2 weeks, which is the same thing as 25 million per year, so it’s clearly above the background rate,” Dr. Offit said.
Studies suggest possible mechanism
On April 9, the New England Journal of Medicine published a detailed evaluation of the 11 patients in Germany and Austria who developed the rare clots after their Vaxzevria vaccines.
The study detected rare antibodies to a signaling protein called platelet factor 4, which helps to coordinate clot formation.
These same type of antibodies form in some people given the blood thinning drug heparin. In those reactions, which are also exceptionally rare, the same type of syndrome develops, leading to large, devastating clots that consume circulating platelets.
It’s not yet clear whether people who develop reactions to the vaccines already have some platelet factor 4 antibodies before they are vaccinated, or whether the vaccines somehow spur the body to make these antibodies, which then launch a kind of autoimmune attack.
The researchers on the paper gave the syndrome a name, vaccine-induced thrombotic thrombocytopenia (VITT).
It’s also not clear why more cases seem to be in women than in men. Andrew Eisenberger, MD, an associate professor of hematology and oncology at Columbia University, New York, said the most common causes of cerebral venous sinus thrombosis have to do with conditions that raise estrogen levels, like pregnancy and hormonal contraception.
“Estrogen naturally leads to changes in several clotting proteins in the blood that may predispose to abnormal blood clotting in a few different sites in the body,” he said. “The clotting changes we are encountering with some of COVID-19 vaccines are likely to be synergistic with the effects of estrogen on the blood.”
No matter the cause, the CDC on April 13 alerted doctors to keep a high index of suspicion for VITT in patients who have received the Johnson & Johnson vaccination within the last 2 weeks. In those patients, the usual course of treatment with blood thinning drugs like heparin may be harmful.
Symptoms to watch for include severe headache or backache, new neurologic symptoms, severe abdominal pain, shortness of breath, leg swelling, tiny red spots on the skin, or easy bruising.
Grappling with evidence
The CDC’s Advisory Committee on Immunization Practices will meet today in an emergency session to review the cases and see if any changes are needed to use of the J&J vaccine in the United States.
Last week, for example, the United Kingdom restricted the use of the AstraZeneca vaccine in people aged younger than 30 years, saying the risks and benefits of vaccination are “more finely balanced” for this age group.
With cases of COVID-19 rising again in the United States, and the Johnson & Johnson vaccine currently the most convenient form of protection against the virus, the committee will have to weigh the risks of that infection against the risk of rare clots caused by vaccination.
They will also likely have to rule out whether any of the cases had COVID. At least one study has reported CVST clots in three patients with confirmed COVID infections. In Europe, COVID infection did not seem to play a role in the formation of the clots with low platelets.
Hilda Bastian, PhD, a clinical trials expert who cofounded the Cochrane Collaboration, said it won’t be an easy task. Much will depend on how certain the committee members feel they know about all the events linked to the vaccine.
“That’s the really, really hard issue from my point of view for them right this moment. Have we missed any? Or how many are we likely to have missed?” asked Dr. Bastian, who lives in Australia.
“In a country that size with that fragmented [of] a health care system, how sure can you be that you know them all? That’s going to be a really difficult situation for them to grapple with, the quality of information that they’ve got,” she said.
A version of this article first appeared on Medscape.com.
on April 14, 2021, after the CDC and Food and Drug Administration recommended that states hold off on using it pending a detailed review of six cases of the same kind of rare but serious event – a blood clot in the vessels that drain blood from the brain combined with a large drop in platelets, which increases the risk for bleeding.
This combination can lead to severe strokes that can lead to brain damage or death. Among the six cases reported, which came to light over the past 3 weeks, one person died, according to the CDC. All six were women and ranged in age from 18 to 48 years.
According to a report from the Vaccine Adverse Event Reporting System (VAERS), which is maintained by the Department of Health & Human Services, the woman who died was 45. She developed a gradually worsening headache about a week after receiving the Johnson & Johnson vaccine.
On March 17, the day she came to the hospital, she was dry heaving. Her headache had suddenly gotten much worse, and the left side of her body was weak, which are signs of a stroke. A CT scan revealed both bleeding in her brain and a clot in her cortical vein. She died the following day.
In addition to VAERS, which accepts reports from anyone, the CDC and FDA are monitoring at least eight other safety systems maintained by hospitals, research centers, long-term care facilities, and insurance companies for signs of trouble with the vaccines. VAERS data is searchable and open to the public. Most of these systems are not publicly available to protect patient privacy. It’s unclear which systems detected the six cases cited by federal regulators.
“These are very serious and potentially fatal problems occurring in a healthy young adult. It’s serious and we need to get to the bottom of it,” said Ed Belongia, MD, director of the Center for Clinical Epidemiology and Population Health at the Marshfield (Wis.) Clinic Research Institute. Dr. Belongia leads a research team that helps the CDC monitor vaccine safety and effectiveness.
“Safety is always the highest priority, and I think what we’ve seen here in the past 24 hours is our vaccine safety monitoring system is working,” he said.
Others agree. “I think what CDC and FDA have detected is a rare, but likely real adverse event associated with this vaccine,” said Paul Offit, MD, director of vaccine education at Children’s Hospital of Philadelphia.
Although much is still unknown about these events, they follow a similar pattern of blood clots reported with the AstraZeneca vaccine in Europe. That vaccine is now sold under the brand name Vaxzevria.
This has experts questioning whether all vaccines of this type may cause these rare clots.
“I think it’s likely a class effect,” said Dr. Offit, who was a member of the FDA advisory committee that reviewed clinical trial data on the J&J vaccine before it was authorized for use.
Adenovirus vaccines scrutinized
Both the Johnson & Johnson and Vaxzevria vaccines use an adenovirus to ferry genetic instructions for making the coronaviruses spike protein into our cells.
Adenoviruses are common, relatively simple viruses that normally cause mild cold or flu symptoms. The ones used in the vaccine are disabled so they can’t make us sick. They’re more like Trojan horses.
Once inside our cells, they release the DNA instructions they carry to make the spike protein of the new coronavirus. Those cells then crank out copies of the spike protein, which then get displayed on the outer surface of the cell membrane where they are recognized by the immune system.
The immune system then makes antibodies and other defenses against the spike so that, when the real coronavirus comes along, our bodies are ready to fight the infection.
There’s no question the vaccine works. In clinical trials, the Johnson & Johnson vaccine was 66% percent effective at preventing against moderate to severe COVID-19 infection, and none of the patients who got COVID-19 after vaccination had to be admitted to the hospital or died.
The idea behind using adenoviruses in vaccines isn’t a new one. In a kind of fight-fire-with-fire approach, the idea is to use a virus, which is good at infecting us, to fight a different kind of virus.
Researchers have been working on the concept for about 10 years, but the COVID-19 vaccines that use this technology are some of the first adenovirus-vector vaccines deployed in humans.
Only one other adenovirus vaccine, for Ebola, has been approved for use in humans. It was approved in Europe last year. Before the Johnson & Johnson vaccine, no other adenovirus vector has been available for use in humans in the United States.
There are six adenovirus-vector vaccines for COVID-19. In addition to AstraZeneca and Johnson & Johnson, there’s the Russian-developed vaccine Sputnik V, along with CanSino from China, and the Covishield vaccine in India.
Adenovirus vaccines are more stable than the mRNA vaccines. That makes them easier to store and transport.
But they have a significant downside, too. Because adenoviruses infect humans out in the world, we already make antibodies against them. So there’s always a danger that our immune systems might recognize and react to the vaccine, rendering it ineffective. For that reason, scientists try to carefully select the adenovirus vectors, or carriers, they use.
The two vaccines under investigation for blood clots are slightly different. The Johnson & Johnson vaccine uses the vector AD26, because most of the population lacks preexisting immunity to it. Vaxzevria uses an adenovirus that infects chimpanzees, called ChAdOx1.
Vaxzevria has been widely used in Europe but has not yet been authorized in the United States.
On April 7, the European Medicines Agency, Europe’s counterpart to the FDA, ruled that unusual blood clots with low blood platelets should be listed as rare side effects on the Vaxzevria vaccine.
The decision came after reviewing 62 cases of cerebral venous sinus thrombosis (CVST) linked to the vaccine and 25 cases of another rare type of clot, called a splanchnic vein thrombosis. Splanchnic veins drain blood from the major organs in the digestive system, including the stomach, liver, and intestines; 18 of those events were fatal.
The reports were culled from reporting in Europe and the United Kingdom, where around 25 million people have received the Vaxzevria vaccine, making these clots exceptionally rare, but serious.
So far, six cases of CVST have been reported in the United States, after more than 7 million doses of the Johnson & Johnson vaccines have been administered.
A key question for U.S. regulators will be the background rate for these types of rare combinations of clots and deplenished platelets. The background rate is the number of events that would be expected to occur naturally in a population of unvaccinated people. On a press call on April 13, Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, was asked about the frequency of this dangerous combination. He said the combination of low platelets and clots was so rare that it was hard to pinpoint, but might be somewhere between 2 and 14 cases per million people over the course of a year.
The first Johnson & Johnson doses were given in early March. That means the six cases came to light within the first few weeks of use of the vaccine in the United States, a very short amount of time.
“These were six cases per million people for 2 weeks, which is the same thing as 25 million per year, so it’s clearly above the background rate,” Dr. Offit said.
Studies suggest possible mechanism
On April 9, the New England Journal of Medicine published a detailed evaluation of the 11 patients in Germany and Austria who developed the rare clots after their Vaxzevria vaccines.
The study detected rare antibodies to a signaling protein called platelet factor 4, which helps to coordinate clot formation.
These same type of antibodies form in some people given the blood thinning drug heparin. In those reactions, which are also exceptionally rare, the same type of syndrome develops, leading to large, devastating clots that consume circulating platelets.
It’s not yet clear whether people who develop reactions to the vaccines already have some platelet factor 4 antibodies before they are vaccinated, or whether the vaccines somehow spur the body to make these antibodies, which then launch a kind of autoimmune attack.
The researchers on the paper gave the syndrome a name, vaccine-induced thrombotic thrombocytopenia (VITT).
It’s also not clear why more cases seem to be in women than in men. Andrew Eisenberger, MD, an associate professor of hematology and oncology at Columbia University, New York, said the most common causes of cerebral venous sinus thrombosis have to do with conditions that raise estrogen levels, like pregnancy and hormonal contraception.
“Estrogen naturally leads to changes in several clotting proteins in the blood that may predispose to abnormal blood clotting in a few different sites in the body,” he said. “The clotting changes we are encountering with some of COVID-19 vaccines are likely to be synergistic with the effects of estrogen on the blood.”
No matter the cause, the CDC on April 13 alerted doctors to keep a high index of suspicion for VITT in patients who have received the Johnson & Johnson vaccination within the last 2 weeks. In those patients, the usual course of treatment with blood thinning drugs like heparin may be harmful.
Symptoms to watch for include severe headache or backache, new neurologic symptoms, severe abdominal pain, shortness of breath, leg swelling, tiny red spots on the skin, or easy bruising.
Grappling with evidence
The CDC’s Advisory Committee on Immunization Practices will meet today in an emergency session to review the cases and see if any changes are needed to use of the J&J vaccine in the United States.
Last week, for example, the United Kingdom restricted the use of the AstraZeneca vaccine in people aged younger than 30 years, saying the risks and benefits of vaccination are “more finely balanced” for this age group.
With cases of COVID-19 rising again in the United States, and the Johnson & Johnson vaccine currently the most convenient form of protection against the virus, the committee will have to weigh the risks of that infection against the risk of rare clots caused by vaccination.
They will also likely have to rule out whether any of the cases had COVID. At least one study has reported CVST clots in three patients with confirmed COVID infections. In Europe, COVID infection did not seem to play a role in the formation of the clots with low platelets.
Hilda Bastian, PhD, a clinical trials expert who cofounded the Cochrane Collaboration, said it won’t be an easy task. Much will depend on how certain the committee members feel they know about all the events linked to the vaccine.
“That’s the really, really hard issue from my point of view for them right this moment. Have we missed any? Or how many are we likely to have missed?” asked Dr. Bastian, who lives in Australia.
“In a country that size with that fragmented [of] a health care system, how sure can you be that you know them all? That’s going to be a really difficult situation for them to grapple with, the quality of information that they’ve got,” she said.
A version of this article first appeared on Medscape.com.
on April 14, 2021, after the CDC and Food and Drug Administration recommended that states hold off on using it pending a detailed review of six cases of the same kind of rare but serious event – a blood clot in the vessels that drain blood from the brain combined with a large drop in platelets, which increases the risk for bleeding.
This combination can lead to severe strokes that can lead to brain damage or death. Among the six cases reported, which came to light over the past 3 weeks, one person died, according to the CDC. All six were women and ranged in age from 18 to 48 years.
According to a report from the Vaccine Adverse Event Reporting System (VAERS), which is maintained by the Department of Health & Human Services, the woman who died was 45. She developed a gradually worsening headache about a week after receiving the Johnson & Johnson vaccine.
On March 17, the day she came to the hospital, she was dry heaving. Her headache had suddenly gotten much worse, and the left side of her body was weak, which are signs of a stroke. A CT scan revealed both bleeding in her brain and a clot in her cortical vein. She died the following day.
In addition to VAERS, which accepts reports from anyone, the CDC and FDA are monitoring at least eight other safety systems maintained by hospitals, research centers, long-term care facilities, and insurance companies for signs of trouble with the vaccines. VAERS data is searchable and open to the public. Most of these systems are not publicly available to protect patient privacy. It’s unclear which systems detected the six cases cited by federal regulators.
“These are very serious and potentially fatal problems occurring in a healthy young adult. It’s serious and we need to get to the bottom of it,” said Ed Belongia, MD, director of the Center for Clinical Epidemiology and Population Health at the Marshfield (Wis.) Clinic Research Institute. Dr. Belongia leads a research team that helps the CDC monitor vaccine safety and effectiveness.
“Safety is always the highest priority, and I think what we’ve seen here in the past 24 hours is our vaccine safety monitoring system is working,” he said.
Others agree. “I think what CDC and FDA have detected is a rare, but likely real adverse event associated with this vaccine,” said Paul Offit, MD, director of vaccine education at Children’s Hospital of Philadelphia.
Although much is still unknown about these events, they follow a similar pattern of blood clots reported with the AstraZeneca vaccine in Europe. That vaccine is now sold under the brand name Vaxzevria.
This has experts questioning whether all vaccines of this type may cause these rare clots.
“I think it’s likely a class effect,” said Dr. Offit, who was a member of the FDA advisory committee that reviewed clinical trial data on the J&J vaccine before it was authorized for use.
Adenovirus vaccines scrutinized
Both the Johnson & Johnson and Vaxzevria vaccines use an adenovirus to ferry genetic instructions for making the coronaviruses spike protein into our cells.
Adenoviruses are common, relatively simple viruses that normally cause mild cold or flu symptoms. The ones used in the vaccine are disabled so they can’t make us sick. They’re more like Trojan horses.
Once inside our cells, they release the DNA instructions they carry to make the spike protein of the new coronavirus. Those cells then crank out copies of the spike protein, which then get displayed on the outer surface of the cell membrane where they are recognized by the immune system.
The immune system then makes antibodies and other defenses against the spike so that, when the real coronavirus comes along, our bodies are ready to fight the infection.
There’s no question the vaccine works. In clinical trials, the Johnson & Johnson vaccine was 66% percent effective at preventing against moderate to severe COVID-19 infection, and none of the patients who got COVID-19 after vaccination had to be admitted to the hospital or died.
The idea behind using adenoviruses in vaccines isn’t a new one. In a kind of fight-fire-with-fire approach, the idea is to use a virus, which is good at infecting us, to fight a different kind of virus.
Researchers have been working on the concept for about 10 years, but the COVID-19 vaccines that use this technology are some of the first adenovirus-vector vaccines deployed in humans.
Only one other adenovirus vaccine, for Ebola, has been approved for use in humans. It was approved in Europe last year. Before the Johnson & Johnson vaccine, no other adenovirus vector has been available for use in humans in the United States.
There are six adenovirus-vector vaccines for COVID-19. In addition to AstraZeneca and Johnson & Johnson, there’s the Russian-developed vaccine Sputnik V, along with CanSino from China, and the Covishield vaccine in India.
Adenovirus vaccines are more stable than the mRNA vaccines. That makes them easier to store and transport.
But they have a significant downside, too. Because adenoviruses infect humans out in the world, we already make antibodies against them. So there’s always a danger that our immune systems might recognize and react to the vaccine, rendering it ineffective. For that reason, scientists try to carefully select the adenovirus vectors, or carriers, they use.
The two vaccines under investigation for blood clots are slightly different. The Johnson & Johnson vaccine uses the vector AD26, because most of the population lacks preexisting immunity to it. Vaxzevria uses an adenovirus that infects chimpanzees, called ChAdOx1.
Vaxzevria has been widely used in Europe but has not yet been authorized in the United States.
On April 7, the European Medicines Agency, Europe’s counterpart to the FDA, ruled that unusual blood clots with low blood platelets should be listed as rare side effects on the Vaxzevria vaccine.
The decision came after reviewing 62 cases of cerebral venous sinus thrombosis (CVST) linked to the vaccine and 25 cases of another rare type of clot, called a splanchnic vein thrombosis. Splanchnic veins drain blood from the major organs in the digestive system, including the stomach, liver, and intestines; 18 of those events were fatal.
The reports were culled from reporting in Europe and the United Kingdom, where around 25 million people have received the Vaxzevria vaccine, making these clots exceptionally rare, but serious.
So far, six cases of CVST have been reported in the United States, after more than 7 million doses of the Johnson & Johnson vaccines have been administered.
A key question for U.S. regulators will be the background rate for these types of rare combinations of clots and deplenished platelets. The background rate is the number of events that would be expected to occur naturally in a population of unvaccinated people. On a press call on April 13, Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, was asked about the frequency of this dangerous combination. He said the combination of low platelets and clots was so rare that it was hard to pinpoint, but might be somewhere between 2 and 14 cases per million people over the course of a year.
The first Johnson & Johnson doses were given in early March. That means the six cases came to light within the first few weeks of use of the vaccine in the United States, a very short amount of time.
“These were six cases per million people for 2 weeks, which is the same thing as 25 million per year, so it’s clearly above the background rate,” Dr. Offit said.
Studies suggest possible mechanism
On April 9, the New England Journal of Medicine published a detailed evaluation of the 11 patients in Germany and Austria who developed the rare clots after their Vaxzevria vaccines.
The study detected rare antibodies to a signaling protein called platelet factor 4, which helps to coordinate clot formation.
These same type of antibodies form in some people given the blood thinning drug heparin. In those reactions, which are also exceptionally rare, the same type of syndrome develops, leading to large, devastating clots that consume circulating platelets.
It’s not yet clear whether people who develop reactions to the vaccines already have some platelet factor 4 antibodies before they are vaccinated, or whether the vaccines somehow spur the body to make these antibodies, which then launch a kind of autoimmune attack.
The researchers on the paper gave the syndrome a name, vaccine-induced thrombotic thrombocytopenia (VITT).
It’s also not clear why more cases seem to be in women than in men. Andrew Eisenberger, MD, an associate professor of hematology and oncology at Columbia University, New York, said the most common causes of cerebral venous sinus thrombosis have to do with conditions that raise estrogen levels, like pregnancy and hormonal contraception.
“Estrogen naturally leads to changes in several clotting proteins in the blood that may predispose to abnormal blood clotting in a few different sites in the body,” he said. “The clotting changes we are encountering with some of COVID-19 vaccines are likely to be synergistic with the effects of estrogen on the blood.”
No matter the cause, the CDC on April 13 alerted doctors to keep a high index of suspicion for VITT in patients who have received the Johnson & Johnson vaccination within the last 2 weeks. In those patients, the usual course of treatment with blood thinning drugs like heparin may be harmful.
Symptoms to watch for include severe headache or backache, new neurologic symptoms, severe abdominal pain, shortness of breath, leg swelling, tiny red spots on the skin, or easy bruising.
Grappling with evidence
The CDC’s Advisory Committee on Immunization Practices will meet today in an emergency session to review the cases and see if any changes are needed to use of the J&J vaccine in the United States.
Last week, for example, the United Kingdom restricted the use of the AstraZeneca vaccine in people aged younger than 30 years, saying the risks and benefits of vaccination are “more finely balanced” for this age group.
With cases of COVID-19 rising again in the United States, and the Johnson & Johnson vaccine currently the most convenient form of protection against the virus, the committee will have to weigh the risks of that infection against the risk of rare clots caused by vaccination.
They will also likely have to rule out whether any of the cases had COVID. At least one study has reported CVST clots in three patients with confirmed COVID infections. In Europe, COVID infection did not seem to play a role in the formation of the clots with low platelets.
Hilda Bastian, PhD, a clinical trials expert who cofounded the Cochrane Collaboration, said it won’t be an easy task. Much will depend on how certain the committee members feel they know about all the events linked to the vaccine.
“That’s the really, really hard issue from my point of view for them right this moment. Have we missed any? Or how many are we likely to have missed?” asked Dr. Bastian, who lives in Australia.
“In a country that size with that fragmented [of] a health care system, how sure can you be that you know them all? That’s going to be a really difficult situation for them to grapple with, the quality of information that they’ve got,” she said.
A version of this article first appeared on Medscape.com.
Hospitalization not rare for children with COVID, study says
Nearly a third of those had severe disease that required mechanical ventilation or admission to an intensive care unit, according to a new study published in JAMA Network Open on April 9.*
That means about 1 in 9 kids with COVID-19 in this cohort needed hospitalization, and about 1 in 28 had severe COVID-19.
“Although most children with COVID-19 experience mild illness, some children develop serious illness that leads to hospitalization, use of invasive mechanical ventilation, and death,” the researchers wrote.
The research team analyzed discharge data from 869 medical facilities in the Premier Healthcare Database Special COVID-19 Release. They looked for COVID-19 patients ages 18 and under who had an in-patient or emergency department visit between March and October 2020.
More than 20,700 children with COVID-19 had an in-patient or an emergency department visit, and 2,430 were hospitalized with COVID-19. Among those, 756 children had severe COVID-19 and were admitted to an intensive care unit or needed mechanical ventilation.
About 53% of the COVID-19 patients were girls, and about 54% were between ages 12-18. In addition, about 29% had at least one chronic condition.
Similar to COVID-19 studies in adults, Hispanic, Latino and Black patients were overrepresented. About 39% of the children were Hispanic or Latino, and 24% were Black. However, the researchers didn’t find an association between severe COVID-19 and race or ethnicity.
The likelihood of severe COVID-19 increased if the patient had at least one chronic condition, was male, or was between ages 2-11.
“Understanding factors associated with severe COVID-19 disease among children could help inform prevention and control strategies,” they added. “Reducing infection risk through community mitigation strategies is critical for protecting children from COVID-19 and preventing poor outcomes.”
As of April 8, more than 3.54 million U.S. children have tested positive for COVID-19, according to the latest report from the American Academy of Pediatrics and Children’s Hospital Association. Cases among children are increasing slightly, with about 73,000 new cases reported during the first week of April.
Children represent about 13.5% of the COVID-19 cases in the country, according to the report. Among the 24 states that provide data, children represented 1% to 3% of all COVID-19 hospitalizations, and less than 2% of all child COVID-19 cases resulted in hospitalization.
“At this time, it appears that severe illness due to COVID-19 is rare among children,” the two groups wrote.
“However, there is an urgent need to collect more data on longer-term impacts of the pandemic on children, including ways the virus may harm the long-term physical health of infected children, as well as its emotional and mental health effects,” they added.
A version of this article first appeared on WebMD.com.
*CORRECTION, 6/7/21 – This story has been corrected to clarify that the patient sample study reflects only those children who presented to an emergency department or received inpatient care for COVID-19 in a hospital network and were included in the Premier Healthcare Database Special COVID-19 Release. A previous version of the story incorrectly implied that 12% of all U.S. children with COVID-19 had required inpatient care.
Nearly a third of those had severe disease that required mechanical ventilation or admission to an intensive care unit, according to a new study published in JAMA Network Open on April 9.*
That means about 1 in 9 kids with COVID-19 in this cohort needed hospitalization, and about 1 in 28 had severe COVID-19.
“Although most children with COVID-19 experience mild illness, some children develop serious illness that leads to hospitalization, use of invasive mechanical ventilation, and death,” the researchers wrote.
The research team analyzed discharge data from 869 medical facilities in the Premier Healthcare Database Special COVID-19 Release. They looked for COVID-19 patients ages 18 and under who had an in-patient or emergency department visit between March and October 2020.
More than 20,700 children with COVID-19 had an in-patient or an emergency department visit, and 2,430 were hospitalized with COVID-19. Among those, 756 children had severe COVID-19 and were admitted to an intensive care unit or needed mechanical ventilation.
About 53% of the COVID-19 patients were girls, and about 54% were between ages 12-18. In addition, about 29% had at least one chronic condition.
Similar to COVID-19 studies in adults, Hispanic, Latino and Black patients were overrepresented. About 39% of the children were Hispanic or Latino, and 24% were Black. However, the researchers didn’t find an association between severe COVID-19 and race or ethnicity.
The likelihood of severe COVID-19 increased if the patient had at least one chronic condition, was male, or was between ages 2-11.
“Understanding factors associated with severe COVID-19 disease among children could help inform prevention and control strategies,” they added. “Reducing infection risk through community mitigation strategies is critical for protecting children from COVID-19 and preventing poor outcomes.”
As of April 8, more than 3.54 million U.S. children have tested positive for COVID-19, according to the latest report from the American Academy of Pediatrics and Children’s Hospital Association. Cases among children are increasing slightly, with about 73,000 new cases reported during the first week of April.
Children represent about 13.5% of the COVID-19 cases in the country, according to the report. Among the 24 states that provide data, children represented 1% to 3% of all COVID-19 hospitalizations, and less than 2% of all child COVID-19 cases resulted in hospitalization.
“At this time, it appears that severe illness due to COVID-19 is rare among children,” the two groups wrote.
“However, there is an urgent need to collect more data on longer-term impacts of the pandemic on children, including ways the virus may harm the long-term physical health of infected children, as well as its emotional and mental health effects,” they added.
A version of this article first appeared on WebMD.com.
*CORRECTION, 6/7/21 – This story has been corrected to clarify that the patient sample study reflects only those children who presented to an emergency department or received inpatient care for COVID-19 in a hospital network and were included in the Premier Healthcare Database Special COVID-19 Release. A previous version of the story incorrectly implied that 12% of all U.S. children with COVID-19 had required inpatient care.
Nearly a third of those had severe disease that required mechanical ventilation or admission to an intensive care unit, according to a new study published in JAMA Network Open on April 9.*
That means about 1 in 9 kids with COVID-19 in this cohort needed hospitalization, and about 1 in 28 had severe COVID-19.
“Although most children with COVID-19 experience mild illness, some children develop serious illness that leads to hospitalization, use of invasive mechanical ventilation, and death,” the researchers wrote.
The research team analyzed discharge data from 869 medical facilities in the Premier Healthcare Database Special COVID-19 Release. They looked for COVID-19 patients ages 18 and under who had an in-patient or emergency department visit between March and October 2020.
More than 20,700 children with COVID-19 had an in-patient or an emergency department visit, and 2,430 were hospitalized with COVID-19. Among those, 756 children had severe COVID-19 and were admitted to an intensive care unit or needed mechanical ventilation.
About 53% of the COVID-19 patients were girls, and about 54% were between ages 12-18. In addition, about 29% had at least one chronic condition.
Similar to COVID-19 studies in adults, Hispanic, Latino and Black patients were overrepresented. About 39% of the children were Hispanic or Latino, and 24% were Black. However, the researchers didn’t find an association between severe COVID-19 and race or ethnicity.
The likelihood of severe COVID-19 increased if the patient had at least one chronic condition, was male, or was between ages 2-11.
“Understanding factors associated with severe COVID-19 disease among children could help inform prevention and control strategies,” they added. “Reducing infection risk through community mitigation strategies is critical for protecting children from COVID-19 and preventing poor outcomes.”
As of April 8, more than 3.54 million U.S. children have tested positive for COVID-19, according to the latest report from the American Academy of Pediatrics and Children’s Hospital Association. Cases among children are increasing slightly, with about 73,000 new cases reported during the first week of April.
Children represent about 13.5% of the COVID-19 cases in the country, according to the report. Among the 24 states that provide data, children represented 1% to 3% of all COVID-19 hospitalizations, and less than 2% of all child COVID-19 cases resulted in hospitalization.
“At this time, it appears that severe illness due to COVID-19 is rare among children,” the two groups wrote.
“However, there is an urgent need to collect more data on longer-term impacts of the pandemic on children, including ways the virus may harm the long-term physical health of infected children, as well as its emotional and mental health effects,” they added.
A version of this article first appeared on WebMD.com.
*CORRECTION, 6/7/21 – This story has been corrected to clarify that the patient sample study reflects only those children who presented to an emergency department or received inpatient care for COVID-19 in a hospital network and were included in the Premier Healthcare Database Special COVID-19 Release. A previous version of the story incorrectly implied that 12% of all U.S. children with COVID-19 had required inpatient care.