User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
How to counsel worried patients about the J&J vaccine news
On April 13, the Centers for Disease Control and Prevention and the Food and Drug Administration issued a joint statement recommending a pause in Johnson & Johnson vaccine administration, pending review of six reported U.S. cases of a rare and severe type of blood clot occurring after receiving the Johnson & Johnson vaccine. To date, more than 6.8 million doses of that vaccine have been given in the United States, so at this point the rate of detected cases of this problem is less than one in a million.
The six cases occurred in women aged 18-48 years, and symptoms occurred 6-13 days after vaccination. In these cases, cerebral venous sinus thrombosis was seen in addition to thrombocytopenia.
Physicians may receive calls from concerned patients who have received a COVID vaccine. However, more than 95% of the vaccine administrations in the United States to date have been the Pfizer and Moderna messenger RNA vaccines. No association between these vaccines and blood clots has been detected. Also, these six cases occurred within 2 weeks of Johnson & Johnson vaccination, so even among those receiving the Johnson & Johnson vaccine, those who are more than 3 weeks out from their vaccination have no need for concern regarding this rare complication.
Physicians should counsel those who have received the Johnson & Johnson vaccine less than 3 weeks ago to watch for easy bruising, gum bleeding, nose bleeds, leg or arm pain or swelling, severe headache or abdominal pain, shortness of breath, or chest pain. If they notice one or more of those symptoms, they should seek medical attention.
The Centers for Disease Control and Prevention will convene a meeting of the Advisory Committee on Immunization Practices on April 14 to review the six U.S. cases of the Johnson & Johnson vaccine and determine their significance.
Several cases of unusual thromboses and thrombocytopenia have been detected after the Oxford AstraZeneca vaccine, which uses the same adenovirus vector technology as the Johnson & Johnson vaccine, but which is not authorized for use in the United States. The Oxford AstraZeneca vaccine uses a recombinant deficient chimpanzee adenovirus to deliver the message to cells to produce antibody against the SARS-CoV-2 spike protein. The Johnson & Johnson vaccine uses a recombinant deficient human adenovirus to deliver this same message.
Two recent reports in the New England Journal of Medicine have reported on thrombosis and thrombocytopenia after the Oxford AstraZeneca vaccine in Europe. Both of these reports identified high levels of IgG antibodies to platelet factor 4–polyanion complexes, similar to the mechanism of heparin-induced thrombocytopenia. The term vaccine-induced immune thrombocytopenia was proposed for this phenomenon. Treatment of this condition involves administration of intravenous immunoglobulin and nonheparin anticoagulants. Recent updates from the World Health Organization report that 169 cases of cerebral venous sinus thrombosis and 53 of splanchnic venous thrombosis occurred after 34 million doses of the Oxford AstraZeneca vaccine was administered in the European Union and United Kingdom.
While this pause in Johnson & Johnson vaccination is disappointing news amid increased cases in parts of the country, the Johnson & Johnson vaccines make up less than 5% of the U.S. vaccine doses administered to date. According to the CDC, more than 122 million Americans have received at least one dose and more than 75 million are fully vaccinated.
Dr. Patterson has received an honorarium from Pfizer for an antifungal symposium and is a subinvestigator for the Novavax vaccine. Her spouse served as a consultant for SCYNEXIS, as a speaker for Gilead Sciences and Basilea, and has received a research grant from the National Institutes of Health for the ACTT remdesivir trial.
A version of this article first appeared on Medscape.com.
On April 13, the Centers for Disease Control and Prevention and the Food and Drug Administration issued a joint statement recommending a pause in Johnson & Johnson vaccine administration, pending review of six reported U.S. cases of a rare and severe type of blood clot occurring after receiving the Johnson & Johnson vaccine. To date, more than 6.8 million doses of that vaccine have been given in the United States, so at this point the rate of detected cases of this problem is less than one in a million.
The six cases occurred in women aged 18-48 years, and symptoms occurred 6-13 days after vaccination. In these cases, cerebral venous sinus thrombosis was seen in addition to thrombocytopenia.
Physicians may receive calls from concerned patients who have received a COVID vaccine. However, more than 95% of the vaccine administrations in the United States to date have been the Pfizer and Moderna messenger RNA vaccines. No association between these vaccines and blood clots has been detected. Also, these six cases occurred within 2 weeks of Johnson & Johnson vaccination, so even among those receiving the Johnson & Johnson vaccine, those who are more than 3 weeks out from their vaccination have no need for concern regarding this rare complication.
Physicians should counsel those who have received the Johnson & Johnson vaccine less than 3 weeks ago to watch for easy bruising, gum bleeding, nose bleeds, leg or arm pain or swelling, severe headache or abdominal pain, shortness of breath, or chest pain. If they notice one or more of those symptoms, they should seek medical attention.
The Centers for Disease Control and Prevention will convene a meeting of the Advisory Committee on Immunization Practices on April 14 to review the six U.S. cases of the Johnson & Johnson vaccine and determine their significance.
Several cases of unusual thromboses and thrombocytopenia have been detected after the Oxford AstraZeneca vaccine, which uses the same adenovirus vector technology as the Johnson & Johnson vaccine, but which is not authorized for use in the United States. The Oxford AstraZeneca vaccine uses a recombinant deficient chimpanzee adenovirus to deliver the message to cells to produce antibody against the SARS-CoV-2 spike protein. The Johnson & Johnson vaccine uses a recombinant deficient human adenovirus to deliver this same message.
Two recent reports in the New England Journal of Medicine have reported on thrombosis and thrombocytopenia after the Oxford AstraZeneca vaccine in Europe. Both of these reports identified high levels of IgG antibodies to platelet factor 4–polyanion complexes, similar to the mechanism of heparin-induced thrombocytopenia. The term vaccine-induced immune thrombocytopenia was proposed for this phenomenon. Treatment of this condition involves administration of intravenous immunoglobulin and nonheparin anticoagulants. Recent updates from the World Health Organization report that 169 cases of cerebral venous sinus thrombosis and 53 of splanchnic venous thrombosis occurred after 34 million doses of the Oxford AstraZeneca vaccine was administered in the European Union and United Kingdom.
While this pause in Johnson & Johnson vaccination is disappointing news amid increased cases in parts of the country, the Johnson & Johnson vaccines make up less than 5% of the U.S. vaccine doses administered to date. According to the CDC, more than 122 million Americans have received at least one dose and more than 75 million are fully vaccinated.
Dr. Patterson has received an honorarium from Pfizer for an antifungal symposium and is a subinvestigator for the Novavax vaccine. Her spouse served as a consultant for SCYNEXIS, as a speaker for Gilead Sciences and Basilea, and has received a research grant from the National Institutes of Health for the ACTT remdesivir trial.
A version of this article first appeared on Medscape.com.
On April 13, the Centers for Disease Control and Prevention and the Food and Drug Administration issued a joint statement recommending a pause in Johnson & Johnson vaccine administration, pending review of six reported U.S. cases of a rare and severe type of blood clot occurring after receiving the Johnson & Johnson vaccine. To date, more than 6.8 million doses of that vaccine have been given in the United States, so at this point the rate of detected cases of this problem is less than one in a million.
The six cases occurred in women aged 18-48 years, and symptoms occurred 6-13 days after vaccination. In these cases, cerebral venous sinus thrombosis was seen in addition to thrombocytopenia.
Physicians may receive calls from concerned patients who have received a COVID vaccine. However, more than 95% of the vaccine administrations in the United States to date have been the Pfizer and Moderna messenger RNA vaccines. No association between these vaccines and blood clots has been detected. Also, these six cases occurred within 2 weeks of Johnson & Johnson vaccination, so even among those receiving the Johnson & Johnson vaccine, those who are more than 3 weeks out from their vaccination have no need for concern regarding this rare complication.
Physicians should counsel those who have received the Johnson & Johnson vaccine less than 3 weeks ago to watch for easy bruising, gum bleeding, nose bleeds, leg or arm pain or swelling, severe headache or abdominal pain, shortness of breath, or chest pain. If they notice one or more of those symptoms, they should seek medical attention.
The Centers for Disease Control and Prevention will convene a meeting of the Advisory Committee on Immunization Practices on April 14 to review the six U.S. cases of the Johnson & Johnson vaccine and determine their significance.
Several cases of unusual thromboses and thrombocytopenia have been detected after the Oxford AstraZeneca vaccine, which uses the same adenovirus vector technology as the Johnson & Johnson vaccine, but which is not authorized for use in the United States. The Oxford AstraZeneca vaccine uses a recombinant deficient chimpanzee adenovirus to deliver the message to cells to produce antibody against the SARS-CoV-2 spike protein. The Johnson & Johnson vaccine uses a recombinant deficient human adenovirus to deliver this same message.
Two recent reports in the New England Journal of Medicine have reported on thrombosis and thrombocytopenia after the Oxford AstraZeneca vaccine in Europe. Both of these reports identified high levels of IgG antibodies to platelet factor 4–polyanion complexes, similar to the mechanism of heparin-induced thrombocytopenia. The term vaccine-induced immune thrombocytopenia was proposed for this phenomenon. Treatment of this condition involves administration of intravenous immunoglobulin and nonheparin anticoagulants. Recent updates from the World Health Organization report that 169 cases of cerebral venous sinus thrombosis and 53 of splanchnic venous thrombosis occurred after 34 million doses of the Oxford AstraZeneca vaccine was administered in the European Union and United Kingdom.
While this pause in Johnson & Johnson vaccination is disappointing news amid increased cases in parts of the country, the Johnson & Johnson vaccines make up less than 5% of the U.S. vaccine doses administered to date. According to the CDC, more than 122 million Americans have received at least one dose and more than 75 million are fully vaccinated.
Dr. Patterson has received an honorarium from Pfizer for an antifungal symposium and is a subinvestigator for the Novavax vaccine. Her spouse served as a consultant for SCYNEXIS, as a speaker for Gilead Sciences and Basilea, and has received a research grant from the National Institutes of Health for the ACTT remdesivir trial.
A version of this article first appeared on Medscape.com.
COVID-19 vaccine failure in patients with blood cancers
COVID vaccines do not work well for patients with hematologic malignancies, new data suggest.
A small study involving 67 such patients shows that nearly half did not produce antibodies and were therefore still at risk of contracting COVID-19, even though they had all received both doses of one of the new mRNA COVID vaccines (Moderna or Pfizer).
“[This] is in stark contrast with the results of phase 1 mRNA vaccine immunogenicity trials, in which robust antibody responses were seen in essentially 100% of participants,” said the authors, led by Mounzer Agha, MD, director of the Mario Lemieux Center for Blood Cancers at the University of Pittsburgh Medical Center’s Hillman Cancer Center.
“Clinicians caring for patients with hematological malignancies and other immunocompromising conditions should be aware of the possibility of COVID-19 vaccine failure,” they emphasized.
“It’s critically important for these patients to be aware of their continued risk [for SARS-CoV-2 infection] and to seek prompt medical attention if they have COVID-19 symptoms, even after vaccination,” Dr. Agha said in a statement.
The study was published online on April 9 as preprint in medRxiv and has not yet undergone peer review.
Antibody responses
The authors analyzed responses in a group of 67 patients who had a hematologic malignancy, including chronic lymphocytic leukemia (CLL), lymphoma, and multiple myeloma. Approximately 45% of the patients were receiving therapy for their cancer at the time of vaccination; the rest were under observation.
All patients received two doses of an mRNA COVID vaccine and so were considered to be fully vaccinated.
Antibody responses for these fully vaccinated patients were then analyzed. The median duration between receipt of the second dose of the vaccine and the antibody test was 23 days.
“In total ... 46.3% ... had a negative antibody result after vaccination and were therefore considered to be vaccine nonresponders,” the authors reported.
The worst responses occurred in patients with CLL, of whom only 23% produced measurable antibodies to either vaccine, although approximately 70% of these patients were not receiving any form of cancer therapy at the time of vaccination.
Older patients were more likely not to have a response to either vaccine compared with younger patients, the investigators added.
In contrast, gender, immunoglobulin G levels, the number of days between the second dose and the measurement of antibodies, and status of cancer therapy did not differ among patients who had a response to the vaccines and those who did not.
“Our findings underscore the importance of adherence to nonpharmaceutical interventions to prevent COVID-19 in hematological malignancy patients,” the authors wrote. This is particularly important, given the fact that among patients with hematologic malignancies who become infected with SARS-CoV-2, the mortality rate is in excess of 30%.
Moreover, among such patients, viral shedding may be prolonged, often lasting several months. As such, “these patients should be advised to wear masks and observe social distancing regardless of vaccination status,” the investigators advised.
As of March 2021, guidance from the Centers for Disease Control and Prevention has allowed gatherings of unmasked people who have been vaccinated and of those at low risk for COVID-19 who have not yet been vaccinated. “As we see more national guidance allowing for unmasked gatherings among vaccinated people, clinicians should counsel their immunocompromised patients about the possibility that COVID-19 vaccines may not fully protect them against SARS-CoV-2,” coauthor Ghady Haidar, MD, assistant professor of medicine at the University of Pittsburgh, said in a statement.
“Our results show that the odds of the vaccine producing an antibody response in people with hematologic malignancies are the equivalent of a coin flip,” he said.
The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
COVID vaccines do not work well for patients with hematologic malignancies, new data suggest.
A small study involving 67 such patients shows that nearly half did not produce antibodies and were therefore still at risk of contracting COVID-19, even though they had all received both doses of one of the new mRNA COVID vaccines (Moderna or Pfizer).
“[This] is in stark contrast with the results of phase 1 mRNA vaccine immunogenicity trials, in which robust antibody responses were seen in essentially 100% of participants,” said the authors, led by Mounzer Agha, MD, director of the Mario Lemieux Center for Blood Cancers at the University of Pittsburgh Medical Center’s Hillman Cancer Center.
“Clinicians caring for patients with hematological malignancies and other immunocompromising conditions should be aware of the possibility of COVID-19 vaccine failure,” they emphasized.
“It’s critically important for these patients to be aware of their continued risk [for SARS-CoV-2 infection] and to seek prompt medical attention if they have COVID-19 symptoms, even after vaccination,” Dr. Agha said in a statement.
The study was published online on April 9 as preprint in medRxiv and has not yet undergone peer review.
Antibody responses
The authors analyzed responses in a group of 67 patients who had a hematologic malignancy, including chronic lymphocytic leukemia (CLL), lymphoma, and multiple myeloma. Approximately 45% of the patients were receiving therapy for their cancer at the time of vaccination; the rest were under observation.
All patients received two doses of an mRNA COVID vaccine and so were considered to be fully vaccinated.
Antibody responses for these fully vaccinated patients were then analyzed. The median duration between receipt of the second dose of the vaccine and the antibody test was 23 days.
“In total ... 46.3% ... had a negative antibody result after vaccination and were therefore considered to be vaccine nonresponders,” the authors reported.
The worst responses occurred in patients with CLL, of whom only 23% produced measurable antibodies to either vaccine, although approximately 70% of these patients were not receiving any form of cancer therapy at the time of vaccination.
Older patients were more likely not to have a response to either vaccine compared with younger patients, the investigators added.
In contrast, gender, immunoglobulin G levels, the number of days between the second dose and the measurement of antibodies, and status of cancer therapy did not differ among patients who had a response to the vaccines and those who did not.
“Our findings underscore the importance of adherence to nonpharmaceutical interventions to prevent COVID-19 in hematological malignancy patients,” the authors wrote. This is particularly important, given the fact that among patients with hematologic malignancies who become infected with SARS-CoV-2, the mortality rate is in excess of 30%.
Moreover, among such patients, viral shedding may be prolonged, often lasting several months. As such, “these patients should be advised to wear masks and observe social distancing regardless of vaccination status,” the investigators advised.
As of March 2021, guidance from the Centers for Disease Control and Prevention has allowed gatherings of unmasked people who have been vaccinated and of those at low risk for COVID-19 who have not yet been vaccinated. “As we see more national guidance allowing for unmasked gatherings among vaccinated people, clinicians should counsel their immunocompromised patients about the possibility that COVID-19 vaccines may not fully protect them against SARS-CoV-2,” coauthor Ghady Haidar, MD, assistant professor of medicine at the University of Pittsburgh, said in a statement.
“Our results show that the odds of the vaccine producing an antibody response in people with hematologic malignancies are the equivalent of a coin flip,” he said.
The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
COVID vaccines do not work well for patients with hematologic malignancies, new data suggest.
A small study involving 67 such patients shows that nearly half did not produce antibodies and were therefore still at risk of contracting COVID-19, even though they had all received both doses of one of the new mRNA COVID vaccines (Moderna or Pfizer).
“[This] is in stark contrast with the results of phase 1 mRNA vaccine immunogenicity trials, in which robust antibody responses were seen in essentially 100% of participants,” said the authors, led by Mounzer Agha, MD, director of the Mario Lemieux Center for Blood Cancers at the University of Pittsburgh Medical Center’s Hillman Cancer Center.
“Clinicians caring for patients with hematological malignancies and other immunocompromising conditions should be aware of the possibility of COVID-19 vaccine failure,” they emphasized.
“It’s critically important for these patients to be aware of their continued risk [for SARS-CoV-2 infection] and to seek prompt medical attention if they have COVID-19 symptoms, even after vaccination,” Dr. Agha said in a statement.
The study was published online on April 9 as preprint in medRxiv and has not yet undergone peer review.
Antibody responses
The authors analyzed responses in a group of 67 patients who had a hematologic malignancy, including chronic lymphocytic leukemia (CLL), lymphoma, and multiple myeloma. Approximately 45% of the patients were receiving therapy for their cancer at the time of vaccination; the rest were under observation.
All patients received two doses of an mRNA COVID vaccine and so were considered to be fully vaccinated.
Antibody responses for these fully vaccinated patients were then analyzed. The median duration between receipt of the second dose of the vaccine and the antibody test was 23 days.
“In total ... 46.3% ... had a negative antibody result after vaccination and were therefore considered to be vaccine nonresponders,” the authors reported.
The worst responses occurred in patients with CLL, of whom only 23% produced measurable antibodies to either vaccine, although approximately 70% of these patients were not receiving any form of cancer therapy at the time of vaccination.
Older patients were more likely not to have a response to either vaccine compared with younger patients, the investigators added.
In contrast, gender, immunoglobulin G levels, the number of days between the second dose and the measurement of antibodies, and status of cancer therapy did not differ among patients who had a response to the vaccines and those who did not.
“Our findings underscore the importance of adherence to nonpharmaceutical interventions to prevent COVID-19 in hematological malignancy patients,” the authors wrote. This is particularly important, given the fact that among patients with hematologic malignancies who become infected with SARS-CoV-2, the mortality rate is in excess of 30%.
Moreover, among such patients, viral shedding may be prolonged, often lasting several months. As such, “these patients should be advised to wear masks and observe social distancing regardless of vaccination status,” the investigators advised.
As of March 2021, guidance from the Centers for Disease Control and Prevention has allowed gatherings of unmasked people who have been vaccinated and of those at low risk for COVID-19 who have not yet been vaccinated. “As we see more national guidance allowing for unmasked gatherings among vaccinated people, clinicians should counsel their immunocompromised patients about the possibility that COVID-19 vaccines may not fully protect them against SARS-CoV-2,” coauthor Ghady Haidar, MD, assistant professor of medicine at the University of Pittsburgh, said in a statement.
“Our results show that the odds of the vaccine producing an antibody response in people with hematologic malignancies are the equivalent of a coin flip,” he said.
The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The cost of pediatric specialization
I suspect that very few of you chose to go into pediatrics as part of a get-rich-quick scheme. But, like me, you may have assumed that by going into medicine you would always have a job buffered from the erratic winds of the economy, an assumption that it turns out did not take into account the risk of a global pandemic.
I also bet that if you chose to subspecialize it was not because you felt you might make more money. I and most of the lay public have always naively assumed that specialists generally make more money because … well, because they spent more time training. You, on the other hand, may have discovered belatedly that becoming a pediatric subspecialist isn’t as lucrative as you thought it might be.
It turns out that, when subjected to some standard money-crunching exercises, the lifetime earning potential of most pediatric subspecialists falls significantly behind that of general pediatricians. In a paper published in the April 2021 issue of Pediatrics, investigators from the departments of neurology and pediatric neurology at Johns Hopkins University have reported that, with the exception of three hospital-based, procedure-oriented specialties (cardiology, critical care, and neonatology) the earning time lost during training is usually not recouped over the course of a subspecialist’s career. This observation may be explained in many cases by the fact that the income generated by most subspecialists is similar to and not greater than that of general pediatricians. Even when the income of a subspecialist is greater, it is generally not enough to allow for catch up for the earning power lost during training. The researchers observed this effect both in academic and nonacademic settings.
It is possible that, as the results of this study become more widely distributed, more pediatricians in training will begin to think a bit more about the bottom line when they are considering fellowship training. I suspect that drift is already underway, and if it continues, we will find more subspecialties experiencing shortages. And the importance of this lack of subspecialists on both a local and national level is not something to ignore.
The authors discuss several possible solutions. One option might be to shorten the subspecialty training period. Obviously, this would raise some concerns about quality. Another might be for the government to begin a program in which student loans were selectively forgiven based on a physician’s decision to pursue a subspecialty that is experiencing a shortage.
Another option might be to subsidize the income of some subspecialists. Although this might have a similar effect as loan forgiveness, as a physician with a longstanding pride in being a generalist I would hate to see subspecialists guaranteed a higher income merely because of the narrower mix of patients they have chosen to see. I have always felt that the challenge faced by a primary care generalist who must be prepared to deal with the breadth of complaints that present themselves at the door is at least as great and in many cases greater than that of a specialist whose patients to a large extent have been presorted.
Another solution that comes to mind is that, instead of shortening fellowship programs, one could restructure basic pediatric training programs to allow physicians who have already chosen to become subspecialists to enter a fellowship program after 2 years of house officer training. Restructuring of this magnitude would not be as simple as lopping off the last year of house officer training. It would require tailoring each physician’s shortened prefellowship learning experience to maximize his or her exposure to clinical situations that will be most relevant to the anticipated subspecialty they have chosen. A plan like this also assumes that a significant number of recent medical school graduates will be ready to make choices during their internship that will channel them into careers that will span decades.
Becoming a generalist was an easy decision for me. Any of the subspecialties I was considering would have meant I would have had to live and work in or near a high-density population. I am and always have been a small town kind of guy.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
I suspect that very few of you chose to go into pediatrics as part of a get-rich-quick scheme. But, like me, you may have assumed that by going into medicine you would always have a job buffered from the erratic winds of the economy, an assumption that it turns out did not take into account the risk of a global pandemic.
I also bet that if you chose to subspecialize it was not because you felt you might make more money. I and most of the lay public have always naively assumed that specialists generally make more money because … well, because they spent more time training. You, on the other hand, may have discovered belatedly that becoming a pediatric subspecialist isn’t as lucrative as you thought it might be.
It turns out that, when subjected to some standard money-crunching exercises, the lifetime earning potential of most pediatric subspecialists falls significantly behind that of general pediatricians. In a paper published in the April 2021 issue of Pediatrics, investigators from the departments of neurology and pediatric neurology at Johns Hopkins University have reported that, with the exception of three hospital-based, procedure-oriented specialties (cardiology, critical care, and neonatology) the earning time lost during training is usually not recouped over the course of a subspecialist’s career. This observation may be explained in many cases by the fact that the income generated by most subspecialists is similar to and not greater than that of general pediatricians. Even when the income of a subspecialist is greater, it is generally not enough to allow for catch up for the earning power lost during training. The researchers observed this effect both in academic and nonacademic settings.
It is possible that, as the results of this study become more widely distributed, more pediatricians in training will begin to think a bit more about the bottom line when they are considering fellowship training. I suspect that drift is already underway, and if it continues, we will find more subspecialties experiencing shortages. And the importance of this lack of subspecialists on both a local and national level is not something to ignore.
The authors discuss several possible solutions. One option might be to shorten the subspecialty training period. Obviously, this would raise some concerns about quality. Another might be for the government to begin a program in which student loans were selectively forgiven based on a physician’s decision to pursue a subspecialty that is experiencing a shortage.
Another option might be to subsidize the income of some subspecialists. Although this might have a similar effect as loan forgiveness, as a physician with a longstanding pride in being a generalist I would hate to see subspecialists guaranteed a higher income merely because of the narrower mix of patients they have chosen to see. I have always felt that the challenge faced by a primary care generalist who must be prepared to deal with the breadth of complaints that present themselves at the door is at least as great and in many cases greater than that of a specialist whose patients to a large extent have been presorted.
Another solution that comes to mind is that, instead of shortening fellowship programs, one could restructure basic pediatric training programs to allow physicians who have already chosen to become subspecialists to enter a fellowship program after 2 years of house officer training. Restructuring of this magnitude would not be as simple as lopping off the last year of house officer training. It would require tailoring each physician’s shortened prefellowship learning experience to maximize his or her exposure to clinical situations that will be most relevant to the anticipated subspecialty they have chosen. A plan like this also assumes that a significant number of recent medical school graduates will be ready to make choices during their internship that will channel them into careers that will span decades.
Becoming a generalist was an easy decision for me. Any of the subspecialties I was considering would have meant I would have had to live and work in or near a high-density population. I am and always have been a small town kind of guy.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
I suspect that very few of you chose to go into pediatrics as part of a get-rich-quick scheme. But, like me, you may have assumed that by going into medicine you would always have a job buffered from the erratic winds of the economy, an assumption that it turns out did not take into account the risk of a global pandemic.
I also bet that if you chose to subspecialize it was not because you felt you might make more money. I and most of the lay public have always naively assumed that specialists generally make more money because … well, because they spent more time training. You, on the other hand, may have discovered belatedly that becoming a pediatric subspecialist isn’t as lucrative as you thought it might be.
It turns out that, when subjected to some standard money-crunching exercises, the lifetime earning potential of most pediatric subspecialists falls significantly behind that of general pediatricians. In a paper published in the April 2021 issue of Pediatrics, investigators from the departments of neurology and pediatric neurology at Johns Hopkins University have reported that, with the exception of three hospital-based, procedure-oriented specialties (cardiology, critical care, and neonatology) the earning time lost during training is usually not recouped over the course of a subspecialist’s career. This observation may be explained in many cases by the fact that the income generated by most subspecialists is similar to and not greater than that of general pediatricians. Even when the income of a subspecialist is greater, it is generally not enough to allow for catch up for the earning power lost during training. The researchers observed this effect both in academic and nonacademic settings.
It is possible that, as the results of this study become more widely distributed, more pediatricians in training will begin to think a bit more about the bottom line when they are considering fellowship training. I suspect that drift is already underway, and if it continues, we will find more subspecialties experiencing shortages. And the importance of this lack of subspecialists on both a local and national level is not something to ignore.
The authors discuss several possible solutions. One option might be to shorten the subspecialty training period. Obviously, this would raise some concerns about quality. Another might be for the government to begin a program in which student loans were selectively forgiven based on a physician’s decision to pursue a subspecialty that is experiencing a shortage.
Another option might be to subsidize the income of some subspecialists. Although this might have a similar effect as loan forgiveness, as a physician with a longstanding pride in being a generalist I would hate to see subspecialists guaranteed a higher income merely because of the narrower mix of patients they have chosen to see. I have always felt that the challenge faced by a primary care generalist who must be prepared to deal with the breadth of complaints that present themselves at the door is at least as great and in many cases greater than that of a specialist whose patients to a large extent have been presorted.
Another solution that comes to mind is that, instead of shortening fellowship programs, one could restructure basic pediatric training programs to allow physicians who have already chosen to become subspecialists to enter a fellowship program after 2 years of house officer training. Restructuring of this magnitude would not be as simple as lopping off the last year of house officer training. It would require tailoring each physician’s shortened prefellowship learning experience to maximize his or her exposure to clinical situations that will be most relevant to the anticipated subspecialty they have chosen. A plan like this also assumes that a significant number of recent medical school graduates will be ready to make choices during their internship that will channel them into careers that will span decades.
Becoming a generalist was an easy decision for me. Any of the subspecialties I was considering would have meant I would have had to live and work in or near a high-density population. I am and always have been a small town kind of guy.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
Data about COVID-19-related skin manifestations in children continue to emerge
Two and stratifying children at risk for serious, systemic illness due to the virus.
In a single-center descriptive study carried out over a 9-month period, researchers in Madrid found that of 50 hospitalized children infected with COVID-19, 21 (42%) had mucocutaneous symptoms, most commonly exanthem, followed by conjunctival hyperemia without secretion and red cracked lips or strawberry tongue. In addition, 18 (36%) fulfilled criteria for Multisystem Inflammatory Syndrome in Children (MIS-C).
“Based on findings in adult patients, the skin manifestations of COVID-19 have been classified under five categories: acral pseudo-chilblain, vesicular eruptions, urticarial lesions, maculopapular eruptions, and livedo or necrosis,” David Andina-Martinez, MD, of Hospital Infantil Universitario Niño Jesús, Madrid, and colleagues wrote in the study, which was published online on April 2 in the Journal of the American Academy of Dermatology.
“Chilblain lesions in healthy children and adolescents have received much attention; these lesions resolve without complications after a few weeks,” they added. “Besides, other cutaneous manifestations of COVID-19 in children have been the matter of case reports or small case series. Nevertheless, the mucocutaneous manifestations in hospitalized children infected with SARS-CoV-2 and their implications on the clinical course have not yet been extensively described.”
In an effort to describe the mucocutaneous manifestations in children hospitalized for COVID-19, the researchers evaluated 50 children up to 18 years of age who were admitted between March 1 and Nov. 30, 2020, to Hospital Infantil Universitario Niño Jesús, which was designated as a pediatric reference center during the peak of the pandemic. The main reasons for admission were respiratory illness (40%) and MIS-C (40%).
Of the 50 patients, 44 (88%) had a positive RT-PCR for SARS-CoV-2 and 6 (12%) met clinical suspicion criteria and had a negative RT-PCR with a positive IgG serology. In 34 patients (68%), a close contact with a suspected or confirmed case of COVID-19 was referred, while the source of the infection remained unknown in the remaining 16 patients (32%).
The researchers reported that 21 patients (42%) had mucocutaneous symptoms, most commonly maculopapular exanthem (86%), conjunctival hyperemia (81%), and red cracked lips or strawberry tongue (43%). In addition, 18 of the 21 patients (86%) fulfilled criteria for MIS-C.
“A tricky thing about MIS-C is that it often manifests 4-5 weeks after a child had COVID-19,” said Christine Ko, MD, professor of dermatology and pathology at Yale University, New Haven, Conn., who was asked to comment on the study. “MIS-C is associated with characteristic bright red lips and a red tongue that might resemble a strawberry. Such oral findings should prompt rapid evaluation for other signs and symptoms. There can be redness of the eyes or other more nonspecific skin findings (large or small areas of redness on the trunk or limbs, sometimes with surface change), but more importantly, fever, a rapid heartbeat, diarrhea, or breathing issues. The risk with MIS-C is a rapid decline in a child’s health, with admission to an intensive care unit.”
Dr. Andina-Martinez and his colleagues also contrast the skin findings of MIS-C, which are not generally on the hands or feet, with the so-called “COVID toe” or finger phenomenon, which has also been associated with SARS-CoV-2, particularly in children. “Only one of the patients in this series had skin involvement of a finger, and it only appeared after recovery from MIS-C,” Dr. Ko noted. “Distinguishing COVID toes from MIS-C is important, as COVID toes has a very good outcome, while MIS-C can have severe consequences, including protracted heart disease.”
In other findings, patients who presented with mucocutaneous signs tended to be older than those without skin signs and they presented at the emergency department with poor general status and extreme tachycardia. They also had higher C-reactive protein and D-dimer levels and lower lymphocyte counts and faced a more than a 10-fold increased risk of being admitted to the PICU, compared with patients who did not have skin signs (OR, 10.24; P = .003).
In a separate study published online on April 7 in JAMA Dermatology, Zachary E. Holcomb, MD, of the combined dermatology residency program at Massachusetts General Hospital, Boston, and colleagues presented what is believed to be the first case report of reactive infectious mucocutaneous eruption (RIME) triggered by SARS-CoV-2. RIME is the preferred term for pediatric patients who present with mucositis and rash (often a scant or even absent skin eruption) triggered by various infectious agents.
The patient, a 17-year-old male, presented to the emergency department with 3 days of mouth pain and nonpainful penile erosions. “One week prior, he experienced transient anosmia and ageusia that had since spontaneously resolved,” the researchers wrote. “At that time, he was tested for SARS-CoV-2 infection via nasopharyngeal polymerase chain reaction (PCR), the results of which were positive.”
At presentation, the patient had no fever, his vital signs were normal, and the physical exam revealed shallow erosions of the vermilion lips and hard palate, circumferential erythematous erosions of the periurethral glans penis, and five small vesicles on the trunk and upper extremities. Serum analysis revealed a normal white blood cell count with mild absolute lymphopenia, slightly elevated creatinine level, normal liver function, slightly elevated C-reactive protein level, and normal ferritin level.
Dr. Holcomb and colleagues made a diagnosis of SARS-CoV-2–associated RIME based on microbiological results, which revealed positive repeated SARS-CoV-2 nasopharyngeal PCR and negative nasopharyngeal PCR testing for Mycoplasma pneumoniae, adenovirus, Chlamydophila pneumoniae, human metapneumovirus, influenza A/B, parainfluenza 1 to 4, rhinovirus, and respiratory syncytial virus. In addition, titers of Mycoplasma pneumoniae IgM levels were negative, but Mycoplasma pneumoniae IgG levels were elevated.
The lesions resolved with 60 mg of oral prednisone taken daily for 4 days. A recurrence of oral mucositis 3 months later responded to 80 mg oral prednisone taken daily for 6 days.
“It’s not surprising that SARS-CoV-2 is yet another trigger for RIME,” said Anna Yasmine Kirkorian, MD, chief of the division of dermatology at Children’s National Hospital, Washington, who was asked to comment about the case report.
“The take-home message is for clinicians to be aware of this association and distinguish these patients from those with MIS-C, because patients with MIS-C require monitoring and urgent systemic treatment. RIME and MIS-C may potentially be distinguished clinically based on the nature of the mucositis (hemorrhagic and erosive in RIME, dry, cracked lips with ‘strawberry tongue’ in MIS-C) but more importantly patients with RIME lack laboratory evidence of severe systemic inflammation,” such as ESR, CRP, or ferritin, she said.
“A final interesting point in this article was the recurrence of mucositis in this patient, which could mean that recurrent mucositis/recurrent RIME might be yet another manifestation of ‘long-COVID’ (now called post-Acute Sequelae of SARS-CoV-2 infection) in some patients,” Dr. Kirkorian added. She noted that the American Academy of Dermatology–International League of Dermatologic Societies COVID-19 Dermatology Registry and articles like these “provide invaluable ‘hot off the presses’ information for clinicians who are facing the protean manifestations of a novel viral epidemic.”
The researchers reported having no financial disclosures.
Two and stratifying children at risk for serious, systemic illness due to the virus.
In a single-center descriptive study carried out over a 9-month period, researchers in Madrid found that of 50 hospitalized children infected with COVID-19, 21 (42%) had mucocutaneous symptoms, most commonly exanthem, followed by conjunctival hyperemia without secretion and red cracked lips or strawberry tongue. In addition, 18 (36%) fulfilled criteria for Multisystem Inflammatory Syndrome in Children (MIS-C).
“Based on findings in adult patients, the skin manifestations of COVID-19 have been classified under five categories: acral pseudo-chilblain, vesicular eruptions, urticarial lesions, maculopapular eruptions, and livedo or necrosis,” David Andina-Martinez, MD, of Hospital Infantil Universitario Niño Jesús, Madrid, and colleagues wrote in the study, which was published online on April 2 in the Journal of the American Academy of Dermatology.
“Chilblain lesions in healthy children and adolescents have received much attention; these lesions resolve without complications after a few weeks,” they added. “Besides, other cutaneous manifestations of COVID-19 in children have been the matter of case reports or small case series. Nevertheless, the mucocutaneous manifestations in hospitalized children infected with SARS-CoV-2 and their implications on the clinical course have not yet been extensively described.”
In an effort to describe the mucocutaneous manifestations in children hospitalized for COVID-19, the researchers evaluated 50 children up to 18 years of age who were admitted between March 1 and Nov. 30, 2020, to Hospital Infantil Universitario Niño Jesús, which was designated as a pediatric reference center during the peak of the pandemic. The main reasons for admission were respiratory illness (40%) and MIS-C (40%).
Of the 50 patients, 44 (88%) had a positive RT-PCR for SARS-CoV-2 and 6 (12%) met clinical suspicion criteria and had a negative RT-PCR with a positive IgG serology. In 34 patients (68%), a close contact with a suspected or confirmed case of COVID-19 was referred, while the source of the infection remained unknown in the remaining 16 patients (32%).
The researchers reported that 21 patients (42%) had mucocutaneous symptoms, most commonly maculopapular exanthem (86%), conjunctival hyperemia (81%), and red cracked lips or strawberry tongue (43%). In addition, 18 of the 21 patients (86%) fulfilled criteria for MIS-C.
“A tricky thing about MIS-C is that it often manifests 4-5 weeks after a child had COVID-19,” said Christine Ko, MD, professor of dermatology and pathology at Yale University, New Haven, Conn., who was asked to comment on the study. “MIS-C is associated with characteristic bright red lips and a red tongue that might resemble a strawberry. Such oral findings should prompt rapid evaluation for other signs and symptoms. There can be redness of the eyes or other more nonspecific skin findings (large or small areas of redness on the trunk or limbs, sometimes with surface change), but more importantly, fever, a rapid heartbeat, diarrhea, or breathing issues. The risk with MIS-C is a rapid decline in a child’s health, with admission to an intensive care unit.”
Dr. Andina-Martinez and his colleagues also contrast the skin findings of MIS-C, which are not generally on the hands or feet, with the so-called “COVID toe” or finger phenomenon, which has also been associated with SARS-CoV-2, particularly in children. “Only one of the patients in this series had skin involvement of a finger, and it only appeared after recovery from MIS-C,” Dr. Ko noted. “Distinguishing COVID toes from MIS-C is important, as COVID toes has a very good outcome, while MIS-C can have severe consequences, including protracted heart disease.”
In other findings, patients who presented with mucocutaneous signs tended to be older than those without skin signs and they presented at the emergency department with poor general status and extreme tachycardia. They also had higher C-reactive protein and D-dimer levels and lower lymphocyte counts and faced a more than a 10-fold increased risk of being admitted to the PICU, compared with patients who did not have skin signs (OR, 10.24; P = .003).
In a separate study published online on April 7 in JAMA Dermatology, Zachary E. Holcomb, MD, of the combined dermatology residency program at Massachusetts General Hospital, Boston, and colleagues presented what is believed to be the first case report of reactive infectious mucocutaneous eruption (RIME) triggered by SARS-CoV-2. RIME is the preferred term for pediatric patients who present with mucositis and rash (often a scant or even absent skin eruption) triggered by various infectious agents.
The patient, a 17-year-old male, presented to the emergency department with 3 days of mouth pain and nonpainful penile erosions. “One week prior, he experienced transient anosmia and ageusia that had since spontaneously resolved,” the researchers wrote. “At that time, he was tested for SARS-CoV-2 infection via nasopharyngeal polymerase chain reaction (PCR), the results of which were positive.”
At presentation, the patient had no fever, his vital signs were normal, and the physical exam revealed shallow erosions of the vermilion lips and hard palate, circumferential erythematous erosions of the periurethral glans penis, and five small vesicles on the trunk and upper extremities. Serum analysis revealed a normal white blood cell count with mild absolute lymphopenia, slightly elevated creatinine level, normal liver function, slightly elevated C-reactive protein level, and normal ferritin level.
Dr. Holcomb and colleagues made a diagnosis of SARS-CoV-2–associated RIME based on microbiological results, which revealed positive repeated SARS-CoV-2 nasopharyngeal PCR and negative nasopharyngeal PCR testing for Mycoplasma pneumoniae, adenovirus, Chlamydophila pneumoniae, human metapneumovirus, influenza A/B, parainfluenza 1 to 4, rhinovirus, and respiratory syncytial virus. In addition, titers of Mycoplasma pneumoniae IgM levels were negative, but Mycoplasma pneumoniae IgG levels were elevated.
The lesions resolved with 60 mg of oral prednisone taken daily for 4 days. A recurrence of oral mucositis 3 months later responded to 80 mg oral prednisone taken daily for 6 days.
“It’s not surprising that SARS-CoV-2 is yet another trigger for RIME,” said Anna Yasmine Kirkorian, MD, chief of the division of dermatology at Children’s National Hospital, Washington, who was asked to comment about the case report.
“The take-home message is for clinicians to be aware of this association and distinguish these patients from those with MIS-C, because patients with MIS-C require monitoring and urgent systemic treatment. RIME and MIS-C may potentially be distinguished clinically based on the nature of the mucositis (hemorrhagic and erosive in RIME, dry, cracked lips with ‘strawberry tongue’ in MIS-C) but more importantly patients with RIME lack laboratory evidence of severe systemic inflammation,” such as ESR, CRP, or ferritin, she said.
“A final interesting point in this article was the recurrence of mucositis in this patient, which could mean that recurrent mucositis/recurrent RIME might be yet another manifestation of ‘long-COVID’ (now called post-Acute Sequelae of SARS-CoV-2 infection) in some patients,” Dr. Kirkorian added. She noted that the American Academy of Dermatology–International League of Dermatologic Societies COVID-19 Dermatology Registry and articles like these “provide invaluable ‘hot off the presses’ information for clinicians who are facing the protean manifestations of a novel viral epidemic.”
The researchers reported having no financial disclosures.
Two and stratifying children at risk for serious, systemic illness due to the virus.
In a single-center descriptive study carried out over a 9-month period, researchers in Madrid found that of 50 hospitalized children infected with COVID-19, 21 (42%) had mucocutaneous symptoms, most commonly exanthem, followed by conjunctival hyperemia without secretion and red cracked lips or strawberry tongue. In addition, 18 (36%) fulfilled criteria for Multisystem Inflammatory Syndrome in Children (MIS-C).
“Based on findings in adult patients, the skin manifestations of COVID-19 have been classified under five categories: acral pseudo-chilblain, vesicular eruptions, urticarial lesions, maculopapular eruptions, and livedo or necrosis,” David Andina-Martinez, MD, of Hospital Infantil Universitario Niño Jesús, Madrid, and colleagues wrote in the study, which was published online on April 2 in the Journal of the American Academy of Dermatology.
“Chilblain lesions in healthy children and adolescents have received much attention; these lesions resolve without complications after a few weeks,” they added. “Besides, other cutaneous manifestations of COVID-19 in children have been the matter of case reports or small case series. Nevertheless, the mucocutaneous manifestations in hospitalized children infected with SARS-CoV-2 and their implications on the clinical course have not yet been extensively described.”
In an effort to describe the mucocutaneous manifestations in children hospitalized for COVID-19, the researchers evaluated 50 children up to 18 years of age who were admitted between March 1 and Nov. 30, 2020, to Hospital Infantil Universitario Niño Jesús, which was designated as a pediatric reference center during the peak of the pandemic. The main reasons for admission were respiratory illness (40%) and MIS-C (40%).
Of the 50 patients, 44 (88%) had a positive RT-PCR for SARS-CoV-2 and 6 (12%) met clinical suspicion criteria and had a negative RT-PCR with a positive IgG serology. In 34 patients (68%), a close contact with a suspected or confirmed case of COVID-19 was referred, while the source of the infection remained unknown in the remaining 16 patients (32%).
The researchers reported that 21 patients (42%) had mucocutaneous symptoms, most commonly maculopapular exanthem (86%), conjunctival hyperemia (81%), and red cracked lips or strawberry tongue (43%). In addition, 18 of the 21 patients (86%) fulfilled criteria for MIS-C.
“A tricky thing about MIS-C is that it often manifests 4-5 weeks after a child had COVID-19,” said Christine Ko, MD, professor of dermatology and pathology at Yale University, New Haven, Conn., who was asked to comment on the study. “MIS-C is associated with characteristic bright red lips and a red tongue that might resemble a strawberry. Such oral findings should prompt rapid evaluation for other signs and symptoms. There can be redness of the eyes or other more nonspecific skin findings (large or small areas of redness on the trunk or limbs, sometimes with surface change), but more importantly, fever, a rapid heartbeat, diarrhea, or breathing issues. The risk with MIS-C is a rapid decline in a child’s health, with admission to an intensive care unit.”
Dr. Andina-Martinez and his colleagues also contrast the skin findings of MIS-C, which are not generally on the hands or feet, with the so-called “COVID toe” or finger phenomenon, which has also been associated with SARS-CoV-2, particularly in children. “Only one of the patients in this series had skin involvement of a finger, and it only appeared after recovery from MIS-C,” Dr. Ko noted. “Distinguishing COVID toes from MIS-C is important, as COVID toes has a very good outcome, while MIS-C can have severe consequences, including protracted heart disease.”
In other findings, patients who presented with mucocutaneous signs tended to be older than those without skin signs and they presented at the emergency department with poor general status and extreme tachycardia. They also had higher C-reactive protein and D-dimer levels and lower lymphocyte counts and faced a more than a 10-fold increased risk of being admitted to the PICU, compared with patients who did not have skin signs (OR, 10.24; P = .003).
In a separate study published online on April 7 in JAMA Dermatology, Zachary E. Holcomb, MD, of the combined dermatology residency program at Massachusetts General Hospital, Boston, and colleagues presented what is believed to be the first case report of reactive infectious mucocutaneous eruption (RIME) triggered by SARS-CoV-2. RIME is the preferred term for pediatric patients who present with mucositis and rash (often a scant or even absent skin eruption) triggered by various infectious agents.
The patient, a 17-year-old male, presented to the emergency department with 3 days of mouth pain and nonpainful penile erosions. “One week prior, he experienced transient anosmia and ageusia that had since spontaneously resolved,” the researchers wrote. “At that time, he was tested for SARS-CoV-2 infection via nasopharyngeal polymerase chain reaction (PCR), the results of which were positive.”
At presentation, the patient had no fever, his vital signs were normal, and the physical exam revealed shallow erosions of the vermilion lips and hard palate, circumferential erythematous erosions of the periurethral glans penis, and five small vesicles on the trunk and upper extremities. Serum analysis revealed a normal white blood cell count with mild absolute lymphopenia, slightly elevated creatinine level, normal liver function, slightly elevated C-reactive protein level, and normal ferritin level.
Dr. Holcomb and colleagues made a diagnosis of SARS-CoV-2–associated RIME based on microbiological results, which revealed positive repeated SARS-CoV-2 nasopharyngeal PCR and negative nasopharyngeal PCR testing for Mycoplasma pneumoniae, adenovirus, Chlamydophila pneumoniae, human metapneumovirus, influenza A/B, parainfluenza 1 to 4, rhinovirus, and respiratory syncytial virus. In addition, titers of Mycoplasma pneumoniae IgM levels were negative, but Mycoplasma pneumoniae IgG levels were elevated.
The lesions resolved with 60 mg of oral prednisone taken daily for 4 days. A recurrence of oral mucositis 3 months later responded to 80 mg oral prednisone taken daily for 6 days.
“It’s not surprising that SARS-CoV-2 is yet another trigger for RIME,” said Anna Yasmine Kirkorian, MD, chief of the division of dermatology at Children’s National Hospital, Washington, who was asked to comment about the case report.
“The take-home message is for clinicians to be aware of this association and distinguish these patients from those with MIS-C, because patients with MIS-C require monitoring and urgent systemic treatment. RIME and MIS-C may potentially be distinguished clinically based on the nature of the mucositis (hemorrhagic and erosive in RIME, dry, cracked lips with ‘strawberry tongue’ in MIS-C) but more importantly patients with RIME lack laboratory evidence of severe systemic inflammation,” such as ESR, CRP, or ferritin, she said.
“A final interesting point in this article was the recurrence of mucositis in this patient, which could mean that recurrent mucositis/recurrent RIME might be yet another manifestation of ‘long-COVID’ (now called post-Acute Sequelae of SARS-CoV-2 infection) in some patients,” Dr. Kirkorian added. She noted that the American Academy of Dermatology–International League of Dermatologic Societies COVID-19 Dermatology Registry and articles like these “provide invaluable ‘hot off the presses’ information for clinicians who are facing the protean manifestations of a novel viral epidemic.”
The researchers reported having no financial disclosures.
FDA to recommend limits on heavy metals in baby foods
The Food and Drug Administration has responded to congressional criticism and launched a multiyear plan to reduce the amount of heavy metals such as mercury and arsenic found in baby food.
Called “Closer to Zero,” the FDA plan calls for continued scientific investigation, establishes acceptable levels of heavy metals, sets up a way to monitor manufacturers’ compliance, and sets “action levels.”
“Although the FDA’s testing shows that children are not at an immediate health risk from exposure to toxic elements at the levels found in foods, we are starting the plan’s work immediately, with both short- and long-term goals for achieving continued improvements in reducing levels of toxic elements in these foods over time,” the FDA said.
However, Closer to Zero will only make recommendations on heavy metal levels.
“Although action levels are not binding, we have seen that, over the years, our guidance on action levels and other actions have contributed to significant reductions of toxic elements in food,” an FDA spokeswoman wrote in a statement, according to the Washington Post.
A congressional panel said in February 2021 that major brands of commercial baby food routinely have high levels of toxic heavy metals. The House Oversight Committee said this leaves babies at risk for serious developmental and neurologic problems.
The committee sharply criticized the FDA for not taking action.
“Despite the well-known risks of harm to babies from toxic heavy metals, FDA has not taken adequate steps to decrease their presence in baby foods,” the committee said. “FDA has not issued thresholds for the vast majority of toxic heavy metals in baby foods and does not require warning labels on any baby food products.”
A version of this article first appeared on WebMD.com.
The Food and Drug Administration has responded to congressional criticism and launched a multiyear plan to reduce the amount of heavy metals such as mercury and arsenic found in baby food.
Called “Closer to Zero,” the FDA plan calls for continued scientific investigation, establishes acceptable levels of heavy metals, sets up a way to monitor manufacturers’ compliance, and sets “action levels.”
“Although the FDA’s testing shows that children are not at an immediate health risk from exposure to toxic elements at the levels found in foods, we are starting the plan’s work immediately, with both short- and long-term goals for achieving continued improvements in reducing levels of toxic elements in these foods over time,” the FDA said.
However, Closer to Zero will only make recommendations on heavy metal levels.
“Although action levels are not binding, we have seen that, over the years, our guidance on action levels and other actions have contributed to significant reductions of toxic elements in food,” an FDA spokeswoman wrote in a statement, according to the Washington Post.
A congressional panel said in February 2021 that major brands of commercial baby food routinely have high levels of toxic heavy metals. The House Oversight Committee said this leaves babies at risk for serious developmental and neurologic problems.
The committee sharply criticized the FDA for not taking action.
“Despite the well-known risks of harm to babies from toxic heavy metals, FDA has not taken adequate steps to decrease their presence in baby foods,” the committee said. “FDA has not issued thresholds for the vast majority of toxic heavy metals in baby foods and does not require warning labels on any baby food products.”
A version of this article first appeared on WebMD.com.
The Food and Drug Administration has responded to congressional criticism and launched a multiyear plan to reduce the amount of heavy metals such as mercury and arsenic found in baby food.
Called “Closer to Zero,” the FDA plan calls for continued scientific investigation, establishes acceptable levels of heavy metals, sets up a way to monitor manufacturers’ compliance, and sets “action levels.”
“Although the FDA’s testing shows that children are not at an immediate health risk from exposure to toxic elements at the levels found in foods, we are starting the plan’s work immediately, with both short- and long-term goals for achieving continued improvements in reducing levels of toxic elements in these foods over time,” the FDA said.
However, Closer to Zero will only make recommendations on heavy metal levels.
“Although action levels are not binding, we have seen that, over the years, our guidance on action levels and other actions have contributed to significant reductions of toxic elements in food,” an FDA spokeswoman wrote in a statement, according to the Washington Post.
A congressional panel said in February 2021 that major brands of commercial baby food routinely have high levels of toxic heavy metals. The House Oversight Committee said this leaves babies at risk for serious developmental and neurologic problems.
The committee sharply criticized the FDA for not taking action.
“Despite the well-known risks of harm to babies from toxic heavy metals, FDA has not taken adequate steps to decrease their presence in baby foods,” the committee said. “FDA has not issued thresholds for the vast majority of toxic heavy metals in baby foods and does not require warning labels on any baby food products.”
A version of this article first appeared on WebMD.com.
Low-calorie diet linked to improved chemo response in leukemia
Children and adolescents with leukemia who were placed on a restrictive diet and exercise regimen concurrent with starting chemotherapy showed responses to treatment that were better than those historically seen in such patients.
This apparently improved response suggests it is possible to boost treatment efficacy without raising the dose – or toxicity – of chemotherapy.
“To our knowledge, this is the first study in any hematologic malignancy to demonstrate potential benefit from caloric restriction via diet and exercise to augment chemotherapy efficacy and improve disease response, the authors reported.
The findings come from the IDEAL pilot trial, conducted in 40 young patients (mean age, 15 years; range, 10-21 years) diagnosed with high-risk B-cell acute lymphoblastic leukemia (B-ALL).
The study was published online April 1 in Blood Advances.
The diet and exercise regimen is a departure from current recommendations for patients with leukemia.
“This was a major paradigm shift – until now, many oncologists encouraged ‘comfort foods’ and increased calories to get through the rigor of chemotherapy,” first author Etan Orgel, MD, of Children’s Hospital Los Angeles and the University of Southern California, also in Los Angeles.
The results from this pilot trial suggest that “the era of encouraging comfort food should be in the past; over-nutrition is likely harmful, and diet and exercise are important tools to harness during chemotherapy,” he said.
Dr. Orgel added that childhood ALL was selected because it is the most common cancer of childhood, but the findings could have potential relevance in other cancer types in children as well as adults.
Commenting on the study, Patrick Brown, MD, director of the pediatric leukemia program at Johns Hopkins University, Baltimore, said the findings are important, albeit preliminary.
“I think the most important contribution of this pilot study is to show that it is possible to change the nutrition and exercise habits of children and adolescents during the initial month of treatment for ALL,” he said in an interview.
“We have to be cautious about the preliminary finding that these changes resulted in deeper remissions – this will need to be confirmed in a larger study,” added Dr. Brown, who was not involved with the research.
Dr. Orgel noted that a prospective, randomized trial, IDEAL-2, is launching later this year to further evaluate the intervention.
Obesity linked to poorer chemotherapy response
Among children and adolescents who start treatment for B-ALL, as many as 40% are overweight or obese, noted the study authors.
Those who are obese have more than a twofold greater risk of having persistent minimal residual disease (MRD) at the end of chemotherapy, considered the strongest patient-level predictor of poor outcome and a common guide for therapy intensification.
The problem is compounded by weight gain that is common during treatment as a result of prolonged chemotherapy and sedentary behavior, they commented.
With studies of obese mice linking calorie and fat restriction to improved survival after chemotherapy, the authors theorized that a calorie- and fat-restrictive diet and exercise could help improve outcomes after chemotherapy in humans.
Participants were enrolled at Children’s Hospital Los Angeles and City of Hope National Medical Center in nearby Duarte. After they were started on chemotherapy, they were placed on a low-carb, low-fat, and low-sugar diet tailored to patient needs and preferences, as well as a moderate daily exercise regimen, and continued on this regimen throughout the 4-week induction phase.
Following the intervention, there were no significant reductions observed in median gain of fat mass at the end of the intervention, compared with baseline (P = .13). However, in the subgroup of patients who were overweight or obese at baseline, the reduction in fat mass was indeed significant versus baseline (+1.5% vs. +9.7% at baseline; P = .02).
Importantly, after adjustment for prognostic factors, adherence to the intervention was associated with a significant reduction in the risk of MRD, compared with recent historical controls who received the same induction therapy at the same institution, but no intervention (odds ratio, 0.30; P = .02).
The intervention was also associated with a lower detectable MRD, compared with the historical controls (OR, 0.16; one-sided P = .002).
“Most importantly, the IDEAL intervention reduced risk of MRD at the end of induction in all patients, irrespective of starting [body mass index] and after accounting for prognostic features,” the authors noted.
Adherence to diet high, exercise low
As many as 82% of study participants achieved the goal of 20% or more caloric deficit throughout the chemotherapy.
“Adherence to the diet was excellent, with caloric deficits and macronutrient goals achieved in nearly all patients, including in the lean group,” the authors reported.
Dr. Orgel added that families embraced the chance to play an active role in the cancer therapy. “In our view, they couldn’t control their disease or their chemotherapy, but this, they could,” he said.
Conversely, adherence to the prescribed exercise was low – just 31.2%, with the inactivity during the first month likely contributed to the similar loss of muscle mass that occurred in both cohorts, Dr. Orgel noted.
“The [low exercise adherence] unfortunately was not a surprise, as it is often difficult to exercise and be active during chemotherapy,” he said.
Key aspects of physical activity will be refined in further studies, Dr. Orgel added.
Insulin sensitivity, adiponectin key factors?
Patients receiving the intervention showed improved insulin sensitivity and reductions in circulating insulin, which are notable in that insulin has been linked to mechanisms that counter chemoresistance, the authors noted.
Furthermore, the decreases in insulin were accompanied by notable elevations in circulating adiponectin, a protein hormone produced and secreted by fat cells.
“Adiponectin was certainly a surprise, as until now it did not appear to play a major role in cancer cell resistance to chemotherapy,” Dr. Orgel said.
“It is too soon to say they are central to the mechanism of the intervention, but the large differences in adiponectin and insulin sensitivity found in children in the trial have definitely highlighted these as important for future study,” he added.
Dr. Orgel, the study coauthors, and Dr. Brown disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Children and adolescents with leukemia who were placed on a restrictive diet and exercise regimen concurrent with starting chemotherapy showed responses to treatment that were better than those historically seen in such patients.
This apparently improved response suggests it is possible to boost treatment efficacy without raising the dose – or toxicity – of chemotherapy.
“To our knowledge, this is the first study in any hematologic malignancy to demonstrate potential benefit from caloric restriction via diet and exercise to augment chemotherapy efficacy and improve disease response, the authors reported.
The findings come from the IDEAL pilot trial, conducted in 40 young patients (mean age, 15 years; range, 10-21 years) diagnosed with high-risk B-cell acute lymphoblastic leukemia (B-ALL).
The study was published online April 1 in Blood Advances.
The diet and exercise regimen is a departure from current recommendations for patients with leukemia.
“This was a major paradigm shift – until now, many oncologists encouraged ‘comfort foods’ and increased calories to get through the rigor of chemotherapy,” first author Etan Orgel, MD, of Children’s Hospital Los Angeles and the University of Southern California, also in Los Angeles.
The results from this pilot trial suggest that “the era of encouraging comfort food should be in the past; over-nutrition is likely harmful, and diet and exercise are important tools to harness during chemotherapy,” he said.
Dr. Orgel added that childhood ALL was selected because it is the most common cancer of childhood, but the findings could have potential relevance in other cancer types in children as well as adults.
Commenting on the study, Patrick Brown, MD, director of the pediatric leukemia program at Johns Hopkins University, Baltimore, said the findings are important, albeit preliminary.
“I think the most important contribution of this pilot study is to show that it is possible to change the nutrition and exercise habits of children and adolescents during the initial month of treatment for ALL,” he said in an interview.
“We have to be cautious about the preliminary finding that these changes resulted in deeper remissions – this will need to be confirmed in a larger study,” added Dr. Brown, who was not involved with the research.
Dr. Orgel noted that a prospective, randomized trial, IDEAL-2, is launching later this year to further evaluate the intervention.
Obesity linked to poorer chemotherapy response
Among children and adolescents who start treatment for B-ALL, as many as 40% are overweight or obese, noted the study authors.
Those who are obese have more than a twofold greater risk of having persistent minimal residual disease (MRD) at the end of chemotherapy, considered the strongest patient-level predictor of poor outcome and a common guide for therapy intensification.
The problem is compounded by weight gain that is common during treatment as a result of prolonged chemotherapy and sedentary behavior, they commented.
With studies of obese mice linking calorie and fat restriction to improved survival after chemotherapy, the authors theorized that a calorie- and fat-restrictive diet and exercise could help improve outcomes after chemotherapy in humans.
Participants were enrolled at Children’s Hospital Los Angeles and City of Hope National Medical Center in nearby Duarte. After they were started on chemotherapy, they were placed on a low-carb, low-fat, and low-sugar diet tailored to patient needs and preferences, as well as a moderate daily exercise regimen, and continued on this regimen throughout the 4-week induction phase.
Following the intervention, there were no significant reductions observed in median gain of fat mass at the end of the intervention, compared with baseline (P = .13). However, in the subgroup of patients who were overweight or obese at baseline, the reduction in fat mass was indeed significant versus baseline (+1.5% vs. +9.7% at baseline; P = .02).
Importantly, after adjustment for prognostic factors, adherence to the intervention was associated with a significant reduction in the risk of MRD, compared with recent historical controls who received the same induction therapy at the same institution, but no intervention (odds ratio, 0.30; P = .02).
The intervention was also associated with a lower detectable MRD, compared with the historical controls (OR, 0.16; one-sided P = .002).
“Most importantly, the IDEAL intervention reduced risk of MRD at the end of induction in all patients, irrespective of starting [body mass index] and after accounting for prognostic features,” the authors noted.
Adherence to diet high, exercise low
As many as 82% of study participants achieved the goal of 20% or more caloric deficit throughout the chemotherapy.
“Adherence to the diet was excellent, with caloric deficits and macronutrient goals achieved in nearly all patients, including in the lean group,” the authors reported.
Dr. Orgel added that families embraced the chance to play an active role in the cancer therapy. “In our view, they couldn’t control their disease or their chemotherapy, but this, they could,” he said.
Conversely, adherence to the prescribed exercise was low – just 31.2%, with the inactivity during the first month likely contributed to the similar loss of muscle mass that occurred in both cohorts, Dr. Orgel noted.
“The [low exercise adherence] unfortunately was not a surprise, as it is often difficult to exercise and be active during chemotherapy,” he said.
Key aspects of physical activity will be refined in further studies, Dr. Orgel added.
Insulin sensitivity, adiponectin key factors?
Patients receiving the intervention showed improved insulin sensitivity and reductions in circulating insulin, which are notable in that insulin has been linked to mechanisms that counter chemoresistance, the authors noted.
Furthermore, the decreases in insulin were accompanied by notable elevations in circulating adiponectin, a protein hormone produced and secreted by fat cells.
“Adiponectin was certainly a surprise, as until now it did not appear to play a major role in cancer cell resistance to chemotherapy,” Dr. Orgel said.
“It is too soon to say they are central to the mechanism of the intervention, but the large differences in adiponectin and insulin sensitivity found in children in the trial have definitely highlighted these as important for future study,” he added.
Dr. Orgel, the study coauthors, and Dr. Brown disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Children and adolescents with leukemia who were placed on a restrictive diet and exercise regimen concurrent with starting chemotherapy showed responses to treatment that were better than those historically seen in such patients.
This apparently improved response suggests it is possible to boost treatment efficacy without raising the dose – or toxicity – of chemotherapy.
“To our knowledge, this is the first study in any hematologic malignancy to demonstrate potential benefit from caloric restriction via diet and exercise to augment chemotherapy efficacy and improve disease response, the authors reported.
The findings come from the IDEAL pilot trial, conducted in 40 young patients (mean age, 15 years; range, 10-21 years) diagnosed with high-risk B-cell acute lymphoblastic leukemia (B-ALL).
The study was published online April 1 in Blood Advances.
The diet and exercise regimen is a departure from current recommendations for patients with leukemia.
“This was a major paradigm shift – until now, many oncologists encouraged ‘comfort foods’ and increased calories to get through the rigor of chemotherapy,” first author Etan Orgel, MD, of Children’s Hospital Los Angeles and the University of Southern California, also in Los Angeles.
The results from this pilot trial suggest that “the era of encouraging comfort food should be in the past; over-nutrition is likely harmful, and diet and exercise are important tools to harness during chemotherapy,” he said.
Dr. Orgel added that childhood ALL was selected because it is the most common cancer of childhood, but the findings could have potential relevance in other cancer types in children as well as adults.
Commenting on the study, Patrick Brown, MD, director of the pediatric leukemia program at Johns Hopkins University, Baltimore, said the findings are important, albeit preliminary.
“I think the most important contribution of this pilot study is to show that it is possible to change the nutrition and exercise habits of children and adolescents during the initial month of treatment for ALL,” he said in an interview.
“We have to be cautious about the preliminary finding that these changes resulted in deeper remissions – this will need to be confirmed in a larger study,” added Dr. Brown, who was not involved with the research.
Dr. Orgel noted that a prospective, randomized trial, IDEAL-2, is launching later this year to further evaluate the intervention.
Obesity linked to poorer chemotherapy response
Among children and adolescents who start treatment for B-ALL, as many as 40% are overweight or obese, noted the study authors.
Those who are obese have more than a twofold greater risk of having persistent minimal residual disease (MRD) at the end of chemotherapy, considered the strongest patient-level predictor of poor outcome and a common guide for therapy intensification.
The problem is compounded by weight gain that is common during treatment as a result of prolonged chemotherapy and sedentary behavior, they commented.
With studies of obese mice linking calorie and fat restriction to improved survival after chemotherapy, the authors theorized that a calorie- and fat-restrictive diet and exercise could help improve outcomes after chemotherapy in humans.
Participants were enrolled at Children’s Hospital Los Angeles and City of Hope National Medical Center in nearby Duarte. After they were started on chemotherapy, they were placed on a low-carb, low-fat, and low-sugar diet tailored to patient needs and preferences, as well as a moderate daily exercise regimen, and continued on this regimen throughout the 4-week induction phase.
Following the intervention, there were no significant reductions observed in median gain of fat mass at the end of the intervention, compared with baseline (P = .13). However, in the subgroup of patients who were overweight or obese at baseline, the reduction in fat mass was indeed significant versus baseline (+1.5% vs. +9.7% at baseline; P = .02).
Importantly, after adjustment for prognostic factors, adherence to the intervention was associated with a significant reduction in the risk of MRD, compared with recent historical controls who received the same induction therapy at the same institution, but no intervention (odds ratio, 0.30; P = .02).
The intervention was also associated with a lower detectable MRD, compared with the historical controls (OR, 0.16; one-sided P = .002).
“Most importantly, the IDEAL intervention reduced risk of MRD at the end of induction in all patients, irrespective of starting [body mass index] and after accounting for prognostic features,” the authors noted.
Adherence to diet high, exercise low
As many as 82% of study participants achieved the goal of 20% or more caloric deficit throughout the chemotherapy.
“Adherence to the diet was excellent, with caloric deficits and macronutrient goals achieved in nearly all patients, including in the lean group,” the authors reported.
Dr. Orgel added that families embraced the chance to play an active role in the cancer therapy. “In our view, they couldn’t control their disease or their chemotherapy, but this, they could,” he said.
Conversely, adherence to the prescribed exercise was low – just 31.2%, with the inactivity during the first month likely contributed to the similar loss of muscle mass that occurred in both cohorts, Dr. Orgel noted.
“The [low exercise adherence] unfortunately was not a surprise, as it is often difficult to exercise and be active during chemotherapy,” he said.
Key aspects of physical activity will be refined in further studies, Dr. Orgel added.
Insulin sensitivity, adiponectin key factors?
Patients receiving the intervention showed improved insulin sensitivity and reductions in circulating insulin, which are notable in that insulin has been linked to mechanisms that counter chemoresistance, the authors noted.
Furthermore, the decreases in insulin were accompanied by notable elevations in circulating adiponectin, a protein hormone produced and secreted by fat cells.
“Adiponectin was certainly a surprise, as until now it did not appear to play a major role in cancer cell resistance to chemotherapy,” Dr. Orgel said.
“It is too soon to say they are central to the mechanism of the intervention, but the large differences in adiponectin and insulin sensitivity found in children in the trial have definitely highlighted these as important for future study,” he added.
Dr. Orgel, the study coauthors, and Dr. Brown disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
COVID-19 in children: New cases on the rise again
The number of new COVID-19 cases in children rose for the third time in the last 4 weeks, reaching the highest point since mid-February, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
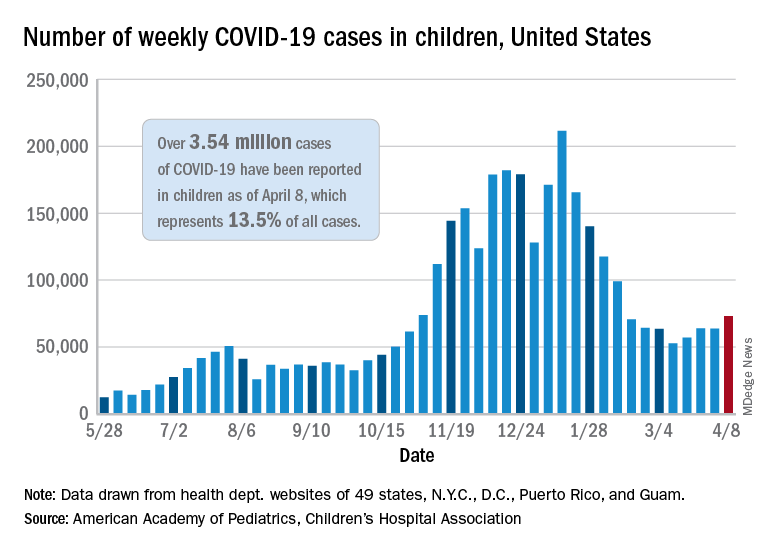
Just over 73,000 cases were reported during the week of April 2-8, up by 14.6% over the previous week. For the latest week, children represented 18.8% of all COVID-19 cases in the United States – also up from the week before and the second-highest proportion seen during the entire pandemic, based on data in the weekly AAP/CHA report.
The 3.54 million children who have been infected with SARS-CoV-2 make up 13.5% of all cases reported in the United States during the pandemic, a figure that climbed again after 2 weeks at 13.4%. The overall rate of infection was just over 4,700 cases per 100,000 children as of April 8, the AAP and CHA said.
State-level data show that Vermont, Michigan, and Maine have been the COVID-19 hotspots over the past 2 weeks. The total number of cases has jumped by almost 19% in Vermont since the week of March 19-25, by 18% in Michigan, and by 12% in Maine, according to the report.
Cumulative data also indicate that the children of Vermont are bearing a greater share of the COVID-19 burden – 21.5% of all cases – than in any other state. North Dakota, meanwhile, has the highest cumulative rate of infection at 9,057 cases per 100,000 children, based on data from 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
The number of COVID-19–related deaths in children increased by 8 during the week of April 2-8 and now stands at 292, just 0.06% of all deaths reported in the 43 states (along with New York City, Puerto Rico, and Guam) that provide age distributions for mortality data, the AAP and CHA said.
The number of new COVID-19 cases in children rose for the third time in the last 4 weeks, reaching the highest point since mid-February, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

Just over 73,000 cases were reported during the week of April 2-8, up by 14.6% over the previous week. For the latest week, children represented 18.8% of all COVID-19 cases in the United States – also up from the week before and the second-highest proportion seen during the entire pandemic, based on data in the weekly AAP/CHA report.
The 3.54 million children who have been infected with SARS-CoV-2 make up 13.5% of all cases reported in the United States during the pandemic, a figure that climbed again after 2 weeks at 13.4%. The overall rate of infection was just over 4,700 cases per 100,000 children as of April 8, the AAP and CHA said.
State-level data show that Vermont, Michigan, and Maine have been the COVID-19 hotspots over the past 2 weeks. The total number of cases has jumped by almost 19% in Vermont since the week of March 19-25, by 18% in Michigan, and by 12% in Maine, according to the report.
Cumulative data also indicate that the children of Vermont are bearing a greater share of the COVID-19 burden – 21.5% of all cases – than in any other state. North Dakota, meanwhile, has the highest cumulative rate of infection at 9,057 cases per 100,000 children, based on data from 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
The number of COVID-19–related deaths in children increased by 8 during the week of April 2-8 and now stands at 292, just 0.06% of all deaths reported in the 43 states (along with New York City, Puerto Rico, and Guam) that provide age distributions for mortality data, the AAP and CHA said.
The number of new COVID-19 cases in children rose for the third time in the last 4 weeks, reaching the highest point since mid-February, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

Just over 73,000 cases were reported during the week of April 2-8, up by 14.6% over the previous week. For the latest week, children represented 18.8% of all COVID-19 cases in the United States – also up from the week before and the second-highest proportion seen during the entire pandemic, based on data in the weekly AAP/CHA report.
The 3.54 million children who have been infected with SARS-CoV-2 make up 13.5% of all cases reported in the United States during the pandemic, a figure that climbed again after 2 weeks at 13.4%. The overall rate of infection was just over 4,700 cases per 100,000 children as of April 8, the AAP and CHA said.
State-level data show that Vermont, Michigan, and Maine have been the COVID-19 hotspots over the past 2 weeks. The total number of cases has jumped by almost 19% in Vermont since the week of March 19-25, by 18% in Michigan, and by 12% in Maine, according to the report.
Cumulative data also indicate that the children of Vermont are bearing a greater share of the COVID-19 burden – 21.5% of all cases – than in any other state. North Dakota, meanwhile, has the highest cumulative rate of infection at 9,057 cases per 100,000 children, based on data from 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
The number of COVID-19–related deaths in children increased by 8 during the week of April 2-8 and now stands at 292, just 0.06% of all deaths reported in the 43 states (along with New York City, Puerto Rico, and Guam) that provide age distributions for mortality data, the AAP and CHA said.
The obesity risk everyone forgets
Clinicians in pediatrics have noticed a troubling pattern emerge during the pandemic, something that is darkly referred to as “the COVID 19,” or the 19 or more pounds that many of our patients have gained in the past year. This phenomenon has underscored many maxims in pediatric weight management: Mainly that frequent snacking, decreased physical activity, and less parental supervision lead to increased weight gain. But could we be missing another lesson this trend is teaching us? What about the relationship between catastrophe and childhood obesity?
Beyond the increased weight gain with lockdowns, I have observed other evidence in my own practice that childhood trauma or adverse experiences increase obesity. Our electronic medical record system gives an alert when a chart with sensitive information is accessed. One example might be if the patient had been seen at a clinic for children who have been abused. I am heartbroken at how often this happens. Academically, I understand the dire statistics about the incidence of child abuse, but the frequency at which I see this pattern is jarring.
Over the years, one striking correlation became clear among my patient population: Children with obesity were more likely to have been seen in the child abuse clinic than normal-weight peers.
I am far from the only one to have observed this relationship. Television shows focusing on severe obesity, such as “My 600-Pound Life,” often show trauma as both a cause and effect of severe obesity. This theme also became apparent on the show “The Biggest Loser,” which highlighted the difficulty of achieving and maintaining substantial weight loss. If even Hollywood has noticed this association, shouldn’t we be much farther ahead?
Pathways to obesity
Adverse childhood experiences (ACE) encompass various causes of child trauma, including abuse or neglect; poverty; household or neighborhood violence; and death, illness, or incarceration of a parent. A pivotal report in 1998 formalized the suspicion that many of us could plainly see: People who suffered ACE have higher incidence of heart disease, COPD, liver disease, incarceration, and drug abuse. For those with six or more ACE, life expectancy averaged 20 years less than those who had none. More recently, a meta-analysis found an odds ratio of 1.46 for adult obesity with known history of childhood trauma.
As a pediatric endocrinologist living in the poorest state of the country, I have clearly observed the correlation between childhood obesity and poverty. While prior generations may have associated child poverty with malnutrition and starvation, we are seeing in modern times that obesity has become a disease of lack. Calorie-dense and processed foods tend to be less expensive, more shelf-stable, and more accessible to people living in both urban and rural food deserts.
I am also a foster mother and have received extensive training in parenting children who have lived through trauma and neglect. For children who have endured food scarcity and deprivation, hoarding food and overeating are expected responses.
But the pathways to abnormal weight gain are myriad and expand beyond binge eating or numbing with food. ACE are particularly troubling because they affect developing brains and the neuroendocrine system; they alter epigenetics and cause heritable changes. Structural brain differences have been evident in the frontopolar cortex, which is linked to centers in the hypothalamus that control appetite. And increased stress raises cortisol release, increases insulin resistance, and alters satiety.
Shifting our approach to treatment
The significant cost of ACE is enormous and affects us all. Health professionals in pediatrics must understand these connections to effectively counsel children and their families dealing with obesity. Handing someone a diet plan and lecturing them about weight loss is never effective, but this common tactic is especially cruel if we do not assess for and address underlying pain. Obviously, blame and shame are ineffective motivators for lifestyle change in any circumstance, but these tactics may be especially harmful in the light of childhood trauma.
Screening for ACE is important in every aspect of pediatric care. The presence of obesity, however, should remind us to be more sensitive to the possibility of causative trauma. Clinicians for adults are not off the hook either. Fully 60% of adults suffered ACE and are dealing with the aftermath.
To improve health outcomes across the board, we must screen for trauma and become educated on trauma-informed care. Perhaps the most important first referral for a child suffering ACE and obesity is to a trained counselor or a social worker. Shepherding children through trauma will be more effective for attaining healthy weight than any remedy I can prescribe as an endocrinologist. Furthermore, this is our necessary role as healers. More than ever, we need to approach chronic diseases, including obesity, with the utmost compassion.
A version of this article first appeared on Medscape.com.
Clinicians in pediatrics have noticed a troubling pattern emerge during the pandemic, something that is darkly referred to as “the COVID 19,” or the 19 or more pounds that many of our patients have gained in the past year. This phenomenon has underscored many maxims in pediatric weight management: Mainly that frequent snacking, decreased physical activity, and less parental supervision lead to increased weight gain. But could we be missing another lesson this trend is teaching us? What about the relationship between catastrophe and childhood obesity?
Beyond the increased weight gain with lockdowns, I have observed other evidence in my own practice that childhood trauma or adverse experiences increase obesity. Our electronic medical record system gives an alert when a chart with sensitive information is accessed. One example might be if the patient had been seen at a clinic for children who have been abused. I am heartbroken at how often this happens. Academically, I understand the dire statistics about the incidence of child abuse, but the frequency at which I see this pattern is jarring.
Over the years, one striking correlation became clear among my patient population: Children with obesity were more likely to have been seen in the child abuse clinic than normal-weight peers.
I am far from the only one to have observed this relationship. Television shows focusing on severe obesity, such as “My 600-Pound Life,” often show trauma as both a cause and effect of severe obesity. This theme also became apparent on the show “The Biggest Loser,” which highlighted the difficulty of achieving and maintaining substantial weight loss. If even Hollywood has noticed this association, shouldn’t we be much farther ahead?
Pathways to obesity
Adverse childhood experiences (ACE) encompass various causes of child trauma, including abuse or neglect; poverty; household or neighborhood violence; and death, illness, or incarceration of a parent. A pivotal report in 1998 formalized the suspicion that many of us could plainly see: People who suffered ACE have higher incidence of heart disease, COPD, liver disease, incarceration, and drug abuse. For those with six or more ACE, life expectancy averaged 20 years less than those who had none. More recently, a meta-analysis found an odds ratio of 1.46 for adult obesity with known history of childhood trauma.
As a pediatric endocrinologist living in the poorest state of the country, I have clearly observed the correlation between childhood obesity and poverty. While prior generations may have associated child poverty with malnutrition and starvation, we are seeing in modern times that obesity has become a disease of lack. Calorie-dense and processed foods tend to be less expensive, more shelf-stable, and more accessible to people living in both urban and rural food deserts.
I am also a foster mother and have received extensive training in parenting children who have lived through trauma and neglect. For children who have endured food scarcity and deprivation, hoarding food and overeating are expected responses.
But the pathways to abnormal weight gain are myriad and expand beyond binge eating or numbing with food. ACE are particularly troubling because they affect developing brains and the neuroendocrine system; they alter epigenetics and cause heritable changes. Structural brain differences have been evident in the frontopolar cortex, which is linked to centers in the hypothalamus that control appetite. And increased stress raises cortisol release, increases insulin resistance, and alters satiety.
Shifting our approach to treatment
The significant cost of ACE is enormous and affects us all. Health professionals in pediatrics must understand these connections to effectively counsel children and their families dealing with obesity. Handing someone a diet plan and lecturing them about weight loss is never effective, but this common tactic is especially cruel if we do not assess for and address underlying pain. Obviously, blame and shame are ineffective motivators for lifestyle change in any circumstance, but these tactics may be especially harmful in the light of childhood trauma.
Screening for ACE is important in every aspect of pediatric care. The presence of obesity, however, should remind us to be more sensitive to the possibility of causative trauma. Clinicians for adults are not off the hook either. Fully 60% of adults suffered ACE and are dealing with the aftermath.
To improve health outcomes across the board, we must screen for trauma and become educated on trauma-informed care. Perhaps the most important first referral for a child suffering ACE and obesity is to a trained counselor or a social worker. Shepherding children through trauma will be more effective for attaining healthy weight than any remedy I can prescribe as an endocrinologist. Furthermore, this is our necessary role as healers. More than ever, we need to approach chronic diseases, including obesity, with the utmost compassion.
A version of this article first appeared on Medscape.com.
Clinicians in pediatrics have noticed a troubling pattern emerge during the pandemic, something that is darkly referred to as “the COVID 19,” or the 19 or more pounds that many of our patients have gained in the past year. This phenomenon has underscored many maxims in pediatric weight management: Mainly that frequent snacking, decreased physical activity, and less parental supervision lead to increased weight gain. But could we be missing another lesson this trend is teaching us? What about the relationship between catastrophe and childhood obesity?
Beyond the increased weight gain with lockdowns, I have observed other evidence in my own practice that childhood trauma or adverse experiences increase obesity. Our electronic medical record system gives an alert when a chart with sensitive information is accessed. One example might be if the patient had been seen at a clinic for children who have been abused. I am heartbroken at how often this happens. Academically, I understand the dire statistics about the incidence of child abuse, but the frequency at which I see this pattern is jarring.
Over the years, one striking correlation became clear among my patient population: Children with obesity were more likely to have been seen in the child abuse clinic than normal-weight peers.
I am far from the only one to have observed this relationship. Television shows focusing on severe obesity, such as “My 600-Pound Life,” often show trauma as both a cause and effect of severe obesity. This theme also became apparent on the show “The Biggest Loser,” which highlighted the difficulty of achieving and maintaining substantial weight loss. If even Hollywood has noticed this association, shouldn’t we be much farther ahead?
Pathways to obesity
Adverse childhood experiences (ACE) encompass various causes of child trauma, including abuse or neglect; poverty; household or neighborhood violence; and death, illness, or incarceration of a parent. A pivotal report in 1998 formalized the suspicion that many of us could plainly see: People who suffered ACE have higher incidence of heart disease, COPD, liver disease, incarceration, and drug abuse. For those with six or more ACE, life expectancy averaged 20 years less than those who had none. More recently, a meta-analysis found an odds ratio of 1.46 for adult obesity with known history of childhood trauma.
As a pediatric endocrinologist living in the poorest state of the country, I have clearly observed the correlation between childhood obesity and poverty. While prior generations may have associated child poverty with malnutrition and starvation, we are seeing in modern times that obesity has become a disease of lack. Calorie-dense and processed foods tend to be less expensive, more shelf-stable, and more accessible to people living in both urban and rural food deserts.
I am also a foster mother and have received extensive training in parenting children who have lived through trauma and neglect. For children who have endured food scarcity and deprivation, hoarding food and overeating are expected responses.
But the pathways to abnormal weight gain are myriad and expand beyond binge eating or numbing with food. ACE are particularly troubling because they affect developing brains and the neuroendocrine system; they alter epigenetics and cause heritable changes. Structural brain differences have been evident in the frontopolar cortex, which is linked to centers in the hypothalamus that control appetite. And increased stress raises cortisol release, increases insulin resistance, and alters satiety.
Shifting our approach to treatment
The significant cost of ACE is enormous and affects us all. Health professionals in pediatrics must understand these connections to effectively counsel children and their families dealing with obesity. Handing someone a diet plan and lecturing them about weight loss is never effective, but this common tactic is especially cruel if we do not assess for and address underlying pain. Obviously, blame and shame are ineffective motivators for lifestyle change in any circumstance, but these tactics may be especially harmful in the light of childhood trauma.
Screening for ACE is important in every aspect of pediatric care. The presence of obesity, however, should remind us to be more sensitive to the possibility of causative trauma. Clinicians for adults are not off the hook either. Fully 60% of adults suffered ACE and are dealing with the aftermath.
To improve health outcomes across the board, we must screen for trauma and become educated on trauma-informed care. Perhaps the most important first referral for a child suffering ACE and obesity is to a trained counselor or a social worker. Shepherding children through trauma will be more effective for attaining healthy weight than any remedy I can prescribe as an endocrinologist. Furthermore, this is our necessary role as healers. More than ever, we need to approach chronic diseases, including obesity, with the utmost compassion.
A version of this article first appeared on Medscape.com.
Helping your patients navigate the coming out process
“Mom, Dad: I’m gay.” Saying these words can be difficult for anyone but especially for adolescents and young adults. The process of coming out is one filled with anticipation, angst, and hopefully relief. However, this process is not a one-time event but rather something that LGBTQ adolescents and young adults have to face every time they meet someone new or are placed in a new situation. They have to decide if that new person can be trusted with their very personal information.
Coming out is a process that begins months to years before the adolescent or young adult utters the words above. The first step in the coming out process is accepting one’s sexual orientation and/or gender identity. This period of time can be somewhat tumultuous, filled with a mix of emotions ranging from fear to excitement. The adolescent or young adult may need support in coming to terms with who they are as their authentic self. This can take the role of a therapist, a trusted friend, or a trusted family member. There may even be times that the adolescent or young adult’s physician is the only person that they are out to besides their friends. Therefore, you can play a very important role in helping your adolescent and young adult patients as they navigate the journey of coming out.
One of the most important ways that physicians can help adolescents and young adults is to spend time alone with them at as many visits as you can. This gives the patient the time to discuss confidential matters with you, including their sexual orientation and/or gender identity. It is possible that the chronic abdominal pain that your adolescent patient is experiencing may not represent an organic abdominal problem but could represent a manifestation of anxiety because that patient is afraid of his/her parent(s) finding out that he/she identifies as LGBTQ. If one of your patients comes out to you, it is important that you validate for your patient that they are normal as who they are. In addition, you can thank your patient for trusting you with that information and let them know that you are there to support them in whatever way they feel appropriate. Just as important is that you work with the adolescent on a plan for their other concerns that respects their right to privacy in regard to their gender identity and/or sexual orientation.
The adolescent or young adult should always be in control of who knows about their gender identity and/or sexual orientation. Ideally, they should also always be the one who shares that information with others. Many times, parents may react positively to finding out that their child identifies as LGBTQ and want to share that information with their friends or family members. Alternatively, the parent could use the patient’s sexual orientation or gender identity negatively against them to their family and/or friends. As the physician, you can help counsel the family that it should always be their child who gets to share that information and when it is shared.
So how can you support your LGBTQ patients as they navigate the coming out process? First, when you find out from your patient that they identify as LGBTQ, ensure that you ask them who knows about their identity. This prevents inadvertent disclosures to the parent/guardian when the patient is not ready for them to know. Second, discuss with the patient if he/she needs any resources related to their sexual orientation and/or gender identity. This includes things such as the names of local LGBTQ youth organizations or the phone number for the Trevor Project suicide hotline, for example. Third, ensure that your office and staff are a welcoming and affirmative environment for your patients. A 2017 survey by the Human Rights Campaign found that only 8% of transgender or gender-diverse adolescents and young adults were out to all of their physicians and only 5% of LGB adolescents and young adults were out to all of their physicians.1 This is likely because of past negative experiences these patients have had with previous physicians. A 2017 study from the Center for American Progress found that 8% of LGB patients and 29% of transgender or gender-diverse patients said that a doctor or health care provider had refused to see them because of their actual or perceived identity.2 Lastly, you could offer to help facilitate a discussion between the patient and his/her parents in relation to his/her sexual orientation and/or gender identity.
In summary, pediatricians can play an important role in the coming out process of their LGBTQ patients. Your office is an important source of support for the physical and mental health of these patients as they navigate this journey. You can also be a strong advocate for these patients to their parents and families. I think that we all can agree that our patients deserve better than only feeling comfortable to be out to 5%-8% of their physicians.
Dr. Cooper is assistant professor of pediatrics at the University of Texas, Dallas, and an adolescent medicine specialist at Children’s Medical Center Dallas. Contact him at [email protected].
References
1. Human Rights Campaign 2018 LGBTQ Youth Report.
2. Mirza SA and Rooney C. “Discrimination prevents LGBTQ people from accessing health care.” Center for American Progress. 2018 Jan 18.
“Mom, Dad: I’m gay.” Saying these words can be difficult for anyone but especially for adolescents and young adults. The process of coming out is one filled with anticipation, angst, and hopefully relief. However, this process is not a one-time event but rather something that LGBTQ adolescents and young adults have to face every time they meet someone new or are placed in a new situation. They have to decide if that new person can be trusted with their very personal information.
Coming out is a process that begins months to years before the adolescent or young adult utters the words above. The first step in the coming out process is accepting one’s sexual orientation and/or gender identity. This period of time can be somewhat tumultuous, filled with a mix of emotions ranging from fear to excitement. The adolescent or young adult may need support in coming to terms with who they are as their authentic self. This can take the role of a therapist, a trusted friend, or a trusted family member. There may even be times that the adolescent or young adult’s physician is the only person that they are out to besides their friends. Therefore, you can play a very important role in helping your adolescent and young adult patients as they navigate the journey of coming out.
One of the most important ways that physicians can help adolescents and young adults is to spend time alone with them at as many visits as you can. This gives the patient the time to discuss confidential matters with you, including their sexual orientation and/or gender identity. It is possible that the chronic abdominal pain that your adolescent patient is experiencing may not represent an organic abdominal problem but could represent a manifestation of anxiety because that patient is afraid of his/her parent(s) finding out that he/she identifies as LGBTQ. If one of your patients comes out to you, it is important that you validate for your patient that they are normal as who they are. In addition, you can thank your patient for trusting you with that information and let them know that you are there to support them in whatever way they feel appropriate. Just as important is that you work with the adolescent on a plan for their other concerns that respects their right to privacy in regard to their gender identity and/or sexual orientation.
The adolescent or young adult should always be in control of who knows about their gender identity and/or sexual orientation. Ideally, they should also always be the one who shares that information with others. Many times, parents may react positively to finding out that their child identifies as LGBTQ and want to share that information with their friends or family members. Alternatively, the parent could use the patient’s sexual orientation or gender identity negatively against them to their family and/or friends. As the physician, you can help counsel the family that it should always be their child who gets to share that information and when it is shared.
So how can you support your LGBTQ patients as they navigate the coming out process? First, when you find out from your patient that they identify as LGBTQ, ensure that you ask them who knows about their identity. This prevents inadvertent disclosures to the parent/guardian when the patient is not ready for them to know. Second, discuss with the patient if he/she needs any resources related to their sexual orientation and/or gender identity. This includes things such as the names of local LGBTQ youth organizations or the phone number for the Trevor Project suicide hotline, for example. Third, ensure that your office and staff are a welcoming and affirmative environment for your patients. A 2017 survey by the Human Rights Campaign found that only 8% of transgender or gender-diverse adolescents and young adults were out to all of their physicians and only 5% of LGB adolescents and young adults were out to all of their physicians.1 This is likely because of past negative experiences these patients have had with previous physicians. A 2017 study from the Center for American Progress found that 8% of LGB patients and 29% of transgender or gender-diverse patients said that a doctor or health care provider had refused to see them because of their actual or perceived identity.2 Lastly, you could offer to help facilitate a discussion between the patient and his/her parents in relation to his/her sexual orientation and/or gender identity.
In summary, pediatricians can play an important role in the coming out process of their LGBTQ patients. Your office is an important source of support for the physical and mental health of these patients as they navigate this journey. You can also be a strong advocate for these patients to their parents and families. I think that we all can agree that our patients deserve better than only feeling comfortable to be out to 5%-8% of their physicians.
Dr. Cooper is assistant professor of pediatrics at the University of Texas, Dallas, and an adolescent medicine specialist at Children’s Medical Center Dallas. Contact him at [email protected].
References
1. Human Rights Campaign 2018 LGBTQ Youth Report.
2. Mirza SA and Rooney C. “Discrimination prevents LGBTQ people from accessing health care.” Center for American Progress. 2018 Jan 18.
“Mom, Dad: I’m gay.” Saying these words can be difficult for anyone but especially for adolescents and young adults. The process of coming out is one filled with anticipation, angst, and hopefully relief. However, this process is not a one-time event but rather something that LGBTQ adolescents and young adults have to face every time they meet someone new or are placed in a new situation. They have to decide if that new person can be trusted with their very personal information.
Coming out is a process that begins months to years before the adolescent or young adult utters the words above. The first step in the coming out process is accepting one’s sexual orientation and/or gender identity. This period of time can be somewhat tumultuous, filled with a mix of emotions ranging from fear to excitement. The adolescent or young adult may need support in coming to terms with who they are as their authentic self. This can take the role of a therapist, a trusted friend, or a trusted family member. There may even be times that the adolescent or young adult’s physician is the only person that they are out to besides their friends. Therefore, you can play a very important role in helping your adolescent and young adult patients as they navigate the journey of coming out.
One of the most important ways that physicians can help adolescents and young adults is to spend time alone with them at as many visits as you can. This gives the patient the time to discuss confidential matters with you, including their sexual orientation and/or gender identity. It is possible that the chronic abdominal pain that your adolescent patient is experiencing may not represent an organic abdominal problem but could represent a manifestation of anxiety because that patient is afraid of his/her parent(s) finding out that he/she identifies as LGBTQ. If one of your patients comes out to you, it is important that you validate for your patient that they are normal as who they are. In addition, you can thank your patient for trusting you with that information and let them know that you are there to support them in whatever way they feel appropriate. Just as important is that you work with the adolescent on a plan for their other concerns that respects their right to privacy in regard to their gender identity and/or sexual orientation.
The adolescent or young adult should always be in control of who knows about their gender identity and/or sexual orientation. Ideally, they should also always be the one who shares that information with others. Many times, parents may react positively to finding out that their child identifies as LGBTQ and want to share that information with their friends or family members. Alternatively, the parent could use the patient’s sexual orientation or gender identity negatively against them to their family and/or friends. As the physician, you can help counsel the family that it should always be their child who gets to share that information and when it is shared.
So how can you support your LGBTQ patients as they navigate the coming out process? First, when you find out from your patient that they identify as LGBTQ, ensure that you ask them who knows about their identity. This prevents inadvertent disclosures to the parent/guardian when the patient is not ready for them to know. Second, discuss with the patient if he/she needs any resources related to their sexual orientation and/or gender identity. This includes things such as the names of local LGBTQ youth organizations or the phone number for the Trevor Project suicide hotline, for example. Third, ensure that your office and staff are a welcoming and affirmative environment for your patients. A 2017 survey by the Human Rights Campaign found that only 8% of transgender or gender-diverse adolescents and young adults were out to all of their physicians and only 5% of LGB adolescents and young adults were out to all of their physicians.1 This is likely because of past negative experiences these patients have had with previous physicians. A 2017 study from the Center for American Progress found that 8% of LGB patients and 29% of transgender or gender-diverse patients said that a doctor or health care provider had refused to see them because of their actual or perceived identity.2 Lastly, you could offer to help facilitate a discussion between the patient and his/her parents in relation to his/her sexual orientation and/or gender identity.
In summary, pediatricians can play an important role in the coming out process of their LGBTQ patients. Your office is an important source of support for the physical and mental health of these patients as they navigate this journey. You can also be a strong advocate for these patients to their parents and families. I think that we all can agree that our patients deserve better than only feeling comfortable to be out to 5%-8% of their physicians.
Dr. Cooper is assistant professor of pediatrics at the University of Texas, Dallas, and an adolescent medicine specialist at Children’s Medical Center Dallas. Contact him at [email protected].
References
1. Human Rights Campaign 2018 LGBTQ Youth Report.
2. Mirza SA and Rooney C. “Discrimination prevents LGBTQ people from accessing health care.” Center for American Progress. 2018 Jan 18.
Family psychoeducation is critical in care of children with disabilities
Dr. Margaret G. Klitzke is a board-certified child and adolescent psychiatrist who has worked across all settings of the Center for Autism and Developmental Disabilities at Bradley Hospital in East Providence, R.I.
I spoke with Dr. Klitzke recently about her work as an outpatient psychiatrist at the center and about the important role of families in the treatment it provides. The center offers highly specialized clinical services for children and adolescents between the ages of 2 and 18 who show signs of serious emotional and behavioral problems in addition to a developmental disability, such as autism, Asperger’s, or intellectual disability.
The center’s model of care emphasizes family involvement. Dr. Klitzke was trained in family interventions by Nathan B. Epstein, MD, and Duane S. Bishop, MD, the originators of the McMaster approach and the problem-centered systems therapy of the family. This training informs much of her work with families.
ALISON M. HERU, MD: Hello, Dr. Klitzke and thank you for agreeing to this interview.
MARGARET G. KLITZKE, DO: My pleasure.
AMH: I admire your dedication to this population of children and adolescents. To me, it seems very hard to work with patients and families where there is significant disability and there is little hope of the patient “getting better.”
MGK: When parents come to us, they have great hopes their children can be helped. They often express understanding and acceptance of the child’s disability, and seek to understand the psychiatric or behavioral issues. These parents are often very dedicated to their children, giving up careers to care for them. But as professionals, we must be sensitive to the role each parent can play and how they can support each other and the family.
AMH: So much of your work focuses on family inclusion and family psychoeducation?
MGK: Yes. An example that stands out is a couple where the mother had become the voice for the family in dealing with professionals, but she was overwhelmed in this role. So, we invited the father in. He explained that medical professionals and school personnel would address their remarks to his wife and that he felt marginalized. We worked with the couple, now always including the father, and he has gone on to become a vocal advocate for children with disabilities. It is inspiring to watch families become advocates – to insist that others see the child’s strengths – not just weaknesses.
AMH: Do you feel that the families ever come to you with too high expectations of what you can do to help their child?
MGK: As a child psychiatrist, one must put oneself in the parents’ shoes. Charlie Zeanah Jr., MD, and others have done wonderful work in attachment. They have identified that parents have fantasies and beliefs about what the child will be like before the child is born. We all have fantasies about our babies before they come to us! For many families, they quickly come to understand that their child is not like other children. This new world of parenting is not what they expected. A mother once gave me a short piece called “Welcome to Holland,” written by a mother whose child has Down syndrome.
AMH: How do you begin to work with these families? There must be such a sense of loss and tragedy in their lives.
MGK: My first goal is to understand what it is like to have a child with developmental disability, not just for the parents but for the siblings, too. I strive to understand what the parents want for their child and how they see themselves as a family. I see us, the health care team, as agents to help the child and the family be the very best they can be.
AMH: How do you deal with parents who are not be on the same page?
MGK: It is important that parents are consistent and are able to work together. Even if they are divorced, I have seen families able to unite around the care of their child with a disability. This is quite an achievement given the high rates of divorce – although most of the families that I have worked with are intact. As in all families, each member has a role in helping the family function well. It means using the strength of each parent to help them become a parenting team.
AMH: What if the parents have unrealistic expectations of their child?
MGK: Yes, there are parents who come to us with unrealistic expectations, such as believing their nonverbal child will talk some day. In such a case, we must be certain that we have exhausted all methods to help this child communicate, and once we have done all we can, then we must accept where that child is; to accept and help the family accept, the child’s weaknesses and acknowledge their strengths. Change what you can and be a support for everything else.
AMH: I find it hard to imagine caring for a severely disabled child. How do these parents do this?
MGK: These are children who are nonverbal, and children who can be very fragile, even medically. What I see are parents who want to connect, who want to find that something inside that child, that special place where there is connection. That place of reciprocity. That is important to us all, helping the family find that place of reciprocal connection.
AMH: What language do you use to discuss this with families?
MGK: I say, “This is the child’s strength and this is the child’s weakness; capitalize on the strengths and let’s shore up their weaknesses.”
AMH: How do you approach the families? Where do you start?
MGK: I meet the family where they are. One cannot with these families or any families stand rigidly 10 feet away, and demand that they change. This never works, and we will be of no help to them. We must understand the family system and how they have arrived at their current place of functioning.
AMH: Can you give an example?
MGK: Yes, for example if a parent is drinking excessively, I help them understand why they are coping that way and see if they are willing to change.
AMH: What keeps you going ?
MGK: I think it comes back to the family work. For me, I believe the families are doing the very best they can. If the family is really impaired in some way, I see it as my job to figure out why that is their pattern of behavior, and I do what I can to help them facilitate change.
AMH: What inspires you about these families?
MGK: These families are able to recognize the strengths and beauty that their children bring them – the strength of these children, their personalities and their wills of steel! They are able to communicate what they need. Siblings, too, make life decisions based on their experiences. They often end up going down the path of caring for such children as professionals.
AMH: Do you have any recommendations for a young child psychiatrist who might be considering working with this population?
MGK: Developmental disabilities in child psychiatry is where medicine, neurology, and child development meet. The advances in genetics and neurology are major gifts to the field. It used to be that I would have to sell the field to medical students and residents. Now they are coming to me saying that they want to work in this area. It is an intellectually rich field in which to work. There is a real change happening. But the place where it becomes really magical is in working with the families.
AMH: What other changes have you seen?
MGK: With the closure of big institutions, it is less of an option for families to walk away. The families now feel that they need to take care of the child.
AMH: What has your career taught you?
MGK: It has taught me patience, to enter every situation without preconceived notions, and that there is something new to learn every day.
References
J Child Adolesc Psychiatry. 1975 Jun 1;14(3):387-421.
Evaluation and Treating Families: The McMaster Approach. Routledge/Taylor & Francis Group, 2005.
Movies to watch
Lorenzo’s Oil, 1992.
My Left Foot, 1989.
Dr. Heru is professor of psychiatry at the University of Colorado at Denver, Aurora. She is editor of “Working With Families in Medical Settings: A Multidisciplinary Guide for Psychiatrists and Other Health Professionals” (Routledge, 2013). She has no conflicts of interest.
Dr. Klitkze is a 1983 graduate of the Texas College of Osteopathic Medicine, and completed her residency and fellowship training at Brown University, Providence, R.I. She is a member of the American Psychiatric Association, the American Academy of Child and Adolescent Psychiatry, and the Rhode Island Medical Society, where she serves on the Physicians’ Health Committee. She is actively involved in teaching medical students, residents, and fellows, and has received several teaching awards from the department of psychiatry and human behavior at Brown.
Dr. Margaret G. Klitzke is a board-certified child and adolescent psychiatrist who has worked across all settings of the Center for Autism and Developmental Disabilities at Bradley Hospital in East Providence, R.I.
I spoke with Dr. Klitzke recently about her work as an outpatient psychiatrist at the center and about the important role of families in the treatment it provides. The center offers highly specialized clinical services for children and adolescents between the ages of 2 and 18 who show signs of serious emotional and behavioral problems in addition to a developmental disability, such as autism, Asperger’s, or intellectual disability.
The center’s model of care emphasizes family involvement. Dr. Klitzke was trained in family interventions by Nathan B. Epstein, MD, and Duane S. Bishop, MD, the originators of the McMaster approach and the problem-centered systems therapy of the family. This training informs much of her work with families.
ALISON M. HERU, MD: Hello, Dr. Klitzke and thank you for agreeing to this interview.
MARGARET G. KLITZKE, DO: My pleasure.
AMH: I admire your dedication to this population of children and adolescents. To me, it seems very hard to work with patients and families where there is significant disability and there is little hope of the patient “getting better.”
MGK: When parents come to us, they have great hopes their children can be helped. They often express understanding and acceptance of the child’s disability, and seek to understand the psychiatric or behavioral issues. These parents are often very dedicated to their children, giving up careers to care for them. But as professionals, we must be sensitive to the role each parent can play and how they can support each other and the family.
AMH: So much of your work focuses on family inclusion and family psychoeducation?
MGK: Yes. An example that stands out is a couple where the mother had become the voice for the family in dealing with professionals, but she was overwhelmed in this role. So, we invited the father in. He explained that medical professionals and school personnel would address their remarks to his wife and that he felt marginalized. We worked with the couple, now always including the father, and he has gone on to become a vocal advocate for children with disabilities. It is inspiring to watch families become advocates – to insist that others see the child’s strengths – not just weaknesses.
AMH: Do you feel that the families ever come to you with too high expectations of what you can do to help their child?
MGK: As a child psychiatrist, one must put oneself in the parents’ shoes. Charlie Zeanah Jr., MD, and others have done wonderful work in attachment. They have identified that parents have fantasies and beliefs about what the child will be like before the child is born. We all have fantasies about our babies before they come to us! For many families, they quickly come to understand that their child is not like other children. This new world of parenting is not what they expected. A mother once gave me a short piece called “Welcome to Holland,” written by a mother whose child has Down syndrome.
AMH: How do you begin to work with these families? There must be such a sense of loss and tragedy in their lives.
MGK: My first goal is to understand what it is like to have a child with developmental disability, not just for the parents but for the siblings, too. I strive to understand what the parents want for their child and how they see themselves as a family. I see us, the health care team, as agents to help the child and the family be the very best they can be.
AMH: How do you deal with parents who are not be on the same page?
MGK: It is important that parents are consistent and are able to work together. Even if they are divorced, I have seen families able to unite around the care of their child with a disability. This is quite an achievement given the high rates of divorce – although most of the families that I have worked with are intact. As in all families, each member has a role in helping the family function well. It means using the strength of each parent to help them become a parenting team.
AMH: What if the parents have unrealistic expectations of their child?
MGK: Yes, there are parents who come to us with unrealistic expectations, such as believing their nonverbal child will talk some day. In such a case, we must be certain that we have exhausted all methods to help this child communicate, and once we have done all we can, then we must accept where that child is; to accept and help the family accept, the child’s weaknesses and acknowledge their strengths. Change what you can and be a support for everything else.
AMH: I find it hard to imagine caring for a severely disabled child. How do these parents do this?
MGK: These are children who are nonverbal, and children who can be very fragile, even medically. What I see are parents who want to connect, who want to find that something inside that child, that special place where there is connection. That place of reciprocity. That is important to us all, helping the family find that place of reciprocal connection.
AMH: What language do you use to discuss this with families?
MGK: I say, “This is the child’s strength and this is the child’s weakness; capitalize on the strengths and let’s shore up their weaknesses.”
AMH: How do you approach the families? Where do you start?
MGK: I meet the family where they are. One cannot with these families or any families stand rigidly 10 feet away, and demand that they change. This never works, and we will be of no help to them. We must understand the family system and how they have arrived at their current place of functioning.
AMH: Can you give an example?
MGK: Yes, for example if a parent is drinking excessively, I help them understand why they are coping that way and see if they are willing to change.
AMH: What keeps you going ?
MGK: I think it comes back to the family work. For me, I believe the families are doing the very best they can. If the family is really impaired in some way, I see it as my job to figure out why that is their pattern of behavior, and I do what I can to help them facilitate change.
AMH: What inspires you about these families?
MGK: These families are able to recognize the strengths and beauty that their children bring them – the strength of these children, their personalities and their wills of steel! They are able to communicate what they need. Siblings, too, make life decisions based on their experiences. They often end up going down the path of caring for such children as professionals.
AMH: Do you have any recommendations for a young child psychiatrist who might be considering working with this population?
MGK: Developmental disabilities in child psychiatry is where medicine, neurology, and child development meet. The advances in genetics and neurology are major gifts to the field. It used to be that I would have to sell the field to medical students and residents. Now they are coming to me saying that they want to work in this area. It is an intellectually rich field in which to work. There is a real change happening. But the place where it becomes really magical is in working with the families.
AMH: What other changes have you seen?
MGK: With the closure of big institutions, it is less of an option for families to walk away. The families now feel that they need to take care of the child.
AMH: What has your career taught you?
MGK: It has taught me patience, to enter every situation without preconceived notions, and that there is something new to learn every day.
References
J Child Adolesc Psychiatry. 1975 Jun 1;14(3):387-421.
Evaluation and Treating Families: The McMaster Approach. Routledge/Taylor & Francis Group, 2005.
Movies to watch
Lorenzo’s Oil, 1992.
My Left Foot, 1989.
Dr. Heru is professor of psychiatry at the University of Colorado at Denver, Aurora. She is editor of “Working With Families in Medical Settings: A Multidisciplinary Guide for Psychiatrists and Other Health Professionals” (Routledge, 2013). She has no conflicts of interest.
Dr. Klitkze is a 1983 graduate of the Texas College of Osteopathic Medicine, and completed her residency and fellowship training at Brown University, Providence, R.I. She is a member of the American Psychiatric Association, the American Academy of Child and Adolescent Psychiatry, and the Rhode Island Medical Society, where she serves on the Physicians’ Health Committee. She is actively involved in teaching medical students, residents, and fellows, and has received several teaching awards from the department of psychiatry and human behavior at Brown.
Dr. Margaret G. Klitzke is a board-certified child and adolescent psychiatrist who has worked across all settings of the Center for Autism and Developmental Disabilities at Bradley Hospital in East Providence, R.I.
I spoke with Dr. Klitzke recently about her work as an outpatient psychiatrist at the center and about the important role of families in the treatment it provides. The center offers highly specialized clinical services for children and adolescents between the ages of 2 and 18 who show signs of serious emotional and behavioral problems in addition to a developmental disability, such as autism, Asperger’s, or intellectual disability.
The center’s model of care emphasizes family involvement. Dr. Klitzke was trained in family interventions by Nathan B. Epstein, MD, and Duane S. Bishop, MD, the originators of the McMaster approach and the problem-centered systems therapy of the family. This training informs much of her work with families.
ALISON M. HERU, MD: Hello, Dr. Klitzke and thank you for agreeing to this interview.
MARGARET G. KLITZKE, DO: My pleasure.
AMH: I admire your dedication to this population of children and adolescents. To me, it seems very hard to work with patients and families where there is significant disability and there is little hope of the patient “getting better.”
MGK: When parents come to us, they have great hopes their children can be helped. They often express understanding and acceptance of the child’s disability, and seek to understand the psychiatric or behavioral issues. These parents are often very dedicated to their children, giving up careers to care for them. But as professionals, we must be sensitive to the role each parent can play and how they can support each other and the family.
AMH: So much of your work focuses on family inclusion and family psychoeducation?
MGK: Yes. An example that stands out is a couple where the mother had become the voice for the family in dealing with professionals, but she was overwhelmed in this role. So, we invited the father in. He explained that medical professionals and school personnel would address their remarks to his wife and that he felt marginalized. We worked with the couple, now always including the father, and he has gone on to become a vocal advocate for children with disabilities. It is inspiring to watch families become advocates – to insist that others see the child’s strengths – not just weaknesses.
AMH: Do you feel that the families ever come to you with too high expectations of what you can do to help their child?
MGK: As a child psychiatrist, one must put oneself in the parents’ shoes. Charlie Zeanah Jr., MD, and others have done wonderful work in attachment. They have identified that parents have fantasies and beliefs about what the child will be like before the child is born. We all have fantasies about our babies before they come to us! For many families, they quickly come to understand that their child is not like other children. This new world of parenting is not what they expected. A mother once gave me a short piece called “Welcome to Holland,” written by a mother whose child has Down syndrome.
AMH: How do you begin to work with these families? There must be such a sense of loss and tragedy in their lives.
MGK: My first goal is to understand what it is like to have a child with developmental disability, not just for the parents but for the siblings, too. I strive to understand what the parents want for their child and how they see themselves as a family. I see us, the health care team, as agents to help the child and the family be the very best they can be.
AMH: How do you deal with parents who are not be on the same page?
MGK: It is important that parents are consistent and are able to work together. Even if they are divorced, I have seen families able to unite around the care of their child with a disability. This is quite an achievement given the high rates of divorce – although most of the families that I have worked with are intact. As in all families, each member has a role in helping the family function well. It means using the strength of each parent to help them become a parenting team.
AMH: What if the parents have unrealistic expectations of their child?
MGK: Yes, there are parents who come to us with unrealistic expectations, such as believing their nonverbal child will talk some day. In such a case, we must be certain that we have exhausted all methods to help this child communicate, and once we have done all we can, then we must accept where that child is; to accept and help the family accept, the child’s weaknesses and acknowledge their strengths. Change what you can and be a support for everything else.
AMH: I find it hard to imagine caring for a severely disabled child. How do these parents do this?
MGK: These are children who are nonverbal, and children who can be very fragile, even medically. What I see are parents who want to connect, who want to find that something inside that child, that special place where there is connection. That place of reciprocity. That is important to us all, helping the family find that place of reciprocal connection.
AMH: What language do you use to discuss this with families?
MGK: I say, “This is the child’s strength and this is the child’s weakness; capitalize on the strengths and let’s shore up their weaknesses.”
AMH: How do you approach the families? Where do you start?
MGK: I meet the family where they are. One cannot with these families or any families stand rigidly 10 feet away, and demand that they change. This never works, and we will be of no help to them. We must understand the family system and how they have arrived at their current place of functioning.
AMH: Can you give an example?
MGK: Yes, for example if a parent is drinking excessively, I help them understand why they are coping that way and see if they are willing to change.
AMH: What keeps you going ?
MGK: I think it comes back to the family work. For me, I believe the families are doing the very best they can. If the family is really impaired in some way, I see it as my job to figure out why that is their pattern of behavior, and I do what I can to help them facilitate change.
AMH: What inspires you about these families?
MGK: These families are able to recognize the strengths and beauty that their children bring them – the strength of these children, their personalities and their wills of steel! They are able to communicate what they need. Siblings, too, make life decisions based on their experiences. They often end up going down the path of caring for such children as professionals.
AMH: Do you have any recommendations for a young child psychiatrist who might be considering working with this population?
MGK: Developmental disabilities in child psychiatry is where medicine, neurology, and child development meet. The advances in genetics and neurology are major gifts to the field. It used to be that I would have to sell the field to medical students and residents. Now they are coming to me saying that they want to work in this area. It is an intellectually rich field in which to work. There is a real change happening. But the place where it becomes really magical is in working with the families.
AMH: What other changes have you seen?
MGK: With the closure of big institutions, it is less of an option for families to walk away. The families now feel that they need to take care of the child.
AMH: What has your career taught you?
MGK: It has taught me patience, to enter every situation without preconceived notions, and that there is something new to learn every day.
References
J Child Adolesc Psychiatry. 1975 Jun 1;14(3):387-421.
Evaluation and Treating Families: The McMaster Approach. Routledge/Taylor & Francis Group, 2005.
Movies to watch
Lorenzo’s Oil, 1992.
My Left Foot, 1989.
Dr. Heru is professor of psychiatry at the University of Colorado at Denver, Aurora. She is editor of “Working With Families in Medical Settings: A Multidisciplinary Guide for Psychiatrists and Other Health Professionals” (Routledge, 2013). She has no conflicts of interest.
Dr. Klitkze is a 1983 graduate of the Texas College of Osteopathic Medicine, and completed her residency and fellowship training at Brown University, Providence, R.I. She is a member of the American Psychiatric Association, the American Academy of Child and Adolescent Psychiatry, and the Rhode Island Medical Society, where she serves on the Physicians’ Health Committee. She is actively involved in teaching medical students, residents, and fellows, and has received several teaching awards from the department of psychiatry and human behavior at Brown.











