User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
CDC recommends use of Pfizer’s COVID vaccine in 12- to 15-year-olds
The Centers for Disease Control and Prevention’s director Rochelle Walensky, MD, signed off on an advisory panel’s recommendation May 12 endorsing the use of the Pfizer-BioNTech COVID-19 vaccine in adolescents aged 12-15 years.
Earlier in the day the CDC’s Advisory Committee on Immunization Practices voted 14-0 in favor of the safety and effectiveness of the vaccine in younger teens.
Dr. Walensky said in an official statement.
The Food and Drug Administration on May 10 issued an emergency use authorization (EUA) for the Pfizer-BioNTech COVID-19 vaccine for the prevention of COVID-19 in individuals 12-15 years old. The FDA first cleared the Pfizer-BioNTech vaccine through an EUA in December 2020 for those ages 16 and older. Pfizer this month also initiated steps with the FDA toward a full approval of its vaccine.
Dr. Walenksy urged parents to seriously consider vaccinating their children.
“Understandably, some parents want more information before their children receive a vaccine,” she said. “I encourage parents with questions to talk to your child’s healthcare provider or your family doctor to learn more about the vaccine.”
Vaccine “safe and effective”
Separately, the American Academy of Pediatrics issued a statement May 12 in support of vaccinating all children ages 12 and older who are eligible for the federally authorized COVID-19 vaccine.
“As a pediatrician and a parent, I have looked forward to getting my own children and patients vaccinated, and I am thrilled that those ages 12 and older can now be protected,” said AAP President Lee Savio Beers, MD, in a statement. “The data continue to show that this vaccine is safe and effective. I urge all parents to call their pediatrician to learn more about how to get their children and teens vaccinated.”
The expanded clearance for the Pfizer vaccine is seen as a critical step for allowing teens to resume activities on which they missed out during the pandemic.
“We’ve seen the harm done to children’s mental and emotional health as they’ve missed out on so many experiences during the pandemic,” Dr. Beers said. “Vaccinating children will protect them and allow them to fully engage in all of the activities – school, sports, socializing with friends and family – that are so important to their health and development.”
A version of this article first appeared on Medscape.com.
The Centers for Disease Control and Prevention’s director Rochelle Walensky, MD, signed off on an advisory panel’s recommendation May 12 endorsing the use of the Pfizer-BioNTech COVID-19 vaccine in adolescents aged 12-15 years.
Earlier in the day the CDC’s Advisory Committee on Immunization Practices voted 14-0 in favor of the safety and effectiveness of the vaccine in younger teens.
Dr. Walensky said in an official statement.
The Food and Drug Administration on May 10 issued an emergency use authorization (EUA) for the Pfizer-BioNTech COVID-19 vaccine for the prevention of COVID-19 in individuals 12-15 years old. The FDA first cleared the Pfizer-BioNTech vaccine through an EUA in December 2020 for those ages 16 and older. Pfizer this month also initiated steps with the FDA toward a full approval of its vaccine.
Dr. Walenksy urged parents to seriously consider vaccinating their children.
“Understandably, some parents want more information before their children receive a vaccine,” she said. “I encourage parents with questions to talk to your child’s healthcare provider or your family doctor to learn more about the vaccine.”
Vaccine “safe and effective”
Separately, the American Academy of Pediatrics issued a statement May 12 in support of vaccinating all children ages 12 and older who are eligible for the federally authorized COVID-19 vaccine.
“As a pediatrician and a parent, I have looked forward to getting my own children and patients vaccinated, and I am thrilled that those ages 12 and older can now be protected,” said AAP President Lee Savio Beers, MD, in a statement. “The data continue to show that this vaccine is safe and effective. I urge all parents to call their pediatrician to learn more about how to get their children and teens vaccinated.”
The expanded clearance for the Pfizer vaccine is seen as a critical step for allowing teens to resume activities on which they missed out during the pandemic.
“We’ve seen the harm done to children’s mental and emotional health as they’ve missed out on so many experiences during the pandemic,” Dr. Beers said. “Vaccinating children will protect them and allow them to fully engage in all of the activities – school, sports, socializing with friends and family – that are so important to their health and development.”
A version of this article first appeared on Medscape.com.
The Centers for Disease Control and Prevention’s director Rochelle Walensky, MD, signed off on an advisory panel’s recommendation May 12 endorsing the use of the Pfizer-BioNTech COVID-19 vaccine in adolescents aged 12-15 years.
Earlier in the day the CDC’s Advisory Committee on Immunization Practices voted 14-0 in favor of the safety and effectiveness of the vaccine in younger teens.
Dr. Walensky said in an official statement.
The Food and Drug Administration on May 10 issued an emergency use authorization (EUA) for the Pfizer-BioNTech COVID-19 vaccine for the prevention of COVID-19 in individuals 12-15 years old. The FDA first cleared the Pfizer-BioNTech vaccine through an EUA in December 2020 for those ages 16 and older. Pfizer this month also initiated steps with the FDA toward a full approval of its vaccine.
Dr. Walenksy urged parents to seriously consider vaccinating their children.
“Understandably, some parents want more information before their children receive a vaccine,” she said. “I encourage parents with questions to talk to your child’s healthcare provider or your family doctor to learn more about the vaccine.”
Vaccine “safe and effective”
Separately, the American Academy of Pediatrics issued a statement May 12 in support of vaccinating all children ages 12 and older who are eligible for the federally authorized COVID-19 vaccine.
“As a pediatrician and a parent, I have looked forward to getting my own children and patients vaccinated, and I am thrilled that those ages 12 and older can now be protected,” said AAP President Lee Savio Beers, MD, in a statement. “The data continue to show that this vaccine is safe and effective. I urge all parents to call their pediatrician to learn more about how to get their children and teens vaccinated.”
The expanded clearance for the Pfizer vaccine is seen as a critical step for allowing teens to resume activities on which they missed out during the pandemic.
“We’ve seen the harm done to children’s mental and emotional health as they’ve missed out on so many experiences during the pandemic,” Dr. Beers said. “Vaccinating children will protect them and allow them to fully engage in all of the activities – school, sports, socializing with friends and family – that are so important to their health and development.”
A version of this article first appeared on Medscape.com.
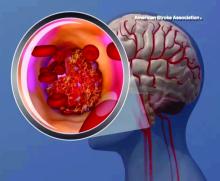
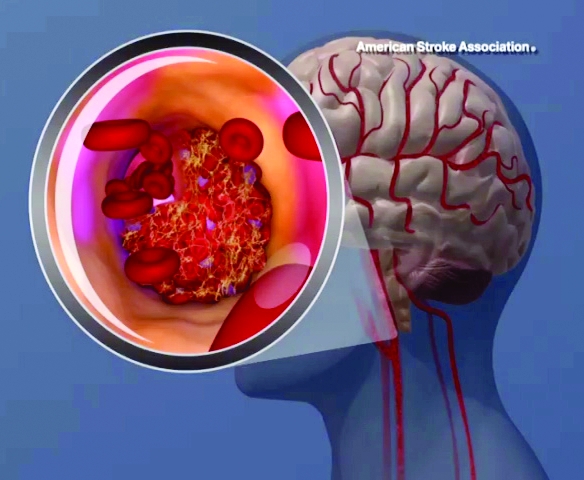
High teen BMI linked to stroke risk in young adulthood
High and even high-normal body mass index (BMI) were linked to increased ischemic stroke risk, regardless of whether or not individuals had diabetes.
Overweight and obese adolescent groups in the study had a roughly two- to threefold increased risk of ischemic stroke, which was apparent even before age 30 years in the study that was based on records of Israeli adolescents evaluated prior to mandatory military service.
These findings highlight the importance of treating and preventing high BMI among adolescence, study coauthor Gilad Twig, MD, MPH, PhD, said in a press release.
“Adults who survive stroke earlier in life face poor functional outcomes, which can lead to unemployment, depression and anxiety,” said Dr. Twig, associate professor in the department of military medicine in The Hebrew University in Jerusalem.
The costs of stroke prevention and care, already high, are expected to become even higher as the adolescent obesity prevalence goes up, fueling further increases in stroke rate, Dr. Twig added.
This is believed to be the first study showing that stroke risk is associated with higher BMI values in both men and women, not just men, Dr. Twig and coauthors said in their article, published May 13, 2021 in the journal Stroke. Previous studies assessing the stroke-BMI relationship in adolescents were based on records of Swedish men evaluated during military conscription at age 18.
In the present study, Dr. Twig and coauthors assessed the linkage between adolescent BMI and first stroke event in 1.9 million male and female adolescents in Israel who were evaluated 1 year prior to mandatory military service, between the years of 1985 and 2013.
They cross-referenced that information with stroke events in a national registry to which all hospitals in Israel are required to report.
The adolescents were about 17 years of age on average at the time of evaluation, 58% were male, and 84% were born in Israel. The mean age at the beginning of follow-up for stroke was about 31 years.
Over the follow-up period, investigators identified 1,088 first stroke events, including 921 ischemic and 167 hemorrhagic strokes.
A gradual increase in stroke rate was seen across BMI categories for ischemic strokes, but not so much for hemorrhagic strokes, investigators found.
Hazard ratios for first ischemic stroke event were 1.4 (95% confidence interval, 1.2-1.6) for the high-normal BMI group, 2.0 (95% CI, 1.6-2.4) for the overweight group, and 3.5 (95% CI, 2.8-4.5) for the obese group after adjusting for age and sex at beginning of follow-up, investigators reported.
When the adjusted results were stratified by presence or absence of diabetes, estimates were similar to what was seen in the overall risk model, they added.
Among those young adults who developed ischemic stroke, 43% smoked, 29% had high blood pressure, 17% had diabetes, and 32% had abnormal lipids at the time of diagnosis, the reported data showed.
The clinical and public health implications of these findings could be substantial, since strokes are associated with worse medical and socioeconomic outcomes in younger as compared with older individuals, according to Dr. Twig and coauthors.
Younger individuals with stroke have a higher risk of recurrent stroke, heart attack, long-term care, or death, they said. Moreover, about half of young-adult stroke survivors have poor functional outcomes, and their risk of unemployment and depression/anxiety is higher than in young individuals without stroke.
One limitation of the study is that follow-up BMI data were not available for all participants. As a result, the contribution of obesity to stroke risk over time could not be assessed, and the independent risk of BMI during adolescence could not be determined. In addition, the authors said the study underrepresents orthodox and ultraorthodox Jewish women, as they are not obligated to serve in the Israeli military.
The study authors had no disclosures related to the study, which was supported by a medical corps Israel Defense Forces research grant.
High and even high-normal body mass index (BMI) were linked to increased ischemic stroke risk, regardless of whether or not individuals had diabetes.
Overweight and obese adolescent groups in the study had a roughly two- to threefold increased risk of ischemic stroke, which was apparent even before age 30 years in the study that was based on records of Israeli adolescents evaluated prior to mandatory military service.
These findings highlight the importance of treating and preventing high BMI among adolescence, study coauthor Gilad Twig, MD, MPH, PhD, said in a press release.
“Adults who survive stroke earlier in life face poor functional outcomes, which can lead to unemployment, depression and anxiety,” said Dr. Twig, associate professor in the department of military medicine in The Hebrew University in Jerusalem.
The costs of stroke prevention and care, already high, are expected to become even higher as the adolescent obesity prevalence goes up, fueling further increases in stroke rate, Dr. Twig added.
This is believed to be the first study showing that stroke risk is associated with higher BMI values in both men and women, not just men, Dr. Twig and coauthors said in their article, published May 13, 2021 in the journal Stroke. Previous studies assessing the stroke-BMI relationship in adolescents were based on records of Swedish men evaluated during military conscription at age 18.
In the present study, Dr. Twig and coauthors assessed the linkage between adolescent BMI and first stroke event in 1.9 million male and female adolescents in Israel who were evaluated 1 year prior to mandatory military service, between the years of 1985 and 2013.
They cross-referenced that information with stroke events in a national registry to which all hospitals in Israel are required to report.
The adolescents were about 17 years of age on average at the time of evaluation, 58% were male, and 84% were born in Israel. The mean age at the beginning of follow-up for stroke was about 31 years.
Over the follow-up period, investigators identified 1,088 first stroke events, including 921 ischemic and 167 hemorrhagic strokes.
A gradual increase in stroke rate was seen across BMI categories for ischemic strokes, but not so much for hemorrhagic strokes, investigators found.
Hazard ratios for first ischemic stroke event were 1.4 (95% confidence interval, 1.2-1.6) for the high-normal BMI group, 2.0 (95% CI, 1.6-2.4) for the overweight group, and 3.5 (95% CI, 2.8-4.5) for the obese group after adjusting for age and sex at beginning of follow-up, investigators reported.
When the adjusted results were stratified by presence or absence of diabetes, estimates were similar to what was seen in the overall risk model, they added.
Among those young adults who developed ischemic stroke, 43% smoked, 29% had high blood pressure, 17% had diabetes, and 32% had abnormal lipids at the time of diagnosis, the reported data showed.
The clinical and public health implications of these findings could be substantial, since strokes are associated with worse medical and socioeconomic outcomes in younger as compared with older individuals, according to Dr. Twig and coauthors.
Younger individuals with stroke have a higher risk of recurrent stroke, heart attack, long-term care, or death, they said. Moreover, about half of young-adult stroke survivors have poor functional outcomes, and their risk of unemployment and depression/anxiety is higher than in young individuals without stroke.
One limitation of the study is that follow-up BMI data were not available for all participants. As a result, the contribution of obesity to stroke risk over time could not be assessed, and the independent risk of BMI during adolescence could not be determined. In addition, the authors said the study underrepresents orthodox and ultraorthodox Jewish women, as they are not obligated to serve in the Israeli military.
The study authors had no disclosures related to the study, which was supported by a medical corps Israel Defense Forces research grant.
High and even high-normal body mass index (BMI) were linked to increased ischemic stroke risk, regardless of whether or not individuals had diabetes.
Overweight and obese adolescent groups in the study had a roughly two- to threefold increased risk of ischemic stroke, which was apparent even before age 30 years in the study that was based on records of Israeli adolescents evaluated prior to mandatory military service.
These findings highlight the importance of treating and preventing high BMI among adolescence, study coauthor Gilad Twig, MD, MPH, PhD, said in a press release.
“Adults who survive stroke earlier in life face poor functional outcomes, which can lead to unemployment, depression and anxiety,” said Dr. Twig, associate professor in the department of military medicine in The Hebrew University in Jerusalem.
The costs of stroke prevention and care, already high, are expected to become even higher as the adolescent obesity prevalence goes up, fueling further increases in stroke rate, Dr. Twig added.
This is believed to be the first study showing that stroke risk is associated with higher BMI values in both men and women, not just men, Dr. Twig and coauthors said in their article, published May 13, 2021 in the journal Stroke. Previous studies assessing the stroke-BMI relationship in adolescents were based on records of Swedish men evaluated during military conscription at age 18.
In the present study, Dr. Twig and coauthors assessed the linkage between adolescent BMI and first stroke event in 1.9 million male and female adolescents in Israel who were evaluated 1 year prior to mandatory military service, between the years of 1985 and 2013.
They cross-referenced that information with stroke events in a national registry to which all hospitals in Israel are required to report.
The adolescents were about 17 years of age on average at the time of evaluation, 58% were male, and 84% were born in Israel. The mean age at the beginning of follow-up for stroke was about 31 years.
Over the follow-up period, investigators identified 1,088 first stroke events, including 921 ischemic and 167 hemorrhagic strokes.
A gradual increase in stroke rate was seen across BMI categories for ischemic strokes, but not so much for hemorrhagic strokes, investigators found.
Hazard ratios for first ischemic stroke event were 1.4 (95% confidence interval, 1.2-1.6) for the high-normal BMI group, 2.0 (95% CI, 1.6-2.4) for the overweight group, and 3.5 (95% CI, 2.8-4.5) for the obese group after adjusting for age and sex at beginning of follow-up, investigators reported.
When the adjusted results were stratified by presence or absence of diabetes, estimates were similar to what was seen in the overall risk model, they added.
Among those young adults who developed ischemic stroke, 43% smoked, 29% had high blood pressure, 17% had diabetes, and 32% had abnormal lipids at the time of diagnosis, the reported data showed.
The clinical and public health implications of these findings could be substantial, since strokes are associated with worse medical and socioeconomic outcomes in younger as compared with older individuals, according to Dr. Twig and coauthors.
Younger individuals with stroke have a higher risk of recurrent stroke, heart attack, long-term care, or death, they said. Moreover, about half of young-adult stroke survivors have poor functional outcomes, and their risk of unemployment and depression/anxiety is higher than in young individuals without stroke.
One limitation of the study is that follow-up BMI data were not available for all participants. As a result, the contribution of obesity to stroke risk over time could not be assessed, and the independent risk of BMI during adolescence could not be determined. In addition, the authors said the study underrepresents orthodox and ultraorthodox Jewish women, as they are not obligated to serve in the Israeli military.
The study authors had no disclosures related to the study, which was supported by a medical corps Israel Defense Forces research grant.
FROM STROKE
Late-breaking news on trajectory of ADHD remission headlines world conference
Most patients will not make a full recovery from attention-deficit/hyperactivity disorder in adulthood. This late-breaking finding headlined the World Congress on ADHD – Virtual Event. Held under the specter of SARS-CoV-2, the virtual program delved into the latest research on ADHD pathophysiology, imaging, genetics, and issues on medical and psychiatric comorbidities.
However, one of the conference’s highlights was a piece of unpublished work on remission patterns by Margaret Sibley, PhD, associate professor of psychiatry and behavioral sciences at the University of Washington, Seattle.
Anywhere from 65% to 67% of young adults have desistant ADHD – meaning that they no longer meet criteria. Only up to 23% experience full remission, said Dr. Sibley during a special late-breaking session. All research on remission and most on persistence consider just one endpoint – nothing is known about longitudinal fluctuations in remission status over time.
Her research sought to answer a key question: Do people fully recover from ADHD?
Using data from the Multimodal Treatment of Attention Deficit Hyperactivity Disorder (MTA) Study, Dr. Sibley prospectively followed over 550 children aged 7-9.9 years with DSM-IV combined-type ADHD over 14 years, until 16 years after baseline, using interviews, questionnaires, and rating scales to track symptoms, impairment, and treatment history.
The researchers also came up with a “winning” definition for full remission, which included three or fewer symptoms of inattention and hyperactive impulsivity from all available reporters, negligible ADHD-related impairment based on preestablished impairment rating thresholds, and discontinuation of medication and behavioral treatments for at least a month prior to assessment.
In the longitudinal results, Dr. Sibley and colleagues reported that the majority (63.8%) demonstrated fluctuations between full or partial remission and ADHD recurrence. Only 9.1% sustained full remission over the course of the study. From these findings, ADHD appears to be a fluctuating disorder. While it continues into adulthood for most people, there may also be periods of remission or “good functioning.”
Most desistance from ADHD represents partial, not full remission, said Dr. Sibley. The results also show that recovery by young adulthood is very rare – most patients with remitted ADHD have recurrences.
These are important findings, said Luis Augusto Rohde, MD, PhD, who co-organized the congress’ scientific program committee with Manfred Gerlach, PhD. It shows that a patient’s ADHD may sometimes be more definitive and at other times, no clear phenotype expression emerges.
COVID’s influence
COVID-19 greatly influenced this year’s program’s agenda, said Dr. Rohde. “There’s a lot of evidence that ADHD patients are at greater risk for COVID-19, which is not a surprise,” said Dr. Rohde, professor of child and adolescent psychiatry at the Federal University of Rio Grande do Sul’s department of psychiatry in Porto Alegre, Brazil.
ADHD is a combination of genetic liability and the demands of the environment. “In times like we are living in right now, if you have increasing demands and stress from the environment, you trigger symptoms in those even with lower genetic liability,” he said. ADHD’s pathophysiology involves attention and executive deficit disorder, which means these patients may not follow strategies to avoid infection.
This shows why COVID was so important to the discussion of program topics, he said.
Two experts addressed this subject head on in a point-counterpoint debate, “Residual effects of the 2019 pandemic will mirror the 1918 pandemic: Will we have lots of new ADHD cases?” James Swanson, PhD, professor of pediatrics at the University of California, Irvine, projected that biological coeffects of COVID-19 will lead to ADHD symptoms, generating potentially 5 million new ADHD cases.
David Coghill, MBChB, MD, a professor of child adolescent mental health at the University of Melbourne, countered that not enough data are available yet to back this hypothesis. “Researchers are asking this question, but clinically we don’t know enough.”
While the COVID virus might not directly lead to more cases of ADHD, this could potentially happen indirectly through environmental agents of the pandemic, offered Dr. Rohde. “We’ve clearly seen in our appointments with families and children that they can’t face the amount of schooling and working from home,” he said.
Novel treatments
The conference also addressed new treatments and nonpharmacologic interventions in the pipeline for ADHD. “We had a chance to discuss the possibilities about new medications that address the problems in the current market and to show the potential usefulness of nonpharma interventions such as neuromodulations in ADHD,” said Dr. Rohde. Speakers discussed strategies ranging from family-based mindfulness interventions to oligoantigenic diets in children with ADHD.
Other researchers are looking at novel digital tools to help patients manage and treat ADHD. Adherence is a major problem in chronic disorders like hypertension, diabetes, epilepsy, and ADHD, said Dr. Rohde. “Due to ADHD symptomatology including inattention, novelty-seeking, executive deficits, and difficulties in persistence, it is an even bigger problem in this disorder.”
Speakers at the “ADHD in the digital age – From pitfalls to challenges” session discussed video game strategies to reduce ADHD impairment, and a texting app to improve adherence. Dr. Rohde talked about the FOCUS app, which fosters collaboration between patients, families, and caregivers to efficiently track ADHD symptoms and help customize treatments.
Studies suggest these tools can significantly improve adherence. They’re also well accepted by patients, said Dr. Rohde. While the expectations are high, digital interventions are not a substitute for medication. “More data is needed to include them as part of the clinical interventions for ADHD.”
Dr. Sibley received book royalties from Guilford Press. Dr. Rohde has received grant or research support from, served as a consultant to, and served on the speakers’ bureau of Bial, Medice, Novartis/Sandoz, Pfizer, and Shire/Takeda in the last 3 years. The ADHD and Juvenile Bipolar Disorder Outpatient Programs chaired by Dr. Rohde have received unrestricted educational and research support from the following pharmaceutical companies in the last 3 years: Novartis/Sandoz and Shire/Takeda. Dr. Rohde has received authorship royalties from Oxford Press and ArtMed and travel grants from Shire to take part in the 2018 APA annual meeting. Dr. Swanson has two patents: (PIXA4), which uses a “time-of-flight” camera to measure growth of infants, and a provisional patent on the mechanism of tolerance to stimulant medication (PATSMTA). He has received travel support from Medice and has done legal review for NLS. Dr. Coghill worked for several pharmaceutical companies but had no disclosures relevant to the session debate on the pandemic.
Most patients will not make a full recovery from attention-deficit/hyperactivity disorder in adulthood. This late-breaking finding headlined the World Congress on ADHD – Virtual Event. Held under the specter of SARS-CoV-2, the virtual program delved into the latest research on ADHD pathophysiology, imaging, genetics, and issues on medical and psychiatric comorbidities.
However, one of the conference’s highlights was a piece of unpublished work on remission patterns by Margaret Sibley, PhD, associate professor of psychiatry and behavioral sciences at the University of Washington, Seattle.
Anywhere from 65% to 67% of young adults have desistant ADHD – meaning that they no longer meet criteria. Only up to 23% experience full remission, said Dr. Sibley during a special late-breaking session. All research on remission and most on persistence consider just one endpoint – nothing is known about longitudinal fluctuations in remission status over time.
Her research sought to answer a key question: Do people fully recover from ADHD?
Using data from the Multimodal Treatment of Attention Deficit Hyperactivity Disorder (MTA) Study, Dr. Sibley prospectively followed over 550 children aged 7-9.9 years with DSM-IV combined-type ADHD over 14 years, until 16 years after baseline, using interviews, questionnaires, and rating scales to track symptoms, impairment, and treatment history.
The researchers also came up with a “winning” definition for full remission, which included three or fewer symptoms of inattention and hyperactive impulsivity from all available reporters, negligible ADHD-related impairment based on preestablished impairment rating thresholds, and discontinuation of medication and behavioral treatments for at least a month prior to assessment.
In the longitudinal results, Dr. Sibley and colleagues reported that the majority (63.8%) demonstrated fluctuations between full or partial remission and ADHD recurrence. Only 9.1% sustained full remission over the course of the study. From these findings, ADHD appears to be a fluctuating disorder. While it continues into adulthood for most people, there may also be periods of remission or “good functioning.”
Most desistance from ADHD represents partial, not full remission, said Dr. Sibley. The results also show that recovery by young adulthood is very rare – most patients with remitted ADHD have recurrences.
These are important findings, said Luis Augusto Rohde, MD, PhD, who co-organized the congress’ scientific program committee with Manfred Gerlach, PhD. It shows that a patient’s ADHD may sometimes be more definitive and at other times, no clear phenotype expression emerges.
COVID’s influence
COVID-19 greatly influenced this year’s program’s agenda, said Dr. Rohde. “There’s a lot of evidence that ADHD patients are at greater risk for COVID-19, which is not a surprise,” said Dr. Rohde, professor of child and adolescent psychiatry at the Federal University of Rio Grande do Sul’s department of psychiatry in Porto Alegre, Brazil.
ADHD is a combination of genetic liability and the demands of the environment. “In times like we are living in right now, if you have increasing demands and stress from the environment, you trigger symptoms in those even with lower genetic liability,” he said. ADHD’s pathophysiology involves attention and executive deficit disorder, which means these patients may not follow strategies to avoid infection.
This shows why COVID was so important to the discussion of program topics, he said.
Two experts addressed this subject head on in a point-counterpoint debate, “Residual effects of the 2019 pandemic will mirror the 1918 pandemic: Will we have lots of new ADHD cases?” James Swanson, PhD, professor of pediatrics at the University of California, Irvine, projected that biological coeffects of COVID-19 will lead to ADHD symptoms, generating potentially 5 million new ADHD cases.
David Coghill, MBChB, MD, a professor of child adolescent mental health at the University of Melbourne, countered that not enough data are available yet to back this hypothesis. “Researchers are asking this question, but clinically we don’t know enough.”
While the COVID virus might not directly lead to more cases of ADHD, this could potentially happen indirectly through environmental agents of the pandemic, offered Dr. Rohde. “We’ve clearly seen in our appointments with families and children that they can’t face the amount of schooling and working from home,” he said.
Novel treatments
The conference also addressed new treatments and nonpharmacologic interventions in the pipeline for ADHD. “We had a chance to discuss the possibilities about new medications that address the problems in the current market and to show the potential usefulness of nonpharma interventions such as neuromodulations in ADHD,” said Dr. Rohde. Speakers discussed strategies ranging from family-based mindfulness interventions to oligoantigenic diets in children with ADHD.
Other researchers are looking at novel digital tools to help patients manage and treat ADHD. Adherence is a major problem in chronic disorders like hypertension, diabetes, epilepsy, and ADHD, said Dr. Rohde. “Due to ADHD symptomatology including inattention, novelty-seeking, executive deficits, and difficulties in persistence, it is an even bigger problem in this disorder.”
Speakers at the “ADHD in the digital age – From pitfalls to challenges” session discussed video game strategies to reduce ADHD impairment, and a texting app to improve adherence. Dr. Rohde talked about the FOCUS app, which fosters collaboration between patients, families, and caregivers to efficiently track ADHD symptoms and help customize treatments.
Studies suggest these tools can significantly improve adherence. They’re also well accepted by patients, said Dr. Rohde. While the expectations are high, digital interventions are not a substitute for medication. “More data is needed to include them as part of the clinical interventions for ADHD.”
Dr. Sibley received book royalties from Guilford Press. Dr. Rohde has received grant or research support from, served as a consultant to, and served on the speakers’ bureau of Bial, Medice, Novartis/Sandoz, Pfizer, and Shire/Takeda in the last 3 years. The ADHD and Juvenile Bipolar Disorder Outpatient Programs chaired by Dr. Rohde have received unrestricted educational and research support from the following pharmaceutical companies in the last 3 years: Novartis/Sandoz and Shire/Takeda. Dr. Rohde has received authorship royalties from Oxford Press and ArtMed and travel grants from Shire to take part in the 2018 APA annual meeting. Dr. Swanson has two patents: (PIXA4), which uses a “time-of-flight” camera to measure growth of infants, and a provisional patent on the mechanism of tolerance to stimulant medication (PATSMTA). He has received travel support from Medice and has done legal review for NLS. Dr. Coghill worked for several pharmaceutical companies but had no disclosures relevant to the session debate on the pandemic.
Most patients will not make a full recovery from attention-deficit/hyperactivity disorder in adulthood. This late-breaking finding headlined the World Congress on ADHD – Virtual Event. Held under the specter of SARS-CoV-2, the virtual program delved into the latest research on ADHD pathophysiology, imaging, genetics, and issues on medical and psychiatric comorbidities.
However, one of the conference’s highlights was a piece of unpublished work on remission patterns by Margaret Sibley, PhD, associate professor of psychiatry and behavioral sciences at the University of Washington, Seattle.
Anywhere from 65% to 67% of young adults have desistant ADHD – meaning that they no longer meet criteria. Only up to 23% experience full remission, said Dr. Sibley during a special late-breaking session. All research on remission and most on persistence consider just one endpoint – nothing is known about longitudinal fluctuations in remission status over time.
Her research sought to answer a key question: Do people fully recover from ADHD?
Using data from the Multimodal Treatment of Attention Deficit Hyperactivity Disorder (MTA) Study, Dr. Sibley prospectively followed over 550 children aged 7-9.9 years with DSM-IV combined-type ADHD over 14 years, until 16 years after baseline, using interviews, questionnaires, and rating scales to track symptoms, impairment, and treatment history.
The researchers also came up with a “winning” definition for full remission, which included three or fewer symptoms of inattention and hyperactive impulsivity from all available reporters, negligible ADHD-related impairment based on preestablished impairment rating thresholds, and discontinuation of medication and behavioral treatments for at least a month prior to assessment.
In the longitudinal results, Dr. Sibley and colleagues reported that the majority (63.8%) demonstrated fluctuations between full or partial remission and ADHD recurrence. Only 9.1% sustained full remission over the course of the study. From these findings, ADHD appears to be a fluctuating disorder. While it continues into adulthood for most people, there may also be periods of remission or “good functioning.”
Most desistance from ADHD represents partial, not full remission, said Dr. Sibley. The results also show that recovery by young adulthood is very rare – most patients with remitted ADHD have recurrences.
These are important findings, said Luis Augusto Rohde, MD, PhD, who co-organized the congress’ scientific program committee with Manfred Gerlach, PhD. It shows that a patient’s ADHD may sometimes be more definitive and at other times, no clear phenotype expression emerges.
COVID’s influence
COVID-19 greatly influenced this year’s program’s agenda, said Dr. Rohde. “There’s a lot of evidence that ADHD patients are at greater risk for COVID-19, which is not a surprise,” said Dr. Rohde, professor of child and adolescent psychiatry at the Federal University of Rio Grande do Sul’s department of psychiatry in Porto Alegre, Brazil.
ADHD is a combination of genetic liability and the demands of the environment. “In times like we are living in right now, if you have increasing demands and stress from the environment, you trigger symptoms in those even with lower genetic liability,” he said. ADHD’s pathophysiology involves attention and executive deficit disorder, which means these patients may not follow strategies to avoid infection.
This shows why COVID was so important to the discussion of program topics, he said.
Two experts addressed this subject head on in a point-counterpoint debate, “Residual effects of the 2019 pandemic will mirror the 1918 pandemic: Will we have lots of new ADHD cases?” James Swanson, PhD, professor of pediatrics at the University of California, Irvine, projected that biological coeffects of COVID-19 will lead to ADHD symptoms, generating potentially 5 million new ADHD cases.
David Coghill, MBChB, MD, a professor of child adolescent mental health at the University of Melbourne, countered that not enough data are available yet to back this hypothesis. “Researchers are asking this question, but clinically we don’t know enough.”
While the COVID virus might not directly lead to more cases of ADHD, this could potentially happen indirectly through environmental agents of the pandemic, offered Dr. Rohde. “We’ve clearly seen in our appointments with families and children that they can’t face the amount of schooling and working from home,” he said.
Novel treatments
The conference also addressed new treatments and nonpharmacologic interventions in the pipeline for ADHD. “We had a chance to discuss the possibilities about new medications that address the problems in the current market and to show the potential usefulness of nonpharma interventions such as neuromodulations in ADHD,” said Dr. Rohde. Speakers discussed strategies ranging from family-based mindfulness interventions to oligoantigenic diets in children with ADHD.
Other researchers are looking at novel digital tools to help patients manage and treat ADHD. Adherence is a major problem in chronic disorders like hypertension, diabetes, epilepsy, and ADHD, said Dr. Rohde. “Due to ADHD symptomatology including inattention, novelty-seeking, executive deficits, and difficulties in persistence, it is an even bigger problem in this disorder.”
Speakers at the “ADHD in the digital age – From pitfalls to challenges” session discussed video game strategies to reduce ADHD impairment, and a texting app to improve adherence. Dr. Rohde talked about the FOCUS app, which fosters collaboration between patients, families, and caregivers to efficiently track ADHD symptoms and help customize treatments.
Studies suggest these tools can significantly improve adherence. They’re also well accepted by patients, said Dr. Rohde. While the expectations are high, digital interventions are not a substitute for medication. “More data is needed to include them as part of the clinical interventions for ADHD.”
Dr. Sibley received book royalties from Guilford Press. Dr. Rohde has received grant or research support from, served as a consultant to, and served on the speakers’ bureau of Bial, Medice, Novartis/Sandoz, Pfizer, and Shire/Takeda in the last 3 years. The ADHD and Juvenile Bipolar Disorder Outpatient Programs chaired by Dr. Rohde have received unrestricted educational and research support from the following pharmaceutical companies in the last 3 years: Novartis/Sandoz and Shire/Takeda. Dr. Rohde has received authorship royalties from Oxford Press and ArtMed and travel grants from Shire to take part in the 2018 APA annual meeting. Dr. Swanson has two patents: (PIXA4), which uses a “time-of-flight” camera to measure growth of infants, and a provisional patent on the mechanism of tolerance to stimulant medication (PATSMTA). He has received travel support from Medice and has done legal review for NLS. Dr. Coghill worked for several pharmaceutical companies but had no disclosures relevant to the session debate on the pandemic.
FROM ADHD 2021
Assessing the cognitive nuances between ADHD and autism
Attention-deficit/hyperactivity disorder and autism spectrum disorder (ASD) often coexist in children and adults, but the range of cognitive abilities can vary widely in these patients. Researchers from around the world are leveraging symptom, cognitive assessment, and neurobiological measures to gain insights on how individuals with ADHD/ASD approach and solve problems.
Several experts discussed the progress of their research during the session, “Overlap and differences of ADHD and autism – new findings of functional imaging and cognition studies” at the World Congress on ADHD – Virtual Event.
“The overlap of these two disorders is a critical issue for our field,” said Sarah Karalunas, PhD, assistant professor of clinical psychology at Purdue University, West Lafayette, Ind., who moderated the session. Clinicians are often asked to make differential diagnoses between these two disorders. Only recently has the DSM-5 allowed their codiagnosis. “There’s increasing recognition that there may be shared cognitive and physiological features that reflect their shared risk and account for the high levels of symptom overlap,” said Dr. Karalunas.
Shared cognitive markers
Under the DSM’s change, “it’s now recognized that an estimated 20%-60% of children with ASD have comorbidities with ADHD, and around 20%-40% of children with ADHD have ASD symptoms,” said Beth Johnson, PhD, a research fellow with the Turner Institute for Brain and Mental Health at Monash University, Melbourne.
The shared overlap on genetic traits and comorbidities such as intellectual disability, anxiety, depression, and oppositional defiant disorder, make it difficult for clinicians to predict clinical outcomes, noted Dr. Johnson.
“We’re now understanding that they’re likely to be multiple autisms and ADHDs, that these symptoms exist on a spectrum of severity or ability,” she said. Dr. Johnson discussed a data-driven subtyping approach based on neurocognitive and symptom profiles in children with ADHD. The aim was to better understand how symptoms are managed across ADHD, ASD and comorbid ASD-ADHD.
As part of this research, her team recruited 295 controls and 117 children with ADHD who underwent clinical phenotyping and also completed working memory tasks, stop signal, and sustained attention tasks.
The researchers divided the children into four stable clusters based on the ADHD rating scale and autism questionnaire data: high ASD/ADHD traits, high ADHD/low ASD, low ADHD/moderate ASD, and low ADHD/ASD. Approximately half of the children with ADHD showed moderate to high ASD symptoms. Looking at neurocognition across the tasks, unsurprisingly, performance was lowest among the high-ASD/ADHD children, with performance on the stop signal being the most pronounced. “Notably, performance on the working memory task worsened with increasing ADHD symptoms,” she reported.
Drift model identifies information processing
Dr. Karalunas has also compared subgroups of ADHD and ASD children. “Our analysis examined whether cognitive impairments in ASD reflect a shared risk mechanism or co-occurring ADHD symptoms and why we see an overlap in these types of impairments,” she said.
Her study included 509 children with ADHD, 97 with ASD, and 301 controls (typical development). All three groups underwent a full cognitive assessment battery that measured attention arousal, basic processing speed, and working memory. Those tasks were collapsed into a series of variables as well as a set of tasks measuring response inhibition, switching, interference control, reward discounting, and measure of reaction time variability.
Four cognitive profiles emerged: a typically developing group, an ADHD group, an ASD group with low levels of ADHD symptoms and an ASD group with high levels of ADHD symptoms.
The ADHD group did worse on many of the tasks than the control group, and the ASD group with low ADHD levels also did poorly relative to the typically developing sample. This shows that autism – even in absence of co-occurring ADHD – demonstrates more cognitive impairment than typically developing kids. The ADHD group with high levels of autism did the most poorly across all of the tasks.
The findings also revealed a symptom severity pattern: the group with fewer symptoms did the best and the group with the most symptoms did the worst. “Overall, this reflects severity of impairment,” said Dr. Karalunas.
To identify measures more specific to either ADHD or autism, Dr. Karalunas and colleagues did a follow-up analysis to characterize cognitive performance. To accomplish this, they applied a drift-diffusion model to the same four cognitive profiles. The model assessed three parameters: drift rate, which relates to the speed or efficiency of information processing, boundary separation or speed accuracy trade-offs (impulsivity), and nondecision time such as motor preparation.
Using the same four cognitive profiles, they found that the ADHD group had slower drift rate relative to the control, although the two groups did not differ on boundary separation, which meant there were no differences on waiting to need to respond. The ADHD group had faster nondecision times. “This is a classic pattern, shown in the literature,” said Dr. Karalunas.
In other results, an interesting pattern began to evolve
Both ASD groups, for example, had much wider boundary separations, which meant they were waiting to be sure before they responded than the ADHD or typically developing groups. In contrast, the two ADHD groups had much faster non-decision times, whereas the two non-ADHD groups had similar nondecisions times.
Unlike the previous analysis, which saw a symptom severity pattern develop, “we’re getting two parameters that seem to track much more specifically to specific symptom domains,” observed Dr. Karalunas.
The results suggest there’s a substantial overlap in cognitive impairments in ADHD/ASD. “But we have pretty strong evidence at this point that these similarities are not accounted for by symptom overlap, especially for things like response and inhibition, working memory and processing speed. These seem to be independently related to ADHD and autism, regardless of the level of comorbid ADHD symptoms in the autism group,” said Dr. Karalunas.
The hope is to expand on these types of analyses to address the interaction of cognition-emotion and social cognition, and empirically define groups based on cognitive performance, she said.
Neurocognitive studies
Researchers have also been studying neural networks to assess ASD and ADHD. Roselyne Chauvin, PhD, a postdoctoral associate at Washington University, St. Louis, discussed the concept of “a task generic connectome,” in which researchers look for a common network between targeted task paradigms to get closer to a common alteration across impairments.
In her research, Dr. Chauvin and colleagues looked at connectivity modulations across three tasks: working memory, reward processing tasks, and stop signal tasks, comparing ADHD patients to siblings and controls. The ADHD group showed reduced sensitivity or a smaller number of connections modulated in the tasks compared with the other groups. Researchers wondered where those missed connections were located.
Dividing the cohorts into task generic and task specific groups, Dr. Chauvin and colleagues found that the ADHD group lacked common processing skills. They were also able to identify reproducible missing circuits in the ADHD participants. Among the cohorts, there was a higher modulation of task-specific edges in the ADHD group.
The ADHD patients seemed to be using more task-tailored alternative strategies that were more challenging and suboptimal.
She also previewed her ongoing work with the EU-AIMS Longitudinal European Autism Project (LEAP) database to study ASD-ADHD comorbidity. In this project, she and her colleagues looked at several tasks: probing emotion processing, inhibitory control, theory of mind, and reward anticipation. Comparing ASD groups with or without ADHD comorbidity or a shared connection, she and her team were able to devise a functional profile predictive of ADHD severity. As an example, “for the connection only used by the ASD with ADHD comorbidity, the more they were using those connections of higher amplitude in the modulation, inside this subset of connection, the higher they would have ADHD severity,” said Dr. Chauvin.
Neural correlates of different behavioral and cognitive profiles haven’t been widely studied, according to Charlotte Tye, PhD, who’s based at the Institute of Psychiatry, Psychology & Neuroscience, King’s College, London. Electroencephalography is a useful technique for understanding the neural correlates of cognitive impairments and teasing apart different models of co-occurrence in ASD and ADHD.
Dr. Tye and colleagues tested this approach in a cohort of boys aged 8-13 years diagnosed with ASD and/or ADHD, measuring EEG while the children did various continuous performance tasks to assess changes in brain activity. Examining P3 amplitude (event-related potential components) they found that children with ADHD or ADHD+ASD showed an attenuated amplitude of the P3, compared with typically developing children and those with ASD.
“This suggests children with an ADHD diagnosis exhibited reduced inhibitory control,” said Dr. Tye. In contrast, children with ASD showed reduced conflict monitoring as indexed by altered N2 amplitude across task conditions.
These, and other studies conducted by Dr. Tye and colleagues indicate that children with ADHD show reduced neural responses during attentional processing, whereas autistic children show typical neural responses, supporting specific profiles.
“Autistic children with a diagnosis of ADHD appear to show the unique patterns of neural responses of autism and ADHD, supporting an additive co-occurrence rather than a distinct condition. This contributes to identification of transdiagnostic subgroups within neurodevelopmental conditions for targeting of personalized intervention, and suggests that children with co-occurring autism and ADHD require support for both conditions,” said Dr. Tye.
An important takeaway from all of these findings is “we can’t look just at how someone does overall on a single test,” said Dr. Karalunas in an interview. “There is a tremendous amount of variability between people who have the same diagnosis, and our research really needs to account for this.”
Attention-deficit/hyperactivity disorder and autism spectrum disorder (ASD) often coexist in children and adults, but the range of cognitive abilities can vary widely in these patients. Researchers from around the world are leveraging symptom, cognitive assessment, and neurobiological measures to gain insights on how individuals with ADHD/ASD approach and solve problems.
Several experts discussed the progress of their research during the session, “Overlap and differences of ADHD and autism – new findings of functional imaging and cognition studies” at the World Congress on ADHD – Virtual Event.
“The overlap of these two disorders is a critical issue for our field,” said Sarah Karalunas, PhD, assistant professor of clinical psychology at Purdue University, West Lafayette, Ind., who moderated the session. Clinicians are often asked to make differential diagnoses between these two disorders. Only recently has the DSM-5 allowed their codiagnosis. “There’s increasing recognition that there may be shared cognitive and physiological features that reflect their shared risk and account for the high levels of symptom overlap,” said Dr. Karalunas.
Shared cognitive markers
Under the DSM’s change, “it’s now recognized that an estimated 20%-60% of children with ASD have comorbidities with ADHD, and around 20%-40% of children with ADHD have ASD symptoms,” said Beth Johnson, PhD, a research fellow with the Turner Institute for Brain and Mental Health at Monash University, Melbourne.
The shared overlap on genetic traits and comorbidities such as intellectual disability, anxiety, depression, and oppositional defiant disorder, make it difficult for clinicians to predict clinical outcomes, noted Dr. Johnson.
“We’re now understanding that they’re likely to be multiple autisms and ADHDs, that these symptoms exist on a spectrum of severity or ability,” she said. Dr. Johnson discussed a data-driven subtyping approach based on neurocognitive and symptom profiles in children with ADHD. The aim was to better understand how symptoms are managed across ADHD, ASD and comorbid ASD-ADHD.
As part of this research, her team recruited 295 controls and 117 children with ADHD who underwent clinical phenotyping and also completed working memory tasks, stop signal, and sustained attention tasks.
The researchers divided the children into four stable clusters based on the ADHD rating scale and autism questionnaire data: high ASD/ADHD traits, high ADHD/low ASD, low ADHD/moderate ASD, and low ADHD/ASD. Approximately half of the children with ADHD showed moderate to high ASD symptoms. Looking at neurocognition across the tasks, unsurprisingly, performance was lowest among the high-ASD/ADHD children, with performance on the stop signal being the most pronounced. “Notably, performance on the working memory task worsened with increasing ADHD symptoms,” she reported.
Drift model identifies information processing
Dr. Karalunas has also compared subgroups of ADHD and ASD children. “Our analysis examined whether cognitive impairments in ASD reflect a shared risk mechanism or co-occurring ADHD symptoms and why we see an overlap in these types of impairments,” she said.
Her study included 509 children with ADHD, 97 with ASD, and 301 controls (typical development). All three groups underwent a full cognitive assessment battery that measured attention arousal, basic processing speed, and working memory. Those tasks were collapsed into a series of variables as well as a set of tasks measuring response inhibition, switching, interference control, reward discounting, and measure of reaction time variability.
Four cognitive profiles emerged: a typically developing group, an ADHD group, an ASD group with low levels of ADHD symptoms and an ASD group with high levels of ADHD symptoms.
The ADHD group did worse on many of the tasks than the control group, and the ASD group with low ADHD levels also did poorly relative to the typically developing sample. This shows that autism – even in absence of co-occurring ADHD – demonstrates more cognitive impairment than typically developing kids. The ADHD group with high levels of autism did the most poorly across all of the tasks.
The findings also revealed a symptom severity pattern: the group with fewer symptoms did the best and the group with the most symptoms did the worst. “Overall, this reflects severity of impairment,” said Dr. Karalunas.
To identify measures more specific to either ADHD or autism, Dr. Karalunas and colleagues did a follow-up analysis to characterize cognitive performance. To accomplish this, they applied a drift-diffusion model to the same four cognitive profiles. The model assessed three parameters: drift rate, which relates to the speed or efficiency of information processing, boundary separation or speed accuracy trade-offs (impulsivity), and nondecision time such as motor preparation.
Using the same four cognitive profiles, they found that the ADHD group had slower drift rate relative to the control, although the two groups did not differ on boundary separation, which meant there were no differences on waiting to need to respond. The ADHD group had faster nondecision times. “This is a classic pattern, shown in the literature,” said Dr. Karalunas.
In other results, an interesting pattern began to evolve
Both ASD groups, for example, had much wider boundary separations, which meant they were waiting to be sure before they responded than the ADHD or typically developing groups. In contrast, the two ADHD groups had much faster non-decision times, whereas the two non-ADHD groups had similar nondecisions times.
Unlike the previous analysis, which saw a symptom severity pattern develop, “we’re getting two parameters that seem to track much more specifically to specific symptom domains,” observed Dr. Karalunas.
The results suggest there’s a substantial overlap in cognitive impairments in ADHD/ASD. “But we have pretty strong evidence at this point that these similarities are not accounted for by symptom overlap, especially for things like response and inhibition, working memory and processing speed. These seem to be independently related to ADHD and autism, regardless of the level of comorbid ADHD symptoms in the autism group,” said Dr. Karalunas.
The hope is to expand on these types of analyses to address the interaction of cognition-emotion and social cognition, and empirically define groups based on cognitive performance, she said.
Neurocognitive studies
Researchers have also been studying neural networks to assess ASD and ADHD. Roselyne Chauvin, PhD, a postdoctoral associate at Washington University, St. Louis, discussed the concept of “a task generic connectome,” in which researchers look for a common network between targeted task paradigms to get closer to a common alteration across impairments.
In her research, Dr. Chauvin and colleagues looked at connectivity modulations across three tasks: working memory, reward processing tasks, and stop signal tasks, comparing ADHD patients to siblings and controls. The ADHD group showed reduced sensitivity or a smaller number of connections modulated in the tasks compared with the other groups. Researchers wondered where those missed connections were located.
Dividing the cohorts into task generic and task specific groups, Dr. Chauvin and colleagues found that the ADHD group lacked common processing skills. They were also able to identify reproducible missing circuits in the ADHD participants. Among the cohorts, there was a higher modulation of task-specific edges in the ADHD group.
The ADHD patients seemed to be using more task-tailored alternative strategies that were more challenging and suboptimal.
She also previewed her ongoing work with the EU-AIMS Longitudinal European Autism Project (LEAP) database to study ASD-ADHD comorbidity. In this project, she and her colleagues looked at several tasks: probing emotion processing, inhibitory control, theory of mind, and reward anticipation. Comparing ASD groups with or without ADHD comorbidity or a shared connection, she and her team were able to devise a functional profile predictive of ADHD severity. As an example, “for the connection only used by the ASD with ADHD comorbidity, the more they were using those connections of higher amplitude in the modulation, inside this subset of connection, the higher they would have ADHD severity,” said Dr. Chauvin.
Neural correlates of different behavioral and cognitive profiles haven’t been widely studied, according to Charlotte Tye, PhD, who’s based at the Institute of Psychiatry, Psychology & Neuroscience, King’s College, London. Electroencephalography is a useful technique for understanding the neural correlates of cognitive impairments and teasing apart different models of co-occurrence in ASD and ADHD.
Dr. Tye and colleagues tested this approach in a cohort of boys aged 8-13 years diagnosed with ASD and/or ADHD, measuring EEG while the children did various continuous performance tasks to assess changes in brain activity. Examining P3 amplitude (event-related potential components) they found that children with ADHD or ADHD+ASD showed an attenuated amplitude of the P3, compared with typically developing children and those with ASD.
“This suggests children with an ADHD diagnosis exhibited reduced inhibitory control,” said Dr. Tye. In contrast, children with ASD showed reduced conflict monitoring as indexed by altered N2 amplitude across task conditions.
These, and other studies conducted by Dr. Tye and colleagues indicate that children with ADHD show reduced neural responses during attentional processing, whereas autistic children show typical neural responses, supporting specific profiles.
“Autistic children with a diagnosis of ADHD appear to show the unique patterns of neural responses of autism and ADHD, supporting an additive co-occurrence rather than a distinct condition. This contributes to identification of transdiagnostic subgroups within neurodevelopmental conditions for targeting of personalized intervention, and suggests that children with co-occurring autism and ADHD require support for both conditions,” said Dr. Tye.
An important takeaway from all of these findings is “we can’t look just at how someone does overall on a single test,” said Dr. Karalunas in an interview. “There is a tremendous amount of variability between people who have the same diagnosis, and our research really needs to account for this.”
Attention-deficit/hyperactivity disorder and autism spectrum disorder (ASD) often coexist in children and adults, but the range of cognitive abilities can vary widely in these patients. Researchers from around the world are leveraging symptom, cognitive assessment, and neurobiological measures to gain insights on how individuals with ADHD/ASD approach and solve problems.
Several experts discussed the progress of their research during the session, “Overlap and differences of ADHD and autism – new findings of functional imaging and cognition studies” at the World Congress on ADHD – Virtual Event.
“The overlap of these two disorders is a critical issue for our field,” said Sarah Karalunas, PhD, assistant professor of clinical psychology at Purdue University, West Lafayette, Ind., who moderated the session. Clinicians are often asked to make differential diagnoses between these two disorders. Only recently has the DSM-5 allowed their codiagnosis. “There’s increasing recognition that there may be shared cognitive and physiological features that reflect their shared risk and account for the high levels of symptom overlap,” said Dr. Karalunas.
Shared cognitive markers
Under the DSM’s change, “it’s now recognized that an estimated 20%-60% of children with ASD have comorbidities with ADHD, and around 20%-40% of children with ADHD have ASD symptoms,” said Beth Johnson, PhD, a research fellow with the Turner Institute for Brain and Mental Health at Monash University, Melbourne.
The shared overlap on genetic traits and comorbidities such as intellectual disability, anxiety, depression, and oppositional defiant disorder, make it difficult for clinicians to predict clinical outcomes, noted Dr. Johnson.
“We’re now understanding that they’re likely to be multiple autisms and ADHDs, that these symptoms exist on a spectrum of severity or ability,” she said. Dr. Johnson discussed a data-driven subtyping approach based on neurocognitive and symptom profiles in children with ADHD. The aim was to better understand how symptoms are managed across ADHD, ASD and comorbid ASD-ADHD.
As part of this research, her team recruited 295 controls and 117 children with ADHD who underwent clinical phenotyping and also completed working memory tasks, stop signal, and sustained attention tasks.
The researchers divided the children into four stable clusters based on the ADHD rating scale and autism questionnaire data: high ASD/ADHD traits, high ADHD/low ASD, low ADHD/moderate ASD, and low ADHD/ASD. Approximately half of the children with ADHD showed moderate to high ASD symptoms. Looking at neurocognition across the tasks, unsurprisingly, performance was lowest among the high-ASD/ADHD children, with performance on the stop signal being the most pronounced. “Notably, performance on the working memory task worsened with increasing ADHD symptoms,” she reported.
Drift model identifies information processing
Dr. Karalunas has also compared subgroups of ADHD and ASD children. “Our analysis examined whether cognitive impairments in ASD reflect a shared risk mechanism or co-occurring ADHD symptoms and why we see an overlap in these types of impairments,” she said.
Her study included 509 children with ADHD, 97 with ASD, and 301 controls (typical development). All three groups underwent a full cognitive assessment battery that measured attention arousal, basic processing speed, and working memory. Those tasks were collapsed into a series of variables as well as a set of tasks measuring response inhibition, switching, interference control, reward discounting, and measure of reaction time variability.
Four cognitive profiles emerged: a typically developing group, an ADHD group, an ASD group with low levels of ADHD symptoms and an ASD group with high levels of ADHD symptoms.
The ADHD group did worse on many of the tasks than the control group, and the ASD group with low ADHD levels also did poorly relative to the typically developing sample. This shows that autism – even in absence of co-occurring ADHD – demonstrates more cognitive impairment than typically developing kids. The ADHD group with high levels of autism did the most poorly across all of the tasks.
The findings also revealed a symptom severity pattern: the group with fewer symptoms did the best and the group with the most symptoms did the worst. “Overall, this reflects severity of impairment,” said Dr. Karalunas.
To identify measures more specific to either ADHD or autism, Dr. Karalunas and colleagues did a follow-up analysis to characterize cognitive performance. To accomplish this, they applied a drift-diffusion model to the same four cognitive profiles. The model assessed three parameters: drift rate, which relates to the speed or efficiency of information processing, boundary separation or speed accuracy trade-offs (impulsivity), and nondecision time such as motor preparation.
Using the same four cognitive profiles, they found that the ADHD group had slower drift rate relative to the control, although the two groups did not differ on boundary separation, which meant there were no differences on waiting to need to respond. The ADHD group had faster nondecision times. “This is a classic pattern, shown in the literature,” said Dr. Karalunas.
In other results, an interesting pattern began to evolve
Both ASD groups, for example, had much wider boundary separations, which meant they were waiting to be sure before they responded than the ADHD or typically developing groups. In contrast, the two ADHD groups had much faster non-decision times, whereas the two non-ADHD groups had similar nondecisions times.
Unlike the previous analysis, which saw a symptom severity pattern develop, “we’re getting two parameters that seem to track much more specifically to specific symptom domains,” observed Dr. Karalunas.
The results suggest there’s a substantial overlap in cognitive impairments in ADHD/ASD. “But we have pretty strong evidence at this point that these similarities are not accounted for by symptom overlap, especially for things like response and inhibition, working memory and processing speed. These seem to be independently related to ADHD and autism, regardless of the level of comorbid ADHD symptoms in the autism group,” said Dr. Karalunas.
The hope is to expand on these types of analyses to address the interaction of cognition-emotion and social cognition, and empirically define groups based on cognitive performance, she said.
Neurocognitive studies
Researchers have also been studying neural networks to assess ASD and ADHD. Roselyne Chauvin, PhD, a postdoctoral associate at Washington University, St. Louis, discussed the concept of “a task generic connectome,” in which researchers look for a common network between targeted task paradigms to get closer to a common alteration across impairments.
In her research, Dr. Chauvin and colleagues looked at connectivity modulations across three tasks: working memory, reward processing tasks, and stop signal tasks, comparing ADHD patients to siblings and controls. The ADHD group showed reduced sensitivity or a smaller number of connections modulated in the tasks compared with the other groups. Researchers wondered where those missed connections were located.
Dividing the cohorts into task generic and task specific groups, Dr. Chauvin and colleagues found that the ADHD group lacked common processing skills. They were also able to identify reproducible missing circuits in the ADHD participants. Among the cohorts, there was a higher modulation of task-specific edges in the ADHD group.
The ADHD patients seemed to be using more task-tailored alternative strategies that were more challenging and suboptimal.
She also previewed her ongoing work with the EU-AIMS Longitudinal European Autism Project (LEAP) database to study ASD-ADHD comorbidity. In this project, she and her colleagues looked at several tasks: probing emotion processing, inhibitory control, theory of mind, and reward anticipation. Comparing ASD groups with or without ADHD comorbidity or a shared connection, she and her team were able to devise a functional profile predictive of ADHD severity. As an example, “for the connection only used by the ASD with ADHD comorbidity, the more they were using those connections of higher amplitude in the modulation, inside this subset of connection, the higher they would have ADHD severity,” said Dr. Chauvin.
Neural correlates of different behavioral and cognitive profiles haven’t been widely studied, according to Charlotte Tye, PhD, who’s based at the Institute of Psychiatry, Psychology & Neuroscience, King’s College, London. Electroencephalography is a useful technique for understanding the neural correlates of cognitive impairments and teasing apart different models of co-occurrence in ASD and ADHD.
Dr. Tye and colleagues tested this approach in a cohort of boys aged 8-13 years diagnosed with ASD and/or ADHD, measuring EEG while the children did various continuous performance tasks to assess changes in brain activity. Examining P3 amplitude (event-related potential components) they found that children with ADHD or ADHD+ASD showed an attenuated amplitude of the P3, compared with typically developing children and those with ASD.
“This suggests children with an ADHD diagnosis exhibited reduced inhibitory control,” said Dr. Tye. In contrast, children with ASD showed reduced conflict monitoring as indexed by altered N2 amplitude across task conditions.
These, and other studies conducted by Dr. Tye and colleagues indicate that children with ADHD show reduced neural responses during attentional processing, whereas autistic children show typical neural responses, supporting specific profiles.
“Autistic children with a diagnosis of ADHD appear to show the unique patterns of neural responses of autism and ADHD, supporting an additive co-occurrence rather than a distinct condition. This contributes to identification of transdiagnostic subgroups within neurodevelopmental conditions for targeting of personalized intervention, and suggests that children with co-occurring autism and ADHD require support for both conditions,” said Dr. Tye.
An important takeaway from all of these findings is “we can’t look just at how someone does overall on a single test,” said Dr. Karalunas in an interview. “There is a tremendous amount of variability between people who have the same diagnosis, and our research really needs to account for this.”
FROM ADHD 2021
Will COVID-19 result in more ADHD cases? A debate
While it’s possible that residual effects of SARS-CoV-2 could lead to an eruption of attention-deficit/hyperactivity disorder (ADHD) cases, a debate at the World Congress on ADHD – Virtual Event underscored the fact that this is still a hypothesis. The bottom line is there needs to be more data, said Luis Augusto Rohde, MD, PhD, cochair of the congress’ scientific program committee and moderator of the session, “Residual effects of the 2019 pandemic will mirror the 1918 pandemic: Will we have lots of new ADHD cases?”
Considering the current pattern of the pandemic, there is not enough evidence for this to be a concern, Dr. Rohde said in an interview.
James Swanson, PhD, professor of pediatrics at the University of California, Irvine, opined that biological co-effects of COVID-19 are likely to have selective effects in children that may produce symptoms representative of ADHD. Using the 1918 Spanish flu pandemic as a historical reference, he estimated that COVID-19 would produce 5 million individuals with new-onset symptoms related to ADHD. “If these cases meet DSM-5 or ICD-11 criteria, there will be lots of new ADHD cases,” he predicted.
David Coghill, MD, a professor of child adolescent mental health at the University of Melbourne, observed that the sums Dr. Swanson presented “are based on maxing out the potential rather than looking at the sums more realistically.”
Could the 1918 pandemic offer clues?
In a commentary, Dr. Swanson and Nora D. Volkow, MD, wrote about “lessons learned” from the 1918 pandemic, and how residual sequelae in that era led to a condition labeled hyperkinetic syndrome in children. “It may be worthwhile to consider the hypothesis that the COVID-19 pandemic may result in a novel etiologic subtype of ADHD that clinicians may recognize in patients in the future,” wrote the commentators.
In survivors of the 1918 pandemic, brain inflammation or encephalitis sometimes emerged as residual sequelae, said Dr. Swanson. In some adult cases, these symptoms were diagnosed as “encephalitis lethargica” (EL) and were associated with Parkinson’s disease. In 1930, based on patients evaluated after 1918, researchers Franz Kramer and Hans Pollnow at Charité Hospital in Berlin described the behavioral manifestation of EL in children as hyperkinetic syndrome, a condition that was characterized by symptoms similar to the properties of ADHD: lack of concentration, insufficient goal orientation, and increased distractibility. “They even reported on autopsy cases that described brain regions that we now know are associated with ADHD from decades of brain imaging studies,” said Dr. Swanson.
COVID-19 rarely results in severe respiratory problems in children but the absolute number requiring hospitalization has accumulated and is now relatively large, said Dr. Swanson. One study of 1,695 severe COVID-19 cases in children and adolescents used MRI and detected neural effects in specific brain regions such as basal ganglia and frontal lobes that previous research had associated with ADHD. Approximately 22% of these rare but severe cases had documented neurologic involvement, and studies of affected children with mild or none of the initial respiratory symptoms of COVID-19 also detected similar selective effects in these brain regions.
A recent survey of medical records of 80 million people that identified 240,000 COVID cases (mostly adults) revealed that a third had neurological and psychiatric sequelae. Dr. Swanson also mentioned an article he wrote more than a decade ago on environmental as well as genetic factors that resulted in etiologic subtypes of ADHD, which provided a model for the impact of COVID-19 on specific brain regions that are associated with ADHD.
So far, the COVID-19 pandemic has produced 150 million cases worldwide and there are about 100 million survivors, setting an estimate of a maximum number of cases with residual sequelae. “I think that severe COVID-19 will probably be related to severe residual sequelae, and that mild or asymptomatic COVID-19 may be associated with less severe residual sequelae, which may resemble ADHD” said Dr. Swanson. If one-third of the cases manifest in some neurologic or psychiatric systems, this means 27 million would have residual sequelae. If 20% have impaired concentration or brain fog, this could result in about 5 million ADHD cases, he said.
Estimates aren’t evidence
The Swanson/Volkow commentary contains a lot of references to “might, could, and may,” said Dr. Coghill. While it’s true that COVID-19 could produce a novel etiologic subtype of ADHD, “the point here is at the moment, all of this is based on hypotheses,” he said.
The Spanish flu did produce mental health consequences – survivors reported depression, sleep disturbances, mental distraction, dizziness, and difficulties coping at work. In the United States, flu death rates from 1918 to 1920 were directly attributed to suicide rates. Unfortunately, these impacts weren’t widely researched, said Dr. Coghill.
It also seems clear that the 1918 Spanish flu outbreak was associated with significant neurological consequences, said Dr. Coghill. By 1919 and 1920, physicians and researchers in the United Kingdom were reporting increases in a variety of symptoms among some patients recovering from flu, such as neuropathy, neurasthenia, meningitis, degenerative changes in nerve cells, and a decline in visual acuity.
The EL cases Dr. Swanson mentioned did coincide with and reach epidemic proportions alongside the Spanish flu. “But still, a causal relationship is far from proven,” said Dr. Coghill.
Sol Levy, MD, described a “disease of criminals” following the 1918 pandemic, in which patients exhibited a high degree of general hyperkinesis, a difficulty in maintaining quiet attitudes, abruptness and clumsiness, and “explosive motor release of all voluntarily inhibited activities.”
However, these impairments suggest a much broader presentation typically seen in ADHD, noted Dr. Coghill.
Neurological complications occur more commonly than initially thought in severe COVID-19, with estimates ranging from 36% to 84%. But in a systematic review of neuropsychiatric complications of severe coronavirus infection, researchers found few psychiatric sequelae of these infections. While they did mention impaired concentration and difficulties with emotional ability, it’s very important to remember that these conditions “are cardinal symptoms of a wide range of psychiatric disorders,” said Dr. Coghill.
Overall, more neurological and neuropsychiatric symptoms largely confine to those with severe COVID-19, meaning they’re much less likely to occur in children and young adults, he said.
If there are severe effects of COVID-19, Dr. Swanson countered that “they might have more ADHD than the complex residual effects [Dr. Coghill] described. I hope that he’s right, but I do think there will be biological co-effects of COVID-19 that will produce symptoms that are more ADHD than other neurological disorders.”
Epigenetic effects
Researchers are now seeing transgenerational and intergenerational effects of potential infection. “So I certainly back high-quality studies looking at the effects of maternal and paternal infection on offspring,” said Dr. Coghill. Establishing clinical cohort studies to follow up on this population would be essential in understanding the risks of SARS-CoV-2. “That might be one way we’ll see an increase in ADHD,” said Dr. Coghill.
The reality is COVID-19 hasn’t been around for that long, and current knowledge about it is limited, he said. Rapid publications, cross-sectional or retrospective data, and poor methodological quality and rigor make generalizability difficult. In addition, limited testing and detection probably underestimate prevalence of neurological and neuropsychiatric complications.
“If history teaches us anything, it is that we should always be measured in how we glean lessons from the past. So let’s not get ahead of ourselves,” he cautioned.
An informal, post-discussion survey of session participants revealed that a slight majority – 55%-60% – expected residual effects of COVID-19 to lead to more ADHD, compared to 40%-45% who didn’t think this would happen.
Dr. Swanson has two patents: (PIXA4), which uses a “time-of-flight” camera to measure growth on infants, and a provisional patent on the mechanism of tolerance to stimulant medication (PATSMTA). Dr. Coghill worked for several pharmaceutical companies but had no disclosures relevant to this debate.
While it’s possible that residual effects of SARS-CoV-2 could lead to an eruption of attention-deficit/hyperactivity disorder (ADHD) cases, a debate at the World Congress on ADHD – Virtual Event underscored the fact that this is still a hypothesis. The bottom line is there needs to be more data, said Luis Augusto Rohde, MD, PhD, cochair of the congress’ scientific program committee and moderator of the session, “Residual effects of the 2019 pandemic will mirror the 1918 pandemic: Will we have lots of new ADHD cases?”
Considering the current pattern of the pandemic, there is not enough evidence for this to be a concern, Dr. Rohde said in an interview.
James Swanson, PhD, professor of pediatrics at the University of California, Irvine, opined that biological co-effects of COVID-19 are likely to have selective effects in children that may produce symptoms representative of ADHD. Using the 1918 Spanish flu pandemic as a historical reference, he estimated that COVID-19 would produce 5 million individuals with new-onset symptoms related to ADHD. “If these cases meet DSM-5 or ICD-11 criteria, there will be lots of new ADHD cases,” he predicted.
David Coghill, MD, a professor of child adolescent mental health at the University of Melbourne, observed that the sums Dr. Swanson presented “are based on maxing out the potential rather than looking at the sums more realistically.”
Could the 1918 pandemic offer clues?
In a commentary, Dr. Swanson and Nora D. Volkow, MD, wrote about “lessons learned” from the 1918 pandemic, and how residual sequelae in that era led to a condition labeled hyperkinetic syndrome in children. “It may be worthwhile to consider the hypothesis that the COVID-19 pandemic may result in a novel etiologic subtype of ADHD that clinicians may recognize in patients in the future,” wrote the commentators.
In survivors of the 1918 pandemic, brain inflammation or encephalitis sometimes emerged as residual sequelae, said Dr. Swanson. In some adult cases, these symptoms were diagnosed as “encephalitis lethargica” (EL) and were associated with Parkinson’s disease. In 1930, based on patients evaluated after 1918, researchers Franz Kramer and Hans Pollnow at Charité Hospital in Berlin described the behavioral manifestation of EL in children as hyperkinetic syndrome, a condition that was characterized by symptoms similar to the properties of ADHD: lack of concentration, insufficient goal orientation, and increased distractibility. “They even reported on autopsy cases that described brain regions that we now know are associated with ADHD from decades of brain imaging studies,” said Dr. Swanson.
COVID-19 rarely results in severe respiratory problems in children but the absolute number requiring hospitalization has accumulated and is now relatively large, said Dr. Swanson. One study of 1,695 severe COVID-19 cases in children and adolescents used MRI and detected neural effects in specific brain regions such as basal ganglia and frontal lobes that previous research had associated with ADHD. Approximately 22% of these rare but severe cases had documented neurologic involvement, and studies of affected children with mild or none of the initial respiratory symptoms of COVID-19 also detected similar selective effects in these brain regions.
A recent survey of medical records of 80 million people that identified 240,000 COVID cases (mostly adults) revealed that a third had neurological and psychiatric sequelae. Dr. Swanson also mentioned an article he wrote more than a decade ago on environmental as well as genetic factors that resulted in etiologic subtypes of ADHD, which provided a model for the impact of COVID-19 on specific brain regions that are associated with ADHD.
So far, the COVID-19 pandemic has produced 150 million cases worldwide and there are about 100 million survivors, setting an estimate of a maximum number of cases with residual sequelae. “I think that severe COVID-19 will probably be related to severe residual sequelae, and that mild or asymptomatic COVID-19 may be associated with less severe residual sequelae, which may resemble ADHD” said Dr. Swanson. If one-third of the cases manifest in some neurologic or psychiatric systems, this means 27 million would have residual sequelae. If 20% have impaired concentration or brain fog, this could result in about 5 million ADHD cases, he said.
Estimates aren’t evidence
The Swanson/Volkow commentary contains a lot of references to “might, could, and may,” said Dr. Coghill. While it’s true that COVID-19 could produce a novel etiologic subtype of ADHD, “the point here is at the moment, all of this is based on hypotheses,” he said.
The Spanish flu did produce mental health consequences – survivors reported depression, sleep disturbances, mental distraction, dizziness, and difficulties coping at work. In the United States, flu death rates from 1918 to 1920 were directly attributed to suicide rates. Unfortunately, these impacts weren’t widely researched, said Dr. Coghill.
It also seems clear that the 1918 Spanish flu outbreak was associated with significant neurological consequences, said Dr. Coghill. By 1919 and 1920, physicians and researchers in the United Kingdom were reporting increases in a variety of symptoms among some patients recovering from flu, such as neuropathy, neurasthenia, meningitis, degenerative changes in nerve cells, and a decline in visual acuity.
The EL cases Dr. Swanson mentioned did coincide with and reach epidemic proportions alongside the Spanish flu. “But still, a causal relationship is far from proven,” said Dr. Coghill.
Sol Levy, MD, described a “disease of criminals” following the 1918 pandemic, in which patients exhibited a high degree of general hyperkinesis, a difficulty in maintaining quiet attitudes, abruptness and clumsiness, and “explosive motor release of all voluntarily inhibited activities.”
However, these impairments suggest a much broader presentation typically seen in ADHD, noted Dr. Coghill.
Neurological complications occur more commonly than initially thought in severe COVID-19, with estimates ranging from 36% to 84%. But in a systematic review of neuropsychiatric complications of severe coronavirus infection, researchers found few psychiatric sequelae of these infections. While they did mention impaired concentration and difficulties with emotional ability, it’s very important to remember that these conditions “are cardinal symptoms of a wide range of psychiatric disorders,” said Dr. Coghill.
Overall, more neurological and neuropsychiatric symptoms largely confine to those with severe COVID-19, meaning they’re much less likely to occur in children and young adults, he said.
If there are severe effects of COVID-19, Dr. Swanson countered that “they might have more ADHD than the complex residual effects [Dr. Coghill] described. I hope that he’s right, but I do think there will be biological co-effects of COVID-19 that will produce symptoms that are more ADHD than other neurological disorders.”
Epigenetic effects
Researchers are now seeing transgenerational and intergenerational effects of potential infection. “So I certainly back high-quality studies looking at the effects of maternal and paternal infection on offspring,” said Dr. Coghill. Establishing clinical cohort studies to follow up on this population would be essential in understanding the risks of SARS-CoV-2. “That might be one way we’ll see an increase in ADHD,” said Dr. Coghill.
The reality is COVID-19 hasn’t been around for that long, and current knowledge about it is limited, he said. Rapid publications, cross-sectional or retrospective data, and poor methodological quality and rigor make generalizability difficult. In addition, limited testing and detection probably underestimate prevalence of neurological and neuropsychiatric complications.
“If history teaches us anything, it is that we should always be measured in how we glean lessons from the past. So let’s not get ahead of ourselves,” he cautioned.
An informal, post-discussion survey of session participants revealed that a slight majority – 55%-60% – expected residual effects of COVID-19 to lead to more ADHD, compared to 40%-45% who didn’t think this would happen.
Dr. Swanson has two patents: (PIXA4), which uses a “time-of-flight” camera to measure growth on infants, and a provisional patent on the mechanism of tolerance to stimulant medication (PATSMTA). Dr. Coghill worked for several pharmaceutical companies but had no disclosures relevant to this debate.
While it’s possible that residual effects of SARS-CoV-2 could lead to an eruption of attention-deficit/hyperactivity disorder (ADHD) cases, a debate at the World Congress on ADHD – Virtual Event underscored the fact that this is still a hypothesis. The bottom line is there needs to be more data, said Luis Augusto Rohde, MD, PhD, cochair of the congress’ scientific program committee and moderator of the session, “Residual effects of the 2019 pandemic will mirror the 1918 pandemic: Will we have lots of new ADHD cases?”
Considering the current pattern of the pandemic, there is not enough evidence for this to be a concern, Dr. Rohde said in an interview.
James Swanson, PhD, professor of pediatrics at the University of California, Irvine, opined that biological co-effects of COVID-19 are likely to have selective effects in children that may produce symptoms representative of ADHD. Using the 1918 Spanish flu pandemic as a historical reference, he estimated that COVID-19 would produce 5 million individuals with new-onset symptoms related to ADHD. “If these cases meet DSM-5 or ICD-11 criteria, there will be lots of new ADHD cases,” he predicted.
David Coghill, MD, a professor of child adolescent mental health at the University of Melbourne, observed that the sums Dr. Swanson presented “are based on maxing out the potential rather than looking at the sums more realistically.”
Could the 1918 pandemic offer clues?
In a commentary, Dr. Swanson and Nora D. Volkow, MD, wrote about “lessons learned” from the 1918 pandemic, and how residual sequelae in that era led to a condition labeled hyperkinetic syndrome in children. “It may be worthwhile to consider the hypothesis that the COVID-19 pandemic may result in a novel etiologic subtype of ADHD that clinicians may recognize in patients in the future,” wrote the commentators.
In survivors of the 1918 pandemic, brain inflammation or encephalitis sometimes emerged as residual sequelae, said Dr. Swanson. In some adult cases, these symptoms were diagnosed as “encephalitis lethargica” (EL) and were associated with Parkinson’s disease. In 1930, based on patients evaluated after 1918, researchers Franz Kramer and Hans Pollnow at Charité Hospital in Berlin described the behavioral manifestation of EL in children as hyperkinetic syndrome, a condition that was characterized by symptoms similar to the properties of ADHD: lack of concentration, insufficient goal orientation, and increased distractibility. “They even reported on autopsy cases that described brain regions that we now know are associated with ADHD from decades of brain imaging studies,” said Dr. Swanson.
COVID-19 rarely results in severe respiratory problems in children but the absolute number requiring hospitalization has accumulated and is now relatively large, said Dr. Swanson. One study of 1,695 severe COVID-19 cases in children and adolescents used MRI and detected neural effects in specific brain regions such as basal ganglia and frontal lobes that previous research had associated with ADHD. Approximately 22% of these rare but severe cases had documented neurologic involvement, and studies of affected children with mild or none of the initial respiratory symptoms of COVID-19 also detected similar selective effects in these brain regions.
A recent survey of medical records of 80 million people that identified 240,000 COVID cases (mostly adults) revealed that a third had neurological and psychiatric sequelae. Dr. Swanson also mentioned an article he wrote more than a decade ago on environmental as well as genetic factors that resulted in etiologic subtypes of ADHD, which provided a model for the impact of COVID-19 on specific brain regions that are associated with ADHD.
So far, the COVID-19 pandemic has produced 150 million cases worldwide and there are about 100 million survivors, setting an estimate of a maximum number of cases with residual sequelae. “I think that severe COVID-19 will probably be related to severe residual sequelae, and that mild or asymptomatic COVID-19 may be associated with less severe residual sequelae, which may resemble ADHD” said Dr. Swanson. If one-third of the cases manifest in some neurologic or psychiatric systems, this means 27 million would have residual sequelae. If 20% have impaired concentration or brain fog, this could result in about 5 million ADHD cases, he said.
Estimates aren’t evidence
The Swanson/Volkow commentary contains a lot of references to “might, could, and may,” said Dr. Coghill. While it’s true that COVID-19 could produce a novel etiologic subtype of ADHD, “the point here is at the moment, all of this is based on hypotheses,” he said.
The Spanish flu did produce mental health consequences – survivors reported depression, sleep disturbances, mental distraction, dizziness, and difficulties coping at work. In the United States, flu death rates from 1918 to 1920 were directly attributed to suicide rates. Unfortunately, these impacts weren’t widely researched, said Dr. Coghill.
It also seems clear that the 1918 Spanish flu outbreak was associated with significant neurological consequences, said Dr. Coghill. By 1919 and 1920, physicians and researchers in the United Kingdom were reporting increases in a variety of symptoms among some patients recovering from flu, such as neuropathy, neurasthenia, meningitis, degenerative changes in nerve cells, and a decline in visual acuity.
The EL cases Dr. Swanson mentioned did coincide with and reach epidemic proportions alongside the Spanish flu. “But still, a causal relationship is far from proven,” said Dr. Coghill.
Sol Levy, MD, described a “disease of criminals” following the 1918 pandemic, in which patients exhibited a high degree of general hyperkinesis, a difficulty in maintaining quiet attitudes, abruptness and clumsiness, and “explosive motor release of all voluntarily inhibited activities.”
However, these impairments suggest a much broader presentation typically seen in ADHD, noted Dr. Coghill.
Neurological complications occur more commonly than initially thought in severe COVID-19, with estimates ranging from 36% to 84%. But in a systematic review of neuropsychiatric complications of severe coronavirus infection, researchers found few psychiatric sequelae of these infections. While they did mention impaired concentration and difficulties with emotional ability, it’s very important to remember that these conditions “are cardinal symptoms of a wide range of psychiatric disorders,” said Dr. Coghill.
Overall, more neurological and neuropsychiatric symptoms largely confine to those with severe COVID-19, meaning they’re much less likely to occur in children and young adults, he said.
If there are severe effects of COVID-19, Dr. Swanson countered that “they might have more ADHD than the complex residual effects [Dr. Coghill] described. I hope that he’s right, but I do think there will be biological co-effects of COVID-19 that will produce symptoms that are more ADHD than other neurological disorders.”
Epigenetic effects
Researchers are now seeing transgenerational and intergenerational effects of potential infection. “So I certainly back high-quality studies looking at the effects of maternal and paternal infection on offspring,” said Dr. Coghill. Establishing clinical cohort studies to follow up on this population would be essential in understanding the risks of SARS-CoV-2. “That might be one way we’ll see an increase in ADHD,” said Dr. Coghill.
The reality is COVID-19 hasn’t been around for that long, and current knowledge about it is limited, he said. Rapid publications, cross-sectional or retrospective data, and poor methodological quality and rigor make generalizability difficult. In addition, limited testing and detection probably underestimate prevalence of neurological and neuropsychiatric complications.
“If history teaches us anything, it is that we should always be measured in how we glean lessons from the past. So let’s not get ahead of ourselves,” he cautioned.
An informal, post-discussion survey of session participants revealed that a slight majority – 55%-60% – expected residual effects of COVID-19 to lead to more ADHD, compared to 40%-45% who didn’t think this would happen.
Dr. Swanson has two patents: (PIXA4), which uses a “time-of-flight” camera to measure growth on infants, and a provisional patent on the mechanism of tolerance to stimulant medication (PATSMTA). Dr. Coghill worked for several pharmaceutical companies but had no disclosures relevant to this debate.
FROM ADHD 2021
Taking a drug holiday: Benefits and risks to children with ADHD
For children with attention-deficit/hyperactivity disorder, taking a weekend or summer break from methylphenidate may have some benefits. A drug “holiday” can help assess whether a drug is still useful and possibly help with drug tolerance, weight gain, and growth suppression. But drug holidays are not without their problems, Lily Hechtman, MD, FRCP, professor at the department of psychiatry, McGill University, Montreal, said during a session at the World Congress on ADHD – Virtual Event.
Ceasing a medication can have repercussions from a health and social standpoint, cautioned Dr. Hechtman, a presenter and moderator of the session, “Unsolved mysteries in the treatment of ADHD with psychostimulants.”
The rate of drug holidays is somewhere between 30% and 40% in ADHD patients. Patients have multiple reasons for taking them, said Dr. Hechtman. The American Academy of Child & Adolescent Psychology as well as the National Institute for Health and Clinical Excellence recommend this method to assess whether a medication is still necessary. Parents may opt for a drug holiday because most would prefer their children to take less medication.
A drug holiday can counteract some of the key side effects of stimulant medication such as decreased appetite and weight loss, and the moodiness and irritability that accompanies the medication, as well as sleep problems.
It may also be used to avoid drug tolerance, the need to increase dosage as medication continues. A 2002 study of 166 children and adolescents treated with methylphenidate revealed that 60% had developed drug tolerance. Drug tolerance increases with duration. “So, the longer the child is on medication, the more likely he or she will develop some drug tolerance,” said Dr. Hechtman.
It is hypothesized that a drug holiday results in the resensitization of the neurons in the brain because they aren’t exposed to the stimulation of dopamine release and dopamine exposure.
The minimum time a patient needs a drug holiday to deal with some drug tolerance is about a month. “Even if you have a drug holiday and your drug tolerance has been decreased, it can reoccur with increasing dosages, once medication resumes” after the holiday, said Dr. Hechtman.
The growth factor
A drug holiday can also address concerns about growth suppression. “Some studies show that drug holidays help with growth suppression and others do not,” said Dr. Hechtman.
The Multimodal Treatment of ADHD (MTA) study, which followed children with ADHD from childhood to adolescence into adulthood, offers some key insights on the effects of treatment on growth.
Over a 10-year period, “you could see that the rate of medication use decreased significantly with time” among participants, said Dr. Hechtman, a coauthor of the MTA research. Only 10% who began the study aged 7-9 years were still using stimulants 10 years later. Looking at short-term effects on growth among these children, those who never went on stimulants to begin with had no growth suppression at all, whereas those who underwent early and consistent treatment experienced the greatest growth suppression.
Comparatively, inconsistently medicated participants had less growth suppression than those who remained on medication. “They were pretty close to the controls,” said Dr. Hechtman.
These patterns continued in a 16-year follow-up, as these patients became adults. Based on the results in the inconsistently treated group, this suggests that drug holidays can limit the effects of growth suppression, at least to a certain extent, said Dr. Hechtman.
Other studies have yielded varying results on the impact of drug holidays on height and weight. “The evidence for the utility of drug holidays for medication side effects is there for decreased appetite and weight, but not so much for decreased height,” summarized Dr. Hechtman.
One recent study of 230 children by James Waxmonsky and colleagues that examined drug holidays on weekends and summers showed that drug holidays did increase weight but interestingly, not height. Older studies Dr. Hechtman cited had inconsistent results on height and weight gain and loss. A 2012 study suggested that drug holidays resulted in a slight improvement in appetite for both weekend and school holidays. But only 9% of the children in the sample (n = 51) saw their appetite return to normal levels.
‘Negative things can happen’
The downside of drug holidays is parents may rationalize that their child is doing fine without the medication, and discontinue it. The process of stopping and starting medication can lead to other problems. “Negative things can happen during drug holidays,” said Dr. Hechtman.
The large variability of doses over the weekend can result in rebound and side effects.
A child may go from a full dose, which could be 50-60 mg of stimulant to zero from Friday to Saturday. As a result they have a lot of rebound on that Saturday. Similarly, they go from zero on Sunday to full dose on the Monday, causing lots of side effects. “Also, they will never have a stable effective dose because of the roller-coaster effect of being on and off the drugs,” she noted.
The lack of consistency and accommodation to the side effects can lead to discontinuation of the medication.
Off medication, the child may be more accident prone or have more injuries. “Their behavior off the medication may be such that it leads to social problems,” Dr. Hechtman continued. Weekend activities that require medication such as homework or school projects, family or religious gatherings, or sports and social activities with family and peers may be affected. If the child is behaving poorly off the medication, they may be expelled from such activities. If it’s a summer drug holiday, they may get kicked out of camp or the swimming pool.
If the child’s condition is already worsening, and a drug holiday takes place on top of this, the child may experience a rebound or relapse, in which the condition looks a lot worse than it did with the drugs.
Do drug holidays matter?
Another session speaker, James Swanson, PhD, who noted that the “emergence of tolerance may limit and eventually undermine initial relative benefit” of stimulants, said there may be instances in which drug holidays may be impractical.
Given the poor adherence to ADHD medication, “most treated ADHD cases stop medication anyway and these patients do not have an opportunity for drug holidays,” he said in an interview.
“If tolerance does emerge, then for long-term treatment the concept of drug holiday seems difficult to evaluate to me,” said Dr. Swanson, director of the Child Development Center at the University of California, Irvine.
Planned medication breaks may not be a good way to evaluate efficacy unless it is performed under “double-blind” conditions, he offered. The MTA used an approach of switching between short periods of time, with and without medication. “We did this to compare medication to placebo and to compare doses of medication to optimize the short-term benefit,” said Dr. Swanson, a coauthor of the MTA study.
Dr. Hechtman receives funding from The Canadian Institutes of Health Research. Dr. Swanson has two patents: (PIXA4), which uses a “time-of-flight” camera to measure growth on infants, and a provisional patent on the mechanism of tolerance to stimulant medication (PATSMTA).
For children with attention-deficit/hyperactivity disorder, taking a weekend or summer break from methylphenidate may have some benefits. A drug “holiday” can help assess whether a drug is still useful and possibly help with drug tolerance, weight gain, and growth suppression. But drug holidays are not without their problems, Lily Hechtman, MD, FRCP, professor at the department of psychiatry, McGill University, Montreal, said during a session at the World Congress on ADHD – Virtual Event.
Ceasing a medication can have repercussions from a health and social standpoint, cautioned Dr. Hechtman, a presenter and moderator of the session, “Unsolved mysteries in the treatment of ADHD with psychostimulants.”
The rate of drug holidays is somewhere between 30% and 40% in ADHD patients. Patients have multiple reasons for taking them, said Dr. Hechtman. The American Academy of Child & Adolescent Psychology as well as the National Institute for Health and Clinical Excellence recommend this method to assess whether a medication is still necessary. Parents may opt for a drug holiday because most would prefer their children to take less medication.
A drug holiday can counteract some of the key side effects of stimulant medication such as decreased appetite and weight loss, and the moodiness and irritability that accompanies the medication, as well as sleep problems.
It may also be used to avoid drug tolerance, the need to increase dosage as medication continues. A 2002 study of 166 children and adolescents treated with methylphenidate revealed that 60% had developed drug tolerance. Drug tolerance increases with duration. “So, the longer the child is on medication, the more likely he or she will develop some drug tolerance,” said Dr. Hechtman.
It is hypothesized that a drug holiday results in the resensitization of the neurons in the brain because they aren’t exposed to the stimulation of dopamine release and dopamine exposure.
The minimum time a patient needs a drug holiday to deal with some drug tolerance is about a month. “Even if you have a drug holiday and your drug tolerance has been decreased, it can reoccur with increasing dosages, once medication resumes” after the holiday, said Dr. Hechtman.
The growth factor
A drug holiday can also address concerns about growth suppression. “Some studies show that drug holidays help with growth suppression and others do not,” said Dr. Hechtman.
The Multimodal Treatment of ADHD (MTA) study, which followed children with ADHD from childhood to adolescence into adulthood, offers some key insights on the effects of treatment on growth.
Over a 10-year period, “you could see that the rate of medication use decreased significantly with time” among participants, said Dr. Hechtman, a coauthor of the MTA research. Only 10% who began the study aged 7-9 years were still using stimulants 10 years later. Looking at short-term effects on growth among these children, those who never went on stimulants to begin with had no growth suppression at all, whereas those who underwent early and consistent treatment experienced the greatest growth suppression.
Comparatively, inconsistently medicated participants had less growth suppression than those who remained on medication. “They were pretty close to the controls,” said Dr. Hechtman.
These patterns continued in a 16-year follow-up, as these patients became adults. Based on the results in the inconsistently treated group, this suggests that drug holidays can limit the effects of growth suppression, at least to a certain extent, said Dr. Hechtman.
Other studies have yielded varying results on the impact of drug holidays on height and weight. “The evidence for the utility of drug holidays for medication side effects is there for decreased appetite and weight, but not so much for decreased height,” summarized Dr. Hechtman.
One recent study of 230 children by James Waxmonsky and colleagues that examined drug holidays on weekends and summers showed that drug holidays did increase weight but interestingly, not height. Older studies Dr. Hechtman cited had inconsistent results on height and weight gain and loss. A 2012 study suggested that drug holidays resulted in a slight improvement in appetite for both weekend and school holidays. But only 9% of the children in the sample (n = 51) saw their appetite return to normal levels.
‘Negative things can happen’
The downside of drug holidays is parents may rationalize that their child is doing fine without the medication, and discontinue it. The process of stopping and starting medication can lead to other problems. “Negative things can happen during drug holidays,” said Dr. Hechtman.
The large variability of doses over the weekend can result in rebound and side effects.
A child may go from a full dose, which could be 50-60 mg of stimulant to zero from Friday to Saturday. As a result they have a lot of rebound on that Saturday. Similarly, they go from zero on Sunday to full dose on the Monday, causing lots of side effects. “Also, they will never have a stable effective dose because of the roller-coaster effect of being on and off the drugs,” she noted.
The lack of consistency and accommodation to the side effects can lead to discontinuation of the medication.
Off medication, the child may be more accident prone or have more injuries. “Their behavior off the medication may be such that it leads to social problems,” Dr. Hechtman continued. Weekend activities that require medication such as homework or school projects, family or religious gatherings, or sports and social activities with family and peers may be affected. If the child is behaving poorly off the medication, they may be expelled from such activities. If it’s a summer drug holiday, they may get kicked out of camp or the swimming pool.
If the child’s condition is already worsening, and a drug holiday takes place on top of this, the child may experience a rebound or relapse, in which the condition looks a lot worse than it did with the drugs.
Do drug holidays matter?
Another session speaker, James Swanson, PhD, who noted that the “emergence of tolerance may limit and eventually undermine initial relative benefit” of stimulants, said there may be instances in which drug holidays may be impractical.
Given the poor adherence to ADHD medication, “most treated ADHD cases stop medication anyway and these patients do not have an opportunity for drug holidays,” he said in an interview.
“If tolerance does emerge, then for long-term treatment the concept of drug holiday seems difficult to evaluate to me,” said Dr. Swanson, director of the Child Development Center at the University of California, Irvine.
Planned medication breaks may not be a good way to evaluate efficacy unless it is performed under “double-blind” conditions, he offered. The MTA used an approach of switching between short periods of time, with and without medication. “We did this to compare medication to placebo and to compare doses of medication to optimize the short-term benefit,” said Dr. Swanson, a coauthor of the MTA study.
Dr. Hechtman receives funding from The Canadian Institutes of Health Research. Dr. Swanson has two patents: (PIXA4), which uses a “time-of-flight” camera to measure growth on infants, and a provisional patent on the mechanism of tolerance to stimulant medication (PATSMTA).
For children with attention-deficit/hyperactivity disorder, taking a weekend or summer break from methylphenidate may have some benefits. A drug “holiday” can help assess whether a drug is still useful and possibly help with drug tolerance, weight gain, and growth suppression. But drug holidays are not without their problems, Lily Hechtman, MD, FRCP, professor at the department of psychiatry, McGill University, Montreal, said during a session at the World Congress on ADHD – Virtual Event.
Ceasing a medication can have repercussions from a health and social standpoint, cautioned Dr. Hechtman, a presenter and moderator of the session, “Unsolved mysteries in the treatment of ADHD with psychostimulants.”
The rate of drug holidays is somewhere between 30% and 40% in ADHD patients. Patients have multiple reasons for taking them, said Dr. Hechtman. The American Academy of Child & Adolescent Psychology as well as the National Institute for Health and Clinical Excellence recommend this method to assess whether a medication is still necessary. Parents may opt for a drug holiday because most would prefer their children to take less medication.
A drug holiday can counteract some of the key side effects of stimulant medication such as decreased appetite and weight loss, and the moodiness and irritability that accompanies the medication, as well as sleep problems.
It may also be used to avoid drug tolerance, the need to increase dosage as medication continues. A 2002 study of 166 children and adolescents treated with methylphenidate revealed that 60% had developed drug tolerance. Drug tolerance increases with duration. “So, the longer the child is on medication, the more likely he or she will develop some drug tolerance,” said Dr. Hechtman.
It is hypothesized that a drug holiday results in the resensitization of the neurons in the brain because they aren’t exposed to the stimulation of dopamine release and dopamine exposure.
The minimum time a patient needs a drug holiday to deal with some drug tolerance is about a month. “Even if you have a drug holiday and your drug tolerance has been decreased, it can reoccur with increasing dosages, once medication resumes” after the holiday, said Dr. Hechtman.
The growth factor
A drug holiday can also address concerns about growth suppression. “Some studies show that drug holidays help with growth suppression and others do not,” said Dr. Hechtman.
The Multimodal Treatment of ADHD (MTA) study, which followed children with ADHD from childhood to adolescence into adulthood, offers some key insights on the effects of treatment on growth.
Over a 10-year period, “you could see that the rate of medication use decreased significantly with time” among participants, said Dr. Hechtman, a coauthor of the MTA research. Only 10% who began the study aged 7-9 years were still using stimulants 10 years later. Looking at short-term effects on growth among these children, those who never went on stimulants to begin with had no growth suppression at all, whereas those who underwent early and consistent treatment experienced the greatest growth suppression.
Comparatively, inconsistently medicated participants had less growth suppression than those who remained on medication. “They were pretty close to the controls,” said Dr. Hechtman.
These patterns continued in a 16-year follow-up, as these patients became adults. Based on the results in the inconsistently treated group, this suggests that drug holidays can limit the effects of growth suppression, at least to a certain extent, said Dr. Hechtman.
Other studies have yielded varying results on the impact of drug holidays on height and weight. “The evidence for the utility of drug holidays for medication side effects is there for decreased appetite and weight, but not so much for decreased height,” summarized Dr. Hechtman.
One recent study of 230 children by James Waxmonsky and colleagues that examined drug holidays on weekends and summers showed that drug holidays did increase weight but interestingly, not height. Older studies Dr. Hechtman cited had inconsistent results on height and weight gain and loss. A 2012 study suggested that drug holidays resulted in a slight improvement in appetite for both weekend and school holidays. But only 9% of the children in the sample (n = 51) saw their appetite return to normal levels.
‘Negative things can happen’
The downside of drug holidays is parents may rationalize that their child is doing fine without the medication, and discontinue it. The process of stopping and starting medication can lead to other problems. “Negative things can happen during drug holidays,” said Dr. Hechtman.
The large variability of doses over the weekend can result in rebound and side effects.
A child may go from a full dose, which could be 50-60 mg of stimulant to zero from Friday to Saturday. As a result they have a lot of rebound on that Saturday. Similarly, they go from zero on Sunday to full dose on the Monday, causing lots of side effects. “Also, they will never have a stable effective dose because of the roller-coaster effect of being on and off the drugs,” she noted.
The lack of consistency and accommodation to the side effects can lead to discontinuation of the medication.
Off medication, the child may be more accident prone or have more injuries. “Their behavior off the medication may be such that it leads to social problems,” Dr. Hechtman continued. Weekend activities that require medication such as homework or school projects, family or religious gatherings, or sports and social activities with family and peers may be affected. If the child is behaving poorly off the medication, they may be expelled from such activities. If it’s a summer drug holiday, they may get kicked out of camp or the swimming pool.
If the child’s condition is already worsening, and a drug holiday takes place on top of this, the child may experience a rebound or relapse, in which the condition looks a lot worse than it did with the drugs.
Do drug holidays matter?
Another session speaker, James Swanson, PhD, who noted that the “emergence of tolerance may limit and eventually undermine initial relative benefit” of stimulants, said there may be instances in which drug holidays may be impractical.
Given the poor adherence to ADHD medication, “most treated ADHD cases stop medication anyway and these patients do not have an opportunity for drug holidays,” he said in an interview.
“If tolerance does emerge, then for long-term treatment the concept of drug holiday seems difficult to evaluate to me,” said Dr. Swanson, director of the Child Development Center at the University of California, Irvine.
Planned medication breaks may not be a good way to evaluate efficacy unless it is performed under “double-blind” conditions, he offered. The MTA used an approach of switching between short periods of time, with and without medication. “We did this to compare medication to placebo and to compare doses of medication to optimize the short-term benefit,” said Dr. Swanson, a coauthor of the MTA study.
Dr. Hechtman receives funding from The Canadian Institutes of Health Research. Dr. Swanson has two patents: (PIXA4), which uses a “time-of-flight” camera to measure growth on infants, and a provisional patent on the mechanism of tolerance to stimulant medication (PATSMTA).
FROM ADHD 2021
Reassuring data on impact of mild COVID-19 on the heart
Six months after mild SARS-CoV-2 infection in a representative health care workforce, no long-term cardiovascular sequelae were detected, compared with a matched SARS-CoV-2 seronegative group.
“Mild COVID-19 left no measurable cardiovascular impact on LV structure, function, scar burden, aortic stiffness, or serum biomarkers,” the researchers reported in an article published online May 8 in JACC: Cardiovascular Imaging.
“We provide societal reassurance and support for the position that screening in asymptomatic individuals following mild disease is not indicated,” first author George Joy, MBBS, University College London, said in presenting the results at EuroCMR, the annual CMR congress of the European Association of Cardiovascular Imaging (EACVI).
Briefing comoderator Leyla Elif Sade, MD, University of Baskent, Ankara, Turkey, said, “This is the hot topic of our time because of obvious reasons and I think [this] study is quite important to avoid unnecessary further testing, surveillance testing, and to avoid a significant burden of health care costs.”
‘Alarming’ early data
Early cardiac magnetic resonance (CMR) studies in patients recovered from mild COVID-19 were “alarming,” Dr. Joy said.
As previously reported, one study showed cardiac abnormalities after mild COVID-19 in up to 78% of patients, with evidence of ongoing myocardial inflammation in 60%. The CMR findings correlated with elevations in troponin T by high-sensitivity assay (hs-TnT).
To investigate further, Dr. Joy and colleagues did a nested case-control study within the COVIDsortium, a prospective study of 731 health care workers from three London hospitals who underwent weekly symptom, polymerase chain reaction, and serology assessment over 4 months during the first wave of the pandemic.
A total of 157 (21.5%) participants seroconverted during the study period.
Six months after infection, 74 seropositive (median age, 39; 62% women) and 75 age-, sex-, and ethnicity-matched seronegative controls underwent cardiovascular phenotyping (comprehensive phantom-calibrated CMR and blood biomarkers). The analysis was blinded, using objective artificial intelligence analytics when available.
The results showed no statistically significant differences between seropositive and seronegative participants in cardiac structure (left ventricular volumes, mass, atrial area), function (ejection fraction, global longitudinal shortening, aortic distensibility), tissue characterization (T1, T2, extracellular volume fraction mapping, late gadolinium enhancement) or biomarkers (troponin, N-terminal pro–B-type natriuretic peptide).
Cardiovascular abnormalities were no more common in seropositive than seronegative otherwise healthy health care workers 6 months post mild SARS-CoV-2 infection. Measured abnormalities were “evenly distributed between both groups,” Dr. Joy said.
Therefore, it’s “important to reassure patients with mild SARS-CoV-2 infection regarding its cardiovascular effects,” Dr. Joy and colleagues concluded.
Limitations and caveats
They caution, however, that the study provides insight only into the short- to medium-term sequelae of patients aged 18-69 with mild COVID-19 who did not require hospitalization and had low numbers of comorbidities.
The study does not address the cardiovascular effects after severe COVID-19 infection requiring hospitalization or in those with multiple comorbid conditions, they noted. It also does not prove that apparently mild SARS-CoV-2 never causes chronic myocarditis.
“The study design would not distinguish between people who had sustained completely healed myocarditis and pericarditis and those in whom the heart had never been affected,” the researchers noted.
They pointed to a recent cross-sectional study of athletes 1-month post mild COVID-19 that found significant pericardial involvement (late enhancement and/or pericardial effusion), although no baseline pre-COVID-19 imaging was performed. In the current study at 6 months post infection the pericardium was normal.
The coauthors of a linked editorial say this study provides “welcome, reassuring information that in healthy individuals who experience mild infection with COVID-19, persisting evidence of cardiovascular complications is very uncommon. The results do not support cardiovascular screening in individuals with mild or asymptomatic infection with COVID-19.”
Colin Berry, PhD, and Kenneth Mangion, PhD, both from University of Glasgow, cautioned that the population is restricted to health care workers; therefore, the findings may not necessarily be generalized to a community population .
“Healthcare workers do not reflect the population of individuals most clinically affected by COVID-19 illness. The severity of acute COVID-19 infection is greatest in older individuals and those with preexisting health problems. Healthcare workers are not representative of the wider, unselected, at-risk, community population,” they pointed out.
Cardiovascular risk factors and concomitant health problems (heart and respiratory disease) may be more common in the community than in health care workers, and prior studies have highlighted their potential impact for disease pathogenesis in COVID-19.
Dr. Berry and Dr. Mangion also noted that women made up nearly two-thirds of the seropositive group. This may reflect a selection bias or may naturally reflect the fact that proportionately more women are asymptomatic or have milder forms of illness, whereas severe SARS-CoV-2 infection requiring hospitalization affects men to a greater degree.
COVIDsortium funding was donated by individuals, charitable trusts, and corporations including Goldman Sachs, Citadel and Citadel Securities, The Guy Foundation, GW Pharmaceuticals, Kusuma Trust, and Jagclif Charitable Trust, and enabled by Barts Charity with support from UCLH Charity. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Six months after mild SARS-CoV-2 infection in a representative health care workforce, no long-term cardiovascular sequelae were detected, compared with a matched SARS-CoV-2 seronegative group.
“Mild COVID-19 left no measurable cardiovascular impact on LV structure, function, scar burden, aortic stiffness, or serum biomarkers,” the researchers reported in an article published online May 8 in JACC: Cardiovascular Imaging.
“We provide societal reassurance and support for the position that screening in asymptomatic individuals following mild disease is not indicated,” first author George Joy, MBBS, University College London, said in presenting the results at EuroCMR, the annual CMR congress of the European Association of Cardiovascular Imaging (EACVI).
Briefing comoderator Leyla Elif Sade, MD, University of Baskent, Ankara, Turkey, said, “This is the hot topic of our time because of obvious reasons and I think [this] study is quite important to avoid unnecessary further testing, surveillance testing, and to avoid a significant burden of health care costs.”
‘Alarming’ early data
Early cardiac magnetic resonance (CMR) studies in patients recovered from mild COVID-19 were “alarming,” Dr. Joy said.
As previously reported, one study showed cardiac abnormalities after mild COVID-19 in up to 78% of patients, with evidence of ongoing myocardial inflammation in 60%. The CMR findings correlated with elevations in troponin T by high-sensitivity assay (hs-TnT).
To investigate further, Dr. Joy and colleagues did a nested case-control study within the COVIDsortium, a prospective study of 731 health care workers from three London hospitals who underwent weekly symptom, polymerase chain reaction, and serology assessment over 4 months during the first wave of the pandemic.
A total of 157 (21.5%) participants seroconverted during the study period.
Six months after infection, 74 seropositive (median age, 39; 62% women) and 75 age-, sex-, and ethnicity-matched seronegative controls underwent cardiovascular phenotyping (comprehensive phantom-calibrated CMR and blood biomarkers). The analysis was blinded, using objective artificial intelligence analytics when available.
The results showed no statistically significant differences between seropositive and seronegative participants in cardiac structure (left ventricular volumes, mass, atrial area), function (ejection fraction, global longitudinal shortening, aortic distensibility), tissue characterization (T1, T2, extracellular volume fraction mapping, late gadolinium enhancement) or biomarkers (troponin, N-terminal pro–B-type natriuretic peptide).
Cardiovascular abnormalities were no more common in seropositive than seronegative otherwise healthy health care workers 6 months post mild SARS-CoV-2 infection. Measured abnormalities were “evenly distributed between both groups,” Dr. Joy said.
Therefore, it’s “important to reassure patients with mild SARS-CoV-2 infection regarding its cardiovascular effects,” Dr. Joy and colleagues concluded.
Limitations and caveats
They caution, however, that the study provides insight only into the short- to medium-term sequelae of patients aged 18-69 with mild COVID-19 who did not require hospitalization and had low numbers of comorbidities.
The study does not address the cardiovascular effects after severe COVID-19 infection requiring hospitalization or in those with multiple comorbid conditions, they noted. It also does not prove that apparently mild SARS-CoV-2 never causes chronic myocarditis.
“The study design would not distinguish between people who had sustained completely healed myocarditis and pericarditis and those in whom the heart had never been affected,” the researchers noted.
They pointed to a recent cross-sectional study of athletes 1-month post mild COVID-19 that found significant pericardial involvement (late enhancement and/or pericardial effusion), although no baseline pre-COVID-19 imaging was performed. In the current study at 6 months post infection the pericardium was normal.
The coauthors of a linked editorial say this study provides “welcome, reassuring information that in healthy individuals who experience mild infection with COVID-19, persisting evidence of cardiovascular complications is very uncommon. The results do not support cardiovascular screening in individuals with mild or asymptomatic infection with COVID-19.”
Colin Berry, PhD, and Kenneth Mangion, PhD, both from University of Glasgow, cautioned that the population is restricted to health care workers; therefore, the findings may not necessarily be generalized to a community population .
“Healthcare workers do not reflect the population of individuals most clinically affected by COVID-19 illness. The severity of acute COVID-19 infection is greatest in older individuals and those with preexisting health problems. Healthcare workers are not representative of the wider, unselected, at-risk, community population,” they pointed out.
Cardiovascular risk factors and concomitant health problems (heart and respiratory disease) may be more common in the community than in health care workers, and prior studies have highlighted their potential impact for disease pathogenesis in COVID-19.
Dr. Berry and Dr. Mangion also noted that women made up nearly two-thirds of the seropositive group. This may reflect a selection bias or may naturally reflect the fact that proportionately more women are asymptomatic or have milder forms of illness, whereas severe SARS-CoV-2 infection requiring hospitalization affects men to a greater degree.
COVIDsortium funding was donated by individuals, charitable trusts, and corporations including Goldman Sachs, Citadel and Citadel Securities, The Guy Foundation, GW Pharmaceuticals, Kusuma Trust, and Jagclif Charitable Trust, and enabled by Barts Charity with support from UCLH Charity. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Six months after mild SARS-CoV-2 infection in a representative health care workforce, no long-term cardiovascular sequelae were detected, compared with a matched SARS-CoV-2 seronegative group.
“Mild COVID-19 left no measurable cardiovascular impact on LV structure, function, scar burden, aortic stiffness, or serum biomarkers,” the researchers reported in an article published online May 8 in JACC: Cardiovascular Imaging.
“We provide societal reassurance and support for the position that screening in asymptomatic individuals following mild disease is not indicated,” first author George Joy, MBBS, University College London, said in presenting the results at EuroCMR, the annual CMR congress of the European Association of Cardiovascular Imaging (EACVI).
Briefing comoderator Leyla Elif Sade, MD, University of Baskent, Ankara, Turkey, said, “This is the hot topic of our time because of obvious reasons and I think [this] study is quite important to avoid unnecessary further testing, surveillance testing, and to avoid a significant burden of health care costs.”
‘Alarming’ early data
Early cardiac magnetic resonance (CMR) studies in patients recovered from mild COVID-19 were “alarming,” Dr. Joy said.
As previously reported, one study showed cardiac abnormalities after mild COVID-19 in up to 78% of patients, with evidence of ongoing myocardial inflammation in 60%. The CMR findings correlated with elevations in troponin T by high-sensitivity assay (hs-TnT).
To investigate further, Dr. Joy and colleagues did a nested case-control study within the COVIDsortium, a prospective study of 731 health care workers from three London hospitals who underwent weekly symptom, polymerase chain reaction, and serology assessment over 4 months during the first wave of the pandemic.
A total of 157 (21.5%) participants seroconverted during the study period.
Six months after infection, 74 seropositive (median age, 39; 62% women) and 75 age-, sex-, and ethnicity-matched seronegative controls underwent cardiovascular phenotyping (comprehensive phantom-calibrated CMR and blood biomarkers). The analysis was blinded, using objective artificial intelligence analytics when available.
The results showed no statistically significant differences between seropositive and seronegative participants in cardiac structure (left ventricular volumes, mass, atrial area), function (ejection fraction, global longitudinal shortening, aortic distensibility), tissue characterization (T1, T2, extracellular volume fraction mapping, late gadolinium enhancement) or biomarkers (troponin, N-terminal pro–B-type natriuretic peptide).
Cardiovascular abnormalities were no more common in seropositive than seronegative otherwise healthy health care workers 6 months post mild SARS-CoV-2 infection. Measured abnormalities were “evenly distributed between both groups,” Dr. Joy said.
Therefore, it’s “important to reassure patients with mild SARS-CoV-2 infection regarding its cardiovascular effects,” Dr. Joy and colleagues concluded.
Limitations and caveats
They caution, however, that the study provides insight only into the short- to medium-term sequelae of patients aged 18-69 with mild COVID-19 who did not require hospitalization and had low numbers of comorbidities.
The study does not address the cardiovascular effects after severe COVID-19 infection requiring hospitalization or in those with multiple comorbid conditions, they noted. It also does not prove that apparently mild SARS-CoV-2 never causes chronic myocarditis.
“The study design would not distinguish between people who had sustained completely healed myocarditis and pericarditis and those in whom the heart had never been affected,” the researchers noted.
They pointed to a recent cross-sectional study of athletes 1-month post mild COVID-19 that found significant pericardial involvement (late enhancement and/or pericardial effusion), although no baseline pre-COVID-19 imaging was performed. In the current study at 6 months post infection the pericardium was normal.
The coauthors of a linked editorial say this study provides “welcome, reassuring information that in healthy individuals who experience mild infection with COVID-19, persisting evidence of cardiovascular complications is very uncommon. The results do not support cardiovascular screening in individuals with mild or asymptomatic infection with COVID-19.”
Colin Berry, PhD, and Kenneth Mangion, PhD, both from University of Glasgow, cautioned that the population is restricted to health care workers; therefore, the findings may not necessarily be generalized to a community population .
“Healthcare workers do not reflect the population of individuals most clinically affected by COVID-19 illness. The severity of acute COVID-19 infection is greatest in older individuals and those with preexisting health problems. Healthcare workers are not representative of the wider, unselected, at-risk, community population,” they pointed out.
Cardiovascular risk factors and concomitant health problems (heart and respiratory disease) may be more common in the community than in health care workers, and prior studies have highlighted their potential impact for disease pathogenesis in COVID-19.
Dr. Berry and Dr. Mangion also noted that women made up nearly two-thirds of the seropositive group. This may reflect a selection bias or may naturally reflect the fact that proportionately more women are asymptomatic or have milder forms of illness, whereas severe SARS-CoV-2 infection requiring hospitalization affects men to a greater degree.
COVIDsortium funding was donated by individuals, charitable trusts, and corporations including Goldman Sachs, Citadel and Citadel Securities, The Guy Foundation, GW Pharmaceuticals, Kusuma Trust, and Jagclif Charitable Trust, and enabled by Barts Charity with support from UCLH Charity. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
What to know about COVID-19 vaccines and skin reactions
The good news is that these side effects tend to be minor and vanish within a few days, Esther Freeman, MD, PhD, said in a presentation at the American Academy of Dermatology Virtual Meeting Experience.
“The reality is actually very reassuring,” Dr. Freeman said, especially in light of what is currently known about when the rashes occur and how anaphylaxis is extremely uncommon. Now, she added, dermatologists can tell patients who had reactions to their initial vaccination that “we know you had this big reaction, and we know that it was upsetting and uncomfortable. But it may not happen the second time around. And if it does, [the reaction is] probably going to be smaller.”
Dr. Freeman, associate professor of dermatology at Harvard Medical School, Boston, highlighted a study published in the Journal of the American Academy of Dermatology that she coauthored with dermatologists across the United States. The researchers tracked 414 cutaneous reactions to the Moderna (83%) and Pfizer (17%) COVID-19 vaccines in a group of patients, which was 90% female, 78% White, and mostly from the United States. Their average age was 44 years. The cases were reported to the AAD–International League of Dermatological Societies registry of COVID-19 cutaneous manifestations.
While most were women, “it’s a little hard to know if this is really going to end up being a true finding,” said Dr. Freeman, the registry’s principal investigator and a member of the AAD’s COVID-19 Ad Hoc Task Force. “If you think about who got vaccinated early, it was health care providers, and the American health care workforce is over 70% female. So I think there’s a little bit of bias here. There may also be a bias because women may be slightly more likely to report or go to their health care provider for a rash.”
Delayed large local reactions were the most common, accounting for 66% (175 cases) of the 267 skin reactions reported after the first Moderna vaccine dose and 30% (31 cases) of the 102 reactions reported after the second dose. These reactions represented 15% (5 cases) of the 34 skin reactions reported after the first Pfizer vaccine dose and 18% (7 cases) of the 40 reactions after the second dose.
There are two peaks with that first dose, Dr. Freeman said. “There’s a peak around day 2 or 3. And there’s another peak around day 7 or 8 with some of these reactions. Only 27% who had a reaction with the first dose had the same reaction with the second.” She added that these reactions “are not cellulitis and don’t require antibiotics.”
Other more common reactions included local injection-site reactions (swelling, erythema, and pain), urticaria (after 24 hours in almost all cases, occurring at a higher rate in patients who received the Pfizer vaccine), and morbilliform eruptions.
Dr. Freeman said that patients may experience redness and swelling in the hands and feet that can be “very uncomfortable.” She described one patient “who was having a hard time actually closing his fist, just because of the amount of swelling and redness in his hand. It did resolve, and it’s important to reassure your patients it will go away.”
According to this study, less common reports of other cutaneous findings with both vaccines included 9 reports of swelling at the site of cosmetic fillers, 8 reports of pernio/chilblains, 10 reports of varicella zoster, 4 reports of herpes simplex flares, 4 pityriasis rosea–like reactions, and 4 rashes in infants of vaccinated breastfeeding mothers.
The study noted that “patients responded well to topical corticosteroids, oral antihistamines, and/or pain-relieving medications. These reactions resolved after a median of 3-4 days.”
It’s important to understand that none of the patients developed anaphylaxis after the second dose even if they’d had a reaction to the first dose, Dr. Freeman said. “But I should point out that we’re talking about reactions that have started more than 4 hours after the vaccine. If a rash such as a urticaria specifically starts within 4 hours of vaccination, that’s in a different category. Those are considered more immediate allergic reactions, and those patients need to be seen by allergy before a second dose.”
Dr. Freeman added that “it’s really interesting to think about how our bodies are really reacting to the vaccine in a way that’s mimicking our body’s reactions to COVID-19.” For example, some patients who got vaccinated developed chilblains similar to the “COVID toes” described in infected patients, apparently as part of the body’s immune response to the virus. “We’ve seen this in patients who actually had COVID and had prior COVID toes and then actually got a flare with their vaccine. And then we’ve also seen it in patients who never had COVID.”
In regard to general advice for patients, she said, “I do still encourage my patients who previously had COVID to go ahead and get the vaccine even if they had a skin manifestation with COVID.”
Shari Lipner, MD, PhD, associate professor of clinical dermatology, Weill Cornell Medicine, New York, said she has have seen only a handful of cases of delayed large local reactions and local injection site reactions after COVID-19 vaccination. “I have seen a significant number of cases of acute urticaria following the first and second doses,” she said in an interview. “However, it is important to keep in mind that we cannot determine cause and effect for the cases of acute urticaria. They may or may not be vaccine related.”
Fortunately, none of the adverse effects she’s seen have been severe. “It is important that dermatologists educate the public and their patients that most people do not develop any skin reaction in response to the vaccine,” she said. In the minority who do, “reactions tend to be mild and are not life-threatening. Many of these skin reactions resolve on their own without treatment.”
She added that “patients with pernio/chilblains or herpes zoster following vaccination should be referred by a board-certified dermatologist for prompt treatment and to avoid sequelae.”
‘COVID vaccine arm’
Delayed local reactions to the Moderna vaccine were also described in a report published online on May 12, 2021, in JAMA Dermatology, after the AAD meeting, in 16 patients referred to the Yale New Haven (Conn.) Hospital Dermatology service who experienced delayed localized cutaneous hypersensitivity reactions a median of 7 days after receiving the vaccine (range, 2-12 days), from Jan. 20 to Feb. 12, 2021. No such cases were reported in Pfizer vaccine recipients.
Of the 16 patients, whose median age was 38 years and who were mostly women, 15 developed the reaction after the first dose, described as “pruritic and variably painful erythematous reactions near the injection site,” which lasted a median of 5 days (range, 1-21 days). After the second dose, 12 of the 16 patients developed injection-site reactions (including one patient who had no reaction after dose 1), a median of 2 days after the vaccine was administered (range, 0-5 days). Histologic results of a biopsy in one patient with a reaction to the second dose “ demonstrated mild predominantly perivascular and focal interstitial mixed infiltrate with lymphocytes and eosinophils consistent with a dermal hypersensitivity reaction,” wrote Alicia J. Little, MD, PhD, of the department of dermatology, Yale University, New Haven, and coauthors.
Compared with immediate hypersensitivity reactions, occurring within 4 hours of vaccination, such as anaphylaxis and urticaria, they concluded that “these delayed localized hypersensitivity reactions are not a contraindication to subsequent vaccination,” and they proposed that they be named “COVID vaccine arm.”
Dr. Freeman reported no disclosures. Dr. Lipner also had no relevant disclosures. Dr. Little reported receiving a grant from the National Center for Advancing Translational Science and a Women’s Health Career Development Award from the Dermatology Foundation while the study was conducted; another author reported equity in Johnson & Johnson in his spouse’s retirement fund outside the submitted work.
The good news is that these side effects tend to be minor and vanish within a few days, Esther Freeman, MD, PhD, said in a presentation at the American Academy of Dermatology Virtual Meeting Experience.
“The reality is actually very reassuring,” Dr. Freeman said, especially in light of what is currently known about when the rashes occur and how anaphylaxis is extremely uncommon. Now, she added, dermatologists can tell patients who had reactions to their initial vaccination that “we know you had this big reaction, and we know that it was upsetting and uncomfortable. But it may not happen the second time around. And if it does, [the reaction is] probably going to be smaller.”
Dr. Freeman, associate professor of dermatology at Harvard Medical School, Boston, highlighted a study published in the Journal of the American Academy of Dermatology that she coauthored with dermatologists across the United States. The researchers tracked 414 cutaneous reactions to the Moderna (83%) and Pfizer (17%) COVID-19 vaccines in a group of patients, which was 90% female, 78% White, and mostly from the United States. Their average age was 44 years. The cases were reported to the AAD–International League of Dermatological Societies registry of COVID-19 cutaneous manifestations.
While most were women, “it’s a little hard to know if this is really going to end up being a true finding,” said Dr. Freeman, the registry’s principal investigator and a member of the AAD’s COVID-19 Ad Hoc Task Force. “If you think about who got vaccinated early, it was health care providers, and the American health care workforce is over 70% female. So I think there’s a little bit of bias here. There may also be a bias because women may be slightly more likely to report or go to their health care provider for a rash.”
Delayed large local reactions were the most common, accounting for 66% (175 cases) of the 267 skin reactions reported after the first Moderna vaccine dose and 30% (31 cases) of the 102 reactions reported after the second dose. These reactions represented 15% (5 cases) of the 34 skin reactions reported after the first Pfizer vaccine dose and 18% (7 cases) of the 40 reactions after the second dose.
There are two peaks with that first dose, Dr. Freeman said. “There’s a peak around day 2 or 3. And there’s another peak around day 7 or 8 with some of these reactions. Only 27% who had a reaction with the first dose had the same reaction with the second.” She added that these reactions “are not cellulitis and don’t require antibiotics.”
Other more common reactions included local injection-site reactions (swelling, erythema, and pain), urticaria (after 24 hours in almost all cases, occurring at a higher rate in patients who received the Pfizer vaccine), and morbilliform eruptions.
Dr. Freeman said that patients may experience redness and swelling in the hands and feet that can be “very uncomfortable.” She described one patient “who was having a hard time actually closing his fist, just because of the amount of swelling and redness in his hand. It did resolve, and it’s important to reassure your patients it will go away.”
According to this study, less common reports of other cutaneous findings with both vaccines included 9 reports of swelling at the site of cosmetic fillers, 8 reports of pernio/chilblains, 10 reports of varicella zoster, 4 reports of herpes simplex flares, 4 pityriasis rosea–like reactions, and 4 rashes in infants of vaccinated breastfeeding mothers.
The study noted that “patients responded well to topical corticosteroids, oral antihistamines, and/or pain-relieving medications. These reactions resolved after a median of 3-4 days.”
It’s important to understand that none of the patients developed anaphylaxis after the second dose even if they’d had a reaction to the first dose, Dr. Freeman said. “But I should point out that we’re talking about reactions that have started more than 4 hours after the vaccine. If a rash such as a urticaria specifically starts within 4 hours of vaccination, that’s in a different category. Those are considered more immediate allergic reactions, and those patients need to be seen by allergy before a second dose.”
Dr. Freeman added that “it’s really interesting to think about how our bodies are really reacting to the vaccine in a way that’s mimicking our body’s reactions to COVID-19.” For example, some patients who got vaccinated developed chilblains similar to the “COVID toes” described in infected patients, apparently as part of the body’s immune response to the virus. “We’ve seen this in patients who actually had COVID and had prior COVID toes and then actually got a flare with their vaccine. And then we’ve also seen it in patients who never had COVID.”
In regard to general advice for patients, she said, “I do still encourage my patients who previously had COVID to go ahead and get the vaccine even if they had a skin manifestation with COVID.”
Shari Lipner, MD, PhD, associate professor of clinical dermatology, Weill Cornell Medicine, New York, said she has have seen only a handful of cases of delayed large local reactions and local injection site reactions after COVID-19 vaccination. “I have seen a significant number of cases of acute urticaria following the first and second doses,” she said in an interview. “However, it is important to keep in mind that we cannot determine cause and effect for the cases of acute urticaria. They may or may not be vaccine related.”
Fortunately, none of the adverse effects she’s seen have been severe. “It is important that dermatologists educate the public and their patients that most people do not develop any skin reaction in response to the vaccine,” she said. In the minority who do, “reactions tend to be mild and are not life-threatening. Many of these skin reactions resolve on their own without treatment.”
She added that “patients with pernio/chilblains or herpes zoster following vaccination should be referred by a board-certified dermatologist for prompt treatment and to avoid sequelae.”
‘COVID vaccine arm’
Delayed local reactions to the Moderna vaccine were also described in a report published online on May 12, 2021, in JAMA Dermatology, after the AAD meeting, in 16 patients referred to the Yale New Haven (Conn.) Hospital Dermatology service who experienced delayed localized cutaneous hypersensitivity reactions a median of 7 days after receiving the vaccine (range, 2-12 days), from Jan. 20 to Feb. 12, 2021. No such cases were reported in Pfizer vaccine recipients.
Of the 16 patients, whose median age was 38 years and who were mostly women, 15 developed the reaction after the first dose, described as “pruritic and variably painful erythematous reactions near the injection site,” which lasted a median of 5 days (range, 1-21 days). After the second dose, 12 of the 16 patients developed injection-site reactions (including one patient who had no reaction after dose 1), a median of 2 days after the vaccine was administered (range, 0-5 days). Histologic results of a biopsy in one patient with a reaction to the second dose “ demonstrated mild predominantly perivascular and focal interstitial mixed infiltrate with lymphocytes and eosinophils consistent with a dermal hypersensitivity reaction,” wrote Alicia J. Little, MD, PhD, of the department of dermatology, Yale University, New Haven, and coauthors.
Compared with immediate hypersensitivity reactions, occurring within 4 hours of vaccination, such as anaphylaxis and urticaria, they concluded that “these delayed localized hypersensitivity reactions are not a contraindication to subsequent vaccination,” and they proposed that they be named “COVID vaccine arm.”
Dr. Freeman reported no disclosures. Dr. Lipner also had no relevant disclosures. Dr. Little reported receiving a grant from the National Center for Advancing Translational Science and a Women’s Health Career Development Award from the Dermatology Foundation while the study was conducted; another author reported equity in Johnson & Johnson in his spouse’s retirement fund outside the submitted work.
The good news is that these side effects tend to be minor and vanish within a few days, Esther Freeman, MD, PhD, said in a presentation at the American Academy of Dermatology Virtual Meeting Experience.
“The reality is actually very reassuring,” Dr. Freeman said, especially in light of what is currently known about when the rashes occur and how anaphylaxis is extremely uncommon. Now, she added, dermatologists can tell patients who had reactions to their initial vaccination that “we know you had this big reaction, and we know that it was upsetting and uncomfortable. But it may not happen the second time around. And if it does, [the reaction is] probably going to be smaller.”
Dr. Freeman, associate professor of dermatology at Harvard Medical School, Boston, highlighted a study published in the Journal of the American Academy of Dermatology that she coauthored with dermatologists across the United States. The researchers tracked 414 cutaneous reactions to the Moderna (83%) and Pfizer (17%) COVID-19 vaccines in a group of patients, which was 90% female, 78% White, and mostly from the United States. Their average age was 44 years. The cases were reported to the AAD–International League of Dermatological Societies registry of COVID-19 cutaneous manifestations.
While most were women, “it’s a little hard to know if this is really going to end up being a true finding,” said Dr. Freeman, the registry’s principal investigator and a member of the AAD’s COVID-19 Ad Hoc Task Force. “If you think about who got vaccinated early, it was health care providers, and the American health care workforce is over 70% female. So I think there’s a little bit of bias here. There may also be a bias because women may be slightly more likely to report or go to their health care provider for a rash.”
Delayed large local reactions were the most common, accounting for 66% (175 cases) of the 267 skin reactions reported after the first Moderna vaccine dose and 30% (31 cases) of the 102 reactions reported after the second dose. These reactions represented 15% (5 cases) of the 34 skin reactions reported after the first Pfizer vaccine dose and 18% (7 cases) of the 40 reactions after the second dose.
There are two peaks with that first dose, Dr. Freeman said. “There’s a peak around day 2 or 3. And there’s another peak around day 7 or 8 with some of these reactions. Only 27% who had a reaction with the first dose had the same reaction with the second.” She added that these reactions “are not cellulitis and don’t require antibiotics.”
Other more common reactions included local injection-site reactions (swelling, erythema, and pain), urticaria (after 24 hours in almost all cases, occurring at a higher rate in patients who received the Pfizer vaccine), and morbilliform eruptions.
Dr. Freeman said that patients may experience redness and swelling in the hands and feet that can be “very uncomfortable.” She described one patient “who was having a hard time actually closing his fist, just because of the amount of swelling and redness in his hand. It did resolve, and it’s important to reassure your patients it will go away.”
According to this study, less common reports of other cutaneous findings with both vaccines included 9 reports of swelling at the site of cosmetic fillers, 8 reports of pernio/chilblains, 10 reports of varicella zoster, 4 reports of herpes simplex flares, 4 pityriasis rosea–like reactions, and 4 rashes in infants of vaccinated breastfeeding mothers.
The study noted that “patients responded well to topical corticosteroids, oral antihistamines, and/or pain-relieving medications. These reactions resolved after a median of 3-4 days.”
It’s important to understand that none of the patients developed anaphylaxis after the second dose even if they’d had a reaction to the first dose, Dr. Freeman said. “But I should point out that we’re talking about reactions that have started more than 4 hours after the vaccine. If a rash such as a urticaria specifically starts within 4 hours of vaccination, that’s in a different category. Those are considered more immediate allergic reactions, and those patients need to be seen by allergy before a second dose.”
Dr. Freeman added that “it’s really interesting to think about how our bodies are really reacting to the vaccine in a way that’s mimicking our body’s reactions to COVID-19.” For example, some patients who got vaccinated developed chilblains similar to the “COVID toes” described in infected patients, apparently as part of the body’s immune response to the virus. “We’ve seen this in patients who actually had COVID and had prior COVID toes and then actually got a flare with their vaccine. And then we’ve also seen it in patients who never had COVID.”
In regard to general advice for patients, she said, “I do still encourage my patients who previously had COVID to go ahead and get the vaccine even if they had a skin manifestation with COVID.”
Shari Lipner, MD, PhD, associate professor of clinical dermatology, Weill Cornell Medicine, New York, said she has have seen only a handful of cases of delayed large local reactions and local injection site reactions after COVID-19 vaccination. “I have seen a significant number of cases of acute urticaria following the first and second doses,” she said in an interview. “However, it is important to keep in mind that we cannot determine cause and effect for the cases of acute urticaria. They may or may not be vaccine related.”
Fortunately, none of the adverse effects she’s seen have been severe. “It is important that dermatologists educate the public and their patients that most people do not develop any skin reaction in response to the vaccine,” she said. In the minority who do, “reactions tend to be mild and are not life-threatening. Many of these skin reactions resolve on their own without treatment.”
She added that “patients with pernio/chilblains or herpes zoster following vaccination should be referred by a board-certified dermatologist for prompt treatment and to avoid sequelae.”
‘COVID vaccine arm’
Delayed local reactions to the Moderna vaccine were also described in a report published online on May 12, 2021, in JAMA Dermatology, after the AAD meeting, in 16 patients referred to the Yale New Haven (Conn.) Hospital Dermatology service who experienced delayed localized cutaneous hypersensitivity reactions a median of 7 days after receiving the vaccine (range, 2-12 days), from Jan. 20 to Feb. 12, 2021. No such cases were reported in Pfizer vaccine recipients.
Of the 16 patients, whose median age was 38 years and who were mostly women, 15 developed the reaction after the first dose, described as “pruritic and variably painful erythematous reactions near the injection site,” which lasted a median of 5 days (range, 1-21 days). After the second dose, 12 of the 16 patients developed injection-site reactions (including one patient who had no reaction after dose 1), a median of 2 days after the vaccine was administered (range, 0-5 days). Histologic results of a biopsy in one patient with a reaction to the second dose “ demonstrated mild predominantly perivascular and focal interstitial mixed infiltrate with lymphocytes and eosinophils consistent with a dermal hypersensitivity reaction,” wrote Alicia J. Little, MD, PhD, of the department of dermatology, Yale University, New Haven, and coauthors.
Compared with immediate hypersensitivity reactions, occurring within 4 hours of vaccination, such as anaphylaxis and urticaria, they concluded that “these delayed localized hypersensitivity reactions are not a contraindication to subsequent vaccination,” and they proposed that they be named “COVID vaccine arm.”
Dr. Freeman reported no disclosures. Dr. Lipner also had no relevant disclosures. Dr. Little reported receiving a grant from the National Center for Advancing Translational Science and a Women’s Health Career Development Award from the Dermatology Foundation while the study was conducted; another author reported equity in Johnson & Johnson in his spouse’s retirement fund outside the submitted work.
FROM AAD VMX 2021
States ready plans to get Pfizer COVID vaccine to younger teens
after the Food and Drug Administration authorized its use in this age group May 10.
Some states hope to start the vaccinations as early as May 13, officials said at an Association of State and Territorial Health Officials news conference.
There are, however, two more steps before shots can reach younger arms. On May 12, the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices is expected to recommend use of the vaccine in this age group. Then CDC Director Rochelle Walensky, MD, must make a final decision to begin vaccinating 12- to 15-year-olds.
Some hoping to start this week
Both the CDC panel and Dr. Walensky are expected to sign off on the vaccine’s use. States have been making plans on how to tailor the vaccination message not just to the patient this time, but to parents and guardians as well, some of whom are hesitant to consent.
Some schools, assuming approval May 12, are ready to start vaccinating in cafeterias and gyms.
Anne Zink, MD, president-elect of the Association of State and Territorial Health Officials and Alaska chief medical officer, told reporters that many of her state’s boroughs and districts have booked in-person vaccines for their schools May 12 as the state has dismissal for summer as early as this week.
Maine is readying four types of distribution sites for the vaccines: primary care offices, Walgreen’s and CVS pharmacies, mass vaccination sites, and schools, said Nirav Shah, MD, current ASTHO president and director of the Maine Center for Disease Control and Prevention.
Starting later this week, he said, the state hopes to host large vaccination clinics for people age 12 and over.
Eliminating barriers
States are working to break down barriers through education and improving access.
In Alaska, many of the drive-through evening vaccination sites are being changed to Pfizer sites so parents just getting off work can take their kids.
It’s also important to get young people to speak to their peers about the importance of vaccines, she said. Some teen groups in Alaska are hosting Zoom calls where they share with children and families why they chose to get vaccinated.
In Maine, Dr. Shah said, “the notion of informed consent applies with equal force to adults as it does with adolescents.” But at least in Maine, it is not required that a parent be on site and present during the vaccination itself.
A parent could sign a form allowing the child to be vaccinated in a school-based clinic. Maine also allows verbal consent so a parent can give consent over the phone, Dr. Shah said.
Dividing vaccine trays
Vaccines going to pediatrician and family medicine offices presents a challenge in that smaller numbers of doses are needed for those venues than at large vaccination sites that get trays of 1,170 Pfizer doses each.
Dr. Shah says states have been talking with federal authorities on the need for smaller packaging.
“Breaking the trays up into smaller lot sizes takes a fair amount of effort,” Dr. Shah said. “We understand that later this month the lot size will be going down to 450.”
But even that will be too much for small offices, he said.
Similarly, an effort is being made in Maine to make sure doctors’ offices are not limited by their refrigeration capabilities. The Pfizer vaccine must be kept at ultra-cold temperatures that many primary care doctors’ offices may not have.
“If they need a cool cube with dry ice, we can furnish that to them,” Dr. Shah said.
Should they be mandated?
Dr. Zink said Alaska generally has high acceptance for recommendations around COVID-19 and has no plans to mandate the COVID-19 vaccines for children.
Umair A. Shah, MD, secretary of health at the Washington State Department of Health, said, “Our number one ability to get people vaccinated is for them to be encouraged to do so, to be incentivized to do so, to do everything we can to make the vaccine choice the easy choice,” including eliminating language, cultural and access barriers.
However, he said, “in higher education, University of Washington and Washington State University have indicated they are going to require COVID vaccines for kids to come back to school. I do think that is something that is increasingly being looked at.”
Though the messages will be tailored differently across the states the bottom line will be the same, Dr. Shah said: The vaccines work and they are safe.
But most critically, “Vaccines are our pathway to moving forward and once and for all ending this pandemic,” he said.
A version of this article first appeared on Medscape.com.
after the Food and Drug Administration authorized its use in this age group May 10.
Some states hope to start the vaccinations as early as May 13, officials said at an Association of State and Territorial Health Officials news conference.
There are, however, two more steps before shots can reach younger arms. On May 12, the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices is expected to recommend use of the vaccine in this age group. Then CDC Director Rochelle Walensky, MD, must make a final decision to begin vaccinating 12- to 15-year-olds.
Some hoping to start this week
Both the CDC panel and Dr. Walensky are expected to sign off on the vaccine’s use. States have been making plans on how to tailor the vaccination message not just to the patient this time, but to parents and guardians as well, some of whom are hesitant to consent.
Some schools, assuming approval May 12, are ready to start vaccinating in cafeterias and gyms.
Anne Zink, MD, president-elect of the Association of State and Territorial Health Officials and Alaska chief medical officer, told reporters that many of her state’s boroughs and districts have booked in-person vaccines for their schools May 12 as the state has dismissal for summer as early as this week.
Maine is readying four types of distribution sites for the vaccines: primary care offices, Walgreen’s and CVS pharmacies, mass vaccination sites, and schools, said Nirav Shah, MD, current ASTHO president and director of the Maine Center for Disease Control and Prevention.
Starting later this week, he said, the state hopes to host large vaccination clinics for people age 12 and over.
Eliminating barriers
States are working to break down barriers through education and improving access.
In Alaska, many of the drive-through evening vaccination sites are being changed to Pfizer sites so parents just getting off work can take their kids.
It’s also important to get young people to speak to their peers about the importance of vaccines, she said. Some teen groups in Alaska are hosting Zoom calls where they share with children and families why they chose to get vaccinated.
In Maine, Dr. Shah said, “the notion of informed consent applies with equal force to adults as it does with adolescents.” But at least in Maine, it is not required that a parent be on site and present during the vaccination itself.
A parent could sign a form allowing the child to be vaccinated in a school-based clinic. Maine also allows verbal consent so a parent can give consent over the phone, Dr. Shah said.
Dividing vaccine trays
Vaccines going to pediatrician and family medicine offices presents a challenge in that smaller numbers of doses are needed for those venues than at large vaccination sites that get trays of 1,170 Pfizer doses each.
Dr. Shah says states have been talking with federal authorities on the need for smaller packaging.
“Breaking the trays up into smaller lot sizes takes a fair amount of effort,” Dr. Shah said. “We understand that later this month the lot size will be going down to 450.”
But even that will be too much for small offices, he said.
Similarly, an effort is being made in Maine to make sure doctors’ offices are not limited by their refrigeration capabilities. The Pfizer vaccine must be kept at ultra-cold temperatures that many primary care doctors’ offices may not have.
“If they need a cool cube with dry ice, we can furnish that to them,” Dr. Shah said.
Should they be mandated?
Dr. Zink said Alaska generally has high acceptance for recommendations around COVID-19 and has no plans to mandate the COVID-19 vaccines for children.
Umair A. Shah, MD, secretary of health at the Washington State Department of Health, said, “Our number one ability to get people vaccinated is for them to be encouraged to do so, to be incentivized to do so, to do everything we can to make the vaccine choice the easy choice,” including eliminating language, cultural and access barriers.
However, he said, “in higher education, University of Washington and Washington State University have indicated they are going to require COVID vaccines for kids to come back to school. I do think that is something that is increasingly being looked at.”
Though the messages will be tailored differently across the states the bottom line will be the same, Dr. Shah said: The vaccines work and they are safe.
But most critically, “Vaccines are our pathway to moving forward and once and for all ending this pandemic,” he said.
A version of this article first appeared on Medscape.com.
after the Food and Drug Administration authorized its use in this age group May 10.
Some states hope to start the vaccinations as early as May 13, officials said at an Association of State and Territorial Health Officials news conference.
There are, however, two more steps before shots can reach younger arms. On May 12, the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices is expected to recommend use of the vaccine in this age group. Then CDC Director Rochelle Walensky, MD, must make a final decision to begin vaccinating 12- to 15-year-olds.
Some hoping to start this week
Both the CDC panel and Dr. Walensky are expected to sign off on the vaccine’s use. States have been making plans on how to tailor the vaccination message not just to the patient this time, but to parents and guardians as well, some of whom are hesitant to consent.
Some schools, assuming approval May 12, are ready to start vaccinating in cafeterias and gyms.
Anne Zink, MD, president-elect of the Association of State and Territorial Health Officials and Alaska chief medical officer, told reporters that many of her state’s boroughs and districts have booked in-person vaccines for their schools May 12 as the state has dismissal for summer as early as this week.
Maine is readying four types of distribution sites for the vaccines: primary care offices, Walgreen’s and CVS pharmacies, mass vaccination sites, and schools, said Nirav Shah, MD, current ASTHO president and director of the Maine Center for Disease Control and Prevention.
Starting later this week, he said, the state hopes to host large vaccination clinics for people age 12 and over.
Eliminating barriers
States are working to break down barriers through education and improving access.
In Alaska, many of the drive-through evening vaccination sites are being changed to Pfizer sites so parents just getting off work can take their kids.
It’s also important to get young people to speak to their peers about the importance of vaccines, she said. Some teen groups in Alaska are hosting Zoom calls where they share with children and families why they chose to get vaccinated.
In Maine, Dr. Shah said, “the notion of informed consent applies with equal force to adults as it does with adolescents.” But at least in Maine, it is not required that a parent be on site and present during the vaccination itself.
A parent could sign a form allowing the child to be vaccinated in a school-based clinic. Maine also allows verbal consent so a parent can give consent over the phone, Dr. Shah said.
Dividing vaccine trays
Vaccines going to pediatrician and family medicine offices presents a challenge in that smaller numbers of doses are needed for those venues than at large vaccination sites that get trays of 1,170 Pfizer doses each.
Dr. Shah says states have been talking with federal authorities on the need for smaller packaging.
“Breaking the trays up into smaller lot sizes takes a fair amount of effort,” Dr. Shah said. “We understand that later this month the lot size will be going down to 450.”
But even that will be too much for small offices, he said.
Similarly, an effort is being made in Maine to make sure doctors’ offices are not limited by their refrigeration capabilities. The Pfizer vaccine must be kept at ultra-cold temperatures that many primary care doctors’ offices may not have.
“If they need a cool cube with dry ice, we can furnish that to them,” Dr. Shah said.
Should they be mandated?
Dr. Zink said Alaska generally has high acceptance for recommendations around COVID-19 and has no plans to mandate the COVID-19 vaccines for children.
Umair A. Shah, MD, secretary of health at the Washington State Department of Health, said, “Our number one ability to get people vaccinated is for them to be encouraged to do so, to be incentivized to do so, to do everything we can to make the vaccine choice the easy choice,” including eliminating language, cultural and access barriers.
However, he said, “in higher education, University of Washington and Washington State University have indicated they are going to require COVID vaccines for kids to come back to school. I do think that is something that is increasingly being looked at.”
Though the messages will be tailored differently across the states the bottom line will be the same, Dr. Shah said: The vaccines work and they are safe.
But most critically, “Vaccines are our pathway to moving forward and once and for all ending this pandemic,” he said.
A version of this article first appeared on Medscape.com.
ADHD in preschool kids: Adrenergic agonists may be a better fit
A new study finds that alpha2-adrenergic agonists may be of benefit and have fewer side effects than stimulant medications for the treatment of attention-deficit/hyperactivity disorder in preschool-age children.
The study was published online May 4 in JAMA.
As part of a retrospective analysis, Elizabeth Harstad, MD, MPH, of Boston Children’s Hospital and colleagues evaluated health record data from 497 preschool-age children with ADHD across seven developmental-behavioral pediatric practices in the United States. Children included in the evaluation were younger than 6 years and were treated for ADHD between Jan. 1, 2013, and July 1, 2017, with either an alpha2-adrenergic agonist or a stimulant.
Overall, 175 children (35%) were prescribed an alpha2-adrenergic agonist (most often guanfacine) as first-line ADHD medication, and 322 children (65%) were prescribed a stimulant (most often a methylphenidate-based preparation). Before any medication regimens were initiated, 62% of children received behavioral therapy.
“These findings suggest that for some children there may be a concern about either how well a stimulant will work or how well a stimulant will be tolerated that is leading clinicians to instead prescribe an alpha2-adrenergic agonist as the first medication tried,” Dr. Harstad said in an interview.
Clinical improvement was noted in 66% of children treated with alpha2-adrenergic agonists (95% confidence interval, 57.5%-73.9%) and in 78% of children treated with stimulants (95% CI, 72.4%-83.4%).
Most adverse effects were more common among children who received stimulants than among those who received alpha2-adrenergic agonists. These adverse effects included difficulty falling asleep (21% vs. 11%), decreased appetite (38% vs. 7%), increased stomachaches (13% vs. 5%), and increased skin picking/repetitive behaviors (11% vs. 5%). Only daytime sleepiness was more frequent among children who received an alpha2-adrenergic agonist rather than a stimulant (38% vs. 3%).
“We also found that for the youngest children (<4 years old), those initiated on alpha2-adrenergic agonists stayed on these medications longer than those initiated on stimulants, which may indicate that they are better tolerated, although more research is needed to confirm this,” Dr. Harstad said.
“While our study focused on how well medications work and how well they are tolerated when used to treat preschool-age children with ADHD, it is important to remember that behavioral therapy is recommended as first-line treatment for ADHD in preschool-age children, not medication,” Dr. Harstad added.
Mark Wolraich, MD, of the University of Oklahoma, echoed that sentiment. “The article mentions that behavioral interventions, in the form of parent training in behavior management, is an effective first-line treatment” and, per the American Academy of Pediatrics guidelines, “is the first line of treatment recommended for preschool-age children before medication should be considered.”
Dr. Wolraich also noted that “neither drug has official FDA [U.S. Food and Drug Administration] approval in this age group” but that “methylphenidate comes the closest to having met the FDA requirements for approval in this age group, which is why the AAP guidelines recommended its use if parent training in behavior management is not sufficient.”
Although Dr. Harstad and colleagues note that the study included a large and diverse sample size from across the United States, they acknowledge that “further research, including from randomized clinical trials, is needed to assess comparative effectiveness of alpha2-adrenergic agonists versus stimulants.”
Funding for the study was provided through a cooperative agreement with the Maternal and Child Health Bureau, the Health Resources and Services Administration, and the U.S. Department of Health & Human Services. Dr. Harstad has reported receiving reported receiving compensation for serving as a medical reviewer for Understood.org and grant funding from the Palmer Family Fund for Autism Research to conduct research related to autism spectrum disorder at Boston Children’s Hospital. Disclosures for the other authors are listed in the original article. Dr. Wolraich has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A new study finds that alpha2-adrenergic agonists may be of benefit and have fewer side effects than stimulant medications for the treatment of attention-deficit/hyperactivity disorder in preschool-age children.
The study was published online May 4 in JAMA.
As part of a retrospective analysis, Elizabeth Harstad, MD, MPH, of Boston Children’s Hospital and colleagues evaluated health record data from 497 preschool-age children with ADHD across seven developmental-behavioral pediatric practices in the United States. Children included in the evaluation were younger than 6 years and were treated for ADHD between Jan. 1, 2013, and July 1, 2017, with either an alpha2-adrenergic agonist or a stimulant.
Overall, 175 children (35%) were prescribed an alpha2-adrenergic agonist (most often guanfacine) as first-line ADHD medication, and 322 children (65%) were prescribed a stimulant (most often a methylphenidate-based preparation). Before any medication regimens were initiated, 62% of children received behavioral therapy.
“These findings suggest that for some children there may be a concern about either how well a stimulant will work or how well a stimulant will be tolerated that is leading clinicians to instead prescribe an alpha2-adrenergic agonist as the first medication tried,” Dr. Harstad said in an interview.
Clinical improvement was noted in 66% of children treated with alpha2-adrenergic agonists (95% confidence interval, 57.5%-73.9%) and in 78% of children treated with stimulants (95% CI, 72.4%-83.4%).
Most adverse effects were more common among children who received stimulants than among those who received alpha2-adrenergic agonists. These adverse effects included difficulty falling asleep (21% vs. 11%), decreased appetite (38% vs. 7%), increased stomachaches (13% vs. 5%), and increased skin picking/repetitive behaviors (11% vs. 5%). Only daytime sleepiness was more frequent among children who received an alpha2-adrenergic agonist rather than a stimulant (38% vs. 3%).
“We also found that for the youngest children (<4 years old), those initiated on alpha2-adrenergic agonists stayed on these medications longer than those initiated on stimulants, which may indicate that they are better tolerated, although more research is needed to confirm this,” Dr. Harstad said.
“While our study focused on how well medications work and how well they are tolerated when used to treat preschool-age children with ADHD, it is important to remember that behavioral therapy is recommended as first-line treatment for ADHD in preschool-age children, not medication,” Dr. Harstad added.
Mark Wolraich, MD, of the University of Oklahoma, echoed that sentiment. “The article mentions that behavioral interventions, in the form of parent training in behavior management, is an effective first-line treatment” and, per the American Academy of Pediatrics guidelines, “is the first line of treatment recommended for preschool-age children before medication should be considered.”
Dr. Wolraich also noted that “neither drug has official FDA [U.S. Food and Drug Administration] approval in this age group” but that “methylphenidate comes the closest to having met the FDA requirements for approval in this age group, which is why the AAP guidelines recommended its use if parent training in behavior management is not sufficient.”
Although Dr. Harstad and colleagues note that the study included a large and diverse sample size from across the United States, they acknowledge that “further research, including from randomized clinical trials, is needed to assess comparative effectiveness of alpha2-adrenergic agonists versus stimulants.”
Funding for the study was provided through a cooperative agreement with the Maternal and Child Health Bureau, the Health Resources and Services Administration, and the U.S. Department of Health & Human Services. Dr. Harstad has reported receiving reported receiving compensation for serving as a medical reviewer for Understood.org and grant funding from the Palmer Family Fund for Autism Research to conduct research related to autism spectrum disorder at Boston Children’s Hospital. Disclosures for the other authors are listed in the original article. Dr. Wolraich has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A new study finds that alpha2-adrenergic agonists may be of benefit and have fewer side effects than stimulant medications for the treatment of attention-deficit/hyperactivity disorder in preschool-age children.
The study was published online May 4 in JAMA.
As part of a retrospective analysis, Elizabeth Harstad, MD, MPH, of Boston Children’s Hospital and colleagues evaluated health record data from 497 preschool-age children with ADHD across seven developmental-behavioral pediatric practices in the United States. Children included in the evaluation were younger than 6 years and were treated for ADHD between Jan. 1, 2013, and July 1, 2017, with either an alpha2-adrenergic agonist or a stimulant.
Overall, 175 children (35%) were prescribed an alpha2-adrenergic agonist (most often guanfacine) as first-line ADHD medication, and 322 children (65%) were prescribed a stimulant (most often a methylphenidate-based preparation). Before any medication regimens were initiated, 62% of children received behavioral therapy.
“These findings suggest that for some children there may be a concern about either how well a stimulant will work or how well a stimulant will be tolerated that is leading clinicians to instead prescribe an alpha2-adrenergic agonist as the first medication tried,” Dr. Harstad said in an interview.
Clinical improvement was noted in 66% of children treated with alpha2-adrenergic agonists (95% confidence interval, 57.5%-73.9%) and in 78% of children treated with stimulants (95% CI, 72.4%-83.4%).
Most adverse effects were more common among children who received stimulants than among those who received alpha2-adrenergic agonists. These adverse effects included difficulty falling asleep (21% vs. 11%), decreased appetite (38% vs. 7%), increased stomachaches (13% vs. 5%), and increased skin picking/repetitive behaviors (11% vs. 5%). Only daytime sleepiness was more frequent among children who received an alpha2-adrenergic agonist rather than a stimulant (38% vs. 3%).
“We also found that for the youngest children (<4 years old), those initiated on alpha2-adrenergic agonists stayed on these medications longer than those initiated on stimulants, which may indicate that they are better tolerated, although more research is needed to confirm this,” Dr. Harstad said.
“While our study focused on how well medications work and how well they are tolerated when used to treat preschool-age children with ADHD, it is important to remember that behavioral therapy is recommended as first-line treatment for ADHD in preschool-age children, not medication,” Dr. Harstad added.
Mark Wolraich, MD, of the University of Oklahoma, echoed that sentiment. “The article mentions that behavioral interventions, in the form of parent training in behavior management, is an effective first-line treatment” and, per the American Academy of Pediatrics guidelines, “is the first line of treatment recommended for preschool-age children before medication should be considered.”
Dr. Wolraich also noted that “neither drug has official FDA [U.S. Food and Drug Administration] approval in this age group” but that “methylphenidate comes the closest to having met the FDA requirements for approval in this age group, which is why the AAP guidelines recommended its use if parent training in behavior management is not sufficient.”
Although Dr. Harstad and colleagues note that the study included a large and diverse sample size from across the United States, they acknowledge that “further research, including from randomized clinical trials, is needed to assess comparative effectiveness of alpha2-adrenergic agonists versus stimulants.”
Funding for the study was provided through a cooperative agreement with the Maternal and Child Health Bureau, the Health Resources and Services Administration, and the U.S. Department of Health & Human Services. Dr. Harstad has reported receiving reported receiving compensation for serving as a medical reviewer for Understood.org and grant funding from the Palmer Family Fund for Autism Research to conduct research related to autism spectrum disorder at Boston Children’s Hospital. Disclosures for the other authors are listed in the original article. Dr. Wolraich has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.