User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
White House announces vaccination plans for younger children
States were allowed to begin preordering the shots this week. But they can’t be delivered into kids’ arms until the FDA and CDC sign off. The shots could be available in early November.
“We know millions of parents have been waiting for COVID-19 vaccine for kids in this age group, and should the FDA and CDC authorize the vaccine, we will be ready to get shots in arms,” Jeff Zients, the White House COVID-19 response coordinator, said at a briefing Oct. 20.
Asked whether announcing plans to deliver a vaccine to children might put pressure on the agencies considering the evidence for their use, Mr. Zients defended the Biden administration’s plans.
“This is the right way to do things: To be operationally ready,” he said. Mr. Zients said they had learned a lesson from the prior administration.
“The decision was made by the FDA and CDC, and the operations weren’t ready. And that meant that adults at the time were not able to receive their vaccines as efficiently, equitably as possible. And this will enable us to be ready for kids,” he said.
Pfizer submitted data to the FDA in late September from its test of the vaccine in 2,200 children. The company said the shots had a favorable safety profile and generated “robust” antibody responses.
An FDA panel is scheduled to meet on Oct. 26 to consider Pfizer’s application. The CDC’s Advisory Committee on Immunization Practices will meet the following week, on Nov. 2 and 3.
Laying the groundwork
Doctors applauded the advance planning.
“Laying this advance groundwork, ensuring supply is available at physician practices, and that a patient’s own physician is available to answer questions, is critical to the continued success of this rollout,” Gerald Harmon, MD, president of the American Medical Association, said in a written statement.
The shots planned for children are 10 micrograms, a smaller dose than is given to adults. To be fully immunized, kids get two doses, spaced about 21 days apart. Vaccines for younger children are packaged in smaller vials and injected through smaller needles, too.
The vaccine for younger children will roll out slightly differently than it has for adults and teens. While adults mostly got their COVID-19 vaccines through pop-up mass vaccination sites, health departments, and other community locations, the strategy to get children immunized against COVID is centered on the offices of pediatricians and primary care doctors.
The White House says 25,000 doctors have already signed up to give the vaccines.
The vaccination campaign will get underway at a tough moment for pediatricians.
The voicemail message at Roswell Pediatrics Center in the suburbs north of Atlanta, for instance, warns parents to be patient.
“Due to the current, new COVID-19 surge, we are experiencing extremely high call volume, as well as suffering from the same staffing shortages that most businesses are having,” the message says, adding that they’re working around the clock to answer questions and return phone calls.
Jesse Hackell, MD, says he knows the feeling. He’s the chief operating officer of Pomona Pediatrics in Pomona, N.Y., and a spokesperson for the American Academy of Pediatrics.
“We’re swamped now by kids who get sent home from school because they sneezed once and they have to be cleared before they can go back to school,” he said. “We’re seeing kids who we don’t need to see in terms of the degree of illness because the school requires them to be cleared [of COVID-19].”
Dr. Hackell has been offering the vaccines to kids ages 12 and up since May. He’s planning to offer it to younger children too.
“Adding the vaccines to it is going to be a challenge, but you know we’ll get up to speed and we’ll make it happen,” he said, adding that pediatricians have done many large-scale vaccination campaigns, like those for the H1N1 influenza vaccine in 2009.
Dr. Hackell helped to draft a new policy in New York that will require COVID-19 vaccines for schoolchildren once they are granted full approval from the FDA. Other states may follow with their own vaccination requirements.
He said ultimately, vaccinating school-age children is going to make them safer, will help prevent the virus from mutating and spreading, and will help society as a whole get back to normal.
“We’re the vaccine experts in pediatrics. This is what we do. It’s a huge part of our practice like no other specialty. If we can’t get it right, how can anyone else be expected to?” he said.
A version of this article first appeared on WebMD.com.
States were allowed to begin preordering the shots this week. But they can’t be delivered into kids’ arms until the FDA and CDC sign off. The shots could be available in early November.
“We know millions of parents have been waiting for COVID-19 vaccine for kids in this age group, and should the FDA and CDC authorize the vaccine, we will be ready to get shots in arms,” Jeff Zients, the White House COVID-19 response coordinator, said at a briefing Oct. 20.
Asked whether announcing plans to deliver a vaccine to children might put pressure on the agencies considering the evidence for their use, Mr. Zients defended the Biden administration’s plans.
“This is the right way to do things: To be operationally ready,” he said. Mr. Zients said they had learned a lesson from the prior administration.
“The decision was made by the FDA and CDC, and the operations weren’t ready. And that meant that adults at the time were not able to receive their vaccines as efficiently, equitably as possible. And this will enable us to be ready for kids,” he said.
Pfizer submitted data to the FDA in late September from its test of the vaccine in 2,200 children. The company said the shots had a favorable safety profile and generated “robust” antibody responses.
An FDA panel is scheduled to meet on Oct. 26 to consider Pfizer’s application. The CDC’s Advisory Committee on Immunization Practices will meet the following week, on Nov. 2 and 3.
Laying the groundwork
Doctors applauded the advance planning.
“Laying this advance groundwork, ensuring supply is available at physician practices, and that a patient’s own physician is available to answer questions, is critical to the continued success of this rollout,” Gerald Harmon, MD, president of the American Medical Association, said in a written statement.
The shots planned for children are 10 micrograms, a smaller dose than is given to adults. To be fully immunized, kids get two doses, spaced about 21 days apart. Vaccines for younger children are packaged in smaller vials and injected through smaller needles, too.
The vaccine for younger children will roll out slightly differently than it has for adults and teens. While adults mostly got their COVID-19 vaccines through pop-up mass vaccination sites, health departments, and other community locations, the strategy to get children immunized against COVID is centered on the offices of pediatricians and primary care doctors.
The White House says 25,000 doctors have already signed up to give the vaccines.
The vaccination campaign will get underway at a tough moment for pediatricians.
The voicemail message at Roswell Pediatrics Center in the suburbs north of Atlanta, for instance, warns parents to be patient.
“Due to the current, new COVID-19 surge, we are experiencing extremely high call volume, as well as suffering from the same staffing shortages that most businesses are having,” the message says, adding that they’re working around the clock to answer questions and return phone calls.
Jesse Hackell, MD, says he knows the feeling. He’s the chief operating officer of Pomona Pediatrics in Pomona, N.Y., and a spokesperson for the American Academy of Pediatrics.
“We’re swamped now by kids who get sent home from school because they sneezed once and they have to be cleared before they can go back to school,” he said. “We’re seeing kids who we don’t need to see in terms of the degree of illness because the school requires them to be cleared [of COVID-19].”
Dr. Hackell has been offering the vaccines to kids ages 12 and up since May. He’s planning to offer it to younger children too.
“Adding the vaccines to it is going to be a challenge, but you know we’ll get up to speed and we’ll make it happen,” he said, adding that pediatricians have done many large-scale vaccination campaigns, like those for the H1N1 influenza vaccine in 2009.
Dr. Hackell helped to draft a new policy in New York that will require COVID-19 vaccines for schoolchildren once they are granted full approval from the FDA. Other states may follow with their own vaccination requirements.
He said ultimately, vaccinating school-age children is going to make them safer, will help prevent the virus from mutating and spreading, and will help society as a whole get back to normal.
“We’re the vaccine experts in pediatrics. This is what we do. It’s a huge part of our practice like no other specialty. If we can’t get it right, how can anyone else be expected to?” he said.
A version of this article first appeared on WebMD.com.
States were allowed to begin preordering the shots this week. But they can’t be delivered into kids’ arms until the FDA and CDC sign off. The shots could be available in early November.
“We know millions of parents have been waiting for COVID-19 vaccine for kids in this age group, and should the FDA and CDC authorize the vaccine, we will be ready to get shots in arms,” Jeff Zients, the White House COVID-19 response coordinator, said at a briefing Oct. 20.
Asked whether announcing plans to deliver a vaccine to children might put pressure on the agencies considering the evidence for their use, Mr. Zients defended the Biden administration’s plans.
“This is the right way to do things: To be operationally ready,” he said. Mr. Zients said they had learned a lesson from the prior administration.
“The decision was made by the FDA and CDC, and the operations weren’t ready. And that meant that adults at the time were not able to receive their vaccines as efficiently, equitably as possible. And this will enable us to be ready for kids,” he said.
Pfizer submitted data to the FDA in late September from its test of the vaccine in 2,200 children. The company said the shots had a favorable safety profile and generated “robust” antibody responses.
An FDA panel is scheduled to meet on Oct. 26 to consider Pfizer’s application. The CDC’s Advisory Committee on Immunization Practices will meet the following week, on Nov. 2 and 3.
Laying the groundwork
Doctors applauded the advance planning.
“Laying this advance groundwork, ensuring supply is available at physician practices, and that a patient’s own physician is available to answer questions, is critical to the continued success of this rollout,” Gerald Harmon, MD, president of the American Medical Association, said in a written statement.
The shots planned for children are 10 micrograms, a smaller dose than is given to adults. To be fully immunized, kids get two doses, spaced about 21 days apart. Vaccines for younger children are packaged in smaller vials and injected through smaller needles, too.
The vaccine for younger children will roll out slightly differently than it has for adults and teens. While adults mostly got their COVID-19 vaccines through pop-up mass vaccination sites, health departments, and other community locations, the strategy to get children immunized against COVID is centered on the offices of pediatricians and primary care doctors.
The White House says 25,000 doctors have already signed up to give the vaccines.
The vaccination campaign will get underway at a tough moment for pediatricians.
The voicemail message at Roswell Pediatrics Center in the suburbs north of Atlanta, for instance, warns parents to be patient.
“Due to the current, new COVID-19 surge, we are experiencing extremely high call volume, as well as suffering from the same staffing shortages that most businesses are having,” the message says, adding that they’re working around the clock to answer questions and return phone calls.
Jesse Hackell, MD, says he knows the feeling. He’s the chief operating officer of Pomona Pediatrics in Pomona, N.Y., and a spokesperson for the American Academy of Pediatrics.
“We’re swamped now by kids who get sent home from school because they sneezed once and they have to be cleared before they can go back to school,” he said. “We’re seeing kids who we don’t need to see in terms of the degree of illness because the school requires them to be cleared [of COVID-19].”
Dr. Hackell has been offering the vaccines to kids ages 12 and up since May. He’s planning to offer it to younger children too.
“Adding the vaccines to it is going to be a challenge, but you know we’ll get up to speed and we’ll make it happen,” he said, adding that pediatricians have done many large-scale vaccination campaigns, like those for the H1N1 influenza vaccine in 2009.
Dr. Hackell helped to draft a new policy in New York that will require COVID-19 vaccines for schoolchildren once they are granted full approval from the FDA. Other states may follow with their own vaccination requirements.
He said ultimately, vaccinating school-age children is going to make them safer, will help prevent the virus from mutating and spreading, and will help society as a whole get back to normal.
“We’re the vaccine experts in pediatrics. This is what we do. It’s a huge part of our practice like no other specialty. If we can’t get it right, how can anyone else be expected to?” he said.
A version of this article first appeared on WebMD.com.
Pediatric organizations declare national emergency in mental health
The American Academy of Pediatrics (AAP), the American Academy of Child and Adolescent Psychiatry (AACAP) and Children’s Hospital Association have declared a national emergency in children’s mental health.
COVID-19 has taken a serious toll, the organizations say, on top of already mounting challenges. Policy changes are urgently needed, they say.
“Today’s declaration is an urgent call to policymakers at all levels of government – we must treat this mental health crisis like the emergency it is,” AAP President Lee Savio Beers, MD, said in a statement.
The Centers for Disease Control and Prevention found that between March and October 2020, emergency department visits for mental health emergencies rose by 24% for children ages 5-11 years and 31% for children ages 12-17 years. ED visits for suspected suicide attempts increased nearly 51% among girls ages 12-17 years of age in early 2021 compared to the same period in 2019.
Recent data in Pediatrics also show a marked increase in loss of a caregiver and sharp disparities by race and ethnicity.
“We found that from April 1, 2020, through June 30, 2021, over 140,000 children in the U.S. experienced the death of a parent or grandparent caregiver. The risk of such loss was 1.1 to 4.5 times higher among children of racial and ethnic minorities, compared to non-Hispanic White children,” researchers wrote.
“We are caring for young people with soaring rates of depression, anxiety, trauma, loneliness, and suicidality that will have lasting impacts on them, their families, their communities, and all of our futures,” said AACAP President Gabrielle A. Carlson, MD.
Among the actions the groups are calling for are the following:
- Increase federal funding to ensure all families can access mental health services.
- Improve access to telemedicine.
- Accelerate integration of mental health care in pediatric primary care.
- Fully fund community-based systems of care that connect families to evidence-based interventions.
- Promote and pay for trauma-informed care services.
- Address workforce challenges so that children can access mental health services wherever they live.
The organizations represent more than 77,000 physician members and more than 200 children’s hospitals.
Jenna Triana, MD, a child and adolescent psychiatrist at the University of Minnesota, Minneapolis, said in an interview that while specific institutions such as the University of Colorado have declared emergencies in pediatric mental health, declaring a national emergency is important.
She said the timing is important because fall is typically a heavy time for pediatric psychiatry with children and adolescents returning to school, and it is especially pronounced with the pandemic.
The usual diagnoses providers are seeing “are all worse,” she said.
“The bar for getting admission to the hospital has been raised because we’re such a limited resource. We’ve had to be so thoughtful about who truly, truly needs admission and who can come up with some kind of safe plan for outside of the hospital,” Dr. Triana said.
“The patients I’m seeing in the hospital – the level of illness I’m seeing is much higher than it was a couple of years ago,” she said.
Now, Dr. Triana said, patients who are depressed and suicidal are seeking help outside the hospital in day-treatment programs or intensive outpatient therapy.
At the hospital, she said, “our wait list is usually around 20 kids sitting in the ER waiting for a patient bed. Kids wait either in the ER or a medical bed sometimes a week or more waiting for inpatient psychiatry.”
She said while she thinks all of the proposed recommendations are good, “I think what’s difficult is the speed at which any of this can happen."
“We’re in crisis now and we’ve been in crisis for months,” she added.
She said the key will be using what’s already in place – telehealth options to ease the burdens and training more primary care providers in mental health triage.
Joanna Quigley, MD, a child and adolescent psychiatrist at the University of Michigan in Ann Arbor, said in an interview, “It’s very powerful that these three groups came together and made a joint effort and statement to really highlight how serious this problem is across the country.”
She said she sees all of the challenges the leaders of the organizations describe.
At Michigan, she said, as elsewhere, specialists are seeing a large increase in the number of children presenting to the children’s psychiatric ED and the children’s ED and increased demand for outpatient services.
Children in need are waiting “several months” to see either therapists or psychiatrists, she said.
Dr. Quigley said primary care offices are seeing more children and children with higher levels of anxiety and depression as well as self-harm and suicidal thoughts in the pandemic.
She noted that it’s challenging to find providers who are accepting new patients and hard to find providers who take certain kinds of insurance, particularly Medicaid, she said.
Change will take strengthening all the areas of support the organizations’ leaders are calling for, she said.
“School-based interventions are so vital, especially for these children who have been away from an in-person setting and were without services for the time that schools were shut down,” she said.
Dr. Quigley and Dr. Triana report no relevant financial relationships.
The American Academy of Pediatrics (AAP), the American Academy of Child and Adolescent Psychiatry (AACAP) and Children’s Hospital Association have declared a national emergency in children’s mental health.
COVID-19 has taken a serious toll, the organizations say, on top of already mounting challenges. Policy changes are urgently needed, they say.
“Today’s declaration is an urgent call to policymakers at all levels of government – we must treat this mental health crisis like the emergency it is,” AAP President Lee Savio Beers, MD, said in a statement.
The Centers for Disease Control and Prevention found that between March and October 2020, emergency department visits for mental health emergencies rose by 24% for children ages 5-11 years and 31% for children ages 12-17 years. ED visits for suspected suicide attempts increased nearly 51% among girls ages 12-17 years of age in early 2021 compared to the same period in 2019.
Recent data in Pediatrics also show a marked increase in loss of a caregiver and sharp disparities by race and ethnicity.
“We found that from April 1, 2020, through June 30, 2021, over 140,000 children in the U.S. experienced the death of a parent or grandparent caregiver. The risk of such loss was 1.1 to 4.5 times higher among children of racial and ethnic minorities, compared to non-Hispanic White children,” researchers wrote.
“We are caring for young people with soaring rates of depression, anxiety, trauma, loneliness, and suicidality that will have lasting impacts on them, their families, their communities, and all of our futures,” said AACAP President Gabrielle A. Carlson, MD.
Among the actions the groups are calling for are the following:
- Increase federal funding to ensure all families can access mental health services.
- Improve access to telemedicine.
- Accelerate integration of mental health care in pediatric primary care.
- Fully fund community-based systems of care that connect families to evidence-based interventions.
- Promote and pay for trauma-informed care services.
- Address workforce challenges so that children can access mental health services wherever they live.
The organizations represent more than 77,000 physician members and more than 200 children’s hospitals.
Jenna Triana, MD, a child and adolescent psychiatrist at the University of Minnesota, Minneapolis, said in an interview that while specific institutions such as the University of Colorado have declared emergencies in pediatric mental health, declaring a national emergency is important.
She said the timing is important because fall is typically a heavy time for pediatric psychiatry with children and adolescents returning to school, and it is especially pronounced with the pandemic.
The usual diagnoses providers are seeing “are all worse,” she said.
“The bar for getting admission to the hospital has been raised because we’re such a limited resource. We’ve had to be so thoughtful about who truly, truly needs admission and who can come up with some kind of safe plan for outside of the hospital,” Dr. Triana said.
“The patients I’m seeing in the hospital – the level of illness I’m seeing is much higher than it was a couple of years ago,” she said.
Now, Dr. Triana said, patients who are depressed and suicidal are seeking help outside the hospital in day-treatment programs or intensive outpatient therapy.
At the hospital, she said, “our wait list is usually around 20 kids sitting in the ER waiting for a patient bed. Kids wait either in the ER or a medical bed sometimes a week or more waiting for inpatient psychiatry.”
She said while she thinks all of the proposed recommendations are good, “I think what’s difficult is the speed at which any of this can happen."
“We’re in crisis now and we’ve been in crisis for months,” she added.
She said the key will be using what’s already in place – telehealth options to ease the burdens and training more primary care providers in mental health triage.
Joanna Quigley, MD, a child and adolescent psychiatrist at the University of Michigan in Ann Arbor, said in an interview, “It’s very powerful that these three groups came together and made a joint effort and statement to really highlight how serious this problem is across the country.”
She said she sees all of the challenges the leaders of the organizations describe.
At Michigan, she said, as elsewhere, specialists are seeing a large increase in the number of children presenting to the children’s psychiatric ED and the children’s ED and increased demand for outpatient services.
Children in need are waiting “several months” to see either therapists or psychiatrists, she said.
Dr. Quigley said primary care offices are seeing more children and children with higher levels of anxiety and depression as well as self-harm and suicidal thoughts in the pandemic.
She noted that it’s challenging to find providers who are accepting new patients and hard to find providers who take certain kinds of insurance, particularly Medicaid, she said.
Change will take strengthening all the areas of support the organizations’ leaders are calling for, she said.
“School-based interventions are so vital, especially for these children who have been away from an in-person setting and were without services for the time that schools were shut down,” she said.
Dr. Quigley and Dr. Triana report no relevant financial relationships.
The American Academy of Pediatrics (AAP), the American Academy of Child and Adolescent Psychiatry (AACAP) and Children’s Hospital Association have declared a national emergency in children’s mental health.
COVID-19 has taken a serious toll, the organizations say, on top of already mounting challenges. Policy changes are urgently needed, they say.
“Today’s declaration is an urgent call to policymakers at all levels of government – we must treat this mental health crisis like the emergency it is,” AAP President Lee Savio Beers, MD, said in a statement.
The Centers for Disease Control and Prevention found that between March and October 2020, emergency department visits for mental health emergencies rose by 24% for children ages 5-11 years and 31% for children ages 12-17 years. ED visits for suspected suicide attempts increased nearly 51% among girls ages 12-17 years of age in early 2021 compared to the same period in 2019.
Recent data in Pediatrics also show a marked increase in loss of a caregiver and sharp disparities by race and ethnicity.
“We found that from April 1, 2020, through June 30, 2021, over 140,000 children in the U.S. experienced the death of a parent or grandparent caregiver. The risk of such loss was 1.1 to 4.5 times higher among children of racial and ethnic minorities, compared to non-Hispanic White children,” researchers wrote.
“We are caring for young people with soaring rates of depression, anxiety, trauma, loneliness, and suicidality that will have lasting impacts on them, their families, their communities, and all of our futures,” said AACAP President Gabrielle A. Carlson, MD.
Among the actions the groups are calling for are the following:
- Increase federal funding to ensure all families can access mental health services.
- Improve access to telemedicine.
- Accelerate integration of mental health care in pediatric primary care.
- Fully fund community-based systems of care that connect families to evidence-based interventions.
- Promote and pay for trauma-informed care services.
- Address workforce challenges so that children can access mental health services wherever they live.
The organizations represent more than 77,000 physician members and more than 200 children’s hospitals.
Jenna Triana, MD, a child and adolescent psychiatrist at the University of Minnesota, Minneapolis, said in an interview that while specific institutions such as the University of Colorado have declared emergencies in pediatric mental health, declaring a national emergency is important.
She said the timing is important because fall is typically a heavy time for pediatric psychiatry with children and adolescents returning to school, and it is especially pronounced with the pandemic.
The usual diagnoses providers are seeing “are all worse,” she said.
“The bar for getting admission to the hospital has been raised because we’re such a limited resource. We’ve had to be so thoughtful about who truly, truly needs admission and who can come up with some kind of safe plan for outside of the hospital,” Dr. Triana said.
“The patients I’m seeing in the hospital – the level of illness I’m seeing is much higher than it was a couple of years ago,” she said.
Now, Dr. Triana said, patients who are depressed and suicidal are seeking help outside the hospital in day-treatment programs or intensive outpatient therapy.
At the hospital, she said, “our wait list is usually around 20 kids sitting in the ER waiting for a patient bed. Kids wait either in the ER or a medical bed sometimes a week or more waiting for inpatient psychiatry.”
She said while she thinks all of the proposed recommendations are good, “I think what’s difficult is the speed at which any of this can happen."
“We’re in crisis now and we’ve been in crisis for months,” she added.
She said the key will be using what’s already in place – telehealth options to ease the burdens and training more primary care providers in mental health triage.
Joanna Quigley, MD, a child and adolescent psychiatrist at the University of Michigan in Ann Arbor, said in an interview, “It’s very powerful that these three groups came together and made a joint effort and statement to really highlight how serious this problem is across the country.”
She said she sees all of the challenges the leaders of the organizations describe.
At Michigan, she said, as elsewhere, specialists are seeing a large increase in the number of children presenting to the children’s psychiatric ED and the children’s ED and increased demand for outpatient services.
Children in need are waiting “several months” to see either therapists or psychiatrists, she said.
Dr. Quigley said primary care offices are seeing more children and children with higher levels of anxiety and depression as well as self-harm and suicidal thoughts in the pandemic.
She noted that it’s challenging to find providers who are accepting new patients and hard to find providers who take certain kinds of insurance, particularly Medicaid, she said.
Change will take strengthening all the areas of support the organizations’ leaders are calling for, she said.
“School-based interventions are so vital, especially for these children who have been away from an in-person setting and were without services for the time that schools were shut down,” she said.
Dr. Quigley and Dr. Triana report no relevant financial relationships.
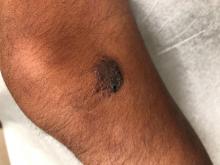
Teen boy’s knee lesion has changed
A biopsy of the lesion was performed which showed an increased number of eccrine glands and blood vessels within the dermis. Some areas showed an increase in adipocytes and smooth muscle bundles. The changes were consistent with eccrine angiomatous hamartoma (EAH).
The boy was referred to vascular laser therapy for treatment of the lesion.
EAH is a rare benign vascular growth characterized by an increased number of mature eccrine glands and blood vessels in the dermis and subcutis. The lesions are mostly present on the extremities, but cases of diffuse congenital lesions and lesions on the face and trunk have also been described. The lesions can be seen at birth or during the first years of life in about half of the cases, and the others tend to occur later in puberty and rarely in adulthood.1
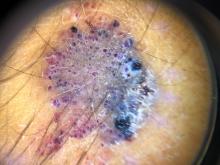
Clinically, EAH lesions present as red, yellow to brown papules and plaques. Different dermoscopic patterns have been described which include the popcorn pattern that presents as yellow, confluent nodules with popcornlike shapes over a background of erythema, and linear arborizing vessels. The spitzoid pattern are brown globules on a background of erythema and pseudoreticular pigmentation around the globules. The verrucous hemangiomalike pattern has a bluish-white hue, reddish-blue or bluish lacunae, as seen in our patient.2-4
Most of the lesions are asymptomatic, but in some patients, they can be associated with pain, hyperhidrosis, and sometimes bleeding. Hyperhidrosis has been reported early in the presentation or during puberty or pregnancy. Our patient had started on amphetamines when hyperhidrosis occurred. Hyperhidrosis is a knowns side effect of this type of medication and may have had a role in the increased sweating noted on the hamartoma.
EAH can clinically look like verrucous hemangiomas, angiokeratomas, and vascular malformations, and histopathology may be needed to differentiate between them. Eccrine nevi and EAH can be similar. Hyperhidrosis is an early and predominant component of eccrine nevi, compared with one-third of EAH.
The exact etiology of this lesion is not known. It is thought to be caused by an abnormal differentiation of the epithelium, adnexal structure, and the mesenchyme during organogenesis.3 No other associated conditions have been described with EAH.
EAH are benign lesions that rarely require treatment. If the lesions are symptomatic or because of cosmetic reasons, they can be removed surgically. There are some reports of successful treatment with pulse dual-wavelength sequential 595- and 1064-nm lasers.5 Botulinum toxin has also been used in cases of symptomatic hyperhidrosis.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. She has no conflicts. Email her at [email protected].
References
1. Smith SD et al. Pediatr Dermatol. 2019 Nov;36(6):909-12.
2. Patterson AT et al. Am J Dermatopathol. 2016;38:413-7.
3. Garcıa-Garcıa SC et al. JAAD Case Rep. 2018;4(2):165-7.
4. Awatef Kelati et al. JAAD Case Rep. 2018;4(8)835-6.
5. Felgueiras J et al. Dermatol Surg. 2015 Mar;41(3):428-30.
A biopsy of the lesion was performed which showed an increased number of eccrine glands and blood vessels within the dermis. Some areas showed an increase in adipocytes and smooth muscle bundles. The changes were consistent with eccrine angiomatous hamartoma (EAH).
The boy was referred to vascular laser therapy for treatment of the lesion.
EAH is a rare benign vascular growth characterized by an increased number of mature eccrine glands and blood vessels in the dermis and subcutis. The lesions are mostly present on the extremities, but cases of diffuse congenital lesions and lesions on the face and trunk have also been described. The lesions can be seen at birth or during the first years of life in about half of the cases, and the others tend to occur later in puberty and rarely in adulthood.1
Clinically, EAH lesions present as red, yellow to brown papules and plaques. Different dermoscopic patterns have been described which include the popcorn pattern that presents as yellow, confluent nodules with popcornlike shapes over a background of erythema, and linear arborizing vessels. The spitzoid pattern are brown globules on a background of erythema and pseudoreticular pigmentation around the globules. The verrucous hemangiomalike pattern has a bluish-white hue, reddish-blue or bluish lacunae, as seen in our patient.2-4
Most of the lesions are asymptomatic, but in some patients, they can be associated with pain, hyperhidrosis, and sometimes bleeding. Hyperhidrosis has been reported early in the presentation or during puberty or pregnancy. Our patient had started on amphetamines when hyperhidrosis occurred. Hyperhidrosis is a knowns side effect of this type of medication and may have had a role in the increased sweating noted on the hamartoma.
EAH can clinically look like verrucous hemangiomas, angiokeratomas, and vascular malformations, and histopathology may be needed to differentiate between them. Eccrine nevi and EAH can be similar. Hyperhidrosis is an early and predominant component of eccrine nevi, compared with one-third of EAH.
The exact etiology of this lesion is not known. It is thought to be caused by an abnormal differentiation of the epithelium, adnexal structure, and the mesenchyme during organogenesis.3 No other associated conditions have been described with EAH.
EAH are benign lesions that rarely require treatment. If the lesions are symptomatic or because of cosmetic reasons, they can be removed surgically. There are some reports of successful treatment with pulse dual-wavelength sequential 595- and 1064-nm lasers.5 Botulinum toxin has also been used in cases of symptomatic hyperhidrosis.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. She has no conflicts. Email her at [email protected].
References
1. Smith SD et al. Pediatr Dermatol. 2019 Nov;36(6):909-12.
2. Patterson AT et al. Am J Dermatopathol. 2016;38:413-7.
3. Garcıa-Garcıa SC et al. JAAD Case Rep. 2018;4(2):165-7.
4. Awatef Kelati et al. JAAD Case Rep. 2018;4(8)835-6.
5. Felgueiras J et al. Dermatol Surg. 2015 Mar;41(3):428-30.
A biopsy of the lesion was performed which showed an increased number of eccrine glands and blood vessels within the dermis. Some areas showed an increase in adipocytes and smooth muscle bundles. The changes were consistent with eccrine angiomatous hamartoma (EAH).
The boy was referred to vascular laser therapy for treatment of the lesion.
EAH is a rare benign vascular growth characterized by an increased number of mature eccrine glands and blood vessels in the dermis and subcutis. The lesions are mostly present on the extremities, but cases of diffuse congenital lesions and lesions on the face and trunk have also been described. The lesions can be seen at birth or during the first years of life in about half of the cases, and the others tend to occur later in puberty and rarely in adulthood.1
Clinically, EAH lesions present as red, yellow to brown papules and plaques. Different dermoscopic patterns have been described which include the popcorn pattern that presents as yellow, confluent nodules with popcornlike shapes over a background of erythema, and linear arborizing vessels. The spitzoid pattern are brown globules on a background of erythema and pseudoreticular pigmentation around the globules. The verrucous hemangiomalike pattern has a bluish-white hue, reddish-blue or bluish lacunae, as seen in our patient.2-4
Most of the lesions are asymptomatic, but in some patients, they can be associated with pain, hyperhidrosis, and sometimes bleeding. Hyperhidrosis has been reported early in the presentation or during puberty or pregnancy. Our patient had started on amphetamines when hyperhidrosis occurred. Hyperhidrosis is a knowns side effect of this type of medication and may have had a role in the increased sweating noted on the hamartoma.
EAH can clinically look like verrucous hemangiomas, angiokeratomas, and vascular malformations, and histopathology may be needed to differentiate between them. Eccrine nevi and EAH can be similar. Hyperhidrosis is an early and predominant component of eccrine nevi, compared with one-third of EAH.
The exact etiology of this lesion is not known. It is thought to be caused by an abnormal differentiation of the epithelium, adnexal structure, and the mesenchyme during organogenesis.3 No other associated conditions have been described with EAH.
EAH are benign lesions that rarely require treatment. If the lesions are symptomatic or because of cosmetic reasons, they can be removed surgically. There are some reports of successful treatment with pulse dual-wavelength sequential 595- and 1064-nm lasers.5 Botulinum toxin has also been used in cases of symptomatic hyperhidrosis.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. She has no conflicts. Email her at [email protected].
References
1. Smith SD et al. Pediatr Dermatol. 2019 Nov;36(6):909-12.
2. Patterson AT et al. Am J Dermatopathol. 2016;38:413-7.
3. Garcıa-Garcıa SC et al. JAAD Case Rep. 2018;4(2):165-7.
4. Awatef Kelati et al. JAAD Case Rep. 2018;4(8)835-6.
5. Felgueiras J et al. Dermatol Surg. 2015 Mar;41(3):428-30.
A 14-year-old male was referred to our pediatric dermatology clinic for evaluation of a lesion on the left knee that appeared at 1 year of age. The lesion has been growing with him and was not symptomatic until 6 months prior to the consultation, when it started bleeding and feeling wet.
He has a history of attention-deficit/hyperactivity disorder managed with dextroamphetamine-amphetamine. The changes noted on the knee lesion seem to occur at the same time that his ADHD medication was started.
On physical exam he had a violaceous circular plaque on the left knee.
On dermoscopy the lesion showed multiple dilated red and violaceous lacunae and whitish blue hue.
DIY nerve stimulation effective in episodic migraine
results from a phase 3 study show.
This is great news for headache patients who want to explore nondrug treatment options, said study investigator Deena E. Kuruvilla, MD, neurologist and headache specialist at the Westport Headache Institute, Connecticut.
She added that such devices “aren’t always part of the conversation when we’re discussing preventive and acute treatments with our patients. Making this a regular part of the conversation might be helpful to patients.”
The findings were presented at ANA 2021: 146th Annual Meeting of the American Neurological Association (ANA), which was held online.
A key therapeutic target
The randomized, double-blind trial compared E-TNS with sham stimulation for the acute treatment of migraine.
The E-TNS device (Verum Cefaly Abortive Program) stimulates the supraorbital nerve in the forehead. “This nerve is a branch of the trigeminal nerve, which is thought to be the key player in migraine pathophysiology,” Dr. Kuruvilla noted.
The device has been cleared by the U.S. Food and Drug Administration for acute and preventive treatment of migraine.
During a run-in period before randomization, patients were asked to keep a detailed headache diary and to become comfortable using the trial device to treat an acute migraine attack at home.
The study enrolled 538 adult patients at 10 centers. The patients were aged 18 to 65 years, and they had been having episodic migraines, with or without aura, for at least a year. The participants had to have received a migraine diagnosis before age 50, and they had to be experiencing an attack of migraine 2 to 8 days per month.
The patients used the device only for a migraine of at least moderate intensity that was accompanied by at least one migraine-associated symptom, such as photophobia, phonophobia, or nausea. They were asked not to take rescue medication prior to or during a therapy session.
Study participants applied either neurostimulation or sham stimulation for a continuous 2-hour period within 4 hours of a migraine attack over the 2-month study period.
The two primary endpoints were pain freedom and freedom from the most bothersome migraine-associated symptoms at 2 hours.
Compared to sham treatment, active stimulation was more effective in achieving pain freedom (P = .043) and freedom from the most bothersome migraine-associated symptom (P = .001) at 2 hours.
“So the study did meet both primary endpoints with statistical significance,” said Dr. Kuruvilla.
The five secondary endpoints included pain relief at 2 hours; absence of all migraine-associated symptoms at 2 hours; use of rescue medication within 24 hours; sustained pain freedom at 24 hours; and sustained pain relief at 24 hours.
All but one of these endpoints reached statistical significance, showing superiority for the active intervention. The only exception was in regard to use of rescue medication.
The most common adverse event (AE) was forehead paresthesia, discomfort, or burning, which was more common in the active-treatment group than in the sham-treatment group (P = .009). There were four cases of nausea or vomiting in the active-treatment group and none in the sham-treatment group. There were no serious AEs.
Available over the counter
Both moderators of the headache poster tour that featured this study – Justin C. McArthur, MBBS, from Johns Hopkins University, Baltimore, and Steven Galetta, MD, from NYU Grossman School of Medicine – praised the presentation.
Dr. Galetta questioned whether patients were receiving preventive therapies. Dr. Kuruvilla said that the patients were allowed to enter the trial while taking preventive therapies, including antiepileptic treatments, blood pressure medications, and antidepressants, but that they had to be receiving stable doses.
The investigators didn’t distinguish between participants who were taking preventive therapies and those who weren’t, she said. “The aim was really to look at acute treatment for migraine,” and patients taking such medication “had been stable on their regimen for a pretty prolonged period of time.”
Dr. McArthur asked about the origin of the nausea some patients experienced.
It was difficult to determine whether the nausea was an aspect of an individual patient’s migraine attack or was an effect of the stimulation, said Dr. Kuruvilla. She noted that some patients found the vibrating sensation from the device uncomfortable and that nausea could be associated with pain at the site.
The device costs $300 to $400 (U.S.) and is available over the counter.
Dr. Kuruvilla is a consultant for Cefaly, Neurolief, Theranica, Now What Media, and Kx Advisors. She is on the speakers bureau for AbbVie/Allergan, Amgen/Novartis, Lilly, the American Headache Society, Biohaven, and CME meeting, and she is on an advisory board at AbbVie/Allergan, Lilly, Theranica, and Amgen/Novartis. She is editor and associate editor of Healthline and is an author for WebMD/Medscape, Healthline.
A version of this article first appeared on Medscape.com.
results from a phase 3 study show.
This is great news for headache patients who want to explore nondrug treatment options, said study investigator Deena E. Kuruvilla, MD, neurologist and headache specialist at the Westport Headache Institute, Connecticut.
She added that such devices “aren’t always part of the conversation when we’re discussing preventive and acute treatments with our patients. Making this a regular part of the conversation might be helpful to patients.”
The findings were presented at ANA 2021: 146th Annual Meeting of the American Neurological Association (ANA), which was held online.
A key therapeutic target
The randomized, double-blind trial compared E-TNS with sham stimulation for the acute treatment of migraine.
The E-TNS device (Verum Cefaly Abortive Program) stimulates the supraorbital nerve in the forehead. “This nerve is a branch of the trigeminal nerve, which is thought to be the key player in migraine pathophysiology,” Dr. Kuruvilla noted.
The device has been cleared by the U.S. Food and Drug Administration for acute and preventive treatment of migraine.
During a run-in period before randomization, patients were asked to keep a detailed headache diary and to become comfortable using the trial device to treat an acute migraine attack at home.
The study enrolled 538 adult patients at 10 centers. The patients were aged 18 to 65 years, and they had been having episodic migraines, with or without aura, for at least a year. The participants had to have received a migraine diagnosis before age 50, and they had to be experiencing an attack of migraine 2 to 8 days per month.
The patients used the device only for a migraine of at least moderate intensity that was accompanied by at least one migraine-associated symptom, such as photophobia, phonophobia, or nausea. They were asked not to take rescue medication prior to or during a therapy session.
Study participants applied either neurostimulation or sham stimulation for a continuous 2-hour period within 4 hours of a migraine attack over the 2-month study period.
The two primary endpoints were pain freedom and freedom from the most bothersome migraine-associated symptoms at 2 hours.
Compared to sham treatment, active stimulation was more effective in achieving pain freedom (P = .043) and freedom from the most bothersome migraine-associated symptom (P = .001) at 2 hours.
“So the study did meet both primary endpoints with statistical significance,” said Dr. Kuruvilla.
The five secondary endpoints included pain relief at 2 hours; absence of all migraine-associated symptoms at 2 hours; use of rescue medication within 24 hours; sustained pain freedom at 24 hours; and sustained pain relief at 24 hours.
All but one of these endpoints reached statistical significance, showing superiority for the active intervention. The only exception was in regard to use of rescue medication.
The most common adverse event (AE) was forehead paresthesia, discomfort, or burning, which was more common in the active-treatment group than in the sham-treatment group (P = .009). There were four cases of nausea or vomiting in the active-treatment group and none in the sham-treatment group. There were no serious AEs.
Available over the counter
Both moderators of the headache poster tour that featured this study – Justin C. McArthur, MBBS, from Johns Hopkins University, Baltimore, and Steven Galetta, MD, from NYU Grossman School of Medicine – praised the presentation.
Dr. Galetta questioned whether patients were receiving preventive therapies. Dr. Kuruvilla said that the patients were allowed to enter the trial while taking preventive therapies, including antiepileptic treatments, blood pressure medications, and antidepressants, but that they had to be receiving stable doses.
The investigators didn’t distinguish between participants who were taking preventive therapies and those who weren’t, she said. “The aim was really to look at acute treatment for migraine,” and patients taking such medication “had been stable on their regimen for a pretty prolonged period of time.”
Dr. McArthur asked about the origin of the nausea some patients experienced.
It was difficult to determine whether the nausea was an aspect of an individual patient’s migraine attack or was an effect of the stimulation, said Dr. Kuruvilla. She noted that some patients found the vibrating sensation from the device uncomfortable and that nausea could be associated with pain at the site.
The device costs $300 to $400 (U.S.) and is available over the counter.
Dr. Kuruvilla is a consultant for Cefaly, Neurolief, Theranica, Now What Media, and Kx Advisors. She is on the speakers bureau for AbbVie/Allergan, Amgen/Novartis, Lilly, the American Headache Society, Biohaven, and CME meeting, and she is on an advisory board at AbbVie/Allergan, Lilly, Theranica, and Amgen/Novartis. She is editor and associate editor of Healthline and is an author for WebMD/Medscape, Healthline.
A version of this article first appeared on Medscape.com.
results from a phase 3 study show.
This is great news for headache patients who want to explore nondrug treatment options, said study investigator Deena E. Kuruvilla, MD, neurologist and headache specialist at the Westport Headache Institute, Connecticut.
She added that such devices “aren’t always part of the conversation when we’re discussing preventive and acute treatments with our patients. Making this a regular part of the conversation might be helpful to patients.”
The findings were presented at ANA 2021: 146th Annual Meeting of the American Neurological Association (ANA), which was held online.
A key therapeutic target
The randomized, double-blind trial compared E-TNS with sham stimulation for the acute treatment of migraine.
The E-TNS device (Verum Cefaly Abortive Program) stimulates the supraorbital nerve in the forehead. “This nerve is a branch of the trigeminal nerve, which is thought to be the key player in migraine pathophysiology,” Dr. Kuruvilla noted.
The device has been cleared by the U.S. Food and Drug Administration for acute and preventive treatment of migraine.
During a run-in period before randomization, patients were asked to keep a detailed headache diary and to become comfortable using the trial device to treat an acute migraine attack at home.
The study enrolled 538 adult patients at 10 centers. The patients were aged 18 to 65 years, and they had been having episodic migraines, with or without aura, for at least a year. The participants had to have received a migraine diagnosis before age 50, and they had to be experiencing an attack of migraine 2 to 8 days per month.
The patients used the device only for a migraine of at least moderate intensity that was accompanied by at least one migraine-associated symptom, such as photophobia, phonophobia, or nausea. They were asked not to take rescue medication prior to or during a therapy session.
Study participants applied either neurostimulation or sham stimulation for a continuous 2-hour period within 4 hours of a migraine attack over the 2-month study period.
The two primary endpoints were pain freedom and freedom from the most bothersome migraine-associated symptoms at 2 hours.
Compared to sham treatment, active stimulation was more effective in achieving pain freedom (P = .043) and freedom from the most bothersome migraine-associated symptom (P = .001) at 2 hours.
“So the study did meet both primary endpoints with statistical significance,” said Dr. Kuruvilla.
The five secondary endpoints included pain relief at 2 hours; absence of all migraine-associated symptoms at 2 hours; use of rescue medication within 24 hours; sustained pain freedom at 24 hours; and sustained pain relief at 24 hours.
All but one of these endpoints reached statistical significance, showing superiority for the active intervention. The only exception was in regard to use of rescue medication.
The most common adverse event (AE) was forehead paresthesia, discomfort, or burning, which was more common in the active-treatment group than in the sham-treatment group (P = .009). There were four cases of nausea or vomiting in the active-treatment group and none in the sham-treatment group. There were no serious AEs.
Available over the counter
Both moderators of the headache poster tour that featured this study – Justin C. McArthur, MBBS, from Johns Hopkins University, Baltimore, and Steven Galetta, MD, from NYU Grossman School of Medicine – praised the presentation.
Dr. Galetta questioned whether patients were receiving preventive therapies. Dr. Kuruvilla said that the patients were allowed to enter the trial while taking preventive therapies, including antiepileptic treatments, blood pressure medications, and antidepressants, but that they had to be receiving stable doses.
The investigators didn’t distinguish between participants who were taking preventive therapies and those who weren’t, she said. “The aim was really to look at acute treatment for migraine,” and patients taking such medication “had been stable on their regimen for a pretty prolonged period of time.”
Dr. McArthur asked about the origin of the nausea some patients experienced.
It was difficult to determine whether the nausea was an aspect of an individual patient’s migraine attack or was an effect of the stimulation, said Dr. Kuruvilla. She noted that some patients found the vibrating sensation from the device uncomfortable and that nausea could be associated with pain at the site.
The device costs $300 to $400 (U.S.) and is available over the counter.
Dr. Kuruvilla is a consultant for Cefaly, Neurolief, Theranica, Now What Media, and Kx Advisors. She is on the speakers bureau for AbbVie/Allergan, Amgen/Novartis, Lilly, the American Headache Society, Biohaven, and CME meeting, and she is on an advisory board at AbbVie/Allergan, Lilly, Theranica, and Amgen/Novartis. She is editor and associate editor of Healthline and is an author for WebMD/Medscape, Healthline.
A version of this article first appeared on Medscape.com.
FROM ANA
Children and COVID: Vaccinations lower than ever as cases continue to drop
As the COVID-19 vaccine heads toward approval for children under age 12 years, the number of older children receiving it dropped for the 10th consecutive week, based on data from the Centers for Disease Control and Prevention.

Over 47% of all children aged 12-17 years – that’s close to 12 million eligible individuals – have not received even one dose of COVID-19 vaccine, and less than 44% (about 11.1 million) were fully vaccinated as of Oct. 18, the CDC reported on its COVID Data Tracker.
, when eligibility expanded to include 12- to 15-year-olds, according to the CDC data, which also show that weekly vaccinations have never been lower.
Fortunately, the decline in new cases also continued, as the national total fell for a 6th straight week. There were more than 130,000 child cases reported during the week of Oct. 8-14, compared with 148,000 the previous week and the high of almost 252,000 in late August/early September, the American Academy of Pediatrics and the Children’s Hospital Association said in their weekly COVID-19 report.
That brings the cumulative count to 6.18 million, with children accounting for 16.4% of all cases reported since the start of the pandemic. For the week of Oct. 8-14, children represented 25.5% of all COVID-19 cases in the 46 states with up-to-date online dashboards, the AAP and CHA said, noting that New York has never reported age ranges for cases and that Alabama, Nebraska, and Texas stopped reporting over the summer.
Current data indicate that child cases in California now exceed 671,000, more than any other state, followed by Florida with 439,000 (the state defines a child as someone aged 0-14 years) and Illinois with 301,000. Vermont has the highest proportion of COVID-19 cases occurring in children (24.3%), with Alaska (24.1%) and South Carolina (23.2%) just behind. The highest rate of cases – 15,569 per 100,000 children – can be found in South Carolina, while the lowest is in Hawaii (4,838 per 100,000), the AAP and CHA reported.
The total number of COVID-related deaths in children is 681 as of Oct. 18, according to the CDC, with the AAP/CHA reporting 558 as of Oct. 14, based on data from 45 states, New York City, Puerto Rico, and Guam. The CDC reports 65,655 admissions since Aug. 1, 2020, in children aged 0-17 years, and the AAP/CHA tally 23,582 since May 5, 2020, among children in 24 states and New York City.
As the COVID-19 vaccine heads toward approval for children under age 12 years, the number of older children receiving it dropped for the 10th consecutive week, based on data from the Centers for Disease Control and Prevention.

Over 47% of all children aged 12-17 years – that’s close to 12 million eligible individuals – have not received even one dose of COVID-19 vaccine, and less than 44% (about 11.1 million) were fully vaccinated as of Oct. 18, the CDC reported on its COVID Data Tracker.
, when eligibility expanded to include 12- to 15-year-olds, according to the CDC data, which also show that weekly vaccinations have never been lower.
Fortunately, the decline in new cases also continued, as the national total fell for a 6th straight week. There were more than 130,000 child cases reported during the week of Oct. 8-14, compared with 148,000 the previous week and the high of almost 252,000 in late August/early September, the American Academy of Pediatrics and the Children’s Hospital Association said in their weekly COVID-19 report.
That brings the cumulative count to 6.18 million, with children accounting for 16.4% of all cases reported since the start of the pandemic. For the week of Oct. 8-14, children represented 25.5% of all COVID-19 cases in the 46 states with up-to-date online dashboards, the AAP and CHA said, noting that New York has never reported age ranges for cases and that Alabama, Nebraska, and Texas stopped reporting over the summer.
Current data indicate that child cases in California now exceed 671,000, more than any other state, followed by Florida with 439,000 (the state defines a child as someone aged 0-14 years) and Illinois with 301,000. Vermont has the highest proportion of COVID-19 cases occurring in children (24.3%), with Alaska (24.1%) and South Carolina (23.2%) just behind. The highest rate of cases – 15,569 per 100,000 children – can be found in South Carolina, while the lowest is in Hawaii (4,838 per 100,000), the AAP and CHA reported.
The total number of COVID-related deaths in children is 681 as of Oct. 18, according to the CDC, with the AAP/CHA reporting 558 as of Oct. 14, based on data from 45 states, New York City, Puerto Rico, and Guam. The CDC reports 65,655 admissions since Aug. 1, 2020, in children aged 0-17 years, and the AAP/CHA tally 23,582 since May 5, 2020, among children in 24 states and New York City.
As the COVID-19 vaccine heads toward approval for children under age 12 years, the number of older children receiving it dropped for the 10th consecutive week, based on data from the Centers for Disease Control and Prevention.

Over 47% of all children aged 12-17 years – that’s close to 12 million eligible individuals – have not received even one dose of COVID-19 vaccine, and less than 44% (about 11.1 million) were fully vaccinated as of Oct. 18, the CDC reported on its COVID Data Tracker.
, when eligibility expanded to include 12- to 15-year-olds, according to the CDC data, which also show that weekly vaccinations have never been lower.
Fortunately, the decline in new cases also continued, as the national total fell for a 6th straight week. There were more than 130,000 child cases reported during the week of Oct. 8-14, compared with 148,000 the previous week and the high of almost 252,000 in late August/early September, the American Academy of Pediatrics and the Children’s Hospital Association said in their weekly COVID-19 report.
That brings the cumulative count to 6.18 million, with children accounting for 16.4% of all cases reported since the start of the pandemic. For the week of Oct. 8-14, children represented 25.5% of all COVID-19 cases in the 46 states with up-to-date online dashboards, the AAP and CHA said, noting that New York has never reported age ranges for cases and that Alabama, Nebraska, and Texas stopped reporting over the summer.
Current data indicate that child cases in California now exceed 671,000, more than any other state, followed by Florida with 439,000 (the state defines a child as someone aged 0-14 years) and Illinois with 301,000. Vermont has the highest proportion of COVID-19 cases occurring in children (24.3%), with Alaska (24.1%) and South Carolina (23.2%) just behind. The highest rate of cases – 15,569 per 100,000 children – can be found in South Carolina, while the lowest is in Hawaii (4,838 per 100,000), the AAP and CHA reported.
The total number of COVID-related deaths in children is 681 as of Oct. 18, according to the CDC, with the AAP/CHA reporting 558 as of Oct. 14, based on data from 45 states, New York City, Puerto Rico, and Guam. The CDC reports 65,655 admissions since Aug. 1, 2020, in children aged 0-17 years, and the AAP/CHA tally 23,582 since May 5, 2020, among children in 24 states and New York City.
FDA expands use of HIV drug to young children
The new lower dose is approved for children weighing from at least 14 kg (30 pounds) to 25 kg (55 pounds) who are virologically suppressed or new to antiretroviral therapy.
“Children living with HIV are in need of effective and accessible formulations of antiretroviral therapy,” said Merdad Parsey, MD, PhD, chief medical officer of Gilead Sciences, the company that produces Biktarvy, in a press release. “The New Drug Application approval is an important step in fulfilling Gilead’s commitment to a goal of bringing pediatric formulations of Biktarvy to children living with HIV around the world,” he said.
Although advances in treatment for pregnant women with HIV have lowered the likelihood of perinatal HIV transmission, pediatric HIV remains a global public health challenge. In 2020, about 1.7 million children younger than 15 years were living with HIV worldwide; 850 children become infected every day.
The approval, announced October 18, expands the use of Biktarvy to younger children. The medication was originally approved in February 2018 for treatment-naive or virologically suppressed adults. In June 2019, the FDA approved updating of the label to include pediatric patients weighing at least 25 kg. This new lower dose of Biktarvy is for a three-drug combo containing bictegravir 30 mg, emtricitabine 120 mg, and tenofovir alafenamide 15 mg. It is given once a day in tablet form.
The most recent expanded indication was based on data from an open-label, single-arm study that included 22 virologically suppressed children living with HIV. After switching to Biktarvy, 91% of participants (20 of 22) remained virologically suppressed at 24 weeks. HIV-1 RNA was not collected for two patients because of «pandemic-related study disruption,» the press release said.
“As children living with HIV will be on therapy for the foreseeable future and from such a young age, there are a number of factors I weigh as a clinician when prescribing the right HIV treatment option to my pediatric patients,” said Carina Rodriguez, MD, the division chief of pediatric infectious diseases at the University of South Florida, who was one of the study investigators. “Finding an efficacious treatment option is paramount, but tolerability and safety are keys to ensuring treatment success. With this expanded approval, clinicians can add Biktarvy to their arsenal of options to help ensure these children maintain virologic suppression with a treatment option that makes sense for them.”
A version of this article first appeared on Medscape.com.
The new lower dose is approved for children weighing from at least 14 kg (30 pounds) to 25 kg (55 pounds) who are virologically suppressed or new to antiretroviral therapy.
“Children living with HIV are in need of effective and accessible formulations of antiretroviral therapy,” said Merdad Parsey, MD, PhD, chief medical officer of Gilead Sciences, the company that produces Biktarvy, in a press release. “The New Drug Application approval is an important step in fulfilling Gilead’s commitment to a goal of bringing pediatric formulations of Biktarvy to children living with HIV around the world,” he said.
Although advances in treatment for pregnant women with HIV have lowered the likelihood of perinatal HIV transmission, pediatric HIV remains a global public health challenge. In 2020, about 1.7 million children younger than 15 years were living with HIV worldwide; 850 children become infected every day.
The approval, announced October 18, expands the use of Biktarvy to younger children. The medication was originally approved in February 2018 for treatment-naive or virologically suppressed adults. In June 2019, the FDA approved updating of the label to include pediatric patients weighing at least 25 kg. This new lower dose of Biktarvy is for a three-drug combo containing bictegravir 30 mg, emtricitabine 120 mg, and tenofovir alafenamide 15 mg. It is given once a day in tablet form.
The most recent expanded indication was based on data from an open-label, single-arm study that included 22 virologically suppressed children living with HIV. After switching to Biktarvy, 91% of participants (20 of 22) remained virologically suppressed at 24 weeks. HIV-1 RNA was not collected for two patients because of «pandemic-related study disruption,» the press release said.
“As children living with HIV will be on therapy for the foreseeable future and from such a young age, there are a number of factors I weigh as a clinician when prescribing the right HIV treatment option to my pediatric patients,” said Carina Rodriguez, MD, the division chief of pediatric infectious diseases at the University of South Florida, who was one of the study investigators. “Finding an efficacious treatment option is paramount, but tolerability and safety are keys to ensuring treatment success. With this expanded approval, clinicians can add Biktarvy to their arsenal of options to help ensure these children maintain virologic suppression with a treatment option that makes sense for them.”
A version of this article first appeared on Medscape.com.
The new lower dose is approved for children weighing from at least 14 kg (30 pounds) to 25 kg (55 pounds) who are virologically suppressed or new to antiretroviral therapy.
“Children living with HIV are in need of effective and accessible formulations of antiretroviral therapy,” said Merdad Parsey, MD, PhD, chief medical officer of Gilead Sciences, the company that produces Biktarvy, in a press release. “The New Drug Application approval is an important step in fulfilling Gilead’s commitment to a goal of bringing pediatric formulations of Biktarvy to children living with HIV around the world,” he said.
Although advances in treatment for pregnant women with HIV have lowered the likelihood of perinatal HIV transmission, pediatric HIV remains a global public health challenge. In 2020, about 1.7 million children younger than 15 years were living with HIV worldwide; 850 children become infected every day.
The approval, announced October 18, expands the use of Biktarvy to younger children. The medication was originally approved in February 2018 for treatment-naive or virologically suppressed adults. In June 2019, the FDA approved updating of the label to include pediatric patients weighing at least 25 kg. This new lower dose of Biktarvy is for a three-drug combo containing bictegravir 30 mg, emtricitabine 120 mg, and tenofovir alafenamide 15 mg. It is given once a day in tablet form.
The most recent expanded indication was based on data from an open-label, single-arm study that included 22 virologically suppressed children living with HIV. After switching to Biktarvy, 91% of participants (20 of 22) remained virologically suppressed at 24 weeks. HIV-1 RNA was not collected for two patients because of «pandemic-related study disruption,» the press release said.
“As children living with HIV will be on therapy for the foreseeable future and from such a young age, there are a number of factors I weigh as a clinician when prescribing the right HIV treatment option to my pediatric patients,” said Carina Rodriguez, MD, the division chief of pediatric infectious diseases at the University of South Florida, who was one of the study investigators. “Finding an efficacious treatment option is paramount, but tolerability and safety are keys to ensuring treatment success. With this expanded approval, clinicians can add Biktarvy to their arsenal of options to help ensure these children maintain virologic suppression with a treatment option that makes sense for them.”
A version of this article first appeared on Medscape.com.
National Academies issue guidance for childhood COVID-19 vaccines
While the U.S. Food and Drug Administration has yet to give the green light to COVID-19 vaccination for children who are under age 12, it is expected that approval will be granted. In anticipation of the FDA’s go-ahead, which is expected in the coming weeks, a new “rapid expert consultation” has identified “actionable guidance” that state and local decision-makers can use to communicate with the public. The goal is to build confidence in and promote the uptake of COVID-19 vaccines, especially for parents who are contemplating vaccinating their children.
They note that key factors in decision-making concern vaccine side effects, the efficacy of the vaccine in children, availability of research in their child’s age group, research conducted by the parents themselves, and recommendations by the child’s health care provider.
“One of the reasons that the COVID vaccine only became available for children 12 and over months after it was approved for adults is that it takes time and many, many trial participants who are closely monitored before the vaccine ever reaches the general public,” said Nusheen Ameenuddin, MD, MPH, MPA, an assistant professor of pediatrics at the Mayo Clinic, Rochester, Minn. “We continue to talk to parents about the fact that the vaccines have been very safe and effective in this group, and even though people are concerned about side effects, they are much milder and less frequent than the effects of the disease itself.”
Dr. Ameenuddin noted that the lack of data in this age group can be concerning for parents. “It’s not like other vaccines which have been available for a long time, and the clinical trial data are still limited for this age group,” she said. “But I think the main point that practitioners need to emphasize is that, even though the vaccine is new, the science for this vaccine has been around for about a decade.”
The unique circumstances of a pandemic, she pointed out, allowed for important information about effectiveness, safety, and side effects to be obtained more quickly from clinical trial data.
“We have really good evidence for kids 12 and over, about safety and effectiveness, and even though children are not small adults and have their own unique physiology, this has provided a good starting point to suggest that kids slightly younger will also respond well to the vaccines,” said Dr. Ameenuddin, who is also chair of the American Academy of Pediatrics Council on Communications and Media. “As we learn more, we can start gathering more information about even younger kids to ensure that the right dosage and spacing of vaccines can provide maximum vaccine effectiveness and protection from disease.”
The guidance was published Oct. 13 by the National Academies of Sciences, Engineering, and Medicine.
The rapid expert consultation was produced through the Societal Experts Action Network, an activity of the National Academies that is sponsored by the NASEM and the Alfred P. Sloan Foundation. The goal of SEAN is to connect researchers in the social, behavioral, and economic sciences with decision-makers to respond to policy questions related to the COVID-19 pandemic.
In their expert consultation, the authors emphasize that vaccination is critical for decreasing transmission and controlling infection, as well as limiting the emergence of future serious variants. As of Oct. 3, 2021, about 65% of the U.S. population had received at least one dose of the vaccine, and the rate has begun to lag in many areas of the country. There are a variety of reasons for vaccine hesitancy, they note, including perception of low risks from COVID-19 or of high risks from COVID-19 vaccines, exposure to media, political agendas, lack of confidence in science, and distrust of the medical establishment. The Pfizer/BioNTech vaccine is currently authorized for emergency use for individuals 12 years of age and older and fully approved for those aged 16 and older, while the Moderna and the Johnson & Johnson vaccines are authorized for emergency use for those 18 years of age and older.
Many children between the ages of 12 and 17 have not been vaccinated, and the major concerns reported by parents include not knowing enough about the long-term effects of the COVID-19 vaccine in children (88%), concerns about children experiencing serious side effects (79%), and concerns that the COVID-19 vaccine might negatively affect future fertility (73%).
The National Academies have previously released two other “rapid expert consultations” which have addressed building vaccine confidence, and both reports provide key strategies for communicating information about COVID-19 vaccines. In this paper, the focus was on communicating with parents to gain confidence in the vaccine and address concerns.
Key points
The key strategies highlighted for communicating with parents include the following:
- Emphasizing safety and efficacy: Parents should be informed about the ongoing research and clinical trials that will answer more questions about the vaccine and that there is continued monitoring for any safety risks. Pointing to the safety data from the clinical trials for 12- to 17-year-olds, and the lack of serious adverse events from the vaccine in this age group may help alleviate concerns.
- CalibriEncouraging parents to talk with a primary care provider: Research shows that parents trust family physicians and other health care practitioners to provide them with accurate information about vaccines. Local, state, and national leaders can provide messaging templates and other resources to health care professionals who are engaged in these conversations.
- Leveraging social networks to influence parents’ vaccination decisions: Parents are influenced by their social network connections. It is important to engage these networks, especially with members of their community who are considered trustworthy and influential. Social networks may also be very diverse, and include family members, friends, coworkers, social media, and members of their religious community.
While the guidance states that different groups of parents will require different messaging, they suggest that communication can begin with a focus on the things that vaccination can accomplish. In addition to preventing infection with COVID-19, it will allow children to attend school in person and participate in extracurricular activities such as sports, without risking their health. “One thing I’ve learned over several years of working with vaccine-hesitant parents is that you have to tailor each approach to the individual,” said Dr. Ameenuddin. “Different people have different concerns, and first and foremost, it’s important to listen.”
For some parents, emphasizing that the more people that can be vaccinated and the sooner it can be done, the sooner everyone can return to a normal life is a good approach, she added. “I think it’s important to emphasize both the individual and communal benefits of vaccines, but that won’t necessarily reach every person with concerns. I think it’s important to find out what is most important to individuals and work from there to find a way to connect with that family to encourage vaccination.”
Dr. Ameenuddin has no disclosures.
While the U.S. Food and Drug Administration has yet to give the green light to COVID-19 vaccination for children who are under age 12, it is expected that approval will be granted. In anticipation of the FDA’s go-ahead, which is expected in the coming weeks, a new “rapid expert consultation” has identified “actionable guidance” that state and local decision-makers can use to communicate with the public. The goal is to build confidence in and promote the uptake of COVID-19 vaccines, especially for parents who are contemplating vaccinating their children.
They note that key factors in decision-making concern vaccine side effects, the efficacy of the vaccine in children, availability of research in their child’s age group, research conducted by the parents themselves, and recommendations by the child’s health care provider.
“One of the reasons that the COVID vaccine only became available for children 12 and over months after it was approved for adults is that it takes time and many, many trial participants who are closely monitored before the vaccine ever reaches the general public,” said Nusheen Ameenuddin, MD, MPH, MPA, an assistant professor of pediatrics at the Mayo Clinic, Rochester, Minn. “We continue to talk to parents about the fact that the vaccines have been very safe and effective in this group, and even though people are concerned about side effects, they are much milder and less frequent than the effects of the disease itself.”
Dr. Ameenuddin noted that the lack of data in this age group can be concerning for parents. “It’s not like other vaccines which have been available for a long time, and the clinical trial data are still limited for this age group,” she said. “But I think the main point that practitioners need to emphasize is that, even though the vaccine is new, the science for this vaccine has been around for about a decade.”
The unique circumstances of a pandemic, she pointed out, allowed for important information about effectiveness, safety, and side effects to be obtained more quickly from clinical trial data.
“We have really good evidence for kids 12 and over, about safety and effectiveness, and even though children are not small adults and have their own unique physiology, this has provided a good starting point to suggest that kids slightly younger will also respond well to the vaccines,” said Dr. Ameenuddin, who is also chair of the American Academy of Pediatrics Council on Communications and Media. “As we learn more, we can start gathering more information about even younger kids to ensure that the right dosage and spacing of vaccines can provide maximum vaccine effectiveness and protection from disease.”
The guidance was published Oct. 13 by the National Academies of Sciences, Engineering, and Medicine.
The rapid expert consultation was produced through the Societal Experts Action Network, an activity of the National Academies that is sponsored by the NASEM and the Alfred P. Sloan Foundation. The goal of SEAN is to connect researchers in the social, behavioral, and economic sciences with decision-makers to respond to policy questions related to the COVID-19 pandemic.
In their expert consultation, the authors emphasize that vaccination is critical for decreasing transmission and controlling infection, as well as limiting the emergence of future serious variants. As of Oct. 3, 2021, about 65% of the U.S. population had received at least one dose of the vaccine, and the rate has begun to lag in many areas of the country. There are a variety of reasons for vaccine hesitancy, they note, including perception of low risks from COVID-19 or of high risks from COVID-19 vaccines, exposure to media, political agendas, lack of confidence in science, and distrust of the medical establishment. The Pfizer/BioNTech vaccine is currently authorized for emergency use for individuals 12 years of age and older and fully approved for those aged 16 and older, while the Moderna and the Johnson & Johnson vaccines are authorized for emergency use for those 18 years of age and older.
Many children between the ages of 12 and 17 have not been vaccinated, and the major concerns reported by parents include not knowing enough about the long-term effects of the COVID-19 vaccine in children (88%), concerns about children experiencing serious side effects (79%), and concerns that the COVID-19 vaccine might negatively affect future fertility (73%).
The National Academies have previously released two other “rapid expert consultations” which have addressed building vaccine confidence, and both reports provide key strategies for communicating information about COVID-19 vaccines. In this paper, the focus was on communicating with parents to gain confidence in the vaccine and address concerns.
Key points
The key strategies highlighted for communicating with parents include the following:
- Emphasizing safety and efficacy: Parents should be informed about the ongoing research and clinical trials that will answer more questions about the vaccine and that there is continued monitoring for any safety risks. Pointing to the safety data from the clinical trials for 12- to 17-year-olds, and the lack of serious adverse events from the vaccine in this age group may help alleviate concerns.
- CalibriEncouraging parents to talk with a primary care provider: Research shows that parents trust family physicians and other health care practitioners to provide them with accurate information about vaccines. Local, state, and national leaders can provide messaging templates and other resources to health care professionals who are engaged in these conversations.
- Leveraging social networks to influence parents’ vaccination decisions: Parents are influenced by their social network connections. It is important to engage these networks, especially with members of their community who are considered trustworthy and influential. Social networks may also be very diverse, and include family members, friends, coworkers, social media, and members of their religious community.
While the guidance states that different groups of parents will require different messaging, they suggest that communication can begin with a focus on the things that vaccination can accomplish. In addition to preventing infection with COVID-19, it will allow children to attend school in person and participate in extracurricular activities such as sports, without risking their health. “One thing I’ve learned over several years of working with vaccine-hesitant parents is that you have to tailor each approach to the individual,” said Dr. Ameenuddin. “Different people have different concerns, and first and foremost, it’s important to listen.”
For some parents, emphasizing that the more people that can be vaccinated and the sooner it can be done, the sooner everyone can return to a normal life is a good approach, she added. “I think it’s important to emphasize both the individual and communal benefits of vaccines, but that won’t necessarily reach every person with concerns. I think it’s important to find out what is most important to individuals and work from there to find a way to connect with that family to encourage vaccination.”
Dr. Ameenuddin has no disclosures.
While the U.S. Food and Drug Administration has yet to give the green light to COVID-19 vaccination for children who are under age 12, it is expected that approval will be granted. In anticipation of the FDA’s go-ahead, which is expected in the coming weeks, a new “rapid expert consultation” has identified “actionable guidance” that state and local decision-makers can use to communicate with the public. The goal is to build confidence in and promote the uptake of COVID-19 vaccines, especially for parents who are contemplating vaccinating their children.
They note that key factors in decision-making concern vaccine side effects, the efficacy of the vaccine in children, availability of research in their child’s age group, research conducted by the parents themselves, and recommendations by the child’s health care provider.
“One of the reasons that the COVID vaccine only became available for children 12 and over months after it was approved for adults is that it takes time and many, many trial participants who are closely monitored before the vaccine ever reaches the general public,” said Nusheen Ameenuddin, MD, MPH, MPA, an assistant professor of pediatrics at the Mayo Clinic, Rochester, Minn. “We continue to talk to parents about the fact that the vaccines have been very safe and effective in this group, and even though people are concerned about side effects, they are much milder and less frequent than the effects of the disease itself.”
Dr. Ameenuddin noted that the lack of data in this age group can be concerning for parents. “It’s not like other vaccines which have been available for a long time, and the clinical trial data are still limited for this age group,” she said. “But I think the main point that practitioners need to emphasize is that, even though the vaccine is new, the science for this vaccine has been around for about a decade.”
The unique circumstances of a pandemic, she pointed out, allowed for important information about effectiveness, safety, and side effects to be obtained more quickly from clinical trial data.
“We have really good evidence for kids 12 and over, about safety and effectiveness, and even though children are not small adults and have their own unique physiology, this has provided a good starting point to suggest that kids slightly younger will also respond well to the vaccines,” said Dr. Ameenuddin, who is also chair of the American Academy of Pediatrics Council on Communications and Media. “As we learn more, we can start gathering more information about even younger kids to ensure that the right dosage and spacing of vaccines can provide maximum vaccine effectiveness and protection from disease.”
The guidance was published Oct. 13 by the National Academies of Sciences, Engineering, and Medicine.
The rapid expert consultation was produced through the Societal Experts Action Network, an activity of the National Academies that is sponsored by the NASEM and the Alfred P. Sloan Foundation. The goal of SEAN is to connect researchers in the social, behavioral, and economic sciences with decision-makers to respond to policy questions related to the COVID-19 pandemic.
In their expert consultation, the authors emphasize that vaccination is critical for decreasing transmission and controlling infection, as well as limiting the emergence of future serious variants. As of Oct. 3, 2021, about 65% of the U.S. population had received at least one dose of the vaccine, and the rate has begun to lag in many areas of the country. There are a variety of reasons for vaccine hesitancy, they note, including perception of low risks from COVID-19 or of high risks from COVID-19 vaccines, exposure to media, political agendas, lack of confidence in science, and distrust of the medical establishment. The Pfizer/BioNTech vaccine is currently authorized for emergency use for individuals 12 years of age and older and fully approved for those aged 16 and older, while the Moderna and the Johnson & Johnson vaccines are authorized for emergency use for those 18 years of age and older.
Many children between the ages of 12 and 17 have not been vaccinated, and the major concerns reported by parents include not knowing enough about the long-term effects of the COVID-19 vaccine in children (88%), concerns about children experiencing serious side effects (79%), and concerns that the COVID-19 vaccine might negatively affect future fertility (73%).
The National Academies have previously released two other “rapid expert consultations” which have addressed building vaccine confidence, and both reports provide key strategies for communicating information about COVID-19 vaccines. In this paper, the focus was on communicating with parents to gain confidence in the vaccine and address concerns.
Key points
The key strategies highlighted for communicating with parents include the following:
- Emphasizing safety and efficacy: Parents should be informed about the ongoing research and clinical trials that will answer more questions about the vaccine and that there is continued monitoring for any safety risks. Pointing to the safety data from the clinical trials for 12- to 17-year-olds, and the lack of serious adverse events from the vaccine in this age group may help alleviate concerns.
- CalibriEncouraging parents to talk with a primary care provider: Research shows that parents trust family physicians and other health care practitioners to provide them with accurate information about vaccines. Local, state, and national leaders can provide messaging templates and other resources to health care professionals who are engaged in these conversations.
- Leveraging social networks to influence parents’ vaccination decisions: Parents are influenced by their social network connections. It is important to engage these networks, especially with members of their community who are considered trustworthy and influential. Social networks may also be very diverse, and include family members, friends, coworkers, social media, and members of their religious community.
While the guidance states that different groups of parents will require different messaging, they suggest that communication can begin with a focus on the things that vaccination can accomplish. In addition to preventing infection with COVID-19, it will allow children to attend school in person and participate in extracurricular activities such as sports, without risking their health. “One thing I’ve learned over several years of working with vaccine-hesitant parents is that you have to tailor each approach to the individual,” said Dr. Ameenuddin. “Different people have different concerns, and first and foremost, it’s important to listen.”
For some parents, emphasizing that the more people that can be vaccinated and the sooner it can be done, the sooner everyone can return to a normal life is a good approach, she added. “I think it’s important to emphasize both the individual and communal benefits of vaccines, but that won’t necessarily reach every person with concerns. I think it’s important to find out what is most important to individuals and work from there to find a way to connect with that family to encourage vaccination.”
Dr. Ameenuddin has no disclosures.
PA defends against license suspension for COVID treatment
The suspension stemmed from allegations against Scott C. Miller, PA-C, by at least six COVID patients, including some who weren’t his patients or whom he never examined and a few who later died from the virus, according to the Washington Medical Commission.
“Miller’s treatment of COVID-19 patients fell below the standard of care,” the suspension report states. “Miller began a public campaign promoting ivermectin as a curative for COVID-19, and prescribing it without adequate examination to at least one person, with no reliable clinical studies that establish its efficacy in preventing or treating COVID-19.”
Mr. Miller has until early November to respond to the allegations. On his clinic’s website, Mr. Miller stated, “In response to the charges, I want to reassure all of you that the initial attacks against me have been brought on by a small handful of people that have no ties to our medical practice, and by pharmacies and hospitals that have a zero tolerance policy on family members asking that I help them advocate for loved ones that have been admitted and written off in our current system of dismissiveness and neglect.”
Mr. Miller also expressed gratitude for the support he has received recently. A GoFundMe campaign to raise money for Mr. Miller’s legal fund had raised more than $59,000 at press time. His GoFundMe page had been shared 2,400 times. He has more than 550 followers and more than 400 donors.
“I don’t know that I have the words to adequately describe the deep sense of love and connection I have received from you, the families I serve, and those that have reached out to me in this deeply challenging time,” he wrote on the clinic website.
Mr. Miller has spoken publicly about his anti-mask views and his support for ivermectin, according to the commission report. As part of the suspension, he was charged with making “misleading representations regarding the efficacy of non-FDA approved treatment and mask use.”
In one case that was cited in the report, a 39-year-old patient contacted the pediatric clinic, and Mr. Miller spoke with the patient by phone. The patient reported that he had tested positive for COVID. Mr. Miller advised the patient to take supplements, including vitamin D and C, zinc, and melatonin, and he prescribed ivermectin, dexamethasone, and azithromycin. He did not perform an exam, verify the information that the patient had provided, advise the patient regarding interactions, or order follow-up testing, the report states.
Other charges against Mr. Miller include harassing hospital staff by making threatening statements about hospitals and doctors who treat COVID-19 patients and misrepresenting his original 2013 license application. He denied on the application that he was being investigated by another licensing board. At the time, the California Physician Assistant Board was investigating him for providing medical care and prescribing without a supervising doctor’s authorization and without conducting physical exams, among other charges.
A version of this article first appeared on Medscape.com.
The suspension stemmed from allegations against Scott C. Miller, PA-C, by at least six COVID patients, including some who weren’t his patients or whom he never examined and a few who later died from the virus, according to the Washington Medical Commission.
“Miller’s treatment of COVID-19 patients fell below the standard of care,” the suspension report states. “Miller began a public campaign promoting ivermectin as a curative for COVID-19, and prescribing it without adequate examination to at least one person, with no reliable clinical studies that establish its efficacy in preventing or treating COVID-19.”
Mr. Miller has until early November to respond to the allegations. On his clinic’s website, Mr. Miller stated, “In response to the charges, I want to reassure all of you that the initial attacks against me have been brought on by a small handful of people that have no ties to our medical practice, and by pharmacies and hospitals that have a zero tolerance policy on family members asking that I help them advocate for loved ones that have been admitted and written off in our current system of dismissiveness and neglect.”
Mr. Miller also expressed gratitude for the support he has received recently. A GoFundMe campaign to raise money for Mr. Miller’s legal fund had raised more than $59,000 at press time. His GoFundMe page had been shared 2,400 times. He has more than 550 followers and more than 400 donors.
“I don’t know that I have the words to adequately describe the deep sense of love and connection I have received from you, the families I serve, and those that have reached out to me in this deeply challenging time,” he wrote on the clinic website.
Mr. Miller has spoken publicly about his anti-mask views and his support for ivermectin, according to the commission report. As part of the suspension, he was charged with making “misleading representations regarding the efficacy of non-FDA approved treatment and mask use.”
In one case that was cited in the report, a 39-year-old patient contacted the pediatric clinic, and Mr. Miller spoke with the patient by phone. The patient reported that he had tested positive for COVID. Mr. Miller advised the patient to take supplements, including vitamin D and C, zinc, and melatonin, and he prescribed ivermectin, dexamethasone, and azithromycin. He did not perform an exam, verify the information that the patient had provided, advise the patient regarding interactions, or order follow-up testing, the report states.
Other charges against Mr. Miller include harassing hospital staff by making threatening statements about hospitals and doctors who treat COVID-19 patients and misrepresenting his original 2013 license application. He denied on the application that he was being investigated by another licensing board. At the time, the California Physician Assistant Board was investigating him for providing medical care and prescribing without a supervising doctor’s authorization and without conducting physical exams, among other charges.
A version of this article first appeared on Medscape.com.
The suspension stemmed from allegations against Scott C. Miller, PA-C, by at least six COVID patients, including some who weren’t his patients or whom he never examined and a few who later died from the virus, according to the Washington Medical Commission.
“Miller’s treatment of COVID-19 patients fell below the standard of care,” the suspension report states. “Miller began a public campaign promoting ivermectin as a curative for COVID-19, and prescribing it without adequate examination to at least one person, with no reliable clinical studies that establish its efficacy in preventing or treating COVID-19.”
Mr. Miller has until early November to respond to the allegations. On his clinic’s website, Mr. Miller stated, “In response to the charges, I want to reassure all of you that the initial attacks against me have been brought on by a small handful of people that have no ties to our medical practice, and by pharmacies and hospitals that have a zero tolerance policy on family members asking that I help them advocate for loved ones that have been admitted and written off in our current system of dismissiveness and neglect.”
Mr. Miller also expressed gratitude for the support he has received recently. A GoFundMe campaign to raise money for Mr. Miller’s legal fund had raised more than $59,000 at press time. His GoFundMe page had been shared 2,400 times. He has more than 550 followers and more than 400 donors.
“I don’t know that I have the words to adequately describe the deep sense of love and connection I have received from you, the families I serve, and those that have reached out to me in this deeply challenging time,” he wrote on the clinic website.
Mr. Miller has spoken publicly about his anti-mask views and his support for ivermectin, according to the commission report. As part of the suspension, he was charged with making “misleading representations regarding the efficacy of non-FDA approved treatment and mask use.”
In one case that was cited in the report, a 39-year-old patient contacted the pediatric clinic, and Mr. Miller spoke with the patient by phone. The patient reported that he had tested positive for COVID. Mr. Miller advised the patient to take supplements, including vitamin D and C, zinc, and melatonin, and he prescribed ivermectin, dexamethasone, and azithromycin. He did not perform an exam, verify the information that the patient had provided, advise the patient regarding interactions, or order follow-up testing, the report states.
Other charges against Mr. Miller include harassing hospital staff by making threatening statements about hospitals and doctors who treat COVID-19 patients and misrepresenting his original 2013 license application. He denied on the application that he was being investigated by another licensing board. At the time, the California Physician Assistant Board was investigating him for providing medical care and prescribing without a supervising doctor’s authorization and without conducting physical exams, among other charges.
A version of this article first appeared on Medscape.com.
States can reserve COVID shots for kids 5-11 this week
States can preorder COVID-19 vaccine doses for younger children this week as they begin to set up vaccination campaigns for ages 5-11.
Vaccine advisory groups for the FDA and CDC are scheduled to discuss and approve the Pfizer shot for kids in the next three weeks. To help states and cities prepare for the rollout, the CDC issued guidance on how to set up expanded vaccination programs.
Immunization program managers can begin ordering doses on Wednesday, according to the guidance. The vials won’t be delivered until the FDA and CDC authorize the shot, but registering now will help federal officials ship doses quickly once they’re available.
Pharmacies in every state will be able to give COVID-19 shots to children, but they can only use doses that are prepared specifically for children. Ages 5-11 will need a 10-microgram dose, which is one-third of the dose administered to ages 12 and older. The guidance warns that doctors should not try to split up or fraction the adult doses.
The CDC guidance also recommends that pediatricians and family practice doctors should serve as primary places to give shots to kids. The document mentions other options, such as vaccination clinics at schools, but doesn’t endorse them as the first choice for vaccinating kids.
The CDC hasn’t yet addressed questions around whether kids should be required to get vaccinated to attend school. The decision will likely be left to state and city officials.
Federal health officials aren’t yet sure how many parents and guardians will seek shots for their younger kids right away, the AP reported. Demand may be high at first for some families, but it may not be as high as when shots first became available for adults, Marcus Plescia, MD, chief medical officer of the Association of State and Territorial Health Officials, told The Associated Press.
“We’re going to have potentially a very busy, and perhaps modestly chaotic time,” he said.
When vaccines were first authorized for adults, hospitals and pharmacies received priority for ordering shots. Some doctors felt left out. This time, however, the CDC has said that pediatricians will receive higher priority and be able to receive shipments quickly.
As the vaccine rollout begins, health officials should consider logistical concerns to address racial and economic disparities for younger kids, Richard Besser, MD, president and CEO of the Robert Wood Johnson Foundation and a former acting director of the CDC, told the AP.
If parents or guardians can’t leave work to take their kids to a pharmacy or doctor’s office, for instance, their kids may not receive a shot quickly – or at all.
“It’s really important that we recognize the barriers to vaccinations,” he said.
A version of this article first appeared on WebMD.com.
States can preorder COVID-19 vaccine doses for younger children this week as they begin to set up vaccination campaigns for ages 5-11.
Vaccine advisory groups for the FDA and CDC are scheduled to discuss and approve the Pfizer shot for kids in the next three weeks. To help states and cities prepare for the rollout, the CDC issued guidance on how to set up expanded vaccination programs.
Immunization program managers can begin ordering doses on Wednesday, according to the guidance. The vials won’t be delivered until the FDA and CDC authorize the shot, but registering now will help federal officials ship doses quickly once they’re available.
Pharmacies in every state will be able to give COVID-19 shots to children, but they can only use doses that are prepared specifically for children. Ages 5-11 will need a 10-microgram dose, which is one-third of the dose administered to ages 12 and older. The guidance warns that doctors should not try to split up or fraction the adult doses.
The CDC guidance also recommends that pediatricians and family practice doctors should serve as primary places to give shots to kids. The document mentions other options, such as vaccination clinics at schools, but doesn’t endorse them as the first choice for vaccinating kids.
The CDC hasn’t yet addressed questions around whether kids should be required to get vaccinated to attend school. The decision will likely be left to state and city officials.
Federal health officials aren’t yet sure how many parents and guardians will seek shots for their younger kids right away, the AP reported. Demand may be high at first for some families, but it may not be as high as when shots first became available for adults, Marcus Plescia, MD, chief medical officer of the Association of State and Territorial Health Officials, told The Associated Press.
“We’re going to have potentially a very busy, and perhaps modestly chaotic time,” he said.
When vaccines were first authorized for adults, hospitals and pharmacies received priority for ordering shots. Some doctors felt left out. This time, however, the CDC has said that pediatricians will receive higher priority and be able to receive shipments quickly.
As the vaccine rollout begins, health officials should consider logistical concerns to address racial and economic disparities for younger kids, Richard Besser, MD, president and CEO of the Robert Wood Johnson Foundation and a former acting director of the CDC, told the AP.
If parents or guardians can’t leave work to take their kids to a pharmacy or doctor’s office, for instance, their kids may not receive a shot quickly – or at all.
“It’s really important that we recognize the barriers to vaccinations,” he said.
A version of this article first appeared on WebMD.com.
States can preorder COVID-19 vaccine doses for younger children this week as they begin to set up vaccination campaigns for ages 5-11.
Vaccine advisory groups for the FDA and CDC are scheduled to discuss and approve the Pfizer shot for kids in the next three weeks. To help states and cities prepare for the rollout, the CDC issued guidance on how to set up expanded vaccination programs.
Immunization program managers can begin ordering doses on Wednesday, according to the guidance. The vials won’t be delivered until the FDA and CDC authorize the shot, but registering now will help federal officials ship doses quickly once they’re available.
Pharmacies in every state will be able to give COVID-19 shots to children, but they can only use doses that are prepared specifically for children. Ages 5-11 will need a 10-microgram dose, which is one-third of the dose administered to ages 12 and older. The guidance warns that doctors should not try to split up or fraction the adult doses.
The CDC guidance also recommends that pediatricians and family practice doctors should serve as primary places to give shots to kids. The document mentions other options, such as vaccination clinics at schools, but doesn’t endorse them as the first choice for vaccinating kids.
The CDC hasn’t yet addressed questions around whether kids should be required to get vaccinated to attend school. The decision will likely be left to state and city officials.
Federal health officials aren’t yet sure how many parents and guardians will seek shots for their younger kids right away, the AP reported. Demand may be high at first for some families, but it may not be as high as when shots first became available for adults, Marcus Plescia, MD, chief medical officer of the Association of State and Territorial Health Officials, told The Associated Press.
“We’re going to have potentially a very busy, and perhaps modestly chaotic time,” he said.
When vaccines were first authorized for adults, hospitals and pharmacies received priority for ordering shots. Some doctors felt left out. This time, however, the CDC has said that pediatricians will receive higher priority and be able to receive shipments quickly.
As the vaccine rollout begins, health officials should consider logistical concerns to address racial and economic disparities for younger kids, Richard Besser, MD, president and CEO of the Robert Wood Johnson Foundation and a former acting director of the CDC, told the AP.
If parents or guardians can’t leave work to take their kids to a pharmacy or doctor’s office, for instance, their kids may not receive a shot quickly – or at all.
“It’s really important that we recognize the barriers to vaccinations,” he said.
A version of this article first appeared on WebMD.com.
FDA approves cell-based flu shot for ages 6 months and older
The Food and Drug Administration has approved the Flucelvax quadrivalent vaccine for use in children aged 6 months and older, according to a statement from manufacturer Seqirus.
“This approval officially allows all eligible Americans to receive a cell-based influenza vaccine, increasing the potential for greater vaccine effectiveness,” according to the company.
The Centers for Disease Control and Prevention currently recommends annual influenza vaccination for all individuals aged 6 months and older without contraindications.
Flucelvax is manufactured using a cell-based process that yields a more precise match to the WHO-selected influenza strains for a given year. This process avoids the variation associated with traditional egg-based vaccines, and offers the potential for greater vaccine effectiveness, according to the company.
The approval was based in part on data from a phase 3 randomized, controlled noninferiority study of children aged 6-47 months. The data are the first for a cell-based flu vaccine in this age group, and were presented at the Pediatric Academic Societies meeting in 2021.
In the immunogenicity study of children aged 6 months through 3 years, described in the package insert, 1,597 children received Flucelvax quadrivalent and 805 received a control quadrivalent vaccine. After 28 days, Flucelvax showed noninferiority to the control quadrivalent against four influenza strains.
The most common side effects with Flucelvax quadrivalent vaccine overall are pain, redness, swelling, or a hardened area at the injection site, headache, low energy, muscle aches, and malaise. Additional side effects reported in children include tenderness or bruising at the injection site, sleepiness, diarrhea, changes in eating habits, and irritability. The vaccine is contraindicated for individuals with allergies to any of its ingredients.
Additional efficacy data on Flucelvax for children and adolescents aged 2-18 years were recently published in The New England Journal of Medicine.
Full prescribing information for Flucelvax is available here.
The FDA approval letter is available here.[email protected]
The Food and Drug Administration has approved the Flucelvax quadrivalent vaccine for use in children aged 6 months and older, according to a statement from manufacturer Seqirus.
“This approval officially allows all eligible Americans to receive a cell-based influenza vaccine, increasing the potential for greater vaccine effectiveness,” according to the company.
The Centers for Disease Control and Prevention currently recommends annual influenza vaccination for all individuals aged 6 months and older without contraindications.
Flucelvax is manufactured using a cell-based process that yields a more precise match to the WHO-selected influenza strains for a given year. This process avoids the variation associated with traditional egg-based vaccines, and offers the potential for greater vaccine effectiveness, according to the company.
The approval was based in part on data from a phase 3 randomized, controlled noninferiority study of children aged 6-47 months. The data are the first for a cell-based flu vaccine in this age group, and were presented at the Pediatric Academic Societies meeting in 2021.
In the immunogenicity study of children aged 6 months through 3 years, described in the package insert, 1,597 children received Flucelvax quadrivalent and 805 received a control quadrivalent vaccine. After 28 days, Flucelvax showed noninferiority to the control quadrivalent against four influenza strains.
The most common side effects with Flucelvax quadrivalent vaccine overall are pain, redness, swelling, or a hardened area at the injection site, headache, low energy, muscle aches, and malaise. Additional side effects reported in children include tenderness or bruising at the injection site, sleepiness, diarrhea, changes in eating habits, and irritability. The vaccine is contraindicated for individuals with allergies to any of its ingredients.
Additional efficacy data on Flucelvax for children and adolescents aged 2-18 years were recently published in The New England Journal of Medicine.
Full prescribing information for Flucelvax is available here.
The FDA approval letter is available here.[email protected]
The Food and Drug Administration has approved the Flucelvax quadrivalent vaccine for use in children aged 6 months and older, according to a statement from manufacturer Seqirus.
“This approval officially allows all eligible Americans to receive a cell-based influenza vaccine, increasing the potential for greater vaccine effectiveness,” according to the company.
The Centers for Disease Control and Prevention currently recommends annual influenza vaccination for all individuals aged 6 months and older without contraindications.
Flucelvax is manufactured using a cell-based process that yields a more precise match to the WHO-selected influenza strains for a given year. This process avoids the variation associated with traditional egg-based vaccines, and offers the potential for greater vaccine effectiveness, according to the company.
The approval was based in part on data from a phase 3 randomized, controlled noninferiority study of children aged 6-47 months. The data are the first for a cell-based flu vaccine in this age group, and were presented at the Pediatric Academic Societies meeting in 2021.
In the immunogenicity study of children aged 6 months through 3 years, described in the package insert, 1,597 children received Flucelvax quadrivalent and 805 received a control quadrivalent vaccine. After 28 days, Flucelvax showed noninferiority to the control quadrivalent against four influenza strains.
The most common side effects with Flucelvax quadrivalent vaccine overall are pain, redness, swelling, or a hardened area at the injection site, headache, low energy, muscle aches, and malaise. Additional side effects reported in children include tenderness or bruising at the injection site, sleepiness, diarrhea, changes in eating habits, and irritability. The vaccine is contraindicated for individuals with allergies to any of its ingredients.
Additional efficacy data on Flucelvax for children and adolescents aged 2-18 years were recently published in The New England Journal of Medicine.
Full prescribing information for Flucelvax is available here.
The FDA approval letter is available here.[email protected]