User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Study finds nadolol noninferior to propranolol for infantile hemangiomas
according to a study published in JAMA Pediatrics.
“In our experience, nadolol is preferable to propranolol given its observed efficacy and similar safety profile [and] its more predictable metabolism that does not involve the liver,” lead author Elena Pope, MD, told this news organization. “In addition, the fact that nadolol is less lipophilic than propranolol makes it less likely to cross the blood-brain barrier and potentially affect the central nervous system,” added Dr. Pope, who is head of the division of pediatric dermatology at the Hospital for Sick Children, Toronto, and professor of pediatric medicine at the University of Toronto.
The prospective double-blind, randomized noninferiority study was conducted between 2016 and 2020 at two tertiary academic pediatric dermatology clinics in Ontario, Canada. It included 71 infants with a corrected gestational age of 1-6 months whose hemangiomas were greater than 1.5 cm on the face or 3 cm or greater on another body part and had the potential to cause functional impairment or cosmetic disfigurement.
Patients were randomized to either nadolol (oral suspension, 10 mg/mL) or propranolol (oral suspension, 5 mg/mL) beginning at a dose of 0.5 mg/kg per day twice a day and titrated weekly by 0.5 mg/kg per day until the maximum dose of 2 mg/kg per day. The dose was then adjusted until week 24, based on patient weight and clinical response, after which parents could choose to continue the infant on the assigned medication or switch to the other one. Follow-up visits occurred every 2 months after that until week 52.
For the main study outcome, measured by visual analog scale (VAS) scores at week 24, the between-group differences of IH size and color from baseline were 8.8 and 17.1, respectively, in favor of the nadolol group, the researchers report, with similar results seen at week 52. Safety data were similar for both treatments, “demonstrating that nadolol was noninferior to propranolol,” they write.
Additionally, the mean size involution, compared with baseline was 97.9% in the nadolol group and 89.1% in the propranolol group, and the mean color fading was 94.5% in the nadolol group, compared with 80.5% in the propranolol group. During the study, nadolol was also “59% faster in achieving 75% shrinkage of IH, compared with propranolol (P = .02) and 105% faster in achieving 100% shrinkage (P = .07),” they add.
“A considerable portion of patients experienced at least one mild adverse event (77.1% vs. 94.4% at 0-24 weeks and 84.2% vs. 74.2% at 24-52 weeks in the nadolol group vs. the propranolol group, respectively), with a median of two in each intervention group,” they noted, adding that while these numbers are high, they are similar to those in previous clinical trials.
“The efficacy data coupled with a more predictable pharmacokinetic profile and lower chance of crossing the blood-brain barrier may make nadolol a favorable alternative intervention in patients with IHs,” the authors conclude. However, they add that “further studies are needed to prove superiority over propranolol.”
Asked to comment on the results, Ilona J. Frieden, MD, director of the Birthmarks & Vascular Anomalies Center at the University of California, San Francisco, said that while this is a “very interesting study and deserves further consideration,” the findings do not reach the level at which they would change guidelines. “The vast majority of patients being treated with a systemic medication for IH are in fact getting propranolol,” said Dr. Frieden, coauthor of the American Academy of Pediatrics Clinical Practice Guideline for the Management of Infantile Hemangiomas.
“Though this study – designed as a noninferiority study – does seem to show slightly better outcomes from nadolol versus propranolol … it is a relatively small study,” she told this news organization. “Infantile hemangiomas are a very heterogeneous group, and larger studies and longer-term outcome data would be needed to truly compare the two modalities of treatment.”
Concern over the safety of nadolol was raised in a case report published in Pediatrics, which described the death of a 10-week-old girl 7 weeks after starting nadolol for IH. The infant was found to have an elevated postmortem cardiac blood nadolol level of 0.94 mg/L. “Although we debated the conclusion of that report in terms of death attribution to nadolol, one practical pearl is to instruct the parents to discontinue nadolol if the baby has no bowel movements for more than 3 days,” Dr. Pope advised.
The author of that case report, Eric McGillis, MD, program director of clinical pharmacology and toxicology and an emergency physician at Alberta Health Services, in Calgary, Alt., said the conclusion of his report has been taken out of context. “We acknowledge that our case report, like any case report, cannot prove causation,” he told this news organization. “We hypothesized that nadolol may have contributed to the death of the infant based on the limited pharmacokinetic data currently available for nadolol in infants. Nadolol is largely eliminated in the feces and infants may have infrequent stooling based on diet and other factors; therefore, nadolol may accumulate,” he noted.
The infant in the case report did not have a bowel movement for 10 days “and had an elevated postmortem cardiac nadolol concentration in the absence of another obvious cause of death. More pharmacokinetic studies on nadolol in this population are needed to substantiate our hypothesis. However, in the meantime, we agree that having parents monitor stool output for dose adjustments makes practical sense and can potentially reduce harm.”
Dr. Pope presented the results of the study earlier this year at the annual meeting of the Society for Pediatric Dermatology.
The study was supported by Physician Services, Ont. Dr. Pope has reported serving as an advisory board member for Boehringer Ingelheim, Novartis, Sanofi Genzyme, and Timber. Other authors have reported receiving personal fees from Pierre Fabre during the conduct of the study, as well as personal fees from Amgen, Ipsen, Novartis, Pfizer, and Sanofi Genzyme; grants from AbbVie, Clementia, Mayne Pharma, and Sanofi Genzyme; and grants and personal fees from Venthera. One author has a patent for a new topical treatment of IH. Dr. Frieden has reported being a consultant for Pfizer (data safety board), Novartis, and Venthera. Dr. McGillis has reported no relevant financial relationships.
Commentary by Lawrence W. Eichenfield, MD
The treatment of functionally significant and deforming hemangiomas has been revolutionized by propranolol, developed after the observation by Christine Léauté-Labrèze, MD, that a child who developed hypertension as a side effect of systemic steroids for a nasal hemangioma and was prescribed propranolol for the hypertension had rapid shrinkage of the hemangioma. The study by Pope and colleagues assesses nadolol as an alternative to propranolol, showing noninferiority and in some parameters improved outcomes and speed of response. The drug appeared to be fairly well tolerated in the study, though there is a prior published case report of a death from nadolol use for hemangioma treatment from a different Canadian center. Nadolol may be an important alternative to propranolol; however, propranolol remains the only FDA-approved medication for infantile hemangiomas and the generally recommended medication in the American Academy of Pediatrics guidelines for management of infantile hemangiomas.
Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children's Hospital-San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. He disclosed that he has served as an investigator and/or consultant to AbbVie, Lilly, Pfizer, Regeneron, Sanofi-Genzyme, and Verrica.
A version of this article first appeared on Medscape.com.
This article was updated 6/18/22.
according to a study published in JAMA Pediatrics.
“In our experience, nadolol is preferable to propranolol given its observed efficacy and similar safety profile [and] its more predictable metabolism that does not involve the liver,” lead author Elena Pope, MD, told this news organization. “In addition, the fact that nadolol is less lipophilic than propranolol makes it less likely to cross the blood-brain barrier and potentially affect the central nervous system,” added Dr. Pope, who is head of the division of pediatric dermatology at the Hospital for Sick Children, Toronto, and professor of pediatric medicine at the University of Toronto.
The prospective double-blind, randomized noninferiority study was conducted between 2016 and 2020 at two tertiary academic pediatric dermatology clinics in Ontario, Canada. It included 71 infants with a corrected gestational age of 1-6 months whose hemangiomas were greater than 1.5 cm on the face or 3 cm or greater on another body part and had the potential to cause functional impairment or cosmetic disfigurement.
Patients were randomized to either nadolol (oral suspension, 10 mg/mL) or propranolol (oral suspension, 5 mg/mL) beginning at a dose of 0.5 mg/kg per day twice a day and titrated weekly by 0.5 mg/kg per day until the maximum dose of 2 mg/kg per day. The dose was then adjusted until week 24, based on patient weight and clinical response, after which parents could choose to continue the infant on the assigned medication or switch to the other one. Follow-up visits occurred every 2 months after that until week 52.
For the main study outcome, measured by visual analog scale (VAS) scores at week 24, the between-group differences of IH size and color from baseline were 8.8 and 17.1, respectively, in favor of the nadolol group, the researchers report, with similar results seen at week 52. Safety data were similar for both treatments, “demonstrating that nadolol was noninferior to propranolol,” they write.
Additionally, the mean size involution, compared with baseline was 97.9% in the nadolol group and 89.1% in the propranolol group, and the mean color fading was 94.5% in the nadolol group, compared with 80.5% in the propranolol group. During the study, nadolol was also “59% faster in achieving 75% shrinkage of IH, compared with propranolol (P = .02) and 105% faster in achieving 100% shrinkage (P = .07),” they add.
“A considerable portion of patients experienced at least one mild adverse event (77.1% vs. 94.4% at 0-24 weeks and 84.2% vs. 74.2% at 24-52 weeks in the nadolol group vs. the propranolol group, respectively), with a median of two in each intervention group,” they noted, adding that while these numbers are high, they are similar to those in previous clinical trials.
“The efficacy data coupled with a more predictable pharmacokinetic profile and lower chance of crossing the blood-brain barrier may make nadolol a favorable alternative intervention in patients with IHs,” the authors conclude. However, they add that “further studies are needed to prove superiority over propranolol.”
Asked to comment on the results, Ilona J. Frieden, MD, director of the Birthmarks & Vascular Anomalies Center at the University of California, San Francisco, said that while this is a “very interesting study and deserves further consideration,” the findings do not reach the level at which they would change guidelines. “The vast majority of patients being treated with a systemic medication for IH are in fact getting propranolol,” said Dr. Frieden, coauthor of the American Academy of Pediatrics Clinical Practice Guideline for the Management of Infantile Hemangiomas.
“Though this study – designed as a noninferiority study – does seem to show slightly better outcomes from nadolol versus propranolol … it is a relatively small study,” she told this news organization. “Infantile hemangiomas are a very heterogeneous group, and larger studies and longer-term outcome data would be needed to truly compare the two modalities of treatment.”
Concern over the safety of nadolol was raised in a case report published in Pediatrics, which described the death of a 10-week-old girl 7 weeks after starting nadolol for IH. The infant was found to have an elevated postmortem cardiac blood nadolol level of 0.94 mg/L. “Although we debated the conclusion of that report in terms of death attribution to nadolol, one practical pearl is to instruct the parents to discontinue nadolol if the baby has no bowel movements for more than 3 days,” Dr. Pope advised.
The author of that case report, Eric McGillis, MD, program director of clinical pharmacology and toxicology and an emergency physician at Alberta Health Services, in Calgary, Alt., said the conclusion of his report has been taken out of context. “We acknowledge that our case report, like any case report, cannot prove causation,” he told this news organization. “We hypothesized that nadolol may have contributed to the death of the infant based on the limited pharmacokinetic data currently available for nadolol in infants. Nadolol is largely eliminated in the feces and infants may have infrequent stooling based on diet and other factors; therefore, nadolol may accumulate,” he noted.
The infant in the case report did not have a bowel movement for 10 days “and had an elevated postmortem cardiac nadolol concentration in the absence of another obvious cause of death. More pharmacokinetic studies on nadolol in this population are needed to substantiate our hypothesis. However, in the meantime, we agree that having parents monitor stool output for dose adjustments makes practical sense and can potentially reduce harm.”
Dr. Pope presented the results of the study earlier this year at the annual meeting of the Society for Pediatric Dermatology.
The study was supported by Physician Services, Ont. Dr. Pope has reported serving as an advisory board member for Boehringer Ingelheim, Novartis, Sanofi Genzyme, and Timber. Other authors have reported receiving personal fees from Pierre Fabre during the conduct of the study, as well as personal fees from Amgen, Ipsen, Novartis, Pfizer, and Sanofi Genzyme; grants from AbbVie, Clementia, Mayne Pharma, and Sanofi Genzyme; and grants and personal fees from Venthera. One author has a patent for a new topical treatment of IH. Dr. Frieden has reported being a consultant for Pfizer (data safety board), Novartis, and Venthera. Dr. McGillis has reported no relevant financial relationships.
Commentary by Lawrence W. Eichenfield, MD
The treatment of functionally significant and deforming hemangiomas has been revolutionized by propranolol, developed after the observation by Christine Léauté-Labrèze, MD, that a child who developed hypertension as a side effect of systemic steroids for a nasal hemangioma and was prescribed propranolol for the hypertension had rapid shrinkage of the hemangioma. The study by Pope and colleagues assesses nadolol as an alternative to propranolol, showing noninferiority and in some parameters improved outcomes and speed of response. The drug appeared to be fairly well tolerated in the study, though there is a prior published case report of a death from nadolol use for hemangioma treatment from a different Canadian center. Nadolol may be an important alternative to propranolol; however, propranolol remains the only FDA-approved medication for infantile hemangiomas and the generally recommended medication in the American Academy of Pediatrics guidelines for management of infantile hemangiomas.
Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children's Hospital-San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. He disclosed that he has served as an investigator and/or consultant to AbbVie, Lilly, Pfizer, Regeneron, Sanofi-Genzyme, and Verrica.
A version of this article first appeared on Medscape.com.
This article was updated 6/18/22.
according to a study published in JAMA Pediatrics.
“In our experience, nadolol is preferable to propranolol given its observed efficacy and similar safety profile [and] its more predictable metabolism that does not involve the liver,” lead author Elena Pope, MD, told this news organization. “In addition, the fact that nadolol is less lipophilic than propranolol makes it less likely to cross the blood-brain barrier and potentially affect the central nervous system,” added Dr. Pope, who is head of the division of pediatric dermatology at the Hospital for Sick Children, Toronto, and professor of pediatric medicine at the University of Toronto.
The prospective double-blind, randomized noninferiority study was conducted between 2016 and 2020 at two tertiary academic pediatric dermatology clinics in Ontario, Canada. It included 71 infants with a corrected gestational age of 1-6 months whose hemangiomas were greater than 1.5 cm on the face or 3 cm or greater on another body part and had the potential to cause functional impairment or cosmetic disfigurement.
Patients were randomized to either nadolol (oral suspension, 10 mg/mL) or propranolol (oral suspension, 5 mg/mL) beginning at a dose of 0.5 mg/kg per day twice a day and titrated weekly by 0.5 mg/kg per day until the maximum dose of 2 mg/kg per day. The dose was then adjusted until week 24, based on patient weight and clinical response, after which parents could choose to continue the infant on the assigned medication or switch to the other one. Follow-up visits occurred every 2 months after that until week 52.
For the main study outcome, measured by visual analog scale (VAS) scores at week 24, the between-group differences of IH size and color from baseline were 8.8 and 17.1, respectively, in favor of the nadolol group, the researchers report, with similar results seen at week 52. Safety data were similar for both treatments, “demonstrating that nadolol was noninferior to propranolol,” they write.
Additionally, the mean size involution, compared with baseline was 97.9% in the nadolol group and 89.1% in the propranolol group, and the mean color fading was 94.5% in the nadolol group, compared with 80.5% in the propranolol group. During the study, nadolol was also “59% faster in achieving 75% shrinkage of IH, compared with propranolol (P = .02) and 105% faster in achieving 100% shrinkage (P = .07),” they add.
“A considerable portion of patients experienced at least one mild adverse event (77.1% vs. 94.4% at 0-24 weeks and 84.2% vs. 74.2% at 24-52 weeks in the nadolol group vs. the propranolol group, respectively), with a median of two in each intervention group,” they noted, adding that while these numbers are high, they are similar to those in previous clinical trials.
“The efficacy data coupled with a more predictable pharmacokinetic profile and lower chance of crossing the blood-brain barrier may make nadolol a favorable alternative intervention in patients with IHs,” the authors conclude. However, they add that “further studies are needed to prove superiority over propranolol.”
Asked to comment on the results, Ilona J. Frieden, MD, director of the Birthmarks & Vascular Anomalies Center at the University of California, San Francisco, said that while this is a “very interesting study and deserves further consideration,” the findings do not reach the level at which they would change guidelines. “The vast majority of patients being treated with a systemic medication for IH are in fact getting propranolol,” said Dr. Frieden, coauthor of the American Academy of Pediatrics Clinical Practice Guideline for the Management of Infantile Hemangiomas.
“Though this study – designed as a noninferiority study – does seem to show slightly better outcomes from nadolol versus propranolol … it is a relatively small study,” she told this news organization. “Infantile hemangiomas are a very heterogeneous group, and larger studies and longer-term outcome data would be needed to truly compare the two modalities of treatment.”
Concern over the safety of nadolol was raised in a case report published in Pediatrics, which described the death of a 10-week-old girl 7 weeks after starting nadolol for IH. The infant was found to have an elevated postmortem cardiac blood nadolol level of 0.94 mg/L. “Although we debated the conclusion of that report in terms of death attribution to nadolol, one practical pearl is to instruct the parents to discontinue nadolol if the baby has no bowel movements for more than 3 days,” Dr. Pope advised.
The author of that case report, Eric McGillis, MD, program director of clinical pharmacology and toxicology and an emergency physician at Alberta Health Services, in Calgary, Alt., said the conclusion of his report has been taken out of context. “We acknowledge that our case report, like any case report, cannot prove causation,” he told this news organization. “We hypothesized that nadolol may have contributed to the death of the infant based on the limited pharmacokinetic data currently available for nadolol in infants. Nadolol is largely eliminated in the feces and infants may have infrequent stooling based on diet and other factors; therefore, nadolol may accumulate,” he noted.
The infant in the case report did not have a bowel movement for 10 days “and had an elevated postmortem cardiac nadolol concentration in the absence of another obvious cause of death. More pharmacokinetic studies on nadolol in this population are needed to substantiate our hypothesis. However, in the meantime, we agree that having parents monitor stool output for dose adjustments makes practical sense and can potentially reduce harm.”
Dr. Pope presented the results of the study earlier this year at the annual meeting of the Society for Pediatric Dermatology.
The study was supported by Physician Services, Ont. Dr. Pope has reported serving as an advisory board member for Boehringer Ingelheim, Novartis, Sanofi Genzyme, and Timber. Other authors have reported receiving personal fees from Pierre Fabre during the conduct of the study, as well as personal fees from Amgen, Ipsen, Novartis, Pfizer, and Sanofi Genzyme; grants from AbbVie, Clementia, Mayne Pharma, and Sanofi Genzyme; and grants and personal fees from Venthera. One author has a patent for a new topical treatment of IH. Dr. Frieden has reported being a consultant for Pfizer (data safety board), Novartis, and Venthera. Dr. McGillis has reported no relevant financial relationships.
Commentary by Lawrence W. Eichenfield, MD
The treatment of functionally significant and deforming hemangiomas has been revolutionized by propranolol, developed after the observation by Christine Léauté-Labrèze, MD, that a child who developed hypertension as a side effect of systemic steroids for a nasal hemangioma and was prescribed propranolol for the hypertension had rapid shrinkage of the hemangioma. The study by Pope and colleagues assesses nadolol as an alternative to propranolol, showing noninferiority and in some parameters improved outcomes and speed of response. The drug appeared to be fairly well tolerated in the study, though there is a prior published case report of a death from nadolol use for hemangioma treatment from a different Canadian center. Nadolol may be an important alternative to propranolol; however, propranolol remains the only FDA-approved medication for infantile hemangiomas and the generally recommended medication in the American Academy of Pediatrics guidelines for management of infantile hemangiomas.
Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children's Hospital-San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. He disclosed that he has served as an investigator and/or consultant to AbbVie, Lilly, Pfizer, Regeneron, Sanofi-Genzyme, and Verrica.
A version of this article first appeared on Medscape.com.
This article was updated 6/18/22.
FROM JAMA PEDIATRICS
Did prior authorization refusals lead to this patient’s death?
Ramy Sedhom, MD, a medical oncologist and a palliative care physician at Penn Medicine Princeton Health in Plainsboro, N.J., will always wonder if prior authorization refusals led to his patient’s death.
The patient had advanced gastric cancer and the insurer initially denied a PET scan to rule out metastatic disease. When the scan was eventually allowed, it revealed that the cancer had spread.
Standard treatment would have been difficult for the patient, an older individual with comorbidities. But Dr. Sedhom knew that a European study had reported equal efficacy and fewer side effects with a reduced chemotherapy regimen, and he thought that was the best approach in this situation.
The insurer disagreed with Dr. Sedhom’s decision and, while the two argued, the patient’s symptoms worsened. He was admitted to the hospital, where he experienced a decline in function, common for older patients. “Long story short, he was never able to seek treatment and then transitioned to hospice,” Dr. Sedhom said. “It was one of those situations where there was a 3- to 4-week delay in what should have been standard care.”
. Nearly 4 years after major organizations — American Hospital Association, America’s Health Insurance Plans, American Medical Association, Blue Cross Blue Shield Association, and others — signed a consensus statement agreeing to improve the prior authorization process, physicians say little progress has been made.
Indeed, 83% of physicians say that the number of prior authorizations required for prescription medications and medical services has increased over the last 5 years, according to survey results released earlier this year.
“It’s decidedly worse — there’s no question about it,” said Andrew R. Spector, MD, a neurologist and sleep medicine specialist at Duke Health in Durham, N.C. “Drugs that I used to get without prior authorizations now require them.”
When Vignesh I. Doraiswamy, MD, an internal medicine hospitalist at the Ohio State University Wexner Medical Center in Columbus, discharged a patient with Clostridioides difficile infection, he followed clinical guidelines to prescribe vancomycin for 10 to 14 days. “And the insurance company said, ‘Well, yeah, we only authorize about 5 days,’ which just makes no sense,” Dr. Doraiswamy said. “There’s nowhere in any literature that says 5 days is sufficient. What worries me is that is the standard of care we are supposed to give and yet we are unable to.”
Yash B. Jobanputra, MD, a cardiology fellow at Saint Vincent Hospital in Worcester, Mass., laments that prior authorization is used in situations that simply do not make common sense. During his residency, a woman who had tested positive for the BRCA gene mutation with a strong family history of breast cancer needed a breast ultrasound and an MRI scan every 6 months to 1 year. Despite the documentation that she was at extremely high risk for developing breast cancer, he had to go through prior authorization every time she was due for new images.
“I had to call the insurance company, they would put me on hold, I would wait to speak to a physician — and the end response would be, ‘Yeah, this is what needs to be done,’” he said. “But having established her positive status once should be enough really. I shouldn’t have to go through the circus all over again.”
Prior authorization is also being used for routine diagnostics, such as a Holter monitor for patients complaining of heart palpitations. “Depending on the insurance, for some patients we can give it to them in the clinic right away,” Dr. Jobanputra said. “Whereas some others we have to wait until we get prior authorization from the insurance company and the patient has to come back again to the hospital to get the monitor. That is a delay in patient care.”
The delays also extend to emergency care, Dr. Doraiswamy said. He cites the example of a heart attack patient who needed an emergency heart catheterization but ran into a prior authorization delay. “I just said, ‘Try your best not to get stressed’ which is not easy for a patient finding out their stay wasn’t covered when they had just been through a heart attack,” he said. “Then I spent 20 to 30 minutes — most of it on hold — to answer the question ‘Why did this patient need to get admitted?’ “
Physicians feel disrespected because that type of prior authorization hassle is just busywork. “Rarely is a valid stay that was initially denied, not eventually accepted,” Dr. Doraiswamy said. “But why couldn’t they have just seen that the guy had a heart attack and he obviously needed to be in the hospital?”
For Dr. Spector, the Duke Health sleep medicine specialist, prior authorization is not just a speed bump, it’s a full stop. Insurers have started mandating a multiple sleep latency test (MSLT) to confirm narcolepsy before covering medication to treat the condition. “We know that the MSLT is very often wrong,” he said. “There are a lot of times we’re dealing with patients with narcolepsy who simply don’t meet the testing criteria that the insurance requires, and payers will not accept our clinical judgment.”
In his view, the prior authorization landscape is worsening — and not only because a “faulty test” is being used to deny treatment. “The appeal process is worse,” Dr. Spector said. “I used to be able to get on the phone and do a peer-to-peer review with a physician who I could reason with… but that doesn’t happen anymore. There is virtually no way to bypass these blanket rules.”
Other survey findings also stand in direct contradiction of the 2018 consensus agreement:
A large majority (87%) of physicians report that prior authorization interferes with continuity of care, even though the industry groups agreed that patients should be protected from treatment disruption when there is a formulary or treatment-coverage change.
Despite a consensus to encourage transparency and easy accessibility of prior authorization requirements, 68% of physicians reported that it is difficult to determine whether a prescription medication requires prior authorization, and 58% report that it’s difficult for medical services.
Phone and fax are the most commonly used methods for completing prior authorizations, despite agreement that electronic prior authorization, using existing national standard transactions, should be accelerated. Fewer than one quarter of physicians said that their electronic health record system supports electronic prior authorization for prescription medications.
Dr. Spector wants to see legislation that forces insurers to live up to some of the tenets of the 2018 consensus statement. In September, a new Texas law went into effect, exempting physicians from prior authorization if, during the previous six months, 90% of their treatments met an insurer›s medical necessity criteria. In January, the recently approved Prior Authorization Reform Act in Illinois will reduce the number of services subject to prior authorization, mandate a prior authorization decision within 5 days, and set disciplinary measures for health plans that do not comply, among other things.
“What gives me hope is that at least somewhere in the country, somebody is doing something,” Dr. Spector said. “And if it goes well, maybe other insurers will adopt it. I’m really hoping they demonstrate that the money they can save on the administration of all the appeals and prior authorization paperwork can actually go into caring for patients.”
In addition to state-level action, reform may also be advancing at the federal level. In October, a bill was introduced in the U.S. Senate that mirrors a prior authorization reform bill introduced in the House of Representatives last May. Both bills have broad bipartisan support; the House bill has more than 235 co-sponsors.
In an interview with this news organization, Rep. Ami Bera, MD, (D-CA) said it is “very realistic” that the bill will become law during this session of Congress. “We do think this bill will get marked up in committee and hopefully we can get it to the floor either as a stand-alone bill where we know we have the votes to pass it or as part of a larger legislative package,” he said.
If approved, the Improving Seniors’ Timely Access to Care Act of 2021 would require that Medicare Advantage plans minimize the use of prior authorization for routinely approved services; require real-time decisions for certain requests; report the extent of their use of prior authorization and their rate of approvals or denials, among other things; and establish an electronic prior authorization system.
Medicare Advantage plans are private insurers that are regulated by the Centers for Medicare & Medicaid Services (CMS), which will create the specific rules and penalties associated with the reforms, if they become law. “One would presume that a condition of being a Medicare Advantage plan is that you’re going to have to comply with these new regulations,” said Katie Orrico, senior vice president of health policy and advocacy for the American Association of Neurological Surgeons and Congress of Neurological Surgeons (AANS/CNS). “So they will have some amount of teeth in the form of a mandate.”
The AANS and CNS are part of the Regulatory Relief Coalition, a group of 14 national physician specialty organizations. Winning prior authorization reform in the Medicare Advantage plans is part of its bigger strategy. “If those commercial plans have to follow a set of rules and processes for Medicare, then why not just expand those same processes to all other parts of their business?” Ms. Orrico said.
Despite his frustration with their prior authorization processes, Dr. Doraiswamy, the Ohio State hospitalist, agrees that working to improve insurers’ practices is the best way forward. “It’s so easy to make them look like these evil, giant conglomerations that exist solely to suck money and not care about anyone’s health, but I don’t know if that’s necessarily the case,” he said. “We really have to figure out how best to work with insurance companies to make sure that, while they are profit-generating institutions, that [profit] shouldn’t come at the cost of patient care.”
A version of this article first appeared on Medscape.com.
Ramy Sedhom, MD, a medical oncologist and a palliative care physician at Penn Medicine Princeton Health in Plainsboro, N.J., will always wonder if prior authorization refusals led to his patient’s death.
The patient had advanced gastric cancer and the insurer initially denied a PET scan to rule out metastatic disease. When the scan was eventually allowed, it revealed that the cancer had spread.
Standard treatment would have been difficult for the patient, an older individual with comorbidities. But Dr. Sedhom knew that a European study had reported equal efficacy and fewer side effects with a reduced chemotherapy regimen, and he thought that was the best approach in this situation.
The insurer disagreed with Dr. Sedhom’s decision and, while the two argued, the patient’s symptoms worsened. He was admitted to the hospital, where he experienced a decline in function, common for older patients. “Long story short, he was never able to seek treatment and then transitioned to hospice,” Dr. Sedhom said. “It was one of those situations where there was a 3- to 4-week delay in what should have been standard care.”
. Nearly 4 years after major organizations — American Hospital Association, America’s Health Insurance Plans, American Medical Association, Blue Cross Blue Shield Association, and others — signed a consensus statement agreeing to improve the prior authorization process, physicians say little progress has been made.
Indeed, 83% of physicians say that the number of prior authorizations required for prescription medications and medical services has increased over the last 5 years, according to survey results released earlier this year.
“It’s decidedly worse — there’s no question about it,” said Andrew R. Spector, MD, a neurologist and sleep medicine specialist at Duke Health in Durham, N.C. “Drugs that I used to get without prior authorizations now require them.”
When Vignesh I. Doraiswamy, MD, an internal medicine hospitalist at the Ohio State University Wexner Medical Center in Columbus, discharged a patient with Clostridioides difficile infection, he followed clinical guidelines to prescribe vancomycin for 10 to 14 days. “And the insurance company said, ‘Well, yeah, we only authorize about 5 days,’ which just makes no sense,” Dr. Doraiswamy said. “There’s nowhere in any literature that says 5 days is sufficient. What worries me is that is the standard of care we are supposed to give and yet we are unable to.”
Yash B. Jobanputra, MD, a cardiology fellow at Saint Vincent Hospital in Worcester, Mass., laments that prior authorization is used in situations that simply do not make common sense. During his residency, a woman who had tested positive for the BRCA gene mutation with a strong family history of breast cancer needed a breast ultrasound and an MRI scan every 6 months to 1 year. Despite the documentation that she was at extremely high risk for developing breast cancer, he had to go through prior authorization every time she was due for new images.
“I had to call the insurance company, they would put me on hold, I would wait to speak to a physician — and the end response would be, ‘Yeah, this is what needs to be done,’” he said. “But having established her positive status once should be enough really. I shouldn’t have to go through the circus all over again.”
Prior authorization is also being used for routine diagnostics, such as a Holter monitor for patients complaining of heart palpitations. “Depending on the insurance, for some patients we can give it to them in the clinic right away,” Dr. Jobanputra said. “Whereas some others we have to wait until we get prior authorization from the insurance company and the patient has to come back again to the hospital to get the monitor. That is a delay in patient care.”
The delays also extend to emergency care, Dr. Doraiswamy said. He cites the example of a heart attack patient who needed an emergency heart catheterization but ran into a prior authorization delay. “I just said, ‘Try your best not to get stressed’ which is not easy for a patient finding out their stay wasn’t covered when they had just been through a heart attack,” he said. “Then I spent 20 to 30 minutes — most of it on hold — to answer the question ‘Why did this patient need to get admitted?’ “
Physicians feel disrespected because that type of prior authorization hassle is just busywork. “Rarely is a valid stay that was initially denied, not eventually accepted,” Dr. Doraiswamy said. “But why couldn’t they have just seen that the guy had a heart attack and he obviously needed to be in the hospital?”
For Dr. Spector, the Duke Health sleep medicine specialist, prior authorization is not just a speed bump, it’s a full stop. Insurers have started mandating a multiple sleep latency test (MSLT) to confirm narcolepsy before covering medication to treat the condition. “We know that the MSLT is very often wrong,” he said. “There are a lot of times we’re dealing with patients with narcolepsy who simply don’t meet the testing criteria that the insurance requires, and payers will not accept our clinical judgment.”
In his view, the prior authorization landscape is worsening — and not only because a “faulty test” is being used to deny treatment. “The appeal process is worse,” Dr. Spector said. “I used to be able to get on the phone and do a peer-to-peer review with a physician who I could reason with… but that doesn’t happen anymore. There is virtually no way to bypass these blanket rules.”
Other survey findings also stand in direct contradiction of the 2018 consensus agreement:
A large majority (87%) of physicians report that prior authorization interferes with continuity of care, even though the industry groups agreed that patients should be protected from treatment disruption when there is a formulary or treatment-coverage change.
Despite a consensus to encourage transparency and easy accessibility of prior authorization requirements, 68% of physicians reported that it is difficult to determine whether a prescription medication requires prior authorization, and 58% report that it’s difficult for medical services.
Phone and fax are the most commonly used methods for completing prior authorizations, despite agreement that electronic prior authorization, using existing national standard transactions, should be accelerated. Fewer than one quarter of physicians said that their electronic health record system supports electronic prior authorization for prescription medications.
Dr. Spector wants to see legislation that forces insurers to live up to some of the tenets of the 2018 consensus statement. In September, a new Texas law went into effect, exempting physicians from prior authorization if, during the previous six months, 90% of their treatments met an insurer›s medical necessity criteria. In January, the recently approved Prior Authorization Reform Act in Illinois will reduce the number of services subject to prior authorization, mandate a prior authorization decision within 5 days, and set disciplinary measures for health plans that do not comply, among other things.
“What gives me hope is that at least somewhere in the country, somebody is doing something,” Dr. Spector said. “And if it goes well, maybe other insurers will adopt it. I’m really hoping they demonstrate that the money they can save on the administration of all the appeals and prior authorization paperwork can actually go into caring for patients.”
In addition to state-level action, reform may also be advancing at the federal level. In October, a bill was introduced in the U.S. Senate that mirrors a prior authorization reform bill introduced in the House of Representatives last May. Both bills have broad bipartisan support; the House bill has more than 235 co-sponsors.
In an interview with this news organization, Rep. Ami Bera, MD, (D-CA) said it is “very realistic” that the bill will become law during this session of Congress. “We do think this bill will get marked up in committee and hopefully we can get it to the floor either as a stand-alone bill where we know we have the votes to pass it or as part of a larger legislative package,” he said.
If approved, the Improving Seniors’ Timely Access to Care Act of 2021 would require that Medicare Advantage plans minimize the use of prior authorization for routinely approved services; require real-time decisions for certain requests; report the extent of their use of prior authorization and their rate of approvals or denials, among other things; and establish an electronic prior authorization system.
Medicare Advantage plans are private insurers that are regulated by the Centers for Medicare & Medicaid Services (CMS), which will create the specific rules and penalties associated with the reforms, if they become law. “One would presume that a condition of being a Medicare Advantage plan is that you’re going to have to comply with these new regulations,” said Katie Orrico, senior vice president of health policy and advocacy for the American Association of Neurological Surgeons and Congress of Neurological Surgeons (AANS/CNS). “So they will have some amount of teeth in the form of a mandate.”
The AANS and CNS are part of the Regulatory Relief Coalition, a group of 14 national physician specialty organizations. Winning prior authorization reform in the Medicare Advantage plans is part of its bigger strategy. “If those commercial plans have to follow a set of rules and processes for Medicare, then why not just expand those same processes to all other parts of their business?” Ms. Orrico said.
Despite his frustration with their prior authorization processes, Dr. Doraiswamy, the Ohio State hospitalist, agrees that working to improve insurers’ practices is the best way forward. “It’s so easy to make them look like these evil, giant conglomerations that exist solely to suck money and not care about anyone’s health, but I don’t know if that’s necessarily the case,” he said. “We really have to figure out how best to work with insurance companies to make sure that, while they are profit-generating institutions, that [profit] shouldn’t come at the cost of patient care.”
A version of this article first appeared on Medscape.com.
Ramy Sedhom, MD, a medical oncologist and a palliative care physician at Penn Medicine Princeton Health in Plainsboro, N.J., will always wonder if prior authorization refusals led to his patient’s death.
The patient had advanced gastric cancer and the insurer initially denied a PET scan to rule out metastatic disease. When the scan was eventually allowed, it revealed that the cancer had spread.
Standard treatment would have been difficult for the patient, an older individual with comorbidities. But Dr. Sedhom knew that a European study had reported equal efficacy and fewer side effects with a reduced chemotherapy regimen, and he thought that was the best approach in this situation.
The insurer disagreed with Dr. Sedhom’s decision and, while the two argued, the patient’s symptoms worsened. He was admitted to the hospital, where he experienced a decline in function, common for older patients. “Long story short, he was never able to seek treatment and then transitioned to hospice,” Dr. Sedhom said. “It was one of those situations where there was a 3- to 4-week delay in what should have been standard care.”
. Nearly 4 years after major organizations — American Hospital Association, America’s Health Insurance Plans, American Medical Association, Blue Cross Blue Shield Association, and others — signed a consensus statement agreeing to improve the prior authorization process, physicians say little progress has been made.
Indeed, 83% of physicians say that the number of prior authorizations required for prescription medications and medical services has increased over the last 5 years, according to survey results released earlier this year.
“It’s decidedly worse — there’s no question about it,” said Andrew R. Spector, MD, a neurologist and sleep medicine specialist at Duke Health in Durham, N.C. “Drugs that I used to get without prior authorizations now require them.”
When Vignesh I. Doraiswamy, MD, an internal medicine hospitalist at the Ohio State University Wexner Medical Center in Columbus, discharged a patient with Clostridioides difficile infection, he followed clinical guidelines to prescribe vancomycin for 10 to 14 days. “And the insurance company said, ‘Well, yeah, we only authorize about 5 days,’ which just makes no sense,” Dr. Doraiswamy said. “There’s nowhere in any literature that says 5 days is sufficient. What worries me is that is the standard of care we are supposed to give and yet we are unable to.”
Yash B. Jobanputra, MD, a cardiology fellow at Saint Vincent Hospital in Worcester, Mass., laments that prior authorization is used in situations that simply do not make common sense. During his residency, a woman who had tested positive for the BRCA gene mutation with a strong family history of breast cancer needed a breast ultrasound and an MRI scan every 6 months to 1 year. Despite the documentation that she was at extremely high risk for developing breast cancer, he had to go through prior authorization every time she was due for new images.
“I had to call the insurance company, they would put me on hold, I would wait to speak to a physician — and the end response would be, ‘Yeah, this is what needs to be done,’” he said. “But having established her positive status once should be enough really. I shouldn’t have to go through the circus all over again.”
Prior authorization is also being used for routine diagnostics, such as a Holter monitor for patients complaining of heart palpitations. “Depending on the insurance, for some patients we can give it to them in the clinic right away,” Dr. Jobanputra said. “Whereas some others we have to wait until we get prior authorization from the insurance company and the patient has to come back again to the hospital to get the monitor. That is a delay in patient care.”
The delays also extend to emergency care, Dr. Doraiswamy said. He cites the example of a heart attack patient who needed an emergency heart catheterization but ran into a prior authorization delay. “I just said, ‘Try your best not to get stressed’ which is not easy for a patient finding out their stay wasn’t covered when they had just been through a heart attack,” he said. “Then I spent 20 to 30 minutes — most of it on hold — to answer the question ‘Why did this patient need to get admitted?’ “
Physicians feel disrespected because that type of prior authorization hassle is just busywork. “Rarely is a valid stay that was initially denied, not eventually accepted,” Dr. Doraiswamy said. “But why couldn’t they have just seen that the guy had a heart attack and he obviously needed to be in the hospital?”
For Dr. Spector, the Duke Health sleep medicine specialist, prior authorization is not just a speed bump, it’s a full stop. Insurers have started mandating a multiple sleep latency test (MSLT) to confirm narcolepsy before covering medication to treat the condition. “We know that the MSLT is very often wrong,” he said. “There are a lot of times we’re dealing with patients with narcolepsy who simply don’t meet the testing criteria that the insurance requires, and payers will not accept our clinical judgment.”
In his view, the prior authorization landscape is worsening — and not only because a “faulty test” is being used to deny treatment. “The appeal process is worse,” Dr. Spector said. “I used to be able to get on the phone and do a peer-to-peer review with a physician who I could reason with… but that doesn’t happen anymore. There is virtually no way to bypass these blanket rules.”
Other survey findings also stand in direct contradiction of the 2018 consensus agreement:
A large majority (87%) of physicians report that prior authorization interferes with continuity of care, even though the industry groups agreed that patients should be protected from treatment disruption when there is a formulary or treatment-coverage change.
Despite a consensus to encourage transparency and easy accessibility of prior authorization requirements, 68% of physicians reported that it is difficult to determine whether a prescription medication requires prior authorization, and 58% report that it’s difficult for medical services.
Phone and fax are the most commonly used methods for completing prior authorizations, despite agreement that electronic prior authorization, using existing national standard transactions, should be accelerated. Fewer than one quarter of physicians said that their electronic health record system supports electronic prior authorization for prescription medications.
Dr. Spector wants to see legislation that forces insurers to live up to some of the tenets of the 2018 consensus statement. In September, a new Texas law went into effect, exempting physicians from prior authorization if, during the previous six months, 90% of their treatments met an insurer›s medical necessity criteria. In January, the recently approved Prior Authorization Reform Act in Illinois will reduce the number of services subject to prior authorization, mandate a prior authorization decision within 5 days, and set disciplinary measures for health plans that do not comply, among other things.
“What gives me hope is that at least somewhere in the country, somebody is doing something,” Dr. Spector said. “And if it goes well, maybe other insurers will adopt it. I’m really hoping they demonstrate that the money they can save on the administration of all the appeals and prior authorization paperwork can actually go into caring for patients.”
In addition to state-level action, reform may also be advancing at the federal level. In October, a bill was introduced in the U.S. Senate that mirrors a prior authorization reform bill introduced in the House of Representatives last May. Both bills have broad bipartisan support; the House bill has more than 235 co-sponsors.
In an interview with this news organization, Rep. Ami Bera, MD, (D-CA) said it is “very realistic” that the bill will become law during this session of Congress. “We do think this bill will get marked up in committee and hopefully we can get it to the floor either as a stand-alone bill where we know we have the votes to pass it or as part of a larger legislative package,” he said.
If approved, the Improving Seniors’ Timely Access to Care Act of 2021 would require that Medicare Advantage plans minimize the use of prior authorization for routinely approved services; require real-time decisions for certain requests; report the extent of their use of prior authorization and their rate of approvals or denials, among other things; and establish an electronic prior authorization system.
Medicare Advantage plans are private insurers that are regulated by the Centers for Medicare & Medicaid Services (CMS), which will create the specific rules and penalties associated with the reforms, if they become law. “One would presume that a condition of being a Medicare Advantage plan is that you’re going to have to comply with these new regulations,” said Katie Orrico, senior vice president of health policy and advocacy for the American Association of Neurological Surgeons and Congress of Neurological Surgeons (AANS/CNS). “So they will have some amount of teeth in the form of a mandate.”
The AANS and CNS are part of the Regulatory Relief Coalition, a group of 14 national physician specialty organizations. Winning prior authorization reform in the Medicare Advantage plans is part of its bigger strategy. “If those commercial plans have to follow a set of rules and processes for Medicare, then why not just expand those same processes to all other parts of their business?” Ms. Orrico said.
Despite his frustration with their prior authorization processes, Dr. Doraiswamy, the Ohio State hospitalist, agrees that working to improve insurers’ practices is the best way forward. “It’s so easy to make them look like these evil, giant conglomerations that exist solely to suck money and not care about anyone’s health, but I don’t know if that’s necessarily the case,” he said. “We really have to figure out how best to work with insurance companies to make sure that, while they are profit-generating institutions, that [profit] shouldn’t come at the cost of patient care.”
A version of this article first appeared on Medscape.com.
Does vitamin D benefit only those who are deficient?
, suggests a new large-scale analysis.
Data on more than 380,000 participants gathered from 35 studies showed that, overall, there is no significant relationship between 25(OH)D concentrations, a clinical indicator of vitamin D status, and the incidence of coronary heart disease (CHD), stroke, or all-cause death, in a Mendelian randomization analysis.
However, Stephen Burgess, PhD, and colleagues showed that, in vitamin D–deficient individuals, each 10 nmol/L increase in 25(OH)D concentrations reduced the risk of all-cause mortality by 31%.
The research, published in The Lancet Diabetes & Endocrinology, also suggests there was a nonsignificant link between 25(OH)D concentrations and stroke and CHD, but again, only in vitamin D deficient individuals.
In an accompanying editorial, Guillaume Butler-Laporte, MD, and J. Brent Richards, MD, praise the researchers on their study methodology.
They add that the results “could have important public health and clinical consequences” and will “allow clinicians to better weigh the potential benefits of supplementation against its risk,” such as financial cost, “for better patient care – particularly among those with frank vitamin D deficiency.”
They continue: “Given that vitamin D deficiency is relatively common and vitamin D supplementation is safe, the rationale exists to test the effect of vitamin D supplementation in those with deficiency in large-scale randomized controlled trials.”
However, Dr. Butler-Laporte and Dr. Richards, of the Lady Davis Institute, Jewish General Hospital, Montreal, also note the study has several limitations, including the fact that the lifetime exposure to lower vitamin D levels captured by Mendelian randomization may result in larger effect sizes than in conventional trials.
Prior RCTS underpowered to detect effects of vitamin D supplements
“There are several potential mechanisms by which vitamin D could be protective for cardiovascular mortality, including mechanisms linking low vitamin D status with hyperparathyroidism and low serum calcium and phosphate,” write Dr. Burgess of the MRC Biostatistics Unit, University of Cambridge (England), and coauthors.
They also highlight that vitamin D is “further implicated in endothelial cell function” and affects the transcription of genes linked to cell division and apoptosis, providing “potential mechanisms implicating vitamin D for cancer.”
The researchers note that, while epidemiologic studies have “consistently” found a link between 25(OH)D levels and increased risk of cardiovascular disease, all-cause mortality, and other chronic diseases, several large trials of vitamin D supplementation have reported “null results.”
They argue, however, that many of these trials have recruited individuals “irrespective of baseline 25(OH)D concentration” and have been underpowered to detect the effects of supplementation.
To overcome these limitations, the team gathered data from the UK Biobank, the European Prospective Investigation Into Cancer and Nutrition Cardiovascular Disease (EPIC-CVD) study, 31 studies from the Vitamin D Studies Collaboration (VitDSC), and two Copenhagen population-based studies.
They first performed an observational study that included 384,721 individuals from the UK Biobank and 26,336 from EPIC-CVD who had a valid 25(OH)D measurement and no previously known cardiovascular disease at baseline.
Researchers also included 67,992 participants from the VitDSC studies who did not have previously known cardiovascular disease. They analyzed 25(OH)D concentrations, conventional cardiovascular risk factors, and major incident cardiovascular morbidity and mortality using individual participant data.
The results showed that, at low 25(OH)D concentrations, there was an inverse association between 25(OH)D and incident CHD, stroke, and all-cause mortality.
Next, the team conducted a Mendelian randomization analysis on 333,002 individuals from the UK Biobank and 26,336 from EPIC-CVD who were of European ancestry and had both a valid 25(OH)D measurement and genetic data that passed quality-control steps.
Information on 31,362 participants in the Copenhagen population-based studies was also included, giving a total of 386,406 individuals, of whom 33,546 had CHD, 18,166 had a stroke, and 27,885 died.
The mean age of participants ranged from 54.8 to 57.5 years, and between 53.4% and 55.4% were female.
Up to 7% of study participants were vitamin D deficient
The 25(OH)D analysis indicated that 3.9% of UK Biobank and 3.7% of Copenhagen study participants were deficient, compared with 6.9% in EPIC-CVD.
Across the full range of 25(OH)D concentrations, there was no significant association between genetically predicted 25(OH)D levels and CHD, stroke, or all-cause mortality.
However, restricting the analysis to individuals deemed vitamin D deficient (25[OH]D concentration < 25 nmol/L) revealed there was “strong evidence” for an inverse association with all-cause mortality, at an odds ratio per 10 nmol/L increase in genetically predicted 25(OH)D concentration of 0.69 (P < .0001), the team notes.
There were also nonsignificant associations between being in the deficient stratum and CHD, at an odds ratio of 0.89 (P = .14), and stroke, at an odds ratio of 0.85 (P = .09).
Further analysis suggests the association between 25(OH)D concentrations and all-cause mortality has a “clear threshold shape,” the researchers say, with evidence of an inverse association at concentrations below 40 nmol/L and null associations above that threshold.
They acknowledge, however, that their study has several potential limitations, including the assumption in their Mendelian randomization that the “only causal pathway from the genetic variants to the outcome is via 25(OH)D concentrations.”
Moreover, the genetic variants may affect 25(OH)D concentrations in a different way from “dietary supplementation or other clinical interventions.”
They also concede that their study was limited to middle-aged participants of European ancestries, which means the findings “might not be applicable to other populations.”
The study was funded by the British Heart Foundation, Medical Research Council, National Institute for Health Research, Health Data Research UK, Cancer Research UK, and International Agency for Research on Cancer. Dr. Burgess has reported no relevant financial relationships. Disclosures for the other authors are listed with the article.
A version of this article first appeared on Medscape.com.
, suggests a new large-scale analysis.
Data on more than 380,000 participants gathered from 35 studies showed that, overall, there is no significant relationship between 25(OH)D concentrations, a clinical indicator of vitamin D status, and the incidence of coronary heart disease (CHD), stroke, or all-cause death, in a Mendelian randomization analysis.
However, Stephen Burgess, PhD, and colleagues showed that, in vitamin D–deficient individuals, each 10 nmol/L increase in 25(OH)D concentrations reduced the risk of all-cause mortality by 31%.
The research, published in The Lancet Diabetes & Endocrinology, also suggests there was a nonsignificant link between 25(OH)D concentrations and stroke and CHD, but again, only in vitamin D deficient individuals.
In an accompanying editorial, Guillaume Butler-Laporte, MD, and J. Brent Richards, MD, praise the researchers on their study methodology.
They add that the results “could have important public health and clinical consequences” and will “allow clinicians to better weigh the potential benefits of supplementation against its risk,” such as financial cost, “for better patient care – particularly among those with frank vitamin D deficiency.”
They continue: “Given that vitamin D deficiency is relatively common and vitamin D supplementation is safe, the rationale exists to test the effect of vitamin D supplementation in those with deficiency in large-scale randomized controlled trials.”
However, Dr. Butler-Laporte and Dr. Richards, of the Lady Davis Institute, Jewish General Hospital, Montreal, also note the study has several limitations, including the fact that the lifetime exposure to lower vitamin D levels captured by Mendelian randomization may result in larger effect sizes than in conventional trials.
Prior RCTS underpowered to detect effects of vitamin D supplements
“There are several potential mechanisms by which vitamin D could be protective for cardiovascular mortality, including mechanisms linking low vitamin D status with hyperparathyroidism and low serum calcium and phosphate,” write Dr. Burgess of the MRC Biostatistics Unit, University of Cambridge (England), and coauthors.
They also highlight that vitamin D is “further implicated in endothelial cell function” and affects the transcription of genes linked to cell division and apoptosis, providing “potential mechanisms implicating vitamin D for cancer.”
The researchers note that, while epidemiologic studies have “consistently” found a link between 25(OH)D levels and increased risk of cardiovascular disease, all-cause mortality, and other chronic diseases, several large trials of vitamin D supplementation have reported “null results.”
They argue, however, that many of these trials have recruited individuals “irrespective of baseline 25(OH)D concentration” and have been underpowered to detect the effects of supplementation.
To overcome these limitations, the team gathered data from the UK Biobank, the European Prospective Investigation Into Cancer and Nutrition Cardiovascular Disease (EPIC-CVD) study, 31 studies from the Vitamin D Studies Collaboration (VitDSC), and two Copenhagen population-based studies.
They first performed an observational study that included 384,721 individuals from the UK Biobank and 26,336 from EPIC-CVD who had a valid 25(OH)D measurement and no previously known cardiovascular disease at baseline.
Researchers also included 67,992 participants from the VitDSC studies who did not have previously known cardiovascular disease. They analyzed 25(OH)D concentrations, conventional cardiovascular risk factors, and major incident cardiovascular morbidity and mortality using individual participant data.
The results showed that, at low 25(OH)D concentrations, there was an inverse association between 25(OH)D and incident CHD, stroke, and all-cause mortality.
Next, the team conducted a Mendelian randomization analysis on 333,002 individuals from the UK Biobank and 26,336 from EPIC-CVD who were of European ancestry and had both a valid 25(OH)D measurement and genetic data that passed quality-control steps.
Information on 31,362 participants in the Copenhagen population-based studies was also included, giving a total of 386,406 individuals, of whom 33,546 had CHD, 18,166 had a stroke, and 27,885 died.
The mean age of participants ranged from 54.8 to 57.5 years, and between 53.4% and 55.4% were female.
Up to 7% of study participants were vitamin D deficient
The 25(OH)D analysis indicated that 3.9% of UK Biobank and 3.7% of Copenhagen study participants were deficient, compared with 6.9% in EPIC-CVD.
Across the full range of 25(OH)D concentrations, there was no significant association between genetically predicted 25(OH)D levels and CHD, stroke, or all-cause mortality.
However, restricting the analysis to individuals deemed vitamin D deficient (25[OH]D concentration < 25 nmol/L) revealed there was “strong evidence” for an inverse association with all-cause mortality, at an odds ratio per 10 nmol/L increase in genetically predicted 25(OH)D concentration of 0.69 (P < .0001), the team notes.
There were also nonsignificant associations between being in the deficient stratum and CHD, at an odds ratio of 0.89 (P = .14), and stroke, at an odds ratio of 0.85 (P = .09).
Further analysis suggests the association between 25(OH)D concentrations and all-cause mortality has a “clear threshold shape,” the researchers say, with evidence of an inverse association at concentrations below 40 nmol/L and null associations above that threshold.
They acknowledge, however, that their study has several potential limitations, including the assumption in their Mendelian randomization that the “only causal pathway from the genetic variants to the outcome is via 25(OH)D concentrations.”
Moreover, the genetic variants may affect 25(OH)D concentrations in a different way from “dietary supplementation or other clinical interventions.”
They also concede that their study was limited to middle-aged participants of European ancestries, which means the findings “might not be applicable to other populations.”
The study was funded by the British Heart Foundation, Medical Research Council, National Institute for Health Research, Health Data Research UK, Cancer Research UK, and International Agency for Research on Cancer. Dr. Burgess has reported no relevant financial relationships. Disclosures for the other authors are listed with the article.
A version of this article first appeared on Medscape.com.
, suggests a new large-scale analysis.
Data on more than 380,000 participants gathered from 35 studies showed that, overall, there is no significant relationship between 25(OH)D concentrations, a clinical indicator of vitamin D status, and the incidence of coronary heart disease (CHD), stroke, or all-cause death, in a Mendelian randomization analysis.
However, Stephen Burgess, PhD, and colleagues showed that, in vitamin D–deficient individuals, each 10 nmol/L increase in 25(OH)D concentrations reduced the risk of all-cause mortality by 31%.
The research, published in The Lancet Diabetes & Endocrinology, also suggests there was a nonsignificant link between 25(OH)D concentrations and stroke and CHD, but again, only in vitamin D deficient individuals.
In an accompanying editorial, Guillaume Butler-Laporte, MD, and J. Brent Richards, MD, praise the researchers on their study methodology.
They add that the results “could have important public health and clinical consequences” and will “allow clinicians to better weigh the potential benefits of supplementation against its risk,” such as financial cost, “for better patient care – particularly among those with frank vitamin D deficiency.”
They continue: “Given that vitamin D deficiency is relatively common and vitamin D supplementation is safe, the rationale exists to test the effect of vitamin D supplementation in those with deficiency in large-scale randomized controlled trials.”
However, Dr. Butler-Laporte and Dr. Richards, of the Lady Davis Institute, Jewish General Hospital, Montreal, also note the study has several limitations, including the fact that the lifetime exposure to lower vitamin D levels captured by Mendelian randomization may result in larger effect sizes than in conventional trials.
Prior RCTS underpowered to detect effects of vitamin D supplements
“There are several potential mechanisms by which vitamin D could be protective for cardiovascular mortality, including mechanisms linking low vitamin D status with hyperparathyroidism and low serum calcium and phosphate,” write Dr. Burgess of the MRC Biostatistics Unit, University of Cambridge (England), and coauthors.
They also highlight that vitamin D is “further implicated in endothelial cell function” and affects the transcription of genes linked to cell division and apoptosis, providing “potential mechanisms implicating vitamin D for cancer.”
The researchers note that, while epidemiologic studies have “consistently” found a link between 25(OH)D levels and increased risk of cardiovascular disease, all-cause mortality, and other chronic diseases, several large trials of vitamin D supplementation have reported “null results.”
They argue, however, that many of these trials have recruited individuals “irrespective of baseline 25(OH)D concentration” and have been underpowered to detect the effects of supplementation.
To overcome these limitations, the team gathered data from the UK Biobank, the European Prospective Investigation Into Cancer and Nutrition Cardiovascular Disease (EPIC-CVD) study, 31 studies from the Vitamin D Studies Collaboration (VitDSC), and two Copenhagen population-based studies.
They first performed an observational study that included 384,721 individuals from the UK Biobank and 26,336 from EPIC-CVD who had a valid 25(OH)D measurement and no previously known cardiovascular disease at baseline.
Researchers also included 67,992 participants from the VitDSC studies who did not have previously known cardiovascular disease. They analyzed 25(OH)D concentrations, conventional cardiovascular risk factors, and major incident cardiovascular morbidity and mortality using individual participant data.
The results showed that, at low 25(OH)D concentrations, there was an inverse association between 25(OH)D and incident CHD, stroke, and all-cause mortality.
Next, the team conducted a Mendelian randomization analysis on 333,002 individuals from the UK Biobank and 26,336 from EPIC-CVD who were of European ancestry and had both a valid 25(OH)D measurement and genetic data that passed quality-control steps.
Information on 31,362 participants in the Copenhagen population-based studies was also included, giving a total of 386,406 individuals, of whom 33,546 had CHD, 18,166 had a stroke, and 27,885 died.
The mean age of participants ranged from 54.8 to 57.5 years, and between 53.4% and 55.4% were female.
Up to 7% of study participants were vitamin D deficient
The 25(OH)D analysis indicated that 3.9% of UK Biobank and 3.7% of Copenhagen study participants were deficient, compared with 6.9% in EPIC-CVD.
Across the full range of 25(OH)D concentrations, there was no significant association between genetically predicted 25(OH)D levels and CHD, stroke, or all-cause mortality.
However, restricting the analysis to individuals deemed vitamin D deficient (25[OH]D concentration < 25 nmol/L) revealed there was “strong evidence” for an inverse association with all-cause mortality, at an odds ratio per 10 nmol/L increase in genetically predicted 25(OH)D concentration of 0.69 (P < .0001), the team notes.
There were also nonsignificant associations between being in the deficient stratum and CHD, at an odds ratio of 0.89 (P = .14), and stroke, at an odds ratio of 0.85 (P = .09).
Further analysis suggests the association between 25(OH)D concentrations and all-cause mortality has a “clear threshold shape,” the researchers say, with evidence of an inverse association at concentrations below 40 nmol/L and null associations above that threshold.
They acknowledge, however, that their study has several potential limitations, including the assumption in their Mendelian randomization that the “only causal pathway from the genetic variants to the outcome is via 25(OH)D concentrations.”
Moreover, the genetic variants may affect 25(OH)D concentrations in a different way from “dietary supplementation or other clinical interventions.”
They also concede that their study was limited to middle-aged participants of European ancestries, which means the findings “might not be applicable to other populations.”
The study was funded by the British Heart Foundation, Medical Research Council, National Institute for Health Research, Health Data Research UK, Cancer Research UK, and International Agency for Research on Cancer. Dr. Burgess has reported no relevant financial relationships. Disclosures for the other authors are listed with the article.
A version of this article first appeared on Medscape.com.
U.S. obesity rates soar in early adulthood
Obesity rates among “emerging adults” aged 18-25 have soared in the United States in recent decades with the mean body mass index (BMI) for these young adults now in the overweight category, according to research highlighting troubling trends in an often-overlooked age group.
While similar patterns have been observed in other age groups, including adolescents (ages 12-19) and young adults (ages 20-39) across recent decades, emerging adulthood tends to get less attention in the evaluation of obesity trends.
“Emerging adulthood may be a key period for preventing and treating obesity given that habits formed during this period often persist through the remainder of the life course,” write the authors of the study, which was published online Nov. 23 in JAMA.
“There is an urgent need for research on risk factors contributing to obesity during this developmental stage to inform the design of interventions as well as policies aimed at prevention,” they add.
They found that by 2018 a third of all young adults had obesity, compared with just 6% at the beginning of the study periods in 1976.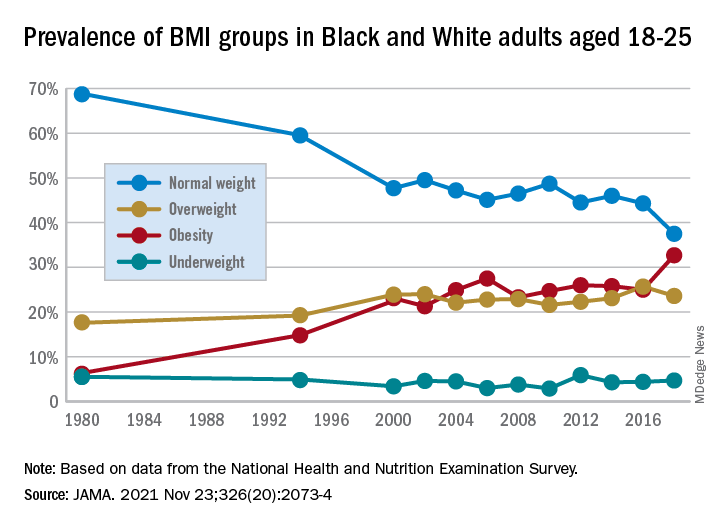
Studying the ages of transition
The findings are from an analysis of 8,015 emerging adults aged 18-25 in the cross-sectional National Health and Nutrition Examination Survey (NHANES), including NHANES II (1976-1980), NHANES III (1988-1994), and the continuous NHANES cycles from 1999 through 2018.
About half (3,965) of participants were female, 3,037 were non-Hispanic Black, and 2,386 met the criteria for household poverty.
The results showed substantial increases in mean BMI among emerging adults from a level in the normal range, at 23.1 kg/m2, in 1976-1980, increasing to 27.7 kg/m2 (overweight) in 2017-2018 (P = .006).
The prevalence of obesity (BMI 30.0 kg/m2 or higher) in the emerging adult age group soared from 6.2% between 1976-1980 to 32.7% in 2017-2018 (P = .007).
Meanwhile, the rate of those with normal/healthy weight (BMI 18.5-24.9 kg/m2) dropped from 68.7% to 37.5% (P = .005) over the same period.
Sensitivity analyses that were limited to continuous NHANES cycles showed similar results.
First author Alejandra Ellison-Barnes, MD, MPH, said the trends are consistent with rising obesity rates in the population as a whole – other studies have shown increases in obesity among children, adolescents, and adults over the same period – but are nevertheless striking, she stressed.
Young adults now fall into overweight category
“While we were not surprised by the general trend, given what is known about the increasing prevalence of obesity in both children and adults, we were surprised by the magnitude of the increase in prevalence and that the mean BMI in this age group now falls in the overweight range,” Dr. Ellison-Barnes, of the Division of General Internal Medicine, Johns Hopkins University School of Medicine, Baltimore, told this news organization.
She said she is not aware of other studies that have looked at obesity trends specifically among emerging adults.
However, considering the substantial life changes and growing independence, the life stage is important to understand in terms of dietary/lifestyle patterns.
“We theorize that emerging adulthood is a critical period for obesity development given that it is a time when individuals are often undergoing major life transitions such as leaving home, attending higher education, entering the workforce, and developing new relationships,” she emphasized.
As far as causes are concerned, “societal and cultural trends in these areas over the past several decades may have played a role in the observed changes,” she speculated.
The study population was limited to non-Hispanic Black and non-Hispanic White individuals due to changes in how NHANES assessed race and ethnicity over time. Therefore, a study limitation is that the patterns observed may not be generalizable to other races and ethnicities, the authors note.
However, considering the influence lifestyle changes can have, early adulthood “may be an ideal time to intervene in the clinical setting to prevent, manage, or reverse obesity to prevent adverse health outcomes in the future,” Dr. Ellison-Barnes said.
Dr. Ellison-Barnes has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Obesity rates among “emerging adults” aged 18-25 have soared in the United States in recent decades with the mean body mass index (BMI) for these young adults now in the overweight category, according to research highlighting troubling trends in an often-overlooked age group.
While similar patterns have been observed in other age groups, including adolescents (ages 12-19) and young adults (ages 20-39) across recent decades, emerging adulthood tends to get less attention in the evaluation of obesity trends.
“Emerging adulthood may be a key period for preventing and treating obesity given that habits formed during this period often persist through the remainder of the life course,” write the authors of the study, which was published online Nov. 23 in JAMA.
“There is an urgent need for research on risk factors contributing to obesity during this developmental stage to inform the design of interventions as well as policies aimed at prevention,” they add.
They found that by 2018 a third of all young adults had obesity, compared with just 6% at the beginning of the study periods in 1976.
Studying the ages of transition
The findings are from an analysis of 8,015 emerging adults aged 18-25 in the cross-sectional National Health and Nutrition Examination Survey (NHANES), including NHANES II (1976-1980), NHANES III (1988-1994), and the continuous NHANES cycles from 1999 through 2018.
About half (3,965) of participants were female, 3,037 were non-Hispanic Black, and 2,386 met the criteria for household poverty.
The results showed substantial increases in mean BMI among emerging adults from a level in the normal range, at 23.1 kg/m2, in 1976-1980, increasing to 27.7 kg/m2 (overweight) in 2017-2018 (P = .006).
The prevalence of obesity (BMI 30.0 kg/m2 or higher) in the emerging adult age group soared from 6.2% between 1976-1980 to 32.7% in 2017-2018 (P = .007).
Meanwhile, the rate of those with normal/healthy weight (BMI 18.5-24.9 kg/m2) dropped from 68.7% to 37.5% (P = .005) over the same period.
Sensitivity analyses that were limited to continuous NHANES cycles showed similar results.
First author Alejandra Ellison-Barnes, MD, MPH, said the trends are consistent with rising obesity rates in the population as a whole – other studies have shown increases in obesity among children, adolescents, and adults over the same period – but are nevertheless striking, she stressed.
Young adults now fall into overweight category
“While we were not surprised by the general trend, given what is known about the increasing prevalence of obesity in both children and adults, we were surprised by the magnitude of the increase in prevalence and that the mean BMI in this age group now falls in the overweight range,” Dr. Ellison-Barnes, of the Division of General Internal Medicine, Johns Hopkins University School of Medicine, Baltimore, told this news organization.
She said she is not aware of other studies that have looked at obesity trends specifically among emerging adults.
However, considering the substantial life changes and growing independence, the life stage is important to understand in terms of dietary/lifestyle patterns.
“We theorize that emerging adulthood is a critical period for obesity development given that it is a time when individuals are often undergoing major life transitions such as leaving home, attending higher education, entering the workforce, and developing new relationships,” she emphasized.
As far as causes are concerned, “societal and cultural trends in these areas over the past several decades may have played a role in the observed changes,” she speculated.
The study population was limited to non-Hispanic Black and non-Hispanic White individuals due to changes in how NHANES assessed race and ethnicity over time. Therefore, a study limitation is that the patterns observed may not be generalizable to other races and ethnicities, the authors note.
However, considering the influence lifestyle changes can have, early adulthood “may be an ideal time to intervene in the clinical setting to prevent, manage, or reverse obesity to prevent adverse health outcomes in the future,” Dr. Ellison-Barnes said.
Dr. Ellison-Barnes has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Obesity rates among “emerging adults” aged 18-25 have soared in the United States in recent decades with the mean body mass index (BMI) for these young adults now in the overweight category, according to research highlighting troubling trends in an often-overlooked age group.
While similar patterns have been observed in other age groups, including adolescents (ages 12-19) and young adults (ages 20-39) across recent decades, emerging adulthood tends to get less attention in the evaluation of obesity trends.
“Emerging adulthood may be a key period for preventing and treating obesity given that habits formed during this period often persist through the remainder of the life course,” write the authors of the study, which was published online Nov. 23 in JAMA.
“There is an urgent need for research on risk factors contributing to obesity during this developmental stage to inform the design of interventions as well as policies aimed at prevention,” they add.
They found that by 2018 a third of all young adults had obesity, compared with just 6% at the beginning of the study periods in 1976.
Studying the ages of transition
The findings are from an analysis of 8,015 emerging adults aged 18-25 in the cross-sectional National Health and Nutrition Examination Survey (NHANES), including NHANES II (1976-1980), NHANES III (1988-1994), and the continuous NHANES cycles from 1999 through 2018.
About half (3,965) of participants were female, 3,037 were non-Hispanic Black, and 2,386 met the criteria for household poverty.
The results showed substantial increases in mean BMI among emerging adults from a level in the normal range, at 23.1 kg/m2, in 1976-1980, increasing to 27.7 kg/m2 (overweight) in 2017-2018 (P = .006).
The prevalence of obesity (BMI 30.0 kg/m2 or higher) in the emerging adult age group soared from 6.2% between 1976-1980 to 32.7% in 2017-2018 (P = .007).
Meanwhile, the rate of those with normal/healthy weight (BMI 18.5-24.9 kg/m2) dropped from 68.7% to 37.5% (P = .005) over the same period.
Sensitivity analyses that were limited to continuous NHANES cycles showed similar results.
First author Alejandra Ellison-Barnes, MD, MPH, said the trends are consistent with rising obesity rates in the population as a whole – other studies have shown increases in obesity among children, adolescents, and adults over the same period – but are nevertheless striking, she stressed.
Young adults now fall into overweight category
“While we were not surprised by the general trend, given what is known about the increasing prevalence of obesity in both children and adults, we were surprised by the magnitude of the increase in prevalence and that the mean BMI in this age group now falls in the overweight range,” Dr. Ellison-Barnes, of the Division of General Internal Medicine, Johns Hopkins University School of Medicine, Baltimore, told this news organization.
She said she is not aware of other studies that have looked at obesity trends specifically among emerging adults.
However, considering the substantial life changes and growing independence, the life stage is important to understand in terms of dietary/lifestyle patterns.
“We theorize that emerging adulthood is a critical period for obesity development given that it is a time when individuals are often undergoing major life transitions such as leaving home, attending higher education, entering the workforce, and developing new relationships,” she emphasized.
As far as causes are concerned, “societal and cultural trends in these areas over the past several decades may have played a role in the observed changes,” she speculated.
The study population was limited to non-Hispanic Black and non-Hispanic White individuals due to changes in how NHANES assessed race and ethnicity over time. Therefore, a study limitation is that the patterns observed may not be generalizable to other races and ethnicities, the authors note.
However, considering the influence lifestyle changes can have, early adulthood “may be an ideal time to intervene in the clinical setting to prevent, manage, or reverse obesity to prevent adverse health outcomes in the future,” Dr. Ellison-Barnes said.
Dr. Ellison-Barnes has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Big drop in U.S. cervical cancer rates, mortality in younger women
The analysis adds to a growing body of evidence demonstrating vaccine-associated changes in cervical cancer incidence and mortality.
Previous data from the United Kingdom, published earlier in November, showed that cervical cancer rates were 87% lower among girls who received the HPV vaccine compared to previously unvaccinated generations. Based on the analysis, the authors concluded that the UK’s HPV immunization program “almost eliminated cervical cancer” in women born since September 1995.
The latest study, published Nov. 29 in JAMA Pediatrics , reports a 38% drop in cervical cancer incidence and a 43% decline in mortality among young women and girls after HPV vaccination was introduced in the United States.
“These results are encouraging,” Peter Sasieni, MD, of King’s College London, and senior author on the U.K. study, told this news organization in an email.
The difference in incidence rates between the U.K. and U.S. studies, Dr. Sasieni explained, is likely due to HPV vaccine coverage not expanding as significantly in the United States as it has in the United Kingdom, and “thus one would anticipate a lower impact on the population in the U.S.”
In the U.S. analysis, Justin Barnes, MD, a radiation oncology resident at Washington University, St. Louis, and colleagues examined cervical cancer incidence between January 2001 and December 2017 using Surveillance, Epidemiology, and End Results and National Program of Cancer Registries data as well as mortality data from the National Center for Health Statistics.
Dr. Barnes and colleagues then compared changes in cervical cancer incidence and mortality between prevaccination years (January 2001 to December 2005) and postvaccination years (January 2010 to December 2017) among three age cohorts – 15-24 years, 25-29 years, and 30-39 years.
“The older 2 groups were included as comparison, given their low vaccination rates,” Dr. Barnes and colleagues explained.
Results show that between the prevaccination and postvaccination periods, the incidence of cervical cancer dropped by 38% in the youngest cohort and by only 16% in the middle-aged group and 8% in the oldest cohort.
Women and girls in the youngest group saw a striking drop in mortality: a 43% decline, which translated to a mortality rate of 0.6 per 100,000.
On the other hand, the authors report a 4.7% decline in mortality in the oldest group and a 4.3% increase in mortality in the middle-aged group – translating to a mortality rate of 1.89 per 100,000 and 0.57 per 100,000, respectively.
Overall, “these nationwide data showed decreased cervical cancer incidence and mortality among women and girls aged 15-24 years after HPV vaccine introduction,” Dr. Barnes and colleagues wrote. The changes in cervical cancer incidence and mortality observed in the youngest age group “were greater than changes in those aged 25 to 29 years and 30 to 39 years, suggesting possible associations with HPV vaccination.”
This analysis lines up with previous evidence from U.S. epidemiologic data, which “have shown decreased cervical cancer incidence after vaccine implementation in women and girls aged 15 to 24 years but not older women.”
Although “the number of deaths and hence the number of potentially averted deaths in young women and girls was small,” the study adds to the current literature by “providing suggestive evidence for vaccine-associated decreases in cervical cancer mortality,” investigators concluded.
The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The analysis adds to a growing body of evidence demonstrating vaccine-associated changes in cervical cancer incidence and mortality.
Previous data from the United Kingdom, published earlier in November, showed that cervical cancer rates were 87% lower among girls who received the HPV vaccine compared to previously unvaccinated generations. Based on the analysis, the authors concluded that the UK’s HPV immunization program “almost eliminated cervical cancer” in women born since September 1995.
The latest study, published Nov. 29 in JAMA Pediatrics , reports a 38% drop in cervical cancer incidence and a 43% decline in mortality among young women and girls after HPV vaccination was introduced in the United States.
“These results are encouraging,” Peter Sasieni, MD, of King’s College London, and senior author on the U.K. study, told this news organization in an email.
The difference in incidence rates between the U.K. and U.S. studies, Dr. Sasieni explained, is likely due to HPV vaccine coverage not expanding as significantly in the United States as it has in the United Kingdom, and “thus one would anticipate a lower impact on the population in the U.S.”
In the U.S. analysis, Justin Barnes, MD, a radiation oncology resident at Washington University, St. Louis, and colleagues examined cervical cancer incidence between January 2001 and December 2017 using Surveillance, Epidemiology, and End Results and National Program of Cancer Registries data as well as mortality data from the National Center for Health Statistics.
Dr. Barnes and colleagues then compared changes in cervical cancer incidence and mortality between prevaccination years (January 2001 to December 2005) and postvaccination years (January 2010 to December 2017) among three age cohorts – 15-24 years, 25-29 years, and 30-39 years.
“The older 2 groups were included as comparison, given their low vaccination rates,” Dr. Barnes and colleagues explained.
Results show that between the prevaccination and postvaccination periods, the incidence of cervical cancer dropped by 38% in the youngest cohort and by only 16% in the middle-aged group and 8% in the oldest cohort.
Women and girls in the youngest group saw a striking drop in mortality: a 43% decline, which translated to a mortality rate of 0.6 per 100,000.
On the other hand, the authors report a 4.7% decline in mortality in the oldest group and a 4.3% increase in mortality in the middle-aged group – translating to a mortality rate of 1.89 per 100,000 and 0.57 per 100,000, respectively.
Overall, “these nationwide data showed decreased cervical cancer incidence and mortality among women and girls aged 15-24 years after HPV vaccine introduction,” Dr. Barnes and colleagues wrote. The changes in cervical cancer incidence and mortality observed in the youngest age group “were greater than changes in those aged 25 to 29 years and 30 to 39 years, suggesting possible associations with HPV vaccination.”
This analysis lines up with previous evidence from U.S. epidemiologic data, which “have shown decreased cervical cancer incidence after vaccine implementation in women and girls aged 15 to 24 years but not older women.”
Although “the number of deaths and hence the number of potentially averted deaths in young women and girls was small,” the study adds to the current literature by “providing suggestive evidence for vaccine-associated decreases in cervical cancer mortality,” investigators concluded.
The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The analysis adds to a growing body of evidence demonstrating vaccine-associated changes in cervical cancer incidence and mortality.
Previous data from the United Kingdom, published earlier in November, showed that cervical cancer rates were 87% lower among girls who received the HPV vaccine compared to previously unvaccinated generations. Based on the analysis, the authors concluded that the UK’s HPV immunization program “almost eliminated cervical cancer” in women born since September 1995.
The latest study, published Nov. 29 in JAMA Pediatrics , reports a 38% drop in cervical cancer incidence and a 43% decline in mortality among young women and girls after HPV vaccination was introduced in the United States.
“These results are encouraging,” Peter Sasieni, MD, of King’s College London, and senior author on the U.K. study, told this news organization in an email.
The difference in incidence rates between the U.K. and U.S. studies, Dr. Sasieni explained, is likely due to HPV vaccine coverage not expanding as significantly in the United States as it has in the United Kingdom, and “thus one would anticipate a lower impact on the population in the U.S.”
In the U.S. analysis, Justin Barnes, MD, a radiation oncology resident at Washington University, St. Louis, and colleagues examined cervical cancer incidence between January 2001 and December 2017 using Surveillance, Epidemiology, and End Results and National Program of Cancer Registries data as well as mortality data from the National Center for Health Statistics.
Dr. Barnes and colleagues then compared changes in cervical cancer incidence and mortality between prevaccination years (January 2001 to December 2005) and postvaccination years (January 2010 to December 2017) among three age cohorts – 15-24 years, 25-29 years, and 30-39 years.
“The older 2 groups were included as comparison, given their low vaccination rates,” Dr. Barnes and colleagues explained.
Results show that between the prevaccination and postvaccination periods, the incidence of cervical cancer dropped by 38% in the youngest cohort and by only 16% in the middle-aged group and 8% in the oldest cohort.
Women and girls in the youngest group saw a striking drop in mortality: a 43% decline, which translated to a mortality rate of 0.6 per 100,000.
On the other hand, the authors report a 4.7% decline in mortality in the oldest group and a 4.3% increase in mortality in the middle-aged group – translating to a mortality rate of 1.89 per 100,000 and 0.57 per 100,000, respectively.
Overall, “these nationwide data showed decreased cervical cancer incidence and mortality among women and girls aged 15-24 years after HPV vaccine introduction,” Dr. Barnes and colleagues wrote. The changes in cervical cancer incidence and mortality observed in the youngest age group “were greater than changes in those aged 25 to 29 years and 30 to 39 years, suggesting possible associations with HPV vaccination.”
This analysis lines up with previous evidence from U.S. epidemiologic data, which “have shown decreased cervical cancer incidence after vaccine implementation in women and girls aged 15 to 24 years but not older women.”
Although “the number of deaths and hence the number of potentially averted deaths in young women and girls was small,” the study adds to the current literature by “providing suggestive evidence for vaccine-associated decreases in cervical cancer mortality,” investigators concluded.
The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA PEDIATRICS
Pfizer COVID vaccine is 100% effective in adolescents: Study
Pfizer announced on Nov. 22 that its COVID-19 vaccine provided long-term protection against the virus in a late-stage clinical trial among adolescents ages 12-15.
A two-dose series was 100% effective against COVID-19, which was measured between 7 days and 4 months after the second dose.
“As the global health community works to increase the number of vaccinated people around the world, these additional data provide further confidence in our vaccine safety and effectiveness profile in adolescents,” Albert Bourla, PhD, chairman and CEO of Pfizer, said in a statement.
The clinical trial researchers found no serious safety concerns while following patients for 6 months. The adverse events were consistent with other clinical safety data for the vaccine, the company said.
Pfizer will incorporate the data into its submissions for full regulatory approval of the vaccine for ages 12-15 in the United States and worldwide.
The company will request clearance for a 30-mcg dose of the vaccines for ages 12 and older. The shot received FDA emergency use authorization for ages 12-15 in May and full approval for ages 16 and older in August.
The study included 2,228 clinical trial participants who were monitored between November 2020 and September 2021. There were 30 confirmed symptomatic cases of COVID-19 in the placebo group that didn’t receive the vaccine and 0 COVID-19 cases among the vaccinated group.
The efficacy was consistently high across gender, race, ethnicity, and health conditions, the company said.
“This is especially important as we see rates of COVID-19 climbing in this age group in some regions, while vaccine uptake has slowed,” Mr. Bourla said. “We look forward to sharing these data with the FDA and other regulators.”
A version of this article first appeared on WebMD.com.
Pfizer announced on Nov. 22 that its COVID-19 vaccine provided long-term protection against the virus in a late-stage clinical trial among adolescents ages 12-15.
A two-dose series was 100% effective against COVID-19, which was measured between 7 days and 4 months after the second dose.
“As the global health community works to increase the number of vaccinated people around the world, these additional data provide further confidence in our vaccine safety and effectiveness profile in adolescents,” Albert Bourla, PhD, chairman and CEO of Pfizer, said in a statement.
The clinical trial researchers found no serious safety concerns while following patients for 6 months. The adverse events were consistent with other clinical safety data for the vaccine, the company said.
Pfizer will incorporate the data into its submissions for full regulatory approval of the vaccine for ages 12-15 in the United States and worldwide.
The company will request clearance for a 30-mcg dose of the vaccines for ages 12 and older. The shot received FDA emergency use authorization for ages 12-15 in May and full approval for ages 16 and older in August.
The study included 2,228 clinical trial participants who were monitored between November 2020 and September 2021. There were 30 confirmed symptomatic cases of COVID-19 in the placebo group that didn’t receive the vaccine and 0 COVID-19 cases among the vaccinated group.
The efficacy was consistently high across gender, race, ethnicity, and health conditions, the company said.
“This is especially important as we see rates of COVID-19 climbing in this age group in some regions, while vaccine uptake has slowed,” Mr. Bourla said. “We look forward to sharing these data with the FDA and other regulators.”
A version of this article first appeared on WebMD.com.
Pfizer announced on Nov. 22 that its COVID-19 vaccine provided long-term protection against the virus in a late-stage clinical trial among adolescents ages 12-15.
A two-dose series was 100% effective against COVID-19, which was measured between 7 days and 4 months after the second dose.
“As the global health community works to increase the number of vaccinated people around the world, these additional data provide further confidence in our vaccine safety and effectiveness profile in adolescents,” Albert Bourla, PhD, chairman and CEO of Pfizer, said in a statement.
The clinical trial researchers found no serious safety concerns while following patients for 6 months. The adverse events were consistent with other clinical safety data for the vaccine, the company said.
Pfizer will incorporate the data into its submissions for full regulatory approval of the vaccine for ages 12-15 in the United States and worldwide.
The company will request clearance for a 30-mcg dose of the vaccines for ages 12 and older. The shot received FDA emergency use authorization for ages 12-15 in May and full approval for ages 16 and older in August.
The study included 2,228 clinical trial participants who were monitored between November 2020 and September 2021. There were 30 confirmed symptomatic cases of COVID-19 in the placebo group that didn’t receive the vaccine and 0 COVID-19 cases among the vaccinated group.
The efficacy was consistently high across gender, race, ethnicity, and health conditions, the company said.
“This is especially important as we see rates of COVID-19 climbing in this age group in some regions, while vaccine uptake has slowed,” Mr. Bourla said. “We look forward to sharing these data with the FDA and other regulators.”
A version of this article first appeared on WebMD.com.
Surveillance for measles is a victim of the COVID pandemic
Although the estimated annual number of measles deaths decreased 94% from 2000 to 2020, the COVID-19 pandemic took a toll on both measles vaccination and surveillance, according to a recent report in Morbidity and Mortality Weekly Report.
The number of World Health Organization member states that achieved more than 90% coverage with the first dose of the measles vaccine (MCV1) declined 37% from 2019 to 2020. In 2020, 23 million infants did not receive MCV1 through routine immunization services, and another 93 million were affected by the postponement of mass immunizations or supplementary immunization activities because of the pandemic. Also, endemic transmission was reestablished in nine countries that had previously eliminated measles.
But perhaps the most overlooked aspect of COVID-19 is its effect on surveillance.
“The entire COVID pandemic really put a lot of strain on the surveillance systems, not only for measles but for all vaccine-preventable disease, because there’s a lot of overlap in the staff who work for surveillance,” said Katrina Kretsinger, MD, a medical epidemiologist at the Centers for Disease Control and Prevention, who contributed to the MMWR report.
Because of the stress on the systems, a lot fewer specimens were tested, she said in an interview. And it’s not just measles that is at risk. This has had an impact on the Global Polio Eradication Initiative, which lost staff.
In addition, many vaccination campaigns “were postponed and curtailed throughout 2020,” Dr. Kretsinger said. The strengthening of surveillance systems – and immunization systems, more broadly – needs to be a priority.
“It’s not clear that the children who were missed during that year were subsequently caught up,” she explained. Having a “cohort of children who have missed measles vaccine creates the reservoir of susceptibility that will provide the nidus for the next big outbreak.”
Measles is the indicator disease. That could mean a resurgence of other vaccine-preventable diseases as well.
This report “was written by some of the world’s experts in measles, and it raises concerns about potential resurgence of measles,” said Walter Orenstein, MD, professor of medicine, epidemiology, global health, and pediatrics at Emory University, Atlanta. “Measles is sort of a canary in the coal mine. If you look at vaccine-preventable diseases, measles is probably the most contagious, so the herd-immunity threshold is highest. Usually on the order of 92%-94% immunity is needed to stop transmission.”
“Measles is the indicator disease,” he said in an interview. “That could mean a resurgence of other vaccine-preventable diseases as well.” Outbreaks don’t just affect the countries where infections are occurring, they “also affect our own domestic health security.”
“Some sort of periodic intensified routine immunization” would be helpful, said Dr. Kretsinger, who recommends “going through and selectively doing some sort of intensified efforts to catch children up early for the entire range of vaccines that they may have missed.”
“Some of these capture campaigns in areas that are thought to have the major problem would be very, very important,” agreed Dr. Orenstein. “A school entry check is one way of trying to look at kids, let’s say at 4-6 years of age, in schools around the world,” offering doses if they’re unvaccinated or inadequately vaccinated. “Another is to try to improve surveillance and try to understand if the cases are vaccine failure or failure to vaccinate.”
“Where the health systems are the most fragile is where those gaps will be the last to be filled, if they are at all, and where we have the basic concerns,” Dr. Kretsinger explained.
“Years ago, WHO recognized that vaccine hesitancy is a top global health threat,” said Dr. Orenstein. “People may not see these diseases so they don’t mean much to them. Since vaccines, we’re victims of our own success.” There’s also a lot of incorrect information circulating.
“We need to realize – and it’s been shown with COVID – that a decision not to vaccinate is not just a decision for your own child. It’s a community decision,” he pointed out. “It’s not my freedom to drive drunk, because not only do I put myself at risk, but others can’t control the car. We have speed limits and other examples where we restrict personal choice because it can adversely affect individuals.”
“My favorite line is vaccines don’t save lives, vaccinations save lives,” Dr. Orenstein said. “The vaccine dose that remains in the vial is 0% effective, no matter what the clinical trials show. And the issue, I think, is that we need to determine how to convince the hesitant to get confident enough to accept vaccination. For that, there is behavioral research; there’s a whole bunch of things that need to be supported. Just purchasing the vaccine doesn’t get it into the bodies.”
Dr. Kretsinger and Dr. Orenstein disclosed no relevant financial relationships .
A version of this article first appeared on Medscape.com.
Although the estimated annual number of measles deaths decreased 94% from 2000 to 2020, the COVID-19 pandemic took a toll on both measles vaccination and surveillance, according to a recent report in Morbidity and Mortality Weekly Report.
The number of World Health Organization member states that achieved more than 90% coverage with the first dose of the measles vaccine (MCV1) declined 37% from 2019 to 2020. In 2020, 23 million infants did not receive MCV1 through routine immunization services, and another 93 million were affected by the postponement of mass immunizations or supplementary immunization activities because of the pandemic. Also, endemic transmission was reestablished in nine countries that had previously eliminated measles.
But perhaps the most overlooked aspect of COVID-19 is its effect on surveillance.
“The entire COVID pandemic really put a lot of strain on the surveillance systems, not only for measles but for all vaccine-preventable disease, because there’s a lot of overlap in the staff who work for surveillance,” said Katrina Kretsinger, MD, a medical epidemiologist at the Centers for Disease Control and Prevention, who contributed to the MMWR report.
Because of the stress on the systems, a lot fewer specimens were tested, she said in an interview. And it’s not just measles that is at risk. This has had an impact on the Global Polio Eradication Initiative, which lost staff.
In addition, many vaccination campaigns “were postponed and curtailed throughout 2020,” Dr. Kretsinger said. The strengthening of surveillance systems – and immunization systems, more broadly – needs to be a priority.
“It’s not clear that the children who were missed during that year were subsequently caught up,” she explained. Having a “cohort of children who have missed measles vaccine creates the reservoir of susceptibility that will provide the nidus for the next big outbreak.”
Measles is the indicator disease. That could mean a resurgence of other vaccine-preventable diseases as well.
This report “was written by some of the world’s experts in measles, and it raises concerns about potential resurgence of measles,” said Walter Orenstein, MD, professor of medicine, epidemiology, global health, and pediatrics at Emory University, Atlanta. “Measles is sort of a canary in the coal mine. If you look at vaccine-preventable diseases, measles is probably the most contagious, so the herd-immunity threshold is highest. Usually on the order of 92%-94% immunity is needed to stop transmission.”
“Measles is the indicator disease,” he said in an interview. “That could mean a resurgence of other vaccine-preventable diseases as well.” Outbreaks don’t just affect the countries where infections are occurring, they “also affect our own domestic health security.”
“Some sort of periodic intensified routine immunization” would be helpful, said Dr. Kretsinger, who recommends “going through and selectively doing some sort of intensified efforts to catch children up early for the entire range of vaccines that they may have missed.”
“Some of these capture campaigns in areas that are thought to have the major problem would be very, very important,” agreed Dr. Orenstein. “A school entry check is one way of trying to look at kids, let’s say at 4-6 years of age, in schools around the world,” offering doses if they’re unvaccinated or inadequately vaccinated. “Another is to try to improve surveillance and try to understand if the cases are vaccine failure or failure to vaccinate.”
“Where the health systems are the most fragile is where those gaps will be the last to be filled, if they are at all, and where we have the basic concerns,” Dr. Kretsinger explained.
“Years ago, WHO recognized that vaccine hesitancy is a top global health threat,” said Dr. Orenstein. “People may not see these diseases so they don’t mean much to them. Since vaccines, we’re victims of our own success.” There’s also a lot of incorrect information circulating.
“We need to realize – and it’s been shown with COVID – that a decision not to vaccinate is not just a decision for your own child. It’s a community decision,” he pointed out. “It’s not my freedom to drive drunk, because not only do I put myself at risk, but others can’t control the car. We have speed limits and other examples where we restrict personal choice because it can adversely affect individuals.”
“My favorite line is vaccines don’t save lives, vaccinations save lives,” Dr. Orenstein said. “The vaccine dose that remains in the vial is 0% effective, no matter what the clinical trials show. And the issue, I think, is that we need to determine how to convince the hesitant to get confident enough to accept vaccination. For that, there is behavioral research; there’s a whole bunch of things that need to be supported. Just purchasing the vaccine doesn’t get it into the bodies.”
Dr. Kretsinger and Dr. Orenstein disclosed no relevant financial relationships .
A version of this article first appeared on Medscape.com.
Although the estimated annual number of measles deaths decreased 94% from 2000 to 2020, the COVID-19 pandemic took a toll on both measles vaccination and surveillance, according to a recent report in Morbidity and Mortality Weekly Report.
The number of World Health Organization member states that achieved more than 90% coverage with the first dose of the measles vaccine (MCV1) declined 37% from 2019 to 2020. In 2020, 23 million infants did not receive MCV1 through routine immunization services, and another 93 million were affected by the postponement of mass immunizations or supplementary immunization activities because of the pandemic. Also, endemic transmission was reestablished in nine countries that had previously eliminated measles.
But perhaps the most overlooked aspect of COVID-19 is its effect on surveillance.
“The entire COVID pandemic really put a lot of strain on the surveillance systems, not only for measles but for all vaccine-preventable disease, because there’s a lot of overlap in the staff who work for surveillance,” said Katrina Kretsinger, MD, a medical epidemiologist at the Centers for Disease Control and Prevention, who contributed to the MMWR report.
Because of the stress on the systems, a lot fewer specimens were tested, she said in an interview. And it’s not just measles that is at risk. This has had an impact on the Global Polio Eradication Initiative, which lost staff.
In addition, many vaccination campaigns “were postponed and curtailed throughout 2020,” Dr. Kretsinger said. The strengthening of surveillance systems – and immunization systems, more broadly – needs to be a priority.
“It’s not clear that the children who were missed during that year were subsequently caught up,” she explained. Having a “cohort of children who have missed measles vaccine creates the reservoir of susceptibility that will provide the nidus for the next big outbreak.”
Measles is the indicator disease. That could mean a resurgence of other vaccine-preventable diseases as well.
This report “was written by some of the world’s experts in measles, and it raises concerns about potential resurgence of measles,” said Walter Orenstein, MD, professor of medicine, epidemiology, global health, and pediatrics at Emory University, Atlanta. “Measles is sort of a canary in the coal mine. If you look at vaccine-preventable diseases, measles is probably the most contagious, so the herd-immunity threshold is highest. Usually on the order of 92%-94% immunity is needed to stop transmission.”
“Measles is the indicator disease,” he said in an interview. “That could mean a resurgence of other vaccine-preventable diseases as well.” Outbreaks don’t just affect the countries where infections are occurring, they “also affect our own domestic health security.”
“Some sort of periodic intensified routine immunization” would be helpful, said Dr. Kretsinger, who recommends “going through and selectively doing some sort of intensified efforts to catch children up early for the entire range of vaccines that they may have missed.”
“Some of these capture campaigns in areas that are thought to have the major problem would be very, very important,” agreed Dr. Orenstein. “A school entry check is one way of trying to look at kids, let’s say at 4-6 years of age, in schools around the world,” offering doses if they’re unvaccinated or inadequately vaccinated. “Another is to try to improve surveillance and try to understand if the cases are vaccine failure or failure to vaccinate.”
“Where the health systems are the most fragile is where those gaps will be the last to be filled, if they are at all, and where we have the basic concerns,” Dr. Kretsinger explained.
“Years ago, WHO recognized that vaccine hesitancy is a top global health threat,” said Dr. Orenstein. “People may not see these diseases so they don’t mean much to them. Since vaccines, we’re victims of our own success.” There’s also a lot of incorrect information circulating.
“We need to realize – and it’s been shown with COVID – that a decision not to vaccinate is not just a decision for your own child. It’s a community decision,” he pointed out. “It’s not my freedom to drive drunk, because not only do I put myself at risk, but others can’t control the car. We have speed limits and other examples where we restrict personal choice because it can adversely affect individuals.”
“My favorite line is vaccines don’t save lives, vaccinations save lives,” Dr. Orenstein said. “The vaccine dose that remains in the vial is 0% effective, no matter what the clinical trials show. And the issue, I think, is that we need to determine how to convince the hesitant to get confident enough to accept vaccination. For that, there is behavioral research; there’s a whole bunch of things that need to be supported. Just purchasing the vaccine doesn’t get it into the bodies.”
Dr. Kretsinger and Dr. Orenstein disclosed no relevant financial relationships .
A version of this article first appeared on Medscape.com.
Fueling an ‘already raging fire’: Fifth COVID surge approaches
“A significant rise in cases just before Thanksgiving is not what we want to be seeing,” said Stephen Kissler, PhD, a postdoctoral researcher and data modeler at the Harvard TH Chan School of Public Health in Boston.
Dr. Kissler said he’d rather see increases in daily cases coming 2 weeks after busy travel periods, as that would mean they could come back down as people returned to their routines.
Seeing big increases in cases ahead of the holidays, he said, “is sort of like adding fuel to an already raging fire.”
Last winter, vaccines hadn’t been rolled out as the nation prepared for Thanksgiving. COVID-19 was burning through family gatherings.
But now that two-thirds of Americans over age 5 are fully vaccinated and booster doses are approved for all adults, will a rise in cases translate, once again, into a strain on our still thinly stretched healthcare system?
Experts say the vaccines are keeping people out of the hospital, which will help. And new antiviral pills are coming that seem to be able to cut a COVID-19 infection off at the knees, at least according to early data. A U.S. Food and Drug Administration panel meets next week to discuss the first application for a pill by Merck.
But experts caution that the coming surge will almost certainly tax hospitals again, especially in areas with lower vaccination rates.
And even states where blood testing shows that significant numbers of people have antibodies after a COVID-19 infection aren’t out of the woods, in part because we still don’t know how long the immunity generated by infection may last.
“Erosion of immunity”
“It’s hard to know how much risk is out there,” said Jeffrey Shaman, PhD, professor of environmental health sciences at Columbia University’s Mailman School of Public Health in New York City, who has been modeling the trajectory of the pandemic.
“We’re estimating, unfortunately, and we have for many weeks now, that there is an erosion of immunity,” Dr. Shaman said. “I think it could get bad. How bad? I’m not sure.”
Ali Mokdad, PhD, a professor of health metrics sciences at the University of Washington’s Institute for Health Metrics and Evaluation in Seattle, agrees.
Because there are so few studies on how long immunity from natural infection lasts, Dr. Mokdad and his colleagues are assuming that waning immunity after infection happens at least as quickly as it does after vaccination.
Their model is predicting that the average number of daily cases will peak at around 100,000, with another 100,000 going undetected, and will stay at that level until the end of January, as some states recover from their surges and others pick up steam.
While the number of daily deaths won’t climb to the heights seen during the summer surge, Dr. Mokdad said their model is predicting that daily deaths will climb again to about 1,200 a day.
“We are almost there right now, and it will be with us for a while,” he said. “We are predicting 881,000 deaths by March 1.”
The United States has currently recorded 773,000 COVID-19 deaths, so Dr. Mokdad is predicting about 120,000 more deaths between now and then.
He said his model shows that more than half of those deaths could be prevented if 95% of Americans wore their masks while in close proximity to strangers.
Currently, only about 36% of Americans are consistently wearing masks, according to surveys. While people are moving around more now, mobility is at prepandemic levels in some states.
“The rise that you are seeing right now is high mobility and low mask wearing in the United States,” Dr. Mokdad said.
The solution, he said, is for all adults to get another dose of vaccine — he doesn’t like calling it a booster.
“Because they’re vaccinated and they have two doses they have a false sense of security that they are protected. We needed to come ahead of it immediately and say you need a third dose, and we were late to do so,” Dr. Mokdad said.
A version of this article first appeared on Medscape.com.
“A significant rise in cases just before Thanksgiving is not what we want to be seeing,” said Stephen Kissler, PhD, a postdoctoral researcher and data modeler at the Harvard TH Chan School of Public Health in Boston.
Dr. Kissler said he’d rather see increases in daily cases coming 2 weeks after busy travel periods, as that would mean they could come back down as people returned to their routines.
Seeing big increases in cases ahead of the holidays, he said, “is sort of like adding fuel to an already raging fire.”
Last winter, vaccines hadn’t been rolled out as the nation prepared for Thanksgiving. COVID-19 was burning through family gatherings.
But now that two-thirds of Americans over age 5 are fully vaccinated and booster doses are approved for all adults, will a rise in cases translate, once again, into a strain on our still thinly stretched healthcare system?
Experts say the vaccines are keeping people out of the hospital, which will help. And new antiviral pills are coming that seem to be able to cut a COVID-19 infection off at the knees, at least according to early data. A U.S. Food and Drug Administration panel meets next week to discuss the first application for a pill by Merck.
But experts caution that the coming surge will almost certainly tax hospitals again, especially in areas with lower vaccination rates.
And even states where blood testing shows that significant numbers of people have antibodies after a COVID-19 infection aren’t out of the woods, in part because we still don’t know how long the immunity generated by infection may last.
“Erosion of immunity”
“It’s hard to know how much risk is out there,” said Jeffrey Shaman, PhD, professor of environmental health sciences at Columbia University’s Mailman School of Public Health in New York City, who has been modeling the trajectory of the pandemic.
“We’re estimating, unfortunately, and we have for many weeks now, that there is an erosion of immunity,” Dr. Shaman said. “I think it could get bad. How bad? I’m not sure.”
Ali Mokdad, PhD, a professor of health metrics sciences at the University of Washington’s Institute for Health Metrics and Evaluation in Seattle, agrees.
Because there are so few studies on how long immunity from natural infection lasts, Dr. Mokdad and his colleagues are assuming that waning immunity after infection happens at least as quickly as it does after vaccination.
Their model is predicting that the average number of daily cases will peak at around 100,000, with another 100,000 going undetected, and will stay at that level until the end of January, as some states recover from their surges and others pick up steam.
While the number of daily deaths won’t climb to the heights seen during the summer surge, Dr. Mokdad said their model is predicting that daily deaths will climb again to about 1,200 a day.
“We are almost there right now, and it will be with us for a while,” he said. “We are predicting 881,000 deaths by March 1.”
The United States has currently recorded 773,000 COVID-19 deaths, so Dr. Mokdad is predicting about 120,000 more deaths between now and then.
He said his model shows that more than half of those deaths could be prevented if 95% of Americans wore their masks while in close proximity to strangers.
Currently, only about 36% of Americans are consistently wearing masks, according to surveys. While people are moving around more now, mobility is at prepandemic levels in some states.
“The rise that you are seeing right now is high mobility and low mask wearing in the United States,” Dr. Mokdad said.
The solution, he said, is for all adults to get another dose of vaccine — he doesn’t like calling it a booster.
“Because they’re vaccinated and they have two doses they have a false sense of security that they are protected. We needed to come ahead of it immediately and say you need a third dose, and we were late to do so,” Dr. Mokdad said.
A version of this article first appeared on Medscape.com.
“A significant rise in cases just before Thanksgiving is not what we want to be seeing,” said Stephen Kissler, PhD, a postdoctoral researcher and data modeler at the Harvard TH Chan School of Public Health in Boston.
Dr. Kissler said he’d rather see increases in daily cases coming 2 weeks after busy travel periods, as that would mean they could come back down as people returned to their routines.
Seeing big increases in cases ahead of the holidays, he said, “is sort of like adding fuel to an already raging fire.”
Last winter, vaccines hadn’t been rolled out as the nation prepared for Thanksgiving. COVID-19 was burning through family gatherings.
But now that two-thirds of Americans over age 5 are fully vaccinated and booster doses are approved for all adults, will a rise in cases translate, once again, into a strain on our still thinly stretched healthcare system?
Experts say the vaccines are keeping people out of the hospital, which will help. And new antiviral pills are coming that seem to be able to cut a COVID-19 infection off at the knees, at least according to early data. A U.S. Food and Drug Administration panel meets next week to discuss the first application for a pill by Merck.
But experts caution that the coming surge will almost certainly tax hospitals again, especially in areas with lower vaccination rates.
And even states where blood testing shows that significant numbers of people have antibodies after a COVID-19 infection aren’t out of the woods, in part because we still don’t know how long the immunity generated by infection may last.
“Erosion of immunity”
“It’s hard to know how much risk is out there,” said Jeffrey Shaman, PhD, professor of environmental health sciences at Columbia University’s Mailman School of Public Health in New York City, who has been modeling the trajectory of the pandemic.
“We’re estimating, unfortunately, and we have for many weeks now, that there is an erosion of immunity,” Dr. Shaman said. “I think it could get bad. How bad? I’m not sure.”
Ali Mokdad, PhD, a professor of health metrics sciences at the University of Washington’s Institute for Health Metrics and Evaluation in Seattle, agrees.
Because there are so few studies on how long immunity from natural infection lasts, Dr. Mokdad and his colleagues are assuming that waning immunity after infection happens at least as quickly as it does after vaccination.
Their model is predicting that the average number of daily cases will peak at around 100,000, with another 100,000 going undetected, and will stay at that level until the end of January, as some states recover from their surges and others pick up steam.
While the number of daily deaths won’t climb to the heights seen during the summer surge, Dr. Mokdad said their model is predicting that daily deaths will climb again to about 1,200 a day.
“We are almost there right now, and it will be with us for a while,” he said. “We are predicting 881,000 deaths by March 1.”
The United States has currently recorded 773,000 COVID-19 deaths, so Dr. Mokdad is predicting about 120,000 more deaths between now and then.
He said his model shows that more than half of those deaths could be prevented if 95% of Americans wore their masks while in close proximity to strangers.
Currently, only about 36% of Americans are consistently wearing masks, according to surveys. While people are moving around more now, mobility is at prepandemic levels in some states.
“The rise that you are seeing right now is high mobility and low mask wearing in the United States,” Dr. Mokdad said.
The solution, he said, is for all adults to get another dose of vaccine — he doesn’t like calling it a booster.
“Because they’re vaccinated and they have two doses they have a false sense of security that they are protected. We needed to come ahead of it immediately and say you need a third dose, and we were late to do so,” Dr. Mokdad said.
A version of this article first appeared on Medscape.com.
30% of docs say they don’t want own kids 5-11 to get COVID vaccine
A Medscape
Among physician respondents who have children in that age group, 30% said they would not want their children to be vaccinated; 9% were unsure. For nurses/advanced practice registered nurses (APRNs), more (45%) said they did not want their kids to get the COVID-19 vaccine; 13% were unsure. Among pharmacists, 31% said they would not get them vaccinated and 9% were unsure.
Clinicians were more likely to want vaccinations for their kids 5-11 than were 510 consumers polled by WebMD at the same time. Overall, 49% of the consumers who had kids that age did not want them to get the COVID-19 vaccine.
On November 2, Centers for Disease Control and Prevention (CDC) Director Rochelle P. Walensky, MD, MPH, endorsed the CDC Advisory Committee on Immunization Practices’ recommendation that children 5-11 be vaccinated with the Pfizer-BioNTech pediatric vaccine. That decision expanded vaccine recommendations to about 28 million children in the United States.
The CDC states that, in clinical trials, the Pfizer vaccine had more than 90% efficacy in preventing laboratory-confirmed COVID-19 infection in children 5 to 15 years old, and that the immune response in children ages 5-15 equaled the immune response in people 16 to 25 years old.
The Medscape poll, fielded from November 3 to November 11, included 325 physicians, 793 nurses/APRNs, and 151 pharmacists.
How safe is the vaccine?
Clinicians were asked how confident they were that the vaccine is safe for that age group, and 66% of physicians, 52% of nurses/APRNs, and 66% of pharmacists said they were somewhat or very confident.
Among consumers overall in the WebMD poll, 56% said they were confident or somewhat confident that the vaccine is safe in that age group.
Among adolescents and young adults, rare cases of myocarditis and pericarditis in adolescents and young adults have been reported. According to the CDC, “[I]n one study, the risk of myocarditis after the second dose of Pfizer-BioNTech in the week following vaccination was around 54 cases per million doses administered to males ages 12-17 years.”
Known and potential benefits of COVID-19 vaccination outweigh the risks, including the possible risk for myocarditis or pericarditis, the CDC states.
Across clinician types, women edged out their male counterparts on confidence in the vaccine’ s safety: 71% vs 65% among physicians, 55% vs 45% among nurses/APRNs, and 68% vs 60% among pharmacists.
Among both physicians and nurses, younger physicians (under 45) tended to have greater confidence in the vaccine’ s safety: 72% vs 64% (physicians), 54% vs 51% (nurses/APRNs), and 71% vs 59% (pharmacists).
The difference in confidence was clear between vaccinated and unvaccinated physicians. All of the unvaccinated physicians who responded to the poll said they had no confidence in the vaccine for kids. Among unvaccinated nurses/APRNs, 2% were somewhat confident in the vaccine for kids under 12.
Knowledge about smaller dosage
The clinicians were asked about whether they were aware, before reading the poll question, that the Pfizer vaccine for children and the proposed Moderna vaccine for children in this age group (5-11) would have a different dosage.
The dose for kids 5-11 is 10 micrograms rather than 30 micrograms for people at least 12 years old. Children 5-11 receive a second dose 21 days or more after their first shot. The formulation comes with an orange cap, and a smaller needle is used.
Knowledge on the lower dose was highest among pharmacists (91% said they knew), followed by physicians (84%) and nurses (79%).
The poll also asked whether the COVID-19 vaccine should be added to the list of childhood immunizations. Responses varied widely and uncertainty was evident.
Notably, female physicians were more likely to say it should be added to the list of immunizations than were their male counterparts: 46% vs 35% (physicians), 26% vs 22% (nurses/APRNs), and 33% vs 30% (pharmacists).
A version of this article first appeared on Medscape.com.
A Medscape
Among physician respondents who have children in that age group, 30% said they would not want their children to be vaccinated; 9% were unsure. For nurses/advanced practice registered nurses (APRNs), more (45%) said they did not want their kids to get the COVID-19 vaccine; 13% were unsure. Among pharmacists, 31% said they would not get them vaccinated and 9% were unsure.
Clinicians were more likely to want vaccinations for their kids 5-11 than were 510 consumers polled by WebMD at the same time. Overall, 49% of the consumers who had kids that age did not want them to get the COVID-19 vaccine.
On November 2, Centers for Disease Control and Prevention (CDC) Director Rochelle P. Walensky, MD, MPH, endorsed the CDC Advisory Committee on Immunization Practices’ recommendation that children 5-11 be vaccinated with the Pfizer-BioNTech pediatric vaccine. That decision expanded vaccine recommendations to about 28 million children in the United States.
The CDC states that, in clinical trials, the Pfizer vaccine had more than 90% efficacy in preventing laboratory-confirmed COVID-19 infection in children 5 to 15 years old, and that the immune response in children ages 5-15 equaled the immune response in people 16 to 25 years old.
The Medscape poll, fielded from November 3 to November 11, included 325 physicians, 793 nurses/APRNs, and 151 pharmacists.
How safe is the vaccine?
Clinicians were asked how confident they were that the vaccine is safe for that age group, and 66% of physicians, 52% of nurses/APRNs, and 66% of pharmacists said they were somewhat or very confident.
Among consumers overall in the WebMD poll, 56% said they were confident or somewhat confident that the vaccine is safe in that age group.
Among adolescents and young adults, rare cases of myocarditis and pericarditis in adolescents and young adults have been reported. According to the CDC, “[I]n one study, the risk of myocarditis after the second dose of Pfizer-BioNTech in the week following vaccination was around 54 cases per million doses administered to males ages 12-17 years.”
Known and potential benefits of COVID-19 vaccination outweigh the risks, including the possible risk for myocarditis or pericarditis, the CDC states.
Across clinician types, women edged out their male counterparts on confidence in the vaccine’ s safety: 71% vs 65% among physicians, 55% vs 45% among nurses/APRNs, and 68% vs 60% among pharmacists.
Among both physicians and nurses, younger physicians (under 45) tended to have greater confidence in the vaccine’ s safety: 72% vs 64% (physicians), 54% vs 51% (nurses/APRNs), and 71% vs 59% (pharmacists).
The difference in confidence was clear between vaccinated and unvaccinated physicians. All of the unvaccinated physicians who responded to the poll said they had no confidence in the vaccine for kids. Among unvaccinated nurses/APRNs, 2% were somewhat confident in the vaccine for kids under 12.
Knowledge about smaller dosage
The clinicians were asked about whether they were aware, before reading the poll question, that the Pfizer vaccine for children and the proposed Moderna vaccine for children in this age group (5-11) would have a different dosage.
The dose for kids 5-11 is 10 micrograms rather than 30 micrograms for people at least 12 years old. Children 5-11 receive a second dose 21 days or more after their first shot. The formulation comes with an orange cap, and a smaller needle is used.
Knowledge on the lower dose was highest among pharmacists (91% said they knew), followed by physicians (84%) and nurses (79%).
The poll also asked whether the COVID-19 vaccine should be added to the list of childhood immunizations. Responses varied widely and uncertainty was evident.
Notably, female physicians were more likely to say it should be added to the list of immunizations than were their male counterparts: 46% vs 35% (physicians), 26% vs 22% (nurses/APRNs), and 33% vs 30% (pharmacists).
A version of this article first appeared on Medscape.com.
A Medscape
Among physician respondents who have children in that age group, 30% said they would not want their children to be vaccinated; 9% were unsure. For nurses/advanced practice registered nurses (APRNs), more (45%) said they did not want their kids to get the COVID-19 vaccine; 13% were unsure. Among pharmacists, 31% said they would not get them vaccinated and 9% were unsure.
Clinicians were more likely to want vaccinations for their kids 5-11 than were 510 consumers polled by WebMD at the same time. Overall, 49% of the consumers who had kids that age did not want them to get the COVID-19 vaccine.
On November 2, Centers for Disease Control and Prevention (CDC) Director Rochelle P. Walensky, MD, MPH, endorsed the CDC Advisory Committee on Immunization Practices’ recommendation that children 5-11 be vaccinated with the Pfizer-BioNTech pediatric vaccine. That decision expanded vaccine recommendations to about 28 million children in the United States.
The CDC states that, in clinical trials, the Pfizer vaccine had more than 90% efficacy in preventing laboratory-confirmed COVID-19 infection in children 5 to 15 years old, and that the immune response in children ages 5-15 equaled the immune response in people 16 to 25 years old.
The Medscape poll, fielded from November 3 to November 11, included 325 physicians, 793 nurses/APRNs, and 151 pharmacists.
How safe is the vaccine?
Clinicians were asked how confident they were that the vaccine is safe for that age group, and 66% of physicians, 52% of nurses/APRNs, and 66% of pharmacists said they were somewhat or very confident.
Among consumers overall in the WebMD poll, 56% said they were confident or somewhat confident that the vaccine is safe in that age group.
Among adolescents and young adults, rare cases of myocarditis and pericarditis in adolescents and young adults have been reported. According to the CDC, “[I]n one study, the risk of myocarditis after the second dose of Pfizer-BioNTech in the week following vaccination was around 54 cases per million doses administered to males ages 12-17 years.”
Known and potential benefits of COVID-19 vaccination outweigh the risks, including the possible risk for myocarditis or pericarditis, the CDC states.
Across clinician types, women edged out their male counterparts on confidence in the vaccine’ s safety: 71% vs 65% among physicians, 55% vs 45% among nurses/APRNs, and 68% vs 60% among pharmacists.
Among both physicians and nurses, younger physicians (under 45) tended to have greater confidence in the vaccine’ s safety: 72% vs 64% (physicians), 54% vs 51% (nurses/APRNs), and 71% vs 59% (pharmacists).
The difference in confidence was clear between vaccinated and unvaccinated physicians. All of the unvaccinated physicians who responded to the poll said they had no confidence in the vaccine for kids. Among unvaccinated nurses/APRNs, 2% were somewhat confident in the vaccine for kids under 12.
Knowledge about smaller dosage
The clinicians were asked about whether they were aware, before reading the poll question, that the Pfizer vaccine for children and the proposed Moderna vaccine for children in this age group (5-11) would have a different dosage.
The dose for kids 5-11 is 10 micrograms rather than 30 micrograms for people at least 12 years old. Children 5-11 receive a second dose 21 days or more after their first shot. The formulation comes with an orange cap, and a smaller needle is used.
Knowledge on the lower dose was highest among pharmacists (91% said they knew), followed by physicians (84%) and nurses (79%).
The poll also asked whether the COVID-19 vaccine should be added to the list of childhood immunizations. Responses varied widely and uncertainty was evident.
Notably, female physicians were more likely to say it should be added to the list of immunizations than were their male counterparts: 46% vs 35% (physicians), 26% vs 22% (nurses/APRNs), and 33% vs 30% (pharmacists).
A version of this article first appeared on Medscape.com.
High-poverty areas host more firearm-related youth deaths
Higher poverty concentration at the county level significantly increased the risk of firearm-related deaths in children and youth aged 5-24 years in the United States, based on a review of approximately 67,000 fatalities.
Firearms are the second-leading cause of death in children and young adults in the United States, according to data from the Centers for Disease Control and Prevention, wrote Jefferson T. Barrett, MD, of The Children’s Hospital at Montefiore, New York, and colleagues. County-level poverty has been associated with increased injury mortality in children, but the association between county-level poverty and firearm-related mortality in particular has not been well studied.
In a cross-sectional study published in JAMA Pediatrics, 67,905 firearm-related deaths in children and youth aged 5-24 years that occurred between Jan. 1, 2007, and Dec. 31, 2016 were analyzed. The deaths included 42,512 homicides (62.6%), 23,034 suicides (33.9%), and 1,627 unintentional deaths (2.4%).
County poverty data were acquired from the U.S. Census Bureau. County-level poverty was divided into five categories based on percentage of the population living below the federal poverty level: 0%-4.9%, 5%-9.9%, 10%-14.9%, 15%-19.9%, and 20% or more.
Overall, 88.6% of the total deaths were in males. Notably, 44.8% of total firearm-related deaths and 63.9% of homicides occurred in non-Hispanic Blacks, who make up only 14% of the youth population in the United States, the researchers wrote.
The total number of firearm-related deaths was 248 in the lowest quintile of poverty concentration, followed by 6,841, 18,551, 27,305, and 14,960 in the remaining quintiles.
In a multivariate regression model that included demographics, urban versus rural, and statewide firearm prevalence, youth in counties with the highest quintile of poverty concentration had an increased rate of total firearm-related deaths (adjusted incidence rate ratio, 2.29), as well as increased rates of homicides, suicides, and unintentional deaths (aIRR, 3.55, 1.45, and 9.32, respectively), compared with those living in the lowest quintile of poverty concentration. Individuals in the highest poverty quintile accounted for 22.0% of total firearm-related deaths, 25.5% of homicides, 15.3% of suicides, and 25.1% of unintentional deaths.
The researchers also calculated the population-attributable fraction (PAF) and years of potential life lost. “The PAF represents the proportion of deaths associated with a particular exposure, which was concentrated county poverty in this study,” they explained. The PAF for all firearm-related deaths was 0.51, PAFs for homicides, suicides, and unintentional deaths were 0.66, 0.30, and 0.86, respectively. The PAF calculation translated to 34,292 firearm-related deaths that may not have occurred if youth in all counties had the same risk as those in counties with the lowest poverty concentration.
“Over the 10-year study period, we observed 3,833,105 years of potential life lost in youth aged 5-24 years from firearm-related deaths,” the researchers wrote.
The study findings were limited by several factors including the potential bias of a cross-section design, and inability to account for all the ways that county-level poverty might increase the risk of firearm-related death in children and teens, the researchers noted. Other potential limitations include possible misclassification of death, lack of data on individual family incomes, shifts in counties in the poverty categories over time, and the use of statewide, rather than countywide, estimates of firearm ownership.
However, the results are consistent with those of previous studies, and add that “mortality rates were consistent even after controlling for demographic variables, county urbanicity, and statewide firearm prevalence,” the researchers concluded.
Address structural racism to reduce disparities
“Firearm-related homicides among youth aged 5-24 years are among the causes of death with the greatest disparities,” based on CDC fatal injury reports, wrote Alice M. Ellyson, PhD, Frederick P. Rivara, MD, and Ali Rowhani-Rahbar, MD, all of the University of Washington, Seattle, in an accompanying editorial.
The current study builds on previous research, including studies showing an association between income inequality and firearm-related homicide, they said. More research is needed to determine how to intervene in the pathways between poverty and firearm-related death. For example, if access to high-quality health care is a factor, programs to increase access to health insurance, such as the Affordable Care Act and Children’s Health Insurance Program, or to increase access to high-quality trauma care may help reduce firearm-related death in youth.
“The study of where, how, and why racism operates as a factor in both poverty and firearm-related death must continue, especially considering the disparities consistently documented in Alaska Native or American Indian, Black, and Hispanic communities,” the editorialists wrote.
“Key potential mechanisms for reducing the consequences of poverty for firearm-related death are often denied to racial and ethnic minority groups through a variety of structures, policies, and systems in health care, employment, housing, transportation, and education,” they emphasized, and the impact of racism, not only on the pathways to poverty, but also on mediators between poverty and firearm-related death, must be explored.
Findings spotlight need to for poverty programs
The study was an interesting look at the specific relationship between poverty and firearm-related deaths in people aged younger than 25 years in the United States, Tim Joos, MD, of Seattle said in an interview.
“Although America is not a poor country, the combination of poverty within America and its unique gun culture seems to prove deadly for its youth,” Dr. Joos said. “The strongest relationship is between firearm-related homicide and poverty, but unintentional firearm deaths and poverty also are clearly linked, whereas the link between firearm-related suicide and poverty appears to be present, but small.”.
In the current study, “the authors note that firearm deaths are the second-leading cause of death among all people ages 15-24 years,” said Dr. Joos. “Many of us have followed children from infancy just to have them meet this untimely end as adolescents, wishing we had a vaccine or other remedy in our toolbelt for this particular scourge.
“As our country currently debates the size of the social safety net, this study is one of many that suggests government programs aimed at poverty alleviation would substantially contribute to the health of American youth,” Dr. Joos added.
The study received no outside funding. Lead author Dr. Barrett had no financial conflicts to disclose. Dr. Ellyson disclosed funds from the CDC, the state of Washington, and the Grandmothers Against Gun Violence Foundation for research outside the submitted work. Dr. Rivara disclosed funds from the National Institutes of Health, the State of Washington, and the National Collaborative on Gun Violence Research for research outside the submitted work. Dr. Rowhani-Rahbar disclosed funds from the CDC, National Institutes of Health, National Collaborative on Gun Violence Research, Fund for a Safer Future, and state of Washington for research outside the submitted work. Dr. Joos had no financial conflicts to disclose, but serves on the editorial advisory board of Pediatric News.
Higher poverty concentration at the county level significantly increased the risk of firearm-related deaths in children and youth aged 5-24 years in the United States, based on a review of approximately 67,000 fatalities.
Firearms are the second-leading cause of death in children and young adults in the United States, according to data from the Centers for Disease Control and Prevention, wrote Jefferson T. Barrett, MD, of The Children’s Hospital at Montefiore, New York, and colleagues. County-level poverty has been associated with increased injury mortality in children, but the association between county-level poverty and firearm-related mortality in particular has not been well studied.
In a cross-sectional study published in JAMA Pediatrics, 67,905 firearm-related deaths in children and youth aged 5-24 years that occurred between Jan. 1, 2007, and Dec. 31, 2016 were analyzed. The deaths included 42,512 homicides (62.6%), 23,034 suicides (33.9%), and 1,627 unintentional deaths (2.4%).
County poverty data were acquired from the U.S. Census Bureau. County-level poverty was divided into five categories based on percentage of the population living below the federal poverty level: 0%-4.9%, 5%-9.9%, 10%-14.9%, 15%-19.9%, and 20% or more.
Overall, 88.6% of the total deaths were in males. Notably, 44.8% of total firearm-related deaths and 63.9% of homicides occurred in non-Hispanic Blacks, who make up only 14% of the youth population in the United States, the researchers wrote.
The total number of firearm-related deaths was 248 in the lowest quintile of poverty concentration, followed by 6,841, 18,551, 27,305, and 14,960 in the remaining quintiles.
In a multivariate regression model that included demographics, urban versus rural, and statewide firearm prevalence, youth in counties with the highest quintile of poverty concentration had an increased rate of total firearm-related deaths (adjusted incidence rate ratio, 2.29), as well as increased rates of homicides, suicides, and unintentional deaths (aIRR, 3.55, 1.45, and 9.32, respectively), compared with those living in the lowest quintile of poverty concentration. Individuals in the highest poverty quintile accounted for 22.0% of total firearm-related deaths, 25.5% of homicides, 15.3% of suicides, and 25.1% of unintentional deaths.
The researchers also calculated the population-attributable fraction (PAF) and years of potential life lost. “The PAF represents the proportion of deaths associated with a particular exposure, which was concentrated county poverty in this study,” they explained. The PAF for all firearm-related deaths was 0.51, PAFs for homicides, suicides, and unintentional deaths were 0.66, 0.30, and 0.86, respectively. The PAF calculation translated to 34,292 firearm-related deaths that may not have occurred if youth in all counties had the same risk as those in counties with the lowest poverty concentration.
“Over the 10-year study period, we observed 3,833,105 years of potential life lost in youth aged 5-24 years from firearm-related deaths,” the researchers wrote.
The study findings were limited by several factors including the potential bias of a cross-section design, and inability to account for all the ways that county-level poverty might increase the risk of firearm-related death in children and teens, the researchers noted. Other potential limitations include possible misclassification of death, lack of data on individual family incomes, shifts in counties in the poverty categories over time, and the use of statewide, rather than countywide, estimates of firearm ownership.
However, the results are consistent with those of previous studies, and add that “mortality rates were consistent even after controlling for demographic variables, county urbanicity, and statewide firearm prevalence,” the researchers concluded.
Address structural racism to reduce disparities
“Firearm-related homicides among youth aged 5-24 years are among the causes of death with the greatest disparities,” based on CDC fatal injury reports, wrote Alice M. Ellyson, PhD, Frederick P. Rivara, MD, and Ali Rowhani-Rahbar, MD, all of the University of Washington, Seattle, in an accompanying editorial.
The current study builds on previous research, including studies showing an association between income inequality and firearm-related homicide, they said. More research is needed to determine how to intervene in the pathways between poverty and firearm-related death. For example, if access to high-quality health care is a factor, programs to increase access to health insurance, such as the Affordable Care Act and Children’s Health Insurance Program, or to increase access to high-quality trauma care may help reduce firearm-related death in youth.
“The study of where, how, and why racism operates as a factor in both poverty and firearm-related death must continue, especially considering the disparities consistently documented in Alaska Native or American Indian, Black, and Hispanic communities,” the editorialists wrote.
“Key potential mechanisms for reducing the consequences of poverty for firearm-related death are often denied to racial and ethnic minority groups through a variety of structures, policies, and systems in health care, employment, housing, transportation, and education,” they emphasized, and the impact of racism, not only on the pathways to poverty, but also on mediators between poverty and firearm-related death, must be explored.
Findings spotlight need to for poverty programs
The study was an interesting look at the specific relationship between poverty and firearm-related deaths in people aged younger than 25 years in the United States, Tim Joos, MD, of Seattle said in an interview.
“Although America is not a poor country, the combination of poverty within America and its unique gun culture seems to prove deadly for its youth,” Dr. Joos said. “The strongest relationship is between firearm-related homicide and poverty, but unintentional firearm deaths and poverty also are clearly linked, whereas the link between firearm-related suicide and poverty appears to be present, but small.”.
In the current study, “the authors note that firearm deaths are the second-leading cause of death among all people ages 15-24 years,” said Dr. Joos. “Many of us have followed children from infancy just to have them meet this untimely end as adolescents, wishing we had a vaccine or other remedy in our toolbelt for this particular scourge.
“As our country currently debates the size of the social safety net, this study is one of many that suggests government programs aimed at poverty alleviation would substantially contribute to the health of American youth,” Dr. Joos added.
The study received no outside funding. Lead author Dr. Barrett had no financial conflicts to disclose. Dr. Ellyson disclosed funds from the CDC, the state of Washington, and the Grandmothers Against Gun Violence Foundation for research outside the submitted work. Dr. Rivara disclosed funds from the National Institutes of Health, the State of Washington, and the National Collaborative on Gun Violence Research for research outside the submitted work. Dr. Rowhani-Rahbar disclosed funds from the CDC, National Institutes of Health, National Collaborative on Gun Violence Research, Fund for a Safer Future, and state of Washington for research outside the submitted work. Dr. Joos had no financial conflicts to disclose, but serves on the editorial advisory board of Pediatric News.
Higher poverty concentration at the county level significantly increased the risk of firearm-related deaths in children and youth aged 5-24 years in the United States, based on a review of approximately 67,000 fatalities.
Firearms are the second-leading cause of death in children and young adults in the United States, according to data from the Centers for Disease Control and Prevention, wrote Jefferson T. Barrett, MD, of The Children’s Hospital at Montefiore, New York, and colleagues. County-level poverty has been associated with increased injury mortality in children, but the association between county-level poverty and firearm-related mortality in particular has not been well studied.
In a cross-sectional study published in JAMA Pediatrics, 67,905 firearm-related deaths in children and youth aged 5-24 years that occurred between Jan. 1, 2007, and Dec. 31, 2016 were analyzed. The deaths included 42,512 homicides (62.6%), 23,034 suicides (33.9%), and 1,627 unintentional deaths (2.4%).
County poverty data were acquired from the U.S. Census Bureau. County-level poverty was divided into five categories based on percentage of the population living below the federal poverty level: 0%-4.9%, 5%-9.9%, 10%-14.9%, 15%-19.9%, and 20% or more.
Overall, 88.6% of the total deaths were in males. Notably, 44.8% of total firearm-related deaths and 63.9% of homicides occurred in non-Hispanic Blacks, who make up only 14% of the youth population in the United States, the researchers wrote.
The total number of firearm-related deaths was 248 in the lowest quintile of poverty concentration, followed by 6,841, 18,551, 27,305, and 14,960 in the remaining quintiles.
In a multivariate regression model that included demographics, urban versus rural, and statewide firearm prevalence, youth in counties with the highest quintile of poverty concentration had an increased rate of total firearm-related deaths (adjusted incidence rate ratio, 2.29), as well as increased rates of homicides, suicides, and unintentional deaths (aIRR, 3.55, 1.45, and 9.32, respectively), compared with those living in the lowest quintile of poverty concentration. Individuals in the highest poverty quintile accounted for 22.0% of total firearm-related deaths, 25.5% of homicides, 15.3% of suicides, and 25.1% of unintentional deaths.
The researchers also calculated the population-attributable fraction (PAF) and years of potential life lost. “The PAF represents the proportion of deaths associated with a particular exposure, which was concentrated county poverty in this study,” they explained. The PAF for all firearm-related deaths was 0.51, PAFs for homicides, suicides, and unintentional deaths were 0.66, 0.30, and 0.86, respectively. The PAF calculation translated to 34,292 firearm-related deaths that may not have occurred if youth in all counties had the same risk as those in counties with the lowest poverty concentration.
“Over the 10-year study period, we observed 3,833,105 years of potential life lost in youth aged 5-24 years from firearm-related deaths,” the researchers wrote.
The study findings were limited by several factors including the potential bias of a cross-section design, and inability to account for all the ways that county-level poverty might increase the risk of firearm-related death in children and teens, the researchers noted. Other potential limitations include possible misclassification of death, lack of data on individual family incomes, shifts in counties in the poverty categories over time, and the use of statewide, rather than countywide, estimates of firearm ownership.
However, the results are consistent with those of previous studies, and add that “mortality rates were consistent even after controlling for demographic variables, county urbanicity, and statewide firearm prevalence,” the researchers concluded.
Address structural racism to reduce disparities
“Firearm-related homicides among youth aged 5-24 years are among the causes of death with the greatest disparities,” based on CDC fatal injury reports, wrote Alice M. Ellyson, PhD, Frederick P. Rivara, MD, and Ali Rowhani-Rahbar, MD, all of the University of Washington, Seattle, in an accompanying editorial.
The current study builds on previous research, including studies showing an association between income inequality and firearm-related homicide, they said. More research is needed to determine how to intervene in the pathways between poverty and firearm-related death. For example, if access to high-quality health care is a factor, programs to increase access to health insurance, such as the Affordable Care Act and Children’s Health Insurance Program, or to increase access to high-quality trauma care may help reduce firearm-related death in youth.
“The study of where, how, and why racism operates as a factor in both poverty and firearm-related death must continue, especially considering the disparities consistently documented in Alaska Native or American Indian, Black, and Hispanic communities,” the editorialists wrote.
“Key potential mechanisms for reducing the consequences of poverty for firearm-related death are often denied to racial and ethnic minority groups through a variety of structures, policies, and systems in health care, employment, housing, transportation, and education,” they emphasized, and the impact of racism, not only on the pathways to poverty, but also on mediators between poverty and firearm-related death, must be explored.
Findings spotlight need to for poverty programs
The study was an interesting look at the specific relationship between poverty and firearm-related deaths in people aged younger than 25 years in the United States, Tim Joos, MD, of Seattle said in an interview.
“Although America is not a poor country, the combination of poverty within America and its unique gun culture seems to prove deadly for its youth,” Dr. Joos said. “The strongest relationship is between firearm-related homicide and poverty, but unintentional firearm deaths and poverty also are clearly linked, whereas the link between firearm-related suicide and poverty appears to be present, but small.”.
In the current study, “the authors note that firearm deaths are the second-leading cause of death among all people ages 15-24 years,” said Dr. Joos. “Many of us have followed children from infancy just to have them meet this untimely end as adolescents, wishing we had a vaccine or other remedy in our toolbelt for this particular scourge.
“As our country currently debates the size of the social safety net, this study is one of many that suggests government programs aimed at poverty alleviation would substantially contribute to the health of American youth,” Dr. Joos added.
The study received no outside funding. Lead author Dr. Barrett had no financial conflicts to disclose. Dr. Ellyson disclosed funds from the CDC, the state of Washington, and the Grandmothers Against Gun Violence Foundation for research outside the submitted work. Dr. Rivara disclosed funds from the National Institutes of Health, the State of Washington, and the National Collaborative on Gun Violence Research for research outside the submitted work. Dr. Rowhani-Rahbar disclosed funds from the CDC, National Institutes of Health, National Collaborative on Gun Violence Research, Fund for a Safer Future, and state of Washington for research outside the submitted work. Dr. Joos had no financial conflicts to disclose, but serves on the editorial advisory board of Pediatric News.
FROM JAMA PEDIATRICS


