User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
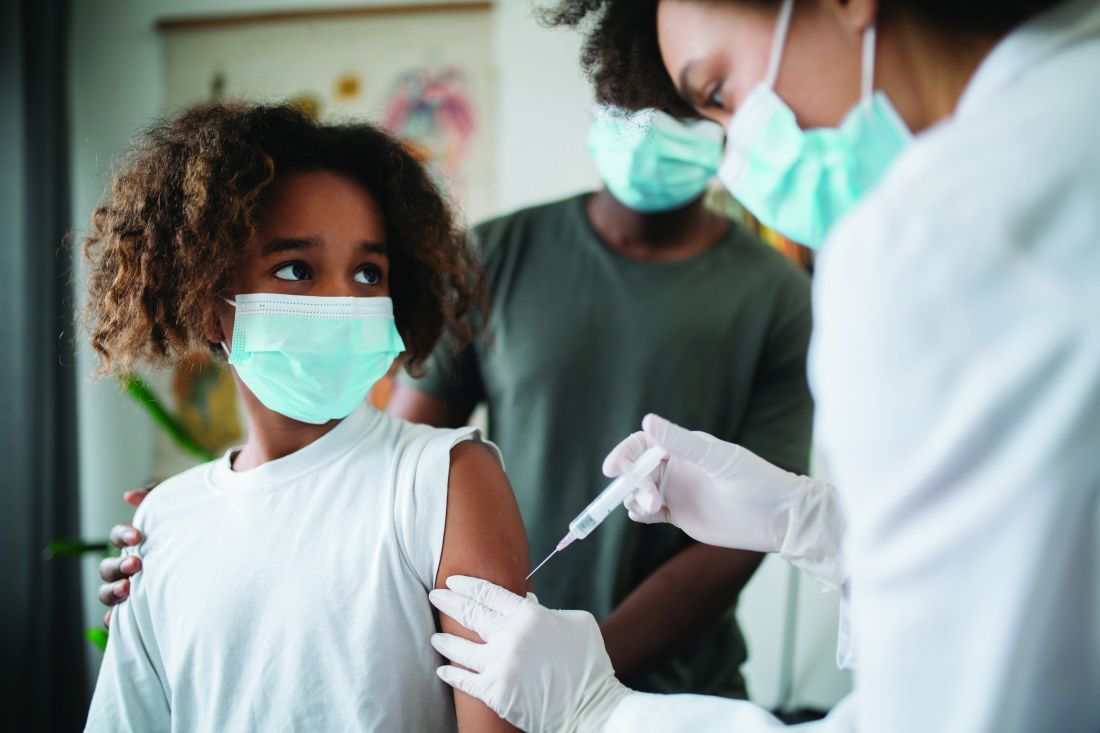
RSV resurgence likely in wake of COVID-19
The impact of respiratory syncytial virus (RSV)will likely be greater in 2021 and 2022 in the United States than in previous years as a result of the ongoing COVID-19 pandemic, based on data from a simulation-modeling study involving approximately 19 million individuals.
Although RSV usually follows consistent patterns of timing and duration, the disease all but disappeared starting in March 2020 after the introduction of measures to mitigate the spread of COVID-19, Zhe Zheng, MBBS, of Yale University, New Haven, Conn., and colleagues wrote.
However, lifting of mitigation measures has resulted in emergence of RSV in various parts of the world in early 2021, and trends may be similar in the United States, but data are needed to plan for prophylaxis and hospital use, they noted.
In a study published in JAMA Network Open, the researchers developed a simulation model for epidemics of RSV based on historical data. They acquired inpatient records from New York during 2005-2014 and from California during 2003-2011. The primary clinical outcome was the estimated monthly hospitalizations for RSV.
The simulated study population was 19.45 million individuals. After evaluating several scenarios including continued low transmission associated with social distancing and other mitigation measures, the researchers focused on the likely scenario that introduction of RSV from other regions would likely spark RSV epidemics in the United States.
They determined that spring and summer 2021 would show an increase in hospitalizations for RSV. Overall, higher rates of virus introduction from other regions were associated with more intense spring and summer RSV epidemics, with the trade-off of smaller winter epidemics. In the model, the expected RSV epidemic in spring and summer 2021 in New York was small, with a peak incidence of 419 hospitalizations per 100,000 people in April; by contrast, for states with less seasonal variability, such as Florida, the model predicted a larger summer epidemic.
In the model, the mean age of hospitalization for children younger than 5 years for January 2022 was expected to be 1.17 years, compared with 0.84 years in January 2019, the researchers noted.
Across all age groups, the greatest relative increase in the incidence of RSV infection was predicted for children aged 1-4 years (ranging from 82% to 86%), as were lower respiratory infections (87%-101%) and hospitalization (99%-119%), compared with prepandemic levels.
Hospitalizations for children aged 1 year were predicted to double compared with prepandemic seasons; 707 per 100,000 children per year for 2021 and 2022 versus 355 per 100,000 children per year in a typical prepandemic season. However, the largest incidence of lower respiratory infections (30,075 per 100,000) was predicted for infants aged 3-5 months, and the largest incidence of hospitalizations (3,116 per 100,000) was predicted for infants younger than 3 months.
“Without virus importation, the risk of RSV infections across all age groups in the winter of 2021 and 2022 would be greater, as more susceptible individuals were spared from infections in the absence of summer epidemics,” the researchers noted.
The older mean hospitalization age seen in the model was similar to the reported median patient age in Australia both before the pandemic and during the reemergent RSV epidemic.
“This makes intuitive sense, since many children born in 2020 were spared from RSV infection due to the low virus activity; these children will be older when they get infected for the first time during the reemergent epidemics,” the researchers wrote. “Consequently, stakeholders should consider modifying prophylaxis guidelines to include high-risk infants less than 2 years of age for the 2021-2022 season.”
The study findings were limited by several factors including the lack of data on level of virus introduction or on the impact of lack of boosting on infants with only transplacentally acquired RSV antibodies, the researchers noted. Other limitations include the use of historical data and the lack of data on values outside those included in the model, as well as the inability to control for other factors that could influence RSV, such as vaccines or long-lasting antibodies.
However, the results suggest that the rate of imported infections is associated with RSV hospitalizations, and the model effectively captured the RSV epidemics in the United States in spring and summer 2021.
Models can guide clinical preparations
“Health care simulation modeling is a growing field, with very exciting implications,” Lenore Jarvis, MD, of George Washington University, Washington, said in an interview. The field has the potential ability to influence health care in a data-driven way, including, but not limited to, staffing and other hospital operations, as well as patient care decision-making. “In short, accurate modeling and predictions can help us to make informed health care decisions that can lead to increased quality of care, potential cost savings, and even to help save lives,” she said.
Although the details of transmission modeling were not mentioned in the study, the authors evaluated the performances of several models and scenarios. “Scenario 4, for example, was focused on in particular because it best captured the observed dynamics [for RSV] that emerged during the spring and summer of 2021,” Dr. Jarvis said.
“Pediatricians can speak to these trends firsthand. A decrease in expected RSV infections and hospitalizations in 2020, followed by an unprecedented and early increase in RSV infections and severity in 2021, and the factors that the authors account for make sense, such as reintroduction of RSV from other regions and low immunity in the population,” she said. “It also makes sense that, in these transmission modeling scenarios, the expected mean age of hospitalization because of RSV increased with a temporary (hopefully) increase in RSV hospitalizations in the 2021 season, and potentially the 2022 RSV season.”
As for additional research, Dr. Jarvis said she would like to see follow-up data on the RSV transmission modeling. “For example, with scenario 4, does this scenario continue to perform well in other time periods, such as the winter? If the modeling continues to be accurate during other periods of evaluation and reevaluation, this modeling could be very useful in helping pediatric clinics and hospitals to prepare for RSV care and hospital capacity management.”
The study was supported by grants to various researchers from the National Institute of Allergy and Infectious Diseases/National Institutes of Health, the National Center for Advancing Translational Science at the National Institutes of Health, and NIH Roadmap for Medical Research. Lead author Ms. Zheng had no financial conflicts to disclose. Her study coauthors disclosed relationships with companies including AbbVie, Merck, Pfizer, GlaxoSmithKline, MedImmune, and Janssen. Dr. Jarvis had no financial conflicts to disclose and serves on the Pediatric News editorial advisory board.
The impact of respiratory syncytial virus (RSV)will likely be greater in 2021 and 2022 in the United States than in previous years as a result of the ongoing COVID-19 pandemic, based on data from a simulation-modeling study involving approximately 19 million individuals.
Although RSV usually follows consistent patterns of timing and duration, the disease all but disappeared starting in March 2020 after the introduction of measures to mitigate the spread of COVID-19, Zhe Zheng, MBBS, of Yale University, New Haven, Conn., and colleagues wrote.
However, lifting of mitigation measures has resulted in emergence of RSV in various parts of the world in early 2021, and trends may be similar in the United States, but data are needed to plan for prophylaxis and hospital use, they noted.
In a study published in JAMA Network Open, the researchers developed a simulation model for epidemics of RSV based on historical data. They acquired inpatient records from New York during 2005-2014 and from California during 2003-2011. The primary clinical outcome was the estimated monthly hospitalizations for RSV.
The simulated study population was 19.45 million individuals. After evaluating several scenarios including continued low transmission associated with social distancing and other mitigation measures, the researchers focused on the likely scenario that introduction of RSV from other regions would likely spark RSV epidemics in the United States.
They determined that spring and summer 2021 would show an increase in hospitalizations for RSV. Overall, higher rates of virus introduction from other regions were associated with more intense spring and summer RSV epidemics, with the trade-off of smaller winter epidemics. In the model, the expected RSV epidemic in spring and summer 2021 in New York was small, with a peak incidence of 419 hospitalizations per 100,000 people in April; by contrast, for states with less seasonal variability, such as Florida, the model predicted a larger summer epidemic.
In the model, the mean age of hospitalization for children younger than 5 years for January 2022 was expected to be 1.17 years, compared with 0.84 years in January 2019, the researchers noted.
Across all age groups, the greatest relative increase in the incidence of RSV infection was predicted for children aged 1-4 years (ranging from 82% to 86%), as were lower respiratory infections (87%-101%) and hospitalization (99%-119%), compared with prepandemic levels.
Hospitalizations for children aged 1 year were predicted to double compared with prepandemic seasons; 707 per 100,000 children per year for 2021 and 2022 versus 355 per 100,000 children per year in a typical prepandemic season. However, the largest incidence of lower respiratory infections (30,075 per 100,000) was predicted for infants aged 3-5 months, and the largest incidence of hospitalizations (3,116 per 100,000) was predicted for infants younger than 3 months.
“Without virus importation, the risk of RSV infections across all age groups in the winter of 2021 and 2022 would be greater, as more susceptible individuals were spared from infections in the absence of summer epidemics,” the researchers noted.
The older mean hospitalization age seen in the model was similar to the reported median patient age in Australia both before the pandemic and during the reemergent RSV epidemic.
“This makes intuitive sense, since many children born in 2020 were spared from RSV infection due to the low virus activity; these children will be older when they get infected for the first time during the reemergent epidemics,” the researchers wrote. “Consequently, stakeholders should consider modifying prophylaxis guidelines to include high-risk infants less than 2 years of age for the 2021-2022 season.”
The study findings were limited by several factors including the lack of data on level of virus introduction or on the impact of lack of boosting on infants with only transplacentally acquired RSV antibodies, the researchers noted. Other limitations include the use of historical data and the lack of data on values outside those included in the model, as well as the inability to control for other factors that could influence RSV, such as vaccines or long-lasting antibodies.
However, the results suggest that the rate of imported infections is associated with RSV hospitalizations, and the model effectively captured the RSV epidemics in the United States in spring and summer 2021.
Models can guide clinical preparations
“Health care simulation modeling is a growing field, with very exciting implications,” Lenore Jarvis, MD, of George Washington University, Washington, said in an interview. The field has the potential ability to influence health care in a data-driven way, including, but not limited to, staffing and other hospital operations, as well as patient care decision-making. “In short, accurate modeling and predictions can help us to make informed health care decisions that can lead to increased quality of care, potential cost savings, and even to help save lives,” she said.
Although the details of transmission modeling were not mentioned in the study, the authors evaluated the performances of several models and scenarios. “Scenario 4, for example, was focused on in particular because it best captured the observed dynamics [for RSV] that emerged during the spring and summer of 2021,” Dr. Jarvis said.
“Pediatricians can speak to these trends firsthand. A decrease in expected RSV infections and hospitalizations in 2020, followed by an unprecedented and early increase in RSV infections and severity in 2021, and the factors that the authors account for make sense, such as reintroduction of RSV from other regions and low immunity in the population,” she said. “It also makes sense that, in these transmission modeling scenarios, the expected mean age of hospitalization because of RSV increased with a temporary (hopefully) increase in RSV hospitalizations in the 2021 season, and potentially the 2022 RSV season.”
As for additional research, Dr. Jarvis said she would like to see follow-up data on the RSV transmission modeling. “For example, with scenario 4, does this scenario continue to perform well in other time periods, such as the winter? If the modeling continues to be accurate during other periods of evaluation and reevaluation, this modeling could be very useful in helping pediatric clinics and hospitals to prepare for RSV care and hospital capacity management.”
The study was supported by grants to various researchers from the National Institute of Allergy and Infectious Diseases/National Institutes of Health, the National Center for Advancing Translational Science at the National Institutes of Health, and NIH Roadmap for Medical Research. Lead author Ms. Zheng had no financial conflicts to disclose. Her study coauthors disclosed relationships with companies including AbbVie, Merck, Pfizer, GlaxoSmithKline, MedImmune, and Janssen. Dr. Jarvis had no financial conflicts to disclose and serves on the Pediatric News editorial advisory board.
The impact of respiratory syncytial virus (RSV)will likely be greater in 2021 and 2022 in the United States than in previous years as a result of the ongoing COVID-19 pandemic, based on data from a simulation-modeling study involving approximately 19 million individuals.
Although RSV usually follows consistent patterns of timing and duration, the disease all but disappeared starting in March 2020 after the introduction of measures to mitigate the spread of COVID-19, Zhe Zheng, MBBS, of Yale University, New Haven, Conn., and colleagues wrote.
However, lifting of mitigation measures has resulted in emergence of RSV in various parts of the world in early 2021, and trends may be similar in the United States, but data are needed to plan for prophylaxis and hospital use, they noted.
In a study published in JAMA Network Open, the researchers developed a simulation model for epidemics of RSV based on historical data. They acquired inpatient records from New York during 2005-2014 and from California during 2003-2011. The primary clinical outcome was the estimated monthly hospitalizations for RSV.
The simulated study population was 19.45 million individuals. After evaluating several scenarios including continued low transmission associated with social distancing and other mitigation measures, the researchers focused on the likely scenario that introduction of RSV from other regions would likely spark RSV epidemics in the United States.
They determined that spring and summer 2021 would show an increase in hospitalizations for RSV. Overall, higher rates of virus introduction from other regions were associated with more intense spring and summer RSV epidemics, with the trade-off of smaller winter epidemics. In the model, the expected RSV epidemic in spring and summer 2021 in New York was small, with a peak incidence of 419 hospitalizations per 100,000 people in April; by contrast, for states with less seasonal variability, such as Florida, the model predicted a larger summer epidemic.
In the model, the mean age of hospitalization for children younger than 5 years for January 2022 was expected to be 1.17 years, compared with 0.84 years in January 2019, the researchers noted.
Across all age groups, the greatest relative increase in the incidence of RSV infection was predicted for children aged 1-4 years (ranging from 82% to 86%), as were lower respiratory infections (87%-101%) and hospitalization (99%-119%), compared with prepandemic levels.
Hospitalizations for children aged 1 year were predicted to double compared with prepandemic seasons; 707 per 100,000 children per year for 2021 and 2022 versus 355 per 100,000 children per year in a typical prepandemic season. However, the largest incidence of lower respiratory infections (30,075 per 100,000) was predicted for infants aged 3-5 months, and the largest incidence of hospitalizations (3,116 per 100,000) was predicted for infants younger than 3 months.
“Without virus importation, the risk of RSV infections across all age groups in the winter of 2021 and 2022 would be greater, as more susceptible individuals were spared from infections in the absence of summer epidemics,” the researchers noted.
The older mean hospitalization age seen in the model was similar to the reported median patient age in Australia both before the pandemic and during the reemergent RSV epidemic.
“This makes intuitive sense, since many children born in 2020 were spared from RSV infection due to the low virus activity; these children will be older when they get infected for the first time during the reemergent epidemics,” the researchers wrote. “Consequently, stakeholders should consider modifying prophylaxis guidelines to include high-risk infants less than 2 years of age for the 2021-2022 season.”
The study findings were limited by several factors including the lack of data on level of virus introduction or on the impact of lack of boosting on infants with only transplacentally acquired RSV antibodies, the researchers noted. Other limitations include the use of historical data and the lack of data on values outside those included in the model, as well as the inability to control for other factors that could influence RSV, such as vaccines or long-lasting antibodies.
However, the results suggest that the rate of imported infections is associated with RSV hospitalizations, and the model effectively captured the RSV epidemics in the United States in spring and summer 2021.
Models can guide clinical preparations
“Health care simulation modeling is a growing field, with very exciting implications,” Lenore Jarvis, MD, of George Washington University, Washington, said in an interview. The field has the potential ability to influence health care in a data-driven way, including, but not limited to, staffing and other hospital operations, as well as patient care decision-making. “In short, accurate modeling and predictions can help us to make informed health care decisions that can lead to increased quality of care, potential cost savings, and even to help save lives,” she said.
Although the details of transmission modeling were not mentioned in the study, the authors evaluated the performances of several models and scenarios. “Scenario 4, for example, was focused on in particular because it best captured the observed dynamics [for RSV] that emerged during the spring and summer of 2021,” Dr. Jarvis said.
“Pediatricians can speak to these trends firsthand. A decrease in expected RSV infections and hospitalizations in 2020, followed by an unprecedented and early increase in RSV infections and severity in 2021, and the factors that the authors account for make sense, such as reintroduction of RSV from other regions and low immunity in the population,” she said. “It also makes sense that, in these transmission modeling scenarios, the expected mean age of hospitalization because of RSV increased with a temporary (hopefully) increase in RSV hospitalizations in the 2021 season, and potentially the 2022 RSV season.”
As for additional research, Dr. Jarvis said she would like to see follow-up data on the RSV transmission modeling. “For example, with scenario 4, does this scenario continue to perform well in other time periods, such as the winter? If the modeling continues to be accurate during other periods of evaluation and reevaluation, this modeling could be very useful in helping pediatric clinics and hospitals to prepare for RSV care and hospital capacity management.”
The study was supported by grants to various researchers from the National Institute of Allergy and Infectious Diseases/National Institutes of Health, the National Center for Advancing Translational Science at the National Institutes of Health, and NIH Roadmap for Medical Research. Lead author Ms. Zheng had no financial conflicts to disclose. Her study coauthors disclosed relationships with companies including AbbVie, Merck, Pfizer, GlaxoSmithKline, MedImmune, and Janssen. Dr. Jarvis had no financial conflicts to disclose and serves on the Pediatric News editorial advisory board.
FROM JAMA NETWORK OPEN
Ophthalmologist who developed medical botox dies at 89
his family confirmed to National Public Radio.
Four decades ago, Alan Brown Scott, MD, a native of Berkeley, Calif., turned the drug, once a deadly poison, into a revolutionary treatment for obscure eye diseases. It later became a well-known blockbuster treatment for reducing the appearance of wrinkles and treating hyperhidrosis (excessive sweating). Other approved medical uses include treatment of overactive bladder and urinary incontinence.
According to the American Society of Plastic Surgeons, its popularity for cosmetic use was boosted further during the pandemic and it was the No. 1 minimally invasive cosmetic procedure performed in 2020. Among the 13.3 million procedures, 4.4 million involved Botox.
According to Bloomberg Businessweek, Ed Schantz, who was working in the military’s biological weapons program, was the one to first send the toxin to Dr. Scott, who wanted to explore its properties for medical use.
The same Bloomberg article also noted that the original botulinum toxin itself “is so powerful that a tiny amount can suffocate a person by paralyzing the muscles used for breathing.”
Dr. Scott was looking for a way to help his patients avoid extensive surgeries.
“Specifically, he was aiming to treat people with strabismus, or cross-eyes, and blepharospasm, which is an uncontrollable closure of eyes. Today, it’s also used as a treatment to help with migraines, hair loss, and drooling,” NPR reported.
The New York Times once described Botox as “medicine’s answer to duct tape.”
Dr. Scott was the executive director of the Smith-Kettlewell Eye Research Institute in San Francisco when he did his pioneering research with botulinum toxin in the 1970s and 1980s, according to a 2002 article in SFGate.
In 1991, Dr. Scott sold the drug to Allergan, when it was called Oculinum. The next year, the name was officially changed to Botox.
In 2002, Dr. Scott told SFGate, when asked about the more popular use for the drug, “I think that’s a charming, slightly frivolous use,” adding, “but it’s not along the lines of what I was into, applications for serious disorders.”
According to Scientific American in 2016, Dr. Scott, then age 83, kept working on the noncosmetic benefits of botulism-toxin injections for eye-related disorders at the Strabismus Research Foundation,
He told Scientific American he was proud that his efforts “are directly helpful to people.”
“There are interesting and difficult problems still to be solved, and I’m a practicing physician and I see them every day,” he said.
Dr. Scott’s daughter, Ann Scott, told NPR: “He definitely loved his work and he was also a really great father.” She said her dad involved his children in his research and work.
She added, “He was a really calm, more of a quiet reserved person,” and said he was committed to teaching his students, many of them international students.
“That was what he really loved,” she said.
Dr. Scott, who died Dec. 16, was in intensive care for the last 10 days from an unspecified illness, his daughter told NPR.
A version of this article first appeared on Medscape.com.
his family confirmed to National Public Radio.
Four decades ago, Alan Brown Scott, MD, a native of Berkeley, Calif., turned the drug, once a deadly poison, into a revolutionary treatment for obscure eye diseases. It later became a well-known blockbuster treatment for reducing the appearance of wrinkles and treating hyperhidrosis (excessive sweating). Other approved medical uses include treatment of overactive bladder and urinary incontinence.
According to the American Society of Plastic Surgeons, its popularity for cosmetic use was boosted further during the pandemic and it was the No. 1 minimally invasive cosmetic procedure performed in 2020. Among the 13.3 million procedures, 4.4 million involved Botox.
According to Bloomberg Businessweek, Ed Schantz, who was working in the military’s biological weapons program, was the one to first send the toxin to Dr. Scott, who wanted to explore its properties for medical use.
The same Bloomberg article also noted that the original botulinum toxin itself “is so powerful that a tiny amount can suffocate a person by paralyzing the muscles used for breathing.”
Dr. Scott was looking for a way to help his patients avoid extensive surgeries.
“Specifically, he was aiming to treat people with strabismus, or cross-eyes, and blepharospasm, which is an uncontrollable closure of eyes. Today, it’s also used as a treatment to help with migraines, hair loss, and drooling,” NPR reported.
The New York Times once described Botox as “medicine’s answer to duct tape.”
Dr. Scott was the executive director of the Smith-Kettlewell Eye Research Institute in San Francisco when he did his pioneering research with botulinum toxin in the 1970s and 1980s, according to a 2002 article in SFGate.
In 1991, Dr. Scott sold the drug to Allergan, when it was called Oculinum. The next year, the name was officially changed to Botox.
In 2002, Dr. Scott told SFGate, when asked about the more popular use for the drug, “I think that’s a charming, slightly frivolous use,” adding, “but it’s not along the lines of what I was into, applications for serious disorders.”
According to Scientific American in 2016, Dr. Scott, then age 83, kept working on the noncosmetic benefits of botulism-toxin injections for eye-related disorders at the Strabismus Research Foundation,
He told Scientific American he was proud that his efforts “are directly helpful to people.”
“There are interesting and difficult problems still to be solved, and I’m a practicing physician and I see them every day,” he said.
Dr. Scott’s daughter, Ann Scott, told NPR: “He definitely loved his work and he was also a really great father.” She said her dad involved his children in his research and work.
She added, “He was a really calm, more of a quiet reserved person,” and said he was committed to teaching his students, many of them international students.
“That was what he really loved,” she said.
Dr. Scott, who died Dec. 16, was in intensive care for the last 10 days from an unspecified illness, his daughter told NPR.
A version of this article first appeared on Medscape.com.
his family confirmed to National Public Radio.
Four decades ago, Alan Brown Scott, MD, a native of Berkeley, Calif., turned the drug, once a deadly poison, into a revolutionary treatment for obscure eye diseases. It later became a well-known blockbuster treatment for reducing the appearance of wrinkles and treating hyperhidrosis (excessive sweating). Other approved medical uses include treatment of overactive bladder and urinary incontinence.
According to the American Society of Plastic Surgeons, its popularity for cosmetic use was boosted further during the pandemic and it was the No. 1 minimally invasive cosmetic procedure performed in 2020. Among the 13.3 million procedures, 4.4 million involved Botox.
According to Bloomberg Businessweek, Ed Schantz, who was working in the military’s biological weapons program, was the one to first send the toxin to Dr. Scott, who wanted to explore its properties for medical use.
The same Bloomberg article also noted that the original botulinum toxin itself “is so powerful that a tiny amount can suffocate a person by paralyzing the muscles used for breathing.”
Dr. Scott was looking for a way to help his patients avoid extensive surgeries.
“Specifically, he was aiming to treat people with strabismus, or cross-eyes, and blepharospasm, which is an uncontrollable closure of eyes. Today, it’s also used as a treatment to help with migraines, hair loss, and drooling,” NPR reported.
The New York Times once described Botox as “medicine’s answer to duct tape.”
Dr. Scott was the executive director of the Smith-Kettlewell Eye Research Institute in San Francisco when he did his pioneering research with botulinum toxin in the 1970s and 1980s, according to a 2002 article in SFGate.
In 1991, Dr. Scott sold the drug to Allergan, when it was called Oculinum. The next year, the name was officially changed to Botox.
In 2002, Dr. Scott told SFGate, when asked about the more popular use for the drug, “I think that’s a charming, slightly frivolous use,” adding, “but it’s not along the lines of what I was into, applications for serious disorders.”
According to Scientific American in 2016, Dr. Scott, then age 83, kept working on the noncosmetic benefits of botulism-toxin injections for eye-related disorders at the Strabismus Research Foundation,
He told Scientific American he was proud that his efforts “are directly helpful to people.”
“There are interesting and difficult problems still to be solved, and I’m a practicing physician and I see them every day,” he said.
Dr. Scott’s daughter, Ann Scott, told NPR: “He definitely loved his work and he was also a really great father.” She said her dad involved his children in his research and work.
She added, “He was a really calm, more of a quiet reserved person,” and said he was committed to teaching his students, many of them international students.
“That was what he really loved,” she said.
Dr. Scott, who died Dec. 16, was in intensive care for the last 10 days from an unspecified illness, his daughter told NPR.
A version of this article first appeared on Medscape.com.
Visceral fat may help ID heart risk in obese youth
The amount of fat surrounding abdominal organs may help clinicians identify cardiovascular risk in young people with obesity, researchers have found.
Severely overweight children and young adults showed a subtle association between visceral fat and arterial stiffness independent of body mass index (BMI). The association was not present in those of healthy weight, possibly because their visceral fat stores are too small to have a detectable effect on cardiovascular health, according to the researchers, who reported their findings in the latest issue of Pediatric Obesity.
“Those kids with greater visceral fat had stiffer arteries, which can overtax and overstress the system and lead to unfortunate consequences in terms of cardiovascular health down the line,” senior author Joseph M. Kindler, PhD, an assistant professor of nutritional sciences at the University of Georgia, Athens, told this news organization.
The data came from cross-sectional measurements in 605 youth (67% female, 56% non-Black) aged 10-23 years at Cincinnati Children’s Hospital Medical Center. The sample included 236 individuals of healthy weight, 224 with obesity, and 145 with type 2 diabetes.
Visceral fat was assessed with dual-energy x-ray absorptiometry (DXA), a widely used test of bone mineral density screening to assess fracture risk. Carotid-femoral pulse wave velocity (PWV) was used to gauge arterial stiffness, a subclinical sign of cardiovascular disease.
Visceral fat was associated with PWV in all three groups of study subjects (P < .05), the researchers found, whereas the amount of subcutaneous fat was linked to arterial stiffness in obese youth and those with obesity but not those whose weight was considered healthy.
The amount of fat was associated with an additional 1.6% of the variability in arterial stiffness in youth with obesity after accounting for BMI. Subcutaneous fat, meanwhile, did not appear to affect PWV, the researchers found. “In youth with healthy weight, visceral fat, subcutaneous fat, BMI, and waist circumference were not significantly associated with PWV in any analyses,” they write.
The researchers cited a paucity of data on the relationship between visceral fat and cardiovascular disease in children with obesity. Although BMI is a reliable and readily available indicator of risk for disease, DXA “might give us a little more information,” Dr. Kindler, a nutritionist and bone biologist, said. As for clinical use to supplement BMI and waist circumference, he said, “maybe there’s room for visceral fat, but we do need a lot more science to back those decisions down the line.”
For example, what normal visceral fat accumulation during childhood looks like is unknown, he said.
Rigorous longitudinal studies are needed to establish cause and effect, but the new findings offer “a potential connection between visceral fat and cardiovascular disease risk in youth in a relatively large sample,” Wei Shen, MD, MPH, the associate director of the body composition unit at the New York Obesity Nutrition Research Center at Columbia University, New York, said.
Ideally, said Dr. Shen, who was not involved in the latest study, it would be “more credible to use the most accurate measure of visceral fat, the volumetric measurement of visceral fat using MRI” to establish a causal relationship with cardiovascular risk. However, MRI is more expensive and less accessible than DXA. To assess visceral fat in the clinic, “waist circumference may still be a good choice, as it is so convenient to use,” she added.
Dr. Kindler and his colleagues highlighted the need to examine the effect of excess visceral fat as well as intrahepatic fat on youth with type 2 diabetes, who experience cardiovascular complications independent of whether they are obese. In the new study, the positive association between visceral fat and arterial stiffness did not differ between youth with obesity and normal glucose control and those with obesity and type 2 diabetes.
Funding came from the Endocrine Fellows Foundation, the National Institutes of Health, and the University of Georgia Obesity Initiative. Dr. Kindler and Dr. Shen have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The amount of fat surrounding abdominal organs may help clinicians identify cardiovascular risk in young people with obesity, researchers have found.
Severely overweight children and young adults showed a subtle association between visceral fat and arterial stiffness independent of body mass index (BMI). The association was not present in those of healthy weight, possibly because their visceral fat stores are too small to have a detectable effect on cardiovascular health, according to the researchers, who reported their findings in the latest issue of Pediatric Obesity.
“Those kids with greater visceral fat had stiffer arteries, which can overtax and overstress the system and lead to unfortunate consequences in terms of cardiovascular health down the line,” senior author Joseph M. Kindler, PhD, an assistant professor of nutritional sciences at the University of Georgia, Athens, told this news organization.
The data came from cross-sectional measurements in 605 youth (67% female, 56% non-Black) aged 10-23 years at Cincinnati Children’s Hospital Medical Center. The sample included 236 individuals of healthy weight, 224 with obesity, and 145 with type 2 diabetes.
Visceral fat was assessed with dual-energy x-ray absorptiometry (DXA), a widely used test of bone mineral density screening to assess fracture risk. Carotid-femoral pulse wave velocity (PWV) was used to gauge arterial stiffness, a subclinical sign of cardiovascular disease.
Visceral fat was associated with PWV in all three groups of study subjects (P < .05), the researchers found, whereas the amount of subcutaneous fat was linked to arterial stiffness in obese youth and those with obesity but not those whose weight was considered healthy.
The amount of fat was associated with an additional 1.6% of the variability in arterial stiffness in youth with obesity after accounting for BMI. Subcutaneous fat, meanwhile, did not appear to affect PWV, the researchers found. “In youth with healthy weight, visceral fat, subcutaneous fat, BMI, and waist circumference were not significantly associated with PWV in any analyses,” they write.
The researchers cited a paucity of data on the relationship between visceral fat and cardiovascular disease in children with obesity. Although BMI is a reliable and readily available indicator of risk for disease, DXA “might give us a little more information,” Dr. Kindler, a nutritionist and bone biologist, said. As for clinical use to supplement BMI and waist circumference, he said, “maybe there’s room for visceral fat, but we do need a lot more science to back those decisions down the line.”
For example, what normal visceral fat accumulation during childhood looks like is unknown, he said.
Rigorous longitudinal studies are needed to establish cause and effect, but the new findings offer “a potential connection between visceral fat and cardiovascular disease risk in youth in a relatively large sample,” Wei Shen, MD, MPH, the associate director of the body composition unit at the New York Obesity Nutrition Research Center at Columbia University, New York, said.
Ideally, said Dr. Shen, who was not involved in the latest study, it would be “more credible to use the most accurate measure of visceral fat, the volumetric measurement of visceral fat using MRI” to establish a causal relationship with cardiovascular risk. However, MRI is more expensive and less accessible than DXA. To assess visceral fat in the clinic, “waist circumference may still be a good choice, as it is so convenient to use,” she added.
Dr. Kindler and his colleagues highlighted the need to examine the effect of excess visceral fat as well as intrahepatic fat on youth with type 2 diabetes, who experience cardiovascular complications independent of whether they are obese. In the new study, the positive association between visceral fat and arterial stiffness did not differ between youth with obesity and normal glucose control and those with obesity and type 2 diabetes.
Funding came from the Endocrine Fellows Foundation, the National Institutes of Health, and the University of Georgia Obesity Initiative. Dr. Kindler and Dr. Shen have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The amount of fat surrounding abdominal organs may help clinicians identify cardiovascular risk in young people with obesity, researchers have found.
Severely overweight children and young adults showed a subtle association between visceral fat and arterial stiffness independent of body mass index (BMI). The association was not present in those of healthy weight, possibly because their visceral fat stores are too small to have a detectable effect on cardiovascular health, according to the researchers, who reported their findings in the latest issue of Pediatric Obesity.
“Those kids with greater visceral fat had stiffer arteries, which can overtax and overstress the system and lead to unfortunate consequences in terms of cardiovascular health down the line,” senior author Joseph M. Kindler, PhD, an assistant professor of nutritional sciences at the University of Georgia, Athens, told this news organization.
The data came from cross-sectional measurements in 605 youth (67% female, 56% non-Black) aged 10-23 years at Cincinnati Children’s Hospital Medical Center. The sample included 236 individuals of healthy weight, 224 with obesity, and 145 with type 2 diabetes.
Visceral fat was assessed with dual-energy x-ray absorptiometry (DXA), a widely used test of bone mineral density screening to assess fracture risk. Carotid-femoral pulse wave velocity (PWV) was used to gauge arterial stiffness, a subclinical sign of cardiovascular disease.
Visceral fat was associated with PWV in all three groups of study subjects (P < .05), the researchers found, whereas the amount of subcutaneous fat was linked to arterial stiffness in obese youth and those with obesity but not those whose weight was considered healthy.
The amount of fat was associated with an additional 1.6% of the variability in arterial stiffness in youth with obesity after accounting for BMI. Subcutaneous fat, meanwhile, did not appear to affect PWV, the researchers found. “In youth with healthy weight, visceral fat, subcutaneous fat, BMI, and waist circumference were not significantly associated with PWV in any analyses,” they write.
The researchers cited a paucity of data on the relationship between visceral fat and cardiovascular disease in children with obesity. Although BMI is a reliable and readily available indicator of risk for disease, DXA “might give us a little more information,” Dr. Kindler, a nutritionist and bone biologist, said. As for clinical use to supplement BMI and waist circumference, he said, “maybe there’s room for visceral fat, but we do need a lot more science to back those decisions down the line.”
For example, what normal visceral fat accumulation during childhood looks like is unknown, he said.
Rigorous longitudinal studies are needed to establish cause and effect, but the new findings offer “a potential connection between visceral fat and cardiovascular disease risk in youth in a relatively large sample,” Wei Shen, MD, MPH, the associate director of the body composition unit at the New York Obesity Nutrition Research Center at Columbia University, New York, said.
Ideally, said Dr. Shen, who was not involved in the latest study, it would be “more credible to use the most accurate measure of visceral fat, the volumetric measurement of visceral fat using MRI” to establish a causal relationship with cardiovascular risk. However, MRI is more expensive and less accessible than DXA. To assess visceral fat in the clinic, “waist circumference may still be a good choice, as it is so convenient to use,” she added.
Dr. Kindler and his colleagues highlighted the need to examine the effect of excess visceral fat as well as intrahepatic fat on youth with type 2 diabetes, who experience cardiovascular complications independent of whether they are obese. In the new study, the positive association between visceral fat and arterial stiffness did not differ between youth with obesity and normal glucose control and those with obesity and type 2 diabetes.
Funding came from the Endocrine Fellows Foundation, the National Institutes of Health, and the University of Georgia Obesity Initiative. Dr. Kindler and Dr. Shen have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AAP updates guidance on HIV testing and prophylaxis in youth
Pediatricians should take a more proactive role in protecting children and adolescents from HIV infections, according to updated guidance from the American Academy of Pediatrics. The comprehensive new recommendations stress winning the trust and confidence of pediatric patients and reaffirm support for testing and treating adolescents without parental consent where state laws allow.
While the number of HIV-infected people in the United States remains high, most sexually active youth do not believe they are at risk and have never been tested, noted authors Katherine K. Hsu, MD, MPH, of the Massachusetts Department of Public Health and Boston University Medical Center, and Natella Yurievna Rakhmanina, MD, PhD, of Children’s National Hospital and George Washington University, both in Washington.
That is a knowledge gap that pediatricians are well situated to fill. “Pediatricians can play a key role in preventing and controlling HIV infection by promoting risk-reduction counseling and offering routine HIV testing and prophylaxis to adolescent and young adult (youth) patients,” they wrote on Dec. 20, 2021, in their study published in Pediatrics.
Key components of youth encounters, they stressed, is creating safe environments for obtaining an accurate sexual and reproductive health assessment and providing nonstigmatizing risk counseling.
According to Dr. Rakhmanina, major barriers to addressing preventive HIV counseling have included pediatricians’ lack of time, cultural differences, adolescents’ inaccurate responses, discomfort discussing sexual issues, and adolescents’ fear of parent or caregiver notification. Other concerns have been lack of adequate payment and insufficient training in how to talk to adolescents about sexual and reproductive issues.
According to the Centers for Disease Control and Prevention, at year end in 2018 an estimated 1,173,900 people age 13 or older were living with HIV infection in the United States, of whom 47,800 (4%) were adolescents and young adults 13-24 years of age.
These estimates include diagnosed and undiagnosed individuals. Between 2014 and 2018, new diagnoses of HIV infection accounted for 21% (7,817 of 37,515) of all new HIV diagnoses in the United States.
The new AAP clinical report updates policy statements from 2001 and again 2011 that encouraged HIV testing of all sexually active youth.
It reflects changes in epidemiology, advances in diagnostic testing with improved immunoassays, and updated recommendations for HIV testing and postexposure prophylaxis (PEP), as well as new guidance for pre-exposure prophylaxis (PrEP).
A 2017 study found that the 2011 HIV testing guidelines was associated with only a slight increase in HIV screening and a shift toward testing younger people and away from testing on the basis of risk.
Against this backdrop of persistent HIV infection and to-date modest uptake of earlier guidance, the 2021 statement made 14 main recommendations to pediatricians. Among these:
- Foster open discussion of gender and sexual orientation and behavior, as well as reproductive health issues.
- Recognize the clinical presentation of the acute retroviral syndrome, which can present as syndromes resembling infectious mononucleosis and influenza.
- Consider including virologic testing in the diagnostic workup of sexually active youth.
- Consider routine HIV screening for all youth 15 years or older at least once and rescreening high-risk youth. Those at higher risk should be rescreened at least annually, and potentially as frequently as every 3-6 months.
- Youth at substantial risk should be routinely offered PrEP, while PEP with antiretroviral drugs is indicated after unsafe exposures such as unsafe sexual activity, unsafe needle use, or sexual violence. Survivors of sexual violence should have baseline HIV testing and sexually transmitted infection (STI) screening and treatment. They should also be offered mental health and other supportive counseling.
- Test youth who request HIV screening at any time even in the absence of reported risk factors. Although parent or guardian involvement is preferable, in most legal settings the adolescent’s consent should suffice for testing and treatment.
- For youth with a positive HIV test, facilitate and confirm prompt linkage to age-appropriate HIV specialty care.
Will the current report’s recommendations be met with greater uptake than previous iterations? Yes, according to Maria E. Trent, MD, MPH, chief of the division of adolescent/young adult medicine at Johns Hopkins University, Baltimore, but a fundamental first step will be the establishment of honesty and confidentiality. “Pediatricians are essential stakeholders in HIV prevention and intervention efforts in the United States. Recent data, however, suggest that pediatricians often struggle to create the essential alone time with adolescents and young adults to conduct critical sexual health conversations that allow for adequate STI/HIV risk screening,” said Dr. Trent, who was not involved in the report. “Consistently creating that space will be the first task for ensuring adherence to these recommendations.”
Strategies to optimize risk screening for clinical decision support, such as confidential online previsit questionnaires that link to the electronic medical record, may facilitate discussions during the visit while maintaining clinician efficiency, she added.
Furthermore, while one-time general HIV screening during adolescence will be an easy goal, “integrating annual testing, biomedical intervention for PrEP/PEP, and ongoing follow-up and testing for those on biomedical intervention may present practical but not insurmountable challenges,” Dr. Trent said.
When pediatricians recognize that care is suboptimal in practice, ensuring that pediatricians have established linkages to adolescent-friendly services for free or low-cost HIV testing, PrEP/PEP, and HIV management will prevent gaps in care, Dr. Trent continued. “The most exciting development in health care is that telemedicine can now be used to work with young people, giving the practicing pediatrician more opportunities and flexibility to deliver and triage care.”
Will any of the guidelines such as an adolescent’s right to independent consent be considered unacceptable by parents? “While this part of the recommendations is not new, the thought that their adolescent can initiate and receive confidential care for HIV prevention or intervention without their knowledge or consent may initially be challenging to process,” Dr. Trent said. “Ultimately, what I’ve observed in practice is that parents are relieved and often proud of their young person for taking the initiative to engage in self-care to maintain their health and relieved to be involved as a critical support person.”
She added that pediatricians need to make their practice policies clear and have information available for parents on state laws related to confidential care. “They also need to carefully use the electronic health record to avoid errors in disclosures to proxies without patient consent.”
Dr. Rakhmanina agreed there will likely be greater adherence to this round of recommendations. “The culture of addressing sexual and reproductive health issues among adolescents in the U.S. is changing among pediatric providers, and we start seeing more champions of PrEP and HIV testing in our communities,” she said.
This study received no external funding. The authors had no financial relationships or potential conflicts of interest to disclose. Dr. Trent disclosed no competing interests relevant to her comments.
Pediatricians should take a more proactive role in protecting children and adolescents from HIV infections, according to updated guidance from the American Academy of Pediatrics. The comprehensive new recommendations stress winning the trust and confidence of pediatric patients and reaffirm support for testing and treating adolescents without parental consent where state laws allow.
While the number of HIV-infected people in the United States remains high, most sexually active youth do not believe they are at risk and have never been tested, noted authors Katherine K. Hsu, MD, MPH, of the Massachusetts Department of Public Health and Boston University Medical Center, and Natella Yurievna Rakhmanina, MD, PhD, of Children’s National Hospital and George Washington University, both in Washington.
That is a knowledge gap that pediatricians are well situated to fill. “Pediatricians can play a key role in preventing and controlling HIV infection by promoting risk-reduction counseling and offering routine HIV testing and prophylaxis to adolescent and young adult (youth) patients,” they wrote on Dec. 20, 2021, in their study published in Pediatrics.
Key components of youth encounters, they stressed, is creating safe environments for obtaining an accurate sexual and reproductive health assessment and providing nonstigmatizing risk counseling.
According to Dr. Rakhmanina, major barriers to addressing preventive HIV counseling have included pediatricians’ lack of time, cultural differences, adolescents’ inaccurate responses, discomfort discussing sexual issues, and adolescents’ fear of parent or caregiver notification. Other concerns have been lack of adequate payment and insufficient training in how to talk to adolescents about sexual and reproductive issues.
According to the Centers for Disease Control and Prevention, at year end in 2018 an estimated 1,173,900 people age 13 or older were living with HIV infection in the United States, of whom 47,800 (4%) were adolescents and young adults 13-24 years of age.
These estimates include diagnosed and undiagnosed individuals. Between 2014 and 2018, new diagnoses of HIV infection accounted for 21% (7,817 of 37,515) of all new HIV diagnoses in the United States.
The new AAP clinical report updates policy statements from 2001 and again 2011 that encouraged HIV testing of all sexually active youth.
It reflects changes in epidemiology, advances in diagnostic testing with improved immunoassays, and updated recommendations for HIV testing and postexposure prophylaxis (PEP), as well as new guidance for pre-exposure prophylaxis (PrEP).
A 2017 study found that the 2011 HIV testing guidelines was associated with only a slight increase in HIV screening and a shift toward testing younger people and away from testing on the basis of risk.
Against this backdrop of persistent HIV infection and to-date modest uptake of earlier guidance, the 2021 statement made 14 main recommendations to pediatricians. Among these:
- Foster open discussion of gender and sexual orientation and behavior, as well as reproductive health issues.
- Recognize the clinical presentation of the acute retroviral syndrome, which can present as syndromes resembling infectious mononucleosis and influenza.
- Consider including virologic testing in the diagnostic workup of sexually active youth.
- Consider routine HIV screening for all youth 15 years or older at least once and rescreening high-risk youth. Those at higher risk should be rescreened at least annually, and potentially as frequently as every 3-6 months.
- Youth at substantial risk should be routinely offered PrEP, while PEP with antiretroviral drugs is indicated after unsafe exposures such as unsafe sexual activity, unsafe needle use, or sexual violence. Survivors of sexual violence should have baseline HIV testing and sexually transmitted infection (STI) screening and treatment. They should also be offered mental health and other supportive counseling.
- Test youth who request HIV screening at any time even in the absence of reported risk factors. Although parent or guardian involvement is preferable, in most legal settings the adolescent’s consent should suffice for testing and treatment.
- For youth with a positive HIV test, facilitate and confirm prompt linkage to age-appropriate HIV specialty care.
Will the current report’s recommendations be met with greater uptake than previous iterations? Yes, according to Maria E. Trent, MD, MPH, chief of the division of adolescent/young adult medicine at Johns Hopkins University, Baltimore, but a fundamental first step will be the establishment of honesty and confidentiality. “Pediatricians are essential stakeholders in HIV prevention and intervention efforts in the United States. Recent data, however, suggest that pediatricians often struggle to create the essential alone time with adolescents and young adults to conduct critical sexual health conversations that allow for adequate STI/HIV risk screening,” said Dr. Trent, who was not involved in the report. “Consistently creating that space will be the first task for ensuring adherence to these recommendations.”
Strategies to optimize risk screening for clinical decision support, such as confidential online previsit questionnaires that link to the electronic medical record, may facilitate discussions during the visit while maintaining clinician efficiency, she added.
Furthermore, while one-time general HIV screening during adolescence will be an easy goal, “integrating annual testing, biomedical intervention for PrEP/PEP, and ongoing follow-up and testing for those on biomedical intervention may present practical but not insurmountable challenges,” Dr. Trent said.
When pediatricians recognize that care is suboptimal in practice, ensuring that pediatricians have established linkages to adolescent-friendly services for free or low-cost HIV testing, PrEP/PEP, and HIV management will prevent gaps in care, Dr. Trent continued. “The most exciting development in health care is that telemedicine can now be used to work with young people, giving the practicing pediatrician more opportunities and flexibility to deliver and triage care.”
Will any of the guidelines such as an adolescent’s right to independent consent be considered unacceptable by parents? “While this part of the recommendations is not new, the thought that their adolescent can initiate and receive confidential care for HIV prevention or intervention without their knowledge or consent may initially be challenging to process,” Dr. Trent said. “Ultimately, what I’ve observed in practice is that parents are relieved and often proud of their young person for taking the initiative to engage in self-care to maintain their health and relieved to be involved as a critical support person.”
She added that pediatricians need to make their practice policies clear and have information available for parents on state laws related to confidential care. “They also need to carefully use the electronic health record to avoid errors in disclosures to proxies without patient consent.”
Dr. Rakhmanina agreed there will likely be greater adherence to this round of recommendations. “The culture of addressing sexual and reproductive health issues among adolescents in the U.S. is changing among pediatric providers, and we start seeing more champions of PrEP and HIV testing in our communities,” she said.
This study received no external funding. The authors had no financial relationships or potential conflicts of interest to disclose. Dr. Trent disclosed no competing interests relevant to her comments.
Pediatricians should take a more proactive role in protecting children and adolescents from HIV infections, according to updated guidance from the American Academy of Pediatrics. The comprehensive new recommendations stress winning the trust and confidence of pediatric patients and reaffirm support for testing and treating adolescents without parental consent where state laws allow.
While the number of HIV-infected people in the United States remains high, most sexually active youth do not believe they are at risk and have never been tested, noted authors Katherine K. Hsu, MD, MPH, of the Massachusetts Department of Public Health and Boston University Medical Center, and Natella Yurievna Rakhmanina, MD, PhD, of Children’s National Hospital and George Washington University, both in Washington.
That is a knowledge gap that pediatricians are well situated to fill. “Pediatricians can play a key role in preventing and controlling HIV infection by promoting risk-reduction counseling and offering routine HIV testing and prophylaxis to adolescent and young adult (youth) patients,” they wrote on Dec. 20, 2021, in their study published in Pediatrics.
Key components of youth encounters, they stressed, is creating safe environments for obtaining an accurate sexual and reproductive health assessment and providing nonstigmatizing risk counseling.
According to Dr. Rakhmanina, major barriers to addressing preventive HIV counseling have included pediatricians’ lack of time, cultural differences, adolescents’ inaccurate responses, discomfort discussing sexual issues, and adolescents’ fear of parent or caregiver notification. Other concerns have been lack of adequate payment and insufficient training in how to talk to adolescents about sexual and reproductive issues.
According to the Centers for Disease Control and Prevention, at year end in 2018 an estimated 1,173,900 people age 13 or older were living with HIV infection in the United States, of whom 47,800 (4%) were adolescents and young adults 13-24 years of age.
These estimates include diagnosed and undiagnosed individuals. Between 2014 and 2018, new diagnoses of HIV infection accounted for 21% (7,817 of 37,515) of all new HIV diagnoses in the United States.
The new AAP clinical report updates policy statements from 2001 and again 2011 that encouraged HIV testing of all sexually active youth.
It reflects changes in epidemiology, advances in diagnostic testing with improved immunoassays, and updated recommendations for HIV testing and postexposure prophylaxis (PEP), as well as new guidance for pre-exposure prophylaxis (PrEP).
A 2017 study found that the 2011 HIV testing guidelines was associated with only a slight increase in HIV screening and a shift toward testing younger people and away from testing on the basis of risk.
Against this backdrop of persistent HIV infection and to-date modest uptake of earlier guidance, the 2021 statement made 14 main recommendations to pediatricians. Among these:
- Foster open discussion of gender and sexual orientation and behavior, as well as reproductive health issues.
- Recognize the clinical presentation of the acute retroviral syndrome, which can present as syndromes resembling infectious mononucleosis and influenza.
- Consider including virologic testing in the diagnostic workup of sexually active youth.
- Consider routine HIV screening for all youth 15 years or older at least once and rescreening high-risk youth. Those at higher risk should be rescreened at least annually, and potentially as frequently as every 3-6 months.
- Youth at substantial risk should be routinely offered PrEP, while PEP with antiretroviral drugs is indicated after unsafe exposures such as unsafe sexual activity, unsafe needle use, or sexual violence. Survivors of sexual violence should have baseline HIV testing and sexually transmitted infection (STI) screening and treatment. They should also be offered mental health and other supportive counseling.
- Test youth who request HIV screening at any time even in the absence of reported risk factors. Although parent or guardian involvement is preferable, in most legal settings the adolescent’s consent should suffice for testing and treatment.
- For youth with a positive HIV test, facilitate and confirm prompt linkage to age-appropriate HIV specialty care.
Will the current report’s recommendations be met with greater uptake than previous iterations? Yes, according to Maria E. Trent, MD, MPH, chief of the division of adolescent/young adult medicine at Johns Hopkins University, Baltimore, but a fundamental first step will be the establishment of honesty and confidentiality. “Pediatricians are essential stakeholders in HIV prevention and intervention efforts in the United States. Recent data, however, suggest that pediatricians often struggle to create the essential alone time with adolescents and young adults to conduct critical sexual health conversations that allow for adequate STI/HIV risk screening,” said Dr. Trent, who was not involved in the report. “Consistently creating that space will be the first task for ensuring adherence to these recommendations.”
Strategies to optimize risk screening for clinical decision support, such as confidential online previsit questionnaires that link to the electronic medical record, may facilitate discussions during the visit while maintaining clinician efficiency, she added.
Furthermore, while one-time general HIV screening during adolescence will be an easy goal, “integrating annual testing, biomedical intervention for PrEP/PEP, and ongoing follow-up and testing for those on biomedical intervention may present practical but not insurmountable challenges,” Dr. Trent said.
When pediatricians recognize that care is suboptimal in practice, ensuring that pediatricians have established linkages to adolescent-friendly services for free or low-cost HIV testing, PrEP/PEP, and HIV management will prevent gaps in care, Dr. Trent continued. “The most exciting development in health care is that telemedicine can now be used to work with young people, giving the practicing pediatrician more opportunities and flexibility to deliver and triage care.”
Will any of the guidelines such as an adolescent’s right to independent consent be considered unacceptable by parents? “While this part of the recommendations is not new, the thought that their adolescent can initiate and receive confidential care for HIV prevention or intervention without their knowledge or consent may initially be challenging to process,” Dr. Trent said. “Ultimately, what I’ve observed in practice is that parents are relieved and often proud of their young person for taking the initiative to engage in self-care to maintain their health and relieved to be involved as a critical support person.”
She added that pediatricians need to make their practice policies clear and have information available for parents on state laws related to confidential care. “They also need to carefully use the electronic health record to avoid errors in disclosures to proxies without patient consent.”
Dr. Rakhmanina agreed there will likely be greater adherence to this round of recommendations. “The culture of addressing sexual and reproductive health issues among adolescents in the U.S. is changing among pediatric providers, and we start seeing more champions of PrEP and HIV testing in our communities,” she said.
This study received no external funding. The authors had no financial relationships or potential conflicts of interest to disclose. Dr. Trent disclosed no competing interests relevant to her comments.
FROM PEDIATRICS
Voxelotor for sickle cell anemia now down to 4-year-olds
The indication had previously been for patients 12 years old and up, the FDA said in an announcement.
Voxelotor (Oxbryta) was originally approved for sickle cell disease in November 2019 and was described as the first drug that directly inhibits sickle hemoglobin polymerization, the root cause of the disease. It binds and stabilizes hemoglobin to prevent red blood cells from sickling and being destroyed.
Approval for the new indication of use in children down to age 4 was based on data from a phase 2 trial that involved 45 children aged 4-11 years; the results show that 36% had an increase in hemoglobin greater than 1 g/dL by week 24, the FDA said.
“Complications of [sickle cell disease] that can cause irreversible organ damage are known to begin in the first few years of life, which is why earlier intervention is critical,” commented Ted Love, MD, president and CEO of Global Blood Therapeutics, the manufacturer, in a press release.
The company is studying voxelotor in children as young as 9 months old.
The agent was granted an accelerated approval by the FDA, so continued approval depends on additional data to confirm that increases in hemoglobin have clinical benefit.
With the new approvals, voxelotor is now available in 500-mg tablets and the 300-mg tablets for oral suspension. Dosing for ages 12 years and up is 1,500 mg once daily. Dosing for children 4 to up to 12 years old is weight based.
The most common side effects are headache, vomiting, diarrhea, abdominal pain, nausea, rash, and fever.
A version of this article first appeared on Medscape.com.
The indication had previously been for patients 12 years old and up, the FDA said in an announcement.
Voxelotor (Oxbryta) was originally approved for sickle cell disease in November 2019 and was described as the first drug that directly inhibits sickle hemoglobin polymerization, the root cause of the disease. It binds and stabilizes hemoglobin to prevent red blood cells from sickling and being destroyed.
Approval for the new indication of use in children down to age 4 was based on data from a phase 2 trial that involved 45 children aged 4-11 years; the results show that 36% had an increase in hemoglobin greater than 1 g/dL by week 24, the FDA said.
“Complications of [sickle cell disease] that can cause irreversible organ damage are known to begin in the first few years of life, which is why earlier intervention is critical,” commented Ted Love, MD, president and CEO of Global Blood Therapeutics, the manufacturer, in a press release.
The company is studying voxelotor in children as young as 9 months old.
The agent was granted an accelerated approval by the FDA, so continued approval depends on additional data to confirm that increases in hemoglobin have clinical benefit.
With the new approvals, voxelotor is now available in 500-mg tablets and the 300-mg tablets for oral suspension. Dosing for ages 12 years and up is 1,500 mg once daily. Dosing for children 4 to up to 12 years old is weight based.
The most common side effects are headache, vomiting, diarrhea, abdominal pain, nausea, rash, and fever.
A version of this article first appeared on Medscape.com.
The indication had previously been for patients 12 years old and up, the FDA said in an announcement.
Voxelotor (Oxbryta) was originally approved for sickle cell disease in November 2019 and was described as the first drug that directly inhibits sickle hemoglobin polymerization, the root cause of the disease. It binds and stabilizes hemoglobin to prevent red blood cells from sickling and being destroyed.
Approval for the new indication of use in children down to age 4 was based on data from a phase 2 trial that involved 45 children aged 4-11 years; the results show that 36% had an increase in hemoglobin greater than 1 g/dL by week 24, the FDA said.
“Complications of [sickle cell disease] that can cause irreversible organ damage are known to begin in the first few years of life, which is why earlier intervention is critical,” commented Ted Love, MD, president and CEO of Global Blood Therapeutics, the manufacturer, in a press release.
The company is studying voxelotor in children as young as 9 months old.
The agent was granted an accelerated approval by the FDA, so continued approval depends on additional data to confirm that increases in hemoglobin have clinical benefit.
With the new approvals, voxelotor is now available in 500-mg tablets and the 300-mg tablets for oral suspension. Dosing for ages 12 years and up is 1,500 mg once daily. Dosing for children 4 to up to 12 years old is weight based.
The most common side effects are headache, vomiting, diarrhea, abdominal pain, nausea, rash, and fever.
A version of this article first appeared on Medscape.com.
Emergency docs cite ‘dire’ situation as COVID grows, nurses scarce
With emergency departments straining to keep up with the latest COVID surge, the American College of Emergency Physicians
The organization said that it is “very concerned that nursing shortages in emergency departments can complicate patient access to care and add to incredible levels of stress already on physician-led care teams,” according to a press release.
ACEP President Gillian Schmitz, MD, told this news organization, “The situation is dire in many emergency departments around the country. Emergency physicians are seeing more patients with fewer resources and less staff.
“Emergency physicians in the hardest hit communities are scrambling to locate available experts, exhausting federal support, and doing all they can to adapt to the demands of the current surge – everyone is being stretched to their limit.”
The Emergency Nurses Association (ENA) agrees with ACEP’s call for a team approach to stemming the shortage.
ENA President Ron Kraus, MSN, RN, said in an interview, “The pandemic has only amplified several long-standing issues impacting emergency nurses, such as workplace violence, a healthy work environment, and concerns about staffing shortages and the pipeline of new nurses. That said, we can’t lose focus on what’s most important in these challenging moments – ensuring every patient receives the high quality of care.”
The responsibility falls on the “collaborative effort” of the emergency department with emergency nurses playing a pivotal role, he said. But the stress, fatigue, and burnout driving nurses away from their jobs “should not be viewed as added inconvenience to anyone during a pandemic, but as a long-term threat to our health care system.”
ACEP’s press release stated that with fewer nurses available in the emergency department, team members are clocking extra hours, caring for more patients, and stretched to take on additional clinical and nonclinical duties.
“I am hearing from colleagues from Washington state to Michigan to New York that this is the worst they have seen since the beginning of the pandemic,” Dr. Schmitz said. “Everyone available is filling gaps as best they can, but the current path for many frontline workers is not sustainable,” she said in the release.
Meanwhile, ACEP is also tackling violence in the emergency department and has initiatives to protect the mental health of those working on the front lines, the release states.
“Emergency physicians will continue to do everything necessary to treat patients,” Dr. Schmitz said in the release, “but it will take a collaborative effort with legislators, policymakers and health system leaders to strengthen care teams, improve access and address capacity concerns with solutions that can save lives right now and in the months ahead.”
Dr. Schmitz stated that in Washington state, ICUs are at 97% to 100% capacity and less than 30 pediatric inpatient beds are available in the western part of the state.
“In Michigan and New York, several emergency departments are overflowing, and doctors are being called in to triage people in the waiting room because all of the emergency department beds are holding admissions. There are scenarios where entire hospitals are backing up into the emergency department and waiting room and we are physically running out of space and nursing staff.”
ACEP represents its 40,000 emergency physician members.
A version of this article first appeared on Medscape.com.
With emergency departments straining to keep up with the latest COVID surge, the American College of Emergency Physicians
The organization said that it is “very concerned that nursing shortages in emergency departments can complicate patient access to care and add to incredible levels of stress already on physician-led care teams,” according to a press release.
ACEP President Gillian Schmitz, MD, told this news organization, “The situation is dire in many emergency departments around the country. Emergency physicians are seeing more patients with fewer resources and less staff.
“Emergency physicians in the hardest hit communities are scrambling to locate available experts, exhausting federal support, and doing all they can to adapt to the demands of the current surge – everyone is being stretched to their limit.”
The Emergency Nurses Association (ENA) agrees with ACEP’s call for a team approach to stemming the shortage.
ENA President Ron Kraus, MSN, RN, said in an interview, “The pandemic has only amplified several long-standing issues impacting emergency nurses, such as workplace violence, a healthy work environment, and concerns about staffing shortages and the pipeline of new nurses. That said, we can’t lose focus on what’s most important in these challenging moments – ensuring every patient receives the high quality of care.”
The responsibility falls on the “collaborative effort” of the emergency department with emergency nurses playing a pivotal role, he said. But the stress, fatigue, and burnout driving nurses away from their jobs “should not be viewed as added inconvenience to anyone during a pandemic, but as a long-term threat to our health care system.”
ACEP’s press release stated that with fewer nurses available in the emergency department, team members are clocking extra hours, caring for more patients, and stretched to take on additional clinical and nonclinical duties.
“I am hearing from colleagues from Washington state to Michigan to New York that this is the worst they have seen since the beginning of the pandemic,” Dr. Schmitz said. “Everyone available is filling gaps as best they can, but the current path for many frontline workers is not sustainable,” she said in the release.
Meanwhile, ACEP is also tackling violence in the emergency department and has initiatives to protect the mental health of those working on the front lines, the release states.
“Emergency physicians will continue to do everything necessary to treat patients,” Dr. Schmitz said in the release, “but it will take a collaborative effort with legislators, policymakers and health system leaders to strengthen care teams, improve access and address capacity concerns with solutions that can save lives right now and in the months ahead.”
Dr. Schmitz stated that in Washington state, ICUs are at 97% to 100% capacity and less than 30 pediatric inpatient beds are available in the western part of the state.
“In Michigan and New York, several emergency departments are overflowing, and doctors are being called in to triage people in the waiting room because all of the emergency department beds are holding admissions. There are scenarios where entire hospitals are backing up into the emergency department and waiting room and we are physically running out of space and nursing staff.”
ACEP represents its 40,000 emergency physician members.
A version of this article first appeared on Medscape.com.
With emergency departments straining to keep up with the latest COVID surge, the American College of Emergency Physicians
The organization said that it is “very concerned that nursing shortages in emergency departments can complicate patient access to care and add to incredible levels of stress already on physician-led care teams,” according to a press release.
ACEP President Gillian Schmitz, MD, told this news organization, “The situation is dire in many emergency departments around the country. Emergency physicians are seeing more patients with fewer resources and less staff.
“Emergency physicians in the hardest hit communities are scrambling to locate available experts, exhausting federal support, and doing all they can to adapt to the demands of the current surge – everyone is being stretched to their limit.”
The Emergency Nurses Association (ENA) agrees with ACEP’s call for a team approach to stemming the shortage.
ENA President Ron Kraus, MSN, RN, said in an interview, “The pandemic has only amplified several long-standing issues impacting emergency nurses, such as workplace violence, a healthy work environment, and concerns about staffing shortages and the pipeline of new nurses. That said, we can’t lose focus on what’s most important in these challenging moments – ensuring every patient receives the high quality of care.”
The responsibility falls on the “collaborative effort” of the emergency department with emergency nurses playing a pivotal role, he said. But the stress, fatigue, and burnout driving nurses away from their jobs “should not be viewed as added inconvenience to anyone during a pandemic, but as a long-term threat to our health care system.”
ACEP’s press release stated that with fewer nurses available in the emergency department, team members are clocking extra hours, caring for more patients, and stretched to take on additional clinical and nonclinical duties.
“I am hearing from colleagues from Washington state to Michigan to New York that this is the worst they have seen since the beginning of the pandemic,” Dr. Schmitz said. “Everyone available is filling gaps as best they can, but the current path for many frontline workers is not sustainable,” she said in the release.
Meanwhile, ACEP is also tackling violence in the emergency department and has initiatives to protect the mental health of those working on the front lines, the release states.
“Emergency physicians will continue to do everything necessary to treat patients,” Dr. Schmitz said in the release, “but it will take a collaborative effort with legislators, policymakers and health system leaders to strengthen care teams, improve access and address capacity concerns with solutions that can save lives right now and in the months ahead.”
Dr. Schmitz stated that in Washington state, ICUs are at 97% to 100% capacity and less than 30 pediatric inpatient beds are available in the western part of the state.
“In Michigan and New York, several emergency departments are overflowing, and doctors are being called in to triage people in the waiting room because all of the emergency department beds are holding admissions. There are scenarios where entire hospitals are backing up into the emergency department and waiting room and we are physically running out of space and nursing staff.”
ACEP represents its 40,000 emergency physician members.
A version of this article first appeared on Medscape.com.
CDC supports ‘test-to-stay’ for COVID- exposed students
The Centers for Disease Control and Prevention has announced that in the following days.
The new guidance, known as the “test-to-stay” protocol, would reduce the number of children who are expected to stay home as a close contact to someone who tested positive for the virus.
“Test-to-stay is an encouraging public health practice to keep our children in schools,” Rochelle Walensky, MD, director of the CDC, said during a White House press briefing.
When a COVID-19 case is identified in a school, the test-to-stay strategy allows schools to implement regular testing rather than quarantine close contacts. If the contacts don’t experience symptoms and test negative at least twice in a seven-day period, they can continue in-person learning. If they test positive, then they are required to isolate.
In recent months, the CDC has collaborated with several school districts across the United States to evaluate test-to-stay programs. On Dec. 17, the CDC published two studies in its Morbidity and Mortality Weekly Report that demonstrated the effectiveness of these programs in limiting the spread of the virus while also keeping students in class.
“CDC is updating our materials to help schools and parents know how to best implement this promising and now-proven practice, along with our multi-layer prevention strategies that will help keep our children in the classroom safely,” Dr. Walensky said. “These studies demonstrated that test-to-stay works to keep unvaccinated children in school safely.”
In one study, researchers analyzed data for public schools in Los Angeles County between Aug. 16 and Oct. 31, where 432 schools implemented test-to-stay and 1,635 did not.
The Los Angeles County Department of Public Health found that COVID-19 cases did not increase among the schools that used the protocol, as compared with schools that didn’t.
Before test-to-stay was implemented, the average daily number of cases was 10 cases per 100,000 students in districts that later adopted the protocol and 20 cases per 100,000 students in districts that didn’t. After the program was implemented, average daily case rates declined in all school districts but remained lower in test-to-stay districts, with 6 cases per 100,000 students as compared with 11 cases per 100,000 students in districts that didn’t do the protocol.
In addition, schools that didn’t use the test-to-stay program “lost substantial in-person school days,” researchers wrote. At the same time, implementing the program “requires resources that might be currently unavailable for some schools,” they added, noting that “a higher percentage of disadvantaged schools” didn’t do the protocol.
The program requires personnel who can track which students need to be tested, their results and when they can come off the list of close contacts, officials told CNN. This can be a challenge for overstretched school nursing staff.
In another study published last week, researchers analyzed data between Aug. 9 and Oct. 29 for 90 schools across 31 districts in Lake County, Ill., that implemented test-to-stay programs. During that time, the schools reported 258 COVID-19 cases and 1,664 close contacts.
The Lake County Health Department examined the number of close contacts that later tested positive and whether the virus further spread from the close contacts to other people. They found that 16 of the close contacts tested positive and that these were all students. No one appeared to transmit the virus to others at school, but nine cases were identified among household contacts.
Overall, study authors wrote, the test-to-stay protocol preserved in-person learning days for students. In addition, regular testing, masking, and physical distancing led to lower virus transmission in school.
“The test-to-stay-programs are really good at balancing the costs and benefits,” Zoe McLaren, a health policy expert at the University of Maryland at Baltimore, told The New York Times.
“What the test-to-stay program does is help us keep COVID cases down, while also trying to make sure we keep kids in school as much as possible, which I think is really important,” she said.
A version of this article first appeared on WebMD.com.
The Centers for Disease Control and Prevention has announced that in the following days.
The new guidance, known as the “test-to-stay” protocol, would reduce the number of children who are expected to stay home as a close contact to someone who tested positive for the virus.
“Test-to-stay is an encouraging public health practice to keep our children in schools,” Rochelle Walensky, MD, director of the CDC, said during a White House press briefing.
When a COVID-19 case is identified in a school, the test-to-stay strategy allows schools to implement regular testing rather than quarantine close contacts. If the contacts don’t experience symptoms and test negative at least twice in a seven-day period, they can continue in-person learning. If they test positive, then they are required to isolate.
In recent months, the CDC has collaborated with several school districts across the United States to evaluate test-to-stay programs. On Dec. 17, the CDC published two studies in its Morbidity and Mortality Weekly Report that demonstrated the effectiveness of these programs in limiting the spread of the virus while also keeping students in class.
“CDC is updating our materials to help schools and parents know how to best implement this promising and now-proven practice, along with our multi-layer prevention strategies that will help keep our children in the classroom safely,” Dr. Walensky said. “These studies demonstrated that test-to-stay works to keep unvaccinated children in school safely.”
In one study, researchers analyzed data for public schools in Los Angeles County between Aug. 16 and Oct. 31, where 432 schools implemented test-to-stay and 1,635 did not.
The Los Angeles County Department of Public Health found that COVID-19 cases did not increase among the schools that used the protocol, as compared with schools that didn’t.
Before test-to-stay was implemented, the average daily number of cases was 10 cases per 100,000 students in districts that later adopted the protocol and 20 cases per 100,000 students in districts that didn’t. After the program was implemented, average daily case rates declined in all school districts but remained lower in test-to-stay districts, with 6 cases per 100,000 students as compared with 11 cases per 100,000 students in districts that didn’t do the protocol.
In addition, schools that didn’t use the test-to-stay program “lost substantial in-person school days,” researchers wrote. At the same time, implementing the program “requires resources that might be currently unavailable for some schools,” they added, noting that “a higher percentage of disadvantaged schools” didn’t do the protocol.
The program requires personnel who can track which students need to be tested, their results and when they can come off the list of close contacts, officials told CNN. This can be a challenge for overstretched school nursing staff.
In another study published last week, researchers analyzed data between Aug. 9 and Oct. 29 for 90 schools across 31 districts in Lake County, Ill., that implemented test-to-stay programs. During that time, the schools reported 258 COVID-19 cases and 1,664 close contacts.
The Lake County Health Department examined the number of close contacts that later tested positive and whether the virus further spread from the close contacts to other people. They found that 16 of the close contacts tested positive and that these were all students. No one appeared to transmit the virus to others at school, but nine cases were identified among household contacts.
Overall, study authors wrote, the test-to-stay protocol preserved in-person learning days for students. In addition, regular testing, masking, and physical distancing led to lower virus transmission in school.
“The test-to-stay-programs are really good at balancing the costs and benefits,” Zoe McLaren, a health policy expert at the University of Maryland at Baltimore, told The New York Times.
“What the test-to-stay program does is help us keep COVID cases down, while also trying to make sure we keep kids in school as much as possible, which I think is really important,” she said.
A version of this article first appeared on WebMD.com.
The Centers for Disease Control and Prevention has announced that in the following days.
The new guidance, known as the “test-to-stay” protocol, would reduce the number of children who are expected to stay home as a close contact to someone who tested positive for the virus.
“Test-to-stay is an encouraging public health practice to keep our children in schools,” Rochelle Walensky, MD, director of the CDC, said during a White House press briefing.
When a COVID-19 case is identified in a school, the test-to-stay strategy allows schools to implement regular testing rather than quarantine close contacts. If the contacts don’t experience symptoms and test negative at least twice in a seven-day period, they can continue in-person learning. If they test positive, then they are required to isolate.
In recent months, the CDC has collaborated with several school districts across the United States to evaluate test-to-stay programs. On Dec. 17, the CDC published two studies in its Morbidity and Mortality Weekly Report that demonstrated the effectiveness of these programs in limiting the spread of the virus while also keeping students in class.
“CDC is updating our materials to help schools and parents know how to best implement this promising and now-proven practice, along with our multi-layer prevention strategies that will help keep our children in the classroom safely,” Dr. Walensky said. “These studies demonstrated that test-to-stay works to keep unvaccinated children in school safely.”
In one study, researchers analyzed data for public schools in Los Angeles County between Aug. 16 and Oct. 31, where 432 schools implemented test-to-stay and 1,635 did not.
The Los Angeles County Department of Public Health found that COVID-19 cases did not increase among the schools that used the protocol, as compared with schools that didn’t.
Before test-to-stay was implemented, the average daily number of cases was 10 cases per 100,000 students in districts that later adopted the protocol and 20 cases per 100,000 students in districts that didn’t. After the program was implemented, average daily case rates declined in all school districts but remained lower in test-to-stay districts, with 6 cases per 100,000 students as compared with 11 cases per 100,000 students in districts that didn’t do the protocol.
In addition, schools that didn’t use the test-to-stay program “lost substantial in-person school days,” researchers wrote. At the same time, implementing the program “requires resources that might be currently unavailable for some schools,” they added, noting that “a higher percentage of disadvantaged schools” didn’t do the protocol.
The program requires personnel who can track which students need to be tested, their results and when they can come off the list of close contacts, officials told CNN. This can be a challenge for overstretched school nursing staff.
In another study published last week, researchers analyzed data between Aug. 9 and Oct. 29 for 90 schools across 31 districts in Lake County, Ill., that implemented test-to-stay programs. During that time, the schools reported 258 COVID-19 cases and 1,664 close contacts.
The Lake County Health Department examined the number of close contacts that later tested positive and whether the virus further spread from the close contacts to other people. They found that 16 of the close contacts tested positive and that these were all students. No one appeared to transmit the virus to others at school, but nine cases were identified among household contacts.
Overall, study authors wrote, the test-to-stay protocol preserved in-person learning days for students. In addition, regular testing, masking, and physical distancing led to lower virus transmission in school.
“The test-to-stay-programs are really good at balancing the costs and benefits,” Zoe McLaren, a health policy expert at the University of Maryland at Baltimore, told The New York Times.
“What the test-to-stay program does is help us keep COVID cases down, while also trying to make sure we keep kids in school as much as possible, which I think is really important,” she said.
A version of this article first appeared on WebMD.com.
Pfizer COVID vaccine for younger children hits snag
Pfizer’s COVID-19 vaccine for children ages 2 to 5 years old fizzled in clinical trials, the company said on Friday, signaling a further delay in getting a vaccine to preschoolers just as Omicron bears down on the U.S.
In a news release, Pfizer reported that while its 3-microgram dose – which is less than one-third of the dose given to older children – generated a protective immune response in babies and toddlers ages 6 to 24 months, it didn’t generate adequate immunity in children ages 2 to 5.
The company plans to change its clinical trial to add a third dose for younger children in hopes of improving those results. It also plans to test a third dose of its 10-microgram vaccine for children ages 5 to 12.
If the trials are successful, Pfizer said it would submit data to the FDA for an emergency use authorization (EUA) in the first half of 2022.
That pushes the timeline of getting a vaccine to younger children back by several months. In November, Anthony Fauci, MD, head of the National Institute of Allergy Infectious Diseases, predicted a vaccine would be ready for preschoolers by spring.
“On one hand, parents are understandably disappointed,” said Jill Foster, MD, a pediatric infectious disease doctor at the University of Minnesota Medical School. “On the other, it shows that the system for testing vaccines is working. Children are not little adults and have complex immune systems, so it’s not just a matter of making the dose smaller and expecting that it will work,” she said, noting that data from Moderna’s KidCOVE study in preschoolers is pending.
Until there’s a vaccine, Dr. Foster says parents should protect babies and toddlers by making sure everyone around them is vaccinated, promote the use of face masks for everyone around them and for all children over age 2, and continue to avoid crowded gatherings, particularly those that are indoors.
“Hand sanitizer is important, but this virus, especially the Omicron variant, is very easily spread through the air, so keep the air clear of virus as much as possible,” she said.
While the youngest children are still waiting for an effective vaccine, there was reassuring news Dec. 16 about the safety of Pfizer’s vaccine for school-aged kids – those ages 5 through 11.
Out of more than 7 million doses given since this vaccine was authorized for emergency use in late October, most reactions to the vaccine – including arm pain, swelling, and fatigue – have been mild and gone away quickly, without the need to miss school or see a doctor, the CDC reported to a meeting of its Advisory Committee on Immunization Practices, or ACIP.
Many experts had been waiting to see if this vaccine would cause rare cases of heart inflammation called myocarditis, as a higher dose did in teens and young adults.
The news on this front was excellent. About 6 weeks after this vaccine became available, the CDC says there have been only eight confirmed cases of myocarditis in this age group. Six more cases are under investigation.
To put this risk into context, data collected by the American Academy of Pediatrics and the Children’s Hospital Association shows that about 1% of children who test positive for COVID-19 are hospitalized for their infections, while the risk of getting a case of myocarditis after vaccination is .0002%, making it about 5,000 times more likely that a child would need to be hospitalized for COVID-19 than for myocarditis after vaccination.
John Su, MD, who is a member of the CDC’s Vaccine Safety Team, reported there had been two deaths in children after a COVID-19 vaccination. Both were girls, ages 5 and 6. Both had complicated medical histories for several medical disorders. It’s not clear their deaths were linked to the vaccine, and the causes of their deaths are still under investigation.
A version of this article first appeared on WebMD.com.
Pfizer’s COVID-19 vaccine for children ages 2 to 5 years old fizzled in clinical trials, the company said on Friday, signaling a further delay in getting a vaccine to preschoolers just as Omicron bears down on the U.S.
In a news release, Pfizer reported that while its 3-microgram dose – which is less than one-third of the dose given to older children – generated a protective immune response in babies and toddlers ages 6 to 24 months, it didn’t generate adequate immunity in children ages 2 to 5.
The company plans to change its clinical trial to add a third dose for younger children in hopes of improving those results. It also plans to test a third dose of its 10-microgram vaccine for children ages 5 to 12.
If the trials are successful, Pfizer said it would submit data to the FDA for an emergency use authorization (EUA) in the first half of 2022.
That pushes the timeline of getting a vaccine to younger children back by several months. In November, Anthony Fauci, MD, head of the National Institute of Allergy Infectious Diseases, predicted a vaccine would be ready for preschoolers by spring.
“On one hand, parents are understandably disappointed,” said Jill Foster, MD, a pediatric infectious disease doctor at the University of Minnesota Medical School. “On the other, it shows that the system for testing vaccines is working. Children are not little adults and have complex immune systems, so it’s not just a matter of making the dose smaller and expecting that it will work,” she said, noting that data from Moderna’s KidCOVE study in preschoolers is pending.
Until there’s a vaccine, Dr. Foster says parents should protect babies and toddlers by making sure everyone around them is vaccinated, promote the use of face masks for everyone around them and for all children over age 2, and continue to avoid crowded gatherings, particularly those that are indoors.
“Hand sanitizer is important, but this virus, especially the Omicron variant, is very easily spread through the air, so keep the air clear of virus as much as possible,” she said.
While the youngest children are still waiting for an effective vaccine, there was reassuring news Dec. 16 about the safety of Pfizer’s vaccine for school-aged kids – those ages 5 through 11.
Out of more than 7 million doses given since this vaccine was authorized for emergency use in late October, most reactions to the vaccine – including arm pain, swelling, and fatigue – have been mild and gone away quickly, without the need to miss school or see a doctor, the CDC reported to a meeting of its Advisory Committee on Immunization Practices, or ACIP.
Many experts had been waiting to see if this vaccine would cause rare cases of heart inflammation called myocarditis, as a higher dose did in teens and young adults.
The news on this front was excellent. About 6 weeks after this vaccine became available, the CDC says there have been only eight confirmed cases of myocarditis in this age group. Six more cases are under investigation.
To put this risk into context, data collected by the American Academy of Pediatrics and the Children’s Hospital Association shows that about 1% of children who test positive for COVID-19 are hospitalized for their infections, while the risk of getting a case of myocarditis after vaccination is .0002%, making it about 5,000 times more likely that a child would need to be hospitalized for COVID-19 than for myocarditis after vaccination.
John Su, MD, who is a member of the CDC’s Vaccine Safety Team, reported there had been two deaths in children after a COVID-19 vaccination. Both were girls, ages 5 and 6. Both had complicated medical histories for several medical disorders. It’s not clear their deaths were linked to the vaccine, and the causes of their deaths are still under investigation.
A version of this article first appeared on WebMD.com.
Pfizer’s COVID-19 vaccine for children ages 2 to 5 years old fizzled in clinical trials, the company said on Friday, signaling a further delay in getting a vaccine to preschoolers just as Omicron bears down on the U.S.
In a news release, Pfizer reported that while its 3-microgram dose – which is less than one-third of the dose given to older children – generated a protective immune response in babies and toddlers ages 6 to 24 months, it didn’t generate adequate immunity in children ages 2 to 5.
The company plans to change its clinical trial to add a third dose for younger children in hopes of improving those results. It also plans to test a third dose of its 10-microgram vaccine for children ages 5 to 12.
If the trials are successful, Pfizer said it would submit data to the FDA for an emergency use authorization (EUA) in the first half of 2022.
That pushes the timeline of getting a vaccine to younger children back by several months. In November, Anthony Fauci, MD, head of the National Institute of Allergy Infectious Diseases, predicted a vaccine would be ready for preschoolers by spring.
“On one hand, parents are understandably disappointed,” said Jill Foster, MD, a pediatric infectious disease doctor at the University of Minnesota Medical School. “On the other, it shows that the system for testing vaccines is working. Children are not little adults and have complex immune systems, so it’s not just a matter of making the dose smaller and expecting that it will work,” she said, noting that data from Moderna’s KidCOVE study in preschoolers is pending.
Until there’s a vaccine, Dr. Foster says parents should protect babies and toddlers by making sure everyone around them is vaccinated, promote the use of face masks for everyone around them and for all children over age 2, and continue to avoid crowded gatherings, particularly those that are indoors.
“Hand sanitizer is important, but this virus, especially the Omicron variant, is very easily spread through the air, so keep the air clear of virus as much as possible,” she said.
While the youngest children are still waiting for an effective vaccine, there was reassuring news Dec. 16 about the safety of Pfizer’s vaccine for school-aged kids – those ages 5 through 11.
Out of more than 7 million doses given since this vaccine was authorized for emergency use in late October, most reactions to the vaccine – including arm pain, swelling, and fatigue – have been mild and gone away quickly, without the need to miss school or see a doctor, the CDC reported to a meeting of its Advisory Committee on Immunization Practices, or ACIP.
Many experts had been waiting to see if this vaccine would cause rare cases of heart inflammation called myocarditis, as a higher dose did in teens and young adults.
The news on this front was excellent. About 6 weeks after this vaccine became available, the CDC says there have been only eight confirmed cases of myocarditis in this age group. Six more cases are under investigation.
To put this risk into context, data collected by the American Academy of Pediatrics and the Children’s Hospital Association shows that about 1% of children who test positive for COVID-19 are hospitalized for their infections, while the risk of getting a case of myocarditis after vaccination is .0002%, making it about 5,000 times more likely that a child would need to be hospitalized for COVID-19 than for myocarditis after vaccination.
John Su, MD, who is a member of the CDC’s Vaccine Safety Team, reported there had been two deaths in children after a COVID-19 vaccination. Both were girls, ages 5 and 6. Both had complicated medical histories for several medical disorders. It’s not clear their deaths were linked to the vaccine, and the causes of their deaths are still under investigation.
A version of this article first appeared on WebMD.com.
COVID cases spike as questions remain about Omicron’s threat
The best way to stay protected is by getting vaccinated and boosted, they said.
“For the unvaccinated, you’re looking at a winter of severe illness and death – for yourselves, families, and the hospitals who may soon overwhelm,” White House COVID-19 Response Coordinator Jeff Zients said at a news briefing Dec. 17. “We need the American people to do their part.”
The Omicron variant has been detected in at least 39 states and 75 countries, according to CDC director Rochelle Walensky, MD.
The strain is more transmissible than the already highly infectious Delta variant, and although there was early evidence that it caused more mild disease, she said that is likely because many of those infected have been vaccinated and boosted.
“Although Delta continues to circulate widely in the United States, Omicron is increasing rapidly and we expect it to become the dominant strain in the United States, as it has in other countries, in the coming weeks,” Dr. Walensky said.
The United States is averaging close to 1,300 deaths from COVID-19 each day. New cases, deaths, and hospitalizations are higher now than in the previous winter – before vaccines were so widely available. The New York Times reported on Dec. 17 that new infections in Connecticut and Maine have grown 150% in the past 2 weeks, and Ohio and Indiana are seeing hospitalization rates nearing the worst of 2020-2021’s winter surge.
Dueling reports released recently gave cause for relief and concern about Omicron.
A study from South Africa released on Dec. 14 shows lower hospitalizations during the first 3 weeks of the Omicron wave than during earlier waves from other variants. That’s the good news.
The concerning news is out of the United Kingdom, where Imperial College London reported Dec. 17 that the risk of reinfection with COVID-19 from Omicron is more than 5 times as high and that cases of Omicron-based COVID-19 are doubling every 2 days.
What’s more, the study “finds no evidence of Omicron having lower severity than Delta, judged by either the proportion of people testing positive who report symptoms, or by the proportion of cases seeking hospital care after infection. However, hospitalization data remains very limited at this time,” the researchers said.
“We have no evidence that the virus itself is more mild,” Eric Topol, MD, executive vice president of Scripps Research and editor-in-chief of Medscape, told PBS NewsHour. “Until we have that, we have to assume that people who don’t have any protection are highly vulnerable to getting very ill.”
The White House COVID-19 team continues to urge parents and guardians to get their children vaccinated, especially in anticipation of a post-holiday spike. Dr. Walensky said the CDC’s vaccine advisory board met on Dec. 16 to continue the safety discussion about COVID-19 vaccinations in children.
So far, 20 million children under 17 and 5 million under 11 have received their shots.
“Looking specifically at vaccine safety data from over 50,000 children 5-11 years old, we found no evidence of serious safety concerns,” Dr. Walensky said.
Top infectious disease expert Anthony S. Fauci, MD, highlighted the importance of getting vaccinated and boosted to avoid serious disease from Delta and Omicron.
“We’re in a situation where we are now facing a very important Delta surge and we are looking over our shoulder at an oncoming Omicron surge,” he said. “The optimum protection is fully vaccinated plus a boost.”
A version of this article first appeared on WebMD.com.
The best way to stay protected is by getting vaccinated and boosted, they said.
“For the unvaccinated, you’re looking at a winter of severe illness and death – for yourselves, families, and the hospitals who may soon overwhelm,” White House COVID-19 Response Coordinator Jeff Zients said at a news briefing Dec. 17. “We need the American people to do their part.”
The Omicron variant has been detected in at least 39 states and 75 countries, according to CDC director Rochelle Walensky, MD.
The strain is more transmissible than the already highly infectious Delta variant, and although there was early evidence that it caused more mild disease, she said that is likely because many of those infected have been vaccinated and boosted.
“Although Delta continues to circulate widely in the United States, Omicron is increasing rapidly and we expect it to become the dominant strain in the United States, as it has in other countries, in the coming weeks,” Dr. Walensky said.
The United States is averaging close to 1,300 deaths from COVID-19 each day. New cases, deaths, and hospitalizations are higher now than in the previous winter – before vaccines were so widely available. The New York Times reported on Dec. 17 that new infections in Connecticut and Maine have grown 150% in the past 2 weeks, and Ohio and Indiana are seeing hospitalization rates nearing the worst of 2020-2021’s winter surge.
Dueling reports released recently gave cause for relief and concern about Omicron.
A study from South Africa released on Dec. 14 shows lower hospitalizations during the first 3 weeks of the Omicron wave than during earlier waves from other variants. That’s the good news.
The concerning news is out of the United Kingdom, where Imperial College London reported Dec. 17 that the risk of reinfection with COVID-19 from Omicron is more than 5 times as high and that cases of Omicron-based COVID-19 are doubling every 2 days.
What’s more, the study “finds no evidence of Omicron having lower severity than Delta, judged by either the proportion of people testing positive who report symptoms, or by the proportion of cases seeking hospital care after infection. However, hospitalization data remains very limited at this time,” the researchers said.
“We have no evidence that the virus itself is more mild,” Eric Topol, MD, executive vice president of Scripps Research and editor-in-chief of Medscape, told PBS NewsHour. “Until we have that, we have to assume that people who don’t have any protection are highly vulnerable to getting very ill.”
The White House COVID-19 team continues to urge parents and guardians to get their children vaccinated, especially in anticipation of a post-holiday spike. Dr. Walensky said the CDC’s vaccine advisory board met on Dec. 16 to continue the safety discussion about COVID-19 vaccinations in children.
So far, 20 million children under 17 and 5 million under 11 have received their shots.
“Looking specifically at vaccine safety data from over 50,000 children 5-11 years old, we found no evidence of serious safety concerns,” Dr. Walensky said.
Top infectious disease expert Anthony S. Fauci, MD, highlighted the importance of getting vaccinated and boosted to avoid serious disease from Delta and Omicron.
“We’re in a situation where we are now facing a very important Delta surge and we are looking over our shoulder at an oncoming Omicron surge,” he said. “The optimum protection is fully vaccinated plus a boost.”
A version of this article first appeared on WebMD.com.
The best way to stay protected is by getting vaccinated and boosted, they said.
“For the unvaccinated, you’re looking at a winter of severe illness and death – for yourselves, families, and the hospitals who may soon overwhelm,” White House COVID-19 Response Coordinator Jeff Zients said at a news briefing Dec. 17. “We need the American people to do their part.”
The Omicron variant has been detected in at least 39 states and 75 countries, according to CDC director Rochelle Walensky, MD.
The strain is more transmissible than the already highly infectious Delta variant, and although there was early evidence that it caused more mild disease, she said that is likely because many of those infected have been vaccinated and boosted.
“Although Delta continues to circulate widely in the United States, Omicron is increasing rapidly and we expect it to become the dominant strain in the United States, as it has in other countries, in the coming weeks,” Dr. Walensky said.
The United States is averaging close to 1,300 deaths from COVID-19 each day. New cases, deaths, and hospitalizations are higher now than in the previous winter – before vaccines were so widely available. The New York Times reported on Dec. 17 that new infections in Connecticut and Maine have grown 150% in the past 2 weeks, and Ohio and Indiana are seeing hospitalization rates nearing the worst of 2020-2021’s winter surge.
Dueling reports released recently gave cause for relief and concern about Omicron.
A study from South Africa released on Dec. 14 shows lower hospitalizations during the first 3 weeks of the Omicron wave than during earlier waves from other variants. That’s the good news.
The concerning news is out of the United Kingdom, where Imperial College London reported Dec. 17 that the risk of reinfection with COVID-19 from Omicron is more than 5 times as high and that cases of Omicron-based COVID-19 are doubling every 2 days.
What’s more, the study “finds no evidence of Omicron having lower severity than Delta, judged by either the proportion of people testing positive who report symptoms, or by the proportion of cases seeking hospital care after infection. However, hospitalization data remains very limited at this time,” the researchers said.
“We have no evidence that the virus itself is more mild,” Eric Topol, MD, executive vice president of Scripps Research and editor-in-chief of Medscape, told PBS NewsHour. “Until we have that, we have to assume that people who don’t have any protection are highly vulnerable to getting very ill.”
The White House COVID-19 team continues to urge parents and guardians to get their children vaccinated, especially in anticipation of a post-holiday spike. Dr. Walensky said the CDC’s vaccine advisory board met on Dec. 16 to continue the safety discussion about COVID-19 vaccinations in children.
So far, 20 million children under 17 and 5 million under 11 have received their shots.
“Looking specifically at vaccine safety data from over 50,000 children 5-11 years old, we found no evidence of serious safety concerns,” Dr. Walensky said.
Top infectious disease expert Anthony S. Fauci, MD, highlighted the importance of getting vaccinated and boosted to avoid serious disease from Delta and Omicron.
“We’re in a situation where we are now facing a very important Delta surge and we are looking over our shoulder at an oncoming Omicron surge,” he said. “The optimum protection is fully vaccinated plus a boost.”
A version of this article first appeared on WebMD.com.
Small myocarditis risk now seen for adenovirus-based COVID-19 vaccine
The first large population study to investigate the association between different COVID-19 vaccines types and cardiac effects and adverse events shows a small increase in the risk for acute myocarditis with both the mRNA-based vaccines and – in what may a first in the literature – an adenovirus-vector vaccine.
The excess risk was seen following the first dose of the ChAdOc1 (AstraZeneca/Oxford), the adenovirus-based vaccine, and the mRNA-based BNT162b2 (Pfizer/BioNTech). It was observed after first and second doses of the mRNA-1273 (Moderna) vaccine.
The incidence rate ratios for myocarditis 1-7 days after the first AstraZeneca, Pfizer, and Moderna injections were 1.76, 1.45, and 8.38, respectively, and 23.1 after the second dose of the Moderna vaccine.
“There’s a bit more uncertainty and worry about mRNA vaccines because it’s quite a new vector for vaccination and, therefore, there’s been more focus on the potential side effects,” said Nicholas Mills, MD.
“But it doesn’t surprise me the signal is present for all types of vaccines because they’re designed to generate a systemic immune response and that is, unfortunately, where you can cause small risks for immune-mediated illnesses like myocarditis,” Dr. Mills, from the University of Edinburgh, told this news organization. Dr. Mills is a coauthor on the study, published Dec. 14 in Nature Medicine.
To put the risks in context, the group estimated between 1 and 10 additional myocarditis hospitalizations or deaths per 1 million people vaccinated, but 40 excess myocarditis events per million following a positive SARS-CoV-2 test result.
As reported, rates of excess myocarditis events associated with a first dose were 2 per million injections of the AstraZeneca vaccine, 1 per million for the Pfizer vaccine, and 6 per million with the Moderna vaccine.
Following a second dose, there were 10 additional myocarditis events per million people receiving the Moderna vaccine and none among recipients of the AstraZeneca or Pfizer vaccines.
“It was particularly seen within the first 7 days of the first dose, which is very consistent with what we see in people who have viral myocarditis,” Dr. Mills said. “So it looks like a real signal but it’s very small.”
The results are in line with previous studies of the Pfizer vaccine in Israel and studies of the Moderna vaccine in the United States, Biykem Bozkurt, MD, PhD, professor of medicine at Baylor College of Medicine, Houston, told this news organization.
“What this paper does is confirm that cardiovascular complications – and they are only looking at a small component of those cardiovascular complications – are markedly higher with the COVID-19 infection than with the vaccines,” she said.
It also adds a new twist to the search for the mechanisms of myocarditis, which has focused on the immunogenicity of the RNA in the Pfizer and Moderna vaccines but also hypothesized that molecular mimicry between the SARS-CoV-2 spike glycoprotein and cell antigens, antibody production against cardiac proteins, and testosterone may play a role.
“But now it doesn’t look like the risk is solely confined to the mRNA vaccine platform because it’s also happening with the adenovirus,” Dr. Bozkurt said. “The mechanisms require future experimental and clinical research and we’ll need more granular data with cohorts that are closely followed up as well as subclinical follow-up.”
James de Lemos, MD, professor of medicine at the University of Texas Southwestern Medical Center, Dallas, and cochair of the American Heart Association’s COVID-19 CVD Registry, said he was also not surprised by a myocarditis signal with AstraZeneca’s adenovirus vaccine.
“Looking at relative risks has biological implications, but the clinical and public health implications are that the absolute risk with the adenovirus is trivial. And you see that with their estimations of absolute risk where it’s literally sort of a needle in the haystack of 1 or 2 per million,” he said in an interview.
Large-scale data
The investigators examined the rates of hospital admission or death from myocarditis, pericarditis, and cardiac arrhythmia in the 28 days following SARS-CoV-2 vaccination or infection by linking the English National Immunisation Database of COVID-19 vaccination with a national patient-level health care database of 38.6 million people, aged 16 years or older, vaccinated from Dec.1, 2020, to Aug. 24, 2021.
The number of people admitted to the hospital or who died during the study period was 1,615 for myocarditis, 1,574 for pericarditis, and 385,508 for cardiac arrhythmia.
There was no evidence of an increased risk for pericarditis or cardiac arrhythmia following vaccination, except for arrhythmia in the 28 days following a second dose of the Moderna vaccine (IRR, 1.46).
In contrast, the risk was increased for pericarditis (IRR, 2.79) and cardiac arrhythmia (IRR, 5.35) in the 28 days following a positive SARS-CoV-2 test result.
Although the scale of the analysis allows for more precise estimates than what’s been possible in smaller data sets, there is the challenge of diagnosing COVID-19 from billing codes and the potential for ascertainment bias, noted Dr. de Lemos.
“Having said that, I think it’s a really important study, because it’s the first study to put the incidence in context in the same general population the risks of myocarditis with various vaccines and with COVID-19,” he said.
“That’s really important and provides a lot of reassurance for those who are trying to balance the risks and benefits of vaccination.”
Analyses by sex and age
A subgroup analysis by age showed increased risks for myocarditis with the mRNA vaccines only in those younger than 40, whereas no association was found with the Oxford adenovirus vaccine.
“We’re not seeing any signal here that would make us change the recommendation for vaccination in children as a consequence of this risk,” Dr. Mills said during a press briefing.
Dr. Bozkurt pointed out, however, that the estimated excess in myocarditis events following a second dose of the Moderna vaccine in these younger adults reportedly exceeded that for SARS-CoV-2 infection (15 per million vs. 10 per million).
“For that age group, it’s concerning and needs further clarification. This hasn’t been seen before,” she said.
The average age was 39 years for those receiving two doses of the Moderna vaccine and 55 for recipients of the Pfizer and Oxford vaccines. The Moderna vaccine wasn’t rolled out until April 2021 in the United Kingdom, the authors noted, so the number of patients who received this vaccine is lower.
Although reports have suggested young males are at greater risk for myocarditis after vaccination, an analysis by sex found that women had an increased risk for myocarditis after a first dose of the AstraZeneca (IRR, 1.40) and Pfizer (IRR, 1.54) vaccines and following a positive COVID-19 test result (IRR, 11.00).
“Women being at increased risk is rather a new message,” Dr. Bozkurt said. “But the incidence rate ratios are being compared against the unvaccinated, so when you see the increase in women, it doesn’t mean it’s increased against men. It would be helpful for sex-specific incidence rate ratios to be reported for younger age subgroups, such as ages 16-20 and 20-30, to determine whether there’s an increased risk for males compared to females at younger ages.”
Age and sex differences are huge questions, but “I think we’ll learn a lot about myocarditis in general from what is going to be an explosion of research into the vaccine-associated causes,” Dr. de Lemos said.
“That will help us understand myocarditis more broadly and prepare us for the next generation of vaccines, which inevitably will be mRNA based.”
Dr. Mills reported having no relevant disclosures. Dr. Bozkurt reported consulting for Bayer and scPharmaceuticals and serving on a clinical-events committee for a trial supported by Abbott Pharmaceuticals and on a data and safety monitoring board for a trial supported by Liva Nova Pharmaceuticals. Dr. De Lemos reported having no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
The first large population study to investigate the association between different COVID-19 vaccines types and cardiac effects and adverse events shows a small increase in the risk for acute myocarditis with both the mRNA-based vaccines and – in what may a first in the literature – an adenovirus-vector vaccine.
The excess risk was seen following the first dose of the ChAdOc1 (AstraZeneca/Oxford), the adenovirus-based vaccine, and the mRNA-based BNT162b2 (Pfizer/BioNTech). It was observed after first and second doses of the mRNA-1273 (Moderna) vaccine.
The incidence rate ratios for myocarditis 1-7 days after the first AstraZeneca, Pfizer, and Moderna injections were 1.76, 1.45, and 8.38, respectively, and 23.1 after the second dose of the Moderna vaccine.
“There’s a bit more uncertainty and worry about mRNA vaccines because it’s quite a new vector for vaccination and, therefore, there’s been more focus on the potential side effects,” said Nicholas Mills, MD.
“But it doesn’t surprise me the signal is present for all types of vaccines because they’re designed to generate a systemic immune response and that is, unfortunately, where you can cause small risks for immune-mediated illnesses like myocarditis,” Dr. Mills, from the University of Edinburgh, told this news organization. Dr. Mills is a coauthor on the study, published Dec. 14 in Nature Medicine.
To put the risks in context, the group estimated between 1 and 10 additional myocarditis hospitalizations or deaths per 1 million people vaccinated, but 40 excess myocarditis events per million following a positive SARS-CoV-2 test result.
As reported, rates of excess myocarditis events associated with a first dose were 2 per million injections of the AstraZeneca vaccine, 1 per million for the Pfizer vaccine, and 6 per million with the Moderna vaccine.
Following a second dose, there were 10 additional myocarditis events per million people receiving the Moderna vaccine and none among recipients of the AstraZeneca or Pfizer vaccines.
“It was particularly seen within the first 7 days of the first dose, which is very consistent with what we see in people who have viral myocarditis,” Dr. Mills said. “So it looks like a real signal but it’s very small.”
The results are in line with previous studies of the Pfizer vaccine in Israel and studies of the Moderna vaccine in the United States, Biykem Bozkurt, MD, PhD, professor of medicine at Baylor College of Medicine, Houston, told this news organization.
“What this paper does is confirm that cardiovascular complications – and they are only looking at a small component of those cardiovascular complications – are markedly higher with the COVID-19 infection than with the vaccines,” she said.
It also adds a new twist to the search for the mechanisms of myocarditis, which has focused on the immunogenicity of the RNA in the Pfizer and Moderna vaccines but also hypothesized that molecular mimicry between the SARS-CoV-2 spike glycoprotein and cell antigens, antibody production against cardiac proteins, and testosterone may play a role.
“But now it doesn’t look like the risk is solely confined to the mRNA vaccine platform because it’s also happening with the adenovirus,” Dr. Bozkurt said. “The mechanisms require future experimental and clinical research and we’ll need more granular data with cohorts that are closely followed up as well as subclinical follow-up.”
James de Lemos, MD, professor of medicine at the University of Texas Southwestern Medical Center, Dallas, and cochair of the American Heart Association’s COVID-19 CVD Registry, said he was also not surprised by a myocarditis signal with AstraZeneca’s adenovirus vaccine.
“Looking at relative risks has biological implications, but the clinical and public health implications are that the absolute risk with the adenovirus is trivial. And you see that with their estimations of absolute risk where it’s literally sort of a needle in the haystack of 1 or 2 per million,” he said in an interview.
Large-scale data
The investigators examined the rates of hospital admission or death from myocarditis, pericarditis, and cardiac arrhythmia in the 28 days following SARS-CoV-2 vaccination or infection by linking the English National Immunisation Database of COVID-19 vaccination with a national patient-level health care database of 38.6 million people, aged 16 years or older, vaccinated from Dec.1, 2020, to Aug. 24, 2021.
The number of people admitted to the hospital or who died during the study period was 1,615 for myocarditis, 1,574 for pericarditis, and 385,508 for cardiac arrhythmia.
There was no evidence of an increased risk for pericarditis or cardiac arrhythmia following vaccination, except for arrhythmia in the 28 days following a second dose of the Moderna vaccine (IRR, 1.46).
In contrast, the risk was increased for pericarditis (IRR, 2.79) and cardiac arrhythmia (IRR, 5.35) in the 28 days following a positive SARS-CoV-2 test result.
Although the scale of the analysis allows for more precise estimates than what’s been possible in smaller data sets, there is the challenge of diagnosing COVID-19 from billing codes and the potential for ascertainment bias, noted Dr. de Lemos.
“Having said that, I think it’s a really important study, because it’s the first study to put the incidence in context in the same general population the risks of myocarditis with various vaccines and with COVID-19,” he said.
“That’s really important and provides a lot of reassurance for those who are trying to balance the risks and benefits of vaccination.”
Analyses by sex and age
A subgroup analysis by age showed increased risks for myocarditis with the mRNA vaccines only in those younger than 40, whereas no association was found with the Oxford adenovirus vaccine.
“We’re not seeing any signal here that would make us change the recommendation for vaccination in children as a consequence of this risk,” Dr. Mills said during a press briefing.
Dr. Bozkurt pointed out, however, that the estimated excess in myocarditis events following a second dose of the Moderna vaccine in these younger adults reportedly exceeded that for SARS-CoV-2 infection (15 per million vs. 10 per million).
“For that age group, it’s concerning and needs further clarification. This hasn’t been seen before,” she said.
The average age was 39 years for those receiving two doses of the Moderna vaccine and 55 for recipients of the Pfizer and Oxford vaccines. The Moderna vaccine wasn’t rolled out until April 2021 in the United Kingdom, the authors noted, so the number of patients who received this vaccine is lower.
Although reports have suggested young males are at greater risk for myocarditis after vaccination, an analysis by sex found that women had an increased risk for myocarditis after a first dose of the AstraZeneca (IRR, 1.40) and Pfizer (IRR, 1.54) vaccines and following a positive COVID-19 test result (IRR, 11.00).
“Women being at increased risk is rather a new message,” Dr. Bozkurt said. “But the incidence rate ratios are being compared against the unvaccinated, so when you see the increase in women, it doesn’t mean it’s increased against men. It would be helpful for sex-specific incidence rate ratios to be reported for younger age subgroups, such as ages 16-20 and 20-30, to determine whether there’s an increased risk for males compared to females at younger ages.”
Age and sex differences are huge questions, but “I think we’ll learn a lot about myocarditis in general from what is going to be an explosion of research into the vaccine-associated causes,” Dr. de Lemos said.
“That will help us understand myocarditis more broadly and prepare us for the next generation of vaccines, which inevitably will be mRNA based.”
Dr. Mills reported having no relevant disclosures. Dr. Bozkurt reported consulting for Bayer and scPharmaceuticals and serving on a clinical-events committee for a trial supported by Abbott Pharmaceuticals and on a data and safety monitoring board for a trial supported by Liva Nova Pharmaceuticals. Dr. De Lemos reported having no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
The first large population study to investigate the association between different COVID-19 vaccines types and cardiac effects and adverse events shows a small increase in the risk for acute myocarditis with both the mRNA-based vaccines and – in what may a first in the literature – an adenovirus-vector vaccine.
The excess risk was seen following the first dose of the ChAdOc1 (AstraZeneca/Oxford), the adenovirus-based vaccine, and the mRNA-based BNT162b2 (Pfizer/BioNTech). It was observed after first and second doses of the mRNA-1273 (Moderna) vaccine.
The incidence rate ratios for myocarditis 1-7 days after the first AstraZeneca, Pfizer, and Moderna injections were 1.76, 1.45, and 8.38, respectively, and 23.1 after the second dose of the Moderna vaccine.
“There’s a bit more uncertainty and worry about mRNA vaccines because it’s quite a new vector for vaccination and, therefore, there’s been more focus on the potential side effects,” said Nicholas Mills, MD.
“But it doesn’t surprise me the signal is present for all types of vaccines because they’re designed to generate a systemic immune response and that is, unfortunately, where you can cause small risks for immune-mediated illnesses like myocarditis,” Dr. Mills, from the University of Edinburgh, told this news organization. Dr. Mills is a coauthor on the study, published Dec. 14 in Nature Medicine.
To put the risks in context, the group estimated between 1 and 10 additional myocarditis hospitalizations or deaths per 1 million people vaccinated, but 40 excess myocarditis events per million following a positive SARS-CoV-2 test result.
As reported, rates of excess myocarditis events associated with a first dose were 2 per million injections of the AstraZeneca vaccine, 1 per million for the Pfizer vaccine, and 6 per million with the Moderna vaccine.
Following a second dose, there were 10 additional myocarditis events per million people receiving the Moderna vaccine and none among recipients of the AstraZeneca or Pfizer vaccines.
“It was particularly seen within the first 7 days of the first dose, which is very consistent with what we see in people who have viral myocarditis,” Dr. Mills said. “So it looks like a real signal but it’s very small.”
The results are in line with previous studies of the Pfizer vaccine in Israel and studies of the Moderna vaccine in the United States, Biykem Bozkurt, MD, PhD, professor of medicine at Baylor College of Medicine, Houston, told this news organization.
“What this paper does is confirm that cardiovascular complications – and they are only looking at a small component of those cardiovascular complications – are markedly higher with the COVID-19 infection than with the vaccines,” she said.
It also adds a new twist to the search for the mechanisms of myocarditis, which has focused on the immunogenicity of the RNA in the Pfizer and Moderna vaccines but also hypothesized that molecular mimicry between the SARS-CoV-2 spike glycoprotein and cell antigens, antibody production against cardiac proteins, and testosterone may play a role.
“But now it doesn’t look like the risk is solely confined to the mRNA vaccine platform because it’s also happening with the adenovirus,” Dr. Bozkurt said. “The mechanisms require future experimental and clinical research and we’ll need more granular data with cohorts that are closely followed up as well as subclinical follow-up.”
James de Lemos, MD, professor of medicine at the University of Texas Southwestern Medical Center, Dallas, and cochair of the American Heart Association’s COVID-19 CVD Registry, said he was also not surprised by a myocarditis signal with AstraZeneca’s adenovirus vaccine.
“Looking at relative risks has biological implications, but the clinical and public health implications are that the absolute risk with the adenovirus is trivial. And you see that with their estimations of absolute risk where it’s literally sort of a needle in the haystack of 1 or 2 per million,” he said in an interview.
Large-scale data
The investigators examined the rates of hospital admission or death from myocarditis, pericarditis, and cardiac arrhythmia in the 28 days following SARS-CoV-2 vaccination or infection by linking the English National Immunisation Database of COVID-19 vaccination with a national patient-level health care database of 38.6 million people, aged 16 years or older, vaccinated from Dec.1, 2020, to Aug. 24, 2021.
The number of people admitted to the hospital or who died during the study period was 1,615 for myocarditis, 1,574 for pericarditis, and 385,508 for cardiac arrhythmia.
There was no evidence of an increased risk for pericarditis or cardiac arrhythmia following vaccination, except for arrhythmia in the 28 days following a second dose of the Moderna vaccine (IRR, 1.46).
In contrast, the risk was increased for pericarditis (IRR, 2.79) and cardiac arrhythmia (IRR, 5.35) in the 28 days following a positive SARS-CoV-2 test result.
Although the scale of the analysis allows for more precise estimates than what’s been possible in smaller data sets, there is the challenge of diagnosing COVID-19 from billing codes and the potential for ascertainment bias, noted Dr. de Lemos.
“Having said that, I think it’s a really important study, because it’s the first study to put the incidence in context in the same general population the risks of myocarditis with various vaccines and with COVID-19,” he said.
“That’s really important and provides a lot of reassurance for those who are trying to balance the risks and benefits of vaccination.”
Analyses by sex and age
A subgroup analysis by age showed increased risks for myocarditis with the mRNA vaccines only in those younger than 40, whereas no association was found with the Oxford adenovirus vaccine.
“We’re not seeing any signal here that would make us change the recommendation for vaccination in children as a consequence of this risk,” Dr. Mills said during a press briefing.
Dr. Bozkurt pointed out, however, that the estimated excess in myocarditis events following a second dose of the Moderna vaccine in these younger adults reportedly exceeded that for SARS-CoV-2 infection (15 per million vs. 10 per million).
“For that age group, it’s concerning and needs further clarification. This hasn’t been seen before,” she said.
The average age was 39 years for those receiving two doses of the Moderna vaccine and 55 for recipients of the Pfizer and Oxford vaccines. The Moderna vaccine wasn’t rolled out until April 2021 in the United Kingdom, the authors noted, so the number of patients who received this vaccine is lower.
Although reports have suggested young males are at greater risk for myocarditis after vaccination, an analysis by sex found that women had an increased risk for myocarditis after a first dose of the AstraZeneca (IRR, 1.40) and Pfizer (IRR, 1.54) vaccines and following a positive COVID-19 test result (IRR, 11.00).
“Women being at increased risk is rather a new message,” Dr. Bozkurt said. “But the incidence rate ratios are being compared against the unvaccinated, so when you see the increase in women, it doesn’t mean it’s increased against men. It would be helpful for sex-specific incidence rate ratios to be reported for younger age subgroups, such as ages 16-20 and 20-30, to determine whether there’s an increased risk for males compared to females at younger ages.”
Age and sex differences are huge questions, but “I think we’ll learn a lot about myocarditis in general from what is going to be an explosion of research into the vaccine-associated causes,” Dr. de Lemos said.
“That will help us understand myocarditis more broadly and prepare us for the next generation of vaccines, which inevitably will be mRNA based.”
Dr. Mills reported having no relevant disclosures. Dr. Bozkurt reported consulting for Bayer and scPharmaceuticals and serving on a clinical-events committee for a trial supported by Abbott Pharmaceuticals and on a data and safety monitoring board for a trial supported by Liva Nova Pharmaceuticals. Dr. De Lemos reported having no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
FROM NATURE MEDICINE