User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Children and COVID: New cases down again, but still ‘extremely high’
The indication of an Omicron decline has become a trend: New cases of COVID-19 in children were down for a second consecutive week in the United States, according to the American Academy of Pediatrics and the Children’s Hospital Association.
but the nearly 632,000 cases reported were down by 22% from the previous week and by 45% from what appears to be the peak of the Omicron surge during the week of Jan. 14-20, the AAP/CHA data show.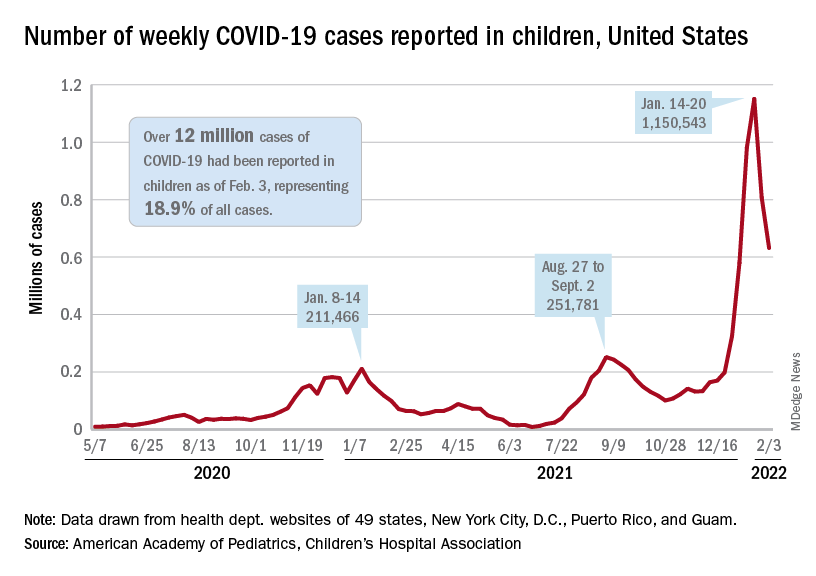
To put the effect of the Delta and Omicron variants into some sort of perspective, the total number of COVID-19 cases among children passed 5 million at the beginning of September 2021, about a year and a half into the pandemic. In the last 5 months, the cumulative count has more than doubled and now stands at 12 million, the AAP and CHA said in their weekly COVID report.
Hospital admissions and emergency department visits followed the same downward trend over the last week. The rate of new hospitalizations fell to 0.81 per 100,000 children aged 0-17 years as of Feb. 2 (down from a peak of 1.25 per 100,000 on Jan. 15), and ED visits with diagnosed COVID-19 dropped to 1.8% (peak was 14.1%), 1.9% (peak was 14.3%), and 3.4% (peak was 14%) of all visits for children aged 16-17, 12-15, and 0-11 years, respectively, the Centers for Disease Control and Prevention reported.
The vaccination response
The surge of infections brought about by the Omicron variant, however, did not translate into increased vaccination, at least for the youngest eligible children. Vaccine initiation rose slightly among children aged 5-11 in early and mid-January but, by early February, new vaccinations had declined to their lowest point since approval in early November of 2021, the AAP said in its weekly COVID vaccination report.
As a result, the 5- to 11-year-olds are well behind the pace set by those aged 12-15 for the first 3 months of their vaccination experience. Through the first 13 weeks after the COVID vaccine was approved for children aged 12-15 in early May, 44.5% had received at least one dose and 32.3% were fully vaccinated. Among children aged 5-11, the corresponding figures through 13 weeks were 31% and 22.5%, according to CDC data.
The vaccination reaction to Omicron was somewhat more robust for children aged 12-17, compared with the younger group, but initiations dropped at the same time that new cases began to decline. In terms of total volume, the response among 12- to 17-year-olds was much smaller than that seen in July and August of 2021 as the Delta surge was hitting the United States, the AAP vaccination report shows.
All those vaccinations add up to this: Over 16.8 million children aged 12-17 and almost 9 million aged 5-11 had received at least one dose of vaccine as of Feb. 7, which works out to 66.6% of the older group and 31.2% of the younger cohort. Almost 14.3 million (56.4%) of those aged 12-17 are fully vaccinated, as are 6.6 million (22.9%) of the 5- to 11-year-olds, the CDC said on its COVID Data Tracker.
The indication of an Omicron decline has become a trend: New cases of COVID-19 in children were down for a second consecutive week in the United States, according to the American Academy of Pediatrics and the Children’s Hospital Association.
but the nearly 632,000 cases reported were down by 22% from the previous week and by 45% from what appears to be the peak of the Omicron surge during the week of Jan. 14-20, the AAP/CHA data show.
To put the effect of the Delta and Omicron variants into some sort of perspective, the total number of COVID-19 cases among children passed 5 million at the beginning of September 2021, about a year and a half into the pandemic. In the last 5 months, the cumulative count has more than doubled and now stands at 12 million, the AAP and CHA said in their weekly COVID report.
Hospital admissions and emergency department visits followed the same downward trend over the last week. The rate of new hospitalizations fell to 0.81 per 100,000 children aged 0-17 years as of Feb. 2 (down from a peak of 1.25 per 100,000 on Jan. 15), and ED visits with diagnosed COVID-19 dropped to 1.8% (peak was 14.1%), 1.9% (peak was 14.3%), and 3.4% (peak was 14%) of all visits for children aged 16-17, 12-15, and 0-11 years, respectively, the Centers for Disease Control and Prevention reported.
The vaccination response
The surge of infections brought about by the Omicron variant, however, did not translate into increased vaccination, at least for the youngest eligible children. Vaccine initiation rose slightly among children aged 5-11 in early and mid-January but, by early February, new vaccinations had declined to their lowest point since approval in early November of 2021, the AAP said in its weekly COVID vaccination report.
As a result, the 5- to 11-year-olds are well behind the pace set by those aged 12-15 for the first 3 months of their vaccination experience. Through the first 13 weeks after the COVID vaccine was approved for children aged 12-15 in early May, 44.5% had received at least one dose and 32.3% were fully vaccinated. Among children aged 5-11, the corresponding figures through 13 weeks were 31% and 22.5%, according to CDC data.
The vaccination reaction to Omicron was somewhat more robust for children aged 12-17, compared with the younger group, but initiations dropped at the same time that new cases began to decline. In terms of total volume, the response among 12- to 17-year-olds was much smaller than that seen in July and August of 2021 as the Delta surge was hitting the United States, the AAP vaccination report shows.
All those vaccinations add up to this: Over 16.8 million children aged 12-17 and almost 9 million aged 5-11 had received at least one dose of vaccine as of Feb. 7, which works out to 66.6% of the older group and 31.2% of the younger cohort. Almost 14.3 million (56.4%) of those aged 12-17 are fully vaccinated, as are 6.6 million (22.9%) of the 5- to 11-year-olds, the CDC said on its COVID Data Tracker.
The indication of an Omicron decline has become a trend: New cases of COVID-19 in children were down for a second consecutive week in the United States, according to the American Academy of Pediatrics and the Children’s Hospital Association.
but the nearly 632,000 cases reported were down by 22% from the previous week and by 45% from what appears to be the peak of the Omicron surge during the week of Jan. 14-20, the AAP/CHA data show.
To put the effect of the Delta and Omicron variants into some sort of perspective, the total number of COVID-19 cases among children passed 5 million at the beginning of September 2021, about a year and a half into the pandemic. In the last 5 months, the cumulative count has more than doubled and now stands at 12 million, the AAP and CHA said in their weekly COVID report.
Hospital admissions and emergency department visits followed the same downward trend over the last week. The rate of new hospitalizations fell to 0.81 per 100,000 children aged 0-17 years as of Feb. 2 (down from a peak of 1.25 per 100,000 on Jan. 15), and ED visits with diagnosed COVID-19 dropped to 1.8% (peak was 14.1%), 1.9% (peak was 14.3%), and 3.4% (peak was 14%) of all visits for children aged 16-17, 12-15, and 0-11 years, respectively, the Centers for Disease Control and Prevention reported.
The vaccination response
The surge of infections brought about by the Omicron variant, however, did not translate into increased vaccination, at least for the youngest eligible children. Vaccine initiation rose slightly among children aged 5-11 in early and mid-January but, by early February, new vaccinations had declined to their lowest point since approval in early November of 2021, the AAP said in its weekly COVID vaccination report.
As a result, the 5- to 11-year-olds are well behind the pace set by those aged 12-15 for the first 3 months of their vaccination experience. Through the first 13 weeks after the COVID vaccine was approved for children aged 12-15 in early May, 44.5% had received at least one dose and 32.3% were fully vaccinated. Among children aged 5-11, the corresponding figures through 13 weeks were 31% and 22.5%, according to CDC data.
The vaccination reaction to Omicron was somewhat more robust for children aged 12-17, compared with the younger group, but initiations dropped at the same time that new cases began to decline. In terms of total volume, the response among 12- to 17-year-olds was much smaller than that seen in July and August of 2021 as the Delta surge was hitting the United States, the AAP vaccination report shows.
All those vaccinations add up to this: Over 16.8 million children aged 12-17 and almost 9 million aged 5-11 had received at least one dose of vaccine as of Feb. 7, which works out to 66.6% of the older group and 31.2% of the younger cohort. Almost 14.3 million (56.4%) of those aged 12-17 are fully vaccinated, as are 6.6 million (22.9%) of the 5- to 11-year-olds, the CDC said on its COVID Data Tracker.
Picture warnings on sugary drinks could help fight childhood obesity
Sugary beverages – juice, soda, decadent lattes, sports drinks, and more – are the leading source of both calories and added sugar in the American diet, according to the Harvard T. H. Chan School of Public Health. But new research published in PLoS Medicine has found that
The new study, from researchers at the University of North Carolina at Chapel Hill, found that parents were 17% less likely to buy sugary drinks for their children when the beverages had the graphical – and graphic – warnings on the products.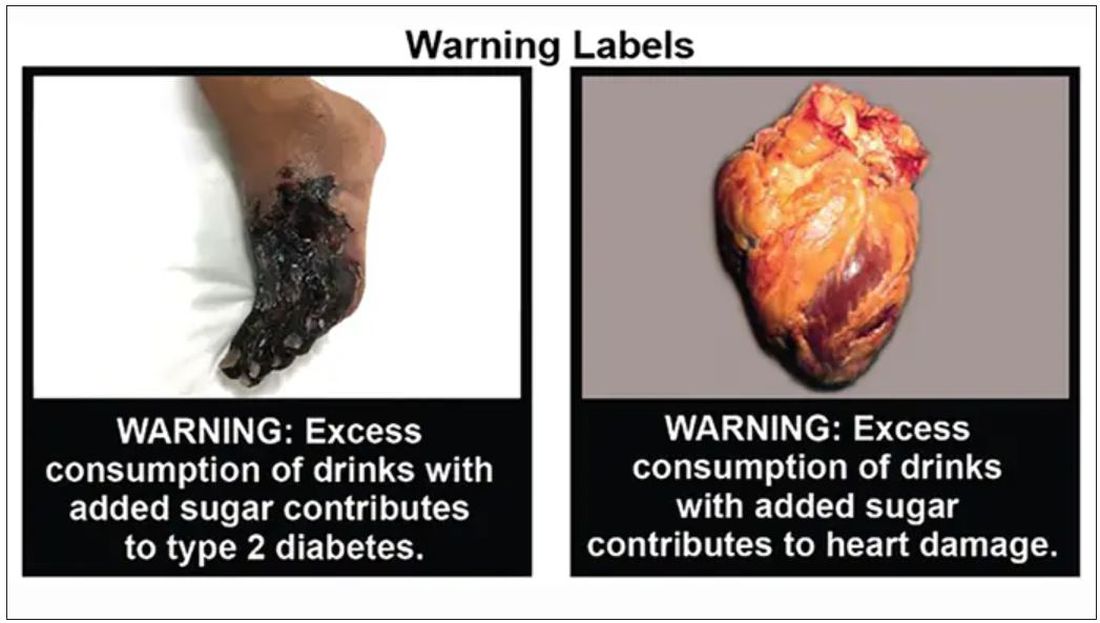
The researchers turned a laboratory setting into a “minimart,” and parents were told to choose one drink and snack for their children, along with one household item (to disguise the purpose of the study).
Some parents were presented with sweetened drinks with images on the products reflecting type 2 diabetes and heart damage. Others were shown sugary drinks with a barcode label and no picture warning.
Forty-five percent of parents chose sugary drinks for their children when the products had no picture warning, but only 28% of parents chose sugary beverages with the cautionary images.
“When people make choices about what food to buy, they are juggling dozens of factors, like taste, cost, and advertising, and are looking at many products at once,” said Lindsey Smith Taillie, PhD, a nutrition researcher and the senior author of the paper.
“Showing that warnings can cut through the noise of everything else that’s happening in a food store is powerful evidence that they would help reduce sugary drink purchases in the real world.”
Children are particularly prone to overindulging on sugar, largely due to companies’ frequent marketing displays of pleasurable-looking and seemingly “thirst-quenching” sweet beverages.
Drink packaging also can be misleading.
Fruits and vegetables displayed on the front of many beverages often lead parents to buy what they believe are “healthy” options, when these drinks could be packed with sugar, according to a study published online Jan. 22 in the journal Appetite.
Parents are often “doing the best with what information they have,” so more education about nutrition, through picture warning labels, for example, would make a difference, said Caroline Fausel, a paleo food blogger, podcaster, and author of “Prep, Cook, Freeze: A Paleo Meal Planning Cookbook.”
Healthier choices on the rise
The American Beverage Association, an industry trade group, shared the current steps that major companies are taking to help lower Americans’ sugar intake.
Pepsi, Coca-Cola, and Keurig Dr Pepper joined forces in 2014 to create the Balance Calories Initiative, which aims to reduce beverage calories in the national diet.
Coca-Cola now offers 250 beverages with zero to low calories, and Keurig Dr Pepper has 158 products with 40 calories or less. Pepsi sells 7.5-ounce mini-cans, along with various other sizes, to encourage portion control.
“Beverage companies are fully transparent about the calories and sugar in our products, and we are offering more choices with less sugar than ever before,” William Dermody, vice president of media and public affairs for the American Beverage Association, said in a statement. “We agree that too much sugar is not good for anyone, and clear information about beverages is most helpful to consumers.”
Other big companies also are taking strides to lower sugar content in their products.
Kraft Heinz, which owns the popular line of Capri Sun drinks, has publicly shared its efforts to ramp up the nutritional value of its products.
The company has a goal to slash 60 million pounds of total sugar in Kraft Heinz products globally by 2025.
“As more people become aware of the harm that excessive sugar can cause in the body, my hope is that they continue to choose healthier alternatives,” Ms. Fausel said.
Creating new patterns
For children who consume sweetened juices and sodas regularly, the transition to healthier options might be challenging at first.
“Change can involve tantrums and unhappiness, and right now parents are at their max living pandemic parenting life,” said Jennifer Anderson, a registered dietitian and CEO of Kids Eat in Color, LLC, a resource for improving child nutrition and health through innovative education, meal plans, and tools. “Kids can get used to having sugary drinks, and they don’t want to give them up.”
One way to help make the switch is by having only water and milk as options while children are up and about, a technique that works particularly well for younger children, she said.
“This sort of ‘quiet restriction’ helps kids learn to love the healthier option without feeling deprived,” Ms. Anderson said. “They will eventually learn about juice, soda, chocolate milk, sports drinks, and more, but you can let them learn about those foods at a slower pace when you rarely or don’t serve them at home.”
The researchers reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Sugary beverages – juice, soda, decadent lattes, sports drinks, and more – are the leading source of both calories and added sugar in the American diet, according to the Harvard T. H. Chan School of Public Health. But new research published in PLoS Medicine has found that
The new study, from researchers at the University of North Carolina at Chapel Hill, found that parents were 17% less likely to buy sugary drinks for their children when the beverages had the graphical – and graphic – warnings on the products.
The researchers turned a laboratory setting into a “minimart,” and parents were told to choose one drink and snack for their children, along with one household item (to disguise the purpose of the study).
Some parents were presented with sweetened drinks with images on the products reflecting type 2 diabetes and heart damage. Others were shown sugary drinks with a barcode label and no picture warning.
Forty-five percent of parents chose sugary drinks for their children when the products had no picture warning, but only 28% of parents chose sugary beverages with the cautionary images.
“When people make choices about what food to buy, they are juggling dozens of factors, like taste, cost, and advertising, and are looking at many products at once,” said Lindsey Smith Taillie, PhD, a nutrition researcher and the senior author of the paper.
“Showing that warnings can cut through the noise of everything else that’s happening in a food store is powerful evidence that they would help reduce sugary drink purchases in the real world.”
Children are particularly prone to overindulging on sugar, largely due to companies’ frequent marketing displays of pleasurable-looking and seemingly “thirst-quenching” sweet beverages.
Drink packaging also can be misleading.
Fruits and vegetables displayed on the front of many beverages often lead parents to buy what they believe are “healthy” options, when these drinks could be packed with sugar, according to a study published online Jan. 22 in the journal Appetite.
Parents are often “doing the best with what information they have,” so more education about nutrition, through picture warning labels, for example, would make a difference, said Caroline Fausel, a paleo food blogger, podcaster, and author of “Prep, Cook, Freeze: A Paleo Meal Planning Cookbook.”
Healthier choices on the rise
The American Beverage Association, an industry trade group, shared the current steps that major companies are taking to help lower Americans’ sugar intake.
Pepsi, Coca-Cola, and Keurig Dr Pepper joined forces in 2014 to create the Balance Calories Initiative, which aims to reduce beverage calories in the national diet.
Coca-Cola now offers 250 beverages with zero to low calories, and Keurig Dr Pepper has 158 products with 40 calories or less. Pepsi sells 7.5-ounce mini-cans, along with various other sizes, to encourage portion control.
“Beverage companies are fully transparent about the calories and sugar in our products, and we are offering more choices with less sugar than ever before,” William Dermody, vice president of media and public affairs for the American Beverage Association, said in a statement. “We agree that too much sugar is not good for anyone, and clear information about beverages is most helpful to consumers.”
Other big companies also are taking strides to lower sugar content in their products.
Kraft Heinz, which owns the popular line of Capri Sun drinks, has publicly shared its efforts to ramp up the nutritional value of its products.
The company has a goal to slash 60 million pounds of total sugar in Kraft Heinz products globally by 2025.
“As more people become aware of the harm that excessive sugar can cause in the body, my hope is that they continue to choose healthier alternatives,” Ms. Fausel said.
Creating new patterns
For children who consume sweetened juices and sodas regularly, the transition to healthier options might be challenging at first.
“Change can involve tantrums and unhappiness, and right now parents are at their max living pandemic parenting life,” said Jennifer Anderson, a registered dietitian and CEO of Kids Eat in Color, LLC, a resource for improving child nutrition and health through innovative education, meal plans, and tools. “Kids can get used to having sugary drinks, and they don’t want to give them up.”
One way to help make the switch is by having only water and milk as options while children are up and about, a technique that works particularly well for younger children, she said.
“This sort of ‘quiet restriction’ helps kids learn to love the healthier option without feeling deprived,” Ms. Anderson said. “They will eventually learn about juice, soda, chocolate milk, sports drinks, and more, but you can let them learn about those foods at a slower pace when you rarely or don’t serve them at home.”
The researchers reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Sugary beverages – juice, soda, decadent lattes, sports drinks, and more – are the leading source of both calories and added sugar in the American diet, according to the Harvard T. H. Chan School of Public Health. But new research published in PLoS Medicine has found that
The new study, from researchers at the University of North Carolina at Chapel Hill, found that parents were 17% less likely to buy sugary drinks for their children when the beverages had the graphical – and graphic – warnings on the products.
The researchers turned a laboratory setting into a “minimart,” and parents were told to choose one drink and snack for their children, along with one household item (to disguise the purpose of the study).
Some parents were presented with sweetened drinks with images on the products reflecting type 2 diabetes and heart damage. Others were shown sugary drinks with a barcode label and no picture warning.
Forty-five percent of parents chose sugary drinks for their children when the products had no picture warning, but only 28% of parents chose sugary beverages with the cautionary images.
“When people make choices about what food to buy, they are juggling dozens of factors, like taste, cost, and advertising, and are looking at many products at once,” said Lindsey Smith Taillie, PhD, a nutrition researcher and the senior author of the paper.
“Showing that warnings can cut through the noise of everything else that’s happening in a food store is powerful evidence that they would help reduce sugary drink purchases in the real world.”
Children are particularly prone to overindulging on sugar, largely due to companies’ frequent marketing displays of pleasurable-looking and seemingly “thirst-quenching” sweet beverages.
Drink packaging also can be misleading.
Fruits and vegetables displayed on the front of many beverages often lead parents to buy what they believe are “healthy” options, when these drinks could be packed with sugar, according to a study published online Jan. 22 in the journal Appetite.
Parents are often “doing the best with what information they have,” so more education about nutrition, through picture warning labels, for example, would make a difference, said Caroline Fausel, a paleo food blogger, podcaster, and author of “Prep, Cook, Freeze: A Paleo Meal Planning Cookbook.”
Healthier choices on the rise
The American Beverage Association, an industry trade group, shared the current steps that major companies are taking to help lower Americans’ sugar intake.
Pepsi, Coca-Cola, and Keurig Dr Pepper joined forces in 2014 to create the Balance Calories Initiative, which aims to reduce beverage calories in the national diet.
Coca-Cola now offers 250 beverages with zero to low calories, and Keurig Dr Pepper has 158 products with 40 calories or less. Pepsi sells 7.5-ounce mini-cans, along with various other sizes, to encourage portion control.
“Beverage companies are fully transparent about the calories and sugar in our products, and we are offering more choices with less sugar than ever before,” William Dermody, vice president of media and public affairs for the American Beverage Association, said in a statement. “We agree that too much sugar is not good for anyone, and clear information about beverages is most helpful to consumers.”
Other big companies also are taking strides to lower sugar content in their products.
Kraft Heinz, which owns the popular line of Capri Sun drinks, has publicly shared its efforts to ramp up the nutritional value of its products.
The company has a goal to slash 60 million pounds of total sugar in Kraft Heinz products globally by 2025.
“As more people become aware of the harm that excessive sugar can cause in the body, my hope is that they continue to choose healthier alternatives,” Ms. Fausel said.
Creating new patterns
For children who consume sweetened juices and sodas regularly, the transition to healthier options might be challenging at first.
“Change can involve tantrums and unhappiness, and right now parents are at their max living pandemic parenting life,” said Jennifer Anderson, a registered dietitian and CEO of Kids Eat in Color, LLC, a resource for improving child nutrition and health through innovative education, meal plans, and tools. “Kids can get used to having sugary drinks, and they don’t want to give them up.”
One way to help make the switch is by having only water and milk as options while children are up and about, a technique that works particularly well for younger children, she said.
“This sort of ‘quiet restriction’ helps kids learn to love the healthier option without feeling deprived,” Ms. Anderson said. “They will eventually learn about juice, soda, chocolate milk, sports drinks, and more, but you can let them learn about those foods at a slower pace when you rarely or don’t serve them at home.”
The researchers reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM PLOS MEDICINE
Absolute increase in Kawasaki CV risk remains small in long-term follow-up
Vasculitis of the coronary arteries is a well-recognized acute complication of Kawasaki disease, but the long-term risk of cardiovascular (CV) sequelae does not appear to be clinically meaningful for most patients, according to results from an analysis of data presented at the annual meeting of the Canadian Rheumatology Association.
For patients and parents, these data provide “a message of reassurance,” according to Jennifer J.Y. Lee, MD, a pediatric rheumatologist affiliated with the Hospital for Sick Children, Toronto.
The long-term outcomes were characterized as reassuring even though rates of hypertension, major adverse cardiac events (MACE), and death from CV events were higher in patients with Kawasaki disease relative to controls in a retrospective data-linkage study. In fact, these differences were highly statistically significant, but the absolute differences were extremely small.
For this analysis, the 1,174 patients diagnosed with Kawasaki disease at Dr. Lee’s institution between 1991 and 2008 were compared in a 10:1 ratio to 11,740 controls matched for factors such as age, sex, ethnicity, and geographic region. The median follow-up period was 20 years, and the maximum was 28 years.
Adjusted CV risks are significant
In an adjusted Cox proportional hazard ratio model, patients in the Kawasaki group had a more than twofold increase in risk for hypertension (aHR, 2.3; P < .0001) and all-cause mortality (aHR, 2.5; P = .009). They also had more than a 10-fold increase in risk for MACE (aHR, 10.3; P < .0001).
These statistics belie the clinical relevance, according to Dr. Lee. Because of the very low rates of all the measured events in both groups, there was just one more case of hypertension per 1,250 patient-years of follow-up, one more case of MACE per 833 patient-years of follow-up, and one more death for 3,846 patient years of follow-up.
Moreover, when these outcomes were graphed over time, most events occurred during the acute period or in the initial years of follow-up.
“There was not a constant increase in risk of these outcomes over time for patients with Kawasaki disease relative to the controls,” Dr. Lee reported. “The long-term prognosis for Kawasaki patients remains favorable.”
European group reports similar results
Similar results from a single-center experience were published 3 years ago. In that study, 207 Kawasaki patients treated at the University of Lausanne (Switzerland) were followed for 30 years. Complications after the acute phase were characterized as “rare.”
For example, only three patients (1.4%) had a subsequent episode of myocardial ischemia. All three had developed a coronary aneurysm during the acute phase of Kawasaki disease. The authors of that study reported that children who had not received immunoglobulins during the acute phase or who developed Kawasaki disease outside of the usual age range were more likely to have subsequent events, such as disease recurrence.
Other studies of long-term CV outcomes in patients with Kawasaki disease generally show similar data, according to James T. Gaensbauer, MD, a pediatric infectious disease specialist at the Mayo Clinic, Rochester, Minn.
“I generally agree with the premise that major complications are rare when you compare a cohort of patients with Kawasaki disease with the general population,” Dr. Gaensbauer said. However, he added, “I do not think you can say no one needs to worry.”
Severity of acute disease might matter
During the acute phase of Kawasaki disease, the arterial damage varies. As suggested in the University of Lausanne follow-up, patients with significant coronary aneurysms do appear to be at greater risk of long-term complications. Dr. Gaensbauer cited a statement from the American Heart Association that noted a higher risk of CV sequelae from Kawasaki disease with a greater or more severe coronary aneurysm or in the face of other evidence of damage to the arterial tree.
“The clinical course within the first 2 years of Kawasaki disease appears to be important for risk of CV complications after this time,” Dr. Gaensbauer said.
The absolute risk of CV events in patients with a more complicated acute course of Kawasaki disease remains incompletely understood, but Dr. Gaensbauer said that there are several sets of data, including these new data from the Hospital for Sick Children, that suggest that the overall prognosis is good. However, he cautioned that this reassurance does not necessarily apply to children with a difficult acute course.
According to the 2017 AHA statement on Kawasaki disease, risk stratification based on echocardiography and other measures after the acute phase of Kawasaki disease are reasonable to determine if long-term follow-up is needed. In those without abnormalities, it is reasonable to forgo further cardiology assessment.
Dr. Lee and Dr. Gaensbauer reported having no potential conflicts of interest.
Vasculitis of the coronary arteries is a well-recognized acute complication of Kawasaki disease, but the long-term risk of cardiovascular (CV) sequelae does not appear to be clinically meaningful for most patients, according to results from an analysis of data presented at the annual meeting of the Canadian Rheumatology Association.
For patients and parents, these data provide “a message of reassurance,” according to Jennifer J.Y. Lee, MD, a pediatric rheumatologist affiliated with the Hospital for Sick Children, Toronto.
The long-term outcomes were characterized as reassuring even though rates of hypertension, major adverse cardiac events (MACE), and death from CV events were higher in patients with Kawasaki disease relative to controls in a retrospective data-linkage study. In fact, these differences were highly statistically significant, but the absolute differences were extremely small.
For this analysis, the 1,174 patients diagnosed with Kawasaki disease at Dr. Lee’s institution between 1991 and 2008 were compared in a 10:1 ratio to 11,740 controls matched for factors such as age, sex, ethnicity, and geographic region. The median follow-up period was 20 years, and the maximum was 28 years.
Adjusted CV risks are significant
In an adjusted Cox proportional hazard ratio model, patients in the Kawasaki group had a more than twofold increase in risk for hypertension (aHR, 2.3; P < .0001) and all-cause mortality (aHR, 2.5; P = .009). They also had more than a 10-fold increase in risk for MACE (aHR, 10.3; P < .0001).
These statistics belie the clinical relevance, according to Dr. Lee. Because of the very low rates of all the measured events in both groups, there was just one more case of hypertension per 1,250 patient-years of follow-up, one more case of MACE per 833 patient-years of follow-up, and one more death for 3,846 patient years of follow-up.
Moreover, when these outcomes were graphed over time, most events occurred during the acute period or in the initial years of follow-up.
“There was not a constant increase in risk of these outcomes over time for patients with Kawasaki disease relative to the controls,” Dr. Lee reported. “The long-term prognosis for Kawasaki patients remains favorable.”
European group reports similar results
Similar results from a single-center experience were published 3 years ago. In that study, 207 Kawasaki patients treated at the University of Lausanne (Switzerland) were followed for 30 years. Complications after the acute phase were characterized as “rare.”
For example, only three patients (1.4%) had a subsequent episode of myocardial ischemia. All three had developed a coronary aneurysm during the acute phase of Kawasaki disease. The authors of that study reported that children who had not received immunoglobulins during the acute phase or who developed Kawasaki disease outside of the usual age range were more likely to have subsequent events, such as disease recurrence.
Other studies of long-term CV outcomes in patients with Kawasaki disease generally show similar data, according to James T. Gaensbauer, MD, a pediatric infectious disease specialist at the Mayo Clinic, Rochester, Minn.
“I generally agree with the premise that major complications are rare when you compare a cohort of patients with Kawasaki disease with the general population,” Dr. Gaensbauer said. However, he added, “I do not think you can say no one needs to worry.”
Severity of acute disease might matter
During the acute phase of Kawasaki disease, the arterial damage varies. As suggested in the University of Lausanne follow-up, patients with significant coronary aneurysms do appear to be at greater risk of long-term complications. Dr. Gaensbauer cited a statement from the American Heart Association that noted a higher risk of CV sequelae from Kawasaki disease with a greater or more severe coronary aneurysm or in the face of other evidence of damage to the arterial tree.
“The clinical course within the first 2 years of Kawasaki disease appears to be important for risk of CV complications after this time,” Dr. Gaensbauer said.
The absolute risk of CV events in patients with a more complicated acute course of Kawasaki disease remains incompletely understood, but Dr. Gaensbauer said that there are several sets of data, including these new data from the Hospital for Sick Children, that suggest that the overall prognosis is good. However, he cautioned that this reassurance does not necessarily apply to children with a difficult acute course.
According to the 2017 AHA statement on Kawasaki disease, risk stratification based on echocardiography and other measures after the acute phase of Kawasaki disease are reasonable to determine if long-term follow-up is needed. In those without abnormalities, it is reasonable to forgo further cardiology assessment.
Dr. Lee and Dr. Gaensbauer reported having no potential conflicts of interest.
Vasculitis of the coronary arteries is a well-recognized acute complication of Kawasaki disease, but the long-term risk of cardiovascular (CV) sequelae does not appear to be clinically meaningful for most patients, according to results from an analysis of data presented at the annual meeting of the Canadian Rheumatology Association.
For patients and parents, these data provide “a message of reassurance,” according to Jennifer J.Y. Lee, MD, a pediatric rheumatologist affiliated with the Hospital for Sick Children, Toronto.
The long-term outcomes were characterized as reassuring even though rates of hypertension, major adverse cardiac events (MACE), and death from CV events were higher in patients with Kawasaki disease relative to controls in a retrospective data-linkage study. In fact, these differences were highly statistically significant, but the absolute differences were extremely small.
For this analysis, the 1,174 patients diagnosed with Kawasaki disease at Dr. Lee’s institution between 1991 and 2008 were compared in a 10:1 ratio to 11,740 controls matched for factors such as age, sex, ethnicity, and geographic region. The median follow-up period was 20 years, and the maximum was 28 years.
Adjusted CV risks are significant
In an adjusted Cox proportional hazard ratio model, patients in the Kawasaki group had a more than twofold increase in risk for hypertension (aHR, 2.3; P < .0001) and all-cause mortality (aHR, 2.5; P = .009). They also had more than a 10-fold increase in risk for MACE (aHR, 10.3; P < .0001).
These statistics belie the clinical relevance, according to Dr. Lee. Because of the very low rates of all the measured events in both groups, there was just one more case of hypertension per 1,250 patient-years of follow-up, one more case of MACE per 833 patient-years of follow-up, and one more death for 3,846 patient years of follow-up.
Moreover, when these outcomes were graphed over time, most events occurred during the acute period or in the initial years of follow-up.
“There was not a constant increase in risk of these outcomes over time for patients with Kawasaki disease relative to the controls,” Dr. Lee reported. “The long-term prognosis for Kawasaki patients remains favorable.”
European group reports similar results
Similar results from a single-center experience were published 3 years ago. In that study, 207 Kawasaki patients treated at the University of Lausanne (Switzerland) were followed for 30 years. Complications after the acute phase were characterized as “rare.”
For example, only three patients (1.4%) had a subsequent episode of myocardial ischemia. All three had developed a coronary aneurysm during the acute phase of Kawasaki disease. The authors of that study reported that children who had not received immunoglobulins during the acute phase or who developed Kawasaki disease outside of the usual age range were more likely to have subsequent events, such as disease recurrence.
Other studies of long-term CV outcomes in patients with Kawasaki disease generally show similar data, according to James T. Gaensbauer, MD, a pediatric infectious disease specialist at the Mayo Clinic, Rochester, Minn.
“I generally agree with the premise that major complications are rare when you compare a cohort of patients with Kawasaki disease with the general population,” Dr. Gaensbauer said. However, he added, “I do not think you can say no one needs to worry.”
Severity of acute disease might matter
During the acute phase of Kawasaki disease, the arterial damage varies. As suggested in the University of Lausanne follow-up, patients with significant coronary aneurysms do appear to be at greater risk of long-term complications. Dr. Gaensbauer cited a statement from the American Heart Association that noted a higher risk of CV sequelae from Kawasaki disease with a greater or more severe coronary aneurysm or in the face of other evidence of damage to the arterial tree.
“The clinical course within the first 2 years of Kawasaki disease appears to be important for risk of CV complications after this time,” Dr. Gaensbauer said.
The absolute risk of CV events in patients with a more complicated acute course of Kawasaki disease remains incompletely understood, but Dr. Gaensbauer said that there are several sets of data, including these new data from the Hospital for Sick Children, that suggest that the overall prognosis is good. However, he cautioned that this reassurance does not necessarily apply to children with a difficult acute course.
According to the 2017 AHA statement on Kawasaki disease, risk stratification based on echocardiography and other measures after the acute phase of Kawasaki disease are reasonable to determine if long-term follow-up is needed. In those without abnormalities, it is reasonable to forgo further cardiology assessment.
Dr. Lee and Dr. Gaensbauer reported having no potential conflicts of interest.
FROM THE ANNUAL MEETING OF THE CANADIAN RHEUMATOLOGY ASSOCIATION
Q&A: Long COVID symptoms, management, and where we’re headed
Long COVID continues to be a moving target – continuously evolving and still surprising doctors and patients who have sometimes incapacitating long-term symptoms.
Little about the disorder seems predictable at this point. People can have long COVID after asymptomatic, mild, or severe COVID-19, for example. And when a person gets long COVID – also known as long-haul COVID – symptoms can vary widely.
To address all the uncertainty, the New York State Department of Health gathered experts in primary care, pediatrics, physical medicine, rehabilitation, and pulmonology to answer some pressing questions.
New York in 2020 was the first epicenter of the pandemic in the United States, making it also the center of the long COVID epidemic, says Emily Lutterloh, MD, director of the Division of Epidemiology at the New York State Department of Health.
What do you do when you’re seeing a patient with long COVID for the first time?
The first exam varies because there are so many different ways long COVID presents itself, says Benjamin Abramoff, MD, a physical medicine and rehabilitation specialist at Penn Medicine in Philadelphia.
I’ve now been seriously ill with #LongCovid for 11 months. I was never hospitalized. I didn’t even have a “mild” covid case. Instead, I developed Long Covid from an asymptomatic infection.
I’m far from unique. Up to 1/5 of asymptomatic patients go on to have long-term symptoms.
— Ravi Veriah Jacques (@RaviHVJ) February 3, 2022
Assessing their previous and current care also helps to direct their ongoing management, says Zijian Chen, MD, medical director of the Center for Post-COVID Care at Mount Sinai Health System in New York.
Can vaccination help people with long COVID?
Anything that we can do to help prevent people from being critically ill or being hospitalized with COVID-19 is helpful to prevent long COVID, says Dr. Abramoff, who is also director of the long COVID clinic at the University of Pennsylvania, Philadelphia.
“So that’s something I always discuss with patients. In some research, sometimes patients do feel better after the vaccine,” he says.
What kind of therapies do you find helpful for your patients?
Rehabilitation is a key part of recovery from long COVID, Dr. Abramoff says. “It is very important to make this very patient-specific.”
“We have patients that are working. They’re already going to the gym in some cases but don’t feel like they have the same endurance,” he says. “And then we have patients who are so crippled by their fatigue that they can’t get out of bed.”
1/ What is #LongCOVID?!
A disabling malady from ongoing inflammation, autoimmunity, & potential viral reservoirs (GI, brain?)
NEW DATA: The Lungs “light up” on special MRI Scans 3 to 9 months later in patients never hospitalized for COVID.https://t.co/I2kyZ4cK5F pic.twitter.com/dL1P67L2DK
— WesElyMD (@WesElyMD) February 2, 2022
An exercise program can help people who have long COVID.
“There’s a big role for therapy services in the recovery of these patients,” says John Baratta, MD, of the department of physical medicine and rehabilitation at the University of North Carolina at Chapel Hill.
But the limited number of long COVID clinics can mean some people are unable to get to therapists trained on the needs of patients with lingering COVID symptoms. Educating community physical and occupational therapists is one solution.
How long does it take for people with long COVID to recover and get back to 100% if they can?
Specific numbers aren’t really available, Dr. Baratta says.
“But I can tell you the general trend that I see is that a lot of patients have a gradual improvement of symptoms. The slow but steady improvement with time may be the body’s natural healing process, a result of medical interventions, or both.”
It can help to reassure people with long COVID that they will not be discharged from care until they feel they’ve maximized their health, says Sharagim Kemp, DO, medical director of the COVID Recovery Program for Nuvance Health, a health system in New York and Connecticut.
It’s essential to set realistic recovery expectations and tell patients that not everyone will return to 100% of their pre-COVID functioning, she says.
“Once we are able to help them reset their expectations, there’s almost an accelerated recovery because they are not putting that pressure on themselves anymore,” Dr. Kemp says.
What are the most common symptoms you’re seeing in long COVID?
It’s helpful to think of long COVID as a very broad umbrella term, Dr. Abramoff says.
Echoing what many others have observed, fatigue, cognitive dysfunction or “brain fog,“ and shortness of breath or troubled breathing appear to be the most common symptoms, he says.
Some reported vague symptoms, Dr. Kemp says.
People may go to the doctor “not even realizing that they had COVID. That’s one of the important points here – to have a high index of suspicion for patients who come in with multiple symptoms,” she says.
For this reason, patients can report symptoms that don’t necessarily fit into any specialty, says Sarah J. Ryan, MD, an internal medicine doctor at Columbia University Irving Medical Center in New York. People say they are “just not themselves” or they are tired after their COVID-19 recovery.
Is there a connection between severe COVID cases and severe long COVID?
“It’s not like that at all. I would say that more than 80% of the patients that we see had mild to moderate illness and they were not hospitalized,” Dr. Baratta says.
Long COVID is a bit different in children and teenagers, says Ixsy Ramirez, MD, a pediatric pulmonologist at University of Michigan Health, Ann Arbor. Most patients in the long COVID clinic at the University of Michigan were previously healthy, and not children with asthma or other lung conditions as one might expect. In fact, many are student athletes, or were before they had long COVID.
In this population, shortness of breath is most common, followed by chest pain and fatigue. Unfortunately, the symptoms are so serious for many kids that their performance is limited, even if they can return to competitive play.
Are there defined criteria you use to diagnose long COVID? How do you give someone a diagnosis?
That’s an ever-evolving question, Dr. Kemp says. The generally accepted definition centers on persistent or new symptoms 4 weeks or more after the original COVID-19 illness, but there are exceptions.
Researchers are working on lab tests to help confirm the diagnosis. But without a definitive blood biomarker, getting to the diagnosis requires “some thorough detective work,” Dr. Ryan says.
Do you bring in mental health providers to help with treatment?
“We focus on mental health quite a bit actually,” says, Dr. Chen, cofounder of his institution’s COVID recovery clinic. Mount Sinai offers one-on-one and group mental health services, for example.
“Personally, I’ve seen patients that I did not expect to have such severe mental health changes” with long COVID.
One of the most powerful accounts and testimonies I have seen on what most #LongCovid patients experience when interacting with their doctors.
“I did not fit in a box, so they chose not to see me, even worse they made me feel like it was my fault for not fitting in their box” pic.twitter.com/7GQLBucuO5
— charlos (@loscharlos) February 3, 2022
Examples include severe depression, cases of acute psychosis, hallucinations, and other problems “that are really unexpected after a viral illness.”
Stony Brook University Hospital in New York has a long COVID clinic staffed by multiple primary care doctors who do exams and refer patients to services. A bonus of offering psychological services to all post-COVID patients is doctors get a more complete picture of each person and a better understanding of what they are going through, says Abigail Chua, MD, a pulmonologist at Stony Brook.
Some empathy is essential, Dr. Baratta says. “It’s important to recognize that a lot of these patients present with a sense of grief or loss for their prior life.”
What does the future hold?
A simple test to diagnose long COVID, combined with an effective treatment that helps people feel better within a week, would be ideal, Dr. Abramoff says.
“That would be lovely. But you know, we’re just not at that point.”
And it would be helpful to start identifying subtypes of long COVID so diagnosis and treatment can be more targeted, Dr. Abramoff says. Otherwise, “It’s going to be a very challenging approach to try to treat all of our patients with long COVID symptoms the same way.”
Good clinical trials likewise are needed to address all the subtleties of long COVID.
A number of long COVID centers are collaborating on research to find out more, Dr. Chen says. Actions include setting up a bank of tissue samples from people with long COVID so researchers can continue to figure out the condition.
One goal, Dr. Chen says, would be the ability to treat long COVID rather than just its symptoms.
Long COVID emphasizes the need to prevent people from getting COVID in the first place, Dr. Ramirez says. This will continue to be important, particularly when some people dismiss the seriousness of COVID, comparing it to a cold if they get it. That attitude discounts the large number of people who unfortunately go on to develop long-term, often debilitating, symptoms.
A version of this article first appeared on WebMD.com.
Long COVID continues to be a moving target – continuously evolving and still surprising doctors and patients who have sometimes incapacitating long-term symptoms.
Little about the disorder seems predictable at this point. People can have long COVID after asymptomatic, mild, or severe COVID-19, for example. And when a person gets long COVID – also known as long-haul COVID – symptoms can vary widely.
To address all the uncertainty, the New York State Department of Health gathered experts in primary care, pediatrics, physical medicine, rehabilitation, and pulmonology to answer some pressing questions.
New York in 2020 was the first epicenter of the pandemic in the United States, making it also the center of the long COVID epidemic, says Emily Lutterloh, MD, director of the Division of Epidemiology at the New York State Department of Health.
What do you do when you’re seeing a patient with long COVID for the first time?
The first exam varies because there are so many different ways long COVID presents itself, says Benjamin Abramoff, MD, a physical medicine and rehabilitation specialist at Penn Medicine in Philadelphia.
I’ve now been seriously ill with #LongCovid for 11 months. I was never hospitalized. I didn’t even have a “mild” covid case. Instead, I developed Long Covid from an asymptomatic infection.
I’m far from unique. Up to 1/5 of asymptomatic patients go on to have long-term symptoms.
— Ravi Veriah Jacques (@RaviHVJ) February 3, 2022
Assessing their previous and current care also helps to direct their ongoing management, says Zijian Chen, MD, medical director of the Center for Post-COVID Care at Mount Sinai Health System in New York.
Can vaccination help people with long COVID?
Anything that we can do to help prevent people from being critically ill or being hospitalized with COVID-19 is helpful to prevent long COVID, says Dr. Abramoff, who is also director of the long COVID clinic at the University of Pennsylvania, Philadelphia.
“So that’s something I always discuss with patients. In some research, sometimes patients do feel better after the vaccine,” he says.
What kind of therapies do you find helpful for your patients?
Rehabilitation is a key part of recovery from long COVID, Dr. Abramoff says. “It is very important to make this very patient-specific.”
“We have patients that are working. They’re already going to the gym in some cases but don’t feel like they have the same endurance,” he says. “And then we have patients who are so crippled by their fatigue that they can’t get out of bed.”
1/ What is #LongCOVID?!
A disabling malady from ongoing inflammation, autoimmunity, & potential viral reservoirs (GI, brain?)
NEW DATA: The Lungs “light up” on special MRI Scans 3 to 9 months later in patients never hospitalized for COVID.https://t.co/I2kyZ4cK5F pic.twitter.com/dL1P67L2DK
— WesElyMD (@WesElyMD) February 2, 2022
An exercise program can help people who have long COVID.
“There’s a big role for therapy services in the recovery of these patients,” says John Baratta, MD, of the department of physical medicine and rehabilitation at the University of North Carolina at Chapel Hill.
But the limited number of long COVID clinics can mean some people are unable to get to therapists trained on the needs of patients with lingering COVID symptoms. Educating community physical and occupational therapists is one solution.
How long does it take for people with long COVID to recover and get back to 100% if they can?
Specific numbers aren’t really available, Dr. Baratta says.
“But I can tell you the general trend that I see is that a lot of patients have a gradual improvement of symptoms. The slow but steady improvement with time may be the body’s natural healing process, a result of medical interventions, or both.”
It can help to reassure people with long COVID that they will not be discharged from care until they feel they’ve maximized their health, says Sharagim Kemp, DO, medical director of the COVID Recovery Program for Nuvance Health, a health system in New York and Connecticut.
It’s essential to set realistic recovery expectations and tell patients that not everyone will return to 100% of their pre-COVID functioning, she says.
“Once we are able to help them reset their expectations, there’s almost an accelerated recovery because they are not putting that pressure on themselves anymore,” Dr. Kemp says.
What are the most common symptoms you’re seeing in long COVID?
It’s helpful to think of long COVID as a very broad umbrella term, Dr. Abramoff says.
Echoing what many others have observed, fatigue, cognitive dysfunction or “brain fog,“ and shortness of breath or troubled breathing appear to be the most common symptoms, he says.
Some reported vague symptoms, Dr. Kemp says.
People may go to the doctor “not even realizing that they had COVID. That’s one of the important points here – to have a high index of suspicion for patients who come in with multiple symptoms,” she says.
For this reason, patients can report symptoms that don’t necessarily fit into any specialty, says Sarah J. Ryan, MD, an internal medicine doctor at Columbia University Irving Medical Center in New York. People say they are “just not themselves” or they are tired after their COVID-19 recovery.
Is there a connection between severe COVID cases and severe long COVID?
“It’s not like that at all. I would say that more than 80% of the patients that we see had mild to moderate illness and they were not hospitalized,” Dr. Baratta says.
Long COVID is a bit different in children and teenagers, says Ixsy Ramirez, MD, a pediatric pulmonologist at University of Michigan Health, Ann Arbor. Most patients in the long COVID clinic at the University of Michigan were previously healthy, and not children with asthma or other lung conditions as one might expect. In fact, many are student athletes, or were before they had long COVID.
In this population, shortness of breath is most common, followed by chest pain and fatigue. Unfortunately, the symptoms are so serious for many kids that their performance is limited, even if they can return to competitive play.
Are there defined criteria you use to diagnose long COVID? How do you give someone a diagnosis?
That’s an ever-evolving question, Dr. Kemp says. The generally accepted definition centers on persistent or new symptoms 4 weeks or more after the original COVID-19 illness, but there are exceptions.
Researchers are working on lab tests to help confirm the diagnosis. But without a definitive blood biomarker, getting to the diagnosis requires “some thorough detective work,” Dr. Ryan says.
Do you bring in mental health providers to help with treatment?
“We focus on mental health quite a bit actually,” says, Dr. Chen, cofounder of his institution’s COVID recovery clinic. Mount Sinai offers one-on-one and group mental health services, for example.
“Personally, I’ve seen patients that I did not expect to have such severe mental health changes” with long COVID.
One of the most powerful accounts and testimonies I have seen on what most #LongCovid patients experience when interacting with their doctors.
“I did not fit in a box, so they chose not to see me, even worse they made me feel like it was my fault for not fitting in their box” pic.twitter.com/7GQLBucuO5
— charlos (@loscharlos) February 3, 2022
Examples include severe depression, cases of acute psychosis, hallucinations, and other problems “that are really unexpected after a viral illness.”
Stony Brook University Hospital in New York has a long COVID clinic staffed by multiple primary care doctors who do exams and refer patients to services. A bonus of offering psychological services to all post-COVID patients is doctors get a more complete picture of each person and a better understanding of what they are going through, says Abigail Chua, MD, a pulmonologist at Stony Brook.
Some empathy is essential, Dr. Baratta says. “It’s important to recognize that a lot of these patients present with a sense of grief or loss for their prior life.”
What does the future hold?
A simple test to diagnose long COVID, combined with an effective treatment that helps people feel better within a week, would be ideal, Dr. Abramoff says.
“That would be lovely. But you know, we’re just not at that point.”
And it would be helpful to start identifying subtypes of long COVID so diagnosis and treatment can be more targeted, Dr. Abramoff says. Otherwise, “It’s going to be a very challenging approach to try to treat all of our patients with long COVID symptoms the same way.”
Good clinical trials likewise are needed to address all the subtleties of long COVID.
A number of long COVID centers are collaborating on research to find out more, Dr. Chen says. Actions include setting up a bank of tissue samples from people with long COVID so researchers can continue to figure out the condition.
One goal, Dr. Chen says, would be the ability to treat long COVID rather than just its symptoms.
Long COVID emphasizes the need to prevent people from getting COVID in the first place, Dr. Ramirez says. This will continue to be important, particularly when some people dismiss the seriousness of COVID, comparing it to a cold if they get it. That attitude discounts the large number of people who unfortunately go on to develop long-term, often debilitating, symptoms.
A version of this article first appeared on WebMD.com.
Long COVID continues to be a moving target – continuously evolving and still surprising doctors and patients who have sometimes incapacitating long-term symptoms.
Little about the disorder seems predictable at this point. People can have long COVID after asymptomatic, mild, or severe COVID-19, for example. And when a person gets long COVID – also known as long-haul COVID – symptoms can vary widely.
To address all the uncertainty, the New York State Department of Health gathered experts in primary care, pediatrics, physical medicine, rehabilitation, and pulmonology to answer some pressing questions.
New York in 2020 was the first epicenter of the pandemic in the United States, making it also the center of the long COVID epidemic, says Emily Lutterloh, MD, director of the Division of Epidemiology at the New York State Department of Health.
What do you do when you’re seeing a patient with long COVID for the first time?
The first exam varies because there are so many different ways long COVID presents itself, says Benjamin Abramoff, MD, a physical medicine and rehabilitation specialist at Penn Medicine in Philadelphia.
I’ve now been seriously ill with #LongCovid for 11 months. I was never hospitalized. I didn’t even have a “mild” covid case. Instead, I developed Long Covid from an asymptomatic infection.
I’m far from unique. Up to 1/5 of asymptomatic patients go on to have long-term symptoms.
— Ravi Veriah Jacques (@RaviHVJ) February 3, 2022
Assessing their previous and current care also helps to direct their ongoing management, says Zijian Chen, MD, medical director of the Center for Post-COVID Care at Mount Sinai Health System in New York.
Can vaccination help people with long COVID?
Anything that we can do to help prevent people from being critically ill or being hospitalized with COVID-19 is helpful to prevent long COVID, says Dr. Abramoff, who is also director of the long COVID clinic at the University of Pennsylvania, Philadelphia.
“So that’s something I always discuss with patients. In some research, sometimes patients do feel better after the vaccine,” he says.
What kind of therapies do you find helpful for your patients?
Rehabilitation is a key part of recovery from long COVID, Dr. Abramoff says. “It is very important to make this very patient-specific.”
“We have patients that are working. They’re already going to the gym in some cases but don’t feel like they have the same endurance,” he says. “And then we have patients who are so crippled by their fatigue that they can’t get out of bed.”
1/ What is #LongCOVID?!
A disabling malady from ongoing inflammation, autoimmunity, & potential viral reservoirs (GI, brain?)
NEW DATA: The Lungs “light up” on special MRI Scans 3 to 9 months later in patients never hospitalized for COVID.https://t.co/I2kyZ4cK5F pic.twitter.com/dL1P67L2DK
— WesElyMD (@WesElyMD) February 2, 2022
An exercise program can help people who have long COVID.
“There’s a big role for therapy services in the recovery of these patients,” says John Baratta, MD, of the department of physical medicine and rehabilitation at the University of North Carolina at Chapel Hill.
But the limited number of long COVID clinics can mean some people are unable to get to therapists trained on the needs of patients with lingering COVID symptoms. Educating community physical and occupational therapists is one solution.
How long does it take for people with long COVID to recover and get back to 100% if they can?
Specific numbers aren’t really available, Dr. Baratta says.
“But I can tell you the general trend that I see is that a lot of patients have a gradual improvement of symptoms. The slow but steady improvement with time may be the body’s natural healing process, a result of medical interventions, or both.”
It can help to reassure people with long COVID that they will not be discharged from care until they feel they’ve maximized their health, says Sharagim Kemp, DO, medical director of the COVID Recovery Program for Nuvance Health, a health system in New York and Connecticut.
It’s essential to set realistic recovery expectations and tell patients that not everyone will return to 100% of their pre-COVID functioning, she says.
“Once we are able to help them reset their expectations, there’s almost an accelerated recovery because they are not putting that pressure on themselves anymore,” Dr. Kemp says.
What are the most common symptoms you’re seeing in long COVID?
It’s helpful to think of long COVID as a very broad umbrella term, Dr. Abramoff says.
Echoing what many others have observed, fatigue, cognitive dysfunction or “brain fog,“ and shortness of breath or troubled breathing appear to be the most common symptoms, he says.
Some reported vague symptoms, Dr. Kemp says.
People may go to the doctor “not even realizing that they had COVID. That’s one of the important points here – to have a high index of suspicion for patients who come in with multiple symptoms,” she says.
For this reason, patients can report symptoms that don’t necessarily fit into any specialty, says Sarah J. Ryan, MD, an internal medicine doctor at Columbia University Irving Medical Center in New York. People say they are “just not themselves” or they are tired after their COVID-19 recovery.
Is there a connection between severe COVID cases and severe long COVID?
“It’s not like that at all. I would say that more than 80% of the patients that we see had mild to moderate illness and they were not hospitalized,” Dr. Baratta says.
Long COVID is a bit different in children and teenagers, says Ixsy Ramirez, MD, a pediatric pulmonologist at University of Michigan Health, Ann Arbor. Most patients in the long COVID clinic at the University of Michigan were previously healthy, and not children with asthma or other lung conditions as one might expect. In fact, many are student athletes, or were before they had long COVID.
In this population, shortness of breath is most common, followed by chest pain and fatigue. Unfortunately, the symptoms are so serious for many kids that their performance is limited, even if they can return to competitive play.
Are there defined criteria you use to diagnose long COVID? How do you give someone a diagnosis?
That’s an ever-evolving question, Dr. Kemp says. The generally accepted definition centers on persistent or new symptoms 4 weeks or more after the original COVID-19 illness, but there are exceptions.
Researchers are working on lab tests to help confirm the diagnosis. But without a definitive blood biomarker, getting to the diagnosis requires “some thorough detective work,” Dr. Ryan says.
Do you bring in mental health providers to help with treatment?
“We focus on mental health quite a bit actually,” says, Dr. Chen, cofounder of his institution’s COVID recovery clinic. Mount Sinai offers one-on-one and group mental health services, for example.
“Personally, I’ve seen patients that I did not expect to have such severe mental health changes” with long COVID.
One of the most powerful accounts and testimonies I have seen on what most #LongCovid patients experience when interacting with their doctors.
“I did not fit in a box, so they chose not to see me, even worse they made me feel like it was my fault for not fitting in their box” pic.twitter.com/7GQLBucuO5
— charlos (@loscharlos) February 3, 2022
Examples include severe depression, cases of acute psychosis, hallucinations, and other problems “that are really unexpected after a viral illness.”
Stony Brook University Hospital in New York has a long COVID clinic staffed by multiple primary care doctors who do exams and refer patients to services. A bonus of offering psychological services to all post-COVID patients is doctors get a more complete picture of each person and a better understanding of what they are going through, says Abigail Chua, MD, a pulmonologist at Stony Brook.
Some empathy is essential, Dr. Baratta says. “It’s important to recognize that a lot of these patients present with a sense of grief or loss for their prior life.”
What does the future hold?
A simple test to diagnose long COVID, combined with an effective treatment that helps people feel better within a week, would be ideal, Dr. Abramoff says.
“That would be lovely. But you know, we’re just not at that point.”
And it would be helpful to start identifying subtypes of long COVID so diagnosis and treatment can be more targeted, Dr. Abramoff says. Otherwise, “It’s going to be a very challenging approach to try to treat all of our patients with long COVID symptoms the same way.”
Good clinical trials likewise are needed to address all the subtleties of long COVID.
A number of long COVID centers are collaborating on research to find out more, Dr. Chen says. Actions include setting up a bank of tissue samples from people with long COVID so researchers can continue to figure out the condition.
One goal, Dr. Chen says, would be the ability to treat long COVID rather than just its symptoms.
Long COVID emphasizes the need to prevent people from getting COVID in the first place, Dr. Ramirez says. This will continue to be important, particularly when some people dismiss the seriousness of COVID, comparing it to a cold if they get it. That attitude discounts the large number of people who unfortunately go on to develop long-term, often debilitating, symptoms.
A version of this article first appeared on WebMD.com.
Two emerging drugs exacerbating opioid crisis
Two illicit drugs are contributing to a sharp rise in fentanyl-related deaths, a new study from the Centers for Disease Control and Prevention shows.
Para-fluorofentanyl, a schedule I substance often found in heroin packets and counterfeit pills, is making a comeback on the illicit drug market, Jordan Trecki, PhD, and associates reported in the Centers for Disease Control and Prevention’s Morbidity and Mortality Weekly Report (2022 Jan 28;71[4]:153-5). U.S. medical examiner reports and national law enforcement seizure data point to a rise in encounters of this drug along with metonitazene, a benzimidazole-opioid, in combination with fentanyl.
On their own, para-fluorofentanyl and metonitazene can kill the user through respiratory depression. Combinations of these substances and other opioids, including fentanyl-related compounds or adulterants, “pose an even greater potential harm to the patient than previously observed,” reported Dr. Trecki, a pharmacologist affiliated with the Drug Enforcement Administration, and colleagues.
Opioids contribute to about 75% of all U.S. drug overdose deaths, which rose by 28.5% during 2020-2021, according to the National Center for Health Statistics. And fentanyl is replacing heroin as the primary drug of use, said addiction specialist Brian Fuehrlein, MD, PhD, in an interview.
“For patients with stimulant use disorder and even cannabis use disorder, fentanyl is becoming more and more common as an adulterant in those substances, often resulting in inadvertent use. Hence, fentanyl and fentanyl-like drugs and fentanyl analogues are becoming increasingly common and important,” said Dr. Fuehrlein, director of the psychiatric emergency room at the VA Connecticut Healthcare System. He was not involved with the MMWR study.
Tennessee data reflect national problem
Recent data from a medical examiner in Knoxville, Tenn., illustrate what might be happening nationwide with those two emerging substances.
Over the last 2 years, the Knox County Regional Forensic Center has identified para-fluorofentanyl in the toxicology results of drug overdose victims, and metonitazene – either on its own or in combination with fentanyl and para-fluorofentanyl. Fentanyl appeared in 562 or 73% of 770 unintentional drug overdose deaths from November 2020 to August 2021. Forty-eight of these cases involved para-fluorofentanyl, and 26 involved metonitazene.
“Although the percentage of law enforcement encounters with these substances in Tennessee decreased relative to the national total percentage within this time frame, the increase in encounters both within Tennessee and nationally reflect an increased distribution of para-fluorofentanyl and metonitazene throughout the United States,” the authors reported.
How to identify substances, manage overdoses
The authors encouraged physicians, labs, and medical examiners to be on the lookout for these two substances either in the emergency department or when identifying the cause of drug overdose deaths.
They also advised that stronger opioids, such as fentanyl, para-fluorofentanyl, metonitazene, or other benzimidazoles may warrant additional doses of the opioid-reversal drug naloxone.
While he hasn’t personally seen any of these drugs in his practice, “I would assume that these are on the rise due to inexpensive cost to manufacture and potency of effect,” said Dr. Fuehrlein, also an associate professor of psychiatry at Yale University, New Haven, Conn.
The need for additional naloxone to manage acute overdoses is a key takeaway of the MMWR paper, he added. Clinicians should also educate patients about harm reduction strategies to avoid overdose death when using potentially powerful and unknown drugs. “Things like start low and go slow, buy from the same supplier, do not use opioids with alcohol or benzos, have Narcan available, do not use alone, etc.”
Dr. Fuehrlein had no disclosures.
Two illicit drugs are contributing to a sharp rise in fentanyl-related deaths, a new study from the Centers for Disease Control and Prevention shows.
Para-fluorofentanyl, a schedule I substance often found in heroin packets and counterfeit pills, is making a comeback on the illicit drug market, Jordan Trecki, PhD, and associates reported in the Centers for Disease Control and Prevention’s Morbidity and Mortality Weekly Report (2022 Jan 28;71[4]:153-5). U.S. medical examiner reports and national law enforcement seizure data point to a rise in encounters of this drug along with metonitazene, a benzimidazole-opioid, in combination with fentanyl.
On their own, para-fluorofentanyl and metonitazene can kill the user through respiratory depression. Combinations of these substances and other opioids, including fentanyl-related compounds or adulterants, “pose an even greater potential harm to the patient than previously observed,” reported Dr. Trecki, a pharmacologist affiliated with the Drug Enforcement Administration, and colleagues.
Opioids contribute to about 75% of all U.S. drug overdose deaths, which rose by 28.5% during 2020-2021, according to the National Center for Health Statistics. And fentanyl is replacing heroin as the primary drug of use, said addiction specialist Brian Fuehrlein, MD, PhD, in an interview.
“For patients with stimulant use disorder and even cannabis use disorder, fentanyl is becoming more and more common as an adulterant in those substances, often resulting in inadvertent use. Hence, fentanyl and fentanyl-like drugs and fentanyl analogues are becoming increasingly common and important,” said Dr. Fuehrlein, director of the psychiatric emergency room at the VA Connecticut Healthcare System. He was not involved with the MMWR study.
Tennessee data reflect national problem
Recent data from a medical examiner in Knoxville, Tenn., illustrate what might be happening nationwide with those two emerging substances.
Over the last 2 years, the Knox County Regional Forensic Center has identified para-fluorofentanyl in the toxicology results of drug overdose victims, and metonitazene – either on its own or in combination with fentanyl and para-fluorofentanyl. Fentanyl appeared in 562 or 73% of 770 unintentional drug overdose deaths from November 2020 to August 2021. Forty-eight of these cases involved para-fluorofentanyl, and 26 involved metonitazene.
“Although the percentage of law enforcement encounters with these substances in Tennessee decreased relative to the national total percentage within this time frame, the increase in encounters both within Tennessee and nationally reflect an increased distribution of para-fluorofentanyl and metonitazene throughout the United States,” the authors reported.
How to identify substances, manage overdoses
The authors encouraged physicians, labs, and medical examiners to be on the lookout for these two substances either in the emergency department or when identifying the cause of drug overdose deaths.
They also advised that stronger opioids, such as fentanyl, para-fluorofentanyl, metonitazene, or other benzimidazoles may warrant additional doses of the opioid-reversal drug naloxone.
While he hasn’t personally seen any of these drugs in his practice, “I would assume that these are on the rise due to inexpensive cost to manufacture and potency of effect,” said Dr. Fuehrlein, also an associate professor of psychiatry at Yale University, New Haven, Conn.
The need for additional naloxone to manage acute overdoses is a key takeaway of the MMWR paper, he added. Clinicians should also educate patients about harm reduction strategies to avoid overdose death when using potentially powerful and unknown drugs. “Things like start low and go slow, buy from the same supplier, do not use opioids with alcohol or benzos, have Narcan available, do not use alone, etc.”
Dr. Fuehrlein had no disclosures.
Two illicit drugs are contributing to a sharp rise in fentanyl-related deaths, a new study from the Centers for Disease Control and Prevention shows.
Para-fluorofentanyl, a schedule I substance often found in heroin packets and counterfeit pills, is making a comeback on the illicit drug market, Jordan Trecki, PhD, and associates reported in the Centers for Disease Control and Prevention’s Morbidity and Mortality Weekly Report (2022 Jan 28;71[4]:153-5). U.S. medical examiner reports and national law enforcement seizure data point to a rise in encounters of this drug along with metonitazene, a benzimidazole-opioid, in combination with fentanyl.
On their own, para-fluorofentanyl and metonitazene can kill the user through respiratory depression. Combinations of these substances and other opioids, including fentanyl-related compounds or adulterants, “pose an even greater potential harm to the patient than previously observed,” reported Dr. Trecki, a pharmacologist affiliated with the Drug Enforcement Administration, and colleagues.
Opioids contribute to about 75% of all U.S. drug overdose deaths, which rose by 28.5% during 2020-2021, according to the National Center for Health Statistics. And fentanyl is replacing heroin as the primary drug of use, said addiction specialist Brian Fuehrlein, MD, PhD, in an interview.
“For patients with stimulant use disorder and even cannabis use disorder, fentanyl is becoming more and more common as an adulterant in those substances, often resulting in inadvertent use. Hence, fentanyl and fentanyl-like drugs and fentanyl analogues are becoming increasingly common and important,” said Dr. Fuehrlein, director of the psychiatric emergency room at the VA Connecticut Healthcare System. He was not involved with the MMWR study.
Tennessee data reflect national problem
Recent data from a medical examiner in Knoxville, Tenn., illustrate what might be happening nationwide with those two emerging substances.
Over the last 2 years, the Knox County Regional Forensic Center has identified para-fluorofentanyl in the toxicology results of drug overdose victims, and metonitazene – either on its own or in combination with fentanyl and para-fluorofentanyl. Fentanyl appeared in 562 or 73% of 770 unintentional drug overdose deaths from November 2020 to August 2021. Forty-eight of these cases involved para-fluorofentanyl, and 26 involved metonitazene.
“Although the percentage of law enforcement encounters with these substances in Tennessee decreased relative to the national total percentage within this time frame, the increase in encounters both within Tennessee and nationally reflect an increased distribution of para-fluorofentanyl and metonitazene throughout the United States,” the authors reported.
How to identify substances, manage overdoses
The authors encouraged physicians, labs, and medical examiners to be on the lookout for these two substances either in the emergency department or when identifying the cause of drug overdose deaths.
They also advised that stronger opioids, such as fentanyl, para-fluorofentanyl, metonitazene, or other benzimidazoles may warrant additional doses of the opioid-reversal drug naloxone.
While he hasn’t personally seen any of these drugs in his practice, “I would assume that these are on the rise due to inexpensive cost to manufacture and potency of effect,” said Dr. Fuehrlein, also an associate professor of psychiatry at Yale University, New Haven, Conn.
The need for additional naloxone to manage acute overdoses is a key takeaway of the MMWR paper, he added. Clinicians should also educate patients about harm reduction strategies to avoid overdose death when using potentially powerful and unknown drugs. “Things like start low and go slow, buy from the same supplier, do not use opioids with alcohol or benzos, have Narcan available, do not use alone, etc.”
Dr. Fuehrlein had no disclosures.
Referrals to gender clinics in Sweden drop after media coverage
Media coverage of transgender health care judged to be “negative” was associated with a drop of around 30% in referral rates to gender identity clinics in Sweden among young people under age 19, a new study indicates.
Malin Indremo, MS, from the department of neuroscience, Uppsala (Sweden) University, and colleagues explored the effect of the documentaries, “The Trans Train and Teenage Girls,” which they explain was a “Swedish public service television show” representing “investigative journalism.” The two-part documentary series was aired in Sweden in April 2019 and October 2019, respectively, and is now available in English on YouTube.
In their article, published online in JAMA Network Open, the authors said they consider “The Trans Train” programs to be “negative” media coverage because the “documentaries addressed the distinct increase among adolescents referred to gender identity clinics in recent years. Two young adults who regretted their transition and parents of transgender individuals who questioned the clinics’ assessments of their children were interviewed, and concerns were raised about whether gender-confirming treatments are based on sufficient scientific evidence.”
The programs, they suggest, may have influenced and jeopardized young transgender individuals’ access to transgender-specific health care.
Stella O’Malley, a U.K.-based psychotherapist specializing in transgender care and executive director of Genspect, an international organization that provides support to the parents of young people who are questioning their gender, expressed her disappointment with the study’s conclusions.
“I’m really surprised and disappointed that the researchers believe that negative coverage is the reason for a drop in referrals when it is more accurate to say that the information provided by ‘The Trans Train’ documentaries was concerning and suggests that further critical analysis and a review needs to be carried out on the clinics in question,” she said in an interview.
Ms. O’Malley herself made a documentary for Channel 4 in the United Kingdom, broadcast in 2018, called: “Trans Kids: It’s Time to Talk.”
Rapidly increasing numbers of youth, especially girls, question gender
As Ms. Indremo and coauthors explained – and as has been widely reported by this news organization – “the number of referrals to gender identity clinics have rapidly increased worldwide” in recent years, and this “has been especially prominent in adolescents and young adults.”
In addition, they acknowledged, “there has been a shift in gender ratio, with a preponderance toward individuals who were assigned female at birth (AFAB).”
This was the topic of “The Trans Train” programs, and in fact, following their broadcast, Ms. Indremo and colleagues noted that “an intense debate in national media [in Sweden] arose from the documentaries.”
Their research aimed to explore the association between both “positive” and “negative” media coverage and the number of referrals to gender identity clinics for young people (under aged 19) respectively. Data from the six gender clinics in Sweden were included between January 2017 and December 2019.
In the period studied, the clinics received 1,784 referrals, including 613 referrals in 2017, 663 referrals in 2018, and 508 referrals in 2019.
From the age-specific data that included 1,674 referrals, 359 individuals (21.4%) were younger than 13 years and 1,315 individuals (78.6%) were aged 13-18 years. From the assigned sex-specific data that included 1,435 referrals, 1,034 individuals (72.1%) were AFAB and 401 individuals (27.9%) were assigned male at birth (AMAB). Information on sex assigned at birth was lacking from one clinic, which was excluded from the analysis.
When they examined data for the 3 months following the airing of the first part of “The Trans Train” documentary series (in April 2019), they found that referrals to gender clinics fell by 25.4% overall, compared with the 3 months before part 1 was screened. Specifically, they fell by 25.3% for young people aged 13-18 years and by 32.2% for those born female.
In the extended analyses of 6 months following part 1, a decrease of total referrals by 30.7% was observed, while referrals for AFAB individuals decreased by 37.4% and referrals for individuals aged 13-18 years decreased by 27.7%. A decrease of referrals by 41.7% for children aged younger than 13 years was observed in the 6-month analysis, as well as a decrease of 8.2% among AMAB individuals.
“The Trans Train” documentaries, Ms. Indremo and colleagues said, “were criticized for being negatively biased and giving an oversimplified picture of transgender health care.”
Did the nature of the trans train documentaries influence referrals?
In an invited commentary published in JAMA Network Open, Ken C. Pang, PhD, from the Murdoch Children’s Research Institute, Melbourne, and colleagues noted: “Although the mechanisms underlying this decrease [in referrals] were not formally explored in their study, the authors reasonably speculated that both parents and referring health professionals may have been less likely to support a child or adolescent’s attendance at a specialist pediatric gender clinic following the documentaries.”
Dr. Pang and colleagues went on to say it is “the ... responsibility of media organizations in ensuring that stories depicting health care for transgender and gender diverse (TGD) young people are fair, balanced, nuanced, and accurate.”
Often, media reports have “fallen short of these standards and lacked the voices of TGD young people who have benefited from gender-affirming care or the perspectives of health professionals with expertise in providing such care,” they added.
“For example, some [media reports] have suggested that the growing number of referrals to such clinics is not owing to greater awareness of gender diversity and empowerment of TGD young people but is instead being driven by other factors such as peer influence, while others have warned that the use of gender-affirming hormonal interventions in TGD young people represents an undue risk,” they continue.
Ms. Indremo and colleagues didn’t see any drop-in referrals after the second part of the series, aired in October 2019, but they say this was likely because referrals were “already lowered” by the airing of the first part of the documentaries.
Nor did they see an increase in referrals following what they say was a “positive” media event in the form of a story about a professional Swedish handball player who announced the decision to quit his career to seek care for gender dysphoria.
“One may assume that a single news event is not significant enough to influence referral counts,” they suggested, noting also that Sweden represents “a society where there is already a relatively high level of awareness of gender identity issues.”
“Our results point to a differential association of media attention depending on the tone of the media content,” they observed.
Dr. Pang and coauthors noted it would be “helpful to examine whether similar media coverage in other countries has been associated with similar decreases in referral numbers and whether particular types of media stories are more prone to having this association.”
Parents and doctors debate treatment of gender dysphoria
In Sweden, custodians’ permission as well as custodians’ help is needed for minors to access care for gender dysphoria, said Ms. Indremo and coauthors. “It is possible that the content of the documentaries contributed to a higher custodian barrier to having their children referred for assessment, believing it may not be in the best interest of their child. This would highly impact young transgender individuals’ possibilities to access care.”
They also acknowledge that health care practitioners who refer young people to specialist clinics might also have been influenced by the documentaries, noting “some commentators argued that all treatments for gender dysphoria be stopped, and that ‘all health care given at the gender identity clinics was an experiment lacking scientific basis.’ ”
In April 2021, Angela Sämfjord, MD, child and adolescent psychiatrist at Sahlgrenska University Hospital, Gothenburg, Sweden, who started a child and adolescent clinic – the Lundstrom Gender Clinic – told this news organization she had reevaluated her approach even prior to “The Trans Train” documentaries and had resigned in 2018 because of her own fears about the lack of evidence for hormonal and surgical treatments of youth with gender dysphoria.
Following the debate that ensued after the airing of “The Trans Train” programs, the Swedish National Board of Health and Welfare published new recommendations in March 2021, which reflected a significant change in direction for the evaluation of gender dysphoria in minors, emphasizing the requirement for a thorough mental health assessment.
And in May 2021, Karolinska Children’s Hospital, which houses one of the leading gender identity clinics in Sweden, announced it would stop the routine medical treatment of children with gender dysphoria under the age of 18, which meant a total ban on the prescribing of puberty blockers and cross-sex hormones to minors. Such treatment could henceforth only be carried out within the setting of a clinical trial approved by the EPM (Ethical Review Agency/Swedish Institutional Review Board), it said.
The remaining five gender identity clinics in Sweden decided upon their own rules, but in general, they have become much more cautious regarding medical treatment of minors within the past year. Also, there is a desire in Sweden to reduce the number of gender identity clinics for minors from the current six to perhaps a maximum of three nationwide.
However, neither Ms. Indremo and colleagues nor Dr. Pang and colleagues mentioned the subsequent change to the Swedish NBHW recommendations on evaluation of gender dysphoria in minors in JAMA articles.
New NBHW recommendations about medical treatment of gender dysphoria with puberty blockers and cross-sex hormones for minors were due to be issued in 2021 but have been delayed.
Debate in other countries
Sweden is not alone in discussing this issue. In 2020, Finland became the first country in the world to issue new guidelines that concluded there is a lack of quality evidence to support the use of hormonal interventions in adolescents with gender dysphoria.
This issue has been hotly debated in the United Kingdom – not least with the Keira Bell court case and two National Institute for Health and Clinical Excellence evidence reviews concluding there is a lack of data to support the use of puberty-blocking agents and “cross-sex” hormones in youth with gender dysphoria.
And a number of U.S. states are attempting to outlaw the medical and surgical treatment of gender dysphoria in minors. Even health care professionals who have been treating young people with gender dysphoria for years – some of whom are transgender themselves – have started to speak out and are questioning what they call “sloppy care” given to many such youth.
Indeed, a recent survey shows that detransitioners – individuals who suffer from gender dysphoria, transition to the opposite sex but then regret their decision and detransition – are getting short shrift when it comes to care, with over half of the 100 surveyed saying they feel they did not receive adequate evaluation from a doctor or mental health professional before starting to transition.
And new draft standards of care for treating people with gender dysphoria by the World Professional Association for Transgender Health have drawn criticism from experts.
‘First do no harm’
In their conclusion, Dr. Pang and coauthors said that, with respect to the media coverage of young people with gender dysphoria, “who are, after all, one of the most vulnerable subgroups within our society, perhaps our media should recall one of the core tenets of health care and ensure their stories ‘first, do no harm.’”
However, in a commentary recently published in Child and Adolescent Mental Health, Alison Clayton, MBBS, from the University of Melbourne, and coauthors again pointed out that evidence reviews of the use of puberty blockers in young people with gender dysphoria show “there is very low certainty of the benefits of puberty blockers, an unknown risk of harm, and there is need for more rigorous research.”
“The clinically prudent thing to do, if we aim to ‘first, do no harm,’ is to proceed with extreme caution, especially given the rapidly rising case numbers and novel gender dysphoria presentations,” Clayton and colleagues concluded.
Ms. Indremo and coauthors reported no relevant financial relationships. Dr. Pang reported being a member of the Australian Professional Association for Trans Health and its research committee. One commentary coauthor has reported being a member of WPATH.
A version of this article first appeared on Medscape.com.
Media coverage of transgender health care judged to be “negative” was associated with a drop of around 30% in referral rates to gender identity clinics in Sweden among young people under age 19, a new study indicates.
Malin Indremo, MS, from the department of neuroscience, Uppsala (Sweden) University, and colleagues explored the effect of the documentaries, “The Trans Train and Teenage Girls,” which they explain was a “Swedish public service television show” representing “investigative journalism.” The two-part documentary series was aired in Sweden in April 2019 and October 2019, respectively, and is now available in English on YouTube.
In their article, published online in JAMA Network Open, the authors said they consider “The Trans Train” programs to be “negative” media coverage because the “documentaries addressed the distinct increase among adolescents referred to gender identity clinics in recent years. Two young adults who regretted their transition and parents of transgender individuals who questioned the clinics’ assessments of their children were interviewed, and concerns were raised about whether gender-confirming treatments are based on sufficient scientific evidence.”
The programs, they suggest, may have influenced and jeopardized young transgender individuals’ access to transgender-specific health care.
Stella O’Malley, a U.K.-based psychotherapist specializing in transgender care and executive director of Genspect, an international organization that provides support to the parents of young people who are questioning their gender, expressed her disappointment with the study’s conclusions.
“I’m really surprised and disappointed that the researchers believe that negative coverage is the reason for a drop in referrals when it is more accurate to say that the information provided by ‘The Trans Train’ documentaries was concerning and suggests that further critical analysis and a review needs to be carried out on the clinics in question,” she said in an interview.
Ms. O’Malley herself made a documentary for Channel 4 in the United Kingdom, broadcast in 2018, called: “Trans Kids: It’s Time to Talk.”
Rapidly increasing numbers of youth, especially girls, question gender
As Ms. Indremo and coauthors explained – and as has been widely reported by this news organization – “the number of referrals to gender identity clinics have rapidly increased worldwide” in recent years, and this “has been especially prominent in adolescents and young adults.”
In addition, they acknowledged, “there has been a shift in gender ratio, with a preponderance toward individuals who were assigned female at birth (AFAB).”
This was the topic of “The Trans Train” programs, and in fact, following their broadcast, Ms. Indremo and colleagues noted that “an intense debate in national media [in Sweden] arose from the documentaries.”
Their research aimed to explore the association between both “positive” and “negative” media coverage and the number of referrals to gender identity clinics for young people (under aged 19) respectively. Data from the six gender clinics in Sweden were included between January 2017 and December 2019.
In the period studied, the clinics received 1,784 referrals, including 613 referrals in 2017, 663 referrals in 2018, and 508 referrals in 2019.
From the age-specific data that included 1,674 referrals, 359 individuals (21.4%) were younger than 13 years and 1,315 individuals (78.6%) were aged 13-18 years. From the assigned sex-specific data that included 1,435 referrals, 1,034 individuals (72.1%) were AFAB and 401 individuals (27.9%) were assigned male at birth (AMAB). Information on sex assigned at birth was lacking from one clinic, which was excluded from the analysis.
When they examined data for the 3 months following the airing of the first part of “The Trans Train” documentary series (in April 2019), they found that referrals to gender clinics fell by 25.4% overall, compared with the 3 months before part 1 was screened. Specifically, they fell by 25.3% for young people aged 13-18 years and by 32.2% for those born female.
In the extended analyses of 6 months following part 1, a decrease of total referrals by 30.7% was observed, while referrals for AFAB individuals decreased by 37.4% and referrals for individuals aged 13-18 years decreased by 27.7%. A decrease of referrals by 41.7% for children aged younger than 13 years was observed in the 6-month analysis, as well as a decrease of 8.2% among AMAB individuals.
“The Trans Train” documentaries, Ms. Indremo and colleagues said, “were criticized for being negatively biased and giving an oversimplified picture of transgender health care.”
Did the nature of the trans train documentaries influence referrals?
In an invited commentary published in JAMA Network Open, Ken C. Pang, PhD, from the Murdoch Children’s Research Institute, Melbourne, and colleagues noted: “Although the mechanisms underlying this decrease [in referrals] were not formally explored in their study, the authors reasonably speculated that both parents and referring health professionals may have been less likely to support a child or adolescent’s attendance at a specialist pediatric gender clinic following the documentaries.”
Dr. Pang and colleagues went on to say it is “the ... responsibility of media organizations in ensuring that stories depicting health care for transgender and gender diverse (TGD) young people are fair, balanced, nuanced, and accurate.”
Often, media reports have “fallen short of these standards and lacked the voices of TGD young people who have benefited from gender-affirming care or the perspectives of health professionals with expertise in providing such care,” they added.
“For example, some [media reports] have suggested that the growing number of referrals to such clinics is not owing to greater awareness of gender diversity and empowerment of TGD young people but is instead being driven by other factors such as peer influence, while others have warned that the use of gender-affirming hormonal interventions in TGD young people represents an undue risk,” they continue.
Ms. Indremo and colleagues didn’t see any drop-in referrals after the second part of the series, aired in October 2019, but they say this was likely because referrals were “already lowered” by the airing of the first part of the documentaries.
Nor did they see an increase in referrals following what they say was a “positive” media event in the form of a story about a professional Swedish handball player who announced the decision to quit his career to seek care for gender dysphoria.
“One may assume that a single news event is not significant enough to influence referral counts,” they suggested, noting also that Sweden represents “a society where there is already a relatively high level of awareness of gender identity issues.”
“Our results point to a differential association of media attention depending on the tone of the media content,” they observed.
Dr. Pang and coauthors noted it would be “helpful to examine whether similar media coverage in other countries has been associated with similar decreases in referral numbers and whether particular types of media stories are more prone to having this association.”
Parents and doctors debate treatment of gender dysphoria
In Sweden, custodians’ permission as well as custodians’ help is needed for minors to access care for gender dysphoria, said Ms. Indremo and coauthors. “It is possible that the content of the documentaries contributed to a higher custodian barrier to having their children referred for assessment, believing it may not be in the best interest of their child. This would highly impact young transgender individuals’ possibilities to access care.”
They also acknowledge that health care practitioners who refer young people to specialist clinics might also have been influenced by the documentaries, noting “some commentators argued that all treatments for gender dysphoria be stopped, and that ‘all health care given at the gender identity clinics was an experiment lacking scientific basis.’ ”
In April 2021, Angela Sämfjord, MD, child and adolescent psychiatrist at Sahlgrenska University Hospital, Gothenburg, Sweden, who started a child and adolescent clinic – the Lundstrom Gender Clinic – told this news organization she had reevaluated her approach even prior to “The Trans Train” documentaries and had resigned in 2018 because of her own fears about the lack of evidence for hormonal and surgical treatments of youth with gender dysphoria.
Following the debate that ensued after the airing of “The Trans Train” programs, the Swedish National Board of Health and Welfare published new recommendations in March 2021, which reflected a significant change in direction for the evaluation of gender dysphoria in minors, emphasizing the requirement for a thorough mental health assessment.
And in May 2021, Karolinska Children’s Hospital, which houses one of the leading gender identity clinics in Sweden, announced it would stop the routine medical treatment of children with gender dysphoria under the age of 18, which meant a total ban on the prescribing of puberty blockers and cross-sex hormones to minors. Such treatment could henceforth only be carried out within the setting of a clinical trial approved by the EPM (Ethical Review Agency/Swedish Institutional Review Board), it said.
The remaining five gender identity clinics in Sweden decided upon their own rules, but in general, they have become much more cautious regarding medical treatment of minors within the past year. Also, there is a desire in Sweden to reduce the number of gender identity clinics for minors from the current six to perhaps a maximum of three nationwide.
However, neither Ms. Indremo and colleagues nor Dr. Pang and colleagues mentioned the subsequent change to the Swedish NBHW recommendations on evaluation of gender dysphoria in minors in JAMA articles.
New NBHW recommendations about medical treatment of gender dysphoria with puberty blockers and cross-sex hormones for minors were due to be issued in 2021 but have been delayed.
Debate in other countries
Sweden is not alone in discussing this issue. In 2020, Finland became the first country in the world to issue new guidelines that concluded there is a lack of quality evidence to support the use of hormonal interventions in adolescents with gender dysphoria.
This issue has been hotly debated in the United Kingdom – not least with the Keira Bell court case and two National Institute for Health and Clinical Excellence evidence reviews concluding there is a lack of data to support the use of puberty-blocking agents and “cross-sex” hormones in youth with gender dysphoria.
And a number of U.S. states are attempting to outlaw the medical and surgical treatment of gender dysphoria in minors. Even health care professionals who have been treating young people with gender dysphoria for years – some of whom are transgender themselves – have started to speak out and are questioning what they call “sloppy care” given to many such youth.
Indeed, a recent survey shows that detransitioners – individuals who suffer from gender dysphoria, transition to the opposite sex but then regret their decision and detransition – are getting short shrift when it comes to care, with over half of the 100 surveyed saying they feel they did not receive adequate evaluation from a doctor or mental health professional before starting to transition.
And new draft standards of care for treating people with gender dysphoria by the World Professional Association for Transgender Health have drawn criticism from experts.
‘First do no harm’
In their conclusion, Dr. Pang and coauthors said that, with respect to the media coverage of young people with gender dysphoria, “who are, after all, one of the most vulnerable subgroups within our society, perhaps our media should recall one of the core tenets of health care and ensure their stories ‘first, do no harm.’”
However, in a commentary recently published in Child and Adolescent Mental Health, Alison Clayton, MBBS, from the University of Melbourne, and coauthors again pointed out that evidence reviews of the use of puberty blockers in young people with gender dysphoria show “there is very low certainty of the benefits of puberty blockers, an unknown risk of harm, and there is need for more rigorous research.”
“The clinically prudent thing to do, if we aim to ‘first, do no harm,’ is to proceed with extreme caution, especially given the rapidly rising case numbers and novel gender dysphoria presentations,” Clayton and colleagues concluded.
Ms. Indremo and coauthors reported no relevant financial relationships. Dr. Pang reported being a member of the Australian Professional Association for Trans Health and its research committee. One commentary coauthor has reported being a member of WPATH.
A version of this article first appeared on Medscape.com.
Media coverage of transgender health care judged to be “negative” was associated with a drop of around 30% in referral rates to gender identity clinics in Sweden among young people under age 19, a new study indicates.
Malin Indremo, MS, from the department of neuroscience, Uppsala (Sweden) University, and colleagues explored the effect of the documentaries, “The Trans Train and Teenage Girls,” which they explain was a “Swedish public service television show” representing “investigative journalism.” The two-part documentary series was aired in Sweden in April 2019 and October 2019, respectively, and is now available in English on YouTube.
In their article, published online in JAMA Network Open, the authors said they consider “The Trans Train” programs to be “negative” media coverage because the “documentaries addressed the distinct increase among adolescents referred to gender identity clinics in recent years. Two young adults who regretted their transition and parents of transgender individuals who questioned the clinics’ assessments of their children were interviewed, and concerns were raised about whether gender-confirming treatments are based on sufficient scientific evidence.”
The programs, they suggest, may have influenced and jeopardized young transgender individuals’ access to transgender-specific health care.
Stella O’Malley, a U.K.-based psychotherapist specializing in transgender care and executive director of Genspect, an international organization that provides support to the parents of young people who are questioning their gender, expressed her disappointment with the study’s conclusions.
“I’m really surprised and disappointed that the researchers believe that negative coverage is the reason for a drop in referrals when it is more accurate to say that the information provided by ‘The Trans Train’ documentaries was concerning and suggests that further critical analysis and a review needs to be carried out on the clinics in question,” she said in an interview.
Ms. O’Malley herself made a documentary for Channel 4 in the United Kingdom, broadcast in 2018, called: “Trans Kids: It’s Time to Talk.”
Rapidly increasing numbers of youth, especially girls, question gender
As Ms. Indremo and coauthors explained – and as has been widely reported by this news organization – “the number of referrals to gender identity clinics have rapidly increased worldwide” in recent years, and this “has been especially prominent in adolescents and young adults.”
In addition, they acknowledged, “there has been a shift in gender ratio, with a preponderance toward individuals who were assigned female at birth (AFAB).”
This was the topic of “The Trans Train” programs, and in fact, following their broadcast, Ms. Indremo and colleagues noted that “an intense debate in national media [in Sweden] arose from the documentaries.”
Their research aimed to explore the association between both “positive” and “negative” media coverage and the number of referrals to gender identity clinics for young people (under aged 19) respectively. Data from the six gender clinics in Sweden were included between January 2017 and December 2019.
In the period studied, the clinics received 1,784 referrals, including 613 referrals in 2017, 663 referrals in 2018, and 508 referrals in 2019.
From the age-specific data that included 1,674 referrals, 359 individuals (21.4%) were younger than 13 years and 1,315 individuals (78.6%) were aged 13-18 years. From the assigned sex-specific data that included 1,435 referrals, 1,034 individuals (72.1%) were AFAB and 401 individuals (27.9%) were assigned male at birth (AMAB). Information on sex assigned at birth was lacking from one clinic, which was excluded from the analysis.
When they examined data for the 3 months following the airing of the first part of “The Trans Train” documentary series (in April 2019), they found that referrals to gender clinics fell by 25.4% overall, compared with the 3 months before part 1 was screened. Specifically, they fell by 25.3% for young people aged 13-18 years and by 32.2% for those born female.
In the extended analyses of 6 months following part 1, a decrease of total referrals by 30.7% was observed, while referrals for AFAB individuals decreased by 37.4% and referrals for individuals aged 13-18 years decreased by 27.7%. A decrease of referrals by 41.7% for children aged younger than 13 years was observed in the 6-month analysis, as well as a decrease of 8.2% among AMAB individuals.
“The Trans Train” documentaries, Ms. Indremo and colleagues said, “were criticized for being negatively biased and giving an oversimplified picture of transgender health care.”
Did the nature of the trans train documentaries influence referrals?
In an invited commentary published in JAMA Network Open, Ken C. Pang, PhD, from the Murdoch Children’s Research Institute, Melbourne, and colleagues noted: “Although the mechanisms underlying this decrease [in referrals] were not formally explored in their study, the authors reasonably speculated that both parents and referring health professionals may have been less likely to support a child or adolescent’s attendance at a specialist pediatric gender clinic following the documentaries.”
Dr. Pang and colleagues went on to say it is “the ... responsibility of media organizations in ensuring that stories depicting health care for transgender and gender diverse (TGD) young people are fair, balanced, nuanced, and accurate.”
Often, media reports have “fallen short of these standards and lacked the voices of TGD young people who have benefited from gender-affirming care or the perspectives of health professionals with expertise in providing such care,” they added.
“For example, some [media reports] have suggested that the growing number of referrals to such clinics is not owing to greater awareness of gender diversity and empowerment of TGD young people but is instead being driven by other factors such as peer influence, while others have warned that the use of gender-affirming hormonal interventions in TGD young people represents an undue risk,” they continue.
Ms. Indremo and colleagues didn’t see any drop-in referrals after the second part of the series, aired in October 2019, but they say this was likely because referrals were “already lowered” by the airing of the first part of the documentaries.
Nor did they see an increase in referrals following what they say was a “positive” media event in the form of a story about a professional Swedish handball player who announced the decision to quit his career to seek care for gender dysphoria.
“One may assume that a single news event is not significant enough to influence referral counts,” they suggested, noting also that Sweden represents “a society where there is already a relatively high level of awareness of gender identity issues.”
“Our results point to a differential association of media attention depending on the tone of the media content,” they observed.
Dr. Pang and coauthors noted it would be “helpful to examine whether similar media coverage in other countries has been associated with similar decreases in referral numbers and whether particular types of media stories are more prone to having this association.”
Parents and doctors debate treatment of gender dysphoria
In Sweden, custodians’ permission as well as custodians’ help is needed for minors to access care for gender dysphoria, said Ms. Indremo and coauthors. “It is possible that the content of the documentaries contributed to a higher custodian barrier to having their children referred for assessment, believing it may not be in the best interest of their child. This would highly impact young transgender individuals’ possibilities to access care.”
They also acknowledge that health care practitioners who refer young people to specialist clinics might also have been influenced by the documentaries, noting “some commentators argued that all treatments for gender dysphoria be stopped, and that ‘all health care given at the gender identity clinics was an experiment lacking scientific basis.’ ”
In April 2021, Angela Sämfjord, MD, child and adolescent psychiatrist at Sahlgrenska University Hospital, Gothenburg, Sweden, who started a child and adolescent clinic – the Lundstrom Gender Clinic – told this news organization she had reevaluated her approach even prior to “The Trans Train” documentaries and had resigned in 2018 because of her own fears about the lack of evidence for hormonal and surgical treatments of youth with gender dysphoria.
Following the debate that ensued after the airing of “The Trans Train” programs, the Swedish National Board of Health and Welfare published new recommendations in March 2021, which reflected a significant change in direction for the evaluation of gender dysphoria in minors, emphasizing the requirement for a thorough mental health assessment.
And in May 2021, Karolinska Children’s Hospital, which houses one of the leading gender identity clinics in Sweden, announced it would stop the routine medical treatment of children with gender dysphoria under the age of 18, which meant a total ban on the prescribing of puberty blockers and cross-sex hormones to minors. Such treatment could henceforth only be carried out within the setting of a clinical trial approved by the EPM (Ethical Review Agency/Swedish Institutional Review Board), it said.
The remaining five gender identity clinics in Sweden decided upon their own rules, but in general, they have become much more cautious regarding medical treatment of minors within the past year. Also, there is a desire in Sweden to reduce the number of gender identity clinics for minors from the current six to perhaps a maximum of three nationwide.
However, neither Ms. Indremo and colleagues nor Dr. Pang and colleagues mentioned the subsequent change to the Swedish NBHW recommendations on evaluation of gender dysphoria in minors in JAMA articles.
New NBHW recommendations about medical treatment of gender dysphoria with puberty blockers and cross-sex hormones for minors were due to be issued in 2021 but have been delayed.
Debate in other countries
Sweden is not alone in discussing this issue. In 2020, Finland became the first country in the world to issue new guidelines that concluded there is a lack of quality evidence to support the use of hormonal interventions in adolescents with gender dysphoria.
This issue has been hotly debated in the United Kingdom – not least with the Keira Bell court case and two National Institute for Health and Clinical Excellence evidence reviews concluding there is a lack of data to support the use of puberty-blocking agents and “cross-sex” hormones in youth with gender dysphoria.
And a number of U.S. states are attempting to outlaw the medical and surgical treatment of gender dysphoria in minors. Even health care professionals who have been treating young people with gender dysphoria for years – some of whom are transgender themselves – have started to speak out and are questioning what they call “sloppy care” given to many such youth.
Indeed, a recent survey shows that detransitioners – individuals who suffer from gender dysphoria, transition to the opposite sex but then regret their decision and detransition – are getting short shrift when it comes to care, with over half of the 100 surveyed saying they feel they did not receive adequate evaluation from a doctor or mental health professional before starting to transition.
And new draft standards of care for treating people with gender dysphoria by the World Professional Association for Transgender Health have drawn criticism from experts.
‘First do no harm’
In their conclusion, Dr. Pang and coauthors said that, with respect to the media coverage of young people with gender dysphoria, “who are, after all, one of the most vulnerable subgroups within our society, perhaps our media should recall one of the core tenets of health care and ensure their stories ‘first, do no harm.’”
However, in a commentary recently published in Child and Adolescent Mental Health, Alison Clayton, MBBS, from the University of Melbourne, and coauthors again pointed out that evidence reviews of the use of puberty blockers in young people with gender dysphoria show “there is very low certainty of the benefits of puberty blockers, an unknown risk of harm, and there is need for more rigorous research.”
“The clinically prudent thing to do, if we aim to ‘first, do no harm,’ is to proceed with extreme caution, especially given the rapidly rising case numbers and novel gender dysphoria presentations,” Clayton and colleagues concluded.
Ms. Indremo and coauthors reported no relevant financial relationships. Dr. Pang reported being a member of the Australian Professional Association for Trans Health and its research committee. One commentary coauthor has reported being a member of WPATH.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
Motor function restored in three men after complete paralysis from spinal cord injury
(SCI), new research shows.
The study demonstrated that an epidural electrical stimulation (EES) system developed specifically for spinal cord injuries enabled three men with complete paralysis to stand, walk, cycle, swim, and move their torso within 1 day.
“Thanks to this technology, we have been able to target individuals with the most serious spinal cord injury, meaning those with clinically complete spinal cord injury, with no sensation and no movement in the legs,” Grégoire Courtine, PhD, professor of neuroscience and neurotechnology at the Swiss Federal Institute of Technology, University Hospital Lausanne (Switzerland), and the University of Lausanne, told reporters attending a press briefing.
The study was published online Feb. 7, 2022, in Nature Medicine.
More rapid, precise, effective
SCIs involve severed connections between the brain and extremities. To compensate for these lost connections, researchers have investigated stem cell therapy, brain-machine interfaces, and powered exoskeletons.
However, these approaches aren’t yet ready for prime time.
In the meantime, researchers discovered even patients with a “complete” injury may have low-functioning connections and started investigating epidural stimulators designed to treat chronic pain. Recent studies – including three published in 2018 – showed promise for these pain-related stimulators in patients with incomplete SCI.
But using such “repurposed” technology meant the electrode array was relatively narrow and short, “so we could not target all the regions of the spinal cord involving control of leg and trunk movements,” said Dr. Courtine. With the newer technology “we are much more precise, effective, and more rapid in delivering therapy.”
To develop this new approach, the researchers designed a paddle lead with an arrangement of electrodes that targets sacral, lumbar, and low-thoracic dorsal roots involved in leg and trunk movements. They also established a personalized computational framework that allows for optimal surgical placement of this paddle lead.
In addition, they developed software that renders the configuration of individualized activity–dependent stimulation programs rapid, simple, and predictable.
They tested these neurotechnologies in three men with complete sensorimotor paralysis as part of an ongoing clinical trial. The participants, aged 29, 32, and 41 years, suffered an SCI from a motor bike accident 3, 9, and 1 year before enrollment.
All three patients exhibited complete sensorimotor paralysis. They were unable to take any step, and muscles remained quiescent during these attempts.
A neurosurgeon implanted electrodes along the spinal cord of study subjects. Wires from these electrodes were connected to a neurostimulator implanted under the skin in the abdomen.
The men can select different activity-based programs from a tablet that sends signals to the implanted device.
Personalized approach
Within a single day of the surgery, the participants were able to stand, walk, cycle, swim, and control trunk movements.
“It was not perfect at the very beginning, but they could train very early on to have a more fluid gait,” said study investigator neurosurgeon Joceylyne Bloch, MD, associate professor, University of Lausanne and University Hospital Lausanne.
At this stage, not all paralyzed patients are eligible for the procedure. Dr. Bloch explained that at least 6 cm of healthy spinal cord under the lesion is needed to implant the electrodes.
“There’s a huge variability of spinal cord anatomy between individuals. That’s why it’s important to study each person individually and to have individual models in order to be precise.”
Researchers envision having “a library of electrode arrays,” added Dr. Courtine. With preoperative imaging of the individual’s spinal cord, “the neurosurgeon can select the more appropriate electrode array for that specific patient.”
Dr. Courtine noted recovery of sensation with the system differs from one individual to another. One study participant, Michel Roccati, now 30, told the briefing he feels a contraction in his muscle during the stimulation.
Currently, only individuals whose injury is more than a year old are included in the study to ensure patients have “a stable lesion” and reached “a plateau of recovery,” said Dr. Bloch. However, animal models show intervening earlier might boost the benefits.
A patient’s age can influence the outcome, as younger patients are likely in better condition and more motivated than older patients, said Dr. Bloch. However, she noted patients closing in on 50 years have responded well to the therapy.
Such stimulation systems may prove useful in treating conditions typically associated with SCI, such as hypertension and bladder control, and perhaps also in patients with Parkinson’s disease, said Dr. Courtine.
The researchers plan to conduct another study that will include a next-generation pulse generator with features that make the stimulation even more effective and user friendly. A voice recognition system could eventually be connected to the system.
“The next step is a minicomputer that you implant in the body that communicates in real time with an external iPhone,” said Dr. Courtine.
ONWARD Medical, which developed the technology, has received a breakthrough device designation from the Food and Drug Administration. The company is in discussions with the FDA to carry out a clinical trial of the device in the United States.
A ‘huge step forward’
Peter J. Grahn, PhD, assistant professor, department of physical medicine and rehabilitation and department of neurologic surgery, Mayo Clinic, Rochester, Minn., an author of one of the 2018 studies, said this technology “is a huge step forward” and “really pushes the field.”
Compared with the device used in his study that’s designed to treat neuropathic pain, this new system “is much more capable of dynamic stimulation,” said Dr. Grahn. “You can tailor the stimulation based on which area of the spinal cord you want to target during a specific function.”
There has been “a lot of hope and hype” recently around stem cells and biological molecules that were supposed to be “magic pills” to cure spinal cord dysfunction, said Dr. Grahn. “I don’t think this is one of those.”
However, he questioned the researchers’ use of the word “walking.”
“They say independent stepping or walking is restored on day 1, but the graphs show day 1 function is having over 60% of their body weight supported when they’re taking these steps,” he said.
In addition, the “big question” is how this technology can “be distilled down” into an approach “applicable across rehabilitation centers,” said Dr. Grahn.
The study was supported by numerous organizations, including ONWARD Medical. Dr. Courtine and Dr. Bloch hold various patents in relation with the present work. Dr. Courtine is a consultant with ONWARD Medical, and he and Dr. Bloch are shareholders of ONWARD Medical, a company with direct relationships with the presented work. Dr. Grahn reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
(SCI), new research shows.
The study demonstrated that an epidural electrical stimulation (EES) system developed specifically for spinal cord injuries enabled three men with complete paralysis to stand, walk, cycle, swim, and move their torso within 1 day.
“Thanks to this technology, we have been able to target individuals with the most serious spinal cord injury, meaning those with clinically complete spinal cord injury, with no sensation and no movement in the legs,” Grégoire Courtine, PhD, professor of neuroscience and neurotechnology at the Swiss Federal Institute of Technology, University Hospital Lausanne (Switzerland), and the University of Lausanne, told reporters attending a press briefing.
The study was published online Feb. 7, 2022, in Nature Medicine.
More rapid, precise, effective
SCIs involve severed connections between the brain and extremities. To compensate for these lost connections, researchers have investigated stem cell therapy, brain-machine interfaces, and powered exoskeletons.
However, these approaches aren’t yet ready for prime time.
In the meantime, researchers discovered even patients with a “complete” injury may have low-functioning connections and started investigating epidural stimulators designed to treat chronic pain. Recent studies – including three published in 2018 – showed promise for these pain-related stimulators in patients with incomplete SCI.
But using such “repurposed” technology meant the electrode array was relatively narrow and short, “so we could not target all the regions of the spinal cord involving control of leg and trunk movements,” said Dr. Courtine. With the newer technology “we are much more precise, effective, and more rapid in delivering therapy.”
To develop this new approach, the researchers designed a paddle lead with an arrangement of electrodes that targets sacral, lumbar, and low-thoracic dorsal roots involved in leg and trunk movements. They also established a personalized computational framework that allows for optimal surgical placement of this paddle lead.
In addition, they developed software that renders the configuration of individualized activity–dependent stimulation programs rapid, simple, and predictable.
They tested these neurotechnologies in three men with complete sensorimotor paralysis as part of an ongoing clinical trial. The participants, aged 29, 32, and 41 years, suffered an SCI from a motor bike accident 3, 9, and 1 year before enrollment.
All three patients exhibited complete sensorimotor paralysis. They were unable to take any step, and muscles remained quiescent during these attempts.
A neurosurgeon implanted electrodes along the spinal cord of study subjects. Wires from these electrodes were connected to a neurostimulator implanted under the skin in the abdomen.
The men can select different activity-based programs from a tablet that sends signals to the implanted device.
Personalized approach
Within a single day of the surgery, the participants were able to stand, walk, cycle, swim, and control trunk movements.
“It was not perfect at the very beginning, but they could train very early on to have a more fluid gait,” said study investigator neurosurgeon Joceylyne Bloch, MD, associate professor, University of Lausanne and University Hospital Lausanne.
At this stage, not all paralyzed patients are eligible for the procedure. Dr. Bloch explained that at least 6 cm of healthy spinal cord under the lesion is needed to implant the electrodes.
“There’s a huge variability of spinal cord anatomy between individuals. That’s why it’s important to study each person individually and to have individual models in order to be precise.”
Researchers envision having “a library of electrode arrays,” added Dr. Courtine. With preoperative imaging of the individual’s spinal cord, “the neurosurgeon can select the more appropriate electrode array for that specific patient.”
Dr. Courtine noted recovery of sensation with the system differs from one individual to another. One study participant, Michel Roccati, now 30, told the briefing he feels a contraction in his muscle during the stimulation.
Currently, only individuals whose injury is more than a year old are included in the study to ensure patients have “a stable lesion” and reached “a plateau of recovery,” said Dr. Bloch. However, animal models show intervening earlier might boost the benefits.
A patient’s age can influence the outcome, as younger patients are likely in better condition and more motivated than older patients, said Dr. Bloch. However, she noted patients closing in on 50 years have responded well to the therapy.
Such stimulation systems may prove useful in treating conditions typically associated with SCI, such as hypertension and bladder control, and perhaps also in patients with Parkinson’s disease, said Dr. Courtine.
The researchers plan to conduct another study that will include a next-generation pulse generator with features that make the stimulation even more effective and user friendly. A voice recognition system could eventually be connected to the system.
“The next step is a minicomputer that you implant in the body that communicates in real time with an external iPhone,” said Dr. Courtine.
ONWARD Medical, which developed the technology, has received a breakthrough device designation from the Food and Drug Administration. The company is in discussions with the FDA to carry out a clinical trial of the device in the United States.
A ‘huge step forward’
Peter J. Grahn, PhD, assistant professor, department of physical medicine and rehabilitation and department of neurologic surgery, Mayo Clinic, Rochester, Minn., an author of one of the 2018 studies, said this technology “is a huge step forward” and “really pushes the field.”
Compared with the device used in his study that’s designed to treat neuropathic pain, this new system “is much more capable of dynamic stimulation,” said Dr. Grahn. “You can tailor the stimulation based on which area of the spinal cord you want to target during a specific function.”
There has been “a lot of hope and hype” recently around stem cells and biological molecules that were supposed to be “magic pills” to cure spinal cord dysfunction, said Dr. Grahn. “I don’t think this is one of those.”
However, he questioned the researchers’ use of the word “walking.”
“They say independent stepping or walking is restored on day 1, but the graphs show day 1 function is having over 60% of their body weight supported when they’re taking these steps,” he said.
In addition, the “big question” is how this technology can “be distilled down” into an approach “applicable across rehabilitation centers,” said Dr. Grahn.
The study was supported by numerous organizations, including ONWARD Medical. Dr. Courtine and Dr. Bloch hold various patents in relation with the present work. Dr. Courtine is a consultant with ONWARD Medical, and he and Dr. Bloch are shareholders of ONWARD Medical, a company with direct relationships with the presented work. Dr. Grahn reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
(SCI), new research shows.
The study demonstrated that an epidural electrical stimulation (EES) system developed specifically for spinal cord injuries enabled three men with complete paralysis to stand, walk, cycle, swim, and move their torso within 1 day.
“Thanks to this technology, we have been able to target individuals with the most serious spinal cord injury, meaning those with clinically complete spinal cord injury, with no sensation and no movement in the legs,” Grégoire Courtine, PhD, professor of neuroscience and neurotechnology at the Swiss Federal Institute of Technology, University Hospital Lausanne (Switzerland), and the University of Lausanne, told reporters attending a press briefing.
The study was published online Feb. 7, 2022, in Nature Medicine.
More rapid, precise, effective
SCIs involve severed connections between the brain and extremities. To compensate for these lost connections, researchers have investigated stem cell therapy, brain-machine interfaces, and powered exoskeletons.
However, these approaches aren’t yet ready for prime time.
In the meantime, researchers discovered even patients with a “complete” injury may have low-functioning connections and started investigating epidural stimulators designed to treat chronic pain. Recent studies – including three published in 2018 – showed promise for these pain-related stimulators in patients with incomplete SCI.
But using such “repurposed” technology meant the electrode array was relatively narrow and short, “so we could not target all the regions of the spinal cord involving control of leg and trunk movements,” said Dr. Courtine. With the newer technology “we are much more precise, effective, and more rapid in delivering therapy.”
To develop this new approach, the researchers designed a paddle lead with an arrangement of electrodes that targets sacral, lumbar, and low-thoracic dorsal roots involved in leg and trunk movements. They also established a personalized computational framework that allows for optimal surgical placement of this paddle lead.
In addition, they developed software that renders the configuration of individualized activity–dependent stimulation programs rapid, simple, and predictable.
They tested these neurotechnologies in three men with complete sensorimotor paralysis as part of an ongoing clinical trial. The participants, aged 29, 32, and 41 years, suffered an SCI from a motor bike accident 3, 9, and 1 year before enrollment.
All three patients exhibited complete sensorimotor paralysis. They were unable to take any step, and muscles remained quiescent during these attempts.
A neurosurgeon implanted electrodes along the spinal cord of study subjects. Wires from these electrodes were connected to a neurostimulator implanted under the skin in the abdomen.
The men can select different activity-based programs from a tablet that sends signals to the implanted device.
Personalized approach
Within a single day of the surgery, the participants were able to stand, walk, cycle, swim, and control trunk movements.
“It was not perfect at the very beginning, but they could train very early on to have a more fluid gait,” said study investigator neurosurgeon Joceylyne Bloch, MD, associate professor, University of Lausanne and University Hospital Lausanne.
At this stage, not all paralyzed patients are eligible for the procedure. Dr. Bloch explained that at least 6 cm of healthy spinal cord under the lesion is needed to implant the electrodes.
“There’s a huge variability of spinal cord anatomy between individuals. That’s why it’s important to study each person individually and to have individual models in order to be precise.”
Researchers envision having “a library of electrode arrays,” added Dr. Courtine. With preoperative imaging of the individual’s spinal cord, “the neurosurgeon can select the more appropriate electrode array for that specific patient.”
Dr. Courtine noted recovery of sensation with the system differs from one individual to another. One study participant, Michel Roccati, now 30, told the briefing he feels a contraction in his muscle during the stimulation.
Currently, only individuals whose injury is more than a year old are included in the study to ensure patients have “a stable lesion” and reached “a plateau of recovery,” said Dr. Bloch. However, animal models show intervening earlier might boost the benefits.
A patient’s age can influence the outcome, as younger patients are likely in better condition and more motivated than older patients, said Dr. Bloch. However, she noted patients closing in on 50 years have responded well to the therapy.
Such stimulation systems may prove useful in treating conditions typically associated with SCI, such as hypertension and bladder control, and perhaps also in patients with Parkinson’s disease, said Dr. Courtine.
The researchers plan to conduct another study that will include a next-generation pulse generator with features that make the stimulation even more effective and user friendly. A voice recognition system could eventually be connected to the system.
“The next step is a minicomputer that you implant in the body that communicates in real time with an external iPhone,” said Dr. Courtine.
ONWARD Medical, which developed the technology, has received a breakthrough device designation from the Food and Drug Administration. The company is in discussions with the FDA to carry out a clinical trial of the device in the United States.
A ‘huge step forward’
Peter J. Grahn, PhD, assistant professor, department of physical medicine and rehabilitation and department of neurologic surgery, Mayo Clinic, Rochester, Minn., an author of one of the 2018 studies, said this technology “is a huge step forward” and “really pushes the field.”
Compared with the device used in his study that’s designed to treat neuropathic pain, this new system “is much more capable of dynamic stimulation,” said Dr. Grahn. “You can tailor the stimulation based on which area of the spinal cord you want to target during a specific function.”
There has been “a lot of hope and hype” recently around stem cells and biological molecules that were supposed to be “magic pills” to cure spinal cord dysfunction, said Dr. Grahn. “I don’t think this is one of those.”
However, he questioned the researchers’ use of the word “walking.”
“They say independent stepping or walking is restored on day 1, but the graphs show day 1 function is having over 60% of their body weight supported when they’re taking these steps,” he said.
In addition, the “big question” is how this technology can “be distilled down” into an approach “applicable across rehabilitation centers,” said Dr. Grahn.
The study was supported by numerous organizations, including ONWARD Medical. Dr. Courtine and Dr. Bloch hold various patents in relation with the present work. Dr. Courtine is a consultant with ONWARD Medical, and he and Dr. Bloch are shareholders of ONWARD Medical, a company with direct relationships with the presented work. Dr. Grahn reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM NATURE MEDICINE
FDA approves first-ever drug for cold agglutinin disease
A new drug has become the first and only treatment for people with cold agglutinin disease (CAD) that is approved by the U.S. Food and Drug Administration.
CAD is a rare autoimmune hemolytic anemia, affecting about 5,000 people in the United States. It is caused by antibodies binding to the surface of red blood cells, which starts a process that causes the body’s immune system to mistakenly attack healthy red blood cells and cause their rupture (hemolysis). This can lead to severe anemia, which is often treated by blood transfusions.
“For people living with cold agglutinin disease, it is as if their body’s immune system is waging a war on itself. The relentless destruction of healthy red blood cells is a daily, silent reality for people with CAD. For the first time, we have a treatment that targets complement-mediated hemolysis, which is the underlying cause of the red blood cell destruction in many CAD patients,” commented Catherine Broome, MD, associate professor of medicine at Georgetown University Lombardi Comprehensive Cancer Center, in a company press release.
Dr. Broome was the principal investigator in the CARDINAL study, which was the basis for the new approval. In this pivotal study, patients treated with sutimlimab had an improvement in anemia as measured by hemoglobin (Hgb) and bilirubin levels, she commented in the company statement.
The CARDINAL study was a 26-week open-label, single-arm phase 3 study conducted in 24 patients with CAD who had recent history of blood transfusion.
The primary efficacy endpoint was a composite defined as the proportion of patients who achieved normalization of Hgb level greater than or equal to 12 g/dL or demonstrated an increase from baseline in Hgb level greater than or equal to 2 g/dL at the treatment assessment time point (mean value from weeks 23, 25, and 26) and no blood transfusion from weeks 5 through 26.
More than half of the patients (13 of 24, 54%) met the composite primary endpoint criteria, with 17 of 24 (71%) patients remaining transfusion-free after week 5. Most patients (22 of 24, 92%) did not use other CAD-related treatments.
For the secondary measures on disease process, patients enrolled in the trial experienced a mean increase in Hgb level of 2.29 g/dL at week 3 and 3.18 g/dL at the 26-week treatment assessment time points (increasing from the mean baseline level of 8.6 g/dL). In addition, there was a mean reduction in bilirubin levels (n = 14) of -2.23 mg/dL from a mean baseline level of 3.23 mg/dL.
The most common adverse reactions occurring in 10% or more of patients were respiratory tract infection, viral infection, diarrhea, dyspepsia, cough, arthralgia, arthritis, and peripheral edema. Serious adverse reactions were reported in 3 of 24 (13%) patients, and these included streptococcal sepsis and staphylococcal wound infection (n = 1), arthralgia (n = 1), and respiratory tract infection (n = 1).
None of the adverse reactions led to discontinuation of the drug, the company noted. Dosage interruptions due to an adverse reaction occurred in 4 of 24 (17%) patients who received the drug.
The recommended dose is based on body weight (6,500 mg for people 39-75 kg and 7,500 mg for people greater than 75 kg). The drug is administered intravenously weekly for the first 2 weeks with administration every 2 weeks thereafter.
Full prescribing information is available here.
The product is a humanized monoclonal antibody that is designed to selectively target and inhibit C1s in the classical complement pathway, which is part of the innate immune system. By blocking C1s, Enjaymo inhibits the activation of the complement cascade in the immune system and inhibits C1-activated hemolysis in CAD to prevent the abnormal destruction of healthy red blood cells. Enjaymo does not inhibit the lectin and alternative pathways, the company noted.
In the U.S., Enjaymo received FDA breakthrough therapy and orphan drug designation, as well as priority review. The product is awaiting approval in Europe and Japan.
Sanofi says the product is expected to be available in the United States in the coming weeks, with a list price, or wholesale acquisition cost, of $1,800 per vial. Actual costs to patients are generally anticipated to be lower, as the list price does not reflect insurance coverage, copay support, or financial assistance from patient support programs. The company offers support for eligible patients on 1-833-223-2428.
A version of this article first appeared on Medscape.com.
A new drug has become the first and only treatment for people with cold agglutinin disease (CAD) that is approved by the U.S. Food and Drug Administration.
CAD is a rare autoimmune hemolytic anemia, affecting about 5,000 people in the United States. It is caused by antibodies binding to the surface of red blood cells, which starts a process that causes the body’s immune system to mistakenly attack healthy red blood cells and cause their rupture (hemolysis). This can lead to severe anemia, which is often treated by blood transfusions.
“For people living with cold agglutinin disease, it is as if their body’s immune system is waging a war on itself. The relentless destruction of healthy red blood cells is a daily, silent reality for people with CAD. For the first time, we have a treatment that targets complement-mediated hemolysis, which is the underlying cause of the red blood cell destruction in many CAD patients,” commented Catherine Broome, MD, associate professor of medicine at Georgetown University Lombardi Comprehensive Cancer Center, in a company press release.
Dr. Broome was the principal investigator in the CARDINAL study, which was the basis for the new approval. In this pivotal study, patients treated with sutimlimab had an improvement in anemia as measured by hemoglobin (Hgb) and bilirubin levels, she commented in the company statement.
The CARDINAL study was a 26-week open-label, single-arm phase 3 study conducted in 24 patients with CAD who had recent history of blood transfusion.
The primary efficacy endpoint was a composite defined as the proportion of patients who achieved normalization of Hgb level greater than or equal to 12 g/dL or demonstrated an increase from baseline in Hgb level greater than or equal to 2 g/dL at the treatment assessment time point (mean value from weeks 23, 25, and 26) and no blood transfusion from weeks 5 through 26.
More than half of the patients (13 of 24, 54%) met the composite primary endpoint criteria, with 17 of 24 (71%) patients remaining transfusion-free after week 5. Most patients (22 of 24, 92%) did not use other CAD-related treatments.
For the secondary measures on disease process, patients enrolled in the trial experienced a mean increase in Hgb level of 2.29 g/dL at week 3 and 3.18 g/dL at the 26-week treatment assessment time points (increasing from the mean baseline level of 8.6 g/dL). In addition, there was a mean reduction in bilirubin levels (n = 14) of -2.23 mg/dL from a mean baseline level of 3.23 mg/dL.
The most common adverse reactions occurring in 10% or more of patients were respiratory tract infection, viral infection, diarrhea, dyspepsia, cough, arthralgia, arthritis, and peripheral edema. Serious adverse reactions were reported in 3 of 24 (13%) patients, and these included streptococcal sepsis and staphylococcal wound infection (n = 1), arthralgia (n = 1), and respiratory tract infection (n = 1).
None of the adverse reactions led to discontinuation of the drug, the company noted. Dosage interruptions due to an adverse reaction occurred in 4 of 24 (17%) patients who received the drug.
The recommended dose is based on body weight (6,500 mg for people 39-75 kg and 7,500 mg for people greater than 75 kg). The drug is administered intravenously weekly for the first 2 weeks with administration every 2 weeks thereafter.
Full prescribing information is available here.
The product is a humanized monoclonal antibody that is designed to selectively target and inhibit C1s in the classical complement pathway, which is part of the innate immune system. By blocking C1s, Enjaymo inhibits the activation of the complement cascade in the immune system and inhibits C1-activated hemolysis in CAD to prevent the abnormal destruction of healthy red blood cells. Enjaymo does not inhibit the lectin and alternative pathways, the company noted.
In the U.S., Enjaymo received FDA breakthrough therapy and orphan drug designation, as well as priority review. The product is awaiting approval in Europe and Japan.
Sanofi says the product is expected to be available in the United States in the coming weeks, with a list price, or wholesale acquisition cost, of $1,800 per vial. Actual costs to patients are generally anticipated to be lower, as the list price does not reflect insurance coverage, copay support, or financial assistance from patient support programs. The company offers support for eligible patients on 1-833-223-2428.
A version of this article first appeared on Medscape.com.
A new drug has become the first and only treatment for people with cold agglutinin disease (CAD) that is approved by the U.S. Food and Drug Administration.
CAD is a rare autoimmune hemolytic anemia, affecting about 5,000 people in the United States. It is caused by antibodies binding to the surface of red blood cells, which starts a process that causes the body’s immune system to mistakenly attack healthy red blood cells and cause their rupture (hemolysis). This can lead to severe anemia, which is often treated by blood transfusions.
“For people living with cold agglutinin disease, it is as if their body’s immune system is waging a war on itself. The relentless destruction of healthy red blood cells is a daily, silent reality for people with CAD. For the first time, we have a treatment that targets complement-mediated hemolysis, which is the underlying cause of the red blood cell destruction in many CAD patients,” commented Catherine Broome, MD, associate professor of medicine at Georgetown University Lombardi Comprehensive Cancer Center, in a company press release.
Dr. Broome was the principal investigator in the CARDINAL study, which was the basis for the new approval. In this pivotal study, patients treated with sutimlimab had an improvement in anemia as measured by hemoglobin (Hgb) and bilirubin levels, she commented in the company statement.
The CARDINAL study was a 26-week open-label, single-arm phase 3 study conducted in 24 patients with CAD who had recent history of blood transfusion.
The primary efficacy endpoint was a composite defined as the proportion of patients who achieved normalization of Hgb level greater than or equal to 12 g/dL or demonstrated an increase from baseline in Hgb level greater than or equal to 2 g/dL at the treatment assessment time point (mean value from weeks 23, 25, and 26) and no blood transfusion from weeks 5 through 26.
More than half of the patients (13 of 24, 54%) met the composite primary endpoint criteria, with 17 of 24 (71%) patients remaining transfusion-free after week 5. Most patients (22 of 24, 92%) did not use other CAD-related treatments.
For the secondary measures on disease process, patients enrolled in the trial experienced a mean increase in Hgb level of 2.29 g/dL at week 3 and 3.18 g/dL at the 26-week treatment assessment time points (increasing from the mean baseline level of 8.6 g/dL). In addition, there was a mean reduction in bilirubin levels (n = 14) of -2.23 mg/dL from a mean baseline level of 3.23 mg/dL.
The most common adverse reactions occurring in 10% or more of patients were respiratory tract infection, viral infection, diarrhea, dyspepsia, cough, arthralgia, arthritis, and peripheral edema. Serious adverse reactions were reported in 3 of 24 (13%) patients, and these included streptococcal sepsis and staphylococcal wound infection (n = 1), arthralgia (n = 1), and respiratory tract infection (n = 1).
None of the adverse reactions led to discontinuation of the drug, the company noted. Dosage interruptions due to an adverse reaction occurred in 4 of 24 (17%) patients who received the drug.
The recommended dose is based on body weight (6,500 mg for people 39-75 kg and 7,500 mg for people greater than 75 kg). The drug is administered intravenously weekly for the first 2 weeks with administration every 2 weeks thereafter.
Full prescribing information is available here.
The product is a humanized monoclonal antibody that is designed to selectively target and inhibit C1s in the classical complement pathway, which is part of the innate immune system. By blocking C1s, Enjaymo inhibits the activation of the complement cascade in the immune system and inhibits C1-activated hemolysis in CAD to prevent the abnormal destruction of healthy red blood cells. Enjaymo does not inhibit the lectin and alternative pathways, the company noted.
In the U.S., Enjaymo received FDA breakthrough therapy and orphan drug designation, as well as priority review. The product is awaiting approval in Europe and Japan.
Sanofi says the product is expected to be available in the United States in the coming weeks, with a list price, or wholesale acquisition cost, of $1,800 per vial. Actual costs to patients are generally anticipated to be lower, as the list price does not reflect insurance coverage, copay support, or financial assistance from patient support programs. The company offers support for eligible patients on 1-833-223-2428.
A version of this article first appeared on Medscape.com.
Infant bronchiolitis subtype may predict asthma risk
Bronchiolitis is the leading cause of infant hospitalizations in the United States and Europe, and almost one-third of these patients go on to develop asthma later in childhood.
But a multinational team of researchers has presented evidence that could avoid that outcome. They identified four different subtypes of bronchiolitis along with a decision tree that can determine which infants are most likely to develop asthma as they get older.
Reporting in the journal eClinical Medicine, Michimasa Fujiogi, MD, of Massachusetts General Hospital and Harvard University, Boston, and colleagues analyzed three multicenter prospective cohort studies that included a combined 3,081 infants hospitalized with severe bronchiolitis.
“This study added a base for the early identification of high-risk patients during early infancy,” Dr. Fujiogi said in an interview. “Using the prediction rule of this study, it is possible to identify groups at high risk of asthma during a critical period of airway development – early infancy.”
The researchers identified four clinically distinct and reproducible profiles of infants hospitalized for bronchiolitis:
- A: characterized by a history of breathing problems and eczema, rhinovirus infection, and low prevalence of respiratory syncytial virus (RSV) infection.
- B: characterized by the classic symptoms of wheezing and cough at presentation, a low prevalence of previous breathing problems and rhinovirus infection, and a high likelihood of RSV infection.
- C: the most severe group, characterized by inadequate oral intake, severe retraction at presentation, and longer hospital stays.
- D: the least ill group, with little history of breathing problems but inadequate oral intake with no or mild retraction.
Infants with profile A had the highest risk for developing asthma – more than 250% greater than with typical bronchiolitis. They were also older and were more likely to have parents who had asthma – and none had solo-RSV infection. In the overall analysis, the risk for developing asthma by age 6 or 7 was 23%.
The researchers stated that the decision tree accurately predicts the high-risk profile with high degrees of sensitivity and specificity. The decision tree used four predictors that together defined infants with profile A: RSV infection status, previous breathing problems, eczema, and parental asthma.
“Our data would facilitate the development of profile-specific prevention strategies for asthma – for example, modification of host response, prophylaxis for severe viral infection – by identifying asthma risk groups early in infancy,” Dr. Fujiogi said.
The study received funding from the National Institutes of Health. Dr. Fujiogi and coauthors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Bronchiolitis is the leading cause of infant hospitalizations in the United States and Europe, and almost one-third of these patients go on to develop asthma later in childhood.
But a multinational team of researchers has presented evidence that could avoid that outcome. They identified four different subtypes of bronchiolitis along with a decision tree that can determine which infants are most likely to develop asthma as they get older.
Reporting in the journal eClinical Medicine, Michimasa Fujiogi, MD, of Massachusetts General Hospital and Harvard University, Boston, and colleagues analyzed three multicenter prospective cohort studies that included a combined 3,081 infants hospitalized with severe bronchiolitis.
“This study added a base for the early identification of high-risk patients during early infancy,” Dr. Fujiogi said in an interview. “Using the prediction rule of this study, it is possible to identify groups at high risk of asthma during a critical period of airway development – early infancy.”
The researchers identified four clinically distinct and reproducible profiles of infants hospitalized for bronchiolitis:
- A: characterized by a history of breathing problems and eczema, rhinovirus infection, and low prevalence of respiratory syncytial virus (RSV) infection.
- B: characterized by the classic symptoms of wheezing and cough at presentation, a low prevalence of previous breathing problems and rhinovirus infection, and a high likelihood of RSV infection.
- C: the most severe group, characterized by inadequate oral intake, severe retraction at presentation, and longer hospital stays.
- D: the least ill group, with little history of breathing problems but inadequate oral intake with no or mild retraction.
Infants with profile A had the highest risk for developing asthma – more than 250% greater than with typical bronchiolitis. They were also older and were more likely to have parents who had asthma – and none had solo-RSV infection. In the overall analysis, the risk for developing asthma by age 6 or 7 was 23%.
The researchers stated that the decision tree accurately predicts the high-risk profile with high degrees of sensitivity and specificity. The decision tree used four predictors that together defined infants with profile A: RSV infection status, previous breathing problems, eczema, and parental asthma.
“Our data would facilitate the development of profile-specific prevention strategies for asthma – for example, modification of host response, prophylaxis for severe viral infection – by identifying asthma risk groups early in infancy,” Dr. Fujiogi said.
The study received funding from the National Institutes of Health. Dr. Fujiogi and coauthors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Bronchiolitis is the leading cause of infant hospitalizations in the United States and Europe, and almost one-third of these patients go on to develop asthma later in childhood.
But a multinational team of researchers has presented evidence that could avoid that outcome. They identified four different subtypes of bronchiolitis along with a decision tree that can determine which infants are most likely to develop asthma as they get older.
Reporting in the journal eClinical Medicine, Michimasa Fujiogi, MD, of Massachusetts General Hospital and Harvard University, Boston, and colleagues analyzed three multicenter prospective cohort studies that included a combined 3,081 infants hospitalized with severe bronchiolitis.
“This study added a base for the early identification of high-risk patients during early infancy,” Dr. Fujiogi said in an interview. “Using the prediction rule of this study, it is possible to identify groups at high risk of asthma during a critical period of airway development – early infancy.”
The researchers identified four clinically distinct and reproducible profiles of infants hospitalized for bronchiolitis:
- A: characterized by a history of breathing problems and eczema, rhinovirus infection, and low prevalence of respiratory syncytial virus (RSV) infection.
- B: characterized by the classic symptoms of wheezing and cough at presentation, a low prevalence of previous breathing problems and rhinovirus infection, and a high likelihood of RSV infection.
- C: the most severe group, characterized by inadequate oral intake, severe retraction at presentation, and longer hospital stays.
- D: the least ill group, with little history of breathing problems but inadequate oral intake with no or mild retraction.
Infants with profile A had the highest risk for developing asthma – more than 250% greater than with typical bronchiolitis. They were also older and were more likely to have parents who had asthma – and none had solo-RSV infection. In the overall analysis, the risk for developing asthma by age 6 or 7 was 23%.
The researchers stated that the decision tree accurately predicts the high-risk profile with high degrees of sensitivity and specificity. The decision tree used four predictors that together defined infants with profile A: RSV infection status, previous breathing problems, eczema, and parental asthma.
“Our data would facilitate the development of profile-specific prevention strategies for asthma – for example, modification of host response, prophylaxis for severe viral infection – by identifying asthma risk groups early in infancy,” Dr. Fujiogi said.
The study received funding from the National Institutes of Health. Dr. Fujiogi and coauthors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Questions about optimal dosing of isotretinoin persist, expert says
Although the to this day, according to Diane M. Thiboutot, MD.
These include, what is the ideal daily dose of isotretinoin? What is the ideal cumulative dose of isotretinoin to minimize relapse of acne? What is the ideal duration of isotretinoin therapy? How do you define relapse?
“Initially, it was recommended as 1–mg/kg per day dosing,” Dr. Thiboutot, professor of dermatology at Penn State University, Hershey, said during the Orlando Dermatology Aesthetic and Clinical Conference. “As time went on and we became familiar with flares that some patients can have on that dose, it was recommended to start treatment at a dose of 0.5 mg/kg per day. Then there were trends for low dosing, intermittent dosing, and some of the more recent medical literature is talking about high dosing.”
Clinical studies
A multicenter study of 150 patients published in 1984 found that rates of relapse (retreatment needed) were 42% of patients of patients treated with 0.1 mg/kg daily, 20% in patients treated with 0.5 mg/kg daily, and 10% in patients treated with 1.0 mg/kg daily.
In a later study, researchers who followed 299 patients for 5 years post isotretinoin treatment found that there were more relapses in the 0.5-mg/kg group versus those treated with 1.0 mg/kg. Factors that contributed to the need for more treatment courses included lower-dose regimens (0.1 mg/kg and 0.5 mg/kg), having severe acne, being a female over the age of 25 at the onset of therapy, and having a prolonged history of acne.
More recently, investigators who conducted a single-center study of 1,453 patients treated with isotretinoin defined relapse as the need for a second course of isotretinoin. “They found that neither daily nor cumulative dosages influenced relapse of acne vulgaris in patients treated with varying doses of isotretinoin as long as treatment was continued for 2 or more months after the acne had completely resolved,” said Dr. Thiboutot, a past president of the American Acne and Rosacea Society.
“The current evidence underpinning the 120-150 mg/kg cumulative threshold–dosing regimen is equivocal and is based on two low-grade studies,” she noted. “Cumulative doses required for clearance appear lower for acne of mild to moderate severity and higher for more severe acne. Future investigations should use clinically relevant endpoints as end of treatment criteria and define treatment success in acne accurately.”
Other studies have looked at whether higher doses of isotretinoin could reduce treatment failures in patients with acne, Dr. Thiboutot said. In a retrospective chart review of 102 patients with acne who had been treated with isotretinoin for at least 4 consecutive months and followed for over a year, 45.1% required further treatment and 15.7% received a second course of isotretinoin. Cumulative dose (mg/kg), follow-up period, duration of treatment, and daily dose during the last month of treatment were not significantly different between those who relapsed and those who did not relapse.
However, “while the cumulative dose of isotretinoin did not significantly impact acne relapse, patients who received a higher cumulative dose were less likely to require a second course of treatment,” the authors wrote, adding that “female patients had a higher risk of needing retrial regardless of their cumulative dose.” They commented that “prescribing a higher dose per weight may result in less severe acne recurrences and the need for further isotretinoin therapy.”
Another study evaluated 116 patients who were treated to clearance with dosing at the discretion of the provider and defined relapse as subsequent treatment with an oral or topical agent. In the lower-dose treatment (less than 220 mg/kg; mean of 170 mg/kg) group, the relapse rate was 47.4%, compared with 26.9% in the higher-dose (greater than 220 mg/kg) group (P = .03). Cheilitis and xerosis during treatment was reported in nearly all patients in both treatment groups, but retinoid dermatitis was significantly more common among those in the higher-dose group (53.8% vs. 31.6%; P = .02). There were no significant differences in other adverse events between the two groups.
According to Dr. Thiboutot, variables to consider in selection of a daily dose include the presence of intense inflammation, cysts, nodules, potential difference in side-effect profiles with ethnicity, and an individual’s degree of side effects and their level of comfort. “What are some of the concerns with higher doses? Those of us who have treated a lot of acne patients have had the unfortunate occurrence where you start someone on isotretinoin and their acne explodes, as do their cysts and nodules. Once that happens it’s a bad situation and it takes you a while to get past it.”
Acne fulminans
Dr. Thiboutot was part of a panel of experts who assembled guidelines on the classification, management, and prevention of acne fulminans, published in 2017. “Acne fulminans can be induced by isotretinoin or it can occur on its own. If you have someone who has a lot of inflammation, a lot of cysts and nodules, it’s probably best to start them on a lower dose with or without prednisone,” she said.
Age at treatment is another variable that can affect the duration of response to isotretinoin. “If the patient is very young when they need isotretinoin, it’s highly likely that they will need it again,” Dr. Thiboutot said. “Also, adult females often need a repeat dose of isotretinoin. The cumulative dose is important, and the presence of truncal acne is a factor affecting duration of response. Truncal acne takes longer to clear, and it oftentimes needs a longer treatment course.”
Data from the iPledge database indicate that 37% of 10- to 11-year-olds needed a repeat course of isotretinoin, “and as you get older, the need for retreatment is less,” she said.
High-dose versus low-dose isotretinoin
A slow, low-dose approach to the use of isotretinoin in practice can minimize side effects and cystic flares, according to Dr. Thiboutot. “The cons are that you could have a longer treatment duration, you might need more patient visits, and it creates more prolonged drug exposure in patients who can become pregnant,” she said.
“The pros of a high-dose strategy include a shorter treatment duration and potential costs savings. The cons are that there is an increased risk of side effects and a risk of cystic flare. It seems that the general agreement is to treat until clearance and then treat for another 2 months. A clear definition of acne relapse is needed as well as prospective studies to optimize dosing.”
Dr. Thiboutot disclosed that she is a consultant Galderma and Novartis. She has also performed clinical trials for Galderma, Cassiopea, and Foamix.
Commentary by Robert Sidbury, MD, MPH
Isotretinoin has been around for a long time – 40-plus years, to be exact. Despite this cumulative experience, ideal dosing and duration remain uncertain. Many providers have evolved from a “one size fits all” approach (for example, 1 mg/kg of body weight per day for 4-6 months) to a more tailored and nuanced strategy. Dr. Thiboutot validates the need to individualize therapy and helps identify patients at higher risk for relapse. Younger patients are at greater risk for repeat courses of isotretinoin – Dr. Thiboutot cites a study showing 37% of 10- to 11-year-olds required a second course of isotretinoin. This could simply be a proxy for severity, which also augurs a more protracted course. Adult women and those with truncal acne also trend toward longer courses. Knowing these risk factors will help practitioners counsel proper expectations, tailor treatment courses, and thread the always challenging isotretinoin needle: Should one dose “low and slow” and court a longer risk window, or higher and faster, sometimes leading to a dryer disaster?
Dr. Sidbury is chief of dermatology at Seattle Children's Hospital and professor, department of pediatrics, University of Washington, Seattle. He is a site principal investigator for dupilumab trials, for which the hospital has a contract with Regeneron.
This article was updated 6/10/22.
Although the to this day, according to Diane M. Thiboutot, MD.
These include, what is the ideal daily dose of isotretinoin? What is the ideal cumulative dose of isotretinoin to minimize relapse of acne? What is the ideal duration of isotretinoin therapy? How do you define relapse?
“Initially, it was recommended as 1–mg/kg per day dosing,” Dr. Thiboutot, professor of dermatology at Penn State University, Hershey, said during the Orlando Dermatology Aesthetic and Clinical Conference. “As time went on and we became familiar with flares that some patients can have on that dose, it was recommended to start treatment at a dose of 0.5 mg/kg per day. Then there were trends for low dosing, intermittent dosing, and some of the more recent medical literature is talking about high dosing.”
Clinical studies
A multicenter study of 150 patients published in 1984 found that rates of relapse (retreatment needed) were 42% of patients of patients treated with 0.1 mg/kg daily, 20% in patients treated with 0.5 mg/kg daily, and 10% in patients treated with 1.0 mg/kg daily.
In a later study, researchers who followed 299 patients for 5 years post isotretinoin treatment found that there were more relapses in the 0.5-mg/kg group versus those treated with 1.0 mg/kg. Factors that contributed to the need for more treatment courses included lower-dose regimens (0.1 mg/kg and 0.5 mg/kg), having severe acne, being a female over the age of 25 at the onset of therapy, and having a prolonged history of acne.
More recently, investigators who conducted a single-center study of 1,453 patients treated with isotretinoin defined relapse as the need for a second course of isotretinoin. “They found that neither daily nor cumulative dosages influenced relapse of acne vulgaris in patients treated with varying doses of isotretinoin as long as treatment was continued for 2 or more months after the acne had completely resolved,” said Dr. Thiboutot, a past president of the American Acne and Rosacea Society.
“The current evidence underpinning the 120-150 mg/kg cumulative threshold–dosing regimen is equivocal and is based on two low-grade studies,” she noted. “Cumulative doses required for clearance appear lower for acne of mild to moderate severity and higher for more severe acne. Future investigations should use clinically relevant endpoints as end of treatment criteria and define treatment success in acne accurately.”
Other studies have looked at whether higher doses of isotretinoin could reduce treatment failures in patients with acne, Dr. Thiboutot said. In a retrospective chart review of 102 patients with acne who had been treated with isotretinoin for at least 4 consecutive months and followed for over a year, 45.1% required further treatment and 15.7% received a second course of isotretinoin. Cumulative dose (mg/kg), follow-up period, duration of treatment, and daily dose during the last month of treatment were not significantly different between those who relapsed and those who did not relapse.
However, “while the cumulative dose of isotretinoin did not significantly impact acne relapse, patients who received a higher cumulative dose were less likely to require a second course of treatment,” the authors wrote, adding that “female patients had a higher risk of needing retrial regardless of their cumulative dose.” They commented that “prescribing a higher dose per weight may result in less severe acne recurrences and the need for further isotretinoin therapy.”
Another study evaluated 116 patients who were treated to clearance with dosing at the discretion of the provider and defined relapse as subsequent treatment with an oral or topical agent. In the lower-dose treatment (less than 220 mg/kg; mean of 170 mg/kg) group, the relapse rate was 47.4%, compared with 26.9% in the higher-dose (greater than 220 mg/kg) group (P = .03). Cheilitis and xerosis during treatment was reported in nearly all patients in both treatment groups, but retinoid dermatitis was significantly more common among those in the higher-dose group (53.8% vs. 31.6%; P = .02). There were no significant differences in other adverse events between the two groups.
According to Dr. Thiboutot, variables to consider in selection of a daily dose include the presence of intense inflammation, cysts, nodules, potential difference in side-effect profiles with ethnicity, and an individual’s degree of side effects and their level of comfort. “What are some of the concerns with higher doses? Those of us who have treated a lot of acne patients have had the unfortunate occurrence where you start someone on isotretinoin and their acne explodes, as do their cysts and nodules. Once that happens it’s a bad situation and it takes you a while to get past it.”
Acne fulminans
Dr. Thiboutot was part of a panel of experts who assembled guidelines on the classification, management, and prevention of acne fulminans, published in 2017. “Acne fulminans can be induced by isotretinoin or it can occur on its own. If you have someone who has a lot of inflammation, a lot of cysts and nodules, it’s probably best to start them on a lower dose with or without prednisone,” she said.
Age at treatment is another variable that can affect the duration of response to isotretinoin. “If the patient is very young when they need isotretinoin, it’s highly likely that they will need it again,” Dr. Thiboutot said. “Also, adult females often need a repeat dose of isotretinoin. The cumulative dose is important, and the presence of truncal acne is a factor affecting duration of response. Truncal acne takes longer to clear, and it oftentimes needs a longer treatment course.”
Data from the iPledge database indicate that 37% of 10- to 11-year-olds needed a repeat course of isotretinoin, “and as you get older, the need for retreatment is less,” she said.
High-dose versus low-dose isotretinoin
A slow, low-dose approach to the use of isotretinoin in practice can minimize side effects and cystic flares, according to Dr. Thiboutot. “The cons are that you could have a longer treatment duration, you might need more patient visits, and it creates more prolonged drug exposure in patients who can become pregnant,” she said.
“The pros of a high-dose strategy include a shorter treatment duration and potential costs savings. The cons are that there is an increased risk of side effects and a risk of cystic flare. It seems that the general agreement is to treat until clearance and then treat for another 2 months. A clear definition of acne relapse is needed as well as prospective studies to optimize dosing.”
Dr. Thiboutot disclosed that she is a consultant Galderma and Novartis. She has also performed clinical trials for Galderma, Cassiopea, and Foamix.
Commentary by Robert Sidbury, MD, MPH
Isotretinoin has been around for a long time – 40-plus years, to be exact. Despite this cumulative experience, ideal dosing and duration remain uncertain. Many providers have evolved from a “one size fits all” approach (for example, 1 mg/kg of body weight per day for 4-6 months) to a more tailored and nuanced strategy. Dr. Thiboutot validates the need to individualize therapy and helps identify patients at higher risk for relapse. Younger patients are at greater risk for repeat courses of isotretinoin – Dr. Thiboutot cites a study showing 37% of 10- to 11-year-olds required a second course of isotretinoin. This could simply be a proxy for severity, which also augurs a more protracted course. Adult women and those with truncal acne also trend toward longer courses. Knowing these risk factors will help practitioners counsel proper expectations, tailor treatment courses, and thread the always challenging isotretinoin needle: Should one dose “low and slow” and court a longer risk window, or higher and faster, sometimes leading to a dryer disaster?
Dr. Sidbury is chief of dermatology at Seattle Children's Hospital and professor, department of pediatrics, University of Washington, Seattle. He is a site principal investigator for dupilumab trials, for which the hospital has a contract with Regeneron.
This article was updated 6/10/22.
Although the to this day, according to Diane M. Thiboutot, MD.
These include, what is the ideal daily dose of isotretinoin? What is the ideal cumulative dose of isotretinoin to minimize relapse of acne? What is the ideal duration of isotretinoin therapy? How do you define relapse?
“Initially, it was recommended as 1–mg/kg per day dosing,” Dr. Thiboutot, professor of dermatology at Penn State University, Hershey, said during the Orlando Dermatology Aesthetic and Clinical Conference. “As time went on and we became familiar with flares that some patients can have on that dose, it was recommended to start treatment at a dose of 0.5 mg/kg per day. Then there were trends for low dosing, intermittent dosing, and some of the more recent medical literature is talking about high dosing.”
Clinical studies
A multicenter study of 150 patients published in 1984 found that rates of relapse (retreatment needed) were 42% of patients of patients treated with 0.1 mg/kg daily, 20% in patients treated with 0.5 mg/kg daily, and 10% in patients treated with 1.0 mg/kg daily.
In a later study, researchers who followed 299 patients for 5 years post isotretinoin treatment found that there were more relapses in the 0.5-mg/kg group versus those treated with 1.0 mg/kg. Factors that contributed to the need for more treatment courses included lower-dose regimens (0.1 mg/kg and 0.5 mg/kg), having severe acne, being a female over the age of 25 at the onset of therapy, and having a prolonged history of acne.
More recently, investigators who conducted a single-center study of 1,453 patients treated with isotretinoin defined relapse as the need for a second course of isotretinoin. “They found that neither daily nor cumulative dosages influenced relapse of acne vulgaris in patients treated with varying doses of isotretinoin as long as treatment was continued for 2 or more months after the acne had completely resolved,” said Dr. Thiboutot, a past president of the American Acne and Rosacea Society.
“The current evidence underpinning the 120-150 mg/kg cumulative threshold–dosing regimen is equivocal and is based on two low-grade studies,” she noted. “Cumulative doses required for clearance appear lower for acne of mild to moderate severity and higher for more severe acne. Future investigations should use clinically relevant endpoints as end of treatment criteria and define treatment success in acne accurately.”
Other studies have looked at whether higher doses of isotretinoin could reduce treatment failures in patients with acne, Dr. Thiboutot said. In a retrospective chart review of 102 patients with acne who had been treated with isotretinoin for at least 4 consecutive months and followed for over a year, 45.1% required further treatment and 15.7% received a second course of isotretinoin. Cumulative dose (mg/kg), follow-up period, duration of treatment, and daily dose during the last month of treatment were not significantly different between those who relapsed and those who did not relapse.
However, “while the cumulative dose of isotretinoin did not significantly impact acne relapse, patients who received a higher cumulative dose were less likely to require a second course of treatment,” the authors wrote, adding that “female patients had a higher risk of needing retrial regardless of their cumulative dose.” They commented that “prescribing a higher dose per weight may result in less severe acne recurrences and the need for further isotretinoin therapy.”
Another study evaluated 116 patients who were treated to clearance with dosing at the discretion of the provider and defined relapse as subsequent treatment with an oral or topical agent. In the lower-dose treatment (less than 220 mg/kg; mean of 170 mg/kg) group, the relapse rate was 47.4%, compared with 26.9% in the higher-dose (greater than 220 mg/kg) group (P = .03). Cheilitis and xerosis during treatment was reported in nearly all patients in both treatment groups, but retinoid dermatitis was significantly more common among those in the higher-dose group (53.8% vs. 31.6%; P = .02). There were no significant differences in other adverse events between the two groups.
According to Dr. Thiboutot, variables to consider in selection of a daily dose include the presence of intense inflammation, cysts, nodules, potential difference in side-effect profiles with ethnicity, and an individual’s degree of side effects and their level of comfort. “What are some of the concerns with higher doses? Those of us who have treated a lot of acne patients have had the unfortunate occurrence where you start someone on isotretinoin and their acne explodes, as do their cysts and nodules. Once that happens it’s a bad situation and it takes you a while to get past it.”
Acne fulminans
Dr. Thiboutot was part of a panel of experts who assembled guidelines on the classification, management, and prevention of acne fulminans, published in 2017. “Acne fulminans can be induced by isotretinoin or it can occur on its own. If you have someone who has a lot of inflammation, a lot of cysts and nodules, it’s probably best to start them on a lower dose with or without prednisone,” she said.
Age at treatment is another variable that can affect the duration of response to isotretinoin. “If the patient is very young when they need isotretinoin, it’s highly likely that they will need it again,” Dr. Thiboutot said. “Also, adult females often need a repeat dose of isotretinoin. The cumulative dose is important, and the presence of truncal acne is a factor affecting duration of response. Truncal acne takes longer to clear, and it oftentimes needs a longer treatment course.”
Data from the iPledge database indicate that 37% of 10- to 11-year-olds needed a repeat course of isotretinoin, “and as you get older, the need for retreatment is less,” she said.
High-dose versus low-dose isotretinoin
A slow, low-dose approach to the use of isotretinoin in practice can minimize side effects and cystic flares, according to Dr. Thiboutot. “The cons are that you could have a longer treatment duration, you might need more patient visits, and it creates more prolonged drug exposure in patients who can become pregnant,” she said.
“The pros of a high-dose strategy include a shorter treatment duration and potential costs savings. The cons are that there is an increased risk of side effects and a risk of cystic flare. It seems that the general agreement is to treat until clearance and then treat for another 2 months. A clear definition of acne relapse is needed as well as prospective studies to optimize dosing.”
Dr. Thiboutot disclosed that she is a consultant Galderma and Novartis. She has also performed clinical trials for Galderma, Cassiopea, and Foamix.
Commentary by Robert Sidbury, MD, MPH
Isotretinoin has been around for a long time – 40-plus years, to be exact. Despite this cumulative experience, ideal dosing and duration remain uncertain. Many providers have evolved from a “one size fits all” approach (for example, 1 mg/kg of body weight per day for 4-6 months) to a more tailored and nuanced strategy. Dr. Thiboutot validates the need to individualize therapy and helps identify patients at higher risk for relapse. Younger patients are at greater risk for repeat courses of isotretinoin – Dr. Thiboutot cites a study showing 37% of 10- to 11-year-olds required a second course of isotretinoin. This could simply be a proxy for severity, which also augurs a more protracted course. Adult women and those with truncal acne also trend toward longer courses. Knowing these risk factors will help practitioners counsel proper expectations, tailor treatment courses, and thread the always challenging isotretinoin needle: Should one dose “low and slow” and court a longer risk window, or higher and faster, sometimes leading to a dryer disaster?
Dr. Sidbury is chief of dermatology at Seattle Children's Hospital and professor, department of pediatrics, University of Washington, Seattle. He is a site principal investigator for dupilumab trials, for which the hospital has a contract with Regeneron.
This article was updated 6/10/22.
FROM ODAC 2022



