User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Endometriosis not linked with preterm birth, new study finds
These new findings, which were published online in JAMA Network Open, suggest that changing monitoring strategies to prevent preterm birth for women with the disease may not be necessary.
The research team, led by Louis Marcellin, MD, PhD, with the department of obstetrics and gynecology at Université de Paris, also found that disease phenotype or whether the preterm birth was induced or spontaneous did not appear to alter the result.
Those results differ from previous research. Data on the phenotypes and their link with preterm birth have been scarce, but previous studies have shown the risk for preterm birth is more pronounced in women who have deep endometriosis than in women with ovarian endometriosis.
Dr. Marcellin said in an interview that “little is known about the impact of endometriosis on obstetric outcomes. In contrast to previous studies, we reported no differences in the risk for preterm delivery between women with endometriosis (34 of 470 [7.2%]) and those without endometriosis (53 of 881 [6.0%]), even when adjusted for multiple factors.”
The authors accounted for mother’s age, body mass index before pregnancy, birth country, number of times the woman had given birth, previous cesarean delivery, and history of preterm birth. After adjusting for potential confounders, endometriosis was not associated with preterm birth (adjusted odds ratio, 1.07; 95% confidence interval, 0.64-1.77).
The researchers found no differences among preterm births based on a mother’s endometriosis phenotype. Those phenotypes include Isolated superficial peritoneal endometriosis, ovarian endometrioma, and deep endometriosis.
“Monitoring pregnancy beyond the normal protocols or changing management strategies may not be warranted in cases of endometriosis,” Dr. Marcellin said.
More research on endometriosis’ potential link to birth outcomes is needed.
An expert not involved with the study said the new paper highlights important new avenues of research but should not be seen as the final word on the connection between endometriosis and preterm birth.
Of the 1,351 study participants (mean age, 32.9 years) who had a singleton delivery after 22 weeks’ gestation, 470 were assigned to the endometriosis group, and 881 were assigned to the control group.
The authors concluded that “pregnant women with endometriosis should not be considered to have an exceptionally high risk for preterm birth. However, further studies are needed to examine the potential for other adverse perinatal outcomes or specific but rare complications.”
Daniela Carusi, MD, said the difficulty with the study’s design is that “premature birth is not one problem or one disease.”
Many very different problems can all end with premature birth. Sometimes it’s an infection or inflammation or bleeding in the uterus or hypertension in the mother, for example, and all those things can lead to a preterm birth, she explained.
“This study inherently lumps all those things together,” said Dr. Carusi, who is director of surgical obstetrics and placental abnormalities in the department of obstetrics and gynecology at Brigham and Women’s Hospital, Boston. “It’s quite possible endometriosis can have a big impact in one of those areas and no impact in the other areas, but the study design wouldn’t be able to pick that up.”
Editorialists: Results challenge findings of previous studies
In an accompanying commentary, Liisu Saavalainen, MD, PhD, and Oskari Heikinheimo, MD, PhD, both with the department of obstetrics and gynecology, Helsinki University Hospital, wrote that several previous studies have suggested that women with endometriosis have a slightly higher risk for preterm birth.
Those studies were mostly retrospective and differed in the way they classified endometriosis and the way they selected patients, the editorialists write. Also, most women in these studies typically had subfertility, they added.
The study by Dr. Marcellin and colleagues differs from previous related research in that was prospective and assessed the risk for preterm delivery in women both with endometriosis and those without endometriosis from several maternity centers in France. The women with endometriosis were classified according to the severity of their disease.
The editorialists wrote: “The novel results by Marcellin et al. challenge the findings of most previous studies on this topic. These results are valuable and comforting. However, they are also likely to trigger new studies on the pregnancy risks associated with different types of endometriosis. That is good news.”
Dr. Carusi said the study was well done and included a notably large size. Further complimenting the research, she said it’s important to talk about this little-discussed pregnancy complication. There’s been much more focus for women with endometriosis and their physicians on getting pregnant and on talking about the length of their term.
The study leaves some things unanswered.
The study was funded by research grants from the French Ministry of Health and was sponsored by the Département de la Recherche Clinique et du Développement de l’Assistance Publique–Hôpitaux de Paris. Dr. Carusi reported no relevant financial relationships. One study coauthor reported receiving personal fees from Bioserinity and Ferring outside the submitted work. No other disclosures were reported.
A version of this article first appeared on Medscape.com.
These new findings, which were published online in JAMA Network Open, suggest that changing monitoring strategies to prevent preterm birth for women with the disease may not be necessary.
The research team, led by Louis Marcellin, MD, PhD, with the department of obstetrics and gynecology at Université de Paris, also found that disease phenotype or whether the preterm birth was induced or spontaneous did not appear to alter the result.
Those results differ from previous research. Data on the phenotypes and their link with preterm birth have been scarce, but previous studies have shown the risk for preterm birth is more pronounced in women who have deep endometriosis than in women with ovarian endometriosis.
Dr. Marcellin said in an interview that “little is known about the impact of endometriosis on obstetric outcomes. In contrast to previous studies, we reported no differences in the risk for preterm delivery between women with endometriosis (34 of 470 [7.2%]) and those without endometriosis (53 of 881 [6.0%]), even when adjusted for multiple factors.”
The authors accounted for mother’s age, body mass index before pregnancy, birth country, number of times the woman had given birth, previous cesarean delivery, and history of preterm birth. After adjusting for potential confounders, endometriosis was not associated with preterm birth (adjusted odds ratio, 1.07; 95% confidence interval, 0.64-1.77).
The researchers found no differences among preterm births based on a mother’s endometriosis phenotype. Those phenotypes include Isolated superficial peritoneal endometriosis, ovarian endometrioma, and deep endometriosis.
“Monitoring pregnancy beyond the normal protocols or changing management strategies may not be warranted in cases of endometriosis,” Dr. Marcellin said.
More research on endometriosis’ potential link to birth outcomes is needed.
An expert not involved with the study said the new paper highlights important new avenues of research but should not be seen as the final word on the connection between endometriosis and preterm birth.
Of the 1,351 study participants (mean age, 32.9 years) who had a singleton delivery after 22 weeks’ gestation, 470 were assigned to the endometriosis group, and 881 were assigned to the control group.
The authors concluded that “pregnant women with endometriosis should not be considered to have an exceptionally high risk for preterm birth. However, further studies are needed to examine the potential for other adverse perinatal outcomes or specific but rare complications.”
Daniela Carusi, MD, said the difficulty with the study’s design is that “premature birth is not one problem or one disease.”
Many very different problems can all end with premature birth. Sometimes it’s an infection or inflammation or bleeding in the uterus or hypertension in the mother, for example, and all those things can lead to a preterm birth, she explained.
“This study inherently lumps all those things together,” said Dr. Carusi, who is director of surgical obstetrics and placental abnormalities in the department of obstetrics and gynecology at Brigham and Women’s Hospital, Boston. “It’s quite possible endometriosis can have a big impact in one of those areas and no impact in the other areas, but the study design wouldn’t be able to pick that up.”
Editorialists: Results challenge findings of previous studies
In an accompanying commentary, Liisu Saavalainen, MD, PhD, and Oskari Heikinheimo, MD, PhD, both with the department of obstetrics and gynecology, Helsinki University Hospital, wrote that several previous studies have suggested that women with endometriosis have a slightly higher risk for preterm birth.
Those studies were mostly retrospective and differed in the way they classified endometriosis and the way they selected patients, the editorialists write. Also, most women in these studies typically had subfertility, they added.
The study by Dr. Marcellin and colleagues differs from previous related research in that was prospective and assessed the risk for preterm delivery in women both with endometriosis and those without endometriosis from several maternity centers in France. The women with endometriosis were classified according to the severity of their disease.
The editorialists wrote: “The novel results by Marcellin et al. challenge the findings of most previous studies on this topic. These results are valuable and comforting. However, they are also likely to trigger new studies on the pregnancy risks associated with different types of endometriosis. That is good news.”
Dr. Carusi said the study was well done and included a notably large size. Further complimenting the research, she said it’s important to talk about this little-discussed pregnancy complication. There’s been much more focus for women with endometriosis and their physicians on getting pregnant and on talking about the length of their term.
The study leaves some things unanswered.
The study was funded by research grants from the French Ministry of Health and was sponsored by the Département de la Recherche Clinique et du Développement de l’Assistance Publique–Hôpitaux de Paris. Dr. Carusi reported no relevant financial relationships. One study coauthor reported receiving personal fees from Bioserinity and Ferring outside the submitted work. No other disclosures were reported.
A version of this article first appeared on Medscape.com.
These new findings, which were published online in JAMA Network Open, suggest that changing monitoring strategies to prevent preterm birth for women with the disease may not be necessary.
The research team, led by Louis Marcellin, MD, PhD, with the department of obstetrics and gynecology at Université de Paris, also found that disease phenotype or whether the preterm birth was induced or spontaneous did not appear to alter the result.
Those results differ from previous research. Data on the phenotypes and their link with preterm birth have been scarce, but previous studies have shown the risk for preterm birth is more pronounced in women who have deep endometriosis than in women with ovarian endometriosis.
Dr. Marcellin said in an interview that “little is known about the impact of endometriosis on obstetric outcomes. In contrast to previous studies, we reported no differences in the risk for preterm delivery between women with endometriosis (34 of 470 [7.2%]) and those without endometriosis (53 of 881 [6.0%]), even when adjusted for multiple factors.”
The authors accounted for mother’s age, body mass index before pregnancy, birth country, number of times the woman had given birth, previous cesarean delivery, and history of preterm birth. After adjusting for potential confounders, endometriosis was not associated with preterm birth (adjusted odds ratio, 1.07; 95% confidence interval, 0.64-1.77).
The researchers found no differences among preterm births based on a mother’s endometriosis phenotype. Those phenotypes include Isolated superficial peritoneal endometriosis, ovarian endometrioma, and deep endometriosis.
“Monitoring pregnancy beyond the normal protocols or changing management strategies may not be warranted in cases of endometriosis,” Dr. Marcellin said.
More research on endometriosis’ potential link to birth outcomes is needed.
An expert not involved with the study said the new paper highlights important new avenues of research but should not be seen as the final word on the connection between endometriosis and preterm birth.
Of the 1,351 study participants (mean age, 32.9 years) who had a singleton delivery after 22 weeks’ gestation, 470 were assigned to the endometriosis group, and 881 were assigned to the control group.
The authors concluded that “pregnant women with endometriosis should not be considered to have an exceptionally high risk for preterm birth. However, further studies are needed to examine the potential for other adverse perinatal outcomes or specific but rare complications.”
Daniela Carusi, MD, said the difficulty with the study’s design is that “premature birth is not one problem or one disease.”
Many very different problems can all end with premature birth. Sometimes it’s an infection or inflammation or bleeding in the uterus or hypertension in the mother, for example, and all those things can lead to a preterm birth, she explained.
“This study inherently lumps all those things together,” said Dr. Carusi, who is director of surgical obstetrics and placental abnormalities in the department of obstetrics and gynecology at Brigham and Women’s Hospital, Boston. “It’s quite possible endometriosis can have a big impact in one of those areas and no impact in the other areas, but the study design wouldn’t be able to pick that up.”
Editorialists: Results challenge findings of previous studies
In an accompanying commentary, Liisu Saavalainen, MD, PhD, and Oskari Heikinheimo, MD, PhD, both with the department of obstetrics and gynecology, Helsinki University Hospital, wrote that several previous studies have suggested that women with endometriosis have a slightly higher risk for preterm birth.
Those studies were mostly retrospective and differed in the way they classified endometriosis and the way they selected patients, the editorialists write. Also, most women in these studies typically had subfertility, they added.
The study by Dr. Marcellin and colleagues differs from previous related research in that was prospective and assessed the risk for preterm delivery in women both with endometriosis and those without endometriosis from several maternity centers in France. The women with endometriosis were classified according to the severity of their disease.
The editorialists wrote: “The novel results by Marcellin et al. challenge the findings of most previous studies on this topic. These results are valuable and comforting. However, they are also likely to trigger new studies on the pregnancy risks associated with different types of endometriosis. That is good news.”
Dr. Carusi said the study was well done and included a notably large size. Further complimenting the research, she said it’s important to talk about this little-discussed pregnancy complication. There’s been much more focus for women with endometriosis and their physicians on getting pregnant and on talking about the length of their term.
The study leaves some things unanswered.
The study was funded by research grants from the French Ministry of Health and was sponsored by the Département de la Recherche Clinique et du Développement de l’Assistance Publique–Hôpitaux de Paris. Dr. Carusi reported no relevant financial relationships. One study coauthor reported receiving personal fees from Bioserinity and Ferring outside the submitted work. No other disclosures were reported.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
If you’ve got 3 seconds, then you’ve got time to work out
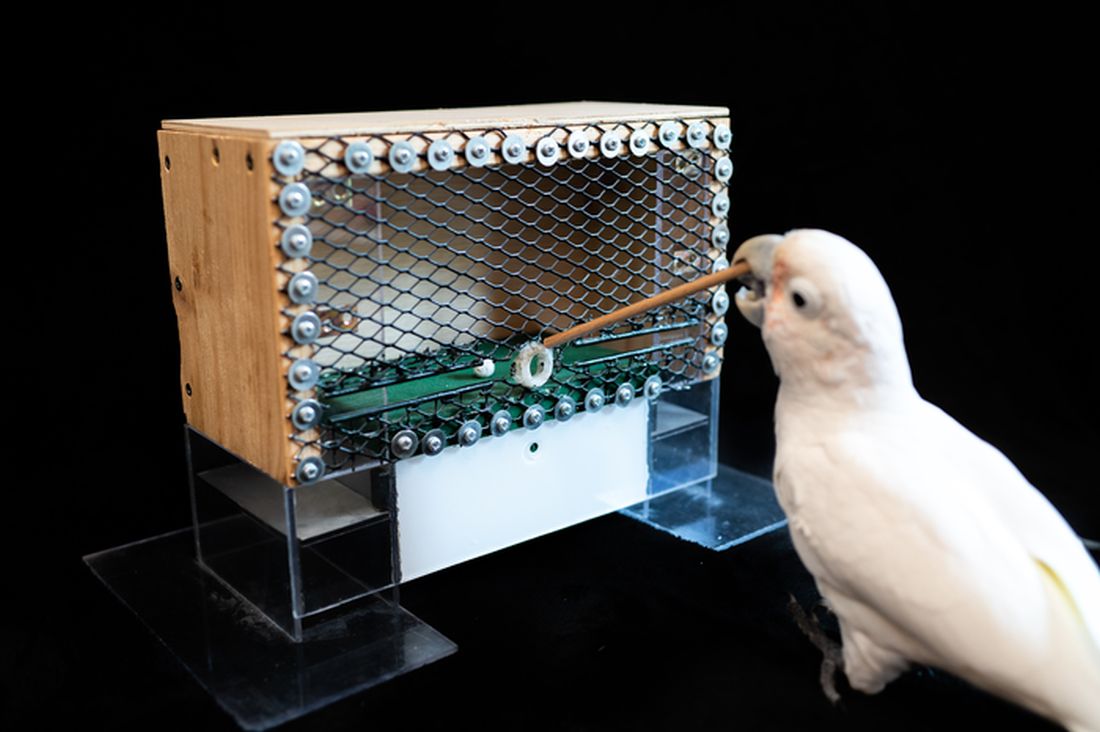
Goffin’s cockatoo? More like golfin’ cockatoo
Can birds play golf? Of course not; it’s ridiculous. Humans can barely play golf, and we invented the sport. Anyway, moving on to “Brian retraction injury after elective aneurysm clipping.”
Hang on, we’re now hearing that a group of researchers, as part of a large international project comparing children’s innovation and problem-solving skills with those of cockatoos, have in fact taught a group of Goffin’s cockatoos how to play golf. Huh. What an oddly specific project. All right, fine, I guess we’ll go with the golf-playing birds.
Golf may seem very simple at its core. It is, essentially, whacking a ball with a stick. But the Scots who invented the game were undertaking a complex project involving combined usage of multiple tools, and until now, only primates were thought to be capable of utilizing compound tools to play games such as golf.
For this latest research, published in Scientific Reports, our intrepid birds were given a rudimentary form of golf to play (featuring a stick, a ball, and a closed box to get the ball through). Putting the ball through the hole gave the bird a reward. Not every cockatoo was able to hole out, but three did, with each inventing a unique way to manipulate the stick to hit the ball.
As entertaining as it would be to simply teach some birds how to play golf, we do loop back around to medical relevance. While children are perfectly capable of using tools, young children in particular are actually quite bad at using tools to solve novel solutions. Present a 5-year-old with a stick, a ball, and a hole, and that child might not figure out what the cockatoos did. The research really does give insight into the psychology behind the development of complex tools and technology by our ancient ancestors, according to the researchers.
We’re not entirely convinced this isn’t an elaborate ploy to get a bird out onto the PGA Tour. The LOTME staff can see the future headline already: “Painted bunting wins Valspar Championship in epic playoff.”
Work out now, sweat never
Okay, show of hands: Who’s familiar with “Name that tune?” The TV game show got a reboot last year, but some of us are old enough to remember the 1970s version hosted by national treasure Tom Kennedy.
The contestants try to identify a song as quickly as possible, claiming that they “can name that tune in five notes.” Or four notes, or three. Well, welcome to “Name that exercise study.”
Senior author Masatoshi Nakamura, PhD, and associates gathered together 39 students from Niigata (Japan) University of Health and Welfare and had them perform one isometric, concentric, or eccentric bicep curl with a dumbbell for 3 seconds a day at maximum effort for 5 days a week, over 4 weeks. And yes, we did say 3 seconds.
“Lifting the weight sees the bicep in concentric contraction, lowering the weight sees it in eccentric contraction, while holding the weight parallel to the ground is isometric,” they explained in a statement on Eurekalert.
The three exercise groups were compared with a group that did no exercise, and after 4 weeks of rigorous but brief science, the group doing eccentric contractions had the best results, as their overall muscle strength increased by 11.5%. After a total of just 60 seconds of exercise in 4 weeks. That’s 60 seconds. In 4 weeks.
Big news, but maybe we can do better. “Tom, we can do that exercise in 2 seconds.”
And one! And two! Whoa, feel the burn.
Tingling over anxiety
Apparently there are two kinds of people in this world. Those who love ASMR and those who just don’t get it.
ASMR, for those who don’t know, is the autonomous sensory meridian response. An online community has surfaced, with video creators making tapping sounds, whispering, or brushing mannequin hair to elicit “a pleasant tingling sensation originating from the scalp and neck which can spread to the rest of the body” from viewers, Charlotte M. Eid and associates said in PLOS One.
The people who are into these types of videos are more likely to have higher levels of neuroticism than those who aren’t, which gives ASMR the potential to be a nontraditional form of treatment for anxiety and/or neuroticism, they suggested.
The research involved a group of 64 volunteers who watched an ASMR video meant to trigger the tingles and then completed questionnaires to evaluate their levels of neuroticism, trait anxiety, and state anxiety, said Ms. Eid and associates of Northumbria University in Newcastle-upon-Tyne, England.
The people who had a history of producing tingles from ASMR videos in the past had higher levels of anxiety, compared with those who didn’t. Those who responded to triggers also received some benefit from the video in the study, reporting lower levels of neuroticism and anxiety after watching, the investigators found.
Although people who didn’t have a history of tingles didn’t feel any reduction in anxiety after the video, that didn’t stop the people who weren’t familiar with the genre from catching tingles.
So if you find yourself a little high strung or anxious, or if you can’t sleep, consider watching a person pretending to give you a makeover or using fingernails to tap on books for some relaxation. Don’t knock it until you try it!
Living in the past? Not so far-fetched
It’s usually an insult when people tell us to stop living in the past, but the joke’s on them because we really do live in the past. By 15 seconds, to be exact, according to researchers from the University of California, Berkeley.
But wait, did you just read that last sentence 15 seconds ago, even though it feels like real time? Did we just type these words now, or 15 seconds ago?
Think of your brain as a web page you’re constantly refreshing. We are constantly seeing new pictures, images, and colors, and your brain is responsible for keeping everything in chronological order. This new research suggests that our brains show us images from 15 seconds prior. Is your mind blown yet?
“One could say our brain is procrastinating. It’s too much work to constantly update images, so it sticks to the past because the past is a good predictor of the present. We recycle information from the past because it’s faster, more efficient and less work,” senior author David Whitney explained in a statement from the university.
It seems like the 15-second rule helps us not lose our minds by keeping a steady flow of information, but it could be a bit dangerous if someone, such as a surgeon, needs to see things with extreme precision.
And now we are definitely feeling a bit anxious about our upcoming heart/spleen/gallbladder replacement. … Where’s that link to the ASMR video?
Goffin’s cockatoo? More like golfin’ cockatoo
Can birds play golf? Of course not; it’s ridiculous. Humans can barely play golf, and we invented the sport. Anyway, moving on to “Brian retraction injury after elective aneurysm clipping.”
Hang on, we’re now hearing that a group of researchers, as part of a large international project comparing children’s innovation and problem-solving skills with those of cockatoos, have in fact taught a group of Goffin’s cockatoos how to play golf. Huh. What an oddly specific project. All right, fine, I guess we’ll go with the golf-playing birds.
Golf may seem very simple at its core. It is, essentially, whacking a ball with a stick. But the Scots who invented the game were undertaking a complex project involving combined usage of multiple tools, and until now, only primates were thought to be capable of utilizing compound tools to play games such as golf.
For this latest research, published in Scientific Reports, our intrepid birds were given a rudimentary form of golf to play (featuring a stick, a ball, and a closed box to get the ball through). Putting the ball through the hole gave the bird a reward. Not every cockatoo was able to hole out, but three did, with each inventing a unique way to manipulate the stick to hit the ball.
As entertaining as it would be to simply teach some birds how to play golf, we do loop back around to medical relevance. While children are perfectly capable of using tools, young children in particular are actually quite bad at using tools to solve novel solutions. Present a 5-year-old with a stick, a ball, and a hole, and that child might not figure out what the cockatoos did. The research really does give insight into the psychology behind the development of complex tools and technology by our ancient ancestors, according to the researchers.
We’re not entirely convinced this isn’t an elaborate ploy to get a bird out onto the PGA Tour. The LOTME staff can see the future headline already: “Painted bunting wins Valspar Championship in epic playoff.”
Work out now, sweat never
Okay, show of hands: Who’s familiar with “Name that tune?” The TV game show got a reboot last year, but some of us are old enough to remember the 1970s version hosted by national treasure Tom Kennedy.
The contestants try to identify a song as quickly as possible, claiming that they “can name that tune in five notes.” Or four notes, or three. Well, welcome to “Name that exercise study.”
Senior author Masatoshi Nakamura, PhD, and associates gathered together 39 students from Niigata (Japan) University of Health and Welfare and had them perform one isometric, concentric, or eccentric bicep curl with a dumbbell for 3 seconds a day at maximum effort for 5 days a week, over 4 weeks. And yes, we did say 3 seconds.
“Lifting the weight sees the bicep in concentric contraction, lowering the weight sees it in eccentric contraction, while holding the weight parallel to the ground is isometric,” they explained in a statement on Eurekalert.
The three exercise groups were compared with a group that did no exercise, and after 4 weeks of rigorous but brief science, the group doing eccentric contractions had the best results, as their overall muscle strength increased by 11.5%. After a total of just 60 seconds of exercise in 4 weeks. That’s 60 seconds. In 4 weeks.
Big news, but maybe we can do better. “Tom, we can do that exercise in 2 seconds.”
And one! And two! Whoa, feel the burn.
Tingling over anxiety
Apparently there are two kinds of people in this world. Those who love ASMR and those who just don’t get it.
ASMR, for those who don’t know, is the autonomous sensory meridian response. An online community has surfaced, with video creators making tapping sounds, whispering, or brushing mannequin hair to elicit “a pleasant tingling sensation originating from the scalp and neck which can spread to the rest of the body” from viewers, Charlotte M. Eid and associates said in PLOS One.
The people who are into these types of videos are more likely to have higher levels of neuroticism than those who aren’t, which gives ASMR the potential to be a nontraditional form of treatment for anxiety and/or neuroticism, they suggested.
The research involved a group of 64 volunteers who watched an ASMR video meant to trigger the tingles and then completed questionnaires to evaluate their levels of neuroticism, trait anxiety, and state anxiety, said Ms. Eid and associates of Northumbria University in Newcastle-upon-Tyne, England.
The people who had a history of producing tingles from ASMR videos in the past had higher levels of anxiety, compared with those who didn’t. Those who responded to triggers also received some benefit from the video in the study, reporting lower levels of neuroticism and anxiety after watching, the investigators found.
Although people who didn’t have a history of tingles didn’t feel any reduction in anxiety after the video, that didn’t stop the people who weren’t familiar with the genre from catching tingles.
So if you find yourself a little high strung or anxious, or if you can’t sleep, consider watching a person pretending to give you a makeover or using fingernails to tap on books for some relaxation. Don’t knock it until you try it!
Living in the past? Not so far-fetched
It’s usually an insult when people tell us to stop living in the past, but the joke’s on them because we really do live in the past. By 15 seconds, to be exact, according to researchers from the University of California, Berkeley.
But wait, did you just read that last sentence 15 seconds ago, even though it feels like real time? Did we just type these words now, or 15 seconds ago?
Think of your brain as a web page you’re constantly refreshing. We are constantly seeing new pictures, images, and colors, and your brain is responsible for keeping everything in chronological order. This new research suggests that our brains show us images from 15 seconds prior. Is your mind blown yet?
“One could say our brain is procrastinating. It’s too much work to constantly update images, so it sticks to the past because the past is a good predictor of the present. We recycle information from the past because it’s faster, more efficient and less work,” senior author David Whitney explained in a statement from the university.
It seems like the 15-second rule helps us not lose our minds by keeping a steady flow of information, but it could be a bit dangerous if someone, such as a surgeon, needs to see things with extreme precision.
And now we are definitely feeling a bit anxious about our upcoming heart/spleen/gallbladder replacement. … Where’s that link to the ASMR video?
Goffin’s cockatoo? More like golfin’ cockatoo
Can birds play golf? Of course not; it’s ridiculous. Humans can barely play golf, and we invented the sport. Anyway, moving on to “Brian retraction injury after elective aneurysm clipping.”
Hang on, we’re now hearing that a group of researchers, as part of a large international project comparing children’s innovation and problem-solving skills with those of cockatoos, have in fact taught a group of Goffin’s cockatoos how to play golf. Huh. What an oddly specific project. All right, fine, I guess we’ll go with the golf-playing birds.
Golf may seem very simple at its core. It is, essentially, whacking a ball with a stick. But the Scots who invented the game were undertaking a complex project involving combined usage of multiple tools, and until now, only primates were thought to be capable of utilizing compound tools to play games such as golf.
For this latest research, published in Scientific Reports, our intrepid birds were given a rudimentary form of golf to play (featuring a stick, a ball, and a closed box to get the ball through). Putting the ball through the hole gave the bird a reward. Not every cockatoo was able to hole out, but three did, with each inventing a unique way to manipulate the stick to hit the ball.
As entertaining as it would be to simply teach some birds how to play golf, we do loop back around to medical relevance. While children are perfectly capable of using tools, young children in particular are actually quite bad at using tools to solve novel solutions. Present a 5-year-old with a stick, a ball, and a hole, and that child might not figure out what the cockatoos did. The research really does give insight into the psychology behind the development of complex tools and technology by our ancient ancestors, according to the researchers.
We’re not entirely convinced this isn’t an elaborate ploy to get a bird out onto the PGA Tour. The LOTME staff can see the future headline already: “Painted bunting wins Valspar Championship in epic playoff.”
Work out now, sweat never
Okay, show of hands: Who’s familiar with “Name that tune?” The TV game show got a reboot last year, but some of us are old enough to remember the 1970s version hosted by national treasure Tom Kennedy.
The contestants try to identify a song as quickly as possible, claiming that they “can name that tune in five notes.” Or four notes, or three. Well, welcome to “Name that exercise study.”
Senior author Masatoshi Nakamura, PhD, and associates gathered together 39 students from Niigata (Japan) University of Health and Welfare and had them perform one isometric, concentric, or eccentric bicep curl with a dumbbell for 3 seconds a day at maximum effort for 5 days a week, over 4 weeks. And yes, we did say 3 seconds.
“Lifting the weight sees the bicep in concentric contraction, lowering the weight sees it in eccentric contraction, while holding the weight parallel to the ground is isometric,” they explained in a statement on Eurekalert.
The three exercise groups were compared with a group that did no exercise, and after 4 weeks of rigorous but brief science, the group doing eccentric contractions had the best results, as their overall muscle strength increased by 11.5%. After a total of just 60 seconds of exercise in 4 weeks. That’s 60 seconds. In 4 weeks.
Big news, but maybe we can do better. “Tom, we can do that exercise in 2 seconds.”
And one! And two! Whoa, feel the burn.
Tingling over anxiety
Apparently there are two kinds of people in this world. Those who love ASMR and those who just don’t get it.
ASMR, for those who don’t know, is the autonomous sensory meridian response. An online community has surfaced, with video creators making tapping sounds, whispering, or brushing mannequin hair to elicit “a pleasant tingling sensation originating from the scalp and neck which can spread to the rest of the body” from viewers, Charlotte M. Eid and associates said in PLOS One.
The people who are into these types of videos are more likely to have higher levels of neuroticism than those who aren’t, which gives ASMR the potential to be a nontraditional form of treatment for anxiety and/or neuroticism, they suggested.
The research involved a group of 64 volunteers who watched an ASMR video meant to trigger the tingles and then completed questionnaires to evaluate their levels of neuroticism, trait anxiety, and state anxiety, said Ms. Eid and associates of Northumbria University in Newcastle-upon-Tyne, England.
The people who had a history of producing tingles from ASMR videos in the past had higher levels of anxiety, compared with those who didn’t. Those who responded to triggers also received some benefit from the video in the study, reporting lower levels of neuroticism and anxiety after watching, the investigators found.
Although people who didn’t have a history of tingles didn’t feel any reduction in anxiety after the video, that didn’t stop the people who weren’t familiar with the genre from catching tingles.
So if you find yourself a little high strung or anxious, or if you can’t sleep, consider watching a person pretending to give you a makeover or using fingernails to tap on books for some relaxation. Don’t knock it until you try it!
Living in the past? Not so far-fetched
It’s usually an insult when people tell us to stop living in the past, but the joke’s on them because we really do live in the past. By 15 seconds, to be exact, according to researchers from the University of California, Berkeley.
But wait, did you just read that last sentence 15 seconds ago, even though it feels like real time? Did we just type these words now, or 15 seconds ago?
Think of your brain as a web page you’re constantly refreshing. We are constantly seeing new pictures, images, and colors, and your brain is responsible for keeping everything in chronological order. This new research suggests that our brains show us images from 15 seconds prior. Is your mind blown yet?
“One could say our brain is procrastinating. It’s too much work to constantly update images, so it sticks to the past because the past is a good predictor of the present. We recycle information from the past because it’s faster, more efficient and less work,” senior author David Whitney explained in a statement from the university.
It seems like the 15-second rule helps us not lose our minds by keeping a steady flow of information, but it could be a bit dangerous if someone, such as a surgeon, needs to see things with extreme precision.
And now we are definitely feeling a bit anxious about our upcoming heart/spleen/gallbladder replacement. … Where’s that link to the ASMR video?
Substantial numbers of U.S. youth report vaping cannabis
Adolescents and young adults who use e-cigarettes reported vaping cannabis, according to selected data from the national Population Assessment of Tobacco and Health (PATH) study.
Ruoyan Sun, PhD, an assistant professor at the University of Alabama at Birmingham, and colleagues examined results of PATH’s wave5 survey conducted from December 2018 to November 2019. PATH is a National Institutes of Health–Food and Drug Administration collaboration begun in 2013.
Their analysis, published online Feb. 7, 2022, in JAMA Pediatrics, evaluated the frequency of cannabis vaping across several age groups: 164 respondents ages 12-14; 919 participants ages 15-17; and 3,038 participants ages 18-24. Respondents included for analysis reported electronic nicotine product consumption in the past 30 days. In response to the question “When you have used an electronic product, how often were you using it to smoke marijuana, marijuana concentrates, marijuana waxes, THC, or hash oils?” 35.0% (95% confidence interval, 29.3%-41.2%) of current e-smokers aged 12-14 years said they had done so, as did 51.3% (95% CI, 47.7%-54.9%) of those aged 15-17 years and 54.6% (95% CI, 52.5%-56.7%) of young adults aged 18-24.
The prevalence of those who reported vaping cannabis every time they vaped was 3.1% (95% CI, 1.3%-6.9%) of youths aged 12-14 years, 6.7% (95% CI, 5.3%-8.6%) of youths aged 15-17 years, and 10.3% (95% CI, 9.0%-11.6%) of young adults aged 18-24.
Among children ages 12-14, 65% said they never vaped cannabis, while 48.7% and 45.4%, respectively, in the two older groups said they did.
“This is a very important finding and it mirrors what some of us have already seen in practice,” said pediatric pulmonologist S. Christy Sadreameli, MD, MHS, an assistant professor of pediatrics at John Hopkins University, Baltimore. “It is important for pediatricians to realize that dual use of cannabis and nicotine vaping, and exclusive use of cannabis vaping, are not uncommon. It informs how we ask questions and how we counsel our patients.” Dr. Sadreameli was not involved in the PATH study.
Overall, the survey participants were 56% male, with 24% of respondents identifying as Hispanic, 8% as non-Hispanic Black, 58% as non-Hispanic White, and 10% as of other race/ethnicity. The weighted proportion of current e-cigarette use was 3.0% (95% CI, 2.6%-3.4%) in youths ages 12-14 years, 14.4% (95% CI, 13.5%-15.3%) in those 15-17 years, and 26.2% (95% CI, 25.3%-27.1%) in young adults.
Other recent national surveys such as the National Institute on Drug Abuses’s Monitoring the Future are reporting a growing prevalence of youth cannabis vaping, Dr. Sun said. For example, the prevalence of cannabis vaping in the past 12-month period among grade 12 students grew from 9.5% in 2017 to 22.1% in 2020. Vaping cannabis was more prevalent among Hispanic teens than other ethnicities.
Vaping devices such as e-cigarettes, vaping pens, e-cigars, and e-hookahs can be used to inhale multiple substances, including nicotine, cannabis, and opium, Dr. Sun noted in an interview. “So in addition to asking about the behavior of vaping itself, pediatricians could pay more attention to what is being vaped in these devices.”
According to Dr. Sadreameli, vaping more than one substance at a time could potentially work synergistically to cause more harm, compared with one product alone. “The other aspect to consider is that vaping multiple types of products may increase the chance of harm from other components of the mixture,” she said. For instance, a lot of the e-cigarette or vaping use-associated lung injury (EVALI) cases have been linked to vitamin E acetate, which was found in certain cannabis formulations. “Anecdotally, most EVALI patients I’ve met seemed to report use of multiple products, including cannabis-containing and nicotine-containing products.”
Dr. Sadreameli added that some cannabis vapers will have other issues. “For example, there is a severe vomiting syndrome I’ve seen, which is induced by cannabis and improved by cessation from cannabis,” she said. “It is important for pediatricians to ask the right questions of their patients in order to better understand what they may be experiencing, provide counseling, and to help them.”
A related issue is cessation, she said. “For those working to achieve cessation from nicotine-based products, sometimes nicotine replacement therapies are helpful. However, cessation from cannabis-containing products is going to look different.”
Although the study did not yield information on the prevalence simultaneous nicotine/cannabis vaping, the authors suggested that some vapers may be combining substances. Previous studies may have modestly overestimated the prevalence of nicotine vaping given their finding that some current e-cigarette users reported vaping cannabis every time they vaped and may be vaping cannabis exclusively. “However, if some current users vaped nicotine and cannabis simultaneously, then overestimation of nicotine vaping would be smaller,” they wrote.
Future surveys on this area should contain detailed questions on nicotine and cannabis vaping, including the substance being vaped and the frequency and intensity of use, Dr. Sun said. “In addition, these surveys could examine some other substances that are being vaped, such as opium and cocaine.”
The PATH study is supported by the NIH, National Institute on Drug Abuse, Department of Health & Human Services, and the FDA’s Center for Tobacco Products. The authors and Dr. Sadreameli had no competing interests to disclose.
Adolescents and young adults who use e-cigarettes reported vaping cannabis, according to selected data from the national Population Assessment of Tobacco and Health (PATH) study.
Ruoyan Sun, PhD, an assistant professor at the University of Alabama at Birmingham, and colleagues examined results of PATH’s wave5 survey conducted from December 2018 to November 2019. PATH is a National Institutes of Health–Food and Drug Administration collaboration begun in 2013.
Their analysis, published online Feb. 7, 2022, in JAMA Pediatrics, evaluated the frequency of cannabis vaping across several age groups: 164 respondents ages 12-14; 919 participants ages 15-17; and 3,038 participants ages 18-24. Respondents included for analysis reported electronic nicotine product consumption in the past 30 days. In response to the question “When you have used an electronic product, how often were you using it to smoke marijuana, marijuana concentrates, marijuana waxes, THC, or hash oils?” 35.0% (95% confidence interval, 29.3%-41.2%) of current e-smokers aged 12-14 years said they had done so, as did 51.3% (95% CI, 47.7%-54.9%) of those aged 15-17 years and 54.6% (95% CI, 52.5%-56.7%) of young adults aged 18-24.
The prevalence of those who reported vaping cannabis every time they vaped was 3.1% (95% CI, 1.3%-6.9%) of youths aged 12-14 years, 6.7% (95% CI, 5.3%-8.6%) of youths aged 15-17 years, and 10.3% (95% CI, 9.0%-11.6%) of young adults aged 18-24.
Among children ages 12-14, 65% said they never vaped cannabis, while 48.7% and 45.4%, respectively, in the two older groups said they did.
“This is a very important finding and it mirrors what some of us have already seen in practice,” said pediatric pulmonologist S. Christy Sadreameli, MD, MHS, an assistant professor of pediatrics at John Hopkins University, Baltimore. “It is important for pediatricians to realize that dual use of cannabis and nicotine vaping, and exclusive use of cannabis vaping, are not uncommon. It informs how we ask questions and how we counsel our patients.” Dr. Sadreameli was not involved in the PATH study.
Overall, the survey participants were 56% male, with 24% of respondents identifying as Hispanic, 8% as non-Hispanic Black, 58% as non-Hispanic White, and 10% as of other race/ethnicity. The weighted proportion of current e-cigarette use was 3.0% (95% CI, 2.6%-3.4%) in youths ages 12-14 years, 14.4% (95% CI, 13.5%-15.3%) in those 15-17 years, and 26.2% (95% CI, 25.3%-27.1%) in young adults.
Other recent national surveys such as the National Institute on Drug Abuses’s Monitoring the Future are reporting a growing prevalence of youth cannabis vaping, Dr. Sun said. For example, the prevalence of cannabis vaping in the past 12-month period among grade 12 students grew from 9.5% in 2017 to 22.1% in 2020. Vaping cannabis was more prevalent among Hispanic teens than other ethnicities.
Vaping devices such as e-cigarettes, vaping pens, e-cigars, and e-hookahs can be used to inhale multiple substances, including nicotine, cannabis, and opium, Dr. Sun noted in an interview. “So in addition to asking about the behavior of vaping itself, pediatricians could pay more attention to what is being vaped in these devices.”
According to Dr. Sadreameli, vaping more than one substance at a time could potentially work synergistically to cause more harm, compared with one product alone. “The other aspect to consider is that vaping multiple types of products may increase the chance of harm from other components of the mixture,” she said. For instance, a lot of the e-cigarette or vaping use-associated lung injury (EVALI) cases have been linked to vitamin E acetate, which was found in certain cannabis formulations. “Anecdotally, most EVALI patients I’ve met seemed to report use of multiple products, including cannabis-containing and nicotine-containing products.”
Dr. Sadreameli added that some cannabis vapers will have other issues. “For example, there is a severe vomiting syndrome I’ve seen, which is induced by cannabis and improved by cessation from cannabis,” she said. “It is important for pediatricians to ask the right questions of their patients in order to better understand what they may be experiencing, provide counseling, and to help them.”
A related issue is cessation, she said. “For those working to achieve cessation from nicotine-based products, sometimes nicotine replacement therapies are helpful. However, cessation from cannabis-containing products is going to look different.”
Although the study did not yield information on the prevalence simultaneous nicotine/cannabis vaping, the authors suggested that some vapers may be combining substances. Previous studies may have modestly overestimated the prevalence of nicotine vaping given their finding that some current e-cigarette users reported vaping cannabis every time they vaped and may be vaping cannabis exclusively. “However, if some current users vaped nicotine and cannabis simultaneously, then overestimation of nicotine vaping would be smaller,” they wrote.
Future surveys on this area should contain detailed questions on nicotine and cannabis vaping, including the substance being vaped and the frequency and intensity of use, Dr. Sun said. “In addition, these surveys could examine some other substances that are being vaped, such as opium and cocaine.”
The PATH study is supported by the NIH, National Institute on Drug Abuse, Department of Health & Human Services, and the FDA’s Center for Tobacco Products. The authors and Dr. Sadreameli had no competing interests to disclose.
Adolescents and young adults who use e-cigarettes reported vaping cannabis, according to selected data from the national Population Assessment of Tobacco and Health (PATH) study.
Ruoyan Sun, PhD, an assistant professor at the University of Alabama at Birmingham, and colleagues examined results of PATH’s wave5 survey conducted from December 2018 to November 2019. PATH is a National Institutes of Health–Food and Drug Administration collaboration begun in 2013.
Their analysis, published online Feb. 7, 2022, in JAMA Pediatrics, evaluated the frequency of cannabis vaping across several age groups: 164 respondents ages 12-14; 919 participants ages 15-17; and 3,038 participants ages 18-24. Respondents included for analysis reported electronic nicotine product consumption in the past 30 days. In response to the question “When you have used an electronic product, how often were you using it to smoke marijuana, marijuana concentrates, marijuana waxes, THC, or hash oils?” 35.0% (95% confidence interval, 29.3%-41.2%) of current e-smokers aged 12-14 years said they had done so, as did 51.3% (95% CI, 47.7%-54.9%) of those aged 15-17 years and 54.6% (95% CI, 52.5%-56.7%) of young adults aged 18-24.
The prevalence of those who reported vaping cannabis every time they vaped was 3.1% (95% CI, 1.3%-6.9%) of youths aged 12-14 years, 6.7% (95% CI, 5.3%-8.6%) of youths aged 15-17 years, and 10.3% (95% CI, 9.0%-11.6%) of young adults aged 18-24.
Among children ages 12-14, 65% said they never vaped cannabis, while 48.7% and 45.4%, respectively, in the two older groups said they did.
“This is a very important finding and it mirrors what some of us have already seen in practice,” said pediatric pulmonologist S. Christy Sadreameli, MD, MHS, an assistant professor of pediatrics at John Hopkins University, Baltimore. “It is important for pediatricians to realize that dual use of cannabis and nicotine vaping, and exclusive use of cannabis vaping, are not uncommon. It informs how we ask questions and how we counsel our patients.” Dr. Sadreameli was not involved in the PATH study.
Overall, the survey participants were 56% male, with 24% of respondents identifying as Hispanic, 8% as non-Hispanic Black, 58% as non-Hispanic White, and 10% as of other race/ethnicity. The weighted proportion of current e-cigarette use was 3.0% (95% CI, 2.6%-3.4%) in youths ages 12-14 years, 14.4% (95% CI, 13.5%-15.3%) in those 15-17 years, and 26.2% (95% CI, 25.3%-27.1%) in young adults.
Other recent national surveys such as the National Institute on Drug Abuses’s Monitoring the Future are reporting a growing prevalence of youth cannabis vaping, Dr. Sun said. For example, the prevalence of cannabis vaping in the past 12-month period among grade 12 students grew from 9.5% in 2017 to 22.1% in 2020. Vaping cannabis was more prevalent among Hispanic teens than other ethnicities.
Vaping devices such as e-cigarettes, vaping pens, e-cigars, and e-hookahs can be used to inhale multiple substances, including nicotine, cannabis, and opium, Dr. Sun noted in an interview. “So in addition to asking about the behavior of vaping itself, pediatricians could pay more attention to what is being vaped in these devices.”
According to Dr. Sadreameli, vaping more than one substance at a time could potentially work synergistically to cause more harm, compared with one product alone. “The other aspect to consider is that vaping multiple types of products may increase the chance of harm from other components of the mixture,” she said. For instance, a lot of the e-cigarette or vaping use-associated lung injury (EVALI) cases have been linked to vitamin E acetate, which was found in certain cannabis formulations. “Anecdotally, most EVALI patients I’ve met seemed to report use of multiple products, including cannabis-containing and nicotine-containing products.”
Dr. Sadreameli added that some cannabis vapers will have other issues. “For example, there is a severe vomiting syndrome I’ve seen, which is induced by cannabis and improved by cessation from cannabis,” she said. “It is important for pediatricians to ask the right questions of their patients in order to better understand what they may be experiencing, provide counseling, and to help them.”
A related issue is cessation, she said. “For those working to achieve cessation from nicotine-based products, sometimes nicotine replacement therapies are helpful. However, cessation from cannabis-containing products is going to look different.”
Although the study did not yield information on the prevalence simultaneous nicotine/cannabis vaping, the authors suggested that some vapers may be combining substances. Previous studies may have modestly overestimated the prevalence of nicotine vaping given their finding that some current e-cigarette users reported vaping cannabis every time they vaped and may be vaping cannabis exclusively. “However, if some current users vaped nicotine and cannabis simultaneously, then overestimation of nicotine vaping would be smaller,” they wrote.
Future surveys on this area should contain detailed questions on nicotine and cannabis vaping, including the substance being vaped and the frequency and intensity of use, Dr. Sun said. “In addition, these surveys could examine some other substances that are being vaped, such as opium and cocaine.”
The PATH study is supported by the NIH, National Institute on Drug Abuse, Department of Health & Human Services, and the FDA’s Center for Tobacco Products. The authors and Dr. Sadreameli had no competing interests to disclose.
FROM JAMA PEDIATRICS
Expert shares workup pearls for children with severe atopic dermatitis
“Many patients who have failed topical steroids have never had adequate treatment,” Anna Yasmine Kirkorian, MD, chief of dermatology at National Children’s Hospital in Washington, said during the ODAC Dermatology, Aesthetic & Surgical Conference. “There is no lower age limit on the use of topical corticosteroids, and low potency corticosteroids are inadequate to treat severe eczema. The idea that only over-the-counter 2.5% hydrocortisone cream is necessary is not true,” she added.
“You also want to scrutinize the vehicle,” she said, noting that children are often prescribed cream formulations that hurt when applied, so parents stop applying them. “Ointments are generally the vehicles of choice in childhood,” she added.
It is generally not advised to use topical and oral antibiotics in children with AD, unless there are clear signs of infection. “If they’re just slightly oozy, don’t use them,” she continued. “Of course, every child or adult with eczema has Staph aureus on them, but most of the time, what you need to do is repair the barrier. We know that from data and common sense. When we repair their barrier, their rates of infection decrease.”
A focal area with pustules and pus should be cultured and treated, Dr. Kirkorian said. “Monotherapy with antibiotics is going to do nothing for you.” In cases of children with failure to thrive, she recommends referral to pediatric dermatology, allergy/immunology, GI, or genetics, as appropriate.
For children with severe AD, Dr. Kirkorian favors a rescue plan with a one-pound jar of triamcinolone ointment 0.1%. She recommends application of the ointment to all areas, including the face and scalp once nightly for 2 weeks, with a follow-up appointment at the end of that time. “If you just give people medicine and ask them to come back in 6 months, they are not able to comply with that and they don’t have faith that it’s going to work,” explained Dr. Kirkorian, associate professor of dermatology and pediatrics at George Washington University, Washington. At the end of 2 weeks, “the majority will have improved dramatically, and then you can implement maintenance therapy with topical calcineurin inhibitors, crisaborole, or possibly topical ruxolitinib.”
Some clinicians prescribe oral antihistamines for AD, but Dr. Kirkorian said that data supporting their use are limited and antihistamines are not approved for use in children younger than 6 months of age. Sedating antihistamines will induce sleep, “but do not provide durable night-long sleep,” and routine use may have an impact on learning and school performance. In addition, exposure to antihistamines in children under age 2 may be associated with development of ADHD at school age.
The interleukin-4 receptor alpha antagonist dupilumab (Dupixent) is approved by the Food and Drug Administration for moderate to severe AD in patients ages 6 and older. But obtaining it for patients can be tricky, she said, as this requires documented failure of corticosteroids, calcineurin inhibitors, crisaborole ointment, and phototherapy (if prescribed). Patients are often obligated to do step therapy with an off-label drug such as cyclosporine or methotrexate for 3 months, and they need to demonstrate responses with objective measures of severity such as the SCORAD (SCORing Atopic Dermatitis) and the validated Investigator Global Assessment.
“Most of my patients carry insurance that does not approve dupilumab without failure of a prior off-label systemic immunosuppressant medication,” Dr. Kirkorian said. Cyclosporine is her first choice for a systemic immunosuppressant “because it has a fast onset of action, it’s effective for treatment of atopic dermatitis, and safe for short-term use,” she said. “I don’t think that methotrexate works well for eczema. It can take weeks and weeks to work.”
She typically starts patients on a 5 mg/kg dose of cyclosporine. Baseline tests include CBC, CMP (comprehensive metabolic panel), lipids, and vitals. She repeats the labs at 1 month, and includes a blood pressure check. Potential adverse effects of cyclosporine include infections (including opportunistic infections), cytopenias, hypertension, nephrotoxicity, hepatotoxicity, neurotoxicity (including posterior reversible encephalopathy syndrome), electrolyte disturbance, lymphoma, and cutaneous malignancy.
“The good news is that we generally don’t see the adverse effects with short-term use,” Dr. Kirkorian said. “We will see some hypertrichosis and gingival hypertrophy, which resolves with cessation of therapy. There are serious side effects if you use it for long enough.”
As for methotrexate, “it is still a very important drug in pediatric dermatology, particularly in other conditions such as psoriasis,” she said. “The problem is that weekly dosing of methotrexate poses a greater risk of dosing errors. People aren’t really triggered to think of a once-weekly medication. If you do use it, give them a short supply to make sure that they come back, and that they don’t give it daily accidentally.”
Practical tips she offered for prescribing cyclosporine include supplying a patient handout with information on all adverse effects, dosing information, vaccination information, and pregnancy precautions, with contact information (a patient portal or on-call number) for the treating clinician in case a patient develops adverse effects. Administration of live vaccines while patients are on cyclosporine is not recommended.
When transitioning patients from cyclosporine or methotrexate to dupilumab, Dr. Kirkorian recommends tapering the immunosuppressant dose by half every 2 weeks to complete cessation by week 8 of treatment. For patients who experience a severe baseline flare once the immunosuppressant is tapered, despite the switch to dupilumab, she recommends restarting methotrexate at a full dose and then reducing the dose every 2 weeks until the lowest effective dose (2.5-5 mg weekly) is reached.
“Waning efficacy is real,” she said. “We can add methotrexate to recapture efficacy. Check for superimposed allergic contact dermatitis.”
With upadacitinib (Rinvoq), an oral Janus kinase (JAK) inhibitor recently approved for treating refractory, moderate to severe AD in patients 12 years of age and older, is the risk profile acceptable to parents and physicians? “I think the answer is yes,” Dr. Kirkorian said. “But we’re going to have to think through that very carefully. It’s going to be exciting to see how this drug changes management in our patients.”
Dr. Kirkorian disclosed that she is a member of the advisory board for Verrica Pharmaceuticals.
“Many patients who have failed topical steroids have never had adequate treatment,” Anna Yasmine Kirkorian, MD, chief of dermatology at National Children’s Hospital in Washington, said during the ODAC Dermatology, Aesthetic & Surgical Conference. “There is no lower age limit on the use of topical corticosteroids, and low potency corticosteroids are inadequate to treat severe eczema. The idea that only over-the-counter 2.5% hydrocortisone cream is necessary is not true,” she added.
“You also want to scrutinize the vehicle,” she said, noting that children are often prescribed cream formulations that hurt when applied, so parents stop applying them. “Ointments are generally the vehicles of choice in childhood,” she added.
It is generally not advised to use topical and oral antibiotics in children with AD, unless there are clear signs of infection. “If they’re just slightly oozy, don’t use them,” she continued. “Of course, every child or adult with eczema has Staph aureus on them, but most of the time, what you need to do is repair the barrier. We know that from data and common sense. When we repair their barrier, their rates of infection decrease.”
A focal area with pustules and pus should be cultured and treated, Dr. Kirkorian said. “Monotherapy with antibiotics is going to do nothing for you.” In cases of children with failure to thrive, she recommends referral to pediatric dermatology, allergy/immunology, GI, or genetics, as appropriate.
For children with severe AD, Dr. Kirkorian favors a rescue plan with a one-pound jar of triamcinolone ointment 0.1%. She recommends application of the ointment to all areas, including the face and scalp once nightly for 2 weeks, with a follow-up appointment at the end of that time. “If you just give people medicine and ask them to come back in 6 months, they are not able to comply with that and they don’t have faith that it’s going to work,” explained Dr. Kirkorian, associate professor of dermatology and pediatrics at George Washington University, Washington. At the end of 2 weeks, “the majority will have improved dramatically, and then you can implement maintenance therapy with topical calcineurin inhibitors, crisaborole, or possibly topical ruxolitinib.”
Some clinicians prescribe oral antihistamines for AD, but Dr. Kirkorian said that data supporting their use are limited and antihistamines are not approved for use in children younger than 6 months of age. Sedating antihistamines will induce sleep, “but do not provide durable night-long sleep,” and routine use may have an impact on learning and school performance. In addition, exposure to antihistamines in children under age 2 may be associated with development of ADHD at school age.
The interleukin-4 receptor alpha antagonist dupilumab (Dupixent) is approved by the Food and Drug Administration for moderate to severe AD in patients ages 6 and older. But obtaining it for patients can be tricky, she said, as this requires documented failure of corticosteroids, calcineurin inhibitors, crisaborole ointment, and phototherapy (if prescribed). Patients are often obligated to do step therapy with an off-label drug such as cyclosporine or methotrexate for 3 months, and they need to demonstrate responses with objective measures of severity such as the SCORAD (SCORing Atopic Dermatitis) and the validated Investigator Global Assessment.
“Most of my patients carry insurance that does not approve dupilumab without failure of a prior off-label systemic immunosuppressant medication,” Dr. Kirkorian said. Cyclosporine is her first choice for a systemic immunosuppressant “because it has a fast onset of action, it’s effective for treatment of atopic dermatitis, and safe for short-term use,” she said. “I don’t think that methotrexate works well for eczema. It can take weeks and weeks to work.”
She typically starts patients on a 5 mg/kg dose of cyclosporine. Baseline tests include CBC, CMP (comprehensive metabolic panel), lipids, and vitals. She repeats the labs at 1 month, and includes a blood pressure check. Potential adverse effects of cyclosporine include infections (including opportunistic infections), cytopenias, hypertension, nephrotoxicity, hepatotoxicity, neurotoxicity (including posterior reversible encephalopathy syndrome), electrolyte disturbance, lymphoma, and cutaneous malignancy.
“The good news is that we generally don’t see the adverse effects with short-term use,” Dr. Kirkorian said. “We will see some hypertrichosis and gingival hypertrophy, which resolves with cessation of therapy. There are serious side effects if you use it for long enough.”
As for methotrexate, “it is still a very important drug in pediatric dermatology, particularly in other conditions such as psoriasis,” she said. “The problem is that weekly dosing of methotrexate poses a greater risk of dosing errors. People aren’t really triggered to think of a once-weekly medication. If you do use it, give them a short supply to make sure that they come back, and that they don’t give it daily accidentally.”
Practical tips she offered for prescribing cyclosporine include supplying a patient handout with information on all adverse effects, dosing information, vaccination information, and pregnancy precautions, with contact information (a patient portal or on-call number) for the treating clinician in case a patient develops adverse effects. Administration of live vaccines while patients are on cyclosporine is not recommended.
When transitioning patients from cyclosporine or methotrexate to dupilumab, Dr. Kirkorian recommends tapering the immunosuppressant dose by half every 2 weeks to complete cessation by week 8 of treatment. For patients who experience a severe baseline flare once the immunosuppressant is tapered, despite the switch to dupilumab, she recommends restarting methotrexate at a full dose and then reducing the dose every 2 weeks until the lowest effective dose (2.5-5 mg weekly) is reached.
“Waning efficacy is real,” she said. “We can add methotrexate to recapture efficacy. Check for superimposed allergic contact dermatitis.”
With upadacitinib (Rinvoq), an oral Janus kinase (JAK) inhibitor recently approved for treating refractory, moderate to severe AD in patients 12 years of age and older, is the risk profile acceptable to parents and physicians? “I think the answer is yes,” Dr. Kirkorian said. “But we’re going to have to think through that very carefully. It’s going to be exciting to see how this drug changes management in our patients.”
Dr. Kirkorian disclosed that she is a member of the advisory board for Verrica Pharmaceuticals.
“Many patients who have failed topical steroids have never had adequate treatment,” Anna Yasmine Kirkorian, MD, chief of dermatology at National Children’s Hospital in Washington, said during the ODAC Dermatology, Aesthetic & Surgical Conference. “There is no lower age limit on the use of topical corticosteroids, and low potency corticosteroids are inadequate to treat severe eczema. The idea that only over-the-counter 2.5% hydrocortisone cream is necessary is not true,” she added.
“You also want to scrutinize the vehicle,” she said, noting that children are often prescribed cream formulations that hurt when applied, so parents stop applying them. “Ointments are generally the vehicles of choice in childhood,” she added.
It is generally not advised to use topical and oral antibiotics in children with AD, unless there are clear signs of infection. “If they’re just slightly oozy, don’t use them,” she continued. “Of course, every child or adult with eczema has Staph aureus on them, but most of the time, what you need to do is repair the barrier. We know that from data and common sense. When we repair their barrier, their rates of infection decrease.”
A focal area with pustules and pus should be cultured and treated, Dr. Kirkorian said. “Monotherapy with antibiotics is going to do nothing for you.” In cases of children with failure to thrive, she recommends referral to pediatric dermatology, allergy/immunology, GI, or genetics, as appropriate.
For children with severe AD, Dr. Kirkorian favors a rescue plan with a one-pound jar of triamcinolone ointment 0.1%. She recommends application of the ointment to all areas, including the face and scalp once nightly for 2 weeks, with a follow-up appointment at the end of that time. “If you just give people medicine and ask them to come back in 6 months, they are not able to comply with that and they don’t have faith that it’s going to work,” explained Dr. Kirkorian, associate professor of dermatology and pediatrics at George Washington University, Washington. At the end of 2 weeks, “the majority will have improved dramatically, and then you can implement maintenance therapy with topical calcineurin inhibitors, crisaborole, or possibly topical ruxolitinib.”
Some clinicians prescribe oral antihistamines for AD, but Dr. Kirkorian said that data supporting their use are limited and antihistamines are not approved for use in children younger than 6 months of age. Sedating antihistamines will induce sleep, “but do not provide durable night-long sleep,” and routine use may have an impact on learning and school performance. In addition, exposure to antihistamines in children under age 2 may be associated with development of ADHD at school age.
The interleukin-4 receptor alpha antagonist dupilumab (Dupixent) is approved by the Food and Drug Administration for moderate to severe AD in patients ages 6 and older. But obtaining it for patients can be tricky, she said, as this requires documented failure of corticosteroids, calcineurin inhibitors, crisaborole ointment, and phototherapy (if prescribed). Patients are often obligated to do step therapy with an off-label drug such as cyclosporine or methotrexate for 3 months, and they need to demonstrate responses with objective measures of severity such as the SCORAD (SCORing Atopic Dermatitis) and the validated Investigator Global Assessment.
“Most of my patients carry insurance that does not approve dupilumab without failure of a prior off-label systemic immunosuppressant medication,” Dr. Kirkorian said. Cyclosporine is her first choice for a systemic immunosuppressant “because it has a fast onset of action, it’s effective for treatment of atopic dermatitis, and safe for short-term use,” she said. “I don’t think that methotrexate works well for eczema. It can take weeks and weeks to work.”
She typically starts patients on a 5 mg/kg dose of cyclosporine. Baseline tests include CBC, CMP (comprehensive metabolic panel), lipids, and vitals. She repeats the labs at 1 month, and includes a blood pressure check. Potential adverse effects of cyclosporine include infections (including opportunistic infections), cytopenias, hypertension, nephrotoxicity, hepatotoxicity, neurotoxicity (including posterior reversible encephalopathy syndrome), electrolyte disturbance, lymphoma, and cutaneous malignancy.
“The good news is that we generally don’t see the adverse effects with short-term use,” Dr. Kirkorian said. “We will see some hypertrichosis and gingival hypertrophy, which resolves with cessation of therapy. There are serious side effects if you use it for long enough.”
As for methotrexate, “it is still a very important drug in pediatric dermatology, particularly in other conditions such as psoriasis,” she said. “The problem is that weekly dosing of methotrexate poses a greater risk of dosing errors. People aren’t really triggered to think of a once-weekly medication. If you do use it, give them a short supply to make sure that they come back, and that they don’t give it daily accidentally.”
Practical tips she offered for prescribing cyclosporine include supplying a patient handout with information on all adverse effects, dosing information, vaccination information, and pregnancy precautions, with contact information (a patient portal or on-call number) for the treating clinician in case a patient develops adverse effects. Administration of live vaccines while patients are on cyclosporine is not recommended.
When transitioning patients from cyclosporine or methotrexate to dupilumab, Dr. Kirkorian recommends tapering the immunosuppressant dose by half every 2 weeks to complete cessation by week 8 of treatment. For patients who experience a severe baseline flare once the immunosuppressant is tapered, despite the switch to dupilumab, she recommends restarting methotrexate at a full dose and then reducing the dose every 2 weeks until the lowest effective dose (2.5-5 mg weekly) is reached.
“Waning efficacy is real,” she said. “We can add methotrexate to recapture efficacy. Check for superimposed allergic contact dermatitis.”
With upadacitinib (Rinvoq), an oral Janus kinase (JAK) inhibitor recently approved for treating refractory, moderate to severe AD in patients 12 years of age and older, is the risk profile acceptable to parents and physicians? “I think the answer is yes,” Dr. Kirkorian said. “But we’re going to have to think through that very carefully. It’s going to be exciting to see how this drug changes management in our patients.”
Dr. Kirkorian disclosed that she is a member of the advisory board for Verrica Pharmaceuticals.
FROM ODAC 2022
Agreement reached for research definition of ‘long COVID’ in children and young people
Long COVID can affect adults, young people, and children, and now for the first time, in a landmark study accepted for publication in the Archives of Disease in Childhood, formal agreement has been made on a research definition for post–acute COVID-19, or “long COVID” as it is commonly known, in children and young people.
The researchers charged themselves with a single objective – to derive a research definition for long COVID (post–acute COVID-19) in children and young people to allow comparisons between research studies. Specifically, so studies on prevalence, course, and outcome of long COVID in this age group can be reliably compared, because to date there has been no consensus. In fact, the authors pointed out how the “slew of definitions” currently used all differ in number, type, and duration of symptoms, which hampers research efforts. In addition, the lack of definition consensus has contributed to very wide reported variations in the estimated prevalence of long COVID in children of 1%-51%, with the authors saying that a “consistently applied definition of long COVID will help reduce the variability of prevalence estimates.”
Statements sequentially whittled down
“Using robust consensus methodology,” the authors said, “we derived a research definition for long COVID in children and young people.”
To achieve the definition consensus, a three-phase online Delphi process was used, followed by a virtual consensus meeting. The 123 participants registered to take part in the study included 23 people (19%) in a lived experience panel, 50 (42%) in the researcher or researcher/service delivery combined panel and 47 (39%) in the service delivery panel. Of 120 registered participants, 105 (88%) completed phase 1, 86 eligible participants (82% of those completing phase 1) completed phase 2 and 77 eligible participants (90% of those completing phase 2) completed phase 3. Seventeen participants attended and voted at the consensus meeting – 4 (23%) from the service delivery panel, 11 (65%) from the researcher panel, and 2 (12%) from the lived experience panel.
Presented with 49 statements in each phase, participants scored these from 1-9 based on how important they were perceived to be with regards inclusion in the research definition of long COVID in children and young people. Having been sequentially whittled down in three phases, 10 statements were discussed at the consensus meeting, and a panel of eight 11- to 17-year-olds affected by long COVID also reviewed the statements to reach a final agreement.
Five of the statements were agreed to be included in the definition, which stated that long COVID in children and young people is a condition in which a child or young person has symptoms (at least one of which is a physical symptom) that have continued or developed after a diagnosis of COVID-19 (confirmed with one or more positive COVID tests); impact their physical, mental, or social well-being; are interfering with some aspect of daily living (for example, school, work, home, or relationships); and persist for a minimum duration of 12 weeks after initial testing for COVID-19 (even if symptoms have waxed and waned over that period).
David Strain, MBChB, MD, chair of the BMA board of science and clinical senior lecturer and honorary consultant, University of Exeter (England), told the Science Media Centre: “A Delphi study builds a consensus from the world’s experts by presenting a series of statements and continuing to refine them until there is agreement as to what the definition of pediatric long COVID should be.” He added: “This is vitally important in order to align the global research effort into long COVID.”
Reassuringly similar
From the agreed five statements, a further research definition was proposed to align with the World Health Organization definition for adults: “Post–COVID-19 condition occurs in young people with a history of confirmed SARS CoV-2 infection, with at least one persisting physical symptom for a minimum duration of 12 weeks after initial testing that cannot be explained by an alternative diagnosis. The symptoms have an impact on everyday functioning, may continue or develop after COVID-19 infection, and may fluctuate or relapse over time.”
The authors concluded: “This is the first research definition of long COVID (post–COVID-19 condition) in children and young people and complements the clinical case definition in adults proposed by WHO,” adding that the two definitions are “reassuringly similar.”
They reiterated how widespread adoption of this definition would allow comparisons between studies such that a core outcome set can be developed and the prevalence, course and outcome of long COVID in children and young people can be reliably evaluated, which “will substantially help strengthen the evidence base on this debilitating condition.”
In addition, the authors said that a consistently applied definition of long COVID will help to provide a “more accurate picture on the true impact of the condition.”
The researchers emphasized the need to differentiate between a clinical case definition and a research definition of long COVID and explained: “It is understandable that the patient groups representing people with long COVID are concerned about a definition that could restrict access to services that are needed.”
They went on to say that in their view the decision whether a child or young person can see a health care professional, access any support needed, or be referred, investigated, or treated for long COVID should be a “shared decision involving the young person, their carers, and clinicians.”
Dr. Strain reinforced that it was important that the definition was a research one and not a clinical one, pointing out that the 12-week period in the research definition “does not necessarily mean that a child or young person should need to wait 3 months before being offered help or assistance from their health care team, indeed a 3-month delay in offering support to a child or young person, at this vitally important period of their educational development, could have lasting long-term impacts.”
A version of this article first appeared on Medscape.co.uk.
Long COVID can affect adults, young people, and children, and now for the first time, in a landmark study accepted for publication in the Archives of Disease in Childhood, formal agreement has been made on a research definition for post–acute COVID-19, or “long COVID” as it is commonly known, in children and young people.
The researchers charged themselves with a single objective – to derive a research definition for long COVID (post–acute COVID-19) in children and young people to allow comparisons between research studies. Specifically, so studies on prevalence, course, and outcome of long COVID in this age group can be reliably compared, because to date there has been no consensus. In fact, the authors pointed out how the “slew of definitions” currently used all differ in number, type, and duration of symptoms, which hampers research efforts. In addition, the lack of definition consensus has contributed to very wide reported variations in the estimated prevalence of long COVID in children of 1%-51%, with the authors saying that a “consistently applied definition of long COVID will help reduce the variability of prevalence estimates.”
Statements sequentially whittled down
“Using robust consensus methodology,” the authors said, “we derived a research definition for long COVID in children and young people.”
To achieve the definition consensus, a three-phase online Delphi process was used, followed by a virtual consensus meeting. The 123 participants registered to take part in the study included 23 people (19%) in a lived experience panel, 50 (42%) in the researcher or researcher/service delivery combined panel and 47 (39%) in the service delivery panel. Of 120 registered participants, 105 (88%) completed phase 1, 86 eligible participants (82% of those completing phase 1) completed phase 2 and 77 eligible participants (90% of those completing phase 2) completed phase 3. Seventeen participants attended and voted at the consensus meeting – 4 (23%) from the service delivery panel, 11 (65%) from the researcher panel, and 2 (12%) from the lived experience panel.
Presented with 49 statements in each phase, participants scored these from 1-9 based on how important they were perceived to be with regards inclusion in the research definition of long COVID in children and young people. Having been sequentially whittled down in three phases, 10 statements were discussed at the consensus meeting, and a panel of eight 11- to 17-year-olds affected by long COVID also reviewed the statements to reach a final agreement.
Five of the statements were agreed to be included in the definition, which stated that long COVID in children and young people is a condition in which a child or young person has symptoms (at least one of which is a physical symptom) that have continued or developed after a diagnosis of COVID-19 (confirmed with one or more positive COVID tests); impact their physical, mental, or social well-being; are interfering with some aspect of daily living (for example, school, work, home, or relationships); and persist for a minimum duration of 12 weeks after initial testing for COVID-19 (even if symptoms have waxed and waned over that period).
David Strain, MBChB, MD, chair of the BMA board of science and clinical senior lecturer and honorary consultant, University of Exeter (England), told the Science Media Centre: “A Delphi study builds a consensus from the world’s experts by presenting a series of statements and continuing to refine them until there is agreement as to what the definition of pediatric long COVID should be.” He added: “This is vitally important in order to align the global research effort into long COVID.”
Reassuringly similar
From the agreed five statements, a further research definition was proposed to align with the World Health Organization definition for adults: “Post–COVID-19 condition occurs in young people with a history of confirmed SARS CoV-2 infection, with at least one persisting physical symptom for a minimum duration of 12 weeks after initial testing that cannot be explained by an alternative diagnosis. The symptoms have an impact on everyday functioning, may continue or develop after COVID-19 infection, and may fluctuate or relapse over time.”
The authors concluded: “This is the first research definition of long COVID (post–COVID-19 condition) in children and young people and complements the clinical case definition in adults proposed by WHO,” adding that the two definitions are “reassuringly similar.”
They reiterated how widespread adoption of this definition would allow comparisons between studies such that a core outcome set can be developed and the prevalence, course and outcome of long COVID in children and young people can be reliably evaluated, which “will substantially help strengthen the evidence base on this debilitating condition.”
In addition, the authors said that a consistently applied definition of long COVID will help to provide a “more accurate picture on the true impact of the condition.”
The researchers emphasized the need to differentiate between a clinical case definition and a research definition of long COVID and explained: “It is understandable that the patient groups representing people with long COVID are concerned about a definition that could restrict access to services that are needed.”
They went on to say that in their view the decision whether a child or young person can see a health care professional, access any support needed, or be referred, investigated, or treated for long COVID should be a “shared decision involving the young person, their carers, and clinicians.”
Dr. Strain reinforced that it was important that the definition was a research one and not a clinical one, pointing out that the 12-week period in the research definition “does not necessarily mean that a child or young person should need to wait 3 months before being offered help or assistance from their health care team, indeed a 3-month delay in offering support to a child or young person, at this vitally important period of their educational development, could have lasting long-term impacts.”
A version of this article first appeared on Medscape.co.uk.
Long COVID can affect adults, young people, and children, and now for the first time, in a landmark study accepted for publication in the Archives of Disease in Childhood, formal agreement has been made on a research definition for post–acute COVID-19, or “long COVID” as it is commonly known, in children and young people.
The researchers charged themselves with a single objective – to derive a research definition for long COVID (post–acute COVID-19) in children and young people to allow comparisons between research studies. Specifically, so studies on prevalence, course, and outcome of long COVID in this age group can be reliably compared, because to date there has been no consensus. In fact, the authors pointed out how the “slew of definitions” currently used all differ in number, type, and duration of symptoms, which hampers research efforts. In addition, the lack of definition consensus has contributed to very wide reported variations in the estimated prevalence of long COVID in children of 1%-51%, with the authors saying that a “consistently applied definition of long COVID will help reduce the variability of prevalence estimates.”
Statements sequentially whittled down
“Using robust consensus methodology,” the authors said, “we derived a research definition for long COVID in children and young people.”
To achieve the definition consensus, a three-phase online Delphi process was used, followed by a virtual consensus meeting. The 123 participants registered to take part in the study included 23 people (19%) in a lived experience panel, 50 (42%) in the researcher or researcher/service delivery combined panel and 47 (39%) in the service delivery panel. Of 120 registered participants, 105 (88%) completed phase 1, 86 eligible participants (82% of those completing phase 1) completed phase 2 and 77 eligible participants (90% of those completing phase 2) completed phase 3. Seventeen participants attended and voted at the consensus meeting – 4 (23%) from the service delivery panel, 11 (65%) from the researcher panel, and 2 (12%) from the lived experience panel.
Presented with 49 statements in each phase, participants scored these from 1-9 based on how important they were perceived to be with regards inclusion in the research definition of long COVID in children and young people. Having been sequentially whittled down in three phases, 10 statements were discussed at the consensus meeting, and a panel of eight 11- to 17-year-olds affected by long COVID also reviewed the statements to reach a final agreement.
Five of the statements were agreed to be included in the definition, which stated that long COVID in children and young people is a condition in which a child or young person has symptoms (at least one of which is a physical symptom) that have continued or developed after a diagnosis of COVID-19 (confirmed with one or more positive COVID tests); impact their physical, mental, or social well-being; are interfering with some aspect of daily living (for example, school, work, home, or relationships); and persist for a minimum duration of 12 weeks after initial testing for COVID-19 (even if symptoms have waxed and waned over that period).
David Strain, MBChB, MD, chair of the BMA board of science and clinical senior lecturer and honorary consultant, University of Exeter (England), told the Science Media Centre: “A Delphi study builds a consensus from the world’s experts by presenting a series of statements and continuing to refine them until there is agreement as to what the definition of pediatric long COVID should be.” He added: “This is vitally important in order to align the global research effort into long COVID.”
Reassuringly similar
From the agreed five statements, a further research definition was proposed to align with the World Health Organization definition for adults: “Post–COVID-19 condition occurs in young people with a history of confirmed SARS CoV-2 infection, with at least one persisting physical symptom for a minimum duration of 12 weeks after initial testing that cannot be explained by an alternative diagnosis. The symptoms have an impact on everyday functioning, may continue or develop after COVID-19 infection, and may fluctuate or relapse over time.”
The authors concluded: “This is the first research definition of long COVID (post–COVID-19 condition) in children and young people and complements the clinical case definition in adults proposed by WHO,” adding that the two definitions are “reassuringly similar.”
They reiterated how widespread adoption of this definition would allow comparisons between studies such that a core outcome set can be developed and the prevalence, course and outcome of long COVID in children and young people can be reliably evaluated, which “will substantially help strengthen the evidence base on this debilitating condition.”
In addition, the authors said that a consistently applied definition of long COVID will help to provide a “more accurate picture on the true impact of the condition.”
The researchers emphasized the need to differentiate between a clinical case definition and a research definition of long COVID and explained: “It is understandable that the patient groups representing people with long COVID are concerned about a definition that could restrict access to services that are needed.”
They went on to say that in their view the decision whether a child or young person can see a health care professional, access any support needed, or be referred, investigated, or treated for long COVID should be a “shared decision involving the young person, their carers, and clinicians.”
Dr. Strain reinforced that it was important that the definition was a research one and not a clinical one, pointing out that the 12-week period in the research definition “does not necessarily mean that a child or young person should need to wait 3 months before being offered help or assistance from their health care team, indeed a 3-month delay in offering support to a child or young person, at this vitally important period of their educational development, could have lasting long-term impacts.”
A version of this article first appeared on Medscape.co.uk.
FROM THE ARCHIVES OF DISEASE IN CHILDHOOD
Promising leads to crack long COVID discovered
It’s a story of promise at a time of urgent need.
They proposed many theories on what might be driving long COVID. A role for a virus “cryptic reservoir” that could reactivate at any time, “viral remnants” that trigger chronic inflammation, and action by “autoimmune antibodies” that cause ongoing symptoms are possibilities.
In fact, it’s likely that research will show long COVID is a condition with more than one cause, the experts said during a recent webinar.
People might experience post-infection problems, including organ damage that takes time to heal after initial COVID-19 illness. Or they may be living with post-immune factors, including ongoing immune system responses triggered by autoantibodies.
Determining the cause or causes of long COVID is essential for treatment. For example, if one person’s symptoms persist because of an overactive immune system, “we need to provide immunosuppressant therapies,” Akiko Iwasaki, PhD, said. “But we don’t want to give that to someone who has a persistent virus reservoir,” meaning remnants of the virus remain in their bodies.
Interestingly, a study preprint, which has not been peer reviewed, found dogs were accurate more than half the time in sniffing out long COVID, said Dr. Iwasaki, professor of immunobiology and developmental biology at Yale University, New Haven, Conn.
The dogs were tasked with identifying 45 people with long COVID versus 188 people without it. The findings suggest the presence of a unique chemical in the sweat of people with long COVID that could someday lead to a diagnostic test.
Viral persistence possible
If one of the main theories holds, it could be that the coronavirus somehow remains in the body in some form for some people after COVID-19.
Mady Hornig, MD, agreed this is a possibility that needs to be investigated further.
“A weakened immune response to an infection may mean that you have cryptic reservoirs of virus that are continuing to cause symptoms,” she said during the briefing. Dr. Hornig is a doctor-scientist specializing in epidemiology at Columbia University, New York.
“That may explain why some patients with long COVID feel better after vaccination,” because the vaccine creates a strong antibody response to fight COVID-19, Dr. Iwasaki said.
Researchers are unearthing additional potential factors contributing to long COVID.
Viral persistence could also reactivate other dormant viruses in the body, such as Epstein-Barr virus (EBV), said Lawrence Purpura, MD, MPH, an infectious disease specialist at New York Presbyterian/Columbia University. Reactivation of Epstein-Barr is one of four identifying signs of long COVID revealed in a Jan. 25 study published in the journal Cell.
Immune overactivation also possible?
For other people with long COVID, it’s not the virus sticking around but the body’s reaction that’s the issue.
Investigators suggest autoimmunity plays a role, and they point to the presence of autoantibodies, for example.
When these autoantibodies persist, they can cause tissue and organ damage over time.
Other investigators are proposing “immune exhaustion” in long COVID because of similarities to chronic fatigue syndrome, Dr. Hornig said.
“It should be ‘all hands on deck’ for research into long COVID,” she said. “The number of disabled individuals who will likely qualify for a diagnosis of [chronic fatigue syndrome] is growing by the second.”
Forging ahead on future research
It’s clear there is more work to do. There are investigators working on banking tissue samples from people with long COVID to learn more, for example.
Also, finding a biomarker unique to long COVID could vastly improve the precision of diagnosing long COVID, especially if the dog sniffing option does not pan out.
Of the thousands of biomarker possibilities, Dr. Hornig said, “maybe that’s one or two that ultimately make a real impact on patient care. So it’s going to be critical to find those quickly, translate them, and make them available.”
In the meantime, some answers might come from a large study sponsored by the National Institutes of Health. The NIH is funding the “Researching COVID to Enhance Recovery” project using $470 million from the American Rescue Plan. Investigators at NYU Langone Health are leading the effort and plan to share the wealth by funding more than 100 researchers at more than 30 institutions to create a “metacohort” to study long COVID. More information is available at recovercovid.org.
“Fortunately, through the global research effort, we are now really starting to expand our understanding of how long COVID manifests, how common it is, and what the underlying mechanisms may be,” Dr. Purpura said.
A version of this article first appeared on WebMD.com.
It’s a story of promise at a time of urgent need.
They proposed many theories on what might be driving long COVID. A role for a virus “cryptic reservoir” that could reactivate at any time, “viral remnants” that trigger chronic inflammation, and action by “autoimmune antibodies” that cause ongoing symptoms are possibilities.
In fact, it’s likely that research will show long COVID is a condition with more than one cause, the experts said during a recent webinar.
People might experience post-infection problems, including organ damage that takes time to heal after initial COVID-19 illness. Or they may be living with post-immune factors, including ongoing immune system responses triggered by autoantibodies.
Determining the cause or causes of long COVID is essential for treatment. For example, if one person’s symptoms persist because of an overactive immune system, “we need to provide immunosuppressant therapies,” Akiko Iwasaki, PhD, said. “But we don’t want to give that to someone who has a persistent virus reservoir,” meaning remnants of the virus remain in their bodies.
Interestingly, a study preprint, which has not been peer reviewed, found dogs were accurate more than half the time in sniffing out long COVID, said Dr. Iwasaki, professor of immunobiology and developmental biology at Yale University, New Haven, Conn.
The dogs were tasked with identifying 45 people with long COVID versus 188 people without it. The findings suggest the presence of a unique chemical in the sweat of people with long COVID that could someday lead to a diagnostic test.
Viral persistence possible
If one of the main theories holds, it could be that the coronavirus somehow remains in the body in some form for some people after COVID-19.
Mady Hornig, MD, agreed this is a possibility that needs to be investigated further.
“A weakened immune response to an infection may mean that you have cryptic reservoirs of virus that are continuing to cause symptoms,” she said during the briefing. Dr. Hornig is a doctor-scientist specializing in epidemiology at Columbia University, New York.
“That may explain why some patients with long COVID feel better after vaccination,” because the vaccine creates a strong antibody response to fight COVID-19, Dr. Iwasaki said.
Researchers are unearthing additional potential factors contributing to long COVID.
Viral persistence could also reactivate other dormant viruses in the body, such as Epstein-Barr virus (EBV), said Lawrence Purpura, MD, MPH, an infectious disease specialist at New York Presbyterian/Columbia University. Reactivation of Epstein-Barr is one of four identifying signs of long COVID revealed in a Jan. 25 study published in the journal Cell.
Immune overactivation also possible?
For other people with long COVID, it’s not the virus sticking around but the body’s reaction that’s the issue.
Investigators suggest autoimmunity plays a role, and they point to the presence of autoantibodies, for example.
When these autoantibodies persist, they can cause tissue and organ damage over time.
Other investigators are proposing “immune exhaustion” in long COVID because of similarities to chronic fatigue syndrome, Dr. Hornig said.
“It should be ‘all hands on deck’ for research into long COVID,” she said. “The number of disabled individuals who will likely qualify for a diagnosis of [chronic fatigue syndrome] is growing by the second.”
Forging ahead on future research
It’s clear there is more work to do. There are investigators working on banking tissue samples from people with long COVID to learn more, for example.
Also, finding a biomarker unique to long COVID could vastly improve the precision of diagnosing long COVID, especially if the dog sniffing option does not pan out.
Of the thousands of biomarker possibilities, Dr. Hornig said, “maybe that’s one or two that ultimately make a real impact on patient care. So it’s going to be critical to find those quickly, translate them, and make them available.”
In the meantime, some answers might come from a large study sponsored by the National Institutes of Health. The NIH is funding the “Researching COVID to Enhance Recovery” project using $470 million from the American Rescue Plan. Investigators at NYU Langone Health are leading the effort and plan to share the wealth by funding more than 100 researchers at more than 30 institutions to create a “metacohort” to study long COVID. More information is available at recovercovid.org.
“Fortunately, through the global research effort, we are now really starting to expand our understanding of how long COVID manifests, how common it is, and what the underlying mechanisms may be,” Dr. Purpura said.
A version of this article first appeared on WebMD.com.
It’s a story of promise at a time of urgent need.
They proposed many theories on what might be driving long COVID. A role for a virus “cryptic reservoir” that could reactivate at any time, “viral remnants” that trigger chronic inflammation, and action by “autoimmune antibodies” that cause ongoing symptoms are possibilities.
In fact, it’s likely that research will show long COVID is a condition with more than one cause, the experts said during a recent webinar.
People might experience post-infection problems, including organ damage that takes time to heal after initial COVID-19 illness. Or they may be living with post-immune factors, including ongoing immune system responses triggered by autoantibodies.
Determining the cause or causes of long COVID is essential for treatment. For example, if one person’s symptoms persist because of an overactive immune system, “we need to provide immunosuppressant therapies,” Akiko Iwasaki, PhD, said. “But we don’t want to give that to someone who has a persistent virus reservoir,” meaning remnants of the virus remain in their bodies.
Interestingly, a study preprint, which has not been peer reviewed, found dogs were accurate more than half the time in sniffing out long COVID, said Dr. Iwasaki, professor of immunobiology and developmental biology at Yale University, New Haven, Conn.
The dogs were tasked with identifying 45 people with long COVID versus 188 people without it. The findings suggest the presence of a unique chemical in the sweat of people with long COVID that could someday lead to a diagnostic test.
Viral persistence possible
If one of the main theories holds, it could be that the coronavirus somehow remains in the body in some form for some people after COVID-19.
Mady Hornig, MD, agreed this is a possibility that needs to be investigated further.
“A weakened immune response to an infection may mean that you have cryptic reservoirs of virus that are continuing to cause symptoms,” she said during the briefing. Dr. Hornig is a doctor-scientist specializing in epidemiology at Columbia University, New York.
“That may explain why some patients with long COVID feel better after vaccination,” because the vaccine creates a strong antibody response to fight COVID-19, Dr. Iwasaki said.
Researchers are unearthing additional potential factors contributing to long COVID.
Viral persistence could also reactivate other dormant viruses in the body, such as Epstein-Barr virus (EBV), said Lawrence Purpura, MD, MPH, an infectious disease specialist at New York Presbyterian/Columbia University. Reactivation of Epstein-Barr is one of four identifying signs of long COVID revealed in a Jan. 25 study published in the journal Cell.
Immune overactivation also possible?
For other people with long COVID, it’s not the virus sticking around but the body’s reaction that’s the issue.
Investigators suggest autoimmunity plays a role, and they point to the presence of autoantibodies, for example.
When these autoantibodies persist, they can cause tissue and organ damage over time.
Other investigators are proposing “immune exhaustion” in long COVID because of similarities to chronic fatigue syndrome, Dr. Hornig said.
“It should be ‘all hands on deck’ for research into long COVID,” she said. “The number of disabled individuals who will likely qualify for a diagnosis of [chronic fatigue syndrome] is growing by the second.”
Forging ahead on future research
It’s clear there is more work to do. There are investigators working on banking tissue samples from people with long COVID to learn more, for example.
Also, finding a biomarker unique to long COVID could vastly improve the precision of diagnosing long COVID, especially if the dog sniffing option does not pan out.
Of the thousands of biomarker possibilities, Dr. Hornig said, “maybe that’s one or two that ultimately make a real impact on patient care. So it’s going to be critical to find those quickly, translate them, and make them available.”
In the meantime, some answers might come from a large study sponsored by the National Institutes of Health. The NIH is funding the “Researching COVID to Enhance Recovery” project using $470 million from the American Rescue Plan. Investigators at NYU Langone Health are leading the effort and plan to share the wealth by funding more than 100 researchers at more than 30 institutions to create a “metacohort” to study long COVID. More information is available at recovercovid.org.
“Fortunately, through the global research effort, we are now really starting to expand our understanding of how long COVID manifests, how common it is, and what the underlying mechanisms may be,” Dr. Purpura said.
A version of this article first appeared on WebMD.com.
Potential new neuromodulation treatment for migraines
Most people avoid smartphones when they have a migraine headache, but a noninvasive treatment for episodic migraines may change that.
that can help ease migraine pain.
Tina Montgomery, 58, has suffered from migraines since childhood and spent years looking for something to help manage them. Doctors consider her a “chronic” sufferer in that she has more than 14 migraines a month (fewer than 14 is considered “episodic”). Prescription antidepressants, anticonvulsants, and botulinum toxin shots as preventive treatments helped a little but not enough.
A few years ago, she found some relief using a new preventive injectable medication that targets a peptide known as CGRP, combined with an oral CGRP rescue medication, ubrogepant (Ubrelvy). However, by early 2021, Ms. Montgomery’s chronic migraines were back as she faced stress from the pandemic and her role as a caregiver for her aging parents.
“I was going through so much medication. I just didn’t feel good taking so much,” she said.
Looking for relief, she read about Nerivio, a wearable migraine treatment device that uses remote electrical neuromodulation (REN). She mentioned the device to her neurologist, and he agreed she might benefit from trying it out. Today, she uses the device whenever she feels a migraine may be imminent, she said.
“It really helps me stave off migraines I feel coming on and the milder ones where I would normally hesitate to use prescription medication because [insurance] limits the number of pills they give you in a month,” she said. “I follow through with the Nerivio treatment and usually find that my migraine doesn’t fully develop or is completely gone, and I don’t get a migraine at all.”
Taking it on the arm
The device works by stimulating nerves at the back of the arm right around the triceps. “Those nerve fibers relay information to the brain stem [so it can] work its magic and use the brain’s own natural mechanisms for reducing pain,” said Brian M. Grosberg, MD, director of the Hartford Healthcare Ayer Neuroscience Institute Headache Center, West Hartford, Conn.
These mechanisms are like a bait-and-switch for the brain, said Britany Klenofsky, MD, assistant professor of neurology, Icahn School of Medicine at Mount Sinai, New York. “You’re trying to stimulate pain somewhere else [on the body] to tell the brain to protect itself and release [the neurotransmitter] serotonin,” she said. “You do this by putting the device on your arm, an area that’s away from the head where the pain is actively occurring, turning the device on, and increasing the stimulation to a nearly painful stimulus.”
This pseudo pain prompts the brain to release serotonin, the feel-good hormone along with norepinephrine and noradrenaline. The device works best when it’s used as soon as a migraine starts, so patients should hook up Nerivio within the first 20-30 minutes of onset of pain, said Dr. Grosberg, who was an investigator on the double-blind treatment study that led to FDA clearance. If patients wait too long, the device may not work.
This is why as soon as Ms. Montgomery feels a migraine aura (there are six types of migraine auras, including visual changes and muscle weakness) that occurs right before a migraine strikes, she puts the device armband on her upper arm and launches its smartphone app. Then she turns on the device for a 45-minute treatment, which begins with what she characterizes as tingling and vibration sensations on her arm. She turns up the intensity of the sensations, which are mild electric currents, until they are well-felt but not painful.
Ms. Montgomery said she can use the device and multitask since there’s no need for her to lie down or sit in a darkened room. And since it is worn on the arm, she can wear it under a shirtsleeve while working or out in public without anyone noticing. She also uses the app’s migraine diary and guided meditation to help reduce the anxiety that often accompanies her migraines.
The device is approved for adolescents and adults and can be used for both episodic and chronic migraines. From an efficacy standpoint, the device provides relief about as well as a commonly used pharmaceutical class of drugs, triptans. About 37% of people with episodic migraine achieved complete freedom from pain 2 hours after their treatment. In addition, about two-thirds of people reported pain relief after 2 hours, which is better success than people find with many prescription and nonprescription drugs.
A separate study looked at acute treatment for chronic migraine sufferers and found nearly 60% of people using the device found relief and 21% said they were pain-free after 2 hours. Almost two-thirds of those who experienced pain relief were pain-free 24 hours after the treatment.
Finding the perfect patient
There are other FDA-cleared noninvasive devices to treat migraines. One device, CEFALY, is an external trigeminal nerve stimulation device that sits on the forehead. Another device, SpringTMS, uses transcranial magnetic stimulation on the back of the head. A third option, the gammaCore Sapphire, is placed on the neck to stimulate the vagus nerve. All three have been cleared by the FDA to work as preventive and acute treatments for migraine.
Theranica, the company that developed Nerivio, is trying to boost use of the device by allowing patients to get a prescription via telehealth visits with a physician.
The company, as well as the companies behind the other neuromodulation devices, are marketing their treatments to children ages 12 and up since nonpharmacologic options are often preferable for parents, said Thomas Berk, MD, a clinical associate professor in the division of headache at NYU Langone Health in New York.
Dr. Berk said the devices could be appealing for those people who don’t want or can’t take medication, such as pregnant women or those who don’t respond well to drugs. “[They] could also be used by somebody who needs something in addition to a medication,” he said.
For now, people like Ms. Montgomery say they are happy to have another tool in their migraine arsenal. “Overall, I’m taking less medication because I haven’t had to have my Ubrelvy refilled as often as I used to,” she said. “It’s really helped me manage changes and stresses in my life.”
A version of this article first appeared on Medscape.com.
Most people avoid smartphones when they have a migraine headache, but a noninvasive treatment for episodic migraines may change that.
that can help ease migraine pain.
Tina Montgomery, 58, has suffered from migraines since childhood and spent years looking for something to help manage them. Doctors consider her a “chronic” sufferer in that she has more than 14 migraines a month (fewer than 14 is considered “episodic”). Prescription antidepressants, anticonvulsants, and botulinum toxin shots as preventive treatments helped a little but not enough.
A few years ago, she found some relief using a new preventive injectable medication that targets a peptide known as CGRP, combined with an oral CGRP rescue medication, ubrogepant (Ubrelvy). However, by early 2021, Ms. Montgomery’s chronic migraines were back as she faced stress from the pandemic and her role as a caregiver for her aging parents.
“I was going through so much medication. I just didn’t feel good taking so much,” she said.
Looking for relief, she read about Nerivio, a wearable migraine treatment device that uses remote electrical neuromodulation (REN). She mentioned the device to her neurologist, and he agreed she might benefit from trying it out. Today, she uses the device whenever she feels a migraine may be imminent, she said.
“It really helps me stave off migraines I feel coming on and the milder ones where I would normally hesitate to use prescription medication because [insurance] limits the number of pills they give you in a month,” she said. “I follow through with the Nerivio treatment and usually find that my migraine doesn’t fully develop or is completely gone, and I don’t get a migraine at all.”
Taking it on the arm
The device works by stimulating nerves at the back of the arm right around the triceps. “Those nerve fibers relay information to the brain stem [so it can] work its magic and use the brain’s own natural mechanisms for reducing pain,” said Brian M. Grosberg, MD, director of the Hartford Healthcare Ayer Neuroscience Institute Headache Center, West Hartford, Conn.
These mechanisms are like a bait-and-switch for the brain, said Britany Klenofsky, MD, assistant professor of neurology, Icahn School of Medicine at Mount Sinai, New York. “You’re trying to stimulate pain somewhere else [on the body] to tell the brain to protect itself and release [the neurotransmitter] serotonin,” she said. “You do this by putting the device on your arm, an area that’s away from the head where the pain is actively occurring, turning the device on, and increasing the stimulation to a nearly painful stimulus.”
This pseudo pain prompts the brain to release serotonin, the feel-good hormone along with norepinephrine and noradrenaline. The device works best when it’s used as soon as a migraine starts, so patients should hook up Nerivio within the first 20-30 minutes of onset of pain, said Dr. Grosberg, who was an investigator on the double-blind treatment study that led to FDA clearance. If patients wait too long, the device may not work.
This is why as soon as Ms. Montgomery feels a migraine aura (there are six types of migraine auras, including visual changes and muscle weakness) that occurs right before a migraine strikes, she puts the device armband on her upper arm and launches its smartphone app. Then she turns on the device for a 45-minute treatment, which begins with what she characterizes as tingling and vibration sensations on her arm. She turns up the intensity of the sensations, which are mild electric currents, until they are well-felt but not painful.
Ms. Montgomery said she can use the device and multitask since there’s no need for her to lie down or sit in a darkened room. And since it is worn on the arm, she can wear it under a shirtsleeve while working or out in public without anyone noticing. She also uses the app’s migraine diary and guided meditation to help reduce the anxiety that often accompanies her migraines.
The device is approved for adolescents and adults and can be used for both episodic and chronic migraines. From an efficacy standpoint, the device provides relief about as well as a commonly used pharmaceutical class of drugs, triptans. About 37% of people with episodic migraine achieved complete freedom from pain 2 hours after their treatment. In addition, about two-thirds of people reported pain relief after 2 hours, which is better success than people find with many prescription and nonprescription drugs.
A separate study looked at acute treatment for chronic migraine sufferers and found nearly 60% of people using the device found relief and 21% said they were pain-free after 2 hours. Almost two-thirds of those who experienced pain relief were pain-free 24 hours after the treatment.
Finding the perfect patient
There are other FDA-cleared noninvasive devices to treat migraines. One device, CEFALY, is an external trigeminal nerve stimulation device that sits on the forehead. Another device, SpringTMS, uses transcranial magnetic stimulation on the back of the head. A third option, the gammaCore Sapphire, is placed on the neck to stimulate the vagus nerve. All three have been cleared by the FDA to work as preventive and acute treatments for migraine.
Theranica, the company that developed Nerivio, is trying to boost use of the device by allowing patients to get a prescription via telehealth visits with a physician.
The company, as well as the companies behind the other neuromodulation devices, are marketing their treatments to children ages 12 and up since nonpharmacologic options are often preferable for parents, said Thomas Berk, MD, a clinical associate professor in the division of headache at NYU Langone Health in New York.
Dr. Berk said the devices could be appealing for those people who don’t want or can’t take medication, such as pregnant women or those who don’t respond well to drugs. “[They] could also be used by somebody who needs something in addition to a medication,” he said.
For now, people like Ms. Montgomery say they are happy to have another tool in their migraine arsenal. “Overall, I’m taking less medication because I haven’t had to have my Ubrelvy refilled as often as I used to,” she said. “It’s really helped me manage changes and stresses in my life.”
A version of this article first appeared on Medscape.com.
Most people avoid smartphones when they have a migraine headache, but a noninvasive treatment for episodic migraines may change that.
that can help ease migraine pain.
Tina Montgomery, 58, has suffered from migraines since childhood and spent years looking for something to help manage them. Doctors consider her a “chronic” sufferer in that she has more than 14 migraines a month (fewer than 14 is considered “episodic”). Prescription antidepressants, anticonvulsants, and botulinum toxin shots as preventive treatments helped a little but not enough.
A few years ago, she found some relief using a new preventive injectable medication that targets a peptide known as CGRP, combined with an oral CGRP rescue medication, ubrogepant (Ubrelvy). However, by early 2021, Ms. Montgomery’s chronic migraines were back as she faced stress from the pandemic and her role as a caregiver for her aging parents.
“I was going through so much medication. I just didn’t feel good taking so much,” she said.
Looking for relief, she read about Nerivio, a wearable migraine treatment device that uses remote electrical neuromodulation (REN). She mentioned the device to her neurologist, and he agreed she might benefit from trying it out. Today, she uses the device whenever she feels a migraine may be imminent, she said.
“It really helps me stave off migraines I feel coming on and the milder ones where I would normally hesitate to use prescription medication because [insurance] limits the number of pills they give you in a month,” she said. “I follow through with the Nerivio treatment and usually find that my migraine doesn’t fully develop or is completely gone, and I don’t get a migraine at all.”
Taking it on the arm
The device works by stimulating nerves at the back of the arm right around the triceps. “Those nerve fibers relay information to the brain stem [so it can] work its magic and use the brain’s own natural mechanisms for reducing pain,” said Brian M. Grosberg, MD, director of the Hartford Healthcare Ayer Neuroscience Institute Headache Center, West Hartford, Conn.
These mechanisms are like a bait-and-switch for the brain, said Britany Klenofsky, MD, assistant professor of neurology, Icahn School of Medicine at Mount Sinai, New York. “You’re trying to stimulate pain somewhere else [on the body] to tell the brain to protect itself and release [the neurotransmitter] serotonin,” she said. “You do this by putting the device on your arm, an area that’s away from the head where the pain is actively occurring, turning the device on, and increasing the stimulation to a nearly painful stimulus.”
This pseudo pain prompts the brain to release serotonin, the feel-good hormone along with norepinephrine and noradrenaline. The device works best when it’s used as soon as a migraine starts, so patients should hook up Nerivio within the first 20-30 minutes of onset of pain, said Dr. Grosberg, who was an investigator on the double-blind treatment study that led to FDA clearance. If patients wait too long, the device may not work.
This is why as soon as Ms. Montgomery feels a migraine aura (there are six types of migraine auras, including visual changes and muscle weakness) that occurs right before a migraine strikes, she puts the device armband on her upper arm and launches its smartphone app. Then she turns on the device for a 45-minute treatment, which begins with what she characterizes as tingling and vibration sensations on her arm. She turns up the intensity of the sensations, which are mild electric currents, until they are well-felt but not painful.
Ms. Montgomery said she can use the device and multitask since there’s no need for her to lie down or sit in a darkened room. And since it is worn on the arm, she can wear it under a shirtsleeve while working or out in public without anyone noticing. She also uses the app’s migraine diary and guided meditation to help reduce the anxiety that often accompanies her migraines.
The device is approved for adolescents and adults and can be used for both episodic and chronic migraines. From an efficacy standpoint, the device provides relief about as well as a commonly used pharmaceutical class of drugs, triptans. About 37% of people with episodic migraine achieved complete freedom from pain 2 hours after their treatment. In addition, about two-thirds of people reported pain relief after 2 hours, which is better success than people find with many prescription and nonprescription drugs.
A separate study looked at acute treatment for chronic migraine sufferers and found nearly 60% of people using the device found relief and 21% said they were pain-free after 2 hours. Almost two-thirds of those who experienced pain relief were pain-free 24 hours after the treatment.
Finding the perfect patient
There are other FDA-cleared noninvasive devices to treat migraines. One device, CEFALY, is an external trigeminal nerve stimulation device that sits on the forehead. Another device, SpringTMS, uses transcranial magnetic stimulation on the back of the head. A third option, the gammaCore Sapphire, is placed on the neck to stimulate the vagus nerve. All three have been cleared by the FDA to work as preventive and acute treatments for migraine.
Theranica, the company that developed Nerivio, is trying to boost use of the device by allowing patients to get a prescription via telehealth visits with a physician.
The company, as well as the companies behind the other neuromodulation devices, are marketing their treatments to children ages 12 and up since nonpharmacologic options are often preferable for parents, said Thomas Berk, MD, a clinical associate professor in the division of headache at NYU Langone Health in New York.
Dr. Berk said the devices could be appealing for those people who don’t want or can’t take medication, such as pregnant women or those who don’t respond well to drugs. “[They] could also be used by somebody who needs something in addition to a medication,” he said.
For now, people like Ms. Montgomery say they are happy to have another tool in their migraine arsenal. “Overall, I’m taking less medication because I haven’t had to have my Ubrelvy refilled as often as I used to,” she said. “It’s really helped me manage changes and stresses in my life.”
A version of this article first appeared on Medscape.com.
Endocrine Society and others to FDA: Restrict BPA
The chemical is used to make plastics in items such as food containers, pitchers, and inner linings of metal products. Small amounts of BPA can leak into food and beverages.
The petition points to a December 2021 report by the European Food Safety Authority titled: “Re-evaluation of the risks to public health related to the presence of bisphenol A (BPA) in foodstuffs,” which summarizes evidence gathered since 2013.
It concludes that “there is a health concern from BPA exposure for all age groups.” Specific concerns include harm to the immune system and male and female reproductive systems.
Average American exposed to 5,000 times the safe level of BPA
The EFSA established a new “tolerable daily intake” of BPA of 0.04 ng/kg of body weight per day. By contrast, in 2014 the FDA estimated that the mean BPA intake for the U.S. population older than 2 years was 200 ng/kg bw/day and that the 90th percentile for BPA intake was 500 ng/kg of body weight per day.
“Using FDA’s own exposure estimates, the average American is exposed to more than 5000 times the safe level of 0.04 ng BPA/kg [body weight per day] set by the EFSA expert panel. Without a doubt, these values constitute a high health risk and support the conclusion that uses of BPA are not safe ... Given the magnitude of the overexposure, we request an expedited review by FDA,” the petition reads.
In addition to the Endocrine Society, which has long warned about the dangers of endocrine-disrupting chemicals, other signatories to the petition include the Environmental Defense Fund, Breast Cancer Prevention Partners, Clean Water Action/Clean Water Fund, Consumer Reports, Environmental Working Group, Healthy Babies Bright Futures, and the former director of the National Institute of Environmental Health Sciences and National Toxicology Program.
In a statement, Endocrine Society BPA expert Heather Patisaul, PhD, of North Carolina University, Raleigh, said the report’s findings “are extremely concerning and prove the point that even very low levels of BPA exposure can be harmful and lead to issues with reproductive health, breast cancer risk, behavior, and metabolism.”
“The FDA needs to acknowledge the science behind endocrine-disrupting chemicals and act accordingly to protect public health,” she urged.
The FDA is expected to decide within the next few days whether to open a docket to accept comments.
A final decision could take 6 months or longer, an Endocrine Society spokesperson told this news organization.
A version of this article first appeared on Medscape.com.
The chemical is used to make plastics in items such as food containers, pitchers, and inner linings of metal products. Small amounts of BPA can leak into food and beverages.
The petition points to a December 2021 report by the European Food Safety Authority titled: “Re-evaluation of the risks to public health related to the presence of bisphenol A (BPA) in foodstuffs,” which summarizes evidence gathered since 2013.
It concludes that “there is a health concern from BPA exposure for all age groups.” Specific concerns include harm to the immune system and male and female reproductive systems.
Average American exposed to 5,000 times the safe level of BPA
The EFSA established a new “tolerable daily intake” of BPA of 0.04 ng/kg of body weight per day. By contrast, in 2014 the FDA estimated that the mean BPA intake for the U.S. population older than 2 years was 200 ng/kg bw/day and that the 90th percentile for BPA intake was 500 ng/kg of body weight per day.
“Using FDA’s own exposure estimates, the average American is exposed to more than 5000 times the safe level of 0.04 ng BPA/kg [body weight per day] set by the EFSA expert panel. Without a doubt, these values constitute a high health risk and support the conclusion that uses of BPA are not safe ... Given the magnitude of the overexposure, we request an expedited review by FDA,” the petition reads.
In addition to the Endocrine Society, which has long warned about the dangers of endocrine-disrupting chemicals, other signatories to the petition include the Environmental Defense Fund, Breast Cancer Prevention Partners, Clean Water Action/Clean Water Fund, Consumer Reports, Environmental Working Group, Healthy Babies Bright Futures, and the former director of the National Institute of Environmental Health Sciences and National Toxicology Program.
In a statement, Endocrine Society BPA expert Heather Patisaul, PhD, of North Carolina University, Raleigh, said the report’s findings “are extremely concerning and prove the point that even very low levels of BPA exposure can be harmful and lead to issues with reproductive health, breast cancer risk, behavior, and metabolism.”
“The FDA needs to acknowledge the science behind endocrine-disrupting chemicals and act accordingly to protect public health,” she urged.
The FDA is expected to decide within the next few days whether to open a docket to accept comments.
A final decision could take 6 months or longer, an Endocrine Society spokesperson told this news organization.
A version of this article first appeared on Medscape.com.
The chemical is used to make plastics in items such as food containers, pitchers, and inner linings of metal products. Small amounts of BPA can leak into food and beverages.
The petition points to a December 2021 report by the European Food Safety Authority titled: “Re-evaluation of the risks to public health related to the presence of bisphenol A (BPA) in foodstuffs,” which summarizes evidence gathered since 2013.
It concludes that “there is a health concern from BPA exposure for all age groups.” Specific concerns include harm to the immune system and male and female reproductive systems.
Average American exposed to 5,000 times the safe level of BPA
The EFSA established a new “tolerable daily intake” of BPA of 0.04 ng/kg of body weight per day. By contrast, in 2014 the FDA estimated that the mean BPA intake for the U.S. population older than 2 years was 200 ng/kg bw/day and that the 90th percentile for BPA intake was 500 ng/kg of body weight per day.
“Using FDA’s own exposure estimates, the average American is exposed to more than 5000 times the safe level of 0.04 ng BPA/kg [body weight per day] set by the EFSA expert panel. Without a doubt, these values constitute a high health risk and support the conclusion that uses of BPA are not safe ... Given the magnitude of the overexposure, we request an expedited review by FDA,” the petition reads.
In addition to the Endocrine Society, which has long warned about the dangers of endocrine-disrupting chemicals, other signatories to the petition include the Environmental Defense Fund, Breast Cancer Prevention Partners, Clean Water Action/Clean Water Fund, Consumer Reports, Environmental Working Group, Healthy Babies Bright Futures, and the former director of the National Institute of Environmental Health Sciences and National Toxicology Program.
In a statement, Endocrine Society BPA expert Heather Patisaul, PhD, of North Carolina University, Raleigh, said the report’s findings “are extremely concerning and prove the point that even very low levels of BPA exposure can be harmful and lead to issues with reproductive health, breast cancer risk, behavior, and metabolism.”
“The FDA needs to acknowledge the science behind endocrine-disrupting chemicals and act accordingly to protect public health,” she urged.
The FDA is expected to decide within the next few days whether to open a docket to accept comments.
A final decision could take 6 months or longer, an Endocrine Society spokesperson told this news organization.
A version of this article first appeared on Medscape.com.
Cystic fibrosis in retreat, but still unbeaten
In 1938, the year that cystic fibrosis (CF) was first described clinically, four of five children born with the disease did not live past their first birthdays.
In 2019, the median age at death for patients enrolled in the Cystic Fibrosis Foundation (CFF) registry was 32 years, and the predicted life expectancy for patients with CF who were born from 2015 through 2019 was 46 years.
Those numbers reflect the remarkable progress made in the past 4 decades in the care of patients with CF, but also highlight the obstacles ahead, given that the predicted life expectancy for the overall U.S. population in 2019 (pre–COVID-19) was 78.9 years.
Julie Desch, MD, is a CF survivor who has beaten the odds and then some. At age 61, the retired surgical pathologist is a CF patient advocate, speaker, and a board member of the Cystic Fibrosis Research Institute, a not-for-profit organization that funds CF research and offers education, advocacy, and psychosocial support for persons with CF and their families and caregivers.
In an interview, Dr. Desch said that while there has been remarkable progress in her lifetime in the field of CF research and treatment, particularly in the development of drugs that modulate function of the underlying cause of approximately 90% of CF cases, there are still many CF patients who cannot benefit from these therapies.
“There are still 10% of people who don’t make a protein to be modified, so that’s a huge unmet need,” she said.
Genetic disorder
CF is a chronic autosomal recessive disorder with multiorgan and multisystem manifestations. It is caused by mutations in the CFTR gene, which codes for the protein CF transmembrane conductance regulator. CFTR controls transport of chloride ions across cell membranes, specifically the apical membrane of epithelial cells in tissues of the airways, intestinal tract, pancreas, kidneys, sweat glands, and the reproductive system, notably the vas deferens in males.
The F508 deletion (F508del) mutation is the most common, occurring in approximately 70% of persons with CF. It is a class 2-type protein processing mutation, leading to defects in cellular processing, protein stability, and chloride channel gating defects.
The CFTR protein also secretes bicarbonate to regulate the pH of airway surface liquid, and inhibits the epithelial sodium channel, which mediates passive sodium transport across apical membranes of sodium-absorbing epithelial cells in the kidneys, intestine, and airways.
CF typically presents with the buildup in the lungs of abnormally viscous and sticky mucus leading to frequent, severe infections, particularly with Pseudomonas aeruginosa, progressive lung damage and, prior to the development of effective disease management, to premature death. The phenotype often includes malnutrition due to malabsorption, and failure to thrive.
Diagnosis
In all 50 U.S. states and the District of Columbia, newborns are screened for CF with an assay for immunoreactive trypsinogen (IRT) an indirect marker for pancreatic injury that is elevated in serum in most newborns with CF, but also detected in premature infants or those delivered under stressful circumstances. In some states newborns are tested only for IRT, with a diagnosis confirmed with a sweat chloride test and/or a CFTR mutation panel.
Treatment
There is no cure for CF, but the discovery of the gene in 1989 by Canadian and U.S. investigators has led to life-prolonging therapeutic interventions, specifically the development of CFTR modulators.
CFTR modulators include potentiators such as ivacaftor (Kalydeco), and correctors such as lumacaftor and tezacaftor (available in the combination Orkambi), and most recently in the triple combination of elexacaftor, tezacaftor, and ivacaftor (Trikafta; ETI).
Neil Sweezey, MD, FRCPC, a CF expert at The Hospital for Sick Children (SickKids) in Toronto, told this news organization that the ideal therapy for CF, genetic correction of the underlying mutations, is still not feasible, but that CFTR modulators are a close second.
“For 90% of patients, the three-drug combination Trikafta has been shown to be quite safe, quite tolerable, and quite remarkably beneficial,” he said.
In a study reported at CHEST 2021 by investigators from Nationwide Children’s Hospital in Columbus, Ohio, 32 adults who were started on the triple combination had significantly improved in forced expiratory volume in 1 second (FEV1), gain in body mass index, decreased sweat chloride and decreased colonization by Pseudomonas species. In addition, patients had significant improvements in blood inflammatory markers.
Christopher H. Goss, MD, FCCP, professor of pulmonary critical care and sleep medicine and professor of pediatrics at the University of Washington in Seattle, agreed that with the availability of the triple combination, “these are extraordinary times. An astounding fact is that most patients have complete resolution of cough, and the exacerbation rates have just plummeted,” he said in an interview.
Some of the reductions in exacerbations may be attributable to the COVID-19 pandemic, he noted, because patients in isolation have less exposure to circulating respiratory viruses.
“But it has been miraculous, and the clinical effect is certainly still more astounding than the effects of ivacaftor, which was the first truly breakthrough drug. Weight goes up, well-being increases, and the population lung function has shifted up to better grade lung function, in the entire population,” he said.
In addition, the need for lung and heart transplantation has sharply declined.
“I had a patient who had decided to forgo transplantation, despite absolutely horrible lung function, and he’s now bowling and leading a very productive life, when before he had been preparing for end of life,” Dr. Goss said.
Dr. Sweezey emphasized that as with all medications, patients being started on the triple combination require close monitoring for potential adverse events that might require dose modification or, for a small number of patients, withdrawal.
Burden of care
CFTR modulators have reduced but not eliminated the need for some patients to have mucolytic therapy, which may include dornase alfa, a recombinant human deoxyribonuclease (DNase) that reduces the viscosity of lung secretions, hypertonic saline inhaled twice daily (for patients 12 and older), mannitol, and physical manipulations to help patients clear mucus. This can include both manual percussion and the use of devices for high-frequency chest wall oscillation.
The complex nature of CF often requires a combination of other therapies to address comorbidities. These therapies may include infection prophylaxis and treatment with antibiotics and antifungals, nutrition support, and therapy for CF-related complications, including gastrointestinal issues, liver diseases, diabetes, and osteopenia that may be related to poor nutrient absorption, chronic inflammation, or other sequelae of CF.
In addition, patients often require frequent CF care center visits – ideally a minimum of every 3 months – which can result in significant loss of work or school time.
“Outcomes for patients in the long run have been absolutely proven to be best if they’re followed in big, established, multidisciplinary well-organized CF centers,” Dr. Sweezey said. “In the United States and Canada if you’re looked after on a regular basis, which means quarterly, every 3 months – whether you need it or not, you really do need it – and if the patients are seen and assessed and checked every 3 months all of their lives, they have small changes caught early, whether it’s an infection you can slap down with medication or a nutrition problem that may be affecting a child’s growth and development.”
“We’re really kind of at a pivotal moment in CF, where we realize things are changing,” said A. Whitney Brown, MD, senior director for clinical affairs at the Cystic Fibrosis Foundation, and an adult CF and lung transplant physician in the Inova Advanced Lung Disease Program in Falls Church, Va.
“Patient needs and interest have evolved, because of the pandemic and because of the highly effective modulator therapy, but we want to take great effort to study it in a rigorous way, to make sure that as we are agile and adapt the care model, that we can maintain the same quality outcomes that we have traditionally done,” she said in an interview.
The Lancet Respiratory Medicine Commission on the future of CF care states that models of care “need to consider management approaches (including disease monitoring) to maintain health and delay lung transplantation, while minimizing the burden of care for patients and their families.”
‘A great problem to have’
One of the most significant changes in CF care has been the growing population of CF patients like Dr. Desch who are living well into adulthood, with some approaching Medicare eligibility.
With the advent of triple therapy and CFTR modulators being started earlier in life, lung function can be preserved, damage to other organs can be minimized, and life expectancy for patients with CF will continue to improve.
“We’re anticipating that there may be some needs in the aging CF population that are different than what we have historically had,” Dr. Brown said. “Will there be geriatric providers that need to become experts in CF care? That’s a great problem to have,” she said.
Dr. Goss agreed, noting that CF is steadily shifting from a near uniformly fatal disease to a chronic disorder that in many cases can be managed “with a complex regimen of novel drugs, much like HIV.”
He noted that there are multiple drug interactions with the triple combination, “so it’s really important that people don’t start a CF patient on a drug without consulting a pharmacist, because you can totally inactivate ETI, or augment it dramatically, and we’ve seen both happen.”
Cost and access
All experts interviewed for this article agreed that while the care of patients with CF has improved exponentially over the last few decades, there are still troubling inequities in care.
One of the largest impediments is the cost of care, with the triple combination costing more than $300,000 per year.
“Clearly patients aren’t paying that, but insurance companies are, and that’s causing all kinds of trickle-down effects that definitely affect patients. The patients like myself who are able to have insurance that covers it benefit, but there are so many people that don’t,” Dr. Desch said.
Dr. Sweezey noted that prior to the advent of ETI, patients with CF in Canada had better outcomes and longer life expectancy than did similar patients in the United States because of universal access to care and coordinated services under Canada’s health care system, compared with the highly fragmented and inefficient U.S. system. He added that the wider availability of ETI in the United States vs. Canada may begin to narrow that gap, however.
As noted before, there is a substantial proportion of patients – an estimated 10% – who have CFTR mutations that are not correctable by currently available CFTR modulators, and these patients are at significant risk for irreversible airway complications and lung damage.
In addition, although CF occurs most frequently among people of White ancestry, the disease does not respect distinctions of race or ethnicity.
“It’s not just [Whites] – a lot of people from different racial backgrounds, ethnic backgrounds, are not being diagnosed or are not being diagnosed soon enough to have effective care early enough,” Dr. Desch said.
That statement is supported by the Lancet Respiratory Medicine Commission on the future of cystic fibrosis care, whose members noted in 2019 that “epidemiological studies in the past 2 decades have shown that cystic fibrosis occurs and is more frequent than was previously thought in populations of non-European descent, and the disease is now recognized in many regions of the world.”
The commission members noted that the costs of adequate CF care may be beyond the reach of many patients in developing nations.
Still, if the substantial barriers of cost and access can be overcome, the future will continue to look brighter for patients with CF. As Dr. Sweezey put it: “There are studies that are pushing lower age limits for using these modulators, and as the evidence builds for the efficacy and safety at younger ages, I think all of us are hoping that we’ll end up being able to use either the current or future modulators to actually prevent trouble in CF, rather than trying to come along and fix it after it’s been there.”
Dr. Brown disclosed advisory board activity for Vertex that ended prior to her joining the CF Foundation. Dr. Desch, Dr. Goss, and Dr. Sweezey reported no relevant conflicts of interest.
In 1938, the year that cystic fibrosis (CF) was first described clinically, four of five children born with the disease did not live past their first birthdays.
In 2019, the median age at death for patients enrolled in the Cystic Fibrosis Foundation (CFF) registry was 32 years, and the predicted life expectancy for patients with CF who were born from 2015 through 2019 was 46 years.
Those numbers reflect the remarkable progress made in the past 4 decades in the care of patients with CF, but also highlight the obstacles ahead, given that the predicted life expectancy for the overall U.S. population in 2019 (pre–COVID-19) was 78.9 years.
Julie Desch, MD, is a CF survivor who has beaten the odds and then some. At age 61, the retired surgical pathologist is a CF patient advocate, speaker, and a board member of the Cystic Fibrosis Research Institute, a not-for-profit organization that funds CF research and offers education, advocacy, and psychosocial support for persons with CF and their families and caregivers.
In an interview, Dr. Desch said that while there has been remarkable progress in her lifetime in the field of CF research and treatment, particularly in the development of drugs that modulate function of the underlying cause of approximately 90% of CF cases, there are still many CF patients who cannot benefit from these therapies.
“There are still 10% of people who don’t make a protein to be modified, so that’s a huge unmet need,” she said.
Genetic disorder
CF is a chronic autosomal recessive disorder with multiorgan and multisystem manifestations. It is caused by mutations in the CFTR gene, which codes for the protein CF transmembrane conductance regulator. CFTR controls transport of chloride ions across cell membranes, specifically the apical membrane of epithelial cells in tissues of the airways, intestinal tract, pancreas, kidneys, sweat glands, and the reproductive system, notably the vas deferens in males.
The F508 deletion (F508del) mutation is the most common, occurring in approximately 70% of persons with CF. It is a class 2-type protein processing mutation, leading to defects in cellular processing, protein stability, and chloride channel gating defects.
The CFTR protein also secretes bicarbonate to regulate the pH of airway surface liquid, and inhibits the epithelial sodium channel, which mediates passive sodium transport across apical membranes of sodium-absorbing epithelial cells in the kidneys, intestine, and airways.
CF typically presents with the buildup in the lungs of abnormally viscous and sticky mucus leading to frequent, severe infections, particularly with Pseudomonas aeruginosa, progressive lung damage and, prior to the development of effective disease management, to premature death. The phenotype often includes malnutrition due to malabsorption, and failure to thrive.
Diagnosis
In all 50 U.S. states and the District of Columbia, newborns are screened for CF with an assay for immunoreactive trypsinogen (IRT) an indirect marker for pancreatic injury that is elevated in serum in most newborns with CF, but also detected in premature infants or those delivered under stressful circumstances. In some states newborns are tested only for IRT, with a diagnosis confirmed with a sweat chloride test and/or a CFTR mutation panel.
Treatment
There is no cure for CF, but the discovery of the gene in 1989 by Canadian and U.S. investigators has led to life-prolonging therapeutic interventions, specifically the development of CFTR modulators.
CFTR modulators include potentiators such as ivacaftor (Kalydeco), and correctors such as lumacaftor and tezacaftor (available in the combination Orkambi), and most recently in the triple combination of elexacaftor, tezacaftor, and ivacaftor (Trikafta; ETI).
Neil Sweezey, MD, FRCPC, a CF expert at The Hospital for Sick Children (SickKids) in Toronto, told this news organization that the ideal therapy for CF, genetic correction of the underlying mutations, is still not feasible, but that CFTR modulators are a close second.
“For 90% of patients, the three-drug combination Trikafta has been shown to be quite safe, quite tolerable, and quite remarkably beneficial,” he said.
In a study reported at CHEST 2021 by investigators from Nationwide Children’s Hospital in Columbus, Ohio, 32 adults who were started on the triple combination had significantly improved in forced expiratory volume in 1 second (FEV1), gain in body mass index, decreased sweat chloride and decreased colonization by Pseudomonas species. In addition, patients had significant improvements in blood inflammatory markers.
Christopher H. Goss, MD, FCCP, professor of pulmonary critical care and sleep medicine and professor of pediatrics at the University of Washington in Seattle, agreed that with the availability of the triple combination, “these are extraordinary times. An astounding fact is that most patients have complete resolution of cough, and the exacerbation rates have just plummeted,” he said in an interview.
Some of the reductions in exacerbations may be attributable to the COVID-19 pandemic, he noted, because patients in isolation have less exposure to circulating respiratory viruses.
“But it has been miraculous, and the clinical effect is certainly still more astounding than the effects of ivacaftor, which was the first truly breakthrough drug. Weight goes up, well-being increases, and the population lung function has shifted up to better grade lung function, in the entire population,” he said.
In addition, the need for lung and heart transplantation has sharply declined.
“I had a patient who had decided to forgo transplantation, despite absolutely horrible lung function, and he’s now bowling and leading a very productive life, when before he had been preparing for end of life,” Dr. Goss said.
Dr. Sweezey emphasized that as with all medications, patients being started on the triple combination require close monitoring for potential adverse events that might require dose modification or, for a small number of patients, withdrawal.
Burden of care
CFTR modulators have reduced but not eliminated the need for some patients to have mucolytic therapy, which may include dornase alfa, a recombinant human deoxyribonuclease (DNase) that reduces the viscosity of lung secretions, hypertonic saline inhaled twice daily (for patients 12 and older), mannitol, and physical manipulations to help patients clear mucus. This can include both manual percussion and the use of devices for high-frequency chest wall oscillation.
The complex nature of CF often requires a combination of other therapies to address comorbidities. These therapies may include infection prophylaxis and treatment with antibiotics and antifungals, nutrition support, and therapy for CF-related complications, including gastrointestinal issues, liver diseases, diabetes, and osteopenia that may be related to poor nutrient absorption, chronic inflammation, or other sequelae of CF.
In addition, patients often require frequent CF care center visits – ideally a minimum of every 3 months – which can result in significant loss of work or school time.
“Outcomes for patients in the long run have been absolutely proven to be best if they’re followed in big, established, multidisciplinary well-organized CF centers,” Dr. Sweezey said. “In the United States and Canada if you’re looked after on a regular basis, which means quarterly, every 3 months – whether you need it or not, you really do need it – and if the patients are seen and assessed and checked every 3 months all of their lives, they have small changes caught early, whether it’s an infection you can slap down with medication or a nutrition problem that may be affecting a child’s growth and development.”
“We’re really kind of at a pivotal moment in CF, where we realize things are changing,” said A. Whitney Brown, MD, senior director for clinical affairs at the Cystic Fibrosis Foundation, and an adult CF and lung transplant physician in the Inova Advanced Lung Disease Program in Falls Church, Va.
“Patient needs and interest have evolved, because of the pandemic and because of the highly effective modulator therapy, but we want to take great effort to study it in a rigorous way, to make sure that as we are agile and adapt the care model, that we can maintain the same quality outcomes that we have traditionally done,” she said in an interview.
The Lancet Respiratory Medicine Commission on the future of CF care states that models of care “need to consider management approaches (including disease monitoring) to maintain health and delay lung transplantation, while minimizing the burden of care for patients and their families.”
‘A great problem to have’
One of the most significant changes in CF care has been the growing population of CF patients like Dr. Desch who are living well into adulthood, with some approaching Medicare eligibility.
With the advent of triple therapy and CFTR modulators being started earlier in life, lung function can be preserved, damage to other organs can be minimized, and life expectancy for patients with CF will continue to improve.
“We’re anticipating that there may be some needs in the aging CF population that are different than what we have historically had,” Dr. Brown said. “Will there be geriatric providers that need to become experts in CF care? That’s a great problem to have,” she said.
Dr. Goss agreed, noting that CF is steadily shifting from a near uniformly fatal disease to a chronic disorder that in many cases can be managed “with a complex regimen of novel drugs, much like HIV.”
He noted that there are multiple drug interactions with the triple combination, “so it’s really important that people don’t start a CF patient on a drug without consulting a pharmacist, because you can totally inactivate ETI, or augment it dramatically, and we’ve seen both happen.”
Cost and access
All experts interviewed for this article agreed that while the care of patients with CF has improved exponentially over the last few decades, there are still troubling inequities in care.
One of the largest impediments is the cost of care, with the triple combination costing more than $300,000 per year.
“Clearly patients aren’t paying that, but insurance companies are, and that’s causing all kinds of trickle-down effects that definitely affect patients. The patients like myself who are able to have insurance that covers it benefit, but there are so many people that don’t,” Dr. Desch said.
Dr. Sweezey noted that prior to the advent of ETI, patients with CF in Canada had better outcomes and longer life expectancy than did similar patients in the United States because of universal access to care and coordinated services under Canada’s health care system, compared with the highly fragmented and inefficient U.S. system. He added that the wider availability of ETI in the United States vs. Canada may begin to narrow that gap, however.
As noted before, there is a substantial proportion of patients – an estimated 10% – who have CFTR mutations that are not correctable by currently available CFTR modulators, and these patients are at significant risk for irreversible airway complications and lung damage.
In addition, although CF occurs most frequently among people of White ancestry, the disease does not respect distinctions of race or ethnicity.
“It’s not just [Whites] – a lot of people from different racial backgrounds, ethnic backgrounds, are not being diagnosed or are not being diagnosed soon enough to have effective care early enough,” Dr. Desch said.
That statement is supported by the Lancet Respiratory Medicine Commission on the future of cystic fibrosis care, whose members noted in 2019 that “epidemiological studies in the past 2 decades have shown that cystic fibrosis occurs and is more frequent than was previously thought in populations of non-European descent, and the disease is now recognized in many regions of the world.”
The commission members noted that the costs of adequate CF care may be beyond the reach of many patients in developing nations.
Still, if the substantial barriers of cost and access can be overcome, the future will continue to look brighter for patients with CF. As Dr. Sweezey put it: “There are studies that are pushing lower age limits for using these modulators, and as the evidence builds for the efficacy and safety at younger ages, I think all of us are hoping that we’ll end up being able to use either the current or future modulators to actually prevent trouble in CF, rather than trying to come along and fix it after it’s been there.”
Dr. Brown disclosed advisory board activity for Vertex that ended prior to her joining the CF Foundation. Dr. Desch, Dr. Goss, and Dr. Sweezey reported no relevant conflicts of interest.
In 1938, the year that cystic fibrosis (CF) was first described clinically, four of five children born with the disease did not live past their first birthdays.
In 2019, the median age at death for patients enrolled in the Cystic Fibrosis Foundation (CFF) registry was 32 years, and the predicted life expectancy for patients with CF who were born from 2015 through 2019 was 46 years.
Those numbers reflect the remarkable progress made in the past 4 decades in the care of patients with CF, but also highlight the obstacles ahead, given that the predicted life expectancy for the overall U.S. population in 2019 (pre–COVID-19) was 78.9 years.
Julie Desch, MD, is a CF survivor who has beaten the odds and then some. At age 61, the retired surgical pathologist is a CF patient advocate, speaker, and a board member of the Cystic Fibrosis Research Institute, a not-for-profit organization that funds CF research and offers education, advocacy, and psychosocial support for persons with CF and their families and caregivers.
In an interview, Dr. Desch said that while there has been remarkable progress in her lifetime in the field of CF research and treatment, particularly in the development of drugs that modulate function of the underlying cause of approximately 90% of CF cases, there are still many CF patients who cannot benefit from these therapies.
“There are still 10% of people who don’t make a protein to be modified, so that’s a huge unmet need,” she said.
Genetic disorder
CF is a chronic autosomal recessive disorder with multiorgan and multisystem manifestations. It is caused by mutations in the CFTR gene, which codes for the protein CF transmembrane conductance regulator. CFTR controls transport of chloride ions across cell membranes, specifically the apical membrane of epithelial cells in tissues of the airways, intestinal tract, pancreas, kidneys, sweat glands, and the reproductive system, notably the vas deferens in males.
The F508 deletion (F508del) mutation is the most common, occurring in approximately 70% of persons with CF. It is a class 2-type protein processing mutation, leading to defects in cellular processing, protein stability, and chloride channel gating defects.
The CFTR protein also secretes bicarbonate to regulate the pH of airway surface liquid, and inhibits the epithelial sodium channel, which mediates passive sodium transport across apical membranes of sodium-absorbing epithelial cells in the kidneys, intestine, and airways.
CF typically presents with the buildup in the lungs of abnormally viscous and sticky mucus leading to frequent, severe infections, particularly with Pseudomonas aeruginosa, progressive lung damage and, prior to the development of effective disease management, to premature death. The phenotype often includes malnutrition due to malabsorption, and failure to thrive.
Diagnosis
In all 50 U.S. states and the District of Columbia, newborns are screened for CF with an assay for immunoreactive trypsinogen (IRT) an indirect marker for pancreatic injury that is elevated in serum in most newborns with CF, but also detected in premature infants or those delivered under stressful circumstances. In some states newborns are tested only for IRT, with a diagnosis confirmed with a sweat chloride test and/or a CFTR mutation panel.
Treatment
There is no cure for CF, but the discovery of the gene in 1989 by Canadian and U.S. investigators has led to life-prolonging therapeutic interventions, specifically the development of CFTR modulators.
CFTR modulators include potentiators such as ivacaftor (Kalydeco), and correctors such as lumacaftor and tezacaftor (available in the combination Orkambi), and most recently in the triple combination of elexacaftor, tezacaftor, and ivacaftor (Trikafta; ETI).
Neil Sweezey, MD, FRCPC, a CF expert at The Hospital for Sick Children (SickKids) in Toronto, told this news organization that the ideal therapy for CF, genetic correction of the underlying mutations, is still not feasible, but that CFTR modulators are a close second.
“For 90% of patients, the three-drug combination Trikafta has been shown to be quite safe, quite tolerable, and quite remarkably beneficial,” he said.
In a study reported at CHEST 2021 by investigators from Nationwide Children’s Hospital in Columbus, Ohio, 32 adults who were started on the triple combination had significantly improved in forced expiratory volume in 1 second (FEV1), gain in body mass index, decreased sweat chloride and decreased colonization by Pseudomonas species. In addition, patients had significant improvements in blood inflammatory markers.
Christopher H. Goss, MD, FCCP, professor of pulmonary critical care and sleep medicine and professor of pediatrics at the University of Washington in Seattle, agreed that with the availability of the triple combination, “these are extraordinary times. An astounding fact is that most patients have complete resolution of cough, and the exacerbation rates have just plummeted,” he said in an interview.
Some of the reductions in exacerbations may be attributable to the COVID-19 pandemic, he noted, because patients in isolation have less exposure to circulating respiratory viruses.
“But it has been miraculous, and the clinical effect is certainly still more astounding than the effects of ivacaftor, which was the first truly breakthrough drug. Weight goes up, well-being increases, and the population lung function has shifted up to better grade lung function, in the entire population,” he said.
In addition, the need for lung and heart transplantation has sharply declined.
“I had a patient who had decided to forgo transplantation, despite absolutely horrible lung function, and he’s now bowling and leading a very productive life, when before he had been preparing for end of life,” Dr. Goss said.
Dr. Sweezey emphasized that as with all medications, patients being started on the triple combination require close monitoring for potential adverse events that might require dose modification or, for a small number of patients, withdrawal.
Burden of care
CFTR modulators have reduced but not eliminated the need for some patients to have mucolytic therapy, which may include dornase alfa, a recombinant human deoxyribonuclease (DNase) that reduces the viscosity of lung secretions, hypertonic saline inhaled twice daily (for patients 12 and older), mannitol, and physical manipulations to help patients clear mucus. This can include both manual percussion and the use of devices for high-frequency chest wall oscillation.
The complex nature of CF often requires a combination of other therapies to address comorbidities. These therapies may include infection prophylaxis and treatment with antibiotics and antifungals, nutrition support, and therapy for CF-related complications, including gastrointestinal issues, liver diseases, diabetes, and osteopenia that may be related to poor nutrient absorption, chronic inflammation, or other sequelae of CF.
In addition, patients often require frequent CF care center visits – ideally a minimum of every 3 months – which can result in significant loss of work or school time.
“Outcomes for patients in the long run have been absolutely proven to be best if they’re followed in big, established, multidisciplinary well-organized CF centers,” Dr. Sweezey said. “In the United States and Canada if you’re looked after on a regular basis, which means quarterly, every 3 months – whether you need it or not, you really do need it – and if the patients are seen and assessed and checked every 3 months all of their lives, they have small changes caught early, whether it’s an infection you can slap down with medication or a nutrition problem that may be affecting a child’s growth and development.”
“We’re really kind of at a pivotal moment in CF, where we realize things are changing,” said A. Whitney Brown, MD, senior director for clinical affairs at the Cystic Fibrosis Foundation, and an adult CF and lung transplant physician in the Inova Advanced Lung Disease Program in Falls Church, Va.
“Patient needs and interest have evolved, because of the pandemic and because of the highly effective modulator therapy, but we want to take great effort to study it in a rigorous way, to make sure that as we are agile and adapt the care model, that we can maintain the same quality outcomes that we have traditionally done,” she said in an interview.
The Lancet Respiratory Medicine Commission on the future of CF care states that models of care “need to consider management approaches (including disease monitoring) to maintain health and delay lung transplantation, while minimizing the burden of care for patients and their families.”
‘A great problem to have’
One of the most significant changes in CF care has been the growing population of CF patients like Dr. Desch who are living well into adulthood, with some approaching Medicare eligibility.
With the advent of triple therapy and CFTR modulators being started earlier in life, lung function can be preserved, damage to other organs can be minimized, and life expectancy for patients with CF will continue to improve.
“We’re anticipating that there may be some needs in the aging CF population that are different than what we have historically had,” Dr. Brown said. “Will there be geriatric providers that need to become experts in CF care? That’s a great problem to have,” she said.
Dr. Goss agreed, noting that CF is steadily shifting from a near uniformly fatal disease to a chronic disorder that in many cases can be managed “with a complex regimen of novel drugs, much like HIV.”
He noted that there are multiple drug interactions with the triple combination, “so it’s really important that people don’t start a CF patient on a drug without consulting a pharmacist, because you can totally inactivate ETI, or augment it dramatically, and we’ve seen both happen.”
Cost and access
All experts interviewed for this article agreed that while the care of patients with CF has improved exponentially over the last few decades, there are still troubling inequities in care.
One of the largest impediments is the cost of care, with the triple combination costing more than $300,000 per year.
“Clearly patients aren’t paying that, but insurance companies are, and that’s causing all kinds of trickle-down effects that definitely affect patients. The patients like myself who are able to have insurance that covers it benefit, but there are so many people that don’t,” Dr. Desch said.
Dr. Sweezey noted that prior to the advent of ETI, patients with CF in Canada had better outcomes and longer life expectancy than did similar patients in the United States because of universal access to care and coordinated services under Canada’s health care system, compared with the highly fragmented and inefficient U.S. system. He added that the wider availability of ETI in the United States vs. Canada may begin to narrow that gap, however.
As noted before, there is a substantial proportion of patients – an estimated 10% – who have CFTR mutations that are not correctable by currently available CFTR modulators, and these patients are at significant risk for irreversible airway complications and lung damage.
In addition, although CF occurs most frequently among people of White ancestry, the disease does not respect distinctions of race or ethnicity.
“It’s not just [Whites] – a lot of people from different racial backgrounds, ethnic backgrounds, are not being diagnosed or are not being diagnosed soon enough to have effective care early enough,” Dr. Desch said.
That statement is supported by the Lancet Respiratory Medicine Commission on the future of cystic fibrosis care, whose members noted in 2019 that “epidemiological studies in the past 2 decades have shown that cystic fibrosis occurs and is more frequent than was previously thought in populations of non-European descent, and the disease is now recognized in many regions of the world.”
The commission members noted that the costs of adequate CF care may be beyond the reach of many patients in developing nations.
Still, if the substantial barriers of cost and access can be overcome, the future will continue to look brighter for patients with CF. As Dr. Sweezey put it: “There are studies that are pushing lower age limits for using these modulators, and as the evidence builds for the efficacy and safety at younger ages, I think all of us are hoping that we’ll end up being able to use either the current or future modulators to actually prevent trouble in CF, rather than trying to come along and fix it after it’s been there.”
Dr. Brown disclosed advisory board activity for Vertex that ended prior to her joining the CF Foundation. Dr. Desch, Dr. Goss, and Dr. Sweezey reported no relevant conflicts of interest.
USDA announces stricter standards for school nutrition
The U.S. Department of Agriculture has announced new changes to school nutrition standards for the next 2 school years, which will reinstate health goals that were rolled back during the Trump administration.
The Biden administration is also tightening rules for fat and salt content in foods after restrictions were eased during the pandemic, according to the Washington Post.
“Nutritious school meals give America’s children the foundation for successful, healthy lives,” Tom Vilsack, the U.S. agriculture secretary, said in a statement on Feb. 4.
“We applaud schools’ heroic efforts throughout the challenges of this pandemic to continue serving kids the most nutritious meals possible,” he said. “The standards we’re putting in place of the next 2 school years will help schools transition to a future that builds on the tremendous strides they’ve made improving school meal nutrition over the past decade.”
For the 2022-2023 school year, schools and childcare providers will be required to offer low-fat or nonfat unflavored milks and limit the fat in sweet flavored milks. In addition, at least 80% of the grains served during school breakfasts and lunches each week must be considered rich in whole grains.
For the 2023-2024 school year, the weekly sodium limit for school lunches will be decreased by 10%.
The changes mark a shift from the Trump administration, which eased policies on whole grains, nonfat milk, and sodium, the newspaper reported. Then the pandemic forced additional changes as school districts scrambled to package meals for students. The USDA granted extra flexibility and eased some guidelines to ensure that children could be fed while schools were closed or focused on remote learning.
Now the USDA is updating the nutrition standards to “give schools clear expectations for gradual transition from current pandemic operations to more nutritious meals,” Stacy Dean, the USDA’s deputy undersecretary for food, nutrition, and consumer services, told reporters.
The Biden administration’s changes represent a shift back to Obama-era nutrition standards from 2012, according to the Post. But some nutrition advocates have said the new changes don’t address enough issues, such as added sugars. Fruit and vegetable requirements, for instance, will remain the same as the 2012 standards.
That said, some advocates have said the transition could be tough as schools move out of pandemic-era protocols. The School Nutrition Association, which represents school food service manufacturers and professionals, has urged Congress to provide additional support and waiver extensions for the next school year.
“School nutrition professionals are frantic just trying to get enough food on the tray for our students amid relentless supply chain disruptions and labor shortages,” Beth Wallace, the association’s president, told the Washington Post.
The shift will likely require a balancing act and slow transition. The USDA has been consulting with stakeholders for months to determine how to move toward stricter school nutrition standards while also acknowledging the pandemic, supply chain disruptions, and labor shortages.
“This approach is really going to help move forward the nutrition of the meals and allows the schools to continue to function effectively,” Geri Henchy, director of nutrition policy at the Food Research and Action Center, told the Post.
“Schools can’t make big changes at this point because of the supply chain and staffing,” she said. “They have a lot of waivers at this point that are helping them, and this balances the needs of all the different sectors.”
The USDA plans to issue a proposed rule in fall 2022 to update nutrition standards for the future, the department said in its announcement, which would be finalized for the 2024-2025 school year.
A version of this article first appeared on WebMD.com.
The U.S. Department of Agriculture has announced new changes to school nutrition standards for the next 2 school years, which will reinstate health goals that were rolled back during the Trump administration.
The Biden administration is also tightening rules for fat and salt content in foods after restrictions were eased during the pandemic, according to the Washington Post.
“Nutritious school meals give America’s children the foundation for successful, healthy lives,” Tom Vilsack, the U.S. agriculture secretary, said in a statement on Feb. 4.
“We applaud schools’ heroic efforts throughout the challenges of this pandemic to continue serving kids the most nutritious meals possible,” he said. “The standards we’re putting in place of the next 2 school years will help schools transition to a future that builds on the tremendous strides they’ve made improving school meal nutrition over the past decade.”
For the 2022-2023 school year, schools and childcare providers will be required to offer low-fat or nonfat unflavored milks and limit the fat in sweet flavored milks. In addition, at least 80% of the grains served during school breakfasts and lunches each week must be considered rich in whole grains.
For the 2023-2024 school year, the weekly sodium limit for school lunches will be decreased by 10%.
The changes mark a shift from the Trump administration, which eased policies on whole grains, nonfat milk, and sodium, the newspaper reported. Then the pandemic forced additional changes as school districts scrambled to package meals for students. The USDA granted extra flexibility and eased some guidelines to ensure that children could be fed while schools were closed or focused on remote learning.
Now the USDA is updating the nutrition standards to “give schools clear expectations for gradual transition from current pandemic operations to more nutritious meals,” Stacy Dean, the USDA’s deputy undersecretary for food, nutrition, and consumer services, told reporters.
The Biden administration’s changes represent a shift back to Obama-era nutrition standards from 2012, according to the Post. But some nutrition advocates have said the new changes don’t address enough issues, such as added sugars. Fruit and vegetable requirements, for instance, will remain the same as the 2012 standards.
That said, some advocates have said the transition could be tough as schools move out of pandemic-era protocols. The School Nutrition Association, which represents school food service manufacturers and professionals, has urged Congress to provide additional support and waiver extensions for the next school year.
“School nutrition professionals are frantic just trying to get enough food on the tray for our students amid relentless supply chain disruptions and labor shortages,” Beth Wallace, the association’s president, told the Washington Post.
The shift will likely require a balancing act and slow transition. The USDA has been consulting with stakeholders for months to determine how to move toward stricter school nutrition standards while also acknowledging the pandemic, supply chain disruptions, and labor shortages.
“This approach is really going to help move forward the nutrition of the meals and allows the schools to continue to function effectively,” Geri Henchy, director of nutrition policy at the Food Research and Action Center, told the Post.
“Schools can’t make big changes at this point because of the supply chain and staffing,” she said. “They have a lot of waivers at this point that are helping them, and this balances the needs of all the different sectors.”
The USDA plans to issue a proposed rule in fall 2022 to update nutrition standards for the future, the department said in its announcement, which would be finalized for the 2024-2025 school year.
A version of this article first appeared on WebMD.com.
The U.S. Department of Agriculture has announced new changes to school nutrition standards for the next 2 school years, which will reinstate health goals that were rolled back during the Trump administration.
The Biden administration is also tightening rules for fat and salt content in foods after restrictions were eased during the pandemic, according to the Washington Post.
“Nutritious school meals give America’s children the foundation for successful, healthy lives,” Tom Vilsack, the U.S. agriculture secretary, said in a statement on Feb. 4.
“We applaud schools’ heroic efforts throughout the challenges of this pandemic to continue serving kids the most nutritious meals possible,” he said. “The standards we’re putting in place of the next 2 school years will help schools transition to a future that builds on the tremendous strides they’ve made improving school meal nutrition over the past decade.”
For the 2022-2023 school year, schools and childcare providers will be required to offer low-fat or nonfat unflavored milks and limit the fat in sweet flavored milks. In addition, at least 80% of the grains served during school breakfasts and lunches each week must be considered rich in whole grains.
For the 2023-2024 school year, the weekly sodium limit for school lunches will be decreased by 10%.
The changes mark a shift from the Trump administration, which eased policies on whole grains, nonfat milk, and sodium, the newspaper reported. Then the pandemic forced additional changes as school districts scrambled to package meals for students. The USDA granted extra flexibility and eased some guidelines to ensure that children could be fed while schools were closed or focused on remote learning.
Now the USDA is updating the nutrition standards to “give schools clear expectations for gradual transition from current pandemic operations to more nutritious meals,” Stacy Dean, the USDA’s deputy undersecretary for food, nutrition, and consumer services, told reporters.
The Biden administration’s changes represent a shift back to Obama-era nutrition standards from 2012, according to the Post. But some nutrition advocates have said the new changes don’t address enough issues, such as added sugars. Fruit and vegetable requirements, for instance, will remain the same as the 2012 standards.
That said, some advocates have said the transition could be tough as schools move out of pandemic-era protocols. The School Nutrition Association, which represents school food service manufacturers and professionals, has urged Congress to provide additional support and waiver extensions for the next school year.
“School nutrition professionals are frantic just trying to get enough food on the tray for our students amid relentless supply chain disruptions and labor shortages,” Beth Wallace, the association’s president, told the Washington Post.
The shift will likely require a balancing act and slow transition. The USDA has been consulting with stakeholders for months to determine how to move toward stricter school nutrition standards while also acknowledging the pandemic, supply chain disruptions, and labor shortages.
“This approach is really going to help move forward the nutrition of the meals and allows the schools to continue to function effectively,” Geri Henchy, director of nutrition policy at the Food Research and Action Center, told the Post.
“Schools can’t make big changes at this point because of the supply chain and staffing,” she said. “They have a lot of waivers at this point that are helping them, and this balances the needs of all the different sectors.”
The USDA plans to issue a proposed rule in fall 2022 to update nutrition standards for the future, the department said in its announcement, which would be finalized for the 2024-2025 school year.
A version of this article first appeared on WebMD.com.