User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Medical boards pressured to let it slide when doctors spread COVID misinformation
Tennessee’s Board of Medical Examiners unanimously adopted in September 2021 a statement that said doctors spreading COVID misinformation – such as suggesting that vaccines contain microchips – could jeopardize their license to practice.
“I’m very glad that we’re taking this step,” Dr. Stephen Loyd, MD, the panel’s vice president, said at the time. “If you’re spreading this willful misinformation, for me it’s going to be really hard to do anything other than put you on probation or take your license for a year. There has to be a message sent for this. It’s not okay.”
The board’s statement was posted on a government website.
The growing tension in Tennessee between conservative lawmakers and the state’s medical board may be the most prominent example in the country. But the Federation of State Medical Boards, which created the language adopted by at least 15 state boards, is tracking legislation introduced by Republicans in at least 14 states that would restrict a medical board’s authority to discipline doctors for their advice on COVID.
Humayun Chaudhry, DO, the federation’s CEO, called it “an unwelcome trend.” The nonprofit association, based in Euless, Tex., said the statement is merely a COVID-specific restatement of an existing rule: that doctors who engage in behavior that puts patients at risk could face disciplinary action.
Although doctors have leeway to decide which treatments to provide, the medical boards that oversee them have broad authority over licensing. Often, doctors are investigated for violating guidelines on prescribing high-powered drugs. But physicians are sometimes punished for other “unprofessional conduct.” In 2013, Tennessee’s board fined U.S. Rep. Scott DesJarlais for separately having sexual relations with two female patients more than a decade earlier.
Still, stopping doctors from sharing unsound medical advice has proved challenging. Even defining misinformation has been difficult. And during the pandemic, resistance from some state legislatures is complicating the effort.
A relatively small group of physicians peddle COVID misinformation, but many of them associate with America’s Frontline Doctors. Its founder, Simone Gold, MD, has claimed patients are dying from COVID treatments, not the virus itself. Sherri Tenpenny, DO, said in a legislative hearing in Ohio that the COVID vaccine could magnetize patients. Stella Immanuel, MD, has pushed hydroxychloroquine as a COVID cure in Texas, although clinical trials showed that it had no benefit. None of them agreed to requests for comment.
The Texas Medical Board fined Dr. Immanuel $500 for not informing a patient of the risks associated with using hydroxychloroquine as an off-label COVID treatment.
In Tennessee, state lawmakers called a special legislative session in October to address COVID restrictions, and Republican Gov. Bill Lee signed a sweeping package of bills that push back against pandemic rules. One included language directed at the medical board’s recent COVID policy statement, making it more difficult for the panel to investigate complaints about physicians’ advice on COVID vaccines or treatments.
In November, Republican state Rep. John Ragan sent the medical board a letter demanding that the statement be deleted from the state’s website. Rep. Ragan leads a legislative panel that had raised the prospect of defunding the state’s health department over its promotion of COVID vaccines to teens.
Among his demands, Rep. Ragan listed 20 questions he wanted the medical board to answer in writing, including why the misinformation “policy” was proposed nearly two years into the pandemic, which scholars would determine what constitutes misinformation, and how was the “policy” not an infringement on the doctor-patient relationship.
“If you fail to act promptly, your organization will be required to appear before the Joint Government Operations Committee to explain your inaction,” Rep. Ragan wrote in the letter, obtained by Kaiser Health News and Nashville Public Radio.
In response to a request for comment, Rep. Ragan said that “any executive agency, including Board of Medical Examiners, that refuses to follow the law is subject to dissolution.”
He set a deadline of Dec. 7.
In Florida, a Republican-sponsored bill making its way through the state legislature proposes to ban medical boards from revoking or threatening to revoke doctors’ licenses for what they say unless “direct physical harm” of a patient occurred. If the publicized complaint can’t be proved, the board could owe a doctor up to $1.5 million in damages.
Although Florida’s medical board has not adopted the Federation of State Medical Boards’ COVID misinformation statement, the panel has considered misinformation complaints against physicians, including the state’s surgeon general, Joseph Ladapo, MD, PhD.
Dr. Chaudhry said he’s surprised just how many COVID-related complaints are being filed across the country. Often, boards do not publicize investigations before a violation of ethics or standards is confirmed. But in response to a survey by the federation in late 2021, two-thirds of state boards reported an increase in misinformation complaints. And the federation said 12 boards had taken action against a licensed physician.
“At the end of the day, if a physician who is licensed engages in activity that causes harm, the state medical boards are the ones that historically have been set up to look into the situation and make a judgment about what happened or didn’t happen,” Dr. Chaudhry said. “And if you start to chip away at that, it becomes a slippery slope.”
The Georgia Composite Medical Board adopted a version of the federation’s misinformation guidance in early November and has been receiving 10-20 complaints each month, said Debi Dalton, MD, the chairperson. Two months in, no one had been sanctioned.
Dr. Dalton said that even putting out a misinformation policy leaves some “gray” area. Generally, physicians are expected to follow the “consensus,” rather than “the newest information that pops up on social media,” she said.
“We expect physicians to think ethically, professionally, and with the safety of patients in mind,” Dr. Dalton said.
A few physician groups are resisting attempts to root out misinformation, including the Association of American Physicians and Surgeons, known for its stands against government regulation.
Some medical boards have opted against taking a public stand against misinformation.
The Alabama Board of Medical Examiners discussed signing on to the federation’s statement, according to the minutes from an October meeting. But after debating the potential legal ramifications in a private executive session, the board opted not to act.
In Tennessee, the Board of Medical Examiners met on the day Rep. Ragan had set as the deadline and voted to remove the misinformation statement from its website to avoid being called into a legislative hearing. But then, in late January, the board decided to stick with the policy – although it did not republish the statement online immediately – and more specifically defined misinformation, calling it “content that is false, inaccurate or misleading, even if spread unintentionally.”
Board members acknowledged they would likely get more pushback from lawmakers but said they wanted to protect their profession from interference.
“Doctors who are putting forth good evidence-based medicine deserve the protection of this board so they can actually say: ‘Hey, I’m in line with this guideline, and this is a source of truth,’” said Melanie Blake, MD, the board’s president. “We should be a source of truth.”
The medical board was looking into nearly 30 open complaints related to COVID when its misinformation statement came down from its website. As of early February, no Tennessee physician had faced disciplinary action.
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation. This story is part of a partnership that includes Nashville Public Radio, NPR, and KHN.
Tennessee’s Board of Medical Examiners unanimously adopted in September 2021 a statement that said doctors spreading COVID misinformation – such as suggesting that vaccines contain microchips – could jeopardize their license to practice.
“I’m very glad that we’re taking this step,” Dr. Stephen Loyd, MD, the panel’s vice president, said at the time. “If you’re spreading this willful misinformation, for me it’s going to be really hard to do anything other than put you on probation or take your license for a year. There has to be a message sent for this. It’s not okay.”
The board’s statement was posted on a government website.
The growing tension in Tennessee between conservative lawmakers and the state’s medical board may be the most prominent example in the country. But the Federation of State Medical Boards, which created the language adopted by at least 15 state boards, is tracking legislation introduced by Republicans in at least 14 states that would restrict a medical board’s authority to discipline doctors for their advice on COVID.
Humayun Chaudhry, DO, the federation’s CEO, called it “an unwelcome trend.” The nonprofit association, based in Euless, Tex., said the statement is merely a COVID-specific restatement of an existing rule: that doctors who engage in behavior that puts patients at risk could face disciplinary action.
Although doctors have leeway to decide which treatments to provide, the medical boards that oversee them have broad authority over licensing. Often, doctors are investigated for violating guidelines on prescribing high-powered drugs. But physicians are sometimes punished for other “unprofessional conduct.” In 2013, Tennessee’s board fined U.S. Rep. Scott DesJarlais for separately having sexual relations with two female patients more than a decade earlier.
Still, stopping doctors from sharing unsound medical advice has proved challenging. Even defining misinformation has been difficult. And during the pandemic, resistance from some state legislatures is complicating the effort.
A relatively small group of physicians peddle COVID misinformation, but many of them associate with America’s Frontline Doctors. Its founder, Simone Gold, MD, has claimed patients are dying from COVID treatments, not the virus itself. Sherri Tenpenny, DO, said in a legislative hearing in Ohio that the COVID vaccine could magnetize patients. Stella Immanuel, MD, has pushed hydroxychloroquine as a COVID cure in Texas, although clinical trials showed that it had no benefit. None of them agreed to requests for comment.
The Texas Medical Board fined Dr. Immanuel $500 for not informing a patient of the risks associated with using hydroxychloroquine as an off-label COVID treatment.
In Tennessee, state lawmakers called a special legislative session in October to address COVID restrictions, and Republican Gov. Bill Lee signed a sweeping package of bills that push back against pandemic rules. One included language directed at the medical board’s recent COVID policy statement, making it more difficult for the panel to investigate complaints about physicians’ advice on COVID vaccines or treatments.
In November, Republican state Rep. John Ragan sent the medical board a letter demanding that the statement be deleted from the state’s website. Rep. Ragan leads a legislative panel that had raised the prospect of defunding the state’s health department over its promotion of COVID vaccines to teens.
Among his demands, Rep. Ragan listed 20 questions he wanted the medical board to answer in writing, including why the misinformation “policy” was proposed nearly two years into the pandemic, which scholars would determine what constitutes misinformation, and how was the “policy” not an infringement on the doctor-patient relationship.
“If you fail to act promptly, your organization will be required to appear before the Joint Government Operations Committee to explain your inaction,” Rep. Ragan wrote in the letter, obtained by Kaiser Health News and Nashville Public Radio.
In response to a request for comment, Rep. Ragan said that “any executive agency, including Board of Medical Examiners, that refuses to follow the law is subject to dissolution.”
He set a deadline of Dec. 7.
In Florida, a Republican-sponsored bill making its way through the state legislature proposes to ban medical boards from revoking or threatening to revoke doctors’ licenses for what they say unless “direct physical harm” of a patient occurred. If the publicized complaint can’t be proved, the board could owe a doctor up to $1.5 million in damages.
Although Florida’s medical board has not adopted the Federation of State Medical Boards’ COVID misinformation statement, the panel has considered misinformation complaints against physicians, including the state’s surgeon general, Joseph Ladapo, MD, PhD.
Dr. Chaudhry said he’s surprised just how many COVID-related complaints are being filed across the country. Often, boards do not publicize investigations before a violation of ethics or standards is confirmed. But in response to a survey by the federation in late 2021, two-thirds of state boards reported an increase in misinformation complaints. And the federation said 12 boards had taken action against a licensed physician.
“At the end of the day, if a physician who is licensed engages in activity that causes harm, the state medical boards are the ones that historically have been set up to look into the situation and make a judgment about what happened or didn’t happen,” Dr. Chaudhry said. “And if you start to chip away at that, it becomes a slippery slope.”
The Georgia Composite Medical Board adopted a version of the federation’s misinformation guidance in early November and has been receiving 10-20 complaints each month, said Debi Dalton, MD, the chairperson. Two months in, no one had been sanctioned.
Dr. Dalton said that even putting out a misinformation policy leaves some “gray” area. Generally, physicians are expected to follow the “consensus,” rather than “the newest information that pops up on social media,” she said.
“We expect physicians to think ethically, professionally, and with the safety of patients in mind,” Dr. Dalton said.
A few physician groups are resisting attempts to root out misinformation, including the Association of American Physicians and Surgeons, known for its stands against government regulation.
Some medical boards have opted against taking a public stand against misinformation.
The Alabama Board of Medical Examiners discussed signing on to the federation’s statement, according to the minutes from an October meeting. But after debating the potential legal ramifications in a private executive session, the board opted not to act.
In Tennessee, the Board of Medical Examiners met on the day Rep. Ragan had set as the deadline and voted to remove the misinformation statement from its website to avoid being called into a legislative hearing. But then, in late January, the board decided to stick with the policy – although it did not republish the statement online immediately – and more specifically defined misinformation, calling it “content that is false, inaccurate or misleading, even if spread unintentionally.”
Board members acknowledged they would likely get more pushback from lawmakers but said they wanted to protect their profession from interference.
“Doctors who are putting forth good evidence-based medicine deserve the protection of this board so they can actually say: ‘Hey, I’m in line with this guideline, and this is a source of truth,’” said Melanie Blake, MD, the board’s president. “We should be a source of truth.”
The medical board was looking into nearly 30 open complaints related to COVID when its misinformation statement came down from its website. As of early February, no Tennessee physician had faced disciplinary action.
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation. This story is part of a partnership that includes Nashville Public Radio, NPR, and KHN.
Tennessee’s Board of Medical Examiners unanimously adopted in September 2021 a statement that said doctors spreading COVID misinformation – such as suggesting that vaccines contain microchips – could jeopardize their license to practice.
“I’m very glad that we’re taking this step,” Dr. Stephen Loyd, MD, the panel’s vice president, said at the time. “If you’re spreading this willful misinformation, for me it’s going to be really hard to do anything other than put you on probation or take your license for a year. There has to be a message sent for this. It’s not okay.”
The board’s statement was posted on a government website.
The growing tension in Tennessee between conservative lawmakers and the state’s medical board may be the most prominent example in the country. But the Federation of State Medical Boards, which created the language adopted by at least 15 state boards, is tracking legislation introduced by Republicans in at least 14 states that would restrict a medical board’s authority to discipline doctors for their advice on COVID.
Humayun Chaudhry, DO, the federation’s CEO, called it “an unwelcome trend.” The nonprofit association, based in Euless, Tex., said the statement is merely a COVID-specific restatement of an existing rule: that doctors who engage in behavior that puts patients at risk could face disciplinary action.
Although doctors have leeway to decide which treatments to provide, the medical boards that oversee them have broad authority over licensing. Often, doctors are investigated for violating guidelines on prescribing high-powered drugs. But physicians are sometimes punished for other “unprofessional conduct.” In 2013, Tennessee’s board fined U.S. Rep. Scott DesJarlais for separately having sexual relations with two female patients more than a decade earlier.
Still, stopping doctors from sharing unsound medical advice has proved challenging. Even defining misinformation has been difficult. And during the pandemic, resistance from some state legislatures is complicating the effort.
A relatively small group of physicians peddle COVID misinformation, but many of them associate with America’s Frontline Doctors. Its founder, Simone Gold, MD, has claimed patients are dying from COVID treatments, not the virus itself. Sherri Tenpenny, DO, said in a legislative hearing in Ohio that the COVID vaccine could magnetize patients. Stella Immanuel, MD, has pushed hydroxychloroquine as a COVID cure in Texas, although clinical trials showed that it had no benefit. None of them agreed to requests for comment.
The Texas Medical Board fined Dr. Immanuel $500 for not informing a patient of the risks associated with using hydroxychloroquine as an off-label COVID treatment.
In Tennessee, state lawmakers called a special legislative session in October to address COVID restrictions, and Republican Gov. Bill Lee signed a sweeping package of bills that push back against pandemic rules. One included language directed at the medical board’s recent COVID policy statement, making it more difficult for the panel to investigate complaints about physicians’ advice on COVID vaccines or treatments.
In November, Republican state Rep. John Ragan sent the medical board a letter demanding that the statement be deleted from the state’s website. Rep. Ragan leads a legislative panel that had raised the prospect of defunding the state’s health department over its promotion of COVID vaccines to teens.
Among his demands, Rep. Ragan listed 20 questions he wanted the medical board to answer in writing, including why the misinformation “policy” was proposed nearly two years into the pandemic, which scholars would determine what constitutes misinformation, and how was the “policy” not an infringement on the doctor-patient relationship.
“If you fail to act promptly, your organization will be required to appear before the Joint Government Operations Committee to explain your inaction,” Rep. Ragan wrote in the letter, obtained by Kaiser Health News and Nashville Public Radio.
In response to a request for comment, Rep. Ragan said that “any executive agency, including Board of Medical Examiners, that refuses to follow the law is subject to dissolution.”
He set a deadline of Dec. 7.
In Florida, a Republican-sponsored bill making its way through the state legislature proposes to ban medical boards from revoking or threatening to revoke doctors’ licenses for what they say unless “direct physical harm” of a patient occurred. If the publicized complaint can’t be proved, the board could owe a doctor up to $1.5 million in damages.
Although Florida’s medical board has not adopted the Federation of State Medical Boards’ COVID misinformation statement, the panel has considered misinformation complaints against physicians, including the state’s surgeon general, Joseph Ladapo, MD, PhD.
Dr. Chaudhry said he’s surprised just how many COVID-related complaints are being filed across the country. Often, boards do not publicize investigations before a violation of ethics or standards is confirmed. But in response to a survey by the federation in late 2021, two-thirds of state boards reported an increase in misinformation complaints. And the federation said 12 boards had taken action against a licensed physician.
“At the end of the day, if a physician who is licensed engages in activity that causes harm, the state medical boards are the ones that historically have been set up to look into the situation and make a judgment about what happened or didn’t happen,” Dr. Chaudhry said. “And if you start to chip away at that, it becomes a slippery slope.”
The Georgia Composite Medical Board adopted a version of the federation’s misinformation guidance in early November and has been receiving 10-20 complaints each month, said Debi Dalton, MD, the chairperson. Two months in, no one had been sanctioned.
Dr. Dalton said that even putting out a misinformation policy leaves some “gray” area. Generally, physicians are expected to follow the “consensus,” rather than “the newest information that pops up on social media,” she said.
“We expect physicians to think ethically, professionally, and with the safety of patients in mind,” Dr. Dalton said.
A few physician groups are resisting attempts to root out misinformation, including the Association of American Physicians and Surgeons, known for its stands against government regulation.
Some medical boards have opted against taking a public stand against misinformation.
The Alabama Board of Medical Examiners discussed signing on to the federation’s statement, according to the minutes from an October meeting. But after debating the potential legal ramifications in a private executive session, the board opted not to act.
In Tennessee, the Board of Medical Examiners met on the day Rep. Ragan had set as the deadline and voted to remove the misinformation statement from its website to avoid being called into a legislative hearing. But then, in late January, the board decided to stick with the policy – although it did not republish the statement online immediately – and more specifically defined misinformation, calling it “content that is false, inaccurate or misleading, even if spread unintentionally.”
Board members acknowledged they would likely get more pushback from lawmakers but said they wanted to protect their profession from interference.
“Doctors who are putting forth good evidence-based medicine deserve the protection of this board so they can actually say: ‘Hey, I’m in line with this guideline, and this is a source of truth,’” said Melanie Blake, MD, the board’s president. “We should be a source of truth.”
The medical board was looking into nearly 30 open complaints related to COVID when its misinformation statement came down from its website. As of early February, no Tennessee physician had faced disciplinary action.
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation. This story is part of a partnership that includes Nashville Public Radio, NPR, and KHN.
16 toddlers with HIV at birth had no detectable virus 2 years later
Hours after their births, 34 infants began a three-drug combination HIV treatment. Now, 2 years later, a third of those toddlers have tested negative for HIV antibodies and have no detectable HIV DNA in their blood. The children aren’t cured of HIV, but as many as 16 of them may be candidates to stop treatment and see if they are in fact in HIV remission.
If one or more are,
At the Conference on Retroviruses and Opportunistic Infections, Deborah Persaud, MD, interim director of pediatric infectious diseases and professor of pediatrics at Johns Hopkins University School of Medicine, Baltimore, Md., told this news organization that the evidence suggests that more U.S. clinicians should start infants at high risk for HIV on presumptive treatment – not only to potentially prevent transmission but also to set the child up for the lowest possible viral reservoir, the first step to HIV remission.
The three-drug preemptive treatment is “not uniformly practiced,” Dr. Persaud said in an interview. “We’re at a point now where we don’t have to wait to see if we have remission” to act on these findings, she said. “The question is, should this now become standard of care for in-utero infected infants?”
Every year, about 150 infants are born with HIV in the United States, according to the Elizabeth Glaser Pediatric AIDS Foundation. Current U.S. perinatal treatment guidelines already suggest either treatment with one or more HIV drugs at birth to attempt preventing transmission or initiating three-drug regimens for infants at high risk for perinatally acquired HIV. In this case “high risk” is defined as infants born to:
- people who haven’t received any HIV treatment before delivery or during delivery,
- people who did receive treatment but failed to achieve undetectable viral loads, or
- people who acquire HIV during pregnancy, or who otherwise weren’t diagnosed until after birth.
Trying to replicate the Mississippi baby
The Mississippi baby did eventually relapse. But ever since Dr. Persaud reported the case of that 2-year-old who went into treatment-free remission in 2013, she has been trying to figure out how to duplicate that initial success. There were several factors in that remission, but one piece researchers could control was starting treatment very early – before HIV blood tests even come back positive. So, in this trial, researchers enrolled 440 infants in Africa and Asia at high risk for in utero HIV transmission.
All 440 of those infants received their first doses of the three-drug preemptive treatment within 24 hours of birth. Of those 440 infants, 34 tested positive for HIV and remained in the trial.*
Meanwhile, in North America, South America, and African countries, another 20 infants enrolled in the trial – not as part of the protocol but because their clinicians had been influenced by the news of the Mississippi baby, Dr. Persaud said, and decided on their own to start high-risk infants on three-drug regimens preemptively.
“We wanted to take advantage of those real-world situations of infants being treated outside the clinical trials,” Dr. Persaud said.
Now there were 54 infants trying this very early treatment. In Cohort One, they started their first drug cocktail 7 hours after delivery. In Cohort Two, their first antiretroviral combination treatment was at 32.8 hours of life, and they enrolled in the trial at 8 days. Then researchers followed the infants closely, adding on lopinavir and ritonavir when age-appropriate.
Meeting milestones
To continue in the trial and be considered for treatment interruption, infants had to meet certain milestones. At 24 weeks, HIV RNA needed to be below 200 copies per milliliter. Then their HIV RNA needed to stay below 200 copies consistently until week 48. At week 48, they had to have an HIV RNA that was even lower – below 20 or 40 copies – with “target not detected” in the test in HIV RNA. That’s a sign that there weren’t even any trace levels of viral nucleic acid RNA in the blood to indicate HIV. Then, from week 48 on, they had to maintain that level of viral suppression until age 2.
At that point, not only did they need to maintain that level of viral suppression, they also needed to have a negative HIV antibody test and a PCR test for total HIV DNA, which had to be undetectable down to the limit of 4 copies per 106 – that is, there were fewer than 4 copies of the virus out of 1 million cells tested. Only then would they be considered for treatment interruption.
“After week 28 there was no leeway,” Dr. Persaud said. Then “they had to have nothing detectable from the first year of age. We thought the best shot at remission were cases that achieved very good and strict virologic control.”
Criteria for consideration
Of the 34 infants in Cohort One, 24 infants made it past the first hurdle at 24 weeks and 6 had PCR tests that found no cell-associated HIV DNA. In Cohort Two, 15 made it past the week-24 hurdle and 4 had no detectable HIV DNA via PCR test.
Now, more than 2 years out from study initiation, Dr. Persaud and colleagues are evaluating each child to see if any still meet the requirements for treatment interruption. The COVID-19 pandemic has delayed their evaluations, and it’s possible that fewer children now meet the requirements. But Dr. Persaud said there are still candidates left. An analysis suggests that up to 30% of the children, or 16, were candidates at 2 years.
“We have kids who are eligible for [antiretroviral therapy] cessation years out from this, which I think is really important,” she said in an interview. “It’s not game over.”
And although 30% is not an overwhelming victory, Dr. Persaud said the team’s goal was “to identify an N of 1 to replicate the Mississippi baby.” The study team, led by Ellen Chadwick, MD, of Northwestern University’s Feinberg School of Medicine, Chicago, and a member of the board that creates HIV perinatal treatment guidelines, is starting a new trial, using more modern, integrase inhibitor-based, three-drug regimens for infants and pairing them with broadly neutralizing antibodies. The combination used in this trial included zidovudine, or AZT.
If one of the children is able to go off treatment, it would be the first step toward creating a functional cure for HIV, starting with the youngest people affected by the virus.
“This trial convinces me that very early treatment was the key strategy that led to remission in the Mississippi baby,” Dr. Persaud said in an interview. “We’re confirming here that the first step toward remission and cure is reducing reservoirs. We’ve got that here. Whether we need more on top of that – therapeutic vaccines, immunotherapies, or a better regimen to start out with – needs to be determined.”
The presentation was met with excitement and questions. For instance, if very early treatment works, why does it work for just 30% of the children?
Were some of the children able to control HIV on their own because they were rare post-treatment controllers? And was 30% really a victory? Others were convinced of it.
“Amazing outcome to have 30% so well suppressed after 2 years with CA-DNA not detected,” commented Hermione Lyall, MBChB, a pediatric infectious disease doctor at Imperial College Healthcare NHS Trust in the United Kingdom, in the virtual chat.
As for whether the study should change practice, Elaine Abrams, MD, professor of epidemiology and pediatrics at Columbia University Medical Center, New York, and CROI cochair, said that this study proves that the three-drug regimen is at the very least safe to start immediately.
Whether it should become standard of care everywhere is still up for discussion, she told this news organization.
“It very much depends on what you’re trying to achieve,” she said. “Postnatal prophylaxis is provided to reduce the risk of acquiring infection. That’s a different objective than early treatment. If you have 1,000 high-risk babies, how many are likely to turn out to have HIV infection? And how many of those will you be treating with three drugs and actually making this impact by doing so? And how many babies are going to be getting possibly extra treatment that they don’t need?”
Regardless, what’s clear is that treatment is essential – for mother and infant, said Carl Dieffenbach, PhD, director of the division of AIDS at the National Institute of Allergy and Infectious Diseases. It needs to start, he said, by making sure all mothers know their HIV status and have access early in pregnancy to the treatment that can prevent transmission.
“So much of what’s wrong in the world is about implementation of health care,” he said in an interview. Still, “if you could demonstrate that early treatment to the mother plus early treatment to the babies [is efficacious], we could really talk about an HIV-free generation of kids.”
The study was funded by the National Institutes of Health. Dr. Persaud, Dr. Dieffenbach, Dr. Abrams, and Dr. Lyall all report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Correction, 2/16/22: An earlier version of this article misstated the number that tested positive for HIV and remained in the trial.
This article was updated 2/16/22.
Hours after their births, 34 infants began a three-drug combination HIV treatment. Now, 2 years later, a third of those toddlers have tested negative for HIV antibodies and have no detectable HIV DNA in their blood. The children aren’t cured of HIV, but as many as 16 of them may be candidates to stop treatment and see if they are in fact in HIV remission.
If one or more are,
At the Conference on Retroviruses and Opportunistic Infections, Deborah Persaud, MD, interim director of pediatric infectious diseases and professor of pediatrics at Johns Hopkins University School of Medicine, Baltimore, Md., told this news organization that the evidence suggests that more U.S. clinicians should start infants at high risk for HIV on presumptive treatment – not only to potentially prevent transmission but also to set the child up for the lowest possible viral reservoir, the first step to HIV remission.
The three-drug preemptive treatment is “not uniformly practiced,” Dr. Persaud said in an interview. “We’re at a point now where we don’t have to wait to see if we have remission” to act on these findings, she said. “The question is, should this now become standard of care for in-utero infected infants?”
Every year, about 150 infants are born with HIV in the United States, according to the Elizabeth Glaser Pediatric AIDS Foundation. Current U.S. perinatal treatment guidelines already suggest either treatment with one or more HIV drugs at birth to attempt preventing transmission or initiating three-drug regimens for infants at high risk for perinatally acquired HIV. In this case “high risk” is defined as infants born to:
- people who haven’t received any HIV treatment before delivery or during delivery,
- people who did receive treatment but failed to achieve undetectable viral loads, or
- people who acquire HIV during pregnancy, or who otherwise weren’t diagnosed until after birth.
Trying to replicate the Mississippi baby
The Mississippi baby did eventually relapse. But ever since Dr. Persaud reported the case of that 2-year-old who went into treatment-free remission in 2013, she has been trying to figure out how to duplicate that initial success. There were several factors in that remission, but one piece researchers could control was starting treatment very early – before HIV blood tests even come back positive. So, in this trial, researchers enrolled 440 infants in Africa and Asia at high risk for in utero HIV transmission.
All 440 of those infants received their first doses of the three-drug preemptive treatment within 24 hours of birth. Of those 440 infants, 34 tested positive for HIV and remained in the trial.*
Meanwhile, in North America, South America, and African countries, another 20 infants enrolled in the trial – not as part of the protocol but because their clinicians had been influenced by the news of the Mississippi baby, Dr. Persaud said, and decided on their own to start high-risk infants on three-drug regimens preemptively.
“We wanted to take advantage of those real-world situations of infants being treated outside the clinical trials,” Dr. Persaud said.
Now there were 54 infants trying this very early treatment. In Cohort One, they started their first drug cocktail 7 hours after delivery. In Cohort Two, their first antiretroviral combination treatment was at 32.8 hours of life, and they enrolled in the trial at 8 days. Then researchers followed the infants closely, adding on lopinavir and ritonavir when age-appropriate.
Meeting milestones
To continue in the trial and be considered for treatment interruption, infants had to meet certain milestones. At 24 weeks, HIV RNA needed to be below 200 copies per milliliter. Then their HIV RNA needed to stay below 200 copies consistently until week 48. At week 48, they had to have an HIV RNA that was even lower – below 20 or 40 copies – with “target not detected” in the test in HIV RNA. That’s a sign that there weren’t even any trace levels of viral nucleic acid RNA in the blood to indicate HIV. Then, from week 48 on, they had to maintain that level of viral suppression until age 2.
At that point, not only did they need to maintain that level of viral suppression, they also needed to have a negative HIV antibody test and a PCR test for total HIV DNA, which had to be undetectable down to the limit of 4 copies per 106 – that is, there were fewer than 4 copies of the virus out of 1 million cells tested. Only then would they be considered for treatment interruption.
“After week 28 there was no leeway,” Dr. Persaud said. Then “they had to have nothing detectable from the first year of age. We thought the best shot at remission were cases that achieved very good and strict virologic control.”
Criteria for consideration
Of the 34 infants in Cohort One, 24 infants made it past the first hurdle at 24 weeks and 6 had PCR tests that found no cell-associated HIV DNA. In Cohort Two, 15 made it past the week-24 hurdle and 4 had no detectable HIV DNA via PCR test.
Now, more than 2 years out from study initiation, Dr. Persaud and colleagues are evaluating each child to see if any still meet the requirements for treatment interruption. The COVID-19 pandemic has delayed their evaluations, and it’s possible that fewer children now meet the requirements. But Dr. Persaud said there are still candidates left. An analysis suggests that up to 30% of the children, or 16, were candidates at 2 years.
“We have kids who are eligible for [antiretroviral therapy] cessation years out from this, which I think is really important,” she said in an interview. “It’s not game over.”
And although 30% is not an overwhelming victory, Dr. Persaud said the team’s goal was “to identify an N of 1 to replicate the Mississippi baby.” The study team, led by Ellen Chadwick, MD, of Northwestern University’s Feinberg School of Medicine, Chicago, and a member of the board that creates HIV perinatal treatment guidelines, is starting a new trial, using more modern, integrase inhibitor-based, three-drug regimens for infants and pairing them with broadly neutralizing antibodies. The combination used in this trial included zidovudine, or AZT.
If one of the children is able to go off treatment, it would be the first step toward creating a functional cure for HIV, starting with the youngest people affected by the virus.
“This trial convinces me that very early treatment was the key strategy that led to remission in the Mississippi baby,” Dr. Persaud said in an interview. “We’re confirming here that the first step toward remission and cure is reducing reservoirs. We’ve got that here. Whether we need more on top of that – therapeutic vaccines, immunotherapies, or a better regimen to start out with – needs to be determined.”
The presentation was met with excitement and questions. For instance, if very early treatment works, why does it work for just 30% of the children?
Were some of the children able to control HIV on their own because they were rare post-treatment controllers? And was 30% really a victory? Others were convinced of it.
“Amazing outcome to have 30% so well suppressed after 2 years with CA-DNA not detected,” commented Hermione Lyall, MBChB, a pediatric infectious disease doctor at Imperial College Healthcare NHS Trust in the United Kingdom, in the virtual chat.
As for whether the study should change practice, Elaine Abrams, MD, professor of epidemiology and pediatrics at Columbia University Medical Center, New York, and CROI cochair, said that this study proves that the three-drug regimen is at the very least safe to start immediately.
Whether it should become standard of care everywhere is still up for discussion, she told this news organization.
“It very much depends on what you’re trying to achieve,” she said. “Postnatal prophylaxis is provided to reduce the risk of acquiring infection. That’s a different objective than early treatment. If you have 1,000 high-risk babies, how many are likely to turn out to have HIV infection? And how many of those will you be treating with three drugs and actually making this impact by doing so? And how many babies are going to be getting possibly extra treatment that they don’t need?”
Regardless, what’s clear is that treatment is essential – for mother and infant, said Carl Dieffenbach, PhD, director of the division of AIDS at the National Institute of Allergy and Infectious Diseases. It needs to start, he said, by making sure all mothers know their HIV status and have access early in pregnancy to the treatment that can prevent transmission.
“So much of what’s wrong in the world is about implementation of health care,” he said in an interview. Still, “if you could demonstrate that early treatment to the mother plus early treatment to the babies [is efficacious], we could really talk about an HIV-free generation of kids.”
The study was funded by the National Institutes of Health. Dr. Persaud, Dr. Dieffenbach, Dr. Abrams, and Dr. Lyall all report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Correction, 2/16/22: An earlier version of this article misstated the number that tested positive for HIV and remained in the trial.
This article was updated 2/16/22.
Hours after their births, 34 infants began a three-drug combination HIV treatment. Now, 2 years later, a third of those toddlers have tested negative for HIV antibodies and have no detectable HIV DNA in their blood. The children aren’t cured of HIV, but as many as 16 of them may be candidates to stop treatment and see if they are in fact in HIV remission.
If one or more are,
At the Conference on Retroviruses and Opportunistic Infections, Deborah Persaud, MD, interim director of pediatric infectious diseases and professor of pediatrics at Johns Hopkins University School of Medicine, Baltimore, Md., told this news organization that the evidence suggests that more U.S. clinicians should start infants at high risk for HIV on presumptive treatment – not only to potentially prevent transmission but also to set the child up for the lowest possible viral reservoir, the first step to HIV remission.
The three-drug preemptive treatment is “not uniformly practiced,” Dr. Persaud said in an interview. “We’re at a point now where we don’t have to wait to see if we have remission” to act on these findings, she said. “The question is, should this now become standard of care for in-utero infected infants?”
Every year, about 150 infants are born with HIV in the United States, according to the Elizabeth Glaser Pediatric AIDS Foundation. Current U.S. perinatal treatment guidelines already suggest either treatment with one or more HIV drugs at birth to attempt preventing transmission or initiating three-drug regimens for infants at high risk for perinatally acquired HIV. In this case “high risk” is defined as infants born to:
- people who haven’t received any HIV treatment before delivery or during delivery,
- people who did receive treatment but failed to achieve undetectable viral loads, or
- people who acquire HIV during pregnancy, or who otherwise weren’t diagnosed until after birth.
Trying to replicate the Mississippi baby
The Mississippi baby did eventually relapse. But ever since Dr. Persaud reported the case of that 2-year-old who went into treatment-free remission in 2013, she has been trying to figure out how to duplicate that initial success. There were several factors in that remission, but one piece researchers could control was starting treatment very early – before HIV blood tests even come back positive. So, in this trial, researchers enrolled 440 infants in Africa and Asia at high risk for in utero HIV transmission.
All 440 of those infants received their first doses of the three-drug preemptive treatment within 24 hours of birth. Of those 440 infants, 34 tested positive for HIV and remained in the trial.*
Meanwhile, in North America, South America, and African countries, another 20 infants enrolled in the trial – not as part of the protocol but because their clinicians had been influenced by the news of the Mississippi baby, Dr. Persaud said, and decided on their own to start high-risk infants on three-drug regimens preemptively.
“We wanted to take advantage of those real-world situations of infants being treated outside the clinical trials,” Dr. Persaud said.
Now there were 54 infants trying this very early treatment. In Cohort One, they started their first drug cocktail 7 hours after delivery. In Cohort Two, their first antiretroviral combination treatment was at 32.8 hours of life, and they enrolled in the trial at 8 days. Then researchers followed the infants closely, adding on lopinavir and ritonavir when age-appropriate.
Meeting milestones
To continue in the trial and be considered for treatment interruption, infants had to meet certain milestones. At 24 weeks, HIV RNA needed to be below 200 copies per milliliter. Then their HIV RNA needed to stay below 200 copies consistently until week 48. At week 48, they had to have an HIV RNA that was even lower – below 20 or 40 copies – with “target not detected” in the test in HIV RNA. That’s a sign that there weren’t even any trace levels of viral nucleic acid RNA in the blood to indicate HIV. Then, from week 48 on, they had to maintain that level of viral suppression until age 2.
At that point, not only did they need to maintain that level of viral suppression, they also needed to have a negative HIV antibody test and a PCR test for total HIV DNA, which had to be undetectable down to the limit of 4 copies per 106 – that is, there were fewer than 4 copies of the virus out of 1 million cells tested. Only then would they be considered for treatment interruption.
“After week 28 there was no leeway,” Dr. Persaud said. Then “they had to have nothing detectable from the first year of age. We thought the best shot at remission were cases that achieved very good and strict virologic control.”
Criteria for consideration
Of the 34 infants in Cohort One, 24 infants made it past the first hurdle at 24 weeks and 6 had PCR tests that found no cell-associated HIV DNA. In Cohort Two, 15 made it past the week-24 hurdle and 4 had no detectable HIV DNA via PCR test.
Now, more than 2 years out from study initiation, Dr. Persaud and colleagues are evaluating each child to see if any still meet the requirements for treatment interruption. The COVID-19 pandemic has delayed their evaluations, and it’s possible that fewer children now meet the requirements. But Dr. Persaud said there are still candidates left. An analysis suggests that up to 30% of the children, or 16, were candidates at 2 years.
“We have kids who are eligible for [antiretroviral therapy] cessation years out from this, which I think is really important,” she said in an interview. “It’s not game over.”
And although 30% is not an overwhelming victory, Dr. Persaud said the team’s goal was “to identify an N of 1 to replicate the Mississippi baby.” The study team, led by Ellen Chadwick, MD, of Northwestern University’s Feinberg School of Medicine, Chicago, and a member of the board that creates HIV perinatal treatment guidelines, is starting a new trial, using more modern, integrase inhibitor-based, three-drug regimens for infants and pairing them with broadly neutralizing antibodies. The combination used in this trial included zidovudine, or AZT.
If one of the children is able to go off treatment, it would be the first step toward creating a functional cure for HIV, starting with the youngest people affected by the virus.
“This trial convinces me that very early treatment was the key strategy that led to remission in the Mississippi baby,” Dr. Persaud said in an interview. “We’re confirming here that the first step toward remission and cure is reducing reservoirs. We’ve got that here. Whether we need more on top of that – therapeutic vaccines, immunotherapies, or a better regimen to start out with – needs to be determined.”
The presentation was met with excitement and questions. For instance, if very early treatment works, why does it work for just 30% of the children?
Were some of the children able to control HIV on their own because they were rare post-treatment controllers? And was 30% really a victory? Others were convinced of it.
“Amazing outcome to have 30% so well suppressed after 2 years with CA-DNA not detected,” commented Hermione Lyall, MBChB, a pediatric infectious disease doctor at Imperial College Healthcare NHS Trust in the United Kingdom, in the virtual chat.
As for whether the study should change practice, Elaine Abrams, MD, professor of epidemiology and pediatrics at Columbia University Medical Center, New York, and CROI cochair, said that this study proves that the three-drug regimen is at the very least safe to start immediately.
Whether it should become standard of care everywhere is still up for discussion, she told this news organization.
“It very much depends on what you’re trying to achieve,” she said. “Postnatal prophylaxis is provided to reduce the risk of acquiring infection. That’s a different objective than early treatment. If you have 1,000 high-risk babies, how many are likely to turn out to have HIV infection? And how many of those will you be treating with three drugs and actually making this impact by doing so? And how many babies are going to be getting possibly extra treatment that they don’t need?”
Regardless, what’s clear is that treatment is essential – for mother and infant, said Carl Dieffenbach, PhD, director of the division of AIDS at the National Institute of Allergy and Infectious Diseases. It needs to start, he said, by making sure all mothers know their HIV status and have access early in pregnancy to the treatment that can prevent transmission.
“So much of what’s wrong in the world is about implementation of health care,” he said in an interview. Still, “if you could demonstrate that early treatment to the mother plus early treatment to the babies [is efficacious], we could really talk about an HIV-free generation of kids.”
The study was funded by the National Institutes of Health. Dr. Persaud, Dr. Dieffenbach, Dr. Abrams, and Dr. Lyall all report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Correction, 2/16/22: An earlier version of this article misstated the number that tested positive for HIV and remained in the trial.
This article was updated 2/16/22.
FROM CROI 22
New study shows natural immunity to COVID has enduring strength
It’s a matter of quality, not quantity. That’s the gist of a new Israeli study that shows that unvaccinated people with a prior SARS-CoV-2 infection create antibodies that are more effective in the long run compared with others who were vaccinated but never infected.
“While the quantity of antibodies decreases with time in both COVID-19 recovered patients and vaccinated individuals, ” lead author Carmit Cohen, PhD, said in an interview.
This difference could explain why previously infected patients appear to be better protected against a new infection than those who have only been vaccinated, according to a news release attached to the research.
One key caveat: This research does not include people from the later part of the pandemic.
This means there is a catch in terms of timing, William Schaffner, MD, Vanderbilt University School of Medicine, Nashville, Tenn., said when asked to comment on the study: “The study involved only the early COVID strains – it has no information on either the Delta or Omicron variants. Thus, the results primarily are of scientific or historical interest but are not immediately relevant to the current situation.”
The findings come from an early release of a study to be presented at the European Congress of Clinical Microbiology & Infectious Diseases in April.
An unexpected finding of the study showed that obese people had better protection – a higher and more sustained immune response – compared with overweight and normal-weight individuals.
“The results in the obese group were indeed unexpected and need further research to confirm or dispute,” Dr. Schaffner said. “Obesity does predispose to more severe disease.”
A focus on earlier strains
Dr. Cohen – a senior research assistant in infectious disease prevention at the Sheba Medical Center in Ramat Gan, Israel – and her colleagues recruited participants between March 25, 2020 and Nov. 25, 2020 and completed analysis in April 2021. This means they assessed people with a history of infection from the original, the Alpha, and some Beta strains of SARS-CoV-2.
Dr. Cohen indicated that the next phase of their research will examine innate and acquired immune responses to the more recent Delta and Omicron variants.
The investigators analyzed the antibody-induced immune response up to 1 year in 130 COVID-19 recovered but unvaccinated individuals versus up to 8 months among 402 others matched by age and body mass index (BMI) and without previous infection who received two doses of the Pfizer vaccine.
The numbers of antibodies a month after vaccination were higher than those in the COVID-19 recovered patients. However, these numbers also declined more steeply in the vaccinated group, they note.
To assess the antibody performance, the investigators used the avidity index. This assay measures antibody function based on the strength of the interactions between the antibody and the viral antigen.
They found that the avidity index was higher in vaccinated individuals than in recovered patients initially but changes over time. At up to 6 months, the index did not significantly change in vaccinated individuals, whereas it gradually increased in recovered patients. This increase would potentially protect them from reinfection, the authors note.
These findings stand in stark contrast to an Oct. 29, 2021, Centers for Disease Control and Prevention study that found that COVID-19 vaccines provided five times the protection of natural immunity.
Those results, published in the organization’s Morbidity and Mortality Weekly Report, suggest that vaccination helps people mount a higher, stronger, and more consistent level of immunity against COVID-19 hospitalization than infection alone for at least 6 months.
Protection linked to obesity
Another finding that ran against the scientific grain was the data about obesity.
There was a higher and more persistent antibody performance among people with a BMI of 30 kg/m2.
This could relate to greater disease severity and/or a more pronounced initial response to infection among the obese group.
“Our hypothesis is that patients with obesity begin with a more pronounced response – reflected also by the disease manifestation – and the trend of decline is similar, therefore the kinetics of immune response remain higher throughout the study,” Dr. Cohen said.
“The results in the obese group were indeed unexpected and need further research to confirm or dispute,” said Dr. Schaffner, who is also the current medical director of the National Foundation for Infectious Diseases. “Obesity does predispose to more severe disease.”
Before the boosters
Along with using participants from only the earlier part of the pandemic, another limitation of the study was that the vaccinated group had only two doses of vaccine; boosters were not given during the time of the study, Dr. Schaffner said.
“Again, not the current situation.”
“That said, the strength and duration of natural immunity provided by the early variants was solid for up to a year, confirming previous reports,” he said.
A version of this article first appeared on Medscape.com.
It’s a matter of quality, not quantity. That’s the gist of a new Israeli study that shows that unvaccinated people with a prior SARS-CoV-2 infection create antibodies that are more effective in the long run compared with others who were vaccinated but never infected.
“While the quantity of antibodies decreases with time in both COVID-19 recovered patients and vaccinated individuals, ” lead author Carmit Cohen, PhD, said in an interview.
This difference could explain why previously infected patients appear to be better protected against a new infection than those who have only been vaccinated, according to a news release attached to the research.
One key caveat: This research does not include people from the later part of the pandemic.
This means there is a catch in terms of timing, William Schaffner, MD, Vanderbilt University School of Medicine, Nashville, Tenn., said when asked to comment on the study: “The study involved only the early COVID strains – it has no information on either the Delta or Omicron variants. Thus, the results primarily are of scientific or historical interest but are not immediately relevant to the current situation.”
The findings come from an early release of a study to be presented at the European Congress of Clinical Microbiology & Infectious Diseases in April.
An unexpected finding of the study showed that obese people had better protection – a higher and more sustained immune response – compared with overweight and normal-weight individuals.
“The results in the obese group were indeed unexpected and need further research to confirm or dispute,” Dr. Schaffner said. “Obesity does predispose to more severe disease.”
A focus on earlier strains
Dr. Cohen – a senior research assistant in infectious disease prevention at the Sheba Medical Center in Ramat Gan, Israel – and her colleagues recruited participants between March 25, 2020 and Nov. 25, 2020 and completed analysis in April 2021. This means they assessed people with a history of infection from the original, the Alpha, and some Beta strains of SARS-CoV-2.
Dr. Cohen indicated that the next phase of their research will examine innate and acquired immune responses to the more recent Delta and Omicron variants.
The investigators analyzed the antibody-induced immune response up to 1 year in 130 COVID-19 recovered but unvaccinated individuals versus up to 8 months among 402 others matched by age and body mass index (BMI) and without previous infection who received two doses of the Pfizer vaccine.
The numbers of antibodies a month after vaccination were higher than those in the COVID-19 recovered patients. However, these numbers also declined more steeply in the vaccinated group, they note.
To assess the antibody performance, the investigators used the avidity index. This assay measures antibody function based on the strength of the interactions between the antibody and the viral antigen.
They found that the avidity index was higher in vaccinated individuals than in recovered patients initially but changes over time. At up to 6 months, the index did not significantly change in vaccinated individuals, whereas it gradually increased in recovered patients. This increase would potentially protect them from reinfection, the authors note.
These findings stand in stark contrast to an Oct. 29, 2021, Centers for Disease Control and Prevention study that found that COVID-19 vaccines provided five times the protection of natural immunity.
Those results, published in the organization’s Morbidity and Mortality Weekly Report, suggest that vaccination helps people mount a higher, stronger, and more consistent level of immunity against COVID-19 hospitalization than infection alone for at least 6 months.
Protection linked to obesity
Another finding that ran against the scientific grain was the data about obesity.
There was a higher and more persistent antibody performance among people with a BMI of 30 kg/m2.
This could relate to greater disease severity and/or a more pronounced initial response to infection among the obese group.
“Our hypothesis is that patients with obesity begin with a more pronounced response – reflected also by the disease manifestation – and the trend of decline is similar, therefore the kinetics of immune response remain higher throughout the study,” Dr. Cohen said.
“The results in the obese group were indeed unexpected and need further research to confirm or dispute,” said Dr. Schaffner, who is also the current medical director of the National Foundation for Infectious Diseases. “Obesity does predispose to more severe disease.”
Before the boosters
Along with using participants from only the earlier part of the pandemic, another limitation of the study was that the vaccinated group had only two doses of vaccine; boosters were not given during the time of the study, Dr. Schaffner said.
“Again, not the current situation.”
“That said, the strength and duration of natural immunity provided by the early variants was solid for up to a year, confirming previous reports,” he said.
A version of this article first appeared on Medscape.com.
It’s a matter of quality, not quantity. That’s the gist of a new Israeli study that shows that unvaccinated people with a prior SARS-CoV-2 infection create antibodies that are more effective in the long run compared with others who were vaccinated but never infected.
“While the quantity of antibodies decreases with time in both COVID-19 recovered patients and vaccinated individuals, ” lead author Carmit Cohen, PhD, said in an interview.
This difference could explain why previously infected patients appear to be better protected against a new infection than those who have only been vaccinated, according to a news release attached to the research.
One key caveat: This research does not include people from the later part of the pandemic.
This means there is a catch in terms of timing, William Schaffner, MD, Vanderbilt University School of Medicine, Nashville, Tenn., said when asked to comment on the study: “The study involved only the early COVID strains – it has no information on either the Delta or Omicron variants. Thus, the results primarily are of scientific or historical interest but are not immediately relevant to the current situation.”
The findings come from an early release of a study to be presented at the European Congress of Clinical Microbiology & Infectious Diseases in April.
An unexpected finding of the study showed that obese people had better protection – a higher and more sustained immune response – compared with overweight and normal-weight individuals.
“The results in the obese group were indeed unexpected and need further research to confirm or dispute,” Dr. Schaffner said. “Obesity does predispose to more severe disease.”
A focus on earlier strains
Dr. Cohen – a senior research assistant in infectious disease prevention at the Sheba Medical Center in Ramat Gan, Israel – and her colleagues recruited participants between March 25, 2020 and Nov. 25, 2020 and completed analysis in April 2021. This means they assessed people with a history of infection from the original, the Alpha, and some Beta strains of SARS-CoV-2.
Dr. Cohen indicated that the next phase of their research will examine innate and acquired immune responses to the more recent Delta and Omicron variants.
The investigators analyzed the antibody-induced immune response up to 1 year in 130 COVID-19 recovered but unvaccinated individuals versus up to 8 months among 402 others matched by age and body mass index (BMI) and without previous infection who received two doses of the Pfizer vaccine.
The numbers of antibodies a month after vaccination were higher than those in the COVID-19 recovered patients. However, these numbers also declined more steeply in the vaccinated group, they note.
To assess the antibody performance, the investigators used the avidity index. This assay measures antibody function based on the strength of the interactions between the antibody and the viral antigen.
They found that the avidity index was higher in vaccinated individuals than in recovered patients initially but changes over time. At up to 6 months, the index did not significantly change in vaccinated individuals, whereas it gradually increased in recovered patients. This increase would potentially protect them from reinfection, the authors note.
These findings stand in stark contrast to an Oct. 29, 2021, Centers for Disease Control and Prevention study that found that COVID-19 vaccines provided five times the protection of natural immunity.
Those results, published in the organization’s Morbidity and Mortality Weekly Report, suggest that vaccination helps people mount a higher, stronger, and more consistent level of immunity against COVID-19 hospitalization than infection alone for at least 6 months.
Protection linked to obesity
Another finding that ran against the scientific grain was the data about obesity.
There was a higher and more persistent antibody performance among people with a BMI of 30 kg/m2.
This could relate to greater disease severity and/or a more pronounced initial response to infection among the obese group.
“Our hypothesis is that patients with obesity begin with a more pronounced response – reflected also by the disease manifestation – and the trend of decline is similar, therefore the kinetics of immune response remain higher throughout the study,” Dr. Cohen said.
“The results in the obese group were indeed unexpected and need further research to confirm or dispute,” said Dr. Schaffner, who is also the current medical director of the National Foundation for Infectious Diseases. “Obesity does predispose to more severe disease.”
Before the boosters
Along with using participants from only the earlier part of the pandemic, another limitation of the study was that the vaccinated group had only two doses of vaccine; boosters were not given during the time of the study, Dr. Schaffner said.
“Again, not the current situation.”
“That said, the strength and duration of natural immunity provided by the early variants was solid for up to a year, confirming previous reports,” he said.
A version of this article first appeared on Medscape.com.
To a perfect day
Motionless, every Olympic skater starts off perfectly. Once the music starts, it’s up to them whether they will continue on perfectly or not. In this way, you’re just like an Olympic skater. Each day, a skating program. The music starts the moment your foot touches the floor in the morning. It’s up to you if the rest of the day will continue on flawlessly or not. To this point, I’ve yet to have a perfect day.
If I’m honest, my “perfect day” streak typically ends once I’ve made coffee. By then, I’ll have spilled a few grains of grounds or clinked mugs together when taking one from the cupboard. (D’oh!) Hardly ever can I make it to backing out of the driveway, let alone through a patient encounter. I’ve had a few procedures that when complete I’ve thought, “well, that looks great.” I can remember encounters that went brilliantly despite a high technical difficulty. I’ve also tagged a 7-iron shot 160 downwind yards to within inches of the cup. But I’ve hardly ever done anything in my life perfectly.
What does it mean to be perfect? Well, there have been 23 perfect baseball games. In 1972, the Miami Dolphins had the only perfect NFL season, 14-0 (although my 2007 Patriots went 18-0 before losing to the – ugh – Giants). Every year, several hundred students score a perfect 1600 on the SAT. In an underground vault somewhere in France is a perfect sphere, a perfectly spherical 1-kg mass of pure silicon. There are at least 51 perfect numbers. And model Bella Hadid’s exactly 1.62-ratioed face is said to be perfectly beautiful. But yet, U.S. skater Nathan Chen’s seemingly flawless 113.97-point short program in Beijing, still imperfect.
Attempting a perfect day or perfect surgery or a perfect pour over coffee is a fun game, but perfectionism has an insidious side. Some of us feel this way every day: We must do it exactly right, every time. Even an insignificant imperfection or error feels like failure. A 3.90 GPA is a fail. 515 on the MCAT, not nearly good enough. For them, the burden of perfection is crushing. It is hard for some to recognize that even if your performance could not be improved, the outcome can still be flawed. A chip in the ice, a patient showing up late, an interviewer with an agenda, a missed referee call can all flub up an otherwise flawless day. It isn’t necessary to abandon hope, all ye who live in the real world. Although achieving perfection is usually impossible, reward comes from the pursuit of perfection, not from holding it. It is called perfectionistic striving and in contrast to perfectionistic concerns, it is associated with resilience and positive mood. To do so you must combine giving your all with acceptance of whatever the outcome.
Keith Jarrett is one of the greatest jazz pianists of all time. He is a true perfectionist, precise in his standards and exacting in expectations. In 1975 in Cologne, Germany, he agreed to play at the behest of a teenage girl who arranged to have him perform at the opera house. Except, there was a miscommunication and only a small, broken rehearsal piano was available. As the story goes, she approached him as he waited to be taken back to his hotel, the concert was canceled and she somehow convinced him to play on the nearly unplayable instrument. The result is the Köln Concert, one of the greatest jazz performances in history. It was perfectly imperfect.
Yes, even the 1-kg sphere has femtogram quantities of other elements mixed in – the universal standard for perfect is itself, imperfect. It doesn’t matter. It’s the pursuit of such that makes life worthwhile. There’s always tomorrow. Have your coffee grinders ready.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected]
Motionless, every Olympic skater starts off perfectly. Once the music starts, it’s up to them whether they will continue on perfectly or not. In this way, you’re just like an Olympic skater. Each day, a skating program. The music starts the moment your foot touches the floor in the morning. It’s up to you if the rest of the day will continue on flawlessly or not. To this point, I’ve yet to have a perfect day.
If I’m honest, my “perfect day” streak typically ends once I’ve made coffee. By then, I’ll have spilled a few grains of grounds or clinked mugs together when taking one from the cupboard. (D’oh!) Hardly ever can I make it to backing out of the driveway, let alone through a patient encounter. I’ve had a few procedures that when complete I’ve thought, “well, that looks great.” I can remember encounters that went brilliantly despite a high technical difficulty. I’ve also tagged a 7-iron shot 160 downwind yards to within inches of the cup. But I’ve hardly ever done anything in my life perfectly.
What does it mean to be perfect? Well, there have been 23 perfect baseball games. In 1972, the Miami Dolphins had the only perfect NFL season, 14-0 (although my 2007 Patriots went 18-0 before losing to the – ugh – Giants). Every year, several hundred students score a perfect 1600 on the SAT. In an underground vault somewhere in France is a perfect sphere, a perfectly spherical 1-kg mass of pure silicon. There are at least 51 perfect numbers. And model Bella Hadid’s exactly 1.62-ratioed face is said to be perfectly beautiful. But yet, U.S. skater Nathan Chen’s seemingly flawless 113.97-point short program in Beijing, still imperfect.
Attempting a perfect day or perfect surgery or a perfect pour over coffee is a fun game, but perfectionism has an insidious side. Some of us feel this way every day: We must do it exactly right, every time. Even an insignificant imperfection or error feels like failure. A 3.90 GPA is a fail. 515 on the MCAT, not nearly good enough. For them, the burden of perfection is crushing. It is hard for some to recognize that even if your performance could not be improved, the outcome can still be flawed. A chip in the ice, a patient showing up late, an interviewer with an agenda, a missed referee call can all flub up an otherwise flawless day. It isn’t necessary to abandon hope, all ye who live in the real world. Although achieving perfection is usually impossible, reward comes from the pursuit of perfection, not from holding it. It is called perfectionistic striving and in contrast to perfectionistic concerns, it is associated with resilience and positive mood. To do so you must combine giving your all with acceptance of whatever the outcome.
Keith Jarrett is one of the greatest jazz pianists of all time. He is a true perfectionist, precise in his standards and exacting in expectations. In 1975 in Cologne, Germany, he agreed to play at the behest of a teenage girl who arranged to have him perform at the opera house. Except, there was a miscommunication and only a small, broken rehearsal piano was available. As the story goes, she approached him as he waited to be taken back to his hotel, the concert was canceled and she somehow convinced him to play on the nearly unplayable instrument. The result is the Köln Concert, one of the greatest jazz performances in history. It was perfectly imperfect.
Yes, even the 1-kg sphere has femtogram quantities of other elements mixed in – the universal standard for perfect is itself, imperfect. It doesn’t matter. It’s the pursuit of such that makes life worthwhile. There’s always tomorrow. Have your coffee grinders ready.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected]
Motionless, every Olympic skater starts off perfectly. Once the music starts, it’s up to them whether they will continue on perfectly or not. In this way, you’re just like an Olympic skater. Each day, a skating program. The music starts the moment your foot touches the floor in the morning. It’s up to you if the rest of the day will continue on flawlessly or not. To this point, I’ve yet to have a perfect day.
If I’m honest, my “perfect day” streak typically ends once I’ve made coffee. By then, I’ll have spilled a few grains of grounds or clinked mugs together when taking one from the cupboard. (D’oh!) Hardly ever can I make it to backing out of the driveway, let alone through a patient encounter. I’ve had a few procedures that when complete I’ve thought, “well, that looks great.” I can remember encounters that went brilliantly despite a high technical difficulty. I’ve also tagged a 7-iron shot 160 downwind yards to within inches of the cup. But I’ve hardly ever done anything in my life perfectly.
What does it mean to be perfect? Well, there have been 23 perfect baseball games. In 1972, the Miami Dolphins had the only perfect NFL season, 14-0 (although my 2007 Patriots went 18-0 before losing to the – ugh – Giants). Every year, several hundred students score a perfect 1600 on the SAT. In an underground vault somewhere in France is a perfect sphere, a perfectly spherical 1-kg mass of pure silicon. There are at least 51 perfect numbers. And model Bella Hadid’s exactly 1.62-ratioed face is said to be perfectly beautiful. But yet, U.S. skater Nathan Chen’s seemingly flawless 113.97-point short program in Beijing, still imperfect.
Attempting a perfect day or perfect surgery or a perfect pour over coffee is a fun game, but perfectionism has an insidious side. Some of us feel this way every day: We must do it exactly right, every time. Even an insignificant imperfection or error feels like failure. A 3.90 GPA is a fail. 515 on the MCAT, not nearly good enough. For them, the burden of perfection is crushing. It is hard for some to recognize that even if your performance could not be improved, the outcome can still be flawed. A chip in the ice, a patient showing up late, an interviewer with an agenda, a missed referee call can all flub up an otherwise flawless day. It isn’t necessary to abandon hope, all ye who live in the real world. Although achieving perfection is usually impossible, reward comes from the pursuit of perfection, not from holding it. It is called perfectionistic striving and in contrast to perfectionistic concerns, it is associated with resilience and positive mood. To do so you must combine giving your all with acceptance of whatever the outcome.
Keith Jarrett is one of the greatest jazz pianists of all time. He is a true perfectionist, precise in his standards and exacting in expectations. In 1975 in Cologne, Germany, he agreed to play at the behest of a teenage girl who arranged to have him perform at the opera house. Except, there was a miscommunication and only a small, broken rehearsal piano was available. As the story goes, she approached him as he waited to be taken back to his hotel, the concert was canceled and she somehow convinced him to play on the nearly unplayable instrument. The result is the Köln Concert, one of the greatest jazz performances in history. It was perfectly imperfect.
Yes, even the 1-kg sphere has femtogram quantities of other elements mixed in – the universal standard for perfect is itself, imperfect. It doesn’t matter. It’s the pursuit of such that makes life worthwhile. There’s always tomorrow. Have your coffee grinders ready.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected]
A third person living with HIV has been cured by transplant
In a first, If she remains off treatment without any hint of HIV, she would be only the third person in the world – after the Berlin Patient and the London Patient – to be cured through a transplant.
“Her own virus could not infect her cells,” said Yvonne Bryson, MD, chief of pediatric infectious diseases at the University of California, Los Angeles, who presented the study at the Conference on Retroviruses and Opportunistic Infections, which both presenters and the audience attended remotely.
The middle-aged New York woman of mixed race, who has asked that her specific race and age not be shared to protect her privacy, was diagnosed with HIV in 2013 when she was still in the very early stages of infection. She started treatment immediately and quickly achieved an undetectable viral load. An undetectable viral load not only prevents someone from transmitting HIV to others but also reduces or eliminates HIV replication, which means fewer variants and less time for the virus to infiltrate cells where it can hide.
But in 2017, she was diagnosed with leukemia. As a last resort to cure her of the cancer, she received a combination of adult stem cells from a relative’s blood that closely matched her own and umbilical cord blood obtained from a cord blood bank. That particular sample of cord blood was selected for its genetic mutation against the CCR5 receptor on immune cells, CD4 T cells. That mutation makes the immune system resistant to HIV.
The two previous HIV cures, of Berlin Patient Timothy Ray Brown and London Patient Adam Castillejo, also used stem cell transplantation with a CCR5 mutation, but theirs were bone marrow transplants. Bone marrow transplants are more arduous than cord blood transplants, which are commonly used in pediatric cancer treatment.
In this case, the physicians treating her used both.
“This allows the adult cells to accelerate and grow up until the cord blood takes over,” said Dr. Bryson. During her presentation, Dr. Bryson pointed to two types of data: First, she presented data showing the level of HIV in the patient’s blood. Soon after HIV diagnosis and treatment, her viral load dropped to undetectable levels. She had a spike of virus when she received the transplant, but then it went back to undetectable and has stayed that way ever since.
Meanwhile, following the transplant, her immune system started rebuilding itself using the new, HIV-resistant cells provided in the transplant. As her care team watched, no graft-versus-host (GVH) disease, a common side effect of stem cell transplants, emerged. In fact, the transplant went so well that she was discharged early from the hospital.
One hundred days after the transplant, the immune system contained within the cord blood had taken over. Her CD4 immune cells returned to normal levels a little more than a year after the transplant. By 27 months, she decided to stop all HIV treatment to see if the transplant had worked.
This was the real test. But as Dr. Bryson and colleagues continued to watch her HIV viral load and her CD4 counts and search for infectious virus, they didn’t find any. She tested negative for HIV by antibody test. Dr. Bryson grew 75 million of her cells in a lab to look for any HIV. None. Aside from one blip in detectable HIV DNA at 14 weeks, researchers never found HIV in the patient again.
“Her cells are resistant to HIV now – both her own strains and laboratory strains,” Dr. Bryson told this news organization. “It’s been 14 months since then. She has no rebound and no detectable virus.”
The presentation drew as raucous as praise gets in a virtual environment. The comments began pouring in.
“Impressive results,” wrote Jim Hoxie, MD, professor emeritus at the University of Pennsylvania, Philadelphia.
“Exciting case,” wrote Allison Agwu, MD, a professor of pediatrics at Johns Hopkins University, Baltimore.
And Dennis Copertino, a research specialist at Weill Cornell Medicine, New York, wrote: “Thank you so much for translating this important cure strategy to people of color.”
Most donors with CCR5 mutations are White, Dr. Bryson said, suggesting that this approach, in a mixed-race woman, could expand the pool of people living with HIV and cancer who are good candidates for the approach.
But other observers had questions, ones that may require more research to answer. Some asked why this woman’s virus, after transplantation, wasn’t just immune to viruses with CCR5 but also another variant, called CXCR4, that one wouldn’t expect. Luis Montaner, DVM, director of the Immunopathogenesis Laboratory at the Wistar Institute in Philadelphia, wondered whether it was more than the blood that had cleared HIV. Did it get into the tissue, too? That question has not yet been answered.
For Carl Dieffenbach, PhD, director of the division of AIDS at the National Institute of Allergy and Infectious Diseases, the lack of GVH disease was a powerful and hopeful finding.
“There’s been this ongoing hypothesis that maybe graft-versus-host disease was needed at some level to help clear out every last single CD4+ T cell that may or may not have been harboring replication-competent virus,” Dr. Dieffenbach said in an interview. “But there was no GVH disease. That’s incredible. It’s a wonderful thing.”
Now the challenge is to move from a single case to making cure available to other people living with HIV.
The case also got cure researchers thinking.
Dr. Montaner called the case “an encouraging roadmap supporting anti-CCR5 strategies by CRISPR Cas9,” studies that are now underway.
Steven Deeks, MD, called the case “perhaps a model for how we might do this using a person’s own cells. Because we were never really going to be transplanting cells from another person as a scalable cure.”
For people living with HIV, particularly women of color, the results raise hopes and questions. Nina Martinez knows something about being a “first.” In 2019, she was the first American woman of color living with HIV to donate a kidney to another person living with the virus. To her, the excitement over the first woman of color being cured of HIV just shines a light on how very White and male HIV cure studies have been until now.
“For me, I’m not looking for a cure in which the successful step forward is me getting cancer,” she said in an interview. “I’m looking at, what’s going to be sustainable? I want to know what’s going to work for a group of people.”
Gina Marie Brown, a social worker living with HIV in New Orleans, is also thinking of groups of people.
“Every time we get a breakthrough, it’s like the sun is taken from behind the clouds a little more,” said Ms. Brown. “I think about people in the South, who bear a huge burden of HIV. I think about trans women. I think about Black women, and gay, bisexual, and same-gender-loving men. This could really impact HIV – in the same way that PrEP [pre-exposure prophylaxis] has, the same way that one pill once a day has.”
When Ms. Brown was diagnosed with HIV 22 years ago, she started to plan her funeral.
“That’s how much I thought HIV was a death sentence,” she told this news organization. “Oh my goodness! Glad you stuck around, Gina.”
The study was funded by the National Institutes of Health. Dr. Bryson, Dr. Dieffenbach, Dr. Deeks, and Dr. Montaner disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In a first, If she remains off treatment without any hint of HIV, she would be only the third person in the world – after the Berlin Patient and the London Patient – to be cured through a transplant.
“Her own virus could not infect her cells,” said Yvonne Bryson, MD, chief of pediatric infectious diseases at the University of California, Los Angeles, who presented the study at the Conference on Retroviruses and Opportunistic Infections, which both presenters and the audience attended remotely.
The middle-aged New York woman of mixed race, who has asked that her specific race and age not be shared to protect her privacy, was diagnosed with HIV in 2013 when she was still in the very early stages of infection. She started treatment immediately and quickly achieved an undetectable viral load. An undetectable viral load not only prevents someone from transmitting HIV to others but also reduces or eliminates HIV replication, which means fewer variants and less time for the virus to infiltrate cells where it can hide.
But in 2017, she was diagnosed with leukemia. As a last resort to cure her of the cancer, she received a combination of adult stem cells from a relative’s blood that closely matched her own and umbilical cord blood obtained from a cord blood bank. That particular sample of cord blood was selected for its genetic mutation against the CCR5 receptor on immune cells, CD4 T cells. That mutation makes the immune system resistant to HIV.
The two previous HIV cures, of Berlin Patient Timothy Ray Brown and London Patient Adam Castillejo, also used stem cell transplantation with a CCR5 mutation, but theirs were bone marrow transplants. Bone marrow transplants are more arduous than cord blood transplants, which are commonly used in pediatric cancer treatment.
In this case, the physicians treating her used both.
“This allows the adult cells to accelerate and grow up until the cord blood takes over,” said Dr. Bryson. During her presentation, Dr. Bryson pointed to two types of data: First, she presented data showing the level of HIV in the patient’s blood. Soon after HIV diagnosis and treatment, her viral load dropped to undetectable levels. She had a spike of virus when she received the transplant, but then it went back to undetectable and has stayed that way ever since.
Meanwhile, following the transplant, her immune system started rebuilding itself using the new, HIV-resistant cells provided in the transplant. As her care team watched, no graft-versus-host (GVH) disease, a common side effect of stem cell transplants, emerged. In fact, the transplant went so well that she was discharged early from the hospital.
One hundred days after the transplant, the immune system contained within the cord blood had taken over. Her CD4 immune cells returned to normal levels a little more than a year after the transplant. By 27 months, she decided to stop all HIV treatment to see if the transplant had worked.
This was the real test. But as Dr. Bryson and colleagues continued to watch her HIV viral load and her CD4 counts and search for infectious virus, they didn’t find any. She tested negative for HIV by antibody test. Dr. Bryson grew 75 million of her cells in a lab to look for any HIV. None. Aside from one blip in detectable HIV DNA at 14 weeks, researchers never found HIV in the patient again.
“Her cells are resistant to HIV now – both her own strains and laboratory strains,” Dr. Bryson told this news organization. “It’s been 14 months since then. She has no rebound and no detectable virus.”
The presentation drew as raucous as praise gets in a virtual environment. The comments began pouring in.
“Impressive results,” wrote Jim Hoxie, MD, professor emeritus at the University of Pennsylvania, Philadelphia.
“Exciting case,” wrote Allison Agwu, MD, a professor of pediatrics at Johns Hopkins University, Baltimore.
And Dennis Copertino, a research specialist at Weill Cornell Medicine, New York, wrote: “Thank you so much for translating this important cure strategy to people of color.”
Most donors with CCR5 mutations are White, Dr. Bryson said, suggesting that this approach, in a mixed-race woman, could expand the pool of people living with HIV and cancer who are good candidates for the approach.
But other observers had questions, ones that may require more research to answer. Some asked why this woman’s virus, after transplantation, wasn’t just immune to viruses with CCR5 but also another variant, called CXCR4, that one wouldn’t expect. Luis Montaner, DVM, director of the Immunopathogenesis Laboratory at the Wistar Institute in Philadelphia, wondered whether it was more than the blood that had cleared HIV. Did it get into the tissue, too? That question has not yet been answered.
For Carl Dieffenbach, PhD, director of the division of AIDS at the National Institute of Allergy and Infectious Diseases, the lack of GVH disease was a powerful and hopeful finding.
“There’s been this ongoing hypothesis that maybe graft-versus-host disease was needed at some level to help clear out every last single CD4+ T cell that may or may not have been harboring replication-competent virus,” Dr. Dieffenbach said in an interview. “But there was no GVH disease. That’s incredible. It’s a wonderful thing.”
Now the challenge is to move from a single case to making cure available to other people living with HIV.
The case also got cure researchers thinking.
Dr. Montaner called the case “an encouraging roadmap supporting anti-CCR5 strategies by CRISPR Cas9,” studies that are now underway.
Steven Deeks, MD, called the case “perhaps a model for how we might do this using a person’s own cells. Because we were never really going to be transplanting cells from another person as a scalable cure.”
For people living with HIV, particularly women of color, the results raise hopes and questions. Nina Martinez knows something about being a “first.” In 2019, she was the first American woman of color living with HIV to donate a kidney to another person living with the virus. To her, the excitement over the first woman of color being cured of HIV just shines a light on how very White and male HIV cure studies have been until now.
“For me, I’m not looking for a cure in which the successful step forward is me getting cancer,” she said in an interview. “I’m looking at, what’s going to be sustainable? I want to know what’s going to work for a group of people.”
Gina Marie Brown, a social worker living with HIV in New Orleans, is also thinking of groups of people.
“Every time we get a breakthrough, it’s like the sun is taken from behind the clouds a little more,” said Ms. Brown. “I think about people in the South, who bear a huge burden of HIV. I think about trans women. I think about Black women, and gay, bisexual, and same-gender-loving men. This could really impact HIV – in the same way that PrEP [pre-exposure prophylaxis] has, the same way that one pill once a day has.”
When Ms. Brown was diagnosed with HIV 22 years ago, she started to plan her funeral.
“That’s how much I thought HIV was a death sentence,” she told this news organization. “Oh my goodness! Glad you stuck around, Gina.”
The study was funded by the National Institutes of Health. Dr. Bryson, Dr. Dieffenbach, Dr. Deeks, and Dr. Montaner disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In a first, If she remains off treatment without any hint of HIV, she would be only the third person in the world – after the Berlin Patient and the London Patient – to be cured through a transplant.
“Her own virus could not infect her cells,” said Yvonne Bryson, MD, chief of pediatric infectious diseases at the University of California, Los Angeles, who presented the study at the Conference on Retroviruses and Opportunistic Infections, which both presenters and the audience attended remotely.
The middle-aged New York woman of mixed race, who has asked that her specific race and age not be shared to protect her privacy, was diagnosed with HIV in 2013 when she was still in the very early stages of infection. She started treatment immediately and quickly achieved an undetectable viral load. An undetectable viral load not only prevents someone from transmitting HIV to others but also reduces or eliminates HIV replication, which means fewer variants and less time for the virus to infiltrate cells where it can hide.
But in 2017, she was diagnosed with leukemia. As a last resort to cure her of the cancer, she received a combination of adult stem cells from a relative’s blood that closely matched her own and umbilical cord blood obtained from a cord blood bank. That particular sample of cord blood was selected for its genetic mutation against the CCR5 receptor on immune cells, CD4 T cells. That mutation makes the immune system resistant to HIV.
The two previous HIV cures, of Berlin Patient Timothy Ray Brown and London Patient Adam Castillejo, also used stem cell transplantation with a CCR5 mutation, but theirs were bone marrow transplants. Bone marrow transplants are more arduous than cord blood transplants, which are commonly used in pediatric cancer treatment.
In this case, the physicians treating her used both.
“This allows the adult cells to accelerate and grow up until the cord blood takes over,” said Dr. Bryson. During her presentation, Dr. Bryson pointed to two types of data: First, she presented data showing the level of HIV in the patient’s blood. Soon after HIV diagnosis and treatment, her viral load dropped to undetectable levels. She had a spike of virus when she received the transplant, but then it went back to undetectable and has stayed that way ever since.
Meanwhile, following the transplant, her immune system started rebuilding itself using the new, HIV-resistant cells provided in the transplant. As her care team watched, no graft-versus-host (GVH) disease, a common side effect of stem cell transplants, emerged. In fact, the transplant went so well that she was discharged early from the hospital.
One hundred days after the transplant, the immune system contained within the cord blood had taken over. Her CD4 immune cells returned to normal levels a little more than a year after the transplant. By 27 months, she decided to stop all HIV treatment to see if the transplant had worked.
This was the real test. But as Dr. Bryson and colleagues continued to watch her HIV viral load and her CD4 counts and search for infectious virus, they didn’t find any. She tested negative for HIV by antibody test. Dr. Bryson grew 75 million of her cells in a lab to look for any HIV. None. Aside from one blip in detectable HIV DNA at 14 weeks, researchers never found HIV in the patient again.
“Her cells are resistant to HIV now – both her own strains and laboratory strains,” Dr. Bryson told this news organization. “It’s been 14 months since then. She has no rebound and no detectable virus.”
The presentation drew as raucous as praise gets in a virtual environment. The comments began pouring in.
“Impressive results,” wrote Jim Hoxie, MD, professor emeritus at the University of Pennsylvania, Philadelphia.
“Exciting case,” wrote Allison Agwu, MD, a professor of pediatrics at Johns Hopkins University, Baltimore.
And Dennis Copertino, a research specialist at Weill Cornell Medicine, New York, wrote: “Thank you so much for translating this important cure strategy to people of color.”
Most donors with CCR5 mutations are White, Dr. Bryson said, suggesting that this approach, in a mixed-race woman, could expand the pool of people living with HIV and cancer who are good candidates for the approach.
But other observers had questions, ones that may require more research to answer. Some asked why this woman’s virus, after transplantation, wasn’t just immune to viruses with CCR5 but also another variant, called CXCR4, that one wouldn’t expect. Luis Montaner, DVM, director of the Immunopathogenesis Laboratory at the Wistar Institute in Philadelphia, wondered whether it was more than the blood that had cleared HIV. Did it get into the tissue, too? That question has not yet been answered.
For Carl Dieffenbach, PhD, director of the division of AIDS at the National Institute of Allergy and Infectious Diseases, the lack of GVH disease was a powerful and hopeful finding.
“There’s been this ongoing hypothesis that maybe graft-versus-host disease was needed at some level to help clear out every last single CD4+ T cell that may or may not have been harboring replication-competent virus,” Dr. Dieffenbach said in an interview. “But there was no GVH disease. That’s incredible. It’s a wonderful thing.”
Now the challenge is to move from a single case to making cure available to other people living with HIV.
The case also got cure researchers thinking.
Dr. Montaner called the case “an encouraging roadmap supporting anti-CCR5 strategies by CRISPR Cas9,” studies that are now underway.
Steven Deeks, MD, called the case “perhaps a model for how we might do this using a person’s own cells. Because we were never really going to be transplanting cells from another person as a scalable cure.”
For people living with HIV, particularly women of color, the results raise hopes and questions. Nina Martinez knows something about being a “first.” In 2019, she was the first American woman of color living with HIV to donate a kidney to another person living with the virus. To her, the excitement over the first woman of color being cured of HIV just shines a light on how very White and male HIV cure studies have been until now.
“For me, I’m not looking for a cure in which the successful step forward is me getting cancer,” she said in an interview. “I’m looking at, what’s going to be sustainable? I want to know what’s going to work for a group of people.”
Gina Marie Brown, a social worker living with HIV in New Orleans, is also thinking of groups of people.
“Every time we get a breakthrough, it’s like the sun is taken from behind the clouds a little more,” said Ms. Brown. “I think about people in the South, who bear a huge burden of HIV. I think about trans women. I think about Black women, and gay, bisexual, and same-gender-loving men. This could really impact HIV – in the same way that PrEP [pre-exposure prophylaxis] has, the same way that one pill once a day has.”
When Ms. Brown was diagnosed with HIV 22 years ago, she started to plan her funeral.
“That’s how much I thought HIV was a death sentence,” she told this news organization. “Oh my goodness! Glad you stuck around, Gina.”
The study was funded by the National Institutes of Health. Dr. Bryson, Dr. Dieffenbach, Dr. Deeks, and Dr. Montaner disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM CROI 2022
Eighteen-year study shows inconsistencies in treating, classifying JIA
“Children are not little adults” is a common refrain in pediatric medicine, but when it comes to a condition like juvenile idiopathic arthritis (JIA), rheumatologists might be better off treating pediatric and adult rheumatic disease more similarly.
A recent study published in Arthritis Care & Research followed children diagnosed with JIA for 18 years. Although not the first long-term study to examine children with JIA, it is unique in that it took place “during a time where biologic DMARDs [disease-modifying antirheumatic drugs] were emerging as a fundamental therapy in the management of children with JIA,” said Dawn M. Wahezi, MD, chief of the division of pediatric rheumatology at the Children’s Hospital at Montefiore in New York, who was not involved with the study.
Additionally, the study highlights the International League of Associations for Rheumatology (ILAR) consensus-based classification criteria as an imperfect method to categorize patients with JIA.
Mia Glerup, MD, PhD, of the department of pediatrics at Aarhus (Denmark) University Hospital and colleagues prospectively analyzed 373 patients from Denmark, Norway, Sweden, and Finland with new-onset JIA between 1997 and 2000 and evaluated them at baseline, 8 years, and 18 years. At each visit, the researchers collected data on demographics, disease activity, ILAR category, treatment, and blood samples.
Patients in the cohort were mostly girls (66.7%) with a median age of 5.9 years at onset. Approximately one-third (34.8%) of patients were antinuclear antibody (ANA) positive and 21.6% were HLA-B27 positive. The most common JIA categories at baseline were persistent oligoarthritis (53.9%), polyarticular rheumatoid factor (RF) negative (21.1%), and undifferentiated arthritis (10.2%).
Dr. Glerup and colleagues found that the proportion of patients not receiving DMARDs declined from 73.2% at baseline to 59.7% at 8 years, and then rose again to 70% at 18 years (risk ratio, 1.3; P = .003). The group of 103 patients who used conventional DMARDs (cDMARDs) either as monotherapy or in combination with a biologic DMARD (bDMARD) at 8 years dwindled to 44 (42.7%) at 18 years (RR, 0.4; P < .001), whereas 32 of 52 patients (61.5%) using bDMARDs at 8 years were still taking them at 18 years (RR, 0.6; P = .02). Across the whole study, 14.7% of patients never received any JIA treatment, and 33 of 85 patients (38.8%) on continuous DMARDs developed uveitis during the study period.
Overall, 62.7% of patients received DMARDs at least once, including 89.7% with polyarticular RF negative, 77.3% with oligoarticular extended, 76.9% with systemic, 75.7% with juvenile enthesitis-related arthritis (ERA), 66.7% with polyarticular RF-positive, 65.2% with juvenile psoriatic arthritis (JPsA), 58.9% with undifferentiated JIA, and 27.6% of patients with persistent oligoarticular disease.
The median number of active joints dropped from 3 (range, 1-30) at baseline to 0 at 8 years (range, 0-13), whereas the median cumulative number of affected joints rose from 3 at baseline (range, 1-30) to 6 at 8 years (range, 1-41). At last follow-up, the median number of active joints was 0 (range, 0-5) and median cumulative number of affected joints was 7 (range, 1-47). The percentage of patients in remission barely changed from 52% at 8 years to 51% at 18.
Some patients also changed ILAR categories during the study period, with 7% shifting between baseline and 8 years, and 11% shifting between 8-year and 18-year follow-up. Compared with baseline, by the 18-year follow-up time point there was a significant decrease in the number of patients categorized as oligoarticular (230 vs. 197 patients; P = .02), a significant increase in patients in the psoriatic ILAR category (8 vs. 28 patients; P < .001), and a nonsignificant increase in the number of patients in the undifferentiated category (45 vs. 63 patients; P = .06).
“Almost half of the changes in the distribution between the ILAR categories were caused by updated information on heredity in a first-degree relative obtained at the follow-up visits,” Dr. Glerup and colleagues write.
The results of the long-term study show that patients are “likely to remain in remission – with the converse also evident, as patients still with evidence of disease activity at 8 years after disease onset were more likely to have refractory disease,” Dr. Wahezi said.
Commenting on the study’s findings, Lisa F. Imundo, MD, director of adolescent rheumatology at Columbia University Medical Center in New York, said they are “great news to be able to give parents of young kids with arthritis.” However, she questioned whether the results are generalizable to populations of patients “who are in the worst prognostic group.”
For example, a substantial proportion of patients were classified under the oligoarticular category. “That’s already a group that we know from experience tends to have a better outcome than some of the other groups of JIA,” she said.
“That kind of weaves its way through the whole study, because then they show a lot of patients have come off their medication. Patients who had more severe disease in more joints would be less likely, I think, to just stop their medication and stop going to doctors,” Dr. Imundo explained.
Although the study is valuable for its long-term follow-up, there is also a question of generalizability across a more diverse ethnic and racial group. The authors do not elaborate on the racial breakdown of their patients, Dr. Imundo said, “so we’re going to have to assume that the vast majority are going to [have] Caucasian Nordic ethnic background, and that goes along with them having this high percentage of HLA-B27 positivity, which is a gene that’s more prevalent in northern European populations.”
Jonathan Hausmann, MD, a pediatric and adult rheumatologist at Boston Children’s Hospital, Boston,, told this news organization that he believes the overall conclusions from the study – that JIA persists over time and that ILAR classification is a somewhat imprecise measure of assessing JIA types in children – would be generalizable to other groups.
However, long-term registries evaluating JIA in more diverse populations, such as the Childhood Arthritis and Rheumatology Research Alliance (CARRA) registry, could confirm these results, said Dr. Hausmann, who is a registry informatics associate with CARRA and was not associated with the research.
Long-term management of JIA
In an accompanying editorial, Jaime Guzman, MD, MSc, and Ross E. Petty, MD, PhD, of British Columbia Children’s Hospital and the University of British Columbia, Vancouver, said a rheumatologist’s interpretation of the study would be tied to what they learned about children with arthritis in medical school. They would see the glass as “half full” if children who achieved remission stayed in remission if they learned that a child might end up outgrowing JIA but potentially develop lifelong disability, whereas others may focus on the outcome of approximately half of patients not achieving remission.
“When I was going through medical school, I remember learning that JIA is a disease of children, and typically, they outgrow it as they become adults,” Dr. Hausmann said. “I think this study and many other studies have shown that that’s actually not the case – that, in fact, it may be a majority of kids continue having active disease even through adulthood.”
If a rheumatologist knows JIA is likely to continue into adulthood, “that’s huge,” Dr. Hausmann said. “That means when we first diagnose patients with JIA as kids, we need to set expectations with the families that this may not just go away; this may be something that could be more lifelong.”
Education on the part of the patient, their parents, and their clinician on the expected trajectory of the disease is critical so that children can continue their own care as they transition to adulthood, Dr. Hausmann explained. “The earlier the kids develop the skills to discuss their medicines, their side effects, the better they’ll be able to transition to adult medicine,” he said.
For the patients who go into remission and stay in remission, the message is also important. “To have the reassurance that a lot of those kids won’t be having active joint symptoms or need to be on medication, that’s a huge positive message that can get out there, so I think that’s great,” Dr. Imundo said.
Time to move on from ILAR classification?
Another big takeaway from the study was how patients’ ILAR classification changed across the 18-year follow-up. First proposed in 1995, the JIA ILAR classification has been revised several times for clarification purposes. In its current form, the ILAR classification considers a patient’s history when categorizing JIA types but also includes factors such as immediate family history. This system of assessing JIA has been criticized and there are initiatives to create a new JIA classification system to replace it.
“The ILAR criteria were designed to classify patients 6 months after disease onset in an attempt to find some commonality in clinical phenotypes, prognosis, and suggested management,” Dr. Wahezi said. “While there continues to be debate as to whether we can improve our classification of JIA patients, it is not surprising that phenotypes may evolve over time as new clinical features develop. As pediatric rheumatologists, we are well accustomed to having to modify management plans as children manifest with new clinical features over time.”
Although the percentage of patients who switched ILAR classifications over the study period was “much higher” than she would have thought, Dr. Imundo said it was the reasons provided in the study that seemed odd to her. “The classification scheme relies on your family history, like someone else in your family now has psoriasis, so your arthritis classification changes,” she explained.
“We want to head toward a much more unified classification scheme, a simpler one. We now understand that some of the diseases that we see in pediatrics are really the equivalent or same disease in adults,” she said.
“Most of the pediatric categories of JIA have distinct adult correlates,” Dr. Hausmann agreed. RF-positive polyarthritis in children and rheumatoid arthritis in adults are correlated, as are systemic JIA and adult-onset Still’s disease, he explained. “That has been borne out also by genetic susceptibility studies that the genetic predispositions to systemic arthritis in children is the same as the genetic predisposition to adult-onset Still’s disease in adults. By and large, there are a lot of similarities between the two.
“I think we need to incorporate some of that knowledge in better classifying kids with JIA so that we can find the best treatments and the best outcomes, and we can provide information to families about the expected course of the disease over time so that can inform our discussions.”
Some pediatric rheumatologists accept the classification system is flawed, but not all concur with the degree to which these problems impact patient care. “While the ILAR classification criteria may be subject to criticism, it does provide general context and prognostic implications for patients and families,” Dr. Wahezi said.
“The medicines certainly are very similar across the JIA categories, so the implications are not as broad” when classification changes,” Dr. Hausmann said. “But it certainly shows that there are things that we still don’t know. I think classification is actually pretty important because it might give you a sense of how persistent the disease will be.”
Dr. Imundo said the ILAR classification’s “time is limited,” and rheumatologists may soon need to adopt a new way of classifying children with rheumatic disease – “a more data-driven, genetics-driven scheme.”
“These categories are so imperfect, and the patients are changing. I feel like that says to me, let’s find something that’s more predictive that really helps us a little better than what we have now,” she said.
The study had no specific funding. The authors of the study and the editorial have disclosed no relevant financial relationships. Dr. Hausmann reports receiving salary support from CARRA. Dr. Imundo and Dr. Wahezi have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
“Children are not little adults” is a common refrain in pediatric medicine, but when it comes to a condition like juvenile idiopathic arthritis (JIA), rheumatologists might be better off treating pediatric and adult rheumatic disease more similarly.
A recent study published in Arthritis Care & Research followed children diagnosed with JIA for 18 years. Although not the first long-term study to examine children with JIA, it is unique in that it took place “during a time where biologic DMARDs [disease-modifying antirheumatic drugs] were emerging as a fundamental therapy in the management of children with JIA,” said Dawn M. Wahezi, MD, chief of the division of pediatric rheumatology at the Children’s Hospital at Montefiore in New York, who was not involved with the study.
Additionally, the study highlights the International League of Associations for Rheumatology (ILAR) consensus-based classification criteria as an imperfect method to categorize patients with JIA.
Mia Glerup, MD, PhD, of the department of pediatrics at Aarhus (Denmark) University Hospital and colleagues prospectively analyzed 373 patients from Denmark, Norway, Sweden, and Finland with new-onset JIA between 1997 and 2000 and evaluated them at baseline, 8 years, and 18 years. At each visit, the researchers collected data on demographics, disease activity, ILAR category, treatment, and blood samples.
Patients in the cohort were mostly girls (66.7%) with a median age of 5.9 years at onset. Approximately one-third (34.8%) of patients were antinuclear antibody (ANA) positive and 21.6% were HLA-B27 positive. The most common JIA categories at baseline were persistent oligoarthritis (53.9%), polyarticular rheumatoid factor (RF) negative (21.1%), and undifferentiated arthritis (10.2%).
Dr. Glerup and colleagues found that the proportion of patients not receiving DMARDs declined from 73.2% at baseline to 59.7% at 8 years, and then rose again to 70% at 18 years (risk ratio, 1.3; P = .003). The group of 103 patients who used conventional DMARDs (cDMARDs) either as monotherapy or in combination with a biologic DMARD (bDMARD) at 8 years dwindled to 44 (42.7%) at 18 years (RR, 0.4; P < .001), whereas 32 of 52 patients (61.5%) using bDMARDs at 8 years were still taking them at 18 years (RR, 0.6; P = .02). Across the whole study, 14.7% of patients never received any JIA treatment, and 33 of 85 patients (38.8%) on continuous DMARDs developed uveitis during the study period.
Overall, 62.7% of patients received DMARDs at least once, including 89.7% with polyarticular RF negative, 77.3% with oligoarticular extended, 76.9% with systemic, 75.7% with juvenile enthesitis-related arthritis (ERA), 66.7% with polyarticular RF-positive, 65.2% with juvenile psoriatic arthritis (JPsA), 58.9% with undifferentiated JIA, and 27.6% of patients with persistent oligoarticular disease.
The median number of active joints dropped from 3 (range, 1-30) at baseline to 0 at 8 years (range, 0-13), whereas the median cumulative number of affected joints rose from 3 at baseline (range, 1-30) to 6 at 8 years (range, 1-41). At last follow-up, the median number of active joints was 0 (range, 0-5) and median cumulative number of affected joints was 7 (range, 1-47). The percentage of patients in remission barely changed from 52% at 8 years to 51% at 18.
Some patients also changed ILAR categories during the study period, with 7% shifting between baseline and 8 years, and 11% shifting between 8-year and 18-year follow-up. Compared with baseline, by the 18-year follow-up time point there was a significant decrease in the number of patients categorized as oligoarticular (230 vs. 197 patients; P = .02), a significant increase in patients in the psoriatic ILAR category (8 vs. 28 patients; P < .001), and a nonsignificant increase in the number of patients in the undifferentiated category (45 vs. 63 patients; P = .06).
“Almost half of the changes in the distribution between the ILAR categories were caused by updated information on heredity in a first-degree relative obtained at the follow-up visits,” Dr. Glerup and colleagues write.
The results of the long-term study show that patients are “likely to remain in remission – with the converse also evident, as patients still with evidence of disease activity at 8 years after disease onset were more likely to have refractory disease,” Dr. Wahezi said.
Commenting on the study’s findings, Lisa F. Imundo, MD, director of adolescent rheumatology at Columbia University Medical Center in New York, said they are “great news to be able to give parents of young kids with arthritis.” However, she questioned whether the results are generalizable to populations of patients “who are in the worst prognostic group.”
For example, a substantial proportion of patients were classified under the oligoarticular category. “That’s already a group that we know from experience tends to have a better outcome than some of the other groups of JIA,” she said.
“That kind of weaves its way through the whole study, because then they show a lot of patients have come off their medication. Patients who had more severe disease in more joints would be less likely, I think, to just stop their medication and stop going to doctors,” Dr. Imundo explained.
Although the study is valuable for its long-term follow-up, there is also a question of generalizability across a more diverse ethnic and racial group. The authors do not elaborate on the racial breakdown of their patients, Dr. Imundo said, “so we’re going to have to assume that the vast majority are going to [have] Caucasian Nordic ethnic background, and that goes along with them having this high percentage of HLA-B27 positivity, which is a gene that’s more prevalent in northern European populations.”
Jonathan Hausmann, MD, a pediatric and adult rheumatologist at Boston Children’s Hospital, Boston,, told this news organization that he believes the overall conclusions from the study – that JIA persists over time and that ILAR classification is a somewhat imprecise measure of assessing JIA types in children – would be generalizable to other groups.
However, long-term registries evaluating JIA in more diverse populations, such as the Childhood Arthritis and Rheumatology Research Alliance (CARRA) registry, could confirm these results, said Dr. Hausmann, who is a registry informatics associate with CARRA and was not associated with the research.
Long-term management of JIA
In an accompanying editorial, Jaime Guzman, MD, MSc, and Ross E. Petty, MD, PhD, of British Columbia Children’s Hospital and the University of British Columbia, Vancouver, said a rheumatologist’s interpretation of the study would be tied to what they learned about children with arthritis in medical school. They would see the glass as “half full” if children who achieved remission stayed in remission if they learned that a child might end up outgrowing JIA but potentially develop lifelong disability, whereas others may focus on the outcome of approximately half of patients not achieving remission.
“When I was going through medical school, I remember learning that JIA is a disease of children, and typically, they outgrow it as they become adults,” Dr. Hausmann said. “I think this study and many other studies have shown that that’s actually not the case – that, in fact, it may be a majority of kids continue having active disease even through adulthood.”
If a rheumatologist knows JIA is likely to continue into adulthood, “that’s huge,” Dr. Hausmann said. “That means when we first diagnose patients with JIA as kids, we need to set expectations with the families that this may not just go away; this may be something that could be more lifelong.”
Education on the part of the patient, their parents, and their clinician on the expected trajectory of the disease is critical so that children can continue their own care as they transition to adulthood, Dr. Hausmann explained. “The earlier the kids develop the skills to discuss their medicines, their side effects, the better they’ll be able to transition to adult medicine,” he said.
For the patients who go into remission and stay in remission, the message is also important. “To have the reassurance that a lot of those kids won’t be having active joint symptoms or need to be on medication, that’s a huge positive message that can get out there, so I think that’s great,” Dr. Imundo said.
Time to move on from ILAR classification?
Another big takeaway from the study was how patients’ ILAR classification changed across the 18-year follow-up. First proposed in 1995, the JIA ILAR classification has been revised several times for clarification purposes. In its current form, the ILAR classification considers a patient’s history when categorizing JIA types but also includes factors such as immediate family history. This system of assessing JIA has been criticized and there are initiatives to create a new JIA classification system to replace it.
“The ILAR criteria were designed to classify patients 6 months after disease onset in an attempt to find some commonality in clinical phenotypes, prognosis, and suggested management,” Dr. Wahezi said. “While there continues to be debate as to whether we can improve our classification of JIA patients, it is not surprising that phenotypes may evolve over time as new clinical features develop. As pediatric rheumatologists, we are well accustomed to having to modify management plans as children manifest with new clinical features over time.”
Although the percentage of patients who switched ILAR classifications over the study period was “much higher” than she would have thought, Dr. Imundo said it was the reasons provided in the study that seemed odd to her. “The classification scheme relies on your family history, like someone else in your family now has psoriasis, so your arthritis classification changes,” she explained.
“We want to head toward a much more unified classification scheme, a simpler one. We now understand that some of the diseases that we see in pediatrics are really the equivalent or same disease in adults,” she said.
“Most of the pediatric categories of JIA have distinct adult correlates,” Dr. Hausmann agreed. RF-positive polyarthritis in children and rheumatoid arthritis in adults are correlated, as are systemic JIA and adult-onset Still’s disease, he explained. “That has been borne out also by genetic susceptibility studies that the genetic predispositions to systemic arthritis in children is the same as the genetic predisposition to adult-onset Still’s disease in adults. By and large, there are a lot of similarities between the two.
“I think we need to incorporate some of that knowledge in better classifying kids with JIA so that we can find the best treatments and the best outcomes, and we can provide information to families about the expected course of the disease over time so that can inform our discussions.”
Some pediatric rheumatologists accept the classification system is flawed, but not all concur with the degree to which these problems impact patient care. “While the ILAR classification criteria may be subject to criticism, it does provide general context and prognostic implications for patients and families,” Dr. Wahezi said.
“The medicines certainly are very similar across the JIA categories, so the implications are not as broad” when classification changes,” Dr. Hausmann said. “But it certainly shows that there are things that we still don’t know. I think classification is actually pretty important because it might give you a sense of how persistent the disease will be.”
Dr. Imundo said the ILAR classification’s “time is limited,” and rheumatologists may soon need to adopt a new way of classifying children with rheumatic disease – “a more data-driven, genetics-driven scheme.”
“These categories are so imperfect, and the patients are changing. I feel like that says to me, let’s find something that’s more predictive that really helps us a little better than what we have now,” she said.
The study had no specific funding. The authors of the study and the editorial have disclosed no relevant financial relationships. Dr. Hausmann reports receiving salary support from CARRA. Dr. Imundo and Dr. Wahezi have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
“Children are not little adults” is a common refrain in pediatric medicine, but when it comes to a condition like juvenile idiopathic arthritis (JIA), rheumatologists might be better off treating pediatric and adult rheumatic disease more similarly.
A recent study published in Arthritis Care & Research followed children diagnosed with JIA for 18 years. Although not the first long-term study to examine children with JIA, it is unique in that it took place “during a time where biologic DMARDs [disease-modifying antirheumatic drugs] were emerging as a fundamental therapy in the management of children with JIA,” said Dawn M. Wahezi, MD, chief of the division of pediatric rheumatology at the Children’s Hospital at Montefiore in New York, who was not involved with the study.
Additionally, the study highlights the International League of Associations for Rheumatology (ILAR) consensus-based classification criteria as an imperfect method to categorize patients with JIA.
Mia Glerup, MD, PhD, of the department of pediatrics at Aarhus (Denmark) University Hospital and colleagues prospectively analyzed 373 patients from Denmark, Norway, Sweden, and Finland with new-onset JIA between 1997 and 2000 and evaluated them at baseline, 8 years, and 18 years. At each visit, the researchers collected data on demographics, disease activity, ILAR category, treatment, and blood samples.
Patients in the cohort were mostly girls (66.7%) with a median age of 5.9 years at onset. Approximately one-third (34.8%) of patients were antinuclear antibody (ANA) positive and 21.6% were HLA-B27 positive. The most common JIA categories at baseline were persistent oligoarthritis (53.9%), polyarticular rheumatoid factor (RF) negative (21.1%), and undifferentiated arthritis (10.2%).
Dr. Glerup and colleagues found that the proportion of patients not receiving DMARDs declined from 73.2% at baseline to 59.7% at 8 years, and then rose again to 70% at 18 years (risk ratio, 1.3; P = .003). The group of 103 patients who used conventional DMARDs (cDMARDs) either as monotherapy or in combination with a biologic DMARD (bDMARD) at 8 years dwindled to 44 (42.7%) at 18 years (RR, 0.4; P < .001), whereas 32 of 52 patients (61.5%) using bDMARDs at 8 years were still taking them at 18 years (RR, 0.6; P = .02). Across the whole study, 14.7% of patients never received any JIA treatment, and 33 of 85 patients (38.8%) on continuous DMARDs developed uveitis during the study period.
Overall, 62.7% of patients received DMARDs at least once, including 89.7% with polyarticular RF negative, 77.3% with oligoarticular extended, 76.9% with systemic, 75.7% with juvenile enthesitis-related arthritis (ERA), 66.7% with polyarticular RF-positive, 65.2% with juvenile psoriatic arthritis (JPsA), 58.9% with undifferentiated JIA, and 27.6% of patients with persistent oligoarticular disease.
The median number of active joints dropped from 3 (range, 1-30) at baseline to 0 at 8 years (range, 0-13), whereas the median cumulative number of affected joints rose from 3 at baseline (range, 1-30) to 6 at 8 years (range, 1-41). At last follow-up, the median number of active joints was 0 (range, 0-5) and median cumulative number of affected joints was 7 (range, 1-47). The percentage of patients in remission barely changed from 52% at 8 years to 51% at 18.
Some patients also changed ILAR categories during the study period, with 7% shifting between baseline and 8 years, and 11% shifting between 8-year and 18-year follow-up. Compared with baseline, by the 18-year follow-up time point there was a significant decrease in the number of patients categorized as oligoarticular (230 vs. 197 patients; P = .02), a significant increase in patients in the psoriatic ILAR category (8 vs. 28 patients; P < .001), and a nonsignificant increase in the number of patients in the undifferentiated category (45 vs. 63 patients; P = .06).
“Almost half of the changes in the distribution between the ILAR categories were caused by updated information on heredity in a first-degree relative obtained at the follow-up visits,” Dr. Glerup and colleagues write.
The results of the long-term study show that patients are “likely to remain in remission – with the converse also evident, as patients still with evidence of disease activity at 8 years after disease onset were more likely to have refractory disease,” Dr. Wahezi said.
Commenting on the study’s findings, Lisa F. Imundo, MD, director of adolescent rheumatology at Columbia University Medical Center in New York, said they are “great news to be able to give parents of young kids with arthritis.” However, she questioned whether the results are generalizable to populations of patients “who are in the worst prognostic group.”
For example, a substantial proportion of patients were classified under the oligoarticular category. “That’s already a group that we know from experience tends to have a better outcome than some of the other groups of JIA,” she said.
“That kind of weaves its way through the whole study, because then they show a lot of patients have come off their medication. Patients who had more severe disease in more joints would be less likely, I think, to just stop their medication and stop going to doctors,” Dr. Imundo explained.
Although the study is valuable for its long-term follow-up, there is also a question of generalizability across a more diverse ethnic and racial group. The authors do not elaborate on the racial breakdown of their patients, Dr. Imundo said, “so we’re going to have to assume that the vast majority are going to [have] Caucasian Nordic ethnic background, and that goes along with them having this high percentage of HLA-B27 positivity, which is a gene that’s more prevalent in northern European populations.”
Jonathan Hausmann, MD, a pediatric and adult rheumatologist at Boston Children’s Hospital, Boston,, told this news organization that he believes the overall conclusions from the study – that JIA persists over time and that ILAR classification is a somewhat imprecise measure of assessing JIA types in children – would be generalizable to other groups.
However, long-term registries evaluating JIA in more diverse populations, such as the Childhood Arthritis and Rheumatology Research Alliance (CARRA) registry, could confirm these results, said Dr. Hausmann, who is a registry informatics associate with CARRA and was not associated with the research.
Long-term management of JIA
In an accompanying editorial, Jaime Guzman, MD, MSc, and Ross E. Petty, MD, PhD, of British Columbia Children’s Hospital and the University of British Columbia, Vancouver, said a rheumatologist’s interpretation of the study would be tied to what they learned about children with arthritis in medical school. They would see the glass as “half full” if children who achieved remission stayed in remission if they learned that a child might end up outgrowing JIA but potentially develop lifelong disability, whereas others may focus on the outcome of approximately half of patients not achieving remission.
“When I was going through medical school, I remember learning that JIA is a disease of children, and typically, they outgrow it as they become adults,” Dr. Hausmann said. “I think this study and many other studies have shown that that’s actually not the case – that, in fact, it may be a majority of kids continue having active disease even through adulthood.”
If a rheumatologist knows JIA is likely to continue into adulthood, “that’s huge,” Dr. Hausmann said. “That means when we first diagnose patients with JIA as kids, we need to set expectations with the families that this may not just go away; this may be something that could be more lifelong.”
Education on the part of the patient, their parents, and their clinician on the expected trajectory of the disease is critical so that children can continue their own care as they transition to adulthood, Dr. Hausmann explained. “The earlier the kids develop the skills to discuss their medicines, their side effects, the better they’ll be able to transition to adult medicine,” he said.
For the patients who go into remission and stay in remission, the message is also important. “To have the reassurance that a lot of those kids won’t be having active joint symptoms or need to be on medication, that’s a huge positive message that can get out there, so I think that’s great,” Dr. Imundo said.
Time to move on from ILAR classification?
Another big takeaway from the study was how patients’ ILAR classification changed across the 18-year follow-up. First proposed in 1995, the JIA ILAR classification has been revised several times for clarification purposes. In its current form, the ILAR classification considers a patient’s history when categorizing JIA types but also includes factors such as immediate family history. This system of assessing JIA has been criticized and there are initiatives to create a new JIA classification system to replace it.
“The ILAR criteria were designed to classify patients 6 months after disease onset in an attempt to find some commonality in clinical phenotypes, prognosis, and suggested management,” Dr. Wahezi said. “While there continues to be debate as to whether we can improve our classification of JIA patients, it is not surprising that phenotypes may evolve over time as new clinical features develop. As pediatric rheumatologists, we are well accustomed to having to modify management plans as children manifest with new clinical features over time.”
Although the percentage of patients who switched ILAR classifications over the study period was “much higher” than she would have thought, Dr. Imundo said it was the reasons provided in the study that seemed odd to her. “The classification scheme relies on your family history, like someone else in your family now has psoriasis, so your arthritis classification changes,” she explained.
“We want to head toward a much more unified classification scheme, a simpler one. We now understand that some of the diseases that we see in pediatrics are really the equivalent or same disease in adults,” she said.
“Most of the pediatric categories of JIA have distinct adult correlates,” Dr. Hausmann agreed. RF-positive polyarthritis in children and rheumatoid arthritis in adults are correlated, as are systemic JIA and adult-onset Still’s disease, he explained. “That has been borne out also by genetic susceptibility studies that the genetic predispositions to systemic arthritis in children is the same as the genetic predisposition to adult-onset Still’s disease in adults. By and large, there are a lot of similarities between the two.
“I think we need to incorporate some of that knowledge in better classifying kids with JIA so that we can find the best treatments and the best outcomes, and we can provide information to families about the expected course of the disease over time so that can inform our discussions.”
Some pediatric rheumatologists accept the classification system is flawed, but not all concur with the degree to which these problems impact patient care. “While the ILAR classification criteria may be subject to criticism, it does provide general context and prognostic implications for patients and families,” Dr. Wahezi said.
“The medicines certainly are very similar across the JIA categories, so the implications are not as broad” when classification changes,” Dr. Hausmann said. “But it certainly shows that there are things that we still don’t know. I think classification is actually pretty important because it might give you a sense of how persistent the disease will be.”
Dr. Imundo said the ILAR classification’s “time is limited,” and rheumatologists may soon need to adopt a new way of classifying children with rheumatic disease – “a more data-driven, genetics-driven scheme.”
“These categories are so imperfect, and the patients are changing. I feel like that says to me, let’s find something that’s more predictive that really helps us a little better than what we have now,” she said.
The study had no specific funding. The authors of the study and the editorial have disclosed no relevant financial relationships. Dr. Hausmann reports receiving salary support from CARRA. Dr. Imundo and Dr. Wahezi have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ARTHRITIS CARE & RESEARCH
Tips for connecting with your patients
It is a tough time to be a doctor. With the stresses of the pandemic, the continued unfettered rise of insurance company BS, and so many medical groups being bought up that we often don’t even know who makes the decisions, the patient can sometimes be hidden in the equation.
Be curious
When physicians are curious about why patients have symptoms, how those symptoms will affect their lives, and how worried the patient is about them, patients feel cared about.
Ascertaining how concerned patients are about their symptoms will help you make decisions on whether symptoms you are not concerned about actually need to be treated.
Limit use of EHRs when possible
Use of the electronic health record during visits is essential, but focusing on it too much can put a barrier between the physician and the patient.
Marmor and colleagues found there is an inverse relationship between time spent on the EHR by a patient’s physician and the patient’s satisfaction.1
Eye contact with the patient is important, especially when patients are sharing concerns they are scared about and upsetting experiences. There can be awkward pauses when looking things up on the EHR. Fill those pauses by explaining to the patient what you are doing, or chatting with the patient.
Consider teaching medical students
When a medical student works with you, it doubles the time the patient gets with a concerned listener. Students also can do a great job with timely follow-up and checking in with worried patients.
By having the student present in the clinic room, with the patient present, the patient can really feel heard. The student shares all the details the patient shared, and now their physician is hearing an organized, thoughtful report of the patients concerns.
In fact, I was involved in a study that showed that patients preferred in room presentations, and that they were more satisfied when students presented in the room.2
Use healing words
Some words carry loaded emotions. The word chronic, for example, has negative connotations, whereas the term persisting does not.
I will often ask patients how long they have been suffering from a symptom to imply my concern for what they are going through. The term “chief complaint” is outdated, and upsets patients when they see it in their medical record.
As a patient of mine once said to me: “I never complained about that problem, I just brought it to your attention.” No one wants to be seen as a complainer. Substituting the word concern for complaint works well.
Explain as you examine
People love to hear the term normal. When you are examining a patient, let them know when findings are normal.
I also find it helpful to explain to patients why I am doing certain physical exam maneuvers. This helps them assess how thorough we are in our thought process.
When patients feel their physicians are thorough, they have more confidence in them.
In summary
- Be curious.
- Do not overly focus on the EHR.
- Consider teaching a medical student.
- Be careful of word choice.
- “Overexplain” the physical exam.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and serves as 3rd-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at [email protected].
References
1. Marmor RA et al. Appl Clin Inform. 2018 Jan;9(1):11-4.
2. Rogers HD et al. Acad Med. 2003 Sep;78(9):945-9.
It is a tough time to be a doctor. With the stresses of the pandemic, the continued unfettered rise of insurance company BS, and so many medical groups being bought up that we often don’t even know who makes the decisions, the patient can sometimes be hidden in the equation.
Be curious
When physicians are curious about why patients have symptoms, how those symptoms will affect their lives, and how worried the patient is about them, patients feel cared about.
Ascertaining how concerned patients are about their symptoms will help you make decisions on whether symptoms you are not concerned about actually need to be treated.
Limit use of EHRs when possible
Use of the electronic health record during visits is essential, but focusing on it too much can put a barrier between the physician and the patient.
Marmor and colleagues found there is an inverse relationship between time spent on the EHR by a patient’s physician and the patient’s satisfaction.1
Eye contact with the patient is important, especially when patients are sharing concerns they are scared about and upsetting experiences. There can be awkward pauses when looking things up on the EHR. Fill those pauses by explaining to the patient what you are doing, or chatting with the patient.
Consider teaching medical students
When a medical student works with you, it doubles the time the patient gets with a concerned listener. Students also can do a great job with timely follow-up and checking in with worried patients.
By having the student present in the clinic room, with the patient present, the patient can really feel heard. The student shares all the details the patient shared, and now their physician is hearing an organized, thoughtful report of the patients concerns.
In fact, I was involved in a study that showed that patients preferred in room presentations, and that they were more satisfied when students presented in the room.2
Use healing words
Some words carry loaded emotions. The word chronic, for example, has negative connotations, whereas the term persisting does not.
I will often ask patients how long they have been suffering from a symptom to imply my concern for what they are going through. The term “chief complaint” is outdated, and upsets patients when they see it in their medical record.
As a patient of mine once said to me: “I never complained about that problem, I just brought it to your attention.” No one wants to be seen as a complainer. Substituting the word concern for complaint works well.
Explain as you examine
People love to hear the term normal. When you are examining a patient, let them know when findings are normal.
I also find it helpful to explain to patients why I am doing certain physical exam maneuvers. This helps them assess how thorough we are in our thought process.
When patients feel their physicians are thorough, they have more confidence in them.
In summary
- Be curious.
- Do not overly focus on the EHR.
- Consider teaching a medical student.
- Be careful of word choice.
- “Overexplain” the physical exam.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and serves as 3rd-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at [email protected].
References
1. Marmor RA et al. Appl Clin Inform. 2018 Jan;9(1):11-4.
2. Rogers HD et al. Acad Med. 2003 Sep;78(9):945-9.
It is a tough time to be a doctor. With the stresses of the pandemic, the continued unfettered rise of insurance company BS, and so many medical groups being bought up that we often don’t even know who makes the decisions, the patient can sometimes be hidden in the equation.
Be curious
When physicians are curious about why patients have symptoms, how those symptoms will affect their lives, and how worried the patient is about them, patients feel cared about.
Ascertaining how concerned patients are about their symptoms will help you make decisions on whether symptoms you are not concerned about actually need to be treated.
Limit use of EHRs when possible
Use of the electronic health record during visits is essential, but focusing on it too much can put a barrier between the physician and the patient.
Marmor and colleagues found there is an inverse relationship between time spent on the EHR by a patient’s physician and the patient’s satisfaction.1
Eye contact with the patient is important, especially when patients are sharing concerns they are scared about and upsetting experiences. There can be awkward pauses when looking things up on the EHR. Fill those pauses by explaining to the patient what you are doing, or chatting with the patient.
Consider teaching medical students
When a medical student works with you, it doubles the time the patient gets with a concerned listener. Students also can do a great job with timely follow-up and checking in with worried patients.
By having the student present in the clinic room, with the patient present, the patient can really feel heard. The student shares all the details the patient shared, and now their physician is hearing an organized, thoughtful report of the patients concerns.
In fact, I was involved in a study that showed that patients preferred in room presentations, and that they were more satisfied when students presented in the room.2
Use healing words
Some words carry loaded emotions. The word chronic, for example, has negative connotations, whereas the term persisting does not.
I will often ask patients how long they have been suffering from a symptom to imply my concern for what they are going through. The term “chief complaint” is outdated, and upsets patients when they see it in their medical record.
As a patient of mine once said to me: “I never complained about that problem, I just brought it to your attention.” No one wants to be seen as a complainer. Substituting the word concern for complaint works well.
Explain as you examine
People love to hear the term normal. When you are examining a patient, let them know when findings are normal.
I also find it helpful to explain to patients why I am doing certain physical exam maneuvers. This helps them assess how thorough we are in our thought process.
When patients feel their physicians are thorough, they have more confidence in them.
In summary
- Be curious.
- Do not overly focus on the EHR.
- Consider teaching a medical student.
- Be careful of word choice.
- “Overexplain” the physical exam.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and serves as 3rd-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at [email protected].
References
1. Marmor RA et al. Appl Clin Inform. 2018 Jan;9(1):11-4.
2. Rogers HD et al. Acad Med. 2003 Sep;78(9):945-9.
A 19-month-old vaccinated female with 2 days of rash
Acute hemorrhagic edema of infancy (AHEI) is a leukocytoclastic vasculitis that typically affects children between 4 months and 2 years of age.1 Etiology is unknown but the majority of cases are preceded by infections, vaccinations, or certain medications.2
AHEI is a self-limited disease that runs a benign course with spontaneous resolution within days to 3 weeks.3 Classic presentation involves acute onset of fever, purpura, ecchymosis, and inflammatory edema. Edema is often the first sign, and may involve the face, ears, scrotum, or extremities. Hemorrhagic lesions may vary in size but often coalesce and present in a distinctive “cockade” or rosette pattern with scalloped borders. Systemic manifestations are rare, but renal and joint involvement may occur.4 Despite the dramatic and sometimes extensive appearance of the dermatologic manifestations, patients with AHEI are usually not in significant distress.
Diagnosis is clinical, but skin biopsy may show leukocytoclastic vasculitis of the superficial small vessels with infiltrations of neutrophils, extravasation of red blood cells, and fibrinoid necrosis.5 In most cases, immunofluorescence is negative for perivascular IgA deposition. Treatment is symptomatic as the disease resolves spontaneously. Recurrence is uncommon but may occur, and usually occurs early.
What is on the differential?
Kawasaki disease. Similar to AHEI, patients with Kawasaki disease also may present with facial and extremity edema. However, patients with Kawasaki disease appear sicker, have associated lymphadenopathy, conjunctivitis, and fever longer than 5 days. The lack of elevated inflammatory markers, acute-onset, classic dermatologic lesions, and nontoxic appearance in our patient rule out Kawasaki disease and make AHEI more likely.
IgA vasculitis/Henoch-Schönlein purpura. The distinction between AHEI and Henoch-Schönlein purpura is among the most challenging. AHEI commonly afflicts younger children ranging from 4 months to 2 years, whereas Henoch-Schönlein purpura occurs in older children from 3 to 6 years of age. Visceral involvement is rare in AHEI, but frequently presents in Henoch-Schönlein purpura with gastrointestinal and renal complications. Although our patient had both mild renal involvement and a distribution primarily on the buttocks and lower limbs, similar to the classic distribution of Henoch-Schönlein purpura, the younger age and lack of gastrointestinal and arthritic manifestations make AHEI more likely.
Gianotti-Crosti syndrome. Gianotti-Crosti syndrome, also known as papulovesicular acrodermatitis of childhood, mainly affects children between the ages of 6 months and 12 years. Like AHEI, Gianotti-Crosti is a self-limiting condition likely triggered by viral infection or immunization. However, Gianotti-Crosti is characterized by a papular rash that may last for several weeks. Neither AHEI nor Gianotti-Crosti are pruritic, but patients with Gianotti-Crosti tend to have either inguinal or axillary lymphadenopathy. Our patient’s large, coalescing dusky red patches and edematous plaques without lymphadenopathy are more consistent with AHEI.
Erythema multiforme. Erythema multiforme is an acute, immune-mediated condition characterized by distinctive target-like lesions on the skin often accompanied by erosions or bullae. Unlike AHEI, erythema multiforme can involve the oral, genital, and/or ocular mucosae. Erythema multiforme is rare before the age of 4 years. Although the targetoid or annular purpuric configuration of erythema multiforme may present similarly to AHEI in some cases, the young age of our patient and the lack of mucosal involvement make AHEI more likely.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. Ms. Kleinman is a pediatric dermatology research associate at the University of California, San Diego, and Rady Children’s Hospital, San Diego. Neither Dr. Matiz nor Ms. Kleinman has any relevant financial disclosures.
References
1. Savino F et al. Pediatr Dermatol. 2013;30(6):e149-e152.
2. Carboni E et al. F1000Res. 2019;8:1771. 2019 Oct 17.
3. Fiore E et al. J Am Acad Dermatol. 2008;59(4):684-95.
4. Watanabe T and Sato Y. Pediatr Nephrol. 2007;22(11):1979-81.
5. Cunha DF et al. Autops Case Rep. 2015;5(3):37-41.
Acute hemorrhagic edema of infancy (AHEI) is a leukocytoclastic vasculitis that typically affects children between 4 months and 2 years of age.1 Etiology is unknown but the majority of cases are preceded by infections, vaccinations, or certain medications.2
AHEI is a self-limited disease that runs a benign course with spontaneous resolution within days to 3 weeks.3 Classic presentation involves acute onset of fever, purpura, ecchymosis, and inflammatory edema. Edema is often the first sign, and may involve the face, ears, scrotum, or extremities. Hemorrhagic lesions may vary in size but often coalesce and present in a distinctive “cockade” or rosette pattern with scalloped borders. Systemic manifestations are rare, but renal and joint involvement may occur.4 Despite the dramatic and sometimes extensive appearance of the dermatologic manifestations, patients with AHEI are usually not in significant distress.
Diagnosis is clinical, but skin biopsy may show leukocytoclastic vasculitis of the superficial small vessels with infiltrations of neutrophils, extravasation of red blood cells, and fibrinoid necrosis.5 In most cases, immunofluorescence is negative for perivascular IgA deposition. Treatment is symptomatic as the disease resolves spontaneously. Recurrence is uncommon but may occur, and usually occurs early.
What is on the differential?
Kawasaki disease. Similar to AHEI, patients with Kawasaki disease also may present with facial and extremity edema. However, patients with Kawasaki disease appear sicker, have associated lymphadenopathy, conjunctivitis, and fever longer than 5 days. The lack of elevated inflammatory markers, acute-onset, classic dermatologic lesions, and nontoxic appearance in our patient rule out Kawasaki disease and make AHEI more likely.
IgA vasculitis/Henoch-Schönlein purpura. The distinction between AHEI and Henoch-Schönlein purpura is among the most challenging. AHEI commonly afflicts younger children ranging from 4 months to 2 years, whereas Henoch-Schönlein purpura occurs in older children from 3 to 6 years of age. Visceral involvement is rare in AHEI, but frequently presents in Henoch-Schönlein purpura with gastrointestinal and renal complications. Although our patient had both mild renal involvement and a distribution primarily on the buttocks and lower limbs, similar to the classic distribution of Henoch-Schönlein purpura, the younger age and lack of gastrointestinal and arthritic manifestations make AHEI more likely.
Gianotti-Crosti syndrome. Gianotti-Crosti syndrome, also known as papulovesicular acrodermatitis of childhood, mainly affects children between the ages of 6 months and 12 years. Like AHEI, Gianotti-Crosti is a self-limiting condition likely triggered by viral infection or immunization. However, Gianotti-Crosti is characterized by a papular rash that may last for several weeks. Neither AHEI nor Gianotti-Crosti are pruritic, but patients with Gianotti-Crosti tend to have either inguinal or axillary lymphadenopathy. Our patient’s large, coalescing dusky red patches and edematous plaques without lymphadenopathy are more consistent with AHEI.
Erythema multiforme. Erythema multiforme is an acute, immune-mediated condition characterized by distinctive target-like lesions on the skin often accompanied by erosions or bullae. Unlike AHEI, erythema multiforme can involve the oral, genital, and/or ocular mucosae. Erythema multiforme is rare before the age of 4 years. Although the targetoid or annular purpuric configuration of erythema multiforme may present similarly to AHEI in some cases, the young age of our patient and the lack of mucosal involvement make AHEI more likely.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. Ms. Kleinman is a pediatric dermatology research associate at the University of California, San Diego, and Rady Children’s Hospital, San Diego. Neither Dr. Matiz nor Ms. Kleinman has any relevant financial disclosures.
References
1. Savino F et al. Pediatr Dermatol. 2013;30(6):e149-e152.
2. Carboni E et al. F1000Res. 2019;8:1771. 2019 Oct 17.
3. Fiore E et al. J Am Acad Dermatol. 2008;59(4):684-95.
4. Watanabe T and Sato Y. Pediatr Nephrol. 2007;22(11):1979-81.
5. Cunha DF et al. Autops Case Rep. 2015;5(3):37-41.
Acute hemorrhagic edema of infancy (AHEI) is a leukocytoclastic vasculitis that typically affects children between 4 months and 2 years of age.1 Etiology is unknown but the majority of cases are preceded by infections, vaccinations, or certain medications.2
AHEI is a self-limited disease that runs a benign course with spontaneous resolution within days to 3 weeks.3 Classic presentation involves acute onset of fever, purpura, ecchymosis, and inflammatory edema. Edema is often the first sign, and may involve the face, ears, scrotum, or extremities. Hemorrhagic lesions may vary in size but often coalesce and present in a distinctive “cockade” or rosette pattern with scalloped borders. Systemic manifestations are rare, but renal and joint involvement may occur.4 Despite the dramatic and sometimes extensive appearance of the dermatologic manifestations, patients with AHEI are usually not in significant distress.
Diagnosis is clinical, but skin biopsy may show leukocytoclastic vasculitis of the superficial small vessels with infiltrations of neutrophils, extravasation of red blood cells, and fibrinoid necrosis.5 In most cases, immunofluorescence is negative for perivascular IgA deposition. Treatment is symptomatic as the disease resolves spontaneously. Recurrence is uncommon but may occur, and usually occurs early.
What is on the differential?
Kawasaki disease. Similar to AHEI, patients with Kawasaki disease also may present with facial and extremity edema. However, patients with Kawasaki disease appear sicker, have associated lymphadenopathy, conjunctivitis, and fever longer than 5 days. The lack of elevated inflammatory markers, acute-onset, classic dermatologic lesions, and nontoxic appearance in our patient rule out Kawasaki disease and make AHEI more likely.
IgA vasculitis/Henoch-Schönlein purpura. The distinction between AHEI and Henoch-Schönlein purpura is among the most challenging. AHEI commonly afflicts younger children ranging from 4 months to 2 years, whereas Henoch-Schönlein purpura occurs in older children from 3 to 6 years of age. Visceral involvement is rare in AHEI, but frequently presents in Henoch-Schönlein purpura with gastrointestinal and renal complications. Although our patient had both mild renal involvement and a distribution primarily on the buttocks and lower limbs, similar to the classic distribution of Henoch-Schönlein purpura, the younger age and lack of gastrointestinal and arthritic manifestations make AHEI more likely.
Gianotti-Crosti syndrome. Gianotti-Crosti syndrome, also known as papulovesicular acrodermatitis of childhood, mainly affects children between the ages of 6 months and 12 years. Like AHEI, Gianotti-Crosti is a self-limiting condition likely triggered by viral infection or immunization. However, Gianotti-Crosti is characterized by a papular rash that may last for several weeks. Neither AHEI nor Gianotti-Crosti are pruritic, but patients with Gianotti-Crosti tend to have either inguinal or axillary lymphadenopathy. Our patient’s large, coalescing dusky red patches and edematous plaques without lymphadenopathy are more consistent with AHEI.
Erythema multiforme. Erythema multiforme is an acute, immune-mediated condition characterized by distinctive target-like lesions on the skin often accompanied by erosions or bullae. Unlike AHEI, erythema multiforme can involve the oral, genital, and/or ocular mucosae. Erythema multiforme is rare before the age of 4 years. Although the targetoid or annular purpuric configuration of erythema multiforme may present similarly to AHEI in some cases, the young age of our patient and the lack of mucosal involvement make AHEI more likely.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. Ms. Kleinman is a pediatric dermatology research associate at the University of California, San Diego, and Rady Children’s Hospital, San Diego. Neither Dr. Matiz nor Ms. Kleinman has any relevant financial disclosures.
References
1. Savino F et al. Pediatr Dermatol. 2013;30(6):e149-e152.
2. Carboni E et al. F1000Res. 2019;8:1771. 2019 Oct 17.
3. Fiore E et al. J Am Acad Dermatol. 2008;59(4):684-95.
4. Watanabe T and Sato Y. Pediatr Nephrol. 2007;22(11):1979-81.
5. Cunha DF et al. Autops Case Rep. 2015;5(3):37-41.
Children and COVID: Weekly cases down by more than half
A third consecutive week of declines in new COVID-19 cases among children has brought the weekly count down by 74% since the Omicron surge peaked in mid-January, based on data from the American Academy of Pediatrics and the Children’s Hospital Association.
and by 74% from the peak of 1.15 million cases recorded for the week of Jan. 14-20, the AAP and CHA said in their weekly COVID report. They also noted that the weekly tally was still higher than anything seen during the Delta surge.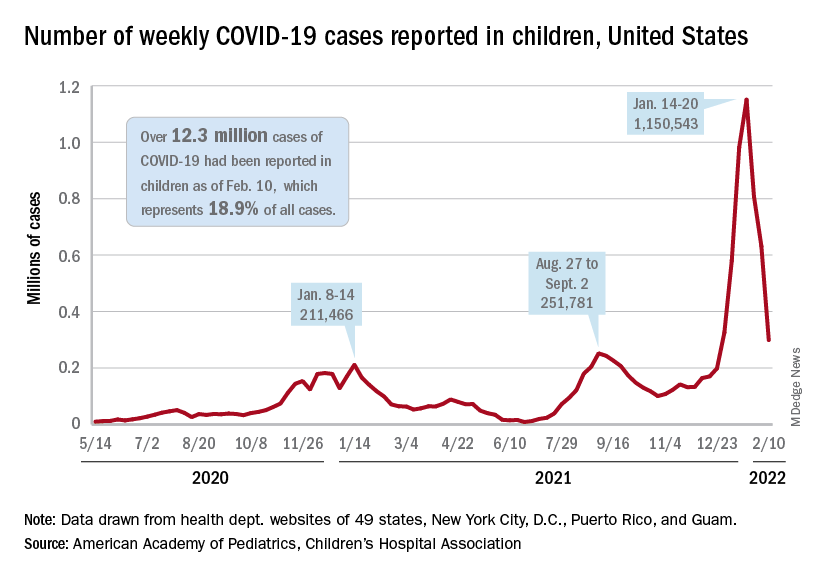
The total number of pediatric cases was over 12.3 million as of Feb. 10, with children representing 18.9% of cases in all ages, according to the AAP/CHA report. The Centers for Disease Control and Prevention puts the two measures at 10.4 million and 17.3% on its COVID Data Tracker, based on availability of age data for 59.6 million total cases as of Feb. 14. The CDC also reported that 1,282 children have died from COVID-19 so far, which is about 0.17% of all deaths with age data available.
The AAP and CHA have been collecting data from state and territorial health departments, which have not always been consistently available over the course of the pandemic. Also, the CDC defines children as those under age 18 years, but that upper boundary varies from 14 to 20 among the states.
The decline of the Omicron variant also can be seen in new admissions of children with confirmed COVID-19, which continued to drop. The 7-day average of 435 admissions per day for the week of Feb. 6-12 was less than half of the peak seen in mid-January, when it reached 914 per day. The daily admission rate on Feb. 12 was 0.60 per 100,000 children aged 0-17 years – again, less than half the peak rate of 1.25 reported on Jan. 16, CDC data show.
The fading threat of Omicron also seems to be reflected in recent vaccination trends. Both initial doses and completions declined for the fourth consecutive week (Feb. 3-9) among children aged 5-11 years, while initiations held steady for 12- to 17-year-olds but completions declined for the third straight week, the AAP said in its separate vaccination report, which is based on data from the CDC.
As of Feb. 14, almost 32% of children aged 5-11 – that’s almost 9.2 million individuals – had received at least one dose of the COVID-19 vaccine and just over 24% (6.9 million) were fully vaccinated, the CDC reported. For children aged 12-17, the corresponding figures are 67% (16.9 million) and 57% (14.4 million). Newly available data from the CDC also indicate that 19.5% (2.8 million) of children aged 12-17 have received a booster dose.
A third consecutive week of declines in new COVID-19 cases among children has brought the weekly count down by 74% since the Omicron surge peaked in mid-January, based on data from the American Academy of Pediatrics and the Children’s Hospital Association.
and by 74% from the peak of 1.15 million cases recorded for the week of Jan. 14-20, the AAP and CHA said in their weekly COVID report. They also noted that the weekly tally was still higher than anything seen during the Delta surge.
The total number of pediatric cases was over 12.3 million as of Feb. 10, with children representing 18.9% of cases in all ages, according to the AAP/CHA report. The Centers for Disease Control and Prevention puts the two measures at 10.4 million and 17.3% on its COVID Data Tracker, based on availability of age data for 59.6 million total cases as of Feb. 14. The CDC also reported that 1,282 children have died from COVID-19 so far, which is about 0.17% of all deaths with age data available.
The AAP and CHA have been collecting data from state and territorial health departments, which have not always been consistently available over the course of the pandemic. Also, the CDC defines children as those under age 18 years, but that upper boundary varies from 14 to 20 among the states.
The decline of the Omicron variant also can be seen in new admissions of children with confirmed COVID-19, which continued to drop. The 7-day average of 435 admissions per day for the week of Feb. 6-12 was less than half of the peak seen in mid-January, when it reached 914 per day. The daily admission rate on Feb. 12 was 0.60 per 100,000 children aged 0-17 years – again, less than half the peak rate of 1.25 reported on Jan. 16, CDC data show.
The fading threat of Omicron also seems to be reflected in recent vaccination trends. Both initial doses and completions declined for the fourth consecutive week (Feb. 3-9) among children aged 5-11 years, while initiations held steady for 12- to 17-year-olds but completions declined for the third straight week, the AAP said in its separate vaccination report, which is based on data from the CDC.
As of Feb. 14, almost 32% of children aged 5-11 – that’s almost 9.2 million individuals – had received at least one dose of the COVID-19 vaccine and just over 24% (6.9 million) were fully vaccinated, the CDC reported. For children aged 12-17, the corresponding figures are 67% (16.9 million) and 57% (14.4 million). Newly available data from the CDC also indicate that 19.5% (2.8 million) of children aged 12-17 have received a booster dose.
A third consecutive week of declines in new COVID-19 cases among children has brought the weekly count down by 74% since the Omicron surge peaked in mid-January, based on data from the American Academy of Pediatrics and the Children’s Hospital Association.
and by 74% from the peak of 1.15 million cases recorded for the week of Jan. 14-20, the AAP and CHA said in their weekly COVID report. They also noted that the weekly tally was still higher than anything seen during the Delta surge.
The total number of pediatric cases was over 12.3 million as of Feb. 10, with children representing 18.9% of cases in all ages, according to the AAP/CHA report. The Centers for Disease Control and Prevention puts the two measures at 10.4 million and 17.3% on its COVID Data Tracker, based on availability of age data for 59.6 million total cases as of Feb. 14. The CDC also reported that 1,282 children have died from COVID-19 so far, which is about 0.17% of all deaths with age data available.
The AAP and CHA have been collecting data from state and territorial health departments, which have not always been consistently available over the course of the pandemic. Also, the CDC defines children as those under age 18 years, but that upper boundary varies from 14 to 20 among the states.
The decline of the Omicron variant also can be seen in new admissions of children with confirmed COVID-19, which continued to drop. The 7-day average of 435 admissions per day for the week of Feb. 6-12 was less than half of the peak seen in mid-January, when it reached 914 per day. The daily admission rate on Feb. 12 was 0.60 per 100,000 children aged 0-17 years – again, less than half the peak rate of 1.25 reported on Jan. 16, CDC data show.
The fading threat of Omicron also seems to be reflected in recent vaccination trends. Both initial doses and completions declined for the fourth consecutive week (Feb. 3-9) among children aged 5-11 years, while initiations held steady for 12- to 17-year-olds but completions declined for the third straight week, the AAP said in its separate vaccination report, which is based on data from the CDC.
As of Feb. 14, almost 32% of children aged 5-11 – that’s almost 9.2 million individuals – had received at least one dose of the COVID-19 vaccine and just over 24% (6.9 million) were fully vaccinated, the CDC reported. For children aged 12-17, the corresponding figures are 67% (16.9 million) and 57% (14.4 million). Newly available data from the CDC also indicate that 19.5% (2.8 million) of children aged 12-17 have received a booster dose.
Growth hormone therapy for certain children may help them reach their potential
“Dr. Lilley, you’ll always be my favorite doctor; you helped me grow.”
These were the parting words from the last patient that I treated during my endocrinology fellowship. I had watched this young man grow from a prepubertal 17-year-old to a young man who had reached his predicted family height as I treated his delayed puberty caused by Kallmann syndrome, a problem that had been missed for years. It was the appropriate bookend for my chosen specialty.
Watching children grow and develop into who they were meant to be is one of my favorite things about endocrinology, as well as forming meaningful relationships with families. Treating detectable deficiencies in logical and measurable ways is also extremely satisfying.
Too little testosterone? That’s a problem I can solve. Too much thyroid hormone? There’s a blocker for that! Endocrinology can be a straightforward field, and when all goes well, everyone leaves happy.
Except when they don’t.
Gatekeepers for treatment for children’s growth
“Nice to meet you. We’re here to get growth hormone.”
“We’re here because his pediatrician made us come. We’ve already decided we’re not going to put hormones into his body.”
These are common statements I hear when I first meet new patients whose parents are concerned about their children’s growth. Pediatric endocrinologists, after all, are the usual gatekeepers for this treatment.
Growth hormone (GH) often makes the news for controversial reasons – most commonly for its abuse by elite athletes hoping to exploit its anabolic effects – causing parents to have varied opinions about its possible use in their children.
Some refuse endocrinology referrals at all owing to concerns that we will push daily injections on their children. Others demand referrals for their children of average height, hoping for every perceived advantage.
GH deficiency (GHD) – a condition where the pituitary gland fails to produce enough GH – can occur because of congenital pituitary malformations; anatomic, surgical, or traumatic interruptions to the gland; or enzyme deficiencies leading to faulty production.
GHD is just one reason for poor growth, however.
Growth is one of the most important indicators of health in children. A waning growth rate may be an early symptom of serious problems. In my clinic, I’ve diagnosed severe hypothyroidism in a marathon runner, a brain tumor, celiac disease in a teenager with no gastrointestinal complaints, autoimmune hepatitis, and several other diseases needing treatment in children who show no symptoms other than poor growth.
Barriers to normal growth
Sometimes, the die is cast for children to have barriers to normal growth. Several genetic conditions can lead to poor GH production or response, and GH treatment is often necessary to approximate normal height.
These may include:
- Turner syndrome (in females who are missing an X chromosome in whole or part) should be considered in every girl with abnormally short stature; mosaic forms of the condition may be subtle and lack classic features.
- Noonan syndrome is important to detect owing to the possibility of cardiac or renal malformations that may also occur in this condition, caused by a mutation in one of the genes in the RAS-MAPK pathway.
- Russell-Silver syndrome can cause intrauterine GH restriction and has been traced to uniparental disomy of chromosome 7 or duplications, mutations, or methylation defects in chromosome 11.
- Individuals with Prader-Willi syndrome, which is characterized by low muscle tone, hyperphagia, and hypogonadism, have demonstrated dramatic benefits from GH therapy, primarily in maintaining a normal body mass index.
Children who are small for their gestational age may be GH resistant, and those who do not catch up to their growth curve by the age of 2 years may require GH treatment to reach their height potential.
GH therapy isn’t entirely benign. Rare adverse effects of overtreatment can include slipped capital femoral epiphysis (a fracture to the growth plate) and pseudotumor cerebri (idiopathic intracranial hypertension).
Overtreatment can cause acromegaly, which results in coarsened features and large hands and feet.
When is GH therapy warranted?
“Growth hormone therapy has been denied by her insurer. They want you to fill out an appeal.”
Insurance approval in the United States can be a herculean effort because GH is expensive: Out-of-pocket costs are prohibitive for most people without insurance assistance, ranging from $7,000 to $30,000 annually.
Pediatric endocrinologists aren’t in the business of cosmetic endocrinology. Treatment of idiopathic short stature has been controversial since this became an indication for GH treatment.
GH isn’t always necessary. Diagnosing the underlying cause for poor growth is the most important step.
Often, we find constitutional delay of growth and puberty, or “late bloomers.” This condition is characterized by a delayed bone age (growth plates more open than expected for age) and delayed pubertal onset. These children will often reach a normal height despite starting as some of the smallest of their peers.
However, GH plays other roles in the body than simply propelling height. Children with congenital GHD will require GH treatment to prevent hypoglycemia, especially in infancy.
GH is needed even in adults with fused growth plates for normal lipid metabolism, bone accrual, and maintaining normal muscle mass.
I have noticed marked improvements in muscle tone in many children with developmental delays who are treated with GH, and research supports cognitive benefits for certain populations.
The most common regimens for GH focus on treatment via subcutaneous injection nightly, when GH is naturally produced; sometimes, injections are given six nights out of seven to provide a break or for splitting time between households.
Newer once-weekly formulations have recently received approval, as reported by this news organization, and are coming into use.
Pediatric endocrinologists measure height and follow growth factors closely with visits every 3-6 months. GH levels are not useful outside of provocative diagnostic (stimulation) testing.
Insulinlike growth factor 1 or insulinlike growth factor binding protein levels are analyzed per Tanner stage of puberty to assess appropriate response and to make dose adjustments.
Annual standardized films of the left hand help predict progress and anticipated adult height. Treatment usually persists through puberty until growth plates are closed; if true GHD is noticed, much smaller doses are continued through adulthood.
Regardless, conversations about GH happen with your friendly local pediatric endocrinologist.
We are thrilled to help shepherd patients through their growing age to meet their potential. For more information about GH treatment for children, the MAGIC Foundation is the perfect place to start.
Dr. Lilley is director of the pediatric diabetes and lipid program, Mississippi Center for Advanced Medicine, Madison. She disclosed no relevant conflicts of interest. A version of this article first appeared on Medscape.com.
“Dr. Lilley, you’ll always be my favorite doctor; you helped me grow.”
These were the parting words from the last patient that I treated during my endocrinology fellowship. I had watched this young man grow from a prepubertal 17-year-old to a young man who had reached his predicted family height as I treated his delayed puberty caused by Kallmann syndrome, a problem that had been missed for years. It was the appropriate bookend for my chosen specialty.
Watching children grow and develop into who they were meant to be is one of my favorite things about endocrinology, as well as forming meaningful relationships with families. Treating detectable deficiencies in logical and measurable ways is also extremely satisfying.
Too little testosterone? That’s a problem I can solve. Too much thyroid hormone? There’s a blocker for that! Endocrinology can be a straightforward field, and when all goes well, everyone leaves happy.
Except when they don’t.
Gatekeepers for treatment for children’s growth
“Nice to meet you. We’re here to get growth hormone.”
“We’re here because his pediatrician made us come. We’ve already decided we’re not going to put hormones into his body.”
These are common statements I hear when I first meet new patients whose parents are concerned about their children’s growth. Pediatric endocrinologists, after all, are the usual gatekeepers for this treatment.
Growth hormone (GH) often makes the news for controversial reasons – most commonly for its abuse by elite athletes hoping to exploit its anabolic effects – causing parents to have varied opinions about its possible use in their children.
Some refuse endocrinology referrals at all owing to concerns that we will push daily injections on their children. Others demand referrals for their children of average height, hoping for every perceived advantage.
GH deficiency (GHD) – a condition where the pituitary gland fails to produce enough GH – can occur because of congenital pituitary malformations; anatomic, surgical, or traumatic interruptions to the gland; or enzyme deficiencies leading to faulty production.
GHD is just one reason for poor growth, however.
Growth is one of the most important indicators of health in children. A waning growth rate may be an early symptom of serious problems. In my clinic, I’ve diagnosed severe hypothyroidism in a marathon runner, a brain tumor, celiac disease in a teenager with no gastrointestinal complaints, autoimmune hepatitis, and several other diseases needing treatment in children who show no symptoms other than poor growth.
Barriers to normal growth
Sometimes, the die is cast for children to have barriers to normal growth. Several genetic conditions can lead to poor GH production or response, and GH treatment is often necessary to approximate normal height.
These may include:
- Turner syndrome (in females who are missing an X chromosome in whole or part) should be considered in every girl with abnormally short stature; mosaic forms of the condition may be subtle and lack classic features.
- Noonan syndrome is important to detect owing to the possibility of cardiac or renal malformations that may also occur in this condition, caused by a mutation in one of the genes in the RAS-MAPK pathway.
- Russell-Silver syndrome can cause intrauterine GH restriction and has been traced to uniparental disomy of chromosome 7 or duplications, mutations, or methylation defects in chromosome 11.
- Individuals with Prader-Willi syndrome, which is characterized by low muscle tone, hyperphagia, and hypogonadism, have demonstrated dramatic benefits from GH therapy, primarily in maintaining a normal body mass index.
Children who are small for their gestational age may be GH resistant, and those who do not catch up to their growth curve by the age of 2 years may require GH treatment to reach their height potential.
GH therapy isn’t entirely benign. Rare adverse effects of overtreatment can include slipped capital femoral epiphysis (a fracture to the growth plate) and pseudotumor cerebri (idiopathic intracranial hypertension).
Overtreatment can cause acromegaly, which results in coarsened features and large hands and feet.
When is GH therapy warranted?
“Growth hormone therapy has been denied by her insurer. They want you to fill out an appeal.”
Insurance approval in the United States can be a herculean effort because GH is expensive: Out-of-pocket costs are prohibitive for most people without insurance assistance, ranging from $7,000 to $30,000 annually.
Pediatric endocrinologists aren’t in the business of cosmetic endocrinology. Treatment of idiopathic short stature has been controversial since this became an indication for GH treatment.
GH isn’t always necessary. Diagnosing the underlying cause for poor growth is the most important step.
Often, we find constitutional delay of growth and puberty, or “late bloomers.” This condition is characterized by a delayed bone age (growth plates more open than expected for age) and delayed pubertal onset. These children will often reach a normal height despite starting as some of the smallest of their peers.
However, GH plays other roles in the body than simply propelling height. Children with congenital GHD will require GH treatment to prevent hypoglycemia, especially in infancy.
GH is needed even in adults with fused growth plates for normal lipid metabolism, bone accrual, and maintaining normal muscle mass.
I have noticed marked improvements in muscle tone in many children with developmental delays who are treated with GH, and research supports cognitive benefits for certain populations.
The most common regimens for GH focus on treatment via subcutaneous injection nightly, when GH is naturally produced; sometimes, injections are given six nights out of seven to provide a break or for splitting time between households.
Newer once-weekly formulations have recently received approval, as reported by this news organization, and are coming into use.
Pediatric endocrinologists measure height and follow growth factors closely with visits every 3-6 months. GH levels are not useful outside of provocative diagnostic (stimulation) testing.
Insulinlike growth factor 1 or insulinlike growth factor binding protein levels are analyzed per Tanner stage of puberty to assess appropriate response and to make dose adjustments.
Annual standardized films of the left hand help predict progress and anticipated adult height. Treatment usually persists through puberty until growth plates are closed; if true GHD is noticed, much smaller doses are continued through adulthood.
Regardless, conversations about GH happen with your friendly local pediatric endocrinologist.
We are thrilled to help shepherd patients through their growing age to meet their potential. For more information about GH treatment for children, the MAGIC Foundation is the perfect place to start.
Dr. Lilley is director of the pediatric diabetes and lipid program, Mississippi Center for Advanced Medicine, Madison. She disclosed no relevant conflicts of interest. A version of this article first appeared on Medscape.com.
“Dr. Lilley, you’ll always be my favorite doctor; you helped me grow.”
These were the parting words from the last patient that I treated during my endocrinology fellowship. I had watched this young man grow from a prepubertal 17-year-old to a young man who had reached his predicted family height as I treated his delayed puberty caused by Kallmann syndrome, a problem that had been missed for years. It was the appropriate bookend for my chosen specialty.
Watching children grow and develop into who they were meant to be is one of my favorite things about endocrinology, as well as forming meaningful relationships with families. Treating detectable deficiencies in logical and measurable ways is also extremely satisfying.
Too little testosterone? That’s a problem I can solve. Too much thyroid hormone? There’s a blocker for that! Endocrinology can be a straightforward field, and when all goes well, everyone leaves happy.
Except when they don’t.
Gatekeepers for treatment for children’s growth
“Nice to meet you. We’re here to get growth hormone.”
“We’re here because his pediatrician made us come. We’ve already decided we’re not going to put hormones into his body.”
These are common statements I hear when I first meet new patients whose parents are concerned about their children’s growth. Pediatric endocrinologists, after all, are the usual gatekeepers for this treatment.
Growth hormone (GH) often makes the news for controversial reasons – most commonly for its abuse by elite athletes hoping to exploit its anabolic effects – causing parents to have varied opinions about its possible use in their children.
Some refuse endocrinology referrals at all owing to concerns that we will push daily injections on their children. Others demand referrals for their children of average height, hoping for every perceived advantage.
GH deficiency (GHD) – a condition where the pituitary gland fails to produce enough GH – can occur because of congenital pituitary malformations; anatomic, surgical, or traumatic interruptions to the gland; or enzyme deficiencies leading to faulty production.
GHD is just one reason for poor growth, however.
Growth is one of the most important indicators of health in children. A waning growth rate may be an early symptom of serious problems. In my clinic, I’ve diagnosed severe hypothyroidism in a marathon runner, a brain tumor, celiac disease in a teenager with no gastrointestinal complaints, autoimmune hepatitis, and several other diseases needing treatment in children who show no symptoms other than poor growth.
Barriers to normal growth
Sometimes, the die is cast for children to have barriers to normal growth. Several genetic conditions can lead to poor GH production or response, and GH treatment is often necessary to approximate normal height.
These may include:
- Turner syndrome (in females who are missing an X chromosome in whole or part) should be considered in every girl with abnormally short stature; mosaic forms of the condition may be subtle and lack classic features.
- Noonan syndrome is important to detect owing to the possibility of cardiac or renal malformations that may also occur in this condition, caused by a mutation in one of the genes in the RAS-MAPK pathway.
- Russell-Silver syndrome can cause intrauterine GH restriction and has been traced to uniparental disomy of chromosome 7 or duplications, mutations, or methylation defects in chromosome 11.
- Individuals with Prader-Willi syndrome, which is characterized by low muscle tone, hyperphagia, and hypogonadism, have demonstrated dramatic benefits from GH therapy, primarily in maintaining a normal body mass index.
Children who are small for their gestational age may be GH resistant, and those who do not catch up to their growth curve by the age of 2 years may require GH treatment to reach their height potential.
GH therapy isn’t entirely benign. Rare adverse effects of overtreatment can include slipped capital femoral epiphysis (a fracture to the growth plate) and pseudotumor cerebri (idiopathic intracranial hypertension).
Overtreatment can cause acromegaly, which results in coarsened features and large hands and feet.
When is GH therapy warranted?
“Growth hormone therapy has been denied by her insurer. They want you to fill out an appeal.”
Insurance approval in the United States can be a herculean effort because GH is expensive: Out-of-pocket costs are prohibitive for most people without insurance assistance, ranging from $7,000 to $30,000 annually.
Pediatric endocrinologists aren’t in the business of cosmetic endocrinology. Treatment of idiopathic short stature has been controversial since this became an indication for GH treatment.
GH isn’t always necessary. Diagnosing the underlying cause for poor growth is the most important step.
Often, we find constitutional delay of growth and puberty, or “late bloomers.” This condition is characterized by a delayed bone age (growth plates more open than expected for age) and delayed pubertal onset. These children will often reach a normal height despite starting as some of the smallest of their peers.
However, GH plays other roles in the body than simply propelling height. Children with congenital GHD will require GH treatment to prevent hypoglycemia, especially in infancy.
GH is needed even in adults with fused growth plates for normal lipid metabolism, bone accrual, and maintaining normal muscle mass.
I have noticed marked improvements in muscle tone in many children with developmental delays who are treated with GH, and research supports cognitive benefits for certain populations.
The most common regimens for GH focus on treatment via subcutaneous injection nightly, when GH is naturally produced; sometimes, injections are given six nights out of seven to provide a break or for splitting time between households.
Newer once-weekly formulations have recently received approval, as reported by this news organization, and are coming into use.
Pediatric endocrinologists measure height and follow growth factors closely with visits every 3-6 months. GH levels are not useful outside of provocative diagnostic (stimulation) testing.
Insulinlike growth factor 1 or insulinlike growth factor binding protein levels are analyzed per Tanner stage of puberty to assess appropriate response and to make dose adjustments.
Annual standardized films of the left hand help predict progress and anticipated adult height. Treatment usually persists through puberty until growth plates are closed; if true GHD is noticed, much smaller doses are continued through adulthood.
Regardless, conversations about GH happen with your friendly local pediatric endocrinologist.
We are thrilled to help shepherd patients through their growing age to meet their potential. For more information about GH treatment for children, the MAGIC Foundation is the perfect place to start.
Dr. Lilley is director of the pediatric diabetes and lipid program, Mississippi Center for Advanced Medicine, Madison. She disclosed no relevant conflicts of interest. A version of this article first appeared on Medscape.com.