User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Ultra-processed: Doctors debate whether putting this label on foods is useful
The NOVA system divides foods into “fresh or minimally processed,” such as strawberries or steel-cut oats; “processed culinary ingredients,” such as olive oil; “processed foods,” such as cheeses; and “ultra-processed foods.” UPFs are defined as “industrial formulations made by deconstructing natural food into its chemical constituents, modifying them and recombining them with additives into products liable to displace all other NOVA food groups.”
According to doctors who presented during the meeting, ultra-processed foods are drawing increased attention, because researchers have been examining them in National Institutes of Health–funded studies and journalists have been writing about them.
During the debate session at the meeting, some experts said that, with obesity and poor health skyrocketing, increased awareness and labeling of UPFs can only be a good thing. In contrast others noted at the meeting that the classification system that has come to be used for identifying UPFs – the NOVA Food Classification system – is too mushy, confusing, and, ultimately unhelpful.
Carlos Monteiro, MD, PhD, professor of nutrition and public health at the University of Sao Paolo, was part of the group favoring the NOVA system’s classifying certain foods as UPFs, during the debate. He drew attention to the extent to which the world’s population is getting its calories from UPFs.
Mexico and France get about 30% of calories from these foods. In Canada, it’s 48%. And in the United States, it’s 57%, Dr. Monteiro said.
Studies have found that UPFs, many of which are designed to be exceedingly flavorful and intended to replace consumption of unprocessed whole foods, lead to more overall energy intake, more added sugar in the diet, and less fiber and protein intake, he said.
To further support his arguments, Dr. Monteiro pointed to studies suggesting that it is not just the resulting change in the nutritional intake that is unhealthy, but the UPF manufacturing process itself. When adjusting for fat, sugar, and sodium intake, for example, health outcomes associated with UPFs remain poor, he explained.
“I’m sorry,” he said in the debate. “If you don’t reduce this, you don’t reduce your obesity, your diabetes prevalence.”
A study presented by Jacqueline Vernarelli, PhD, during a different session at the meeting suggested there may be other downsides to consuming UPFs. This research, which was based on the U.S. National Youth Fitness Survey, found that poorer locomotor skills among children aged 3-5 and poorer cardiovascular fitness among those aged 12-15 were associated with getting more calories from UPFs.
Those with lower cardiovascular fitness consumed 1,234 calories a day from UPFs, and those with higher cardiovascular fitness consumed 1,007 calories a day from UPFs (P = .002), according to the new research.
“It’s notable here that, although these differences are significant, both groups are consuming a pretty high proportion of their diet from ultra-processed foods,” said Dr. Vernarelli, associate professor of public health at Sacred Heart University, Fairfield, Conn., during her presentation.
In the debate session, Arne Astrup, MD, PhD, senior project director at the Healthy Weight Center at the Novo Nordisk Foundation, Hellerup, Denmark, presented an opposing view.
He said the definition of UPFs makes it too difficult to categorize many foods, pointing to a study from this year in which about 150 nutrition experts, doctors, and dietitians classified 120 foods. Only three marketed foods and one generic food were classified the same by all the evaluators.
Referring to the study Dr. Astrup cited, Dr. Monteiro said it was a mere “exercise,” and the experts involved in it had conflicts of interest.
Dr. Astrup touted this study’s size and its appearance in the peer-reviewed journal the European Journal of Clinical Nutrition.
Defending his point of view, Dr. Astrup said, “The definition and classification is so ambiguous, and the risk of misclassification is so extremely high, I think we really miss the basic requirement of science, namely that we know what we are talking about,” he said.
If you take an unprocessed food, and insert a “little additive … suddenly it’s an ultra-processed food,” he added.
UPF definition doesn’t flag some unhealthy foods
Susan Roberts, PhD, professor of nutrition at Tufts University, Boston, was a discussant at the debate and touched on the merits of both sides. She noted that the UPF definition doesn’t flag some “clearly unhealthy foods,” such as table sugar, but does flag some healthy ones, such as plant-based burgers – to which Dr. Monteiro said that the system was not a system meant to divide foods into healthy and unhealthy groups, during the debate session.
The inclusion of both healthy and unhealthy foods in NOVA’s definition of a UPF is a serious problem, Dr. Roberts said.
“It’s almost like it’s an emotional classification designed to get at the food industry rather than focusing on health – and I think that’s asking for trouble because it’s just going to be such a mess to tell consumers, ‘Well, this ultra-processed food is healthy and this one isn’t,’ ” she said. What’s happening is the term ultra-processed is being used interchangeably with unhealthy.
The discussion that the UPF classification has generated is useful, Dr. Roberts continued. “This definition grew out of that recognition that we’re engaged in an unprecedented experiment of how unhealthy can you make the world without having a major catastrophe.”
She added that the UPF concept deserves a more formalized and rigorous evaluation.
“This is an important topic for the future of public health, and I think it needs big committees to address it seriously,” she said. “I think we should not be dealing with this individually in different labs.”
Doctor’s take on usefulness of discussing UPF concept with patients
Mark Corkins, MD, who did not participate in the debate at the meeting, said he talks to parents and children about nutrition at every office visit in which he sees a child with an unhealthy weight.
“Persistence wears down resistance,” said the chair of the American Academy of Pediatrics nutrition committee, in an interview.“A consistent message – you say the same thing and you say it multiple times.”
The idea of “ultra-processed foods” plays a role in those conversations, but largely in the background. It’s a topic that’s important for pediatric health, Dr. Corkins said – but he doesn’t make it the focal point.
“It’s not a direct attack on ultra-processed foods that usually I take as my direction,” said Dr. Corkins, who is also chief of pediatric gastroenterology at Le Bonheur Children’s Hospital in Memphis, Tenn.. “What I try to focus on, and what I think the American Academy of Pediatrics would focus on, is that we need to focus on making the diet better.”
He added, “Parents are aware – they don’t call it ultra-processed food, they call it junk food.”
Dr. Corkins continued that he is reluctant to directly challenge parents on feeding their children unhealthy foods – ultra-processed or not – lest he shame them and harm the relationship.
“Guilt as a motivator isn’t really highly successful,” he said, in an interview.
Dr. Astrup reported advisory committee or board member involvement with Green Leaf Medical and RNPC, France. Dr. Roberts reported advisory committee or board member involvement with Danone, and an ownership interest in Instinct Health Science. Dr. Monteiro and Dr. Corkins reported no relevant disclosures.
The NOVA system divides foods into “fresh or minimally processed,” such as strawberries or steel-cut oats; “processed culinary ingredients,” such as olive oil; “processed foods,” such as cheeses; and “ultra-processed foods.” UPFs are defined as “industrial formulations made by deconstructing natural food into its chemical constituents, modifying them and recombining them with additives into products liable to displace all other NOVA food groups.”
According to doctors who presented during the meeting, ultra-processed foods are drawing increased attention, because researchers have been examining them in National Institutes of Health–funded studies and journalists have been writing about them.
During the debate session at the meeting, some experts said that, with obesity and poor health skyrocketing, increased awareness and labeling of UPFs can only be a good thing. In contrast others noted at the meeting that the classification system that has come to be used for identifying UPFs – the NOVA Food Classification system – is too mushy, confusing, and, ultimately unhelpful.
Carlos Monteiro, MD, PhD, professor of nutrition and public health at the University of Sao Paolo, was part of the group favoring the NOVA system’s classifying certain foods as UPFs, during the debate. He drew attention to the extent to which the world’s population is getting its calories from UPFs.
Mexico and France get about 30% of calories from these foods. In Canada, it’s 48%. And in the United States, it’s 57%, Dr. Monteiro said.
Studies have found that UPFs, many of which are designed to be exceedingly flavorful and intended to replace consumption of unprocessed whole foods, lead to more overall energy intake, more added sugar in the diet, and less fiber and protein intake, he said.
To further support his arguments, Dr. Monteiro pointed to studies suggesting that it is not just the resulting change in the nutritional intake that is unhealthy, but the UPF manufacturing process itself. When adjusting for fat, sugar, and sodium intake, for example, health outcomes associated with UPFs remain poor, he explained.
“I’m sorry,” he said in the debate. “If you don’t reduce this, you don’t reduce your obesity, your diabetes prevalence.”
A study presented by Jacqueline Vernarelli, PhD, during a different session at the meeting suggested there may be other downsides to consuming UPFs. This research, which was based on the U.S. National Youth Fitness Survey, found that poorer locomotor skills among children aged 3-5 and poorer cardiovascular fitness among those aged 12-15 were associated with getting more calories from UPFs.
Those with lower cardiovascular fitness consumed 1,234 calories a day from UPFs, and those with higher cardiovascular fitness consumed 1,007 calories a day from UPFs (P = .002), according to the new research.
“It’s notable here that, although these differences are significant, both groups are consuming a pretty high proportion of their diet from ultra-processed foods,” said Dr. Vernarelli, associate professor of public health at Sacred Heart University, Fairfield, Conn., during her presentation.
In the debate session, Arne Astrup, MD, PhD, senior project director at the Healthy Weight Center at the Novo Nordisk Foundation, Hellerup, Denmark, presented an opposing view.
He said the definition of UPFs makes it too difficult to categorize many foods, pointing to a study from this year in which about 150 nutrition experts, doctors, and dietitians classified 120 foods. Only three marketed foods and one generic food were classified the same by all the evaluators.
Referring to the study Dr. Astrup cited, Dr. Monteiro said it was a mere “exercise,” and the experts involved in it had conflicts of interest.
Dr. Astrup touted this study’s size and its appearance in the peer-reviewed journal the European Journal of Clinical Nutrition.
Defending his point of view, Dr. Astrup said, “The definition and classification is so ambiguous, and the risk of misclassification is so extremely high, I think we really miss the basic requirement of science, namely that we know what we are talking about,” he said.
If you take an unprocessed food, and insert a “little additive … suddenly it’s an ultra-processed food,” he added.
UPF definition doesn’t flag some unhealthy foods
Susan Roberts, PhD, professor of nutrition at Tufts University, Boston, was a discussant at the debate and touched on the merits of both sides. She noted that the UPF definition doesn’t flag some “clearly unhealthy foods,” such as table sugar, but does flag some healthy ones, such as plant-based burgers – to which Dr. Monteiro said that the system was not a system meant to divide foods into healthy and unhealthy groups, during the debate session.
The inclusion of both healthy and unhealthy foods in NOVA’s definition of a UPF is a serious problem, Dr. Roberts said.
“It’s almost like it’s an emotional classification designed to get at the food industry rather than focusing on health – and I think that’s asking for trouble because it’s just going to be such a mess to tell consumers, ‘Well, this ultra-processed food is healthy and this one isn’t,’ ” she said. What’s happening is the term ultra-processed is being used interchangeably with unhealthy.
The discussion that the UPF classification has generated is useful, Dr. Roberts continued. “This definition grew out of that recognition that we’re engaged in an unprecedented experiment of how unhealthy can you make the world without having a major catastrophe.”
She added that the UPF concept deserves a more formalized and rigorous evaluation.
“This is an important topic for the future of public health, and I think it needs big committees to address it seriously,” she said. “I think we should not be dealing with this individually in different labs.”
Doctor’s take on usefulness of discussing UPF concept with patients
Mark Corkins, MD, who did not participate in the debate at the meeting, said he talks to parents and children about nutrition at every office visit in which he sees a child with an unhealthy weight.
“Persistence wears down resistance,” said the chair of the American Academy of Pediatrics nutrition committee, in an interview.“A consistent message – you say the same thing and you say it multiple times.”
The idea of “ultra-processed foods” plays a role in those conversations, but largely in the background. It’s a topic that’s important for pediatric health, Dr. Corkins said – but he doesn’t make it the focal point.
“It’s not a direct attack on ultra-processed foods that usually I take as my direction,” said Dr. Corkins, who is also chief of pediatric gastroenterology at Le Bonheur Children’s Hospital in Memphis, Tenn.. “What I try to focus on, and what I think the American Academy of Pediatrics would focus on, is that we need to focus on making the diet better.”
He added, “Parents are aware – they don’t call it ultra-processed food, they call it junk food.”
Dr. Corkins continued that he is reluctant to directly challenge parents on feeding their children unhealthy foods – ultra-processed or not – lest he shame them and harm the relationship.
“Guilt as a motivator isn’t really highly successful,” he said, in an interview.
Dr. Astrup reported advisory committee or board member involvement with Green Leaf Medical and RNPC, France. Dr. Roberts reported advisory committee or board member involvement with Danone, and an ownership interest in Instinct Health Science. Dr. Monteiro and Dr. Corkins reported no relevant disclosures.
The NOVA system divides foods into “fresh or minimally processed,” such as strawberries or steel-cut oats; “processed culinary ingredients,” such as olive oil; “processed foods,” such as cheeses; and “ultra-processed foods.” UPFs are defined as “industrial formulations made by deconstructing natural food into its chemical constituents, modifying them and recombining them with additives into products liable to displace all other NOVA food groups.”
According to doctors who presented during the meeting, ultra-processed foods are drawing increased attention, because researchers have been examining them in National Institutes of Health–funded studies and journalists have been writing about them.
During the debate session at the meeting, some experts said that, with obesity and poor health skyrocketing, increased awareness and labeling of UPFs can only be a good thing. In contrast others noted at the meeting that the classification system that has come to be used for identifying UPFs – the NOVA Food Classification system – is too mushy, confusing, and, ultimately unhelpful.
Carlos Monteiro, MD, PhD, professor of nutrition and public health at the University of Sao Paolo, was part of the group favoring the NOVA system’s classifying certain foods as UPFs, during the debate. He drew attention to the extent to which the world’s population is getting its calories from UPFs.
Mexico and France get about 30% of calories from these foods. In Canada, it’s 48%. And in the United States, it’s 57%, Dr. Monteiro said.
Studies have found that UPFs, many of which are designed to be exceedingly flavorful and intended to replace consumption of unprocessed whole foods, lead to more overall energy intake, more added sugar in the diet, and less fiber and protein intake, he said.
To further support his arguments, Dr. Monteiro pointed to studies suggesting that it is not just the resulting change in the nutritional intake that is unhealthy, but the UPF manufacturing process itself. When adjusting for fat, sugar, and sodium intake, for example, health outcomes associated with UPFs remain poor, he explained.
“I’m sorry,” he said in the debate. “If you don’t reduce this, you don’t reduce your obesity, your diabetes prevalence.”
A study presented by Jacqueline Vernarelli, PhD, during a different session at the meeting suggested there may be other downsides to consuming UPFs. This research, which was based on the U.S. National Youth Fitness Survey, found that poorer locomotor skills among children aged 3-5 and poorer cardiovascular fitness among those aged 12-15 were associated with getting more calories from UPFs.
Those with lower cardiovascular fitness consumed 1,234 calories a day from UPFs, and those with higher cardiovascular fitness consumed 1,007 calories a day from UPFs (P = .002), according to the new research.
“It’s notable here that, although these differences are significant, both groups are consuming a pretty high proportion of their diet from ultra-processed foods,” said Dr. Vernarelli, associate professor of public health at Sacred Heart University, Fairfield, Conn., during her presentation.
In the debate session, Arne Astrup, MD, PhD, senior project director at the Healthy Weight Center at the Novo Nordisk Foundation, Hellerup, Denmark, presented an opposing view.
He said the definition of UPFs makes it too difficult to categorize many foods, pointing to a study from this year in which about 150 nutrition experts, doctors, and dietitians classified 120 foods. Only three marketed foods and one generic food were classified the same by all the evaluators.
Referring to the study Dr. Astrup cited, Dr. Monteiro said it was a mere “exercise,” and the experts involved in it had conflicts of interest.
Dr. Astrup touted this study’s size and its appearance in the peer-reviewed journal the European Journal of Clinical Nutrition.
Defending his point of view, Dr. Astrup said, “The definition and classification is so ambiguous, and the risk of misclassification is so extremely high, I think we really miss the basic requirement of science, namely that we know what we are talking about,” he said.
If you take an unprocessed food, and insert a “little additive … suddenly it’s an ultra-processed food,” he added.
UPF definition doesn’t flag some unhealthy foods
Susan Roberts, PhD, professor of nutrition at Tufts University, Boston, was a discussant at the debate and touched on the merits of both sides. She noted that the UPF definition doesn’t flag some “clearly unhealthy foods,” such as table sugar, but does flag some healthy ones, such as plant-based burgers – to which Dr. Monteiro said that the system was not a system meant to divide foods into healthy and unhealthy groups, during the debate session.
The inclusion of both healthy and unhealthy foods in NOVA’s definition of a UPF is a serious problem, Dr. Roberts said.
“It’s almost like it’s an emotional classification designed to get at the food industry rather than focusing on health – and I think that’s asking for trouble because it’s just going to be such a mess to tell consumers, ‘Well, this ultra-processed food is healthy and this one isn’t,’ ” she said. What’s happening is the term ultra-processed is being used interchangeably with unhealthy.
The discussion that the UPF classification has generated is useful, Dr. Roberts continued. “This definition grew out of that recognition that we’re engaged in an unprecedented experiment of how unhealthy can you make the world without having a major catastrophe.”
She added that the UPF concept deserves a more formalized and rigorous evaluation.
“This is an important topic for the future of public health, and I think it needs big committees to address it seriously,” she said. “I think we should not be dealing with this individually in different labs.”
Doctor’s take on usefulness of discussing UPF concept with patients
Mark Corkins, MD, who did not participate in the debate at the meeting, said he talks to parents and children about nutrition at every office visit in which he sees a child with an unhealthy weight.
“Persistence wears down resistance,” said the chair of the American Academy of Pediatrics nutrition committee, in an interview.“A consistent message – you say the same thing and you say it multiple times.”
The idea of “ultra-processed foods” plays a role in those conversations, but largely in the background. It’s a topic that’s important for pediatric health, Dr. Corkins said – but he doesn’t make it the focal point.
“It’s not a direct attack on ultra-processed foods that usually I take as my direction,” said Dr. Corkins, who is also chief of pediatric gastroenterology at Le Bonheur Children’s Hospital in Memphis, Tenn.. “What I try to focus on, and what I think the American Academy of Pediatrics would focus on, is that we need to focus on making the diet better.”
He added, “Parents are aware – they don’t call it ultra-processed food, they call it junk food.”
Dr. Corkins continued that he is reluctant to directly challenge parents on feeding their children unhealthy foods – ultra-processed or not – lest he shame them and harm the relationship.
“Guilt as a motivator isn’t really highly successful,” he said, in an interview.
Dr. Astrup reported advisory committee or board member involvement with Green Leaf Medical and RNPC, France. Dr. Roberts reported advisory committee or board member involvement with Danone, and an ownership interest in Instinct Health Science. Dr. Monteiro and Dr. Corkins reported no relevant disclosures.
FROM NUTRITION 2022
FDA authorizes COVID vaccines in kids as young as 6 months
, one of the final steps in a long-awaited authorization process to extend protection to the youngest of Americans.
The agency’s move comes after a closely watched FDA advisory group vote earlier this week, which resulted in a unanimous vote in favor of the FDA authorizing both vaccines in this age group.
“The FDA’s evaluation and analysis of the safety, effectiveness, and manufacturing data of these vaccines was rigorous and comprehensive, supporting the EUAs,” the agency said in a news release.
The data show that the “known and potential benefits” of the vaccines outweigh any potential risks, the agency said.
The Moderna vaccine is authorized as a two-dose primary series in children 6 months to 17 years of age. The Pfizer vaccine is now authorized as a three-dose primary series in children 6 months up to 4 years of age. Pfizer’s vaccine was already authorized in children 5 years old and older.
Now all eyes are on the Centers for Disease Control and Prevention, which is expected to decide on the final regulatory hurdle at a meeting June 18. The CDC’s Advisory Committee on Immunization Practices has scheduled a vote on whether to give the vaccines the green light.
If ACIP gives the OK, CDC Director Rochelle Walensky, MD, MPH, is expected to issue recommendations for use shortly thereafter.
Following these final regulatory steps, parents could start bringing their children to pediatricians, family doctors, or local pharmacies for vaccination as early as June 20.
A version of this article first appeared on WebMD.com.
, one of the final steps in a long-awaited authorization process to extend protection to the youngest of Americans.
The agency’s move comes after a closely watched FDA advisory group vote earlier this week, which resulted in a unanimous vote in favor of the FDA authorizing both vaccines in this age group.
“The FDA’s evaluation and analysis of the safety, effectiveness, and manufacturing data of these vaccines was rigorous and comprehensive, supporting the EUAs,” the agency said in a news release.
The data show that the “known and potential benefits” of the vaccines outweigh any potential risks, the agency said.
The Moderna vaccine is authorized as a two-dose primary series in children 6 months to 17 years of age. The Pfizer vaccine is now authorized as a three-dose primary series in children 6 months up to 4 years of age. Pfizer’s vaccine was already authorized in children 5 years old and older.
Now all eyes are on the Centers for Disease Control and Prevention, which is expected to decide on the final regulatory hurdle at a meeting June 18. The CDC’s Advisory Committee on Immunization Practices has scheduled a vote on whether to give the vaccines the green light.
If ACIP gives the OK, CDC Director Rochelle Walensky, MD, MPH, is expected to issue recommendations for use shortly thereafter.
Following these final regulatory steps, parents could start bringing their children to pediatricians, family doctors, or local pharmacies for vaccination as early as June 20.
A version of this article first appeared on WebMD.com.
, one of the final steps in a long-awaited authorization process to extend protection to the youngest of Americans.
The agency’s move comes after a closely watched FDA advisory group vote earlier this week, which resulted in a unanimous vote in favor of the FDA authorizing both vaccines in this age group.
“The FDA’s evaluation and analysis of the safety, effectiveness, and manufacturing data of these vaccines was rigorous and comprehensive, supporting the EUAs,” the agency said in a news release.
The data show that the “known and potential benefits” of the vaccines outweigh any potential risks, the agency said.
The Moderna vaccine is authorized as a two-dose primary series in children 6 months to 17 years of age. The Pfizer vaccine is now authorized as a three-dose primary series in children 6 months up to 4 years of age. Pfizer’s vaccine was already authorized in children 5 years old and older.
Now all eyes are on the Centers for Disease Control and Prevention, which is expected to decide on the final regulatory hurdle at a meeting June 18. The CDC’s Advisory Committee on Immunization Practices has scheduled a vote on whether to give the vaccines the green light.
If ACIP gives the OK, CDC Director Rochelle Walensky, MD, MPH, is expected to issue recommendations for use shortly thereafter.
Following these final regulatory steps, parents could start bringing their children to pediatricians, family doctors, or local pharmacies for vaccination as early as June 20.
A version of this article first appeared on WebMD.com.
A doctor’s missed diagnosis results in mega award
, according to a story from WCCO CBS Minnesota, among other news outlets. The award has been called the largest judgment of its kind in Minnesota history.
In January 2017, Nepalese immigrant Anuj Thapa was playing in an indoor soccer game at St. Cloud State University when another player tackled him. His left leg badly injured, Mr. Thapa was taken by ambulance to CentraCare’s St. Cloud Hospital. The orthopedic surgeon on call that day was Chad Holien, MD, who is affiliated with St. Cloud Orthopedics, a private clinic in nearby Sartell, Minn. Following preparations, and with the help of a physician assistant, Dr. Holien operated on the patient’s broken leg.
But Mr. Thapa experienced post-surgical complications – severe pain, numbness, burning, and muscle issues. Despite the complications, he was discharged from the hospital that afternoon and sent home.
Six days later, Mr. Thapa returned to St. Cloud Hospital, still complaining of severe pain. A second orthopedic surgeon operated and found that Mr. Thapa had “acute compartment syndrome,” the result of internal pressure that had built up in his leg muscles.
Over time, Mr. Thapa underwent more than 20 surgeries on his leg to deal with the ongoing pain and other complications, according to WCCO.
In 2019, he filed a medical malpractice suit in U.S. district court against St. Cloud Orthopedics, the private practice that employed the surgeon and the PA. (Under Minnesota law, an employer is responsible for the actions of its employees.)
In his complaint, Mr. Thapa alleged that in treating him, “the defendants departed from accepted standards of medical practice.” Among other things, he claimed that Dr. Holien and the PA had not properly evaluated his postoperative symptoms, failed to diagnose and treat his compartment syndrome, and improperly discharged him from the hospital. These lapses, Mr. Thapa said, led to his “severe, permanent, and disabling injuries.”
The federal jury agreed. After a weeklong trial, it awarded the plaintiff $100 million for future “pain, disability, disfigurement, embarrassment, and emotional distress.” It also gave him $10 million for past suffering and a little more than $1 million for past and future medical bills.
In a postverdict statement, Mr. Thapa’s attorney said that, while the surgeon and PA are undoubtedly good providers, they made mistakes in this case.
A defense attorney for St. Cloud Orthopedics disputes this: “We maintain the care provided in this case was in accordance with accepted standards of care.”
At press time, the defense had not determined whether to appeal the jury’s $111 million verdict. “St. Cloud continues to support its providers,” said the clinic’s defense attorney. “We are evaluating our options regarding this verdict.”
The content contained in this article is for informational purposes only and does not constitute legal advice. Reliance on any information provided in this article is solely at your own risk.
A version of this article first appeared on Medscape.com.
, according to a story from WCCO CBS Minnesota, among other news outlets. The award has been called the largest judgment of its kind in Minnesota history.
In January 2017, Nepalese immigrant Anuj Thapa was playing in an indoor soccer game at St. Cloud State University when another player tackled him. His left leg badly injured, Mr. Thapa was taken by ambulance to CentraCare’s St. Cloud Hospital. The orthopedic surgeon on call that day was Chad Holien, MD, who is affiliated with St. Cloud Orthopedics, a private clinic in nearby Sartell, Minn. Following preparations, and with the help of a physician assistant, Dr. Holien operated on the patient’s broken leg.
But Mr. Thapa experienced post-surgical complications – severe pain, numbness, burning, and muscle issues. Despite the complications, he was discharged from the hospital that afternoon and sent home.
Six days later, Mr. Thapa returned to St. Cloud Hospital, still complaining of severe pain. A second orthopedic surgeon operated and found that Mr. Thapa had “acute compartment syndrome,” the result of internal pressure that had built up in his leg muscles.
Over time, Mr. Thapa underwent more than 20 surgeries on his leg to deal with the ongoing pain and other complications, according to WCCO.
In 2019, he filed a medical malpractice suit in U.S. district court against St. Cloud Orthopedics, the private practice that employed the surgeon and the PA. (Under Minnesota law, an employer is responsible for the actions of its employees.)
In his complaint, Mr. Thapa alleged that in treating him, “the defendants departed from accepted standards of medical practice.” Among other things, he claimed that Dr. Holien and the PA had not properly evaluated his postoperative symptoms, failed to diagnose and treat his compartment syndrome, and improperly discharged him from the hospital. These lapses, Mr. Thapa said, led to his “severe, permanent, and disabling injuries.”
The federal jury agreed. After a weeklong trial, it awarded the plaintiff $100 million for future “pain, disability, disfigurement, embarrassment, and emotional distress.” It also gave him $10 million for past suffering and a little more than $1 million for past and future medical bills.
In a postverdict statement, Mr. Thapa’s attorney said that, while the surgeon and PA are undoubtedly good providers, they made mistakes in this case.
A defense attorney for St. Cloud Orthopedics disputes this: “We maintain the care provided in this case was in accordance with accepted standards of care.”
At press time, the defense had not determined whether to appeal the jury’s $111 million verdict. “St. Cloud continues to support its providers,” said the clinic’s defense attorney. “We are evaluating our options regarding this verdict.”
The content contained in this article is for informational purposes only and does not constitute legal advice. Reliance on any information provided in this article is solely at your own risk.
A version of this article first appeared on Medscape.com.
, according to a story from WCCO CBS Minnesota, among other news outlets. The award has been called the largest judgment of its kind in Minnesota history.
In January 2017, Nepalese immigrant Anuj Thapa was playing in an indoor soccer game at St. Cloud State University when another player tackled him. His left leg badly injured, Mr. Thapa was taken by ambulance to CentraCare’s St. Cloud Hospital. The orthopedic surgeon on call that day was Chad Holien, MD, who is affiliated with St. Cloud Orthopedics, a private clinic in nearby Sartell, Minn. Following preparations, and with the help of a physician assistant, Dr. Holien operated on the patient’s broken leg.
But Mr. Thapa experienced post-surgical complications – severe pain, numbness, burning, and muscle issues. Despite the complications, he was discharged from the hospital that afternoon and sent home.
Six days later, Mr. Thapa returned to St. Cloud Hospital, still complaining of severe pain. A second orthopedic surgeon operated and found that Mr. Thapa had “acute compartment syndrome,” the result of internal pressure that had built up in his leg muscles.
Over time, Mr. Thapa underwent more than 20 surgeries on his leg to deal with the ongoing pain and other complications, according to WCCO.
In 2019, he filed a medical malpractice suit in U.S. district court against St. Cloud Orthopedics, the private practice that employed the surgeon and the PA. (Under Minnesota law, an employer is responsible for the actions of its employees.)
In his complaint, Mr. Thapa alleged that in treating him, “the defendants departed from accepted standards of medical practice.” Among other things, he claimed that Dr. Holien and the PA had not properly evaluated his postoperative symptoms, failed to diagnose and treat his compartment syndrome, and improperly discharged him from the hospital. These lapses, Mr. Thapa said, led to his “severe, permanent, and disabling injuries.”
The federal jury agreed. After a weeklong trial, it awarded the plaintiff $100 million for future “pain, disability, disfigurement, embarrassment, and emotional distress.” It also gave him $10 million for past suffering and a little more than $1 million for past and future medical bills.
In a postverdict statement, Mr. Thapa’s attorney said that, while the surgeon and PA are undoubtedly good providers, they made mistakes in this case.
A defense attorney for St. Cloud Orthopedics disputes this: “We maintain the care provided in this case was in accordance with accepted standards of care.”
At press time, the defense had not determined whether to appeal the jury’s $111 million verdict. “St. Cloud continues to support its providers,” said the clinic’s defense attorney. “We are evaluating our options regarding this verdict.”
The content contained in this article is for informational purposes only and does not constitute legal advice. Reliance on any information provided in this article is solely at your own risk.
A version of this article first appeared on Medscape.com.
Past COVID-19 infection could play role in childhood hepatitis
There may be a link between the recent unexplained cases of hepatitis in children and prior coronavirus infections, according to new research from Israel.
The study involves five children in Israel who had mild cases of COVID-19 who went on to develop hepatitis; two of these children required liver transplants. But clinicians are cautious about drawing conclusions from such a small study.
“All you can say is that these five cases seem to have proximity to COVID-19, and COVID-19 may be able to cause pediatric liver complications,” said Nancy Reau, MD, section chief of hepatology at Rush University in Chicago. She was not involved with the study.
While COVID-19 could be one explanation for these hepatitis cases, it is also possible that the two are unrelated, said William Balistreri, MD, director emeritus of the Pediatric Liver Care Center at Cincinnati Children’s Hospital Medical Center. He also is unaffiliated with the study.
Hepatitis is rare in children, and between 30% and 50% of these pediatric cases have no known cause, according to the CDC.
Since April 2022, children with hepatitis of an unexplained cause have garnered global attention. The United Kingdom now has 240 confirmed cases, the United States is investigating 290 cases, and Israel has reported 12 cases to the World Health Organization. Many investigators think that these liver problems could be related to adenovirus – a common infection in children that normally causes cold or flu-like symptoms – because more than half of global cases tested for the virus have been positive, according to the WHO. About 12% of children with unexplained hepatitis have tested positive for SARS-CoV-2, the virus that causes COVID-19, but investigators are considering the possibility that some cases may be related to prior infections.
The study documents five patients, 3-months to 13 years old, with prior coronavirus infection who later developed hepatitis. All were treated at Schneider Children’s Medical Hospital in Petah Tikva, Israel, during 2021. The paper was published in the Journal of Pediatric Gastroenterology and Nutrition. Two patients, a 3-month-old and 5-month-old, needed liver transplants. The other three patients (two 8-year-olds and a 13-year-old) were treated with steroids. None of the five children had received any vaccinations against COVID-19. The time between COVID-19 infection and liver problems ranged from 21 to 130 days.
“It took time to be convinced that this could be COVID-related,” said senior study author Orith Waisbourd-Zinman, MD, director of pediatric liver disease service at Schneider Children’s Medical Hospital. “It’s something that wasn’t described.”
Sudden-onset hepatitis after COVID-19 has been recorded in adults, and the virus has been associated with multisystem inflammatory syndrome in children (MIS-C). The condition causes inflammation through the body, including the heart, lungs, and kidneys.
“We know that COVID can be mischievous, and children are no more exempt from that than adults,” Dr. Reau said.
Liver samples taken from these five patients did not test positive for COVID-19, similar to how liver samples have tested negative for adenovirus in more recent hepatitis cases around the world. Dr. Waisbourd-Zinman suggested that in these patients, hepatitis may have been brought on by an inflammatory response that was triggered by the virus.
Still, there are notable differences between these five cases and current cases internationally. These five children became sick during the period of December 2020 to September 2021, whereas all current counted cases in the United Kingdom occurred after January 2022. The first cases in the United States took place in October 2021. It could be that there were similar hepatitis cases before that were not identified, Dr. Reau said.
The ages of the Israeli children with hepatitis also differ from the cases seen globally. More than three-fourths of these reported hepatitis cases occurred in children under 5, the WHO reports, though affected individuals have been as young as 1-month-old up to 16 years old. In the United Kingdom, which accounts for about a third of cases reported to the WHO, most children with unexplained hepatitis have been between 3 and 5 years old.
More research is needed to tease out any relationship between prior COVID-19 infection and liver inflammation, Dr. Balistreri said.
“I’m not sure what to make of any of it yet. We know that SARS-CoV-2 can alter immune responses ... so it wouldn’t surprise me,” if COVID-19 and these hepatitis cases were linked, he said. “It’s just that we need more information.”
A version of this article first appeared on WebMD.com.
There may be a link between the recent unexplained cases of hepatitis in children and prior coronavirus infections, according to new research from Israel.
The study involves five children in Israel who had mild cases of COVID-19 who went on to develop hepatitis; two of these children required liver transplants. But clinicians are cautious about drawing conclusions from such a small study.
“All you can say is that these five cases seem to have proximity to COVID-19, and COVID-19 may be able to cause pediatric liver complications,” said Nancy Reau, MD, section chief of hepatology at Rush University in Chicago. She was not involved with the study.
While COVID-19 could be one explanation for these hepatitis cases, it is also possible that the two are unrelated, said William Balistreri, MD, director emeritus of the Pediatric Liver Care Center at Cincinnati Children’s Hospital Medical Center. He also is unaffiliated with the study.
Hepatitis is rare in children, and between 30% and 50% of these pediatric cases have no known cause, according to the CDC.
Since April 2022, children with hepatitis of an unexplained cause have garnered global attention. The United Kingdom now has 240 confirmed cases, the United States is investigating 290 cases, and Israel has reported 12 cases to the World Health Organization. Many investigators think that these liver problems could be related to adenovirus – a common infection in children that normally causes cold or flu-like symptoms – because more than half of global cases tested for the virus have been positive, according to the WHO. About 12% of children with unexplained hepatitis have tested positive for SARS-CoV-2, the virus that causes COVID-19, but investigators are considering the possibility that some cases may be related to prior infections.
The study documents five patients, 3-months to 13 years old, with prior coronavirus infection who later developed hepatitis. All were treated at Schneider Children’s Medical Hospital in Petah Tikva, Israel, during 2021. The paper was published in the Journal of Pediatric Gastroenterology and Nutrition. Two patients, a 3-month-old and 5-month-old, needed liver transplants. The other three patients (two 8-year-olds and a 13-year-old) were treated with steroids. None of the five children had received any vaccinations against COVID-19. The time between COVID-19 infection and liver problems ranged from 21 to 130 days.
“It took time to be convinced that this could be COVID-related,” said senior study author Orith Waisbourd-Zinman, MD, director of pediatric liver disease service at Schneider Children’s Medical Hospital. “It’s something that wasn’t described.”
Sudden-onset hepatitis after COVID-19 has been recorded in adults, and the virus has been associated with multisystem inflammatory syndrome in children (MIS-C). The condition causes inflammation through the body, including the heart, lungs, and kidneys.
“We know that COVID can be mischievous, and children are no more exempt from that than adults,” Dr. Reau said.
Liver samples taken from these five patients did not test positive for COVID-19, similar to how liver samples have tested negative for adenovirus in more recent hepatitis cases around the world. Dr. Waisbourd-Zinman suggested that in these patients, hepatitis may have been brought on by an inflammatory response that was triggered by the virus.
Still, there are notable differences between these five cases and current cases internationally. These five children became sick during the period of December 2020 to September 2021, whereas all current counted cases in the United Kingdom occurred after January 2022. The first cases in the United States took place in October 2021. It could be that there were similar hepatitis cases before that were not identified, Dr. Reau said.
The ages of the Israeli children with hepatitis also differ from the cases seen globally. More than three-fourths of these reported hepatitis cases occurred in children under 5, the WHO reports, though affected individuals have been as young as 1-month-old up to 16 years old. In the United Kingdom, which accounts for about a third of cases reported to the WHO, most children with unexplained hepatitis have been between 3 and 5 years old.
More research is needed to tease out any relationship between prior COVID-19 infection and liver inflammation, Dr. Balistreri said.
“I’m not sure what to make of any of it yet. We know that SARS-CoV-2 can alter immune responses ... so it wouldn’t surprise me,” if COVID-19 and these hepatitis cases were linked, he said. “It’s just that we need more information.”
A version of this article first appeared on WebMD.com.
There may be a link between the recent unexplained cases of hepatitis in children and prior coronavirus infections, according to new research from Israel.
The study involves five children in Israel who had mild cases of COVID-19 who went on to develop hepatitis; two of these children required liver transplants. But clinicians are cautious about drawing conclusions from such a small study.
“All you can say is that these five cases seem to have proximity to COVID-19, and COVID-19 may be able to cause pediatric liver complications,” said Nancy Reau, MD, section chief of hepatology at Rush University in Chicago. She was not involved with the study.
While COVID-19 could be one explanation for these hepatitis cases, it is also possible that the two are unrelated, said William Balistreri, MD, director emeritus of the Pediatric Liver Care Center at Cincinnati Children’s Hospital Medical Center. He also is unaffiliated with the study.
Hepatitis is rare in children, and between 30% and 50% of these pediatric cases have no known cause, according to the CDC.
Since April 2022, children with hepatitis of an unexplained cause have garnered global attention. The United Kingdom now has 240 confirmed cases, the United States is investigating 290 cases, and Israel has reported 12 cases to the World Health Organization. Many investigators think that these liver problems could be related to adenovirus – a common infection in children that normally causes cold or flu-like symptoms – because more than half of global cases tested for the virus have been positive, according to the WHO. About 12% of children with unexplained hepatitis have tested positive for SARS-CoV-2, the virus that causes COVID-19, but investigators are considering the possibility that some cases may be related to prior infections.
The study documents five patients, 3-months to 13 years old, with prior coronavirus infection who later developed hepatitis. All were treated at Schneider Children’s Medical Hospital in Petah Tikva, Israel, during 2021. The paper was published in the Journal of Pediatric Gastroenterology and Nutrition. Two patients, a 3-month-old and 5-month-old, needed liver transplants. The other three patients (two 8-year-olds and a 13-year-old) were treated with steroids. None of the five children had received any vaccinations against COVID-19. The time between COVID-19 infection and liver problems ranged from 21 to 130 days.
“It took time to be convinced that this could be COVID-related,” said senior study author Orith Waisbourd-Zinman, MD, director of pediatric liver disease service at Schneider Children’s Medical Hospital. “It’s something that wasn’t described.”
Sudden-onset hepatitis after COVID-19 has been recorded in adults, and the virus has been associated with multisystem inflammatory syndrome in children (MIS-C). The condition causes inflammation through the body, including the heart, lungs, and kidneys.
“We know that COVID can be mischievous, and children are no more exempt from that than adults,” Dr. Reau said.
Liver samples taken from these five patients did not test positive for COVID-19, similar to how liver samples have tested negative for adenovirus in more recent hepatitis cases around the world. Dr. Waisbourd-Zinman suggested that in these patients, hepatitis may have been brought on by an inflammatory response that was triggered by the virus.
Still, there are notable differences between these five cases and current cases internationally. These five children became sick during the period of December 2020 to September 2021, whereas all current counted cases in the United Kingdom occurred after January 2022. The first cases in the United States took place in October 2021. It could be that there were similar hepatitis cases before that were not identified, Dr. Reau said.
The ages of the Israeli children with hepatitis also differ from the cases seen globally. More than three-fourths of these reported hepatitis cases occurred in children under 5, the WHO reports, though affected individuals have been as young as 1-month-old up to 16 years old. In the United Kingdom, which accounts for about a third of cases reported to the WHO, most children with unexplained hepatitis have been between 3 and 5 years old.
More research is needed to tease out any relationship between prior COVID-19 infection and liver inflammation, Dr. Balistreri said.
“I’m not sure what to make of any of it yet. We know that SARS-CoV-2 can alter immune responses ... so it wouldn’t surprise me,” if COVID-19 and these hepatitis cases were linked, he said. “It’s just that we need more information.”
A version of this article first appeared on WebMD.com.
Biomarkers may help to predict persistent oligoarticular JIA
Ongoing research in patients with oligoarticular juvenile idiopathic arthritis (JIA) so far suggests that a set of biomarkers in synovial fluid may help to predict which patients may be more likely to stay with persistent oligoarticular disease rather than progress to polyarticular disease, according to new research presented at the annual scientific meeting of the Childhood Arthritis and Rheumatology Research Alliance, held virtually this year. Identifying biomarkers in synovial fluid or possibly serum could aid families and physicians in being more proactive in treatment protocols, said AnneMarie C. Brescia, MD, of Nemours Children’s Hospital in Wilmington, Del.
“JIA carries the risk of permanent joint damage and disability, which can result when joint involvement evolves from oligoarticular into a polyarticular course, termed extended oligoarticular disease,” Dr. Brescia told attendees. “Since disease progression increases the risk for disability, early prediction of this course is essential.”
This group – those whose oligoarticular disease will begin recruiting joints and ultimately become extended oligoarticular JIA – is “very important because they have been shown to have worse health-related quality of life and greater risk of needing a joint replacement than even polyarticular [JIA],” Dr. Brescia said. “So, our lab has really focused on trying to predict who will fall in this group.”
Melissa Oliver, MD, assistant professor of clinical pediatrics in the division of pediatric rheumatology at Indiana University in Indianapolis, was not involved in the study but agreed that having highly sensitive and specific biomarkers could be particularly helpful in clinical care.
“Biomarkers can help guide treatment decisions and help physicians and their patients share the decision-making about next choices and when to change,” Dr. Oliver told this news organization. “If a provider and parent know that their child has these markers in their serum or synovial fluid that may predict extension of their disease, then they may be more aggressive upfront with therapy.”
The study aimed to determine whether differential levels of synovial fluid proteins could be used to predict whether JIA would evolve into an extended course before it became clinically evident. Although early aggressive treatment is common with rheumatoid arthritis and can lead to remission, JIA treatment paradigms tend to be more reactive, Dr. Brescia said.
“It would be better to switch to proactive, that if we’re able to predict that this patient may have a more difficult course with extension to polyarticular, we could be prepared, we could inform the parents, and it would just help us have a more proactive approach,” she said.
The researchers used antibody arrays to detect the following inflammatory mediators in blinded samples: CD14, interleukin (IL)-1-alpha, IL-3, IL-5, IL-6, vascular endothelial growth factor (VEGF), and angiogenin. They analyzed 37 samples with persistent disease and 32 samples from disease that had not yet extended but would become extended in that patient. The samples came from patients who were taking no medicines or only NSAIDs. The researchers assessed the sensitivity and specificity of each biomarker. Sensitivity referred the biomarker’s ability to correctly indicate that the sample would extend, and specificity referred to the biomarker’s accuracy in determining that the disease in the sample would remain persistent.
Combining samples from cohorts at Nemours Children’s Health (14 persistent and 7 extended-to-be) and Cincinnati Children’s Hospital (23 persistent and 25 extended-to-be) yielded the following results: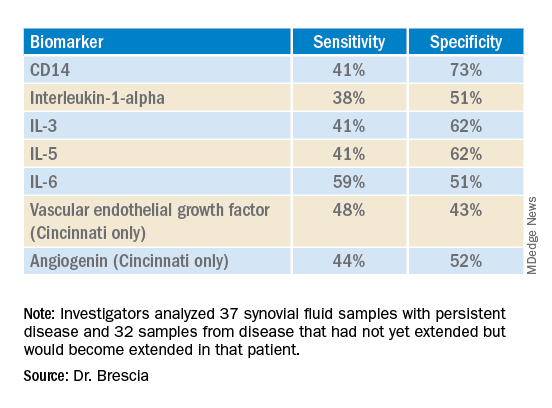
The findings revealed that the selected biomarkers were more accurate at predicting whose disease would remain persistent than predicting those that would extend, Dr. Brescia said. CD14 was the most specific biomarker, and IL-6 was the most sensitive biomarker in both groups.
When the researchers translated the findings from ELISA to the Luminex platform, positive results in synovial fluid for all these biomarkers were also positive in serum samples. Although the differences between persistent and extended-to-be samples did not reach statistical significance using Luminex, the pattern was the same for each biomarker.
“Luminex is more sensitive than ELISA. We believe that conducting an LDA [linear discriminant analysis] using these Luminex measurements will allow us to determine new cutoffs or new protein levels that are appropriate for Luminex to predict who will extend,” Dr. Brescia said. “It’s also our goal to develop a serum panel because ... being able to detect these markers in serum would expand the applicability of these markers to more patients.”
Dr. Brescia then described the group’s work in defining clinically relevant subpopulations of patients based on fibroblast-like synoviocytes (FLS) cells in the synovial intimal lining that produce inflammatory cytokines.
“Our compelling, single-cell, RNA sequencing preliminary data revealing multiple subpopulations within the total FLS population supports our hypothesis that distinct FLS subpopulations correlate with clinical outcome,” said Dr. Brescia. They looked at the percentage of chondrocyte-like, fibroblast-like, and smooth muscle-like subpopulations in samples from patients with oligoarticular JIA, extended-to-be JIA, and polyarticular JIA. Chondrocytes occurred in the largest proportion, and polyarticular JIA FLS had the largest percentage of chondrocytes, compared with the other two subpopulation groups.
“This is a work in progress,” Dr. Brescia said, “so hopefully you’ll hear about it next year.” In response to an attendee’s question, she said she believes identifying reliable biomarkers will eventually lead to refining treatment paradigms.
“I think it will at least change the guidance we can provide parents about making next choices and how quickly to accelerate to those next choices,” Dr. Brescia said. For example, if a child’s serum or synovial fluid has markers that show a very high likelihood of extension, the parent may decide to proceed to the next level medication sooner. “I do think it will push both parents and doctors to be a little more proactive instead of reactive when the poor patient comes back with 13 joints involved when they had just been an oligo for years.”
Dr. Oliver noted the promise of CD14 and IL-6 in potentially predicting which patients’ disease will stay persistent but cautioned that it’s still early in evaluating these biomarkers, especially with the limited patient samples in this study.
“I think these results are promising, and it’s great that there are groups out there working on this,” Dr. Oliver said. “Once we have a reliable, highly sensitive and specific biomarker, that will definitely help providers, parents, and patients be more informed.”
The research was supported by the Open Net Foundation, the Arthritis Foundation, Delaware Community Foundation, the Delaware Clinical and Translational Research (DE-CTR) ACCEL Program, the Nancy Taylor Foundation for Chronic Diseases, and CARRA. Dr. Brescia and Dr. Oliver have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Ongoing research in patients with oligoarticular juvenile idiopathic arthritis (JIA) so far suggests that a set of biomarkers in synovial fluid may help to predict which patients may be more likely to stay with persistent oligoarticular disease rather than progress to polyarticular disease, according to new research presented at the annual scientific meeting of the Childhood Arthritis and Rheumatology Research Alliance, held virtually this year. Identifying biomarkers in synovial fluid or possibly serum could aid families and physicians in being more proactive in treatment protocols, said AnneMarie C. Brescia, MD, of Nemours Children’s Hospital in Wilmington, Del.
“JIA carries the risk of permanent joint damage and disability, which can result when joint involvement evolves from oligoarticular into a polyarticular course, termed extended oligoarticular disease,” Dr. Brescia told attendees. “Since disease progression increases the risk for disability, early prediction of this course is essential.”
This group – those whose oligoarticular disease will begin recruiting joints and ultimately become extended oligoarticular JIA – is “very important because they have been shown to have worse health-related quality of life and greater risk of needing a joint replacement than even polyarticular [JIA],” Dr. Brescia said. “So, our lab has really focused on trying to predict who will fall in this group.”
Melissa Oliver, MD, assistant professor of clinical pediatrics in the division of pediatric rheumatology at Indiana University in Indianapolis, was not involved in the study but agreed that having highly sensitive and specific biomarkers could be particularly helpful in clinical care.
“Biomarkers can help guide treatment decisions and help physicians and their patients share the decision-making about next choices and when to change,” Dr. Oliver told this news organization. “If a provider and parent know that their child has these markers in their serum or synovial fluid that may predict extension of their disease, then they may be more aggressive upfront with therapy.”
The study aimed to determine whether differential levels of synovial fluid proteins could be used to predict whether JIA would evolve into an extended course before it became clinically evident. Although early aggressive treatment is common with rheumatoid arthritis and can lead to remission, JIA treatment paradigms tend to be more reactive, Dr. Brescia said.
“It would be better to switch to proactive, that if we’re able to predict that this patient may have a more difficult course with extension to polyarticular, we could be prepared, we could inform the parents, and it would just help us have a more proactive approach,” she said.
The researchers used antibody arrays to detect the following inflammatory mediators in blinded samples: CD14, interleukin (IL)-1-alpha, IL-3, IL-5, IL-6, vascular endothelial growth factor (VEGF), and angiogenin. They analyzed 37 samples with persistent disease and 32 samples from disease that had not yet extended but would become extended in that patient. The samples came from patients who were taking no medicines or only NSAIDs. The researchers assessed the sensitivity and specificity of each biomarker. Sensitivity referred the biomarker’s ability to correctly indicate that the sample would extend, and specificity referred to the biomarker’s accuracy in determining that the disease in the sample would remain persistent.
Combining samples from cohorts at Nemours Children’s Health (14 persistent and 7 extended-to-be) and Cincinnati Children’s Hospital (23 persistent and 25 extended-to-be) yielded the following results:
The findings revealed that the selected biomarkers were more accurate at predicting whose disease would remain persistent than predicting those that would extend, Dr. Brescia said. CD14 was the most specific biomarker, and IL-6 was the most sensitive biomarker in both groups.
When the researchers translated the findings from ELISA to the Luminex platform, positive results in synovial fluid for all these biomarkers were also positive in serum samples. Although the differences between persistent and extended-to-be samples did not reach statistical significance using Luminex, the pattern was the same for each biomarker.
“Luminex is more sensitive than ELISA. We believe that conducting an LDA [linear discriminant analysis] using these Luminex measurements will allow us to determine new cutoffs or new protein levels that are appropriate for Luminex to predict who will extend,” Dr. Brescia said. “It’s also our goal to develop a serum panel because ... being able to detect these markers in serum would expand the applicability of these markers to more patients.”
Dr. Brescia then described the group’s work in defining clinically relevant subpopulations of patients based on fibroblast-like synoviocytes (FLS) cells in the synovial intimal lining that produce inflammatory cytokines.
“Our compelling, single-cell, RNA sequencing preliminary data revealing multiple subpopulations within the total FLS population supports our hypothesis that distinct FLS subpopulations correlate with clinical outcome,” said Dr. Brescia. They looked at the percentage of chondrocyte-like, fibroblast-like, and smooth muscle-like subpopulations in samples from patients with oligoarticular JIA, extended-to-be JIA, and polyarticular JIA. Chondrocytes occurred in the largest proportion, and polyarticular JIA FLS had the largest percentage of chondrocytes, compared with the other two subpopulation groups.
“This is a work in progress,” Dr. Brescia said, “so hopefully you’ll hear about it next year.” In response to an attendee’s question, she said she believes identifying reliable biomarkers will eventually lead to refining treatment paradigms.
“I think it will at least change the guidance we can provide parents about making next choices and how quickly to accelerate to those next choices,” Dr. Brescia said. For example, if a child’s serum or synovial fluid has markers that show a very high likelihood of extension, the parent may decide to proceed to the next level medication sooner. “I do think it will push both parents and doctors to be a little more proactive instead of reactive when the poor patient comes back with 13 joints involved when they had just been an oligo for years.”
Dr. Oliver noted the promise of CD14 and IL-6 in potentially predicting which patients’ disease will stay persistent but cautioned that it’s still early in evaluating these biomarkers, especially with the limited patient samples in this study.
“I think these results are promising, and it’s great that there are groups out there working on this,” Dr. Oliver said. “Once we have a reliable, highly sensitive and specific biomarker, that will definitely help providers, parents, and patients be more informed.”
The research was supported by the Open Net Foundation, the Arthritis Foundation, Delaware Community Foundation, the Delaware Clinical and Translational Research (DE-CTR) ACCEL Program, the Nancy Taylor Foundation for Chronic Diseases, and CARRA. Dr. Brescia and Dr. Oliver have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Ongoing research in patients with oligoarticular juvenile idiopathic arthritis (JIA) so far suggests that a set of biomarkers in synovial fluid may help to predict which patients may be more likely to stay with persistent oligoarticular disease rather than progress to polyarticular disease, according to new research presented at the annual scientific meeting of the Childhood Arthritis and Rheumatology Research Alliance, held virtually this year. Identifying biomarkers in synovial fluid or possibly serum could aid families and physicians in being more proactive in treatment protocols, said AnneMarie C. Brescia, MD, of Nemours Children’s Hospital in Wilmington, Del.
“JIA carries the risk of permanent joint damage and disability, which can result when joint involvement evolves from oligoarticular into a polyarticular course, termed extended oligoarticular disease,” Dr. Brescia told attendees. “Since disease progression increases the risk for disability, early prediction of this course is essential.”
This group – those whose oligoarticular disease will begin recruiting joints and ultimately become extended oligoarticular JIA – is “very important because they have been shown to have worse health-related quality of life and greater risk of needing a joint replacement than even polyarticular [JIA],” Dr. Brescia said. “So, our lab has really focused on trying to predict who will fall in this group.”
Melissa Oliver, MD, assistant professor of clinical pediatrics in the division of pediatric rheumatology at Indiana University in Indianapolis, was not involved in the study but agreed that having highly sensitive and specific biomarkers could be particularly helpful in clinical care.
“Biomarkers can help guide treatment decisions and help physicians and their patients share the decision-making about next choices and when to change,” Dr. Oliver told this news organization. “If a provider and parent know that their child has these markers in their serum or synovial fluid that may predict extension of their disease, then they may be more aggressive upfront with therapy.”
The study aimed to determine whether differential levels of synovial fluid proteins could be used to predict whether JIA would evolve into an extended course before it became clinically evident. Although early aggressive treatment is common with rheumatoid arthritis and can lead to remission, JIA treatment paradigms tend to be more reactive, Dr. Brescia said.
“It would be better to switch to proactive, that if we’re able to predict that this patient may have a more difficult course with extension to polyarticular, we could be prepared, we could inform the parents, and it would just help us have a more proactive approach,” she said.
The researchers used antibody arrays to detect the following inflammatory mediators in blinded samples: CD14, interleukin (IL)-1-alpha, IL-3, IL-5, IL-6, vascular endothelial growth factor (VEGF), and angiogenin. They analyzed 37 samples with persistent disease and 32 samples from disease that had not yet extended but would become extended in that patient. The samples came from patients who were taking no medicines or only NSAIDs. The researchers assessed the sensitivity and specificity of each biomarker. Sensitivity referred the biomarker’s ability to correctly indicate that the sample would extend, and specificity referred to the biomarker’s accuracy in determining that the disease in the sample would remain persistent.
Combining samples from cohorts at Nemours Children’s Health (14 persistent and 7 extended-to-be) and Cincinnati Children’s Hospital (23 persistent and 25 extended-to-be) yielded the following results:
The findings revealed that the selected biomarkers were more accurate at predicting whose disease would remain persistent than predicting those that would extend, Dr. Brescia said. CD14 was the most specific biomarker, and IL-6 was the most sensitive biomarker in both groups.
When the researchers translated the findings from ELISA to the Luminex platform, positive results in synovial fluid for all these biomarkers were also positive in serum samples. Although the differences between persistent and extended-to-be samples did not reach statistical significance using Luminex, the pattern was the same for each biomarker.
“Luminex is more sensitive than ELISA. We believe that conducting an LDA [linear discriminant analysis] using these Luminex measurements will allow us to determine new cutoffs or new protein levels that are appropriate for Luminex to predict who will extend,” Dr. Brescia said. “It’s also our goal to develop a serum panel because ... being able to detect these markers in serum would expand the applicability of these markers to more patients.”
Dr. Brescia then described the group’s work in defining clinically relevant subpopulations of patients based on fibroblast-like synoviocytes (FLS) cells in the synovial intimal lining that produce inflammatory cytokines.
“Our compelling, single-cell, RNA sequencing preliminary data revealing multiple subpopulations within the total FLS population supports our hypothesis that distinct FLS subpopulations correlate with clinical outcome,” said Dr. Brescia. They looked at the percentage of chondrocyte-like, fibroblast-like, and smooth muscle-like subpopulations in samples from patients with oligoarticular JIA, extended-to-be JIA, and polyarticular JIA. Chondrocytes occurred in the largest proportion, and polyarticular JIA FLS had the largest percentage of chondrocytes, compared with the other two subpopulation groups.
“This is a work in progress,” Dr. Brescia said, “so hopefully you’ll hear about it next year.” In response to an attendee’s question, she said she believes identifying reliable biomarkers will eventually lead to refining treatment paradigms.
“I think it will at least change the guidance we can provide parents about making next choices and how quickly to accelerate to those next choices,” Dr. Brescia said. For example, if a child’s serum or synovial fluid has markers that show a very high likelihood of extension, the parent may decide to proceed to the next level medication sooner. “I do think it will push both parents and doctors to be a little more proactive instead of reactive when the poor patient comes back with 13 joints involved when they had just been an oligo for years.”
Dr. Oliver noted the promise of CD14 and IL-6 in potentially predicting which patients’ disease will stay persistent but cautioned that it’s still early in evaluating these biomarkers, especially with the limited patient samples in this study.
“I think these results are promising, and it’s great that there are groups out there working on this,” Dr. Oliver said. “Once we have a reliable, highly sensitive and specific biomarker, that will definitely help providers, parents, and patients be more informed.”
The research was supported by the Open Net Foundation, the Arthritis Foundation, Delaware Community Foundation, the Delaware Clinical and Translational Research (DE-CTR) ACCEL Program, the Nancy Taylor Foundation for Chronic Diseases, and CARRA. Dr. Brescia and Dr. Oliver have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM CARRA 2022
Monkeypox: What’s a pediatrician to do?
Not long ago, a pediatrician working in a local urgent care clinic called me about a teenage girl with a pruritic rash. She described vesicles and pustules located primarily on the face and arms with no surrounding cellulitis or other exam findings.
“She probably has impetigo,” my colleague said. “But I took a travel and exposure history and learned that her grandma had recently returned home from visiting family in the Congo. Do you think I need to worry about monkeypox?”
While most pediatricians in the United States have never seen a case of monkeypox, the virus is not new. An orthopox, it belongs to the same genus that includes smallpox and cowpox viruses. It was discovered in 1958 when two colonies of monkeys kept for research developed pox-like rashes. The earliest human case was reported in 1970 in the Democratic Republic of Congo and now the virus is endemic in some counties in Central and West Africa.
Monkeypox virus is a zoonotic disease – it can spread from animals to people. Rodents and other small mammals – not monkeys – are thought to be the most likely reservoir. The virus typically spreads from person to person through close contact with skin or respiratory secretions or contact with contaminated fomites. Typical infection begins with fever, lymphadenopathy, and flulike symptoms that include headache and malaise. One to four days after the onset of fever, the characteristic rash begins as macular lesions that evolve into papules, then vesicles, and finally pustules. Pustular lesions are deep-seated, well circumscribed, and are usually the same size and in the same stage of development on a given body site. The rash often starts on the face or the mouth, and then moves to the extremities, including the palms and soles. Over time, the lesions umbilicate and ultimately crust over.
On May 20, the Centers for Disease Control and Prevention issued a Health Advisory describing a case of monkeypox in a patient in Massachusetts. A single case normally wouldn’t cause too much alarm. In fact, there were two cases reported in the United States in 2021, both in travelers returning to the United States from Nigeria, a country in which the virus is endemic. No transmissions from these individuals to close contacts were identified.
The Massachusetts case was remarkable for two reasons. It occurred in an individual who had recently returned from a trip to Canada, which is not a country in which the virus is endemic. Additionally, it occurred in the context of a global outbreak of monkey pox that has, to date, disproportionately affected individuals who identify as men who have sex with men. Patients have often lacked the characteristic prodrome and many have had rash localized to the perianal and genital area, with or without symptoms of proctitis (anorectal pain, tenesmus, and bleeding). Clinically, some lesions mimicked sexually transmitted infections that the occur in the anogenital area, including herpes, syphilis, and lymphogranuloma venereum.
As of May 31, 2022, 17 persons in nine states had been diagnosed with presumed monkeypox virus infection. They ranged in age from 28 to 61 years and 16/17 identified as MSM. Fourteen reported international travel in the 3 weeks before developing symptoms. As of June 12, that number had grown to 53, while worldwide the number of confirmed and suspected cases reached 1,584. Up-to-date case counts are available at https://ourworldindata.org/monkeypox.
Back on the phone, my colleague laughed a little nervously. “I guess I’m not really worried about monkeypox in my patient.” She paused and then asked, “This isn’t going to be the next pandemic, is it?”
Public health experts at the Centers for Disease Control and Prevention and the World Health Organization have been reassuring in that regard. Two vaccines are available for the prevention of monkeypox. JYNNEOS is a nonreplicating live viral vaccine licensed as a two-dose series to prevent both monkeypox and smallpox. ACAM 2000 is a live Vaccinia virus preparation licensed to prevent smallpox. These vaccines are effective when given before exposure but are thought to also beneficial when given as postexposure prophylaxis. According to the CDC, vaccination within 4 days of exposure can prevent the development of disease. Vaccination within 14 days of exposure may not prevent the development of disease but may lessen symptoms. Treatment is generally supportive but antiviral therapy could be considered for individuals with severe disease. Tecovirmat is Food and Drug Administration approved for the treatment of smallpox but is available under nonresearch Expanded Access Investigational New Drug (EA-IND) protocol for the treatment of children and adults with severe orthopox infections, including monkeypox.
So, what’s a pediatrician to do? Take a good travel history, as my colleague did, because that is good medicine. At this point in an outbreak though, a lack of travel does not exclude the diagnosis. Perform a thorough exam of skin and mucosal areas. When there are rashes in the genital or perianal area, consider the possibility of monkeypox in addition to typical sexually transmitted infections. Ask about exposure to other persons with similar rashes, as well as close or intimate contact with a persons in a social network experiencing monkeypox infections. This includes MSM who meet partners through an online website, app, or at social events. Monkeypox can also be spread through contact with an animal (dead or alive) that is an African endemic species or use of a product derived from such animals. Public health experts encourage clinicians to be alert for rash illnesses consistent with monkeypox, regardless of a patient’s gender or sexual orientation, history of international travel, or specific risk factors.
Pediatricians see many kids with rashes, and while cases of monkeypox climb daily, the disease is still very rare. Given the media coverage of the outbreak, pediatricians should be prepared for questions from patients and their parents. Clinicians who suspect a case of monkeypox should contact their local or state health department for guidance and the need for testing. Tips for recognizing monkeypox and distinguishing it from more common viral illnesses such as chicken pox are available at www.cdc.gov/poxvirus/monkeypox/clinicians/clinical-recognition.html.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She said she had no relevant financial disclosures. Email her at [email protected].
Not long ago, a pediatrician working in a local urgent care clinic called me about a teenage girl with a pruritic rash. She described vesicles and pustules located primarily on the face and arms with no surrounding cellulitis or other exam findings.
“She probably has impetigo,” my colleague said. “But I took a travel and exposure history and learned that her grandma had recently returned home from visiting family in the Congo. Do you think I need to worry about monkeypox?”
While most pediatricians in the United States have never seen a case of monkeypox, the virus is not new. An orthopox, it belongs to the same genus that includes smallpox and cowpox viruses. It was discovered in 1958 when two colonies of monkeys kept for research developed pox-like rashes. The earliest human case was reported in 1970 in the Democratic Republic of Congo and now the virus is endemic in some counties in Central and West Africa.
Monkeypox virus is a zoonotic disease – it can spread from animals to people. Rodents and other small mammals – not monkeys – are thought to be the most likely reservoir. The virus typically spreads from person to person through close contact with skin or respiratory secretions or contact with contaminated fomites. Typical infection begins with fever, lymphadenopathy, and flulike symptoms that include headache and malaise. One to four days after the onset of fever, the characteristic rash begins as macular lesions that evolve into papules, then vesicles, and finally pustules. Pustular lesions are deep-seated, well circumscribed, and are usually the same size and in the same stage of development on a given body site. The rash often starts on the face or the mouth, and then moves to the extremities, including the palms and soles. Over time, the lesions umbilicate and ultimately crust over.
On May 20, the Centers for Disease Control and Prevention issued a Health Advisory describing a case of monkeypox in a patient in Massachusetts. A single case normally wouldn’t cause too much alarm. In fact, there were two cases reported in the United States in 2021, both in travelers returning to the United States from Nigeria, a country in which the virus is endemic. No transmissions from these individuals to close contacts were identified.
The Massachusetts case was remarkable for two reasons. It occurred in an individual who had recently returned from a trip to Canada, which is not a country in which the virus is endemic. Additionally, it occurred in the context of a global outbreak of monkey pox that has, to date, disproportionately affected individuals who identify as men who have sex with men. Patients have often lacked the characteristic prodrome and many have had rash localized to the perianal and genital area, with or without symptoms of proctitis (anorectal pain, tenesmus, and bleeding). Clinically, some lesions mimicked sexually transmitted infections that the occur in the anogenital area, including herpes, syphilis, and lymphogranuloma venereum.
As of May 31, 2022, 17 persons in nine states had been diagnosed with presumed monkeypox virus infection. They ranged in age from 28 to 61 years and 16/17 identified as MSM. Fourteen reported international travel in the 3 weeks before developing symptoms. As of June 12, that number had grown to 53, while worldwide the number of confirmed and suspected cases reached 1,584. Up-to-date case counts are available at https://ourworldindata.org/monkeypox.
Back on the phone, my colleague laughed a little nervously. “I guess I’m not really worried about monkeypox in my patient.” She paused and then asked, “This isn’t going to be the next pandemic, is it?”
Public health experts at the Centers for Disease Control and Prevention and the World Health Organization have been reassuring in that regard. Two vaccines are available for the prevention of monkeypox. JYNNEOS is a nonreplicating live viral vaccine licensed as a two-dose series to prevent both monkeypox and smallpox. ACAM 2000 is a live Vaccinia virus preparation licensed to prevent smallpox. These vaccines are effective when given before exposure but are thought to also beneficial when given as postexposure prophylaxis. According to the CDC, vaccination within 4 days of exposure can prevent the development of disease. Vaccination within 14 days of exposure may not prevent the development of disease but may lessen symptoms. Treatment is generally supportive but antiviral therapy could be considered for individuals with severe disease. Tecovirmat is Food and Drug Administration approved for the treatment of smallpox but is available under nonresearch Expanded Access Investigational New Drug (EA-IND) protocol for the treatment of children and adults with severe orthopox infections, including monkeypox.
So, what’s a pediatrician to do? Take a good travel history, as my colleague did, because that is good medicine. At this point in an outbreak though, a lack of travel does not exclude the diagnosis. Perform a thorough exam of skin and mucosal areas. When there are rashes in the genital or perianal area, consider the possibility of monkeypox in addition to typical sexually transmitted infections. Ask about exposure to other persons with similar rashes, as well as close or intimate contact with a persons in a social network experiencing monkeypox infections. This includes MSM who meet partners through an online website, app, or at social events. Monkeypox can also be spread through contact with an animal (dead or alive) that is an African endemic species or use of a product derived from such animals. Public health experts encourage clinicians to be alert for rash illnesses consistent with monkeypox, regardless of a patient’s gender or sexual orientation, history of international travel, or specific risk factors.
Pediatricians see many kids with rashes, and while cases of monkeypox climb daily, the disease is still very rare. Given the media coverage of the outbreak, pediatricians should be prepared for questions from patients and their parents. Clinicians who suspect a case of monkeypox should contact their local or state health department for guidance and the need for testing. Tips for recognizing monkeypox and distinguishing it from more common viral illnesses such as chicken pox are available at www.cdc.gov/poxvirus/monkeypox/clinicians/clinical-recognition.html.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She said she had no relevant financial disclosures. Email her at [email protected].
Not long ago, a pediatrician working in a local urgent care clinic called me about a teenage girl with a pruritic rash. She described vesicles and pustules located primarily on the face and arms with no surrounding cellulitis or other exam findings.
“She probably has impetigo,” my colleague said. “But I took a travel and exposure history and learned that her grandma had recently returned home from visiting family in the Congo. Do you think I need to worry about monkeypox?”
While most pediatricians in the United States have never seen a case of monkeypox, the virus is not new. An orthopox, it belongs to the same genus that includes smallpox and cowpox viruses. It was discovered in 1958 when two colonies of monkeys kept for research developed pox-like rashes. The earliest human case was reported in 1970 in the Democratic Republic of Congo and now the virus is endemic in some counties in Central and West Africa.
Monkeypox virus is a zoonotic disease – it can spread from animals to people. Rodents and other small mammals – not monkeys – are thought to be the most likely reservoir. The virus typically spreads from person to person through close contact with skin or respiratory secretions or contact with contaminated fomites. Typical infection begins with fever, lymphadenopathy, and flulike symptoms that include headache and malaise. One to four days after the onset of fever, the characteristic rash begins as macular lesions that evolve into papules, then vesicles, and finally pustules. Pustular lesions are deep-seated, well circumscribed, and are usually the same size and in the same stage of development on a given body site. The rash often starts on the face or the mouth, and then moves to the extremities, including the palms and soles. Over time, the lesions umbilicate and ultimately crust over.
On May 20, the Centers for Disease Control and Prevention issued a Health Advisory describing a case of monkeypox in a patient in Massachusetts. A single case normally wouldn’t cause too much alarm. In fact, there were two cases reported in the United States in 2021, both in travelers returning to the United States from Nigeria, a country in which the virus is endemic. No transmissions from these individuals to close contacts were identified.
The Massachusetts case was remarkable for two reasons. It occurred in an individual who had recently returned from a trip to Canada, which is not a country in which the virus is endemic. Additionally, it occurred in the context of a global outbreak of monkey pox that has, to date, disproportionately affected individuals who identify as men who have sex with men. Patients have often lacked the characteristic prodrome and many have had rash localized to the perianal and genital area, with or without symptoms of proctitis (anorectal pain, tenesmus, and bleeding). Clinically, some lesions mimicked sexually transmitted infections that the occur in the anogenital area, including herpes, syphilis, and lymphogranuloma venereum.
As of May 31, 2022, 17 persons in nine states had been diagnosed with presumed monkeypox virus infection. They ranged in age from 28 to 61 years and 16/17 identified as MSM. Fourteen reported international travel in the 3 weeks before developing symptoms. As of June 12, that number had grown to 53, while worldwide the number of confirmed and suspected cases reached 1,584. Up-to-date case counts are available at https://ourworldindata.org/monkeypox.
Back on the phone, my colleague laughed a little nervously. “I guess I’m not really worried about monkeypox in my patient.” She paused and then asked, “This isn’t going to be the next pandemic, is it?”
Public health experts at the Centers for Disease Control and Prevention and the World Health Organization have been reassuring in that regard. Two vaccines are available for the prevention of monkeypox. JYNNEOS is a nonreplicating live viral vaccine licensed as a two-dose series to prevent both monkeypox and smallpox. ACAM 2000 is a live Vaccinia virus preparation licensed to prevent smallpox. These vaccines are effective when given before exposure but are thought to also beneficial when given as postexposure prophylaxis. According to the CDC, vaccination within 4 days of exposure can prevent the development of disease. Vaccination within 14 days of exposure may not prevent the development of disease but may lessen symptoms. Treatment is generally supportive but antiviral therapy could be considered for individuals with severe disease. Tecovirmat is Food and Drug Administration approved for the treatment of smallpox but is available under nonresearch Expanded Access Investigational New Drug (EA-IND) protocol for the treatment of children and adults with severe orthopox infections, including monkeypox.
So, what’s a pediatrician to do? Take a good travel history, as my colleague did, because that is good medicine. At this point in an outbreak though, a lack of travel does not exclude the diagnosis. Perform a thorough exam of skin and mucosal areas. When there are rashes in the genital or perianal area, consider the possibility of monkeypox in addition to typical sexually transmitted infections. Ask about exposure to other persons with similar rashes, as well as close or intimate contact with a persons in a social network experiencing monkeypox infections. This includes MSM who meet partners through an online website, app, or at social events. Monkeypox can also be spread through contact with an animal (dead or alive) that is an African endemic species or use of a product derived from such animals. Public health experts encourage clinicians to be alert for rash illnesses consistent with monkeypox, regardless of a patient’s gender or sexual orientation, history of international travel, or specific risk factors.
Pediatricians see many kids with rashes, and while cases of monkeypox climb daily, the disease is still very rare. Given the media coverage of the outbreak, pediatricians should be prepared for questions from patients and their parents. Clinicians who suspect a case of monkeypox should contact their local or state health department for guidance and the need for testing. Tips for recognizing monkeypox and distinguishing it from more common viral illnesses such as chicken pox are available at www.cdc.gov/poxvirus/monkeypox/clinicians/clinical-recognition.html.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She said she had no relevant financial disclosures. Email her at [email protected].
Biden boosts LGBTQIA+ protections, bans conversion therapy
President Joe Biden issued an executive order on June 15 banning conversion therapy and offering other LBGTQIA+ protections as part of White House efforts to advance equality during Pride Month.
“My order will use the full force of the federal government to end inhumane practices of conversion therapy,” President Biden said in a speech before signing the order. “This is the first time the federal government is making a coordinated effort against this dangerous and discredited practice.”
Conversion therapy is any emotional or physical therapy used to “cure” or “repair” a person’s attraction to the same sex, or their gender identity and expression. Providers claim these therapies can make someone heterosexual or “straight.” But there’s no evidence to support this.
Medical and mental health experts have rejected conversion therapy practices as dangerous and discriminatory for decades.
The executive order also addresses:
- The LGBTQIA+ youth mental health crisis, in part by expanding suicide prevention resources for that at-risk population.
- Discrimination within the foster care system against LGBTQIA+ children and parents.
- Discrimination, poverty and isolation challenges faced by LGBTQIA+ seniors.
- Efforts to strengthen federal data collection in this population to counter homelessness, housing insecurity and barriers to health care access.
Enforcement of executive order will rely on legal experts, including the Justice Department.
President Biden’s order comes at a time when multiple states are promoting or passing anti-LGBTQIA+ laws.
“I don’t have to tell you about the ultra-MAGA agenda attacking our freedoms. There are more than 300 discriminatory bills introduced in states across this country,” President Biden said. “In Texas, they are knocking on front doors to investigate parents who are raising transgender children, and in Florida they are going after Mickey Mouse for God’s sake.”
First Lady Jill Biden, PhD, said the order will not solve all problems. “Prejudice and discrimination still lurk. We will not let the progress we fought for slip away. Pride is a celebration of the courage it takes to stand up for what’s right.”
The American Psychiatric Association applauded President Biden’s action. This executive order will “protect the mental health of LGBTQ+ people, particularly children. APA has long condemned the practice of so-called ‘conversion therapy’ and we welcome the federal government’s efforts to raise public awareness about its harms, alongside other practices that will help to end it.”
The goal of the order is to “improve the health, wellbeing, and safety of countless families across the country,” senior White House administration officials said in a June 15 media call. “And they will send a powerful signal from the president of the United States to LGBTQIA+ kids across the country – who may be feeling scared and hopeless – that their president has their back.”
Biden also called on Congress to pass the Equality Act “to enshrine the long overdue civil rights to protect all Americans.”
The event was held in the East Room of the White House at a Pride event attended by Vice President Kamala Harris and her husband, the first lady, Transportation Secretary Pete Buttigieg, and hundreds of LGBTQIA+ leaders.
Guidance on starting transgender treatment
In other LGBTQIA+-related news, an international group focusing on transgender health lowered the minimum ages they recommend for starting hormone therapy or surgery for transgender youth.
The World Professional Association for Transgender Health said that hormones could be started at 14, 2 years earlier than the group’s previous advice. The association also said some surgeries can be performed at age 15 or 17, a year or so earlier than their previous recommendations.
The group acknowledged potential risks but said it is unethical and harmful to withhold early treatment, according to a report from The Associated Press.
Transgender treatment for teens has been a controversial issue, with experts disagreeing about whether teenagers can fully understand the ramifications of such life-altering decisions.
During the White House background media call, senior administration officials pointed to existing policy regarding transgender care. “We’ve already put out guidance through HHS about civil rights protections and making clear that the denial of medical care based on someone’s gender identity is discriminatory and have invited the members of the public to file complaints with the Office of Civil Rights.”
A version of this article first appeared on WebMD.com.
President Joe Biden issued an executive order on June 15 banning conversion therapy and offering other LBGTQIA+ protections as part of White House efforts to advance equality during Pride Month.
“My order will use the full force of the federal government to end inhumane practices of conversion therapy,” President Biden said in a speech before signing the order. “This is the first time the federal government is making a coordinated effort against this dangerous and discredited practice.”
Conversion therapy is any emotional or physical therapy used to “cure” or “repair” a person’s attraction to the same sex, or their gender identity and expression. Providers claim these therapies can make someone heterosexual or “straight.” But there’s no evidence to support this.
Medical and mental health experts have rejected conversion therapy practices as dangerous and discriminatory for decades.
The executive order also addresses:
- The LGBTQIA+ youth mental health crisis, in part by expanding suicide prevention resources for that at-risk population.
- Discrimination within the foster care system against LGBTQIA+ children and parents.
- Discrimination, poverty and isolation challenges faced by LGBTQIA+ seniors.
- Efforts to strengthen federal data collection in this population to counter homelessness, housing insecurity and barriers to health care access.
Enforcement of executive order will rely on legal experts, including the Justice Department.
President Biden’s order comes at a time when multiple states are promoting or passing anti-LGBTQIA+ laws.
“I don’t have to tell you about the ultra-MAGA agenda attacking our freedoms. There are more than 300 discriminatory bills introduced in states across this country,” President Biden said. “In Texas, they are knocking on front doors to investigate parents who are raising transgender children, and in Florida they are going after Mickey Mouse for God’s sake.”
First Lady Jill Biden, PhD, said the order will not solve all problems. “Prejudice and discrimination still lurk. We will not let the progress we fought for slip away. Pride is a celebration of the courage it takes to stand up for what’s right.”
The American Psychiatric Association applauded President Biden’s action. This executive order will “protect the mental health of LGBTQ+ people, particularly children. APA has long condemned the practice of so-called ‘conversion therapy’ and we welcome the federal government’s efforts to raise public awareness about its harms, alongside other practices that will help to end it.”
The goal of the order is to “improve the health, wellbeing, and safety of countless families across the country,” senior White House administration officials said in a June 15 media call. “And they will send a powerful signal from the president of the United States to LGBTQIA+ kids across the country – who may be feeling scared and hopeless – that their president has their back.”
Biden also called on Congress to pass the Equality Act “to enshrine the long overdue civil rights to protect all Americans.”
The event was held in the East Room of the White House at a Pride event attended by Vice President Kamala Harris and her husband, the first lady, Transportation Secretary Pete Buttigieg, and hundreds of LGBTQIA+ leaders.
Guidance on starting transgender treatment
In other LGBTQIA+-related news, an international group focusing on transgender health lowered the minimum ages they recommend for starting hormone therapy or surgery for transgender youth.
The World Professional Association for Transgender Health said that hormones could be started at 14, 2 years earlier than the group’s previous advice. The association also said some surgeries can be performed at age 15 or 17, a year or so earlier than their previous recommendations.
The group acknowledged potential risks but said it is unethical and harmful to withhold early treatment, according to a report from The Associated Press.
Transgender treatment for teens has been a controversial issue, with experts disagreeing about whether teenagers can fully understand the ramifications of such life-altering decisions.
During the White House background media call, senior administration officials pointed to existing policy regarding transgender care. “We’ve already put out guidance through HHS about civil rights protections and making clear that the denial of medical care based on someone’s gender identity is discriminatory and have invited the members of the public to file complaints with the Office of Civil Rights.”
A version of this article first appeared on WebMD.com.
President Joe Biden issued an executive order on June 15 banning conversion therapy and offering other LBGTQIA+ protections as part of White House efforts to advance equality during Pride Month.
“My order will use the full force of the federal government to end inhumane practices of conversion therapy,” President Biden said in a speech before signing the order. “This is the first time the federal government is making a coordinated effort against this dangerous and discredited practice.”
Conversion therapy is any emotional or physical therapy used to “cure” or “repair” a person’s attraction to the same sex, or their gender identity and expression. Providers claim these therapies can make someone heterosexual or “straight.” But there’s no evidence to support this.
Medical and mental health experts have rejected conversion therapy practices as dangerous and discriminatory for decades.
The executive order also addresses:
- The LGBTQIA+ youth mental health crisis, in part by expanding suicide prevention resources for that at-risk population.
- Discrimination within the foster care system against LGBTQIA+ children and parents.
- Discrimination, poverty and isolation challenges faced by LGBTQIA+ seniors.
- Efforts to strengthen federal data collection in this population to counter homelessness, housing insecurity and barriers to health care access.
Enforcement of executive order will rely on legal experts, including the Justice Department.
President Biden’s order comes at a time when multiple states are promoting or passing anti-LGBTQIA+ laws.
“I don’t have to tell you about the ultra-MAGA agenda attacking our freedoms. There are more than 300 discriminatory bills introduced in states across this country,” President Biden said. “In Texas, they are knocking on front doors to investigate parents who are raising transgender children, and in Florida they are going after Mickey Mouse for God’s sake.”
First Lady Jill Biden, PhD, said the order will not solve all problems. “Prejudice and discrimination still lurk. We will not let the progress we fought for slip away. Pride is a celebration of the courage it takes to stand up for what’s right.”
The American Psychiatric Association applauded President Biden’s action. This executive order will “protect the mental health of LGBTQ+ people, particularly children. APA has long condemned the practice of so-called ‘conversion therapy’ and we welcome the federal government’s efforts to raise public awareness about its harms, alongside other practices that will help to end it.”
The goal of the order is to “improve the health, wellbeing, and safety of countless families across the country,” senior White House administration officials said in a June 15 media call. “And they will send a powerful signal from the president of the United States to LGBTQIA+ kids across the country – who may be feeling scared and hopeless – that their president has their back.”
Biden also called on Congress to pass the Equality Act “to enshrine the long overdue civil rights to protect all Americans.”
The event was held in the East Room of the White House at a Pride event attended by Vice President Kamala Harris and her husband, the first lady, Transportation Secretary Pete Buttigieg, and hundreds of LGBTQIA+ leaders.
Guidance on starting transgender treatment
In other LGBTQIA+-related news, an international group focusing on transgender health lowered the minimum ages they recommend for starting hormone therapy or surgery for transgender youth.
The World Professional Association for Transgender Health said that hormones could be started at 14, 2 years earlier than the group’s previous advice. The association also said some surgeries can be performed at age 15 or 17, a year or so earlier than their previous recommendations.
The group acknowledged potential risks but said it is unethical and harmful to withhold early treatment, according to a report from The Associated Press.
Transgender treatment for teens has been a controversial issue, with experts disagreeing about whether teenagers can fully understand the ramifications of such life-altering decisions.
During the White House background media call, senior administration officials pointed to existing policy regarding transgender care. “We’ve already put out guidance through HHS about civil rights protections and making clear that the denial of medical care based on someone’s gender identity is discriminatory and have invited the members of the public to file complaints with the Office of Civil Rights.”
A version of this article first appeared on WebMD.com.
Pediatric hepatitis has not increased during pandemic: CDC
The number of pediatric hepatitis cases has remained steady since 2017, new research from the Centers for Disease Control and Prevention suggests, despite the recent investigation into children with hepatitis of unknown cause. The study also found that there was no indication of elevated rates of adenovirus type 40/41 infection in children.
But Rohit Kohli, MBBS, MS, chief of the Division of Gastroenterology, Hepatology, and Nutrition at the Children’s Hospital Los Angeles, California, says that although the study is “well-designed and robust,” that does not mean that these hepatitis cases of unknown origin are no longer a concern. He was not involved with the CDC research. “As a clinician, I’m still worried,” he said. “Why I feel like this is not conclusive is that there are other data from entities like the United Kingdom Health Security Agency that are incongruent with [these findings],” he said.
The research was published in the CDC’s Morbidity and Mortality Weekly Report.
In November 2021, the Alabama Department of Public Health began an investigation with the CDC after a cluster of children were admitted to a children’s hospital in the state with severe hepatitis, who all tested positive for adenovirus. When the United Kingdom’s Health Security Agency announced an investigation into similar cases in early April 2022, the CDC decided to expand their search nationally.
Now, as of June 15, the agency is investigating 290 cases in 41 states and U.S. territories. Worldwide, 650 cases in 33 countries have been reported, according to the most recent update by the World Health Organization on May 27, 2022. At least 38 patients have needed liver transplants, and nine deaths have been reported to WHO.
In its most recent press call on the topic, the CDC announced that it’s aware of six deaths in the United States through May 20, 2022. The COVID-19 vaccine has been ruled out as a potential cause because the majority of affected children are unvaccinated or are too young to receive the vaccine. Adenovirus infection remains a leading suspect in these sick children because the virus has been detected in 60.8% of tested cases, WHO reports.
Investigators have detected an increase in reported pediatric hepatitis cases, compared with prior years in the United Kingdom, but it was not clear whether that same pattern would be found in the United States. Neither pediatric hepatitis nor adenovirus type 40/41 are reportable conditions in the United States. In the May 20 CDC press call, Umesh Parashar, MD, chief of the CDC’s Viral Gastroenteritis Branch, said that an estimated 1,500-2,000 children aged younger than 10 are hospitalized in the United States for hepatitis every year. “That’s a fairly large number,” he said, and it might make it difficult to detect a small increase in cases.
To better estimate trends in pediatric hepatitis and adenovirus infection in the United States, investigators collected available data on emergency department (ED) visits, hospitalizations, and liver transplants associated with hepatitis in children as well as adenovirus stool testing results. Researchers used four large databases: the National Syndromic Surveillance Program; the Premier Healthcare Database Special Release; the Organ Procurement and Transplant Network; and Labcorp, which is a large commercial lab network.
To account for changes in health care utilization in the first year of the COVID-19 pandemic, the team compared hepatitis-associated ED visits, hospitalizations, and liver transplants from October 2021 to March 2022 versus the same months (January to March and October to December) in 2017, 2018, and 2019. For adenovirus stool testing, results from October 2021 to March 2022 were compared with the same calendar months (October to March) from 2017-2018, 2018-2019, and 2019-2020, to help control for seasonality.
Investigators found no statistically significant increases in the outcomes during October 2021 to March 2022 versus pre-pandemic years:
- Weekly ED visits with hepatitis-associated discharge codes
- Hepatitis-associated monthly hospitalizations in children aged 0-4 years (22 vs. 19.5; P = .26)
- Hepatitis-associated monthly hospitalization in children aged 5-11 years (12 vs. 10.5; P = .42)
- Monthly liver transplants (5 vs. 4; P = .19)
- Percentage of stool specimens positive for adenovirus types 40/41, though the number of specimens tested was highest in March 2022
The authors acknowledged that pediatric hepatitis is rare, so it may be difficult tease out small changes in the number of cases. Also, data on hospitalizations and liver transplants have a 2- to 3-month reporting delay, so the case counts for March 2022 “might be underreported,” they wrote. Mr. Kohli noted that because hepatitis and adenovirus are not reportable conditions, the analysis relied on retrospective data from insurance companies and electronic medical records. Retrospective data are inherently limited, compared with prospective analyses, he said, and it’s possible that certain cases could be included in more than one database and thus be double-counted, whereas other cases could be missed entirely.
These findings also conflict with data from the United Kingdom, which in May reported that the average number of hepatitis cases had increased, compared with previous years, he said. More data are needed, he said, and he is involved with a study with the North American Society for Pediatric Gastroenterology and the American Association for the Study of Liver Diseases that is also collecting data to try to understand whether there has been an uptick in pediatric hepatitis cases. The study will collect patient data directly from hospitals as well as include additional pathology data, such as biopsy results.
“We should not be inhibited to look further academically – and public health–wise – while we take into cognizance this very good, robust attempt from the CDC,” he said.
A version of this article first appeared on Medscape.com.
The number of pediatric hepatitis cases has remained steady since 2017, new research from the Centers for Disease Control and Prevention suggests, despite the recent investigation into children with hepatitis of unknown cause. The study also found that there was no indication of elevated rates of adenovirus type 40/41 infection in children.
But Rohit Kohli, MBBS, MS, chief of the Division of Gastroenterology, Hepatology, and Nutrition at the Children’s Hospital Los Angeles, California, says that although the study is “well-designed and robust,” that does not mean that these hepatitis cases of unknown origin are no longer a concern. He was not involved with the CDC research. “As a clinician, I’m still worried,” he said. “Why I feel like this is not conclusive is that there are other data from entities like the United Kingdom Health Security Agency that are incongruent with [these findings],” he said.
The research was published in the CDC’s Morbidity and Mortality Weekly Report.
In November 2021, the Alabama Department of Public Health began an investigation with the CDC after a cluster of children were admitted to a children’s hospital in the state with severe hepatitis, who all tested positive for adenovirus. When the United Kingdom’s Health Security Agency announced an investigation into similar cases in early April 2022, the CDC decided to expand their search nationally.
Now, as of June 15, the agency is investigating 290 cases in 41 states and U.S. territories. Worldwide, 650 cases in 33 countries have been reported, according to the most recent update by the World Health Organization on May 27, 2022. At least 38 patients have needed liver transplants, and nine deaths have been reported to WHO.
In its most recent press call on the topic, the CDC announced that it’s aware of six deaths in the United States through May 20, 2022. The COVID-19 vaccine has been ruled out as a potential cause because the majority of affected children are unvaccinated or are too young to receive the vaccine. Adenovirus infection remains a leading suspect in these sick children because the virus has been detected in 60.8% of tested cases, WHO reports.
Investigators have detected an increase in reported pediatric hepatitis cases, compared with prior years in the United Kingdom, but it was not clear whether that same pattern would be found in the United States. Neither pediatric hepatitis nor adenovirus type 40/41 are reportable conditions in the United States. In the May 20 CDC press call, Umesh Parashar, MD, chief of the CDC’s Viral Gastroenteritis Branch, said that an estimated 1,500-2,000 children aged younger than 10 are hospitalized in the United States for hepatitis every year. “That’s a fairly large number,” he said, and it might make it difficult to detect a small increase in cases.
To better estimate trends in pediatric hepatitis and adenovirus infection in the United States, investigators collected available data on emergency department (ED) visits, hospitalizations, and liver transplants associated with hepatitis in children as well as adenovirus stool testing results. Researchers used four large databases: the National Syndromic Surveillance Program; the Premier Healthcare Database Special Release; the Organ Procurement and Transplant Network; and Labcorp, which is a large commercial lab network.
To account for changes in health care utilization in the first year of the COVID-19 pandemic, the team compared hepatitis-associated ED visits, hospitalizations, and liver transplants from October 2021 to March 2022 versus the same months (January to March and October to December) in 2017, 2018, and 2019. For adenovirus stool testing, results from October 2021 to March 2022 were compared with the same calendar months (October to March) from 2017-2018, 2018-2019, and 2019-2020, to help control for seasonality.
Investigators found no statistically significant increases in the outcomes during October 2021 to March 2022 versus pre-pandemic years:
- Weekly ED visits with hepatitis-associated discharge codes
- Hepatitis-associated monthly hospitalizations in children aged 0-4 years (22 vs. 19.5; P = .26)
- Hepatitis-associated monthly hospitalization in children aged 5-11 years (12 vs. 10.5; P = .42)
- Monthly liver transplants (5 vs. 4; P = .19)
- Percentage of stool specimens positive for adenovirus types 40/41, though the number of specimens tested was highest in March 2022
The authors acknowledged that pediatric hepatitis is rare, so it may be difficult tease out small changes in the number of cases. Also, data on hospitalizations and liver transplants have a 2- to 3-month reporting delay, so the case counts for March 2022 “might be underreported,” they wrote. Mr. Kohli noted that because hepatitis and adenovirus are not reportable conditions, the analysis relied on retrospective data from insurance companies and electronic medical records. Retrospective data are inherently limited, compared with prospective analyses, he said, and it’s possible that certain cases could be included in more than one database and thus be double-counted, whereas other cases could be missed entirely.
These findings also conflict with data from the United Kingdom, which in May reported that the average number of hepatitis cases had increased, compared with previous years, he said. More data are needed, he said, and he is involved with a study with the North American Society for Pediatric Gastroenterology and the American Association for the Study of Liver Diseases that is also collecting data to try to understand whether there has been an uptick in pediatric hepatitis cases. The study will collect patient data directly from hospitals as well as include additional pathology data, such as biopsy results.
“We should not be inhibited to look further academically – and public health–wise – while we take into cognizance this very good, robust attempt from the CDC,” he said.
A version of this article first appeared on Medscape.com.
The number of pediatric hepatitis cases has remained steady since 2017, new research from the Centers for Disease Control and Prevention suggests, despite the recent investigation into children with hepatitis of unknown cause. The study also found that there was no indication of elevated rates of adenovirus type 40/41 infection in children.
But Rohit Kohli, MBBS, MS, chief of the Division of Gastroenterology, Hepatology, and Nutrition at the Children’s Hospital Los Angeles, California, says that although the study is “well-designed and robust,” that does not mean that these hepatitis cases of unknown origin are no longer a concern. He was not involved with the CDC research. “As a clinician, I’m still worried,” he said. “Why I feel like this is not conclusive is that there are other data from entities like the United Kingdom Health Security Agency that are incongruent with [these findings],” he said.
The research was published in the CDC’s Morbidity and Mortality Weekly Report.
In November 2021, the Alabama Department of Public Health began an investigation with the CDC after a cluster of children were admitted to a children’s hospital in the state with severe hepatitis, who all tested positive for adenovirus. When the United Kingdom’s Health Security Agency announced an investigation into similar cases in early April 2022, the CDC decided to expand their search nationally.
Now, as of June 15, the agency is investigating 290 cases in 41 states and U.S. territories. Worldwide, 650 cases in 33 countries have been reported, according to the most recent update by the World Health Organization on May 27, 2022. At least 38 patients have needed liver transplants, and nine deaths have been reported to WHO.
In its most recent press call on the topic, the CDC announced that it’s aware of six deaths in the United States through May 20, 2022. The COVID-19 vaccine has been ruled out as a potential cause because the majority of affected children are unvaccinated or are too young to receive the vaccine. Adenovirus infection remains a leading suspect in these sick children because the virus has been detected in 60.8% of tested cases, WHO reports.
Investigators have detected an increase in reported pediatric hepatitis cases, compared with prior years in the United Kingdom, but it was not clear whether that same pattern would be found in the United States. Neither pediatric hepatitis nor adenovirus type 40/41 are reportable conditions in the United States. In the May 20 CDC press call, Umesh Parashar, MD, chief of the CDC’s Viral Gastroenteritis Branch, said that an estimated 1,500-2,000 children aged younger than 10 are hospitalized in the United States for hepatitis every year. “That’s a fairly large number,” he said, and it might make it difficult to detect a small increase in cases.
To better estimate trends in pediatric hepatitis and adenovirus infection in the United States, investigators collected available data on emergency department (ED) visits, hospitalizations, and liver transplants associated with hepatitis in children as well as adenovirus stool testing results. Researchers used four large databases: the National Syndromic Surveillance Program; the Premier Healthcare Database Special Release; the Organ Procurement and Transplant Network; and Labcorp, which is a large commercial lab network.
To account for changes in health care utilization in the first year of the COVID-19 pandemic, the team compared hepatitis-associated ED visits, hospitalizations, and liver transplants from October 2021 to March 2022 versus the same months (January to March and October to December) in 2017, 2018, and 2019. For adenovirus stool testing, results from October 2021 to March 2022 were compared with the same calendar months (October to March) from 2017-2018, 2018-2019, and 2019-2020, to help control for seasonality.
Investigators found no statistically significant increases in the outcomes during October 2021 to March 2022 versus pre-pandemic years:
- Weekly ED visits with hepatitis-associated discharge codes
- Hepatitis-associated monthly hospitalizations in children aged 0-4 years (22 vs. 19.5; P = .26)
- Hepatitis-associated monthly hospitalization in children aged 5-11 years (12 vs. 10.5; P = .42)
- Monthly liver transplants (5 vs. 4; P = .19)
- Percentage of stool specimens positive for adenovirus types 40/41, though the number of specimens tested was highest in March 2022
The authors acknowledged that pediatric hepatitis is rare, so it may be difficult tease out small changes in the number of cases. Also, data on hospitalizations and liver transplants have a 2- to 3-month reporting delay, so the case counts for March 2022 “might be underreported,” they wrote. Mr. Kohli noted that because hepatitis and adenovirus are not reportable conditions, the analysis relied on retrospective data from insurance companies and electronic medical records. Retrospective data are inherently limited, compared with prospective analyses, he said, and it’s possible that certain cases could be included in more than one database and thus be double-counted, whereas other cases could be missed entirely.
These findings also conflict with data from the United Kingdom, which in May reported that the average number of hepatitis cases had increased, compared with previous years, he said. More data are needed, he said, and he is involved with a study with the North American Society for Pediatric Gastroenterology and the American Association for the Study of Liver Diseases that is also collecting data to try to understand whether there has been an uptick in pediatric hepatitis cases. The study will collect patient data directly from hospitals as well as include additional pathology data, such as biopsy results.
“We should not be inhibited to look further academically – and public health–wise – while we take into cognizance this very good, robust attempt from the CDC,” he said.
A version of this article first appeared on Medscape.com.
FROM MMWR
Fisher-Price, feds issue baby rocker warning after 13 deaths
Heads up, parents: Fisher-Price and the Consumer Product Safety Commission said on June 14 that, between 2009 and 2022, at least 13 infants died after falling asleep in the company’s rockers.
The deaths were linked to the Fisher-Price Infant-to-Toddler Rockers and the Newborn-to-Toddler Rockers, according to a statement from the CPSC and Fisher-Price.
The CPSC and Fisher-Price reminded parents and caregivers that products, namely “rockers, gliders, soothers, and swings,” should not be used for infant sleep and that parents and caregivers “should not leave infants in these products unsupervised, unrestrained, or with bedding material, due to the risk of suffocation.”
In 2019, the CPSC issued a recall for the Fisher-Price Rock ‘n Play Sleeper after more than 30 infant fatalities occurred after its 2009 introduction. And in 2021, a similar recall occurred after four infants, all of whom were under 4 months old, died between April 2019 and February 2020, according to The Associated Press.
The CPSC’s warning on the rockers was delayed because of a 1981 Gag Rule that prevented the agency from issuing a warning when they first became aware of the infant deaths associated with the rockers; the rule blocks the agency from doing so “without first seeking permission from the product’s maker,” CPSC Commissioner Richard Trumka said in the statement.
“When CPSC needs to warn the public about a pattern of death and injury tied to a product, it should be able to quickly issue that warning to prevent further loss of life. ... Here, the Gag Rule delayed our message to the public by 2 months.”
A new safety regulation enacted by the CPSC will take effect to prevent further harm from infant sleep products. Beginning June 23, 2022, all infant sleep products must have a sleep surface angle of 10 degrees or less, according to the agency. The Safe Sleep for Babies Act signed into law in 2021 follows the CPSC’s industry recommendations, according to NPR.
A version of this article first appeared on WebMD.com.
Heads up, parents: Fisher-Price and the Consumer Product Safety Commission said on June 14 that, between 2009 and 2022, at least 13 infants died after falling asleep in the company’s rockers.
The deaths were linked to the Fisher-Price Infant-to-Toddler Rockers and the Newborn-to-Toddler Rockers, according to a statement from the CPSC and Fisher-Price.
The CPSC and Fisher-Price reminded parents and caregivers that products, namely “rockers, gliders, soothers, and swings,” should not be used for infant sleep and that parents and caregivers “should not leave infants in these products unsupervised, unrestrained, or with bedding material, due to the risk of suffocation.”
In 2019, the CPSC issued a recall for the Fisher-Price Rock ‘n Play Sleeper after more than 30 infant fatalities occurred after its 2009 introduction. And in 2021, a similar recall occurred after four infants, all of whom were under 4 months old, died between April 2019 and February 2020, according to The Associated Press.
The CPSC’s warning on the rockers was delayed because of a 1981 Gag Rule that prevented the agency from issuing a warning when they first became aware of the infant deaths associated with the rockers; the rule blocks the agency from doing so “without first seeking permission from the product’s maker,” CPSC Commissioner Richard Trumka said in the statement.
“When CPSC needs to warn the public about a pattern of death and injury tied to a product, it should be able to quickly issue that warning to prevent further loss of life. ... Here, the Gag Rule delayed our message to the public by 2 months.”
A new safety regulation enacted by the CPSC will take effect to prevent further harm from infant sleep products. Beginning June 23, 2022, all infant sleep products must have a sleep surface angle of 10 degrees or less, according to the agency. The Safe Sleep for Babies Act signed into law in 2021 follows the CPSC’s industry recommendations, according to NPR.
A version of this article first appeared on WebMD.com.
Heads up, parents: Fisher-Price and the Consumer Product Safety Commission said on June 14 that, between 2009 and 2022, at least 13 infants died after falling asleep in the company’s rockers.
The deaths were linked to the Fisher-Price Infant-to-Toddler Rockers and the Newborn-to-Toddler Rockers, according to a statement from the CPSC and Fisher-Price.
The CPSC and Fisher-Price reminded parents and caregivers that products, namely “rockers, gliders, soothers, and swings,” should not be used for infant sleep and that parents and caregivers “should not leave infants in these products unsupervised, unrestrained, or with bedding material, due to the risk of suffocation.”
In 2019, the CPSC issued a recall for the Fisher-Price Rock ‘n Play Sleeper after more than 30 infant fatalities occurred after its 2009 introduction. And in 2021, a similar recall occurred after four infants, all of whom were under 4 months old, died between April 2019 and February 2020, according to The Associated Press.
The CPSC’s warning on the rockers was delayed because of a 1981 Gag Rule that prevented the agency from issuing a warning when they first became aware of the infant deaths associated with the rockers; the rule blocks the agency from doing so “without first seeking permission from the product’s maker,” CPSC Commissioner Richard Trumka said in the statement.
“When CPSC needs to warn the public about a pattern of death and injury tied to a product, it should be able to quickly issue that warning to prevent further loss of life. ... Here, the Gag Rule delayed our message to the public by 2 months.”
A new safety regulation enacted by the CPSC will take effect to prevent further harm from infant sleep products. Beginning June 23, 2022, all infant sleep products must have a sleep surface angle of 10 degrees or less, according to the agency. The Safe Sleep for Babies Act signed into law in 2021 follows the CPSC’s industry recommendations, according to NPR.
A version of this article first appeared on WebMD.com.
Updated pediatric uveitis recommendations advise on expanded treatment options
Glucocorticoids should be bridging therapies in the treatment of juvenile idiopathic arthritis–associated uveitis (JIAU) and idiopathic chronic anterior uveitis (CAU), according to recently released recommendations from the Multinational Interdisciplinary Working Group for Uveitis in Childhood (MIWGUC).
The recommendations cover literature from December 2014 to June 2020 and represent an update of previously published treatment guidelines from 2018. The MIWGUC work group that formulated the new recommendations consisted of eight pediatric rheumatologists and eight ophthalmologists with expertise in pediatric uveitis.
One major shift from the previous guidelines is the lack of distinction between JIAU and CAU, said lead author Ivan Foeldvari, MD, head of the Hamburg (Germany) Center for Pediatric and Adolescent Rheumatology.
“We are considering these two conditions equivalent regarding the ophthalmological presentation,” Dr. Foeldvari said in an interview.
These guidelines have also expanded possible treatment options for these conditions in light of data from clinical trials that have pointed to new options, Dr. Foeldvari noted.
The guidelines also present new options, compared with the 2019 American College of Rheumatology/Arthritis Foundation JIA-associated uveitis guideline. The data cutoff for that guideline was 2014. “Many key papers were published since 2014,” Dr. Foeldvari said.
Another major change is in the escalation of therapy, he noted.
“We view glucocorticoids as a bridging agent, which is very important to emphasize,” Dr. Foeldvari said. “We do not want oral glucocorticoids used as a monotherapy. If you consider a child who has severe uveitis and you want to give an oral glucocorticoid treatment, then it should be considered only for bridging. We suggest to start a DMARD [disease-modifying antirheumatic drug].”
The specific recommendation is that methotrexate be the first DMARD that clinicians choose after using glucocorticoids as a bridging therapy; adalimumab is recommended as the next treatment choice for patients who do not respond to methotrexate.
The working group also calls for limited use of topical glucocorticoids in the affected eye, he said.
“We recommend no more than two or three drops long term in the eye, because there are studies that show continuous local therapy is the main reason that children may develop blindness,” Dr. Foeldvari said. “With respect to oral corticosteroids, they have a lot of systemic effects. Those effects include a high risk of infection, weight gain, and growth disturbance.”
The new recommendations can guide treatment decisions for rheumatologists and ophthalmologists alike, according to Daniel J. Lovell, MD, MPH, the Joseph E. Levinson Endowed Chair of Pediatric Rheumatology and professor of pediatrics at the University of Cincinnati and Cincinnati Children’s Hospital Medical Center. He was one of the authors of the 2019 ACR/Arthritis Foundation guideline.
“We [rheumatologists] comanage these patients with ophthalmologists,” Dr. Lovell said in an interview. “Ophthalmologists are oftentimes not as experienced in using biologics or methotrexate in terms of monitoring for safety and dosing.”
Dr. Lovell pointed out that the key message from this set of recommendations is to curb the use of topical steroids.
“Topical steroids should be used sparingly and as monotherapy for a very short period of time,” Dr. Lovell said. “Any guidelines agree that if eye inflammation is still present at 3 months, we need to move beyond topical steroid monotherapy.”
These new recommendations from MIWGUC are fairly consistent with the 2019 ACR/Arthritis Foundation guideline, he noted.
“The differences are very minor,” Dr. Lovell said. “In both instances, systemic corticosteroids should be bridging therapy. If you have a patient who needs systemic corticosteroids in addition to topical at the same time, you should be talking about adding other anti-inflammatory treatments, such as traditional and/or biologic DMARDs. Both MIWGUC and the ACR guidelines agree on that.”
The 2019 ACR/Arthritis Foundation guideline did not mention rituximab as an option, nor Janus kinase (JAK) inhibitors, Dr. Lovell said, noting there was no literature on JAK inhibitors as a possible option for JIAU when the guideline was being formulated.
Both sets of guidelines point out that there is a dearth of literature with respect to determining the safe dose of maintenance topical corticosteroids, Dr. Lovell said.
“The ACR 2019 guidelines state you should add systemic therapy if there is persistent eye inflammation despite use of up to two drops per day of topical corticosteroids, while the European [MIWGUC] guideline states you can allow up to three drops,” he said. “In both instances, they are quoting the same two sources. Both guidelines indicate that the literature is very scant as to defining a true, safe dose of topical ocular corticosteroids. They differ by one drop allowed per day. In both instances, in the presence of active uveitis, at 3 months on topical steroid monotherapy, both [guidelines] strongly recommend adding systemic therapy.”
Marinka Twilt, MD, MSCE, PhD, associate professor in the department of pediatrics at the University of Calgary (Alta.), noted in an interview that these latest recommendations from MIWGUC have included consensus views on what to do if certain medications fail to lead to remission, which is not addressed in the 2019 ACR/Arthritis Foundation guideline.
“The new manuscript provides consensus on the use of abatacept, JAK inhibitors, and rituximab if patients are refractory to adalimumab and tocilizumab, which is not discussed in the 2019 recommendations,” Dr. Twilt said.
She also pointed out that these recommendations suggest adalimumab as treatment before infliximab, whereas the 2019 guideline did not recommend using one or the other first.
In compiling the recommendations, the authors received no outside financial support. Dr. Foeldvari is a member of advisory boards for Lilly, Pfizer, Novartis, and Medac. Dr. Lovell and Dr. Twilt disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Glucocorticoids should be bridging therapies in the treatment of juvenile idiopathic arthritis–associated uveitis (JIAU) and idiopathic chronic anterior uveitis (CAU), according to recently released recommendations from the Multinational Interdisciplinary Working Group for Uveitis in Childhood (MIWGUC).
The recommendations cover literature from December 2014 to June 2020 and represent an update of previously published treatment guidelines from 2018. The MIWGUC work group that formulated the new recommendations consisted of eight pediatric rheumatologists and eight ophthalmologists with expertise in pediatric uveitis.
One major shift from the previous guidelines is the lack of distinction between JIAU and CAU, said lead author Ivan Foeldvari, MD, head of the Hamburg (Germany) Center for Pediatric and Adolescent Rheumatology.
“We are considering these two conditions equivalent regarding the ophthalmological presentation,” Dr. Foeldvari said in an interview.
These guidelines have also expanded possible treatment options for these conditions in light of data from clinical trials that have pointed to new options, Dr. Foeldvari noted.
The guidelines also present new options, compared with the 2019 American College of Rheumatology/Arthritis Foundation JIA-associated uveitis guideline. The data cutoff for that guideline was 2014. “Many key papers were published since 2014,” Dr. Foeldvari said.
Another major change is in the escalation of therapy, he noted.
“We view glucocorticoids as a bridging agent, which is very important to emphasize,” Dr. Foeldvari said. “We do not want oral glucocorticoids used as a monotherapy. If you consider a child who has severe uveitis and you want to give an oral glucocorticoid treatment, then it should be considered only for bridging. We suggest to start a DMARD [disease-modifying antirheumatic drug].”
The specific recommendation is that methotrexate be the first DMARD that clinicians choose after using glucocorticoids as a bridging therapy; adalimumab is recommended as the next treatment choice for patients who do not respond to methotrexate.
The working group also calls for limited use of topical glucocorticoids in the affected eye, he said.
“We recommend no more than two or three drops long term in the eye, because there are studies that show continuous local therapy is the main reason that children may develop blindness,” Dr. Foeldvari said. “With respect to oral corticosteroids, they have a lot of systemic effects. Those effects include a high risk of infection, weight gain, and growth disturbance.”
The new recommendations can guide treatment decisions for rheumatologists and ophthalmologists alike, according to Daniel J. Lovell, MD, MPH, the Joseph E. Levinson Endowed Chair of Pediatric Rheumatology and professor of pediatrics at the University of Cincinnati and Cincinnati Children’s Hospital Medical Center. He was one of the authors of the 2019 ACR/Arthritis Foundation guideline.
“We [rheumatologists] comanage these patients with ophthalmologists,” Dr. Lovell said in an interview. “Ophthalmologists are oftentimes not as experienced in using biologics or methotrexate in terms of monitoring for safety and dosing.”
Dr. Lovell pointed out that the key message from this set of recommendations is to curb the use of topical steroids.
“Topical steroids should be used sparingly and as monotherapy for a very short period of time,” Dr. Lovell said. “Any guidelines agree that if eye inflammation is still present at 3 months, we need to move beyond topical steroid monotherapy.”
These new recommendations from MIWGUC are fairly consistent with the 2019 ACR/Arthritis Foundation guideline, he noted.
“The differences are very minor,” Dr. Lovell said. “In both instances, systemic corticosteroids should be bridging therapy. If you have a patient who needs systemic corticosteroids in addition to topical at the same time, you should be talking about adding other anti-inflammatory treatments, such as traditional and/or biologic DMARDs. Both MIWGUC and the ACR guidelines agree on that.”
The 2019 ACR/Arthritis Foundation guideline did not mention rituximab as an option, nor Janus kinase (JAK) inhibitors, Dr. Lovell said, noting there was no literature on JAK inhibitors as a possible option for JIAU when the guideline was being formulated.
Both sets of guidelines point out that there is a dearth of literature with respect to determining the safe dose of maintenance topical corticosteroids, Dr. Lovell said.
“The ACR 2019 guidelines state you should add systemic therapy if there is persistent eye inflammation despite use of up to two drops per day of topical corticosteroids, while the European [MIWGUC] guideline states you can allow up to three drops,” he said. “In both instances, they are quoting the same two sources. Both guidelines indicate that the literature is very scant as to defining a true, safe dose of topical ocular corticosteroids. They differ by one drop allowed per day. In both instances, in the presence of active uveitis, at 3 months on topical steroid monotherapy, both [guidelines] strongly recommend adding systemic therapy.”
Marinka Twilt, MD, MSCE, PhD, associate professor in the department of pediatrics at the University of Calgary (Alta.), noted in an interview that these latest recommendations from MIWGUC have included consensus views on what to do if certain medications fail to lead to remission, which is not addressed in the 2019 ACR/Arthritis Foundation guideline.
“The new manuscript provides consensus on the use of abatacept, JAK inhibitors, and rituximab if patients are refractory to adalimumab and tocilizumab, which is not discussed in the 2019 recommendations,” Dr. Twilt said.
She also pointed out that these recommendations suggest adalimumab as treatment before infliximab, whereas the 2019 guideline did not recommend using one or the other first.
In compiling the recommendations, the authors received no outside financial support. Dr. Foeldvari is a member of advisory boards for Lilly, Pfizer, Novartis, and Medac. Dr. Lovell and Dr. Twilt disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Glucocorticoids should be bridging therapies in the treatment of juvenile idiopathic arthritis–associated uveitis (JIAU) and idiopathic chronic anterior uveitis (CAU), according to recently released recommendations from the Multinational Interdisciplinary Working Group for Uveitis in Childhood (MIWGUC).
The recommendations cover literature from December 2014 to June 2020 and represent an update of previously published treatment guidelines from 2018. The MIWGUC work group that formulated the new recommendations consisted of eight pediatric rheumatologists and eight ophthalmologists with expertise in pediatric uveitis.
One major shift from the previous guidelines is the lack of distinction between JIAU and CAU, said lead author Ivan Foeldvari, MD, head of the Hamburg (Germany) Center for Pediatric and Adolescent Rheumatology.
“We are considering these two conditions equivalent regarding the ophthalmological presentation,” Dr. Foeldvari said in an interview.
These guidelines have also expanded possible treatment options for these conditions in light of data from clinical trials that have pointed to new options, Dr. Foeldvari noted.
The guidelines also present new options, compared with the 2019 American College of Rheumatology/Arthritis Foundation JIA-associated uveitis guideline. The data cutoff for that guideline was 2014. “Many key papers were published since 2014,” Dr. Foeldvari said.
Another major change is in the escalation of therapy, he noted.
“We view glucocorticoids as a bridging agent, which is very important to emphasize,” Dr. Foeldvari said. “We do not want oral glucocorticoids used as a monotherapy. If you consider a child who has severe uveitis and you want to give an oral glucocorticoid treatment, then it should be considered only for bridging. We suggest to start a DMARD [disease-modifying antirheumatic drug].”
The specific recommendation is that methotrexate be the first DMARD that clinicians choose after using glucocorticoids as a bridging therapy; adalimumab is recommended as the next treatment choice for patients who do not respond to methotrexate.
The working group also calls for limited use of topical glucocorticoids in the affected eye, he said.
“We recommend no more than two or three drops long term in the eye, because there are studies that show continuous local therapy is the main reason that children may develop blindness,” Dr. Foeldvari said. “With respect to oral corticosteroids, they have a lot of systemic effects. Those effects include a high risk of infection, weight gain, and growth disturbance.”
The new recommendations can guide treatment decisions for rheumatologists and ophthalmologists alike, according to Daniel J. Lovell, MD, MPH, the Joseph E. Levinson Endowed Chair of Pediatric Rheumatology and professor of pediatrics at the University of Cincinnati and Cincinnati Children’s Hospital Medical Center. He was one of the authors of the 2019 ACR/Arthritis Foundation guideline.
“We [rheumatologists] comanage these patients with ophthalmologists,” Dr. Lovell said in an interview. “Ophthalmologists are oftentimes not as experienced in using biologics or methotrexate in terms of monitoring for safety and dosing.”
Dr. Lovell pointed out that the key message from this set of recommendations is to curb the use of topical steroids.
“Topical steroids should be used sparingly and as monotherapy for a very short period of time,” Dr. Lovell said. “Any guidelines agree that if eye inflammation is still present at 3 months, we need to move beyond topical steroid monotherapy.”
These new recommendations from MIWGUC are fairly consistent with the 2019 ACR/Arthritis Foundation guideline, he noted.
“The differences are very minor,” Dr. Lovell said. “In both instances, systemic corticosteroids should be bridging therapy. If you have a patient who needs systemic corticosteroids in addition to topical at the same time, you should be talking about adding other anti-inflammatory treatments, such as traditional and/or biologic DMARDs. Both MIWGUC and the ACR guidelines agree on that.”
The 2019 ACR/Arthritis Foundation guideline did not mention rituximab as an option, nor Janus kinase (JAK) inhibitors, Dr. Lovell said, noting there was no literature on JAK inhibitors as a possible option for JIAU when the guideline was being formulated.
Both sets of guidelines point out that there is a dearth of literature with respect to determining the safe dose of maintenance topical corticosteroids, Dr. Lovell said.
“The ACR 2019 guidelines state you should add systemic therapy if there is persistent eye inflammation despite use of up to two drops per day of topical corticosteroids, while the European [MIWGUC] guideline states you can allow up to three drops,” he said. “In both instances, they are quoting the same two sources. Both guidelines indicate that the literature is very scant as to defining a true, safe dose of topical ocular corticosteroids. They differ by one drop allowed per day. In both instances, in the presence of active uveitis, at 3 months on topical steroid monotherapy, both [guidelines] strongly recommend adding systemic therapy.”
Marinka Twilt, MD, MSCE, PhD, associate professor in the department of pediatrics at the University of Calgary (Alta.), noted in an interview that these latest recommendations from MIWGUC have included consensus views on what to do if certain medications fail to lead to remission, which is not addressed in the 2019 ACR/Arthritis Foundation guideline.
“The new manuscript provides consensus on the use of abatacept, JAK inhibitors, and rituximab if patients are refractory to adalimumab and tocilizumab, which is not discussed in the 2019 recommendations,” Dr. Twilt said.
She also pointed out that these recommendations suggest adalimumab as treatment before infliximab, whereas the 2019 guideline did not recommend using one or the other first.
In compiling the recommendations, the authors received no outside financial support. Dr. Foeldvari is a member of advisory boards for Lilly, Pfizer, Novartis, and Medac. Dr. Lovell and Dr. Twilt disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ARTHRITIS CARE RESEARCH





