User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
FDA orders Juul to stop selling E-cigarettes
The marketing denial order covers all the company’s products in the United States, which means Juul must stop distributing the products and remove everything on the market. That includes the Juul device and flavor replacement pods in the tobacco and menthol flavors.
“Today’s action is further progress on the FDA’s commitment to ensuring that all e-cigarette and electronic nicotine delivery system products currently being marketed to consumers meet our public health standards,” Robert Califf, MD, the FDA commissioner, said in the announcement.
“The agency has dedicated significant resources to review products from the companies that account for most of the U.S. market,” he said. “We recognize these make up a significant part of the available products and many have played a disproportionate role in the rise in youth vaping.”
The marketing denial order covers only the commercial distribution and retail sale of Juul’s products and doesn’t restrict consumer possession or use. The FDA “cannot and will not” enforce actions against consumers, the agency said.
The order comes after a 2-year review of the company’s application seeking authorization to continue selling non–fruit-flavored products, such as menthol and tobacco. The FDA determined the application “lacked sufficient evidence regarding the toxicological profile of the products to demonstrate that marketing of the products would be appropriate for the protection of the public health.”
Some of Juul’s study findings raised concerns because of “insufficient and conflicting data,” the FDA said, including potentially harmful chemicals leaching from the Juul liquid replacement pods.
“To date, the FDA has not received clinical information to suggest an immediate hazard associated with the use of the JUUL device or JUUL pods,” the agency said. “However, the [orders] issued today reflect FDA’s determination that there is insufficient evidence to assess the potential toxicological risks of using the JUUL products.”
Juul is expected to appeal the FDA’s decision, according to The New York Times.
In recent years, the FDA has reviewed marketing applications from Juul and other e-cigarette companies as anti-tobacco groups have called for new rules to limit products that led to a surge in youth vaping during the past decade. At the same time, advocates of e-cigarettes and nicotine-delivery devices have said the products help adult smokers to quit cigarettes and other tobacco products.
Juul, in particular, has been blamed for fueling the surge in underage vaping due to fruity flavors and hip marketing, according to The Wall Street Journal. The company removed sweet and fruity flavors from shelves in 2019 and has been trying to repair its reputation by limiting its marketing and focusing on adult cigarette smokers.
In 2020, all e-cigarette manufacturers in the United States were required to submit their products for FDA review to stay on the market, the newspaper reported. The agency has been weighing the potential benefits for adult cigarette smokers against the harms for young people.
The FDA banned the sale of fruit- and mint-flavored cartridges and juice pods in 2020, but menthol and tobacco-flavored products were left on the market, according to USA Today. In September 2021, the agency also banned the sale of hundreds of thousands of vaping and e-cigarette products but didn’t rule on Juul.
Meanwhile, the FDA has cleared Reynolds American and NJOY Holdings – two of Juul’s biggest rivals – to keep tobacco-flavored products on the market. Industry experts expected Juul to receive similar clearance, the Journal reported.
Juul, which was at the top of the U.S. e-cigarette market in 2018, has moved to second place behind Reynolds’s Vuse brand, the newspaper reported. The United States represents most of the company’s revenue, though its products are also available in Canada, the United Kingdom, France, Italy, and the Philippines.
Underage vaping has fallen in the United States since federal restrictions raised the legal purchase age for tobacco products to 21 and banned the sale of sweet and fruity cartridges, according to the Journal. Juul’s popularity has also dropped among youth, with other products such as Puff Bar, Vuse, and Smok becoming more popular among e-cigarette users in high school.
In a separate decision announced this week, the FDA is also moving forward with a plan to reduce the amount of nicotine in cigarettes. The decision, which has been years in the making, is aimed at prompting millions of cigarette users to quit smoking or switch to alternatives such as e-cigarettes, as well as limit the number of users who pick up smoking at an early age.
A version of this article first appeared on WebMD.com .
The marketing denial order covers all the company’s products in the United States, which means Juul must stop distributing the products and remove everything on the market. That includes the Juul device and flavor replacement pods in the tobacco and menthol flavors.
“Today’s action is further progress on the FDA’s commitment to ensuring that all e-cigarette and electronic nicotine delivery system products currently being marketed to consumers meet our public health standards,” Robert Califf, MD, the FDA commissioner, said in the announcement.
“The agency has dedicated significant resources to review products from the companies that account for most of the U.S. market,” he said. “We recognize these make up a significant part of the available products and many have played a disproportionate role in the rise in youth vaping.”
The marketing denial order covers only the commercial distribution and retail sale of Juul’s products and doesn’t restrict consumer possession or use. The FDA “cannot and will not” enforce actions against consumers, the agency said.
The order comes after a 2-year review of the company’s application seeking authorization to continue selling non–fruit-flavored products, such as menthol and tobacco. The FDA determined the application “lacked sufficient evidence regarding the toxicological profile of the products to demonstrate that marketing of the products would be appropriate for the protection of the public health.”
Some of Juul’s study findings raised concerns because of “insufficient and conflicting data,” the FDA said, including potentially harmful chemicals leaching from the Juul liquid replacement pods.
“To date, the FDA has not received clinical information to suggest an immediate hazard associated with the use of the JUUL device or JUUL pods,” the agency said. “However, the [orders] issued today reflect FDA’s determination that there is insufficient evidence to assess the potential toxicological risks of using the JUUL products.”
Juul is expected to appeal the FDA’s decision, according to The New York Times.
In recent years, the FDA has reviewed marketing applications from Juul and other e-cigarette companies as anti-tobacco groups have called for new rules to limit products that led to a surge in youth vaping during the past decade. At the same time, advocates of e-cigarettes and nicotine-delivery devices have said the products help adult smokers to quit cigarettes and other tobacco products.
Juul, in particular, has been blamed for fueling the surge in underage vaping due to fruity flavors and hip marketing, according to The Wall Street Journal. The company removed sweet and fruity flavors from shelves in 2019 and has been trying to repair its reputation by limiting its marketing and focusing on adult cigarette smokers.
In 2020, all e-cigarette manufacturers in the United States were required to submit their products for FDA review to stay on the market, the newspaper reported. The agency has been weighing the potential benefits for adult cigarette smokers against the harms for young people.
The FDA banned the sale of fruit- and mint-flavored cartridges and juice pods in 2020, but menthol and tobacco-flavored products were left on the market, according to USA Today. In September 2021, the agency also banned the sale of hundreds of thousands of vaping and e-cigarette products but didn’t rule on Juul.
Meanwhile, the FDA has cleared Reynolds American and NJOY Holdings – two of Juul’s biggest rivals – to keep tobacco-flavored products on the market. Industry experts expected Juul to receive similar clearance, the Journal reported.
Juul, which was at the top of the U.S. e-cigarette market in 2018, has moved to second place behind Reynolds’s Vuse brand, the newspaper reported. The United States represents most of the company’s revenue, though its products are also available in Canada, the United Kingdom, France, Italy, and the Philippines.
Underage vaping has fallen in the United States since federal restrictions raised the legal purchase age for tobacco products to 21 and banned the sale of sweet and fruity cartridges, according to the Journal. Juul’s popularity has also dropped among youth, with other products such as Puff Bar, Vuse, and Smok becoming more popular among e-cigarette users in high school.
In a separate decision announced this week, the FDA is also moving forward with a plan to reduce the amount of nicotine in cigarettes. The decision, which has been years in the making, is aimed at prompting millions of cigarette users to quit smoking or switch to alternatives such as e-cigarettes, as well as limit the number of users who pick up smoking at an early age.
A version of this article first appeared on WebMD.com .
The marketing denial order covers all the company’s products in the United States, which means Juul must stop distributing the products and remove everything on the market. That includes the Juul device and flavor replacement pods in the tobacco and menthol flavors.
“Today’s action is further progress on the FDA’s commitment to ensuring that all e-cigarette and electronic nicotine delivery system products currently being marketed to consumers meet our public health standards,” Robert Califf, MD, the FDA commissioner, said in the announcement.
“The agency has dedicated significant resources to review products from the companies that account for most of the U.S. market,” he said. “We recognize these make up a significant part of the available products and many have played a disproportionate role in the rise in youth vaping.”
The marketing denial order covers only the commercial distribution and retail sale of Juul’s products and doesn’t restrict consumer possession or use. The FDA “cannot and will not” enforce actions against consumers, the agency said.
The order comes after a 2-year review of the company’s application seeking authorization to continue selling non–fruit-flavored products, such as menthol and tobacco. The FDA determined the application “lacked sufficient evidence regarding the toxicological profile of the products to demonstrate that marketing of the products would be appropriate for the protection of the public health.”
Some of Juul’s study findings raised concerns because of “insufficient and conflicting data,” the FDA said, including potentially harmful chemicals leaching from the Juul liquid replacement pods.
“To date, the FDA has not received clinical information to suggest an immediate hazard associated with the use of the JUUL device or JUUL pods,” the agency said. “However, the [orders] issued today reflect FDA’s determination that there is insufficient evidence to assess the potential toxicological risks of using the JUUL products.”
Juul is expected to appeal the FDA’s decision, according to The New York Times.
In recent years, the FDA has reviewed marketing applications from Juul and other e-cigarette companies as anti-tobacco groups have called for new rules to limit products that led to a surge in youth vaping during the past decade. At the same time, advocates of e-cigarettes and nicotine-delivery devices have said the products help adult smokers to quit cigarettes and other tobacco products.
Juul, in particular, has been blamed for fueling the surge in underage vaping due to fruity flavors and hip marketing, according to The Wall Street Journal. The company removed sweet and fruity flavors from shelves in 2019 and has been trying to repair its reputation by limiting its marketing and focusing on adult cigarette smokers.
In 2020, all e-cigarette manufacturers in the United States were required to submit their products for FDA review to stay on the market, the newspaper reported. The agency has been weighing the potential benefits for adult cigarette smokers against the harms for young people.
The FDA banned the sale of fruit- and mint-flavored cartridges and juice pods in 2020, but menthol and tobacco-flavored products were left on the market, according to USA Today. In September 2021, the agency also banned the sale of hundreds of thousands of vaping and e-cigarette products but didn’t rule on Juul.
Meanwhile, the FDA has cleared Reynolds American and NJOY Holdings – two of Juul’s biggest rivals – to keep tobacco-flavored products on the market. Industry experts expected Juul to receive similar clearance, the Journal reported.
Juul, which was at the top of the U.S. e-cigarette market in 2018, has moved to second place behind Reynolds’s Vuse brand, the newspaper reported. The United States represents most of the company’s revenue, though its products are also available in Canada, the United Kingdom, France, Italy, and the Philippines.
Underage vaping has fallen in the United States since federal restrictions raised the legal purchase age for tobacco products to 21 and banned the sale of sweet and fruity cartridges, according to the Journal. Juul’s popularity has also dropped among youth, with other products such as Puff Bar, Vuse, and Smok becoming more popular among e-cigarette users in high school.
In a separate decision announced this week, the FDA is also moving forward with a plan to reduce the amount of nicotine in cigarettes. The decision, which has been years in the making, is aimed at prompting millions of cigarette users to quit smoking or switch to alternatives such as e-cigarettes, as well as limit the number of users who pick up smoking at an early age.
A version of this article first appeared on WebMD.com .
Introduce allergens early, say French allergists
Although in many cases, food-allergen tolerance can be achieved with oral immunotherapy, primary prevention of food allergies remains crucial, according to the French Society of Allergology. In new recommendations that were presented at a session of the Congress of French Pediatric Societies, the academic society advocated early introduction of allergens for all children, starting at 4 months of age.
The latest prevention data from two major studies, LEAP and EAT, have prompted European and French experts to rethink their stance on food diversification. The new French proposals were recently published under the coordination of Dominique Sabouraud-Leclerc, MD, pediatrics department, Reims (France) University Hospital, on behalf of the Food Allergy Working Group of the French Society of Allergology.
For all newborns, regardless of whether they have a history of atopic or nonatopic dermatitis, food diversification is now recommended from 4 months of age instead of 6 months, as was previously recommended. If the child does not develop atopic dermatitis or develops only a mild form, peanuts, eggs, and nuts may be introduced at home.
However, if the child experiences severe atopic dermatitis, an allergy testing panel for peanuts, nuts, eggs, and cow’s milk proteins should be performed. An oral food challenge may be conducted at the allergist’s discretion.
Regarding peanuts, the working group proposed introducing a purée in the form of either a mixture of peanuts/hazelnuts/cashew nuts (1 level teaspoon five times a week; 2 g of protein/food per week) or a 100% peanut paste (1 scant teaspoon four times a week; 2 g of peanut protein/week). If the family is worried, the allergist can suggest monitoring the child in the clinic waiting room for 30 minutes after the first dose.
“We shouldn’t delay the introduction of the primary allergens anymore, regardless of whether children are at risk for a food allergy, and particularly a peanut allergy,” explained Stéphanie Lejeune, MD, pediatric pulmonologist and allergist at Lille (France) Regional University Hospital, who presented these new findings at the congress. “In fact, if we only target at-risk children, we overlook children with no family history who will nevertheless develop food allergies. The idea is to introduce everything, especially peanuts, between 4 and 6 months of age and to no longer do so gradually, one food after another, as was being done until now, beginning at 6 months and over. We must give priority to regularity over quantity.”
Although this approach is based on clinical trials, no real-life data are currently available.
LEAP and EAT studies support early introduction of peanuts
A study from 2021 summed up the risk factors for peanut allergy. About 61% of infants (4-11 months) had atopic dermatitis, 18% had a food allergy, 62% had a first-degree relative with a peanut allergy, and 11% had a confirmed peanut allergy. The risk of peanut allergy increased with age and severe eczema.
In 2015, the LEAP study, which was conducted in the United Kingdom with 640 infants aged 4-11 months who had risk factors for peanut allergy, revolutionized peanut-allergy primary prevention. Regardless of whether the children were sensitized or not, the number of children who developed a peanut allergy was systematically lower in the group that ingested the allergen in comparison with the “avoidance” group.
Additionally, the LEAP-ON study showed that protection against peanut allergy persisted for 12 months after cessation of consumption between ages 5 and 6 years among children who had consumed peanuts previously.
Early diversification in the general population was investigated in the EAT study, which involved 1303 breastfed infants. Of these infants, 24% had atopic dermatitis (median SCORAD score, 7.5). They were divided into two arms: avoidance and breast feeding until 6 months (standard introduction) or early introduction at 3 months (boiled egg, milk, peanuts, sesame, white fish, wheat, 2 g of protein twice a week). In the per-protocol analysis, there were 13 cases of peanut allergy in the standard introduction group; there were no cases in the early introduction group.
A version of this article first appeared on Medscape.com.
Although in many cases, food-allergen tolerance can be achieved with oral immunotherapy, primary prevention of food allergies remains crucial, according to the French Society of Allergology. In new recommendations that were presented at a session of the Congress of French Pediatric Societies, the academic society advocated early introduction of allergens for all children, starting at 4 months of age.
The latest prevention data from two major studies, LEAP and EAT, have prompted European and French experts to rethink their stance on food diversification. The new French proposals were recently published under the coordination of Dominique Sabouraud-Leclerc, MD, pediatrics department, Reims (France) University Hospital, on behalf of the Food Allergy Working Group of the French Society of Allergology.
For all newborns, regardless of whether they have a history of atopic or nonatopic dermatitis, food diversification is now recommended from 4 months of age instead of 6 months, as was previously recommended. If the child does not develop atopic dermatitis or develops only a mild form, peanuts, eggs, and nuts may be introduced at home.
However, if the child experiences severe atopic dermatitis, an allergy testing panel for peanuts, nuts, eggs, and cow’s milk proteins should be performed. An oral food challenge may be conducted at the allergist’s discretion.
Regarding peanuts, the working group proposed introducing a purée in the form of either a mixture of peanuts/hazelnuts/cashew nuts (1 level teaspoon five times a week; 2 g of protein/food per week) or a 100% peanut paste (1 scant teaspoon four times a week; 2 g of peanut protein/week). If the family is worried, the allergist can suggest monitoring the child in the clinic waiting room for 30 minutes after the first dose.
“We shouldn’t delay the introduction of the primary allergens anymore, regardless of whether children are at risk for a food allergy, and particularly a peanut allergy,” explained Stéphanie Lejeune, MD, pediatric pulmonologist and allergist at Lille (France) Regional University Hospital, who presented these new findings at the congress. “In fact, if we only target at-risk children, we overlook children with no family history who will nevertheless develop food allergies. The idea is to introduce everything, especially peanuts, between 4 and 6 months of age and to no longer do so gradually, one food after another, as was being done until now, beginning at 6 months and over. We must give priority to regularity over quantity.”
Although this approach is based on clinical trials, no real-life data are currently available.
LEAP and EAT studies support early introduction of peanuts
A study from 2021 summed up the risk factors for peanut allergy. About 61% of infants (4-11 months) had atopic dermatitis, 18% had a food allergy, 62% had a first-degree relative with a peanut allergy, and 11% had a confirmed peanut allergy. The risk of peanut allergy increased with age and severe eczema.
In 2015, the LEAP study, which was conducted in the United Kingdom with 640 infants aged 4-11 months who had risk factors for peanut allergy, revolutionized peanut-allergy primary prevention. Regardless of whether the children were sensitized or not, the number of children who developed a peanut allergy was systematically lower in the group that ingested the allergen in comparison with the “avoidance” group.
Additionally, the LEAP-ON study showed that protection against peanut allergy persisted for 12 months after cessation of consumption between ages 5 and 6 years among children who had consumed peanuts previously.
Early diversification in the general population was investigated in the EAT study, which involved 1303 breastfed infants. Of these infants, 24% had atopic dermatitis (median SCORAD score, 7.5). They were divided into two arms: avoidance and breast feeding until 6 months (standard introduction) or early introduction at 3 months (boiled egg, milk, peanuts, sesame, white fish, wheat, 2 g of protein twice a week). In the per-protocol analysis, there were 13 cases of peanut allergy in the standard introduction group; there were no cases in the early introduction group.
A version of this article first appeared on Medscape.com.
Although in many cases, food-allergen tolerance can be achieved with oral immunotherapy, primary prevention of food allergies remains crucial, according to the French Society of Allergology. In new recommendations that were presented at a session of the Congress of French Pediatric Societies, the academic society advocated early introduction of allergens for all children, starting at 4 months of age.
The latest prevention data from two major studies, LEAP and EAT, have prompted European and French experts to rethink their stance on food diversification. The new French proposals were recently published under the coordination of Dominique Sabouraud-Leclerc, MD, pediatrics department, Reims (France) University Hospital, on behalf of the Food Allergy Working Group of the French Society of Allergology.
For all newborns, regardless of whether they have a history of atopic or nonatopic dermatitis, food diversification is now recommended from 4 months of age instead of 6 months, as was previously recommended. If the child does not develop atopic dermatitis or develops only a mild form, peanuts, eggs, and nuts may be introduced at home.
However, if the child experiences severe atopic dermatitis, an allergy testing panel for peanuts, nuts, eggs, and cow’s milk proteins should be performed. An oral food challenge may be conducted at the allergist’s discretion.
Regarding peanuts, the working group proposed introducing a purée in the form of either a mixture of peanuts/hazelnuts/cashew nuts (1 level teaspoon five times a week; 2 g of protein/food per week) or a 100% peanut paste (1 scant teaspoon four times a week; 2 g of peanut protein/week). If the family is worried, the allergist can suggest monitoring the child in the clinic waiting room for 30 minutes after the first dose.
“We shouldn’t delay the introduction of the primary allergens anymore, regardless of whether children are at risk for a food allergy, and particularly a peanut allergy,” explained Stéphanie Lejeune, MD, pediatric pulmonologist and allergist at Lille (France) Regional University Hospital, who presented these new findings at the congress. “In fact, if we only target at-risk children, we overlook children with no family history who will nevertheless develop food allergies. The idea is to introduce everything, especially peanuts, between 4 and 6 months of age and to no longer do so gradually, one food after another, as was being done until now, beginning at 6 months and over. We must give priority to regularity over quantity.”
Although this approach is based on clinical trials, no real-life data are currently available.
LEAP and EAT studies support early introduction of peanuts
A study from 2021 summed up the risk factors for peanut allergy. About 61% of infants (4-11 months) had atopic dermatitis, 18% had a food allergy, 62% had a first-degree relative with a peanut allergy, and 11% had a confirmed peanut allergy. The risk of peanut allergy increased with age and severe eczema.
In 2015, the LEAP study, which was conducted in the United Kingdom with 640 infants aged 4-11 months who had risk factors for peanut allergy, revolutionized peanut-allergy primary prevention. Regardless of whether the children were sensitized or not, the number of children who developed a peanut allergy was systematically lower in the group that ingested the allergen in comparison with the “avoidance” group.
Additionally, the LEAP-ON study showed that protection against peanut allergy persisted for 12 months after cessation of consumption between ages 5 and 6 years among children who had consumed peanuts previously.
Early diversification in the general population was investigated in the EAT study, which involved 1303 breastfed infants. Of these infants, 24% had atopic dermatitis (median SCORAD score, 7.5). They were divided into two arms: avoidance and breast feeding until 6 months (standard introduction) or early introduction at 3 months (boiled egg, milk, peanuts, sesame, white fish, wheat, 2 g of protein twice a week). In the per-protocol analysis, there were 13 cases of peanut allergy in the standard introduction group; there were no cases in the early introduction group.
A version of this article first appeared on Medscape.com.
Artificial intelligence: The Netflix of cancer treatment
Chemotherapy, now streaming at just $15.99 a month!
It’s a lazy Sunday and you flip on Netflix, looking for something new to watch. There’s an almost-overwhelming number of shows out there, but right at the top of the recommended list is something that strikes your fancy right away. The algorithm behind the scenes is doing its job well, winnowing the universe of content right down to the few things you’ll find relevant, based on what you’ve watched and liked in the past.
Now, the almighty content algorithm is coming for something a little more useful than binge watching obscure 80s sitcoms: cancer treatment.
By plugging the fully sequenced genomes of nearly 10,000 patients with 33 different types of cancer into an algorithm powered by the same sort of artificial intelligence used by Netflix, researchers from London and San Diego found 21 common faults in the chromosomes of tumors, which they called copy number signatures. While cancer is a complex disease, when faults occur in those copy number signatures, the results were similar across the board. If X genetic defect occurs within a tumor, Y result will happen, even across cancer types. For example, tumors whose chromosomes had shattered and reformed had by far the worst disease outcomes.
The eventual hope is that, just as Netflix can predict what you’ll want to watch based on what you’ve already seen, oncologists will be able to predict the course of a cancer, based on the tumor’s early genetic traits, and get ahead of future genetic degradation to prevent the worst outcomes. A sort of “Oh, your tumor has enjoyed The Office. Might we suggest a treatment of 30 Rock” situation. Further research will be required to determine whether or not the cancer algorithm can get us part 2 of “Stranger Things 4” a week early.
Pay criminals, cut crime?
What is the best method for punishing those who commit wrongdoing? Fines? Jail time? Actually, no. A recent study says that financial compensation works best.
In other words, pay them for their actions. Really.
Psychologist Tage S. Rai, PhD, of the University of California, San Diego, Rady School of Management, found that people who hurt others or commit crimes are actually doing it because they think it’s the right thing to do. The results of this study say play at the angle of their morality. When that’s compromised, the offender is less likely to do it again.
Four different experiments were conducted using an online economics game with nearly 1,500 participants. Dr. Rai found that providing a monetary bonus for inflicting a punishment on a third party within the game cut the participants’ willingness to do it again by 50%.
“People punish others to signal their own goodness and receiving compensation might make it seem as though they’re driven by greed rather than justice,” he said.
The big deterrent, though, was negative judgment from peers. People in the study were even more hesitant to inflict harm and gain a profit if they thought they were going to be judged for it.
So maybe the answer to cutting crime isn’t as simple as slapping on a fine. It’s slapping on shame and paying them for it.
A conspiracy of chronobiologic proportions
The Golden State Warriors just won the NBA championship – that much is true – but we’ve got some news that you didn’t get from ESPN. The kind of news that their “partners” from the NBA didn’t want them to report. Unlike most conspiracy theories, however, this one has some science behind it.
In this case, science in the form of a study published in Frontiers in Physiology says that jet lag had a greater effect on the Boston Celtics than it did on the Warriors.
“Eastward travel – where the destination time is later than the origin time – requires the athlete to shorten their day (known as a phase advance). During phase advance, athletes often struggle to fall asleep at an earlier bedtime, leading to sleep loss and, consequently, potential impaired physiological performance and motivation the next day,” senior author Elise Facer-Childs, PhD, of Monash University, Melbourne, said in written statement.
Dr. Facer-Childs and associates took a very close look at 10 seasons’ worth of NBA games – 11,481 games, to be exact – and found “that eastward (but not westward) jet lag was associated with impaired performance for home (but not away) teams.” The existence of a pro-Western bias against teams that traveled eastward for their home games was clear:
- The chance of winning for eastern teams was reduced by 6.0%.
- They grabbed 1.3 fewer rebounds per game.
- Their field goal percentage was 1.2% lower.
And here’s the final nail in the conspiracy coffin: The NBA knew about the jet lag effect and changed the schedule of the finals in 2014 in a way that makes it worse. Before that, the higher-seeded team got two home games, then the lower-seeded team had three at home, followed by two more at the home of the higher seed. Now it’s a 2-2-1-1-1 arrangement that leads to more travel and, of course, more jet lag.
The study was published during the championship series, so the investigators suggested that the Celtics “might benefit from chronobiology-informed strategies designed to mitigate eastward jet lag symptomatology.”
So there you have it, sports fans/conspiracy theorists: You can’t chase Steph Curry around the court for 48 minutes without the right chronobiology-informed strategy. Everyone knows that.
Being hungry can alter your ‘type’
Fasting and being hungry can be a dangerous mix for becoming “hangry” and irritable, but did you know being hungry can also affect your attraction to other people?
Evidence has shown that being hungry can affect important things such as decision-making, memory, cognition, and function. It might affect decision-making in the sense that those six tacos at Taco Bell might win out over grilled chicken breast and veggies at home, but can hunger make you think that the person you just swiped right on isn’t really your type after all?
We’ll leave that up to Valentina Cazzato of Liverpool (England) John Moores University and associates, whose study involved 44 people, of whom 21 were women in their early 20s. The participants were shown computer-generated images of men and women of different sizes. The same background was used for each picture and all the expressions of the models were neutral. Participants were asked to rate each image on how much they liked it. One study was done on participants who had been fasting for 12 hours, and the second was done on those who had just eaten something.
The subjects generally preferred slim models over more rounded ones, but not after fasting. When they were hungry, they found the round human bodies and faces more attractive. So, yes, it’s definitely possible that hunger can alter your attraction to others.
“Future work might seek to elucidate the relationship between physiological states of hunger and shifts in appreciation of the human bodies and whether this relationship might be mediated by individual traits associated with to beholder’s body adiposity,” said researchers.
Chemotherapy, now streaming at just $15.99 a month!
It’s a lazy Sunday and you flip on Netflix, looking for something new to watch. There’s an almost-overwhelming number of shows out there, but right at the top of the recommended list is something that strikes your fancy right away. The algorithm behind the scenes is doing its job well, winnowing the universe of content right down to the few things you’ll find relevant, based on what you’ve watched and liked in the past.
Now, the almighty content algorithm is coming for something a little more useful than binge watching obscure 80s sitcoms: cancer treatment.
By plugging the fully sequenced genomes of nearly 10,000 patients with 33 different types of cancer into an algorithm powered by the same sort of artificial intelligence used by Netflix, researchers from London and San Diego found 21 common faults in the chromosomes of tumors, which they called copy number signatures. While cancer is a complex disease, when faults occur in those copy number signatures, the results were similar across the board. If X genetic defect occurs within a tumor, Y result will happen, even across cancer types. For example, tumors whose chromosomes had shattered and reformed had by far the worst disease outcomes.
The eventual hope is that, just as Netflix can predict what you’ll want to watch based on what you’ve already seen, oncologists will be able to predict the course of a cancer, based on the tumor’s early genetic traits, and get ahead of future genetic degradation to prevent the worst outcomes. A sort of “Oh, your tumor has enjoyed The Office. Might we suggest a treatment of 30 Rock” situation. Further research will be required to determine whether or not the cancer algorithm can get us part 2 of “Stranger Things 4” a week early.
Pay criminals, cut crime?
What is the best method for punishing those who commit wrongdoing? Fines? Jail time? Actually, no. A recent study says that financial compensation works best.
In other words, pay them for their actions. Really.
Psychologist Tage S. Rai, PhD, of the University of California, San Diego, Rady School of Management, found that people who hurt others or commit crimes are actually doing it because they think it’s the right thing to do. The results of this study say play at the angle of their morality. When that’s compromised, the offender is less likely to do it again.
Four different experiments were conducted using an online economics game with nearly 1,500 participants. Dr. Rai found that providing a monetary bonus for inflicting a punishment on a third party within the game cut the participants’ willingness to do it again by 50%.
“People punish others to signal their own goodness and receiving compensation might make it seem as though they’re driven by greed rather than justice,” he said.
The big deterrent, though, was negative judgment from peers. People in the study were even more hesitant to inflict harm and gain a profit if they thought they were going to be judged for it.
So maybe the answer to cutting crime isn’t as simple as slapping on a fine. It’s slapping on shame and paying them for it.
A conspiracy of chronobiologic proportions
The Golden State Warriors just won the NBA championship – that much is true – but we’ve got some news that you didn’t get from ESPN. The kind of news that their “partners” from the NBA didn’t want them to report. Unlike most conspiracy theories, however, this one has some science behind it.
In this case, science in the form of a study published in Frontiers in Physiology says that jet lag had a greater effect on the Boston Celtics than it did on the Warriors.
“Eastward travel – where the destination time is later than the origin time – requires the athlete to shorten their day (known as a phase advance). During phase advance, athletes often struggle to fall asleep at an earlier bedtime, leading to sleep loss and, consequently, potential impaired physiological performance and motivation the next day,” senior author Elise Facer-Childs, PhD, of Monash University, Melbourne, said in written statement.
Dr. Facer-Childs and associates took a very close look at 10 seasons’ worth of NBA games – 11,481 games, to be exact – and found “that eastward (but not westward) jet lag was associated with impaired performance for home (but not away) teams.” The existence of a pro-Western bias against teams that traveled eastward for their home games was clear:
- The chance of winning for eastern teams was reduced by 6.0%.
- They grabbed 1.3 fewer rebounds per game.
- Their field goal percentage was 1.2% lower.
And here’s the final nail in the conspiracy coffin: The NBA knew about the jet lag effect and changed the schedule of the finals in 2014 in a way that makes it worse. Before that, the higher-seeded team got two home games, then the lower-seeded team had three at home, followed by two more at the home of the higher seed. Now it’s a 2-2-1-1-1 arrangement that leads to more travel and, of course, more jet lag.
The study was published during the championship series, so the investigators suggested that the Celtics “might benefit from chronobiology-informed strategies designed to mitigate eastward jet lag symptomatology.”
So there you have it, sports fans/conspiracy theorists: You can’t chase Steph Curry around the court for 48 minutes without the right chronobiology-informed strategy. Everyone knows that.
Being hungry can alter your ‘type’
Fasting and being hungry can be a dangerous mix for becoming “hangry” and irritable, but did you know being hungry can also affect your attraction to other people?
Evidence has shown that being hungry can affect important things such as decision-making, memory, cognition, and function. It might affect decision-making in the sense that those six tacos at Taco Bell might win out over grilled chicken breast and veggies at home, but can hunger make you think that the person you just swiped right on isn’t really your type after all?
We’ll leave that up to Valentina Cazzato of Liverpool (England) John Moores University and associates, whose study involved 44 people, of whom 21 were women in their early 20s. The participants were shown computer-generated images of men and women of different sizes. The same background was used for each picture and all the expressions of the models were neutral. Participants were asked to rate each image on how much they liked it. One study was done on participants who had been fasting for 12 hours, and the second was done on those who had just eaten something.
The subjects generally preferred slim models over more rounded ones, but not after fasting. When they were hungry, they found the round human bodies and faces more attractive. So, yes, it’s definitely possible that hunger can alter your attraction to others.
“Future work might seek to elucidate the relationship between physiological states of hunger and shifts in appreciation of the human bodies and whether this relationship might be mediated by individual traits associated with to beholder’s body adiposity,” said researchers.
Chemotherapy, now streaming at just $15.99 a month!
It’s a lazy Sunday and you flip on Netflix, looking for something new to watch. There’s an almost-overwhelming number of shows out there, but right at the top of the recommended list is something that strikes your fancy right away. The algorithm behind the scenes is doing its job well, winnowing the universe of content right down to the few things you’ll find relevant, based on what you’ve watched and liked in the past.
Now, the almighty content algorithm is coming for something a little more useful than binge watching obscure 80s sitcoms: cancer treatment.
By plugging the fully sequenced genomes of nearly 10,000 patients with 33 different types of cancer into an algorithm powered by the same sort of artificial intelligence used by Netflix, researchers from London and San Diego found 21 common faults in the chromosomes of tumors, which they called copy number signatures. While cancer is a complex disease, when faults occur in those copy number signatures, the results were similar across the board. If X genetic defect occurs within a tumor, Y result will happen, even across cancer types. For example, tumors whose chromosomes had shattered and reformed had by far the worst disease outcomes.
The eventual hope is that, just as Netflix can predict what you’ll want to watch based on what you’ve already seen, oncologists will be able to predict the course of a cancer, based on the tumor’s early genetic traits, and get ahead of future genetic degradation to prevent the worst outcomes. A sort of “Oh, your tumor has enjoyed The Office. Might we suggest a treatment of 30 Rock” situation. Further research will be required to determine whether or not the cancer algorithm can get us part 2 of “Stranger Things 4” a week early.
Pay criminals, cut crime?
What is the best method for punishing those who commit wrongdoing? Fines? Jail time? Actually, no. A recent study says that financial compensation works best.
In other words, pay them for their actions. Really.
Psychologist Tage S. Rai, PhD, of the University of California, San Diego, Rady School of Management, found that people who hurt others or commit crimes are actually doing it because they think it’s the right thing to do. The results of this study say play at the angle of their morality. When that’s compromised, the offender is less likely to do it again.
Four different experiments were conducted using an online economics game with nearly 1,500 participants. Dr. Rai found that providing a monetary bonus for inflicting a punishment on a third party within the game cut the participants’ willingness to do it again by 50%.
“People punish others to signal their own goodness and receiving compensation might make it seem as though they’re driven by greed rather than justice,” he said.
The big deterrent, though, was negative judgment from peers. People in the study were even more hesitant to inflict harm and gain a profit if they thought they were going to be judged for it.
So maybe the answer to cutting crime isn’t as simple as slapping on a fine. It’s slapping on shame and paying them for it.
A conspiracy of chronobiologic proportions
The Golden State Warriors just won the NBA championship – that much is true – but we’ve got some news that you didn’t get from ESPN. The kind of news that their “partners” from the NBA didn’t want them to report. Unlike most conspiracy theories, however, this one has some science behind it.
In this case, science in the form of a study published in Frontiers in Physiology says that jet lag had a greater effect on the Boston Celtics than it did on the Warriors.
“Eastward travel – where the destination time is later than the origin time – requires the athlete to shorten their day (known as a phase advance). During phase advance, athletes often struggle to fall asleep at an earlier bedtime, leading to sleep loss and, consequently, potential impaired physiological performance and motivation the next day,” senior author Elise Facer-Childs, PhD, of Monash University, Melbourne, said in written statement.
Dr. Facer-Childs and associates took a very close look at 10 seasons’ worth of NBA games – 11,481 games, to be exact – and found “that eastward (but not westward) jet lag was associated with impaired performance for home (but not away) teams.” The existence of a pro-Western bias against teams that traveled eastward for their home games was clear:
- The chance of winning for eastern teams was reduced by 6.0%.
- They grabbed 1.3 fewer rebounds per game.
- Their field goal percentage was 1.2% lower.
And here’s the final nail in the conspiracy coffin: The NBA knew about the jet lag effect and changed the schedule of the finals in 2014 in a way that makes it worse. Before that, the higher-seeded team got two home games, then the lower-seeded team had three at home, followed by two more at the home of the higher seed. Now it’s a 2-2-1-1-1 arrangement that leads to more travel and, of course, more jet lag.
The study was published during the championship series, so the investigators suggested that the Celtics “might benefit from chronobiology-informed strategies designed to mitigate eastward jet lag symptomatology.”
So there you have it, sports fans/conspiracy theorists: You can’t chase Steph Curry around the court for 48 minutes without the right chronobiology-informed strategy. Everyone knows that.
Being hungry can alter your ‘type’
Fasting and being hungry can be a dangerous mix for becoming “hangry” and irritable, but did you know being hungry can also affect your attraction to other people?
Evidence has shown that being hungry can affect important things such as decision-making, memory, cognition, and function. It might affect decision-making in the sense that those six tacos at Taco Bell might win out over grilled chicken breast and veggies at home, but can hunger make you think that the person you just swiped right on isn’t really your type after all?
We’ll leave that up to Valentina Cazzato of Liverpool (England) John Moores University and associates, whose study involved 44 people, of whom 21 were women in their early 20s. The participants were shown computer-generated images of men and women of different sizes. The same background was used for each picture and all the expressions of the models were neutral. Participants were asked to rate each image on how much they liked it. One study was done on participants who had been fasting for 12 hours, and the second was done on those who had just eaten something.
The subjects generally preferred slim models over more rounded ones, but not after fasting. When they were hungry, they found the round human bodies and faces more attractive. So, yes, it’s definitely possible that hunger can alter your attraction to others.
“Future work might seek to elucidate the relationship between physiological states of hunger and shifts in appreciation of the human bodies and whether this relationship might be mediated by individual traits associated with to beholder’s body adiposity,” said researchers.
Provider recommendation key to boosting teen HPV vaccines
Human papilloma virus (HPV) vaccination coverage of at least one dose significantly increased in U.S. adolescents from 56.1% in 2015 to 75.4% in 2020, according to the National Immunization Survey–Teen (NIS-Teen).
The telephone survey, conducted among the parents or guardians of children ages 13-17, found a faster increase in coverage among males than females: 4.7 percentage points annually versus 2.7 percentage points annually. With yearly overall survey samples ranging from 21,875 to 17,970, these coverage differences between males and females narrowed over the 5 years of the survey period.
The difference between coverage among males and females decreased from 13 to 3 percentage points. Traditionally, parents of boys have been less likely to vaccinate their sons against HPV.
Despite the increase in uptake, however, in 2020 about 25% of adolescents had not received at least one dose of HPV vaccine. “Targeted strategies are needed to increase coverage and narrow down inequalities,” Peng-jun Lu, MD, PhD, of the National Center for Immunization and Respiratory Diseases at the Centers for Disease Control and Prevention in Atlanta, and colleagues wrote in Pediatrics.
In other NIS-Teen findings:
- Coverage in 2020 was 73.7% for males and 76.8% for females (P < .05).
- Coverage rose to 80.7% for those with a provider recommendation but was only 51.7% for those without one (P < .05).
- The rate was 80.3% for those with a well-child visit at age 11-12 years and 64.8% for those without (P < .05).
- In multivariable logistic regression, the main characteristics independently associated with a higher likelihood of vaccination included a provider recommendation, age 16-17 years, and being non-Hispanic Black, Hispanic, American Indian, or Alaskan Native.
- Other predictors of vaccination included having Medicaid insurance and having a mother who was widowed, divorced, or separated, or had no more than a high school education.
- Also predictive was having two or more provider contacts in the past 12 months, a well-child visit at age 11-12 years, and one or two vaccine providers (P < .05).
- Coverage among adolescents living in non-metropolitan statistical areas was significantly lower than those living in MSA principal cities in all years assessed (P < .05).
Provider recommendation remains significant and has historically been highly associated with HPV vaccination. In the 2012 NIS-Teen, for example, 15% of parents not intending to have their daughters vaccinated in the next 12 months cited the lack of a provider recommendation.
“To increase HPV vaccination coverage and further reduce HPV-related morbidity and mortality, providers, parents, and adolescents should use every health care visit as a chance to review vaccination histories and ensure that every adolescent receives the HPV vaccine and other needed vaccines,” Dr. Lu and associates wrote. But 18.5% of parents in the survey received no provider recommendation.
“Of note, we found that teenagers who had mothers with more education or who live in more rural communities had a lower likelihood of receiving vaccination against HPV,” Dr. Lu told this news organization. “Further research should be conducted to better understand these findings.”
According to Margaret E. Thew, DNP, FNP-BC, director of adolescent medicine at the Medical College of Wisconsin in Milwaukee, several studies have highlighted resistance to the vaccine among better-educated parents. “Parents with higher education associate the HPV vaccine with sexual activity and consequently refuse,” said Ms. Thew, who was not involved in the NIS-Teen study. “They mistakenly assume that their children are not sexually active and they lack the understanding that HPV is one of the biggest causes of oral cancer.”
The increased uptake among males was encouraging, said Ms. Thew.
Sharing her perspective on the survey-based study but not involved in it, Melissa B. Gilkey, PhD, associate professor of health behavior at the University of North Carolina in Chapel Hill, said the study is important for characterizing national trends in HPV vaccination coverage using high-quality data. “The almost 20-percentage-point jump in HPV vaccination coverage from 2015 to 2020 speaks to the hard work of primary care doctors and nurses, health departments, the CDC, and other government agencies, and public health researchers,” she told this news organization. “We’ve long understood how critical primary care is, but these data are a powerful reminder that if we want to increase HPV vaccination rates, we need to be supporting primary care doctors and nurses.”
Dr. Gilkey added that effective interventions are available to help primary care teams recommend the HPV vaccine and address parents’ vaccination concerns effectively. “However, there remains an urgent need to roll out these interventions nationally.”
This is especially true in the context of the COVID-19 pandemic, which has disrupted well-child visits and led to a decline in HPV vaccination coverage, she said. “We can’t afford to lose our hard-won gains in HPV vaccination coverage, so supporting provider recommendations and well-child visits is more important now than ever.”
According to Dr. Lu, providers should routinely recommend the vaccine and highlight the importance of vaccination in preventing HPV-related cancers. “Additionally, health care providers, parents, and adolescents should use every health care visit as a chance to review vaccination histories and ensure that every adolescent receives HPV vaccine and other needed vaccines.”
This study had no external funding. The authors had no potential conflicts of interest to disclose. Dr. Gilkey is co-principal investigator of a CDC-funded study evaluating a model for improving HPV vaccine coverage in primary care settings. Ms. Thew disclosed no potential conflicts of interest.
Human papilloma virus (HPV) vaccination coverage of at least one dose significantly increased in U.S. adolescents from 56.1% in 2015 to 75.4% in 2020, according to the National Immunization Survey–Teen (NIS-Teen).
The telephone survey, conducted among the parents or guardians of children ages 13-17, found a faster increase in coverage among males than females: 4.7 percentage points annually versus 2.7 percentage points annually. With yearly overall survey samples ranging from 21,875 to 17,970, these coverage differences between males and females narrowed over the 5 years of the survey period.
The difference between coverage among males and females decreased from 13 to 3 percentage points. Traditionally, parents of boys have been less likely to vaccinate their sons against HPV.
Despite the increase in uptake, however, in 2020 about 25% of adolescents had not received at least one dose of HPV vaccine. “Targeted strategies are needed to increase coverage and narrow down inequalities,” Peng-jun Lu, MD, PhD, of the National Center for Immunization and Respiratory Diseases at the Centers for Disease Control and Prevention in Atlanta, and colleagues wrote in Pediatrics.
In other NIS-Teen findings:
- Coverage in 2020 was 73.7% for males and 76.8% for females (P < .05).
- Coverage rose to 80.7% for those with a provider recommendation but was only 51.7% for those without one (P < .05).
- The rate was 80.3% for those with a well-child visit at age 11-12 years and 64.8% for those without (P < .05).
- In multivariable logistic regression, the main characteristics independently associated with a higher likelihood of vaccination included a provider recommendation, age 16-17 years, and being non-Hispanic Black, Hispanic, American Indian, or Alaskan Native.
- Other predictors of vaccination included having Medicaid insurance and having a mother who was widowed, divorced, or separated, or had no more than a high school education.
- Also predictive was having two or more provider contacts in the past 12 months, a well-child visit at age 11-12 years, and one or two vaccine providers (P < .05).
- Coverage among adolescents living in non-metropolitan statistical areas was significantly lower than those living in MSA principal cities in all years assessed (P < .05).
Provider recommendation remains significant and has historically been highly associated with HPV vaccination. In the 2012 NIS-Teen, for example, 15% of parents not intending to have their daughters vaccinated in the next 12 months cited the lack of a provider recommendation.
“To increase HPV vaccination coverage and further reduce HPV-related morbidity and mortality, providers, parents, and adolescents should use every health care visit as a chance to review vaccination histories and ensure that every adolescent receives the HPV vaccine and other needed vaccines,” Dr. Lu and associates wrote. But 18.5% of parents in the survey received no provider recommendation.
“Of note, we found that teenagers who had mothers with more education or who live in more rural communities had a lower likelihood of receiving vaccination against HPV,” Dr. Lu told this news organization. “Further research should be conducted to better understand these findings.”
According to Margaret E. Thew, DNP, FNP-BC, director of adolescent medicine at the Medical College of Wisconsin in Milwaukee, several studies have highlighted resistance to the vaccine among better-educated parents. “Parents with higher education associate the HPV vaccine with sexual activity and consequently refuse,” said Ms. Thew, who was not involved in the NIS-Teen study. “They mistakenly assume that their children are not sexually active and they lack the understanding that HPV is one of the biggest causes of oral cancer.”
The increased uptake among males was encouraging, said Ms. Thew.
Sharing her perspective on the survey-based study but not involved in it, Melissa B. Gilkey, PhD, associate professor of health behavior at the University of North Carolina in Chapel Hill, said the study is important for characterizing national trends in HPV vaccination coverage using high-quality data. “The almost 20-percentage-point jump in HPV vaccination coverage from 2015 to 2020 speaks to the hard work of primary care doctors and nurses, health departments, the CDC, and other government agencies, and public health researchers,” she told this news organization. “We’ve long understood how critical primary care is, but these data are a powerful reminder that if we want to increase HPV vaccination rates, we need to be supporting primary care doctors and nurses.”
Dr. Gilkey added that effective interventions are available to help primary care teams recommend the HPV vaccine and address parents’ vaccination concerns effectively. “However, there remains an urgent need to roll out these interventions nationally.”
This is especially true in the context of the COVID-19 pandemic, which has disrupted well-child visits and led to a decline in HPV vaccination coverage, she said. “We can’t afford to lose our hard-won gains in HPV vaccination coverage, so supporting provider recommendations and well-child visits is more important now than ever.”
According to Dr. Lu, providers should routinely recommend the vaccine and highlight the importance of vaccination in preventing HPV-related cancers. “Additionally, health care providers, parents, and adolescents should use every health care visit as a chance to review vaccination histories and ensure that every adolescent receives HPV vaccine and other needed vaccines.”
This study had no external funding. The authors had no potential conflicts of interest to disclose. Dr. Gilkey is co-principal investigator of a CDC-funded study evaluating a model for improving HPV vaccine coverage in primary care settings. Ms. Thew disclosed no potential conflicts of interest.
Human papilloma virus (HPV) vaccination coverage of at least one dose significantly increased in U.S. adolescents from 56.1% in 2015 to 75.4% in 2020, according to the National Immunization Survey–Teen (NIS-Teen).
The telephone survey, conducted among the parents or guardians of children ages 13-17, found a faster increase in coverage among males than females: 4.7 percentage points annually versus 2.7 percentage points annually. With yearly overall survey samples ranging from 21,875 to 17,970, these coverage differences between males and females narrowed over the 5 years of the survey period.
The difference between coverage among males and females decreased from 13 to 3 percentage points. Traditionally, parents of boys have been less likely to vaccinate their sons against HPV.
Despite the increase in uptake, however, in 2020 about 25% of adolescents had not received at least one dose of HPV vaccine. “Targeted strategies are needed to increase coverage and narrow down inequalities,” Peng-jun Lu, MD, PhD, of the National Center for Immunization and Respiratory Diseases at the Centers for Disease Control and Prevention in Atlanta, and colleagues wrote in Pediatrics.
In other NIS-Teen findings:
- Coverage in 2020 was 73.7% for males and 76.8% for females (P < .05).
- Coverage rose to 80.7% for those with a provider recommendation but was only 51.7% for those without one (P < .05).
- The rate was 80.3% for those with a well-child visit at age 11-12 years and 64.8% for those without (P < .05).
- In multivariable logistic regression, the main characteristics independently associated with a higher likelihood of vaccination included a provider recommendation, age 16-17 years, and being non-Hispanic Black, Hispanic, American Indian, or Alaskan Native.
- Other predictors of vaccination included having Medicaid insurance and having a mother who was widowed, divorced, or separated, or had no more than a high school education.
- Also predictive was having two or more provider contacts in the past 12 months, a well-child visit at age 11-12 years, and one or two vaccine providers (P < .05).
- Coverage among adolescents living in non-metropolitan statistical areas was significantly lower than those living in MSA principal cities in all years assessed (P < .05).
Provider recommendation remains significant and has historically been highly associated with HPV vaccination. In the 2012 NIS-Teen, for example, 15% of parents not intending to have their daughters vaccinated in the next 12 months cited the lack of a provider recommendation.
“To increase HPV vaccination coverage and further reduce HPV-related morbidity and mortality, providers, parents, and adolescents should use every health care visit as a chance to review vaccination histories and ensure that every adolescent receives the HPV vaccine and other needed vaccines,” Dr. Lu and associates wrote. But 18.5% of parents in the survey received no provider recommendation.
“Of note, we found that teenagers who had mothers with more education or who live in more rural communities had a lower likelihood of receiving vaccination against HPV,” Dr. Lu told this news organization. “Further research should be conducted to better understand these findings.”
According to Margaret E. Thew, DNP, FNP-BC, director of adolescent medicine at the Medical College of Wisconsin in Milwaukee, several studies have highlighted resistance to the vaccine among better-educated parents. “Parents with higher education associate the HPV vaccine with sexual activity and consequently refuse,” said Ms. Thew, who was not involved in the NIS-Teen study. “They mistakenly assume that their children are not sexually active and they lack the understanding that HPV is one of the biggest causes of oral cancer.”
The increased uptake among males was encouraging, said Ms. Thew.
Sharing her perspective on the survey-based study but not involved in it, Melissa B. Gilkey, PhD, associate professor of health behavior at the University of North Carolina in Chapel Hill, said the study is important for characterizing national trends in HPV vaccination coverage using high-quality data. “The almost 20-percentage-point jump in HPV vaccination coverage from 2015 to 2020 speaks to the hard work of primary care doctors and nurses, health departments, the CDC, and other government agencies, and public health researchers,” she told this news organization. “We’ve long understood how critical primary care is, but these data are a powerful reminder that if we want to increase HPV vaccination rates, we need to be supporting primary care doctors and nurses.”
Dr. Gilkey added that effective interventions are available to help primary care teams recommend the HPV vaccine and address parents’ vaccination concerns effectively. “However, there remains an urgent need to roll out these interventions nationally.”
This is especially true in the context of the COVID-19 pandemic, which has disrupted well-child visits and led to a decline in HPV vaccination coverage, she said. “We can’t afford to lose our hard-won gains in HPV vaccination coverage, so supporting provider recommendations and well-child visits is more important now than ever.”
According to Dr. Lu, providers should routinely recommend the vaccine and highlight the importance of vaccination in preventing HPV-related cancers. “Additionally, health care providers, parents, and adolescents should use every health care visit as a chance to review vaccination histories and ensure that every adolescent receives HPV vaccine and other needed vaccines.”
This study had no external funding. The authors had no potential conflicts of interest to disclose. Dr. Gilkey is co-principal investigator of a CDC-funded study evaluating a model for improving HPV vaccine coverage in primary care settings. Ms. Thew disclosed no potential conflicts of interest.
FROM PEDIATRICS
Study provides consensus on lab monitoring during isotretinoin therapy
For generally ideally within a month prior to the start of treatment, and a second time at peak dose.
Other tests such as complete blood cell counts and basic metabolic panels as well as specific laboratory tests such as LDL and HDL cholesterol should not be routinely monitored.
Those are key conclusions from a Delphi consensus study that included 22 acne experts from five continents and was published in JAMA Dermatology.
“Our results apply findings from recent literature and are in accordance with recent studies that have recommended against excessive laboratory monitoring,” senior corresponding author Arash Mostaghimi, MD, MPA, MPH, and coauthors wrote. “For instance, several studies in both teenagers and adults have shown that routine complete blood cell count laboratory tests are unnecessary without suspicion of an underlying abnormality and that rare abnormalities, if present, either resolved or remained stable without clinical impact on treatment. Likewise, liver function tests and lipid panels ordered at baseline and after 2 months of therapy were deemed sufficient if the clinical context and results do not suggest potential abnormalities.”
The authors also noted that, while published acne management guidelines exist, such as the American Academy of Dermatology work group guidelines and the National Institute for Health and Care Excellence guideline, “the specific recommendations surrounding laboratory monitoring frequency are nonstandardized and often nonspecific.”
To establish a consensus for isotretinoin laboratory monitoring, Dr. Mostaghimi, of the department of dermatology at Brigham and Women’s Hospital, Boston, and colleagues used a Delphi process to administer four rounds of electronic surveys to 22 board-certified dermatologists between 2021 and 2022. The primary outcome measured was whether participants could reach consensus on key isotretinoin lab monitoring parameters. Responses that failed to reach a threshold of 70% indicated no consensus.
The surveyed dermatologists had been in practice for a mean of 23.7 years, 54.5% were female, 54.5% practiced in an academic setting, and 63.9% were based in North America. They reached consensus for checking ALT within a month prior to initiation (89.5%) and at peak dose (89.5%), but not checking monthly (76.2%) or after completing treatment (73.7%). They also reached consensus on checking triglycerides within a month prior to initiation (89.5%) and at peak dose (78.9%) but not to check monthly (84.2%) or after completing treatment (73.7%).
Meanwhile, consensus was achieved for not checking complete blood cell count or basic metabolic panel parameters at any point during isotretinoin treatment (all > 70%), as well as not checking gamma-glutamyl transferase (78.9%), bilirubin (81.0%), albumin (72.7%), total protein (72.7%), LDL cholesterol (73.7%), HDL cholesterol (73.7%), or C-reactive protein (77.3%).
“Additional research is required to determine best practices for laboratory measures that did not reach consensus,” the authors wrote. The study results “are intended to guide appropriate clinical decision-making,” they added. “Although our recommendations cannot replace clinical judgment based on the unique circumstances of individual patients, we believe they provide a framework for management of a typical, otherwise healthy patient being treated with isotretinoin for acne. More routine monitoring, or reduced monitoring, should be considered on a case-by-case basis accounting for the unique medical history, circumstances, and baseline abnormalities, if present, of each patient.”
“Practicing dermatologists, including myself, routinely check blood laboratory values during isotretinoin treatment,” said Lawrence J. Green, MD, clinical professor of dermatology at George Washington University, Washington, who was asked to comment on the study. “Even though just a small number of U.S.-based and international acne researchers were involved in this Delphi consensus statement, this article still makes us practicing clinicians feel more comfortable in checking fewer lab chemistries and also less frequently checking labs when we use isotretinoin.
“That said, I don’t think most of us are ready, because of legal reasons, to do that infrequent monitoring” during isotretinoin therapy, Dr. Green added. “I think most dermatologists do not routinely perform CBCs anymore, but we still feel obligated to check triglycerides and liver function more frequently” than recommended in the new study.
Dr. Mostaghimi reported receiving grants and personal fees from Pfizer, personal fees from Eli Lilly, personal fees and licensing from Concert, personal fees from Bioniz, holds equity and advisory board membership from Hims & Hers and Figure 1, personal fees from Digital Diagnostics, and personal fees from AbbVie outside the submitted work. Other authors reported serving as an adviser, a speaker consultant, investigator, and/or board member, or having received honoraria from different pharmaceutical companies; several authors had no disclosures. Dr. Green disclosed that he is a speaker, consultant, or investigator for numerous pharmaceutical companies.
For generally ideally within a month prior to the start of treatment, and a second time at peak dose.
Other tests such as complete blood cell counts and basic metabolic panels as well as specific laboratory tests such as LDL and HDL cholesterol should not be routinely monitored.
Those are key conclusions from a Delphi consensus study that included 22 acne experts from five continents and was published in JAMA Dermatology.
“Our results apply findings from recent literature and are in accordance with recent studies that have recommended against excessive laboratory monitoring,” senior corresponding author Arash Mostaghimi, MD, MPA, MPH, and coauthors wrote. “For instance, several studies in both teenagers and adults have shown that routine complete blood cell count laboratory tests are unnecessary without suspicion of an underlying abnormality and that rare abnormalities, if present, either resolved or remained stable without clinical impact on treatment. Likewise, liver function tests and lipid panels ordered at baseline and after 2 months of therapy were deemed sufficient if the clinical context and results do not suggest potential abnormalities.”
The authors also noted that, while published acne management guidelines exist, such as the American Academy of Dermatology work group guidelines and the National Institute for Health and Care Excellence guideline, “the specific recommendations surrounding laboratory monitoring frequency are nonstandardized and often nonspecific.”
To establish a consensus for isotretinoin laboratory monitoring, Dr. Mostaghimi, of the department of dermatology at Brigham and Women’s Hospital, Boston, and colleagues used a Delphi process to administer four rounds of electronic surveys to 22 board-certified dermatologists between 2021 and 2022. The primary outcome measured was whether participants could reach consensus on key isotretinoin lab monitoring parameters. Responses that failed to reach a threshold of 70% indicated no consensus.
The surveyed dermatologists had been in practice for a mean of 23.7 years, 54.5% were female, 54.5% practiced in an academic setting, and 63.9% were based in North America. They reached consensus for checking ALT within a month prior to initiation (89.5%) and at peak dose (89.5%), but not checking monthly (76.2%) or after completing treatment (73.7%). They also reached consensus on checking triglycerides within a month prior to initiation (89.5%) and at peak dose (78.9%) but not to check monthly (84.2%) or after completing treatment (73.7%).
Meanwhile, consensus was achieved for not checking complete blood cell count or basic metabolic panel parameters at any point during isotretinoin treatment (all > 70%), as well as not checking gamma-glutamyl transferase (78.9%), bilirubin (81.0%), albumin (72.7%), total protein (72.7%), LDL cholesterol (73.7%), HDL cholesterol (73.7%), or C-reactive protein (77.3%).
“Additional research is required to determine best practices for laboratory measures that did not reach consensus,” the authors wrote. The study results “are intended to guide appropriate clinical decision-making,” they added. “Although our recommendations cannot replace clinical judgment based on the unique circumstances of individual patients, we believe they provide a framework for management of a typical, otherwise healthy patient being treated with isotretinoin for acne. More routine monitoring, or reduced monitoring, should be considered on a case-by-case basis accounting for the unique medical history, circumstances, and baseline abnormalities, if present, of each patient.”
“Practicing dermatologists, including myself, routinely check blood laboratory values during isotretinoin treatment,” said Lawrence J. Green, MD, clinical professor of dermatology at George Washington University, Washington, who was asked to comment on the study. “Even though just a small number of U.S.-based and international acne researchers were involved in this Delphi consensus statement, this article still makes us practicing clinicians feel more comfortable in checking fewer lab chemistries and also less frequently checking labs when we use isotretinoin.
“That said, I don’t think most of us are ready, because of legal reasons, to do that infrequent monitoring” during isotretinoin therapy, Dr. Green added. “I think most dermatologists do not routinely perform CBCs anymore, but we still feel obligated to check triglycerides and liver function more frequently” than recommended in the new study.
Dr. Mostaghimi reported receiving grants and personal fees from Pfizer, personal fees from Eli Lilly, personal fees and licensing from Concert, personal fees from Bioniz, holds equity and advisory board membership from Hims & Hers and Figure 1, personal fees from Digital Diagnostics, and personal fees from AbbVie outside the submitted work. Other authors reported serving as an adviser, a speaker consultant, investigator, and/or board member, or having received honoraria from different pharmaceutical companies; several authors had no disclosures. Dr. Green disclosed that he is a speaker, consultant, or investigator for numerous pharmaceutical companies.
For generally ideally within a month prior to the start of treatment, and a second time at peak dose.
Other tests such as complete blood cell counts and basic metabolic panels as well as specific laboratory tests such as LDL and HDL cholesterol should not be routinely monitored.
Those are key conclusions from a Delphi consensus study that included 22 acne experts from five continents and was published in JAMA Dermatology.
“Our results apply findings from recent literature and are in accordance with recent studies that have recommended against excessive laboratory monitoring,” senior corresponding author Arash Mostaghimi, MD, MPA, MPH, and coauthors wrote. “For instance, several studies in both teenagers and adults have shown that routine complete blood cell count laboratory tests are unnecessary without suspicion of an underlying abnormality and that rare abnormalities, if present, either resolved or remained stable without clinical impact on treatment. Likewise, liver function tests and lipid panels ordered at baseline and after 2 months of therapy were deemed sufficient if the clinical context and results do not suggest potential abnormalities.”
The authors also noted that, while published acne management guidelines exist, such as the American Academy of Dermatology work group guidelines and the National Institute for Health and Care Excellence guideline, “the specific recommendations surrounding laboratory monitoring frequency are nonstandardized and often nonspecific.”
To establish a consensus for isotretinoin laboratory monitoring, Dr. Mostaghimi, of the department of dermatology at Brigham and Women’s Hospital, Boston, and colleagues used a Delphi process to administer four rounds of electronic surveys to 22 board-certified dermatologists between 2021 and 2022. The primary outcome measured was whether participants could reach consensus on key isotretinoin lab monitoring parameters. Responses that failed to reach a threshold of 70% indicated no consensus.
The surveyed dermatologists had been in practice for a mean of 23.7 years, 54.5% were female, 54.5% practiced in an academic setting, and 63.9% were based in North America. They reached consensus for checking ALT within a month prior to initiation (89.5%) and at peak dose (89.5%), but not checking monthly (76.2%) or after completing treatment (73.7%). They also reached consensus on checking triglycerides within a month prior to initiation (89.5%) and at peak dose (78.9%) but not to check monthly (84.2%) or after completing treatment (73.7%).
Meanwhile, consensus was achieved for not checking complete blood cell count or basic metabolic panel parameters at any point during isotretinoin treatment (all > 70%), as well as not checking gamma-glutamyl transferase (78.9%), bilirubin (81.0%), albumin (72.7%), total protein (72.7%), LDL cholesterol (73.7%), HDL cholesterol (73.7%), or C-reactive protein (77.3%).
“Additional research is required to determine best practices for laboratory measures that did not reach consensus,” the authors wrote. The study results “are intended to guide appropriate clinical decision-making,” they added. “Although our recommendations cannot replace clinical judgment based on the unique circumstances of individual patients, we believe they provide a framework for management of a typical, otherwise healthy patient being treated with isotretinoin for acne. More routine monitoring, or reduced monitoring, should be considered on a case-by-case basis accounting for the unique medical history, circumstances, and baseline abnormalities, if present, of each patient.”
“Practicing dermatologists, including myself, routinely check blood laboratory values during isotretinoin treatment,” said Lawrence J. Green, MD, clinical professor of dermatology at George Washington University, Washington, who was asked to comment on the study. “Even though just a small number of U.S.-based and international acne researchers were involved in this Delphi consensus statement, this article still makes us practicing clinicians feel more comfortable in checking fewer lab chemistries and also less frequently checking labs when we use isotretinoin.
“That said, I don’t think most of us are ready, because of legal reasons, to do that infrequent monitoring” during isotretinoin therapy, Dr. Green added. “I think most dermatologists do not routinely perform CBCs anymore, but we still feel obligated to check triglycerides and liver function more frequently” than recommended in the new study.
Dr. Mostaghimi reported receiving grants and personal fees from Pfizer, personal fees from Eli Lilly, personal fees and licensing from Concert, personal fees from Bioniz, holds equity and advisory board membership from Hims & Hers and Figure 1, personal fees from Digital Diagnostics, and personal fees from AbbVie outside the submitted work. Other authors reported serving as an adviser, a speaker consultant, investigator, and/or board member, or having received honoraria from different pharmaceutical companies; several authors had no disclosures. Dr. Green disclosed that he is a speaker, consultant, or investigator for numerous pharmaceutical companies.
FROM JAMA DERMATOLOGY
Milk allergy frequently overdiagnosed
According to a consensus study, many infants in some countries are misdiagnosed with allergy to cow, sheep, or goat milk, and they’re prescribed specialized formulas they don’t need.
“Milk allergy overdiagnosis is common in some regions and can potentially harm mothers and infants,” the authors write in Clinical & Experimental Allergy. “These new consensus recommendations on the safe detection and management of milk allergy in children under 2 years aim to reduce harms associated with milk allergy overdiagnosis.”
“This guidance, developed by experts without commercial ties to the formula industry, aims to reduce milk allergy overdiagnosis and [to] support ... breastfeeding and less use of specialized formula, compared with current guidelines,” they add.
Up to 1% of European infants 2 years of age and younger are considered allergic to cow’s milk. Prescriptions for specialized formula for bottle-fed infants allergic to cow’s milk in Australia, England, and Norway have grown to over 10 times the expected volumes.
Lead study author Hilary I. Allen, National Heart and Lung Institute, Imperial College London, and her colleagues on several continents developed practical guidance for providers on safely detecting and managing milk allergy in infants.
Due to lack of high-certainty research evidence in this area, they used the Delphi consensus method.
The study involved two rounds of anonymous consensus-building surveys and one formal meeting in 2021.
The team identified experts from diverse geographic and cultural settings by searching medical databases for the term “milk hypersensitivity.” They asked those experts to recommend colleagues. The researchers also contacted experts with ties to international professional organizations, such as the International Board of Lactation Consultant Examiners, as well as societies associated with the World Allergy Organization.
The 17 study participants included clinicians and researchers in general practice, health visiting, lactation support, midwifery, nutrition, and relevant areas of pediatrics from Africa, Asia, Australia, Europe, the Middle East, and North America. Experts with recent conflicts of interest with the breastmilk substitute (formula) industry were excluded from the study. Five authors of earlier milk allergy guidelines and seven parents contributed feedback.
In each survey round, participants used a nine-point scale to rank the importance of each proposed statement that addressed prevention of overdiagnosis or underdiagnosis, support of breastfeeding women, and the role of specialized formula products.
Based on the number of total points participants assigned, each statement was classified as “essential,” “recommended,” “no consensus,” or “excluded” due to lack of relevance.
The experts agreed on 38 essential statements in several categories, including:
- Maternal dietary restriction is often not necessary to manage milk allergy
- In infants with chronic symptoms who are exclusively breastfed, milk allergy diagnosis should be considered only in specific, rare circumstances
- Milk allergy diagnosis does not usually need to be considered for stool changes, aversive feeding, or occasional spots of blood in stool, if not related in time with milk protein ingestion
The consensus recommendations provide more restrictive criteria than earlier guidelines for detecting milk allergy, fewer maternal dietary exclusions, and less use of specialized formula.
During an infant formula shortage in the U.S., a timely study
Jodi A. Shroba, MSN, APRN, CPNP, coordinator for the Food Allergy Program, Children’s Mercy Kansas City, Missouri, welcomed the study’s engagement of specialists in various fields and avoidance of bias from formula companies.
“Food allergies have received a lot of attention, especially through websites and social media,” Ms. Shroba, who was not involved in the study, told this news organization in an email. “Unfortunately, a lot of that information is incorrect and can lead to misunderstanding and misdiagnosis.”
“This article helps guide practitioners through identifying the concerning symptoms of milk allergy versus normal infant symptoms,” she said. “It can help providers discern when testing, elimination diets, and changes in formula are warranted.
“This guidance emphasizes the reproducibility and specificity of symptoms, which are key elements of a food allergy diagnosis,” she explained. “By eliminating unnecessary milk allergy labeling, we can keep infants on appropriate diets for their age, such as breastfeeding or milk-based formulas. Proper diagnosis can also reduce unnecessary financial strain of specialty formulas, stress to the family regarding feedings, and a restrictive diet for the breastfeeding mother.”
The study will be useful to a wide range of health care providers, Jennifer Anne Dantzer, MD, assistant professor of pediatrics, Johns Hopkins Medicine, Baltimore, said in an email.
“With the current formula shortage, there has perhaps never been a more important time to do this study and provide additional guidance on who does or does not need special formula,” noted Dr. Dantzer, who also was not involved in the study. “A milk allergy diagnosis impacts the child and the family, so it is very important to avoid overdiagnosis and to support the breastfeeding mother.”
“These findings should provide reassurance that dietary exclusions for the breastfeeding mother are not needed for most children with milk allergy,” she said. “If a milk allergy is suspected, the child should be referred to an allergist.”
The authors recommend further related research into the safety and effectiveness of using the guidance in practice.
One coauthor reports financial relationships with a biotech company. Ms. Allen and her remaining coauthors, as well as Ms. Shroba and Dr. Dantzer, report no relevant financial relationships. The study was funded through fellowships.
A version of this article first appeared on Medscape.com.
According to a consensus study, many infants in some countries are misdiagnosed with allergy to cow, sheep, or goat milk, and they’re prescribed specialized formulas they don’t need.
“Milk allergy overdiagnosis is common in some regions and can potentially harm mothers and infants,” the authors write in Clinical & Experimental Allergy. “These new consensus recommendations on the safe detection and management of milk allergy in children under 2 years aim to reduce harms associated with milk allergy overdiagnosis.”
“This guidance, developed by experts without commercial ties to the formula industry, aims to reduce milk allergy overdiagnosis and [to] support ... breastfeeding and less use of specialized formula, compared with current guidelines,” they add.
Up to 1% of European infants 2 years of age and younger are considered allergic to cow’s milk. Prescriptions for specialized formula for bottle-fed infants allergic to cow’s milk in Australia, England, and Norway have grown to over 10 times the expected volumes.
Lead study author Hilary I. Allen, National Heart and Lung Institute, Imperial College London, and her colleagues on several continents developed practical guidance for providers on safely detecting and managing milk allergy in infants.
Due to lack of high-certainty research evidence in this area, they used the Delphi consensus method.
The study involved two rounds of anonymous consensus-building surveys and one formal meeting in 2021.
The team identified experts from diverse geographic and cultural settings by searching medical databases for the term “milk hypersensitivity.” They asked those experts to recommend colleagues. The researchers also contacted experts with ties to international professional organizations, such as the International Board of Lactation Consultant Examiners, as well as societies associated with the World Allergy Organization.
The 17 study participants included clinicians and researchers in general practice, health visiting, lactation support, midwifery, nutrition, and relevant areas of pediatrics from Africa, Asia, Australia, Europe, the Middle East, and North America. Experts with recent conflicts of interest with the breastmilk substitute (formula) industry were excluded from the study. Five authors of earlier milk allergy guidelines and seven parents contributed feedback.
In each survey round, participants used a nine-point scale to rank the importance of each proposed statement that addressed prevention of overdiagnosis or underdiagnosis, support of breastfeeding women, and the role of specialized formula products.
Based on the number of total points participants assigned, each statement was classified as “essential,” “recommended,” “no consensus,” or “excluded” due to lack of relevance.
The experts agreed on 38 essential statements in several categories, including:
- Maternal dietary restriction is often not necessary to manage milk allergy
- In infants with chronic symptoms who are exclusively breastfed, milk allergy diagnosis should be considered only in specific, rare circumstances
- Milk allergy diagnosis does not usually need to be considered for stool changes, aversive feeding, or occasional spots of blood in stool, if not related in time with milk protein ingestion
The consensus recommendations provide more restrictive criteria than earlier guidelines for detecting milk allergy, fewer maternal dietary exclusions, and less use of specialized formula.
During an infant formula shortage in the U.S., a timely study
Jodi A. Shroba, MSN, APRN, CPNP, coordinator for the Food Allergy Program, Children’s Mercy Kansas City, Missouri, welcomed the study’s engagement of specialists in various fields and avoidance of bias from formula companies.
“Food allergies have received a lot of attention, especially through websites and social media,” Ms. Shroba, who was not involved in the study, told this news organization in an email. “Unfortunately, a lot of that information is incorrect and can lead to misunderstanding and misdiagnosis.”
“This article helps guide practitioners through identifying the concerning symptoms of milk allergy versus normal infant symptoms,” she said. “It can help providers discern when testing, elimination diets, and changes in formula are warranted.
“This guidance emphasizes the reproducibility and specificity of symptoms, which are key elements of a food allergy diagnosis,” she explained. “By eliminating unnecessary milk allergy labeling, we can keep infants on appropriate diets for their age, such as breastfeeding or milk-based formulas. Proper diagnosis can also reduce unnecessary financial strain of specialty formulas, stress to the family regarding feedings, and a restrictive diet for the breastfeeding mother.”
The study will be useful to a wide range of health care providers, Jennifer Anne Dantzer, MD, assistant professor of pediatrics, Johns Hopkins Medicine, Baltimore, said in an email.
“With the current formula shortage, there has perhaps never been a more important time to do this study and provide additional guidance on who does or does not need special formula,” noted Dr. Dantzer, who also was not involved in the study. “A milk allergy diagnosis impacts the child and the family, so it is very important to avoid overdiagnosis and to support the breastfeeding mother.”
“These findings should provide reassurance that dietary exclusions for the breastfeeding mother are not needed for most children with milk allergy,” she said. “If a milk allergy is suspected, the child should be referred to an allergist.”
The authors recommend further related research into the safety and effectiveness of using the guidance in practice.
One coauthor reports financial relationships with a biotech company. Ms. Allen and her remaining coauthors, as well as Ms. Shroba and Dr. Dantzer, report no relevant financial relationships. The study was funded through fellowships.
A version of this article first appeared on Medscape.com.
According to a consensus study, many infants in some countries are misdiagnosed with allergy to cow, sheep, or goat milk, and they’re prescribed specialized formulas they don’t need.
“Milk allergy overdiagnosis is common in some regions and can potentially harm mothers and infants,” the authors write in Clinical & Experimental Allergy. “These new consensus recommendations on the safe detection and management of milk allergy in children under 2 years aim to reduce harms associated with milk allergy overdiagnosis.”
“This guidance, developed by experts without commercial ties to the formula industry, aims to reduce milk allergy overdiagnosis and [to] support ... breastfeeding and less use of specialized formula, compared with current guidelines,” they add.
Up to 1% of European infants 2 years of age and younger are considered allergic to cow’s milk. Prescriptions for specialized formula for bottle-fed infants allergic to cow’s milk in Australia, England, and Norway have grown to over 10 times the expected volumes.
Lead study author Hilary I. Allen, National Heart and Lung Institute, Imperial College London, and her colleagues on several continents developed practical guidance for providers on safely detecting and managing milk allergy in infants.
Due to lack of high-certainty research evidence in this area, they used the Delphi consensus method.
The study involved two rounds of anonymous consensus-building surveys and one formal meeting in 2021.
The team identified experts from diverse geographic and cultural settings by searching medical databases for the term “milk hypersensitivity.” They asked those experts to recommend colleagues. The researchers also contacted experts with ties to international professional organizations, such as the International Board of Lactation Consultant Examiners, as well as societies associated with the World Allergy Organization.
The 17 study participants included clinicians and researchers in general practice, health visiting, lactation support, midwifery, nutrition, and relevant areas of pediatrics from Africa, Asia, Australia, Europe, the Middle East, and North America. Experts with recent conflicts of interest with the breastmilk substitute (formula) industry were excluded from the study. Five authors of earlier milk allergy guidelines and seven parents contributed feedback.
In each survey round, participants used a nine-point scale to rank the importance of each proposed statement that addressed prevention of overdiagnosis or underdiagnosis, support of breastfeeding women, and the role of specialized formula products.
Based on the number of total points participants assigned, each statement was classified as “essential,” “recommended,” “no consensus,” or “excluded” due to lack of relevance.
The experts agreed on 38 essential statements in several categories, including:
- Maternal dietary restriction is often not necessary to manage milk allergy
- In infants with chronic symptoms who are exclusively breastfed, milk allergy diagnosis should be considered only in specific, rare circumstances
- Milk allergy diagnosis does not usually need to be considered for stool changes, aversive feeding, or occasional spots of blood in stool, if not related in time with milk protein ingestion
The consensus recommendations provide more restrictive criteria than earlier guidelines for detecting milk allergy, fewer maternal dietary exclusions, and less use of specialized formula.
During an infant formula shortage in the U.S., a timely study
Jodi A. Shroba, MSN, APRN, CPNP, coordinator for the Food Allergy Program, Children’s Mercy Kansas City, Missouri, welcomed the study’s engagement of specialists in various fields and avoidance of bias from formula companies.
“Food allergies have received a lot of attention, especially through websites and social media,” Ms. Shroba, who was not involved in the study, told this news organization in an email. “Unfortunately, a lot of that information is incorrect and can lead to misunderstanding and misdiagnosis.”
“This article helps guide practitioners through identifying the concerning symptoms of milk allergy versus normal infant symptoms,” she said. “It can help providers discern when testing, elimination diets, and changes in formula are warranted.
“This guidance emphasizes the reproducibility and specificity of symptoms, which are key elements of a food allergy diagnosis,” she explained. “By eliminating unnecessary milk allergy labeling, we can keep infants on appropriate diets for their age, such as breastfeeding or milk-based formulas. Proper diagnosis can also reduce unnecessary financial strain of specialty formulas, stress to the family regarding feedings, and a restrictive diet for the breastfeeding mother.”
The study will be useful to a wide range of health care providers, Jennifer Anne Dantzer, MD, assistant professor of pediatrics, Johns Hopkins Medicine, Baltimore, said in an email.
“With the current formula shortage, there has perhaps never been a more important time to do this study and provide additional guidance on who does or does not need special formula,” noted Dr. Dantzer, who also was not involved in the study. “A milk allergy diagnosis impacts the child and the family, so it is very important to avoid overdiagnosis and to support the breastfeeding mother.”
“These findings should provide reassurance that dietary exclusions for the breastfeeding mother are not needed for most children with milk allergy,” she said. “If a milk allergy is suspected, the child should be referred to an allergist.”
The authors recommend further related research into the safety and effectiveness of using the guidance in practice.
One coauthor reports financial relationships with a biotech company. Ms. Allen and her remaining coauthors, as well as Ms. Shroba and Dr. Dantzer, report no relevant financial relationships. The study was funded through fellowships.
A version of this article first appeared on Medscape.com.
Pediatric obesity disparities widen
Lower levels of household income and education in the United States are associated with higher rates of adolescent obesity. These socioeconomic disparities “have widened during the last two decades,” new research shows.
Because obesity in adolescence has immediate and long-term health consequences, this phenomenon “may exacerbate socioeconomic disparities in chronic diseases into adulthood,” study author Ryunosuke Goto, MD, of University of Tokyo Hospital, and colleagues reported in JAMA Pediatrics.
Groups with higher rates of obesity may also be less likely to access treatment, said Kyung E. Rhee, MD, professor of pediatrics at University of California, San Diego School of Medicine, who was not involved in the new analysis.
“These are the families who have a harder time getting to the doctor’s office or getting to programs because they are working multiple jobs, or they don’t have as much flexibility,” Dr. Rhee told this news organization.
20 years of data
A recent study showed a relationship between socioeconomic status (SES) and weight in adults. Research examining current trends in adolescents has been limited, however, according to the authors of the new study.
To address this gap, Dr. Goto and colleagues looked at obesity trends among approximately 20,000 U.S. children aged 10-19 years using cross-sectional data from the 1999-2018 National Health and Nutrition Examination Surveys.
They compared the prevalence of obesity among participants whose household income was 138% of the federal poverty level or less versus those with higher levels of household income. They also examined obesity prevalence according to whether the head of household had graduated college.
Relative to higher-income households, adolescents from lower-income households were more likely to be non-Hispanic Black (21.7% vs. 10.4%) or Hispanic (30.6% vs. 13.4%) and to have an unmarried parent (54.5% vs. 23%). They were also more likely to have obesity (22.8% vs. 17.3%).
The prevalence of obesity likewise was higher among adolescents whose head of household did not have a college degree (21.8% vs. 11.6%).
In an analysis that adjusted for race, ethnicity, height, and marital status of the head of household, the prevalence of obesity increased over 20 years, particularly among adolescents from lower-income homes, the researchers reported.
Lower income was associated with an increase in obesity prevalence of 4.2 percentage points, and less education was associated with an increase in obesity prevalence of 9 percentage points.
By 2015-2018, the gap in obesity prevalence between low-income households and higher-income households was 6.4 percentage points more than it had been during 1999-2002 (95% confidence interval, 1.5-11.4). “When we assessed linear trends, the gap in obesity prevalence by income and education increased by an average of 1.5 (95% CI, 0.4-2.6) and 1.1 (95% CI, 0.0-2.3) percentage points every 4 years, respectively,” according to the researchers.
How to treat
Separately, researchers are studying ways to help treat patients with obesity and increase access to treatment. To that end, Dr. Rhee and colleagues developed a new program called Guided Self-Help Obesity Treatment in the Doctor’s Office (GOT Doc).
The guided self-help program was designed to provide similar resources as a leading treatment approach – family-based treatment – but in a less intensive, more accessible way.
Results from a randomized trial comparing this guided self-help approach with family-based treatment were published in Pediatrics.
The trial included 159 children and their parents. The children had an average age of 9.6 years and body mass index z-score of 2.1. Participants were primarily Latinx and from lower income neighborhoods.
Whereas family-based treatment included hour-long sessions at an academic center, the guided self-help program featured a 20-minute session in the office where patients typically see their primary care physician.
Both programs covered how to self-monitor food intake, set healthy goals, and modify the home environment to promote behavioral change. They also discussed body image, bullying, and emotional health. The program is framed around developing a healthy lifestyle rather than weight loss itself, Dr. Rhee said.
Children in both groups had significant reductions in their body mass index percentiles after the 6-month treatment programs. The reductions were largely maintained at 6-month follow-up.
Families in the guided self-help program, however, had a 67% lower risk of dropping out of the study and reported greater satisfaction and convenience. They attended more than half of the treatment sessions, whereas participants assigned to family-based treatment attended 1 in 5 sessions, on average.
The trial was conducted before the COVID-19 pandemic. Next, the researchers plan to test delivery of a guided self-help program via video calls, Dr. Rhee said.
Having options readily available for families who are interested in treatment for obesity proved valuable to clinicians, Dr. Rhee said. “They could then just refer them down the hall to the interventionist who was there, who was going to then work with the family to make these changes,” she said.
The study by Dr. Goto and colleagues was supported by grants from the Japan Society for the Promotion of Science. The trial by Dr. Rhee et al. was supported by a grant from the Health Resources and Services Administration. Neither research team had conflict of interest disclosures.
A version of this article first appeared on Medscape.com.
Lower levels of household income and education in the United States are associated with higher rates of adolescent obesity. These socioeconomic disparities “have widened during the last two decades,” new research shows.
Because obesity in adolescence has immediate and long-term health consequences, this phenomenon “may exacerbate socioeconomic disparities in chronic diseases into adulthood,” study author Ryunosuke Goto, MD, of University of Tokyo Hospital, and colleagues reported in JAMA Pediatrics.
Groups with higher rates of obesity may also be less likely to access treatment, said Kyung E. Rhee, MD, professor of pediatrics at University of California, San Diego School of Medicine, who was not involved in the new analysis.
“These are the families who have a harder time getting to the doctor’s office or getting to programs because they are working multiple jobs, or they don’t have as much flexibility,” Dr. Rhee told this news organization.
20 years of data
A recent study showed a relationship between socioeconomic status (SES) and weight in adults. Research examining current trends in adolescents has been limited, however, according to the authors of the new study.
To address this gap, Dr. Goto and colleagues looked at obesity trends among approximately 20,000 U.S. children aged 10-19 years using cross-sectional data from the 1999-2018 National Health and Nutrition Examination Surveys.
They compared the prevalence of obesity among participants whose household income was 138% of the federal poverty level or less versus those with higher levels of household income. They also examined obesity prevalence according to whether the head of household had graduated college.
Relative to higher-income households, adolescents from lower-income households were more likely to be non-Hispanic Black (21.7% vs. 10.4%) or Hispanic (30.6% vs. 13.4%) and to have an unmarried parent (54.5% vs. 23%). They were also more likely to have obesity (22.8% vs. 17.3%).
The prevalence of obesity likewise was higher among adolescents whose head of household did not have a college degree (21.8% vs. 11.6%).
In an analysis that adjusted for race, ethnicity, height, and marital status of the head of household, the prevalence of obesity increased over 20 years, particularly among adolescents from lower-income homes, the researchers reported.
Lower income was associated with an increase in obesity prevalence of 4.2 percentage points, and less education was associated with an increase in obesity prevalence of 9 percentage points.
By 2015-2018, the gap in obesity prevalence between low-income households and higher-income households was 6.4 percentage points more than it had been during 1999-2002 (95% confidence interval, 1.5-11.4). “When we assessed linear trends, the gap in obesity prevalence by income and education increased by an average of 1.5 (95% CI, 0.4-2.6) and 1.1 (95% CI, 0.0-2.3) percentage points every 4 years, respectively,” according to the researchers.
How to treat
Separately, researchers are studying ways to help treat patients with obesity and increase access to treatment. To that end, Dr. Rhee and colleagues developed a new program called Guided Self-Help Obesity Treatment in the Doctor’s Office (GOT Doc).
The guided self-help program was designed to provide similar resources as a leading treatment approach – family-based treatment – but in a less intensive, more accessible way.
Results from a randomized trial comparing this guided self-help approach with family-based treatment were published in Pediatrics.
The trial included 159 children and their parents. The children had an average age of 9.6 years and body mass index z-score of 2.1. Participants were primarily Latinx and from lower income neighborhoods.
Whereas family-based treatment included hour-long sessions at an academic center, the guided self-help program featured a 20-minute session in the office where patients typically see their primary care physician.
Both programs covered how to self-monitor food intake, set healthy goals, and modify the home environment to promote behavioral change. They also discussed body image, bullying, and emotional health. The program is framed around developing a healthy lifestyle rather than weight loss itself, Dr. Rhee said.
Children in both groups had significant reductions in their body mass index percentiles after the 6-month treatment programs. The reductions were largely maintained at 6-month follow-up.
Families in the guided self-help program, however, had a 67% lower risk of dropping out of the study and reported greater satisfaction and convenience. They attended more than half of the treatment sessions, whereas participants assigned to family-based treatment attended 1 in 5 sessions, on average.
The trial was conducted before the COVID-19 pandemic. Next, the researchers plan to test delivery of a guided self-help program via video calls, Dr. Rhee said.
Having options readily available for families who are interested in treatment for obesity proved valuable to clinicians, Dr. Rhee said. “They could then just refer them down the hall to the interventionist who was there, who was going to then work with the family to make these changes,” she said.
The study by Dr. Goto and colleagues was supported by grants from the Japan Society for the Promotion of Science. The trial by Dr. Rhee et al. was supported by a grant from the Health Resources and Services Administration. Neither research team had conflict of interest disclosures.
A version of this article first appeared on Medscape.com.
Lower levels of household income and education in the United States are associated with higher rates of adolescent obesity. These socioeconomic disparities “have widened during the last two decades,” new research shows.
Because obesity in adolescence has immediate and long-term health consequences, this phenomenon “may exacerbate socioeconomic disparities in chronic diseases into adulthood,” study author Ryunosuke Goto, MD, of University of Tokyo Hospital, and colleagues reported in JAMA Pediatrics.
Groups with higher rates of obesity may also be less likely to access treatment, said Kyung E. Rhee, MD, professor of pediatrics at University of California, San Diego School of Medicine, who was not involved in the new analysis.
“These are the families who have a harder time getting to the doctor’s office or getting to programs because they are working multiple jobs, or they don’t have as much flexibility,” Dr. Rhee told this news organization.
20 years of data
A recent study showed a relationship between socioeconomic status (SES) and weight in adults. Research examining current trends in adolescents has been limited, however, according to the authors of the new study.
To address this gap, Dr. Goto and colleagues looked at obesity trends among approximately 20,000 U.S. children aged 10-19 years using cross-sectional data from the 1999-2018 National Health and Nutrition Examination Surveys.
They compared the prevalence of obesity among participants whose household income was 138% of the federal poverty level or less versus those with higher levels of household income. They also examined obesity prevalence according to whether the head of household had graduated college.
Relative to higher-income households, adolescents from lower-income households were more likely to be non-Hispanic Black (21.7% vs. 10.4%) or Hispanic (30.6% vs. 13.4%) and to have an unmarried parent (54.5% vs. 23%). They were also more likely to have obesity (22.8% vs. 17.3%).
The prevalence of obesity likewise was higher among adolescents whose head of household did not have a college degree (21.8% vs. 11.6%).
In an analysis that adjusted for race, ethnicity, height, and marital status of the head of household, the prevalence of obesity increased over 20 years, particularly among adolescents from lower-income homes, the researchers reported.
Lower income was associated with an increase in obesity prevalence of 4.2 percentage points, and less education was associated with an increase in obesity prevalence of 9 percentage points.
By 2015-2018, the gap in obesity prevalence between low-income households and higher-income households was 6.4 percentage points more than it had been during 1999-2002 (95% confidence interval, 1.5-11.4). “When we assessed linear trends, the gap in obesity prevalence by income and education increased by an average of 1.5 (95% CI, 0.4-2.6) and 1.1 (95% CI, 0.0-2.3) percentage points every 4 years, respectively,” according to the researchers.
How to treat
Separately, researchers are studying ways to help treat patients with obesity and increase access to treatment. To that end, Dr. Rhee and colleagues developed a new program called Guided Self-Help Obesity Treatment in the Doctor’s Office (GOT Doc).
The guided self-help program was designed to provide similar resources as a leading treatment approach – family-based treatment – but in a less intensive, more accessible way.
Results from a randomized trial comparing this guided self-help approach with family-based treatment were published in Pediatrics.
The trial included 159 children and their parents. The children had an average age of 9.6 years and body mass index z-score of 2.1. Participants were primarily Latinx and from lower income neighborhoods.
Whereas family-based treatment included hour-long sessions at an academic center, the guided self-help program featured a 20-minute session in the office where patients typically see their primary care physician.
Both programs covered how to self-monitor food intake, set healthy goals, and modify the home environment to promote behavioral change. They also discussed body image, bullying, and emotional health. The program is framed around developing a healthy lifestyle rather than weight loss itself, Dr. Rhee said.
Children in both groups had significant reductions in their body mass index percentiles after the 6-month treatment programs. The reductions were largely maintained at 6-month follow-up.
Families in the guided self-help program, however, had a 67% lower risk of dropping out of the study and reported greater satisfaction and convenience. They attended more than half of the treatment sessions, whereas participants assigned to family-based treatment attended 1 in 5 sessions, on average.
The trial was conducted before the COVID-19 pandemic. Next, the researchers plan to test delivery of a guided self-help program via video calls, Dr. Rhee said.
Having options readily available for families who are interested in treatment for obesity proved valuable to clinicians, Dr. Rhee said. “They could then just refer them down the hall to the interventionist who was there, who was going to then work with the family to make these changes,” she said.
The study by Dr. Goto and colleagues was supported by grants from the Japan Society for the Promotion of Science. The trial by Dr. Rhee et al. was supported by a grant from the Health Resources and Services Administration. Neither research team had conflict of interest disclosures.
A version of this article first appeared on Medscape.com.
Children and COVID: Vaccines now available to all ages
The COVID-19 prevention effort in children enters its next phase as June draws to a close, while new pediatric cases continued on a downward trend and hospitalizations continued to rise.
The COVID-19 vaccines from Pfizer-BioNTech and Moderna were approved for use in children as young as 6 months, the Centers for Disease Control and Prevention announced on June 18.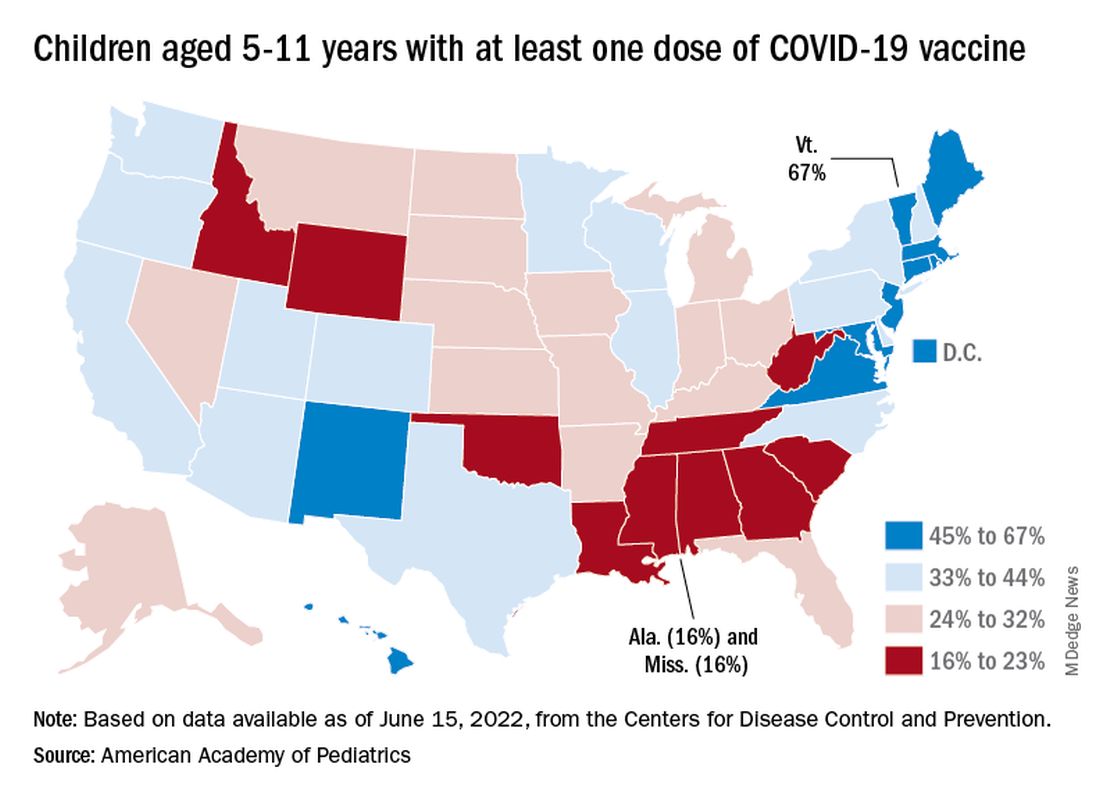
“We know millions of parents and caregivers are eager to get their young children vaccinated. ... I encourage parents and caregivers with questions to talk to their doctor, nurse, or local pharmacist to learn more about the benefits of vaccinations,” CDC Director Rochelle P. Walensky, MD, MPH, said in a written statement.
There are, however, indications that many parents are not that eager. Another 11% said “they will only do so if they are required,” Kaiser noted.
The vaccination experience with children aged 5-11 years seems to agree with those numbers. As of June 16, more than 7 months after the vaccine became available, just over 36% had received at least one dose and about 30% were fully vaccinated, CDC data show.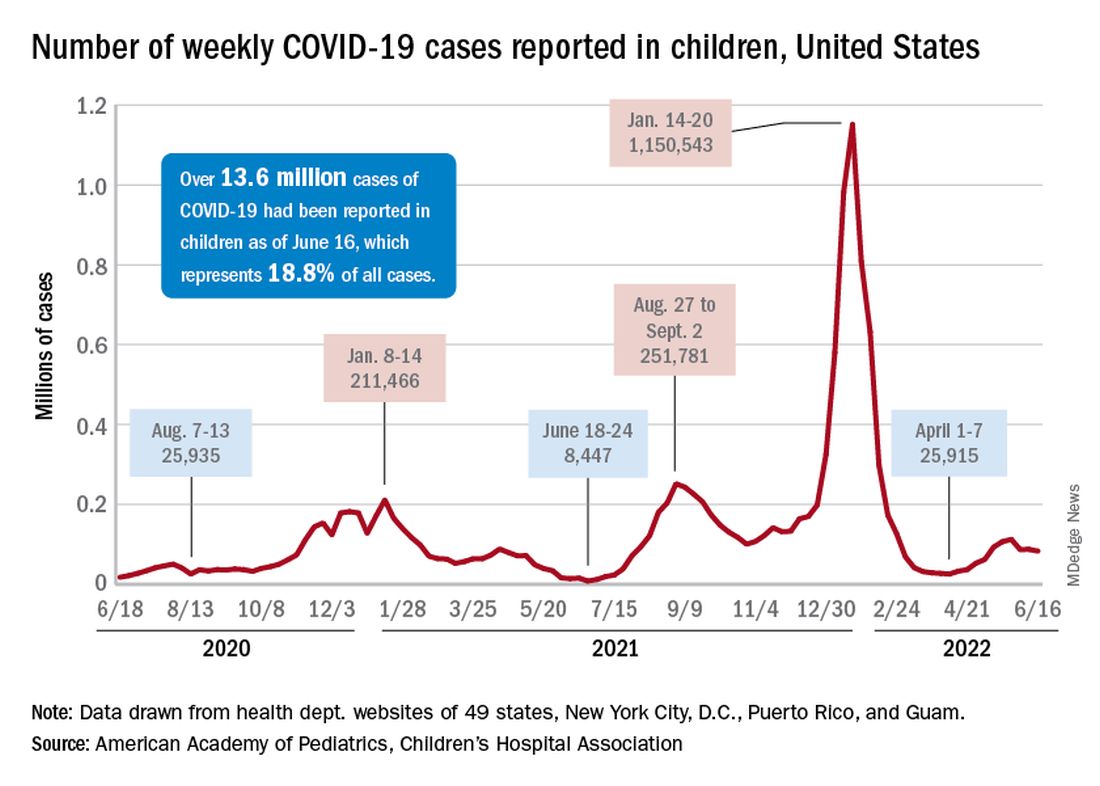
There are, according to the American Academy of Pediatrics, still five states where less than 20% of eligible 5- to 11-year-olds have received an initial vaccination. Among children aged 12-17, uptake has been much higher: 70% have received at least one dose and 60% are fully vaccinated, the CDC said.
Trends for new cases, hospitalizations diverging
COVID incidence in children, meanwhile, dropped for the second time in 3 weeks. There were 83,000 new cases reported during June 10-16, a decline of 4.8% from the previous week, according to the AAP and the Children’s Hospital Association.
New cases had risen by a very slight 0.31% during the week of June 3-9 after dropping 22% the week before (May 27 to June 2). Total cases in children have surpassed 13.6 million, which represents 18.8% of cases in all ages since the start of the pandemic, the AAP and CHA said in their weekly COVID report.
New admissions of children with confirmed COVID-19, however, have continued to climb since early to mid April. On June 16, the rate for children aged 0-17 years was up to 0.31 per 100,000, compared with the 0.13 per 100,000 recorded as late as April 11, the CDC said on its COVID Data Tracker.
The COVID-19 prevention effort in children enters its next phase as June draws to a close, while new pediatric cases continued on a downward trend and hospitalizations continued to rise.
The COVID-19 vaccines from Pfizer-BioNTech and Moderna were approved for use in children as young as 6 months, the Centers for Disease Control and Prevention announced on June 18.
“We know millions of parents and caregivers are eager to get their young children vaccinated. ... I encourage parents and caregivers with questions to talk to their doctor, nurse, or local pharmacist to learn more about the benefits of vaccinations,” CDC Director Rochelle P. Walensky, MD, MPH, said in a written statement.
There are, however, indications that many parents are not that eager. Another 11% said “they will only do so if they are required,” Kaiser noted.
The vaccination experience with children aged 5-11 years seems to agree with those numbers. As of June 16, more than 7 months after the vaccine became available, just over 36% had received at least one dose and about 30% were fully vaccinated, CDC data show.
There are, according to the American Academy of Pediatrics, still five states where less than 20% of eligible 5- to 11-year-olds have received an initial vaccination. Among children aged 12-17, uptake has been much higher: 70% have received at least one dose and 60% are fully vaccinated, the CDC said.
Trends for new cases, hospitalizations diverging
COVID incidence in children, meanwhile, dropped for the second time in 3 weeks. There were 83,000 new cases reported during June 10-16, a decline of 4.8% from the previous week, according to the AAP and the Children’s Hospital Association.
New cases had risen by a very slight 0.31% during the week of June 3-9 after dropping 22% the week before (May 27 to June 2). Total cases in children have surpassed 13.6 million, which represents 18.8% of cases in all ages since the start of the pandemic, the AAP and CHA said in their weekly COVID report.
New admissions of children with confirmed COVID-19, however, have continued to climb since early to mid April. On June 16, the rate for children aged 0-17 years was up to 0.31 per 100,000, compared with the 0.13 per 100,000 recorded as late as April 11, the CDC said on its COVID Data Tracker.
The COVID-19 prevention effort in children enters its next phase as June draws to a close, while new pediatric cases continued on a downward trend and hospitalizations continued to rise.
The COVID-19 vaccines from Pfizer-BioNTech and Moderna were approved for use in children as young as 6 months, the Centers for Disease Control and Prevention announced on June 18.
“We know millions of parents and caregivers are eager to get their young children vaccinated. ... I encourage parents and caregivers with questions to talk to their doctor, nurse, or local pharmacist to learn more about the benefits of vaccinations,” CDC Director Rochelle P. Walensky, MD, MPH, said in a written statement.
There are, however, indications that many parents are not that eager. Another 11% said “they will only do so if they are required,” Kaiser noted.
The vaccination experience with children aged 5-11 years seems to agree with those numbers. As of June 16, more than 7 months after the vaccine became available, just over 36% had received at least one dose and about 30% were fully vaccinated, CDC data show.
There are, according to the American Academy of Pediatrics, still five states where less than 20% of eligible 5- to 11-year-olds have received an initial vaccination. Among children aged 12-17, uptake has been much higher: 70% have received at least one dose and 60% are fully vaccinated, the CDC said.
Trends for new cases, hospitalizations diverging
COVID incidence in children, meanwhile, dropped for the second time in 3 weeks. There were 83,000 new cases reported during June 10-16, a decline of 4.8% from the previous week, according to the AAP and the Children’s Hospital Association.
New cases had risen by a very slight 0.31% during the week of June 3-9 after dropping 22% the week before (May 27 to June 2). Total cases in children have surpassed 13.6 million, which represents 18.8% of cases in all ages since the start of the pandemic, the AAP and CHA said in their weekly COVID report.
New admissions of children with confirmed COVID-19, however, have continued to climb since early to mid April. On June 16, the rate for children aged 0-17 years was up to 0.31 per 100,000, compared with the 0.13 per 100,000 recorded as late as April 11, the CDC said on its COVID Data Tracker.
Biden moves to limit nicotine levels in cigarettes
The Department of Health and Human Services posted a notice that details plans for a new rule to create a maximum allowed amount of nicotine in certain tobacco products. The Food and Drug Administration would take the action, the notice said, “to reduce addictiveness to certain tobacco products, thus giving addicted users a greater ability to quit.” The product standard would also help keep nonsmokers interested in trying tobacco, mainly youth, from starting to smoke and become regulars.
“Lowering nicotine levels to minimally addictive or non-addictive levels would decrease the likelihood that future generations of young people become addicted to cigarettes and help more currently addicted smokers to quit,” FDA Commissioner Robert Califf, MD, said in a statement.
The FDA, in charge of regulating cigarettes, issues a proposed rule when changes are discussed. That would be followed by a period for public comments before a final rule could be issued.
The proposed rule was first reported by The Washington Post.
The FDA in 2018 published a study in the New England Journal of Medicine that estimated that a potential limit on nicotine in cigarettes could, by the year 2100, prevent more than 33 million people from becoming regular smokers, and prevent the deaths of more than 8 million people from tobacco-related illnesses.
The action to reduce nicotine levels would fit in with President Joe Biden’s goal of reducing cancer death rates by half over 25 years. Each year, according to the American Cancer Society, about 480,000 deaths (about 1 in 5) are related to smoking. Currently, about 34 million American adults still smoke cigarettes.
Matthew Myers, president of the Campaign for Tobacco-Free Kids, called the proposed rule a “truly game-changing proposal.”
“There is no other single action our country can take that would prevent more young people from becoming addicted to tobacco or have a greater impact on reducing deaths from cancer, cardiovascular disease and respiratory disease,” Mr. Myers said in a statement.
However, he said, “these gains will only be realized if the administration and the FDA demonstrate a full-throated commitment to finalizing and implementing this proposal.”
The FDA proposed the nicotine reduction strategy in talks with the White House and the Department of Health and Human Services early in 2021, according to the Post.
Earlier this year, the FDA issued a proposed rule to ban menthol flavoring in cigarettes. The agency is accepting public comments though July 5.
The action of reducing nicotine levels would likely take years to complete, Mitch Zeller, JD, recently retired director of the FDA Center for Tobacco Products, told the Post.
In 2018, the FDA issued a proposed ruling to set a standard for maximum nicotine levels in cigarettes.
Advocates say the action of slashing nicotine, the active – and addictive – ingredient in cigarettes, would save millions of lives for generations to come. Opponents liken it to the prohibition of alcohol in the 1920s and predict the action will fail.
Others say that if limits are put on nicotine levels, adults should have greater access to noncombustible alternatives.
A version of this article first appeared on WebMD.com.
The Department of Health and Human Services posted a notice that details plans for a new rule to create a maximum allowed amount of nicotine in certain tobacco products. The Food and Drug Administration would take the action, the notice said, “to reduce addictiveness to certain tobacco products, thus giving addicted users a greater ability to quit.” The product standard would also help keep nonsmokers interested in trying tobacco, mainly youth, from starting to smoke and become regulars.
“Lowering nicotine levels to minimally addictive or non-addictive levels would decrease the likelihood that future generations of young people become addicted to cigarettes and help more currently addicted smokers to quit,” FDA Commissioner Robert Califf, MD, said in a statement.
The FDA, in charge of regulating cigarettes, issues a proposed rule when changes are discussed. That would be followed by a period for public comments before a final rule could be issued.
The proposed rule was first reported by The Washington Post.
The FDA in 2018 published a study in the New England Journal of Medicine that estimated that a potential limit on nicotine in cigarettes could, by the year 2100, prevent more than 33 million people from becoming regular smokers, and prevent the deaths of more than 8 million people from tobacco-related illnesses.
The action to reduce nicotine levels would fit in with President Joe Biden’s goal of reducing cancer death rates by half over 25 years. Each year, according to the American Cancer Society, about 480,000 deaths (about 1 in 5) are related to smoking. Currently, about 34 million American adults still smoke cigarettes.
Matthew Myers, president of the Campaign for Tobacco-Free Kids, called the proposed rule a “truly game-changing proposal.”
“There is no other single action our country can take that would prevent more young people from becoming addicted to tobacco or have a greater impact on reducing deaths from cancer, cardiovascular disease and respiratory disease,” Mr. Myers said in a statement.
However, he said, “these gains will only be realized if the administration and the FDA demonstrate a full-throated commitment to finalizing and implementing this proposal.”
The FDA proposed the nicotine reduction strategy in talks with the White House and the Department of Health and Human Services early in 2021, according to the Post.
Earlier this year, the FDA issued a proposed rule to ban menthol flavoring in cigarettes. The agency is accepting public comments though July 5.
The action of reducing nicotine levels would likely take years to complete, Mitch Zeller, JD, recently retired director of the FDA Center for Tobacco Products, told the Post.
In 2018, the FDA issued a proposed ruling to set a standard for maximum nicotine levels in cigarettes.
Advocates say the action of slashing nicotine, the active – and addictive – ingredient in cigarettes, would save millions of lives for generations to come. Opponents liken it to the prohibition of alcohol in the 1920s and predict the action will fail.
Others say that if limits are put on nicotine levels, adults should have greater access to noncombustible alternatives.
A version of this article first appeared on WebMD.com.
The Department of Health and Human Services posted a notice that details plans for a new rule to create a maximum allowed amount of nicotine in certain tobacco products. The Food and Drug Administration would take the action, the notice said, “to reduce addictiveness to certain tobacco products, thus giving addicted users a greater ability to quit.” The product standard would also help keep nonsmokers interested in trying tobacco, mainly youth, from starting to smoke and become regulars.
“Lowering nicotine levels to minimally addictive or non-addictive levels would decrease the likelihood that future generations of young people become addicted to cigarettes and help more currently addicted smokers to quit,” FDA Commissioner Robert Califf, MD, said in a statement.
The FDA, in charge of regulating cigarettes, issues a proposed rule when changes are discussed. That would be followed by a period for public comments before a final rule could be issued.
The proposed rule was first reported by The Washington Post.
The FDA in 2018 published a study in the New England Journal of Medicine that estimated that a potential limit on nicotine in cigarettes could, by the year 2100, prevent more than 33 million people from becoming regular smokers, and prevent the deaths of more than 8 million people from tobacco-related illnesses.
The action to reduce nicotine levels would fit in with President Joe Biden’s goal of reducing cancer death rates by half over 25 years. Each year, according to the American Cancer Society, about 480,000 deaths (about 1 in 5) are related to smoking. Currently, about 34 million American adults still smoke cigarettes.
Matthew Myers, president of the Campaign for Tobacco-Free Kids, called the proposed rule a “truly game-changing proposal.”
“There is no other single action our country can take that would prevent more young people from becoming addicted to tobacco or have a greater impact on reducing deaths from cancer, cardiovascular disease and respiratory disease,” Mr. Myers said in a statement.
However, he said, “these gains will only be realized if the administration and the FDA demonstrate a full-throated commitment to finalizing and implementing this proposal.”
The FDA proposed the nicotine reduction strategy in talks with the White House and the Department of Health and Human Services early in 2021, according to the Post.
Earlier this year, the FDA issued a proposed rule to ban menthol flavoring in cigarettes. The agency is accepting public comments though July 5.
The action of reducing nicotine levels would likely take years to complete, Mitch Zeller, JD, recently retired director of the FDA Center for Tobacco Products, told the Post.
In 2018, the FDA issued a proposed ruling to set a standard for maximum nicotine levels in cigarettes.
Advocates say the action of slashing nicotine, the active – and addictive – ingredient in cigarettes, would save millions of lives for generations to come. Opponents liken it to the prohibition of alcohol in the 1920s and predict the action will fail.
Others say that if limits are put on nicotine levels, adults should have greater access to noncombustible alternatives.
A version of this article first appeared on WebMD.com.
Moderate activity versus sweat equity
It’s no secret that the fitness level of all age groups in our country is poor. A recent study in Pediatrics sharpens the focus on the question of how we might address the problem in the teenage population. Based in England, the investigators placed wrist accelerometers on their 13- and 14-year-old subjects who were then assessed using shuttle runs at progressively faster speeds.
The researchers found that the participants’ cardiorespiratory fitness improved as the subjects’ time doing vigorous activity increased up to 20 minutes and then plateaued. The study authors could not prove that the vigorous activity caused the increased in fitness. However, they were impressed by the plateau phenomenon and suggest that this might suggest a change in the recommendations by the World Health Organization and U.S. Department of Health & Human Services which currently call for 60 minutes of moderate to vigorous physical activity per day for adolescents
At first blush a shift down to 20 minutes of vigorous activity would appear to be workable and achievable. This would be particularly true for public school systems that are already struggling to get any kind of activity shoehorned into their schedules that are already crammed in an attempt to address mandated academic achievement goals. Freeing up an additional 40 minutes of the school day and yielding improved cardiorespiratory fitness sounds like a win-win.
But, let’s take a deep breath and for a few moments return to the world of reality. First, how many school systems are providing that 60 minutes of moderate activity (let’s forget the vigorous piece for the moment) included in the current WHO/HHS recommendations? Next, let’s take a look at what “vigorous” activity means. There are variety of definitions but in general they include sweating, flushing, and dyspnea to the point of having difficulty speaking.
Let’s just focus on the “sweating” part. To me that sounds like an activity that would require some wardrobe alteration at a minimum and very likely a locker room and a shower. Those can be fightin’ words for many teenagers. Even if a school can provide adequate locker room and shower infrastructure change-ups and showers are time-gobbling activities. And, more realistically, what are the chances of getting body image–challenged adolescents to willingly take advantage of them? You don’t have to talk to very many adults before you will hear stories of discomfort and embarrassment resulting from forced locker room and shower experiences. When I was a teenager the only way you could flunk physical education was to refuse to go in the locker room and “change up.” I think or at least hope that physical educators are more sensitive to the fragility of their adolescents students. But, the bottom line is that creating a curriculum that will improve cardiorespiratory fitness is fraught with challenges most school systems can’t address. It’s sad but true.
So, where does that leave us? This new study from England may be helpful for families who are caught in a time crunch and looking improve their fitness or for the physical educator who would like to help his/her motivated students get on a healthier track. But, this study should not prompt us to throw up our hands and toss out the current recommendations of an hour of moderate activity. As unrealistic as it may be for most school systems it allows for the injection of physical activity into academic settings where creative educators can offer things like walking lectures and field trips. It all boils down to the fact that some activity is better than none at all with or without the sweat equity.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
It’s no secret that the fitness level of all age groups in our country is poor. A recent study in Pediatrics sharpens the focus on the question of how we might address the problem in the teenage population. Based in England, the investigators placed wrist accelerometers on their 13- and 14-year-old subjects who were then assessed using shuttle runs at progressively faster speeds.
The researchers found that the participants’ cardiorespiratory fitness improved as the subjects’ time doing vigorous activity increased up to 20 minutes and then plateaued. The study authors could not prove that the vigorous activity caused the increased in fitness. However, they were impressed by the plateau phenomenon and suggest that this might suggest a change in the recommendations by the World Health Organization and U.S. Department of Health & Human Services which currently call for 60 minutes of moderate to vigorous physical activity per day for adolescents
At first blush a shift down to 20 minutes of vigorous activity would appear to be workable and achievable. This would be particularly true for public school systems that are already struggling to get any kind of activity shoehorned into their schedules that are already crammed in an attempt to address mandated academic achievement goals. Freeing up an additional 40 minutes of the school day and yielding improved cardiorespiratory fitness sounds like a win-win.
But, let’s take a deep breath and for a few moments return to the world of reality. First, how many school systems are providing that 60 minutes of moderate activity (let’s forget the vigorous piece for the moment) included in the current WHO/HHS recommendations? Next, let’s take a look at what “vigorous” activity means. There are variety of definitions but in general they include sweating, flushing, and dyspnea to the point of having difficulty speaking.
Let’s just focus on the “sweating” part. To me that sounds like an activity that would require some wardrobe alteration at a minimum and very likely a locker room and a shower. Those can be fightin’ words for many teenagers. Even if a school can provide adequate locker room and shower infrastructure change-ups and showers are time-gobbling activities. And, more realistically, what are the chances of getting body image–challenged adolescents to willingly take advantage of them? You don’t have to talk to very many adults before you will hear stories of discomfort and embarrassment resulting from forced locker room and shower experiences. When I was a teenager the only way you could flunk physical education was to refuse to go in the locker room and “change up.” I think or at least hope that physical educators are more sensitive to the fragility of their adolescents students. But, the bottom line is that creating a curriculum that will improve cardiorespiratory fitness is fraught with challenges most school systems can’t address. It’s sad but true.
So, where does that leave us? This new study from England may be helpful for families who are caught in a time crunch and looking improve their fitness or for the physical educator who would like to help his/her motivated students get on a healthier track. But, this study should not prompt us to throw up our hands and toss out the current recommendations of an hour of moderate activity. As unrealistic as it may be for most school systems it allows for the injection of physical activity into academic settings where creative educators can offer things like walking lectures and field trips. It all boils down to the fact that some activity is better than none at all with or without the sweat equity.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
It’s no secret that the fitness level of all age groups in our country is poor. A recent study in Pediatrics sharpens the focus on the question of how we might address the problem in the teenage population. Based in England, the investigators placed wrist accelerometers on their 13- and 14-year-old subjects who were then assessed using shuttle runs at progressively faster speeds.
The researchers found that the participants’ cardiorespiratory fitness improved as the subjects’ time doing vigorous activity increased up to 20 minutes and then plateaued. The study authors could not prove that the vigorous activity caused the increased in fitness. However, they were impressed by the plateau phenomenon and suggest that this might suggest a change in the recommendations by the World Health Organization and U.S. Department of Health & Human Services which currently call for 60 minutes of moderate to vigorous physical activity per day for adolescents
At first blush a shift down to 20 minutes of vigorous activity would appear to be workable and achievable. This would be particularly true for public school systems that are already struggling to get any kind of activity shoehorned into their schedules that are already crammed in an attempt to address mandated academic achievement goals. Freeing up an additional 40 minutes of the school day and yielding improved cardiorespiratory fitness sounds like a win-win.
But, let’s take a deep breath and for a few moments return to the world of reality. First, how many school systems are providing that 60 minutes of moderate activity (let’s forget the vigorous piece for the moment) included in the current WHO/HHS recommendations? Next, let’s take a look at what “vigorous” activity means. There are variety of definitions but in general they include sweating, flushing, and dyspnea to the point of having difficulty speaking.
Let’s just focus on the “sweating” part. To me that sounds like an activity that would require some wardrobe alteration at a minimum and very likely a locker room and a shower. Those can be fightin’ words for many teenagers. Even if a school can provide adequate locker room and shower infrastructure change-ups and showers are time-gobbling activities. And, more realistically, what are the chances of getting body image–challenged adolescents to willingly take advantage of them? You don’t have to talk to very many adults before you will hear stories of discomfort and embarrassment resulting from forced locker room and shower experiences. When I was a teenager the only way you could flunk physical education was to refuse to go in the locker room and “change up.” I think or at least hope that physical educators are more sensitive to the fragility of their adolescents students. But, the bottom line is that creating a curriculum that will improve cardiorespiratory fitness is fraught with challenges most school systems can’t address. It’s sad but true.
So, where does that leave us? This new study from England may be helpful for families who are caught in a time crunch and looking improve their fitness or for the physical educator who would like to help his/her motivated students get on a healthier track. But, this study should not prompt us to throw up our hands and toss out the current recommendations of an hour of moderate activity. As unrealistic as it may be for most school systems it allows for the injection of physical activity into academic settings where creative educators can offer things like walking lectures and field trips. It all boils down to the fact that some activity is better than none at all with or without the sweat equity.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].










