User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Abbott to start making Similac baby formula again
Abbott Nutrition is resuming production of Similac, its leading baby formula, at a Michigan plant that was shut down earlier in 2022 because of contamination concerns.
The company closed the plant in February, which triggered a national shortage of baby formula amid pandemic-related supply chain issues that created a lack of formula ingredients.
“We know that the nationwide infant formula shortage has been difficult for the families we serve, and while restarting Similac production in Michigan is an important milestone, we won’t rest until this product is back on shelves,” Robert Ford, chairman and CEO of Abbott, said in a statement on Aug. 26.
“Making infant formula is a responsibility we take very seriously, and parents can feel confident in the quality and safety of Similac and other Abbott formulas,” he said. “We are committed to re-earning the trust parents and health care providers have placed in us for decades.”
Abbott estimated that it will take about 6 weeks for Similac products to ship to stores. Production has restarted, which will be followed by “enhanced” testing before and after the formula is made.
In February, Abbott voluntarily recalled batches of three formulas after the Food and Drug Administration received consumer complaints about infants becoming sick. Four babies who consumed formulas from the Michigan plant got bacterial infections, and at least two babies died.
The illnesses were linked to Cronobacter sakazakii – bacteria that can lead to life-threatening infections and inflammation of the brain and spine.
After investigations at the plant, Abbott said there is no conclusive evidence to link the formula to the illnesses. No samples of the recalled product tested positive for the bacteria, and in all four cases, unopened containers of formula in the infants’ homes tested negative for the bacteria.
At the same time, FDA officials said in May that the Michigan plant had a leaking roof, water pooling on the floor, and cracks in production equipment that could allow bacteria to grow, according to The New York Times.
Abbott agreed with the federal government to create new safeguards, such as hiring a qualified expert to oversee improvements at the plant and notify the FDA if any issues were identified, the newspaper reported.
On July 1, the company restarted production of EleCare, a specialty formula, and later resumed production of some metabolic formulas. These products will begin to ship in coming weeks, the company said.
Since July, C. sakazakii has been found in a couple of batches of formula.
“In those cases, we found the issue, addressed it and no affected product has been or will be distributed,” Abbott said in the statement. “This confirms our quality systems work.”
In August, Abbott will supply the United States with more than 8 million pounds of infant formula, which is higher than the levels in August 2021, the company said. To ensure that people in the federal Special Supplemental Nutrition Program for Women, Infants and Children have access to formula, the company is extending rebates until the end of October.
“Restarting a large manufacturing facility after a several-month shutdown is a complex process, and it takes time to ensure that equipment, processes and production are functioning smoothly and sustainably,” the company said in the statement. “There have been – and likely will be – stops and starts from time to time. We’ve experienced events like severe weather, we’ve had to make mechanical adjustments, and we’ve had to discard some early production batches that didn’t meet our standards.”
A version of this article first appeared on WebMD.com.
Abbott Nutrition is resuming production of Similac, its leading baby formula, at a Michigan plant that was shut down earlier in 2022 because of contamination concerns.
The company closed the plant in February, which triggered a national shortage of baby formula amid pandemic-related supply chain issues that created a lack of formula ingredients.
“We know that the nationwide infant formula shortage has been difficult for the families we serve, and while restarting Similac production in Michigan is an important milestone, we won’t rest until this product is back on shelves,” Robert Ford, chairman and CEO of Abbott, said in a statement on Aug. 26.
“Making infant formula is a responsibility we take very seriously, and parents can feel confident in the quality and safety of Similac and other Abbott formulas,” he said. “We are committed to re-earning the trust parents and health care providers have placed in us for decades.”
Abbott estimated that it will take about 6 weeks for Similac products to ship to stores. Production has restarted, which will be followed by “enhanced” testing before and after the formula is made.
In February, Abbott voluntarily recalled batches of three formulas after the Food and Drug Administration received consumer complaints about infants becoming sick. Four babies who consumed formulas from the Michigan plant got bacterial infections, and at least two babies died.
The illnesses were linked to Cronobacter sakazakii – bacteria that can lead to life-threatening infections and inflammation of the brain and spine.
After investigations at the plant, Abbott said there is no conclusive evidence to link the formula to the illnesses. No samples of the recalled product tested positive for the bacteria, and in all four cases, unopened containers of formula in the infants’ homes tested negative for the bacteria.
At the same time, FDA officials said in May that the Michigan plant had a leaking roof, water pooling on the floor, and cracks in production equipment that could allow bacteria to grow, according to The New York Times.
Abbott agreed with the federal government to create new safeguards, such as hiring a qualified expert to oversee improvements at the plant and notify the FDA if any issues were identified, the newspaper reported.
On July 1, the company restarted production of EleCare, a specialty formula, and later resumed production of some metabolic formulas. These products will begin to ship in coming weeks, the company said.
Since July, C. sakazakii has been found in a couple of batches of formula.
“In those cases, we found the issue, addressed it and no affected product has been or will be distributed,” Abbott said in the statement. “This confirms our quality systems work.”
In August, Abbott will supply the United States with more than 8 million pounds of infant formula, which is higher than the levels in August 2021, the company said. To ensure that people in the federal Special Supplemental Nutrition Program for Women, Infants and Children have access to formula, the company is extending rebates until the end of October.
“Restarting a large manufacturing facility after a several-month shutdown is a complex process, and it takes time to ensure that equipment, processes and production are functioning smoothly and sustainably,” the company said in the statement. “There have been – and likely will be – stops and starts from time to time. We’ve experienced events like severe weather, we’ve had to make mechanical adjustments, and we’ve had to discard some early production batches that didn’t meet our standards.”
A version of this article first appeared on WebMD.com.
Abbott Nutrition is resuming production of Similac, its leading baby formula, at a Michigan plant that was shut down earlier in 2022 because of contamination concerns.
The company closed the plant in February, which triggered a national shortage of baby formula amid pandemic-related supply chain issues that created a lack of formula ingredients.
“We know that the nationwide infant formula shortage has been difficult for the families we serve, and while restarting Similac production in Michigan is an important milestone, we won’t rest until this product is back on shelves,” Robert Ford, chairman and CEO of Abbott, said in a statement on Aug. 26.
“Making infant formula is a responsibility we take very seriously, and parents can feel confident in the quality and safety of Similac and other Abbott formulas,” he said. “We are committed to re-earning the trust parents and health care providers have placed in us for decades.”
Abbott estimated that it will take about 6 weeks for Similac products to ship to stores. Production has restarted, which will be followed by “enhanced” testing before and after the formula is made.
In February, Abbott voluntarily recalled batches of three formulas after the Food and Drug Administration received consumer complaints about infants becoming sick. Four babies who consumed formulas from the Michigan plant got bacterial infections, and at least two babies died.
The illnesses were linked to Cronobacter sakazakii – bacteria that can lead to life-threatening infections and inflammation of the brain and spine.
After investigations at the plant, Abbott said there is no conclusive evidence to link the formula to the illnesses. No samples of the recalled product tested positive for the bacteria, and in all four cases, unopened containers of formula in the infants’ homes tested negative for the bacteria.
At the same time, FDA officials said in May that the Michigan plant had a leaking roof, water pooling on the floor, and cracks in production equipment that could allow bacteria to grow, according to The New York Times.
Abbott agreed with the federal government to create new safeguards, such as hiring a qualified expert to oversee improvements at the plant and notify the FDA if any issues were identified, the newspaper reported.
On July 1, the company restarted production of EleCare, a specialty formula, and later resumed production of some metabolic formulas. These products will begin to ship in coming weeks, the company said.
Since July, C. sakazakii has been found in a couple of batches of formula.
“In those cases, we found the issue, addressed it and no affected product has been or will be distributed,” Abbott said in the statement. “This confirms our quality systems work.”
In August, Abbott will supply the United States with more than 8 million pounds of infant formula, which is higher than the levels in August 2021, the company said. To ensure that people in the federal Special Supplemental Nutrition Program for Women, Infants and Children have access to formula, the company is extending rebates until the end of October.
“Restarting a large manufacturing facility after a several-month shutdown is a complex process, and it takes time to ensure that equipment, processes and production are functioning smoothly and sustainably,” the company said in the statement. “There have been – and likely will be – stops and starts from time to time. We’ve experienced events like severe weather, we’ve had to make mechanical adjustments, and we’ve had to discard some early production batches that didn’t meet our standards.”
A version of this article first appeared on WebMD.com.
FDA authorizes updated COVID boosters to target newest variants
The agency cited data to support the safety and efficacy of this next generation of mRNA vaccines targeted toward variants of concern.
The Pfizer EUA corresponds to the company’s combination booster shot that includes the original COVID-19 vaccine as well as a vaccine specifically designed to protect against the most recent Omicron variants, BA.4 and BA.5.
The Moderna combination vaccine will contain both the firm’s original COVID-19 vaccine and a vaccine to protect specifically against Omicron BA.4 and BA.5 subvariants.
As of Aug. 27, BA.4 and BA.4.6 account for about 11% of circulating variants and BA.5 accounts for almost all the remaining 89%, Centers for Disease Control and Prevention data show.
The next step will be review of the scientific data by the CDC’s Advisory Committee on Immunization Practices, which is set to meet Sept. 1 and 2. The final hurdle before distribution of the new vaccines will be sign-off on CDC recommendations for use by agency Director Rochelle Walensky, MD.
This is a developing story. A version of this article first appeared on WebMD.com.
The agency cited data to support the safety and efficacy of this next generation of mRNA vaccines targeted toward variants of concern.
The Pfizer EUA corresponds to the company’s combination booster shot that includes the original COVID-19 vaccine as well as a vaccine specifically designed to protect against the most recent Omicron variants, BA.4 and BA.5.
The Moderna combination vaccine will contain both the firm’s original COVID-19 vaccine and a vaccine to protect specifically against Omicron BA.4 and BA.5 subvariants.
As of Aug. 27, BA.4 and BA.4.6 account for about 11% of circulating variants and BA.5 accounts for almost all the remaining 89%, Centers for Disease Control and Prevention data show.
The next step will be review of the scientific data by the CDC’s Advisory Committee on Immunization Practices, which is set to meet Sept. 1 and 2. The final hurdle before distribution of the new vaccines will be sign-off on CDC recommendations for use by agency Director Rochelle Walensky, MD.
This is a developing story. A version of this article first appeared on WebMD.com.
The agency cited data to support the safety and efficacy of this next generation of mRNA vaccines targeted toward variants of concern.
The Pfizer EUA corresponds to the company’s combination booster shot that includes the original COVID-19 vaccine as well as a vaccine specifically designed to protect against the most recent Omicron variants, BA.4 and BA.5.
The Moderna combination vaccine will contain both the firm’s original COVID-19 vaccine and a vaccine to protect specifically against Omicron BA.4 and BA.5 subvariants.
As of Aug. 27, BA.4 and BA.4.6 account for about 11% of circulating variants and BA.5 accounts for almost all the remaining 89%, Centers for Disease Control and Prevention data show.
The next step will be review of the scientific data by the CDC’s Advisory Committee on Immunization Practices, which is set to meet Sept. 1 and 2. The final hurdle before distribution of the new vaccines will be sign-off on CDC recommendations for use by agency Director Rochelle Walensky, MD.
This is a developing story. A version of this article first appeared on WebMD.com.
Mother’s fat metabolism in early pregnancy linked to baby’s weight
A baby’s weight and neurodevelopment in the first 2 years of life could be influenced by maternal fat metabolism in the early stages of pregnancy, according to a study.
Patterns of fetal abdominal growth were associated with maternal lipid metabolites that tracked newborn growth, adiposity, and development into childhood and could be identified as early as the 5th month of pregnancy, according to researchers at the University of Oxford, working with colleagues at the University of California.
These fetal growth patterns were also associated with blood flow and nutrient transfer by the placenta, demonstrating a complex interaction between maternal and fetal nutrition early in pregnancy, with implications for postnatal weight and health in later life, they suggested.
Stephen Kennedy, MD, professor of reproductive medicine at the University of Oxford, who co-led the investigation, said it had “provided valuable new insights into the biological origins of childhood obesity, which is one of the most pressing public health issues facing governments around the world.”
International study
The prospective observational study, published in The Lancet Diabetes and Endocrinology, involved 3,598 pregnant women from six countries – Brazil, Kenya, Pakistan, South Africa, Thailand, and the United Kingdom – aged 18 and older and with a BMI of less than 35 kg/m2. The women were monitored using regular fetal ultrasound scans and metabolomic analysis of early pregnancy maternal blood and umbilical cord venous blood at the time of birth.
Their infants, who were singletons, and conceived naturally, were then followed for 2 years to assess their growth and development.
Fetal abdominal circumference growth was found to accelerate or decelerate within “a crucial 20-25 week gestational age window” that followed 4 trajectories of faltering growth, early accelerating growth, late accelerating growth, or median growth. These traits were matched by fetus-placenta blood flow patterns throughout pregnancy and different growth, adiposity, vision, and neurodevelopment outcomes in early childhood, researchers said.
Overall, 709 maternal metabolites had a positive effect for the faltering growth phenotype, and 54 for the early accelerating growth phenotype, whilst 31 had a negative effect for the faltering growth phenotype and 76 for the early accelerating growth phenotype.
The maternal metabolite signatures included 5-hydroxy-eicosatetraenoic acid and 11 phosphatidylcholines linked to oxylipin or saturated fatty acid sidechains. The fungicide, chlorothalonil, was “highly abundant” in the early accelerating growth phenotype group.
‘A unique insight’
Aris Papageorghiou, professor of Fetal Medicine at the University of Oxford, who co-led the research, said: “This study provides evidence of distinct patterns of fetal abdominal growth and placental transfer and how they relate to longer term health. The finding of an association with maternal lipid metabolism early in pregnancy also provides unique insights into how the mother’s health and diet influence her child’s adiposity.”
First author José Villar, MD, professor of perinatal medicine at Oxford, said: “The study complements our previous work that identified fetal head growth trajectories associated with different developmental, behavioral, visual, and growth outcomes at 2 years of age.” Taken together, “the growth of babies’ bodies and brain[s] track separately and early – while still within the womb,” he said.
According to Dr. Kennedy, the latest results “could contribute to earlier identification of infants at risk of obesity” and urged policymakers to “take notice of these findings in their efforts to prevent the oncoming epidemic of obesity, with all its likely adverse social and economic consequences.”
Funding for the study was provided by the Bill and Melinda Gates Foundation.
A version of this article first appeared on Medscape UK.
A baby’s weight and neurodevelopment in the first 2 years of life could be influenced by maternal fat metabolism in the early stages of pregnancy, according to a study.
Patterns of fetal abdominal growth were associated with maternal lipid metabolites that tracked newborn growth, adiposity, and development into childhood and could be identified as early as the 5th month of pregnancy, according to researchers at the University of Oxford, working with colleagues at the University of California.
These fetal growth patterns were also associated with blood flow and nutrient transfer by the placenta, demonstrating a complex interaction between maternal and fetal nutrition early in pregnancy, with implications for postnatal weight and health in later life, they suggested.
Stephen Kennedy, MD, professor of reproductive medicine at the University of Oxford, who co-led the investigation, said it had “provided valuable new insights into the biological origins of childhood obesity, which is one of the most pressing public health issues facing governments around the world.”
International study
The prospective observational study, published in The Lancet Diabetes and Endocrinology, involved 3,598 pregnant women from six countries – Brazil, Kenya, Pakistan, South Africa, Thailand, and the United Kingdom – aged 18 and older and with a BMI of less than 35 kg/m2. The women were monitored using regular fetal ultrasound scans and metabolomic analysis of early pregnancy maternal blood and umbilical cord venous blood at the time of birth.
Their infants, who were singletons, and conceived naturally, were then followed for 2 years to assess their growth and development.
Fetal abdominal circumference growth was found to accelerate or decelerate within “a crucial 20-25 week gestational age window” that followed 4 trajectories of faltering growth, early accelerating growth, late accelerating growth, or median growth. These traits were matched by fetus-placenta blood flow patterns throughout pregnancy and different growth, adiposity, vision, and neurodevelopment outcomes in early childhood, researchers said.
Overall, 709 maternal metabolites had a positive effect for the faltering growth phenotype, and 54 for the early accelerating growth phenotype, whilst 31 had a negative effect for the faltering growth phenotype and 76 for the early accelerating growth phenotype.
The maternal metabolite signatures included 5-hydroxy-eicosatetraenoic acid and 11 phosphatidylcholines linked to oxylipin or saturated fatty acid sidechains. The fungicide, chlorothalonil, was “highly abundant” in the early accelerating growth phenotype group.
‘A unique insight’
Aris Papageorghiou, professor of Fetal Medicine at the University of Oxford, who co-led the research, said: “This study provides evidence of distinct patterns of fetal abdominal growth and placental transfer and how they relate to longer term health. The finding of an association with maternal lipid metabolism early in pregnancy also provides unique insights into how the mother’s health and diet influence her child’s adiposity.”
First author José Villar, MD, professor of perinatal medicine at Oxford, said: “The study complements our previous work that identified fetal head growth trajectories associated with different developmental, behavioral, visual, and growth outcomes at 2 years of age.” Taken together, “the growth of babies’ bodies and brain[s] track separately and early – while still within the womb,” he said.
According to Dr. Kennedy, the latest results “could contribute to earlier identification of infants at risk of obesity” and urged policymakers to “take notice of these findings in their efforts to prevent the oncoming epidemic of obesity, with all its likely adverse social and economic consequences.”
Funding for the study was provided by the Bill and Melinda Gates Foundation.
A version of this article first appeared on Medscape UK.
A baby’s weight and neurodevelopment in the first 2 years of life could be influenced by maternal fat metabolism in the early stages of pregnancy, according to a study.
Patterns of fetal abdominal growth were associated with maternal lipid metabolites that tracked newborn growth, adiposity, and development into childhood and could be identified as early as the 5th month of pregnancy, according to researchers at the University of Oxford, working with colleagues at the University of California.
These fetal growth patterns were also associated with blood flow and nutrient transfer by the placenta, demonstrating a complex interaction between maternal and fetal nutrition early in pregnancy, with implications for postnatal weight and health in later life, they suggested.
Stephen Kennedy, MD, professor of reproductive medicine at the University of Oxford, who co-led the investigation, said it had “provided valuable new insights into the biological origins of childhood obesity, which is one of the most pressing public health issues facing governments around the world.”
International study
The prospective observational study, published in The Lancet Diabetes and Endocrinology, involved 3,598 pregnant women from six countries – Brazil, Kenya, Pakistan, South Africa, Thailand, and the United Kingdom – aged 18 and older and with a BMI of less than 35 kg/m2. The women were monitored using regular fetal ultrasound scans and metabolomic analysis of early pregnancy maternal blood and umbilical cord venous blood at the time of birth.
Their infants, who were singletons, and conceived naturally, were then followed for 2 years to assess their growth and development.
Fetal abdominal circumference growth was found to accelerate or decelerate within “a crucial 20-25 week gestational age window” that followed 4 trajectories of faltering growth, early accelerating growth, late accelerating growth, or median growth. These traits were matched by fetus-placenta blood flow patterns throughout pregnancy and different growth, adiposity, vision, and neurodevelopment outcomes in early childhood, researchers said.
Overall, 709 maternal metabolites had a positive effect for the faltering growth phenotype, and 54 for the early accelerating growth phenotype, whilst 31 had a negative effect for the faltering growth phenotype and 76 for the early accelerating growth phenotype.
The maternal metabolite signatures included 5-hydroxy-eicosatetraenoic acid and 11 phosphatidylcholines linked to oxylipin or saturated fatty acid sidechains. The fungicide, chlorothalonil, was “highly abundant” in the early accelerating growth phenotype group.
‘A unique insight’
Aris Papageorghiou, professor of Fetal Medicine at the University of Oxford, who co-led the research, said: “This study provides evidence of distinct patterns of fetal abdominal growth and placental transfer and how they relate to longer term health. The finding of an association with maternal lipid metabolism early in pregnancy also provides unique insights into how the mother’s health and diet influence her child’s adiposity.”
First author José Villar, MD, professor of perinatal medicine at Oxford, said: “The study complements our previous work that identified fetal head growth trajectories associated with different developmental, behavioral, visual, and growth outcomes at 2 years of age.” Taken together, “the growth of babies’ bodies and brain[s] track separately and early – while still within the womb,” he said.
According to Dr. Kennedy, the latest results “could contribute to earlier identification of infants at risk of obesity” and urged policymakers to “take notice of these findings in their efforts to prevent the oncoming epidemic of obesity, with all its likely adverse social and economic consequences.”
Funding for the study was provided by the Bill and Melinda Gates Foundation.
A version of this article first appeared on Medscape UK.
How much do we really know about gender dysphoria?
At the risk of losing a digit or two I am going to dip my toes into the murky waters of gender-affirming care, sometimes referred to as trans care. Recently, Moira Szilagyi, MD, PhD, president of the American Academy of Pediatrics, released two statements, one in the Aug. 22, 2022, Wall Street Journal, the other summarized in the Aug. 25, 2022, AAP Daily Briefing, in which she attempts to clarify the academy’s position on gender-affirming care. They were well-worded and heroic attempts to clear the air. I fear these explanations will do little to encourage informed and courteous discussions between those entrenched on either side of a disagreement that is unfortunately being played out on media outlets and state legislatures instead of the offices of primary care physicians and specialists where it belongs.
The current mess is an example of what can happen when there is a paucity of reliable data, a superabundance of emotion, and a system that feeds on instant news and sound bites with little understanding of how science should work.
Some of the turmoil is a response to the notion that in certain situations gender dysphoria may be a condition that can be learned or mimicked from exposure to other gender-dysphoric individuals. Two papers anchor either side of the debate. The first paper was published in 2018 by a then–Brown University health expert who hypothesized the existence of a condition which she labeled “rapid-onset gender dysphoria [ROGD]”. One can imagine that “social contagion” might be considered as one of the potential contributors to this hypothesized condition. Unfortunately, the publication of the paper ignited a firestorm of criticism from a segment of the population that advocates for the transgender community, prompting the university and the online publisher to backpedal and reevaluate the quality of the research on which the paper was based.
One of the concerns voiced at the time of publication was that the research could be used to support the transphobic agenda by some state legislatures hoping to ban gender-affirming care. How large a role the paper played in the current spate of legislation in is unclear. I suspect it has been small. But, one can’t deny the potential exists.
Leaping forward to 2022, the second paper was published in the August issue of Pediatrics, in which the authors attempted to test the ROGD hypothesis and question the inference of social contagion.
The investigators found that in 2017 and 2019 the birth ratios of transgender-diverse (TGD) individuals did not favor assigned female-sex-at-birth (AFAB) individuals. They also discovered that in their sample overall there was a decrease in the percentage of adolescents who self-identified as TGD. Not surprisingly, “bullying victimization and suicidality were higher among TGD youth when compared with their cisgender peers.” The authors concluded that their findings were “incongruent with an ROGD hypothesis that posits social contagion” nor should it be used to restrict access to gender-affirming care.
There you have it. Are we any closer to understanding gender dysphoria and its origins? I don’t think so. The media is somewhat less confused. The NBC News online presence headline on Aug. 3, 2022, reads “‘Social contagion’ isn’t causing more youths to be transgender, study finds.”
My sense is that the general population perceives an increase in the prevalence of gender dysphoria. It is very likely that this perception is primarily a reflection of a more compassionate and educated attitude in a significant portion of the population making it less challenging for gender-dysphoric youth to surface. However, it should not surprise us that some parents and observers are concerned that a percentage of this increased prevalence is the result of social contagion. Nor should it surprise us that some advocates for the trans population feel threatened by this hypothesis.
Neither of these studies really answers the question of whether some cases of gender dysphoria are the result of social contagion. Both were small samples using methodology that has been called into question. The bottom line is that we need more studies and must remain open to considering their results. That’s how science should work.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
At the risk of losing a digit or two I am going to dip my toes into the murky waters of gender-affirming care, sometimes referred to as trans care. Recently, Moira Szilagyi, MD, PhD, president of the American Academy of Pediatrics, released two statements, one in the Aug. 22, 2022, Wall Street Journal, the other summarized in the Aug. 25, 2022, AAP Daily Briefing, in which she attempts to clarify the academy’s position on gender-affirming care. They were well-worded and heroic attempts to clear the air. I fear these explanations will do little to encourage informed and courteous discussions between those entrenched on either side of a disagreement that is unfortunately being played out on media outlets and state legislatures instead of the offices of primary care physicians and specialists where it belongs.
The current mess is an example of what can happen when there is a paucity of reliable data, a superabundance of emotion, and a system that feeds on instant news and sound bites with little understanding of how science should work.
Some of the turmoil is a response to the notion that in certain situations gender dysphoria may be a condition that can be learned or mimicked from exposure to other gender-dysphoric individuals. Two papers anchor either side of the debate. The first paper was published in 2018 by a then–Brown University health expert who hypothesized the existence of a condition which she labeled “rapid-onset gender dysphoria [ROGD]”. One can imagine that “social contagion” might be considered as one of the potential contributors to this hypothesized condition. Unfortunately, the publication of the paper ignited a firestorm of criticism from a segment of the population that advocates for the transgender community, prompting the university and the online publisher to backpedal and reevaluate the quality of the research on which the paper was based.
One of the concerns voiced at the time of publication was that the research could be used to support the transphobic agenda by some state legislatures hoping to ban gender-affirming care. How large a role the paper played in the current spate of legislation in is unclear. I suspect it has been small. But, one can’t deny the potential exists.
Leaping forward to 2022, the second paper was published in the August issue of Pediatrics, in which the authors attempted to test the ROGD hypothesis and question the inference of social contagion.
The investigators found that in 2017 and 2019 the birth ratios of transgender-diverse (TGD) individuals did not favor assigned female-sex-at-birth (AFAB) individuals. They also discovered that in their sample overall there was a decrease in the percentage of adolescents who self-identified as TGD. Not surprisingly, “bullying victimization and suicidality were higher among TGD youth when compared with their cisgender peers.” The authors concluded that their findings were “incongruent with an ROGD hypothesis that posits social contagion” nor should it be used to restrict access to gender-affirming care.
There you have it. Are we any closer to understanding gender dysphoria and its origins? I don’t think so. The media is somewhat less confused. The NBC News online presence headline on Aug. 3, 2022, reads “‘Social contagion’ isn’t causing more youths to be transgender, study finds.”
My sense is that the general population perceives an increase in the prevalence of gender dysphoria. It is very likely that this perception is primarily a reflection of a more compassionate and educated attitude in a significant portion of the population making it less challenging for gender-dysphoric youth to surface. However, it should not surprise us that some parents and observers are concerned that a percentage of this increased prevalence is the result of social contagion. Nor should it surprise us that some advocates for the trans population feel threatened by this hypothesis.
Neither of these studies really answers the question of whether some cases of gender dysphoria are the result of social contagion. Both were small samples using methodology that has been called into question. The bottom line is that we need more studies and must remain open to considering their results. That’s how science should work.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
At the risk of losing a digit or two I am going to dip my toes into the murky waters of gender-affirming care, sometimes referred to as trans care. Recently, Moira Szilagyi, MD, PhD, president of the American Academy of Pediatrics, released two statements, one in the Aug. 22, 2022, Wall Street Journal, the other summarized in the Aug. 25, 2022, AAP Daily Briefing, in which she attempts to clarify the academy’s position on gender-affirming care. They were well-worded and heroic attempts to clear the air. I fear these explanations will do little to encourage informed and courteous discussions between those entrenched on either side of a disagreement that is unfortunately being played out on media outlets and state legislatures instead of the offices of primary care physicians and specialists where it belongs.
The current mess is an example of what can happen when there is a paucity of reliable data, a superabundance of emotion, and a system that feeds on instant news and sound bites with little understanding of how science should work.
Some of the turmoil is a response to the notion that in certain situations gender dysphoria may be a condition that can be learned or mimicked from exposure to other gender-dysphoric individuals. Two papers anchor either side of the debate. The first paper was published in 2018 by a then–Brown University health expert who hypothesized the existence of a condition which she labeled “rapid-onset gender dysphoria [ROGD]”. One can imagine that “social contagion” might be considered as one of the potential contributors to this hypothesized condition. Unfortunately, the publication of the paper ignited a firestorm of criticism from a segment of the population that advocates for the transgender community, prompting the university and the online publisher to backpedal and reevaluate the quality of the research on which the paper was based.
One of the concerns voiced at the time of publication was that the research could be used to support the transphobic agenda by some state legislatures hoping to ban gender-affirming care. How large a role the paper played in the current spate of legislation in is unclear. I suspect it has been small. But, one can’t deny the potential exists.
Leaping forward to 2022, the second paper was published in the August issue of Pediatrics, in which the authors attempted to test the ROGD hypothesis and question the inference of social contagion.
The investigators found that in 2017 and 2019 the birth ratios of transgender-diverse (TGD) individuals did not favor assigned female-sex-at-birth (AFAB) individuals. They also discovered that in their sample overall there was a decrease in the percentage of adolescents who self-identified as TGD. Not surprisingly, “bullying victimization and suicidality were higher among TGD youth when compared with their cisgender peers.” The authors concluded that their findings were “incongruent with an ROGD hypothesis that posits social contagion” nor should it be used to restrict access to gender-affirming care.
There you have it. Are we any closer to understanding gender dysphoria and its origins? I don’t think so. The media is somewhat less confused. The NBC News online presence headline on Aug. 3, 2022, reads “‘Social contagion’ isn’t causing more youths to be transgender, study finds.”
My sense is that the general population perceives an increase in the prevalence of gender dysphoria. It is very likely that this perception is primarily a reflection of a more compassionate and educated attitude in a significant portion of the population making it less challenging for gender-dysphoric youth to surface. However, it should not surprise us that some parents and observers are concerned that a percentage of this increased prevalence is the result of social contagion. Nor should it surprise us that some advocates for the trans population feel threatened by this hypothesis.
Neither of these studies really answers the question of whether some cases of gender dysphoria are the result of social contagion. Both were small samples using methodology that has been called into question. The bottom line is that we need more studies and must remain open to considering their results. That’s how science should work.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
Children and COVID: New cases increase; hospital admissions could follow
New cases of COVID-19 in children were up again after 2 weeks of declines, and preliminary data suggest that hospitalizations may be on the rise as well.
, based on data collected by the American Academy of Pediatrics and the Children’s Hospital Association from state and territorial health departments.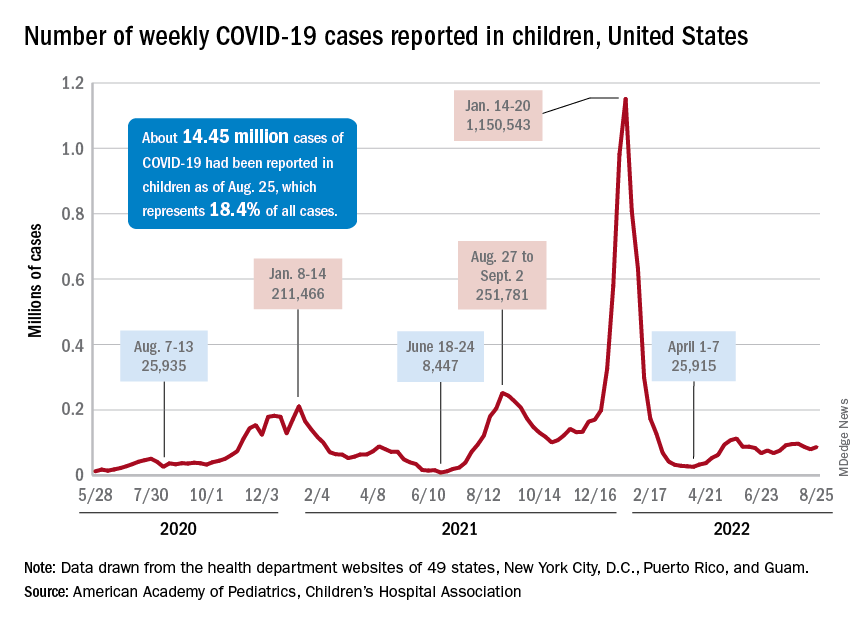
A similar increase seems to be reflected by hospital-level data. The latest 7-day (Aug. 21-27) average is 305 new admissions with diagnosed COVID per day among children aged 0-17 years, compared with 290 per day for the week of Aug. 14-20, the Centers for Disease Control and Prevention reported, while also noting the potential for reporting delays in the most recent 7-day period.
Daily hospital admissions for COVID had been headed downward through the first half of August, falling from 0.46 per 100,000 population at the end of July to 0.40 on Aug. 19, the CDC said on its COVID Data Tracker. Since then, however, admissions have gone the other way, with the preliminary nature of the latest data suggesting that the numbers will be even higher as more hospitals report over the next few days.
Vaccine initiations continue to fall
Initiations among school-age children have fallen for 3 consecutive weeks since Aug. 3, when numbers receiving their first vaccinations reached late-summer highs for those aged 5-11 and 12-17 years. Children under age 5, included in the CDC data for the first time on Aug. 11 as separate groups – under 2 years and 2-4 years – have had vaccine initiations drop by 8.0% and 19.8% over the 2 following weeks, the CDC said.
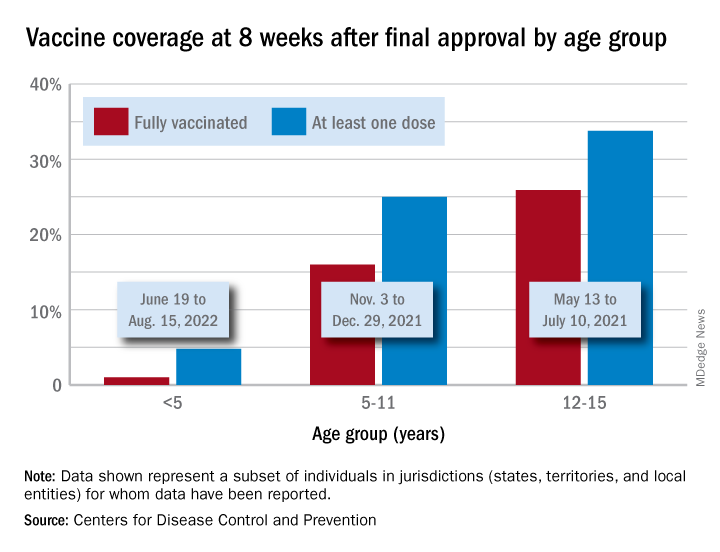
Through their first 8 weeks of vaccine eligibility (June 19 to Aug. 15), 4.8% of children under 5 years of age had received a first vaccination and 1.0% were fully vaccinated. For the two other age groups (5-11 and 12-15) who became eligible after the very first emergency authorization back in 2020, the respective proportions were 25.0% and 16.0% (5-11) and 33.8% and 26.1% (12-15) through the first 8 weeks, according to CDC data.
New cases of COVID-19 in children were up again after 2 weeks of declines, and preliminary data suggest that hospitalizations may be on the rise as well.
, based on data collected by the American Academy of Pediatrics and the Children’s Hospital Association from state and territorial health departments.
A similar increase seems to be reflected by hospital-level data. The latest 7-day (Aug. 21-27) average is 305 new admissions with diagnosed COVID per day among children aged 0-17 years, compared with 290 per day for the week of Aug. 14-20, the Centers for Disease Control and Prevention reported, while also noting the potential for reporting delays in the most recent 7-day period.
Daily hospital admissions for COVID had been headed downward through the first half of August, falling from 0.46 per 100,000 population at the end of July to 0.40 on Aug. 19, the CDC said on its COVID Data Tracker. Since then, however, admissions have gone the other way, with the preliminary nature of the latest data suggesting that the numbers will be even higher as more hospitals report over the next few days.
Vaccine initiations continue to fall
Initiations among school-age children have fallen for 3 consecutive weeks since Aug. 3, when numbers receiving their first vaccinations reached late-summer highs for those aged 5-11 and 12-17 years. Children under age 5, included in the CDC data for the first time on Aug. 11 as separate groups – under 2 years and 2-4 years – have had vaccine initiations drop by 8.0% and 19.8% over the 2 following weeks, the CDC said.

Through their first 8 weeks of vaccine eligibility (June 19 to Aug. 15), 4.8% of children under 5 years of age had received a first vaccination and 1.0% were fully vaccinated. For the two other age groups (5-11 and 12-15) who became eligible after the very first emergency authorization back in 2020, the respective proportions were 25.0% and 16.0% (5-11) and 33.8% and 26.1% (12-15) through the first 8 weeks, according to CDC data.
New cases of COVID-19 in children were up again after 2 weeks of declines, and preliminary data suggest that hospitalizations may be on the rise as well.
, based on data collected by the American Academy of Pediatrics and the Children’s Hospital Association from state and territorial health departments.
A similar increase seems to be reflected by hospital-level data. The latest 7-day (Aug. 21-27) average is 305 new admissions with diagnosed COVID per day among children aged 0-17 years, compared with 290 per day for the week of Aug. 14-20, the Centers for Disease Control and Prevention reported, while also noting the potential for reporting delays in the most recent 7-day period.
Daily hospital admissions for COVID had been headed downward through the first half of August, falling from 0.46 per 100,000 population at the end of July to 0.40 on Aug. 19, the CDC said on its COVID Data Tracker. Since then, however, admissions have gone the other way, with the preliminary nature of the latest data suggesting that the numbers will be even higher as more hospitals report over the next few days.
Vaccine initiations continue to fall
Initiations among school-age children have fallen for 3 consecutive weeks since Aug. 3, when numbers receiving their first vaccinations reached late-summer highs for those aged 5-11 and 12-17 years. Children under age 5, included in the CDC data for the first time on Aug. 11 as separate groups – under 2 years and 2-4 years – have had vaccine initiations drop by 8.0% and 19.8% over the 2 following weeks, the CDC said.

Through their first 8 weeks of vaccine eligibility (June 19 to Aug. 15), 4.8% of children under 5 years of age had received a first vaccination and 1.0% were fully vaccinated. For the two other age groups (5-11 and 12-15) who became eligible after the very first emergency authorization back in 2020, the respective proportions were 25.0% and 16.0% (5-11) and 33.8% and 26.1% (12-15) through the first 8 weeks, according to CDC data.
How do you live with COVID? One doctor’s personal experience
Early in 2020, Anne Peters, MD, caught COVID-19. The author of Medscape’s “Peters on Diabetes” column was sick in March 2020 before state-mandated lockdowns, and well before there were any vaccines.
She remembers sitting in a small exam room with two patients who had flown to her Los Angeles office from New York. The elderly couple had hearing difficulties, so Dr. Peters sat close to them, putting on a continuous glucose monitor. “At that time, we didn’t think of COVID-19 as being in L.A.,” Dr. Peters recalled, “so I think we were not terribly consistent at mask-wearing due to the need to educate.”
“Several days later, I got COVID, but I didn’t know I had COVID per se. I felt crappy, had a terrible sore throat, lost my sense of taste and smell [which was not yet described as a COVID symptom], was completely exhausted, but had no fever or cough, which were the only criteria for getting COVID tested at the time. I didn’t know I had been exposed until 2 weeks later, when the patient’s assistant returned the sensor warning us to ‘be careful’ with it because the patient and his wife were recovering from COVID.”
That early battle with COVID-19 was just the beginning of what would become a 2-year struggle, including familial loss amid her own health problems and concerns about the under-resourced patients she cares for. Here, she shares her journey through the pandemic with this news organization.
Question: Thanks for talking to us. Let’s discuss your journey over these past 2.5 years.
Answer: Everybody has their own COVID story because we all went through this together. Some of us have worse COVID stories, and some of us have better ones, but all have been impacted.
I’m not a sick person. I’m a very healthy person but COVID made me so unwell for 2 years. The brain fog and fatigue were nothing compared to the autonomic neuropathy that affected my heart. It was really limiting for me. And I still don’t know the long-term implications, looking 20-30 years from now.
Q: When you initially had COVID, what were your symptoms? What was the impact?
A: I had all the symptoms of COVID, except for a cough and fever. I lost my sense of taste and smell. I had a horrible headache, a sore throat, and I was exhausted. I couldn’t get tested because I didn’t have the right symptoms.
Despite being sick, I never stopped working but just switched to telemedicine. I also took my regular monthly trip to our cabin in Montana. I unknowingly flew on a plane with COVID. I wore a well-fitted N95 mask, so I don’t think I gave anybody COVID. I didn’t give COVID to my partner, Eric, which is hard to believe as – at 77 – he’s older than me. He has diabetes, heart disease, and every other high-risk characteristic. If he’d gotten COVID back then, it would have been terrible, as there were no treatments, but luckily he didn’t get it.
Q: When were you officially diagnosed?
A: Two or 3 months after I thought I might have had COVID, I checked my antibodies, which tested strongly positive for a prior COVID infection. That was when I knew all the symptoms I’d had were due to the disease.
Q: Not only were you dealing with your own illness, but also that of those close to you. Can you talk about that?
A: In April 2020, my mother who was in her 90s and otherwise healthy except for dementia, got COVID. She could have gotten it from me. I visited often but wore a mask. She had all the horrible pulmonary symptoms. In her advance directive, she didn’t want to be hospitalized so I kept her in her home. She died from COVID in her own bed. It was fairly brutal, but at least I kept her where she felt comforted.
My 91-year-old dad was living in a different residential facility. Throughout COVID he had become very depressed because his social patterns had changed. Prior to COVID, they all ate together, but during the pandemic they were unable to. He missed his social connections, disliked being isolated in his room, hated everyone in masks.
He was a bit demented, but not so much that he couldn’t communicate with me or remember where his grandson was going to law school. I wasn’t allowed inside the facility, which was hard on him. I hadn’t told him his wife died because the hospice social workers advised me that I shouldn’t give him news that he couldn’t process readily until I could spend time with him. Unfortunately, that time never came. In December 2020, he got COVID. One of the people in that facility had gone to the hospital, came back, and tested negative, but actually had COVID and gave it to my dad. The guy who gave it to my dad didn’t die but my dad was terribly ill. He died 2 weeks short of getting his vaccine. He was coherent enough to have a conversation. I asked him: ‘Do you want to go to the hospital?’ And he said: ‘No, because it would be too scary,’ since he couldn’t be with me. I put him on hospice and held his hand as he died from pulmonary COVID, which was awful. I couldn’t give him enough morphine or valium to ease his breathing. But his last words to me were “I love you,” and at the very end he seemed peaceful, which was a blessing.
I got an autopsy, because he wanted one. Nothing else was wrong with him other than COVID. It destroyed his lungs. The rest of him was fine – no heart disease, cancer, or anything else. He died of COVID-19, the same as my mother.
That same week, my aunt, my only surviving older relative, who was in Des Moines, Iowa, died of COVID-19. All three family members died before the vaccine came out.
It was hard to lose my parents. I’m the only surviving child because my sister died in her 20s. It’s not been an easy pandemic. But what pandemic is easy? I just happened to have lost more people than most. Ironically, my grandfather was one of the legionnaires at the Bellevue-Stratford Hotel in Philadelphia in 1976 and died of Legionnaire’s disease before we knew what was causing the outbreak.
Q: Were you still struggling with COVID?
A: COVID impacted my whole body. I lost a lot of weight. I didn’t want to eat, and my gastrointestinal system was not happy. It took a while for my sense of taste and smell to come back. Nothing tasted good. I’m not a foodie; I don’t really care about food. We could get takeout or whatever, but none of it appealed to me. I’m not so sure it was a taste thing, I just didn’t feel like eating.
I didn’t realize I had “brain fog” per se, because I felt stressed and overwhelmed by the pandemic and my patients’ concerns. But one day, about 3 months after I had developed COVID, I woke up without the fog. Which made me aware that I hadn’t been feeling right up until that point.
The worst symptoms, however, were cardiac. I noticed also immediately that my heart rate went up very quickly with minimal exertion. My pulse has always been in the 55-60 bpm range, and suddenly just walking across a room made it go up to over 140 bpm. If I did any aerobic activity, it went up over 160 and would be associated with dyspnea and chest pain. I believed these were all post-COVID symptoms and felt validated when reports of others having similar issues were published in the literature.
Q: Did you continue seeing patients?
A: Yes, of course. Patients never needed their doctors more. In East L.A., where patients don’t have easy access to telemedicine, I kept going into clinic throughout the pandemic. In the more affluent Westside of Los Angeles, we switched to telemedicine, which was quite effective for most. However, because diabetes was associated with an increased risk of hospitalization and death from COVID, my patients were understandably afraid. I’ve never been busier, but (like all health care providers), I became more of a COVID provider than a diabetologist.
Q: Do you feel your battle with COVID impacted your work?
A: It didn’t affect me at work. If I was sitting still, I was fine. Sitting at home at a desk, I didn’t notice any symptoms. But as a habitual stair-user, I would be gasping for breath in the stairwell because I couldn’t go up the stairs to my office as I once could.
I think you empathize more with people who had COVID (when you’ve had it yourself). There was such a huge patient burden. And I think that’s been the thing that’s affected health care providers the most – no matter what specialty we’re in – that nobody has answers.
Q: What happened after you had your vaccine?
A: The vaccine itself was fine. I didn’t have any reaction to the first two doses. But the first booster made my cardiac issues worse.
By this point, my cardiac problems stopped me from exercising. I even went to the ER with chest pain once because I was having palpitations and chest pressure caused by simply taking my morning shower. Fortunately, I wasn’t having an MI, but I certainly wasn’t “normal.”
My measure of my fitness is the cross-country skiing trail I use in Montana. I know exactly how far I can ski. Usually I can do the loop in 35 minutes. After COVID, I lasted 10 minutes. I would be tachycardic, short of breath with chest pain radiating down my left arm. I would rest and try to keep going. But with each rest period, I only got worse. I would be laying in the snow and strangers would ask if I needed help.
Q: What helped you?
A: I’ve read a lot about long COVID and have tried to learn from the experts. Of course, I never went to a doctor directly, although I did ask colleagues for advice. What I learned was to never push myself. I forced myself to create an exercise schedule where I only exercised three times a week with rest days in between. When exercising, the second my heart rate went above 140 bpm, I stopped until I could get it back down. I would push against this new limit, even though my limit was low.
Additionally, I worked on my breathing patterns and did meditative breathing for 10 minutes twice daily using a commercially available app.
Although progress was slow, I did improve, and by June 2022, I seemed back to normal. I was not as fit as I was prior to COVID and needed to improve, but the tachycardic response to exercise and cardiac symptoms were gone. I felt like my normal self. Normal enough to go on a spot packing trip in the Sierras in August. (Horses carried us and a mule carried the gear over the 12,000-foot pass into the mountains, and then left my friend and me high in the Sierras for a week.) We were camped above 10,000 feet and every day hiked up to another high mountain lake where we fly-fished for trout that we ate for dinner. The hikes were a challenge, but not abnormally so. Not as they would have been while I had long COVID.
Q: What is the current atmosphere in your clinic?
A: COVID is much milder now in my vaccinated patients, but I feel most health care providers are exhausted. Many of my staff left when COVID hit because they didn’t want to keep working. It made practicing medicine exhausting. There’s been a shortage of nurses, a shortage of everything. We’ve been required to do a whole lot more than we ever did before. It’s much harder to be a doctor. This pandemic is the first time I’ve ever thought of quitting. Granted, I lost my whole family, or at least the older generation, but it’s just been almost overwhelming.
On the plus side, almost every one of my patients has been vaccinated, because early on, people would ask: “Do you trust this vaccine?” I would reply: “I saw my parents die from COVID when they weren’t vaccinated, so you’re getting vaccinated. This is real and the vaccines help.” It made me very good at convincing people to get vaccines because I knew what it was like to see someone dying from COVID up close.
Q: What advice do you have for those struggling with the COVID pandemic?
A: People need to decide what their own risk is for getting sick and how many times they want to get COVID. At this point, I want people to go out, but safely. In the beginning, when my patients said, “can I go visit my granddaughter?” I said, “no,” but that was before we had the vaccine. Now I feel it is safe to go out using common sense. I still have my patients wear masks on planes. I still have patients try to eat outside as much as possible. And I tell people to take the precautions that make sense, but I tell them to go out and do things because life is short.
I had a patient in his 70s who has many risk factors like heart disease and diabetes. His granddaughter’s Bat Mitzvah in Florida was coming up. He asked: “Can I go?” I told him “Yes,” but to be safe – to wear an N95 mask on the plane and at the event, and stay in his own hotel room, rather than with the whole family. I said, “You need to do this.” Earlier in the pandemic, I saw people who literally died from loneliness and isolation.
He and his wife flew there. He sent me a picture of himself with his granddaughter. When he returned, he showed me a handwritten note from her that said, “I love you so much. Everyone else canceled, which made me cry. You’re the only one who came. You have no idea how much this meant to me.”
He’s back in L.A., and he didn’t get COVID. He said, “It was the best thing I’ve done in years.” That’s what I need to help people with, navigating this world with COVID and assessing risks and benefits. As with all of medicine, my advice is individualized. My advice changes based on the major circulating variant and the rates of the virus in the population, as well as the risk factors of the individual.
Q: What are you doing now?
A: I’m trying to avoid getting COVID again, or another booster. I could get pre-exposure monoclonal antibodies but am waiting to do anything further until I see what happens over the fall and winter. I still wear a mask inside but now do a mix of in-person and telemedicine visits. I still try to go to outdoor restaurants, which is easy in California. But I’m flying to see my son in New York and plan to go to Europe this fall for a meeting. I also go to my cabin in Montana every month to get my “dose” of the wilderness. Overall, I travel for conferences and speaking engagements much less because I have learned the joy of staying home.
Thinking back on my life as a doctor, my career began as an intern at Stanford rotating through Ward 5B, the AIDS unit at San Francisco General Hospital, and will likely end with COVID. In spite of all our medical advances, my generation of physicians, much as many generations before us, has a front-row seat to the vulnerability of humans to infectious diseases and how far we still need to go to protect our patients from communicable illness.
A version of this article first appeared on Medscape.com.
Anne L. Peters, MD, is a professor of medicine at the University of Southern California, Los Angeles, and director of the USC clinical diabetes programs. She has published more than 200 articles, reviews, and abstracts; three books on diabetes; and has been an investigator for more than 40 research studies. She has spoken internationally at over 400 programs and serves on many committees of several professional organizations.
Early in 2020, Anne Peters, MD, caught COVID-19. The author of Medscape’s “Peters on Diabetes” column was sick in March 2020 before state-mandated lockdowns, and well before there were any vaccines.
She remembers sitting in a small exam room with two patients who had flown to her Los Angeles office from New York. The elderly couple had hearing difficulties, so Dr. Peters sat close to them, putting on a continuous glucose monitor. “At that time, we didn’t think of COVID-19 as being in L.A.,” Dr. Peters recalled, “so I think we were not terribly consistent at mask-wearing due to the need to educate.”
“Several days later, I got COVID, but I didn’t know I had COVID per se. I felt crappy, had a terrible sore throat, lost my sense of taste and smell [which was not yet described as a COVID symptom], was completely exhausted, but had no fever or cough, which were the only criteria for getting COVID tested at the time. I didn’t know I had been exposed until 2 weeks later, when the patient’s assistant returned the sensor warning us to ‘be careful’ with it because the patient and his wife were recovering from COVID.”
That early battle with COVID-19 was just the beginning of what would become a 2-year struggle, including familial loss amid her own health problems and concerns about the under-resourced patients she cares for. Here, she shares her journey through the pandemic with this news organization.
Question: Thanks for talking to us. Let’s discuss your journey over these past 2.5 years.
Answer: Everybody has their own COVID story because we all went through this together. Some of us have worse COVID stories, and some of us have better ones, but all have been impacted.
I’m not a sick person. I’m a very healthy person but COVID made me so unwell for 2 years. The brain fog and fatigue were nothing compared to the autonomic neuropathy that affected my heart. It was really limiting for me. And I still don’t know the long-term implications, looking 20-30 years from now.
Q: When you initially had COVID, what were your symptoms? What was the impact?
A: I had all the symptoms of COVID, except for a cough and fever. I lost my sense of taste and smell. I had a horrible headache, a sore throat, and I was exhausted. I couldn’t get tested because I didn’t have the right symptoms.
Despite being sick, I never stopped working but just switched to telemedicine. I also took my regular monthly trip to our cabin in Montana. I unknowingly flew on a plane with COVID. I wore a well-fitted N95 mask, so I don’t think I gave anybody COVID. I didn’t give COVID to my partner, Eric, which is hard to believe as – at 77 – he’s older than me. He has diabetes, heart disease, and every other high-risk characteristic. If he’d gotten COVID back then, it would have been terrible, as there were no treatments, but luckily he didn’t get it.
Q: When were you officially diagnosed?
A: Two or 3 months after I thought I might have had COVID, I checked my antibodies, which tested strongly positive for a prior COVID infection. That was when I knew all the symptoms I’d had were due to the disease.
Q: Not only were you dealing with your own illness, but also that of those close to you. Can you talk about that?
A: In April 2020, my mother who was in her 90s and otherwise healthy except for dementia, got COVID. She could have gotten it from me. I visited often but wore a mask. She had all the horrible pulmonary symptoms. In her advance directive, she didn’t want to be hospitalized so I kept her in her home. She died from COVID in her own bed. It was fairly brutal, but at least I kept her where she felt comforted.
My 91-year-old dad was living in a different residential facility. Throughout COVID he had become very depressed because his social patterns had changed. Prior to COVID, they all ate together, but during the pandemic they were unable to. He missed his social connections, disliked being isolated in his room, hated everyone in masks.
He was a bit demented, but not so much that he couldn’t communicate with me or remember where his grandson was going to law school. I wasn’t allowed inside the facility, which was hard on him. I hadn’t told him his wife died because the hospice social workers advised me that I shouldn’t give him news that he couldn’t process readily until I could spend time with him. Unfortunately, that time never came. In December 2020, he got COVID. One of the people in that facility had gone to the hospital, came back, and tested negative, but actually had COVID and gave it to my dad. The guy who gave it to my dad didn’t die but my dad was terribly ill. He died 2 weeks short of getting his vaccine. He was coherent enough to have a conversation. I asked him: ‘Do you want to go to the hospital?’ And he said: ‘No, because it would be too scary,’ since he couldn’t be with me. I put him on hospice and held his hand as he died from pulmonary COVID, which was awful. I couldn’t give him enough morphine or valium to ease his breathing. But his last words to me were “I love you,” and at the very end he seemed peaceful, which was a blessing.
I got an autopsy, because he wanted one. Nothing else was wrong with him other than COVID. It destroyed his lungs. The rest of him was fine – no heart disease, cancer, or anything else. He died of COVID-19, the same as my mother.
That same week, my aunt, my only surviving older relative, who was in Des Moines, Iowa, died of COVID-19. All three family members died before the vaccine came out.
It was hard to lose my parents. I’m the only surviving child because my sister died in her 20s. It’s not been an easy pandemic. But what pandemic is easy? I just happened to have lost more people than most. Ironically, my grandfather was one of the legionnaires at the Bellevue-Stratford Hotel in Philadelphia in 1976 and died of Legionnaire’s disease before we knew what was causing the outbreak.
Q: Were you still struggling with COVID?
A: COVID impacted my whole body. I lost a lot of weight. I didn’t want to eat, and my gastrointestinal system was not happy. It took a while for my sense of taste and smell to come back. Nothing tasted good. I’m not a foodie; I don’t really care about food. We could get takeout or whatever, but none of it appealed to me. I’m not so sure it was a taste thing, I just didn’t feel like eating.
I didn’t realize I had “brain fog” per se, because I felt stressed and overwhelmed by the pandemic and my patients’ concerns. But one day, about 3 months after I had developed COVID, I woke up without the fog. Which made me aware that I hadn’t been feeling right up until that point.
The worst symptoms, however, were cardiac. I noticed also immediately that my heart rate went up very quickly with minimal exertion. My pulse has always been in the 55-60 bpm range, and suddenly just walking across a room made it go up to over 140 bpm. If I did any aerobic activity, it went up over 160 and would be associated with dyspnea and chest pain. I believed these were all post-COVID symptoms and felt validated when reports of others having similar issues were published in the literature.
Q: Did you continue seeing patients?
A: Yes, of course. Patients never needed their doctors more. In East L.A., where patients don’t have easy access to telemedicine, I kept going into clinic throughout the pandemic. In the more affluent Westside of Los Angeles, we switched to telemedicine, which was quite effective for most. However, because diabetes was associated with an increased risk of hospitalization and death from COVID, my patients were understandably afraid. I’ve never been busier, but (like all health care providers), I became more of a COVID provider than a diabetologist.
Q: Do you feel your battle with COVID impacted your work?
A: It didn’t affect me at work. If I was sitting still, I was fine. Sitting at home at a desk, I didn’t notice any symptoms. But as a habitual stair-user, I would be gasping for breath in the stairwell because I couldn’t go up the stairs to my office as I once could.
I think you empathize more with people who had COVID (when you’ve had it yourself). There was such a huge patient burden. And I think that’s been the thing that’s affected health care providers the most – no matter what specialty we’re in – that nobody has answers.
Q: What happened after you had your vaccine?
A: The vaccine itself was fine. I didn’t have any reaction to the first two doses. But the first booster made my cardiac issues worse.
By this point, my cardiac problems stopped me from exercising. I even went to the ER with chest pain once because I was having palpitations and chest pressure caused by simply taking my morning shower. Fortunately, I wasn’t having an MI, but I certainly wasn’t “normal.”
My measure of my fitness is the cross-country skiing trail I use in Montana. I know exactly how far I can ski. Usually I can do the loop in 35 minutes. After COVID, I lasted 10 minutes. I would be tachycardic, short of breath with chest pain radiating down my left arm. I would rest and try to keep going. But with each rest period, I only got worse. I would be laying in the snow and strangers would ask if I needed help.
Q: What helped you?
A: I’ve read a lot about long COVID and have tried to learn from the experts. Of course, I never went to a doctor directly, although I did ask colleagues for advice. What I learned was to never push myself. I forced myself to create an exercise schedule where I only exercised three times a week with rest days in between. When exercising, the second my heart rate went above 140 bpm, I stopped until I could get it back down. I would push against this new limit, even though my limit was low.
Additionally, I worked on my breathing patterns and did meditative breathing for 10 minutes twice daily using a commercially available app.
Although progress was slow, I did improve, and by June 2022, I seemed back to normal. I was not as fit as I was prior to COVID and needed to improve, but the tachycardic response to exercise and cardiac symptoms were gone. I felt like my normal self. Normal enough to go on a spot packing trip in the Sierras in August. (Horses carried us and a mule carried the gear over the 12,000-foot pass into the mountains, and then left my friend and me high in the Sierras for a week.) We were camped above 10,000 feet and every day hiked up to another high mountain lake where we fly-fished for trout that we ate for dinner. The hikes were a challenge, but not abnormally so. Not as they would have been while I had long COVID.
Q: What is the current atmosphere in your clinic?
A: COVID is much milder now in my vaccinated patients, but I feel most health care providers are exhausted. Many of my staff left when COVID hit because they didn’t want to keep working. It made practicing medicine exhausting. There’s been a shortage of nurses, a shortage of everything. We’ve been required to do a whole lot more than we ever did before. It’s much harder to be a doctor. This pandemic is the first time I’ve ever thought of quitting. Granted, I lost my whole family, or at least the older generation, but it’s just been almost overwhelming.
On the plus side, almost every one of my patients has been vaccinated, because early on, people would ask: “Do you trust this vaccine?” I would reply: “I saw my parents die from COVID when they weren’t vaccinated, so you’re getting vaccinated. This is real and the vaccines help.” It made me very good at convincing people to get vaccines because I knew what it was like to see someone dying from COVID up close.
Q: What advice do you have for those struggling with the COVID pandemic?
A: People need to decide what their own risk is for getting sick and how many times they want to get COVID. At this point, I want people to go out, but safely. In the beginning, when my patients said, “can I go visit my granddaughter?” I said, “no,” but that was before we had the vaccine. Now I feel it is safe to go out using common sense. I still have my patients wear masks on planes. I still have patients try to eat outside as much as possible. And I tell people to take the precautions that make sense, but I tell them to go out and do things because life is short.
I had a patient in his 70s who has many risk factors like heart disease and diabetes. His granddaughter’s Bat Mitzvah in Florida was coming up. He asked: “Can I go?” I told him “Yes,” but to be safe – to wear an N95 mask on the plane and at the event, and stay in his own hotel room, rather than with the whole family. I said, “You need to do this.” Earlier in the pandemic, I saw people who literally died from loneliness and isolation.
He and his wife flew there. He sent me a picture of himself with his granddaughter. When he returned, he showed me a handwritten note from her that said, “I love you so much. Everyone else canceled, which made me cry. You’re the only one who came. You have no idea how much this meant to me.”
He’s back in L.A., and he didn’t get COVID. He said, “It was the best thing I’ve done in years.” That’s what I need to help people with, navigating this world with COVID and assessing risks and benefits. As with all of medicine, my advice is individualized. My advice changes based on the major circulating variant and the rates of the virus in the population, as well as the risk factors of the individual.
Q: What are you doing now?
A: I’m trying to avoid getting COVID again, or another booster. I could get pre-exposure monoclonal antibodies but am waiting to do anything further until I see what happens over the fall and winter. I still wear a mask inside but now do a mix of in-person and telemedicine visits. I still try to go to outdoor restaurants, which is easy in California. But I’m flying to see my son in New York and plan to go to Europe this fall for a meeting. I also go to my cabin in Montana every month to get my “dose” of the wilderness. Overall, I travel for conferences and speaking engagements much less because I have learned the joy of staying home.
Thinking back on my life as a doctor, my career began as an intern at Stanford rotating through Ward 5B, the AIDS unit at San Francisco General Hospital, and will likely end with COVID. In spite of all our medical advances, my generation of physicians, much as many generations before us, has a front-row seat to the vulnerability of humans to infectious diseases and how far we still need to go to protect our patients from communicable illness.
A version of this article first appeared on Medscape.com.
Anne L. Peters, MD, is a professor of medicine at the University of Southern California, Los Angeles, and director of the USC clinical diabetes programs. She has published more than 200 articles, reviews, and abstracts; three books on diabetes; and has been an investigator for more than 40 research studies. She has spoken internationally at over 400 programs and serves on many committees of several professional organizations.
Early in 2020, Anne Peters, MD, caught COVID-19. The author of Medscape’s “Peters on Diabetes” column was sick in March 2020 before state-mandated lockdowns, and well before there were any vaccines.
She remembers sitting in a small exam room with two patients who had flown to her Los Angeles office from New York. The elderly couple had hearing difficulties, so Dr. Peters sat close to them, putting on a continuous glucose monitor. “At that time, we didn’t think of COVID-19 as being in L.A.,” Dr. Peters recalled, “so I think we were not terribly consistent at mask-wearing due to the need to educate.”
“Several days later, I got COVID, but I didn’t know I had COVID per se. I felt crappy, had a terrible sore throat, lost my sense of taste and smell [which was not yet described as a COVID symptom], was completely exhausted, but had no fever or cough, which were the only criteria for getting COVID tested at the time. I didn’t know I had been exposed until 2 weeks later, when the patient’s assistant returned the sensor warning us to ‘be careful’ with it because the patient and his wife were recovering from COVID.”
That early battle with COVID-19 was just the beginning of what would become a 2-year struggle, including familial loss amid her own health problems and concerns about the under-resourced patients she cares for. Here, she shares her journey through the pandemic with this news organization.
Question: Thanks for talking to us. Let’s discuss your journey over these past 2.5 years.
Answer: Everybody has their own COVID story because we all went through this together. Some of us have worse COVID stories, and some of us have better ones, but all have been impacted.
I’m not a sick person. I’m a very healthy person but COVID made me so unwell for 2 years. The brain fog and fatigue were nothing compared to the autonomic neuropathy that affected my heart. It was really limiting for me. And I still don’t know the long-term implications, looking 20-30 years from now.
Q: When you initially had COVID, what were your symptoms? What was the impact?
A: I had all the symptoms of COVID, except for a cough and fever. I lost my sense of taste and smell. I had a horrible headache, a sore throat, and I was exhausted. I couldn’t get tested because I didn’t have the right symptoms.
Despite being sick, I never stopped working but just switched to telemedicine. I also took my regular monthly trip to our cabin in Montana. I unknowingly flew on a plane with COVID. I wore a well-fitted N95 mask, so I don’t think I gave anybody COVID. I didn’t give COVID to my partner, Eric, which is hard to believe as – at 77 – he’s older than me. He has diabetes, heart disease, and every other high-risk characteristic. If he’d gotten COVID back then, it would have been terrible, as there were no treatments, but luckily he didn’t get it.
Q: When were you officially diagnosed?
A: Two or 3 months after I thought I might have had COVID, I checked my antibodies, which tested strongly positive for a prior COVID infection. That was when I knew all the symptoms I’d had were due to the disease.
Q: Not only were you dealing with your own illness, but also that of those close to you. Can you talk about that?
A: In April 2020, my mother who was in her 90s and otherwise healthy except for dementia, got COVID. She could have gotten it from me. I visited often but wore a mask. She had all the horrible pulmonary symptoms. In her advance directive, she didn’t want to be hospitalized so I kept her in her home. She died from COVID in her own bed. It was fairly brutal, but at least I kept her where she felt comforted.
My 91-year-old dad was living in a different residential facility. Throughout COVID he had become very depressed because his social patterns had changed. Prior to COVID, they all ate together, but during the pandemic they were unable to. He missed his social connections, disliked being isolated in his room, hated everyone in masks.
He was a bit demented, but not so much that he couldn’t communicate with me or remember where his grandson was going to law school. I wasn’t allowed inside the facility, which was hard on him. I hadn’t told him his wife died because the hospice social workers advised me that I shouldn’t give him news that he couldn’t process readily until I could spend time with him. Unfortunately, that time never came. In December 2020, he got COVID. One of the people in that facility had gone to the hospital, came back, and tested negative, but actually had COVID and gave it to my dad. The guy who gave it to my dad didn’t die but my dad was terribly ill. He died 2 weeks short of getting his vaccine. He was coherent enough to have a conversation. I asked him: ‘Do you want to go to the hospital?’ And he said: ‘No, because it would be too scary,’ since he couldn’t be with me. I put him on hospice and held his hand as he died from pulmonary COVID, which was awful. I couldn’t give him enough morphine or valium to ease his breathing. But his last words to me were “I love you,” and at the very end he seemed peaceful, which was a blessing.
I got an autopsy, because he wanted one. Nothing else was wrong with him other than COVID. It destroyed his lungs. The rest of him was fine – no heart disease, cancer, or anything else. He died of COVID-19, the same as my mother.
That same week, my aunt, my only surviving older relative, who was in Des Moines, Iowa, died of COVID-19. All three family members died before the vaccine came out.
It was hard to lose my parents. I’m the only surviving child because my sister died in her 20s. It’s not been an easy pandemic. But what pandemic is easy? I just happened to have lost more people than most. Ironically, my grandfather was one of the legionnaires at the Bellevue-Stratford Hotel in Philadelphia in 1976 and died of Legionnaire’s disease before we knew what was causing the outbreak.
Q: Were you still struggling with COVID?
A: COVID impacted my whole body. I lost a lot of weight. I didn’t want to eat, and my gastrointestinal system was not happy. It took a while for my sense of taste and smell to come back. Nothing tasted good. I’m not a foodie; I don’t really care about food. We could get takeout or whatever, but none of it appealed to me. I’m not so sure it was a taste thing, I just didn’t feel like eating.
I didn’t realize I had “brain fog” per se, because I felt stressed and overwhelmed by the pandemic and my patients’ concerns. But one day, about 3 months after I had developed COVID, I woke up without the fog. Which made me aware that I hadn’t been feeling right up until that point.
The worst symptoms, however, were cardiac. I noticed also immediately that my heart rate went up very quickly with minimal exertion. My pulse has always been in the 55-60 bpm range, and suddenly just walking across a room made it go up to over 140 bpm. If I did any aerobic activity, it went up over 160 and would be associated with dyspnea and chest pain. I believed these were all post-COVID symptoms and felt validated when reports of others having similar issues were published in the literature.
Q: Did you continue seeing patients?
A: Yes, of course. Patients never needed their doctors more. In East L.A., where patients don’t have easy access to telemedicine, I kept going into clinic throughout the pandemic. In the more affluent Westside of Los Angeles, we switched to telemedicine, which was quite effective for most. However, because diabetes was associated with an increased risk of hospitalization and death from COVID, my patients were understandably afraid. I’ve never been busier, but (like all health care providers), I became more of a COVID provider than a diabetologist.
Q: Do you feel your battle with COVID impacted your work?
A: It didn’t affect me at work. If I was sitting still, I was fine. Sitting at home at a desk, I didn’t notice any symptoms. But as a habitual stair-user, I would be gasping for breath in the stairwell because I couldn’t go up the stairs to my office as I once could.
I think you empathize more with people who had COVID (when you’ve had it yourself). There was such a huge patient burden. And I think that’s been the thing that’s affected health care providers the most – no matter what specialty we’re in – that nobody has answers.
Q: What happened after you had your vaccine?
A: The vaccine itself was fine. I didn’t have any reaction to the first two doses. But the first booster made my cardiac issues worse.
By this point, my cardiac problems stopped me from exercising. I even went to the ER with chest pain once because I was having palpitations and chest pressure caused by simply taking my morning shower. Fortunately, I wasn’t having an MI, but I certainly wasn’t “normal.”
My measure of my fitness is the cross-country skiing trail I use in Montana. I know exactly how far I can ski. Usually I can do the loop in 35 minutes. After COVID, I lasted 10 minutes. I would be tachycardic, short of breath with chest pain radiating down my left arm. I would rest and try to keep going. But with each rest period, I only got worse. I would be laying in the snow and strangers would ask if I needed help.
Q: What helped you?
A: I’ve read a lot about long COVID and have tried to learn from the experts. Of course, I never went to a doctor directly, although I did ask colleagues for advice. What I learned was to never push myself. I forced myself to create an exercise schedule where I only exercised three times a week with rest days in between. When exercising, the second my heart rate went above 140 bpm, I stopped until I could get it back down. I would push against this new limit, even though my limit was low.
Additionally, I worked on my breathing patterns and did meditative breathing for 10 minutes twice daily using a commercially available app.
Although progress was slow, I did improve, and by June 2022, I seemed back to normal. I was not as fit as I was prior to COVID and needed to improve, but the tachycardic response to exercise and cardiac symptoms were gone. I felt like my normal self. Normal enough to go on a spot packing trip in the Sierras in August. (Horses carried us and a mule carried the gear over the 12,000-foot pass into the mountains, and then left my friend and me high in the Sierras for a week.) We were camped above 10,000 feet and every day hiked up to another high mountain lake where we fly-fished for trout that we ate for dinner. The hikes were a challenge, but not abnormally so. Not as they would have been while I had long COVID.
Q: What is the current atmosphere in your clinic?
A: COVID is much milder now in my vaccinated patients, but I feel most health care providers are exhausted. Many of my staff left when COVID hit because they didn’t want to keep working. It made practicing medicine exhausting. There’s been a shortage of nurses, a shortage of everything. We’ve been required to do a whole lot more than we ever did before. It’s much harder to be a doctor. This pandemic is the first time I’ve ever thought of quitting. Granted, I lost my whole family, or at least the older generation, but it’s just been almost overwhelming.
On the plus side, almost every one of my patients has been vaccinated, because early on, people would ask: “Do you trust this vaccine?” I would reply: “I saw my parents die from COVID when they weren’t vaccinated, so you’re getting vaccinated. This is real and the vaccines help.” It made me very good at convincing people to get vaccines because I knew what it was like to see someone dying from COVID up close.
Q: What advice do you have for those struggling with the COVID pandemic?
A: People need to decide what their own risk is for getting sick and how many times they want to get COVID. At this point, I want people to go out, but safely. In the beginning, when my patients said, “can I go visit my granddaughter?” I said, “no,” but that was before we had the vaccine. Now I feel it is safe to go out using common sense. I still have my patients wear masks on planes. I still have patients try to eat outside as much as possible. And I tell people to take the precautions that make sense, but I tell them to go out and do things because life is short.
I had a patient in his 70s who has many risk factors like heart disease and diabetes. His granddaughter’s Bat Mitzvah in Florida was coming up. He asked: “Can I go?” I told him “Yes,” but to be safe – to wear an N95 mask on the plane and at the event, and stay in his own hotel room, rather than with the whole family. I said, “You need to do this.” Earlier in the pandemic, I saw people who literally died from loneliness and isolation.
He and his wife flew there. He sent me a picture of himself with his granddaughter. When he returned, he showed me a handwritten note from her that said, “I love you so much. Everyone else canceled, which made me cry. You’re the only one who came. You have no idea how much this meant to me.”
He’s back in L.A., and he didn’t get COVID. He said, “It was the best thing I’ve done in years.” That’s what I need to help people with, navigating this world with COVID and assessing risks and benefits. As with all of medicine, my advice is individualized. My advice changes based on the major circulating variant and the rates of the virus in the population, as well as the risk factors of the individual.
Q: What are you doing now?
A: I’m trying to avoid getting COVID again, or another booster. I could get pre-exposure monoclonal antibodies but am waiting to do anything further until I see what happens over the fall and winter. I still wear a mask inside but now do a mix of in-person and telemedicine visits. I still try to go to outdoor restaurants, which is easy in California. But I’m flying to see my son in New York and plan to go to Europe this fall for a meeting. I also go to my cabin in Montana every month to get my “dose” of the wilderness. Overall, I travel for conferences and speaking engagements much less because I have learned the joy of staying home.
Thinking back on my life as a doctor, my career began as an intern at Stanford rotating through Ward 5B, the AIDS unit at San Francisco General Hospital, and will likely end with COVID. In spite of all our medical advances, my generation of physicians, much as many generations before us, has a front-row seat to the vulnerability of humans to infectious diseases and how far we still need to go to protect our patients from communicable illness.
A version of this article first appeared on Medscape.com.
Anne L. Peters, MD, is a professor of medicine at the University of Southern California, Los Angeles, and director of the USC clinical diabetes programs. She has published more than 200 articles, reviews, and abstracts; three books on diabetes; and has been an investigator for more than 40 research studies. She has spoken internationally at over 400 programs and serves on many committees of several professional organizations.
Chlorophyll water can trigger pseudoporphyria, expert warns
PORTLAND, ORE. – If a child presents with pseudoporphyria – a bullous photodermatosis with the clinical and histological features of porphyria cutanea tarda (PCT) but with normal porphyrins – chlorophyll water could be the culprit.
Commercially available, green pigment–infused chlorophyll water is marketed with claims that it supports cancer prevention and digestive health, facilitates weight loss, and improves skin complexion. “It also absorbs light, so lo and behold, if your patient is photosensitive, they might get pseudoporphyria,” Robert Sidbury, MD, MPH, chief of the division of dermatology at Seattle Children’s Hospital, said at the annual meeting of the Pacific Dermatologic Association.
This was one of the clinical pearls he shared during his presentation.
Dr. Sidbury added that the risk of photosensitivity increases in children who are taking other medications such as doxycycline, methotrexate, or even naproxen. At least two cases of pseudoporphyria following self-medication with chlorophyll have been described in the dermatology literature.
Is it SSSS or SJS?
Another clinical pearl that Dr. Sidbury shared at the meeting related to staphylococcal scalded skin syndrome (SSSS), which causes reddening and blistering of the skin that makes it appear scalded or burned. To rule out Stevens-Johnson Syndrome (SJS) in a child who presents with such skin manifestations, he routinely performs the unscientific lollipop test, which he learned from Bernard A. “Buddy” Cohen, MD, professor of dermatology and pediatrics at Johns Hopkins University, Baltimore.
“If they eat it, it’s Staph scalded skin,” said Dr. Sidbury, who is also professor of pediatrics at the University of Washington, Seattle. “If they don’t, it’s likely SJS. It’s not the most specific test, but it’s easy to do, because there’s no mucous membrane involvement in Staph scalded skin.”
In a poster presented during the 2022 annual meeting of the Society for Pediatric Dermatology, Sarah Cipriano, MD, MPH, and colleagues at the University of Utah, Salt Lake City, retrospectively study 85 patients aged younger than 18 years diagnosed with SSSS between Jan. 1, 2010, and Aug. 21, 2021. They found that ancillary blood cultures and CSF cultures did not improve diagnostic precision in SSSS patients.
“They don’t add anything unless there’s an indication beyond the Staph scalded skin,” said Dr. Sidbury, who was not involved in the study. “The researchers also found that clindamycin does not improve outcomes in these patients, so avoid using it.” Instead, a first-generation cephalosporin is indicated, and an alternate diagnosis should be considered if the patient does not improve within 48 hours.
Dr. Sidbury disclosed that he has conducted research for Regeneron, Galderma, and UCB. He is also an adviser for Leo Pharmaceuticals and a speaker for Biersdorf.
PORTLAND, ORE. – If a child presents with pseudoporphyria – a bullous photodermatosis with the clinical and histological features of porphyria cutanea tarda (PCT) but with normal porphyrins – chlorophyll water could be the culprit.
Commercially available, green pigment–infused chlorophyll water is marketed with claims that it supports cancer prevention and digestive health, facilitates weight loss, and improves skin complexion. “It also absorbs light, so lo and behold, if your patient is photosensitive, they might get pseudoporphyria,” Robert Sidbury, MD, MPH, chief of the division of dermatology at Seattle Children’s Hospital, said at the annual meeting of the Pacific Dermatologic Association.
This was one of the clinical pearls he shared during his presentation.
Dr. Sidbury added that the risk of photosensitivity increases in children who are taking other medications such as doxycycline, methotrexate, or even naproxen. At least two cases of pseudoporphyria following self-medication with chlorophyll have been described in the dermatology literature.
Is it SSSS or SJS?
Another clinical pearl that Dr. Sidbury shared at the meeting related to staphylococcal scalded skin syndrome (SSSS), which causes reddening and blistering of the skin that makes it appear scalded or burned. To rule out Stevens-Johnson Syndrome (SJS) in a child who presents with such skin manifestations, he routinely performs the unscientific lollipop test, which he learned from Bernard A. “Buddy” Cohen, MD, professor of dermatology and pediatrics at Johns Hopkins University, Baltimore.
“If they eat it, it’s Staph scalded skin,” said Dr. Sidbury, who is also professor of pediatrics at the University of Washington, Seattle. “If they don’t, it’s likely SJS. It’s not the most specific test, but it’s easy to do, because there’s no mucous membrane involvement in Staph scalded skin.”
In a poster presented during the 2022 annual meeting of the Society for Pediatric Dermatology, Sarah Cipriano, MD, MPH, and colleagues at the University of Utah, Salt Lake City, retrospectively study 85 patients aged younger than 18 years diagnosed with SSSS between Jan. 1, 2010, and Aug. 21, 2021. They found that ancillary blood cultures and CSF cultures did not improve diagnostic precision in SSSS patients.
“They don’t add anything unless there’s an indication beyond the Staph scalded skin,” said Dr. Sidbury, who was not involved in the study. “The researchers also found that clindamycin does not improve outcomes in these patients, so avoid using it.” Instead, a first-generation cephalosporin is indicated, and an alternate diagnosis should be considered if the patient does not improve within 48 hours.
Dr. Sidbury disclosed that he has conducted research for Regeneron, Galderma, and UCB. He is also an adviser for Leo Pharmaceuticals and a speaker for Biersdorf.
PORTLAND, ORE. – If a child presents with pseudoporphyria – a bullous photodermatosis with the clinical and histological features of porphyria cutanea tarda (PCT) but with normal porphyrins – chlorophyll water could be the culprit.
Commercially available, green pigment–infused chlorophyll water is marketed with claims that it supports cancer prevention and digestive health, facilitates weight loss, and improves skin complexion. “It also absorbs light, so lo and behold, if your patient is photosensitive, they might get pseudoporphyria,” Robert Sidbury, MD, MPH, chief of the division of dermatology at Seattle Children’s Hospital, said at the annual meeting of the Pacific Dermatologic Association.
This was one of the clinical pearls he shared during his presentation.
Dr. Sidbury added that the risk of photosensitivity increases in children who are taking other medications such as doxycycline, methotrexate, or even naproxen. At least two cases of pseudoporphyria following self-medication with chlorophyll have been described in the dermatology literature.
Is it SSSS or SJS?
Another clinical pearl that Dr. Sidbury shared at the meeting related to staphylococcal scalded skin syndrome (SSSS), which causes reddening and blistering of the skin that makes it appear scalded or burned. To rule out Stevens-Johnson Syndrome (SJS) in a child who presents with such skin manifestations, he routinely performs the unscientific lollipop test, which he learned from Bernard A. “Buddy” Cohen, MD, professor of dermatology and pediatrics at Johns Hopkins University, Baltimore.
“If they eat it, it’s Staph scalded skin,” said Dr. Sidbury, who is also professor of pediatrics at the University of Washington, Seattle. “If they don’t, it’s likely SJS. It’s not the most specific test, but it’s easy to do, because there’s no mucous membrane involvement in Staph scalded skin.”
In a poster presented during the 2022 annual meeting of the Society for Pediatric Dermatology, Sarah Cipriano, MD, MPH, and colleagues at the University of Utah, Salt Lake City, retrospectively study 85 patients aged younger than 18 years diagnosed with SSSS between Jan. 1, 2010, and Aug. 21, 2021. They found that ancillary blood cultures and CSF cultures did not improve diagnostic precision in SSSS patients.
“They don’t add anything unless there’s an indication beyond the Staph scalded skin,” said Dr. Sidbury, who was not involved in the study. “The researchers also found that clindamycin does not improve outcomes in these patients, so avoid using it.” Instead, a first-generation cephalosporin is indicated, and an alternate diagnosis should be considered if the patient does not improve within 48 hours.
Dr. Sidbury disclosed that he has conducted research for Regeneron, Galderma, and UCB. He is also an adviser for Leo Pharmaceuticals and a speaker for Biersdorf.
AT PDA 2022
VTE risk not elevated in AD patients on JAK inhibitors: Study
, according to a new systemic review and meta-analysis, published online in JAMA Dermatology.
“These findings may provide a reference for clinicians in prescribing JAK inhibitors for patients with AD,” Tai-Li Chen, MD, of Taipei (Taiwan) Veterans General Hospital, Taipei, and colleagues wrote in the study.
The results shed some welcome light on treatment for this dermatologic population, for whom enthusiasm about JAK inhibitors was dampened by the addition of a boxed warning to the labels of JAK inhibitors last year, required by the Food and Drug Administration. The warning, which describes an increased risk of “serious heart-related events such as heart attack or stroke, cancer, blood clots, and death” was triggered by results of the ORAL Surveillance study of patients with rheumatoid arthritis (RA) treated with tofacitinib.
The boxed warning is also included in the labels of topical ruxolitinib, a JAK inhibitor approved by the FDA for mild to moderate AD in 2021, and in the labels of two oral JAK inhibitors, upadacitinib and abrocitinib, approved by the FDA for treating moderate to severe AD in January 2022.
Despite the new findings, some dermatologists are still urging caution.
“All the JAK inhibitor trials are short term. I still think the precautionary principle applies and we need to counsel on the risks of JAKs,” tweeted Aaron Drucker, MD, a dermatologist at Women’s College Hospital, and associate professor at the University of Toronto. “It is great to have these as options for our patients. But we need to be aware of the risks associated with this class of medications, counsel patients about them when we are informing them of the risks and benefits of treatment options, and wait for more data specific to this population to make even more informed decisions,” he told this news organization.
The meta-analysis examined both the risk of incident VTE in untreated patients with AD compared with non-AD patients, as well as the risk of VTE in AD patients treated with JAK inhibitors compared with those on either placebo or dupilumab. Four JAK inhibitors were studied: abrocitinib, baricitinib (under FDA review for AD), upadacitinib, and SHR0302 (in clinical trials).
Two studies (458,206 participants) found the overall incidence rate of VTE for patients with AD was 0.23 events per 100 patient-years. The risk was did not differ from that in non-AD patients (pooled hazard ratio [HR], 0.95; 95% confidence interval [CI], 0.62-1.45).
Another 15 studies included 8,787 participants with AD and found no significant differences in the rates of VTE in AD patients treated with JAK inhibitors (0.05%) versus those treated with placebo or dupilumab (0.03%). However “with the increasing applications of JAK inhibitors in AD, more clinical data are needed to identify patients at high risk for VTE,” noted the authors.
“We need more, long-term data,” agreed Dr. Drucker, adding that a major issue is the short-term nature of AD trials to date (generally up to 16 weeks), which “don’t provide adequate reassurance.” He said although the FDA’s boxed warning was prompted by a trial in RA patients treated with tofacitinib (a less selective JAK inhibitor than those approved by the FDA for AD), and the same risks have not been demonstrated specifically for the JAK inhibitors used for a patients with AD, he still remains cautious.
While agreeing on the need for more long-term data, Andrew Blauvelt, MD, MBA, president of Oregon Medical Research Center, Portland, said that the new findings should “provide reassurance” to dermatologists and are “consonant with recent published meta-analyses reporting no increased VTE risk in patients with psoriasis, RA, or inflammatory bowel disease treated with JAK inhibitors” in Arthritis & Rheumatology, and Mayo Clinic Proceedings.
In an interview, Dr. Blauvelt said that safety profiles emerging for the newer JAK inhibitors, which block JAK 1/2, have been overshadowed by the older RA data for tofacitinib – which is a JAK 1/3 inhibitor, “despite emerging long-term, monotherapy, clinical study data for dermatologic diseases showing no or rare risks of developing severe adverse events outlined in the boxed warnings.”
Both Dr. Blauvelt and Dr. Drucker pointed out that people with RA tend to have more comorbidities than those with AD that would predispose them to adverse events. In fact, “approximately 75% of patients in the ORAL Surveillance study were also on concomitant methotrexate and/or prednisone, which can greatly confound safety results,” said Dr. Blauvelt.
The study authors did not report any disclosures. No funding source for the study was provided. Dr. Drucker has no relevant disclosures. Dr. Blauvelt has been a clinical study investigator in trials for AD treatments, including JAK inhibitors; his disclosures include serving as a speaker, scientific adviser, and/or clinical study investigator for multiple companies including AbbVie, Arcutis, Bristol-Myers Squibb, Pfizer, Incyte, Regeneron, Sanofi Genzyme, and UCB Pharma.
, according to a new systemic review and meta-analysis, published online in JAMA Dermatology.
“These findings may provide a reference for clinicians in prescribing JAK inhibitors for patients with AD,” Tai-Li Chen, MD, of Taipei (Taiwan) Veterans General Hospital, Taipei, and colleagues wrote in the study.
The results shed some welcome light on treatment for this dermatologic population, for whom enthusiasm about JAK inhibitors was dampened by the addition of a boxed warning to the labels of JAK inhibitors last year, required by the Food and Drug Administration. The warning, which describes an increased risk of “serious heart-related events such as heart attack or stroke, cancer, blood clots, and death” was triggered by results of the ORAL Surveillance study of patients with rheumatoid arthritis (RA) treated with tofacitinib.
The boxed warning is also included in the labels of topical ruxolitinib, a JAK inhibitor approved by the FDA for mild to moderate AD in 2021, and in the labels of two oral JAK inhibitors, upadacitinib and abrocitinib, approved by the FDA for treating moderate to severe AD in January 2022.
Despite the new findings, some dermatologists are still urging caution.
“All the JAK inhibitor trials are short term. I still think the precautionary principle applies and we need to counsel on the risks of JAKs,” tweeted Aaron Drucker, MD, a dermatologist at Women’s College Hospital, and associate professor at the University of Toronto. “It is great to have these as options for our patients. But we need to be aware of the risks associated with this class of medications, counsel patients about them when we are informing them of the risks and benefits of treatment options, and wait for more data specific to this population to make even more informed decisions,” he told this news organization.
The meta-analysis examined both the risk of incident VTE in untreated patients with AD compared with non-AD patients, as well as the risk of VTE in AD patients treated with JAK inhibitors compared with those on either placebo or dupilumab. Four JAK inhibitors were studied: abrocitinib, baricitinib (under FDA review for AD), upadacitinib, and SHR0302 (in clinical trials).
Two studies (458,206 participants) found the overall incidence rate of VTE for patients with AD was 0.23 events per 100 patient-years. The risk was did not differ from that in non-AD patients (pooled hazard ratio [HR], 0.95; 95% confidence interval [CI], 0.62-1.45).
Another 15 studies included 8,787 participants with AD and found no significant differences in the rates of VTE in AD patients treated with JAK inhibitors (0.05%) versus those treated with placebo or dupilumab (0.03%). However “with the increasing applications of JAK inhibitors in AD, more clinical data are needed to identify patients at high risk for VTE,” noted the authors.
“We need more, long-term data,” agreed Dr. Drucker, adding that a major issue is the short-term nature of AD trials to date (generally up to 16 weeks), which “don’t provide adequate reassurance.” He said although the FDA’s boxed warning was prompted by a trial in RA patients treated with tofacitinib (a less selective JAK inhibitor than those approved by the FDA for AD), and the same risks have not been demonstrated specifically for the JAK inhibitors used for a patients with AD, he still remains cautious.
While agreeing on the need for more long-term data, Andrew Blauvelt, MD, MBA, president of Oregon Medical Research Center, Portland, said that the new findings should “provide reassurance” to dermatologists and are “consonant with recent published meta-analyses reporting no increased VTE risk in patients with psoriasis, RA, or inflammatory bowel disease treated with JAK inhibitors” in Arthritis & Rheumatology, and Mayo Clinic Proceedings.
In an interview, Dr. Blauvelt said that safety profiles emerging for the newer JAK inhibitors, which block JAK 1/2, have been overshadowed by the older RA data for tofacitinib – which is a JAK 1/3 inhibitor, “despite emerging long-term, monotherapy, clinical study data for dermatologic diseases showing no or rare risks of developing severe adverse events outlined in the boxed warnings.”
Both Dr. Blauvelt and Dr. Drucker pointed out that people with RA tend to have more comorbidities than those with AD that would predispose them to adverse events. In fact, “approximately 75% of patients in the ORAL Surveillance study were also on concomitant methotrexate and/or prednisone, which can greatly confound safety results,” said Dr. Blauvelt.
The study authors did not report any disclosures. No funding source for the study was provided. Dr. Drucker has no relevant disclosures. Dr. Blauvelt has been a clinical study investigator in trials for AD treatments, including JAK inhibitors; his disclosures include serving as a speaker, scientific adviser, and/or clinical study investigator for multiple companies including AbbVie, Arcutis, Bristol-Myers Squibb, Pfizer, Incyte, Regeneron, Sanofi Genzyme, and UCB Pharma.
, according to a new systemic review and meta-analysis, published online in JAMA Dermatology.
“These findings may provide a reference for clinicians in prescribing JAK inhibitors for patients with AD,” Tai-Li Chen, MD, of Taipei (Taiwan) Veterans General Hospital, Taipei, and colleagues wrote in the study.
The results shed some welcome light on treatment for this dermatologic population, for whom enthusiasm about JAK inhibitors was dampened by the addition of a boxed warning to the labels of JAK inhibitors last year, required by the Food and Drug Administration. The warning, which describes an increased risk of “serious heart-related events such as heart attack or stroke, cancer, blood clots, and death” was triggered by results of the ORAL Surveillance study of patients with rheumatoid arthritis (RA) treated with tofacitinib.
The boxed warning is also included in the labels of topical ruxolitinib, a JAK inhibitor approved by the FDA for mild to moderate AD in 2021, and in the labels of two oral JAK inhibitors, upadacitinib and abrocitinib, approved by the FDA for treating moderate to severe AD in January 2022.
Despite the new findings, some dermatologists are still urging caution.
“All the JAK inhibitor trials are short term. I still think the precautionary principle applies and we need to counsel on the risks of JAKs,” tweeted Aaron Drucker, MD, a dermatologist at Women’s College Hospital, and associate professor at the University of Toronto. “It is great to have these as options for our patients. But we need to be aware of the risks associated with this class of medications, counsel patients about them when we are informing them of the risks and benefits of treatment options, and wait for more data specific to this population to make even more informed decisions,” he told this news organization.
The meta-analysis examined both the risk of incident VTE in untreated patients with AD compared with non-AD patients, as well as the risk of VTE in AD patients treated with JAK inhibitors compared with those on either placebo or dupilumab. Four JAK inhibitors were studied: abrocitinib, baricitinib (under FDA review for AD), upadacitinib, and SHR0302 (in clinical trials).
Two studies (458,206 participants) found the overall incidence rate of VTE for patients with AD was 0.23 events per 100 patient-years. The risk was did not differ from that in non-AD patients (pooled hazard ratio [HR], 0.95; 95% confidence interval [CI], 0.62-1.45).
Another 15 studies included 8,787 participants with AD and found no significant differences in the rates of VTE in AD patients treated with JAK inhibitors (0.05%) versus those treated with placebo or dupilumab (0.03%). However “with the increasing applications of JAK inhibitors in AD, more clinical data are needed to identify patients at high risk for VTE,” noted the authors.
“We need more, long-term data,” agreed Dr. Drucker, adding that a major issue is the short-term nature of AD trials to date (generally up to 16 weeks), which “don’t provide adequate reassurance.” He said although the FDA’s boxed warning was prompted by a trial in RA patients treated with tofacitinib (a less selective JAK inhibitor than those approved by the FDA for AD), and the same risks have not been demonstrated specifically for the JAK inhibitors used for a patients with AD, he still remains cautious.
While agreeing on the need for more long-term data, Andrew Blauvelt, MD, MBA, president of Oregon Medical Research Center, Portland, said that the new findings should “provide reassurance” to dermatologists and are “consonant with recent published meta-analyses reporting no increased VTE risk in patients with psoriasis, RA, or inflammatory bowel disease treated with JAK inhibitors” in Arthritis & Rheumatology, and Mayo Clinic Proceedings.
In an interview, Dr. Blauvelt said that safety profiles emerging for the newer JAK inhibitors, which block JAK 1/2, have been overshadowed by the older RA data for tofacitinib – which is a JAK 1/3 inhibitor, “despite emerging long-term, monotherapy, clinical study data for dermatologic diseases showing no or rare risks of developing severe adverse events outlined in the boxed warnings.”
Both Dr. Blauvelt and Dr. Drucker pointed out that people with RA tend to have more comorbidities than those with AD that would predispose them to adverse events. In fact, “approximately 75% of patients in the ORAL Surveillance study were also on concomitant methotrexate and/or prednisone, which can greatly confound safety results,” said Dr. Blauvelt.
The study authors did not report any disclosures. No funding source for the study was provided. Dr. Drucker has no relevant disclosures. Dr. Blauvelt has been a clinical study investigator in trials for AD treatments, including JAK inhibitors; his disclosures include serving as a speaker, scientific adviser, and/or clinical study investigator for multiple companies including AbbVie, Arcutis, Bristol-Myers Squibb, Pfizer, Incyte, Regeneron, Sanofi Genzyme, and UCB Pharma.
FROM JAMA DERMATOLOGY
How docs in firearm-friendly states talk gun safety
Samuel Mathis, MD, tries to cover a lot of ground during a wellness exam for his patients. Nutrition, immunizations, dental hygiene, and staying safe at school are a few of the topics on his list. And the Texas pediatrician asks one more question of children and their parents: “Are there any firearms in the house?”
If the answer is “yes,” Dr. Mathis discusses safety courses and other ideas with the families. “Rather than ask a bunch of questions, often I will say it’s recommended to keep them locked up and don’t forget toddlers can climb heights that you never would have envisioned,” said Dr. Mathis, an assistant professor at the University of Texas Medical Branch, Galveston.
Dr. Mathis said some of his physician colleagues are wary of bringing up the topic of guns in a state that leads the nation with more than 1 million registered firearms. “My discussion is more on firearm responsibility and just making sure they are taking extra steps to keep themselves and everyone around them safe. That works much better in these discussions.”
Gun safety: Public health concern, not politics
The statistics tell why:
- Unintentional shooting deaths by children rose by nearly one third in a 3-month period in 2020, compared with the same period in 2019.
- Of every 10 gun deaths in the United States, 6 are by suicide.
- As of July 28, 372 mass shootings have occured.
- Firearms now represent the leading cause of death among the nation’s youth.
In 2018, the editors of Annals of Internal Medicine urged physicians in the United States to sign a pledge to talk with their patients about guns in the home. To date, at least 3,664 have done so.
In 2019, the American Academy of Family Medicine, with other leading physician and public health organizations, issued a “call to action,” recommending ways to reduce firearm-related injury and death in the United States. Physicians can and should address the issue, it said, by counseling patients about firearm safety.
“This is just another part of healthcare,” said Sarah C. Nosal, MD, a member of the board of directors of the AAFP, who practices at the Urban Horizons Family Health Center, New York.
Dr. Nosal said she asks about firearms during every well-child visit. She also focuses on patients with a history of depression or suicide attempts and those who have experienced domestic violence.
Are physicians counseling patients about gun safety?
A 2018 survey of physicians found that 73% of the 71 who responded agreed to discuss gun safety with at-risk patients. But just 5% said they always talk to those at-risk patients, according to Melanie G. Hagen, MD, professor of internal medicine at the University of Florida, Gainesville, who led the study. While the overwhelming majority agreed that gun safety is a public health issue, only 55% said they felt comfortable initiating conversations about firearms with their patients.
Have things changed since then? “Probably not,” Dr. Hagen said in an interview. She cited some reasons, at least in her state.
One obstacle is that many people, including physicians, believe that Florida’s physician gag law, which prohibited physicians from asking about a patient’s firearm ownership, was still in effect. The law, passed in 2011, was overturned in 2017. In her survey, 76% said they were aware it had been overturned. But that awareness appears not to be universal, she said.
In a 2020 report about physician involvement in promoting gun safety, researchers noted four main challenges: lingering fears about the overturned law and potential liability from violating it, feeling unprepared, worry that patients don’t want to discuss the topic, and lack of time to talk about it during a rushed office visit.
But recent research suggests that patients are often open to talking about gun safety, and another study found that if physicians are given educational materials on firearm safety, more will counsel patients about gun safety.
Are patients and parents receptive?
Parents welcome discussion from health care providers about gun safety, according to a study from the University of Pennsylvania, Philadelphia.
Researchers asked roughly 100 parents to watch a short video about a firearm safety program designed to prevent accidents and suicides from guns. The program, still under study, involves a discussion between a parent and a pediatrician, with information given on secure storage of guns and the offering of a free cable lock.
The parents, about equally divided between gun owners and non–gun owners, said they were open to discussion about firearm safety, especially when the conversation involves their child’s pediatrician. Among the gun owners, only one in three said all their firearms were locked, unloaded, and stored properly. But after getting the safety information, 64% said they would change the way they stored their firearms.
A different program that offered pediatricians educational materials on firearm safety, as well as free firearm locks for distribution, increased the likelihood that the physicians would counsel patients on gun safety, other researchers reported.
Getting the conversation started
Some patients “bristle” when they’re asked about guns, Dr. Hagen said. Focusing on the “why” of the question can soften their response. One of her patients, a man in his 80s, had worked as a prison guard. After he was diagnosed with clinical depression, she asked him if he ever thought about ending his life. He said yes.
“And in Florida, I know a lot of people have guns,” she said. The state ranks second in the nation, with more than a half million registered weapons.
When Dr. Hagen asked him if he had firearms at home, he balked. Why did she need to know? “People do get defensive,” she said. “Luckily, I had a good relationship with this man, and he was willing to listen to me. If it’s someone I have a good relationship with, and I have this initial bristling, if I say: ‘I’m worried about you, I’m worried about your safety,’ that changes the entire conversation.”
She talked through the best plan for this patient, and he agreed to give his weapons to his son to keep.
Likewise, she talks with family members of dementia patients, urging them to be sure the weapons are stored and locked to prevent tragic accidents.
Dr. Nosal said reading the room is key. “Often, we are having the conversation with a parent with a child present,” she said. “Perhaps that is not the conversation the parent or guardian wanted to have with the child present.” In such a situation, she suggests asking the parent if they would talk about it solo.
“It can be a challenge to know the appropriate way to start the conversation,” Dr. Mathis said. The topic is not taught in medical school, although many experts think it should be. Dr. Hagen recently delivered a lecture to medical students about how to broach the topic with patients. She said she hopes it will become a regular event.
“It really comes down to being willing to be open and just ask that first question in a nonjudgmental way,” Dr. Mathis said. It helps, too, he said, for physicians to remember what he always tries to keep in mind: “My job isn’t politics, my job is health.”
Among the points Dr. Hagen makes in her lecture about talking to patients about guns are the following:
- Every day, more than 110 Americans are killed with guns.
- Gun violence accounts for just 1%-2% of those deaths, but mass shootings serve to shine a light on the issue of gun safety.
- 110,000 firearm injuries a year require medical or legal attention. Each year, more than 1,200 children in this country die from gun-related injuries.
- More than 33,000 people, on average, die in the United States each year from gun violence, including more than 21,000 from suicide.
- About 31% of all U.S. households have firearms; 22% of U.S. adults own one or more.
- Guns are 70% less likely to be stored locked and unloaded in homes where suicides or unintentional gun injuries occur.
- Action points: Identify risk, counsel patients at risk, act when someone is in imminent danger (such as unsafe practices or suicide threats).
- Focus on identifying adults who have a risk of inflicting violence on self or others.
- Focus on health and well-being with all; be conversational and educational.
- Clinicians should ask five crucial questions, all with an “L,” if firearms are in the home: Is it Loaded? Locked? Are Little children present? Is the owner feeling Low? Are they Learned [educated] in gun safety?
A version of this article first appeared on Medscape.com.
Samuel Mathis, MD, tries to cover a lot of ground during a wellness exam for his patients. Nutrition, immunizations, dental hygiene, and staying safe at school are a few of the topics on his list. And the Texas pediatrician asks one more question of children and their parents: “Are there any firearms in the house?”
If the answer is “yes,” Dr. Mathis discusses safety courses and other ideas with the families. “Rather than ask a bunch of questions, often I will say it’s recommended to keep them locked up and don’t forget toddlers can climb heights that you never would have envisioned,” said Dr. Mathis, an assistant professor at the University of Texas Medical Branch, Galveston.
Dr. Mathis said some of his physician colleagues are wary of bringing up the topic of guns in a state that leads the nation with more than 1 million registered firearms. “My discussion is more on firearm responsibility and just making sure they are taking extra steps to keep themselves and everyone around them safe. That works much better in these discussions.”
Gun safety: Public health concern, not politics
The statistics tell why:
- Unintentional shooting deaths by children rose by nearly one third in a 3-month period in 2020, compared with the same period in 2019.
- Of every 10 gun deaths in the United States, 6 are by suicide.
- As of July 28, 372 mass shootings have occured.
- Firearms now represent the leading cause of death among the nation’s youth.
In 2018, the editors of Annals of Internal Medicine urged physicians in the United States to sign a pledge to talk with their patients about guns in the home. To date, at least 3,664 have done so.
In 2019, the American Academy of Family Medicine, with other leading physician and public health organizations, issued a “call to action,” recommending ways to reduce firearm-related injury and death in the United States. Physicians can and should address the issue, it said, by counseling patients about firearm safety.
“This is just another part of healthcare,” said Sarah C. Nosal, MD, a member of the board of directors of the AAFP, who practices at the Urban Horizons Family Health Center, New York.
Dr. Nosal said she asks about firearms during every well-child visit. She also focuses on patients with a history of depression or suicide attempts and those who have experienced domestic violence.
Are physicians counseling patients about gun safety?
A 2018 survey of physicians found that 73% of the 71 who responded agreed to discuss gun safety with at-risk patients. But just 5% said they always talk to those at-risk patients, according to Melanie G. Hagen, MD, professor of internal medicine at the University of Florida, Gainesville, who led the study. While the overwhelming majority agreed that gun safety is a public health issue, only 55% said they felt comfortable initiating conversations about firearms with their patients.
Have things changed since then? “Probably not,” Dr. Hagen said in an interview. She cited some reasons, at least in her state.
One obstacle is that many people, including physicians, believe that Florida’s physician gag law, which prohibited physicians from asking about a patient’s firearm ownership, was still in effect. The law, passed in 2011, was overturned in 2017. In her survey, 76% said they were aware it had been overturned. But that awareness appears not to be universal, she said.
In a 2020 report about physician involvement in promoting gun safety, researchers noted four main challenges: lingering fears about the overturned law and potential liability from violating it, feeling unprepared, worry that patients don’t want to discuss the topic, and lack of time to talk about it during a rushed office visit.
But recent research suggests that patients are often open to talking about gun safety, and another study found that if physicians are given educational materials on firearm safety, more will counsel patients about gun safety.
Are patients and parents receptive?
Parents welcome discussion from health care providers about gun safety, according to a study from the University of Pennsylvania, Philadelphia.
Researchers asked roughly 100 parents to watch a short video about a firearm safety program designed to prevent accidents and suicides from guns. The program, still under study, involves a discussion between a parent and a pediatrician, with information given on secure storage of guns and the offering of a free cable lock.
The parents, about equally divided between gun owners and non–gun owners, said they were open to discussion about firearm safety, especially when the conversation involves their child’s pediatrician. Among the gun owners, only one in three said all their firearms were locked, unloaded, and stored properly. But after getting the safety information, 64% said they would change the way they stored their firearms.
A different program that offered pediatricians educational materials on firearm safety, as well as free firearm locks for distribution, increased the likelihood that the physicians would counsel patients on gun safety, other researchers reported.
Getting the conversation started
Some patients “bristle” when they’re asked about guns, Dr. Hagen said. Focusing on the “why” of the question can soften their response. One of her patients, a man in his 80s, had worked as a prison guard. After he was diagnosed with clinical depression, she asked him if he ever thought about ending his life. He said yes.
“And in Florida, I know a lot of people have guns,” she said. The state ranks second in the nation, with more than a half million registered weapons.
When Dr. Hagen asked him if he had firearms at home, he balked. Why did she need to know? “People do get defensive,” she said. “Luckily, I had a good relationship with this man, and he was willing to listen to me. If it’s someone I have a good relationship with, and I have this initial bristling, if I say: ‘I’m worried about you, I’m worried about your safety,’ that changes the entire conversation.”
She talked through the best plan for this patient, and he agreed to give his weapons to his son to keep.
Likewise, she talks with family members of dementia patients, urging them to be sure the weapons are stored and locked to prevent tragic accidents.
Dr. Nosal said reading the room is key. “Often, we are having the conversation with a parent with a child present,” she said. “Perhaps that is not the conversation the parent or guardian wanted to have with the child present.” In such a situation, she suggests asking the parent if they would talk about it solo.
“It can be a challenge to know the appropriate way to start the conversation,” Dr. Mathis said. The topic is not taught in medical school, although many experts think it should be. Dr. Hagen recently delivered a lecture to medical students about how to broach the topic with patients. She said she hopes it will become a regular event.
“It really comes down to being willing to be open and just ask that first question in a nonjudgmental way,” Dr. Mathis said. It helps, too, he said, for physicians to remember what he always tries to keep in mind: “My job isn’t politics, my job is health.”
Among the points Dr. Hagen makes in her lecture about talking to patients about guns are the following:
- Every day, more than 110 Americans are killed with guns.
- Gun violence accounts for just 1%-2% of those deaths, but mass shootings serve to shine a light on the issue of gun safety.
- 110,000 firearm injuries a year require medical or legal attention. Each year, more than 1,200 children in this country die from gun-related injuries.
- More than 33,000 people, on average, die in the United States each year from gun violence, including more than 21,000 from suicide.
- About 31% of all U.S. households have firearms; 22% of U.S. adults own one or more.
- Guns are 70% less likely to be stored locked and unloaded in homes where suicides or unintentional gun injuries occur.
- Action points: Identify risk, counsel patients at risk, act when someone is in imminent danger (such as unsafe practices or suicide threats).
- Focus on identifying adults who have a risk of inflicting violence on self or others.
- Focus on health and well-being with all; be conversational and educational.
- Clinicians should ask five crucial questions, all with an “L,” if firearms are in the home: Is it Loaded? Locked? Are Little children present? Is the owner feeling Low? Are they Learned [educated] in gun safety?
A version of this article first appeared on Medscape.com.
Samuel Mathis, MD, tries to cover a lot of ground during a wellness exam for his patients. Nutrition, immunizations, dental hygiene, and staying safe at school are a few of the topics on his list. And the Texas pediatrician asks one more question of children and their parents: “Are there any firearms in the house?”
If the answer is “yes,” Dr. Mathis discusses safety courses and other ideas with the families. “Rather than ask a bunch of questions, often I will say it’s recommended to keep them locked up and don’t forget toddlers can climb heights that you never would have envisioned,” said Dr. Mathis, an assistant professor at the University of Texas Medical Branch, Galveston.
Dr. Mathis said some of his physician colleagues are wary of bringing up the topic of guns in a state that leads the nation with more than 1 million registered firearms. “My discussion is more on firearm responsibility and just making sure they are taking extra steps to keep themselves and everyone around them safe. That works much better in these discussions.”
Gun safety: Public health concern, not politics
The statistics tell why:
- Unintentional shooting deaths by children rose by nearly one third in a 3-month period in 2020, compared with the same period in 2019.
- Of every 10 gun deaths in the United States, 6 are by suicide.
- As of July 28, 372 mass shootings have occured.
- Firearms now represent the leading cause of death among the nation’s youth.
In 2018, the editors of Annals of Internal Medicine urged physicians in the United States to sign a pledge to talk with their patients about guns in the home. To date, at least 3,664 have done so.
In 2019, the American Academy of Family Medicine, with other leading physician and public health organizations, issued a “call to action,” recommending ways to reduce firearm-related injury and death in the United States. Physicians can and should address the issue, it said, by counseling patients about firearm safety.
“This is just another part of healthcare,” said Sarah C. Nosal, MD, a member of the board of directors of the AAFP, who practices at the Urban Horizons Family Health Center, New York.
Dr. Nosal said she asks about firearms during every well-child visit. She also focuses on patients with a history of depression or suicide attempts and those who have experienced domestic violence.
Are physicians counseling patients about gun safety?
A 2018 survey of physicians found that 73% of the 71 who responded agreed to discuss gun safety with at-risk patients. But just 5% said they always talk to those at-risk patients, according to Melanie G. Hagen, MD, professor of internal medicine at the University of Florida, Gainesville, who led the study. While the overwhelming majority agreed that gun safety is a public health issue, only 55% said they felt comfortable initiating conversations about firearms with their patients.
Have things changed since then? “Probably not,” Dr. Hagen said in an interview. She cited some reasons, at least in her state.
One obstacle is that many people, including physicians, believe that Florida’s physician gag law, which prohibited physicians from asking about a patient’s firearm ownership, was still in effect. The law, passed in 2011, was overturned in 2017. In her survey, 76% said they were aware it had been overturned. But that awareness appears not to be universal, she said.
In a 2020 report about physician involvement in promoting gun safety, researchers noted four main challenges: lingering fears about the overturned law and potential liability from violating it, feeling unprepared, worry that patients don’t want to discuss the topic, and lack of time to talk about it during a rushed office visit.
But recent research suggests that patients are often open to talking about gun safety, and another study found that if physicians are given educational materials on firearm safety, more will counsel patients about gun safety.
Are patients and parents receptive?
Parents welcome discussion from health care providers about gun safety, according to a study from the University of Pennsylvania, Philadelphia.
Researchers asked roughly 100 parents to watch a short video about a firearm safety program designed to prevent accidents and suicides from guns. The program, still under study, involves a discussion between a parent and a pediatrician, with information given on secure storage of guns and the offering of a free cable lock.
The parents, about equally divided between gun owners and non–gun owners, said they were open to discussion about firearm safety, especially when the conversation involves their child’s pediatrician. Among the gun owners, only one in three said all their firearms were locked, unloaded, and stored properly. But after getting the safety information, 64% said they would change the way they stored their firearms.
A different program that offered pediatricians educational materials on firearm safety, as well as free firearm locks for distribution, increased the likelihood that the physicians would counsel patients on gun safety, other researchers reported.
Getting the conversation started
Some patients “bristle” when they’re asked about guns, Dr. Hagen said. Focusing on the “why” of the question can soften their response. One of her patients, a man in his 80s, had worked as a prison guard. After he was diagnosed with clinical depression, she asked him if he ever thought about ending his life. He said yes.
“And in Florida, I know a lot of people have guns,” she said. The state ranks second in the nation, with more than a half million registered weapons.
When Dr. Hagen asked him if he had firearms at home, he balked. Why did she need to know? “People do get defensive,” she said. “Luckily, I had a good relationship with this man, and he was willing to listen to me. If it’s someone I have a good relationship with, and I have this initial bristling, if I say: ‘I’m worried about you, I’m worried about your safety,’ that changes the entire conversation.”
She talked through the best plan for this patient, and he agreed to give his weapons to his son to keep.
Likewise, she talks with family members of dementia patients, urging them to be sure the weapons are stored and locked to prevent tragic accidents.
Dr. Nosal said reading the room is key. “Often, we are having the conversation with a parent with a child present,” she said. “Perhaps that is not the conversation the parent or guardian wanted to have with the child present.” In such a situation, she suggests asking the parent if they would talk about it solo.
“It can be a challenge to know the appropriate way to start the conversation,” Dr. Mathis said. The topic is not taught in medical school, although many experts think it should be. Dr. Hagen recently delivered a lecture to medical students about how to broach the topic with patients. She said she hopes it will become a regular event.
“It really comes down to being willing to be open and just ask that first question in a nonjudgmental way,” Dr. Mathis said. It helps, too, he said, for physicians to remember what he always tries to keep in mind: “My job isn’t politics, my job is health.”
Among the points Dr. Hagen makes in her lecture about talking to patients about guns are the following:
- Every day, more than 110 Americans are killed with guns.
- Gun violence accounts for just 1%-2% of those deaths, but mass shootings serve to shine a light on the issue of gun safety.
- 110,000 firearm injuries a year require medical or legal attention. Each year, more than 1,200 children in this country die from gun-related injuries.
- More than 33,000 people, on average, die in the United States each year from gun violence, including more than 21,000 from suicide.
- About 31% of all U.S. households have firearms; 22% of U.S. adults own one or more.
- Guns are 70% less likely to be stored locked and unloaded in homes where suicides or unintentional gun injuries occur.
- Action points: Identify risk, counsel patients at risk, act when someone is in imminent danger (such as unsafe practices or suicide threats).
- Focus on identifying adults who have a risk of inflicting violence on self or others.
- Focus on health and well-being with all; be conversational and educational.
- Clinicians should ask five crucial questions, all with an “L,” if firearms are in the home: Is it Loaded? Locked? Are Little children present? Is the owner feeling Low? Are they Learned [educated] in gun safety?
A version of this article first appeared on Medscape.com.
Infographic: Is physician behavior on social media really so bad?
The medical profession is held to a high standard of personal conduct, so physicians keep a sharp eye out for how fellow doctors behave. That goes for social media as well as in-person conduct.
(and it’s not as egregious as you might think). If you’re interested in delving deeper into the data, check out the Medscape Physicians Behaving Badly Report 2022.
A version of this article first appeared on Medscape.com.
The medical profession is held to a high standard of personal conduct, so physicians keep a sharp eye out for how fellow doctors behave. That goes for social media as well as in-person conduct.
(and it’s not as egregious as you might think). If you’re interested in delving deeper into the data, check out the Medscape Physicians Behaving Badly Report 2022.

A version of this article first appeared on Medscape.com.
The medical profession is held to a high standard of personal conduct, so physicians keep a sharp eye out for how fellow doctors behave. That goes for social media as well as in-person conduct.
(and it’s not as egregious as you might think). If you’re interested in delving deeper into the data, check out the Medscape Physicians Behaving Badly Report 2022.

A version of this article first appeared on Medscape.com.








