User login
News and Views that Matter to Pediatricians
The leading independent newspaper covering news and commentary in pediatrics.
AHA targets rising prevalence of obstructive sleep apnea in children
Obstructive sleep apnea is becoming more common in children and adolescents as the prevalence of obesity increases, but it may also be a preventable risk factor for cardiovascular disease, according to a new scientific statement from the American Heart Association.
The statement focuses on the links between OSA and CVD risk factors in children and adolescents, and reviews diagnostic strategies and treatments. The writing committee reported that 1%-6% of children and adolescents have OSA, as do up to 60% of adolescents considered obese.
The statement was created by the AHA’s Atherosclerosis, Hypertension, and Obesity in the Young subcommittee of the Council on Cardiovascular Disease in the Young and was published online in the Journal of the American Heart Association.
Carissa M. Baker-Smith, MD, chair of the writing group chair and director of pediatric preventive cardiology at Nemours Cardiac Center, Alfred I. duPont Hospital for Children, Wilmington, Del., explained the rationale for issuing the statement at this time, noting that the relationship between OSA and CVD in adults is well documented.
“There has been less focus on the importance of recognizing and treating sleep apnea in youth,” she said in an interview. “Thus, we felt that it was vitally important to get the word out to parents and to providers that paying attention to the quality and duration of your child’s sleep is vitally important to a child’s long-term heart health. Risk factors for heart disease, when present in childhood, can persist into adulthood.”
Clarity on polysomnography
For making the diagnosis of OSA in children, the statement provides clarity on the use of polysomnography and the role of the apnea-hypopnea index, which is lower in children with OSA than in adults. “One controversy, or at least as I saw it, was whether or not polysomnography testing is always required to make the diagnosis of OSA and before proceeding with tonsil and adenoid removal among children for whom enlarged tonsils and adenoids are present,” Dr. Baker-Smith said. “Polysomnography testing is not always needed before an ear, nose, and throat surgeon may recommend surgery.”
The statement also noted that history and physical examination may not yield enough reliable information to distinguish OSA from snoring.
In areas where sleep laboratories that work with children aren’t available, alternative tests such as daytime nap polysomnography, nocturnal oximetry, and nocturnal video recording may be used – with a caveat. “These alternative tests have weaker positive and negative predictive values when compared with polysomnography,” the writing committee noted. Home sleep apnea tests aren’t recommended in children. Questionnaires “are useful as screening, but not as diagnostic tools.”
Pediatric patients being evaluated for OSA should also be screened for hypertension and metabolic syndrome, as well as central nervous system and behavioral disorders. Diagnosing OSA in children and adolescents requires “a high index of suspicion,” the committee wrote.
Pediatricians and pediatric cardiologists should exercise that high index of suspicion when receiving referrals for cardiac evaluations for attention deficit hyperactivity disorder medication, Dr. Baker-Smith said. “Take the time to ask about a child’s sleep – snoring, apnea, etc. – especially if the child has obesity, difficulty focusing during the day, and if there is evidence of systemic hypertension or other signs of metabolic syndrome,” she said.
Risk factors for OSA in children
The statement also reviewed risk factors for OSA, among them obesity, particularly among children younger than 6 years. Other risk factors include upper and lower airway disease, hypotonia, parental history of hyperplasia of the adenoids and tonsils, craniofacial malformations, and neuromuscular disorders. However, the committee cited “limited data” to support that children with congenital heart disease may be at greater risk for OSA and sleep-disordered breathing (SDB).
Black children are at significantly greater risk, and socioeconomic factors “may be potential confounders,” the committee stated. Other risk factors include allergic rhinitis and sickle cell disease.
But the statement underscores that “obesity is the main risk factor” for OSA in children and adolescents, and that the presence of increased inflammation may explain this relationship. Steroids may alleviate these symptoms, even in nonobese children, and removal of the adenoids or tonsils is an option to reduce inflammation in children with OSA.
“Obesity is a significant risk factor for sleep disturbances and obstructive sleep apnea, and the severity of sleep apnea may be improved by weight-loss interventions, which then improves metabolic syndrome factors such as insulin sensitivity,” Dr. Baker-Smith said. “We need to increase awareness about how the rising prevalence of obesity may be impacting sleep quality in kids and recognize sleep-disordered breathing as something that could contribute to risks for hypertension and later cardiovascular disease.”
Children in whom OSA is suspected should also undergo screening for metabolic syndrome, and central nervous system and behavioral disorders.
Cardiovascular risks
The statement explores the connection between cardiovascular complications and SDB and OSA in depth.
“Inadequate sleep duration of < 5 hours per night in children and adolescents has been linked to an increased risk of hypertension and is also associated with an increased prevalence of obesity,” the committee wrote.
However, the statement left one question hanging: whether OSA alone or obesity cause higher BP in younger patients with OSA. But the committee concluded that BP levels increase with the severity of OSA, although the effects can vary with age. OSA in children peaks between ages 2 and 8, corresponding to the peak prevalence of hypertrophy of the tonsils and adenoids. Children aged 10-11 with more severe OSA may have BP dysregulation, while older adolescents develop higher sustained BP. Obesity may be a confounder for daytime BP elevations, while nighttime hypertension depends less on obesity and more on OSA severity.
“OSA is associated with abnormal BP in youth and, in particular, higher nighttime blood pressures and loss of the normal decline in BP that should occur during sleep,” Dr. Baker-Smith said. “Children with OSA appear to have higher BP than controls during both sleep and wake times, and BP levels increase with increasing severity of OSA.”
Nonetheless, children with OSA are at greater risk for other cardiovascular problems. Left ventricular hypertrophy may be a secondary outcome. “The presence of obstructive sleep apnea in children is associated with an 11-fold increased risk for LVH in children, a relationship not seen in the presence of primary snoring alone,” Dr. Baker-Smith said.
Dr. Baker-Smith had no relevant disclosures. Coauthor Amal Isaiah, MD, is coinventor of an imaging system for sleep apnea and receives royalties from the University of Maryland. The other coauthors have no relevant financial relationships to disclose.
Obstructive sleep apnea is becoming more common in children and adolescents as the prevalence of obesity increases, but it may also be a preventable risk factor for cardiovascular disease, according to a new scientific statement from the American Heart Association.
The statement focuses on the links between OSA and CVD risk factors in children and adolescents, and reviews diagnostic strategies and treatments. The writing committee reported that 1%-6% of children and adolescents have OSA, as do up to 60% of adolescents considered obese.
The statement was created by the AHA’s Atherosclerosis, Hypertension, and Obesity in the Young subcommittee of the Council on Cardiovascular Disease in the Young and was published online in the Journal of the American Heart Association.
Carissa M. Baker-Smith, MD, chair of the writing group chair and director of pediatric preventive cardiology at Nemours Cardiac Center, Alfred I. duPont Hospital for Children, Wilmington, Del., explained the rationale for issuing the statement at this time, noting that the relationship between OSA and CVD in adults is well documented.
“There has been less focus on the importance of recognizing and treating sleep apnea in youth,” she said in an interview. “Thus, we felt that it was vitally important to get the word out to parents and to providers that paying attention to the quality and duration of your child’s sleep is vitally important to a child’s long-term heart health. Risk factors for heart disease, when present in childhood, can persist into adulthood.”
Clarity on polysomnography
For making the diagnosis of OSA in children, the statement provides clarity on the use of polysomnography and the role of the apnea-hypopnea index, which is lower in children with OSA than in adults. “One controversy, or at least as I saw it, was whether or not polysomnography testing is always required to make the diagnosis of OSA and before proceeding with tonsil and adenoid removal among children for whom enlarged tonsils and adenoids are present,” Dr. Baker-Smith said. “Polysomnography testing is not always needed before an ear, nose, and throat surgeon may recommend surgery.”
The statement also noted that history and physical examination may not yield enough reliable information to distinguish OSA from snoring.
In areas where sleep laboratories that work with children aren’t available, alternative tests such as daytime nap polysomnography, nocturnal oximetry, and nocturnal video recording may be used – with a caveat. “These alternative tests have weaker positive and negative predictive values when compared with polysomnography,” the writing committee noted. Home sleep apnea tests aren’t recommended in children. Questionnaires “are useful as screening, but not as diagnostic tools.”
Pediatric patients being evaluated for OSA should also be screened for hypertension and metabolic syndrome, as well as central nervous system and behavioral disorders. Diagnosing OSA in children and adolescents requires “a high index of suspicion,” the committee wrote.
Pediatricians and pediatric cardiologists should exercise that high index of suspicion when receiving referrals for cardiac evaluations for attention deficit hyperactivity disorder medication, Dr. Baker-Smith said. “Take the time to ask about a child’s sleep – snoring, apnea, etc. – especially if the child has obesity, difficulty focusing during the day, and if there is evidence of systemic hypertension or other signs of metabolic syndrome,” she said.
Risk factors for OSA in children
The statement also reviewed risk factors for OSA, among them obesity, particularly among children younger than 6 years. Other risk factors include upper and lower airway disease, hypotonia, parental history of hyperplasia of the adenoids and tonsils, craniofacial malformations, and neuromuscular disorders. However, the committee cited “limited data” to support that children with congenital heart disease may be at greater risk for OSA and sleep-disordered breathing (SDB).
Black children are at significantly greater risk, and socioeconomic factors “may be potential confounders,” the committee stated. Other risk factors include allergic rhinitis and sickle cell disease.
But the statement underscores that “obesity is the main risk factor” for OSA in children and adolescents, and that the presence of increased inflammation may explain this relationship. Steroids may alleviate these symptoms, even in nonobese children, and removal of the adenoids or tonsils is an option to reduce inflammation in children with OSA.
“Obesity is a significant risk factor for sleep disturbances and obstructive sleep apnea, and the severity of sleep apnea may be improved by weight-loss interventions, which then improves metabolic syndrome factors such as insulin sensitivity,” Dr. Baker-Smith said. “We need to increase awareness about how the rising prevalence of obesity may be impacting sleep quality in kids and recognize sleep-disordered breathing as something that could contribute to risks for hypertension and later cardiovascular disease.”
Children in whom OSA is suspected should also undergo screening for metabolic syndrome, and central nervous system and behavioral disorders.
Cardiovascular risks
The statement explores the connection between cardiovascular complications and SDB and OSA in depth.
“Inadequate sleep duration of < 5 hours per night in children and adolescents has been linked to an increased risk of hypertension and is also associated with an increased prevalence of obesity,” the committee wrote.
However, the statement left one question hanging: whether OSA alone or obesity cause higher BP in younger patients with OSA. But the committee concluded that BP levels increase with the severity of OSA, although the effects can vary with age. OSA in children peaks between ages 2 and 8, corresponding to the peak prevalence of hypertrophy of the tonsils and adenoids. Children aged 10-11 with more severe OSA may have BP dysregulation, while older adolescents develop higher sustained BP. Obesity may be a confounder for daytime BP elevations, while nighttime hypertension depends less on obesity and more on OSA severity.
“OSA is associated with abnormal BP in youth and, in particular, higher nighttime blood pressures and loss of the normal decline in BP that should occur during sleep,” Dr. Baker-Smith said. “Children with OSA appear to have higher BP than controls during both sleep and wake times, and BP levels increase with increasing severity of OSA.”
Nonetheless, children with OSA are at greater risk for other cardiovascular problems. Left ventricular hypertrophy may be a secondary outcome. “The presence of obstructive sleep apnea in children is associated with an 11-fold increased risk for LVH in children, a relationship not seen in the presence of primary snoring alone,” Dr. Baker-Smith said.
Dr. Baker-Smith had no relevant disclosures. Coauthor Amal Isaiah, MD, is coinventor of an imaging system for sleep apnea and receives royalties from the University of Maryland. The other coauthors have no relevant financial relationships to disclose.
Obstructive sleep apnea is becoming more common in children and adolescents as the prevalence of obesity increases, but it may also be a preventable risk factor for cardiovascular disease, according to a new scientific statement from the American Heart Association.
The statement focuses on the links between OSA and CVD risk factors in children and adolescents, and reviews diagnostic strategies and treatments. The writing committee reported that 1%-6% of children and adolescents have OSA, as do up to 60% of adolescents considered obese.
The statement was created by the AHA’s Atherosclerosis, Hypertension, and Obesity in the Young subcommittee of the Council on Cardiovascular Disease in the Young and was published online in the Journal of the American Heart Association.
Carissa M. Baker-Smith, MD, chair of the writing group chair and director of pediatric preventive cardiology at Nemours Cardiac Center, Alfred I. duPont Hospital for Children, Wilmington, Del., explained the rationale for issuing the statement at this time, noting that the relationship between OSA and CVD in adults is well documented.
“There has been less focus on the importance of recognizing and treating sleep apnea in youth,” she said in an interview. “Thus, we felt that it was vitally important to get the word out to parents and to providers that paying attention to the quality and duration of your child’s sleep is vitally important to a child’s long-term heart health. Risk factors for heart disease, when present in childhood, can persist into adulthood.”
Clarity on polysomnography
For making the diagnosis of OSA in children, the statement provides clarity on the use of polysomnography and the role of the apnea-hypopnea index, which is lower in children with OSA than in adults. “One controversy, or at least as I saw it, was whether or not polysomnography testing is always required to make the diagnosis of OSA and before proceeding with tonsil and adenoid removal among children for whom enlarged tonsils and adenoids are present,” Dr. Baker-Smith said. “Polysomnography testing is not always needed before an ear, nose, and throat surgeon may recommend surgery.”
The statement also noted that history and physical examination may not yield enough reliable information to distinguish OSA from snoring.
In areas where sleep laboratories that work with children aren’t available, alternative tests such as daytime nap polysomnography, nocturnal oximetry, and nocturnal video recording may be used – with a caveat. “These alternative tests have weaker positive and negative predictive values when compared with polysomnography,” the writing committee noted. Home sleep apnea tests aren’t recommended in children. Questionnaires “are useful as screening, but not as diagnostic tools.”
Pediatric patients being evaluated for OSA should also be screened for hypertension and metabolic syndrome, as well as central nervous system and behavioral disorders. Diagnosing OSA in children and adolescents requires “a high index of suspicion,” the committee wrote.
Pediatricians and pediatric cardiologists should exercise that high index of suspicion when receiving referrals for cardiac evaluations for attention deficit hyperactivity disorder medication, Dr. Baker-Smith said. “Take the time to ask about a child’s sleep – snoring, apnea, etc. – especially if the child has obesity, difficulty focusing during the day, and if there is evidence of systemic hypertension or other signs of metabolic syndrome,” she said.
Risk factors for OSA in children
The statement also reviewed risk factors for OSA, among them obesity, particularly among children younger than 6 years. Other risk factors include upper and lower airway disease, hypotonia, parental history of hyperplasia of the adenoids and tonsils, craniofacial malformations, and neuromuscular disorders. However, the committee cited “limited data” to support that children with congenital heart disease may be at greater risk for OSA and sleep-disordered breathing (SDB).
Black children are at significantly greater risk, and socioeconomic factors “may be potential confounders,” the committee stated. Other risk factors include allergic rhinitis and sickle cell disease.
But the statement underscores that “obesity is the main risk factor” for OSA in children and adolescents, and that the presence of increased inflammation may explain this relationship. Steroids may alleviate these symptoms, even in nonobese children, and removal of the adenoids or tonsils is an option to reduce inflammation in children with OSA.
“Obesity is a significant risk factor for sleep disturbances and obstructive sleep apnea, and the severity of sleep apnea may be improved by weight-loss interventions, which then improves metabolic syndrome factors such as insulin sensitivity,” Dr. Baker-Smith said. “We need to increase awareness about how the rising prevalence of obesity may be impacting sleep quality in kids and recognize sleep-disordered breathing as something that could contribute to risks for hypertension and later cardiovascular disease.”
Children in whom OSA is suspected should also undergo screening for metabolic syndrome, and central nervous system and behavioral disorders.
Cardiovascular risks
The statement explores the connection between cardiovascular complications and SDB and OSA in depth.
“Inadequate sleep duration of < 5 hours per night in children and adolescents has been linked to an increased risk of hypertension and is also associated with an increased prevalence of obesity,” the committee wrote.
However, the statement left one question hanging: whether OSA alone or obesity cause higher BP in younger patients with OSA. But the committee concluded that BP levels increase with the severity of OSA, although the effects can vary with age. OSA in children peaks between ages 2 and 8, corresponding to the peak prevalence of hypertrophy of the tonsils and adenoids. Children aged 10-11 with more severe OSA may have BP dysregulation, while older adolescents develop higher sustained BP. Obesity may be a confounder for daytime BP elevations, while nighttime hypertension depends less on obesity and more on OSA severity.
“OSA is associated with abnormal BP in youth and, in particular, higher nighttime blood pressures and loss of the normal decline in BP that should occur during sleep,” Dr. Baker-Smith said. “Children with OSA appear to have higher BP than controls during both sleep and wake times, and BP levels increase with increasing severity of OSA.”
Nonetheless, children with OSA are at greater risk for other cardiovascular problems. Left ventricular hypertrophy may be a secondary outcome. “The presence of obstructive sleep apnea in children is associated with an 11-fold increased risk for LVH in children, a relationship not seen in the presence of primary snoring alone,” Dr. Baker-Smith said.
Dr. Baker-Smith had no relevant disclosures. Coauthor Amal Isaiah, MD, is coinventor of an imaging system for sleep apnea and receives royalties from the University of Maryland. The other coauthors have no relevant financial relationships to disclose.
FROM JOURNAL OF THE AMERICAN HEART ASSOCIATION
Not so fast food
As long as I can remember, children have been notoriously wasteful when dining in school cafeterias. Even those children who bring their own food often return home in the afternoon with their lunches half eaten. Not surprisingly, the food tossed out is often the healthier portion of the meal. Schools have tried a variety of strategies to curb this wastage, including using volunteer student monitors to police and encourage ecologically based recycling.
The authors of a recent study published on JAMA Network Open observed that when elementary and middle-school students were allowed a 20-minute seated lunch period they consumed more food and there was significantly less waste of fruits and vegetable compared with when the students’ lunch period was limited to 10 minutes. Interestingly, there was no difference in the beverage and entrée consumption when the lunch period was doubled.
The authors postulate that younger children may not have acquired the dexterity to feed themselves optimally in the shorter lunch period. I’m not sure I buy that argument. It may be simply that the children ate and drank their favorites first and needed a bit more time to allow their little guts to move things along. But, regardless of the explanation, the investigators’ observations deserve further study.
When I was in high school our lunch period was a full hour, which allowed me to make the half mile walk to home and back to eat a home-prepared meal. The noon hour was when school clubs and committees met and there was a full schedule of diversions to fill out the hour. I don’t recall the seated portion of the lunch period having any time restriction.
By the time my own children were in middle school, lunch periods lasted no longer than 20 minutes. I was not surprised to learn from this recent study that in some schools the seated lunch period has been shortened to 10 minutes. In some cases the truncated lunch periods are a response to space and time limitations. I fear that occasionally, educators and administrators have found it so difficult to keep young children who are accustomed to watching television while they eat engaged that the periods have been shortened to minimize the chaos.
Here in Maine, the governor has just announced plans to offer free breakfast and lunch to every student in response to a federal initiative. If we intend to make nutrition a cornerstone of the educational process this study from the University of Illinois at Urbana-Champaign suggests that we must do more than simply provide the food at no cost. We must somehow carve out more time in the day for the children to eat a healthy diet.
But, where is this time going to come from? Many school systems have already cannibalized physical education to the point that most children are not getting a healthy amount of exercise. It is unfortunate that we have come to expect public school systems to solve all of our societal ills and compensate for less-than-healthy home environments. But that is the reality. If we think nutrition and physical activity are important components of our children’s educations then we must make the time necessary to provide them.
Will this mean longer school days? And will those longer days cost money? You bet they will, but that may be the price we have to pay for healthier, better educated children.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
As long as I can remember, children have been notoriously wasteful when dining in school cafeterias. Even those children who bring their own food often return home in the afternoon with their lunches half eaten. Not surprisingly, the food tossed out is often the healthier portion of the meal. Schools have tried a variety of strategies to curb this wastage, including using volunteer student monitors to police and encourage ecologically based recycling.
The authors of a recent study published on JAMA Network Open observed that when elementary and middle-school students were allowed a 20-minute seated lunch period they consumed more food and there was significantly less waste of fruits and vegetable compared with when the students’ lunch period was limited to 10 minutes. Interestingly, there was no difference in the beverage and entrée consumption when the lunch period was doubled.
The authors postulate that younger children may not have acquired the dexterity to feed themselves optimally in the shorter lunch period. I’m not sure I buy that argument. It may be simply that the children ate and drank their favorites first and needed a bit more time to allow their little guts to move things along. But, regardless of the explanation, the investigators’ observations deserve further study.
When I was in high school our lunch period was a full hour, which allowed me to make the half mile walk to home and back to eat a home-prepared meal. The noon hour was when school clubs and committees met and there was a full schedule of diversions to fill out the hour. I don’t recall the seated portion of the lunch period having any time restriction.
By the time my own children were in middle school, lunch periods lasted no longer than 20 minutes. I was not surprised to learn from this recent study that in some schools the seated lunch period has been shortened to 10 minutes. In some cases the truncated lunch periods are a response to space and time limitations. I fear that occasionally, educators and administrators have found it so difficult to keep young children who are accustomed to watching television while they eat engaged that the periods have been shortened to minimize the chaos.
Here in Maine, the governor has just announced plans to offer free breakfast and lunch to every student in response to a federal initiative. If we intend to make nutrition a cornerstone of the educational process this study from the University of Illinois at Urbana-Champaign suggests that we must do more than simply provide the food at no cost. We must somehow carve out more time in the day for the children to eat a healthy diet.
But, where is this time going to come from? Many school systems have already cannibalized physical education to the point that most children are not getting a healthy amount of exercise. It is unfortunate that we have come to expect public school systems to solve all of our societal ills and compensate for less-than-healthy home environments. But that is the reality. If we think nutrition and physical activity are important components of our children’s educations then we must make the time necessary to provide them.
Will this mean longer school days? And will those longer days cost money? You bet they will, but that may be the price we have to pay for healthier, better educated children.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
As long as I can remember, children have been notoriously wasteful when dining in school cafeterias. Even those children who bring their own food often return home in the afternoon with their lunches half eaten. Not surprisingly, the food tossed out is often the healthier portion of the meal. Schools have tried a variety of strategies to curb this wastage, including using volunteer student monitors to police and encourage ecologically based recycling.
The authors of a recent study published on JAMA Network Open observed that when elementary and middle-school students were allowed a 20-minute seated lunch period they consumed more food and there was significantly less waste of fruits and vegetable compared with when the students’ lunch period was limited to 10 minutes. Interestingly, there was no difference in the beverage and entrée consumption when the lunch period was doubled.
The authors postulate that younger children may not have acquired the dexterity to feed themselves optimally in the shorter lunch period. I’m not sure I buy that argument. It may be simply that the children ate and drank their favorites first and needed a bit more time to allow their little guts to move things along. But, regardless of the explanation, the investigators’ observations deserve further study.
When I was in high school our lunch period was a full hour, which allowed me to make the half mile walk to home and back to eat a home-prepared meal. The noon hour was when school clubs and committees met and there was a full schedule of diversions to fill out the hour. I don’t recall the seated portion of the lunch period having any time restriction.
By the time my own children were in middle school, lunch periods lasted no longer than 20 minutes. I was not surprised to learn from this recent study that in some schools the seated lunch period has been shortened to 10 minutes. In some cases the truncated lunch periods are a response to space and time limitations. I fear that occasionally, educators and administrators have found it so difficult to keep young children who are accustomed to watching television while they eat engaged that the periods have been shortened to minimize the chaos.
Here in Maine, the governor has just announced plans to offer free breakfast and lunch to every student in response to a federal initiative. If we intend to make nutrition a cornerstone of the educational process this study from the University of Illinois at Urbana-Champaign suggests that we must do more than simply provide the food at no cost. We must somehow carve out more time in the day for the children to eat a healthy diet.
But, where is this time going to come from? Many school systems have already cannibalized physical education to the point that most children are not getting a healthy amount of exercise. It is unfortunate that we have come to expect public school systems to solve all of our societal ills and compensate for less-than-healthy home environments. But that is the reality. If we think nutrition and physical activity are important components of our children’s educations then we must make the time necessary to provide them.
Will this mean longer school days? And will those longer days cost money? You bet they will, but that may be the price we have to pay for healthier, better educated children.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
Children and COVID: New cases soar to near-record level
Weekly cases of COVID-19 in children jumped by nearly 50% in the United States, posting the highest count since hitting a pandemic high back in mid-January, a new report shows.
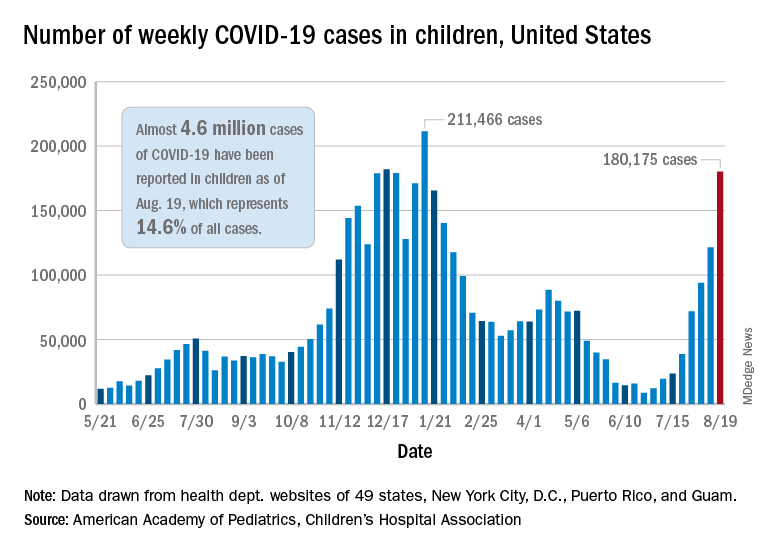
The latest weekly figure represents a 48% increase over the previous week and an increase of over 2,000% in the 8 weeks since the national count dropped to a low of 8,500 cases for the week of June 18-24, the American Academy of Pediatrics and the Children’s Hospital Association said in their weekly COVID report.
Vaccinations, in the meantime, appear to be headed in the opposite direction. Vaccine initiations were down for the second consecutive week, falling by 18% among 12- to 15-year-olds and by 15% in those aged 16-17 years, according to data from the Centers for Disease Control and Prevention.
Nationally, about 47% of children aged 12-15 and 56% of those aged 16-17 have received at least one dose of COVID vaccine as of Aug. 23, with 34% and 44%, respectively, reaching full vaccination. The total number of children with at least one dose is 11.6 million, including a relatively small number (about 200,000) of children under age 12 years, the CDC said on its COVID Data Tracker.
At the state level, vaccination is a source of considerable disparity. In Vermont, 73% of children aged 12-17 had received at least one dose by Aug. 18, and 63% were fully vaccinated. In Wyoming, however, just 25% of children had received at least one dose (17% are fully vaccinated), while Alabama has a lowest-in-the-nation full vaccination rate of 14%, based on a separate AAP analysis of CDC data.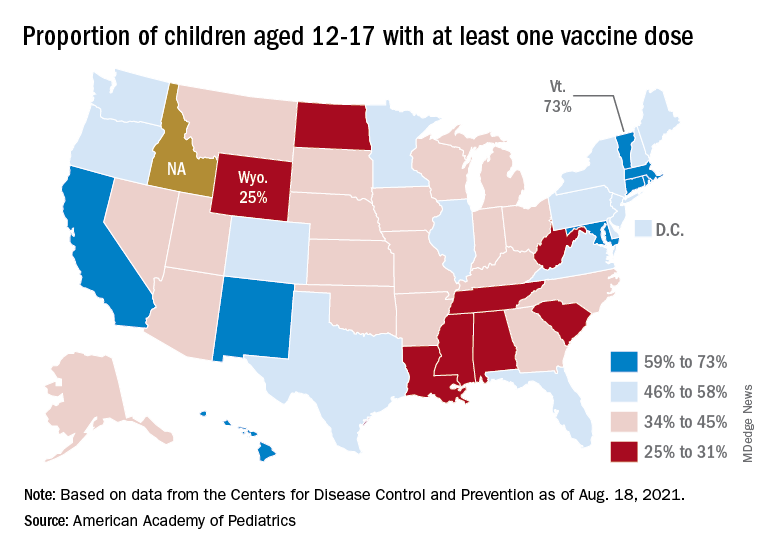
There are seven states in which over 60% of 12- to 17-year-olds have at least started the vaccine regimen and five states where less than 30% have received at least one dose, the AAP noted.
Back on the incidence side of the pandemic, Mississippi and Hawaii had the largest increases in new cases over the past 2 weeks, followed by Florida and West Virginia. Cumulative figures show that California has had the most cases overall in children (550,337), Vermont has the highest proportion of all cases in children (22.9%), and Rhode Island has the highest rate of cases per 100,000 (10,636), the AAP and CHA said in the joint report based on data from 49 states, the District of Columbia, New York City, Puerto Rico, and Guam.
Add up all those jurisdictions, and it works out to 4.6 million children infected with SARS-CoV-2 as of Aug. 19, with children representing 14.6% of all cases since the start of the pandemic. There have been over 18,000 hospitalizations so far, which is just 2.3% of the total for all ages in the 23 states (and New York City) that are reporting such data on their health department websites, the AAP and CHA said.
The number of COVID-related deaths in children is now 402 after the largest 1-week increase (24) since late May of 2020, when the AAP/CHA coverage began. Mortality data by age are available from 44 states, New York City, Puerto Rico, and Guam.
Weekly cases of COVID-19 in children jumped by nearly 50% in the United States, posting the highest count since hitting a pandemic high back in mid-January, a new report shows.

The latest weekly figure represents a 48% increase over the previous week and an increase of over 2,000% in the 8 weeks since the national count dropped to a low of 8,500 cases for the week of June 18-24, the American Academy of Pediatrics and the Children’s Hospital Association said in their weekly COVID report.
Vaccinations, in the meantime, appear to be headed in the opposite direction. Vaccine initiations were down for the second consecutive week, falling by 18% among 12- to 15-year-olds and by 15% in those aged 16-17 years, according to data from the Centers for Disease Control and Prevention.
Nationally, about 47% of children aged 12-15 and 56% of those aged 16-17 have received at least one dose of COVID vaccine as of Aug. 23, with 34% and 44%, respectively, reaching full vaccination. The total number of children with at least one dose is 11.6 million, including a relatively small number (about 200,000) of children under age 12 years, the CDC said on its COVID Data Tracker.
At the state level, vaccination is a source of considerable disparity. In Vermont, 73% of children aged 12-17 had received at least one dose by Aug. 18, and 63% were fully vaccinated. In Wyoming, however, just 25% of children had received at least one dose (17% are fully vaccinated), while Alabama has a lowest-in-the-nation full vaccination rate of 14%, based on a separate AAP analysis of CDC data.
There are seven states in which over 60% of 12- to 17-year-olds have at least started the vaccine regimen and five states where less than 30% have received at least one dose, the AAP noted.
Back on the incidence side of the pandemic, Mississippi and Hawaii had the largest increases in new cases over the past 2 weeks, followed by Florida and West Virginia. Cumulative figures show that California has had the most cases overall in children (550,337), Vermont has the highest proportion of all cases in children (22.9%), and Rhode Island has the highest rate of cases per 100,000 (10,636), the AAP and CHA said in the joint report based on data from 49 states, the District of Columbia, New York City, Puerto Rico, and Guam.
Add up all those jurisdictions, and it works out to 4.6 million children infected with SARS-CoV-2 as of Aug. 19, with children representing 14.6% of all cases since the start of the pandemic. There have been over 18,000 hospitalizations so far, which is just 2.3% of the total for all ages in the 23 states (and New York City) that are reporting such data on their health department websites, the AAP and CHA said.
The number of COVID-related deaths in children is now 402 after the largest 1-week increase (24) since late May of 2020, when the AAP/CHA coverage began. Mortality data by age are available from 44 states, New York City, Puerto Rico, and Guam.
Weekly cases of COVID-19 in children jumped by nearly 50% in the United States, posting the highest count since hitting a pandemic high back in mid-January, a new report shows.

The latest weekly figure represents a 48% increase over the previous week and an increase of over 2,000% in the 8 weeks since the national count dropped to a low of 8,500 cases for the week of June 18-24, the American Academy of Pediatrics and the Children’s Hospital Association said in their weekly COVID report.
Vaccinations, in the meantime, appear to be headed in the opposite direction. Vaccine initiations were down for the second consecutive week, falling by 18% among 12- to 15-year-olds and by 15% in those aged 16-17 years, according to data from the Centers for Disease Control and Prevention.
Nationally, about 47% of children aged 12-15 and 56% of those aged 16-17 have received at least one dose of COVID vaccine as of Aug. 23, with 34% and 44%, respectively, reaching full vaccination. The total number of children with at least one dose is 11.6 million, including a relatively small number (about 200,000) of children under age 12 years, the CDC said on its COVID Data Tracker.
At the state level, vaccination is a source of considerable disparity. In Vermont, 73% of children aged 12-17 had received at least one dose by Aug. 18, and 63% were fully vaccinated. In Wyoming, however, just 25% of children had received at least one dose (17% are fully vaccinated), while Alabama has a lowest-in-the-nation full vaccination rate of 14%, based on a separate AAP analysis of CDC data.
There are seven states in which over 60% of 12- to 17-year-olds have at least started the vaccine regimen and five states where less than 30% have received at least one dose, the AAP noted.
Back on the incidence side of the pandemic, Mississippi and Hawaii had the largest increases in new cases over the past 2 weeks, followed by Florida and West Virginia. Cumulative figures show that California has had the most cases overall in children (550,337), Vermont has the highest proportion of all cases in children (22.9%), and Rhode Island has the highest rate of cases per 100,000 (10,636), the AAP and CHA said in the joint report based on data from 49 states, the District of Columbia, New York City, Puerto Rico, and Guam.
Add up all those jurisdictions, and it works out to 4.6 million children infected with SARS-CoV-2 as of Aug. 19, with children representing 14.6% of all cases since the start of the pandemic. There have been over 18,000 hospitalizations so far, which is just 2.3% of the total for all ages in the 23 states (and New York City) that are reporting such data on their health department websites, the AAP and CHA said.
The number of COVID-related deaths in children is now 402 after the largest 1-week increase (24) since late May of 2020, when the AAP/CHA coverage began. Mortality data by age are available from 44 states, New York City, Puerto Rico, and Guam.
Prevalence of youth-onset diabetes climbing, type 2 disease more so in racial/ethnic minorities
The prevalence of youth-onset diabetes in the United States rose significantly from 2001 to 2017, with rates of type 2 diabetes climbing disproportionately among racial/ethnic minorities, according to investigators.
In individuals aged 19 years or younger, prevalence rates of type 1 and type 2 diabetes increased 45.1% and 95.3%, respectively, reported lead author Jean M. Lawrence, ScD, MPH, MSSA, program director of the division of diabetes, endocrinology, and metabolic diseases at the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, Md., and colleagues.
“Elucidating differences in diabetes prevalence trends by diabetes type and demographic characteristics is essential to describe the burden of disease and to estimate current and future resource needs,” Dr. Lawrence and colleagues wrote in JAMA.
The retrospective analysis was a part of the ongoing SEARCH study, which includes data from individuals in six areas across the United States: Colorado, California, Ohio, South Carolina, Washington state, and Arizona/New Mexico (Indian Health Services). In the present report, three prevalence years were evaluated: 2001, 2009, and 2017. For each year, approximately 3.5 million youths were included. Findings were reported in terms of diabetes type, race/ethnicity, age at diagnosis, and sex.
Absolute prevalence of type 1 diabetes per 1,000 youths increased from 1.48 in 2001, to 1.93 in 2009, and finally 2.15 in 2017. Across the 16-year period, this represents an absolute increase of 0.67 (95% confidence interval, 0.64-0.70), and a relative increase of 45.1% (95% CI, 40.0%-50.4%). In absolute terms, prevalence increased most among non-Hispanic White (0.93 per 1,000) and non-Hispanic Black (0.89 per 1,000) youths.
While type 2 diabetes was comparatively less common than type 1 diabetes, absolute prevalence per 1,000 youths increased to a greater degree, rising from 0.34 in 2001 to 0.46 in 2009 and to 0.67 in 2017. This amounts to relative increase across the period of 95.3% (95% CI, 77.0%-115.4%). Absolute increases were disproportionate among racial/ethnic minorities, particularly Black and Hispanic youths, who had absolute increases per 1,000 youths of 0.85 (95% CI, 0.74-0.97) and 0.57 (95% CI, 0.51-0.64), respectively, compared with 0.05 (95% CI, 0.03-0.07) for White youths.
“Increases [among Black and Hispanic youths] were not linear,” the investigators noted. “Hispanic youths had a significantly greater increase in the first interval compared with the second interval, while Black youths had no significant increase in the first interval and a significant increase in the second interval.”
Dr. Lawrence and colleagues offered several possible factors driving these trends in type 2 diabetes.
“Changes in anthropometric risk factors appear to play a significant role,” they wrote, noting that “Black and Mexican American teenagers experienced the greatest increase in prevalence of obesity/severe obesity from 1999 to 2018, which may contribute to race and ethnicity differences. Other contributing factors may include increases in exposure to maternal obesity and diabetes (gestational and type 2 diabetes) and exposure to environmental chemicals.”
According to Megan Kelsey, MD, associate professor of pediatric endocrinology, director of lifestyle medicine endocrinology, and medical director of the bariatric surgery center at Children’s Hospital Colorado, Aurora, the increased rates of type 2 diabetes reported by the study are alarming, yet they pale in comparison with what’s been happening since the pandemic began.
“Individual institutions have reported anywhere between a 50% – which is basically what we’re seeing at our hospital – to a 300% increase in new diagnoses [of type 2 diabetes] in a single-year time period,” Dr. Kelsey said in an interview. “So what is reported [in the present study] doesn’t even get at what’s been going on over the past year and a half.”
Dr. Kelsey offered some speculative drivers of this recent surge in cases, including stress, weight gain caused by sedentary behavior and more access to food, and the possibility that SARS-CoV-2 may infect pancreatic islet beta cells, thereby interfering with insulin production.
Type 2 diabetes is particularly concerning among young people, Dr. Kelsey noted, as it is more challenging to manage than adult-onset disease.
Young patients “also develop complications much sooner than you’d expect,” she added. “So we really need to understand why these rates are increasing, how we can identify kids at risk, and how we can better prevent it, so we aren’t stuck with a disease that’s really difficult to treat.”
To this end, the NIH recently opened applications for investigators to participate in a prospective longitudinal study of youth-onset type 2 diabetes. Young people at risk of diabetes will be followed through puberty, a period of increased risk, according to Dr. Kelsey.
“The goal will be to take kids who don’t yet have [type 2] diabetes, but are at risk, and try to better understand, as some of them progress to developing diabetes, what is going on,” Dr. Kelsey said. “What are other factors that we can use to better predict who’s going to develop diabetes? And can we use the information from this [upcoming] study to understand how to better prevent it? Because nothing that has been tried so far has worked.”
The study was supported by the Centers for Disease Control and Prevention, NIDDK, and others. The investigators and Dr. Kelsey reported no conflicts of interest.
The prevalence of youth-onset diabetes in the United States rose significantly from 2001 to 2017, with rates of type 2 diabetes climbing disproportionately among racial/ethnic minorities, according to investigators.
In individuals aged 19 years or younger, prevalence rates of type 1 and type 2 diabetes increased 45.1% and 95.3%, respectively, reported lead author Jean M. Lawrence, ScD, MPH, MSSA, program director of the division of diabetes, endocrinology, and metabolic diseases at the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, Md., and colleagues.
“Elucidating differences in diabetes prevalence trends by diabetes type and demographic characteristics is essential to describe the burden of disease and to estimate current and future resource needs,” Dr. Lawrence and colleagues wrote in JAMA.
The retrospective analysis was a part of the ongoing SEARCH study, which includes data from individuals in six areas across the United States: Colorado, California, Ohio, South Carolina, Washington state, and Arizona/New Mexico (Indian Health Services). In the present report, three prevalence years were evaluated: 2001, 2009, and 2017. For each year, approximately 3.5 million youths were included. Findings were reported in terms of diabetes type, race/ethnicity, age at diagnosis, and sex.
Absolute prevalence of type 1 diabetes per 1,000 youths increased from 1.48 in 2001, to 1.93 in 2009, and finally 2.15 in 2017. Across the 16-year period, this represents an absolute increase of 0.67 (95% confidence interval, 0.64-0.70), and a relative increase of 45.1% (95% CI, 40.0%-50.4%). In absolute terms, prevalence increased most among non-Hispanic White (0.93 per 1,000) and non-Hispanic Black (0.89 per 1,000) youths.
While type 2 diabetes was comparatively less common than type 1 diabetes, absolute prevalence per 1,000 youths increased to a greater degree, rising from 0.34 in 2001 to 0.46 in 2009 and to 0.67 in 2017. This amounts to relative increase across the period of 95.3% (95% CI, 77.0%-115.4%). Absolute increases were disproportionate among racial/ethnic minorities, particularly Black and Hispanic youths, who had absolute increases per 1,000 youths of 0.85 (95% CI, 0.74-0.97) and 0.57 (95% CI, 0.51-0.64), respectively, compared with 0.05 (95% CI, 0.03-0.07) for White youths.
“Increases [among Black and Hispanic youths] were not linear,” the investigators noted. “Hispanic youths had a significantly greater increase in the first interval compared with the second interval, while Black youths had no significant increase in the first interval and a significant increase in the second interval.”
Dr. Lawrence and colleagues offered several possible factors driving these trends in type 2 diabetes.
“Changes in anthropometric risk factors appear to play a significant role,” they wrote, noting that “Black and Mexican American teenagers experienced the greatest increase in prevalence of obesity/severe obesity from 1999 to 2018, which may contribute to race and ethnicity differences. Other contributing factors may include increases in exposure to maternal obesity and diabetes (gestational and type 2 diabetes) and exposure to environmental chemicals.”
According to Megan Kelsey, MD, associate professor of pediatric endocrinology, director of lifestyle medicine endocrinology, and medical director of the bariatric surgery center at Children’s Hospital Colorado, Aurora, the increased rates of type 2 diabetes reported by the study are alarming, yet they pale in comparison with what’s been happening since the pandemic began.
“Individual institutions have reported anywhere between a 50% – which is basically what we’re seeing at our hospital – to a 300% increase in new diagnoses [of type 2 diabetes] in a single-year time period,” Dr. Kelsey said in an interview. “So what is reported [in the present study] doesn’t even get at what’s been going on over the past year and a half.”
Dr. Kelsey offered some speculative drivers of this recent surge in cases, including stress, weight gain caused by sedentary behavior and more access to food, and the possibility that SARS-CoV-2 may infect pancreatic islet beta cells, thereby interfering with insulin production.
Type 2 diabetes is particularly concerning among young people, Dr. Kelsey noted, as it is more challenging to manage than adult-onset disease.
Young patients “also develop complications much sooner than you’d expect,” she added. “So we really need to understand why these rates are increasing, how we can identify kids at risk, and how we can better prevent it, so we aren’t stuck with a disease that’s really difficult to treat.”
To this end, the NIH recently opened applications for investigators to participate in a prospective longitudinal study of youth-onset type 2 diabetes. Young people at risk of diabetes will be followed through puberty, a period of increased risk, according to Dr. Kelsey.
“The goal will be to take kids who don’t yet have [type 2] diabetes, but are at risk, and try to better understand, as some of them progress to developing diabetes, what is going on,” Dr. Kelsey said. “What are other factors that we can use to better predict who’s going to develop diabetes? And can we use the information from this [upcoming] study to understand how to better prevent it? Because nothing that has been tried so far has worked.”
The study was supported by the Centers for Disease Control and Prevention, NIDDK, and others. The investigators and Dr. Kelsey reported no conflicts of interest.
The prevalence of youth-onset diabetes in the United States rose significantly from 2001 to 2017, with rates of type 2 diabetes climbing disproportionately among racial/ethnic minorities, according to investigators.
In individuals aged 19 years or younger, prevalence rates of type 1 and type 2 diabetes increased 45.1% and 95.3%, respectively, reported lead author Jean M. Lawrence, ScD, MPH, MSSA, program director of the division of diabetes, endocrinology, and metabolic diseases at the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, Md., and colleagues.
“Elucidating differences in diabetes prevalence trends by diabetes type and demographic characteristics is essential to describe the burden of disease and to estimate current and future resource needs,” Dr. Lawrence and colleagues wrote in JAMA.
The retrospective analysis was a part of the ongoing SEARCH study, which includes data from individuals in six areas across the United States: Colorado, California, Ohio, South Carolina, Washington state, and Arizona/New Mexico (Indian Health Services). In the present report, three prevalence years were evaluated: 2001, 2009, and 2017. For each year, approximately 3.5 million youths were included. Findings were reported in terms of diabetes type, race/ethnicity, age at diagnosis, and sex.
Absolute prevalence of type 1 diabetes per 1,000 youths increased from 1.48 in 2001, to 1.93 in 2009, and finally 2.15 in 2017. Across the 16-year period, this represents an absolute increase of 0.67 (95% confidence interval, 0.64-0.70), and a relative increase of 45.1% (95% CI, 40.0%-50.4%). In absolute terms, prevalence increased most among non-Hispanic White (0.93 per 1,000) and non-Hispanic Black (0.89 per 1,000) youths.
While type 2 diabetes was comparatively less common than type 1 diabetes, absolute prevalence per 1,000 youths increased to a greater degree, rising from 0.34 in 2001 to 0.46 in 2009 and to 0.67 in 2017. This amounts to relative increase across the period of 95.3% (95% CI, 77.0%-115.4%). Absolute increases were disproportionate among racial/ethnic minorities, particularly Black and Hispanic youths, who had absolute increases per 1,000 youths of 0.85 (95% CI, 0.74-0.97) and 0.57 (95% CI, 0.51-0.64), respectively, compared with 0.05 (95% CI, 0.03-0.07) for White youths.
“Increases [among Black and Hispanic youths] were not linear,” the investigators noted. “Hispanic youths had a significantly greater increase in the first interval compared with the second interval, while Black youths had no significant increase in the first interval and a significant increase in the second interval.”
Dr. Lawrence and colleagues offered several possible factors driving these trends in type 2 diabetes.
“Changes in anthropometric risk factors appear to play a significant role,” they wrote, noting that “Black and Mexican American teenagers experienced the greatest increase in prevalence of obesity/severe obesity from 1999 to 2018, which may contribute to race and ethnicity differences. Other contributing factors may include increases in exposure to maternal obesity and diabetes (gestational and type 2 diabetes) and exposure to environmental chemicals.”
According to Megan Kelsey, MD, associate professor of pediatric endocrinology, director of lifestyle medicine endocrinology, and medical director of the bariatric surgery center at Children’s Hospital Colorado, Aurora, the increased rates of type 2 diabetes reported by the study are alarming, yet they pale in comparison with what’s been happening since the pandemic began.
“Individual institutions have reported anywhere between a 50% – which is basically what we’re seeing at our hospital – to a 300% increase in new diagnoses [of type 2 diabetes] in a single-year time period,” Dr. Kelsey said in an interview. “So what is reported [in the present study] doesn’t even get at what’s been going on over the past year and a half.”
Dr. Kelsey offered some speculative drivers of this recent surge in cases, including stress, weight gain caused by sedentary behavior and more access to food, and the possibility that SARS-CoV-2 may infect pancreatic islet beta cells, thereby interfering with insulin production.
Type 2 diabetes is particularly concerning among young people, Dr. Kelsey noted, as it is more challenging to manage than adult-onset disease.
Young patients “also develop complications much sooner than you’d expect,” she added. “So we really need to understand why these rates are increasing, how we can identify kids at risk, and how we can better prevent it, so we aren’t stuck with a disease that’s really difficult to treat.”
To this end, the NIH recently opened applications for investigators to participate in a prospective longitudinal study of youth-onset type 2 diabetes. Young people at risk of diabetes will be followed through puberty, a period of increased risk, according to Dr. Kelsey.
“The goal will be to take kids who don’t yet have [type 2] diabetes, but are at risk, and try to better understand, as some of them progress to developing diabetes, what is going on,” Dr. Kelsey said. “What are other factors that we can use to better predict who’s going to develop diabetes? And can we use the information from this [upcoming] study to understand how to better prevent it? Because nothing that has been tried so far has worked.”
The study was supported by the Centers for Disease Control and Prevention, NIDDK, and others. The investigators and Dr. Kelsey reported no conflicts of interest.
FROM JAMA
Prevalence of high-risk HPV types dwindled since vaccine approval
Young women who received the quadrivalent human papillomavirus (HPV) vaccine had fewer and fewer infections with high-risk HPV strains covered by the vaccine year after year, but the incidence of high-risk strains that were not covered by the vaccine increased over the same 12-year period, researchers report in a study published August 23 in JAMA Open Network.
“One of the unique contributions that this study provides is the evaluation of a real-world example of the HPV infection rates following immunization in a population of adolescent girls and young adult women at a single health center in a large U.S. city, reflecting strong evidence of vaccine effectiveness,” write Nicolas F. Schlecht, PhD, a professor of oncology at Roswell Park Comprehensive Cancer Center, Buffalo, and his colleagues. “Previous surveillance studies from the U.S. have involved older women and populations with relatively low vaccine coverage.”
In addition to supporting the value of continuing to vaccinate teens against HPV, the findings underscore the importance of continuing to screen women for cervical cancer, Dr. Schlecht said in an interview.
“HPV has not and is not going away,” he said. “We need to keep on our toes with screening and other measures to continue to prevent the development of cervix cancer,” including monitoring different high-risk HPV types and keeping a close eye on cervical precancer rates, particularly CIN3 and cervix cancer, he said. “The vaccines are definitely a good thing. Just getting rid of HPV16 is an amazing accomplishment.”
Kevin Ault, MD, a professor of ob/gyn and academic specialist director of clinical and translational research at the University of Kansas, Kansas City, told this news organization that other studies have had similar findings, but this one is larger with longer follow-up.
“The take-home message is that vaccines work, and this is especially true for the HPV vaccine,” said Dr. Ault, who was not involved in the research. “The vaccine prevents HPV infections and the consequences of these infections, such as cervical cancer. The results are consistent with other studies in different settings, so they are likely generalizable.”
The researchers collected data from October 2007, shortly after the vaccine was approved, through September 2019 on sexually active adolescent and young women aged 13 to 21 years who had received the HPV vaccine and had agreed to follow-up assessments every 6 months until they turned 26. Each follow-up included the collecting of samples of cervical and anal cells for polymerase chain reaction testing for the presence of HPV types.
More than half of the 1,453 participants were Hispanic (58.8%), and half were Black (50.4%), including 15% Hispanic and Black patients. The average age of the participants was 18 years. They were tracked for a median 2.4 years. Nearly half the participants (48%) received the HPV vaccine prior to sexual debut.
For the longitudinal study, the researchers adjusted for participants’ age, the year they received the vaccine, and the years since they were vaccinated. They also tracked breakthrough infections for the four types of HPV covered by the vaccine in participants who received the vaccine before sexual debut.
“We evaluated whether infection rates for HPV have changed since the administration of the vaccine by assessing longitudinally the probability of HPV detection over time among vaccinated participants while adjusting for changes in cohort characteristics over time,” the researchers write. In their statistical analysis, they made adjustments for the number of vaccine doses participants received before their first study visit, age at sexual debut, age at first vaccine dose, number of sexual partners in the preceding 6 months, consistency of condom use during sex, history of a positive chlamydia test, and, for anal HPV analyses, whether the participants had had anal sex in the previous 6 months.
The average age at first intercourse remained steady at 15 years throughout the study, but the average age of vaccination dropped from 18 years in 2008 to 12 years in 2019 (P < .001). More than half the participants (64%) had had at least three lifetime sexual partners at baseline.
After adjustment for age, the researchers found that the incidence of the four HPV types covered by the vaccine – HPV-6, HPV-11, HPV-16, and HPV-18 – dropped more each year, shifting from 9.1% from 2008-2010 to 4.7% from 2017-2019. The effect was even greater among those vaccinated prior to sexual debut; for those patients, the incidence of the four vaccine types dropped from 8.8% to 1.7% over the course of the study. Declines over time also occurred for anal types HPV-31 (adjusted odds ratio [aOR] = 0.76) and HPV-45 (aOR = 0.77). Those vaccinated prior to any sexual intercourse had 19% lower odds of infection per year with a vaccine-covered HPV type.
“We were really excited to see that the types targeted by the vaccines were considerably lower over time in our population,” Dr. Schlecht told this news organization. “This is an important observation, since most of these types are the most worrisome for cervical cancer.”
They were surprised, however, to see overall HPV prevalence increase over time, particularly with the high-risk HPV types that were not covered by the quadrivalent vaccine.
Prevalence of cervical high-risk types not in the vaccine increased from 25.1% from 2008-2010 to 30.5% from 2017-2019. Odds of detection of high-risk HPV types not covered by the vaccine increased 8% each year, particularly for HPV-56 and HPV-68; anal HPV types increased 11% each year. Neither age nor recent number of sexual partners affected the findings.
“The underlying mechanisms for the observed increased detection of specific non-vaccine HPV types over time are not yet clear.”
“We hope this doesn’t translate into some increase in cervical neoplasia that is unanticipated,” Dr. Schlecht said. He noted that the attributable risks for cancer associated with nonvaccine high-risk HPV types remain low. “Theoretical concerns are one thing; actual data is what drives the show,” he said.
The research was funded by the National Institutes of Health and the Icahn School of Medicine at Mount Sinai, New York. Dr. Schlecht has served on advisory boards for Merck, GlaxoSmithKline (GSK), and PDS Biotechnology. One author previously served on a GSK advisory board, and another worked with Merck on an early vaccine trial. Dr. Ault has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Young women who received the quadrivalent human papillomavirus (HPV) vaccine had fewer and fewer infections with high-risk HPV strains covered by the vaccine year after year, but the incidence of high-risk strains that were not covered by the vaccine increased over the same 12-year period, researchers report in a study published August 23 in JAMA Open Network.
“One of the unique contributions that this study provides is the evaluation of a real-world example of the HPV infection rates following immunization in a population of adolescent girls and young adult women at a single health center in a large U.S. city, reflecting strong evidence of vaccine effectiveness,” write Nicolas F. Schlecht, PhD, a professor of oncology at Roswell Park Comprehensive Cancer Center, Buffalo, and his colleagues. “Previous surveillance studies from the U.S. have involved older women and populations with relatively low vaccine coverage.”
In addition to supporting the value of continuing to vaccinate teens against HPV, the findings underscore the importance of continuing to screen women for cervical cancer, Dr. Schlecht said in an interview.
“HPV has not and is not going away,” he said. “We need to keep on our toes with screening and other measures to continue to prevent the development of cervix cancer,” including monitoring different high-risk HPV types and keeping a close eye on cervical precancer rates, particularly CIN3 and cervix cancer, he said. “The vaccines are definitely a good thing. Just getting rid of HPV16 is an amazing accomplishment.”
Kevin Ault, MD, a professor of ob/gyn and academic specialist director of clinical and translational research at the University of Kansas, Kansas City, told this news organization that other studies have had similar findings, but this one is larger with longer follow-up.
“The take-home message is that vaccines work, and this is especially true for the HPV vaccine,” said Dr. Ault, who was not involved in the research. “The vaccine prevents HPV infections and the consequences of these infections, such as cervical cancer. The results are consistent with other studies in different settings, so they are likely generalizable.”
The researchers collected data from October 2007, shortly after the vaccine was approved, through September 2019 on sexually active adolescent and young women aged 13 to 21 years who had received the HPV vaccine and had agreed to follow-up assessments every 6 months until they turned 26. Each follow-up included the collecting of samples of cervical and anal cells for polymerase chain reaction testing for the presence of HPV types.
More than half of the 1,453 participants were Hispanic (58.8%), and half were Black (50.4%), including 15% Hispanic and Black patients. The average age of the participants was 18 years. They were tracked for a median 2.4 years. Nearly half the participants (48%) received the HPV vaccine prior to sexual debut.
For the longitudinal study, the researchers adjusted for participants’ age, the year they received the vaccine, and the years since they were vaccinated. They also tracked breakthrough infections for the four types of HPV covered by the vaccine in participants who received the vaccine before sexual debut.
“We evaluated whether infection rates for HPV have changed since the administration of the vaccine by assessing longitudinally the probability of HPV detection over time among vaccinated participants while adjusting for changes in cohort characteristics over time,” the researchers write. In their statistical analysis, they made adjustments for the number of vaccine doses participants received before their first study visit, age at sexual debut, age at first vaccine dose, number of sexual partners in the preceding 6 months, consistency of condom use during sex, history of a positive chlamydia test, and, for anal HPV analyses, whether the participants had had anal sex in the previous 6 months.
The average age at first intercourse remained steady at 15 years throughout the study, but the average age of vaccination dropped from 18 years in 2008 to 12 years in 2019 (P < .001). More than half the participants (64%) had had at least three lifetime sexual partners at baseline.
After adjustment for age, the researchers found that the incidence of the four HPV types covered by the vaccine – HPV-6, HPV-11, HPV-16, and HPV-18 – dropped more each year, shifting from 9.1% from 2008-2010 to 4.7% from 2017-2019. The effect was even greater among those vaccinated prior to sexual debut; for those patients, the incidence of the four vaccine types dropped from 8.8% to 1.7% over the course of the study. Declines over time also occurred for anal types HPV-31 (adjusted odds ratio [aOR] = 0.76) and HPV-45 (aOR = 0.77). Those vaccinated prior to any sexual intercourse had 19% lower odds of infection per year with a vaccine-covered HPV type.
“We were really excited to see that the types targeted by the vaccines were considerably lower over time in our population,” Dr. Schlecht told this news organization. “This is an important observation, since most of these types are the most worrisome for cervical cancer.”
They were surprised, however, to see overall HPV prevalence increase over time, particularly with the high-risk HPV types that were not covered by the quadrivalent vaccine.
Prevalence of cervical high-risk types not in the vaccine increased from 25.1% from 2008-2010 to 30.5% from 2017-2019. Odds of detection of high-risk HPV types not covered by the vaccine increased 8% each year, particularly for HPV-56 and HPV-68; anal HPV types increased 11% each year. Neither age nor recent number of sexual partners affected the findings.
“The underlying mechanisms for the observed increased detection of specific non-vaccine HPV types over time are not yet clear.”
“We hope this doesn’t translate into some increase in cervical neoplasia that is unanticipated,” Dr. Schlecht said. He noted that the attributable risks for cancer associated with nonvaccine high-risk HPV types remain low. “Theoretical concerns are one thing; actual data is what drives the show,” he said.
The research was funded by the National Institutes of Health and the Icahn School of Medicine at Mount Sinai, New York. Dr. Schlecht has served on advisory boards for Merck, GlaxoSmithKline (GSK), and PDS Biotechnology. One author previously served on a GSK advisory board, and another worked with Merck on an early vaccine trial. Dr. Ault has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Young women who received the quadrivalent human papillomavirus (HPV) vaccine had fewer and fewer infections with high-risk HPV strains covered by the vaccine year after year, but the incidence of high-risk strains that were not covered by the vaccine increased over the same 12-year period, researchers report in a study published August 23 in JAMA Open Network.
“One of the unique contributions that this study provides is the evaluation of a real-world example of the HPV infection rates following immunization in a population of adolescent girls and young adult women at a single health center in a large U.S. city, reflecting strong evidence of vaccine effectiveness,” write Nicolas F. Schlecht, PhD, a professor of oncology at Roswell Park Comprehensive Cancer Center, Buffalo, and his colleagues. “Previous surveillance studies from the U.S. have involved older women and populations with relatively low vaccine coverage.”
In addition to supporting the value of continuing to vaccinate teens against HPV, the findings underscore the importance of continuing to screen women for cervical cancer, Dr. Schlecht said in an interview.
“HPV has not and is not going away,” he said. “We need to keep on our toes with screening and other measures to continue to prevent the development of cervix cancer,” including monitoring different high-risk HPV types and keeping a close eye on cervical precancer rates, particularly CIN3 and cervix cancer, he said. “The vaccines are definitely a good thing. Just getting rid of HPV16 is an amazing accomplishment.”
Kevin Ault, MD, a professor of ob/gyn and academic specialist director of clinical and translational research at the University of Kansas, Kansas City, told this news organization that other studies have had similar findings, but this one is larger with longer follow-up.
“The take-home message is that vaccines work, and this is especially true for the HPV vaccine,” said Dr. Ault, who was not involved in the research. “The vaccine prevents HPV infections and the consequences of these infections, such as cervical cancer. The results are consistent with other studies in different settings, so they are likely generalizable.”
The researchers collected data from October 2007, shortly after the vaccine was approved, through September 2019 on sexually active adolescent and young women aged 13 to 21 years who had received the HPV vaccine and had agreed to follow-up assessments every 6 months until they turned 26. Each follow-up included the collecting of samples of cervical and anal cells for polymerase chain reaction testing for the presence of HPV types.
More than half of the 1,453 participants were Hispanic (58.8%), and half were Black (50.4%), including 15% Hispanic and Black patients. The average age of the participants was 18 years. They were tracked for a median 2.4 years. Nearly half the participants (48%) received the HPV vaccine prior to sexual debut.
For the longitudinal study, the researchers adjusted for participants’ age, the year they received the vaccine, and the years since they were vaccinated. They also tracked breakthrough infections for the four types of HPV covered by the vaccine in participants who received the vaccine before sexual debut.
“We evaluated whether infection rates for HPV have changed since the administration of the vaccine by assessing longitudinally the probability of HPV detection over time among vaccinated participants while adjusting for changes in cohort characteristics over time,” the researchers write. In their statistical analysis, they made adjustments for the number of vaccine doses participants received before their first study visit, age at sexual debut, age at first vaccine dose, number of sexual partners in the preceding 6 months, consistency of condom use during sex, history of a positive chlamydia test, and, for anal HPV analyses, whether the participants had had anal sex in the previous 6 months.
The average age at first intercourse remained steady at 15 years throughout the study, but the average age of vaccination dropped from 18 years in 2008 to 12 years in 2019 (P < .001). More than half the participants (64%) had had at least three lifetime sexual partners at baseline.
After adjustment for age, the researchers found that the incidence of the four HPV types covered by the vaccine – HPV-6, HPV-11, HPV-16, and HPV-18 – dropped more each year, shifting from 9.1% from 2008-2010 to 4.7% from 2017-2019. The effect was even greater among those vaccinated prior to sexual debut; for those patients, the incidence of the four vaccine types dropped from 8.8% to 1.7% over the course of the study. Declines over time also occurred for anal types HPV-31 (adjusted odds ratio [aOR] = 0.76) and HPV-45 (aOR = 0.77). Those vaccinated prior to any sexual intercourse had 19% lower odds of infection per year with a vaccine-covered HPV type.
“We were really excited to see that the types targeted by the vaccines were considerably lower over time in our population,” Dr. Schlecht told this news organization. “This is an important observation, since most of these types are the most worrisome for cervical cancer.”
They were surprised, however, to see overall HPV prevalence increase over time, particularly with the high-risk HPV types that were not covered by the quadrivalent vaccine.
Prevalence of cervical high-risk types not in the vaccine increased from 25.1% from 2008-2010 to 30.5% from 2017-2019. Odds of detection of high-risk HPV types not covered by the vaccine increased 8% each year, particularly for HPV-56 and HPV-68; anal HPV types increased 11% each year. Neither age nor recent number of sexual partners affected the findings.
“The underlying mechanisms for the observed increased detection of specific non-vaccine HPV types over time are not yet clear.”
“We hope this doesn’t translate into some increase in cervical neoplasia that is unanticipated,” Dr. Schlecht said. He noted that the attributable risks for cancer associated with nonvaccine high-risk HPV types remain low. “Theoretical concerns are one thing; actual data is what drives the show,” he said.
The research was funded by the National Institutes of Health and the Icahn School of Medicine at Mount Sinai, New York. Dr. Schlecht has served on advisory boards for Merck, GlaxoSmithKline (GSK), and PDS Biotechnology. One author previously served on a GSK advisory board, and another worked with Merck on an early vaccine trial. Dr. Ault has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Nearly 1 in 5 parents put off care for their kids in pandemic
Many families delayed much-needed health care for their children out of fears that they may be exposed to SARS-CoV-2, according to data from the Urban Institute April 2021 Health Reform Monitoring Survey.
Data from 9,067 adults aged 18 to 64 years indicate that nearly 1 in 5 parents delayed or did not get care for their children in the past 12 months because of fear of exposure to the virus.
“It’s not surprising given the timing of the survey – April 2021 – when many people couldn’t get a vaccine yet and were reporting delayed care because of concerns about exposure during the past 30 days,” study author Dulce Gonzalez, BA, a research associate in the Health Policy Center at the Urban Institute, said in an interview.
In a previous survey that the Urban Institute conducted in September 2020, 28.8% of parents reported delaying or forgoing one or more types of health care for their children because of virus concerns or health care practitioner service limits.
These concerns still affect parents’ decision making when it comes to their child’s health. Nearly 1 in 10 parents reported that they had skipped doctor’s appointments for their children in the past 30 days. More than 1 in 10 adults forwent their own health care in the past month for the same reason.
“I think it’s important for parents to understand that health care workers and health care facilities are equipped to prevent infections from spreading,” Mundeep Kainth, DO, MPH, who was not involved in the study, told this news organization. “COVID-19 is not the first infection that we’ve seen in the medical setting, and we definitely are well aware of how it can spread and have been taking many precautions.”
The most common type of delayed or forgone care was dental care (5.3%), followed by well-child visits (4.0%) and general or specialist visits (3.2%). About 3% of parents said their child had missed out on immunizations. Nearly 6% of parents said their child had missed out on multiple types of care.
One reason dental care is the most commonly skipped type of care is because people might not consider dental care to be as urgent as other types of care, Ms. Gonzalez said. However, oral health can affect a person’s overall wellness.
Dr. Kainth, an infection disease specialist at Cohen Children’s Medical Center, New Hyde Park, New York, said the lack of immunization because of COVID-19 can have adverse health effects on children and could possibly lead to outbreaks in schools and day care settings. In the Urban Institute’s 2020 survey, 18.5% of parents said putting off their child’s health care worsened their child’s health, and 15.6% said it limited their children’s ability to go to school or day care.
“We are already concerned that we will have pockets of [vaccine-preventable] infections that we normally did not see before in communities where they are not vaccinating their children at high enough numbers,” Dr. Kainth said. “It is a little concerning that there’s probably a lot of catch up to be done for particular vaccines that are specifically for those entering day care and school.”
The current survey also found that parents with incomes below 250% of the federal poverty level were more likely than those with higher incomes to have put off care for their children in the past 30 days. More than 12% of families living in poverty put off care for their children, compared with 6.5% of those with higher incomes. They were also more likely to delay or forgo multiple types of care, at 8.1% versus 3.3%. Parents with lower family incomes were also more likely to report that their children had unmet needs for dental care, checkups, or other preventive care.
“We know that lower-income parents could be more exposed to costs they might not be able to afford if they were to get sick,” Ms. Gonzalez said. “Low-income adults have been disproportionately affected by job loss during the pandemic. They are also more likely to live in communities that have faced the largest health impacts of COVID-19.”
“There’s also advantages to the pediatrician visit that are not just about providing care but also providing guidance and advice to families and parents who are maybe struggling with certain issues that are above and beyond just the medical advice,” Dr. Kainth explained.
“That is probably the most tragic part of hearing that parents and kids are not going to the well visits, because that’s where families get a lot of support. And I think at this time, we probably need that more than ever,” she continued.
The authors said the findings highlight the importance of increasing rates of COVID-19 vaccinations among eligible adolescents and encouraging vaccinations for children younger than 12 when they become eligible, not only to protect them from COVID-19 but also to help families feel comfortable obtaining care.
The study was funded by the Robert Wood Johnson Foundation. The authors and Dr. Kainth have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Many families delayed much-needed health care for their children out of fears that they may be exposed to SARS-CoV-2, according to data from the Urban Institute April 2021 Health Reform Monitoring Survey.
Data from 9,067 adults aged 18 to 64 years indicate that nearly 1 in 5 parents delayed or did not get care for their children in the past 12 months because of fear of exposure to the virus.
“It’s not surprising given the timing of the survey – April 2021 – when many people couldn’t get a vaccine yet and were reporting delayed care because of concerns about exposure during the past 30 days,” study author Dulce Gonzalez, BA, a research associate in the Health Policy Center at the Urban Institute, said in an interview.
In a previous survey that the Urban Institute conducted in September 2020, 28.8% of parents reported delaying or forgoing one or more types of health care for their children because of virus concerns or health care practitioner service limits.
These concerns still affect parents’ decision making when it comes to their child’s health. Nearly 1 in 10 parents reported that they had skipped doctor’s appointments for their children in the past 30 days. More than 1 in 10 adults forwent their own health care in the past month for the same reason.
“I think it’s important for parents to understand that health care workers and health care facilities are equipped to prevent infections from spreading,” Mundeep Kainth, DO, MPH, who was not involved in the study, told this news organization. “COVID-19 is not the first infection that we’ve seen in the medical setting, and we definitely are well aware of how it can spread and have been taking many precautions.”
The most common type of delayed or forgone care was dental care (5.3%), followed by well-child visits (4.0%) and general or specialist visits (3.2%). About 3% of parents said their child had missed out on immunizations. Nearly 6% of parents said their child had missed out on multiple types of care.
One reason dental care is the most commonly skipped type of care is because people might not consider dental care to be as urgent as other types of care, Ms. Gonzalez said. However, oral health can affect a person’s overall wellness.
Dr. Kainth, an infection disease specialist at Cohen Children’s Medical Center, New Hyde Park, New York, said the lack of immunization because of COVID-19 can have adverse health effects on children and could possibly lead to outbreaks in schools and day care settings. In the Urban Institute’s 2020 survey, 18.5% of parents said putting off their child’s health care worsened their child’s health, and 15.6% said it limited their children’s ability to go to school or day care.
“We are already concerned that we will have pockets of [vaccine-preventable] infections that we normally did not see before in communities where they are not vaccinating their children at high enough numbers,” Dr. Kainth said. “It is a little concerning that there’s probably a lot of catch up to be done for particular vaccines that are specifically for those entering day care and school.”
The current survey also found that parents with incomes below 250% of the federal poverty level were more likely than those with higher incomes to have put off care for their children in the past 30 days. More than 12% of families living in poverty put off care for their children, compared with 6.5% of those with higher incomes. They were also more likely to delay or forgo multiple types of care, at 8.1% versus 3.3%. Parents with lower family incomes were also more likely to report that their children had unmet needs for dental care, checkups, or other preventive care.
“We know that lower-income parents could be more exposed to costs they might not be able to afford if they were to get sick,” Ms. Gonzalez said. “Low-income adults have been disproportionately affected by job loss during the pandemic. They are also more likely to live in communities that have faced the largest health impacts of COVID-19.”
“There’s also advantages to the pediatrician visit that are not just about providing care but also providing guidance and advice to families and parents who are maybe struggling with certain issues that are above and beyond just the medical advice,” Dr. Kainth explained.
“That is probably the most tragic part of hearing that parents and kids are not going to the well visits, because that’s where families get a lot of support. And I think at this time, we probably need that more than ever,” she continued.
The authors said the findings highlight the importance of increasing rates of COVID-19 vaccinations among eligible adolescents and encouraging vaccinations for children younger than 12 when they become eligible, not only to protect them from COVID-19 but also to help families feel comfortable obtaining care.
The study was funded by the Robert Wood Johnson Foundation. The authors and Dr. Kainth have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Many families delayed much-needed health care for their children out of fears that they may be exposed to SARS-CoV-2, according to data from the Urban Institute April 2021 Health Reform Monitoring Survey.
Data from 9,067 adults aged 18 to 64 years indicate that nearly 1 in 5 parents delayed or did not get care for their children in the past 12 months because of fear of exposure to the virus.
“It’s not surprising given the timing of the survey – April 2021 – when many people couldn’t get a vaccine yet and were reporting delayed care because of concerns about exposure during the past 30 days,” study author Dulce Gonzalez, BA, a research associate in the Health Policy Center at the Urban Institute, said in an interview.
In a previous survey that the Urban Institute conducted in September 2020, 28.8% of parents reported delaying or forgoing one or more types of health care for their children because of virus concerns or health care practitioner service limits.
These concerns still affect parents’ decision making when it comes to their child’s health. Nearly 1 in 10 parents reported that they had skipped doctor’s appointments for their children in the past 30 days. More than 1 in 10 adults forwent their own health care in the past month for the same reason.
“I think it’s important for parents to understand that health care workers and health care facilities are equipped to prevent infections from spreading,” Mundeep Kainth, DO, MPH, who was not involved in the study, told this news organization. “COVID-19 is not the first infection that we’ve seen in the medical setting, and we definitely are well aware of how it can spread and have been taking many precautions.”
The most common type of delayed or forgone care was dental care (5.3%), followed by well-child visits (4.0%) and general or specialist visits (3.2%). About 3% of parents said their child had missed out on immunizations. Nearly 6% of parents said their child had missed out on multiple types of care.
One reason dental care is the most commonly skipped type of care is because people might not consider dental care to be as urgent as other types of care, Ms. Gonzalez said. However, oral health can affect a person’s overall wellness.
Dr. Kainth, an infection disease specialist at Cohen Children’s Medical Center, New Hyde Park, New York, said the lack of immunization because of COVID-19 can have adverse health effects on children and could possibly lead to outbreaks in schools and day care settings. In the Urban Institute’s 2020 survey, 18.5% of parents said putting off their child’s health care worsened their child’s health, and 15.6% said it limited their children’s ability to go to school or day care.
“We are already concerned that we will have pockets of [vaccine-preventable] infections that we normally did not see before in communities where they are not vaccinating their children at high enough numbers,” Dr. Kainth said. “It is a little concerning that there’s probably a lot of catch up to be done for particular vaccines that are specifically for those entering day care and school.”
The current survey also found that parents with incomes below 250% of the federal poverty level were more likely than those with higher incomes to have put off care for their children in the past 30 days. More than 12% of families living in poverty put off care for their children, compared with 6.5% of those with higher incomes. They were also more likely to delay or forgo multiple types of care, at 8.1% versus 3.3%. Parents with lower family incomes were also more likely to report that their children had unmet needs for dental care, checkups, or other preventive care.
“We know that lower-income parents could be more exposed to costs they might not be able to afford if they were to get sick,” Ms. Gonzalez said. “Low-income adults have been disproportionately affected by job loss during the pandemic. They are also more likely to live in communities that have faced the largest health impacts of COVID-19.”
“There’s also advantages to the pediatrician visit that are not just about providing care but also providing guidance and advice to families and parents who are maybe struggling with certain issues that are above and beyond just the medical advice,” Dr. Kainth explained.
“That is probably the most tragic part of hearing that parents and kids are not going to the well visits, because that’s where families get a lot of support. And I think at this time, we probably need that more than ever,” she continued.
The authors said the findings highlight the importance of increasing rates of COVID-19 vaccinations among eligible adolescents and encouraging vaccinations for children younger than 12 when they become eligible, not only to protect them from COVID-19 but also to help families feel comfortable obtaining care.
The study was funded by the Robert Wood Johnson Foundation. The authors and Dr. Kainth have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Health care workers eager for COVID booster shots
As COVID vaccine boosters move closer to reality, most physicians and nurses are ready and willing to get another shot in the arm, according to a new Medscape survey.
Altogether, 93% of physicians and 87% of nurses/advanced practice nurses (APNs) said they wanted to get a booster, although the timing of when they wanted the shots differed somewhat between the two groups surveyed Aug. 4-15.
Among the 732 physicians polled, 50% wanted to get their shot immediately, compared with 38% of the 1,193 nurses/APNs who responded, while 44% of physicians and 50% of nurses/APNs said that they would wait until the vaccine booster was authorized and recommended.
At this point in time, almost all of the health care workers surveyed – 98% of physicians and 94% of nurses/APNs – have been fully vaccinated against COVID-19. A small proportion of each group, however, received the Johnson & Johnson vaccine (1% of physicians and 3% of nurses) and are not included in the current plan for booster shots.
The Medscape survey sample did include one group that is already eligible for a third dose: About 20% of physicians and 26% of nurses/ANPs said they have a condition or take a medication that compromises their immune system.
Respondents’ experiences with patient requests for boosters suggest a somewhat lower level of interest. About two-thirds of the health care workers (69% of physicians and 63% of nurses) said that patients frequently or sometimes asked about COVID boosters, compared with 13% (physicians) and 19% (nurses) who said their patients had never asked.
Interest lower among general population
In a separate survey conducted by WebMD, 82% of those who have been at least partially vaccinated said they want to get a COVID vaccine booster (14% immediately and 68% after authorization and recommendation). Of the remaining vaccinees, 7% said they do not want to get a booster and 11% were unsure.
The full sample of 592 respondents surveyed Aug. 5-10, however, included 19% who do not plan to get vaccinated and 6% who are planning to be vaccinated but have not yet done so.
The proportion of immunocompromised individuals in the two survey groups was similar, with about 25% of those in the WebMD survey reporting they have a condition or take a medication that compromises their immune system. Those respondents were more than twice as likely to want to get a booster immediately, compared to those with an uncompromised immune system (24% vs. 11%).
The distribution of vaccines received by brand was also comparable between the two groups surveyed. Of health care workers and readers, over half of each group received the Pfizer/BioNTech vaccine (59% vs. 54%), followed by Moderna (38% vs. 40%) and Johnson & Johnson (3% vs. 5%).
A version of this article first appeared on Medscape.com.
As COVID vaccine boosters move closer to reality, most physicians and nurses are ready and willing to get another shot in the arm, according to a new Medscape survey.
Altogether, 93% of physicians and 87% of nurses/advanced practice nurses (APNs) said they wanted to get a booster, although the timing of when they wanted the shots differed somewhat between the two groups surveyed Aug. 4-15.
Among the 732 physicians polled, 50% wanted to get their shot immediately, compared with 38% of the 1,193 nurses/APNs who responded, while 44% of physicians and 50% of nurses/APNs said that they would wait until the vaccine booster was authorized and recommended.
At this point in time, almost all of the health care workers surveyed – 98% of physicians and 94% of nurses/APNs – have been fully vaccinated against COVID-19. A small proportion of each group, however, received the Johnson & Johnson vaccine (1% of physicians and 3% of nurses) and are not included in the current plan for booster shots.
The Medscape survey sample did include one group that is already eligible for a third dose: About 20% of physicians and 26% of nurses/ANPs said they have a condition or take a medication that compromises their immune system.
Respondents’ experiences with patient requests for boosters suggest a somewhat lower level of interest. About two-thirds of the health care workers (69% of physicians and 63% of nurses) said that patients frequently or sometimes asked about COVID boosters, compared with 13% (physicians) and 19% (nurses) who said their patients had never asked.
Interest lower among general population
In a separate survey conducted by WebMD, 82% of those who have been at least partially vaccinated said they want to get a COVID vaccine booster (14% immediately and 68% after authorization and recommendation). Of the remaining vaccinees, 7% said they do not want to get a booster and 11% were unsure.
The full sample of 592 respondents surveyed Aug. 5-10, however, included 19% who do not plan to get vaccinated and 6% who are planning to be vaccinated but have not yet done so.
The proportion of immunocompromised individuals in the two survey groups was similar, with about 25% of those in the WebMD survey reporting they have a condition or take a medication that compromises their immune system. Those respondents were more than twice as likely to want to get a booster immediately, compared to those with an uncompromised immune system (24% vs. 11%).
The distribution of vaccines received by brand was also comparable between the two groups surveyed. Of health care workers and readers, over half of each group received the Pfizer/BioNTech vaccine (59% vs. 54%), followed by Moderna (38% vs. 40%) and Johnson & Johnson (3% vs. 5%).
A version of this article first appeared on Medscape.com.
As COVID vaccine boosters move closer to reality, most physicians and nurses are ready and willing to get another shot in the arm, according to a new Medscape survey.
Altogether, 93% of physicians and 87% of nurses/advanced practice nurses (APNs) said they wanted to get a booster, although the timing of when they wanted the shots differed somewhat between the two groups surveyed Aug. 4-15.
Among the 732 physicians polled, 50% wanted to get their shot immediately, compared with 38% of the 1,193 nurses/APNs who responded, while 44% of physicians and 50% of nurses/APNs said that they would wait until the vaccine booster was authorized and recommended.
At this point in time, almost all of the health care workers surveyed – 98% of physicians and 94% of nurses/APNs – have been fully vaccinated against COVID-19. A small proportion of each group, however, received the Johnson & Johnson vaccine (1% of physicians and 3% of nurses) and are not included in the current plan for booster shots.
The Medscape survey sample did include one group that is already eligible for a third dose: About 20% of physicians and 26% of nurses/ANPs said they have a condition or take a medication that compromises their immune system.
Respondents’ experiences with patient requests for boosters suggest a somewhat lower level of interest. About two-thirds of the health care workers (69% of physicians and 63% of nurses) said that patients frequently or sometimes asked about COVID boosters, compared with 13% (physicians) and 19% (nurses) who said their patients had never asked.
Interest lower among general population
In a separate survey conducted by WebMD, 82% of those who have been at least partially vaccinated said they want to get a COVID vaccine booster (14% immediately and 68% after authorization and recommendation). Of the remaining vaccinees, 7% said they do not want to get a booster and 11% were unsure.
The full sample of 592 respondents surveyed Aug. 5-10, however, included 19% who do not plan to get vaccinated and 6% who are planning to be vaccinated but have not yet done so.
The proportion of immunocompromised individuals in the two survey groups was similar, with about 25% of those in the WebMD survey reporting they have a condition or take a medication that compromises their immune system. Those respondents were more than twice as likely to want to get a booster immediately, compared to those with an uncompromised immune system (24% vs. 11%).
The distribution of vaccines received by brand was also comparable between the two groups surveyed. Of health care workers and readers, over half of each group received the Pfizer/BioNTech vaccine (59% vs. 54%), followed by Moderna (38% vs. 40%) and Johnson & Johnson (3% vs. 5%).
A version of this article first appeared on Medscape.com.
Q&A: Get flu shot early this year? Same time as COVID vaccine?
With first-time COVID-19 immunizations continuing and the plan to offer booster vaccines to most Americans starting next month, what are the considerations for getting COVID-19 and flu shots at the same time?
This news organization asked Andrew T. Pavia, MD, for his advice. He is the George and Esther Gross Presidential Professor and chief of the division of pediatric infectious diseases at the University of Utah, Salt Lake City, and a fellow of the Infectious Diseases Society of America.
Q: With COVID-19 cases surging, is it a good idea to get the flu shot early this season?
Dr. Pavia: I don’t think there is a rush to do it in August, but it is a good idea to get a flu shot this season. The consequences of getting the flu while COVID is circulating are serious.
Q: What are the implications?
There are some we know and some we don’t know. If you develop flu-like symptoms, you’re going to have to get tested. You’re going to have to stay home quite a bit longer if you get a definitive (positive COVID-19) test than you would simply with flu symptoms. Also, you’re probably going to miss work when your workplace is very stressed or your children are stressed by having COVID circulating in schools.
The part we know less about are the implications of getting the flu and COVID together. There is some reason to believe if you get them together, the illness will be more severe. We are seeing that with RSV (respiratory syncytial virus) and parainfluenza and COVID coinfections in children. They appear to be quite severe.
But for flu, we just don’t have the data yet. That’s because there really was no cocirculation of COVID and influenza with the exception of parts of China for a brief part of February and March.
Q: Will the planned administration of booster COVID-19 shots this fall affect the number of people who get the flu vaccine or how it’s distributed?
It creates a lot of logistical challenges, particularly for hospitals and other places that need to vaccinate a large number of their employees for flu and that will need to give COVID boosters at about the same time period. It also creates logistical challenges for doctors’ offices.
But we don’t know of any reason why you can’t give the two shots together.
Q: Is it possible flu season will be more severe because we isolated and wore masks, etc., last winter? Any science behind that?
The more you study flu, the less you can predict, and I’ve been studying flu for a long time. There are reasons that might suggest a severe flu season – there has been limited immunity, and some people are not wearing masks effectively and they are gathering again. Those are things we believe protected us from influenza last season.
But we have not seen flu emerge yet. Normally we look to Australia, New Zealand, and South Africa during their winter – which is our summer – to get some idea of what is over the horizon for the Northern Hemisphere. Flu activity in Australia has been very modest this year.
That might mean flu may not show up for a while, but I would be loathe to make a prediction.
Q: What are the chances we’ll see a flu outbreak like we’re seeing with RSV, which is normally a winter illness?
The fact that we had a summer RSV surge just gives you an idea of how the normal epidemiology of viral infections has been disrupted. It means anything could happen with influenza. It could show up late summer or fall or wait until next spring.
We really don’t understand how those interactions work. When a new flu strain emerges, it often ignores the traditional behavior and shows up in the spring or fall. It happened in the 2009 pandemic, it happened in 1918.
The one thing I would safely predict about the next flu wave is that it will surprise us.
Q: Are you hopeful that combination vaccines in development from a number of companies, such as Moderna, Novavax, and Vivaldi, will be effective?
It is beginning to look like COVID will be with us for the foreseeable future – maybe as a seasonal virus or maybe as an ongoing pandemic. We are going to need to protect (ourselves) simultaneously against the flu and COVID. A single shot is a great way to do that – nobody wants two needles; nobody wants two trips to get vaccinated.
An effective combination vaccine would be a really great tool.
We have to wait to see what the science shows us, because they are quite different viruses. We won’t know if a combination vaccine works well and has acceptable side effects until we do those studies.
Q. Do you know at this point whether the side effects from two vaccines would be additive? Is there any way to predict that?
There is no way to predict. There are so many things that go into whether someone has side effects that we don’t understand. With fairly reactogenic vaccines like the mRNA vaccines, lots of people have no side effects whatsoever and others are really uncomfortable for 24 hours.
Flu is generally a better tolerated vaccine. There are still people who get muscle aches and very sore arms. I don’t think we can predict if getting two will be additive or just the same as getting one vaccine.
Q: Other than convenience and the benefit for people who are needle-phobic, are there any other advantages of combining them into one shot?
The logistics alone are enough to justify having one effective product if we can make one. It should reduce the overall cost of administration and reduce time off from work.
The combination vaccines given by pediatricians have been very successful. They reduce the number of needles for kids and make it much easier for parents and the pediatricians administering them. The same principle should apply to adults, who sometimes are less brave about needles than kids are.
Historically, combined vaccines in general have worked as well as vaccines given alone, but there have been exceptions. We just have to see what the products look like.
Q: For now, the flu vaccine and COVID-19 vaccine are single products. If you get them separately, is it better to put some time between the two?
We don’t know. There are studies that probably won’t be out in time to decide in September. They are looking at whether you get an equivalent immune response if you give them together or apart.
For now, I would say the advantage of getting them together is if you do get side effects, you’ll only get them once – one day to suffer through them. Also, it’s one trip to the doctor.
The potential advantage of separating them is that is how we developed and tested the vaccines. If you do react to them, side effects could be milder, but it will be on two separate days.
I would recommend doing whatever works so that you get both vaccines in a timely manner.
I’m going to get my flu shot as soon as it’s available. If I’m due for a COVID booster at that time, I would probably do them together.
Q: Do you foresee a point in the future when the predominant strain of SARS-CoV-2 will be one of the components of a flu vaccine, like we did in the past with H1N1, etc?
It really remains to be seen, but it is very conceivable it could happen. The same companies that developed COVID-19 vaccines are working on flu vaccines.
Q: Any other advice for people concerned about getting immunized against both COVID-19 and influenza in the coming months?
There is no side effect of the vaccine that begins to approach the risk you face from either disease. It’s really one of the best things you can do to protect yourself is to get vaccinated.
In the case of flu, the vaccine is only modestly effective, but it still saves tens of thousands of lives each year. The SARS-CoV-2 vaccine is a much better vaccine and a deadlier disease.
Dr. Pavia consulted for GlaxoSmithKline on influenza testing.
A version of this article first appeared on Medscape.com.
With first-time COVID-19 immunizations continuing and the plan to offer booster vaccines to most Americans starting next month, what are the considerations for getting COVID-19 and flu shots at the same time?
This news organization asked Andrew T. Pavia, MD, for his advice. He is the George and Esther Gross Presidential Professor and chief of the division of pediatric infectious diseases at the University of Utah, Salt Lake City, and a fellow of the Infectious Diseases Society of America.
Q: With COVID-19 cases surging, is it a good idea to get the flu shot early this season?
Dr. Pavia: I don’t think there is a rush to do it in August, but it is a good idea to get a flu shot this season. The consequences of getting the flu while COVID is circulating are serious.
Q: What are the implications?
There are some we know and some we don’t know. If you develop flu-like symptoms, you’re going to have to get tested. You’re going to have to stay home quite a bit longer if you get a definitive (positive COVID-19) test than you would simply with flu symptoms. Also, you’re probably going to miss work when your workplace is very stressed or your children are stressed by having COVID circulating in schools.
The part we know less about are the implications of getting the flu and COVID together. There is some reason to believe if you get them together, the illness will be more severe. We are seeing that with RSV (respiratory syncytial virus) and parainfluenza and COVID coinfections in children. They appear to be quite severe.
But for flu, we just don’t have the data yet. That’s because there really was no cocirculation of COVID and influenza with the exception of parts of China for a brief part of February and March.
Q: Will the planned administration of booster COVID-19 shots this fall affect the number of people who get the flu vaccine or how it’s distributed?
It creates a lot of logistical challenges, particularly for hospitals and other places that need to vaccinate a large number of their employees for flu and that will need to give COVID boosters at about the same time period. It also creates logistical challenges for doctors’ offices.
But we don’t know of any reason why you can’t give the two shots together.
Q: Is it possible flu season will be more severe because we isolated and wore masks, etc., last winter? Any science behind that?
The more you study flu, the less you can predict, and I’ve been studying flu for a long time. There are reasons that might suggest a severe flu season – there has been limited immunity, and some people are not wearing masks effectively and they are gathering again. Those are things we believe protected us from influenza last season.
But we have not seen flu emerge yet. Normally we look to Australia, New Zealand, and South Africa during their winter – which is our summer – to get some idea of what is over the horizon for the Northern Hemisphere. Flu activity in Australia has been very modest this year.
That might mean flu may not show up for a while, but I would be loathe to make a prediction.
Q: What are the chances we’ll see a flu outbreak like we’re seeing with RSV, which is normally a winter illness?
The fact that we had a summer RSV surge just gives you an idea of how the normal epidemiology of viral infections has been disrupted. It means anything could happen with influenza. It could show up late summer or fall or wait until next spring.
We really don’t understand how those interactions work. When a new flu strain emerges, it often ignores the traditional behavior and shows up in the spring or fall. It happened in the 2009 pandemic, it happened in 1918.
The one thing I would safely predict about the next flu wave is that it will surprise us.
Q: Are you hopeful that combination vaccines in development from a number of companies, such as Moderna, Novavax, and Vivaldi, will be effective?
It is beginning to look like COVID will be with us for the foreseeable future – maybe as a seasonal virus or maybe as an ongoing pandemic. We are going to need to protect (ourselves) simultaneously against the flu and COVID. A single shot is a great way to do that – nobody wants two needles; nobody wants two trips to get vaccinated.
An effective combination vaccine would be a really great tool.
We have to wait to see what the science shows us, because they are quite different viruses. We won’t know if a combination vaccine works well and has acceptable side effects until we do those studies.
Q. Do you know at this point whether the side effects from two vaccines would be additive? Is there any way to predict that?
There is no way to predict. There are so many things that go into whether someone has side effects that we don’t understand. With fairly reactogenic vaccines like the mRNA vaccines, lots of people have no side effects whatsoever and others are really uncomfortable for 24 hours.
Flu is generally a better tolerated vaccine. There are still people who get muscle aches and very sore arms. I don’t think we can predict if getting two will be additive or just the same as getting one vaccine.
Q: Other than convenience and the benefit for people who are needle-phobic, are there any other advantages of combining them into one shot?
The logistics alone are enough to justify having one effective product if we can make one. It should reduce the overall cost of administration and reduce time off from work.
The combination vaccines given by pediatricians have been very successful. They reduce the number of needles for kids and make it much easier for parents and the pediatricians administering them. The same principle should apply to adults, who sometimes are less brave about needles than kids are.
Historically, combined vaccines in general have worked as well as vaccines given alone, but there have been exceptions. We just have to see what the products look like.
Q: For now, the flu vaccine and COVID-19 vaccine are single products. If you get them separately, is it better to put some time between the two?
We don’t know. There are studies that probably won’t be out in time to decide in September. They are looking at whether you get an equivalent immune response if you give them together or apart.
For now, I would say the advantage of getting them together is if you do get side effects, you’ll only get them once – one day to suffer through them. Also, it’s one trip to the doctor.
The potential advantage of separating them is that is how we developed and tested the vaccines. If you do react to them, side effects could be milder, but it will be on two separate days.
I would recommend doing whatever works so that you get both vaccines in a timely manner.
I’m going to get my flu shot as soon as it’s available. If I’m due for a COVID booster at that time, I would probably do them together.
Q: Do you foresee a point in the future when the predominant strain of SARS-CoV-2 will be one of the components of a flu vaccine, like we did in the past with H1N1, etc?
It really remains to be seen, but it is very conceivable it could happen. The same companies that developed COVID-19 vaccines are working on flu vaccines.
Q: Any other advice for people concerned about getting immunized against both COVID-19 and influenza in the coming months?
There is no side effect of the vaccine that begins to approach the risk you face from either disease. It’s really one of the best things you can do to protect yourself is to get vaccinated.
In the case of flu, the vaccine is only modestly effective, but it still saves tens of thousands of lives each year. The SARS-CoV-2 vaccine is a much better vaccine and a deadlier disease.
Dr. Pavia consulted for GlaxoSmithKline on influenza testing.
A version of this article first appeared on Medscape.com.
With first-time COVID-19 immunizations continuing and the plan to offer booster vaccines to most Americans starting next month, what are the considerations for getting COVID-19 and flu shots at the same time?
This news organization asked Andrew T. Pavia, MD, for his advice. He is the George and Esther Gross Presidential Professor and chief of the division of pediatric infectious diseases at the University of Utah, Salt Lake City, and a fellow of the Infectious Diseases Society of America.
Q: With COVID-19 cases surging, is it a good idea to get the flu shot early this season?
Dr. Pavia: I don’t think there is a rush to do it in August, but it is a good idea to get a flu shot this season. The consequences of getting the flu while COVID is circulating are serious.
Q: What are the implications?
There are some we know and some we don’t know. If you develop flu-like symptoms, you’re going to have to get tested. You’re going to have to stay home quite a bit longer if you get a definitive (positive COVID-19) test than you would simply with flu symptoms. Also, you’re probably going to miss work when your workplace is very stressed or your children are stressed by having COVID circulating in schools.
The part we know less about are the implications of getting the flu and COVID together. There is some reason to believe if you get them together, the illness will be more severe. We are seeing that with RSV (respiratory syncytial virus) and parainfluenza and COVID coinfections in children. They appear to be quite severe.
But for flu, we just don’t have the data yet. That’s because there really was no cocirculation of COVID and influenza with the exception of parts of China for a brief part of February and March.
Q: Will the planned administration of booster COVID-19 shots this fall affect the number of people who get the flu vaccine or how it’s distributed?
It creates a lot of logistical challenges, particularly for hospitals and other places that need to vaccinate a large number of their employees for flu and that will need to give COVID boosters at about the same time period. It also creates logistical challenges for doctors’ offices.
But we don’t know of any reason why you can’t give the two shots together.
Q: Is it possible flu season will be more severe because we isolated and wore masks, etc., last winter? Any science behind that?
The more you study flu, the less you can predict, and I’ve been studying flu for a long time. There are reasons that might suggest a severe flu season – there has been limited immunity, and some people are not wearing masks effectively and they are gathering again. Those are things we believe protected us from influenza last season.
But we have not seen flu emerge yet. Normally we look to Australia, New Zealand, and South Africa during their winter – which is our summer – to get some idea of what is over the horizon for the Northern Hemisphere. Flu activity in Australia has been very modest this year.
That might mean flu may not show up for a while, but I would be loathe to make a prediction.
Q: What are the chances we’ll see a flu outbreak like we’re seeing with RSV, which is normally a winter illness?
The fact that we had a summer RSV surge just gives you an idea of how the normal epidemiology of viral infections has been disrupted. It means anything could happen with influenza. It could show up late summer or fall or wait until next spring.
We really don’t understand how those interactions work. When a new flu strain emerges, it often ignores the traditional behavior and shows up in the spring or fall. It happened in the 2009 pandemic, it happened in 1918.
The one thing I would safely predict about the next flu wave is that it will surprise us.
Q: Are you hopeful that combination vaccines in development from a number of companies, such as Moderna, Novavax, and Vivaldi, will be effective?
It is beginning to look like COVID will be with us for the foreseeable future – maybe as a seasonal virus or maybe as an ongoing pandemic. We are going to need to protect (ourselves) simultaneously against the flu and COVID. A single shot is a great way to do that – nobody wants two needles; nobody wants two trips to get vaccinated.
An effective combination vaccine would be a really great tool.
We have to wait to see what the science shows us, because they are quite different viruses. We won’t know if a combination vaccine works well and has acceptable side effects until we do those studies.
Q. Do you know at this point whether the side effects from two vaccines would be additive? Is there any way to predict that?
There is no way to predict. There are so many things that go into whether someone has side effects that we don’t understand. With fairly reactogenic vaccines like the mRNA vaccines, lots of people have no side effects whatsoever and others are really uncomfortable for 24 hours.
Flu is generally a better tolerated vaccine. There are still people who get muscle aches and very sore arms. I don’t think we can predict if getting two will be additive or just the same as getting one vaccine.
Q: Other than convenience and the benefit for people who are needle-phobic, are there any other advantages of combining them into one shot?
The logistics alone are enough to justify having one effective product if we can make one. It should reduce the overall cost of administration and reduce time off from work.
The combination vaccines given by pediatricians have been very successful. They reduce the number of needles for kids and make it much easier for parents and the pediatricians administering them. The same principle should apply to adults, who sometimes are less brave about needles than kids are.
Historically, combined vaccines in general have worked as well as vaccines given alone, but there have been exceptions. We just have to see what the products look like.
Q: For now, the flu vaccine and COVID-19 vaccine are single products. If you get them separately, is it better to put some time between the two?
We don’t know. There are studies that probably won’t be out in time to decide in September. They are looking at whether you get an equivalent immune response if you give them together or apart.
For now, I would say the advantage of getting them together is if you do get side effects, you’ll only get them once – one day to suffer through them. Also, it’s one trip to the doctor.
The potential advantage of separating them is that is how we developed and tested the vaccines. If you do react to them, side effects could be milder, but it will be on two separate days.
I would recommend doing whatever works so that you get both vaccines in a timely manner.
I’m going to get my flu shot as soon as it’s available. If I’m due for a COVID booster at that time, I would probably do them together.
Q: Do you foresee a point in the future when the predominant strain of SARS-CoV-2 will be one of the components of a flu vaccine, like we did in the past with H1N1, etc?
It really remains to be seen, but it is very conceivable it could happen. The same companies that developed COVID-19 vaccines are working on flu vaccines.
Q: Any other advice for people concerned about getting immunized against both COVID-19 and influenza in the coming months?
There is no side effect of the vaccine that begins to approach the risk you face from either disease. It’s really one of the best things you can do to protect yourself is to get vaccinated.
In the case of flu, the vaccine is only modestly effective, but it still saves tens of thousands of lives each year. The SARS-CoV-2 vaccine is a much better vaccine and a deadlier disease.
Dr. Pavia consulted for GlaxoSmithKline on influenza testing.
A version of this article first appeared on Medscape.com.
Plastic barriers may not stop COVID-19 spread, experts say
Plastic barriers that separate people in stores, restaurants, and classrooms may not be as effective at stopping the spread of COVID-19 as originally thought, according to The New York Times.
Scientists who study air flow, ventilation, and aerosol droplets say the barriers may not help, and in fact, could make the situation worse by blocking normal air flow, the newspaper reported.
Typically, as people interact and breathe in a room, currents and ventilation systems recirculate the air and disperse the exhaled particles. With plastic barriers, however, particles could get trapped in “dead zones” and build up.
“If you have a forest of barriers in a classroom, it’s going to interfere with proper ventilation of that room,” Linsey Marr, professor of civil and environmental engineering at Virginia Tech, told the newspaper.
“Everybody’s aerosols are going to be trapped and stuck there and building up, and they will end up spreading beyond your own desk,” she said.
Several variables factor into the efficacy of plastic barriers, The New York Times reported. Shields may stop big respiratory droplets from coughs and sneezes, for instance, but they may not do much to prevent small aerosol particles from viruses such as COVID-19 from spreading.
“We have shown this effect of blocking larger particles, but also that the smaller aerosols travel over the screen and become mixed in the room air within about 5 minutes,” Catherine Noakes, professor of environment engineering at the University of Leeds, told the newspaper.
“This means if people are interacting for more than a few minutes, they would likely be exposed to the virus regardless of the screen,” she said.
The effectiveness of plastic barriers likely also depends on the location and setup, the newspaper reported. A bus driver with a large barrier, for instance, may be able to avoid inhaling the particles that passengers are exhaling. A bank cashier or store clerk behind a large barrier may also be partly protected.
Even still, scientists say more research is needed. For instance, taller barriers are more likely to be effective. However, a large number of barriers in one room could likely block air flow.
Researchers have recommended that schools and offices focus on ventilation, masks, and vaccines to slow the spread of the coronavirus.
“Air flow in rooms is pretty complicated,” Richard Corsi, dean of engineering at the University of California at Davis, told the newspaper.
“Every room is different in terms of the arrangement of furniture, the height of the walls and ceilings, the vents, where the bookshelves are,” he said. “All of these things have a huge impact on the actual flow and air distribution in a room.”
A version of this article first appeared on WebMD.com.
Plastic barriers that separate people in stores, restaurants, and classrooms may not be as effective at stopping the spread of COVID-19 as originally thought, according to The New York Times.
Scientists who study air flow, ventilation, and aerosol droplets say the barriers may not help, and in fact, could make the situation worse by blocking normal air flow, the newspaper reported.
Typically, as people interact and breathe in a room, currents and ventilation systems recirculate the air and disperse the exhaled particles. With plastic barriers, however, particles could get trapped in “dead zones” and build up.
“If you have a forest of barriers in a classroom, it’s going to interfere with proper ventilation of that room,” Linsey Marr, professor of civil and environmental engineering at Virginia Tech, told the newspaper.
“Everybody’s aerosols are going to be trapped and stuck there and building up, and they will end up spreading beyond your own desk,” she said.
Several variables factor into the efficacy of plastic barriers, The New York Times reported. Shields may stop big respiratory droplets from coughs and sneezes, for instance, but they may not do much to prevent small aerosol particles from viruses such as COVID-19 from spreading.
“We have shown this effect of blocking larger particles, but also that the smaller aerosols travel over the screen and become mixed in the room air within about 5 minutes,” Catherine Noakes, professor of environment engineering at the University of Leeds, told the newspaper.
“This means if people are interacting for more than a few minutes, they would likely be exposed to the virus regardless of the screen,” she said.
The effectiveness of plastic barriers likely also depends on the location and setup, the newspaper reported. A bus driver with a large barrier, for instance, may be able to avoid inhaling the particles that passengers are exhaling. A bank cashier or store clerk behind a large barrier may also be partly protected.
Even still, scientists say more research is needed. For instance, taller barriers are more likely to be effective. However, a large number of barriers in one room could likely block air flow.
Researchers have recommended that schools and offices focus on ventilation, masks, and vaccines to slow the spread of the coronavirus.
“Air flow in rooms is pretty complicated,” Richard Corsi, dean of engineering at the University of California at Davis, told the newspaper.
“Every room is different in terms of the arrangement of furniture, the height of the walls and ceilings, the vents, where the bookshelves are,” he said. “All of these things have a huge impact on the actual flow and air distribution in a room.”
A version of this article first appeared on WebMD.com.
Plastic barriers that separate people in stores, restaurants, and classrooms may not be as effective at stopping the spread of COVID-19 as originally thought, according to The New York Times.
Scientists who study air flow, ventilation, and aerosol droplets say the barriers may not help, and in fact, could make the situation worse by blocking normal air flow, the newspaper reported.
Typically, as people interact and breathe in a room, currents and ventilation systems recirculate the air and disperse the exhaled particles. With plastic barriers, however, particles could get trapped in “dead zones” and build up.
“If you have a forest of barriers in a classroom, it’s going to interfere with proper ventilation of that room,” Linsey Marr, professor of civil and environmental engineering at Virginia Tech, told the newspaper.
“Everybody’s aerosols are going to be trapped and stuck there and building up, and they will end up spreading beyond your own desk,” she said.
Several variables factor into the efficacy of plastic barriers, The New York Times reported. Shields may stop big respiratory droplets from coughs and sneezes, for instance, but they may not do much to prevent small aerosol particles from viruses such as COVID-19 from spreading.
“We have shown this effect of blocking larger particles, but also that the smaller aerosols travel over the screen and become mixed in the room air within about 5 minutes,” Catherine Noakes, professor of environment engineering at the University of Leeds, told the newspaper.
“This means if people are interacting for more than a few minutes, they would likely be exposed to the virus regardless of the screen,” she said.
The effectiveness of plastic barriers likely also depends on the location and setup, the newspaper reported. A bus driver with a large barrier, for instance, may be able to avoid inhaling the particles that passengers are exhaling. A bank cashier or store clerk behind a large barrier may also be partly protected.
Even still, scientists say more research is needed. For instance, taller barriers are more likely to be effective. However, a large number of barriers in one room could likely block air flow.
Researchers have recommended that schools and offices focus on ventilation, masks, and vaccines to slow the spread of the coronavirus.
“Air flow in rooms is pretty complicated,” Richard Corsi, dean of engineering at the University of California at Davis, told the newspaper.
“Every room is different in terms of the arrangement of furniture, the height of the walls and ceilings, the vents, where the bookshelves are,” he said. “All of these things have a huge impact on the actual flow and air distribution in a room.”
A version of this article first appeared on WebMD.com.
FDA fully approves Pfizer COVID-19 vaccine
The Food and Drug Administration has granted a biological license application, more commonly known as “full approval,” to the Pfizer-BioNTech COVID-19 vaccine.
It is the first COVID-19 vaccine to be fully licensed in the United States. It will be marketed under the trade name Comirnaty.
The approval applies to individuals ages 16 years and older. The vaccine is still available for emergency use for those ages 12-15.
The FDA’s stamp of approval is somewhat anticlimactic, following months of real-world use and millions of doses doled out to the general population. It comes after months of scrutiny by the agency of the clinical trial data.
Still, the approval puts the vaccines on firmer legal footing and is expected to spur a raft of new vaccination requirements by employers, schools, and universities.
“The FDA approval is the gold standard,” President Joe Biden said from the White House. “Those who have been waiting for full approval should go and get your shot now.”
“It could save your life or the lives of those you love,” he said.
Biden also called on businesses to mandate COVID vaccines for their employees.
Indeed, soon after the approval was announced, Defense Secretary Lloyd Austin said the vaccines would be required for all 1.4 million active duty service members.
Public health advocates have seen full approval as an important tool to increase U.S. vaccination rates and had criticized the FDA for taking so long to grant the license.
In a news briefing on the approval, Peter Marks, MD, director of the FDA’s Center for Biologics Evaluation and Research, said the agency had not dragged its feet.
Marks noted that his team had reviewed tens of thousands of pages of clinical trial data -- down to the level of individual patients. They also inspected clinical trial sites and manufacturing facilities, and reviewed information gathered after the vaccines were authorized for use.
“It’s been 97 days since Pfizer completed the role of its [application for approval] and the clock started, which means that we completed this in about 40% of the normal clock time for a submission of this magnitude,” he said. “People worked day and night.”
The agency resisted pressure to speed up its process, saying a thorough review was necessary to ensure public confidence.
“While millions of people have already safely received COVID-19 vaccines, we recognize that for some, the FDA approval of a vaccine may now instill additional confidence to get vaccinated. Today’s milestone puts us one step closer to altering the course of this pandemic in the U.S.,” acting FDA Commissioner Janet Woodcock said in a FDA news release.
Experts agreed the move would increase public confidence.
“I don't expect a big line outside of vaccination sites this afternoon or tomorrow morning, but it will persuade some,” said William Schaffner, MD, a professor of infectious diseases at Vanderbilt University in Nashville.
A recent Kaiser Family Foundation poll found that 3 in 10 unvaccinated adults said they would be more likely to get vaccinated if the vaccines were given full approval.
More importantly, Schaffner said, the FDA’s approval would lay the groundwork for vaccine mandates. “I think those kinds of mandates are going to be necessary to get us up over 80% vaccinated.”
In granting the approval, the agency reviewed a record amount of data from more than 40,000 people who took part in clinical trials. About 12,000 recipients have been followed for at least 6 months, the agency said.
The FDA also reviewed safety data collected since it issued its emergency use authorization for the shots in December.
Based on the results from the clinical trials, the vaccine was 91% effective at preventing COVID-19 disease. But that estimate came from data collected before the Delta variant became widespread.
The most commonly reported side effects in the clinical trials were pain, redness and swelling at the injection site, fatigue, headache, muscle or joint pain, chills, and fever.
The FDA said the vaccine is effective in preventing COVID-19 and potentially serious outcomes, including hospitalization and death.
Based on safety data reviewed since the two-dose vaccine was approved, the FDA said the data demonstrates a higher risk for heart inflammation -- clinically known as myocarditis or pericarditis -- especially within 7 days after the second dose of the shots. The risk is highest for men under age 40, compared to women and older men.
The prescription information includes warnings about these risks. The FDA said the drugmakers must continue to study the risks and long-term effects on people who have myocarditis after vaccination.
A version of this article first appeared on Medscape.com.
This article was updated on 8/24/21.
The Food and Drug Administration has granted a biological license application, more commonly known as “full approval,” to the Pfizer-BioNTech COVID-19 vaccine.
It is the first COVID-19 vaccine to be fully licensed in the United States. It will be marketed under the trade name Comirnaty.
The approval applies to individuals ages 16 years and older. The vaccine is still available for emergency use for those ages 12-15.
The FDA’s stamp of approval is somewhat anticlimactic, following months of real-world use and millions of doses doled out to the general population. It comes after months of scrutiny by the agency of the clinical trial data.
Still, the approval puts the vaccines on firmer legal footing and is expected to spur a raft of new vaccination requirements by employers, schools, and universities.
“The FDA approval is the gold standard,” President Joe Biden said from the White House. “Those who have been waiting for full approval should go and get your shot now.”
“It could save your life or the lives of those you love,” he said.
Biden also called on businesses to mandate COVID vaccines for their employees.
Indeed, soon after the approval was announced, Defense Secretary Lloyd Austin said the vaccines would be required for all 1.4 million active duty service members.
Public health advocates have seen full approval as an important tool to increase U.S. vaccination rates and had criticized the FDA for taking so long to grant the license.
In a news briefing on the approval, Peter Marks, MD, director of the FDA’s Center for Biologics Evaluation and Research, said the agency had not dragged its feet.
Marks noted that his team had reviewed tens of thousands of pages of clinical trial data -- down to the level of individual patients. They also inspected clinical trial sites and manufacturing facilities, and reviewed information gathered after the vaccines were authorized for use.
“It’s been 97 days since Pfizer completed the role of its [application for approval] and the clock started, which means that we completed this in about 40% of the normal clock time for a submission of this magnitude,” he said. “People worked day and night.”
The agency resisted pressure to speed up its process, saying a thorough review was necessary to ensure public confidence.
“While millions of people have already safely received COVID-19 vaccines, we recognize that for some, the FDA approval of a vaccine may now instill additional confidence to get vaccinated. Today’s milestone puts us one step closer to altering the course of this pandemic in the U.S.,” acting FDA Commissioner Janet Woodcock said in a FDA news release.
Experts agreed the move would increase public confidence.
“I don't expect a big line outside of vaccination sites this afternoon or tomorrow morning, but it will persuade some,” said William Schaffner, MD, a professor of infectious diseases at Vanderbilt University in Nashville.
A recent Kaiser Family Foundation poll found that 3 in 10 unvaccinated adults said they would be more likely to get vaccinated if the vaccines were given full approval.
More importantly, Schaffner said, the FDA’s approval would lay the groundwork for vaccine mandates. “I think those kinds of mandates are going to be necessary to get us up over 80% vaccinated.”
In granting the approval, the agency reviewed a record amount of data from more than 40,000 people who took part in clinical trials. About 12,000 recipients have been followed for at least 6 months, the agency said.
The FDA also reviewed safety data collected since it issued its emergency use authorization for the shots in December.
Based on the results from the clinical trials, the vaccine was 91% effective at preventing COVID-19 disease. But that estimate came from data collected before the Delta variant became widespread.
The most commonly reported side effects in the clinical trials were pain, redness and swelling at the injection site, fatigue, headache, muscle or joint pain, chills, and fever.
The FDA said the vaccine is effective in preventing COVID-19 and potentially serious outcomes, including hospitalization and death.
Based on safety data reviewed since the two-dose vaccine was approved, the FDA said the data demonstrates a higher risk for heart inflammation -- clinically known as myocarditis or pericarditis -- especially within 7 days after the second dose of the shots. The risk is highest for men under age 40, compared to women and older men.
The prescription information includes warnings about these risks. The FDA said the drugmakers must continue to study the risks and long-term effects on people who have myocarditis after vaccination.
A version of this article first appeared on Medscape.com.
This article was updated on 8/24/21.
The Food and Drug Administration has granted a biological license application, more commonly known as “full approval,” to the Pfizer-BioNTech COVID-19 vaccine.
It is the first COVID-19 vaccine to be fully licensed in the United States. It will be marketed under the trade name Comirnaty.
The approval applies to individuals ages 16 years and older. The vaccine is still available for emergency use for those ages 12-15.
The FDA’s stamp of approval is somewhat anticlimactic, following months of real-world use and millions of doses doled out to the general population. It comes after months of scrutiny by the agency of the clinical trial data.
Still, the approval puts the vaccines on firmer legal footing and is expected to spur a raft of new vaccination requirements by employers, schools, and universities.
“The FDA approval is the gold standard,” President Joe Biden said from the White House. “Those who have been waiting for full approval should go and get your shot now.”
“It could save your life or the lives of those you love,” he said.
Biden also called on businesses to mandate COVID vaccines for their employees.
Indeed, soon after the approval was announced, Defense Secretary Lloyd Austin said the vaccines would be required for all 1.4 million active duty service members.
Public health advocates have seen full approval as an important tool to increase U.S. vaccination rates and had criticized the FDA for taking so long to grant the license.
In a news briefing on the approval, Peter Marks, MD, director of the FDA’s Center for Biologics Evaluation and Research, said the agency had not dragged its feet.
Marks noted that his team had reviewed tens of thousands of pages of clinical trial data -- down to the level of individual patients. They also inspected clinical trial sites and manufacturing facilities, and reviewed information gathered after the vaccines were authorized for use.
“It’s been 97 days since Pfizer completed the role of its [application for approval] and the clock started, which means that we completed this in about 40% of the normal clock time for a submission of this magnitude,” he said. “People worked day and night.”
The agency resisted pressure to speed up its process, saying a thorough review was necessary to ensure public confidence.
“While millions of people have already safely received COVID-19 vaccines, we recognize that for some, the FDA approval of a vaccine may now instill additional confidence to get vaccinated. Today’s milestone puts us one step closer to altering the course of this pandemic in the U.S.,” acting FDA Commissioner Janet Woodcock said in a FDA news release.
Experts agreed the move would increase public confidence.
“I don't expect a big line outside of vaccination sites this afternoon or tomorrow morning, but it will persuade some,” said William Schaffner, MD, a professor of infectious diseases at Vanderbilt University in Nashville.
A recent Kaiser Family Foundation poll found that 3 in 10 unvaccinated adults said they would be more likely to get vaccinated if the vaccines were given full approval.
More importantly, Schaffner said, the FDA’s approval would lay the groundwork for vaccine mandates. “I think those kinds of mandates are going to be necessary to get us up over 80% vaccinated.”
In granting the approval, the agency reviewed a record amount of data from more than 40,000 people who took part in clinical trials. About 12,000 recipients have been followed for at least 6 months, the agency said.
The FDA also reviewed safety data collected since it issued its emergency use authorization for the shots in December.
Based on the results from the clinical trials, the vaccine was 91% effective at preventing COVID-19 disease. But that estimate came from data collected before the Delta variant became widespread.
The most commonly reported side effects in the clinical trials were pain, redness and swelling at the injection site, fatigue, headache, muscle or joint pain, chills, and fever.
The FDA said the vaccine is effective in preventing COVID-19 and potentially serious outcomes, including hospitalization and death.
Based on safety data reviewed since the two-dose vaccine was approved, the FDA said the data demonstrates a higher risk for heart inflammation -- clinically known as myocarditis or pericarditis -- especially within 7 days after the second dose of the shots. The risk is highest for men under age 40, compared to women and older men.
The prescription information includes warnings about these risks. The FDA said the drugmakers must continue to study the risks and long-term effects on people who have myocarditis after vaccination.
A version of this article first appeared on Medscape.com.
This article was updated on 8/24/21.





