User login
News and Views that Matter to Pediatricians
The leading independent newspaper covering news and commentary in pediatrics.
Decline in child COVID may signal end of latest surge
A second consecutive week of falling COVID-19 cases in children, along with continued declines in new admissions, may indicate that the latest surge has peaked.
Children made up over 25% of all new cases each week over that 3-week period covering the end of August and the first half of September, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
New hospitalizations in children aged 0-17 years peaked on Sept. 4 – when the rate reached 0.51 per 100,000 population – and were down to 0.47 as of Sept. 11, the latest date for which data should be considered reliable, the Centers for Disease Control and Prevention said.
The CDC’s data largely agree with the AAP/CHA report, showing that cases peaked during the week of Aug. 22-28. Cases per 100,000 for children that week looked like this: 154.7 (age 0-4 years), 276.6 (5-11 years), 320.0 (12-15), and 334.1 (16-17). The highest rates that week among adults were 288.6 per 100,000 in 30- to 39-year-olds and 286.5 for those aged 18-29, the CDC said on its COVID Data Tracker.
By the week of Sept. 5-11 – reporting delays can affect more recent data – the rates in children were down more than 20% in each of the four age groups, according to the CDC.
Vaccinations among children, unfortunately, continue to decline. Vaccine initiations for 12- to 15-year-olds slipped from 199,000 (Sept. 7-13) to 179,000 during the week of Sept. 14-20, while the 16- to 17-year-olds went from almost 83,000 down to 75,000. Initiations have dropped for 6 straight weeks in both age groups, based on the CDC data.
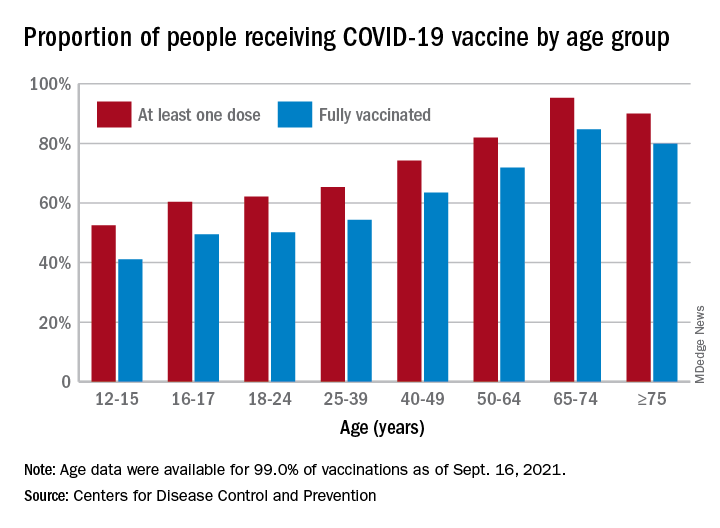
Despite those declines, however, the 16- and 17-year-olds just passed a couple of vaccination milestones. More than 60% – 60.9%, to be exact – have now received at least one dose of COVID vaccine, and 50.3% can be considered fully vaccinated. For those aged 12-15, the corresponding figures are 53.1% and 42.0%, the CDC reported.
When children under age 12 years are included – through clinical trial involvement or incorrect birth dates – the CDC data put the total count of Americans under age 18 who have received at least one dose of vaccine at almost 12.8 million, with vaccination complete in 10.3 million.
Total cases, as calculated by the APA and CHA, are now over 5.5 million, although that figure includes cases in individuals as old as 20 years, since many states differ from the CDC on the age range for a child. The CDC’s COVID Data Tracker put the total for children aged 0-17 at nearly 4.6 million.
The total number of COVID-related deaths in children is 480 as of Sept. 16, the AAP and CHA said, based on data from 45 states, New York, City, Puerto Rico, and Guam, but the CDC provides a higher number, 548, since the pandemic began. Children aged 0-4 years represent the largest share (32.3%) of those 548 deaths, followed by the 12- to 15-year-olds (26.5%), based on the CDC data.
A second consecutive week of falling COVID-19 cases in children, along with continued declines in new admissions, may indicate that the latest surge has peaked.
Children made up over 25% of all new cases each week over that 3-week period covering the end of August and the first half of September, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
New hospitalizations in children aged 0-17 years peaked on Sept. 4 – when the rate reached 0.51 per 100,000 population – and were down to 0.47 as of Sept. 11, the latest date for which data should be considered reliable, the Centers for Disease Control and Prevention said.
The CDC’s data largely agree with the AAP/CHA report, showing that cases peaked during the week of Aug. 22-28. Cases per 100,000 for children that week looked like this: 154.7 (age 0-4 years), 276.6 (5-11 years), 320.0 (12-15), and 334.1 (16-17). The highest rates that week among adults were 288.6 per 100,000 in 30- to 39-year-olds and 286.5 for those aged 18-29, the CDC said on its COVID Data Tracker.
By the week of Sept. 5-11 – reporting delays can affect more recent data – the rates in children were down more than 20% in each of the four age groups, according to the CDC.
Vaccinations among children, unfortunately, continue to decline. Vaccine initiations for 12- to 15-year-olds slipped from 199,000 (Sept. 7-13) to 179,000 during the week of Sept. 14-20, while the 16- to 17-year-olds went from almost 83,000 down to 75,000. Initiations have dropped for 6 straight weeks in both age groups, based on the CDC data.
Despite those declines, however, the 16- and 17-year-olds just passed a couple of vaccination milestones. More than 60% – 60.9%, to be exact – have now received at least one dose of COVID vaccine, and 50.3% can be considered fully vaccinated. For those aged 12-15, the corresponding figures are 53.1% and 42.0%, the CDC reported.
When children under age 12 years are included – through clinical trial involvement or incorrect birth dates – the CDC data put the total count of Americans under age 18 who have received at least one dose of vaccine at almost 12.8 million, with vaccination complete in 10.3 million.
Total cases, as calculated by the APA and CHA, are now over 5.5 million, although that figure includes cases in individuals as old as 20 years, since many states differ from the CDC on the age range for a child. The CDC’s COVID Data Tracker put the total for children aged 0-17 at nearly 4.6 million.
The total number of COVID-related deaths in children is 480 as of Sept. 16, the AAP and CHA said, based on data from 45 states, New York, City, Puerto Rico, and Guam, but the CDC provides a higher number, 548, since the pandemic began. Children aged 0-4 years represent the largest share (32.3%) of those 548 deaths, followed by the 12- to 15-year-olds (26.5%), based on the CDC data.
A second consecutive week of falling COVID-19 cases in children, along with continued declines in new admissions, may indicate that the latest surge has peaked.
Children made up over 25% of all new cases each week over that 3-week period covering the end of August and the first half of September, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
New hospitalizations in children aged 0-17 years peaked on Sept. 4 – when the rate reached 0.51 per 100,000 population – and were down to 0.47 as of Sept. 11, the latest date for which data should be considered reliable, the Centers for Disease Control and Prevention said.
The CDC’s data largely agree with the AAP/CHA report, showing that cases peaked during the week of Aug. 22-28. Cases per 100,000 for children that week looked like this: 154.7 (age 0-4 years), 276.6 (5-11 years), 320.0 (12-15), and 334.1 (16-17). The highest rates that week among adults were 288.6 per 100,000 in 30- to 39-year-olds and 286.5 for those aged 18-29, the CDC said on its COVID Data Tracker.
By the week of Sept. 5-11 – reporting delays can affect more recent data – the rates in children were down more than 20% in each of the four age groups, according to the CDC.
Vaccinations among children, unfortunately, continue to decline. Vaccine initiations for 12- to 15-year-olds slipped from 199,000 (Sept. 7-13) to 179,000 during the week of Sept. 14-20, while the 16- to 17-year-olds went from almost 83,000 down to 75,000. Initiations have dropped for 6 straight weeks in both age groups, based on the CDC data.
Despite those declines, however, the 16- and 17-year-olds just passed a couple of vaccination milestones. More than 60% – 60.9%, to be exact – have now received at least one dose of COVID vaccine, and 50.3% can be considered fully vaccinated. For those aged 12-15, the corresponding figures are 53.1% and 42.0%, the CDC reported.
When children under age 12 years are included – through clinical trial involvement or incorrect birth dates – the CDC data put the total count of Americans under age 18 who have received at least one dose of vaccine at almost 12.8 million, with vaccination complete in 10.3 million.
Total cases, as calculated by the APA and CHA, are now over 5.5 million, although that figure includes cases in individuals as old as 20 years, since many states differ from the CDC on the age range for a child. The CDC’s COVID Data Tracker put the total for children aged 0-17 at nearly 4.6 million.
The total number of COVID-related deaths in children is 480 as of Sept. 16, the AAP and CHA said, based on data from 45 states, New York, City, Puerto Rico, and Guam, but the CDC provides a higher number, 548, since the pandemic began. Children aged 0-4 years represent the largest share (32.3%) of those 548 deaths, followed by the 12- to 15-year-olds (26.5%), based on the CDC data.
COVID-19 claims more than 675,000 U.S. lives, surpassing the 1918 flu
, according to data collected by Johns Hopkins University.
Although the raw numbers match, epidemiologists point out that 675,000 deaths in 1918 was a much greater proportion of the population. In 1918, the U.S. population was 105 million, less than one third of what it is today.
The AIDS pandemic of the 1980s remains the deadliest of the 20th Century, claiming the lives of 700,000 Americans. But at our current pace of 2,000 COVID deaths a day, we could quickly eclipse that death toll, too.
Even though the 1918 epidemic is often called the “Spanish Flu,” there is no universal consensus regarding where the virus originated, according to the Centers for Disease Control and Prevention.
Still, the almost incomprehensible loss harkens back to a time when medicine and technology were far less advanced than they are today.
In 1918, the United States didn’t have access to a vaccine, or near real-time tools to trace the spread and communicate the threat.
In some ways, the United States has failed to learn from the mistakes of the past.
There are many similarities between the two pandemics. In the spring of 1918, when the first wave of influenza hit, the United States and its allies were nearing victory in Europe in World War I. Just this summer the United States has ended its longest war, the conflict in Afghanistan, as COVID cases surge.
In both pandemics, hospitals and funeral homes were overrun and makeshift clinics were opened where space was available. Mask mandates were installed; schools, churches, and theaters closed; and social distancing was encouraged.
As is the case today, different jurisdictions took different steps to fight the pandemic and some were more successful than others.
According to History.com, in 1918, Philadelphia’s mayor said a popular annual parade could be held, and an estimated 200,000 people attended. In less than 2 weeks, more than 1,000 local residents were dead. But in St. Louis, public gatherings were banned, schools and theaters closed, and the death toll there was one eighth of Philadelphia’s.
Just as in 1918, America has at times continued to fan the flames of the epidemic by relaxing restrictions too quickly and relying on unproven treatments. Poor communication allowed younger people to feel that they wouldn’t necessarily face the worst consequences of the virus, contributing to a false sense of security in the age group that was fueling the spread.
“A lot of the mistakes that we definitely fell into in 1918, we hoped we wouldn’t fall into in 2020,” epidemiologist Stephen Kissler, PhD, of the Harvard T.H. Chan School of Public Health, told CNN. “We did.”
A version of this article first appeared on Medscape.com.
, according to data collected by Johns Hopkins University.
Although the raw numbers match, epidemiologists point out that 675,000 deaths in 1918 was a much greater proportion of the population. In 1918, the U.S. population was 105 million, less than one third of what it is today.
The AIDS pandemic of the 1980s remains the deadliest of the 20th Century, claiming the lives of 700,000 Americans. But at our current pace of 2,000 COVID deaths a day, we could quickly eclipse that death toll, too.
Even though the 1918 epidemic is often called the “Spanish Flu,” there is no universal consensus regarding where the virus originated, according to the Centers for Disease Control and Prevention.
Still, the almost incomprehensible loss harkens back to a time when medicine and technology were far less advanced than they are today.
In 1918, the United States didn’t have access to a vaccine, or near real-time tools to trace the spread and communicate the threat.
In some ways, the United States has failed to learn from the mistakes of the past.
There are many similarities between the two pandemics. In the spring of 1918, when the first wave of influenza hit, the United States and its allies were nearing victory in Europe in World War I. Just this summer the United States has ended its longest war, the conflict in Afghanistan, as COVID cases surge.
In both pandemics, hospitals and funeral homes were overrun and makeshift clinics were opened where space was available. Mask mandates were installed; schools, churches, and theaters closed; and social distancing was encouraged.
As is the case today, different jurisdictions took different steps to fight the pandemic and some were more successful than others.
According to History.com, in 1918, Philadelphia’s mayor said a popular annual parade could be held, and an estimated 200,000 people attended. In less than 2 weeks, more than 1,000 local residents were dead. But in St. Louis, public gatherings were banned, schools and theaters closed, and the death toll there was one eighth of Philadelphia’s.
Just as in 1918, America has at times continued to fan the flames of the epidemic by relaxing restrictions too quickly and relying on unproven treatments. Poor communication allowed younger people to feel that they wouldn’t necessarily face the worst consequences of the virus, contributing to a false sense of security in the age group that was fueling the spread.
“A lot of the mistakes that we definitely fell into in 1918, we hoped we wouldn’t fall into in 2020,” epidemiologist Stephen Kissler, PhD, of the Harvard T.H. Chan School of Public Health, told CNN. “We did.”
A version of this article first appeared on Medscape.com.
, according to data collected by Johns Hopkins University.
Although the raw numbers match, epidemiologists point out that 675,000 deaths in 1918 was a much greater proportion of the population. In 1918, the U.S. population was 105 million, less than one third of what it is today.
The AIDS pandemic of the 1980s remains the deadliest of the 20th Century, claiming the lives of 700,000 Americans. But at our current pace of 2,000 COVID deaths a day, we could quickly eclipse that death toll, too.
Even though the 1918 epidemic is often called the “Spanish Flu,” there is no universal consensus regarding where the virus originated, according to the Centers for Disease Control and Prevention.
Still, the almost incomprehensible loss harkens back to a time when medicine and technology were far less advanced than they are today.
In 1918, the United States didn’t have access to a vaccine, or near real-time tools to trace the spread and communicate the threat.
In some ways, the United States has failed to learn from the mistakes of the past.
There are many similarities between the two pandemics. In the spring of 1918, when the first wave of influenza hit, the United States and its allies were nearing victory in Europe in World War I. Just this summer the United States has ended its longest war, the conflict in Afghanistan, as COVID cases surge.
In both pandemics, hospitals and funeral homes were overrun and makeshift clinics were opened where space was available. Mask mandates were installed; schools, churches, and theaters closed; and social distancing was encouraged.
As is the case today, different jurisdictions took different steps to fight the pandemic and some were more successful than others.
According to History.com, in 1918, Philadelphia’s mayor said a popular annual parade could be held, and an estimated 200,000 people attended. In less than 2 weeks, more than 1,000 local residents were dead. But in St. Louis, public gatherings were banned, schools and theaters closed, and the death toll there was one eighth of Philadelphia’s.
Just as in 1918, America has at times continued to fan the flames of the epidemic by relaxing restrictions too quickly and relying on unproven treatments. Poor communication allowed younger people to feel that they wouldn’t necessarily face the worst consequences of the virus, contributing to a false sense of security in the age group that was fueling the spread.
“A lot of the mistakes that we definitely fell into in 1918, we hoped we wouldn’t fall into in 2020,” epidemiologist Stephen Kissler, PhD, of the Harvard T.H. Chan School of Public Health, told CNN. “We did.”
A version of this article first appeared on Medscape.com.
HPV vaccine safety concerns up 80% from 2015 to 2018
Despite a decrease in reported adverse events after receiving the human papillomavirus (HPV) vaccine, among parents of unvaccinated adolescents, concerns about the vaccine’s safety rose 80% from 2015 to 2018, according to research published September 17 in JAMA Network Open.
Since its approval in 2006 by the U.S. Food and Drug Administration, uptake of the HPV vaccine has consistently lagged behind that of other routine vaccinations. According to the most recent data from the Centers for Disease Control and Prevention, released September 3, 58.6% of adolescents were considered up to date with their HPV vaccinations in 2020.
Trials prior to the vaccine’s FDA approval as well as an abundance of clinical and observational evidence after it hit the market demonstrate the vaccine’s efficacy and safety, said lead author Kalyani Sonawane, PhD, an assistant professor of management, policy, and community health at the UTHealth School of Public Health, in Houston, Texas, in an interview. Still, recent research suggests that safety concerns are a main reason why parents are hesitant to have their children vaccinated, she noted.
In the study, Dr. Sonawane and colleagues analyzed data from National Immunization Survey-Teen (NIS-Teen) from 2015 through 2018. NIS-Teen is a random-digit-dialed telephone survey conducted annually by the CDC to monitor routine vaccination coverage among adolescents aged 13 to 17. The researchers identified 39,364 adolescents who had not received any HPV shots and reviewed the caregivers’ reasons for vaccine hesitancy. The research team also reviewed the Vaccine Adverse Event Reporting System (VAERS). They identified 16,621 reports that listed the HPV vaccine from 2015 through 2018.
The top five reasons caregivers cited for avoiding the HPV vaccine were the following:
- not needed or necessary
- safety concerns
- not recommended
- lack of knowledge
- not sexually active
Of these, safety concerns were the only factor that increased during the study period. They increased from 13.0% in 2015 to 23.4% in 2018. Concerns over vaccine safety rose in 30 states, with increases of over 200% in California, Hawaii, South Dakota, and Mississippi.
The proportion of unvaccinated adolescents whose caregivers thought the HPV vaccine was not needed or necessary remained steady at around 25%. Those whose caregivers listed “not recommended,” “lack of knowledge,” and “not sexually active” as reasons for avoiding vaccination decreased over the study period.
The reporting rate for adverse events following HPV vaccination decreased from 44.7 per 100,000 doses in 2015 to 29.4 per 100,000 doses in 2018. Of the reported 16,621 adverse events following HPV vaccination that occurred over the study period, 4.6% were serious, resulting in hospitalizations, disability, life-threatening events, or death. From 2015 through 2018, reporting rates for serious adverse events remained level at around 0.3 events per 100,000 doses.
This mismatch between increasing vaccine safety concerns and decreasing adverse events suggests that disinformation may be driving these concerns more than scientific fact, Nosayaba Osazuwa-Peters, PhD, MPH, an assistant professor in head and neck surgery and communication sciences at the Duke University School of Medicine, in Durham, North Carolina, told this news organization. He co-wrote an invited commentary on the study and was not involved with the research. Although there have always been people who are hesitant to receive vaccinations, he said, social media and the internet have undoubtedly played a role in spreading concern.
Dr. Sonawane agreed. Online, “there are a lot of antivaccine groups that are making unwarranted claims about the vaccine’s safety,” such as that the HPV vaccine causes autism or fertility problems in women, she said. “We believe that this growing antivaccine movement in the U.S. and across the globe – which the World Health Organization has declared as one of the biggest threats right now – is also contributing to safety concerns among U.S. parents, particularly HPV vaccine safety.”
Although the study did not address strategies to combat this misinformation, Dr. Osazuwa-Peters said clinicians need to improve their communication with parents and patients. One way to do that, he said, is by bolstering an online presence and by countering vaccine disinformation with evidence-based responses on the internet. Most people get their medical information online. “Many people are just afraid because they don’t trust the messages coming from health care,” he said. “So, we need to a better job of not just providing the facts but providing the facts in a way that the end users can understand and appreciate.”
Dr. Sonawane and Dr. Osazuwa-Peters report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Despite a decrease in reported adverse events after receiving the human papillomavirus (HPV) vaccine, among parents of unvaccinated adolescents, concerns about the vaccine’s safety rose 80% from 2015 to 2018, according to research published September 17 in JAMA Network Open.
Since its approval in 2006 by the U.S. Food and Drug Administration, uptake of the HPV vaccine has consistently lagged behind that of other routine vaccinations. According to the most recent data from the Centers for Disease Control and Prevention, released September 3, 58.6% of adolescents were considered up to date with their HPV vaccinations in 2020.
Trials prior to the vaccine’s FDA approval as well as an abundance of clinical and observational evidence after it hit the market demonstrate the vaccine’s efficacy and safety, said lead author Kalyani Sonawane, PhD, an assistant professor of management, policy, and community health at the UTHealth School of Public Health, in Houston, Texas, in an interview. Still, recent research suggests that safety concerns are a main reason why parents are hesitant to have their children vaccinated, she noted.
In the study, Dr. Sonawane and colleagues analyzed data from National Immunization Survey-Teen (NIS-Teen) from 2015 through 2018. NIS-Teen is a random-digit-dialed telephone survey conducted annually by the CDC to monitor routine vaccination coverage among adolescents aged 13 to 17. The researchers identified 39,364 adolescents who had not received any HPV shots and reviewed the caregivers’ reasons for vaccine hesitancy. The research team also reviewed the Vaccine Adverse Event Reporting System (VAERS). They identified 16,621 reports that listed the HPV vaccine from 2015 through 2018.
The top five reasons caregivers cited for avoiding the HPV vaccine were the following:
- not needed or necessary
- safety concerns
- not recommended
- lack of knowledge
- not sexually active
Of these, safety concerns were the only factor that increased during the study period. They increased from 13.0% in 2015 to 23.4% in 2018. Concerns over vaccine safety rose in 30 states, with increases of over 200% in California, Hawaii, South Dakota, and Mississippi.
The proportion of unvaccinated adolescents whose caregivers thought the HPV vaccine was not needed or necessary remained steady at around 25%. Those whose caregivers listed “not recommended,” “lack of knowledge,” and “not sexually active” as reasons for avoiding vaccination decreased over the study period.
The reporting rate for adverse events following HPV vaccination decreased from 44.7 per 100,000 doses in 2015 to 29.4 per 100,000 doses in 2018. Of the reported 16,621 adverse events following HPV vaccination that occurred over the study period, 4.6% were serious, resulting in hospitalizations, disability, life-threatening events, or death. From 2015 through 2018, reporting rates for serious adverse events remained level at around 0.3 events per 100,000 doses.
This mismatch between increasing vaccine safety concerns and decreasing adverse events suggests that disinformation may be driving these concerns more than scientific fact, Nosayaba Osazuwa-Peters, PhD, MPH, an assistant professor in head and neck surgery and communication sciences at the Duke University School of Medicine, in Durham, North Carolina, told this news organization. He co-wrote an invited commentary on the study and was not involved with the research. Although there have always been people who are hesitant to receive vaccinations, he said, social media and the internet have undoubtedly played a role in spreading concern.
Dr. Sonawane agreed. Online, “there are a lot of antivaccine groups that are making unwarranted claims about the vaccine’s safety,” such as that the HPV vaccine causes autism or fertility problems in women, she said. “We believe that this growing antivaccine movement in the U.S. and across the globe – which the World Health Organization has declared as one of the biggest threats right now – is also contributing to safety concerns among U.S. parents, particularly HPV vaccine safety.”
Although the study did not address strategies to combat this misinformation, Dr. Osazuwa-Peters said clinicians need to improve their communication with parents and patients. One way to do that, he said, is by bolstering an online presence and by countering vaccine disinformation with evidence-based responses on the internet. Most people get their medical information online. “Many people are just afraid because they don’t trust the messages coming from health care,” he said. “So, we need to a better job of not just providing the facts but providing the facts in a way that the end users can understand and appreciate.”
Dr. Sonawane and Dr. Osazuwa-Peters report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Despite a decrease in reported adverse events after receiving the human papillomavirus (HPV) vaccine, among parents of unvaccinated adolescents, concerns about the vaccine’s safety rose 80% from 2015 to 2018, according to research published September 17 in JAMA Network Open.
Since its approval in 2006 by the U.S. Food and Drug Administration, uptake of the HPV vaccine has consistently lagged behind that of other routine vaccinations. According to the most recent data from the Centers for Disease Control and Prevention, released September 3, 58.6% of adolescents were considered up to date with their HPV vaccinations in 2020.
Trials prior to the vaccine’s FDA approval as well as an abundance of clinical and observational evidence after it hit the market demonstrate the vaccine’s efficacy and safety, said lead author Kalyani Sonawane, PhD, an assistant professor of management, policy, and community health at the UTHealth School of Public Health, in Houston, Texas, in an interview. Still, recent research suggests that safety concerns are a main reason why parents are hesitant to have their children vaccinated, she noted.
In the study, Dr. Sonawane and colleagues analyzed data from National Immunization Survey-Teen (NIS-Teen) from 2015 through 2018. NIS-Teen is a random-digit-dialed telephone survey conducted annually by the CDC to monitor routine vaccination coverage among adolescents aged 13 to 17. The researchers identified 39,364 adolescents who had not received any HPV shots and reviewed the caregivers’ reasons for vaccine hesitancy. The research team also reviewed the Vaccine Adverse Event Reporting System (VAERS). They identified 16,621 reports that listed the HPV vaccine from 2015 through 2018.
The top five reasons caregivers cited for avoiding the HPV vaccine were the following:
- not needed or necessary
- safety concerns
- not recommended
- lack of knowledge
- not sexually active
Of these, safety concerns were the only factor that increased during the study period. They increased from 13.0% in 2015 to 23.4% in 2018. Concerns over vaccine safety rose in 30 states, with increases of over 200% in California, Hawaii, South Dakota, and Mississippi.
The proportion of unvaccinated adolescents whose caregivers thought the HPV vaccine was not needed or necessary remained steady at around 25%. Those whose caregivers listed “not recommended,” “lack of knowledge,” and “not sexually active” as reasons for avoiding vaccination decreased over the study period.
The reporting rate for adverse events following HPV vaccination decreased from 44.7 per 100,000 doses in 2015 to 29.4 per 100,000 doses in 2018. Of the reported 16,621 adverse events following HPV vaccination that occurred over the study period, 4.6% were serious, resulting in hospitalizations, disability, life-threatening events, or death. From 2015 through 2018, reporting rates for serious adverse events remained level at around 0.3 events per 100,000 doses.
This mismatch between increasing vaccine safety concerns and decreasing adverse events suggests that disinformation may be driving these concerns more than scientific fact, Nosayaba Osazuwa-Peters, PhD, MPH, an assistant professor in head and neck surgery and communication sciences at the Duke University School of Medicine, in Durham, North Carolina, told this news organization. He co-wrote an invited commentary on the study and was not involved with the research. Although there have always been people who are hesitant to receive vaccinations, he said, social media and the internet have undoubtedly played a role in spreading concern.
Dr. Sonawane agreed. Online, “there are a lot of antivaccine groups that are making unwarranted claims about the vaccine’s safety,” such as that the HPV vaccine causes autism or fertility problems in women, she said. “We believe that this growing antivaccine movement in the U.S. and across the globe – which the World Health Organization has declared as one of the biggest threats right now – is also contributing to safety concerns among U.S. parents, particularly HPV vaccine safety.”
Although the study did not address strategies to combat this misinformation, Dr. Osazuwa-Peters said clinicians need to improve their communication with parents and patients. One way to do that, he said, is by bolstering an online presence and by countering vaccine disinformation with evidence-based responses on the internet. Most people get their medical information online. “Many people are just afraid because they don’t trust the messages coming from health care,” he said. “So, we need to a better job of not just providing the facts but providing the facts in a way that the end users can understand and appreciate.”
Dr. Sonawane and Dr. Osazuwa-Peters report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Adolescent immunizations and protecting our children from COVID-19
I began thinking of a topic for this column weeks ago determined to discuss anything except COVID-19. Yet, news reports from all sources blasted daily reminders of rising COVID-19 cases overall and specifically in children.
In August, school resumed for many of our patients and the battle over mandating masks for school attendance was in full swing. The fact that it is a Centers for Disease Control and Prevention recommendation supported by both the American Academy of Pediatrics and the Pediatric Infectious Disease Society fell on deaf ears. One day, I heard a report that over 25,000 students attending Texas public schools were diagnosed with COVID-19 between Aug. 23 and Aug. 29. This peak in activity occurred just 2 weeks after the start of school and led to the closure of 45 school districts. Texas does not have a monopoly on these rising cases. Delta, a more contagious variant, began circulating in June 2021 and by July it was the most predominant. Emergency department visits and hospitalizations have increased nationwide. During the latter 2 weeks of August 2021, COVID-19–related ED visits and hospitalizations for persons aged 0-17 years were 3.4 and 3.7 times higher in states with the lowest vaccination coverage, compared with states with high vaccination coverage (MMWR Morb Mortal Wkly Rep. 2021;70:1249-54). Specifically, the rates of hospitalization the week ending Aug. 14, 2021, were nearly 5 times the rates for the week ending June 26, 2021, for 0- to 17-year-olds and nearly 10 times the rates for children 0-4 years of age. Hospitalization rates were 10.1 times higher for unimmunized adolescents than for fully vaccinated ones (MMWR Morb Mortal Wkly Rep. 2021;70:1255-60).
Multiple elected state leaders have opposed interventions such as mandating masks in school, and our children are paying for it. These leaders have relinquished their responsibility to local school boards. Several have reinforced the no-mask mandate while others have had the courage and insight to ignore state government leaders and have established mask mandates.
How is this lack of enforcement of national recommendations affecting our patients? Let’s look at two neighboring school districts in Texas. School districts have COVID-19 dashboards that are updated daily and accessible to the general public. School District A requires masks for school entry. It serves 196,171 students and has 27,195 teachers and staff. Since school opened in August, 1,606 cumulative cases of COVID-19 in students (0.8%) and 282 in staff (1%) have been reported. Fifty-five percent of the student cases occurred in elementary schools. In contrast, School District B located in the adjacent county serves 64,517 students and has 3,906 teachers and staff with no mask mandate. Since August, there have been 4,506 cumulative COVID-19 cases in students (6.9%) and 578 (14.7%) in staff. Information regarding the specific school type was not provided; however, the dashboard indicates that 2,924 cases (64.8%) occurred in children younger than 11 years of age. County data indicate 62% of those older than 12 years of age were fully vaccinated in District A, compared with 54% of persons older than 12 years in District B. The county COVID-19 positivity rate in District A is 17.6% and in District B it is 20%. Both counties are experiencing increased COVID-19 activity yet have had strikingly different outcomes in the student/staff population. While supporting the case for wearing masks to prevent disease transmission, one can’t ignore the adolescents who were infected and vaccine eligible (District A: 706; District B: 1,582). Their vaccination status could not be determined.
As pediatricians we have played an integral part in the elimination of diseases through educating and administering vaccinations. Adolescents are relatively healthy, thus limiting the number of encounters with them. The majority complete the 11-year visit; however, many fail to return for the 16- to 18-year visit.
So how are we doing? CDC data from 10 U.S. jurisdictions demonstrated a substantial decrease in vaccine administration between March and May of 2020, compared with the same period in 2018 and 2019. A decline was anticipated because of the nationwide lockdown. Doses of HPV administered declined almost 64% and 71% for 9- to 12-year-olds and 13- to 17-year-olds, respectively. Tdap administration declined 66% and 61% for the same respective age groups. Although administered doses increased between June and September of 2020, it was not sufficient to achieve catch-up coverage. Compared to the same period in 2018-2019, administration of the HPV vaccine declined 12.8% and 28% (ages 9-12 and ages 13-17) and for Tdap it was 21% and 30% lower (ages 9-12 and ages 13-17) (MMWR Morb Mortal Wkly Rep. 2021;70:840-5).
Now, we have another adolescent vaccine to discuss and encourage our patients to receive. We also need to address their concerns and/or to at least direct them to a reliable source to obtain accurate information. For the first time, a recommended vaccine may not be available at their medical home. Many don’t know where to go to receive it (http://www.vaccines.gov). Results of a Kaiser Family Foundation COVID-19 survey (August 2021) indicated that parents trusted their pediatricians most often (78%) for vaccine advice. The respondents voiced concern about trusting the location where the child would be immunized and long-term effects especially related to fertility. Parents who received communications regarding the benefits of vaccination were twice as likely to have their adolescents immunized. Finally, remember: Like parent, like child. An immunized parent is more likely to immunize the adolescent. (See Fig. 1.)
It is beyond the scope of this column to discuss the psychosocial aspects of this disease: children experiencing the death of teachers, classmates, family members, and those viewing the vitriol between pro- and antimask proponents often exhibited on school premises. And let’s not forget the child who wants to wear a mask but may be ostracized or bullied for doing so.
Our job is to do our very best to advocate for and to protect our patients by promoting mandatory masks at schools and encouraging vaccination of adolescents as we patiently wait for vaccines to become available for all of our children.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures.
I began thinking of a topic for this column weeks ago determined to discuss anything except COVID-19. Yet, news reports from all sources blasted daily reminders of rising COVID-19 cases overall and specifically in children.
In August, school resumed for many of our patients and the battle over mandating masks for school attendance was in full swing. The fact that it is a Centers for Disease Control and Prevention recommendation supported by both the American Academy of Pediatrics and the Pediatric Infectious Disease Society fell on deaf ears. One day, I heard a report that over 25,000 students attending Texas public schools were diagnosed with COVID-19 between Aug. 23 and Aug. 29. This peak in activity occurred just 2 weeks after the start of school and led to the closure of 45 school districts. Texas does not have a monopoly on these rising cases. Delta, a more contagious variant, began circulating in June 2021 and by July it was the most predominant. Emergency department visits and hospitalizations have increased nationwide. During the latter 2 weeks of August 2021, COVID-19–related ED visits and hospitalizations for persons aged 0-17 years were 3.4 and 3.7 times higher in states with the lowest vaccination coverage, compared with states with high vaccination coverage (MMWR Morb Mortal Wkly Rep. 2021;70:1249-54). Specifically, the rates of hospitalization the week ending Aug. 14, 2021, were nearly 5 times the rates for the week ending June 26, 2021, for 0- to 17-year-olds and nearly 10 times the rates for children 0-4 years of age. Hospitalization rates were 10.1 times higher for unimmunized adolescents than for fully vaccinated ones (MMWR Morb Mortal Wkly Rep. 2021;70:1255-60).
Multiple elected state leaders have opposed interventions such as mandating masks in school, and our children are paying for it. These leaders have relinquished their responsibility to local school boards. Several have reinforced the no-mask mandate while others have had the courage and insight to ignore state government leaders and have established mask mandates.
How is this lack of enforcement of national recommendations affecting our patients? Let’s look at two neighboring school districts in Texas. School districts have COVID-19 dashboards that are updated daily and accessible to the general public. School District A requires masks for school entry. It serves 196,171 students and has 27,195 teachers and staff. Since school opened in August, 1,606 cumulative cases of COVID-19 in students (0.8%) and 282 in staff (1%) have been reported. Fifty-five percent of the student cases occurred in elementary schools. In contrast, School District B located in the adjacent county serves 64,517 students and has 3,906 teachers and staff with no mask mandate. Since August, there have been 4,506 cumulative COVID-19 cases in students (6.9%) and 578 (14.7%) in staff. Information regarding the specific school type was not provided; however, the dashboard indicates that 2,924 cases (64.8%) occurred in children younger than 11 years of age. County data indicate 62% of those older than 12 years of age were fully vaccinated in District A, compared with 54% of persons older than 12 years in District B. The county COVID-19 positivity rate in District A is 17.6% and in District B it is 20%. Both counties are experiencing increased COVID-19 activity yet have had strikingly different outcomes in the student/staff population. While supporting the case for wearing masks to prevent disease transmission, one can’t ignore the adolescents who were infected and vaccine eligible (District A: 706; District B: 1,582). Their vaccination status could not be determined.
As pediatricians we have played an integral part in the elimination of diseases through educating and administering vaccinations. Adolescents are relatively healthy, thus limiting the number of encounters with them. The majority complete the 11-year visit; however, many fail to return for the 16- to 18-year visit.
So how are we doing? CDC data from 10 U.S. jurisdictions demonstrated a substantial decrease in vaccine administration between March and May of 2020, compared with the same period in 2018 and 2019. A decline was anticipated because of the nationwide lockdown. Doses of HPV administered declined almost 64% and 71% for 9- to 12-year-olds and 13- to 17-year-olds, respectively. Tdap administration declined 66% and 61% for the same respective age groups. Although administered doses increased between June and September of 2020, it was not sufficient to achieve catch-up coverage. Compared to the same period in 2018-2019, administration of the HPV vaccine declined 12.8% and 28% (ages 9-12 and ages 13-17) and for Tdap it was 21% and 30% lower (ages 9-12 and ages 13-17) (MMWR Morb Mortal Wkly Rep. 2021;70:840-5).
Now, we have another adolescent vaccine to discuss and encourage our patients to receive. We also need to address their concerns and/or to at least direct them to a reliable source to obtain accurate information. For the first time, a recommended vaccine may not be available at their medical home. Many don’t know where to go to receive it (http://www.vaccines.gov). Results of a Kaiser Family Foundation COVID-19 survey (August 2021) indicated that parents trusted their pediatricians most often (78%) for vaccine advice. The respondents voiced concern about trusting the location where the child would be immunized and long-term effects especially related to fertility. Parents who received communications regarding the benefits of vaccination were twice as likely to have their adolescents immunized. Finally, remember: Like parent, like child. An immunized parent is more likely to immunize the adolescent. (See Fig. 1.)

It is beyond the scope of this column to discuss the psychosocial aspects of this disease: children experiencing the death of teachers, classmates, family members, and those viewing the vitriol between pro- and antimask proponents often exhibited on school premises. And let’s not forget the child who wants to wear a mask but may be ostracized or bullied for doing so.
Our job is to do our very best to advocate for and to protect our patients by promoting mandatory masks at schools and encouraging vaccination of adolescents as we patiently wait for vaccines to become available for all of our children.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures.
I began thinking of a topic for this column weeks ago determined to discuss anything except COVID-19. Yet, news reports from all sources blasted daily reminders of rising COVID-19 cases overall and specifically in children.
In August, school resumed for many of our patients and the battle over mandating masks for school attendance was in full swing. The fact that it is a Centers for Disease Control and Prevention recommendation supported by both the American Academy of Pediatrics and the Pediatric Infectious Disease Society fell on deaf ears. One day, I heard a report that over 25,000 students attending Texas public schools were diagnosed with COVID-19 between Aug. 23 and Aug. 29. This peak in activity occurred just 2 weeks after the start of school and led to the closure of 45 school districts. Texas does not have a monopoly on these rising cases. Delta, a more contagious variant, began circulating in June 2021 and by July it was the most predominant. Emergency department visits and hospitalizations have increased nationwide. During the latter 2 weeks of August 2021, COVID-19–related ED visits and hospitalizations for persons aged 0-17 years were 3.4 and 3.7 times higher in states with the lowest vaccination coverage, compared with states with high vaccination coverage (MMWR Morb Mortal Wkly Rep. 2021;70:1249-54). Specifically, the rates of hospitalization the week ending Aug. 14, 2021, were nearly 5 times the rates for the week ending June 26, 2021, for 0- to 17-year-olds and nearly 10 times the rates for children 0-4 years of age. Hospitalization rates were 10.1 times higher for unimmunized adolescents than for fully vaccinated ones (MMWR Morb Mortal Wkly Rep. 2021;70:1255-60).
Multiple elected state leaders have opposed interventions such as mandating masks in school, and our children are paying for it. These leaders have relinquished their responsibility to local school boards. Several have reinforced the no-mask mandate while others have had the courage and insight to ignore state government leaders and have established mask mandates.
How is this lack of enforcement of national recommendations affecting our patients? Let’s look at two neighboring school districts in Texas. School districts have COVID-19 dashboards that are updated daily and accessible to the general public. School District A requires masks for school entry. It serves 196,171 students and has 27,195 teachers and staff. Since school opened in August, 1,606 cumulative cases of COVID-19 in students (0.8%) and 282 in staff (1%) have been reported. Fifty-five percent of the student cases occurred in elementary schools. In contrast, School District B located in the adjacent county serves 64,517 students and has 3,906 teachers and staff with no mask mandate. Since August, there have been 4,506 cumulative COVID-19 cases in students (6.9%) and 578 (14.7%) in staff. Information regarding the specific school type was not provided; however, the dashboard indicates that 2,924 cases (64.8%) occurred in children younger than 11 years of age. County data indicate 62% of those older than 12 years of age were fully vaccinated in District A, compared with 54% of persons older than 12 years in District B. The county COVID-19 positivity rate in District A is 17.6% and in District B it is 20%. Both counties are experiencing increased COVID-19 activity yet have had strikingly different outcomes in the student/staff population. While supporting the case for wearing masks to prevent disease transmission, one can’t ignore the adolescents who were infected and vaccine eligible (District A: 706; District B: 1,582). Their vaccination status could not be determined.
As pediatricians we have played an integral part in the elimination of diseases through educating and administering vaccinations. Adolescents are relatively healthy, thus limiting the number of encounters with them. The majority complete the 11-year visit; however, many fail to return for the 16- to 18-year visit.
So how are we doing? CDC data from 10 U.S. jurisdictions demonstrated a substantial decrease in vaccine administration between March and May of 2020, compared with the same period in 2018 and 2019. A decline was anticipated because of the nationwide lockdown. Doses of HPV administered declined almost 64% and 71% for 9- to 12-year-olds and 13- to 17-year-olds, respectively. Tdap administration declined 66% and 61% for the same respective age groups. Although administered doses increased between June and September of 2020, it was not sufficient to achieve catch-up coverage. Compared to the same period in 2018-2019, administration of the HPV vaccine declined 12.8% and 28% (ages 9-12 and ages 13-17) and for Tdap it was 21% and 30% lower (ages 9-12 and ages 13-17) (MMWR Morb Mortal Wkly Rep. 2021;70:840-5).
Now, we have another adolescent vaccine to discuss and encourage our patients to receive. We also need to address their concerns and/or to at least direct them to a reliable source to obtain accurate information. For the first time, a recommended vaccine may not be available at their medical home. Many don’t know where to go to receive it (http://www.vaccines.gov). Results of a Kaiser Family Foundation COVID-19 survey (August 2021) indicated that parents trusted their pediatricians most often (78%) for vaccine advice. The respondents voiced concern about trusting the location where the child would be immunized and long-term effects especially related to fertility. Parents who received communications regarding the benefits of vaccination were twice as likely to have their adolescents immunized. Finally, remember: Like parent, like child. An immunized parent is more likely to immunize the adolescent. (See Fig. 1.)

It is beyond the scope of this column to discuss the psychosocial aspects of this disease: children experiencing the death of teachers, classmates, family members, and those viewing the vitriol between pro- and antimask proponents often exhibited on school premises. And let’s not forget the child who wants to wear a mask but may be ostracized or bullied for doing so.
Our job is to do our very best to advocate for and to protect our patients by promoting mandatory masks at schools and encouraging vaccination of adolescents as we patiently wait for vaccines to become available for all of our children.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures.
COVID vaccine is safe, effective for children aged 5-11, Pfizer says
With record numbers of COVID-19 cases being reported in kids, Pfizer and its partner BioNTech have announced that their mRNA vaccine for COVID-19 is safe and appears to generate a protective immune response in children as young as 5.
The companies have been testing a lower dose of the vaccine -- just 10 milligrams -- in children between the ages of 5 and 11. That’s one-third the dose given to adults.
In a clinical trial that included more than 2,200 children, Pfizer says two doses of the vaccines given 3 weeks apart generated a high level of neutralizing antibodies, comparable to the level seen in older children who get a higher dose of the vaccine.
On the advice of its vaccine advisory committee, the Food and Drug Administration asked vaccine makers to include more children in these studies earlier this year.
Rather than testing whether the vaccines are preventing COVID-19 illness in children, as they did in adults, the pharmaceutical companies that make the COVID-19 vaccines are looking at the antibody levels generated by the vaccines instead. The FDA has approved the approach in hopes of speeding vaccines to children, who are now back in school full time in most parts of the United States.
With that in mind, Evan Anderson, MD, a doctor with Children’s Healthcare of Atlanta who is an investigator for the trial — and is therefore kept in the dark about its results — said it’s important to keep in mind that the company didn’t share any efficacy data today.
“We don’t know whether there were cases of COVID-19 among children that were enrolled in the study and how those compared in those who received placebo versus those that received vaccine,” he said.
The company says side effects seen in the trial are comparable to those seen in older children. The company said there were no cases of heart inflammation called myocarditis observed. Pfizer says they plan to send their data to the FDA as soon as possible.
The company says side effects seen in the trial are comparable to those seen in older children. Pfizer says they plan to send their data to the FDA as soon as possible.
“We are pleased to be able to submit data to regulatory authorities for this group of school-aged children before the start of the winter season,” Ugur Sahin, MD, CEO and co-founder of BioNTech, said in a news release. “The safety profile and immunogenicity data in children aged 5 to 11 years vaccinated at a lower dose are consistent with those we have observed with our vaccine in other older populations at a higher dose.”
When asked how soon the FDA might act on Pfizer’s application, Anderson said others had speculated about timelines of 4 to 6 weeks, but he also noted that the FDA could still exercise its authority to ask the company for more information, which could slow the process down.
“As a parent myself, I would love to see that timeline occurring quickly. However, I do want the FDA to fully review the data and ask the necessary questions,” he said. “It’s a little speculative to get too definitive with timelines.”
A version of this article first appeared on WebMD.com.
With record numbers of COVID-19 cases being reported in kids, Pfizer and its partner BioNTech have announced that their mRNA vaccine for COVID-19 is safe and appears to generate a protective immune response in children as young as 5.
The companies have been testing a lower dose of the vaccine -- just 10 milligrams -- in children between the ages of 5 and 11. That’s one-third the dose given to adults.
In a clinical trial that included more than 2,200 children, Pfizer says two doses of the vaccines given 3 weeks apart generated a high level of neutralizing antibodies, comparable to the level seen in older children who get a higher dose of the vaccine.
On the advice of its vaccine advisory committee, the Food and Drug Administration asked vaccine makers to include more children in these studies earlier this year.
Rather than testing whether the vaccines are preventing COVID-19 illness in children, as they did in adults, the pharmaceutical companies that make the COVID-19 vaccines are looking at the antibody levels generated by the vaccines instead. The FDA has approved the approach in hopes of speeding vaccines to children, who are now back in school full time in most parts of the United States.
With that in mind, Evan Anderson, MD, a doctor with Children’s Healthcare of Atlanta who is an investigator for the trial — and is therefore kept in the dark about its results — said it’s important to keep in mind that the company didn’t share any efficacy data today.
“We don’t know whether there were cases of COVID-19 among children that were enrolled in the study and how those compared in those who received placebo versus those that received vaccine,” he said.
The company says side effects seen in the trial are comparable to those seen in older children. The company said there were no cases of heart inflammation called myocarditis observed. Pfizer says they plan to send their data to the FDA as soon as possible.
The company says side effects seen in the trial are comparable to those seen in older children. Pfizer says they plan to send their data to the FDA as soon as possible.
“We are pleased to be able to submit data to regulatory authorities for this group of school-aged children before the start of the winter season,” Ugur Sahin, MD, CEO and co-founder of BioNTech, said in a news release. “The safety profile and immunogenicity data in children aged 5 to 11 years vaccinated at a lower dose are consistent with those we have observed with our vaccine in other older populations at a higher dose.”
When asked how soon the FDA might act on Pfizer’s application, Anderson said others had speculated about timelines of 4 to 6 weeks, but he also noted that the FDA could still exercise its authority to ask the company for more information, which could slow the process down.
“As a parent myself, I would love to see that timeline occurring quickly. However, I do want the FDA to fully review the data and ask the necessary questions,” he said. “It’s a little speculative to get too definitive with timelines.”
A version of this article first appeared on WebMD.com.
With record numbers of COVID-19 cases being reported in kids, Pfizer and its partner BioNTech have announced that their mRNA vaccine for COVID-19 is safe and appears to generate a protective immune response in children as young as 5.
The companies have been testing a lower dose of the vaccine -- just 10 milligrams -- in children between the ages of 5 and 11. That’s one-third the dose given to adults.
In a clinical trial that included more than 2,200 children, Pfizer says two doses of the vaccines given 3 weeks apart generated a high level of neutralizing antibodies, comparable to the level seen in older children who get a higher dose of the vaccine.
On the advice of its vaccine advisory committee, the Food and Drug Administration asked vaccine makers to include more children in these studies earlier this year.
Rather than testing whether the vaccines are preventing COVID-19 illness in children, as they did in adults, the pharmaceutical companies that make the COVID-19 vaccines are looking at the antibody levels generated by the vaccines instead. The FDA has approved the approach in hopes of speeding vaccines to children, who are now back in school full time in most parts of the United States.
With that in mind, Evan Anderson, MD, a doctor with Children’s Healthcare of Atlanta who is an investigator for the trial — and is therefore kept in the dark about its results — said it’s important to keep in mind that the company didn’t share any efficacy data today.
“We don’t know whether there were cases of COVID-19 among children that were enrolled in the study and how those compared in those who received placebo versus those that received vaccine,” he said.
The company says side effects seen in the trial are comparable to those seen in older children. The company said there were no cases of heart inflammation called myocarditis observed. Pfizer says they plan to send their data to the FDA as soon as possible.
The company says side effects seen in the trial are comparable to those seen in older children. Pfizer says they plan to send their data to the FDA as soon as possible.
“We are pleased to be able to submit data to regulatory authorities for this group of school-aged children before the start of the winter season,” Ugur Sahin, MD, CEO and co-founder of BioNTech, said in a news release. “The safety profile and immunogenicity data in children aged 5 to 11 years vaccinated at a lower dose are consistent with those we have observed with our vaccine in other older populations at a higher dose.”
When asked how soon the FDA might act on Pfizer’s application, Anderson said others had speculated about timelines of 4 to 6 weeks, but he also noted that the FDA could still exercise its authority to ask the company for more information, which could slow the process down.
“As a parent myself, I would love to see that timeline occurring quickly. However, I do want the FDA to fully review the data and ask the necessary questions,” he said. “It’s a little speculative to get too definitive with timelines.”
A version of this article first appeared on WebMD.com.
Parent-led intervention linked with decreased autism symptoms in at-risk infants
These findings, which were published in JAMA Pediatrics, were the first evidence worldwide that a preemptive intervention during infancy could lead to such a significant improvement in children’s social development, resulting in “three times fewer diagnoses of autism at age 3,” said lead author Andrew Whitehouse, PhD, in a statement.
“No trial of a preemptive infant intervention, applied prior to diagnosis, has to date shown such an effect to impact diagnostic outcomes – until now,” he said.
Study intervention is a nontraditonal approach
Dr. Whitehouse, who is professor of Autism Research at Telethon Kids and University of Western Australia, and Director of CliniKids in Perth, said the intervention is a departure from traditional approaches. “Traditionally, therapy seeks to train children to learn ‘typical’ behaviors,” he said in an email. “The difference of this therapy is that we help parents understand the unique abilities of their baby, and to use these strengths as a foundation for future development.”
Dr. Whitehouse’s study included 103 children (aged approximately 12 months), who displayed at least three of five behaviors indicating a high likelihood of ASD as defined by the Social Attention and Communication Surveillance–Revised (SACS-R) 12-month checklist. The infants were randomized to receive either usual care or the intervention, which is called the iBASIS–Video Interaction to Promote Positive Parenting (iBASIS-VIPP). Usual care was delivered by community physicians, whereas the intervention involved 10 sessions delivered at home by a trained therapist.
“The iBASIS-VIPP uses video-feedback as a means of helping parents recognize their baby’s communication cues so they can respond in a way that builds their social communication development,” Dr. Whitehouse explained in an interview. “The therapist then provides guidance to the parent as to how their baby is communicating with them, and they can communicate back to have back-and-forth conversations.”
“We know these back-and-forth conversations are crucial to support early social communication development, and are a precursor to more complex skills, such as verbal language,” he added.
Reassessment of the children at age 3 years showed a “small but enduring” benefit of the intervention, noted the authors. Children in the intervention group had a reduction in ASD symptom severity (P = .04), and reduced odds of ASD classification, compared with children receiving usual care (6.7% vs. 20.5%; odds ratio, 0.18; P = .02).
The findings provide “initial evidence of efficacy for a new clinical model that uses a specific developmentally focused intervention,” noted the authors. “The children falling below the diagnostic threshold still had developmental difficulties, but by working with each child’s unique differences, rather than trying to counter them, the therapy has effectively supported their development through the early childhood years,” noted Dr. Whitehouse in a statement.
Other research has shown benefits of new study approach
This is a “solid” study, “but, as acknowledged by the authors, the main effects are small in magnitude, and longer-term outcomes will be important to capture,” said Jessica Brian, PhD, C Psych, associate professor in the department of pediatrics at the University of Toronto, colead at the Autism Research Centre, and psychologist and clinician-investigator at Holland Bloorview Kids Rehab Hospital in Toronto.
Dr. Brian said she and her coauthors of a paper published in Autism Research and others have shown that the kind of approach used in the new study can be helpful for enhancing different areas of toddler development, but “the specific finding of reduced likelihood of a clinical ASD diagnosis is a bit different.”
The goal of reducing the likelihood of an ASD diagnosis “needs to be considered carefully, from the perspective of autism acceptance,” she added. “From an acceptance lens, the primary objective of early intervention in ASD might be better positioned as aiming to enhance or support a young child’s development, help them make developmental progress, build on their strengths, optimize outcomes, or reduce impairment. … I think the authors do a good job of balancing this perspective.”
New study shows value of parent-mediated interventions
Overall, Dr. Brian, who coauthored the Canadian Paediatric Society’s position statement on ASD diagnosis, lauded the findings as good news.
“It shows the value of using parent-mediated interventions, which are far less costly and are more resource-efficient than most therapist-delivered models.”
“In cases where parent-mediated approaches are made available to families prior to diagnosis, there is potential for strong effects, when the brain is most amenable to learning. Such models may also be an ideal fit before diagnosis, since they are less resource-intensive than therapist-delivered models, which may only be funded by governments once a diagnosis is confirmed,” she said.
“Finally, parent-mediated programs have the potential to support parents during what, for many families, is a particularly challenging time as they identify their child’s developmental differences or receive a diagnosis. Such programs have potential to increase parents’ confidence in parenting a young child with unique learning needs.”
What Dr. Brian thought was missing from the paper was acknowledgment that, “despite early developmental gains from parent-mediated interventions, it is likely that most children with ASD will need additional supports throughout development.”
This study was sponsored by the Telethon Kids Institute. Dr. Whitehouse reported no conflicts of interest. Dr. Brian codeveloped a parent-mediated intervention for toddlers with probable or confirmed ASD (the Social ABCs), for which she does not receive any royalties.
These findings, which were published in JAMA Pediatrics, were the first evidence worldwide that a preemptive intervention during infancy could lead to such a significant improvement in children’s social development, resulting in “three times fewer diagnoses of autism at age 3,” said lead author Andrew Whitehouse, PhD, in a statement.
“No trial of a preemptive infant intervention, applied prior to diagnosis, has to date shown such an effect to impact diagnostic outcomes – until now,” he said.
Study intervention is a nontraditonal approach
Dr. Whitehouse, who is professor of Autism Research at Telethon Kids and University of Western Australia, and Director of CliniKids in Perth, said the intervention is a departure from traditional approaches. “Traditionally, therapy seeks to train children to learn ‘typical’ behaviors,” he said in an email. “The difference of this therapy is that we help parents understand the unique abilities of their baby, and to use these strengths as a foundation for future development.”
Dr. Whitehouse’s study included 103 children (aged approximately 12 months), who displayed at least three of five behaviors indicating a high likelihood of ASD as defined by the Social Attention and Communication Surveillance–Revised (SACS-R) 12-month checklist. The infants were randomized to receive either usual care or the intervention, which is called the iBASIS–Video Interaction to Promote Positive Parenting (iBASIS-VIPP). Usual care was delivered by community physicians, whereas the intervention involved 10 sessions delivered at home by a trained therapist.
“The iBASIS-VIPP uses video-feedback as a means of helping parents recognize their baby’s communication cues so they can respond in a way that builds their social communication development,” Dr. Whitehouse explained in an interview. “The therapist then provides guidance to the parent as to how their baby is communicating with them, and they can communicate back to have back-and-forth conversations.”
“We know these back-and-forth conversations are crucial to support early social communication development, and are a precursor to more complex skills, such as verbal language,” he added.
Reassessment of the children at age 3 years showed a “small but enduring” benefit of the intervention, noted the authors. Children in the intervention group had a reduction in ASD symptom severity (P = .04), and reduced odds of ASD classification, compared with children receiving usual care (6.7% vs. 20.5%; odds ratio, 0.18; P = .02).
The findings provide “initial evidence of efficacy for a new clinical model that uses a specific developmentally focused intervention,” noted the authors. “The children falling below the diagnostic threshold still had developmental difficulties, but by working with each child’s unique differences, rather than trying to counter them, the therapy has effectively supported their development through the early childhood years,” noted Dr. Whitehouse in a statement.
Other research has shown benefits of new study approach
This is a “solid” study, “but, as acknowledged by the authors, the main effects are small in magnitude, and longer-term outcomes will be important to capture,” said Jessica Brian, PhD, C Psych, associate professor in the department of pediatrics at the University of Toronto, colead at the Autism Research Centre, and psychologist and clinician-investigator at Holland Bloorview Kids Rehab Hospital in Toronto.
Dr. Brian said she and her coauthors of a paper published in Autism Research and others have shown that the kind of approach used in the new study can be helpful for enhancing different areas of toddler development, but “the specific finding of reduced likelihood of a clinical ASD diagnosis is a bit different.”
The goal of reducing the likelihood of an ASD diagnosis “needs to be considered carefully, from the perspective of autism acceptance,” she added. “From an acceptance lens, the primary objective of early intervention in ASD might be better positioned as aiming to enhance or support a young child’s development, help them make developmental progress, build on their strengths, optimize outcomes, or reduce impairment. … I think the authors do a good job of balancing this perspective.”
New study shows value of parent-mediated interventions
Overall, Dr. Brian, who coauthored the Canadian Paediatric Society’s position statement on ASD diagnosis, lauded the findings as good news.
“It shows the value of using parent-mediated interventions, which are far less costly and are more resource-efficient than most therapist-delivered models.”
“In cases where parent-mediated approaches are made available to families prior to diagnosis, there is potential for strong effects, when the brain is most amenable to learning. Such models may also be an ideal fit before diagnosis, since they are less resource-intensive than therapist-delivered models, which may only be funded by governments once a diagnosis is confirmed,” she said.
“Finally, parent-mediated programs have the potential to support parents during what, for many families, is a particularly challenging time as they identify their child’s developmental differences or receive a diagnosis. Such programs have potential to increase parents’ confidence in parenting a young child with unique learning needs.”
What Dr. Brian thought was missing from the paper was acknowledgment that, “despite early developmental gains from parent-mediated interventions, it is likely that most children with ASD will need additional supports throughout development.”
This study was sponsored by the Telethon Kids Institute. Dr. Whitehouse reported no conflicts of interest. Dr. Brian codeveloped a parent-mediated intervention for toddlers with probable or confirmed ASD (the Social ABCs), for which she does not receive any royalties.
These findings, which were published in JAMA Pediatrics, were the first evidence worldwide that a preemptive intervention during infancy could lead to such a significant improvement in children’s social development, resulting in “three times fewer diagnoses of autism at age 3,” said lead author Andrew Whitehouse, PhD, in a statement.
“No trial of a preemptive infant intervention, applied prior to diagnosis, has to date shown such an effect to impact diagnostic outcomes – until now,” he said.
Study intervention is a nontraditonal approach
Dr. Whitehouse, who is professor of Autism Research at Telethon Kids and University of Western Australia, and Director of CliniKids in Perth, said the intervention is a departure from traditional approaches. “Traditionally, therapy seeks to train children to learn ‘typical’ behaviors,” he said in an email. “The difference of this therapy is that we help parents understand the unique abilities of their baby, and to use these strengths as a foundation for future development.”
Dr. Whitehouse’s study included 103 children (aged approximately 12 months), who displayed at least three of five behaviors indicating a high likelihood of ASD as defined by the Social Attention and Communication Surveillance–Revised (SACS-R) 12-month checklist. The infants were randomized to receive either usual care or the intervention, which is called the iBASIS–Video Interaction to Promote Positive Parenting (iBASIS-VIPP). Usual care was delivered by community physicians, whereas the intervention involved 10 sessions delivered at home by a trained therapist.
“The iBASIS-VIPP uses video-feedback as a means of helping parents recognize their baby’s communication cues so they can respond in a way that builds their social communication development,” Dr. Whitehouse explained in an interview. “The therapist then provides guidance to the parent as to how their baby is communicating with them, and they can communicate back to have back-and-forth conversations.”
“We know these back-and-forth conversations are crucial to support early social communication development, and are a precursor to more complex skills, such as verbal language,” he added.
Reassessment of the children at age 3 years showed a “small but enduring” benefit of the intervention, noted the authors. Children in the intervention group had a reduction in ASD symptom severity (P = .04), and reduced odds of ASD classification, compared with children receiving usual care (6.7% vs. 20.5%; odds ratio, 0.18; P = .02).
The findings provide “initial evidence of efficacy for a new clinical model that uses a specific developmentally focused intervention,” noted the authors. “The children falling below the diagnostic threshold still had developmental difficulties, but by working with each child’s unique differences, rather than trying to counter them, the therapy has effectively supported their development through the early childhood years,” noted Dr. Whitehouse in a statement.
Other research has shown benefits of new study approach
This is a “solid” study, “but, as acknowledged by the authors, the main effects are small in magnitude, and longer-term outcomes will be important to capture,” said Jessica Brian, PhD, C Psych, associate professor in the department of pediatrics at the University of Toronto, colead at the Autism Research Centre, and psychologist and clinician-investigator at Holland Bloorview Kids Rehab Hospital in Toronto.
Dr. Brian said she and her coauthors of a paper published in Autism Research and others have shown that the kind of approach used in the new study can be helpful for enhancing different areas of toddler development, but “the specific finding of reduced likelihood of a clinical ASD diagnosis is a bit different.”
The goal of reducing the likelihood of an ASD diagnosis “needs to be considered carefully, from the perspective of autism acceptance,” she added. “From an acceptance lens, the primary objective of early intervention in ASD might be better positioned as aiming to enhance or support a young child’s development, help them make developmental progress, build on their strengths, optimize outcomes, or reduce impairment. … I think the authors do a good job of balancing this perspective.”
New study shows value of parent-mediated interventions
Overall, Dr. Brian, who coauthored the Canadian Paediatric Society’s position statement on ASD diagnosis, lauded the findings as good news.
“It shows the value of using parent-mediated interventions, which are far less costly and are more resource-efficient than most therapist-delivered models.”
“In cases where parent-mediated approaches are made available to families prior to diagnosis, there is potential for strong effects, when the brain is most amenable to learning. Such models may also be an ideal fit before diagnosis, since they are less resource-intensive than therapist-delivered models, which may only be funded by governments once a diagnosis is confirmed,” she said.
“Finally, parent-mediated programs have the potential to support parents during what, for many families, is a particularly challenging time as they identify their child’s developmental differences or receive a diagnosis. Such programs have potential to increase parents’ confidence in parenting a young child with unique learning needs.”
What Dr. Brian thought was missing from the paper was acknowledgment that, “despite early developmental gains from parent-mediated interventions, it is likely that most children with ASD will need additional supports throughout development.”
This study was sponsored by the Telethon Kids Institute. Dr. Whitehouse reported no conflicts of interest. Dr. Brian codeveloped a parent-mediated intervention for toddlers with probable or confirmed ASD (the Social ABCs), for which she does not receive any royalties.
FROM JAMA PEDIATRICS
European agency recommends two new adalimumab biosimilars
The European Medicines Agency’s Committee for Medicinal Products for Human Use recommended marketing authorization this week for two new adalimumab biosimilars, Hukyndra and Libmyris.
The biosimilars, both developed by STADA Arzneimittel AG, will be available as a 40-mg solution for injection in a pre-filled syringe and pre-filled pen and 80-mg solution for injection in a pre-filled syringe. Both biosimilars will have 15 indications:
- rheumatoid arthritis
- polyarticular juvenile idiopathic arthritis
- enthesitis-related arthritis
- ankylosing spondylitis
- axial spondyloarthritis without radiographic evidence of ankylosing spondylitis
- psoriatic arthritis
- chronic plaque psoriasis (adults and children)
- hidradenitis suppurativa
- Crohn’s disease (adults and children)
- ulcerative colitis (adults and children)
- uveitis (adults and children)
Data show that both Hukyndra and Libmyris are highly similar to the reference product Humira (adalimumab), a monoclonal antibody to tumor necrosis factor alpha, and have comparable quality, safety, and efficacy.
A version of this article first appeared on Medscape.com.
The European Medicines Agency’s Committee for Medicinal Products for Human Use recommended marketing authorization this week for two new adalimumab biosimilars, Hukyndra and Libmyris.
The biosimilars, both developed by STADA Arzneimittel AG, will be available as a 40-mg solution for injection in a pre-filled syringe and pre-filled pen and 80-mg solution for injection in a pre-filled syringe. Both biosimilars will have 15 indications:
- rheumatoid arthritis
- polyarticular juvenile idiopathic arthritis
- enthesitis-related arthritis
- ankylosing spondylitis
- axial spondyloarthritis without radiographic evidence of ankylosing spondylitis
- psoriatic arthritis
- chronic plaque psoriasis (adults and children)
- hidradenitis suppurativa
- Crohn’s disease (adults and children)
- ulcerative colitis (adults and children)
- uveitis (adults and children)
Data show that both Hukyndra and Libmyris are highly similar to the reference product Humira (adalimumab), a monoclonal antibody to tumor necrosis factor alpha, and have comparable quality, safety, and efficacy.
A version of this article first appeared on Medscape.com.
The European Medicines Agency’s Committee for Medicinal Products for Human Use recommended marketing authorization this week for two new adalimumab biosimilars, Hukyndra and Libmyris.
The biosimilars, both developed by STADA Arzneimittel AG, will be available as a 40-mg solution for injection in a pre-filled syringe and pre-filled pen and 80-mg solution for injection in a pre-filled syringe. Both biosimilars will have 15 indications:
- rheumatoid arthritis
- polyarticular juvenile idiopathic arthritis
- enthesitis-related arthritis
- ankylosing spondylitis
- axial spondyloarthritis without radiographic evidence of ankylosing spondylitis
- psoriatic arthritis
- chronic plaque psoriasis (adults and children)
- hidradenitis suppurativa
- Crohn’s disease (adults and children)
- ulcerative colitis (adults and children)
- uveitis (adults and children)
Data show that both Hukyndra and Libmyris are highly similar to the reference product Humira (adalimumab), a monoclonal antibody to tumor necrosis factor alpha, and have comparable quality, safety, and efficacy.
A version of this article first appeared on Medscape.com.
FDA panel backs Pfizer's COVID booster for 65 and older, those at high risk
An expert panel that advises the Food and Drug Administration on its regulatory decisions voted Sept. 17 against recommending third doses of Pfizer’s COVID-19 vaccine for younger Americans.
But they didn’t kill the idea of booster shots completely.
In a dramatic, last-minute pivot, the 18 members of the FDA’s Vaccines and Related Biological Products Advisory Committee unanimously voted to recommend the FDA make boosters available for seniors and others at high risk of severe outcomes from COVID-19, including health care workers.
The 16-2 vote was a rebuttal to Pfizer’s initial request. The company had asked the FDA to allow it to offer third doses to all Americans over the age of 16 at least six months after their second shot.
The company requested an amendment to the full approval the FDA granted in August. That is the typical way boosters are authorized in the U.S., but it requires a higher bar of evidence and more regulatory scrutiny than the agency had been able to give since Pfizer filed for the change just days after its vaccine was granted full approval.
The committee’s actions were also a rebuff to the Biden administration, which announced before the FDA approved them that boosters would be rolled out to the general public Sept. 20. The announcement triggered the resignations of two of the agency’s top vaccine reviewers, who both participated in the Sept. 17 meeting.
After initially voting against Pfizer’s request to amend its license, the committee then worked on the fly with FDA officials to craft a strategy that would allow third doses to be offered under an emergency use authorization (EUA).
An EUA requires a lower standard of evidence and is more specific. It will restrict third doses to a more defined population than a change to the license would. It will also require Pfizer to continue to monitor the safety of third doses as they begin to be administered.
“This should demonstrate to the public that the members of this committee are independent of the FDA and that we do, in fact, bring our voices to the table when we are asked to serve on this committee,” said Archana Chattergee, MD, a pediatric infectious disease specialist who is dean of the Chicago Medical School at Rosalind Franklin University in Illinois.
The FDA doesn’t have to follow the committee’s recommendation, but almost certainly will, though regulators said they may still make some changes.
“We are not bound at FDA by your vote, we can tweak this,” said Peter Marks, MD, director of the Center for Biologics Evaluation and Research at the FDA. Dr. Marks participated in the meeting and helped to draft the revised proposal.
If the FDA issues the recommended EUA, a council of independent advisors to the CDC will make specific recommendations about how the third doses should be given. After the CDC director weighs in, boosters will begin rolling out to the public.
Moderna submitted data to the FDA on Sept. 1 in support of adding a booster dose to its regimen. The agency has not yet scheduled a public review of that data.
The Biden administration is prepared to administer shots as soon as they get the green light, Surgeon General Vivek Murthy, MD, said at a White House briefing earlier Sept. 17.
"This process is consistent with what we outlined in August where our goals were to stay ahead of the virus," Dr. Murthy said. "Our goal then and now is to protect the health and well-being of the public. As soon as the FDA and CDC complete their evaluations, we will be ready to move forward accordingly."
He added, "We've used this time since our August announcement to communicate and coordinate with pharmacy partners, nursing homes, states, and localities."
White House COVID-19 Response Coordinator Jeff Zients said vaccine supply is "in good shape for all Americans to get boosters as recommended."
Taking cues from Israel
In considering Pfizer’s original request, the committee overwhelmingly felt that they didn’t have enough information to say that the benefits of an additional dose of vaccine in 16- and 17-year-olds would outweigh its risk. Teens have the highest risk of rare heart inflammation after vaccination, a side effect known as myocarditis. It is not known how the vaccines are causing these cases of heart swelling. Most who have been diagnosed with the condition have recovered, though some have needed hospital care.
Pfizer didn’t include 16- and 17-year-olds in its studies of boosters, which included about 300 people between the ages of 18 and 55. The company acknowledged that gap in its data but pointed to FDA guidance that said evidence from adults could be extrapolated to teens.
“We don’t know that much about risks,” said committee member Eric Rubin, MD, who is editor-in-chief of the New England Journal of Medicine.
Much of the data on the potential benefits and harms of third Pfizer doses comes from Israel, which first began rolling out boosters to older adults in July.
In a highly anticipated presentation, Sharon Alroy-Preis, Israel’s director of public health services, joined the meeting to describe Israel’s experience with boosters.
Israel began to see a third surge of COVID-19 cases in December.
“This was after having two waves and two lockdowns,” Ms. Alroy-Preis said. By the third surge, she said, Israelis were tired.
“We decided on a lockdown, but the compliance of the public wasn’t as it was in the previous two waves,” she said.
Then the vaccine arrived. Israel started vaccinations as soon as the FDA approved it, and they quickly vaccinated a high percentage of their population, about 3 months faster than the rest of the world.
All vaccinations are reported and tracked by the Ministry of Health, so the country is able to keep close tabs on how well the shots are working.
As vaccines rolled out, cases fell dramatically. The pandemic seemed to be behind them. Delta arrived in March. By June, their cases were doubling every 10 days, despite about 80% of their most vulnerable adults being fully vaccinated, she said.
Most concerning was that about 60% of severe cases were breakthrough cases in fully vaccinated individuals.
“We had to stop and figure out, was this a Delta issue,” she said. “Or was this a waning immunity issue.”
“We had some clue that it might not be the Delta variant, at least not alone,” she said.
People who had originally been first in line for the vaccines, seniors and health care workers, were having the highest rates of breakthrough infections. People further away from their second dose were more likely to get a breakthrough infection.
Ms. Alroy-Preis said that if they had not started booster doses in July, their hospitals would have been overwhelmed. They had projected that they would have 2,000 cases in the hospital each day.
Boosters have helped to flatten the curve, though they are still seeing a significant number of infections.
Data from Israel presented at the meeting show boosters are largely safe and effective at reducing severe outcomes in seniors. Israeli experience also showed that third doses, which generate very high levels of neutralizing antibodies—the first and fastest line of the body’s immune defense - -may also slow transmission of the virus.
Key differences in the U.S.
The benefit of slowing down the explosive spread of a highly contagious virus was tantalizing, but many members noted that circumstances in Israel are very different than in the United States. Israel went into its current Delta surge already having high levels of vaccination in its population. They also relied on the Pfizer vaccine almost exclusively for their campaign.
The United States used a different mix of vaccines – Pfizer, Moderna, and Johnson & Johnson -- and doesn’t have the same high level of vaccination coverage of its population.
In the United States, transmission is mainly being driven by unvaccinated people, Dr. Rubin noted.
“That really means the primary benefit is going to be in reducing disease,” he said, “And we know the people who are going to benefit from that … and those are the kinds of people the FDA has already approved a third dose for,” he said, referring to those with underlying health conditions.
But Israel only began vaccinating younger people a few weeks ago. Most are still within a window where rare risks like myocarditis could appear, Rubin noted.
He and other members of the committee said they wished they had more information about the safety of third doses in younger adults.
“We don’t have that right now, and I don’t think I would be comfortable giving it to a 16-year-old,” he said.
At the same time, the primary benefit for third doses would be in preventing severe disease, and overall, data from the United States and other countries show that two doses of the vaccines remain highly effective at preventing hospitalization and death.
Asked why Israel began to see more severe cases in fully vaccinated people, the CDC’s Sara Oliver, MD, a disease detective with the CDC, said it was probably due to a mix of factors including the fact that Israel defines severe cases a little differently.
In the United States, a severe case is generally a person who has to be hospitalized or who has died from the infection. In Israel, a person with a severe case is someone who has an elevated respiratory rate and someone who has a blood oxygen level less than 94%. In the United States, that kind of patient wouldn’t necessarily be hospitalized.
In the end, one of the two committee members who wanted full approval for Pfizer’s third doses said he was satisfied with the outcome.
Mark Sawyer, MD, a professor of pediatrics and infectious disease at the University of California at San Diego, said he voted yes on the first question because he thought full approval was the best way to give doctors the flexibility to prescribe the shots to vulnerable individuals.
“I’m really glad we authorized a vaccine for a third dose, and I plan to go out and get my vaccine this afternoon,” Dr. Sawyer said, noting that he was at high risk as a health care provider.
This article was updated 9/19/21.
A version of this article first appeared on Medscape.com.
An expert panel that advises the Food and Drug Administration on its regulatory decisions voted Sept. 17 against recommending third doses of Pfizer’s COVID-19 vaccine for younger Americans.
But they didn’t kill the idea of booster shots completely.
In a dramatic, last-minute pivot, the 18 members of the FDA’s Vaccines and Related Biological Products Advisory Committee unanimously voted to recommend the FDA make boosters available for seniors and others at high risk of severe outcomes from COVID-19, including health care workers.
The 16-2 vote was a rebuttal to Pfizer’s initial request. The company had asked the FDA to allow it to offer third doses to all Americans over the age of 16 at least six months after their second shot.
The company requested an amendment to the full approval the FDA granted in August. That is the typical way boosters are authorized in the U.S., but it requires a higher bar of evidence and more regulatory scrutiny than the agency had been able to give since Pfizer filed for the change just days after its vaccine was granted full approval.
The committee’s actions were also a rebuff to the Biden administration, which announced before the FDA approved them that boosters would be rolled out to the general public Sept. 20. The announcement triggered the resignations of two of the agency’s top vaccine reviewers, who both participated in the Sept. 17 meeting.
After initially voting against Pfizer’s request to amend its license, the committee then worked on the fly with FDA officials to craft a strategy that would allow third doses to be offered under an emergency use authorization (EUA).
An EUA requires a lower standard of evidence and is more specific. It will restrict third doses to a more defined population than a change to the license would. It will also require Pfizer to continue to monitor the safety of third doses as they begin to be administered.
“This should demonstrate to the public that the members of this committee are independent of the FDA and that we do, in fact, bring our voices to the table when we are asked to serve on this committee,” said Archana Chattergee, MD, a pediatric infectious disease specialist who is dean of the Chicago Medical School at Rosalind Franklin University in Illinois.
The FDA doesn’t have to follow the committee’s recommendation, but almost certainly will, though regulators said they may still make some changes.
“We are not bound at FDA by your vote, we can tweak this,” said Peter Marks, MD, director of the Center for Biologics Evaluation and Research at the FDA. Dr. Marks participated in the meeting and helped to draft the revised proposal.
If the FDA issues the recommended EUA, a council of independent advisors to the CDC will make specific recommendations about how the third doses should be given. After the CDC director weighs in, boosters will begin rolling out to the public.
Moderna submitted data to the FDA on Sept. 1 in support of adding a booster dose to its regimen. The agency has not yet scheduled a public review of that data.
The Biden administration is prepared to administer shots as soon as they get the green light, Surgeon General Vivek Murthy, MD, said at a White House briefing earlier Sept. 17.
"This process is consistent with what we outlined in August where our goals were to stay ahead of the virus," Dr. Murthy said. "Our goal then and now is to protect the health and well-being of the public. As soon as the FDA and CDC complete their evaluations, we will be ready to move forward accordingly."
He added, "We've used this time since our August announcement to communicate and coordinate with pharmacy partners, nursing homes, states, and localities."
White House COVID-19 Response Coordinator Jeff Zients said vaccine supply is "in good shape for all Americans to get boosters as recommended."
Taking cues from Israel
In considering Pfizer’s original request, the committee overwhelmingly felt that they didn’t have enough information to say that the benefits of an additional dose of vaccine in 16- and 17-year-olds would outweigh its risk. Teens have the highest risk of rare heart inflammation after vaccination, a side effect known as myocarditis. It is not known how the vaccines are causing these cases of heart swelling. Most who have been diagnosed with the condition have recovered, though some have needed hospital care.
Pfizer didn’t include 16- and 17-year-olds in its studies of boosters, which included about 300 people between the ages of 18 and 55. The company acknowledged that gap in its data but pointed to FDA guidance that said evidence from adults could be extrapolated to teens.
“We don’t know that much about risks,” said committee member Eric Rubin, MD, who is editor-in-chief of the New England Journal of Medicine.
Much of the data on the potential benefits and harms of third Pfizer doses comes from Israel, which first began rolling out boosters to older adults in July.
In a highly anticipated presentation, Sharon Alroy-Preis, Israel’s director of public health services, joined the meeting to describe Israel’s experience with boosters.
Israel began to see a third surge of COVID-19 cases in December.
“This was after having two waves and two lockdowns,” Ms. Alroy-Preis said. By the third surge, she said, Israelis were tired.
“We decided on a lockdown, but the compliance of the public wasn’t as it was in the previous two waves,” she said.
Then the vaccine arrived. Israel started vaccinations as soon as the FDA approved it, and they quickly vaccinated a high percentage of their population, about 3 months faster than the rest of the world.
All vaccinations are reported and tracked by the Ministry of Health, so the country is able to keep close tabs on how well the shots are working.
As vaccines rolled out, cases fell dramatically. The pandemic seemed to be behind them. Delta arrived in March. By June, their cases were doubling every 10 days, despite about 80% of their most vulnerable adults being fully vaccinated, she said.
Most concerning was that about 60% of severe cases were breakthrough cases in fully vaccinated individuals.
“We had to stop and figure out, was this a Delta issue,” she said. “Or was this a waning immunity issue.”
“We had some clue that it might not be the Delta variant, at least not alone,” she said.
People who had originally been first in line for the vaccines, seniors and health care workers, were having the highest rates of breakthrough infections. People further away from their second dose were more likely to get a breakthrough infection.
Ms. Alroy-Preis said that if they had not started booster doses in July, their hospitals would have been overwhelmed. They had projected that they would have 2,000 cases in the hospital each day.
Boosters have helped to flatten the curve, though they are still seeing a significant number of infections.
Data from Israel presented at the meeting show boosters are largely safe and effective at reducing severe outcomes in seniors. Israeli experience also showed that third doses, which generate very high levels of neutralizing antibodies—the first and fastest line of the body’s immune defense - -may also slow transmission of the virus.
Key differences in the U.S.
The benefit of slowing down the explosive spread of a highly contagious virus was tantalizing, but many members noted that circumstances in Israel are very different than in the United States. Israel went into its current Delta surge already having high levels of vaccination in its population. They also relied on the Pfizer vaccine almost exclusively for their campaign.
The United States used a different mix of vaccines – Pfizer, Moderna, and Johnson & Johnson -- and doesn’t have the same high level of vaccination coverage of its population.
In the United States, transmission is mainly being driven by unvaccinated people, Dr. Rubin noted.
“That really means the primary benefit is going to be in reducing disease,” he said, “And we know the people who are going to benefit from that … and those are the kinds of people the FDA has already approved a third dose for,” he said, referring to those with underlying health conditions.
But Israel only began vaccinating younger people a few weeks ago. Most are still within a window where rare risks like myocarditis could appear, Rubin noted.
He and other members of the committee said they wished they had more information about the safety of third doses in younger adults.
“We don’t have that right now, and I don’t think I would be comfortable giving it to a 16-year-old,” he said.
At the same time, the primary benefit for third doses would be in preventing severe disease, and overall, data from the United States and other countries show that two doses of the vaccines remain highly effective at preventing hospitalization and death.
Asked why Israel began to see more severe cases in fully vaccinated people, the CDC’s Sara Oliver, MD, a disease detective with the CDC, said it was probably due to a mix of factors including the fact that Israel defines severe cases a little differently.
In the United States, a severe case is generally a person who has to be hospitalized or who has died from the infection. In Israel, a person with a severe case is someone who has an elevated respiratory rate and someone who has a blood oxygen level less than 94%. In the United States, that kind of patient wouldn’t necessarily be hospitalized.
In the end, one of the two committee members who wanted full approval for Pfizer’s third doses said he was satisfied with the outcome.
Mark Sawyer, MD, a professor of pediatrics and infectious disease at the University of California at San Diego, said he voted yes on the first question because he thought full approval was the best way to give doctors the flexibility to prescribe the shots to vulnerable individuals.
“I’m really glad we authorized a vaccine for a third dose, and I plan to go out and get my vaccine this afternoon,” Dr. Sawyer said, noting that he was at high risk as a health care provider.
This article was updated 9/19/21.
A version of this article first appeared on Medscape.com.
An expert panel that advises the Food and Drug Administration on its regulatory decisions voted Sept. 17 against recommending third doses of Pfizer’s COVID-19 vaccine for younger Americans.
But they didn’t kill the idea of booster shots completely.
In a dramatic, last-minute pivot, the 18 members of the FDA’s Vaccines and Related Biological Products Advisory Committee unanimously voted to recommend the FDA make boosters available for seniors and others at high risk of severe outcomes from COVID-19, including health care workers.
The 16-2 vote was a rebuttal to Pfizer’s initial request. The company had asked the FDA to allow it to offer third doses to all Americans over the age of 16 at least six months after their second shot.
The company requested an amendment to the full approval the FDA granted in August. That is the typical way boosters are authorized in the U.S., but it requires a higher bar of evidence and more regulatory scrutiny than the agency had been able to give since Pfizer filed for the change just days after its vaccine was granted full approval.
The committee’s actions were also a rebuff to the Biden administration, which announced before the FDA approved them that boosters would be rolled out to the general public Sept. 20. The announcement triggered the resignations of two of the agency’s top vaccine reviewers, who both participated in the Sept. 17 meeting.
After initially voting against Pfizer’s request to amend its license, the committee then worked on the fly with FDA officials to craft a strategy that would allow third doses to be offered under an emergency use authorization (EUA).
An EUA requires a lower standard of evidence and is more specific. It will restrict third doses to a more defined population than a change to the license would. It will also require Pfizer to continue to monitor the safety of third doses as they begin to be administered.
“This should demonstrate to the public that the members of this committee are independent of the FDA and that we do, in fact, bring our voices to the table when we are asked to serve on this committee,” said Archana Chattergee, MD, a pediatric infectious disease specialist who is dean of the Chicago Medical School at Rosalind Franklin University in Illinois.
The FDA doesn’t have to follow the committee’s recommendation, but almost certainly will, though regulators said they may still make some changes.
“We are not bound at FDA by your vote, we can tweak this,” said Peter Marks, MD, director of the Center for Biologics Evaluation and Research at the FDA. Dr. Marks participated in the meeting and helped to draft the revised proposal.
If the FDA issues the recommended EUA, a council of independent advisors to the CDC will make specific recommendations about how the third doses should be given. After the CDC director weighs in, boosters will begin rolling out to the public.
Moderna submitted data to the FDA on Sept. 1 in support of adding a booster dose to its regimen. The agency has not yet scheduled a public review of that data.
The Biden administration is prepared to administer shots as soon as they get the green light, Surgeon General Vivek Murthy, MD, said at a White House briefing earlier Sept. 17.
"This process is consistent with what we outlined in August where our goals were to stay ahead of the virus," Dr. Murthy said. "Our goal then and now is to protect the health and well-being of the public. As soon as the FDA and CDC complete their evaluations, we will be ready to move forward accordingly."
He added, "We've used this time since our August announcement to communicate and coordinate with pharmacy partners, nursing homes, states, and localities."
White House COVID-19 Response Coordinator Jeff Zients said vaccine supply is "in good shape for all Americans to get boosters as recommended."
Taking cues from Israel
In considering Pfizer’s original request, the committee overwhelmingly felt that they didn’t have enough information to say that the benefits of an additional dose of vaccine in 16- and 17-year-olds would outweigh its risk. Teens have the highest risk of rare heart inflammation after vaccination, a side effect known as myocarditis. It is not known how the vaccines are causing these cases of heart swelling. Most who have been diagnosed with the condition have recovered, though some have needed hospital care.
Pfizer didn’t include 16- and 17-year-olds in its studies of boosters, which included about 300 people between the ages of 18 and 55. The company acknowledged that gap in its data but pointed to FDA guidance that said evidence from adults could be extrapolated to teens.
“We don’t know that much about risks,” said committee member Eric Rubin, MD, who is editor-in-chief of the New England Journal of Medicine.
Much of the data on the potential benefits and harms of third Pfizer doses comes from Israel, which first began rolling out boosters to older adults in July.
In a highly anticipated presentation, Sharon Alroy-Preis, Israel’s director of public health services, joined the meeting to describe Israel’s experience with boosters.
Israel began to see a third surge of COVID-19 cases in December.
“This was after having two waves and two lockdowns,” Ms. Alroy-Preis said. By the third surge, she said, Israelis were tired.
“We decided on a lockdown, but the compliance of the public wasn’t as it was in the previous two waves,” she said.
Then the vaccine arrived. Israel started vaccinations as soon as the FDA approved it, and they quickly vaccinated a high percentage of their population, about 3 months faster than the rest of the world.
All vaccinations are reported and tracked by the Ministry of Health, so the country is able to keep close tabs on how well the shots are working.
As vaccines rolled out, cases fell dramatically. The pandemic seemed to be behind them. Delta arrived in March. By June, their cases were doubling every 10 days, despite about 80% of their most vulnerable adults being fully vaccinated, she said.
Most concerning was that about 60% of severe cases were breakthrough cases in fully vaccinated individuals.
“We had to stop and figure out, was this a Delta issue,” she said. “Or was this a waning immunity issue.”
“We had some clue that it might not be the Delta variant, at least not alone,” she said.
People who had originally been first in line for the vaccines, seniors and health care workers, were having the highest rates of breakthrough infections. People further away from their second dose were more likely to get a breakthrough infection.
Ms. Alroy-Preis said that if they had not started booster doses in July, their hospitals would have been overwhelmed. They had projected that they would have 2,000 cases in the hospital each day.
Boosters have helped to flatten the curve, though they are still seeing a significant number of infections.
Data from Israel presented at the meeting show boosters are largely safe and effective at reducing severe outcomes in seniors. Israeli experience also showed that third doses, which generate very high levels of neutralizing antibodies—the first and fastest line of the body’s immune defense - -may also slow transmission of the virus.
Key differences in the U.S.
The benefit of slowing down the explosive spread of a highly contagious virus was tantalizing, but many members noted that circumstances in Israel are very different than in the United States. Israel went into its current Delta surge already having high levels of vaccination in its population. They also relied on the Pfizer vaccine almost exclusively for their campaign.
The United States used a different mix of vaccines – Pfizer, Moderna, and Johnson & Johnson -- and doesn’t have the same high level of vaccination coverage of its population.
In the United States, transmission is mainly being driven by unvaccinated people, Dr. Rubin noted.
“That really means the primary benefit is going to be in reducing disease,” he said, “And we know the people who are going to benefit from that … and those are the kinds of people the FDA has already approved a third dose for,” he said, referring to those with underlying health conditions.
But Israel only began vaccinating younger people a few weeks ago. Most are still within a window where rare risks like myocarditis could appear, Rubin noted.
He and other members of the committee said they wished they had more information about the safety of third doses in younger adults.
“We don’t have that right now, and I don’t think I would be comfortable giving it to a 16-year-old,” he said.
At the same time, the primary benefit for third doses would be in preventing severe disease, and overall, data from the United States and other countries show that two doses of the vaccines remain highly effective at preventing hospitalization and death.
Asked why Israel began to see more severe cases in fully vaccinated people, the CDC’s Sara Oliver, MD, a disease detective with the CDC, said it was probably due to a mix of factors including the fact that Israel defines severe cases a little differently.
In the United States, a severe case is generally a person who has to be hospitalized or who has died from the infection. In Israel, a person with a severe case is someone who has an elevated respiratory rate and someone who has a blood oxygen level less than 94%. In the United States, that kind of patient wouldn’t necessarily be hospitalized.
In the end, one of the two committee members who wanted full approval for Pfizer’s third doses said he was satisfied with the outcome.
Mark Sawyer, MD, a professor of pediatrics and infectious disease at the University of California at San Diego, said he voted yes on the first question because he thought full approval was the best way to give doctors the flexibility to prescribe the shots to vulnerable individuals.
“I’m really glad we authorized a vaccine for a third dose, and I plan to go out and get my vaccine this afternoon,” Dr. Sawyer said, noting that he was at high risk as a health care provider.
This article was updated 9/19/21.
A version of this article first appeared on Medscape.com.
Higher than standard vitamin D dose provides no added benefits to children’s neurodevelopment
Prescribing higher doses of vitamin D may not provide any additional benefits to children’s brain development, a new study suggests.
New research published online in JAMA found that there were no differences in children’s developmental milestones or social-emotional problems when given a higher daily dose of 1,200 IU of vitamin D versus the standard dose of 400 IU.
Although past studies have looked into the relationship between vitamin D and neurodevelopment in children, the findings were inconsistent. A 2019 study published in Psychoneuroendocrinology found that vitamin D deficiency could be a biological risk factor for psychiatric disorders and that vitamin D acts as a neurosteroid with direct effect on brain development. However, a 2021 study published in Global Pediatric Health found no significant association between vitamin D levels and neurodevelopmental status in children at 2 years old.
Researchers of the current study said they expected to find a positive association between higher vitamin D levels and neurodevelopment.
“Our results highlight that the current recommendations, set forth mainly on the basis of bone health, also support healthy brain development,” said study author Kati Heinonen, PhD, associate professor of psychology and welfare sciences at Tampere (Finland) University. “Our results also point out that higher than currently recommended levels do not add to the benefits received from the vitamin D supplements.”
For the study, Dr. Heinonen and colleagues analyzed data from a double-blind, randomized clinical trial involving healthy infants born full-term between Jan. 1, 2013, and June 30, 2014, at a maternity hospital in Helsinki. They got follow-up information on 404 infants who were randomized to receive 400 IU of oral vitamin D supplements daily and 397 infants who received 1,200 IU of vitamin D supplements from 2 weeks to 24 months of age.
Researchers found no differences between the 400-IU group and the 1,200-IU group in the mean adjusted Ages and Stages Questionnaire total score at 12 months, a questionnaire that’s used to measure communication, problem solving, gross motor skills, fine motor skills, and personal and social skills. However, they did find that children receiving 1,200 IU of vitamin D supplementation had better developmental milestone scores in communication and problem-solving skills at 12 months.
Furthermore, they also found that higher vitamin D concentrations were associated with fewer sleeping problems at 24 months.
The researcher’s findings did not surprise Francis E. Rushton Jr., MD, a clinical professor of pediatrics at the University of South Carolina, Columbia, who was not involved in the study. “This study reveals that more is not always better,” Dr. Rushton said in an interview.
Dr. Rushton, who is also the medical director of the Quality Through Innovation in Pediatrics network, said other ways to enhance early brain development include initiatives like infant home visitation and language enrichment programs like Reach Out and Read.
Dr. Heinonen noted that the study’s findings might be different if it had been conducted on infants from a different country.
“We have to remember that the participants were from northern European countries where several food products are also fortified by vitamin D,” Dr. Heinonen explained. “Thus, direct recommendations of the amount of the supplementation given for children from 2 weeks to 2 years in other countries should not be done on the basis of our study.”
Researchers also observed that the children receiving 1,200 IU of vitamin D supplementation had a risk of scoring higher on the externalizing symptoms scale at 24 months, meaning these infants are more likely to lose their temper and become physically aggressive.
“We could not fully exclude potential disadvantageous effects of higher doses. Even if minimal, the potential nonbeneficial effects of higher than standard doses warrant further studies,” she said.
Researchers said more studies are needed that follow children up to school age and adolescence, when higher cognitive abilities develop, to understand the long-term outcomes of early vitamin D supplementation.
Prescribing higher doses of vitamin D may not provide any additional benefits to children’s brain development, a new study suggests.
New research published online in JAMA found that there were no differences in children’s developmental milestones or social-emotional problems when given a higher daily dose of 1,200 IU of vitamin D versus the standard dose of 400 IU.
Although past studies have looked into the relationship between vitamin D and neurodevelopment in children, the findings were inconsistent. A 2019 study published in Psychoneuroendocrinology found that vitamin D deficiency could be a biological risk factor for psychiatric disorders and that vitamin D acts as a neurosteroid with direct effect on brain development. However, a 2021 study published in Global Pediatric Health found no significant association between vitamin D levels and neurodevelopmental status in children at 2 years old.
Researchers of the current study said they expected to find a positive association between higher vitamin D levels and neurodevelopment.
“Our results highlight that the current recommendations, set forth mainly on the basis of bone health, also support healthy brain development,” said study author Kati Heinonen, PhD, associate professor of psychology and welfare sciences at Tampere (Finland) University. “Our results also point out that higher than currently recommended levels do not add to the benefits received from the vitamin D supplements.”
For the study, Dr. Heinonen and colleagues analyzed data from a double-blind, randomized clinical trial involving healthy infants born full-term between Jan. 1, 2013, and June 30, 2014, at a maternity hospital in Helsinki. They got follow-up information on 404 infants who were randomized to receive 400 IU of oral vitamin D supplements daily and 397 infants who received 1,200 IU of vitamin D supplements from 2 weeks to 24 months of age.
Researchers found no differences between the 400-IU group and the 1,200-IU group in the mean adjusted Ages and Stages Questionnaire total score at 12 months, a questionnaire that’s used to measure communication, problem solving, gross motor skills, fine motor skills, and personal and social skills. However, they did find that children receiving 1,200 IU of vitamin D supplementation had better developmental milestone scores in communication and problem-solving skills at 12 months.
Furthermore, they also found that higher vitamin D concentrations were associated with fewer sleeping problems at 24 months.
The researcher’s findings did not surprise Francis E. Rushton Jr., MD, a clinical professor of pediatrics at the University of South Carolina, Columbia, who was not involved in the study. “This study reveals that more is not always better,” Dr. Rushton said in an interview.
Dr. Rushton, who is also the medical director of the Quality Through Innovation in Pediatrics network, said other ways to enhance early brain development include initiatives like infant home visitation and language enrichment programs like Reach Out and Read.
Dr. Heinonen noted that the study’s findings might be different if it had been conducted on infants from a different country.
“We have to remember that the participants were from northern European countries where several food products are also fortified by vitamin D,” Dr. Heinonen explained. “Thus, direct recommendations of the amount of the supplementation given for children from 2 weeks to 2 years in other countries should not be done on the basis of our study.”
Researchers also observed that the children receiving 1,200 IU of vitamin D supplementation had a risk of scoring higher on the externalizing symptoms scale at 24 months, meaning these infants are more likely to lose their temper and become physically aggressive.
“We could not fully exclude potential disadvantageous effects of higher doses. Even if minimal, the potential nonbeneficial effects of higher than standard doses warrant further studies,” she said.
Researchers said more studies are needed that follow children up to school age and adolescence, when higher cognitive abilities develop, to understand the long-term outcomes of early vitamin D supplementation.
Prescribing higher doses of vitamin D may not provide any additional benefits to children’s brain development, a new study suggests.
New research published online in JAMA found that there were no differences in children’s developmental milestones or social-emotional problems when given a higher daily dose of 1,200 IU of vitamin D versus the standard dose of 400 IU.
Although past studies have looked into the relationship between vitamin D and neurodevelopment in children, the findings were inconsistent. A 2019 study published in Psychoneuroendocrinology found that vitamin D deficiency could be a biological risk factor for psychiatric disorders and that vitamin D acts as a neurosteroid with direct effect on brain development. However, a 2021 study published in Global Pediatric Health found no significant association between vitamin D levels and neurodevelopmental status in children at 2 years old.
Researchers of the current study said they expected to find a positive association between higher vitamin D levels and neurodevelopment.
“Our results highlight that the current recommendations, set forth mainly on the basis of bone health, also support healthy brain development,” said study author Kati Heinonen, PhD, associate professor of psychology and welfare sciences at Tampere (Finland) University. “Our results also point out that higher than currently recommended levels do not add to the benefits received from the vitamin D supplements.”
For the study, Dr. Heinonen and colleagues analyzed data from a double-blind, randomized clinical trial involving healthy infants born full-term between Jan. 1, 2013, and June 30, 2014, at a maternity hospital in Helsinki. They got follow-up information on 404 infants who were randomized to receive 400 IU of oral vitamin D supplements daily and 397 infants who received 1,200 IU of vitamin D supplements from 2 weeks to 24 months of age.
Researchers found no differences between the 400-IU group and the 1,200-IU group in the mean adjusted Ages and Stages Questionnaire total score at 12 months, a questionnaire that’s used to measure communication, problem solving, gross motor skills, fine motor skills, and personal and social skills. However, they did find that children receiving 1,200 IU of vitamin D supplementation had better developmental milestone scores in communication and problem-solving skills at 12 months.
Furthermore, they also found that higher vitamin D concentrations were associated with fewer sleeping problems at 24 months.
The researcher’s findings did not surprise Francis E. Rushton Jr., MD, a clinical professor of pediatrics at the University of South Carolina, Columbia, who was not involved in the study. “This study reveals that more is not always better,” Dr. Rushton said in an interview.
Dr. Rushton, who is also the medical director of the Quality Through Innovation in Pediatrics network, said other ways to enhance early brain development include initiatives like infant home visitation and language enrichment programs like Reach Out and Read.
Dr. Heinonen noted that the study’s findings might be different if it had been conducted on infants from a different country.
“We have to remember that the participants were from northern European countries where several food products are also fortified by vitamin D,” Dr. Heinonen explained. “Thus, direct recommendations of the amount of the supplementation given for children from 2 weeks to 2 years in other countries should not be done on the basis of our study.”
Researchers also observed that the children receiving 1,200 IU of vitamin D supplementation had a risk of scoring higher on the externalizing symptoms scale at 24 months, meaning these infants are more likely to lose their temper and become physically aggressive.
“We could not fully exclude potential disadvantageous effects of higher doses. Even if minimal, the potential nonbeneficial effects of higher than standard doses warrant further studies,” she said.
Researchers said more studies are needed that follow children up to school age and adolescence, when higher cognitive abilities develop, to understand the long-term outcomes of early vitamin D supplementation.
FROM JAMA
‘Empathy fatigue’ in clinicians rises with latest COVID-19 surge
Heidi Erickson, MD, is tired. As a pulmonary and critical care physician at Hennepin Healthcare in Minneapolis, she has been providing care for patients with COVID-19 since the start of the pandemic.
It was exhausting from the beginning, as she and her colleagues scrambled to understand how to deal with this new disease. But lately, she has noticed a different kind of exhaustion arising from the knowledge that with vaccines widely available, the latest surge was preventable.
Her intensive care unit is currently as full as it has ever been with COVID-19 patients, many of them young adults and most of them unvaccinated. After the recent death of one patient, an unvaccinated man with teenage children, she had to face his family’s questions about why ivermectin, an antiparasitic medication that was falsely promoted as a COVID-19 treatment, was not administered.
“I’m fatigued because I’m working more than ever, but more people don’t have to die,” Dr. Erickson said in an interview . “It’s been very hard physically, mentally, emotionally.”
Amid yet another surge in COVID-19 cases around the United States, clinicians are speaking out about their growing frustration with this preventable crisis.
Some are using the terms “empathy fatigue” and “compassion fatigue” – a sense that they are losing empathy for unvaccinated individuals who are fueling the pandemic.
Dr. Erickson says she is frustrated not by individual patients but by a system that has allowed disinformation to proliferate. Experts say these types of feelings fit into a widespread pattern of physician burnout that has taken a new turn at this stage of the pandemic.
Paradoxical choices
Empathy is a cornerstone of what clinicians do, and the ability to understand and share a patient’s feelings is an essential skill for providing effective care, says Kaz Nelson, MD, a psychiatrist at the University of Minnesota, Minneapolis.
Practitioners face paradoxical situations all the time, she notes. These include individuals who break bones and go skydiving again, people who have high cholesterol but continue to eat fried foods, and those with advanced lung cancer who continue to smoke.
To treat patients with compassion, practitioners learn to set aside judgment by acknowledging the complexity of human behavior. They may lament the addictive nature of nicotine and advertising that targets children, for example, while still listening and caring.
Empathy requires high-level brain function, but as stress levels rise, brain function that drives empathy tends to shut down. It’s a survival mechanism, Dr. Nelson says.
When health care workers feel overwhelmed, trapped, or threatened by patients demanding unproven treatments or by ICUs with more patients than ventilators, they may experience a fight-or-flight response that makes them defensive, frustrated, angry, or uncaring, notes Mona Masood, DO, a Philadelphia-area psychiatrist and founder of Physician Support Line, a free mental health hotline for doctors.
Some clinicians have taken to Twitter and other social media platforms to post about these types of experiences.
These feelings, which have been brewing for months, have been exacerbated by the complexity of the current situation. Clinicians see a disconnect between what is and what could be, Dr. Nelson notes.
“Prior to vaccines, there weren’t other options, and so we had toxic stress and we had fatigue, but we could still maintain little bits of empathy by saying, ‘You know, people didn’t choose to get infected, and we are in a pandemic.’ We could kind of hate the virus. Now with access to vaccines, that last connection to empathy is removed for many people,” she says.
Self-preservation vs. empathy
Compassion fatigue or empathy fatigue is just one reaction to feeling completely maxed out and overstressed, Dr. Nelson says. Anger at society, such as what Dr. Erickson experienced, is another response.
Practitioners may also feel as if they are just going through the motions of their job, or they might disassociate, ceasing to feel that their patients are human. Plenty of doctors and nurses have cried in their cars after shifts and have posted tearful videos on social media.
Early in the pandemic, Dr. Masood says, physicians who called the support hotline expressed sadness and grief. Now, she had her colleagues hear frustration and anger, along with guilt and shame for having feelings they believe they shouldn’t be having, especially toward patients. They may feel unprofessional or worse – unworthy of being physicians, she says.
One recent caller to the hotline was a long-time ICU physician who had been told so many times by patients that ivermectin was the only medicine that would cure them that he began to doubt himself, says Dr. Masood. This caller needed to be reassured by another physician that he was doing the right thing.
Another emergency department physician told Dr. Masood about a young child who had arrived at the hospital with COVID-19 symptoms. When asked whether the family had been exposed to anyone with COVID-19, the child’s parent lied so that they could be triaged faster.
The physician, who needed to step away from the situation, reached out to Dr. Masood to express her frustration so that she wouldn’t “let it out” on the patient.
“It’s hard to have empathy for people who, for all intents and purposes, are very self-centered,” Dr. Masood says. “We’re at a place where we’re having to choose between self-preservation and empathy.”
How to cope
To help practitioners cope, Dr. Masood offers words that describe what they’re experiencing. She often hears clinicians say things such as, “This is a type of burnout that I feel to my bones,” or “This makes me want to quit,” or “I feel like I’m at the end of my rope.”
She encourages them to consider the terms “empathy fatigue,” and “moral injury” in order to reconcile how their sense of responsibility to take care of people is compromised by factors outside of their control.
It is not shameful to acknowledge that they experience emotions, including difficult ones such as frustration, anger, sadness, and anxiety, Dr. Masood adds.
Being frustrated with a patient doesn’t make someone a bad doctor, and admitting those emotions is the first step toward dealing with them, she says.
before they cause a sense of callousness or other consequences that become harder to heal from as time goes on.
“We’re trained to just go, go, go and sometimes not pause and check in,” she says. Clinicians who open up are likely to find they are not the only ones feeling tired or frustrated right now, she adds.
“Connect with peers and colleagues, because chances are, they can relate,” Dr. Nelson says.
A version of this article first appeared on Medscape.com.
Heidi Erickson, MD, is tired. As a pulmonary and critical care physician at Hennepin Healthcare in Minneapolis, she has been providing care for patients with COVID-19 since the start of the pandemic.
It was exhausting from the beginning, as she and her colleagues scrambled to understand how to deal with this new disease. But lately, she has noticed a different kind of exhaustion arising from the knowledge that with vaccines widely available, the latest surge was preventable.
Her intensive care unit is currently as full as it has ever been with COVID-19 patients, many of them young adults and most of them unvaccinated. After the recent death of one patient, an unvaccinated man with teenage children, she had to face his family’s questions about why ivermectin, an antiparasitic medication that was falsely promoted as a COVID-19 treatment, was not administered.
“I’m fatigued because I’m working more than ever, but more people don’t have to die,” Dr. Erickson said in an interview . “It’s been very hard physically, mentally, emotionally.”
Amid yet another surge in COVID-19 cases around the United States, clinicians are speaking out about their growing frustration with this preventable crisis.
Some are using the terms “empathy fatigue” and “compassion fatigue” – a sense that they are losing empathy for unvaccinated individuals who are fueling the pandemic.
Dr. Erickson says she is frustrated not by individual patients but by a system that has allowed disinformation to proliferate. Experts say these types of feelings fit into a widespread pattern of physician burnout that has taken a new turn at this stage of the pandemic.
Paradoxical choices
Empathy is a cornerstone of what clinicians do, and the ability to understand and share a patient’s feelings is an essential skill for providing effective care, says Kaz Nelson, MD, a psychiatrist at the University of Minnesota, Minneapolis.
Practitioners face paradoxical situations all the time, she notes. These include individuals who break bones and go skydiving again, people who have high cholesterol but continue to eat fried foods, and those with advanced lung cancer who continue to smoke.
To treat patients with compassion, practitioners learn to set aside judgment by acknowledging the complexity of human behavior. They may lament the addictive nature of nicotine and advertising that targets children, for example, while still listening and caring.
Empathy requires high-level brain function, but as stress levels rise, brain function that drives empathy tends to shut down. It’s a survival mechanism, Dr. Nelson says.
When health care workers feel overwhelmed, trapped, or threatened by patients demanding unproven treatments or by ICUs with more patients than ventilators, they may experience a fight-or-flight response that makes them defensive, frustrated, angry, or uncaring, notes Mona Masood, DO, a Philadelphia-area psychiatrist and founder of Physician Support Line, a free mental health hotline for doctors.
Some clinicians have taken to Twitter and other social media platforms to post about these types of experiences.
These feelings, which have been brewing for months, have been exacerbated by the complexity of the current situation. Clinicians see a disconnect between what is and what could be, Dr. Nelson notes.
“Prior to vaccines, there weren’t other options, and so we had toxic stress and we had fatigue, but we could still maintain little bits of empathy by saying, ‘You know, people didn’t choose to get infected, and we are in a pandemic.’ We could kind of hate the virus. Now with access to vaccines, that last connection to empathy is removed for many people,” she says.
Self-preservation vs. empathy
Compassion fatigue or empathy fatigue is just one reaction to feeling completely maxed out and overstressed, Dr. Nelson says. Anger at society, such as what Dr. Erickson experienced, is another response.
Practitioners may also feel as if they are just going through the motions of their job, or they might disassociate, ceasing to feel that their patients are human. Plenty of doctors and nurses have cried in their cars after shifts and have posted tearful videos on social media.
Early in the pandemic, Dr. Masood says, physicians who called the support hotline expressed sadness and grief. Now, she had her colleagues hear frustration and anger, along with guilt and shame for having feelings they believe they shouldn’t be having, especially toward patients. They may feel unprofessional or worse – unworthy of being physicians, she says.
One recent caller to the hotline was a long-time ICU physician who had been told so many times by patients that ivermectin was the only medicine that would cure them that he began to doubt himself, says Dr. Masood. This caller needed to be reassured by another physician that he was doing the right thing.
Another emergency department physician told Dr. Masood about a young child who had arrived at the hospital with COVID-19 symptoms. When asked whether the family had been exposed to anyone with COVID-19, the child’s parent lied so that they could be triaged faster.
The physician, who needed to step away from the situation, reached out to Dr. Masood to express her frustration so that she wouldn’t “let it out” on the patient.
“It’s hard to have empathy for people who, for all intents and purposes, are very self-centered,” Dr. Masood says. “We’re at a place where we’re having to choose between self-preservation and empathy.”
How to cope
To help practitioners cope, Dr. Masood offers words that describe what they’re experiencing. She often hears clinicians say things such as, “This is a type of burnout that I feel to my bones,” or “This makes me want to quit,” or “I feel like I’m at the end of my rope.”
She encourages them to consider the terms “empathy fatigue,” and “moral injury” in order to reconcile how their sense of responsibility to take care of people is compromised by factors outside of their control.
It is not shameful to acknowledge that they experience emotions, including difficult ones such as frustration, anger, sadness, and anxiety, Dr. Masood adds.
Being frustrated with a patient doesn’t make someone a bad doctor, and admitting those emotions is the first step toward dealing with them, she says.
before they cause a sense of callousness or other consequences that become harder to heal from as time goes on.
“We’re trained to just go, go, go and sometimes not pause and check in,” she says. Clinicians who open up are likely to find they are not the only ones feeling tired or frustrated right now, she adds.
“Connect with peers and colleagues, because chances are, they can relate,” Dr. Nelson says.
A version of this article first appeared on Medscape.com.
Heidi Erickson, MD, is tired. As a pulmonary and critical care physician at Hennepin Healthcare in Minneapolis, she has been providing care for patients with COVID-19 since the start of the pandemic.
It was exhausting from the beginning, as she and her colleagues scrambled to understand how to deal with this new disease. But lately, she has noticed a different kind of exhaustion arising from the knowledge that with vaccines widely available, the latest surge was preventable.
Her intensive care unit is currently as full as it has ever been with COVID-19 patients, many of them young adults and most of them unvaccinated. After the recent death of one patient, an unvaccinated man with teenage children, she had to face his family’s questions about why ivermectin, an antiparasitic medication that was falsely promoted as a COVID-19 treatment, was not administered.
“I’m fatigued because I’m working more than ever, but more people don’t have to die,” Dr. Erickson said in an interview . “It’s been very hard physically, mentally, emotionally.”
Amid yet another surge in COVID-19 cases around the United States, clinicians are speaking out about their growing frustration with this preventable crisis.
Some are using the terms “empathy fatigue” and “compassion fatigue” – a sense that they are losing empathy for unvaccinated individuals who are fueling the pandemic.
Dr. Erickson says she is frustrated not by individual patients but by a system that has allowed disinformation to proliferate. Experts say these types of feelings fit into a widespread pattern of physician burnout that has taken a new turn at this stage of the pandemic.
Paradoxical choices
Empathy is a cornerstone of what clinicians do, and the ability to understand and share a patient’s feelings is an essential skill for providing effective care, says Kaz Nelson, MD, a psychiatrist at the University of Minnesota, Minneapolis.
Practitioners face paradoxical situations all the time, she notes. These include individuals who break bones and go skydiving again, people who have high cholesterol but continue to eat fried foods, and those with advanced lung cancer who continue to smoke.
To treat patients with compassion, practitioners learn to set aside judgment by acknowledging the complexity of human behavior. They may lament the addictive nature of nicotine and advertising that targets children, for example, while still listening and caring.
Empathy requires high-level brain function, but as stress levels rise, brain function that drives empathy tends to shut down. It’s a survival mechanism, Dr. Nelson says.
When health care workers feel overwhelmed, trapped, or threatened by patients demanding unproven treatments or by ICUs with more patients than ventilators, they may experience a fight-or-flight response that makes them defensive, frustrated, angry, or uncaring, notes Mona Masood, DO, a Philadelphia-area psychiatrist and founder of Physician Support Line, a free mental health hotline for doctors.
Some clinicians have taken to Twitter and other social media platforms to post about these types of experiences.
These feelings, which have been brewing for months, have been exacerbated by the complexity of the current situation. Clinicians see a disconnect between what is and what could be, Dr. Nelson notes.
“Prior to vaccines, there weren’t other options, and so we had toxic stress and we had fatigue, but we could still maintain little bits of empathy by saying, ‘You know, people didn’t choose to get infected, and we are in a pandemic.’ We could kind of hate the virus. Now with access to vaccines, that last connection to empathy is removed for many people,” she says.
Self-preservation vs. empathy
Compassion fatigue or empathy fatigue is just one reaction to feeling completely maxed out and overstressed, Dr. Nelson says. Anger at society, such as what Dr. Erickson experienced, is another response.
Practitioners may also feel as if they are just going through the motions of their job, or they might disassociate, ceasing to feel that their patients are human. Plenty of doctors and nurses have cried in their cars after shifts and have posted tearful videos on social media.
Early in the pandemic, Dr. Masood says, physicians who called the support hotline expressed sadness and grief. Now, she had her colleagues hear frustration and anger, along with guilt and shame for having feelings they believe they shouldn’t be having, especially toward patients. They may feel unprofessional or worse – unworthy of being physicians, she says.
One recent caller to the hotline was a long-time ICU physician who had been told so many times by patients that ivermectin was the only medicine that would cure them that he began to doubt himself, says Dr. Masood. This caller needed to be reassured by another physician that he was doing the right thing.
Another emergency department physician told Dr. Masood about a young child who had arrived at the hospital with COVID-19 symptoms. When asked whether the family had been exposed to anyone with COVID-19, the child’s parent lied so that they could be triaged faster.
The physician, who needed to step away from the situation, reached out to Dr. Masood to express her frustration so that she wouldn’t “let it out” on the patient.
“It’s hard to have empathy for people who, for all intents and purposes, are very self-centered,” Dr. Masood says. “We’re at a place where we’re having to choose between self-preservation and empathy.”
How to cope
To help practitioners cope, Dr. Masood offers words that describe what they’re experiencing. She often hears clinicians say things such as, “This is a type of burnout that I feel to my bones,” or “This makes me want to quit,” or “I feel like I’m at the end of my rope.”
She encourages them to consider the terms “empathy fatigue,” and “moral injury” in order to reconcile how their sense of responsibility to take care of people is compromised by factors outside of their control.
It is not shameful to acknowledge that they experience emotions, including difficult ones such as frustration, anger, sadness, and anxiety, Dr. Masood adds.
Being frustrated with a patient doesn’t make someone a bad doctor, and admitting those emotions is the first step toward dealing with them, she says.
before they cause a sense of callousness or other consequences that become harder to heal from as time goes on.
“We’re trained to just go, go, go and sometimes not pause and check in,” she says. Clinicians who open up are likely to find they are not the only ones feeling tired or frustrated right now, she adds.
“Connect with peers and colleagues, because chances are, they can relate,” Dr. Nelson says.
A version of this article first appeared on Medscape.com.





