User login
News and Views that Matter to Pediatricians
The leading independent newspaper covering news and commentary in pediatrics.
Federal judge pauses strict Texas abortion law
Robert Pitman, a federal district court judge in Austin, sided with the Biden administration and granted the Justice Department’s request to halt enforcement of the new law. The Biden administration sued to stop the law, and Mr. Pitman’s decision pauses the law while it moves through federal courts, The New York Times reported.
In a 113-page ruling, Mr. Pitman criticized the law, also known as Senate Bill 8, for delegating enforcement to private individuals, who can sue anyone who performs abortions or “aids and abets” them. Plaintiffs are encouraged to file lawsuits because they recover legal fees and $10,000 if they win.
“From the moment S.B. 8 went into effect, women have been unlawfully prevented from exercising control over their own lives in ways that are protected by the Constitution,” Mr. Pitman wrote in the ruling.
“This court will not sanction one more day of this offensive deprivation of such an important right,” he said.
The Texas law bans abortions once fetal cardiac activity can be detected, which is usually around 6 weeks of pregnancy. Women may not know they’re pregnant yet during that time frame.
It’s not yet clear what effect Mr. Pitman’s ruling will have on women in Texas, the Times reported. Since the law went into effect about a month ago, women have sought abortion providers in other states. Mr. Pitman’s ruling pauses the enforcement of the law, but clinics may still face retroactive lawsuits for abortions provided while the law was temporarily suspended.
Whole Woman’s Health, which operates four clinics in Texas, said Wednesday that it was “making plans to resume abortion care up to 18 weeks as soon as possible,” the newspaper reported. The Center for Reproductive Rights also said that clinics “hope to resume full abortion services as soon as they are able.”
Late Oct. 6, Texas said it would appeal the ruling. The U.S. Court of Appeals for the Fifth Circuit is one of the most conservative in the country, according to the Times, and another decision could come soon.
A version of this article first appeared on WebMD.com.
Robert Pitman, a federal district court judge in Austin, sided with the Biden administration and granted the Justice Department’s request to halt enforcement of the new law. The Biden administration sued to stop the law, and Mr. Pitman’s decision pauses the law while it moves through federal courts, The New York Times reported.
In a 113-page ruling, Mr. Pitman criticized the law, also known as Senate Bill 8, for delegating enforcement to private individuals, who can sue anyone who performs abortions or “aids and abets” them. Plaintiffs are encouraged to file lawsuits because they recover legal fees and $10,000 if they win.
“From the moment S.B. 8 went into effect, women have been unlawfully prevented from exercising control over their own lives in ways that are protected by the Constitution,” Mr. Pitman wrote in the ruling.
“This court will not sanction one more day of this offensive deprivation of such an important right,” he said.
The Texas law bans abortions once fetal cardiac activity can be detected, which is usually around 6 weeks of pregnancy. Women may not know they’re pregnant yet during that time frame.
It’s not yet clear what effect Mr. Pitman’s ruling will have on women in Texas, the Times reported. Since the law went into effect about a month ago, women have sought abortion providers in other states. Mr. Pitman’s ruling pauses the enforcement of the law, but clinics may still face retroactive lawsuits for abortions provided while the law was temporarily suspended.
Whole Woman’s Health, which operates four clinics in Texas, said Wednesday that it was “making plans to resume abortion care up to 18 weeks as soon as possible,” the newspaper reported. The Center for Reproductive Rights also said that clinics “hope to resume full abortion services as soon as they are able.”
Late Oct. 6, Texas said it would appeal the ruling. The U.S. Court of Appeals for the Fifth Circuit is one of the most conservative in the country, according to the Times, and another decision could come soon.
A version of this article first appeared on WebMD.com.
Robert Pitman, a federal district court judge in Austin, sided with the Biden administration and granted the Justice Department’s request to halt enforcement of the new law. The Biden administration sued to stop the law, and Mr. Pitman’s decision pauses the law while it moves through federal courts, The New York Times reported.
In a 113-page ruling, Mr. Pitman criticized the law, also known as Senate Bill 8, for delegating enforcement to private individuals, who can sue anyone who performs abortions or “aids and abets” them. Plaintiffs are encouraged to file lawsuits because they recover legal fees and $10,000 if they win.
“From the moment S.B. 8 went into effect, women have been unlawfully prevented from exercising control over their own lives in ways that are protected by the Constitution,” Mr. Pitman wrote in the ruling.
“This court will not sanction one more day of this offensive deprivation of such an important right,” he said.
The Texas law bans abortions once fetal cardiac activity can be detected, which is usually around 6 weeks of pregnancy. Women may not know they’re pregnant yet during that time frame.
It’s not yet clear what effect Mr. Pitman’s ruling will have on women in Texas, the Times reported. Since the law went into effect about a month ago, women have sought abortion providers in other states. Mr. Pitman’s ruling pauses the enforcement of the law, but clinics may still face retroactive lawsuits for abortions provided while the law was temporarily suspended.
Whole Woman’s Health, which operates four clinics in Texas, said Wednesday that it was “making plans to resume abortion care up to 18 weeks as soon as possible,” the newspaper reported. The Center for Reproductive Rights also said that clinics “hope to resume full abortion services as soon as they are able.”
Late Oct. 6, Texas said it would appeal the ruling. The U.S. Court of Appeals for the Fifth Circuit is one of the most conservative in the country, according to the Times, and another decision could come soon.
A version of this article first appeared on WebMD.com.
Pfizer asks FDA to authorize COVID vaccine for kids 5-11
The request comes after the drugmaker submitted clinical trial data to the FDA on Sept. 28. Pfizer said the study of 2,268 children showed the vaccine was safe and produced a robust immune response.
Participants in the studies received a lower dose of the vaccine, 10 micrograms. Their response 2 weeks after a second dose was reportedly equal to the immune protection in a control group of 16- to 25-year-olds who received the fully approved 30-microgram doses.
Currently, the Pfizer EUA applies to 12- to 15-year-olds and people eligible for a Pfizer booster shot. The drugmaker received full FDA approval for the vaccine for Americans 16 years and older in August.
The filing for authorization in 5- to 11-year-olds comes as overall cases of COVID-19 in the United States continue to decline. The decrease includes a drop in new cases in children for the fourth consecutive week, according to analysis of data from the American Academy of Pediatrics and the Children’s Hospital Association.
The next step is an FDA decision on whether to expand the current emergency use authorization (EUA) for teenagers to the younger age group.
Timing of any official word from the agency is unknown. But possibly in anticipation of today’s filing, the FDA already scheduled a meeting of its Vaccines and Related Biological Products Advisory Committee for Oct. 25.
A version of this article first appeared on WebMD.com.
The request comes after the drugmaker submitted clinical trial data to the FDA on Sept. 28. Pfizer said the study of 2,268 children showed the vaccine was safe and produced a robust immune response.
Participants in the studies received a lower dose of the vaccine, 10 micrograms. Their response 2 weeks after a second dose was reportedly equal to the immune protection in a control group of 16- to 25-year-olds who received the fully approved 30-microgram doses.
Currently, the Pfizer EUA applies to 12- to 15-year-olds and people eligible for a Pfizer booster shot. The drugmaker received full FDA approval for the vaccine for Americans 16 years and older in August.
The filing for authorization in 5- to 11-year-olds comes as overall cases of COVID-19 in the United States continue to decline. The decrease includes a drop in new cases in children for the fourth consecutive week, according to analysis of data from the American Academy of Pediatrics and the Children’s Hospital Association.
The next step is an FDA decision on whether to expand the current emergency use authorization (EUA) for teenagers to the younger age group.
Timing of any official word from the agency is unknown. But possibly in anticipation of today’s filing, the FDA already scheduled a meeting of its Vaccines and Related Biological Products Advisory Committee for Oct. 25.
A version of this article first appeared on WebMD.com.
The request comes after the drugmaker submitted clinical trial data to the FDA on Sept. 28. Pfizer said the study of 2,268 children showed the vaccine was safe and produced a robust immune response.
Participants in the studies received a lower dose of the vaccine, 10 micrograms. Their response 2 weeks after a second dose was reportedly equal to the immune protection in a control group of 16- to 25-year-olds who received the fully approved 30-microgram doses.
Currently, the Pfizer EUA applies to 12- to 15-year-olds and people eligible for a Pfizer booster shot. The drugmaker received full FDA approval for the vaccine for Americans 16 years and older in August.
The filing for authorization in 5- to 11-year-olds comes as overall cases of COVID-19 in the United States continue to decline. The decrease includes a drop in new cases in children for the fourth consecutive week, according to analysis of data from the American Academy of Pediatrics and the Children’s Hospital Association.
The next step is an FDA decision on whether to expand the current emergency use authorization (EUA) for teenagers to the younger age group.
Timing of any official word from the agency is unknown. But possibly in anticipation of today’s filing, the FDA already scheduled a meeting of its Vaccines and Related Biological Products Advisory Committee for Oct. 25.
A version of this article first appeared on WebMD.com.
Case reports underscore risk of cerebral edema, AFCE in children with COVID-19
according to pediatric neurologists who are urging colleagues to watch out for similar cases.
At least one other child in the United States has died after becoming infected with the virus and developing cerebral edema. “The rapid and devastating clinical course in both of these cases highlights the need for early recognition of a cerebral edema and AFCE as potential complications of COVID-19 in pediatric patients,” the neurologists wrote.
The case was highlighted in a poster presented at the annual meeting of the Child Neurology Society and in a report published earlier this year in Child Neurology Open.
According to pediatric neurologist Timothy Gershon, MD, PhD , of the University of North Carolina at Chapel Hill, the child appeared in clinic in July 2020. She had been healthy but was suffering from 1 day of fever, seizure-like activity (generalized convulsions and drooling), anorexia, and lethargy.
The girl, who was subsequently diagnosed with COVID-19, deteriorated in the hospital. “She received IV dexamethasone in attempts to reduce cerebral edema,” the neurologists wrote. “Regarding immunomodulatory therapy, she received intravenous immunoglobulin (2 g/kg), anakinra, and hydrocortisone; despite approval for remdesivir and COVID-19 convalescent plasma, these were ultimately withheld due to poor prognosis.”
Brain death examinations at 24 and 48 hours after cardiac arrest were consistent with brain death, they reported.
Neurologists believe the patient suffered from AFCE, “an often fatal pediatric clinical entity consisting of fever, encephalopathy, and new-onset seizures followed by rapid, diffuse, and medically-refractory cerebral edema.” They add that “AFCE occurs as a rare complication of a variety of common pediatric infections, and a CNS [central nervous system] pathogen is identified in only a minority of cases, suggesting a para-infectious mechanism of edema.”
Neurologists offered a case definition of the “recently recognized” AFCE earlier this year.
“This was an extremely rare rapid progression to cerebral edema. I think it was related to the patient’s COVID infection, but why this patient got it and others don’t is unknown,” Dr. Gershon said in an interview. “The full spectrum of neurological complications of COVID were not yet known [at the time]. We didn’t know, and still don’t know, what the causative links are between COVID and suddenly having seizures and brain swelling.”
He said he’d treat a similar patient differently now and give dexamethasone earlier in the clinical course, although “there is no data to tell us if any therapy could have reversed it.” Specifically, he said, “I’d give dexamethasone at the first sign of brain involvement, using the dosing recommended for cerebral edema, and try to get the MRI earlier in the course.”
Dr. Gershon and colleagues noted another case of fatal cerebral edema in a child, a 7-year-old boy who was treated in New York state. That case “shows that fatal cerebral edema may complicate pediatric multisystem inflammatory syndrome,” they wrote.
Pediatric critical care specialist Preetha Krishnan, MD, of Randall Children’s Hospital at Legacy Emanuel in Portland, Ore., helped develop the new definition of AFCE. In an interview, she said AFCE is difficult to diagnose because the signs/symptoms – such as fever, altered sensorium, and seizures – are found in other conditions such as febrile status epilepticus with a viral illness.
“The key to recognition of AFCE is that unlike other disease processes, these children have rapid neurologic progression,” she said. “In addition, many of our AFCE patients also had vomiting and/or headache, which in retrospect was likely an indication of elevated ICP [intracranial pressure] rather than viral infection.”
She added that “if a child with fever, seizures, and encephalopathy has cerebral edema on imaging and/or has neurologic progression, AFCE should be considered. Most of our cases of AFCE had fulminant progression within the first 3 days of their head imaging noting cerebral edema. There are other neurologic diseases, such as acute necrotizing encephalopathy of childhood, that also have progressive signs/symptoms, but head imaging and lab work should help differentiate many of these etiologies.”
In regard to treatment, she said, “our unit would likely err on the side of providing as much neuroprotective measures as is reasonable, such as maintaining normothermia, consideration of hyperosmolar therapy, maintaining normocarbia and normoxemia, managing seizures, etc. I would recommend getting the entire neurocritical care team involved in the management discussion. This varies by center, but will likely include neurology, ID [infectious disease], possibly neurosurgery, and PICU.”
As for the new case report, Krishnan said COVID-19 has been linked to neurologic complications, “so it does not surprise me that AFCE is part of the neurologic spectrum of disease.”
No funding was reported, and the authors report no relevant disclosures. Dr. Krishnan has no disclosures.
according to pediatric neurologists who are urging colleagues to watch out for similar cases.
At least one other child in the United States has died after becoming infected with the virus and developing cerebral edema. “The rapid and devastating clinical course in both of these cases highlights the need for early recognition of a cerebral edema and AFCE as potential complications of COVID-19 in pediatric patients,” the neurologists wrote.
The case was highlighted in a poster presented at the annual meeting of the Child Neurology Society and in a report published earlier this year in Child Neurology Open.
According to pediatric neurologist Timothy Gershon, MD, PhD , of the University of North Carolina at Chapel Hill, the child appeared in clinic in July 2020. She had been healthy but was suffering from 1 day of fever, seizure-like activity (generalized convulsions and drooling), anorexia, and lethargy.
The girl, who was subsequently diagnosed with COVID-19, deteriorated in the hospital. “She received IV dexamethasone in attempts to reduce cerebral edema,” the neurologists wrote. “Regarding immunomodulatory therapy, she received intravenous immunoglobulin (2 g/kg), anakinra, and hydrocortisone; despite approval for remdesivir and COVID-19 convalescent plasma, these were ultimately withheld due to poor prognosis.”
Brain death examinations at 24 and 48 hours after cardiac arrest were consistent with brain death, they reported.
Neurologists believe the patient suffered from AFCE, “an often fatal pediatric clinical entity consisting of fever, encephalopathy, and new-onset seizures followed by rapid, diffuse, and medically-refractory cerebral edema.” They add that “AFCE occurs as a rare complication of a variety of common pediatric infections, and a CNS [central nervous system] pathogen is identified in only a minority of cases, suggesting a para-infectious mechanism of edema.”
Neurologists offered a case definition of the “recently recognized” AFCE earlier this year.
“This was an extremely rare rapid progression to cerebral edema. I think it was related to the patient’s COVID infection, but why this patient got it and others don’t is unknown,” Dr. Gershon said in an interview. “The full spectrum of neurological complications of COVID were not yet known [at the time]. We didn’t know, and still don’t know, what the causative links are between COVID and suddenly having seizures and brain swelling.”
He said he’d treat a similar patient differently now and give dexamethasone earlier in the clinical course, although “there is no data to tell us if any therapy could have reversed it.” Specifically, he said, “I’d give dexamethasone at the first sign of brain involvement, using the dosing recommended for cerebral edema, and try to get the MRI earlier in the course.”
Dr. Gershon and colleagues noted another case of fatal cerebral edema in a child, a 7-year-old boy who was treated in New York state. That case “shows that fatal cerebral edema may complicate pediatric multisystem inflammatory syndrome,” they wrote.
Pediatric critical care specialist Preetha Krishnan, MD, of Randall Children’s Hospital at Legacy Emanuel in Portland, Ore., helped develop the new definition of AFCE. In an interview, she said AFCE is difficult to diagnose because the signs/symptoms – such as fever, altered sensorium, and seizures – are found in other conditions such as febrile status epilepticus with a viral illness.
“The key to recognition of AFCE is that unlike other disease processes, these children have rapid neurologic progression,” she said. “In addition, many of our AFCE patients also had vomiting and/or headache, which in retrospect was likely an indication of elevated ICP [intracranial pressure] rather than viral infection.”
She added that “if a child with fever, seizures, and encephalopathy has cerebral edema on imaging and/or has neurologic progression, AFCE should be considered. Most of our cases of AFCE had fulminant progression within the first 3 days of their head imaging noting cerebral edema. There are other neurologic diseases, such as acute necrotizing encephalopathy of childhood, that also have progressive signs/symptoms, but head imaging and lab work should help differentiate many of these etiologies.”
In regard to treatment, she said, “our unit would likely err on the side of providing as much neuroprotective measures as is reasonable, such as maintaining normothermia, consideration of hyperosmolar therapy, maintaining normocarbia and normoxemia, managing seizures, etc. I would recommend getting the entire neurocritical care team involved in the management discussion. This varies by center, but will likely include neurology, ID [infectious disease], possibly neurosurgery, and PICU.”
As for the new case report, Krishnan said COVID-19 has been linked to neurologic complications, “so it does not surprise me that AFCE is part of the neurologic spectrum of disease.”
No funding was reported, and the authors report no relevant disclosures. Dr. Krishnan has no disclosures.
according to pediatric neurologists who are urging colleagues to watch out for similar cases.
At least one other child in the United States has died after becoming infected with the virus and developing cerebral edema. “The rapid and devastating clinical course in both of these cases highlights the need for early recognition of a cerebral edema and AFCE as potential complications of COVID-19 in pediatric patients,” the neurologists wrote.
The case was highlighted in a poster presented at the annual meeting of the Child Neurology Society and in a report published earlier this year in Child Neurology Open.
According to pediatric neurologist Timothy Gershon, MD, PhD , of the University of North Carolina at Chapel Hill, the child appeared in clinic in July 2020. She had been healthy but was suffering from 1 day of fever, seizure-like activity (generalized convulsions and drooling), anorexia, and lethargy.
The girl, who was subsequently diagnosed with COVID-19, deteriorated in the hospital. “She received IV dexamethasone in attempts to reduce cerebral edema,” the neurologists wrote. “Regarding immunomodulatory therapy, she received intravenous immunoglobulin (2 g/kg), anakinra, and hydrocortisone; despite approval for remdesivir and COVID-19 convalescent plasma, these were ultimately withheld due to poor prognosis.”
Brain death examinations at 24 and 48 hours after cardiac arrest were consistent with brain death, they reported.
Neurologists believe the patient suffered from AFCE, “an often fatal pediatric clinical entity consisting of fever, encephalopathy, and new-onset seizures followed by rapid, diffuse, and medically-refractory cerebral edema.” They add that “AFCE occurs as a rare complication of a variety of common pediatric infections, and a CNS [central nervous system] pathogen is identified in only a minority of cases, suggesting a para-infectious mechanism of edema.”
Neurologists offered a case definition of the “recently recognized” AFCE earlier this year.
“This was an extremely rare rapid progression to cerebral edema. I think it was related to the patient’s COVID infection, but why this patient got it and others don’t is unknown,” Dr. Gershon said in an interview. “The full spectrum of neurological complications of COVID were not yet known [at the time]. We didn’t know, and still don’t know, what the causative links are between COVID and suddenly having seizures and brain swelling.”
He said he’d treat a similar patient differently now and give dexamethasone earlier in the clinical course, although “there is no data to tell us if any therapy could have reversed it.” Specifically, he said, “I’d give dexamethasone at the first sign of brain involvement, using the dosing recommended for cerebral edema, and try to get the MRI earlier in the course.”
Dr. Gershon and colleagues noted another case of fatal cerebral edema in a child, a 7-year-old boy who was treated in New York state. That case “shows that fatal cerebral edema may complicate pediatric multisystem inflammatory syndrome,” they wrote.
Pediatric critical care specialist Preetha Krishnan, MD, of Randall Children’s Hospital at Legacy Emanuel in Portland, Ore., helped develop the new definition of AFCE. In an interview, she said AFCE is difficult to diagnose because the signs/symptoms – such as fever, altered sensorium, and seizures – are found in other conditions such as febrile status epilepticus with a viral illness.
“The key to recognition of AFCE is that unlike other disease processes, these children have rapid neurologic progression,” she said. “In addition, many of our AFCE patients also had vomiting and/or headache, which in retrospect was likely an indication of elevated ICP [intracranial pressure] rather than viral infection.”
She added that “if a child with fever, seizures, and encephalopathy has cerebral edema on imaging and/or has neurologic progression, AFCE should be considered. Most of our cases of AFCE had fulminant progression within the first 3 days of their head imaging noting cerebral edema. There are other neurologic diseases, such as acute necrotizing encephalopathy of childhood, that also have progressive signs/symptoms, but head imaging and lab work should help differentiate many of these etiologies.”
In regard to treatment, she said, “our unit would likely err on the side of providing as much neuroprotective measures as is reasonable, such as maintaining normothermia, consideration of hyperosmolar therapy, maintaining normocarbia and normoxemia, managing seizures, etc. I would recommend getting the entire neurocritical care team involved in the management discussion. This varies by center, but will likely include neurology, ID [infectious disease], possibly neurosurgery, and PICU.”
As for the new case report, Krishnan said COVID-19 has been linked to neurologic complications, “so it does not surprise me that AFCE is part of the neurologic spectrum of disease.”
No funding was reported, and the authors report no relevant disclosures. Dr. Krishnan has no disclosures.
FROM CNS 2021
Maternal SSRI use linked to more encephalopathy in newborns, risk still small
, although the overall risk remains extremely low, a new study finds.
The findings were presented in a poster at the 50th annual meeting of the Child Neurology Society.
“Our work showed that neonates exposed to SSRI in utero had higher risks of neonatal encephalopathy even when adjusting for confounders such as maternal mental health disorders and age. SSRIs could cause side effects such as encephalopathy in neonates, and these risks need to be balanced carefully with the potential benefits of treatment to the mother,” study lead author Marie Cornet, MD, a neonatology fellow with Benioff Children’s Hospital at the University of California, San Francisco, said in an interview.
According to Dr. Cornet, “we know that SSRI exposure in utero is associated with increased risks of respiratory distress at birth, need for positive-pressure ventilation, and an abnormal neurologic exam.” The researchers launched the new study to determine if the estimated 4%-8% of pregnant women who take SSRIs may be putting their newborns at greater risk of NE.
The researchers retrospectively tracked 305,426 infants who were born in the Kaiser Permanente Northern California health system (≥35 weeks) from 2011 to 2019. The mothers had an average age of 31 years, and approximately 34.7% were White, 34.7% of unknown race, 23.3% Asian, and 6.2% Black.
The researchers defined NE as a “5-minute APGAR score <7 and abnormal level of consciousness, activity, tone, or reflexes.”
A total of 8,024 infants (2.6%) had mothers who used SSRIs in the third trimester, and 510 (0.17%) were determined to have had NE.
After adjustment for maternal depression or anxiety, maternal age, race, and hospital, exposed neonates had 2.7 times higher odds of NE (odds ratio, 2.7).
Each 25 mg per day increase in the dose of SSRIs, as equalized to doses of sertraline (Zoloft), was linked to a significant 31% increase in the odds of developing NE (OR, 1.31).
The study doesn’t examine the benefits of SSRI treatment in pregnancy. Those taking SSRIs were much more likely to have depression during pregnancy (76.5% vs. 13.5%) and anxiety (56.7% vs. 6.9%), compared with those who did not take the drug.
The possible connection between SSRIs and NE is unclear. “SSRIs may contribute to NE by a withdrawal mechanism or by a toxicity mechanism. It is also possible that SSRIs themselves are not responsible for encephalopathy, or that the severity of maternal mental health is itself responsible for increased neonatal encephalopathy,” Dr. Cornet said. “However, we believe we adjusted our results thoroughly for that. Furthermore, in this cohort, neonates born from mothers with untreated depression were not at higher risk of encephalopathy than neonates born to mothers without depression.”
She added: “When infants have acidosis or require prolonged resuscitation after birth, they get treated with therapeutic hypothermia. This invasive treatment was shown to decrease mortality and morbidity in neonates with hypoxic-ischemic encephalopathy. However, therapeutic hypothermia may not be helpful in infants with encephalopathy due to other causes than acute hypoxia-ischemia, such as infection, inflammation, genetic conditions, or exposure to toxins. In the case of SSRIs, our results show that, while neonates often have encephalopathy, this encephalopathy is often mild and self-resolved. We did not see a statistically significant increase in acidosis or treatment with therapeutic hypothermia.”
In the future neurologists should consider SSRI use as a potential cause in cases of NE, Dr. Cornet said. “If there are no signs of hypoxic-ischemic encephalopathy – no evidence of acidosis, acute perinatal event – treatment with therapeutic hypothermia may not be indicated.”
Dr. Cornet said more research is in the works. “Studying the long-term side effect of SSRIs on neonatal brain development and injury is essential,” she said. “We plan to compare brain injury in neonates exposed and unexposed to SSRIs and examine long-term outcomes to assess if the effect of SSRI exposure is transient or has a lasting impact.”
This study was funded by the Thrasher Early Career Research Grant and by the Newborn Brain Research Innovation Award at UCSF. The authors have no relevant disclosures.
, although the overall risk remains extremely low, a new study finds.
The findings were presented in a poster at the 50th annual meeting of the Child Neurology Society.
“Our work showed that neonates exposed to SSRI in utero had higher risks of neonatal encephalopathy even when adjusting for confounders such as maternal mental health disorders and age. SSRIs could cause side effects such as encephalopathy in neonates, and these risks need to be balanced carefully with the potential benefits of treatment to the mother,” study lead author Marie Cornet, MD, a neonatology fellow with Benioff Children’s Hospital at the University of California, San Francisco, said in an interview.
According to Dr. Cornet, “we know that SSRI exposure in utero is associated with increased risks of respiratory distress at birth, need for positive-pressure ventilation, and an abnormal neurologic exam.” The researchers launched the new study to determine if the estimated 4%-8% of pregnant women who take SSRIs may be putting their newborns at greater risk of NE.
The researchers retrospectively tracked 305,426 infants who were born in the Kaiser Permanente Northern California health system (≥35 weeks) from 2011 to 2019. The mothers had an average age of 31 years, and approximately 34.7% were White, 34.7% of unknown race, 23.3% Asian, and 6.2% Black.
The researchers defined NE as a “5-minute APGAR score <7 and abnormal level of consciousness, activity, tone, or reflexes.”
A total of 8,024 infants (2.6%) had mothers who used SSRIs in the third trimester, and 510 (0.17%) were determined to have had NE.
After adjustment for maternal depression or anxiety, maternal age, race, and hospital, exposed neonates had 2.7 times higher odds of NE (odds ratio, 2.7).
Each 25 mg per day increase in the dose of SSRIs, as equalized to doses of sertraline (Zoloft), was linked to a significant 31% increase in the odds of developing NE (OR, 1.31).
The study doesn’t examine the benefits of SSRI treatment in pregnancy. Those taking SSRIs were much more likely to have depression during pregnancy (76.5% vs. 13.5%) and anxiety (56.7% vs. 6.9%), compared with those who did not take the drug.
The possible connection between SSRIs and NE is unclear. “SSRIs may contribute to NE by a withdrawal mechanism or by a toxicity mechanism. It is also possible that SSRIs themselves are not responsible for encephalopathy, or that the severity of maternal mental health is itself responsible for increased neonatal encephalopathy,” Dr. Cornet said. “However, we believe we adjusted our results thoroughly for that. Furthermore, in this cohort, neonates born from mothers with untreated depression were not at higher risk of encephalopathy than neonates born to mothers without depression.”
She added: “When infants have acidosis or require prolonged resuscitation after birth, they get treated with therapeutic hypothermia. This invasive treatment was shown to decrease mortality and morbidity in neonates with hypoxic-ischemic encephalopathy. However, therapeutic hypothermia may not be helpful in infants with encephalopathy due to other causes than acute hypoxia-ischemia, such as infection, inflammation, genetic conditions, or exposure to toxins. In the case of SSRIs, our results show that, while neonates often have encephalopathy, this encephalopathy is often mild and self-resolved. We did not see a statistically significant increase in acidosis or treatment with therapeutic hypothermia.”
In the future neurologists should consider SSRI use as a potential cause in cases of NE, Dr. Cornet said. “If there are no signs of hypoxic-ischemic encephalopathy – no evidence of acidosis, acute perinatal event – treatment with therapeutic hypothermia may not be indicated.”
Dr. Cornet said more research is in the works. “Studying the long-term side effect of SSRIs on neonatal brain development and injury is essential,” she said. “We plan to compare brain injury in neonates exposed and unexposed to SSRIs and examine long-term outcomes to assess if the effect of SSRI exposure is transient or has a lasting impact.”
This study was funded by the Thrasher Early Career Research Grant and by the Newborn Brain Research Innovation Award at UCSF. The authors have no relevant disclosures.
, although the overall risk remains extremely low, a new study finds.
The findings were presented in a poster at the 50th annual meeting of the Child Neurology Society.
“Our work showed that neonates exposed to SSRI in utero had higher risks of neonatal encephalopathy even when adjusting for confounders such as maternal mental health disorders and age. SSRIs could cause side effects such as encephalopathy in neonates, and these risks need to be balanced carefully with the potential benefits of treatment to the mother,” study lead author Marie Cornet, MD, a neonatology fellow with Benioff Children’s Hospital at the University of California, San Francisco, said in an interview.
According to Dr. Cornet, “we know that SSRI exposure in utero is associated with increased risks of respiratory distress at birth, need for positive-pressure ventilation, and an abnormal neurologic exam.” The researchers launched the new study to determine if the estimated 4%-8% of pregnant women who take SSRIs may be putting their newborns at greater risk of NE.
The researchers retrospectively tracked 305,426 infants who were born in the Kaiser Permanente Northern California health system (≥35 weeks) from 2011 to 2019. The mothers had an average age of 31 years, and approximately 34.7% were White, 34.7% of unknown race, 23.3% Asian, and 6.2% Black.
The researchers defined NE as a “5-minute APGAR score <7 and abnormal level of consciousness, activity, tone, or reflexes.”
A total of 8,024 infants (2.6%) had mothers who used SSRIs in the third trimester, and 510 (0.17%) were determined to have had NE.
After adjustment for maternal depression or anxiety, maternal age, race, and hospital, exposed neonates had 2.7 times higher odds of NE (odds ratio, 2.7).
Each 25 mg per day increase in the dose of SSRIs, as equalized to doses of sertraline (Zoloft), was linked to a significant 31% increase in the odds of developing NE (OR, 1.31).
The study doesn’t examine the benefits of SSRI treatment in pregnancy. Those taking SSRIs were much more likely to have depression during pregnancy (76.5% vs. 13.5%) and anxiety (56.7% vs. 6.9%), compared with those who did not take the drug.
The possible connection between SSRIs and NE is unclear. “SSRIs may contribute to NE by a withdrawal mechanism or by a toxicity mechanism. It is also possible that SSRIs themselves are not responsible for encephalopathy, or that the severity of maternal mental health is itself responsible for increased neonatal encephalopathy,” Dr. Cornet said. “However, we believe we adjusted our results thoroughly for that. Furthermore, in this cohort, neonates born from mothers with untreated depression were not at higher risk of encephalopathy than neonates born to mothers without depression.”
She added: “When infants have acidosis or require prolonged resuscitation after birth, they get treated with therapeutic hypothermia. This invasive treatment was shown to decrease mortality and morbidity in neonates with hypoxic-ischemic encephalopathy. However, therapeutic hypothermia may not be helpful in infants with encephalopathy due to other causes than acute hypoxia-ischemia, such as infection, inflammation, genetic conditions, or exposure to toxins. In the case of SSRIs, our results show that, while neonates often have encephalopathy, this encephalopathy is often mild and self-resolved. We did not see a statistically significant increase in acidosis or treatment with therapeutic hypothermia.”
In the future neurologists should consider SSRI use as a potential cause in cases of NE, Dr. Cornet said. “If there are no signs of hypoxic-ischemic encephalopathy – no evidence of acidosis, acute perinatal event – treatment with therapeutic hypothermia may not be indicated.”
Dr. Cornet said more research is in the works. “Studying the long-term side effect of SSRIs on neonatal brain development and injury is essential,” she said. “We plan to compare brain injury in neonates exposed and unexposed to SSRIs and examine long-term outcomes to assess if the effect of SSRI exposure is transient or has a lasting impact.”
This study was funded by the Thrasher Early Career Research Grant and by the Newborn Brain Research Innovation Award at UCSF. The authors have no relevant disclosures.
FROM CNS 2021
Merck’s new COVID-19 pill: ‘Game changer’ or just one more tool?
Soon after Merck announced on Oct. 1 that it would ask federal regulators for emergency use authorization (EUA) for its auspicious new COVID-19 pill, the accolades began.
Former Food and Drug Administration chief Scott Gottlieb, MD, told CNBC the drug was “a profound game changer.” Top infectious disease expert Anthony S. Fauci, MD, called the early data “impressive.” The World Health Organization termed it “certainly good news,” while saying it awaits more data.
Merck, partnering with Ridgeback Biotherapeutics on the investigational oral antiviral medicine molnupiravir, plans to submit applications to regulatory agencies worldwide, hoping to deliver the first oral antiviral medication for COVID-19.
Interim clinical trial results show that the drug may slash the risk for hospitalization or death by 50% in those with mild to moderate COVID-19.
When the results were found to be so favorable, the study was halted at the recommendation of an independent data-monitoring committee and in consultation with the FDA.
“This anticipated drug has gotten a little more hype than it deserves,” said William Schaffner, MD, professor of preventive medicine and infectious disease specialist at Vanderbilt University Medical Center in Nashville, Tenn. He and others suggest a reality check.
“It’s not exactly a home run, like penicillin for strep throat,” agreed Carl Fichtenbaum, MD, professor of infectious diseases at the University of Cincinnati, who is investigating a similar pill for a rival company, Atea, partnering with Roche.
“But it is encouraging,” he said. “It will probably be an incremental improvement on what we have.” The fact that it can be taken at home is a plus: “Anything we can do to keep people from getting sicker is a good thing.”
“The data show in this higher risk group [those who were studied had at least one risk factor for severe COVID-19, such as age or a medical condition], it reduces the risk of advancing to severe disease by 50%,” Dr. Schaffner said. While that’s a clear benefit for half, it of course leaves the other half without benefit, he said.
Others critiqued the predicted cost of the drug. The U.S. government has already agreed to pay about $700 per patient, according to a new report from Harvard T. H. Chan School of Public Health, Boston, and King’s College Hospital, London. That analysis concluded that the actual cost of production for the 5-day course is $17.74.
“We fully expect that having an oral treatment that reduces the risk of hospitalizations will be significantly cost effective for society,” Melissa Moody, a Merck spokesperson, told this news organization. “We are optimistic that molnupiravir can become an important medicine as part of the global effort to fight the pandemic.”
Merck expects to produce 10 million courses of treatment by the end of the year, with additional doses expected to be produced in 2022, according to a company press release. Earlier in 2021, Merck finalized its agreement with the U.S. government to supply about 1.7 million courses of the drug at the $700 price, once an EUA or FDA approval is given.
Merck also has supply and purchase agreements with other governments worldwide, pending regulatory approval.
Study details
Details about the study findings came from a Merck press release. In the planned interim analysis, Merck and Ridgeback evaluated data from 775 patients initially enrolled in the phase 3 MOVe-OUT trial.
All adults had lab-confirmed mild to moderate COVID-19, and reported onset of symptoms within 5 days of being randomly assigned to the drug or placebo. All had at least one risk factor linked with poor disease outcome (such as older age or obesity).
The drug is a ribonucleoside and works by creating mutations in the virus’s genome, halting the ability of the virus to replicate.
Through day 29 of the study, the drug reduced the risk or hospitalization or death by about 50%. While 7.3% of those who received the drug either died or were hospitalized by day 29, 14.1% of those on placebo did, a statistically significant difference (P = .0012).
Side effects were similar in both groups, with 35% of the drug-treated and 40% of the placebo group reporting some side effect, Merck reported. Adverse drug-related events were 12% in the drug group and 11% in the placebo group. While 1.3% of the drug-treated group quit the study because of an adverse event, 3.4% of the placebo group quit.
Pros, cons, and unknowns
The ability to take the drug orally, and at home, is a definite plus, Dr. Schaffner said, compared with the monoclonal antibody treatment currently approved that must be given intravenously or subcutaneously and in certain locations.
More people could be reached and helped with the option of an at-home, oral medicine, he and others agreed.
The regimen for molnupiravir is four pills, two times daily, for 5 days, even if symptoms are mild. As with other prescription drugs, “there will always be folks who don’t comply completely” with the prescribed regimen, Dr. Schaffner said. With this pill, that might be especially true if the symptoms are very mild.
The 50% reduction is not as effective as the benefit often quoted for monoclonal antibody treatment. In clinical trials of Regeneron’s monoclonal antibody treatment, the regimen reduced COVID-19–related hospitalization or death in high-risk patients by 70%.
Even so, the new pill could change the pandemic’s course, others say. “I think molnupiravir has the potential to change how we take care of people who have COVID and risk factors for developing severe disease,” Rajesh Tim Gandhi, MD, an infectious disease physician at Massachusetts General Hospital and Harvard Medical School in Boston, told this news organization.
“What we’ll need to do, however, is make sure that people get tested quickly after they develop symptoms and, if they’re confirmed to have COVID, start on the pills within 5 days of developing symptoms,” he said, while warning that more data are needed about the drug and the trial results.
Another concern is that the promise of a pill will stall vaccination rates, with some people figuring why get vaccinated when they can obtain the pill if they do get sick.
Relying on treatment alone won’t work, Dr. Schaffner said. “Let’s [also] focus on prevention, which is the vaccine. We have to keep working both sides of the street.”
Dr. Gandhi added: “It’s important to remember that even though molnupiravir reduced the likelihood of hospitalization and death, a number of people who received the drug still got sick enough to end up in the hospital.”
Also unknown, he said, is how severe their disease was and whether they will develop long COVID.
The Merck study included only unvaccinated people. Might it work for those vaccinated people who get a breakthrough infection? “From a purely scientific perspective, there is no reason to believe molnupiravir would not work in people who are vaccinated, but the overall efficacy on top of the vaccine is likely dependent on how well they were able to mount a protective immune response to the vaccine,” Ms. Moody said. Still, Merck believes the pill could be of benefit for these infections too, she added.
As for the expected cost, Ms. Moody said that the company takes into account a number of factors in setting pricing, “but fundamentally we look at the impact of the disease, the benefits that the drug delivers to patients and to society, and at supporting ongoing drug development.”
On Merck’s heels: Pfizer, Roche, Atea
Pfizer is studying an antiviral pill, PF-07321332, a protease inhibitor that blocks the protease enzymes and halts replication of the virus.
In addition to studying the drug in infected patients at high risk of severe illness and in those at typical risk, Pfizer launched a phase 2-3 study in late September that will enroll people who live in the same household as a person with a confirmed, symptomatic COVID-19 infection to see if the drug can prevent disease in those who have been exposed.
Atea and Roche’s COVID pill, AT527, is in phase 3 trials as well. AT527 is an inhibitor of polymerase, an enzyme many viruses have, to stop replications. Atea is evaluating the drug to reduce disease “burden” and for both pre- and postexposure prevention.
Big picture: Role of COVID-19 pills
It may be necessary to target the coronavirus with more than one antiviral agent, said Dr. Fichtenbaum, a principal investigator for the AT527 trials.
“Sometimes viruses require two or three active agents to control their replication,” he said, citing information gleaned from other viral research, such as HIV. For control of HIV infection, a cocktail or combination of antivirals is often recommended.
That may well be the case for COVID-19, Dr. Fichtenbaum said. The goal would be to attack the virus at more than one pathway.
A version of this article first appeared on Medscape.com.
Soon after Merck announced on Oct. 1 that it would ask federal regulators for emergency use authorization (EUA) for its auspicious new COVID-19 pill, the accolades began.
Former Food and Drug Administration chief Scott Gottlieb, MD, told CNBC the drug was “a profound game changer.” Top infectious disease expert Anthony S. Fauci, MD, called the early data “impressive.” The World Health Organization termed it “certainly good news,” while saying it awaits more data.
Merck, partnering with Ridgeback Biotherapeutics on the investigational oral antiviral medicine molnupiravir, plans to submit applications to regulatory agencies worldwide, hoping to deliver the first oral antiviral medication for COVID-19.
Interim clinical trial results show that the drug may slash the risk for hospitalization or death by 50% in those with mild to moderate COVID-19.
When the results were found to be so favorable, the study was halted at the recommendation of an independent data-monitoring committee and in consultation with the FDA.
“This anticipated drug has gotten a little more hype than it deserves,” said William Schaffner, MD, professor of preventive medicine and infectious disease specialist at Vanderbilt University Medical Center in Nashville, Tenn. He and others suggest a reality check.
“It’s not exactly a home run, like penicillin for strep throat,” agreed Carl Fichtenbaum, MD, professor of infectious diseases at the University of Cincinnati, who is investigating a similar pill for a rival company, Atea, partnering with Roche.
“But it is encouraging,” he said. “It will probably be an incremental improvement on what we have.” The fact that it can be taken at home is a plus: “Anything we can do to keep people from getting sicker is a good thing.”
“The data show in this higher risk group [those who were studied had at least one risk factor for severe COVID-19, such as age or a medical condition], it reduces the risk of advancing to severe disease by 50%,” Dr. Schaffner said. While that’s a clear benefit for half, it of course leaves the other half without benefit, he said.
Others critiqued the predicted cost of the drug. The U.S. government has already agreed to pay about $700 per patient, according to a new report from Harvard T. H. Chan School of Public Health, Boston, and King’s College Hospital, London. That analysis concluded that the actual cost of production for the 5-day course is $17.74.
“We fully expect that having an oral treatment that reduces the risk of hospitalizations will be significantly cost effective for society,” Melissa Moody, a Merck spokesperson, told this news organization. “We are optimistic that molnupiravir can become an important medicine as part of the global effort to fight the pandemic.”
Merck expects to produce 10 million courses of treatment by the end of the year, with additional doses expected to be produced in 2022, according to a company press release. Earlier in 2021, Merck finalized its agreement with the U.S. government to supply about 1.7 million courses of the drug at the $700 price, once an EUA or FDA approval is given.
Merck also has supply and purchase agreements with other governments worldwide, pending regulatory approval.
Study details
Details about the study findings came from a Merck press release. In the planned interim analysis, Merck and Ridgeback evaluated data from 775 patients initially enrolled in the phase 3 MOVe-OUT trial.
All adults had lab-confirmed mild to moderate COVID-19, and reported onset of symptoms within 5 days of being randomly assigned to the drug or placebo. All had at least one risk factor linked with poor disease outcome (such as older age or obesity).
The drug is a ribonucleoside and works by creating mutations in the virus’s genome, halting the ability of the virus to replicate.
Through day 29 of the study, the drug reduced the risk or hospitalization or death by about 50%. While 7.3% of those who received the drug either died or were hospitalized by day 29, 14.1% of those on placebo did, a statistically significant difference (P = .0012).
Side effects were similar in both groups, with 35% of the drug-treated and 40% of the placebo group reporting some side effect, Merck reported. Adverse drug-related events were 12% in the drug group and 11% in the placebo group. While 1.3% of the drug-treated group quit the study because of an adverse event, 3.4% of the placebo group quit.
Pros, cons, and unknowns
The ability to take the drug orally, and at home, is a definite plus, Dr. Schaffner said, compared with the monoclonal antibody treatment currently approved that must be given intravenously or subcutaneously and in certain locations.
More people could be reached and helped with the option of an at-home, oral medicine, he and others agreed.
The regimen for molnupiravir is four pills, two times daily, for 5 days, even if symptoms are mild. As with other prescription drugs, “there will always be folks who don’t comply completely” with the prescribed regimen, Dr. Schaffner said. With this pill, that might be especially true if the symptoms are very mild.
The 50% reduction is not as effective as the benefit often quoted for monoclonal antibody treatment. In clinical trials of Regeneron’s monoclonal antibody treatment, the regimen reduced COVID-19–related hospitalization or death in high-risk patients by 70%.
Even so, the new pill could change the pandemic’s course, others say. “I think molnupiravir has the potential to change how we take care of people who have COVID and risk factors for developing severe disease,” Rajesh Tim Gandhi, MD, an infectious disease physician at Massachusetts General Hospital and Harvard Medical School in Boston, told this news organization.
“What we’ll need to do, however, is make sure that people get tested quickly after they develop symptoms and, if they’re confirmed to have COVID, start on the pills within 5 days of developing symptoms,” he said, while warning that more data are needed about the drug and the trial results.
Another concern is that the promise of a pill will stall vaccination rates, with some people figuring why get vaccinated when they can obtain the pill if they do get sick.
Relying on treatment alone won’t work, Dr. Schaffner said. “Let’s [also] focus on prevention, which is the vaccine. We have to keep working both sides of the street.”
Dr. Gandhi added: “It’s important to remember that even though molnupiravir reduced the likelihood of hospitalization and death, a number of people who received the drug still got sick enough to end up in the hospital.”
Also unknown, he said, is how severe their disease was and whether they will develop long COVID.
The Merck study included only unvaccinated people. Might it work for those vaccinated people who get a breakthrough infection? “From a purely scientific perspective, there is no reason to believe molnupiravir would not work in people who are vaccinated, but the overall efficacy on top of the vaccine is likely dependent on how well they were able to mount a protective immune response to the vaccine,” Ms. Moody said. Still, Merck believes the pill could be of benefit for these infections too, she added.
As for the expected cost, Ms. Moody said that the company takes into account a number of factors in setting pricing, “but fundamentally we look at the impact of the disease, the benefits that the drug delivers to patients and to society, and at supporting ongoing drug development.”
On Merck’s heels: Pfizer, Roche, Atea
Pfizer is studying an antiviral pill, PF-07321332, a protease inhibitor that blocks the protease enzymes and halts replication of the virus.
In addition to studying the drug in infected patients at high risk of severe illness and in those at typical risk, Pfizer launched a phase 2-3 study in late September that will enroll people who live in the same household as a person with a confirmed, symptomatic COVID-19 infection to see if the drug can prevent disease in those who have been exposed.
Atea and Roche’s COVID pill, AT527, is in phase 3 trials as well. AT527 is an inhibitor of polymerase, an enzyme many viruses have, to stop replications. Atea is evaluating the drug to reduce disease “burden” and for both pre- and postexposure prevention.
Big picture: Role of COVID-19 pills
It may be necessary to target the coronavirus with more than one antiviral agent, said Dr. Fichtenbaum, a principal investigator for the AT527 trials.
“Sometimes viruses require two or three active agents to control their replication,” he said, citing information gleaned from other viral research, such as HIV. For control of HIV infection, a cocktail or combination of antivirals is often recommended.
That may well be the case for COVID-19, Dr. Fichtenbaum said. The goal would be to attack the virus at more than one pathway.
A version of this article first appeared on Medscape.com.
Soon after Merck announced on Oct. 1 that it would ask federal regulators for emergency use authorization (EUA) for its auspicious new COVID-19 pill, the accolades began.
Former Food and Drug Administration chief Scott Gottlieb, MD, told CNBC the drug was “a profound game changer.” Top infectious disease expert Anthony S. Fauci, MD, called the early data “impressive.” The World Health Organization termed it “certainly good news,” while saying it awaits more data.
Merck, partnering with Ridgeback Biotherapeutics on the investigational oral antiviral medicine molnupiravir, plans to submit applications to regulatory agencies worldwide, hoping to deliver the first oral antiviral medication for COVID-19.
Interim clinical trial results show that the drug may slash the risk for hospitalization or death by 50% in those with mild to moderate COVID-19.
When the results were found to be so favorable, the study was halted at the recommendation of an independent data-monitoring committee and in consultation with the FDA.
“This anticipated drug has gotten a little more hype than it deserves,” said William Schaffner, MD, professor of preventive medicine and infectious disease specialist at Vanderbilt University Medical Center in Nashville, Tenn. He and others suggest a reality check.
“It’s not exactly a home run, like penicillin for strep throat,” agreed Carl Fichtenbaum, MD, professor of infectious diseases at the University of Cincinnati, who is investigating a similar pill for a rival company, Atea, partnering with Roche.
“But it is encouraging,” he said. “It will probably be an incremental improvement on what we have.” The fact that it can be taken at home is a plus: “Anything we can do to keep people from getting sicker is a good thing.”
“The data show in this higher risk group [those who were studied had at least one risk factor for severe COVID-19, such as age or a medical condition], it reduces the risk of advancing to severe disease by 50%,” Dr. Schaffner said. While that’s a clear benefit for half, it of course leaves the other half without benefit, he said.
Others critiqued the predicted cost of the drug. The U.S. government has already agreed to pay about $700 per patient, according to a new report from Harvard T. H. Chan School of Public Health, Boston, and King’s College Hospital, London. That analysis concluded that the actual cost of production for the 5-day course is $17.74.
“We fully expect that having an oral treatment that reduces the risk of hospitalizations will be significantly cost effective for society,” Melissa Moody, a Merck spokesperson, told this news organization. “We are optimistic that molnupiravir can become an important medicine as part of the global effort to fight the pandemic.”
Merck expects to produce 10 million courses of treatment by the end of the year, with additional doses expected to be produced in 2022, according to a company press release. Earlier in 2021, Merck finalized its agreement with the U.S. government to supply about 1.7 million courses of the drug at the $700 price, once an EUA or FDA approval is given.
Merck also has supply and purchase agreements with other governments worldwide, pending regulatory approval.
Study details
Details about the study findings came from a Merck press release. In the planned interim analysis, Merck and Ridgeback evaluated data from 775 patients initially enrolled in the phase 3 MOVe-OUT trial.
All adults had lab-confirmed mild to moderate COVID-19, and reported onset of symptoms within 5 days of being randomly assigned to the drug or placebo. All had at least one risk factor linked with poor disease outcome (such as older age or obesity).
The drug is a ribonucleoside and works by creating mutations in the virus’s genome, halting the ability of the virus to replicate.
Through day 29 of the study, the drug reduced the risk or hospitalization or death by about 50%. While 7.3% of those who received the drug either died or were hospitalized by day 29, 14.1% of those on placebo did, a statistically significant difference (P = .0012).
Side effects were similar in both groups, with 35% of the drug-treated and 40% of the placebo group reporting some side effect, Merck reported. Adverse drug-related events were 12% in the drug group and 11% in the placebo group. While 1.3% of the drug-treated group quit the study because of an adverse event, 3.4% of the placebo group quit.
Pros, cons, and unknowns
The ability to take the drug orally, and at home, is a definite plus, Dr. Schaffner said, compared with the monoclonal antibody treatment currently approved that must be given intravenously or subcutaneously and in certain locations.
More people could be reached and helped with the option of an at-home, oral medicine, he and others agreed.
The regimen for molnupiravir is four pills, two times daily, for 5 days, even if symptoms are mild. As with other prescription drugs, “there will always be folks who don’t comply completely” with the prescribed regimen, Dr. Schaffner said. With this pill, that might be especially true if the symptoms are very mild.
The 50% reduction is not as effective as the benefit often quoted for monoclonal antibody treatment. In clinical trials of Regeneron’s monoclonal antibody treatment, the regimen reduced COVID-19–related hospitalization or death in high-risk patients by 70%.
Even so, the new pill could change the pandemic’s course, others say. “I think molnupiravir has the potential to change how we take care of people who have COVID and risk factors for developing severe disease,” Rajesh Tim Gandhi, MD, an infectious disease physician at Massachusetts General Hospital and Harvard Medical School in Boston, told this news organization.
“What we’ll need to do, however, is make sure that people get tested quickly after they develop symptoms and, if they’re confirmed to have COVID, start on the pills within 5 days of developing symptoms,” he said, while warning that more data are needed about the drug and the trial results.
Another concern is that the promise of a pill will stall vaccination rates, with some people figuring why get vaccinated when they can obtain the pill if they do get sick.
Relying on treatment alone won’t work, Dr. Schaffner said. “Let’s [also] focus on prevention, which is the vaccine. We have to keep working both sides of the street.”
Dr. Gandhi added: “It’s important to remember that even though molnupiravir reduced the likelihood of hospitalization and death, a number of people who received the drug still got sick enough to end up in the hospital.”
Also unknown, he said, is how severe their disease was and whether they will develop long COVID.
The Merck study included only unvaccinated people. Might it work for those vaccinated people who get a breakthrough infection? “From a purely scientific perspective, there is no reason to believe molnupiravir would not work in people who are vaccinated, but the overall efficacy on top of the vaccine is likely dependent on how well they were able to mount a protective immune response to the vaccine,” Ms. Moody said. Still, Merck believes the pill could be of benefit for these infections too, she added.
As for the expected cost, Ms. Moody said that the company takes into account a number of factors in setting pricing, “but fundamentally we look at the impact of the disease, the benefits that the drug delivers to patients and to society, and at supporting ongoing drug development.”
On Merck’s heels: Pfizer, Roche, Atea
Pfizer is studying an antiviral pill, PF-07321332, a protease inhibitor that blocks the protease enzymes and halts replication of the virus.
In addition to studying the drug in infected patients at high risk of severe illness and in those at typical risk, Pfizer launched a phase 2-3 study in late September that will enroll people who live in the same household as a person with a confirmed, symptomatic COVID-19 infection to see if the drug can prevent disease in those who have been exposed.
Atea and Roche’s COVID pill, AT527, is in phase 3 trials as well. AT527 is an inhibitor of polymerase, an enzyme many viruses have, to stop replications. Atea is evaluating the drug to reduce disease “burden” and for both pre- and postexposure prevention.
Big picture: Role of COVID-19 pills
It may be necessary to target the coronavirus with more than one antiviral agent, said Dr. Fichtenbaum, a principal investigator for the AT527 trials.
“Sometimes viruses require two or three active agents to control their replication,” he said, citing information gleaned from other viral research, such as HIV. For control of HIV infection, a cocktail or combination of antivirals is often recommended.
That may well be the case for COVID-19, Dr. Fichtenbaum said. The goal would be to attack the virus at more than one pathway.
A version of this article first appeared on Medscape.com.
Cold viruses thrived in kids as other viruses faded in 2020
The common-cold viruses rhinovirus (RV) and enterovirus (EV) continued to circulate among children during the COVID-19 pandemic while there were sharp declines in influenza, respiratory syncytial virus (RSV), and other respiratory viruses, new data indicate.
Researchers used data from the Centers for Disease Control and Prevention’s New Vaccine Surveillance Network. The cases involved 37,676 children in seven geographically diverse U.S. medical centers between December 2016 and January 2021. Patients presented to emergency departments or were hospitalized with RV, EV, and other acute respiratory viruses.
The investigators found that the percentage of children in whom RV/EV was detected from March 2020 to January 2021 was similar to the percentage during the same months in 2017-2018 and 2019-2020. However, the proportion of children infected with influenza, RSV, and other respiratory viruses combined dropped significantly in comparison to the three prior seasons.

Danielle Rankin, MPH, lead author of the study and a doctoral candidate in pediatric infectious disease at Vanderbilt University, in Nashville, Tenn., presented the study on Sept. 30 during a press conference at IDWeek 2021, an annual scientific meeting on infectious diseases.
“Reasoning for rhinovirus and enterovirus circulation is unknown but may be attributed to a number of factors, such as different transmission routes or the prolonged survival of the virus on surfaces,” Ms. Rankin said. “Improved understanding of these persistent factors of RV/EV and the role of nonpharmaceutical interventions on transmission dynamics can further guide future prevention recommendations and guidelines.”
Coauthor Claire Midgley, PhD, an epidemiologist in the Division of Viral Diseases at the CDC, told reporters that further studies will assess why RV and EV remained during the pandemic and which virus types within the RV/EV group persisted.
“We do know that the virus can spread through secretions on people’s hands,” she said. “Washing kids’ hands regularly and trying not to touch your face where possible is a really effective way to prevent transmission,” Dr. Midgley said.
“The more we understand about all of these factors, the better we can inform prevention measures.”
Andrew T. Pavia, MD, chief, division of pediatric infectious diseases, University of Utah, Salt Lake City, who was not involved in the study, told this news organization that rhinoviruses can persist in the nose for a very long time, especially in younger children, which increases the opportunities for transmission.
“Very young children who are unable to wear masks or are unlikely to wear them well may be acting as the reservoir, allowing transmission in households,” he said. “There is also an enormous pool of diverse rhinoviruses, so past colds provide limited immunity, as everyone has found out from experience.”
Martha Perry, MD, associate professor at the University of North Carolina at Chapel Hill and chief of adolescent medicine, told this news organization that some of the differences in the prevalence of viruses may be because of their seasonality.
“Times when there were more mask mandates were times when RSV and influenza are more prevalent,” said Dr. Perry, who was not involved with the study. “We were masking more intently during those times, and there was loosening of restrictions when we see more enterovirus, particularly because that tends to be more of a summer/fall virus.”
She agreed that the differences may result from the way the viruses are transmitted.
“Perhaps masks were helping with RSV and influenza, but perhaps there was not as much hand washing or cleansing as needed to prevent the spread of rhinovirus and enterovirus, because those are viruses that require a bit more hand washing,” Dr. Perry said. “They are less aerosolized and better spread with hand-to-hand contact.”
Dr. Perry added that on the flip side, “it’s really exciting that there are ways we can prevent RSV and influenza, which tend to cause more severe infection.”
Ms. Rankin said limitations of the study include the fact that from March 2020 to January 2021, health care–seeking behaviors may have changed because of the pandemic and that the study does not include the frequency of respiratory viruses in the outpatient setting.
The sharp 2020-2021 decline in RSV reported in the study may have reversed after many of the COVID-19 restrictions were lifted this summer.
This news organization reported in June of this year that the CDC has issued a health advisory to notify clinicians and caregivers about an increase in cases of interseasonal RSV in parts of the southern United States.
The CDC has urged broader testing for RSV among patients presenting with acute respiratory illness who test negative for SARS-CoV-2.
The study’s authors, Ms. Pavia, and Dr. Perry have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The common-cold viruses rhinovirus (RV) and enterovirus (EV) continued to circulate among children during the COVID-19 pandemic while there were sharp declines in influenza, respiratory syncytial virus (RSV), and other respiratory viruses, new data indicate.
Researchers used data from the Centers for Disease Control and Prevention’s New Vaccine Surveillance Network. The cases involved 37,676 children in seven geographically diverse U.S. medical centers between December 2016 and January 2021. Patients presented to emergency departments or were hospitalized with RV, EV, and other acute respiratory viruses.
The investigators found that the percentage of children in whom RV/EV was detected from March 2020 to January 2021 was similar to the percentage during the same months in 2017-2018 and 2019-2020. However, the proportion of children infected with influenza, RSV, and other respiratory viruses combined dropped significantly in comparison to the three prior seasons.

Danielle Rankin, MPH, lead author of the study and a doctoral candidate in pediatric infectious disease at Vanderbilt University, in Nashville, Tenn., presented the study on Sept. 30 during a press conference at IDWeek 2021, an annual scientific meeting on infectious diseases.
“Reasoning for rhinovirus and enterovirus circulation is unknown but may be attributed to a number of factors, such as different transmission routes or the prolonged survival of the virus on surfaces,” Ms. Rankin said. “Improved understanding of these persistent factors of RV/EV and the role of nonpharmaceutical interventions on transmission dynamics can further guide future prevention recommendations and guidelines.”
Coauthor Claire Midgley, PhD, an epidemiologist in the Division of Viral Diseases at the CDC, told reporters that further studies will assess why RV and EV remained during the pandemic and which virus types within the RV/EV group persisted.
“We do know that the virus can spread through secretions on people’s hands,” she said. “Washing kids’ hands regularly and trying not to touch your face where possible is a really effective way to prevent transmission,” Dr. Midgley said.
“The more we understand about all of these factors, the better we can inform prevention measures.”
Andrew T. Pavia, MD, chief, division of pediatric infectious diseases, University of Utah, Salt Lake City, who was not involved in the study, told this news organization that rhinoviruses can persist in the nose for a very long time, especially in younger children, which increases the opportunities for transmission.
“Very young children who are unable to wear masks or are unlikely to wear them well may be acting as the reservoir, allowing transmission in households,” he said. “There is also an enormous pool of diverse rhinoviruses, so past colds provide limited immunity, as everyone has found out from experience.”
Martha Perry, MD, associate professor at the University of North Carolina at Chapel Hill and chief of adolescent medicine, told this news organization that some of the differences in the prevalence of viruses may be because of their seasonality.
“Times when there were more mask mandates were times when RSV and influenza are more prevalent,” said Dr. Perry, who was not involved with the study. “We were masking more intently during those times, and there was loosening of restrictions when we see more enterovirus, particularly because that tends to be more of a summer/fall virus.”
She agreed that the differences may result from the way the viruses are transmitted.
“Perhaps masks were helping with RSV and influenza, but perhaps there was not as much hand washing or cleansing as needed to prevent the spread of rhinovirus and enterovirus, because those are viruses that require a bit more hand washing,” Dr. Perry said. “They are less aerosolized and better spread with hand-to-hand contact.”
Dr. Perry added that on the flip side, “it’s really exciting that there are ways we can prevent RSV and influenza, which tend to cause more severe infection.”
Ms. Rankin said limitations of the study include the fact that from March 2020 to January 2021, health care–seeking behaviors may have changed because of the pandemic and that the study does not include the frequency of respiratory viruses in the outpatient setting.
The sharp 2020-2021 decline in RSV reported in the study may have reversed after many of the COVID-19 restrictions were lifted this summer.
This news organization reported in June of this year that the CDC has issued a health advisory to notify clinicians and caregivers about an increase in cases of interseasonal RSV in parts of the southern United States.
The CDC has urged broader testing for RSV among patients presenting with acute respiratory illness who test negative for SARS-CoV-2.
The study’s authors, Ms. Pavia, and Dr. Perry have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The common-cold viruses rhinovirus (RV) and enterovirus (EV) continued to circulate among children during the COVID-19 pandemic while there were sharp declines in influenza, respiratory syncytial virus (RSV), and other respiratory viruses, new data indicate.
Researchers used data from the Centers for Disease Control and Prevention’s New Vaccine Surveillance Network. The cases involved 37,676 children in seven geographically diverse U.S. medical centers between December 2016 and January 2021. Patients presented to emergency departments or were hospitalized with RV, EV, and other acute respiratory viruses.
The investigators found that the percentage of children in whom RV/EV was detected from March 2020 to January 2021 was similar to the percentage during the same months in 2017-2018 and 2019-2020. However, the proportion of children infected with influenza, RSV, and other respiratory viruses combined dropped significantly in comparison to the three prior seasons.

Danielle Rankin, MPH, lead author of the study and a doctoral candidate in pediatric infectious disease at Vanderbilt University, in Nashville, Tenn., presented the study on Sept. 30 during a press conference at IDWeek 2021, an annual scientific meeting on infectious diseases.
“Reasoning for rhinovirus and enterovirus circulation is unknown but may be attributed to a number of factors, such as different transmission routes or the prolonged survival of the virus on surfaces,” Ms. Rankin said. “Improved understanding of these persistent factors of RV/EV and the role of nonpharmaceutical interventions on transmission dynamics can further guide future prevention recommendations and guidelines.”
Coauthor Claire Midgley, PhD, an epidemiologist in the Division of Viral Diseases at the CDC, told reporters that further studies will assess why RV and EV remained during the pandemic and which virus types within the RV/EV group persisted.
“We do know that the virus can spread through secretions on people’s hands,” she said. “Washing kids’ hands regularly and trying not to touch your face where possible is a really effective way to prevent transmission,” Dr. Midgley said.
“The more we understand about all of these factors, the better we can inform prevention measures.”
Andrew T. Pavia, MD, chief, division of pediatric infectious diseases, University of Utah, Salt Lake City, who was not involved in the study, told this news organization that rhinoviruses can persist in the nose for a very long time, especially in younger children, which increases the opportunities for transmission.
“Very young children who are unable to wear masks or are unlikely to wear them well may be acting as the reservoir, allowing transmission in households,” he said. “There is also an enormous pool of diverse rhinoviruses, so past colds provide limited immunity, as everyone has found out from experience.”
Martha Perry, MD, associate professor at the University of North Carolina at Chapel Hill and chief of adolescent medicine, told this news organization that some of the differences in the prevalence of viruses may be because of their seasonality.
“Times when there were more mask mandates were times when RSV and influenza are more prevalent,” said Dr. Perry, who was not involved with the study. “We were masking more intently during those times, and there was loosening of restrictions when we see more enterovirus, particularly because that tends to be more of a summer/fall virus.”
She agreed that the differences may result from the way the viruses are transmitted.
“Perhaps masks were helping with RSV and influenza, but perhaps there was not as much hand washing or cleansing as needed to prevent the spread of rhinovirus and enterovirus, because those are viruses that require a bit more hand washing,” Dr. Perry said. “They are less aerosolized and better spread with hand-to-hand contact.”
Dr. Perry added that on the flip side, “it’s really exciting that there are ways we can prevent RSV and influenza, which tend to cause more severe infection.”
Ms. Rankin said limitations of the study include the fact that from March 2020 to January 2021, health care–seeking behaviors may have changed because of the pandemic and that the study does not include the frequency of respiratory viruses in the outpatient setting.
The sharp 2020-2021 decline in RSV reported in the study may have reversed after many of the COVID-19 restrictions were lifted this summer.
This news organization reported in June of this year that the CDC has issued a health advisory to notify clinicians and caregivers about an increase in cases of interseasonal RSV in parts of the southern United States.
The CDC has urged broader testing for RSV among patients presenting with acute respiratory illness who test negative for SARS-CoV-2.
The study’s authors, Ms. Pavia, and Dr. Perry have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Web of antimicrobials doesn’t hold water
Music plus mushrooms equals therapy
Magic mushrooms have been used recreationally and medicinally for thousands of years, but researchers have found adding music could be a game changer in antidepressant treatment.
The ingredient that makes these mushrooms so magical is psilocybin. It works well for the clinical treatment of mental health conditions and some forms of depression because the “trip” can be contained to one work day, making it easy to administer under supervision. With the accompaniment of music, scientists have found that psilocybin evokes emotion.
This recent study, presented at the European College of Neuropsychopharmacology Congress in Lisbon, tested participants’ emotional response to music before and after the psilocybin. Ketanserin, an antihypertensive drug, was used to test against the effects of psilocybin. The scientist played Mozart and Elgar and found that participants on psilocybin had an emotional response increase of 60%. That response was even greater, compared with ketanserin, which actually lessened the emotional response to music.
“This shows that combination of psilocybin and music has a strong emotional effect, and we believe that this will be important for the therapeutic application of psychedelics if they are approved for clinical use,” said lead researcher Dea Siggaard Stenbæk of the University of Copenhagen.
Professor David J. Nutt of Imperial College in London, who was not involved in the study, said that it supports the use of music for treatment efficacy with psychedelics and suggested that the next step is to “optimise this approach probably through individualising and personalising music tracks in therapy.”
Cue the 1960s LSD music montage.
Chicken ‘white striping is not a disease’
Have you ever sliced open a new pack of chicken breasts to start dinner and noticed white fatty lines running through the chicken? Maybe you thought it was just some extra fat to trim off, but the Humane League calls it “white striping disease.”
Chicken is the No. 1 meat consumed by Americans, so it’s not surprising that chickens are factory farmed and raised to be ready for slaughter quickly, according to CBSNews.com, which reported that the Humane League claims white striping is found in 70% of the chicken in popular grocery stores. The league expressed concern for the chickens’ welfare as they are bred to grow bigger quickly, which is causing the white striping and increasing the fat content of the meat by as much as 224%.
The National Chicken Council told CBS that the league’s findings were unscientific. A spokesperson said, “White striping is not a disease. It is a quality factor in chicken breast meat caused by deposits of fat in the muscle during the bird’s growth and development.” He went on to say that severe white striping happens in 3%-6% of birds, which are mostly used in further processed products, not in chicken breast packages.
Somehow, that’s not making us feel any better.
The itsy bitsy spider lets us all down
Most people do not like spiders. That’s too bad, because spiders are generally nothing but helpful little creatures that prey upon annoying flies and other pests. Then there’s the silk they produce. The ancient Romans used it to treat conditions such as warts and skin lesions. Spiders wrap their eggs in silk to protect them from harmful bacteria.
Of course, we can hardly trust the medical opinions of people from 2,000 years ago, but modern-day studies have not definitively proved whether or not spider silk has any antimicrobial properties.
To settle the matter once and for all, researchers from Denmark built a silk-harvesting machine using the most famous of Danish inventions: Legos. The contraption, sort of a paddle wheel, pulled the silk from several different species of spider pinned down by the researchers. The silk was then tested against three different bacteria species, including good old Escherichia coli.
Unfortunately for our spider friends, their silk has no antimicrobial activity. The researchers suspected that any such activity seen in previous studies was actually caused by improper control for the solvents used to extract the silk; those solvents can have antimicrobial properties on their own. As for protecting their eggs, rather than killing bacteria, the silk likely provides a physical barrier alone.
It is bad news for spiders on the benefit-to-humanity front, but look at the bright side: If their silk had antimicrobial activity, we’d have to start farming them to acquire more silk. And that’s no good. Spiders deserve to roam free, hunt as they please, and drop down on your head from the ceiling.
Anxiety and allergies: Cause, effect, confusion
We’re big fans of science, but as longtime, totally impartial (Science rules!) observers of science’s medical realm, we can see that the day-to-day process of practicing the scientific method occasionally gets a bit messy. And no, we’re not talking about COVID-19.
We’re talking allergies. We’re talking mental health. We’re talking allergic disease and mental health.
We’re talking about a pair of press releases we came across during our never-ending search for material to educate, entertain, and astound our fabulously wonderful and loyal readers. (We say that, of course, in the most impartial way possible.)
The first release was titled, “Allergies including asthma and hay fever not linked to mental health traits” and covered research from the University of Bristol (England). The investigators were trying to determine if “allergic diseases actually causes mental health traits including anxiety, depression, bipolar disorder, and schizophrenia, or vice versa,” according to the release.
What they found, however, was “little evidence of a causal relationship between the onset of allergic disease and mental health.” Again, this is the press release talking.
The second release seemed to suggest the exact opposite: “Study uncovers link between allergies and mental health conditions.” That got our attention. A little more reading revealed that “people with asthma, atopic dermatitis, and hay fever also had a higher likelihood of having depression, anxiety, bipolar disorder, or neuroticism.”
One of the investigators was quoted as saying, “Establishing whether allergic disease causes mental health problems, or vice versa, is important to ensure that resources and treatment strategies are targeted appropriately.”
Did you notice the “vice versa”? Did you notice that it appeared in quotes from both releases? We did, so we took a closer look at the source. The second release covered a group of investigators from the University of Bristol – the same group, and the same study, in fact, as the first one.
So there you have it. One study, two press releases, and one confused journalist. Thank you, science.
Music plus mushrooms equals therapy
Magic mushrooms have been used recreationally and medicinally for thousands of years, but researchers have found adding music could be a game changer in antidepressant treatment.
The ingredient that makes these mushrooms so magical is psilocybin. It works well for the clinical treatment of mental health conditions and some forms of depression because the “trip” can be contained to one work day, making it easy to administer under supervision. With the accompaniment of music, scientists have found that psilocybin evokes emotion.
This recent study, presented at the European College of Neuropsychopharmacology Congress in Lisbon, tested participants’ emotional response to music before and after the psilocybin. Ketanserin, an antihypertensive drug, was used to test against the effects of psilocybin. The scientist played Mozart and Elgar and found that participants on psilocybin had an emotional response increase of 60%. That response was even greater, compared with ketanserin, which actually lessened the emotional response to music.
“This shows that combination of psilocybin and music has a strong emotional effect, and we believe that this will be important for the therapeutic application of psychedelics if they are approved for clinical use,” said lead researcher Dea Siggaard Stenbæk of the University of Copenhagen.
Professor David J. Nutt of Imperial College in London, who was not involved in the study, said that it supports the use of music for treatment efficacy with psychedelics and suggested that the next step is to “optimise this approach probably through individualising and personalising music tracks in therapy.”
Cue the 1960s LSD music montage.
Chicken ‘white striping is not a disease’
Have you ever sliced open a new pack of chicken breasts to start dinner and noticed white fatty lines running through the chicken? Maybe you thought it was just some extra fat to trim off, but the Humane League calls it “white striping disease.”
Chicken is the No. 1 meat consumed by Americans, so it’s not surprising that chickens are factory farmed and raised to be ready for slaughter quickly, according to CBSNews.com, which reported that the Humane League claims white striping is found in 70% of the chicken in popular grocery stores. The league expressed concern for the chickens’ welfare as they are bred to grow bigger quickly, which is causing the white striping and increasing the fat content of the meat by as much as 224%.
The National Chicken Council told CBS that the league’s findings were unscientific. A spokesperson said, “White striping is not a disease. It is a quality factor in chicken breast meat caused by deposits of fat in the muscle during the bird’s growth and development.” He went on to say that severe white striping happens in 3%-6% of birds, which are mostly used in further processed products, not in chicken breast packages.
Somehow, that’s not making us feel any better.
The itsy bitsy spider lets us all down
Most people do not like spiders. That’s too bad, because spiders are generally nothing but helpful little creatures that prey upon annoying flies and other pests. Then there’s the silk they produce. The ancient Romans used it to treat conditions such as warts and skin lesions. Spiders wrap their eggs in silk to protect them from harmful bacteria.
Of course, we can hardly trust the medical opinions of people from 2,000 years ago, but modern-day studies have not definitively proved whether or not spider silk has any antimicrobial properties.
To settle the matter once and for all, researchers from Denmark built a silk-harvesting machine using the most famous of Danish inventions: Legos. The contraption, sort of a paddle wheel, pulled the silk from several different species of spider pinned down by the researchers. The silk was then tested against three different bacteria species, including good old Escherichia coli.
Unfortunately for our spider friends, their silk has no antimicrobial activity. The researchers suspected that any such activity seen in previous studies was actually caused by improper control for the solvents used to extract the silk; those solvents can have antimicrobial properties on their own. As for protecting their eggs, rather than killing bacteria, the silk likely provides a physical barrier alone.
It is bad news for spiders on the benefit-to-humanity front, but look at the bright side: If their silk had antimicrobial activity, we’d have to start farming them to acquire more silk. And that’s no good. Spiders deserve to roam free, hunt as they please, and drop down on your head from the ceiling.
Anxiety and allergies: Cause, effect, confusion
We’re big fans of science, but as longtime, totally impartial (Science rules!) observers of science’s medical realm, we can see that the day-to-day process of practicing the scientific method occasionally gets a bit messy. And no, we’re not talking about COVID-19.
We’re talking allergies. We’re talking mental health. We’re talking allergic disease and mental health.
We’re talking about a pair of press releases we came across during our never-ending search for material to educate, entertain, and astound our fabulously wonderful and loyal readers. (We say that, of course, in the most impartial way possible.)
The first release was titled, “Allergies including asthma and hay fever not linked to mental health traits” and covered research from the University of Bristol (England). The investigators were trying to determine if “allergic diseases actually causes mental health traits including anxiety, depression, bipolar disorder, and schizophrenia, or vice versa,” according to the release.
What they found, however, was “little evidence of a causal relationship between the onset of allergic disease and mental health.” Again, this is the press release talking.
The second release seemed to suggest the exact opposite: “Study uncovers link between allergies and mental health conditions.” That got our attention. A little more reading revealed that “people with asthma, atopic dermatitis, and hay fever also had a higher likelihood of having depression, anxiety, bipolar disorder, or neuroticism.”
One of the investigators was quoted as saying, “Establishing whether allergic disease causes mental health problems, or vice versa, is important to ensure that resources and treatment strategies are targeted appropriately.”
Did you notice the “vice versa”? Did you notice that it appeared in quotes from both releases? We did, so we took a closer look at the source. The second release covered a group of investigators from the University of Bristol – the same group, and the same study, in fact, as the first one.
So there you have it. One study, two press releases, and one confused journalist. Thank you, science.
Music plus mushrooms equals therapy
Magic mushrooms have been used recreationally and medicinally for thousands of years, but researchers have found adding music could be a game changer in antidepressant treatment.
The ingredient that makes these mushrooms so magical is psilocybin. It works well for the clinical treatment of mental health conditions and some forms of depression because the “trip” can be contained to one work day, making it easy to administer under supervision. With the accompaniment of music, scientists have found that psilocybin evokes emotion.
This recent study, presented at the European College of Neuropsychopharmacology Congress in Lisbon, tested participants’ emotional response to music before and after the psilocybin. Ketanserin, an antihypertensive drug, was used to test against the effects of psilocybin. The scientist played Mozart and Elgar and found that participants on psilocybin had an emotional response increase of 60%. That response was even greater, compared with ketanserin, which actually lessened the emotional response to music.
“This shows that combination of psilocybin and music has a strong emotional effect, and we believe that this will be important for the therapeutic application of psychedelics if they are approved for clinical use,” said lead researcher Dea Siggaard Stenbæk of the University of Copenhagen.
Professor David J. Nutt of Imperial College in London, who was not involved in the study, said that it supports the use of music for treatment efficacy with psychedelics and suggested that the next step is to “optimise this approach probably through individualising and personalising music tracks in therapy.”
Cue the 1960s LSD music montage.
Chicken ‘white striping is not a disease’
Have you ever sliced open a new pack of chicken breasts to start dinner and noticed white fatty lines running through the chicken? Maybe you thought it was just some extra fat to trim off, but the Humane League calls it “white striping disease.”
Chicken is the No. 1 meat consumed by Americans, so it’s not surprising that chickens are factory farmed and raised to be ready for slaughter quickly, according to CBSNews.com, which reported that the Humane League claims white striping is found in 70% of the chicken in popular grocery stores. The league expressed concern for the chickens’ welfare as they are bred to grow bigger quickly, which is causing the white striping and increasing the fat content of the meat by as much as 224%.
The National Chicken Council told CBS that the league’s findings were unscientific. A spokesperson said, “White striping is not a disease. It is a quality factor in chicken breast meat caused by deposits of fat in the muscle during the bird’s growth and development.” He went on to say that severe white striping happens in 3%-6% of birds, which are mostly used in further processed products, not in chicken breast packages.
Somehow, that’s not making us feel any better.
The itsy bitsy spider lets us all down
Most people do not like spiders. That’s too bad, because spiders are generally nothing but helpful little creatures that prey upon annoying flies and other pests. Then there’s the silk they produce. The ancient Romans used it to treat conditions such as warts and skin lesions. Spiders wrap their eggs in silk to protect them from harmful bacteria.
Of course, we can hardly trust the medical opinions of people from 2,000 years ago, but modern-day studies have not definitively proved whether or not spider silk has any antimicrobial properties.
To settle the matter once and for all, researchers from Denmark built a silk-harvesting machine using the most famous of Danish inventions: Legos. The contraption, sort of a paddle wheel, pulled the silk from several different species of spider pinned down by the researchers. The silk was then tested against three different bacteria species, including good old Escherichia coli.
Unfortunately for our spider friends, their silk has no antimicrobial activity. The researchers suspected that any such activity seen in previous studies was actually caused by improper control for the solvents used to extract the silk; those solvents can have antimicrobial properties on their own. As for protecting their eggs, rather than killing bacteria, the silk likely provides a physical barrier alone.
It is bad news for spiders on the benefit-to-humanity front, but look at the bright side: If their silk had antimicrobial activity, we’d have to start farming them to acquire more silk. And that’s no good. Spiders deserve to roam free, hunt as they please, and drop down on your head from the ceiling.
Anxiety and allergies: Cause, effect, confusion
We’re big fans of science, but as longtime, totally impartial (Science rules!) observers of science’s medical realm, we can see that the day-to-day process of practicing the scientific method occasionally gets a bit messy. And no, we’re not talking about COVID-19.
We’re talking allergies. We’re talking mental health. We’re talking allergic disease and mental health.
We’re talking about a pair of press releases we came across during our never-ending search for material to educate, entertain, and astound our fabulously wonderful and loyal readers. (We say that, of course, in the most impartial way possible.)
The first release was titled, “Allergies including asthma and hay fever not linked to mental health traits” and covered research from the University of Bristol (England). The investigators were trying to determine if “allergic diseases actually causes mental health traits including anxiety, depression, bipolar disorder, and schizophrenia, or vice versa,” according to the release.
What they found, however, was “little evidence of a causal relationship between the onset of allergic disease and mental health.” Again, this is the press release talking.
The second release seemed to suggest the exact opposite: “Study uncovers link between allergies and mental health conditions.” That got our attention. A little more reading revealed that “people with asthma, atopic dermatitis, and hay fever also had a higher likelihood of having depression, anxiety, bipolar disorder, or neuroticism.”
One of the investigators was quoted as saying, “Establishing whether allergic disease causes mental health problems, or vice versa, is important to ensure that resources and treatment strategies are targeted appropriately.”
Did you notice the “vice versa”? Did you notice that it appeared in quotes from both releases? We did, so we took a closer look at the source. The second release covered a group of investigators from the University of Bristol – the same group, and the same study, in fact, as the first one.
So there you have it. One study, two press releases, and one confused journalist. Thank you, science.
Handheld device highly sensitive in detecting amblyopia; can be used in children as young as 2 years of age
A handheld vision screening device to test for amblyopia and strabismus has been found to have a sensitivity of 100%, a specificity of 85%, and a median acquisition time of 28 seconds, according to a study published in the Journal of American Association for Pediatric Ophthalmology and Strabismus.
The prospective study involved 300 children recruited from two Kaiser Permanente Southern California pediatric clinics. The patients, aged 24-72 months, were first screened by trained research staff for amblyopia and strabismus using the device, called the Pediatric Vision Scanner (PVS). They were subsequently screened by a pediatric ophthalmologist who was masked to the previous screening results and who then performed a comprehensive eye examination.
With the gold-standard ophthalmologist examination, six children (2%) were identified as having amblyopia and/or strabismus. Using the PVS, all six children with amblyopia and/or strabismus were identified, yielding 100% sensitivity. PVS findings were normal for 45 children (15%), yielding a specificity rate of 85%. The positive predictive value was 26.0% (95% confidence interval, 12.4%-32.4%), and the negative predictive value was 100% (95% CI, 97.1%-100%).
The findings suggest that the device could be used to screen for amblyopia, according to Shaival S. Shah, MD, the study’s first author, who is a pediatric ophthalmologist and regional section lead of pediatric ophthalmology, Southern California Permanente Medical Group.
“A strength of this device is that it is user friendly and easy to use and very quick, which is essential when working with young children,” said Dr. Shah in an interview. He noted that the device could be used for children as young as 2 years.
Dr. Shah pointed out that the children were recruited from a pediatrician’s office and reflect more of a “real-world setting” than had they been recruited from a pediatric ophthalmology clinic.
Dr. Shah added that, with a negative predictive value of 100%, the device is highly reliable at informing the clinician that amblyopia is not present. “It did have a positive predictive value of 26%, which needs to be considered when deciding one’s vision screening strategy,” he said.
A limitation of the study is that there was no head-to-head comparison with another screening device, noted Dr. Shah. “While it may have been more useful to include another vision screening device to have a head-to-head comparison, we did not do this to limit complexity and cost.”
Michael J. Wan, MD, FRCSC, pediatric ophthalmologist, Sick Kids Hospital, Toronto, and assistant professor at the University of Toronto, told this news organization that the device has multiple strengths, including quick acquisition time and excellent detection rate of amblyopia and strabismus in children as young as 2 years.
“It is highly reliable at informing the clinician that amblyopia is not present,” said Dr. Wan, who was not involved in the study. “The PVS uses an elegant mechanism to test for amblyopia directly (as opposed to other screening devices, which only detect risk factors). This study demonstrates the impressive diagnostic accuracy of this approach. With a study population of 300 children, the PVS had a sensitivity of 100% and specificity of 85% (over 90% in cooperative children). This means that the PVS would detect essentially all cases of amblyopia and strabismus while minimizing the number of unnecessary referrals and examinations.”
He added that, although the study included children as young as 2 years, only 2.5% of the children were unable to complete the PVS test. “Detecting amblyopia in children at an age when treatment is still effective has been a longstanding goal in pediatric ophthalmology,” said Dr. Wan, who described the technology as user friendly. “Based on this study, the search for an accurate and practical pediatric vision screening device appears to be over.”
Dr. Wan said it would be useful to replicate this study with a different population to confirm the findings.
Dr. Shah and Dr. Wan disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A handheld vision screening device to test for amblyopia and strabismus has been found to have a sensitivity of 100%, a specificity of 85%, and a median acquisition time of 28 seconds, according to a study published in the Journal of American Association for Pediatric Ophthalmology and Strabismus.
The prospective study involved 300 children recruited from two Kaiser Permanente Southern California pediatric clinics. The patients, aged 24-72 months, were first screened by trained research staff for amblyopia and strabismus using the device, called the Pediatric Vision Scanner (PVS). They were subsequently screened by a pediatric ophthalmologist who was masked to the previous screening results and who then performed a comprehensive eye examination.
With the gold-standard ophthalmologist examination, six children (2%) were identified as having amblyopia and/or strabismus. Using the PVS, all six children with amblyopia and/or strabismus were identified, yielding 100% sensitivity. PVS findings were normal for 45 children (15%), yielding a specificity rate of 85%. The positive predictive value was 26.0% (95% confidence interval, 12.4%-32.4%), and the negative predictive value was 100% (95% CI, 97.1%-100%).
The findings suggest that the device could be used to screen for amblyopia, according to Shaival S. Shah, MD, the study’s first author, who is a pediatric ophthalmologist and regional section lead of pediatric ophthalmology, Southern California Permanente Medical Group.
“A strength of this device is that it is user friendly and easy to use and very quick, which is essential when working with young children,” said Dr. Shah in an interview. He noted that the device could be used for children as young as 2 years.
Dr. Shah pointed out that the children were recruited from a pediatrician’s office and reflect more of a “real-world setting” than had they been recruited from a pediatric ophthalmology clinic.
Dr. Shah added that, with a negative predictive value of 100%, the device is highly reliable at informing the clinician that amblyopia is not present. “It did have a positive predictive value of 26%, which needs to be considered when deciding one’s vision screening strategy,” he said.
A limitation of the study is that there was no head-to-head comparison with another screening device, noted Dr. Shah. “While it may have been more useful to include another vision screening device to have a head-to-head comparison, we did not do this to limit complexity and cost.”
Michael J. Wan, MD, FRCSC, pediatric ophthalmologist, Sick Kids Hospital, Toronto, and assistant professor at the University of Toronto, told this news organization that the device has multiple strengths, including quick acquisition time and excellent detection rate of amblyopia and strabismus in children as young as 2 years.
“It is highly reliable at informing the clinician that amblyopia is not present,” said Dr. Wan, who was not involved in the study. “The PVS uses an elegant mechanism to test for amblyopia directly (as opposed to other screening devices, which only detect risk factors). This study demonstrates the impressive diagnostic accuracy of this approach. With a study population of 300 children, the PVS had a sensitivity of 100% and specificity of 85% (over 90% in cooperative children). This means that the PVS would detect essentially all cases of amblyopia and strabismus while minimizing the number of unnecessary referrals and examinations.”
He added that, although the study included children as young as 2 years, only 2.5% of the children were unable to complete the PVS test. “Detecting amblyopia in children at an age when treatment is still effective has been a longstanding goal in pediatric ophthalmology,” said Dr. Wan, who described the technology as user friendly. “Based on this study, the search for an accurate and practical pediatric vision screening device appears to be over.”
Dr. Wan said it would be useful to replicate this study with a different population to confirm the findings.
Dr. Shah and Dr. Wan disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A handheld vision screening device to test for amblyopia and strabismus has been found to have a sensitivity of 100%, a specificity of 85%, and a median acquisition time of 28 seconds, according to a study published in the Journal of American Association for Pediatric Ophthalmology and Strabismus.
The prospective study involved 300 children recruited from two Kaiser Permanente Southern California pediatric clinics. The patients, aged 24-72 months, were first screened by trained research staff for amblyopia and strabismus using the device, called the Pediatric Vision Scanner (PVS). They were subsequently screened by a pediatric ophthalmologist who was masked to the previous screening results and who then performed a comprehensive eye examination.
With the gold-standard ophthalmologist examination, six children (2%) were identified as having amblyopia and/or strabismus. Using the PVS, all six children with amblyopia and/or strabismus were identified, yielding 100% sensitivity. PVS findings were normal for 45 children (15%), yielding a specificity rate of 85%. The positive predictive value was 26.0% (95% confidence interval, 12.4%-32.4%), and the negative predictive value was 100% (95% CI, 97.1%-100%).
The findings suggest that the device could be used to screen for amblyopia, according to Shaival S. Shah, MD, the study’s first author, who is a pediatric ophthalmologist and regional section lead of pediatric ophthalmology, Southern California Permanente Medical Group.
“A strength of this device is that it is user friendly and easy to use and very quick, which is essential when working with young children,” said Dr. Shah in an interview. He noted that the device could be used for children as young as 2 years.
Dr. Shah pointed out that the children were recruited from a pediatrician’s office and reflect more of a “real-world setting” than had they been recruited from a pediatric ophthalmology clinic.
Dr. Shah added that, with a negative predictive value of 100%, the device is highly reliable at informing the clinician that amblyopia is not present. “It did have a positive predictive value of 26%, which needs to be considered when deciding one’s vision screening strategy,” he said.
A limitation of the study is that there was no head-to-head comparison with another screening device, noted Dr. Shah. “While it may have been more useful to include another vision screening device to have a head-to-head comparison, we did not do this to limit complexity and cost.”
Michael J. Wan, MD, FRCSC, pediatric ophthalmologist, Sick Kids Hospital, Toronto, and assistant professor at the University of Toronto, told this news organization that the device has multiple strengths, including quick acquisition time and excellent detection rate of amblyopia and strabismus in children as young as 2 years.
“It is highly reliable at informing the clinician that amblyopia is not present,” said Dr. Wan, who was not involved in the study. “The PVS uses an elegant mechanism to test for amblyopia directly (as opposed to other screening devices, which only detect risk factors). This study demonstrates the impressive diagnostic accuracy of this approach. With a study population of 300 children, the PVS had a sensitivity of 100% and specificity of 85% (over 90% in cooperative children). This means that the PVS would detect essentially all cases of amblyopia and strabismus while minimizing the number of unnecessary referrals and examinations.”
He added that, although the study included children as young as 2 years, only 2.5% of the children were unable to complete the PVS test. “Detecting amblyopia in children at an age when treatment is still effective has been a longstanding goal in pediatric ophthalmology,” said Dr. Wan, who described the technology as user friendly. “Based on this study, the search for an accurate and practical pediatric vision screening device appears to be over.”
Dr. Wan said it would be useful to replicate this study with a different population to confirm the findings.
Dr. Shah and Dr. Wan disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FDA clears first mobile rapid test for concussion
, the company has announced.
Eye-Sync is a virtual reality eye-tracking platform that provides objective measurements to aid in the assessment of concussion. It’s the first mobile, rapid test for concussion that has been cleared by the FDA, the company said.
As reported by this news organization, Eye-Sync received breakthrough designation from the FDA for this indication in March 2019.
The FDA initially cleared the Eye-Sync platform for recording, viewing, and analyzing eye movements to help clinicians identify visual tracking impairment.
The Eye-Sync technology uses a series of 60-second eye tracking assessments, neurocognitive batteries, symptom inventories, and standardized patient inventories to identify the type and severity of impairment after concussion.
“The platform generates customizable and interpretive reports that support clinical decision making and offers visual and vestibular therapies to remedy deficits and monitor improvement over time,” the company said.
In support of the application for use in concussion, SyncThink enrolled 1,655 children and adults into a clinical study that collected comprehensive patient and concussion-related data for over 12 months.
The company used these data to develop proprietary algorithms and deep learning models to identify a positive or negative indication of concussion.
The study showed that Eye-Sinc had sensitivity greater than 82% and specificity greater than 93%, “thereby providing clinicians with significant and actionable data when evaluating individuals with concussion,” the company said in a news release.
“The outcome of this study very clearly shows the effectiveness of our technology at detecting concussion and definitively demonstrates the clinical utility of Eye-Sinc,” SyncThink Chief Clinical Officer Scott Anderson said in the release.
“It also shows that the future of concussion diagnosis is no longer purely symptom-based but that of a technology driven multi-modal approach,” Mr. Anderson said.
A version of this article first appeared on Medscape.com.
, the company has announced.
Eye-Sync is a virtual reality eye-tracking platform that provides objective measurements to aid in the assessment of concussion. It’s the first mobile, rapid test for concussion that has been cleared by the FDA, the company said.
As reported by this news organization, Eye-Sync received breakthrough designation from the FDA for this indication in March 2019.
The FDA initially cleared the Eye-Sync platform for recording, viewing, and analyzing eye movements to help clinicians identify visual tracking impairment.
The Eye-Sync technology uses a series of 60-second eye tracking assessments, neurocognitive batteries, symptom inventories, and standardized patient inventories to identify the type and severity of impairment after concussion.
“The platform generates customizable and interpretive reports that support clinical decision making and offers visual and vestibular therapies to remedy deficits and monitor improvement over time,” the company said.
In support of the application for use in concussion, SyncThink enrolled 1,655 children and adults into a clinical study that collected comprehensive patient and concussion-related data for over 12 months.
The company used these data to develop proprietary algorithms and deep learning models to identify a positive or negative indication of concussion.
The study showed that Eye-Sinc had sensitivity greater than 82% and specificity greater than 93%, “thereby providing clinicians with significant and actionable data when evaluating individuals with concussion,” the company said in a news release.
“The outcome of this study very clearly shows the effectiveness of our technology at detecting concussion and definitively demonstrates the clinical utility of Eye-Sinc,” SyncThink Chief Clinical Officer Scott Anderson said in the release.
“It also shows that the future of concussion diagnosis is no longer purely symptom-based but that of a technology driven multi-modal approach,” Mr. Anderson said.
A version of this article first appeared on Medscape.com.
, the company has announced.
Eye-Sync is a virtual reality eye-tracking platform that provides objective measurements to aid in the assessment of concussion. It’s the first mobile, rapid test for concussion that has been cleared by the FDA, the company said.
As reported by this news organization, Eye-Sync received breakthrough designation from the FDA for this indication in March 2019.
The FDA initially cleared the Eye-Sync platform for recording, viewing, and analyzing eye movements to help clinicians identify visual tracking impairment.
The Eye-Sync technology uses a series of 60-second eye tracking assessments, neurocognitive batteries, symptom inventories, and standardized patient inventories to identify the type and severity of impairment after concussion.
“The platform generates customizable and interpretive reports that support clinical decision making and offers visual and vestibular therapies to remedy deficits and monitor improvement over time,” the company said.
In support of the application for use in concussion, SyncThink enrolled 1,655 children and adults into a clinical study that collected comprehensive patient and concussion-related data for over 12 months.
The company used these data to develop proprietary algorithms and deep learning models to identify a positive or negative indication of concussion.
The study showed that Eye-Sinc had sensitivity greater than 82% and specificity greater than 93%, “thereby providing clinicians with significant and actionable data when evaluating individuals with concussion,” the company said in a news release.
“The outcome of this study very clearly shows the effectiveness of our technology at detecting concussion and definitively demonstrates the clinical utility of Eye-Sinc,” SyncThink Chief Clinical Officer Scott Anderson said in the release.
“It also shows that the future of concussion diagnosis is no longer purely symptom-based but that of a technology driven multi-modal approach,” Mr. Anderson said.
A version of this article first appeared on Medscape.com.
Children and COVID: Decline of summer surge continues
The continuing decline in COVID-19 incidence suggests the latest surge has peaked as new cases in children dropped for the 4th consecutive week, based on data from the American Academy of Pediatrics and the Children’s Hospital Association.
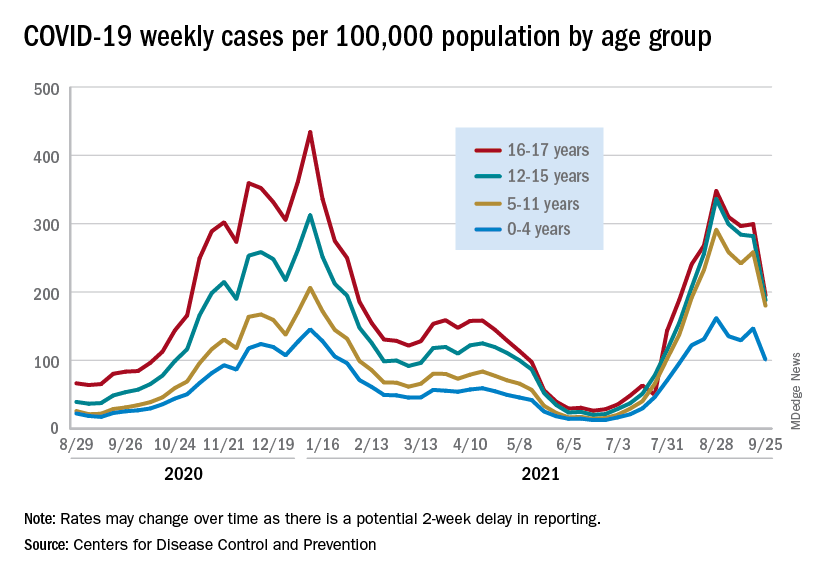
Preliminary data from the Centers for Disease Control and Prevention, however, show an uptick in new cases in late September, largely among younger children, that may indicate otherwise. Those data have a potential 2-week reporting delay, the CDC said on its COVID Data Tracker, so the most recent points on the graph (see above) could still go up.
. Those new cases made up almost 27% of all cases for the week, and the nearly 5.9 million child cases that have been reported since the start of the pandemic represent 16.2% of cases among Americans of all ages, the two groups said in their weekly COVID-19 report.
The CDC data on new cases by age group suggest that younger children have borne a heavier burden in the summer surge of COVID than they did last winter. The rate of new cases was not as high for 16- and 17-year-olds in the summer, but the other age groups all reached higher peaks than in the winter, including the 12- to 15-year-olds, who have been getting vaccinated since May, according to the COVID Data Tracker.
With vaccination approval getting closer for children under age 12 years, initiation in those already eligible continues to slide. Those aged 12-15 made up just 6.9% of new vaccinations during the 2 weeks from Sept. 21 to Oct. 4, and that figure has been dropping since July 13-26, when it was 14.1%. Vaccine initiation among 16- and 17-year-olds over that time has dropped by almost half, from 5.4% to 2.9%, the CDC data show.
All the vaccinations so far add up to this: Almost 55% of those aged 12-15 have gotten at least one dose of COVID vaccine, as have over 62% of those aged 16-17, and 52% of the older group is fully vaccinated, as is 44% of the younger group. Altogether, 10.8 million children were fully vaccinated as of Oct. 4, including those under 12 who may be participating in clinical trials or had a birth date entered incorrectly, the CDC said.
The continuing decline in COVID-19 incidence suggests the latest surge has peaked as new cases in children dropped for the 4th consecutive week, based on data from the American Academy of Pediatrics and the Children’s Hospital Association.

Preliminary data from the Centers for Disease Control and Prevention, however, show an uptick in new cases in late September, largely among younger children, that may indicate otherwise. Those data have a potential 2-week reporting delay, the CDC said on its COVID Data Tracker, so the most recent points on the graph (see above) could still go up.
. Those new cases made up almost 27% of all cases for the week, and the nearly 5.9 million child cases that have been reported since the start of the pandemic represent 16.2% of cases among Americans of all ages, the two groups said in their weekly COVID-19 report.
The CDC data on new cases by age group suggest that younger children have borne a heavier burden in the summer surge of COVID than they did last winter. The rate of new cases was not as high for 16- and 17-year-olds in the summer, but the other age groups all reached higher peaks than in the winter, including the 12- to 15-year-olds, who have been getting vaccinated since May, according to the COVID Data Tracker.
With vaccination approval getting closer for children under age 12 years, initiation in those already eligible continues to slide. Those aged 12-15 made up just 6.9% of new vaccinations during the 2 weeks from Sept. 21 to Oct. 4, and that figure has been dropping since July 13-26, when it was 14.1%. Vaccine initiation among 16- and 17-year-olds over that time has dropped by almost half, from 5.4% to 2.9%, the CDC data show.
All the vaccinations so far add up to this: Almost 55% of those aged 12-15 have gotten at least one dose of COVID vaccine, as have over 62% of those aged 16-17, and 52% of the older group is fully vaccinated, as is 44% of the younger group. Altogether, 10.8 million children were fully vaccinated as of Oct. 4, including those under 12 who may be participating in clinical trials or had a birth date entered incorrectly, the CDC said.
The continuing decline in COVID-19 incidence suggests the latest surge has peaked as new cases in children dropped for the 4th consecutive week, based on data from the American Academy of Pediatrics and the Children’s Hospital Association.

Preliminary data from the Centers for Disease Control and Prevention, however, show an uptick in new cases in late September, largely among younger children, that may indicate otherwise. Those data have a potential 2-week reporting delay, the CDC said on its COVID Data Tracker, so the most recent points on the graph (see above) could still go up.
. Those new cases made up almost 27% of all cases for the week, and the nearly 5.9 million child cases that have been reported since the start of the pandemic represent 16.2% of cases among Americans of all ages, the two groups said in their weekly COVID-19 report.
The CDC data on new cases by age group suggest that younger children have borne a heavier burden in the summer surge of COVID than they did last winter. The rate of new cases was not as high for 16- and 17-year-olds in the summer, but the other age groups all reached higher peaks than in the winter, including the 12- to 15-year-olds, who have been getting vaccinated since May, according to the COVID Data Tracker.
With vaccination approval getting closer for children under age 12 years, initiation in those already eligible continues to slide. Those aged 12-15 made up just 6.9% of new vaccinations during the 2 weeks from Sept. 21 to Oct. 4, and that figure has been dropping since July 13-26, when it was 14.1%. Vaccine initiation among 16- and 17-year-olds over that time has dropped by almost half, from 5.4% to 2.9%, the CDC data show.
All the vaccinations so far add up to this: Almost 55% of those aged 12-15 have gotten at least one dose of COVID vaccine, as have over 62% of those aged 16-17, and 52% of the older group is fully vaccinated, as is 44% of the younger group. Altogether, 10.8 million children were fully vaccinated as of Oct. 4, including those under 12 who may be participating in clinical trials or had a birth date entered incorrectly, the CDC said.




