User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
Experts highlight benefits and offer caveats for first postpartum depression pill
For the first time, the Food and Drug Administration approved a pill taken once daily for 14 days to help women manage the often strong, sometimes overpowering symptoms of postpartum depression.
for a condition that affects an estimated 1 in 8 women in the United States. What will it mean for easing symptoms such as hopelessness, crankiness, and lack of interest in bonding with the baby or, in the case of multiples, babies – and in some cases, thoughts of death or suicide?
A fast-acting option
“We don’t have many oral medications that are fast-acting antidepressants, so this is incredibly exciting,” said Sarah Oreck, MD, a psychiatrist in private practice in Los Angeles who specializes in reproductive psychiatry. The rapid response is likely because the medication targets the hormonal mechanism underlying postpartum depression, she added.
Zuranolone (Zurzuvae, Biogen/Sage) is different from most other antidepressants – it is designed to be taken for a shorter period. Also, Because zuranolone is a pill, it is more convenient to take than the other FDA-approved treatment, the IV infusion brexanolone (Zulresso, Sage).
“It’s obviously game changing to have something in pill form. The infusion has to be done at an infusion center to monitor people for any complications,” said Kimberly Yonkers, MD, a psychiatrist specializing in women’s health, a Distinguished Life Fellow of the American Psychiatric Association (APA), and the Katz Family Chair of Psychiatry at the University of Massachusetts Chan Medical School/UMass Memorial Medical Center in Worcester.
Women may experience improvement in postpartum depression in as soon as 3 days after starting the medication. In contrast, “typical antidepressants can take up to 2 weeks before patients notice a difference and 4 to 8 weeks to see a full response. A fast-acting pill that can be taken orally could be an ideal option for the 15% to 20% of women who experience postpartum depression,” said Priya Gopalan, MD, a psychiatrist with UPMC Western Psychiatric Hospital and Magee-Womens Hospital in Pittsburgh.
The medical community, and reproductive psychiatrists in particular, has always suspected differences in the biological underpinnings of postpartum depression and major depressive disorder, Dr. Oreck said. “We know that postpartum depression looks different from major depressive disorder and that hormonal shifts during pregnancy and postpartum are a huge risk factor for postpartum depression,” she said.
Although selective serotonin reuptake inhibitors (SSRIs) are helpful and currently the standard of care for treating moderate to severe postpartum depression in combination with therapy, Dr. Oreck added, early studies suggest that zuranolone may work faster and potentially be more effective than SSRIs in treating the condition.
Zuranolone is a version of a naturally occurring hormone called allopregnanolone, a metabolite of progesterone. Concentrations of allopregnanolone rise dramatically during pregnancy and then drop precipitously after childbirth. Zuranolone works through modulating GABA-A, a neurotransmitter implicated in the development of depression.
“It is encouraging that postpartum individuals may now have more options to manage a debilitating condition that affects them and their families,” said Christopher Zahn, MD, interim CEO and chief of clinical practice and health equity and quality for the American College of Obstetricians and Gynecologists (ACOG).
ACOG recommends women be screened for depression at least three times – during early pregnancy, later in pregnancy, and again after delivery. A decision to start this or any other medicine should be individualized and based on shared decision-making between a patient and doctor, Dr. Zahn added.
The cost of zuranolone is not yet known. Dr. Yonkers said cost of the infusion can serve as a cautionary tale for the manufacturer. Some reports put the infusion cost at $34,000. “Cost is going to be an important component to this. The previous intervention was priced so high that it was not affordable to many people and it was difficult to access.”
Beyond ‘baby blues’
The APA has changed the name from “postpartum depression” to “peripartum depression” because evidence suggests feelings and symptoms also can start late in pregnancy. “It means you don’t have to wait until somebody delivers to screen for depression. We have to recognize that depression can occur during pregnancy,” Dr. Yonkers said. “In fact it is not uncommon during the third trimester.”
No matter when it starts, the condition can be “very serious,” particularly if the person already experiences depression, including bipolar disorder, Dr. Yonkers added.
Postpartum depression “is more than just ‘baby blues.’ It is a potentially debilitating illness that causes feelings of intense sadness and worthlessness, making it difficult to care for and bond with your newborn,” Dr. Gopalan said.
Can be a medical emergency
Severe postpartum depression requires immediate attention and treatment.
“One of the things we have to be cautious about is for people with previous predisposition to hurt themselves,” Dr. Yonkers said. “It is therefore important to consider somebody’s medical and behavioral health history as well.
“For an individual with recurring depression or severe episodes of depression, this may not be sufficient, because they are just going to get these 14 days of therapy,” Dr. Yonkers said. “They may need ongoing antidepressants.
“It may not be the right pill for everybody,” Dr. Yonkers added. She recommended everyone be followed closely during and after treatment “to make sure they are responding and to monitor for relapse.”
The science that led to approval
The clinical trials showed early response in patients with severe postpartum depression. Researchers conducted two studies of women who developed a major depressive episode in the third trimester of pregnancy or within 4 weeks of delivery. They found women who took zuranolone once in the evening for 14 days “showed significantly more improvement in their symptoms compared to those in the placebo group.”
The antidepressant effect lasted at least 4 weeks after stopping the medication.
Drowsiness, dizziness, diarrhea, fatigue, nasopharyngitis, and urinary tract infection were the most common side effects. The label has a boxed warning noting that the medication can affect a person’s ability to drive and perform other potentially hazardous activities. Use of zuranolone may also cause suicidal thoughts and behavior, according to an FDA news release announcing the approval.
The start of more help for mothers?
Zuranolone is not a cure-all. As with most psychiatric prescriptions, the medication likely will work best in conjunction with behavioral health treatments such as psychotherapy, use of other medications, behavioral management, support groups, and self-care tools such as meditation, exercise, and yoga, Dr. Gopalan said.
Dr. Oreck said she hopes this first pill approval will lead to more discoveries. “I hope this is the beginning of more innovation and development of novel treatments that can target women’s mental health issues specifically – female reproductive hormones impact mental health in unique ways and it’s exciting to finally see research and development dollars dedicated to them,” she said. “The FDA approval of this pill provides the potential to improve the lives of millions of Americans suffering from postpartum depression.”
Dr. Oreck, Dr. Yonkers, Dr. Gopalan, and Dr. Zahn have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
For the first time, the Food and Drug Administration approved a pill taken once daily for 14 days to help women manage the often strong, sometimes overpowering symptoms of postpartum depression.
for a condition that affects an estimated 1 in 8 women in the United States. What will it mean for easing symptoms such as hopelessness, crankiness, and lack of interest in bonding with the baby or, in the case of multiples, babies – and in some cases, thoughts of death or suicide?
A fast-acting option
“We don’t have many oral medications that are fast-acting antidepressants, so this is incredibly exciting,” said Sarah Oreck, MD, a psychiatrist in private practice in Los Angeles who specializes in reproductive psychiatry. The rapid response is likely because the medication targets the hormonal mechanism underlying postpartum depression, she added.
Zuranolone (Zurzuvae, Biogen/Sage) is different from most other antidepressants – it is designed to be taken for a shorter period. Also, Because zuranolone is a pill, it is more convenient to take than the other FDA-approved treatment, the IV infusion brexanolone (Zulresso, Sage).
“It’s obviously game changing to have something in pill form. The infusion has to be done at an infusion center to monitor people for any complications,” said Kimberly Yonkers, MD, a psychiatrist specializing in women’s health, a Distinguished Life Fellow of the American Psychiatric Association (APA), and the Katz Family Chair of Psychiatry at the University of Massachusetts Chan Medical School/UMass Memorial Medical Center in Worcester.
Women may experience improvement in postpartum depression in as soon as 3 days after starting the medication. In contrast, “typical antidepressants can take up to 2 weeks before patients notice a difference and 4 to 8 weeks to see a full response. A fast-acting pill that can be taken orally could be an ideal option for the 15% to 20% of women who experience postpartum depression,” said Priya Gopalan, MD, a psychiatrist with UPMC Western Psychiatric Hospital and Magee-Womens Hospital in Pittsburgh.
The medical community, and reproductive psychiatrists in particular, has always suspected differences in the biological underpinnings of postpartum depression and major depressive disorder, Dr. Oreck said. “We know that postpartum depression looks different from major depressive disorder and that hormonal shifts during pregnancy and postpartum are a huge risk factor for postpartum depression,” she said.
Although selective serotonin reuptake inhibitors (SSRIs) are helpful and currently the standard of care for treating moderate to severe postpartum depression in combination with therapy, Dr. Oreck added, early studies suggest that zuranolone may work faster and potentially be more effective than SSRIs in treating the condition.
Zuranolone is a version of a naturally occurring hormone called allopregnanolone, a metabolite of progesterone. Concentrations of allopregnanolone rise dramatically during pregnancy and then drop precipitously after childbirth. Zuranolone works through modulating GABA-A, a neurotransmitter implicated in the development of depression.
“It is encouraging that postpartum individuals may now have more options to manage a debilitating condition that affects them and their families,” said Christopher Zahn, MD, interim CEO and chief of clinical practice and health equity and quality for the American College of Obstetricians and Gynecologists (ACOG).
ACOG recommends women be screened for depression at least three times – during early pregnancy, later in pregnancy, and again after delivery. A decision to start this or any other medicine should be individualized and based on shared decision-making between a patient and doctor, Dr. Zahn added.
The cost of zuranolone is not yet known. Dr. Yonkers said cost of the infusion can serve as a cautionary tale for the manufacturer. Some reports put the infusion cost at $34,000. “Cost is going to be an important component to this. The previous intervention was priced so high that it was not affordable to many people and it was difficult to access.”
Beyond ‘baby blues’
The APA has changed the name from “postpartum depression” to “peripartum depression” because evidence suggests feelings and symptoms also can start late in pregnancy. “It means you don’t have to wait until somebody delivers to screen for depression. We have to recognize that depression can occur during pregnancy,” Dr. Yonkers said. “In fact it is not uncommon during the third trimester.”
No matter when it starts, the condition can be “very serious,” particularly if the person already experiences depression, including bipolar disorder, Dr. Yonkers added.
Postpartum depression “is more than just ‘baby blues.’ It is a potentially debilitating illness that causes feelings of intense sadness and worthlessness, making it difficult to care for and bond with your newborn,” Dr. Gopalan said.
Can be a medical emergency
Severe postpartum depression requires immediate attention and treatment.
“One of the things we have to be cautious about is for people with previous predisposition to hurt themselves,” Dr. Yonkers said. “It is therefore important to consider somebody’s medical and behavioral health history as well.
“For an individual with recurring depression or severe episodes of depression, this may not be sufficient, because they are just going to get these 14 days of therapy,” Dr. Yonkers said. “They may need ongoing antidepressants.
“It may not be the right pill for everybody,” Dr. Yonkers added. She recommended everyone be followed closely during and after treatment “to make sure they are responding and to monitor for relapse.”
The science that led to approval
The clinical trials showed early response in patients with severe postpartum depression. Researchers conducted two studies of women who developed a major depressive episode in the third trimester of pregnancy or within 4 weeks of delivery. They found women who took zuranolone once in the evening for 14 days “showed significantly more improvement in their symptoms compared to those in the placebo group.”
The antidepressant effect lasted at least 4 weeks after stopping the medication.
Drowsiness, dizziness, diarrhea, fatigue, nasopharyngitis, and urinary tract infection were the most common side effects. The label has a boxed warning noting that the medication can affect a person’s ability to drive and perform other potentially hazardous activities. Use of zuranolone may also cause suicidal thoughts and behavior, according to an FDA news release announcing the approval.
The start of more help for mothers?
Zuranolone is not a cure-all. As with most psychiatric prescriptions, the medication likely will work best in conjunction with behavioral health treatments such as psychotherapy, use of other medications, behavioral management, support groups, and self-care tools such as meditation, exercise, and yoga, Dr. Gopalan said.
Dr. Oreck said she hopes this first pill approval will lead to more discoveries. “I hope this is the beginning of more innovation and development of novel treatments that can target women’s mental health issues specifically – female reproductive hormones impact mental health in unique ways and it’s exciting to finally see research and development dollars dedicated to them,” she said. “The FDA approval of this pill provides the potential to improve the lives of millions of Americans suffering from postpartum depression.”
Dr. Oreck, Dr. Yonkers, Dr. Gopalan, and Dr. Zahn have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
For the first time, the Food and Drug Administration approved a pill taken once daily for 14 days to help women manage the often strong, sometimes overpowering symptoms of postpartum depression.
for a condition that affects an estimated 1 in 8 women in the United States. What will it mean for easing symptoms such as hopelessness, crankiness, and lack of interest in bonding with the baby or, in the case of multiples, babies – and in some cases, thoughts of death or suicide?
A fast-acting option
“We don’t have many oral medications that are fast-acting antidepressants, so this is incredibly exciting,” said Sarah Oreck, MD, a psychiatrist in private practice in Los Angeles who specializes in reproductive psychiatry. The rapid response is likely because the medication targets the hormonal mechanism underlying postpartum depression, she added.
Zuranolone (Zurzuvae, Biogen/Sage) is different from most other antidepressants – it is designed to be taken for a shorter period. Also, Because zuranolone is a pill, it is more convenient to take than the other FDA-approved treatment, the IV infusion brexanolone (Zulresso, Sage).
“It’s obviously game changing to have something in pill form. The infusion has to be done at an infusion center to monitor people for any complications,” said Kimberly Yonkers, MD, a psychiatrist specializing in women’s health, a Distinguished Life Fellow of the American Psychiatric Association (APA), and the Katz Family Chair of Psychiatry at the University of Massachusetts Chan Medical School/UMass Memorial Medical Center in Worcester.
Women may experience improvement in postpartum depression in as soon as 3 days after starting the medication. In contrast, “typical antidepressants can take up to 2 weeks before patients notice a difference and 4 to 8 weeks to see a full response. A fast-acting pill that can be taken orally could be an ideal option for the 15% to 20% of women who experience postpartum depression,” said Priya Gopalan, MD, a psychiatrist with UPMC Western Psychiatric Hospital and Magee-Womens Hospital in Pittsburgh.
The medical community, and reproductive psychiatrists in particular, has always suspected differences in the biological underpinnings of postpartum depression and major depressive disorder, Dr. Oreck said. “We know that postpartum depression looks different from major depressive disorder and that hormonal shifts during pregnancy and postpartum are a huge risk factor for postpartum depression,” she said.
Although selective serotonin reuptake inhibitors (SSRIs) are helpful and currently the standard of care for treating moderate to severe postpartum depression in combination with therapy, Dr. Oreck added, early studies suggest that zuranolone may work faster and potentially be more effective than SSRIs in treating the condition.
Zuranolone is a version of a naturally occurring hormone called allopregnanolone, a metabolite of progesterone. Concentrations of allopregnanolone rise dramatically during pregnancy and then drop precipitously after childbirth. Zuranolone works through modulating GABA-A, a neurotransmitter implicated in the development of depression.
“It is encouraging that postpartum individuals may now have more options to manage a debilitating condition that affects them and their families,” said Christopher Zahn, MD, interim CEO and chief of clinical practice and health equity and quality for the American College of Obstetricians and Gynecologists (ACOG).
ACOG recommends women be screened for depression at least three times – during early pregnancy, later in pregnancy, and again after delivery. A decision to start this or any other medicine should be individualized and based on shared decision-making between a patient and doctor, Dr. Zahn added.
The cost of zuranolone is not yet known. Dr. Yonkers said cost of the infusion can serve as a cautionary tale for the manufacturer. Some reports put the infusion cost at $34,000. “Cost is going to be an important component to this. The previous intervention was priced so high that it was not affordable to many people and it was difficult to access.”
Beyond ‘baby blues’
The APA has changed the name from “postpartum depression” to “peripartum depression” because evidence suggests feelings and symptoms also can start late in pregnancy. “It means you don’t have to wait until somebody delivers to screen for depression. We have to recognize that depression can occur during pregnancy,” Dr. Yonkers said. “In fact it is not uncommon during the third trimester.”
No matter when it starts, the condition can be “very serious,” particularly if the person already experiences depression, including bipolar disorder, Dr. Yonkers added.
Postpartum depression “is more than just ‘baby blues.’ It is a potentially debilitating illness that causes feelings of intense sadness and worthlessness, making it difficult to care for and bond with your newborn,” Dr. Gopalan said.
Can be a medical emergency
Severe postpartum depression requires immediate attention and treatment.
“One of the things we have to be cautious about is for people with previous predisposition to hurt themselves,” Dr. Yonkers said. “It is therefore important to consider somebody’s medical and behavioral health history as well.
“For an individual with recurring depression or severe episodes of depression, this may not be sufficient, because they are just going to get these 14 days of therapy,” Dr. Yonkers said. “They may need ongoing antidepressants.
“It may not be the right pill for everybody,” Dr. Yonkers added. She recommended everyone be followed closely during and after treatment “to make sure they are responding and to monitor for relapse.”
The science that led to approval
The clinical trials showed early response in patients with severe postpartum depression. Researchers conducted two studies of women who developed a major depressive episode in the third trimester of pregnancy or within 4 weeks of delivery. They found women who took zuranolone once in the evening for 14 days “showed significantly more improvement in their symptoms compared to those in the placebo group.”
The antidepressant effect lasted at least 4 weeks after stopping the medication.
Drowsiness, dizziness, diarrhea, fatigue, nasopharyngitis, and urinary tract infection were the most common side effects. The label has a boxed warning noting that the medication can affect a person’s ability to drive and perform other potentially hazardous activities. Use of zuranolone may also cause suicidal thoughts and behavior, according to an FDA news release announcing the approval.
The start of more help for mothers?
Zuranolone is not a cure-all. As with most psychiatric prescriptions, the medication likely will work best in conjunction with behavioral health treatments such as psychotherapy, use of other medications, behavioral management, support groups, and self-care tools such as meditation, exercise, and yoga, Dr. Gopalan said.
Dr. Oreck said she hopes this first pill approval will lead to more discoveries. “I hope this is the beginning of more innovation and development of novel treatments that can target women’s mental health issues specifically – female reproductive hormones impact mental health in unique ways and it’s exciting to finally see research and development dollars dedicated to them,” she said. “The FDA approval of this pill provides the potential to improve the lives of millions of Americans suffering from postpartum depression.”
Dr. Oreck, Dr. Yonkers, Dr. Gopalan, and Dr. Zahn have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Ontario case shows potential supplement risk for consumers
A woman’s quest to become pregnant resulted in lead poisoning from an Ayurvedic treatment. The case triggered the seizure of pills from an Ontario natural-products clinic and the issuance of government warnings about the risks of products from this business, according to a new report.
, including the presence of lead and other metals in Ayurvedic products, according to the report.
“When consumer products may be contaminated with lead, or when lead exposure is linked to sources in the community, involving public health can facilitate broader actions to reduce and prevent exposures to other people at risk,” wrote report author Julian Gitelman, MD, MPH, a resident physician at the University of Toronto Dalla Lana School of Public Health, and colleagues.
Their case study was published in the Canadian Medical Association Journal.
The researchers detailed what happened after a 39-year-old woman sought medical care for abdominal pain, constipation, nausea, and vomiting. The woman underwent a series of tests, including colonoscopy, laparoscopy, and biopsies of bone marrow and ovarian cysts.
Only later did clinicians home in on the cause of her ailments: the Ayurvedic medications that the patient had been taking daily for more than a year for infertility. Her daily regimen had varied, ranging from a few pills to a dozen pills.
Heavy metals are sometimes intentionally added to Ayurvedic supplements for perceived healing properties, wrote the authors. They cited a previous study of a sample of Ayurvedic pills bought on the Internet from manufacturers based in the United States and India that showed that 21% contained lead, mercury, or arsenic.
A case report published last year in German Medical Weekly raised the same issue.
Melatonin gummies
Regulators in many countries struggle to help consumers understand the risks of natural health supplements, and the challenge extends well beyond Ayurvedic products.
There has been a “huge and very troubling increase” in U.S. poison control calls associated with gummy-bear products containing melatonin, said Canadian Senator Stan Kutcher, MD, at a May 11 meeting of Canada’s Standing Senate Committee on Social Affairs, Science, and Technology.
In April, JAMA published a U.S. analysis of melatonin gummy products, Dr. Kutcher noted. In this research letter, investigators reported that one product did not contain detectable levels of melatonin but did contain 31.3 mg of cannabidiol.
In other products, the quantity of melatonin ranged from 74% to 347% of the labeled quantity. A previous Canadian study of 16 melatonin brands found that the actual dose of melatonin ranged from 17% to 478% of the declared quantity, the letter noted.
The May 11 Senate meeting provided a forum for many of the recurring debates about supplements, which also are known as natural health products.
Barry Power, PharmD, editor in chief for the Canadian Pharmacists Association, said that his group was disappointed when Canada excluded natural health products from Vanessa’s Law, which was passed in 2014. This law sought to improve the reporting of adverse reactions to drugs.
“We’re glad this is being revisited now,” Dr. Power told the Senate committee. “Although natural health products are often seen as low risk, we need to keep in mind that ‘low risk’ does not mean ‘no risk,’ and ‘natural’ does not mean ‘safe.’ ”
In contrast, Aaron Skelton, chief executive of the Canadian Health Food Association, spoke against this bid to expand the reach of Vanessa’s Law into natural health products. Canadian lawmakers attached provisions regarding increased oversight of natural health products to a budget package instead of considering them as part of a stand-alone bill.
“Our concern is that the powers that are being discussed have not been reviewed and debated,” Mr. Skelton told Dr. Kutcher. “The potential for overreach and unnecessary regulation is significant, and that deserves debate.”
“Profits should not trump Canadians’ health,” answered Dr. Kutcher, who earlier served as head of the psychiatry department at Dalhousie University in Halifax, N.S.
By June, Vanessa’s Law had been expanded with provisions that address natural health products, including the reporting of products that present a serious risk to consumers.
Educating consumers
Many consumers overestimate the level of government regulation of supplements, said Pieter A. Cohen, MD, leader of the Supplement Research Program at Cambridge Health Alliance in Massachusetts. Dr. Cohen was the lead author of the JAMA research letter about melatonin products.
Supplements often share shelves in pharmacies with medicines that are subject to more strict regulation, which causes confusion.
“It’s really hard to wrap your brain around [the fact] that a health product is being sold in pharmacies in the United States and it’s not being vetted by the FDA [U.S. Food and Drug Administration]”, Dr. Cohen said in an interview
The confusion extends across borders. Many consumers in other countries will assume that the FDA performed premarket screening of U.S.-made supplements, but that is not the case, he said.
People who want to take supplements should look for reputable sources of information about them, such as the website of the National Institutes of Health’s Office of Dietary Supplements, Dr. Cohen said. But patients often forget or fail to do this, which can create medical puzzles, such as the case of the woman in the Ontario case study, said Peter Lurie, MD, MPH, executive director of the nonprofit Center for Science in the Public Interest, which has pressed for increased regulation of supplements.
Clinicians need to keep in mind that patients may need prodding to reveal what supplements they are taking, he said.
“They just think of them as different, somehow not the province of the doctor,” Dr. Lurie said. “For others, they are concerned that the doctors will disapprove. So, they hide it.”
A version of this article first appeared on Medscape.com.
A woman’s quest to become pregnant resulted in lead poisoning from an Ayurvedic treatment. The case triggered the seizure of pills from an Ontario natural-products clinic and the issuance of government warnings about the risks of products from this business, according to a new report.
, including the presence of lead and other metals in Ayurvedic products, according to the report.
“When consumer products may be contaminated with lead, or when lead exposure is linked to sources in the community, involving public health can facilitate broader actions to reduce and prevent exposures to other people at risk,” wrote report author Julian Gitelman, MD, MPH, a resident physician at the University of Toronto Dalla Lana School of Public Health, and colleagues.
Their case study was published in the Canadian Medical Association Journal.
The researchers detailed what happened after a 39-year-old woman sought medical care for abdominal pain, constipation, nausea, and vomiting. The woman underwent a series of tests, including colonoscopy, laparoscopy, and biopsies of bone marrow and ovarian cysts.
Only later did clinicians home in on the cause of her ailments: the Ayurvedic medications that the patient had been taking daily for more than a year for infertility. Her daily regimen had varied, ranging from a few pills to a dozen pills.
Heavy metals are sometimes intentionally added to Ayurvedic supplements for perceived healing properties, wrote the authors. They cited a previous study of a sample of Ayurvedic pills bought on the Internet from manufacturers based in the United States and India that showed that 21% contained lead, mercury, or arsenic.
A case report published last year in German Medical Weekly raised the same issue.
Melatonin gummies
Regulators in many countries struggle to help consumers understand the risks of natural health supplements, and the challenge extends well beyond Ayurvedic products.
There has been a “huge and very troubling increase” in U.S. poison control calls associated with gummy-bear products containing melatonin, said Canadian Senator Stan Kutcher, MD, at a May 11 meeting of Canada’s Standing Senate Committee on Social Affairs, Science, and Technology.
In April, JAMA published a U.S. analysis of melatonin gummy products, Dr. Kutcher noted. In this research letter, investigators reported that one product did not contain detectable levels of melatonin but did contain 31.3 mg of cannabidiol.
In other products, the quantity of melatonin ranged from 74% to 347% of the labeled quantity. A previous Canadian study of 16 melatonin brands found that the actual dose of melatonin ranged from 17% to 478% of the declared quantity, the letter noted.
The May 11 Senate meeting provided a forum for many of the recurring debates about supplements, which also are known as natural health products.
Barry Power, PharmD, editor in chief for the Canadian Pharmacists Association, said that his group was disappointed when Canada excluded natural health products from Vanessa’s Law, which was passed in 2014. This law sought to improve the reporting of adverse reactions to drugs.
“We’re glad this is being revisited now,” Dr. Power told the Senate committee. “Although natural health products are often seen as low risk, we need to keep in mind that ‘low risk’ does not mean ‘no risk,’ and ‘natural’ does not mean ‘safe.’ ”
In contrast, Aaron Skelton, chief executive of the Canadian Health Food Association, spoke against this bid to expand the reach of Vanessa’s Law into natural health products. Canadian lawmakers attached provisions regarding increased oversight of natural health products to a budget package instead of considering them as part of a stand-alone bill.
“Our concern is that the powers that are being discussed have not been reviewed and debated,” Mr. Skelton told Dr. Kutcher. “The potential for overreach and unnecessary regulation is significant, and that deserves debate.”
“Profits should not trump Canadians’ health,” answered Dr. Kutcher, who earlier served as head of the psychiatry department at Dalhousie University in Halifax, N.S.
By June, Vanessa’s Law had been expanded with provisions that address natural health products, including the reporting of products that present a serious risk to consumers.
Educating consumers
Many consumers overestimate the level of government regulation of supplements, said Pieter A. Cohen, MD, leader of the Supplement Research Program at Cambridge Health Alliance in Massachusetts. Dr. Cohen was the lead author of the JAMA research letter about melatonin products.
Supplements often share shelves in pharmacies with medicines that are subject to more strict regulation, which causes confusion.
“It’s really hard to wrap your brain around [the fact] that a health product is being sold in pharmacies in the United States and it’s not being vetted by the FDA [U.S. Food and Drug Administration]”, Dr. Cohen said in an interview
The confusion extends across borders. Many consumers in other countries will assume that the FDA performed premarket screening of U.S.-made supplements, but that is not the case, he said.
People who want to take supplements should look for reputable sources of information about them, such as the website of the National Institutes of Health’s Office of Dietary Supplements, Dr. Cohen said. But patients often forget or fail to do this, which can create medical puzzles, such as the case of the woman in the Ontario case study, said Peter Lurie, MD, MPH, executive director of the nonprofit Center for Science in the Public Interest, which has pressed for increased regulation of supplements.
Clinicians need to keep in mind that patients may need prodding to reveal what supplements they are taking, he said.
“They just think of them as different, somehow not the province of the doctor,” Dr. Lurie said. “For others, they are concerned that the doctors will disapprove. So, they hide it.”
A version of this article first appeared on Medscape.com.
A woman’s quest to become pregnant resulted in lead poisoning from an Ayurvedic treatment. The case triggered the seizure of pills from an Ontario natural-products clinic and the issuance of government warnings about the risks of products from this business, according to a new report.
, including the presence of lead and other metals in Ayurvedic products, according to the report.
“When consumer products may be contaminated with lead, or when lead exposure is linked to sources in the community, involving public health can facilitate broader actions to reduce and prevent exposures to other people at risk,” wrote report author Julian Gitelman, MD, MPH, a resident physician at the University of Toronto Dalla Lana School of Public Health, and colleagues.
Their case study was published in the Canadian Medical Association Journal.
The researchers detailed what happened after a 39-year-old woman sought medical care for abdominal pain, constipation, nausea, and vomiting. The woman underwent a series of tests, including colonoscopy, laparoscopy, and biopsies of bone marrow and ovarian cysts.
Only later did clinicians home in on the cause of her ailments: the Ayurvedic medications that the patient had been taking daily for more than a year for infertility. Her daily regimen had varied, ranging from a few pills to a dozen pills.
Heavy metals are sometimes intentionally added to Ayurvedic supplements for perceived healing properties, wrote the authors. They cited a previous study of a sample of Ayurvedic pills bought on the Internet from manufacturers based in the United States and India that showed that 21% contained lead, mercury, or arsenic.
A case report published last year in German Medical Weekly raised the same issue.
Melatonin gummies
Regulators in many countries struggle to help consumers understand the risks of natural health supplements, and the challenge extends well beyond Ayurvedic products.
There has been a “huge and very troubling increase” in U.S. poison control calls associated with gummy-bear products containing melatonin, said Canadian Senator Stan Kutcher, MD, at a May 11 meeting of Canada’s Standing Senate Committee on Social Affairs, Science, and Technology.
In April, JAMA published a U.S. analysis of melatonin gummy products, Dr. Kutcher noted. In this research letter, investigators reported that one product did not contain detectable levels of melatonin but did contain 31.3 mg of cannabidiol.
In other products, the quantity of melatonin ranged from 74% to 347% of the labeled quantity. A previous Canadian study of 16 melatonin brands found that the actual dose of melatonin ranged from 17% to 478% of the declared quantity, the letter noted.
The May 11 Senate meeting provided a forum for many of the recurring debates about supplements, which also are known as natural health products.
Barry Power, PharmD, editor in chief for the Canadian Pharmacists Association, said that his group was disappointed when Canada excluded natural health products from Vanessa’s Law, which was passed in 2014. This law sought to improve the reporting of adverse reactions to drugs.
“We’re glad this is being revisited now,” Dr. Power told the Senate committee. “Although natural health products are often seen as low risk, we need to keep in mind that ‘low risk’ does not mean ‘no risk,’ and ‘natural’ does not mean ‘safe.’ ”
In contrast, Aaron Skelton, chief executive of the Canadian Health Food Association, spoke against this bid to expand the reach of Vanessa’s Law into natural health products. Canadian lawmakers attached provisions regarding increased oversight of natural health products to a budget package instead of considering them as part of a stand-alone bill.
“Our concern is that the powers that are being discussed have not been reviewed and debated,” Mr. Skelton told Dr. Kutcher. “The potential for overreach and unnecessary regulation is significant, and that deserves debate.”
“Profits should not trump Canadians’ health,” answered Dr. Kutcher, who earlier served as head of the psychiatry department at Dalhousie University in Halifax, N.S.
By June, Vanessa’s Law had been expanded with provisions that address natural health products, including the reporting of products that present a serious risk to consumers.
Educating consumers
Many consumers overestimate the level of government regulation of supplements, said Pieter A. Cohen, MD, leader of the Supplement Research Program at Cambridge Health Alliance in Massachusetts. Dr. Cohen was the lead author of the JAMA research letter about melatonin products.
Supplements often share shelves in pharmacies with medicines that are subject to more strict regulation, which causes confusion.
“It’s really hard to wrap your brain around [the fact] that a health product is being sold in pharmacies in the United States and it’s not being vetted by the FDA [U.S. Food and Drug Administration]”, Dr. Cohen said in an interview
The confusion extends across borders. Many consumers in other countries will assume that the FDA performed premarket screening of U.S.-made supplements, but that is not the case, he said.
People who want to take supplements should look for reputable sources of information about them, such as the website of the National Institutes of Health’s Office of Dietary Supplements, Dr. Cohen said. But patients often forget or fail to do this, which can create medical puzzles, such as the case of the woman in the Ontario case study, said Peter Lurie, MD, MPH, executive director of the nonprofit Center for Science in the Public Interest, which has pressed for increased regulation of supplements.
Clinicians need to keep in mind that patients may need prodding to reveal what supplements they are taking, he said.
“They just think of them as different, somehow not the province of the doctor,” Dr. Lurie said. “For others, they are concerned that the doctors will disapprove. So, they hide it.”
A version of this article first appeared on Medscape.com.
FROM THE CANADIAN MEDICAL ASSOCIATION JOURNAL
Older women risk overdiagnosis with mammograms: Study
Women who continued breast cancer screenings when they reached age 70 had no lower chance of dying from the disease, and just getting a mammogram could instead set them on a path toward unnecessary risks, according to a new study from Yale University.
The findings, published in Annals of Internal Medicine, suggest that , meaning that the cancer found during the screening would not have caused symptoms in a person’s lifetime. (For context, the average life expectancy of a woman in the U.S. is 79 years, according to the Centers for Disease Control and Prevention.)
Overdiagnosis can be harmful because it carries the risks of complications from overtreatment, plus financial and emotional hardships and unnecessary use of limited resources.
For the study, researchers analyzed data for 54,635 women aged 70 and older and compared the rate of breast cancer diagnosis and death among women who did and did not have mammograms during a 15-year follow-up period.
The rate of breast cancer in the study among women aged 70-74 was 6% for women who were screened and 4% for women who were not screened. The researchers estimated that 31% of the cases were potentially overdiagnosed. Among women aged 75-84, breast cancer was found in 5% of women who were screened, compared to less than 3% of unscreened women. Their estimated overdiagnosis rate was 47%. Finally, 3% of women aged 85 and older who were screened had breast cancer detected, compared with 1% of women in the unscreened group. For the older group, the overdiagnosis rate was 54%.
“While our study focused on overdiagnosis, it is important to acknowledge that overdiagnosis is just one of many considerations when deciding whether to continue screening,” researcher and Yale assistant professor of medicine Ilana Richman, MD, said in a statement. “A patient’s preferences and values, personal risk factors, and the overall balance of risks and benefits from screening are also important to take into account when making screening decisions.”
A version of this article first appeared on WebMD.com.
Women who continued breast cancer screenings when they reached age 70 had no lower chance of dying from the disease, and just getting a mammogram could instead set them on a path toward unnecessary risks, according to a new study from Yale University.
The findings, published in Annals of Internal Medicine, suggest that , meaning that the cancer found during the screening would not have caused symptoms in a person’s lifetime. (For context, the average life expectancy of a woman in the U.S. is 79 years, according to the Centers for Disease Control and Prevention.)
Overdiagnosis can be harmful because it carries the risks of complications from overtreatment, plus financial and emotional hardships and unnecessary use of limited resources.
For the study, researchers analyzed data for 54,635 women aged 70 and older and compared the rate of breast cancer diagnosis and death among women who did and did not have mammograms during a 15-year follow-up period.
The rate of breast cancer in the study among women aged 70-74 was 6% for women who were screened and 4% for women who were not screened. The researchers estimated that 31% of the cases were potentially overdiagnosed. Among women aged 75-84, breast cancer was found in 5% of women who were screened, compared to less than 3% of unscreened women. Their estimated overdiagnosis rate was 47%. Finally, 3% of women aged 85 and older who were screened had breast cancer detected, compared with 1% of women in the unscreened group. For the older group, the overdiagnosis rate was 54%.
“While our study focused on overdiagnosis, it is important to acknowledge that overdiagnosis is just one of many considerations when deciding whether to continue screening,” researcher and Yale assistant professor of medicine Ilana Richman, MD, said in a statement. “A patient’s preferences and values, personal risk factors, and the overall balance of risks and benefits from screening are also important to take into account when making screening decisions.”
A version of this article first appeared on WebMD.com.
Women who continued breast cancer screenings when they reached age 70 had no lower chance of dying from the disease, and just getting a mammogram could instead set them on a path toward unnecessary risks, according to a new study from Yale University.
The findings, published in Annals of Internal Medicine, suggest that , meaning that the cancer found during the screening would not have caused symptoms in a person’s lifetime. (For context, the average life expectancy of a woman in the U.S. is 79 years, according to the Centers for Disease Control and Prevention.)
Overdiagnosis can be harmful because it carries the risks of complications from overtreatment, plus financial and emotional hardships and unnecessary use of limited resources.
For the study, researchers analyzed data for 54,635 women aged 70 and older and compared the rate of breast cancer diagnosis and death among women who did and did not have mammograms during a 15-year follow-up period.
The rate of breast cancer in the study among women aged 70-74 was 6% for women who were screened and 4% for women who were not screened. The researchers estimated that 31% of the cases were potentially overdiagnosed. Among women aged 75-84, breast cancer was found in 5% of women who were screened, compared to less than 3% of unscreened women. Their estimated overdiagnosis rate was 47%. Finally, 3% of women aged 85 and older who were screened had breast cancer detected, compared with 1% of women in the unscreened group. For the older group, the overdiagnosis rate was 54%.
“While our study focused on overdiagnosis, it is important to acknowledge that overdiagnosis is just one of many considerations when deciding whether to continue screening,” researcher and Yale assistant professor of medicine Ilana Richman, MD, said in a statement. “A patient’s preferences and values, personal risk factors, and the overall balance of risks and benefits from screening are also important to take into account when making screening decisions.”
A version of this article first appeared on WebMD.com.
FROM ANNALS OF INTERNAL MEDICINE
On the best way to exercise
This transcript has been edited for clarity.
I’m going to talk about something important to a lot of us, based on a new study that has just come out that promises to tell us the right way to exercise. This is a major issue as we think about the best ways to stay healthy.
There are basically two main types of exercise that exercise physiologists think about. There are aerobic exercises: the cardiovascular things like running on a treadmill or outside. Then there are muscle-strengthening exercises: lifting weights, calisthenics, and so on. And of course, plenty of exercises do both at the same time.
It seems that the era of aerobic exercise as the main way to improve health was the 1980s and early 1990s. Then we started to increasingly recognize that muscle-strengthening exercise was really important too. We’ve got a ton of data on the benefits of cardiovascular and aerobic exercise (a reduced risk for cardiovascular disease, cancer, and all-cause mortality, and even improved cognitive function) across a variety of study designs, including cohort studies, but also some randomized controlled trials where people were randomized to aerobic activity.
We’re starting to get more data on the benefits of muscle-strengthening exercises, although it hasn’t been in the zeitgeist as much. Obviously, this increases strength and may reduce visceral fat, increase anaerobic capacity and muscle mass, and therefore [increase the] basal metabolic rate. What is really interesting about muscle strengthening is that muscle just takes up more energy at rest, so building bigger muscles increases your basal energy expenditure and increases insulin sensitivity because muscle is a good insulin sensitizer.
So, do you do both? Do you do one? Do you do the other? What’s the right answer here?
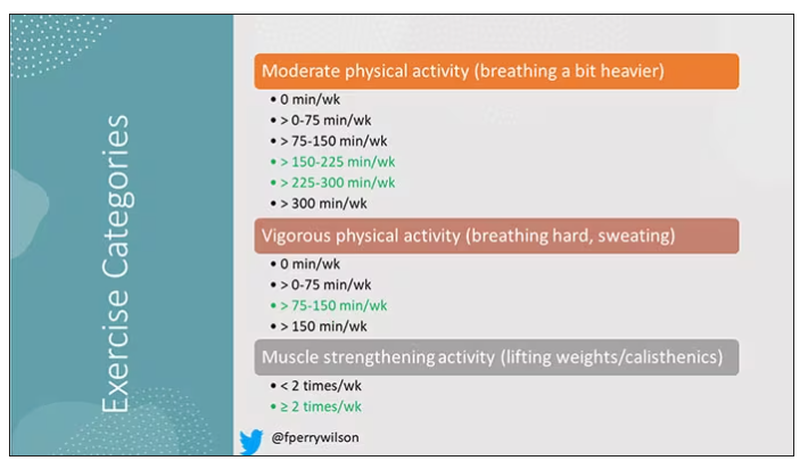
it depends on who you ask. The Center for Disease Control and Prevention’s recommendation, which changes from time to time, is that you should do at least 150 minutes a week of moderate-intensity aerobic activity. Anything that gets your heart beating faster counts here. So that’s 30 minutes, 5 days a week. They also say you can do 75 minutes a week of vigorous-intensity aerobic activity – something that really gets your heart rate up and you are breaking a sweat. Now they also recommend at least 2 days a week of a muscle-strengthening activity that makes your muscles work harder than usual, whether that’s push-ups or lifting weights or something like that.
The World Health Organization is similar. They don’t target 150 minutes a week. They actually say at least 150 and up to 300 minutes of moderate-intensity physical activity or 75-150 minutes of vigorous intensity aerobic physical activity. They are setting the floor, whereas the CDC sets its target and then they go a bit higher. They also recommend 2 days of muscle strengthening per week for optimal health.
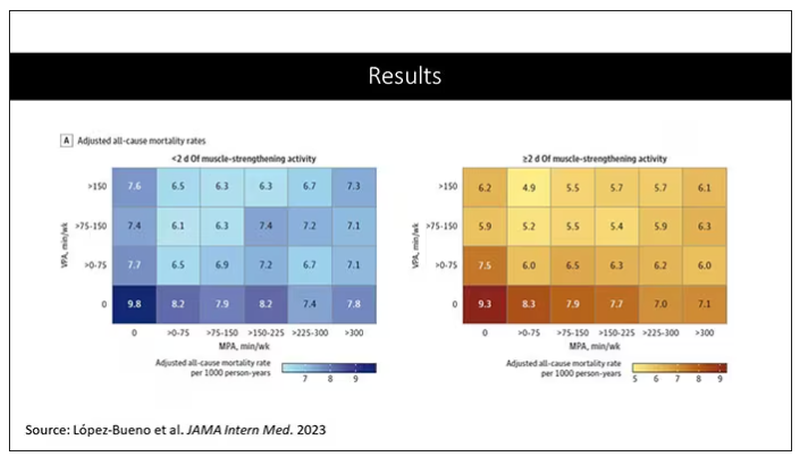
But what do the data show? Why am I talking about this? It’s because of this new study in JAMA Internal Medicine by Ruben Lopez Bueno and colleagues. I’m going to focus on all-cause mortality for brevity, but the results are broadly similar.
The data source is the U.S. National Health Interview Survey. A total of 500,705 people took part in the survey and answered a slew of questions (including self-reports on their exercise amounts), with a median follow-up of about 10 years looking for things like cardiovascular deaths, cancer deaths, and so on.
The survey classified people into different exercise categories – how much time they spent doing moderate physical activity (MPA), vigorous physical activity (VPA), or muscle-strengthening activity (MSA).
There are six categories based on duration of MPA (the WHO targets are highlighted in green), four categories based on length of time of VPA, and two categories of MSA (≥ or < two times per week). This gives a total of 48 possible combinations of exercise you could do in a typical week.
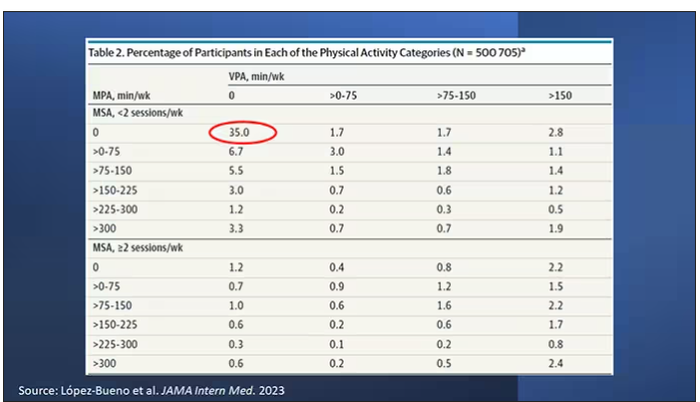
Here are the percentages of people who fell into each of these 48 potential categories. The largest is the 35% of people who fell into the “nothing” category (no MPA, no VPA, and less than two sessions per week of MSA). These “nothing” people are going to be a reference category moving forward.
So who are these people? On the far left are the 361,000 people (the vast majority) who don’t hit that 150 minutes a week of MPA or 75 minutes a week of VPA, and they don’t do 2 days a week of MSA. The other three categories are increasing amounts of exercise. Younger people seem to be doing more exercise at the higher ends, and men are more likely to be doing exercise at the higher end. There are also some interesting findings from the alcohol drinking survey. The people who do more exercise are more likely to be current drinkers. This is interesting. I confirmed these data with the investigator. This might suggest one of the reasons why some studies have shown that drinkers have better outcomes in terms of either cardiovascular or cognitive outcomes over time. There’s a lot of conflicting data there, but in part, it might be that healthier people might drink more alcohol. It could be a socioeconomic phenomenon as well.
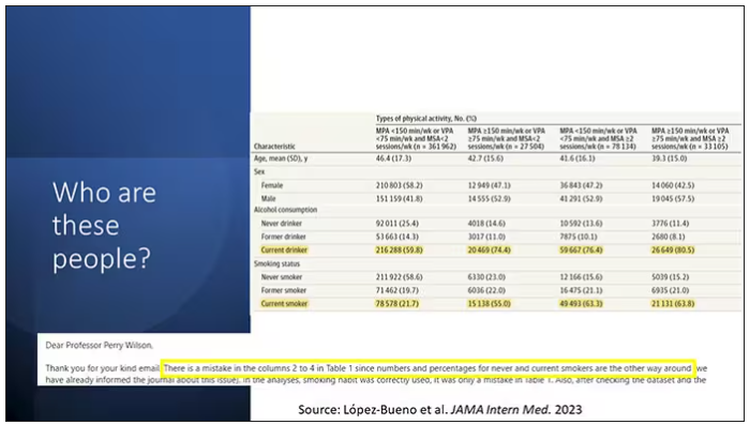
Now, what blew my mind were these smoker numbers, but don’t get too excited about it. What it looks like from the table in JAMA Internal Medicine is that 20% of the people who don’t do much exercise smoke, and then something like 60% of the people who do more exercise smoke. That can’t be right. So I checked with the lead study author. There is a mistake in these columns for smoking. They were supposed to flip the “never smoker” and “current smoker” numbers. You can actually see that just 15.2% of those who exercise a lot are current smokers, not 63.8%. This has been fixed online, but just in case you saw this and you were as confused as I was that these incredibly healthy smokers are out there exercising all the time, it was just a typo.
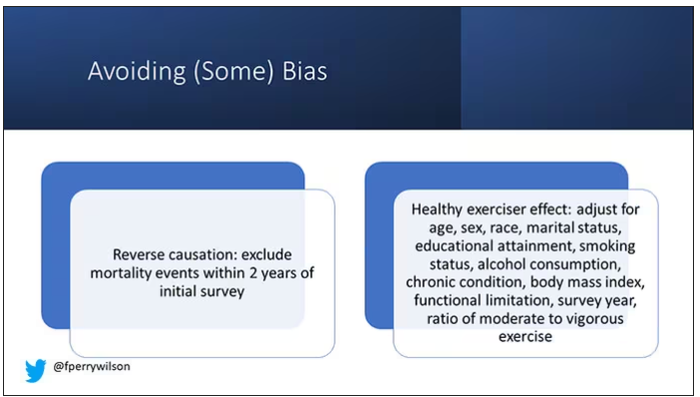
There is bias here. One of the big ones is called reverse causation bias. This is what might happen if, let’s say you’re already sick, you have cancer, you have some serious cardiovascular disease, or heart failure. You can’t exercise that much. You physically can’t do it. And then if you die, we wouldn’t find that exercise is beneficial. We would see that sicker people aren’t as able to exercise. The investigators got around this a bit by excluding mortality events within 2 years of the initial survey. Anyone who died within 2 years after saying how often they exercised was not included in this analysis.
This is known as the healthy exerciser or healthy user effect. Sometimes this means that people who exercise a lot probably do other healthy things; they might eat better or get out in the sun more. Researchers try to get around this through multivariable adjustment. They adjust for age, sex, race, marital status, etc. No adjustment is perfect. There’s always residual confounding. But this is probably the best you can do with the dataset like the one they had access to.
Let’s go to the results, which are nicely heat-mapped in the paper. They’re divided into people who have less or more than 2 days of MSA. Our reference groups that we want to pay attention to are the people who don’t do anything. The highest mortality of 9.8 individuals per 1,000 person-years is seen in the group that reported no moderate physical activity, no VPA, and less than 2 days a week of MSA.
As you move up and to the right (more VPA and MPA), you see lower numbers. The lowest number was 4.9 among people who reported more than 150 minutes per week of VPA and 2 days of MSA.
Looking at these data, the benefit, or the bang for your buck is higher for VPA than for MPA. Getting 2 days of MSA does have a tendency to reduce overall mortality. This is not necessarily causal, but it is rather potent and consistent across all the different groups.
So, what are we supposed to do here? I think the most clear finding from the study is that anything is better than nothing. This study suggests that if you are going to get activity, push on the vigorous activity if you’re physically able to do it. And of course, layering in the MSA as well seems to be associated with benefit.
Like everything in life, there’s no one simple solution. It’s a mix. But telling ourselves and our patients to get out there if you can and break a sweat as often as you can during the week, and take a couple of days to get those muscles a little bigger, may increase insulin sensitivity and basal metabolic rate – is it guaranteed to extend life? No. This is an observational study. We can’t say; we don’t have causal data here, but it’s unlikely to cause much harm. I’m particularly happy that people are doing a much better job now of really dissecting out the kinds of physical activity that are beneficial. It turns out that all of it is, and probably a mixture is best.
Dr. Wilson is associate professor, department of medicine, and interim director, program of applied translational research, Yale University, New Haven, Conn. He disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
I’m going to talk about something important to a lot of us, based on a new study that has just come out that promises to tell us the right way to exercise. This is a major issue as we think about the best ways to stay healthy.
There are basically two main types of exercise that exercise physiologists think about. There are aerobic exercises: the cardiovascular things like running on a treadmill or outside. Then there are muscle-strengthening exercises: lifting weights, calisthenics, and so on. And of course, plenty of exercises do both at the same time.
It seems that the era of aerobic exercise as the main way to improve health was the 1980s and early 1990s. Then we started to increasingly recognize that muscle-strengthening exercise was really important too. We’ve got a ton of data on the benefits of cardiovascular and aerobic exercise (a reduced risk for cardiovascular disease, cancer, and all-cause mortality, and even improved cognitive function) across a variety of study designs, including cohort studies, but also some randomized controlled trials where people were randomized to aerobic activity.
We’re starting to get more data on the benefits of muscle-strengthening exercises, although it hasn’t been in the zeitgeist as much. Obviously, this increases strength and may reduce visceral fat, increase anaerobic capacity and muscle mass, and therefore [increase the] basal metabolic rate. What is really interesting about muscle strengthening is that muscle just takes up more energy at rest, so building bigger muscles increases your basal energy expenditure and increases insulin sensitivity because muscle is a good insulin sensitizer.
So, do you do both? Do you do one? Do you do the other? What’s the right answer here?
it depends on who you ask. The Center for Disease Control and Prevention’s recommendation, which changes from time to time, is that you should do at least 150 minutes a week of moderate-intensity aerobic activity. Anything that gets your heart beating faster counts here. So that’s 30 minutes, 5 days a week. They also say you can do 75 minutes a week of vigorous-intensity aerobic activity – something that really gets your heart rate up and you are breaking a sweat. Now they also recommend at least 2 days a week of a muscle-strengthening activity that makes your muscles work harder than usual, whether that’s push-ups or lifting weights or something like that.
The World Health Organization is similar. They don’t target 150 minutes a week. They actually say at least 150 and up to 300 minutes of moderate-intensity physical activity or 75-150 minutes of vigorous intensity aerobic physical activity. They are setting the floor, whereas the CDC sets its target and then they go a bit higher. They also recommend 2 days of muscle strengthening per week for optimal health.
But what do the data show? Why am I talking about this? It’s because of this new study in JAMA Internal Medicine by Ruben Lopez Bueno and colleagues. I’m going to focus on all-cause mortality for brevity, but the results are broadly similar.
The data source is the U.S. National Health Interview Survey. A total of 500,705 people took part in the survey and answered a slew of questions (including self-reports on their exercise amounts), with a median follow-up of about 10 years looking for things like cardiovascular deaths, cancer deaths, and so on.
The survey classified people into different exercise categories – how much time they spent doing moderate physical activity (MPA), vigorous physical activity (VPA), or muscle-strengthening activity (MSA).
There are six categories based on duration of MPA (the WHO targets are highlighted in green), four categories based on length of time of VPA, and two categories of MSA (≥ or < two times per week). This gives a total of 48 possible combinations of exercise you could do in a typical week.
Here are the percentages of people who fell into each of these 48 potential categories. The largest is the 35% of people who fell into the “nothing” category (no MPA, no VPA, and less than two sessions per week of MSA). These “nothing” people are going to be a reference category moving forward.
So who are these people? On the far left are the 361,000 people (the vast majority) who don’t hit that 150 minutes a week of MPA or 75 minutes a week of VPA, and they don’t do 2 days a week of MSA. The other three categories are increasing amounts of exercise. Younger people seem to be doing more exercise at the higher ends, and men are more likely to be doing exercise at the higher end. There are also some interesting findings from the alcohol drinking survey. The people who do more exercise are more likely to be current drinkers. This is interesting. I confirmed these data with the investigator. This might suggest one of the reasons why some studies have shown that drinkers have better outcomes in terms of either cardiovascular or cognitive outcomes over time. There’s a lot of conflicting data there, but in part, it might be that healthier people might drink more alcohol. It could be a socioeconomic phenomenon as well.
Now, what blew my mind were these smoker numbers, but don’t get too excited about it. What it looks like from the table in JAMA Internal Medicine is that 20% of the people who don’t do much exercise smoke, and then something like 60% of the people who do more exercise smoke. That can’t be right. So I checked with the lead study author. There is a mistake in these columns for smoking. They were supposed to flip the “never smoker” and “current smoker” numbers. You can actually see that just 15.2% of those who exercise a lot are current smokers, not 63.8%. This has been fixed online, but just in case you saw this and you were as confused as I was that these incredibly healthy smokers are out there exercising all the time, it was just a typo.
There is bias here. One of the big ones is called reverse causation bias. This is what might happen if, let’s say you’re already sick, you have cancer, you have some serious cardiovascular disease, or heart failure. You can’t exercise that much. You physically can’t do it. And then if you die, we wouldn’t find that exercise is beneficial. We would see that sicker people aren’t as able to exercise. The investigators got around this a bit by excluding mortality events within 2 years of the initial survey. Anyone who died within 2 years after saying how often they exercised was not included in this analysis.
This is known as the healthy exerciser or healthy user effect. Sometimes this means that people who exercise a lot probably do other healthy things; they might eat better or get out in the sun more. Researchers try to get around this through multivariable adjustment. They adjust for age, sex, race, marital status, etc. No adjustment is perfect. There’s always residual confounding. But this is probably the best you can do with the dataset like the one they had access to.
Let’s go to the results, which are nicely heat-mapped in the paper. They’re divided into people who have less or more than 2 days of MSA. Our reference groups that we want to pay attention to are the people who don’t do anything. The highest mortality of 9.8 individuals per 1,000 person-years is seen in the group that reported no moderate physical activity, no VPA, and less than 2 days a week of MSA.
As you move up and to the right (more VPA and MPA), you see lower numbers. The lowest number was 4.9 among people who reported more than 150 minutes per week of VPA and 2 days of MSA.
Looking at these data, the benefit, or the bang for your buck is higher for VPA than for MPA. Getting 2 days of MSA does have a tendency to reduce overall mortality. This is not necessarily causal, but it is rather potent and consistent across all the different groups.
So, what are we supposed to do here? I think the most clear finding from the study is that anything is better than nothing. This study suggests that if you are going to get activity, push on the vigorous activity if you’re physically able to do it. And of course, layering in the MSA as well seems to be associated with benefit.
Like everything in life, there’s no one simple solution. It’s a mix. But telling ourselves and our patients to get out there if you can and break a sweat as often as you can during the week, and take a couple of days to get those muscles a little bigger, may increase insulin sensitivity and basal metabolic rate – is it guaranteed to extend life? No. This is an observational study. We can’t say; we don’t have causal data here, but it’s unlikely to cause much harm. I’m particularly happy that people are doing a much better job now of really dissecting out the kinds of physical activity that are beneficial. It turns out that all of it is, and probably a mixture is best.
Dr. Wilson is associate professor, department of medicine, and interim director, program of applied translational research, Yale University, New Haven, Conn. He disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
I’m going to talk about something important to a lot of us, based on a new study that has just come out that promises to tell us the right way to exercise. This is a major issue as we think about the best ways to stay healthy.
There are basically two main types of exercise that exercise physiologists think about. There are aerobic exercises: the cardiovascular things like running on a treadmill or outside. Then there are muscle-strengthening exercises: lifting weights, calisthenics, and so on. And of course, plenty of exercises do both at the same time.
It seems that the era of aerobic exercise as the main way to improve health was the 1980s and early 1990s. Then we started to increasingly recognize that muscle-strengthening exercise was really important too. We’ve got a ton of data on the benefits of cardiovascular and aerobic exercise (a reduced risk for cardiovascular disease, cancer, and all-cause mortality, and even improved cognitive function) across a variety of study designs, including cohort studies, but also some randomized controlled trials where people were randomized to aerobic activity.
We’re starting to get more data on the benefits of muscle-strengthening exercises, although it hasn’t been in the zeitgeist as much. Obviously, this increases strength and may reduce visceral fat, increase anaerobic capacity and muscle mass, and therefore [increase the] basal metabolic rate. What is really interesting about muscle strengthening is that muscle just takes up more energy at rest, so building bigger muscles increases your basal energy expenditure and increases insulin sensitivity because muscle is a good insulin sensitizer.
So, do you do both? Do you do one? Do you do the other? What’s the right answer here?
it depends on who you ask. The Center for Disease Control and Prevention’s recommendation, which changes from time to time, is that you should do at least 150 minutes a week of moderate-intensity aerobic activity. Anything that gets your heart beating faster counts here. So that’s 30 minutes, 5 days a week. They also say you can do 75 minutes a week of vigorous-intensity aerobic activity – something that really gets your heart rate up and you are breaking a sweat. Now they also recommend at least 2 days a week of a muscle-strengthening activity that makes your muscles work harder than usual, whether that’s push-ups or lifting weights or something like that.
The World Health Organization is similar. They don’t target 150 minutes a week. They actually say at least 150 and up to 300 minutes of moderate-intensity physical activity or 75-150 minutes of vigorous intensity aerobic physical activity. They are setting the floor, whereas the CDC sets its target and then they go a bit higher. They also recommend 2 days of muscle strengthening per week for optimal health.
But what do the data show? Why am I talking about this? It’s because of this new study in JAMA Internal Medicine by Ruben Lopez Bueno and colleagues. I’m going to focus on all-cause mortality for brevity, but the results are broadly similar.
The data source is the U.S. National Health Interview Survey. A total of 500,705 people took part in the survey and answered a slew of questions (including self-reports on their exercise amounts), with a median follow-up of about 10 years looking for things like cardiovascular deaths, cancer deaths, and so on.
The survey classified people into different exercise categories – how much time they spent doing moderate physical activity (MPA), vigorous physical activity (VPA), or muscle-strengthening activity (MSA).
There are six categories based on duration of MPA (the WHO targets are highlighted in green), four categories based on length of time of VPA, and two categories of MSA (≥ or < two times per week). This gives a total of 48 possible combinations of exercise you could do in a typical week.
Here are the percentages of people who fell into each of these 48 potential categories. The largest is the 35% of people who fell into the “nothing” category (no MPA, no VPA, and less than two sessions per week of MSA). These “nothing” people are going to be a reference category moving forward.
So who are these people? On the far left are the 361,000 people (the vast majority) who don’t hit that 150 minutes a week of MPA or 75 minutes a week of VPA, and they don’t do 2 days a week of MSA. The other three categories are increasing amounts of exercise. Younger people seem to be doing more exercise at the higher ends, and men are more likely to be doing exercise at the higher end. There are also some interesting findings from the alcohol drinking survey. The people who do more exercise are more likely to be current drinkers. This is interesting. I confirmed these data with the investigator. This might suggest one of the reasons why some studies have shown that drinkers have better outcomes in terms of either cardiovascular or cognitive outcomes over time. There’s a lot of conflicting data there, but in part, it might be that healthier people might drink more alcohol. It could be a socioeconomic phenomenon as well.
Now, what blew my mind were these smoker numbers, but don’t get too excited about it. What it looks like from the table in JAMA Internal Medicine is that 20% of the people who don’t do much exercise smoke, and then something like 60% of the people who do more exercise smoke. That can’t be right. So I checked with the lead study author. There is a mistake in these columns for smoking. They were supposed to flip the “never smoker” and “current smoker” numbers. You can actually see that just 15.2% of those who exercise a lot are current smokers, not 63.8%. This has been fixed online, but just in case you saw this and you were as confused as I was that these incredibly healthy smokers are out there exercising all the time, it was just a typo.
There is bias here. One of the big ones is called reverse causation bias. This is what might happen if, let’s say you’re already sick, you have cancer, you have some serious cardiovascular disease, or heart failure. You can’t exercise that much. You physically can’t do it. And then if you die, we wouldn’t find that exercise is beneficial. We would see that sicker people aren’t as able to exercise. The investigators got around this a bit by excluding mortality events within 2 years of the initial survey. Anyone who died within 2 years after saying how often they exercised was not included in this analysis.
This is known as the healthy exerciser or healthy user effect. Sometimes this means that people who exercise a lot probably do other healthy things; they might eat better or get out in the sun more. Researchers try to get around this through multivariable adjustment. They adjust for age, sex, race, marital status, etc. No adjustment is perfect. There’s always residual confounding. But this is probably the best you can do with the dataset like the one they had access to.
Let’s go to the results, which are nicely heat-mapped in the paper. They’re divided into people who have less or more than 2 days of MSA. Our reference groups that we want to pay attention to are the people who don’t do anything. The highest mortality of 9.8 individuals per 1,000 person-years is seen in the group that reported no moderate physical activity, no VPA, and less than 2 days a week of MSA.
As you move up and to the right (more VPA and MPA), you see lower numbers. The lowest number was 4.9 among people who reported more than 150 minutes per week of VPA and 2 days of MSA.
Looking at these data, the benefit, or the bang for your buck is higher for VPA than for MPA. Getting 2 days of MSA does have a tendency to reduce overall mortality. This is not necessarily causal, but it is rather potent and consistent across all the different groups.
So, what are we supposed to do here? I think the most clear finding from the study is that anything is better than nothing. This study suggests that if you are going to get activity, push on the vigorous activity if you’re physically able to do it. And of course, layering in the MSA as well seems to be associated with benefit.
Like everything in life, there’s no one simple solution. It’s a mix. But telling ourselves and our patients to get out there if you can and break a sweat as often as you can during the week, and take a couple of days to get those muscles a little bigger, may increase insulin sensitivity and basal metabolic rate – is it guaranteed to extend life? No. This is an observational study. We can’t say; we don’t have causal data here, but it’s unlikely to cause much harm. I’m particularly happy that people are doing a much better job now of really dissecting out the kinds of physical activity that are beneficial. It turns out that all of it is, and probably a mixture is best.
Dr. Wilson is associate professor, department of medicine, and interim director, program of applied translational research, Yale University, New Haven, Conn. He disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Does tamoxifen use increase the risk of endometrial cancer in premenopausal patients?
Ryu KJ, Kim MS, Lee JY, et al. Risk of endometrial polyps, hyperplasia, carcinoma, and uterine cancer after tamoxifen treatment in premenopausal women with breast cancer. JAMA Netw Open. 2022;5:e2243951.
EXPERT COMMENTARY
Tamoxifen is a selective estrogen receptor modulator (SERM) approved by the US Food and Drug Administration (FDA) for both adjuvant treatment of invasive or metastatic breast cancer with hormone receptor (HR)–positive tumors (duration, 5 to 10 years) and for reduction of future breast cancers in certain high-risk individuals (duration, 5 years). It is also occasionally used for non-FDA approved indications, such as cyclic mastodynia.
Because breast cancer is among the most frequently diagnosed cancers in the United States (297,790 new cases expected in 2023) and approximately 80% are HR-positive tumors that will require hormonal adjuvant therapy,1 physicians and other gynecologic clinicians should have a working understanding of tamoxifen, including the risks and benefits associated with its use. Among the recognized serious adverse effects of tamoxifen is the increased risk of endometrial cancer in menopausal patients. This adverse effect creates a potential conundrum for clinicians who may be managing patients with tamoxifen to treat or prevent breast cancer, while also increasing the risk of another cancer. Prior prospective studies of tamoxifen have demonstrated a statistically and clinically significant increased risk of endometrial cancer in menopausal patients but not in premenopausal patients.
A recent study challenged those previous findings, suggesting that the risk of endometrial cancer is similar in both premenopausal and postmenopausal patients taking tamoxifen for treatment of breast cancer.2
Details of the study
The study by Ryu and colleagues used data from the Korean National Health Insurance Service, which covers 97% of the Korean population.2 The authors selected patients being treated for invasive breast cancer from January 1, 2003, through December 31, 2018, who were between the ages of 20 and 50 years when the breast cancer diagnosis was first made. Patients with a diagnostic code entered into their electronic health record that was consistent with menopausal status were excluded, along with any patients with a current or prior history of aromatase inhibitor use (for which one must be naturally, medically, or surgically menopausal to use). Based on these exclusions, the study cohort was then assumed to be premenopausal.
The study group included patients diagnosed with invasive breast cancer who were treated with adjuvant hormonal therapy with tamoxifen (n = 34,637), and the control group included patients with invasive breast cancer who were not treated with adjuvant hormonal therapy (n = 43,683). The primary study end point was the finding of endometrial or uterine pathology, including endometrial polyps, endometrial hyperplasia, endometrial cancer, and other uterine malignant neoplasms not originating in the endometrium (for example, uterine sarcomas).
Because this was a retrospective cohort study that included all eligible patients, the 2 groups were not matched. The treatment group was statistically older, had a higher body mass index (BMI) and a larger waist circumference, were more likely to be hypertensive, and included more patients with diabetes than the control group—all known risk factors for endometrial cancer. However, after adjusting for these 4 factors, an increased risk of endometrial cancer remained in the tamoxifen group compared with the control group (hazard ratio [HR], 3.77; 95% confidence interval [CI], 3.04–4.66). In addition, tamoxifen use was independently associated with an increased risk of endometrial polyps (HR, 3.90; 95% CI, 3.65–4.16), endometrial hyperplasia (HR, 5.56; 95% CI, 5.06–6.12), and other uterine cancers (HR, 2.27; 95% CI, 1.54–3.33). In a subgroup analysis, the risk for endometrial cancer was not higher in patients treated for more than 5 years of tamoxifen compared with those treated for 5 years or less.
Study strengths and limitations
A major strength of this study was the large number of study participants (n = 34,637 tamoxifen; n = 43,683 control), the long duration of follow-up (up to 15 years), and use of a single source of data with coverage of nearly the entire population of Korea. While the 2 study populations (tamoxifen vs no tamoxifen) were initially unbalanced in terms of endometrial cancer risk (age, BMI, concurrent diagnoses of hypertension and diabetes), the authors corrected for this with a multivariate analysis.
Furthermore, while the likely homogeneity of the study population may not make the results generalizable, the authors noted that Korean patients have a higher tendency toward early-onset breast cancer. This observation could make this cohort better suited for a study on premenopausal effects of tamoxifen.
Limitations. These data are provocative as they conflict with level 1 evidence based on multiple well-designed, double-blind, placebo-controlled randomized trials in which tamoxifen use for 5 years did not demonstrate a statistically increased risk of endometrial cancer in patients younger than age 50.3-5 Because of the importance of the question and the implications for many premenopausal women being treated with tamoxifen, we carefully evaluated the study methodology to better understand this discrepancy.
Continue to: Methodological concerns...
Methodological concerns
In the study by Ryu and colleagues, we found the definition of premenopausal to be problematic. Ultimately, if patients did not have a diagnosis of menopause in the problem summary list, they were assumed to be premenopausal if they were between the ages of 20 and 50 and not taking an aromatase inhibitor. However, important considerations in this population include the cancer stage and treatment regimens that can and do directly impact menopausal status.
Data demonstrate that early-onset breast cancer tends to be associated with more biologically aggressive characteristics that frequently require adjuvant or neoadjuvant chemotherapy.6,7 This chemotherapy regimen is comprised most commonly of Adriamycin (doxorubicin), paclitaxel, and cyclophosphamide. Cyclophosphamide is an alkylating agent that is a known gonadotoxin, and it often renders patients either temporarily or permanently menopausal due to chemotherapy-induced ovarian failure. Prior studies have demonstrated that for patients in their 40s, approximately 90% of those treated with cyclophosphamide-containing chemo-therapy for breast cancer will experience chemotherapy-induced amenorrhea (CIA).8 Although some patients in their 40s with CIA will resume ovarian function, the majority will not.8,9
Due to the lack of reliability in diagnosing CIA, blood levels of estradiol and follicle stimulating hormone are often necessary for confirmation and, even so, may be only temporary. One prospective analysis of 4 randomized neoadjuvant/adjuvant breast cancer trials used this approach and demonstrated that 85.1% of the study cohort experienced chemotherapy-induced ovarian failure at the end of their treatment, with some fluctuating back to premenopausal hormonal levels at 6 and 12 months.10
Furthermore, in the study by Ryu and colleagues, there is no description or confirmation of menstrual patterns in the study group to support the diagnosis of ongoing premenopausal status. Data on CIA and loss of ovarian function, therefore, are critical to the accurate categorization of patients as premenopausal or menopausal in this study. The study also relied on consistent and accurate recording of appropriate medical codes to capture a patient’s menopausal status, which is unclear for this particular population and health system.
In evaluating prior research, multiple studies demonstrated no increased risk of endometrial cancer in premenopausal women taking tamoxifen for breast cancer prevention (TABLE).3,5 These breast cancer prevention trials have several major advantages in assessing tamoxifen-associated endometrial cancer risk for premenopausal patients compared with the current study:
- Both studies were prospective double-blind, placebo-controlled randomized clinical breast cancer prevention trials with carefully designed and measured outcomes.
- Since these were breast cancer prevention trials, administration of gonadotoxic chemotherapy was not a concern. As a result, miscategorizing patients with chemotherapy-induced menopause as premenopausal would not be expected, and premature menopause would not be expected at a higher rate than the general population.
- Careful histories were required prior to study entry and throughout the study, including data on menopausal status and menstrual and uterine bleeding histories.11
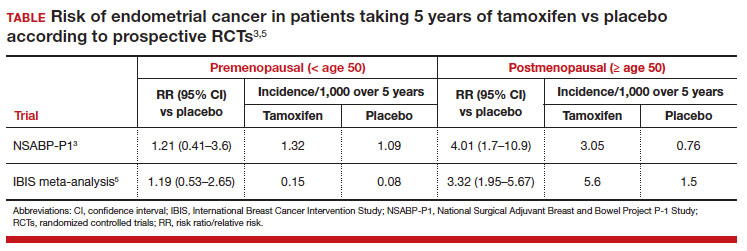
In these prevention trials, the effect of tamoxifen on uterine pathology demonstratedrepeatable evidence that there was a statistically significant increased risk of endometrial cancer in postmenopausal women, but there was no similar increased risk of endometrial cancer in premenopausal women (TABLE).3,5 Interestingly, the magnitude of the endometrial cancer risk found in the premenopausal patients in the study by Ryu and colleagues (RR, 3.77) is comparable to that of the menopausal group in the prevention trials, raising concern that many or most of the patients in the treatment group assumed to be premenopausal may have indeed been “menopausal” for some or all the time they were taking tamoxifen due to the possible aforementioned reasons. ●
While the data from the study by Ryu and colleagues are provocative, the findings that premenopausal women are at an increased risk of endometrial cancer do not agree with those of well-designed previous trials. Our concerns about categorization bias (that is, women in the treatment group may have been menopausal for some or all the time they were taking tamoxifen but were not formally diagnosed) make the conclusion that endometrial cancer risk is increased in truly premenopausal women somewhat specious. In a Committee Opinion (last endorsed in 2020), the American College of Obstetricians and Gynecologists (ACOG) stated the following: “Postmenopausal women taking tamoxifen should be closely monitored for symptoms of endometrial hyperplasia or cancer. Premenopausal women treated with tamoxifen have no known increased risk of uterine cancer and as such require no additional monitoring beyond routine gynecologic care.”12 Based on multiple previously published studies with solid level 1 evidence and the challenges with the current study design, we continue to agree with this ACOG statement.
VERSHA PLEASANT, MD, MPH; MARK D. PEARLMAN, MD
- Siegel RL, Miller KD, Wagle NS, et al. Cancer statistics, 2023. CA Cancer J Clin. 2023;73:17-48.
- Ryu KJ, Kim MS, Lee JY, et al. Risk of endometrial polyps, hyperplasia, carcinoma, and uterine cancer after tamoxifen treatment in premenopausal women with breast cancer. JAMA Netw Open. 2022;5:e2243951-e.
- Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998;90:1371-1388.
- Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for the prevention of breast cancer: current status of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 2005;97:1652-1662.
- Iqbal J, Ginsburg OM, Wijeratne TD, et al. Endometrial cancer and venous thromboembolism in women under age 50 who take tamoxifen for prevention of breast cancer: a systematic review. Cancer Treat Rev. 2012;38:318-328.
- Kumar R, Abreu C, Toi M, et al. Oncobiology and treatment of breast cancer in young women. Cancer Metastasis Rev. 2022;41:749-770.
- Tesch ME, Partidge AH. Treatment of breast cancer in young adults. Am Soc Clin Oncol Educ Book. 2022;42:1-12.
- Han HS, Ro J, Lee KS, et al. Analysis of chemotherapy-induced amenorrhea rates by three different anthracycline and taxane containing regimens for early breast cancer. Breast Cancer Res Treat. 2009;115:335-342.
- Henry NL, Xia R, Banerjee M, et al. Predictors of recovery of ovarian function during aromatase inhibitor therapy. Ann Oncol. 2013;24:2011-2016.
- Furlanetto J, Marme F, Seiler S, et al. Chemotherapy-induced ovarian failure in young women with early breast cancer: prospective analysis of four randomised neoadjuvant/ adjuvant breast cancer trials. Eur J Cancer. 2021;152: 193-203.
- Runowicz CD, Costantino JP, Wickerham DL, et al. Gynecologic conditions in participants in the NSABP breast cancer prevention study of tamoxifen and raloxifene (STAR). Am J Obstet Gynecol. 2011;205:535.e1-535.e5.
- American College of Obstetricians and Gynecologists. Committee opinion no. 601: tamoxifen and uterine cancer. Obstet Gynecol. 2014;123:1394-1397.
Ryu KJ, Kim MS, Lee JY, et al. Risk of endometrial polyps, hyperplasia, carcinoma, and uterine cancer after tamoxifen treatment in premenopausal women with breast cancer. JAMA Netw Open. 2022;5:e2243951.
EXPERT COMMENTARY
Tamoxifen is a selective estrogen receptor modulator (SERM) approved by the US Food and Drug Administration (FDA) for both adjuvant treatment of invasive or metastatic breast cancer with hormone receptor (HR)–positive tumors (duration, 5 to 10 years) and for reduction of future breast cancers in certain high-risk individuals (duration, 5 years). It is also occasionally used for non-FDA approved indications, such as cyclic mastodynia.
Because breast cancer is among the most frequently diagnosed cancers in the United States (297,790 new cases expected in 2023) and approximately 80% are HR-positive tumors that will require hormonal adjuvant therapy,1 physicians and other gynecologic clinicians should have a working understanding of tamoxifen, including the risks and benefits associated with its use. Among the recognized serious adverse effects of tamoxifen is the increased risk of endometrial cancer in menopausal patients. This adverse effect creates a potential conundrum for clinicians who may be managing patients with tamoxifen to treat or prevent breast cancer, while also increasing the risk of another cancer. Prior prospective studies of tamoxifen have demonstrated a statistically and clinically significant increased risk of endometrial cancer in menopausal patients but not in premenopausal patients.
A recent study challenged those previous findings, suggesting that the risk of endometrial cancer is similar in both premenopausal and postmenopausal patients taking tamoxifen for treatment of breast cancer.2
Details of the study
The study by Ryu and colleagues used data from the Korean National Health Insurance Service, which covers 97% of the Korean population.2 The authors selected patients being treated for invasive breast cancer from January 1, 2003, through December 31, 2018, who were between the ages of 20 and 50 years when the breast cancer diagnosis was first made. Patients with a diagnostic code entered into their electronic health record that was consistent with menopausal status were excluded, along with any patients with a current or prior history of aromatase inhibitor use (for which one must be naturally, medically, or surgically menopausal to use). Based on these exclusions, the study cohort was then assumed to be premenopausal.
The study group included patients diagnosed with invasive breast cancer who were treated with adjuvant hormonal therapy with tamoxifen (n = 34,637), and the control group included patients with invasive breast cancer who were not treated with adjuvant hormonal therapy (n = 43,683). The primary study end point was the finding of endometrial or uterine pathology, including endometrial polyps, endometrial hyperplasia, endometrial cancer, and other uterine malignant neoplasms not originating in the endometrium (for example, uterine sarcomas).
Because this was a retrospective cohort study that included all eligible patients, the 2 groups were not matched. The treatment group was statistically older, had a higher body mass index (BMI) and a larger waist circumference, were more likely to be hypertensive, and included more patients with diabetes than the control group—all known risk factors for endometrial cancer. However, after adjusting for these 4 factors, an increased risk of endometrial cancer remained in the tamoxifen group compared with the control group (hazard ratio [HR], 3.77; 95% confidence interval [CI], 3.04–4.66). In addition, tamoxifen use was independently associated with an increased risk of endometrial polyps (HR, 3.90; 95% CI, 3.65–4.16), endometrial hyperplasia (HR, 5.56; 95% CI, 5.06–6.12), and other uterine cancers (HR, 2.27; 95% CI, 1.54–3.33). In a subgroup analysis, the risk for endometrial cancer was not higher in patients treated for more than 5 years of tamoxifen compared with those treated for 5 years or less.
Study strengths and limitations
A major strength of this study was the large number of study participants (n = 34,637 tamoxifen; n = 43,683 control), the long duration of follow-up (up to 15 years), and use of a single source of data with coverage of nearly the entire population of Korea. While the 2 study populations (tamoxifen vs no tamoxifen) were initially unbalanced in terms of endometrial cancer risk (age, BMI, concurrent diagnoses of hypertension and diabetes), the authors corrected for this with a multivariate analysis.
Furthermore, while the likely homogeneity of the study population may not make the results generalizable, the authors noted that Korean patients have a higher tendency toward early-onset breast cancer. This observation could make this cohort better suited for a study on premenopausal effects of tamoxifen.
Limitations. These data are provocative as they conflict with level 1 evidence based on multiple well-designed, double-blind, placebo-controlled randomized trials in which tamoxifen use for 5 years did not demonstrate a statistically increased risk of endometrial cancer in patients younger than age 50.3-5 Because of the importance of the question and the implications for many premenopausal women being treated with tamoxifen, we carefully evaluated the study methodology to better understand this discrepancy.
Continue to: Methodological concerns...
Methodological concerns
In the study by Ryu and colleagues, we found the definition of premenopausal to be problematic. Ultimately, if patients did not have a diagnosis of menopause in the problem summary list, they were assumed to be premenopausal if they were between the ages of 20 and 50 and not taking an aromatase inhibitor. However, important considerations in this population include the cancer stage and treatment regimens that can and do directly impact menopausal status.
Data demonstrate that early-onset breast cancer tends to be associated with more biologically aggressive characteristics that frequently require adjuvant or neoadjuvant chemotherapy.6,7 This chemotherapy regimen is comprised most commonly of Adriamycin (doxorubicin), paclitaxel, and cyclophosphamide. Cyclophosphamide is an alkylating agent that is a known gonadotoxin, and it often renders patients either temporarily or permanently menopausal due to chemotherapy-induced ovarian failure. Prior studies have demonstrated that for patients in their 40s, approximately 90% of those treated with cyclophosphamide-containing chemo-therapy for breast cancer will experience chemotherapy-induced amenorrhea (CIA).8 Although some patients in their 40s with CIA will resume ovarian function, the majority will not.8,9
Due to the lack of reliability in diagnosing CIA, blood levels of estradiol and follicle stimulating hormone are often necessary for confirmation and, even so, may be only temporary. One prospective analysis of 4 randomized neoadjuvant/adjuvant breast cancer trials used this approach and demonstrated that 85.1% of the study cohort experienced chemotherapy-induced ovarian failure at the end of their treatment, with some fluctuating back to premenopausal hormonal levels at 6 and 12 months.10
Furthermore, in the study by Ryu and colleagues, there is no description or confirmation of menstrual patterns in the study group to support the diagnosis of ongoing premenopausal status. Data on CIA and loss of ovarian function, therefore, are critical to the accurate categorization of patients as premenopausal or menopausal in this study. The study also relied on consistent and accurate recording of appropriate medical codes to capture a patient’s menopausal status, which is unclear for this particular population and health system.
In evaluating prior research, multiple studies demonstrated no increased risk of endometrial cancer in premenopausal women taking tamoxifen for breast cancer prevention (TABLE).3,5 These breast cancer prevention trials have several major advantages in assessing tamoxifen-associated endometrial cancer risk for premenopausal patients compared with the current study:
- Both studies were prospective double-blind, placebo-controlled randomized clinical breast cancer prevention trials with carefully designed and measured outcomes.
- Since these were breast cancer prevention trials, administration of gonadotoxic chemotherapy was not a concern. As a result, miscategorizing patients with chemotherapy-induced menopause as premenopausal would not be expected, and premature menopause would not be expected at a higher rate than the general population.
- Careful histories were required prior to study entry and throughout the study, including data on menopausal status and menstrual and uterine bleeding histories.11

In these prevention trials, the effect of tamoxifen on uterine pathology demonstratedrepeatable evidence that there was a statistically significant increased risk of endometrial cancer in postmenopausal women, but there was no similar increased risk of endometrial cancer in premenopausal women (TABLE).3,5 Interestingly, the magnitude of the endometrial cancer risk found in the premenopausal patients in the study by Ryu and colleagues (RR, 3.77) is comparable to that of the menopausal group in the prevention trials, raising concern that many or most of the patients in the treatment group assumed to be premenopausal may have indeed been “menopausal” for some or all the time they were taking tamoxifen due to the possible aforementioned reasons. ●
While the data from the study by Ryu and colleagues are provocative, the findings that premenopausal women are at an increased risk of endometrial cancer do not agree with those of well-designed previous trials. Our concerns about categorization bias (that is, women in the treatment group may have been menopausal for some or all the time they were taking tamoxifen but were not formally diagnosed) make the conclusion that endometrial cancer risk is increased in truly premenopausal women somewhat specious. In a Committee Opinion (last endorsed in 2020), the American College of Obstetricians and Gynecologists (ACOG) stated the following: “Postmenopausal women taking tamoxifen should be closely monitored for symptoms of endometrial hyperplasia or cancer. Premenopausal women treated with tamoxifen have no known increased risk of uterine cancer and as such require no additional monitoring beyond routine gynecologic care.”12 Based on multiple previously published studies with solid level 1 evidence and the challenges with the current study design, we continue to agree with this ACOG statement.
VERSHA PLEASANT, MD, MPH; MARK D. PEARLMAN, MD
Ryu KJ, Kim MS, Lee JY, et al. Risk of endometrial polyps, hyperplasia, carcinoma, and uterine cancer after tamoxifen treatment in premenopausal women with breast cancer. JAMA Netw Open. 2022;5:e2243951.
EXPERT COMMENTARY
Tamoxifen is a selective estrogen receptor modulator (SERM) approved by the US Food and Drug Administration (FDA) for both adjuvant treatment of invasive or metastatic breast cancer with hormone receptor (HR)–positive tumors (duration, 5 to 10 years) and for reduction of future breast cancers in certain high-risk individuals (duration, 5 years). It is also occasionally used for non-FDA approved indications, such as cyclic mastodynia.
Because breast cancer is among the most frequently diagnosed cancers in the United States (297,790 new cases expected in 2023) and approximately 80% are HR-positive tumors that will require hormonal adjuvant therapy,1 physicians and other gynecologic clinicians should have a working understanding of tamoxifen, including the risks and benefits associated with its use. Among the recognized serious adverse effects of tamoxifen is the increased risk of endometrial cancer in menopausal patients. This adverse effect creates a potential conundrum for clinicians who may be managing patients with tamoxifen to treat or prevent breast cancer, while also increasing the risk of another cancer. Prior prospective studies of tamoxifen have demonstrated a statistically and clinically significant increased risk of endometrial cancer in menopausal patients but not in premenopausal patients.
A recent study challenged those previous findings, suggesting that the risk of endometrial cancer is similar in both premenopausal and postmenopausal patients taking tamoxifen for treatment of breast cancer.2
Details of the study
The study by Ryu and colleagues used data from the Korean National Health Insurance Service, which covers 97% of the Korean population.2 The authors selected patients being treated for invasive breast cancer from January 1, 2003, through December 31, 2018, who were between the ages of 20 and 50 years when the breast cancer diagnosis was first made. Patients with a diagnostic code entered into their electronic health record that was consistent with menopausal status were excluded, along with any patients with a current or prior history of aromatase inhibitor use (for which one must be naturally, medically, or surgically menopausal to use). Based on these exclusions, the study cohort was then assumed to be premenopausal.
The study group included patients diagnosed with invasive breast cancer who were treated with adjuvant hormonal therapy with tamoxifen (n = 34,637), and the control group included patients with invasive breast cancer who were not treated with adjuvant hormonal therapy (n = 43,683). The primary study end point was the finding of endometrial or uterine pathology, including endometrial polyps, endometrial hyperplasia, endometrial cancer, and other uterine malignant neoplasms not originating in the endometrium (for example, uterine sarcomas).
Because this was a retrospective cohort study that included all eligible patients, the 2 groups were not matched. The treatment group was statistically older, had a higher body mass index (BMI) and a larger waist circumference, were more likely to be hypertensive, and included more patients with diabetes than the control group—all known risk factors for endometrial cancer. However, after adjusting for these 4 factors, an increased risk of endometrial cancer remained in the tamoxifen group compared with the control group (hazard ratio [HR], 3.77; 95% confidence interval [CI], 3.04–4.66). In addition, tamoxifen use was independently associated with an increased risk of endometrial polyps (HR, 3.90; 95% CI, 3.65–4.16), endometrial hyperplasia (HR, 5.56; 95% CI, 5.06–6.12), and other uterine cancers (HR, 2.27; 95% CI, 1.54–3.33). In a subgroup analysis, the risk for endometrial cancer was not higher in patients treated for more than 5 years of tamoxifen compared with those treated for 5 years or less.
Study strengths and limitations
A major strength of this study was the large number of study participants (n = 34,637 tamoxifen; n = 43,683 control), the long duration of follow-up (up to 15 years), and use of a single source of data with coverage of nearly the entire population of Korea. While the 2 study populations (tamoxifen vs no tamoxifen) were initially unbalanced in terms of endometrial cancer risk (age, BMI, concurrent diagnoses of hypertension and diabetes), the authors corrected for this with a multivariate analysis.
Furthermore, while the likely homogeneity of the study population may not make the results generalizable, the authors noted that Korean patients have a higher tendency toward early-onset breast cancer. This observation could make this cohort better suited for a study on premenopausal effects of tamoxifen.
Limitations. These data are provocative as they conflict with level 1 evidence based on multiple well-designed, double-blind, placebo-controlled randomized trials in which tamoxifen use for 5 years did not demonstrate a statistically increased risk of endometrial cancer in patients younger than age 50.3-5 Because of the importance of the question and the implications for many premenopausal women being treated with tamoxifen, we carefully evaluated the study methodology to better understand this discrepancy.
Continue to: Methodological concerns...
Methodological concerns
In the study by Ryu and colleagues, we found the definition of premenopausal to be problematic. Ultimately, if patients did not have a diagnosis of menopause in the problem summary list, they were assumed to be premenopausal if they were between the ages of 20 and 50 and not taking an aromatase inhibitor. However, important considerations in this population include the cancer stage and treatment regimens that can and do directly impact menopausal status.
Data demonstrate that early-onset breast cancer tends to be associated with more biologically aggressive characteristics that frequently require adjuvant or neoadjuvant chemotherapy.6,7 This chemotherapy regimen is comprised most commonly of Adriamycin (doxorubicin), paclitaxel, and cyclophosphamide. Cyclophosphamide is an alkylating agent that is a known gonadotoxin, and it often renders patients either temporarily or permanently menopausal due to chemotherapy-induced ovarian failure. Prior studies have demonstrated that for patients in their 40s, approximately 90% of those treated with cyclophosphamide-containing chemo-therapy for breast cancer will experience chemotherapy-induced amenorrhea (CIA).8 Although some patients in their 40s with CIA will resume ovarian function, the majority will not.8,9
Due to the lack of reliability in diagnosing CIA, blood levels of estradiol and follicle stimulating hormone are often necessary for confirmation and, even so, may be only temporary. One prospective analysis of 4 randomized neoadjuvant/adjuvant breast cancer trials used this approach and demonstrated that 85.1% of the study cohort experienced chemotherapy-induced ovarian failure at the end of their treatment, with some fluctuating back to premenopausal hormonal levels at 6 and 12 months.10
Furthermore, in the study by Ryu and colleagues, there is no description or confirmation of menstrual patterns in the study group to support the diagnosis of ongoing premenopausal status. Data on CIA and loss of ovarian function, therefore, are critical to the accurate categorization of patients as premenopausal or menopausal in this study. The study also relied on consistent and accurate recording of appropriate medical codes to capture a patient’s menopausal status, which is unclear for this particular population and health system.
In evaluating prior research, multiple studies demonstrated no increased risk of endometrial cancer in premenopausal women taking tamoxifen for breast cancer prevention (TABLE).3,5 These breast cancer prevention trials have several major advantages in assessing tamoxifen-associated endometrial cancer risk for premenopausal patients compared with the current study:
- Both studies were prospective double-blind, placebo-controlled randomized clinical breast cancer prevention trials with carefully designed and measured outcomes.
- Since these were breast cancer prevention trials, administration of gonadotoxic chemotherapy was not a concern. As a result, miscategorizing patients with chemotherapy-induced menopause as premenopausal would not be expected, and premature menopause would not be expected at a higher rate than the general population.
- Careful histories were required prior to study entry and throughout the study, including data on menopausal status and menstrual and uterine bleeding histories.11

In these prevention trials, the effect of tamoxifen on uterine pathology demonstratedrepeatable evidence that there was a statistically significant increased risk of endometrial cancer in postmenopausal women, but there was no similar increased risk of endometrial cancer in premenopausal women (TABLE).3,5 Interestingly, the magnitude of the endometrial cancer risk found in the premenopausal patients in the study by Ryu and colleagues (RR, 3.77) is comparable to that of the menopausal group in the prevention trials, raising concern that many or most of the patients in the treatment group assumed to be premenopausal may have indeed been “menopausal” for some or all the time they were taking tamoxifen due to the possible aforementioned reasons. ●
While the data from the study by Ryu and colleagues are provocative, the findings that premenopausal women are at an increased risk of endometrial cancer do not agree with those of well-designed previous trials. Our concerns about categorization bias (that is, women in the treatment group may have been menopausal for some or all the time they were taking tamoxifen but were not formally diagnosed) make the conclusion that endometrial cancer risk is increased in truly premenopausal women somewhat specious. In a Committee Opinion (last endorsed in 2020), the American College of Obstetricians and Gynecologists (ACOG) stated the following: “Postmenopausal women taking tamoxifen should be closely monitored for symptoms of endometrial hyperplasia or cancer. Premenopausal women treated with tamoxifen have no known increased risk of uterine cancer and as such require no additional monitoring beyond routine gynecologic care.”12 Based on multiple previously published studies with solid level 1 evidence and the challenges with the current study design, we continue to agree with this ACOG statement.
VERSHA PLEASANT, MD, MPH; MARK D. PEARLMAN, MD
- Siegel RL, Miller KD, Wagle NS, et al. Cancer statistics, 2023. CA Cancer J Clin. 2023;73:17-48.
- Ryu KJ, Kim MS, Lee JY, et al. Risk of endometrial polyps, hyperplasia, carcinoma, and uterine cancer after tamoxifen treatment in premenopausal women with breast cancer. JAMA Netw Open. 2022;5:e2243951-e.
- Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998;90:1371-1388.
- Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for the prevention of breast cancer: current status of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 2005;97:1652-1662.
- Iqbal J, Ginsburg OM, Wijeratne TD, et al. Endometrial cancer and venous thromboembolism in women under age 50 who take tamoxifen for prevention of breast cancer: a systematic review. Cancer Treat Rev. 2012;38:318-328.
- Kumar R, Abreu C, Toi M, et al. Oncobiology and treatment of breast cancer in young women. Cancer Metastasis Rev. 2022;41:749-770.
- Tesch ME, Partidge AH. Treatment of breast cancer in young adults. Am Soc Clin Oncol Educ Book. 2022;42:1-12.
- Han HS, Ro J, Lee KS, et al. Analysis of chemotherapy-induced amenorrhea rates by three different anthracycline and taxane containing regimens for early breast cancer. Breast Cancer Res Treat. 2009;115:335-342.
- Henry NL, Xia R, Banerjee M, et al. Predictors of recovery of ovarian function during aromatase inhibitor therapy. Ann Oncol. 2013;24:2011-2016.
- Furlanetto J, Marme F, Seiler S, et al. Chemotherapy-induced ovarian failure in young women with early breast cancer: prospective analysis of four randomised neoadjuvant/ adjuvant breast cancer trials. Eur J Cancer. 2021;152: 193-203.
- Runowicz CD, Costantino JP, Wickerham DL, et al. Gynecologic conditions in participants in the NSABP breast cancer prevention study of tamoxifen and raloxifene (STAR). Am J Obstet Gynecol. 2011;205:535.e1-535.e5.
- American College of Obstetricians and Gynecologists. Committee opinion no. 601: tamoxifen and uterine cancer. Obstet Gynecol. 2014;123:1394-1397.
- Siegel RL, Miller KD, Wagle NS, et al. Cancer statistics, 2023. CA Cancer J Clin. 2023;73:17-48.
- Ryu KJ, Kim MS, Lee JY, et al. Risk of endometrial polyps, hyperplasia, carcinoma, and uterine cancer after tamoxifen treatment in premenopausal women with breast cancer. JAMA Netw Open. 2022;5:e2243951-e.
- Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998;90:1371-1388.
- Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for the prevention of breast cancer: current status of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 2005;97:1652-1662.
- Iqbal J, Ginsburg OM, Wijeratne TD, et al. Endometrial cancer and venous thromboembolism in women under age 50 who take tamoxifen for prevention of breast cancer: a systematic review. Cancer Treat Rev. 2012;38:318-328.
- Kumar R, Abreu C, Toi M, et al. Oncobiology and treatment of breast cancer in young women. Cancer Metastasis Rev. 2022;41:749-770.
- Tesch ME, Partidge AH. Treatment of breast cancer in young adults. Am Soc Clin Oncol Educ Book. 2022;42:1-12.
- Han HS, Ro J, Lee KS, et al. Analysis of chemotherapy-induced amenorrhea rates by three different anthracycline and taxane containing regimens for early breast cancer. Breast Cancer Res Treat. 2009;115:335-342.
- Henry NL, Xia R, Banerjee M, et al. Predictors of recovery of ovarian function during aromatase inhibitor therapy. Ann Oncol. 2013;24:2011-2016.
- Furlanetto J, Marme F, Seiler S, et al. Chemotherapy-induced ovarian failure in young women with early breast cancer: prospective analysis of four randomised neoadjuvant/ adjuvant breast cancer trials. Eur J Cancer. 2021;152: 193-203.
- Runowicz CD, Costantino JP, Wickerham DL, et al. Gynecologic conditions in participants in the NSABP breast cancer prevention study of tamoxifen and raloxifene (STAR). Am J Obstet Gynecol. 2011;205:535.e1-535.e5.
- American College of Obstetricians and Gynecologists. Committee opinion no. 601: tamoxifen and uterine cancer. Obstet Gynecol. 2014;123:1394-1397.
We asked doctors using AI scribes: Just how good are they?
Andrea Partida, DO, an obstetrician and gynecologist in Enid, Okla., loves her new assistant.
The 15 or 20 minutes she used to spend on documentation for each patient visit is now 3. The 2-3 hours she’d spend charting outside clinic hours is maybe 1.
All that time saved allows her to see two to five more patients a day, provide better care to each patient, and get more involved in hospital leadership at Integris Health, where she works.
“I have a better work-life balance with my family,” Dr. Partida said. “I leave work at work and get home earlier.”
You’ve probably figured out the plot twist: Dr. Partida’s assistant is not a person – it’s artificial intelligence (AI).
Dr. Partida uses IRIS, a tool from OnPoint Healthcare Partners, part of a fast-growing niche of AI medical scribes designed to automate onerous data entry. The evolution of generative AI – specifically, large language models, such as ChatGPT – has led to a rapid explosion of these tools. Other companies in the space include Abridge, Ambience Healthcare, Augmedix, DeepScribe, Nuance (part of Microsoft), and Suki. The newest kid on the block, Amazon Web Services, announced the launch of HealthScribe in July.
These tools – some of which are already on the market, with more on the way – record patient visits and generate notes for treatment and billing. Earlier iterations combine AI with offsite human scribes who provide quality control. But more and more are fully automated, no human required. Some also offer video recording and foreign language translation.
The promise is alluring: Ease your workload and reclaim hours in your day so you can spend more time with patients or try that “work-life balance” thing you’ve heard so much about.
But do these tools fulfill that promise?
According to Dr. Partida and other doctors who spoke with this news organization, the answer is a resounding yes.
A tech solution for a tech problem
“I believe a lot of doctors see patients for free. They get paid to do paperwork,” said Anthony J. Mazzarelli, MD, JD, MBE, co-president and CEO of Cooper University Health Care, in Camden, N.J.
Indeed, for every hour U.S. clinicians spend with their patients, they may spend 2 more hours documenting in electronic health records (EHRs), estimates show. About half of doctors, especially those in primary care, report feeling burned out, and some 42% say they want to quit clinical practice.
Enter AI scribes.
“The holy grail in medicine right now is improving burnout while also maintaining or improving productivity and quality,” said Patricia Garcia, MD, associate clinical information officer for ambulatory care at Stanford (Calif.) Health Care. “These ambient digital scribes have the potential to do just that.”
While anyone can buy these products, their use has been mostly limited to pilot programs and early adopters so far, said Dr. Garcia, who has been helping to pilot Nuance’s digital scribe, DAX, at Stanford.
But that’s expected to change quickly. “I don’t think the time horizon is a decade,” Dr. Garcia said. “I think within a matter of 2 or 3 years, these tools will be pervasive throughout health care.”
Since introducing these tools at Cooper, “our doctors’ paperwork burden is significantly lighter,” said Dr. Mazzarelli, who decides which technologies Cooper should invest in and who monitors their results. In Cooper studies, physicians who used DAX more than half the time spent 43% less time working on notes.
“They spend more time connecting with their patients, talking with them, and looking them in the eye,” Dr. Mazzarelli said. That, in turn, seems to improve patient outcomes, reduce doctor burnout and turnover, and lower costs.
The AI scribes, by virtue of eliminating the distraction of note taking, also allow doctors to give their full attention to the patient. “The patient relationship is the most important aspect of medicine,” said Raul Ayala, MD, MHCM, a family medicine physician at Adventist Health, in Hanford, Calif., who uses Augmedix. The digital scribe “helps us strengthen that relationship.”
What’s it like to use an AI medical scribe?
The scribes feature hardware (typically a smartphone or tablet) and software built on automatic speech recognition, natural language processing, and machine learning. Download an app to your device, and you’re ready to go. Use it to record in-person or telehealth visits.
In the first week, a company may help train you to use the hardware and software. You’ll likely start by using it for a few patient visits per day, ramping up gradually. Dr. Partida said she was comfortable using the system for all her patients in 6 weeks.
Each day, Dr. Partida logs in to a dedicated smartphone or tablet, opens the app, and reviews her schedule, including details she needs to prepare for each patient.
At the start of each patient visit, Dr. Partida taps the app icon to begin recording and lays the device nearby. She can pause as needed. At the end of the visit, she taps the icon again to stop recording.
The AI listens, creates the note, and updates relevant data in the EHR. The note includes patient problems, assessment, treatment plan, patient history, orders, and tasks for staff, along with medications, referrals, and preauthorizations. A human scribe, who is also a physician, reviews the information for accuracy and edits it as needed. By the next morning, the data are ready for Dr. Partida to review.
Fully automated versions can generate notes much faster. Jack Shilling, MD, MBA, an orthopedic surgeon at Cooper University Health Care, in Voorhees, N.J., uses DAX. A new feature called DAX Express – which uses OpenAI’s GPT-4 but no humans – provides him with a draft of his clinical notes in just seconds.
How accurate are AI notes?
The accuracy of those notes remains an open question, Dr. Garcia said – mostly because accuracy can be hard to define.
“If you asked five docs to write a note based on the same patient encounter, you’d get five different notes,” Dr. Garcia said. “That makes it hard to assess these technologies in a scientifically rigorous way.”
Still, the onus is on the physician to review the notes and edit them as needed, Dr. Garcia said. How light or heavy those edits are can depend on your unique preferences.
Dr. Shilling said he may need to lightly edit transcripts of his conversations with patients. “When someone tells me how long their knee hurts, slight variability in their transcribed words is tolerable,” he said. But for some things – such as physical exam notes and x-ray readings – he dictates directly into the device, speaking at a closer range and being less conversational, more exact in his speech.
Should you let patients know they’re being recorded?
The federal Health Insurance Portability and Accountability Act (HIPAA) does not require providers to inform patients that their face-to-face conversations are being recorded, said Daniel Lebovic, JD, corporate legal counsel at Compliancy Group, in Greenlawn, N.Y., a company that helps providers adhere to HIPAA rules.
But make sure you know the laws in your state and the policies at your health care practice. State laws may require providers to inform patients and to get patients’ consent in advance of being recorded.
All the doctors who spoke to this news organization said their patients are informed that they’ll be recorded and that they can opt out if they wish.
How much do AI scribes cost?
As the marketplace for these tools expands, companies are offering more products and services at different price points that target a range of organizations, from large health care systems to small private practices.
Price models vary, said Dr. Garcia. Some are based on the number of users, others on the number of notes, and still others on minutes.
Amazon’s HealthScribe is priced at 10 cents per minute. For 1,000 consultation transcripts per month, with each call averaging 15 minutes, it would take 15,000 minutes at a total cost of $1,500 for the month.
In general, the rapidly growing competition in this space could mean prices become more affordable, Dr. Garcia said. “It’s good that so many are getting into this game, because that means the price will come down and it will be a lot more accessible to everybody.”
A version of this article appeared on Medscape.com.
Andrea Partida, DO, an obstetrician and gynecologist in Enid, Okla., loves her new assistant.
The 15 or 20 minutes she used to spend on documentation for each patient visit is now 3. The 2-3 hours she’d spend charting outside clinic hours is maybe 1.
All that time saved allows her to see two to five more patients a day, provide better care to each patient, and get more involved in hospital leadership at Integris Health, where she works.
“I have a better work-life balance with my family,” Dr. Partida said. “I leave work at work and get home earlier.”
You’ve probably figured out the plot twist: Dr. Partida’s assistant is not a person – it’s artificial intelligence (AI).
Dr. Partida uses IRIS, a tool from OnPoint Healthcare Partners, part of a fast-growing niche of AI medical scribes designed to automate onerous data entry. The evolution of generative AI – specifically, large language models, such as ChatGPT – has led to a rapid explosion of these tools. Other companies in the space include Abridge, Ambience Healthcare, Augmedix, DeepScribe, Nuance (part of Microsoft), and Suki. The newest kid on the block, Amazon Web Services, announced the launch of HealthScribe in July.
These tools – some of which are already on the market, with more on the way – record patient visits and generate notes for treatment and billing. Earlier iterations combine AI with offsite human scribes who provide quality control. But more and more are fully automated, no human required. Some also offer video recording and foreign language translation.
The promise is alluring: Ease your workload and reclaim hours in your day so you can spend more time with patients or try that “work-life balance” thing you’ve heard so much about.
But do these tools fulfill that promise?
According to Dr. Partida and other doctors who spoke with this news organization, the answer is a resounding yes.
A tech solution for a tech problem
“I believe a lot of doctors see patients for free. They get paid to do paperwork,” said Anthony J. Mazzarelli, MD, JD, MBE, co-president and CEO of Cooper University Health Care, in Camden, N.J.
Indeed, for every hour U.S. clinicians spend with their patients, they may spend 2 more hours documenting in electronic health records (EHRs), estimates show. About half of doctors, especially those in primary care, report feeling burned out, and some 42% say they want to quit clinical practice.
Enter AI scribes.
“The holy grail in medicine right now is improving burnout while also maintaining or improving productivity and quality,” said Patricia Garcia, MD, associate clinical information officer for ambulatory care at Stanford (Calif.) Health Care. “These ambient digital scribes have the potential to do just that.”
While anyone can buy these products, their use has been mostly limited to pilot programs and early adopters so far, said Dr. Garcia, who has been helping to pilot Nuance’s digital scribe, DAX, at Stanford.
But that’s expected to change quickly. “I don’t think the time horizon is a decade,” Dr. Garcia said. “I think within a matter of 2 or 3 years, these tools will be pervasive throughout health care.”
Since introducing these tools at Cooper, “our doctors’ paperwork burden is significantly lighter,” said Dr. Mazzarelli, who decides which technologies Cooper should invest in and who monitors their results. In Cooper studies, physicians who used DAX more than half the time spent 43% less time working on notes.
“They spend more time connecting with their patients, talking with them, and looking them in the eye,” Dr. Mazzarelli said. That, in turn, seems to improve patient outcomes, reduce doctor burnout and turnover, and lower costs.
The AI scribes, by virtue of eliminating the distraction of note taking, also allow doctors to give their full attention to the patient. “The patient relationship is the most important aspect of medicine,” said Raul Ayala, MD, MHCM, a family medicine physician at Adventist Health, in Hanford, Calif., who uses Augmedix. The digital scribe “helps us strengthen that relationship.”
What’s it like to use an AI medical scribe?
The scribes feature hardware (typically a smartphone or tablet) and software built on automatic speech recognition, natural language processing, and machine learning. Download an app to your device, and you’re ready to go. Use it to record in-person or telehealth visits.
In the first week, a company may help train you to use the hardware and software. You’ll likely start by using it for a few patient visits per day, ramping up gradually. Dr. Partida said she was comfortable using the system for all her patients in 6 weeks.
Each day, Dr. Partida logs in to a dedicated smartphone or tablet, opens the app, and reviews her schedule, including details she needs to prepare for each patient.
At the start of each patient visit, Dr. Partida taps the app icon to begin recording and lays the device nearby. She can pause as needed. At the end of the visit, she taps the icon again to stop recording.
The AI listens, creates the note, and updates relevant data in the EHR. The note includes patient problems, assessment, treatment plan, patient history, orders, and tasks for staff, along with medications, referrals, and preauthorizations. A human scribe, who is also a physician, reviews the information for accuracy and edits it as needed. By the next morning, the data are ready for Dr. Partida to review.
Fully automated versions can generate notes much faster. Jack Shilling, MD, MBA, an orthopedic surgeon at Cooper University Health Care, in Voorhees, N.J., uses DAX. A new feature called DAX Express – which uses OpenAI’s GPT-4 but no humans – provides him with a draft of his clinical notes in just seconds.
How accurate are AI notes?
The accuracy of those notes remains an open question, Dr. Garcia said – mostly because accuracy can be hard to define.
“If you asked five docs to write a note based on the same patient encounter, you’d get five different notes,” Dr. Garcia said. “That makes it hard to assess these technologies in a scientifically rigorous way.”
Still, the onus is on the physician to review the notes and edit them as needed, Dr. Garcia said. How light or heavy those edits are can depend on your unique preferences.
Dr. Shilling said he may need to lightly edit transcripts of his conversations with patients. “When someone tells me how long their knee hurts, slight variability in their transcribed words is tolerable,” he said. But for some things – such as physical exam notes and x-ray readings – he dictates directly into the device, speaking at a closer range and being less conversational, more exact in his speech.
Should you let patients know they’re being recorded?
The federal Health Insurance Portability and Accountability Act (HIPAA) does not require providers to inform patients that their face-to-face conversations are being recorded, said Daniel Lebovic, JD, corporate legal counsel at Compliancy Group, in Greenlawn, N.Y., a company that helps providers adhere to HIPAA rules.
But make sure you know the laws in your state and the policies at your health care practice. State laws may require providers to inform patients and to get patients’ consent in advance of being recorded.
All the doctors who spoke to this news organization said their patients are informed that they’ll be recorded and that they can opt out if they wish.
How much do AI scribes cost?
As the marketplace for these tools expands, companies are offering more products and services at different price points that target a range of organizations, from large health care systems to small private practices.
Price models vary, said Dr. Garcia. Some are based on the number of users, others on the number of notes, and still others on minutes.
Amazon’s HealthScribe is priced at 10 cents per minute. For 1,000 consultation transcripts per month, with each call averaging 15 minutes, it would take 15,000 minutes at a total cost of $1,500 for the month.
In general, the rapidly growing competition in this space could mean prices become more affordable, Dr. Garcia said. “It’s good that so many are getting into this game, because that means the price will come down and it will be a lot more accessible to everybody.”
A version of this article appeared on Medscape.com.
Andrea Partida, DO, an obstetrician and gynecologist in Enid, Okla., loves her new assistant.
The 15 or 20 minutes she used to spend on documentation for each patient visit is now 3. The 2-3 hours she’d spend charting outside clinic hours is maybe 1.
All that time saved allows her to see two to five more patients a day, provide better care to each patient, and get more involved in hospital leadership at Integris Health, where she works.
“I have a better work-life balance with my family,” Dr. Partida said. “I leave work at work and get home earlier.”
You’ve probably figured out the plot twist: Dr. Partida’s assistant is not a person – it’s artificial intelligence (AI).
Dr. Partida uses IRIS, a tool from OnPoint Healthcare Partners, part of a fast-growing niche of AI medical scribes designed to automate onerous data entry. The evolution of generative AI – specifically, large language models, such as ChatGPT – has led to a rapid explosion of these tools. Other companies in the space include Abridge, Ambience Healthcare, Augmedix, DeepScribe, Nuance (part of Microsoft), and Suki. The newest kid on the block, Amazon Web Services, announced the launch of HealthScribe in July.
These tools – some of which are already on the market, with more on the way – record patient visits and generate notes for treatment and billing. Earlier iterations combine AI with offsite human scribes who provide quality control. But more and more are fully automated, no human required. Some also offer video recording and foreign language translation.
The promise is alluring: Ease your workload and reclaim hours in your day so you can spend more time with patients or try that “work-life balance” thing you’ve heard so much about.
But do these tools fulfill that promise?
According to Dr. Partida and other doctors who spoke with this news organization, the answer is a resounding yes.
A tech solution for a tech problem
“I believe a lot of doctors see patients for free. They get paid to do paperwork,” said Anthony J. Mazzarelli, MD, JD, MBE, co-president and CEO of Cooper University Health Care, in Camden, N.J.
Indeed, for every hour U.S. clinicians spend with their patients, they may spend 2 more hours documenting in electronic health records (EHRs), estimates show. About half of doctors, especially those in primary care, report feeling burned out, and some 42% say they want to quit clinical practice.
Enter AI scribes.
“The holy grail in medicine right now is improving burnout while also maintaining or improving productivity and quality,” said Patricia Garcia, MD, associate clinical information officer for ambulatory care at Stanford (Calif.) Health Care. “These ambient digital scribes have the potential to do just that.”
While anyone can buy these products, their use has been mostly limited to pilot programs and early adopters so far, said Dr. Garcia, who has been helping to pilot Nuance’s digital scribe, DAX, at Stanford.
But that’s expected to change quickly. “I don’t think the time horizon is a decade,” Dr. Garcia said. “I think within a matter of 2 or 3 years, these tools will be pervasive throughout health care.”
Since introducing these tools at Cooper, “our doctors’ paperwork burden is significantly lighter,” said Dr. Mazzarelli, who decides which technologies Cooper should invest in and who monitors their results. In Cooper studies, physicians who used DAX more than half the time spent 43% less time working on notes.
“They spend more time connecting with their patients, talking with them, and looking them in the eye,” Dr. Mazzarelli said. That, in turn, seems to improve patient outcomes, reduce doctor burnout and turnover, and lower costs.
The AI scribes, by virtue of eliminating the distraction of note taking, also allow doctors to give their full attention to the patient. “The patient relationship is the most important aspect of medicine,” said Raul Ayala, MD, MHCM, a family medicine physician at Adventist Health, in Hanford, Calif., who uses Augmedix. The digital scribe “helps us strengthen that relationship.”
What’s it like to use an AI medical scribe?
The scribes feature hardware (typically a smartphone or tablet) and software built on automatic speech recognition, natural language processing, and machine learning. Download an app to your device, and you’re ready to go. Use it to record in-person or telehealth visits.
In the first week, a company may help train you to use the hardware and software. You’ll likely start by using it for a few patient visits per day, ramping up gradually. Dr. Partida said she was comfortable using the system for all her patients in 6 weeks.
Each day, Dr. Partida logs in to a dedicated smartphone or tablet, opens the app, and reviews her schedule, including details she needs to prepare for each patient.
At the start of each patient visit, Dr. Partida taps the app icon to begin recording and lays the device nearby. She can pause as needed. At the end of the visit, she taps the icon again to stop recording.
The AI listens, creates the note, and updates relevant data in the EHR. The note includes patient problems, assessment, treatment plan, patient history, orders, and tasks for staff, along with medications, referrals, and preauthorizations. A human scribe, who is also a physician, reviews the information for accuracy and edits it as needed. By the next morning, the data are ready for Dr. Partida to review.
Fully automated versions can generate notes much faster. Jack Shilling, MD, MBA, an orthopedic surgeon at Cooper University Health Care, in Voorhees, N.J., uses DAX. A new feature called DAX Express – which uses OpenAI’s GPT-4 but no humans – provides him with a draft of his clinical notes in just seconds.
How accurate are AI notes?
The accuracy of those notes remains an open question, Dr. Garcia said – mostly because accuracy can be hard to define.
“If you asked five docs to write a note based on the same patient encounter, you’d get five different notes,” Dr. Garcia said. “That makes it hard to assess these technologies in a scientifically rigorous way.”
Still, the onus is on the physician to review the notes and edit them as needed, Dr. Garcia said. How light or heavy those edits are can depend on your unique preferences.
Dr. Shilling said he may need to lightly edit transcripts of his conversations with patients. “When someone tells me how long their knee hurts, slight variability in their transcribed words is tolerable,” he said. But for some things – such as physical exam notes and x-ray readings – he dictates directly into the device, speaking at a closer range and being less conversational, more exact in his speech.
Should you let patients know they’re being recorded?
The federal Health Insurance Portability and Accountability Act (HIPAA) does not require providers to inform patients that their face-to-face conversations are being recorded, said Daniel Lebovic, JD, corporate legal counsel at Compliancy Group, in Greenlawn, N.Y., a company that helps providers adhere to HIPAA rules.
But make sure you know the laws in your state and the policies at your health care practice. State laws may require providers to inform patients and to get patients’ consent in advance of being recorded.
All the doctors who spoke to this news organization said their patients are informed that they’ll be recorded and that they can opt out if they wish.
How much do AI scribes cost?
As the marketplace for these tools expands, companies are offering more products and services at different price points that target a range of organizations, from large health care systems to small private practices.
Price models vary, said Dr. Garcia. Some are based on the number of users, others on the number of notes, and still others on minutes.
Amazon’s HealthScribe is priced at 10 cents per minute. For 1,000 consultation transcripts per month, with each call averaging 15 minutes, it would take 15,000 minutes at a total cost of $1,500 for the month.
In general, the rapidly growing competition in this space could mean prices become more affordable, Dr. Garcia said. “It’s good that so many are getting into this game, because that means the price will come down and it will be a lot more accessible to everybody.”
A version of this article appeared on Medscape.com.
U.S. has new dominant COVID variant called EG.5
Called “Eris” among avid COVID trackers, the strain EG.5 now accounts for 17% of all U.S. COVID infections, according to the latest Centers for Disease Control and Prevention estimates. That’s up from 12% the week prior.
EG.5 has been rising worldwide, just weeks after the World Health Organization added the strain to its official monitoring list. In the United Kingdom, it now accounts for 1 in 10 COVID cases, The Independent reported.
EG.5 is a descendant of the XBB strains that have dominated tracking lists in recent months. It has the same makeup as XBB.1.9.2 but carries an extra spike mutation, according to a summary published by the Center for Infectious Disease Research and Policy at the University of Minnesota. The spike protein is the part of the virus that allows it to enter human cells. But there’s no indication so far that EG.5 is more contagious or severe than other recent variants, according to the CIDRAP summary and a recent podcast from the American Medical Association. The CDC said that current vaccines protect against the variant.
U.S. hospitals saw a 12% increase in COVID admissions during the week ending on July 22, with 8,047 people being admitted because of the virus, up from an all-time low of 6,306 the week of June 24. In 17 states, the past-week increase in hospitalizations was 20% or greater. In Minnesota, the rate jumped by 50%, and in West Virginia, it jumped by 63%. Meanwhile, deaths reached their lowest weekly rate ever for the week of data ending July 29, with just 176 deaths reported by the CDC.
A version of this article first appeared on WebMD.com.
Called “Eris” among avid COVID trackers, the strain EG.5 now accounts for 17% of all U.S. COVID infections, according to the latest Centers for Disease Control and Prevention estimates. That’s up from 12% the week prior.
EG.5 has been rising worldwide, just weeks after the World Health Organization added the strain to its official monitoring list. In the United Kingdom, it now accounts for 1 in 10 COVID cases, The Independent reported.
EG.5 is a descendant of the XBB strains that have dominated tracking lists in recent months. It has the same makeup as XBB.1.9.2 but carries an extra spike mutation, according to a summary published by the Center for Infectious Disease Research and Policy at the University of Minnesota. The spike protein is the part of the virus that allows it to enter human cells. But there’s no indication so far that EG.5 is more contagious or severe than other recent variants, according to the CIDRAP summary and a recent podcast from the American Medical Association. The CDC said that current vaccines protect against the variant.
U.S. hospitals saw a 12% increase in COVID admissions during the week ending on July 22, with 8,047 people being admitted because of the virus, up from an all-time low of 6,306 the week of June 24. In 17 states, the past-week increase in hospitalizations was 20% or greater. In Minnesota, the rate jumped by 50%, and in West Virginia, it jumped by 63%. Meanwhile, deaths reached their lowest weekly rate ever for the week of data ending July 29, with just 176 deaths reported by the CDC.
A version of this article first appeared on WebMD.com.
Called “Eris” among avid COVID trackers, the strain EG.5 now accounts for 17% of all U.S. COVID infections, according to the latest Centers for Disease Control and Prevention estimates. That’s up from 12% the week prior.
EG.5 has been rising worldwide, just weeks after the World Health Organization added the strain to its official monitoring list. In the United Kingdom, it now accounts for 1 in 10 COVID cases, The Independent reported.
EG.5 is a descendant of the XBB strains that have dominated tracking lists in recent months. It has the same makeup as XBB.1.9.2 but carries an extra spike mutation, according to a summary published by the Center for Infectious Disease Research and Policy at the University of Minnesota. The spike protein is the part of the virus that allows it to enter human cells. But there’s no indication so far that EG.5 is more contagious or severe than other recent variants, according to the CIDRAP summary and a recent podcast from the American Medical Association. The CDC said that current vaccines protect against the variant.
U.S. hospitals saw a 12% increase in COVID admissions during the week ending on July 22, with 8,047 people being admitted because of the virus, up from an all-time low of 6,306 the week of June 24. In 17 states, the past-week increase in hospitalizations was 20% or greater. In Minnesota, the rate jumped by 50%, and in West Virginia, it jumped by 63%. Meanwhile, deaths reached their lowest weekly rate ever for the week of data ending July 29, with just 176 deaths reported by the CDC.
A version of this article first appeared on WebMD.com.
Unveiling the potential of prediction models in obstetrics
In the dawn of artificial intelligence’s potential to inform clinical practice, the importance of understanding the intent and interpretation of prediction tools is vital. In medicine, informed decision-making promotes patient autonomy and can lead to improved patient satisfaction and engagement in their own care.
This shared understanding enables patients to make informed choices about their care, reducing anxiety and increasing confidence in medical decision-making.
In obstetric clinical practice, prediction tools have been created to assess risk of primary cesarean delivery in gestational diabetes,1 cesarean delivery in hypertensive disorders of pregnancy,2 and failed induction of labor in nulliparous patients with an unfavorable cervix.3 By assessing a patient’s risk profile, clinicians can identify high-risk individuals who may require closer monitoring, early interventions, or specialized care. This allows for more timely interventions to optimize maternal and fetal health outcomes.
Other prediction tools are created to better elucidate to patients their individual risk of an outcome that may be modifiable, aiding physician counseling on mitigating factors to improve overall results. A relevant example is the American Diabetes Association’s risk of type 2 diabetes calculator used for counseling patients on risk reduction. This model includes both preexisting (ethnicity, family history, age, sex assigned at birth) and modifiable risk factors (body mass index, hypertension, physical activity) to predict risk of type 2 diabetes and is widely used in clinical practice to encourage integration of lifestyle changes to decrease risk.4 This model highlights the utility of prediction tools in counseling, providing quantitative data to clinicians to discuss a patient’s individual risk and how to mitigate that risk.
While predictive models clearly have many advantages and potential to improve personalized medicine, concerns have been raised that their interpretation and application can sometimes have unintended consequences as the complexity of these models can lead to variation in understanding among clinicians that impact decision-making. Different clinicians may assign different levels of importance to the predicted risks, resulting in differences in treatment plans and interventions. This variability can lead to disparities in care and outcomes, as patients with similar risk profiles may receive different management approaches based on the interpreting clinician.
Providers may either overly rely on prediction models or completely disregard them, depending on their level of trust or skepticism. Overreliance on prediction models may lead to the neglect of important clinical information or intuition, while disregarding the models may result in missed opportunities for early intervention or appropriate risk stratification. Achieving a balance between clinical judgment and the use of prediction models is crucial for optimal decision-making.
An example of how misinterpretation of the role of prediction tools in patient counseling can have far reaching consequences is the vaginal birth after cesarean (VBAC) calculator where race and ethnicity naturalized racial differences and likely contributed to cesarean overuse in Black pregnant people as non-White race was associated with a decreased chance of successful VBAC. Although the authors of the study that created the VBAC calculator intended it to be used as an adjunct to counseling, institutions and providers used low calculator scores to discourage or prohibit pregnant people from attempting a trial of labor after cesarean (TOLAC). This highlighted the importance of contextualizing the intent of prediction models within the broader clinical setting and individual patient circumstances and preferences.
This gap between intent and interpretation and subsequent application is influenced by individual clinician experience, training, personal biases, and subjective judgment. These subjective elements can introduce inconsistencies and variability in the utilization of prediction tools, leading to potential discrepancies in patient care. Inadequate understanding of prediction models and their statistical concepts can contribute to misinterpretation. It is this bias that prevents prediction models from serving their true purpose: to inform clinical decision-making, improve patient outcomes, and optimize resource allocation.
Clinicians may struggle with concepts such as predictive accuracy, overfitting, calibration, and external validation. Educational initiatives and enhanced training in statistical literacy can empower clinicians to better comprehend and apply prediction models in their practice. Researchers should make it clear that models should not be used in isolation, but rather integrated with clinical expertise and patient preferences. Understanding the limitations of prediction models and incorporating additional clinical information is essential.
Prediction models in obstetrics should undergo continuous evaluation and improvement to enhance their reliability and applicability. Regular updates, external validation, and recalibration are necessary to account for evolving clinical practices, changes in patient populations, and emerging evidence. Engaging clinicians in the evaluation process can foster ownership and promote a sense of trust in the models.
As machine learning and artificial intelligence improve the accuracy of prediction models, there is potential to revolutionize obstetric care by enabling more accurate individualized risk assessment and decision-making. Machine learning has the potential to significantly enhance prediction models in obstetrics by leveraging complex algorithms and advanced computational techniques. However, the unpredictable nature of clinician interpretation poses challenges to the effective utilization of these models.
By emphasizing communication, collaboration, education, and continuous evaluation, we can bridge the gap between prediction models and clinician interpretation that optimizes their use. This concerted effort will ultimately lead to improved patient care, enhanced clinical outcomes, and a more harmonious integration of these tools into obstetric practice.
Dr. Ramos is assistant professor of maternal fetal medicine and associate principal investigator at the Mother Infant Research Institute, Tufts University and Tufts Medical Center, Boston.
References
1. Ramos SZ et al. Predicting primary cesarean delivery in pregnancies complicated by gestational diabetes mellitus. Am J Obstet Gynecol. 2023 Jun 7;S0002-9378(23)00371-X. doi: 10.1016/j.ajog.2023.06.002.
2. Beninati MJ et al. Prediction model for vaginal birth after induction of labor in women with hypertensive disorders of pregnancy. Obstet Gynecol. 2020 Aug;136(2):402-410. doi: 10.1097/AOG.0000000000003938.
3. Levine LD et al. A validated calculator to estimate risk of cesarean after an induction of labor with an unfavorable cervix. Am J Obstet Gynecol. 2018 Feb;218(2):254.e1-254.e7. doi: 10.1016/j.ajog.2017.11.603.
4. American Diabetes Association. Our 60-Second Type 2 Diabetes Risk Test.
In the dawn of artificial intelligence’s potential to inform clinical practice, the importance of understanding the intent and interpretation of prediction tools is vital. In medicine, informed decision-making promotes patient autonomy and can lead to improved patient satisfaction and engagement in their own care.
This shared understanding enables patients to make informed choices about their care, reducing anxiety and increasing confidence in medical decision-making.
In obstetric clinical practice, prediction tools have been created to assess risk of primary cesarean delivery in gestational diabetes,1 cesarean delivery in hypertensive disorders of pregnancy,2 and failed induction of labor in nulliparous patients with an unfavorable cervix.3 By assessing a patient’s risk profile, clinicians can identify high-risk individuals who may require closer monitoring, early interventions, or specialized care. This allows for more timely interventions to optimize maternal and fetal health outcomes.
Other prediction tools are created to better elucidate to patients their individual risk of an outcome that may be modifiable, aiding physician counseling on mitigating factors to improve overall results. A relevant example is the American Diabetes Association’s risk of type 2 diabetes calculator used for counseling patients on risk reduction. This model includes both preexisting (ethnicity, family history, age, sex assigned at birth) and modifiable risk factors (body mass index, hypertension, physical activity) to predict risk of type 2 diabetes and is widely used in clinical practice to encourage integration of lifestyle changes to decrease risk.4 This model highlights the utility of prediction tools in counseling, providing quantitative data to clinicians to discuss a patient’s individual risk and how to mitigate that risk.
While predictive models clearly have many advantages and potential to improve personalized medicine, concerns have been raised that their interpretation and application can sometimes have unintended consequences as the complexity of these models can lead to variation in understanding among clinicians that impact decision-making. Different clinicians may assign different levels of importance to the predicted risks, resulting in differences in treatment plans and interventions. This variability can lead to disparities in care and outcomes, as patients with similar risk profiles may receive different management approaches based on the interpreting clinician.
Providers may either overly rely on prediction models or completely disregard them, depending on their level of trust or skepticism. Overreliance on prediction models may lead to the neglect of important clinical information or intuition, while disregarding the models may result in missed opportunities for early intervention or appropriate risk stratification. Achieving a balance between clinical judgment and the use of prediction models is crucial for optimal decision-making.
An example of how misinterpretation of the role of prediction tools in patient counseling can have far reaching consequences is the vaginal birth after cesarean (VBAC) calculator where race and ethnicity naturalized racial differences and likely contributed to cesarean overuse in Black pregnant people as non-White race was associated with a decreased chance of successful VBAC. Although the authors of the study that created the VBAC calculator intended it to be used as an adjunct to counseling, institutions and providers used low calculator scores to discourage or prohibit pregnant people from attempting a trial of labor after cesarean (TOLAC). This highlighted the importance of contextualizing the intent of prediction models within the broader clinical setting and individual patient circumstances and preferences.
This gap between intent and interpretation and subsequent application is influenced by individual clinician experience, training, personal biases, and subjective judgment. These subjective elements can introduce inconsistencies and variability in the utilization of prediction tools, leading to potential discrepancies in patient care. Inadequate understanding of prediction models and their statistical concepts can contribute to misinterpretation. It is this bias that prevents prediction models from serving their true purpose: to inform clinical decision-making, improve patient outcomes, and optimize resource allocation.
Clinicians may struggle with concepts such as predictive accuracy, overfitting, calibration, and external validation. Educational initiatives and enhanced training in statistical literacy can empower clinicians to better comprehend and apply prediction models in their practice. Researchers should make it clear that models should not be used in isolation, but rather integrated with clinical expertise and patient preferences. Understanding the limitations of prediction models and incorporating additional clinical information is essential.
Prediction models in obstetrics should undergo continuous evaluation and improvement to enhance their reliability and applicability. Regular updates, external validation, and recalibration are necessary to account for evolving clinical practices, changes in patient populations, and emerging evidence. Engaging clinicians in the evaluation process can foster ownership and promote a sense of trust in the models.
As machine learning and artificial intelligence improve the accuracy of prediction models, there is potential to revolutionize obstetric care by enabling more accurate individualized risk assessment and decision-making. Machine learning has the potential to significantly enhance prediction models in obstetrics by leveraging complex algorithms and advanced computational techniques. However, the unpredictable nature of clinician interpretation poses challenges to the effective utilization of these models.
By emphasizing communication, collaboration, education, and continuous evaluation, we can bridge the gap between prediction models and clinician interpretation that optimizes their use. This concerted effort will ultimately lead to improved patient care, enhanced clinical outcomes, and a more harmonious integration of these tools into obstetric practice.
Dr. Ramos is assistant professor of maternal fetal medicine and associate principal investigator at the Mother Infant Research Institute, Tufts University and Tufts Medical Center, Boston.
References
1. Ramos SZ et al. Predicting primary cesarean delivery in pregnancies complicated by gestational diabetes mellitus. Am J Obstet Gynecol. 2023 Jun 7;S0002-9378(23)00371-X. doi: 10.1016/j.ajog.2023.06.002.
2. Beninati MJ et al. Prediction model for vaginal birth after induction of labor in women with hypertensive disorders of pregnancy. Obstet Gynecol. 2020 Aug;136(2):402-410. doi: 10.1097/AOG.0000000000003938.
3. Levine LD et al. A validated calculator to estimate risk of cesarean after an induction of labor with an unfavorable cervix. Am J Obstet Gynecol. 2018 Feb;218(2):254.e1-254.e7. doi: 10.1016/j.ajog.2017.11.603.
4. American Diabetes Association. Our 60-Second Type 2 Diabetes Risk Test.
In the dawn of artificial intelligence’s potential to inform clinical practice, the importance of understanding the intent and interpretation of prediction tools is vital. In medicine, informed decision-making promotes patient autonomy and can lead to improved patient satisfaction and engagement in their own care.
This shared understanding enables patients to make informed choices about their care, reducing anxiety and increasing confidence in medical decision-making.
In obstetric clinical practice, prediction tools have been created to assess risk of primary cesarean delivery in gestational diabetes,1 cesarean delivery in hypertensive disorders of pregnancy,2 and failed induction of labor in nulliparous patients with an unfavorable cervix.3 By assessing a patient’s risk profile, clinicians can identify high-risk individuals who may require closer monitoring, early interventions, or specialized care. This allows for more timely interventions to optimize maternal and fetal health outcomes.
Other prediction tools are created to better elucidate to patients their individual risk of an outcome that may be modifiable, aiding physician counseling on mitigating factors to improve overall results. A relevant example is the American Diabetes Association’s risk of type 2 diabetes calculator used for counseling patients on risk reduction. This model includes both preexisting (ethnicity, family history, age, sex assigned at birth) and modifiable risk factors (body mass index, hypertension, physical activity) to predict risk of type 2 diabetes and is widely used in clinical practice to encourage integration of lifestyle changes to decrease risk.4 This model highlights the utility of prediction tools in counseling, providing quantitative data to clinicians to discuss a patient’s individual risk and how to mitigate that risk.
While predictive models clearly have many advantages and potential to improve personalized medicine, concerns have been raised that their interpretation and application can sometimes have unintended consequences as the complexity of these models can lead to variation in understanding among clinicians that impact decision-making. Different clinicians may assign different levels of importance to the predicted risks, resulting in differences in treatment plans and interventions. This variability can lead to disparities in care and outcomes, as patients with similar risk profiles may receive different management approaches based on the interpreting clinician.
Providers may either overly rely on prediction models or completely disregard them, depending on their level of trust or skepticism. Overreliance on prediction models may lead to the neglect of important clinical information or intuition, while disregarding the models may result in missed opportunities for early intervention or appropriate risk stratification. Achieving a balance between clinical judgment and the use of prediction models is crucial for optimal decision-making.
An example of how misinterpretation of the role of prediction tools in patient counseling can have far reaching consequences is the vaginal birth after cesarean (VBAC) calculator where race and ethnicity naturalized racial differences and likely contributed to cesarean overuse in Black pregnant people as non-White race was associated with a decreased chance of successful VBAC. Although the authors of the study that created the VBAC calculator intended it to be used as an adjunct to counseling, institutions and providers used low calculator scores to discourage or prohibit pregnant people from attempting a trial of labor after cesarean (TOLAC). This highlighted the importance of contextualizing the intent of prediction models within the broader clinical setting and individual patient circumstances and preferences.
This gap between intent and interpretation and subsequent application is influenced by individual clinician experience, training, personal biases, and subjective judgment. These subjective elements can introduce inconsistencies and variability in the utilization of prediction tools, leading to potential discrepancies in patient care. Inadequate understanding of prediction models and their statistical concepts can contribute to misinterpretation. It is this bias that prevents prediction models from serving their true purpose: to inform clinical decision-making, improve patient outcomes, and optimize resource allocation.
Clinicians may struggle with concepts such as predictive accuracy, overfitting, calibration, and external validation. Educational initiatives and enhanced training in statistical literacy can empower clinicians to better comprehend and apply prediction models in their practice. Researchers should make it clear that models should not be used in isolation, but rather integrated with clinical expertise and patient preferences. Understanding the limitations of prediction models and incorporating additional clinical information is essential.
Prediction models in obstetrics should undergo continuous evaluation and improvement to enhance their reliability and applicability. Regular updates, external validation, and recalibration are necessary to account for evolving clinical practices, changes in patient populations, and emerging evidence. Engaging clinicians in the evaluation process can foster ownership and promote a sense of trust in the models.
As machine learning and artificial intelligence improve the accuracy of prediction models, there is potential to revolutionize obstetric care by enabling more accurate individualized risk assessment and decision-making. Machine learning has the potential to significantly enhance prediction models in obstetrics by leveraging complex algorithms and advanced computational techniques. However, the unpredictable nature of clinician interpretation poses challenges to the effective utilization of these models.
By emphasizing communication, collaboration, education, and continuous evaluation, we can bridge the gap between prediction models and clinician interpretation that optimizes their use. This concerted effort will ultimately lead to improved patient care, enhanced clinical outcomes, and a more harmonious integration of these tools into obstetric practice.
Dr. Ramos is assistant professor of maternal fetal medicine and associate principal investigator at the Mother Infant Research Institute, Tufts University and Tufts Medical Center, Boston.
References
1. Ramos SZ et al. Predicting primary cesarean delivery in pregnancies complicated by gestational diabetes mellitus. Am J Obstet Gynecol. 2023 Jun 7;S0002-9378(23)00371-X. doi: 10.1016/j.ajog.2023.06.002.
2. Beninati MJ et al. Prediction model for vaginal birth after induction of labor in women with hypertensive disorders of pregnancy. Obstet Gynecol. 2020 Aug;136(2):402-410. doi: 10.1097/AOG.0000000000003938.
3. Levine LD et al. A validated calculator to estimate risk of cesarean after an induction of labor with an unfavorable cervix. Am J Obstet Gynecol. 2018 Feb;218(2):254.e1-254.e7. doi: 10.1016/j.ajog.2017.11.603.
4. American Diabetes Association. Our 60-Second Type 2 Diabetes Risk Test.
Dural-puncture epidural drives faster conversion to cesarean anesthesia
DPE, while not new, has become more popular as an option for initiating labor analgesia, but data comparing DPE with standard epidural in conversion to surgical anesthesia for cesarean deliveries are limited, Nadir Sharawi, MD, of the University of Arkansas for Medical Sciences, Little Rock, and colleagues wrote.
DPE involves no injection of intrathecal drugs, and the potential advantages include easier translocation of epidural medications into the intrathecal space for improved analgesia, but the effects of DPE on the onset and reliability of surgical anesthesia remain unknown, they said.
In a study published in JAMA Network Open, the researchers randomized 70 women scheduled for cesarean delivery of singleton pregnancies to DPE and 70 to a standard epidural. The participants were aged 18 years and older, with a mean age of the 30.1 years; the study was conducted between April 2019 and October 2022 at a single center.
The primary outcome was the time to the loss of sharp sensation at T6, defined as “the start of epidural extension anesthesia (time zero on the stopwatch) to when the patient could no longer feel sharp sensation at T6 (assessed bilaterally at the midclavicular line),” the researchers wrote.
The onset time to surgical anesthesia was faster in the DPE group, compared with the standard group, with a median of 422 seconds versus 655 seconds.
A key secondary outcome was a composite measure of the quality of epidural anesthesia that included failure to achieve a T10 bilateral block preoperatively in the delivery room, failure to achieve a surgical block at T6 within 15 minutes of chloroprocaine administration, requirement for intraoperative analgesia, repeat neuraxial procedure, and conversion to general anesthesia. The composite rates of lower quality anesthesia were significantly less in the DPE group, compared with the standard group (15.7% vs. 36.3%; P = .007).
Additional secondary outcomes included maternal satisfaction and pain score during surgery, adverse events, opioid use in the first 24 hours, maternal vasopressor requirements, epidural block assessments, and neonatal outcomes. No significant differences in these outcomes were noted between the groups, and no instances of local anesthetic systemic toxicity or neurological complications were reported.
The findings were limited by several factors including the study population of women scheduled for cesarean delivery and not in labor, and the inability to detect less frequent complications such as post–dural-puncture headache and accidental dural puncture, the researchers noted.
In addition, the results may vary with the use of other combinations of local anesthetics and opioids. “Chloroprocaine was chosen in this study because of its ease of administration without the need for opioids and other additives along with the low risk of systemic toxic effects, which favors rapid administration for emergent cesarean delivery,” they wrote.
However, the results show an association between DPE within an hour of epidural extension for elective cesarean delivery and a faster onset of anesthesia, improved block quality, and a more favorable ratio of risks versus benefits, compared with the use of standard epidural, the researchers concluded.
No need for general anesthesia?
“There is controversy over whether the dural puncture epidural technique improves labor analgesia when compared to a standard epidural,” Dr. Shawari said in an interview. “However, there are limited data on whether the dural puncture epidural technique decreases the onset time to surgical anesthesia when compared to a standard epidural for cesarean delivery. This is important as a pre-existing epidural is commonly used to convert labor analgesia to surgical anesthesia in the setting of urgent cesarean delivery. A faster onset of epidural anesthesia could potentially avoid the need for general anesthesia in an emergency.”
The researchers were not surprised by the findings given their experience with performing dural puncture epidurals for labor analgesia, Dr. Shawari said. In those cases, DPE provided a faster onset when converting cesarean anesthesia, compared with a standard epidural.
The takeaway from the current study is that DPE also provided “a faster onset and improved quality of anesthesia when compared to standard epidural for elective cesarean delivery,” Dr. Shawari said. However, additional research is needed to confirm the findings for intrapartum cesarean delivery.
Progress in improving pain control
“Adequate pain control during cesarean delivery is incredibly important,” Catherine Albright, MD, a maternal-fetal medicine specialist at the University of Washington, Seattle, said in an interview. “Inadequate pain control leads to the need to provide additional intravenous medications or the need to be put under general anesthesia, which changes the birth experience and is more dangerous for the birthing person and the neonate.
“In my clinical experience, there are many times when patients do not have adequate pain control during a cesarean delivery,” said Dr. Albright, who was not involved in the current study. “I am pleased to see that there is research underway about how to best manage pain on labor and delivery, especially in the setting of conversion from labor anesthesia to cesarean anesthesia.”
The findings may have implications for clinical practice, said Dr. Albright. If the dural puncture epidural can improve cesarean anesthesia following an epidural during labor, rather than anesthesia provided for an elective cesarean), “then I believe it would reduce the number of patients who require additional pain medication, have a poor cesarean experience, and/or need to be put under general anesthesia.”
However, “as noted by the authors, additional research is needed to further determine possible risks and side effects from this technique, and also to ensure that it also works in the setting of labor, rather than for an elective cesarean,” Dr. Albright added.
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Albright had no financial conflicts to disclose.
DPE, while not new, has become more popular as an option for initiating labor analgesia, but data comparing DPE with standard epidural in conversion to surgical anesthesia for cesarean deliveries are limited, Nadir Sharawi, MD, of the University of Arkansas for Medical Sciences, Little Rock, and colleagues wrote.
DPE involves no injection of intrathecal drugs, and the potential advantages include easier translocation of epidural medications into the intrathecal space for improved analgesia, but the effects of DPE on the onset and reliability of surgical anesthesia remain unknown, they said.
In a study published in JAMA Network Open, the researchers randomized 70 women scheduled for cesarean delivery of singleton pregnancies to DPE and 70 to a standard epidural. The participants were aged 18 years and older, with a mean age of the 30.1 years; the study was conducted between April 2019 and October 2022 at a single center.
The primary outcome was the time to the loss of sharp sensation at T6, defined as “the start of epidural extension anesthesia (time zero on the stopwatch) to when the patient could no longer feel sharp sensation at T6 (assessed bilaterally at the midclavicular line),” the researchers wrote.
The onset time to surgical anesthesia was faster in the DPE group, compared with the standard group, with a median of 422 seconds versus 655 seconds.
A key secondary outcome was a composite measure of the quality of epidural anesthesia that included failure to achieve a T10 bilateral block preoperatively in the delivery room, failure to achieve a surgical block at T6 within 15 minutes of chloroprocaine administration, requirement for intraoperative analgesia, repeat neuraxial procedure, and conversion to general anesthesia. The composite rates of lower quality anesthesia were significantly less in the DPE group, compared with the standard group (15.7% vs. 36.3%; P = .007).
Additional secondary outcomes included maternal satisfaction and pain score during surgery, adverse events, opioid use in the first 24 hours, maternal vasopressor requirements, epidural block assessments, and neonatal outcomes. No significant differences in these outcomes were noted between the groups, and no instances of local anesthetic systemic toxicity or neurological complications were reported.
The findings were limited by several factors including the study population of women scheduled for cesarean delivery and not in labor, and the inability to detect less frequent complications such as post–dural-puncture headache and accidental dural puncture, the researchers noted.
In addition, the results may vary with the use of other combinations of local anesthetics and opioids. “Chloroprocaine was chosen in this study because of its ease of administration without the need for opioids and other additives along with the low risk of systemic toxic effects, which favors rapid administration for emergent cesarean delivery,” they wrote.
However, the results show an association between DPE within an hour of epidural extension for elective cesarean delivery and a faster onset of anesthesia, improved block quality, and a more favorable ratio of risks versus benefits, compared with the use of standard epidural, the researchers concluded.
No need for general anesthesia?
“There is controversy over whether the dural puncture epidural technique improves labor analgesia when compared to a standard epidural,” Dr. Shawari said in an interview. “However, there are limited data on whether the dural puncture epidural technique decreases the onset time to surgical anesthesia when compared to a standard epidural for cesarean delivery. This is important as a pre-existing epidural is commonly used to convert labor analgesia to surgical anesthesia in the setting of urgent cesarean delivery. A faster onset of epidural anesthesia could potentially avoid the need for general anesthesia in an emergency.”
The researchers were not surprised by the findings given their experience with performing dural puncture epidurals for labor analgesia, Dr. Shawari said. In those cases, DPE provided a faster onset when converting cesarean anesthesia, compared with a standard epidural.
The takeaway from the current study is that DPE also provided “a faster onset and improved quality of anesthesia when compared to standard epidural for elective cesarean delivery,” Dr. Shawari said. However, additional research is needed to confirm the findings for intrapartum cesarean delivery.
Progress in improving pain control
“Adequate pain control during cesarean delivery is incredibly important,” Catherine Albright, MD, a maternal-fetal medicine specialist at the University of Washington, Seattle, said in an interview. “Inadequate pain control leads to the need to provide additional intravenous medications or the need to be put under general anesthesia, which changes the birth experience and is more dangerous for the birthing person and the neonate.
“In my clinical experience, there are many times when patients do not have adequate pain control during a cesarean delivery,” said Dr. Albright, who was not involved in the current study. “I am pleased to see that there is research underway about how to best manage pain on labor and delivery, especially in the setting of conversion from labor anesthesia to cesarean anesthesia.”
The findings may have implications for clinical practice, said Dr. Albright. If the dural puncture epidural can improve cesarean anesthesia following an epidural during labor, rather than anesthesia provided for an elective cesarean), “then I believe it would reduce the number of patients who require additional pain medication, have a poor cesarean experience, and/or need to be put under general anesthesia.”
However, “as noted by the authors, additional research is needed to further determine possible risks and side effects from this technique, and also to ensure that it also works in the setting of labor, rather than for an elective cesarean,” Dr. Albright added.
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Albright had no financial conflicts to disclose.
DPE, while not new, has become more popular as an option for initiating labor analgesia, but data comparing DPE with standard epidural in conversion to surgical anesthesia for cesarean deliveries are limited, Nadir Sharawi, MD, of the University of Arkansas for Medical Sciences, Little Rock, and colleagues wrote.
DPE involves no injection of intrathecal drugs, and the potential advantages include easier translocation of epidural medications into the intrathecal space for improved analgesia, but the effects of DPE on the onset and reliability of surgical anesthesia remain unknown, they said.
In a study published in JAMA Network Open, the researchers randomized 70 women scheduled for cesarean delivery of singleton pregnancies to DPE and 70 to a standard epidural. The participants were aged 18 years and older, with a mean age of the 30.1 years; the study was conducted between April 2019 and October 2022 at a single center.
The primary outcome was the time to the loss of sharp sensation at T6, defined as “the start of epidural extension anesthesia (time zero on the stopwatch) to when the patient could no longer feel sharp sensation at T6 (assessed bilaterally at the midclavicular line),” the researchers wrote.
The onset time to surgical anesthesia was faster in the DPE group, compared with the standard group, with a median of 422 seconds versus 655 seconds.
A key secondary outcome was a composite measure of the quality of epidural anesthesia that included failure to achieve a T10 bilateral block preoperatively in the delivery room, failure to achieve a surgical block at T6 within 15 minutes of chloroprocaine administration, requirement for intraoperative analgesia, repeat neuraxial procedure, and conversion to general anesthesia. The composite rates of lower quality anesthesia were significantly less in the DPE group, compared with the standard group (15.7% vs. 36.3%; P = .007).
Additional secondary outcomes included maternal satisfaction and pain score during surgery, adverse events, opioid use in the first 24 hours, maternal vasopressor requirements, epidural block assessments, and neonatal outcomes. No significant differences in these outcomes were noted between the groups, and no instances of local anesthetic systemic toxicity or neurological complications were reported.
The findings were limited by several factors including the study population of women scheduled for cesarean delivery and not in labor, and the inability to detect less frequent complications such as post–dural-puncture headache and accidental dural puncture, the researchers noted.
In addition, the results may vary with the use of other combinations of local anesthetics and opioids. “Chloroprocaine was chosen in this study because of its ease of administration without the need for opioids and other additives along with the low risk of systemic toxic effects, which favors rapid administration for emergent cesarean delivery,” they wrote.
However, the results show an association between DPE within an hour of epidural extension for elective cesarean delivery and a faster onset of anesthesia, improved block quality, and a more favorable ratio of risks versus benefits, compared with the use of standard epidural, the researchers concluded.
No need for general anesthesia?
“There is controversy over whether the dural puncture epidural technique improves labor analgesia when compared to a standard epidural,” Dr. Shawari said in an interview. “However, there are limited data on whether the dural puncture epidural technique decreases the onset time to surgical anesthesia when compared to a standard epidural for cesarean delivery. This is important as a pre-existing epidural is commonly used to convert labor analgesia to surgical anesthesia in the setting of urgent cesarean delivery. A faster onset of epidural anesthesia could potentially avoid the need for general anesthesia in an emergency.”
The researchers were not surprised by the findings given their experience with performing dural puncture epidurals for labor analgesia, Dr. Shawari said. In those cases, DPE provided a faster onset when converting cesarean anesthesia, compared with a standard epidural.
The takeaway from the current study is that DPE also provided “a faster onset and improved quality of anesthesia when compared to standard epidural for elective cesarean delivery,” Dr. Shawari said. However, additional research is needed to confirm the findings for intrapartum cesarean delivery.
Progress in improving pain control
“Adequate pain control during cesarean delivery is incredibly important,” Catherine Albright, MD, a maternal-fetal medicine specialist at the University of Washington, Seattle, said in an interview. “Inadequate pain control leads to the need to provide additional intravenous medications or the need to be put under general anesthesia, which changes the birth experience and is more dangerous for the birthing person and the neonate.
“In my clinical experience, there are many times when patients do not have adequate pain control during a cesarean delivery,” said Dr. Albright, who was not involved in the current study. “I am pleased to see that there is research underway about how to best manage pain on labor and delivery, especially in the setting of conversion from labor anesthesia to cesarean anesthesia.”
The findings may have implications for clinical practice, said Dr. Albright. If the dural puncture epidural can improve cesarean anesthesia following an epidural during labor, rather than anesthesia provided for an elective cesarean), “then I believe it would reduce the number of patients who require additional pain medication, have a poor cesarean experience, and/or need to be put under general anesthesia.”
However, “as noted by the authors, additional research is needed to further determine possible risks and side effects from this technique, and also to ensure that it also works in the setting of labor, rather than for an elective cesarean,” Dr. Albright added.
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Albright had no financial conflicts to disclose.
FROM JAMA NETWORK OPEN
Cigna accused of using AI, not doctors, to deny claims: Lawsuit
and forcing providers to bill patients in full.
In a complaint filed recently in California’s eastern district court, plaintiffs and Cigna health plan members Suzanne Kisting-Leung and Ayesha Smiley and their attorneys say that Cigna violates state insurance regulations by failing to conduct a “thorough, fair, and objective” review of their and other members’ claims.
The lawsuit says that, instead, Cigna relies on an algorithm, PxDx, to review and frequently deny medically necessary claims. According to court records, the system allows Cigna’s doctors to “instantly reject claims on medical grounds without ever opening patient files.” With use of the system, the average claims processing time is 1.2 seconds.
Cigna says it uses technology to verify coding on standard, low-cost procedures and to expedite physician reimbursement. In a statement to CBS News, the company called the lawsuit “highly questionable.”
The case highlights growing concerns about AI and its ability to replace humans for tasks and interactions in health care, business, and beyond. Public advocacy law firm Clarkson, which is representing the plaintiffs, has previously sued tech giants Google and ChatGPT creator OpenAI for harvesting Internet users’ personal and professional data to train their AI systems.
According to the complaint, Cigna denied the plaintiffs medically necessary tests, including blood work to screen for vitamin D deficiency and ultrasounds for patients suspected of having ovarian cancer. The plaintiffs’ attempts to appeal were unfruitful, and they were forced to pay out of pocket.
The plaintiff’s attorneys argue that the claims do not undergo more detailed reviews by physicians and employees, as mandated by California insurance laws, and that Cigna benefits by saving on labor costs.
Clarkson is demanding a jury trial and has asked the court to certify the Cigna case as a federal class action, potentially allowing the insurer’s other 2 million health plan members in California to join the lawsuit.
I. Glenn Cohen, JD, deputy dean and professor at Harvard Law School, Cambridge, Mass., said in an interview that this is the first lawsuit he’s aware of in which AI was involved in denying health insurance claims and that it is probably an uphill battle for the plaintiffs.
“In the last 25 years, the U.S. Supreme Court’s decisions have made getting a class action approved more difficult. If allowed to go forward as a class action, which Cigna is likely to vigorously oppose, then the pressure on Cigna to settle the case becomes enormous,” he said.
The allegations come after a recent deep dive by the nonprofit ProPublica uncovered similar claim denial issues. One physician who worked for Cigna told the nonprofit that he and other company doctors essentially rubber-stamped the denials in batches, which took “all of 10 seconds to do 50 at a time.”
In 2022, the American Medical Association and two state physician groups joined another class action against Cigna stemming from allegations that the insurer’s intermediary, Multiplan, intentionally underpaid medical claims. And in March, Cigna’s pharmacy benefit manager, Express Scripts, was accused of conspiring with other PBMs to drive up prescription drug prices for Ohio consumers, violating state antitrust laws.
Mr. Cohen said he expects Cigna to push back in court about the California class size, which the plaintiff’s attorneys hope will encompass all Cigna health plan members in the state.
“The injury is primarily to those whose claims were denied by AI, presumably a much smaller set of individuals and harder to identify,” said Mr. Cohen.
A version of this article first appeared on Medscape.com.
and forcing providers to bill patients in full.
In a complaint filed recently in California’s eastern district court, plaintiffs and Cigna health plan members Suzanne Kisting-Leung and Ayesha Smiley and their attorneys say that Cigna violates state insurance regulations by failing to conduct a “thorough, fair, and objective” review of their and other members’ claims.
The lawsuit says that, instead, Cigna relies on an algorithm, PxDx, to review and frequently deny medically necessary claims. According to court records, the system allows Cigna’s doctors to “instantly reject claims on medical grounds without ever opening patient files.” With use of the system, the average claims processing time is 1.2 seconds.
Cigna says it uses technology to verify coding on standard, low-cost procedures and to expedite physician reimbursement. In a statement to CBS News, the company called the lawsuit “highly questionable.”
The case highlights growing concerns about AI and its ability to replace humans for tasks and interactions in health care, business, and beyond. Public advocacy law firm Clarkson, which is representing the plaintiffs, has previously sued tech giants Google and ChatGPT creator OpenAI for harvesting Internet users’ personal and professional data to train their AI systems.
According to the complaint, Cigna denied the plaintiffs medically necessary tests, including blood work to screen for vitamin D deficiency and ultrasounds for patients suspected of having ovarian cancer. The plaintiffs’ attempts to appeal were unfruitful, and they were forced to pay out of pocket.
The plaintiff’s attorneys argue that the claims do not undergo more detailed reviews by physicians and employees, as mandated by California insurance laws, and that Cigna benefits by saving on labor costs.
Clarkson is demanding a jury trial and has asked the court to certify the Cigna case as a federal class action, potentially allowing the insurer’s other 2 million health plan members in California to join the lawsuit.
I. Glenn Cohen, JD, deputy dean and professor at Harvard Law School, Cambridge, Mass., said in an interview that this is the first lawsuit he’s aware of in which AI was involved in denying health insurance claims and that it is probably an uphill battle for the plaintiffs.
“In the last 25 years, the U.S. Supreme Court’s decisions have made getting a class action approved more difficult. If allowed to go forward as a class action, which Cigna is likely to vigorously oppose, then the pressure on Cigna to settle the case becomes enormous,” he said.
The allegations come after a recent deep dive by the nonprofit ProPublica uncovered similar claim denial issues. One physician who worked for Cigna told the nonprofit that he and other company doctors essentially rubber-stamped the denials in batches, which took “all of 10 seconds to do 50 at a time.”
In 2022, the American Medical Association and two state physician groups joined another class action against Cigna stemming from allegations that the insurer’s intermediary, Multiplan, intentionally underpaid medical claims. And in March, Cigna’s pharmacy benefit manager, Express Scripts, was accused of conspiring with other PBMs to drive up prescription drug prices for Ohio consumers, violating state antitrust laws.
Mr. Cohen said he expects Cigna to push back in court about the California class size, which the plaintiff’s attorneys hope will encompass all Cigna health plan members in the state.
“The injury is primarily to those whose claims were denied by AI, presumably a much smaller set of individuals and harder to identify,” said Mr. Cohen.
A version of this article first appeared on Medscape.com.
and forcing providers to bill patients in full.
In a complaint filed recently in California’s eastern district court, plaintiffs and Cigna health plan members Suzanne Kisting-Leung and Ayesha Smiley and their attorneys say that Cigna violates state insurance regulations by failing to conduct a “thorough, fair, and objective” review of their and other members’ claims.
The lawsuit says that, instead, Cigna relies on an algorithm, PxDx, to review and frequently deny medically necessary claims. According to court records, the system allows Cigna’s doctors to “instantly reject claims on medical grounds without ever opening patient files.” With use of the system, the average claims processing time is 1.2 seconds.
Cigna says it uses technology to verify coding on standard, low-cost procedures and to expedite physician reimbursement. In a statement to CBS News, the company called the lawsuit “highly questionable.”
The case highlights growing concerns about AI and its ability to replace humans for tasks and interactions in health care, business, and beyond. Public advocacy law firm Clarkson, which is representing the plaintiffs, has previously sued tech giants Google and ChatGPT creator OpenAI for harvesting Internet users’ personal and professional data to train their AI systems.
According to the complaint, Cigna denied the plaintiffs medically necessary tests, including blood work to screen for vitamin D deficiency and ultrasounds for patients suspected of having ovarian cancer. The plaintiffs’ attempts to appeal were unfruitful, and they were forced to pay out of pocket.
The plaintiff’s attorneys argue that the claims do not undergo more detailed reviews by physicians and employees, as mandated by California insurance laws, and that Cigna benefits by saving on labor costs.
Clarkson is demanding a jury trial and has asked the court to certify the Cigna case as a federal class action, potentially allowing the insurer’s other 2 million health plan members in California to join the lawsuit.
I. Glenn Cohen, JD, deputy dean and professor at Harvard Law School, Cambridge, Mass., said in an interview that this is the first lawsuit he’s aware of in which AI was involved in denying health insurance claims and that it is probably an uphill battle for the plaintiffs.
“In the last 25 years, the U.S. Supreme Court’s decisions have made getting a class action approved more difficult. If allowed to go forward as a class action, which Cigna is likely to vigorously oppose, then the pressure on Cigna to settle the case becomes enormous,” he said.
The allegations come after a recent deep dive by the nonprofit ProPublica uncovered similar claim denial issues. One physician who worked for Cigna told the nonprofit that he and other company doctors essentially rubber-stamped the denials in batches, which took “all of 10 seconds to do 50 at a time.”
In 2022, the American Medical Association and two state physician groups joined another class action against Cigna stemming from allegations that the insurer’s intermediary, Multiplan, intentionally underpaid medical claims. And in March, Cigna’s pharmacy benefit manager, Express Scripts, was accused of conspiring with other PBMs to drive up prescription drug prices for Ohio consumers, violating state antitrust laws.
Mr. Cohen said he expects Cigna to push back in court about the California class size, which the plaintiff’s attorneys hope will encompass all Cigna health plan members in the state.
“The injury is primarily to those whose claims were denied by AI, presumably a much smaller set of individuals and harder to identify,” said Mr. Cohen.
A version of this article first appeared on Medscape.com.