User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
The role of social media in aesthetic trends
Recently, but I had never heard it before. Not too long afterwards, patients were asking me about it in the office, using the same terminology, and I had several calls about it in one day. When I asked one trusted patient where she’d heard this term, which seemed to be trending, she told me that she had seen it on Instagram, as an ad or a “suggested for you” post.
Whether it’s a different name or term for a cosmetic procedure or laser we use that I’ve never heard before – such as “lip flip” or trap tox (also known as “Barbie Botox”) – many of these trendy terms spread like wildfire on social media. Some of the terms may be marketing tools started and spread by doctors who perform aesthetic procedures, something I don’t recommend as it only creates confusion for patients and practitioners, similar to the confusion consumers face regarding the plethora of over-the-counter skin care options and the marketing terms used for them. Other terms and trends are also started by nonphysician or non–professionally trained providers, sometimes leading to an unsafe or misleading term for an aesthetic procedure.
Over the past few years, several articles about the impact of social media in aesthetics have been published. In one recent paper, published in 2022, Boen and Jerdan noted that 72% of people in the United States use social media, up from 5% of American adults in 2005. In the United States, they note, “YouTube is the most popular platform with 73% of adult users, followed by Facebook (69%), Instagram (37%), SnapChat (24%), and Twitter (22%). Of the sites used daily, Facebook has the most activity (74%), followed by Instagram (64%), SnapChat (63%), YouTube (51%), and Twitter (42%).” They argue that the pros of social media in aesthetic medicine include its use as an educational tool by medical professionals to educate and provide accurate information about cosmetic procedures, and that “providing factual and evidence-based medical information to the public can help to counteract the abundant misinformation that is out there.” The cons include misinformation, no credentialing verification of the provider of the information – essentially anyone can be an “influencer” – as well as the addictive nature of social media for the consumer.
Along the same lines, younger patients tend to rely more on social media in choosing treatments and providers, further perpetuating any anxiety created from misinformation and unrealistic expectations from nonmedical influencers regarding procedures, filters used on photographs, photo editing, etc., in achieving an aesthetic result.
Physicians, particularly fellowship-trained aesthetic and surgical dermatologists, plastic and reconstructive surgeons, oculoplastic surgeons, and ENT facial plastic surgeons, who have the most training, knowledge, and expertise about aesthetic procedures, often have the least amount of time to devote to education via social media, compared with nonmedical influencers. Unless sponsored, they are also not being compensated for using it as an educational tool, except for potential indirect compensation from using it as a marketing tool for themselves and their practices. In contrast, nonmedical influencers often have many followers and time to create content, and in some cases, this is their full-time job.
All in all, most authors agree that social media has been associated with an increased acceptance of cosmetic surgery and procedures. Whether it be a trend seen on social media, or viewing one’s appearance in a filtered or photoediting app, or seeing an image of how another person looks (similar to how people in magazines, films and on television, were viewed in the past), social media has piqued people’s interest in aesthetics. It remains a balance for interested physicians to help keep information about cosmetic procedures presented in a healthy, interesting, professional, and accurate manner, and in a non–time-consuming way.
Dr. Wesley practices dermatology in Beverly Hills, Calif. Write to her at [email protected]. She had no relevant disclosures.
References
Boen M and Jerdan K. Clin Dermatol. 2022 Jan-Feb;40(1):45-8.
Chen J et al. JAMA Facial Plast Surg. 2019 Sep 1;21(5):361-7.
Chopan M et al. Plast Reconstr Surg. 2019 Apr;143(4):1259-65.
Recently, but I had never heard it before. Not too long afterwards, patients were asking me about it in the office, using the same terminology, and I had several calls about it in one day. When I asked one trusted patient where she’d heard this term, which seemed to be trending, she told me that she had seen it on Instagram, as an ad or a “suggested for you” post.
Whether it’s a different name or term for a cosmetic procedure or laser we use that I’ve never heard before – such as “lip flip” or trap tox (also known as “Barbie Botox”) – many of these trendy terms spread like wildfire on social media. Some of the terms may be marketing tools started and spread by doctors who perform aesthetic procedures, something I don’t recommend as it only creates confusion for patients and practitioners, similar to the confusion consumers face regarding the plethora of over-the-counter skin care options and the marketing terms used for them. Other terms and trends are also started by nonphysician or non–professionally trained providers, sometimes leading to an unsafe or misleading term for an aesthetic procedure.
Over the past few years, several articles about the impact of social media in aesthetics have been published. In one recent paper, published in 2022, Boen and Jerdan noted that 72% of people in the United States use social media, up from 5% of American adults in 2005. In the United States, they note, “YouTube is the most popular platform with 73% of adult users, followed by Facebook (69%), Instagram (37%), SnapChat (24%), and Twitter (22%). Of the sites used daily, Facebook has the most activity (74%), followed by Instagram (64%), SnapChat (63%), YouTube (51%), and Twitter (42%).” They argue that the pros of social media in aesthetic medicine include its use as an educational tool by medical professionals to educate and provide accurate information about cosmetic procedures, and that “providing factual and evidence-based medical information to the public can help to counteract the abundant misinformation that is out there.” The cons include misinformation, no credentialing verification of the provider of the information – essentially anyone can be an “influencer” – as well as the addictive nature of social media for the consumer.
Along the same lines, younger patients tend to rely more on social media in choosing treatments and providers, further perpetuating any anxiety created from misinformation and unrealistic expectations from nonmedical influencers regarding procedures, filters used on photographs, photo editing, etc., in achieving an aesthetic result.
Physicians, particularly fellowship-trained aesthetic and surgical dermatologists, plastic and reconstructive surgeons, oculoplastic surgeons, and ENT facial plastic surgeons, who have the most training, knowledge, and expertise about aesthetic procedures, often have the least amount of time to devote to education via social media, compared with nonmedical influencers. Unless sponsored, they are also not being compensated for using it as an educational tool, except for potential indirect compensation from using it as a marketing tool for themselves and their practices. In contrast, nonmedical influencers often have many followers and time to create content, and in some cases, this is their full-time job.
All in all, most authors agree that social media has been associated with an increased acceptance of cosmetic surgery and procedures. Whether it be a trend seen on social media, or viewing one’s appearance in a filtered or photoediting app, or seeing an image of how another person looks (similar to how people in magazines, films and on television, were viewed in the past), social media has piqued people’s interest in aesthetics. It remains a balance for interested physicians to help keep information about cosmetic procedures presented in a healthy, interesting, professional, and accurate manner, and in a non–time-consuming way.
Dr. Wesley practices dermatology in Beverly Hills, Calif. Write to her at [email protected]. She had no relevant disclosures.
References
Boen M and Jerdan K. Clin Dermatol. 2022 Jan-Feb;40(1):45-8.
Chen J et al. JAMA Facial Plast Surg. 2019 Sep 1;21(5):361-7.
Chopan M et al. Plast Reconstr Surg. 2019 Apr;143(4):1259-65.
Recently, but I had never heard it before. Not too long afterwards, patients were asking me about it in the office, using the same terminology, and I had several calls about it in one day. When I asked one trusted patient where she’d heard this term, which seemed to be trending, she told me that she had seen it on Instagram, as an ad or a “suggested for you” post.
Whether it’s a different name or term for a cosmetic procedure or laser we use that I’ve never heard before – such as “lip flip” or trap tox (also known as “Barbie Botox”) – many of these trendy terms spread like wildfire on social media. Some of the terms may be marketing tools started and spread by doctors who perform aesthetic procedures, something I don’t recommend as it only creates confusion for patients and practitioners, similar to the confusion consumers face regarding the plethora of over-the-counter skin care options and the marketing terms used for them. Other terms and trends are also started by nonphysician or non–professionally trained providers, sometimes leading to an unsafe or misleading term for an aesthetic procedure.
Over the past few years, several articles about the impact of social media in aesthetics have been published. In one recent paper, published in 2022, Boen and Jerdan noted that 72% of people in the United States use social media, up from 5% of American adults in 2005. In the United States, they note, “YouTube is the most popular platform with 73% of adult users, followed by Facebook (69%), Instagram (37%), SnapChat (24%), and Twitter (22%). Of the sites used daily, Facebook has the most activity (74%), followed by Instagram (64%), SnapChat (63%), YouTube (51%), and Twitter (42%).” They argue that the pros of social media in aesthetic medicine include its use as an educational tool by medical professionals to educate and provide accurate information about cosmetic procedures, and that “providing factual and evidence-based medical information to the public can help to counteract the abundant misinformation that is out there.” The cons include misinformation, no credentialing verification of the provider of the information – essentially anyone can be an “influencer” – as well as the addictive nature of social media for the consumer.
Along the same lines, younger patients tend to rely more on social media in choosing treatments and providers, further perpetuating any anxiety created from misinformation and unrealistic expectations from nonmedical influencers regarding procedures, filters used on photographs, photo editing, etc., in achieving an aesthetic result.
Physicians, particularly fellowship-trained aesthetic and surgical dermatologists, plastic and reconstructive surgeons, oculoplastic surgeons, and ENT facial plastic surgeons, who have the most training, knowledge, and expertise about aesthetic procedures, often have the least amount of time to devote to education via social media, compared with nonmedical influencers. Unless sponsored, they are also not being compensated for using it as an educational tool, except for potential indirect compensation from using it as a marketing tool for themselves and their practices. In contrast, nonmedical influencers often have many followers and time to create content, and in some cases, this is their full-time job.
All in all, most authors agree that social media has been associated with an increased acceptance of cosmetic surgery and procedures. Whether it be a trend seen on social media, or viewing one’s appearance in a filtered or photoediting app, or seeing an image of how another person looks (similar to how people in magazines, films and on television, were viewed in the past), social media has piqued people’s interest in aesthetics. It remains a balance for interested physicians to help keep information about cosmetic procedures presented in a healthy, interesting, professional, and accurate manner, and in a non–time-consuming way.
Dr. Wesley practices dermatology in Beverly Hills, Calif. Write to her at [email protected]. She had no relevant disclosures.
References
Boen M and Jerdan K. Clin Dermatol. 2022 Jan-Feb;40(1):45-8.
Chen J et al. JAMA Facial Plast Surg. 2019 Sep 1;21(5):361-7.
Chopan M et al. Plast Reconstr Surg. 2019 Apr;143(4):1259-65.
PCPs facing increased patient demand for knowledgeable menopause care
In 2017, a survey of 20 U.S. residency programs in family medicine, internal medicine, and ob.gyn. showed that only 6.8% of residents felt they were being adequately prepared to manage menopausal patients effectively, including how to use hormone therapy (HT).
Of the 177 residents who responded to the survey, 102 (56%) were in either family medicine or internal medicine.
“My guess is that there has been no substantial evolution in medical training to this day,” said lead survey study author Juliana Kling, MD, MPH, professor of medicine, chair of women’s health internal medicine, and dean, Mayo Clinic Alix School of Medicine, Scottsdale, Ariz.
The survey showed that overall 98% of residents thought it was important to know about menopause. However, 34% said they wouldn’t recommend HT in a severely symptomatic woman with no contraindications, and 60% said they wouldn’t recommend HT until at least the natural age of menopause in a prematurely menopausal woman. Some even recommended against it.
“Hormone therapy is effective, and for most healthy women younger than 60, the benefits are going to outweigh the risks,” said Dr. Kling. “We need to be comfortable, even in internal medicine, with prescribing hormones for the right women.”
The researchers concluded that “residual ambivalence about [hormone therapy] on the part of educators” may have played a role in curriculums that didn’t acknowledge the clinical relevance of menopause or include current evidence on the use of HT. Physicians should be taught to recognize menopausal symptoms, know the risks and benefits of HT and the alternatives, and how to select suitable candidates, they said.
Up to 80% of women in the United States are affected by menopausal vasomotor symptoms, but only one in four receive treatment, Dr. Kling pointed out. “Women will spend about a third of their lives after menopause, so being prepared to manage the consequences of menopause, such as bone health, vaginal dryness and painful intercourse, and increased cardiovascular disease risk, is critically important to all of us caring for women,” she emphasized. “These aren’t just ‘bothersome symptoms.’ ”
It is estimated that by 2060, there will be 90 million postmenopausal women in the United States. “Given the number of women who will experience symptoms of menopause and the considerable associated burden to their health and to the health care system, it is important to invest in educating future clinicians to provide evidence-based, comprehensive menopause management,” said Dr. Kling and coauthors in a February 2023 review of menopause treatments.
HT is the standard for the treatment of hot flashes and night sweats, and is highly effective for the prevention of bone loss and managing genitourinary syndrome of menopause. Among the alternatives to HT, the nonhormonal pharmacologic fezolinetant (Veozah) was approved by the U.S. Food and Drug Administration last May.
Following the early negative reports from the Women’s Health Initiative study of HT in 2002 and 2004, however, steep declines in HT prescription rates were seen among internists and family medicine practitioners. By 2009, only 18% of all HT prescriptions were written by primary care providers, and today, many remain wary about prescribing HT, despite evidence of its clinical value and safety.
“I think there’s a whole generation of family physicians who were taught that [hormone therapy] is dangerous and still feel very uncomfortable about using it to treat menopausal symptoms,” said Santina J.G. Wheat, MD, MPH, associate professor of family and community medicine at Northwestern University, Chicago. “These are the physicians educating the next generation of physicians,” said Dr. Wheat, who is program director for the McGaw Northwestern Family Medicine Residency Erie Humboldt Park.
Heather Hirsch, MD, an internist who specializes in menopause medicine in Columbus, Ohio, estimates that there are 300 internists among the 1,000 or so health care providers currently certified in menopause medicine through The Menopause Society (formerly the North American Menopause Society or NAMS). With 63 million women in the United States between the ages of 34 and 65, “that adds up to one doctor for several million patients,” she pointed out.
“In my opinion, the impact on menopausal care is profound,” said Jennifer T. Allen, MD, associate professor of obstetrics and gynecology, and director of menopause and midlife health at the Medical College of Georgia, Augusta. “If a physician was not exposed to menopause medicine in medical school or residency and does not choose to learn about menopause after training, then the opportunity to fully care for perimenopausal and postmenopausal women is extinguished.”
Not everyone agrees. “There’s no question that women’s health in general and menopausal issues specifically are a critical part of health care that is typically covered in most family medicine curriculums,” said Neil S. Skolnik, MD, professor of family and community medicine at the Sidney Kimmel Medical College in Philadelphia. “In family medicine, we really do attend to women’s health – particularly women’s health around menopause – as an important part of resident physician training,” emphasized Dr. Skolnik who is also and also associate director of the family medicine residency program at Abington Jefferson Health in Jenkintown, Penn.
"Family physicians are in a unique position to offer female patients effective care at perimenopause and beyond," added Karen L. Smith, MD, a family physician from Raeford, N.C., who is a board member of the American Academy of Family Physicians.*
Even so, many primary care physicians remain unsure about the use of HT, according to William E. Golden, MD, an internist and geriatrician, and professor of medicine and public health at the University of Arkansas for Medical Sciences, Little Rock.
“On the whole area of hot flashes and vasomotor instability, I think we’re in a state of significant flux and confusion,” Dr. Golden said in an interview. “For a long time, a lot of doctors told patients, ‘It’s okay, you’ll age out of it.’ Then the data started showing that the vasomotor symptoms continued for years so physicians began to reevaluate how to manage them. Now, the pendulum has swung back to giving estrogen.”
Many family physicians have been left to their own devices to figure out how to manage menopausal patients, said Dr. Wheat. “When there are significant changes to clinical management – or in the case of HT, a real reversal in how menopausal symptoms are managed – getting information out to physicians can be challenging.”
Meanwhile, patient demand for answers to their questions about menopause and the use of HT is changing the conversation, where it’s taking place, and with whom.
Some media-savvy doctors have taken to TikTok, where a lot of women started educating themselves about menopause during the pandemic. Dr. Hirsch is one of them. She uses the social media platform to talk about menopause and FDA-approved HT, but warned that for every clinician who is certified in menopause medicine “there are five more selling snake oil.”
Mainstream media has also jumped on the menopause bandwagon. The New York Times was one of the first, declaring that “menopause is having a moment.” On Feb. 1, the newspaper stormed the gates of the medical establishment with an article asking why more doctors weren’t offering HT to women experiencing hot flashes, sleeplessness, and pain during sex. The headline: “Women have been misled about menopause.”
On April 5, “The Menopause Talk” was posted to Oprah Daily, along with a menopause curriculum to give viewers “the tools to stay firmly in the driver’s seat as you navigate perimenopause and then menopause.” Popular topics included how to get your sex life back, premature menopause survival, and ways to work with insurers so that treatment is affordable.
“There’s been a sea-change in the culture that’s being driven by patient demand,” said Dr. Kling. “The conversation, colloquially, in the media, and with our patients, is evolving. Menopause is no longer such a taboo topic, and our patients are really demanding that we have answers for them. Clinicians are recognizing that they need better training in menopause and seeking that out.”
Last June, “Transforming Women’s Health” – the Mayo Clinic’s annual CME program held in partnership with The Menopause Society – had record physician attendance. “We’re going to make sure that our trainees are learning the up-to-date recommendations, not the ones from 20 years ago when the initial WHI reports made everyone fearful of hormones,” said Dr. Kling.
Dr. Kling disclosed that she is a medical editor for Everyday Health, and has a relationship with Evolve Medical Education. Dr. Skolnik reported relationships with numerous pharmaceutical companies. He is an MDedge Family Medicine board member. Dr. Golden is an MDedge Internal Medicine board member, and Dr. Wheat is an MDedge Family Medicine board member. Dr. Allen reported having no potential conflicts of interest.
* This story was updated on Sept 18, 2023. The quotation is attributable to Dr. Smith, not Dr. Skolnik.
In 2017, a survey of 20 U.S. residency programs in family medicine, internal medicine, and ob.gyn. showed that only 6.8% of residents felt they were being adequately prepared to manage menopausal patients effectively, including how to use hormone therapy (HT).
Of the 177 residents who responded to the survey, 102 (56%) were in either family medicine or internal medicine.
“My guess is that there has been no substantial evolution in medical training to this day,” said lead survey study author Juliana Kling, MD, MPH, professor of medicine, chair of women’s health internal medicine, and dean, Mayo Clinic Alix School of Medicine, Scottsdale, Ariz.
The survey showed that overall 98% of residents thought it was important to know about menopause. However, 34% said they wouldn’t recommend HT in a severely symptomatic woman with no contraindications, and 60% said they wouldn’t recommend HT until at least the natural age of menopause in a prematurely menopausal woman. Some even recommended against it.
“Hormone therapy is effective, and for most healthy women younger than 60, the benefits are going to outweigh the risks,” said Dr. Kling. “We need to be comfortable, even in internal medicine, with prescribing hormones for the right women.”
The researchers concluded that “residual ambivalence about [hormone therapy] on the part of educators” may have played a role in curriculums that didn’t acknowledge the clinical relevance of menopause or include current evidence on the use of HT. Physicians should be taught to recognize menopausal symptoms, know the risks and benefits of HT and the alternatives, and how to select suitable candidates, they said.
Up to 80% of women in the United States are affected by menopausal vasomotor symptoms, but only one in four receive treatment, Dr. Kling pointed out. “Women will spend about a third of their lives after menopause, so being prepared to manage the consequences of menopause, such as bone health, vaginal dryness and painful intercourse, and increased cardiovascular disease risk, is critically important to all of us caring for women,” she emphasized. “These aren’t just ‘bothersome symptoms.’ ”
It is estimated that by 2060, there will be 90 million postmenopausal women in the United States. “Given the number of women who will experience symptoms of menopause and the considerable associated burden to their health and to the health care system, it is important to invest in educating future clinicians to provide evidence-based, comprehensive menopause management,” said Dr. Kling and coauthors in a February 2023 review of menopause treatments.
HT is the standard for the treatment of hot flashes and night sweats, and is highly effective for the prevention of bone loss and managing genitourinary syndrome of menopause. Among the alternatives to HT, the nonhormonal pharmacologic fezolinetant (Veozah) was approved by the U.S. Food and Drug Administration last May.
Following the early negative reports from the Women’s Health Initiative study of HT in 2002 and 2004, however, steep declines in HT prescription rates were seen among internists and family medicine practitioners. By 2009, only 18% of all HT prescriptions were written by primary care providers, and today, many remain wary about prescribing HT, despite evidence of its clinical value and safety.
“I think there’s a whole generation of family physicians who were taught that [hormone therapy] is dangerous and still feel very uncomfortable about using it to treat menopausal symptoms,” said Santina J.G. Wheat, MD, MPH, associate professor of family and community medicine at Northwestern University, Chicago. “These are the physicians educating the next generation of physicians,” said Dr. Wheat, who is program director for the McGaw Northwestern Family Medicine Residency Erie Humboldt Park.
Heather Hirsch, MD, an internist who specializes in menopause medicine in Columbus, Ohio, estimates that there are 300 internists among the 1,000 or so health care providers currently certified in menopause medicine through The Menopause Society (formerly the North American Menopause Society or NAMS). With 63 million women in the United States between the ages of 34 and 65, “that adds up to one doctor for several million patients,” she pointed out.
“In my opinion, the impact on menopausal care is profound,” said Jennifer T. Allen, MD, associate professor of obstetrics and gynecology, and director of menopause and midlife health at the Medical College of Georgia, Augusta. “If a physician was not exposed to menopause medicine in medical school or residency and does not choose to learn about menopause after training, then the opportunity to fully care for perimenopausal and postmenopausal women is extinguished.”
Not everyone agrees. “There’s no question that women’s health in general and menopausal issues specifically are a critical part of health care that is typically covered in most family medicine curriculums,” said Neil S. Skolnik, MD, professor of family and community medicine at the Sidney Kimmel Medical College in Philadelphia. “In family medicine, we really do attend to women’s health – particularly women’s health around menopause – as an important part of resident physician training,” emphasized Dr. Skolnik who is also and also associate director of the family medicine residency program at Abington Jefferson Health in Jenkintown, Penn.
"Family physicians are in a unique position to offer female patients effective care at perimenopause and beyond," added Karen L. Smith, MD, a family physician from Raeford, N.C., who is a board member of the American Academy of Family Physicians.*
Even so, many primary care physicians remain unsure about the use of HT, according to William E. Golden, MD, an internist and geriatrician, and professor of medicine and public health at the University of Arkansas for Medical Sciences, Little Rock.
“On the whole area of hot flashes and vasomotor instability, I think we’re in a state of significant flux and confusion,” Dr. Golden said in an interview. “For a long time, a lot of doctors told patients, ‘It’s okay, you’ll age out of it.’ Then the data started showing that the vasomotor symptoms continued for years so physicians began to reevaluate how to manage them. Now, the pendulum has swung back to giving estrogen.”
Many family physicians have been left to their own devices to figure out how to manage menopausal patients, said Dr. Wheat. “When there are significant changes to clinical management – or in the case of HT, a real reversal in how menopausal symptoms are managed – getting information out to physicians can be challenging.”
Meanwhile, patient demand for answers to their questions about menopause and the use of HT is changing the conversation, where it’s taking place, and with whom.
Some media-savvy doctors have taken to TikTok, where a lot of women started educating themselves about menopause during the pandemic. Dr. Hirsch is one of them. She uses the social media platform to talk about menopause and FDA-approved HT, but warned that for every clinician who is certified in menopause medicine “there are five more selling snake oil.”
Mainstream media has also jumped on the menopause bandwagon. The New York Times was one of the first, declaring that “menopause is having a moment.” On Feb. 1, the newspaper stormed the gates of the medical establishment with an article asking why more doctors weren’t offering HT to women experiencing hot flashes, sleeplessness, and pain during sex. The headline: “Women have been misled about menopause.”
On April 5, “The Menopause Talk” was posted to Oprah Daily, along with a menopause curriculum to give viewers “the tools to stay firmly in the driver’s seat as you navigate perimenopause and then menopause.” Popular topics included how to get your sex life back, premature menopause survival, and ways to work with insurers so that treatment is affordable.
“There’s been a sea-change in the culture that’s being driven by patient demand,” said Dr. Kling. “The conversation, colloquially, in the media, and with our patients, is evolving. Menopause is no longer such a taboo topic, and our patients are really demanding that we have answers for them. Clinicians are recognizing that they need better training in menopause and seeking that out.”
Last June, “Transforming Women’s Health” – the Mayo Clinic’s annual CME program held in partnership with The Menopause Society – had record physician attendance. “We’re going to make sure that our trainees are learning the up-to-date recommendations, not the ones from 20 years ago when the initial WHI reports made everyone fearful of hormones,” said Dr. Kling.
Dr. Kling disclosed that she is a medical editor for Everyday Health, and has a relationship with Evolve Medical Education. Dr. Skolnik reported relationships with numerous pharmaceutical companies. He is an MDedge Family Medicine board member. Dr. Golden is an MDedge Internal Medicine board member, and Dr. Wheat is an MDedge Family Medicine board member. Dr. Allen reported having no potential conflicts of interest.
* This story was updated on Sept 18, 2023. The quotation is attributable to Dr. Smith, not Dr. Skolnik.
In 2017, a survey of 20 U.S. residency programs in family medicine, internal medicine, and ob.gyn. showed that only 6.8% of residents felt they were being adequately prepared to manage menopausal patients effectively, including how to use hormone therapy (HT).
Of the 177 residents who responded to the survey, 102 (56%) were in either family medicine or internal medicine.
“My guess is that there has been no substantial evolution in medical training to this day,” said lead survey study author Juliana Kling, MD, MPH, professor of medicine, chair of women’s health internal medicine, and dean, Mayo Clinic Alix School of Medicine, Scottsdale, Ariz.
The survey showed that overall 98% of residents thought it was important to know about menopause. However, 34% said they wouldn’t recommend HT in a severely symptomatic woman with no contraindications, and 60% said they wouldn’t recommend HT until at least the natural age of menopause in a prematurely menopausal woman. Some even recommended against it.
“Hormone therapy is effective, and for most healthy women younger than 60, the benefits are going to outweigh the risks,” said Dr. Kling. “We need to be comfortable, even in internal medicine, with prescribing hormones for the right women.”
The researchers concluded that “residual ambivalence about [hormone therapy] on the part of educators” may have played a role in curriculums that didn’t acknowledge the clinical relevance of menopause or include current evidence on the use of HT. Physicians should be taught to recognize menopausal symptoms, know the risks and benefits of HT and the alternatives, and how to select suitable candidates, they said.
Up to 80% of women in the United States are affected by menopausal vasomotor symptoms, but only one in four receive treatment, Dr. Kling pointed out. “Women will spend about a third of their lives after menopause, so being prepared to manage the consequences of menopause, such as bone health, vaginal dryness and painful intercourse, and increased cardiovascular disease risk, is critically important to all of us caring for women,” she emphasized. “These aren’t just ‘bothersome symptoms.’ ”
It is estimated that by 2060, there will be 90 million postmenopausal women in the United States. “Given the number of women who will experience symptoms of menopause and the considerable associated burden to their health and to the health care system, it is important to invest in educating future clinicians to provide evidence-based, comprehensive menopause management,” said Dr. Kling and coauthors in a February 2023 review of menopause treatments.
HT is the standard for the treatment of hot flashes and night sweats, and is highly effective for the prevention of bone loss and managing genitourinary syndrome of menopause. Among the alternatives to HT, the nonhormonal pharmacologic fezolinetant (Veozah) was approved by the U.S. Food and Drug Administration last May.
Following the early negative reports from the Women’s Health Initiative study of HT in 2002 and 2004, however, steep declines in HT prescription rates were seen among internists and family medicine practitioners. By 2009, only 18% of all HT prescriptions were written by primary care providers, and today, many remain wary about prescribing HT, despite evidence of its clinical value and safety.
“I think there’s a whole generation of family physicians who were taught that [hormone therapy] is dangerous and still feel very uncomfortable about using it to treat menopausal symptoms,” said Santina J.G. Wheat, MD, MPH, associate professor of family and community medicine at Northwestern University, Chicago. “These are the physicians educating the next generation of physicians,” said Dr. Wheat, who is program director for the McGaw Northwestern Family Medicine Residency Erie Humboldt Park.
Heather Hirsch, MD, an internist who specializes in menopause medicine in Columbus, Ohio, estimates that there are 300 internists among the 1,000 or so health care providers currently certified in menopause medicine through The Menopause Society (formerly the North American Menopause Society or NAMS). With 63 million women in the United States between the ages of 34 and 65, “that adds up to one doctor for several million patients,” she pointed out.
“In my opinion, the impact on menopausal care is profound,” said Jennifer T. Allen, MD, associate professor of obstetrics and gynecology, and director of menopause and midlife health at the Medical College of Georgia, Augusta. “If a physician was not exposed to menopause medicine in medical school or residency and does not choose to learn about menopause after training, then the opportunity to fully care for perimenopausal and postmenopausal women is extinguished.”
Not everyone agrees. “There’s no question that women’s health in general and menopausal issues specifically are a critical part of health care that is typically covered in most family medicine curriculums,” said Neil S. Skolnik, MD, professor of family and community medicine at the Sidney Kimmel Medical College in Philadelphia. “In family medicine, we really do attend to women’s health – particularly women’s health around menopause – as an important part of resident physician training,” emphasized Dr. Skolnik who is also and also associate director of the family medicine residency program at Abington Jefferson Health in Jenkintown, Penn.
"Family physicians are in a unique position to offer female patients effective care at perimenopause and beyond," added Karen L. Smith, MD, a family physician from Raeford, N.C., who is a board member of the American Academy of Family Physicians.*
Even so, many primary care physicians remain unsure about the use of HT, according to William E. Golden, MD, an internist and geriatrician, and professor of medicine and public health at the University of Arkansas for Medical Sciences, Little Rock.
“On the whole area of hot flashes and vasomotor instability, I think we’re in a state of significant flux and confusion,” Dr. Golden said in an interview. “For a long time, a lot of doctors told patients, ‘It’s okay, you’ll age out of it.’ Then the data started showing that the vasomotor symptoms continued for years so physicians began to reevaluate how to manage them. Now, the pendulum has swung back to giving estrogen.”
Many family physicians have been left to their own devices to figure out how to manage menopausal patients, said Dr. Wheat. “When there are significant changes to clinical management – or in the case of HT, a real reversal in how menopausal symptoms are managed – getting information out to physicians can be challenging.”
Meanwhile, patient demand for answers to their questions about menopause and the use of HT is changing the conversation, where it’s taking place, and with whom.
Some media-savvy doctors have taken to TikTok, where a lot of women started educating themselves about menopause during the pandemic. Dr. Hirsch is one of them. She uses the social media platform to talk about menopause and FDA-approved HT, but warned that for every clinician who is certified in menopause medicine “there are five more selling snake oil.”
Mainstream media has also jumped on the menopause bandwagon. The New York Times was one of the first, declaring that “menopause is having a moment.” On Feb. 1, the newspaper stormed the gates of the medical establishment with an article asking why more doctors weren’t offering HT to women experiencing hot flashes, sleeplessness, and pain during sex. The headline: “Women have been misled about menopause.”
On April 5, “The Menopause Talk” was posted to Oprah Daily, along with a menopause curriculum to give viewers “the tools to stay firmly in the driver’s seat as you navigate perimenopause and then menopause.” Popular topics included how to get your sex life back, premature menopause survival, and ways to work with insurers so that treatment is affordable.
“There’s been a sea-change in the culture that’s being driven by patient demand,” said Dr. Kling. “The conversation, colloquially, in the media, and with our patients, is evolving. Menopause is no longer such a taboo topic, and our patients are really demanding that we have answers for them. Clinicians are recognizing that they need better training in menopause and seeking that out.”
Last June, “Transforming Women’s Health” – the Mayo Clinic’s annual CME program held in partnership with The Menopause Society – had record physician attendance. “We’re going to make sure that our trainees are learning the up-to-date recommendations, not the ones from 20 years ago when the initial WHI reports made everyone fearful of hormones,” said Dr. Kling.
Dr. Kling disclosed that she is a medical editor for Everyday Health, and has a relationship with Evolve Medical Education. Dr. Skolnik reported relationships with numerous pharmaceutical companies. He is an MDedge Family Medicine board member. Dr. Golden is an MDedge Internal Medicine board member, and Dr. Wheat is an MDedge Family Medicine board member. Dr. Allen reported having no potential conflicts of interest.
* This story was updated on Sept 18, 2023. The quotation is attributable to Dr. Smith, not Dr. Skolnik.
Social media use may promote depression in pregnancy
Depressive symptoms among pregnant women have risen in recent years, but the potential impact of social media use on depression in pregnancy has not been well studied, wrote Lotte Muskens, a PhD candidate at Tilburg (the Netherlands) University and colleagues.
In a study published in the Journal of Affective Disorders, the researchers surveyed 697 pregnant women aged 19-42 years who were part of a larger longitudinal prospective study (the Brabant Study) in the Netherlands. The mean age of the participants was 31 years; 96% were employed, 99% had a partner, and 71% had a bachelor’s degree or higher. Depressive symptoms were assessed at 12, 20, and 28 weeks of pregnancy using the Dutch version of the 10-item Edinburgh Depression Scale (EDS).
The researchers categorized the participants into trajectories of depressive symptoms during pregnancy, with 489 identified as low stable (mean EDS scores 2.8-3.0), 183 as intermediate stable (mean EDS scores 8.4-8.8), and 25 as high stable (mean EDS scores 15.1-16.9).
Problematic SMU was identified using the six-item Bergen Social Media Addiction Scale (BSMAS) at 12 weeks of pregnancy; scores ranged from 6 to 30, with higher scores representing more problematic SMU.
The mean BSMAS scores were 9.0, 10.7, and 12.6 for the low-stable, intermediate-stable, and high-stable depression groups, respectively.
Data on social media use (SMU) were collected at 12 weeks of pregnancy. Social media was defined as common platforms including Facebook, Instagram, LinkedIn, Pinterest, Twitter, and YouTube.
SMU was defined in terms of intensity, measured by time and frequency. Time was measured by asking participants to list how many hours per day they used social media on a scale of 1 (no use of social media) to 9 (7 or more hours per day). Frequency was measured by asking how often participants visited the various social media platforms, on a scale of 1 (no use of social media) to 7 (five or more visits per day). Overall, the participants averaged 1.6 hours per day and 19.5 visits per week on SMU.
Increased time and frequency of SMU was significantly associated with increased odds of being in the high-stable group, compared with the low-stable group in an adjusted analysis (odds ratios, 1.51 and 1.05, respectively; P = .017 and P = .019, respectively).
In addition, problematic SMU (as defined by higher BSMAS scores) remained significantly associated with increased odds of belonging to the intermediate-stable or high-stable classes in an adjusted analysis (odds ratios, 1.17 and 1.31; P < .001 for both).
“While our results suggest that SMU can have negative consequences for pregnant women’s mental wellbeing, it is important to note that SMU during pregnancy may also be helpful for some pregnant women,” as many women, especially first-time mothers, find information and support through social media, the researchers wrote in their discussion.
The findings were limited by several factors, including the variation in group sizes for depressive symptoms, reliance on self-reports, and the collection of data during the COVID-19 pandemic, which may have affected the results, the researchers noted.
However, the results were strengthened by the large sample size and longitudinal design that allowed measurement of trajectories. More research is needed to determine causal relationships, but the data indicate an association between higher levels of depression during pregnancy and more intense and problematic SMU use, and health care providers should discuss SMU in addition to other risk factors for depression in pregnant women, the researchers concluded.
The study received no outside funding. The researchers had no financial conflicts to disclose.
Depressive symptoms among pregnant women have risen in recent years, but the potential impact of social media use on depression in pregnancy has not been well studied, wrote Lotte Muskens, a PhD candidate at Tilburg (the Netherlands) University and colleagues.
In a study published in the Journal of Affective Disorders, the researchers surveyed 697 pregnant women aged 19-42 years who were part of a larger longitudinal prospective study (the Brabant Study) in the Netherlands. The mean age of the participants was 31 years; 96% were employed, 99% had a partner, and 71% had a bachelor’s degree or higher. Depressive symptoms were assessed at 12, 20, and 28 weeks of pregnancy using the Dutch version of the 10-item Edinburgh Depression Scale (EDS).
The researchers categorized the participants into trajectories of depressive symptoms during pregnancy, with 489 identified as low stable (mean EDS scores 2.8-3.0), 183 as intermediate stable (mean EDS scores 8.4-8.8), and 25 as high stable (mean EDS scores 15.1-16.9).
Problematic SMU was identified using the six-item Bergen Social Media Addiction Scale (BSMAS) at 12 weeks of pregnancy; scores ranged from 6 to 30, with higher scores representing more problematic SMU.
The mean BSMAS scores were 9.0, 10.7, and 12.6 for the low-stable, intermediate-stable, and high-stable depression groups, respectively.
Data on social media use (SMU) were collected at 12 weeks of pregnancy. Social media was defined as common platforms including Facebook, Instagram, LinkedIn, Pinterest, Twitter, and YouTube.
SMU was defined in terms of intensity, measured by time and frequency. Time was measured by asking participants to list how many hours per day they used social media on a scale of 1 (no use of social media) to 9 (7 or more hours per day). Frequency was measured by asking how often participants visited the various social media platforms, on a scale of 1 (no use of social media) to 7 (five or more visits per day). Overall, the participants averaged 1.6 hours per day and 19.5 visits per week on SMU.
Increased time and frequency of SMU was significantly associated with increased odds of being in the high-stable group, compared with the low-stable group in an adjusted analysis (odds ratios, 1.51 and 1.05, respectively; P = .017 and P = .019, respectively).
In addition, problematic SMU (as defined by higher BSMAS scores) remained significantly associated with increased odds of belonging to the intermediate-stable or high-stable classes in an adjusted analysis (odds ratios, 1.17 and 1.31; P < .001 for both).
“While our results suggest that SMU can have negative consequences for pregnant women’s mental wellbeing, it is important to note that SMU during pregnancy may also be helpful for some pregnant women,” as many women, especially first-time mothers, find information and support through social media, the researchers wrote in their discussion.
The findings were limited by several factors, including the variation in group sizes for depressive symptoms, reliance on self-reports, and the collection of data during the COVID-19 pandemic, which may have affected the results, the researchers noted.
However, the results were strengthened by the large sample size and longitudinal design that allowed measurement of trajectories. More research is needed to determine causal relationships, but the data indicate an association between higher levels of depression during pregnancy and more intense and problematic SMU use, and health care providers should discuss SMU in addition to other risk factors for depression in pregnant women, the researchers concluded.
The study received no outside funding. The researchers had no financial conflicts to disclose.
Depressive symptoms among pregnant women have risen in recent years, but the potential impact of social media use on depression in pregnancy has not been well studied, wrote Lotte Muskens, a PhD candidate at Tilburg (the Netherlands) University and colleagues.
In a study published in the Journal of Affective Disorders, the researchers surveyed 697 pregnant women aged 19-42 years who were part of a larger longitudinal prospective study (the Brabant Study) in the Netherlands. The mean age of the participants was 31 years; 96% were employed, 99% had a partner, and 71% had a bachelor’s degree or higher. Depressive symptoms were assessed at 12, 20, and 28 weeks of pregnancy using the Dutch version of the 10-item Edinburgh Depression Scale (EDS).
The researchers categorized the participants into trajectories of depressive symptoms during pregnancy, with 489 identified as low stable (mean EDS scores 2.8-3.0), 183 as intermediate stable (mean EDS scores 8.4-8.8), and 25 as high stable (mean EDS scores 15.1-16.9).
Problematic SMU was identified using the six-item Bergen Social Media Addiction Scale (BSMAS) at 12 weeks of pregnancy; scores ranged from 6 to 30, with higher scores representing more problematic SMU.
The mean BSMAS scores were 9.0, 10.7, and 12.6 for the low-stable, intermediate-stable, and high-stable depression groups, respectively.
Data on social media use (SMU) were collected at 12 weeks of pregnancy. Social media was defined as common platforms including Facebook, Instagram, LinkedIn, Pinterest, Twitter, and YouTube.
SMU was defined in terms of intensity, measured by time and frequency. Time was measured by asking participants to list how many hours per day they used social media on a scale of 1 (no use of social media) to 9 (7 or more hours per day). Frequency was measured by asking how often participants visited the various social media platforms, on a scale of 1 (no use of social media) to 7 (five or more visits per day). Overall, the participants averaged 1.6 hours per day and 19.5 visits per week on SMU.
Increased time and frequency of SMU was significantly associated with increased odds of being in the high-stable group, compared with the low-stable group in an adjusted analysis (odds ratios, 1.51 and 1.05, respectively; P = .017 and P = .019, respectively).
In addition, problematic SMU (as defined by higher BSMAS scores) remained significantly associated with increased odds of belonging to the intermediate-stable or high-stable classes in an adjusted analysis (odds ratios, 1.17 and 1.31; P < .001 for both).
“While our results suggest that SMU can have negative consequences for pregnant women’s mental wellbeing, it is important to note that SMU during pregnancy may also be helpful for some pregnant women,” as many women, especially first-time mothers, find information and support through social media, the researchers wrote in their discussion.
The findings were limited by several factors, including the variation in group sizes for depressive symptoms, reliance on self-reports, and the collection of data during the COVID-19 pandemic, which may have affected the results, the researchers noted.
However, the results were strengthened by the large sample size and longitudinal design that allowed measurement of trajectories. More research is needed to determine causal relationships, but the data indicate an association between higher levels of depression during pregnancy and more intense and problematic SMU use, and health care providers should discuss SMU in addition to other risk factors for depression in pregnant women, the researchers concluded.
The study received no outside funding. The researchers had no financial conflicts to disclose.
FROM THE JOURNAL OF AFFECTIVE DISORDERS
Freezing the biological clock: A 2023 update on preserving fertility
Throughout the 20th century, the management of ectopic pregnancy evolved from preserving the life of the mother to preserving fertility by utilizing the conservative treatment of methotrexate and/or tubal surgery. I make this, seemingly obscure, reference to managing ectopic pregnancy to consider an analogous shift over time in the management of patients with cancer. Over the next decade, the number of people who have lived 5 or more years after their cancer diagnosis is projected to increase approximately 30%, to 16.3 million. Due to the improved survival rates following a cancer diagnosis,1 revolutionary developments have been made in fertility preservation to obviate the impact of gonadotoxic therapy. We have evolved, however, from shielding and transposing ovaries to ovarian tissue cryopreservation,2 with rapid implementation.
While advances in reproductive cryopreservation have allowed for the delay, or even potential “prevention” of infertility, assisted reproductive technology (ART) cannot yet claim a “cure” in ensuring procreation. Nevertheless, fertility preservation is a burgeoning field that has transitioned from an experimental label to a standard of care in 2012, as designated by the American Society for Reproductive Medicine (ASRM).3 From the original intention of offering oocyte cryopreservation to women at risk of ovarian failure from impending gonadotoxic cancer treatment, fertility preservation has accelerated to include freezing for nonmedical reasons—eg, planned oocyte cryopreservation (POC), or “social” egg freezing, to ovarian tissue cryopreservation to accommodate the expediency needed for the treatment of certain cancer treatments. Additionally, across the United States, the number of donor egg banks, which allow women an easily accessible option, is rivaling enduring sperm banks. Due to the advanced methodology of vitrification and growing demand for the technology due to increasing IVF cycles, cryopreservation has become a specialized area of reproductive medicine, and a target of venture capital and private equity commercialization. This article will review the latest techniques, appropriate counseling, and cost/benefit ratio of fertility preservation, with an emphasis on POC.
CASE 1 Fertility preservation options for patient with breast cancer
A 37-year-old woman with newly diagnosed hormone receptor−positive breast cancer is referred for a fertility preservation consultation prior to initiating treatment. Her oncologist plans chemotherapy, followed by radiation and a minimum of 5 years of tamoxifen therapy.
What is the best consultation approach for this patient?
Consultation involves understanding several factors
The consultation approach to this patient involves ascertaining her medical, social, and family history, along with her reproductive plans.
Medical history. For the medical component, we must focus on her diagnosis, anticipated treatment with timeline, risks of gonadal toxicity with planned treatments, her current medical stability, and prognosis for expected survival.
Social history. Her age, relationship status, and desired family size address her social history.
Family history. Given that her cancer affects the breast, there is the risk of genetic susceptibility and potential for embryo testing for the BRCA gene.
Reproductive plans. These include her and her partner’s, if applicable, number of desired children and their risk factors for infertility.
Regarding the reproductive timeline, the antihormonal therapy that may be required for her treatment may improve overall survival, but it would delay the time to pregnancy. Consequently, the pursuit of fertility preservation prior to cancer treatment is a multidisciplinary approach that can involve medical oncology, radiation oncology, REI, medical genetics, and often, psychology. Fortunately, evidence continues to support fertility preservation, with or without hormonal ovarian stimulation, for patients with breast cancer. Data, with up to 5 years of follow-up, has indicated that it is safe.4
Continue to: Oncofertility...
Oncofertility
To address the need to maximize the reproductive potential of patients with newly diagnosed cancer, the field of oncofertility combines the specialties of oncology and reproductive medicine. The reproductive risk of cancer treatment is gonadotoxicity, with subsequent iatrogenic primary ovarian insufficiency (POI) and infertility. Alkylating agents (including cyclosphosphamide) have the highest risk for amenorrhea, while antimetabolites (including methotrexate, 5–fluorouracil) have the lowest risk.5 Treating bone marrow/stem cell transplantation using high-dose alkylating agents, with or without whole body irradiation, results in ≥80% amenorrhea. The minimum radiation dose to induce ovarian failure decreases with advancing age, from 18.4 Gy at age 10 years to 6 Gy at age 40 years, due to biologically diminishing ovarian reserve and an increase in the radiosensitivity of oocytes.6 An online tool—using varying factors including age, chemotherapy dose, prior treatment, smoking, and baseline diminished ovarian reserve—is available to help predict the chance of ovarian failure following chemotherapy.7
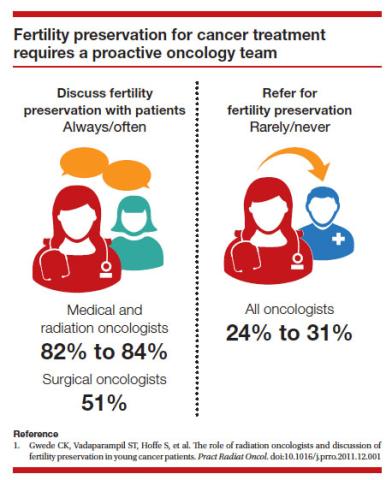
Since 2006, the American Society of Clinical Oncology recommended, as part of the consent prior to therapy, oncologists should address the possibility of infertility with patients “as early in treatment planning as possible” and “...Fertility preservation is an important, if not necessary, consideration when planning cancer treatment in reproductive-age patients.”
Reference
1. Lee SJ, Schover LR, Partridge AH, et al. American Society of Clinical Oncology recommendations on fertility preservation in cancer patients. J Clin Oncol. 2006;24:2917-2931.
Cryopreservation to the rescue
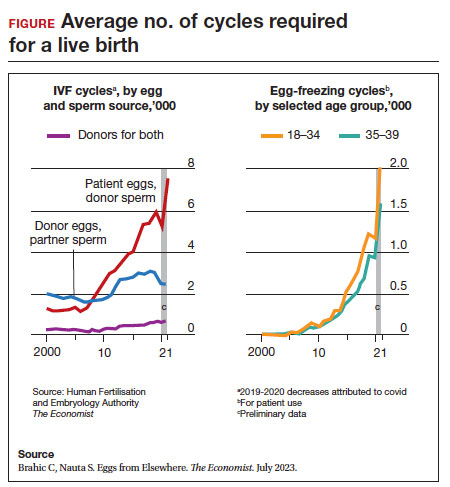
Since 2012, when ASRM removed the experimental designation on oocyte cryopreservation (OC), the number of cycles offered for fertility preservation has increased dramatically (FIGURE),8 initially being used for patients with cancer and now also including women desiring POC.
Ovarian and embryo cryopreservation. Ovarian stimulation and egg retrieval for OC can now occur within 2 weeks due to a random start protocol whereby women can begin ovarian stimulation any day in their cycle (ie, preovulation or postovulation).9
OC followed by thawing for subsequent fertilization and embryo transfer is employed as a matter of routine when patients with infertility utilize frozen eggs from a donor. While there remains debate over better live birth rates with frozen eggs versus fresh eggs, clinic experience may be a critical factor.10
Ovarian tissue cryopreservation. In addition to the fertility preservation procedures of oocytes and embryo cryopreservation, ovarian tissue cryopreservation became a standard option in 2019 when ASRM removed its experimental designation.11 Given the potential time constraints of urgent cancer treatment, ovarian tissue cryopreservation has the advantage of not requiring ovarian stimulation or sexual maturity and is able to be performed while patients are receiving chemotherapy. If successful, ovarian tissue cryopreservation followed by orthotopic transplantation has the potential to restore natural ovarian function and natural conceptions.12 However, despite first successfully being described in 2004, ovarian tissue cryopreservation, which does require subsequent thawing and tissue transplantation, remains less available to patients due to low usage rates, which have resulted in few clinics having adequate proficiency.13,14
Ovarian tissue cryopreservation involves obtaining ovarian cortical tissue, dissecting the tissue into small fragments, and cryopreserving it using either a slow-cool technique or vitrification. Orthotopic transplantation has been the most successful method for using ovarian tissue in humans. Live birth rates are modest.15 In all cancer survivors, particularly those with leukemia, autologous ovarian tissue transplantation may contain malignant cells that could lead to the reintroduction of cancer as the tissue is removed prior to treatment.16
Pregnancy outcomes using embryos created from ovaries recently exposed to chemotherapy in humans is not known, but animal studies suggest that there may be higher rates of miscarriage and birth defects given the severe DNA damage to oocytes of developing follicles.17 Hence, ovarian stimulation should be initiated and completed before the start of chemotherapy.
Continue to: Planned oocyte cryopreservation...
Planned oocyte cryopreservation
With advances in ART, POC offers patients the opportunity to preserve fertility until desired. However, despite its potential benefits, POC compels the discussion of various considerations in addition to oncofertility, such as ethical concerns and insurance coverage.
CASE 2 Woman plans for elective egg freezing
A 32-year-old single, professional woman is advancing in her career and wishes to delay childbearing. She is concerned about the potential for age-related fertility decline and wants to explore the option of elective egg freezing. Emily has no medical conditions that would impair her fertility, but she wants to ensure that she has the option of having biological children in the future. She is unsure about the potential financial burden of the procedure and whether her employer’s insurance covers such elective procedures.
How do you counsel her about her options?
Medical considerations
Approximately 25% of reproductive-aged women have considered POC.18 An analysis revealed POC was more cost-effective than delaying procreation and undergoing IVF with preimplantation genetic testing for aneuploidies at an advanced reproductive age.19
The process of planned oocyte cryopreservation. POC involves ovarian stimulation, usually with parenteral gonadotropins, to produce multiple mature oocytes for same-day cryopreservation following transvaginal retrieval, typically in an office-based surgery center as an outpatient procedure while the patient is under IV sedation. While the procedure has been proven effective, there are inherent risks and limitations. The success rates of subsequent fertility treatments using the cryopreserved eggs are influenced by the woman’s age at the time of freezing, the number of mature oocytes retrieved and vitrified, and the quality of the oocytes following thaw. A recent study reported a 70% live-birth rate in women aged less than 38 years who cryopreserved ≥ 20 mature eggs.20 To increase the number of cryopreserved oocytes, multiple egg retrievals or “batching” may be of benefit for women with diminished ovarian reserve.21
It is important for clinicians to thoroughly assess a patient’s medical history, ovarian reserve (by antral follicle count and levels of anti-müllerian hormone [AMH]), and reproductive goals before recommending proceeding with POC. Of note, AMH is a useful marker for ovarian reserve but has not been shown to predict natural fertility. Its value is in providing a guide to the dosage of ovarian stimulation and an estimation of the number of oocytes to be retrieved. Per ASRM, “Extremely low AMH values should not be used to refuse treatment in IVF.” AMH levels and antral follicle count have only a weak association with such qualitative outcomes as oocyte quality, clinical pregnancy rates, and live birth rates. Complications from egg retrieval, both short and long term, are rare. The inherent risk from POC is the lack of a guaranteed subsequent live birth.22
Ethical and social considerations
POC raises several ethical considerations, including concerns of perpetuating societal pressure on women to defer procreation to prioritize their careers over family planning.23 Despite controversies, POC appears as a chosen strategy against age-related infertility and may allow women to feel that they are more socially, psychologically, and financially stable before pursuing motherhood.24 Open and honest discussions between clinicians and patients are crucial to ensure informed decision making and address these ethical concerns.
Per an ACOG statement from February 2023 (https://www.acog.org/womens-health/faqs/having-a-baby-after-age-35-how-aging-affects-fertility-and-pregnancy) “...egg freezing is recommended mainly for patients having cancer treatment that will affect their future fertility. There is not enough research to recommend routine egg freezing for the sole purpose of delaying childbearing.”
A recent survey of patients who had elected egg freezing at some point included more than 80% who were aged 35 or older, and revealed that 93% of the survey participants had not yet returned to use their frozen oocytes.25 The most common reason cited in the survey for a delay in attempted procreation was lack of a partner. Another reason was undergoing oocyte cryopreservation after an optimal reproductive age, with participants concluding that they felt they had improved their reproductive future after undergoing oocyte cryopreservation and feeling empowered by the process. As part of counseling, women should be informed of the possibility of not utilizing their frozen eggs in the future, whether due to natural conception or other personal reasons.
Continue to: Employer insurance coverage...
Employer insurance coverage
Access to elective egg freezing is largely influenced by insurance coverage. Currently, employer-provided insurance coverage for this procedure varies widely. While some companies offer comprehensive coverage, others provide limited or no coverage at all. The cost of elective egg freezing can range from $10,000 to $15,000, excluding additional expenses such as medications and annual storage fees. The financial burden can create a gap between patients who desire POC and those with an ability to implement the process. The cost can be a significant barrier for many patients considering this option and perpetuates the lack of universal diversity, equity, and inclusion.
CASE 3 Gender dysphoria and fertility preservation
A 22-year-old transgender man is preparing to undergo gender-affirming hormone therapy and surgery. He is concerned about the potential impact of testosterone therapy on his oocytes and wishes to explore options for fertility preservation prior to oophorectomy.26
What are the patient’s options for fertility preservation?
The patient has the fertility preservation options of OC following ovarian stimulation or ovarian tissue cryopreservation at the time of oophorectomy. Preliminary evidence does not demonstrate impairment of ovarian stimulation and oocyte retrieval number with concurrent testosterone exposure. Ethical considerations, in this case, involve respecting the patient’s autonomy, addressing potential conflicts between gender-affirming care and fertility preservation (eg, a risk of dysphoria in transgender patients preserving biological gametes from a prior assigned gender), and ensuring access to fertility preservation services without discrimination. It is essential to provide the patient in this case with comprehensive information regarding the impact of hormone therapy on fertility, the available options, and the potential financial costs involved. Supportive counseling should also be offered to address any psychological or emotional aspects related to fertility preservation for all patients considering this option.
A call for diversity, equity, and inclusion
To improve access to POC, advocating for employer-offered insurance coverage is paramount. Women’s health providers can encourage dialogue between employers, insurers, and policymakers, which can lead to policy changes that prioritize coverage for fertilitypreservation options. This could include mandating coverage for POC as part of comprehensive health care plans or providing tax incentives to employers who offer coverage for these procedures. Furthermore, public awareness campaigns and advocacy efforts can help educate employers about the importance of including fertility preservation coverage in their employee benefits packages.
Conclusion
Just as physicians must recognize their responsibility to patients to distinguish unproven yet promising science from evidence-based and clinically established science, so too must they advise their patients to consider fertility preservation services in a way that is both clinically justified and ethically appropriate. Informed decisions must be made by appropriate counseling of evidence-based medicine to protect the interest of patients. POC provides patients with an opportunity to preserve their fertility and exercise reproductive autonomy. However, access to this procedure is often hindered by limited or nonexistent employer insurance coverage. By recognizing the medical, ethical, and social implications of POC and implementing strategies to improve coverage, collaborative efforts may increase accessibility and defray costs to provide patients with the option of deferring childbearing and preserving their reproductive potential. ●
1. Promptly offer fertility preservation treatment options with sensitivity and clarity.
2. Dedicate ample time and exercise patience during the consultation.
3. Provide education using multiple modalities to help patients assimilate information.
4. Encourage consultation with mental health professionals.
Special considerations for hematologic malignancies:
- Treatment can be associated with significant gonadal toxicity and premature ovarian failure.
- Patients are frequently ill at the time of presentation and ineligible for certain fertility preservation options.
References
1. Ethics Committee of the American Society for Reproductive Medicine. Fertility preservation and reproduction in patients facing gonadotoxic therapies: a committee opinion. Fertil Steril. 2018;110:380-386. doi:10.1016/j.fertnstert.2018.06.012
2. Kim SS, Klemp J, Fabian C. Breast cancer and fertility preservation. Fertil Steril. 2011;95:15351543. doi: 10.1016/j.fertnstert.2011.01.003
- American Cancer Society. Cancer Treatment & Survivorship Facts & Figures 2022-2024. Atlanta, Georgia: American Cancer Society; 2022.
- Oktay K, Karlikaya G. Ovarian function after autologous transplantation of frozen-banked human ovarian tissue. N Engl J Med. 2000;342:1919
- Practice Committees of the American Society for Reproductive Medicine and the Society for Assisted Reproductive Technology. Mature oocyte cryopreservation: a guideline. Fertil Steril. 2013;99:37-43. doi: 10.1016 /j.fertnstert.2012.09.028
- Marklund A, Lekberg T, Hedayati E, et al. Relapse rates and diseasespecific mortality following procedures for fertility preservation at time of breast cancer diagnosis. JAMA Oncol. 2022;8:1438-1446. doi:10.1001 /jamaoncol.2022.3677
- Zhao J, Liu J, Chen K, et al. What lies behind chemotherapy-induced amenorrhea for breast cancer patients: a meta-analysis. Breast Cancer Res Treat. 2014;145:113-128. https://doi.org/10.1007/s10549-014-2914-x
- Wallace WH, Thomson AB, Saran F, et al. Predicting age of ovarian failure after radiation to a field that includes the ovaries. Int J Radiat Oncol Biol Phys. 2005;62:738-744. http://doi.org10.1016/j.ijrobp.2004.11.038
- Chung EH, Acharya CR, Harris BS, et al. Development of a fertility risk calculator to predict individualized chance of hovarian failure after chemotherapy. J Assist Reprod Genetics. 2021;38:3047-3055. https://doi .org/10.1007/s10815-021-02311-0
- Brahic C, Nauta S. Eggs From Elsewhere. The Economist. July 2023.
- Cakmak H, Rosen MP. Random-start ovarian stimulation in patients with cancer. Curr Opin Obstet Gynecol. 2015;27:215-221. doi: 10.1097/ GCO.0000000000000180
- Eaton JL, Truong T, Li YJ, et al. Prevalence of a good perinatal outcome with cryopreserved compared with fresh donor oocytes. Obstet Gynecol. 2020;135:709-716. doi: 10.1097/AOG.0000000000003695
- Practice Committee of the American Society for Reproductive Medicine. Fertility preservation in patients undergoing gonadotoxic therapy or gonadectomy: a committee opinion. Fertil Steril. 2019;112:1022-1033. doi: 10.1016/j.fertnstert.2019.09.013
- Oktay K, Marin L, Bedoschi G, et al. Ovarian transplantation with robotic surgery and a neovascularizing human extracellular matrix scaffold: a case series in comparison to meta-analytic data. Fertil Steril. 2021. doi:https ://doi.org/10.1016/j.fertnstert.2021.08.034
- Donnez J, Dolmans MM, Demylle D, et al. Livebirth after orthotopic transplantation of cryopreserved ovarian tissue. Lancet. 2004;364:1405-1410.
- Hoekman EJ, Louwe LA, Rooijers M, et al. Ovarian tissue cryopreservation: low usage rates and high live-birth rate after transplantation. Acta Obstet Gynecol Scand. 2020;99:213-221. doi: 10.1111/aogs.13735
- Donnez J, Dolmans MM, Diaz C, et al. Ovarian cortex transplantation: time to move on from experimental studies to open clinical application. Fertil Steril. 2015;104:1097-1098. doi: 10.1016/j.fertnstert.2015.08.005
- Rosendahl M, Greve T, Andersen CY. The safety of transplanting cryopreserved ovarian tissue in cancer patients: a review of the literature. J Assist Reprod Genet. 2013;30, 11-24. https://doi.org/10.1007/s10815-012-9912-x
- Soleimani R, Heytens E, Darzynkiewicz Z, et al. Mechanisms of chemotherapyinduced human ovarian aging: double strand DNA breaks and microvascular compromise. Aging (Albany NY). 2011;3:782-793.
- Milman LW, Senapati S, Sammel MD, et al. Assessing reproductive choices of women and the likelihood of oocyte cryopreservation in the era of elective oocyte freezing. Fertil Steril. 2017;107:1214-1222.e3. doi: 10.1016 /j.fertnstert.2017.03.010
- Bakkensen JB, Flannagan KSJ, Mumford SL, et al. A SART data cost-effectiveness analysis of planned oocyte cryopreservation versus in vitro fertilization with preimplantation genetic testing for aneuploidy considering ideal family size. Fertil Steril. 2022;118:875-884. https://doi.org/10.1016/j.fertnstert.2022.07.022
- Cascante SD, Blakemore JK, DeVore S. Fifteen years of autologous oocyte thaw outcomes from a large university-based fertility center. Fertil Steril. 2022;118:158-166. doi: 10.1016/j.fertnstert.2022.04.013
- Cobo A, Garrido N, Crespo J, et al. Accumulation of oocytes: a new strategy for managing low-responder patients. Reprod BioMedicine Online. 2018;37:669675. doi:10.1016/j.rbmo.2018.07.004
- Practice Committee of the American Society for Reproductive Medicine. Testing and interpreting measures of ovarian reserve: a committee opinion. Fertil Steril. 2020;114:1151-1157. doi: 10.1016/j.fertnstert.2020.09
- What you need to know about egg-freezing, the hot new perk at Google, Apple, and Facebook. Business Insider. September 17, 2017. Accessed August 9, 2023. https://www.businessinsider.com/egg-freezing-at-facebook-apple -google-hot-new-perk-2017-9
- Varlas VN, Bors RG, Albu D, et al. Social freezing: pressing pause on fertility. Int J Environ Res Public Health. 2021;18:8088. doi: 10.3390/ijerph18158088
- Hodes-Wertz B, Druckenmiller S, Smith M, et al. What do reproductive-age women who undergo oocyte cryopreservation think about the process as a means to preserve fertility? Fertil Steril. 2013;100:1343-1349. doi: 10.1016 /j.fertnstert.2013.07.201
- Moravek MB, Dixon M, Pena SM, et al. Management of testosterone around ovarian stimulation in transmasculine patients: challenging common practices to meet patient needs-2 case reports. Hum Reprod. 2023;38:482-488. doi: 10.1093/humrep/dead003
Throughout the 20th century, the management of ectopic pregnancy evolved from preserving the life of the mother to preserving fertility by utilizing the conservative treatment of methotrexate and/or tubal surgery. I make this, seemingly obscure, reference to managing ectopic pregnancy to consider an analogous shift over time in the management of patients with cancer. Over the next decade, the number of people who have lived 5 or more years after their cancer diagnosis is projected to increase approximately 30%, to 16.3 million. Due to the improved survival rates following a cancer diagnosis,1 revolutionary developments have been made in fertility preservation to obviate the impact of gonadotoxic therapy. We have evolved, however, from shielding and transposing ovaries to ovarian tissue cryopreservation,2 with rapid implementation.
While advances in reproductive cryopreservation have allowed for the delay, or even potential “prevention” of infertility, assisted reproductive technology (ART) cannot yet claim a “cure” in ensuring procreation. Nevertheless, fertility preservation is a burgeoning field that has transitioned from an experimental label to a standard of care in 2012, as designated by the American Society for Reproductive Medicine (ASRM).3 From the original intention of offering oocyte cryopreservation to women at risk of ovarian failure from impending gonadotoxic cancer treatment, fertility preservation has accelerated to include freezing for nonmedical reasons—eg, planned oocyte cryopreservation (POC), or “social” egg freezing, to ovarian tissue cryopreservation to accommodate the expediency needed for the treatment of certain cancer treatments. Additionally, across the United States, the number of donor egg banks, which allow women an easily accessible option, is rivaling enduring sperm banks. Due to the advanced methodology of vitrification and growing demand for the technology due to increasing IVF cycles, cryopreservation has become a specialized area of reproductive medicine, and a target of venture capital and private equity commercialization. This article will review the latest techniques, appropriate counseling, and cost/benefit ratio of fertility preservation, with an emphasis on POC.
CASE 1 Fertility preservation options for patient with breast cancer
A 37-year-old woman with newly diagnosed hormone receptor−positive breast cancer is referred for a fertility preservation consultation prior to initiating treatment. Her oncologist plans chemotherapy, followed by radiation and a minimum of 5 years of tamoxifen therapy.
What is the best consultation approach for this patient?
Consultation involves understanding several factors
The consultation approach to this patient involves ascertaining her medical, social, and family history, along with her reproductive plans.
Medical history. For the medical component, we must focus on her diagnosis, anticipated treatment with timeline, risks of gonadal toxicity with planned treatments, her current medical stability, and prognosis for expected survival.
Social history. Her age, relationship status, and desired family size address her social history.
Family history. Given that her cancer affects the breast, there is the risk of genetic susceptibility and potential for embryo testing for the BRCA gene.
Reproductive plans. These include her and her partner’s, if applicable, number of desired children and their risk factors for infertility.
Regarding the reproductive timeline, the antihormonal therapy that may be required for her treatment may improve overall survival, but it would delay the time to pregnancy. Consequently, the pursuit of fertility preservation prior to cancer treatment is a multidisciplinary approach that can involve medical oncology, radiation oncology, REI, medical genetics, and often, psychology. Fortunately, evidence continues to support fertility preservation, with or without hormonal ovarian stimulation, for patients with breast cancer. Data, with up to 5 years of follow-up, has indicated that it is safe.4
Continue to: Oncofertility...
Oncofertility
To address the need to maximize the reproductive potential of patients with newly diagnosed cancer, the field of oncofertility combines the specialties of oncology and reproductive medicine. The reproductive risk of cancer treatment is gonadotoxicity, with subsequent iatrogenic primary ovarian insufficiency (POI) and infertility. Alkylating agents (including cyclosphosphamide) have the highest risk for amenorrhea, while antimetabolites (including methotrexate, 5–fluorouracil) have the lowest risk.5 Treating bone marrow/stem cell transplantation using high-dose alkylating agents, with or without whole body irradiation, results in ≥80% amenorrhea. The minimum radiation dose to induce ovarian failure decreases with advancing age, from 18.4 Gy at age 10 years to 6 Gy at age 40 years, due to biologically diminishing ovarian reserve and an increase in the radiosensitivity of oocytes.6 An online tool—using varying factors including age, chemotherapy dose, prior treatment, smoking, and baseline diminished ovarian reserve—is available to help predict the chance of ovarian failure following chemotherapy.7
Since 2006, the American Society of Clinical Oncology recommended, as part of the consent prior to therapy, oncologists should address the possibility of infertility with patients “as early in treatment planning as possible” and “...Fertility preservation is an important, if not necessary, consideration when planning cancer treatment in reproductive-age patients.”
Reference
1. Lee SJ, Schover LR, Partridge AH, et al. American Society of Clinical Oncology recommendations on fertility preservation in cancer patients. J Clin Oncol. 2006;24:2917-2931.
Cryopreservation to the rescue
Since 2012, when ASRM removed the experimental designation on oocyte cryopreservation (OC), the number of cycles offered for fertility preservation has increased dramatically (FIGURE),8 initially being used for patients with cancer and now also including women desiring POC.

Ovarian and embryo cryopreservation. Ovarian stimulation and egg retrieval for OC can now occur within 2 weeks due to a random start protocol whereby women can begin ovarian stimulation any day in their cycle (ie, preovulation or postovulation).9
OC followed by thawing for subsequent fertilization and embryo transfer is employed as a matter of routine when patients with infertility utilize frozen eggs from a donor. While there remains debate over better live birth rates with frozen eggs versus fresh eggs, clinic experience may be a critical factor.10
Ovarian tissue cryopreservation. In addition to the fertility preservation procedures of oocytes and embryo cryopreservation, ovarian tissue cryopreservation became a standard option in 2019 when ASRM removed its experimental designation.11 Given the potential time constraints of urgent cancer treatment, ovarian tissue cryopreservation has the advantage of not requiring ovarian stimulation or sexual maturity and is able to be performed while patients are receiving chemotherapy. If successful, ovarian tissue cryopreservation followed by orthotopic transplantation has the potential to restore natural ovarian function and natural conceptions.12 However, despite first successfully being described in 2004, ovarian tissue cryopreservation, which does require subsequent thawing and tissue transplantation, remains less available to patients due to low usage rates, which have resulted in few clinics having adequate proficiency.13,14
Ovarian tissue cryopreservation involves obtaining ovarian cortical tissue, dissecting the tissue into small fragments, and cryopreserving it using either a slow-cool technique or vitrification. Orthotopic transplantation has been the most successful method for using ovarian tissue in humans. Live birth rates are modest.15 In all cancer survivors, particularly those with leukemia, autologous ovarian tissue transplantation may contain malignant cells that could lead to the reintroduction of cancer as the tissue is removed prior to treatment.16
Pregnancy outcomes using embryos created from ovaries recently exposed to chemotherapy in humans is not known, but animal studies suggest that there may be higher rates of miscarriage and birth defects given the severe DNA damage to oocytes of developing follicles.17 Hence, ovarian stimulation should be initiated and completed before the start of chemotherapy.
Continue to: Planned oocyte cryopreservation...
Planned oocyte cryopreservation
With advances in ART, POC offers patients the opportunity to preserve fertility until desired. However, despite its potential benefits, POC compels the discussion of various considerations in addition to oncofertility, such as ethical concerns and insurance coverage.
CASE 2 Woman plans for elective egg freezing
A 32-year-old single, professional woman is advancing in her career and wishes to delay childbearing. She is concerned about the potential for age-related fertility decline and wants to explore the option of elective egg freezing. Emily has no medical conditions that would impair her fertility, but she wants to ensure that she has the option of having biological children in the future. She is unsure about the potential financial burden of the procedure and whether her employer’s insurance covers such elective procedures.
How do you counsel her about her options?
Medical considerations
Approximately 25% of reproductive-aged women have considered POC.18 An analysis revealed POC was more cost-effective than delaying procreation and undergoing IVF with preimplantation genetic testing for aneuploidies at an advanced reproductive age.19
The process of planned oocyte cryopreservation. POC involves ovarian stimulation, usually with parenteral gonadotropins, to produce multiple mature oocytes for same-day cryopreservation following transvaginal retrieval, typically in an office-based surgery center as an outpatient procedure while the patient is under IV sedation. While the procedure has been proven effective, there are inherent risks and limitations. The success rates of subsequent fertility treatments using the cryopreserved eggs are influenced by the woman’s age at the time of freezing, the number of mature oocytes retrieved and vitrified, and the quality of the oocytes following thaw. A recent study reported a 70% live-birth rate in women aged less than 38 years who cryopreserved ≥ 20 mature eggs.20 To increase the number of cryopreserved oocytes, multiple egg retrievals or “batching” may be of benefit for women with diminished ovarian reserve.21
It is important for clinicians to thoroughly assess a patient’s medical history, ovarian reserve (by antral follicle count and levels of anti-müllerian hormone [AMH]), and reproductive goals before recommending proceeding with POC. Of note, AMH is a useful marker for ovarian reserve but has not been shown to predict natural fertility. Its value is in providing a guide to the dosage of ovarian stimulation and an estimation of the number of oocytes to be retrieved. Per ASRM, “Extremely low AMH values should not be used to refuse treatment in IVF.” AMH levels and antral follicle count have only a weak association with such qualitative outcomes as oocyte quality, clinical pregnancy rates, and live birth rates. Complications from egg retrieval, both short and long term, are rare. The inherent risk from POC is the lack of a guaranteed subsequent live birth.22
Ethical and social considerations
POC raises several ethical considerations, including concerns of perpetuating societal pressure on women to defer procreation to prioritize their careers over family planning.23 Despite controversies, POC appears as a chosen strategy against age-related infertility and may allow women to feel that they are more socially, psychologically, and financially stable before pursuing motherhood.24 Open and honest discussions between clinicians and patients are crucial to ensure informed decision making and address these ethical concerns.
Per an ACOG statement from February 2023 (https://www.acog.org/womens-health/faqs/having-a-baby-after-age-35-how-aging-affects-fertility-and-pregnancy) “...egg freezing is recommended mainly for patients having cancer treatment that will affect their future fertility. There is not enough research to recommend routine egg freezing for the sole purpose of delaying childbearing.”
A recent survey of patients who had elected egg freezing at some point included more than 80% who were aged 35 or older, and revealed that 93% of the survey participants had not yet returned to use their frozen oocytes.25 The most common reason cited in the survey for a delay in attempted procreation was lack of a partner. Another reason was undergoing oocyte cryopreservation after an optimal reproductive age, with participants concluding that they felt they had improved their reproductive future after undergoing oocyte cryopreservation and feeling empowered by the process. As part of counseling, women should be informed of the possibility of not utilizing their frozen eggs in the future, whether due to natural conception or other personal reasons.
Continue to: Employer insurance coverage...
Employer insurance coverage
Access to elective egg freezing is largely influenced by insurance coverage. Currently, employer-provided insurance coverage for this procedure varies widely. While some companies offer comprehensive coverage, others provide limited or no coverage at all. The cost of elective egg freezing can range from $10,000 to $15,000, excluding additional expenses such as medications and annual storage fees. The financial burden can create a gap between patients who desire POC and those with an ability to implement the process. The cost can be a significant barrier for many patients considering this option and perpetuates the lack of universal diversity, equity, and inclusion.
CASE 3 Gender dysphoria and fertility preservation
A 22-year-old transgender man is preparing to undergo gender-affirming hormone therapy and surgery. He is concerned about the potential impact of testosterone therapy on his oocytes and wishes to explore options for fertility preservation prior to oophorectomy.26
What are the patient’s options for fertility preservation?
The patient has the fertility preservation options of OC following ovarian stimulation or ovarian tissue cryopreservation at the time of oophorectomy. Preliminary evidence does not demonstrate impairment of ovarian stimulation and oocyte retrieval number with concurrent testosterone exposure. Ethical considerations, in this case, involve respecting the patient’s autonomy, addressing potential conflicts between gender-affirming care and fertility preservation (eg, a risk of dysphoria in transgender patients preserving biological gametes from a prior assigned gender), and ensuring access to fertility preservation services without discrimination. It is essential to provide the patient in this case with comprehensive information regarding the impact of hormone therapy on fertility, the available options, and the potential financial costs involved. Supportive counseling should also be offered to address any psychological or emotional aspects related to fertility preservation for all patients considering this option.
A call for diversity, equity, and inclusion
To improve access to POC, advocating for employer-offered insurance coverage is paramount. Women’s health providers can encourage dialogue between employers, insurers, and policymakers, which can lead to policy changes that prioritize coverage for fertilitypreservation options. This could include mandating coverage for POC as part of comprehensive health care plans or providing tax incentives to employers who offer coverage for these procedures. Furthermore, public awareness campaigns and advocacy efforts can help educate employers about the importance of including fertility preservation coverage in their employee benefits packages.
Conclusion
Just as physicians must recognize their responsibility to patients to distinguish unproven yet promising science from evidence-based and clinically established science, so too must they advise their patients to consider fertility preservation services in a way that is both clinically justified and ethically appropriate. Informed decisions must be made by appropriate counseling of evidence-based medicine to protect the interest of patients. POC provides patients with an opportunity to preserve their fertility and exercise reproductive autonomy. However, access to this procedure is often hindered by limited or nonexistent employer insurance coverage. By recognizing the medical, ethical, and social implications of POC and implementing strategies to improve coverage, collaborative efforts may increase accessibility and defray costs to provide patients with the option of deferring childbearing and preserving their reproductive potential. ●
1. Promptly offer fertility preservation treatment options with sensitivity and clarity.
2. Dedicate ample time and exercise patience during the consultation.
3. Provide education using multiple modalities to help patients assimilate information.
4. Encourage consultation with mental health professionals.
Special considerations for hematologic malignancies:
- Treatment can be associated with significant gonadal toxicity and premature ovarian failure.
- Patients are frequently ill at the time of presentation and ineligible for certain fertility preservation options.
References
1. Ethics Committee of the American Society for Reproductive Medicine. Fertility preservation and reproduction in patients facing gonadotoxic therapies: a committee opinion. Fertil Steril. 2018;110:380-386. doi:10.1016/j.fertnstert.2018.06.012
2. Kim SS, Klemp J, Fabian C. Breast cancer and fertility preservation. Fertil Steril. 2011;95:15351543. doi: 10.1016/j.fertnstert.2011.01.003
Throughout the 20th century, the management of ectopic pregnancy evolved from preserving the life of the mother to preserving fertility by utilizing the conservative treatment of methotrexate and/or tubal surgery. I make this, seemingly obscure, reference to managing ectopic pregnancy to consider an analogous shift over time in the management of patients with cancer. Over the next decade, the number of people who have lived 5 or more years after their cancer diagnosis is projected to increase approximately 30%, to 16.3 million. Due to the improved survival rates following a cancer diagnosis,1 revolutionary developments have been made in fertility preservation to obviate the impact of gonadotoxic therapy. We have evolved, however, from shielding and transposing ovaries to ovarian tissue cryopreservation,2 with rapid implementation.
While advances in reproductive cryopreservation have allowed for the delay, or even potential “prevention” of infertility, assisted reproductive technology (ART) cannot yet claim a “cure” in ensuring procreation. Nevertheless, fertility preservation is a burgeoning field that has transitioned from an experimental label to a standard of care in 2012, as designated by the American Society for Reproductive Medicine (ASRM).3 From the original intention of offering oocyte cryopreservation to women at risk of ovarian failure from impending gonadotoxic cancer treatment, fertility preservation has accelerated to include freezing for nonmedical reasons—eg, planned oocyte cryopreservation (POC), or “social” egg freezing, to ovarian tissue cryopreservation to accommodate the expediency needed for the treatment of certain cancer treatments. Additionally, across the United States, the number of donor egg banks, which allow women an easily accessible option, is rivaling enduring sperm banks. Due to the advanced methodology of vitrification and growing demand for the technology due to increasing IVF cycles, cryopreservation has become a specialized area of reproductive medicine, and a target of venture capital and private equity commercialization. This article will review the latest techniques, appropriate counseling, and cost/benefit ratio of fertility preservation, with an emphasis on POC.
CASE 1 Fertility preservation options for patient with breast cancer
A 37-year-old woman with newly diagnosed hormone receptor−positive breast cancer is referred for a fertility preservation consultation prior to initiating treatment. Her oncologist plans chemotherapy, followed by radiation and a minimum of 5 years of tamoxifen therapy.
What is the best consultation approach for this patient?
Consultation involves understanding several factors
The consultation approach to this patient involves ascertaining her medical, social, and family history, along with her reproductive plans.
Medical history. For the medical component, we must focus on her diagnosis, anticipated treatment with timeline, risks of gonadal toxicity with planned treatments, her current medical stability, and prognosis for expected survival.
Social history. Her age, relationship status, and desired family size address her social history.
Family history. Given that her cancer affects the breast, there is the risk of genetic susceptibility and potential for embryo testing for the BRCA gene.
Reproductive plans. These include her and her partner’s, if applicable, number of desired children and their risk factors for infertility.
Regarding the reproductive timeline, the antihormonal therapy that may be required for her treatment may improve overall survival, but it would delay the time to pregnancy. Consequently, the pursuit of fertility preservation prior to cancer treatment is a multidisciplinary approach that can involve medical oncology, radiation oncology, REI, medical genetics, and often, psychology. Fortunately, evidence continues to support fertility preservation, with or without hormonal ovarian stimulation, for patients with breast cancer. Data, with up to 5 years of follow-up, has indicated that it is safe.4
Continue to: Oncofertility...
Oncofertility
To address the need to maximize the reproductive potential of patients with newly diagnosed cancer, the field of oncofertility combines the specialties of oncology and reproductive medicine. The reproductive risk of cancer treatment is gonadotoxicity, with subsequent iatrogenic primary ovarian insufficiency (POI) and infertility. Alkylating agents (including cyclosphosphamide) have the highest risk for amenorrhea, while antimetabolites (including methotrexate, 5–fluorouracil) have the lowest risk.5 Treating bone marrow/stem cell transplantation using high-dose alkylating agents, with or without whole body irradiation, results in ≥80% amenorrhea. The minimum radiation dose to induce ovarian failure decreases with advancing age, from 18.4 Gy at age 10 years to 6 Gy at age 40 years, due to biologically diminishing ovarian reserve and an increase in the radiosensitivity of oocytes.6 An online tool—using varying factors including age, chemotherapy dose, prior treatment, smoking, and baseline diminished ovarian reserve—is available to help predict the chance of ovarian failure following chemotherapy.7
Since 2006, the American Society of Clinical Oncology recommended, as part of the consent prior to therapy, oncologists should address the possibility of infertility with patients “as early in treatment planning as possible” and “...Fertility preservation is an important, if not necessary, consideration when planning cancer treatment in reproductive-age patients.”
Reference
1. Lee SJ, Schover LR, Partridge AH, et al. American Society of Clinical Oncology recommendations on fertility preservation in cancer patients. J Clin Oncol. 2006;24:2917-2931.
Cryopreservation to the rescue
Since 2012, when ASRM removed the experimental designation on oocyte cryopreservation (OC), the number of cycles offered for fertility preservation has increased dramatically (FIGURE),8 initially being used for patients with cancer and now also including women desiring POC.

Ovarian and embryo cryopreservation. Ovarian stimulation and egg retrieval for OC can now occur within 2 weeks due to a random start protocol whereby women can begin ovarian stimulation any day in their cycle (ie, preovulation or postovulation).9
OC followed by thawing for subsequent fertilization and embryo transfer is employed as a matter of routine when patients with infertility utilize frozen eggs from a donor. While there remains debate over better live birth rates with frozen eggs versus fresh eggs, clinic experience may be a critical factor.10
Ovarian tissue cryopreservation. In addition to the fertility preservation procedures of oocytes and embryo cryopreservation, ovarian tissue cryopreservation became a standard option in 2019 when ASRM removed its experimental designation.11 Given the potential time constraints of urgent cancer treatment, ovarian tissue cryopreservation has the advantage of not requiring ovarian stimulation or sexual maturity and is able to be performed while patients are receiving chemotherapy. If successful, ovarian tissue cryopreservation followed by orthotopic transplantation has the potential to restore natural ovarian function and natural conceptions.12 However, despite first successfully being described in 2004, ovarian tissue cryopreservation, which does require subsequent thawing and tissue transplantation, remains less available to patients due to low usage rates, which have resulted in few clinics having adequate proficiency.13,14
Ovarian tissue cryopreservation involves obtaining ovarian cortical tissue, dissecting the tissue into small fragments, and cryopreserving it using either a slow-cool technique or vitrification. Orthotopic transplantation has been the most successful method for using ovarian tissue in humans. Live birth rates are modest.15 In all cancer survivors, particularly those with leukemia, autologous ovarian tissue transplantation may contain malignant cells that could lead to the reintroduction of cancer as the tissue is removed prior to treatment.16
Pregnancy outcomes using embryos created from ovaries recently exposed to chemotherapy in humans is not known, but animal studies suggest that there may be higher rates of miscarriage and birth defects given the severe DNA damage to oocytes of developing follicles.17 Hence, ovarian stimulation should be initiated and completed before the start of chemotherapy.
Continue to: Planned oocyte cryopreservation...
Planned oocyte cryopreservation
With advances in ART, POC offers patients the opportunity to preserve fertility until desired. However, despite its potential benefits, POC compels the discussion of various considerations in addition to oncofertility, such as ethical concerns and insurance coverage.
CASE 2 Woman plans for elective egg freezing
A 32-year-old single, professional woman is advancing in her career and wishes to delay childbearing. She is concerned about the potential for age-related fertility decline and wants to explore the option of elective egg freezing. Emily has no medical conditions that would impair her fertility, but she wants to ensure that she has the option of having biological children in the future. She is unsure about the potential financial burden of the procedure and whether her employer’s insurance covers such elective procedures.
How do you counsel her about her options?
Medical considerations
Approximately 25% of reproductive-aged women have considered POC.18 An analysis revealed POC was more cost-effective than delaying procreation and undergoing IVF with preimplantation genetic testing for aneuploidies at an advanced reproductive age.19
The process of planned oocyte cryopreservation. POC involves ovarian stimulation, usually with parenteral gonadotropins, to produce multiple mature oocytes for same-day cryopreservation following transvaginal retrieval, typically in an office-based surgery center as an outpatient procedure while the patient is under IV sedation. While the procedure has been proven effective, there are inherent risks and limitations. The success rates of subsequent fertility treatments using the cryopreserved eggs are influenced by the woman’s age at the time of freezing, the number of mature oocytes retrieved and vitrified, and the quality of the oocytes following thaw. A recent study reported a 70% live-birth rate in women aged less than 38 years who cryopreserved ≥ 20 mature eggs.20 To increase the number of cryopreserved oocytes, multiple egg retrievals or “batching” may be of benefit for women with diminished ovarian reserve.21
It is important for clinicians to thoroughly assess a patient’s medical history, ovarian reserve (by antral follicle count and levels of anti-müllerian hormone [AMH]), and reproductive goals before recommending proceeding with POC. Of note, AMH is a useful marker for ovarian reserve but has not been shown to predict natural fertility. Its value is in providing a guide to the dosage of ovarian stimulation and an estimation of the number of oocytes to be retrieved. Per ASRM, “Extremely low AMH values should not be used to refuse treatment in IVF.” AMH levels and antral follicle count have only a weak association with such qualitative outcomes as oocyte quality, clinical pregnancy rates, and live birth rates. Complications from egg retrieval, both short and long term, are rare. The inherent risk from POC is the lack of a guaranteed subsequent live birth.22
Ethical and social considerations
POC raises several ethical considerations, including concerns of perpetuating societal pressure on women to defer procreation to prioritize their careers over family planning.23 Despite controversies, POC appears as a chosen strategy against age-related infertility and may allow women to feel that they are more socially, psychologically, and financially stable before pursuing motherhood.24 Open and honest discussions between clinicians and patients are crucial to ensure informed decision making and address these ethical concerns.
Per an ACOG statement from February 2023 (https://www.acog.org/womens-health/faqs/having-a-baby-after-age-35-how-aging-affects-fertility-and-pregnancy) “...egg freezing is recommended mainly for patients having cancer treatment that will affect their future fertility. There is not enough research to recommend routine egg freezing for the sole purpose of delaying childbearing.”
A recent survey of patients who had elected egg freezing at some point included more than 80% who were aged 35 or older, and revealed that 93% of the survey participants had not yet returned to use their frozen oocytes.25 The most common reason cited in the survey for a delay in attempted procreation was lack of a partner. Another reason was undergoing oocyte cryopreservation after an optimal reproductive age, with participants concluding that they felt they had improved their reproductive future after undergoing oocyte cryopreservation and feeling empowered by the process. As part of counseling, women should be informed of the possibility of not utilizing their frozen eggs in the future, whether due to natural conception or other personal reasons.
Continue to: Employer insurance coverage...
Employer insurance coverage
Access to elective egg freezing is largely influenced by insurance coverage. Currently, employer-provided insurance coverage for this procedure varies widely. While some companies offer comprehensive coverage, others provide limited or no coverage at all. The cost of elective egg freezing can range from $10,000 to $15,000, excluding additional expenses such as medications and annual storage fees. The financial burden can create a gap between patients who desire POC and those with an ability to implement the process. The cost can be a significant barrier for many patients considering this option and perpetuates the lack of universal diversity, equity, and inclusion.
CASE 3 Gender dysphoria and fertility preservation
A 22-year-old transgender man is preparing to undergo gender-affirming hormone therapy and surgery. He is concerned about the potential impact of testosterone therapy on his oocytes and wishes to explore options for fertility preservation prior to oophorectomy.26
What are the patient’s options for fertility preservation?
The patient has the fertility preservation options of OC following ovarian stimulation or ovarian tissue cryopreservation at the time of oophorectomy. Preliminary evidence does not demonstrate impairment of ovarian stimulation and oocyte retrieval number with concurrent testosterone exposure. Ethical considerations, in this case, involve respecting the patient’s autonomy, addressing potential conflicts between gender-affirming care and fertility preservation (eg, a risk of dysphoria in transgender patients preserving biological gametes from a prior assigned gender), and ensuring access to fertility preservation services without discrimination. It is essential to provide the patient in this case with comprehensive information regarding the impact of hormone therapy on fertility, the available options, and the potential financial costs involved. Supportive counseling should also be offered to address any psychological or emotional aspects related to fertility preservation for all patients considering this option.
A call for diversity, equity, and inclusion
To improve access to POC, advocating for employer-offered insurance coverage is paramount. Women’s health providers can encourage dialogue between employers, insurers, and policymakers, which can lead to policy changes that prioritize coverage for fertilitypreservation options. This could include mandating coverage for POC as part of comprehensive health care plans or providing tax incentives to employers who offer coverage for these procedures. Furthermore, public awareness campaigns and advocacy efforts can help educate employers about the importance of including fertility preservation coverage in their employee benefits packages.
Conclusion
Just as physicians must recognize their responsibility to patients to distinguish unproven yet promising science from evidence-based and clinically established science, so too must they advise their patients to consider fertility preservation services in a way that is both clinically justified and ethically appropriate. Informed decisions must be made by appropriate counseling of evidence-based medicine to protect the interest of patients. POC provides patients with an opportunity to preserve their fertility and exercise reproductive autonomy. However, access to this procedure is often hindered by limited or nonexistent employer insurance coverage. By recognizing the medical, ethical, and social implications of POC and implementing strategies to improve coverage, collaborative efforts may increase accessibility and defray costs to provide patients with the option of deferring childbearing and preserving their reproductive potential. ●
1. Promptly offer fertility preservation treatment options with sensitivity and clarity.
2. Dedicate ample time and exercise patience during the consultation.
3. Provide education using multiple modalities to help patients assimilate information.
4. Encourage consultation with mental health professionals.
Special considerations for hematologic malignancies:
- Treatment can be associated with significant gonadal toxicity and premature ovarian failure.
- Patients are frequently ill at the time of presentation and ineligible for certain fertility preservation options.
References
1. Ethics Committee of the American Society for Reproductive Medicine. Fertility preservation and reproduction in patients facing gonadotoxic therapies: a committee opinion. Fertil Steril. 2018;110:380-386. doi:10.1016/j.fertnstert.2018.06.012
2. Kim SS, Klemp J, Fabian C. Breast cancer and fertility preservation. Fertil Steril. 2011;95:15351543. doi: 10.1016/j.fertnstert.2011.01.003
- American Cancer Society. Cancer Treatment & Survivorship Facts & Figures 2022-2024. Atlanta, Georgia: American Cancer Society; 2022.
- Oktay K, Karlikaya G. Ovarian function after autologous transplantation of frozen-banked human ovarian tissue. N Engl J Med. 2000;342:1919
- Practice Committees of the American Society for Reproductive Medicine and the Society for Assisted Reproductive Technology. Mature oocyte cryopreservation: a guideline. Fertil Steril. 2013;99:37-43. doi: 10.1016 /j.fertnstert.2012.09.028
- Marklund A, Lekberg T, Hedayati E, et al. Relapse rates and diseasespecific mortality following procedures for fertility preservation at time of breast cancer diagnosis. JAMA Oncol. 2022;8:1438-1446. doi:10.1001 /jamaoncol.2022.3677
- Zhao J, Liu J, Chen K, et al. What lies behind chemotherapy-induced amenorrhea for breast cancer patients: a meta-analysis. Breast Cancer Res Treat. 2014;145:113-128. https://doi.org/10.1007/s10549-014-2914-x
- Wallace WH, Thomson AB, Saran F, et al. Predicting age of ovarian failure after radiation to a field that includes the ovaries. Int J Radiat Oncol Biol Phys. 2005;62:738-744. http://doi.org10.1016/j.ijrobp.2004.11.038
- Chung EH, Acharya CR, Harris BS, et al. Development of a fertility risk calculator to predict individualized chance of hovarian failure after chemotherapy. J Assist Reprod Genetics. 2021;38:3047-3055. https://doi .org/10.1007/s10815-021-02311-0
- Brahic C, Nauta S. Eggs From Elsewhere. The Economist. July 2023.
- Cakmak H, Rosen MP. Random-start ovarian stimulation in patients with cancer. Curr Opin Obstet Gynecol. 2015;27:215-221. doi: 10.1097/ GCO.0000000000000180
- Eaton JL, Truong T, Li YJ, et al. Prevalence of a good perinatal outcome with cryopreserved compared with fresh donor oocytes. Obstet Gynecol. 2020;135:709-716. doi: 10.1097/AOG.0000000000003695
- Practice Committee of the American Society for Reproductive Medicine. Fertility preservation in patients undergoing gonadotoxic therapy or gonadectomy: a committee opinion. Fertil Steril. 2019;112:1022-1033. doi: 10.1016/j.fertnstert.2019.09.013
- Oktay K, Marin L, Bedoschi G, et al. Ovarian transplantation with robotic surgery and a neovascularizing human extracellular matrix scaffold: a case series in comparison to meta-analytic data. Fertil Steril. 2021. doi:https ://doi.org/10.1016/j.fertnstert.2021.08.034
- Donnez J, Dolmans MM, Demylle D, et al. Livebirth after orthotopic transplantation of cryopreserved ovarian tissue. Lancet. 2004;364:1405-1410.
- Hoekman EJ, Louwe LA, Rooijers M, et al. Ovarian tissue cryopreservation: low usage rates and high live-birth rate after transplantation. Acta Obstet Gynecol Scand. 2020;99:213-221. doi: 10.1111/aogs.13735
- Donnez J, Dolmans MM, Diaz C, et al. Ovarian cortex transplantation: time to move on from experimental studies to open clinical application. Fertil Steril. 2015;104:1097-1098. doi: 10.1016/j.fertnstert.2015.08.005
- Rosendahl M, Greve T, Andersen CY. The safety of transplanting cryopreserved ovarian tissue in cancer patients: a review of the literature. J Assist Reprod Genet. 2013;30, 11-24. https://doi.org/10.1007/s10815-012-9912-x
- Soleimani R, Heytens E, Darzynkiewicz Z, et al. Mechanisms of chemotherapyinduced human ovarian aging: double strand DNA breaks and microvascular compromise. Aging (Albany NY). 2011;3:782-793.
- Milman LW, Senapati S, Sammel MD, et al. Assessing reproductive choices of women and the likelihood of oocyte cryopreservation in the era of elective oocyte freezing. Fertil Steril. 2017;107:1214-1222.e3. doi: 10.1016 /j.fertnstert.2017.03.010
- Bakkensen JB, Flannagan KSJ, Mumford SL, et al. A SART data cost-effectiveness analysis of planned oocyte cryopreservation versus in vitro fertilization with preimplantation genetic testing for aneuploidy considering ideal family size. Fertil Steril. 2022;118:875-884. https://doi.org/10.1016/j.fertnstert.2022.07.022
- Cascante SD, Blakemore JK, DeVore S. Fifteen years of autologous oocyte thaw outcomes from a large university-based fertility center. Fertil Steril. 2022;118:158-166. doi: 10.1016/j.fertnstert.2022.04.013
- Cobo A, Garrido N, Crespo J, et al. Accumulation of oocytes: a new strategy for managing low-responder patients. Reprod BioMedicine Online. 2018;37:669675. doi:10.1016/j.rbmo.2018.07.004
- Practice Committee of the American Society for Reproductive Medicine. Testing and interpreting measures of ovarian reserve: a committee opinion. Fertil Steril. 2020;114:1151-1157. doi: 10.1016/j.fertnstert.2020.09
- What you need to know about egg-freezing, the hot new perk at Google, Apple, and Facebook. Business Insider. September 17, 2017. Accessed August 9, 2023. https://www.businessinsider.com/egg-freezing-at-facebook-apple -google-hot-new-perk-2017-9
- Varlas VN, Bors RG, Albu D, et al. Social freezing: pressing pause on fertility. Int J Environ Res Public Health. 2021;18:8088. doi: 10.3390/ijerph18158088
- Hodes-Wertz B, Druckenmiller S, Smith M, et al. What do reproductive-age women who undergo oocyte cryopreservation think about the process as a means to preserve fertility? Fertil Steril. 2013;100:1343-1349. doi: 10.1016 /j.fertnstert.2013.07.201
- Moravek MB, Dixon M, Pena SM, et al. Management of testosterone around ovarian stimulation in transmasculine patients: challenging common practices to meet patient needs-2 case reports. Hum Reprod. 2023;38:482-488. doi: 10.1093/humrep/dead003
- American Cancer Society. Cancer Treatment & Survivorship Facts & Figures 2022-2024. Atlanta, Georgia: American Cancer Society; 2022.
- Oktay K, Karlikaya G. Ovarian function after autologous transplantation of frozen-banked human ovarian tissue. N Engl J Med. 2000;342:1919
- Practice Committees of the American Society for Reproductive Medicine and the Society for Assisted Reproductive Technology. Mature oocyte cryopreservation: a guideline. Fertil Steril. 2013;99:37-43. doi: 10.1016 /j.fertnstert.2012.09.028
- Marklund A, Lekberg T, Hedayati E, et al. Relapse rates and diseasespecific mortality following procedures for fertility preservation at time of breast cancer diagnosis. JAMA Oncol. 2022;8:1438-1446. doi:10.1001 /jamaoncol.2022.3677
- Zhao J, Liu J, Chen K, et al. What lies behind chemotherapy-induced amenorrhea for breast cancer patients: a meta-analysis. Breast Cancer Res Treat. 2014;145:113-128. https://doi.org/10.1007/s10549-014-2914-x
- Wallace WH, Thomson AB, Saran F, et al. Predicting age of ovarian failure after radiation to a field that includes the ovaries. Int J Radiat Oncol Biol Phys. 2005;62:738-744. http://doi.org10.1016/j.ijrobp.2004.11.038
- Chung EH, Acharya CR, Harris BS, et al. Development of a fertility risk calculator to predict individualized chance of hovarian failure after chemotherapy. J Assist Reprod Genetics. 2021;38:3047-3055. https://doi .org/10.1007/s10815-021-02311-0
- Brahic C, Nauta S. Eggs From Elsewhere. The Economist. July 2023.
- Cakmak H, Rosen MP. Random-start ovarian stimulation in patients with cancer. Curr Opin Obstet Gynecol. 2015;27:215-221. doi: 10.1097/ GCO.0000000000000180
- Eaton JL, Truong T, Li YJ, et al. Prevalence of a good perinatal outcome with cryopreserved compared with fresh donor oocytes. Obstet Gynecol. 2020;135:709-716. doi: 10.1097/AOG.0000000000003695
- Practice Committee of the American Society for Reproductive Medicine. Fertility preservation in patients undergoing gonadotoxic therapy or gonadectomy: a committee opinion. Fertil Steril. 2019;112:1022-1033. doi: 10.1016/j.fertnstert.2019.09.013
- Oktay K, Marin L, Bedoschi G, et al. Ovarian transplantation with robotic surgery and a neovascularizing human extracellular matrix scaffold: a case series in comparison to meta-analytic data. Fertil Steril. 2021. doi:https ://doi.org/10.1016/j.fertnstert.2021.08.034
- Donnez J, Dolmans MM, Demylle D, et al. Livebirth after orthotopic transplantation of cryopreserved ovarian tissue. Lancet. 2004;364:1405-1410.
- Hoekman EJ, Louwe LA, Rooijers M, et al. Ovarian tissue cryopreservation: low usage rates and high live-birth rate after transplantation. Acta Obstet Gynecol Scand. 2020;99:213-221. doi: 10.1111/aogs.13735
- Donnez J, Dolmans MM, Diaz C, et al. Ovarian cortex transplantation: time to move on from experimental studies to open clinical application. Fertil Steril. 2015;104:1097-1098. doi: 10.1016/j.fertnstert.2015.08.005
- Rosendahl M, Greve T, Andersen CY. The safety of transplanting cryopreserved ovarian tissue in cancer patients: a review of the literature. J Assist Reprod Genet. 2013;30, 11-24. https://doi.org/10.1007/s10815-012-9912-x
- Soleimani R, Heytens E, Darzynkiewicz Z, et al. Mechanisms of chemotherapyinduced human ovarian aging: double strand DNA breaks and microvascular compromise. Aging (Albany NY). 2011;3:782-793.
- Milman LW, Senapati S, Sammel MD, et al. Assessing reproductive choices of women and the likelihood of oocyte cryopreservation in the era of elective oocyte freezing. Fertil Steril. 2017;107:1214-1222.e3. doi: 10.1016 /j.fertnstert.2017.03.010
- Bakkensen JB, Flannagan KSJ, Mumford SL, et al. A SART data cost-effectiveness analysis of planned oocyte cryopreservation versus in vitro fertilization with preimplantation genetic testing for aneuploidy considering ideal family size. Fertil Steril. 2022;118:875-884. https://doi.org/10.1016/j.fertnstert.2022.07.022
- Cascante SD, Blakemore JK, DeVore S. Fifteen years of autologous oocyte thaw outcomes from a large university-based fertility center. Fertil Steril. 2022;118:158-166. doi: 10.1016/j.fertnstert.2022.04.013
- Cobo A, Garrido N, Crespo J, et al. Accumulation of oocytes: a new strategy for managing low-responder patients. Reprod BioMedicine Online. 2018;37:669675. doi:10.1016/j.rbmo.2018.07.004
- Practice Committee of the American Society for Reproductive Medicine. Testing and interpreting measures of ovarian reserve: a committee opinion. Fertil Steril. 2020;114:1151-1157. doi: 10.1016/j.fertnstert.2020.09
- What you need to know about egg-freezing, the hot new perk at Google, Apple, and Facebook. Business Insider. September 17, 2017. Accessed August 9, 2023. https://www.businessinsider.com/egg-freezing-at-facebook-apple -google-hot-new-perk-2017-9
- Varlas VN, Bors RG, Albu D, et al. Social freezing: pressing pause on fertility. Int J Environ Res Public Health. 2021;18:8088. doi: 10.3390/ijerph18158088
- Hodes-Wertz B, Druckenmiller S, Smith M, et al. What do reproductive-age women who undergo oocyte cryopreservation think about the process as a means to preserve fertility? Fertil Steril. 2013;100:1343-1349. doi: 10.1016 /j.fertnstert.2013.07.201
- Moravek MB, Dixon M, Pena SM, et al. Management of testosterone around ovarian stimulation in transmasculine patients: challenging common practices to meet patient needs-2 case reports. Hum Reprod. 2023;38:482-488. doi: 10.1093/humrep/dead003
Can skin bleaching lead to cancer?
SINGAPORE –
This question was posed by Ousmane Faye, MD, PhD, director general of Mali’s Bamako Dermatology Hospital, at the World Congress of Dermatology.
Dr. Faye explored the issue during a hot topics session at the meeting, prefacing that it was an important question to ask because “in West Africa, skin bleaching is very common.”
“There are many local names” for skin bleaching, he said. “For example, in Senegal, it’s called xessal; in Mali and Ivory Coast, its name is caco; in South Africa, there are many names, like ukutsheyisa.”
Skin bleaching refers to the cosmetic misuse of topical agents to change one’s natural skin color. It’s a centuries-old practice that people, mainly women, adopt “to increase attractiveness and self-esteem,” explained Dr. Faye.
To demonstrate how pervasive skin bleaching is on the continent, he presented a slide that summarized figures from six studies spanning the past 2 decades. Prevalence ranged from 25% in Mali (based on a 1993 survey of 210 women) to a high of 79.25% in Benin (from a sample size of 511 women in 2019). In other studies of women in Burkina Faso and Togo, the figures were 44.3% and 58.9%, respectively. The most recently conducted study, which involved 2,689 Senegalese women and was published in 2022, found that nearly 6 in 10 (59.2%) respondents used skin-lightening products.
But skin bleaching isn’t just limited to Africa, said session moderator Omar Lupi, MD, PhD, associate professor of dermatology at the Federal University of the State of Rio de Janeiro, when approached for an independent comment. “It’s a traditional practice around the world. Maybe not in the developed countries, but it’s quite common in Africa, in South America, and in Asia.”
His sentiments are echoed in a meta-analysis that was published in the International Journal of Dermatology in 2019. The work examined 68 studies involving more than 67,000 people across Africa, Asia, Europe, the Middle East, and North America. It found that the pooled lifetime prevalence of skin bleaching was 27.7% (95% confidence interval, 19.6-37.5; P < .01).
“This is an important and interesting topic because our world is shrinking,” Dr. Lupi told this news organization. “Even in countries that don’t have bleaching as a common situation, we now have patients who are migrating from one part [of the world] to another, so bleaching is something that can knock on your door and you need to be prepared.”
Misuse leads to complications
The issue is pertinent to dermatologists because skin bleaching is associated with a wide range of complications. Take, for example, topical steroids, which are the most common products used for bleaching, said Dr. Faye in his talk.
“Clobetasol can suppress the hypothalamic-pituitary-adrenal (HPA) function,” he said, referring to the body’s main stress response system. “It can also foster skin infection, including bacterial, fungal, viral, and parasitic infection.”
In addition, topical steroids that are misused as skin lighteners have been reported to cause stretch marks, skin atrophy, inflammatory acne, and even metabolic disorders such as diabetes and hypertension, said Dr. Faye.
To further his point, he cited a 2021 prospective case-control study conducted across five sub-Saharan countries, which found that the use of “voluntary cosmetic depigmentation” significantly increased a person’s risk for necrotizing fasciitis of the lower limbs (odds ratio, 2.29; 95% CI, 1.19-3.73; P = .0226).
Similarly, mercury, another substance found in products commonly used to bleach skin, has been associated with problems ranging from rashes to renal toxicity. And because it’s so incredibly harmful, mercury is also known to cause neurologic abnormalities.
Apart from causing certain conditions, prolonged use of skin-lightening products can change the way existing diseases present themselves as well as their severity, added Dr. Faye.
An increased risk
But what about skin bleaching’s link with cancer? “Skin cancer on Black skin is uncommon, yet it occurs in skin-bleaching women,” said Dr. Faye.
“Since 2000, we have had some cases of skin cancer associated with skin bleaching,” he continued, adding that squamous cell carcinoma (SCC) is the most frequent type of cancer observed.
If you look at what’s been published on the topic so far, you’ll see that “all the cases of skin cancer are located over the neck or some exposed area when skin bleaching products are used for more than 10 years,” said Dr. Faye. “And most of the time, the age of the patient ranges from 30 to 60 years.”
The first known case in Africa was reported in a 58-year-old woman from Ghana, who had been using skin bleaching products for close to 30 years. The patient presented with tumors on her face, neck, and arms.
Dr. Faye then proceeded to share more than 10 such carcinoma cases. “These previous reports strongly suggest a relationship between skin bleaching and skin cancers,” said Dr. Faye.
Indeed, there have been reports and publications in the literature that support his observation, including one last year, which found that use of the tyrosinase inhibitor hydroquinone was associated with approximately a threefold increased risk for skin cancer.
For some, including Brazil’s Dr. Lupi, Dr. Faye’s talk was enlightening: “I didn’t know about this relationship [of bleaching] with skin cancer, it was something new for me.”
But the prevalence of SCC is very low, compared with that of skin bleaching, Dr. Faye acknowledged. Moreover, the cancer observed in the cases reported could have resulted from a number of reasons, including exposure to harmful ultraviolet rays from the sun and genetic predisposition in addition to the use of bleaching products such as hydroquinone. “Other causes of skin cancer are not excluded,” he said.
To further explore the link between skin bleaching and cancer, “we need case-control studies to provide more evidence,” he added. Until then, dermatologists “should keep on promoting messages” to prevent SCC from occurring. This includes encouraging the use of proper sun protection in addition to discouraging the practice of skin bleaching, which still persists despite more than 10 African nations banning the use of toxic skin-lightening products.
Dr. Faye and Dr. Lupi report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
SINGAPORE –
This question was posed by Ousmane Faye, MD, PhD, director general of Mali’s Bamako Dermatology Hospital, at the World Congress of Dermatology.
Dr. Faye explored the issue during a hot topics session at the meeting, prefacing that it was an important question to ask because “in West Africa, skin bleaching is very common.”
“There are many local names” for skin bleaching, he said. “For example, in Senegal, it’s called xessal; in Mali and Ivory Coast, its name is caco; in South Africa, there are many names, like ukutsheyisa.”
Skin bleaching refers to the cosmetic misuse of topical agents to change one’s natural skin color. It’s a centuries-old practice that people, mainly women, adopt “to increase attractiveness and self-esteem,” explained Dr. Faye.
To demonstrate how pervasive skin bleaching is on the continent, he presented a slide that summarized figures from six studies spanning the past 2 decades. Prevalence ranged from 25% in Mali (based on a 1993 survey of 210 women) to a high of 79.25% in Benin (from a sample size of 511 women in 2019). In other studies of women in Burkina Faso and Togo, the figures were 44.3% and 58.9%, respectively. The most recently conducted study, which involved 2,689 Senegalese women and was published in 2022, found that nearly 6 in 10 (59.2%) respondents used skin-lightening products.
But skin bleaching isn’t just limited to Africa, said session moderator Omar Lupi, MD, PhD, associate professor of dermatology at the Federal University of the State of Rio de Janeiro, when approached for an independent comment. “It’s a traditional practice around the world. Maybe not in the developed countries, but it’s quite common in Africa, in South America, and in Asia.”
His sentiments are echoed in a meta-analysis that was published in the International Journal of Dermatology in 2019. The work examined 68 studies involving more than 67,000 people across Africa, Asia, Europe, the Middle East, and North America. It found that the pooled lifetime prevalence of skin bleaching was 27.7% (95% confidence interval, 19.6-37.5; P < .01).
“This is an important and interesting topic because our world is shrinking,” Dr. Lupi told this news organization. “Even in countries that don’t have bleaching as a common situation, we now have patients who are migrating from one part [of the world] to another, so bleaching is something that can knock on your door and you need to be prepared.”
Misuse leads to complications
The issue is pertinent to dermatologists because skin bleaching is associated with a wide range of complications. Take, for example, topical steroids, which are the most common products used for bleaching, said Dr. Faye in his talk.
“Clobetasol can suppress the hypothalamic-pituitary-adrenal (HPA) function,” he said, referring to the body’s main stress response system. “It can also foster skin infection, including bacterial, fungal, viral, and parasitic infection.”
In addition, topical steroids that are misused as skin lighteners have been reported to cause stretch marks, skin atrophy, inflammatory acne, and even metabolic disorders such as diabetes and hypertension, said Dr. Faye.
To further his point, he cited a 2021 prospective case-control study conducted across five sub-Saharan countries, which found that the use of “voluntary cosmetic depigmentation” significantly increased a person’s risk for necrotizing fasciitis of the lower limbs (odds ratio, 2.29; 95% CI, 1.19-3.73; P = .0226).
Similarly, mercury, another substance found in products commonly used to bleach skin, has been associated with problems ranging from rashes to renal toxicity. And because it’s so incredibly harmful, mercury is also known to cause neurologic abnormalities.
Apart from causing certain conditions, prolonged use of skin-lightening products can change the way existing diseases present themselves as well as their severity, added Dr. Faye.
An increased risk
But what about skin bleaching’s link with cancer? “Skin cancer on Black skin is uncommon, yet it occurs in skin-bleaching women,” said Dr. Faye.
“Since 2000, we have had some cases of skin cancer associated with skin bleaching,” he continued, adding that squamous cell carcinoma (SCC) is the most frequent type of cancer observed.
If you look at what’s been published on the topic so far, you’ll see that “all the cases of skin cancer are located over the neck or some exposed area when skin bleaching products are used for more than 10 years,” said Dr. Faye. “And most of the time, the age of the patient ranges from 30 to 60 years.”
The first known case in Africa was reported in a 58-year-old woman from Ghana, who had been using skin bleaching products for close to 30 years. The patient presented with tumors on her face, neck, and arms.
Dr. Faye then proceeded to share more than 10 such carcinoma cases. “These previous reports strongly suggest a relationship between skin bleaching and skin cancers,” said Dr. Faye.
Indeed, there have been reports and publications in the literature that support his observation, including one last year, which found that use of the tyrosinase inhibitor hydroquinone was associated with approximately a threefold increased risk for skin cancer.
For some, including Brazil’s Dr. Lupi, Dr. Faye’s talk was enlightening: “I didn’t know about this relationship [of bleaching] with skin cancer, it was something new for me.”
But the prevalence of SCC is very low, compared with that of skin bleaching, Dr. Faye acknowledged. Moreover, the cancer observed in the cases reported could have resulted from a number of reasons, including exposure to harmful ultraviolet rays from the sun and genetic predisposition in addition to the use of bleaching products such as hydroquinone. “Other causes of skin cancer are not excluded,” he said.
To further explore the link between skin bleaching and cancer, “we need case-control studies to provide more evidence,” he added. Until then, dermatologists “should keep on promoting messages” to prevent SCC from occurring. This includes encouraging the use of proper sun protection in addition to discouraging the practice of skin bleaching, which still persists despite more than 10 African nations banning the use of toxic skin-lightening products.
Dr. Faye and Dr. Lupi report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
SINGAPORE –
This question was posed by Ousmane Faye, MD, PhD, director general of Mali’s Bamako Dermatology Hospital, at the World Congress of Dermatology.
Dr. Faye explored the issue during a hot topics session at the meeting, prefacing that it was an important question to ask because “in West Africa, skin bleaching is very common.”
“There are many local names” for skin bleaching, he said. “For example, in Senegal, it’s called xessal; in Mali and Ivory Coast, its name is caco; in South Africa, there are many names, like ukutsheyisa.”
Skin bleaching refers to the cosmetic misuse of topical agents to change one’s natural skin color. It’s a centuries-old practice that people, mainly women, adopt “to increase attractiveness and self-esteem,” explained Dr. Faye.
To demonstrate how pervasive skin bleaching is on the continent, he presented a slide that summarized figures from six studies spanning the past 2 decades. Prevalence ranged from 25% in Mali (based on a 1993 survey of 210 women) to a high of 79.25% in Benin (from a sample size of 511 women in 2019). In other studies of women in Burkina Faso and Togo, the figures were 44.3% and 58.9%, respectively. The most recently conducted study, which involved 2,689 Senegalese women and was published in 2022, found that nearly 6 in 10 (59.2%) respondents used skin-lightening products.
But skin bleaching isn’t just limited to Africa, said session moderator Omar Lupi, MD, PhD, associate professor of dermatology at the Federal University of the State of Rio de Janeiro, when approached for an independent comment. “It’s a traditional practice around the world. Maybe not in the developed countries, but it’s quite common in Africa, in South America, and in Asia.”
His sentiments are echoed in a meta-analysis that was published in the International Journal of Dermatology in 2019. The work examined 68 studies involving more than 67,000 people across Africa, Asia, Europe, the Middle East, and North America. It found that the pooled lifetime prevalence of skin bleaching was 27.7% (95% confidence interval, 19.6-37.5; P < .01).
“This is an important and interesting topic because our world is shrinking,” Dr. Lupi told this news organization. “Even in countries that don’t have bleaching as a common situation, we now have patients who are migrating from one part [of the world] to another, so bleaching is something that can knock on your door and you need to be prepared.”
Misuse leads to complications
The issue is pertinent to dermatologists because skin bleaching is associated with a wide range of complications. Take, for example, topical steroids, which are the most common products used for bleaching, said Dr. Faye in his talk.
“Clobetasol can suppress the hypothalamic-pituitary-adrenal (HPA) function,” he said, referring to the body’s main stress response system. “It can also foster skin infection, including bacterial, fungal, viral, and parasitic infection.”
In addition, topical steroids that are misused as skin lighteners have been reported to cause stretch marks, skin atrophy, inflammatory acne, and even metabolic disorders such as diabetes and hypertension, said Dr. Faye.
To further his point, he cited a 2021 prospective case-control study conducted across five sub-Saharan countries, which found that the use of “voluntary cosmetic depigmentation” significantly increased a person’s risk for necrotizing fasciitis of the lower limbs (odds ratio, 2.29; 95% CI, 1.19-3.73; P = .0226).
Similarly, mercury, another substance found in products commonly used to bleach skin, has been associated with problems ranging from rashes to renal toxicity. And because it’s so incredibly harmful, mercury is also known to cause neurologic abnormalities.
Apart from causing certain conditions, prolonged use of skin-lightening products can change the way existing diseases present themselves as well as their severity, added Dr. Faye.
An increased risk
But what about skin bleaching’s link with cancer? “Skin cancer on Black skin is uncommon, yet it occurs in skin-bleaching women,” said Dr. Faye.
“Since 2000, we have had some cases of skin cancer associated with skin bleaching,” he continued, adding that squamous cell carcinoma (SCC) is the most frequent type of cancer observed.
If you look at what’s been published on the topic so far, you’ll see that “all the cases of skin cancer are located over the neck or some exposed area when skin bleaching products are used for more than 10 years,” said Dr. Faye. “And most of the time, the age of the patient ranges from 30 to 60 years.”
The first known case in Africa was reported in a 58-year-old woman from Ghana, who had been using skin bleaching products for close to 30 years. The patient presented with tumors on her face, neck, and arms.
Dr. Faye then proceeded to share more than 10 such carcinoma cases. “These previous reports strongly suggest a relationship between skin bleaching and skin cancers,” said Dr. Faye.
Indeed, there have been reports and publications in the literature that support his observation, including one last year, which found that use of the tyrosinase inhibitor hydroquinone was associated with approximately a threefold increased risk for skin cancer.
For some, including Brazil’s Dr. Lupi, Dr. Faye’s talk was enlightening: “I didn’t know about this relationship [of bleaching] with skin cancer, it was something new for me.”
But the prevalence of SCC is very low, compared with that of skin bleaching, Dr. Faye acknowledged. Moreover, the cancer observed in the cases reported could have resulted from a number of reasons, including exposure to harmful ultraviolet rays from the sun and genetic predisposition in addition to the use of bleaching products such as hydroquinone. “Other causes of skin cancer are not excluded,” he said.
To further explore the link between skin bleaching and cancer, “we need case-control studies to provide more evidence,” he added. Until then, dermatologists “should keep on promoting messages” to prevent SCC from occurring. This includes encouraging the use of proper sun protection in addition to discouraging the practice of skin bleaching, which still persists despite more than 10 African nations banning the use of toxic skin-lightening products.
Dr. Faye and Dr. Lupi report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT WCD 2023
12 steps to closing your practice without problems
Whether you’ve decided to retire, relocate, or work for your local hospital, unwinding your practice will take time.
“Doctors shouldn’t assume everything takes care of itself. Many don’t think about compliance issues, patient abandonment, or accounts receivable that they need to keep open to collect from billing, which can occur months after the dates of service,” said David Zetter, president of Zetter HealthCare management consultants in Pennsylvania.
Debra Phairas, president of Practice and Liability Consultants, LLC, in California, suggests doctors start planning for the closing of their practice at least 90-120 days from their closing date.
“Many people and entities need to be notified,” said Ms. Phairas. The list includes patients, payers, vendors, employees, licensing boards, and federal and state agencies.
Medical societies may have specific bylaws that apply; malpractice carriers have rules about how long you should retain medical records; and some state laws require that you communicate that you’re closing in a newspaper, Mr. Zetter added.
Ms. Phairas recommends that physicians decide first whether they will sell their practice or if they’ll just shut it down. If they sell and the buyer is a doctor, they may want to provide transition assistance such as introducing patients and staff, she said. Otherwise, doctors may need to terminate their staff.
After doctors make that decision, Mr. Zetter and Ms. Phairas recommend taking these 12 steps to ensure that the process goes smoothly.
What to do 60-90 days out
1. Check your insurance contracts. The Centers for Medicare & Medicaid Services requires physicians to notify them 90 days after deciding to retire or withdraw from Medicare or Medicaid. Other payers may also require 90 days’ notice to terminate their contracts.
You’ll also need to provide payers with a forwarding address for sending payments after the office closes, and notify your malpractice insurance carrier and any other contracted insurance carriers such as workers’ compensation or employee benefit plans.
2. Buy “tail” coverage. Doctors can be sued for malpractice years after they close their practice so this provides coverage against claims reported after the liability policy expires.
3. Check your hospital contracts. Most hospitals where you have privileges require 90 days’ notice that you are closing the practice.
4. Arrange for safe storage of medical records. If you are selling your practice to another physician, that doctor can take charge of them, as long as you obtain a patient’s consent to transfer the medical records, said Ms. Phairas. Otherwise, the practice is required to make someone the guardian of the records after the practice closes, said Mr. Zetter. This allows patients at a later date to obtain copies of their records at a cost.
“This usually means printing all the records to PDF to be retained; otherwise, doctors have to continue to pay the license fee for the EMR software to access the records, and no practice is going to continue to pay this indefinitely,” said Mr. Zetter.
Check with your malpractice insurance carrier for how long they require medical records to be retained, which may vary for adult and pediatric records.
Ms. Phairas also advises doctors to keep their original records. “The biggest mistake doctors can make is to give patients all their records. Your chart is your best defense weapon in a liability claim.”
What to do 30-60 days out
5. Tell your staff. They should not hear that you’re retiring or leaving the practice from other people, said Ms. Phairas. But timing is important. “If you notify them too soon, they may look for another job. I recommend telling them about 45 days out and just before you notify patients, although you may want to tell the office manager sooner.”
Doctors may need help closing the practice and should consider offering the employees a severance bonus to stay until the end, said Ms. Phairas. If they do leave sooner, then you can hire temporary staff.
6. Notify patients to avoid any claims of abandonment. You should notify all active patients, which, depending on your state, can be any patient the physician has treated sometime in the past 12-36 months.
Some state laws require the notice to be published as an advertisement in the local newspaper and will say how far in advance it needs to be published and how long the ad needs to run. Notification also should be posted throughout the practice, and patients who call or visit should be given oral reminders.
“Your biggest expense will be mailing a letter to all patients,” said Mr. Zetter. The letter should include:
- The date of closing.
- The name(s) of the physicians taking over the practice (if applicable).
- Local physicians who would be willing to accept new patients.
- Instructions for how patients can obtain or transfer medical records (with a deadline for submitting record requests).
- How to contact the practice if patients and families have any concerns about the closing.
7. Notify your professional associations. These include your state medical board, credentialing organizations, and professional memberships. It’s critical to renew your license even if you plan to practice in other states. He recalled that one doctor let his license lapse and the medical board notified Medicaid that he was no longer licensed. “CMS went after him because he didn’t notify them that he was no longer operating in Washington. CMS shut him down in every state/territory. This interventional radiologist spent 3 years with two attorneys to get it resolved,” said Mr. Zetter.
8. Terminate any leases with landlords or try to negotiate renting the office space on a month-to-month basis until you close or sell, suggests Ms. Phairas. If the practice owns the space, the partners will need to decide if the space will be sold or leased to a new business.
What to do 30 days out
9. Notify referring physicians of when you plan to close your practice so they don’t send new patients after that date.
10. Send a letter to the Drug Enforcement Agency to deactivate your license if you plan not to write another prescription and after you have safely disposed of prescription drugs following the federal guidelines. Destroy all prescription pads and contact drug representatives to determine what to do with unused samples, if needed.
11. Notify all vendors. Inform medical suppliers, office suppliers, collection agencies, laundry services, housekeeping services, hazardous waste disposal services, and any other vendors. Make sure to request a final statement from them so you can close out your accounts.
12. Process your accounts receivable to collect money owed to you. Consider employing a collection agency or staff member to reconcile accounts after the practice has closed.
Mr. Zetter also suggested retaining a certified accountant to handle the expenses for shutting down the business and to handle your future tax returns. “If you shut down the practice in 2023, you will still have to file a tax return for that year in 2024,” he said.
A version of this article first appeared on Medscape.com.
Whether you’ve decided to retire, relocate, or work for your local hospital, unwinding your practice will take time.
“Doctors shouldn’t assume everything takes care of itself. Many don’t think about compliance issues, patient abandonment, or accounts receivable that they need to keep open to collect from billing, which can occur months after the dates of service,” said David Zetter, president of Zetter HealthCare management consultants in Pennsylvania.
Debra Phairas, president of Practice and Liability Consultants, LLC, in California, suggests doctors start planning for the closing of their practice at least 90-120 days from their closing date.
“Many people and entities need to be notified,” said Ms. Phairas. The list includes patients, payers, vendors, employees, licensing boards, and federal and state agencies.
Medical societies may have specific bylaws that apply; malpractice carriers have rules about how long you should retain medical records; and some state laws require that you communicate that you’re closing in a newspaper, Mr. Zetter added.
Ms. Phairas recommends that physicians decide first whether they will sell their practice or if they’ll just shut it down. If they sell and the buyer is a doctor, they may want to provide transition assistance such as introducing patients and staff, she said. Otherwise, doctors may need to terminate their staff.
After doctors make that decision, Mr. Zetter and Ms. Phairas recommend taking these 12 steps to ensure that the process goes smoothly.
What to do 60-90 days out
1. Check your insurance contracts. The Centers for Medicare & Medicaid Services requires physicians to notify them 90 days after deciding to retire or withdraw from Medicare or Medicaid. Other payers may also require 90 days’ notice to terminate their contracts.
You’ll also need to provide payers with a forwarding address for sending payments after the office closes, and notify your malpractice insurance carrier and any other contracted insurance carriers such as workers’ compensation or employee benefit plans.
2. Buy “tail” coverage. Doctors can be sued for malpractice years after they close their practice so this provides coverage against claims reported after the liability policy expires.
3. Check your hospital contracts. Most hospitals where you have privileges require 90 days’ notice that you are closing the practice.
4. Arrange for safe storage of medical records. If you are selling your practice to another physician, that doctor can take charge of them, as long as you obtain a patient’s consent to transfer the medical records, said Ms. Phairas. Otherwise, the practice is required to make someone the guardian of the records after the practice closes, said Mr. Zetter. This allows patients at a later date to obtain copies of their records at a cost.
“This usually means printing all the records to PDF to be retained; otherwise, doctors have to continue to pay the license fee for the EMR software to access the records, and no practice is going to continue to pay this indefinitely,” said Mr. Zetter.
Check with your malpractice insurance carrier for how long they require medical records to be retained, which may vary for adult and pediatric records.
Ms. Phairas also advises doctors to keep their original records. “The biggest mistake doctors can make is to give patients all their records. Your chart is your best defense weapon in a liability claim.”
What to do 30-60 days out
5. Tell your staff. They should not hear that you’re retiring or leaving the practice from other people, said Ms. Phairas. But timing is important. “If you notify them too soon, they may look for another job. I recommend telling them about 45 days out and just before you notify patients, although you may want to tell the office manager sooner.”
Doctors may need help closing the practice and should consider offering the employees a severance bonus to stay until the end, said Ms. Phairas. If they do leave sooner, then you can hire temporary staff.
6. Notify patients to avoid any claims of abandonment. You should notify all active patients, which, depending on your state, can be any patient the physician has treated sometime in the past 12-36 months.
Some state laws require the notice to be published as an advertisement in the local newspaper and will say how far in advance it needs to be published and how long the ad needs to run. Notification also should be posted throughout the practice, and patients who call or visit should be given oral reminders.
“Your biggest expense will be mailing a letter to all patients,” said Mr. Zetter. The letter should include:
- The date of closing.
- The name(s) of the physicians taking over the practice (if applicable).
- Local physicians who would be willing to accept new patients.
- Instructions for how patients can obtain or transfer medical records (with a deadline for submitting record requests).
- How to contact the practice if patients and families have any concerns about the closing.
7. Notify your professional associations. These include your state medical board, credentialing organizations, and professional memberships. It’s critical to renew your license even if you plan to practice in other states. He recalled that one doctor let his license lapse and the medical board notified Medicaid that he was no longer licensed. “CMS went after him because he didn’t notify them that he was no longer operating in Washington. CMS shut him down in every state/territory. This interventional radiologist spent 3 years with two attorneys to get it resolved,” said Mr. Zetter.
8. Terminate any leases with landlords or try to negotiate renting the office space on a month-to-month basis until you close or sell, suggests Ms. Phairas. If the practice owns the space, the partners will need to decide if the space will be sold or leased to a new business.
What to do 30 days out
9. Notify referring physicians of when you plan to close your practice so they don’t send new patients after that date.
10. Send a letter to the Drug Enforcement Agency to deactivate your license if you plan not to write another prescription and after you have safely disposed of prescription drugs following the federal guidelines. Destroy all prescription pads and contact drug representatives to determine what to do with unused samples, if needed.
11. Notify all vendors. Inform medical suppliers, office suppliers, collection agencies, laundry services, housekeeping services, hazardous waste disposal services, and any other vendors. Make sure to request a final statement from them so you can close out your accounts.
12. Process your accounts receivable to collect money owed to you. Consider employing a collection agency or staff member to reconcile accounts after the practice has closed.
Mr. Zetter also suggested retaining a certified accountant to handle the expenses for shutting down the business and to handle your future tax returns. “If you shut down the practice in 2023, you will still have to file a tax return for that year in 2024,” he said.
A version of this article first appeared on Medscape.com.
Whether you’ve decided to retire, relocate, or work for your local hospital, unwinding your practice will take time.
“Doctors shouldn’t assume everything takes care of itself. Many don’t think about compliance issues, patient abandonment, or accounts receivable that they need to keep open to collect from billing, which can occur months after the dates of service,” said David Zetter, president of Zetter HealthCare management consultants in Pennsylvania.
Debra Phairas, president of Practice and Liability Consultants, LLC, in California, suggests doctors start planning for the closing of their practice at least 90-120 days from their closing date.
“Many people and entities need to be notified,” said Ms. Phairas. The list includes patients, payers, vendors, employees, licensing boards, and federal and state agencies.
Medical societies may have specific bylaws that apply; malpractice carriers have rules about how long you should retain medical records; and some state laws require that you communicate that you’re closing in a newspaper, Mr. Zetter added.
Ms. Phairas recommends that physicians decide first whether they will sell their practice or if they’ll just shut it down. If they sell and the buyer is a doctor, they may want to provide transition assistance such as introducing patients and staff, she said. Otherwise, doctors may need to terminate their staff.
After doctors make that decision, Mr. Zetter and Ms. Phairas recommend taking these 12 steps to ensure that the process goes smoothly.
What to do 60-90 days out
1. Check your insurance contracts. The Centers for Medicare & Medicaid Services requires physicians to notify them 90 days after deciding to retire or withdraw from Medicare or Medicaid. Other payers may also require 90 days’ notice to terminate their contracts.
You’ll also need to provide payers with a forwarding address for sending payments after the office closes, and notify your malpractice insurance carrier and any other contracted insurance carriers such as workers’ compensation or employee benefit plans.
2. Buy “tail” coverage. Doctors can be sued for malpractice years after they close their practice so this provides coverage against claims reported after the liability policy expires.
3. Check your hospital contracts. Most hospitals where you have privileges require 90 days’ notice that you are closing the practice.
4. Arrange for safe storage of medical records. If you are selling your practice to another physician, that doctor can take charge of them, as long as you obtain a patient’s consent to transfer the medical records, said Ms. Phairas. Otherwise, the practice is required to make someone the guardian of the records after the practice closes, said Mr. Zetter. This allows patients at a later date to obtain copies of their records at a cost.
“This usually means printing all the records to PDF to be retained; otherwise, doctors have to continue to pay the license fee for the EMR software to access the records, and no practice is going to continue to pay this indefinitely,” said Mr. Zetter.
Check with your malpractice insurance carrier for how long they require medical records to be retained, which may vary for adult and pediatric records.
Ms. Phairas also advises doctors to keep their original records. “The biggest mistake doctors can make is to give patients all their records. Your chart is your best defense weapon in a liability claim.”
What to do 30-60 days out
5. Tell your staff. They should not hear that you’re retiring or leaving the practice from other people, said Ms. Phairas. But timing is important. “If you notify them too soon, they may look for another job. I recommend telling them about 45 days out and just before you notify patients, although you may want to tell the office manager sooner.”
Doctors may need help closing the practice and should consider offering the employees a severance bonus to stay until the end, said Ms. Phairas. If they do leave sooner, then you can hire temporary staff.
6. Notify patients to avoid any claims of abandonment. You should notify all active patients, which, depending on your state, can be any patient the physician has treated sometime in the past 12-36 months.
Some state laws require the notice to be published as an advertisement in the local newspaper and will say how far in advance it needs to be published and how long the ad needs to run. Notification also should be posted throughout the practice, and patients who call or visit should be given oral reminders.
“Your biggest expense will be mailing a letter to all patients,” said Mr. Zetter. The letter should include:
- The date of closing.
- The name(s) of the physicians taking over the practice (if applicable).
- Local physicians who would be willing to accept new patients.
- Instructions for how patients can obtain or transfer medical records (with a deadline for submitting record requests).
- How to contact the practice if patients and families have any concerns about the closing.
7. Notify your professional associations. These include your state medical board, credentialing organizations, and professional memberships. It’s critical to renew your license even if you plan to practice in other states. He recalled that one doctor let his license lapse and the medical board notified Medicaid that he was no longer licensed. “CMS went after him because he didn’t notify them that he was no longer operating in Washington. CMS shut him down in every state/territory. This interventional radiologist spent 3 years with two attorneys to get it resolved,” said Mr. Zetter.
8. Terminate any leases with landlords or try to negotiate renting the office space on a month-to-month basis until you close or sell, suggests Ms. Phairas. If the practice owns the space, the partners will need to decide if the space will be sold or leased to a new business.
What to do 30 days out
9. Notify referring physicians of when you plan to close your practice so they don’t send new patients after that date.
10. Send a letter to the Drug Enforcement Agency to deactivate your license if you plan not to write another prescription and after you have safely disposed of prescription drugs following the federal guidelines. Destroy all prescription pads and contact drug representatives to determine what to do with unused samples, if needed.
11. Notify all vendors. Inform medical suppliers, office suppliers, collection agencies, laundry services, housekeeping services, hazardous waste disposal services, and any other vendors. Make sure to request a final statement from them so you can close out your accounts.
12. Process your accounts receivable to collect money owed to you. Consider employing a collection agency or staff member to reconcile accounts after the practice has closed.
Mr. Zetter also suggested retaining a certified accountant to handle the expenses for shutting down the business and to handle your future tax returns. “If you shut down the practice in 2023, you will still have to file a tax return for that year in 2024,” he said.
A version of this article first appeared on Medscape.com.
FDA panel deems phenylephrine ineffective
The Nonprescription Drug Advisory Committee discussed the efficacy and pharmacokinetic data for phenylephrine. The committee’s next move is to determine if the drug’s status as Generally Recognized as Safe and Effective should be revoked. This would mean manufacturers would have to come up with new formulations, or products containing the drug would be removed from store shelves. NDAC did not disclose a timeline for assessing GRASE status.
The vote that formally declared phenylephrine ineffective was in line with a review of pharmacology and clinical data presented by the FDA on Sept. 11, which found that the oral bioavailability of the drug is less than 1%, compared with 38%, a number often cited in the literature and based on outdated technology.
A mechanism potentially responsible for inefficacy may be the half-life of phenylephrine.
“The half-life of the parent phenylephrine is much shorter than that of total phenylephrine, suggesting that the duration of action for active parent phenylephrine is far shorter than the monographed dosing interval of every 4 hours and is therefore open to question,” the review states.
The side effects of phenylephrine include headaches, insomnia, and nervousness. At higher doses, it can increase blood pressure.
The review also found that original studies used to support the efficacy of phenylephrine were inconclusive at best and contained potential methodological, statistical, and data integrity issues.
Pseudoephedrine is the only other nonprescription oral nasal decongestant on the retail market but is only available behind the counter due to its use as a potential narcotic.
Manufacturers have used phenylephrine instead of pseudoephedrine in many products due to this limitation.
Revoking the GRASE status of phenylephrine would leave patients without an over-the-counter option.
According to the FDA review, 242 million packages or bottles of phenylephrine products were sold in 2022, resulting in $1.76 billion in sales. A little over 50 million packages of pseudoephedrine were sold that same year, resulting in $542 million in sales.
“I think there’s a huge potential for consumer concern,” Diane B. Ginsburg, PhD, MS, RPh, the pharmacy practice division associate dean for Healthcare Partnerships at The University of Texas at Austin, said during the panel.
She said patients may be confused and concerned about the panel vote, especially those who feel they have benefitted from phenylephrine products. In the event of GRASE removal, she advised reassuring patients that phenylephrine is being pulled from shelves due to inefficacy rather than immediate health risks.
“The real positive here to me is the opportunity from an educational perspective to show consumers the fact that there are a lot more ways to treat” conditions that present with the symptom of congestion, such as rhinitis.
According to the FDA review, “most consumers may simply need instruction on the alternatives, including how to obtain ‘behind-the-counter’ pseudoephedrine or to use alternative treatments, including intranasal decongestants (including intranasal phenylephrine), intranasal steroids, intranasal antihistamines, or intranasal saline products.”
Despite these complications, “there are a number of potential benefits that would be derived by changing the GRASE status of oral phenylephrine.”
These include avoiding unnecessary costs of taking an ineffective drug, potential allergic reactions and side effects, and the risks of patients taking a higher dosage.
A version of this article appeared on Medscape.com.
The Nonprescription Drug Advisory Committee discussed the efficacy and pharmacokinetic data for phenylephrine. The committee’s next move is to determine if the drug’s status as Generally Recognized as Safe and Effective should be revoked. This would mean manufacturers would have to come up with new formulations, or products containing the drug would be removed from store shelves. NDAC did not disclose a timeline for assessing GRASE status.
The vote that formally declared phenylephrine ineffective was in line with a review of pharmacology and clinical data presented by the FDA on Sept. 11, which found that the oral bioavailability of the drug is less than 1%, compared with 38%, a number often cited in the literature and based on outdated technology.
A mechanism potentially responsible for inefficacy may be the half-life of phenylephrine.
“The half-life of the parent phenylephrine is much shorter than that of total phenylephrine, suggesting that the duration of action for active parent phenylephrine is far shorter than the monographed dosing interval of every 4 hours and is therefore open to question,” the review states.
The side effects of phenylephrine include headaches, insomnia, and nervousness. At higher doses, it can increase blood pressure.
The review also found that original studies used to support the efficacy of phenylephrine were inconclusive at best and contained potential methodological, statistical, and data integrity issues.
Pseudoephedrine is the only other nonprescription oral nasal decongestant on the retail market but is only available behind the counter due to its use as a potential narcotic.
Manufacturers have used phenylephrine instead of pseudoephedrine in many products due to this limitation.
Revoking the GRASE status of phenylephrine would leave patients without an over-the-counter option.
According to the FDA review, 242 million packages or bottles of phenylephrine products were sold in 2022, resulting in $1.76 billion in sales. A little over 50 million packages of pseudoephedrine were sold that same year, resulting in $542 million in sales.
“I think there’s a huge potential for consumer concern,” Diane B. Ginsburg, PhD, MS, RPh, the pharmacy practice division associate dean for Healthcare Partnerships at The University of Texas at Austin, said during the panel.
She said patients may be confused and concerned about the panel vote, especially those who feel they have benefitted from phenylephrine products. In the event of GRASE removal, she advised reassuring patients that phenylephrine is being pulled from shelves due to inefficacy rather than immediate health risks.
“The real positive here to me is the opportunity from an educational perspective to show consumers the fact that there are a lot more ways to treat” conditions that present with the symptom of congestion, such as rhinitis.
According to the FDA review, “most consumers may simply need instruction on the alternatives, including how to obtain ‘behind-the-counter’ pseudoephedrine or to use alternative treatments, including intranasal decongestants (including intranasal phenylephrine), intranasal steroids, intranasal antihistamines, or intranasal saline products.”
Despite these complications, “there are a number of potential benefits that would be derived by changing the GRASE status of oral phenylephrine.”
These include avoiding unnecessary costs of taking an ineffective drug, potential allergic reactions and side effects, and the risks of patients taking a higher dosage.
A version of this article appeared on Medscape.com.
The Nonprescription Drug Advisory Committee discussed the efficacy and pharmacokinetic data for phenylephrine. The committee’s next move is to determine if the drug’s status as Generally Recognized as Safe and Effective should be revoked. This would mean manufacturers would have to come up with new formulations, or products containing the drug would be removed from store shelves. NDAC did not disclose a timeline for assessing GRASE status.
The vote that formally declared phenylephrine ineffective was in line with a review of pharmacology and clinical data presented by the FDA on Sept. 11, which found that the oral bioavailability of the drug is less than 1%, compared with 38%, a number often cited in the literature and based on outdated technology.
A mechanism potentially responsible for inefficacy may be the half-life of phenylephrine.
“The half-life of the parent phenylephrine is much shorter than that of total phenylephrine, suggesting that the duration of action for active parent phenylephrine is far shorter than the monographed dosing interval of every 4 hours and is therefore open to question,” the review states.
The side effects of phenylephrine include headaches, insomnia, and nervousness. At higher doses, it can increase blood pressure.
The review also found that original studies used to support the efficacy of phenylephrine were inconclusive at best and contained potential methodological, statistical, and data integrity issues.
Pseudoephedrine is the only other nonprescription oral nasal decongestant on the retail market but is only available behind the counter due to its use as a potential narcotic.
Manufacturers have used phenylephrine instead of pseudoephedrine in many products due to this limitation.
Revoking the GRASE status of phenylephrine would leave patients without an over-the-counter option.
According to the FDA review, 242 million packages or bottles of phenylephrine products were sold in 2022, resulting in $1.76 billion in sales. A little over 50 million packages of pseudoephedrine were sold that same year, resulting in $542 million in sales.
“I think there’s a huge potential for consumer concern,” Diane B. Ginsburg, PhD, MS, RPh, the pharmacy practice division associate dean for Healthcare Partnerships at The University of Texas at Austin, said during the panel.
She said patients may be confused and concerned about the panel vote, especially those who feel they have benefitted from phenylephrine products. In the event of GRASE removal, she advised reassuring patients that phenylephrine is being pulled from shelves due to inefficacy rather than immediate health risks.
“The real positive here to me is the opportunity from an educational perspective to show consumers the fact that there are a lot more ways to treat” conditions that present with the symptom of congestion, such as rhinitis.
According to the FDA review, “most consumers may simply need instruction on the alternatives, including how to obtain ‘behind-the-counter’ pseudoephedrine or to use alternative treatments, including intranasal decongestants (including intranasal phenylephrine), intranasal steroids, intranasal antihistamines, or intranasal saline products.”
Despite these complications, “there are a number of potential benefits that would be derived by changing the GRASE status of oral phenylephrine.”
These include avoiding unnecessary costs of taking an ineffective drug, potential allergic reactions and side effects, and the risks of patients taking a higher dosage.
A version of this article appeared on Medscape.com.
Stress, insomnia tied to increased AFib risk for older women
TOPLINE:
Eight psychosocial factors, grouped into two distinct clusters, are significantly associated with risk for atrial fibrillation in postmenopausal women, with insomnia and stressful life events (SLEs) being the most strongly associated with AFib, a large new study has found.
METHODOLOGY:
- In addition to traditional risk factors such as obesity, advanced age, ethnicity, smoking, alcohol, hypertension, diabetes, coronary artery disease, heart failure, and emotional and psychological distress may also affect AFib.
- The study included 83,736 postmenopausal women in the Women’s Health Initiative (mean age, 63.9 years; 88.1% White) who did not have AFib at baseline.
- From questionnaires, researchers collected information on psychosocial stressors and used hierarchical cluster analysis to identify patterns of psychosocial predictors.
- They separated these clusters into quartiles, identified associations between psychosocial exposure variables, and adjusted for traditional risk factors.
- Over an average follow-up of 10.5 years, 23,954 participants (28.6%) developed incident AFib.
TAKEAWAY:
- The analysis generated two clusters of distinct psychosocial variables that were significantly associated with AFib: the Stress Cluster, including SLEs, depressive symptoms, and insomnia; and the Strain Cluster, including three personality traits: optimism, cynical hostility, and emotional expressiveness; and two social measures: social support, and social strain.
- Those in the highest quartiles of both the Stress Cluster and the Strain Cluster had greater rates of AFib, compared with those in the lowest quartiles.
- In a final model, the association between SLEs (hazard ratio, 1.02; 95% confidence interval, 1.01-1.04) and insomnia (HR, 1.04; 95% CI, 1.03-1.06) were most strongly linked to increased incidence of AFib, and a sensitivity analysis using snoring as a surrogate marker for sleep apnea didn’t change this outcome, supporting the independent effect of insomnia on AFib.
- In subgroup analyses, the Stress Cluster had a stronger association with AFib incidence in younger (50-69 years) versus older women (70-79 years), and in non-Hispanic White and Asian women versus Hispanic and non-Hispanic Black women.
IN PRACTICE:
The results support the hypothesis that psychosocial predictors account for additional risk for AFib “above and beyond” traditional risk factors, the authors wrote. Identifying and addressing sex-specific, modifiable risk factors, including insomnia, “may help reduce the burden of AF[ib] in aging women.”
SOURCE:
The study was conducted by Susan X. Zhao, MD, division of cardiology, department of medicine, Santa Clara Valley Medical Center, San Jose, Calif., and colleagues. It was published online in the Journal of the American Heart Association.
LIMITATIONS:
The psychometric questionnaires were administered only at study entry, but psychosocial variables may change over time. Data on sleep apnea and other sleep disorders, which may confound the relationship between insomnia and AFib, were not available, and although the study included a sensitivity analysis controlling for snoring, this is an imperfect surrogate for sleep apnea. Generalizability to other demographic, racial, and ethnic groups is limited.
DISCLOSURES:
The Women’s Health Initiative program is funded by the National Heart, Lung, and Blood Institute; National Institutes of Health; and the Department of Health & Human Services. The authors have no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
Eight psychosocial factors, grouped into two distinct clusters, are significantly associated with risk for atrial fibrillation in postmenopausal women, with insomnia and stressful life events (SLEs) being the most strongly associated with AFib, a large new study has found.
METHODOLOGY:
- In addition to traditional risk factors such as obesity, advanced age, ethnicity, smoking, alcohol, hypertension, diabetes, coronary artery disease, heart failure, and emotional and psychological distress may also affect AFib.
- The study included 83,736 postmenopausal women in the Women’s Health Initiative (mean age, 63.9 years; 88.1% White) who did not have AFib at baseline.
- From questionnaires, researchers collected information on psychosocial stressors and used hierarchical cluster analysis to identify patterns of psychosocial predictors.
- They separated these clusters into quartiles, identified associations between psychosocial exposure variables, and adjusted for traditional risk factors.
- Over an average follow-up of 10.5 years, 23,954 participants (28.6%) developed incident AFib.
TAKEAWAY:
- The analysis generated two clusters of distinct psychosocial variables that were significantly associated with AFib: the Stress Cluster, including SLEs, depressive symptoms, and insomnia; and the Strain Cluster, including three personality traits: optimism, cynical hostility, and emotional expressiveness; and two social measures: social support, and social strain.
- Those in the highest quartiles of both the Stress Cluster and the Strain Cluster had greater rates of AFib, compared with those in the lowest quartiles.
- In a final model, the association between SLEs (hazard ratio, 1.02; 95% confidence interval, 1.01-1.04) and insomnia (HR, 1.04; 95% CI, 1.03-1.06) were most strongly linked to increased incidence of AFib, and a sensitivity analysis using snoring as a surrogate marker for sleep apnea didn’t change this outcome, supporting the independent effect of insomnia on AFib.
- In subgroup analyses, the Stress Cluster had a stronger association with AFib incidence in younger (50-69 years) versus older women (70-79 years), and in non-Hispanic White and Asian women versus Hispanic and non-Hispanic Black women.
IN PRACTICE:
The results support the hypothesis that psychosocial predictors account for additional risk for AFib “above and beyond” traditional risk factors, the authors wrote. Identifying and addressing sex-specific, modifiable risk factors, including insomnia, “may help reduce the burden of AF[ib] in aging women.”
SOURCE:
The study was conducted by Susan X. Zhao, MD, division of cardiology, department of medicine, Santa Clara Valley Medical Center, San Jose, Calif., and colleagues. It was published online in the Journal of the American Heart Association.
LIMITATIONS:
The psychometric questionnaires were administered only at study entry, but psychosocial variables may change over time. Data on sleep apnea and other sleep disorders, which may confound the relationship between insomnia and AFib, were not available, and although the study included a sensitivity analysis controlling for snoring, this is an imperfect surrogate for sleep apnea. Generalizability to other demographic, racial, and ethnic groups is limited.
DISCLOSURES:
The Women’s Health Initiative program is funded by the National Heart, Lung, and Blood Institute; National Institutes of Health; and the Department of Health & Human Services. The authors have no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
Eight psychosocial factors, grouped into two distinct clusters, are significantly associated with risk for atrial fibrillation in postmenopausal women, with insomnia and stressful life events (SLEs) being the most strongly associated with AFib, a large new study has found.
METHODOLOGY:
- In addition to traditional risk factors such as obesity, advanced age, ethnicity, smoking, alcohol, hypertension, diabetes, coronary artery disease, heart failure, and emotional and psychological distress may also affect AFib.
- The study included 83,736 postmenopausal women in the Women’s Health Initiative (mean age, 63.9 years; 88.1% White) who did not have AFib at baseline.
- From questionnaires, researchers collected information on psychosocial stressors and used hierarchical cluster analysis to identify patterns of psychosocial predictors.
- They separated these clusters into quartiles, identified associations between psychosocial exposure variables, and adjusted for traditional risk factors.
- Over an average follow-up of 10.5 years, 23,954 participants (28.6%) developed incident AFib.
TAKEAWAY:
- The analysis generated two clusters of distinct psychosocial variables that were significantly associated with AFib: the Stress Cluster, including SLEs, depressive symptoms, and insomnia; and the Strain Cluster, including three personality traits: optimism, cynical hostility, and emotional expressiveness; and two social measures: social support, and social strain.
- Those in the highest quartiles of both the Stress Cluster and the Strain Cluster had greater rates of AFib, compared with those in the lowest quartiles.
- In a final model, the association between SLEs (hazard ratio, 1.02; 95% confidence interval, 1.01-1.04) and insomnia (HR, 1.04; 95% CI, 1.03-1.06) were most strongly linked to increased incidence of AFib, and a sensitivity analysis using snoring as a surrogate marker for sleep apnea didn’t change this outcome, supporting the independent effect of insomnia on AFib.
- In subgroup analyses, the Stress Cluster had a stronger association with AFib incidence in younger (50-69 years) versus older women (70-79 years), and in non-Hispanic White and Asian women versus Hispanic and non-Hispanic Black women.
IN PRACTICE:
The results support the hypothesis that psychosocial predictors account for additional risk for AFib “above and beyond” traditional risk factors, the authors wrote. Identifying and addressing sex-specific, modifiable risk factors, including insomnia, “may help reduce the burden of AF[ib] in aging women.”
SOURCE:
The study was conducted by Susan X. Zhao, MD, division of cardiology, department of medicine, Santa Clara Valley Medical Center, San Jose, Calif., and colleagues. It was published online in the Journal of the American Heart Association.
LIMITATIONS:
The psychometric questionnaires were administered only at study entry, but psychosocial variables may change over time. Data on sleep apnea and other sleep disorders, which may confound the relationship between insomnia and AFib, were not available, and although the study included a sensitivity analysis controlling for snoring, this is an imperfect surrogate for sleep apnea. Generalizability to other demographic, racial, and ethnic groups is limited.
DISCLOSURES:
The Women’s Health Initiative program is funded by the National Heart, Lung, and Blood Institute; National Institutes of Health; and the Department of Health & Human Services. The authors have no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
FROM JOURNAL OF THE AMERICAN HEART ASSOCIATION
Disenfranchised grief: What it looks like, where it goes
What happens to grief when those around you don’t understand it? Where does it go? How do you process it?
Disenfranchised grief, when someone or society more generally doesn’t see a loss as worthy of mourning, can deprive people of experiencing or processing their sadness. This grief, which may be triggered by the death of an ex-spouse, a pet, a failed adoption, can be painful and long-lasting.
Suzanne Cole, MD: ‘I didn’t feel the right to grieve’
During the COVID-19 pandemic, my little sister unexpectedly died. Though she was not one of the nearly 7 million people who died of the virus, in 2021 she became another type of statistic: one of the 109,699 people in the United State who died from a drug overdose. Hers was from fentanyl laced with methamphetamines.
Her death unraveled me. I felt deep guilt that I could not pull her from the sweeping current that had wrenched her from mainstream society into the underbelly of sex work and toward the solace of mind-altering drugs.
But I did not feel the right to grieve for her as I have grieved for other loved ones who were not blamed for their exit from this world. My sister was living a sordid life on the fringes of society. My grief felt invalid, undeserved. Yet, in the eyes of other “upstanding citizens,” her life was not as worth grieving – or so I thought. I tucked my sorrow into a small corner of my soul so no one would see, and I carried on.
To this day, the shame I feel robbed me of the ability to freely talk about her or share the searing pain I feel. Tears still prick my eyes when I think of her, but I have become adept at swallowing them, shaking off the waves of grief as though nothing happened. Even now, I cannot shake the pervasive feeling that my silent tears don’t deserve to be wept.
Don S. Dizon, MD: Working through tragedy
As a medical student, I worked with an outpatient physician as part of a third-year rotation. When we met, the first thing that struck me was how disheveled he looked. His clothes were wrinkled, and his pants were baggy. He took cigarette breaks, which I found disturbing.
But I quickly came to admire him. Despite my first impression, he was the type of doctor I aspired to be. He didn’t need to look at a patient’s chart to recall who they were. He just knew them. He greeted patients warmly, asked about their family. He even remembered the special occasions his patients had mentioned since their past visit. He epitomized empathy and connectedness.
Spending one day in clinic brought to light the challenges of forming such bonds with patients. A man came into the cancer clinic reporting chest pain and was triaged to an exam room. Soon after, the patient was found unresponsive on the floor. Nurses were yelling for help, and the doctor ran in and started CPR while minutes ticked by waiting for an ambulance that could take him to the ED.
By the time help arrived, the patient was blue.
He had died in the clinic in the middle of the day, as the waiting room filled. After the body was taken away, the doctor went into the bathroom. About 20 minutes later, he came out, eyes bloodshot, and continued with the rest of his day, ensuring each patient was seen and cared for.
As a medical student, it hit me how hard it must be to see something so tragic like the end of a life and then continue with your day as if nothing had happened. This is an experience of grief I later came to know well after nearly 30 years treating patients with advanced cancers: compartmentalizing it and carrying on.
A space for grieving: The Schwartz Center Rounds
Disenfranchised grief, the grief that is hard to share and often seems wrong to feel in the first place, can be triggered in many situations. Losing a person others don’t believe deserve to be grieved, such as an abusive partner or someone who committed a crime; losing someone you cared for in a professional role; a loss experienced in a breakup or same-sex partnership, if that relationship was not accepted by one’s family; loss from infertility, miscarriage, stillbirth, or failed adoption; loss that may be taboo or stigmatized, such as deaths via suicide or abortion; and loss of a job, home, or possession that you treasure.
Many of us have had similar situations or will, and the feeling that no one understands the need to mourn can be paralyzing and alienating. In the early days, intense, crushing feelings can cause intrusive, distracting thoughts, and over time, that grief can linger and find a permanent place in our minds.
More and more, though, we are being given opportunities to reflect on these sad moments.
The Schwartz Rounds are an example of such an opportunity. In these rounds, we gather to talk about the experience of caring for people, not the science of medicine.
During one particularly powerful rounds, I spoke to my colleagues about my initial meeting with a patient who was very sick. I detailed the experience of telling her children and her at that initial consult how I thought she was dying and that I did not recommend therapy. I remember how they cried. And I remembered how powerless I felt.
As I recalled that memory during Schwartz Rounds, I could not stop from crying. The unfairness of being a physician meeting someone for the first time and having to tell them such bad news overwhelmed me.
Even more poignant, I had the chance to reconnect with this woman’s children, who were present that day, not as audience members but as participants. Their presence may have brought my emotions to the surface more strongly. In that moment, I could show them the feelings I had bottled up for the sake of professionalism. Ultimately, I felt relieved, freer somehow, as if this burden my soul was carrying had been lifted.
Although we are both grateful for forums like this, these opportunities to share and express the grief we may have hidden away are not as common as they should be.
As physicians, we may express grief by shedding tears at the bedside of a patient nearing the end of life or through the anxiety we feel when our patient suffers a severe reaction to treatment. But we tend to put it away, to go on with our day, because there are others to be seen and cared for and more work to be done. Somehow, we move forward, shedding tears in one room and celebrating victories in another.
We need to create more spaces to express and feel grief, so we don’t get lost in it. Because understanding how grief impacts us, as people and as providers, is one of the most important realizations we can make as we go about our time-honored profession as healers.
Dr. Dizon is the director of women’s cancers at Lifespan Cancer Institute, director of medical oncology at Rhode Island Hospital, and a professor of medicine at Brown University, all in Providence. He reported conflicts of interest with Regeneron, AstraZeneca, Clovis, Bristol-Myers Squibb, and Kazia.
A version of this article first appeared on Medscape.com.
What happens to grief when those around you don’t understand it? Where does it go? How do you process it?
Disenfranchised grief, when someone or society more generally doesn’t see a loss as worthy of mourning, can deprive people of experiencing or processing their sadness. This grief, which may be triggered by the death of an ex-spouse, a pet, a failed adoption, can be painful and long-lasting.
Suzanne Cole, MD: ‘I didn’t feel the right to grieve’
During the COVID-19 pandemic, my little sister unexpectedly died. Though she was not one of the nearly 7 million people who died of the virus, in 2021 she became another type of statistic: one of the 109,699 people in the United State who died from a drug overdose. Hers was from fentanyl laced with methamphetamines.
Her death unraveled me. I felt deep guilt that I could not pull her from the sweeping current that had wrenched her from mainstream society into the underbelly of sex work and toward the solace of mind-altering drugs.
But I did not feel the right to grieve for her as I have grieved for other loved ones who were not blamed for their exit from this world. My sister was living a sordid life on the fringes of society. My grief felt invalid, undeserved. Yet, in the eyes of other “upstanding citizens,” her life was not as worth grieving – or so I thought. I tucked my sorrow into a small corner of my soul so no one would see, and I carried on.
To this day, the shame I feel robbed me of the ability to freely talk about her or share the searing pain I feel. Tears still prick my eyes when I think of her, but I have become adept at swallowing them, shaking off the waves of grief as though nothing happened. Even now, I cannot shake the pervasive feeling that my silent tears don’t deserve to be wept.
Don S. Dizon, MD: Working through tragedy
As a medical student, I worked with an outpatient physician as part of a third-year rotation. When we met, the first thing that struck me was how disheveled he looked. His clothes were wrinkled, and his pants were baggy. He took cigarette breaks, which I found disturbing.
But I quickly came to admire him. Despite my first impression, he was the type of doctor I aspired to be. He didn’t need to look at a patient’s chart to recall who they were. He just knew them. He greeted patients warmly, asked about their family. He even remembered the special occasions his patients had mentioned since their past visit. He epitomized empathy and connectedness.
Spending one day in clinic brought to light the challenges of forming such bonds with patients. A man came into the cancer clinic reporting chest pain and was triaged to an exam room. Soon after, the patient was found unresponsive on the floor. Nurses were yelling for help, and the doctor ran in and started CPR while minutes ticked by waiting for an ambulance that could take him to the ED.
By the time help arrived, the patient was blue.
He had died in the clinic in the middle of the day, as the waiting room filled. After the body was taken away, the doctor went into the bathroom. About 20 minutes later, he came out, eyes bloodshot, and continued with the rest of his day, ensuring each patient was seen and cared for.
As a medical student, it hit me how hard it must be to see something so tragic like the end of a life and then continue with your day as if nothing had happened. This is an experience of grief I later came to know well after nearly 30 years treating patients with advanced cancers: compartmentalizing it and carrying on.
A space for grieving: The Schwartz Center Rounds
Disenfranchised grief, the grief that is hard to share and often seems wrong to feel in the first place, can be triggered in many situations. Losing a person others don’t believe deserve to be grieved, such as an abusive partner or someone who committed a crime; losing someone you cared for in a professional role; a loss experienced in a breakup or same-sex partnership, if that relationship was not accepted by one’s family; loss from infertility, miscarriage, stillbirth, or failed adoption; loss that may be taboo or stigmatized, such as deaths via suicide or abortion; and loss of a job, home, or possession that you treasure.
Many of us have had similar situations or will, and the feeling that no one understands the need to mourn can be paralyzing and alienating. In the early days, intense, crushing feelings can cause intrusive, distracting thoughts, and over time, that grief can linger and find a permanent place in our minds.
More and more, though, we are being given opportunities to reflect on these sad moments.
The Schwartz Rounds are an example of such an opportunity. In these rounds, we gather to talk about the experience of caring for people, not the science of medicine.
During one particularly powerful rounds, I spoke to my colleagues about my initial meeting with a patient who was very sick. I detailed the experience of telling her children and her at that initial consult how I thought she was dying and that I did not recommend therapy. I remember how they cried. And I remembered how powerless I felt.
As I recalled that memory during Schwartz Rounds, I could not stop from crying. The unfairness of being a physician meeting someone for the first time and having to tell them such bad news overwhelmed me.
Even more poignant, I had the chance to reconnect with this woman’s children, who were present that day, not as audience members but as participants. Their presence may have brought my emotions to the surface more strongly. In that moment, I could show them the feelings I had bottled up for the sake of professionalism. Ultimately, I felt relieved, freer somehow, as if this burden my soul was carrying had been lifted.
Although we are both grateful for forums like this, these opportunities to share and express the grief we may have hidden away are not as common as they should be.
As physicians, we may express grief by shedding tears at the bedside of a patient nearing the end of life or through the anxiety we feel when our patient suffers a severe reaction to treatment. But we tend to put it away, to go on with our day, because there are others to be seen and cared for and more work to be done. Somehow, we move forward, shedding tears in one room and celebrating victories in another.
We need to create more spaces to express and feel grief, so we don’t get lost in it. Because understanding how grief impacts us, as people and as providers, is one of the most important realizations we can make as we go about our time-honored profession as healers.
Dr. Dizon is the director of women’s cancers at Lifespan Cancer Institute, director of medical oncology at Rhode Island Hospital, and a professor of medicine at Brown University, all in Providence. He reported conflicts of interest with Regeneron, AstraZeneca, Clovis, Bristol-Myers Squibb, and Kazia.
A version of this article first appeared on Medscape.com.
What happens to grief when those around you don’t understand it? Where does it go? How do you process it?
Disenfranchised grief, when someone or society more generally doesn’t see a loss as worthy of mourning, can deprive people of experiencing or processing their sadness. This grief, which may be triggered by the death of an ex-spouse, a pet, a failed adoption, can be painful and long-lasting.
Suzanne Cole, MD: ‘I didn’t feel the right to grieve’
During the COVID-19 pandemic, my little sister unexpectedly died. Though she was not one of the nearly 7 million people who died of the virus, in 2021 she became another type of statistic: one of the 109,699 people in the United State who died from a drug overdose. Hers was from fentanyl laced with methamphetamines.
Her death unraveled me. I felt deep guilt that I could not pull her from the sweeping current that had wrenched her from mainstream society into the underbelly of sex work and toward the solace of mind-altering drugs.
But I did not feel the right to grieve for her as I have grieved for other loved ones who were not blamed for their exit from this world. My sister was living a sordid life on the fringes of society. My grief felt invalid, undeserved. Yet, in the eyes of other “upstanding citizens,” her life was not as worth grieving – or so I thought. I tucked my sorrow into a small corner of my soul so no one would see, and I carried on.
To this day, the shame I feel robbed me of the ability to freely talk about her or share the searing pain I feel. Tears still prick my eyes when I think of her, but I have become adept at swallowing them, shaking off the waves of grief as though nothing happened. Even now, I cannot shake the pervasive feeling that my silent tears don’t deserve to be wept.
Don S. Dizon, MD: Working through tragedy
As a medical student, I worked with an outpatient physician as part of a third-year rotation. When we met, the first thing that struck me was how disheveled he looked. His clothes were wrinkled, and his pants were baggy. He took cigarette breaks, which I found disturbing.
But I quickly came to admire him. Despite my first impression, he was the type of doctor I aspired to be. He didn’t need to look at a patient’s chart to recall who they were. He just knew them. He greeted patients warmly, asked about their family. He even remembered the special occasions his patients had mentioned since their past visit. He epitomized empathy and connectedness.
Spending one day in clinic brought to light the challenges of forming such bonds with patients. A man came into the cancer clinic reporting chest pain and was triaged to an exam room. Soon after, the patient was found unresponsive on the floor. Nurses were yelling for help, and the doctor ran in and started CPR while minutes ticked by waiting for an ambulance that could take him to the ED.
By the time help arrived, the patient was blue.
He had died in the clinic in the middle of the day, as the waiting room filled. After the body was taken away, the doctor went into the bathroom. About 20 minutes later, he came out, eyes bloodshot, and continued with the rest of his day, ensuring each patient was seen and cared for.
As a medical student, it hit me how hard it must be to see something so tragic like the end of a life and then continue with your day as if nothing had happened. This is an experience of grief I later came to know well after nearly 30 years treating patients with advanced cancers: compartmentalizing it and carrying on.
A space for grieving: The Schwartz Center Rounds
Disenfranchised grief, the grief that is hard to share and often seems wrong to feel in the first place, can be triggered in many situations. Losing a person others don’t believe deserve to be grieved, such as an abusive partner or someone who committed a crime; losing someone you cared for in a professional role; a loss experienced in a breakup or same-sex partnership, if that relationship was not accepted by one’s family; loss from infertility, miscarriage, stillbirth, or failed adoption; loss that may be taboo or stigmatized, such as deaths via suicide or abortion; and loss of a job, home, or possession that you treasure.
Many of us have had similar situations or will, and the feeling that no one understands the need to mourn can be paralyzing and alienating. In the early days, intense, crushing feelings can cause intrusive, distracting thoughts, and over time, that grief can linger and find a permanent place in our minds.
More and more, though, we are being given opportunities to reflect on these sad moments.
The Schwartz Rounds are an example of such an opportunity. In these rounds, we gather to talk about the experience of caring for people, not the science of medicine.
During one particularly powerful rounds, I spoke to my colleagues about my initial meeting with a patient who was very sick. I detailed the experience of telling her children and her at that initial consult how I thought she was dying and that I did not recommend therapy. I remember how they cried. And I remembered how powerless I felt.
As I recalled that memory during Schwartz Rounds, I could not stop from crying. The unfairness of being a physician meeting someone for the first time and having to tell them such bad news overwhelmed me.
Even more poignant, I had the chance to reconnect with this woman’s children, who were present that day, not as audience members but as participants. Their presence may have brought my emotions to the surface more strongly. In that moment, I could show them the feelings I had bottled up for the sake of professionalism. Ultimately, I felt relieved, freer somehow, as if this burden my soul was carrying had been lifted.
Although we are both grateful for forums like this, these opportunities to share and express the grief we may have hidden away are not as common as they should be.
As physicians, we may express grief by shedding tears at the bedside of a patient nearing the end of life or through the anxiety we feel when our patient suffers a severe reaction to treatment. But we tend to put it away, to go on with our day, because there are others to be seen and cared for and more work to be done. Somehow, we move forward, shedding tears in one room and celebrating victories in another.
We need to create more spaces to express and feel grief, so we don’t get lost in it. Because understanding how grief impacts us, as people and as providers, is one of the most important realizations we can make as we go about our time-honored profession as healers.
Dr. Dizon is the director of women’s cancers at Lifespan Cancer Institute, director of medical oncology at Rhode Island Hospital, and a professor of medicine at Brown University, all in Providence. He reported conflicts of interest with Regeneron, AstraZeneca, Clovis, Bristol-Myers Squibb, and Kazia.
A version of this article first appeared on Medscape.com.
Does remote blood pressure monitoring improve patient outcomes postpartum?
Hirshberg A, Zhu Y, Smith-McLallen A, et al. Association of a remote blood pressure monitoring program with postpartum adverse outcomes. Obstet Gynecol. 2023;141:1163-1170. doi:10.1097/AOG.0000000000005197.
EXPERT COMMENTARY
Courtney Bisson, MD, is a Maternal-Fetal Medicine Fellow, University of Chicago/NorthShore University HealthSystem, Chicago, Illinois.
Sarosh Rana, MD, MPH, is Professor of Obstetrics and Gynecology and Section Chief, Maternal-Fetal Medicine, University of Chicago.
Hypertensive disorders of pregnancy account for a significant amount of morbidity during pregnancy and postpartum. In the pregnant population, data have shown that the implementation of a standardized blood pressure education program, provision of a blood pressure cuff, and assistance with postpartum follow-up result in improved blood pressures and postpartum follow-up for up to 6 weeks. In the nonpregnant population, literature suggests that RBPM in patients with hypertension results in improved outcomes, although the long-term impact of RBPM in the postpartum population remains unclear.
Recently, Hirshberg and colleagues published the results of a retrospective cohort study that assessed the impact of RBPM with text message reminders for 10 days postpartum on a composite of adverse maternal outcomes, readmissions, and follow-up within 1 year postpartum.1

Details of the study
The retrospective cohort study was conducted during 2017–2021 based on insurance claims of patients with hypertensive disorders of pregnancy who were enrolled in a twice-daily text message–based RBPM program for 10 days postpartum.
Data from 1,700 patients enrolled in RBPM were compared with that of propensity score matched controls that included 2,297 women not enrolled in RBPM. Of these controls, 1,276 patients (cohort C) simultaneously received care at other institutions without RBPM, and 1,021 patients (cohort A) received care at the same institution prior to implementation of RBPM.
Results. Patients in the RBPM group were found to have a significantly lower rate of composite adverse maternal outcomes compared with their matched cohorts in the year after delivery. (Individual adverse outcomes included stroke, disseminated intravascular coagulation, eclampsia, pulmonary edema, renal injury or liver failure, HELLP [hemolysis, elevated liver enzymes, low platelet count] syndrome, myocardial infarction, and cardiomyopathy.) Rates were 2.9% versus 4.7% (odds ratio [OR], 0.61; 95% confidence interval [CI], 0.40–0.98) in the RBPM group compared with cohort A; rates in the RBPM group compared with cohort C were 3.2% versus 4.5% (OR, 0.71; 95% CI, 0.47–1.07).
Although not statistically significant, rates of emergency department visits and readmissions also were lower in the RBPM patients. Those enrolled in the RBPM program were more likely to have follow-up with cardiologists or specialist visits within 6 months postpartum. Fewer emergency department visits and readmissions resulted in lower health care utilization costs.
Study strengths and limitations
This study’s strength lies in its design and implementation of standardized protocols that allowed assessment of clinically meaningful outcomes postpartum. Although the program for RBPM was for only 10 days postpartum, it showed effects beyond the timeframe of the direct care. No such prior data exist evaluating a program’s effectiveness in improving postpartum clinical outcomes and costs through 1 year postdelivery.
Study limitations include residual bias from unobserved confounders, analysis of only 1 payer type, lack of patient level data, and evaluation of disparity. ●
Previous work by Suresh and colleagues illustrated that a standardized postpartum blood pressure monitoring quality improvement initiative resulted in better blood pressures, improved postpartum visit adherence, and reduced disparity.2 The study by Hirshberg and colleagues furthers these findings, illustrating how uniform protocols surrounding preeclampsia management in the postpartum setting could further improve morbidity and mortality in the year following childbirth. Such protocols should be incorporated hospital-wide in standard obstetrical management.
COURTNEY BISSON, MD; SAROSH RANA, MD, MPH
- Hirshberg A, Zhu Y, Smith-McLallen A, et al. Association of a remote blood pressure monitoring program with postpartum adverse outcomes. Obstet Gynecol. 2023;141:1163-1170. doi:10.1097/AOG.0000000000005197.
- Suresh SC, Duncan C, Kaur H, et al. Postpartum outcomes with systematic treatment and management of postpartum hypertension. Obstet Gynecol. 2021;138:777-787. doi:10.1097 /AOG.0000000000004574.
Hirshberg A, Zhu Y, Smith-McLallen A, et al. Association of a remote blood pressure monitoring program with postpartum adverse outcomes. Obstet Gynecol. 2023;141:1163-1170. doi:10.1097/AOG.0000000000005197.
EXPERT COMMENTARY
Courtney Bisson, MD, is a Maternal-Fetal Medicine Fellow, University of Chicago/NorthShore University HealthSystem, Chicago, Illinois.
Sarosh Rana, MD, MPH, is Professor of Obstetrics and Gynecology and Section Chief, Maternal-Fetal Medicine, University of Chicago.
Hypertensive disorders of pregnancy account for a significant amount of morbidity during pregnancy and postpartum. In the pregnant population, data have shown that the implementation of a standardized blood pressure education program, provision of a blood pressure cuff, and assistance with postpartum follow-up result in improved blood pressures and postpartum follow-up for up to 6 weeks. In the nonpregnant population, literature suggests that RBPM in patients with hypertension results in improved outcomes, although the long-term impact of RBPM in the postpartum population remains unclear.
Recently, Hirshberg and colleagues published the results of a retrospective cohort study that assessed the impact of RBPM with text message reminders for 10 days postpartum on a composite of adverse maternal outcomes, readmissions, and follow-up within 1 year postpartum.1

Details of the study
The retrospective cohort study was conducted during 2017–2021 based on insurance claims of patients with hypertensive disorders of pregnancy who were enrolled in a twice-daily text message–based RBPM program for 10 days postpartum.
Data from 1,700 patients enrolled in RBPM were compared with that of propensity score matched controls that included 2,297 women not enrolled in RBPM. Of these controls, 1,276 patients (cohort C) simultaneously received care at other institutions without RBPM, and 1,021 patients (cohort A) received care at the same institution prior to implementation of RBPM.
Results. Patients in the RBPM group were found to have a significantly lower rate of composite adverse maternal outcomes compared with their matched cohorts in the year after delivery. (Individual adverse outcomes included stroke, disseminated intravascular coagulation, eclampsia, pulmonary edema, renal injury or liver failure, HELLP [hemolysis, elevated liver enzymes, low platelet count] syndrome, myocardial infarction, and cardiomyopathy.) Rates were 2.9% versus 4.7% (odds ratio [OR], 0.61; 95% confidence interval [CI], 0.40–0.98) in the RBPM group compared with cohort A; rates in the RBPM group compared with cohort C were 3.2% versus 4.5% (OR, 0.71; 95% CI, 0.47–1.07).
Although not statistically significant, rates of emergency department visits and readmissions also were lower in the RBPM patients. Those enrolled in the RBPM program were more likely to have follow-up with cardiologists or specialist visits within 6 months postpartum. Fewer emergency department visits and readmissions resulted in lower health care utilization costs.
Study strengths and limitations
This study’s strength lies in its design and implementation of standardized protocols that allowed assessment of clinically meaningful outcomes postpartum. Although the program for RBPM was for only 10 days postpartum, it showed effects beyond the timeframe of the direct care. No such prior data exist evaluating a program’s effectiveness in improving postpartum clinical outcomes and costs through 1 year postdelivery.
Study limitations include residual bias from unobserved confounders, analysis of only 1 payer type, lack of patient level data, and evaluation of disparity. ●
Previous work by Suresh and colleagues illustrated that a standardized postpartum blood pressure monitoring quality improvement initiative resulted in better blood pressures, improved postpartum visit adherence, and reduced disparity.2 The study by Hirshberg and colleagues furthers these findings, illustrating how uniform protocols surrounding preeclampsia management in the postpartum setting could further improve morbidity and mortality in the year following childbirth. Such protocols should be incorporated hospital-wide in standard obstetrical management.
COURTNEY BISSON, MD; SAROSH RANA, MD, MPH
Hirshberg A, Zhu Y, Smith-McLallen A, et al. Association of a remote blood pressure monitoring program with postpartum adverse outcomes. Obstet Gynecol. 2023;141:1163-1170. doi:10.1097/AOG.0000000000005197.
EXPERT COMMENTARY
Courtney Bisson, MD, is a Maternal-Fetal Medicine Fellow, University of Chicago/NorthShore University HealthSystem, Chicago, Illinois.
Sarosh Rana, MD, MPH, is Professor of Obstetrics and Gynecology and Section Chief, Maternal-Fetal Medicine, University of Chicago.
Hypertensive disorders of pregnancy account for a significant amount of morbidity during pregnancy and postpartum. In the pregnant population, data have shown that the implementation of a standardized blood pressure education program, provision of a blood pressure cuff, and assistance with postpartum follow-up result in improved blood pressures and postpartum follow-up for up to 6 weeks. In the nonpregnant population, literature suggests that RBPM in patients with hypertension results in improved outcomes, although the long-term impact of RBPM in the postpartum population remains unclear.
Recently, Hirshberg and colleagues published the results of a retrospective cohort study that assessed the impact of RBPM with text message reminders for 10 days postpartum on a composite of adverse maternal outcomes, readmissions, and follow-up within 1 year postpartum.1

Details of the study
The retrospective cohort study was conducted during 2017–2021 based on insurance claims of patients with hypertensive disorders of pregnancy who were enrolled in a twice-daily text message–based RBPM program for 10 days postpartum.
Data from 1,700 patients enrolled in RBPM were compared with that of propensity score matched controls that included 2,297 women not enrolled in RBPM. Of these controls, 1,276 patients (cohort C) simultaneously received care at other institutions without RBPM, and 1,021 patients (cohort A) received care at the same institution prior to implementation of RBPM.
Results. Patients in the RBPM group were found to have a significantly lower rate of composite adverse maternal outcomes compared with their matched cohorts in the year after delivery. (Individual adverse outcomes included stroke, disseminated intravascular coagulation, eclampsia, pulmonary edema, renal injury or liver failure, HELLP [hemolysis, elevated liver enzymes, low platelet count] syndrome, myocardial infarction, and cardiomyopathy.) Rates were 2.9% versus 4.7% (odds ratio [OR], 0.61; 95% confidence interval [CI], 0.40–0.98) in the RBPM group compared with cohort A; rates in the RBPM group compared with cohort C were 3.2% versus 4.5% (OR, 0.71; 95% CI, 0.47–1.07).
Although not statistically significant, rates of emergency department visits and readmissions also were lower in the RBPM patients. Those enrolled in the RBPM program were more likely to have follow-up with cardiologists or specialist visits within 6 months postpartum. Fewer emergency department visits and readmissions resulted in lower health care utilization costs.
Study strengths and limitations
This study’s strength lies in its design and implementation of standardized protocols that allowed assessment of clinically meaningful outcomes postpartum. Although the program for RBPM was for only 10 days postpartum, it showed effects beyond the timeframe of the direct care. No such prior data exist evaluating a program’s effectiveness in improving postpartum clinical outcomes and costs through 1 year postdelivery.
Study limitations include residual bias from unobserved confounders, analysis of only 1 payer type, lack of patient level data, and evaluation of disparity. ●
Previous work by Suresh and colleagues illustrated that a standardized postpartum blood pressure monitoring quality improvement initiative resulted in better blood pressures, improved postpartum visit adherence, and reduced disparity.2 The study by Hirshberg and colleagues furthers these findings, illustrating how uniform protocols surrounding preeclampsia management in the postpartum setting could further improve morbidity and mortality in the year following childbirth. Such protocols should be incorporated hospital-wide in standard obstetrical management.
COURTNEY BISSON, MD; SAROSH RANA, MD, MPH
- Hirshberg A, Zhu Y, Smith-McLallen A, et al. Association of a remote blood pressure monitoring program with postpartum adverse outcomes. Obstet Gynecol. 2023;141:1163-1170. doi:10.1097/AOG.0000000000005197.
- Suresh SC, Duncan C, Kaur H, et al. Postpartum outcomes with systematic treatment and management of postpartum hypertension. Obstet Gynecol. 2021;138:777-787. doi:10.1097 /AOG.0000000000004574.
- Hirshberg A, Zhu Y, Smith-McLallen A, et al. Association of a remote blood pressure monitoring program with postpartum adverse outcomes. Obstet Gynecol. 2023;141:1163-1170. doi:10.1097/AOG.0000000000005197.
- Suresh SC, Duncan C, Kaur H, et al. Postpartum outcomes with systematic treatment and management of postpartum hypertension. Obstet Gynecol. 2021;138:777-787. doi:10.1097 /AOG.0000000000004574.