User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
The Supreme Court 2020‒2021: What will affect ObGyns?
The Supreme Court’s usual processes were disrupted this term. The COVID-19 pandemic required audio hearings rather than in-person, and it resulted in a number of emergency legal appeals. As the Court began its regular sessions on October 5, 2020, there were only 8 justices—Justice Ruth Bader Ginsburg had passed away and Amy Coney Barrett had not yet been confirmed by the Senate. The Court decided many important cases this term, including dealing with the delivery of drugs to induce abortions, a Centers for Disease Control and Prevention (CDC) moratorium on housing evictions, yet another case on the Affordable Care Act, state laws concerning pharmacy benefit managers, and the Hologic and Minerva endometrial ablation systems patents. After considering these cases, we also will briefly look at other cases of general interest.
Abortion
Patient access to mifepristone
In May 2020, the American College of Obstetricians and Gynecologists (ACOG) was the named plaintiff in a lawsuit against the US Food and Drug Administration (FDA) regarding the drugs mifepristone and misoprostol that are used to induce medical abortions.1 The case was filed by the American Civil Liberties Union on behalf of ACOG and others2,3 and raised the issue of patients’ access to these medications. The basic claim of the case was that during the pandemic, the FDA’s regulation of mifepristone was unconstitutional in that they imposed an undue burden on the decision of women to have an abortion.4 (Although misoprostol is a part of the medical abortion regimen, it is not subject to special regulation and was not part of the litigation.)
The FDA regulation of mifepristone, begun in 2000 but modified since then, includes 3 elements to assure safe use:
- prescribers must have special training or certification
- the drug can be dispensed to patients only in a hospital, clinic, or medical office under the supervision of a certified health care provider (known as the “in-person dispensing requirement” because retail pharmacy or mail distribution are prohibited)
- the health care provider must review a “patient agreement form” with the patient and have the patient sign the consent form in the provider’s presence.5
The pandemic made fulfilling these requirements substantially more burdensome and difficult. The question was whether the FDA was constitutionally required to modify its regulations during a pandemic to take account of the undue burden of the regulation created by the pandemic. That is, the question was not whether the FDA could have or should have chosen to make the modification, but whether it was required to do so.
In July 2020, a federal district court in Maryland held that the FDA regulation was an unconstitutional burden on the abortion rights of women during the pandemic and issued a preliminary injunction to stop the FDA from enforcing the in-person dispensing and signature rules. The district judge applied the injunction to Maryland, but also made it a nationwide injunction. (The issue of district court nationwide injunctions is considered in, “District court ‘nationwide injunctions’”).
The FDA asked the Fourth Circuit Court of Appeals to stay the enforcement of the injunction, which the appeals court denied. The FDA then appealed to the Supreme Court, asking it to stay the injunction. In October 2020, the Court announced that it was holding the FDA’s request “in abeyance” to allow the district court to consider a motion by the FDA to dissolve or change the injunction. It gave the district court 40 days in which to act. That decision by the Court was in the “Shadow Docket” (see sidebar on page XX), so the exact vote of the Court in October is not clear, but 2 Justices (Alito and Thomas) dissented and would have stayed the injunction.6 Over the next 40 days, the district court did not withdraw its nationwide injunction.
Thus, on January 12, 2021, the case was again before the Supreme Court, which let the FDA’s regulations regarding mifepristone remain in place by lifting the district court’s injunction. Most of the justices supporting the stay did not write to explain their decision, although their dissent in the earlier cases may have served that purpose. (Maryland was permitting many kinds of activity that were more risky than visiting a clinic—indoor dining, with open hair salons, gyms, and casinos.)7 Chief Justice Roberts wrote a concurrence to indicate that, in his view, the issue was not whether the FDA’s regulations placed an undue burden on a right to an abortion generally, but that “My view is that courts owe significant deference” to the public health authorities (here meaning the FDA). Justices Sotomayor and Kagan dissented, saying that the issue was the undue burden on women, given the difficulties of the pandemic, particularly going to medical facilities during the COVID-19 pandemic.8
The injunction, sought by ACOG and others, was issued by the district court and was in effect for several months before it was dissolved by the Supreme Court. Following the change in presidential administrations, in April 2021 the FDA announced that it was going to “exercise enforcement discretion with respect to the in-person dispensing requirement…during the COVID-19 public health emergency.”9
Continue to: The Texas abortion case...
The Texas abortion case
The Court, on September 1, 2021, declined to block a Texas abortion statute from taking effect.10 This law precludes abortions after a fetal heartbeat is present at about 6 weeks of gestation. The Fifth Circuit declined to grant an injunction delaying implementation of the Texas law, and the Court did not reverse that decision.
Over the years, a variety of states have placed limitations on abortion, and those almost always have been enjoined by federal courts before they went into effect. However, the Texas statute, which undoubtedly is unconstitutional, was creatively constructed to avoid an early injunction.11 The statute does not allow state officials to enforce the new law, but rather it allows almost any private citizen to seek monetary damages from anyone performing an abortion or who “aids and abets” an abortion. Thus, it is difficult to tailor a lawsuit before this law is enforced. First, courts do not enjoin laws; they usually enjoin individuals from enforcing the law, and in this case it is difficult to know which individuals will be enforcing the laws and what their decisions might be. There also are some questions about the degree to which federal courts can enjoin state courts from deciding lawsuits under state law. For these procedural reasons, the majority of the Court found that those attacking the Texas law had not met their burden of showing that that they would win their case.
Even 3 of the dissenting justices said the defendants may be right that “existing doctrines preclude judicial intervention,” but that the consequences are such that the Court should delay the law until there is time for briefing and argument. The other 3 dissenting justices thought there would be ways of getting around the clever roadblock Texas had erected for the federal courts.
There has been some commentary that this case portends the abandonment of Roe v Wade and Casey,12 but that conclusion does not seem warranted by this case. The Court has accepted a Mississippi abortion law to be heard next term.13 In addition, the Texas statute is likely to be back in federal court once a private individual has filed a claim for money from an abortion provider (and likely even before that).
COVID-19 cases
The Supreme Court decided several cases related to COVID-19, including adjustments to election procedures, church services, and CDC eviction moratoria. As a general matter early in the pandemic, the Court deferred to government authorities, generally upholding government actions. Chief Justice Roberts emphasized the importance of the Court deferring to government officials in emergencies. As the pandemic progressed into 2021, however, the Court became less and less sympathetic to government actions that were not consistent, permitted by existing law, or reasonably necessary. For example, regulations of churches that were inconsistent with the regulation of similar organizations were struck down.14
Among the most interesting of the summer 2021 cases was the CDC eviction moratorium that essentially prohibited landlords nationwide from evicting tenants for nonpayment of rent. When the challenges to these CDC regulations first reached the Court, the moratorium was about to expire; in a 5-4 decision, the Court did not enjoin the CDC from continuing that policy. Justice Kavanaugh (the fifth vote) warned that “clear and specific congressional authorization…would be necessary to extend the moratorium past July 31.”15 Despite telling the Court that the moratorium would expire on July 31, just 3 days after the expiration and without any congressional authorization, the CDC reinstated what was practically the same moratorium.16 On August 26, the Court struck down the reinstated regulation, probably by a 6-3 margin. (Because this case arose in the “Shadow Docket,” the vote of some justices is not certain).17
Continue to: The Affordable Care Act...
The Affordable Care Act
The Affordable Care Act was challenged in the Court for the third time.18 In this term’s case, several states argued that when Congress essentially eliminated the penalty/tax for not purchasing insurance coverage, there was no longer a constitutional basis for the individual mandate. With that centerpiece gone, they claimed, the whole statute should be declared unconstitutional.
Along with many other specialty groups, ACOG joined an amicus curiae brief sponsored by the American Medical Association (AMA).19 An amicus brief is one not filed by the parties to the case, but by organizations or individuals who have information that may be of use to the Court in considering the case. Among other things, the filing of an amicus brief indicates the interest of the organization in the outcome of the case. In this case, the crux of the amicus was that even if the individual mandate currently is not constitutional, the Court should sever that provision and retain the rest of the ACA.
Despite some wild predictions about what the Court might do, it did not decide any substantive issue. Rather, it found that none of the parties to the case had “standing” to challenge the constitutionality of the ACA. Therefore, in effect, the Court dismissed the case without deciding the substantive legal issues.
Pharmacy Benefit Managers
The powerful Pharmacy Benefit Managers (PBMs) are a hidden part of the health care system; however, in recent years there has been increasing regulatory attention paid to them. Some states have begun regulating aspects of PBMs. In this term, the Court considered an Arkansas law that sought to protect local pharmacies from PBM pricing practices.20 The AMA filed an amicus brief in the case which made legal arguments, most of which had been made by the parties to the litigation.21
PBMs generally tell pharmacies how much they will reimburse the pharmacy for filling a prescription for a particular drug. In some instances, PBMs will set a reimbursement price that is lower than the wholesale price at which local pharmacies can purchase the drug. The Arkansas law prohibited PBMs in the state from reimbursing pharmacies for less than the wholesale cost the pharmacy paid for the drug.
The claim of the PBMs was that the Arkansas law violated the Employee Retirement Income Security Act (ERISA). In part, this act preempts state law that relates to fringe benefit plans. States have the authority to regulate insurance, but ERISA limits what they can do when the insurance relates to fringe benefits. The Court held that ERISA does not preempt the Arkansas law or similar state laws in other states. Because the state law was not preempted by the state law, the Arkansas regulation was upheld. The fact that this was a unanimous decision (8-0, because Justice Barrett was not on the Court when the case was heard) suggests that states may have leeway in additional regulations of PBMs, and it would not be surprising to see more of that state regulation in the future.
Continue to: Patent uncertainty...
Patent uncertainty
Csaba Truckai invented and patented the NovaSure System ablation device with a “moisture permeable” head. He sold his company and the related patents, which eventually were purchased by Hologic. Over time, Hologic added claims to the original patent. In the meantime, Truckai went on to invent another device, the Minerva Endometrial Ablation System (MEAS), which had a “moisture impermeable” head. (Note that the “Minerva Surgical, Inc.” involved in this case is not related to the company “Minerva Industries,” which some identified as a “patent troll.”)22
Hologic sued Minerva, claiming that Truckai’s second device (MEAS) infringed on its patent for the first device (NovaSure). Truckai’s defense was that the patent on NovaSure was invalid. Hologic felt that since Truckai had obtained that patent and then sold it, it was improper for him now to claim it was invalid. There is a doctrine for that: assignor estoppel—the person who sold (assigned) the patent is prevented from later claiming it was invalid. The question in this case was whether assignor estoppel is part of the patent law of the United States. It is not in the patent statutes, so it is a court-determined part of the law.
In a 5-4 decision this Term, the Court held that assignor estoppel is recognized, but that it is narrow.23 The Court identified several exceptions to assignor estoppel, notably for this case, including the situation in which the purchaser of the patent, after the purchase, returns to the Patent and Trademark Office to expand (amend) the patent’s claims. In that case, the seller could not be estopped by the amended terms of the patent. Minerva claimed that it was attacking the expanded patent that included changes made after it sold the patent. The Court, therefore, returned the case to the Federal Circuit to apply the principles it laid out about assignor estoppel.
Biotech and other fast-moving fields frequently have new technology building on slightly earlier technology. The current patent system often leaves uncertainty about who owns which part of a valid patent. This uncertainty is a drag on innovation, and the patent system is supposed to spur innovation. Assignor estoppel is likely to create additional complexity and uncertainty in some patents, which is regrettable.
Review of the Term
In addition to the other disruptions of the Term, during the first part of the Term, Amy Coney Barrett was not yet confirmed by the Senate, so there were only 8 justices until October 27. She did not participate in those cases that were heard before she joined the Court. The consensus is that the Court heard 67 cases: 57 were formally briefed and argued along with 8 summary reversals and 2 religious cases in the Shadow Docket. In my opinion, this undercounts both the number and the importance of the Shadow Docket cases, but the following data use the 67 case convention.24
The Court was unanimous in 43% of the cases, including some of the most divisive issues. That unanimity reflects very narrow decisions. There were (by conventional count) only eight 5-4 opinions (12%), an unusually low number. Justice Kavanaugh is viewed as the “median” justice. He was in the majority in 97% of all cases. Chief Justice Roberts and Justice Barrett were in the majority 91%, and Justice Gorsuch 90%. As for the other justices, they were in the majority (all cases) most of the time: Justice Alito, 83%; Justice Thomas, 81%; Justice Breyer, 76%; Justice Kagan, 75%; and Justice Sotomayor, 69%. In “divided cases” (when unanimous cases are removed), the percentages are: Justice Kavanaugh, 95%; Chief Justice Roberts and Justice Barrett, 84%; Justice Gorsuch, 82%; Justice Alito, 70%; Justice Thomas, 66%; Justice Breyer, 58%; Justice Kagan, 55%; and Justice Sotomayor, 45%.
When the term began, many Court watchers expected a relatively uninteresting term, dealing with many technical legal details. In fact, it turned out to be more interesting and important than expected, even with narrow holdings in important cases. Part of the secret of the term was that a lot of the real action was in the Shadow Docket. The end of the term is sometimes the moment when a justice announces a plan to retire. Many commentators expected Justice Breyer might announce—he has been under pressure to do so, to allow President Biden to nominate and a Democratic Senate to confirm a progressive justice. However, he did not do so. It is possible that he will announce his retirement to be effective when his successor is confirmed, but that is pure speculation.
Continue to: Next Term...
Next Term
The next term began on Monday, October 4, 2021. With the considerable current activity in the Shadow Docket, there was not much of a summer break. The coming term looks extraordinary. The headline case is an abortion case from Mississippi, Dobbs v Jackson Women’s Health Organization.25 The legal question is the constitutionality of Mississippi law that prohibits most abortions after 15 weeks of gestation. The Texas abortion law will also be back before the Court. As we saw this term, big cases may produce very narrow results, but this case has the potential for being a notable abortion decision.
In a different case the Court will decide whether a state attorney general can step in to defend an abortion law when the state health secretary does not do so.26
The Court also has accepted 3 cases dealing with reimbursement for health services. One deals with whether or not the Department of Health and Human Services can set reimbursement rates without good survey data regarding costs,27 another involves the calculation of additional payments for hospitals that serve a “disproportionate number of low-income patients,”28 and the third whether state Medicaid programs can take funds from an injured beneficiary’s tort recovery to cover future Medicaid costs.29
In other cases, the Court will review a gun control law from New York. The Court’s earlier Second Amendment cases involved guns in the home used for self-defense, but this case raises the question of whether a state can practically preclude “concealed-carry licenses.”30 Many experts believe the Court will accept a case dealing with racial preferences in college admissions, perhaps the Harvard case in which the claim is discrimination against Asian Americans.31
The ACOG mifepristone case was interesting, in part because the federal district court issued a nationwide injunction against the Americans with Disabilities Act, enforcing its rules anywhere in the country. The effect of these orders is for a single district judge to create the “law of the land,” at least until that is reviewed—which can take months. The advantage of the nationwide injunction is that it avoids having to repeatedly litigate the same issues in multiple courts around the country. The downside is that plaintiffs can seek out a nonrepresentative judge or circuit and receive an injunction that would be granted by few other circuits. In addition, a nationwide injunction can apply to specific circumstances that are not before the court issuing the injunction. In the mifepristone case, for example, 10 states requested to intervene in the ACOG case. The court rejected the request, but the nationwide injunction applied to those states.1
Although federal judges have had the authority to issue nationwide injunctions for years, they are becoming much more common. One reason is the ease of forum shopping noted earlier—organizations can cherry-pick district courts and circuits sympathetic to their views. Both left- and right-leaning organizations have learned this lesson, so left-leaning groups are likely to file in specific districts in the Ninth Circuit, and right-leaning groups to districts in the Fifth Circuit.
If the current trend of increasing nationwide injunctions continues, either the rules for the federal courts or congressional action may be required to reduce some of the abuses by both sides of the political spectrum.
The ACOG mifepristone case was interesting, in part because the federal district court issued a nationwide injunction against the Americans with Disabilities Act, enforcing its rules anywhere in the country. The effect of these orders is for a single district judge to create the “law of the land,” at least until that is reviewed—which can take months. The advantage of the nationwide injunction is that it avoids having to repeatedly litigate the same issues in multiple courts around the country. The downside is that plaintiffs can seek out a nonrepresentative judge or circuit and receive an injunction that would be granted by few other circuits. In addition, a nationwide injunction can apply to specific circumstances that are not before the court issuing the injunction. In the mifepristone case, for example, 10 states requested to intervene in the ACOG case. The court rejected the request, but the nationwide injunction applied to those states.1
Although federal judges have had the authority to issue nationwide injunctions for years, they are becoming much more common. One reason is the ease of forum shopping noted earlier—organizations can cherry-pick district courts and circuits sympathetic to their views. Both left- and right-leaning organizations have learned this lesson, so left-leaning groups are likely to file in specific districts in the Ninth Circuit, and right-leaning groups to districts in the Fifth Circuit.
If the current trend of increasing nationwide injunctions continues, either the rules for the federal courts or congressional action may be required to reduce some of the abuses by both sides of the political spectrum. Reference Am. Coll. of Obstetricians & Gynecologists v. United States FDA, 467 F. Supp. 3d 282, 284 (D. Md. 2020).
Reference
1. Am. Coll. of Obstetricians & Gynecologists v. United States FDA, 467 F. Supp. 3d 282, 284 (D. Md. 2020).
The “Shadow Docket”
The ACOG mifepristone decisions do not appear on the Supreme Court’s “Court Opinions” website.1 They appear in what has become known in recent years as “The Shadow Docket,” an informal term that includes many orders of the Court and statements of individual justices regarding some cases.2 There are hundreds of orders by the Court each Term, there is nothing particularly shadowy about any of these items—they are all publicly available on the Court’s website and later in paper format. It is, however, a little harder to find and much harder to sort through than the major opinions. In some cases, it is not possible to tell what the vote was, how each justice voted, and what the reasoning of the Court was. In a few cases it is difficult to know exactly what the Court was holding or otherwise leaves some confusion about what the law actually is.3
The part of the Shadow Docket that is most intriguing for commentators, and where the ACOG cases appear, is the “Opinions Relating to Orders.”4 These are a variety of opinions, some written by the Court and many by individual justices. It also includes the action of the Court in some cases in which there was not full briefing or oral argument. The statements by justices often are to dissent from the denial of cert of decisions of the Court. These opinions have become much more common over the years. In this past term, there were approximately 60 such opinions related to about 50 cases. In part, this relates to the number of pandemic cases that could not wait for a Court decision going through the extended ordinary process. Although the Shadow Docket has been of interest to academic observers and Court watchers for years, this year it has attracted the attention of Congress.5
References
1. Opinions of the Court. Supreme Court website. https://www.supremecourt.gov/opinions/slipopinion/20#list. Accessed October 10, 2021.
2. Baude W. Foreword: the Supreme Court’s Shadow Docket, 9 N.Y.U. J.L. & Liberty 1 (2015).
3. Vladeck SI. The Solicitor General and the Shadow Docket, 133 Harvard Law Review. 123 (2019).
4. Opinions relating to orders. Supreme Court website. https://www.supremecourt.gov/opinions/relatingtoorders/20#list. Accessed October 10, 2021.
5. The Supreme Court’s Shadow Docket: Hearing Before the Subcommittee on Courts, Intellectual Property and the Internet of the H. Committee on the Judiciary, 117th Congress (2021).
- American College of Obstetricians & Gynecologists v. United States FDA, 472 F. Supp. 3d 183 (D. Md. 2020).
- Michael Kunzelman, Doctors Sue to Block FDA Abortion Pill Rule During Pandemic, (May 29, 2020).
- ACLU, American College Of Obstetricians And Gynecologists V. U.S. Food And Drug Administration, https://www.aclu.org/cases/american-college-obstetricians-and-gynecologists-v-us-food-and-drug-administration. Updated February 12, 2021. Accessed August 27, 2021.
- Whole Woman’s Health v Hellerstedt, 579 US ___ (2016), 136 S Ct 2292.
- 2016 Clinical Review at 39, 47, 49, Opp’n Mot. PI Ex. 19, ECF No. 62-11.
- American College of Obstetricians and Gynecologists v FDA (I), decided October 8, 2020.
- October 8, 2020, dissenting opinion by Justice Alito.
- January 12, 2021, dissenting opinion by Justice Sotomayor.
- Questions and answers on Mifeprex. U.S. Food and Drug Administration website. Published April 13, 2021. https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/questions-and-answers-mifeprex. Accessed October 9, 2021.
- Whole Woman’s Health v Jackson, decided September 1, 2021.
- Texas Senate Bill 8, relating to abortion, including abortions after detection of unborn child’s heartbeat; authorizing a private civil right of action. LegiScan website. https://legiscan.com/TX/text/SB8/id/2395961. Accessed October 9, 2021.
- Planned Parenthood of Southeastern Pennsylvania v Casey, 505 U. S. 833 (1992); Roe v Wade, 410 U. S. 113 (1973).
- Dobbs v Jackson Women’s Health Organization, No. 19-1392.
- Roman Catholic Diocese of Brooklyn v Cuomo, decided November 25, 2020.
- Alabama Association of Realtors v Department of Health and Human Services, decided June 29, 2021.
- Temporary halt in residential evictions in communities with substantial or high levels of community transmission of COVID-19 to prevent the further spread of COVID-19. August 6, 2021. https://www.federalregister.gov/documents/2021/08/06/2021-16945/temporary-halt-in-residential-evictions-in-communities-with-substantial-or-high-transmission-of.
- Alabama Association of Realtors v Department of Health and Human Services, decided August 26, 2021.
- California v Texas, decided June 17, 2021.
- Brief of Amici Curiae American Medical Association, American Academy of Allergy, Asthma and Immunology, Aerospace Medical Association, American Academy of Family Physicians, American Academy of Pediatrics, American College of Cardiology, American College of Emergency Physicians, American College of Medical Genetics and Genomics, American College of Obstetricians and Gynecologists, American College of Physicians, American College of Radiation Oncology, American College of Radiology, American Psychiatric Association, American Society of Gastrointestinal Endoscopy, American Society of Hematology, American Society of Metabolic and Bariatric Surgery, Endocrine Society, GLMA: Health Professionals Advancing LGBTQ Equality, Renal Physicians Association, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology in Support of Petitioners, in California v. Texas. May 13, 2020. https://www.supremecourt.gov/DocketPDF/19/19-840/143469/20200513150051995_19-840%20Amici%20Brief%20AMA.pdf. Accessed October 9, 2021.
- Rutledge v Pharmaceutical Care Management Association, decided December 10, 2020.
- Brief of the American Medical Association, The Arkansas Medical Society, and The Litigation Center of the American Medical Association and the State Medical Societies as Amici Curiae in Support of Petitioner in Rutledge v Pharmaceutical Care Management Association. March 2, 2020. https://www.supremecourt.gov/DocketPDF/18/18-540/134670/20200302163622018_Rutledge%20v.%20PCMA%20Amicus%20Brief%20of%20AMA%20et%20al.pdf. Accessed October 9, 2021.
- Apple quietly settles patent lawsuit, promptly gets hit with another one. TechCrunch website. Published July 30, 2010. https://techcrunch.com/2010/07/30/apple-minerva-emblaze/. Accessed October 9, 2021.
- Minerva Surgical, Inc. v Hologic, Inc., decided June 29, 2021.
- Stat pack. SCOTUS Blog website. Published July 6, 2021. https://www.scotusblog.com/wp-content/uploads/2021/07/Final-Stat-Pack-7.6.21.pdf. Accessed October 9, 2021.
- Dobbs v Jackson Women’s Health Organization, No. 19-1392.
- Cameron v. EMW Women’s Surgical Center, https://www.scotusblog.com/case-files/cases/cameron-v-emw-womens-surgical-center-p-s-c/. Accessed August 28, 2021.
- American Hospital Association v Becerra, No. 20-1114.
- Becerra v Empire Health Foundation, No. 20-1312.
- Gallardo v Marstiller, No. 20-1263.
- New York State Rifle & Pistol Association Inc. v Corlett, No. 20-843.
- Students for Fair Admissions v President & Fellows of Harvard College, No. 20-1199.
The Supreme Court’s usual processes were disrupted this term. The COVID-19 pandemic required audio hearings rather than in-person, and it resulted in a number of emergency legal appeals. As the Court began its regular sessions on October 5, 2020, there were only 8 justices—Justice Ruth Bader Ginsburg had passed away and Amy Coney Barrett had not yet been confirmed by the Senate. The Court decided many important cases this term, including dealing with the delivery of drugs to induce abortions, a Centers for Disease Control and Prevention (CDC) moratorium on housing evictions, yet another case on the Affordable Care Act, state laws concerning pharmacy benefit managers, and the Hologic and Minerva endometrial ablation systems patents. After considering these cases, we also will briefly look at other cases of general interest.
Abortion
Patient access to mifepristone
In May 2020, the American College of Obstetricians and Gynecologists (ACOG) was the named plaintiff in a lawsuit against the US Food and Drug Administration (FDA) regarding the drugs mifepristone and misoprostol that are used to induce medical abortions.1 The case was filed by the American Civil Liberties Union on behalf of ACOG and others2,3 and raised the issue of patients’ access to these medications. The basic claim of the case was that during the pandemic, the FDA’s regulation of mifepristone was unconstitutional in that they imposed an undue burden on the decision of women to have an abortion.4 (Although misoprostol is a part of the medical abortion regimen, it is not subject to special regulation and was not part of the litigation.)
The FDA regulation of mifepristone, begun in 2000 but modified since then, includes 3 elements to assure safe use:
- prescribers must have special training or certification
- the drug can be dispensed to patients only in a hospital, clinic, or medical office under the supervision of a certified health care provider (known as the “in-person dispensing requirement” because retail pharmacy or mail distribution are prohibited)
- the health care provider must review a “patient agreement form” with the patient and have the patient sign the consent form in the provider’s presence.5
The pandemic made fulfilling these requirements substantially more burdensome and difficult. The question was whether the FDA was constitutionally required to modify its regulations during a pandemic to take account of the undue burden of the regulation created by the pandemic. That is, the question was not whether the FDA could have or should have chosen to make the modification, but whether it was required to do so.
In July 2020, a federal district court in Maryland held that the FDA regulation was an unconstitutional burden on the abortion rights of women during the pandemic and issued a preliminary injunction to stop the FDA from enforcing the in-person dispensing and signature rules. The district judge applied the injunction to Maryland, but also made it a nationwide injunction. (The issue of district court nationwide injunctions is considered in, “District court ‘nationwide injunctions’”).
The FDA asked the Fourth Circuit Court of Appeals to stay the enforcement of the injunction, which the appeals court denied. The FDA then appealed to the Supreme Court, asking it to stay the injunction. In October 2020, the Court announced that it was holding the FDA’s request “in abeyance” to allow the district court to consider a motion by the FDA to dissolve or change the injunction. It gave the district court 40 days in which to act. That decision by the Court was in the “Shadow Docket” (see sidebar on page XX), so the exact vote of the Court in October is not clear, but 2 Justices (Alito and Thomas) dissented and would have stayed the injunction.6 Over the next 40 days, the district court did not withdraw its nationwide injunction.
Thus, on January 12, 2021, the case was again before the Supreme Court, which let the FDA’s regulations regarding mifepristone remain in place by lifting the district court’s injunction. Most of the justices supporting the stay did not write to explain their decision, although their dissent in the earlier cases may have served that purpose. (Maryland was permitting many kinds of activity that were more risky than visiting a clinic—indoor dining, with open hair salons, gyms, and casinos.)7 Chief Justice Roberts wrote a concurrence to indicate that, in his view, the issue was not whether the FDA’s regulations placed an undue burden on a right to an abortion generally, but that “My view is that courts owe significant deference” to the public health authorities (here meaning the FDA). Justices Sotomayor and Kagan dissented, saying that the issue was the undue burden on women, given the difficulties of the pandemic, particularly going to medical facilities during the COVID-19 pandemic.8
The injunction, sought by ACOG and others, was issued by the district court and was in effect for several months before it was dissolved by the Supreme Court. Following the change in presidential administrations, in April 2021 the FDA announced that it was going to “exercise enforcement discretion with respect to the in-person dispensing requirement…during the COVID-19 public health emergency.”9
Continue to: The Texas abortion case...
The Texas abortion case
The Court, on September 1, 2021, declined to block a Texas abortion statute from taking effect.10 This law precludes abortions after a fetal heartbeat is present at about 6 weeks of gestation. The Fifth Circuit declined to grant an injunction delaying implementation of the Texas law, and the Court did not reverse that decision.
Over the years, a variety of states have placed limitations on abortion, and those almost always have been enjoined by federal courts before they went into effect. However, the Texas statute, which undoubtedly is unconstitutional, was creatively constructed to avoid an early injunction.11 The statute does not allow state officials to enforce the new law, but rather it allows almost any private citizen to seek monetary damages from anyone performing an abortion or who “aids and abets” an abortion. Thus, it is difficult to tailor a lawsuit before this law is enforced. First, courts do not enjoin laws; they usually enjoin individuals from enforcing the law, and in this case it is difficult to know which individuals will be enforcing the laws and what their decisions might be. There also are some questions about the degree to which federal courts can enjoin state courts from deciding lawsuits under state law. For these procedural reasons, the majority of the Court found that those attacking the Texas law had not met their burden of showing that that they would win their case.
Even 3 of the dissenting justices said the defendants may be right that “existing doctrines preclude judicial intervention,” but that the consequences are such that the Court should delay the law until there is time for briefing and argument. The other 3 dissenting justices thought there would be ways of getting around the clever roadblock Texas had erected for the federal courts.
There has been some commentary that this case portends the abandonment of Roe v Wade and Casey,12 but that conclusion does not seem warranted by this case. The Court has accepted a Mississippi abortion law to be heard next term.13 In addition, the Texas statute is likely to be back in federal court once a private individual has filed a claim for money from an abortion provider (and likely even before that).
COVID-19 cases
The Supreme Court decided several cases related to COVID-19, including adjustments to election procedures, church services, and CDC eviction moratoria. As a general matter early in the pandemic, the Court deferred to government authorities, generally upholding government actions. Chief Justice Roberts emphasized the importance of the Court deferring to government officials in emergencies. As the pandemic progressed into 2021, however, the Court became less and less sympathetic to government actions that were not consistent, permitted by existing law, or reasonably necessary. For example, regulations of churches that were inconsistent with the regulation of similar organizations were struck down.14
Among the most interesting of the summer 2021 cases was the CDC eviction moratorium that essentially prohibited landlords nationwide from evicting tenants for nonpayment of rent. When the challenges to these CDC regulations first reached the Court, the moratorium was about to expire; in a 5-4 decision, the Court did not enjoin the CDC from continuing that policy. Justice Kavanaugh (the fifth vote) warned that “clear and specific congressional authorization…would be necessary to extend the moratorium past July 31.”15 Despite telling the Court that the moratorium would expire on July 31, just 3 days after the expiration and without any congressional authorization, the CDC reinstated what was practically the same moratorium.16 On August 26, the Court struck down the reinstated regulation, probably by a 6-3 margin. (Because this case arose in the “Shadow Docket,” the vote of some justices is not certain).17
Continue to: The Affordable Care Act...
The Affordable Care Act
The Affordable Care Act was challenged in the Court for the third time.18 In this term’s case, several states argued that when Congress essentially eliminated the penalty/tax for not purchasing insurance coverage, there was no longer a constitutional basis for the individual mandate. With that centerpiece gone, they claimed, the whole statute should be declared unconstitutional.
Along with many other specialty groups, ACOG joined an amicus curiae brief sponsored by the American Medical Association (AMA).19 An amicus brief is one not filed by the parties to the case, but by organizations or individuals who have information that may be of use to the Court in considering the case. Among other things, the filing of an amicus brief indicates the interest of the organization in the outcome of the case. In this case, the crux of the amicus was that even if the individual mandate currently is not constitutional, the Court should sever that provision and retain the rest of the ACA.
Despite some wild predictions about what the Court might do, it did not decide any substantive issue. Rather, it found that none of the parties to the case had “standing” to challenge the constitutionality of the ACA. Therefore, in effect, the Court dismissed the case without deciding the substantive legal issues.
Pharmacy Benefit Managers
The powerful Pharmacy Benefit Managers (PBMs) are a hidden part of the health care system; however, in recent years there has been increasing regulatory attention paid to them. Some states have begun regulating aspects of PBMs. In this term, the Court considered an Arkansas law that sought to protect local pharmacies from PBM pricing practices.20 The AMA filed an amicus brief in the case which made legal arguments, most of which had been made by the parties to the litigation.21
PBMs generally tell pharmacies how much they will reimburse the pharmacy for filling a prescription for a particular drug. In some instances, PBMs will set a reimbursement price that is lower than the wholesale price at which local pharmacies can purchase the drug. The Arkansas law prohibited PBMs in the state from reimbursing pharmacies for less than the wholesale cost the pharmacy paid for the drug.
The claim of the PBMs was that the Arkansas law violated the Employee Retirement Income Security Act (ERISA). In part, this act preempts state law that relates to fringe benefit plans. States have the authority to regulate insurance, but ERISA limits what they can do when the insurance relates to fringe benefits. The Court held that ERISA does not preempt the Arkansas law or similar state laws in other states. Because the state law was not preempted by the state law, the Arkansas regulation was upheld. The fact that this was a unanimous decision (8-0, because Justice Barrett was not on the Court when the case was heard) suggests that states may have leeway in additional regulations of PBMs, and it would not be surprising to see more of that state regulation in the future.
Continue to: Patent uncertainty...
Patent uncertainty
Csaba Truckai invented and patented the NovaSure System ablation device with a “moisture permeable” head. He sold his company and the related patents, which eventually were purchased by Hologic. Over time, Hologic added claims to the original patent. In the meantime, Truckai went on to invent another device, the Minerva Endometrial Ablation System (MEAS), which had a “moisture impermeable” head. (Note that the “Minerva Surgical, Inc.” involved in this case is not related to the company “Minerva Industries,” which some identified as a “patent troll.”)22
Hologic sued Minerva, claiming that Truckai’s second device (MEAS) infringed on its patent for the first device (NovaSure). Truckai’s defense was that the patent on NovaSure was invalid. Hologic felt that since Truckai had obtained that patent and then sold it, it was improper for him now to claim it was invalid. There is a doctrine for that: assignor estoppel—the person who sold (assigned) the patent is prevented from later claiming it was invalid. The question in this case was whether assignor estoppel is part of the patent law of the United States. It is not in the patent statutes, so it is a court-determined part of the law.
In a 5-4 decision this Term, the Court held that assignor estoppel is recognized, but that it is narrow.23 The Court identified several exceptions to assignor estoppel, notably for this case, including the situation in which the purchaser of the patent, after the purchase, returns to the Patent and Trademark Office to expand (amend) the patent’s claims. In that case, the seller could not be estopped by the amended terms of the patent. Minerva claimed that it was attacking the expanded patent that included changes made after it sold the patent. The Court, therefore, returned the case to the Federal Circuit to apply the principles it laid out about assignor estoppel.
Biotech and other fast-moving fields frequently have new technology building on slightly earlier technology. The current patent system often leaves uncertainty about who owns which part of a valid patent. This uncertainty is a drag on innovation, and the patent system is supposed to spur innovation. Assignor estoppel is likely to create additional complexity and uncertainty in some patents, which is regrettable.
Review of the Term
In addition to the other disruptions of the Term, during the first part of the Term, Amy Coney Barrett was not yet confirmed by the Senate, so there were only 8 justices until October 27. She did not participate in those cases that were heard before she joined the Court. The consensus is that the Court heard 67 cases: 57 were formally briefed and argued along with 8 summary reversals and 2 religious cases in the Shadow Docket. In my opinion, this undercounts both the number and the importance of the Shadow Docket cases, but the following data use the 67 case convention.24
The Court was unanimous in 43% of the cases, including some of the most divisive issues. That unanimity reflects very narrow decisions. There were (by conventional count) only eight 5-4 opinions (12%), an unusually low number. Justice Kavanaugh is viewed as the “median” justice. He was in the majority in 97% of all cases. Chief Justice Roberts and Justice Barrett were in the majority 91%, and Justice Gorsuch 90%. As for the other justices, they were in the majority (all cases) most of the time: Justice Alito, 83%; Justice Thomas, 81%; Justice Breyer, 76%; Justice Kagan, 75%; and Justice Sotomayor, 69%. In “divided cases” (when unanimous cases are removed), the percentages are: Justice Kavanaugh, 95%; Chief Justice Roberts and Justice Barrett, 84%; Justice Gorsuch, 82%; Justice Alito, 70%; Justice Thomas, 66%; Justice Breyer, 58%; Justice Kagan, 55%; and Justice Sotomayor, 45%.
When the term began, many Court watchers expected a relatively uninteresting term, dealing with many technical legal details. In fact, it turned out to be more interesting and important than expected, even with narrow holdings in important cases. Part of the secret of the term was that a lot of the real action was in the Shadow Docket. The end of the term is sometimes the moment when a justice announces a plan to retire. Many commentators expected Justice Breyer might announce—he has been under pressure to do so, to allow President Biden to nominate and a Democratic Senate to confirm a progressive justice. However, he did not do so. It is possible that he will announce his retirement to be effective when his successor is confirmed, but that is pure speculation.
Continue to: Next Term...
Next Term
The next term began on Monday, October 4, 2021. With the considerable current activity in the Shadow Docket, there was not much of a summer break. The coming term looks extraordinary. The headline case is an abortion case from Mississippi, Dobbs v Jackson Women’s Health Organization.25 The legal question is the constitutionality of Mississippi law that prohibits most abortions after 15 weeks of gestation. The Texas abortion law will also be back before the Court. As we saw this term, big cases may produce very narrow results, but this case has the potential for being a notable abortion decision.
In a different case the Court will decide whether a state attorney general can step in to defend an abortion law when the state health secretary does not do so.26
The Court also has accepted 3 cases dealing with reimbursement for health services. One deals with whether or not the Department of Health and Human Services can set reimbursement rates without good survey data regarding costs,27 another involves the calculation of additional payments for hospitals that serve a “disproportionate number of low-income patients,”28 and the third whether state Medicaid programs can take funds from an injured beneficiary’s tort recovery to cover future Medicaid costs.29
In other cases, the Court will review a gun control law from New York. The Court’s earlier Second Amendment cases involved guns in the home used for self-defense, but this case raises the question of whether a state can practically preclude “concealed-carry licenses.”30 Many experts believe the Court will accept a case dealing with racial preferences in college admissions, perhaps the Harvard case in which the claim is discrimination against Asian Americans.31
The ACOG mifepristone case was interesting, in part because the federal district court issued a nationwide injunction against the Americans with Disabilities Act, enforcing its rules anywhere in the country. The effect of these orders is for a single district judge to create the “law of the land,” at least until that is reviewed—which can take months. The advantage of the nationwide injunction is that it avoids having to repeatedly litigate the same issues in multiple courts around the country. The downside is that plaintiffs can seek out a nonrepresentative judge or circuit and receive an injunction that would be granted by few other circuits. In addition, a nationwide injunction can apply to specific circumstances that are not before the court issuing the injunction. In the mifepristone case, for example, 10 states requested to intervene in the ACOG case. The court rejected the request, but the nationwide injunction applied to those states.1
Although federal judges have had the authority to issue nationwide injunctions for years, they are becoming much more common. One reason is the ease of forum shopping noted earlier—organizations can cherry-pick district courts and circuits sympathetic to their views. Both left- and right-leaning organizations have learned this lesson, so left-leaning groups are likely to file in specific districts in the Ninth Circuit, and right-leaning groups to districts in the Fifth Circuit.
If the current trend of increasing nationwide injunctions continues, either the rules for the federal courts or congressional action may be required to reduce some of the abuses by both sides of the political spectrum.
The ACOG mifepristone case was interesting, in part because the federal district court issued a nationwide injunction against the Americans with Disabilities Act, enforcing its rules anywhere in the country. The effect of these orders is for a single district judge to create the “law of the land,” at least until that is reviewed—which can take months. The advantage of the nationwide injunction is that it avoids having to repeatedly litigate the same issues in multiple courts around the country. The downside is that plaintiffs can seek out a nonrepresentative judge or circuit and receive an injunction that would be granted by few other circuits. In addition, a nationwide injunction can apply to specific circumstances that are not before the court issuing the injunction. In the mifepristone case, for example, 10 states requested to intervene in the ACOG case. The court rejected the request, but the nationwide injunction applied to those states.1
Although federal judges have had the authority to issue nationwide injunctions for years, they are becoming much more common. One reason is the ease of forum shopping noted earlier—organizations can cherry-pick district courts and circuits sympathetic to their views. Both left- and right-leaning organizations have learned this lesson, so left-leaning groups are likely to file in specific districts in the Ninth Circuit, and right-leaning groups to districts in the Fifth Circuit.
If the current trend of increasing nationwide injunctions continues, either the rules for the federal courts or congressional action may be required to reduce some of the abuses by both sides of the political spectrum. Reference Am. Coll. of Obstetricians & Gynecologists v. United States FDA, 467 F. Supp. 3d 282, 284 (D. Md. 2020).
Reference
1. Am. Coll. of Obstetricians & Gynecologists v. United States FDA, 467 F. Supp. 3d 282, 284 (D. Md. 2020).
The “Shadow Docket”
The ACOG mifepristone decisions do not appear on the Supreme Court’s “Court Opinions” website.1 They appear in what has become known in recent years as “The Shadow Docket,” an informal term that includes many orders of the Court and statements of individual justices regarding some cases.2 There are hundreds of orders by the Court each Term, there is nothing particularly shadowy about any of these items—they are all publicly available on the Court’s website and later in paper format. It is, however, a little harder to find and much harder to sort through than the major opinions. In some cases, it is not possible to tell what the vote was, how each justice voted, and what the reasoning of the Court was. In a few cases it is difficult to know exactly what the Court was holding or otherwise leaves some confusion about what the law actually is.3
The part of the Shadow Docket that is most intriguing for commentators, and where the ACOG cases appear, is the “Opinions Relating to Orders.”4 These are a variety of opinions, some written by the Court and many by individual justices. It also includes the action of the Court in some cases in which there was not full briefing or oral argument. The statements by justices often are to dissent from the denial of cert of decisions of the Court. These opinions have become much more common over the years. In this past term, there were approximately 60 such opinions related to about 50 cases. In part, this relates to the number of pandemic cases that could not wait for a Court decision going through the extended ordinary process. Although the Shadow Docket has been of interest to academic observers and Court watchers for years, this year it has attracted the attention of Congress.5
References
1. Opinions of the Court. Supreme Court website. https://www.supremecourt.gov/opinions/slipopinion/20#list. Accessed October 10, 2021.
2. Baude W. Foreword: the Supreme Court’s Shadow Docket, 9 N.Y.U. J.L. & Liberty 1 (2015).
3. Vladeck SI. The Solicitor General and the Shadow Docket, 133 Harvard Law Review. 123 (2019).
4. Opinions relating to orders. Supreme Court website. https://www.supremecourt.gov/opinions/relatingtoorders/20#list. Accessed October 10, 2021.
5. The Supreme Court’s Shadow Docket: Hearing Before the Subcommittee on Courts, Intellectual Property and the Internet of the H. Committee on the Judiciary, 117th Congress (2021).
The Supreme Court’s usual processes were disrupted this term. The COVID-19 pandemic required audio hearings rather than in-person, and it resulted in a number of emergency legal appeals. As the Court began its regular sessions on October 5, 2020, there were only 8 justices—Justice Ruth Bader Ginsburg had passed away and Amy Coney Barrett had not yet been confirmed by the Senate. The Court decided many important cases this term, including dealing with the delivery of drugs to induce abortions, a Centers for Disease Control and Prevention (CDC) moratorium on housing evictions, yet another case on the Affordable Care Act, state laws concerning pharmacy benefit managers, and the Hologic and Minerva endometrial ablation systems patents. After considering these cases, we also will briefly look at other cases of general interest.
Abortion
Patient access to mifepristone
In May 2020, the American College of Obstetricians and Gynecologists (ACOG) was the named plaintiff in a lawsuit against the US Food and Drug Administration (FDA) regarding the drugs mifepristone and misoprostol that are used to induce medical abortions.1 The case was filed by the American Civil Liberties Union on behalf of ACOG and others2,3 and raised the issue of patients’ access to these medications. The basic claim of the case was that during the pandemic, the FDA’s regulation of mifepristone was unconstitutional in that they imposed an undue burden on the decision of women to have an abortion.4 (Although misoprostol is a part of the medical abortion regimen, it is not subject to special regulation and was not part of the litigation.)
The FDA regulation of mifepristone, begun in 2000 but modified since then, includes 3 elements to assure safe use:
- prescribers must have special training or certification
- the drug can be dispensed to patients only in a hospital, clinic, or medical office under the supervision of a certified health care provider (known as the “in-person dispensing requirement” because retail pharmacy or mail distribution are prohibited)
- the health care provider must review a “patient agreement form” with the patient and have the patient sign the consent form in the provider’s presence.5
The pandemic made fulfilling these requirements substantially more burdensome and difficult. The question was whether the FDA was constitutionally required to modify its regulations during a pandemic to take account of the undue burden of the regulation created by the pandemic. That is, the question was not whether the FDA could have or should have chosen to make the modification, but whether it was required to do so.
In July 2020, a federal district court in Maryland held that the FDA regulation was an unconstitutional burden on the abortion rights of women during the pandemic and issued a preliminary injunction to stop the FDA from enforcing the in-person dispensing and signature rules. The district judge applied the injunction to Maryland, but also made it a nationwide injunction. (The issue of district court nationwide injunctions is considered in, “District court ‘nationwide injunctions’”).
The FDA asked the Fourth Circuit Court of Appeals to stay the enforcement of the injunction, which the appeals court denied. The FDA then appealed to the Supreme Court, asking it to stay the injunction. In October 2020, the Court announced that it was holding the FDA’s request “in abeyance” to allow the district court to consider a motion by the FDA to dissolve or change the injunction. It gave the district court 40 days in which to act. That decision by the Court was in the “Shadow Docket” (see sidebar on page XX), so the exact vote of the Court in October is not clear, but 2 Justices (Alito and Thomas) dissented and would have stayed the injunction.6 Over the next 40 days, the district court did not withdraw its nationwide injunction.
Thus, on January 12, 2021, the case was again before the Supreme Court, which let the FDA’s regulations regarding mifepristone remain in place by lifting the district court’s injunction. Most of the justices supporting the stay did not write to explain their decision, although their dissent in the earlier cases may have served that purpose. (Maryland was permitting many kinds of activity that were more risky than visiting a clinic—indoor dining, with open hair salons, gyms, and casinos.)7 Chief Justice Roberts wrote a concurrence to indicate that, in his view, the issue was not whether the FDA’s regulations placed an undue burden on a right to an abortion generally, but that “My view is that courts owe significant deference” to the public health authorities (here meaning the FDA). Justices Sotomayor and Kagan dissented, saying that the issue was the undue burden on women, given the difficulties of the pandemic, particularly going to medical facilities during the COVID-19 pandemic.8
The injunction, sought by ACOG and others, was issued by the district court and was in effect for several months before it was dissolved by the Supreme Court. Following the change in presidential administrations, in April 2021 the FDA announced that it was going to “exercise enforcement discretion with respect to the in-person dispensing requirement…during the COVID-19 public health emergency.”9
Continue to: The Texas abortion case...
The Texas abortion case
The Court, on September 1, 2021, declined to block a Texas abortion statute from taking effect.10 This law precludes abortions after a fetal heartbeat is present at about 6 weeks of gestation. The Fifth Circuit declined to grant an injunction delaying implementation of the Texas law, and the Court did not reverse that decision.
Over the years, a variety of states have placed limitations on abortion, and those almost always have been enjoined by federal courts before they went into effect. However, the Texas statute, which undoubtedly is unconstitutional, was creatively constructed to avoid an early injunction.11 The statute does not allow state officials to enforce the new law, but rather it allows almost any private citizen to seek monetary damages from anyone performing an abortion or who “aids and abets” an abortion. Thus, it is difficult to tailor a lawsuit before this law is enforced. First, courts do not enjoin laws; they usually enjoin individuals from enforcing the law, and in this case it is difficult to know which individuals will be enforcing the laws and what their decisions might be. There also are some questions about the degree to which federal courts can enjoin state courts from deciding lawsuits under state law. For these procedural reasons, the majority of the Court found that those attacking the Texas law had not met their burden of showing that that they would win their case.
Even 3 of the dissenting justices said the defendants may be right that “existing doctrines preclude judicial intervention,” but that the consequences are such that the Court should delay the law until there is time for briefing and argument. The other 3 dissenting justices thought there would be ways of getting around the clever roadblock Texas had erected for the federal courts.
There has been some commentary that this case portends the abandonment of Roe v Wade and Casey,12 but that conclusion does not seem warranted by this case. The Court has accepted a Mississippi abortion law to be heard next term.13 In addition, the Texas statute is likely to be back in federal court once a private individual has filed a claim for money from an abortion provider (and likely even before that).
COVID-19 cases
The Supreme Court decided several cases related to COVID-19, including adjustments to election procedures, church services, and CDC eviction moratoria. As a general matter early in the pandemic, the Court deferred to government authorities, generally upholding government actions. Chief Justice Roberts emphasized the importance of the Court deferring to government officials in emergencies. As the pandemic progressed into 2021, however, the Court became less and less sympathetic to government actions that were not consistent, permitted by existing law, or reasonably necessary. For example, regulations of churches that were inconsistent with the regulation of similar organizations were struck down.14
Among the most interesting of the summer 2021 cases was the CDC eviction moratorium that essentially prohibited landlords nationwide from evicting tenants for nonpayment of rent. When the challenges to these CDC regulations first reached the Court, the moratorium was about to expire; in a 5-4 decision, the Court did not enjoin the CDC from continuing that policy. Justice Kavanaugh (the fifth vote) warned that “clear and specific congressional authorization…would be necessary to extend the moratorium past July 31.”15 Despite telling the Court that the moratorium would expire on July 31, just 3 days after the expiration and without any congressional authorization, the CDC reinstated what was practically the same moratorium.16 On August 26, the Court struck down the reinstated regulation, probably by a 6-3 margin. (Because this case arose in the “Shadow Docket,” the vote of some justices is not certain).17
Continue to: The Affordable Care Act...
The Affordable Care Act
The Affordable Care Act was challenged in the Court for the third time.18 In this term’s case, several states argued that when Congress essentially eliminated the penalty/tax for not purchasing insurance coverage, there was no longer a constitutional basis for the individual mandate. With that centerpiece gone, they claimed, the whole statute should be declared unconstitutional.
Along with many other specialty groups, ACOG joined an amicus curiae brief sponsored by the American Medical Association (AMA).19 An amicus brief is one not filed by the parties to the case, but by organizations or individuals who have information that may be of use to the Court in considering the case. Among other things, the filing of an amicus brief indicates the interest of the organization in the outcome of the case. In this case, the crux of the amicus was that even if the individual mandate currently is not constitutional, the Court should sever that provision and retain the rest of the ACA.
Despite some wild predictions about what the Court might do, it did not decide any substantive issue. Rather, it found that none of the parties to the case had “standing” to challenge the constitutionality of the ACA. Therefore, in effect, the Court dismissed the case without deciding the substantive legal issues.
Pharmacy Benefit Managers
The powerful Pharmacy Benefit Managers (PBMs) are a hidden part of the health care system; however, in recent years there has been increasing regulatory attention paid to them. Some states have begun regulating aspects of PBMs. In this term, the Court considered an Arkansas law that sought to protect local pharmacies from PBM pricing practices.20 The AMA filed an amicus brief in the case which made legal arguments, most of which had been made by the parties to the litigation.21
PBMs generally tell pharmacies how much they will reimburse the pharmacy for filling a prescription for a particular drug. In some instances, PBMs will set a reimbursement price that is lower than the wholesale price at which local pharmacies can purchase the drug. The Arkansas law prohibited PBMs in the state from reimbursing pharmacies for less than the wholesale cost the pharmacy paid for the drug.
The claim of the PBMs was that the Arkansas law violated the Employee Retirement Income Security Act (ERISA). In part, this act preempts state law that relates to fringe benefit plans. States have the authority to regulate insurance, but ERISA limits what they can do when the insurance relates to fringe benefits. The Court held that ERISA does not preempt the Arkansas law or similar state laws in other states. Because the state law was not preempted by the state law, the Arkansas regulation was upheld. The fact that this was a unanimous decision (8-0, because Justice Barrett was not on the Court when the case was heard) suggests that states may have leeway in additional regulations of PBMs, and it would not be surprising to see more of that state regulation in the future.
Continue to: Patent uncertainty...
Patent uncertainty
Csaba Truckai invented and patented the NovaSure System ablation device with a “moisture permeable” head. He sold his company and the related patents, which eventually were purchased by Hologic. Over time, Hologic added claims to the original patent. In the meantime, Truckai went on to invent another device, the Minerva Endometrial Ablation System (MEAS), which had a “moisture impermeable” head. (Note that the “Minerva Surgical, Inc.” involved in this case is not related to the company “Minerva Industries,” which some identified as a “patent troll.”)22
Hologic sued Minerva, claiming that Truckai’s second device (MEAS) infringed on its patent for the first device (NovaSure). Truckai’s defense was that the patent on NovaSure was invalid. Hologic felt that since Truckai had obtained that patent and then sold it, it was improper for him now to claim it was invalid. There is a doctrine for that: assignor estoppel—the person who sold (assigned) the patent is prevented from later claiming it was invalid. The question in this case was whether assignor estoppel is part of the patent law of the United States. It is not in the patent statutes, so it is a court-determined part of the law.
In a 5-4 decision this Term, the Court held that assignor estoppel is recognized, but that it is narrow.23 The Court identified several exceptions to assignor estoppel, notably for this case, including the situation in which the purchaser of the patent, after the purchase, returns to the Patent and Trademark Office to expand (amend) the patent’s claims. In that case, the seller could not be estopped by the amended terms of the patent. Minerva claimed that it was attacking the expanded patent that included changes made after it sold the patent. The Court, therefore, returned the case to the Federal Circuit to apply the principles it laid out about assignor estoppel.
Biotech and other fast-moving fields frequently have new technology building on slightly earlier technology. The current patent system often leaves uncertainty about who owns which part of a valid patent. This uncertainty is a drag on innovation, and the patent system is supposed to spur innovation. Assignor estoppel is likely to create additional complexity and uncertainty in some patents, which is regrettable.
Review of the Term
In addition to the other disruptions of the Term, during the first part of the Term, Amy Coney Barrett was not yet confirmed by the Senate, so there were only 8 justices until October 27. She did not participate in those cases that were heard before she joined the Court. The consensus is that the Court heard 67 cases: 57 were formally briefed and argued along with 8 summary reversals and 2 religious cases in the Shadow Docket. In my opinion, this undercounts both the number and the importance of the Shadow Docket cases, but the following data use the 67 case convention.24
The Court was unanimous in 43% of the cases, including some of the most divisive issues. That unanimity reflects very narrow decisions. There were (by conventional count) only eight 5-4 opinions (12%), an unusually low number. Justice Kavanaugh is viewed as the “median” justice. He was in the majority in 97% of all cases. Chief Justice Roberts and Justice Barrett were in the majority 91%, and Justice Gorsuch 90%. As for the other justices, they were in the majority (all cases) most of the time: Justice Alito, 83%; Justice Thomas, 81%; Justice Breyer, 76%; Justice Kagan, 75%; and Justice Sotomayor, 69%. In “divided cases” (when unanimous cases are removed), the percentages are: Justice Kavanaugh, 95%; Chief Justice Roberts and Justice Barrett, 84%; Justice Gorsuch, 82%; Justice Alito, 70%; Justice Thomas, 66%; Justice Breyer, 58%; Justice Kagan, 55%; and Justice Sotomayor, 45%.
When the term began, many Court watchers expected a relatively uninteresting term, dealing with many technical legal details. In fact, it turned out to be more interesting and important than expected, even with narrow holdings in important cases. Part of the secret of the term was that a lot of the real action was in the Shadow Docket. The end of the term is sometimes the moment when a justice announces a plan to retire. Many commentators expected Justice Breyer might announce—he has been under pressure to do so, to allow President Biden to nominate and a Democratic Senate to confirm a progressive justice. However, he did not do so. It is possible that he will announce his retirement to be effective when his successor is confirmed, but that is pure speculation.
Continue to: Next Term...
Next Term
The next term began on Monday, October 4, 2021. With the considerable current activity in the Shadow Docket, there was not much of a summer break. The coming term looks extraordinary. The headline case is an abortion case from Mississippi, Dobbs v Jackson Women’s Health Organization.25 The legal question is the constitutionality of Mississippi law that prohibits most abortions after 15 weeks of gestation. The Texas abortion law will also be back before the Court. As we saw this term, big cases may produce very narrow results, but this case has the potential for being a notable abortion decision.
In a different case the Court will decide whether a state attorney general can step in to defend an abortion law when the state health secretary does not do so.26
The Court also has accepted 3 cases dealing with reimbursement for health services. One deals with whether or not the Department of Health and Human Services can set reimbursement rates without good survey data regarding costs,27 another involves the calculation of additional payments for hospitals that serve a “disproportionate number of low-income patients,”28 and the third whether state Medicaid programs can take funds from an injured beneficiary’s tort recovery to cover future Medicaid costs.29
In other cases, the Court will review a gun control law from New York. The Court’s earlier Second Amendment cases involved guns in the home used for self-defense, but this case raises the question of whether a state can practically preclude “concealed-carry licenses.”30 Many experts believe the Court will accept a case dealing with racial preferences in college admissions, perhaps the Harvard case in which the claim is discrimination against Asian Americans.31
The ACOG mifepristone case was interesting, in part because the federal district court issued a nationwide injunction against the Americans with Disabilities Act, enforcing its rules anywhere in the country. The effect of these orders is for a single district judge to create the “law of the land,” at least until that is reviewed—which can take months. The advantage of the nationwide injunction is that it avoids having to repeatedly litigate the same issues in multiple courts around the country. The downside is that plaintiffs can seek out a nonrepresentative judge or circuit and receive an injunction that would be granted by few other circuits. In addition, a nationwide injunction can apply to specific circumstances that are not before the court issuing the injunction. In the mifepristone case, for example, 10 states requested to intervene in the ACOG case. The court rejected the request, but the nationwide injunction applied to those states.1
Although federal judges have had the authority to issue nationwide injunctions for years, they are becoming much more common. One reason is the ease of forum shopping noted earlier—organizations can cherry-pick district courts and circuits sympathetic to their views. Both left- and right-leaning organizations have learned this lesson, so left-leaning groups are likely to file in specific districts in the Ninth Circuit, and right-leaning groups to districts in the Fifth Circuit.
If the current trend of increasing nationwide injunctions continues, either the rules for the federal courts or congressional action may be required to reduce some of the abuses by both sides of the political spectrum.
The ACOG mifepristone case was interesting, in part because the federal district court issued a nationwide injunction against the Americans with Disabilities Act, enforcing its rules anywhere in the country. The effect of these orders is for a single district judge to create the “law of the land,” at least until that is reviewed—which can take months. The advantage of the nationwide injunction is that it avoids having to repeatedly litigate the same issues in multiple courts around the country. The downside is that plaintiffs can seek out a nonrepresentative judge or circuit and receive an injunction that would be granted by few other circuits. In addition, a nationwide injunction can apply to specific circumstances that are not before the court issuing the injunction. In the mifepristone case, for example, 10 states requested to intervene in the ACOG case. The court rejected the request, but the nationwide injunction applied to those states.1
Although federal judges have had the authority to issue nationwide injunctions for years, they are becoming much more common. One reason is the ease of forum shopping noted earlier—organizations can cherry-pick district courts and circuits sympathetic to their views. Both left- and right-leaning organizations have learned this lesson, so left-leaning groups are likely to file in specific districts in the Ninth Circuit, and right-leaning groups to districts in the Fifth Circuit.
If the current trend of increasing nationwide injunctions continues, either the rules for the federal courts or congressional action may be required to reduce some of the abuses by both sides of the political spectrum. Reference Am. Coll. of Obstetricians & Gynecologists v. United States FDA, 467 F. Supp. 3d 282, 284 (D. Md. 2020).
Reference
1. Am. Coll. of Obstetricians & Gynecologists v. United States FDA, 467 F. Supp. 3d 282, 284 (D. Md. 2020).
The “Shadow Docket”
The ACOG mifepristone decisions do not appear on the Supreme Court’s “Court Opinions” website.1 They appear in what has become known in recent years as “The Shadow Docket,” an informal term that includes many orders of the Court and statements of individual justices regarding some cases.2 There are hundreds of orders by the Court each Term, there is nothing particularly shadowy about any of these items—they are all publicly available on the Court’s website and later in paper format. It is, however, a little harder to find and much harder to sort through than the major opinions. In some cases, it is not possible to tell what the vote was, how each justice voted, and what the reasoning of the Court was. In a few cases it is difficult to know exactly what the Court was holding or otherwise leaves some confusion about what the law actually is.3
The part of the Shadow Docket that is most intriguing for commentators, and where the ACOG cases appear, is the “Opinions Relating to Orders.”4 These are a variety of opinions, some written by the Court and many by individual justices. It also includes the action of the Court in some cases in which there was not full briefing or oral argument. The statements by justices often are to dissent from the denial of cert of decisions of the Court. These opinions have become much more common over the years. In this past term, there were approximately 60 such opinions related to about 50 cases. In part, this relates to the number of pandemic cases that could not wait for a Court decision going through the extended ordinary process. Although the Shadow Docket has been of interest to academic observers and Court watchers for years, this year it has attracted the attention of Congress.5
References
1. Opinions of the Court. Supreme Court website. https://www.supremecourt.gov/opinions/slipopinion/20#list. Accessed October 10, 2021.
2. Baude W. Foreword: the Supreme Court’s Shadow Docket, 9 N.Y.U. J.L. & Liberty 1 (2015).
3. Vladeck SI. The Solicitor General and the Shadow Docket, 133 Harvard Law Review. 123 (2019).
4. Opinions relating to orders. Supreme Court website. https://www.supremecourt.gov/opinions/relatingtoorders/20#list. Accessed October 10, 2021.
5. The Supreme Court’s Shadow Docket: Hearing Before the Subcommittee on Courts, Intellectual Property and the Internet of the H. Committee on the Judiciary, 117th Congress (2021).
- American College of Obstetricians & Gynecologists v. United States FDA, 472 F. Supp. 3d 183 (D. Md. 2020).
- Michael Kunzelman, Doctors Sue to Block FDA Abortion Pill Rule During Pandemic, (May 29, 2020).
- ACLU, American College Of Obstetricians And Gynecologists V. U.S. Food And Drug Administration, https://www.aclu.org/cases/american-college-obstetricians-and-gynecologists-v-us-food-and-drug-administration. Updated February 12, 2021. Accessed August 27, 2021.
- Whole Woman’s Health v Hellerstedt, 579 US ___ (2016), 136 S Ct 2292.
- 2016 Clinical Review at 39, 47, 49, Opp’n Mot. PI Ex. 19, ECF No. 62-11.
- American College of Obstetricians and Gynecologists v FDA (I), decided October 8, 2020.
- October 8, 2020, dissenting opinion by Justice Alito.
- January 12, 2021, dissenting opinion by Justice Sotomayor.
- Questions and answers on Mifeprex. U.S. Food and Drug Administration website. Published April 13, 2021. https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/questions-and-answers-mifeprex. Accessed October 9, 2021.
- Whole Woman’s Health v Jackson, decided September 1, 2021.
- Texas Senate Bill 8, relating to abortion, including abortions after detection of unborn child’s heartbeat; authorizing a private civil right of action. LegiScan website. https://legiscan.com/TX/text/SB8/id/2395961. Accessed October 9, 2021.
- Planned Parenthood of Southeastern Pennsylvania v Casey, 505 U. S. 833 (1992); Roe v Wade, 410 U. S. 113 (1973).
- Dobbs v Jackson Women’s Health Organization, No. 19-1392.
- Roman Catholic Diocese of Brooklyn v Cuomo, decided November 25, 2020.
- Alabama Association of Realtors v Department of Health and Human Services, decided June 29, 2021.
- Temporary halt in residential evictions in communities with substantial or high levels of community transmission of COVID-19 to prevent the further spread of COVID-19. August 6, 2021. https://www.federalregister.gov/documents/2021/08/06/2021-16945/temporary-halt-in-residential-evictions-in-communities-with-substantial-or-high-transmission-of.
- Alabama Association of Realtors v Department of Health and Human Services, decided August 26, 2021.
- California v Texas, decided June 17, 2021.
- Brief of Amici Curiae American Medical Association, American Academy of Allergy, Asthma and Immunology, Aerospace Medical Association, American Academy of Family Physicians, American Academy of Pediatrics, American College of Cardiology, American College of Emergency Physicians, American College of Medical Genetics and Genomics, American College of Obstetricians and Gynecologists, American College of Physicians, American College of Radiation Oncology, American College of Radiology, American Psychiatric Association, American Society of Gastrointestinal Endoscopy, American Society of Hematology, American Society of Metabolic and Bariatric Surgery, Endocrine Society, GLMA: Health Professionals Advancing LGBTQ Equality, Renal Physicians Association, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology in Support of Petitioners, in California v. Texas. May 13, 2020. https://www.supremecourt.gov/DocketPDF/19/19-840/143469/20200513150051995_19-840%20Amici%20Brief%20AMA.pdf. Accessed October 9, 2021.
- Rutledge v Pharmaceutical Care Management Association, decided December 10, 2020.
- Brief of the American Medical Association, The Arkansas Medical Society, and The Litigation Center of the American Medical Association and the State Medical Societies as Amici Curiae in Support of Petitioner in Rutledge v Pharmaceutical Care Management Association. March 2, 2020. https://www.supremecourt.gov/DocketPDF/18/18-540/134670/20200302163622018_Rutledge%20v.%20PCMA%20Amicus%20Brief%20of%20AMA%20et%20al.pdf. Accessed October 9, 2021.
- Apple quietly settles patent lawsuit, promptly gets hit with another one. TechCrunch website. Published July 30, 2010. https://techcrunch.com/2010/07/30/apple-minerva-emblaze/. Accessed October 9, 2021.
- Minerva Surgical, Inc. v Hologic, Inc., decided June 29, 2021.
- Stat pack. SCOTUS Blog website. Published July 6, 2021. https://www.scotusblog.com/wp-content/uploads/2021/07/Final-Stat-Pack-7.6.21.pdf. Accessed October 9, 2021.
- Dobbs v Jackson Women’s Health Organization, No. 19-1392.
- Cameron v. EMW Women’s Surgical Center, https://www.scotusblog.com/case-files/cases/cameron-v-emw-womens-surgical-center-p-s-c/. Accessed August 28, 2021.
- American Hospital Association v Becerra, No. 20-1114.
- Becerra v Empire Health Foundation, No. 20-1312.
- Gallardo v Marstiller, No. 20-1263.
- New York State Rifle & Pistol Association Inc. v Corlett, No. 20-843.
- Students for Fair Admissions v President & Fellows of Harvard College, No. 20-1199.
- American College of Obstetricians & Gynecologists v. United States FDA, 472 F. Supp. 3d 183 (D. Md. 2020).
- Michael Kunzelman, Doctors Sue to Block FDA Abortion Pill Rule During Pandemic, (May 29, 2020).
- ACLU, American College Of Obstetricians And Gynecologists V. U.S. Food And Drug Administration, https://www.aclu.org/cases/american-college-obstetricians-and-gynecologists-v-us-food-and-drug-administration. Updated February 12, 2021. Accessed August 27, 2021.
- Whole Woman’s Health v Hellerstedt, 579 US ___ (2016), 136 S Ct 2292.
- 2016 Clinical Review at 39, 47, 49, Opp’n Mot. PI Ex. 19, ECF No. 62-11.
- American College of Obstetricians and Gynecologists v FDA (I), decided October 8, 2020.
- October 8, 2020, dissenting opinion by Justice Alito.
- January 12, 2021, dissenting opinion by Justice Sotomayor.
- Questions and answers on Mifeprex. U.S. Food and Drug Administration website. Published April 13, 2021. https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/questions-and-answers-mifeprex. Accessed October 9, 2021.
- Whole Woman’s Health v Jackson, decided September 1, 2021.
- Texas Senate Bill 8, relating to abortion, including abortions after detection of unborn child’s heartbeat; authorizing a private civil right of action. LegiScan website. https://legiscan.com/TX/text/SB8/id/2395961. Accessed October 9, 2021.
- Planned Parenthood of Southeastern Pennsylvania v Casey, 505 U. S. 833 (1992); Roe v Wade, 410 U. S. 113 (1973).
- Dobbs v Jackson Women’s Health Organization, No. 19-1392.
- Roman Catholic Diocese of Brooklyn v Cuomo, decided November 25, 2020.
- Alabama Association of Realtors v Department of Health and Human Services, decided June 29, 2021.
- Temporary halt in residential evictions in communities with substantial or high levels of community transmission of COVID-19 to prevent the further spread of COVID-19. August 6, 2021. https://www.federalregister.gov/documents/2021/08/06/2021-16945/temporary-halt-in-residential-evictions-in-communities-with-substantial-or-high-transmission-of.
- Alabama Association of Realtors v Department of Health and Human Services, decided August 26, 2021.
- California v Texas, decided June 17, 2021.
- Brief of Amici Curiae American Medical Association, American Academy of Allergy, Asthma and Immunology, Aerospace Medical Association, American Academy of Family Physicians, American Academy of Pediatrics, American College of Cardiology, American College of Emergency Physicians, American College of Medical Genetics and Genomics, American College of Obstetricians and Gynecologists, American College of Physicians, American College of Radiation Oncology, American College of Radiology, American Psychiatric Association, American Society of Gastrointestinal Endoscopy, American Society of Hematology, American Society of Metabolic and Bariatric Surgery, Endocrine Society, GLMA: Health Professionals Advancing LGBTQ Equality, Renal Physicians Association, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology in Support of Petitioners, in California v. Texas. May 13, 2020. https://www.supremecourt.gov/DocketPDF/19/19-840/143469/20200513150051995_19-840%20Amici%20Brief%20AMA.pdf. Accessed October 9, 2021.
- Rutledge v Pharmaceutical Care Management Association, decided December 10, 2020.
- Brief of the American Medical Association, The Arkansas Medical Society, and The Litigation Center of the American Medical Association and the State Medical Societies as Amici Curiae in Support of Petitioner in Rutledge v Pharmaceutical Care Management Association. March 2, 2020. https://www.supremecourt.gov/DocketPDF/18/18-540/134670/20200302163622018_Rutledge%20v.%20PCMA%20Amicus%20Brief%20of%20AMA%20et%20al.pdf. Accessed October 9, 2021.
- Apple quietly settles patent lawsuit, promptly gets hit with another one. TechCrunch website. Published July 30, 2010. https://techcrunch.com/2010/07/30/apple-minerva-emblaze/. Accessed October 9, 2021.
- Minerva Surgical, Inc. v Hologic, Inc., decided June 29, 2021.
- Stat pack. SCOTUS Blog website. Published July 6, 2021. https://www.scotusblog.com/wp-content/uploads/2021/07/Final-Stat-Pack-7.6.21.pdf. Accessed October 9, 2021.
- Dobbs v Jackson Women’s Health Organization, No. 19-1392.
- Cameron v. EMW Women’s Surgical Center, https://www.scotusblog.com/case-files/cases/cameron-v-emw-womens-surgical-center-p-s-c/. Accessed August 28, 2021.
- American Hospital Association v Becerra, No. 20-1114.
- Becerra v Empire Health Foundation, No. 20-1312.
- Gallardo v Marstiller, No. 20-1263.
- New York State Rifle & Pistol Association Inc. v Corlett, No. 20-843.
- Students for Fair Admissions v President & Fellows of Harvard College, No. 20-1199.
COVID vaccines’ protection dropped sharply over 6 months: Study
, a study of almost 800,000 veterans found.
The study, published in the journal Science ., says the three vaccines offered about the same protection against the virus in March, when the Delta variant was first detected in the United States, but that changed 6 months later.
The Moderna two-dose vaccine went from being 89% effective in March to 58% effective in September, according to a story about the study in theLos Angeles Times.
Meanwhile, the Pfizer/BioNTech vaccine went from being 87% effective to 45% effective over the same time period.
The Johnson & Johnson vaccine showed the biggest drop -- from 86% effectiveness to 13% over those 6 months.
“In summary, although vaccination remains protective against SARS-CoV-2 infection, protection waned as the Delta variant emerged in the U.S., and this decline did not differ by age,” the study said.
The three vaccines also lost effectiveness in the ability to protect against death in veterans 65 and over after only 3 months, the Los Angeles Times reported.
Compared to unvaccinated veterans in that age group, veterans who got the Moderna vaccine and had a breakthrough case were 76% less likely to die of COVID-19 by July.
The protection was 70% for Pfizer/BioNTech vaccine recipients and 52% for J&J vaccine recipients for the same age group, compared to unvaccinated veterans, according to the newspaper.
For veterans under 65, the protectiveness against a fatal case of COVID was 84% for Pfizer/BioNTech recipients, 82% for Moderna recipients, and 73% for J&J recipients, compared to unvaccinated veterans in that age group.
The study confirms the need for booster vaccines and protective measures such as vaccine passports, vaccine mandates, masking, hand-washing, and social distancing, the researchers said.
Of the veterans studied, about 500,000 were vaccinated and 300,000 were not. Researchers noted that the study population had 6 times as many men as women. About 48% of the study group was 65 or older, 29% was 50-64, while 24% was under 50.
Researchers from the Public Health Institute in Oakland, the Veterans Affairs Medical Center in San Francisco, and the University of Texas Health Science Center conducted the study.
A version of this article first appeared on WebMD.com.
, a study of almost 800,000 veterans found.
The study, published in the journal Science ., says the three vaccines offered about the same protection against the virus in March, when the Delta variant was first detected in the United States, but that changed 6 months later.
The Moderna two-dose vaccine went from being 89% effective in March to 58% effective in September, according to a story about the study in theLos Angeles Times.
Meanwhile, the Pfizer/BioNTech vaccine went from being 87% effective to 45% effective over the same time period.
The Johnson & Johnson vaccine showed the biggest drop -- from 86% effectiveness to 13% over those 6 months.
“In summary, although vaccination remains protective against SARS-CoV-2 infection, protection waned as the Delta variant emerged in the U.S., and this decline did not differ by age,” the study said.
The three vaccines also lost effectiveness in the ability to protect against death in veterans 65 and over after only 3 months, the Los Angeles Times reported.
Compared to unvaccinated veterans in that age group, veterans who got the Moderna vaccine and had a breakthrough case were 76% less likely to die of COVID-19 by July.
The protection was 70% for Pfizer/BioNTech vaccine recipients and 52% for J&J vaccine recipients for the same age group, compared to unvaccinated veterans, according to the newspaper.
For veterans under 65, the protectiveness against a fatal case of COVID was 84% for Pfizer/BioNTech recipients, 82% for Moderna recipients, and 73% for J&J recipients, compared to unvaccinated veterans in that age group.
The study confirms the need for booster vaccines and protective measures such as vaccine passports, vaccine mandates, masking, hand-washing, and social distancing, the researchers said.
Of the veterans studied, about 500,000 were vaccinated and 300,000 were not. Researchers noted that the study population had 6 times as many men as women. About 48% of the study group was 65 or older, 29% was 50-64, while 24% was under 50.
Researchers from the Public Health Institute in Oakland, the Veterans Affairs Medical Center in San Francisco, and the University of Texas Health Science Center conducted the study.
A version of this article first appeared on WebMD.com.
, a study of almost 800,000 veterans found.
The study, published in the journal Science ., says the three vaccines offered about the same protection against the virus in March, when the Delta variant was first detected in the United States, but that changed 6 months later.
The Moderna two-dose vaccine went from being 89% effective in March to 58% effective in September, according to a story about the study in theLos Angeles Times.
Meanwhile, the Pfizer/BioNTech vaccine went from being 87% effective to 45% effective over the same time period.
The Johnson & Johnson vaccine showed the biggest drop -- from 86% effectiveness to 13% over those 6 months.
“In summary, although vaccination remains protective against SARS-CoV-2 infection, protection waned as the Delta variant emerged in the U.S., and this decline did not differ by age,” the study said.
The three vaccines also lost effectiveness in the ability to protect against death in veterans 65 and over after only 3 months, the Los Angeles Times reported.
Compared to unvaccinated veterans in that age group, veterans who got the Moderna vaccine and had a breakthrough case were 76% less likely to die of COVID-19 by July.
The protection was 70% for Pfizer/BioNTech vaccine recipients and 52% for J&J vaccine recipients for the same age group, compared to unvaccinated veterans, according to the newspaper.
For veterans under 65, the protectiveness against a fatal case of COVID was 84% for Pfizer/BioNTech recipients, 82% for Moderna recipients, and 73% for J&J recipients, compared to unvaccinated veterans in that age group.
The study confirms the need for booster vaccines and protective measures such as vaccine passports, vaccine mandates, masking, hand-washing, and social distancing, the researchers said.
Of the veterans studied, about 500,000 were vaccinated and 300,000 were not. Researchers noted that the study population had 6 times as many men as women. About 48% of the study group was 65 or older, 29% was 50-64, while 24% was under 50.
Researchers from the Public Health Institute in Oakland, the Veterans Affairs Medical Center in San Francisco, and the University of Texas Health Science Center conducted the study.
A version of this article first appeared on WebMD.com.
FROM SCIENCE
Vitamin D and omega-3 supplements reduce autoimmune disease risk
For those of us who cannot sit in the sun and fish all day, the next best thing for preventing autoimmune diseases may be supplementation with vitamin D and fish oil-derived omega-3 fatty acids, results of a large prospective randomized trial suggest.
Among nearly 26,000 adults enrolled in a randomized trial designed primarily to study the effects of vitamin D and omega-3 supplementation on incident cancer and cardiovascular disease, 5, and 5 years of omega-3 fatty acid supplementation was associated with an 18% reduction in confirmed and probable incident autoimmune diseases, reported Karen H. Costenbader, MD, MPH, of Brigham & Women’s Hospital in Boston.
“The clinical importance of these results is very high, given that these are nontoxic, well-tolerated supplements, and that there are no other known effective therapies to reduce the incidence of autoimmune diseases,” she said during the virtual annual meeting of the American College of Rheumatology.
“People do have to take the supplements a long time to start to see the reduction in risk, especially for vitamin D, but they make biological sense, and autoimmune diseases develop slowly over time, so taking it today isn’t going to reduce risk of developing something tomorrow,” Dr. Costenbader said in an interview.
“These supplements have other health benefits. Obviously, fish oil is anti-inflammatory, and vitamin D is good for osteoporosis prevention, especially in our patients who take glucocorticoids. People who are otherwise healthy and have a family history of autoimmune disease might also consider starting to take these supplements,” she said.
After watching her presentation, session co-moderator Gregg Silverman, MD, from the NYU Langone School of Medicine in New York, who was not involved in the study, commented “I’m going to [nutrition store] GNC to get some vitamins.”
When asked for comment, the other session moderator, Tracy Frech, MD, of Vanderbilt University, Nashville, said, “I think Dr. Costenbader’s work is very important and her presentation excellent. My current practice is replacement of vitamin D in all autoimmune disease patients with low levels and per bone health guidelines. Additionally, I discuss omega-3 supplementation with Sjögren’s [syndrome] patients as a consideration.”
Evidence base
Dr. Costenbader noted that in a 2013 observational study from France, vitamin D derived through ultraviolet (UV) light exposure was associated with a lower risk for incident Crohn’s disease but not ulcerative colitis, and in two analyses of data in 2014 from the Nurses’ Health Study, both high plasma levels of 25-OH vitamin D and geographic residence in areas of high UV exposure were associated with a decreased incidence of rheumatoid arthritis (RA).
Other observational studies have supported omega-3 fatty acids for their anti-inflammatory properties, including a 2005 Danish prospective cohort study showing a lower risk for RA in participants who reported higher levels of fatty fish intake. In a separate study conducted in 2017, healthy volunteers with higher omega-3 fatty acid/total lipid proportions in red blood cell membranes had a lower prevalence of anti-cyclic citrullinated peptide (anti-CCP) antibodies and rheumatoid factor and a lower incidence of progression to inflammatory arthritis, she said.
Ancillary study
Despite the evidence, however, there have been no prospective randomized trials to test the effects of either vitamin D or omega-3 fatty acid supplementation on the incidence of autoimmune disease over time.
To rectify this, Dr. Costenbader and colleagues piggybacked an ancillary study onto the Vitamin D and Omega-3 Trial (VITAL), which had primary outcomes of cancer and cardiovascular disease incidence.
A total of 25,871 participants were enrolled, including 12,786 men aged 50 and older, and 13,085 women aged 55 and older.
The study had a 2 x 2 factorial design, with patients randomly assigned to vitamin D 2,000 IU/day or placebo, and then further randomized to either 1 g/day omega-3 fatty acids or placebo in both the vitamin D and placebo primary randomization arms.
At baseline 16,956 participants were assayed for 25-OH vitamin D and plasma omega 3 index, the ratio of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) to total fatty acids. Participants self-reported baseline and all incident autoimmune diseases annually, with the reports confirmed by medical record review and disease criteria whenever possible.
Results
At 5 years of follow-up, confirmed incident autoimmune diseases had occurred in 123 patients in the active vitamin D group, compared with 155 in the placebo vitamin D group, translating into a hazard ratio (HR) for vitamin D of 0.78 (P = .045).
In the active omega-3 arm, 130 participants developed an autoimmune disease, compared with 148 in the placebo omega-3 arm, which translated into a nonsignificant HR of 0.85.
There was no statistical interaction between the two supplements. The investigators did observe an interaction between vitamin D and body mass index, with the effect stronger among participants with low BMI (P = .02). There also was an interaction between omega-3 fatty acids with a family history of autoimmune disease (P = .03).
In multivariate analysis adjusted for age, sex, race, and other supplement arm, vitamin D alone was associated with an HR for incident autoimmune disease of 0.68 (P = .02), omega-3 alone was associated with a nonsignificant HR of 0.74, and the combination was associated with an HR of 0.69 (P = .03).
Dr. Costenbader and colleagues acknowledged that the study was limited by the lack of a high-risk or nutritionally-deficient population, where the effects of supplementation might be larger; the restriction of the sample to older adults; and to the difficulty of confirming incident autoimmune thyroid disease from patient reports.
Cheryl Koehn, an arthritis patient advocate from Vancouver, Canada, who was not involved in the study, commented in the “chat” section of the presentation that her rheumatologist “has recommended vitamin D for years now. Says basically everyone north of Boston is vitamin D deficient. I take 1,000 IU per day. Been taking it for years.” Ms. Koehn is the founder and president of Arthritis Consumer Experts, a website that provides education to those with arthritis.
“Agreed. I tell every patient to take vitamin D supplement,” commented Fatma Dedeoglu, MD, a rheumatologist at Boston Children’s Hospital.
A version of this article first appeared on Medscape.com.
For those of us who cannot sit in the sun and fish all day, the next best thing for preventing autoimmune diseases may be supplementation with vitamin D and fish oil-derived omega-3 fatty acids, results of a large prospective randomized trial suggest.
Among nearly 26,000 adults enrolled in a randomized trial designed primarily to study the effects of vitamin D and omega-3 supplementation on incident cancer and cardiovascular disease, 5, and 5 years of omega-3 fatty acid supplementation was associated with an 18% reduction in confirmed and probable incident autoimmune diseases, reported Karen H. Costenbader, MD, MPH, of Brigham & Women’s Hospital in Boston.
“The clinical importance of these results is very high, given that these are nontoxic, well-tolerated supplements, and that there are no other known effective therapies to reduce the incidence of autoimmune diseases,” she said during the virtual annual meeting of the American College of Rheumatology.
“People do have to take the supplements a long time to start to see the reduction in risk, especially for vitamin D, but they make biological sense, and autoimmune diseases develop slowly over time, so taking it today isn’t going to reduce risk of developing something tomorrow,” Dr. Costenbader said in an interview.
“These supplements have other health benefits. Obviously, fish oil is anti-inflammatory, and vitamin D is good for osteoporosis prevention, especially in our patients who take glucocorticoids. People who are otherwise healthy and have a family history of autoimmune disease might also consider starting to take these supplements,” she said.
After watching her presentation, session co-moderator Gregg Silverman, MD, from the NYU Langone School of Medicine in New York, who was not involved in the study, commented “I’m going to [nutrition store] GNC to get some vitamins.”
When asked for comment, the other session moderator, Tracy Frech, MD, of Vanderbilt University, Nashville, said, “I think Dr. Costenbader’s work is very important and her presentation excellent. My current practice is replacement of vitamin D in all autoimmune disease patients with low levels and per bone health guidelines. Additionally, I discuss omega-3 supplementation with Sjögren’s [syndrome] patients as a consideration.”
Evidence base
Dr. Costenbader noted that in a 2013 observational study from France, vitamin D derived through ultraviolet (UV) light exposure was associated with a lower risk for incident Crohn’s disease but not ulcerative colitis, and in two analyses of data in 2014 from the Nurses’ Health Study, both high plasma levels of 25-OH vitamin D and geographic residence in areas of high UV exposure were associated with a decreased incidence of rheumatoid arthritis (RA).
Other observational studies have supported omega-3 fatty acids for their anti-inflammatory properties, including a 2005 Danish prospective cohort study showing a lower risk for RA in participants who reported higher levels of fatty fish intake. In a separate study conducted in 2017, healthy volunteers with higher omega-3 fatty acid/total lipid proportions in red blood cell membranes had a lower prevalence of anti-cyclic citrullinated peptide (anti-CCP) antibodies and rheumatoid factor and a lower incidence of progression to inflammatory arthritis, she said.
Ancillary study
Despite the evidence, however, there have been no prospective randomized trials to test the effects of either vitamin D or omega-3 fatty acid supplementation on the incidence of autoimmune disease over time.
To rectify this, Dr. Costenbader and colleagues piggybacked an ancillary study onto the Vitamin D and Omega-3 Trial (VITAL), which had primary outcomes of cancer and cardiovascular disease incidence.
A total of 25,871 participants were enrolled, including 12,786 men aged 50 and older, and 13,085 women aged 55 and older.
The study had a 2 x 2 factorial design, with patients randomly assigned to vitamin D 2,000 IU/day or placebo, and then further randomized to either 1 g/day omega-3 fatty acids or placebo in both the vitamin D and placebo primary randomization arms.
At baseline 16,956 participants were assayed for 25-OH vitamin D and plasma omega 3 index, the ratio of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) to total fatty acids. Participants self-reported baseline and all incident autoimmune diseases annually, with the reports confirmed by medical record review and disease criteria whenever possible.
Results
At 5 years of follow-up, confirmed incident autoimmune diseases had occurred in 123 patients in the active vitamin D group, compared with 155 in the placebo vitamin D group, translating into a hazard ratio (HR) for vitamin D of 0.78 (P = .045).
In the active omega-3 arm, 130 participants developed an autoimmune disease, compared with 148 in the placebo omega-3 arm, which translated into a nonsignificant HR of 0.85.
There was no statistical interaction between the two supplements. The investigators did observe an interaction between vitamin D and body mass index, with the effect stronger among participants with low BMI (P = .02). There also was an interaction between omega-3 fatty acids with a family history of autoimmune disease (P = .03).
In multivariate analysis adjusted for age, sex, race, and other supplement arm, vitamin D alone was associated with an HR for incident autoimmune disease of 0.68 (P = .02), omega-3 alone was associated with a nonsignificant HR of 0.74, and the combination was associated with an HR of 0.69 (P = .03).
Dr. Costenbader and colleagues acknowledged that the study was limited by the lack of a high-risk or nutritionally-deficient population, where the effects of supplementation might be larger; the restriction of the sample to older adults; and to the difficulty of confirming incident autoimmune thyroid disease from patient reports.
Cheryl Koehn, an arthritis patient advocate from Vancouver, Canada, who was not involved in the study, commented in the “chat” section of the presentation that her rheumatologist “has recommended vitamin D for years now. Says basically everyone north of Boston is vitamin D deficient. I take 1,000 IU per day. Been taking it for years.” Ms. Koehn is the founder and president of Arthritis Consumer Experts, a website that provides education to those with arthritis.
“Agreed. I tell every patient to take vitamin D supplement,” commented Fatma Dedeoglu, MD, a rheumatologist at Boston Children’s Hospital.
A version of this article first appeared on Medscape.com.
For those of us who cannot sit in the sun and fish all day, the next best thing for preventing autoimmune diseases may be supplementation with vitamin D and fish oil-derived omega-3 fatty acids, results of a large prospective randomized trial suggest.
Among nearly 26,000 adults enrolled in a randomized trial designed primarily to study the effects of vitamin D and omega-3 supplementation on incident cancer and cardiovascular disease, 5, and 5 years of omega-3 fatty acid supplementation was associated with an 18% reduction in confirmed and probable incident autoimmune diseases, reported Karen H. Costenbader, MD, MPH, of Brigham & Women’s Hospital in Boston.
“The clinical importance of these results is very high, given that these are nontoxic, well-tolerated supplements, and that there are no other known effective therapies to reduce the incidence of autoimmune diseases,” she said during the virtual annual meeting of the American College of Rheumatology.
“People do have to take the supplements a long time to start to see the reduction in risk, especially for vitamin D, but they make biological sense, and autoimmune diseases develop slowly over time, so taking it today isn’t going to reduce risk of developing something tomorrow,” Dr. Costenbader said in an interview.
“These supplements have other health benefits. Obviously, fish oil is anti-inflammatory, and vitamin D is good for osteoporosis prevention, especially in our patients who take glucocorticoids. People who are otherwise healthy and have a family history of autoimmune disease might also consider starting to take these supplements,” she said.
After watching her presentation, session co-moderator Gregg Silverman, MD, from the NYU Langone School of Medicine in New York, who was not involved in the study, commented “I’m going to [nutrition store] GNC to get some vitamins.”
When asked for comment, the other session moderator, Tracy Frech, MD, of Vanderbilt University, Nashville, said, “I think Dr. Costenbader’s work is very important and her presentation excellent. My current practice is replacement of vitamin D in all autoimmune disease patients with low levels and per bone health guidelines. Additionally, I discuss omega-3 supplementation with Sjögren’s [syndrome] patients as a consideration.”
Evidence base
Dr. Costenbader noted that in a 2013 observational study from France, vitamin D derived through ultraviolet (UV) light exposure was associated with a lower risk for incident Crohn’s disease but not ulcerative colitis, and in two analyses of data in 2014 from the Nurses’ Health Study, both high plasma levels of 25-OH vitamin D and geographic residence in areas of high UV exposure were associated with a decreased incidence of rheumatoid arthritis (RA).
Other observational studies have supported omega-3 fatty acids for their anti-inflammatory properties, including a 2005 Danish prospective cohort study showing a lower risk for RA in participants who reported higher levels of fatty fish intake. In a separate study conducted in 2017, healthy volunteers with higher omega-3 fatty acid/total lipid proportions in red blood cell membranes had a lower prevalence of anti-cyclic citrullinated peptide (anti-CCP) antibodies and rheumatoid factor and a lower incidence of progression to inflammatory arthritis, she said.
Ancillary study
Despite the evidence, however, there have been no prospective randomized trials to test the effects of either vitamin D or omega-3 fatty acid supplementation on the incidence of autoimmune disease over time.
To rectify this, Dr. Costenbader and colleagues piggybacked an ancillary study onto the Vitamin D and Omega-3 Trial (VITAL), which had primary outcomes of cancer and cardiovascular disease incidence.
A total of 25,871 participants were enrolled, including 12,786 men aged 50 and older, and 13,085 women aged 55 and older.
The study had a 2 x 2 factorial design, with patients randomly assigned to vitamin D 2,000 IU/day or placebo, and then further randomized to either 1 g/day omega-3 fatty acids or placebo in both the vitamin D and placebo primary randomization arms.
At baseline 16,956 participants were assayed for 25-OH vitamin D and plasma omega 3 index, the ratio of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) to total fatty acids. Participants self-reported baseline and all incident autoimmune diseases annually, with the reports confirmed by medical record review and disease criteria whenever possible.
Results
At 5 years of follow-up, confirmed incident autoimmune diseases had occurred in 123 patients in the active vitamin D group, compared with 155 in the placebo vitamin D group, translating into a hazard ratio (HR) for vitamin D of 0.78 (P = .045).
In the active omega-3 arm, 130 participants developed an autoimmune disease, compared with 148 in the placebo omega-3 arm, which translated into a nonsignificant HR of 0.85.
There was no statistical interaction between the two supplements. The investigators did observe an interaction between vitamin D and body mass index, with the effect stronger among participants with low BMI (P = .02). There also was an interaction between omega-3 fatty acids with a family history of autoimmune disease (P = .03).
In multivariate analysis adjusted for age, sex, race, and other supplement arm, vitamin D alone was associated with an HR for incident autoimmune disease of 0.68 (P = .02), omega-3 alone was associated with a nonsignificant HR of 0.74, and the combination was associated with an HR of 0.69 (P = .03).
Dr. Costenbader and colleagues acknowledged that the study was limited by the lack of a high-risk or nutritionally-deficient population, where the effects of supplementation might be larger; the restriction of the sample to older adults; and to the difficulty of confirming incident autoimmune thyroid disease from patient reports.
Cheryl Koehn, an arthritis patient advocate from Vancouver, Canada, who was not involved in the study, commented in the “chat” section of the presentation that her rheumatologist “has recommended vitamin D for years now. Says basically everyone north of Boston is vitamin D deficient. I take 1,000 IU per day. Been taking it for years.” Ms. Koehn is the founder and president of Arthritis Consumer Experts, a website that provides education to those with arthritis.
“Agreed. I tell every patient to take vitamin D supplement,” commented Fatma Dedeoglu, MD, a rheumatologist at Boston Children’s Hospital.
A version of this article first appeared on Medscape.com.
FROM ACR 2021
Detransitioners received poor evaluation when transitioning
Over half of people who believed they were transgender, transitioned to the opposite sex, but then regretted it and transitioned back – known as detransitioners – felt they did not receive adequate evaluation from a doctor or mental health professional before starting transition, new research indicates.
In what is thought to be the first study to ask whether detransitioners informed their original clinicians of their regret at transitioning, only 24 of the 100 surveyed said they had done so.
This strongly suggests that records on detransition may understate the real numbers, said Lisa Littman, MD, MPH, president of The Institute for Comprehensive Gender Dysphoria Research (ICGDR), who is the sole author of the study, published in Archives of Sexual Behavior.
She stressed that the findings illustrate the complexity surrounding gender dysphoria. “We need to recognize that there are many different types of experiences around gender dysphoria, transition, and detransition,” she told this news organization.
She said there is some resistance among certain health care professionals, and in society in general, to the idea that transitioning is not always successful.
‘We need to understand why this is happening’
“Detransition exists and we need to understand why this is happening,” Dr. Littman emphasized.
She observed that some supporters of “rapid transition” do not want to accept that transitioning helps some individuals but harms others.
“In the end, our goals should be providing the right treatment for the right patient, and without a thorough evaluation, clinicians are at serious risk of giving patients the wrong treatment,” she urged.
She noted that, despite some individuals feeling better after transition, these people still felt inclined to detransition because of discrimination and pressure.
“Individuals should not be pressured to detransition, nor should they be pressured to transition. Both types of pressure were reported by respondents.”
The recently recognized shift from mostly natal males to natal females seeking to transition was borne out by her study data, with the proportion of natal girls who detransitioned at 69%.
‘Shedding light’ on often ignored population
Asked to comment on the study, Laura Edwards-Leeper, PhD, a clinical psychologist from Beaverton, Ore., who specializes in gender-diverse and transgender children, welcomed Dr. Littman’s study.
It is, said Dr. Edwards-Leeper, a “critical preliminary step toward shedding light on this often-ignored and dismissed population of individuals who deserve support, compassion, and sometimes medical intervention from health care providers.”
She added that multiple online reports attest to detransitioners feeling they had not received adequate evaluation prior to medically transitioning, as well as many who expressed feeling too ashamed or angry to return to their same clinicians to detransition.
“Littman’s study provides quantitative support for both of these reported experiences, further emphasizing the importance of the field taking a closer look at the processes currently in place for those experiencing gender dysphoria,” said Dr. Edwards-Leeper.
And Miroslav L. Djordjevic, MD, PhD, professor of surgery/urology, University of Belgrade (Serbia), who is a specialist in urogenital reconstructive surgery and has performed over 2,000 gender-reassignment surgeries in transgender individuals, has recently seen many cases of regret after such surgeries, with requests for reversal operations.
“Despite the fact that medical detransition is relatively safe and without severe consequences, surgical detransition presents one of the most difficult issues in transgender medicine,” Dr. Djordjevic told this news organization.
Commending Dr. Littman on her study, he drew attention to some of the bioethical questions that arise relating to those who detransition.
“I ask what happened in the period before medical transitioning? Was there proper psychological care during medical transitioning? Who confirmed their desire for detransition – the same professionals who did the transition?” or someone else, he continued. “And who accepted these individuals for gender-affirming surgery and what were the criteria for this decision?”
Substantial study of reasons for both transitioning and detransitioning
In her article, Dr. Littman describes a 100-strong population of individuals (66 Americans, 9 British, 9 Canadian, 4 Australians, and 12 from “other” nations), ranging in age from 18 years to over 60 years with a mean age of 29.2 years, who had experienced gender dysphoria, chosen to undergo medical and/or surgical transition, and then detransitioned by discontinuing medications, having reversal surgery, or both.
Participants completed a 115-question survey providing data including age at first experience of gender dysphoria, when participants first sought transitioning care and from whom, and whether they felt pressured to do so. Friendship group dynamics were also explored.
Various narratives of participants’ transitioning-detransitioning experiences were gathered and grouped, for example, those related to discrimination pressures, experiences of trauma or mental health conditions prior to transition, and reports of internalized homophobia.
Dr. Edwards-Leeper observed that the study offers a more extensive assessment of reasons for detransitioning than any other prior research in the field, which has been sparse.
A survey published in April found that detransitioners report significant unmet medical and psychological needs, and a lack of compassion and help from medical and mental health practitioners.
But another 2021 study concluded most detransitioners only reverted to their birth sex because of societal or family pressure, discrimination, or shift to a nonbinary identity.
“However, [Dr.] Littman’s study found that only a small percentage actually detransitioned for that reason [23%], whereas the majority detransitioned because of a change in how the individual understood being a male or female, resulting in becoming comfortable in their assigned gender [60%],” noted Dr. Edwards-Leeper.
Reasons for detransitioning
Asked to expand upon the motives for detransition identified in her study, Dr. Littman told this news organization: “We found remarkable breadth in the reasons given for detransitioning.”
“I believe that we were able to capture the diversity of experiences around detransition because we reached out to communities that were strongly ‘protransition’ – like the World Professional Association for Transgender Health – and communities where individuals might be more skeptical about transition being universally beneficial, like detransition forums,” she said.
Speaking to the complexity of the experiences, 87% selected more than one reason for detransitioning.
The most common reason (60%) was becoming more comfortable identifying with their birth sex, followed by having concerns about potential medical complications from transitioning (49.0%).
Regarding those who became more comfortable with their natal sex, Dr. Littman noted that the finding adds “further support that gender dysphoria is not always permanent.”
She added that, “because most gender-dysphoric youth who are allowed to go through puberty grow up to be lesbian, gay, or bisexual (LGB) nontransgender adults, intervening too soon with medical treatments risks derailing their development as LGB individuals.”
Internalized homophobia or difficulty accepting themselves as lesbian, gay, or bisexual was reported by 23% of participants as a reason for transition and subsequent detransition.
“For these people, transitioning could be interpreted as an attempt to escape the reality of being same-sex attracted and detransitioning was part of accepting themselves as homosexual or bisexual,” explained Dr. Littman.
“Exploring their distress and discomfort around sexual orientation issues may have been more helpful to them than medical and surgical transition or at least an important part of exploration,” she added in the article.
Societal pressure, friends, and social media also play a role
The latest first-hand reports also support prior work by Dr. Littman when she first identified the concept she termed rapid-onset gender dysphoria (ROGD) to describe a sudden transgender identification, usually in the early teenage years, and with no prior indication of any gender questioning.
ROGD, Dr. Littman believes, is strongly related to psychosocial factors, such as trauma, mental health problems, or social influence contributing to the development of gender dysphoria.
The current study found that 58% of respondents expressed the belief that the cause of their gender dysphoria was something specific, such as trauma, abuse, or a mental health condition, with respondents suggesting that transitioning prevented, or delayed, them from addressing their underlying mental health conditions.
One participant is quoted as saying: “I was deeply uncomfortable with my secondary sex characteristics, which I now understand was a result of childhood trauma and associating my secondary sex characteristics with those events.”
Reflecting on their previous identification as transgender, more than a third of respondents reported that someone else told them their feelings meant they were transgender, and they believed them.
“This speaks to the effect social influence can have on people’s interpretation of their own feelings and their development of a transgender identity,” Dr. Littman remarked.
“Participants also listed several social media sources that encouraged them to believe that transitioning would help them,” she added.
Several friendship group dynamics suggestive of social influence were reported by a subset of respondents, including the fact that their friendship groups mocked people who were not transgender and their popularity increased when they announced they were going to transition.
Pendulum has swung too far the other way
Natal females, who in recent years have made up most referrals, were younger than natal males when they sought transition and decided to detransition; and they stayed “transitioned” for a shorter period than natal males. They were also more likely to have experienced a trauma less than 1 year before the onset of gender dysphoria and were more likely to have felt pressured to transition.
“Because the females in the study transitioned more recently than the males, they may have experienced a culture where there is more of a ‘push’ to transition,” Dr. Littman pointed out.
She added that, “20 years ago, gender-dysphoric patients were most likely to be underdiagnosed and undertreated. Now, the pendulum has swung the other way and patients are, in my opinion, more likely to be overdiagnosed and overtreated. I think we need to aim for somewhere between these two extremes and prioritize people getting the right treatment for the right reason for their distress.”
Dr. Djordjevic added that, with colleagues from Belgrade and the Netherlands, he has published accounts of the experiences of seven individuals who showed regret after gender-affirming surgery.
All of them were born male, “and we confirmed the very poor evaluation and transition process they underwent. We conclude that clinicians should be aware that not everyone with gender identity disorders need or want all elements of hormonal or surgical therapy,” he told this news organization.
Dr. Edwards-Leeper said that more long-term longitudinal studies are needed that follow individuals who undergo transition under different models of care.
“My prediction is that those who first engage in supportive, gender exploratory therapy, followed by comprehensive assessment, will have the best outcomes, perhaps even if they ultimately detransition, as these individuals will know that they did not jump into irreversible interventions too quickly and had time to make the best decision for themselves at the time,” she concluded.
A version of this article first appeared on Medscape.com.
Over half of people who believed they were transgender, transitioned to the opposite sex, but then regretted it and transitioned back – known as detransitioners – felt they did not receive adequate evaluation from a doctor or mental health professional before starting transition, new research indicates.
In what is thought to be the first study to ask whether detransitioners informed their original clinicians of their regret at transitioning, only 24 of the 100 surveyed said they had done so.
This strongly suggests that records on detransition may understate the real numbers, said Lisa Littman, MD, MPH, president of The Institute for Comprehensive Gender Dysphoria Research (ICGDR), who is the sole author of the study, published in Archives of Sexual Behavior.
She stressed that the findings illustrate the complexity surrounding gender dysphoria. “We need to recognize that there are many different types of experiences around gender dysphoria, transition, and detransition,” she told this news organization.
She said there is some resistance among certain health care professionals, and in society in general, to the idea that transitioning is not always successful.
‘We need to understand why this is happening’
“Detransition exists and we need to understand why this is happening,” Dr. Littman emphasized.
She observed that some supporters of “rapid transition” do not want to accept that transitioning helps some individuals but harms others.
“In the end, our goals should be providing the right treatment for the right patient, and without a thorough evaluation, clinicians are at serious risk of giving patients the wrong treatment,” she urged.
She noted that, despite some individuals feeling better after transition, these people still felt inclined to detransition because of discrimination and pressure.
“Individuals should not be pressured to detransition, nor should they be pressured to transition. Both types of pressure were reported by respondents.”
The recently recognized shift from mostly natal males to natal females seeking to transition was borne out by her study data, with the proportion of natal girls who detransitioned at 69%.
‘Shedding light’ on often ignored population
Asked to comment on the study, Laura Edwards-Leeper, PhD, a clinical psychologist from Beaverton, Ore., who specializes in gender-diverse and transgender children, welcomed Dr. Littman’s study.
It is, said Dr. Edwards-Leeper, a “critical preliminary step toward shedding light on this often-ignored and dismissed population of individuals who deserve support, compassion, and sometimes medical intervention from health care providers.”
She added that multiple online reports attest to detransitioners feeling they had not received adequate evaluation prior to medically transitioning, as well as many who expressed feeling too ashamed or angry to return to their same clinicians to detransition.
“Littman’s study provides quantitative support for both of these reported experiences, further emphasizing the importance of the field taking a closer look at the processes currently in place for those experiencing gender dysphoria,” said Dr. Edwards-Leeper.
And Miroslav L. Djordjevic, MD, PhD, professor of surgery/urology, University of Belgrade (Serbia), who is a specialist in urogenital reconstructive surgery and has performed over 2,000 gender-reassignment surgeries in transgender individuals, has recently seen many cases of regret after such surgeries, with requests for reversal operations.
“Despite the fact that medical detransition is relatively safe and without severe consequences, surgical detransition presents one of the most difficult issues in transgender medicine,” Dr. Djordjevic told this news organization.
Commending Dr. Littman on her study, he drew attention to some of the bioethical questions that arise relating to those who detransition.
“I ask what happened in the period before medical transitioning? Was there proper psychological care during medical transitioning? Who confirmed their desire for detransition – the same professionals who did the transition?” or someone else, he continued. “And who accepted these individuals for gender-affirming surgery and what were the criteria for this decision?”
Substantial study of reasons for both transitioning and detransitioning
In her article, Dr. Littman describes a 100-strong population of individuals (66 Americans, 9 British, 9 Canadian, 4 Australians, and 12 from “other” nations), ranging in age from 18 years to over 60 years with a mean age of 29.2 years, who had experienced gender dysphoria, chosen to undergo medical and/or surgical transition, and then detransitioned by discontinuing medications, having reversal surgery, or both.
Participants completed a 115-question survey providing data including age at first experience of gender dysphoria, when participants first sought transitioning care and from whom, and whether they felt pressured to do so. Friendship group dynamics were also explored.
Various narratives of participants’ transitioning-detransitioning experiences were gathered and grouped, for example, those related to discrimination pressures, experiences of trauma or mental health conditions prior to transition, and reports of internalized homophobia.
Dr. Edwards-Leeper observed that the study offers a more extensive assessment of reasons for detransitioning than any other prior research in the field, which has been sparse.
A survey published in April found that detransitioners report significant unmet medical and psychological needs, and a lack of compassion and help from medical and mental health practitioners.
But another 2021 study concluded most detransitioners only reverted to their birth sex because of societal or family pressure, discrimination, or shift to a nonbinary identity.
“However, [Dr.] Littman’s study found that only a small percentage actually detransitioned for that reason [23%], whereas the majority detransitioned because of a change in how the individual understood being a male or female, resulting in becoming comfortable in their assigned gender [60%],” noted Dr. Edwards-Leeper.
Reasons for detransitioning
Asked to expand upon the motives for detransition identified in her study, Dr. Littman told this news organization: “We found remarkable breadth in the reasons given for detransitioning.”
“I believe that we were able to capture the diversity of experiences around detransition because we reached out to communities that were strongly ‘protransition’ – like the World Professional Association for Transgender Health – and communities where individuals might be more skeptical about transition being universally beneficial, like detransition forums,” she said.
Speaking to the complexity of the experiences, 87% selected more than one reason for detransitioning.
The most common reason (60%) was becoming more comfortable identifying with their birth sex, followed by having concerns about potential medical complications from transitioning (49.0%).
Regarding those who became more comfortable with their natal sex, Dr. Littman noted that the finding adds “further support that gender dysphoria is not always permanent.”
She added that, “because most gender-dysphoric youth who are allowed to go through puberty grow up to be lesbian, gay, or bisexual (LGB) nontransgender adults, intervening too soon with medical treatments risks derailing their development as LGB individuals.”
Internalized homophobia or difficulty accepting themselves as lesbian, gay, or bisexual was reported by 23% of participants as a reason for transition and subsequent detransition.
“For these people, transitioning could be interpreted as an attempt to escape the reality of being same-sex attracted and detransitioning was part of accepting themselves as homosexual or bisexual,” explained Dr. Littman.
“Exploring their distress and discomfort around sexual orientation issues may have been more helpful to them than medical and surgical transition or at least an important part of exploration,” she added in the article.
Societal pressure, friends, and social media also play a role
The latest first-hand reports also support prior work by Dr. Littman when she first identified the concept she termed rapid-onset gender dysphoria (ROGD) to describe a sudden transgender identification, usually in the early teenage years, and with no prior indication of any gender questioning.
ROGD, Dr. Littman believes, is strongly related to psychosocial factors, such as trauma, mental health problems, or social influence contributing to the development of gender dysphoria.
The current study found that 58% of respondents expressed the belief that the cause of their gender dysphoria was something specific, such as trauma, abuse, or a mental health condition, with respondents suggesting that transitioning prevented, or delayed, them from addressing their underlying mental health conditions.
One participant is quoted as saying: “I was deeply uncomfortable with my secondary sex characteristics, which I now understand was a result of childhood trauma and associating my secondary sex characteristics with those events.”
Reflecting on their previous identification as transgender, more than a third of respondents reported that someone else told them their feelings meant they were transgender, and they believed them.
“This speaks to the effect social influence can have on people’s interpretation of their own feelings and their development of a transgender identity,” Dr. Littman remarked.
“Participants also listed several social media sources that encouraged them to believe that transitioning would help them,” she added.
Several friendship group dynamics suggestive of social influence were reported by a subset of respondents, including the fact that their friendship groups mocked people who were not transgender and their popularity increased when they announced they were going to transition.
Pendulum has swung too far the other way
Natal females, who in recent years have made up most referrals, were younger than natal males when they sought transition and decided to detransition; and they stayed “transitioned” for a shorter period than natal males. They were also more likely to have experienced a trauma less than 1 year before the onset of gender dysphoria and were more likely to have felt pressured to transition.
“Because the females in the study transitioned more recently than the males, they may have experienced a culture where there is more of a ‘push’ to transition,” Dr. Littman pointed out.
She added that, “20 years ago, gender-dysphoric patients were most likely to be underdiagnosed and undertreated. Now, the pendulum has swung the other way and patients are, in my opinion, more likely to be overdiagnosed and overtreated. I think we need to aim for somewhere between these two extremes and prioritize people getting the right treatment for the right reason for their distress.”
Dr. Djordjevic added that, with colleagues from Belgrade and the Netherlands, he has published accounts of the experiences of seven individuals who showed regret after gender-affirming surgery.
All of them were born male, “and we confirmed the very poor evaluation and transition process they underwent. We conclude that clinicians should be aware that not everyone with gender identity disorders need or want all elements of hormonal or surgical therapy,” he told this news organization.
Dr. Edwards-Leeper said that more long-term longitudinal studies are needed that follow individuals who undergo transition under different models of care.
“My prediction is that those who first engage in supportive, gender exploratory therapy, followed by comprehensive assessment, will have the best outcomes, perhaps even if they ultimately detransition, as these individuals will know that they did not jump into irreversible interventions too quickly and had time to make the best decision for themselves at the time,” she concluded.
A version of this article first appeared on Medscape.com.
Over half of people who believed they were transgender, transitioned to the opposite sex, but then regretted it and transitioned back – known as detransitioners – felt they did not receive adequate evaluation from a doctor or mental health professional before starting transition, new research indicates.
In what is thought to be the first study to ask whether detransitioners informed their original clinicians of their regret at transitioning, only 24 of the 100 surveyed said they had done so.
This strongly suggests that records on detransition may understate the real numbers, said Lisa Littman, MD, MPH, president of The Institute for Comprehensive Gender Dysphoria Research (ICGDR), who is the sole author of the study, published in Archives of Sexual Behavior.
She stressed that the findings illustrate the complexity surrounding gender dysphoria. “We need to recognize that there are many different types of experiences around gender dysphoria, transition, and detransition,” she told this news organization.
She said there is some resistance among certain health care professionals, and in society in general, to the idea that transitioning is not always successful.
‘We need to understand why this is happening’
“Detransition exists and we need to understand why this is happening,” Dr. Littman emphasized.
She observed that some supporters of “rapid transition” do not want to accept that transitioning helps some individuals but harms others.
“In the end, our goals should be providing the right treatment for the right patient, and without a thorough evaluation, clinicians are at serious risk of giving patients the wrong treatment,” she urged.
She noted that, despite some individuals feeling better after transition, these people still felt inclined to detransition because of discrimination and pressure.
“Individuals should not be pressured to detransition, nor should they be pressured to transition. Both types of pressure were reported by respondents.”
The recently recognized shift from mostly natal males to natal females seeking to transition was borne out by her study data, with the proportion of natal girls who detransitioned at 69%.
‘Shedding light’ on often ignored population
Asked to comment on the study, Laura Edwards-Leeper, PhD, a clinical psychologist from Beaverton, Ore., who specializes in gender-diverse and transgender children, welcomed Dr. Littman’s study.
It is, said Dr. Edwards-Leeper, a “critical preliminary step toward shedding light on this often-ignored and dismissed population of individuals who deserve support, compassion, and sometimes medical intervention from health care providers.”
She added that multiple online reports attest to detransitioners feeling they had not received adequate evaluation prior to medically transitioning, as well as many who expressed feeling too ashamed or angry to return to their same clinicians to detransition.
“Littman’s study provides quantitative support for both of these reported experiences, further emphasizing the importance of the field taking a closer look at the processes currently in place for those experiencing gender dysphoria,” said Dr. Edwards-Leeper.
And Miroslav L. Djordjevic, MD, PhD, professor of surgery/urology, University of Belgrade (Serbia), who is a specialist in urogenital reconstructive surgery and has performed over 2,000 gender-reassignment surgeries in transgender individuals, has recently seen many cases of regret after such surgeries, with requests for reversal operations.
“Despite the fact that medical detransition is relatively safe and without severe consequences, surgical detransition presents one of the most difficult issues in transgender medicine,” Dr. Djordjevic told this news organization.
Commending Dr. Littman on her study, he drew attention to some of the bioethical questions that arise relating to those who detransition.
“I ask what happened in the period before medical transitioning? Was there proper psychological care during medical transitioning? Who confirmed their desire for detransition – the same professionals who did the transition?” or someone else, he continued. “And who accepted these individuals for gender-affirming surgery and what were the criteria for this decision?”
Substantial study of reasons for both transitioning and detransitioning
In her article, Dr. Littman describes a 100-strong population of individuals (66 Americans, 9 British, 9 Canadian, 4 Australians, and 12 from “other” nations), ranging in age from 18 years to over 60 years with a mean age of 29.2 years, who had experienced gender dysphoria, chosen to undergo medical and/or surgical transition, and then detransitioned by discontinuing medications, having reversal surgery, or both.
Participants completed a 115-question survey providing data including age at first experience of gender dysphoria, when participants first sought transitioning care and from whom, and whether they felt pressured to do so. Friendship group dynamics were also explored.
Various narratives of participants’ transitioning-detransitioning experiences were gathered and grouped, for example, those related to discrimination pressures, experiences of trauma or mental health conditions prior to transition, and reports of internalized homophobia.
Dr. Edwards-Leeper observed that the study offers a more extensive assessment of reasons for detransitioning than any other prior research in the field, which has been sparse.
A survey published in April found that detransitioners report significant unmet medical and psychological needs, and a lack of compassion and help from medical and mental health practitioners.
But another 2021 study concluded most detransitioners only reverted to their birth sex because of societal or family pressure, discrimination, or shift to a nonbinary identity.
“However, [Dr.] Littman’s study found that only a small percentage actually detransitioned for that reason [23%], whereas the majority detransitioned because of a change in how the individual understood being a male or female, resulting in becoming comfortable in their assigned gender [60%],” noted Dr. Edwards-Leeper.
Reasons for detransitioning
Asked to expand upon the motives for detransition identified in her study, Dr. Littman told this news organization: “We found remarkable breadth in the reasons given for detransitioning.”
“I believe that we were able to capture the diversity of experiences around detransition because we reached out to communities that were strongly ‘protransition’ – like the World Professional Association for Transgender Health – and communities where individuals might be more skeptical about transition being universally beneficial, like detransition forums,” she said.
Speaking to the complexity of the experiences, 87% selected more than one reason for detransitioning.
The most common reason (60%) was becoming more comfortable identifying with their birth sex, followed by having concerns about potential medical complications from transitioning (49.0%).
Regarding those who became more comfortable with their natal sex, Dr. Littman noted that the finding adds “further support that gender dysphoria is not always permanent.”
She added that, “because most gender-dysphoric youth who are allowed to go through puberty grow up to be lesbian, gay, or bisexual (LGB) nontransgender adults, intervening too soon with medical treatments risks derailing their development as LGB individuals.”
Internalized homophobia or difficulty accepting themselves as lesbian, gay, or bisexual was reported by 23% of participants as a reason for transition and subsequent detransition.
“For these people, transitioning could be interpreted as an attempt to escape the reality of being same-sex attracted and detransitioning was part of accepting themselves as homosexual or bisexual,” explained Dr. Littman.
“Exploring their distress and discomfort around sexual orientation issues may have been more helpful to them than medical and surgical transition or at least an important part of exploration,” she added in the article.
Societal pressure, friends, and social media also play a role
The latest first-hand reports also support prior work by Dr. Littman when she first identified the concept she termed rapid-onset gender dysphoria (ROGD) to describe a sudden transgender identification, usually in the early teenage years, and with no prior indication of any gender questioning.
ROGD, Dr. Littman believes, is strongly related to psychosocial factors, such as trauma, mental health problems, or social influence contributing to the development of gender dysphoria.
The current study found that 58% of respondents expressed the belief that the cause of their gender dysphoria was something specific, such as trauma, abuse, or a mental health condition, with respondents suggesting that transitioning prevented, or delayed, them from addressing their underlying mental health conditions.
One participant is quoted as saying: “I was deeply uncomfortable with my secondary sex characteristics, which I now understand was a result of childhood trauma and associating my secondary sex characteristics with those events.”
Reflecting on their previous identification as transgender, more than a third of respondents reported that someone else told them their feelings meant they were transgender, and they believed them.
“This speaks to the effect social influence can have on people’s interpretation of their own feelings and their development of a transgender identity,” Dr. Littman remarked.
“Participants also listed several social media sources that encouraged them to believe that transitioning would help them,” she added.
Several friendship group dynamics suggestive of social influence were reported by a subset of respondents, including the fact that their friendship groups mocked people who were not transgender and their popularity increased when they announced they were going to transition.
Pendulum has swung too far the other way
Natal females, who in recent years have made up most referrals, were younger than natal males when they sought transition and decided to detransition; and they stayed “transitioned” for a shorter period than natal males. They were also more likely to have experienced a trauma less than 1 year before the onset of gender dysphoria and were more likely to have felt pressured to transition.
“Because the females in the study transitioned more recently than the males, they may have experienced a culture where there is more of a ‘push’ to transition,” Dr. Littman pointed out.
She added that, “20 years ago, gender-dysphoric patients were most likely to be underdiagnosed and undertreated. Now, the pendulum has swung the other way and patients are, in my opinion, more likely to be overdiagnosed and overtreated. I think we need to aim for somewhere between these two extremes and prioritize people getting the right treatment for the right reason for their distress.”
Dr. Djordjevic added that, with colleagues from Belgrade and the Netherlands, he has published accounts of the experiences of seven individuals who showed regret after gender-affirming surgery.
All of them were born male, “and we confirmed the very poor evaluation and transition process they underwent. We conclude that clinicians should be aware that not everyone with gender identity disorders need or want all elements of hormonal or surgical therapy,” he told this news organization.
Dr. Edwards-Leeper said that more long-term longitudinal studies are needed that follow individuals who undergo transition under different models of care.
“My prediction is that those who first engage in supportive, gender exploratory therapy, followed by comprehensive assessment, will have the best outcomes, perhaps even if they ultimately detransition, as these individuals will know that they did not jump into irreversible interventions too quickly and had time to make the best decision for themselves at the time,” she concluded.
A version of this article first appeared on Medscape.com.
Pfizer says its COVID-19 pill is highly effective
, according to the drug’s maker, Pfizer.
The drug -- called Paxlovid -- was 89% effective, compared to a placebo, at preventing hospitalization or death in patients with COVID-19 who were at high risk of severe complications. The company says it plans to ask the FDA to authorize the drug for emergency use.
The medication appears to work so well that Pfizer has stopped enrollment in the trial of the drug, which works by blocking an enzyme called a protease that the new coronavirus needs to make more copies of itself.
Stopping a clinical trial is a rare action that’s typically taken when a therapy appears to be very effective or clearly dangerous. In both those cases, it’s considered unethical to continue a clinical trial where people are randomly assigned either an active drug or a placebo, when safer or more effective options are available to them.
In this case, the company said in a news release that the move was recommended by an independent panel of advisers who are overseeing the trial, called a data safety monitoring committee, and done in consultation with the FDA.
“Today’s news is a real game-changer in the global efforts to halt the devastation of this pandemic,” said Albert Bourla, PhD, Pfizer chairman and chief executive officer. “These data suggest that our oral antiviral candidate, if approved or authorized by regulatory authorities, has the potential to save patients’ lives, reduce the severity of COVID-19 infections, and eliminate up to nine out of ten hospitalizations.”
In a randomized clinical trial that included more than 1,900 patients who tested positive for COVID-19 and were at risk for having severe complications for their infections, those who received Paxlovid within 3 days of the start of their symptoms were 89% less likely to be hospitalized than those who got a placebo pill -- three patients out of 389 who got the drug were hospitalized, compared with 27 out of 385 who got the placebo. Among patients who got the drug within 5 days of the start of their symptoms, six out of 607 were hospitalized within 28 days, compared to 41 out of 612 who got the placebo.
There were no deaths over the course of a month in patients who took Paxlovid, but 10 deaths in the group that got the placebo.
The news comes on the heels of an announcement in October by the drug company Merck that its experimental antiviral pill, molnupiravir, reduced the risk of hospitalization or death by 50% in patients with mild to moderate COVID, compared to a placebo.
The United Kingdom became the first country to authorize the use of molnupiravir, which is brand-named Lagevrio.
Stephen Griffin, PhD, an associate professor of medicine at the University of Leeds, hailed the success of both new antiviral pills.
“They both demonstrate that, with appropriate investment, the development of bespoke direct-acting antiviral drugs targeting SARS-CoV2 was eminently feasible and has ultimately proven far more successful than repurposing other drugs with questionable antiviral effects,” said Dr. Griffin, who was not involved in the development of either drug.
“The success of these antivirals potentially marks a new era in our ability to prevent the severe consequences of SARS-CoV2 infection, and is also a vital element for the care of clinically vulnerable people who may be unable to either receive or respond to vaccines,” he said.
A version of this article first appeared on WebMD.com.
, according to the drug’s maker, Pfizer.
The drug -- called Paxlovid -- was 89% effective, compared to a placebo, at preventing hospitalization or death in patients with COVID-19 who were at high risk of severe complications. The company says it plans to ask the FDA to authorize the drug for emergency use.
The medication appears to work so well that Pfizer has stopped enrollment in the trial of the drug, which works by blocking an enzyme called a protease that the new coronavirus needs to make more copies of itself.
Stopping a clinical trial is a rare action that’s typically taken when a therapy appears to be very effective or clearly dangerous. In both those cases, it’s considered unethical to continue a clinical trial where people are randomly assigned either an active drug or a placebo, when safer or more effective options are available to them.
In this case, the company said in a news release that the move was recommended by an independent panel of advisers who are overseeing the trial, called a data safety monitoring committee, and done in consultation with the FDA.
“Today’s news is a real game-changer in the global efforts to halt the devastation of this pandemic,” said Albert Bourla, PhD, Pfizer chairman and chief executive officer. “These data suggest that our oral antiviral candidate, if approved or authorized by regulatory authorities, has the potential to save patients’ lives, reduce the severity of COVID-19 infections, and eliminate up to nine out of ten hospitalizations.”
In a randomized clinical trial that included more than 1,900 patients who tested positive for COVID-19 and were at risk for having severe complications for their infections, those who received Paxlovid within 3 days of the start of their symptoms were 89% less likely to be hospitalized than those who got a placebo pill -- three patients out of 389 who got the drug were hospitalized, compared with 27 out of 385 who got the placebo. Among patients who got the drug within 5 days of the start of their symptoms, six out of 607 were hospitalized within 28 days, compared to 41 out of 612 who got the placebo.
There were no deaths over the course of a month in patients who took Paxlovid, but 10 deaths in the group that got the placebo.
The news comes on the heels of an announcement in October by the drug company Merck that its experimental antiviral pill, molnupiravir, reduced the risk of hospitalization or death by 50% in patients with mild to moderate COVID, compared to a placebo.
The United Kingdom became the first country to authorize the use of molnupiravir, which is brand-named Lagevrio.
Stephen Griffin, PhD, an associate professor of medicine at the University of Leeds, hailed the success of both new antiviral pills.
“They both demonstrate that, with appropriate investment, the development of bespoke direct-acting antiviral drugs targeting SARS-CoV2 was eminently feasible and has ultimately proven far more successful than repurposing other drugs with questionable antiviral effects,” said Dr. Griffin, who was not involved in the development of either drug.
“The success of these antivirals potentially marks a new era in our ability to prevent the severe consequences of SARS-CoV2 infection, and is also a vital element for the care of clinically vulnerable people who may be unable to either receive or respond to vaccines,” he said.
A version of this article first appeared on WebMD.com.
, according to the drug’s maker, Pfizer.
The drug -- called Paxlovid -- was 89% effective, compared to a placebo, at preventing hospitalization or death in patients with COVID-19 who were at high risk of severe complications. The company says it plans to ask the FDA to authorize the drug for emergency use.
The medication appears to work so well that Pfizer has stopped enrollment in the trial of the drug, which works by blocking an enzyme called a protease that the new coronavirus needs to make more copies of itself.
Stopping a clinical trial is a rare action that’s typically taken when a therapy appears to be very effective or clearly dangerous. In both those cases, it’s considered unethical to continue a clinical trial where people are randomly assigned either an active drug or a placebo, when safer or more effective options are available to them.
In this case, the company said in a news release that the move was recommended by an independent panel of advisers who are overseeing the trial, called a data safety monitoring committee, and done in consultation with the FDA.
“Today’s news is a real game-changer in the global efforts to halt the devastation of this pandemic,” said Albert Bourla, PhD, Pfizer chairman and chief executive officer. “These data suggest that our oral antiviral candidate, if approved or authorized by regulatory authorities, has the potential to save patients’ lives, reduce the severity of COVID-19 infections, and eliminate up to nine out of ten hospitalizations.”
In a randomized clinical trial that included more than 1,900 patients who tested positive for COVID-19 and were at risk for having severe complications for their infections, those who received Paxlovid within 3 days of the start of their symptoms were 89% less likely to be hospitalized than those who got a placebo pill -- three patients out of 389 who got the drug were hospitalized, compared with 27 out of 385 who got the placebo. Among patients who got the drug within 5 days of the start of their symptoms, six out of 607 were hospitalized within 28 days, compared to 41 out of 612 who got the placebo.
There were no deaths over the course of a month in patients who took Paxlovid, but 10 deaths in the group that got the placebo.
The news comes on the heels of an announcement in October by the drug company Merck that its experimental antiviral pill, molnupiravir, reduced the risk of hospitalization or death by 50% in patients with mild to moderate COVID, compared to a placebo.
The United Kingdom became the first country to authorize the use of molnupiravir, which is brand-named Lagevrio.
Stephen Griffin, PhD, an associate professor of medicine at the University of Leeds, hailed the success of both new antiviral pills.
“They both demonstrate that, with appropriate investment, the development of bespoke direct-acting antiviral drugs targeting SARS-CoV2 was eminently feasible and has ultimately proven far more successful than repurposing other drugs with questionable antiviral effects,” said Dr. Griffin, who was not involved in the development of either drug.
“The success of these antivirals potentially marks a new era in our ability to prevent the severe consequences of SARS-CoV2 infection, and is also a vital element for the care of clinically vulnerable people who may be unable to either receive or respond to vaccines,” he said.
A version of this article first appeared on WebMD.com.
Success of HPV vaccination: ‘Dramatic’ reduction in cervical cancer
Among young women who received the HPV vaccine when they were 12-13 years old (before their sexual debut), cervical cancer rates are 87% lower than among previous nonvaccinated generations.
“It’s been incredible to see the impact of HPV vaccination, and now we can prove it prevented hundreds of women from developing cancer in England,” senior author Peter Sasieni, MD, King’s College London, said in a statement. “To see the real-life impact of the vaccine has been truly rewarding.”
“This study provides the first direct evidence of the impact of the UK HPV vaccination campaign on cervical cancer incidence, showing a large reduction in cervical cancer rates in vaccinated cohorts,” Kate Soldan, MD, U.K. Health Security Agency, London, commented in a statement.
Vanessa Saliba, MD, a consultant epidemiologist for the U.K. Health Security Agency, agreed, saying that “these remarkable findings confirm that the HPV vaccine saves lives by dramatically reducing cervical cancer rates among women.
“This reminds us that vaccines are one of the most important tools we have to help us live longer, healthier lives,” she added.
The study was published online Nov. 3, 2021, in The Lancet.
Approached for comment on the new study, Maurice Markman, MD, president, Medicine and Science Cancer Treatment Centers of America, noted that the results of the English study are very similar to those of a Swedish study of the quadrivalent vaccine alone.
“You can put any superlatives you want in here, but these are stunningly positive results,” Dr. Markman said in an interview. He said that, as an oncologist who has been treating cervical cancer for 40 years, particularly patients with advanced cervical cancer, “I can tell you this is one of the most devastating diseases to women, and the ability to eliminate this cancer with something as simple as a vaccine is the goal of cancer therapy, and it’s been remarkably successful.
“I can only emphasize the critical importance of all parents to see that their children who are eligible for the vaccine receive it. This is a cancer prevention strategy that is unbelievably, remarkably effective and safe,” Dr. Markman added.
National vaccination program
The national HPV vaccination program in England began in 2008. Initially, the bivalent Cervarix vaccine against HPV 16 and 18 was used. HPV 16 and 18 are responsible for 70% to 80% of all cervical cancers in England, the researchers note in their article.
In 2012, the program switched to the quadrivalent HPV vaccine (Gardasil), which is effective against two additional HPV types, HPV 6 and 11. Those strains cause genital warts.
The prevention program originally recommended a three-dose regimen in which both HPV vaccines were used. Currently, two doses are given to girls younger than 15 years. In addition, a single dose of the HPV vaccine provides good protection against persistent infection. The efficacy rate of a single dose is similar to that of three doses, the authors comment.
Population-based registry
The new data come from a population-based cancer registry that shows the incidence of cervical cancer and noninvasive cervical carcinoma (CIN3) in England between January 2006 and June 2019.
The study included seven cohorts of women who were aged 20-64 years at the end of 2019. Three of these cohorts composed the vaccinated population.
The team reports that overall, from January 2006 to June 2019, there were 27,946 cases of cervical cancer and 318,058 cases of CIN3.
In the three vaccinated cohorts, there were around 450 fewer cases of cervical cancer and 17,200 fewer cases of CIN3 than would be expected in a nonvaccinated population.
The three vaccinated cohorts had been eligible to receive Cervarix when they were aged 12-13 years. A catch-up scheme aimed at 14- to 16-year-olds and 16- to 18-year-olds. Most of these persons were vaccinated through a school vaccination program.
The team analyzed the data for each of these cohorts.
Among the cohort eligible for vaccination at 12-13 years of age, 89% received at least one dose of the HPV vaccine; 85% received three shots and were fully vaccinated. Among these persons, the rate of cervical cancer was 87% lower than expected in a nonvaccinated population, and the rate of CIN3 was 97% lower than expected.
For the cohort that was eligible to be vaccinated between the ages of 14 and 16 years, the corresponding reductions were 62% for cervical cancer and 75% for CIN3.
For the cohort eligible for vaccination between the ages of 16 and 18 years (of whom 60% had received at least one dose and 45% were fully vaccinated), the corresponding reduction were 34% for cervical cancer and 39% for CIN3.
The authors acknowledge some limitations with the study, principally that cervical cancer is rare in young women, and these vaccinated populations are still young. The youngest would have been vaccinated at age 12 in 2008 and so would be only 23 years old in 2019, when the follow-up in this current study ended. The authors emphasize that because the vaccinated populations are still young, it is too early to assess the full impact of HPV vaccination on cervical cancer rates.
Editorial commentary
“The relative reductions in cervical cancer, expected as a result of the HPV vaccination program, support the anticipated vaccine effectiveness,” commented two authors of an accompanying editorial, Maggie Cruickshank, MD, University of Aberdeen (Scotland), and Mihaela Grigore, MD, University of Medicine and Pharmacy, Lasi, Romania.
“The scale of the HPV vaccination effect reported by this study should also stimulate vaccination programs in low-income and middle-income countries where the problem of cervical cancer is a far greater public health issue than in those with well established systems of vaccination and screening,” they comment.
“The most important issue, besides the availability of the vaccine ... is the education of the population to accept the vaccination because a high rate of immunization is a key element of success,” they emphasize. “Even in a wealthy country, such as England with free access to HPV immunization, uptake has not reached the 90% vaccination target of girls aged 15 years set by WHO [World Health Organization].”
The authors and editorialists disclosed no relevant financial relationships. Dr. Markman is a regular contributor to Medscape Oncology. He has received income of $250 or more from Genentech, AstraZeneca, Celgene, Clovis, and Amgen.
A version of this article first appeared on Medscape.com.
Among young women who received the HPV vaccine when they were 12-13 years old (before their sexual debut), cervical cancer rates are 87% lower than among previous nonvaccinated generations.
“It’s been incredible to see the impact of HPV vaccination, and now we can prove it prevented hundreds of women from developing cancer in England,” senior author Peter Sasieni, MD, King’s College London, said in a statement. “To see the real-life impact of the vaccine has been truly rewarding.”
“This study provides the first direct evidence of the impact of the UK HPV vaccination campaign on cervical cancer incidence, showing a large reduction in cervical cancer rates in vaccinated cohorts,” Kate Soldan, MD, U.K. Health Security Agency, London, commented in a statement.
Vanessa Saliba, MD, a consultant epidemiologist for the U.K. Health Security Agency, agreed, saying that “these remarkable findings confirm that the HPV vaccine saves lives by dramatically reducing cervical cancer rates among women.
“This reminds us that vaccines are one of the most important tools we have to help us live longer, healthier lives,” she added.
The study was published online Nov. 3, 2021, in The Lancet.
Approached for comment on the new study, Maurice Markman, MD, president, Medicine and Science Cancer Treatment Centers of America, noted that the results of the English study are very similar to those of a Swedish study of the quadrivalent vaccine alone.
“You can put any superlatives you want in here, but these are stunningly positive results,” Dr. Markman said in an interview. He said that, as an oncologist who has been treating cervical cancer for 40 years, particularly patients with advanced cervical cancer, “I can tell you this is one of the most devastating diseases to women, and the ability to eliminate this cancer with something as simple as a vaccine is the goal of cancer therapy, and it’s been remarkably successful.
“I can only emphasize the critical importance of all parents to see that their children who are eligible for the vaccine receive it. This is a cancer prevention strategy that is unbelievably, remarkably effective and safe,” Dr. Markman added.
National vaccination program
The national HPV vaccination program in England began in 2008. Initially, the bivalent Cervarix vaccine against HPV 16 and 18 was used. HPV 16 and 18 are responsible for 70% to 80% of all cervical cancers in England, the researchers note in their article.
In 2012, the program switched to the quadrivalent HPV vaccine (Gardasil), which is effective against two additional HPV types, HPV 6 and 11. Those strains cause genital warts.
The prevention program originally recommended a three-dose regimen in which both HPV vaccines were used. Currently, two doses are given to girls younger than 15 years. In addition, a single dose of the HPV vaccine provides good protection against persistent infection. The efficacy rate of a single dose is similar to that of three doses, the authors comment.
Population-based registry
The new data come from a population-based cancer registry that shows the incidence of cervical cancer and noninvasive cervical carcinoma (CIN3) in England between January 2006 and June 2019.
The study included seven cohorts of women who were aged 20-64 years at the end of 2019. Three of these cohorts composed the vaccinated population.
The team reports that overall, from January 2006 to June 2019, there were 27,946 cases of cervical cancer and 318,058 cases of CIN3.
In the three vaccinated cohorts, there were around 450 fewer cases of cervical cancer and 17,200 fewer cases of CIN3 than would be expected in a nonvaccinated population.
The three vaccinated cohorts had been eligible to receive Cervarix when they were aged 12-13 years. A catch-up scheme aimed at 14- to 16-year-olds and 16- to 18-year-olds. Most of these persons were vaccinated through a school vaccination program.
The team analyzed the data for each of these cohorts.
Among the cohort eligible for vaccination at 12-13 years of age, 89% received at least one dose of the HPV vaccine; 85% received three shots and were fully vaccinated. Among these persons, the rate of cervical cancer was 87% lower than expected in a nonvaccinated population, and the rate of CIN3 was 97% lower than expected.
For the cohort that was eligible to be vaccinated between the ages of 14 and 16 years, the corresponding reductions were 62% for cervical cancer and 75% for CIN3.
For the cohort eligible for vaccination between the ages of 16 and 18 years (of whom 60% had received at least one dose and 45% were fully vaccinated), the corresponding reduction were 34% for cervical cancer and 39% for CIN3.
The authors acknowledge some limitations with the study, principally that cervical cancer is rare in young women, and these vaccinated populations are still young. The youngest would have been vaccinated at age 12 in 2008 and so would be only 23 years old in 2019, when the follow-up in this current study ended. The authors emphasize that because the vaccinated populations are still young, it is too early to assess the full impact of HPV vaccination on cervical cancer rates.
Editorial commentary
“The relative reductions in cervical cancer, expected as a result of the HPV vaccination program, support the anticipated vaccine effectiveness,” commented two authors of an accompanying editorial, Maggie Cruickshank, MD, University of Aberdeen (Scotland), and Mihaela Grigore, MD, University of Medicine and Pharmacy, Lasi, Romania.
“The scale of the HPV vaccination effect reported by this study should also stimulate vaccination programs in low-income and middle-income countries where the problem of cervical cancer is a far greater public health issue than in those with well established systems of vaccination and screening,” they comment.
“The most important issue, besides the availability of the vaccine ... is the education of the population to accept the vaccination because a high rate of immunization is a key element of success,” they emphasize. “Even in a wealthy country, such as England with free access to HPV immunization, uptake has not reached the 90% vaccination target of girls aged 15 years set by WHO [World Health Organization].”
The authors and editorialists disclosed no relevant financial relationships. Dr. Markman is a regular contributor to Medscape Oncology. He has received income of $250 or more from Genentech, AstraZeneca, Celgene, Clovis, and Amgen.
A version of this article first appeared on Medscape.com.
Among young women who received the HPV vaccine when they were 12-13 years old (before their sexual debut), cervical cancer rates are 87% lower than among previous nonvaccinated generations.
“It’s been incredible to see the impact of HPV vaccination, and now we can prove it prevented hundreds of women from developing cancer in England,” senior author Peter Sasieni, MD, King’s College London, said in a statement. “To see the real-life impact of the vaccine has been truly rewarding.”
“This study provides the first direct evidence of the impact of the UK HPV vaccination campaign on cervical cancer incidence, showing a large reduction in cervical cancer rates in vaccinated cohorts,” Kate Soldan, MD, U.K. Health Security Agency, London, commented in a statement.
Vanessa Saliba, MD, a consultant epidemiologist for the U.K. Health Security Agency, agreed, saying that “these remarkable findings confirm that the HPV vaccine saves lives by dramatically reducing cervical cancer rates among women.
“This reminds us that vaccines are one of the most important tools we have to help us live longer, healthier lives,” she added.
The study was published online Nov. 3, 2021, in The Lancet.
Approached for comment on the new study, Maurice Markman, MD, president, Medicine and Science Cancer Treatment Centers of America, noted that the results of the English study are very similar to those of a Swedish study of the quadrivalent vaccine alone.
“You can put any superlatives you want in here, but these are stunningly positive results,” Dr. Markman said in an interview. He said that, as an oncologist who has been treating cervical cancer for 40 years, particularly patients with advanced cervical cancer, “I can tell you this is one of the most devastating diseases to women, and the ability to eliminate this cancer with something as simple as a vaccine is the goal of cancer therapy, and it’s been remarkably successful.
“I can only emphasize the critical importance of all parents to see that their children who are eligible for the vaccine receive it. This is a cancer prevention strategy that is unbelievably, remarkably effective and safe,” Dr. Markman added.
National vaccination program
The national HPV vaccination program in England began in 2008. Initially, the bivalent Cervarix vaccine against HPV 16 and 18 was used. HPV 16 and 18 are responsible for 70% to 80% of all cervical cancers in England, the researchers note in their article.
In 2012, the program switched to the quadrivalent HPV vaccine (Gardasil), which is effective against two additional HPV types, HPV 6 and 11. Those strains cause genital warts.
The prevention program originally recommended a three-dose regimen in which both HPV vaccines were used. Currently, two doses are given to girls younger than 15 years. In addition, a single dose of the HPV vaccine provides good protection against persistent infection. The efficacy rate of a single dose is similar to that of three doses, the authors comment.
Population-based registry
The new data come from a population-based cancer registry that shows the incidence of cervical cancer and noninvasive cervical carcinoma (CIN3) in England between January 2006 and June 2019.
The study included seven cohorts of women who were aged 20-64 years at the end of 2019. Three of these cohorts composed the vaccinated population.
The team reports that overall, from January 2006 to June 2019, there were 27,946 cases of cervical cancer and 318,058 cases of CIN3.
In the three vaccinated cohorts, there were around 450 fewer cases of cervical cancer and 17,200 fewer cases of CIN3 than would be expected in a nonvaccinated population.
The three vaccinated cohorts had been eligible to receive Cervarix when they were aged 12-13 years. A catch-up scheme aimed at 14- to 16-year-olds and 16- to 18-year-olds. Most of these persons were vaccinated through a school vaccination program.
The team analyzed the data for each of these cohorts.
Among the cohort eligible for vaccination at 12-13 years of age, 89% received at least one dose of the HPV vaccine; 85% received three shots and were fully vaccinated. Among these persons, the rate of cervical cancer was 87% lower than expected in a nonvaccinated population, and the rate of CIN3 was 97% lower than expected.
For the cohort that was eligible to be vaccinated between the ages of 14 and 16 years, the corresponding reductions were 62% for cervical cancer and 75% for CIN3.
For the cohort eligible for vaccination between the ages of 16 and 18 years (of whom 60% had received at least one dose and 45% were fully vaccinated), the corresponding reduction were 34% for cervical cancer and 39% for CIN3.
The authors acknowledge some limitations with the study, principally that cervical cancer is rare in young women, and these vaccinated populations are still young. The youngest would have been vaccinated at age 12 in 2008 and so would be only 23 years old in 2019, when the follow-up in this current study ended. The authors emphasize that because the vaccinated populations are still young, it is too early to assess the full impact of HPV vaccination on cervical cancer rates.
Editorial commentary
“The relative reductions in cervical cancer, expected as a result of the HPV vaccination program, support the anticipated vaccine effectiveness,” commented two authors of an accompanying editorial, Maggie Cruickshank, MD, University of Aberdeen (Scotland), and Mihaela Grigore, MD, University of Medicine and Pharmacy, Lasi, Romania.
“The scale of the HPV vaccination effect reported by this study should also stimulate vaccination programs in low-income and middle-income countries where the problem of cervical cancer is a far greater public health issue than in those with well established systems of vaccination and screening,” they comment.
“The most important issue, besides the availability of the vaccine ... is the education of the population to accept the vaccination because a high rate of immunization is a key element of success,” they emphasize. “Even in a wealthy country, such as England with free access to HPV immunization, uptake has not reached the 90% vaccination target of girls aged 15 years set by WHO [World Health Organization].”
The authors and editorialists disclosed no relevant financial relationships. Dr. Markman is a regular contributor to Medscape Oncology. He has received income of $250 or more from Genentech, AstraZeneca, Celgene, Clovis, and Amgen.
A version of this article first appeared on Medscape.com.
Ivermectin–COVID-19 study retracted; authors blame file mix-up
The paper, “Effects of a Single Dose of Ivermectin on Viral and Clinical Outcomes in Asymptomatic SARS-CoV-2 Infected Subjects: A Pilot Clinical Trial in Lebanon,” appeared in the journal Viruses in May. According to the abstract: “A randomized controlled trial was conducted in 100 asymptomatic Lebanese subjects that have tested positive for SARS-CoV2. Fifty patients received standard preventive treatment, mainly supplements, and the experimental group received a single dose (according to body weight) of ivermectin, in addition to the same supplements the control group received.”
Results results results … and: “Ivermectin appears to be efficacious in providing clinical benefits in a randomized treatment of asymptomatic SARS-CoV-2-positive subjects, effectively resulting in fewer symptoms, lower viral load and reduced hospital admissions. However, larger-scale trials are warranted for this conclusion to be further cemented.”
However, in early October, the BBC reported — in a larger piece about the concerns about ivermectin-Covid-19 research — that the study “was found to have blocks of details of 11 patients that had been copied and pasted repeatedly – suggesting many of the trial’s apparent patients didn’t really exist.”
The study’s authors told the BBC that the ‘original set of data was rigged, sabotaged or mistakenly entered in the final file’ and that they have submitted a retraction to the scientific journal which published it.
That’s not quite what the retraction notice states: “The journal retracts the article, Effects of a Single Dose of Ivermectin on Viral and Clinical Outcomes in Asymptomatic SARS-CoV-2 Infected Subjects: A Pilot Clinical Trial in Lebanon [ 1 ], cited above. Following publication, the authors contacted the editorial office regarding an error between files used for the statistical analysis. Adhering to our complaints procedure, an investigation was conducted that confirmed the error reported by the authors.
This retraction was approved by the Editor in Chief of the journal. The authors agreed to this retraction.”
Ali Samaha, of Lebanese University in Beirut, and the lead author of the study, told us: “It was brought to our attention that we have used wrong file for our paper. We informed immediately the journal and we have run investigations. After revising the raw data we realised that a file that was used to train a research assistant was sent by mistake for analysis. Re-analysing the original data , the conclusions of the paper remained valid. For our transparency we asked for retraction.”
About that BBC report? Samaha said: “The BBC article was generated before the report of independent reviewers who confirmed an innocent mistake by using wrong file.”
Samaha added that he and his colleagues are now considering whether to resubmit the paper.
The article has been cited four times, according to Clarivate Analytics’ Web of Science — including in this meta-analysis published in June in the American Journal of Therapeutics , which concluded that: “Moderate-certainty evidence finds that large reductions in COVID-19 deaths are possible using ivermectin. Using ivermectin early in the clinical course may reduce numbers progressing to severe disease. The apparent safety and low cost suggest that ivermectin is likely to have a significant impact on the SARS-CoV-2 pandemic globally.”
That article was a social media darling, receiving more than 45,000 tweets and pickups in 90 news outlets, according to Altmetrics, which ranks it No. 7 among all papers published at that time.
A version of this article first appeared on Retraction Watch.
The paper, “Effects of a Single Dose of Ivermectin on Viral and Clinical Outcomes in Asymptomatic SARS-CoV-2 Infected Subjects: A Pilot Clinical Trial in Lebanon,” appeared in the journal Viruses in May. According to the abstract: “A randomized controlled trial was conducted in 100 asymptomatic Lebanese subjects that have tested positive for SARS-CoV2. Fifty patients received standard preventive treatment, mainly supplements, and the experimental group received a single dose (according to body weight) of ivermectin, in addition to the same supplements the control group received.”
Results results results … and: “Ivermectin appears to be efficacious in providing clinical benefits in a randomized treatment of asymptomatic SARS-CoV-2-positive subjects, effectively resulting in fewer symptoms, lower viral load and reduced hospital admissions. However, larger-scale trials are warranted for this conclusion to be further cemented.”
However, in early October, the BBC reported — in a larger piece about the concerns about ivermectin-Covid-19 research — that the study “was found to have blocks of details of 11 patients that had been copied and pasted repeatedly – suggesting many of the trial’s apparent patients didn’t really exist.”
The study’s authors told the BBC that the ‘original set of data was rigged, sabotaged or mistakenly entered in the final file’ and that they have submitted a retraction to the scientific journal which published it.
That’s not quite what the retraction notice states: “The journal retracts the article, Effects of a Single Dose of Ivermectin on Viral and Clinical Outcomes in Asymptomatic SARS-CoV-2 Infected Subjects: A Pilot Clinical Trial in Lebanon [ 1 ], cited above. Following publication, the authors contacted the editorial office regarding an error between files used for the statistical analysis. Adhering to our complaints procedure, an investigation was conducted that confirmed the error reported by the authors.
This retraction was approved by the Editor in Chief of the journal. The authors agreed to this retraction.”
Ali Samaha, of Lebanese University in Beirut, and the lead author of the study, told us: “It was brought to our attention that we have used wrong file for our paper. We informed immediately the journal and we have run investigations. After revising the raw data we realised that a file that was used to train a research assistant was sent by mistake for analysis. Re-analysing the original data , the conclusions of the paper remained valid. For our transparency we asked for retraction.”
About that BBC report? Samaha said: “The BBC article was generated before the report of independent reviewers who confirmed an innocent mistake by using wrong file.”
Samaha added that he and his colleagues are now considering whether to resubmit the paper.
The article has been cited four times, according to Clarivate Analytics’ Web of Science — including in this meta-analysis published in June in the American Journal of Therapeutics , which concluded that: “Moderate-certainty evidence finds that large reductions in COVID-19 deaths are possible using ivermectin. Using ivermectin early in the clinical course may reduce numbers progressing to severe disease. The apparent safety and low cost suggest that ivermectin is likely to have a significant impact on the SARS-CoV-2 pandemic globally.”
That article was a social media darling, receiving more than 45,000 tweets and pickups in 90 news outlets, according to Altmetrics, which ranks it No. 7 among all papers published at that time.
A version of this article first appeared on Retraction Watch.
The paper, “Effects of a Single Dose of Ivermectin on Viral and Clinical Outcomes in Asymptomatic SARS-CoV-2 Infected Subjects: A Pilot Clinical Trial in Lebanon,” appeared in the journal Viruses in May. According to the abstract: “A randomized controlled trial was conducted in 100 asymptomatic Lebanese subjects that have tested positive for SARS-CoV2. Fifty patients received standard preventive treatment, mainly supplements, and the experimental group received a single dose (according to body weight) of ivermectin, in addition to the same supplements the control group received.”
Results results results … and: “Ivermectin appears to be efficacious in providing clinical benefits in a randomized treatment of asymptomatic SARS-CoV-2-positive subjects, effectively resulting in fewer symptoms, lower viral load and reduced hospital admissions. However, larger-scale trials are warranted for this conclusion to be further cemented.”
However, in early October, the BBC reported — in a larger piece about the concerns about ivermectin-Covid-19 research — that the study “was found to have blocks of details of 11 patients that had been copied and pasted repeatedly – suggesting many of the trial’s apparent patients didn’t really exist.”
The study’s authors told the BBC that the ‘original set of data was rigged, sabotaged or mistakenly entered in the final file’ and that they have submitted a retraction to the scientific journal which published it.
That’s not quite what the retraction notice states: “The journal retracts the article, Effects of a Single Dose of Ivermectin on Viral and Clinical Outcomes in Asymptomatic SARS-CoV-2 Infected Subjects: A Pilot Clinical Trial in Lebanon [ 1 ], cited above. Following publication, the authors contacted the editorial office regarding an error between files used for the statistical analysis. Adhering to our complaints procedure, an investigation was conducted that confirmed the error reported by the authors.
This retraction was approved by the Editor in Chief of the journal. The authors agreed to this retraction.”
Ali Samaha, of Lebanese University in Beirut, and the lead author of the study, told us: “It was brought to our attention that we have used wrong file for our paper. We informed immediately the journal and we have run investigations. After revising the raw data we realised that a file that was used to train a research assistant was sent by mistake for analysis. Re-analysing the original data , the conclusions of the paper remained valid. For our transparency we asked for retraction.”
About that BBC report? Samaha said: “The BBC article was generated before the report of independent reviewers who confirmed an innocent mistake by using wrong file.”
Samaha added that he and his colleagues are now considering whether to resubmit the paper.
The article has been cited four times, according to Clarivate Analytics’ Web of Science — including in this meta-analysis published in June in the American Journal of Therapeutics , which concluded that: “Moderate-certainty evidence finds that large reductions in COVID-19 deaths are possible using ivermectin. Using ivermectin early in the clinical course may reduce numbers progressing to severe disease. The apparent safety and low cost suggest that ivermectin is likely to have a significant impact on the SARS-CoV-2 pandemic globally.”
That article was a social media darling, receiving more than 45,000 tweets and pickups in 90 news outlets, according to Altmetrics, which ranks it No. 7 among all papers published at that time.
A version of this article first appeared on Retraction Watch.
AHA dietary guidance cites structural challenges to heart-healthy patterns
In a new scientific statement on diet and lifestyle recommendations, the American Heart Association is highlighting, for the first time, structural challenges that impede the adoption of heart-healthy dietary patterns.
This is in addition to stressing aspects of diet that improve cardiovascular health and reduce cardiovascular risk, with an emphasis on dietary patterns and food-based guidance beyond naming individual foods or nutrients.
The 2021 Dietary Guidance to Improve Cardiovascular Health scientific statement, developed under Alice H. Lichtenstein, DSc, chair of the AHA writing group, provides 10 evidence-based guidance recommendations to promote cardiometabolic health.
“The way to make heart-healthy choices every day,” said Dr. Lichtenstein, of the Jean Mayer USDA Human Nutrition Research Center on Aging at Tufts University in Boston, in a statement, “is to step back, look at the environment in which you eat, whether it be at home, at work, during social interaction, and then identify what the best choices are. And if there are no good choices, then think about how you can modify your environment so that there are good choices.”
The statement, published in Circulation, underscores growing evidence that nutrition-related chronic diseases have maternal-nutritional origins, and that prevention of pediatric obesity is a key to preserving and prolonging ideal cardiovascular health.
The features are as follows:
- Adjust energy intake and expenditure to achieve and maintain a healthy body weight. To counter the shift toward higher energy intake and more sedentary lifestyles over the past 3 decades, the statement recommends at least 150 minutes of moderate physical activity per week, adjusted for individual’s age, activity level, sex, and size.
- Eat plenty of fruits and vegetables; choose a wide variety. Observational and intervention studies document that dietary patterns rich in varied fruits and vegetables, with the exception of white potatoes, are linked to a lower risk of cardiovascular disease (CVD). Also, whole fruits and vegetables, which more readily provide fiber and satiety, are preferred over juices.
- Choose whole grain foods and products made mostly with whole grains rather than refined grains. Evidence from observational, interventional, and clinical studies confirm the benefits of frequent consumption of whole grains over infrequent consumption or over refined grains in terms of CVD risk, coronary heart disease (CHD), stroke, metabolic syndrome, cardiometabolic risk factors, laxation, and gut microbiota.
- Choose healthy sources of protein, mostly from plants (legumes and nuts).
- Higher intake of legumes, which are rich in protein and fiber, is associated with lower CVD risk, while higher nut intake is associated with lower risks of CVD, CHD, and stroke mortality/incidence. Replacing animal-source foods with plant-source whole foods, beyond health benefits, lowers the diet’s carbon footprint. Meat alternatives are often ultraprocessed and evidence on their short- and long-term health effects is limited. Unsaturated fats are preferred, as are lean, nonprocessed meats.
- Use liquid plant oils rather than tropical oils (coconut, palm, and palm kernel), animal fats (butter and lard), and partially hydrogenated fats. Saturated and trans fats (animal and dairy fats, and partially hydrogenated fat) should be replaced with nontropical liquid plant oils. Evidence supports cardiovascular benefits of dietary unsaturated fats, especially polyunsaturated fats primarily from plant oils (e.g. soybean, corn, safflower and sunflower oils, walnuts, and flax seeds).
- Choose minimally processed foods instead of ultraprocessed foods. Because of their proven association with adverse health outcomes, including overweight and obesity, cardiometabolic disorders (type 2 diabetes, CVD), and all-cause mortality, the consumption of many ultraprocessed foods is of concern. Ultraprocessed foods include artificial colors and flavors and preservatives that promote shelf stability, preserve texture, and increase palatability. A general principle is to emphasize unprocessed or minimally processed foods.
- Minimize intake of beverages and foods with added sugars. Added sugars (commonly glucose, dextrose, sucrose, corn syrup, honey, maple syrup, and concentrated fruit juice) are tied to elevated risk for type 2 diabetes, high cholesterol, and excess body weight. Findings from meta-analyses on body weight and metabolic outcomes for replacing added sugars with low-energy sweeteners are mixed, and the possibility of reverse causality has been raised.
- Choose and prepare foods with little or no salt. In general, the effects of sodium reduction on blood pressure tend to be higher in Black people, middle-aged and older people, and those with hypertension. In the United States, the main combined sources of sodium intake are processed foods, those prepared outside the home, packaged foods, and restaurant foods. Potassium-enriched salts are a promising alternative.
- If you don’t drink alcohol, don’t start; if you choose to drink, limit intake.
- While relationships between alcohol intake and cardiovascular outcomes are complex, the 2020 Dietary Guidelines Advisory Committee recently concluded that those who do drink should consume no more than one drink per day and should not drink alcohol in binges; the 2020 Dietary Guidelines for Americans continues to recommend no more than one drink per day for women and two drinks per day for men.
- Adhere to the guidance regardless in all settings. Food-based dietary guidance applies to all foods and beverages, regardless of where prepared, procured, and consumed. Policies should be enacted that encourage healthier default options (for example, whole grains, minimized sodium and sugar content).
Recognizing impediments
The AHA/ASA scientific statement closes with the declaration: “Creating an environment that facilitates, rather than impedes, adherence to heart-healthy dietary patterns among all individuals is a public health imperative.” It points to the National Institutes of Health’s 2020-2030 Strategic Plan for National Institutes of Health Nutrition Research, which focuses on precision nutrition as a means “to determine the impact on health of not only what individuals eat, but also of why, when, and how they eat throughout the life course.”
Ultimately, precision nutrition may provide personalized diets for CVD prevention. But the “food environment,” often conditioned by “rampant nutrition misinformation” through local, state, and federal practices and policies, may impede the adoption of heart-healthy dietary patterns. Factors such as targeted food marketing (for example, of processed food and beverages in minority neighborhoods), structural racism, neighborhood segregation, unhealthy built environments, and food insecurity create environments in which unhealthy foods are the default option.”
These factors compound adverse dietary and health effects, and underscore a need to “directly combat nutrition misinformation among the public and health care professionals.” They also explain why, despite widespread knowledge of heart-healthy dietary pattern components, little progress has been made in achieving dietary goals in the United States.
Dr. Lichtenstein’s office, in response to a request regarding AHA advocacy and consumer programs, provided the following links: Voices for Healthy Kids initiative site and choosing healthier processed foods and one on fresh, frozen, and canned fruits and vegetables.
Dr. Lichtenstein had no disclosures. Disclosures for the writing group members are included in the statement.
In a new scientific statement on diet and lifestyle recommendations, the American Heart Association is highlighting, for the first time, structural challenges that impede the adoption of heart-healthy dietary patterns.
This is in addition to stressing aspects of diet that improve cardiovascular health and reduce cardiovascular risk, with an emphasis on dietary patterns and food-based guidance beyond naming individual foods or nutrients.
The 2021 Dietary Guidance to Improve Cardiovascular Health scientific statement, developed under Alice H. Lichtenstein, DSc, chair of the AHA writing group, provides 10 evidence-based guidance recommendations to promote cardiometabolic health.
“The way to make heart-healthy choices every day,” said Dr. Lichtenstein, of the Jean Mayer USDA Human Nutrition Research Center on Aging at Tufts University in Boston, in a statement, “is to step back, look at the environment in which you eat, whether it be at home, at work, during social interaction, and then identify what the best choices are. And if there are no good choices, then think about how you can modify your environment so that there are good choices.”
The statement, published in Circulation, underscores growing evidence that nutrition-related chronic diseases have maternal-nutritional origins, and that prevention of pediatric obesity is a key to preserving and prolonging ideal cardiovascular health.
The features are as follows:
- Adjust energy intake and expenditure to achieve and maintain a healthy body weight. To counter the shift toward higher energy intake and more sedentary lifestyles over the past 3 decades, the statement recommends at least 150 minutes of moderate physical activity per week, adjusted for individual’s age, activity level, sex, and size.
- Eat plenty of fruits and vegetables; choose a wide variety. Observational and intervention studies document that dietary patterns rich in varied fruits and vegetables, with the exception of white potatoes, are linked to a lower risk of cardiovascular disease (CVD). Also, whole fruits and vegetables, which more readily provide fiber and satiety, are preferred over juices.
- Choose whole grain foods and products made mostly with whole grains rather than refined grains. Evidence from observational, interventional, and clinical studies confirm the benefits of frequent consumption of whole grains over infrequent consumption or over refined grains in terms of CVD risk, coronary heart disease (CHD), stroke, metabolic syndrome, cardiometabolic risk factors, laxation, and gut microbiota.
- Choose healthy sources of protein, mostly from plants (legumes and nuts).
- Higher intake of legumes, which are rich in protein and fiber, is associated with lower CVD risk, while higher nut intake is associated with lower risks of CVD, CHD, and stroke mortality/incidence. Replacing animal-source foods with plant-source whole foods, beyond health benefits, lowers the diet’s carbon footprint. Meat alternatives are often ultraprocessed and evidence on their short- and long-term health effects is limited. Unsaturated fats are preferred, as are lean, nonprocessed meats.
- Use liquid plant oils rather than tropical oils (coconut, palm, and palm kernel), animal fats (butter and lard), and partially hydrogenated fats. Saturated and trans fats (animal and dairy fats, and partially hydrogenated fat) should be replaced with nontropical liquid plant oils. Evidence supports cardiovascular benefits of dietary unsaturated fats, especially polyunsaturated fats primarily from plant oils (e.g. soybean, corn, safflower and sunflower oils, walnuts, and flax seeds).
- Choose minimally processed foods instead of ultraprocessed foods. Because of their proven association with adverse health outcomes, including overweight and obesity, cardiometabolic disorders (type 2 diabetes, CVD), and all-cause mortality, the consumption of many ultraprocessed foods is of concern. Ultraprocessed foods include artificial colors and flavors and preservatives that promote shelf stability, preserve texture, and increase palatability. A general principle is to emphasize unprocessed or minimally processed foods.
- Minimize intake of beverages and foods with added sugars. Added sugars (commonly glucose, dextrose, sucrose, corn syrup, honey, maple syrup, and concentrated fruit juice) are tied to elevated risk for type 2 diabetes, high cholesterol, and excess body weight. Findings from meta-analyses on body weight and metabolic outcomes for replacing added sugars with low-energy sweeteners are mixed, and the possibility of reverse causality has been raised.
- Choose and prepare foods with little or no salt. In general, the effects of sodium reduction on blood pressure tend to be higher in Black people, middle-aged and older people, and those with hypertension. In the United States, the main combined sources of sodium intake are processed foods, those prepared outside the home, packaged foods, and restaurant foods. Potassium-enriched salts are a promising alternative.
- If you don’t drink alcohol, don’t start; if you choose to drink, limit intake.
- While relationships between alcohol intake and cardiovascular outcomes are complex, the 2020 Dietary Guidelines Advisory Committee recently concluded that those who do drink should consume no more than one drink per day and should not drink alcohol in binges; the 2020 Dietary Guidelines for Americans continues to recommend no more than one drink per day for women and two drinks per day for men.
- Adhere to the guidance regardless in all settings. Food-based dietary guidance applies to all foods and beverages, regardless of where prepared, procured, and consumed. Policies should be enacted that encourage healthier default options (for example, whole grains, minimized sodium and sugar content).
Recognizing impediments
The AHA/ASA scientific statement closes with the declaration: “Creating an environment that facilitates, rather than impedes, adherence to heart-healthy dietary patterns among all individuals is a public health imperative.” It points to the National Institutes of Health’s 2020-2030 Strategic Plan for National Institutes of Health Nutrition Research, which focuses on precision nutrition as a means “to determine the impact on health of not only what individuals eat, but also of why, when, and how they eat throughout the life course.”
Ultimately, precision nutrition may provide personalized diets for CVD prevention. But the “food environment,” often conditioned by “rampant nutrition misinformation” through local, state, and federal practices and policies, may impede the adoption of heart-healthy dietary patterns. Factors such as targeted food marketing (for example, of processed food and beverages in minority neighborhoods), structural racism, neighborhood segregation, unhealthy built environments, and food insecurity create environments in which unhealthy foods are the default option.”
These factors compound adverse dietary and health effects, and underscore a need to “directly combat nutrition misinformation among the public and health care professionals.” They also explain why, despite widespread knowledge of heart-healthy dietary pattern components, little progress has been made in achieving dietary goals in the United States.
Dr. Lichtenstein’s office, in response to a request regarding AHA advocacy and consumer programs, provided the following links: Voices for Healthy Kids initiative site and choosing healthier processed foods and one on fresh, frozen, and canned fruits and vegetables.
Dr. Lichtenstein had no disclosures. Disclosures for the writing group members are included in the statement.
In a new scientific statement on diet and lifestyle recommendations, the American Heart Association is highlighting, for the first time, structural challenges that impede the adoption of heart-healthy dietary patterns.
This is in addition to stressing aspects of diet that improve cardiovascular health and reduce cardiovascular risk, with an emphasis on dietary patterns and food-based guidance beyond naming individual foods or nutrients.
The 2021 Dietary Guidance to Improve Cardiovascular Health scientific statement, developed under Alice H. Lichtenstein, DSc, chair of the AHA writing group, provides 10 evidence-based guidance recommendations to promote cardiometabolic health.
“The way to make heart-healthy choices every day,” said Dr. Lichtenstein, of the Jean Mayer USDA Human Nutrition Research Center on Aging at Tufts University in Boston, in a statement, “is to step back, look at the environment in which you eat, whether it be at home, at work, during social interaction, and then identify what the best choices are. And if there are no good choices, then think about how you can modify your environment so that there are good choices.”
The statement, published in Circulation, underscores growing evidence that nutrition-related chronic diseases have maternal-nutritional origins, and that prevention of pediatric obesity is a key to preserving and prolonging ideal cardiovascular health.
The features are as follows:
- Adjust energy intake and expenditure to achieve and maintain a healthy body weight. To counter the shift toward higher energy intake and more sedentary lifestyles over the past 3 decades, the statement recommends at least 150 minutes of moderate physical activity per week, adjusted for individual’s age, activity level, sex, and size.
- Eat plenty of fruits and vegetables; choose a wide variety. Observational and intervention studies document that dietary patterns rich in varied fruits and vegetables, with the exception of white potatoes, are linked to a lower risk of cardiovascular disease (CVD). Also, whole fruits and vegetables, which more readily provide fiber and satiety, are preferred over juices.
- Choose whole grain foods and products made mostly with whole grains rather than refined grains. Evidence from observational, interventional, and clinical studies confirm the benefits of frequent consumption of whole grains over infrequent consumption or over refined grains in terms of CVD risk, coronary heart disease (CHD), stroke, metabolic syndrome, cardiometabolic risk factors, laxation, and gut microbiota.
- Choose healthy sources of protein, mostly from plants (legumes and nuts).
- Higher intake of legumes, which are rich in protein and fiber, is associated with lower CVD risk, while higher nut intake is associated with lower risks of CVD, CHD, and stroke mortality/incidence. Replacing animal-source foods with plant-source whole foods, beyond health benefits, lowers the diet’s carbon footprint. Meat alternatives are often ultraprocessed and evidence on their short- and long-term health effects is limited. Unsaturated fats are preferred, as are lean, nonprocessed meats.
- Use liquid plant oils rather than tropical oils (coconut, palm, and palm kernel), animal fats (butter and lard), and partially hydrogenated fats. Saturated and trans fats (animal and dairy fats, and partially hydrogenated fat) should be replaced with nontropical liquid plant oils. Evidence supports cardiovascular benefits of dietary unsaturated fats, especially polyunsaturated fats primarily from plant oils (e.g. soybean, corn, safflower and sunflower oils, walnuts, and flax seeds).
- Choose minimally processed foods instead of ultraprocessed foods. Because of their proven association with adverse health outcomes, including overweight and obesity, cardiometabolic disorders (type 2 diabetes, CVD), and all-cause mortality, the consumption of many ultraprocessed foods is of concern. Ultraprocessed foods include artificial colors and flavors and preservatives that promote shelf stability, preserve texture, and increase palatability. A general principle is to emphasize unprocessed or minimally processed foods.
- Minimize intake of beverages and foods with added sugars. Added sugars (commonly glucose, dextrose, sucrose, corn syrup, honey, maple syrup, and concentrated fruit juice) are tied to elevated risk for type 2 diabetes, high cholesterol, and excess body weight. Findings from meta-analyses on body weight and metabolic outcomes for replacing added sugars with low-energy sweeteners are mixed, and the possibility of reverse causality has been raised.
- Choose and prepare foods with little or no salt. In general, the effects of sodium reduction on blood pressure tend to be higher in Black people, middle-aged and older people, and those with hypertension. In the United States, the main combined sources of sodium intake are processed foods, those prepared outside the home, packaged foods, and restaurant foods. Potassium-enriched salts are a promising alternative.
- If you don’t drink alcohol, don’t start; if you choose to drink, limit intake.
- While relationships between alcohol intake and cardiovascular outcomes are complex, the 2020 Dietary Guidelines Advisory Committee recently concluded that those who do drink should consume no more than one drink per day and should not drink alcohol in binges; the 2020 Dietary Guidelines for Americans continues to recommend no more than one drink per day for women and two drinks per day for men.
- Adhere to the guidance regardless in all settings. Food-based dietary guidance applies to all foods and beverages, regardless of where prepared, procured, and consumed. Policies should be enacted that encourage healthier default options (for example, whole grains, minimized sodium and sugar content).
Recognizing impediments
The AHA/ASA scientific statement closes with the declaration: “Creating an environment that facilitates, rather than impedes, adherence to heart-healthy dietary patterns among all individuals is a public health imperative.” It points to the National Institutes of Health’s 2020-2030 Strategic Plan for National Institutes of Health Nutrition Research, which focuses on precision nutrition as a means “to determine the impact on health of not only what individuals eat, but also of why, when, and how they eat throughout the life course.”
Ultimately, precision nutrition may provide personalized diets for CVD prevention. But the “food environment,” often conditioned by “rampant nutrition misinformation” through local, state, and federal practices and policies, may impede the adoption of heart-healthy dietary patterns. Factors such as targeted food marketing (for example, of processed food and beverages in minority neighborhoods), structural racism, neighborhood segregation, unhealthy built environments, and food insecurity create environments in which unhealthy foods are the default option.”
These factors compound adverse dietary and health effects, and underscore a need to “directly combat nutrition misinformation among the public and health care professionals.” They also explain why, despite widespread knowledge of heart-healthy dietary pattern components, little progress has been made in achieving dietary goals in the United States.
Dr. Lichtenstein’s office, in response to a request regarding AHA advocacy and consumer programs, provided the following links: Voices for Healthy Kids initiative site and choosing healthier processed foods and one on fresh, frozen, and canned fruits and vegetables.
Dr. Lichtenstein had no disclosures. Disclosures for the writing group members are included in the statement.
FROM CIRCULATION
Feds launch COVID-19 worker vaccine mandates
The Biden administration on Nov. 4 unveiled its rule to require most of the country’s larger employers to mandate workers be fully vaccinated against COVID-19, but set a Jan. 4 deadline, avoiding the busy holiday season.
The White House also shifted the time lines for earlier mandates applying to federal workers and contractors to Jan. 4. And the same deadline applies to a new separate rule for health care workers.
The new rules are meant to preempt “any inconsistent state or local laws,” including bans and limits on employers’ authority to require vaccination, masks, or testing, the White House said in a statement.
The rule on employers from the Occupational Safety and Health Administration will apply to organizations with 100 or more employees. These employers will need to make sure each worker is fully vaccinated or tests for COVID-19 on at least a weekly basis. The OSHA rule will also require that employers provide paid time for employees to get vaccinated and ensure that all unvaccinated workers wear a face mask in the workplace. This rule will cover 84 million employees. The OSHA rule will not apply to workplaces covered by either the Centers for Medicare & Medicaid Services rule or the federal contractor vaccination requirement
“The virus will not go away by itself, or because we wish it away: We have to act,” President Joe Biden said in a statement. “Vaccination is the single best pathway out of this pandemic.”
Mandates were not the preferred route to managing the pandemic, he said.
“Too many people remain unvaccinated for us to get out of this pandemic for good,” he said. “So I instituted requirements – and they are working.”
The White House said 70% percent of U.S. adults are now fully vaccinated – up from less than 1% when Mr. Biden took office in January.
The CMS vaccine rule is meant to cover more than 17 million workers and about 76,000 medical care sites, including hospitals, ambulatory surgery centers, nursing homes, dialysis facilities, home health agencies, and long-term care facilities. The rule will apply to employees whether their positions involve patient care or not.
Unlike the OSHA mandate, the one for health care workers will not offer the option of frequent COVID-19 testing instead of vaccination. There is a “higher bar” for health care workers, given their role in treating patients, so the mandate allows only for vaccination or limited exemptions, a senior administration official said on Nov. 3 during a call with reporters.
The CMS rule includes a “range of remedies,” including penalties and denial of payment for health care facilities that fail to meet the vaccine mandate. CMS could theoretically cut off hospitals and other medical organizations for failure to comply, but that would be a “last resort,” a senior administration official said. CMS will instead work with health care facilities to help them comply with the federal rule on vaccination of medical workers.
The new CMS rules apply only to Medicare- and Medicaid-certified centers and organizations. The rule does not directly apply to other health care entities, such as doctor’s offices, that are not regulated by CMS.
“Most states have separate licensing requirements for health care staff and health care providers that would be applicable to physician office staff and other staff in small health care entities that are not subject to vaccination requirements under this IFC,” CMS said in the rule.
A version of this article first appeared on WebMD.com.
The Biden administration on Nov. 4 unveiled its rule to require most of the country’s larger employers to mandate workers be fully vaccinated against COVID-19, but set a Jan. 4 deadline, avoiding the busy holiday season.
The White House also shifted the time lines for earlier mandates applying to federal workers and contractors to Jan. 4. And the same deadline applies to a new separate rule for health care workers.
The new rules are meant to preempt “any inconsistent state or local laws,” including bans and limits on employers’ authority to require vaccination, masks, or testing, the White House said in a statement.
The rule on employers from the Occupational Safety and Health Administration will apply to organizations with 100 or more employees. These employers will need to make sure each worker is fully vaccinated or tests for COVID-19 on at least a weekly basis. The OSHA rule will also require that employers provide paid time for employees to get vaccinated and ensure that all unvaccinated workers wear a face mask in the workplace. This rule will cover 84 million employees. The OSHA rule will not apply to workplaces covered by either the Centers for Medicare & Medicaid Services rule or the federal contractor vaccination requirement
“The virus will not go away by itself, or because we wish it away: We have to act,” President Joe Biden said in a statement. “Vaccination is the single best pathway out of this pandemic.”
Mandates were not the preferred route to managing the pandemic, he said.
“Too many people remain unvaccinated for us to get out of this pandemic for good,” he said. “So I instituted requirements – and they are working.”
The White House said 70% percent of U.S. adults are now fully vaccinated – up from less than 1% when Mr. Biden took office in January.
The CMS vaccine rule is meant to cover more than 17 million workers and about 76,000 medical care sites, including hospitals, ambulatory surgery centers, nursing homes, dialysis facilities, home health agencies, and long-term care facilities. The rule will apply to employees whether their positions involve patient care or not.
Unlike the OSHA mandate, the one for health care workers will not offer the option of frequent COVID-19 testing instead of vaccination. There is a “higher bar” for health care workers, given their role in treating patients, so the mandate allows only for vaccination or limited exemptions, a senior administration official said on Nov. 3 during a call with reporters.
The CMS rule includes a “range of remedies,” including penalties and denial of payment for health care facilities that fail to meet the vaccine mandate. CMS could theoretically cut off hospitals and other medical organizations for failure to comply, but that would be a “last resort,” a senior administration official said. CMS will instead work with health care facilities to help them comply with the federal rule on vaccination of medical workers.
The new CMS rules apply only to Medicare- and Medicaid-certified centers and organizations. The rule does not directly apply to other health care entities, such as doctor’s offices, that are not regulated by CMS.
“Most states have separate licensing requirements for health care staff and health care providers that would be applicable to physician office staff and other staff in small health care entities that are not subject to vaccination requirements under this IFC,” CMS said in the rule.
A version of this article first appeared on WebMD.com.
The Biden administration on Nov. 4 unveiled its rule to require most of the country’s larger employers to mandate workers be fully vaccinated against COVID-19, but set a Jan. 4 deadline, avoiding the busy holiday season.
The White House also shifted the time lines for earlier mandates applying to federal workers and contractors to Jan. 4. And the same deadline applies to a new separate rule for health care workers.
The new rules are meant to preempt “any inconsistent state or local laws,” including bans and limits on employers’ authority to require vaccination, masks, or testing, the White House said in a statement.
The rule on employers from the Occupational Safety and Health Administration will apply to organizations with 100 or more employees. These employers will need to make sure each worker is fully vaccinated or tests for COVID-19 on at least a weekly basis. The OSHA rule will also require that employers provide paid time for employees to get vaccinated and ensure that all unvaccinated workers wear a face mask in the workplace. This rule will cover 84 million employees. The OSHA rule will not apply to workplaces covered by either the Centers for Medicare & Medicaid Services rule or the federal contractor vaccination requirement
“The virus will not go away by itself, or because we wish it away: We have to act,” President Joe Biden said in a statement. “Vaccination is the single best pathway out of this pandemic.”
Mandates were not the preferred route to managing the pandemic, he said.
“Too many people remain unvaccinated for us to get out of this pandemic for good,” he said. “So I instituted requirements – and they are working.”
The White House said 70% percent of U.S. adults are now fully vaccinated – up from less than 1% when Mr. Biden took office in January.
The CMS vaccine rule is meant to cover more than 17 million workers and about 76,000 medical care sites, including hospitals, ambulatory surgery centers, nursing homes, dialysis facilities, home health agencies, and long-term care facilities. The rule will apply to employees whether their positions involve patient care or not.
Unlike the OSHA mandate, the one for health care workers will not offer the option of frequent COVID-19 testing instead of vaccination. There is a “higher bar” for health care workers, given their role in treating patients, so the mandate allows only for vaccination or limited exemptions, a senior administration official said on Nov. 3 during a call with reporters.
The CMS rule includes a “range of remedies,” including penalties and denial of payment for health care facilities that fail to meet the vaccine mandate. CMS could theoretically cut off hospitals and other medical organizations for failure to comply, but that would be a “last resort,” a senior administration official said. CMS will instead work with health care facilities to help them comply with the federal rule on vaccination of medical workers.
The new CMS rules apply only to Medicare- and Medicaid-certified centers and organizations. The rule does not directly apply to other health care entities, such as doctor’s offices, that are not regulated by CMS.
“Most states have separate licensing requirements for health care staff and health care providers that would be applicable to physician office staff and other staff in small health care entities that are not subject to vaccination requirements under this IFC,” CMS said in the rule.
A version of this article first appeared on WebMD.com.
How to screen for prediabetes and type 2 diabetes in an ObGyn practice
The prevalence of T2DM is on the rise in the United States, and T2DM is currently the 7th leading cause of death.1 In a study of 28,143 participants in the US National Health and Nutrition Examination Survey (NHANES) who were 18 years or older, the prevalence of diabetes increased from 9.8% to 14.3% between 2000 and 2008.2 About 24% of the participants had undiagnosed diabetes prior to the testing they received as a study participant.2 People from minority groups have a higher rate of T2DM than non-Hispanic White people. Using data from 2018, the Centers for Disease Control and Prevention reported that the prevalence of diagnosed diabetes was highest among American Indians/Alaska Natives (14.7%), people of Hispanic origin (12.5%), and non-Hispanic Blacks (11.7%), followed by non-Hispanic Asians (9.2%) and non-Hispanic Whites (7.5%).1 Diabetes is a major risk factor for myocardial infarction, stroke, renal failure, retinopathy, peripheral vascular disease, and neuropathy.1 Early detection and treatment of both prediabetes and diabetes may improve health and reduce these preventable complications, saving lives, preventing heart and renal failure and blindness.
T2DM is caused by a combination of insulin resistance and insufficient pancreatic secretion of insulin to overcome the insulin resistance.3 In young adults with insulin resistance, pancreatic secretion of insulin is often sufficient to overcome the insulin resistance resulting in normal glucose levels and persistently increased insulin concentration. As individuals with insulin resistance age, pancreatic secretion of insulin may decline, resulting in insufficient production of insulin and rising glucose levels. Many individuals experience a prolonged stage of prediabetes that may be present for decades prior to transitioning to T2DM. In 2020, 35% of US adults were reported to have prediabetes.1
Screening for diabetes mellitus
The US Preventive Services Task Force (USPSTF) recently recommended that all adults aged 35 to 70 years who are overweight or obese be screened for T2DM (B recommendation).4 Screening for diabetes will also result in detecting many people with prediabetes. The criteria for diagnosing diabetes and prediabetes are presented in the TABLE. Based on cohort studies, the USPSTF noted that screening every 3 years is a reasonable approach.4 They also recommended that people diagnosed with prediabetes should initiate preventive measures, including optimizing diet, weight loss, exercise, and in some cases, medication treatment such as metformin.5
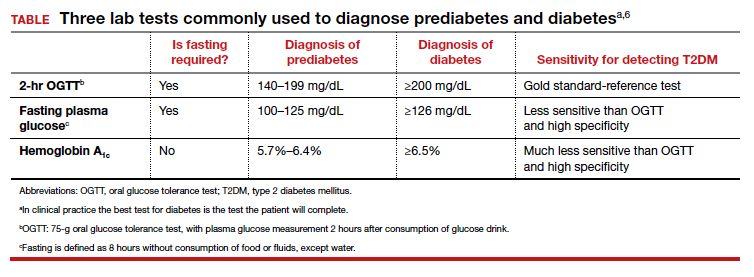
Approaches to the diagnosis of diabetes and prediabetes
Three laboratory tests are widely utilized for the diagnosis of prediabetes and diabetes: measurement of a plasma glucose 2 hours following consumption of oral glucose 75 g (2-hr oral glucose tolerance test [OGTT]), measurement of a fasting plasma glucose, and measurement of hemoglobin A1c (see Table).6In clinical practice, the best diabetes screening test is the test the patient will complete. Most evidence indicates that, compared with the 2-hr OGTT, a hemoglobin A1c measurement is specific for diagnosing T2DM, but not sensitive. In other words, if the hemoglobin A1c is ≥6.5%, the glucose measurement 2 hours following an OGTT will very likely be ≥200 mg/dL. But if the hemoglobin A1c is between 5.7% and 6.5%, the person might be diagnosed with T2DM if they had a 2-hr OGTT.6
In one study, 1,241 nondiabetic, overweight, or obese participants had all 3 tests to diagnose T2DM.7 The 2-hr OGTT diagnosed T2DM in 148 participants (12%). However, the hemoglobin A1c test only diagnosed T2DM in 78 of the 148 participants who were diagnosed with T2DM based on the 2-hr OGTT, missing 47% of the cases of T2DM. In this study, using the 2-hr OGTT as the “gold standard” reference test, the hemoglobin A1c test had a sensitivity of 53% and specificity of 97%.7
In clinical practice one approach is to explain to the patient the pros and cons of the 3 tests for T2DM and ask them to select the test they prefer to complete. In a high-risk population, including people with obesity, completing any of the 3 tests is better than not testing for diabetes. It also should be noted that, among people who have a normal body mass index (BMI), a “prediabetes” diagnosis is controversial. Compared with obese persons with prediabetes, people with a normal BMI and prediabetes diagnosed by a blood test progress to diabetes at a much lower rate. The value of diagnosing prediabetes after 70 years of age is also controversial because few people in this situation progress to diabetes.8 Clinicians should be cautious about diagnosing prediabetes in lean or elderly people.
The reliability of the hemoglobin A1c test is reduced in conditions associated with increased red blood cell turnover, including sickle cell disease, pregnancy (second and third trimesters), hemodialysis, recent blood transfusions or erythropoietin therapy. In these clinical situations, only blood glucose measurements should be used to diagnose prediabetes and T2DM.6 It should be noted that concordance among any of the 3 tests is not perfect.6
Continue to: A 2-step approach to diagnosing T2DM...
A 2-step approach to diagnosing T2DM
An alternative to relying on a single test for T2DM is to use a 2-step approach for screening. The first step is a hemoglobin A1c measurement, which neither requires fasting nor waiting for 2 hours for post–glucose load blood draw. If the hemoglobin A1c result is ≥6.5%, a T2DM diagnosis can be made, with no additional testing. If the hemoglobin A1c result is 5.7% to 6.4%, the person probably has either prediabetes or diabetes and can be offered a 2-hr OGTT to definitively determine if T2DM is the proper diagnosis. If the hemoglobin A1c test is <5.7%, it is unlikely that the person has T2DM or prediabetes at the time of the test. In this situation, the testing could be repeated in 3 years. Using a 2-step approach reduces the number of people who are tested with a 2-hr OGTT and detects more cases of T2DM than a 1-step approach that relies on a hemoglobin A1c measurement alone.
Treatment of prediabetes is warranted in people at high risk for developing diabetes
It is better to prevent diabetes among people with a high risk of diabetes than to treat diabetes once it is established. People with prediabetes who are overweight or obese are at high risk for developing diabetes. Prediabetes is diagnosed by a fasting plasma glucose level of 100 to 125 mg/dL or a hemoglobin A1c measurement of 5.7% to 6.4%.
High-quality randomized clinical trials have definitively demonstrated that, among people at high risk for developing diabetes, lifestyle modification and metformin treatment reduce the risk of developing diabetes. In the Diabetes Prevention Program (DPP) 3,234 people with a high risk of diabetes, mean BMI 34 kg/m2, were randomly assigned to 1 of 3 groups9:
- a control group
- metformin (850 mg twice daily) or
- lifestyle modification that included exercise (moderate intensity exercise for 150 minutes per week and weight loss (7% of body weight using a low-calorie, low-fat diet).
At 2.8 years of follow-up the incidence of diabetes was 11%, 7.8%, and 4.8% per 100 person-years in the people assigned to the control, metformin, and lifestyle modification groups, respectively.9 In the DPP study, compared with the control group, metformin was most effective in decreasing the risk of transitioning to diabetes in people who had a BMI ≥35 kg/m2 (53% reduction in risk) or a BMI from 30 to 35 kg/m2 (16% reduction in risk).9 Metformin was not as effective at preventing the transition to diabetes in people who had a normal BMI or who were overweight (3% reduction).9
In the Finnish Diabetes Prevention Study, 522 obese people with impaired glucose tolerance were randomly assigned to lifestyle modification or a control group. After 4 years, the cumulative incidence of diabetes was 11% and 23% in the lifestyle modification and control groups, respectively.10 A meta-analysis of 23 randomized clinical trials reported that, among people with a high risk of developing diabetes, compared with no intervention (control group), lifestyle modification, including dieting, exercising, and weight loss significantly reduced the risk of developing diabetes (pooled relative risk [RR], 0.78; 95% confidence interval [CI], 0.69‒0.88).5
In clinical practice, offering a patient at high risk for diabetes a suite of options, including5,9,10:
- a formal nutrition consult with the goal of targeting a 7% reduction in weight
- recommending moderate intensity exercise, 150 minutes weekly
- metformin treatment, if the patient is obese
would reduce the patient’s risk of developing diabetes.
Treatment of T2DM is complex
For people with T2DM, a widely recommended treatment goal is to reduce the hemoglobin A1c measurement to ≤7%. Initial treatment includes a comprehensive diabetes self-management education program, weight loss using diet and exercise, and metformin treatment. Metformin may be associated with an increased risk of lactic acidosis, especially in people with renal insufficiency. The US Food and Drug Administration (FDA) recommends against initiating metformin therapy for people with an estimated glomerular filtration rate (eGFR) of 30 to 45 mL/min/1.73 m2. The FDA determined that metformin is contraindicated in people with an eGFR of <30 mL/min/1.73 m2.11 Many people with T2DM will require treatment with multiple pharmacologic agents to achieve a hemoglobin A1c ≤7%. In addition to metformin, pharmacologic agents used to treat T2DM include insulin, sulfonylureas, glucagon-like peptide-1(GLP-1) receptor agonists, a sodium glucose cotransporter (SGLT2) inhibitor, dipeptidyl peptidase-4 (DPP-4) inhibitors, or an alpha-glucosidase inhibitor. Given the complexity of managing T2DM over a lifetime, most individuals with T2DM receive their diabetes care from a primary care clinician or subspecialist in endocrinology.
Experts predict that, within the next 8 years, the prevalence of obesity among adults in the United States will be approximately 50%.12 The US health care system has not been effective in controlling the obesity epidemic. Our failure to control the obesity epidemic will result in an increase in the prevalence of prediabetes and T2DM, leading to a rise in cardiovascular, renal, and eye disease. The diagnosis of prediabetes and diabetes is within the scope of practice of obstetrics and gynecology. The treatment of prediabetes is also within the scope of ObGyns, who have both expertise and familiarity in the diagnosis of gestational diabetes, a form of prediabetes. ●
- Centers for Disease Control and Prevention. National Diabetes Statistics Report. 2020. https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. Accessed October 26, 2021.
- Wang L, Li X, Wang Z, et al. Trends in prevalence of diabetes and control of risk factors in diabetes among U.S. adults, 1999-2018. JAMA. 2021;326:1-13. doi: 10.1001/jama.2021.9883.
- Type 2 diabetes. Centers for Disease Control and Prevention website. . Last reviewed August 10, 2021 Accessed October 27, 2021.
- US Preventive Services Task Force. Screening for prediabetes and diabetes. US Preventive Services Task Force Recommendation Statement. JAMA. 2021;326:736-743. doi: 10.1001/jama.2021.12531.
- Jonas D, Crotty K, Yun JD, et al. Screening for prediabetes and type 2 diabetes mellitus: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2021;326:744-760. doi: 10.1001/jama.2021.10403.
- American Diabetes Association. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes‒2020. Diabetes Care. 2020;43(suppl 1):S14-S31. doi: 10.2337/dc20-S002.
- Meijnikman AS, De Block CE, Dirinck E, et al. Not performing an OGTT results in significant under diagnosis of (pre)diabetes in a high-risk adult Caucasian population. Int J Obes. 2017;41:1615-1620. doi: 10.1038/ijo.2017.165.
- Rooney MR, Rawlings AM, Pankow JS, et al. Risk of progression to diabetes among older adults with prediabetes. JAMA Intern Med. 2021;181:511-519. doi: 10.1001/jamainternmed.2020.8774.
- Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393-403. doi: 10.1056/NEJMoa012512.
- Tuomilehto J, Lindström J, Eriksson JG, et al; Finnish Diabetes Prevention Study Group. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344:1343-1350. doi: 10.1056/NEJM200105033441801.
- Glucophage [package insert]. Princeton, NJ: Bristol Meyers Squibb; April 2017. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017020357s037s039,021202s021s023lbl.pdf. Accessed October 27, 2021.
- Ward ZJ, Bleich SN, Cradock AL, et al. Projected U.S. state-level prevalence of adult obesity and severe obesity. N Engl J Med. 2019;381;2440-2450. doi: 10.1056/NEJMc1917339.
The prevalence of T2DM is on the rise in the United States, and T2DM is currently the 7th leading cause of death.1 In a study of 28,143 participants in the US National Health and Nutrition Examination Survey (NHANES) who were 18 years or older, the prevalence of diabetes increased from 9.8% to 14.3% between 2000 and 2008.2 About 24% of the participants had undiagnosed diabetes prior to the testing they received as a study participant.2 People from minority groups have a higher rate of T2DM than non-Hispanic White people. Using data from 2018, the Centers for Disease Control and Prevention reported that the prevalence of diagnosed diabetes was highest among American Indians/Alaska Natives (14.7%), people of Hispanic origin (12.5%), and non-Hispanic Blacks (11.7%), followed by non-Hispanic Asians (9.2%) and non-Hispanic Whites (7.5%).1 Diabetes is a major risk factor for myocardial infarction, stroke, renal failure, retinopathy, peripheral vascular disease, and neuropathy.1 Early detection and treatment of both prediabetes and diabetes may improve health and reduce these preventable complications, saving lives, preventing heart and renal failure and blindness.
T2DM is caused by a combination of insulin resistance and insufficient pancreatic secretion of insulin to overcome the insulin resistance.3 In young adults with insulin resistance, pancreatic secretion of insulin is often sufficient to overcome the insulin resistance resulting in normal glucose levels and persistently increased insulin concentration. As individuals with insulin resistance age, pancreatic secretion of insulin may decline, resulting in insufficient production of insulin and rising glucose levels. Many individuals experience a prolonged stage of prediabetes that may be present for decades prior to transitioning to T2DM. In 2020, 35% of US adults were reported to have prediabetes.1
Screening for diabetes mellitus
The US Preventive Services Task Force (USPSTF) recently recommended that all adults aged 35 to 70 years who are overweight or obese be screened for T2DM (B recommendation).4 Screening for diabetes will also result in detecting many people with prediabetes. The criteria for diagnosing diabetes and prediabetes are presented in the TABLE. Based on cohort studies, the USPSTF noted that screening every 3 years is a reasonable approach.4 They also recommended that people diagnosed with prediabetes should initiate preventive measures, including optimizing diet, weight loss, exercise, and in some cases, medication treatment such as metformin.5

Approaches to the diagnosis of diabetes and prediabetes
Three laboratory tests are widely utilized for the diagnosis of prediabetes and diabetes: measurement of a plasma glucose 2 hours following consumption of oral glucose 75 g (2-hr oral glucose tolerance test [OGTT]), measurement of a fasting plasma glucose, and measurement of hemoglobin A1c (see Table).6In clinical practice, the best diabetes screening test is the test the patient will complete. Most evidence indicates that, compared with the 2-hr OGTT, a hemoglobin A1c measurement is specific for diagnosing T2DM, but not sensitive. In other words, if the hemoglobin A1c is ≥6.5%, the glucose measurement 2 hours following an OGTT will very likely be ≥200 mg/dL. But if the hemoglobin A1c is between 5.7% and 6.5%, the person might be diagnosed with T2DM if they had a 2-hr OGTT.6
In one study, 1,241 nondiabetic, overweight, or obese participants had all 3 tests to diagnose T2DM.7 The 2-hr OGTT diagnosed T2DM in 148 participants (12%). However, the hemoglobin A1c test only diagnosed T2DM in 78 of the 148 participants who were diagnosed with T2DM based on the 2-hr OGTT, missing 47% of the cases of T2DM. In this study, using the 2-hr OGTT as the “gold standard” reference test, the hemoglobin A1c test had a sensitivity of 53% and specificity of 97%.7
In clinical practice one approach is to explain to the patient the pros and cons of the 3 tests for T2DM and ask them to select the test they prefer to complete. In a high-risk population, including people with obesity, completing any of the 3 tests is better than not testing for diabetes. It also should be noted that, among people who have a normal body mass index (BMI), a “prediabetes” diagnosis is controversial. Compared with obese persons with prediabetes, people with a normal BMI and prediabetes diagnosed by a blood test progress to diabetes at a much lower rate. The value of diagnosing prediabetes after 70 years of age is also controversial because few people in this situation progress to diabetes.8 Clinicians should be cautious about diagnosing prediabetes in lean or elderly people.
The reliability of the hemoglobin A1c test is reduced in conditions associated with increased red blood cell turnover, including sickle cell disease, pregnancy (second and third trimesters), hemodialysis, recent blood transfusions or erythropoietin therapy. In these clinical situations, only blood glucose measurements should be used to diagnose prediabetes and T2DM.6 It should be noted that concordance among any of the 3 tests is not perfect.6
Continue to: A 2-step approach to diagnosing T2DM...
A 2-step approach to diagnosing T2DM
An alternative to relying on a single test for T2DM is to use a 2-step approach for screening. The first step is a hemoglobin A1c measurement, which neither requires fasting nor waiting for 2 hours for post–glucose load blood draw. If the hemoglobin A1c result is ≥6.5%, a T2DM diagnosis can be made, with no additional testing. If the hemoglobin A1c result is 5.7% to 6.4%, the person probably has either prediabetes or diabetes and can be offered a 2-hr OGTT to definitively determine if T2DM is the proper diagnosis. If the hemoglobin A1c test is <5.7%, it is unlikely that the person has T2DM or prediabetes at the time of the test. In this situation, the testing could be repeated in 3 years. Using a 2-step approach reduces the number of people who are tested with a 2-hr OGTT and detects more cases of T2DM than a 1-step approach that relies on a hemoglobin A1c measurement alone.
Treatment of prediabetes is warranted in people at high risk for developing diabetes
It is better to prevent diabetes among people with a high risk of diabetes than to treat diabetes once it is established. People with prediabetes who are overweight or obese are at high risk for developing diabetes. Prediabetes is diagnosed by a fasting plasma glucose level of 100 to 125 mg/dL or a hemoglobin A1c measurement of 5.7% to 6.4%.
High-quality randomized clinical trials have definitively demonstrated that, among people at high risk for developing diabetes, lifestyle modification and metformin treatment reduce the risk of developing diabetes. In the Diabetes Prevention Program (DPP) 3,234 people with a high risk of diabetes, mean BMI 34 kg/m2, were randomly assigned to 1 of 3 groups9:
- a control group
- metformin (850 mg twice daily) or
- lifestyle modification that included exercise (moderate intensity exercise for 150 minutes per week and weight loss (7% of body weight using a low-calorie, low-fat diet).
At 2.8 years of follow-up the incidence of diabetes was 11%, 7.8%, and 4.8% per 100 person-years in the people assigned to the control, metformin, and lifestyle modification groups, respectively.9 In the DPP study, compared with the control group, metformin was most effective in decreasing the risk of transitioning to diabetes in people who had a BMI ≥35 kg/m2 (53% reduction in risk) or a BMI from 30 to 35 kg/m2 (16% reduction in risk).9 Metformin was not as effective at preventing the transition to diabetes in people who had a normal BMI or who were overweight (3% reduction).9
In the Finnish Diabetes Prevention Study, 522 obese people with impaired glucose tolerance were randomly assigned to lifestyle modification or a control group. After 4 years, the cumulative incidence of diabetes was 11% and 23% in the lifestyle modification and control groups, respectively.10 A meta-analysis of 23 randomized clinical trials reported that, among people with a high risk of developing diabetes, compared with no intervention (control group), lifestyle modification, including dieting, exercising, and weight loss significantly reduced the risk of developing diabetes (pooled relative risk [RR], 0.78; 95% confidence interval [CI], 0.69‒0.88).5
In clinical practice, offering a patient at high risk for diabetes a suite of options, including5,9,10:
- a formal nutrition consult with the goal of targeting a 7% reduction in weight
- recommending moderate intensity exercise, 150 minutes weekly
- metformin treatment, if the patient is obese
would reduce the patient’s risk of developing diabetes.
Treatment of T2DM is complex
For people with T2DM, a widely recommended treatment goal is to reduce the hemoglobin A1c measurement to ≤7%. Initial treatment includes a comprehensive diabetes self-management education program, weight loss using diet and exercise, and metformin treatment. Metformin may be associated with an increased risk of lactic acidosis, especially in people with renal insufficiency. The US Food and Drug Administration (FDA) recommends against initiating metformin therapy for people with an estimated glomerular filtration rate (eGFR) of 30 to 45 mL/min/1.73 m2. The FDA determined that metformin is contraindicated in people with an eGFR of <30 mL/min/1.73 m2.11 Many people with T2DM will require treatment with multiple pharmacologic agents to achieve a hemoglobin A1c ≤7%. In addition to metformin, pharmacologic agents used to treat T2DM include insulin, sulfonylureas, glucagon-like peptide-1(GLP-1) receptor agonists, a sodium glucose cotransporter (SGLT2) inhibitor, dipeptidyl peptidase-4 (DPP-4) inhibitors, or an alpha-glucosidase inhibitor. Given the complexity of managing T2DM over a lifetime, most individuals with T2DM receive their diabetes care from a primary care clinician or subspecialist in endocrinology.
Experts predict that, within the next 8 years, the prevalence of obesity among adults in the United States will be approximately 50%.12 The US health care system has not been effective in controlling the obesity epidemic. Our failure to control the obesity epidemic will result in an increase in the prevalence of prediabetes and T2DM, leading to a rise in cardiovascular, renal, and eye disease. The diagnosis of prediabetes and diabetes is within the scope of practice of obstetrics and gynecology. The treatment of prediabetes is also within the scope of ObGyns, who have both expertise and familiarity in the diagnosis of gestational diabetes, a form of prediabetes. ●
The prevalence of T2DM is on the rise in the United States, and T2DM is currently the 7th leading cause of death.1 In a study of 28,143 participants in the US National Health and Nutrition Examination Survey (NHANES) who were 18 years or older, the prevalence of diabetes increased from 9.8% to 14.3% between 2000 and 2008.2 About 24% of the participants had undiagnosed diabetes prior to the testing they received as a study participant.2 People from minority groups have a higher rate of T2DM than non-Hispanic White people. Using data from 2018, the Centers for Disease Control and Prevention reported that the prevalence of diagnosed diabetes was highest among American Indians/Alaska Natives (14.7%), people of Hispanic origin (12.5%), and non-Hispanic Blacks (11.7%), followed by non-Hispanic Asians (9.2%) and non-Hispanic Whites (7.5%).1 Diabetes is a major risk factor for myocardial infarction, stroke, renal failure, retinopathy, peripheral vascular disease, and neuropathy.1 Early detection and treatment of both prediabetes and diabetes may improve health and reduce these preventable complications, saving lives, preventing heart and renal failure and blindness.
T2DM is caused by a combination of insulin resistance and insufficient pancreatic secretion of insulin to overcome the insulin resistance.3 In young adults with insulin resistance, pancreatic secretion of insulin is often sufficient to overcome the insulin resistance resulting in normal glucose levels and persistently increased insulin concentration. As individuals with insulin resistance age, pancreatic secretion of insulin may decline, resulting in insufficient production of insulin and rising glucose levels. Many individuals experience a prolonged stage of prediabetes that may be present for decades prior to transitioning to T2DM. In 2020, 35% of US adults were reported to have prediabetes.1
Screening for diabetes mellitus
The US Preventive Services Task Force (USPSTF) recently recommended that all adults aged 35 to 70 years who are overweight or obese be screened for T2DM (B recommendation).4 Screening for diabetes will also result in detecting many people with prediabetes. The criteria for diagnosing diabetes and prediabetes are presented in the TABLE. Based on cohort studies, the USPSTF noted that screening every 3 years is a reasonable approach.4 They also recommended that people diagnosed with prediabetes should initiate preventive measures, including optimizing diet, weight loss, exercise, and in some cases, medication treatment such as metformin.5

Approaches to the diagnosis of diabetes and prediabetes
Three laboratory tests are widely utilized for the diagnosis of prediabetes and diabetes: measurement of a plasma glucose 2 hours following consumption of oral glucose 75 g (2-hr oral glucose tolerance test [OGTT]), measurement of a fasting plasma glucose, and measurement of hemoglobin A1c (see Table).6In clinical practice, the best diabetes screening test is the test the patient will complete. Most evidence indicates that, compared with the 2-hr OGTT, a hemoglobin A1c measurement is specific for diagnosing T2DM, but not sensitive. In other words, if the hemoglobin A1c is ≥6.5%, the glucose measurement 2 hours following an OGTT will very likely be ≥200 mg/dL. But if the hemoglobin A1c is between 5.7% and 6.5%, the person might be diagnosed with T2DM if they had a 2-hr OGTT.6
In one study, 1,241 nondiabetic, overweight, or obese participants had all 3 tests to diagnose T2DM.7 The 2-hr OGTT diagnosed T2DM in 148 participants (12%). However, the hemoglobin A1c test only diagnosed T2DM in 78 of the 148 participants who were diagnosed with T2DM based on the 2-hr OGTT, missing 47% of the cases of T2DM. In this study, using the 2-hr OGTT as the “gold standard” reference test, the hemoglobin A1c test had a sensitivity of 53% and specificity of 97%.7
In clinical practice one approach is to explain to the patient the pros and cons of the 3 tests for T2DM and ask them to select the test they prefer to complete. In a high-risk population, including people with obesity, completing any of the 3 tests is better than not testing for diabetes. It also should be noted that, among people who have a normal body mass index (BMI), a “prediabetes” diagnosis is controversial. Compared with obese persons with prediabetes, people with a normal BMI and prediabetes diagnosed by a blood test progress to diabetes at a much lower rate. The value of diagnosing prediabetes after 70 years of age is also controversial because few people in this situation progress to diabetes.8 Clinicians should be cautious about diagnosing prediabetes in lean or elderly people.
The reliability of the hemoglobin A1c test is reduced in conditions associated with increased red blood cell turnover, including sickle cell disease, pregnancy (second and third trimesters), hemodialysis, recent blood transfusions or erythropoietin therapy. In these clinical situations, only blood glucose measurements should be used to diagnose prediabetes and T2DM.6 It should be noted that concordance among any of the 3 tests is not perfect.6
Continue to: A 2-step approach to diagnosing T2DM...
A 2-step approach to diagnosing T2DM
An alternative to relying on a single test for T2DM is to use a 2-step approach for screening. The first step is a hemoglobin A1c measurement, which neither requires fasting nor waiting for 2 hours for post–glucose load blood draw. If the hemoglobin A1c result is ≥6.5%, a T2DM diagnosis can be made, with no additional testing. If the hemoglobin A1c result is 5.7% to 6.4%, the person probably has either prediabetes or diabetes and can be offered a 2-hr OGTT to definitively determine if T2DM is the proper diagnosis. If the hemoglobin A1c test is <5.7%, it is unlikely that the person has T2DM or prediabetes at the time of the test. In this situation, the testing could be repeated in 3 years. Using a 2-step approach reduces the number of people who are tested with a 2-hr OGTT and detects more cases of T2DM than a 1-step approach that relies on a hemoglobin A1c measurement alone.
Treatment of prediabetes is warranted in people at high risk for developing diabetes
It is better to prevent diabetes among people with a high risk of diabetes than to treat diabetes once it is established. People with prediabetes who are overweight or obese are at high risk for developing diabetes. Prediabetes is diagnosed by a fasting plasma glucose level of 100 to 125 mg/dL or a hemoglobin A1c measurement of 5.7% to 6.4%.
High-quality randomized clinical trials have definitively demonstrated that, among people at high risk for developing diabetes, lifestyle modification and metformin treatment reduce the risk of developing diabetes. In the Diabetes Prevention Program (DPP) 3,234 people with a high risk of diabetes, mean BMI 34 kg/m2, were randomly assigned to 1 of 3 groups9:
- a control group
- metformin (850 mg twice daily) or
- lifestyle modification that included exercise (moderate intensity exercise for 150 minutes per week and weight loss (7% of body weight using a low-calorie, low-fat diet).
At 2.8 years of follow-up the incidence of diabetes was 11%, 7.8%, and 4.8% per 100 person-years in the people assigned to the control, metformin, and lifestyle modification groups, respectively.9 In the DPP study, compared with the control group, metformin was most effective in decreasing the risk of transitioning to diabetes in people who had a BMI ≥35 kg/m2 (53% reduction in risk) or a BMI from 30 to 35 kg/m2 (16% reduction in risk).9 Metformin was not as effective at preventing the transition to diabetes in people who had a normal BMI or who were overweight (3% reduction).9
In the Finnish Diabetes Prevention Study, 522 obese people with impaired glucose tolerance were randomly assigned to lifestyle modification or a control group. After 4 years, the cumulative incidence of diabetes was 11% and 23% in the lifestyle modification and control groups, respectively.10 A meta-analysis of 23 randomized clinical trials reported that, among people with a high risk of developing diabetes, compared with no intervention (control group), lifestyle modification, including dieting, exercising, and weight loss significantly reduced the risk of developing diabetes (pooled relative risk [RR], 0.78; 95% confidence interval [CI], 0.69‒0.88).5
In clinical practice, offering a patient at high risk for diabetes a suite of options, including5,9,10:
- a formal nutrition consult with the goal of targeting a 7% reduction in weight
- recommending moderate intensity exercise, 150 minutes weekly
- metformin treatment, if the patient is obese
would reduce the patient’s risk of developing diabetes.
Treatment of T2DM is complex
For people with T2DM, a widely recommended treatment goal is to reduce the hemoglobin A1c measurement to ≤7%. Initial treatment includes a comprehensive diabetes self-management education program, weight loss using diet and exercise, and metformin treatment. Metformin may be associated with an increased risk of lactic acidosis, especially in people with renal insufficiency. The US Food and Drug Administration (FDA) recommends against initiating metformin therapy for people with an estimated glomerular filtration rate (eGFR) of 30 to 45 mL/min/1.73 m2. The FDA determined that metformin is contraindicated in people with an eGFR of <30 mL/min/1.73 m2.11 Many people with T2DM will require treatment with multiple pharmacologic agents to achieve a hemoglobin A1c ≤7%. In addition to metformin, pharmacologic agents used to treat T2DM include insulin, sulfonylureas, glucagon-like peptide-1(GLP-1) receptor agonists, a sodium glucose cotransporter (SGLT2) inhibitor, dipeptidyl peptidase-4 (DPP-4) inhibitors, or an alpha-glucosidase inhibitor. Given the complexity of managing T2DM over a lifetime, most individuals with T2DM receive their diabetes care from a primary care clinician or subspecialist in endocrinology.
Experts predict that, within the next 8 years, the prevalence of obesity among adults in the United States will be approximately 50%.12 The US health care system has not been effective in controlling the obesity epidemic. Our failure to control the obesity epidemic will result in an increase in the prevalence of prediabetes and T2DM, leading to a rise in cardiovascular, renal, and eye disease. The diagnosis of prediabetes and diabetes is within the scope of practice of obstetrics and gynecology. The treatment of prediabetes is also within the scope of ObGyns, who have both expertise and familiarity in the diagnosis of gestational diabetes, a form of prediabetes. ●
- Centers for Disease Control and Prevention. National Diabetes Statistics Report. 2020. https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. Accessed October 26, 2021.
- Wang L, Li X, Wang Z, et al. Trends in prevalence of diabetes and control of risk factors in diabetes among U.S. adults, 1999-2018. JAMA. 2021;326:1-13. doi: 10.1001/jama.2021.9883.
- Type 2 diabetes. Centers for Disease Control and Prevention website. . Last reviewed August 10, 2021 Accessed October 27, 2021.
- US Preventive Services Task Force. Screening for prediabetes and diabetes. US Preventive Services Task Force Recommendation Statement. JAMA. 2021;326:736-743. doi: 10.1001/jama.2021.12531.
- Jonas D, Crotty K, Yun JD, et al. Screening for prediabetes and type 2 diabetes mellitus: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2021;326:744-760. doi: 10.1001/jama.2021.10403.
- American Diabetes Association. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes‒2020. Diabetes Care. 2020;43(suppl 1):S14-S31. doi: 10.2337/dc20-S002.
- Meijnikman AS, De Block CE, Dirinck E, et al. Not performing an OGTT results in significant under diagnosis of (pre)diabetes in a high-risk adult Caucasian population. Int J Obes. 2017;41:1615-1620. doi: 10.1038/ijo.2017.165.
- Rooney MR, Rawlings AM, Pankow JS, et al. Risk of progression to diabetes among older adults with prediabetes. JAMA Intern Med. 2021;181:511-519. doi: 10.1001/jamainternmed.2020.8774.
- Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393-403. doi: 10.1056/NEJMoa012512.
- Tuomilehto J, Lindström J, Eriksson JG, et al; Finnish Diabetes Prevention Study Group. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344:1343-1350. doi: 10.1056/NEJM200105033441801.
- Glucophage [package insert]. Princeton, NJ: Bristol Meyers Squibb; April 2017. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017020357s037s039,021202s021s023lbl.pdf. Accessed October 27, 2021.
- Ward ZJ, Bleich SN, Cradock AL, et al. Projected U.S. state-level prevalence of adult obesity and severe obesity. N Engl J Med. 2019;381;2440-2450. doi: 10.1056/NEJMc1917339.
- Centers for Disease Control and Prevention. National Diabetes Statistics Report. 2020. https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. Accessed October 26, 2021.
- Wang L, Li X, Wang Z, et al. Trends in prevalence of diabetes and control of risk factors in diabetes among U.S. adults, 1999-2018. JAMA. 2021;326:1-13. doi: 10.1001/jama.2021.9883.
- Type 2 diabetes. Centers for Disease Control and Prevention website. . Last reviewed August 10, 2021 Accessed October 27, 2021.
- US Preventive Services Task Force. Screening for prediabetes and diabetes. US Preventive Services Task Force Recommendation Statement. JAMA. 2021;326:736-743. doi: 10.1001/jama.2021.12531.
- Jonas D, Crotty K, Yun JD, et al. Screening for prediabetes and type 2 diabetes mellitus: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2021;326:744-760. doi: 10.1001/jama.2021.10403.
- American Diabetes Association. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes‒2020. Diabetes Care. 2020;43(suppl 1):S14-S31. doi: 10.2337/dc20-S002.
- Meijnikman AS, De Block CE, Dirinck E, et al. Not performing an OGTT results in significant under diagnosis of (pre)diabetes in a high-risk adult Caucasian population. Int J Obes. 2017;41:1615-1620. doi: 10.1038/ijo.2017.165.
- Rooney MR, Rawlings AM, Pankow JS, et al. Risk of progression to diabetes among older adults with prediabetes. JAMA Intern Med. 2021;181:511-519. doi: 10.1001/jamainternmed.2020.8774.
- Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393-403. doi: 10.1056/NEJMoa012512.
- Tuomilehto J, Lindström J, Eriksson JG, et al; Finnish Diabetes Prevention Study Group. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344:1343-1350. doi: 10.1056/NEJM200105033441801.
- Glucophage [package insert]. Princeton, NJ: Bristol Meyers Squibb; April 2017. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017020357s037s039,021202s021s023lbl.pdf. Accessed October 27, 2021.
- Ward ZJ, Bleich SN, Cradock AL, et al. Projected U.S. state-level prevalence of adult obesity and severe obesity. N Engl J Med. 2019;381;2440-2450. doi: 10.1056/NEJMc1917339.