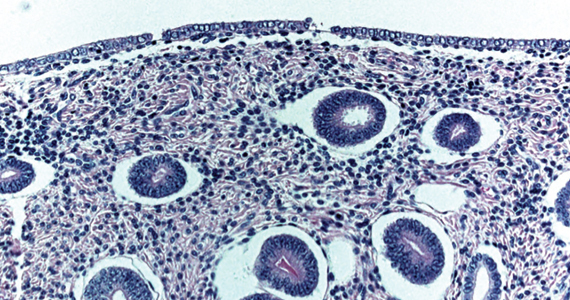
User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
Denosumab boosts bone strength in glucocorticoid users
Bone strength and microarchitecture remained stronger at 24 months after treatment with denosumab compared to risedronate, in a study of 110 adults using glucocorticoids.
Patients using glucocorticoids are at increased risk for vertebral and nonvertebral fractures at both the start of treatment or as treatment continues, wrote Piet Geusens, MD, of Maastricht University, the Netherlands, and colleagues.
Imaging data collected via high-resolution peripheral quantitative computed tomography (HR-pQCT) allow for the assessment of bone microarchitecture and strength, but specific data comparing the impact of bone treatment in patients using glucocorticoids are lacking, they said.
In a study published in the Journal of Bone and Mineral Research, the researchers identified a subset of 56 patients randomized to denosumab and 54 to risedronate patients out of a total of 590 patients who were enrolled in a phase 3 randomized, controlled trial of denosumab vs. risedronate for bone mineral density. The main results of the larger trial – presented at EULAR 2018 – showed greater increases in bone strength with denosumab over risedronate in patients receiving glucocorticoids.
In the current study, the researchers reviewed HR-pQCT scans of the distal radius and tibia at baseline, 12 months, and 24 months. Bone strength and microarchitecture were defined in terms of failure load (FL) as a primary outcome. Patients also were divided into subpopulations of those initiating glucocorticoid treatment (GC-I) and continuing treatment (GC-C).
Baseline characteristics were mainly balanced among the treatment groups within the GC-I and GC-C categories.
Among the GC-I patients, in the denosumab group, FL increased significantly from baseline to 12 months at the radius at tibia (1.8% and 1.7%, respectively) but did not change significantly in the risedronate group, which translated to a significant treatment difference between the drugs of 3.3% for radius and 2.5% for tibia.
At 24 months, the radius measure of FL was unchanged from baseline in denosumab patients but significantly decreased in risedronate patients, with a difference of –4.1%, which translated to a significant between-treatment difference at the radius of 5.6% (P < .001). Changes at the tibia were not significantly different between the groups at 24 months.
Among the GC-C patients, FL was unchanged from baseline to 12 months for both the denosumab and risedronate groups. However, FL significantly increased with denosumab (4.3%) and remained unchanged in the risedronate group.
The researchers also found significant differences between denosumab and risedronate in percentage changes in cortical bone mineral density, and less prominent changes and differences in trabecular bone mineral density.
The study findings were limited by several factors including the use of the HR-pQCT scanner, which limits the measurement of trabecular microarchitecture, and the use of only standard HR-pQCT parameters, which do not allow insight into endosteal changes, and the inability to correct for multiplicity of data, the researchers noted.
However, the results support the superiority of denosumab over risedronate for preventing FL and total bone mineral density loss at the radius and tibia in new glucocorticoid users, and for increasing FL and total bone mineral density at the radius in long-term glucocorticoid users, they said.
Denosumab therefore could be a useful therapeutic option and could inform decision-making in patients initiating GC-therapy or on long-term GC-therapy, they concluded.
The study was supported by Amgen. Dr. Geusens disclosed grants from Amgen, Celgene, Lilly, Merck, Pfizer, Roche, UCB, Fresenius, Mylan, and Sandoz, and grants and other funding from AbbVie, outside the current study.
Bone strength and microarchitecture remained stronger at 24 months after treatment with denosumab compared to risedronate, in a study of 110 adults using glucocorticoids.
Patients using glucocorticoids are at increased risk for vertebral and nonvertebral fractures at both the start of treatment or as treatment continues, wrote Piet Geusens, MD, of Maastricht University, the Netherlands, and colleagues.
Imaging data collected via high-resolution peripheral quantitative computed tomography (HR-pQCT) allow for the assessment of bone microarchitecture and strength, but specific data comparing the impact of bone treatment in patients using glucocorticoids are lacking, they said.
In a study published in the Journal of Bone and Mineral Research, the researchers identified a subset of 56 patients randomized to denosumab and 54 to risedronate patients out of a total of 590 patients who were enrolled in a phase 3 randomized, controlled trial of denosumab vs. risedronate for bone mineral density. The main results of the larger trial – presented at EULAR 2018 – showed greater increases in bone strength with denosumab over risedronate in patients receiving glucocorticoids.
In the current study, the researchers reviewed HR-pQCT scans of the distal radius and tibia at baseline, 12 months, and 24 months. Bone strength and microarchitecture were defined in terms of failure load (FL) as a primary outcome. Patients also were divided into subpopulations of those initiating glucocorticoid treatment (GC-I) and continuing treatment (GC-C).
Baseline characteristics were mainly balanced among the treatment groups within the GC-I and GC-C categories.
Among the GC-I patients, in the denosumab group, FL increased significantly from baseline to 12 months at the radius at tibia (1.8% and 1.7%, respectively) but did not change significantly in the risedronate group, which translated to a significant treatment difference between the drugs of 3.3% for radius and 2.5% for tibia.
At 24 months, the radius measure of FL was unchanged from baseline in denosumab patients but significantly decreased in risedronate patients, with a difference of –4.1%, which translated to a significant between-treatment difference at the radius of 5.6% (P < .001). Changes at the tibia were not significantly different between the groups at 24 months.
Among the GC-C patients, FL was unchanged from baseline to 12 months for both the denosumab and risedronate groups. However, FL significantly increased with denosumab (4.3%) and remained unchanged in the risedronate group.
The researchers also found significant differences between denosumab and risedronate in percentage changes in cortical bone mineral density, and less prominent changes and differences in trabecular bone mineral density.
The study findings were limited by several factors including the use of the HR-pQCT scanner, which limits the measurement of trabecular microarchitecture, and the use of only standard HR-pQCT parameters, which do not allow insight into endosteal changes, and the inability to correct for multiplicity of data, the researchers noted.
However, the results support the superiority of denosumab over risedronate for preventing FL and total bone mineral density loss at the radius and tibia in new glucocorticoid users, and for increasing FL and total bone mineral density at the radius in long-term glucocorticoid users, they said.
Denosumab therefore could be a useful therapeutic option and could inform decision-making in patients initiating GC-therapy or on long-term GC-therapy, they concluded.
The study was supported by Amgen. Dr. Geusens disclosed grants from Amgen, Celgene, Lilly, Merck, Pfizer, Roche, UCB, Fresenius, Mylan, and Sandoz, and grants and other funding from AbbVie, outside the current study.
Bone strength and microarchitecture remained stronger at 24 months after treatment with denosumab compared to risedronate, in a study of 110 adults using glucocorticoids.
Patients using glucocorticoids are at increased risk for vertebral and nonvertebral fractures at both the start of treatment or as treatment continues, wrote Piet Geusens, MD, of Maastricht University, the Netherlands, and colleagues.
Imaging data collected via high-resolution peripheral quantitative computed tomography (HR-pQCT) allow for the assessment of bone microarchitecture and strength, but specific data comparing the impact of bone treatment in patients using glucocorticoids are lacking, they said.
In a study published in the Journal of Bone and Mineral Research, the researchers identified a subset of 56 patients randomized to denosumab and 54 to risedronate patients out of a total of 590 patients who were enrolled in a phase 3 randomized, controlled trial of denosumab vs. risedronate for bone mineral density. The main results of the larger trial – presented at EULAR 2018 – showed greater increases in bone strength with denosumab over risedronate in patients receiving glucocorticoids.
In the current study, the researchers reviewed HR-pQCT scans of the distal radius and tibia at baseline, 12 months, and 24 months. Bone strength and microarchitecture were defined in terms of failure load (FL) as a primary outcome. Patients also were divided into subpopulations of those initiating glucocorticoid treatment (GC-I) and continuing treatment (GC-C).
Baseline characteristics were mainly balanced among the treatment groups within the GC-I and GC-C categories.
Among the GC-I patients, in the denosumab group, FL increased significantly from baseline to 12 months at the radius at tibia (1.8% and 1.7%, respectively) but did not change significantly in the risedronate group, which translated to a significant treatment difference between the drugs of 3.3% for radius and 2.5% for tibia.
At 24 months, the radius measure of FL was unchanged from baseline in denosumab patients but significantly decreased in risedronate patients, with a difference of –4.1%, which translated to a significant between-treatment difference at the radius of 5.6% (P < .001). Changes at the tibia were not significantly different between the groups at 24 months.
Among the GC-C patients, FL was unchanged from baseline to 12 months for both the denosumab and risedronate groups. However, FL significantly increased with denosumab (4.3%) and remained unchanged in the risedronate group.
The researchers also found significant differences between denosumab and risedronate in percentage changes in cortical bone mineral density, and less prominent changes and differences in trabecular bone mineral density.
The study findings were limited by several factors including the use of the HR-pQCT scanner, which limits the measurement of trabecular microarchitecture, and the use of only standard HR-pQCT parameters, which do not allow insight into endosteal changes, and the inability to correct for multiplicity of data, the researchers noted.
However, the results support the superiority of denosumab over risedronate for preventing FL and total bone mineral density loss at the radius and tibia in new glucocorticoid users, and for increasing FL and total bone mineral density at the radius in long-term glucocorticoid users, they said.
Denosumab therefore could be a useful therapeutic option and could inform decision-making in patients initiating GC-therapy or on long-term GC-therapy, they concluded.
The study was supported by Amgen. Dr. Geusens disclosed grants from Amgen, Celgene, Lilly, Merck, Pfizer, Roche, UCB, Fresenius, Mylan, and Sandoz, and grants and other funding from AbbVie, outside the current study.
FROM THE JOURNAL OF BONE AND MINERAL RESEARCH
COVID cases rising in about half of states
About half the states have reported increases in COVID cases fueled by the Omicron subvariant, Axios reported. Alaska, Vermont, and Rhode Island had the highest increases, with more than 20 new cases per 100,000 people.
Nationally, the statistics are encouraging, with the 7-day average of daily cases around 26,000 on April 6, down from around 41,000 on March 6, according to the Centers for Disease Control and Prevention. The number of deaths has dropped to an average of around 600 a day, down 34% from 2 weeks ago.
National health officials have said some spots would have a lot of COVID cases.
“Looking across the country, we see that 95% of counties are reporting low COVID-19 community levels, which represent over 97% of the U.S. population,” CDC Director Rochelle Walensky, MD, said April 5 at a White House news briefing.
“If we look more closely at the local level, we find a handful of counties where we are seeing increases in both cases and markers of more severe disease, like hospitalizations and in-patient bed capacity, which have resulted in an increased COVID-19 community level in some areas.”
Meanwhile, the Commonwealth Fund issued a report April 8 saying the U.S. vaccine program had prevented an estimated 2.2 million deaths and 17 million hospitalizations.
If the vaccine program didn’t exist, the United States would have had another 66 million COVID infections and spent about $900 billion more on health care, the foundation said.
The United States has reported about 982,000 COVID-related deaths so far with about 80 million COVID cases, according to the CDC.
“Our findings highlight the profound and ongoing impact of the vaccination program in reducing infections, hospitalizations, and deaths,” the Commonwealth Fund said.
“Investing in vaccination programs also has produced substantial cost savings – approximately the size of one-fifth of annual national health expenditures – by dramatically reducing the amount spent on COVID-19 hospitalizations.”
A version of this article first appeared on WebMD.com.
About half the states have reported increases in COVID cases fueled by the Omicron subvariant, Axios reported. Alaska, Vermont, and Rhode Island had the highest increases, with more than 20 new cases per 100,000 people.
Nationally, the statistics are encouraging, with the 7-day average of daily cases around 26,000 on April 6, down from around 41,000 on March 6, according to the Centers for Disease Control and Prevention. The number of deaths has dropped to an average of around 600 a day, down 34% from 2 weeks ago.
National health officials have said some spots would have a lot of COVID cases.
“Looking across the country, we see that 95% of counties are reporting low COVID-19 community levels, which represent over 97% of the U.S. population,” CDC Director Rochelle Walensky, MD, said April 5 at a White House news briefing.
“If we look more closely at the local level, we find a handful of counties where we are seeing increases in both cases and markers of more severe disease, like hospitalizations and in-patient bed capacity, which have resulted in an increased COVID-19 community level in some areas.”
Meanwhile, the Commonwealth Fund issued a report April 8 saying the U.S. vaccine program had prevented an estimated 2.2 million deaths and 17 million hospitalizations.
If the vaccine program didn’t exist, the United States would have had another 66 million COVID infections and spent about $900 billion more on health care, the foundation said.
The United States has reported about 982,000 COVID-related deaths so far with about 80 million COVID cases, according to the CDC.
“Our findings highlight the profound and ongoing impact of the vaccination program in reducing infections, hospitalizations, and deaths,” the Commonwealth Fund said.
“Investing in vaccination programs also has produced substantial cost savings – approximately the size of one-fifth of annual national health expenditures – by dramatically reducing the amount spent on COVID-19 hospitalizations.”
A version of this article first appeared on WebMD.com.
About half the states have reported increases in COVID cases fueled by the Omicron subvariant, Axios reported. Alaska, Vermont, and Rhode Island had the highest increases, with more than 20 new cases per 100,000 people.
Nationally, the statistics are encouraging, with the 7-day average of daily cases around 26,000 on April 6, down from around 41,000 on March 6, according to the Centers for Disease Control and Prevention. The number of deaths has dropped to an average of around 600 a day, down 34% from 2 weeks ago.
National health officials have said some spots would have a lot of COVID cases.
“Looking across the country, we see that 95% of counties are reporting low COVID-19 community levels, which represent over 97% of the U.S. population,” CDC Director Rochelle Walensky, MD, said April 5 at a White House news briefing.
“If we look more closely at the local level, we find a handful of counties where we are seeing increases in both cases and markers of more severe disease, like hospitalizations and in-patient bed capacity, which have resulted in an increased COVID-19 community level in some areas.”
Meanwhile, the Commonwealth Fund issued a report April 8 saying the U.S. vaccine program had prevented an estimated 2.2 million deaths and 17 million hospitalizations.
If the vaccine program didn’t exist, the United States would have had another 66 million COVID infections and spent about $900 billion more on health care, the foundation said.
The United States has reported about 982,000 COVID-related deaths so far with about 80 million COVID cases, according to the CDC.
“Our findings highlight the profound and ongoing impact of the vaccination program in reducing infections, hospitalizations, and deaths,” the Commonwealth Fund said.
“Investing in vaccination programs also has produced substantial cost savings – approximately the size of one-fifth of annual national health expenditures – by dramatically reducing the amount spent on COVID-19 hospitalizations.”
A version of this article first appeared on WebMD.com.
Infectious disease pop quiz: Clinical challenge #22 for the ObGyn
In a pregnant woman who has a life-threatening allergy to penicillin, what is the most appropriate treatment for syphilis?
Continue to the answer...
This patient should be admitted to the hospital and rapidly desensitized to penicillin. She then can be treated with the appropriate dose of penicillin, given her stage of syphilis. Of note, in the future, the patient’s allergy to penicillin will return, despite the brief period of desensitization.
- Duff P. Maternal and perinatal infections: bacterial. In: Landon MB, Galan HL, Jauniaux ERM, et al. Gabbe’s Obstetrics: Normal and Problem Pregnancies. 8th ed. Elsevier; 2021:1124-1146.
- Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore TJ, et al. Creasy & Resnik’s Maternal-Fetal Medicine: Principles and Practice. 8th ed. Elsevier; 2019:862-919.
In a pregnant woman who has a life-threatening allergy to penicillin, what is the most appropriate treatment for syphilis?
Continue to the answer...
This patient should be admitted to the hospital and rapidly desensitized to penicillin. She then can be treated with the appropriate dose of penicillin, given her stage of syphilis. Of note, in the future, the patient’s allergy to penicillin will return, despite the brief period of desensitization.
In a pregnant woman who has a life-threatening allergy to penicillin, what is the most appropriate treatment for syphilis?
Continue to the answer...
This patient should be admitted to the hospital and rapidly desensitized to penicillin. She then can be treated with the appropriate dose of penicillin, given her stage of syphilis. Of note, in the future, the patient’s allergy to penicillin will return, despite the brief period of desensitization.
- Duff P. Maternal and perinatal infections: bacterial. In: Landon MB, Galan HL, Jauniaux ERM, et al. Gabbe’s Obstetrics: Normal and Problem Pregnancies. 8th ed. Elsevier; 2021:1124-1146.
- Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore TJ, et al. Creasy & Resnik’s Maternal-Fetal Medicine: Principles and Practice. 8th ed. Elsevier; 2019:862-919.
- Duff P. Maternal and perinatal infections: bacterial. In: Landon MB, Galan HL, Jauniaux ERM, et al. Gabbe’s Obstetrics: Normal and Problem Pregnancies. 8th ed. Elsevier; 2021:1124-1146.
- Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore TJ, et al. Creasy & Resnik’s Maternal-Fetal Medicine: Principles and Practice. 8th ed. Elsevier; 2019:862-919.
Suing patients: Medical, ethical, and legal considerations
Although it is common to read about patients suing their hospitals, there has been increasing public and political attention given to hospitals suing their patients to collect unpaid hospital bills. KH’s story began with an emergency appendectomy. She did not have health insurance to cover the $14,000 hospital bill. The family was unable to pay the bill, and the nonprofit hospital sued them for that bill, plus some additional expenses (totaling about $17,000), plus interest was accumulating at 9% per year. The hospital won a judgment, and it garnished the husband’s pay (10% of after-taxes pay, in this case) and placed a lien on the family’s home. Years later—because of interest and additional hospital bills—the family had paid $20,000, but still owed $26,000.1
The extent of the problem
This is neither a hypothetical case nor a rare event. Studies and press reports have noted dozens of examples of hospital collection excesses. One study found that unpaid medical bill lawsuits increased by 37% in Wisconsin between 2001 and 2018, with 5% of hospitals accounting for 25% of the lawsuits.2 Another report found almost “31,000 civil cases filed by 139 hospitals in 26 New York counties from 2015 to 2019.”3 Similar to the Wisconsin report, a small number of health care providers accounted for the majority of lawsuits. In another example, one Missouri nonprofit hospital, Heartland (rebranded “Mosaic”), created its own for-profit debt collection agency (Northwest Financial Services), which filed 11,000 lawsuits from 2009 to 2013, resulting in 6,000 wage garnishments.1 The Wall Street Journal, among others, has reported for years on the difficulties created by lawsuits against patients.4 Axios and Johns Hopkins reported that “medical debt comprises 58% of all debt collections in the United States.” And although some collection actions declined early in the pandemic, it did not appear to last.5,6
Inconsistent collection policies. Collection policies vary greatly from hospital to hospital, with an increasing number of hospitals demanding up-front payments (before services). Many of these health care institutions persuade patients to put medical debt on their credit cards, sometimes as part of an up-front (before service) process.7 If using a standard credit card, this comes with a very high interest rate. There are some special health-related credit cards, such as CareCredit, that generally have better interest rates. These cards offer no-interest short-term loans, with significant interest for longer-term loans. Thus, failure to repay the full amount when due means that the “deferred interest” (about 27%) must be paid.8 Also any of the problems patients have repaying a credit card (or other loan), of course, are no longer directly related to the hospital. These “indirect collections” still burden patients with medical debt.
Where you go matters. Because there is no common collection policy or practice among hospitals, choosing the wrong hospital may result in a lawsuit. A careful study of lawsuits for medical debt or garnishments related to that debt in 2017 in Virginia showed how being treated at certain hospitals dramatically changed the odds of wage garnishment for unpaid bills.9 It revealed that 29,286 hospital lawsuits were filed to collect medical debt—9,232 of which were wage garnishments (the most aggressive form of debt collection). Five hospitals alone accounted for the majority of garnishments in the state. Notably, nonprofit hospitals accounted for 71% of the garnishment cases. On the other hand, about 50% of the hospitals in the study did not file any lawsuits to garnish wages for medical debt.9
Why is there so much hospital debt?
One would think the Affordable Care Act (ACA) and other reforms would mean fewer people do not have health insurance—and the problems experienced by the patient in the case above. Indeed, the number of insured has increased in the United States, including through the expansion of Medicaid. Nonetheless, in 2020, the Census Bureau reported that 28 million people did not have health insurance for any part of the year; that figure would be higher if those who had insurance for only part of the year were included.10
One reason for medical debt is the very high level of “under” insurance—that is, even with health insurance, copays for significant medical bills exceed what the patient can pay. Nearly half of adults (excluding the elderly) were enrolled in high-deductible health plans (in 2017).11 Among most employment-based plans, deductibles and co-pays have been going up for a decade.12 Overall, 20% of employer-provided plans had deductibles in excess of $3,000 ($5,000 for families).13 Of course, many families do not have anywhere near the resources to pay high deductibles, and that represents likely medical debt. The more modest copays of Medicare (often 20%) can be enough to push some elderly individuals beyond their capacity to pay.
“Out-of-network” care also may result in large hospital charges—and debt. Emergency care, for example, may be sought from the closest provider, even though out of network, and the insurance company may refuse to pay the charges. Another surprise form of billing is when a health care insurance company tentatively approves coverage and then after the patient receives care, determines it was unnecessary. In that case, even in-network charges may be denied, with the patient left to pay all the charges.
Continue to: How medical debt affects patients...
How medical debt affects patients
For patients, medical debt places pressure on their financial circumstances. Bankruptcy has a profound financial impact, and approximately two-thirds of bankruptcies are related to medical care costs and debt, including “indirect collection.”14 Even when the financial effect is not so devastating, it is often substantial, as the above case demonstrated. In a 2018 survey, almost 30% of those with health insurance had medical debts in some form of collection action, and 25% of those individuals said they did not know they owed the money.15 The same survey found that 20% of respondents had medical debt that adversely affected their credit scores and access to credit.15
At work, although employers are not supposed to treat employees adversely because of garnishment, some employers may not adhere to that rule. Furthermore, employees may believe or be concerned that the very existence of garnishment may penalize them at their current job or make it difficult to move to a better one.16
Lastly, patients with medical debt may be reluctant to seek needed medical care. They may be concerned about adding more medical debt or embarrassed or afraid that they would not be welcome at the hospital where they owe money.7
Public perception of hospitals
Lawsuits against patients also have a negative effect on hospitals—and it is not limited to the relatively few institutions that file many of these lawsuits each year. Press reports about lawsuits against patients garner great public interest and anger, and this tarnishes the image of heath care facilities in general because many people often do not distinguish the actions of a few institutions.
The sensitivity of health care organizations to bad publicity from debt collection practices was seen in a follow-up study of the previously discussed Virginia data. In the year following this report, there was a 59% decrease in the number of lawsuits filed, including a 66% decrease in garnishments.17 Eleven hospitals in the state that had been filing debt lawsuits stopped doing so.17
Medical debt: The obligation of nonprofit hospitals
The response seen in the Virginia follow-up study may also reflect well-founded concern from board members about political consequences and even taxation problems. The majority of hospitals, including those in these studies, are nonprofit institutions with an Internal Revenue Service (IRS) 501(c)(3) “tax-exempt” status. (Note, “nonprofit” does not mean that the organization does not make a profit, but that the profit does not accrue to individuals.) The “nonprofit” status is usually granted by states, but the federal tax-exempt status is granted by the IRS. This status exempts the institutions from paying most federal taxes, and (perhaps most importantly) qualifies donors to receive tax deductions (and similar benefits) for donations made to these hospitals. This important tax treatment is granted based on the theory that their services are so valuable to the public that advancing their work through the tax exemption ultimately benefits the public more than the tax revenue would.
In return for these benefits, the organization has obligations to work in the public interest. For years, hospitals have been criticized for not providing sufficient public benefits (compared, for example, with for-profit hospitals) to justify the tax exemption. That criticism caused the IRS to begin requiring a special Form 990, Schedule H, which is attached to the usual 501(c)(3) informational tax return, “to provide information on the activities and policies of, and community benefit provided by, its hospital facilities and other non-hospital health care facilities.”18 Part III of Schedule H asks, in part, about bad debt and collection practices.
Then the ACA Section 501(r) enhanced the obligation of nonprofit health facilities to provide charitable care in two ways. First, they must have, and make available, policies to provide free and discounted care; and second, they cannot sue for payment until they make an individualized determination as to whether the patient should have received discounted care or financial assistance.19
Thus aggressive collection practices (which should include “indirect collection”) invite special scrutiny by local officials and the IRS. In the longer-term, concern that tax-exempt hospitals are not truly operating in the public interest is undoubtedly amplified by these aggressive debt collection practices. How can a hospital claim it is truly operating in the public interest when it sues dozens of modest-income individuals each year?
Regulating medical debt and its collection
The No Surprises Act
In December 2020, Congress adopted the No Surprises Act to address some of the problems of patient debt.20 Among other things, the act protects patients “from receiving surprise medical bills when they receive most emergency services,” or when they are in an in-network hospital but receive services from out-of-network providers (such as anesthesia and radiology).21 Several states also have similar legislation, so the federal law specifically states that where state laws are more protective of patients, the state’s higher protections apply, and vice versa. The act took effect on January 1, 2022, though there is an “interim final” regulation that will be subject to change, and there is already litigation over those regulations.22 The real complexity of the rules will arise through the regulations, which are likely to change several times over the next few years. To help with this, the American Medical Association has an extensive toolkit for health care providers.23
Continue to: Additional regulations...
Additional regulations
Both the federal government and most states are likely to take additional action to reduce hospital debt lawsuits. Some proposals sound simple enough but would have significant complications. For example, governments could prohibit all lawsuits that collect hospital debt.7 Such a regulation would mean that paying hospital debts would essentially become optional. Imagine the millionaire who does not want to pay a $25,000 hospital charge; or patients with other debts who would pay those off before the hospital debt. The regulation might have income or asset limits on debt collection lawsuits and the like, but it quickly becomes complicated. Furthermore, to protect themselves, hospitals would undoubtedly become much more aggressive about requiring up-front payments—which would force the debt or prepayment onto credit cards or similar debt obligations that are not subject to the no collection lawsuit rule.
Public reporting. The follow-up study in Virginia17 suggests that requiring public reporting of the number of cases filed by or on behalf of (directly or indirectly) each hospital may help. Hospitals would, of course, have incentives to make their figures look better, perhaps by selling the debt to an agency that would be able to file suit in its name rather than the hospital’s name. These might be little more than indirect collections. For reporting purposes, any form of transferring debt might be considered filing a lawsuit. The problem, noted earlier, about requiring prepayment or credit cards would also exist.
Get the board involved. A different approach would be to ensure that a hospital’s board of trustees is involved in setting and overseeing debt collection policies. For example, the law might require boards to annually consider and adopt specific debt collection practices—including indirect collection efforts. Boards should already be doing something similar to this, but regulation might be an inexpensive way to ensure it is done—and in a manner consistent with the organization’s values. Another suggestion is to require the board to approve any legal action against specific patients.7 By making sure this is not just another item on the consent agenda, the oversight would probably reduce automatic debt collection processes.
Expand IRS reporting requirements for nonprofits. Indeed, for nonprofit hospitals with 501(c)(3) obligations, the Form 990, Schedule H already provides some information about collection actions and uncompensated care, and this is enhanced by the ACA Section 501(r). These could be expanded and perhaps include “indirect” collections. The IRS could “flag” hospitals with high total litigation and similar collection actions, and ask the hospital to provide a detailed explanation for each action and how it was consistent with the obligation to serve the public (thereby justifying the exempt taxation status, an idea proposed by the US Government Accountability Office in 2020).24
Ensure the hospital’s actions reflect their mission and values
Hospitals are created to provide medical care for people and to improve the human condition. Those who lead them should, and generally do, share that purpose. The apparent collection policies that have garnered negative public attention suggest that some of these institutions have lost focus of their ultimate mission and values. The boards and executives of these health care institutions, as well as the medical professionals and attorneys who serve them, should be continuously guided by those values.
Important decisions—including collection and prepayment processes—reflect the values of the institution. Failure to ensure these procedures are in line with the organization’s mission is an embarrassment to all health care facilities, including the majority of hospitals that do not engage in these aggressive collection practices. Not addressing these issues will likely result in political and legal action—blunt and inefficient instruments—to limit what the public sees as wrongdoing. ●
- Kiel P. From the E.R. to the courtroom: how nonprofit hospitals are seizing patients’ wages. ProPublica. December 19, 2014. Accessed March 21, 2022. https://www.propublica.org/article/how-nonprofit-hospitals-are-seizing-patients-wages
- Cooper Z, Han J, Mahoney N. Hospital lawsuits over unpaid bills increased by 37 percent in Wisconsin from 2001 to 2018. Health Affairs. 2021;40:1830-1835. Accessed March 21, 2022. https://www.healthaffairs.org/doi/full/10.1377 /hlthaff.2021.01130
- LaMantia J. New York hospitals have filed thousands of lawsuits against patients. Modern Healthcare. March 13, 2020. Accessed March 21, 2022. https://www.modernhealthcare .com/legal/new-york-hospitals-have-filed-thousands -lawsuits-against-patients
- Armour S. When patients can’t pay, many hospitals are suing. Wall Street Journal. June 25, 2019. Accessed March 21, 2022. https://www.wsj.com/articles/nonprofit-hospitals-criticized-for-debt-collection-tactics-11561467600
- McGhee M, Chase W. How America’s top hospitals hound patients with predatory billing. Axios. Accessed March 21, 2022. https://www.axios.com/hospital-billing
- Owens C. Public spotlight on hospital lawsuits may slow them down. June 14, 2021. Accessed March 22, 2022. https:// www.axios.com/hospital-lawsuits-slowing-down-media -35ce395a-9fe3-4b23-b815-d7b06cce2773.html
- Buck ID. When hospitals sue patients. Hastings L.J. 2022;73:191-232, at 209-211. Accessed March 21, 2022. https:// repository.uchastings.edu/cgi/viewcontent.cgi?article =3961&context=hastings_law_journal
- Lagasse J. Healthcare turns to zero-interest loans to give patients a better reason to pay. Healthcare Finance. May 3, 2017. Accessed March 21, 2022. https://www.healthcarefinancenews.com/news/healthcare-turns-zero-interest-loans-give-patients-better-reason-pay#:~:text=Zero%2Dinterest%20loans%20are%20finding,of%20the%20patient%2Dprovider%20relationship.
- Bruhn WE, Rutkow L, Wang P, et al. Prevalence and characteristics of Virginia hospitals suing patients and garnishing wages for unpaid medical bills. JAMA. 2019;322:691-692. doi:10.1001/jama.2019.9144
- Keisler-Starkey K, Bunch LN. Health insurance coverage in the United States: 2020. September 14, 2021. United States Census Bureau Current Population Reports, P60-274. US Government Publishing Office; September 2021. Accessed March 21, 2022. https://www.census.gov/content/dam /Census/library/publications/2021/demo/p60-274.pdf
- Cohen RA, Zammitti EP. High-deductible health plan enrollment among adults aged 18-64 with employment-based insurance coverage. NCHS Data Brief, No. 317. August 2018. Accessed March 21, 2022. https://www.cdc.gov/nchs/data/databriefs/db317.pdf
- Kaiser Family Foundation. Employer health benefits: 2020 summary of findings. Accessed March 21, 2022. https://www.kff.org/report-section/ehbs-2020-summary-of-findings/
- Picchi A. Higher health insurance deductibles a sickening trend for Americans. CBS NEWS. June 13, 2019. Accessed March 21, 2022. https://www.cbsnews.com/news/high-health-insurance-deductibles-a-sickening-trend-thats -causing-financial-hardship/
- Himmelstein DU, Lawless RM, Thorne D, Foohey P, Woolhandler S. Medical bankruptcy: still common despite the Affordable Care Act. Am J Public Health. 2019;109:431-433. doi:10.2105/AJPH.2018.304901
- Rosato D. What medical debt does to your credit score. Consumer Reports. July 26, 2018. Accessed March 21, 2022. https://www.consumerreports.org/credit-scores-reports/what-medical-debt-does-to-your-credit-score/
- State laws on wage garnishments. Nolo web site. https://www.nolo.com/legal-encyclopedia/free-books/employee-rights-book/chapter2-9.html. Accessed April 1, 2022.
- Patruzo JGR, Hashim F, Dun C, et al. Trends in hospital lawsuits filed against patients for unpaid bills following published research about their activity. JAMA Network Open. 2021;4:e2121926. doi:10.1001/jamanetworkopen.2021.21926
- About Schedule H (Form 990), hospitals. IRS. Updated June 10, 2021. Accessed March 21, 2022. https://www.irs.gov/forms-pubs/about-schedule-h-form-990
- Requirements for 501(c)(3) hospitals under the Affordable Care Act – Section 501(r). Updated September 9, 2021. Accessed March 21, 2022. https://www.irs.gov/charities-non-profits/charitable-organizations/requirements-for-501c3-hospitals-under-the-affordable-care-act-section-501r
- Pub. L. No. 116-260, 134 Stat. 1182, Division BB, § 109.
- Fact sheet. No Surprises: understand your rights against surprise medical bills. Centers for Medicare and Medicaid Services. January 3, 2022. Accessed March 21, 2022. https://www.cms.gov/newsroom/fact-sheets/no-surprises-understand-your-rights-against-surprise-medical-bills
- Implementation of the No Surprises Act. Accessed March 21, 2022. https://www.ama-assn.org/delivering-care/patient-support-advocacy/implementation-no-surprises-act
- American Medical Association. Toolkit for physicians: preparing for implementation of the No Surprises Act. January 2022. Accessed March 21, 2022. https://www.ama-assn.org/system/files/ama-nsa-toolkit.pdf
- US Government Accountability Office. Tax administration: opportunities exist to improve oversight of hospitals’ taxexempt status. September 2020. Accessed March 21, 2022. https://www.gao.gov/assets/gao-20-679.pdf
Although it is common to read about patients suing their hospitals, there has been increasing public and political attention given to hospitals suing their patients to collect unpaid hospital bills. KH’s story began with an emergency appendectomy. She did not have health insurance to cover the $14,000 hospital bill. The family was unable to pay the bill, and the nonprofit hospital sued them for that bill, plus some additional expenses (totaling about $17,000), plus interest was accumulating at 9% per year. The hospital won a judgment, and it garnished the husband’s pay (10% of after-taxes pay, in this case) and placed a lien on the family’s home. Years later—because of interest and additional hospital bills—the family had paid $20,000, but still owed $26,000.1
The extent of the problem
This is neither a hypothetical case nor a rare event. Studies and press reports have noted dozens of examples of hospital collection excesses. One study found that unpaid medical bill lawsuits increased by 37% in Wisconsin between 2001 and 2018, with 5% of hospitals accounting for 25% of the lawsuits.2 Another report found almost “31,000 civil cases filed by 139 hospitals in 26 New York counties from 2015 to 2019.”3 Similar to the Wisconsin report, a small number of health care providers accounted for the majority of lawsuits. In another example, one Missouri nonprofit hospital, Heartland (rebranded “Mosaic”), created its own for-profit debt collection agency (Northwest Financial Services), which filed 11,000 lawsuits from 2009 to 2013, resulting in 6,000 wage garnishments.1 The Wall Street Journal, among others, has reported for years on the difficulties created by lawsuits against patients.4 Axios and Johns Hopkins reported that “medical debt comprises 58% of all debt collections in the United States.” And although some collection actions declined early in the pandemic, it did not appear to last.5,6
Inconsistent collection policies. Collection policies vary greatly from hospital to hospital, with an increasing number of hospitals demanding up-front payments (before services). Many of these health care institutions persuade patients to put medical debt on their credit cards, sometimes as part of an up-front (before service) process.7 If using a standard credit card, this comes with a very high interest rate. There are some special health-related credit cards, such as CareCredit, that generally have better interest rates. These cards offer no-interest short-term loans, with significant interest for longer-term loans. Thus, failure to repay the full amount when due means that the “deferred interest” (about 27%) must be paid.8 Also any of the problems patients have repaying a credit card (or other loan), of course, are no longer directly related to the hospital. These “indirect collections” still burden patients with medical debt.
Where you go matters. Because there is no common collection policy or practice among hospitals, choosing the wrong hospital may result in a lawsuit. A careful study of lawsuits for medical debt or garnishments related to that debt in 2017 in Virginia showed how being treated at certain hospitals dramatically changed the odds of wage garnishment for unpaid bills.9 It revealed that 29,286 hospital lawsuits were filed to collect medical debt—9,232 of which were wage garnishments (the most aggressive form of debt collection). Five hospitals alone accounted for the majority of garnishments in the state. Notably, nonprofit hospitals accounted for 71% of the garnishment cases. On the other hand, about 50% of the hospitals in the study did not file any lawsuits to garnish wages for medical debt.9
Why is there so much hospital debt?
One would think the Affordable Care Act (ACA) and other reforms would mean fewer people do not have health insurance—and the problems experienced by the patient in the case above. Indeed, the number of insured has increased in the United States, including through the expansion of Medicaid. Nonetheless, in 2020, the Census Bureau reported that 28 million people did not have health insurance for any part of the year; that figure would be higher if those who had insurance for only part of the year were included.10
One reason for medical debt is the very high level of “under” insurance—that is, even with health insurance, copays for significant medical bills exceed what the patient can pay. Nearly half of adults (excluding the elderly) were enrolled in high-deductible health plans (in 2017).11 Among most employment-based plans, deductibles and co-pays have been going up for a decade.12 Overall, 20% of employer-provided plans had deductibles in excess of $3,000 ($5,000 for families).13 Of course, many families do not have anywhere near the resources to pay high deductibles, and that represents likely medical debt. The more modest copays of Medicare (often 20%) can be enough to push some elderly individuals beyond their capacity to pay.
“Out-of-network” care also may result in large hospital charges—and debt. Emergency care, for example, may be sought from the closest provider, even though out of network, and the insurance company may refuse to pay the charges. Another surprise form of billing is when a health care insurance company tentatively approves coverage and then after the patient receives care, determines it was unnecessary. In that case, even in-network charges may be denied, with the patient left to pay all the charges.
Continue to: How medical debt affects patients...
How medical debt affects patients
For patients, medical debt places pressure on their financial circumstances. Bankruptcy has a profound financial impact, and approximately two-thirds of bankruptcies are related to medical care costs and debt, including “indirect collection.”14 Even when the financial effect is not so devastating, it is often substantial, as the above case demonstrated. In a 2018 survey, almost 30% of those with health insurance had medical debts in some form of collection action, and 25% of those individuals said they did not know they owed the money.15 The same survey found that 20% of respondents had medical debt that adversely affected their credit scores and access to credit.15
At work, although employers are not supposed to treat employees adversely because of garnishment, some employers may not adhere to that rule. Furthermore, employees may believe or be concerned that the very existence of garnishment may penalize them at their current job or make it difficult to move to a better one.16
Lastly, patients with medical debt may be reluctant to seek needed medical care. They may be concerned about adding more medical debt or embarrassed or afraid that they would not be welcome at the hospital where they owe money.7
Public perception of hospitals
Lawsuits against patients also have a negative effect on hospitals—and it is not limited to the relatively few institutions that file many of these lawsuits each year. Press reports about lawsuits against patients garner great public interest and anger, and this tarnishes the image of heath care facilities in general because many people often do not distinguish the actions of a few institutions.
The sensitivity of health care organizations to bad publicity from debt collection practices was seen in a follow-up study of the previously discussed Virginia data. In the year following this report, there was a 59% decrease in the number of lawsuits filed, including a 66% decrease in garnishments.17 Eleven hospitals in the state that had been filing debt lawsuits stopped doing so.17
Medical debt: The obligation of nonprofit hospitals
The response seen in the Virginia follow-up study may also reflect well-founded concern from board members about political consequences and even taxation problems. The majority of hospitals, including those in these studies, are nonprofit institutions with an Internal Revenue Service (IRS) 501(c)(3) “tax-exempt” status. (Note, “nonprofit” does not mean that the organization does not make a profit, but that the profit does not accrue to individuals.) The “nonprofit” status is usually granted by states, but the federal tax-exempt status is granted by the IRS. This status exempts the institutions from paying most federal taxes, and (perhaps most importantly) qualifies donors to receive tax deductions (and similar benefits) for donations made to these hospitals. This important tax treatment is granted based on the theory that their services are so valuable to the public that advancing their work through the tax exemption ultimately benefits the public more than the tax revenue would.
In return for these benefits, the organization has obligations to work in the public interest. For years, hospitals have been criticized for not providing sufficient public benefits (compared, for example, with for-profit hospitals) to justify the tax exemption. That criticism caused the IRS to begin requiring a special Form 990, Schedule H, which is attached to the usual 501(c)(3) informational tax return, “to provide information on the activities and policies of, and community benefit provided by, its hospital facilities and other non-hospital health care facilities.”18 Part III of Schedule H asks, in part, about bad debt and collection practices.
Then the ACA Section 501(r) enhanced the obligation of nonprofit health facilities to provide charitable care in two ways. First, they must have, and make available, policies to provide free and discounted care; and second, they cannot sue for payment until they make an individualized determination as to whether the patient should have received discounted care or financial assistance.19
Thus aggressive collection practices (which should include “indirect collection”) invite special scrutiny by local officials and the IRS. In the longer-term, concern that tax-exempt hospitals are not truly operating in the public interest is undoubtedly amplified by these aggressive debt collection practices. How can a hospital claim it is truly operating in the public interest when it sues dozens of modest-income individuals each year?
Regulating medical debt and its collection
The No Surprises Act
In December 2020, Congress adopted the No Surprises Act to address some of the problems of patient debt.20 Among other things, the act protects patients “from receiving surprise medical bills when they receive most emergency services,” or when they are in an in-network hospital but receive services from out-of-network providers (such as anesthesia and radiology).21 Several states also have similar legislation, so the federal law specifically states that where state laws are more protective of patients, the state’s higher protections apply, and vice versa. The act took effect on January 1, 2022, though there is an “interim final” regulation that will be subject to change, and there is already litigation over those regulations.22 The real complexity of the rules will arise through the regulations, which are likely to change several times over the next few years. To help with this, the American Medical Association has an extensive toolkit for health care providers.23
Continue to: Additional regulations...
Additional regulations
Both the federal government and most states are likely to take additional action to reduce hospital debt lawsuits. Some proposals sound simple enough but would have significant complications. For example, governments could prohibit all lawsuits that collect hospital debt.7 Such a regulation would mean that paying hospital debts would essentially become optional. Imagine the millionaire who does not want to pay a $25,000 hospital charge; or patients with other debts who would pay those off before the hospital debt. The regulation might have income or asset limits on debt collection lawsuits and the like, but it quickly becomes complicated. Furthermore, to protect themselves, hospitals would undoubtedly become much more aggressive about requiring up-front payments—which would force the debt or prepayment onto credit cards or similar debt obligations that are not subject to the no collection lawsuit rule.
Public reporting. The follow-up study in Virginia17 suggests that requiring public reporting of the number of cases filed by or on behalf of (directly or indirectly) each hospital may help. Hospitals would, of course, have incentives to make their figures look better, perhaps by selling the debt to an agency that would be able to file suit in its name rather than the hospital’s name. These might be little more than indirect collections. For reporting purposes, any form of transferring debt might be considered filing a lawsuit. The problem, noted earlier, about requiring prepayment or credit cards would also exist.
Get the board involved. A different approach would be to ensure that a hospital’s board of trustees is involved in setting and overseeing debt collection policies. For example, the law might require boards to annually consider and adopt specific debt collection practices—including indirect collection efforts. Boards should already be doing something similar to this, but regulation might be an inexpensive way to ensure it is done—and in a manner consistent with the organization’s values. Another suggestion is to require the board to approve any legal action against specific patients.7 By making sure this is not just another item on the consent agenda, the oversight would probably reduce automatic debt collection processes.
Expand IRS reporting requirements for nonprofits. Indeed, for nonprofit hospitals with 501(c)(3) obligations, the Form 990, Schedule H already provides some information about collection actions and uncompensated care, and this is enhanced by the ACA Section 501(r). These could be expanded and perhaps include “indirect” collections. The IRS could “flag” hospitals with high total litigation and similar collection actions, and ask the hospital to provide a detailed explanation for each action and how it was consistent with the obligation to serve the public (thereby justifying the exempt taxation status, an idea proposed by the US Government Accountability Office in 2020).24
Ensure the hospital’s actions reflect their mission and values
Hospitals are created to provide medical care for people and to improve the human condition. Those who lead them should, and generally do, share that purpose. The apparent collection policies that have garnered negative public attention suggest that some of these institutions have lost focus of their ultimate mission and values. The boards and executives of these health care institutions, as well as the medical professionals and attorneys who serve them, should be continuously guided by those values.
Important decisions—including collection and prepayment processes—reflect the values of the institution. Failure to ensure these procedures are in line with the organization’s mission is an embarrassment to all health care facilities, including the majority of hospitals that do not engage in these aggressive collection practices. Not addressing these issues will likely result in political and legal action—blunt and inefficient instruments—to limit what the public sees as wrongdoing. ●
Although it is common to read about patients suing their hospitals, there has been increasing public and political attention given to hospitals suing their patients to collect unpaid hospital bills. KH’s story began with an emergency appendectomy. She did not have health insurance to cover the $14,000 hospital bill. The family was unable to pay the bill, and the nonprofit hospital sued them for that bill, plus some additional expenses (totaling about $17,000), plus interest was accumulating at 9% per year. The hospital won a judgment, and it garnished the husband’s pay (10% of after-taxes pay, in this case) and placed a lien on the family’s home. Years later—because of interest and additional hospital bills—the family had paid $20,000, but still owed $26,000.1
The extent of the problem
This is neither a hypothetical case nor a rare event. Studies and press reports have noted dozens of examples of hospital collection excesses. One study found that unpaid medical bill lawsuits increased by 37% in Wisconsin between 2001 and 2018, with 5% of hospitals accounting for 25% of the lawsuits.2 Another report found almost “31,000 civil cases filed by 139 hospitals in 26 New York counties from 2015 to 2019.”3 Similar to the Wisconsin report, a small number of health care providers accounted for the majority of lawsuits. In another example, one Missouri nonprofit hospital, Heartland (rebranded “Mosaic”), created its own for-profit debt collection agency (Northwest Financial Services), which filed 11,000 lawsuits from 2009 to 2013, resulting in 6,000 wage garnishments.1 The Wall Street Journal, among others, has reported for years on the difficulties created by lawsuits against patients.4 Axios and Johns Hopkins reported that “medical debt comprises 58% of all debt collections in the United States.” And although some collection actions declined early in the pandemic, it did not appear to last.5,6
Inconsistent collection policies. Collection policies vary greatly from hospital to hospital, with an increasing number of hospitals demanding up-front payments (before services). Many of these health care institutions persuade patients to put medical debt on their credit cards, sometimes as part of an up-front (before service) process.7 If using a standard credit card, this comes with a very high interest rate. There are some special health-related credit cards, such as CareCredit, that generally have better interest rates. These cards offer no-interest short-term loans, with significant interest for longer-term loans. Thus, failure to repay the full amount when due means that the “deferred interest” (about 27%) must be paid.8 Also any of the problems patients have repaying a credit card (or other loan), of course, are no longer directly related to the hospital. These “indirect collections” still burden patients with medical debt.
Where you go matters. Because there is no common collection policy or practice among hospitals, choosing the wrong hospital may result in a lawsuit. A careful study of lawsuits for medical debt or garnishments related to that debt in 2017 in Virginia showed how being treated at certain hospitals dramatically changed the odds of wage garnishment for unpaid bills.9 It revealed that 29,286 hospital lawsuits were filed to collect medical debt—9,232 of which were wage garnishments (the most aggressive form of debt collection). Five hospitals alone accounted for the majority of garnishments in the state. Notably, nonprofit hospitals accounted for 71% of the garnishment cases. On the other hand, about 50% of the hospitals in the study did not file any lawsuits to garnish wages for medical debt.9
Why is there so much hospital debt?
One would think the Affordable Care Act (ACA) and other reforms would mean fewer people do not have health insurance—and the problems experienced by the patient in the case above. Indeed, the number of insured has increased in the United States, including through the expansion of Medicaid. Nonetheless, in 2020, the Census Bureau reported that 28 million people did not have health insurance for any part of the year; that figure would be higher if those who had insurance for only part of the year were included.10
One reason for medical debt is the very high level of “under” insurance—that is, even with health insurance, copays for significant medical bills exceed what the patient can pay. Nearly half of adults (excluding the elderly) were enrolled in high-deductible health plans (in 2017).11 Among most employment-based plans, deductibles and co-pays have been going up for a decade.12 Overall, 20% of employer-provided plans had deductibles in excess of $3,000 ($5,000 for families).13 Of course, many families do not have anywhere near the resources to pay high deductibles, and that represents likely medical debt. The more modest copays of Medicare (often 20%) can be enough to push some elderly individuals beyond their capacity to pay.
“Out-of-network” care also may result in large hospital charges—and debt. Emergency care, for example, may be sought from the closest provider, even though out of network, and the insurance company may refuse to pay the charges. Another surprise form of billing is when a health care insurance company tentatively approves coverage and then after the patient receives care, determines it was unnecessary. In that case, even in-network charges may be denied, with the patient left to pay all the charges.
Continue to: How medical debt affects patients...
How medical debt affects patients
For patients, medical debt places pressure on their financial circumstances. Bankruptcy has a profound financial impact, and approximately two-thirds of bankruptcies are related to medical care costs and debt, including “indirect collection.”14 Even when the financial effect is not so devastating, it is often substantial, as the above case demonstrated. In a 2018 survey, almost 30% of those with health insurance had medical debts in some form of collection action, and 25% of those individuals said they did not know they owed the money.15 The same survey found that 20% of respondents had medical debt that adversely affected their credit scores and access to credit.15
At work, although employers are not supposed to treat employees adversely because of garnishment, some employers may not adhere to that rule. Furthermore, employees may believe or be concerned that the very existence of garnishment may penalize them at their current job or make it difficult to move to a better one.16
Lastly, patients with medical debt may be reluctant to seek needed medical care. They may be concerned about adding more medical debt or embarrassed or afraid that they would not be welcome at the hospital where they owe money.7
Public perception of hospitals
Lawsuits against patients also have a negative effect on hospitals—and it is not limited to the relatively few institutions that file many of these lawsuits each year. Press reports about lawsuits against patients garner great public interest and anger, and this tarnishes the image of heath care facilities in general because many people often do not distinguish the actions of a few institutions.
The sensitivity of health care organizations to bad publicity from debt collection practices was seen in a follow-up study of the previously discussed Virginia data. In the year following this report, there was a 59% decrease in the number of lawsuits filed, including a 66% decrease in garnishments.17 Eleven hospitals in the state that had been filing debt lawsuits stopped doing so.17
Medical debt: The obligation of nonprofit hospitals
The response seen in the Virginia follow-up study may also reflect well-founded concern from board members about political consequences and even taxation problems. The majority of hospitals, including those in these studies, are nonprofit institutions with an Internal Revenue Service (IRS) 501(c)(3) “tax-exempt” status. (Note, “nonprofit” does not mean that the organization does not make a profit, but that the profit does not accrue to individuals.) The “nonprofit” status is usually granted by states, but the federal tax-exempt status is granted by the IRS. This status exempts the institutions from paying most federal taxes, and (perhaps most importantly) qualifies donors to receive tax deductions (and similar benefits) for donations made to these hospitals. This important tax treatment is granted based on the theory that their services are so valuable to the public that advancing their work through the tax exemption ultimately benefits the public more than the tax revenue would.
In return for these benefits, the organization has obligations to work in the public interest. For years, hospitals have been criticized for not providing sufficient public benefits (compared, for example, with for-profit hospitals) to justify the tax exemption. That criticism caused the IRS to begin requiring a special Form 990, Schedule H, which is attached to the usual 501(c)(3) informational tax return, “to provide information on the activities and policies of, and community benefit provided by, its hospital facilities and other non-hospital health care facilities.”18 Part III of Schedule H asks, in part, about bad debt and collection practices.
Then the ACA Section 501(r) enhanced the obligation of nonprofit health facilities to provide charitable care in two ways. First, they must have, and make available, policies to provide free and discounted care; and second, they cannot sue for payment until they make an individualized determination as to whether the patient should have received discounted care or financial assistance.19
Thus aggressive collection practices (which should include “indirect collection”) invite special scrutiny by local officials and the IRS. In the longer-term, concern that tax-exempt hospitals are not truly operating in the public interest is undoubtedly amplified by these aggressive debt collection practices. How can a hospital claim it is truly operating in the public interest when it sues dozens of modest-income individuals each year?
Regulating medical debt and its collection
The No Surprises Act
In December 2020, Congress adopted the No Surprises Act to address some of the problems of patient debt.20 Among other things, the act protects patients “from receiving surprise medical bills when they receive most emergency services,” or when they are in an in-network hospital but receive services from out-of-network providers (such as anesthesia and radiology).21 Several states also have similar legislation, so the federal law specifically states that where state laws are more protective of patients, the state’s higher protections apply, and vice versa. The act took effect on January 1, 2022, though there is an “interim final” regulation that will be subject to change, and there is already litigation over those regulations.22 The real complexity of the rules will arise through the regulations, which are likely to change several times over the next few years. To help with this, the American Medical Association has an extensive toolkit for health care providers.23
Continue to: Additional regulations...
Additional regulations
Both the federal government and most states are likely to take additional action to reduce hospital debt lawsuits. Some proposals sound simple enough but would have significant complications. For example, governments could prohibit all lawsuits that collect hospital debt.7 Such a regulation would mean that paying hospital debts would essentially become optional. Imagine the millionaire who does not want to pay a $25,000 hospital charge; or patients with other debts who would pay those off before the hospital debt. The regulation might have income or asset limits on debt collection lawsuits and the like, but it quickly becomes complicated. Furthermore, to protect themselves, hospitals would undoubtedly become much more aggressive about requiring up-front payments—which would force the debt or prepayment onto credit cards or similar debt obligations that are not subject to the no collection lawsuit rule.
Public reporting. The follow-up study in Virginia17 suggests that requiring public reporting of the number of cases filed by or on behalf of (directly or indirectly) each hospital may help. Hospitals would, of course, have incentives to make their figures look better, perhaps by selling the debt to an agency that would be able to file suit in its name rather than the hospital’s name. These might be little more than indirect collections. For reporting purposes, any form of transferring debt might be considered filing a lawsuit. The problem, noted earlier, about requiring prepayment or credit cards would also exist.
Get the board involved. A different approach would be to ensure that a hospital’s board of trustees is involved in setting and overseeing debt collection policies. For example, the law might require boards to annually consider and adopt specific debt collection practices—including indirect collection efforts. Boards should already be doing something similar to this, but regulation might be an inexpensive way to ensure it is done—and in a manner consistent with the organization’s values. Another suggestion is to require the board to approve any legal action against specific patients.7 By making sure this is not just another item on the consent agenda, the oversight would probably reduce automatic debt collection processes.
Expand IRS reporting requirements for nonprofits. Indeed, for nonprofit hospitals with 501(c)(3) obligations, the Form 990, Schedule H already provides some information about collection actions and uncompensated care, and this is enhanced by the ACA Section 501(r). These could be expanded and perhaps include “indirect” collections. The IRS could “flag” hospitals with high total litigation and similar collection actions, and ask the hospital to provide a detailed explanation for each action and how it was consistent with the obligation to serve the public (thereby justifying the exempt taxation status, an idea proposed by the US Government Accountability Office in 2020).24
Ensure the hospital’s actions reflect their mission and values
Hospitals are created to provide medical care for people and to improve the human condition. Those who lead them should, and generally do, share that purpose. The apparent collection policies that have garnered negative public attention suggest that some of these institutions have lost focus of their ultimate mission and values. The boards and executives of these health care institutions, as well as the medical professionals and attorneys who serve them, should be continuously guided by those values.
Important decisions—including collection and prepayment processes—reflect the values of the institution. Failure to ensure these procedures are in line with the organization’s mission is an embarrassment to all health care facilities, including the majority of hospitals that do not engage in these aggressive collection practices. Not addressing these issues will likely result in political and legal action—blunt and inefficient instruments—to limit what the public sees as wrongdoing. ●
- Kiel P. From the E.R. to the courtroom: how nonprofit hospitals are seizing patients’ wages. ProPublica. December 19, 2014. Accessed March 21, 2022. https://www.propublica.org/article/how-nonprofit-hospitals-are-seizing-patients-wages
- Cooper Z, Han J, Mahoney N. Hospital lawsuits over unpaid bills increased by 37 percent in Wisconsin from 2001 to 2018. Health Affairs. 2021;40:1830-1835. Accessed March 21, 2022. https://www.healthaffairs.org/doi/full/10.1377 /hlthaff.2021.01130
- LaMantia J. New York hospitals have filed thousands of lawsuits against patients. Modern Healthcare. March 13, 2020. Accessed March 21, 2022. https://www.modernhealthcare .com/legal/new-york-hospitals-have-filed-thousands -lawsuits-against-patients
- Armour S. When patients can’t pay, many hospitals are suing. Wall Street Journal. June 25, 2019. Accessed March 21, 2022. https://www.wsj.com/articles/nonprofit-hospitals-criticized-for-debt-collection-tactics-11561467600
- McGhee M, Chase W. How America’s top hospitals hound patients with predatory billing. Axios. Accessed March 21, 2022. https://www.axios.com/hospital-billing
- Owens C. Public spotlight on hospital lawsuits may slow them down. June 14, 2021. Accessed March 22, 2022. https:// www.axios.com/hospital-lawsuits-slowing-down-media -35ce395a-9fe3-4b23-b815-d7b06cce2773.html
- Buck ID. When hospitals sue patients. Hastings L.J. 2022;73:191-232, at 209-211. Accessed March 21, 2022. https:// repository.uchastings.edu/cgi/viewcontent.cgi?article =3961&context=hastings_law_journal
- Lagasse J. Healthcare turns to zero-interest loans to give patients a better reason to pay. Healthcare Finance. May 3, 2017. Accessed March 21, 2022. https://www.healthcarefinancenews.com/news/healthcare-turns-zero-interest-loans-give-patients-better-reason-pay#:~:text=Zero%2Dinterest%20loans%20are%20finding,of%20the%20patient%2Dprovider%20relationship.
- Bruhn WE, Rutkow L, Wang P, et al. Prevalence and characteristics of Virginia hospitals suing patients and garnishing wages for unpaid medical bills. JAMA. 2019;322:691-692. doi:10.1001/jama.2019.9144
- Keisler-Starkey K, Bunch LN. Health insurance coverage in the United States: 2020. September 14, 2021. United States Census Bureau Current Population Reports, P60-274. US Government Publishing Office; September 2021. Accessed March 21, 2022. https://www.census.gov/content/dam /Census/library/publications/2021/demo/p60-274.pdf
- Cohen RA, Zammitti EP. High-deductible health plan enrollment among adults aged 18-64 with employment-based insurance coverage. NCHS Data Brief, No. 317. August 2018. Accessed March 21, 2022. https://www.cdc.gov/nchs/data/databriefs/db317.pdf
- Kaiser Family Foundation. Employer health benefits: 2020 summary of findings. Accessed March 21, 2022. https://www.kff.org/report-section/ehbs-2020-summary-of-findings/
- Picchi A. Higher health insurance deductibles a sickening trend for Americans. CBS NEWS. June 13, 2019. Accessed March 21, 2022. https://www.cbsnews.com/news/high-health-insurance-deductibles-a-sickening-trend-thats -causing-financial-hardship/
- Himmelstein DU, Lawless RM, Thorne D, Foohey P, Woolhandler S. Medical bankruptcy: still common despite the Affordable Care Act. Am J Public Health. 2019;109:431-433. doi:10.2105/AJPH.2018.304901
- Rosato D. What medical debt does to your credit score. Consumer Reports. July 26, 2018. Accessed March 21, 2022. https://www.consumerreports.org/credit-scores-reports/what-medical-debt-does-to-your-credit-score/
- State laws on wage garnishments. Nolo web site. https://www.nolo.com/legal-encyclopedia/free-books/employee-rights-book/chapter2-9.html. Accessed April 1, 2022.
- Patruzo JGR, Hashim F, Dun C, et al. Trends in hospital lawsuits filed against patients for unpaid bills following published research about their activity. JAMA Network Open. 2021;4:e2121926. doi:10.1001/jamanetworkopen.2021.21926
- About Schedule H (Form 990), hospitals. IRS. Updated June 10, 2021. Accessed March 21, 2022. https://www.irs.gov/forms-pubs/about-schedule-h-form-990
- Requirements for 501(c)(3) hospitals under the Affordable Care Act – Section 501(r). Updated September 9, 2021. Accessed March 21, 2022. https://www.irs.gov/charities-non-profits/charitable-organizations/requirements-for-501c3-hospitals-under-the-affordable-care-act-section-501r
- Pub. L. No. 116-260, 134 Stat. 1182, Division BB, § 109.
- Fact sheet. No Surprises: understand your rights against surprise medical bills. Centers for Medicare and Medicaid Services. January 3, 2022. Accessed March 21, 2022. https://www.cms.gov/newsroom/fact-sheets/no-surprises-understand-your-rights-against-surprise-medical-bills
- Implementation of the No Surprises Act. Accessed March 21, 2022. https://www.ama-assn.org/delivering-care/patient-support-advocacy/implementation-no-surprises-act
- American Medical Association. Toolkit for physicians: preparing for implementation of the No Surprises Act. January 2022. Accessed March 21, 2022. https://www.ama-assn.org/system/files/ama-nsa-toolkit.pdf
- US Government Accountability Office. Tax administration: opportunities exist to improve oversight of hospitals’ taxexempt status. September 2020. Accessed March 21, 2022. https://www.gao.gov/assets/gao-20-679.pdf
- Kiel P. From the E.R. to the courtroom: how nonprofit hospitals are seizing patients’ wages. ProPublica. December 19, 2014. Accessed March 21, 2022. https://www.propublica.org/article/how-nonprofit-hospitals-are-seizing-patients-wages
- Cooper Z, Han J, Mahoney N. Hospital lawsuits over unpaid bills increased by 37 percent in Wisconsin from 2001 to 2018. Health Affairs. 2021;40:1830-1835. Accessed March 21, 2022. https://www.healthaffairs.org/doi/full/10.1377 /hlthaff.2021.01130
- LaMantia J. New York hospitals have filed thousands of lawsuits against patients. Modern Healthcare. March 13, 2020. Accessed March 21, 2022. https://www.modernhealthcare .com/legal/new-york-hospitals-have-filed-thousands -lawsuits-against-patients
- Armour S. When patients can’t pay, many hospitals are suing. Wall Street Journal. June 25, 2019. Accessed March 21, 2022. https://www.wsj.com/articles/nonprofit-hospitals-criticized-for-debt-collection-tactics-11561467600
- McGhee M, Chase W. How America’s top hospitals hound patients with predatory billing. Axios. Accessed March 21, 2022. https://www.axios.com/hospital-billing
- Owens C. Public spotlight on hospital lawsuits may slow them down. June 14, 2021. Accessed March 22, 2022. https:// www.axios.com/hospital-lawsuits-slowing-down-media -35ce395a-9fe3-4b23-b815-d7b06cce2773.html
- Buck ID. When hospitals sue patients. Hastings L.J. 2022;73:191-232, at 209-211. Accessed March 21, 2022. https:// repository.uchastings.edu/cgi/viewcontent.cgi?article =3961&context=hastings_law_journal
- Lagasse J. Healthcare turns to zero-interest loans to give patients a better reason to pay. Healthcare Finance. May 3, 2017. Accessed March 21, 2022. https://www.healthcarefinancenews.com/news/healthcare-turns-zero-interest-loans-give-patients-better-reason-pay#:~:text=Zero%2Dinterest%20loans%20are%20finding,of%20the%20patient%2Dprovider%20relationship.
- Bruhn WE, Rutkow L, Wang P, et al. Prevalence and characteristics of Virginia hospitals suing patients and garnishing wages for unpaid medical bills. JAMA. 2019;322:691-692. doi:10.1001/jama.2019.9144
- Keisler-Starkey K, Bunch LN. Health insurance coverage in the United States: 2020. September 14, 2021. United States Census Bureau Current Population Reports, P60-274. US Government Publishing Office; September 2021. Accessed March 21, 2022. https://www.census.gov/content/dam /Census/library/publications/2021/demo/p60-274.pdf
- Cohen RA, Zammitti EP. High-deductible health plan enrollment among adults aged 18-64 with employment-based insurance coverage. NCHS Data Brief, No. 317. August 2018. Accessed March 21, 2022. https://www.cdc.gov/nchs/data/databriefs/db317.pdf
- Kaiser Family Foundation. Employer health benefits: 2020 summary of findings. Accessed March 21, 2022. https://www.kff.org/report-section/ehbs-2020-summary-of-findings/
- Picchi A. Higher health insurance deductibles a sickening trend for Americans. CBS NEWS. June 13, 2019. Accessed March 21, 2022. https://www.cbsnews.com/news/high-health-insurance-deductibles-a-sickening-trend-thats -causing-financial-hardship/
- Himmelstein DU, Lawless RM, Thorne D, Foohey P, Woolhandler S. Medical bankruptcy: still common despite the Affordable Care Act. Am J Public Health. 2019;109:431-433. doi:10.2105/AJPH.2018.304901
- Rosato D. What medical debt does to your credit score. Consumer Reports. July 26, 2018. Accessed March 21, 2022. https://www.consumerreports.org/credit-scores-reports/what-medical-debt-does-to-your-credit-score/
- State laws on wage garnishments. Nolo web site. https://www.nolo.com/legal-encyclopedia/free-books/employee-rights-book/chapter2-9.html. Accessed April 1, 2022.
- Patruzo JGR, Hashim F, Dun C, et al. Trends in hospital lawsuits filed against patients for unpaid bills following published research about their activity. JAMA Network Open. 2021;4:e2121926. doi:10.1001/jamanetworkopen.2021.21926
- About Schedule H (Form 990), hospitals. IRS. Updated June 10, 2021. Accessed March 21, 2022. https://www.irs.gov/forms-pubs/about-schedule-h-form-990
- Requirements for 501(c)(3) hospitals under the Affordable Care Act – Section 501(r). Updated September 9, 2021. Accessed March 21, 2022. https://www.irs.gov/charities-non-profits/charitable-organizations/requirements-for-501c3-hospitals-under-the-affordable-care-act-section-501r
- Pub. L. No. 116-260, 134 Stat. 1182, Division BB, § 109.
- Fact sheet. No Surprises: understand your rights against surprise medical bills. Centers for Medicare and Medicaid Services. January 3, 2022. Accessed March 21, 2022. https://www.cms.gov/newsroom/fact-sheets/no-surprises-understand-your-rights-against-surprise-medical-bills
- Implementation of the No Surprises Act. Accessed March 21, 2022. https://www.ama-assn.org/delivering-care/patient-support-advocacy/implementation-no-surprises-act
- American Medical Association. Toolkit for physicians: preparing for implementation of the No Surprises Act. January 2022. Accessed March 21, 2022. https://www.ama-assn.org/system/files/ama-nsa-toolkit.pdf
- US Government Accountability Office. Tax administration: opportunities exist to improve oversight of hospitals’ taxexempt status. September 2020. Accessed March 21, 2022. https://www.gao.gov/assets/gao-20-679.pdf
Infectious disease pop quiz: Clinical challenges for the ObGyn
In this question-and-answer article (the third in a series), our objective is to reinforce for the clinician several practical points of management for common infectious diseases. The principal references for the answers to the questions are 2 textbook chapters written by Dr. Duff.1,2 Other pertinent references are included in the text.
21. What prophylactic antibiotic should be administered intrapartum to a pregnant woman who is colonized with group B streptococci but who has a mild allergy to penicillin?
In this situation, the drug of choice is intravenous cefazolin, 2 g initially then 1 g every 8 hours until delivery. For patients with a severe allergy to penicillin, the drugs of choice are either clindamycin, 900 mg intravenously every 8 hours (if sensitivity of the organism is confirmed), or vancomycin, 20 mg/kg intravenously every 8 hours (maximum of 2 g per single dose).
22. In a pregnant woman who has a life-threatening allergy to penicillin, what is the most appropriate treatment for syphilis?
This patient should be admitted to the hospital and rapidly desensitized to penicillin. She then can be treated with the appropriate dose of penicillin, given her stage of syphilis. Of note, in the future, the patient’s allergy to penicillin will return, despite the brief period of desensitization.
23. What are the most common organisms that cause chorioamnionitis and puerperal endometritis?
Chorioamnionitis and puerperal endometritis are polymicrobial, mixed aerobic-anaerobic infections. The dominant organisms are anaerobic gram-negative bacilli (Bacteroides and Prevotella species); anaerobic gram-positive cocci (Peptococcus species and Peptostreptococcus species); aerobic gram-negative bacilli (principally, Escherichia coli, Klebsiella pneumoniae, and Proteus species); and aerobic gram-positive cocci (enterococci, staphylococci, and group B streptococci). ●
- Duff P. Maternal and perinatal infections: bacterial. In: Landon MB, Galan HL, Jauniaux ERM, et al. Gabbe’s Obstetrics: Normal and Problem Pregnancies. 8th ed. Elsevier; 2021:1124-1146.
- Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore TJ, et al. Creasy & Resnik’s Maternal-Fetal Medicine: Principles and Practice. 8th ed. Elsevier; 2019:862-919.
In this question-and-answer article (the third in a series), our objective is to reinforce for the clinician several practical points of management for common infectious diseases. The principal references for the answers to the questions are 2 textbook chapters written by Dr. Duff.1,2 Other pertinent references are included in the text.
21. What prophylactic antibiotic should be administered intrapartum to a pregnant woman who is colonized with group B streptococci but who has a mild allergy to penicillin?
In this situation, the drug of choice is intravenous cefazolin, 2 g initially then 1 g every 8 hours until delivery. For patients with a severe allergy to penicillin, the drugs of choice are either clindamycin, 900 mg intravenously every 8 hours (if sensitivity of the organism is confirmed), or vancomycin, 20 mg/kg intravenously every 8 hours (maximum of 2 g per single dose).
22. In a pregnant woman who has a life-threatening allergy to penicillin, what is the most appropriate treatment for syphilis?
This patient should be admitted to the hospital and rapidly desensitized to penicillin. She then can be treated with the appropriate dose of penicillin, given her stage of syphilis. Of note, in the future, the patient’s allergy to penicillin will return, despite the brief period of desensitization.
23. What are the most common organisms that cause chorioamnionitis and puerperal endometritis?
Chorioamnionitis and puerperal endometritis are polymicrobial, mixed aerobic-anaerobic infections. The dominant organisms are anaerobic gram-negative bacilli (Bacteroides and Prevotella species); anaerobic gram-positive cocci (Peptococcus species and Peptostreptococcus species); aerobic gram-negative bacilli (principally, Escherichia coli, Klebsiella pneumoniae, and Proteus species); and aerobic gram-positive cocci (enterococci, staphylococci, and group B streptococci). ●
In this question-and-answer article (the third in a series), our objective is to reinforce for the clinician several practical points of management for common infectious diseases. The principal references for the answers to the questions are 2 textbook chapters written by Dr. Duff.1,2 Other pertinent references are included in the text.
21. What prophylactic antibiotic should be administered intrapartum to a pregnant woman who is colonized with group B streptococci but who has a mild allergy to penicillin?
In this situation, the drug of choice is intravenous cefazolin, 2 g initially then 1 g every 8 hours until delivery. For patients with a severe allergy to penicillin, the drugs of choice are either clindamycin, 900 mg intravenously every 8 hours (if sensitivity of the organism is confirmed), or vancomycin, 20 mg/kg intravenously every 8 hours (maximum of 2 g per single dose).
22. In a pregnant woman who has a life-threatening allergy to penicillin, what is the most appropriate treatment for syphilis?
This patient should be admitted to the hospital and rapidly desensitized to penicillin. She then can be treated with the appropriate dose of penicillin, given her stage of syphilis. Of note, in the future, the patient’s allergy to penicillin will return, despite the brief period of desensitization.
23. What are the most common organisms that cause chorioamnionitis and puerperal endometritis?
Chorioamnionitis and puerperal endometritis are polymicrobial, mixed aerobic-anaerobic infections. The dominant organisms are anaerobic gram-negative bacilli (Bacteroides and Prevotella species); anaerobic gram-positive cocci (Peptococcus species and Peptostreptococcus species); aerobic gram-negative bacilli (principally, Escherichia coli, Klebsiella pneumoniae, and Proteus species); and aerobic gram-positive cocci (enterococci, staphylococci, and group B streptococci). ●
- Duff P. Maternal and perinatal infections: bacterial. In: Landon MB, Galan HL, Jauniaux ERM, et al. Gabbe’s Obstetrics: Normal and Problem Pregnancies. 8th ed. Elsevier; 2021:1124-1146.
- Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore TJ, et al. Creasy & Resnik’s Maternal-Fetal Medicine: Principles and Practice. 8th ed. Elsevier; 2019:862-919.
- Duff P. Maternal and perinatal infections: bacterial. In: Landon MB, Galan HL, Jauniaux ERM, et al. Gabbe’s Obstetrics: Normal and Problem Pregnancies. 8th ed. Elsevier; 2021:1124-1146.
- Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore TJ, et al. Creasy & Resnik’s Maternal-Fetal Medicine: Principles and Practice. 8th ed. Elsevier; 2019:862-919.
SERMs revisited: Can they improve menopausal care?
Selective estrogen receptor modulators (SERMs) are unique synthetic compounds that bind to the estrogen receptor and initiate either estrogenic agonistic or antagonistic activity, depending on the confirmational change they produce on binding to the receptor. Many SERMs have come to market, others have not. Unlike estrogens, which regardless of dose or route of administration all carry risks as a boxed warning on the label, referred to as class labeling,1 various SERMs exert various effects in some tissues (uterus, vagina) while they have apparent class properties in others (bone, breast).2
The first SERM, for all practical purposes, was tamoxifen (although clomiphene citrate is often considered a SERM). Tamoxifen was approved by the US Food and Drug Administration (FDA) in 1978 for the treatment of breast cancer and, subsequently, for breast cancer risk reduction. It became the most widely prescribed anticancer drug worldwide.
Subsequently, when data showed that tamoxifen could produce a small number of endometrial cancers and a larger number of endometrial polyps,3,4 there was renewed interest in raloxifene. In preclinical animal studies, raloxifene behaved differently than tamoxifen in the uterus. After clinical trials with raloxifene showed uterine safety,5 the drug was FDA approved for prevention of osteoporosis in 1997, for treatment of osteoporosis in 1999, and for breast cancer risk reduction in 2009. Most clinicians are familiar with these 2 SERMs, which have been in clinical use for more than 4 and 2 decades, respectively.
Ospemifene: A third-generation SERM and its indications
Hormone deficiency from menopause causes vulvovaginal and urogenital changes as well as a multitude of symptoms and signs, including vulvar and vaginal thinning, loss of rugal folds, diminished elasticity, increased pH, and most notably dyspareunia. The nomenclature that previously described vulvovaginal atrophy (VVA) has been expanded to include genitourinary syndrome of menopause (GSM).6 Unfortunately, many health care providers do not ask patients about GSM symptoms, and few women report their symptoms to their clinician.7 Furthermore, although low-dose local estrogens applied vaginally have been the mainstay of therapy for VVA/GSM, only 7% of symptomatic women use any pharmacologic agent,8 mainly because of fear of estrogens due to the class labeling mentioned above.
Ospemifene, a newer SERM, improved superficial cells and reduced parabasal cells as seen on a maturation index compared with placebo, according to results of multiple phase 3 clinical trials9,10; it also lowered vaginal pH and improved most bothersome symptoms (original studies were for dyspareunia). As a result, the FDA approved ospemifene for treatment of moderate to severe dyspareunia from VVA of menopause.
Subsequent studies allowed for a broadened indication to include treatment of moderate to severe dryness due to menopause.11 The ospemifene label contains a boxed warning that states, “In the endometrium, [ospemifene] has estrogen agonistic effects.”12 Although ospemifene is not an estrogen (it’s a SERM), the label goes on to state, “There is an increased risk of endometrial cancer in a woman with a uterus who uses unopposed estrogens.” This statement caused The Medical Letter to initially suggest that patients who receive ospemifene also should receive a progestational agent—a suggestion they later retracted.13,14
To understand why the ospemifene labeling might be worded in such a way, one must review the data regarding the poorly named entity “weakly proliferative endometrium.” The package labeling combines any proliferative endometrium (“weakly” plus “actively” plus “disordered”) that occurred in the clinical trial. Thus, 86.1 per 1,000 of the ospemifene-treated patients (vs 13.3 per 1,000 of those taking placebo) had any one of the proliferative types. The problem is that “actively proliferative” endometrial glands will have mitotic activity in virtually every nucleus of the gland as well as abundant glandular progression (FIGURE 1), whereas “weakly proliferative” is actually closer to inactive or atrophic endometrium with an occasional mitotic figure in only a few nuclei of each gland (FIGURE 2).
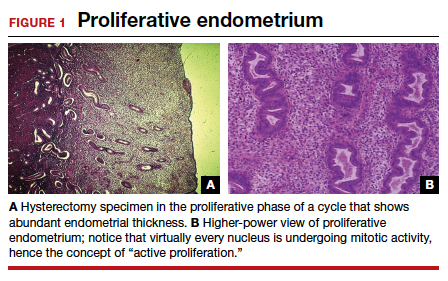
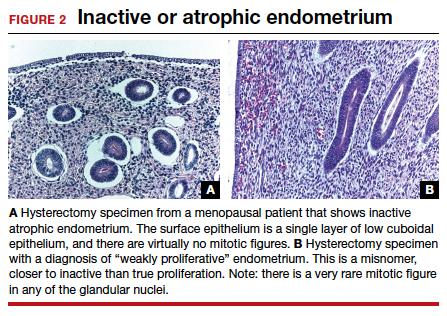
In addition, at 1 year, the incidence of active proliferation with ospemifene was 1%.15 In examining the uterine safety study for raloxifene, both doses of that agent had an active proliferation incidence of 3% at 1 year.5 Furthermore, that study had an estrogen-only arm in which, at end point, the incidence of endometrial proliferation was 39%, and hyperplasia, 23%!5 It therefore is evident that, in the endometrium, ospemifene is much more like the SERM raloxifene than it is like estrogen. The American College of Obstetricians and Gynecologists (ACOG) endorsed ospemifene (level A evidence) as a first-line therapy for dyspareunia, noting absent endometrial stimulation.16
Continue to: Ospemifene effects on breast and bone...
Ospemifene effects on breast and bone
Although ospemifene is approved for treatment of moderate to severe VVA/GSM, it has other SERM effects typical of its class. The label currently states that ospemifene “has not been adequately studied in women with breast cancer; therefore, it should not be used in women with known or suspected breast cancer.”12 We know that tamoxifen reduced breast cancer 49% in high-risk women in the Breast Cancer Prevention Trial (BCPT).17 We also know that in the Multiple Outcomes of Raloxifene Evaluation (MORE) trial, raloxifene reduced breast cancer 77% in osteoporotic women,18 and in the Study of Tamoxifen and Raloxifene (STAR) trial, it performed virtually identically to tamoxifen in breast cancer prevention.19 Previous studies demonstrated that ospemifene inhibits breast cancer cell growth in in vitro cultures as well as in animal studies20 and inhibits proliferation of human breast tissue epithelial cells,21 with breast effects similar to those seen with tamoxifen and raloxifene.
Thus, although one would not choose ospemifene as a primary treatment or risk-reducing agent for a patient with breast cancer, the direction of its activity in breast tissue is indisputable and is likely the reason that in the European Union (unlike in the United States) it is approved to treat dyspareunia from VVA/GSM in women with a prior history of breast cancer.
Virtually all SERMs have estrogen agonistic activity in bone. Bone is a dynamic organ, constantly being laid down and taken away (resorption). Estrogen and SERMs are potent antiresorptives in bone metabolism. Ospemifene effectively reduced bone loss in ovariectomized rats, with activity comparable to that of estradiol and raloxifene.22 Clinical data from 3 phase 1 or 2 clinical trials found that ospemifene 60 mg/day had a positive effect on biochemical markers for bone turnover in healthy postmenopausal women, with significant improvements relative to placebo and effects comparable to those of raloxifene.23 Actual fracture or bone mineral density (BMD) data in postmenopausal women are lacking, but there is a good correlation between biochemical markers for bone turnover and the occurrence of fracture.24 Once again, women who need treatment for osteoporosis should not be treated primarily with ospemifene, but women who use ospemifene for dyspareunia can expect positive activity on bone metabolism.
Clinical application
Ospemifene is an oral SERM approved for the treatment of moderate to severe dyspareunia as well as dryness from VVA due to menopause. In addition, it appears one can safely surmise that the direction of ospemifene’s activity in bone and breast is virtually indisputable. The magnitude of that activity, however, is unstudied. Therefore, in selecting an agent to treat women with dyspareunia or vaginal dryness from VVA of menopause, determining any potential add-on benefit for that particular patient in either bone and/or breast is clinically appropriate.
The SERM bazedoxifene
A meta-analysis of 4 randomized, placebo-controlled trials showed that another SERM, bazedoxifene, can significantly decrease the incidence of vertebral fracture in postmenopausal women at follow-up of 3 and 7 years.25 That meta-analysis also confirmed the long-term favorable safety and tolerability of bazedoxifene, with no increase in adverse events, serious adverse events, myocardial infarction, stroke, venous thromboembolic events, or breast carcinoma in patients using bazedoxifene. However, bazedoxifene use did result in an increased incidence of hot flushes and leg cramps across 7 years.25 Bazedoxifene is available in a 20-mg dose for treatment of postmenopausal osteoporosis in Israel and a number of European Union countries.
Continue to: Enter the concept of tissue-selective estrogen complex (TSEC)...
Enter the concept of tissue-selective estrogen complex (TSEC)
Some postmenopausal women are extremely intolerant of any progestogen added to estrogen therapy to confer endometrial protection in those with a uterus. According to the results of a clinical trial of postmenopausal women, bazedoxifene is the only SERM shown to decrease endometrial thickness compared with placebo.26 This is the basis for thinking that perhaps a SERM like bazedoxifene, instead of a progestogen, could be used to confer endometrial protection.
A further consideration comes out of the evaluation of data derived from the 2 arms of the Women’s Health Initiative (WHI).27 In the arm that combined conjugated estrogen with medroxyprogesterone acetate through 11.3 years, there was a 25% increase in the incidence of invasive breast cancer, which was statistically significant. Contrast that with the arm in hysterectomized women who received only conjugated estrogen (often inaccurately referred to as the “estrogen only” arm of the WHI). In that study arm, the relative risk of invasive breast cancer was reduced 23%, also statistically significant. Thus, the culprit in the breast cancer incidence difference in these 2 arms appears to be the addition of the progestogen medroxyprogesterone acetate.27
Since the progestogen was used only for endometrial protection, could such endometrial protection be provided by a SERM like bazedoxifene? Preclinical trials showed that a combination of bazedoxifene and conjugated estrogen (in various estrogen doses) resulted in uterine wet weight in an ovariectomized rat model that was no different than that with placebo.28
In terms of effects on breast, preclinical models showed that conjugated estrogen use resulted in less mammary duct elongation and end bud proliferation than estradiol by itself, and that the combination of conjugated estrogen and bazedoxifene resulted in mammary duct elongation and end bud proliferation that was similar to that in the ovariectomized animals and considerably less than a combination of estradiol with bazedoxifene.29
Five phase 3 studies known as the SMART (Selective estrogens, Menopause, And Response to Therapy) trials were then conducted. Collectively, these studies examined the frequency and severity of vasomotor symptoms (VMS), BMD, bone turnover markers, lipid profiles, sleep, quality of life, breast density, and endometrial safety with conjugated estrogen/bazedoxifene treatment.30 Based on these trials with more than 7,500 women, in 2013 the FDA approved a compound of conjugated estrogen 0.45 mg and bazedoxifene 20 mg (Duavee in the United States and Duavive outside the United States).
The incidence of endometrial hyperplasia at 12 months was consistently less than 1%, which is the FDA guidance for approval of hormone therapies. The incidence of bleeding or spotting with conjugated estrogen/bazedoxifene (FIGURE 3) in each 4-week interval over 12 months mirror-imaged that of placebo and ranged from 3.9% in the first 4-week interval to 1.7% in the last 4 weeks, compared with conjugated estrogen 0.45 mg/medroxyprogesterone acetate 1.5 mg, which had a 20.8% incidence of bleeding or spotting in the first 4-week interval and was still at an 8.8% incidence in the last 4 weeks.31 This is extremely relevant in clinical practice. There was no difference from placebo in breast cancer incidence, breast pain or tenderness, abnormal mammograms, or breast density at month 12.32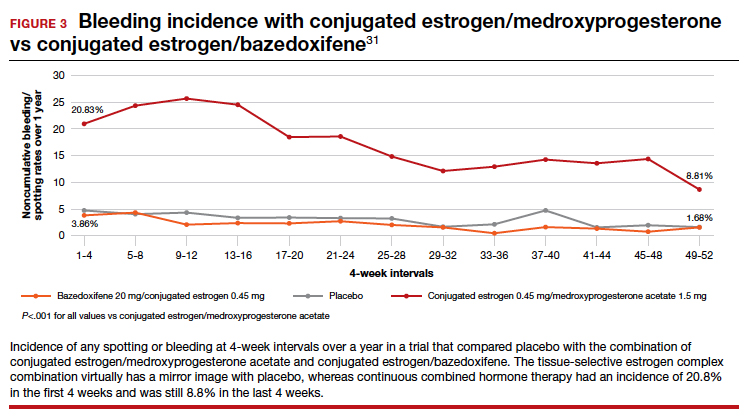
In terms of frequency of VMS, there was a 74% reduction from baseline at 12 weeks compared with placebo (P<.001), as well as a 37% reduction in the VMS severity score (P<.001).32 Statistically significant improvements occurred in lumbar spine and hip BMD (P<.01) for women who were 1 to 5 years since menopause as well as for those who were more than 5 years since menopause.33
Packaging issue puts TSEC on back order
In May 2020, Pfizer voluntarily recalled its conjugated estrogen/bazedoxifene product after identifying a “flaw in the drug’s foil laminate pouch that introduced oxygen and lowered the dissolution rate of active pharmaceutical ingredient bazedoxifene acetate.”34 The manufacturer then wrote a letter to health care professionals in September 2021 stating, “Duavee continues to be out of stock due to an unexpected and complex packaging issue, resulting in manufacturing delays. This has nothing to do with the safety or quality of the product itself but could affect product stability throughout its shelf life… Given regulatory approval timelines for any new packaging, it is unlikely that Duavee will return to stock in 2022.”35
Other TSECs?
The conjugated estrogen/bazedoxifene combination is the first FDA-approved TSEC. Other attempts have been made to achieve similar results with combined raloxifene and 17β-estradiol.36 That study was meant to be a 52-week treatment trial with either raloxifene 60 mg alone or in combination with 17β-estradiol 1 mg per day to assess effects on VMS and endometrial safety. The study was stopped early because signs of endometrial stimulation were observed in the raloxifene plus estradiol group. Thus, one cannot combine any estrogen with any SERM and assume similar results.
Clinical application
The combination of conjugated estrogen/bazedoxifene is approved for treatment of VMS of menopause as well as prevention of osteoporosis. Although it is not approved for treatment of moderate to severe VVA, in younger women who initiate treatment it should prevent the development of moderate to severe symptoms of VVA.
Finally, this drug should be protective of the breast. Conjugated estrogen has clearly shown a reduction in breast cancer incidence and mortality, and bazedoxifene is a SERM. All SERMs have, as a class effect, been shown to be antiestrogens in breast tissue, and abundant preclinical data point in that direction.
This combination of conjugated estrogen/bazedoxifene, when it is once again clinically available, may well provide a new paradigm of hormone therapy that is progestogen free and has a benefit/risk ratio that tilts toward its benefits.
Potential for wider therapeutic benefits
Newer SERMs like ospemifene, approved for treatment of VVA/GSM, and bazedoxifene/conjugated estrogen combination, approved for treatment of VMS and prevention of bone loss, have other beneficial properties that can and should result in their more widespread use. ●
- Stuenkel CA. More evidence why the product labeling for low-dose vaginal estrogen should be changed? Menopause. 2018;25:4-6.
- Goldstein SR. Not all SERMs are created equal. Menopause. 2006;13:325-327.
- Neven P, De Muylder X, Van Belle Y, et al. Hysteroscopic follow-up during tamoxifen treatment. Eur J Obstet Gynecol Reprod Biol. 1990;35:235-238.
- Schwartz LB, Snyder J, Horan C, et al. The use of transvaginal ultrasound and saline infusion sonohysterography for the evaluation of asymptomatic postmenopausal breast cancer patients on tamoxifen. Ultrasound Obstet Gynecol. 1998;11:48-53.
- Goldstein SR, Scheele WH, Rajagopalan SK, et al. A 12-month comparative study of raloxifene, estrogen, and placebo on the postmenopausal endometrium. Obstet Gynecol. 2000;95:95-103.
- Portman DJ, Gass MLS. Vulvovaginal Atrophy Terminology Consensus Conference Panel. Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the International Society for the Study of Women’s Sexual Health and the North American Menopause Society. Menopause. 2014;21:1063-1068.
- Parish SJ, Nappi RE, Krychman ML, et al. Impact of vulvovaginal health on postmenopausal women: a review of surveys on symptoms of vulvovaginal atrophy. Int J Womens Health. 2013;5:437-447.
- Kingsberg SA, Krychman M, Graham S, et al. The Women’s EMPOWER Survey: identifying women’s perceptions on vulvar and vaginal atrophy and its treatment. J Sex Med. 2017;14:413-424.
- Bachmann GA, Komi JO; Ospemifene Study Group. Ospemifene effectively treats vulvovaginal atrophy in postmenopausal women: results from a pivotal phase 3 study. Menopause. 2010;17:480-486.
- Portman DJ, Bachmann GA, Simon JA; Ospemifene Study Group. Ospemifene, a novel selective estrogen receptor modulator for treating dyspareunia associated with postmenopausal vulvar and vaginal atrophy. Menopause. 2013;20:623-630.
- Archer DF, Goldstein SR, Simon JA, et al. Efficacy and safety of ospemifene in postmenopausal women with moderateto-severe vaginal dryness: a phase 3, randomized, doubleblind, placebo-controlled, multicenter trial. Menopause. 2019;26:611-621.
- Osphena. Package insert. Shionogi Inc; 2018.
- Ospemifene (Osphena) for dyspareunia. Med Lett Drugs Ther. 2013;55:55-56.
- Addendum: Ospemifene (Osphena) for dyspareunia (Med Lett Drugs Ther 2013;55:55). Med Lett Drugs Ther. 2013;55:84.
- Goldstein SR, Bachmann G, Lin V, et al. Endometrial safety profile of ospemifene 60 mg when used for long-term treatment of vulvar and vaginal atrophy for up to 1 year. Abstract. Climacteric. 2011;14(suppl 1):S57.
- ACOG practice bulletin no. 141: management of menopausal symptoms. Obstet Gynecol. 2014;123:202-216.
- Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998;90:1371-1388.
- Cummings SR, Eckert S, Krueger KA, et al. The effect of raloxifene on risk of breast cancer in postmenopausal women: results from the MORE randomized trial. Multiple Outcomes of Raloxifene Evaluation. JAMA. 1999;281:2189-2197.
- Vogel VG, Costantino JP, Wickerham DL, et al; National Surgical Adjuvant Breast and Bowel Project (NSABP). Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA. 2006;295:2727-2741.
- Qu Q, Zheng H, Dahllund J, et al. Selective estrogenic effects of a novel triphenylethylene compound, FC1271a, on bone, cholesterol level, and reproductive tissues in intact and ovariectomized rats. Endocrinology. 2000;141:809-820.
- Eigeliene N, Kangas L, Hellmer C, et al. Effects of ospemifene, a novel selective estrogen-receptor modulator, on human breast tissue ex vivo. Menopause. 2016;23:719-730.
- Kangas L, Unkila M. Tissue selectivity of ospemifene: pharmacologic profile and clinical implications. Steroids. 2013;78:1273-1280.
- Constantine GD, Kagan R, Miller PD. Effects of ospemifene on bone parameters including clinical biomarkers in postmenopausal women. Menopause. 2016;23:638-644.
- Gerdhem P, Ivaska KK, Alatalo SL, et al. Biochemical markers of bone metabolism and prediction of fracture in elderly women. J Bone Miner Res. 2004;19:386-393.
- Peng L, Luo Q, Lu H. Efficacy and safety of bazedoxifene in postmenopausal women with osteoporosis: a systematic review and meta-analysis. Medicine. 2017;96(49):e8659.
- Ronkin S, Northington R, Baracat E, et al. Endometrial effects of bazedoxifene acetate, a novel selective estrogen receptor modulator, in postmenopausal women. Obstet Gynecol. 2005;105:1397-1404.
- Anderson GL, Chlebowski RT, Aragaki AK, et al. Conjugated equine oestrogen and breast cancer incidence and mortality in postmenopausal women with hysterectomy: extended follow-up of the Women’s Health Initiative randomized placebo-controlled trial. Lancet Oncol. 2012;13:476-486.
- Kharode Y, Bodine PV, Miller CP, et al. The pairing of a selective estrogen receptor modulator, bazedoxifene, with conjugated estrogens as a new paradigm for the treatment of menopausal symptoms and osteoporosis prevention. Endocrinology. 2008;149:6084-6091.
- Song Y, Santen RJ, Wang JP, et al. Effects of the conjugated equine estrogen/bazedoxifene tissue-selective estrogen complex (TSEC) on mammary gland and breast cancer in mice. Endocrinology. 2012;153:5706-5715.
- Umland EM, Karel L, Santoro N. Bazedoxifene and conjugated equine estrogen: a combination product for the management of vasomotor symptoms and osteoporosis prevention associated with menopause. Pharmacotherapy. 2016;36:548-561.
- Kagan R, Goldstein SR, Pickar JH, et al. Patient considerations in the management of menopausal symptoms: role of conjugated estrogens with bazedoxifene. Ther Clin Risk Manag. 2016;12:549–562.
- Pinkerton JV, Harvey JA, Pan K, et al. Breast effects of bazedoxifene-conjugated estrogens: a randomized controlled trial. Obstet Gynecol. 2013;121:959-968.
- Lindsay R, Gallagher JC, Kagan R, et al. Efficacy of tissue-selective estrogen complex of bazedoxifene/ conjugated estrogens for osteoporosis prevention in at-risk postmenopausal women. Fertil Steril. 2009;92:1045-1052.
- Fierce Pharma. Pfizer continues recalls of menopause drug Duavee on faulty packaging concerns. https:// www.fiercepharma.com/manufacturing/pfizer-recallsmenopause-drug-duavive-uk-due-to-faulty-packagingworries. June 9, 2020. Accessed February 8, 2022.
- Pfizer. Letter to health care provider. Subject: Duavee (conjugated estrogens/bazedoxifene) extended drug shortage. September 10, 2021.
- Stovall DW, Utian WH, Gass MLS, et al. The effects of combined raloxifene and oral estrogen on vasomotor symptoms and endometrial safety. Menopause. 2007; 14(3 pt 1):510-517.
Selective estrogen receptor modulators (SERMs) are unique synthetic compounds that bind to the estrogen receptor and initiate either estrogenic agonistic or antagonistic activity, depending on the confirmational change they produce on binding to the receptor. Many SERMs have come to market, others have not. Unlike estrogens, which regardless of dose or route of administration all carry risks as a boxed warning on the label, referred to as class labeling,1 various SERMs exert various effects in some tissues (uterus, vagina) while they have apparent class properties in others (bone, breast).2
The first SERM, for all practical purposes, was tamoxifen (although clomiphene citrate is often considered a SERM). Tamoxifen was approved by the US Food and Drug Administration (FDA) in 1978 for the treatment of breast cancer and, subsequently, for breast cancer risk reduction. It became the most widely prescribed anticancer drug worldwide.
Subsequently, when data showed that tamoxifen could produce a small number of endometrial cancers and a larger number of endometrial polyps,3,4 there was renewed interest in raloxifene. In preclinical animal studies, raloxifene behaved differently than tamoxifen in the uterus. After clinical trials with raloxifene showed uterine safety,5 the drug was FDA approved for prevention of osteoporosis in 1997, for treatment of osteoporosis in 1999, and for breast cancer risk reduction in 2009. Most clinicians are familiar with these 2 SERMs, which have been in clinical use for more than 4 and 2 decades, respectively.
Ospemifene: A third-generation SERM and its indications
Hormone deficiency from menopause causes vulvovaginal and urogenital changes as well as a multitude of symptoms and signs, including vulvar and vaginal thinning, loss of rugal folds, diminished elasticity, increased pH, and most notably dyspareunia. The nomenclature that previously described vulvovaginal atrophy (VVA) has been expanded to include genitourinary syndrome of menopause (GSM).6 Unfortunately, many health care providers do not ask patients about GSM symptoms, and few women report their symptoms to their clinician.7 Furthermore, although low-dose local estrogens applied vaginally have been the mainstay of therapy for VVA/GSM, only 7% of symptomatic women use any pharmacologic agent,8 mainly because of fear of estrogens due to the class labeling mentioned above.
Ospemifene, a newer SERM, improved superficial cells and reduced parabasal cells as seen on a maturation index compared with placebo, according to results of multiple phase 3 clinical trials9,10; it also lowered vaginal pH and improved most bothersome symptoms (original studies were for dyspareunia). As a result, the FDA approved ospemifene for treatment of moderate to severe dyspareunia from VVA of menopause.
Subsequent studies allowed for a broadened indication to include treatment of moderate to severe dryness due to menopause.11 The ospemifene label contains a boxed warning that states, “In the endometrium, [ospemifene] has estrogen agonistic effects.”12 Although ospemifene is not an estrogen (it’s a SERM), the label goes on to state, “There is an increased risk of endometrial cancer in a woman with a uterus who uses unopposed estrogens.” This statement caused The Medical Letter to initially suggest that patients who receive ospemifene also should receive a progestational agent—a suggestion they later retracted.13,14
To understand why the ospemifene labeling might be worded in such a way, one must review the data regarding the poorly named entity “weakly proliferative endometrium.” The package labeling combines any proliferative endometrium (“weakly” plus “actively” plus “disordered”) that occurred in the clinical trial. Thus, 86.1 per 1,000 of the ospemifene-treated patients (vs 13.3 per 1,000 of those taking placebo) had any one of the proliferative types. The problem is that “actively proliferative” endometrial glands will have mitotic activity in virtually every nucleus of the gland as well as abundant glandular progression (FIGURE 1), whereas “weakly proliferative” is actually closer to inactive or atrophic endometrium with an occasional mitotic figure in only a few nuclei of each gland (FIGURE 2).


In addition, at 1 year, the incidence of active proliferation with ospemifene was 1%.15 In examining the uterine safety study for raloxifene, both doses of that agent had an active proliferation incidence of 3% at 1 year.5 Furthermore, that study had an estrogen-only arm in which, at end point, the incidence of endometrial proliferation was 39%, and hyperplasia, 23%!5 It therefore is evident that, in the endometrium, ospemifene is much more like the SERM raloxifene than it is like estrogen. The American College of Obstetricians and Gynecologists (ACOG) endorsed ospemifene (level A evidence) as a first-line therapy for dyspareunia, noting absent endometrial stimulation.16
Continue to: Ospemifene effects on breast and bone...
Ospemifene effects on breast and bone
Although ospemifene is approved for treatment of moderate to severe VVA/GSM, it has other SERM effects typical of its class. The label currently states that ospemifene “has not been adequately studied in women with breast cancer; therefore, it should not be used in women with known or suspected breast cancer.”12 We know that tamoxifen reduced breast cancer 49% in high-risk women in the Breast Cancer Prevention Trial (BCPT).17 We also know that in the Multiple Outcomes of Raloxifene Evaluation (MORE) trial, raloxifene reduced breast cancer 77% in osteoporotic women,18 and in the Study of Tamoxifen and Raloxifene (STAR) trial, it performed virtually identically to tamoxifen in breast cancer prevention.19 Previous studies demonstrated that ospemifene inhibits breast cancer cell growth in in vitro cultures as well as in animal studies20 and inhibits proliferation of human breast tissue epithelial cells,21 with breast effects similar to those seen with tamoxifen and raloxifene.
Thus, although one would not choose ospemifene as a primary treatment or risk-reducing agent for a patient with breast cancer, the direction of its activity in breast tissue is indisputable and is likely the reason that in the European Union (unlike in the United States) it is approved to treat dyspareunia from VVA/GSM in women with a prior history of breast cancer.
Virtually all SERMs have estrogen agonistic activity in bone. Bone is a dynamic organ, constantly being laid down and taken away (resorption). Estrogen and SERMs are potent antiresorptives in bone metabolism. Ospemifene effectively reduced bone loss in ovariectomized rats, with activity comparable to that of estradiol and raloxifene.22 Clinical data from 3 phase 1 or 2 clinical trials found that ospemifene 60 mg/day had a positive effect on biochemical markers for bone turnover in healthy postmenopausal women, with significant improvements relative to placebo and effects comparable to those of raloxifene.23 Actual fracture or bone mineral density (BMD) data in postmenopausal women are lacking, but there is a good correlation between biochemical markers for bone turnover and the occurrence of fracture.24 Once again, women who need treatment for osteoporosis should not be treated primarily with ospemifene, but women who use ospemifene for dyspareunia can expect positive activity on bone metabolism.
Clinical application
Ospemifene is an oral SERM approved for the treatment of moderate to severe dyspareunia as well as dryness from VVA due to menopause. In addition, it appears one can safely surmise that the direction of ospemifene’s activity in bone and breast is virtually indisputable. The magnitude of that activity, however, is unstudied. Therefore, in selecting an agent to treat women with dyspareunia or vaginal dryness from VVA of menopause, determining any potential add-on benefit for that particular patient in either bone and/or breast is clinically appropriate.
The SERM bazedoxifene
A meta-analysis of 4 randomized, placebo-controlled trials showed that another SERM, bazedoxifene, can significantly decrease the incidence of vertebral fracture in postmenopausal women at follow-up of 3 and 7 years.25 That meta-analysis also confirmed the long-term favorable safety and tolerability of bazedoxifene, with no increase in adverse events, serious adverse events, myocardial infarction, stroke, venous thromboembolic events, or breast carcinoma in patients using bazedoxifene. However, bazedoxifene use did result in an increased incidence of hot flushes and leg cramps across 7 years.25 Bazedoxifene is available in a 20-mg dose for treatment of postmenopausal osteoporosis in Israel and a number of European Union countries.
Continue to: Enter the concept of tissue-selective estrogen complex (TSEC)...
Enter the concept of tissue-selective estrogen complex (TSEC)
Some postmenopausal women are extremely intolerant of any progestogen added to estrogen therapy to confer endometrial protection in those with a uterus. According to the results of a clinical trial of postmenopausal women, bazedoxifene is the only SERM shown to decrease endometrial thickness compared with placebo.26 This is the basis for thinking that perhaps a SERM like bazedoxifene, instead of a progestogen, could be used to confer endometrial protection.
A further consideration comes out of the evaluation of data derived from the 2 arms of the Women’s Health Initiative (WHI).27 In the arm that combined conjugated estrogen with medroxyprogesterone acetate through 11.3 years, there was a 25% increase in the incidence of invasive breast cancer, which was statistically significant. Contrast that with the arm in hysterectomized women who received only conjugated estrogen (often inaccurately referred to as the “estrogen only” arm of the WHI). In that study arm, the relative risk of invasive breast cancer was reduced 23%, also statistically significant. Thus, the culprit in the breast cancer incidence difference in these 2 arms appears to be the addition of the progestogen medroxyprogesterone acetate.27
Since the progestogen was used only for endometrial protection, could such endometrial protection be provided by a SERM like bazedoxifene? Preclinical trials showed that a combination of bazedoxifene and conjugated estrogen (in various estrogen doses) resulted in uterine wet weight in an ovariectomized rat model that was no different than that with placebo.28
In terms of effects on breast, preclinical models showed that conjugated estrogen use resulted in less mammary duct elongation and end bud proliferation than estradiol by itself, and that the combination of conjugated estrogen and bazedoxifene resulted in mammary duct elongation and end bud proliferation that was similar to that in the ovariectomized animals and considerably less than a combination of estradiol with bazedoxifene.29
Five phase 3 studies known as the SMART (Selective estrogens, Menopause, And Response to Therapy) trials were then conducted. Collectively, these studies examined the frequency and severity of vasomotor symptoms (VMS), BMD, bone turnover markers, lipid profiles, sleep, quality of life, breast density, and endometrial safety with conjugated estrogen/bazedoxifene treatment.30 Based on these trials with more than 7,500 women, in 2013 the FDA approved a compound of conjugated estrogen 0.45 mg and bazedoxifene 20 mg (Duavee in the United States and Duavive outside the United States).
The incidence of endometrial hyperplasia at 12 months was consistently less than 1%, which is the FDA guidance for approval of hormone therapies. The incidence of bleeding or spotting with conjugated estrogen/bazedoxifene (FIGURE 3) in each 4-week interval over 12 months mirror-imaged that of placebo and ranged from 3.9% in the first 4-week interval to 1.7% in the last 4 weeks, compared with conjugated estrogen 0.45 mg/medroxyprogesterone acetate 1.5 mg, which had a 20.8% incidence of bleeding or spotting in the first 4-week interval and was still at an 8.8% incidence in the last 4 weeks.31 This is extremely relevant in clinical practice. There was no difference from placebo in breast cancer incidence, breast pain or tenderness, abnormal mammograms, or breast density at month 12.32
In terms of frequency of VMS, there was a 74% reduction from baseline at 12 weeks compared with placebo (P<.001), as well as a 37% reduction in the VMS severity score (P<.001).32 Statistically significant improvements occurred in lumbar spine and hip BMD (P<.01) for women who were 1 to 5 years since menopause as well as for those who were more than 5 years since menopause.33
Packaging issue puts TSEC on back order
In May 2020, Pfizer voluntarily recalled its conjugated estrogen/bazedoxifene product after identifying a “flaw in the drug’s foil laminate pouch that introduced oxygen and lowered the dissolution rate of active pharmaceutical ingredient bazedoxifene acetate.”34 The manufacturer then wrote a letter to health care professionals in September 2021 stating, “Duavee continues to be out of stock due to an unexpected and complex packaging issue, resulting in manufacturing delays. This has nothing to do with the safety or quality of the product itself but could affect product stability throughout its shelf life… Given regulatory approval timelines for any new packaging, it is unlikely that Duavee will return to stock in 2022.”35
Other TSECs?
The conjugated estrogen/bazedoxifene combination is the first FDA-approved TSEC. Other attempts have been made to achieve similar results with combined raloxifene and 17β-estradiol.36 That study was meant to be a 52-week treatment trial with either raloxifene 60 mg alone or in combination with 17β-estradiol 1 mg per day to assess effects on VMS and endometrial safety. The study was stopped early because signs of endometrial stimulation were observed in the raloxifene plus estradiol group. Thus, one cannot combine any estrogen with any SERM and assume similar results.
Clinical application
The combination of conjugated estrogen/bazedoxifene is approved for treatment of VMS of menopause as well as prevention of osteoporosis. Although it is not approved for treatment of moderate to severe VVA, in younger women who initiate treatment it should prevent the development of moderate to severe symptoms of VVA.
Finally, this drug should be protective of the breast. Conjugated estrogen has clearly shown a reduction in breast cancer incidence and mortality, and bazedoxifene is a SERM. All SERMs have, as a class effect, been shown to be antiestrogens in breast tissue, and abundant preclinical data point in that direction.
This combination of conjugated estrogen/bazedoxifene, when it is once again clinically available, may well provide a new paradigm of hormone therapy that is progestogen free and has a benefit/risk ratio that tilts toward its benefits.
Potential for wider therapeutic benefits
Newer SERMs like ospemifene, approved for treatment of VVA/GSM, and bazedoxifene/conjugated estrogen combination, approved for treatment of VMS and prevention of bone loss, have other beneficial properties that can and should result in their more widespread use. ●
Selective estrogen receptor modulators (SERMs) are unique synthetic compounds that bind to the estrogen receptor and initiate either estrogenic agonistic or antagonistic activity, depending on the confirmational change they produce on binding to the receptor. Many SERMs have come to market, others have not. Unlike estrogens, which regardless of dose or route of administration all carry risks as a boxed warning on the label, referred to as class labeling,1 various SERMs exert various effects in some tissues (uterus, vagina) while they have apparent class properties in others (bone, breast).2
The first SERM, for all practical purposes, was tamoxifen (although clomiphene citrate is often considered a SERM). Tamoxifen was approved by the US Food and Drug Administration (FDA) in 1978 for the treatment of breast cancer and, subsequently, for breast cancer risk reduction. It became the most widely prescribed anticancer drug worldwide.
Subsequently, when data showed that tamoxifen could produce a small number of endometrial cancers and a larger number of endometrial polyps,3,4 there was renewed interest in raloxifene. In preclinical animal studies, raloxifene behaved differently than tamoxifen in the uterus. After clinical trials with raloxifene showed uterine safety,5 the drug was FDA approved for prevention of osteoporosis in 1997, for treatment of osteoporosis in 1999, and for breast cancer risk reduction in 2009. Most clinicians are familiar with these 2 SERMs, which have been in clinical use for more than 4 and 2 decades, respectively.
Ospemifene: A third-generation SERM and its indications
Hormone deficiency from menopause causes vulvovaginal and urogenital changes as well as a multitude of symptoms and signs, including vulvar and vaginal thinning, loss of rugal folds, diminished elasticity, increased pH, and most notably dyspareunia. The nomenclature that previously described vulvovaginal atrophy (VVA) has been expanded to include genitourinary syndrome of menopause (GSM).6 Unfortunately, many health care providers do not ask patients about GSM symptoms, and few women report their symptoms to their clinician.7 Furthermore, although low-dose local estrogens applied vaginally have been the mainstay of therapy for VVA/GSM, only 7% of symptomatic women use any pharmacologic agent,8 mainly because of fear of estrogens due to the class labeling mentioned above.
Ospemifene, a newer SERM, improved superficial cells and reduced parabasal cells as seen on a maturation index compared with placebo, according to results of multiple phase 3 clinical trials9,10; it also lowered vaginal pH and improved most bothersome symptoms (original studies were for dyspareunia). As a result, the FDA approved ospemifene for treatment of moderate to severe dyspareunia from VVA of menopause.
Subsequent studies allowed for a broadened indication to include treatment of moderate to severe dryness due to menopause.11 The ospemifene label contains a boxed warning that states, “In the endometrium, [ospemifene] has estrogen agonistic effects.”12 Although ospemifene is not an estrogen (it’s a SERM), the label goes on to state, “There is an increased risk of endometrial cancer in a woman with a uterus who uses unopposed estrogens.” This statement caused The Medical Letter to initially suggest that patients who receive ospemifene also should receive a progestational agent—a suggestion they later retracted.13,14
To understand why the ospemifene labeling might be worded in such a way, one must review the data regarding the poorly named entity “weakly proliferative endometrium.” The package labeling combines any proliferative endometrium (“weakly” plus “actively” plus “disordered”) that occurred in the clinical trial. Thus, 86.1 per 1,000 of the ospemifene-treated patients (vs 13.3 per 1,000 of those taking placebo) had any one of the proliferative types. The problem is that “actively proliferative” endometrial glands will have mitotic activity in virtually every nucleus of the gland as well as abundant glandular progression (FIGURE 1), whereas “weakly proliferative” is actually closer to inactive or atrophic endometrium with an occasional mitotic figure in only a few nuclei of each gland (FIGURE 2).


In addition, at 1 year, the incidence of active proliferation with ospemifene was 1%.15 In examining the uterine safety study for raloxifene, both doses of that agent had an active proliferation incidence of 3% at 1 year.5 Furthermore, that study had an estrogen-only arm in which, at end point, the incidence of endometrial proliferation was 39%, and hyperplasia, 23%!5 It therefore is evident that, in the endometrium, ospemifene is much more like the SERM raloxifene than it is like estrogen. The American College of Obstetricians and Gynecologists (ACOG) endorsed ospemifene (level A evidence) as a first-line therapy for dyspareunia, noting absent endometrial stimulation.16
Continue to: Ospemifene effects on breast and bone...
Ospemifene effects on breast and bone
Although ospemifene is approved for treatment of moderate to severe VVA/GSM, it has other SERM effects typical of its class. The label currently states that ospemifene “has not been adequately studied in women with breast cancer; therefore, it should not be used in women with known or suspected breast cancer.”12 We know that tamoxifen reduced breast cancer 49% in high-risk women in the Breast Cancer Prevention Trial (BCPT).17 We also know that in the Multiple Outcomes of Raloxifene Evaluation (MORE) trial, raloxifene reduced breast cancer 77% in osteoporotic women,18 and in the Study of Tamoxifen and Raloxifene (STAR) trial, it performed virtually identically to tamoxifen in breast cancer prevention.19 Previous studies demonstrated that ospemifene inhibits breast cancer cell growth in in vitro cultures as well as in animal studies20 and inhibits proliferation of human breast tissue epithelial cells,21 with breast effects similar to those seen with tamoxifen and raloxifene.
Thus, although one would not choose ospemifene as a primary treatment or risk-reducing agent for a patient with breast cancer, the direction of its activity in breast tissue is indisputable and is likely the reason that in the European Union (unlike in the United States) it is approved to treat dyspareunia from VVA/GSM in women with a prior history of breast cancer.
Virtually all SERMs have estrogen agonistic activity in bone. Bone is a dynamic organ, constantly being laid down and taken away (resorption). Estrogen and SERMs are potent antiresorptives in bone metabolism. Ospemifene effectively reduced bone loss in ovariectomized rats, with activity comparable to that of estradiol and raloxifene.22 Clinical data from 3 phase 1 or 2 clinical trials found that ospemifene 60 mg/day had a positive effect on biochemical markers for bone turnover in healthy postmenopausal women, with significant improvements relative to placebo and effects comparable to those of raloxifene.23 Actual fracture or bone mineral density (BMD) data in postmenopausal women are lacking, but there is a good correlation between biochemical markers for bone turnover and the occurrence of fracture.24 Once again, women who need treatment for osteoporosis should not be treated primarily with ospemifene, but women who use ospemifene for dyspareunia can expect positive activity on bone metabolism.
Clinical application
Ospemifene is an oral SERM approved for the treatment of moderate to severe dyspareunia as well as dryness from VVA due to menopause. In addition, it appears one can safely surmise that the direction of ospemifene’s activity in bone and breast is virtually indisputable. The magnitude of that activity, however, is unstudied. Therefore, in selecting an agent to treat women with dyspareunia or vaginal dryness from VVA of menopause, determining any potential add-on benefit for that particular patient in either bone and/or breast is clinically appropriate.
The SERM bazedoxifene
A meta-analysis of 4 randomized, placebo-controlled trials showed that another SERM, bazedoxifene, can significantly decrease the incidence of vertebral fracture in postmenopausal women at follow-up of 3 and 7 years.25 That meta-analysis also confirmed the long-term favorable safety and tolerability of bazedoxifene, with no increase in adverse events, serious adverse events, myocardial infarction, stroke, venous thromboembolic events, or breast carcinoma in patients using bazedoxifene. However, bazedoxifene use did result in an increased incidence of hot flushes and leg cramps across 7 years.25 Bazedoxifene is available in a 20-mg dose for treatment of postmenopausal osteoporosis in Israel and a number of European Union countries.
Continue to: Enter the concept of tissue-selective estrogen complex (TSEC)...
Enter the concept of tissue-selective estrogen complex (TSEC)
Some postmenopausal women are extremely intolerant of any progestogen added to estrogen therapy to confer endometrial protection in those with a uterus. According to the results of a clinical trial of postmenopausal women, bazedoxifene is the only SERM shown to decrease endometrial thickness compared with placebo.26 This is the basis for thinking that perhaps a SERM like bazedoxifene, instead of a progestogen, could be used to confer endometrial protection.
A further consideration comes out of the evaluation of data derived from the 2 arms of the Women’s Health Initiative (WHI).27 In the arm that combined conjugated estrogen with medroxyprogesterone acetate through 11.3 years, there was a 25% increase in the incidence of invasive breast cancer, which was statistically significant. Contrast that with the arm in hysterectomized women who received only conjugated estrogen (often inaccurately referred to as the “estrogen only” arm of the WHI). In that study arm, the relative risk of invasive breast cancer was reduced 23%, also statistically significant. Thus, the culprit in the breast cancer incidence difference in these 2 arms appears to be the addition of the progestogen medroxyprogesterone acetate.27
Since the progestogen was used only for endometrial protection, could such endometrial protection be provided by a SERM like bazedoxifene? Preclinical trials showed that a combination of bazedoxifene and conjugated estrogen (in various estrogen doses) resulted in uterine wet weight in an ovariectomized rat model that was no different than that with placebo.28
In terms of effects on breast, preclinical models showed that conjugated estrogen use resulted in less mammary duct elongation and end bud proliferation than estradiol by itself, and that the combination of conjugated estrogen and bazedoxifene resulted in mammary duct elongation and end bud proliferation that was similar to that in the ovariectomized animals and considerably less than a combination of estradiol with bazedoxifene.29
Five phase 3 studies known as the SMART (Selective estrogens, Menopause, And Response to Therapy) trials were then conducted. Collectively, these studies examined the frequency and severity of vasomotor symptoms (VMS), BMD, bone turnover markers, lipid profiles, sleep, quality of life, breast density, and endometrial safety with conjugated estrogen/bazedoxifene treatment.30 Based on these trials with more than 7,500 women, in 2013 the FDA approved a compound of conjugated estrogen 0.45 mg and bazedoxifene 20 mg (Duavee in the United States and Duavive outside the United States).
The incidence of endometrial hyperplasia at 12 months was consistently less than 1%, which is the FDA guidance for approval of hormone therapies. The incidence of bleeding or spotting with conjugated estrogen/bazedoxifene (FIGURE 3) in each 4-week interval over 12 months mirror-imaged that of placebo and ranged from 3.9% in the first 4-week interval to 1.7% in the last 4 weeks, compared with conjugated estrogen 0.45 mg/medroxyprogesterone acetate 1.5 mg, which had a 20.8% incidence of bleeding or spotting in the first 4-week interval and was still at an 8.8% incidence in the last 4 weeks.31 This is extremely relevant in clinical practice. There was no difference from placebo in breast cancer incidence, breast pain or tenderness, abnormal mammograms, or breast density at month 12.32
In terms of frequency of VMS, there was a 74% reduction from baseline at 12 weeks compared with placebo (P<.001), as well as a 37% reduction in the VMS severity score (P<.001).32 Statistically significant improvements occurred in lumbar spine and hip BMD (P<.01) for women who were 1 to 5 years since menopause as well as for those who were more than 5 years since menopause.33
Packaging issue puts TSEC on back order
In May 2020, Pfizer voluntarily recalled its conjugated estrogen/bazedoxifene product after identifying a “flaw in the drug’s foil laminate pouch that introduced oxygen and lowered the dissolution rate of active pharmaceutical ingredient bazedoxifene acetate.”34 The manufacturer then wrote a letter to health care professionals in September 2021 stating, “Duavee continues to be out of stock due to an unexpected and complex packaging issue, resulting in manufacturing delays. This has nothing to do with the safety or quality of the product itself but could affect product stability throughout its shelf life… Given regulatory approval timelines for any new packaging, it is unlikely that Duavee will return to stock in 2022.”35
Other TSECs?
The conjugated estrogen/bazedoxifene combination is the first FDA-approved TSEC. Other attempts have been made to achieve similar results with combined raloxifene and 17β-estradiol.36 That study was meant to be a 52-week treatment trial with either raloxifene 60 mg alone or in combination with 17β-estradiol 1 mg per day to assess effects on VMS and endometrial safety. The study was stopped early because signs of endometrial stimulation were observed in the raloxifene plus estradiol group. Thus, one cannot combine any estrogen with any SERM and assume similar results.
Clinical application
The combination of conjugated estrogen/bazedoxifene is approved for treatment of VMS of menopause as well as prevention of osteoporosis. Although it is not approved for treatment of moderate to severe VVA, in younger women who initiate treatment it should prevent the development of moderate to severe symptoms of VVA.
Finally, this drug should be protective of the breast. Conjugated estrogen has clearly shown a reduction in breast cancer incidence and mortality, and bazedoxifene is a SERM. All SERMs have, as a class effect, been shown to be antiestrogens in breast tissue, and abundant preclinical data point in that direction.
This combination of conjugated estrogen/bazedoxifene, when it is once again clinically available, may well provide a new paradigm of hormone therapy that is progestogen free and has a benefit/risk ratio that tilts toward its benefits.
Potential for wider therapeutic benefits
Newer SERMs like ospemifene, approved for treatment of VVA/GSM, and bazedoxifene/conjugated estrogen combination, approved for treatment of VMS and prevention of bone loss, have other beneficial properties that can and should result in their more widespread use. ●
- Stuenkel CA. More evidence why the product labeling for low-dose vaginal estrogen should be changed? Menopause. 2018;25:4-6.
- Goldstein SR. Not all SERMs are created equal. Menopause. 2006;13:325-327.
- Neven P, De Muylder X, Van Belle Y, et al. Hysteroscopic follow-up during tamoxifen treatment. Eur J Obstet Gynecol Reprod Biol. 1990;35:235-238.
- Schwartz LB, Snyder J, Horan C, et al. The use of transvaginal ultrasound and saline infusion sonohysterography for the evaluation of asymptomatic postmenopausal breast cancer patients on tamoxifen. Ultrasound Obstet Gynecol. 1998;11:48-53.
- Goldstein SR, Scheele WH, Rajagopalan SK, et al. A 12-month comparative study of raloxifene, estrogen, and placebo on the postmenopausal endometrium. Obstet Gynecol. 2000;95:95-103.
- Portman DJ, Gass MLS. Vulvovaginal Atrophy Terminology Consensus Conference Panel. Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the International Society for the Study of Women’s Sexual Health and the North American Menopause Society. Menopause. 2014;21:1063-1068.
- Parish SJ, Nappi RE, Krychman ML, et al. Impact of vulvovaginal health on postmenopausal women: a review of surveys on symptoms of vulvovaginal atrophy. Int J Womens Health. 2013;5:437-447.
- Kingsberg SA, Krychman M, Graham S, et al. The Women’s EMPOWER Survey: identifying women’s perceptions on vulvar and vaginal atrophy and its treatment. J Sex Med. 2017;14:413-424.
- Bachmann GA, Komi JO; Ospemifene Study Group. Ospemifene effectively treats vulvovaginal atrophy in postmenopausal women: results from a pivotal phase 3 study. Menopause. 2010;17:480-486.
- Portman DJ, Bachmann GA, Simon JA; Ospemifene Study Group. Ospemifene, a novel selective estrogen receptor modulator for treating dyspareunia associated with postmenopausal vulvar and vaginal atrophy. Menopause. 2013;20:623-630.
- Archer DF, Goldstein SR, Simon JA, et al. Efficacy and safety of ospemifene in postmenopausal women with moderateto-severe vaginal dryness: a phase 3, randomized, doubleblind, placebo-controlled, multicenter trial. Menopause. 2019;26:611-621.
- Osphena. Package insert. Shionogi Inc; 2018.
- Ospemifene (Osphena) for dyspareunia. Med Lett Drugs Ther. 2013;55:55-56.
- Addendum: Ospemifene (Osphena) for dyspareunia (Med Lett Drugs Ther 2013;55:55). Med Lett Drugs Ther. 2013;55:84.
- Goldstein SR, Bachmann G, Lin V, et al. Endometrial safety profile of ospemifene 60 mg when used for long-term treatment of vulvar and vaginal atrophy for up to 1 year. Abstract. Climacteric. 2011;14(suppl 1):S57.
- ACOG practice bulletin no. 141: management of menopausal symptoms. Obstet Gynecol. 2014;123:202-216.
- Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998;90:1371-1388.
- Cummings SR, Eckert S, Krueger KA, et al. The effect of raloxifene on risk of breast cancer in postmenopausal women: results from the MORE randomized trial. Multiple Outcomes of Raloxifene Evaluation. JAMA. 1999;281:2189-2197.
- Vogel VG, Costantino JP, Wickerham DL, et al; National Surgical Adjuvant Breast and Bowel Project (NSABP). Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA. 2006;295:2727-2741.
- Qu Q, Zheng H, Dahllund J, et al. Selective estrogenic effects of a novel triphenylethylene compound, FC1271a, on bone, cholesterol level, and reproductive tissues in intact and ovariectomized rats. Endocrinology. 2000;141:809-820.
- Eigeliene N, Kangas L, Hellmer C, et al. Effects of ospemifene, a novel selective estrogen-receptor modulator, on human breast tissue ex vivo. Menopause. 2016;23:719-730.
- Kangas L, Unkila M. Tissue selectivity of ospemifene: pharmacologic profile and clinical implications. Steroids. 2013;78:1273-1280.
- Constantine GD, Kagan R, Miller PD. Effects of ospemifene on bone parameters including clinical biomarkers in postmenopausal women. Menopause. 2016;23:638-644.
- Gerdhem P, Ivaska KK, Alatalo SL, et al. Biochemical markers of bone metabolism and prediction of fracture in elderly women. J Bone Miner Res. 2004;19:386-393.
- Peng L, Luo Q, Lu H. Efficacy and safety of bazedoxifene in postmenopausal women with osteoporosis: a systematic review and meta-analysis. Medicine. 2017;96(49):e8659.
- Ronkin S, Northington R, Baracat E, et al. Endometrial effects of bazedoxifene acetate, a novel selective estrogen receptor modulator, in postmenopausal women. Obstet Gynecol. 2005;105:1397-1404.
- Anderson GL, Chlebowski RT, Aragaki AK, et al. Conjugated equine oestrogen and breast cancer incidence and mortality in postmenopausal women with hysterectomy: extended follow-up of the Women’s Health Initiative randomized placebo-controlled trial. Lancet Oncol. 2012;13:476-486.
- Kharode Y, Bodine PV, Miller CP, et al. The pairing of a selective estrogen receptor modulator, bazedoxifene, with conjugated estrogens as a new paradigm for the treatment of menopausal symptoms and osteoporosis prevention. Endocrinology. 2008;149:6084-6091.
- Song Y, Santen RJ, Wang JP, et al. Effects of the conjugated equine estrogen/bazedoxifene tissue-selective estrogen complex (TSEC) on mammary gland and breast cancer in mice. Endocrinology. 2012;153:5706-5715.
- Umland EM, Karel L, Santoro N. Bazedoxifene and conjugated equine estrogen: a combination product for the management of vasomotor symptoms and osteoporosis prevention associated with menopause. Pharmacotherapy. 2016;36:548-561.
- Kagan R, Goldstein SR, Pickar JH, et al. Patient considerations in the management of menopausal symptoms: role of conjugated estrogens with bazedoxifene. Ther Clin Risk Manag. 2016;12:549–562.
- Pinkerton JV, Harvey JA, Pan K, et al. Breast effects of bazedoxifene-conjugated estrogens: a randomized controlled trial. Obstet Gynecol. 2013;121:959-968.
- Lindsay R, Gallagher JC, Kagan R, et al. Efficacy of tissue-selective estrogen complex of bazedoxifene/ conjugated estrogens for osteoporosis prevention in at-risk postmenopausal women. Fertil Steril. 2009;92:1045-1052.
- Fierce Pharma. Pfizer continues recalls of menopause drug Duavee on faulty packaging concerns. https:// www.fiercepharma.com/manufacturing/pfizer-recallsmenopause-drug-duavive-uk-due-to-faulty-packagingworries. June 9, 2020. Accessed February 8, 2022.
- Pfizer. Letter to health care provider. Subject: Duavee (conjugated estrogens/bazedoxifene) extended drug shortage. September 10, 2021.
- Stovall DW, Utian WH, Gass MLS, et al. The effects of combined raloxifene and oral estrogen on vasomotor symptoms and endometrial safety. Menopause. 2007; 14(3 pt 1):510-517.
- Stuenkel CA. More evidence why the product labeling for low-dose vaginal estrogen should be changed? Menopause. 2018;25:4-6.
- Goldstein SR. Not all SERMs are created equal. Menopause. 2006;13:325-327.
- Neven P, De Muylder X, Van Belle Y, et al. Hysteroscopic follow-up during tamoxifen treatment. Eur J Obstet Gynecol Reprod Biol. 1990;35:235-238.
- Schwartz LB, Snyder J, Horan C, et al. The use of transvaginal ultrasound and saline infusion sonohysterography for the evaluation of asymptomatic postmenopausal breast cancer patients on tamoxifen. Ultrasound Obstet Gynecol. 1998;11:48-53.
- Goldstein SR, Scheele WH, Rajagopalan SK, et al. A 12-month comparative study of raloxifene, estrogen, and placebo on the postmenopausal endometrium. Obstet Gynecol. 2000;95:95-103.
- Portman DJ, Gass MLS. Vulvovaginal Atrophy Terminology Consensus Conference Panel. Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the International Society for the Study of Women’s Sexual Health and the North American Menopause Society. Menopause. 2014;21:1063-1068.
- Parish SJ, Nappi RE, Krychman ML, et al. Impact of vulvovaginal health on postmenopausal women: a review of surveys on symptoms of vulvovaginal atrophy. Int J Womens Health. 2013;5:437-447.
- Kingsberg SA, Krychman M, Graham S, et al. The Women’s EMPOWER Survey: identifying women’s perceptions on vulvar and vaginal atrophy and its treatment. J Sex Med. 2017;14:413-424.
- Bachmann GA, Komi JO; Ospemifene Study Group. Ospemifene effectively treats vulvovaginal atrophy in postmenopausal women: results from a pivotal phase 3 study. Menopause. 2010;17:480-486.
- Portman DJ, Bachmann GA, Simon JA; Ospemifene Study Group. Ospemifene, a novel selective estrogen receptor modulator for treating dyspareunia associated with postmenopausal vulvar and vaginal atrophy. Menopause. 2013;20:623-630.
- Archer DF, Goldstein SR, Simon JA, et al. Efficacy and safety of ospemifene in postmenopausal women with moderateto-severe vaginal dryness: a phase 3, randomized, doubleblind, placebo-controlled, multicenter trial. Menopause. 2019;26:611-621.
- Osphena. Package insert. Shionogi Inc; 2018.
- Ospemifene (Osphena) for dyspareunia. Med Lett Drugs Ther. 2013;55:55-56.
- Addendum: Ospemifene (Osphena) for dyspareunia (Med Lett Drugs Ther 2013;55:55). Med Lett Drugs Ther. 2013;55:84.
- Goldstein SR, Bachmann G, Lin V, et al. Endometrial safety profile of ospemifene 60 mg when used for long-term treatment of vulvar and vaginal atrophy for up to 1 year. Abstract. Climacteric. 2011;14(suppl 1):S57.
- ACOG practice bulletin no. 141: management of menopausal symptoms. Obstet Gynecol. 2014;123:202-216.
- Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998;90:1371-1388.
- Cummings SR, Eckert S, Krueger KA, et al. The effect of raloxifene on risk of breast cancer in postmenopausal women: results from the MORE randomized trial. Multiple Outcomes of Raloxifene Evaluation. JAMA. 1999;281:2189-2197.
- Vogel VG, Costantino JP, Wickerham DL, et al; National Surgical Adjuvant Breast and Bowel Project (NSABP). Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA. 2006;295:2727-2741.
- Qu Q, Zheng H, Dahllund J, et al. Selective estrogenic effects of a novel triphenylethylene compound, FC1271a, on bone, cholesterol level, and reproductive tissues in intact and ovariectomized rats. Endocrinology. 2000;141:809-820.
- Eigeliene N, Kangas L, Hellmer C, et al. Effects of ospemifene, a novel selective estrogen-receptor modulator, on human breast tissue ex vivo. Menopause. 2016;23:719-730.
- Kangas L, Unkila M. Tissue selectivity of ospemifene: pharmacologic profile and clinical implications. Steroids. 2013;78:1273-1280.
- Constantine GD, Kagan R, Miller PD. Effects of ospemifene on bone parameters including clinical biomarkers in postmenopausal women. Menopause. 2016;23:638-644.
- Gerdhem P, Ivaska KK, Alatalo SL, et al. Biochemical markers of bone metabolism and prediction of fracture in elderly women. J Bone Miner Res. 2004;19:386-393.
- Peng L, Luo Q, Lu H. Efficacy and safety of bazedoxifene in postmenopausal women with osteoporosis: a systematic review and meta-analysis. Medicine. 2017;96(49):e8659.
- Ronkin S, Northington R, Baracat E, et al. Endometrial effects of bazedoxifene acetate, a novel selective estrogen receptor modulator, in postmenopausal women. Obstet Gynecol. 2005;105:1397-1404.
- Anderson GL, Chlebowski RT, Aragaki AK, et al. Conjugated equine oestrogen and breast cancer incidence and mortality in postmenopausal women with hysterectomy: extended follow-up of the Women’s Health Initiative randomized placebo-controlled trial. Lancet Oncol. 2012;13:476-486.
- Kharode Y, Bodine PV, Miller CP, et al. The pairing of a selective estrogen receptor modulator, bazedoxifene, with conjugated estrogens as a new paradigm for the treatment of menopausal symptoms and osteoporosis prevention. Endocrinology. 2008;149:6084-6091.
- Song Y, Santen RJ, Wang JP, et al. Effects of the conjugated equine estrogen/bazedoxifene tissue-selective estrogen complex (TSEC) on mammary gland and breast cancer in mice. Endocrinology. 2012;153:5706-5715.
- Umland EM, Karel L, Santoro N. Bazedoxifene and conjugated equine estrogen: a combination product for the management of vasomotor symptoms and osteoporosis prevention associated with menopause. Pharmacotherapy. 2016;36:548-561.
- Kagan R, Goldstein SR, Pickar JH, et al. Patient considerations in the management of menopausal symptoms: role of conjugated estrogens with bazedoxifene. Ther Clin Risk Manag. 2016;12:549–562.
- Pinkerton JV, Harvey JA, Pan K, et al. Breast effects of bazedoxifene-conjugated estrogens: a randomized controlled trial. Obstet Gynecol. 2013;121:959-968.
- Lindsay R, Gallagher JC, Kagan R, et al. Efficacy of tissue-selective estrogen complex of bazedoxifene/ conjugated estrogens for osteoporosis prevention in at-risk postmenopausal women. Fertil Steril. 2009;92:1045-1052.
- Fierce Pharma. Pfizer continues recalls of menopause drug Duavee on faulty packaging concerns. https:// www.fiercepharma.com/manufacturing/pfizer-recallsmenopause-drug-duavive-uk-due-to-faulty-packagingworries. June 9, 2020. Accessed February 8, 2022.
- Pfizer. Letter to health care provider. Subject: Duavee (conjugated estrogens/bazedoxifene) extended drug shortage. September 10, 2021.
- Stovall DW, Utian WH, Gass MLS, et al. The effects of combined raloxifene and oral estrogen on vasomotor symptoms and endometrial safety. Menopause. 2007; 14(3 pt 1):510-517.
What placental mechanisms protect the fetus from harm in the setting of maternal COVID-19 infection?
Taglauer ES, Wachman EM, Juttukonda L, et al. Acute severe acute respiratory syndrome coronavirus 2 infection in pregnancy is associated with placental angiotensin-converting enzyme 2 shedding. Am J Pathol. 2022;192:595-603. doi.org/10.1016/j.ajpath.2021.12.011
EXPERT COMMENTARY
Although transmission of SARS-CoV-2 virus from an infected mother to her fetus is rare, placental infection with SARS-CoV-2 can occur and has been observed in association with placental damage and adverse pregnancy outcomes, including stillbirth.1 Understanding what mechanisms of defense protect the placenta and fetus from direct SARS-CoV-2 infection at the maternal-fetal interface, as well as the factors that might disturb or enhance that protection, is critical to gaining a deeper understanding of the potential impact of maternal COVID-19 on fetal well-being.
Details of the study
In a cohort of 24 pregnant individuals, Taglauer and colleagues investigated levels of placental angiotensin-converting enzyme (ACE)-2, placental ADAM17 (a disintegrin and metalloprotease domain 17) activity, and maternal serum soluble ACE2 in samples obtained at delivery from individuals with a history of second trimester COVID-19 infection, early third trimester COVID-19 infection, and no history of COVID-19 infection.
Results. Maternal COVID-19 infection in the early third trimester of pregnancy resulted in lower ACE2 protein levels in the placenta at delivery, higher ACE2 gene expression, and an increase in ADAM17 activity, compared with infection in the second trimester of pregnancy and compared with noninfected controls.
The authors postulated that increased ADAM17 activity—the enzyme responsible for ACE2 cleavage and shedding—may be responsible for lower ACE2 protein levels. Soluble ACE2 levels in maternal blood at delivery were increased in individuals with third trimester COVID-19 infection, although the source of soluble ACE2 (placental or otherwise) could not be determined with the methods employed. Levels of placental estrogen were no different between groups, which suggests that estrogen is not responsible for the observed differences.
Study strengths and limitations
ACE2 is the main receptor for the SARS-CoV-2 virus and facilitates viral entry into the cell.2 Placental villous cells that are in direct contact with maternal blood express the ACE2 protein, rendering them potentially vulnerable to SARS-CoV-2 infection.3 In this study, the authors observed lower placental ACE2 protein in term placentas from recent (early third trimester) but not remote (second trimester) maternal SARS-CoV-2 infection, arguably the result of the observed increase in ADAM17 cleavage activity. Prior studies have shown conflicting results, with equal or higher ACE2 levels noted in the setting of maternal COVID-19 infection, which may be related to differences in COVID-19 disease severity, gestational age of infection, and/or fetal sex in these cohorts.4-6
The concept that increased placental ACE2 shedding represents a protective defense mechanism that might last weeks beyond the acute infectious period is intriguing, but it requires further study. Observed differences in third but not second trimester COVID-19 infections could indicate either 1) an effect of maternal COVID-19 infection that lasts for several weeks but eventually normalizes over time, in the case of a remote infection; or 2) that second trimester maternal COVID-19 infection does not have the same pronounced effect on ACE2 levels as does third trimester infection. Observational studies of the human placenta are not able to answer this question, as directly sampling the placenta at the time of the exposure (or repeated sampling over time) in ongoing pregnancies is neither practical nor ethical. Further studies using animal or cellular models of SARS-CoV-2 infection in pregnancy may be necessary to fully understand the clinical relevance of these findings.
The study by Taglauer and colleagues provides a compelling argument for exploring how immune defenses at the maternal-fetal interface evolve over time and vary by trimester of exposure. ●
As the number of pregnancies exposed to COVID-19 continues to grow worldwide, how immune defenses at the maternal-fetal interface protect against fetal infection remains an important area of investigation.
LYDIA L. SHOOK, MD
- Pregnant people are at increased risk of more severe COVID-19 illness.
- The risk of stillbirth is 2- to 4-fold higher in women with COVID-19 infection during pregnancy.1
- COVID-19 vaccination is recommended for all people who are pregnant, lactating, or considering pregnancy.
- Pregnant and recently pregnant people up to 6 weeks postpartum should receive a third “booster” dose of a COVID-19 mRNA vaccine following completion of their initial COVID-19 vaccine or vaccine series.
- The mRNA COVID-19 vaccines are preferred over the Johnson & Johnson/Janssen COVID-19 vaccine for pregnant and lactating individuals for primary series and booster vaccination.
- Completion of a 2-dose mRNA COVID-19 vaccination series during pregnancy might help prevent COVID-19 hospitalization among infants <6 months.2
aVaccine recommendations adapted from: ACOG practice advisory: COVID-19 vaccination considerations for obstetric-gynecologic care. Last updated March 2, 2022. https://www.acog.org/clinical/ clinical-guidance/practice-advisory/articles/2020/12/covid-19-vaccination-considerations-for-obstetricgynecologic-care. Accessed March 21, 2022.
References
1. DeSisto CL, Wallace B, Simeone RM, et al. Risk for stillbirth among women with and without COVID-19 at delivery hospitalization—United States, March 2020–September 2021. MMWR Morbid Mortal Wkly Rep. 2021;70:1640-1645.
2. Halasa NB, Olson SM, Staat MA, et al; Overcoming COVID-19 Investigators; Overcoming COVID-19 Network. Effectiveness of maternal vaccination with mRNA COVID-19 vaccine during pregnancy against COVID-19–associated hospitalization in infants aged <6 months—17 states, July 2021–January 2022. MMWR Morbid Mortal Wkly Rep. 2022;71:264-270.
- Schwartz DA, Avvad-Portari E, Babál, et al. Placental tissue destruction and insufficiency from COVID-19 causes stillbirth and neonatal death from hypoxic-ischemic injury: a study of 68 cases with SARS-CoV-2 placentitis from 12 countries. Arch Pathol Lab Med. February 10, 2022. doi:10.5858/arpa.2022- 0029-SA.
- Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181: 271-280.e8.
- Hecht JL, Quade B, Deshpande V, et al. SARS-CoV-2 can infect the placenta and is not associated with specific placental histopathology: a series of 19 placentas from COVID-19- positive mothers. Mod Pathol. 2020;33:2092-2103.
- Mourad M, Jacob T, Sadovsky E, et al. Placental response to maternal SARS-CoV-2 infection. Sci Rep. 2021;11:14390.
- Lu-Culligan A, Chavan AR, Vijayakumar P, et al. Maternal respiratory SARS-CoV-2 infection in pregnancy is associated with a robust inflammatory response at the maternal-fetal interface. Med (N Y). 2021;2:591-610.e10.
- Shook LL, Bordt EA, Meinsohn MC, et al. Placental expression of ACE2 and TMPRSS2 in maternal severe acute respiratory syndrome coronavirus 2 infection: are placental defenses mediated by fetal sex? J Infect Dis. 2021;224(suppl 6):S659.
Taglauer ES, Wachman EM, Juttukonda L, et al. Acute severe acute respiratory syndrome coronavirus 2 infection in pregnancy is associated with placental angiotensin-converting enzyme 2 shedding. Am J Pathol. 2022;192:595-603. doi.org/10.1016/j.ajpath.2021.12.011
EXPERT COMMENTARY
Although transmission of SARS-CoV-2 virus from an infected mother to her fetus is rare, placental infection with SARS-CoV-2 can occur and has been observed in association with placental damage and adverse pregnancy outcomes, including stillbirth.1 Understanding what mechanisms of defense protect the placenta and fetus from direct SARS-CoV-2 infection at the maternal-fetal interface, as well as the factors that might disturb or enhance that protection, is critical to gaining a deeper understanding of the potential impact of maternal COVID-19 on fetal well-being.
Details of the study
In a cohort of 24 pregnant individuals, Taglauer and colleagues investigated levels of placental angiotensin-converting enzyme (ACE)-2, placental ADAM17 (a disintegrin and metalloprotease domain 17) activity, and maternal serum soluble ACE2 in samples obtained at delivery from individuals with a history of second trimester COVID-19 infection, early third trimester COVID-19 infection, and no history of COVID-19 infection.
Results. Maternal COVID-19 infection in the early third trimester of pregnancy resulted in lower ACE2 protein levels in the placenta at delivery, higher ACE2 gene expression, and an increase in ADAM17 activity, compared with infection in the second trimester of pregnancy and compared with noninfected controls.
The authors postulated that increased ADAM17 activity—the enzyme responsible for ACE2 cleavage and shedding—may be responsible for lower ACE2 protein levels. Soluble ACE2 levels in maternal blood at delivery were increased in individuals with third trimester COVID-19 infection, although the source of soluble ACE2 (placental or otherwise) could not be determined with the methods employed. Levels of placental estrogen were no different between groups, which suggests that estrogen is not responsible for the observed differences.
Study strengths and limitations
ACE2 is the main receptor for the SARS-CoV-2 virus and facilitates viral entry into the cell.2 Placental villous cells that are in direct contact with maternal blood express the ACE2 protein, rendering them potentially vulnerable to SARS-CoV-2 infection.3 In this study, the authors observed lower placental ACE2 protein in term placentas from recent (early third trimester) but not remote (second trimester) maternal SARS-CoV-2 infection, arguably the result of the observed increase in ADAM17 cleavage activity. Prior studies have shown conflicting results, with equal or higher ACE2 levels noted in the setting of maternal COVID-19 infection, which may be related to differences in COVID-19 disease severity, gestational age of infection, and/or fetal sex in these cohorts.4-6
The concept that increased placental ACE2 shedding represents a protective defense mechanism that might last weeks beyond the acute infectious period is intriguing, but it requires further study. Observed differences in third but not second trimester COVID-19 infections could indicate either 1) an effect of maternal COVID-19 infection that lasts for several weeks but eventually normalizes over time, in the case of a remote infection; or 2) that second trimester maternal COVID-19 infection does not have the same pronounced effect on ACE2 levels as does third trimester infection. Observational studies of the human placenta are not able to answer this question, as directly sampling the placenta at the time of the exposure (or repeated sampling over time) in ongoing pregnancies is neither practical nor ethical. Further studies using animal or cellular models of SARS-CoV-2 infection in pregnancy may be necessary to fully understand the clinical relevance of these findings.
The study by Taglauer and colleagues provides a compelling argument for exploring how immune defenses at the maternal-fetal interface evolve over time and vary by trimester of exposure. ●
As the number of pregnancies exposed to COVID-19 continues to grow worldwide, how immune defenses at the maternal-fetal interface protect against fetal infection remains an important area of investigation.
LYDIA L. SHOOK, MD
- Pregnant people are at increased risk of more severe COVID-19 illness.
- The risk of stillbirth is 2- to 4-fold higher in women with COVID-19 infection during pregnancy.1
- COVID-19 vaccination is recommended for all people who are pregnant, lactating, or considering pregnancy.
- Pregnant and recently pregnant people up to 6 weeks postpartum should receive a third “booster” dose of a COVID-19 mRNA vaccine following completion of their initial COVID-19 vaccine or vaccine series.
- The mRNA COVID-19 vaccines are preferred over the Johnson & Johnson/Janssen COVID-19 vaccine for pregnant and lactating individuals for primary series and booster vaccination.
- Completion of a 2-dose mRNA COVID-19 vaccination series during pregnancy might help prevent COVID-19 hospitalization among infants <6 months.2
aVaccine recommendations adapted from: ACOG practice advisory: COVID-19 vaccination considerations for obstetric-gynecologic care. Last updated March 2, 2022. https://www.acog.org/clinical/ clinical-guidance/practice-advisory/articles/2020/12/covid-19-vaccination-considerations-for-obstetricgynecologic-care. Accessed March 21, 2022.
References
1. DeSisto CL, Wallace B, Simeone RM, et al. Risk for stillbirth among women with and without COVID-19 at delivery hospitalization—United States, March 2020–September 2021. MMWR Morbid Mortal Wkly Rep. 2021;70:1640-1645.
2. Halasa NB, Olson SM, Staat MA, et al; Overcoming COVID-19 Investigators; Overcoming COVID-19 Network. Effectiveness of maternal vaccination with mRNA COVID-19 vaccine during pregnancy against COVID-19–associated hospitalization in infants aged <6 months—17 states, July 2021–January 2022. MMWR Morbid Mortal Wkly Rep. 2022;71:264-270.
Taglauer ES, Wachman EM, Juttukonda L, et al. Acute severe acute respiratory syndrome coronavirus 2 infection in pregnancy is associated with placental angiotensin-converting enzyme 2 shedding. Am J Pathol. 2022;192:595-603. doi.org/10.1016/j.ajpath.2021.12.011
EXPERT COMMENTARY
Although transmission of SARS-CoV-2 virus from an infected mother to her fetus is rare, placental infection with SARS-CoV-2 can occur and has been observed in association with placental damage and adverse pregnancy outcomes, including stillbirth.1 Understanding what mechanisms of defense protect the placenta and fetus from direct SARS-CoV-2 infection at the maternal-fetal interface, as well as the factors that might disturb or enhance that protection, is critical to gaining a deeper understanding of the potential impact of maternal COVID-19 on fetal well-being.
Details of the study
In a cohort of 24 pregnant individuals, Taglauer and colleagues investigated levels of placental angiotensin-converting enzyme (ACE)-2, placental ADAM17 (a disintegrin and metalloprotease domain 17) activity, and maternal serum soluble ACE2 in samples obtained at delivery from individuals with a history of second trimester COVID-19 infection, early third trimester COVID-19 infection, and no history of COVID-19 infection.
Results. Maternal COVID-19 infection in the early third trimester of pregnancy resulted in lower ACE2 protein levels in the placenta at delivery, higher ACE2 gene expression, and an increase in ADAM17 activity, compared with infection in the second trimester of pregnancy and compared with noninfected controls.
The authors postulated that increased ADAM17 activity—the enzyme responsible for ACE2 cleavage and shedding—may be responsible for lower ACE2 protein levels. Soluble ACE2 levels in maternal blood at delivery were increased in individuals with third trimester COVID-19 infection, although the source of soluble ACE2 (placental or otherwise) could not be determined with the methods employed. Levels of placental estrogen were no different between groups, which suggests that estrogen is not responsible for the observed differences.
Study strengths and limitations
ACE2 is the main receptor for the SARS-CoV-2 virus and facilitates viral entry into the cell.2 Placental villous cells that are in direct contact with maternal blood express the ACE2 protein, rendering them potentially vulnerable to SARS-CoV-2 infection.3 In this study, the authors observed lower placental ACE2 protein in term placentas from recent (early third trimester) but not remote (second trimester) maternal SARS-CoV-2 infection, arguably the result of the observed increase in ADAM17 cleavage activity. Prior studies have shown conflicting results, with equal or higher ACE2 levels noted in the setting of maternal COVID-19 infection, which may be related to differences in COVID-19 disease severity, gestational age of infection, and/or fetal sex in these cohorts.4-6
The concept that increased placental ACE2 shedding represents a protective defense mechanism that might last weeks beyond the acute infectious period is intriguing, but it requires further study. Observed differences in third but not second trimester COVID-19 infections could indicate either 1) an effect of maternal COVID-19 infection that lasts for several weeks but eventually normalizes over time, in the case of a remote infection; or 2) that second trimester maternal COVID-19 infection does not have the same pronounced effect on ACE2 levels as does third trimester infection. Observational studies of the human placenta are not able to answer this question, as directly sampling the placenta at the time of the exposure (or repeated sampling over time) in ongoing pregnancies is neither practical nor ethical. Further studies using animal or cellular models of SARS-CoV-2 infection in pregnancy may be necessary to fully understand the clinical relevance of these findings.
The study by Taglauer and colleagues provides a compelling argument for exploring how immune defenses at the maternal-fetal interface evolve over time and vary by trimester of exposure. ●
As the number of pregnancies exposed to COVID-19 continues to grow worldwide, how immune defenses at the maternal-fetal interface protect against fetal infection remains an important area of investigation.
LYDIA L. SHOOK, MD
- Pregnant people are at increased risk of more severe COVID-19 illness.
- The risk of stillbirth is 2- to 4-fold higher in women with COVID-19 infection during pregnancy.1
- COVID-19 vaccination is recommended for all people who are pregnant, lactating, or considering pregnancy.
- Pregnant and recently pregnant people up to 6 weeks postpartum should receive a third “booster” dose of a COVID-19 mRNA vaccine following completion of their initial COVID-19 vaccine or vaccine series.
- The mRNA COVID-19 vaccines are preferred over the Johnson & Johnson/Janssen COVID-19 vaccine for pregnant and lactating individuals for primary series and booster vaccination.
- Completion of a 2-dose mRNA COVID-19 vaccination series during pregnancy might help prevent COVID-19 hospitalization among infants <6 months.2
aVaccine recommendations adapted from: ACOG practice advisory: COVID-19 vaccination considerations for obstetric-gynecologic care. Last updated March 2, 2022. https://www.acog.org/clinical/ clinical-guidance/practice-advisory/articles/2020/12/covid-19-vaccination-considerations-for-obstetricgynecologic-care. Accessed March 21, 2022.
References
1. DeSisto CL, Wallace B, Simeone RM, et al. Risk for stillbirth among women with and without COVID-19 at delivery hospitalization—United States, March 2020–September 2021. MMWR Morbid Mortal Wkly Rep. 2021;70:1640-1645.
2. Halasa NB, Olson SM, Staat MA, et al; Overcoming COVID-19 Investigators; Overcoming COVID-19 Network. Effectiveness of maternal vaccination with mRNA COVID-19 vaccine during pregnancy against COVID-19–associated hospitalization in infants aged <6 months—17 states, July 2021–January 2022. MMWR Morbid Mortal Wkly Rep. 2022;71:264-270.
- Schwartz DA, Avvad-Portari E, Babál, et al. Placental tissue destruction and insufficiency from COVID-19 causes stillbirth and neonatal death from hypoxic-ischemic injury: a study of 68 cases with SARS-CoV-2 placentitis from 12 countries. Arch Pathol Lab Med. February 10, 2022. doi:10.5858/arpa.2022- 0029-SA.
- Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181: 271-280.e8.
- Hecht JL, Quade B, Deshpande V, et al. SARS-CoV-2 can infect the placenta and is not associated with specific placental histopathology: a series of 19 placentas from COVID-19- positive mothers. Mod Pathol. 2020;33:2092-2103.
- Mourad M, Jacob T, Sadovsky E, et al. Placental response to maternal SARS-CoV-2 infection. Sci Rep. 2021;11:14390.
- Lu-Culligan A, Chavan AR, Vijayakumar P, et al. Maternal respiratory SARS-CoV-2 infection in pregnancy is associated with a robust inflammatory response at the maternal-fetal interface. Med (N Y). 2021;2:591-610.e10.
- Shook LL, Bordt EA, Meinsohn MC, et al. Placental expression of ACE2 and TMPRSS2 in maternal severe acute respiratory syndrome coronavirus 2 infection: are placental defenses mediated by fetal sex? J Infect Dis. 2021;224(suppl 6):S659.
- Schwartz DA, Avvad-Portari E, Babál, et al. Placental tissue destruction and insufficiency from COVID-19 causes stillbirth and neonatal death from hypoxic-ischemic injury: a study of 68 cases with SARS-CoV-2 placentitis from 12 countries. Arch Pathol Lab Med. February 10, 2022. doi:10.5858/arpa.2022- 0029-SA.
- Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181: 271-280.e8.
- Hecht JL, Quade B, Deshpande V, et al. SARS-CoV-2 can infect the placenta and is not associated with specific placental histopathology: a series of 19 placentas from COVID-19- positive mothers. Mod Pathol. 2020;33:2092-2103.
- Mourad M, Jacob T, Sadovsky E, et al. Placental response to maternal SARS-CoV-2 infection. Sci Rep. 2021;11:14390.
- Lu-Culligan A, Chavan AR, Vijayakumar P, et al. Maternal respiratory SARS-CoV-2 infection in pregnancy is associated with a robust inflammatory response at the maternal-fetal interface. Med (N Y). 2021;2:591-610.e10.
- Shook LL, Bordt EA, Meinsohn MC, et al. Placental expression of ACE2 and TMPRSS2 in maternal severe acute respiratory syndrome coronavirus 2 infection: are placental defenses mediated by fetal sex? J Infect Dis. 2021;224(suppl 6):S659.
Protease inhibitors increase small-for-gestational-age but not other pregnancy risks
Pregnant women with HIV can be reassured that protease inhibitors are safer than previously thought in terms of risk to the fetus, according to research from the National Perinatal Epidemiology Unit (NPEU) at Oxford Population Health, a research institute based at the University of Oxford (England).
Antiretroviral therapy (ART) is recommended for all pregnant women living with HIV and plays a crucial role both in improving maternal health and in reducing transmission of HIV from mother to child. However, there has been a critical lack of evidence about the effects of ART on the risk of adverse pregnancy outcomes, with particular concern about protease inhibitors.
Current guidelines recommend that protease inhibitor-based therapies should be used in pregnancy only if first-line treatments (such as integrase and reverse-transcriptase based treatments) are either unsuitable or unavailable. These guidelines also often advise against the use of a specific protease inhibitor, lopinavir/ritonavir, citing an increased risk of preterm birth. However, such advice may restrict treatment options for pregnant women with HIV on the basis of limited evidence.
Largest review to date
The NPEU researchers, therefore, conducted the largest systematic review to date of adverse perinatal outcomes after a range of antiretroviral therapies. It included 34 cohort studies published between 1980 and 2020 and involving over 57,000 pregnant women with HIV in 22 different countries. The review, published in eClinicalMedicine, looked for evidence of 11 perinatal outcomes:
- Preterm birth, very preterm birth, and spontaneous preterm birth
- Low birth weight, very low birth weight, term low birth weight, and preterm low birth weight
- Small for gestational age and very small for gestational age
- Stillbirth, and neonatal death
Using pairwise random-effects meta-analyses, researchers compared protease inhibitor versus non-protease inhibitor-based ART, as well as specifically looking at the comparative risks associated with different protease inhibitor regimens.
They found that protease inhibitor-based ART significantly increased the risk of small or very small for gestational age babies, with relative risks of 1.24 (95% confidence interval, 1.08-1.43; I2 = 66.7%) and 1.40 (95% CI, 1.09-1.81; I2 = 0.0%), respectively. However there were no significant differences in other adverse pregnancy outcomes for protease inhibitors, compared with other therapies.
In addition, researchers found no significant differences in perinatal outcomes between ART regimens containing lopinavir/ritonavir, atazanavir/ritonavir, or darunavir/ritonavir, which are the most frequently used protease inhibitors.
No increased risk of preterm birth
Senior author Dr. Joris Hemelaar, senior clinical research fellow at the NPEU and honorary consultant in obstetrics at the John Radcliffe Hospital, Oxford (England), said: “Antiretroviral therapy in pregnancy has clear benefits for maternal health and prevention of HIV transmission to the child, but our study has shown for the first time that protease inhibitors are associated with babies being small or very small for their gestational age.”
“However, there was no increased risk of preterm birth, or any other adverse pregnancy outcomes. This means protease inhibitors remain an important option for pregnant women living with HIV if other treatments are unsuitable, for example due to drug resistance, or unavailable. The evidence presented here indicates that the commonly used protease inhibitors atazanavir, lopinavir, and darunavir are comparable with regard to perinatal outcomes, which should inform international treatment guidelines.”
Over 70% of the studies assessed were conducted in high-income countries, and Dr. Hemelaar added that there is an urgent need for more research on pregnancy outcomes after different ART in low- to middle-income countries, where the burden of HIV is highest.
Professor Yvonne Gilleece, a spokesperson for the British HIV Association (BHIVA) and immediate past chair of the BHIVA guidelines on the management of HIV in pregnancy and the postpartum period commented: “Pregnancy is a unique life situation in which we must consider the safety of both the birthing parent and the baby. Due to ongoing under-representation of all women in clinical trials, but particularly pregnant women, we do not have enough evidence on which to base all our management decisions. This systematic review includes large numbers of pregnant women living with HIV and can, therefore, improve an informed discussion regarding the safety of the use of protease inhibitors during pregnancy.”
Dr. Hemelaar told Medscape UK: “Many international treatment guidelines cite adverse pregnancy outcomes, in particular preterm birth, associated with protease inhibitor (PI)-drugs as a reason for caution for their use in pregnancy. However, PI drugs are not associated with preterm birth in our analysis. This suggests that PI drugs may not be as detrimental as previously thought (and we found no differences between different PI drugs used), and, hence, these drugs may have a more favourable profile for use in pregnancy.
“However, many other aspects of treatment, including the extent to which the virus can be suppressed, adverse drug effects, adherence to drug prescriptions, antiretroviral drug resistance, drug interactions, drug cost, and availability, should also be taken into account by clinicians and guideline development committees.”
A version of this article first appeared on Medscape UK.
Pregnant women with HIV can be reassured that protease inhibitors are safer than previously thought in terms of risk to the fetus, according to research from the National Perinatal Epidemiology Unit (NPEU) at Oxford Population Health, a research institute based at the University of Oxford (England).
Antiretroviral therapy (ART) is recommended for all pregnant women living with HIV and plays a crucial role both in improving maternal health and in reducing transmission of HIV from mother to child. However, there has been a critical lack of evidence about the effects of ART on the risk of adverse pregnancy outcomes, with particular concern about protease inhibitors.
Current guidelines recommend that protease inhibitor-based therapies should be used in pregnancy only if first-line treatments (such as integrase and reverse-transcriptase based treatments) are either unsuitable or unavailable. These guidelines also often advise against the use of a specific protease inhibitor, lopinavir/ritonavir, citing an increased risk of preterm birth. However, such advice may restrict treatment options for pregnant women with HIV on the basis of limited evidence.
Largest review to date
The NPEU researchers, therefore, conducted the largest systematic review to date of adverse perinatal outcomes after a range of antiretroviral therapies. It included 34 cohort studies published between 1980 and 2020 and involving over 57,000 pregnant women with HIV in 22 different countries. The review, published in eClinicalMedicine, looked for evidence of 11 perinatal outcomes:
- Preterm birth, very preterm birth, and spontaneous preterm birth
- Low birth weight, very low birth weight, term low birth weight, and preterm low birth weight
- Small for gestational age and very small for gestational age
- Stillbirth, and neonatal death
Using pairwise random-effects meta-analyses, researchers compared protease inhibitor versus non-protease inhibitor-based ART, as well as specifically looking at the comparative risks associated with different protease inhibitor regimens.
They found that protease inhibitor-based ART significantly increased the risk of small or very small for gestational age babies, with relative risks of 1.24 (95% confidence interval, 1.08-1.43; I2 = 66.7%) and 1.40 (95% CI, 1.09-1.81; I2 = 0.0%), respectively. However there were no significant differences in other adverse pregnancy outcomes for protease inhibitors, compared with other therapies.
In addition, researchers found no significant differences in perinatal outcomes between ART regimens containing lopinavir/ritonavir, atazanavir/ritonavir, or darunavir/ritonavir, which are the most frequently used protease inhibitors.
No increased risk of preterm birth
Senior author Dr. Joris Hemelaar, senior clinical research fellow at the NPEU and honorary consultant in obstetrics at the John Radcliffe Hospital, Oxford (England), said: “Antiretroviral therapy in pregnancy has clear benefits for maternal health and prevention of HIV transmission to the child, but our study has shown for the first time that protease inhibitors are associated with babies being small or very small for their gestational age.”
“However, there was no increased risk of preterm birth, or any other adverse pregnancy outcomes. This means protease inhibitors remain an important option for pregnant women living with HIV if other treatments are unsuitable, for example due to drug resistance, or unavailable. The evidence presented here indicates that the commonly used protease inhibitors atazanavir, lopinavir, and darunavir are comparable with regard to perinatal outcomes, which should inform international treatment guidelines.”
Over 70% of the studies assessed were conducted in high-income countries, and Dr. Hemelaar added that there is an urgent need for more research on pregnancy outcomes after different ART in low- to middle-income countries, where the burden of HIV is highest.
Professor Yvonne Gilleece, a spokesperson for the British HIV Association (BHIVA) and immediate past chair of the BHIVA guidelines on the management of HIV in pregnancy and the postpartum period commented: “Pregnancy is a unique life situation in which we must consider the safety of both the birthing parent and the baby. Due to ongoing under-representation of all women in clinical trials, but particularly pregnant women, we do not have enough evidence on which to base all our management decisions. This systematic review includes large numbers of pregnant women living with HIV and can, therefore, improve an informed discussion regarding the safety of the use of protease inhibitors during pregnancy.”
Dr. Hemelaar told Medscape UK: “Many international treatment guidelines cite adverse pregnancy outcomes, in particular preterm birth, associated with protease inhibitor (PI)-drugs as a reason for caution for their use in pregnancy. However, PI drugs are not associated with preterm birth in our analysis. This suggests that PI drugs may not be as detrimental as previously thought (and we found no differences between different PI drugs used), and, hence, these drugs may have a more favourable profile for use in pregnancy.
“However, many other aspects of treatment, including the extent to which the virus can be suppressed, adverse drug effects, adherence to drug prescriptions, antiretroviral drug resistance, drug interactions, drug cost, and availability, should also be taken into account by clinicians and guideline development committees.”
A version of this article first appeared on Medscape UK.
Pregnant women with HIV can be reassured that protease inhibitors are safer than previously thought in terms of risk to the fetus, according to research from the National Perinatal Epidemiology Unit (NPEU) at Oxford Population Health, a research institute based at the University of Oxford (England).
Antiretroviral therapy (ART) is recommended for all pregnant women living with HIV and plays a crucial role both in improving maternal health and in reducing transmission of HIV from mother to child. However, there has been a critical lack of evidence about the effects of ART on the risk of adverse pregnancy outcomes, with particular concern about protease inhibitors.
Current guidelines recommend that protease inhibitor-based therapies should be used in pregnancy only if first-line treatments (such as integrase and reverse-transcriptase based treatments) are either unsuitable or unavailable. These guidelines also often advise against the use of a specific protease inhibitor, lopinavir/ritonavir, citing an increased risk of preterm birth. However, such advice may restrict treatment options for pregnant women with HIV on the basis of limited evidence.
Largest review to date
The NPEU researchers, therefore, conducted the largest systematic review to date of adverse perinatal outcomes after a range of antiretroviral therapies. It included 34 cohort studies published between 1980 and 2020 and involving over 57,000 pregnant women with HIV in 22 different countries. The review, published in eClinicalMedicine, looked for evidence of 11 perinatal outcomes:
- Preterm birth, very preterm birth, and spontaneous preterm birth
- Low birth weight, very low birth weight, term low birth weight, and preterm low birth weight
- Small for gestational age and very small for gestational age
- Stillbirth, and neonatal death
Using pairwise random-effects meta-analyses, researchers compared protease inhibitor versus non-protease inhibitor-based ART, as well as specifically looking at the comparative risks associated with different protease inhibitor regimens.
They found that protease inhibitor-based ART significantly increased the risk of small or very small for gestational age babies, with relative risks of 1.24 (95% confidence interval, 1.08-1.43; I2 = 66.7%) and 1.40 (95% CI, 1.09-1.81; I2 = 0.0%), respectively. However there were no significant differences in other adverse pregnancy outcomes for protease inhibitors, compared with other therapies.
In addition, researchers found no significant differences in perinatal outcomes between ART regimens containing lopinavir/ritonavir, atazanavir/ritonavir, or darunavir/ritonavir, which are the most frequently used protease inhibitors.
No increased risk of preterm birth
Senior author Dr. Joris Hemelaar, senior clinical research fellow at the NPEU and honorary consultant in obstetrics at the John Radcliffe Hospital, Oxford (England), said: “Antiretroviral therapy in pregnancy has clear benefits for maternal health and prevention of HIV transmission to the child, but our study has shown for the first time that protease inhibitors are associated with babies being small or very small for their gestational age.”
“However, there was no increased risk of preterm birth, or any other adverse pregnancy outcomes. This means protease inhibitors remain an important option for pregnant women living with HIV if other treatments are unsuitable, for example due to drug resistance, or unavailable. The evidence presented here indicates that the commonly used protease inhibitors atazanavir, lopinavir, and darunavir are comparable with regard to perinatal outcomes, which should inform international treatment guidelines.”
Over 70% of the studies assessed were conducted in high-income countries, and Dr. Hemelaar added that there is an urgent need for more research on pregnancy outcomes after different ART in low- to middle-income countries, where the burden of HIV is highest.
Professor Yvonne Gilleece, a spokesperson for the British HIV Association (BHIVA) and immediate past chair of the BHIVA guidelines on the management of HIV in pregnancy and the postpartum period commented: “Pregnancy is a unique life situation in which we must consider the safety of both the birthing parent and the baby. Due to ongoing under-representation of all women in clinical trials, but particularly pregnant women, we do not have enough evidence on which to base all our management decisions. This systematic review includes large numbers of pregnant women living with HIV and can, therefore, improve an informed discussion regarding the safety of the use of protease inhibitors during pregnancy.”
Dr. Hemelaar told Medscape UK: “Many international treatment guidelines cite adverse pregnancy outcomes, in particular preterm birth, associated with protease inhibitor (PI)-drugs as a reason for caution for their use in pregnancy. However, PI drugs are not associated with preterm birth in our analysis. This suggests that PI drugs may not be as detrimental as previously thought (and we found no differences between different PI drugs used), and, hence, these drugs may have a more favourable profile for use in pregnancy.
“However, many other aspects of treatment, including the extent to which the virus can be suppressed, adverse drug effects, adherence to drug prescriptions, antiretroviral drug resistance, drug interactions, drug cost, and availability, should also be taken into account by clinicians and guideline development committees.”
A version of this article first appeared on Medscape UK.
FROM ECLINICALMEDICINE
Study suggests keto diet increases tumor growth in ovarian cancer
A ketogenic diet fed to mice with epithelial ovarian cancer led to significantly increased tumor growth and gut microbiome alterations, according to study recently presented at the annual meeting of the Society of Gynecologic Oncology.
“The keto diet is very popular, especially among patients who believe it may treat cancer by starving tumors of the fuel they need to grow, altering the immune system, and other anticancer effects,” said study leader Mariam AlHilli, MD, of the Cleveland Clinic.
The findings are surprising because in other studies the high-fat, zero-carb ketogenic diet has demonstrated tumor-suppressing effects. It has been under study as a possible adjuvant therapy for other cancers, such as glioblastoma, colon cancer, prostate cancer, and pancreatic cancer.
“While we don’t know yet whether these findings extend to patients, the results in animals indicate that instead of being protective, the keto diet appears to promote ovarian cancer growth and progression,” Dr. AlHilli said. In the present study, tumor bearing mice were fed a keto diet consisting of 10% protein, 0% carbohydrates, and 90% fat, while the high-fat diet was 10% protein, 15% carbohydrates, and 75% fat. The control diet consisted of 10% protein, 77% carbohydrates, and 13% fat. Epithelial ovarian cancer tumor growth was monitored weekly.
Over the 6- to 10-week course of study, a 9.1-fold increase from baseline in tumor growth was observed in the keto diet-fed mice (n = 20). Among mice fed a high-fat diet (n = 20) that included some carbohydrates, tumor growth increased 2.0-fold from baseline, and among control group mice (n = 20) fed a low-fat, high carbohydrate diet, tumor growth increased 3.1-fold.
The investigators observed several hallmarks of tumor progression: tumor associated macrophages were enriched significantly, activated lymphoid cells (natural killer cells) were significantly reduced (P < .001), and M2:M1 polarization trended higher. Also, in keto diet–fed mice, gene set enrichment analysis revealed that epithelial ovarian cancer tumors had increased angiogenesis and inflammatory responses, enhanced epithelial-to-mesenchymal transition phenotype, and altered lipid metabolism. Compared with high-fat diet–fed mice, the keto-fed mice had increases in lipid catalytic activity and catabolism, as well as decreases in lipid synthesis.
“The tumor increase could be mediated by the gut microbiome or by gene alterations or by metabolite levels that influence tumor growth. It’s possible that each cancer type is different. The composition of the diet may be a factor, as well as how tumors metabolize fat and ketones,” Dr. AlHilli said.
The results need to be confirmed in preclinical animal studies and in additional models, she added.
The study was funded by a K12 Grant and internal funding from Cleveland Clinic. Dr. AlHilli declared no relevant disclosures.
A ketogenic diet fed to mice with epithelial ovarian cancer led to significantly increased tumor growth and gut microbiome alterations, according to study recently presented at the annual meeting of the Society of Gynecologic Oncology.
“The keto diet is very popular, especially among patients who believe it may treat cancer by starving tumors of the fuel they need to grow, altering the immune system, and other anticancer effects,” said study leader Mariam AlHilli, MD, of the Cleveland Clinic.
The findings are surprising because in other studies the high-fat, zero-carb ketogenic diet has demonstrated tumor-suppressing effects. It has been under study as a possible adjuvant therapy for other cancers, such as glioblastoma, colon cancer, prostate cancer, and pancreatic cancer.
“While we don’t know yet whether these findings extend to patients, the results in animals indicate that instead of being protective, the keto diet appears to promote ovarian cancer growth and progression,” Dr. AlHilli said. In the present study, tumor bearing mice were fed a keto diet consisting of 10% protein, 0% carbohydrates, and 90% fat, while the high-fat diet was 10% protein, 15% carbohydrates, and 75% fat. The control diet consisted of 10% protein, 77% carbohydrates, and 13% fat. Epithelial ovarian cancer tumor growth was monitored weekly.
Over the 6- to 10-week course of study, a 9.1-fold increase from baseline in tumor growth was observed in the keto diet-fed mice (n = 20). Among mice fed a high-fat diet (n = 20) that included some carbohydrates, tumor growth increased 2.0-fold from baseline, and among control group mice (n = 20) fed a low-fat, high carbohydrate diet, tumor growth increased 3.1-fold.
The investigators observed several hallmarks of tumor progression: tumor associated macrophages were enriched significantly, activated lymphoid cells (natural killer cells) were significantly reduced (P < .001), and M2:M1 polarization trended higher. Also, in keto diet–fed mice, gene set enrichment analysis revealed that epithelial ovarian cancer tumors had increased angiogenesis and inflammatory responses, enhanced epithelial-to-mesenchymal transition phenotype, and altered lipid metabolism. Compared with high-fat diet–fed mice, the keto-fed mice had increases in lipid catalytic activity and catabolism, as well as decreases in lipid synthesis.
“The tumor increase could be mediated by the gut microbiome or by gene alterations or by metabolite levels that influence tumor growth. It’s possible that each cancer type is different. The composition of the diet may be a factor, as well as how tumors metabolize fat and ketones,” Dr. AlHilli said.
The results need to be confirmed in preclinical animal studies and in additional models, she added.
The study was funded by a K12 Grant and internal funding from Cleveland Clinic. Dr. AlHilli declared no relevant disclosures.
A ketogenic diet fed to mice with epithelial ovarian cancer led to significantly increased tumor growth and gut microbiome alterations, according to study recently presented at the annual meeting of the Society of Gynecologic Oncology.
“The keto diet is very popular, especially among patients who believe it may treat cancer by starving tumors of the fuel they need to grow, altering the immune system, and other anticancer effects,” said study leader Mariam AlHilli, MD, of the Cleveland Clinic.
The findings are surprising because in other studies the high-fat, zero-carb ketogenic diet has demonstrated tumor-suppressing effects. It has been under study as a possible adjuvant therapy for other cancers, such as glioblastoma, colon cancer, prostate cancer, and pancreatic cancer.
“While we don’t know yet whether these findings extend to patients, the results in animals indicate that instead of being protective, the keto diet appears to promote ovarian cancer growth and progression,” Dr. AlHilli said. In the present study, tumor bearing mice were fed a keto diet consisting of 10% protein, 0% carbohydrates, and 90% fat, while the high-fat diet was 10% protein, 15% carbohydrates, and 75% fat. The control diet consisted of 10% protein, 77% carbohydrates, and 13% fat. Epithelial ovarian cancer tumor growth was monitored weekly.
Over the 6- to 10-week course of study, a 9.1-fold increase from baseline in tumor growth was observed in the keto diet-fed mice (n = 20). Among mice fed a high-fat diet (n = 20) that included some carbohydrates, tumor growth increased 2.0-fold from baseline, and among control group mice (n = 20) fed a low-fat, high carbohydrate diet, tumor growth increased 3.1-fold.
The investigators observed several hallmarks of tumor progression: tumor associated macrophages were enriched significantly, activated lymphoid cells (natural killer cells) were significantly reduced (P < .001), and M2:M1 polarization trended higher. Also, in keto diet–fed mice, gene set enrichment analysis revealed that epithelial ovarian cancer tumors had increased angiogenesis and inflammatory responses, enhanced epithelial-to-mesenchymal transition phenotype, and altered lipid metabolism. Compared with high-fat diet–fed mice, the keto-fed mice had increases in lipid catalytic activity and catabolism, as well as decreases in lipid synthesis.
“The tumor increase could be mediated by the gut microbiome or by gene alterations or by metabolite levels that influence tumor growth. It’s possible that each cancer type is different. The composition of the diet may be a factor, as well as how tumors metabolize fat and ketones,” Dr. AlHilli said.
The results need to be confirmed in preclinical animal studies and in additional models, she added.
The study was funded by a K12 Grant and internal funding from Cleveland Clinic. Dr. AlHilli declared no relevant disclosures.
FROM SGO 2022
AI model predicts ovarian cancer responses
The model, using still-frame images from pretreatment laparoscopic surgical videos, had an overall accuracy rate of 93%, according to the pilot study’s first author, Deanna Glassman, MD, an oncologic fellow at the University of Texas MD Anderson Cancer Center, Houston.
Dr. Glassman described her research in a presentation given at the annual meeting of the Society of Gynecologic Oncology.
While the AI model successfully identified all excellent-response patients, it did classify about a third of patients with poor responses as excellent responses. The smaller number of images in the poor-response category, Dr. Glassman speculated, may explain the misclassification.
Researchers took 435 representative still-frame images from pretreatment laparoscopic surgical videos of 113 patients with pathologically proven high-grade serous ovarian cancer. Using 70% of the images to train the model, they used 10% for validation and 20% for the actual testing. They developed the AI model with images from four anatomical locations (diaphragm, omentum, peritoneum, and pelvis), training it using deep learning and neural networks to extract morphological disease patterns for correlation with either of two outcomes: excellent response or poor response. An excellent response was defined as progression-free survival of 12 months or more, and poor response as PFS of 6 months or less. In the retrospective study of images, after excluding 32 gray-zone patients, 75 patients (66%) had durable responses to therapy and 6 (5%) had poor responses.
The PFS was 19 months in the excellent-response group and 3 months in the poor-response group.
Clinicians have often observed differences in gross morphology within the single histologic diagnosis of high-grade serous ovarian cancer. The research intent was to determine if AI could detect these distinct morphological patterns in the still frame images taken at the time of laparoscopy, and correlate them with the eventual clinical outcomes. Dr. Glassman and colleagues are currently validating the model with a much larger cohort, and will look into clinical testing.
“The big-picture goal,” Dr. Glassman said in an interview, “would be to utilize the model to predict which patients would do well with traditional standard of care treatments and those who wouldn’t do well so that we can personalize the treatment plan for those patients with alternative agents and therapies.”
Once validated, the model could also be employed to identify patterns of disease in other gynecologic cancers or distinguish between viable and necrosed malignant tissue.
The study’s predominant limitation was the small sample size which is being addressed in a larger ongoing study.
Funding was provided by a T32 grant, MD Anderson Cancer Center Support Grant, MD Anderson Ovarian Cancer Moon Shot, SPORE in Ovarian Cancer, the American Cancer Society, and the Ovarian Cancer Research Alliance. Dr. Glassman declared no relevant financial relationships.
The model, using still-frame images from pretreatment laparoscopic surgical videos, had an overall accuracy rate of 93%, according to the pilot study’s first author, Deanna Glassman, MD, an oncologic fellow at the University of Texas MD Anderson Cancer Center, Houston.
Dr. Glassman described her research in a presentation given at the annual meeting of the Society of Gynecologic Oncology.
While the AI model successfully identified all excellent-response patients, it did classify about a third of patients with poor responses as excellent responses. The smaller number of images in the poor-response category, Dr. Glassman speculated, may explain the misclassification.
Researchers took 435 representative still-frame images from pretreatment laparoscopic surgical videos of 113 patients with pathologically proven high-grade serous ovarian cancer. Using 70% of the images to train the model, they used 10% for validation and 20% for the actual testing. They developed the AI model with images from four anatomical locations (diaphragm, omentum, peritoneum, and pelvis), training it using deep learning and neural networks to extract morphological disease patterns for correlation with either of two outcomes: excellent response or poor response. An excellent response was defined as progression-free survival of 12 months or more, and poor response as PFS of 6 months or less. In the retrospective study of images, after excluding 32 gray-zone patients, 75 patients (66%) had durable responses to therapy and 6 (5%) had poor responses.
The PFS was 19 months in the excellent-response group and 3 months in the poor-response group.
Clinicians have often observed differences in gross morphology within the single histologic diagnosis of high-grade serous ovarian cancer. The research intent was to determine if AI could detect these distinct morphological patterns in the still frame images taken at the time of laparoscopy, and correlate them with the eventual clinical outcomes. Dr. Glassman and colleagues are currently validating the model with a much larger cohort, and will look into clinical testing.
“The big-picture goal,” Dr. Glassman said in an interview, “would be to utilize the model to predict which patients would do well with traditional standard of care treatments and those who wouldn’t do well so that we can personalize the treatment plan for those patients with alternative agents and therapies.”
Once validated, the model could also be employed to identify patterns of disease in other gynecologic cancers or distinguish between viable and necrosed malignant tissue.
The study’s predominant limitation was the small sample size which is being addressed in a larger ongoing study.
Funding was provided by a T32 grant, MD Anderson Cancer Center Support Grant, MD Anderson Ovarian Cancer Moon Shot, SPORE in Ovarian Cancer, the American Cancer Society, and the Ovarian Cancer Research Alliance. Dr. Glassman declared no relevant financial relationships.
The model, using still-frame images from pretreatment laparoscopic surgical videos, had an overall accuracy rate of 93%, according to the pilot study’s first author, Deanna Glassman, MD, an oncologic fellow at the University of Texas MD Anderson Cancer Center, Houston.
Dr. Glassman described her research in a presentation given at the annual meeting of the Society of Gynecologic Oncology.
While the AI model successfully identified all excellent-response patients, it did classify about a third of patients with poor responses as excellent responses. The smaller number of images in the poor-response category, Dr. Glassman speculated, may explain the misclassification.
Researchers took 435 representative still-frame images from pretreatment laparoscopic surgical videos of 113 patients with pathologically proven high-grade serous ovarian cancer. Using 70% of the images to train the model, they used 10% for validation and 20% for the actual testing. They developed the AI model with images from four anatomical locations (diaphragm, omentum, peritoneum, and pelvis), training it using deep learning and neural networks to extract morphological disease patterns for correlation with either of two outcomes: excellent response or poor response. An excellent response was defined as progression-free survival of 12 months or more, and poor response as PFS of 6 months or less. In the retrospective study of images, after excluding 32 gray-zone patients, 75 patients (66%) had durable responses to therapy and 6 (5%) had poor responses.
The PFS was 19 months in the excellent-response group and 3 months in the poor-response group.
Clinicians have often observed differences in gross morphology within the single histologic diagnosis of high-grade serous ovarian cancer. The research intent was to determine if AI could detect these distinct morphological patterns in the still frame images taken at the time of laparoscopy, and correlate them with the eventual clinical outcomes. Dr. Glassman and colleagues are currently validating the model with a much larger cohort, and will look into clinical testing.
“The big-picture goal,” Dr. Glassman said in an interview, “would be to utilize the model to predict which patients would do well with traditional standard of care treatments and those who wouldn’t do well so that we can personalize the treatment plan for those patients with alternative agents and therapies.”
Once validated, the model could also be employed to identify patterns of disease in other gynecologic cancers or distinguish between viable and necrosed malignant tissue.
The study’s predominant limitation was the small sample size which is being addressed in a larger ongoing study.
Funding was provided by a T32 grant, MD Anderson Cancer Center Support Grant, MD Anderson Ovarian Cancer Moon Shot, SPORE in Ovarian Cancer, the American Cancer Society, and the Ovarian Cancer Research Alliance. Dr. Glassman declared no relevant financial relationships.
FROM SGO 2022