User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
The enemy of carcinogenic fumes is my friendly begonia
Sowing the seeds of cancer prevention
Are you looking to add to your quality of life, even though pets are not your speed? Might we suggest something with lower maintenance? Something a little greener?
Indoor plants can purify the air that comes from outside. Researchers at the University of Technology Sydney, in partnership with the plantscaping company Ambius, showed that a “green wall” made up of mixed indoor plants was able to suck up 97% of “the most toxic compounds” from the air in just 8 hours. We’re talking about lung-irritating, headache-inducing, cancer risk–boosting compounds from gasoline fumes, including benzene.
Public health initiatives often strive to reduce cardiovascular and obesity risks, but breathing seems pretty important too. According to the World Health Organization, household air pollution is responsible for about 2.5 million global premature deaths each year. And since 2020 we’ve become accustomed to spending more time inside and at home.
“This new research proves that plants should not just be seen as ‘nice to have,’ but rather a crucial part of every workplace wellness plan,” Ambius General Manager Johan Hodgson said in statement released by the university.
So don’t spend hundreds of dollars on a fancy air filtration system when a wall of plants can do that for next to nothing. Find what works for you and your space and become a plant parent today! Your lungs will thank you.
But officer, I had to swerve to miss the duodenal ampulla
Tiny video capsule endoscopes have been around for many years, but they have one big weakness: The ingestible cameras’ journey through the GI tract is passively driven by gravity and the natural movement of the body, so they often miss potential problem areas.
Not anymore. That flaw has been addressed by medical technology company AnX Robotica, which has taken endoscopy to the next level by adding that wondrous directional control device of the modern electronic age, a joystick.
The new system “uses an external magnet and hand-held video game style joysticks to move the capsule in three dimensions,” which allows physicians to “remotely drive a miniature video capsule to all regions of the stomach to visualize and photograph potential problem areas,” according to Andrew C. Meltzer, MD, of George Washington University and associates, who conducted a pilot study funded by AnX Robotica.
The video capsule provided a 95% rate of visualization in the stomachs of 40 patients who were examined at a medical office building by an emergency medicine physician who had no previous specialty training in endoscopy. “Capsules were driven by the ER physician and then the study reports were reviewed by an attending gastroenterologist who was physically off site,” the investigators said in a written statement.
The capsule operator did receive some additional training, and development of artificial intelligence to self-drive the capsule is in the works, but for now, we’re talking about a device controlled by a human using a joystick. And we all know that 50-year-olds are not especially known for their joystick skills. For that we need real experts. Yup, we need to put those joystick-controlled capsule endoscopes in the hands of teenage gamers. Who wants to go first?
Maybe AI isn’t ready for the big time after all
“How long before some intrepid stockholder says: ‘Hey, instead of paying doctors, why don’t we just use the free robot instead?’ ” Those words appeared on LOTME but a month ago. After all, the AI is supposed to be smarter and more empathetic than a doctor. And did we mention it’s free? Or at least extremely cheap. Cheaper than, say, a group of recently unionized health care workers.
In early May, the paid employees manning the National Eating Disorders Association emergency hotline voted to unionize, as they felt overwhelmed and underpaid. Apparently, paying six people an extra few thousand a year was too much for NEDA’s leadership, as they decided a few weeks later to fire those workers, fully closing down the hotline. Instead of talking to a real person, people “calling in” for support would be met with Tessa, a wellness chatbot that would hopefully guide them through their crisis. Key word, hopefully.
In perhaps the least surprising twist of the year, NEDA was forced to walk back its decision about a week after its initial announcement. It all started with a viral Instagram post from a woman who called in and received the following advice from Tessa: Lose 1-2 pounds a week, count calories and work for a 500- to 1,000-calorie deficit, weigh herself weekly, and restrict her diet. Unfortunately, all of these suggestions were things that led to the development of the woman’s eating disorder.
Naturally, NEDA responded in good grace, accusing the woman of lying. A NEDA vice president even left some nasty comments on the post, but hastily deleted them a day later when NEDA announced it was shutting down Tessa “until further notice for a complete investigation.” NEDA’s CEO insisted they hadn’t seen that behavior from Tessa before, calling it a “bug” and insisting the bot would only be down temporarily until the triggers causing the bug were fixed.
In the aftermath, several doctors and psychologists chimed in, terming the rush to automate human roles dangerous and risky. After all, much of what makes these hotlines effective is the volunteers speaking from their own experience. An unsupervised bot doesn’t seem to have what it takes to deal with a mental health crisis, but we’re betting that Tessa will be back. As a wise cephalopod once said: Nobody gives a care about the fate of labor as long as they can get their instant gratification.
You can’t spell existential without s-t-e-n-t
This week, we’re including a special “bonus” item that, to be honest, has nothing to do with stents. That’s why our editor is making us call this a “bonus” (and making us use quote marks, too): It doesn’t really have anything to do with stents or health care or those who practice health care. Actually, his exact words were, “You can’t just give the readers someone else’s ****ing list and expect to get paid for it.” Did we mention that he looks like Jack Nicklaus but acts like BoJack Horseman?
Anywaaay, we’re pretty sure that the list in question – “America’s Top 10 Most Googled Existential Questions” – says something about the human condition, just not about stents:
1. Why is the sky blue?
2. What do dreams mean?
3. What is the meaning of life?
4. Why am I so tired?
5. Who am I?
6. What is love?
7. Is a hot dog a sandwich?
8. What came first, the chicken or the egg?
9. What should I do?
10. Do animals have souls?
Sowing the seeds of cancer prevention
Are you looking to add to your quality of life, even though pets are not your speed? Might we suggest something with lower maintenance? Something a little greener?
Indoor plants can purify the air that comes from outside. Researchers at the University of Technology Sydney, in partnership with the plantscaping company Ambius, showed that a “green wall” made up of mixed indoor plants was able to suck up 97% of “the most toxic compounds” from the air in just 8 hours. We’re talking about lung-irritating, headache-inducing, cancer risk–boosting compounds from gasoline fumes, including benzene.
Public health initiatives often strive to reduce cardiovascular and obesity risks, but breathing seems pretty important too. According to the World Health Organization, household air pollution is responsible for about 2.5 million global premature deaths each year. And since 2020 we’ve become accustomed to spending more time inside and at home.
“This new research proves that plants should not just be seen as ‘nice to have,’ but rather a crucial part of every workplace wellness plan,” Ambius General Manager Johan Hodgson said in statement released by the university.
So don’t spend hundreds of dollars on a fancy air filtration system when a wall of plants can do that for next to nothing. Find what works for you and your space and become a plant parent today! Your lungs will thank you.
But officer, I had to swerve to miss the duodenal ampulla
Tiny video capsule endoscopes have been around for many years, but they have one big weakness: The ingestible cameras’ journey through the GI tract is passively driven by gravity and the natural movement of the body, so they often miss potential problem areas.
Not anymore. That flaw has been addressed by medical technology company AnX Robotica, which has taken endoscopy to the next level by adding that wondrous directional control device of the modern electronic age, a joystick.
The new system “uses an external magnet and hand-held video game style joysticks to move the capsule in three dimensions,” which allows physicians to “remotely drive a miniature video capsule to all regions of the stomach to visualize and photograph potential problem areas,” according to Andrew C. Meltzer, MD, of George Washington University and associates, who conducted a pilot study funded by AnX Robotica.
The video capsule provided a 95% rate of visualization in the stomachs of 40 patients who were examined at a medical office building by an emergency medicine physician who had no previous specialty training in endoscopy. “Capsules were driven by the ER physician and then the study reports were reviewed by an attending gastroenterologist who was physically off site,” the investigators said in a written statement.
The capsule operator did receive some additional training, and development of artificial intelligence to self-drive the capsule is in the works, but for now, we’re talking about a device controlled by a human using a joystick. And we all know that 50-year-olds are not especially known for their joystick skills. For that we need real experts. Yup, we need to put those joystick-controlled capsule endoscopes in the hands of teenage gamers. Who wants to go first?
Maybe AI isn’t ready for the big time after all
“How long before some intrepid stockholder says: ‘Hey, instead of paying doctors, why don’t we just use the free robot instead?’ ” Those words appeared on LOTME but a month ago. After all, the AI is supposed to be smarter and more empathetic than a doctor. And did we mention it’s free? Or at least extremely cheap. Cheaper than, say, a group of recently unionized health care workers.
In early May, the paid employees manning the National Eating Disorders Association emergency hotline voted to unionize, as they felt overwhelmed and underpaid. Apparently, paying six people an extra few thousand a year was too much for NEDA’s leadership, as they decided a few weeks later to fire those workers, fully closing down the hotline. Instead of talking to a real person, people “calling in” for support would be met with Tessa, a wellness chatbot that would hopefully guide them through their crisis. Key word, hopefully.
In perhaps the least surprising twist of the year, NEDA was forced to walk back its decision about a week after its initial announcement. It all started with a viral Instagram post from a woman who called in and received the following advice from Tessa: Lose 1-2 pounds a week, count calories and work for a 500- to 1,000-calorie deficit, weigh herself weekly, and restrict her diet. Unfortunately, all of these suggestions were things that led to the development of the woman’s eating disorder.
Naturally, NEDA responded in good grace, accusing the woman of lying. A NEDA vice president even left some nasty comments on the post, but hastily deleted them a day later when NEDA announced it was shutting down Tessa “until further notice for a complete investigation.” NEDA’s CEO insisted they hadn’t seen that behavior from Tessa before, calling it a “bug” and insisting the bot would only be down temporarily until the triggers causing the bug were fixed.
In the aftermath, several doctors and psychologists chimed in, terming the rush to automate human roles dangerous and risky. After all, much of what makes these hotlines effective is the volunteers speaking from their own experience. An unsupervised bot doesn’t seem to have what it takes to deal with a mental health crisis, but we’re betting that Tessa will be back. As a wise cephalopod once said: Nobody gives a care about the fate of labor as long as they can get their instant gratification.
You can’t spell existential without s-t-e-n-t
This week, we’re including a special “bonus” item that, to be honest, has nothing to do with stents. That’s why our editor is making us call this a “bonus” (and making us use quote marks, too): It doesn’t really have anything to do with stents or health care or those who practice health care. Actually, his exact words were, “You can’t just give the readers someone else’s ****ing list and expect to get paid for it.” Did we mention that he looks like Jack Nicklaus but acts like BoJack Horseman?
Anywaaay, we’re pretty sure that the list in question – “America’s Top 10 Most Googled Existential Questions” – says something about the human condition, just not about stents:
1. Why is the sky blue?
2. What do dreams mean?
3. What is the meaning of life?
4. Why am I so tired?
5. Who am I?
6. What is love?
7. Is a hot dog a sandwich?
8. What came first, the chicken or the egg?
9. What should I do?
10. Do animals have souls?
Sowing the seeds of cancer prevention
Are you looking to add to your quality of life, even though pets are not your speed? Might we suggest something with lower maintenance? Something a little greener?
Indoor plants can purify the air that comes from outside. Researchers at the University of Technology Sydney, in partnership with the plantscaping company Ambius, showed that a “green wall” made up of mixed indoor plants was able to suck up 97% of “the most toxic compounds” from the air in just 8 hours. We’re talking about lung-irritating, headache-inducing, cancer risk–boosting compounds from gasoline fumes, including benzene.
Public health initiatives often strive to reduce cardiovascular and obesity risks, but breathing seems pretty important too. According to the World Health Organization, household air pollution is responsible for about 2.5 million global premature deaths each year. And since 2020 we’ve become accustomed to spending more time inside and at home.
“This new research proves that plants should not just be seen as ‘nice to have,’ but rather a crucial part of every workplace wellness plan,” Ambius General Manager Johan Hodgson said in statement released by the university.
So don’t spend hundreds of dollars on a fancy air filtration system when a wall of plants can do that for next to nothing. Find what works for you and your space and become a plant parent today! Your lungs will thank you.
But officer, I had to swerve to miss the duodenal ampulla
Tiny video capsule endoscopes have been around for many years, but they have one big weakness: The ingestible cameras’ journey through the GI tract is passively driven by gravity and the natural movement of the body, so they often miss potential problem areas.
Not anymore. That flaw has been addressed by medical technology company AnX Robotica, which has taken endoscopy to the next level by adding that wondrous directional control device of the modern electronic age, a joystick.
The new system “uses an external magnet and hand-held video game style joysticks to move the capsule in three dimensions,” which allows physicians to “remotely drive a miniature video capsule to all regions of the stomach to visualize and photograph potential problem areas,” according to Andrew C. Meltzer, MD, of George Washington University and associates, who conducted a pilot study funded by AnX Robotica.
The video capsule provided a 95% rate of visualization in the stomachs of 40 patients who were examined at a medical office building by an emergency medicine physician who had no previous specialty training in endoscopy. “Capsules were driven by the ER physician and then the study reports were reviewed by an attending gastroenterologist who was physically off site,” the investigators said in a written statement.
The capsule operator did receive some additional training, and development of artificial intelligence to self-drive the capsule is in the works, but for now, we’re talking about a device controlled by a human using a joystick. And we all know that 50-year-olds are not especially known for their joystick skills. For that we need real experts. Yup, we need to put those joystick-controlled capsule endoscopes in the hands of teenage gamers. Who wants to go first?
Maybe AI isn’t ready for the big time after all
“How long before some intrepid stockholder says: ‘Hey, instead of paying doctors, why don’t we just use the free robot instead?’ ” Those words appeared on LOTME but a month ago. After all, the AI is supposed to be smarter and more empathetic than a doctor. And did we mention it’s free? Or at least extremely cheap. Cheaper than, say, a group of recently unionized health care workers.
In early May, the paid employees manning the National Eating Disorders Association emergency hotline voted to unionize, as they felt overwhelmed and underpaid. Apparently, paying six people an extra few thousand a year was too much for NEDA’s leadership, as they decided a few weeks later to fire those workers, fully closing down the hotline. Instead of talking to a real person, people “calling in” for support would be met with Tessa, a wellness chatbot that would hopefully guide them through their crisis. Key word, hopefully.
In perhaps the least surprising twist of the year, NEDA was forced to walk back its decision about a week after its initial announcement. It all started with a viral Instagram post from a woman who called in and received the following advice from Tessa: Lose 1-2 pounds a week, count calories and work for a 500- to 1,000-calorie deficit, weigh herself weekly, and restrict her diet. Unfortunately, all of these suggestions were things that led to the development of the woman’s eating disorder.
Naturally, NEDA responded in good grace, accusing the woman of lying. A NEDA vice president even left some nasty comments on the post, but hastily deleted them a day later when NEDA announced it was shutting down Tessa “until further notice for a complete investigation.” NEDA’s CEO insisted they hadn’t seen that behavior from Tessa before, calling it a “bug” and insisting the bot would only be down temporarily until the triggers causing the bug were fixed.
In the aftermath, several doctors and psychologists chimed in, terming the rush to automate human roles dangerous and risky. After all, much of what makes these hotlines effective is the volunteers speaking from their own experience. An unsupervised bot doesn’t seem to have what it takes to deal with a mental health crisis, but we’re betting that Tessa will be back. As a wise cephalopod once said: Nobody gives a care about the fate of labor as long as they can get their instant gratification.
You can’t spell existential without s-t-e-n-t
This week, we’re including a special “bonus” item that, to be honest, has nothing to do with stents. That’s why our editor is making us call this a “bonus” (and making us use quote marks, too): It doesn’t really have anything to do with stents or health care or those who practice health care. Actually, his exact words were, “You can’t just give the readers someone else’s ****ing list and expect to get paid for it.” Did we mention that he looks like Jack Nicklaus but acts like BoJack Horseman?
Anywaaay, we’re pretty sure that the list in question – “America’s Top 10 Most Googled Existential Questions” – says something about the human condition, just not about stents:
1. Why is the sky blue?
2. What do dreams mean?
3. What is the meaning of life?
4. Why am I so tired?
5. Who am I?
6. What is love?
7. Is a hot dog a sandwich?
8. What came first, the chicken or the egg?
9. What should I do?
10. Do animals have souls?
Therapeutic hypothermia to treat neonatal encephalopathy improves childhood outcomes
Therapeutic hypothermia (TH) for moderate and severe neonatal encephalopathy has been shown to reduce the risk of newborn death, major neurodevelopmental disability, developmental delay, and cerebral palsy.1 It is estimated that 8 newborns with moderate or severe neonatal encephalopathy need to be treated with TH to prevent 1 case of cerebral palsy.1 The key elements of TH include:
- initiate hypothermia within 6 hoursof birth
- cool the newborn to a core temperature of 33.5˚ C to 34.5˚ C (92.3˚ F to 94.1˚ F) for 72 hours
- obtain brain ultrasonography to assess for intracranial hemorrhage
- obtain sequential MRI studies to assess brain structure and function
- initiate EEG monitoring for seizure activity.
During hypothermia the newborn is sedated, and oral feedings are reduced. During TH, important physiological goals are to maintain normal oxygenation, blood pressure, fluid balance, and glucose levels.1,2
TH: The basics
Most of the major published randomized clinical trials used the following inclusion criteria to initiate TH2:
- gestational age at birth of ≥ 35 weeks
- neonate is within 6 hours of birth
- an Apgar score ≤ 5 at 10 minutes of life or prolonged resuscitation at birth or umbilical artery cord pH < 7.1 or neonatal blood gas within 60 minutes of life < 7.1
- moderate to severe encephalopathy or the presence of seizures
- absence of recognizable congenital abnormalities at birth.
However, in some institutions, expert neonatologists have developed more liberal criteria for the initiation of TH, to be considered on a case-by-case basis. These more inclusive criteria, which will result in more newborns being treated with TH, include3:
- gestational age at birth of ≥ 34 weeks
- neonate is within 12 hours of birth
- a sentinel event at birth or Apgar score ≤ 5 at 10 minutes of life or prolonged resuscitation or umbilical artery cord pH < 7.1 or neonatal blood gas within 60 minutes of life < 7.1 or postnatal cardiopulmonary failure
- moderate to severe encephalopathy or concern for the presence of seizures.
Birth at a gestational age ≤ 34 weeks is a contraindication to TH. Relative contraindications to initiation of TH include: birth weight < 1,750 g, severe congenital anomaly, major genetic disorders, known severe metabolic disorders, major intracranial hemorrhage, severe septicemia, and uncorrectable coagulopathy.3 Adverse outcomes of TH include thrombocytopenia, cardiac arrythmia, and fat necrosis.4
Diagnosing neonatal encephalopathy
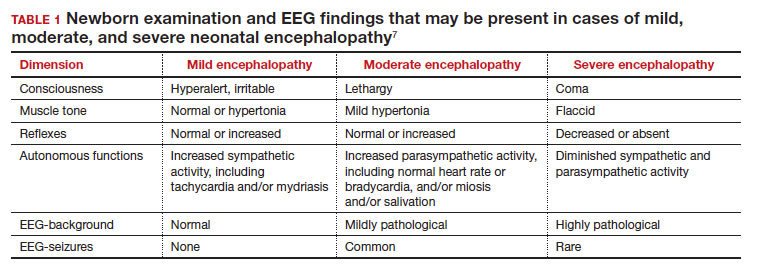
Neonatal encephalopathy is a clinical diagnosis, defined as abnormal neurologic function in the first few days of life in an infant born at ≥ 35 weeks’ gestation. It is divided into 3 categories: mild (Stage 1), moderate (Stage 2), and severe (Stage 3).5,6 Institutions vary in the criteria used to differentiate mild from moderate neonatal encephalopathy, the two most frequent forms of encephalopathy. Newborns with mild encephalopathy are not routinely treated with TH because TH has not been shown to be helpful in this setting. Institutions with liberal criteria for diagnosing moderate encephalopathy will initiate TH in more cases. Involvement of a pediatric neurologist in the diagnosis of moderate encephalopathy may help confirm the diagnosis made by the primary neonatologist and provide an independent, second opinion about whether the newborn should be diagnosed with mild or moderate encephalopathy, a clinically important distinction. Physical examination and EEG findings associated with cases of mild, moderate, and severe encephalopathy are presented in TABLE 1.7
Continue: Obstetric factors that may be associated with neonatal encephalopathy...
Obstetric factors that may be associated with neonatal encephalopathy
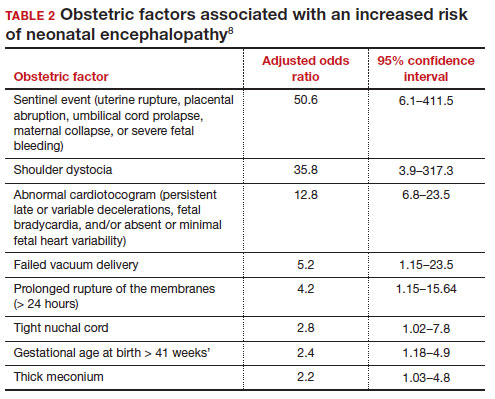
In a retrospective case-control study that included 405 newborns at ≥ 35 weeks’ gestational age with neonatal encephalopathy thought to be due to hypoxia, 8 obstetric factors were identified as being associated with an increased risk of neonatal encephalopathy, including (TABLE 2)8:
1. an obstetric sentinel event (uterine rupture, placental abruption, umbilical cord prolapse, maternal collapse, or severe fetal bleeding)
2. shoulder dystocia
3. abnormal cardiotocogram (persistent late or variable decelerations, fetal bradycardia, and/or absent or minimal fetal heart variability)
4. failed vacuum delivery
5. prolonged rupture of the membranes (> 24 hours)
6. tight nuchal cord
7. gestational age at birth > 41 weeks
8. thick meconium.
Similar findings have been reported by other investigators analyzing the obstetric risk factors for neonatal encephalopathy.7,9
Genetic causes of neonatal seizures and neonatal encephalopathy
Many neonatologists practice with the belief that for a newborn with encephalopathy in the setting of a sentinel labor event, a low Apgar score at 5 minutes, an umbilical cord artery pH < 7.00, and/or an elevated lactate level, the diagnosis of hypoxic ischemic encephalopathy is warranted. However, there are many causes of neonatal encephalopathy not related to intrapartum events. For example, neonatal encephalopathy and seizures may be caused by infectious, vascular, metabolic, medications, or congenital problems.10
There are genetic disorders that can be associated with both neonatal seizures and encephalopathy, suggesting that in some cases the primary cause of the encephalopathy is a genetic problem, not management of labor. Mutations in the potassium channel and sodium channel genes are well recognized causes of neonatal seizures.11,12 Cerebral palsy, a childhood outcome that may follow neonatal encephalopathy, also has numerous etiologies, including genetic causes. Among 1,345 children with cerebral palsy referred for exome sequencing, investigators reported that a genetic abnormality was identified in 33% of the cases.13 Mutations in 86 genes were identified in multiple children. Similar results have been reported in other cohorts.14-16 Maintaining an open mind about the causes of a case of neonatal encephalopathy and not jumping to a conclusion before completing an evaluation is an optimal approach.
Parent’s evolving emotional and intellectual reaction to the initiation of TH
Initiation of TH for a newborn with encephalopathy catalyzes parents to wonder, “How did my baby develop an encephalopathy?”, “Did my obstetrician’s management of labor and delivery contribute to the outcome?” and “What is the prognosis for my baby?” These are difficult questions with high emotional valence for both patients and clinicians. Obstetricians and neonatologists should collaborate to provide consistent responses to these questions.
The presence of a low umbilical cord artery pH and high lactate in combination with a low Apgar score at 5 minutes may lead the neonatologist to diagnose hypoxic-ischemic encephalopathy in the medical record. The diagnosis of brain hypoxia and ischemia in a newborn may be interpreted by parents as meaning that labor events caused or contributed to the encephalopathy. During the 72 hours of TH, the newborn is sedated and separated from the parents, causing additional emotional stress and uncertainty. When a baby is transferred from a community hospital to a neonatal intensive care unit (NICU) at a tertiary center, the parents may be geographically separated from their baby during a critical period of time, adding to their anxiety. At some point during the care process most newborns treated with TH will have an EEG, brain ultrasound, and brain magnetic resonance imaging (MRI). These data will be discussed with the parent(s) and may cause confusion and additional stress.
The optimal approach to communicating with parents whose newborn is treated with TH continues to evolve. Best practices may include17-20:
- in-person, regular multidisciplinary family meetings with the parents, including neonatologists, obstetricians, social service specialists and mental health experts when possible
- providing emotional support to parents, recognizing the psychological trauma of the clinical events
- encouraging parents to have physical contact with the newborn during TH
- elevating the role of the parents in the care process by having them participate in care events such as diapering the newborn
- ensuring that clinicians do not blame other clinicians for the clinical outcome
- communicating the results and interpretation of advanced physiological monitoring and imaging studies, with an emphasis on clarity, recognizing the limitations of the studies
- providing educational materials for parents about TH, early intervention programs, and support resources.
Coordinated and consistent communication with the parents is often difficult to facilitate due to many factors, including the unique perspectives and vocabularies of clinicians from different specialties and the difficulty of coordinating communications with all those involved over multiple shifts and sites of care. In terms of vocabulary, neonatologists are comfortable with making a diagnosis of hypoxic-ischemic encephalopathy in a newborn, but obstetricians would prefer that neonatologists use the more generic diagnosis of encephalopathy, holding judgment on the cause until additional data are available. In terms of coordinating communication over multiple shifts and sites of care, interactions between an obstetrician and their patient typically occurs in the postpartum unit, while interactions between neonatologists and parents occur in the NICU.
Parents of a baby with neonatal encephalopathy undergoing TH may have numerous traumatic experiences during the care process. For weeks or months after birth, they may recall or dream about the absence of sounds from their newborn at birth, the resuscitation events including chest compressions and intubation, the shivering of the baby during TH, and the jarring pivot from the expectation of holding and bonding with a healthy newborn to the reality of a sick newborn requiring intensive care. Obstetricians are also traumatized by these events and support from peers and mental health experts may help them recognize, explore, and adapt to the trauma. Neonatologists believe that TH can help improve the childhood outcomes of newborns with encephalopathy, a goal endorsed by all clinicians and family members. ●
- Jacobs SE, Berg M, Hunt R, et al. Cooling for newborns with hypoxic ischemic encephalopathy. Cochrane Database Syst Rev. 2013;CD003311.
- Committee on Fetus and Newborn; Papile E, Baley JE, Benitz W, et al. Hypothermia and neonatal encephalopathy. Pediatrics. 2014;133:1146-1150.
- Academic Medical Center Patient Safety Organization. Therapeutic hypothermia in neonates. Recommendations of the neonatal encephalopathy task force. 2016. https://www.rmf.harvard. edu/-/media/Files/_Global/KC/PDFs/Guide lines/crico_neonates.pdf. Accessed May 25, 2023.
- Zhang W, Ma J, Danzeng Q, et al. Safety of moderate hypothermia for perinatal hypoxic-ischemic encephalopathy: a meta-analysis. Pediatr Neurol. 2017;74:51-61.
- Sarnat HB, Sarnat MS. Neonatal encephalopathy following fetal distress: a clinical and electroencephalographic study. Arch Neurol. 1976;33:696-705.
- Thompson CM, Puterman AS, Linley LL, et al. The value of a scoring system for hypoxic ischemic encephalopathy in predicting neurodevelopmental outcome. Acta Pediatr. 1997;86:757-761.
- Lundgren C, Brudin L, Wanby AS, et al. Ante- and intrapartum risk factors for neonatal hypoxic ischemic encephalopathy. J Matern Fetal Neonatal Med. 2018;31:1595-1601.
- Martinez-Biarge M, Diez-Sebastian J, Wusthoff CJ, et al. Antepartum and intrapartum factors preceding neonatal hypoxic-ischemic encephalopathy. Pediatrics. 2013;132:e952-e959.
- Lorain P, Bower A, Gottardi E, et al. Risk factors for hypoxic-ischemic encephalopathy in cases of severe acidosis: a case-control study. Acta Obstet Gynecol Scand. 2022;101:471-478.
- Russ JB, Simmons R, Glass HC. Neonatal encephalopathy: beyond hypoxic-ischemic encephalopathy. Neo Reviews. 2021;22:e148-e162.
- Allen NM, Mannion M, Conroy J, et al. The variable phenotypes of KCNQ-related epilepsy. Epilepsia. 2014;55:e99-e105.
- Zibro J, Shellhaas RA. Neonatal seizures: diagnosis, etiologies and management. Semin Neurol. 2020;40:246-256.
- Moreno-De-Luca A, Millan F, Peacreta DR, et al. Molecular diagnostic yield of exome sequencing in patients with cerebral palsy. JAMA. 2021;325:467-475.
- Srivastava S, Lewis SA, Cohen JS, et al. Molecular diagnostic yield of exome sequencing and chromosomal microarray in cerebral palsy. A systematic review and meta-analysis. JAMA Neurology. 2022;79:1287-1295.
- Gonzalez-Mantilla PJ, Hu Y, Myers SM, et al. Diagnostic yield of exome sequencing in cerebral palsy and implications for genetic testing guidelines. A systematic review and meta-analysis. JAMA Pediatr. Epub March 6, 2023.
- van Eyk C, MacLennon SC, MacLennan AH. All patients with cerebral palsy diagnosis merit genomic sequencing. JAMA Pediatr. Epub March 6, 2023.
- Craig AK, James C, Bainter J, et al. Parental perceptions of neonatal therapeutic hypothermia; emotional and healing experiences. J Matern Fetal Neonatal Med. 2020;33:2889-2896. doi: 10.1080/14767058.2018.1563592.
- Sagaser A, Pilon B, Goeller A, et al. Parent experience of hypoxic-ischemic encephalopathy and hypothermia: a call for trauma informed care. Am J Perinatol. Epub March 4, 2022.
- Cascio A, Ferrand A, Racine E, et al. Discussing brain magnetic resonance imaging results for neonates with hypoxic-ischemic encephalopathy treated with hypothermia: a challenge for clinicians and parents. E Neurological Sci. 2022;29:100424.
- Thyagarajan B, Baral V, Gunda R, et al. Parental perceptions of hypothermia treatment for neonatal hypoxic-ischaemic encephalopathy. J Matern Fetal Neonatal Med. 2018;31:2527-2533.
Therapeutic hypothermia (TH) for moderate and severe neonatal encephalopathy has been shown to reduce the risk of newborn death, major neurodevelopmental disability, developmental delay, and cerebral palsy.1 It is estimated that 8 newborns with moderate or severe neonatal encephalopathy need to be treated with TH to prevent 1 case of cerebral palsy.1 The key elements of TH include:
- initiate hypothermia within 6 hoursof birth
- cool the newborn to a core temperature of 33.5˚ C to 34.5˚ C (92.3˚ F to 94.1˚ F) for 72 hours
- obtain brain ultrasonography to assess for intracranial hemorrhage
- obtain sequential MRI studies to assess brain structure and function
- initiate EEG monitoring for seizure activity.
During hypothermia the newborn is sedated, and oral feedings are reduced. During TH, important physiological goals are to maintain normal oxygenation, blood pressure, fluid balance, and glucose levels.1,2
TH: The basics
Most of the major published randomized clinical trials used the following inclusion criteria to initiate TH2:
- gestational age at birth of ≥ 35 weeks
- neonate is within 6 hours of birth
- an Apgar score ≤ 5 at 10 minutes of life or prolonged resuscitation at birth or umbilical artery cord pH < 7.1 or neonatal blood gas within 60 minutes of life < 7.1
- moderate to severe encephalopathy or the presence of seizures
- absence of recognizable congenital abnormalities at birth.
However, in some institutions, expert neonatologists have developed more liberal criteria for the initiation of TH, to be considered on a case-by-case basis. These more inclusive criteria, which will result in more newborns being treated with TH, include3:
- gestational age at birth of ≥ 34 weeks
- neonate is within 12 hours of birth
- a sentinel event at birth or Apgar score ≤ 5 at 10 minutes of life or prolonged resuscitation or umbilical artery cord pH < 7.1 or neonatal blood gas within 60 minutes of life < 7.1 or postnatal cardiopulmonary failure
- moderate to severe encephalopathy or concern for the presence of seizures.
Birth at a gestational age ≤ 34 weeks is a contraindication to TH. Relative contraindications to initiation of TH include: birth weight < 1,750 g, severe congenital anomaly, major genetic disorders, known severe metabolic disorders, major intracranial hemorrhage, severe septicemia, and uncorrectable coagulopathy.3 Adverse outcomes of TH include thrombocytopenia, cardiac arrythmia, and fat necrosis.4
Diagnosing neonatal encephalopathy
Neonatal encephalopathy is a clinical diagnosis, defined as abnormal neurologic function in the first few days of life in an infant born at ≥ 35 weeks’ gestation. It is divided into 3 categories: mild (Stage 1), moderate (Stage 2), and severe (Stage 3).5,6 Institutions vary in the criteria used to differentiate mild from moderate neonatal encephalopathy, the two most frequent forms of encephalopathy. Newborns with mild encephalopathy are not routinely treated with TH because TH has not been shown to be helpful in this setting. Institutions with liberal criteria for diagnosing moderate encephalopathy will initiate TH in more cases. Involvement of a pediatric neurologist in the diagnosis of moderate encephalopathy may help confirm the diagnosis made by the primary neonatologist and provide an independent, second opinion about whether the newborn should be diagnosed with mild or moderate encephalopathy, a clinically important distinction. Physical examination and EEG findings associated with cases of mild, moderate, and severe encephalopathy are presented in TABLE 1.7

Continue: Obstetric factors that may be associated with neonatal encephalopathy...
Obstetric factors that may be associated with neonatal encephalopathy
In a retrospective case-control study that included 405 newborns at ≥ 35 weeks’ gestational age with neonatal encephalopathy thought to be due to hypoxia, 8 obstetric factors were identified as being associated with an increased risk of neonatal encephalopathy, including (TABLE 2)8:
1. an obstetric sentinel event (uterine rupture, placental abruption, umbilical cord prolapse, maternal collapse, or severe fetal bleeding)
2. shoulder dystocia
3. abnormal cardiotocogram (persistent late or variable decelerations, fetal bradycardia, and/or absent or minimal fetal heart variability)
4. failed vacuum delivery
5. prolonged rupture of the membranes (> 24 hours)
6. tight nuchal cord
7. gestational age at birth > 41 weeks
8. thick meconium.

Similar findings have been reported by other investigators analyzing the obstetric risk factors for neonatal encephalopathy.7,9
Genetic causes of neonatal seizures and neonatal encephalopathy
Many neonatologists practice with the belief that for a newborn with encephalopathy in the setting of a sentinel labor event, a low Apgar score at 5 minutes, an umbilical cord artery pH < 7.00, and/or an elevated lactate level, the diagnosis of hypoxic ischemic encephalopathy is warranted. However, there are many causes of neonatal encephalopathy not related to intrapartum events. For example, neonatal encephalopathy and seizures may be caused by infectious, vascular, metabolic, medications, or congenital problems.10
There are genetic disorders that can be associated with both neonatal seizures and encephalopathy, suggesting that in some cases the primary cause of the encephalopathy is a genetic problem, not management of labor. Mutations in the potassium channel and sodium channel genes are well recognized causes of neonatal seizures.11,12 Cerebral palsy, a childhood outcome that may follow neonatal encephalopathy, also has numerous etiologies, including genetic causes. Among 1,345 children with cerebral palsy referred for exome sequencing, investigators reported that a genetic abnormality was identified in 33% of the cases.13 Mutations in 86 genes were identified in multiple children. Similar results have been reported in other cohorts.14-16 Maintaining an open mind about the causes of a case of neonatal encephalopathy and not jumping to a conclusion before completing an evaluation is an optimal approach.
Parent’s evolving emotional and intellectual reaction to the initiation of TH
Initiation of TH for a newborn with encephalopathy catalyzes parents to wonder, “How did my baby develop an encephalopathy?”, “Did my obstetrician’s management of labor and delivery contribute to the outcome?” and “What is the prognosis for my baby?” These are difficult questions with high emotional valence for both patients and clinicians. Obstetricians and neonatologists should collaborate to provide consistent responses to these questions.
The presence of a low umbilical cord artery pH and high lactate in combination with a low Apgar score at 5 minutes may lead the neonatologist to diagnose hypoxic-ischemic encephalopathy in the medical record. The diagnosis of brain hypoxia and ischemia in a newborn may be interpreted by parents as meaning that labor events caused or contributed to the encephalopathy. During the 72 hours of TH, the newborn is sedated and separated from the parents, causing additional emotional stress and uncertainty. When a baby is transferred from a community hospital to a neonatal intensive care unit (NICU) at a tertiary center, the parents may be geographically separated from their baby during a critical period of time, adding to their anxiety. At some point during the care process most newborns treated with TH will have an EEG, brain ultrasound, and brain magnetic resonance imaging (MRI). These data will be discussed with the parent(s) and may cause confusion and additional stress.
The optimal approach to communicating with parents whose newborn is treated with TH continues to evolve. Best practices may include17-20:
- in-person, regular multidisciplinary family meetings with the parents, including neonatologists, obstetricians, social service specialists and mental health experts when possible
- providing emotional support to parents, recognizing the psychological trauma of the clinical events
- encouraging parents to have physical contact with the newborn during TH
- elevating the role of the parents in the care process by having them participate in care events such as diapering the newborn
- ensuring that clinicians do not blame other clinicians for the clinical outcome
- communicating the results and interpretation of advanced physiological monitoring and imaging studies, with an emphasis on clarity, recognizing the limitations of the studies
- providing educational materials for parents about TH, early intervention programs, and support resources.
Coordinated and consistent communication with the parents is often difficult to facilitate due to many factors, including the unique perspectives and vocabularies of clinicians from different specialties and the difficulty of coordinating communications with all those involved over multiple shifts and sites of care. In terms of vocabulary, neonatologists are comfortable with making a diagnosis of hypoxic-ischemic encephalopathy in a newborn, but obstetricians would prefer that neonatologists use the more generic diagnosis of encephalopathy, holding judgment on the cause until additional data are available. In terms of coordinating communication over multiple shifts and sites of care, interactions between an obstetrician and their patient typically occurs in the postpartum unit, while interactions between neonatologists and parents occur in the NICU.
Parents of a baby with neonatal encephalopathy undergoing TH may have numerous traumatic experiences during the care process. For weeks or months after birth, they may recall or dream about the absence of sounds from their newborn at birth, the resuscitation events including chest compressions and intubation, the shivering of the baby during TH, and the jarring pivot from the expectation of holding and bonding with a healthy newborn to the reality of a sick newborn requiring intensive care. Obstetricians are also traumatized by these events and support from peers and mental health experts may help them recognize, explore, and adapt to the trauma. Neonatologists believe that TH can help improve the childhood outcomes of newborns with encephalopathy, a goal endorsed by all clinicians and family members. ●
Therapeutic hypothermia (TH) for moderate and severe neonatal encephalopathy has been shown to reduce the risk of newborn death, major neurodevelopmental disability, developmental delay, and cerebral palsy.1 It is estimated that 8 newborns with moderate or severe neonatal encephalopathy need to be treated with TH to prevent 1 case of cerebral palsy.1 The key elements of TH include:
- initiate hypothermia within 6 hoursof birth
- cool the newborn to a core temperature of 33.5˚ C to 34.5˚ C (92.3˚ F to 94.1˚ F) for 72 hours
- obtain brain ultrasonography to assess for intracranial hemorrhage
- obtain sequential MRI studies to assess brain structure and function
- initiate EEG monitoring for seizure activity.
During hypothermia the newborn is sedated, and oral feedings are reduced. During TH, important physiological goals are to maintain normal oxygenation, blood pressure, fluid balance, and glucose levels.1,2
TH: The basics
Most of the major published randomized clinical trials used the following inclusion criteria to initiate TH2:
- gestational age at birth of ≥ 35 weeks
- neonate is within 6 hours of birth
- an Apgar score ≤ 5 at 10 minutes of life or prolonged resuscitation at birth or umbilical artery cord pH < 7.1 or neonatal blood gas within 60 minutes of life < 7.1
- moderate to severe encephalopathy or the presence of seizures
- absence of recognizable congenital abnormalities at birth.
However, in some institutions, expert neonatologists have developed more liberal criteria for the initiation of TH, to be considered on a case-by-case basis. These more inclusive criteria, which will result in more newborns being treated with TH, include3:
- gestational age at birth of ≥ 34 weeks
- neonate is within 12 hours of birth
- a sentinel event at birth or Apgar score ≤ 5 at 10 minutes of life or prolonged resuscitation or umbilical artery cord pH < 7.1 or neonatal blood gas within 60 minutes of life < 7.1 or postnatal cardiopulmonary failure
- moderate to severe encephalopathy or concern for the presence of seizures.
Birth at a gestational age ≤ 34 weeks is a contraindication to TH. Relative contraindications to initiation of TH include: birth weight < 1,750 g, severe congenital anomaly, major genetic disorders, known severe metabolic disorders, major intracranial hemorrhage, severe septicemia, and uncorrectable coagulopathy.3 Adverse outcomes of TH include thrombocytopenia, cardiac arrythmia, and fat necrosis.4
Diagnosing neonatal encephalopathy
Neonatal encephalopathy is a clinical diagnosis, defined as abnormal neurologic function in the first few days of life in an infant born at ≥ 35 weeks’ gestation. It is divided into 3 categories: mild (Stage 1), moderate (Stage 2), and severe (Stage 3).5,6 Institutions vary in the criteria used to differentiate mild from moderate neonatal encephalopathy, the two most frequent forms of encephalopathy. Newborns with mild encephalopathy are not routinely treated with TH because TH has not been shown to be helpful in this setting. Institutions with liberal criteria for diagnosing moderate encephalopathy will initiate TH in more cases. Involvement of a pediatric neurologist in the diagnosis of moderate encephalopathy may help confirm the diagnosis made by the primary neonatologist and provide an independent, second opinion about whether the newborn should be diagnosed with mild or moderate encephalopathy, a clinically important distinction. Physical examination and EEG findings associated with cases of mild, moderate, and severe encephalopathy are presented in TABLE 1.7

Continue: Obstetric factors that may be associated with neonatal encephalopathy...
Obstetric factors that may be associated with neonatal encephalopathy
In a retrospective case-control study that included 405 newborns at ≥ 35 weeks’ gestational age with neonatal encephalopathy thought to be due to hypoxia, 8 obstetric factors were identified as being associated with an increased risk of neonatal encephalopathy, including (TABLE 2)8:
1. an obstetric sentinel event (uterine rupture, placental abruption, umbilical cord prolapse, maternal collapse, or severe fetal bleeding)
2. shoulder dystocia
3. abnormal cardiotocogram (persistent late or variable decelerations, fetal bradycardia, and/or absent or minimal fetal heart variability)
4. failed vacuum delivery
5. prolonged rupture of the membranes (> 24 hours)
6. tight nuchal cord
7. gestational age at birth > 41 weeks
8. thick meconium.

Similar findings have been reported by other investigators analyzing the obstetric risk factors for neonatal encephalopathy.7,9
Genetic causes of neonatal seizures and neonatal encephalopathy
Many neonatologists practice with the belief that for a newborn with encephalopathy in the setting of a sentinel labor event, a low Apgar score at 5 minutes, an umbilical cord artery pH < 7.00, and/or an elevated lactate level, the diagnosis of hypoxic ischemic encephalopathy is warranted. However, there are many causes of neonatal encephalopathy not related to intrapartum events. For example, neonatal encephalopathy and seizures may be caused by infectious, vascular, metabolic, medications, or congenital problems.10
There are genetic disorders that can be associated with both neonatal seizures and encephalopathy, suggesting that in some cases the primary cause of the encephalopathy is a genetic problem, not management of labor. Mutations in the potassium channel and sodium channel genes are well recognized causes of neonatal seizures.11,12 Cerebral palsy, a childhood outcome that may follow neonatal encephalopathy, also has numerous etiologies, including genetic causes. Among 1,345 children with cerebral palsy referred for exome sequencing, investigators reported that a genetic abnormality was identified in 33% of the cases.13 Mutations in 86 genes were identified in multiple children. Similar results have been reported in other cohorts.14-16 Maintaining an open mind about the causes of a case of neonatal encephalopathy and not jumping to a conclusion before completing an evaluation is an optimal approach.
Parent’s evolving emotional and intellectual reaction to the initiation of TH
Initiation of TH for a newborn with encephalopathy catalyzes parents to wonder, “How did my baby develop an encephalopathy?”, “Did my obstetrician’s management of labor and delivery contribute to the outcome?” and “What is the prognosis for my baby?” These are difficult questions with high emotional valence for both patients and clinicians. Obstetricians and neonatologists should collaborate to provide consistent responses to these questions.
The presence of a low umbilical cord artery pH and high lactate in combination with a low Apgar score at 5 minutes may lead the neonatologist to diagnose hypoxic-ischemic encephalopathy in the medical record. The diagnosis of brain hypoxia and ischemia in a newborn may be interpreted by parents as meaning that labor events caused or contributed to the encephalopathy. During the 72 hours of TH, the newborn is sedated and separated from the parents, causing additional emotional stress and uncertainty. When a baby is transferred from a community hospital to a neonatal intensive care unit (NICU) at a tertiary center, the parents may be geographically separated from their baby during a critical period of time, adding to their anxiety. At some point during the care process most newborns treated with TH will have an EEG, brain ultrasound, and brain magnetic resonance imaging (MRI). These data will be discussed with the parent(s) and may cause confusion and additional stress.
The optimal approach to communicating with parents whose newborn is treated with TH continues to evolve. Best practices may include17-20:
- in-person, regular multidisciplinary family meetings with the parents, including neonatologists, obstetricians, social service specialists and mental health experts when possible
- providing emotional support to parents, recognizing the psychological trauma of the clinical events
- encouraging parents to have physical contact with the newborn during TH
- elevating the role of the parents in the care process by having them participate in care events such as diapering the newborn
- ensuring that clinicians do not blame other clinicians for the clinical outcome
- communicating the results and interpretation of advanced physiological monitoring and imaging studies, with an emphasis on clarity, recognizing the limitations of the studies
- providing educational materials for parents about TH, early intervention programs, and support resources.
Coordinated and consistent communication with the parents is often difficult to facilitate due to many factors, including the unique perspectives and vocabularies of clinicians from different specialties and the difficulty of coordinating communications with all those involved over multiple shifts and sites of care. In terms of vocabulary, neonatologists are comfortable with making a diagnosis of hypoxic-ischemic encephalopathy in a newborn, but obstetricians would prefer that neonatologists use the more generic diagnosis of encephalopathy, holding judgment on the cause until additional data are available. In terms of coordinating communication over multiple shifts and sites of care, interactions between an obstetrician and their patient typically occurs in the postpartum unit, while interactions between neonatologists and parents occur in the NICU.
Parents of a baby with neonatal encephalopathy undergoing TH may have numerous traumatic experiences during the care process. For weeks or months after birth, they may recall or dream about the absence of sounds from their newborn at birth, the resuscitation events including chest compressions and intubation, the shivering of the baby during TH, and the jarring pivot from the expectation of holding and bonding with a healthy newborn to the reality of a sick newborn requiring intensive care. Obstetricians are also traumatized by these events and support from peers and mental health experts may help them recognize, explore, and adapt to the trauma. Neonatologists believe that TH can help improve the childhood outcomes of newborns with encephalopathy, a goal endorsed by all clinicians and family members. ●
- Jacobs SE, Berg M, Hunt R, et al. Cooling for newborns with hypoxic ischemic encephalopathy. Cochrane Database Syst Rev. 2013;CD003311.
- Committee on Fetus and Newborn; Papile E, Baley JE, Benitz W, et al. Hypothermia and neonatal encephalopathy. Pediatrics. 2014;133:1146-1150.
- Academic Medical Center Patient Safety Organization. Therapeutic hypothermia in neonates. Recommendations of the neonatal encephalopathy task force. 2016. https://www.rmf.harvard. edu/-/media/Files/_Global/KC/PDFs/Guide lines/crico_neonates.pdf. Accessed May 25, 2023.
- Zhang W, Ma J, Danzeng Q, et al. Safety of moderate hypothermia for perinatal hypoxic-ischemic encephalopathy: a meta-analysis. Pediatr Neurol. 2017;74:51-61.
- Sarnat HB, Sarnat MS. Neonatal encephalopathy following fetal distress: a clinical and electroencephalographic study. Arch Neurol. 1976;33:696-705.
- Thompson CM, Puterman AS, Linley LL, et al. The value of a scoring system for hypoxic ischemic encephalopathy in predicting neurodevelopmental outcome. Acta Pediatr. 1997;86:757-761.
- Lundgren C, Brudin L, Wanby AS, et al. Ante- and intrapartum risk factors for neonatal hypoxic ischemic encephalopathy. J Matern Fetal Neonatal Med. 2018;31:1595-1601.
- Martinez-Biarge M, Diez-Sebastian J, Wusthoff CJ, et al. Antepartum and intrapartum factors preceding neonatal hypoxic-ischemic encephalopathy. Pediatrics. 2013;132:e952-e959.
- Lorain P, Bower A, Gottardi E, et al. Risk factors for hypoxic-ischemic encephalopathy in cases of severe acidosis: a case-control study. Acta Obstet Gynecol Scand. 2022;101:471-478.
- Russ JB, Simmons R, Glass HC. Neonatal encephalopathy: beyond hypoxic-ischemic encephalopathy. Neo Reviews. 2021;22:e148-e162.
- Allen NM, Mannion M, Conroy J, et al. The variable phenotypes of KCNQ-related epilepsy. Epilepsia. 2014;55:e99-e105.
- Zibro J, Shellhaas RA. Neonatal seizures: diagnosis, etiologies and management. Semin Neurol. 2020;40:246-256.
- Moreno-De-Luca A, Millan F, Peacreta DR, et al. Molecular diagnostic yield of exome sequencing in patients with cerebral palsy. JAMA. 2021;325:467-475.
- Srivastava S, Lewis SA, Cohen JS, et al. Molecular diagnostic yield of exome sequencing and chromosomal microarray in cerebral palsy. A systematic review and meta-analysis. JAMA Neurology. 2022;79:1287-1295.
- Gonzalez-Mantilla PJ, Hu Y, Myers SM, et al. Diagnostic yield of exome sequencing in cerebral palsy and implications for genetic testing guidelines. A systematic review and meta-analysis. JAMA Pediatr. Epub March 6, 2023.
- van Eyk C, MacLennon SC, MacLennan AH. All patients with cerebral palsy diagnosis merit genomic sequencing. JAMA Pediatr. Epub March 6, 2023.
- Craig AK, James C, Bainter J, et al. Parental perceptions of neonatal therapeutic hypothermia; emotional and healing experiences. J Matern Fetal Neonatal Med. 2020;33:2889-2896. doi: 10.1080/14767058.2018.1563592.
- Sagaser A, Pilon B, Goeller A, et al. Parent experience of hypoxic-ischemic encephalopathy and hypothermia: a call for trauma informed care. Am J Perinatol. Epub March 4, 2022.
- Cascio A, Ferrand A, Racine E, et al. Discussing brain magnetic resonance imaging results for neonates with hypoxic-ischemic encephalopathy treated with hypothermia: a challenge for clinicians and parents. E Neurological Sci. 2022;29:100424.
- Thyagarajan B, Baral V, Gunda R, et al. Parental perceptions of hypothermia treatment for neonatal hypoxic-ischaemic encephalopathy. J Matern Fetal Neonatal Med. 2018;31:2527-2533.
- Jacobs SE, Berg M, Hunt R, et al. Cooling for newborns with hypoxic ischemic encephalopathy. Cochrane Database Syst Rev. 2013;CD003311.
- Committee on Fetus and Newborn; Papile E, Baley JE, Benitz W, et al. Hypothermia and neonatal encephalopathy. Pediatrics. 2014;133:1146-1150.
- Academic Medical Center Patient Safety Organization. Therapeutic hypothermia in neonates. Recommendations of the neonatal encephalopathy task force. 2016. https://www.rmf.harvard. edu/-/media/Files/_Global/KC/PDFs/Guide lines/crico_neonates.pdf. Accessed May 25, 2023.
- Zhang W, Ma J, Danzeng Q, et al. Safety of moderate hypothermia for perinatal hypoxic-ischemic encephalopathy: a meta-analysis. Pediatr Neurol. 2017;74:51-61.
- Sarnat HB, Sarnat MS. Neonatal encephalopathy following fetal distress: a clinical and electroencephalographic study. Arch Neurol. 1976;33:696-705.
- Thompson CM, Puterman AS, Linley LL, et al. The value of a scoring system for hypoxic ischemic encephalopathy in predicting neurodevelopmental outcome. Acta Pediatr. 1997;86:757-761.
- Lundgren C, Brudin L, Wanby AS, et al. Ante- and intrapartum risk factors for neonatal hypoxic ischemic encephalopathy. J Matern Fetal Neonatal Med. 2018;31:1595-1601.
- Martinez-Biarge M, Diez-Sebastian J, Wusthoff CJ, et al. Antepartum and intrapartum factors preceding neonatal hypoxic-ischemic encephalopathy. Pediatrics. 2013;132:e952-e959.
- Lorain P, Bower A, Gottardi E, et al. Risk factors for hypoxic-ischemic encephalopathy in cases of severe acidosis: a case-control study. Acta Obstet Gynecol Scand. 2022;101:471-478.
- Russ JB, Simmons R, Glass HC. Neonatal encephalopathy: beyond hypoxic-ischemic encephalopathy. Neo Reviews. 2021;22:e148-e162.
- Allen NM, Mannion M, Conroy J, et al. The variable phenotypes of KCNQ-related epilepsy. Epilepsia. 2014;55:e99-e105.
- Zibro J, Shellhaas RA. Neonatal seizures: diagnosis, etiologies and management. Semin Neurol. 2020;40:246-256.
- Moreno-De-Luca A, Millan F, Peacreta DR, et al. Molecular diagnostic yield of exome sequencing in patients with cerebral palsy. JAMA. 2021;325:467-475.
- Srivastava S, Lewis SA, Cohen JS, et al. Molecular diagnostic yield of exome sequencing and chromosomal microarray in cerebral palsy. A systematic review and meta-analysis. JAMA Neurology. 2022;79:1287-1295.
- Gonzalez-Mantilla PJ, Hu Y, Myers SM, et al. Diagnostic yield of exome sequencing in cerebral palsy and implications for genetic testing guidelines. A systematic review and meta-analysis. JAMA Pediatr. Epub March 6, 2023.
- van Eyk C, MacLennon SC, MacLennan AH. All patients with cerebral palsy diagnosis merit genomic sequencing. JAMA Pediatr. Epub March 6, 2023.
- Craig AK, James C, Bainter J, et al. Parental perceptions of neonatal therapeutic hypothermia; emotional and healing experiences. J Matern Fetal Neonatal Med. 2020;33:2889-2896. doi: 10.1080/14767058.2018.1563592.
- Sagaser A, Pilon B, Goeller A, et al. Parent experience of hypoxic-ischemic encephalopathy and hypothermia: a call for trauma informed care. Am J Perinatol. Epub March 4, 2022.
- Cascio A, Ferrand A, Racine E, et al. Discussing brain magnetic resonance imaging results for neonates with hypoxic-ischemic encephalopathy treated with hypothermia: a challenge for clinicians and parents. E Neurological Sci. 2022;29:100424.
- Thyagarajan B, Baral V, Gunda R, et al. Parental perceptions of hypothermia treatment for neonatal hypoxic-ischaemic encephalopathy. J Matern Fetal Neonatal Med. 2018;31:2527-2533.
Can cffDNA technology be used to determine the underlying cause of pregnancy loss to better inform future pregnancy planning?
Hartwig TJ, Ambye L, Gruhn JR, et al. Cell-free fetal DNA for genetic evaluation in Copenhagen Pregnancy Loss Study (COPL): a prospective cohort study. Lancet. 2023;401:762-771. https://doi.org/10.1016/S0140-6736(22)02610-1.
Expert Commentary
A devastating outcome for women, pregnancy loss is directly proportional to maternal age, estimated to occur in approximately 15% of clinically recognized pregnancies and 30% of preclinical pregnancies.1 Approximately 80% of pregnancy losses occur in the first trimester.2 The frequency of clinically recognized early pregnancy loss for women aged 20–30 years is 9% to 17%, and these rates increase sharply, from 20% at age 35 years to 40% at age 40 years, and 80% at age 45 years. Recurrent pregnancy loss (RPL), defined as the spontaneous loss of 2 or more clinically recognized pregnancies, affects less than 5% of women.3 Genetic testing using chromosomal microarray analysis (CMA) has identified aneuploidy in about 55% of cases of miscarriage.4
Following ASRM guidelines for the evaluation of RPL, which consists of analyzing parental chromosomal abnormalities, congenital and acquired uterine anomalies, endocrine imbalances, and autoimmune factors (including antiphospholipid syndrome), no explainable cause is determined in 50% of cases.3 Recently, it has been shown that more than 90% of patients with RPL will have a probable or definitive cause identified when CMA testing on miscarriage tissue with the ASRM evaluation guidelines.5
Details of the study
In this prospective cohort study from Denmark, the authors analyzed maternal serum for cell-free fetal DNA (cffDNA) to determine the ploidy status of the pregnancy loss. One thousand women older than age 18 were included (those who demonstrated an ultrasound-confirmed intrauterine pregnancy loss prior to 22 weeks’ gestation). Maternal blood was obtained while pregnancy tissue was in situ or within 24 hours of passage of products of conception (POC), then analyzed by genome-wide sequencing of cffDNA.
For the first 333 recruited women (validation phase), direct sequencing of the POC was performed for sensitivity and specificity. Following the elimination of inconclusive samples, 302 of the 333 cases demonstrated a sensitivity of 85% and specificity of 93%. In the subsequent evaluation of 667 women, researchers analyzed maternal serum from the gestational age of fetuses ranging from 35 days to 149 days.
Results. In total, nearly 90% of cases yielded conclusive results, with 50% euploid, 46% aneuploid, and 4% multiple aneuploidies. Earlier gestational ages (less than 7 weeks) had a no-call rate (ie, inconclusive) of approximately 50% (only based on 16 patients), with results typically obtained in maternal serum following passage of POC; in pregnancies at gestational ages past 7 weeks, the no-call rate was about 10%. In general, the longer the time after the pregnancy tissue passed, the higher likelihood of a no-call result.
Applying the technology of single-nucleotide polymorphism (SNP)-based CMA can improve identification of fetal and/or maternal sources as causes of pregnancy loss with accuracy, but it does require collection of POC. Of note, samples were deficient in this study, the authors cite, in one-third of the cases. Given this limitation of collection, the authors argue for use of the noninvasive method of cffDNA, obtained from maternal serum.
Study strengths and weaknesses
Several weaknesses of this study are highlighted. Of the validation cohort, one-third of pregnancy tissue could not be analyzed due to insufficient collection. Only 73% of cases allowed for DNA isolation from fetal tissue or chorionic villi; in 27% of cases samples were labeled “unknown tissue.” In those cases classified as unknown, 70% were further determined to be maternal. When all female and monosomy cases were excluded in an effort to assuredly reduce the risk of contamination with maternal DNA, sensitivity of the cffDNA testing process declined to 78%. Another limitation was the required short window for maternal blood sampling (within 24 hours) and its impact on the no-call rate.
The authors note an association with later-life morbidity in patients with a history of pregnancy loss and RPL (including cardiovascular disease, type 2 diabetes, and mental health disorders), thereby arguing for cffDNA-based testing versus no causal testing; however, no treatment has been proven to be effective at reducing pregnancy loss. ●
The best management course for unexplained RPL is uncertain. Despite its use for a euploid miscarriage or parental chromosomal structural rearrangement, in vitro fertilization with preimplantation genetic testing remains an unproven modality.6,7 Given that approximately 70% of human conceptions never achieve viability, and 50% fail spontaneously before being detected,8 the authors’ findings demonstrate peripheral maternal blood can provide a reasonably high sensitivity and specificity for fetal ploidy status when compared with direct sequencing of pregnancy tissue. As fetal aneuploidy offers a higher percentage of subsequent successful pregnancy outcomes, cffDNA may offer reassurance, or direct further testing, following a pregnancy loss. As an application of their results, evaluation may be deferred for an aneuploid miscarriage.
—MARK P. TROLICE, MD, MBA
- Brown S. Miscarriage and its associations. Semin Reprod Med. 2008;26:391-400. doi: 10.1055/s-0028-1087105.
- Wang X, Chen C , Wang L, et al. Conception, early pregnancy loss, and time to clinical pregnancy: a population-based prospective study. Fertil Steril. 2003;79:577-584.
- Evaluation and treatment of recurrent pregnancy loss: a committee opinion. Practice Committee of the American Society for Reproductive Medicine. Fertil Steril. 2012;98: 1103-1111.
- Papas RS, Kutteh WH. Genetic testing for aneuploidy in patients who have had multiple miscarriages: a review of current literature. Appl Clin Genet. 2021;14:321-329. https://doi.org/10.2147/tacg.s320778.
- Popescu F, Jaslow FC, Kutteh WH. Recurrent pregnancy loss evaluation combined with 24-chromosome microarray of miscarriage tissue provides a probable or definite cause of pregnancy loss in over 90% of patients. Hum Reprod. 2018;33:579-587. https://doi.org/10.1093/humrep/dey021.
- Dahdouh EM, Balayla J, Garcia-Velasco JA, et al. PGT-A for recurrent pregnancy loss: evidence is growing but the issue is not resolved. Hum Reprod. 2021;36:2805-2806. https://doi.org/10.1093/humrep/deab194.
- Iews M, Tan J, Taskin O, et al. Does preimplantation genetic diagnosis improve reproductive outcome in couples with recurrent pregnancy loss owing to structural chromosomal rearrangement? A systematic review. Reproductive Bio Medicine Online. 2018;36:677-685. https://doi.org/10.1016 /j.rbmo.2018.03.005.
- Papas RS, Kutteh WH. Genetic testing for aneuploidy in patients who have had multiple miscarriages: a review of current literature. Appl Clin Genet. 2021;14:321-329. https://doi.org/10.2147/TACG.S320778.
Hartwig TJ, Ambye L, Gruhn JR, et al. Cell-free fetal DNA for genetic evaluation in Copenhagen Pregnancy Loss Study (COPL): a prospective cohort study. Lancet. 2023;401:762-771. https://doi.org/10.1016/S0140-6736(22)02610-1.
Expert Commentary
A devastating outcome for women, pregnancy loss is directly proportional to maternal age, estimated to occur in approximately 15% of clinically recognized pregnancies and 30% of preclinical pregnancies.1 Approximately 80% of pregnancy losses occur in the first trimester.2 The frequency of clinically recognized early pregnancy loss for women aged 20–30 years is 9% to 17%, and these rates increase sharply, from 20% at age 35 years to 40% at age 40 years, and 80% at age 45 years. Recurrent pregnancy loss (RPL), defined as the spontaneous loss of 2 or more clinically recognized pregnancies, affects less than 5% of women.3 Genetic testing using chromosomal microarray analysis (CMA) has identified aneuploidy in about 55% of cases of miscarriage.4
Following ASRM guidelines for the evaluation of RPL, which consists of analyzing parental chromosomal abnormalities, congenital and acquired uterine anomalies, endocrine imbalances, and autoimmune factors (including antiphospholipid syndrome), no explainable cause is determined in 50% of cases.3 Recently, it has been shown that more than 90% of patients with RPL will have a probable or definitive cause identified when CMA testing on miscarriage tissue with the ASRM evaluation guidelines.5
Details of the study
In this prospective cohort study from Denmark, the authors analyzed maternal serum for cell-free fetal DNA (cffDNA) to determine the ploidy status of the pregnancy loss. One thousand women older than age 18 were included (those who demonstrated an ultrasound-confirmed intrauterine pregnancy loss prior to 22 weeks’ gestation). Maternal blood was obtained while pregnancy tissue was in situ or within 24 hours of passage of products of conception (POC), then analyzed by genome-wide sequencing of cffDNA.
For the first 333 recruited women (validation phase), direct sequencing of the POC was performed for sensitivity and specificity. Following the elimination of inconclusive samples, 302 of the 333 cases demonstrated a sensitivity of 85% and specificity of 93%. In the subsequent evaluation of 667 women, researchers analyzed maternal serum from the gestational age of fetuses ranging from 35 days to 149 days.
Results. In total, nearly 90% of cases yielded conclusive results, with 50% euploid, 46% aneuploid, and 4% multiple aneuploidies. Earlier gestational ages (less than 7 weeks) had a no-call rate (ie, inconclusive) of approximately 50% (only based on 16 patients), with results typically obtained in maternal serum following passage of POC; in pregnancies at gestational ages past 7 weeks, the no-call rate was about 10%. In general, the longer the time after the pregnancy tissue passed, the higher likelihood of a no-call result.
Applying the technology of single-nucleotide polymorphism (SNP)-based CMA can improve identification of fetal and/or maternal sources as causes of pregnancy loss with accuracy, but it does require collection of POC. Of note, samples were deficient in this study, the authors cite, in one-third of the cases. Given this limitation of collection, the authors argue for use of the noninvasive method of cffDNA, obtained from maternal serum.
Study strengths and weaknesses
Several weaknesses of this study are highlighted. Of the validation cohort, one-third of pregnancy tissue could not be analyzed due to insufficient collection. Only 73% of cases allowed for DNA isolation from fetal tissue or chorionic villi; in 27% of cases samples were labeled “unknown tissue.” In those cases classified as unknown, 70% were further determined to be maternal. When all female and monosomy cases were excluded in an effort to assuredly reduce the risk of contamination with maternal DNA, sensitivity of the cffDNA testing process declined to 78%. Another limitation was the required short window for maternal blood sampling (within 24 hours) and its impact on the no-call rate.
The authors note an association with later-life morbidity in patients with a history of pregnancy loss and RPL (including cardiovascular disease, type 2 diabetes, and mental health disorders), thereby arguing for cffDNA-based testing versus no causal testing; however, no treatment has been proven to be effective at reducing pregnancy loss. ●
The best management course for unexplained RPL is uncertain. Despite its use for a euploid miscarriage or parental chromosomal structural rearrangement, in vitro fertilization with preimplantation genetic testing remains an unproven modality.6,7 Given that approximately 70% of human conceptions never achieve viability, and 50% fail spontaneously before being detected,8 the authors’ findings demonstrate peripheral maternal blood can provide a reasonably high sensitivity and specificity for fetal ploidy status when compared with direct sequencing of pregnancy tissue. As fetal aneuploidy offers a higher percentage of subsequent successful pregnancy outcomes, cffDNA may offer reassurance, or direct further testing, following a pregnancy loss. As an application of their results, evaluation may be deferred for an aneuploid miscarriage.
—MARK P. TROLICE, MD, MBA
Hartwig TJ, Ambye L, Gruhn JR, et al. Cell-free fetal DNA for genetic evaluation in Copenhagen Pregnancy Loss Study (COPL): a prospective cohort study. Lancet. 2023;401:762-771. https://doi.org/10.1016/S0140-6736(22)02610-1.
Expert Commentary
A devastating outcome for women, pregnancy loss is directly proportional to maternal age, estimated to occur in approximately 15% of clinically recognized pregnancies and 30% of preclinical pregnancies.1 Approximately 80% of pregnancy losses occur in the first trimester.2 The frequency of clinically recognized early pregnancy loss for women aged 20–30 years is 9% to 17%, and these rates increase sharply, from 20% at age 35 years to 40% at age 40 years, and 80% at age 45 years. Recurrent pregnancy loss (RPL), defined as the spontaneous loss of 2 or more clinically recognized pregnancies, affects less than 5% of women.3 Genetic testing using chromosomal microarray analysis (CMA) has identified aneuploidy in about 55% of cases of miscarriage.4
Following ASRM guidelines for the evaluation of RPL, which consists of analyzing parental chromosomal abnormalities, congenital and acquired uterine anomalies, endocrine imbalances, and autoimmune factors (including antiphospholipid syndrome), no explainable cause is determined in 50% of cases.3 Recently, it has been shown that more than 90% of patients with RPL will have a probable or definitive cause identified when CMA testing on miscarriage tissue with the ASRM evaluation guidelines.5
Details of the study
In this prospective cohort study from Denmark, the authors analyzed maternal serum for cell-free fetal DNA (cffDNA) to determine the ploidy status of the pregnancy loss. One thousand women older than age 18 were included (those who demonstrated an ultrasound-confirmed intrauterine pregnancy loss prior to 22 weeks’ gestation). Maternal blood was obtained while pregnancy tissue was in situ or within 24 hours of passage of products of conception (POC), then analyzed by genome-wide sequencing of cffDNA.
For the first 333 recruited women (validation phase), direct sequencing of the POC was performed for sensitivity and specificity. Following the elimination of inconclusive samples, 302 of the 333 cases demonstrated a sensitivity of 85% and specificity of 93%. In the subsequent evaluation of 667 women, researchers analyzed maternal serum from the gestational age of fetuses ranging from 35 days to 149 days.
Results. In total, nearly 90% of cases yielded conclusive results, with 50% euploid, 46% aneuploid, and 4% multiple aneuploidies. Earlier gestational ages (less than 7 weeks) had a no-call rate (ie, inconclusive) of approximately 50% (only based on 16 patients), with results typically obtained in maternal serum following passage of POC; in pregnancies at gestational ages past 7 weeks, the no-call rate was about 10%. In general, the longer the time after the pregnancy tissue passed, the higher likelihood of a no-call result.
Applying the technology of single-nucleotide polymorphism (SNP)-based CMA can improve identification of fetal and/or maternal sources as causes of pregnancy loss with accuracy, but it does require collection of POC. Of note, samples were deficient in this study, the authors cite, in one-third of the cases. Given this limitation of collection, the authors argue for use of the noninvasive method of cffDNA, obtained from maternal serum.
Study strengths and weaknesses
Several weaknesses of this study are highlighted. Of the validation cohort, one-third of pregnancy tissue could not be analyzed due to insufficient collection. Only 73% of cases allowed for DNA isolation from fetal tissue or chorionic villi; in 27% of cases samples were labeled “unknown tissue.” In those cases classified as unknown, 70% were further determined to be maternal. When all female and monosomy cases were excluded in an effort to assuredly reduce the risk of contamination with maternal DNA, sensitivity of the cffDNA testing process declined to 78%. Another limitation was the required short window for maternal blood sampling (within 24 hours) and its impact on the no-call rate.
The authors note an association with later-life morbidity in patients with a history of pregnancy loss and RPL (including cardiovascular disease, type 2 diabetes, and mental health disorders), thereby arguing for cffDNA-based testing versus no causal testing; however, no treatment has been proven to be effective at reducing pregnancy loss. ●
The best management course for unexplained RPL is uncertain. Despite its use for a euploid miscarriage or parental chromosomal structural rearrangement, in vitro fertilization with preimplantation genetic testing remains an unproven modality.6,7 Given that approximately 70% of human conceptions never achieve viability, and 50% fail spontaneously before being detected,8 the authors’ findings demonstrate peripheral maternal blood can provide a reasonably high sensitivity and specificity for fetal ploidy status when compared with direct sequencing of pregnancy tissue. As fetal aneuploidy offers a higher percentage of subsequent successful pregnancy outcomes, cffDNA may offer reassurance, or direct further testing, following a pregnancy loss. As an application of their results, evaluation may be deferred for an aneuploid miscarriage.
—MARK P. TROLICE, MD, MBA
- Brown S. Miscarriage and its associations. Semin Reprod Med. 2008;26:391-400. doi: 10.1055/s-0028-1087105.
- Wang X, Chen C , Wang L, et al. Conception, early pregnancy loss, and time to clinical pregnancy: a population-based prospective study. Fertil Steril. 2003;79:577-584.
- Evaluation and treatment of recurrent pregnancy loss: a committee opinion. Practice Committee of the American Society for Reproductive Medicine. Fertil Steril. 2012;98: 1103-1111.
- Papas RS, Kutteh WH. Genetic testing for aneuploidy in patients who have had multiple miscarriages: a review of current literature. Appl Clin Genet. 2021;14:321-329. https://doi.org/10.2147/tacg.s320778.
- Popescu F, Jaslow FC, Kutteh WH. Recurrent pregnancy loss evaluation combined with 24-chromosome microarray of miscarriage tissue provides a probable or definite cause of pregnancy loss in over 90% of patients. Hum Reprod. 2018;33:579-587. https://doi.org/10.1093/humrep/dey021.
- Dahdouh EM, Balayla J, Garcia-Velasco JA, et al. PGT-A for recurrent pregnancy loss: evidence is growing but the issue is not resolved. Hum Reprod. 2021;36:2805-2806. https://doi.org/10.1093/humrep/deab194.
- Iews M, Tan J, Taskin O, et al. Does preimplantation genetic diagnosis improve reproductive outcome in couples with recurrent pregnancy loss owing to structural chromosomal rearrangement? A systematic review. Reproductive Bio Medicine Online. 2018;36:677-685. https://doi.org/10.1016 /j.rbmo.2018.03.005.
- Papas RS, Kutteh WH. Genetic testing for aneuploidy in patients who have had multiple miscarriages: a review of current literature. Appl Clin Genet. 2021;14:321-329. https://doi.org/10.2147/TACG.S320778.
- Brown S. Miscarriage and its associations. Semin Reprod Med. 2008;26:391-400. doi: 10.1055/s-0028-1087105.
- Wang X, Chen C , Wang L, et al. Conception, early pregnancy loss, and time to clinical pregnancy: a population-based prospective study. Fertil Steril. 2003;79:577-584.
- Evaluation and treatment of recurrent pregnancy loss: a committee opinion. Practice Committee of the American Society for Reproductive Medicine. Fertil Steril. 2012;98: 1103-1111.
- Papas RS, Kutteh WH. Genetic testing for aneuploidy in patients who have had multiple miscarriages: a review of current literature. Appl Clin Genet. 2021;14:321-329. https://doi.org/10.2147/tacg.s320778.
- Popescu F, Jaslow FC, Kutteh WH. Recurrent pregnancy loss evaluation combined with 24-chromosome microarray of miscarriage tissue provides a probable or definite cause of pregnancy loss in over 90% of patients. Hum Reprod. 2018;33:579-587. https://doi.org/10.1093/humrep/dey021.
- Dahdouh EM, Balayla J, Garcia-Velasco JA, et al. PGT-A for recurrent pregnancy loss: evidence is growing but the issue is not resolved. Hum Reprod. 2021;36:2805-2806. https://doi.org/10.1093/humrep/deab194.
- Iews M, Tan J, Taskin O, et al. Does preimplantation genetic diagnosis improve reproductive outcome in couples with recurrent pregnancy loss owing to structural chromosomal rearrangement? A systematic review. Reproductive Bio Medicine Online. 2018;36:677-685. https://doi.org/10.1016 /j.rbmo.2018.03.005.
- Papas RS, Kutteh WH. Genetic testing for aneuploidy in patients who have had multiple miscarriages: a review of current literature. Appl Clin Genet. 2021;14:321-329. https://doi.org/10.2147/TACG.S320778.
ER+/HER2– breast cancer: Is first or second line CDK4/6 inhibitor therapy better?
That was the conclusion of the phase 3 SONIA study, which was presented at the annual meeting of the American Society of Clinical Oncology.
The benefit from first line therapy is not maintained and almost completely disappears when patients in the control arm cross over to receive CDK4/6 inhibition in second line,” said Gabe Sonke, MD, PhD, during his presentation at the meeting.
CDK4/6 inhibitors have shown benefit in both the first-and second-line setting, according to Dr. Sonke, who is a medical oncologist at the Netherlands Cancer Institute, Amsterdam. He added that most guidelines suggest use of CDK4/6 inhibitors in the first line, but there hasn’t been a direct comparison between use in the first and second line.
“Many patients do very well on endocrine therapy alone [in the first line]. Combination treatment leads to a higher risk of the emergence of resistant patterns such as ESR1 mutations, and CDK4/6 inhibitors also come with added costs and toxicities. Given the absence of comparative data between first line and second line, we designed the SONIA trial,” said Dr. Sonke.
Study methods and results
The researchers recruited 1,050 pre- and postmenopausal women who were randomized to a nonsteroidal AI in the first line followed by second-line CDK4/6i plus the estrogen receptor antagonist fulvestrant, or a nonsteroidal AI plus a CDK4/6i in the first line and fulvestrant in the second line. The most commonly used CDK4/6i was palbociclib at 91%, followed by ribociclib at 8%, and abemaciclib at 1%.
After a median follow-up of 37.3 months, the median duration of CDK4/6i exposure was 24.6 months in the first-line CDK4/6i group and 8.1 months in the second-line CDK4/6i group.
The median PFS during first-line therapy was 24.7 months in the first-line CDK4/6i group and 16.1 months in the second-line CDK4/6i group (hazard ratio, 0.59; P < .0001), which was consistent with the results seen in CDK4/6i pivotal trials in the first-line setting, according to Dr. Sonke. However, PFS after two lines of therapy was not significantly different between the groups (31.0 months vs. 26.8 months, respectively; HR, 0.87; P =.10).
The safety profile was similar to what had been seen in previous trials with respect to adverse events like bone marrow and liver function abnormalities and fatigue, but there were 42% more grade 3 or higher adverse events in the first-line CDK4/6i group than in the second-line CDK4/6i group. Dr. Sonke estimated that the increase in costs related to adverse events amounted to about $200,000 per patient receiving CDK4/6i as first line.
There were no significant differences between the two groups in quality of life measurement.
Subgroup analyses of patient categories including prior adjuvant or neoadjuvant chemotherapy or endocrine therapy, de novo metastatic disease, visceral disease, bone-only disease, and treatment with palbociclib or ribociclib showed no difference in outcome for first- versus second-line CDK4/6i treatment.
Are CDK4/6i costs and side effects worth it?
The findings challenge the need for using CDK4/6 inhibitors as first-line treatment in this population, according to Dr. Sonke, who also raised the following related questions.
“If you were a patient, would you consider a treatment that offers no improvement in quality of life and does not improve overall survival? As a doctor or nurse, would you recommend such a treatment to your patient that nearly doubles the incidence of side effects? And if you were responsible for covering the costs of this treatment, whether as an individual or health care insurance, would you consider it worth $200,000?”
For many patients, particularly in the first line setting where resistance mechanisms are less prevalent, endocrine therapy alone remains an excellent option,” said Dr. Sonke during his presentation.
During the discussion portion of the session, Daniel Stover, MD, who is an associate professor of translational therapeutics at Ohio State University Comprehensive Cancer Center, Columbus, pointed out that the lack of differences in the subanalyses leaves little guidance for physicians.
“We really have a limited signal on who can delay CDK4/6 inhibitors. I think one of the most important outcomes of this study is the focus on the patient, as there were substantially fewer adverse events and of course we need to think about financial toxicity as well,” he said. “I think one of the things that is perhaps most exciting to think about is who are the very good risk patients who can delay CDK4/6 inhibitor [therapy]? I think for the majority of patients, endocrine therapy plus CDK4/6 inhibitor is still the appropriate treatment, but I would argue we need additional biomarkers, be it RNA-based biomarkers, novel PET imaging, or perhaps [circulating tumor] DNA dynamics.”
Do cost savings and reduced side effects outweigh first-line PFS benefit?
During the question-and-answer session, William Sikov, MD, spoke up from the audience in support of Dr. Sonke’s conclusions.
“Clearly there are still patients who benefit from that approach, but I think that we have reached an inflection point: I posit that the question has now changed. [We should not ask] why a certain patient should not receive a CDK4/6 inhibitor, but why a certain patient should receive a CDK4/6 inhibitor in the first-line setting,” said Dr. Sikov, who is professor of medicine at Brown University, Providence, R.I.
Dr. Sonke agreed that first-line CDK4/6i is appropriate for some patients, and later echoed the need for biomarkers, but he said that researchers have so far had little luck in identifying any.
“Of course, it’s a shared decision-making between the patient and a doctor, but I think the baseline would be for all of us to consider first line single-agent endocrine therapy,” he said.
Session comoderator Michael Danso, MD, praised the trial but questioned whether the strategy would be adopted in places like the United States, where cost savings is not a major emphasis.
“Progression-free survival is so significant in the first line setting that I can’t imagine that many oncologists in the U.S. will adopt this approach. The other thing is that this was [almost] all palbociclib, so the question remains, would having a different cyclin dependent kinase inhibitor result in the same results? I think the jury’s still out,” said Dr. Danso, who is the research director at Virginia Oncology Associates, Norfolk.
The study was funded by the Dutch government and Dutch Health Insurers. Dr. Sonke has consulted for or advised Biovica, Novartis, and Seagen. He has received research support through his institution from Agendia, AstraZeneca/Merck, Merck Sharp & Dohme, Novartis, Roche, and Seagen. Dr. Sikov has been a speaker for Lilly. Dr. Danso has received honoraria from Amgen and has consulted or advised Immunomedics, Novartis, Pfizer, and Seagen.
That was the conclusion of the phase 3 SONIA study, which was presented at the annual meeting of the American Society of Clinical Oncology.
The benefit from first line therapy is not maintained and almost completely disappears when patients in the control arm cross over to receive CDK4/6 inhibition in second line,” said Gabe Sonke, MD, PhD, during his presentation at the meeting.
CDK4/6 inhibitors have shown benefit in both the first-and second-line setting, according to Dr. Sonke, who is a medical oncologist at the Netherlands Cancer Institute, Amsterdam. He added that most guidelines suggest use of CDK4/6 inhibitors in the first line, but there hasn’t been a direct comparison between use in the first and second line.
“Many patients do very well on endocrine therapy alone [in the first line]. Combination treatment leads to a higher risk of the emergence of resistant patterns such as ESR1 mutations, and CDK4/6 inhibitors also come with added costs and toxicities. Given the absence of comparative data between first line and second line, we designed the SONIA trial,” said Dr. Sonke.
Study methods and results
The researchers recruited 1,050 pre- and postmenopausal women who were randomized to a nonsteroidal AI in the first line followed by second-line CDK4/6i plus the estrogen receptor antagonist fulvestrant, or a nonsteroidal AI plus a CDK4/6i in the first line and fulvestrant in the second line. The most commonly used CDK4/6i was palbociclib at 91%, followed by ribociclib at 8%, and abemaciclib at 1%.
After a median follow-up of 37.3 months, the median duration of CDK4/6i exposure was 24.6 months in the first-line CDK4/6i group and 8.1 months in the second-line CDK4/6i group.
The median PFS during first-line therapy was 24.7 months in the first-line CDK4/6i group and 16.1 months in the second-line CDK4/6i group (hazard ratio, 0.59; P < .0001), which was consistent with the results seen in CDK4/6i pivotal trials in the first-line setting, according to Dr. Sonke. However, PFS after two lines of therapy was not significantly different between the groups (31.0 months vs. 26.8 months, respectively; HR, 0.87; P =.10).
The safety profile was similar to what had been seen in previous trials with respect to adverse events like bone marrow and liver function abnormalities and fatigue, but there were 42% more grade 3 or higher adverse events in the first-line CDK4/6i group than in the second-line CDK4/6i group. Dr. Sonke estimated that the increase in costs related to adverse events amounted to about $200,000 per patient receiving CDK4/6i as first line.
There were no significant differences between the two groups in quality of life measurement.
Subgroup analyses of patient categories including prior adjuvant or neoadjuvant chemotherapy or endocrine therapy, de novo metastatic disease, visceral disease, bone-only disease, and treatment with palbociclib or ribociclib showed no difference in outcome for first- versus second-line CDK4/6i treatment.
Are CDK4/6i costs and side effects worth it?
The findings challenge the need for using CDK4/6 inhibitors as first-line treatment in this population, according to Dr. Sonke, who also raised the following related questions.
“If you were a patient, would you consider a treatment that offers no improvement in quality of life and does not improve overall survival? As a doctor or nurse, would you recommend such a treatment to your patient that nearly doubles the incidence of side effects? And if you were responsible for covering the costs of this treatment, whether as an individual or health care insurance, would you consider it worth $200,000?”
For many patients, particularly in the first line setting where resistance mechanisms are less prevalent, endocrine therapy alone remains an excellent option,” said Dr. Sonke during his presentation.
During the discussion portion of the session, Daniel Stover, MD, who is an associate professor of translational therapeutics at Ohio State University Comprehensive Cancer Center, Columbus, pointed out that the lack of differences in the subanalyses leaves little guidance for physicians.
“We really have a limited signal on who can delay CDK4/6 inhibitors. I think one of the most important outcomes of this study is the focus on the patient, as there were substantially fewer adverse events and of course we need to think about financial toxicity as well,” he said. “I think one of the things that is perhaps most exciting to think about is who are the very good risk patients who can delay CDK4/6 inhibitor [therapy]? I think for the majority of patients, endocrine therapy plus CDK4/6 inhibitor is still the appropriate treatment, but I would argue we need additional biomarkers, be it RNA-based biomarkers, novel PET imaging, or perhaps [circulating tumor] DNA dynamics.”
Do cost savings and reduced side effects outweigh first-line PFS benefit?
During the question-and-answer session, William Sikov, MD, spoke up from the audience in support of Dr. Sonke’s conclusions.
“Clearly there are still patients who benefit from that approach, but I think that we have reached an inflection point: I posit that the question has now changed. [We should not ask] why a certain patient should not receive a CDK4/6 inhibitor, but why a certain patient should receive a CDK4/6 inhibitor in the first-line setting,” said Dr. Sikov, who is professor of medicine at Brown University, Providence, R.I.
Dr. Sonke agreed that first-line CDK4/6i is appropriate for some patients, and later echoed the need for biomarkers, but he said that researchers have so far had little luck in identifying any.
“Of course, it’s a shared decision-making between the patient and a doctor, but I think the baseline would be for all of us to consider first line single-agent endocrine therapy,” he said.
Session comoderator Michael Danso, MD, praised the trial but questioned whether the strategy would be adopted in places like the United States, where cost savings is not a major emphasis.
“Progression-free survival is so significant in the first line setting that I can’t imagine that many oncologists in the U.S. will adopt this approach. The other thing is that this was [almost] all palbociclib, so the question remains, would having a different cyclin dependent kinase inhibitor result in the same results? I think the jury’s still out,” said Dr. Danso, who is the research director at Virginia Oncology Associates, Norfolk.
The study was funded by the Dutch government and Dutch Health Insurers. Dr. Sonke has consulted for or advised Biovica, Novartis, and Seagen. He has received research support through his institution from Agendia, AstraZeneca/Merck, Merck Sharp & Dohme, Novartis, Roche, and Seagen. Dr. Sikov has been a speaker for Lilly. Dr. Danso has received honoraria from Amgen and has consulted or advised Immunomedics, Novartis, Pfizer, and Seagen.
That was the conclusion of the phase 3 SONIA study, which was presented at the annual meeting of the American Society of Clinical Oncology.
The benefit from first line therapy is not maintained and almost completely disappears when patients in the control arm cross over to receive CDK4/6 inhibition in second line,” said Gabe Sonke, MD, PhD, during his presentation at the meeting.
CDK4/6 inhibitors have shown benefit in both the first-and second-line setting, according to Dr. Sonke, who is a medical oncologist at the Netherlands Cancer Institute, Amsterdam. He added that most guidelines suggest use of CDK4/6 inhibitors in the first line, but there hasn’t been a direct comparison between use in the first and second line.
“Many patients do very well on endocrine therapy alone [in the first line]. Combination treatment leads to a higher risk of the emergence of resistant patterns such as ESR1 mutations, and CDK4/6 inhibitors also come with added costs and toxicities. Given the absence of comparative data between first line and second line, we designed the SONIA trial,” said Dr. Sonke.
Study methods and results
The researchers recruited 1,050 pre- and postmenopausal women who were randomized to a nonsteroidal AI in the first line followed by second-line CDK4/6i plus the estrogen receptor antagonist fulvestrant, or a nonsteroidal AI plus a CDK4/6i in the first line and fulvestrant in the second line. The most commonly used CDK4/6i was palbociclib at 91%, followed by ribociclib at 8%, and abemaciclib at 1%.
After a median follow-up of 37.3 months, the median duration of CDK4/6i exposure was 24.6 months in the first-line CDK4/6i group and 8.1 months in the second-line CDK4/6i group.
The median PFS during first-line therapy was 24.7 months in the first-line CDK4/6i group and 16.1 months in the second-line CDK4/6i group (hazard ratio, 0.59; P < .0001), which was consistent with the results seen in CDK4/6i pivotal trials in the first-line setting, according to Dr. Sonke. However, PFS after two lines of therapy was not significantly different between the groups (31.0 months vs. 26.8 months, respectively; HR, 0.87; P =.10).
The safety profile was similar to what had been seen in previous trials with respect to adverse events like bone marrow and liver function abnormalities and fatigue, but there were 42% more grade 3 or higher adverse events in the first-line CDK4/6i group than in the second-line CDK4/6i group. Dr. Sonke estimated that the increase in costs related to adverse events amounted to about $200,000 per patient receiving CDK4/6i as first line.
There were no significant differences between the two groups in quality of life measurement.
Subgroup analyses of patient categories including prior adjuvant or neoadjuvant chemotherapy or endocrine therapy, de novo metastatic disease, visceral disease, bone-only disease, and treatment with palbociclib or ribociclib showed no difference in outcome for first- versus second-line CDK4/6i treatment.
Are CDK4/6i costs and side effects worth it?
The findings challenge the need for using CDK4/6 inhibitors as first-line treatment in this population, according to Dr. Sonke, who also raised the following related questions.
“If you were a patient, would you consider a treatment that offers no improvement in quality of life and does not improve overall survival? As a doctor or nurse, would you recommend such a treatment to your patient that nearly doubles the incidence of side effects? And if you were responsible for covering the costs of this treatment, whether as an individual or health care insurance, would you consider it worth $200,000?”
For many patients, particularly in the first line setting where resistance mechanisms are less prevalent, endocrine therapy alone remains an excellent option,” said Dr. Sonke during his presentation.
During the discussion portion of the session, Daniel Stover, MD, who is an associate professor of translational therapeutics at Ohio State University Comprehensive Cancer Center, Columbus, pointed out that the lack of differences in the subanalyses leaves little guidance for physicians.
“We really have a limited signal on who can delay CDK4/6 inhibitors. I think one of the most important outcomes of this study is the focus on the patient, as there were substantially fewer adverse events and of course we need to think about financial toxicity as well,” he said. “I think one of the things that is perhaps most exciting to think about is who are the very good risk patients who can delay CDK4/6 inhibitor [therapy]? I think for the majority of patients, endocrine therapy plus CDK4/6 inhibitor is still the appropriate treatment, but I would argue we need additional biomarkers, be it RNA-based biomarkers, novel PET imaging, or perhaps [circulating tumor] DNA dynamics.”
Do cost savings and reduced side effects outweigh first-line PFS benefit?
During the question-and-answer session, William Sikov, MD, spoke up from the audience in support of Dr. Sonke’s conclusions.
“Clearly there are still patients who benefit from that approach, but I think that we have reached an inflection point: I posit that the question has now changed. [We should not ask] why a certain patient should not receive a CDK4/6 inhibitor, but why a certain patient should receive a CDK4/6 inhibitor in the first-line setting,” said Dr. Sikov, who is professor of medicine at Brown University, Providence, R.I.
Dr. Sonke agreed that first-line CDK4/6i is appropriate for some patients, and later echoed the need for biomarkers, but he said that researchers have so far had little luck in identifying any.
“Of course, it’s a shared decision-making between the patient and a doctor, but I think the baseline would be for all of us to consider first line single-agent endocrine therapy,” he said.
Session comoderator Michael Danso, MD, praised the trial but questioned whether the strategy would be adopted in places like the United States, where cost savings is not a major emphasis.
“Progression-free survival is so significant in the first line setting that I can’t imagine that many oncologists in the U.S. will adopt this approach. The other thing is that this was [almost] all palbociclib, so the question remains, would having a different cyclin dependent kinase inhibitor result in the same results? I think the jury’s still out,” said Dr. Danso, who is the research director at Virginia Oncology Associates, Norfolk.
The study was funded by the Dutch government and Dutch Health Insurers. Dr. Sonke has consulted for or advised Biovica, Novartis, and Seagen. He has received research support through his institution from Agendia, AstraZeneca/Merck, Merck Sharp & Dohme, Novartis, Roche, and Seagen. Dr. Sikov has been a speaker for Lilly. Dr. Danso has received honoraria from Amgen and has consulted or advised Immunomedics, Novartis, Pfizer, and Seagen.
AT ASCO 2023
How has cannabis legalization affected pregnant mothers?
A population-based study shows that the rate of cannabis-related acute care use during pregnancy increased from 11 per 100,000 pregnancies before legalization to 20 per 100,000 pregnancies afterward: an increase of 82%. Absolute increases were small, however.
“Our findings are consistent with studies highlighting that cannabis use during pregnancy has been increasing in North America, and this study suggests that cannabis legalization might contribute to and accelerate such trends,” study author Daniel Myran, MD, MPH, a public health and preventive medicine physician at the University of Ottawa in Ontario, said in an interview.
The study was published online in the Canadian Medical Association Journal.
Risks for newborns
In a 2019 study, 7% of U.S. women reported using cannabis during pregnancy during 2016-2017, which was double the rate of 3.4% for 2002-2003.
Dr. Myran and colleagues hypothesized that legalizing nonmedical cannabis has affected the drug’s use during pregnancy in Ontario. “We also hypothesized that hospital care for cannabis use would be associated with adverse neonatal outcomes, even after adjusting for other important risk factors that may differ between people with and without cannabis use,” he said.
The researchers’ repeated cross-sectional analysis evaluated changes in the number of pregnant people who received acute care from January 2015 to July 2021 among all patients who were eligible for Ontario’s public health coverage. The final study cohort included 691,242 pregnant patients, of whom 533 had at least one pregnancy with cannabis-related acute care visits. These mothers had a mean age of 24 years vs. 30 for their counterparts with no such visits.
Using segmented regression, the researchers compared changes in the quarterly rate of pregnant people with acute care related to cannabis use (the primary outcome) with those of acute care for mental health conditions or for noncannabis substance use (the control conditions).
“Severe morning sickness was a major risk factor for care in the emergency department or hospital for cannabis use,” said Dr. Myran. “Prior work has found that people who use cannabis during pregnancy often state that it was used to manage challenging symptoms of pregnancy such as morning sickness.”
Most acute care events (72.2%) were emergency department visits. The most common reasons for acute care were harmful cannabis use (57.6%), followed by cannabis dependence or withdrawal (21.5%), and acute cannabis intoxication (12.8%).
Compared with pregnancies without acute care, those with acute care related to cannabis had higher rates of adverse neonatal outcomes such as birth before 37 weeks’ gestational age (16.9% vs. 7.2%), birth weight at or below the bottom fifth percentile after adjustment for gestational age (12.1% vs. 4.4%), and neonatal intensive care unit admission in the first 28 days of life (31.5% vs. 13%).
An adjusted analysis found that patients younger than 35 years and those living in rural settings or the lowest-income neighborhoods had higher odds of acute cannabis-related care during pregnancy. Patients who received acute care for any substance use or schizophrenia before pregnancy or who accessed outpatient mental health services before pregnancy had higher risk for cannabis-related acute care during pregnancy. Mothers receiving acute care for cannabis also had higher risk for acute care for hyperemesis gravidarum during pregnancy (30.9%).
The rate of acute care for other types of substance use such as alcohol and opioids did not change after cannabis legalization, and acute care for mental health conditions such as anxiety and depression during pregnancy declined by 14%, Dr. Myran noted.
“Physicians who care for pregnant people should consider increasing screening for cannabis use during pregnancy,” said Dr. Myran. “In addition, repeated nonstigmatizing screening and counseling may be indicated for higher-risk groups identified in the study, including pregnancies with severe morning sickness.”
The U.S. perspective
Commenting on the study, M. Camille Hoffman, MD, MSc, a maternal-fetal medicine specialist at the University of Colorado in Aurora, said that the findings likely indicate that legalization has made cannabis users less reluctant to come forward for urgent care. “They cannot really claim that this is equivalent to more use, just that more people are willing to present,” she said. Dr. Hoffman was not involved in the study.
The Canadian results do not align perfectly with what is seen in the United States. “It does suggest that there may be more cannabinoid hyperemesis being coded as hyperemesis gravidarum, which is a pregnancy-specific condition vs. a cannabis-dependence-related one,” said Dr. Hoffman.
Literature in the United States often includes tobacco use as a covariate, she added. “This study does not appear to do that,” she said. “Rather, it uses any substance use. Because of this, it is difficult to really know the contribution of cannabis to the adverse pregnancy outcomes vs. the combination of tobacco and cannabis.”
Finally, she pointed out, the proportion of those presenting for acute care for substance use in the 2 years before conception was 22% for acute care visits for cannabis vs 1% for no acute care visits. “This suggests to me that this was a highly vulnerable group before the legalization of cannabis as well. The overall absolute difference is nine in total per 100,000 – hardly enough to draw any real conclusions. Again, maybe those nine were simply more willing to come forth with concerns with cannabis being legal.”
There is no known safe level of cannabis consumption, and its use by pregnant women has been linked to later neurodevelopmental issues in their offspring. A 2022 U.S. study suggested that cannabis exposure in the womb may leave children later in life at risk for autism, psychiatric disorders, and problematic substance abuse, particularly as they enter peak periods of vulnerability in late adolescence.
As to the impact of legalization in certain U.S. states, a 2022 study found that women perceived legalization to mean greater access to cannabis, increased acceptance of use, and greater trust in cannabis retailers. In line with Dr. Hoffman’s view, this study suggested that legalization made pregnant women more willing to discuss cannabis use during pregnancy honestly with their care providers.
In the United States, prenatal cannabis use is still included in definitions of child abuse or neglect and can lead to termination of parental rights, even in states with full legalization.
“These findings highlight the need for ongoing monitoring of markers of cannabis use during pregnancy after legalization,” said Dr. Myran. He also called for effective policies in regions with legal cannabis, such as increased warning labels on cannabis products.
This study was supported by the Canadian Institutes of Health Research and the University of Ottawa site of ICES, which is funded by an annual grant from the Ontario Ministry of Health and Ministry of Long-Term Care. Dr. Myran reports a speaker fee from McMaster University. Dr. Hoffman reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A population-based study shows that the rate of cannabis-related acute care use during pregnancy increased from 11 per 100,000 pregnancies before legalization to 20 per 100,000 pregnancies afterward: an increase of 82%. Absolute increases were small, however.
“Our findings are consistent with studies highlighting that cannabis use during pregnancy has been increasing in North America, and this study suggests that cannabis legalization might contribute to and accelerate such trends,” study author Daniel Myran, MD, MPH, a public health and preventive medicine physician at the University of Ottawa in Ontario, said in an interview.
The study was published online in the Canadian Medical Association Journal.
Risks for newborns
In a 2019 study, 7% of U.S. women reported using cannabis during pregnancy during 2016-2017, which was double the rate of 3.4% for 2002-2003.
Dr. Myran and colleagues hypothesized that legalizing nonmedical cannabis has affected the drug’s use during pregnancy in Ontario. “We also hypothesized that hospital care for cannabis use would be associated with adverse neonatal outcomes, even after adjusting for other important risk factors that may differ between people with and without cannabis use,” he said.
The researchers’ repeated cross-sectional analysis evaluated changes in the number of pregnant people who received acute care from January 2015 to July 2021 among all patients who were eligible for Ontario’s public health coverage. The final study cohort included 691,242 pregnant patients, of whom 533 had at least one pregnancy with cannabis-related acute care visits. These mothers had a mean age of 24 years vs. 30 for their counterparts with no such visits.
Using segmented regression, the researchers compared changes in the quarterly rate of pregnant people with acute care related to cannabis use (the primary outcome) with those of acute care for mental health conditions or for noncannabis substance use (the control conditions).
“Severe morning sickness was a major risk factor for care in the emergency department or hospital for cannabis use,” said Dr. Myran. “Prior work has found that people who use cannabis during pregnancy often state that it was used to manage challenging symptoms of pregnancy such as morning sickness.”
Most acute care events (72.2%) were emergency department visits. The most common reasons for acute care were harmful cannabis use (57.6%), followed by cannabis dependence or withdrawal (21.5%), and acute cannabis intoxication (12.8%).
Compared with pregnancies without acute care, those with acute care related to cannabis had higher rates of adverse neonatal outcomes such as birth before 37 weeks’ gestational age (16.9% vs. 7.2%), birth weight at or below the bottom fifth percentile after adjustment for gestational age (12.1% vs. 4.4%), and neonatal intensive care unit admission in the first 28 days of life (31.5% vs. 13%).
An adjusted analysis found that patients younger than 35 years and those living in rural settings or the lowest-income neighborhoods had higher odds of acute cannabis-related care during pregnancy. Patients who received acute care for any substance use or schizophrenia before pregnancy or who accessed outpatient mental health services before pregnancy had higher risk for cannabis-related acute care during pregnancy. Mothers receiving acute care for cannabis also had higher risk for acute care for hyperemesis gravidarum during pregnancy (30.9%).
The rate of acute care for other types of substance use such as alcohol and opioids did not change after cannabis legalization, and acute care for mental health conditions such as anxiety and depression during pregnancy declined by 14%, Dr. Myran noted.
“Physicians who care for pregnant people should consider increasing screening for cannabis use during pregnancy,” said Dr. Myran. “In addition, repeated nonstigmatizing screening and counseling may be indicated for higher-risk groups identified in the study, including pregnancies with severe morning sickness.”
The U.S. perspective
Commenting on the study, M. Camille Hoffman, MD, MSc, a maternal-fetal medicine specialist at the University of Colorado in Aurora, said that the findings likely indicate that legalization has made cannabis users less reluctant to come forward for urgent care. “They cannot really claim that this is equivalent to more use, just that more people are willing to present,” she said. Dr. Hoffman was not involved in the study.
The Canadian results do not align perfectly with what is seen in the United States. “It does suggest that there may be more cannabinoid hyperemesis being coded as hyperemesis gravidarum, which is a pregnancy-specific condition vs. a cannabis-dependence-related one,” said Dr. Hoffman.
Literature in the United States often includes tobacco use as a covariate, she added. “This study does not appear to do that,” she said. “Rather, it uses any substance use. Because of this, it is difficult to really know the contribution of cannabis to the adverse pregnancy outcomes vs. the combination of tobacco and cannabis.”
Finally, she pointed out, the proportion of those presenting for acute care for substance use in the 2 years before conception was 22% for acute care visits for cannabis vs 1% for no acute care visits. “This suggests to me that this was a highly vulnerable group before the legalization of cannabis as well. The overall absolute difference is nine in total per 100,000 – hardly enough to draw any real conclusions. Again, maybe those nine were simply more willing to come forth with concerns with cannabis being legal.”
There is no known safe level of cannabis consumption, and its use by pregnant women has been linked to later neurodevelopmental issues in their offspring. A 2022 U.S. study suggested that cannabis exposure in the womb may leave children later in life at risk for autism, psychiatric disorders, and problematic substance abuse, particularly as they enter peak periods of vulnerability in late adolescence.
As to the impact of legalization in certain U.S. states, a 2022 study found that women perceived legalization to mean greater access to cannabis, increased acceptance of use, and greater trust in cannabis retailers. In line with Dr. Hoffman’s view, this study suggested that legalization made pregnant women more willing to discuss cannabis use during pregnancy honestly with their care providers.
In the United States, prenatal cannabis use is still included in definitions of child abuse or neglect and can lead to termination of parental rights, even in states with full legalization.
“These findings highlight the need for ongoing monitoring of markers of cannabis use during pregnancy after legalization,” said Dr. Myran. He also called for effective policies in regions with legal cannabis, such as increased warning labels on cannabis products.
This study was supported by the Canadian Institutes of Health Research and the University of Ottawa site of ICES, which is funded by an annual grant from the Ontario Ministry of Health and Ministry of Long-Term Care. Dr. Myran reports a speaker fee from McMaster University. Dr. Hoffman reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A population-based study shows that the rate of cannabis-related acute care use during pregnancy increased from 11 per 100,000 pregnancies before legalization to 20 per 100,000 pregnancies afterward: an increase of 82%. Absolute increases were small, however.
“Our findings are consistent with studies highlighting that cannabis use during pregnancy has been increasing in North America, and this study suggests that cannabis legalization might contribute to and accelerate such trends,” study author Daniel Myran, MD, MPH, a public health and preventive medicine physician at the University of Ottawa in Ontario, said in an interview.
The study was published online in the Canadian Medical Association Journal.
Risks for newborns
In a 2019 study, 7% of U.S. women reported using cannabis during pregnancy during 2016-2017, which was double the rate of 3.4% for 2002-2003.
Dr. Myran and colleagues hypothesized that legalizing nonmedical cannabis has affected the drug’s use during pregnancy in Ontario. “We also hypothesized that hospital care for cannabis use would be associated with adverse neonatal outcomes, even after adjusting for other important risk factors that may differ between people with and without cannabis use,” he said.
The researchers’ repeated cross-sectional analysis evaluated changes in the number of pregnant people who received acute care from January 2015 to July 2021 among all patients who were eligible for Ontario’s public health coverage. The final study cohort included 691,242 pregnant patients, of whom 533 had at least one pregnancy with cannabis-related acute care visits. These mothers had a mean age of 24 years vs. 30 for their counterparts with no such visits.
Using segmented regression, the researchers compared changes in the quarterly rate of pregnant people with acute care related to cannabis use (the primary outcome) with those of acute care for mental health conditions or for noncannabis substance use (the control conditions).
“Severe morning sickness was a major risk factor for care in the emergency department or hospital for cannabis use,” said Dr. Myran. “Prior work has found that people who use cannabis during pregnancy often state that it was used to manage challenging symptoms of pregnancy such as morning sickness.”
Most acute care events (72.2%) were emergency department visits. The most common reasons for acute care were harmful cannabis use (57.6%), followed by cannabis dependence or withdrawal (21.5%), and acute cannabis intoxication (12.8%).
Compared with pregnancies without acute care, those with acute care related to cannabis had higher rates of adverse neonatal outcomes such as birth before 37 weeks’ gestational age (16.9% vs. 7.2%), birth weight at or below the bottom fifth percentile after adjustment for gestational age (12.1% vs. 4.4%), and neonatal intensive care unit admission in the first 28 days of life (31.5% vs. 13%).
An adjusted analysis found that patients younger than 35 years and those living in rural settings or the lowest-income neighborhoods had higher odds of acute cannabis-related care during pregnancy. Patients who received acute care for any substance use or schizophrenia before pregnancy or who accessed outpatient mental health services before pregnancy had higher risk for cannabis-related acute care during pregnancy. Mothers receiving acute care for cannabis also had higher risk for acute care for hyperemesis gravidarum during pregnancy (30.9%).
The rate of acute care for other types of substance use such as alcohol and opioids did not change after cannabis legalization, and acute care for mental health conditions such as anxiety and depression during pregnancy declined by 14%, Dr. Myran noted.
“Physicians who care for pregnant people should consider increasing screening for cannabis use during pregnancy,” said Dr. Myran. “In addition, repeated nonstigmatizing screening and counseling may be indicated for higher-risk groups identified in the study, including pregnancies with severe morning sickness.”
The U.S. perspective
Commenting on the study, M. Camille Hoffman, MD, MSc, a maternal-fetal medicine specialist at the University of Colorado in Aurora, said that the findings likely indicate that legalization has made cannabis users less reluctant to come forward for urgent care. “They cannot really claim that this is equivalent to more use, just that more people are willing to present,” she said. Dr. Hoffman was not involved in the study.
The Canadian results do not align perfectly with what is seen in the United States. “It does suggest that there may be more cannabinoid hyperemesis being coded as hyperemesis gravidarum, which is a pregnancy-specific condition vs. a cannabis-dependence-related one,” said Dr. Hoffman.
Literature in the United States often includes tobacco use as a covariate, she added. “This study does not appear to do that,” she said. “Rather, it uses any substance use. Because of this, it is difficult to really know the contribution of cannabis to the adverse pregnancy outcomes vs. the combination of tobacco and cannabis.”
Finally, she pointed out, the proportion of those presenting for acute care for substance use in the 2 years before conception was 22% for acute care visits for cannabis vs 1% for no acute care visits. “This suggests to me that this was a highly vulnerable group before the legalization of cannabis as well. The overall absolute difference is nine in total per 100,000 – hardly enough to draw any real conclusions. Again, maybe those nine were simply more willing to come forth with concerns with cannabis being legal.”
There is no known safe level of cannabis consumption, and its use by pregnant women has been linked to later neurodevelopmental issues in their offspring. A 2022 U.S. study suggested that cannabis exposure in the womb may leave children later in life at risk for autism, psychiatric disorders, and problematic substance abuse, particularly as they enter peak periods of vulnerability in late adolescence.
As to the impact of legalization in certain U.S. states, a 2022 study found that women perceived legalization to mean greater access to cannabis, increased acceptance of use, and greater trust in cannabis retailers. In line with Dr. Hoffman’s view, this study suggested that legalization made pregnant women more willing to discuss cannabis use during pregnancy honestly with their care providers.
In the United States, prenatal cannabis use is still included in definitions of child abuse or neglect and can lead to termination of parental rights, even in states with full legalization.
“These findings highlight the need for ongoing monitoring of markers of cannabis use during pregnancy after legalization,” said Dr. Myran. He also called for effective policies in regions with legal cannabis, such as increased warning labels on cannabis products.
This study was supported by the Canadian Institutes of Health Research and the University of Ottawa site of ICES, which is funded by an annual grant from the Ontario Ministry of Health and Ministry of Long-Term Care. Dr. Myran reports a speaker fee from McMaster University. Dr. Hoffman reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM CMAJ
How can we make medical training less ‘toxic’?
This transcript has been edited for clarity.
Robert D. Glatter, MD: Welcome. I’m Dr. Robert Glatter, medical adviser for Medscape Emergency Medicine. Joining me to discuss ways to address and reform the toxic culture associated with medical training is Dr. Amy Faith Ho, senior vice president of clinical informatics and analytics at Integrative Emergency Services in Dallas. Also joining us is Dr. Júlia Loyola Ferreira, a pediatric surgeon originally from Brazil, now practicing at Montreal Children’s and focused on advocacy for gender equity and patient-centered care.
Welcome to both of you. Thanks so much for joining me.
Amy Faith Ho, MD, MPH: Thanks so much for having us, Rob.
Dr. Glatter: Amy, I noticed a tweet recently where you talked about how your career choice was affected by the toxic environment in medical school, affecting your choice of residency. Can you elaborate on that?
Dr. Ho: In this instance, what we’re talking about is gender, but it can be directed toward any number of other groups as well.
What you’re alluding to is a tweet by Stanford Surgery Group showing the next residency class, and what was really stunning about this residency class was that it was almost all females. And this was something that took off on social media.
When I saw this, I was really brought back to one of my personal experiences that I chose to share, which was basically that, as a medical student, I really wanted to be a surgeon. I’m an emergency medicine doctor now, so you know that didn’t happen.
The story that I was sharing was that when I was a third-year medical student rotating on surgery, we had a male attending who was very well known at that school at the time who basically would take the female medical students, and instead of clinic, he would round us up. He would have us sit around him in the workplace room while everyone else was seeing patients, and he would have you look at news clippings of himself. He would tell you stories about himself, like he was holding court for the ladies.
It was this very weird culture where my takeaway as a med student was like, “Wow, this is kind of abusive patriarchy that is supported,” because everyone knew about it and was complicit. Even though I really liked surgery, this was just one instance and one example of where you see this culture that really resonates into the rest of life that I didn’t really want to be a part of.
I went into emergency medicine and loved it. It’s also highly procedural, and I was very happy with where I was. What was really interesting about this tweet to me, though, is that it really took off and garnered hundreds of thousands of views on a very niche topic, because what was most revealing is that everyone has a story like this.
It is not just surgery. It is definitely not just one specialty and it is not just one school. It is an endemic problem in medicine. Not only does it change the lives of young women, but it also says so much about the complicity and the culture that we have in medicine that many people were upset about just the same way I was.
Medical training experience in other countries vs. the United States
Dr. Glatter: Júlia, I want to hear about your experience in medical school, surgery, and then fellowship training and up to the present, if possible.
Júlia Loyola Ferreira, MD: In Brazil, as in many countries now, women have made up the majority of the medical students since 2010. It’s a more female-friendly environment when you’re going through medical school, and I was lucky enough to do rotations in areas of surgery where people were friendly to women.
I lived in this tiny bubble that also gave me the privilege of not facing some things that I can imagine that people in Brazil in different areas and smaller towns face. In Brazil, people try to not talk about this gender agenda. This is something that’s being talked about outside Brazil. But in Brazil, we are years back. People are not really engaging on this conversation. I thought it was going to be hard for me as a woman, because Brazil has around 20% female surgeons.
I knew it was going to be challenging, but I had no idea how bad it was. When I started and things started happening, the list was big. I have an example of everything that is written about – microaggression, implicit bias, discrimination, harassment.
Every time I would try to speak about it and talk to someone, I would be strongly gaslighted. It was the whole training, the whole 5 years. People would say, “Oh, I don’t think it was like that. I think you were overreacting.” People would come with all these different answers for what I was experiencing, and that was frustrating. That was even harder because I had to cope with everything that was happening and I had no one to turn to. I had no mentors.
When I looked up to women who were in surgery, they would be tougher on us young surgeons than the men and they would tell us that we should not complain because in their time it was even harder. Now, it’s getting better and we are supposed to accept whatever comes.
That was at least a little bit of what I experienced in my training. It was only after I finished and started to do research about it that I really encountered a field of people who would echo what I was trying to say to many people in different hospitals that I attended to.
That was the key for me to get out of that situation of being gaslighted and of not being able to really talk about it. Suddenly, I started to publish things about Brazil that nobody was even writing or studying. That gave me a large amount of responsibility, but also motivation to keep going and to see the change.
Valuing women in medicine
Dr. Glatter: This is a very important point that you’re raising about the environment of women being hard on other women. We know that men can be very difficult on and also judgmental toward their trainees.
Amy, how would you respond to that? Was your experience similar in emergency medicine training?
Dr. Ho: I actually don’t feel like it was. I think what Júlia is alluding to is this “mean girls” idea, of “I went through it and thus you have to go through it.” I think you do see this in many specialties. One of the classic ones we hear about, and I don’t want to speak to it too much because it’s not my specialty, is ob.gyn., where it is a very female-dominant surgery group. There’s almost a hazing level that you hear about in some of the more malignant workplaces.
I think that you speak to two really important things. Number one is the numbers game. As you were saying, Brazil actually has many women. That’s awesome. That’s actually different from the United States, especially for the historic, existing workplace and less so for the medical students and for residents. I think step one is having minorities like women just present and there.
Step two is actually including and valuing them. While I think it’s really easy to move away from the women discussion, because there are women when you look around in medicine, it doesn’t mean that women are actually being heard, that they’re actually being accepted, or that their viewpoints are being listened to. A big part of it is normalizing not only seeing women in medicine but also normalizing the narrative of women in medicine.
It’s not just about motherhood; it’s about things like normalizing talking about advancement, academic promotions, pay, culture, being called things like “too reactive,” “anxious,” or “too assertive.” These are all classic things that we hear about when we talk about women.
That’s why we’re looking to not only conversations like this, but also structured ways for women to discuss being women in medicine. There are many women in medicine groups in emergency medicine, including: Females Working in Emergency Medicine (FemInEM); the American College of Emergency Physicians (ACEP) and Society for Academic Emergency Medicine (SAEM) women’s groups, which are American Association of Women Emergency Physicians (AAWEP) and Academy for Women in Academic Emergency Medicine (AWAEM), respectively; and the American Medical Women’s Association (AMWA), which is the American Medical Association’s offshoot.
All of these groups are geared toward normalizing women in medicine, normalizing the narrative of women in medicine, and then working on mentoring and educating so that we can advance our initiatives.
Gender balance is not gender equity
Dr. Glatter: Amy, you bring up a very critical point that mentoring is sort of the antidote to gender-based discrimination. Júlia had written a paper back in November of 2022 that was published in the Journal of Surgical Research talking exactly about this and how important it is to develop mentoring. Part of her research showed that about 20% of medical students who took the survey, about 1,000 people, had mentors, which was very disturbing.
Dr. Loyola Ferreira: Mentorship is one of the ways of changing the reality about gender-based discrimination. Amy’s comment was very strong and we need to really keep saying it, which is that gender balance is not gender equity.
The idea of having more women is not the same as women being recognized as equals, as able as men, and as valued as men. To change this very long culture of male domination, we need support, and this support comes from mentorship.
Although I didn’t have one, I feel that since I started being a mentor for some students, it changed not only them but myself. It gave me strength to keep going, studying, publishing, and going further with this discussion. I feel like the relationship was as good for them as it is for me. That’s how things change.
Diversity, equity, and inclusion training
Dr. Glatter: We’re talking about the reality of gender equity in terms of the ability to have equal respect, recognition, opportunities, and access. That’s really an important point to realize, and for our audience, to understand that gender equity is not gender balance.
Amy, I want to talk about medical school curriculums. Are there advances that you’re aware of being made at certain schools, programs, even in residencies, to enforce these things and make it a priority?
Dr. Ho: We’re really lucky that, as a culture in the United States, medical training is certainly very geared toward diversity. Some of that is certainly unofficial. Some of that just means when they’re looking at a medical school class or looking at rank lists for residency, that they’re cognizant of the different backgrounds that people have. That’s still a step. That is a step, that we’re at least acknowledging it.
There are multiple medical schools and residencies that have more formal unconscious-bias training or diversity, equity, and inclusion (DEI) training, both of which are excellent not only for us in the workplace but also for our patients. Almost all of us will see patients of highly diverse backgrounds. I think the biggest push is looking toward the criteria that we use for selecting trainees and students into our programs. Historically, it’s been MCAT, GPA, and so on.
We’ve really started to ask the question of, are these sorts of “objective criteria” actually biased in institutional ways? They talk about this all the time where GPAs will bias against students from underrepresented minorities (URM). I think all medical students and residencies have really acknowledged that. Although there are still test cutoffs, we are putting an inquisitive eye to what those mean, why they exist, and what are the other things that we should consider. This is all very heartening from what I’m seeing in medical training.
Dr. Glatter: There’s no formal rating system for DEI curriculums right now, like ranking of this school, or this program has more advanced recognition in terms of DEI?
Dr. Ho: No, but on the flip side, the U.S. News & World Report was classically one of the major rankings for medical schools. What we saw fairly recently was that very high-tier schools like Harvard and University of Chicago pulled out of that ranking because that ranking did not acknowledge the value of diversity. That was an incredible stance for medical schools to take, to say, “Hey, you are not evaluating an important criterion of ours.”
Dr. Glatter: That’s a great point. Júlia, where are we now in Brazil in terms of awareness of DEI and curriculum in schools and training programs?
Dr. Loyola Ferreira: Our reality is not as good as in the U.S., unfortunately. I don’t see much discussion on residency programs or medical schools at the moment. I see many students bringing it out and trying to make their schools engage in that discussion. This is something that is coming from the bottom up and not from the top down. I think it can lead to change as well. It is a step and it’s a beginning. Institutions should take the responsibility of doing this from the beginning. This is something where Brazil is still years behind you guys.
Dr. Glatter: It’s unfortunate, but certainly it’s important to hear that. What about in Canada and certainly your institution, McGill, where you just completed a master’s degree?
Dr. Loyola Ferreira: Canada is very much like the U.S. This is something that is really happening and it’s happening fast. I see, at least at McGill, a large amount of DEI inclusion and everything on this discussion. They have institutional courses for us to do as students, and we are all obliged to do many courses, which I think is really educating, especially for people with different cultures and backgrounds.
Dr. Glatter: Amy, where do you think we are in emergency medicine to look at the other side of it? Comparing surgery with emergency medicine, do you think we’re well advanced in terms of DEI, inclusion criteria, respect, and dignity, or are we really far off?
Dr. Ho: I may be biased, but I think emergency medicine is one of the best in terms of this, and I think there are a couple of reasons for it. One is that we are an inherently team-based organization. The attending, the residents, and the students all work in line with one another. There’s less of a hierarchy.
The same is true for our nurses, pharmacists, techs, and EMS. We all work together as a team. Because of that fairly flat structure, it’s really easy for us to value one another as individuals with our diverse backgrounds. In a way, that’s harder for specialties that are more hierarchical, and I think surgery is certainly one of the most hierarchical.
The second reason why emergency medicine is fairly well off in this is that we’re, by nature, a safety-net specialty. We see patients of all-comers, all walks, all backgrounds. I think we both recognize the value of physician-patient concordance. When we share characteristics with our patients, we recognize that value immediately at the bedside.
It exposes us to so much diversity. I see a refugee one day and the next patient is someone who is incarcerated. The next patient after that is an important businessman in society. That diversity and whiplash in the type of patients that we see back-to-back helps us see the playing field in a really flat, diverse way. Because of that, I think our culture is much better, as is our understanding of the value and importance of diversity not only for our programs, but also for our patients.
Do female doctors have better patient outcomes?
Dr. Glatter: Specialties working together in the emergency department is so important. Building that team and that togetherness is so critical. Júlia, would you agree?
Dr. Loyola Ferreira: Definitely. Something Amy said that is beautiful is that you recognize yourself in these patients. In surgery, we are taught to try to be away from the patients and not to put ourselves in the same position. We are taught to be less engaging, and this is not good. The good thing is when we really have patient-centered care, when we listen to them, and when we are involved with them.
I saw a publication showing that female and male surgeons treating similar patients had the same surgical outcomes. Women are as good as men technically to do surgery and have the same surgical outcomes. However, there is research showing that surgical teams with greater representation of women have improved surgical outcomes because of patient-centered care and the way women conduct bedside attention to patients. And they have better patient experience measures afterward. That is not only from the women who are treating the patients, but the whole environment. Women end up bringing men [into the conversation] and this better improves patient-centered care, and that makes the whole team a better team attending patients. Definitely, we are in the moment of patient experience and satisfaction, and increasing women is a way of achieving better patient satisfaction and experience.
Dr. Ho: There’s much to be said about having female clinicians available for patients. It doesn’t have to be just for female patients, although again, concordance between physicians and patients is certainly beneficial. Besides outcomes benefit, there’s even just a communication benefit. The way that women and men communicate is inherently different. The way women and men experience certain things is also inherently different.
A classic example of this is women who are experiencing a heart attack may not actually have chest pain but present with nausea. As a female who’s sensitive to this, when I see a woman throwing up, I am very attuned to something actually being wrong, knowing that they may not present with classic pain for a syndrome, but actually may be presenting with nausea instead. It doesn’t have to be a woman who takes that knowledge and turns it into something at the bedside. It certainly doesn’t have to, but it is just a natural, easy thing to step into as a female.
While I’m really careful to not step into this “women are better than men” or “men are better than women” argument, there’s something to be said about how the availability of female clinicians for all patients, not just female patients, can have benefit. Again, it’s shown in studies with cardiovascular outcomes and cardiologists, it’s certainly shown in ob.gyn., particularly for underrepresented minorities as well for maternal outcomes of Black mothers. It’s certainly shown again in patient satisfaction, which is concordance.
There is a profound level of research already on this that goes beyond just the idea of stacking the bench and putting more women in there. That’s not the value. We’re not just here to check off the box. We’re here to actually lend some value to our patients and, again, to one another as well.
Dr. Glatter: Absolutely. These are excellent points. The point you make about patient presentation is so vital. The fact that women have nausea sometimes in ACS presentations, the research never was really attentive to this. It was biased. The symptoms that women may have that are not “typical” for ACS weren’t included in patient presentations. Educating everyone about, overall, the types of presentations that we can recognize is vital and important.
Dr. Ho: Yes. It’s worth saying that, when you look at how medicine and research developed, classically, who were the research participants? They were often White men. They were college students who, historically, because women were not allowed to go to college, were men.
I say that not to fault the institution, because that was the culture of our history, but to just say it is okay to question things. It is okay to realize that someone’s presenting outside of the box and that maybe we actually need to reframe what even created the walls of the box in the first place.
Dr. Glatter: Thank you again for joining us. I truly appreciate your insight and expertise.
Dr. Glatter is assistant professor of emergency medicine, department of emergency medicine, Hofstra/Northwell, New York. Dr. Ho is senior vice president of clinical informatics & analytics, department of emergency medicine, Integrative Emergency Services, Dallas. Dr. Loyola Ferreira is a master of science candidate, department of experimental surgery, McGill University, Montreal. They reported that they had no conflicts of interest.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Robert D. Glatter, MD: Welcome. I’m Dr. Robert Glatter, medical adviser for Medscape Emergency Medicine. Joining me to discuss ways to address and reform the toxic culture associated with medical training is Dr. Amy Faith Ho, senior vice president of clinical informatics and analytics at Integrative Emergency Services in Dallas. Also joining us is Dr. Júlia Loyola Ferreira, a pediatric surgeon originally from Brazil, now practicing at Montreal Children’s and focused on advocacy for gender equity and patient-centered care.
Welcome to both of you. Thanks so much for joining me.
Amy Faith Ho, MD, MPH: Thanks so much for having us, Rob.
Dr. Glatter: Amy, I noticed a tweet recently where you talked about how your career choice was affected by the toxic environment in medical school, affecting your choice of residency. Can you elaborate on that?
Dr. Ho: In this instance, what we’re talking about is gender, but it can be directed toward any number of other groups as well.
What you’re alluding to is a tweet by Stanford Surgery Group showing the next residency class, and what was really stunning about this residency class was that it was almost all females. And this was something that took off on social media.
When I saw this, I was really brought back to one of my personal experiences that I chose to share, which was basically that, as a medical student, I really wanted to be a surgeon. I’m an emergency medicine doctor now, so you know that didn’t happen.
The story that I was sharing was that when I was a third-year medical student rotating on surgery, we had a male attending who was very well known at that school at the time who basically would take the female medical students, and instead of clinic, he would round us up. He would have us sit around him in the workplace room while everyone else was seeing patients, and he would have you look at news clippings of himself. He would tell you stories about himself, like he was holding court for the ladies.
It was this very weird culture where my takeaway as a med student was like, “Wow, this is kind of abusive patriarchy that is supported,” because everyone knew about it and was complicit. Even though I really liked surgery, this was just one instance and one example of where you see this culture that really resonates into the rest of life that I didn’t really want to be a part of.
I went into emergency medicine and loved it. It’s also highly procedural, and I was very happy with where I was. What was really interesting about this tweet to me, though, is that it really took off and garnered hundreds of thousands of views on a very niche topic, because what was most revealing is that everyone has a story like this.
It is not just surgery. It is definitely not just one specialty and it is not just one school. It is an endemic problem in medicine. Not only does it change the lives of young women, but it also says so much about the complicity and the culture that we have in medicine that many people were upset about just the same way I was.
Medical training experience in other countries vs. the United States
Dr. Glatter: Júlia, I want to hear about your experience in medical school, surgery, and then fellowship training and up to the present, if possible.
Júlia Loyola Ferreira, MD: In Brazil, as in many countries now, women have made up the majority of the medical students since 2010. It’s a more female-friendly environment when you’re going through medical school, and I was lucky enough to do rotations in areas of surgery where people were friendly to women.
I lived in this tiny bubble that also gave me the privilege of not facing some things that I can imagine that people in Brazil in different areas and smaller towns face. In Brazil, people try to not talk about this gender agenda. This is something that’s being talked about outside Brazil. But in Brazil, we are years back. People are not really engaging on this conversation. I thought it was going to be hard for me as a woman, because Brazil has around 20% female surgeons.
I knew it was going to be challenging, but I had no idea how bad it was. When I started and things started happening, the list was big. I have an example of everything that is written about – microaggression, implicit bias, discrimination, harassment.
Every time I would try to speak about it and talk to someone, I would be strongly gaslighted. It was the whole training, the whole 5 years. People would say, “Oh, I don’t think it was like that. I think you were overreacting.” People would come with all these different answers for what I was experiencing, and that was frustrating. That was even harder because I had to cope with everything that was happening and I had no one to turn to. I had no mentors.
When I looked up to women who were in surgery, they would be tougher on us young surgeons than the men and they would tell us that we should not complain because in their time it was even harder. Now, it’s getting better and we are supposed to accept whatever comes.
That was at least a little bit of what I experienced in my training. It was only after I finished and started to do research about it that I really encountered a field of people who would echo what I was trying to say to many people in different hospitals that I attended to.
That was the key for me to get out of that situation of being gaslighted and of not being able to really talk about it. Suddenly, I started to publish things about Brazil that nobody was even writing or studying. That gave me a large amount of responsibility, but also motivation to keep going and to see the change.
Valuing women in medicine
Dr. Glatter: This is a very important point that you’re raising about the environment of women being hard on other women. We know that men can be very difficult on and also judgmental toward their trainees.
Amy, how would you respond to that? Was your experience similar in emergency medicine training?
Dr. Ho: I actually don’t feel like it was. I think what Júlia is alluding to is this “mean girls” idea, of “I went through it and thus you have to go through it.” I think you do see this in many specialties. One of the classic ones we hear about, and I don’t want to speak to it too much because it’s not my specialty, is ob.gyn., where it is a very female-dominant surgery group. There’s almost a hazing level that you hear about in some of the more malignant workplaces.
I think that you speak to two really important things. Number one is the numbers game. As you were saying, Brazil actually has many women. That’s awesome. That’s actually different from the United States, especially for the historic, existing workplace and less so for the medical students and for residents. I think step one is having minorities like women just present and there.
Step two is actually including and valuing them. While I think it’s really easy to move away from the women discussion, because there are women when you look around in medicine, it doesn’t mean that women are actually being heard, that they’re actually being accepted, or that their viewpoints are being listened to. A big part of it is normalizing not only seeing women in medicine but also normalizing the narrative of women in medicine.
It’s not just about motherhood; it’s about things like normalizing talking about advancement, academic promotions, pay, culture, being called things like “too reactive,” “anxious,” or “too assertive.” These are all classic things that we hear about when we talk about women.
That’s why we’re looking to not only conversations like this, but also structured ways for women to discuss being women in medicine. There are many women in medicine groups in emergency medicine, including: Females Working in Emergency Medicine (FemInEM); the American College of Emergency Physicians (ACEP) and Society for Academic Emergency Medicine (SAEM) women’s groups, which are American Association of Women Emergency Physicians (AAWEP) and Academy for Women in Academic Emergency Medicine (AWAEM), respectively; and the American Medical Women’s Association (AMWA), which is the American Medical Association’s offshoot.
All of these groups are geared toward normalizing women in medicine, normalizing the narrative of women in medicine, and then working on mentoring and educating so that we can advance our initiatives.
Gender balance is not gender equity
Dr. Glatter: Amy, you bring up a very critical point that mentoring is sort of the antidote to gender-based discrimination. Júlia had written a paper back in November of 2022 that was published in the Journal of Surgical Research talking exactly about this and how important it is to develop mentoring. Part of her research showed that about 20% of medical students who took the survey, about 1,000 people, had mentors, which was very disturbing.
Dr. Loyola Ferreira: Mentorship is one of the ways of changing the reality about gender-based discrimination. Amy’s comment was very strong and we need to really keep saying it, which is that gender balance is not gender equity.
The idea of having more women is not the same as women being recognized as equals, as able as men, and as valued as men. To change this very long culture of male domination, we need support, and this support comes from mentorship.
Although I didn’t have one, I feel that since I started being a mentor for some students, it changed not only them but myself. It gave me strength to keep going, studying, publishing, and going further with this discussion. I feel like the relationship was as good for them as it is for me. That’s how things change.
Diversity, equity, and inclusion training
Dr. Glatter: We’re talking about the reality of gender equity in terms of the ability to have equal respect, recognition, opportunities, and access. That’s really an important point to realize, and for our audience, to understand that gender equity is not gender balance.
Amy, I want to talk about medical school curriculums. Are there advances that you’re aware of being made at certain schools, programs, even in residencies, to enforce these things and make it a priority?
Dr. Ho: We’re really lucky that, as a culture in the United States, medical training is certainly very geared toward diversity. Some of that is certainly unofficial. Some of that just means when they’re looking at a medical school class or looking at rank lists for residency, that they’re cognizant of the different backgrounds that people have. That’s still a step. That is a step, that we’re at least acknowledging it.
There are multiple medical schools and residencies that have more formal unconscious-bias training or diversity, equity, and inclusion (DEI) training, both of which are excellent not only for us in the workplace but also for our patients. Almost all of us will see patients of highly diverse backgrounds. I think the biggest push is looking toward the criteria that we use for selecting trainees and students into our programs. Historically, it’s been MCAT, GPA, and so on.
We’ve really started to ask the question of, are these sorts of “objective criteria” actually biased in institutional ways? They talk about this all the time where GPAs will bias against students from underrepresented minorities (URM). I think all medical students and residencies have really acknowledged that. Although there are still test cutoffs, we are putting an inquisitive eye to what those mean, why they exist, and what are the other things that we should consider. This is all very heartening from what I’m seeing in medical training.
Dr. Glatter: There’s no formal rating system for DEI curriculums right now, like ranking of this school, or this program has more advanced recognition in terms of DEI?
Dr. Ho: No, but on the flip side, the U.S. News & World Report was classically one of the major rankings for medical schools. What we saw fairly recently was that very high-tier schools like Harvard and University of Chicago pulled out of that ranking because that ranking did not acknowledge the value of diversity. That was an incredible stance for medical schools to take, to say, “Hey, you are not evaluating an important criterion of ours.”
Dr. Glatter: That’s a great point. Júlia, where are we now in Brazil in terms of awareness of DEI and curriculum in schools and training programs?
Dr. Loyola Ferreira: Our reality is not as good as in the U.S., unfortunately. I don’t see much discussion on residency programs or medical schools at the moment. I see many students bringing it out and trying to make their schools engage in that discussion. This is something that is coming from the bottom up and not from the top down. I think it can lead to change as well. It is a step and it’s a beginning. Institutions should take the responsibility of doing this from the beginning. This is something where Brazil is still years behind you guys.
Dr. Glatter: It’s unfortunate, but certainly it’s important to hear that. What about in Canada and certainly your institution, McGill, where you just completed a master’s degree?
Dr. Loyola Ferreira: Canada is very much like the U.S. This is something that is really happening and it’s happening fast. I see, at least at McGill, a large amount of DEI inclusion and everything on this discussion. They have institutional courses for us to do as students, and we are all obliged to do many courses, which I think is really educating, especially for people with different cultures and backgrounds.
Dr. Glatter: Amy, where do you think we are in emergency medicine to look at the other side of it? Comparing surgery with emergency medicine, do you think we’re well advanced in terms of DEI, inclusion criteria, respect, and dignity, or are we really far off?
Dr. Ho: I may be biased, but I think emergency medicine is one of the best in terms of this, and I think there are a couple of reasons for it. One is that we are an inherently team-based organization. The attending, the residents, and the students all work in line with one another. There’s less of a hierarchy.
The same is true for our nurses, pharmacists, techs, and EMS. We all work together as a team. Because of that fairly flat structure, it’s really easy for us to value one another as individuals with our diverse backgrounds. In a way, that’s harder for specialties that are more hierarchical, and I think surgery is certainly one of the most hierarchical.
The second reason why emergency medicine is fairly well off in this is that we’re, by nature, a safety-net specialty. We see patients of all-comers, all walks, all backgrounds. I think we both recognize the value of physician-patient concordance. When we share characteristics with our patients, we recognize that value immediately at the bedside.
It exposes us to so much diversity. I see a refugee one day and the next patient is someone who is incarcerated. The next patient after that is an important businessman in society. That diversity and whiplash in the type of patients that we see back-to-back helps us see the playing field in a really flat, diverse way. Because of that, I think our culture is much better, as is our understanding of the value and importance of diversity not only for our programs, but also for our patients.
Do female doctors have better patient outcomes?
Dr. Glatter: Specialties working together in the emergency department is so important. Building that team and that togetherness is so critical. Júlia, would you agree?
Dr. Loyola Ferreira: Definitely. Something Amy said that is beautiful is that you recognize yourself in these patients. In surgery, we are taught to try to be away from the patients and not to put ourselves in the same position. We are taught to be less engaging, and this is not good. The good thing is when we really have patient-centered care, when we listen to them, and when we are involved with them.
I saw a publication showing that female and male surgeons treating similar patients had the same surgical outcomes. Women are as good as men technically to do surgery and have the same surgical outcomes. However, there is research showing that surgical teams with greater representation of women have improved surgical outcomes because of patient-centered care and the way women conduct bedside attention to patients. And they have better patient experience measures afterward. That is not only from the women who are treating the patients, but the whole environment. Women end up bringing men [into the conversation] and this better improves patient-centered care, and that makes the whole team a better team attending patients. Definitely, we are in the moment of patient experience and satisfaction, and increasing women is a way of achieving better patient satisfaction and experience.
Dr. Ho: There’s much to be said about having female clinicians available for patients. It doesn’t have to be just for female patients, although again, concordance between physicians and patients is certainly beneficial. Besides outcomes benefit, there’s even just a communication benefit. The way that women and men communicate is inherently different. The way women and men experience certain things is also inherently different.
A classic example of this is women who are experiencing a heart attack may not actually have chest pain but present with nausea. As a female who’s sensitive to this, when I see a woman throwing up, I am very attuned to something actually being wrong, knowing that they may not present with classic pain for a syndrome, but actually may be presenting with nausea instead. It doesn’t have to be a woman who takes that knowledge and turns it into something at the bedside. It certainly doesn’t have to, but it is just a natural, easy thing to step into as a female.
While I’m really careful to not step into this “women are better than men” or “men are better than women” argument, there’s something to be said about how the availability of female clinicians for all patients, not just female patients, can have benefit. Again, it’s shown in studies with cardiovascular outcomes and cardiologists, it’s certainly shown in ob.gyn., particularly for underrepresented minorities as well for maternal outcomes of Black mothers. It’s certainly shown again in patient satisfaction, which is concordance.
There is a profound level of research already on this that goes beyond just the idea of stacking the bench and putting more women in there. That’s not the value. We’re not just here to check off the box. We’re here to actually lend some value to our patients and, again, to one another as well.
Dr. Glatter: Absolutely. These are excellent points. The point you make about patient presentation is so vital. The fact that women have nausea sometimes in ACS presentations, the research never was really attentive to this. It was biased. The symptoms that women may have that are not “typical” for ACS weren’t included in patient presentations. Educating everyone about, overall, the types of presentations that we can recognize is vital and important.
Dr. Ho: Yes. It’s worth saying that, when you look at how medicine and research developed, classically, who were the research participants? They were often White men. They were college students who, historically, because women were not allowed to go to college, were men.
I say that not to fault the institution, because that was the culture of our history, but to just say it is okay to question things. It is okay to realize that someone’s presenting outside of the box and that maybe we actually need to reframe what even created the walls of the box in the first place.
Dr. Glatter: Thank you again for joining us. I truly appreciate your insight and expertise.
Dr. Glatter is assistant professor of emergency medicine, department of emergency medicine, Hofstra/Northwell, New York. Dr. Ho is senior vice president of clinical informatics & analytics, department of emergency medicine, Integrative Emergency Services, Dallas. Dr. Loyola Ferreira is a master of science candidate, department of experimental surgery, McGill University, Montreal. They reported that they had no conflicts of interest.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Robert D. Glatter, MD: Welcome. I’m Dr. Robert Glatter, medical adviser for Medscape Emergency Medicine. Joining me to discuss ways to address and reform the toxic culture associated with medical training is Dr. Amy Faith Ho, senior vice president of clinical informatics and analytics at Integrative Emergency Services in Dallas. Also joining us is Dr. Júlia Loyola Ferreira, a pediatric surgeon originally from Brazil, now practicing at Montreal Children’s and focused on advocacy for gender equity and patient-centered care.
Welcome to both of you. Thanks so much for joining me.
Amy Faith Ho, MD, MPH: Thanks so much for having us, Rob.
Dr. Glatter: Amy, I noticed a tweet recently where you talked about how your career choice was affected by the toxic environment in medical school, affecting your choice of residency. Can you elaborate on that?
Dr. Ho: In this instance, what we’re talking about is gender, but it can be directed toward any number of other groups as well.
What you’re alluding to is a tweet by Stanford Surgery Group showing the next residency class, and what was really stunning about this residency class was that it was almost all females. And this was something that took off on social media.
When I saw this, I was really brought back to one of my personal experiences that I chose to share, which was basically that, as a medical student, I really wanted to be a surgeon. I’m an emergency medicine doctor now, so you know that didn’t happen.
The story that I was sharing was that when I was a third-year medical student rotating on surgery, we had a male attending who was very well known at that school at the time who basically would take the female medical students, and instead of clinic, he would round us up. He would have us sit around him in the workplace room while everyone else was seeing patients, and he would have you look at news clippings of himself. He would tell you stories about himself, like he was holding court for the ladies.
It was this very weird culture where my takeaway as a med student was like, “Wow, this is kind of abusive patriarchy that is supported,” because everyone knew about it and was complicit. Even though I really liked surgery, this was just one instance and one example of where you see this culture that really resonates into the rest of life that I didn’t really want to be a part of.
I went into emergency medicine and loved it. It’s also highly procedural, and I was very happy with where I was. What was really interesting about this tweet to me, though, is that it really took off and garnered hundreds of thousands of views on a very niche topic, because what was most revealing is that everyone has a story like this.
It is not just surgery. It is definitely not just one specialty and it is not just one school. It is an endemic problem in medicine. Not only does it change the lives of young women, but it also says so much about the complicity and the culture that we have in medicine that many people were upset about just the same way I was.
Medical training experience in other countries vs. the United States
Dr. Glatter: Júlia, I want to hear about your experience in medical school, surgery, and then fellowship training and up to the present, if possible.
Júlia Loyola Ferreira, MD: In Brazil, as in many countries now, women have made up the majority of the medical students since 2010. It’s a more female-friendly environment when you’re going through medical school, and I was lucky enough to do rotations in areas of surgery where people were friendly to women.
I lived in this tiny bubble that also gave me the privilege of not facing some things that I can imagine that people in Brazil in different areas and smaller towns face. In Brazil, people try to not talk about this gender agenda. This is something that’s being talked about outside Brazil. But in Brazil, we are years back. People are not really engaging on this conversation. I thought it was going to be hard for me as a woman, because Brazil has around 20% female surgeons.
I knew it was going to be challenging, but I had no idea how bad it was. When I started and things started happening, the list was big. I have an example of everything that is written about – microaggression, implicit bias, discrimination, harassment.
Every time I would try to speak about it and talk to someone, I would be strongly gaslighted. It was the whole training, the whole 5 years. People would say, “Oh, I don’t think it was like that. I think you were overreacting.” People would come with all these different answers for what I was experiencing, and that was frustrating. That was even harder because I had to cope with everything that was happening and I had no one to turn to. I had no mentors.
When I looked up to women who were in surgery, they would be tougher on us young surgeons than the men and they would tell us that we should not complain because in their time it was even harder. Now, it’s getting better and we are supposed to accept whatever comes.
That was at least a little bit of what I experienced in my training. It was only after I finished and started to do research about it that I really encountered a field of people who would echo what I was trying to say to many people in different hospitals that I attended to.
That was the key for me to get out of that situation of being gaslighted and of not being able to really talk about it. Suddenly, I started to publish things about Brazil that nobody was even writing or studying. That gave me a large amount of responsibility, but also motivation to keep going and to see the change.
Valuing women in medicine
Dr. Glatter: This is a very important point that you’re raising about the environment of women being hard on other women. We know that men can be very difficult on and also judgmental toward their trainees.
Amy, how would you respond to that? Was your experience similar in emergency medicine training?
Dr. Ho: I actually don’t feel like it was. I think what Júlia is alluding to is this “mean girls” idea, of “I went through it and thus you have to go through it.” I think you do see this in many specialties. One of the classic ones we hear about, and I don’t want to speak to it too much because it’s not my specialty, is ob.gyn., where it is a very female-dominant surgery group. There’s almost a hazing level that you hear about in some of the more malignant workplaces.
I think that you speak to two really important things. Number one is the numbers game. As you were saying, Brazil actually has many women. That’s awesome. That’s actually different from the United States, especially for the historic, existing workplace and less so for the medical students and for residents. I think step one is having minorities like women just present and there.
Step two is actually including and valuing them. While I think it’s really easy to move away from the women discussion, because there are women when you look around in medicine, it doesn’t mean that women are actually being heard, that they’re actually being accepted, or that their viewpoints are being listened to. A big part of it is normalizing not only seeing women in medicine but also normalizing the narrative of women in medicine.
It’s not just about motherhood; it’s about things like normalizing talking about advancement, academic promotions, pay, culture, being called things like “too reactive,” “anxious,” or “too assertive.” These are all classic things that we hear about when we talk about women.
That’s why we’re looking to not only conversations like this, but also structured ways for women to discuss being women in medicine. There are many women in medicine groups in emergency medicine, including: Females Working in Emergency Medicine (FemInEM); the American College of Emergency Physicians (ACEP) and Society for Academic Emergency Medicine (SAEM) women’s groups, which are American Association of Women Emergency Physicians (AAWEP) and Academy for Women in Academic Emergency Medicine (AWAEM), respectively; and the American Medical Women’s Association (AMWA), which is the American Medical Association’s offshoot.
All of these groups are geared toward normalizing women in medicine, normalizing the narrative of women in medicine, and then working on mentoring and educating so that we can advance our initiatives.
Gender balance is not gender equity
Dr. Glatter: Amy, you bring up a very critical point that mentoring is sort of the antidote to gender-based discrimination. Júlia had written a paper back in November of 2022 that was published in the Journal of Surgical Research talking exactly about this and how important it is to develop mentoring. Part of her research showed that about 20% of medical students who took the survey, about 1,000 people, had mentors, which was very disturbing.
Dr. Loyola Ferreira: Mentorship is one of the ways of changing the reality about gender-based discrimination. Amy’s comment was very strong and we need to really keep saying it, which is that gender balance is not gender equity.
The idea of having more women is not the same as women being recognized as equals, as able as men, and as valued as men. To change this very long culture of male domination, we need support, and this support comes from mentorship.
Although I didn’t have one, I feel that since I started being a mentor for some students, it changed not only them but myself. It gave me strength to keep going, studying, publishing, and going further with this discussion. I feel like the relationship was as good for them as it is for me. That’s how things change.
Diversity, equity, and inclusion training
Dr. Glatter: We’re talking about the reality of gender equity in terms of the ability to have equal respect, recognition, opportunities, and access. That’s really an important point to realize, and for our audience, to understand that gender equity is not gender balance.
Amy, I want to talk about medical school curriculums. Are there advances that you’re aware of being made at certain schools, programs, even in residencies, to enforce these things and make it a priority?
Dr. Ho: We’re really lucky that, as a culture in the United States, medical training is certainly very geared toward diversity. Some of that is certainly unofficial. Some of that just means when they’re looking at a medical school class or looking at rank lists for residency, that they’re cognizant of the different backgrounds that people have. That’s still a step. That is a step, that we’re at least acknowledging it.
There are multiple medical schools and residencies that have more formal unconscious-bias training or diversity, equity, and inclusion (DEI) training, both of which are excellent not only for us in the workplace but also for our patients. Almost all of us will see patients of highly diverse backgrounds. I think the biggest push is looking toward the criteria that we use for selecting trainees and students into our programs. Historically, it’s been MCAT, GPA, and so on.
We’ve really started to ask the question of, are these sorts of “objective criteria” actually biased in institutional ways? They talk about this all the time where GPAs will bias against students from underrepresented minorities (URM). I think all medical students and residencies have really acknowledged that. Although there are still test cutoffs, we are putting an inquisitive eye to what those mean, why they exist, and what are the other things that we should consider. This is all very heartening from what I’m seeing in medical training.
Dr. Glatter: There’s no formal rating system for DEI curriculums right now, like ranking of this school, or this program has more advanced recognition in terms of DEI?
Dr. Ho: No, but on the flip side, the U.S. News & World Report was classically one of the major rankings for medical schools. What we saw fairly recently was that very high-tier schools like Harvard and University of Chicago pulled out of that ranking because that ranking did not acknowledge the value of diversity. That was an incredible stance for medical schools to take, to say, “Hey, you are not evaluating an important criterion of ours.”
Dr. Glatter: That’s a great point. Júlia, where are we now in Brazil in terms of awareness of DEI and curriculum in schools and training programs?
Dr. Loyola Ferreira: Our reality is not as good as in the U.S., unfortunately. I don’t see much discussion on residency programs or medical schools at the moment. I see many students bringing it out and trying to make their schools engage in that discussion. This is something that is coming from the bottom up and not from the top down. I think it can lead to change as well. It is a step and it’s a beginning. Institutions should take the responsibility of doing this from the beginning. This is something where Brazil is still years behind you guys.
Dr. Glatter: It’s unfortunate, but certainly it’s important to hear that. What about in Canada and certainly your institution, McGill, where you just completed a master’s degree?
Dr. Loyola Ferreira: Canada is very much like the U.S. This is something that is really happening and it’s happening fast. I see, at least at McGill, a large amount of DEI inclusion and everything on this discussion. They have institutional courses for us to do as students, and we are all obliged to do many courses, which I think is really educating, especially for people with different cultures and backgrounds.
Dr. Glatter: Amy, where do you think we are in emergency medicine to look at the other side of it? Comparing surgery with emergency medicine, do you think we’re well advanced in terms of DEI, inclusion criteria, respect, and dignity, or are we really far off?
Dr. Ho: I may be biased, but I think emergency medicine is one of the best in terms of this, and I think there are a couple of reasons for it. One is that we are an inherently team-based organization. The attending, the residents, and the students all work in line with one another. There’s less of a hierarchy.
The same is true for our nurses, pharmacists, techs, and EMS. We all work together as a team. Because of that fairly flat structure, it’s really easy for us to value one another as individuals with our diverse backgrounds. In a way, that’s harder for specialties that are more hierarchical, and I think surgery is certainly one of the most hierarchical.
The second reason why emergency medicine is fairly well off in this is that we’re, by nature, a safety-net specialty. We see patients of all-comers, all walks, all backgrounds. I think we both recognize the value of physician-patient concordance. When we share characteristics with our patients, we recognize that value immediately at the bedside.
It exposes us to so much diversity. I see a refugee one day and the next patient is someone who is incarcerated. The next patient after that is an important businessman in society. That diversity and whiplash in the type of patients that we see back-to-back helps us see the playing field in a really flat, diverse way. Because of that, I think our culture is much better, as is our understanding of the value and importance of diversity not only for our programs, but also for our patients.
Do female doctors have better patient outcomes?
Dr. Glatter: Specialties working together in the emergency department is so important. Building that team and that togetherness is so critical. Júlia, would you agree?
Dr. Loyola Ferreira: Definitely. Something Amy said that is beautiful is that you recognize yourself in these patients. In surgery, we are taught to try to be away from the patients and not to put ourselves in the same position. We are taught to be less engaging, and this is not good. The good thing is when we really have patient-centered care, when we listen to them, and when we are involved with them.
I saw a publication showing that female and male surgeons treating similar patients had the same surgical outcomes. Women are as good as men technically to do surgery and have the same surgical outcomes. However, there is research showing that surgical teams with greater representation of women have improved surgical outcomes because of patient-centered care and the way women conduct bedside attention to patients. And they have better patient experience measures afterward. That is not only from the women who are treating the patients, but the whole environment. Women end up bringing men [into the conversation] and this better improves patient-centered care, and that makes the whole team a better team attending patients. Definitely, we are in the moment of patient experience and satisfaction, and increasing women is a way of achieving better patient satisfaction and experience.
Dr. Ho: There’s much to be said about having female clinicians available for patients. It doesn’t have to be just for female patients, although again, concordance between physicians and patients is certainly beneficial. Besides outcomes benefit, there’s even just a communication benefit. The way that women and men communicate is inherently different. The way women and men experience certain things is also inherently different.
A classic example of this is women who are experiencing a heart attack may not actually have chest pain but present with nausea. As a female who’s sensitive to this, when I see a woman throwing up, I am very attuned to something actually being wrong, knowing that they may not present with classic pain for a syndrome, but actually may be presenting with nausea instead. It doesn’t have to be a woman who takes that knowledge and turns it into something at the bedside. It certainly doesn’t have to, but it is just a natural, easy thing to step into as a female.
While I’m really careful to not step into this “women are better than men” or “men are better than women” argument, there’s something to be said about how the availability of female clinicians for all patients, not just female patients, can have benefit. Again, it’s shown in studies with cardiovascular outcomes and cardiologists, it’s certainly shown in ob.gyn., particularly for underrepresented minorities as well for maternal outcomes of Black mothers. It’s certainly shown again in patient satisfaction, which is concordance.
There is a profound level of research already on this that goes beyond just the idea of stacking the bench and putting more women in there. That’s not the value. We’re not just here to check off the box. We’re here to actually lend some value to our patients and, again, to one another as well.
Dr. Glatter: Absolutely. These are excellent points. The point you make about patient presentation is so vital. The fact that women have nausea sometimes in ACS presentations, the research never was really attentive to this. It was biased. The symptoms that women may have that are not “typical” for ACS weren’t included in patient presentations. Educating everyone about, overall, the types of presentations that we can recognize is vital and important.
Dr. Ho: Yes. It’s worth saying that, when you look at how medicine and research developed, classically, who were the research participants? They were often White men. They were college students who, historically, because women were not allowed to go to college, were men.
I say that not to fault the institution, because that was the culture of our history, but to just say it is okay to question things. It is okay to realize that someone’s presenting outside of the box and that maybe we actually need to reframe what even created the walls of the box in the first place.
Dr. Glatter: Thank you again for joining us. I truly appreciate your insight and expertise.
Dr. Glatter is assistant professor of emergency medicine, department of emergency medicine, Hofstra/Northwell, New York. Dr. Ho is senior vice president of clinical informatics & analytics, department of emergency medicine, Integrative Emergency Services, Dallas. Dr. Loyola Ferreira is a master of science candidate, department of experimental surgery, McGill University, Montreal. They reported that they had no conflicts of interest.
A version of this article first appeared on Medscape.com.
Does weight loss surgery up the risk for bone fractures?
Currently, the two most common types of weight loss surgery performed include sleeve gastrectomy and Roux-en-Y gastric bypass (RYGB). Sleeve gastrectomy involves removing a large portion of the stomach so that its capacity is significantly decreased (to about 20%), reducing the ability to consume large quantities of food. Also, the procedure leads to marked reductions in ghrelin (an appetite-stimulating hormone), and some studies have reported increases in glucagon-like peptide 1 (GLP-1) and peptide YY (PYY), hormones that induce satiety. Gastric bypass involves creating a small stomach pouch and rerouting the small intestine so that it bypasses much of the stomach and also the upper portion of the small intestine. This reduces the amount of food that can be consumed at any time, increases levels of GLP-1 and PYY, and reduces absorption of nutrients with resultant weight loss. Less common bariatric surgeries include gastric banding and biliopancreatic diversion with duodenal switch (BPD-DS). Gastric banding involves placing a ring in the upper portion of the stomach, and the size of the pouch created can be altered by injecting more or less saline through a port inserted under the skin. BPD-DS includes sleeve gastrectomy, resection of a large section of the small intestine, and diversion of the pancreatic and biliary duct to a point below the junction of the ends of the resected gut.
Weight loss surgery is currently recommended for people who have a body mass index greater than or equal to 35 regardless of obesity-related complication and may be considered for those with a BMI greater than or equal to 30. BMI is calculated by dividing the weight (in kilograms) by the height (in meters). In children and adolescents, weight loss surgery should be considered in those with a BMI greater than 120% of the 95th percentile and with a major comorbidity or in those with a BMI greater than 140% of the 95th percentile.
What impact does weight loss surgery have on bone?
Multiple studies in both adults and teenagers have demonstrated that sleeve gastrectomy, RYGB, and BPD-DS (but not gastric banding) are associated with a decrease in bone density, impaired bone structure, and reduced strength estimates over time (Beavers et al; Gagnon, Schafer; Misra, Bredella). The relative risk for fracture after RYGB and BPD-DS is reported to be 1.2-2.3 (that is, 20%-130% more than normal), whereas fracture risk after sleeve gastrectomy is still under study with some conflicting results. Fracture risk starts to increase 2-3 years after surgery and peaks at 5-plus years after surgery. Most of the data for fractures come from studies in adults. With the rising use of weight loss surgery, particularly sleeve gastrectomy, in teenagers, studies are needed to determine fracture risk in this younger age group, who also seem to experience marked reductions in bone density, altered bone structure, and reduced bone strength after bariatric surgery.
What contributes to impaired bone health after weight loss surgery?
The deleterious effect of weight loss surgery on bone appears to be caused by various factors, including the massive and rapid weight loss that occurs after surgery, because body weight has a mechanical loading effect on bone and otherwise promotes bone formation. Weight loss results in mechanical unloading and thus a decrease in bone density. Further, when weight loss occurs, there is loss of both muscle and fat mass, and the reduction in muscle mass is deleterious to bone.
Other possible causes of bone density reduction include reduced absorption of certain nutrients, such as calcium and vitamin D critical for bone mineralization, and alterations in certain hormones that impact bone health. These include increases in parathyroid hormone, which increases bone loss when secreted in excess; increases in PYY (a hormone that reduces bone formation); decreases in ghrelin (a hormone that typically increases bone formation), particularly after sleeve gastrectomy; and decreases in estrone (a kind of estrogen that like other estrogens prevents bone loss). Further, age and gender may modify the bone consequences of surgery as outcomes in postmenopausal women appear to be worse than in younger women and men.
Preventing bone density loss
Given the many benefits of weight loss surgery, what can we do to prevent this decrease in bone density after surgery? It’s important for people undergoing weight loss surgery to be cognizant of this potentially negative outcome and to take appropriate precautions to mitigate this concern.
We should monitor bone density after surgery with the help of dual energy x-ray absorptiometry, starting a few years after surgery, particularly in those who are at greatest risk for fracture, so that we can be proactive about addressing any severe bone loss that warrants pharmacologic intervention.
More general recommendations include optimizing intake of calcium (1,200-1,500 mg/d), vitamin D (2,000-3,000 IUs/d), and protein (60-75 g/d) via diet and/or as supplements and engaging in weight-bearing physical activity because this exerts mechanical loading effects on the skeleton leading to increased bone formation and also increases muscle mass over time, which is beneficial to bone. A progressive resistance training program has been demonstrated to have beneficial effects on bone, and measures should be taken to reduce the risk for falls, which increases after certain kinds of weight loss surgery, such as gastric bypass.
Meeting with a dietitian can help determine any other nutrients that need to be optimized.
Though many hormonal changes after surgery have been linked to reductions in bone density, there are still no recommended hormonal therapies at this time, and more work is required to determine whether specific pharmacologic therapies might help improve bone outcomes after surgery.
Dr. Misra is chief of the division of pediatric endocrinology, Mass General for Children; associate director, Harvard Catalyst Translation and Clinical Research Center; director, Pediatric Endocrine-Sports Endocrine-Neuroendocrine Lab, Mass General Hospital; and professor, department of pediatrics, Harvard Medical School, Boston.
A version of this article originally appeared on Medscape.com.
Currently, the two most common types of weight loss surgery performed include sleeve gastrectomy and Roux-en-Y gastric bypass (RYGB). Sleeve gastrectomy involves removing a large portion of the stomach so that its capacity is significantly decreased (to about 20%), reducing the ability to consume large quantities of food. Also, the procedure leads to marked reductions in ghrelin (an appetite-stimulating hormone), and some studies have reported increases in glucagon-like peptide 1 (GLP-1) and peptide YY (PYY), hormones that induce satiety. Gastric bypass involves creating a small stomach pouch and rerouting the small intestine so that it bypasses much of the stomach and also the upper portion of the small intestine. This reduces the amount of food that can be consumed at any time, increases levels of GLP-1 and PYY, and reduces absorption of nutrients with resultant weight loss. Less common bariatric surgeries include gastric banding and biliopancreatic diversion with duodenal switch (BPD-DS). Gastric banding involves placing a ring in the upper portion of the stomach, and the size of the pouch created can be altered by injecting more or less saline through a port inserted under the skin. BPD-DS includes sleeve gastrectomy, resection of a large section of the small intestine, and diversion of the pancreatic and biliary duct to a point below the junction of the ends of the resected gut.
Weight loss surgery is currently recommended for people who have a body mass index greater than or equal to 35 regardless of obesity-related complication and may be considered for those with a BMI greater than or equal to 30. BMI is calculated by dividing the weight (in kilograms) by the height (in meters). In children and adolescents, weight loss surgery should be considered in those with a BMI greater than 120% of the 95th percentile and with a major comorbidity or in those with a BMI greater than 140% of the 95th percentile.
What impact does weight loss surgery have on bone?
Multiple studies in both adults and teenagers have demonstrated that sleeve gastrectomy, RYGB, and BPD-DS (but not gastric banding) are associated with a decrease in bone density, impaired bone structure, and reduced strength estimates over time (Beavers et al; Gagnon, Schafer; Misra, Bredella). The relative risk for fracture after RYGB and BPD-DS is reported to be 1.2-2.3 (that is, 20%-130% more than normal), whereas fracture risk after sleeve gastrectomy is still under study with some conflicting results. Fracture risk starts to increase 2-3 years after surgery and peaks at 5-plus years after surgery. Most of the data for fractures come from studies in adults. With the rising use of weight loss surgery, particularly sleeve gastrectomy, in teenagers, studies are needed to determine fracture risk in this younger age group, who also seem to experience marked reductions in bone density, altered bone structure, and reduced bone strength after bariatric surgery.
What contributes to impaired bone health after weight loss surgery?
The deleterious effect of weight loss surgery on bone appears to be caused by various factors, including the massive and rapid weight loss that occurs after surgery, because body weight has a mechanical loading effect on bone and otherwise promotes bone formation. Weight loss results in mechanical unloading and thus a decrease in bone density. Further, when weight loss occurs, there is loss of both muscle and fat mass, and the reduction in muscle mass is deleterious to bone.
Other possible causes of bone density reduction include reduced absorption of certain nutrients, such as calcium and vitamin D critical for bone mineralization, and alterations in certain hormones that impact bone health. These include increases in parathyroid hormone, which increases bone loss when secreted in excess; increases in PYY (a hormone that reduces bone formation); decreases in ghrelin (a hormone that typically increases bone formation), particularly after sleeve gastrectomy; and decreases in estrone (a kind of estrogen that like other estrogens prevents bone loss). Further, age and gender may modify the bone consequences of surgery as outcomes in postmenopausal women appear to be worse than in younger women and men.
Preventing bone density loss
Given the many benefits of weight loss surgery, what can we do to prevent this decrease in bone density after surgery? It’s important for people undergoing weight loss surgery to be cognizant of this potentially negative outcome and to take appropriate precautions to mitigate this concern.
We should monitor bone density after surgery with the help of dual energy x-ray absorptiometry, starting a few years after surgery, particularly in those who are at greatest risk for fracture, so that we can be proactive about addressing any severe bone loss that warrants pharmacologic intervention.
More general recommendations include optimizing intake of calcium (1,200-1,500 mg/d), vitamin D (2,000-3,000 IUs/d), and protein (60-75 g/d) via diet and/or as supplements and engaging in weight-bearing physical activity because this exerts mechanical loading effects on the skeleton leading to increased bone formation and also increases muscle mass over time, which is beneficial to bone. A progressive resistance training program has been demonstrated to have beneficial effects on bone, and measures should be taken to reduce the risk for falls, which increases after certain kinds of weight loss surgery, such as gastric bypass.
Meeting with a dietitian can help determine any other nutrients that need to be optimized.
Though many hormonal changes after surgery have been linked to reductions in bone density, there are still no recommended hormonal therapies at this time, and more work is required to determine whether specific pharmacologic therapies might help improve bone outcomes after surgery.
Dr. Misra is chief of the division of pediatric endocrinology, Mass General for Children; associate director, Harvard Catalyst Translation and Clinical Research Center; director, Pediatric Endocrine-Sports Endocrine-Neuroendocrine Lab, Mass General Hospital; and professor, department of pediatrics, Harvard Medical School, Boston.
A version of this article originally appeared on Medscape.com.
Currently, the two most common types of weight loss surgery performed include sleeve gastrectomy and Roux-en-Y gastric bypass (RYGB). Sleeve gastrectomy involves removing a large portion of the stomach so that its capacity is significantly decreased (to about 20%), reducing the ability to consume large quantities of food. Also, the procedure leads to marked reductions in ghrelin (an appetite-stimulating hormone), and some studies have reported increases in glucagon-like peptide 1 (GLP-1) and peptide YY (PYY), hormones that induce satiety. Gastric bypass involves creating a small stomach pouch and rerouting the small intestine so that it bypasses much of the stomach and also the upper portion of the small intestine. This reduces the amount of food that can be consumed at any time, increases levels of GLP-1 and PYY, and reduces absorption of nutrients with resultant weight loss. Less common bariatric surgeries include gastric banding and biliopancreatic diversion with duodenal switch (BPD-DS). Gastric banding involves placing a ring in the upper portion of the stomach, and the size of the pouch created can be altered by injecting more or less saline through a port inserted under the skin. BPD-DS includes sleeve gastrectomy, resection of a large section of the small intestine, and diversion of the pancreatic and biliary duct to a point below the junction of the ends of the resected gut.
Weight loss surgery is currently recommended for people who have a body mass index greater than or equal to 35 regardless of obesity-related complication and may be considered for those with a BMI greater than or equal to 30. BMI is calculated by dividing the weight (in kilograms) by the height (in meters). In children and adolescents, weight loss surgery should be considered in those with a BMI greater than 120% of the 95th percentile and with a major comorbidity or in those with a BMI greater than 140% of the 95th percentile.
What impact does weight loss surgery have on bone?
Multiple studies in both adults and teenagers have demonstrated that sleeve gastrectomy, RYGB, and BPD-DS (but not gastric banding) are associated with a decrease in bone density, impaired bone structure, and reduced strength estimates over time (Beavers et al; Gagnon, Schafer; Misra, Bredella). The relative risk for fracture after RYGB and BPD-DS is reported to be 1.2-2.3 (that is, 20%-130% more than normal), whereas fracture risk after sleeve gastrectomy is still under study with some conflicting results. Fracture risk starts to increase 2-3 years after surgery and peaks at 5-plus years after surgery. Most of the data for fractures come from studies in adults. With the rising use of weight loss surgery, particularly sleeve gastrectomy, in teenagers, studies are needed to determine fracture risk in this younger age group, who also seem to experience marked reductions in bone density, altered bone structure, and reduced bone strength after bariatric surgery.
What contributes to impaired bone health after weight loss surgery?
The deleterious effect of weight loss surgery on bone appears to be caused by various factors, including the massive and rapid weight loss that occurs after surgery, because body weight has a mechanical loading effect on bone and otherwise promotes bone formation. Weight loss results in mechanical unloading and thus a decrease in bone density. Further, when weight loss occurs, there is loss of both muscle and fat mass, and the reduction in muscle mass is deleterious to bone.
Other possible causes of bone density reduction include reduced absorption of certain nutrients, such as calcium and vitamin D critical for bone mineralization, and alterations in certain hormones that impact bone health. These include increases in parathyroid hormone, which increases bone loss when secreted in excess; increases in PYY (a hormone that reduces bone formation); decreases in ghrelin (a hormone that typically increases bone formation), particularly after sleeve gastrectomy; and decreases in estrone (a kind of estrogen that like other estrogens prevents bone loss). Further, age and gender may modify the bone consequences of surgery as outcomes in postmenopausal women appear to be worse than in younger women and men.
Preventing bone density loss
Given the many benefits of weight loss surgery, what can we do to prevent this decrease in bone density after surgery? It’s important for people undergoing weight loss surgery to be cognizant of this potentially negative outcome and to take appropriate precautions to mitigate this concern.
We should monitor bone density after surgery with the help of dual energy x-ray absorptiometry, starting a few years after surgery, particularly in those who are at greatest risk for fracture, so that we can be proactive about addressing any severe bone loss that warrants pharmacologic intervention.
More general recommendations include optimizing intake of calcium (1,200-1,500 mg/d), vitamin D (2,000-3,000 IUs/d), and protein (60-75 g/d) via diet and/or as supplements and engaging in weight-bearing physical activity because this exerts mechanical loading effects on the skeleton leading to increased bone formation and also increases muscle mass over time, which is beneficial to bone. A progressive resistance training program has been demonstrated to have beneficial effects on bone, and measures should be taken to reduce the risk for falls, which increases after certain kinds of weight loss surgery, such as gastric bypass.
Meeting with a dietitian can help determine any other nutrients that need to be optimized.
Though many hormonal changes after surgery have been linked to reductions in bone density, there are still no recommended hormonal therapies at this time, and more work is required to determine whether specific pharmacologic therapies might help improve bone outcomes after surgery.
Dr. Misra is chief of the division of pediatric endocrinology, Mass General for Children; associate director, Harvard Catalyst Translation and Clinical Research Center; director, Pediatric Endocrine-Sports Endocrine-Neuroendocrine Lab, Mass General Hospital; and professor, department of pediatrics, Harvard Medical School, Boston.
A version of this article originally appeared on Medscape.com.
Menopause and long COVID: What women should know
British researchers have noted that women at midlife who have long COVID seem to get specific, and severe, symptoms, including brain fog, fatigue, new-onset dizziness, and difficulty sleeping through the night.
Doctors also think it’s possible that long COVID worsens the symptoms of perimenopause and menopause. Lower levels of estrogen and testosterone appear to be the reason.
“A long COVID theory is that there is a temporary disruption to physiological ovarian steroid hormone production, which could [worsen] symptoms of perimenopause and menopause,” said JoAnn V. Pinkerton, MD, professor of obstetrics at the University of Virginia, Charlottesville, and executive director of the North American Menopause Society.
Long COVID symptoms and menopause symptoms can also be very hard to tell apart.
Another U.K. study cautions that because of this kind of symptom overlap, women at midlife may be misdiagnosed. Research from the North American Menopause Society shows that many women may have trouble recovering from long COVID unless their hormone deficiency is treated.
What are the symptoms of long COVID?
There are over 200 symptoms that have been associated with long COVID, according to the American Medical Association. Some common symptoms are currently defined as the following: feeling extremely tired, feeling depleted after exertion, cognitive issues such as brain fog, heart beating over 100 times a minute, and a loss of sense of smell and taste.
Long COVID symptoms begin a few weeks to a few months after a COVID infection. They can last an indefinite amount of time, but “the hope is that long COVID will not be lifelong,” said Clare Flannery, MD, an endocrinologist and associate professor in the departments of obstetrics, gynecology and reproductive sciences and internal medicine at Yale University, New Haven, Conn.
What are the symptoms of menopause?
Some symptoms of menopause include vaginal infections, irregular bleeding, urinary problems, and sexual problems.
Women in their middle years have other symptoms that can be the same as perimenopause/menopause symptoms.
“Common symptoms of perimenopause and menopause which may also be symptoms ascribed to long COVID include hot flashes, night sweats, disrupted sleep, low mood, depression or anxiety, decreased concentration, memory problems, joint and muscle pains, and headaches,” Dr. Pinkerton said.
Can long COVID actually bring on menopause?
In short: Possibly.
A new study from the Massachusetts Institute of Technology/Patient-Led Research Collaborative/University of California, San Francisco, found that long COVID can cause disruptions to a woman’s menstrual cycle, ovaries, fertility, and menopause itself.
This could be caused by chronic inflammation caused by long COVID on hormones as well. This kind of inflammatory response could explain irregularities in a woman’s menstrual cycle, according to the Newson Health Research and Education study. For instance, “when the body has inflammation, ovulation can happen,” Dr. Flannery said.
The mechanism for how long COVID could spur menopause can also involve a woman’s ovaries.
“Since the theory is that COVID affects the ovary with declines in ovarian reserve and ovarian function, it makes sense that long COVID could bring on symptoms of perimenopause or menopause more acutely or more severely and lengthen the symptoms of the perimenopause and menopausal transition,” Dr. Pinkerton said.
How can hormone replacement therapy benefit women dealing with long COVID during menopause?
Estradiol, the strongest estrogen hormone in a woman’s body, has already been shown to have a positive effect against COVID.
“Estradiol therapy treats symptoms more aggressively in the setting of long COVID,” said Dr. Flannery.
Estradiol is also a form of hormone therapy for menopause symptoms.
“Estradiol has been shown to help hot flashes, night sweats, and sleep and improve mood during perimenopause,” said Dr. Pinkerton. “So it’s likely that perimenopausal or menopausal women with long COVID would see improvements both due to the action of estradiol on the ovary seen during COVID and the improvements in symptoms.”
Estrogen-based hormone therapy has been linked to an increased risk for endometrial, breast, and ovarian cancer, according to the American Cancer Society. This means you should carefully consider how comfortable you are with those additional risks before starting this kind of therapy.
“Which of your symptoms are the most difficult to manage? You may see if you can navigate one to three of them. What are you willing to do for your symptoms? If a woman is willing to favor her sleep for the next 6 months to a year, she may be willing to change how she perceives her risk for cancer,” Dr. Flannery said. “What risk is a woman willing to take? I think if someone has a very low concern about a risk of cancer, and she’s suffering a disrupted life, then taking estradiol in a 1- to 2-year trial period could be critical to help.”
What else can help ease long COVID during menopause?
Getting the COVID vaccine, as well as getting a booster, could help. Not only will this help prevent people from being reinfected with COVID, which can worsen symptoms, but a new Swedish study says there is no evidence that it will cause postmenopausal problems like irregular bleeding.
“Weak and inconsistent associations were observed between SARS-CoV-2 vaccination and healthcare contacts for bleeding in women who are postmenopausal, and even less evidence was recorded of an association for menstrual disturbance or bleeding in women who were premenopausal,” said study coauthor Rickard Ljung, MD, PhD, MPH, professor and acting head of the pharmacoepidemiology and analysis department in the division of use and information of the Swedish Medical Products Agency in Uppsala.
A version of this article first appeared on WebMD.com.
British researchers have noted that women at midlife who have long COVID seem to get specific, and severe, symptoms, including brain fog, fatigue, new-onset dizziness, and difficulty sleeping through the night.
Doctors also think it’s possible that long COVID worsens the symptoms of perimenopause and menopause. Lower levels of estrogen and testosterone appear to be the reason.
“A long COVID theory is that there is a temporary disruption to physiological ovarian steroid hormone production, which could [worsen] symptoms of perimenopause and menopause,” said JoAnn V. Pinkerton, MD, professor of obstetrics at the University of Virginia, Charlottesville, and executive director of the North American Menopause Society.
Long COVID symptoms and menopause symptoms can also be very hard to tell apart.
Another U.K. study cautions that because of this kind of symptom overlap, women at midlife may be misdiagnosed. Research from the North American Menopause Society shows that many women may have trouble recovering from long COVID unless their hormone deficiency is treated.
What are the symptoms of long COVID?
There are over 200 symptoms that have been associated with long COVID, according to the American Medical Association. Some common symptoms are currently defined as the following: feeling extremely tired, feeling depleted after exertion, cognitive issues such as brain fog, heart beating over 100 times a minute, and a loss of sense of smell and taste.
Long COVID symptoms begin a few weeks to a few months after a COVID infection. They can last an indefinite amount of time, but “the hope is that long COVID will not be lifelong,” said Clare Flannery, MD, an endocrinologist and associate professor in the departments of obstetrics, gynecology and reproductive sciences and internal medicine at Yale University, New Haven, Conn.
What are the symptoms of menopause?
Some symptoms of menopause include vaginal infections, irregular bleeding, urinary problems, and sexual problems.
Women in their middle years have other symptoms that can be the same as perimenopause/menopause symptoms.
“Common symptoms of perimenopause and menopause which may also be symptoms ascribed to long COVID include hot flashes, night sweats, disrupted sleep, low mood, depression or anxiety, decreased concentration, memory problems, joint and muscle pains, and headaches,” Dr. Pinkerton said.
Can long COVID actually bring on menopause?
In short: Possibly.
A new study from the Massachusetts Institute of Technology/Patient-Led Research Collaborative/University of California, San Francisco, found that long COVID can cause disruptions to a woman’s menstrual cycle, ovaries, fertility, and menopause itself.
This could be caused by chronic inflammation caused by long COVID on hormones as well. This kind of inflammatory response could explain irregularities in a woman’s menstrual cycle, according to the Newson Health Research and Education study. For instance, “when the body has inflammation, ovulation can happen,” Dr. Flannery said.
The mechanism for how long COVID could spur menopause can also involve a woman’s ovaries.
“Since the theory is that COVID affects the ovary with declines in ovarian reserve and ovarian function, it makes sense that long COVID could bring on symptoms of perimenopause or menopause more acutely or more severely and lengthen the symptoms of the perimenopause and menopausal transition,” Dr. Pinkerton said.
How can hormone replacement therapy benefit women dealing with long COVID during menopause?
Estradiol, the strongest estrogen hormone in a woman’s body, has already been shown to have a positive effect against COVID.
“Estradiol therapy treats symptoms more aggressively in the setting of long COVID,” said Dr. Flannery.
Estradiol is also a form of hormone therapy for menopause symptoms.
“Estradiol has been shown to help hot flashes, night sweats, and sleep and improve mood during perimenopause,” said Dr. Pinkerton. “So it’s likely that perimenopausal or menopausal women with long COVID would see improvements both due to the action of estradiol on the ovary seen during COVID and the improvements in symptoms.”
Estrogen-based hormone therapy has been linked to an increased risk for endometrial, breast, and ovarian cancer, according to the American Cancer Society. This means you should carefully consider how comfortable you are with those additional risks before starting this kind of therapy.
“Which of your symptoms are the most difficult to manage? You may see if you can navigate one to three of them. What are you willing to do for your symptoms? If a woman is willing to favor her sleep for the next 6 months to a year, she may be willing to change how she perceives her risk for cancer,” Dr. Flannery said. “What risk is a woman willing to take? I think if someone has a very low concern about a risk of cancer, and she’s suffering a disrupted life, then taking estradiol in a 1- to 2-year trial period could be critical to help.”
What else can help ease long COVID during menopause?
Getting the COVID vaccine, as well as getting a booster, could help. Not only will this help prevent people from being reinfected with COVID, which can worsen symptoms, but a new Swedish study says there is no evidence that it will cause postmenopausal problems like irregular bleeding.
“Weak and inconsistent associations were observed between SARS-CoV-2 vaccination and healthcare contacts for bleeding in women who are postmenopausal, and even less evidence was recorded of an association for menstrual disturbance or bleeding in women who were premenopausal,” said study coauthor Rickard Ljung, MD, PhD, MPH, professor and acting head of the pharmacoepidemiology and analysis department in the division of use and information of the Swedish Medical Products Agency in Uppsala.
A version of this article first appeared on WebMD.com.
British researchers have noted that women at midlife who have long COVID seem to get specific, and severe, symptoms, including brain fog, fatigue, new-onset dizziness, and difficulty sleeping through the night.
Doctors also think it’s possible that long COVID worsens the symptoms of perimenopause and menopause. Lower levels of estrogen and testosterone appear to be the reason.
“A long COVID theory is that there is a temporary disruption to physiological ovarian steroid hormone production, which could [worsen] symptoms of perimenopause and menopause,” said JoAnn V. Pinkerton, MD, professor of obstetrics at the University of Virginia, Charlottesville, and executive director of the North American Menopause Society.
Long COVID symptoms and menopause symptoms can also be very hard to tell apart.
Another U.K. study cautions that because of this kind of symptom overlap, women at midlife may be misdiagnosed. Research from the North American Menopause Society shows that many women may have trouble recovering from long COVID unless their hormone deficiency is treated.
What are the symptoms of long COVID?
There are over 200 symptoms that have been associated with long COVID, according to the American Medical Association. Some common symptoms are currently defined as the following: feeling extremely tired, feeling depleted after exertion, cognitive issues such as brain fog, heart beating over 100 times a minute, and a loss of sense of smell and taste.
Long COVID symptoms begin a few weeks to a few months after a COVID infection. They can last an indefinite amount of time, but “the hope is that long COVID will not be lifelong,” said Clare Flannery, MD, an endocrinologist and associate professor in the departments of obstetrics, gynecology and reproductive sciences and internal medicine at Yale University, New Haven, Conn.
What are the symptoms of menopause?
Some symptoms of menopause include vaginal infections, irregular bleeding, urinary problems, and sexual problems.
Women in their middle years have other symptoms that can be the same as perimenopause/menopause symptoms.
“Common symptoms of perimenopause and menopause which may also be symptoms ascribed to long COVID include hot flashes, night sweats, disrupted sleep, low mood, depression or anxiety, decreased concentration, memory problems, joint and muscle pains, and headaches,” Dr. Pinkerton said.
Can long COVID actually bring on menopause?
In short: Possibly.
A new study from the Massachusetts Institute of Technology/Patient-Led Research Collaborative/University of California, San Francisco, found that long COVID can cause disruptions to a woman’s menstrual cycle, ovaries, fertility, and menopause itself.
This could be caused by chronic inflammation caused by long COVID on hormones as well. This kind of inflammatory response could explain irregularities in a woman’s menstrual cycle, according to the Newson Health Research and Education study. For instance, “when the body has inflammation, ovulation can happen,” Dr. Flannery said.
The mechanism for how long COVID could spur menopause can also involve a woman’s ovaries.
“Since the theory is that COVID affects the ovary with declines in ovarian reserve and ovarian function, it makes sense that long COVID could bring on symptoms of perimenopause or menopause more acutely or more severely and lengthen the symptoms of the perimenopause and menopausal transition,” Dr. Pinkerton said.
How can hormone replacement therapy benefit women dealing with long COVID during menopause?
Estradiol, the strongest estrogen hormone in a woman’s body, has already been shown to have a positive effect against COVID.
“Estradiol therapy treats symptoms more aggressively in the setting of long COVID,” said Dr. Flannery.
Estradiol is also a form of hormone therapy for menopause symptoms.
“Estradiol has been shown to help hot flashes, night sweats, and sleep and improve mood during perimenopause,” said Dr. Pinkerton. “So it’s likely that perimenopausal or menopausal women with long COVID would see improvements both due to the action of estradiol on the ovary seen during COVID and the improvements in symptoms.”
Estrogen-based hormone therapy has been linked to an increased risk for endometrial, breast, and ovarian cancer, according to the American Cancer Society. This means you should carefully consider how comfortable you are with those additional risks before starting this kind of therapy.
“Which of your symptoms are the most difficult to manage? You may see if you can navigate one to three of them. What are you willing to do for your symptoms? If a woman is willing to favor her sleep for the next 6 months to a year, she may be willing to change how she perceives her risk for cancer,” Dr. Flannery said. “What risk is a woman willing to take? I think if someone has a very low concern about a risk of cancer, and she’s suffering a disrupted life, then taking estradiol in a 1- to 2-year trial period could be critical to help.”
What else can help ease long COVID during menopause?
Getting the COVID vaccine, as well as getting a booster, could help. Not only will this help prevent people from being reinfected with COVID, which can worsen symptoms, but a new Swedish study says there is no evidence that it will cause postmenopausal problems like irregular bleeding.
“Weak and inconsistent associations were observed between SARS-CoV-2 vaccination and healthcare contacts for bleeding in women who are postmenopausal, and even less evidence was recorded of an association for menstrual disturbance or bleeding in women who were premenopausal,” said study coauthor Rickard Ljung, MD, PhD, MPH, professor and acting head of the pharmacoepidemiology and analysis department in the division of use and information of the Swedish Medical Products Agency in Uppsala.
A version of this article first appeared on WebMD.com.
Fibroid characteristics can help us anticipate postpartum hemorrhage
Fibroids, or leiomyomas, are noncancerous monoclonal tumors of the smooth muscle layer of the uterus. Fibroids occur more frequently in Black patients and their prevalence increases with age. The hormonally responsive nature of fibroids, frequently leading to growth with estrogen and progesterone exposure, makes them of particular concern during pregnancy.
Although most patients with fibroids do not have pregnancy complications directly attributable to their fibroids, prior studies have reported several associations, including painful degeneration, early pregnancy loss, preterm birth, placental abruption, malpresentation, and postpartum hemorrhage. Fibroids may predispose to uterine atony and hemorrhage by disrupting or impairing the synchronization and coordination of uterine contractions. Within the current body of literature, it remains less certain whether certain fibroid characteristics are associated with increased hemorrhage risk.
Prior studies evaluating the association between specific fibroid characteristics and postpartum hemorrhage have yielded inconsistent findings. In our study, we evaluated whether certain fibroid characteristics are associated with hemorrhage requiring blood transfusion. Specifically, our goal was to determine whether larger or more numerous fibroids increase the risk of transfusion.
This was a retrospective cohort study spanning 2019-2022. A total of 4,421 patients were included in this study. Fibroid characteristics were collected, including size, number, and location. Fibroid size was classified as small (< 5 cm), medium (5-10 cm), and large (> 10 cm).
In terms of number of fibroids, there was no significant increase in transfusions when comparing one fibroid to multiple fibroids. When assessing fibroid size, however, we did observe a significant incremental increase in rate of transfusions with increasing fibroid size. In terms of fibroid location, patients with fibroids in the lower uterine segment or cervix were about 1.5 times more likely to have hemorrhage requiring transfusion, compared with those without a fibroid in that location.
This study allows practitioners to better risk-stratify patients from the practical perspective of postpartum hemorrhage requiring transfusions. In pregnant patients with fibroids, the specific fibroid characteristics can help us better anticipate clinically significant postpartum hemorrhage. In such patients, it is important to document specific fibroid characteristics, especially the largest fibroid diameter and fibroid location in the lower uterine segment or cervix. This emphasizes the importance of careful sonographic evaluation and consistent documentation of fibroids in pregnant patients.
Our study helps guide more informed counseling and risk stratification in this population, with increasing risk according to fibroid size and location. Patients with high-risk features, that is, medium or large fibroids and those with fibroids located in the lower uterine segment or cervix, should thus receive counseling about their increased risk of hemorrhage. As providers, we can help ameliorate this risk by optimizing hemoglobin levels of those at increased risk prior to delivery, and by ensuring availability of appropriate resources at the time of delivery.
Dr. Yaghoubian is a maternal-fetal medicine fellow at North Shore University Hospital/Long Island Jewish Medical Center in Manhasset, N.Y., and will be joining the faculty at the same institution. Email Dr. Yaghoubian at [email protected].
Fibroids, or leiomyomas, are noncancerous monoclonal tumors of the smooth muscle layer of the uterus. Fibroids occur more frequently in Black patients and their prevalence increases with age. The hormonally responsive nature of fibroids, frequently leading to growth with estrogen and progesterone exposure, makes them of particular concern during pregnancy.
Although most patients with fibroids do not have pregnancy complications directly attributable to their fibroids, prior studies have reported several associations, including painful degeneration, early pregnancy loss, preterm birth, placental abruption, malpresentation, and postpartum hemorrhage. Fibroids may predispose to uterine atony and hemorrhage by disrupting or impairing the synchronization and coordination of uterine contractions. Within the current body of literature, it remains less certain whether certain fibroid characteristics are associated with increased hemorrhage risk.
Prior studies evaluating the association between specific fibroid characteristics and postpartum hemorrhage have yielded inconsistent findings. In our study, we evaluated whether certain fibroid characteristics are associated with hemorrhage requiring blood transfusion. Specifically, our goal was to determine whether larger or more numerous fibroids increase the risk of transfusion.
This was a retrospective cohort study spanning 2019-2022. A total of 4,421 patients were included in this study. Fibroid characteristics were collected, including size, number, and location. Fibroid size was classified as small (< 5 cm), medium (5-10 cm), and large (> 10 cm).
In terms of number of fibroids, there was no significant increase in transfusions when comparing one fibroid to multiple fibroids. When assessing fibroid size, however, we did observe a significant incremental increase in rate of transfusions with increasing fibroid size. In terms of fibroid location, patients with fibroids in the lower uterine segment or cervix were about 1.5 times more likely to have hemorrhage requiring transfusion, compared with those without a fibroid in that location.
This study allows practitioners to better risk-stratify patients from the practical perspective of postpartum hemorrhage requiring transfusions. In pregnant patients with fibroids, the specific fibroid characteristics can help us better anticipate clinically significant postpartum hemorrhage. In such patients, it is important to document specific fibroid characteristics, especially the largest fibroid diameter and fibroid location in the lower uterine segment or cervix. This emphasizes the importance of careful sonographic evaluation and consistent documentation of fibroids in pregnant patients.
Our study helps guide more informed counseling and risk stratification in this population, with increasing risk according to fibroid size and location. Patients with high-risk features, that is, medium or large fibroids and those with fibroids located in the lower uterine segment or cervix, should thus receive counseling about their increased risk of hemorrhage. As providers, we can help ameliorate this risk by optimizing hemoglobin levels of those at increased risk prior to delivery, and by ensuring availability of appropriate resources at the time of delivery.
Dr. Yaghoubian is a maternal-fetal medicine fellow at North Shore University Hospital/Long Island Jewish Medical Center in Manhasset, N.Y., and will be joining the faculty at the same institution. Email Dr. Yaghoubian at [email protected].
Fibroids, or leiomyomas, are noncancerous monoclonal tumors of the smooth muscle layer of the uterus. Fibroids occur more frequently in Black patients and their prevalence increases with age. The hormonally responsive nature of fibroids, frequently leading to growth with estrogen and progesterone exposure, makes them of particular concern during pregnancy.
Although most patients with fibroids do not have pregnancy complications directly attributable to their fibroids, prior studies have reported several associations, including painful degeneration, early pregnancy loss, preterm birth, placental abruption, malpresentation, and postpartum hemorrhage. Fibroids may predispose to uterine atony and hemorrhage by disrupting or impairing the synchronization and coordination of uterine contractions. Within the current body of literature, it remains less certain whether certain fibroid characteristics are associated with increased hemorrhage risk.
Prior studies evaluating the association between specific fibroid characteristics and postpartum hemorrhage have yielded inconsistent findings. In our study, we evaluated whether certain fibroid characteristics are associated with hemorrhage requiring blood transfusion. Specifically, our goal was to determine whether larger or more numerous fibroids increase the risk of transfusion.
This was a retrospective cohort study spanning 2019-2022. A total of 4,421 patients were included in this study. Fibroid characteristics were collected, including size, number, and location. Fibroid size was classified as small (< 5 cm), medium (5-10 cm), and large (> 10 cm).
In terms of number of fibroids, there was no significant increase in transfusions when comparing one fibroid to multiple fibroids. When assessing fibroid size, however, we did observe a significant incremental increase in rate of transfusions with increasing fibroid size. In terms of fibroid location, patients with fibroids in the lower uterine segment or cervix were about 1.5 times more likely to have hemorrhage requiring transfusion, compared with those without a fibroid in that location.
This study allows practitioners to better risk-stratify patients from the practical perspective of postpartum hemorrhage requiring transfusions. In pregnant patients with fibroids, the specific fibroid characteristics can help us better anticipate clinically significant postpartum hemorrhage. In such patients, it is important to document specific fibroid characteristics, especially the largest fibroid diameter and fibroid location in the lower uterine segment or cervix. This emphasizes the importance of careful sonographic evaluation and consistent documentation of fibroids in pregnant patients.
Our study helps guide more informed counseling and risk stratification in this population, with increasing risk according to fibroid size and location. Patients with high-risk features, that is, medium or large fibroids and those with fibroids located in the lower uterine segment or cervix, should thus receive counseling about their increased risk of hemorrhage. As providers, we can help ameliorate this risk by optimizing hemoglobin levels of those at increased risk prior to delivery, and by ensuring availability of appropriate resources at the time of delivery.
Dr. Yaghoubian is a maternal-fetal medicine fellow at North Shore University Hospital/Long Island Jewish Medical Center in Manhasset, N.Y., and will be joining the faculty at the same institution. Email Dr. Yaghoubian at [email protected].
Intervention reduces severe postpartum hemorrhage by 60% in developing nations
Postpartum hemorrhage (PPH) is the leading cause of maternal deaths worldwide, particularly in the least developed and developing countries. Of the 14 million female patients affected each year, approximately 70,000 cases result in death. However, according to a new study conducted by the World Health Organization and the University of Birmingham (England), a simple and affordable strategy may reduce the occurrence of severe cases during vaginal delivery.
These cases are defined as entailing blood loss greater than or equal to 1,000 mL in the 24 hours following delivery. This intervention also substantially reduced the need for blood transfusions, which are often costly and difficult to obtain.
In this trial, 80 secondary-level hospitals in Kenya, Nigeria, South Africa, and Tanzania, in which 210,132 patients underwent vaginal delivery, were randomly assigned to the intervention group or the usual-care group. Researchers identified that, among hospitals and patients with data, a primary outcome event occurred in 1.6% of the patients in the intervention group, compared with 4.3% of those in the usual-care group. In addition, PPH was detected in 93.1% of the patients in the intervention group and in 51.1% of those in the usual-care group. The treatment bundle was used in 91.2% and 19.4%, respectively.
The E-MOTIVE intervention, which is intended for use by health care professionals, consists of three elements:
- A strategy for early detection of PPH, which allows triggering of the “first response” treatment bundle
- A first response bundle called MOTIVE, which is based on WHO guidelines and consists of uterine massage, oxytocic drugs, tranexamic acid, IV fluids, and examination of the genital tract and escalation
- An implementation strategy that focuses on simulation-based training with peer-assisted learning, local E-MOTIVE champions, feedback of actionable data to providers, calibrated drape with trigger line, and MOTIVE emergency trolley or carry case
During a WHO press conference, study author Arri Coomarasamy, MD, said, “This new approach to treating postpartum hemorrhage could radically improve women’s chances of surviving childbirth globally, helping them get the treatment they need when they need it.”
Dr. Coomarasamy, who is also co-director of the WHO Collaborating Centre on Global Women’s Health at the University of Birmingham, added, “Time is of the essence when responding to postpartum bleeding, so interventions that eliminate delays in diagnosis or treatment should be game-changers for maternal health.”
PPH a ‘preventable’ problem
In Brazil, maternal mortality is still one of the most significant challenges in public health. In recent years, the COVID-19 pandemic exacerbated the difficulties and weaknesses in the health care system for pregnant women and new mothers.
The maternal mortality ratio (MMR) is the number of maternal deaths per 100,000 live births from any cause related to or aggravated by pregnancy or its management (excluding accidental or incidental causes) during pregnancy and childbirth or within 42 days of termination of pregnancy. In 2021, the MMR was 113. This figure was almost double the 55.3 maternal deaths per 100,000 live births reported in 2019, which was before the pandemic. Preliminary data from the Brazilian Ministry of Health collected by the Brazilian Obstetric Observatory (OOBr) indicate that the MMR in 2022 decreased to 50.6 maternal deaths per 100,000 live births. However, these numbers could increase, because the maternal mortality committees are still reviewing cases.
Rossana Pulcineli Vieira Francisco, MD, PhD, a professor of obstetrics and gynecology at the University of São Paulo School of Medicine and obstetrics coordinator of the OOBr, affirmed that although these numbers have dropped, they are still much higher than the targets set by health authorities. Brazil is a participant of the UN agreement that aims to reduce MMR to a maximum of 30 maternal deaths per 100,000 live births per year by 2030. “We still have a long way to go to reach this goal within the next 7 years,” Dr. Francisco warned.
She compared rates in Brazil with those of more developed regions. According to the data, the mean MMR in Europe is 13 maternal deaths per 100,000 live births. “Portugal was shocked when maternal deaths surpassed 20 [maternal deaths per 100,000 live births in 2020] amidst the COVID-19 pandemic. The ratio in Brazil, even before the pandemic, was 55,” she said.
“Maternal mortality and infant mortality ratios are powerful indicators of the quality of the health care system,” added the OOBr coordinator, who asserted that investing in primary and prenatal care is essential. Dr. Francisco also pointed out the preventable nature of maternal mortality in Brazil. “The three main causes of direct maternal mortality in Brazil are high blood pressure, postpartum hemorrhage, and infection, particularly in the postpartum period. These issues are all considered preventable.”
Although it is difficult to prevent preeclampsia, hospital care and maternity care measures can significantly reduce the number of deaths caused by this condition. “For high blood pressure, what we most miss is having specialized prenatal care for at-risk women when the problem is diagnosed during pregnancy.”
Regarding PPH, Dr. Francisco calls attention to the importance of training teams to treat the problem. “In Brazil, the lack of training [for professionals] is still a serious problem.”
According to her, investments in rapid response systems are also needed. “As the baby needs nutrients and oxygen, the uterus becomes full of blood vessels at the end of pregnancy. As a result, a PPH leads to significant blood loss. In Brazil, some hospitals don’t even have blood bags. And in some cases, there may not be enough time to get a blood bag from somewhere else.”
Dr. Francisco also points out that, although it may not be feasible for all of Brazil’s health care units to have blood banks, integrated structures could be created to facilitate access to blood in case of emergency.
A grant from the Bill & Melinda Gates Foundation supported the E-MOTIVE project.
A version of this article first appeared on Medscape.com.
Postpartum hemorrhage (PPH) is the leading cause of maternal deaths worldwide, particularly in the least developed and developing countries. Of the 14 million female patients affected each year, approximately 70,000 cases result in death. However, according to a new study conducted by the World Health Organization and the University of Birmingham (England), a simple and affordable strategy may reduce the occurrence of severe cases during vaginal delivery.
These cases are defined as entailing blood loss greater than or equal to 1,000 mL in the 24 hours following delivery. This intervention also substantially reduced the need for blood transfusions, which are often costly and difficult to obtain.
In this trial, 80 secondary-level hospitals in Kenya, Nigeria, South Africa, and Tanzania, in which 210,132 patients underwent vaginal delivery, were randomly assigned to the intervention group or the usual-care group. Researchers identified that, among hospitals and patients with data, a primary outcome event occurred in 1.6% of the patients in the intervention group, compared with 4.3% of those in the usual-care group. In addition, PPH was detected in 93.1% of the patients in the intervention group and in 51.1% of those in the usual-care group. The treatment bundle was used in 91.2% and 19.4%, respectively.
The E-MOTIVE intervention, which is intended for use by health care professionals, consists of three elements:
- A strategy for early detection of PPH, which allows triggering of the “first response” treatment bundle
- A first response bundle called MOTIVE, which is based on WHO guidelines and consists of uterine massage, oxytocic drugs, tranexamic acid, IV fluids, and examination of the genital tract and escalation
- An implementation strategy that focuses on simulation-based training with peer-assisted learning, local E-MOTIVE champions, feedback of actionable data to providers, calibrated drape with trigger line, and MOTIVE emergency trolley or carry case
During a WHO press conference, study author Arri Coomarasamy, MD, said, “This new approach to treating postpartum hemorrhage could radically improve women’s chances of surviving childbirth globally, helping them get the treatment they need when they need it.”
Dr. Coomarasamy, who is also co-director of the WHO Collaborating Centre on Global Women’s Health at the University of Birmingham, added, “Time is of the essence when responding to postpartum bleeding, so interventions that eliminate delays in diagnosis or treatment should be game-changers for maternal health.”
PPH a ‘preventable’ problem
In Brazil, maternal mortality is still one of the most significant challenges in public health. In recent years, the COVID-19 pandemic exacerbated the difficulties and weaknesses in the health care system for pregnant women and new mothers.
The maternal mortality ratio (MMR) is the number of maternal deaths per 100,000 live births from any cause related to or aggravated by pregnancy or its management (excluding accidental or incidental causes) during pregnancy and childbirth or within 42 days of termination of pregnancy. In 2021, the MMR was 113. This figure was almost double the 55.3 maternal deaths per 100,000 live births reported in 2019, which was before the pandemic. Preliminary data from the Brazilian Ministry of Health collected by the Brazilian Obstetric Observatory (OOBr) indicate that the MMR in 2022 decreased to 50.6 maternal deaths per 100,000 live births. However, these numbers could increase, because the maternal mortality committees are still reviewing cases.
Rossana Pulcineli Vieira Francisco, MD, PhD, a professor of obstetrics and gynecology at the University of São Paulo School of Medicine and obstetrics coordinator of the OOBr, affirmed that although these numbers have dropped, they are still much higher than the targets set by health authorities. Brazil is a participant of the UN agreement that aims to reduce MMR to a maximum of 30 maternal deaths per 100,000 live births per year by 2030. “We still have a long way to go to reach this goal within the next 7 years,” Dr. Francisco warned.
She compared rates in Brazil with those of more developed regions. According to the data, the mean MMR in Europe is 13 maternal deaths per 100,000 live births. “Portugal was shocked when maternal deaths surpassed 20 [maternal deaths per 100,000 live births in 2020] amidst the COVID-19 pandemic. The ratio in Brazil, even before the pandemic, was 55,” she said.
“Maternal mortality and infant mortality ratios are powerful indicators of the quality of the health care system,” added the OOBr coordinator, who asserted that investing in primary and prenatal care is essential. Dr. Francisco also pointed out the preventable nature of maternal mortality in Brazil. “The three main causes of direct maternal mortality in Brazil are high blood pressure, postpartum hemorrhage, and infection, particularly in the postpartum period. These issues are all considered preventable.”
Although it is difficult to prevent preeclampsia, hospital care and maternity care measures can significantly reduce the number of deaths caused by this condition. “For high blood pressure, what we most miss is having specialized prenatal care for at-risk women when the problem is diagnosed during pregnancy.”
Regarding PPH, Dr. Francisco calls attention to the importance of training teams to treat the problem. “In Brazil, the lack of training [for professionals] is still a serious problem.”
According to her, investments in rapid response systems are also needed. “As the baby needs nutrients and oxygen, the uterus becomes full of blood vessels at the end of pregnancy. As a result, a PPH leads to significant blood loss. In Brazil, some hospitals don’t even have blood bags. And in some cases, there may not be enough time to get a blood bag from somewhere else.”
Dr. Francisco also points out that, although it may not be feasible for all of Brazil’s health care units to have blood banks, integrated structures could be created to facilitate access to blood in case of emergency.
A grant from the Bill & Melinda Gates Foundation supported the E-MOTIVE project.
A version of this article first appeared on Medscape.com.
Postpartum hemorrhage (PPH) is the leading cause of maternal deaths worldwide, particularly in the least developed and developing countries. Of the 14 million female patients affected each year, approximately 70,000 cases result in death. However, according to a new study conducted by the World Health Organization and the University of Birmingham (England), a simple and affordable strategy may reduce the occurrence of severe cases during vaginal delivery.
These cases are defined as entailing blood loss greater than or equal to 1,000 mL in the 24 hours following delivery. This intervention also substantially reduced the need for blood transfusions, which are often costly and difficult to obtain.
In this trial, 80 secondary-level hospitals in Kenya, Nigeria, South Africa, and Tanzania, in which 210,132 patients underwent vaginal delivery, were randomly assigned to the intervention group or the usual-care group. Researchers identified that, among hospitals and patients with data, a primary outcome event occurred in 1.6% of the patients in the intervention group, compared with 4.3% of those in the usual-care group. In addition, PPH was detected in 93.1% of the patients in the intervention group and in 51.1% of those in the usual-care group. The treatment bundle was used in 91.2% and 19.4%, respectively.
The E-MOTIVE intervention, which is intended for use by health care professionals, consists of three elements:
- A strategy for early detection of PPH, which allows triggering of the “first response” treatment bundle
- A first response bundle called MOTIVE, which is based on WHO guidelines and consists of uterine massage, oxytocic drugs, tranexamic acid, IV fluids, and examination of the genital tract and escalation
- An implementation strategy that focuses on simulation-based training with peer-assisted learning, local E-MOTIVE champions, feedback of actionable data to providers, calibrated drape with trigger line, and MOTIVE emergency trolley or carry case
During a WHO press conference, study author Arri Coomarasamy, MD, said, “This new approach to treating postpartum hemorrhage could radically improve women’s chances of surviving childbirth globally, helping them get the treatment they need when they need it.”
Dr. Coomarasamy, who is also co-director of the WHO Collaborating Centre on Global Women’s Health at the University of Birmingham, added, “Time is of the essence when responding to postpartum bleeding, so interventions that eliminate delays in diagnosis or treatment should be game-changers for maternal health.”
PPH a ‘preventable’ problem
In Brazil, maternal mortality is still one of the most significant challenges in public health. In recent years, the COVID-19 pandemic exacerbated the difficulties and weaknesses in the health care system for pregnant women and new mothers.
The maternal mortality ratio (MMR) is the number of maternal deaths per 100,000 live births from any cause related to or aggravated by pregnancy or its management (excluding accidental or incidental causes) during pregnancy and childbirth or within 42 days of termination of pregnancy. In 2021, the MMR was 113. This figure was almost double the 55.3 maternal deaths per 100,000 live births reported in 2019, which was before the pandemic. Preliminary data from the Brazilian Ministry of Health collected by the Brazilian Obstetric Observatory (OOBr) indicate that the MMR in 2022 decreased to 50.6 maternal deaths per 100,000 live births. However, these numbers could increase, because the maternal mortality committees are still reviewing cases.
Rossana Pulcineli Vieira Francisco, MD, PhD, a professor of obstetrics and gynecology at the University of São Paulo School of Medicine and obstetrics coordinator of the OOBr, affirmed that although these numbers have dropped, they are still much higher than the targets set by health authorities. Brazil is a participant of the UN agreement that aims to reduce MMR to a maximum of 30 maternal deaths per 100,000 live births per year by 2030. “We still have a long way to go to reach this goal within the next 7 years,” Dr. Francisco warned.
She compared rates in Brazil with those of more developed regions. According to the data, the mean MMR in Europe is 13 maternal deaths per 100,000 live births. “Portugal was shocked when maternal deaths surpassed 20 [maternal deaths per 100,000 live births in 2020] amidst the COVID-19 pandemic. The ratio in Brazil, even before the pandemic, was 55,” she said.
“Maternal mortality and infant mortality ratios are powerful indicators of the quality of the health care system,” added the OOBr coordinator, who asserted that investing in primary and prenatal care is essential. Dr. Francisco also pointed out the preventable nature of maternal mortality in Brazil. “The three main causes of direct maternal mortality in Brazil are high blood pressure, postpartum hemorrhage, and infection, particularly in the postpartum period. These issues are all considered preventable.”
Although it is difficult to prevent preeclampsia, hospital care and maternity care measures can significantly reduce the number of deaths caused by this condition. “For high blood pressure, what we most miss is having specialized prenatal care for at-risk women when the problem is diagnosed during pregnancy.”
Regarding PPH, Dr. Francisco calls attention to the importance of training teams to treat the problem. “In Brazil, the lack of training [for professionals] is still a serious problem.”
According to her, investments in rapid response systems are also needed. “As the baby needs nutrients and oxygen, the uterus becomes full of blood vessels at the end of pregnancy. As a result, a PPH leads to significant blood loss. In Brazil, some hospitals don’t even have blood bags. And in some cases, there may not be enough time to get a blood bag from somewhere else.”
Dr. Francisco also points out that, although it may not be feasible for all of Brazil’s health care units to have blood banks, integrated structures could be created to facilitate access to blood in case of emergency.
A grant from the Bill & Melinda Gates Foundation supported the E-MOTIVE project.
A version of this article first appeared on Medscape.com.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE