User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
Breast cancer experts and other HCPs disagree on treatment strategies for early BC
The discrepancy suggests that many providers aren’t aware of the findings of recent landmark trials that formed the basis of the panel’s opinions, said study coauthor Denise A. Yardley, MD, of Tennessee Oncology and Sarah Cannon Research Institute in Nashville, in an interview. The findings, based on responses to a treatment decision tool, were presented in a poster at the annual meeting of the American Society of Clinical Oncology.
Study methods and results
For the new study, researchers analyzed how 547 providers – and the panel – responded to 10 case scenarios in high-risk HER2– early breast cancer between June 2022 and January 2023.
Among the providers surveyed, 72% identified as physicians, including oncologists, hematologists/oncologists, surgery oncologists, radiation oncologists, and pathologists. One percent said they were nurse practitioners or physician assistants, 7% said they were pharmacists, 1% were nurses, and the specific roles of the remaining 19% were unknown, but included medical students, according to Dr. Yardley, who is a breast cancer oncologist.
The study authors developed the free decision tool – available via the medical education company Clinical Care Options – to help oncologists navigate new treatment options for high-risk HER2– early breast cancer. The Food and Drug Administration has recently approved drugs such as abemaciclib, olaparib, and pembrolizumab for the condition.
Health care providers enter details into the tool about their patients along with their intended treatment plans. The tool then shows them recommendations for treatment from a panel of five oncologists with expertise in oncology. The members of the panel based their perspectives on the findings of the KEYNOTE-522 (pembrolizumab), OlympiA (olaparib), and monarchE (abemaciclib) trials.
The oncologists with expertise in breast cancer, who provided recommendations in March 2022, generally agreed about the best treatments, Dr. Yardley said.
The other health care providers surveyed didn’t agree with the breast cancer experts about the best treatment 58.8% of the time.
For example, one scenario describes a HR+, HER2– patient with no deleterious BRCA mutation – or unknown status – who fits the monarchE high-risk criteria. All the breast cancer experts on the panel recommended abemaciclib and endocrine therapy. But 203 providers supported a variety of strategies: endocrine therapy alone (9%), chemotherapy followed by endocrine therapy (49%), and olaparib and endocrine therapy (2%). Only 37% opted for abemaciclib and endocrine therapy, and 4% were uncertain.
Another scenario describes a patient with triple-negative breast cancer with no residual disease after neoadjuvant chemotherapy. All the experts agreed on a strategy of no adjuvant therapy plus observation. Forty percent of 25 providers agreed with this approach, but 24% were uncertain, 12% chose pembrolizumab, and 24% chose capecitabine.
In many cases, providers chose more intensive treatment options than the experts did, Dr. Yardley said.
Overtreatment in cancer is often a reflex for oncologists, she said, although “we’re learning to deescalate these treatment algorithms where there is really no benefit [to extra treatment].”
“It’s a challenge for some of these oncologists who are busy and dealing with multiple solid tumor types to keep up with the nuances of a rapidly changing field,” Dr. Yardley noted.
Many community oncologists aren’t specialists in one type of cancer and must try to keep up with treatment recommendations regarding multiple types, she continued.
Decision tool’s value explained
According to the study, 32% of providers changed their treatment choices in clinical practice after they learned about the expert perspectives via the decision tool; 46% said the expert opinions confirmed that their choices were best practice.
The value of the tool is its ability to help providers make better decisions about patient care, Dr. Yardley said. “There seems to be a need for this kind of support.”
In an interview, University of Pittsburgh Medical Center oncologist Adam M. Brufsky, MD, PhD – who wasn’t involved with the study – said he was surprised by the amount of disagreement between the expert and provider perspectives on treatment. However, he noted that community oncologists – unlike the breast cancer experts – often don’t see just one type of cancer.
“You just have to know so much now as an oncologist,” Dr. Brufsky said. He recommended that colleagues take advantage of decision support tools, such as cancer treatment pathways.
The study was funded by AstraZeneca, Lilly, and Merck Sharp & Dohme. Dr. Yardley has no disclosures, and disclosure information from other authors was not available. Dr. Brufsky discloses consulting support from AstraZeneca, Lilly, and Merck and grants from AstraZeneca.
The discrepancy suggests that many providers aren’t aware of the findings of recent landmark trials that formed the basis of the panel’s opinions, said study coauthor Denise A. Yardley, MD, of Tennessee Oncology and Sarah Cannon Research Institute in Nashville, in an interview. The findings, based on responses to a treatment decision tool, were presented in a poster at the annual meeting of the American Society of Clinical Oncology.
Study methods and results
For the new study, researchers analyzed how 547 providers – and the panel – responded to 10 case scenarios in high-risk HER2– early breast cancer between June 2022 and January 2023.
Among the providers surveyed, 72% identified as physicians, including oncologists, hematologists/oncologists, surgery oncologists, radiation oncologists, and pathologists. One percent said they were nurse practitioners or physician assistants, 7% said they were pharmacists, 1% were nurses, and the specific roles of the remaining 19% were unknown, but included medical students, according to Dr. Yardley, who is a breast cancer oncologist.
The study authors developed the free decision tool – available via the medical education company Clinical Care Options – to help oncologists navigate new treatment options for high-risk HER2– early breast cancer. The Food and Drug Administration has recently approved drugs such as abemaciclib, olaparib, and pembrolizumab for the condition.
Health care providers enter details into the tool about their patients along with their intended treatment plans. The tool then shows them recommendations for treatment from a panel of five oncologists with expertise in oncology. The members of the panel based their perspectives on the findings of the KEYNOTE-522 (pembrolizumab), OlympiA (olaparib), and monarchE (abemaciclib) trials.
The oncologists with expertise in breast cancer, who provided recommendations in March 2022, generally agreed about the best treatments, Dr. Yardley said.
The other health care providers surveyed didn’t agree with the breast cancer experts about the best treatment 58.8% of the time.
For example, one scenario describes a HR+, HER2– patient with no deleterious BRCA mutation – or unknown status – who fits the monarchE high-risk criteria. All the breast cancer experts on the panel recommended abemaciclib and endocrine therapy. But 203 providers supported a variety of strategies: endocrine therapy alone (9%), chemotherapy followed by endocrine therapy (49%), and olaparib and endocrine therapy (2%). Only 37% opted for abemaciclib and endocrine therapy, and 4% were uncertain.
Another scenario describes a patient with triple-negative breast cancer with no residual disease after neoadjuvant chemotherapy. All the experts agreed on a strategy of no adjuvant therapy plus observation. Forty percent of 25 providers agreed with this approach, but 24% were uncertain, 12% chose pembrolizumab, and 24% chose capecitabine.
In many cases, providers chose more intensive treatment options than the experts did, Dr. Yardley said.
Overtreatment in cancer is often a reflex for oncologists, she said, although “we’re learning to deescalate these treatment algorithms where there is really no benefit [to extra treatment].”
“It’s a challenge for some of these oncologists who are busy and dealing with multiple solid tumor types to keep up with the nuances of a rapidly changing field,” Dr. Yardley noted.
Many community oncologists aren’t specialists in one type of cancer and must try to keep up with treatment recommendations regarding multiple types, she continued.
Decision tool’s value explained
According to the study, 32% of providers changed their treatment choices in clinical practice after they learned about the expert perspectives via the decision tool; 46% said the expert opinions confirmed that their choices were best practice.
The value of the tool is its ability to help providers make better decisions about patient care, Dr. Yardley said. “There seems to be a need for this kind of support.”
In an interview, University of Pittsburgh Medical Center oncologist Adam M. Brufsky, MD, PhD – who wasn’t involved with the study – said he was surprised by the amount of disagreement between the expert and provider perspectives on treatment. However, he noted that community oncologists – unlike the breast cancer experts – often don’t see just one type of cancer.
“You just have to know so much now as an oncologist,” Dr. Brufsky said. He recommended that colleagues take advantage of decision support tools, such as cancer treatment pathways.
The study was funded by AstraZeneca, Lilly, and Merck Sharp & Dohme. Dr. Yardley has no disclosures, and disclosure information from other authors was not available. Dr. Brufsky discloses consulting support from AstraZeneca, Lilly, and Merck and grants from AstraZeneca.
The discrepancy suggests that many providers aren’t aware of the findings of recent landmark trials that formed the basis of the panel’s opinions, said study coauthor Denise A. Yardley, MD, of Tennessee Oncology and Sarah Cannon Research Institute in Nashville, in an interview. The findings, based on responses to a treatment decision tool, were presented in a poster at the annual meeting of the American Society of Clinical Oncology.
Study methods and results
For the new study, researchers analyzed how 547 providers – and the panel – responded to 10 case scenarios in high-risk HER2– early breast cancer between June 2022 and January 2023.
Among the providers surveyed, 72% identified as physicians, including oncologists, hematologists/oncologists, surgery oncologists, radiation oncologists, and pathologists. One percent said they were nurse practitioners or physician assistants, 7% said they were pharmacists, 1% were nurses, and the specific roles of the remaining 19% were unknown, but included medical students, according to Dr. Yardley, who is a breast cancer oncologist.
The study authors developed the free decision tool – available via the medical education company Clinical Care Options – to help oncologists navigate new treatment options for high-risk HER2– early breast cancer. The Food and Drug Administration has recently approved drugs such as abemaciclib, olaparib, and pembrolizumab for the condition.
Health care providers enter details into the tool about their patients along with their intended treatment plans. The tool then shows them recommendations for treatment from a panel of five oncologists with expertise in oncology. The members of the panel based their perspectives on the findings of the KEYNOTE-522 (pembrolizumab), OlympiA (olaparib), and monarchE (abemaciclib) trials.
The oncologists with expertise in breast cancer, who provided recommendations in March 2022, generally agreed about the best treatments, Dr. Yardley said.
The other health care providers surveyed didn’t agree with the breast cancer experts about the best treatment 58.8% of the time.
For example, one scenario describes a HR+, HER2– patient with no deleterious BRCA mutation – or unknown status – who fits the monarchE high-risk criteria. All the breast cancer experts on the panel recommended abemaciclib and endocrine therapy. But 203 providers supported a variety of strategies: endocrine therapy alone (9%), chemotherapy followed by endocrine therapy (49%), and olaparib and endocrine therapy (2%). Only 37% opted for abemaciclib and endocrine therapy, and 4% were uncertain.
Another scenario describes a patient with triple-negative breast cancer with no residual disease after neoadjuvant chemotherapy. All the experts agreed on a strategy of no adjuvant therapy plus observation. Forty percent of 25 providers agreed with this approach, but 24% were uncertain, 12% chose pembrolizumab, and 24% chose capecitabine.
In many cases, providers chose more intensive treatment options than the experts did, Dr. Yardley said.
Overtreatment in cancer is often a reflex for oncologists, she said, although “we’re learning to deescalate these treatment algorithms where there is really no benefit [to extra treatment].”
“It’s a challenge for some of these oncologists who are busy and dealing with multiple solid tumor types to keep up with the nuances of a rapidly changing field,” Dr. Yardley noted.
Many community oncologists aren’t specialists in one type of cancer and must try to keep up with treatment recommendations regarding multiple types, she continued.
Decision tool’s value explained
According to the study, 32% of providers changed their treatment choices in clinical practice after they learned about the expert perspectives via the decision tool; 46% said the expert opinions confirmed that their choices were best practice.
The value of the tool is its ability to help providers make better decisions about patient care, Dr. Yardley said. “There seems to be a need for this kind of support.”
In an interview, University of Pittsburgh Medical Center oncologist Adam M. Brufsky, MD, PhD – who wasn’t involved with the study – said he was surprised by the amount of disagreement between the expert and provider perspectives on treatment. However, he noted that community oncologists – unlike the breast cancer experts – often don’t see just one type of cancer.
“You just have to know so much now as an oncologist,” Dr. Brufsky said. He recommended that colleagues take advantage of decision support tools, such as cancer treatment pathways.
The study was funded by AstraZeneca, Lilly, and Merck Sharp & Dohme. Dr. Yardley has no disclosures, and disclosure information from other authors was not available. Dr. Brufsky discloses consulting support from AstraZeneca, Lilly, and Merck and grants from AstraZeneca.
AT ASCO 2023
Hormone therapies still ‘most effective’ in treating menopausal vasomotor symptoms
Despite new options in non–hormone-based treatments,
This recommendation emerged from an updated position statement from the North American Menopause Society in its first review of the scientific literature since 2015. The statement specifically targets nonhormonal management of symptoms such as hot flashes and night sweats, which occur in as many as 80% of menopausal women but are undertreated. The statement appears in the June issue of the Journal of The North American Menopause Society.
“Women with contraindications or objections to hormone treatment should be informed by professionals of evidence-based effective nonhormone treatment options,” stated a NAMS advisory panel led by Chrisandra L. Shufelt, MD, MS, professor and chair of the division of general internal medicine and associate director of the Women’s Health Research Center at the Mayo Clinic in Jacksonville, Fla. The statement is one of multiple NAMS updates performed at regular intervals, said Dr. Shufelt, also past president of NAMS, in an interview. “But the research has changed, and we wanted to make clinicians aware of new medications. One of our interesting findings was more evidence that off-label use of the nonhormonal overactive bladder drug oxybutynin can lower the rate of hot flashes.”
Dr. Shufelt noted that many of the current update’s findings align with previous research, and stressed that the therapeutic recommendations apply specifically to VMS. “Not all menopause-related symptoms are vasomotor, however,” she said. “While a lot of the lifestyle options such as cooling techniques and exercise are not recommended for controlling hot flashes, diet and exercise changes can be beneficial for other health reasons.”
Although it’s the most effective option for VMS, hormone therapy is not suitable for women with contraindications such as a previous blood clot, an estrogen-dependent cancer, a family history of such cancers, or a personal preference against hormone use, Dr. Shufelt added, so nonhormonal alternatives are important to prevent women from wasting time and money on ineffective remedies. “Women need to know what works and what doesn’t,” she said.
Recommended nonhormonal therapies
Based on a rigorous review of the scientific evidence to date, NAMS found the following therapies to be effective: cognitive-behavioral therapy; clinical hypnosis; SSRIs and serotonin-norepinephrine reuptake inhibitors – which yield mild to moderate improvements; gabapentin – which lessens the frequency and severity of hot flashes; fezolinetant (Veozah), a novel first-in-class neurokinin B antagonist that was Food and Drug Administration–approved in May for VSM; and oxybutynin, an antimuscarinic, anticholinergic drug, that reduces moderate to severe VMS, although long-term use in older adults may be linked to cognitive decline, weight loss, and stellate ganglion block.
Therapies that were ineffective, associated with adverse effects (AEs), or lacking adequate evidence of efficacy and thus not recommended for VMS included: paced respiration; supplemental and herbal remedies such as black cohosh, milk thistle, and evening primrose; cooling techniques; trigger avoidance; exercise and yoga; mindfulness-based intervention and relaxation; suvorexant, a dual orexin-receptor antagonist used for insomnia; soy foods, extracts, and the soy metabolite equol; cannabinoids; acupuncture; calibration of neural oscillations; chiropractics; clonidine, an alpha-2 adrenergic agonist that is associated with significant AEs with no recent evidence of benefit over placebo; dietary modification; and pregabalin – which is associated with significant AEs and has controlled-substance prescribing restrictions.
Ultimately, clinicians should individualize menopause care to each patient. For example, “if a patient says that avoiding caffeine in the morning stops her from having hot flashes in the afternoon, that’s fine,” Dr. Shufelt said.
HT still most effective
“This statement is excellent, comprehensive, and evidence-based,” commented Jill M. Rabin MD, vice chair of education and development, obstetrics and gynecology, at Northshore University Hospital/LIJ Medical Center in Manhasset, N.Y., and professor of obstetrics and gynecology at the Donald and Barbara Zucker School of Medicine at Hofstra/Northwell Health in Hempstead, N.Y.
Dr. Rabin, coauthor of Mind Over Bladder was not involved in compiling the statement.
She agreed that hormone therapy is the most effective option for VMS and regularly prescribes it for suitable candidates in different forms depending on the type and severity of menopausal symptoms. As for nonhormonal options, Dr. Rabin added in an interview, some of those not recommended in the current NAMS statement could yet prove to be effective as more data accumulate. Suvorexant may be one to watch, for instance, but currently there are not enough data on its effectiveness.
“It’s really important to keep up on this nonhormonal research,” Dr. Rabin said. “As the population ages, more and more women will be in the peri- and postmenopausal periods and some have medical reasons for not taking hormone therapy.” It’s important to recommend nonhormonal therapies of proven benefit according to current high-level evidence, she said, “but also to keep your ear to the ground about those still under investigation.”
As for the lifestyle and alternative remedies of unproven benefit, Dr. Rabin added, there’s little harm in trying them. “As far as I know, no one’s ever died of relaxation and paced breathing.” In addition, a patient’s interaction with and sense of control over her own physiology provided by these techniques may be beneficial in themselves.
Dr. Shufelt reported grant support from the National Institutes of Health. Numerous authors reported consulting fees from and other financial ties to private-sector companies. Dr. Rabin had no relevant competing interests to disclose with regard to her comments.
Despite new options in non–hormone-based treatments,
This recommendation emerged from an updated position statement from the North American Menopause Society in its first review of the scientific literature since 2015. The statement specifically targets nonhormonal management of symptoms such as hot flashes and night sweats, which occur in as many as 80% of menopausal women but are undertreated. The statement appears in the June issue of the Journal of The North American Menopause Society.
“Women with contraindications or objections to hormone treatment should be informed by professionals of evidence-based effective nonhormone treatment options,” stated a NAMS advisory panel led by Chrisandra L. Shufelt, MD, MS, professor and chair of the division of general internal medicine and associate director of the Women’s Health Research Center at the Mayo Clinic in Jacksonville, Fla. The statement is one of multiple NAMS updates performed at regular intervals, said Dr. Shufelt, also past president of NAMS, in an interview. “But the research has changed, and we wanted to make clinicians aware of new medications. One of our interesting findings was more evidence that off-label use of the nonhormonal overactive bladder drug oxybutynin can lower the rate of hot flashes.”
Dr. Shufelt noted that many of the current update’s findings align with previous research, and stressed that the therapeutic recommendations apply specifically to VMS. “Not all menopause-related symptoms are vasomotor, however,” she said. “While a lot of the lifestyle options such as cooling techniques and exercise are not recommended for controlling hot flashes, diet and exercise changes can be beneficial for other health reasons.”
Although it’s the most effective option for VMS, hormone therapy is not suitable for women with contraindications such as a previous blood clot, an estrogen-dependent cancer, a family history of such cancers, or a personal preference against hormone use, Dr. Shufelt added, so nonhormonal alternatives are important to prevent women from wasting time and money on ineffective remedies. “Women need to know what works and what doesn’t,” she said.
Recommended nonhormonal therapies
Based on a rigorous review of the scientific evidence to date, NAMS found the following therapies to be effective: cognitive-behavioral therapy; clinical hypnosis; SSRIs and serotonin-norepinephrine reuptake inhibitors – which yield mild to moderate improvements; gabapentin – which lessens the frequency and severity of hot flashes; fezolinetant (Veozah), a novel first-in-class neurokinin B antagonist that was Food and Drug Administration–approved in May for VSM; and oxybutynin, an antimuscarinic, anticholinergic drug, that reduces moderate to severe VMS, although long-term use in older adults may be linked to cognitive decline, weight loss, and stellate ganglion block.
Therapies that were ineffective, associated with adverse effects (AEs), or lacking adequate evidence of efficacy and thus not recommended for VMS included: paced respiration; supplemental and herbal remedies such as black cohosh, milk thistle, and evening primrose; cooling techniques; trigger avoidance; exercise and yoga; mindfulness-based intervention and relaxation; suvorexant, a dual orexin-receptor antagonist used for insomnia; soy foods, extracts, and the soy metabolite equol; cannabinoids; acupuncture; calibration of neural oscillations; chiropractics; clonidine, an alpha-2 adrenergic agonist that is associated with significant AEs with no recent evidence of benefit over placebo; dietary modification; and pregabalin – which is associated with significant AEs and has controlled-substance prescribing restrictions.
Ultimately, clinicians should individualize menopause care to each patient. For example, “if a patient says that avoiding caffeine in the morning stops her from having hot flashes in the afternoon, that’s fine,” Dr. Shufelt said.
HT still most effective
“This statement is excellent, comprehensive, and evidence-based,” commented Jill M. Rabin MD, vice chair of education and development, obstetrics and gynecology, at Northshore University Hospital/LIJ Medical Center in Manhasset, N.Y., and professor of obstetrics and gynecology at the Donald and Barbara Zucker School of Medicine at Hofstra/Northwell Health in Hempstead, N.Y.
Dr. Rabin, coauthor of Mind Over Bladder was not involved in compiling the statement.
She agreed that hormone therapy is the most effective option for VMS and regularly prescribes it for suitable candidates in different forms depending on the type and severity of menopausal symptoms. As for nonhormonal options, Dr. Rabin added in an interview, some of those not recommended in the current NAMS statement could yet prove to be effective as more data accumulate. Suvorexant may be one to watch, for instance, but currently there are not enough data on its effectiveness.
“It’s really important to keep up on this nonhormonal research,” Dr. Rabin said. “As the population ages, more and more women will be in the peri- and postmenopausal periods and some have medical reasons for not taking hormone therapy.” It’s important to recommend nonhormonal therapies of proven benefit according to current high-level evidence, she said, “but also to keep your ear to the ground about those still under investigation.”
As for the lifestyle and alternative remedies of unproven benefit, Dr. Rabin added, there’s little harm in trying them. “As far as I know, no one’s ever died of relaxation and paced breathing.” In addition, a patient’s interaction with and sense of control over her own physiology provided by these techniques may be beneficial in themselves.
Dr. Shufelt reported grant support from the National Institutes of Health. Numerous authors reported consulting fees from and other financial ties to private-sector companies. Dr. Rabin had no relevant competing interests to disclose with regard to her comments.
Despite new options in non–hormone-based treatments,
This recommendation emerged from an updated position statement from the North American Menopause Society in its first review of the scientific literature since 2015. The statement specifically targets nonhormonal management of symptoms such as hot flashes and night sweats, which occur in as many as 80% of menopausal women but are undertreated. The statement appears in the June issue of the Journal of The North American Menopause Society.
“Women with contraindications or objections to hormone treatment should be informed by professionals of evidence-based effective nonhormone treatment options,” stated a NAMS advisory panel led by Chrisandra L. Shufelt, MD, MS, professor and chair of the division of general internal medicine and associate director of the Women’s Health Research Center at the Mayo Clinic in Jacksonville, Fla. The statement is one of multiple NAMS updates performed at regular intervals, said Dr. Shufelt, also past president of NAMS, in an interview. “But the research has changed, and we wanted to make clinicians aware of new medications. One of our interesting findings was more evidence that off-label use of the nonhormonal overactive bladder drug oxybutynin can lower the rate of hot flashes.”
Dr. Shufelt noted that many of the current update’s findings align with previous research, and stressed that the therapeutic recommendations apply specifically to VMS. “Not all menopause-related symptoms are vasomotor, however,” she said. “While a lot of the lifestyle options such as cooling techniques and exercise are not recommended for controlling hot flashes, diet and exercise changes can be beneficial for other health reasons.”
Although it’s the most effective option for VMS, hormone therapy is not suitable for women with contraindications such as a previous blood clot, an estrogen-dependent cancer, a family history of such cancers, or a personal preference against hormone use, Dr. Shufelt added, so nonhormonal alternatives are important to prevent women from wasting time and money on ineffective remedies. “Women need to know what works and what doesn’t,” she said.
Recommended nonhormonal therapies
Based on a rigorous review of the scientific evidence to date, NAMS found the following therapies to be effective: cognitive-behavioral therapy; clinical hypnosis; SSRIs and serotonin-norepinephrine reuptake inhibitors – which yield mild to moderate improvements; gabapentin – which lessens the frequency and severity of hot flashes; fezolinetant (Veozah), a novel first-in-class neurokinin B antagonist that was Food and Drug Administration–approved in May for VSM; and oxybutynin, an antimuscarinic, anticholinergic drug, that reduces moderate to severe VMS, although long-term use in older adults may be linked to cognitive decline, weight loss, and stellate ganglion block.
Therapies that were ineffective, associated with adverse effects (AEs), or lacking adequate evidence of efficacy and thus not recommended for VMS included: paced respiration; supplemental and herbal remedies such as black cohosh, milk thistle, and evening primrose; cooling techniques; trigger avoidance; exercise and yoga; mindfulness-based intervention and relaxation; suvorexant, a dual orexin-receptor antagonist used for insomnia; soy foods, extracts, and the soy metabolite equol; cannabinoids; acupuncture; calibration of neural oscillations; chiropractics; clonidine, an alpha-2 adrenergic agonist that is associated with significant AEs with no recent evidence of benefit over placebo; dietary modification; and pregabalin – which is associated with significant AEs and has controlled-substance prescribing restrictions.
Ultimately, clinicians should individualize menopause care to each patient. For example, “if a patient says that avoiding caffeine in the morning stops her from having hot flashes in the afternoon, that’s fine,” Dr. Shufelt said.
HT still most effective
“This statement is excellent, comprehensive, and evidence-based,” commented Jill M. Rabin MD, vice chair of education and development, obstetrics and gynecology, at Northshore University Hospital/LIJ Medical Center in Manhasset, N.Y., and professor of obstetrics and gynecology at the Donald and Barbara Zucker School of Medicine at Hofstra/Northwell Health in Hempstead, N.Y.
Dr. Rabin, coauthor of Mind Over Bladder was not involved in compiling the statement.
She agreed that hormone therapy is the most effective option for VMS and regularly prescribes it for suitable candidates in different forms depending on the type and severity of menopausal symptoms. As for nonhormonal options, Dr. Rabin added in an interview, some of those not recommended in the current NAMS statement could yet prove to be effective as more data accumulate. Suvorexant may be one to watch, for instance, but currently there are not enough data on its effectiveness.
“It’s really important to keep up on this nonhormonal research,” Dr. Rabin said. “As the population ages, more and more women will be in the peri- and postmenopausal periods and some have medical reasons for not taking hormone therapy.” It’s important to recommend nonhormonal therapies of proven benefit according to current high-level evidence, she said, “but also to keep your ear to the ground about those still under investigation.”
As for the lifestyle and alternative remedies of unproven benefit, Dr. Rabin added, there’s little harm in trying them. “As far as I know, no one’s ever died of relaxation and paced breathing.” In addition, a patient’s interaction with and sense of control over her own physiology provided by these techniques may be beneficial in themselves.
Dr. Shufelt reported grant support from the National Institutes of Health. Numerous authors reported consulting fees from and other financial ties to private-sector companies. Dr. Rabin had no relevant competing interests to disclose with regard to her comments.
FROM THE JOURNAL OF THE NORTH AMERICAN MENOPAUSE SOCIETY
WHO advises against nonsugar sweeteners for weight control
These sweeteners include aspartame, acesulfame K, advantame, saccharine, sucralose, stevia, and stevia derivatives.
The recommendation is based on the findings of a systematic review that collected data from 283 studies in adults, children, pregnant women, and mixed populations.
The findings suggest that use of NSSs does not confer any long-term benefit in reducing body fat in adults or children. They also suggest that long-term use of NSSs may have potential undesirable effects.
To clarify, short-term NSS use results in a small reduction in body weight and body mass index in adults without significant effects on other measures of adiposity or cardiometabolic health, including fasting glucose, insulin, blood lipids, and blood pressure.
Conversely, on a long-term basis, results from prospective cohort studies suggest that higher NSS intake is associated with increased risk for type 2 diabetes, cardiovascular diseases, and all-cause mortality in adults (very low– to low-certainty evidence).
Regarding the risk for cancer, results from case-control studies suggest an association between saccharine intake and bladder cancer (very low certainty evidence), but significant associations for other types of cancer were not observed in case-control studies or meta-analysis of prospective cohort studies.
Relatively fewer studies were found for children, and results were largely inconclusive.
Finally, results for pregnant women suggest that higher NSS intake is associated with increased risk for preterm birth (low-certainty evidence) and possibly adiposity in offspring (very low–certainty evidence).
Reducing sugar consumption
“Replacing free sugars with NSS does not help with weight control in the long-term. People need to consider other ways to reduce free sugars intake, such as consuming food with naturally occurring sugars, like fruit, or unsweetened food and beverages,” Francesco Branca, MD, PhD, WHO director of the department of nutrition and food safety, said in a press release.
“NSSs are not essential dietary factors and have no nutritional value. People should reduce the sweetness of the diet altogether, starting early in life, to improve their health,” he added.
Applying the guideline
The recommendation applies to all people except individuals with preexisting diabetes and includes all synthetic and naturally occurring or modified nonnutritive sweeteners, said the WHO.
The recommendation does not apply to personal care and hygiene products containing NSSs, such as toothpaste, skin cream, and medications, or to low-calorie sugars and sugar alcohols (polyols).
Because the link observed in the evidence between NSSs and disease outcomes might be confounded by the baseline characteristics of study participants and complicated patterns of NSS use, the recommendation has been assessed as “conditional” by the WHO.
“This signals that policy decisions based on this recommendation may require substantive discussion in specific country contexts, linked for example to the extent of consumption in different age groups,” said the WHO press release.
This article was translated from the Medscape French Edition . A version of the article appeared on Medscape.com.
These sweeteners include aspartame, acesulfame K, advantame, saccharine, sucralose, stevia, and stevia derivatives.
The recommendation is based on the findings of a systematic review that collected data from 283 studies in adults, children, pregnant women, and mixed populations.
The findings suggest that use of NSSs does not confer any long-term benefit in reducing body fat in adults or children. They also suggest that long-term use of NSSs may have potential undesirable effects.
To clarify, short-term NSS use results in a small reduction in body weight and body mass index in adults without significant effects on other measures of adiposity or cardiometabolic health, including fasting glucose, insulin, blood lipids, and blood pressure.
Conversely, on a long-term basis, results from prospective cohort studies suggest that higher NSS intake is associated with increased risk for type 2 diabetes, cardiovascular diseases, and all-cause mortality in adults (very low– to low-certainty evidence).
Regarding the risk for cancer, results from case-control studies suggest an association between saccharine intake and bladder cancer (very low certainty evidence), but significant associations for other types of cancer were not observed in case-control studies or meta-analysis of prospective cohort studies.
Relatively fewer studies were found for children, and results were largely inconclusive.
Finally, results for pregnant women suggest that higher NSS intake is associated with increased risk for preterm birth (low-certainty evidence) and possibly adiposity in offspring (very low–certainty evidence).
Reducing sugar consumption
“Replacing free sugars with NSS does not help with weight control in the long-term. People need to consider other ways to reduce free sugars intake, such as consuming food with naturally occurring sugars, like fruit, or unsweetened food and beverages,” Francesco Branca, MD, PhD, WHO director of the department of nutrition and food safety, said in a press release.
“NSSs are not essential dietary factors and have no nutritional value. People should reduce the sweetness of the diet altogether, starting early in life, to improve their health,” he added.
Applying the guideline
The recommendation applies to all people except individuals with preexisting diabetes and includes all synthetic and naturally occurring or modified nonnutritive sweeteners, said the WHO.
The recommendation does not apply to personal care and hygiene products containing NSSs, such as toothpaste, skin cream, and medications, or to low-calorie sugars and sugar alcohols (polyols).
Because the link observed in the evidence between NSSs and disease outcomes might be confounded by the baseline characteristics of study participants and complicated patterns of NSS use, the recommendation has been assessed as “conditional” by the WHO.
“This signals that policy decisions based on this recommendation may require substantive discussion in specific country contexts, linked for example to the extent of consumption in different age groups,” said the WHO press release.
This article was translated from the Medscape French Edition . A version of the article appeared on Medscape.com.
These sweeteners include aspartame, acesulfame K, advantame, saccharine, sucralose, stevia, and stevia derivatives.
The recommendation is based on the findings of a systematic review that collected data from 283 studies in adults, children, pregnant women, and mixed populations.
The findings suggest that use of NSSs does not confer any long-term benefit in reducing body fat in adults or children. They also suggest that long-term use of NSSs may have potential undesirable effects.
To clarify, short-term NSS use results in a small reduction in body weight and body mass index in adults without significant effects on other measures of adiposity or cardiometabolic health, including fasting glucose, insulin, blood lipids, and blood pressure.
Conversely, on a long-term basis, results from prospective cohort studies suggest that higher NSS intake is associated with increased risk for type 2 diabetes, cardiovascular diseases, and all-cause mortality in adults (very low– to low-certainty evidence).
Regarding the risk for cancer, results from case-control studies suggest an association between saccharine intake and bladder cancer (very low certainty evidence), but significant associations for other types of cancer were not observed in case-control studies or meta-analysis of prospective cohort studies.
Relatively fewer studies were found for children, and results were largely inconclusive.
Finally, results for pregnant women suggest that higher NSS intake is associated with increased risk for preterm birth (low-certainty evidence) and possibly adiposity in offspring (very low–certainty evidence).
Reducing sugar consumption
“Replacing free sugars with NSS does not help with weight control in the long-term. People need to consider other ways to reduce free sugars intake, such as consuming food with naturally occurring sugars, like fruit, or unsweetened food and beverages,” Francesco Branca, MD, PhD, WHO director of the department of nutrition and food safety, said in a press release.
“NSSs are not essential dietary factors and have no nutritional value. People should reduce the sweetness of the diet altogether, starting early in life, to improve their health,” he added.
Applying the guideline
The recommendation applies to all people except individuals with preexisting diabetes and includes all synthetic and naturally occurring or modified nonnutritive sweeteners, said the WHO.
The recommendation does not apply to personal care and hygiene products containing NSSs, such as toothpaste, skin cream, and medications, or to low-calorie sugars and sugar alcohols (polyols).
Because the link observed in the evidence between NSSs and disease outcomes might be confounded by the baseline characteristics of study participants and complicated patterns of NSS use, the recommendation has been assessed as “conditional” by the WHO.
“This signals that policy decisions based on this recommendation may require substantive discussion in specific country contexts, linked for example to the extent of consumption in different age groups,” said the WHO press release.
This article was translated from the Medscape French Edition . A version of the article appeared on Medscape.com.
How does psoriasis affect fertility and birth outcomes?
in a U.K. cohort study.
Those are key findings from what is believed to be one of the largest studies to investigate fertility and obstetric outcomes in patients with psoriasis.
“Studies that have examined fertility and pregnancy outcomes in women with psoriasis have reported conflicting findings,” lead author Teng-Chou Chen, PhD, of the Centre for Pharmacoepidemiology and Drug Safety at the University of Manchester (England), and colleagues from the Global Psoriasis Atlas wrote in the study, published in JAMA Dermatology. Most of the studies were small, with under 100 women, “and are thus likely underpowered to detect a difference in pregnancy outcomes. The majority of those studies used disease registry data or lacked a matched comparison group and hence were unable to estimate the association of fertility and adverse pregnancy outcomes in women with psoriasis when compared with the general population.”
To determine fertility rates and birth outcomes in female patients with psoriasis, compared with age- and practice-matched patients without psoriasis, the researchers evaluated EHR data from a large U.K. primary care database, the Clinical Practice Research Datalink GOLD, from 1998 to 2019. They limited the analysis to patients aged 15-44 years and used relevant codes from clinical consultations to identify those with psoriasis. Then, for each patient with psoriasis, the researchers selected five comparators without psoriasis from the same primary care practice and matched for year of birth.
Both sets of patients were followed from the index date to age 45 years, death, transfer out of practice, last date of data collection, or end of the study period (Dec. 31, 2019), whichever came first. Pregnancy records were extracted for both sets of patients, and birth outcomes were categorized as pregnancy loss, live birth, stillbirth, and preterm birth. Adverse pregnancy outcomes were also collected. Finally, Dr. Chen and colleagues used a negative binomial model to examine the association between psoriasis and the fertility rate, and they applied logistic regression to compare the association between psoriasis and obstetric outcomes.
The analysis included 63,681 patients with psoriasis and 318,405 comparators whose median age on the index date was 30 years and who were followed for a median of 4.1 years. Among patients with psoriasis, 5.1% met criteria for moderate to severe disease in the follow-up period. The researchers observed that, compared with their age- and practice-matched counterparts, patients with psoriasis were more likely to be current smokers, alcohol drinkers, or overweight on the index date. They were also more often diagnosed with diabetes, hypertension, inflammatory bowel disease, thyroid disorders, and respiratory diseases such as asthma and chronic obstructive pulmonary disease.
Fertility, birth outcomes
When they looked at fertility outcomes, the researchers found that, compared with their matched peers without psoriasis, those with psoriasis had higher rates of fertility (risk ratio, 1.30; 95% confidence interval, 1.27-1.33; P < .001). But after the researchers stratified patients based on psoriasis severity, those with moderate to severe disease had significantly lower rates of fertility (RR, 0.75; 95% CI, 0.69-0.83; P < .001), compared those who did not have psoriasis.
As for adverse birth outcomes, compared with their matched comparators, pregnancies in patients with psoriasis were less likely to end in a live birth (odds ratio, 0.91; 95% CI, 0.88-0.93; P < .001). They also had a higher risk of pregnancy loss (OR, 1.06; 95% CI, 1.03-1.10; P < .001), most during the first trimester, at a gestation period of under 91 days.
In addition to psoriasis, patients younger than age 20 (OR, 2.04; 95% CI, 1.94-2.15; P < .011) and those aged between 20 and 24 years (OR, 1.35; 95% CI, 1.31-1.40; P < .001) had a higher risk of pregnancy loss, compared with those aged between 25 and 34 years.
However, no increases in the risks of antenatal hemorrhage, preeclampsia, or gestational diabetes were observed in patients with psoriasis, and no statistically significant differences in the odds of stillbirth and preterm birth were found between patients with psoriasis and matched comparators who did not have psoriasis.
“The mechanism to link the higher risk of pregnancy loss in patients with psoriasis is not clear, but there might be potential explanations,” the researchers wrote. “Psoriasis is characterized by the increased activity of [interleukin]-17, IL-23, and tumor necrosis factor–alpha. Those proinflammatory cytokines may negatively affect the placenta and cause impaired fetal growth.”
They recommended that further studies “evaluate the effects of better management of psoriasis and close monitoring during pregnancy on pregnancy loss.” In particular, “patients with psoriasis were more likely to have comorbidities that may be related to poor pregnancy outcomes, and hence increased emphasis of managing comorbidities as part of the routine management plan is also warranted.”
Asked to comment on the study, Alexa B. Kimball, MD, MPH, who has been involved with research on this topic, said that she and other investigators had observed some years ago that fertility rates for women with moderate to severe psoriasis might be lower than expected.
This trend was observed in some psoriasis registries, some pregnancy registries, and in clinical practice, Dr. Kimball, professor of dermatology at Harvard Medical School, Boston, said in an interview. “This study clearly demonstrates that lower fertility rates in the moderate to severe psoriasis population occurs and compels further exploration of the reason why.” The reasons could be biologic, she continued, including difficulty conceiving or an increased risk of miscarriage, sociobehavioral issues, or a combination.
“Behavioral examples could include that some women with moderate to severe psoriasis can flare during pregnancy, which might affect their choice” to become pregnant, Dr. Kimball said. “Stigma may also play a role in how women with moderate to severe psoriasis form relationships. Now that there are much better treatments for moderate to severe psoriasis and better knowledge about managing psoriasis during pregnancy, it will also be important to explore whether these trends change over time.”
The study was funded by the International League of Dermatological Societies on behalf of the Global Psoriasis Atlas. Two of the study authors reported receiving consulting fees and grant support from many pharmaceutical companies. Dr. Kimball disclosed that she serves or has served on several Organization of Teratology Information Specialists advisory board pregnancy registries, is a consultant and investigator for Abbvie, Janssen, Lilly, Bristol-Myers Squibb, Moonlake, UCB, and Amgen; has fellowship funding from Janssen; and serves on the board of Almirall.
in a U.K. cohort study.
Those are key findings from what is believed to be one of the largest studies to investigate fertility and obstetric outcomes in patients with psoriasis.
“Studies that have examined fertility and pregnancy outcomes in women with psoriasis have reported conflicting findings,” lead author Teng-Chou Chen, PhD, of the Centre for Pharmacoepidemiology and Drug Safety at the University of Manchester (England), and colleagues from the Global Psoriasis Atlas wrote in the study, published in JAMA Dermatology. Most of the studies were small, with under 100 women, “and are thus likely underpowered to detect a difference in pregnancy outcomes. The majority of those studies used disease registry data or lacked a matched comparison group and hence were unable to estimate the association of fertility and adverse pregnancy outcomes in women with psoriasis when compared with the general population.”
To determine fertility rates and birth outcomes in female patients with psoriasis, compared with age- and practice-matched patients without psoriasis, the researchers evaluated EHR data from a large U.K. primary care database, the Clinical Practice Research Datalink GOLD, from 1998 to 2019. They limited the analysis to patients aged 15-44 years and used relevant codes from clinical consultations to identify those with psoriasis. Then, for each patient with psoriasis, the researchers selected five comparators without psoriasis from the same primary care practice and matched for year of birth.
Both sets of patients were followed from the index date to age 45 years, death, transfer out of practice, last date of data collection, or end of the study period (Dec. 31, 2019), whichever came first. Pregnancy records were extracted for both sets of patients, and birth outcomes were categorized as pregnancy loss, live birth, stillbirth, and preterm birth. Adverse pregnancy outcomes were also collected. Finally, Dr. Chen and colleagues used a negative binomial model to examine the association between psoriasis and the fertility rate, and they applied logistic regression to compare the association between psoriasis and obstetric outcomes.
The analysis included 63,681 patients with psoriasis and 318,405 comparators whose median age on the index date was 30 years and who were followed for a median of 4.1 years. Among patients with psoriasis, 5.1% met criteria for moderate to severe disease in the follow-up period. The researchers observed that, compared with their age- and practice-matched counterparts, patients with psoriasis were more likely to be current smokers, alcohol drinkers, or overweight on the index date. They were also more often diagnosed with diabetes, hypertension, inflammatory bowel disease, thyroid disorders, and respiratory diseases such as asthma and chronic obstructive pulmonary disease.
Fertility, birth outcomes
When they looked at fertility outcomes, the researchers found that, compared with their matched peers without psoriasis, those with psoriasis had higher rates of fertility (risk ratio, 1.30; 95% confidence interval, 1.27-1.33; P < .001). But after the researchers stratified patients based on psoriasis severity, those with moderate to severe disease had significantly lower rates of fertility (RR, 0.75; 95% CI, 0.69-0.83; P < .001), compared those who did not have psoriasis.
As for adverse birth outcomes, compared with their matched comparators, pregnancies in patients with psoriasis were less likely to end in a live birth (odds ratio, 0.91; 95% CI, 0.88-0.93; P < .001). They also had a higher risk of pregnancy loss (OR, 1.06; 95% CI, 1.03-1.10; P < .001), most during the first trimester, at a gestation period of under 91 days.
In addition to psoriasis, patients younger than age 20 (OR, 2.04; 95% CI, 1.94-2.15; P < .011) and those aged between 20 and 24 years (OR, 1.35; 95% CI, 1.31-1.40; P < .001) had a higher risk of pregnancy loss, compared with those aged between 25 and 34 years.
However, no increases in the risks of antenatal hemorrhage, preeclampsia, or gestational diabetes were observed in patients with psoriasis, and no statistically significant differences in the odds of stillbirth and preterm birth were found between patients with psoriasis and matched comparators who did not have psoriasis.
“The mechanism to link the higher risk of pregnancy loss in patients with psoriasis is not clear, but there might be potential explanations,” the researchers wrote. “Psoriasis is characterized by the increased activity of [interleukin]-17, IL-23, and tumor necrosis factor–alpha. Those proinflammatory cytokines may negatively affect the placenta and cause impaired fetal growth.”
They recommended that further studies “evaluate the effects of better management of psoriasis and close monitoring during pregnancy on pregnancy loss.” In particular, “patients with psoriasis were more likely to have comorbidities that may be related to poor pregnancy outcomes, and hence increased emphasis of managing comorbidities as part of the routine management plan is also warranted.”
Asked to comment on the study, Alexa B. Kimball, MD, MPH, who has been involved with research on this topic, said that she and other investigators had observed some years ago that fertility rates for women with moderate to severe psoriasis might be lower than expected.
This trend was observed in some psoriasis registries, some pregnancy registries, and in clinical practice, Dr. Kimball, professor of dermatology at Harvard Medical School, Boston, said in an interview. “This study clearly demonstrates that lower fertility rates in the moderate to severe psoriasis population occurs and compels further exploration of the reason why.” The reasons could be biologic, she continued, including difficulty conceiving or an increased risk of miscarriage, sociobehavioral issues, or a combination.
“Behavioral examples could include that some women with moderate to severe psoriasis can flare during pregnancy, which might affect their choice” to become pregnant, Dr. Kimball said. “Stigma may also play a role in how women with moderate to severe psoriasis form relationships. Now that there are much better treatments for moderate to severe psoriasis and better knowledge about managing psoriasis during pregnancy, it will also be important to explore whether these trends change over time.”
The study was funded by the International League of Dermatological Societies on behalf of the Global Psoriasis Atlas. Two of the study authors reported receiving consulting fees and grant support from many pharmaceutical companies. Dr. Kimball disclosed that she serves or has served on several Organization of Teratology Information Specialists advisory board pregnancy registries, is a consultant and investigator for Abbvie, Janssen, Lilly, Bristol-Myers Squibb, Moonlake, UCB, and Amgen; has fellowship funding from Janssen; and serves on the board of Almirall.
in a U.K. cohort study.
Those are key findings from what is believed to be one of the largest studies to investigate fertility and obstetric outcomes in patients with psoriasis.
“Studies that have examined fertility and pregnancy outcomes in women with psoriasis have reported conflicting findings,” lead author Teng-Chou Chen, PhD, of the Centre for Pharmacoepidemiology and Drug Safety at the University of Manchester (England), and colleagues from the Global Psoriasis Atlas wrote in the study, published in JAMA Dermatology. Most of the studies were small, with under 100 women, “and are thus likely underpowered to detect a difference in pregnancy outcomes. The majority of those studies used disease registry data or lacked a matched comparison group and hence were unable to estimate the association of fertility and adverse pregnancy outcomes in women with psoriasis when compared with the general population.”
To determine fertility rates and birth outcomes in female patients with psoriasis, compared with age- and practice-matched patients without psoriasis, the researchers evaluated EHR data from a large U.K. primary care database, the Clinical Practice Research Datalink GOLD, from 1998 to 2019. They limited the analysis to patients aged 15-44 years and used relevant codes from clinical consultations to identify those with psoriasis. Then, for each patient with psoriasis, the researchers selected five comparators without psoriasis from the same primary care practice and matched for year of birth.
Both sets of patients were followed from the index date to age 45 years, death, transfer out of practice, last date of data collection, or end of the study period (Dec. 31, 2019), whichever came first. Pregnancy records were extracted for both sets of patients, and birth outcomes were categorized as pregnancy loss, live birth, stillbirth, and preterm birth. Adverse pregnancy outcomes were also collected. Finally, Dr. Chen and colleagues used a negative binomial model to examine the association between psoriasis and the fertility rate, and they applied logistic regression to compare the association between psoriasis and obstetric outcomes.
The analysis included 63,681 patients with psoriasis and 318,405 comparators whose median age on the index date was 30 years and who were followed for a median of 4.1 years. Among patients with psoriasis, 5.1% met criteria for moderate to severe disease in the follow-up period. The researchers observed that, compared with their age- and practice-matched counterparts, patients with psoriasis were more likely to be current smokers, alcohol drinkers, or overweight on the index date. They were also more often diagnosed with diabetes, hypertension, inflammatory bowel disease, thyroid disorders, and respiratory diseases such as asthma and chronic obstructive pulmonary disease.
Fertility, birth outcomes
When they looked at fertility outcomes, the researchers found that, compared with their matched peers without psoriasis, those with psoriasis had higher rates of fertility (risk ratio, 1.30; 95% confidence interval, 1.27-1.33; P < .001). But after the researchers stratified patients based on psoriasis severity, those with moderate to severe disease had significantly lower rates of fertility (RR, 0.75; 95% CI, 0.69-0.83; P < .001), compared those who did not have psoriasis.
As for adverse birth outcomes, compared with their matched comparators, pregnancies in patients with psoriasis were less likely to end in a live birth (odds ratio, 0.91; 95% CI, 0.88-0.93; P < .001). They also had a higher risk of pregnancy loss (OR, 1.06; 95% CI, 1.03-1.10; P < .001), most during the first trimester, at a gestation period of under 91 days.
In addition to psoriasis, patients younger than age 20 (OR, 2.04; 95% CI, 1.94-2.15; P < .011) and those aged between 20 and 24 years (OR, 1.35; 95% CI, 1.31-1.40; P < .001) had a higher risk of pregnancy loss, compared with those aged between 25 and 34 years.
However, no increases in the risks of antenatal hemorrhage, preeclampsia, or gestational diabetes were observed in patients with psoriasis, and no statistically significant differences in the odds of stillbirth and preterm birth were found between patients with psoriasis and matched comparators who did not have psoriasis.
“The mechanism to link the higher risk of pregnancy loss in patients with psoriasis is not clear, but there might be potential explanations,” the researchers wrote. “Psoriasis is characterized by the increased activity of [interleukin]-17, IL-23, and tumor necrosis factor–alpha. Those proinflammatory cytokines may negatively affect the placenta and cause impaired fetal growth.”
They recommended that further studies “evaluate the effects of better management of psoriasis and close monitoring during pregnancy on pregnancy loss.” In particular, “patients with psoriasis were more likely to have comorbidities that may be related to poor pregnancy outcomes, and hence increased emphasis of managing comorbidities as part of the routine management plan is also warranted.”
Asked to comment on the study, Alexa B. Kimball, MD, MPH, who has been involved with research on this topic, said that she and other investigators had observed some years ago that fertility rates for women with moderate to severe psoriasis might be lower than expected.
This trend was observed in some psoriasis registries, some pregnancy registries, and in clinical practice, Dr. Kimball, professor of dermatology at Harvard Medical School, Boston, said in an interview. “This study clearly demonstrates that lower fertility rates in the moderate to severe psoriasis population occurs and compels further exploration of the reason why.” The reasons could be biologic, she continued, including difficulty conceiving or an increased risk of miscarriage, sociobehavioral issues, or a combination.
“Behavioral examples could include that some women with moderate to severe psoriasis can flare during pregnancy, which might affect their choice” to become pregnant, Dr. Kimball said. “Stigma may also play a role in how women with moderate to severe psoriasis form relationships. Now that there are much better treatments for moderate to severe psoriasis and better knowledge about managing psoriasis during pregnancy, it will also be important to explore whether these trends change over time.”
The study was funded by the International League of Dermatological Societies on behalf of the Global Psoriasis Atlas. Two of the study authors reported receiving consulting fees and grant support from many pharmaceutical companies. Dr. Kimball disclosed that she serves or has served on several Organization of Teratology Information Specialists advisory board pregnancy registries, is a consultant and investigator for Abbvie, Janssen, Lilly, Bristol-Myers Squibb, Moonlake, UCB, and Amgen; has fellowship funding from Janssen; and serves on the board of Almirall.
FROM JAMA DERMATOLOGY
Kangaroo mother care may cut death risk for premature babies by a third
, according to new research published online in BMJ Global Health.
Starting the contact, which involves mothers carrying the newborn in a sling, within 24 hours of birth and continuing it for at least 8 hours a day both appear to amplify the effect on reducing mortality and infection, the paper states.
Sindhu Sivanandan, MD, with the department of neonatology at Jawaharlal Institute of Postgraduate Medical Education and Research, Puducherry, India, and Mari Jeeva Sankar, MD, in the pediatrics department of the All India Institute of Medical Sciences, New Delhi, looked at existing studies to compare KMC with conventional care and to compare starting the intervention within 24 hours of birth versus a later start.
Their review looked at 31 trials that included 15,559 low-birthweight and preterm infants collectively. Of the 31 trials, 27 studies compared KMC with conventional care and four compared early with late initiation of KMC.
Mortality risk reduction
Analysis showed that, compared with conventional care, KMC appeared to cut mortality risk by 32% (relative risk, 0.68; 95% confidence interval, 0.53-0.86) during birth hospitalization or by 28 days after birth, while it seemed to reduce the risk of severe infection, such as sepsis, by 15% (RR, 0.85; 95% CI, 0.76-0.96; low-certainty evidence.)
That mortality-risk reduction was found regardless of gestational age or weight of the child at enrolment, time of starting KMC, and whether the intervention was started in a hospital or community.
The studies that had compared early with late-initiated KMC showed a reduction in neonatal mortality of 33%.
Low- and middle-income countries have the highest rates of premature births (gestational age of less than 37 weeks) and low birthweight (less than 2,500 grams). Premature births and low birthweight both are key causes of death and disability.
The World Health Organization recommends KMC as the standard of care among low birthweight infants after clinical stabilization. The American Academy of Pediatrics also promotes immediate KMC.
Relevance in the U.S.
Grace Chan, MD, MPH, PhD, an epidemiologist and pediatrician with the Harvard School of Public Health, Boston, said though the practice is promoted by the WHO and AAP, recommendations to families vary widely by providers.
She said the health benefits for KMC are numerous. One of the biggest is that skin-to-skin contact can help transfer heat to newborns who may have trouble regulating their own temperature. That is especially important in cold climates in places where there may be insufficient indoor heat.
She said it’s well-known that preterm babies are at higher risk for apnea, and listening to a mother’s heartbeat may stimulate the child to breathe regularly.
Additionally with KMC, there’s an inherent benefit of a mother or caregiver being able to see any change in a newborn’s color immediately when the baby is held so closely, as opposed to a nurse watching several babies at a time in a neonatal intensive care unit.
This is evidence that starting KMC right away is important, because the risk of death for premature and low-weight newborns is highest in the first 24 hours of life, Dr. Chan noted.
Barriers of time
There are some barriers, she noted, in that mothers or other caregivers caring for several young children may not have the time to carry a child in a sling for 8 or more hours at a time.
The authors conclude that their findings have policy implications, particularly for low- and middle-income countries: “KMC should be provided to all low birth weight and preterm infants irrespective of the settings – both health facilities and at home,” they wrote.
The authors caution that, “very low birth weight, extremely preterm neonates, and severely unstable neonates were often excluded from studies. More evidence is needed before extrapolating the study results in these high-risk groups.”
The study authors and Dr. Chan report no relevant financial relationships.
, according to new research published online in BMJ Global Health.
Starting the contact, which involves mothers carrying the newborn in a sling, within 24 hours of birth and continuing it for at least 8 hours a day both appear to amplify the effect on reducing mortality and infection, the paper states.
Sindhu Sivanandan, MD, with the department of neonatology at Jawaharlal Institute of Postgraduate Medical Education and Research, Puducherry, India, and Mari Jeeva Sankar, MD, in the pediatrics department of the All India Institute of Medical Sciences, New Delhi, looked at existing studies to compare KMC with conventional care and to compare starting the intervention within 24 hours of birth versus a later start.
Their review looked at 31 trials that included 15,559 low-birthweight and preterm infants collectively. Of the 31 trials, 27 studies compared KMC with conventional care and four compared early with late initiation of KMC.
Mortality risk reduction
Analysis showed that, compared with conventional care, KMC appeared to cut mortality risk by 32% (relative risk, 0.68; 95% confidence interval, 0.53-0.86) during birth hospitalization or by 28 days after birth, while it seemed to reduce the risk of severe infection, such as sepsis, by 15% (RR, 0.85; 95% CI, 0.76-0.96; low-certainty evidence.)
That mortality-risk reduction was found regardless of gestational age or weight of the child at enrolment, time of starting KMC, and whether the intervention was started in a hospital or community.
The studies that had compared early with late-initiated KMC showed a reduction in neonatal mortality of 33%.
Low- and middle-income countries have the highest rates of premature births (gestational age of less than 37 weeks) and low birthweight (less than 2,500 grams). Premature births and low birthweight both are key causes of death and disability.
The World Health Organization recommends KMC as the standard of care among low birthweight infants after clinical stabilization. The American Academy of Pediatrics also promotes immediate KMC.
Relevance in the U.S.
Grace Chan, MD, MPH, PhD, an epidemiologist and pediatrician with the Harvard School of Public Health, Boston, said though the practice is promoted by the WHO and AAP, recommendations to families vary widely by providers.
She said the health benefits for KMC are numerous. One of the biggest is that skin-to-skin contact can help transfer heat to newborns who may have trouble regulating their own temperature. That is especially important in cold climates in places where there may be insufficient indoor heat.
She said it’s well-known that preterm babies are at higher risk for apnea, and listening to a mother’s heartbeat may stimulate the child to breathe regularly.
Additionally with KMC, there’s an inherent benefit of a mother or caregiver being able to see any change in a newborn’s color immediately when the baby is held so closely, as opposed to a nurse watching several babies at a time in a neonatal intensive care unit.
This is evidence that starting KMC right away is important, because the risk of death for premature and low-weight newborns is highest in the first 24 hours of life, Dr. Chan noted.
Barriers of time
There are some barriers, she noted, in that mothers or other caregivers caring for several young children may not have the time to carry a child in a sling for 8 or more hours at a time.
The authors conclude that their findings have policy implications, particularly for low- and middle-income countries: “KMC should be provided to all low birth weight and preterm infants irrespective of the settings – both health facilities and at home,” they wrote.
The authors caution that, “very low birth weight, extremely preterm neonates, and severely unstable neonates were often excluded from studies. More evidence is needed before extrapolating the study results in these high-risk groups.”
The study authors and Dr. Chan report no relevant financial relationships.
, according to new research published online in BMJ Global Health.
Starting the contact, which involves mothers carrying the newborn in a sling, within 24 hours of birth and continuing it for at least 8 hours a day both appear to amplify the effect on reducing mortality and infection, the paper states.
Sindhu Sivanandan, MD, with the department of neonatology at Jawaharlal Institute of Postgraduate Medical Education and Research, Puducherry, India, and Mari Jeeva Sankar, MD, in the pediatrics department of the All India Institute of Medical Sciences, New Delhi, looked at existing studies to compare KMC with conventional care and to compare starting the intervention within 24 hours of birth versus a later start.
Their review looked at 31 trials that included 15,559 low-birthweight and preterm infants collectively. Of the 31 trials, 27 studies compared KMC with conventional care and four compared early with late initiation of KMC.
Mortality risk reduction
Analysis showed that, compared with conventional care, KMC appeared to cut mortality risk by 32% (relative risk, 0.68; 95% confidence interval, 0.53-0.86) during birth hospitalization or by 28 days after birth, while it seemed to reduce the risk of severe infection, such as sepsis, by 15% (RR, 0.85; 95% CI, 0.76-0.96; low-certainty evidence.)
That mortality-risk reduction was found regardless of gestational age or weight of the child at enrolment, time of starting KMC, and whether the intervention was started in a hospital or community.
The studies that had compared early with late-initiated KMC showed a reduction in neonatal mortality of 33%.
Low- and middle-income countries have the highest rates of premature births (gestational age of less than 37 weeks) and low birthweight (less than 2,500 grams). Premature births and low birthweight both are key causes of death and disability.
The World Health Organization recommends KMC as the standard of care among low birthweight infants after clinical stabilization. The American Academy of Pediatrics also promotes immediate KMC.
Relevance in the U.S.
Grace Chan, MD, MPH, PhD, an epidemiologist and pediatrician with the Harvard School of Public Health, Boston, said though the practice is promoted by the WHO and AAP, recommendations to families vary widely by providers.
She said the health benefits for KMC are numerous. One of the biggest is that skin-to-skin contact can help transfer heat to newborns who may have trouble regulating their own temperature. That is especially important in cold climates in places where there may be insufficient indoor heat.
She said it’s well-known that preterm babies are at higher risk for apnea, and listening to a mother’s heartbeat may stimulate the child to breathe regularly.
Additionally with KMC, there’s an inherent benefit of a mother or caregiver being able to see any change in a newborn’s color immediately when the baby is held so closely, as opposed to a nurse watching several babies at a time in a neonatal intensive care unit.
This is evidence that starting KMC right away is important, because the risk of death for premature and low-weight newborns is highest in the first 24 hours of life, Dr. Chan noted.
Barriers of time
There are some barriers, she noted, in that mothers or other caregivers caring for several young children may not have the time to carry a child in a sling for 8 or more hours at a time.
The authors conclude that their findings have policy implications, particularly for low- and middle-income countries: “KMC should be provided to all low birth weight and preterm infants irrespective of the settings – both health facilities and at home,” they wrote.
The authors caution that, “very low birth weight, extremely preterm neonates, and severely unstable neonates were often excluded from studies. More evidence is needed before extrapolating the study results in these high-risk groups.”
The study authors and Dr. Chan report no relevant financial relationships.
FROM BMJ GLOBAL HEALTH
Is ChatGPT a friend or foe of medical publishing?
. These tools should not be listed as authors, and researchers must denote how AI-assisted technologies were used, the committee said.
These new guidelines are the latest effort for medical journals to define policies for using these large-scale language models (LLMs) in scientific publication. While these AI-assisted tools can help with tasks such as writing, analyzing data, catching mistakes, and much more, they are also prone to errors, noted Casey Greene, PhD, a professor of biomedical informatics at the University of Colorado at Denver, Aurora. It is also not totally clear how information is stored and processed in these kinds of tools, and who has access to that information, he noted.
At the same time, experts argue that these AI tools could have a positive impact on the field by limiting some of the linguistic disparities in scientific publishing as well as alleviating the burden of some monotonous or mechanical tasks that come along with manuscript writing.
What experts can agree on, though, is that the use of AI tools is here to stay. “This is going to become a common tool,” Dr. Greene said. “I don’t think there’s a way out of that at this point.”
A change in medical publishing
OpenAI released ChatGPT in November 2022. In its own words, ChatGPT is “a deep learning model that has been trained on a massive amount of text data to understand and generate humanlike text.” Enter a question or a prompt, and it will respond. For example, when asked how the AI tool can be used in scientific publishing, ChatGPT responded:
“ChatGPT can aid scientific publishing by generating ideas, clarifying concepts, conducting preliminary literature reviews, providing proofreading and editing suggestions, and assisting with outlining and organizing scientific papers. However, it should be used as a supportive tool alongside domain expertise and validation from trusted scientific sources.”
Just a few months after ChatGPT became available, researchers began using this tool in their own work. One individual, Som Biswas, MD, a radiologist at the University of Tennessee Health Science Center in Memphis, reportedly used ChatGPT to author 16 scientific articles in just 4 months, according to the Daily Beast. Five of these articles have been published in four different journals. Dr. Biswas declined to be interviewed for this article.
There were also reports of papers with ChatGPT as one of the listed authors, which sparked backlash. In response, JAMA, Nature, and Science all published editorials in January outlining their policies for using ChatGPT and other large language models in the scientific authoring process. Editors from the journals of the American College of Cardiology and the American College of Rheumatology also updated their policies to reflect the influence of AI authoring tools.
The consensus is that AI has no place on the author byline.
“We think that’s not appropriate, because coauthorship means that you are taking responsibility for the analysis and the generation of data that are included in a manuscript. A machine that is dictated by AI can’t take responsibility,” said Daniel Solomon, MD, MPH, a rheumatologist at Brigham and Women’s Hospital, Boston, and the editor in chief of the ACR journal Arthritis & Rheumatology.
Issues with AI
One of the big concerns around using AI in writing is that it can generate text that seems plausible but is untrue or not supported by data. For example, Dr. Greene and colleague Milton Pividori, PhD, also of the University of Colorado, were writing a journal article about new software they developed that uses a large language model to revise scientific manuscripts.
“We used the same software to revise that article and at one point, it added a line that noted that the large language model had been fine-tuned on a data set of manuscripts from within the same field. This makes a lot of sense, and is absolutely something you could do, but was not something that we did,” Dr. Greene said. “Without a really careful review of the content, it becomes possible to invent things that were not actually done.”
In another case, ChatGPT falsely stated that a prominent law professor had been accused of sexual assault, citing a Washington Post article that did not exist.
“We live in a society where we are extremely concerned about fake news,” Dr. Pividori added, “and [these kinds of errors] could certainly exacerbate that in the scientific community, which is very concerning because science informs public policy.”
Another issue is the lack of transparency around how large language models like ChatGPT process and store data used to make queries.
“We have no idea how they are recording all the prompts and things that we input into ChatGPT and their systems,” Dr. Pividori said.
OpenAI recently addressed some privacy concerns by allowing users to turn off their chat history with the AI chatbot, so conversations cannot be used to train or improve the company’s models. But Dr. Greene noted that the terms of service “still remain pretty nebulous.”
Dr. Solomon is also concerned with researchers using these AI tools in authoring without knowing how they work. “The thing we are really concerned about is that fact that [LLMs] are a bit of a black box – people don’t really understand the methodologies,” he said.
A positive tool?
But despite these concerns, many think that these types of AI-assisted tools could have a positive impact on medical publishing, particularly for researchers for whom English is not their first language, noted Catherine Gao, MD, a pulmonary and critical care instructor at Northwestern University, Chicago. She recently led research comparing scientific abstracts written by ChatGPT and real abstracts and discovered that reviewers found it “surprisingly difficult” to differentiate the two.
“The majority of research is published in English,” she said in an email. “Responsible use of LLMs can potentially reduce the burden of writing for busy scientists and improve equity for those who are not native English speakers.”
Dr. Pividori agreed, adding that as a non-native English speaker, he spends much more time working on the structure and grammar of sentences when authoring a manuscript, compared with people who speak English as a first language. He noted that these tools can also be used to automate some of the more monotonous tasks that come along with writing manuscripts and allow researchers to focus on the more creative aspects.
In the future, “I want to focus more on the things that only a human can do and let these tools do all the rest of it,” he said.
New rules
But despite how individual researchers feel about LLMs, they agree that these AI tools are here to stay.
“I think that we should anticipate that they will become part of the medical research establishment over time, when we figure out how to use them appropriately,” Dr. Solomon said.
While the debate of how to best use AI in medical publications will continue, journal editors agree that all authors of a manuscript are solely responsible for content in articles that used AI-assisted technology.
“Authors should carefully review and edit the result because AI can generate authoritative-sounding output that can be incorrect, incomplete, or biased,” the ICMJE guidelines state. “Authors should be able to assert that there is no plagiarism in their paper, including in text and images produced by the AI.” This includes appropriate attribution of all cited materials.
The committee also recommends that authors write in both the cover letter and submitted work how AI was used in the manuscript writing process. Recently updated guidelines from the World Association of Medical Editors recommend that all prompts used to generate new text or analytical work should be provided in submitted work. Dr. Greene also noted that if authors used an AI tool to revise their work, they can include a version of the manuscript untouched by LLMs.
It is similar to a preprint, he said, but rather than publishing a version of a paper prior to peer review, someone is showing a version of a manuscript before it was reviewed and revised by AI. “This type of practice could be a path that lets us benefit from these models,” he said, “without having the drawbacks that many are concerned about.”
Dr. Solomon has financial relationships with AbbVie, Amgen, Janssen, CorEvitas, and Moderna. Both Dr. Greene and Dr. Pividori are inventors in the U.S. Provisional Patent Application No. 63/486,706 that the University of Colorado has filed for the “Publishing Infrastructure For AI-Assisted Academic Authoring” invention with the U.S. Patent and Trademark Office. Dr. Greene and Dr. Pividori also received a grant from the Alfred P. Sloan Foundation to improve their AI-based manuscript revision tool. Dr. Gao reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
. These tools should not be listed as authors, and researchers must denote how AI-assisted technologies were used, the committee said.
These new guidelines are the latest effort for medical journals to define policies for using these large-scale language models (LLMs) in scientific publication. While these AI-assisted tools can help with tasks such as writing, analyzing data, catching mistakes, and much more, they are also prone to errors, noted Casey Greene, PhD, a professor of biomedical informatics at the University of Colorado at Denver, Aurora. It is also not totally clear how information is stored and processed in these kinds of tools, and who has access to that information, he noted.
At the same time, experts argue that these AI tools could have a positive impact on the field by limiting some of the linguistic disparities in scientific publishing as well as alleviating the burden of some monotonous or mechanical tasks that come along with manuscript writing.
What experts can agree on, though, is that the use of AI tools is here to stay. “This is going to become a common tool,” Dr. Greene said. “I don’t think there’s a way out of that at this point.”
A change in medical publishing
OpenAI released ChatGPT in November 2022. In its own words, ChatGPT is “a deep learning model that has been trained on a massive amount of text data to understand and generate humanlike text.” Enter a question or a prompt, and it will respond. For example, when asked how the AI tool can be used in scientific publishing, ChatGPT responded:
“ChatGPT can aid scientific publishing by generating ideas, clarifying concepts, conducting preliminary literature reviews, providing proofreading and editing suggestions, and assisting with outlining and organizing scientific papers. However, it should be used as a supportive tool alongside domain expertise and validation from trusted scientific sources.”
Just a few months after ChatGPT became available, researchers began using this tool in their own work. One individual, Som Biswas, MD, a radiologist at the University of Tennessee Health Science Center in Memphis, reportedly used ChatGPT to author 16 scientific articles in just 4 months, according to the Daily Beast. Five of these articles have been published in four different journals. Dr. Biswas declined to be interviewed for this article.
There were also reports of papers with ChatGPT as one of the listed authors, which sparked backlash. In response, JAMA, Nature, and Science all published editorials in January outlining their policies for using ChatGPT and other large language models in the scientific authoring process. Editors from the journals of the American College of Cardiology and the American College of Rheumatology also updated their policies to reflect the influence of AI authoring tools.
The consensus is that AI has no place on the author byline.
“We think that’s not appropriate, because coauthorship means that you are taking responsibility for the analysis and the generation of data that are included in a manuscript. A machine that is dictated by AI can’t take responsibility,” said Daniel Solomon, MD, MPH, a rheumatologist at Brigham and Women’s Hospital, Boston, and the editor in chief of the ACR journal Arthritis & Rheumatology.
Issues with AI
One of the big concerns around using AI in writing is that it can generate text that seems plausible but is untrue or not supported by data. For example, Dr. Greene and colleague Milton Pividori, PhD, also of the University of Colorado, were writing a journal article about new software they developed that uses a large language model to revise scientific manuscripts.
“We used the same software to revise that article and at one point, it added a line that noted that the large language model had been fine-tuned on a data set of manuscripts from within the same field. This makes a lot of sense, and is absolutely something you could do, but was not something that we did,” Dr. Greene said. “Without a really careful review of the content, it becomes possible to invent things that were not actually done.”
In another case, ChatGPT falsely stated that a prominent law professor had been accused of sexual assault, citing a Washington Post article that did not exist.
“We live in a society where we are extremely concerned about fake news,” Dr. Pividori added, “and [these kinds of errors] could certainly exacerbate that in the scientific community, which is very concerning because science informs public policy.”
Another issue is the lack of transparency around how large language models like ChatGPT process and store data used to make queries.
“We have no idea how they are recording all the prompts and things that we input into ChatGPT and their systems,” Dr. Pividori said.
OpenAI recently addressed some privacy concerns by allowing users to turn off their chat history with the AI chatbot, so conversations cannot be used to train or improve the company’s models. But Dr. Greene noted that the terms of service “still remain pretty nebulous.”
Dr. Solomon is also concerned with researchers using these AI tools in authoring without knowing how they work. “The thing we are really concerned about is that fact that [LLMs] are a bit of a black box – people don’t really understand the methodologies,” he said.
A positive tool?
But despite these concerns, many think that these types of AI-assisted tools could have a positive impact on medical publishing, particularly for researchers for whom English is not their first language, noted Catherine Gao, MD, a pulmonary and critical care instructor at Northwestern University, Chicago. She recently led research comparing scientific abstracts written by ChatGPT and real abstracts and discovered that reviewers found it “surprisingly difficult” to differentiate the two.
“The majority of research is published in English,” she said in an email. “Responsible use of LLMs can potentially reduce the burden of writing for busy scientists and improve equity for those who are not native English speakers.”
Dr. Pividori agreed, adding that as a non-native English speaker, he spends much more time working on the structure and grammar of sentences when authoring a manuscript, compared with people who speak English as a first language. He noted that these tools can also be used to automate some of the more monotonous tasks that come along with writing manuscripts and allow researchers to focus on the more creative aspects.
In the future, “I want to focus more on the things that only a human can do and let these tools do all the rest of it,” he said.
New rules
But despite how individual researchers feel about LLMs, they agree that these AI tools are here to stay.
“I think that we should anticipate that they will become part of the medical research establishment over time, when we figure out how to use them appropriately,” Dr. Solomon said.
While the debate of how to best use AI in medical publications will continue, journal editors agree that all authors of a manuscript are solely responsible for content in articles that used AI-assisted technology.
“Authors should carefully review and edit the result because AI can generate authoritative-sounding output that can be incorrect, incomplete, or biased,” the ICMJE guidelines state. “Authors should be able to assert that there is no plagiarism in their paper, including in text and images produced by the AI.” This includes appropriate attribution of all cited materials.
The committee also recommends that authors write in both the cover letter and submitted work how AI was used in the manuscript writing process. Recently updated guidelines from the World Association of Medical Editors recommend that all prompts used to generate new text or analytical work should be provided in submitted work. Dr. Greene also noted that if authors used an AI tool to revise their work, they can include a version of the manuscript untouched by LLMs.
It is similar to a preprint, he said, but rather than publishing a version of a paper prior to peer review, someone is showing a version of a manuscript before it was reviewed and revised by AI. “This type of practice could be a path that lets us benefit from these models,” he said, “without having the drawbacks that many are concerned about.”
Dr. Solomon has financial relationships with AbbVie, Amgen, Janssen, CorEvitas, and Moderna. Both Dr. Greene and Dr. Pividori are inventors in the U.S. Provisional Patent Application No. 63/486,706 that the University of Colorado has filed for the “Publishing Infrastructure For AI-Assisted Academic Authoring” invention with the U.S. Patent and Trademark Office. Dr. Greene and Dr. Pividori also received a grant from the Alfred P. Sloan Foundation to improve their AI-based manuscript revision tool. Dr. Gao reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
. These tools should not be listed as authors, and researchers must denote how AI-assisted technologies were used, the committee said.
These new guidelines are the latest effort for medical journals to define policies for using these large-scale language models (LLMs) in scientific publication. While these AI-assisted tools can help with tasks such as writing, analyzing data, catching mistakes, and much more, they are also prone to errors, noted Casey Greene, PhD, a professor of biomedical informatics at the University of Colorado at Denver, Aurora. It is also not totally clear how information is stored and processed in these kinds of tools, and who has access to that information, he noted.
At the same time, experts argue that these AI tools could have a positive impact on the field by limiting some of the linguistic disparities in scientific publishing as well as alleviating the burden of some monotonous or mechanical tasks that come along with manuscript writing.
What experts can agree on, though, is that the use of AI tools is here to stay. “This is going to become a common tool,” Dr. Greene said. “I don’t think there’s a way out of that at this point.”
A change in medical publishing
OpenAI released ChatGPT in November 2022. In its own words, ChatGPT is “a deep learning model that has been trained on a massive amount of text data to understand and generate humanlike text.” Enter a question or a prompt, and it will respond. For example, when asked how the AI tool can be used in scientific publishing, ChatGPT responded:
“ChatGPT can aid scientific publishing by generating ideas, clarifying concepts, conducting preliminary literature reviews, providing proofreading and editing suggestions, and assisting with outlining and organizing scientific papers. However, it should be used as a supportive tool alongside domain expertise and validation from trusted scientific sources.”
Just a few months after ChatGPT became available, researchers began using this tool in their own work. One individual, Som Biswas, MD, a radiologist at the University of Tennessee Health Science Center in Memphis, reportedly used ChatGPT to author 16 scientific articles in just 4 months, according to the Daily Beast. Five of these articles have been published in four different journals. Dr. Biswas declined to be interviewed for this article.
There were also reports of papers with ChatGPT as one of the listed authors, which sparked backlash. In response, JAMA, Nature, and Science all published editorials in January outlining their policies for using ChatGPT and other large language models in the scientific authoring process. Editors from the journals of the American College of Cardiology and the American College of Rheumatology also updated their policies to reflect the influence of AI authoring tools.
The consensus is that AI has no place on the author byline.
“We think that’s not appropriate, because coauthorship means that you are taking responsibility for the analysis and the generation of data that are included in a manuscript. A machine that is dictated by AI can’t take responsibility,” said Daniel Solomon, MD, MPH, a rheumatologist at Brigham and Women’s Hospital, Boston, and the editor in chief of the ACR journal Arthritis & Rheumatology.
Issues with AI
One of the big concerns around using AI in writing is that it can generate text that seems plausible but is untrue or not supported by data. For example, Dr. Greene and colleague Milton Pividori, PhD, also of the University of Colorado, were writing a journal article about new software they developed that uses a large language model to revise scientific manuscripts.
“We used the same software to revise that article and at one point, it added a line that noted that the large language model had been fine-tuned on a data set of manuscripts from within the same field. This makes a lot of sense, and is absolutely something you could do, but was not something that we did,” Dr. Greene said. “Without a really careful review of the content, it becomes possible to invent things that were not actually done.”
In another case, ChatGPT falsely stated that a prominent law professor had been accused of sexual assault, citing a Washington Post article that did not exist.
“We live in a society where we are extremely concerned about fake news,” Dr. Pividori added, “and [these kinds of errors] could certainly exacerbate that in the scientific community, which is very concerning because science informs public policy.”
Another issue is the lack of transparency around how large language models like ChatGPT process and store data used to make queries.
“We have no idea how they are recording all the prompts and things that we input into ChatGPT and their systems,” Dr. Pividori said.
OpenAI recently addressed some privacy concerns by allowing users to turn off their chat history with the AI chatbot, so conversations cannot be used to train or improve the company’s models. But Dr. Greene noted that the terms of service “still remain pretty nebulous.”
Dr. Solomon is also concerned with researchers using these AI tools in authoring without knowing how they work. “The thing we are really concerned about is that fact that [LLMs] are a bit of a black box – people don’t really understand the methodologies,” he said.
A positive tool?
But despite these concerns, many think that these types of AI-assisted tools could have a positive impact on medical publishing, particularly for researchers for whom English is not their first language, noted Catherine Gao, MD, a pulmonary and critical care instructor at Northwestern University, Chicago. She recently led research comparing scientific abstracts written by ChatGPT and real abstracts and discovered that reviewers found it “surprisingly difficult” to differentiate the two.
“The majority of research is published in English,” she said in an email. “Responsible use of LLMs can potentially reduce the burden of writing for busy scientists and improve equity for those who are not native English speakers.”
Dr. Pividori agreed, adding that as a non-native English speaker, he spends much more time working on the structure and grammar of sentences when authoring a manuscript, compared with people who speak English as a first language. He noted that these tools can also be used to automate some of the more monotonous tasks that come along with writing manuscripts and allow researchers to focus on the more creative aspects.
In the future, “I want to focus more on the things that only a human can do and let these tools do all the rest of it,” he said.
New rules
But despite how individual researchers feel about LLMs, they agree that these AI tools are here to stay.
“I think that we should anticipate that they will become part of the medical research establishment over time, when we figure out how to use them appropriately,” Dr. Solomon said.
While the debate of how to best use AI in medical publications will continue, journal editors agree that all authors of a manuscript are solely responsible for content in articles that used AI-assisted technology.
“Authors should carefully review and edit the result because AI can generate authoritative-sounding output that can be incorrect, incomplete, or biased,” the ICMJE guidelines state. “Authors should be able to assert that there is no plagiarism in their paper, including in text and images produced by the AI.” This includes appropriate attribution of all cited materials.
The committee also recommends that authors write in both the cover letter and submitted work how AI was used in the manuscript writing process. Recently updated guidelines from the World Association of Medical Editors recommend that all prompts used to generate new text or analytical work should be provided in submitted work. Dr. Greene also noted that if authors used an AI tool to revise their work, they can include a version of the manuscript untouched by LLMs.
It is similar to a preprint, he said, but rather than publishing a version of a paper prior to peer review, someone is showing a version of a manuscript before it was reviewed and revised by AI. “This type of practice could be a path that lets us benefit from these models,” he said, “without having the drawbacks that many are concerned about.”
Dr. Solomon has financial relationships with AbbVie, Amgen, Janssen, CorEvitas, and Moderna. Both Dr. Greene and Dr. Pividori are inventors in the U.S. Provisional Patent Application No. 63/486,706 that the University of Colorado has filed for the “Publishing Infrastructure For AI-Assisted Academic Authoring” invention with the U.S. Patent and Trademark Office. Dr. Greene and Dr. Pividori also received a grant from the Alfred P. Sloan Foundation to improve their AI-based manuscript revision tool. Dr. Gao reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Trends in US maternal mortality ratios by race, 2000 – 2020
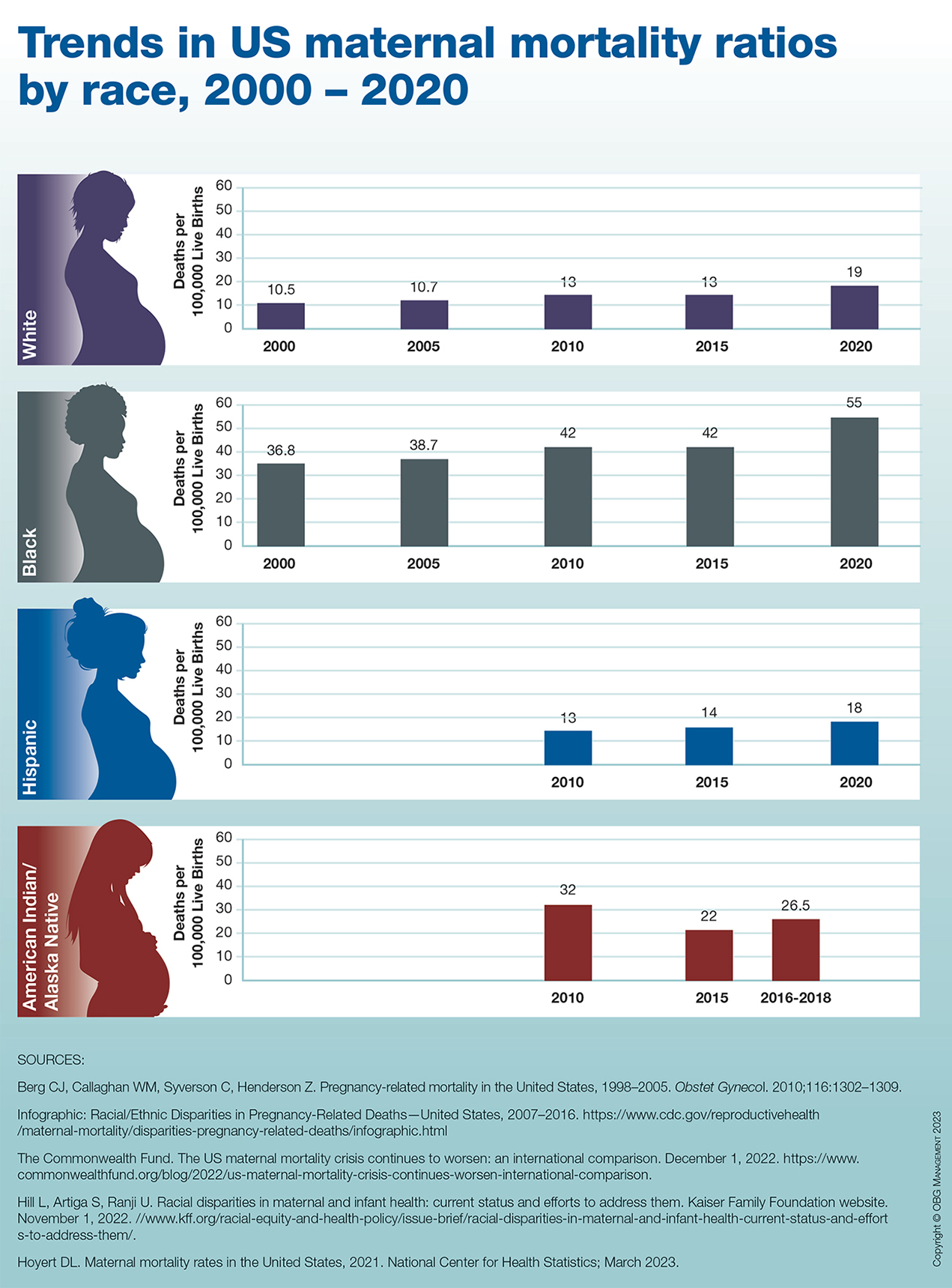


Guide explains nonsurgical management of major hemorrhage
A new guide offers recommendations for the nonsurgical management of major hemorrhage, which is a challenging clinical problem.
Major hemorrhage is a significant cause of death and can occur in a myriad of clinical settings.
“In Ontario, we’ve been collecting quality metrics on major hemorrhages to try and make sure that a higher percentage of patients gets the best possible care when they are experiencing significant bleeding,” author Jeannie Callum, MD, professor and director of transfusion medicine at Kingston (Ont.) Health Sciences Centre and Queen’s University, also in Kingston, said in an interview. “There were some gaps, so this is our effort to get open, clear information out to the emergency doctors, intensive care unit doctors, the surgeons, and everyone else involved in managing major hemorrhage, to help close these gaps.”
The guide was published in the Canadian Medical Association Journal.
Fast care essential
The guide aims to provide answers, based on the latest research, to questions such as when to activate a massive hemorrhage protocol (MHP), which patients should receive tranexamic acid (TXA), which blood products should be transfused before laboratory results are available, how to monitor the effects of blood transfusion, and when fibrinogen concentrate or prothrombin complex concentrate should be given.
Not all recommendations will be followed, Dr. Callum said, especially in rural hospitals with limited resources. But the guide is adaptable, and rural hospitals can create protocols that are customized to their unique circumstances.
Care must be “perfect and fast” in the first hour of major injury, said Dr. Callum. “You need to get a proclotting drug in that first hour if you have a traumatic or postpartum bleed. You have to make sure your clotting factors never fail you throughout your resuscitation. You have to be fast with the transfusion. You have to monitor for the complications of the transfusion, electrolyte disturbances, and the patient’s temperature dropping. It’s a complicated situation that needs a multidisciplinary team.”
Bleeding affects everybody in medicine, from family doctors in smaller institutions who work in emergency departments to obstetricians and surgeons, she added.
“For people under the age of 45, trauma is the most common cause of death. When people die of trauma, they die of bleeding. So many people experience these extreme bleeds. We believe that some of them might be preventable with faster, more standardized, more aggressive care. That’s why we wrote this review,” said Dr. Callum.
Administer TXA quickly
The first recommendation is to ensure that every hospital has a massive hemorrhage protocol. Such a protocol is vital for the emergency department, operating room, and obstetric unit. “Making sure you’ve got a protocol that is updated every 3 years and adjusted to the local hospital context is essential,” said Dr. Callum.
Smaller hospitals will have to adjust their protocols according to the capabilities of their sites. “Some smaller hospitals do not have platelets in stock and get their platelets from another hospital, so you need to adjust your protocol to what you are able to do. Not every hospital can control bleeding in a trauma patient, so your protocol would be to stabilize and call a helicopter. Make sure all of this is detailed so that implementing it becomes automatic,” said Dr. Callum.
An MHP should be activated for patients with uncontrolled hemorrhage who meet the clinical criteria of the local hospital and are expected to need blood product support and red blood cells.
“Lots of people bleed, but not everybody is bleeding enough that they need a code transfusion,” said Dr. Callum. Most patients with gastrointestinal bleeds caused by NSAID use can be managed with uncrossed matched blood from the local blood bank. “But in patients who need the full code transfusion because they are going to need plasma, clotting factor replacement, and many other drugs, that is when the MHP should be activated. Don’t activate it when you don’t need it, because doing so activates the whole hospital and diverts care away from other patients.”
TXA should be administered as soon as possible after onset of hemorrhage in most patients, with the exception of gastrointestinal hemorrhage, where a benefit has not been shown.
TXA has been a major advance in treating massive bleeding, Dr. Callum said. “TXA was invented by a Japanese husband-and-wife research team. We know that it reduces the death rate in trauma and in postpartum hemorrhage, and it reduces the chance of major bleeding with major surgical procedures. We give it routinely in surgical procedures. If a patient gets TXA within 60 minutes of injury, it dramatically reduces the death rate. And it costs $10 per patient. It’s cheap, it’s easy, it has no side effects. It’s just amazing.”
Future research must address several unanswered questions, said Dr. Callum. These questions include whether prehospital transfusion improves patient outcomes, whether whole blood has a role in the early management of major hemorrhage, and what role factor concentrates play in patients with major bleeding.
‘Optimal recommendations’
Commenting on the document, Bourke Tillmann, MD, PhD, trauma team leader at Sunnybrook Health Sciences Centre and the Ross Tilley Burn Center in Toronto, said: “Overall, I think it is a good overview of MHPs as an approach to major hemorrhage.”
The review also is timely, since Ontario released its MHP guidelines in 2021, he added. “I would have liked to see more about the treatment aspects than just an overview of an MHP. But if you are the person overseeing the emergency department or running the blood bank, these protocols are incredibly useful and incredibly important.”
“This report is a nice and thoughtful overview of best practices in many areas, especially trauma, and makes recommendations that are optimal, although they are not necessarily practical in all centers,” Eric L. Legome, MD, professor and chair of emergency medicine at Mount Sinai West and Mount Sinai Morningside, New York, said in an interview.
“If you’re in a small rural hospital with one lab technician, trying to do all of these things, it will not be possible. These are optimal recommendations that people can use to the best of their ability, but they are not standard of care, because some places will not be able to provide this level of care,” he added. “This paper provides practical, reasonable advice that should be looked at as you are trying to implement transfusion policies and processes, with the understanding that it is not necessarily applicable or practical for very small hospitals in very rural centers that might not have access to these types of products and tools, but it’s a reasonable and nicely written paper.”
No outside funding for the guideline was reported. Dr. Callum has received research funding from Canadian Blood Services and Octapharma. She sits on the nominating committee with the Association for the Advancement of Blood & Biotherapies and on the data safety monitoring boards for the Tranexamic Acid for Subdural Hematoma trial and the Fibrinogen Replacement in Trauma trial. Dr. Tillmann and Dr. Legome reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
A new guide offers recommendations for the nonsurgical management of major hemorrhage, which is a challenging clinical problem.
Major hemorrhage is a significant cause of death and can occur in a myriad of clinical settings.
“In Ontario, we’ve been collecting quality metrics on major hemorrhages to try and make sure that a higher percentage of patients gets the best possible care when they are experiencing significant bleeding,” author Jeannie Callum, MD, professor and director of transfusion medicine at Kingston (Ont.) Health Sciences Centre and Queen’s University, also in Kingston, said in an interview. “There were some gaps, so this is our effort to get open, clear information out to the emergency doctors, intensive care unit doctors, the surgeons, and everyone else involved in managing major hemorrhage, to help close these gaps.”
The guide was published in the Canadian Medical Association Journal.
Fast care essential
The guide aims to provide answers, based on the latest research, to questions such as when to activate a massive hemorrhage protocol (MHP), which patients should receive tranexamic acid (TXA), which blood products should be transfused before laboratory results are available, how to monitor the effects of blood transfusion, and when fibrinogen concentrate or prothrombin complex concentrate should be given.
Not all recommendations will be followed, Dr. Callum said, especially in rural hospitals with limited resources. But the guide is adaptable, and rural hospitals can create protocols that are customized to their unique circumstances.
Care must be “perfect and fast” in the first hour of major injury, said Dr. Callum. “You need to get a proclotting drug in that first hour if you have a traumatic or postpartum bleed. You have to make sure your clotting factors never fail you throughout your resuscitation. You have to be fast with the transfusion. You have to monitor for the complications of the transfusion, electrolyte disturbances, and the patient’s temperature dropping. It’s a complicated situation that needs a multidisciplinary team.”
Bleeding affects everybody in medicine, from family doctors in smaller institutions who work in emergency departments to obstetricians and surgeons, she added.
“For people under the age of 45, trauma is the most common cause of death. When people die of trauma, they die of bleeding. So many people experience these extreme bleeds. We believe that some of them might be preventable with faster, more standardized, more aggressive care. That’s why we wrote this review,” said Dr. Callum.
Administer TXA quickly
The first recommendation is to ensure that every hospital has a massive hemorrhage protocol. Such a protocol is vital for the emergency department, operating room, and obstetric unit. “Making sure you’ve got a protocol that is updated every 3 years and adjusted to the local hospital context is essential,” said Dr. Callum.
Smaller hospitals will have to adjust their protocols according to the capabilities of their sites. “Some smaller hospitals do not have platelets in stock and get their platelets from another hospital, so you need to adjust your protocol to what you are able to do. Not every hospital can control bleeding in a trauma patient, so your protocol would be to stabilize and call a helicopter. Make sure all of this is detailed so that implementing it becomes automatic,” said Dr. Callum.
An MHP should be activated for patients with uncontrolled hemorrhage who meet the clinical criteria of the local hospital and are expected to need blood product support and red blood cells.
“Lots of people bleed, but not everybody is bleeding enough that they need a code transfusion,” said Dr. Callum. Most patients with gastrointestinal bleeds caused by NSAID use can be managed with uncrossed matched blood from the local blood bank. “But in patients who need the full code transfusion because they are going to need plasma, clotting factor replacement, and many other drugs, that is when the MHP should be activated. Don’t activate it when you don’t need it, because doing so activates the whole hospital and diverts care away from other patients.”
TXA should be administered as soon as possible after onset of hemorrhage in most patients, with the exception of gastrointestinal hemorrhage, where a benefit has not been shown.
TXA has been a major advance in treating massive bleeding, Dr. Callum said. “TXA was invented by a Japanese husband-and-wife research team. We know that it reduces the death rate in trauma and in postpartum hemorrhage, and it reduces the chance of major bleeding with major surgical procedures. We give it routinely in surgical procedures. If a patient gets TXA within 60 minutes of injury, it dramatically reduces the death rate. And it costs $10 per patient. It’s cheap, it’s easy, it has no side effects. It’s just amazing.”
Future research must address several unanswered questions, said Dr. Callum. These questions include whether prehospital transfusion improves patient outcomes, whether whole blood has a role in the early management of major hemorrhage, and what role factor concentrates play in patients with major bleeding.
‘Optimal recommendations’
Commenting on the document, Bourke Tillmann, MD, PhD, trauma team leader at Sunnybrook Health Sciences Centre and the Ross Tilley Burn Center in Toronto, said: “Overall, I think it is a good overview of MHPs as an approach to major hemorrhage.”
The review also is timely, since Ontario released its MHP guidelines in 2021, he added. “I would have liked to see more about the treatment aspects than just an overview of an MHP. But if you are the person overseeing the emergency department or running the blood bank, these protocols are incredibly useful and incredibly important.”
“This report is a nice and thoughtful overview of best practices in many areas, especially trauma, and makes recommendations that are optimal, although they are not necessarily practical in all centers,” Eric L. Legome, MD, professor and chair of emergency medicine at Mount Sinai West and Mount Sinai Morningside, New York, said in an interview.
“If you’re in a small rural hospital with one lab technician, trying to do all of these things, it will not be possible. These are optimal recommendations that people can use to the best of their ability, but they are not standard of care, because some places will not be able to provide this level of care,” he added. “This paper provides practical, reasonable advice that should be looked at as you are trying to implement transfusion policies and processes, with the understanding that it is not necessarily applicable or practical for very small hospitals in very rural centers that might not have access to these types of products and tools, but it’s a reasonable and nicely written paper.”
No outside funding for the guideline was reported. Dr. Callum has received research funding from Canadian Blood Services and Octapharma. She sits on the nominating committee with the Association for the Advancement of Blood & Biotherapies and on the data safety monitoring boards for the Tranexamic Acid for Subdural Hematoma trial and the Fibrinogen Replacement in Trauma trial. Dr. Tillmann and Dr. Legome reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
A new guide offers recommendations for the nonsurgical management of major hemorrhage, which is a challenging clinical problem.
Major hemorrhage is a significant cause of death and can occur in a myriad of clinical settings.
“In Ontario, we’ve been collecting quality metrics on major hemorrhages to try and make sure that a higher percentage of patients gets the best possible care when they are experiencing significant bleeding,” author Jeannie Callum, MD, professor and director of transfusion medicine at Kingston (Ont.) Health Sciences Centre and Queen’s University, also in Kingston, said in an interview. “There were some gaps, so this is our effort to get open, clear information out to the emergency doctors, intensive care unit doctors, the surgeons, and everyone else involved in managing major hemorrhage, to help close these gaps.”
The guide was published in the Canadian Medical Association Journal.
Fast care essential
The guide aims to provide answers, based on the latest research, to questions such as when to activate a massive hemorrhage protocol (MHP), which patients should receive tranexamic acid (TXA), which blood products should be transfused before laboratory results are available, how to monitor the effects of blood transfusion, and when fibrinogen concentrate or prothrombin complex concentrate should be given.
Not all recommendations will be followed, Dr. Callum said, especially in rural hospitals with limited resources. But the guide is adaptable, and rural hospitals can create protocols that are customized to their unique circumstances.
Care must be “perfect and fast” in the first hour of major injury, said Dr. Callum. “You need to get a proclotting drug in that first hour if you have a traumatic or postpartum bleed. You have to make sure your clotting factors never fail you throughout your resuscitation. You have to be fast with the transfusion. You have to monitor for the complications of the transfusion, electrolyte disturbances, and the patient’s temperature dropping. It’s a complicated situation that needs a multidisciplinary team.”
Bleeding affects everybody in medicine, from family doctors in smaller institutions who work in emergency departments to obstetricians and surgeons, she added.
“For people under the age of 45, trauma is the most common cause of death. When people die of trauma, they die of bleeding. So many people experience these extreme bleeds. We believe that some of them might be preventable with faster, more standardized, more aggressive care. That’s why we wrote this review,” said Dr. Callum.
Administer TXA quickly
The first recommendation is to ensure that every hospital has a massive hemorrhage protocol. Such a protocol is vital for the emergency department, operating room, and obstetric unit. “Making sure you’ve got a protocol that is updated every 3 years and adjusted to the local hospital context is essential,” said Dr. Callum.
Smaller hospitals will have to adjust their protocols according to the capabilities of their sites. “Some smaller hospitals do not have platelets in stock and get their platelets from another hospital, so you need to adjust your protocol to what you are able to do. Not every hospital can control bleeding in a trauma patient, so your protocol would be to stabilize and call a helicopter. Make sure all of this is detailed so that implementing it becomes automatic,” said Dr. Callum.
An MHP should be activated for patients with uncontrolled hemorrhage who meet the clinical criteria of the local hospital and are expected to need blood product support and red blood cells.
“Lots of people bleed, but not everybody is bleeding enough that they need a code transfusion,” said Dr. Callum. Most patients with gastrointestinal bleeds caused by NSAID use can be managed with uncrossed matched blood from the local blood bank. “But in patients who need the full code transfusion because they are going to need plasma, clotting factor replacement, and many other drugs, that is when the MHP should be activated. Don’t activate it when you don’t need it, because doing so activates the whole hospital and diverts care away from other patients.”
TXA should be administered as soon as possible after onset of hemorrhage in most patients, with the exception of gastrointestinal hemorrhage, where a benefit has not been shown.
TXA has been a major advance in treating massive bleeding, Dr. Callum said. “TXA was invented by a Japanese husband-and-wife research team. We know that it reduces the death rate in trauma and in postpartum hemorrhage, and it reduces the chance of major bleeding with major surgical procedures. We give it routinely in surgical procedures. If a patient gets TXA within 60 minutes of injury, it dramatically reduces the death rate. And it costs $10 per patient. It’s cheap, it’s easy, it has no side effects. It’s just amazing.”
Future research must address several unanswered questions, said Dr. Callum. These questions include whether prehospital transfusion improves patient outcomes, whether whole blood has a role in the early management of major hemorrhage, and what role factor concentrates play in patients with major bleeding.
‘Optimal recommendations’
Commenting on the document, Bourke Tillmann, MD, PhD, trauma team leader at Sunnybrook Health Sciences Centre and the Ross Tilley Burn Center in Toronto, said: “Overall, I think it is a good overview of MHPs as an approach to major hemorrhage.”
The review also is timely, since Ontario released its MHP guidelines in 2021, he added. “I would have liked to see more about the treatment aspects than just an overview of an MHP. But if you are the person overseeing the emergency department or running the blood bank, these protocols are incredibly useful and incredibly important.”
“This report is a nice and thoughtful overview of best practices in many areas, especially trauma, and makes recommendations that are optimal, although they are not necessarily practical in all centers,” Eric L. Legome, MD, professor and chair of emergency medicine at Mount Sinai West and Mount Sinai Morningside, New York, said in an interview.
“If you’re in a small rural hospital with one lab technician, trying to do all of these things, it will not be possible. These are optimal recommendations that people can use to the best of their ability, but they are not standard of care, because some places will not be able to provide this level of care,” he added. “This paper provides practical, reasonable advice that should be looked at as you are trying to implement transfusion policies and processes, with the understanding that it is not necessarily applicable or practical for very small hospitals in very rural centers that might not have access to these types of products and tools, but it’s a reasonable and nicely written paper.”
No outside funding for the guideline was reported. Dr. Callum has received research funding from Canadian Blood Services and Octapharma. She sits on the nominating committee with the Association for the Advancement of Blood & Biotherapies and on the data safety monitoring boards for the Tranexamic Acid for Subdural Hematoma trial and the Fibrinogen Replacement in Trauma trial. Dr. Tillmann and Dr. Legome reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM THE CANADIAN MEDICAL ASSOCIATION JOURNAL
Nitroglycerin patches do not improve menopause symptoms
Vasomotor symptoms affect as many as 75% of menopausal women in the United States. Characterized by a sudden onset of flushing, sweating, and chills, symptoms of hot flashes can be managed with hormone therapy, but prolonged use of the treatment poses health risks. In a study published in JAMA Internal Medicine,
METHODOLOGY
- The was a randomized, double-blinded trial involving 134 California women aged 40-62 years.
- Between July 2018 and December 2021, participants self-administered either a nitroglycerin patch at a dosage of 0.2 to 0.6 mg/h or a placebo patch every night.
- Participants were in the late stages of menopause or had already undergone menopause. They reported having seven or more hot flashes per day; at least four were moderate to severe over a 1-week period.
- The primary outcome was a change in the frequency of hot flashes over 5 and 12 weeks.
TAKEAWAY
- Over 5 weeks, the frequency of moderate to severe hot flashes decreased by 3.3 episodes per day in the nitroglycerine group, compared with 2.2 episodes per day in the placebo group (95% CI, −2.2 to 0; P = .05).
- The reduction in overall frequency of hot flashes – either mild, moderate, or severe – over the 5-week period was not statistically significant.
- Over the 12-week period, no statistically significant reductions in hot flashes occurred.
- More than two thirds of participants assigned to the nitroglycerin patches reported having headaches, while three reported chest pain and one had a syncopal episode.
IN PRACTICE
The findings do not support daily use of nitroglycerin patches to treat vasomotor symptoms, the researchers conclude.
“The bottom line is that our study doesn’t allow us to recommend nitroglycerin skin patches as a strategy for consumers to suppress hot flashes in the long term,” Alison Huang, MD, MAS, lead author of the study, said in a press release. “The menopause field is still lacking in effective treatment approaches that don’t involve hormones.”
STUDY DETAILS
The study was led by Alison Huang, MD, MAS, a professor of medicine at the University of California, San Francisco. Two of the authors reported grants from the National Institute on Aging.
LIMITATIONS
Almost 20% of women who used the nitroglycerin patches discontinued treatment before the end of the trial because they could not tolerate the medication, experienced an adverse event, or their symptoms did not improve, according to the researchers. In addition, the 1-week period used to screen for severity and frequency of hot flashes may have been too short to confirm that symptoms were prolonged, which could explain the better-than-expected results in the placebo group.
DISCLOSURES
One author served on the medical advisory board of SomaLogic. Another author is an unpaid consultant to Astellas Pharma. Another author reported grants from the National Institutes of Health.
A version of this article first appeared on Medscape.com.
Vasomotor symptoms affect as many as 75% of menopausal women in the United States. Characterized by a sudden onset of flushing, sweating, and chills, symptoms of hot flashes can be managed with hormone therapy, but prolonged use of the treatment poses health risks. In a study published in JAMA Internal Medicine,
METHODOLOGY
- The was a randomized, double-blinded trial involving 134 California women aged 40-62 years.
- Between July 2018 and December 2021, participants self-administered either a nitroglycerin patch at a dosage of 0.2 to 0.6 mg/h or a placebo patch every night.
- Participants were in the late stages of menopause or had already undergone menopause. They reported having seven or more hot flashes per day; at least four were moderate to severe over a 1-week period.
- The primary outcome was a change in the frequency of hot flashes over 5 and 12 weeks.
TAKEAWAY
- Over 5 weeks, the frequency of moderate to severe hot flashes decreased by 3.3 episodes per day in the nitroglycerine group, compared with 2.2 episodes per day in the placebo group (95% CI, −2.2 to 0; P = .05).
- The reduction in overall frequency of hot flashes – either mild, moderate, or severe – over the 5-week period was not statistically significant.
- Over the 12-week period, no statistically significant reductions in hot flashes occurred.
- More than two thirds of participants assigned to the nitroglycerin patches reported having headaches, while three reported chest pain and one had a syncopal episode.
IN PRACTICE
The findings do not support daily use of nitroglycerin patches to treat vasomotor symptoms, the researchers conclude.
“The bottom line is that our study doesn’t allow us to recommend nitroglycerin skin patches as a strategy for consumers to suppress hot flashes in the long term,” Alison Huang, MD, MAS, lead author of the study, said in a press release. “The menopause field is still lacking in effective treatment approaches that don’t involve hormones.”
STUDY DETAILS
The study was led by Alison Huang, MD, MAS, a professor of medicine at the University of California, San Francisco. Two of the authors reported grants from the National Institute on Aging.
LIMITATIONS
Almost 20% of women who used the nitroglycerin patches discontinued treatment before the end of the trial because they could not tolerate the medication, experienced an adverse event, or their symptoms did not improve, according to the researchers. In addition, the 1-week period used to screen for severity and frequency of hot flashes may have been too short to confirm that symptoms were prolonged, which could explain the better-than-expected results in the placebo group.
DISCLOSURES
One author served on the medical advisory board of SomaLogic. Another author is an unpaid consultant to Astellas Pharma. Another author reported grants from the National Institutes of Health.
A version of this article first appeared on Medscape.com.
Vasomotor symptoms affect as many as 75% of menopausal women in the United States. Characterized by a sudden onset of flushing, sweating, and chills, symptoms of hot flashes can be managed with hormone therapy, but prolonged use of the treatment poses health risks. In a study published in JAMA Internal Medicine,
METHODOLOGY
- The was a randomized, double-blinded trial involving 134 California women aged 40-62 years.
- Between July 2018 and December 2021, participants self-administered either a nitroglycerin patch at a dosage of 0.2 to 0.6 mg/h or a placebo patch every night.
- Participants were in the late stages of menopause or had already undergone menopause. They reported having seven or more hot flashes per day; at least four were moderate to severe over a 1-week period.
- The primary outcome was a change in the frequency of hot flashes over 5 and 12 weeks.
TAKEAWAY
- Over 5 weeks, the frequency of moderate to severe hot flashes decreased by 3.3 episodes per day in the nitroglycerine group, compared with 2.2 episodes per day in the placebo group (95% CI, −2.2 to 0; P = .05).
- The reduction in overall frequency of hot flashes – either mild, moderate, or severe – over the 5-week period was not statistically significant.
- Over the 12-week period, no statistically significant reductions in hot flashes occurred.
- More than two thirds of participants assigned to the nitroglycerin patches reported having headaches, while three reported chest pain and one had a syncopal episode.
IN PRACTICE
The findings do not support daily use of nitroglycerin patches to treat vasomotor symptoms, the researchers conclude.
“The bottom line is that our study doesn’t allow us to recommend nitroglycerin skin patches as a strategy for consumers to suppress hot flashes in the long term,” Alison Huang, MD, MAS, lead author of the study, said in a press release. “The menopause field is still lacking in effective treatment approaches that don’t involve hormones.”
STUDY DETAILS
The study was led by Alison Huang, MD, MAS, a professor of medicine at the University of California, San Francisco. Two of the authors reported grants from the National Institute on Aging.
LIMITATIONS
Almost 20% of women who used the nitroglycerin patches discontinued treatment before the end of the trial because they could not tolerate the medication, experienced an adverse event, or their symptoms did not improve, according to the researchers. In addition, the 1-week period used to screen for severity and frequency of hot flashes may have been too short to confirm that symptoms were prolonged, which could explain the better-than-expected results in the placebo group.
DISCLOSURES
One author served on the medical advisory board of SomaLogic. Another author is an unpaid consultant to Astellas Pharma. Another author reported grants from the National Institutes of Health.
A version of this article first appeared on Medscape.com.
When could you be sued for AI malpractice? You’re likely using it now
The ways in which artificial intelligence (AI) may transform the future of medicine is making headlines across the globe. But chances are, you’re already using AI in your practice every day – you may just not realize it.
And whether you recognize the presence of AI or not, the technology could be putting you in danger of a lawsuit, legal experts say.
“For physicians, AI has also not yet drastically changed or improved the way care is provided or consumed,” said Michael LeTang, chief nursing informatics officer and vice president of risk management and patient safety at Healthcare Risk Advisors, part of TDC Group. “Consequently, it may seem like AI is not present in their work streams, but in reality, it has been utilized in health care for several years. As AI technologies continue to develop and become more sophisticated, we can expect them to play an increasingly significant role in health care.”
Today, most AI applications in health care use narrow AI, which is designed to complete a single task without human assistance, as opposed to artificial general intelligence (AGI), which pertains to human-level reasoning and problem solving across a broad spectrum. Here are some ways doctors are using AI throughout the day – sometimes being aware of its assistance, and sometimes being unaware:
- Many doctors use electronic health records (EHRs) with integrated AI that include computerized clinical decision support tools designed to reduce the risk of diagnostic error and to integrate decision-making in the medication ordering function.
- Cardiologists, pathologists, and dermatologists use AI in the interpretation of vast amounts of images, tracings, and complex patterns.
- Surgeons are using AI-enhanced surgical robotics for orthopedic surgeries, such as joint replacement and spine surgery.
- A growing number of doctors are using ChatGPT to assist in drafting prior authorization letters for insurers. Experts say more doctors are also experimenting with ChatGPT to support medical decision-making.
- Within oncology, physicians use machine learning techniques in the form of computer-aided detection systems for early breast cancer detection.
- AI algorithms are often used by health systems for workflow, staffing optimization, population management, and care coordination.
- Some systems within EHRs use AI to indicate high-risk patients.
- Physicians are using AI applications for the early recognition of sepsis, including EHR-integrated decision tools, such as the Hospital Corporation of America Healthcare’s Sepsis Prediction and Optimization Therapy and the Sepsis Early Risk Assessment algorithm.
- About 30% of radiologists use AI in their practice to analyze x-rays and CT scans.
- Epic Systems recently announced a partnership with Microsoft to integrate ChatGPT into MyChart, Epic’s patient portal system. Pilot hospitals will utilize ChatGPT to automatically generate responses to patient-generated questions sent via the portal.
The growth of AI in health care has been enormous, and it’s only going to continue, said Ravi B. Parikh, MD, an assistant professor in the department of medical ethics and health policy and medicine at the University of Pennsylvania, Philadelphia.
“What’s really critical is that physicians, clinicians, and nurses using AI are provided with the tools to understand how artificial intelligence works and, most importantly, understand that they are still accountable for making the ultimate decision,” Mr. LeTang said, “The information is not always going to be the right thing to do or the most accurate thing to do. They’re still liable for making a bad decision, even if AI is driving that.”
What are the top AI legal dangers of today?
A pressing legal risk is becoming too reliant on the suggestions that AI-based systems provide, which can lead to poor care decisions, said Kenneth Rashbaum, a New York–based cybersecurity attorney with more than 25 years of experience in medical malpractice defense.
This can occur, for example, when using clinical support systems that leverage AI, machine learning, or statistical pattern recognition. Today, clinical support systems are commonly administered through EHRs and other computerized clinical workflows. In general, such systems match a patient’s characteristics to a computerized clinical knowledge base. An assessment or recommendation is then presented to the physician for a decision.
“If the clinician blindly accepts it without considering whether it’s appropriate for this patient at this time with this presentation, the clinician may bear some responsibility if there is an untoward result,” Mr. Rashbaum said.
“A common claim even in the days before the EMR [electronic medical record] and AI, was that the clinician did not take all available information into account in rendering treatment, including history of past and present condition, as reflected in the records, communication with past and other present treating clinicians, lab and radiology results, discussions with the patient, and physical examination findings,” he said. “So, if the clinician relied upon the support prompt to the exclusion of these other sources of information, that could be a very strong argument for the plaintiff.”
Chatbots, such OpenAI’s ChatGPT, are another form of AI raising legal red flags. ChatGPT, trained on a massive set of text data, can carry out conversations, write code, draft emails, and answer any question posed. The chatbot has gained considerable credibility for accurately diagnosing rare conditions in seconds, and it recently passed the U.S. Medical Licensing Examination.
It’s unclear how many doctors are signing onto the ChatGPT website daily, but physicians are actively using the chatbot, particularly for assistance with prior authorization letters and to support decision-making processes in their practices, said Mr. LeTang.
When physicians ask ChatGPT a question, however, they should be mindful that ChatGPT could “hallucinate,” a term that refers to a generated response that sounds plausible but is factually incorrect or is unrelated to the context, explains Harvey Castro, MD, an emergency physician, ChatGPT health care expert, and author of the 2023 book “ChatGPT and Healthcare: Unlocking the Potential of Patient Empowerment.”
Acting on ChatGPT’s response without vetting the information places doctors at serious risk of a lawsuit, he said.
“Sometimes, the response is half true and half false,” he said. “Say, I go outside my specialty of emergency medicine and ask it about a pediatric surgical procedure. It could give me a response that sounds medically correct, but then I ask a pediatric cardiologist, and he says, ‘We don’t even do this. This doesn’t even exist!’ Physicians really have to make sure they are vetting the information provided.”
In response to ChatGPT’s growing usage by health care professionals, hospitals and practices are quickly implementing guidelines, policies, and restrictions that caution physicians about the accuracy of ChatGPT-generated information, adds Mr. LeTang.
Emerging best practices include avoiding the input of patient health information, personally identifiable information, or any data that could be commercially valuable or considered the intellectual property of a hospital or health system, he said.
“Another crucial guideline is not to rely solely on ChatGPT as a definitive source for clinical decision-making; physicians must exercise their professional judgment,” he said. “If best practices are not adhered to, the associated risks are present today. However, these risks may become more significant as AI technologies continue to evolve and become increasingly integrated into health care.”
The potential for misdiagnosis by AI systems and the risk of unnecessary procedures if physicians do not thoroughly evaluate and validate AI predictions are other dangers.
As an example, Mr. LeTang described a case in which a physician documents in the EHR that a patient has presented to the emergency department with chest pains and other signs of a heart attack, and an AI algorithm predicts that the patient is experiencing an active myocardial infarction. If the physician then sends the patient for stenting or an angioplasty without other concrete evidence or tests to confirm the diagnosis, the doctor could later face a misdiagnosis complaint if the costly procedures were unnecessary.
“That’s one of the risks of using artificial intelligence,” he said. “A large percentage of malpractice claims is failure to diagnose, delayed diagnosis, or inaccurate diagnosis. What falls in the category of failure to diagnose is sending a patient for an unnecessary procedure or having an adverse event or bad outcome because of the failure to diagnose.”
So far, no AI lawsuits have been filed, but they may make an appearance soon, said Sue Boisvert, senior patient safety risk manager at The Doctors Company, a national medical liability insurer.
“There are hundreds of AI programs currently in use in health care,” she said. “At some point, a provider will make a decision that is contrary to what the AI recommended. The AI may be wrong, or the provider may be wrong. Either way, the provider will neglect to document their clinical reasoning, a patient will be harmed, and we will have the first AI claim.”
Upcoming AI legal risks to watch for
Lawsuits that allege biased patient care by physicians on the basis of algorithmic bias may also be forthcoming, analysts warn.
Much has been written about algorithmic bias that compounds and worsens inequities in socioeconomic status, ethnicity, sexual orientation, and gender in health systems. In 2019, a groundbreaking article in Science shed light on commonly used algorithms that are considered racially biased and how health care professionals often use such information to make medical decisions.
No claims involving AI bias have come down the pipeline yet, but it’s an area to watch, said Ms. Boisvert. She noted a website that highlights complaints and accusations of AI bias, including in health care.
“We need to be sure the training of the AI is appropriate, current, and broad enough so that there is no bias in the AI when it’s participating in the decision-making,” said Ms. Boisvert. “Imagine if the AI is diagnosing based on a dataset that is not local. It doesn’t represent the population at that particular hospital, and it’s providing inaccurate information to the physicians who are then making decisions about treatment.”
In pain management, for example, there are known differences in how patients experience pain, Ms. Boisvert said. If AI was being used to develop an algorithm for how a particular patient’s postoperative pain should be managed, and the algorithm did not include the differences, the pain control for a certain patient could be inappropriate. A poor outcome resulting from the treatment could lead to a claim against the physician or hospital that used the biased AI system, she said.
In the future, as AI becomes more integrated and accepted in medicine, there may be a risk of legal complaints against doctors for not using AI, said Saurabh Jha, MD, an associate professor of radiology at the University of Pennsylvania, Philadelphia, and a scholar of AI in radiology.
“Ultimately, we might get to a place where AI starts helping physicians detect more or reduce the miss of certain conditions, and it becomes the standard of care,” Dr. Jha said. “For example, if it became part of the standard of care for pulmonary embolism [PE] detection, and you didn’t use it for PE detection, and there was a miss. That could put you at legal risk. We’re not at that stage yet, but that is one future possibility.”
Dr. Parikh envisions an even cloudier liability landscape as the potential grows for AI to control patient care decisions. In such a scenario, rather than just issuing an alert or prediction to a physician, the AI system could trigger an action.
For instance, if an algorithm is trained to predict sepsis and, once triggered, the AI could initiate a nurse-led rapid response or a change in patient care outside the clinician’s control, said Dr. Parikh, who coauthored a recent article on AI and medical liability in The Milbank Quarterly.
“That’s still very much the minority of how AI is being used, but as evidence is growing that AI-based diagnostic tools perform equivalent or even superior to physicians, these autonomous workflows are being considered,” Dr. Parikh said. “When the ultimate action upon the patient is more determined by the AI than what the clinician does, then I think the liability picture gets murkier, and we should be thinking about how we can respond to that from a liability framework.”
How you can prevent AI-related lawsuits
The first step to preventing an AI-related claim is being aware of when and how you are using AI.
Ensure you’re informed about how the AI was trained, Ms. Boisvert stresses.
“Ask questions!” she said. “Is the AI safe? Are the recommendations accurate? Does the AI perform better than current systems? In what way? What databases were used, and did the programmers consider bias? Do I understand how to use the results?”
Never blindly trust the AI but rather view it as a data point in a medical decision, said Dr. Parikh. Ensure that other sources of medical information are properly accessed and that best practices for your specialty are still being followed.
When using any form of AI, document your usage, adds Mr. Rashbaum. A record that clearly outlines how the physician incorporated the AI is critical if a claim later arises in which the doctor is accused of AI-related malpractice, he said.
“Indicating how the AI tool was used, why it was used, and that it was used in conjunction with available clinical information and the clinician’s best judgment could reduce the risk of being found responsible as a result of AI use in a particular case,” he said.
Use chatbots, such as ChatGPT, the way they were intended, as support tools, rather than definitive diagnostic instruments, adds Dr. Castro.
“Doctors should also be well-trained in interpreting and understanding the suggestions provided by ChatGPT and should use their clinical judgment and experience alongside the AI tool for more accurate decision-making,” he said.
In addition, because no AI insurance product exists on the market, physicians and organizations using AI – particularly for direct health care – should evaluate their current insurance or insurance-like products to determine where a claim involving AI might fall and whether the policy would respond, said Ms. Boisvert. The AI vendor/manufacturer will likely have indemnified themselves in the purchase and sale agreement or contract, she said.
It will also become increasingly important for medical practices, hospitals, and health systems to put in place strong data governance strategies, Mr. LeTang said.
“AI relies on good data,” he said. “A data governance strategy is a key component to making sure we understand where the data is coming from, what is represents, how accurate it is, if it’s reproducible, what controls are in place to ensure the right people have the right access, and that if we’re starting to use it to build algorithms, that it’s deidentified.”
While no malpractice claims associated with the use of AI have yet surfaced, this may change as legal courts catch up on the backlog of malpractice claims that were delayed because of COVID-19, and even more so as AI becomes more prevalent in health care, Mr. LeTang said.
“Similar to the attention that autonomous driving systems, like Tesla, receive when the system fails and accidents occur, we can be assured that media outlets will widely publicize AI-related medical adverse events,” he said. “It is crucial for health care professionals, AI developers, and regulatory authorities to work together to ensure the responsible use of AI in health care, with patient safety as the top priority. By doing so, they can mitigate the risks associated with AI implementation and minimize the potential for legal disputes arising from AI-related medical errors.”
A version of this article first appeared on Medscape.com.
The ways in which artificial intelligence (AI) may transform the future of medicine is making headlines across the globe. But chances are, you’re already using AI in your practice every day – you may just not realize it.
And whether you recognize the presence of AI or not, the technology could be putting you in danger of a lawsuit, legal experts say.
“For physicians, AI has also not yet drastically changed or improved the way care is provided or consumed,” said Michael LeTang, chief nursing informatics officer and vice president of risk management and patient safety at Healthcare Risk Advisors, part of TDC Group. “Consequently, it may seem like AI is not present in their work streams, but in reality, it has been utilized in health care for several years. As AI technologies continue to develop and become more sophisticated, we can expect them to play an increasingly significant role in health care.”
Today, most AI applications in health care use narrow AI, which is designed to complete a single task without human assistance, as opposed to artificial general intelligence (AGI), which pertains to human-level reasoning and problem solving across a broad spectrum. Here are some ways doctors are using AI throughout the day – sometimes being aware of its assistance, and sometimes being unaware:
- Many doctors use electronic health records (EHRs) with integrated AI that include computerized clinical decision support tools designed to reduce the risk of diagnostic error and to integrate decision-making in the medication ordering function.
- Cardiologists, pathologists, and dermatologists use AI in the interpretation of vast amounts of images, tracings, and complex patterns.
- Surgeons are using AI-enhanced surgical robotics for orthopedic surgeries, such as joint replacement and spine surgery.
- A growing number of doctors are using ChatGPT to assist in drafting prior authorization letters for insurers. Experts say more doctors are also experimenting with ChatGPT to support medical decision-making.
- Within oncology, physicians use machine learning techniques in the form of computer-aided detection systems for early breast cancer detection.
- AI algorithms are often used by health systems for workflow, staffing optimization, population management, and care coordination.
- Some systems within EHRs use AI to indicate high-risk patients.
- Physicians are using AI applications for the early recognition of sepsis, including EHR-integrated decision tools, such as the Hospital Corporation of America Healthcare’s Sepsis Prediction and Optimization Therapy and the Sepsis Early Risk Assessment algorithm.
- About 30% of radiologists use AI in their practice to analyze x-rays and CT scans.
- Epic Systems recently announced a partnership with Microsoft to integrate ChatGPT into MyChart, Epic’s patient portal system. Pilot hospitals will utilize ChatGPT to automatically generate responses to patient-generated questions sent via the portal.
The growth of AI in health care has been enormous, and it’s only going to continue, said Ravi B. Parikh, MD, an assistant professor in the department of medical ethics and health policy and medicine at the University of Pennsylvania, Philadelphia.
“What’s really critical is that physicians, clinicians, and nurses using AI are provided with the tools to understand how artificial intelligence works and, most importantly, understand that they are still accountable for making the ultimate decision,” Mr. LeTang said, “The information is not always going to be the right thing to do or the most accurate thing to do. They’re still liable for making a bad decision, even if AI is driving that.”
What are the top AI legal dangers of today?
A pressing legal risk is becoming too reliant on the suggestions that AI-based systems provide, which can lead to poor care decisions, said Kenneth Rashbaum, a New York–based cybersecurity attorney with more than 25 years of experience in medical malpractice defense.
This can occur, for example, when using clinical support systems that leverage AI, machine learning, or statistical pattern recognition. Today, clinical support systems are commonly administered through EHRs and other computerized clinical workflows. In general, such systems match a patient’s characteristics to a computerized clinical knowledge base. An assessment or recommendation is then presented to the physician for a decision.
“If the clinician blindly accepts it without considering whether it’s appropriate for this patient at this time with this presentation, the clinician may bear some responsibility if there is an untoward result,” Mr. Rashbaum said.
“A common claim even in the days before the EMR [electronic medical record] and AI, was that the clinician did not take all available information into account in rendering treatment, including history of past and present condition, as reflected in the records, communication with past and other present treating clinicians, lab and radiology results, discussions with the patient, and physical examination findings,” he said. “So, if the clinician relied upon the support prompt to the exclusion of these other sources of information, that could be a very strong argument for the plaintiff.”
Chatbots, such OpenAI’s ChatGPT, are another form of AI raising legal red flags. ChatGPT, trained on a massive set of text data, can carry out conversations, write code, draft emails, and answer any question posed. The chatbot has gained considerable credibility for accurately diagnosing rare conditions in seconds, and it recently passed the U.S. Medical Licensing Examination.
It’s unclear how many doctors are signing onto the ChatGPT website daily, but physicians are actively using the chatbot, particularly for assistance with prior authorization letters and to support decision-making processes in their practices, said Mr. LeTang.
When physicians ask ChatGPT a question, however, they should be mindful that ChatGPT could “hallucinate,” a term that refers to a generated response that sounds plausible but is factually incorrect or is unrelated to the context, explains Harvey Castro, MD, an emergency physician, ChatGPT health care expert, and author of the 2023 book “ChatGPT and Healthcare: Unlocking the Potential of Patient Empowerment.”
Acting on ChatGPT’s response without vetting the information places doctors at serious risk of a lawsuit, he said.
“Sometimes, the response is half true and half false,” he said. “Say, I go outside my specialty of emergency medicine and ask it about a pediatric surgical procedure. It could give me a response that sounds medically correct, but then I ask a pediatric cardiologist, and he says, ‘We don’t even do this. This doesn’t even exist!’ Physicians really have to make sure they are vetting the information provided.”
In response to ChatGPT’s growing usage by health care professionals, hospitals and practices are quickly implementing guidelines, policies, and restrictions that caution physicians about the accuracy of ChatGPT-generated information, adds Mr. LeTang.
Emerging best practices include avoiding the input of patient health information, personally identifiable information, or any data that could be commercially valuable or considered the intellectual property of a hospital or health system, he said.
“Another crucial guideline is not to rely solely on ChatGPT as a definitive source for clinical decision-making; physicians must exercise their professional judgment,” he said. “If best practices are not adhered to, the associated risks are present today. However, these risks may become more significant as AI technologies continue to evolve and become increasingly integrated into health care.”
The potential for misdiagnosis by AI systems and the risk of unnecessary procedures if physicians do not thoroughly evaluate and validate AI predictions are other dangers.
As an example, Mr. LeTang described a case in which a physician documents in the EHR that a patient has presented to the emergency department with chest pains and other signs of a heart attack, and an AI algorithm predicts that the patient is experiencing an active myocardial infarction. If the physician then sends the patient for stenting or an angioplasty without other concrete evidence or tests to confirm the diagnosis, the doctor could later face a misdiagnosis complaint if the costly procedures were unnecessary.
“That’s one of the risks of using artificial intelligence,” he said. “A large percentage of malpractice claims is failure to diagnose, delayed diagnosis, or inaccurate diagnosis. What falls in the category of failure to diagnose is sending a patient for an unnecessary procedure or having an adverse event or bad outcome because of the failure to diagnose.”
So far, no AI lawsuits have been filed, but they may make an appearance soon, said Sue Boisvert, senior patient safety risk manager at The Doctors Company, a national medical liability insurer.
“There are hundreds of AI programs currently in use in health care,” she said. “At some point, a provider will make a decision that is contrary to what the AI recommended. The AI may be wrong, or the provider may be wrong. Either way, the provider will neglect to document their clinical reasoning, a patient will be harmed, and we will have the first AI claim.”
Upcoming AI legal risks to watch for
Lawsuits that allege biased patient care by physicians on the basis of algorithmic bias may also be forthcoming, analysts warn.
Much has been written about algorithmic bias that compounds and worsens inequities in socioeconomic status, ethnicity, sexual orientation, and gender in health systems. In 2019, a groundbreaking article in Science shed light on commonly used algorithms that are considered racially biased and how health care professionals often use such information to make medical decisions.
No claims involving AI bias have come down the pipeline yet, but it’s an area to watch, said Ms. Boisvert. She noted a website that highlights complaints and accusations of AI bias, including in health care.
“We need to be sure the training of the AI is appropriate, current, and broad enough so that there is no bias in the AI when it’s participating in the decision-making,” said Ms. Boisvert. “Imagine if the AI is diagnosing based on a dataset that is not local. It doesn’t represent the population at that particular hospital, and it’s providing inaccurate information to the physicians who are then making decisions about treatment.”
In pain management, for example, there are known differences in how patients experience pain, Ms. Boisvert said. If AI was being used to develop an algorithm for how a particular patient’s postoperative pain should be managed, and the algorithm did not include the differences, the pain control for a certain patient could be inappropriate. A poor outcome resulting from the treatment could lead to a claim against the physician or hospital that used the biased AI system, she said.
In the future, as AI becomes more integrated and accepted in medicine, there may be a risk of legal complaints against doctors for not using AI, said Saurabh Jha, MD, an associate professor of radiology at the University of Pennsylvania, Philadelphia, and a scholar of AI in radiology.
“Ultimately, we might get to a place where AI starts helping physicians detect more or reduce the miss of certain conditions, and it becomes the standard of care,” Dr. Jha said. “For example, if it became part of the standard of care for pulmonary embolism [PE] detection, and you didn’t use it for PE detection, and there was a miss. That could put you at legal risk. We’re not at that stage yet, but that is one future possibility.”
Dr. Parikh envisions an even cloudier liability landscape as the potential grows for AI to control patient care decisions. In such a scenario, rather than just issuing an alert or prediction to a physician, the AI system could trigger an action.
For instance, if an algorithm is trained to predict sepsis and, once triggered, the AI could initiate a nurse-led rapid response or a change in patient care outside the clinician’s control, said Dr. Parikh, who coauthored a recent article on AI and medical liability in The Milbank Quarterly.
“That’s still very much the minority of how AI is being used, but as evidence is growing that AI-based diagnostic tools perform equivalent or even superior to physicians, these autonomous workflows are being considered,” Dr. Parikh said. “When the ultimate action upon the patient is more determined by the AI than what the clinician does, then I think the liability picture gets murkier, and we should be thinking about how we can respond to that from a liability framework.”
How you can prevent AI-related lawsuits
The first step to preventing an AI-related claim is being aware of when and how you are using AI.
Ensure you’re informed about how the AI was trained, Ms. Boisvert stresses.
“Ask questions!” she said. “Is the AI safe? Are the recommendations accurate? Does the AI perform better than current systems? In what way? What databases were used, and did the programmers consider bias? Do I understand how to use the results?”
Never blindly trust the AI but rather view it as a data point in a medical decision, said Dr. Parikh. Ensure that other sources of medical information are properly accessed and that best practices for your specialty are still being followed.
When using any form of AI, document your usage, adds Mr. Rashbaum. A record that clearly outlines how the physician incorporated the AI is critical if a claim later arises in which the doctor is accused of AI-related malpractice, he said.
“Indicating how the AI tool was used, why it was used, and that it was used in conjunction with available clinical information and the clinician’s best judgment could reduce the risk of being found responsible as a result of AI use in a particular case,” he said.
Use chatbots, such as ChatGPT, the way they were intended, as support tools, rather than definitive diagnostic instruments, adds Dr. Castro.
“Doctors should also be well-trained in interpreting and understanding the suggestions provided by ChatGPT and should use their clinical judgment and experience alongside the AI tool for more accurate decision-making,” he said.
In addition, because no AI insurance product exists on the market, physicians and organizations using AI – particularly for direct health care – should evaluate their current insurance or insurance-like products to determine where a claim involving AI might fall and whether the policy would respond, said Ms. Boisvert. The AI vendor/manufacturer will likely have indemnified themselves in the purchase and sale agreement or contract, she said.
It will also become increasingly important for medical practices, hospitals, and health systems to put in place strong data governance strategies, Mr. LeTang said.
“AI relies on good data,” he said. “A data governance strategy is a key component to making sure we understand where the data is coming from, what is represents, how accurate it is, if it’s reproducible, what controls are in place to ensure the right people have the right access, and that if we’re starting to use it to build algorithms, that it’s deidentified.”
While no malpractice claims associated with the use of AI have yet surfaced, this may change as legal courts catch up on the backlog of malpractice claims that were delayed because of COVID-19, and even more so as AI becomes more prevalent in health care, Mr. LeTang said.
“Similar to the attention that autonomous driving systems, like Tesla, receive when the system fails and accidents occur, we can be assured that media outlets will widely publicize AI-related medical adverse events,” he said. “It is crucial for health care professionals, AI developers, and regulatory authorities to work together to ensure the responsible use of AI in health care, with patient safety as the top priority. By doing so, they can mitigate the risks associated with AI implementation and minimize the potential for legal disputes arising from AI-related medical errors.”
A version of this article first appeared on Medscape.com.
The ways in which artificial intelligence (AI) may transform the future of medicine is making headlines across the globe. But chances are, you’re already using AI in your practice every day – you may just not realize it.
And whether you recognize the presence of AI or not, the technology could be putting you in danger of a lawsuit, legal experts say.
“For physicians, AI has also not yet drastically changed or improved the way care is provided or consumed,” said Michael LeTang, chief nursing informatics officer and vice president of risk management and patient safety at Healthcare Risk Advisors, part of TDC Group. “Consequently, it may seem like AI is not present in their work streams, but in reality, it has been utilized in health care for several years. As AI technologies continue to develop and become more sophisticated, we can expect them to play an increasingly significant role in health care.”
Today, most AI applications in health care use narrow AI, which is designed to complete a single task without human assistance, as opposed to artificial general intelligence (AGI), which pertains to human-level reasoning and problem solving across a broad spectrum. Here are some ways doctors are using AI throughout the day – sometimes being aware of its assistance, and sometimes being unaware:
- Many doctors use electronic health records (EHRs) with integrated AI that include computerized clinical decision support tools designed to reduce the risk of diagnostic error and to integrate decision-making in the medication ordering function.
- Cardiologists, pathologists, and dermatologists use AI in the interpretation of vast amounts of images, tracings, and complex patterns.
- Surgeons are using AI-enhanced surgical robotics for orthopedic surgeries, such as joint replacement and spine surgery.
- A growing number of doctors are using ChatGPT to assist in drafting prior authorization letters for insurers. Experts say more doctors are also experimenting with ChatGPT to support medical decision-making.
- Within oncology, physicians use machine learning techniques in the form of computer-aided detection systems for early breast cancer detection.
- AI algorithms are often used by health systems for workflow, staffing optimization, population management, and care coordination.
- Some systems within EHRs use AI to indicate high-risk patients.
- Physicians are using AI applications for the early recognition of sepsis, including EHR-integrated decision tools, such as the Hospital Corporation of America Healthcare’s Sepsis Prediction and Optimization Therapy and the Sepsis Early Risk Assessment algorithm.
- About 30% of radiologists use AI in their practice to analyze x-rays and CT scans.
- Epic Systems recently announced a partnership with Microsoft to integrate ChatGPT into MyChart, Epic’s patient portal system. Pilot hospitals will utilize ChatGPT to automatically generate responses to patient-generated questions sent via the portal.
The growth of AI in health care has been enormous, and it’s only going to continue, said Ravi B. Parikh, MD, an assistant professor in the department of medical ethics and health policy and medicine at the University of Pennsylvania, Philadelphia.
“What’s really critical is that physicians, clinicians, and nurses using AI are provided with the tools to understand how artificial intelligence works and, most importantly, understand that they are still accountable for making the ultimate decision,” Mr. LeTang said, “The information is not always going to be the right thing to do or the most accurate thing to do. They’re still liable for making a bad decision, even if AI is driving that.”
What are the top AI legal dangers of today?
A pressing legal risk is becoming too reliant on the suggestions that AI-based systems provide, which can lead to poor care decisions, said Kenneth Rashbaum, a New York–based cybersecurity attorney with more than 25 years of experience in medical malpractice defense.
This can occur, for example, when using clinical support systems that leverage AI, machine learning, or statistical pattern recognition. Today, clinical support systems are commonly administered through EHRs and other computerized clinical workflows. In general, such systems match a patient’s characteristics to a computerized clinical knowledge base. An assessment or recommendation is then presented to the physician for a decision.
“If the clinician blindly accepts it without considering whether it’s appropriate for this patient at this time with this presentation, the clinician may bear some responsibility if there is an untoward result,” Mr. Rashbaum said.
“A common claim even in the days before the EMR [electronic medical record] and AI, was that the clinician did not take all available information into account in rendering treatment, including history of past and present condition, as reflected in the records, communication with past and other present treating clinicians, lab and radiology results, discussions with the patient, and physical examination findings,” he said. “So, if the clinician relied upon the support prompt to the exclusion of these other sources of information, that could be a very strong argument for the plaintiff.”
Chatbots, such OpenAI’s ChatGPT, are another form of AI raising legal red flags. ChatGPT, trained on a massive set of text data, can carry out conversations, write code, draft emails, and answer any question posed. The chatbot has gained considerable credibility for accurately diagnosing rare conditions in seconds, and it recently passed the U.S. Medical Licensing Examination.
It’s unclear how many doctors are signing onto the ChatGPT website daily, but physicians are actively using the chatbot, particularly for assistance with prior authorization letters and to support decision-making processes in their practices, said Mr. LeTang.
When physicians ask ChatGPT a question, however, they should be mindful that ChatGPT could “hallucinate,” a term that refers to a generated response that sounds plausible but is factually incorrect or is unrelated to the context, explains Harvey Castro, MD, an emergency physician, ChatGPT health care expert, and author of the 2023 book “ChatGPT and Healthcare: Unlocking the Potential of Patient Empowerment.”
Acting on ChatGPT’s response without vetting the information places doctors at serious risk of a lawsuit, he said.
“Sometimes, the response is half true and half false,” he said. “Say, I go outside my specialty of emergency medicine and ask it about a pediatric surgical procedure. It could give me a response that sounds medically correct, but then I ask a pediatric cardiologist, and he says, ‘We don’t even do this. This doesn’t even exist!’ Physicians really have to make sure they are vetting the information provided.”
In response to ChatGPT’s growing usage by health care professionals, hospitals and practices are quickly implementing guidelines, policies, and restrictions that caution physicians about the accuracy of ChatGPT-generated information, adds Mr. LeTang.
Emerging best practices include avoiding the input of patient health information, personally identifiable information, or any data that could be commercially valuable or considered the intellectual property of a hospital or health system, he said.
“Another crucial guideline is not to rely solely on ChatGPT as a definitive source for clinical decision-making; physicians must exercise their professional judgment,” he said. “If best practices are not adhered to, the associated risks are present today. However, these risks may become more significant as AI technologies continue to evolve and become increasingly integrated into health care.”
The potential for misdiagnosis by AI systems and the risk of unnecessary procedures if physicians do not thoroughly evaluate and validate AI predictions are other dangers.
As an example, Mr. LeTang described a case in which a physician documents in the EHR that a patient has presented to the emergency department with chest pains and other signs of a heart attack, and an AI algorithm predicts that the patient is experiencing an active myocardial infarction. If the physician then sends the patient for stenting or an angioplasty without other concrete evidence or tests to confirm the diagnosis, the doctor could later face a misdiagnosis complaint if the costly procedures were unnecessary.
“That’s one of the risks of using artificial intelligence,” he said. “A large percentage of malpractice claims is failure to diagnose, delayed diagnosis, or inaccurate diagnosis. What falls in the category of failure to diagnose is sending a patient for an unnecessary procedure or having an adverse event or bad outcome because of the failure to diagnose.”
So far, no AI lawsuits have been filed, but they may make an appearance soon, said Sue Boisvert, senior patient safety risk manager at The Doctors Company, a national medical liability insurer.
“There are hundreds of AI programs currently in use in health care,” she said. “At some point, a provider will make a decision that is contrary to what the AI recommended. The AI may be wrong, or the provider may be wrong. Either way, the provider will neglect to document their clinical reasoning, a patient will be harmed, and we will have the first AI claim.”
Upcoming AI legal risks to watch for
Lawsuits that allege biased patient care by physicians on the basis of algorithmic bias may also be forthcoming, analysts warn.
Much has been written about algorithmic bias that compounds and worsens inequities in socioeconomic status, ethnicity, sexual orientation, and gender in health systems. In 2019, a groundbreaking article in Science shed light on commonly used algorithms that are considered racially biased and how health care professionals often use such information to make medical decisions.
No claims involving AI bias have come down the pipeline yet, but it’s an area to watch, said Ms. Boisvert. She noted a website that highlights complaints and accusations of AI bias, including in health care.
“We need to be sure the training of the AI is appropriate, current, and broad enough so that there is no bias in the AI when it’s participating in the decision-making,” said Ms. Boisvert. “Imagine if the AI is diagnosing based on a dataset that is not local. It doesn’t represent the population at that particular hospital, and it’s providing inaccurate information to the physicians who are then making decisions about treatment.”
In pain management, for example, there are known differences in how patients experience pain, Ms. Boisvert said. If AI was being used to develop an algorithm for how a particular patient’s postoperative pain should be managed, and the algorithm did not include the differences, the pain control for a certain patient could be inappropriate. A poor outcome resulting from the treatment could lead to a claim against the physician or hospital that used the biased AI system, she said.
In the future, as AI becomes more integrated and accepted in medicine, there may be a risk of legal complaints against doctors for not using AI, said Saurabh Jha, MD, an associate professor of radiology at the University of Pennsylvania, Philadelphia, and a scholar of AI in radiology.
“Ultimately, we might get to a place where AI starts helping physicians detect more or reduce the miss of certain conditions, and it becomes the standard of care,” Dr. Jha said. “For example, if it became part of the standard of care for pulmonary embolism [PE] detection, and you didn’t use it for PE detection, and there was a miss. That could put you at legal risk. We’re not at that stage yet, but that is one future possibility.”
Dr. Parikh envisions an even cloudier liability landscape as the potential grows for AI to control patient care decisions. In such a scenario, rather than just issuing an alert or prediction to a physician, the AI system could trigger an action.
For instance, if an algorithm is trained to predict sepsis and, once triggered, the AI could initiate a nurse-led rapid response or a change in patient care outside the clinician’s control, said Dr. Parikh, who coauthored a recent article on AI and medical liability in The Milbank Quarterly.
“That’s still very much the minority of how AI is being used, but as evidence is growing that AI-based diagnostic tools perform equivalent or even superior to physicians, these autonomous workflows are being considered,” Dr. Parikh said. “When the ultimate action upon the patient is more determined by the AI than what the clinician does, then I think the liability picture gets murkier, and we should be thinking about how we can respond to that from a liability framework.”
How you can prevent AI-related lawsuits
The first step to preventing an AI-related claim is being aware of when and how you are using AI.
Ensure you’re informed about how the AI was trained, Ms. Boisvert stresses.
“Ask questions!” she said. “Is the AI safe? Are the recommendations accurate? Does the AI perform better than current systems? In what way? What databases were used, and did the programmers consider bias? Do I understand how to use the results?”
Never blindly trust the AI but rather view it as a data point in a medical decision, said Dr. Parikh. Ensure that other sources of medical information are properly accessed and that best practices for your specialty are still being followed.
When using any form of AI, document your usage, adds Mr. Rashbaum. A record that clearly outlines how the physician incorporated the AI is critical if a claim later arises in which the doctor is accused of AI-related malpractice, he said.
“Indicating how the AI tool was used, why it was used, and that it was used in conjunction with available clinical information and the clinician’s best judgment could reduce the risk of being found responsible as a result of AI use in a particular case,” he said.
Use chatbots, such as ChatGPT, the way they were intended, as support tools, rather than definitive diagnostic instruments, adds Dr. Castro.
“Doctors should also be well-trained in interpreting and understanding the suggestions provided by ChatGPT and should use their clinical judgment and experience alongside the AI tool for more accurate decision-making,” he said.
In addition, because no AI insurance product exists on the market, physicians and organizations using AI – particularly for direct health care – should evaluate their current insurance or insurance-like products to determine where a claim involving AI might fall and whether the policy would respond, said Ms. Boisvert. The AI vendor/manufacturer will likely have indemnified themselves in the purchase and sale agreement or contract, she said.
It will also become increasingly important for medical practices, hospitals, and health systems to put in place strong data governance strategies, Mr. LeTang said.
“AI relies on good data,” he said. “A data governance strategy is a key component to making sure we understand where the data is coming from, what is represents, how accurate it is, if it’s reproducible, what controls are in place to ensure the right people have the right access, and that if we’re starting to use it to build algorithms, that it’s deidentified.”
While no malpractice claims associated with the use of AI have yet surfaced, this may change as legal courts catch up on the backlog of malpractice claims that were delayed because of COVID-19, and even more so as AI becomes more prevalent in health care, Mr. LeTang said.
“Similar to the attention that autonomous driving systems, like Tesla, receive when the system fails and accidents occur, we can be assured that media outlets will widely publicize AI-related medical adverse events,” he said. “It is crucial for health care professionals, AI developers, and regulatory authorities to work together to ensure the responsible use of AI in health care, with patient safety as the top priority. By doing so, they can mitigate the risks associated with AI implementation and minimize the potential for legal disputes arising from AI-related medical errors.”
A version of this article first appeared on Medscape.com.