User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
News & Perspectives from Ob.Gyn. News
NEWS FROM THE FDA/CDC
New USPSTF draft suggests mammography start at 40, not 50
The US Preventive Services Task Force (USPSTF) on May 9 released a draft recommendation statement and evidence review that provides critical updates to its breast cancer screening recommendations.
The major change: USPSTF proposed reducing the recommended start age for routine screening mammograms from age 50 to age 40. The latest recommendation, which carries a B grade, also calls for screening every other year and sets a cutoff age of 74.The task force’s A and B ratings indicate strong confidence in the evidence for benefit, meaning that clinicians should encourage their patients to get these services as appropriate.
The influential federal advisory panel last updated these recommendations in 2016. At the time, USPSTF recommended routine screening mammograms starting at age 50, and gave a C grade to starting before that.
In the 2016 recommendations, “we felt a woman could start screening in her 40s depending on how she feels about the harms and benefits in an individualized personal decision,” USPSTF member John Wong, MD, chief of clinical decision making and a primary care physician at Tufts Medical Center in Boston, said in an interview. “In this draft recommendation, we now recommend that all women get screened starting at age 40.”
Two major factors prompted the change, explained Dr. Wong. One is that more women are being diagnosed with breast cancer in their 40s. The other is that a growing body of evidence showing that Black women get breast cancer younger, are more likely to die of breast cancer, and would benefit from earlier screening.
“It is now clear that screening every other year starting at age 40 has the potential to save about 20% more lives among all women and there is even greater potential benefit for Black women, who are much more likely to die from breast cancer,” Dr. Wong said.
The American Cancer Society (ACS) called the draft recommendations a “significant positive change,” while noting that the task force recommendations only apply to women at average risk for breast cancer.
FDA approves OTC naloxone, but will cost be a barrier?
The US Food and Drug Administration has approved over-the-counter sales of the overdose reversal agent Narcan (naloxone, Emergent BioSolutions). Greater access to the drug should mean more lives saved. However, it’s unclear how much the nasal spray will cost and whether pharmacies will stock the product openly on shelves.
Currently, major pharmacy chains such as CVS and Walgreens make naloxone available without prescription, but consumers have to ask a pharmacist to dispense the drug.
“The major question is what is it going to cost,” Brian Hurley, MD, MBA, president-elect of the American Society of Addiction Medicine, said in an interview. “In order for people to access it they have to be able to afford it.”
“We won’t accomplish much if people can’t afford to buy Narcan,” said Chuck Ingoglia, president and CEO of the National Council for Mental Wellbeing, in a statement. Still, he applauded the FDA.
“No single approach will end overdose deaths but making Narcan easy to obtain and widely available likely will save countless lives annually,” he said.
“The timeline for availability and price of this OTC product is determined by the manufacturer,” the FDA said in a statement.
Commissioner Robert M. Califf, MD, called for the drug’s manufacturer to “make accessibility to the product a priority by making it available as soon as possible and at an affordable price.”
Emergent BioSolutions did not comment on cost. It said in a statement that the spray “will be available on US shelves and at online retailers by the late summer,” after it has adapted Narcan for direct-to-consumer use, including more consumer-oriented packaging.
Naloxone’s cost varies, depending on geographic location and whether it is generic. According to GoodRX, a box containing two doses of generic naloxone costs $31-$100, depending on location and coupon availability.
A two-dose box of Narcan costs $135-$140. Emergent reported a 14% decline in naloxone sales in 2022—to $373.7 million—blaming it in part on the introduction of generic formulations.
Dr. Hurley said he expects those who purchase Narcan at a drug store will primarily already be shopping there. It may or may not be those who most often experience overdose, such as people leaving incarceration or experiencing homelessness.
Having Narcan available over-the-counter “is an important supplement but it doesn’t replace the existing array of naloxone distribution programs,” Dr. Hurley said.
CONFERENCE COVERAGE
Should you prescribe bioidentical hormones for menopause?
The off-label prescribing of compounded, bioidentical hormone therapy—in pills, creams, or pellets—for symptoms of perimenopause or menopause can put physicians at legal risk because the products lack scientific backing, according to an expert at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists (ACOG).
Clinicians write an estimated 26 to 33 million prescriptions for compounded bioidentical hormone therapy (cBHT) every year, and almost 41% of menopausal women who need treatment try cBHT during their lives. But these drugs lack the approval for this indication from the Food and Drug Administration.
“There is a public perception that this is natural, safer, and anti-aging,” said Robert Kauffman, MD, a professor of obstetrics and gynecology and assistant dean for research at Texas Tech University Health Sciences Center in Amarillo.
Following the 2002 Women’s Health Initiative report showing a link between hormone therapy (HT) and an increase in the incidence of breast cancer, medical schools have slowed or paused instructing trainees on the traditional treatment, Dr. Kauffman said. The association was later determined to be spurious: HT is not associated with a risk for all-cause mortality or deaths from cardiovascular disease or cancer. However, HT still is largely ignored by younger physicians, Dr. Kauffman said, because of unsubstantiated “dangers” such as heart attack, stroke, and deep vein thrombosis.
Once-daily nifedipine sufficient for hypertension in pregnancy
A single 60-mg daily dose of nifedipine appeared similarly effective as taking a 30-mg dose twice daily for treating hypertensive disorders in pregnancy, according to research presented at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
The findings suggest that starting patients on a once-daily 60-mg dose is therefore reasonable, Isabelle Band, BA, a medical student at the Icahn School of Medicine at Mount Sinai, New York, told attendees. Ms. Band said in an interview that there does not appear to be a consensus on the standard of care for nifedipine dosing regimen in this population but that previous in vitro studies have shown increased metabolism of nifedipine in a physiologic state that mimics pregnancy.
“I’ve spoken to some colleagues here who say that they frequently have this debate of which dosing regimen to go with,” Ms. Band said. “I was pleasantly surprised that there was no significant difference between the two dosing regimens because once-daily dosing is less burdensome for patients and will likely improve compliance and convenience for patients.” An additional benefit of once-daily dosing relates to payers because anecdotal reports suggest insurance companies do not tend to approve twice-daily dosing as readily as once-daily dosing, Ms. Band added.
Ms. Band and her colleagues conducted a retrospective chart review of all patients with hypertensive disorders of pregnancy who were admitted to the Mount Sinai Health System between Jan. 1, 2015, and April 30, 2021, and were prescribed nifedipine in a once-daily (60-mg) or twice-daily (two 30-mg) dose. They excluded patients with renal disease and those already taking hypertensives prior to admission.
Among 237 patients who met the criteria, 59% received 60 mg in a twice-daily 30-mg dose, and 41% received 60 mg in a once-daily dose. Among patients requiring an up titration, two-thirds (67%) needed an increase in the nifedipine dose—the most common adjustment—and 20.7% needed both an increase in nifedipine and an additional medication. ●
NEWS FROM THE FDA/CDC
New USPSTF draft suggests mammography start at 40, not 50
The US Preventive Services Task Force (USPSTF) on May 9 released a draft recommendation statement and evidence review that provides critical updates to its breast cancer screening recommendations.
The major change: USPSTF proposed reducing the recommended start age for routine screening mammograms from age 50 to age 40. The latest recommendation, which carries a B grade, also calls for screening every other year and sets a cutoff age of 74.The task force’s A and B ratings indicate strong confidence in the evidence for benefit, meaning that clinicians should encourage their patients to get these services as appropriate.
The influential federal advisory panel last updated these recommendations in 2016. At the time, USPSTF recommended routine screening mammograms starting at age 50, and gave a C grade to starting before that.
In the 2016 recommendations, “we felt a woman could start screening in her 40s depending on how she feels about the harms and benefits in an individualized personal decision,” USPSTF member John Wong, MD, chief of clinical decision making and a primary care physician at Tufts Medical Center in Boston, said in an interview. “In this draft recommendation, we now recommend that all women get screened starting at age 40.”
Two major factors prompted the change, explained Dr. Wong. One is that more women are being diagnosed with breast cancer in their 40s. The other is that a growing body of evidence showing that Black women get breast cancer younger, are more likely to die of breast cancer, and would benefit from earlier screening.
“It is now clear that screening every other year starting at age 40 has the potential to save about 20% more lives among all women and there is even greater potential benefit for Black women, who are much more likely to die from breast cancer,” Dr. Wong said.
The American Cancer Society (ACS) called the draft recommendations a “significant positive change,” while noting that the task force recommendations only apply to women at average risk for breast cancer.
FDA approves OTC naloxone, but will cost be a barrier?
The US Food and Drug Administration has approved over-the-counter sales of the overdose reversal agent Narcan (naloxone, Emergent BioSolutions). Greater access to the drug should mean more lives saved. However, it’s unclear how much the nasal spray will cost and whether pharmacies will stock the product openly on shelves.
Currently, major pharmacy chains such as CVS and Walgreens make naloxone available without prescription, but consumers have to ask a pharmacist to dispense the drug.
“The major question is what is it going to cost,” Brian Hurley, MD, MBA, president-elect of the American Society of Addiction Medicine, said in an interview. “In order for people to access it they have to be able to afford it.”
“We won’t accomplish much if people can’t afford to buy Narcan,” said Chuck Ingoglia, president and CEO of the National Council for Mental Wellbeing, in a statement. Still, he applauded the FDA.
“No single approach will end overdose deaths but making Narcan easy to obtain and widely available likely will save countless lives annually,” he said.
“The timeline for availability and price of this OTC product is determined by the manufacturer,” the FDA said in a statement.
Commissioner Robert M. Califf, MD, called for the drug’s manufacturer to “make accessibility to the product a priority by making it available as soon as possible and at an affordable price.”
Emergent BioSolutions did not comment on cost. It said in a statement that the spray “will be available on US shelves and at online retailers by the late summer,” after it has adapted Narcan for direct-to-consumer use, including more consumer-oriented packaging.
Naloxone’s cost varies, depending on geographic location and whether it is generic. According to GoodRX, a box containing two doses of generic naloxone costs $31-$100, depending on location and coupon availability.
A two-dose box of Narcan costs $135-$140. Emergent reported a 14% decline in naloxone sales in 2022—to $373.7 million—blaming it in part on the introduction of generic formulations.
Dr. Hurley said he expects those who purchase Narcan at a drug store will primarily already be shopping there. It may or may not be those who most often experience overdose, such as people leaving incarceration or experiencing homelessness.
Having Narcan available over-the-counter “is an important supplement but it doesn’t replace the existing array of naloxone distribution programs,” Dr. Hurley said.
CONFERENCE COVERAGE
Should you prescribe bioidentical hormones for menopause?
The off-label prescribing of compounded, bioidentical hormone therapy—in pills, creams, or pellets—for symptoms of perimenopause or menopause can put physicians at legal risk because the products lack scientific backing, according to an expert at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists (ACOG).
Clinicians write an estimated 26 to 33 million prescriptions for compounded bioidentical hormone therapy (cBHT) every year, and almost 41% of menopausal women who need treatment try cBHT during their lives. But these drugs lack the approval for this indication from the Food and Drug Administration.
“There is a public perception that this is natural, safer, and anti-aging,” said Robert Kauffman, MD, a professor of obstetrics and gynecology and assistant dean for research at Texas Tech University Health Sciences Center in Amarillo.
Following the 2002 Women’s Health Initiative report showing a link between hormone therapy (HT) and an increase in the incidence of breast cancer, medical schools have slowed or paused instructing trainees on the traditional treatment, Dr. Kauffman said. The association was later determined to be spurious: HT is not associated with a risk for all-cause mortality or deaths from cardiovascular disease or cancer. However, HT still is largely ignored by younger physicians, Dr. Kauffman said, because of unsubstantiated “dangers” such as heart attack, stroke, and deep vein thrombosis.
Once-daily nifedipine sufficient for hypertension in pregnancy
A single 60-mg daily dose of nifedipine appeared similarly effective as taking a 30-mg dose twice daily for treating hypertensive disorders in pregnancy, according to research presented at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
The findings suggest that starting patients on a once-daily 60-mg dose is therefore reasonable, Isabelle Band, BA, a medical student at the Icahn School of Medicine at Mount Sinai, New York, told attendees. Ms. Band said in an interview that there does not appear to be a consensus on the standard of care for nifedipine dosing regimen in this population but that previous in vitro studies have shown increased metabolism of nifedipine in a physiologic state that mimics pregnancy.
“I’ve spoken to some colleagues here who say that they frequently have this debate of which dosing regimen to go with,” Ms. Band said. “I was pleasantly surprised that there was no significant difference between the two dosing regimens because once-daily dosing is less burdensome for patients and will likely improve compliance and convenience for patients.” An additional benefit of once-daily dosing relates to payers because anecdotal reports suggest insurance companies do not tend to approve twice-daily dosing as readily as once-daily dosing, Ms. Band added.
Ms. Band and her colleagues conducted a retrospective chart review of all patients with hypertensive disorders of pregnancy who were admitted to the Mount Sinai Health System between Jan. 1, 2015, and April 30, 2021, and were prescribed nifedipine in a once-daily (60-mg) or twice-daily (two 30-mg) dose. They excluded patients with renal disease and those already taking hypertensives prior to admission.
Among 237 patients who met the criteria, 59% received 60 mg in a twice-daily 30-mg dose, and 41% received 60 mg in a once-daily dose. Among patients requiring an up titration, two-thirds (67%) needed an increase in the nifedipine dose—the most common adjustment—and 20.7% needed both an increase in nifedipine and an additional medication. ●
NEWS FROM THE FDA/CDC
New USPSTF draft suggests mammography start at 40, not 50
The US Preventive Services Task Force (USPSTF) on May 9 released a draft recommendation statement and evidence review that provides critical updates to its breast cancer screening recommendations.
The major change: USPSTF proposed reducing the recommended start age for routine screening mammograms from age 50 to age 40. The latest recommendation, which carries a B grade, also calls for screening every other year and sets a cutoff age of 74.The task force’s A and B ratings indicate strong confidence in the evidence for benefit, meaning that clinicians should encourage their patients to get these services as appropriate.
The influential federal advisory panel last updated these recommendations in 2016. At the time, USPSTF recommended routine screening mammograms starting at age 50, and gave a C grade to starting before that.
In the 2016 recommendations, “we felt a woman could start screening in her 40s depending on how she feels about the harms and benefits in an individualized personal decision,” USPSTF member John Wong, MD, chief of clinical decision making and a primary care physician at Tufts Medical Center in Boston, said in an interview. “In this draft recommendation, we now recommend that all women get screened starting at age 40.”
Two major factors prompted the change, explained Dr. Wong. One is that more women are being diagnosed with breast cancer in their 40s. The other is that a growing body of evidence showing that Black women get breast cancer younger, are more likely to die of breast cancer, and would benefit from earlier screening.
“It is now clear that screening every other year starting at age 40 has the potential to save about 20% more lives among all women and there is even greater potential benefit for Black women, who are much more likely to die from breast cancer,” Dr. Wong said.
The American Cancer Society (ACS) called the draft recommendations a “significant positive change,” while noting that the task force recommendations only apply to women at average risk for breast cancer.
FDA approves OTC naloxone, but will cost be a barrier?
The US Food and Drug Administration has approved over-the-counter sales of the overdose reversal agent Narcan (naloxone, Emergent BioSolutions). Greater access to the drug should mean more lives saved. However, it’s unclear how much the nasal spray will cost and whether pharmacies will stock the product openly on shelves.
Currently, major pharmacy chains such as CVS and Walgreens make naloxone available without prescription, but consumers have to ask a pharmacist to dispense the drug.
“The major question is what is it going to cost,” Brian Hurley, MD, MBA, president-elect of the American Society of Addiction Medicine, said in an interview. “In order for people to access it they have to be able to afford it.”
“We won’t accomplish much if people can’t afford to buy Narcan,” said Chuck Ingoglia, president and CEO of the National Council for Mental Wellbeing, in a statement. Still, he applauded the FDA.
“No single approach will end overdose deaths but making Narcan easy to obtain and widely available likely will save countless lives annually,” he said.
“The timeline for availability and price of this OTC product is determined by the manufacturer,” the FDA said in a statement.
Commissioner Robert M. Califf, MD, called for the drug’s manufacturer to “make accessibility to the product a priority by making it available as soon as possible and at an affordable price.”
Emergent BioSolutions did not comment on cost. It said in a statement that the spray “will be available on US shelves and at online retailers by the late summer,” after it has adapted Narcan for direct-to-consumer use, including more consumer-oriented packaging.
Naloxone’s cost varies, depending on geographic location and whether it is generic. According to GoodRX, a box containing two doses of generic naloxone costs $31-$100, depending on location and coupon availability.
A two-dose box of Narcan costs $135-$140. Emergent reported a 14% decline in naloxone sales in 2022—to $373.7 million—blaming it in part on the introduction of generic formulations.
Dr. Hurley said he expects those who purchase Narcan at a drug store will primarily already be shopping there. It may or may not be those who most often experience overdose, such as people leaving incarceration or experiencing homelessness.
Having Narcan available over-the-counter “is an important supplement but it doesn’t replace the existing array of naloxone distribution programs,” Dr. Hurley said.
CONFERENCE COVERAGE
Should you prescribe bioidentical hormones for menopause?
The off-label prescribing of compounded, bioidentical hormone therapy—in pills, creams, or pellets—for symptoms of perimenopause or menopause can put physicians at legal risk because the products lack scientific backing, according to an expert at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists (ACOG).
Clinicians write an estimated 26 to 33 million prescriptions for compounded bioidentical hormone therapy (cBHT) every year, and almost 41% of menopausal women who need treatment try cBHT during their lives. But these drugs lack the approval for this indication from the Food and Drug Administration.
“There is a public perception that this is natural, safer, and anti-aging,” said Robert Kauffman, MD, a professor of obstetrics and gynecology and assistant dean for research at Texas Tech University Health Sciences Center in Amarillo.
Following the 2002 Women’s Health Initiative report showing a link between hormone therapy (HT) and an increase in the incidence of breast cancer, medical schools have slowed or paused instructing trainees on the traditional treatment, Dr. Kauffman said. The association was later determined to be spurious: HT is not associated with a risk for all-cause mortality or deaths from cardiovascular disease or cancer. However, HT still is largely ignored by younger physicians, Dr. Kauffman said, because of unsubstantiated “dangers” such as heart attack, stroke, and deep vein thrombosis.
Once-daily nifedipine sufficient for hypertension in pregnancy
A single 60-mg daily dose of nifedipine appeared similarly effective as taking a 30-mg dose twice daily for treating hypertensive disorders in pregnancy, according to research presented at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
The findings suggest that starting patients on a once-daily 60-mg dose is therefore reasonable, Isabelle Band, BA, a medical student at the Icahn School of Medicine at Mount Sinai, New York, told attendees. Ms. Band said in an interview that there does not appear to be a consensus on the standard of care for nifedipine dosing regimen in this population but that previous in vitro studies have shown increased metabolism of nifedipine in a physiologic state that mimics pregnancy.
“I’ve spoken to some colleagues here who say that they frequently have this debate of which dosing regimen to go with,” Ms. Band said. “I was pleasantly surprised that there was no significant difference between the two dosing regimens because once-daily dosing is less burdensome for patients and will likely improve compliance and convenience for patients.” An additional benefit of once-daily dosing relates to payers because anecdotal reports suggest insurance companies do not tend to approve twice-daily dosing as readily as once-daily dosing, Ms. Band added.
Ms. Band and her colleagues conducted a retrospective chart review of all patients with hypertensive disorders of pregnancy who were admitted to the Mount Sinai Health System between Jan. 1, 2015, and April 30, 2021, and were prescribed nifedipine in a once-daily (60-mg) or twice-daily (two 30-mg) dose. They excluded patients with renal disease and those already taking hypertensives prior to admission.
Among 237 patients who met the criteria, 59% received 60 mg in a twice-daily 30-mg dose, and 41% received 60 mg in a once-daily dose. Among patients requiring an up titration, two-thirds (67%) needed an increase in the nifedipine dose—the most common adjustment—and 20.7% needed both an increase in nifedipine and an additional medication. ●
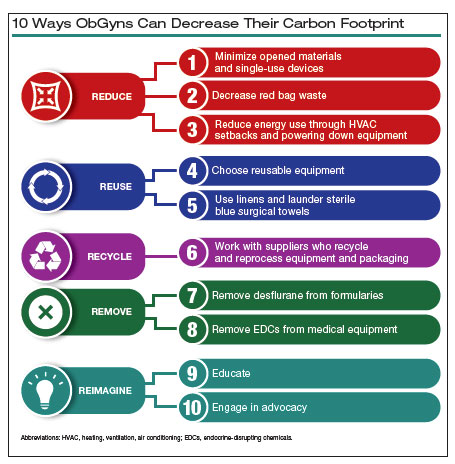
10 ways in which ObGyn care can be more environmentally sustainable
Climate change has been called the biggest health threat of the 21st century.1 The health care sector is a huge contributor to global carbon emissions, accounting for almost double the emissions of global aviation. While other industries and countries are implementing mitigation measures to decrease their emissions, health care is currently on track to double its carbon emissions by 2050, even though it should be carbon neutral by that time to comply with the Paris Climate Agreement.2 There have been some national efforts to curb health care emissions, including the creation of the Office of Climate Change and Health Equity in 2021 and the passage of the Inflation Reduction Act in 2022.3 These are top-down, administrative approaches, and to be successful we will also need clinicians to understand and address this problem.
The negative impacts of heat, air pollution, and exposure to toxic substances on human health have been well documented in multiple regions across multiple specialties.4-7 The United States makes up 27% of the global health care carbon footprint—more emissions than the entire United Kingdom as a country—despite having only 4% of the world’s population.2 Culture and incentives for an overabundance of single-use supplies, not evidence for patient safety, have led to this uniquely American problem. It is evident that our health care industry is an excellent place to implement mitigation measures for carbon emissions that contribute to climate change and can improve health outcomes.
In this article, we recommend 10 practices that can decrease our carbon footprint in ObGyn. We focus on the classic motto of “Reduce, Reuse, Recycle,” while adding “Remove” and “Reimagine” to classify the ways in which we can reduce emissions while not compromising our care to patients.
Reduce
1. Minimize opened materials and single-use devices in the OR and labor and delivery
Health care is a unique setting where a culture of infection prevention and efficiency has led low-cost, single-use supplies to dominate over reusable items. While single-use items can have inexpensive purchasing costs compared to reusable items, the environmental costs required for the production and disposal of the former are often much greater. In operating rooms (ORs) and labor and delivery (LD) units, single-use items are omnipresent. Over the past decade, researchers and clinicians have started to take a closer look at these items and their carbon footprint. One group evaluated hysterectomy through a waste audit and found that the vast majority of waste from all of the cases was Spunbond Meltblown Spunbond, or SMS; plastic materialthat comprises gowns; blue wraps; and drapes; followed by hard plastic material that comprises trays and packaging.8 Moreover, production and manufacturing processes contributed to 95% of the environmental impacts of these items.8
In an effort to be time efficient, OR staff will open sterile surgical packs and individual peel-pack items prior to surgery to minimize having to find items during surgery. However, this creates an inordinate amount of waste. One group of neurosurgeons who evaluated their opened but unused supplies found that 85% of their unused items were individually opened items, leading to a waste of $2.9 million per year.9 Minor procedures like dilation and curettage, cystoscopy, and hysteroscopy do not need such a large sterile field, as these procedures are also safe to perform in the office. Hand surgeons have been quick to lead in this space, particularly with minor procedures such as carpal tunnel release. One division was able to eliminate 2.8 tons of waste and save $13,000 in a 2-year period by reducing the sterile field.10 ObGyns can work with OR and LD staff to create custom packs that minimize unused or underutilized items, helping to reduce both the carbon footprint and health care spending.
Bottom line: ObGyns can help foster a culture of having supplies available but not opened until needed during a case.
Continue to: 2. Decrease regulated medical waste...
2. Decrease regulated medical waste
Health care is unique from other fields in that there are multiple waste streams to consider. Infectious waste and items saturated in blood or capable of causing infection must be placed into regulated medical waste (RMW), or more commonly, red biohazard bags. RMW is autoclaved or incinerated prior to disposal in a landfill. This process is more financially and environmentally costly than general municipal waste (GMW). This process also requires more transport—1 study revealed that GMW traveled 20 km to a landfill for disposal, compared with the 50 km that RMW traveled for sterilized-prior-to-landfill disposal.11
Unfortunately, the vast majority of items placed in RMW are incorrectly triaged and should instead be disposed in GMW.12,13 One study performed in an emergency department revealed that 85% of waste was incorrectly placed in the RMW.12
Bottom line: ObGyns can avoid placing items in RMW that may not qualify and advocate for institution policy changes to remove RMW from places such as waiting rooms, at the patient bedside, or next to scrub sinks.
3. Reduce energy use
ORs and LD units use a lot of energy, and numerous studies have demonstrated that the heating, ventilation, and air conditioning (HVAC) system plays a large role in emissions.8,11 This can easily be fixed by “HVAC setbacks” and powering down rooms when not in use. One institution powered down ORs when not in use and reduced 234 metric tons of CO2 emissions and saved $33,000 per year.14 Transitioning to light-emitting diode (LED) lights reduced energy usage at 1 institution by almost 50%.15 Finally, computers in clinical offices, examination rooms, and administrative offices can be powered down at the end of the day. One study found that in 1 radiology department, 29 computers left on overnight and on weekends emitted 17.7 tons of CO2 emissions in 1 year.16
Bottom line: We as ObGyns can advocate for how energy can be saved outside of surgical cases, including powering down ORs and LD units, transitioning to LED lighting, and powering down workstations.
Reuse
4. Choose reusable equipment
In ObGyn practice, the most commonly used tool is the speculum. Given its omnipresence, the speculum is a great place to start to decrease our carbon footprint. Two studies have evaluated the environmental impact of reusable versus single-use disposable specula, and both demonstrated that the stainless-steel versions have less global warming potential than the acrylic varieties.17,18 Donahue and colleagues17 demonstrated that it only took 2 to 3 pelvic examinations for the cost of stainless-steel specula to break even, even when sterilized in a half-filled autoclave tray. Rodriquez, et al18 revealed that, compared with an acrylic model, the stainless-steel specula had fewer negative impacts in terms of global warming, acidification, respiratory effects, smog, and fossil fuel depletion.18
Bottom line: Strongly consider using stainless-steel specula to reduce costs and carbon emissions.
In addition to specula, ObGyns can choose reusable equipment in the OR. For example, surgeons can use stainless-steel trocars instead of disposable trocars.19 In vaginal cases, Breisky-Navratil retractors can be used instead of disposable self-retaining retractors. Plastic basins that often are included in sterile supply packs can be replaced with stainless-steel basins, which could have profound positive effects on the carbon footprint of gynecologic surgery.8 One study of ObGyns demonstrated that 95% of physicians supported waste-reduction efforts, and 66% supported utilizing reusable surgical tools instead of disposable tools.20
Bottom line: As surgeons, ObGyns have influence over what they want to use in the OR, and they can petition for reusable options over disposable options.
5. Launder the sterile blue towels
Sterile blue towels, which are made of cotton, have the largest environmental footprint compared with other disposable materials, such as plastics, and contribute greatly to toxicity in human health.8,11 Although these towels cannot be laundered and sterilized again for use in a sterile surgical field, they can be laundered and repurposed, including by environmental services to clean hospital rooms. Blue towels should be able to be laundered no matter how saturated in body fluids they are.
Bottom line: ObGyns should strive to always launder the blue towels and educate trainees and other staff in the OR to do the same.
Recycle
6. Recycle and reprocess materials and devices
While recycling is immensely important, it requires a large amount of energy to break down a material to its raw components for manufacturing. It likely reduces our carbon footprint from OR procedures by only 5%.8 However, recycling is still a good way to divert appropriate materials from landfill, saving costs and emissions at the end of a material’s life. One example is sterile blue wrap, which is a petroleum product with a recycling number of 6 and a filtration rating of N99. Blue wrap can be recycled into plastic pellets, or it can be recreated into other hospital supplies, such as gowns.
Bottom line: ObGyns can petition their hospitals to work with suppliers and waste-processing companies who have recycling programs built into their supply chains.
By contrast, reprocessing can have a much larger impact on carbon emissions. Complex items, such as advanced energy devices that can be reprocessed, result in a greater reduction in carbon emissions due to the reuse of their complex materials and manufacturing when compared with such devices that cannot be reprocessed. Recycling and reprocessing programs are already in place for several devices (TABLE). Authors of a systematic review showed that there is no evidence to support the use of single-use supplies and instruments over reprocessed items when considering instrument function, ease of use, patient safety, transmission of infection, or long-term patient outcomes.21
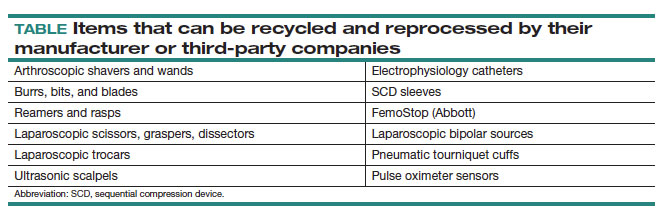
Bottom line: ObGyns can choose to use reprocessed items in ORs instead of single-use devices and educate staff on the safety of these items.
Continue to: Remove...
Remove
7. Remove desflurane and other volatile gases from formularies
Volatile anesthetic gases, such as desflurane, isoflurane, and nitrous oxide, are themselves potent greenhouse gases, comprising a large portion of the carbon emissions that come from the OR.22 Desflurane was developed to have a rapid onset for induction and quick recovery; however, studies have shown no clinical benefit over other gases.23 Furthermore, the costs and greenhouse gas potential are substantial. Desflurane costs 2 to 3 times more and has more than 20 times the global warming potential of the other volatile gases (FIGURE).8 Using 1 hour of desflurane is equivalent to driving 378 miles in a gas-powered vehicle, while the use of isoflurane and sevoflurane create equivalents of only 15 and 8 miles, respectively.23
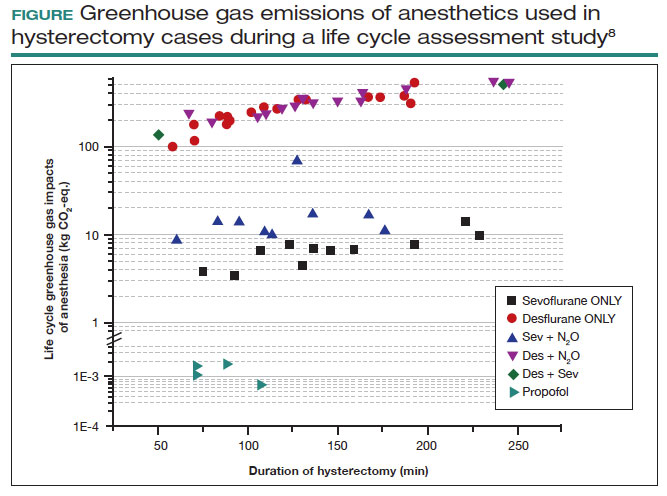
Nitrous oxide is another powerful greenhouse gas that is a direct ozone depletor and can stay in the atmosphere for 114 years.22 Nitrous oxide has limited clinical use in hospitals, but it is often stored in central hospital piping. Most of the impact of nitrous oxide comes through leaks in a poor system design rather than patient delivery. One estimate reveals that more than 13 million liters of nitrous oxide are lost annually from leaks in European hospitals.22 The American Society of Anesthesiologists recommends decommissioning central piping of nitrous oxide in favor of cylinders at the point of care.24
Literature on enhanced recovery after surgery in gynecology promotes the use of propofol over volatile gases for our patients because of the high rate of postoperative nausea and vomiting seen with gases.25 Volatile gases should be a last-choice anesthetic for our patients.
Bottom line: It is critical that ObGyns work with colleagues in anesthesia to develop climate- and patient-friendly protocols for procedures.
8. Remove endocrine-disrupting chemicals from clinical supplies
Endocrine-disrupting chemicals (EDCs) are a type of chemical that alter the hormonal systems of humans, which can result in adverse health effects. Multiple studies and reviews have tied EDCs to reproductive abnormalities, such as the effects of bisphenol A (BPA) on estradiol levels, antral follicle counts, oocyte quality, and implantation rates; phthalates on fibroid burden; triclosan on embryo quality; parabens on live birth rates; and perfluoroalkylsubstances (PFAS or “forever substances”) on hypertensive disorders of pregnancy.5,26,27
What might be most shocking is that these EDCs are incorporated into medical supplies and pharmaceuticals. For example, BPA is known to line dialysis and ointment tubes, parabens are used for their antimicrobial properties in ultrasound gel and hep-locks, and phthalates are found in up to 40% of medical-use plastics and controlled-release medications. Authors of an observational study found that 74% of patients admitted to an LD unit were exposed to EDCs. In a neonatal intensive care unit (NICU), most of the supplies contained an EDC, and urinary BPA levels were elevated in neonates admitted to a NICU, raising concerns about long-term health risks.5
Bottom line: Physicians and health care institutions have an obligation to petition industry partners and suppliers to remove EDCs from their supply chains.
Reimagine
9. Educate
The field of health care sustainability remains in its infancy, but from 2007 to 2019, publications on climate change and health in academia increased by a factor of 8.29 Additionally, through waste audits, quality-improvement projects, and life cycle analyses (analytical tools to evaluate product or process emissions from materials extraction to disposal), we have gained insight into the scope of the problem, with evidence showing that our practices are largely derived from culture. It is time to provide formal education on health care sustainability to medical trainees, staff, and clinicians alike, who desire to see this topic reflected in their formal curricula.30 Start talking about it!
Bottom line: Commentaries, webinars, formal didactics sessions, in-services, and hospital workgroups to introduce this topic are a good way to teach others about the carbon footprint of our care and solutions to minimize it.
10. Engage in advocacy
Physicians have an ethical duty to advocate for change at the local, regional, and national levels if we want to see a better future for our patients, their children, and even ourselves. We should reimagine this work as an important public health initiative.31 Surveys of physicians, including ObGyns, reveal a concern about the sustainability of health care and a commitment to addressing this issue.20 ObGyns are on the frontlines of delivering care every day, so we are poised to implement changes that can impact our patients, especially when we can lead and petition hospital or local committees.20,28,32 There is much to be done, but every voice counts and can make impactful changes at every level. ●
- Costello A, Abbas M, Allen et al. Managing the health effects of climate change: Lancet and University College London Institute for Global Health Commission. Lancet. 2009;373:1693-1733.
- Health care climate footprint report. Health Care Without Harm website. https://www.noharm.org/ClimateFootprintReport. Accessed May 12, 2023.
- Balbus JM, McCannon CJ, Mataka A, et al. After COP26—putting health and equity at the center of the climate movement. N Engl J Med. 2022;386:1295-1297.
- Bekkar B, Pacheco S, Basu R, et al. Association of air pollution and heat exposure with preterm birth, low birth weight, and stillbirth in the US: a systematic review. JAMA Netw Open. 2020;3:e208243.
- Genco M, Anderson-Shaw L, Sargis RM. Unwitting accomplices: endocrine disruptors confounding clinical care. J Clin Endocrinol Metab. 2020;105:e3822-e3827.
- Al-Kindi SG, Sarode A, Zullo M, et al. Ambient air pollution and mortality after cardiac transplantation. J Am Coll Cardiol. 2019;74:30263035.
- Ghosh R, Gauderman WJ, Minor H, et al. Air pollution, weight loss and metabolic benefits of bariatric surgery: a potential model for study of metabolic effects of environmental exposures. Pediatr Obes. 2018;13:312-320.
- Thiel CL, Eckelman M, Guido R, et al. Environmental impacts of surgical procedures: life cycle assessment of hysterectomy in the United States. Environ Sci Technol. 2015;49:1779-1786.
- Zygourakis CC, Yoon S, Valencia V, et al. Operating room waste: disposable supply utilization in neurosurgical procedures. J Neurosurg. 2017;126:620-625.
- van Demark RE, Smith VJS, Fiegen A. Lean and green hand surgery. J Hand Surg. 2018;43:179-181.
- Campion N, Thiel CL, DeBlois J, et al. Life cycle assessment perspectives on delivering an infant in the US. Sci Total Environ. 2012;425:191198.
- Hsu S, Thiel CL, Mello MJ, Slutzman JE. Dumpster diving in the emergency department. West J Emerg Med. 2020;21:1211-1217.
- Mcgain F, Story D, Hendel S. An audit of intensive care unit recyclable waste. Anaesthesia. 2009;64:1299-1302.
- Wormer BA, Augenstein VA, Carpenter CL, et al. The green operating room: simple changes to reduce cost and our carbon footprint. Am Surg. 2013;79:666-671.
- Kagoma Y, Stall N, Rubinstein E, et al. People, planet and profits: the case for greening operating rooms. Can Med Assoc J. 2012;184:19051911.
- McCarthy CJ, Gerstenmaier JF, O’ Neill AC, et al. “EcoRadiology”— pulling the plug on wasted energy in the radiology department. Acad Radiol. 2014;21:1563-1566.
- Donahue LM, Hilton S, Bell SG, et al. A comparative carbon footprint analysis of disposable and reusable vaginal specula. Am J Obstet Gynecol. 2020;223:225.e1-225.e7.
- Rodriguez Morris MI, Hicks A. Life cycle assessment of stainless-steel reusable speculums versus disposable acrylic speculums in a university clinic setting: a case study. Environ Res Commun. 2022;4:025002.
- MacNeill AJ, Lillywhite R, Brown CJ. The impact of surgery on global climate: a carbon footprinting study of operating theatres in three health systems. Lancet Planet Health. 2017;1:e381-e388.
- Thiel C, Duncan P, Woods N. Attitude of US obstetricians and gynaecologists to global warming and medical waste. J Health Serv Res Policy. 2017;22:162-167.
- Siu J, Hill AG, MacCormick AD. Systematic review of reusable versus disposable laparoscopic instruments: costs and safety. ANZ J Surg. 2017;87:28-33.
- Ryan SM, Nielsen CJ. Global warming potential of inhaled anesthetics: application to clinical use. Anesth Analg. 2010;111:92-98.
- Meyer MJ. Desflurane should des-appear: global and financial rationale. Anesth Analg. 2020;131:1317-1322.
- Rollins MD, Arendt KW, Carvalho B, et al. ASA Committee on Obstetric Anesthesia Working Group. Nitrous oxide. American Society of Anesthesiologists website. Accessed May 12, 2023. https://www .asahq.org/about-asa/governance-and-committees/asa-committees /committee-on-obstetric-anesthesia/nitrous-oxide.
- Kalogera E, Dowdy SC. Enhanced recovery pathway in gynecologic surgery: improving outcomes through evidence-based medicine. Obstet Gynecol Clin North Am. 2016;43:551-573.
- Zota AR, Geller RJ, Calafat AM, et al. Phthalates exposure and uterine fibroid burden among women undergoing surgical treatment for fibroids: a preliminary study. Fertil Steril. 2019;111:112-121.
- Bommartio PA, Ferguson KK, Meeker JD, et al. Maternal levels of perfluoroalkyl substances (PFAS) during early pregnancy in relation to preeclampsia subtypes and biomarkers of preeclampsia risk. Environ Health Perspect. 2021;129:107004.
- Azouz S, Boyll P, Swanson M, et al. Managing barriers to recycling in the operating room. Am J Surg. 2019;217:634-638.
- Watts N, Amann M, Arnell N, et al. The 2020 report of The Lancet Countdown on health and climate change: responding to converging crises. Lancet. 2021;397:129-170.
- Ryan EC, Dubrow R, Sherman JD. Medical, nursing, and physician assistant student knowledge and attitudes toward climate change, pollution, and resource conservation in health care. BMC Med Educ. 2020;20:200.
- Giudice LC, Llamas-Clark EF, DeNicola Net al; FIGO Committee on Climate Change and Toxic Environmental Exposures. Climate change, women’s health, and the role of obstetricians and gynecologists in leadership. Int J Gynaecol Obstet. 2021;155:345-356.
- Yates EF, Bowder AN, Roa L, et al. Empowering surgeons, anesthesiologists, and obstetricians to incorporate environmental sustainability in the operating room. Ann Surg. 2021;273:1108-1114.
Climate change has been called the biggest health threat of the 21st century.1 The health care sector is a huge contributor to global carbon emissions, accounting for almost double the emissions of global aviation. While other industries and countries are implementing mitigation measures to decrease their emissions, health care is currently on track to double its carbon emissions by 2050, even though it should be carbon neutral by that time to comply with the Paris Climate Agreement.2 There have been some national efforts to curb health care emissions, including the creation of the Office of Climate Change and Health Equity in 2021 and the passage of the Inflation Reduction Act in 2022.3 These are top-down, administrative approaches, and to be successful we will also need clinicians to understand and address this problem.
The negative impacts of heat, air pollution, and exposure to toxic substances on human health have been well documented in multiple regions across multiple specialties.4-7 The United States makes up 27% of the global health care carbon footprint—more emissions than the entire United Kingdom as a country—despite having only 4% of the world’s population.2 Culture and incentives for an overabundance of single-use supplies, not evidence for patient safety, have led to this uniquely American problem. It is evident that our health care industry is an excellent place to implement mitigation measures for carbon emissions that contribute to climate change and can improve health outcomes.
In this article, we recommend 10 practices that can decrease our carbon footprint in ObGyn. We focus on the classic motto of “Reduce, Reuse, Recycle,” while adding “Remove” and “Reimagine” to classify the ways in which we can reduce emissions while not compromising our care to patients.
Reduce
1. Minimize opened materials and single-use devices in the OR and labor and delivery
Health care is a unique setting where a culture of infection prevention and efficiency has led low-cost, single-use supplies to dominate over reusable items. While single-use items can have inexpensive purchasing costs compared to reusable items, the environmental costs required for the production and disposal of the former are often much greater. In operating rooms (ORs) and labor and delivery (LD) units, single-use items are omnipresent. Over the past decade, researchers and clinicians have started to take a closer look at these items and their carbon footprint. One group evaluated hysterectomy through a waste audit and found that the vast majority of waste from all of the cases was Spunbond Meltblown Spunbond, or SMS; plastic materialthat comprises gowns; blue wraps; and drapes; followed by hard plastic material that comprises trays and packaging.8 Moreover, production and manufacturing processes contributed to 95% of the environmental impacts of these items.8
In an effort to be time efficient, OR staff will open sterile surgical packs and individual peel-pack items prior to surgery to minimize having to find items during surgery. However, this creates an inordinate amount of waste. One group of neurosurgeons who evaluated their opened but unused supplies found that 85% of their unused items were individually opened items, leading to a waste of $2.9 million per year.9 Minor procedures like dilation and curettage, cystoscopy, and hysteroscopy do not need such a large sterile field, as these procedures are also safe to perform in the office. Hand surgeons have been quick to lead in this space, particularly with minor procedures such as carpal tunnel release. One division was able to eliminate 2.8 tons of waste and save $13,000 in a 2-year period by reducing the sterile field.10 ObGyns can work with OR and LD staff to create custom packs that minimize unused or underutilized items, helping to reduce both the carbon footprint and health care spending.
Bottom line: ObGyns can help foster a culture of having supplies available but not opened until needed during a case.
Continue to: 2. Decrease regulated medical waste...
2. Decrease regulated medical waste
Health care is unique from other fields in that there are multiple waste streams to consider. Infectious waste and items saturated in blood or capable of causing infection must be placed into regulated medical waste (RMW), or more commonly, red biohazard bags. RMW is autoclaved or incinerated prior to disposal in a landfill. This process is more financially and environmentally costly than general municipal waste (GMW). This process also requires more transport—1 study revealed that GMW traveled 20 km to a landfill for disposal, compared with the 50 km that RMW traveled for sterilized-prior-to-landfill disposal.11
Unfortunately, the vast majority of items placed in RMW are incorrectly triaged and should instead be disposed in GMW.12,13 One study performed in an emergency department revealed that 85% of waste was incorrectly placed in the RMW.12
Bottom line: ObGyns can avoid placing items in RMW that may not qualify and advocate for institution policy changes to remove RMW from places such as waiting rooms, at the patient bedside, or next to scrub sinks.
3. Reduce energy use
ORs and LD units use a lot of energy, and numerous studies have demonstrated that the heating, ventilation, and air conditioning (HVAC) system plays a large role in emissions.8,11 This can easily be fixed by “HVAC setbacks” and powering down rooms when not in use. One institution powered down ORs when not in use and reduced 234 metric tons of CO2 emissions and saved $33,000 per year.14 Transitioning to light-emitting diode (LED) lights reduced energy usage at 1 institution by almost 50%.15 Finally, computers in clinical offices, examination rooms, and administrative offices can be powered down at the end of the day. One study found that in 1 radiology department, 29 computers left on overnight and on weekends emitted 17.7 tons of CO2 emissions in 1 year.16
Bottom line: We as ObGyns can advocate for how energy can be saved outside of surgical cases, including powering down ORs and LD units, transitioning to LED lighting, and powering down workstations.
Reuse
4. Choose reusable equipment
In ObGyn practice, the most commonly used tool is the speculum. Given its omnipresence, the speculum is a great place to start to decrease our carbon footprint. Two studies have evaluated the environmental impact of reusable versus single-use disposable specula, and both demonstrated that the stainless-steel versions have less global warming potential than the acrylic varieties.17,18 Donahue and colleagues17 demonstrated that it only took 2 to 3 pelvic examinations for the cost of stainless-steel specula to break even, even when sterilized in a half-filled autoclave tray. Rodriquez, et al18 revealed that, compared with an acrylic model, the stainless-steel specula had fewer negative impacts in terms of global warming, acidification, respiratory effects, smog, and fossil fuel depletion.18
Bottom line: Strongly consider using stainless-steel specula to reduce costs and carbon emissions.
In addition to specula, ObGyns can choose reusable equipment in the OR. For example, surgeons can use stainless-steel trocars instead of disposable trocars.19 In vaginal cases, Breisky-Navratil retractors can be used instead of disposable self-retaining retractors. Plastic basins that often are included in sterile supply packs can be replaced with stainless-steel basins, which could have profound positive effects on the carbon footprint of gynecologic surgery.8 One study of ObGyns demonstrated that 95% of physicians supported waste-reduction efforts, and 66% supported utilizing reusable surgical tools instead of disposable tools.20
Bottom line: As surgeons, ObGyns have influence over what they want to use in the OR, and they can petition for reusable options over disposable options.
5. Launder the sterile blue towels
Sterile blue towels, which are made of cotton, have the largest environmental footprint compared with other disposable materials, such as plastics, and contribute greatly to toxicity in human health.8,11 Although these towels cannot be laundered and sterilized again for use in a sterile surgical field, they can be laundered and repurposed, including by environmental services to clean hospital rooms. Blue towels should be able to be laundered no matter how saturated in body fluids they are.
Bottom line: ObGyns should strive to always launder the blue towels and educate trainees and other staff in the OR to do the same.
Recycle
6. Recycle and reprocess materials and devices
While recycling is immensely important, it requires a large amount of energy to break down a material to its raw components for manufacturing. It likely reduces our carbon footprint from OR procedures by only 5%.8 However, recycling is still a good way to divert appropriate materials from landfill, saving costs and emissions at the end of a material’s life. One example is sterile blue wrap, which is a petroleum product with a recycling number of 6 and a filtration rating of N99. Blue wrap can be recycled into plastic pellets, or it can be recreated into other hospital supplies, such as gowns.
Bottom line: ObGyns can petition their hospitals to work with suppliers and waste-processing companies who have recycling programs built into their supply chains.
By contrast, reprocessing can have a much larger impact on carbon emissions. Complex items, such as advanced energy devices that can be reprocessed, result in a greater reduction in carbon emissions due to the reuse of their complex materials and manufacturing when compared with such devices that cannot be reprocessed. Recycling and reprocessing programs are already in place for several devices (TABLE). Authors of a systematic review showed that there is no evidence to support the use of single-use supplies and instruments over reprocessed items when considering instrument function, ease of use, patient safety, transmission of infection, or long-term patient outcomes.21

Bottom line: ObGyns can choose to use reprocessed items in ORs instead of single-use devices and educate staff on the safety of these items.
Continue to: Remove...
Remove
7. Remove desflurane and other volatile gases from formularies
Volatile anesthetic gases, such as desflurane, isoflurane, and nitrous oxide, are themselves potent greenhouse gases, comprising a large portion of the carbon emissions that come from the OR.22 Desflurane was developed to have a rapid onset for induction and quick recovery; however, studies have shown no clinical benefit over other gases.23 Furthermore, the costs and greenhouse gas potential are substantial. Desflurane costs 2 to 3 times more and has more than 20 times the global warming potential of the other volatile gases (FIGURE).8 Using 1 hour of desflurane is equivalent to driving 378 miles in a gas-powered vehicle, while the use of isoflurane and sevoflurane create equivalents of only 15 and 8 miles, respectively.23

Nitrous oxide is another powerful greenhouse gas that is a direct ozone depletor and can stay in the atmosphere for 114 years.22 Nitrous oxide has limited clinical use in hospitals, but it is often stored in central hospital piping. Most of the impact of nitrous oxide comes through leaks in a poor system design rather than patient delivery. One estimate reveals that more than 13 million liters of nitrous oxide are lost annually from leaks in European hospitals.22 The American Society of Anesthesiologists recommends decommissioning central piping of nitrous oxide in favor of cylinders at the point of care.24
Literature on enhanced recovery after surgery in gynecology promotes the use of propofol over volatile gases for our patients because of the high rate of postoperative nausea and vomiting seen with gases.25 Volatile gases should be a last-choice anesthetic for our patients.
Bottom line: It is critical that ObGyns work with colleagues in anesthesia to develop climate- and patient-friendly protocols for procedures.
8. Remove endocrine-disrupting chemicals from clinical supplies
Endocrine-disrupting chemicals (EDCs) are a type of chemical that alter the hormonal systems of humans, which can result in adverse health effects. Multiple studies and reviews have tied EDCs to reproductive abnormalities, such as the effects of bisphenol A (BPA) on estradiol levels, antral follicle counts, oocyte quality, and implantation rates; phthalates on fibroid burden; triclosan on embryo quality; parabens on live birth rates; and perfluoroalkylsubstances (PFAS or “forever substances”) on hypertensive disorders of pregnancy.5,26,27
What might be most shocking is that these EDCs are incorporated into medical supplies and pharmaceuticals. For example, BPA is known to line dialysis and ointment tubes, parabens are used for their antimicrobial properties in ultrasound gel and hep-locks, and phthalates are found in up to 40% of medical-use plastics and controlled-release medications. Authors of an observational study found that 74% of patients admitted to an LD unit were exposed to EDCs. In a neonatal intensive care unit (NICU), most of the supplies contained an EDC, and urinary BPA levels were elevated in neonates admitted to a NICU, raising concerns about long-term health risks.5
Bottom line: Physicians and health care institutions have an obligation to petition industry partners and suppliers to remove EDCs from their supply chains.
Reimagine
9. Educate
The field of health care sustainability remains in its infancy, but from 2007 to 2019, publications on climate change and health in academia increased by a factor of 8.29 Additionally, through waste audits, quality-improvement projects, and life cycle analyses (analytical tools to evaluate product or process emissions from materials extraction to disposal), we have gained insight into the scope of the problem, with evidence showing that our practices are largely derived from culture. It is time to provide formal education on health care sustainability to medical trainees, staff, and clinicians alike, who desire to see this topic reflected in their formal curricula.30 Start talking about it!
Bottom line: Commentaries, webinars, formal didactics sessions, in-services, and hospital workgroups to introduce this topic are a good way to teach others about the carbon footprint of our care and solutions to minimize it.
10. Engage in advocacy
Physicians have an ethical duty to advocate for change at the local, regional, and national levels if we want to see a better future for our patients, their children, and even ourselves. We should reimagine this work as an important public health initiative.31 Surveys of physicians, including ObGyns, reveal a concern about the sustainability of health care and a commitment to addressing this issue.20 ObGyns are on the frontlines of delivering care every day, so we are poised to implement changes that can impact our patients, especially when we can lead and petition hospital or local committees.20,28,32 There is much to be done, but every voice counts and can make impactful changes at every level. ●

Climate change has been called the biggest health threat of the 21st century.1 The health care sector is a huge contributor to global carbon emissions, accounting for almost double the emissions of global aviation. While other industries and countries are implementing mitigation measures to decrease their emissions, health care is currently on track to double its carbon emissions by 2050, even though it should be carbon neutral by that time to comply with the Paris Climate Agreement.2 There have been some national efforts to curb health care emissions, including the creation of the Office of Climate Change and Health Equity in 2021 and the passage of the Inflation Reduction Act in 2022.3 These are top-down, administrative approaches, and to be successful we will also need clinicians to understand and address this problem.
The negative impacts of heat, air pollution, and exposure to toxic substances on human health have been well documented in multiple regions across multiple specialties.4-7 The United States makes up 27% of the global health care carbon footprint—more emissions than the entire United Kingdom as a country—despite having only 4% of the world’s population.2 Culture and incentives for an overabundance of single-use supplies, not evidence for patient safety, have led to this uniquely American problem. It is evident that our health care industry is an excellent place to implement mitigation measures for carbon emissions that contribute to climate change and can improve health outcomes.
In this article, we recommend 10 practices that can decrease our carbon footprint in ObGyn. We focus on the classic motto of “Reduce, Reuse, Recycle,” while adding “Remove” and “Reimagine” to classify the ways in which we can reduce emissions while not compromising our care to patients.
Reduce
1. Minimize opened materials and single-use devices in the OR and labor and delivery
Health care is a unique setting where a culture of infection prevention and efficiency has led low-cost, single-use supplies to dominate over reusable items. While single-use items can have inexpensive purchasing costs compared to reusable items, the environmental costs required for the production and disposal of the former are often much greater. In operating rooms (ORs) and labor and delivery (LD) units, single-use items are omnipresent. Over the past decade, researchers and clinicians have started to take a closer look at these items and their carbon footprint. One group evaluated hysterectomy through a waste audit and found that the vast majority of waste from all of the cases was Spunbond Meltblown Spunbond, or SMS; plastic materialthat comprises gowns; blue wraps; and drapes; followed by hard plastic material that comprises trays and packaging.8 Moreover, production and manufacturing processes contributed to 95% of the environmental impacts of these items.8
In an effort to be time efficient, OR staff will open sterile surgical packs and individual peel-pack items prior to surgery to minimize having to find items during surgery. However, this creates an inordinate amount of waste. One group of neurosurgeons who evaluated their opened but unused supplies found that 85% of their unused items were individually opened items, leading to a waste of $2.9 million per year.9 Minor procedures like dilation and curettage, cystoscopy, and hysteroscopy do not need such a large sterile field, as these procedures are also safe to perform in the office. Hand surgeons have been quick to lead in this space, particularly with minor procedures such as carpal tunnel release. One division was able to eliminate 2.8 tons of waste and save $13,000 in a 2-year period by reducing the sterile field.10 ObGyns can work with OR and LD staff to create custom packs that minimize unused or underutilized items, helping to reduce both the carbon footprint and health care spending.
Bottom line: ObGyns can help foster a culture of having supplies available but not opened until needed during a case.
Continue to: 2. Decrease regulated medical waste...
2. Decrease regulated medical waste
Health care is unique from other fields in that there are multiple waste streams to consider. Infectious waste and items saturated in blood or capable of causing infection must be placed into regulated medical waste (RMW), or more commonly, red biohazard bags. RMW is autoclaved or incinerated prior to disposal in a landfill. This process is more financially and environmentally costly than general municipal waste (GMW). This process also requires more transport—1 study revealed that GMW traveled 20 km to a landfill for disposal, compared with the 50 km that RMW traveled for sterilized-prior-to-landfill disposal.11
Unfortunately, the vast majority of items placed in RMW are incorrectly triaged and should instead be disposed in GMW.12,13 One study performed in an emergency department revealed that 85% of waste was incorrectly placed in the RMW.12
Bottom line: ObGyns can avoid placing items in RMW that may not qualify and advocate for institution policy changes to remove RMW from places such as waiting rooms, at the patient bedside, or next to scrub sinks.
3. Reduce energy use
ORs and LD units use a lot of energy, and numerous studies have demonstrated that the heating, ventilation, and air conditioning (HVAC) system plays a large role in emissions.8,11 This can easily be fixed by “HVAC setbacks” and powering down rooms when not in use. One institution powered down ORs when not in use and reduced 234 metric tons of CO2 emissions and saved $33,000 per year.14 Transitioning to light-emitting diode (LED) lights reduced energy usage at 1 institution by almost 50%.15 Finally, computers in clinical offices, examination rooms, and administrative offices can be powered down at the end of the day. One study found that in 1 radiology department, 29 computers left on overnight and on weekends emitted 17.7 tons of CO2 emissions in 1 year.16
Bottom line: We as ObGyns can advocate for how energy can be saved outside of surgical cases, including powering down ORs and LD units, transitioning to LED lighting, and powering down workstations.
Reuse
4. Choose reusable equipment
In ObGyn practice, the most commonly used tool is the speculum. Given its omnipresence, the speculum is a great place to start to decrease our carbon footprint. Two studies have evaluated the environmental impact of reusable versus single-use disposable specula, and both demonstrated that the stainless-steel versions have less global warming potential than the acrylic varieties.17,18 Donahue and colleagues17 demonstrated that it only took 2 to 3 pelvic examinations for the cost of stainless-steel specula to break even, even when sterilized in a half-filled autoclave tray. Rodriquez, et al18 revealed that, compared with an acrylic model, the stainless-steel specula had fewer negative impacts in terms of global warming, acidification, respiratory effects, smog, and fossil fuel depletion.18
Bottom line: Strongly consider using stainless-steel specula to reduce costs and carbon emissions.
In addition to specula, ObGyns can choose reusable equipment in the OR. For example, surgeons can use stainless-steel trocars instead of disposable trocars.19 In vaginal cases, Breisky-Navratil retractors can be used instead of disposable self-retaining retractors. Plastic basins that often are included in sterile supply packs can be replaced with stainless-steel basins, which could have profound positive effects on the carbon footprint of gynecologic surgery.8 One study of ObGyns demonstrated that 95% of physicians supported waste-reduction efforts, and 66% supported utilizing reusable surgical tools instead of disposable tools.20
Bottom line: As surgeons, ObGyns have influence over what they want to use in the OR, and they can petition for reusable options over disposable options.
5. Launder the sterile blue towels
Sterile blue towels, which are made of cotton, have the largest environmental footprint compared with other disposable materials, such as plastics, and contribute greatly to toxicity in human health.8,11 Although these towels cannot be laundered and sterilized again for use in a sterile surgical field, they can be laundered and repurposed, including by environmental services to clean hospital rooms. Blue towels should be able to be laundered no matter how saturated in body fluids they are.
Bottom line: ObGyns should strive to always launder the blue towels and educate trainees and other staff in the OR to do the same.
Recycle
6. Recycle and reprocess materials and devices
While recycling is immensely important, it requires a large amount of energy to break down a material to its raw components for manufacturing. It likely reduces our carbon footprint from OR procedures by only 5%.8 However, recycling is still a good way to divert appropriate materials from landfill, saving costs and emissions at the end of a material’s life. One example is sterile blue wrap, which is a petroleum product with a recycling number of 6 and a filtration rating of N99. Blue wrap can be recycled into plastic pellets, or it can be recreated into other hospital supplies, such as gowns.
Bottom line: ObGyns can petition their hospitals to work with suppliers and waste-processing companies who have recycling programs built into their supply chains.
By contrast, reprocessing can have a much larger impact on carbon emissions. Complex items, such as advanced energy devices that can be reprocessed, result in a greater reduction in carbon emissions due to the reuse of their complex materials and manufacturing when compared with such devices that cannot be reprocessed. Recycling and reprocessing programs are already in place for several devices (TABLE). Authors of a systematic review showed that there is no evidence to support the use of single-use supplies and instruments over reprocessed items when considering instrument function, ease of use, patient safety, transmission of infection, or long-term patient outcomes.21

Bottom line: ObGyns can choose to use reprocessed items in ORs instead of single-use devices and educate staff on the safety of these items.
Continue to: Remove...
Remove
7. Remove desflurane and other volatile gases from formularies
Volatile anesthetic gases, such as desflurane, isoflurane, and nitrous oxide, are themselves potent greenhouse gases, comprising a large portion of the carbon emissions that come from the OR.22 Desflurane was developed to have a rapid onset for induction and quick recovery; however, studies have shown no clinical benefit over other gases.23 Furthermore, the costs and greenhouse gas potential are substantial. Desflurane costs 2 to 3 times more and has more than 20 times the global warming potential of the other volatile gases (FIGURE).8 Using 1 hour of desflurane is equivalent to driving 378 miles in a gas-powered vehicle, while the use of isoflurane and sevoflurane create equivalents of only 15 and 8 miles, respectively.23

Nitrous oxide is another powerful greenhouse gas that is a direct ozone depletor and can stay in the atmosphere for 114 years.22 Nitrous oxide has limited clinical use in hospitals, but it is often stored in central hospital piping. Most of the impact of nitrous oxide comes through leaks in a poor system design rather than patient delivery. One estimate reveals that more than 13 million liters of nitrous oxide are lost annually from leaks in European hospitals.22 The American Society of Anesthesiologists recommends decommissioning central piping of nitrous oxide in favor of cylinders at the point of care.24
Literature on enhanced recovery after surgery in gynecology promotes the use of propofol over volatile gases for our patients because of the high rate of postoperative nausea and vomiting seen with gases.25 Volatile gases should be a last-choice anesthetic for our patients.
Bottom line: It is critical that ObGyns work with colleagues in anesthesia to develop climate- and patient-friendly protocols for procedures.
8. Remove endocrine-disrupting chemicals from clinical supplies
Endocrine-disrupting chemicals (EDCs) are a type of chemical that alter the hormonal systems of humans, which can result in adverse health effects. Multiple studies and reviews have tied EDCs to reproductive abnormalities, such as the effects of bisphenol A (BPA) on estradiol levels, antral follicle counts, oocyte quality, and implantation rates; phthalates on fibroid burden; triclosan on embryo quality; parabens on live birth rates; and perfluoroalkylsubstances (PFAS or “forever substances”) on hypertensive disorders of pregnancy.5,26,27
What might be most shocking is that these EDCs are incorporated into medical supplies and pharmaceuticals. For example, BPA is known to line dialysis and ointment tubes, parabens are used for their antimicrobial properties in ultrasound gel and hep-locks, and phthalates are found in up to 40% of medical-use plastics and controlled-release medications. Authors of an observational study found that 74% of patients admitted to an LD unit were exposed to EDCs. In a neonatal intensive care unit (NICU), most of the supplies contained an EDC, and urinary BPA levels were elevated in neonates admitted to a NICU, raising concerns about long-term health risks.5
Bottom line: Physicians and health care institutions have an obligation to petition industry partners and suppliers to remove EDCs from their supply chains.
Reimagine
9. Educate
The field of health care sustainability remains in its infancy, but from 2007 to 2019, publications on climate change and health in academia increased by a factor of 8.29 Additionally, through waste audits, quality-improvement projects, and life cycle analyses (analytical tools to evaluate product or process emissions from materials extraction to disposal), we have gained insight into the scope of the problem, with evidence showing that our practices are largely derived from culture. It is time to provide formal education on health care sustainability to medical trainees, staff, and clinicians alike, who desire to see this topic reflected in their formal curricula.30 Start talking about it!
Bottom line: Commentaries, webinars, formal didactics sessions, in-services, and hospital workgroups to introduce this topic are a good way to teach others about the carbon footprint of our care and solutions to minimize it.
10. Engage in advocacy
Physicians have an ethical duty to advocate for change at the local, regional, and national levels if we want to see a better future for our patients, their children, and even ourselves. We should reimagine this work as an important public health initiative.31 Surveys of physicians, including ObGyns, reveal a concern about the sustainability of health care and a commitment to addressing this issue.20 ObGyns are on the frontlines of delivering care every day, so we are poised to implement changes that can impact our patients, especially when we can lead and petition hospital or local committees.20,28,32 There is much to be done, but every voice counts and can make impactful changes at every level. ●

- Costello A, Abbas M, Allen et al. Managing the health effects of climate change: Lancet and University College London Institute for Global Health Commission. Lancet. 2009;373:1693-1733.
- Health care climate footprint report. Health Care Without Harm website. https://www.noharm.org/ClimateFootprintReport. Accessed May 12, 2023.
- Balbus JM, McCannon CJ, Mataka A, et al. After COP26—putting health and equity at the center of the climate movement. N Engl J Med. 2022;386:1295-1297.
- Bekkar B, Pacheco S, Basu R, et al. Association of air pollution and heat exposure with preterm birth, low birth weight, and stillbirth in the US: a systematic review. JAMA Netw Open. 2020;3:e208243.
- Genco M, Anderson-Shaw L, Sargis RM. Unwitting accomplices: endocrine disruptors confounding clinical care. J Clin Endocrinol Metab. 2020;105:e3822-e3827.
- Al-Kindi SG, Sarode A, Zullo M, et al. Ambient air pollution and mortality after cardiac transplantation. J Am Coll Cardiol. 2019;74:30263035.
- Ghosh R, Gauderman WJ, Minor H, et al. Air pollution, weight loss and metabolic benefits of bariatric surgery: a potential model for study of metabolic effects of environmental exposures. Pediatr Obes. 2018;13:312-320.
- Thiel CL, Eckelman M, Guido R, et al. Environmental impacts of surgical procedures: life cycle assessment of hysterectomy in the United States. Environ Sci Technol. 2015;49:1779-1786.
- Zygourakis CC, Yoon S, Valencia V, et al. Operating room waste: disposable supply utilization in neurosurgical procedures. J Neurosurg. 2017;126:620-625.
- van Demark RE, Smith VJS, Fiegen A. Lean and green hand surgery. J Hand Surg. 2018;43:179-181.
- Campion N, Thiel CL, DeBlois J, et al. Life cycle assessment perspectives on delivering an infant in the US. Sci Total Environ. 2012;425:191198.
- Hsu S, Thiel CL, Mello MJ, Slutzman JE. Dumpster diving in the emergency department. West J Emerg Med. 2020;21:1211-1217.
- Mcgain F, Story D, Hendel S. An audit of intensive care unit recyclable waste. Anaesthesia. 2009;64:1299-1302.
- Wormer BA, Augenstein VA, Carpenter CL, et al. The green operating room: simple changes to reduce cost and our carbon footprint. Am Surg. 2013;79:666-671.
- Kagoma Y, Stall N, Rubinstein E, et al. People, planet and profits: the case for greening operating rooms. Can Med Assoc J. 2012;184:19051911.
- McCarthy CJ, Gerstenmaier JF, O’ Neill AC, et al. “EcoRadiology”— pulling the plug on wasted energy in the radiology department. Acad Radiol. 2014;21:1563-1566.
- Donahue LM, Hilton S, Bell SG, et al. A comparative carbon footprint analysis of disposable and reusable vaginal specula. Am J Obstet Gynecol. 2020;223:225.e1-225.e7.
- Rodriguez Morris MI, Hicks A. Life cycle assessment of stainless-steel reusable speculums versus disposable acrylic speculums in a university clinic setting: a case study. Environ Res Commun. 2022;4:025002.
- MacNeill AJ, Lillywhite R, Brown CJ. The impact of surgery on global climate: a carbon footprinting study of operating theatres in three health systems. Lancet Planet Health. 2017;1:e381-e388.
- Thiel C, Duncan P, Woods N. Attitude of US obstetricians and gynaecologists to global warming and medical waste. J Health Serv Res Policy. 2017;22:162-167.
- Siu J, Hill AG, MacCormick AD. Systematic review of reusable versus disposable laparoscopic instruments: costs and safety. ANZ J Surg. 2017;87:28-33.
- Ryan SM, Nielsen CJ. Global warming potential of inhaled anesthetics: application to clinical use. Anesth Analg. 2010;111:92-98.
- Meyer MJ. Desflurane should des-appear: global and financial rationale. Anesth Analg. 2020;131:1317-1322.
- Rollins MD, Arendt KW, Carvalho B, et al. ASA Committee on Obstetric Anesthesia Working Group. Nitrous oxide. American Society of Anesthesiologists website. Accessed May 12, 2023. https://www .asahq.org/about-asa/governance-and-committees/asa-committees /committee-on-obstetric-anesthesia/nitrous-oxide.
- Kalogera E, Dowdy SC. Enhanced recovery pathway in gynecologic surgery: improving outcomes through evidence-based medicine. Obstet Gynecol Clin North Am. 2016;43:551-573.
- Zota AR, Geller RJ, Calafat AM, et al. Phthalates exposure and uterine fibroid burden among women undergoing surgical treatment for fibroids: a preliminary study. Fertil Steril. 2019;111:112-121.
- Bommartio PA, Ferguson KK, Meeker JD, et al. Maternal levels of perfluoroalkyl substances (PFAS) during early pregnancy in relation to preeclampsia subtypes and biomarkers of preeclampsia risk. Environ Health Perspect. 2021;129:107004.
- Azouz S, Boyll P, Swanson M, et al. Managing barriers to recycling in the operating room. Am J Surg. 2019;217:634-638.
- Watts N, Amann M, Arnell N, et al. The 2020 report of The Lancet Countdown on health and climate change: responding to converging crises. Lancet. 2021;397:129-170.
- Ryan EC, Dubrow R, Sherman JD. Medical, nursing, and physician assistant student knowledge and attitudes toward climate change, pollution, and resource conservation in health care. BMC Med Educ. 2020;20:200.
- Giudice LC, Llamas-Clark EF, DeNicola Net al; FIGO Committee on Climate Change and Toxic Environmental Exposures. Climate change, women’s health, and the role of obstetricians and gynecologists in leadership. Int J Gynaecol Obstet. 2021;155:345-356.
- Yates EF, Bowder AN, Roa L, et al. Empowering surgeons, anesthesiologists, and obstetricians to incorporate environmental sustainability in the operating room. Ann Surg. 2021;273:1108-1114.
- Costello A, Abbas M, Allen et al. Managing the health effects of climate change: Lancet and University College London Institute for Global Health Commission. Lancet. 2009;373:1693-1733.
- Health care climate footprint report. Health Care Without Harm website. https://www.noharm.org/ClimateFootprintReport. Accessed May 12, 2023.
- Balbus JM, McCannon CJ, Mataka A, et al. After COP26—putting health and equity at the center of the climate movement. N Engl J Med. 2022;386:1295-1297.
- Bekkar B, Pacheco S, Basu R, et al. Association of air pollution and heat exposure with preterm birth, low birth weight, and stillbirth in the US: a systematic review. JAMA Netw Open. 2020;3:e208243.
- Genco M, Anderson-Shaw L, Sargis RM. Unwitting accomplices: endocrine disruptors confounding clinical care. J Clin Endocrinol Metab. 2020;105:e3822-e3827.
- Al-Kindi SG, Sarode A, Zullo M, et al. Ambient air pollution and mortality after cardiac transplantation. J Am Coll Cardiol. 2019;74:30263035.
- Ghosh R, Gauderman WJ, Minor H, et al. Air pollution, weight loss and metabolic benefits of bariatric surgery: a potential model for study of metabolic effects of environmental exposures. Pediatr Obes. 2018;13:312-320.
- Thiel CL, Eckelman M, Guido R, et al. Environmental impacts of surgical procedures: life cycle assessment of hysterectomy in the United States. Environ Sci Technol. 2015;49:1779-1786.
- Zygourakis CC, Yoon S, Valencia V, et al. Operating room waste: disposable supply utilization in neurosurgical procedures. J Neurosurg. 2017;126:620-625.
- van Demark RE, Smith VJS, Fiegen A. Lean and green hand surgery. J Hand Surg. 2018;43:179-181.
- Campion N, Thiel CL, DeBlois J, et al. Life cycle assessment perspectives on delivering an infant in the US. Sci Total Environ. 2012;425:191198.
- Hsu S, Thiel CL, Mello MJ, Slutzman JE. Dumpster diving in the emergency department. West J Emerg Med. 2020;21:1211-1217.
- Mcgain F, Story D, Hendel S. An audit of intensive care unit recyclable waste. Anaesthesia. 2009;64:1299-1302.
- Wormer BA, Augenstein VA, Carpenter CL, et al. The green operating room: simple changes to reduce cost and our carbon footprint. Am Surg. 2013;79:666-671.
- Kagoma Y, Stall N, Rubinstein E, et al. People, planet and profits: the case for greening operating rooms. Can Med Assoc J. 2012;184:19051911.
- McCarthy CJ, Gerstenmaier JF, O’ Neill AC, et al. “EcoRadiology”— pulling the plug on wasted energy in the radiology department. Acad Radiol. 2014;21:1563-1566.
- Donahue LM, Hilton S, Bell SG, et al. A comparative carbon footprint analysis of disposable and reusable vaginal specula. Am J Obstet Gynecol. 2020;223:225.e1-225.e7.
- Rodriguez Morris MI, Hicks A. Life cycle assessment of stainless-steel reusable speculums versus disposable acrylic speculums in a university clinic setting: a case study. Environ Res Commun. 2022;4:025002.
- MacNeill AJ, Lillywhite R, Brown CJ. The impact of surgery on global climate: a carbon footprinting study of operating theatres in three health systems. Lancet Planet Health. 2017;1:e381-e388.
- Thiel C, Duncan P, Woods N. Attitude of US obstetricians and gynaecologists to global warming and medical waste. J Health Serv Res Policy. 2017;22:162-167.
- Siu J, Hill AG, MacCormick AD. Systematic review of reusable versus disposable laparoscopic instruments: costs and safety. ANZ J Surg. 2017;87:28-33.
- Ryan SM, Nielsen CJ. Global warming potential of inhaled anesthetics: application to clinical use. Anesth Analg. 2010;111:92-98.
- Meyer MJ. Desflurane should des-appear: global and financial rationale. Anesth Analg. 2020;131:1317-1322.
- Rollins MD, Arendt KW, Carvalho B, et al. ASA Committee on Obstetric Anesthesia Working Group. Nitrous oxide. American Society of Anesthesiologists website. Accessed May 12, 2023. https://www .asahq.org/about-asa/governance-and-committees/asa-committees /committee-on-obstetric-anesthesia/nitrous-oxide.
- Kalogera E, Dowdy SC. Enhanced recovery pathway in gynecologic surgery: improving outcomes through evidence-based medicine. Obstet Gynecol Clin North Am. 2016;43:551-573.
- Zota AR, Geller RJ, Calafat AM, et al. Phthalates exposure and uterine fibroid burden among women undergoing surgical treatment for fibroids: a preliminary study. Fertil Steril. 2019;111:112-121.
- Bommartio PA, Ferguson KK, Meeker JD, et al. Maternal levels of perfluoroalkyl substances (PFAS) during early pregnancy in relation to preeclampsia subtypes and biomarkers of preeclampsia risk. Environ Health Perspect. 2021;129:107004.
- Azouz S, Boyll P, Swanson M, et al. Managing barriers to recycling in the operating room. Am J Surg. 2019;217:634-638.
- Watts N, Amann M, Arnell N, et al. The 2020 report of The Lancet Countdown on health and climate change: responding to converging crises. Lancet. 2021;397:129-170.
- Ryan EC, Dubrow R, Sherman JD. Medical, nursing, and physician assistant student knowledge and attitudes toward climate change, pollution, and resource conservation in health care. BMC Med Educ. 2020;20:200.
- Giudice LC, Llamas-Clark EF, DeNicola Net al; FIGO Committee on Climate Change and Toxic Environmental Exposures. Climate change, women’s health, and the role of obstetricians and gynecologists in leadership. Int J Gynaecol Obstet. 2021;155:345-356.
- Yates EF, Bowder AN, Roa L, et al. Empowering surgeons, anesthesiologists, and obstetricians to incorporate environmental sustainability in the operating room. Ann Surg. 2021;273:1108-1114.
Did ob.gyn. residencies take a hit from abortion bans?
Emilee Gibson, MD, recently graduated from Southern Illinois University, Springfield, and starts her ob.gyn. residency at Vanderbilt University Medical Center in Nashville, Tenn., later this month. Abortion is permitted in Illinois but banned in Tennessee, a factor she weighed cautiously when she applied for residencies.
Dr. Gibson told this news organization that medical students, not just those interested in ob.gyn., are starting to think more about what it means to move to a state where it might be difficult to access abortion care. “Just from a personal standpoint, that’s a little scary.”
The Supreme Court’s decision to overturn Roe v. Wade abortion rights last June threatened to derail ob.gyns. in training from pursuing the specialty or locating in states that have banned or limited abortion.
, but some industry leaders, residents, and medical students say it may be too early to judge the full impact of the ruling because most students were already far along in their decision and application for a 2023 residency position.
At this point, some ob.gyn. students are planning careers on the basis of whether they have family ties in a particular state, whether limiting their search might hurt their potential to match in a competitive specialty, and whether their faith in the family planning and abortion training being offered by a program outweighs the drawbacks of being in a state with abortion bans or restrictions.
Lucy Brown, MD, a recent graduate of Indiana University, Indianapolis, said in an interview that she’d be “very nervous” about living and practicing in abortion-restricted Indiana if she were ready to start a family.
Dr. Brown said that she mostly limited applications in the recent Match to ob.gyn. residencies in states that protected abortion rights. Though she applied to a program in her home state of Kentucky, she noted that it – along with a program in Missouri – was very low on her rank list because of their abortion restrictions.
Ultimately, Dr. Brown matched at Johns Hopkins University, Baltimore, where she will receive abortion training and assist with abortions throughout her residency. Maryland’s abortion rights status was a big attraction, she said. “Abortion is integrated into every aspect of the education.”
By the numbers
For students applying to residencies this summer, evaluating the state legislative landscape is a little clearer than it was 1 year ago but is still evolving. As of June 1, 56 ob.gyn. residency programs and more than 1,100 medical residents are in states with the most restrictive bans in the country (19% of all programs), according to the Bixby Center for Reproductive Health at the University of California, San Francisco.
In terms of the latest abortion laws: 14 states banned abortion, 2 states banned abortion between 6 and 12 weeks, and 9 states banned abortion between 15 and 22 weeks, whereas abortion is legal in 25 states and Washington, according to a recent analysis by the Kaiser Family Foundation.
The impact on residencies? The Association of American Medical Colleges recently reported a 2% drop in the number of U.S. MD seniors who applied to residencies and a 5% decline in the number of seniors who applied to ob.gyn. residencies. In states where abortion was banned, the number of senior applicants to ob.gyn. programs dropped by more than 10%, according to AAMC’s Research and Action Institute.
“U.S. MD seniors appear, in general, more likely to avoid states where abortions are banned,” said Atul Grover, MD, PhD, executive director of the Research and Action Institute. “That’s a big difference between states where there are abortion bans and gestational limits and states with no bans or limits; it’s almost twice as large,” Dr. Grover said in an interview. “The question is: Was it a 1-year blip or something that will be the beginning of a trend?”
In a statement to this news organization, officials from the American College of Obstetricians and Gynecologists and the Association of Professors of Gynecology and Obstetrics said that they were aware of the AAMC data but needed to further evaluate the impact of the Dobbs ruling.
A survey released at ACOG’s annual meeting in May found that 58% of third- and fourth-year medical students were unlikely to apply to a residency program in a state with abortion restrictions. Conducted after the Dobbs ruling last year, the survey found that future physicians are choosing where to attend residency according to state abortion policies, indicating that access to abortion care is changing the landscape of medical practice.
“For personal as well as professional reasons, reproductive health care access is now a key factor in residency match decisions as a result of Dobbs,” lead author Ariana Traub, MPH, said. She studies at Emory University, Atlanta, where abortion is restricted.
“Many students, including myself, struggle when trying to decide whether to stay in restricted states where the need is greatest (highest maternal mortality, infant mortality, lower number of physicians), versus going to an unrestricted state” for more comprehensive training and care, Ms. Traub said. “Regardless of this decision, Dobbs and subsequent abortion laws are making students question what matters most and how they can provide the best care.”
In another recently published survey, University of Miami fourth-year student Morgan Levy, MD, MPH, and colleagues found that 77% of students would prefer to apply to a residency program in a state that preserves access to abortion. Ensuring access to those services for themselves or a family member was a key factor, according to the paper published in the Journal of General Internal Medicine.
For Dr. Levy, who recently graduated from a school in abortion-restricted Florida and will soon apply to ob.gyn. residencies, the Dobbs decision made her more committed to becoming an ob.gyn., an interest she’s had since college, she said.
“I do not intend to limit my search,” Dr. Levy said in an interview. “In the states where there are restrictions in place, it’s really important to make sure that people are getting good care,” she said.
Differing perspective
Though survey and anecdotal data show that students and residents expressing hesitation about states with bans or restrictive laws, it appears that most who applied to residency programs during the 2023 Match did not shy away from those states. Almost all the open ob.gyn. residency positions were filled, according to the National Resident Matching Program.
There was no change in how U.S. MD seniors applying for 2023 residency ranked programs on the basis of whether abortion was legal, limited, or banned in the state where a program was based, Donna Lamb, DHSc, MBA, BSN, president and CEO of the NRMP, said.
“We’re seeing what we’ve seen over the past 5 years, and that is a very high fill rate, a very high rate of preference for ob.gyn., and not a heck of a lot of change,” Dr. Lamb said, noting that ob.gyn. programs continue to be very competitive. “We have more applicants than we have positions available,” she said.
In the most recent Match, there were 2,100 applicants (more than half U.S. MD seniors) for about 1,500 slots, with 1,499 initial matches, according to NRMP data. The overall fill rate was 99.7% after the Supplemental Offer and Assistance Program and Electronic Residency Applications process, NRMP reported. The results are similar to what NRMP reported as its previous all-time high year for ob.gyn. placements.
There was a dip in applicants from 2022 to 2023, even though the slots available stayed the same, but it was not markedly different from the previous 5 years, Dr. Lamb said.
“While the Dobbs decision may, indeed, have impacted applicant and application numbers to residency programs, interventions such as signaling may also contribute to the decrease in numbers of applications submitted as well,” AnnaMarie Connolly, MD, ACOG chief of education and academic affairs, and Arthur Ollendorff, MD, APOG president, said in a statement to this news organization.
For the first time in 2022, Match Day applicants were required to “signal” interest in a particular program in an effort to reduce the number of applications and cost to medical students, they noted.
Personal view
When it was time for Dr. Gibson to apply for ob.gyn. residencies, she wondered: Where do you apply in this landscape? But she did not limit her applications: “If I don’t apply to Indiana, Missouri, Tennessee, Wisconsin, Iowa, I’m taking a lot of really great programs off the table.” She did not want to hurt her chances for a match in a competitive specialty, she said.
“Being in Tennessee is going to give me a very different, unique opportunity to hopefully do a lot of advocacy and lobbying and hopefully have my voice heard in maybe a different way than [in Illinois],” Dr. Gibson added.
Cassie Crifase, MPH, a fourth-year student at the University of Wisconsin–Madison applying to ob.gyn. residencies in next year’s Match, said in an interview that she’s concerned about the health risk of living in a state with abortion restrictions. Wisconsin is one of those.
“My list skews toward programs that are in abortion-protected states, but I also am applying to some programs that are in restricted states.” Those states would have to help her meet the Accreditation Council for Graduate Medical Education training requirements. And, she said, she’d want to know if she could still advocate for abortion access in the state.
Sereena Jivraj, a third-year medical student at Texas Christian University in Fort Worth, said that she won’t apply to programs in Alabama, Mississippi, Arkansas, and other nearby states with abortion restrictions. However, Texas is still on her list. “I’m from Texas, my family lives in Texas, and I go to school in Fort Worth, so I have made those connections,” Ms. Jivraj said.
Student advisers generally encourage ob.gyn. hopefuls to apply to 60-100 programs to ensure that they will match, Ms. Jivraj said. “How are you supposed to apply to 100 programs if many of them fall within states with high restrictions?”
What the future holds
Ms. Jivraj said that she’s concerned about what the future holds, especially if the law does not change in Texas. “I don’t want to go to work every day wondering if I’m going to go to jail for something that I say,” she said.
Dr. Crifase has similar fears. “I want to be able to provide the best care for my patients and that would require being able to do those procedures without having to have my first thought be: Is this legal?”
“Things feel very volatile and uncertain,” Pamela Merritt, executive director of the nonprofit Medical Students for Choice in Philadelphia, where abortion is permitted, said. “What we’re asking medical students to do right now is to envision a future in a profession, a lifetime of providing care, where the policies and procedures and standards of the profession are under attack by 26 state legislatures and the federal court system,” she said.
“I don’t think you’re going to see people as willing to take risk.” She added that if someone matches to a program and then has regrets, “You can’t easily jump from residency program to residency program.”
Dr. Levy believes that the impact of the Dobbs decision is “definitely going to be a more common question of applicants to their potential programs.”
Applicants undoubtedly are thinking about how abortion restrictions or bans might affect their own health or that of their partners or families, she said. In a 2022 survey, Dr. Levy and colleagues reported that abortion is not uncommon among physicians, with 11.5% of the 1,566 respondents who had been pregnant saying they had at least one therapeutic abortion.
Students are also considering the potential ramification of a ban on emergency contraception and laws that criminalize physicians’ provision of abortion care, Dr. Levy said. Another complicating factor is individuals’ family ties or roots in specific geographic areas, she said.
Prospective residents will also have a lot of questions about how they will receive family planning training, Dr. Levy commented. “If you’re somewhere that you can’t really provide full-spectrum reproductive health care, then the question will become: How is the program going to provide that training?”
A version of this article first appeared on Medscape.com.
Emilee Gibson, MD, recently graduated from Southern Illinois University, Springfield, and starts her ob.gyn. residency at Vanderbilt University Medical Center in Nashville, Tenn., later this month. Abortion is permitted in Illinois but banned in Tennessee, a factor she weighed cautiously when she applied for residencies.
Dr. Gibson told this news organization that medical students, not just those interested in ob.gyn., are starting to think more about what it means to move to a state where it might be difficult to access abortion care. “Just from a personal standpoint, that’s a little scary.”
The Supreme Court’s decision to overturn Roe v. Wade abortion rights last June threatened to derail ob.gyns. in training from pursuing the specialty or locating in states that have banned or limited abortion.
, but some industry leaders, residents, and medical students say it may be too early to judge the full impact of the ruling because most students were already far along in their decision and application for a 2023 residency position.
At this point, some ob.gyn. students are planning careers on the basis of whether they have family ties in a particular state, whether limiting their search might hurt their potential to match in a competitive specialty, and whether their faith in the family planning and abortion training being offered by a program outweighs the drawbacks of being in a state with abortion bans or restrictions.
Lucy Brown, MD, a recent graduate of Indiana University, Indianapolis, said in an interview that she’d be “very nervous” about living and practicing in abortion-restricted Indiana if she were ready to start a family.
Dr. Brown said that she mostly limited applications in the recent Match to ob.gyn. residencies in states that protected abortion rights. Though she applied to a program in her home state of Kentucky, she noted that it – along with a program in Missouri – was very low on her rank list because of their abortion restrictions.
Ultimately, Dr. Brown matched at Johns Hopkins University, Baltimore, where she will receive abortion training and assist with abortions throughout her residency. Maryland’s abortion rights status was a big attraction, she said. “Abortion is integrated into every aspect of the education.”
By the numbers
For students applying to residencies this summer, evaluating the state legislative landscape is a little clearer than it was 1 year ago but is still evolving. As of June 1, 56 ob.gyn. residency programs and more than 1,100 medical residents are in states with the most restrictive bans in the country (19% of all programs), according to the Bixby Center for Reproductive Health at the University of California, San Francisco.
In terms of the latest abortion laws: 14 states banned abortion, 2 states banned abortion between 6 and 12 weeks, and 9 states banned abortion between 15 and 22 weeks, whereas abortion is legal in 25 states and Washington, according to a recent analysis by the Kaiser Family Foundation.
The impact on residencies? The Association of American Medical Colleges recently reported a 2% drop in the number of U.S. MD seniors who applied to residencies and a 5% decline in the number of seniors who applied to ob.gyn. residencies. In states where abortion was banned, the number of senior applicants to ob.gyn. programs dropped by more than 10%, according to AAMC’s Research and Action Institute.
“U.S. MD seniors appear, in general, more likely to avoid states where abortions are banned,” said Atul Grover, MD, PhD, executive director of the Research and Action Institute. “That’s a big difference between states where there are abortion bans and gestational limits and states with no bans or limits; it’s almost twice as large,” Dr. Grover said in an interview. “The question is: Was it a 1-year blip or something that will be the beginning of a trend?”
In a statement to this news organization, officials from the American College of Obstetricians and Gynecologists and the Association of Professors of Gynecology and Obstetrics said that they were aware of the AAMC data but needed to further evaluate the impact of the Dobbs ruling.
A survey released at ACOG’s annual meeting in May found that 58% of third- and fourth-year medical students were unlikely to apply to a residency program in a state with abortion restrictions. Conducted after the Dobbs ruling last year, the survey found that future physicians are choosing where to attend residency according to state abortion policies, indicating that access to abortion care is changing the landscape of medical practice.
“For personal as well as professional reasons, reproductive health care access is now a key factor in residency match decisions as a result of Dobbs,” lead author Ariana Traub, MPH, said. She studies at Emory University, Atlanta, where abortion is restricted.
“Many students, including myself, struggle when trying to decide whether to stay in restricted states where the need is greatest (highest maternal mortality, infant mortality, lower number of physicians), versus going to an unrestricted state” for more comprehensive training and care, Ms. Traub said. “Regardless of this decision, Dobbs and subsequent abortion laws are making students question what matters most and how they can provide the best care.”
In another recently published survey, University of Miami fourth-year student Morgan Levy, MD, MPH, and colleagues found that 77% of students would prefer to apply to a residency program in a state that preserves access to abortion. Ensuring access to those services for themselves or a family member was a key factor, according to the paper published in the Journal of General Internal Medicine.
For Dr. Levy, who recently graduated from a school in abortion-restricted Florida and will soon apply to ob.gyn. residencies, the Dobbs decision made her more committed to becoming an ob.gyn., an interest she’s had since college, she said.
“I do not intend to limit my search,” Dr. Levy said in an interview. “In the states where there are restrictions in place, it’s really important to make sure that people are getting good care,” she said.
Differing perspective
Though survey and anecdotal data show that students and residents expressing hesitation about states with bans or restrictive laws, it appears that most who applied to residency programs during the 2023 Match did not shy away from those states. Almost all the open ob.gyn. residency positions were filled, according to the National Resident Matching Program.
There was no change in how U.S. MD seniors applying for 2023 residency ranked programs on the basis of whether abortion was legal, limited, or banned in the state where a program was based, Donna Lamb, DHSc, MBA, BSN, president and CEO of the NRMP, said.
“We’re seeing what we’ve seen over the past 5 years, and that is a very high fill rate, a very high rate of preference for ob.gyn., and not a heck of a lot of change,” Dr. Lamb said, noting that ob.gyn. programs continue to be very competitive. “We have more applicants than we have positions available,” she said.
In the most recent Match, there were 2,100 applicants (more than half U.S. MD seniors) for about 1,500 slots, with 1,499 initial matches, according to NRMP data. The overall fill rate was 99.7% after the Supplemental Offer and Assistance Program and Electronic Residency Applications process, NRMP reported. The results are similar to what NRMP reported as its previous all-time high year for ob.gyn. placements.
There was a dip in applicants from 2022 to 2023, even though the slots available stayed the same, but it was not markedly different from the previous 5 years, Dr. Lamb said.
“While the Dobbs decision may, indeed, have impacted applicant and application numbers to residency programs, interventions such as signaling may also contribute to the decrease in numbers of applications submitted as well,” AnnaMarie Connolly, MD, ACOG chief of education and academic affairs, and Arthur Ollendorff, MD, APOG president, said in a statement to this news organization.
For the first time in 2022, Match Day applicants were required to “signal” interest in a particular program in an effort to reduce the number of applications and cost to medical students, they noted.
Personal view
When it was time for Dr. Gibson to apply for ob.gyn. residencies, she wondered: Where do you apply in this landscape? But she did not limit her applications: “If I don’t apply to Indiana, Missouri, Tennessee, Wisconsin, Iowa, I’m taking a lot of really great programs off the table.” She did not want to hurt her chances for a match in a competitive specialty, she said.
“Being in Tennessee is going to give me a very different, unique opportunity to hopefully do a lot of advocacy and lobbying and hopefully have my voice heard in maybe a different way than [in Illinois],” Dr. Gibson added.
Cassie Crifase, MPH, a fourth-year student at the University of Wisconsin–Madison applying to ob.gyn. residencies in next year’s Match, said in an interview that she’s concerned about the health risk of living in a state with abortion restrictions. Wisconsin is one of those.
“My list skews toward programs that are in abortion-protected states, but I also am applying to some programs that are in restricted states.” Those states would have to help her meet the Accreditation Council for Graduate Medical Education training requirements. And, she said, she’d want to know if she could still advocate for abortion access in the state.
Sereena Jivraj, a third-year medical student at Texas Christian University in Fort Worth, said that she won’t apply to programs in Alabama, Mississippi, Arkansas, and other nearby states with abortion restrictions. However, Texas is still on her list. “I’m from Texas, my family lives in Texas, and I go to school in Fort Worth, so I have made those connections,” Ms. Jivraj said.
Student advisers generally encourage ob.gyn. hopefuls to apply to 60-100 programs to ensure that they will match, Ms. Jivraj said. “How are you supposed to apply to 100 programs if many of them fall within states with high restrictions?”
What the future holds
Ms. Jivraj said that she’s concerned about what the future holds, especially if the law does not change in Texas. “I don’t want to go to work every day wondering if I’m going to go to jail for something that I say,” she said.
Dr. Crifase has similar fears. “I want to be able to provide the best care for my patients and that would require being able to do those procedures without having to have my first thought be: Is this legal?”
“Things feel very volatile and uncertain,” Pamela Merritt, executive director of the nonprofit Medical Students for Choice in Philadelphia, where abortion is permitted, said. “What we’re asking medical students to do right now is to envision a future in a profession, a lifetime of providing care, where the policies and procedures and standards of the profession are under attack by 26 state legislatures and the federal court system,” she said.
“I don’t think you’re going to see people as willing to take risk.” She added that if someone matches to a program and then has regrets, “You can’t easily jump from residency program to residency program.”
Dr. Levy believes that the impact of the Dobbs decision is “definitely going to be a more common question of applicants to their potential programs.”
Applicants undoubtedly are thinking about how abortion restrictions or bans might affect their own health or that of their partners or families, she said. In a 2022 survey, Dr. Levy and colleagues reported that abortion is not uncommon among physicians, with 11.5% of the 1,566 respondents who had been pregnant saying they had at least one therapeutic abortion.
Students are also considering the potential ramification of a ban on emergency contraception and laws that criminalize physicians’ provision of abortion care, Dr. Levy said. Another complicating factor is individuals’ family ties or roots in specific geographic areas, she said.
Prospective residents will also have a lot of questions about how they will receive family planning training, Dr. Levy commented. “If you’re somewhere that you can’t really provide full-spectrum reproductive health care, then the question will become: How is the program going to provide that training?”
A version of this article first appeared on Medscape.com.
Emilee Gibson, MD, recently graduated from Southern Illinois University, Springfield, and starts her ob.gyn. residency at Vanderbilt University Medical Center in Nashville, Tenn., later this month. Abortion is permitted in Illinois but banned in Tennessee, a factor she weighed cautiously when she applied for residencies.
Dr. Gibson told this news organization that medical students, not just those interested in ob.gyn., are starting to think more about what it means to move to a state where it might be difficult to access abortion care. “Just from a personal standpoint, that’s a little scary.”
The Supreme Court’s decision to overturn Roe v. Wade abortion rights last June threatened to derail ob.gyns. in training from pursuing the specialty or locating in states that have banned or limited abortion.
, but some industry leaders, residents, and medical students say it may be too early to judge the full impact of the ruling because most students were already far along in their decision and application for a 2023 residency position.
At this point, some ob.gyn. students are planning careers on the basis of whether they have family ties in a particular state, whether limiting their search might hurt their potential to match in a competitive specialty, and whether their faith in the family planning and abortion training being offered by a program outweighs the drawbacks of being in a state with abortion bans or restrictions.
Lucy Brown, MD, a recent graduate of Indiana University, Indianapolis, said in an interview that she’d be “very nervous” about living and practicing in abortion-restricted Indiana if she were ready to start a family.
Dr. Brown said that she mostly limited applications in the recent Match to ob.gyn. residencies in states that protected abortion rights. Though she applied to a program in her home state of Kentucky, she noted that it – along with a program in Missouri – was very low on her rank list because of their abortion restrictions.
Ultimately, Dr. Brown matched at Johns Hopkins University, Baltimore, where she will receive abortion training and assist with abortions throughout her residency. Maryland’s abortion rights status was a big attraction, she said. “Abortion is integrated into every aspect of the education.”
By the numbers
For students applying to residencies this summer, evaluating the state legislative landscape is a little clearer than it was 1 year ago but is still evolving. As of June 1, 56 ob.gyn. residency programs and more than 1,100 medical residents are in states with the most restrictive bans in the country (19% of all programs), according to the Bixby Center for Reproductive Health at the University of California, San Francisco.
In terms of the latest abortion laws: 14 states banned abortion, 2 states banned abortion between 6 and 12 weeks, and 9 states banned abortion between 15 and 22 weeks, whereas abortion is legal in 25 states and Washington, according to a recent analysis by the Kaiser Family Foundation.
The impact on residencies? The Association of American Medical Colleges recently reported a 2% drop in the number of U.S. MD seniors who applied to residencies and a 5% decline in the number of seniors who applied to ob.gyn. residencies. In states where abortion was banned, the number of senior applicants to ob.gyn. programs dropped by more than 10%, according to AAMC’s Research and Action Institute.
“U.S. MD seniors appear, in general, more likely to avoid states where abortions are banned,” said Atul Grover, MD, PhD, executive director of the Research and Action Institute. “That’s a big difference between states where there are abortion bans and gestational limits and states with no bans or limits; it’s almost twice as large,” Dr. Grover said in an interview. “The question is: Was it a 1-year blip or something that will be the beginning of a trend?”
In a statement to this news organization, officials from the American College of Obstetricians and Gynecologists and the Association of Professors of Gynecology and Obstetrics said that they were aware of the AAMC data but needed to further evaluate the impact of the Dobbs ruling.
A survey released at ACOG’s annual meeting in May found that 58% of third- and fourth-year medical students were unlikely to apply to a residency program in a state with abortion restrictions. Conducted after the Dobbs ruling last year, the survey found that future physicians are choosing where to attend residency according to state abortion policies, indicating that access to abortion care is changing the landscape of medical practice.
“For personal as well as professional reasons, reproductive health care access is now a key factor in residency match decisions as a result of Dobbs,” lead author Ariana Traub, MPH, said. She studies at Emory University, Atlanta, where abortion is restricted.
“Many students, including myself, struggle when trying to decide whether to stay in restricted states where the need is greatest (highest maternal mortality, infant mortality, lower number of physicians), versus going to an unrestricted state” for more comprehensive training and care, Ms. Traub said. “Regardless of this decision, Dobbs and subsequent abortion laws are making students question what matters most and how they can provide the best care.”
In another recently published survey, University of Miami fourth-year student Morgan Levy, MD, MPH, and colleagues found that 77% of students would prefer to apply to a residency program in a state that preserves access to abortion. Ensuring access to those services for themselves or a family member was a key factor, according to the paper published in the Journal of General Internal Medicine.
For Dr. Levy, who recently graduated from a school in abortion-restricted Florida and will soon apply to ob.gyn. residencies, the Dobbs decision made her more committed to becoming an ob.gyn., an interest she’s had since college, she said.
“I do not intend to limit my search,” Dr. Levy said in an interview. “In the states where there are restrictions in place, it’s really important to make sure that people are getting good care,” she said.
Differing perspective
Though survey and anecdotal data show that students and residents expressing hesitation about states with bans or restrictive laws, it appears that most who applied to residency programs during the 2023 Match did not shy away from those states. Almost all the open ob.gyn. residency positions were filled, according to the National Resident Matching Program.
There was no change in how U.S. MD seniors applying for 2023 residency ranked programs on the basis of whether abortion was legal, limited, or banned in the state where a program was based, Donna Lamb, DHSc, MBA, BSN, president and CEO of the NRMP, said.
“We’re seeing what we’ve seen over the past 5 years, and that is a very high fill rate, a very high rate of preference for ob.gyn., and not a heck of a lot of change,” Dr. Lamb said, noting that ob.gyn. programs continue to be very competitive. “We have more applicants than we have positions available,” she said.
In the most recent Match, there were 2,100 applicants (more than half U.S. MD seniors) for about 1,500 slots, with 1,499 initial matches, according to NRMP data. The overall fill rate was 99.7% after the Supplemental Offer and Assistance Program and Electronic Residency Applications process, NRMP reported. The results are similar to what NRMP reported as its previous all-time high year for ob.gyn. placements.
There was a dip in applicants from 2022 to 2023, even though the slots available stayed the same, but it was not markedly different from the previous 5 years, Dr. Lamb said.
“While the Dobbs decision may, indeed, have impacted applicant and application numbers to residency programs, interventions such as signaling may also contribute to the decrease in numbers of applications submitted as well,” AnnaMarie Connolly, MD, ACOG chief of education and academic affairs, and Arthur Ollendorff, MD, APOG president, said in a statement to this news organization.
For the first time in 2022, Match Day applicants were required to “signal” interest in a particular program in an effort to reduce the number of applications and cost to medical students, they noted.
Personal view
When it was time for Dr. Gibson to apply for ob.gyn. residencies, she wondered: Where do you apply in this landscape? But she did not limit her applications: “If I don’t apply to Indiana, Missouri, Tennessee, Wisconsin, Iowa, I’m taking a lot of really great programs off the table.” She did not want to hurt her chances for a match in a competitive specialty, she said.
“Being in Tennessee is going to give me a very different, unique opportunity to hopefully do a lot of advocacy and lobbying and hopefully have my voice heard in maybe a different way than [in Illinois],” Dr. Gibson added.
Cassie Crifase, MPH, a fourth-year student at the University of Wisconsin–Madison applying to ob.gyn. residencies in next year’s Match, said in an interview that she’s concerned about the health risk of living in a state with abortion restrictions. Wisconsin is one of those.
“My list skews toward programs that are in abortion-protected states, but I also am applying to some programs that are in restricted states.” Those states would have to help her meet the Accreditation Council for Graduate Medical Education training requirements. And, she said, she’d want to know if she could still advocate for abortion access in the state.
Sereena Jivraj, a third-year medical student at Texas Christian University in Fort Worth, said that she won’t apply to programs in Alabama, Mississippi, Arkansas, and other nearby states with abortion restrictions. However, Texas is still on her list. “I’m from Texas, my family lives in Texas, and I go to school in Fort Worth, so I have made those connections,” Ms. Jivraj said.
Student advisers generally encourage ob.gyn. hopefuls to apply to 60-100 programs to ensure that they will match, Ms. Jivraj said. “How are you supposed to apply to 100 programs if many of them fall within states with high restrictions?”
What the future holds
Ms. Jivraj said that she’s concerned about what the future holds, especially if the law does not change in Texas. “I don’t want to go to work every day wondering if I’m going to go to jail for something that I say,” she said.
Dr. Crifase has similar fears. “I want to be able to provide the best care for my patients and that would require being able to do those procedures without having to have my first thought be: Is this legal?”
“Things feel very volatile and uncertain,” Pamela Merritt, executive director of the nonprofit Medical Students for Choice in Philadelphia, where abortion is permitted, said. “What we’re asking medical students to do right now is to envision a future in a profession, a lifetime of providing care, where the policies and procedures and standards of the profession are under attack by 26 state legislatures and the federal court system,” she said.
“I don’t think you’re going to see people as willing to take risk.” She added that if someone matches to a program and then has regrets, “You can’t easily jump from residency program to residency program.”
Dr. Levy believes that the impact of the Dobbs decision is “definitely going to be a more common question of applicants to their potential programs.”
Applicants undoubtedly are thinking about how abortion restrictions or bans might affect their own health or that of their partners or families, she said. In a 2022 survey, Dr. Levy and colleagues reported that abortion is not uncommon among physicians, with 11.5% of the 1,566 respondents who had been pregnant saying they had at least one therapeutic abortion.
Students are also considering the potential ramification of a ban on emergency contraception and laws that criminalize physicians’ provision of abortion care, Dr. Levy said. Another complicating factor is individuals’ family ties or roots in specific geographic areas, she said.
Prospective residents will also have a lot of questions about how they will receive family planning training, Dr. Levy commented. “If you’re somewhere that you can’t really provide full-spectrum reproductive health care, then the question will become: How is the program going to provide that training?”
A version of this article first appeared on Medscape.com.
The road to weight loss is paved with collusion and sabotage
Three big bumps on the weight-loss journey
The search for the Holy Grail. The destruction of the One Ring. The never-ending struggle to Lose Weight.
Like most legendary quests, weight loss is a journey, and we need support to help us achieve our goal. Maybe it’s gaining a new workout partner or finding a similarly-goaled Facebook Group. For a lot of people, it’s as simple as your friends and family. A recent study, however, suggests that the people closest to you may be your worst weight-loss enemies, and they might not even know it.
Researchers at the University of Surrey reviewed the literature on the positives and negatives of social support when it comes to weight loss and identified three types of negative effects: acts of sabotage, feeding behavior, and collusion.
Let’s start with the softest of intentions and work our way up. Collusion is the least negative. Friends and family may just go with the flow, even if it doesn’t agree with the goals of the person who’s trying to lose weight. It can even happen when health care professionals try to help their patients navigate or avoid obesity, ultimately killing with kindness, so to speak.
Next up, feeding behavior. Maybe you know someone whose love language is cooking. There are also people who share food because they don’t want to waste it or because they’re trying to be polite. They act out of the goodness of their hearts, but they’re putting up roadblocks to someone’s goals. These types of acts are usually one-sided, the researchers found. Remember, it’s okay to say, “No thanks.”
The last method, sabotage, is the most sinister. The saboteur may discourage others from eating healthy, undermine their efforts to be physically active, or take jabs at their confidence or self-esteem. Something as simple as criticizing someone for eating a salad or refusing to go on a walk with them can cause a setback.
“We need to explore this area further to develop interventions which could target family and friends and help them be more supportive in helping those they are close to lose weight,” said lead author Jane Odgen, PhD, of the University of Surrey, Guildford, England.
Like we said before, weight loss is a journey. The right support can only improve the odds of success.
Robots vs. mosquitoes
If there’s one thing robots are bad at, it’s giving solid mental health advice to people in crisis. If there’s one thing robots are very, very good at, it’s causing apocalypses. And joyous day for humanity, this time we’re not the ones being apocalypsed.
Yet.
Taiwan has a big mosquito problem. Not only do the mosquitoes in Taiwan carry dengue – among other dangerous diseases – but they’ve urbanized. Not urbanized in the sense that they’ve acquired a taste for organic coffee and avocado toast (that would be the millennial mosquito, a separate but even more terrifying creature), but more that they’ve adapted to reproduce literally anywhere and everywhere. Taiwanese mosquitoes like to breed in roadside sewer ditches, and this is where our genocidal robot comes in.
To combat the new, dangerous form of street-savvy mosquito, researchers built a robot armed with both insecticide and high-temperature, high-pressure water jets and sent it into the sewers of Kaohsiung City. The robot’s goal was simple: Whenever it came across signs of heavy mosquito breeding – eggs, larvae, pupae, and so on – the robot went to work. Utilizing both its primary weapons, the robot scrubbed numerous breeding sites across the city clean.
The researchers could just sit back and wait to see how effective their robot was. In the immediate aftermath, at various monitoring sites placed alongside the ditches, adult mosquito density fell by two-thirds in areas targeted by the robot. That’s nothing to sniff at, and it does make sense. After all, mosquitoes are quite difficult to kill in their adult stage, why not target them when they’re young and basically immobile?
The researchers saw promise with their mosquito-killing robot, but we’ve noticed a rather large issue. Killing two-thirds of mosquitoes is fine, but the third that’s left will be very angry. Very angry indeed. After all, we’re targeting the mosquito equivalent of children. Let’s hope our mosquito Terminator managed to kill mosquito Sarah Connor, or we’re going to have a big problem on our hands a bit later down the line.
This is knot what you were expecting
Physicians who aren’t surgeons probably don’t realize it, but the big thing that’s been getting between the knot-tying specialists and perfect suturing technique all these years is a lack of physics. Don’t believe us? Well, maybe you’ll believe plastic surgeon Samia Guerid, MD, of Lausanne, Switzerland: “The lack of physics-based analysis has been a limitation.” Nuff said.
That’s not enough for you, is it? Fine, we were warned.
Any surgical knot, Dr. Guerid and associates explained in a written statement, involves the “complex interplay” between six key factors: topology, geometry, elasticity, contact, friction, and polymer plasticity of the suturing filament. The strength of a suture “depends on the tension applied during the tying of the knot, [which] permanently deforms, or stretches the filament, creating a holding force.” Not enough tension and the knot comes undone, while too much snaps the filament.
For the experiment, Dr. Guerid tied a few dozen surgical knots, which were then scanned using x-ray micro–computed tomography to facilitate finite element modeling with a “3D continuum-level constitutive model for elastic-viscoplastic mechanical behavior” – no, we have no idea what that means, either – developed by the research team.
That model, and a great deal of math – so much math – allowed the researchers to define a threshold between loose and tight knots and uncover “relationships between knot strength and pretension, friction, and number of throws,” they said.
But what about the big question? The one about the ideal amount of tension? You may want to sit down. The answer to the ultimate question of the relationship between knot pretension and strength is … Did we mention that the team had its own mathematician? Their predictive model for safe knot-tying is … You’re not going to like this. The best way to teach safe knot-tying to both trainees and robots is … not ready yet.
The secret to targeting the knot tension sweet spot, for now, anyway, is still intuition gained from years of experience. Nobody ever said science was perfect … or easy … or quick.
Three big bumps on the weight-loss journey
The search for the Holy Grail. The destruction of the One Ring. The never-ending struggle to Lose Weight.
Like most legendary quests, weight loss is a journey, and we need support to help us achieve our goal. Maybe it’s gaining a new workout partner or finding a similarly-goaled Facebook Group. For a lot of people, it’s as simple as your friends and family. A recent study, however, suggests that the people closest to you may be your worst weight-loss enemies, and they might not even know it.
Researchers at the University of Surrey reviewed the literature on the positives and negatives of social support when it comes to weight loss and identified three types of negative effects: acts of sabotage, feeding behavior, and collusion.
Let’s start with the softest of intentions and work our way up. Collusion is the least negative. Friends and family may just go with the flow, even if it doesn’t agree with the goals of the person who’s trying to lose weight. It can even happen when health care professionals try to help their patients navigate or avoid obesity, ultimately killing with kindness, so to speak.
Next up, feeding behavior. Maybe you know someone whose love language is cooking. There are also people who share food because they don’t want to waste it or because they’re trying to be polite. They act out of the goodness of their hearts, but they’re putting up roadblocks to someone’s goals. These types of acts are usually one-sided, the researchers found. Remember, it’s okay to say, “No thanks.”
The last method, sabotage, is the most sinister. The saboteur may discourage others from eating healthy, undermine their efforts to be physically active, or take jabs at their confidence or self-esteem. Something as simple as criticizing someone for eating a salad or refusing to go on a walk with them can cause a setback.
“We need to explore this area further to develop interventions which could target family and friends and help them be more supportive in helping those they are close to lose weight,” said lead author Jane Odgen, PhD, of the University of Surrey, Guildford, England.
Like we said before, weight loss is a journey. The right support can only improve the odds of success.
Robots vs. mosquitoes
If there’s one thing robots are bad at, it’s giving solid mental health advice to people in crisis. If there’s one thing robots are very, very good at, it’s causing apocalypses. And joyous day for humanity, this time we’re not the ones being apocalypsed.
Yet.
Taiwan has a big mosquito problem. Not only do the mosquitoes in Taiwan carry dengue – among other dangerous diseases – but they’ve urbanized. Not urbanized in the sense that they’ve acquired a taste for organic coffee and avocado toast (that would be the millennial mosquito, a separate but even more terrifying creature), but more that they’ve adapted to reproduce literally anywhere and everywhere. Taiwanese mosquitoes like to breed in roadside sewer ditches, and this is where our genocidal robot comes in.
To combat the new, dangerous form of street-savvy mosquito, researchers built a robot armed with both insecticide and high-temperature, high-pressure water jets and sent it into the sewers of Kaohsiung City. The robot’s goal was simple: Whenever it came across signs of heavy mosquito breeding – eggs, larvae, pupae, and so on – the robot went to work. Utilizing both its primary weapons, the robot scrubbed numerous breeding sites across the city clean.
The researchers could just sit back and wait to see how effective their robot was. In the immediate aftermath, at various monitoring sites placed alongside the ditches, adult mosquito density fell by two-thirds in areas targeted by the robot. That’s nothing to sniff at, and it does make sense. After all, mosquitoes are quite difficult to kill in their adult stage, why not target them when they’re young and basically immobile?
The researchers saw promise with their mosquito-killing robot, but we’ve noticed a rather large issue. Killing two-thirds of mosquitoes is fine, but the third that’s left will be very angry. Very angry indeed. After all, we’re targeting the mosquito equivalent of children. Let’s hope our mosquito Terminator managed to kill mosquito Sarah Connor, or we’re going to have a big problem on our hands a bit later down the line.
This is knot what you were expecting
Physicians who aren’t surgeons probably don’t realize it, but the big thing that’s been getting between the knot-tying specialists and perfect suturing technique all these years is a lack of physics. Don’t believe us? Well, maybe you’ll believe plastic surgeon Samia Guerid, MD, of Lausanne, Switzerland: “The lack of physics-based analysis has been a limitation.” Nuff said.
That’s not enough for you, is it? Fine, we were warned.
Any surgical knot, Dr. Guerid and associates explained in a written statement, involves the “complex interplay” between six key factors: topology, geometry, elasticity, contact, friction, and polymer plasticity of the suturing filament. The strength of a suture “depends on the tension applied during the tying of the knot, [which] permanently deforms, or stretches the filament, creating a holding force.” Not enough tension and the knot comes undone, while too much snaps the filament.
For the experiment, Dr. Guerid tied a few dozen surgical knots, which were then scanned using x-ray micro–computed tomography to facilitate finite element modeling with a “3D continuum-level constitutive model for elastic-viscoplastic mechanical behavior” – no, we have no idea what that means, either – developed by the research team.
That model, and a great deal of math – so much math – allowed the researchers to define a threshold between loose and tight knots and uncover “relationships between knot strength and pretension, friction, and number of throws,” they said.
But what about the big question? The one about the ideal amount of tension? You may want to sit down. The answer to the ultimate question of the relationship between knot pretension and strength is … Did we mention that the team had its own mathematician? Their predictive model for safe knot-tying is … You’re not going to like this. The best way to teach safe knot-tying to both trainees and robots is … not ready yet.
The secret to targeting the knot tension sweet spot, for now, anyway, is still intuition gained from years of experience. Nobody ever said science was perfect … or easy … or quick.
Three big bumps on the weight-loss journey
The search for the Holy Grail. The destruction of the One Ring. The never-ending struggle to Lose Weight.
Like most legendary quests, weight loss is a journey, and we need support to help us achieve our goal. Maybe it’s gaining a new workout partner or finding a similarly-goaled Facebook Group. For a lot of people, it’s as simple as your friends and family. A recent study, however, suggests that the people closest to you may be your worst weight-loss enemies, and they might not even know it.
Researchers at the University of Surrey reviewed the literature on the positives and negatives of social support when it comes to weight loss and identified three types of negative effects: acts of sabotage, feeding behavior, and collusion.
Let’s start with the softest of intentions and work our way up. Collusion is the least negative. Friends and family may just go with the flow, even if it doesn’t agree with the goals of the person who’s trying to lose weight. It can even happen when health care professionals try to help their patients navigate or avoid obesity, ultimately killing with kindness, so to speak.
Next up, feeding behavior. Maybe you know someone whose love language is cooking. There are also people who share food because they don’t want to waste it or because they’re trying to be polite. They act out of the goodness of their hearts, but they’re putting up roadblocks to someone’s goals. These types of acts are usually one-sided, the researchers found. Remember, it’s okay to say, “No thanks.”
The last method, sabotage, is the most sinister. The saboteur may discourage others from eating healthy, undermine their efforts to be physically active, or take jabs at their confidence or self-esteem. Something as simple as criticizing someone for eating a salad or refusing to go on a walk with them can cause a setback.
“We need to explore this area further to develop interventions which could target family and friends and help them be more supportive in helping those they are close to lose weight,” said lead author Jane Odgen, PhD, of the University of Surrey, Guildford, England.
Like we said before, weight loss is a journey. The right support can only improve the odds of success.
Robots vs. mosquitoes
If there’s one thing robots are bad at, it’s giving solid mental health advice to people in crisis. If there’s one thing robots are very, very good at, it’s causing apocalypses. And joyous day for humanity, this time we’re not the ones being apocalypsed.
Yet.
Taiwan has a big mosquito problem. Not only do the mosquitoes in Taiwan carry dengue – among other dangerous diseases – but they’ve urbanized. Not urbanized in the sense that they’ve acquired a taste for organic coffee and avocado toast (that would be the millennial mosquito, a separate but even more terrifying creature), but more that they’ve adapted to reproduce literally anywhere and everywhere. Taiwanese mosquitoes like to breed in roadside sewer ditches, and this is where our genocidal robot comes in.
To combat the new, dangerous form of street-savvy mosquito, researchers built a robot armed with both insecticide and high-temperature, high-pressure water jets and sent it into the sewers of Kaohsiung City. The robot’s goal was simple: Whenever it came across signs of heavy mosquito breeding – eggs, larvae, pupae, and so on – the robot went to work. Utilizing both its primary weapons, the robot scrubbed numerous breeding sites across the city clean.
The researchers could just sit back and wait to see how effective their robot was. In the immediate aftermath, at various monitoring sites placed alongside the ditches, adult mosquito density fell by two-thirds in areas targeted by the robot. That’s nothing to sniff at, and it does make sense. After all, mosquitoes are quite difficult to kill in their adult stage, why not target them when they’re young and basically immobile?
The researchers saw promise with their mosquito-killing robot, but we’ve noticed a rather large issue. Killing two-thirds of mosquitoes is fine, but the third that’s left will be very angry. Very angry indeed. After all, we’re targeting the mosquito equivalent of children. Let’s hope our mosquito Terminator managed to kill mosquito Sarah Connor, or we’re going to have a big problem on our hands a bit later down the line.
This is knot what you were expecting
Physicians who aren’t surgeons probably don’t realize it, but the big thing that’s been getting between the knot-tying specialists and perfect suturing technique all these years is a lack of physics. Don’t believe us? Well, maybe you’ll believe plastic surgeon Samia Guerid, MD, of Lausanne, Switzerland: “The lack of physics-based analysis has been a limitation.” Nuff said.
That’s not enough for you, is it? Fine, we were warned.
Any surgical knot, Dr. Guerid and associates explained in a written statement, involves the “complex interplay” between six key factors: topology, geometry, elasticity, contact, friction, and polymer plasticity of the suturing filament. The strength of a suture “depends on the tension applied during the tying of the knot, [which] permanently deforms, or stretches the filament, creating a holding force.” Not enough tension and the knot comes undone, while too much snaps the filament.
For the experiment, Dr. Guerid tied a few dozen surgical knots, which were then scanned using x-ray micro–computed tomography to facilitate finite element modeling with a “3D continuum-level constitutive model for elastic-viscoplastic mechanical behavior” – no, we have no idea what that means, either – developed by the research team.
That model, and a great deal of math – so much math – allowed the researchers to define a threshold between loose and tight knots and uncover “relationships between knot strength and pretension, friction, and number of throws,” they said.
But what about the big question? The one about the ideal amount of tension? You may want to sit down. The answer to the ultimate question of the relationship between knot pretension and strength is … Did we mention that the team had its own mathematician? Their predictive model for safe knot-tying is … You’re not going to like this. The best way to teach safe knot-tying to both trainees and robots is … not ready yet.
The secret to targeting the knot tension sweet spot, for now, anyway, is still intuition gained from years of experience. Nobody ever said science was perfect … or easy … or quick.
New bill would provide greater length of time to sue doctors
A bill in the Maine legislature would have the medical malpractice statute of limitations clock start running when a patient discovers the negligence, which could be years after treatment took place. And other states could follow suit with similar bills. What danger does this pose for doctors?
As it stands, the time limit for patients to be able to bring a medical malpractice lawsuit varies by state. For physicians, this extends their period of liability and could potentially increase the number of lawsuits against them.
“The theory behind a statute of limitations is that states want to provide a reasonable, but not indefinite, amount of time for someone to bring a case to court,” says Patrick T. O’Rourke, Esq., adjunct professor at University of Colorado School of Law, Boulder.
Without a statute of limitations, people could bring claims many years after the fact, which makes it harder to obtain and preserve evidence, Mr. O’Rourke says.
In most cases, it isn’t necessary for a patient to know the full extent of their injury or that their physician acted wrongfully or negligently for the statute of limitations to begin running.
Time of injury versus time of discovery
Most states’ laws dictate that the statute of limitations begins at a set time “after the cause of action accrues.” That means that the clock starts ticking from the date of the procedure, surgery, or treatment. In most states, that time is 2 or 3 years.
This can bar some patients from taking any action at all because the statute of limitations ran out. Because of these hurdles, the proposed bill in Maine would extend the statute of limitations.
Proponents of the bill say that patients would still have 3 years to file suit; it just changes when the clock starts. But opponents feel it could open the door to a limitless system in which people have an indefinite time to sue.
Many states already have discovery rules that extend the statute of limitations when the harm was not immediately obvious to the patient. The legal expectation is that patients who have significant pain or unexpected health conditions will seek medical treatment to investigate what’s wrong. Patients who don’t address the situation promptly are not protected by the discovery rule.
“It is the injured person’s obligation, once learning of the injury, to take action to protect their rights,” says Mr. O’Rourke.
Some states have also enacted other claims requirements in medical malpractice cases that are prerequisites for bringing lawsuits that have periods attached to them. For instance, in Florida, parties have 10 days to provide relevant medical records during the investigation period for a malpractice suit, and in Maine, before filing any malpractice action, a plaintiff must file a complaint with a prelitigation screening panel.
Medical malpractice statutes of limitations by state
Although each state has a basic statute of limitations, many states also include clauses for discovery rules. For example, in Vermont, in addition to the 3-year statute of limitations, a patient can pursue legal recourse “2 years from the date the injury is or reasonably should have been discovered, whichever occurs later, but not later than 7 years from the date of the incident.”
In some states, such as Virginia, special extensions apply in cases in which fraud, concealment, or intentional misrepresentation prevented discovery of the injury within the statute of limitations. And in most states, the statute of limitations is much longer for cases in which medical malpractice involves a child, usually at least until the child turns 18.
Statutes of limitations by state
1 Year: California, Kentucky, Louisiana, Ohio, Tennessee
2 Years: Alabama, Alaska, Arizona, Arkansas, Colorado, Connecticut, Delaware, Florida, Georgia, Hawaii, Idaho, Illinois, Indiana, Iowa, Kansas, Michigan, Mississippi, Missouri, Nebraska, New Hampshire, New Jersey, North Dakota, Oklahoma, Oregon, Pennsylvania, South Dakota, Texas, Utah, Virginia, West Virginia, Wyoming
2.5 Years: New York
3 Years: Washington D.C., Maine, Maryland, Massachusetts, Montana, Nevada, New Mexico, North Carolina, Rhode Island, South Carolina, Vermont, Washington, Wisconsin
4 Years: Minnesota
To protect yourself
Mr. O’Rourke says that if your state enacts a law that extends the statute of limitations for medical malpractice, there aren’t any proactive changes you need to make in terms of your day-to-day practice of medicine.
“Physicians should continue to provide care that is consistent with the standards of care for their specialty and ensure that the documentation accurately reflects the care they rendered,” he says.
Always be candid and up-front about a patient’s condition, Mr. O’Rourke says, especially if malpractice is on the table.
“If a physician misleads a patient about the nature or extent of an injury, that could prevent the statute of limitations from beginning to run,” he says. “Being open and honest about an injury doesn’t mean that a physician must admit any fault. The patient is owed timely, accurate, and candid information about their condition.”
Keep good records
If the statute of limitations increases, you’ll need to have access to the medical records for as long as the statute is in place, but this shouldn’t have an effect on your records keeping if you’re up to date with HIPAA compliance, says Mr. O’Rourke.
“I don’t think an extension of the statute should cause physicians to change their practices, particularly with the retention of medical records, which should be maintained consistently with HIPAA requirements irrespective of the limitations period in a particular state,” he adds.
Keep an eye on malpractice insurance rates
It’s possible that your malpractice insurance could go up as a result of laws that increase the statute of limitations. But Mr. O’Rourke thinks it likely won’t be a significant amount.
He says it’s “theoretically possible” that an increase in a limitations period could result in an increase in your malpractice insurance, since some claims that would otherwise have been barred because of time could then proceed, but the increase would be nominal.
“I would expect any increase to be fairly marginal because the majority of claims will already be accounted for on an actuarial basis,” he says. “I also don’t think that the extension of a limitations period would increase the award of damages in a particular case. The injuries should be the same under either limitations period, so the compensable loss should not increase.”
Anything that makes it easier for patients to recover should increase the cost of professional liability insurance, and vice versa, says Charles Silver, McDonald Endowed Chair in Civil Procedure at University of Texas at Austin School of Law and coauthor of “Medical Malpractice Litigation: How It Works – Why Tort Reform Hasn’t Helped.” But the long-term trend across the country is toward declining rates of liability and declining payouts on claims.
“The likelihood of being sued successfully by a former patient is low, as is the risk of having to pay out of pocket to settle a claim,” he says. In 2022, the number of adverse reports nationally was 38,938, and out of those, 10,807 resulted in a payout.
In his research on medical malpractice in Texas, Mr. Silver says physicians who carried $1 million in coverage essentially never faced any personal liability on medical malpractice claims. “[This means] that they never had to write a check to a victim,” he says. “Insurers provided all the money. I suspect that the same is true nationwide.”
Key takeaways
Ultimately, to protect yourself and your practice, you can do the following:
- Know the statute of limitations and discovery rules for your state.
- Review your coverage with your insurer to better understand your liability.
- Keep accurate records for as long as your statute requires.
- Notify your insurer or risk management department as soon as possible in the event of an adverse outcome with a patient, Mr. O’Rourke advises.
“The most important thing a physician can do to avoid being sued, even when negligent, is to treat patients with kindness and respect,” says Mr. Silver. “Patients don’t expect doctors to be perfect, and they rarely sue doctors they like.”
A version of this article first appeared on Medscape.com.
A bill in the Maine legislature would have the medical malpractice statute of limitations clock start running when a patient discovers the negligence, which could be years after treatment took place. And other states could follow suit with similar bills. What danger does this pose for doctors?
As it stands, the time limit for patients to be able to bring a medical malpractice lawsuit varies by state. For physicians, this extends their period of liability and could potentially increase the number of lawsuits against them.
“The theory behind a statute of limitations is that states want to provide a reasonable, but not indefinite, amount of time for someone to bring a case to court,” says Patrick T. O’Rourke, Esq., adjunct professor at University of Colorado School of Law, Boulder.
Without a statute of limitations, people could bring claims many years after the fact, which makes it harder to obtain and preserve evidence, Mr. O’Rourke says.
In most cases, it isn’t necessary for a patient to know the full extent of their injury or that their physician acted wrongfully or negligently for the statute of limitations to begin running.
Time of injury versus time of discovery
Most states’ laws dictate that the statute of limitations begins at a set time “after the cause of action accrues.” That means that the clock starts ticking from the date of the procedure, surgery, or treatment. In most states, that time is 2 or 3 years.
This can bar some patients from taking any action at all because the statute of limitations ran out. Because of these hurdles, the proposed bill in Maine would extend the statute of limitations.
Proponents of the bill say that patients would still have 3 years to file suit; it just changes when the clock starts. But opponents feel it could open the door to a limitless system in which people have an indefinite time to sue.
Many states already have discovery rules that extend the statute of limitations when the harm was not immediately obvious to the patient. The legal expectation is that patients who have significant pain or unexpected health conditions will seek medical treatment to investigate what’s wrong. Patients who don’t address the situation promptly are not protected by the discovery rule.
“It is the injured person’s obligation, once learning of the injury, to take action to protect their rights,” says Mr. O’Rourke.
Some states have also enacted other claims requirements in medical malpractice cases that are prerequisites for bringing lawsuits that have periods attached to them. For instance, in Florida, parties have 10 days to provide relevant medical records during the investigation period for a malpractice suit, and in Maine, before filing any malpractice action, a plaintiff must file a complaint with a prelitigation screening panel.
Medical malpractice statutes of limitations by state
Although each state has a basic statute of limitations, many states also include clauses for discovery rules. For example, in Vermont, in addition to the 3-year statute of limitations, a patient can pursue legal recourse “2 years from the date the injury is or reasonably should have been discovered, whichever occurs later, but not later than 7 years from the date of the incident.”
In some states, such as Virginia, special extensions apply in cases in which fraud, concealment, or intentional misrepresentation prevented discovery of the injury within the statute of limitations. And in most states, the statute of limitations is much longer for cases in which medical malpractice involves a child, usually at least until the child turns 18.
Statutes of limitations by state
1 Year: California, Kentucky, Louisiana, Ohio, Tennessee
2 Years: Alabama, Alaska, Arizona, Arkansas, Colorado, Connecticut, Delaware, Florida, Georgia, Hawaii, Idaho, Illinois, Indiana, Iowa, Kansas, Michigan, Mississippi, Missouri, Nebraska, New Hampshire, New Jersey, North Dakota, Oklahoma, Oregon, Pennsylvania, South Dakota, Texas, Utah, Virginia, West Virginia, Wyoming
2.5 Years: New York
3 Years: Washington D.C., Maine, Maryland, Massachusetts, Montana, Nevada, New Mexico, North Carolina, Rhode Island, South Carolina, Vermont, Washington, Wisconsin
4 Years: Minnesota
To protect yourself
Mr. O’Rourke says that if your state enacts a law that extends the statute of limitations for medical malpractice, there aren’t any proactive changes you need to make in terms of your day-to-day practice of medicine.
“Physicians should continue to provide care that is consistent with the standards of care for their specialty and ensure that the documentation accurately reflects the care they rendered,” he says.
Always be candid and up-front about a patient’s condition, Mr. O’Rourke says, especially if malpractice is on the table.
“If a physician misleads a patient about the nature or extent of an injury, that could prevent the statute of limitations from beginning to run,” he says. “Being open and honest about an injury doesn’t mean that a physician must admit any fault. The patient is owed timely, accurate, and candid information about their condition.”
Keep good records
If the statute of limitations increases, you’ll need to have access to the medical records for as long as the statute is in place, but this shouldn’t have an effect on your records keeping if you’re up to date with HIPAA compliance, says Mr. O’Rourke.
“I don’t think an extension of the statute should cause physicians to change their practices, particularly with the retention of medical records, which should be maintained consistently with HIPAA requirements irrespective of the limitations period in a particular state,” he adds.
Keep an eye on malpractice insurance rates
It’s possible that your malpractice insurance could go up as a result of laws that increase the statute of limitations. But Mr. O’Rourke thinks it likely won’t be a significant amount.
He says it’s “theoretically possible” that an increase in a limitations period could result in an increase in your malpractice insurance, since some claims that would otherwise have been barred because of time could then proceed, but the increase would be nominal.
“I would expect any increase to be fairly marginal because the majority of claims will already be accounted for on an actuarial basis,” he says. “I also don’t think that the extension of a limitations period would increase the award of damages in a particular case. The injuries should be the same under either limitations period, so the compensable loss should not increase.”
Anything that makes it easier for patients to recover should increase the cost of professional liability insurance, and vice versa, says Charles Silver, McDonald Endowed Chair in Civil Procedure at University of Texas at Austin School of Law and coauthor of “Medical Malpractice Litigation: How It Works – Why Tort Reform Hasn’t Helped.” But the long-term trend across the country is toward declining rates of liability and declining payouts on claims.
“The likelihood of being sued successfully by a former patient is low, as is the risk of having to pay out of pocket to settle a claim,” he says. In 2022, the number of adverse reports nationally was 38,938, and out of those, 10,807 resulted in a payout.
In his research on medical malpractice in Texas, Mr. Silver says physicians who carried $1 million in coverage essentially never faced any personal liability on medical malpractice claims. “[This means] that they never had to write a check to a victim,” he says. “Insurers provided all the money. I suspect that the same is true nationwide.”
Key takeaways
Ultimately, to protect yourself and your practice, you can do the following:
- Know the statute of limitations and discovery rules for your state.
- Review your coverage with your insurer to better understand your liability.
- Keep accurate records for as long as your statute requires.
- Notify your insurer or risk management department as soon as possible in the event of an adverse outcome with a patient, Mr. O’Rourke advises.
“The most important thing a physician can do to avoid being sued, even when negligent, is to treat patients with kindness and respect,” says Mr. Silver. “Patients don’t expect doctors to be perfect, and they rarely sue doctors they like.”
A version of this article first appeared on Medscape.com.
A bill in the Maine legislature would have the medical malpractice statute of limitations clock start running when a patient discovers the negligence, which could be years after treatment took place. And other states could follow suit with similar bills. What danger does this pose for doctors?
As it stands, the time limit for patients to be able to bring a medical malpractice lawsuit varies by state. For physicians, this extends their period of liability and could potentially increase the number of lawsuits against them.
“The theory behind a statute of limitations is that states want to provide a reasonable, but not indefinite, amount of time for someone to bring a case to court,” says Patrick T. O’Rourke, Esq., adjunct professor at University of Colorado School of Law, Boulder.
Without a statute of limitations, people could bring claims many years after the fact, which makes it harder to obtain and preserve evidence, Mr. O’Rourke says.
In most cases, it isn’t necessary for a patient to know the full extent of their injury or that their physician acted wrongfully or negligently for the statute of limitations to begin running.
Time of injury versus time of discovery
Most states’ laws dictate that the statute of limitations begins at a set time “after the cause of action accrues.” That means that the clock starts ticking from the date of the procedure, surgery, or treatment. In most states, that time is 2 or 3 years.
This can bar some patients from taking any action at all because the statute of limitations ran out. Because of these hurdles, the proposed bill in Maine would extend the statute of limitations.
Proponents of the bill say that patients would still have 3 years to file suit; it just changes when the clock starts. But opponents feel it could open the door to a limitless system in which people have an indefinite time to sue.
Many states already have discovery rules that extend the statute of limitations when the harm was not immediately obvious to the patient. The legal expectation is that patients who have significant pain or unexpected health conditions will seek medical treatment to investigate what’s wrong. Patients who don’t address the situation promptly are not protected by the discovery rule.
“It is the injured person’s obligation, once learning of the injury, to take action to protect their rights,” says Mr. O’Rourke.
Some states have also enacted other claims requirements in medical malpractice cases that are prerequisites for bringing lawsuits that have periods attached to them. For instance, in Florida, parties have 10 days to provide relevant medical records during the investigation period for a malpractice suit, and in Maine, before filing any malpractice action, a plaintiff must file a complaint with a prelitigation screening panel.
Medical malpractice statutes of limitations by state
Although each state has a basic statute of limitations, many states also include clauses for discovery rules. For example, in Vermont, in addition to the 3-year statute of limitations, a patient can pursue legal recourse “2 years from the date the injury is or reasonably should have been discovered, whichever occurs later, but not later than 7 years from the date of the incident.”
In some states, such as Virginia, special extensions apply in cases in which fraud, concealment, or intentional misrepresentation prevented discovery of the injury within the statute of limitations. And in most states, the statute of limitations is much longer for cases in which medical malpractice involves a child, usually at least until the child turns 18.
Statutes of limitations by state
1 Year: California, Kentucky, Louisiana, Ohio, Tennessee
2 Years: Alabama, Alaska, Arizona, Arkansas, Colorado, Connecticut, Delaware, Florida, Georgia, Hawaii, Idaho, Illinois, Indiana, Iowa, Kansas, Michigan, Mississippi, Missouri, Nebraska, New Hampshire, New Jersey, North Dakota, Oklahoma, Oregon, Pennsylvania, South Dakota, Texas, Utah, Virginia, West Virginia, Wyoming
2.5 Years: New York
3 Years: Washington D.C., Maine, Maryland, Massachusetts, Montana, Nevada, New Mexico, North Carolina, Rhode Island, South Carolina, Vermont, Washington, Wisconsin
4 Years: Minnesota
To protect yourself
Mr. O’Rourke says that if your state enacts a law that extends the statute of limitations for medical malpractice, there aren’t any proactive changes you need to make in terms of your day-to-day practice of medicine.
“Physicians should continue to provide care that is consistent with the standards of care for their specialty and ensure that the documentation accurately reflects the care they rendered,” he says.
Always be candid and up-front about a patient’s condition, Mr. O’Rourke says, especially if malpractice is on the table.
“If a physician misleads a patient about the nature or extent of an injury, that could prevent the statute of limitations from beginning to run,” he says. “Being open and honest about an injury doesn’t mean that a physician must admit any fault. The patient is owed timely, accurate, and candid information about their condition.”
Keep good records
If the statute of limitations increases, you’ll need to have access to the medical records for as long as the statute is in place, but this shouldn’t have an effect on your records keeping if you’re up to date with HIPAA compliance, says Mr. O’Rourke.
“I don’t think an extension of the statute should cause physicians to change their practices, particularly with the retention of medical records, which should be maintained consistently with HIPAA requirements irrespective of the limitations period in a particular state,” he adds.
Keep an eye on malpractice insurance rates
It’s possible that your malpractice insurance could go up as a result of laws that increase the statute of limitations. But Mr. O’Rourke thinks it likely won’t be a significant amount.
He says it’s “theoretically possible” that an increase in a limitations period could result in an increase in your malpractice insurance, since some claims that would otherwise have been barred because of time could then proceed, but the increase would be nominal.
“I would expect any increase to be fairly marginal because the majority of claims will already be accounted for on an actuarial basis,” he says. “I also don’t think that the extension of a limitations period would increase the award of damages in a particular case. The injuries should be the same under either limitations period, so the compensable loss should not increase.”
Anything that makes it easier for patients to recover should increase the cost of professional liability insurance, and vice versa, says Charles Silver, McDonald Endowed Chair in Civil Procedure at University of Texas at Austin School of Law and coauthor of “Medical Malpractice Litigation: How It Works – Why Tort Reform Hasn’t Helped.” But the long-term trend across the country is toward declining rates of liability and declining payouts on claims.
“The likelihood of being sued successfully by a former patient is low, as is the risk of having to pay out of pocket to settle a claim,” he says. In 2022, the number of adverse reports nationally was 38,938, and out of those, 10,807 resulted in a payout.
In his research on medical malpractice in Texas, Mr. Silver says physicians who carried $1 million in coverage essentially never faced any personal liability on medical malpractice claims. “[This means] that they never had to write a check to a victim,” he says. “Insurers provided all the money. I suspect that the same is true nationwide.”
Key takeaways
Ultimately, to protect yourself and your practice, you can do the following:
- Know the statute of limitations and discovery rules for your state.
- Review your coverage with your insurer to better understand your liability.
- Keep accurate records for as long as your statute requires.
- Notify your insurer or risk management department as soon as possible in the event of an adverse outcome with a patient, Mr. O’Rourke advises.
“The most important thing a physician can do to avoid being sued, even when negligent, is to treat patients with kindness and respect,” says Mr. Silver. “Patients don’t expect doctors to be perfect, and they rarely sue doctors they like.”
A version of this article first appeared on Medscape.com.
Protecting your practice data
While data protection is important in any industry, it is particularly critical in health care because in addition to the usual financial records, trade secrets, and other valuable data, confidential patient information is also at risk.
You may think that your computer vendor is responsible for safeguarding your data, but third parties can only do so much. And if your data is compromised, the ultimate responsibility is yours – not to mention the financial loss, and the damage to your practice’s reputation.
In addition to the security vulnerabilities inherent in any system, there are external vulnerabilities, such as weak passwords, viruses, and hacking (either externally or internally). And as hardware becomes more and more portable, there is the increasing risk of theft of platforms and storage media containing confidential data.
A close and ongoing relationship with your hardware and software vendors is essential to good data protection. Your office should have a permanent contact at each company, and you should talk to them regularly. Ask them what sort of firewalls, antivirus software, and other safeguards are in place to protect your system. Whenever they identify a bug or other vulnerability, you should know about it. They should tell you about each software update, what improvements it makes, and what defects it fixes. You should also know about any changes to your data encryption.
Encryption has become an essential component of data protection. It is especially important if you use portable devices such as laptops, pads, or smart phones to store and transport patient information. If you lose one of these devices, or a thumb drive or other storage media, HIPAA will probably not consider it a breach if the data it contains is encrypted.
Encryption isn’t perfect, of course. Log-in credentials can be stolen; and data that is stored in house is can be hacked with malware and phishing techniques, especially if the key to decryption is located on that server. And make sure that employees are not putting any medical data on their own private (unencrypted) devices.
Each employee should have his or her own password, and sharing should be strictly prohibited. Multifactor authentication is becoming increasingly popular for an extra level of security.
Your vendor should require you to change your passwords every few months. If it doesn’t, you need to establish a timetable to do it yourself. All passwords should be strong (no birthdays, pet names, etc.), and they shouldn’t be the same or similar to old passwords.
In some offices, I’ve been surprised to see that every employee has unrestricted access to all practice data. The vulnerabilities of such an arrangement are obvious. There is no reason why receptionists, for example, should have access to medical histories, and insurance people don’t need to know what medications a patient is on. Your vendor can help you design partitions that restrict each employee to only the information they need access to.
Ask if your vendor provides security training for employees. If not, look into hiring a security firm to do it. Regular security training can help employees to recognize data security attacks like phishing, and instills a heightened sense of security awareness and vigilance among staff. They will also gain a better understanding of the role they play in maintaining the overall security of your office.
It goes without saying that third parties, such as business vendors, payers, and managed care providers, should never have access to patient records or other personal health information.
Backing up data
I have written many times about the importance of regularly backing up your data. Industry statistics show that fully 10% of hard drives fail in any given year, and 43% of computer users lose one or more files every year in the form of clinical data, financial records, photos, email, documents, and other important information. Recovery of lost data, when it’s possible at all, can be very expensive.
Even if your EHR vendor backs up your data, you should consider making a separate backup of your own. Backup drives have been known to fail too; and if you decide to switch computer vendors, you don’t want to be at the mercy of the old company that might be reluctant to transfer your data without a hefty payment.
The first rule of backing up is to store your backup drives in a different location from your computers. Unfortunately, that’s a pain; and external drives can be lost or stolen, creating a HIPAA nightmare. So an increasingly popular alternative is automatic remote backup. Several companies offer that service, and the cost is very reasonable for individual computers. Backing up an entire office costs more, depending on how many computers and/or servers you have, but it’s still very reasonable and includes other services, such as operating system and network share support.
The procedure is simple: You create an account and tell the service which files you want copied. Your first backup can take a long time, often days, depending on how much data you are sending and how fast your Internet connection runs. After that the program runs in the background, copying only those files that have changed since the previous backup. Files are encrypted before leaving your computer, and they remain encrypted at the service’s data center, making them HIPAA compliant and, theoretically, only accessible by you.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
While data protection is important in any industry, it is particularly critical in health care because in addition to the usual financial records, trade secrets, and other valuable data, confidential patient information is also at risk.
You may think that your computer vendor is responsible for safeguarding your data, but third parties can only do so much. And if your data is compromised, the ultimate responsibility is yours – not to mention the financial loss, and the damage to your practice’s reputation.
In addition to the security vulnerabilities inherent in any system, there are external vulnerabilities, such as weak passwords, viruses, and hacking (either externally or internally). And as hardware becomes more and more portable, there is the increasing risk of theft of platforms and storage media containing confidential data.
A close and ongoing relationship with your hardware and software vendors is essential to good data protection. Your office should have a permanent contact at each company, and you should talk to them regularly. Ask them what sort of firewalls, antivirus software, and other safeguards are in place to protect your system. Whenever they identify a bug or other vulnerability, you should know about it. They should tell you about each software update, what improvements it makes, and what defects it fixes. You should also know about any changes to your data encryption.
Encryption has become an essential component of data protection. It is especially important if you use portable devices such as laptops, pads, or smart phones to store and transport patient information. If you lose one of these devices, or a thumb drive or other storage media, HIPAA will probably not consider it a breach if the data it contains is encrypted.
Encryption isn’t perfect, of course. Log-in credentials can be stolen; and data that is stored in house is can be hacked with malware and phishing techniques, especially if the key to decryption is located on that server. And make sure that employees are not putting any medical data on their own private (unencrypted) devices.
Each employee should have his or her own password, and sharing should be strictly prohibited. Multifactor authentication is becoming increasingly popular for an extra level of security.
Your vendor should require you to change your passwords every few months. If it doesn’t, you need to establish a timetable to do it yourself. All passwords should be strong (no birthdays, pet names, etc.), and they shouldn’t be the same or similar to old passwords.
In some offices, I’ve been surprised to see that every employee has unrestricted access to all practice data. The vulnerabilities of such an arrangement are obvious. There is no reason why receptionists, for example, should have access to medical histories, and insurance people don’t need to know what medications a patient is on. Your vendor can help you design partitions that restrict each employee to only the information they need access to.
Ask if your vendor provides security training for employees. If not, look into hiring a security firm to do it. Regular security training can help employees to recognize data security attacks like phishing, and instills a heightened sense of security awareness and vigilance among staff. They will also gain a better understanding of the role they play in maintaining the overall security of your office.
It goes without saying that third parties, such as business vendors, payers, and managed care providers, should never have access to patient records or other personal health information.
Backing up data
I have written many times about the importance of regularly backing up your data. Industry statistics show that fully 10% of hard drives fail in any given year, and 43% of computer users lose one or more files every year in the form of clinical data, financial records, photos, email, documents, and other important information. Recovery of lost data, when it’s possible at all, can be very expensive.
Even if your EHR vendor backs up your data, you should consider making a separate backup of your own. Backup drives have been known to fail too; and if you decide to switch computer vendors, you don’t want to be at the mercy of the old company that might be reluctant to transfer your data without a hefty payment.
The first rule of backing up is to store your backup drives in a different location from your computers. Unfortunately, that’s a pain; and external drives can be lost or stolen, creating a HIPAA nightmare. So an increasingly popular alternative is automatic remote backup. Several companies offer that service, and the cost is very reasonable for individual computers. Backing up an entire office costs more, depending on how many computers and/or servers you have, but it’s still very reasonable and includes other services, such as operating system and network share support.
The procedure is simple: You create an account and tell the service which files you want copied. Your first backup can take a long time, often days, depending on how much data you are sending and how fast your Internet connection runs. After that the program runs in the background, copying only those files that have changed since the previous backup. Files are encrypted before leaving your computer, and they remain encrypted at the service’s data center, making them HIPAA compliant and, theoretically, only accessible by you.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
While data protection is important in any industry, it is particularly critical in health care because in addition to the usual financial records, trade secrets, and other valuable data, confidential patient information is also at risk.
You may think that your computer vendor is responsible for safeguarding your data, but third parties can only do so much. And if your data is compromised, the ultimate responsibility is yours – not to mention the financial loss, and the damage to your practice’s reputation.
In addition to the security vulnerabilities inherent in any system, there are external vulnerabilities, such as weak passwords, viruses, and hacking (either externally or internally). And as hardware becomes more and more portable, there is the increasing risk of theft of platforms and storage media containing confidential data.
A close and ongoing relationship with your hardware and software vendors is essential to good data protection. Your office should have a permanent contact at each company, and you should talk to them regularly. Ask them what sort of firewalls, antivirus software, and other safeguards are in place to protect your system. Whenever they identify a bug or other vulnerability, you should know about it. They should tell you about each software update, what improvements it makes, and what defects it fixes. You should also know about any changes to your data encryption.
Encryption has become an essential component of data protection. It is especially important if you use portable devices such as laptops, pads, or smart phones to store and transport patient information. If you lose one of these devices, or a thumb drive or other storage media, HIPAA will probably not consider it a breach if the data it contains is encrypted.
Encryption isn’t perfect, of course. Log-in credentials can be stolen; and data that is stored in house is can be hacked with malware and phishing techniques, especially if the key to decryption is located on that server. And make sure that employees are not putting any medical data on their own private (unencrypted) devices.
Each employee should have his or her own password, and sharing should be strictly prohibited. Multifactor authentication is becoming increasingly popular for an extra level of security.
Your vendor should require you to change your passwords every few months. If it doesn’t, you need to establish a timetable to do it yourself. All passwords should be strong (no birthdays, pet names, etc.), and they shouldn’t be the same or similar to old passwords.
In some offices, I’ve been surprised to see that every employee has unrestricted access to all practice data. The vulnerabilities of such an arrangement are obvious. There is no reason why receptionists, for example, should have access to medical histories, and insurance people don’t need to know what medications a patient is on. Your vendor can help you design partitions that restrict each employee to only the information they need access to.
Ask if your vendor provides security training for employees. If not, look into hiring a security firm to do it. Regular security training can help employees to recognize data security attacks like phishing, and instills a heightened sense of security awareness and vigilance among staff. They will also gain a better understanding of the role they play in maintaining the overall security of your office.
It goes without saying that third parties, such as business vendors, payers, and managed care providers, should never have access to patient records or other personal health information.
Backing up data
I have written many times about the importance of regularly backing up your data. Industry statistics show that fully 10% of hard drives fail in any given year, and 43% of computer users lose one or more files every year in the form of clinical data, financial records, photos, email, documents, and other important information. Recovery of lost data, when it’s possible at all, can be very expensive.
Even if your EHR vendor backs up your data, you should consider making a separate backup of your own. Backup drives have been known to fail too; and if you decide to switch computer vendors, you don’t want to be at the mercy of the old company that might be reluctant to transfer your data without a hefty payment.
The first rule of backing up is to store your backup drives in a different location from your computers. Unfortunately, that’s a pain; and external drives can be lost or stolen, creating a HIPAA nightmare. So an increasingly popular alternative is automatic remote backup. Several companies offer that service, and the cost is very reasonable for individual computers. Backing up an entire office costs more, depending on how many computers and/or servers you have, but it’s still very reasonable and includes other services, such as operating system and network share support.
The procedure is simple: You create an account and tell the service which files you want copied. Your first backup can take a long time, often days, depending on how much data you are sending and how fast your Internet connection runs. After that the program runs in the background, copying only those files that have changed since the previous backup. Files are encrypted before leaving your computer, and they remain encrypted at the service’s data center, making them HIPAA compliant and, theoretically, only accessible by you.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
New study backs up capecitabine dosing practice in metastatic BC
Both progression-free survival (PFS) and overall survival (OS) were similar between the two groups, but patients on the alternative schedule experienced fewer cases of hand-foot syndrome (HFS), diarrhea, and stomatitis, and also had fewer discontinuations and dose modifications.
The Food and Drug Administration–approved dose of capecitabine is 1,250 mg/m2, but 14 days of treatment can lead to significant toxicity, said Qamar Khan, MD, during a presentation of the study at the annual meeting of the American Society of Clinical Oncology. “Mathematical models applied to xenograft [animal model] data suggest that the maximum cytotoxic effect of capecitabine occurs after about 7 days of treatment, beyond which time only toxicity increases,” Dr. Khan said during his talk on the randomized control trial.
The researchers randomized 153 patients to receive a fixed 1,500-mg capecitabine dose twice per day on a 7-day-on, 7-day-off schedule (7/7), or the 1,250–mg/m2 dose twice per day for 14 days followed by 7 days off (14/7). The median age was 60 years, and 85.6% were White, 8.5% were African American, 3.3% were Hispanic, 0.7% were American Indian or Alaskan Native, and 2.0% were other. With respect to disease characteristics, 44% had visceral metastasis, 78% were hormone receptor positive/HER2 negative, and 11% had triple-negative breast cancer. About two-thirds (65%) had received no prior chemotherapy.
Restricted mean survival time (RMST) at 36 months for PFS was 13.9 months in the 7/7 group and 14.6 months in the 14/7 group (difference, 0.7 months; 95.5% CI, –3.14 to 4.57 months). The objective response rate was 8.9% in the 7/7 group and 19.6% in the 14/7 group (P = .11). Median OS was 19.8 months in the 7/7 group and 17.5 months in the 14/7 group (hazard ratio, 0.76; P = .17). The RMST at 47 months for OS was 24.5 months in the 7/7 group and 20.9 months in the 14/7 group (difference, –3.6 months; 95% CI, –8.89 to 1.54 months).
The researchers found no differences in subgroup analyses by visceral metastasis, breast cancer subtype, or number of lines of previous therapy.
The toxicity profile of 7/7 was better with respect to grade 2-4 diarrhea (2.5% vs. 20.5%, P = .0008), grade 2-4 HFS (3.8% vs. 15.1%; P = .0019), and grade 2-4 mucositis (0% vs. 5.5%; P =.0001).
Findings back up clinical practice
“The fixed-dose capecitabine dosing is something that’s been done a lot in practice, because a lot of practitioners recognize that giving the drug for two weeks in a row with a week break is overly toxic, so it’s something we’ve been doing in the community for quite a while,” said Michael Danso, MD, who comoderated the session.
Still, the safety and efficacy data back up that general clinical practice. “There was a randomized trial and colon cancer that didn’t show [equivalent outcomes with the alternate dosing schedule]. So to see that it’s safe and effective in breast cancer is an important [finding],” said Dr. Danso, who is the Research Director at Virginia Oncology Associates, Norfolk.
During the question-and-answer following the talk, Jeffrey Kirshner, MD, a medical oncologist at Hematology-Oncology Associates of Central New York, East Syracuse, noted that his practice has used a similar schedule for years. “I really commend you for doing that study. It really supports what many of us in the real world have been doing for many years. We figured this out empirically, both upfront and when patients can’t tolerate [the 14/7 schedule].”
Fixed dose versus body surface area
Dr. Kirshner also said his practice uses a dose of 1 g/m2 of body surface area on a 7/7 schedule rather than a fixed dose as was done in Dr. Khan’s study. “If you use the higher dose, you might have seen a higher response rate because many of our patients, as you know, have a body surface [BSA] area much greater than 1.5 g/m2.”
Dr. Khan responded that there is little data available on BSA dosing. “We selected 1,500 mg because a lot of people are practicing that, and for convenience, and that most patients who started at a higher dose eventually wound up on a dose of 1,500 mg twice daily.”
Dr. Kirshner also pointed out that the study was conducted in a population with metastatic disease. “I think we need to emphasize that we do not use the 7/7 regimen in a potentially curative setting, such as the CREATE-X regimen for triple-negative [breast cancer].”
Dr. Khan agreed. “I would use the same dose as the CREATE-X trial in the adjuvant setting,” he responded.
Dr. Danso has received honoraria from Amgen and has consulted or advised Immunomedics, Novartis, Pfizer, and Seagen. Dr. Khan and Dr. Kirshner have no relevant financial disclosures.
Both progression-free survival (PFS) and overall survival (OS) were similar between the two groups, but patients on the alternative schedule experienced fewer cases of hand-foot syndrome (HFS), diarrhea, and stomatitis, and also had fewer discontinuations and dose modifications.
The Food and Drug Administration–approved dose of capecitabine is 1,250 mg/m2, but 14 days of treatment can lead to significant toxicity, said Qamar Khan, MD, during a presentation of the study at the annual meeting of the American Society of Clinical Oncology. “Mathematical models applied to xenograft [animal model] data suggest that the maximum cytotoxic effect of capecitabine occurs after about 7 days of treatment, beyond which time only toxicity increases,” Dr. Khan said during his talk on the randomized control trial.
The researchers randomized 153 patients to receive a fixed 1,500-mg capecitabine dose twice per day on a 7-day-on, 7-day-off schedule (7/7), or the 1,250–mg/m2 dose twice per day for 14 days followed by 7 days off (14/7). The median age was 60 years, and 85.6% were White, 8.5% were African American, 3.3% were Hispanic, 0.7% were American Indian or Alaskan Native, and 2.0% were other. With respect to disease characteristics, 44% had visceral metastasis, 78% were hormone receptor positive/HER2 negative, and 11% had triple-negative breast cancer. About two-thirds (65%) had received no prior chemotherapy.
Restricted mean survival time (RMST) at 36 months for PFS was 13.9 months in the 7/7 group and 14.6 months in the 14/7 group (difference, 0.7 months; 95.5% CI, –3.14 to 4.57 months). The objective response rate was 8.9% in the 7/7 group and 19.6% in the 14/7 group (P = .11). Median OS was 19.8 months in the 7/7 group and 17.5 months in the 14/7 group (hazard ratio, 0.76; P = .17). The RMST at 47 months for OS was 24.5 months in the 7/7 group and 20.9 months in the 14/7 group (difference, –3.6 months; 95% CI, –8.89 to 1.54 months).
The researchers found no differences in subgroup analyses by visceral metastasis, breast cancer subtype, or number of lines of previous therapy.
The toxicity profile of 7/7 was better with respect to grade 2-4 diarrhea (2.5% vs. 20.5%, P = .0008), grade 2-4 HFS (3.8% vs. 15.1%; P = .0019), and grade 2-4 mucositis (0% vs. 5.5%; P =.0001).
Findings back up clinical practice
“The fixed-dose capecitabine dosing is something that’s been done a lot in practice, because a lot of practitioners recognize that giving the drug for two weeks in a row with a week break is overly toxic, so it’s something we’ve been doing in the community for quite a while,” said Michael Danso, MD, who comoderated the session.
Still, the safety and efficacy data back up that general clinical practice. “There was a randomized trial and colon cancer that didn’t show [equivalent outcomes with the alternate dosing schedule]. So to see that it’s safe and effective in breast cancer is an important [finding],” said Dr. Danso, who is the Research Director at Virginia Oncology Associates, Norfolk.
During the question-and-answer following the talk, Jeffrey Kirshner, MD, a medical oncologist at Hematology-Oncology Associates of Central New York, East Syracuse, noted that his practice has used a similar schedule for years. “I really commend you for doing that study. It really supports what many of us in the real world have been doing for many years. We figured this out empirically, both upfront and when patients can’t tolerate [the 14/7 schedule].”
Fixed dose versus body surface area
Dr. Kirshner also said his practice uses a dose of 1 g/m2 of body surface area on a 7/7 schedule rather than a fixed dose as was done in Dr. Khan’s study. “If you use the higher dose, you might have seen a higher response rate because many of our patients, as you know, have a body surface [BSA] area much greater than 1.5 g/m2.”
Dr. Khan responded that there is little data available on BSA dosing. “We selected 1,500 mg because a lot of people are practicing that, and for convenience, and that most patients who started at a higher dose eventually wound up on a dose of 1,500 mg twice daily.”
Dr. Kirshner also pointed out that the study was conducted in a population with metastatic disease. “I think we need to emphasize that we do not use the 7/7 regimen in a potentially curative setting, such as the CREATE-X regimen for triple-negative [breast cancer].”
Dr. Khan agreed. “I would use the same dose as the CREATE-X trial in the adjuvant setting,” he responded.
Dr. Danso has received honoraria from Amgen and has consulted or advised Immunomedics, Novartis, Pfizer, and Seagen. Dr. Khan and Dr. Kirshner have no relevant financial disclosures.
Both progression-free survival (PFS) and overall survival (OS) were similar between the two groups, but patients on the alternative schedule experienced fewer cases of hand-foot syndrome (HFS), diarrhea, and stomatitis, and also had fewer discontinuations and dose modifications.
The Food and Drug Administration–approved dose of capecitabine is 1,250 mg/m2, but 14 days of treatment can lead to significant toxicity, said Qamar Khan, MD, during a presentation of the study at the annual meeting of the American Society of Clinical Oncology. “Mathematical models applied to xenograft [animal model] data suggest that the maximum cytotoxic effect of capecitabine occurs after about 7 days of treatment, beyond which time only toxicity increases,” Dr. Khan said during his talk on the randomized control trial.
The researchers randomized 153 patients to receive a fixed 1,500-mg capecitabine dose twice per day on a 7-day-on, 7-day-off schedule (7/7), or the 1,250–mg/m2 dose twice per day for 14 days followed by 7 days off (14/7). The median age was 60 years, and 85.6% were White, 8.5% were African American, 3.3% were Hispanic, 0.7% were American Indian or Alaskan Native, and 2.0% were other. With respect to disease characteristics, 44% had visceral metastasis, 78% were hormone receptor positive/HER2 negative, and 11% had triple-negative breast cancer. About two-thirds (65%) had received no prior chemotherapy.
Restricted mean survival time (RMST) at 36 months for PFS was 13.9 months in the 7/7 group and 14.6 months in the 14/7 group (difference, 0.7 months; 95.5% CI, –3.14 to 4.57 months). The objective response rate was 8.9% in the 7/7 group and 19.6% in the 14/7 group (P = .11). Median OS was 19.8 months in the 7/7 group and 17.5 months in the 14/7 group (hazard ratio, 0.76; P = .17). The RMST at 47 months for OS was 24.5 months in the 7/7 group and 20.9 months in the 14/7 group (difference, –3.6 months; 95% CI, –8.89 to 1.54 months).
The researchers found no differences in subgroup analyses by visceral metastasis, breast cancer subtype, or number of lines of previous therapy.
The toxicity profile of 7/7 was better with respect to grade 2-4 diarrhea (2.5% vs. 20.5%, P = .0008), grade 2-4 HFS (3.8% vs. 15.1%; P = .0019), and grade 2-4 mucositis (0% vs. 5.5%; P =.0001).
Findings back up clinical practice
“The fixed-dose capecitabine dosing is something that’s been done a lot in practice, because a lot of practitioners recognize that giving the drug for two weeks in a row with a week break is overly toxic, so it’s something we’ve been doing in the community for quite a while,” said Michael Danso, MD, who comoderated the session.
Still, the safety and efficacy data back up that general clinical practice. “There was a randomized trial and colon cancer that didn’t show [equivalent outcomes with the alternate dosing schedule]. So to see that it’s safe and effective in breast cancer is an important [finding],” said Dr. Danso, who is the Research Director at Virginia Oncology Associates, Norfolk.
During the question-and-answer following the talk, Jeffrey Kirshner, MD, a medical oncologist at Hematology-Oncology Associates of Central New York, East Syracuse, noted that his practice has used a similar schedule for years. “I really commend you for doing that study. It really supports what many of us in the real world have been doing for many years. We figured this out empirically, both upfront and when patients can’t tolerate [the 14/7 schedule].”
Fixed dose versus body surface area
Dr. Kirshner also said his practice uses a dose of 1 g/m2 of body surface area on a 7/7 schedule rather than a fixed dose as was done in Dr. Khan’s study. “If you use the higher dose, you might have seen a higher response rate because many of our patients, as you know, have a body surface [BSA] area much greater than 1.5 g/m2.”
Dr. Khan responded that there is little data available on BSA dosing. “We selected 1,500 mg because a lot of people are practicing that, and for convenience, and that most patients who started at a higher dose eventually wound up on a dose of 1,500 mg twice daily.”
Dr. Kirshner also pointed out that the study was conducted in a population with metastatic disease. “I think we need to emphasize that we do not use the 7/7 regimen in a potentially curative setting, such as the CREATE-X regimen for triple-negative [breast cancer].”
Dr. Khan agreed. “I would use the same dose as the CREATE-X trial in the adjuvant setting,” he responded.
Dr. Danso has received honoraria from Amgen and has consulted or advised Immunomedics, Novartis, Pfizer, and Seagen. Dr. Khan and Dr. Kirshner have no relevant financial disclosures.
FROM ASCO 2023
In TNBC, repeated biopsies may reveal emergent HER2-low expression
Triple-negative breast cancer (TNBC) is characterized by the absence of hormonal receptors and human epidermal growth factor receptor 2 (HER2) expression. were found to be ineffective in patients with TNBC and known HER2-zero status.
These HER2-low results were of great clinical significance for this patient population, said Yael Bar, MD, PhD, during her presentation of the research, at the annual meeting of the American Society of Clinical Oncology (ASCO).
Previously, the DESTINY-Breast04 trial demonstrated that the antibody-drug conjugate (ADC) trastuzumab deruxtecan (T-DXd) improved progression-free survival (PFS) and overall survival (OS) for patients with HER2-low metastatic breast cancer. “As a result [of the DESTINY-Breast04 findings], T-DXd is now approved for HER2-low but not HER2-zero triple-negative metastatic breast cancer."
“While HER2-low is detected in about 30%-50% of patients with triple-negative breast cancer, several studies have shown that HER2 status is heterogeneous and also dynamic over time, said Dr. Bar, who is an international research fellow in the breast cancer group at Mass General Cancer Center, Boston.
In the new study, Dr. Bar and her co-authors retrospectively identified 512 TNBC patients from 2000 to 2022 from an institutional database. They included core, surgical, or metastatic biopsies. Participants had a mean age of 52 years, with 54% over age 50. They were 83% White, 7% African American, 5% Asian, 3% Hispanic, and 2% other. Stage II was most common at diagnosis at 48%, followed by stage 1 (28%), stage 3 (14%), and stage IV (8%).
Most patients had undergone one (38%) or two (45%) biopsies, while 9% underwent three biopsies, 6% underwent four biopsies, and 2% underwent five or more.
Among all 512 patients in the study, 60% had a HER2-low result on their first biopsy. As of the second biopsy, 73% had at least one HER2-low result, with 13% of the first HER2-low results occurring at the second biopsy. As of the third biopsy, 81% had a HER2-low result, with 9% occurring for the first time. At the fourth biopsy, 86% had a positive result, with 8% occurring for the first time. All patients with five or more biopsies had at least one HER2-low result and none were first-time results.
At the second biopsy, a HER2-low result was detected for 32% of patients for the first time. At the third biopsy, a new HER2-low result was detected in 33%, and at the fourth biopsy, a new HER-2 result was detected in 38%.
The researchers matched early and metastatic biopsies in 71 patients, and 44% had changed status: 68% of those with a status change went HER2-low to HER2-zero, 26% from HER2-zero to HER2-low, and 6% from HER2-low to HER2-positive. Among 50 patients with matched metastatic biopsies, 33% had a change in status, with 63% going from HER2-zero to HER2-low, 31% from HER2-low to HER2-zero, and 6% from HER2-low to HER2-positive.
“We showed here that repeat biopsies can identify new HER2-low results for patients who were previously ineligible for T-DXd; and therefore, we think that a repeat biopsy could be considered if feasible and safe. Also, if a repeat biopsy is performed for any reason, but mainly upon metastatic recurrence, receptors should be retested,” said Dr. Bar.
After Dr. Bar’s presentation, Barbara Pistilli, MD served as a discussant. She noted the increased HER2-low results over successive biopsies. “However, here the question is, are these results related to the changes in the analytical methods over the past 20 years or the changes in the guidelines in terms of definition of HER2 status, or are they more related to a true evolution of HER2 status with the evolution of the disease?” she said during her presentation. Dr. Pistilli is chair of the breast disease committee at Gustave Roussy in Villejuif, France.
She also said that HER2 expression can vary even between different parts of the same tumor and called for alternative methods to following HER2 expression. “I don’t think that we can follow our patients with multiple biopsies over the disease evolution, so we have to find other tools, such as target-positive [circulating tumor cells], or antibody-radiolabeled PET scan in order to better follow the intermetastasis target heterogeneity over time, and finally define what is the optimal ADC sequential strategy for each patient,” said Dr. Pistilli.
Comoderator Michael Danso, MD, also weighed in when asked for comment.
“It was an important trial to show that serial biopsies potentially allow more patients to receive trastuzumab deruxtecan,” said Dr. Danso, who is the research director at Virginia Oncology Associates, Norfolk. However, he pointed out the concerns of a statistician who had spoken up during the question-and-answer session who said that the positive results could simply be the consequence of repeated testing. “If you do a test often enough, statistically you’re going to get a difference in outcome. That was an important point made. Also, if you’re going to get 100% of patients who are eventually going to [develop HER2-low status], the question is, can you just treat everybody with trastuzumab deruxtecan and not do these sequential biopsies? Obviously that is subject to cost; it’s subject to toxicity as well, so you probably want documentation that there is a HER2-low result,” said Dr. Danso.
Dr. Bar has no relevant financial disclosures. Dr. Pistilli has consulted for or advised AstraZeneca, Daiichi Sankyo/UCB Japan, Myriad Genetics, Novartis, PIERRE FABRE, and Puma Biotechnology. She has received research funding through her institution from AstraZeneca, Daiichi Sankyo, Gilead Sciences, Merus, Pfizer, and Puma Biotechnology. She has received travel or accommodation expenses from AstraZeneca, Daiichi Sankyo Europe, MSD Oncology, Novartis, Pfizer, and Pierre Fabre. Dr. Danso has received honoraria from Amgen and has consulted or advised Immunomedics, Novartis, Pfizer, and Seagen.
*This story was updated on 6/13/2023.
Triple-negative breast cancer (TNBC) is characterized by the absence of hormonal receptors and human epidermal growth factor receptor 2 (HER2) expression. were found to be ineffective in patients with TNBC and known HER2-zero status.
These HER2-low results were of great clinical significance for this patient population, said Yael Bar, MD, PhD, during her presentation of the research, at the annual meeting of the American Society of Clinical Oncology (ASCO).
Previously, the DESTINY-Breast04 trial demonstrated that the antibody-drug conjugate (ADC) trastuzumab deruxtecan (T-DXd) improved progression-free survival (PFS) and overall survival (OS) for patients with HER2-low metastatic breast cancer. “As a result [of the DESTINY-Breast04 findings], T-DXd is now approved for HER2-low but not HER2-zero triple-negative metastatic breast cancer."
“While HER2-low is detected in about 30%-50% of patients with triple-negative breast cancer, several studies have shown that HER2 status is heterogeneous and also dynamic over time, said Dr. Bar, who is an international research fellow in the breast cancer group at Mass General Cancer Center, Boston.
In the new study, Dr. Bar and her co-authors retrospectively identified 512 TNBC patients from 2000 to 2022 from an institutional database. They included core, surgical, or metastatic biopsies. Participants had a mean age of 52 years, with 54% over age 50. They were 83% White, 7% African American, 5% Asian, 3% Hispanic, and 2% other. Stage II was most common at diagnosis at 48%, followed by stage 1 (28%), stage 3 (14%), and stage IV (8%).
Most patients had undergone one (38%) or two (45%) biopsies, while 9% underwent three biopsies, 6% underwent four biopsies, and 2% underwent five or more.
Among all 512 patients in the study, 60% had a HER2-low result on their first biopsy. As of the second biopsy, 73% had at least one HER2-low result, with 13% of the first HER2-low results occurring at the second biopsy. As of the third biopsy, 81% had a HER2-low result, with 9% occurring for the first time. At the fourth biopsy, 86% had a positive result, with 8% occurring for the first time. All patients with five or more biopsies had at least one HER2-low result and none were first-time results.
At the second biopsy, a HER2-low result was detected for 32% of patients for the first time. At the third biopsy, a new HER2-low result was detected in 33%, and at the fourth biopsy, a new HER-2 result was detected in 38%.
The researchers matched early and metastatic biopsies in 71 patients, and 44% had changed status: 68% of those with a status change went HER2-low to HER2-zero, 26% from HER2-zero to HER2-low, and 6% from HER2-low to HER2-positive. Among 50 patients with matched metastatic biopsies, 33% had a change in status, with 63% going from HER2-zero to HER2-low, 31% from HER2-low to HER2-zero, and 6% from HER2-low to HER2-positive.
“We showed here that repeat biopsies can identify new HER2-low results for patients who were previously ineligible for T-DXd; and therefore, we think that a repeat biopsy could be considered if feasible and safe. Also, if a repeat biopsy is performed for any reason, but mainly upon metastatic recurrence, receptors should be retested,” said Dr. Bar.
After Dr. Bar’s presentation, Barbara Pistilli, MD served as a discussant. She noted the increased HER2-low results over successive biopsies. “However, here the question is, are these results related to the changes in the analytical methods over the past 20 years or the changes in the guidelines in terms of definition of HER2 status, or are they more related to a true evolution of HER2 status with the evolution of the disease?” she said during her presentation. Dr. Pistilli is chair of the breast disease committee at Gustave Roussy in Villejuif, France.
She also said that HER2 expression can vary even between different parts of the same tumor and called for alternative methods to following HER2 expression. “I don’t think that we can follow our patients with multiple biopsies over the disease evolution, so we have to find other tools, such as target-positive [circulating tumor cells], or antibody-radiolabeled PET scan in order to better follow the intermetastasis target heterogeneity over time, and finally define what is the optimal ADC sequential strategy for each patient,” said Dr. Pistilli.
Comoderator Michael Danso, MD, also weighed in when asked for comment.
“It was an important trial to show that serial biopsies potentially allow more patients to receive trastuzumab deruxtecan,” said Dr. Danso, who is the research director at Virginia Oncology Associates, Norfolk. However, he pointed out the concerns of a statistician who had spoken up during the question-and-answer session who said that the positive results could simply be the consequence of repeated testing. “If you do a test often enough, statistically you’re going to get a difference in outcome. That was an important point made. Also, if you’re going to get 100% of patients who are eventually going to [develop HER2-low status], the question is, can you just treat everybody with trastuzumab deruxtecan and not do these sequential biopsies? Obviously that is subject to cost; it’s subject to toxicity as well, so you probably want documentation that there is a HER2-low result,” said Dr. Danso.
Dr. Bar has no relevant financial disclosures. Dr. Pistilli has consulted for or advised AstraZeneca, Daiichi Sankyo/UCB Japan, Myriad Genetics, Novartis, PIERRE FABRE, and Puma Biotechnology. She has received research funding through her institution from AstraZeneca, Daiichi Sankyo, Gilead Sciences, Merus, Pfizer, and Puma Biotechnology. She has received travel or accommodation expenses from AstraZeneca, Daiichi Sankyo Europe, MSD Oncology, Novartis, Pfizer, and Pierre Fabre. Dr. Danso has received honoraria from Amgen and has consulted or advised Immunomedics, Novartis, Pfizer, and Seagen.
*This story was updated on 6/13/2023.
Triple-negative breast cancer (TNBC) is characterized by the absence of hormonal receptors and human epidermal growth factor receptor 2 (HER2) expression. were found to be ineffective in patients with TNBC and known HER2-zero status.
These HER2-low results were of great clinical significance for this patient population, said Yael Bar, MD, PhD, during her presentation of the research, at the annual meeting of the American Society of Clinical Oncology (ASCO).
Previously, the DESTINY-Breast04 trial demonstrated that the antibody-drug conjugate (ADC) trastuzumab deruxtecan (T-DXd) improved progression-free survival (PFS) and overall survival (OS) for patients with HER2-low metastatic breast cancer. “As a result [of the DESTINY-Breast04 findings], T-DXd is now approved for HER2-low but not HER2-zero triple-negative metastatic breast cancer."
“While HER2-low is detected in about 30%-50% of patients with triple-negative breast cancer, several studies have shown that HER2 status is heterogeneous and also dynamic over time, said Dr. Bar, who is an international research fellow in the breast cancer group at Mass General Cancer Center, Boston.
In the new study, Dr. Bar and her co-authors retrospectively identified 512 TNBC patients from 2000 to 2022 from an institutional database. They included core, surgical, or metastatic biopsies. Participants had a mean age of 52 years, with 54% over age 50. They were 83% White, 7% African American, 5% Asian, 3% Hispanic, and 2% other. Stage II was most common at diagnosis at 48%, followed by stage 1 (28%), stage 3 (14%), and stage IV (8%).
Most patients had undergone one (38%) or two (45%) biopsies, while 9% underwent three biopsies, 6% underwent four biopsies, and 2% underwent five or more.
Among all 512 patients in the study, 60% had a HER2-low result on their first biopsy. As of the second biopsy, 73% had at least one HER2-low result, with 13% of the first HER2-low results occurring at the second biopsy. As of the third biopsy, 81% had a HER2-low result, with 9% occurring for the first time. At the fourth biopsy, 86% had a positive result, with 8% occurring for the first time. All patients with five or more biopsies had at least one HER2-low result and none were first-time results.
At the second biopsy, a HER2-low result was detected for 32% of patients for the first time. At the third biopsy, a new HER2-low result was detected in 33%, and at the fourth biopsy, a new HER-2 result was detected in 38%.
The researchers matched early and metastatic biopsies in 71 patients, and 44% had changed status: 68% of those with a status change went HER2-low to HER2-zero, 26% from HER2-zero to HER2-low, and 6% from HER2-low to HER2-positive. Among 50 patients with matched metastatic biopsies, 33% had a change in status, with 63% going from HER2-zero to HER2-low, 31% from HER2-low to HER2-zero, and 6% from HER2-low to HER2-positive.
“We showed here that repeat biopsies can identify new HER2-low results for patients who were previously ineligible for T-DXd; and therefore, we think that a repeat biopsy could be considered if feasible and safe. Also, if a repeat biopsy is performed for any reason, but mainly upon metastatic recurrence, receptors should be retested,” said Dr. Bar.
After Dr. Bar’s presentation, Barbara Pistilli, MD served as a discussant. She noted the increased HER2-low results over successive biopsies. “However, here the question is, are these results related to the changes in the analytical methods over the past 20 years or the changes in the guidelines in terms of definition of HER2 status, or are they more related to a true evolution of HER2 status with the evolution of the disease?” she said during her presentation. Dr. Pistilli is chair of the breast disease committee at Gustave Roussy in Villejuif, France.
She also said that HER2 expression can vary even between different parts of the same tumor and called for alternative methods to following HER2 expression. “I don’t think that we can follow our patients with multiple biopsies over the disease evolution, so we have to find other tools, such as target-positive [circulating tumor cells], or antibody-radiolabeled PET scan in order to better follow the intermetastasis target heterogeneity over time, and finally define what is the optimal ADC sequential strategy for each patient,” said Dr. Pistilli.
Comoderator Michael Danso, MD, also weighed in when asked for comment.
“It was an important trial to show that serial biopsies potentially allow more patients to receive trastuzumab deruxtecan,” said Dr. Danso, who is the research director at Virginia Oncology Associates, Norfolk. However, he pointed out the concerns of a statistician who had spoken up during the question-and-answer session who said that the positive results could simply be the consequence of repeated testing. “If you do a test often enough, statistically you’re going to get a difference in outcome. That was an important point made. Also, if you’re going to get 100% of patients who are eventually going to [develop HER2-low status], the question is, can you just treat everybody with trastuzumab deruxtecan and not do these sequential biopsies? Obviously that is subject to cost; it’s subject to toxicity as well, so you probably want documentation that there is a HER2-low result,” said Dr. Danso.
Dr. Bar has no relevant financial disclosures. Dr. Pistilli has consulted for or advised AstraZeneca, Daiichi Sankyo/UCB Japan, Myriad Genetics, Novartis, PIERRE FABRE, and Puma Biotechnology. She has received research funding through her institution from AstraZeneca, Daiichi Sankyo, Gilead Sciences, Merus, Pfizer, and Puma Biotechnology. She has received travel or accommodation expenses from AstraZeneca, Daiichi Sankyo Europe, MSD Oncology, Novartis, Pfizer, and Pierre Fabre. Dr. Danso has received honoraria from Amgen and has consulted or advised Immunomedics, Novartis, Pfizer, and Seagen.
*This story was updated on 6/13/2023.
FROM ASCO 2023
Early hysterectomy linked to higher CVD, stroke risk
TOPLINE:
METHODOLOGY:
- Risk of CVD rapidly increases after menopause, possibly owing to loss of protective effects of female sex hormones and hemorheologic changes.
- Results of previous studies of the association between hysterectomy and CVD were mixed.
- Using national health insurance data, this cohort study included 55,539 South Korean women (median age, 45 years) who underwent a hysterectomy and a propensity-matched group of women.
- The primary outcome was CVD, including myocardial infarction (MI), coronary artery revascularization, and stroke.
TAKEAWAY:
- During follow-up of just under 8 years, the hysterectomy group had an increased risk of CVD compared with the non-hysterectomy group (hazard ratio [HR] 1.25; 95% confidence interval [CI], 1.09-1.44; P = .002)
- The incidence of MI and coronary revascularization was comparable between groups, but the risk of stroke was significantly higher among those who had had a hysterectomy (HR, 1.31; 95% CI, 1.12-1.53; P < .001)
- This increase in risk was similar after excluding patients who also underwent adnexal surgery.
IN PRACTICE:
Early hysterectomy was linked to higher CVD risk, especially stroke, but since the CVD incidence wasn’t high, a change in clinical practice may not be needed, said the authors.
STUDY DETAILS:
The study was conducted by Jin-Sung Yuk, MD, PhD, Department of Obstetrics and Gynecology, Sanggye Paik Hospital, Inje University College of Medicine, Seoul, Republic of Korea, and colleagues. It was published online June 12 in JAMA Network Open.
LIMITATIONS:
The study was retrospective and observational and used administrative databases that may be prone to inaccurate coding. The findings may not be generalizable outside Korea.
DISCLOSURES:
The study was supported by a National Research Foundation of Korea grant funded by the Korea government. The authors report no conflicts of interest.
A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Risk of CVD rapidly increases after menopause, possibly owing to loss of protective effects of female sex hormones and hemorheologic changes.
- Results of previous studies of the association between hysterectomy and CVD were mixed.
- Using national health insurance data, this cohort study included 55,539 South Korean women (median age, 45 years) who underwent a hysterectomy and a propensity-matched group of women.
- The primary outcome was CVD, including myocardial infarction (MI), coronary artery revascularization, and stroke.
TAKEAWAY:
- During follow-up of just under 8 years, the hysterectomy group had an increased risk of CVD compared with the non-hysterectomy group (hazard ratio [HR] 1.25; 95% confidence interval [CI], 1.09-1.44; P = .002)
- The incidence of MI and coronary revascularization was comparable between groups, but the risk of stroke was significantly higher among those who had had a hysterectomy (HR, 1.31; 95% CI, 1.12-1.53; P < .001)
- This increase in risk was similar after excluding patients who also underwent adnexal surgery.
IN PRACTICE:
Early hysterectomy was linked to higher CVD risk, especially stroke, but since the CVD incidence wasn’t high, a change in clinical practice may not be needed, said the authors.
STUDY DETAILS:
The study was conducted by Jin-Sung Yuk, MD, PhD, Department of Obstetrics and Gynecology, Sanggye Paik Hospital, Inje University College of Medicine, Seoul, Republic of Korea, and colleagues. It was published online June 12 in JAMA Network Open.
LIMITATIONS:
The study was retrospective and observational and used administrative databases that may be prone to inaccurate coding. The findings may not be generalizable outside Korea.
DISCLOSURES:
The study was supported by a National Research Foundation of Korea grant funded by the Korea government. The authors report no conflicts of interest.
A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Risk of CVD rapidly increases after menopause, possibly owing to loss of protective effects of female sex hormones and hemorheologic changes.
- Results of previous studies of the association between hysterectomy and CVD were mixed.
- Using national health insurance data, this cohort study included 55,539 South Korean women (median age, 45 years) who underwent a hysterectomy and a propensity-matched group of women.
- The primary outcome was CVD, including myocardial infarction (MI), coronary artery revascularization, and stroke.
TAKEAWAY:
- During follow-up of just under 8 years, the hysterectomy group had an increased risk of CVD compared with the non-hysterectomy group (hazard ratio [HR] 1.25; 95% confidence interval [CI], 1.09-1.44; P = .002)
- The incidence of MI and coronary revascularization was comparable between groups, but the risk of stroke was significantly higher among those who had had a hysterectomy (HR, 1.31; 95% CI, 1.12-1.53; P < .001)
- This increase in risk was similar after excluding patients who also underwent adnexal surgery.
IN PRACTICE:
Early hysterectomy was linked to higher CVD risk, especially stroke, but since the CVD incidence wasn’t high, a change in clinical practice may not be needed, said the authors.
STUDY DETAILS:
The study was conducted by Jin-Sung Yuk, MD, PhD, Department of Obstetrics and Gynecology, Sanggye Paik Hospital, Inje University College of Medicine, Seoul, Republic of Korea, and colleagues. It was published online June 12 in JAMA Network Open.
LIMITATIONS:
The study was retrospective and observational and used administrative databases that may be prone to inaccurate coding. The findings may not be generalizable outside Korea.
DISCLOSURES:
The study was supported by a National Research Foundation of Korea grant funded by the Korea government. The authors report no conflicts of interest.
A version of this article first appeared on Medscape.com.
Postpartum IUD insertion: Best practices
CASE 1 Multiparous female with short-interval pregnancies desires contraception
A 24-year-old woman (G4P3) presents for a routine prenatal visit in the third trimester. Her last 2 pregnancies have occurred within 3 months of her prior birth. She endorses feeling overwhelmed with having 4 children under the age of 5 years, and she specifies that she would like to avoid another pregnancy for several years. She plans to breast and bottle feed, and she notes that she tends to forget to take pills. When you look back at her prior charts, you note that she did not return for her last 2 postpartum visits. What can you offer her? What would be a safe contraceptive option for her?
Intrauterine devices (IUDs) are safe, effective, and reported by patients to be satisfactory methods of contraception precisely because they are prone to less user error. The Contraceptive Choice Project demonstrated that patients are more apt to choose them when barriers such as cost and access are removed and nondirective counseling is provided.1 Given that unintended pregnancy rates hover around 48%, the American College of Obstetricians and Gynecologists (ACOG) recommends them as first-line methods for pregnancy prevention.2,3
For repeat pregnancies, the postpartum period is an especially vulnerable time—non-breastfeeding women will ovulate as soon as 25 days after birth, and by 8 weeks 30% will have ovulated.4 Approximately 40% to 57% of women report having unprotected intercourse before 6 weeks postpartum, and nearly 70% of all pregnancies in the first year postpartum are unintended.3,5 Furthermore, patients at highest risk for short-interval pregnancy, such as adolescents, are less likely to return for a postpartum visit.3
Short-interval pregnancies confer greater fetal risk, including risks of low-birth weight, preterm birth, small for gestational age and increased risk of neonatal intensive care unit admission.6 Additionally, maternal health may be compromised during a short-interval pregnancy, particularly in medically complex patients due to increased risks of adverse pregnancy outcomes, such as postpartum bleeding or uterine rupture and disease progression.7 A 2006 meta-analysis by Conde-Agudelo and colleagues found that waiting at least 18 months between pregnancies was optimal for reducing these risks.6
Thus, the immediate postpartum period is an optimal time for addressing contraceptive needs and for preventing short-interval and unintended pregnancy. This article aims to provide evidence supporting the use of immediate postpartum IUDs, as well as their associated risks and barriers to use.
IUD types and routes for immediate postpartum insertion
There are several randomized controlled trials (RCTs) that examine the immediate postpartum use of copper IUDs and levonorgestrel-releasing (LNG) IUDs.8-11 In 2010, Chen and colleagues compared placement of the immediate postpartum IUD following vaginal delivery with interval placement at 6–8 weeks postpartum. Of 51 patients enrolled in each arm, 98% received an IUD immediately postpartum, and 90% received one during their postpartum visit. There were 12 expulsions (24%) in the immediate postpartum IUD group, compared with 2 (4.4%) in the interval group. Expelled IUDs were replaced, and at 6 months both groups had similar rates of IUD use.8
Whitaker and colleagues demonstrated similar findings after randomizing a small group of women who had a cesarean delivery (CD) to interval or immediate placement. There were significantly more expulsions in the post-placental group (20%) than the interval group (0%), but there were more users of the IUD in the post-placental group than in the interval group at 12 months.9
Two RCTs, by Lester and colleagues and Levi et al, demonstrated successful placement of the copper IUD or LNG-IUD following CD, with few expulsions (0% and 8%, respectively). Patients who were randomized to immediate postpartum IUD placement were more likely to receive an IUD than those who were randomized to interval insertion, mostly due to lack of postpartum follow up. Both studies followed patients out to 6 months, and rates of IUD continuation and satisfaction were higher at this time in the immediate postpartum IUD groups.10,11
Continue to: Risks, contraindications, and breastfeeding impact...
Risks, contraindications, and breastfeeding impact
What are the risks of immediate postpartum IUD placement? The highest risk of IUD placement in the immediate postpartum period appears to be expulsion (TABLE 1). In a meta-analysis conducted in 2022, which looked at 11 studies of immediate IUD insertion, the rates of expulsion were between 5% and 27%.3,8,12,13 Results of a study by Cohen and colleagues demonstrated that most expulsions occurred within the first 12 weeks following delivery; of those expulsions that occurred, only 11% went unrecognized.13 Immediate postpartum IUD insertion does not increase the IUD-associated risks of perforation, infection, or immediate postpartum bleeding (although prolonged bleeding may be more common).12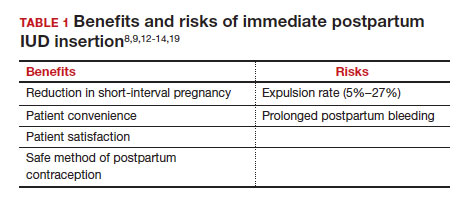
Are there contraindications to placing an IUD immediately postpartum? The main contraindication to immediate postpartum IUD use is peripartum infection, including Triple I, endomyometritis, and puerperal sepsis. Other contraindications include retained placenta requiring manual or surgical removal, uterine anomalies, and other medical contraindications to IUD use as recommended by the US Medical Eligibility Criteria.14
Does immediate IUD placement affect breastfeeding? There is theoretical risk of decreased milk supply or difficulty breastfeeding with initiation of progestin-only methods of contraception in the immediate postpartum period, as the rapid fall in progesterone levels initiates lactogenesis. However, progestin-only methods appear to have limited effect on initiation and continuation of breastfeeding in the immediate postpartum period.15
There were 2 secondary analyses of a pair of RCTs comparing immediate and delayed postpartum IUD use. Results from Levi and colleagues demonstrated no difference between immediate and interval IUD placement groups in the proportion of women who were breastfeeding at 6, 12, and 24 weeks.16 Chen and colleagues’ study was smaller; researchers found that women with interval IUD placement were more likely to be exclusively breastfeeding and continuing to breastfeed at 6 months compared with the immediate postpartum group.17
To better characterize the impact of progestin implants, in a recent meta-analysis, authors examined the use of subcutaneous levonorgestrel rods and found no difference in breastfeeding initiation and continuation rates between women who had them placed immediately versus 6 ̶ 8 weeks postpartum.12
Benefits of immediate postpartum IUD placement
One benefit of immediate postpartum IUD insertion is a reduction in short-interval pregnancies. In a study by Cohen and colleagues13 of young women aged 13 to 22 years choosing immediate postpartum IUDs (82) or implants (162), the authors found that 61% of women retained their IUDs at 12 months postpartum. Because few requested IUD removal over that time frame, the discontinuation rate at 1 year was primarily due to expulsions. Pregnancy rates at 1 year were 7.6% in the IUD group and 1.5% in the implant group. However, the 7.6% rate in the IUD group was lower than in previously studied adolescent control groups: 18.6% of control adolescents (38 of 204) using a contraceptive form other than a postpartum etonogestrel implant had repeat pregnancy at 1 year.13,18
Not only are patients who receive immediate postpartum IUDs more likely to receive them and continue their use, but they are also satisfied with the experience of receiving the IUD and with the method of contraception. A small mixed methods study of 66 patients demonstrated that women were interested in obtaining immediate postpartum contraception to avoid some of the logistical and financial challenges of returning for a postpartum visit. They also felt that the IUD placement was less painful than expected, and they didn’t feel that the insertion process imposed on their birth experience. Many described relief to know that they had a safe and effective contraceptive method upon leaving the hospital.19 Other studies have shown that even among women who expel an IUD following immediate postpartum placement, many choose to replace it in order to continue it as a contraceptive method.8,9,13
Continue to: Instructions for placement...
Instructions for placement
1. Counsel appropriately. Thoroughly counsel patients regarding their options for postpartum contraception, with emphasis on the benefits, risks, and contraindications. Current recommendations to reduce the risk of expulsion are to place the IUD in the delivery room or operating room within 10 minutes of placental delivery.
2. Post ̶ vaginal delivery. Following vaginal delivery, remove the IUD from the inserter, cut the strings to 10 cm and, using either fingers to grasp the wings of the IUD or ring forceps, advance the IUD to the fundus. Ultrasound guidance may be used, but it does not appear to be helpful in preventing expulsion.20
3. Post ̶ cesarean delivery. Once the placenta is delivered, place the IUD using the inserter or a ring forceps at the fundus and guide the strings into the cervix, then close the hysterotomy.
ACOG does recommend formal trainingbefore placing postpartum IUDs. One resource they provide is a free online webinar (https://www.acog.org/education-and-events/webinars/long-acting-reversible-contra ception-overview-and-hands-on-practice-for-residents).3
CASE 1 Resolved
The patient was counseled in the office about her options, and she was most interested in immediate postpartum LNG-IUD placement. She went on to deliver a healthy baby vaginally at 39 weeks. Within 10 minutes of placental delivery, she received an LNG-IUD. She returned to the office 3 months later for STI screening; her examination revealed correct placement and no evidence of expulsion. She expressed that she was happy with her IUD and thankful that she was able to receive it immediately after the birth of her baby.
CASE 2 Nulliparous woman desires IUD for postpartum contraception
A 33-year-old nulliparous woman presents in the third trimester for a routine prenatal visit. She had used the LNG-IUD prior to getting pregnant and reports that she was very happy with it. She knows she wants to wait at least 2 years before trying to get pregnant again, and she would like to resume contraception as soon as it is reasonably safe to do so. She has read that it is possible to get an IUD immediately postpartum and asks about it as a possible option.
What barriers will she face in obtaining an immediate postpartum IUD?
There are many barriers for patients who may be good candidates for immediate postpartum contraception (TABLE 2). Many patients are unaware that it is a safe option, and they often have concerns about such risks as infection, perforation, and effects on breastfeeding. Additionally, providers may not prioritize adequate counseling about postpartum contraception when they face time constraints and a need to counsel about other pregnancy-related topics during the prenatal visit schedule.7,21
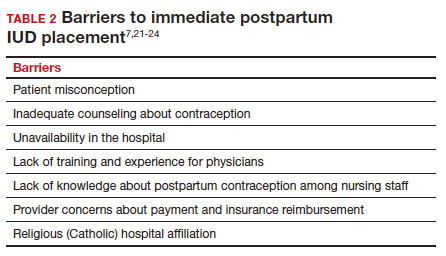
System, hospital, and clinician barriers to immediate postpartum IUD use
Hospital implementation of a successful postpartum IUD program requires pharmacy, intrapartum and postpartum nursing staff, physicians, administration, and billing to be aligned. Hospital administration and pharmacists must stock IUDs in the pharmacy. Hospital nursing staff attitudes toward and knowledge of postpartum contraception can have profound influence on how they discuss safe and effective methods of postpartum contraception with patients who may not have received counseling during prenatal care.22 In a survey of 108 ACOG fellows, nearly 75% of ObGyn physicians did not offer immediate postpartum IUDs; lack of provider training, lack of IUD availability, and concern about cost and payment were found to be common reasons why.21 Additionally, Catholic-affiliated and rural institutions are less likely to offer it, whereas more urban, teaching hospitals are more likely to have programs in place.23 Prior to 2012, immediate postpartum IUD insertions and device costs were part of the global Medicaid obstetric fee in most states, and both hospital systems and individual providers were concerned about loss of revenue.23
In 2015, Washington and colleagues published a decision analysis that examined the cost-effectiveness and cost savings associated with immediate postpartum IUD use. Accounting for expulsion rates, they found that immediate postpartum IUD placement can save $282,540 per 1,000 women over 2 years; additionally, immediate postpartum IUD use can prevent 88 unintended pregnancies per 1,000 women over 2 years.24 Not only do immediate postpartum IUDs have great potential to prevent individual patients from undesired short-interval pregnancies (FIGURE 1), but they can also save the system substantial health care dollars (FIGURE 2).
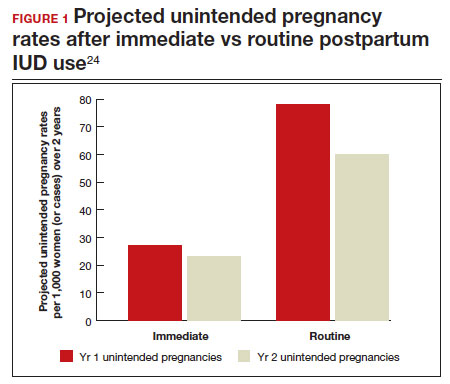
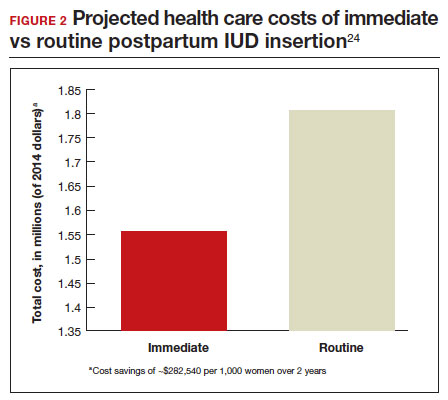
Overcoming barriers
Immediate postpartum IUD implementation is attainable with practice, policy, and institutional changes. Education and training programs geared toward providers and nursing staff can improve understanding of the benefits and risks of immediate postpartum IUD placement. Additionally, clinicians must provide comprehensive, nondirective counseling during the antepartum period, informing patients of all safe and effective options. Expulsion risks should be disclosed, as well as the benefit of not needing to return for a separate postpartum contraception appointment.
Since 2012, many state Medicaid agencies have decoupled reimbursement for inpatient postpartum IUD insertion from the delivery fee. By 2018, more than half of states adopted this practice. Commercial insurers have followed suit in some cases, and as such, both Medicaid and commercially insured patients have had increased access to immediate postpartum IUDs.23 This has translated into increased uptake of immediate postpartum IUDs among both Medicaid and commercially insured patients. Koch et al conducted a retrospective cohort study comparing IUD use in patients 1 year before and 1 year after the policy changes, and they found a 10-fold increase in use of immediate postpartum IUDs.25
While education, counseling, access, and changes in reimbursement may increase access in many hospital systems, some barriers, such as religious affiliation of the hospital system, may be impossible to overcome. A viable alternative to immediate postpartum IUD placement may be early postpartum IUD placement, which could allow patients to coordinate this procedure with 1- or 2-week return routine postpartum visits for CD recovery, mental health screenings, and/or well-baby visits. More data are necessary before recommending this universally, but Averbach and colleagues published a promising meta-analysis that demonstrated no complete expulsions in studies in which IUDs were placed between 2 and 4 weeks postpartum, and only a pooled partial expulsion rate (of immediate postpartum, early inpatient, early outpatient, and interval placement) of 3.7%.4
CASE 2 Resolved
Although the patient was interested in receiving a postpartum LNG-IUD immediately after her vaginal birth, she had to wait until her 6-week postpartum visit. The hospital did not stock IUDs for immediate postpartum IUD use, and her provider, having not been trained on immediate postpartum insertion, did not feel comfortable trying to place it in the immediate postpartum time frame. ●
- Immediate postpartum IUD insertion is a safe and effective method for postpartum contraception for many postpartum women.
- Immediate postpartum IUD insertion can result in increased uptake of postpartum contraception, a reduction in short interval pregnancies, and the opportunity for patients to plan their ideal family size.
- Patients should be thoroughly counseled about the safety of IUD placement and risks of expulsion associated with immediate postpartum placement.
- Successful programs for immediate postpartum IUD insertion incorporate training for providers on proper insertion techniques, education for nursing staff about safety and counseling, on-site IUD supply, and reimbursement that is decoupled from the payment for delivery.
- Winner B, Peipert JF, Zhao Q, et al. Effectiveness of longacting reversible contraception. N Engl J Med. 2012;366:19982007. doi: 10.1056/NEJMoa1110855.
- Bearak J, Popinchalk A, Ganatra B, et al. Unintended pregnancy and abortion by income, region, and the legal status of abortion: estimates from a comprehensive model for 1990-2019. Lancet Glob Health. 2020;8:e1152-e1161. doi: 10.1016/S2214-109X(20)30315-6.
- American College of Obstetricians and Gynecologists’ Committee on Obstetric Practice. Committee Opinion No. 670: Immediate postpartum long-acting reversible contraception. Obstet Gynecol. 2016;128:e32-e37. doi: 10.1097/AOG.0000000000001587.
- Averbach SH, Ermias Y, Jeng G, et al. Expulsion of intrauterine devices after postpartum placement by timing of placement, delivery type, and intrauterine device type: a systematic review and meta-analysis. Am J Obstet Gynecol. 2020;223:177188. doi: 10.1016/j.ajog.2020.02.045.
- Connolly A, Thorp J, Pahel L. Effects of pregnancy and childbirth on postpartum sexual function: a longitudinal prospective study. Int Urogynecol J Pelvic Floor Dysfunct. 2005;16:263-267. doi: 10.1007/s00192-005-1293-6.
- Conde-Agudelo A, Rosas-Bermúdez A, Kafury-Goeta AC. Birth spacing and risk of adverse perinatal outcomes: a meta-analysis. JAMA. 2006;295:1809-1823. doi: 10.1001 /jama.295.15.1809.
- Vricella LK, Gawron LM, Louis JM. Society for MaternalFetal Medicine (SMFM) Consult Series #48: Immediate postpartum long-acting reversible contraception for women at high risk for medical complications. Am J Obstet Gynecol. 2019;220:B2-B12. doi: 10.1016/j.ajog.2019.02.011.
- Chen BA, Reeves MF, Hayes JL, et al. Postplacental or delayed insertion of the levonorgestrel intrauterine device after vaginal delivery: a randomized controlled trial. Obstet Gynecol. 2010;116:1079-1087. doi: 10.1097/AOG.0b013e3181f73fac.
- Whitaker AK, Endres LK, Mistretta SQ, et al. Postplacental insertion of the levonorgestrel intrauterine device after cesarean delivery vs. delayed insertion: a randomized controlled trial. Contraception. 2014;89:534-539. doi: 10.1016/j.contraception.2013.12.007.
- Lester F, Kakaire O, Byamugisha J, et al. Intracesarean insertion of the Copper T380A versus 6 weeks postcesarean: a randomized clinical trial. Contraception. 2015;91:198-203. doi: 10.1016/j.contraception.2014.12.002.
- Levi EE, Stuart GS, Zerden ML, et al. Intrauterine device placement during cesarean delivery and continued use 6 months postpartum: a randomized controlled trial. Obstet Gynecol. 2015;126:5-11. doi: 10.1097/AOG.0000000000000882.
- Sothornwit J, Kaewrudee S, Lumbiganon P, et al. Immediate versus delayed postpartum insertion of contraceptive implant and IUD for contraception. Cochrane Database Syst Rev. 2022;10:CD011913. doi: 10.1002/14651858.CD011913.pub3.
- Cohen R, Sheeder J, Arango N, et al. Twelve-month contraceptive continuation and repeat pregnancy among young mothers choosing postdelivery contraceptive implants or postplacental intrauterine devices. Contraception. 2016;93:178-183. doi: 10.1016/j.contraception.2015.10.001.
- Centers for Disease Control and Prevention (CDC). US Medical Eligibility Criteria for Contraceptive Use, 2010. MMWR Recomm Rep. 2010;59(RR-4):1-86.
- Kapp N, Curtis K, Nanda K. Progestogen-only contraceptive use among breastfeeding women: a systematic review. Contraception. 2010;82:17-37. doi: 10.1016 /j.contraception.2010.02.002.
- Levi EE, Findley MK, Avila K, et al. Placement of levonorgestrel intrauterine device at the time of cesarean delivery and the effect on breastfeeding duration. Breastfeed Med. 2018;13:674679. doi: 10.1089/bfm.2018.0060.
- Chen BA, Reeves MF, Creinin MD, et al. Postplacental or delayed levonorgestrel intrauterine device insertion and breast-feeding duration. Contraception. 2011;84:499-504. doi: 10.1016/j.contraception.2011.01.022.
- Tocce KM, Sheeder JL, Teal SB. Rapid repeat pregnancy in adolescents: do immediate postpartum contraceptive implants make a difference? Am J Obstet Gynecol. 2012;206:481.e1-7. doi: 10.1016/j.ajog.2012.04.015.
- Carr SL, Singh RH, Sussman AL, et al. Women’s experiences with immediate postpartum intrauterine device insertion: a mixed-methods study. Contraception. 2018;97:219-226. doi: 10.1016/j.contraception.2017.10.008.
- Martinez OP, Wilder L, Seal P. Ultrasound-guided compared with non-ultrasound-Guided placement of immediate postpartum intrauterine contraceptive devices. Obstet Gynecol. 2022;140:91-93. doi: 10.1097/AOG.0000000000004828.
- Holden EC, Lai E, Morelli SS, et al. Ongoing barriers to immediate postpartum long-acting reversible contraception: a physician survey. Contracept Reprod Med. 2018;3:23. doi: 10.1186/s40834-018-0078-5.
- Benfield N, Hawkins F, Ray L, et al. Exposure to routine availability of immediate postpartum LARC: effect on attitudes and practices of labor and delivery and postpartum nurses. Contraception. 2018;97:411-414. doi: 10.1016 /j.contraception.2018.01.017.
- Steenland MW, Vatsa R, Pace LE, et al. Immediate postpartum long-acting reversible contraceptive use following statespecific changes in hospital Medicaid reimbursement. JAMA Netw Open. 2022;5:e2237918. doi: 10.1001 /jamanetworkopen.2022.37918.
- Washington CI, Jamshidi R, Thung SF, et al. Timing of postpartum intrauterine device placement: a costeffectiveness analysis. Fertil Steril. 2015;103:131-137. doi: 10.1016/j.fertnstert.2014.09.032
CASE 1 Multiparous female with short-interval pregnancies desires contraception
A 24-year-old woman (G4P3) presents for a routine prenatal visit in the third trimester. Her last 2 pregnancies have occurred within 3 months of her prior birth. She endorses feeling overwhelmed with having 4 children under the age of 5 years, and she specifies that she would like to avoid another pregnancy for several years. She plans to breast and bottle feed, and she notes that she tends to forget to take pills. When you look back at her prior charts, you note that she did not return for her last 2 postpartum visits. What can you offer her? What would be a safe contraceptive option for her?
Intrauterine devices (IUDs) are safe, effective, and reported by patients to be satisfactory methods of contraception precisely because they are prone to less user error. The Contraceptive Choice Project demonstrated that patients are more apt to choose them when barriers such as cost and access are removed and nondirective counseling is provided.1 Given that unintended pregnancy rates hover around 48%, the American College of Obstetricians and Gynecologists (ACOG) recommends them as first-line methods for pregnancy prevention.2,3
For repeat pregnancies, the postpartum period is an especially vulnerable time—non-breastfeeding women will ovulate as soon as 25 days after birth, and by 8 weeks 30% will have ovulated.4 Approximately 40% to 57% of women report having unprotected intercourse before 6 weeks postpartum, and nearly 70% of all pregnancies in the first year postpartum are unintended.3,5 Furthermore, patients at highest risk for short-interval pregnancy, such as adolescents, are less likely to return for a postpartum visit.3
Short-interval pregnancies confer greater fetal risk, including risks of low-birth weight, preterm birth, small for gestational age and increased risk of neonatal intensive care unit admission.6 Additionally, maternal health may be compromised during a short-interval pregnancy, particularly in medically complex patients due to increased risks of adverse pregnancy outcomes, such as postpartum bleeding or uterine rupture and disease progression.7 A 2006 meta-analysis by Conde-Agudelo and colleagues found that waiting at least 18 months between pregnancies was optimal for reducing these risks.6
Thus, the immediate postpartum period is an optimal time for addressing contraceptive needs and for preventing short-interval and unintended pregnancy. This article aims to provide evidence supporting the use of immediate postpartum IUDs, as well as their associated risks and barriers to use.
IUD types and routes for immediate postpartum insertion
There are several randomized controlled trials (RCTs) that examine the immediate postpartum use of copper IUDs and levonorgestrel-releasing (LNG) IUDs.8-11 In 2010, Chen and colleagues compared placement of the immediate postpartum IUD following vaginal delivery with interval placement at 6–8 weeks postpartum. Of 51 patients enrolled in each arm, 98% received an IUD immediately postpartum, and 90% received one during their postpartum visit. There were 12 expulsions (24%) in the immediate postpartum IUD group, compared with 2 (4.4%) in the interval group. Expelled IUDs were replaced, and at 6 months both groups had similar rates of IUD use.8
Whitaker and colleagues demonstrated similar findings after randomizing a small group of women who had a cesarean delivery (CD) to interval or immediate placement. There were significantly more expulsions in the post-placental group (20%) than the interval group (0%), but there were more users of the IUD in the post-placental group than in the interval group at 12 months.9
Two RCTs, by Lester and colleagues and Levi et al, demonstrated successful placement of the copper IUD or LNG-IUD following CD, with few expulsions (0% and 8%, respectively). Patients who were randomized to immediate postpartum IUD placement were more likely to receive an IUD than those who were randomized to interval insertion, mostly due to lack of postpartum follow up. Both studies followed patients out to 6 months, and rates of IUD continuation and satisfaction were higher at this time in the immediate postpartum IUD groups.10,11
Continue to: Risks, contraindications, and breastfeeding impact...
Risks, contraindications, and breastfeeding impact
What are the risks of immediate postpartum IUD placement? The highest risk of IUD placement in the immediate postpartum period appears to be expulsion (TABLE 1). In a meta-analysis conducted in 2022, which looked at 11 studies of immediate IUD insertion, the rates of expulsion were between 5% and 27%.3,8,12,13 Results of a study by Cohen and colleagues demonstrated that most expulsions occurred within the first 12 weeks following delivery; of those expulsions that occurred, only 11% went unrecognized.13 Immediate postpartum IUD insertion does not increase the IUD-associated risks of perforation, infection, or immediate postpartum bleeding (although prolonged bleeding may be more common).12
Are there contraindications to placing an IUD immediately postpartum? The main contraindication to immediate postpartum IUD use is peripartum infection, including Triple I, endomyometritis, and puerperal sepsis. Other contraindications include retained placenta requiring manual or surgical removal, uterine anomalies, and other medical contraindications to IUD use as recommended by the US Medical Eligibility Criteria.14
Does immediate IUD placement affect breastfeeding? There is theoretical risk of decreased milk supply or difficulty breastfeeding with initiation of progestin-only methods of contraception in the immediate postpartum period, as the rapid fall in progesterone levels initiates lactogenesis. However, progestin-only methods appear to have limited effect on initiation and continuation of breastfeeding in the immediate postpartum period.15
There were 2 secondary analyses of a pair of RCTs comparing immediate and delayed postpartum IUD use. Results from Levi and colleagues demonstrated no difference between immediate and interval IUD placement groups in the proportion of women who were breastfeeding at 6, 12, and 24 weeks.16 Chen and colleagues’ study was smaller; researchers found that women with interval IUD placement were more likely to be exclusively breastfeeding and continuing to breastfeed at 6 months compared with the immediate postpartum group.17
To better characterize the impact of progestin implants, in a recent meta-analysis, authors examined the use of subcutaneous levonorgestrel rods and found no difference in breastfeeding initiation and continuation rates between women who had them placed immediately versus 6 ̶ 8 weeks postpartum.12
Benefits of immediate postpartum IUD placement
One benefit of immediate postpartum IUD insertion is a reduction in short-interval pregnancies. In a study by Cohen and colleagues13 of young women aged 13 to 22 years choosing immediate postpartum IUDs (82) or implants (162), the authors found that 61% of women retained their IUDs at 12 months postpartum. Because few requested IUD removal over that time frame, the discontinuation rate at 1 year was primarily due to expulsions. Pregnancy rates at 1 year were 7.6% in the IUD group and 1.5% in the implant group. However, the 7.6% rate in the IUD group was lower than in previously studied adolescent control groups: 18.6% of control adolescents (38 of 204) using a contraceptive form other than a postpartum etonogestrel implant had repeat pregnancy at 1 year.13,18
Not only are patients who receive immediate postpartum IUDs more likely to receive them and continue their use, but they are also satisfied with the experience of receiving the IUD and with the method of contraception. A small mixed methods study of 66 patients demonstrated that women were interested in obtaining immediate postpartum contraception to avoid some of the logistical and financial challenges of returning for a postpartum visit. They also felt that the IUD placement was less painful than expected, and they didn’t feel that the insertion process imposed on their birth experience. Many described relief to know that they had a safe and effective contraceptive method upon leaving the hospital.19 Other studies have shown that even among women who expel an IUD following immediate postpartum placement, many choose to replace it in order to continue it as a contraceptive method.8,9,13
Continue to: Instructions for placement...
Instructions for placement
1. Counsel appropriately. Thoroughly counsel patients regarding their options for postpartum contraception, with emphasis on the benefits, risks, and contraindications. Current recommendations to reduce the risk of expulsion are to place the IUD in the delivery room or operating room within 10 minutes of placental delivery.
2. Post ̶ vaginal delivery. Following vaginal delivery, remove the IUD from the inserter, cut the strings to 10 cm and, using either fingers to grasp the wings of the IUD or ring forceps, advance the IUD to the fundus. Ultrasound guidance may be used, but it does not appear to be helpful in preventing expulsion.20
3. Post ̶ cesarean delivery. Once the placenta is delivered, place the IUD using the inserter or a ring forceps at the fundus and guide the strings into the cervix, then close the hysterotomy.
ACOG does recommend formal trainingbefore placing postpartum IUDs. One resource they provide is a free online webinar (https://www.acog.org/education-and-events/webinars/long-acting-reversible-contra ception-overview-and-hands-on-practice-for-residents).3
CASE 1 Resolved
The patient was counseled in the office about her options, and she was most interested in immediate postpartum LNG-IUD placement. She went on to deliver a healthy baby vaginally at 39 weeks. Within 10 minutes of placental delivery, she received an LNG-IUD. She returned to the office 3 months later for STI screening; her examination revealed correct placement and no evidence of expulsion. She expressed that she was happy with her IUD and thankful that she was able to receive it immediately after the birth of her baby.
CASE 2 Nulliparous woman desires IUD for postpartum contraception
A 33-year-old nulliparous woman presents in the third trimester for a routine prenatal visit. She had used the LNG-IUD prior to getting pregnant and reports that she was very happy with it. She knows she wants to wait at least 2 years before trying to get pregnant again, and she would like to resume contraception as soon as it is reasonably safe to do so. She has read that it is possible to get an IUD immediately postpartum and asks about it as a possible option.
What barriers will she face in obtaining an immediate postpartum IUD?
There are many barriers for patients who may be good candidates for immediate postpartum contraception (TABLE 2). Many patients are unaware that it is a safe option, and they often have concerns about such risks as infection, perforation, and effects on breastfeeding. Additionally, providers may not prioritize adequate counseling about postpartum contraception when they face time constraints and a need to counsel about other pregnancy-related topics during the prenatal visit schedule.7,21

System, hospital, and clinician barriers to immediate postpartum IUD use
Hospital implementation of a successful postpartum IUD program requires pharmacy, intrapartum and postpartum nursing staff, physicians, administration, and billing to be aligned. Hospital administration and pharmacists must stock IUDs in the pharmacy. Hospital nursing staff attitudes toward and knowledge of postpartum contraception can have profound influence on how they discuss safe and effective methods of postpartum contraception with patients who may not have received counseling during prenatal care.22 In a survey of 108 ACOG fellows, nearly 75% of ObGyn physicians did not offer immediate postpartum IUDs; lack of provider training, lack of IUD availability, and concern about cost and payment were found to be common reasons why.21 Additionally, Catholic-affiliated and rural institutions are less likely to offer it, whereas more urban, teaching hospitals are more likely to have programs in place.23 Prior to 2012, immediate postpartum IUD insertions and device costs were part of the global Medicaid obstetric fee in most states, and both hospital systems and individual providers were concerned about loss of revenue.23
In 2015, Washington and colleagues published a decision analysis that examined the cost-effectiveness and cost savings associated with immediate postpartum IUD use. Accounting for expulsion rates, they found that immediate postpartum IUD placement can save $282,540 per 1,000 women over 2 years; additionally, immediate postpartum IUD use can prevent 88 unintended pregnancies per 1,000 women over 2 years.24 Not only do immediate postpartum IUDs have great potential to prevent individual patients from undesired short-interval pregnancies (FIGURE 1), but they can also save the system substantial health care dollars (FIGURE 2).


Overcoming barriers
Immediate postpartum IUD implementation is attainable with practice, policy, and institutional changes. Education and training programs geared toward providers and nursing staff can improve understanding of the benefits and risks of immediate postpartum IUD placement. Additionally, clinicians must provide comprehensive, nondirective counseling during the antepartum period, informing patients of all safe and effective options. Expulsion risks should be disclosed, as well as the benefit of not needing to return for a separate postpartum contraception appointment.
Since 2012, many state Medicaid agencies have decoupled reimbursement for inpatient postpartum IUD insertion from the delivery fee. By 2018, more than half of states adopted this practice. Commercial insurers have followed suit in some cases, and as such, both Medicaid and commercially insured patients have had increased access to immediate postpartum IUDs.23 This has translated into increased uptake of immediate postpartum IUDs among both Medicaid and commercially insured patients. Koch et al conducted a retrospective cohort study comparing IUD use in patients 1 year before and 1 year after the policy changes, and they found a 10-fold increase in use of immediate postpartum IUDs.25
While education, counseling, access, and changes in reimbursement may increase access in many hospital systems, some barriers, such as religious affiliation of the hospital system, may be impossible to overcome. A viable alternative to immediate postpartum IUD placement may be early postpartum IUD placement, which could allow patients to coordinate this procedure with 1- or 2-week return routine postpartum visits for CD recovery, mental health screenings, and/or well-baby visits. More data are necessary before recommending this universally, but Averbach and colleagues published a promising meta-analysis that demonstrated no complete expulsions in studies in which IUDs were placed between 2 and 4 weeks postpartum, and only a pooled partial expulsion rate (of immediate postpartum, early inpatient, early outpatient, and interval placement) of 3.7%.4
CASE 2 Resolved
Although the patient was interested in receiving a postpartum LNG-IUD immediately after her vaginal birth, she had to wait until her 6-week postpartum visit. The hospital did not stock IUDs for immediate postpartum IUD use, and her provider, having not been trained on immediate postpartum insertion, did not feel comfortable trying to place it in the immediate postpartum time frame. ●
- Immediate postpartum IUD insertion is a safe and effective method for postpartum contraception for many postpartum women.
- Immediate postpartum IUD insertion can result in increased uptake of postpartum contraception, a reduction in short interval pregnancies, and the opportunity for patients to plan their ideal family size.
- Patients should be thoroughly counseled about the safety of IUD placement and risks of expulsion associated with immediate postpartum placement.
- Successful programs for immediate postpartum IUD insertion incorporate training for providers on proper insertion techniques, education for nursing staff about safety and counseling, on-site IUD supply, and reimbursement that is decoupled from the payment for delivery.
CASE 1 Multiparous female with short-interval pregnancies desires contraception
A 24-year-old woman (G4P3) presents for a routine prenatal visit in the third trimester. Her last 2 pregnancies have occurred within 3 months of her prior birth. She endorses feeling overwhelmed with having 4 children under the age of 5 years, and she specifies that she would like to avoid another pregnancy for several years. She plans to breast and bottle feed, and she notes that she tends to forget to take pills. When you look back at her prior charts, you note that she did not return for her last 2 postpartum visits. What can you offer her? What would be a safe contraceptive option for her?
Intrauterine devices (IUDs) are safe, effective, and reported by patients to be satisfactory methods of contraception precisely because they are prone to less user error. The Contraceptive Choice Project demonstrated that patients are more apt to choose them when barriers such as cost and access are removed and nondirective counseling is provided.1 Given that unintended pregnancy rates hover around 48%, the American College of Obstetricians and Gynecologists (ACOG) recommends them as first-line methods for pregnancy prevention.2,3
For repeat pregnancies, the postpartum period is an especially vulnerable time—non-breastfeeding women will ovulate as soon as 25 days after birth, and by 8 weeks 30% will have ovulated.4 Approximately 40% to 57% of women report having unprotected intercourse before 6 weeks postpartum, and nearly 70% of all pregnancies in the first year postpartum are unintended.3,5 Furthermore, patients at highest risk for short-interval pregnancy, such as adolescents, are less likely to return for a postpartum visit.3
Short-interval pregnancies confer greater fetal risk, including risks of low-birth weight, preterm birth, small for gestational age and increased risk of neonatal intensive care unit admission.6 Additionally, maternal health may be compromised during a short-interval pregnancy, particularly in medically complex patients due to increased risks of adverse pregnancy outcomes, such as postpartum bleeding or uterine rupture and disease progression.7 A 2006 meta-analysis by Conde-Agudelo and colleagues found that waiting at least 18 months between pregnancies was optimal for reducing these risks.6
Thus, the immediate postpartum period is an optimal time for addressing contraceptive needs and for preventing short-interval and unintended pregnancy. This article aims to provide evidence supporting the use of immediate postpartum IUDs, as well as their associated risks and barriers to use.
IUD types and routes for immediate postpartum insertion
There are several randomized controlled trials (RCTs) that examine the immediate postpartum use of copper IUDs and levonorgestrel-releasing (LNG) IUDs.8-11 In 2010, Chen and colleagues compared placement of the immediate postpartum IUD following vaginal delivery with interval placement at 6–8 weeks postpartum. Of 51 patients enrolled in each arm, 98% received an IUD immediately postpartum, and 90% received one during their postpartum visit. There were 12 expulsions (24%) in the immediate postpartum IUD group, compared with 2 (4.4%) in the interval group. Expelled IUDs were replaced, and at 6 months both groups had similar rates of IUD use.8
Whitaker and colleagues demonstrated similar findings after randomizing a small group of women who had a cesarean delivery (CD) to interval or immediate placement. There were significantly more expulsions in the post-placental group (20%) than the interval group (0%), but there were more users of the IUD in the post-placental group than in the interval group at 12 months.9
Two RCTs, by Lester and colleagues and Levi et al, demonstrated successful placement of the copper IUD or LNG-IUD following CD, with few expulsions (0% and 8%, respectively). Patients who were randomized to immediate postpartum IUD placement were more likely to receive an IUD than those who were randomized to interval insertion, mostly due to lack of postpartum follow up. Both studies followed patients out to 6 months, and rates of IUD continuation and satisfaction were higher at this time in the immediate postpartum IUD groups.10,11
Continue to: Risks, contraindications, and breastfeeding impact...
Risks, contraindications, and breastfeeding impact
What are the risks of immediate postpartum IUD placement? The highest risk of IUD placement in the immediate postpartum period appears to be expulsion (TABLE 1). In a meta-analysis conducted in 2022, which looked at 11 studies of immediate IUD insertion, the rates of expulsion were between 5% and 27%.3,8,12,13 Results of a study by Cohen and colleagues demonstrated that most expulsions occurred within the first 12 weeks following delivery; of those expulsions that occurred, only 11% went unrecognized.13 Immediate postpartum IUD insertion does not increase the IUD-associated risks of perforation, infection, or immediate postpartum bleeding (although prolonged bleeding may be more common).12
Are there contraindications to placing an IUD immediately postpartum? The main contraindication to immediate postpartum IUD use is peripartum infection, including Triple I, endomyometritis, and puerperal sepsis. Other contraindications include retained placenta requiring manual or surgical removal, uterine anomalies, and other medical contraindications to IUD use as recommended by the US Medical Eligibility Criteria.14
Does immediate IUD placement affect breastfeeding? There is theoretical risk of decreased milk supply or difficulty breastfeeding with initiation of progestin-only methods of contraception in the immediate postpartum period, as the rapid fall in progesterone levels initiates lactogenesis. However, progestin-only methods appear to have limited effect on initiation and continuation of breastfeeding in the immediate postpartum period.15
There were 2 secondary analyses of a pair of RCTs comparing immediate and delayed postpartum IUD use. Results from Levi and colleagues demonstrated no difference between immediate and interval IUD placement groups in the proportion of women who were breastfeeding at 6, 12, and 24 weeks.16 Chen and colleagues’ study was smaller; researchers found that women with interval IUD placement were more likely to be exclusively breastfeeding and continuing to breastfeed at 6 months compared with the immediate postpartum group.17
To better characterize the impact of progestin implants, in a recent meta-analysis, authors examined the use of subcutaneous levonorgestrel rods and found no difference in breastfeeding initiation and continuation rates between women who had them placed immediately versus 6 ̶ 8 weeks postpartum.12
Benefits of immediate postpartum IUD placement
One benefit of immediate postpartum IUD insertion is a reduction in short-interval pregnancies. In a study by Cohen and colleagues13 of young women aged 13 to 22 years choosing immediate postpartum IUDs (82) or implants (162), the authors found that 61% of women retained their IUDs at 12 months postpartum. Because few requested IUD removal over that time frame, the discontinuation rate at 1 year was primarily due to expulsions. Pregnancy rates at 1 year were 7.6% in the IUD group and 1.5% in the implant group. However, the 7.6% rate in the IUD group was lower than in previously studied adolescent control groups: 18.6% of control adolescents (38 of 204) using a contraceptive form other than a postpartum etonogestrel implant had repeat pregnancy at 1 year.13,18
Not only are patients who receive immediate postpartum IUDs more likely to receive them and continue their use, but they are also satisfied with the experience of receiving the IUD and with the method of contraception. A small mixed methods study of 66 patients demonstrated that women were interested in obtaining immediate postpartum contraception to avoid some of the logistical and financial challenges of returning for a postpartum visit. They also felt that the IUD placement was less painful than expected, and they didn’t feel that the insertion process imposed on their birth experience. Many described relief to know that they had a safe and effective contraceptive method upon leaving the hospital.19 Other studies have shown that even among women who expel an IUD following immediate postpartum placement, many choose to replace it in order to continue it as a contraceptive method.8,9,13
Continue to: Instructions for placement...
Instructions for placement
1. Counsel appropriately. Thoroughly counsel patients regarding their options for postpartum contraception, with emphasis on the benefits, risks, and contraindications. Current recommendations to reduce the risk of expulsion are to place the IUD in the delivery room or operating room within 10 minutes of placental delivery.
2. Post ̶ vaginal delivery. Following vaginal delivery, remove the IUD from the inserter, cut the strings to 10 cm and, using either fingers to grasp the wings of the IUD or ring forceps, advance the IUD to the fundus. Ultrasound guidance may be used, but it does not appear to be helpful in preventing expulsion.20
3. Post ̶ cesarean delivery. Once the placenta is delivered, place the IUD using the inserter or a ring forceps at the fundus and guide the strings into the cervix, then close the hysterotomy.
ACOG does recommend formal trainingbefore placing postpartum IUDs. One resource they provide is a free online webinar (https://www.acog.org/education-and-events/webinars/long-acting-reversible-contra ception-overview-and-hands-on-practice-for-residents).3
CASE 1 Resolved
The patient was counseled in the office about her options, and she was most interested in immediate postpartum LNG-IUD placement. She went on to deliver a healthy baby vaginally at 39 weeks. Within 10 minutes of placental delivery, she received an LNG-IUD. She returned to the office 3 months later for STI screening; her examination revealed correct placement and no evidence of expulsion. She expressed that she was happy with her IUD and thankful that she was able to receive it immediately after the birth of her baby.
CASE 2 Nulliparous woman desires IUD for postpartum contraception
A 33-year-old nulliparous woman presents in the third trimester for a routine prenatal visit. She had used the LNG-IUD prior to getting pregnant and reports that she was very happy with it. She knows she wants to wait at least 2 years before trying to get pregnant again, and she would like to resume contraception as soon as it is reasonably safe to do so. She has read that it is possible to get an IUD immediately postpartum and asks about it as a possible option.
What barriers will she face in obtaining an immediate postpartum IUD?
There are many barriers for patients who may be good candidates for immediate postpartum contraception (TABLE 2). Many patients are unaware that it is a safe option, and they often have concerns about such risks as infection, perforation, and effects on breastfeeding. Additionally, providers may not prioritize adequate counseling about postpartum contraception when they face time constraints and a need to counsel about other pregnancy-related topics during the prenatal visit schedule.7,21

System, hospital, and clinician barriers to immediate postpartum IUD use
Hospital implementation of a successful postpartum IUD program requires pharmacy, intrapartum and postpartum nursing staff, physicians, administration, and billing to be aligned. Hospital administration and pharmacists must stock IUDs in the pharmacy. Hospital nursing staff attitudes toward and knowledge of postpartum contraception can have profound influence on how they discuss safe and effective methods of postpartum contraception with patients who may not have received counseling during prenatal care.22 In a survey of 108 ACOG fellows, nearly 75% of ObGyn physicians did not offer immediate postpartum IUDs; lack of provider training, lack of IUD availability, and concern about cost and payment were found to be common reasons why.21 Additionally, Catholic-affiliated and rural institutions are less likely to offer it, whereas more urban, teaching hospitals are more likely to have programs in place.23 Prior to 2012, immediate postpartum IUD insertions and device costs were part of the global Medicaid obstetric fee in most states, and both hospital systems and individual providers were concerned about loss of revenue.23
In 2015, Washington and colleagues published a decision analysis that examined the cost-effectiveness and cost savings associated with immediate postpartum IUD use. Accounting for expulsion rates, they found that immediate postpartum IUD placement can save $282,540 per 1,000 women over 2 years; additionally, immediate postpartum IUD use can prevent 88 unintended pregnancies per 1,000 women over 2 years.24 Not only do immediate postpartum IUDs have great potential to prevent individual patients from undesired short-interval pregnancies (FIGURE 1), but they can also save the system substantial health care dollars (FIGURE 2).


Overcoming barriers
Immediate postpartum IUD implementation is attainable with practice, policy, and institutional changes. Education and training programs geared toward providers and nursing staff can improve understanding of the benefits and risks of immediate postpartum IUD placement. Additionally, clinicians must provide comprehensive, nondirective counseling during the antepartum period, informing patients of all safe and effective options. Expulsion risks should be disclosed, as well as the benefit of not needing to return for a separate postpartum contraception appointment.
Since 2012, many state Medicaid agencies have decoupled reimbursement for inpatient postpartum IUD insertion from the delivery fee. By 2018, more than half of states adopted this practice. Commercial insurers have followed suit in some cases, and as such, both Medicaid and commercially insured patients have had increased access to immediate postpartum IUDs.23 This has translated into increased uptake of immediate postpartum IUDs among both Medicaid and commercially insured patients. Koch et al conducted a retrospective cohort study comparing IUD use in patients 1 year before and 1 year after the policy changes, and they found a 10-fold increase in use of immediate postpartum IUDs.25
While education, counseling, access, and changes in reimbursement may increase access in many hospital systems, some barriers, such as religious affiliation of the hospital system, may be impossible to overcome. A viable alternative to immediate postpartum IUD placement may be early postpartum IUD placement, which could allow patients to coordinate this procedure with 1- or 2-week return routine postpartum visits for CD recovery, mental health screenings, and/or well-baby visits. More data are necessary before recommending this universally, but Averbach and colleagues published a promising meta-analysis that demonstrated no complete expulsions in studies in which IUDs were placed between 2 and 4 weeks postpartum, and only a pooled partial expulsion rate (of immediate postpartum, early inpatient, early outpatient, and interval placement) of 3.7%.4
CASE 2 Resolved
Although the patient was interested in receiving a postpartum LNG-IUD immediately after her vaginal birth, she had to wait until her 6-week postpartum visit. The hospital did not stock IUDs for immediate postpartum IUD use, and her provider, having not been trained on immediate postpartum insertion, did not feel comfortable trying to place it in the immediate postpartum time frame. ●
- Immediate postpartum IUD insertion is a safe and effective method for postpartum contraception for many postpartum women.
- Immediate postpartum IUD insertion can result in increased uptake of postpartum contraception, a reduction in short interval pregnancies, and the opportunity for patients to plan their ideal family size.
- Patients should be thoroughly counseled about the safety of IUD placement and risks of expulsion associated with immediate postpartum placement.
- Successful programs for immediate postpartum IUD insertion incorporate training for providers on proper insertion techniques, education for nursing staff about safety and counseling, on-site IUD supply, and reimbursement that is decoupled from the payment for delivery.
- Winner B, Peipert JF, Zhao Q, et al. Effectiveness of longacting reversible contraception. N Engl J Med. 2012;366:19982007. doi: 10.1056/NEJMoa1110855.
- Bearak J, Popinchalk A, Ganatra B, et al. Unintended pregnancy and abortion by income, region, and the legal status of abortion: estimates from a comprehensive model for 1990-2019. Lancet Glob Health. 2020;8:e1152-e1161. doi: 10.1016/S2214-109X(20)30315-6.
- American College of Obstetricians and Gynecologists’ Committee on Obstetric Practice. Committee Opinion No. 670: Immediate postpartum long-acting reversible contraception. Obstet Gynecol. 2016;128:e32-e37. doi: 10.1097/AOG.0000000000001587.
- Averbach SH, Ermias Y, Jeng G, et al. Expulsion of intrauterine devices after postpartum placement by timing of placement, delivery type, and intrauterine device type: a systematic review and meta-analysis. Am J Obstet Gynecol. 2020;223:177188. doi: 10.1016/j.ajog.2020.02.045.
- Connolly A, Thorp J, Pahel L. Effects of pregnancy and childbirth on postpartum sexual function: a longitudinal prospective study. Int Urogynecol J Pelvic Floor Dysfunct. 2005;16:263-267. doi: 10.1007/s00192-005-1293-6.
- Conde-Agudelo A, Rosas-Bermúdez A, Kafury-Goeta AC. Birth spacing and risk of adverse perinatal outcomes: a meta-analysis. JAMA. 2006;295:1809-1823. doi: 10.1001 /jama.295.15.1809.
- Vricella LK, Gawron LM, Louis JM. Society for MaternalFetal Medicine (SMFM) Consult Series #48: Immediate postpartum long-acting reversible contraception for women at high risk for medical complications. Am J Obstet Gynecol. 2019;220:B2-B12. doi: 10.1016/j.ajog.2019.02.011.
- Chen BA, Reeves MF, Hayes JL, et al. Postplacental or delayed insertion of the levonorgestrel intrauterine device after vaginal delivery: a randomized controlled trial. Obstet Gynecol. 2010;116:1079-1087. doi: 10.1097/AOG.0b013e3181f73fac.
- Whitaker AK, Endres LK, Mistretta SQ, et al. Postplacental insertion of the levonorgestrel intrauterine device after cesarean delivery vs. delayed insertion: a randomized controlled trial. Contraception. 2014;89:534-539. doi: 10.1016/j.contraception.2013.12.007.
- Lester F, Kakaire O, Byamugisha J, et al. Intracesarean insertion of the Copper T380A versus 6 weeks postcesarean: a randomized clinical trial. Contraception. 2015;91:198-203. doi: 10.1016/j.contraception.2014.12.002.
- Levi EE, Stuart GS, Zerden ML, et al. Intrauterine device placement during cesarean delivery and continued use 6 months postpartum: a randomized controlled trial. Obstet Gynecol. 2015;126:5-11. doi: 10.1097/AOG.0000000000000882.
- Sothornwit J, Kaewrudee S, Lumbiganon P, et al. Immediate versus delayed postpartum insertion of contraceptive implant and IUD for contraception. Cochrane Database Syst Rev. 2022;10:CD011913. doi: 10.1002/14651858.CD011913.pub3.
- Cohen R, Sheeder J, Arango N, et al. Twelve-month contraceptive continuation and repeat pregnancy among young mothers choosing postdelivery contraceptive implants or postplacental intrauterine devices. Contraception. 2016;93:178-183. doi: 10.1016/j.contraception.2015.10.001.
- Centers for Disease Control and Prevention (CDC). US Medical Eligibility Criteria for Contraceptive Use, 2010. MMWR Recomm Rep. 2010;59(RR-4):1-86.
- Kapp N, Curtis K, Nanda K. Progestogen-only contraceptive use among breastfeeding women: a systematic review. Contraception. 2010;82:17-37. doi: 10.1016 /j.contraception.2010.02.002.
- Levi EE, Findley MK, Avila K, et al. Placement of levonorgestrel intrauterine device at the time of cesarean delivery and the effect on breastfeeding duration. Breastfeed Med. 2018;13:674679. doi: 10.1089/bfm.2018.0060.
- Chen BA, Reeves MF, Creinin MD, et al. Postplacental or delayed levonorgestrel intrauterine device insertion and breast-feeding duration. Contraception. 2011;84:499-504. doi: 10.1016/j.contraception.2011.01.022.
- Tocce KM, Sheeder JL, Teal SB. Rapid repeat pregnancy in adolescents: do immediate postpartum contraceptive implants make a difference? Am J Obstet Gynecol. 2012;206:481.e1-7. doi: 10.1016/j.ajog.2012.04.015.
- Carr SL, Singh RH, Sussman AL, et al. Women’s experiences with immediate postpartum intrauterine device insertion: a mixed-methods study. Contraception. 2018;97:219-226. doi: 10.1016/j.contraception.2017.10.008.
- Martinez OP, Wilder L, Seal P. Ultrasound-guided compared with non-ultrasound-Guided placement of immediate postpartum intrauterine contraceptive devices. Obstet Gynecol. 2022;140:91-93. doi: 10.1097/AOG.0000000000004828.
- Holden EC, Lai E, Morelli SS, et al. Ongoing barriers to immediate postpartum long-acting reversible contraception: a physician survey. Contracept Reprod Med. 2018;3:23. doi: 10.1186/s40834-018-0078-5.
- Benfield N, Hawkins F, Ray L, et al. Exposure to routine availability of immediate postpartum LARC: effect on attitudes and practices of labor and delivery and postpartum nurses. Contraception. 2018;97:411-414. doi: 10.1016 /j.contraception.2018.01.017.
- Steenland MW, Vatsa R, Pace LE, et al. Immediate postpartum long-acting reversible contraceptive use following statespecific changes in hospital Medicaid reimbursement. JAMA Netw Open. 2022;5:e2237918. doi: 10.1001 /jamanetworkopen.2022.37918.
- Washington CI, Jamshidi R, Thung SF, et al. Timing of postpartum intrauterine device placement: a costeffectiveness analysis. Fertil Steril. 2015;103:131-137. doi: 10.1016/j.fertnstert.2014.09.032
- Winner B, Peipert JF, Zhao Q, et al. Effectiveness of longacting reversible contraception. N Engl J Med. 2012;366:19982007. doi: 10.1056/NEJMoa1110855.
- Bearak J, Popinchalk A, Ganatra B, et al. Unintended pregnancy and abortion by income, region, and the legal status of abortion: estimates from a comprehensive model for 1990-2019. Lancet Glob Health. 2020;8:e1152-e1161. doi: 10.1016/S2214-109X(20)30315-6.
- American College of Obstetricians and Gynecologists’ Committee on Obstetric Practice. Committee Opinion No. 670: Immediate postpartum long-acting reversible contraception. Obstet Gynecol. 2016;128:e32-e37. doi: 10.1097/AOG.0000000000001587.
- Averbach SH, Ermias Y, Jeng G, et al. Expulsion of intrauterine devices after postpartum placement by timing of placement, delivery type, and intrauterine device type: a systematic review and meta-analysis. Am J Obstet Gynecol. 2020;223:177188. doi: 10.1016/j.ajog.2020.02.045.
- Connolly A, Thorp J, Pahel L. Effects of pregnancy and childbirth on postpartum sexual function: a longitudinal prospective study. Int Urogynecol J Pelvic Floor Dysfunct. 2005;16:263-267. doi: 10.1007/s00192-005-1293-6.
- Conde-Agudelo A, Rosas-Bermúdez A, Kafury-Goeta AC. Birth spacing and risk of adverse perinatal outcomes: a meta-analysis. JAMA. 2006;295:1809-1823. doi: 10.1001 /jama.295.15.1809.
- Vricella LK, Gawron LM, Louis JM. Society for MaternalFetal Medicine (SMFM) Consult Series #48: Immediate postpartum long-acting reversible contraception for women at high risk for medical complications. Am J Obstet Gynecol. 2019;220:B2-B12. doi: 10.1016/j.ajog.2019.02.011.
- Chen BA, Reeves MF, Hayes JL, et al. Postplacental or delayed insertion of the levonorgestrel intrauterine device after vaginal delivery: a randomized controlled trial. Obstet Gynecol. 2010;116:1079-1087. doi: 10.1097/AOG.0b013e3181f73fac.
- Whitaker AK, Endres LK, Mistretta SQ, et al. Postplacental insertion of the levonorgestrel intrauterine device after cesarean delivery vs. delayed insertion: a randomized controlled trial. Contraception. 2014;89:534-539. doi: 10.1016/j.contraception.2013.12.007.
- Lester F, Kakaire O, Byamugisha J, et al. Intracesarean insertion of the Copper T380A versus 6 weeks postcesarean: a randomized clinical trial. Contraception. 2015;91:198-203. doi: 10.1016/j.contraception.2014.12.002.
- Levi EE, Stuart GS, Zerden ML, et al. Intrauterine device placement during cesarean delivery and continued use 6 months postpartum: a randomized controlled trial. Obstet Gynecol. 2015;126:5-11. doi: 10.1097/AOG.0000000000000882.
- Sothornwit J, Kaewrudee S, Lumbiganon P, et al. Immediate versus delayed postpartum insertion of contraceptive implant and IUD for contraception. Cochrane Database Syst Rev. 2022;10:CD011913. doi: 10.1002/14651858.CD011913.pub3.
- Cohen R, Sheeder J, Arango N, et al. Twelve-month contraceptive continuation and repeat pregnancy among young mothers choosing postdelivery contraceptive implants or postplacental intrauterine devices. Contraception. 2016;93:178-183. doi: 10.1016/j.contraception.2015.10.001.
- Centers for Disease Control and Prevention (CDC). US Medical Eligibility Criteria for Contraceptive Use, 2010. MMWR Recomm Rep. 2010;59(RR-4):1-86.
- Kapp N, Curtis K, Nanda K. Progestogen-only contraceptive use among breastfeeding women: a systematic review. Contraception. 2010;82:17-37. doi: 10.1016 /j.contraception.2010.02.002.
- Levi EE, Findley MK, Avila K, et al. Placement of levonorgestrel intrauterine device at the time of cesarean delivery and the effect on breastfeeding duration. Breastfeed Med. 2018;13:674679. doi: 10.1089/bfm.2018.0060.
- Chen BA, Reeves MF, Creinin MD, et al. Postplacental or delayed levonorgestrel intrauterine device insertion and breast-feeding duration. Contraception. 2011;84:499-504. doi: 10.1016/j.contraception.2011.01.022.
- Tocce KM, Sheeder JL, Teal SB. Rapid repeat pregnancy in adolescents: do immediate postpartum contraceptive implants make a difference? Am J Obstet Gynecol. 2012;206:481.e1-7. doi: 10.1016/j.ajog.2012.04.015.
- Carr SL, Singh RH, Sussman AL, et al. Women’s experiences with immediate postpartum intrauterine device insertion: a mixed-methods study. Contraception. 2018;97:219-226. doi: 10.1016/j.contraception.2017.10.008.
- Martinez OP, Wilder L, Seal P. Ultrasound-guided compared with non-ultrasound-Guided placement of immediate postpartum intrauterine contraceptive devices. Obstet Gynecol. 2022;140:91-93. doi: 10.1097/AOG.0000000000004828.
- Holden EC, Lai E, Morelli SS, et al. Ongoing barriers to immediate postpartum long-acting reversible contraception: a physician survey. Contracept Reprod Med. 2018;3:23. doi: 10.1186/s40834-018-0078-5.
- Benfield N, Hawkins F, Ray L, et al. Exposure to routine availability of immediate postpartum LARC: effect on attitudes and practices of labor and delivery and postpartum nurses. Contraception. 2018;97:411-414. doi: 10.1016 /j.contraception.2018.01.017.
- Steenland MW, Vatsa R, Pace LE, et al. Immediate postpartum long-acting reversible contraceptive use following statespecific changes in hospital Medicaid reimbursement. JAMA Netw Open. 2022;5:e2237918. doi: 10.1001 /jamanetworkopen.2022.37918.
- Washington CI, Jamshidi R, Thung SF, et al. Timing of postpartum intrauterine device placement: a costeffectiveness analysis. Fertil Steril. 2015;103:131-137. doi: 10.1016/j.fertnstert.2014.09.032