User login
Real-time CGM plus insulin pump best for type 1 diabetes
Youth with type 1 diabetes who use real-time continuous glucose monitoring (rtCGM) and an insulin pump spend more time in target glucose range than do those using intermittently scanned CGM (isCGM) and/or multiple daily insulin injections, new data show.
In the multinational cohort study of more than 4,500 people younger than age 21 with type 1 diabetes, those using rtCGM and pumps also spent less time above and below glucose targets and had fewer severe adverse events – either severe hypoglycemia or diabetic ketoacidosis (DKA) – compared with injections and isCGM.
The findings were published online in JAMA Network Open by Klemen Dovc, MD, PhD, assistant professor in the department of pediatric endocrinology, diabetes, and metabolic diseases, University Children’s Hospital, Ljubljana, Slovenia, and colleagues.
“These results underscore the synergistic effect of advanced diabetes technologies that should be more readily available to youths with type 1 diabetes for further improvement of diabetes-related clinical outcomes,” the authors wrote.
Moreover, Dr. Dovc told this news organization: “Clinicians should be aware that there may be differences in effectiveness between different types of devices, and that choosing the right device for each individual may be important for achieving optimal outcomes.”
Real-time CGM + insulin pump = highest time in range
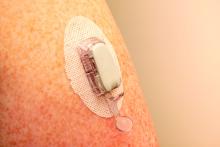
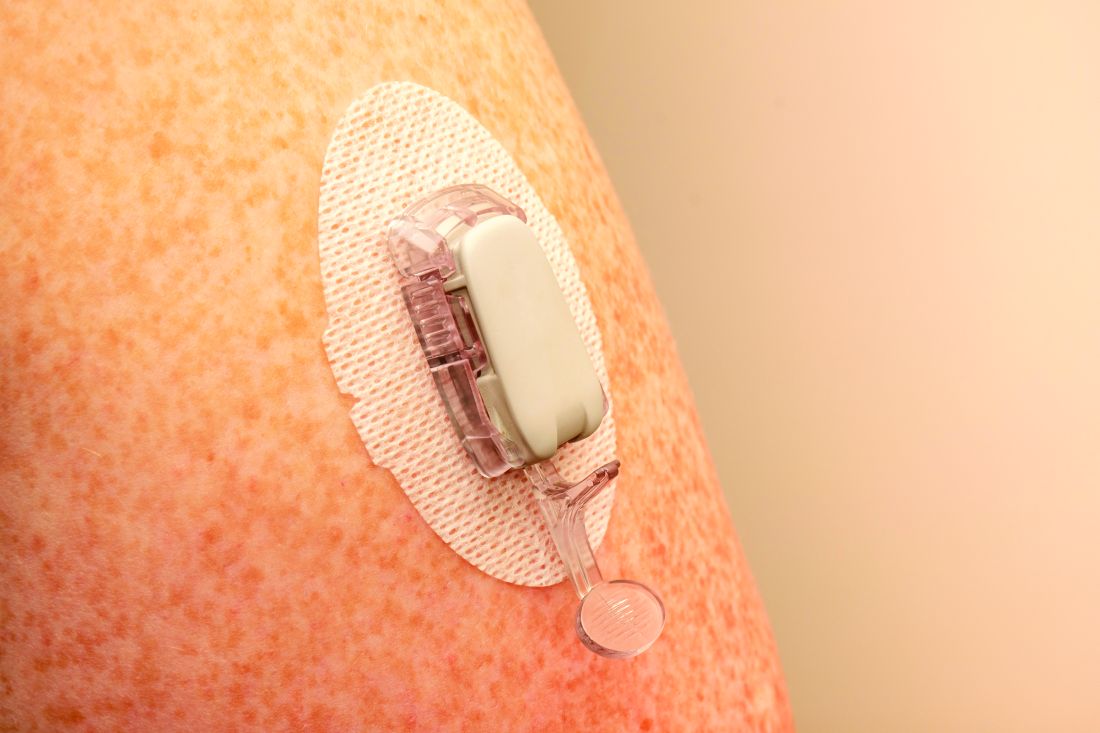
The researchers explained that two modalities of CGM are broadly available: rtCGM, which continuously displays glucose concentration in the interstitial fluid (usually at intervals of 1-5 minutes) on a dedicated receiver or other portable device, such as a smartphone, and provides various adjustable alarms, and isCGM, which displays data on demand when the transmitter is scanned using either a dedicated reader or smartphone-based application.
rtCGMs include devices from Dexcom and Medtronic. The isCGM, or “flash,” generally refers to the Abbott FreeStyle Libre.
The study included individuals younger than 21 years from 34 centers in 21 countries in the SWEET registry, a worldwide network of diabetes care centers for youth, between Jan. 1, 2016, and Dec. 31, 2021.
The researchers didn’t report which particular devices were used in the trial, rather they just divided patients into four groups: 850 used isCGM with a pump, 1,231 used isCGM with multiple daily injections, 2,252 used rtCGM with a pump, and 886 used rtCGM with insulin injections.
After adjustments for sex, age, diabetes duration, and body mass index standard deviation score, rtCGM plus insulin pump was the most likely group to achieve the recommended greater than 70% time in target glycemic range (70-180 mg/dL), with 36.2% achieving it, followed by rtCGM plus injections, at 20.9%, and isCGM plus injections, at 12.5%. Those using isCGM with an insulin pump were the least likely to achieve time in range, at just 11.3%.
Similar trends were seen for the recommended goal of less than 4% of time spent below range (< 70 mg/dL) and less than 25% of time spent above range (> 180 mg/dL). Those using rtCGM with a pump had the highest proportions achieving both of those goals, 73.1% and 32.5%, respectively.
The use of rtCGM, with or without a pump, was associated with lower rates of severe hypoglycemia (2.5% and 2.0%, respectively) than isCGM with or without a pump (5.5% and 5.2%, respectively).
Similarly, the proportion experiencing at least one DKA episode varied from 1.4% for rtCGM plus insulin pump and 0.7% for rtCGM plus injections to 3.0% for isCGM plus pump and 1.5% isCGM plus injections.
Study looked at older technology but results still reflect benefit
Among the rtCGM plus insulin pump group were 264 participants (5% of the total study population) recorded in the database as using automated insulin delivery (AID) systems, also known as the artificial pancreas, although this is likely an undercount as the presence of communication between the two devices was not automatically recorded, Dr. Dovc explained.
Those individuals recorded as using AIDs had a higher unadjusted time in range compared with non-AID users (66.3% vs. 59.0%) and lower time above range (30.1% vs. 37.0%) but didn’t differ in time below range (2.9% vs. 3.0%).
Dr. Dovc told this news organization: “While automated systems are becoming more common, there are still many individuals who do not have access to glucose-responsive devices.” Reasons include lack of reimbursement, or decisions not to use them, he said.
But, he added, “Despite the low reported numbers of AID users, results achieved in the pump with real-time CGM [group] are admirable and approaching recommended consensus targets with a clinically meaningful difference towards all other treatment modalities. As our findings may not be directly applicable to all participants using automated systems, they may still provide useful insights into the factors that influence glycemic control.”
Similarly, the intermittently scanned CGMs used by most in the study, and particularly in the earlier period, didn’t have low- or high-glucose alarms as do later versions. And an even more recent version also doesn’t require scanning either, so is essentially also “real-time.”
Dr. Dovc noted, “in the first half of our observational period only first generation of intermittently-scanned CGM was generally available, and we can speculate that only a small proportion started to use second generation towards the end of our observational period. The exact number of second-generation users was not available in this analysis.”
He acknowledged that because the study was observational and not randomized, patient choice of device could have influenced the outcomes.
“For example, participants who choose to use a more expensive device may have more resources or support available to them, which could influence their ability to manage their diabetes effectively. Additionally, individuals who choose to use a particular device may be more motivated or engaged in their diabetes care, which could also impact their outcomes. It would be important for future studies to explore the impact of device selection on device effectiveness and to control for this potential confounding factor in the analysis.”
This study was supported by the international Better Control in Pediatric and Adolescent Diabetes: Working to Create Centers of Reference (SWEET) corporate members, including Abbott Laboratories, Boehringer Ingelheim, Dexcom, Insulet, Eli Lilly, Medtronic, Sanofi, and the Slovenian National Research Agency. Dr. Dovc disclosed ties with Abbott Laboratories, Medtronic, Novo Nordisk, Eli Lilly, and Pfizer. He served as a member of the European Commission Expert Panel for Medical Devices for Endocrinology and Diabetes.
A version of this article originally appeared on Medscape.com.
Youth with type 1 diabetes who use real-time continuous glucose monitoring (rtCGM) and an insulin pump spend more time in target glucose range than do those using intermittently scanned CGM (isCGM) and/or multiple daily insulin injections, new data show.
In the multinational cohort study of more than 4,500 people younger than age 21 with type 1 diabetes, those using rtCGM and pumps also spent less time above and below glucose targets and had fewer severe adverse events – either severe hypoglycemia or diabetic ketoacidosis (DKA) – compared with injections and isCGM.
The findings were published online in JAMA Network Open by Klemen Dovc, MD, PhD, assistant professor in the department of pediatric endocrinology, diabetes, and metabolic diseases, University Children’s Hospital, Ljubljana, Slovenia, and colleagues.
“These results underscore the synergistic effect of advanced diabetes technologies that should be more readily available to youths with type 1 diabetes for further improvement of diabetes-related clinical outcomes,” the authors wrote.
Moreover, Dr. Dovc told this news organization: “Clinicians should be aware that there may be differences in effectiveness between different types of devices, and that choosing the right device for each individual may be important for achieving optimal outcomes.”
Real-time CGM + insulin pump = highest time in range
The researchers explained that two modalities of CGM are broadly available: rtCGM, which continuously displays glucose concentration in the interstitial fluid (usually at intervals of 1-5 minutes) on a dedicated receiver or other portable device, such as a smartphone, and provides various adjustable alarms, and isCGM, which displays data on demand when the transmitter is scanned using either a dedicated reader or smartphone-based application.
rtCGMs include devices from Dexcom and Medtronic. The isCGM, or “flash,” generally refers to the Abbott FreeStyle Libre.
The study included individuals younger than 21 years from 34 centers in 21 countries in the SWEET registry, a worldwide network of diabetes care centers for youth, between Jan. 1, 2016, and Dec. 31, 2021.
The researchers didn’t report which particular devices were used in the trial, rather they just divided patients into four groups: 850 used isCGM with a pump, 1,231 used isCGM with multiple daily injections, 2,252 used rtCGM with a pump, and 886 used rtCGM with insulin injections.
After adjustments for sex, age, diabetes duration, and body mass index standard deviation score, rtCGM plus insulin pump was the most likely group to achieve the recommended greater than 70% time in target glycemic range (70-180 mg/dL), with 36.2% achieving it, followed by rtCGM plus injections, at 20.9%, and isCGM plus injections, at 12.5%. Those using isCGM with an insulin pump were the least likely to achieve time in range, at just 11.3%.
Similar trends were seen for the recommended goal of less than 4% of time spent below range (< 70 mg/dL) and less than 25% of time spent above range (> 180 mg/dL). Those using rtCGM with a pump had the highest proportions achieving both of those goals, 73.1% and 32.5%, respectively.
The use of rtCGM, with or without a pump, was associated with lower rates of severe hypoglycemia (2.5% and 2.0%, respectively) than isCGM with or without a pump (5.5% and 5.2%, respectively).
Similarly, the proportion experiencing at least one DKA episode varied from 1.4% for rtCGM plus insulin pump and 0.7% for rtCGM plus injections to 3.0% for isCGM plus pump and 1.5% isCGM plus injections.
Study looked at older technology but results still reflect benefit
Among the rtCGM plus insulin pump group were 264 participants (5% of the total study population) recorded in the database as using automated insulin delivery (AID) systems, also known as the artificial pancreas, although this is likely an undercount as the presence of communication between the two devices was not automatically recorded, Dr. Dovc explained.
Those individuals recorded as using AIDs had a higher unadjusted time in range compared with non-AID users (66.3% vs. 59.0%) and lower time above range (30.1% vs. 37.0%) but didn’t differ in time below range (2.9% vs. 3.0%).
Dr. Dovc told this news organization: “While automated systems are becoming more common, there are still many individuals who do not have access to glucose-responsive devices.” Reasons include lack of reimbursement, or decisions not to use them, he said.
But, he added, “Despite the low reported numbers of AID users, results achieved in the pump with real-time CGM [group] are admirable and approaching recommended consensus targets with a clinically meaningful difference towards all other treatment modalities. As our findings may not be directly applicable to all participants using automated systems, they may still provide useful insights into the factors that influence glycemic control.”
Similarly, the intermittently scanned CGMs used by most in the study, and particularly in the earlier period, didn’t have low- or high-glucose alarms as do later versions. And an even more recent version also doesn’t require scanning either, so is essentially also “real-time.”
Dr. Dovc noted, “in the first half of our observational period only first generation of intermittently-scanned CGM was generally available, and we can speculate that only a small proportion started to use second generation towards the end of our observational period. The exact number of second-generation users was not available in this analysis.”
He acknowledged that because the study was observational and not randomized, patient choice of device could have influenced the outcomes.
“For example, participants who choose to use a more expensive device may have more resources or support available to them, which could influence their ability to manage their diabetes effectively. Additionally, individuals who choose to use a particular device may be more motivated or engaged in their diabetes care, which could also impact their outcomes. It would be important for future studies to explore the impact of device selection on device effectiveness and to control for this potential confounding factor in the analysis.”
This study was supported by the international Better Control in Pediatric and Adolescent Diabetes: Working to Create Centers of Reference (SWEET) corporate members, including Abbott Laboratories, Boehringer Ingelheim, Dexcom, Insulet, Eli Lilly, Medtronic, Sanofi, and the Slovenian National Research Agency. Dr. Dovc disclosed ties with Abbott Laboratories, Medtronic, Novo Nordisk, Eli Lilly, and Pfizer. He served as a member of the European Commission Expert Panel for Medical Devices for Endocrinology and Diabetes.
A version of this article originally appeared on Medscape.com.
Youth with type 1 diabetes who use real-time continuous glucose monitoring (rtCGM) and an insulin pump spend more time in target glucose range than do those using intermittently scanned CGM (isCGM) and/or multiple daily insulin injections, new data show.
In the multinational cohort study of more than 4,500 people younger than age 21 with type 1 diabetes, those using rtCGM and pumps also spent less time above and below glucose targets and had fewer severe adverse events – either severe hypoglycemia or diabetic ketoacidosis (DKA) – compared with injections and isCGM.
The findings were published online in JAMA Network Open by Klemen Dovc, MD, PhD, assistant professor in the department of pediatric endocrinology, diabetes, and metabolic diseases, University Children’s Hospital, Ljubljana, Slovenia, and colleagues.
“These results underscore the synergistic effect of advanced diabetes technologies that should be more readily available to youths with type 1 diabetes for further improvement of diabetes-related clinical outcomes,” the authors wrote.
Moreover, Dr. Dovc told this news organization: “Clinicians should be aware that there may be differences in effectiveness between different types of devices, and that choosing the right device for each individual may be important for achieving optimal outcomes.”
Real-time CGM + insulin pump = highest time in range
The researchers explained that two modalities of CGM are broadly available: rtCGM, which continuously displays glucose concentration in the interstitial fluid (usually at intervals of 1-5 minutes) on a dedicated receiver or other portable device, such as a smartphone, and provides various adjustable alarms, and isCGM, which displays data on demand when the transmitter is scanned using either a dedicated reader or smartphone-based application.
rtCGMs include devices from Dexcom and Medtronic. The isCGM, or “flash,” generally refers to the Abbott FreeStyle Libre.
The study included individuals younger than 21 years from 34 centers in 21 countries in the SWEET registry, a worldwide network of diabetes care centers for youth, between Jan. 1, 2016, and Dec. 31, 2021.
The researchers didn’t report which particular devices were used in the trial, rather they just divided patients into four groups: 850 used isCGM with a pump, 1,231 used isCGM with multiple daily injections, 2,252 used rtCGM with a pump, and 886 used rtCGM with insulin injections.
After adjustments for sex, age, diabetes duration, and body mass index standard deviation score, rtCGM plus insulin pump was the most likely group to achieve the recommended greater than 70% time in target glycemic range (70-180 mg/dL), with 36.2% achieving it, followed by rtCGM plus injections, at 20.9%, and isCGM plus injections, at 12.5%. Those using isCGM with an insulin pump were the least likely to achieve time in range, at just 11.3%.
Similar trends were seen for the recommended goal of less than 4% of time spent below range (< 70 mg/dL) and less than 25% of time spent above range (> 180 mg/dL). Those using rtCGM with a pump had the highest proportions achieving both of those goals, 73.1% and 32.5%, respectively.
The use of rtCGM, with or without a pump, was associated with lower rates of severe hypoglycemia (2.5% and 2.0%, respectively) than isCGM with or without a pump (5.5% and 5.2%, respectively).
Similarly, the proportion experiencing at least one DKA episode varied from 1.4% for rtCGM plus insulin pump and 0.7% for rtCGM plus injections to 3.0% for isCGM plus pump and 1.5% isCGM plus injections.
Study looked at older technology but results still reflect benefit
Among the rtCGM plus insulin pump group were 264 participants (5% of the total study population) recorded in the database as using automated insulin delivery (AID) systems, also known as the artificial pancreas, although this is likely an undercount as the presence of communication between the two devices was not automatically recorded, Dr. Dovc explained.
Those individuals recorded as using AIDs had a higher unadjusted time in range compared with non-AID users (66.3% vs. 59.0%) and lower time above range (30.1% vs. 37.0%) but didn’t differ in time below range (2.9% vs. 3.0%).
Dr. Dovc told this news organization: “While automated systems are becoming more common, there are still many individuals who do not have access to glucose-responsive devices.” Reasons include lack of reimbursement, or decisions not to use them, he said.
But, he added, “Despite the low reported numbers of AID users, results achieved in the pump with real-time CGM [group] are admirable and approaching recommended consensus targets with a clinically meaningful difference towards all other treatment modalities. As our findings may not be directly applicable to all participants using automated systems, they may still provide useful insights into the factors that influence glycemic control.”
Similarly, the intermittently scanned CGMs used by most in the study, and particularly in the earlier period, didn’t have low- or high-glucose alarms as do later versions. And an even more recent version also doesn’t require scanning either, so is essentially also “real-time.”
Dr. Dovc noted, “in the first half of our observational period only first generation of intermittently-scanned CGM was generally available, and we can speculate that only a small proportion started to use second generation towards the end of our observational period. The exact number of second-generation users was not available in this analysis.”
He acknowledged that because the study was observational and not randomized, patient choice of device could have influenced the outcomes.
“For example, participants who choose to use a more expensive device may have more resources or support available to them, which could influence their ability to manage their diabetes effectively. Additionally, individuals who choose to use a particular device may be more motivated or engaged in their diabetes care, which could also impact their outcomes. It would be important for future studies to explore the impact of device selection on device effectiveness and to control for this potential confounding factor in the analysis.”
This study was supported by the international Better Control in Pediatric and Adolescent Diabetes: Working to Create Centers of Reference (SWEET) corporate members, including Abbott Laboratories, Boehringer Ingelheim, Dexcom, Insulet, Eli Lilly, Medtronic, Sanofi, and the Slovenian National Research Agency. Dr. Dovc disclosed ties with Abbott Laboratories, Medtronic, Novo Nordisk, Eli Lilly, and Pfizer. He served as a member of the European Commission Expert Panel for Medical Devices for Endocrinology and Diabetes.
A version of this article originally appeared on Medscape.com.
FDA broadens warning on potentially contaminated eye products
The announcement released Wednesday adds to a previous warning issued earlier this month for EzriCare Artificial Tears or Delsam Pharma’s Artificial Tears because of potential bacterial contamination. All three products are manufactured by the same company, Global Pharma Healthcare, based in Tamilnadu, India.
The FDA has faulted the company for multiple violations, including “lack of appropriate microbial testing” and “lack of proper controls concerning tamper-evident packaging,” and has banned imports to the United States.
The updated warning from the FDA did not give additional information about the over-the-counter eye ointment beyond potential bacterial contamination.
On Feb. 1, the CDC issued an alert about an outbreak of a drug-resistant strain of bacteria, Pseudomonas aeruginosa, linked to artificial tear products. To date, 58 patients across 13 states have been identified, and the most commonly reported artificial tear brand was EzriCare Artificial Tears. Five patients had permanent vision loss, and one patient died.
A version of this article first appeared on Medscape.com.
The announcement released Wednesday adds to a previous warning issued earlier this month for EzriCare Artificial Tears or Delsam Pharma’s Artificial Tears because of potential bacterial contamination. All three products are manufactured by the same company, Global Pharma Healthcare, based in Tamilnadu, India.
The FDA has faulted the company for multiple violations, including “lack of appropriate microbial testing” and “lack of proper controls concerning tamper-evident packaging,” and has banned imports to the United States.
The updated warning from the FDA did not give additional information about the over-the-counter eye ointment beyond potential bacterial contamination.
On Feb. 1, the CDC issued an alert about an outbreak of a drug-resistant strain of bacteria, Pseudomonas aeruginosa, linked to artificial tear products. To date, 58 patients across 13 states have been identified, and the most commonly reported artificial tear brand was EzriCare Artificial Tears. Five patients had permanent vision loss, and one patient died.
A version of this article first appeared on Medscape.com.
The announcement released Wednesday adds to a previous warning issued earlier this month for EzriCare Artificial Tears or Delsam Pharma’s Artificial Tears because of potential bacterial contamination. All three products are manufactured by the same company, Global Pharma Healthcare, based in Tamilnadu, India.
The FDA has faulted the company for multiple violations, including “lack of appropriate microbial testing” and “lack of proper controls concerning tamper-evident packaging,” and has banned imports to the United States.
The updated warning from the FDA did not give additional information about the over-the-counter eye ointment beyond potential bacterial contamination.
On Feb. 1, the CDC issued an alert about an outbreak of a drug-resistant strain of bacteria, Pseudomonas aeruginosa, linked to artificial tear products. To date, 58 patients across 13 states have been identified, and the most commonly reported artificial tear brand was EzriCare Artificial Tears. Five patients had permanent vision loss, and one patient died.
A version of this article first appeared on Medscape.com.
How spirituality guides these three doctors
Whether you’re spiritual, religious – or neither – the Medscape Physician Lifestyle & Happiness Report 2023 asked if you have a religious or spiritual belief. Turns out 69% of physicians shared that they have a spiritual or religious practice.
Tapping into the universe
Nick Shamie, MD, an orthopedic surgeon specializing in spine surgery at University of California, Los Angeles, says the constant challenges of making life-and-death decisions offer an opportunity to check in with a higher power.
“Sometimes when I’m going into a tough surgery or have a tough situation, I pause and think about how this isn’t about me and the situation I’m in,” says Dr. Shamie, whose family is Muslim. “It’s about the whole universe. I feel like someone, or some being, is looking over my shoulders, and if my intentions are good, I’ll be fine. The person I’m going to take care of will be fine. That’s how I use my faith.”
Having a belief in something greater than herself also fuels Jill Carnahan, MD, a family medicine physician and functional medicine expert in Boulder, Colo.
“This is key for me as a physician,” says Dr. Carnahan, author of “Unexpected: Finding Resilience Through Functional Medicine, Science, and Faith.” “I urge physicians to think about their source of strength. That’s not necessarily even religious. It could be meditation or being in nature.”
Dr. Carnahan likes to share with patients that there are lessons that can come from being ill – whether treating ill patients or struggling with one’s own illness.
“I like to teach this idea of illness as a teacher,” says Dr. Carnahan, who has Crohn’s disease and is a cancer survivor. “This is tough, but what you’re saying here is that there is meaning or purpose to this experience. It brings awareness to your life that may not have been there before.”
Often illness is our body’s way of getting our attention that our life, relationships, or work needs adjustment. Illness can be a reminder to make changes. “For example, a diagnosis of autoimmunity may be a reminder to take better care of ourselves, or a diagnosis of cancer may cause us to get out of an unhealthy relationship or change jobs to do something more fulfilling, as we have increased awareness of the brevity of life.”
When patients are affected by illness, pain, reduced functionality, and even imminent death, understanding the experience is difficult, and finding any purpose in it may seem impossible. Still, studies show that those who find meaning in the experience cope better with their illness.
Finding that meaning may be a strong driver of survival and may be positively related to hope, belief, and happiness.
Spirituality supports patients
Even if you’re not religious yourself, it can be helpful to support a patient who opts to pray before an arduous procedure, says Sharyar Baradaran, DDS, a periodontist specializing in gum surgery in Beverly Hills, Calif.
“I’ve had patients who go into meditation mode, or they say a prayer before I start surgery,” he says. “I take that opportunity to connect. In that instance, we hold hands. I want them to know that I understand what they’re going through and how they’re trying to find the courage to undergo surgery.”
When Dr. Shamie was a child, his father described religion as embodying the basic tenet of being good to others. “I’ve taken that to heart,” he says. “All religions, all faiths have that as a central premise.”
These doctors agree that when you take the time to stop and hold a patient’s hand, bow your head during their prayer, or acknowledge or speak for a few moments about their faith, especially during a health crisis, surgery, or challenging diagnosis, patients appreciate it and develop an even deeper connection with you.
Dr. Baradaran believes spirituality can play an important role in how health care providers care for patients. Though it may not be widely discussed or reported, and physicians may find little time and space to address patients’ spiritual needs, there is growing sensitivity regarding spirituality in health care. One study found that while physicians understand its importance, nurses are more apt to integrate spirituality into practice.
“No matter the religion, if you’re spiritual, it means you’re listening and being respectful,” says Dr. Baradaran, who is Jewish. “There are times that I’m not familiar with the prayers my patients are saying, but I always take them in, absorb them, and respect them. This allows me to have a deeper connection with them, which is wonderful.”
Dr. Shamie says that he turns to his faith in good times as well as tough ones.
“I see a lot of people who are dealing with very difficult situations, and it’s not their choice to be in this position,” he says. “At those moments, I think to myself how fortunate I am that I’m not experiencing what this individual or family is going through. I do thank God at that time. I appreciate the life I have, and when I witness hardships, it resets my appreciation.”
For Dr. Carnahan, faith is about becoming comfortable with the inevitable uncertainty of life. It’s also about finding ways to tap into the day’s stresses.
“As physicians, we’re workaholics, and one in four of us are burnt out,” she says. “One solution that really works is to step back from the day-to-day grind and find time to pray or meditate or be in nature.”
There are times when a tragedy occurs, and despite your most intense efforts, a patient may die. Those experiences can be crushing to a physician. However, to guide you through the loss of a patient or the daily juggles of managing your practice, Dr. Carnahan suggests finding time every morning to focus on the day ahead and how you connect with the universe.
“I take 15 minutes in the morning and think about how I will bring love to the world,” she says. “If you look for the miracles and the good and the unexpected, that gratitude shift allows your mind to be transformed by what’s happening. It’s often in those moments that you’ll realize again why you went into medicine in the first place.”
Doctors without faith
So, what does this mean if you’re among the 25% of physicians in the Medscape report who do not have a religious or spiritual leaning and aren’t apt to be spiritually minded when it comes to your patients? An article on KevinMD.com points out that atheist physicians are often in the closet about their atheism because they usually bow their heads or keep a respectful silence when a patient or their family offers a prayer request before surgery or a prayer of thanks after a procedure.
The retired atheist physician who wrote the piece reminds us that nonreligious doctors are good people with a high moral compass who may not believe in an afterlife. However, that means they try to make their patients’ quality of life the best they can.
A version of this article first appeared on Medscape.com.
Whether you’re spiritual, religious – or neither – the Medscape Physician Lifestyle & Happiness Report 2023 asked if you have a religious or spiritual belief. Turns out 69% of physicians shared that they have a spiritual or religious practice.
Tapping into the universe
Nick Shamie, MD, an orthopedic surgeon specializing in spine surgery at University of California, Los Angeles, says the constant challenges of making life-and-death decisions offer an opportunity to check in with a higher power.
“Sometimes when I’m going into a tough surgery or have a tough situation, I pause and think about how this isn’t about me and the situation I’m in,” says Dr. Shamie, whose family is Muslim. “It’s about the whole universe. I feel like someone, or some being, is looking over my shoulders, and if my intentions are good, I’ll be fine. The person I’m going to take care of will be fine. That’s how I use my faith.”
Having a belief in something greater than herself also fuels Jill Carnahan, MD, a family medicine physician and functional medicine expert in Boulder, Colo.
“This is key for me as a physician,” says Dr. Carnahan, author of “Unexpected: Finding Resilience Through Functional Medicine, Science, and Faith.” “I urge physicians to think about their source of strength. That’s not necessarily even religious. It could be meditation or being in nature.”
Dr. Carnahan likes to share with patients that there are lessons that can come from being ill – whether treating ill patients or struggling with one’s own illness.
“I like to teach this idea of illness as a teacher,” says Dr. Carnahan, who has Crohn’s disease and is a cancer survivor. “This is tough, but what you’re saying here is that there is meaning or purpose to this experience. It brings awareness to your life that may not have been there before.”
Often illness is our body’s way of getting our attention that our life, relationships, or work needs adjustment. Illness can be a reminder to make changes. “For example, a diagnosis of autoimmunity may be a reminder to take better care of ourselves, or a diagnosis of cancer may cause us to get out of an unhealthy relationship or change jobs to do something more fulfilling, as we have increased awareness of the brevity of life.”
When patients are affected by illness, pain, reduced functionality, and even imminent death, understanding the experience is difficult, and finding any purpose in it may seem impossible. Still, studies show that those who find meaning in the experience cope better with their illness.
Finding that meaning may be a strong driver of survival and may be positively related to hope, belief, and happiness.
Spirituality supports patients
Even if you’re not religious yourself, it can be helpful to support a patient who opts to pray before an arduous procedure, says Sharyar Baradaran, DDS, a periodontist specializing in gum surgery in Beverly Hills, Calif.
“I’ve had patients who go into meditation mode, or they say a prayer before I start surgery,” he says. “I take that opportunity to connect. In that instance, we hold hands. I want them to know that I understand what they’re going through and how they’re trying to find the courage to undergo surgery.”
When Dr. Shamie was a child, his father described religion as embodying the basic tenet of being good to others. “I’ve taken that to heart,” he says. “All religions, all faiths have that as a central premise.”
These doctors agree that when you take the time to stop and hold a patient’s hand, bow your head during their prayer, or acknowledge or speak for a few moments about their faith, especially during a health crisis, surgery, or challenging diagnosis, patients appreciate it and develop an even deeper connection with you.
Dr. Baradaran believes spirituality can play an important role in how health care providers care for patients. Though it may not be widely discussed or reported, and physicians may find little time and space to address patients’ spiritual needs, there is growing sensitivity regarding spirituality in health care. One study found that while physicians understand its importance, nurses are more apt to integrate spirituality into practice.
“No matter the religion, if you’re spiritual, it means you’re listening and being respectful,” says Dr. Baradaran, who is Jewish. “There are times that I’m not familiar with the prayers my patients are saying, but I always take them in, absorb them, and respect them. This allows me to have a deeper connection with them, which is wonderful.”
Dr. Shamie says that he turns to his faith in good times as well as tough ones.
“I see a lot of people who are dealing with very difficult situations, and it’s not their choice to be in this position,” he says. “At those moments, I think to myself how fortunate I am that I’m not experiencing what this individual or family is going through. I do thank God at that time. I appreciate the life I have, and when I witness hardships, it resets my appreciation.”
For Dr. Carnahan, faith is about becoming comfortable with the inevitable uncertainty of life. It’s also about finding ways to tap into the day’s stresses.
“As physicians, we’re workaholics, and one in four of us are burnt out,” she says. “One solution that really works is to step back from the day-to-day grind and find time to pray or meditate or be in nature.”
There are times when a tragedy occurs, and despite your most intense efforts, a patient may die. Those experiences can be crushing to a physician. However, to guide you through the loss of a patient or the daily juggles of managing your practice, Dr. Carnahan suggests finding time every morning to focus on the day ahead and how you connect with the universe.
“I take 15 minutes in the morning and think about how I will bring love to the world,” she says. “If you look for the miracles and the good and the unexpected, that gratitude shift allows your mind to be transformed by what’s happening. It’s often in those moments that you’ll realize again why you went into medicine in the first place.”
Doctors without faith
So, what does this mean if you’re among the 25% of physicians in the Medscape report who do not have a religious or spiritual leaning and aren’t apt to be spiritually minded when it comes to your patients? An article on KevinMD.com points out that atheist physicians are often in the closet about their atheism because they usually bow their heads or keep a respectful silence when a patient or their family offers a prayer request before surgery or a prayer of thanks after a procedure.
The retired atheist physician who wrote the piece reminds us that nonreligious doctors are good people with a high moral compass who may not believe in an afterlife. However, that means they try to make their patients’ quality of life the best they can.
A version of this article first appeared on Medscape.com.
Whether you’re spiritual, religious – or neither – the Medscape Physician Lifestyle & Happiness Report 2023 asked if you have a religious or spiritual belief. Turns out 69% of physicians shared that they have a spiritual or religious practice.
Tapping into the universe
Nick Shamie, MD, an orthopedic surgeon specializing in spine surgery at University of California, Los Angeles, says the constant challenges of making life-and-death decisions offer an opportunity to check in with a higher power.
“Sometimes when I’m going into a tough surgery or have a tough situation, I pause and think about how this isn’t about me and the situation I’m in,” says Dr. Shamie, whose family is Muslim. “It’s about the whole universe. I feel like someone, or some being, is looking over my shoulders, and if my intentions are good, I’ll be fine. The person I’m going to take care of will be fine. That’s how I use my faith.”
Having a belief in something greater than herself also fuels Jill Carnahan, MD, a family medicine physician and functional medicine expert in Boulder, Colo.
“This is key for me as a physician,” says Dr. Carnahan, author of “Unexpected: Finding Resilience Through Functional Medicine, Science, and Faith.” “I urge physicians to think about their source of strength. That’s not necessarily even religious. It could be meditation or being in nature.”
Dr. Carnahan likes to share with patients that there are lessons that can come from being ill – whether treating ill patients or struggling with one’s own illness.
“I like to teach this idea of illness as a teacher,” says Dr. Carnahan, who has Crohn’s disease and is a cancer survivor. “This is tough, but what you’re saying here is that there is meaning or purpose to this experience. It brings awareness to your life that may not have been there before.”
Often illness is our body’s way of getting our attention that our life, relationships, or work needs adjustment. Illness can be a reminder to make changes. “For example, a diagnosis of autoimmunity may be a reminder to take better care of ourselves, or a diagnosis of cancer may cause us to get out of an unhealthy relationship or change jobs to do something more fulfilling, as we have increased awareness of the brevity of life.”
When patients are affected by illness, pain, reduced functionality, and even imminent death, understanding the experience is difficult, and finding any purpose in it may seem impossible. Still, studies show that those who find meaning in the experience cope better with their illness.
Finding that meaning may be a strong driver of survival and may be positively related to hope, belief, and happiness.
Spirituality supports patients
Even if you’re not religious yourself, it can be helpful to support a patient who opts to pray before an arduous procedure, says Sharyar Baradaran, DDS, a periodontist specializing in gum surgery in Beverly Hills, Calif.
“I’ve had patients who go into meditation mode, or they say a prayer before I start surgery,” he says. “I take that opportunity to connect. In that instance, we hold hands. I want them to know that I understand what they’re going through and how they’re trying to find the courage to undergo surgery.”
When Dr. Shamie was a child, his father described religion as embodying the basic tenet of being good to others. “I’ve taken that to heart,” he says. “All religions, all faiths have that as a central premise.”
These doctors agree that when you take the time to stop and hold a patient’s hand, bow your head during their prayer, or acknowledge or speak for a few moments about their faith, especially during a health crisis, surgery, or challenging diagnosis, patients appreciate it and develop an even deeper connection with you.
Dr. Baradaran believes spirituality can play an important role in how health care providers care for patients. Though it may not be widely discussed or reported, and physicians may find little time and space to address patients’ spiritual needs, there is growing sensitivity regarding spirituality in health care. One study found that while physicians understand its importance, nurses are more apt to integrate spirituality into practice.
“No matter the religion, if you’re spiritual, it means you’re listening and being respectful,” says Dr. Baradaran, who is Jewish. “There are times that I’m not familiar with the prayers my patients are saying, but I always take them in, absorb them, and respect them. This allows me to have a deeper connection with them, which is wonderful.”
Dr. Shamie says that he turns to his faith in good times as well as tough ones.
“I see a lot of people who are dealing with very difficult situations, and it’s not their choice to be in this position,” he says. “At those moments, I think to myself how fortunate I am that I’m not experiencing what this individual or family is going through. I do thank God at that time. I appreciate the life I have, and when I witness hardships, it resets my appreciation.”
For Dr. Carnahan, faith is about becoming comfortable with the inevitable uncertainty of life. It’s also about finding ways to tap into the day’s stresses.
“As physicians, we’re workaholics, and one in four of us are burnt out,” she says. “One solution that really works is to step back from the day-to-day grind and find time to pray or meditate or be in nature.”
There are times when a tragedy occurs, and despite your most intense efforts, a patient may die. Those experiences can be crushing to a physician. However, to guide you through the loss of a patient or the daily juggles of managing your practice, Dr. Carnahan suggests finding time every morning to focus on the day ahead and how you connect with the universe.
“I take 15 minutes in the morning and think about how I will bring love to the world,” she says. “If you look for the miracles and the good and the unexpected, that gratitude shift allows your mind to be transformed by what’s happening. It’s often in those moments that you’ll realize again why you went into medicine in the first place.”
Doctors without faith
So, what does this mean if you’re among the 25% of physicians in the Medscape report who do not have a religious or spiritual leaning and aren’t apt to be spiritually minded when it comes to your patients? An article on KevinMD.com points out that atheist physicians are often in the closet about their atheism because they usually bow their heads or keep a respectful silence when a patient or their family offers a prayer request before surgery or a prayer of thanks after a procedure.
The retired atheist physician who wrote the piece reminds us that nonreligious doctors are good people with a high moral compass who may not believe in an afterlife. However, that means they try to make their patients’ quality of life the best they can.
A version of this article first appeared on Medscape.com.
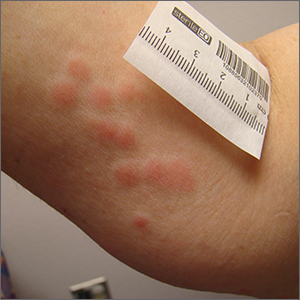
Clustered erythematous limb lesions
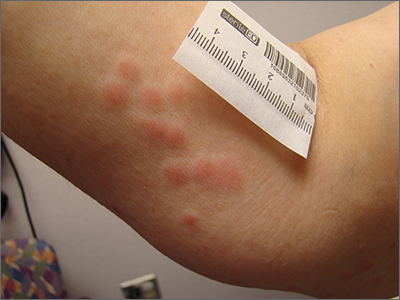
Clustered erythematous macular to papular lesions, especially those that stop at clothing lines, are a frequent manifestation of insect bites. In this case, the lesions lacked a central punctum that is common in many insect bites, so the most likely culprit was bed bugs. It is likely that this patient’s friend inadvertently brought the bed bugs into the home in her luggage or packed belongings. Over time, they spread around the home, causing the patient’s bites and inflammation. When questioned, the patient noted that she could actually see bugs around the couch in her home.
The scientific name for bed bugs is Cimex lectularis. Bed bugs require a blood meal from a host to survive, but they do not remain attached to the human body. Instead, they live in nearby fabrics. Bed bugs are visible to the naked eye when they are in the open, although they usually remain along the seams of fabric, edges of bedding, or in cracks and crevices. Often the feces of bed bugs will collect and be seen as dark spots or streaks on bedding.1
Treatment hinges on the eradication of the bed bugs. The erythematous itching lesions will resolve spontaneously over 1 to 2 weeks. Topical corticosteroids, including hydrocortisone, can be used as necessary to control the itching. Oral antihistamines can also help with itching.
Eradication of all the bed bugs in the home can be difficult and warrant professional extermination services. Washing clothing in hot water of at least 140 °F will kill the insects. Freezing items below –4 °F for at least 2 hours is also effective but may not be possible with home freezers.
It’s worth noting that resistance to insecticides has developed, making chemical eradication difficult. An alternative extermination protocol involves heating an entire home to the required temperatures to eradicate the infestation.1
This patient noted that she had already thrown away the couch, clothes, and bedding where she had seen the insects and had sprayed her apartment with insecticide. She was counseled to contact a professional exterminator to further evaluate the home for any additional areas of infestation and treat if any bed bugs were still in the home. She was also counseled to use loratadine 10 mg/d orally and topical 1% hydrocortisone ointment, as needed, for the itching and inflammation.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Professor and Chair, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine, Kalamazoo.
1. Parola P, Izri A. Bedbugs. N Engl J Med. 2020;382:2230-2237. doi: 10.1056/NEJMcp1905840

Clustered erythematous macular to papular lesions, especially those that stop at clothing lines, are a frequent manifestation of insect bites. In this case, the lesions lacked a central punctum that is common in many insect bites, so the most likely culprit was bed bugs. It is likely that this patient’s friend inadvertently brought the bed bugs into the home in her luggage or packed belongings. Over time, they spread around the home, causing the patient’s bites and inflammation. When questioned, the patient noted that she could actually see bugs around the couch in her home.
The scientific name for bed bugs is Cimex lectularis. Bed bugs require a blood meal from a host to survive, but they do not remain attached to the human body. Instead, they live in nearby fabrics. Bed bugs are visible to the naked eye when they are in the open, although they usually remain along the seams of fabric, edges of bedding, or in cracks and crevices. Often the feces of bed bugs will collect and be seen as dark spots or streaks on bedding.1
Treatment hinges on the eradication of the bed bugs. The erythematous itching lesions will resolve spontaneously over 1 to 2 weeks. Topical corticosteroids, including hydrocortisone, can be used as necessary to control the itching. Oral antihistamines can also help with itching.
Eradication of all the bed bugs in the home can be difficult and warrant professional extermination services. Washing clothing in hot water of at least 140 °F will kill the insects. Freezing items below –4 °F for at least 2 hours is also effective but may not be possible with home freezers.
It’s worth noting that resistance to insecticides has developed, making chemical eradication difficult. An alternative extermination protocol involves heating an entire home to the required temperatures to eradicate the infestation.1
This patient noted that she had already thrown away the couch, clothes, and bedding where she had seen the insects and had sprayed her apartment with insecticide. She was counseled to contact a professional exterminator to further evaluate the home for any additional areas of infestation and treat if any bed bugs were still in the home. She was also counseled to use loratadine 10 mg/d orally and topical 1% hydrocortisone ointment, as needed, for the itching and inflammation.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Professor and Chair, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine, Kalamazoo.

Clustered erythematous macular to papular lesions, especially those that stop at clothing lines, are a frequent manifestation of insect bites. In this case, the lesions lacked a central punctum that is common in many insect bites, so the most likely culprit was bed bugs. It is likely that this patient’s friend inadvertently brought the bed bugs into the home in her luggage or packed belongings. Over time, they spread around the home, causing the patient’s bites and inflammation. When questioned, the patient noted that she could actually see bugs around the couch in her home.
The scientific name for bed bugs is Cimex lectularis. Bed bugs require a blood meal from a host to survive, but they do not remain attached to the human body. Instead, they live in nearby fabrics. Bed bugs are visible to the naked eye when they are in the open, although they usually remain along the seams of fabric, edges of bedding, or in cracks and crevices. Often the feces of bed bugs will collect and be seen as dark spots or streaks on bedding.1
Treatment hinges on the eradication of the bed bugs. The erythematous itching lesions will resolve spontaneously over 1 to 2 weeks. Topical corticosteroids, including hydrocortisone, can be used as necessary to control the itching. Oral antihistamines can also help with itching.
Eradication of all the bed bugs in the home can be difficult and warrant professional extermination services. Washing clothing in hot water of at least 140 °F will kill the insects. Freezing items below –4 °F for at least 2 hours is also effective but may not be possible with home freezers.
It’s worth noting that resistance to insecticides has developed, making chemical eradication difficult. An alternative extermination protocol involves heating an entire home to the required temperatures to eradicate the infestation.1
This patient noted that she had already thrown away the couch, clothes, and bedding where she had seen the insects and had sprayed her apartment with insecticide. She was counseled to contact a professional exterminator to further evaluate the home for any additional areas of infestation and treat if any bed bugs were still in the home. She was also counseled to use loratadine 10 mg/d orally and topical 1% hydrocortisone ointment, as needed, for the itching and inflammation.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Professor and Chair, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine, Kalamazoo.
1. Parola P, Izri A. Bedbugs. N Engl J Med. 2020;382:2230-2237. doi: 10.1056/NEJMcp1905840
1. Parola P, Izri A. Bedbugs. N Engl J Med. 2020;382:2230-2237. doi: 10.1056/NEJMcp1905840
Two cups of coffee increase heart dangers with hypertension
according to researchers at Institute for Global Health Policy Research, Bureau of International Health Cooperation, National Center for Global Health and Medicine, Tokyo.
What to know
People with severely high blood pressure who drink two or more cups of caffeinated coffee each day could double their risk of dying from a heart attack, stroke, or any type of cardiovascular disease.
Too much coffee may raise blood pressure and lead to anxiety, heart palpitations, and difficulty sleeping.
An 8-ounce cup of coffee has 80-100 mg of caffeine, while an 8-ounce cup of green or black tea has 30-50 mg.
Drinking one cup of coffee a day or any amount of green tea was not associated with risk of death across any blood pressure categories, and drinking green tea was not associated with increased risk of death related to cardiovascular disease at any blood pressure level.
Frequent consumers of coffee were more likely to be younger, current smokers, current drinkers, to eat fewer vegetables, and to have higher total cholesterol levels and lower systolic blood pressure regardless of their blood pressure category.
This is a summary of the article “Coffee and Green Tea Consumption and Cardiovascular Disease Mortality Among People With and Without Hypertension,” published in the Journal of the American Heart Association.
A version of this article first appeared on Medscape.com.
according to researchers at Institute for Global Health Policy Research, Bureau of International Health Cooperation, National Center for Global Health and Medicine, Tokyo.
What to know
People with severely high blood pressure who drink two or more cups of caffeinated coffee each day could double their risk of dying from a heart attack, stroke, or any type of cardiovascular disease.
Too much coffee may raise blood pressure and lead to anxiety, heart palpitations, and difficulty sleeping.
An 8-ounce cup of coffee has 80-100 mg of caffeine, while an 8-ounce cup of green or black tea has 30-50 mg.
Drinking one cup of coffee a day or any amount of green tea was not associated with risk of death across any blood pressure categories, and drinking green tea was not associated with increased risk of death related to cardiovascular disease at any blood pressure level.
Frequent consumers of coffee were more likely to be younger, current smokers, current drinkers, to eat fewer vegetables, and to have higher total cholesterol levels and lower systolic blood pressure regardless of their blood pressure category.
This is a summary of the article “Coffee and Green Tea Consumption and Cardiovascular Disease Mortality Among People With and Without Hypertension,” published in the Journal of the American Heart Association.
A version of this article first appeared on Medscape.com.
according to researchers at Institute for Global Health Policy Research, Bureau of International Health Cooperation, National Center for Global Health and Medicine, Tokyo.
What to know
People with severely high blood pressure who drink two or more cups of caffeinated coffee each day could double their risk of dying from a heart attack, stroke, or any type of cardiovascular disease.
Too much coffee may raise blood pressure and lead to anxiety, heart palpitations, and difficulty sleeping.
An 8-ounce cup of coffee has 80-100 mg of caffeine, while an 8-ounce cup of green or black tea has 30-50 mg.
Drinking one cup of coffee a day or any amount of green tea was not associated with risk of death across any blood pressure categories, and drinking green tea was not associated with increased risk of death related to cardiovascular disease at any blood pressure level.
Frequent consumers of coffee were more likely to be younger, current smokers, current drinkers, to eat fewer vegetables, and to have higher total cholesterol levels and lower systolic blood pressure regardless of their blood pressure category.
This is a summary of the article “Coffee and Green Tea Consumption and Cardiovascular Disease Mortality Among People With and Without Hypertension,” published in the Journal of the American Heart Association.
A version of this article first appeared on Medscape.com.
FROM JOURNAL OF AMERICAN HEART ASSOCIATION
AGA guideline defines role of biomarkers in ulcerative colitis
The American Gastroenterological Association (AGA) has released a new clinical practice guideline defining the role of biomarkers in monitoring and managing ulcerative colitis (UC).
, reported lead guideline panelist Siddharth Singh, MD, of University of California San Diego, La Jolla, Calif., and colleagues.
“[I]n routine clinical practice, repeated endoscopic assessment is invasive, expensive, and may be impractical,” the panelists wrote. Their report is in Gastroenterology. “There is an important need for understanding how noninvasive biomarkers may serve as accurate and reliable surrogates for endoscopic assessment of inflammation and whether they can be more readily implemented in a UC care pathway.”
After reviewing relevant randomized controlled trials and observational studies, Dr. Singh and colleagues issued seven conditional recommendations, three of which concern patients in symptomatic remission, and four of which apply to patients with symptomatically active UC.
“The key take-home message is that the routine measurement of noninvasive biomarkers in addition to assessment of patient reported symptoms is critical in evaluating the disease burden of UC,” said Jordan E. Axelrad, MD, MPH, director of clinical and translational research at NYU Langone Health’s Inflammatory Bowel Disease Center, New York. “Many of these recommendations regarding the assessment of disease activity beyond symptoms alone are widely accepted, particularity at tertiary IBD centers; however, this guideline serves to formalize and structure the recommendations, with appropriate test cutoff values, in a simple UC care pathway.”
Recommendations for patients in symptomatic remission
For patients in remission, the guideline advises monitoring both symptoms and biomarkers, with biomarkers measured every 6-12 months.
Asymptomatic patients with normal biomarkers can skip routine endoscopy to evaluate disease activity, according to the guideline, but those with abnormal fecal calprotectin, fecal lactoferrin, or serum C-reactive protein (CRP) are candidates for endoscopic assessment instead of empiric treatment adjustment. Patients may still need periodic colonoscopy for dysplasia surveillance.
“The most important pearl [from the guideline] is that fecal calprotectin less than 150 mcg/g, normal fecal lactoferrin, or normal CRP, can be used to rule out active inflammation in patients in symptomatic remission,” according to Dr. Axelrad.
The guideline suggests that the two fecal biomarkers “may be optimal for monitoring and may be particularly useful in patients where biomarkers have historically correlated with endoscopic disease activity.” In contrast, normal CRP may be insufficient to rule out moderate to severe endoscopic inflammation in patients who recently entered remission following treatment adjustment.
While abnormal biomarkers in asymptomatic patients are sufficient cause for endoscopy, the guideline also suggests that retesting in 3-6 months is a reasonable alternative. If biomarkers are again elevated, then endoscopic evaluation should be considered.
Recommendations for patients with symptomatically active disease
The recommendations for patients with symptomatically active UC follow a similar pathway. The guideline advises an evaluation strategy combining symptoms and biomarkers instead of symptoms alone.
For example, patients with moderate to severe symptoms suggestive of flare and elevated biomarkers are candidates for treatment adjustment without endoscopy.
Still, patient preferences should be considered, Dr. Singh and colleagues noted.
“Patients who place greater value in confirming inflammation, particularly when making significant treatment decisions (such as starting or switching immunosuppressive therapies), and lesser value on the inconvenience of endoscopy, may choose to pursue endoscopic evaluation before treatment adjustment,” they wrote.
For patients with mild symptoms, endoscopy is generally recommended, according to the guideline, unless the patient recently had moderate to severe symptoms and has improved after treatment adjustment; in that case, biomarkers can be used to fine-tune therapy without the need for endoscopy.
Again, providers should engage in shared-decision making, the guideline advises. Patients with mild symptoms but no biomarker results may reasonably elect to undergo endoscopy prior to testing biomarkers, while patients with mild symptoms and normal biomarkers may reasonably elect to retest biomarkers in 3-6 months.
Data remain insufficient to recommend biomarkers over endoscopy
Dr. Singh and colleagues concluded the guideline by highlighting an insufficient level of direct evidence necessary to recommend a biomarker-based treat-to-target strategy over endoscopy-based monitoring strategy, despite indirect evidence suggesting this may be the case.
“[T]here have not been any studies comparing a biomarker-based strategy with an endoscopy-based strategy for assessment and monitoring of endoscopic remission,” they wrote. “This was identified as a knowledge gap by the panel.”
The authors disclosed relationships with Pfizer, AbbVie, Lilly, and others. Dr. Axelrad disclosed relationships with Janssen, AbbVie, Pfizer, and others.
The American Gastroenterological Association (AGA) has released a new clinical practice guideline defining the role of biomarkers in monitoring and managing ulcerative colitis (UC).
, reported lead guideline panelist Siddharth Singh, MD, of University of California San Diego, La Jolla, Calif., and colleagues.
“[I]n routine clinical practice, repeated endoscopic assessment is invasive, expensive, and may be impractical,” the panelists wrote. Their report is in Gastroenterology. “There is an important need for understanding how noninvasive biomarkers may serve as accurate and reliable surrogates for endoscopic assessment of inflammation and whether they can be more readily implemented in a UC care pathway.”
After reviewing relevant randomized controlled trials and observational studies, Dr. Singh and colleagues issued seven conditional recommendations, three of which concern patients in symptomatic remission, and four of which apply to patients with symptomatically active UC.
“The key take-home message is that the routine measurement of noninvasive biomarkers in addition to assessment of patient reported symptoms is critical in evaluating the disease burden of UC,” said Jordan E. Axelrad, MD, MPH, director of clinical and translational research at NYU Langone Health’s Inflammatory Bowel Disease Center, New York. “Many of these recommendations regarding the assessment of disease activity beyond symptoms alone are widely accepted, particularity at tertiary IBD centers; however, this guideline serves to formalize and structure the recommendations, with appropriate test cutoff values, in a simple UC care pathway.”
Recommendations for patients in symptomatic remission
For patients in remission, the guideline advises monitoring both symptoms and biomarkers, with biomarkers measured every 6-12 months.
Asymptomatic patients with normal biomarkers can skip routine endoscopy to evaluate disease activity, according to the guideline, but those with abnormal fecal calprotectin, fecal lactoferrin, or serum C-reactive protein (CRP) are candidates for endoscopic assessment instead of empiric treatment adjustment. Patients may still need periodic colonoscopy for dysplasia surveillance.
“The most important pearl [from the guideline] is that fecal calprotectin less than 150 mcg/g, normal fecal lactoferrin, or normal CRP, can be used to rule out active inflammation in patients in symptomatic remission,” according to Dr. Axelrad.
The guideline suggests that the two fecal biomarkers “may be optimal for monitoring and may be particularly useful in patients where biomarkers have historically correlated with endoscopic disease activity.” In contrast, normal CRP may be insufficient to rule out moderate to severe endoscopic inflammation in patients who recently entered remission following treatment adjustment.
While abnormal biomarkers in asymptomatic patients are sufficient cause for endoscopy, the guideline also suggests that retesting in 3-6 months is a reasonable alternative. If biomarkers are again elevated, then endoscopic evaluation should be considered.
Recommendations for patients with symptomatically active disease
The recommendations for patients with symptomatically active UC follow a similar pathway. The guideline advises an evaluation strategy combining symptoms and biomarkers instead of symptoms alone.
For example, patients with moderate to severe symptoms suggestive of flare and elevated biomarkers are candidates for treatment adjustment without endoscopy.
Still, patient preferences should be considered, Dr. Singh and colleagues noted.
“Patients who place greater value in confirming inflammation, particularly when making significant treatment decisions (such as starting or switching immunosuppressive therapies), and lesser value on the inconvenience of endoscopy, may choose to pursue endoscopic evaluation before treatment adjustment,” they wrote.
For patients with mild symptoms, endoscopy is generally recommended, according to the guideline, unless the patient recently had moderate to severe symptoms and has improved after treatment adjustment; in that case, biomarkers can be used to fine-tune therapy without the need for endoscopy.
Again, providers should engage in shared-decision making, the guideline advises. Patients with mild symptoms but no biomarker results may reasonably elect to undergo endoscopy prior to testing biomarkers, while patients with mild symptoms and normal biomarkers may reasonably elect to retest biomarkers in 3-6 months.
Data remain insufficient to recommend biomarkers over endoscopy
Dr. Singh and colleagues concluded the guideline by highlighting an insufficient level of direct evidence necessary to recommend a biomarker-based treat-to-target strategy over endoscopy-based monitoring strategy, despite indirect evidence suggesting this may be the case.
“[T]here have not been any studies comparing a biomarker-based strategy with an endoscopy-based strategy for assessment and monitoring of endoscopic remission,” they wrote. “This was identified as a knowledge gap by the panel.”
The authors disclosed relationships with Pfizer, AbbVie, Lilly, and others. Dr. Axelrad disclosed relationships with Janssen, AbbVie, Pfizer, and others.
The American Gastroenterological Association (AGA) has released a new clinical practice guideline defining the role of biomarkers in monitoring and managing ulcerative colitis (UC).
, reported lead guideline panelist Siddharth Singh, MD, of University of California San Diego, La Jolla, Calif., and colleagues.
“[I]n routine clinical practice, repeated endoscopic assessment is invasive, expensive, and may be impractical,” the panelists wrote. Their report is in Gastroenterology. “There is an important need for understanding how noninvasive biomarkers may serve as accurate and reliable surrogates for endoscopic assessment of inflammation and whether they can be more readily implemented in a UC care pathway.”
After reviewing relevant randomized controlled trials and observational studies, Dr. Singh and colleagues issued seven conditional recommendations, three of which concern patients in symptomatic remission, and four of which apply to patients with symptomatically active UC.
“The key take-home message is that the routine measurement of noninvasive biomarkers in addition to assessment of patient reported symptoms is critical in evaluating the disease burden of UC,” said Jordan E. Axelrad, MD, MPH, director of clinical and translational research at NYU Langone Health’s Inflammatory Bowel Disease Center, New York. “Many of these recommendations regarding the assessment of disease activity beyond symptoms alone are widely accepted, particularity at tertiary IBD centers; however, this guideline serves to formalize and structure the recommendations, with appropriate test cutoff values, in a simple UC care pathway.”
Recommendations for patients in symptomatic remission
For patients in remission, the guideline advises monitoring both symptoms and biomarkers, with biomarkers measured every 6-12 months.
Asymptomatic patients with normal biomarkers can skip routine endoscopy to evaluate disease activity, according to the guideline, but those with abnormal fecal calprotectin, fecal lactoferrin, or serum C-reactive protein (CRP) are candidates for endoscopic assessment instead of empiric treatment adjustment. Patients may still need periodic colonoscopy for dysplasia surveillance.
“The most important pearl [from the guideline] is that fecal calprotectin less than 150 mcg/g, normal fecal lactoferrin, or normal CRP, can be used to rule out active inflammation in patients in symptomatic remission,” according to Dr. Axelrad.
The guideline suggests that the two fecal biomarkers “may be optimal for monitoring and may be particularly useful in patients where biomarkers have historically correlated with endoscopic disease activity.” In contrast, normal CRP may be insufficient to rule out moderate to severe endoscopic inflammation in patients who recently entered remission following treatment adjustment.
While abnormal biomarkers in asymptomatic patients are sufficient cause for endoscopy, the guideline also suggests that retesting in 3-6 months is a reasonable alternative. If biomarkers are again elevated, then endoscopic evaluation should be considered.
Recommendations for patients with symptomatically active disease
The recommendations for patients with symptomatically active UC follow a similar pathway. The guideline advises an evaluation strategy combining symptoms and biomarkers instead of symptoms alone.
For example, patients with moderate to severe symptoms suggestive of flare and elevated biomarkers are candidates for treatment adjustment without endoscopy.
Still, patient preferences should be considered, Dr. Singh and colleagues noted.
“Patients who place greater value in confirming inflammation, particularly when making significant treatment decisions (such as starting or switching immunosuppressive therapies), and lesser value on the inconvenience of endoscopy, may choose to pursue endoscopic evaluation before treatment adjustment,” they wrote.
For patients with mild symptoms, endoscopy is generally recommended, according to the guideline, unless the patient recently had moderate to severe symptoms and has improved after treatment adjustment; in that case, biomarkers can be used to fine-tune therapy without the need for endoscopy.
Again, providers should engage in shared-decision making, the guideline advises. Patients with mild symptoms but no biomarker results may reasonably elect to undergo endoscopy prior to testing biomarkers, while patients with mild symptoms and normal biomarkers may reasonably elect to retest biomarkers in 3-6 months.
Data remain insufficient to recommend biomarkers over endoscopy
Dr. Singh and colleagues concluded the guideline by highlighting an insufficient level of direct evidence necessary to recommend a biomarker-based treat-to-target strategy over endoscopy-based monitoring strategy, despite indirect evidence suggesting this may be the case.
“[T]here have not been any studies comparing a biomarker-based strategy with an endoscopy-based strategy for assessment and monitoring of endoscopic remission,” they wrote. “This was identified as a knowledge gap by the panel.”
The authors disclosed relationships with Pfizer, AbbVie, Lilly, and others. Dr. Axelrad disclosed relationships with Janssen, AbbVie, Pfizer, and others.
FROM GASTROENTEROLOGY
Zero tolerance for patient bias: Too harsh? Clinicians respond
If a patient refuses care from a health care practitioner because of their race or sex, should their request be accommodated?
In a recent blog on Medscape titled “No, You Can’t See a Different Doctor: We Need Zero Tolerance of Patient Bias,” Cleveland Francis Jr., MD, argued no.
Dr. Francis, who is Black, is a recently retired cardiologist who practiced for 50 years. He is currently Diversity, Equity, and Inclusion Advisor at Inova Heart and Vascular Institute in Falls Church, Va.
When Francis was a medical student and was preparing to take a patient’s history and perform a medical exam, the patient refused and requested a “White doctor,” he recounted.
“I can remember the hurt and embarrassment as if it were yesterday,” he wrote.
The blog, especially the title, drew strong reactions. Close to 500 readers weighed in.
“The title of my blog sounds harsh,” Dr. Francis said, “but in reality, a simple conversation with the patient usually resolves these issues. The difference is that in the old days, there was utter silence, and the wishes of the patient would be granted”
Health care practitioners “should expect to be treated with respect,” he concluded his blog.
Readers agreed on that point, but they debated whether being uncomfortable with a health care practitioner of a different sex or race always constituted “patient bias.”
Some noted that difficulty understanding a practitioner’s accent, for example, is a legitimate reason for asking for another clinician.
Accents and understanding
“If I am struggling to understand you because your accent is too thick or ... because hearing aids can only do so much, I need to ask for someone else,” a reader commented.
Another chimed in: “My elderly parents changed PCPs frequently during the final years of their lives, mainly due to language barriers encountered with foreign-born providers. Due to progressive hearing loss, they simply couldn’t understand them.”
“It is important to remember that there is a Patient Bill of Rights,” she noted, “the first part of which states, ‘You have the right to safe, considerate, and respectful care, provided in a manner consistent with your beliefs.’ ”
A former charge nurse added: “If a request for change was substantive (poor communication, perceived incompetence, trauma history, etc.), I would move mountains to accommodate it, but IMHO [in my humble opinion], the belief in honoring patient preference doesn’t necessarily need to include rearranging the world in order to accommodate racism, sexism, etc.”
Bias against female doctors, male nurses
Many commenters described how they gladly traded when a patient requested a practitioner of the opposite sex.
A female hospitalist related how she contacted the senior male doctor working with her to arrange a patient trade, adding, “I do agree that racial discrimination ought to be discouraged.”
Similarly, a male ICU RN commented: “Over 13 years, I have had a handful of female (usually older) patients request a female nurse. I have always strived to make this happen.”
However, an older woman related how at first she “had some bias against a male nurse touching me and also felt self-conscious,” she said. “So, I tried to relax ... and let him do his job. He was one of the most compassionate, kind, and sensitive nurses I’ve ever had.”
“I think in some cases,” she noted, “some women have had a history of some sort of abuse by a male, whether it’s sexual or psychological,” but in other cases, “it’s often just a personal preference, not a bias.”
A physician assistant (PA) who worked in a rural ED recounted how “there was only one physician and one PA on at any given evening/night shift, both usually White males.”
“Sometimes, you just have to cope as best you can with whomever is available, and in doing so,” he said, “they might just end up being pleasantly surprised.”
Don’t take it personally, move on
“If a patient doesn’t want to see me for whatever reason, then I would rather not treat them,” was a common sentiment.
Patients “should feel comfortable with their provider even if it’s with someone other than myself,” a reader wrote.
A female physician chimed in: “I frequently have older male patients refuse to see me. ... While this is irritating on several levels, I recognize that it is the patient’s choice, sigh, and move on to the next patient.”
“There are many more patients who specifically ask to see me, so I don’t waste my time and energy on being bothered by those who refuse.”
Similarly, a female mental health provider and sometimes patient wrote: “If any patient tells me that they prefer a male ... or someone of a particular race or religion or whatever, I don’t take it personally.”
A female Hispanic doctor chimed in: “Honestly, if a patient does not want to see me due to my race, I’m OK with that. Patients need to feel comfortable with me for the relationship to be therapeutic and effective,” she said.
“Forcing the patient to see me is adding injury to insult to ME! Not to mention increase[d] workload since that patient will take [so] much more time.”
Similarly, an Asian American doctor commented: “There are people who choose not to see me because of my ethnicity. However, I strongly believe that it should always be the patient’s preference. Whatever the reason, do not force the patient to see you in the name of Diversity, Equity, Inclusion, or whatever hurts your feeling. Let the patient go.”
Patient bias vs. patient preference
A physician referring to Dr. Francis’s experience suggested that “perhaps there was an opportunity to explore this misconception directly with the patient. If not, your supervising senior resident or attending should have been informed and brought into the process and conversation.”
“If/when I were rejected by a patient for whatever reason,” another physician commented, “I would gracefully accede, and hope that my colleague would tactfully point out to the patient their error.”
“Having a nurse ask the patient ... what they need style-wise (keeping race, gender, etc., out of it) might help identify whether or not the underlying issue(s) are based on style/needs mismatch match rather than bias,” a reader suggested.
A health care worker commented: “We generally assure patients that we are professionals and think nothing of situations that they might find uncomfortable, but don’t realize that our comfort does not translate to theirs.”
Maybe a different strategy is needed
“Having been the target of bias many times,” a reader said, “I understand the pain that is inflicted. Unfortunately, a patient bias policy, while a good idea, will not prevent patient bias. This is a much larger societal problem. But we can at least tell patients that it is not okay. On the other hand, I would not want to be the provider for a patient who was biased against me and held me in disdain.”
“I do not like Zero Tolerance policies ever. They are too absolute,” another reader commented. “Sometimes, there are reasons and we do have to listen to our patients for why. ... I do not think a policy of zero tolerance will fix the problem of racism.”
“Instead of trying to educate the general public about how not to be jerks,” another reader suggested, “perhaps it would be easier to provide elective classes for doctors and employees who believe themselves to be at-risk for discrimination, providing them with a ‘toolkit’ of strategies for responding to discrimination in the moment, processing it emotionally later on, and reporting the most egregious events through designated channels.”
Another commenter agreed and wrote that, “While we as doctors need and deserve protection, we are also called to act with compassion. So, rather than ask the system for ‘zero-tolerance’ in either direction, we could encourage our health systems to provide education, support, and mediation to any party who feels or fears that they are not being well served. Such a model would include support for physicians who have been the victims of bias and hurt.”
A version of this article originally appeared on Medscape.com.
If a patient refuses care from a health care practitioner because of their race or sex, should their request be accommodated?
In a recent blog on Medscape titled “No, You Can’t See a Different Doctor: We Need Zero Tolerance of Patient Bias,” Cleveland Francis Jr., MD, argued no.
Dr. Francis, who is Black, is a recently retired cardiologist who practiced for 50 years. He is currently Diversity, Equity, and Inclusion Advisor at Inova Heart and Vascular Institute in Falls Church, Va.
When Francis was a medical student and was preparing to take a patient’s history and perform a medical exam, the patient refused and requested a “White doctor,” he recounted.
“I can remember the hurt and embarrassment as if it were yesterday,” he wrote.
The blog, especially the title, drew strong reactions. Close to 500 readers weighed in.
“The title of my blog sounds harsh,” Dr. Francis said, “but in reality, a simple conversation with the patient usually resolves these issues. The difference is that in the old days, there was utter silence, and the wishes of the patient would be granted”
Health care practitioners “should expect to be treated with respect,” he concluded his blog.
Readers agreed on that point, but they debated whether being uncomfortable with a health care practitioner of a different sex or race always constituted “patient bias.”
Some noted that difficulty understanding a practitioner’s accent, for example, is a legitimate reason for asking for another clinician.
Accents and understanding
“If I am struggling to understand you because your accent is too thick or ... because hearing aids can only do so much, I need to ask for someone else,” a reader commented.
Another chimed in: “My elderly parents changed PCPs frequently during the final years of their lives, mainly due to language barriers encountered with foreign-born providers. Due to progressive hearing loss, they simply couldn’t understand them.”
“It is important to remember that there is a Patient Bill of Rights,” she noted, “the first part of which states, ‘You have the right to safe, considerate, and respectful care, provided in a manner consistent with your beliefs.’ ”
A former charge nurse added: “If a request for change was substantive (poor communication, perceived incompetence, trauma history, etc.), I would move mountains to accommodate it, but IMHO [in my humble opinion], the belief in honoring patient preference doesn’t necessarily need to include rearranging the world in order to accommodate racism, sexism, etc.”
Bias against female doctors, male nurses
Many commenters described how they gladly traded when a patient requested a practitioner of the opposite sex.
A female hospitalist related how she contacted the senior male doctor working with her to arrange a patient trade, adding, “I do agree that racial discrimination ought to be discouraged.”
Similarly, a male ICU RN commented: “Over 13 years, I have had a handful of female (usually older) patients request a female nurse. I have always strived to make this happen.”
However, an older woman related how at first she “had some bias against a male nurse touching me and also felt self-conscious,” she said. “So, I tried to relax ... and let him do his job. He was one of the most compassionate, kind, and sensitive nurses I’ve ever had.”
“I think in some cases,” she noted, “some women have had a history of some sort of abuse by a male, whether it’s sexual or psychological,” but in other cases, “it’s often just a personal preference, not a bias.”
A physician assistant (PA) who worked in a rural ED recounted how “there was only one physician and one PA on at any given evening/night shift, both usually White males.”
“Sometimes, you just have to cope as best you can with whomever is available, and in doing so,” he said, “they might just end up being pleasantly surprised.”
Don’t take it personally, move on
“If a patient doesn’t want to see me for whatever reason, then I would rather not treat them,” was a common sentiment.
Patients “should feel comfortable with their provider even if it’s with someone other than myself,” a reader wrote.
A female physician chimed in: “I frequently have older male patients refuse to see me. ... While this is irritating on several levels, I recognize that it is the patient’s choice, sigh, and move on to the next patient.”
“There are many more patients who specifically ask to see me, so I don’t waste my time and energy on being bothered by those who refuse.”
Similarly, a female mental health provider and sometimes patient wrote: “If any patient tells me that they prefer a male ... or someone of a particular race or religion or whatever, I don’t take it personally.”
A female Hispanic doctor chimed in: “Honestly, if a patient does not want to see me due to my race, I’m OK with that. Patients need to feel comfortable with me for the relationship to be therapeutic and effective,” she said.
“Forcing the patient to see me is adding injury to insult to ME! Not to mention increase[d] workload since that patient will take [so] much more time.”
Similarly, an Asian American doctor commented: “There are people who choose not to see me because of my ethnicity. However, I strongly believe that it should always be the patient’s preference. Whatever the reason, do not force the patient to see you in the name of Diversity, Equity, Inclusion, or whatever hurts your feeling. Let the patient go.”
Patient bias vs. patient preference
A physician referring to Dr. Francis’s experience suggested that “perhaps there was an opportunity to explore this misconception directly with the patient. If not, your supervising senior resident or attending should have been informed and brought into the process and conversation.”
“If/when I were rejected by a patient for whatever reason,” another physician commented, “I would gracefully accede, and hope that my colleague would tactfully point out to the patient their error.”
“Having a nurse ask the patient ... what they need style-wise (keeping race, gender, etc., out of it) might help identify whether or not the underlying issue(s) are based on style/needs mismatch match rather than bias,” a reader suggested.
A health care worker commented: “We generally assure patients that we are professionals and think nothing of situations that they might find uncomfortable, but don’t realize that our comfort does not translate to theirs.”
Maybe a different strategy is needed
“Having been the target of bias many times,” a reader said, “I understand the pain that is inflicted. Unfortunately, a patient bias policy, while a good idea, will not prevent patient bias. This is a much larger societal problem. But we can at least tell patients that it is not okay. On the other hand, I would not want to be the provider for a patient who was biased against me and held me in disdain.”
“I do not like Zero Tolerance policies ever. They are too absolute,” another reader commented. “Sometimes, there are reasons and we do have to listen to our patients for why. ... I do not think a policy of zero tolerance will fix the problem of racism.”
“Instead of trying to educate the general public about how not to be jerks,” another reader suggested, “perhaps it would be easier to provide elective classes for doctors and employees who believe themselves to be at-risk for discrimination, providing them with a ‘toolkit’ of strategies for responding to discrimination in the moment, processing it emotionally later on, and reporting the most egregious events through designated channels.”
Another commenter agreed and wrote that, “While we as doctors need and deserve protection, we are also called to act with compassion. So, rather than ask the system for ‘zero-tolerance’ in either direction, we could encourage our health systems to provide education, support, and mediation to any party who feels or fears that they are not being well served. Such a model would include support for physicians who have been the victims of bias and hurt.”
A version of this article originally appeared on Medscape.com.
If a patient refuses care from a health care practitioner because of their race or sex, should their request be accommodated?
In a recent blog on Medscape titled “No, You Can’t See a Different Doctor: We Need Zero Tolerance of Patient Bias,” Cleveland Francis Jr., MD, argued no.
Dr. Francis, who is Black, is a recently retired cardiologist who practiced for 50 years. He is currently Diversity, Equity, and Inclusion Advisor at Inova Heart and Vascular Institute in Falls Church, Va.
When Francis was a medical student and was preparing to take a patient’s history and perform a medical exam, the patient refused and requested a “White doctor,” he recounted.
“I can remember the hurt and embarrassment as if it were yesterday,” he wrote.
The blog, especially the title, drew strong reactions. Close to 500 readers weighed in.
“The title of my blog sounds harsh,” Dr. Francis said, “but in reality, a simple conversation with the patient usually resolves these issues. The difference is that in the old days, there was utter silence, and the wishes of the patient would be granted”
Health care practitioners “should expect to be treated with respect,” he concluded his blog.
Readers agreed on that point, but they debated whether being uncomfortable with a health care practitioner of a different sex or race always constituted “patient bias.”
Some noted that difficulty understanding a practitioner’s accent, for example, is a legitimate reason for asking for another clinician.
Accents and understanding
“If I am struggling to understand you because your accent is too thick or ... because hearing aids can only do so much, I need to ask for someone else,” a reader commented.
Another chimed in: “My elderly parents changed PCPs frequently during the final years of their lives, mainly due to language barriers encountered with foreign-born providers. Due to progressive hearing loss, they simply couldn’t understand them.”
“It is important to remember that there is a Patient Bill of Rights,” she noted, “the first part of which states, ‘You have the right to safe, considerate, and respectful care, provided in a manner consistent with your beliefs.’ ”
A former charge nurse added: “If a request for change was substantive (poor communication, perceived incompetence, trauma history, etc.), I would move mountains to accommodate it, but IMHO [in my humble opinion], the belief in honoring patient preference doesn’t necessarily need to include rearranging the world in order to accommodate racism, sexism, etc.”
Bias against female doctors, male nurses
Many commenters described how they gladly traded when a patient requested a practitioner of the opposite sex.
A female hospitalist related how she contacted the senior male doctor working with her to arrange a patient trade, adding, “I do agree that racial discrimination ought to be discouraged.”
Similarly, a male ICU RN commented: “Over 13 years, I have had a handful of female (usually older) patients request a female nurse. I have always strived to make this happen.”
However, an older woman related how at first she “had some bias against a male nurse touching me and also felt self-conscious,” she said. “So, I tried to relax ... and let him do his job. He was one of the most compassionate, kind, and sensitive nurses I’ve ever had.”
“I think in some cases,” she noted, “some women have had a history of some sort of abuse by a male, whether it’s sexual or psychological,” but in other cases, “it’s often just a personal preference, not a bias.”
A physician assistant (PA) who worked in a rural ED recounted how “there was only one physician and one PA on at any given evening/night shift, both usually White males.”
“Sometimes, you just have to cope as best you can with whomever is available, and in doing so,” he said, “they might just end up being pleasantly surprised.”
Don’t take it personally, move on
“If a patient doesn’t want to see me for whatever reason, then I would rather not treat them,” was a common sentiment.
Patients “should feel comfortable with their provider even if it’s with someone other than myself,” a reader wrote.
A female physician chimed in: “I frequently have older male patients refuse to see me. ... While this is irritating on several levels, I recognize that it is the patient’s choice, sigh, and move on to the next patient.”
“There are many more patients who specifically ask to see me, so I don’t waste my time and energy on being bothered by those who refuse.”
Similarly, a female mental health provider and sometimes patient wrote: “If any patient tells me that they prefer a male ... or someone of a particular race or religion or whatever, I don’t take it personally.”
A female Hispanic doctor chimed in: “Honestly, if a patient does not want to see me due to my race, I’m OK with that. Patients need to feel comfortable with me for the relationship to be therapeutic and effective,” she said.
“Forcing the patient to see me is adding injury to insult to ME! Not to mention increase[d] workload since that patient will take [so] much more time.”
Similarly, an Asian American doctor commented: “There are people who choose not to see me because of my ethnicity. However, I strongly believe that it should always be the patient’s preference. Whatever the reason, do not force the patient to see you in the name of Diversity, Equity, Inclusion, or whatever hurts your feeling. Let the patient go.”
Patient bias vs. patient preference
A physician referring to Dr. Francis’s experience suggested that “perhaps there was an opportunity to explore this misconception directly with the patient. If not, your supervising senior resident or attending should have been informed and brought into the process and conversation.”
“If/when I were rejected by a patient for whatever reason,” another physician commented, “I would gracefully accede, and hope that my colleague would tactfully point out to the patient their error.”
“Having a nurse ask the patient ... what they need style-wise (keeping race, gender, etc., out of it) might help identify whether or not the underlying issue(s) are based on style/needs mismatch match rather than bias,” a reader suggested.
A health care worker commented: “We generally assure patients that we are professionals and think nothing of situations that they might find uncomfortable, but don’t realize that our comfort does not translate to theirs.”
Maybe a different strategy is needed
“Having been the target of bias many times,” a reader said, “I understand the pain that is inflicted. Unfortunately, a patient bias policy, while a good idea, will not prevent patient bias. This is a much larger societal problem. But we can at least tell patients that it is not okay. On the other hand, I would not want to be the provider for a patient who was biased against me and held me in disdain.”
“I do not like Zero Tolerance policies ever. They are too absolute,” another reader commented. “Sometimes, there are reasons and we do have to listen to our patients for why. ... I do not think a policy of zero tolerance will fix the problem of racism.”
“Instead of trying to educate the general public about how not to be jerks,” another reader suggested, “perhaps it would be easier to provide elective classes for doctors and employees who believe themselves to be at-risk for discrimination, providing them with a ‘toolkit’ of strategies for responding to discrimination in the moment, processing it emotionally later on, and reporting the most egregious events through designated channels.”
Another commenter agreed and wrote that, “While we as doctors need and deserve protection, we are also called to act with compassion. So, rather than ask the system for ‘zero-tolerance’ in either direction, we could encourage our health systems to provide education, support, and mediation to any party who feels or fears that they are not being well served. Such a model would include support for physicians who have been the victims of bias and hurt.”
A version of this article originally appeared on Medscape.com.
Silent bradycardia common on loop recorders – pacemaker needed?
Bradycardia is a lot more common than generally believed, but is often asymptomatic and not clinically relevant, and may lead to needless pacemaker therapy, suggests a post hoc analysis of a major study.
The arrhythmia’s presence overall in the randomized LOOP trial predicted an excess risk of syncope and death, and it didn’t matter how it was detected. Bradyarrhythmia revealed incidentally at long-term cardiac rhythm monitoring was no more predictive than when it was picked up in a usual-care setting.
Still, people in the trial with implantable loop recorders (ILR) had six times the chance of being diagnosed with bradyarrhythmias than those in the usual-care control group. LOOP entered older persons in the community without known arrhythmias but with risk factors like diabetes or hypertension.
About 80% of such arrhythmias at ILR monitoring were asymptomatic, compared with less than one-fourth in the usual-care group. Yet pacemaker implantation for bradyarrhythmia was 53% more likely in the ILR group, according to a report published in JAMA Cardiology.
Most participants with asymptomatic bradycardia did not receive treatment for it, yet the study – despite the mostly conservative management – still showed “overtreatment with pacemakers” in the ILR group, observed lead author Søren Zöga Diederichsen, MD, PhD, Copenhagen University Hospital–Rigshospitalet.
Bradyarrhythmia overall predicted later syncope and all-cause and cardiovascular (CV) death, but did so regardless of whether the patient was ILR monitored or received a pacemaker, Diederichsen said in an interview.
“We didn’t see any signal, not even a small signal, toward a health benefit from monitoring and detecting bradycardias, or from acting on them conservatively or implanting pacemakers,” he noted.
The study “emphasizes that you should have symptoms” to justify pacemaker therapy for bradyarrhythmias, regardless of how they were detected, Dr. Diederichsen said.
“Clearly ILRs may identify patients with bradyarrhythmias deserving of treatment” when they are associated with symptoms, an accompanying editorial agreed. In the current analysis, however, “a large proportion of bradycardic events were completely asymptomatic.” Yet bradycardia predicted syncope and CV death in both the ILR and usual care groups, it noted.
“This does raise the question as to whether bradyarrhythmia may be a risk marker for underlying nonarrhythmic conditions to which preventive strategies and treatment should be directed,” wrote editorialists Mark H. Schoenfeld, MD, Yale University, New Haven, Conn., and Kristen K. Patton, MD, University of Washington, Seattle.
“In an aging population with ever-increasing comorbidities, it may become increasingly important to rule out bradycardia as a manifestation of a more sinister underlying disease,” they noted, and to identify “patients who may be particularly vulnerable to adverse outcomes of progressive distal conduction disease.”
The previously published LOOP trial, conducted at four sites in Denmark, compared ILR screening for atrial fibrillation to usual care in 6,004 patients at least 70 years or older, most with hypertension. The main results showed little benefit from screening for atrial fibrillation in prevention of incident stroke or systemic embolism over about 5 years.
The current LOOP analysis, post hoc with all the associated limitations, followed incident bradyarrhythmia in the ILR and usual-care groups; any treatment of the arrhythmia was at physician discretion. The total cohort averaged 75 years in age and 47.3% were women.
The rate of incident bradyarrhythmia was 8.1% overall; it was 20.8% for those with ILR monitoring and 3.8% in the usual care group, for a hazard ratio of 6.21 (95% confidence interval, 5.15-7.48, P < .001).
The arrhythmia was asymptomatic in 23.8% of usual-care patients and 79.8% of those with an ILR.
Bradyarrhythmia was significantly more likely among older patients, male patients, and those with a history of syncope, the group reported.
Pacemakers were implanted for bradyarrhythmia in 2.9% of usual-care patients and 4.5% of those with ILR monitoring for an HR of 1.53 (95% CI, 1.14-2.06, P < .001).
Among usual-care patients, bradyarrhythmia (vs no bradyarrhythmia) was associated with 5.2 times the risk for incident syncope (P < .001). That risk for syncope went up 2.6 times (P = .01) in the ILR group.
The corresponding risks for CV death among controls and among ILR patients increased 4.8 times (P < .001) and by 3.1 (P < .001), respectively. The risks for death from any cause tripled (P < .001) and rose 2.5 times (P < .001) among bradycardic controls and ILR patients, respectively.
Bradyarrhythmia was not significantly related to sudden cardiac death in either group, the report noted.
Given the increasing use of heart rhythm monitoring “inside and outside the clinical setting,” it stated, “bradyarrhythmias are likely to be detected more often, sometimes as an incidental finding. Knowledge about the underlying prevalence and prognostic significance could help guide decisions.”
The study “teaches us a little bit” about the true prevalence of bradyarrhythmias in the general population, including asymptomatic cases that appear to be subclinical or “physiological,” Dr. Diederichsen said in an interview.
It also suggests that such bradycardia will be increasingly observed as use of ILR for arrhythmia screening expands in practice, he predicted. It may also be picked up more often by wearables and other rhythm-monitoring technology used by the public.
In the latter case especially, Dr. Diederichsen said, the current analysis could potentially help alleviate any concerns that bradyarrhythmia without symptoms is something that has to be specifically treated.
Dr. Diederichsen disclosed grants from several Danish research institutions, R. og Hustrus Fond, and Medtronic, as well as receiving personal fees from Vital Beats and Bristol-Myers Squibb/Pfizer. Dr. Schoenfeld reported ownership of stock from Apple. Dr. Patton reported employment as a medical officer for the Food and Drug Administration and serving as associate editor for JAMA Cardiology.
A version of this article originally appeared on Medscape.com.
Bradycardia is a lot more common than generally believed, but is often asymptomatic and not clinically relevant, and may lead to needless pacemaker therapy, suggests a post hoc analysis of a major study.
The arrhythmia’s presence overall in the randomized LOOP trial predicted an excess risk of syncope and death, and it didn’t matter how it was detected. Bradyarrhythmia revealed incidentally at long-term cardiac rhythm monitoring was no more predictive than when it was picked up in a usual-care setting.
Still, people in the trial with implantable loop recorders (ILR) had six times the chance of being diagnosed with bradyarrhythmias than those in the usual-care control group. LOOP entered older persons in the community without known arrhythmias but with risk factors like diabetes or hypertension.
About 80% of such arrhythmias at ILR monitoring were asymptomatic, compared with less than one-fourth in the usual-care group. Yet pacemaker implantation for bradyarrhythmia was 53% more likely in the ILR group, according to a report published in JAMA Cardiology.
Most participants with asymptomatic bradycardia did not receive treatment for it, yet the study – despite the mostly conservative management – still showed “overtreatment with pacemakers” in the ILR group, observed lead author Søren Zöga Diederichsen, MD, PhD, Copenhagen University Hospital–Rigshospitalet.
Bradyarrhythmia overall predicted later syncope and all-cause and cardiovascular (CV) death, but did so regardless of whether the patient was ILR monitored or received a pacemaker, Diederichsen said in an interview.
“We didn’t see any signal, not even a small signal, toward a health benefit from monitoring and detecting bradycardias, or from acting on them conservatively or implanting pacemakers,” he noted.
The study “emphasizes that you should have symptoms” to justify pacemaker therapy for bradyarrhythmias, regardless of how they were detected, Dr. Diederichsen said.
“Clearly ILRs may identify patients with bradyarrhythmias deserving of treatment” when they are associated with symptoms, an accompanying editorial agreed. In the current analysis, however, “a large proportion of bradycardic events were completely asymptomatic.” Yet bradycardia predicted syncope and CV death in both the ILR and usual care groups, it noted.
“This does raise the question as to whether bradyarrhythmia may be a risk marker for underlying nonarrhythmic conditions to which preventive strategies and treatment should be directed,” wrote editorialists Mark H. Schoenfeld, MD, Yale University, New Haven, Conn., and Kristen K. Patton, MD, University of Washington, Seattle.
“In an aging population with ever-increasing comorbidities, it may become increasingly important to rule out bradycardia as a manifestation of a more sinister underlying disease,” they noted, and to identify “patients who may be particularly vulnerable to adverse outcomes of progressive distal conduction disease.”
The previously published LOOP trial, conducted at four sites in Denmark, compared ILR screening for atrial fibrillation to usual care in 6,004 patients at least 70 years or older, most with hypertension. The main results showed little benefit from screening for atrial fibrillation in prevention of incident stroke or systemic embolism over about 5 years.
The current LOOP analysis, post hoc with all the associated limitations, followed incident bradyarrhythmia in the ILR and usual-care groups; any treatment of the arrhythmia was at physician discretion. The total cohort averaged 75 years in age and 47.3% were women.
The rate of incident bradyarrhythmia was 8.1% overall; it was 20.8% for those with ILR monitoring and 3.8% in the usual care group, for a hazard ratio of 6.21 (95% confidence interval, 5.15-7.48, P < .001).
The arrhythmia was asymptomatic in 23.8% of usual-care patients and 79.8% of those with an ILR.
Bradyarrhythmia was significantly more likely among older patients, male patients, and those with a history of syncope, the group reported.
Pacemakers were implanted for bradyarrhythmia in 2.9% of usual-care patients and 4.5% of those with ILR monitoring for an HR of 1.53 (95% CI, 1.14-2.06, P < .001).
Among usual-care patients, bradyarrhythmia (vs no bradyarrhythmia) was associated with 5.2 times the risk for incident syncope (P < .001). That risk for syncope went up 2.6 times (P = .01) in the ILR group.
The corresponding risks for CV death among controls and among ILR patients increased 4.8 times (P < .001) and by 3.1 (P < .001), respectively. The risks for death from any cause tripled (P < .001) and rose 2.5 times (P < .001) among bradycardic controls and ILR patients, respectively.
Bradyarrhythmia was not significantly related to sudden cardiac death in either group, the report noted.
Given the increasing use of heart rhythm monitoring “inside and outside the clinical setting,” it stated, “bradyarrhythmias are likely to be detected more often, sometimes as an incidental finding. Knowledge about the underlying prevalence and prognostic significance could help guide decisions.”
The study “teaches us a little bit” about the true prevalence of bradyarrhythmias in the general population, including asymptomatic cases that appear to be subclinical or “physiological,” Dr. Diederichsen said in an interview.
It also suggests that such bradycardia will be increasingly observed as use of ILR for arrhythmia screening expands in practice, he predicted. It may also be picked up more often by wearables and other rhythm-monitoring technology used by the public.
In the latter case especially, Dr. Diederichsen said, the current analysis could potentially help alleviate any concerns that bradyarrhythmia without symptoms is something that has to be specifically treated.
Dr. Diederichsen disclosed grants from several Danish research institutions, R. og Hustrus Fond, and Medtronic, as well as receiving personal fees from Vital Beats and Bristol-Myers Squibb/Pfizer. Dr. Schoenfeld reported ownership of stock from Apple. Dr. Patton reported employment as a medical officer for the Food and Drug Administration and serving as associate editor for JAMA Cardiology.
A version of this article originally appeared on Medscape.com.
Bradycardia is a lot more common than generally believed, but is often asymptomatic and not clinically relevant, and may lead to needless pacemaker therapy, suggests a post hoc analysis of a major study.
The arrhythmia’s presence overall in the randomized LOOP trial predicted an excess risk of syncope and death, and it didn’t matter how it was detected. Bradyarrhythmia revealed incidentally at long-term cardiac rhythm monitoring was no more predictive than when it was picked up in a usual-care setting.
Still, people in the trial with implantable loop recorders (ILR) had six times the chance of being diagnosed with bradyarrhythmias than those in the usual-care control group. LOOP entered older persons in the community without known arrhythmias but with risk factors like diabetes or hypertension.
About 80% of such arrhythmias at ILR monitoring were asymptomatic, compared with less than one-fourth in the usual-care group. Yet pacemaker implantation for bradyarrhythmia was 53% more likely in the ILR group, according to a report published in JAMA Cardiology.
Most participants with asymptomatic bradycardia did not receive treatment for it, yet the study – despite the mostly conservative management – still showed “overtreatment with pacemakers” in the ILR group, observed lead author Søren Zöga Diederichsen, MD, PhD, Copenhagen University Hospital–Rigshospitalet.
Bradyarrhythmia overall predicted later syncope and all-cause and cardiovascular (CV) death, but did so regardless of whether the patient was ILR monitored or received a pacemaker, Diederichsen said in an interview.
“We didn’t see any signal, not even a small signal, toward a health benefit from monitoring and detecting bradycardias, or from acting on them conservatively or implanting pacemakers,” he noted.
The study “emphasizes that you should have symptoms” to justify pacemaker therapy for bradyarrhythmias, regardless of how they were detected, Dr. Diederichsen said.
“Clearly ILRs may identify patients with bradyarrhythmias deserving of treatment” when they are associated with symptoms, an accompanying editorial agreed. In the current analysis, however, “a large proportion of bradycardic events were completely asymptomatic.” Yet bradycardia predicted syncope and CV death in both the ILR and usual care groups, it noted.
“This does raise the question as to whether bradyarrhythmia may be a risk marker for underlying nonarrhythmic conditions to which preventive strategies and treatment should be directed,” wrote editorialists Mark H. Schoenfeld, MD, Yale University, New Haven, Conn., and Kristen K. Patton, MD, University of Washington, Seattle.
“In an aging population with ever-increasing comorbidities, it may become increasingly important to rule out bradycardia as a manifestation of a more sinister underlying disease,” they noted, and to identify “patients who may be particularly vulnerable to adverse outcomes of progressive distal conduction disease.”
The previously published LOOP trial, conducted at four sites in Denmark, compared ILR screening for atrial fibrillation to usual care in 6,004 patients at least 70 years or older, most with hypertension. The main results showed little benefit from screening for atrial fibrillation in prevention of incident stroke or systemic embolism over about 5 years.
The current LOOP analysis, post hoc with all the associated limitations, followed incident bradyarrhythmia in the ILR and usual-care groups; any treatment of the arrhythmia was at physician discretion. The total cohort averaged 75 years in age and 47.3% were women.
The rate of incident bradyarrhythmia was 8.1% overall; it was 20.8% for those with ILR monitoring and 3.8% in the usual care group, for a hazard ratio of 6.21 (95% confidence interval, 5.15-7.48, P < .001).
The arrhythmia was asymptomatic in 23.8% of usual-care patients and 79.8% of those with an ILR.
Bradyarrhythmia was significantly more likely among older patients, male patients, and those with a history of syncope, the group reported.
Pacemakers were implanted for bradyarrhythmia in 2.9% of usual-care patients and 4.5% of those with ILR monitoring for an HR of 1.53 (95% CI, 1.14-2.06, P < .001).
Among usual-care patients, bradyarrhythmia (vs no bradyarrhythmia) was associated with 5.2 times the risk for incident syncope (P < .001). That risk for syncope went up 2.6 times (P = .01) in the ILR group.
The corresponding risks for CV death among controls and among ILR patients increased 4.8 times (P < .001) and by 3.1 (P < .001), respectively. The risks for death from any cause tripled (P < .001) and rose 2.5 times (P < .001) among bradycardic controls and ILR patients, respectively.
Bradyarrhythmia was not significantly related to sudden cardiac death in either group, the report noted.
Given the increasing use of heart rhythm monitoring “inside and outside the clinical setting,” it stated, “bradyarrhythmias are likely to be detected more often, sometimes as an incidental finding. Knowledge about the underlying prevalence and prognostic significance could help guide decisions.”
The study “teaches us a little bit” about the true prevalence of bradyarrhythmias in the general population, including asymptomatic cases that appear to be subclinical or “physiological,” Dr. Diederichsen said in an interview.
It also suggests that such bradycardia will be increasingly observed as use of ILR for arrhythmia screening expands in practice, he predicted. It may also be picked up more often by wearables and other rhythm-monitoring technology used by the public.
In the latter case especially, Dr. Diederichsen said, the current analysis could potentially help alleviate any concerns that bradyarrhythmia without symptoms is something that has to be specifically treated.
Dr. Diederichsen disclosed grants from several Danish research institutions, R. og Hustrus Fond, and Medtronic, as well as receiving personal fees from Vital Beats and Bristol-Myers Squibb/Pfizer. Dr. Schoenfeld reported ownership of stock from Apple. Dr. Patton reported employment as a medical officer for the Food and Drug Administration and serving as associate editor for JAMA Cardiology.
A version of this article originally appeared on Medscape.com.
FROM JAMA CARDIOLOGY
Cardiologists weigh in on ethically challenging issues
Would you tell a patient about a potentially harmful medical mistake? Would you upcode or overstate a patient’s condition so an insurer will cover it? What about reporting a colleague who seems impaired or engages in sexual harassment or bullying?
In a new survey, this news organization asked more than 4,100 U.S. physicians how they would react to these and other ethically challenging scenarios.
For example, a full 80% of cardiologists responding to the survey said they would reveal a potentially harmful medical mistake to their patient.
This aligns with decades of advice from major medical societies such as the American Medical Association and the American College of Physicians, which endorse disclosing to patients and families any error that could jeopardize the patient’s health.
“Disclosure of close calls should also be made. From a health law context, being upfront with the patient is standard practice,” said Eric Mathison, PhD, a clinical ethicist at University of Toronto.
When it comes to upcoding or overstating a patient’s condition so an insurer will cover it, more than three quarters of cardiologists (78%) viewed this as unacceptable, while 9% felt it was okay and 13% said “it depends.”
Many doctors are willing to stretch coding policies to the limit to support patients and their finances, said Arthur L. Caplan, PhD, NYU professor of bioethics and Medscape blogger. “That’s acceptable advocacy. But most doctors will not say they are willing to commit fraud.”
Okay to breach patient confidentiality?
More than half of cardiologists felt it was okay to breach patient confidentiality when someone’s health could be threatened, 14% felt the opposite, and 29% said it depends.
“I teach that if you know someone faces a direct risk from catching a deadly disease, and you know who that person is, then you have a duty to warn,” Dr. Caplan said. “The disease has to be serious for [breaching confidentiality] to be morally defensible, and your disclosure has to be actionable. Telling your mother won’t achieve a lot” in protecting someone’s health.
In a 2020 ethics survey by this news organization, 72% of cardiologists felt that they could accept a meal or speaking gig from a drug company without its creating any issue for them.
Three years later, only 66% of cardiologists said they could accept a meal or speaking engagement without its influencing their prescribing habits; 21% said they couldn’t and 13% said it depends.
Dr. Caplan thinks that many doctors are deceiving themselves. “We know from business school case studies that even little gifts like calendars and flashlights work. Humans get a sense of debt when they receive gifts. Physicians are no exception. If you get a meal or an invitation to do a talk for a small fee, you may still say, ‘This is nothing to me,’ ” but subconscious favoritism can result, he cautioned.
Support for physician-assisted dying?
Ten states and the District of Columbia now allow physicians to help a terminally ill patient with dying. Fifty percent of cardiologists surveyed support it, 36% are against it, and 14% said it depends. These percentages are roughly the same as in 2020.
Dr. Mathison said the public and physicians are “getting more comfortable with physician-assisted dying. Physicians are seeing it used in practice and hearing from other physicians who are participating.”
However, only 31% of cardiologists felt physician-assisted dying should be allowed for patients in intractable pain; 42% said it should not be legal in this case, and 26% said it depends.
As opposed to physician-assisted dying for terminally ill patients, no U.S. state recognizes the legal right to help end the life of a patient in unending pain. However, Belgium, the Netherlands, and Luxembourg do under certain conditions.
Going public about issues with a cardiologist’s hospital or health care organization became a major issue during the COVID-19 pandemic as some medical professionals struggled to get enough personal protective equipment and made it known.
More than half of cardiologists surveyed (53%) endorsed speaking out if employers don’t provide needed resources; 9% didn’t feel this was appropriate, and 28% said it depends.
Dr. Caplan noted that prominent cases of hospitals firing nurses and doctors who complained over social media may influence cardiologists’ willingness. He also thinks some doctors would ask, “Speak out to whom?” Many cardiologists will aggressively push for resources through the internal chain of command “but don’t think talking to the media is ethical or appropriate.”
The vast majority of cardiologists and physicians overall said they have never failed to report or investigate suspected domestic abuse of a patient.
Both male and female physicians strongly support reporting of abuse cases, said Thomas May, PhD, a bioethicist at Washington State University, Spokane.
This reflects the “tremendous strides society has made in recognizing the impact of abuse and the need for required-reporting policies, because victims are often, if not usually, reticent to come forward. Required reporting is necessary and in the patient’s interests,” Dr. May said.
Romancing a patient?
More than half (58%) of cardiologists felt that having a romantic relationship with a current patient is not okay; 3% were okay with it, and 30% felt it would be okay at least 6 months after the patient-doctor relationship ended.
Dr. May said a romantic relationship is “inappropriate while the professional relationship is active and even for some time afterward. There’s a professional dynamic that needs to be maintained, a sense of objectivity.
“Plus, the physician is in a power relationship to the patient where there’s a sense of gratefulness or vulnerability that makes the patient unable to say no to a personal relationship,” Dr. May said.
Dr. May is not sure 6 months after they stop being your patient is long enough. “I’d think something like 2 years as a minimum. If I were your oncologist and helped save your life, it may never be appropriate,” Dr. May said.
In other ethical questions, one-quarter of cardiologists would report a doctor who seems impaired by drugs, alcohol, or illness, and 62% would do so only after speaking to him/her first.
“Our obligation is to do no harm to patients, and the professional standards and integrity of the profession are at stake,” one survey respondent said.
Another said, “A colleague who recognizes the problem and after private discussion enters a treatment program is often better served than by the often excessively harsh management by the state medical board.”
But when it comes to random alcohol and drug tests for cardiologists, 51% are not in favor, 31% are in favor, and 18% said it depends.
Dr. Caplan thinks that physicians face enough responsibility to patients to warrant such testing randomly but infrequently. “Doctors may feel like they’re being treated unprofessionally, like drug addicts, or question the accuracy of testing,” he noted. But he tilts instead toward “the moral fight to protect patient safety and trying to drive down malpractice costs.”
When it comes to reporting a colleague for sexual harassment or bullying, 71% of cardiologists said yes, they would report such behavior; only 7% would not, while 22% said it depends.
“If we ignore bad behavior such as this by our colleagues, then we are hurting our profession,” one physician said.
A version of this article originally appeared on Medscape.com.
Would you tell a patient about a potentially harmful medical mistake? Would you upcode or overstate a patient’s condition so an insurer will cover it? What about reporting a colleague who seems impaired or engages in sexual harassment or bullying?
In a new survey, this news organization asked more than 4,100 U.S. physicians how they would react to these and other ethically challenging scenarios.
For example, a full 80% of cardiologists responding to the survey said they would reveal a potentially harmful medical mistake to their patient.
This aligns with decades of advice from major medical societies such as the American Medical Association and the American College of Physicians, which endorse disclosing to patients and families any error that could jeopardize the patient’s health.
“Disclosure of close calls should also be made. From a health law context, being upfront with the patient is standard practice,” said Eric Mathison, PhD, a clinical ethicist at University of Toronto.
When it comes to upcoding or overstating a patient’s condition so an insurer will cover it, more than three quarters of cardiologists (78%) viewed this as unacceptable, while 9% felt it was okay and 13% said “it depends.”
Many doctors are willing to stretch coding policies to the limit to support patients and their finances, said Arthur L. Caplan, PhD, NYU professor of bioethics and Medscape blogger. “That’s acceptable advocacy. But most doctors will not say they are willing to commit fraud.”
Okay to breach patient confidentiality?
More than half of cardiologists felt it was okay to breach patient confidentiality when someone’s health could be threatened, 14% felt the opposite, and 29% said it depends.
“I teach that if you know someone faces a direct risk from catching a deadly disease, and you know who that person is, then you have a duty to warn,” Dr. Caplan said. “The disease has to be serious for [breaching confidentiality] to be morally defensible, and your disclosure has to be actionable. Telling your mother won’t achieve a lot” in protecting someone’s health.
In a 2020 ethics survey by this news organization, 72% of cardiologists felt that they could accept a meal or speaking gig from a drug company without its creating any issue for them.
Three years later, only 66% of cardiologists said they could accept a meal or speaking engagement without its influencing their prescribing habits; 21% said they couldn’t and 13% said it depends.
Dr. Caplan thinks that many doctors are deceiving themselves. “We know from business school case studies that even little gifts like calendars and flashlights work. Humans get a sense of debt when they receive gifts. Physicians are no exception. If you get a meal or an invitation to do a talk for a small fee, you may still say, ‘This is nothing to me,’ ” but subconscious favoritism can result, he cautioned.
Support for physician-assisted dying?
Ten states and the District of Columbia now allow physicians to help a terminally ill patient with dying. Fifty percent of cardiologists surveyed support it, 36% are against it, and 14% said it depends. These percentages are roughly the same as in 2020.
Dr. Mathison said the public and physicians are “getting more comfortable with physician-assisted dying. Physicians are seeing it used in practice and hearing from other physicians who are participating.”
However, only 31% of cardiologists felt physician-assisted dying should be allowed for patients in intractable pain; 42% said it should not be legal in this case, and 26% said it depends.
As opposed to physician-assisted dying for terminally ill patients, no U.S. state recognizes the legal right to help end the life of a patient in unending pain. However, Belgium, the Netherlands, and Luxembourg do under certain conditions.
Going public about issues with a cardiologist’s hospital or health care organization became a major issue during the COVID-19 pandemic as some medical professionals struggled to get enough personal protective equipment and made it known.
More than half of cardiologists surveyed (53%) endorsed speaking out if employers don’t provide needed resources; 9% didn’t feel this was appropriate, and 28% said it depends.
Dr. Caplan noted that prominent cases of hospitals firing nurses and doctors who complained over social media may influence cardiologists’ willingness. He also thinks some doctors would ask, “Speak out to whom?” Many cardiologists will aggressively push for resources through the internal chain of command “but don’t think talking to the media is ethical or appropriate.”
The vast majority of cardiologists and physicians overall said they have never failed to report or investigate suspected domestic abuse of a patient.
Both male and female physicians strongly support reporting of abuse cases, said Thomas May, PhD, a bioethicist at Washington State University, Spokane.
This reflects the “tremendous strides society has made in recognizing the impact of abuse and the need for required-reporting policies, because victims are often, if not usually, reticent to come forward. Required reporting is necessary and in the patient’s interests,” Dr. May said.
Romancing a patient?
More than half (58%) of cardiologists felt that having a romantic relationship with a current patient is not okay; 3% were okay with it, and 30% felt it would be okay at least 6 months after the patient-doctor relationship ended.
Dr. May said a romantic relationship is “inappropriate while the professional relationship is active and even for some time afterward. There’s a professional dynamic that needs to be maintained, a sense of objectivity.
“Plus, the physician is in a power relationship to the patient where there’s a sense of gratefulness or vulnerability that makes the patient unable to say no to a personal relationship,” Dr. May said.
Dr. May is not sure 6 months after they stop being your patient is long enough. “I’d think something like 2 years as a minimum. If I were your oncologist and helped save your life, it may never be appropriate,” Dr. May said.
In other ethical questions, one-quarter of cardiologists would report a doctor who seems impaired by drugs, alcohol, or illness, and 62% would do so only after speaking to him/her first.
“Our obligation is to do no harm to patients, and the professional standards and integrity of the profession are at stake,” one survey respondent said.
Another said, “A colleague who recognizes the problem and after private discussion enters a treatment program is often better served than by the often excessively harsh management by the state medical board.”
But when it comes to random alcohol and drug tests for cardiologists, 51% are not in favor, 31% are in favor, and 18% said it depends.
Dr. Caplan thinks that physicians face enough responsibility to patients to warrant such testing randomly but infrequently. “Doctors may feel like they’re being treated unprofessionally, like drug addicts, or question the accuracy of testing,” he noted. But he tilts instead toward “the moral fight to protect patient safety and trying to drive down malpractice costs.”
When it comes to reporting a colleague for sexual harassment or bullying, 71% of cardiologists said yes, they would report such behavior; only 7% would not, while 22% said it depends.
“If we ignore bad behavior such as this by our colleagues, then we are hurting our profession,” one physician said.
A version of this article originally appeared on Medscape.com.
Would you tell a patient about a potentially harmful medical mistake? Would you upcode or overstate a patient’s condition so an insurer will cover it? What about reporting a colleague who seems impaired or engages in sexual harassment or bullying?
In a new survey, this news organization asked more than 4,100 U.S. physicians how they would react to these and other ethically challenging scenarios.
For example, a full 80% of cardiologists responding to the survey said they would reveal a potentially harmful medical mistake to their patient.
This aligns with decades of advice from major medical societies such as the American Medical Association and the American College of Physicians, which endorse disclosing to patients and families any error that could jeopardize the patient’s health.
“Disclosure of close calls should also be made. From a health law context, being upfront with the patient is standard practice,” said Eric Mathison, PhD, a clinical ethicist at University of Toronto.
When it comes to upcoding or overstating a patient’s condition so an insurer will cover it, more than three quarters of cardiologists (78%) viewed this as unacceptable, while 9% felt it was okay and 13% said “it depends.”
Many doctors are willing to stretch coding policies to the limit to support patients and their finances, said Arthur L. Caplan, PhD, NYU professor of bioethics and Medscape blogger. “That’s acceptable advocacy. But most doctors will not say they are willing to commit fraud.”
Okay to breach patient confidentiality?
More than half of cardiologists felt it was okay to breach patient confidentiality when someone’s health could be threatened, 14% felt the opposite, and 29% said it depends.
“I teach that if you know someone faces a direct risk from catching a deadly disease, and you know who that person is, then you have a duty to warn,” Dr. Caplan said. “The disease has to be serious for [breaching confidentiality] to be morally defensible, and your disclosure has to be actionable. Telling your mother won’t achieve a lot” in protecting someone’s health.
In a 2020 ethics survey by this news organization, 72% of cardiologists felt that they could accept a meal or speaking gig from a drug company without its creating any issue for them.
Three years later, only 66% of cardiologists said they could accept a meal or speaking engagement without its influencing their prescribing habits; 21% said they couldn’t and 13% said it depends.
Dr. Caplan thinks that many doctors are deceiving themselves. “We know from business school case studies that even little gifts like calendars and flashlights work. Humans get a sense of debt when they receive gifts. Physicians are no exception. If you get a meal or an invitation to do a talk for a small fee, you may still say, ‘This is nothing to me,’ ” but subconscious favoritism can result, he cautioned.
Support for physician-assisted dying?
Ten states and the District of Columbia now allow physicians to help a terminally ill patient with dying. Fifty percent of cardiologists surveyed support it, 36% are against it, and 14% said it depends. These percentages are roughly the same as in 2020.
Dr. Mathison said the public and physicians are “getting more comfortable with physician-assisted dying. Physicians are seeing it used in practice and hearing from other physicians who are participating.”
However, only 31% of cardiologists felt physician-assisted dying should be allowed for patients in intractable pain; 42% said it should not be legal in this case, and 26% said it depends.
As opposed to physician-assisted dying for terminally ill patients, no U.S. state recognizes the legal right to help end the life of a patient in unending pain. However, Belgium, the Netherlands, and Luxembourg do under certain conditions.
Going public about issues with a cardiologist’s hospital or health care organization became a major issue during the COVID-19 pandemic as some medical professionals struggled to get enough personal protective equipment and made it known.
More than half of cardiologists surveyed (53%) endorsed speaking out if employers don’t provide needed resources; 9% didn’t feel this was appropriate, and 28% said it depends.
Dr. Caplan noted that prominent cases of hospitals firing nurses and doctors who complained over social media may influence cardiologists’ willingness. He also thinks some doctors would ask, “Speak out to whom?” Many cardiologists will aggressively push for resources through the internal chain of command “but don’t think talking to the media is ethical or appropriate.”
The vast majority of cardiologists and physicians overall said they have never failed to report or investigate suspected domestic abuse of a patient.
Both male and female physicians strongly support reporting of abuse cases, said Thomas May, PhD, a bioethicist at Washington State University, Spokane.
This reflects the “tremendous strides society has made in recognizing the impact of abuse and the need for required-reporting policies, because victims are often, if not usually, reticent to come forward. Required reporting is necessary and in the patient’s interests,” Dr. May said.
Romancing a patient?
More than half (58%) of cardiologists felt that having a romantic relationship with a current patient is not okay; 3% were okay with it, and 30% felt it would be okay at least 6 months after the patient-doctor relationship ended.
Dr. May said a romantic relationship is “inappropriate while the professional relationship is active and even for some time afterward. There’s a professional dynamic that needs to be maintained, a sense of objectivity.
“Plus, the physician is in a power relationship to the patient where there’s a sense of gratefulness or vulnerability that makes the patient unable to say no to a personal relationship,” Dr. May said.
Dr. May is not sure 6 months after they stop being your patient is long enough. “I’d think something like 2 years as a minimum. If I were your oncologist and helped save your life, it may never be appropriate,” Dr. May said.
In other ethical questions, one-quarter of cardiologists would report a doctor who seems impaired by drugs, alcohol, or illness, and 62% would do so only after speaking to him/her first.
“Our obligation is to do no harm to patients, and the professional standards and integrity of the profession are at stake,” one survey respondent said.
Another said, “A colleague who recognizes the problem and after private discussion enters a treatment program is often better served than by the often excessively harsh management by the state medical board.”
But when it comes to random alcohol and drug tests for cardiologists, 51% are not in favor, 31% are in favor, and 18% said it depends.
Dr. Caplan thinks that physicians face enough responsibility to patients to warrant such testing randomly but infrequently. “Doctors may feel like they’re being treated unprofessionally, like drug addicts, or question the accuracy of testing,” he noted. But he tilts instead toward “the moral fight to protect patient safety and trying to drive down malpractice costs.”
When it comes to reporting a colleague for sexual harassment or bullying, 71% of cardiologists said yes, they would report such behavior; only 7% would not, while 22% said it depends.
“If we ignore bad behavior such as this by our colleagues, then we are hurting our profession,” one physician said.
A version of this article originally appeared on Medscape.com.
VA Plans to Waive Health Care Copayments for American Indian Veterans
New VA rule proposes to eliminate many copays for Native American veteran.
The US Department of Veterans Affairs (VA) has proposed a new rule that would waive medical copayments incurred on or after January 5, 2022, for eligible American Indian and Alaska Native (AI/AN) veterans.
The policy is intended to encourage veterans to seek regular primary care treatment, the VA says. “It’s no mystery to a lot of people that health care is sometimes hard to come by in many Native American communities,” Travis Trueblood, director of the VA Office of Tribal Health, told reporters in January. “So, this effort by VA will enhance getting people into the facilities, helping them feel welcome and getting them to use those benefits that they've earned.”
Copayments for more than 3 visits to community-based urgent care in any calendar year would still be required. Follow-up care provided by a VA-authorized primary care provider would be exempt from copays. Members of federally recognized tribes are already exempt from copays at Indian Health Service clinics.
Eligibility may be based in part on documentation issued by AI/AN tribal governments to show tribal membership. The VA has proposed the documentation requirement “as this recognizes tribal sovereignty and promotes the Nation-to-Nation relationship that exists between the United States and tribal governments.” The requirement, the notice says, is consistent with the preferences of tribal leaders.
The regulation implements a requirement in the Johnny Isakson and David P. Roe, MD, Veterans Health Care and Benefits Improvement Act of 2020, which prohibited the VA from collecting copayments from AI/AN veterans for hospital care or medical services. Senator Jon Tester (D-MT), chair of the Senate Veterans’ Affairs Committee, and Senator Jerry Moran (R-KS) introduced legislation in 2020 to enact the new policy in January 2022 , which is why the rule is retroactive.
Congress passed the measure as part of a package of veterans’ legislation at the end of 2020, and then-President Donald Trump signed it into law in January 2021. Trueblood said the nature of the federal rulemaking process makes it hard to say exactly when the change will take effect, but that no veteran will be turned away from VA care for not making a copayment, even before the rule is finalized. The VA plans to reimburse eligible veterans who received care in the past year for copayment costs.
“I’m encouraged to see VA answering my call to implement the law and remove burdensome copayments for Native veterans accessing their earned health care,” said Tester in a press release. “The fact is Native veterans have bravely answered the call to duty for generations. And I’ll continue to hold VA accountable in delivering these veterans their long-overdue support.”
New VA rule proposes to eliminate many copays for Native American veteran.
The US Department of Veterans Affairs (VA) has proposed a new rule that would waive medical copayments incurred on or after January 5, 2022, for eligible American Indian and Alaska Native (AI/AN) veterans.
The policy is intended to encourage veterans to seek regular primary care treatment, the VA says. “It’s no mystery to a lot of people that health care is sometimes hard to come by in many Native American communities,” Travis Trueblood, director of the VA Office of Tribal Health, told reporters in January. “So, this effort by VA will enhance getting people into the facilities, helping them feel welcome and getting them to use those benefits that they've earned.”
Copayments for more than 3 visits to community-based urgent care in any calendar year would still be required. Follow-up care provided by a VA-authorized primary care provider would be exempt from copays. Members of federally recognized tribes are already exempt from copays at Indian Health Service clinics.
Eligibility may be based in part on documentation issued by AI/AN tribal governments to show tribal membership. The VA has proposed the documentation requirement “as this recognizes tribal sovereignty and promotes the Nation-to-Nation relationship that exists between the United States and tribal governments.” The requirement, the notice says, is consistent with the preferences of tribal leaders.
The regulation implements a requirement in the Johnny Isakson and David P. Roe, MD, Veterans Health Care and Benefits Improvement Act of 2020, which prohibited the VA from collecting copayments from AI/AN veterans for hospital care or medical services. Senator Jon Tester (D-MT), chair of the Senate Veterans’ Affairs Committee, and Senator Jerry Moran (R-KS) introduced legislation in 2020 to enact the new policy in January 2022 , which is why the rule is retroactive.
Congress passed the measure as part of a package of veterans’ legislation at the end of 2020, and then-President Donald Trump signed it into law in January 2021. Trueblood said the nature of the federal rulemaking process makes it hard to say exactly when the change will take effect, but that no veteran will be turned away from VA care for not making a copayment, even before the rule is finalized. The VA plans to reimburse eligible veterans who received care in the past year for copayment costs.
“I’m encouraged to see VA answering my call to implement the law and remove burdensome copayments for Native veterans accessing their earned health care,” said Tester in a press release. “The fact is Native veterans have bravely answered the call to duty for generations. And I’ll continue to hold VA accountable in delivering these veterans their long-overdue support.”
New VA rule proposes to eliminate many copays for Native American veteran.
The US Department of Veterans Affairs (VA) has proposed a new rule that would waive medical copayments incurred on or after January 5, 2022, for eligible American Indian and Alaska Native (AI/AN) veterans.
The policy is intended to encourage veterans to seek regular primary care treatment, the VA says. “It’s no mystery to a lot of people that health care is sometimes hard to come by in many Native American communities,” Travis Trueblood, director of the VA Office of Tribal Health, told reporters in January. “So, this effort by VA will enhance getting people into the facilities, helping them feel welcome and getting them to use those benefits that they've earned.”
Copayments for more than 3 visits to community-based urgent care in any calendar year would still be required. Follow-up care provided by a VA-authorized primary care provider would be exempt from copays. Members of federally recognized tribes are already exempt from copays at Indian Health Service clinics.
Eligibility may be based in part on documentation issued by AI/AN tribal governments to show tribal membership. The VA has proposed the documentation requirement “as this recognizes tribal sovereignty and promotes the Nation-to-Nation relationship that exists between the United States and tribal governments.” The requirement, the notice says, is consistent with the preferences of tribal leaders.
The regulation implements a requirement in the Johnny Isakson and David P. Roe, MD, Veterans Health Care and Benefits Improvement Act of 2020, which prohibited the VA from collecting copayments from AI/AN veterans for hospital care or medical services. Senator Jon Tester (D-MT), chair of the Senate Veterans’ Affairs Committee, and Senator Jerry Moran (R-KS) introduced legislation in 2020 to enact the new policy in January 2022 , which is why the rule is retroactive.
Congress passed the measure as part of a package of veterans’ legislation at the end of 2020, and then-President Donald Trump signed it into law in January 2021. Trueblood said the nature of the federal rulemaking process makes it hard to say exactly when the change will take effect, but that no veteran will be turned away from VA care for not making a copayment, even before the rule is finalized. The VA plans to reimburse eligible veterans who received care in the past year for copayment costs.
“I’m encouraged to see VA answering my call to implement the law and remove burdensome copayments for Native veterans accessing their earned health care,” said Tester in a press release. “The fact is Native veterans have bravely answered the call to duty for generations. And I’ll continue to hold VA accountable in delivering these veterans their long-overdue support.”