User login
Intranasal zavegepant shows potential as an effective treatment option for acute migraine
Key clinical point: Zavegepant nasal spray was effective in the acute treatment of migraine, with favorable tolerability and safety profiles.
Major finding: At 2 hours post-dose, higher proportions of patients treated with zavegepant vs placebo were free from pain (risk difference 8.8 percentage points; P < .0001) and from their most bothersome symptom (risk difference 8.7 percentage points; P = .0012). Dysgeusia (21% vs 5%), nasal discomfort (4% vs 1%), and nausea (3% vs 1%) were the most common adverse events in the zavegepant vs placebo group.
Study details: Findings are from a phase 3 trial including 1405 patients with ≥1-year history of migraine with or without aura who were randomly assigned to receive zavegepant 10 mg nasal spray (n = 703) or matching placebo (n = 702).
Disclosures: This study was funded by Biohaven Pharmaceuticals. Some authors declared being employees of and holding stocks or stock options in Biohaven Pharmaceuticals. Some others declared ties with various sources, including Biohaven Pharmaceuticals.
Source: Lipton RB et al. Safety, tolerability, and efficacy of zavegepant 10 mg nasal spray for the acute treatment of migraine in the USA: A phase 3, double-blind, randomised, placebo-controlled multicentre trial. Lancet Neurol. 2023;22(3):209-217 (Mar). Doi: 10.1016/S1474-4422(22)00517-8
Key clinical point: Zavegepant nasal spray was effective in the acute treatment of migraine, with favorable tolerability and safety profiles.
Major finding: At 2 hours post-dose, higher proportions of patients treated with zavegepant vs placebo were free from pain (risk difference 8.8 percentage points; P < .0001) and from their most bothersome symptom (risk difference 8.7 percentage points; P = .0012). Dysgeusia (21% vs 5%), nasal discomfort (4% vs 1%), and nausea (3% vs 1%) were the most common adverse events in the zavegepant vs placebo group.
Study details: Findings are from a phase 3 trial including 1405 patients with ≥1-year history of migraine with or without aura who were randomly assigned to receive zavegepant 10 mg nasal spray (n = 703) or matching placebo (n = 702).
Disclosures: This study was funded by Biohaven Pharmaceuticals. Some authors declared being employees of and holding stocks or stock options in Biohaven Pharmaceuticals. Some others declared ties with various sources, including Biohaven Pharmaceuticals.
Source: Lipton RB et al. Safety, tolerability, and efficacy of zavegepant 10 mg nasal spray for the acute treatment of migraine in the USA: A phase 3, double-blind, randomised, placebo-controlled multicentre trial. Lancet Neurol. 2023;22(3):209-217 (Mar). Doi: 10.1016/S1474-4422(22)00517-8
Key clinical point: Zavegepant nasal spray was effective in the acute treatment of migraine, with favorable tolerability and safety profiles.
Major finding: At 2 hours post-dose, higher proportions of patients treated with zavegepant vs placebo were free from pain (risk difference 8.8 percentage points; P < .0001) and from their most bothersome symptom (risk difference 8.7 percentage points; P = .0012). Dysgeusia (21% vs 5%), nasal discomfort (4% vs 1%), and nausea (3% vs 1%) were the most common adverse events in the zavegepant vs placebo group.
Study details: Findings are from a phase 3 trial including 1405 patients with ≥1-year history of migraine with or without aura who were randomly assigned to receive zavegepant 10 mg nasal spray (n = 703) or matching placebo (n = 702).
Disclosures: This study was funded by Biohaven Pharmaceuticals. Some authors declared being employees of and holding stocks or stock options in Biohaven Pharmaceuticals. Some others declared ties with various sources, including Biohaven Pharmaceuticals.
Source: Lipton RB et al. Safety, tolerability, and efficacy of zavegepant 10 mg nasal spray for the acute treatment of migraine in the USA: A phase 3, double-blind, randomised, placebo-controlled multicentre trial. Lancet Neurol. 2023;22(3):209-217 (Mar). Doi: 10.1016/S1474-4422(22)00517-8
Spotting STIs: Vaginal swabs work best
Vaginal swabs are more effective than urine analysis in detecting certain types of sexually transmitted infections, researchers have found.
In the study, which was published online in the Annals of Family Medicine, investigators found that the diagnostic sensitivity of commercially available vaginal swabs was significantly greater than that of urine tests in detecting certain infections, including those caused by Chlamydia trachomatis, Neisseria gonorrhoeae, and Trichomonas vaginalis.
Researchers studied chlamydia and gonorrhea, which are two of the most frequently reported STIs in the United States. Trichomoniasis is the most curable nonviral STI globally, with 156 million cases worldwide in 2016.
The Centers for Disease Control and Prevention has long recommended that vaginal swabs be used to produce optimal samples.
But despite the CDC’s recommendation, urine analysis for these STIs is more commonly used than vaginal swabs among U.S. health care providers.
“We’re using a poor sample type, and we can do better,” said Barbara Van Der Pol, PhD, a professor of medicine and public health at the University of Alabama at Birmingham and an author of the new study, a meta-analysis of 97 studies published between 1995 and 2021.
Vaginal swabs for chlamydia trachomatis had a diagnostic sensitivity of 94.1% (95% confidence interval, 93.2%-94.9%; P < .001), higher than urine testing (86.9%; 95% CI, 85.6%-88.0%; P < .001). The pooled sensitivity estimates for Neisseria gonorrhoeae were 96.5% (95% CI, 94.8%-97.7%; P < .001) for vaginal swabs and 90.7% (95% CI, 88.4%-92.5%; P < .001) for urine specimens.
The difference in pooled sensitivity estimates between vaginal swabs and urine analyses for Trichomonas vaginalis was 98% (95% CI, 97.0%-98.7%; P < .001) for vaginal swabs and 95.1% (95% CI, 93.6%-96.3%) for urine specimens.
STIs included in the study are not typically found in the urethra and appear in urine analyses only if cervical or vaginal cells have dripped into a urine sample. Dr. Van Der Pol and her colleagues estimated that the use of urine samples rather than vaginal swabs may result in more than 400,000 undiagnosed infections annually.
Undiagnosed and untreated STIs can lead to transmissions of the infection as well as infertility and can have negative effects on romantic relationships, according to Dr. Van Der Pol.
Sarah Wood, MD, an attending physician at Children’s Hospital of Philadelphia, said some health care providers may use urine analysis because patients may be more comfortable with this method. The approach also can be more convenient for medical offices: All they must do is hand a specimen container to the patient.
Conversations between clinicians and patients about vaginal swabbing may be considered “sensitive” and the swabbing more invasive, Dr. Wood, an author of an editorial accompanying the journal article, said. Clinicians may also lack awareness that the swab is a more sensitive method of detecting these STIs.
“We all want to do what’s right for our patient, but we often don’t know what’s right for the patient,” Dr. Wood said. “I don’t think people are really aware of a potential real difference in outcomes with one method over the other.”
Dr. Wood advised making STI screening using vaginal swabs more common by “offering universal opt-out screening, so not waiting until you find out if someone’s having sex but just sort of saying, ‘Hey, across our practice, we screen everybody for chlamydia. Is that something that you want to do today?’ That approach sort of takes out the piece of talking about sex, talking about sexual activity.”
Dr. Van Der Pol, who said she has worked in STI diagnostics for 40 years, said she was not surprised by the results and hopes the study changes how samples are collected and used.
“I really hope that it influences practice so that we really start using vaginal swabs, because it gives us better diagnostics for chlamydia and gonorrhea,” Dr. Van Der Pol said.
“Also, then starting to think about comprehensive women’s care in such a way that they actually order other tests on that same sample if a woman is presenting with complaints.”
A version of this article originally appeared on Medscape.com.
Vaginal swabs are more effective than urine analysis in detecting certain types of sexually transmitted infections, researchers have found.
In the study, which was published online in the Annals of Family Medicine, investigators found that the diagnostic sensitivity of commercially available vaginal swabs was significantly greater than that of urine tests in detecting certain infections, including those caused by Chlamydia trachomatis, Neisseria gonorrhoeae, and Trichomonas vaginalis.
Researchers studied chlamydia and gonorrhea, which are two of the most frequently reported STIs in the United States. Trichomoniasis is the most curable nonviral STI globally, with 156 million cases worldwide in 2016.
The Centers for Disease Control and Prevention has long recommended that vaginal swabs be used to produce optimal samples.
But despite the CDC’s recommendation, urine analysis for these STIs is more commonly used than vaginal swabs among U.S. health care providers.
“We’re using a poor sample type, and we can do better,” said Barbara Van Der Pol, PhD, a professor of medicine and public health at the University of Alabama at Birmingham and an author of the new study, a meta-analysis of 97 studies published between 1995 and 2021.
Vaginal swabs for chlamydia trachomatis had a diagnostic sensitivity of 94.1% (95% confidence interval, 93.2%-94.9%; P < .001), higher than urine testing (86.9%; 95% CI, 85.6%-88.0%; P < .001). The pooled sensitivity estimates for Neisseria gonorrhoeae were 96.5% (95% CI, 94.8%-97.7%; P < .001) for vaginal swabs and 90.7% (95% CI, 88.4%-92.5%; P < .001) for urine specimens.
The difference in pooled sensitivity estimates between vaginal swabs and urine analyses for Trichomonas vaginalis was 98% (95% CI, 97.0%-98.7%; P < .001) for vaginal swabs and 95.1% (95% CI, 93.6%-96.3%) for urine specimens.
STIs included in the study are not typically found in the urethra and appear in urine analyses only if cervical or vaginal cells have dripped into a urine sample. Dr. Van Der Pol and her colleagues estimated that the use of urine samples rather than vaginal swabs may result in more than 400,000 undiagnosed infections annually.
Undiagnosed and untreated STIs can lead to transmissions of the infection as well as infertility and can have negative effects on romantic relationships, according to Dr. Van Der Pol.
Sarah Wood, MD, an attending physician at Children’s Hospital of Philadelphia, said some health care providers may use urine analysis because patients may be more comfortable with this method. The approach also can be more convenient for medical offices: All they must do is hand a specimen container to the patient.
Conversations between clinicians and patients about vaginal swabbing may be considered “sensitive” and the swabbing more invasive, Dr. Wood, an author of an editorial accompanying the journal article, said. Clinicians may also lack awareness that the swab is a more sensitive method of detecting these STIs.
“We all want to do what’s right for our patient, but we often don’t know what’s right for the patient,” Dr. Wood said. “I don’t think people are really aware of a potential real difference in outcomes with one method over the other.”
Dr. Wood advised making STI screening using vaginal swabs more common by “offering universal opt-out screening, so not waiting until you find out if someone’s having sex but just sort of saying, ‘Hey, across our practice, we screen everybody for chlamydia. Is that something that you want to do today?’ That approach sort of takes out the piece of talking about sex, talking about sexual activity.”
Dr. Van Der Pol, who said she has worked in STI diagnostics for 40 years, said she was not surprised by the results and hopes the study changes how samples are collected and used.
“I really hope that it influences practice so that we really start using vaginal swabs, because it gives us better diagnostics for chlamydia and gonorrhea,” Dr. Van Der Pol said.
“Also, then starting to think about comprehensive women’s care in such a way that they actually order other tests on that same sample if a woman is presenting with complaints.”
A version of this article originally appeared on Medscape.com.
Vaginal swabs are more effective than urine analysis in detecting certain types of sexually transmitted infections, researchers have found.
In the study, which was published online in the Annals of Family Medicine, investigators found that the diagnostic sensitivity of commercially available vaginal swabs was significantly greater than that of urine tests in detecting certain infections, including those caused by Chlamydia trachomatis, Neisseria gonorrhoeae, and Trichomonas vaginalis.
Researchers studied chlamydia and gonorrhea, which are two of the most frequently reported STIs in the United States. Trichomoniasis is the most curable nonviral STI globally, with 156 million cases worldwide in 2016.
The Centers for Disease Control and Prevention has long recommended that vaginal swabs be used to produce optimal samples.
But despite the CDC’s recommendation, urine analysis for these STIs is more commonly used than vaginal swabs among U.S. health care providers.
“We’re using a poor sample type, and we can do better,” said Barbara Van Der Pol, PhD, a professor of medicine and public health at the University of Alabama at Birmingham and an author of the new study, a meta-analysis of 97 studies published between 1995 and 2021.
Vaginal swabs for chlamydia trachomatis had a diagnostic sensitivity of 94.1% (95% confidence interval, 93.2%-94.9%; P < .001), higher than urine testing (86.9%; 95% CI, 85.6%-88.0%; P < .001). The pooled sensitivity estimates for Neisseria gonorrhoeae were 96.5% (95% CI, 94.8%-97.7%; P < .001) for vaginal swabs and 90.7% (95% CI, 88.4%-92.5%; P < .001) for urine specimens.
The difference in pooled sensitivity estimates between vaginal swabs and urine analyses for Trichomonas vaginalis was 98% (95% CI, 97.0%-98.7%; P < .001) for vaginal swabs and 95.1% (95% CI, 93.6%-96.3%) for urine specimens.
STIs included in the study are not typically found in the urethra and appear in urine analyses only if cervical or vaginal cells have dripped into a urine sample. Dr. Van Der Pol and her colleagues estimated that the use of urine samples rather than vaginal swabs may result in more than 400,000 undiagnosed infections annually.
Undiagnosed and untreated STIs can lead to transmissions of the infection as well as infertility and can have negative effects on romantic relationships, according to Dr. Van Der Pol.
Sarah Wood, MD, an attending physician at Children’s Hospital of Philadelphia, said some health care providers may use urine analysis because patients may be more comfortable with this method. The approach also can be more convenient for medical offices: All they must do is hand a specimen container to the patient.
Conversations between clinicians and patients about vaginal swabbing may be considered “sensitive” and the swabbing more invasive, Dr. Wood, an author of an editorial accompanying the journal article, said. Clinicians may also lack awareness that the swab is a more sensitive method of detecting these STIs.
“We all want to do what’s right for our patient, but we often don’t know what’s right for the patient,” Dr. Wood said. “I don’t think people are really aware of a potential real difference in outcomes with one method over the other.”
Dr. Wood advised making STI screening using vaginal swabs more common by “offering universal opt-out screening, so not waiting until you find out if someone’s having sex but just sort of saying, ‘Hey, across our practice, we screen everybody for chlamydia. Is that something that you want to do today?’ That approach sort of takes out the piece of talking about sex, talking about sexual activity.”
Dr. Van Der Pol, who said she has worked in STI diagnostics for 40 years, said she was not surprised by the results and hopes the study changes how samples are collected and used.
“I really hope that it influences practice so that we really start using vaginal swabs, because it gives us better diagnostics for chlamydia and gonorrhea,” Dr. Van Der Pol said.
“Also, then starting to think about comprehensive women’s care in such a way that they actually order other tests on that same sample if a woman is presenting with complaints.”
A version of this article originally appeared on Medscape.com.
Could a baby’s gut health be an early predictor of future type 1 diabetes?
Microbial biomarkers for type 1 diabetes may be present in infants as young as 12 months old, suggesting the potential to mitigate disease onset by nurturing a healthy gut microbiome early, show data from the Swedish general population.
“Our findings indicate that the gut of infants who go on to develop type 1 diabetes is notably different from healthy babies,” said Malin Bélteky, MD, from the Crown Princess Victoria’s Children’s Hospital, Linköping, Sweden, who jointly led the work, which was recently published in Diabetologia, alongside Patricia L. Milletich, PhD candidate, from the University of Florida, Gainesville.
“This discovery could be used to help identity infants at [the] highest risk of developing type 1 diabetes before or during the first stage of disease and could offer the opportunity to bolster a healthy gut microbiome to prevent the disease from becoming established,” added Dr. Bélteky.
Currently, beta-cell autoantibodies are used to predict disease, which are usually only identifiable between 9 and 36 months of age.
Marian Rewers, MD, PhD, professor of pediatrics & medicine, University of Colorado, Denver, and principal investigator of The Environmental Determinants of Diabetes in the Young (TEDDY) study, welcomed the findings, saying it is a well-designed study from a strong group of investigators.
“While the effective number of cases was very small [n = 16], the results were apparently adjusted for multiple comparisons, and significant differences were noted in the microbiome of cases versus controls at 1 year of age. This was 12 years prior to the average age of type 1 diabetes diagnosis in the cases,” he said.
“The differences in diversity and abundances of specific bacteria need to be interpreted with caution; however, the study results are consistent with several previous reports,” he noted.
Differences in microbial diversity and function
Data were drawn from children participating in the longitudinal, general population All Babies In Southeast Sweden (ABIS) study. Microbiota from stool samples, taken at age 1 year, were sequenced and analyzed to establish diversity, abundance, and functional status of the component bacteria. Questionnaires were completed at birth and at 1 year of age, allowing for the study of environmental factors that might influence the microbiota or type 1 diabetes risk independently. Parent diaries provided information on pregnancy, nutrition, and lifestyle factors.
Of the cohort of 167 children who developed type 1 diabetes by 2020, stool samples were available for 16 of these participants, which were compared with 268 healthy controls. The microbiomes of the 16 infants who later developed type 1 diabetes were compared with 100 iterations of 32 matched control infants (matched by geographical region, siblings at birth, residence type, duration of breastfeeding, and month of stool collection) who didn’t develop type 1 diabetes by the age of 20.
Specific bacteria found in greater abundance in children who later developed type 1 diabetes, compared with those who didn’t, included Firmicutes (Enterococcus, Gemella, and Hungatella), as well as Bacteroides (Bacteroides and Porphyromonas), known to promote inflammation and be involved in the immune response.
Bacteria with greater abundance in children who didn’t develop type 1 diabetes, compared with those who did, were Firmicutes (Anaerostipes, Flavonifractor, and Ruminococcaceae UBA1819, and Eubacterium). These species help maintain metabolic and immune health and produce butyrate, an important short-chain fatty acid that helps prevent inflammation and fuels the cells of the gut lining.
Alistipes were more abundant in infants who didn’t develop type 1 diabetes, and various abundances of Fusicatenibacter were the strongest factors for differentiating future type 1 diabetes, reported the researchers.
“Gut microbial biomarkers at 12 months would benefit the prediction opportunity well before the onset of multiple autoantibodies,” write the authors.
The youngest age at type 1 diabetes diagnosis was aged 1 year, 4 months, and the oldest was aged 21 years, 4 months. The mean age at diagnosis was 13.3 years.
The microbial differences found between infants who go on to develop type 1 diabetes and those who don’t also shed light on interactions between the developing immune system and short-chain fatty acid production and metabolism in childhood autoimmunity, write the authors.
Prior studies have found fewer short-chain fatty acid–producing microbiota in the gut of children with early-onset autoantibody development. This study confirmed these data, finding a decrease in butyrate-producing bacteria (Anaerostipes, Flavonifractor, Ruminococcaceae UBA1819, and Eubacterium) in infants who went on to develop type 1 diabetes. Likewise, a reduction in pyruvate fermentation was found in those infants with future disease.
According to coauthor Eric Triplett, PhD, from the University of Florida, Gainesville: “The autoimmune processes usually begin long before any clinical signs of disease appear, highlighting how differences in the makeup of the infant gut microbiome could shed important light on the complex interaction between the developing immune system, environmental exposures in childhood, and autoimmunity. Studies with much larger cohorts of prospectively traced individuals will be required to establish which are the strongest biomarkers and how effectively they can predict disease.”
The authors and Dr. Rewers have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Microbial biomarkers for type 1 diabetes may be present in infants as young as 12 months old, suggesting the potential to mitigate disease onset by nurturing a healthy gut microbiome early, show data from the Swedish general population.
“Our findings indicate that the gut of infants who go on to develop type 1 diabetes is notably different from healthy babies,” said Malin Bélteky, MD, from the Crown Princess Victoria’s Children’s Hospital, Linköping, Sweden, who jointly led the work, which was recently published in Diabetologia, alongside Patricia L. Milletich, PhD candidate, from the University of Florida, Gainesville.
“This discovery could be used to help identity infants at [the] highest risk of developing type 1 diabetes before or during the first stage of disease and could offer the opportunity to bolster a healthy gut microbiome to prevent the disease from becoming established,” added Dr. Bélteky.
Currently, beta-cell autoantibodies are used to predict disease, which are usually only identifiable between 9 and 36 months of age.
Marian Rewers, MD, PhD, professor of pediatrics & medicine, University of Colorado, Denver, and principal investigator of The Environmental Determinants of Diabetes in the Young (TEDDY) study, welcomed the findings, saying it is a well-designed study from a strong group of investigators.
“While the effective number of cases was very small [n = 16], the results were apparently adjusted for multiple comparisons, and significant differences were noted in the microbiome of cases versus controls at 1 year of age. This was 12 years prior to the average age of type 1 diabetes diagnosis in the cases,” he said.
“The differences in diversity and abundances of specific bacteria need to be interpreted with caution; however, the study results are consistent with several previous reports,” he noted.
Differences in microbial diversity and function
Data were drawn from children participating in the longitudinal, general population All Babies In Southeast Sweden (ABIS) study. Microbiota from stool samples, taken at age 1 year, were sequenced and analyzed to establish diversity, abundance, and functional status of the component bacteria. Questionnaires were completed at birth and at 1 year of age, allowing for the study of environmental factors that might influence the microbiota or type 1 diabetes risk independently. Parent diaries provided information on pregnancy, nutrition, and lifestyle factors.
Of the cohort of 167 children who developed type 1 diabetes by 2020, stool samples were available for 16 of these participants, which were compared with 268 healthy controls. The microbiomes of the 16 infants who later developed type 1 diabetes were compared with 100 iterations of 32 matched control infants (matched by geographical region, siblings at birth, residence type, duration of breastfeeding, and month of stool collection) who didn’t develop type 1 diabetes by the age of 20.
Specific bacteria found in greater abundance in children who later developed type 1 diabetes, compared with those who didn’t, included Firmicutes (Enterococcus, Gemella, and Hungatella), as well as Bacteroides (Bacteroides and Porphyromonas), known to promote inflammation and be involved in the immune response.
Bacteria with greater abundance in children who didn’t develop type 1 diabetes, compared with those who did, were Firmicutes (Anaerostipes, Flavonifractor, and Ruminococcaceae UBA1819, and Eubacterium). These species help maintain metabolic and immune health and produce butyrate, an important short-chain fatty acid that helps prevent inflammation and fuels the cells of the gut lining.
Alistipes were more abundant in infants who didn’t develop type 1 diabetes, and various abundances of Fusicatenibacter were the strongest factors for differentiating future type 1 diabetes, reported the researchers.
“Gut microbial biomarkers at 12 months would benefit the prediction opportunity well before the onset of multiple autoantibodies,” write the authors.
The youngest age at type 1 diabetes diagnosis was aged 1 year, 4 months, and the oldest was aged 21 years, 4 months. The mean age at diagnosis was 13.3 years.
The microbial differences found between infants who go on to develop type 1 diabetes and those who don’t also shed light on interactions between the developing immune system and short-chain fatty acid production and metabolism in childhood autoimmunity, write the authors.
Prior studies have found fewer short-chain fatty acid–producing microbiota in the gut of children with early-onset autoantibody development. This study confirmed these data, finding a decrease in butyrate-producing bacteria (Anaerostipes, Flavonifractor, Ruminococcaceae UBA1819, and Eubacterium) in infants who went on to develop type 1 diabetes. Likewise, a reduction in pyruvate fermentation was found in those infants with future disease.
According to coauthor Eric Triplett, PhD, from the University of Florida, Gainesville: “The autoimmune processes usually begin long before any clinical signs of disease appear, highlighting how differences in the makeup of the infant gut microbiome could shed important light on the complex interaction between the developing immune system, environmental exposures in childhood, and autoimmunity. Studies with much larger cohorts of prospectively traced individuals will be required to establish which are the strongest biomarkers and how effectively they can predict disease.”
The authors and Dr. Rewers have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Microbial biomarkers for type 1 diabetes may be present in infants as young as 12 months old, suggesting the potential to mitigate disease onset by nurturing a healthy gut microbiome early, show data from the Swedish general population.
“Our findings indicate that the gut of infants who go on to develop type 1 diabetes is notably different from healthy babies,” said Malin Bélteky, MD, from the Crown Princess Victoria’s Children’s Hospital, Linköping, Sweden, who jointly led the work, which was recently published in Diabetologia, alongside Patricia L. Milletich, PhD candidate, from the University of Florida, Gainesville.
“This discovery could be used to help identity infants at [the] highest risk of developing type 1 diabetes before or during the first stage of disease and could offer the opportunity to bolster a healthy gut microbiome to prevent the disease from becoming established,” added Dr. Bélteky.
Currently, beta-cell autoantibodies are used to predict disease, which are usually only identifiable between 9 and 36 months of age.
Marian Rewers, MD, PhD, professor of pediatrics & medicine, University of Colorado, Denver, and principal investigator of The Environmental Determinants of Diabetes in the Young (TEDDY) study, welcomed the findings, saying it is a well-designed study from a strong group of investigators.
“While the effective number of cases was very small [n = 16], the results were apparently adjusted for multiple comparisons, and significant differences were noted in the microbiome of cases versus controls at 1 year of age. This was 12 years prior to the average age of type 1 diabetes diagnosis in the cases,” he said.
“The differences in diversity and abundances of specific bacteria need to be interpreted with caution; however, the study results are consistent with several previous reports,” he noted.
Differences in microbial diversity and function
Data were drawn from children participating in the longitudinal, general population All Babies In Southeast Sweden (ABIS) study. Microbiota from stool samples, taken at age 1 year, were sequenced and analyzed to establish diversity, abundance, and functional status of the component bacteria. Questionnaires were completed at birth and at 1 year of age, allowing for the study of environmental factors that might influence the microbiota or type 1 diabetes risk independently. Parent diaries provided information on pregnancy, nutrition, and lifestyle factors.
Of the cohort of 167 children who developed type 1 diabetes by 2020, stool samples were available for 16 of these participants, which were compared with 268 healthy controls. The microbiomes of the 16 infants who later developed type 1 diabetes were compared with 100 iterations of 32 matched control infants (matched by geographical region, siblings at birth, residence type, duration of breastfeeding, and month of stool collection) who didn’t develop type 1 diabetes by the age of 20.
Specific bacteria found in greater abundance in children who later developed type 1 diabetes, compared with those who didn’t, included Firmicutes (Enterococcus, Gemella, and Hungatella), as well as Bacteroides (Bacteroides and Porphyromonas), known to promote inflammation and be involved in the immune response.
Bacteria with greater abundance in children who didn’t develop type 1 diabetes, compared with those who did, were Firmicutes (Anaerostipes, Flavonifractor, and Ruminococcaceae UBA1819, and Eubacterium). These species help maintain metabolic and immune health and produce butyrate, an important short-chain fatty acid that helps prevent inflammation and fuels the cells of the gut lining.
Alistipes were more abundant in infants who didn’t develop type 1 diabetes, and various abundances of Fusicatenibacter were the strongest factors for differentiating future type 1 diabetes, reported the researchers.
“Gut microbial biomarkers at 12 months would benefit the prediction opportunity well before the onset of multiple autoantibodies,” write the authors.
The youngest age at type 1 diabetes diagnosis was aged 1 year, 4 months, and the oldest was aged 21 years, 4 months. The mean age at diagnosis was 13.3 years.
The microbial differences found between infants who go on to develop type 1 diabetes and those who don’t also shed light on interactions between the developing immune system and short-chain fatty acid production and metabolism in childhood autoimmunity, write the authors.
Prior studies have found fewer short-chain fatty acid–producing microbiota in the gut of children with early-onset autoantibody development. This study confirmed these data, finding a decrease in butyrate-producing bacteria (Anaerostipes, Flavonifractor, Ruminococcaceae UBA1819, and Eubacterium) in infants who went on to develop type 1 diabetes. Likewise, a reduction in pyruvate fermentation was found in those infants with future disease.
According to coauthor Eric Triplett, PhD, from the University of Florida, Gainesville: “The autoimmune processes usually begin long before any clinical signs of disease appear, highlighting how differences in the makeup of the infant gut microbiome could shed important light on the complex interaction between the developing immune system, environmental exposures in childhood, and autoimmunity. Studies with much larger cohorts of prospectively traced individuals will be required to establish which are the strongest biomarkers and how effectively they can predict disease.”
The authors and Dr. Rewers have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Tranq-contaminated fentanyl now in 48 states, DEA warns
The DEA warning comes on the heels of a Food and Drug Administration announcement that it would begin more closely monitoring imports of the raw materials and bulk shipments of xylazine, also known as “tranq” and “zombie drug.”
Xylazine was first approved by the FDA in 1972 as a sedative and analgesic for use only in animals, but is increasingly being detected in illicit street drugs, and is often mixed with fentanyl, cocaine, and methamphetamine.
The FDA warned in November that naloxone (Narcan) would not reverse xylazine-related overdoses because the tranquilizer is not an opioid. It does suppress respiration and repeated exposures may lead to dependence and withdrawal, said the agency. Users are also experiencing severe necrosis at injection sites.
“Xylazine is making the deadliest drug threat our country has ever faced, fentanyl, even deadlier,” said DEA Administrator Anne Milgram in a statement. “The DEA Laboratory System is reporting that in 2022 approximately 23% of fentanyl powder and 7% of fentanyl pills seized by the DEA contained xylazine.”
Xylazine use has spread quickly, from its start in the Philadelphia area to the Northeast, the South, and most recently the West.
Citing data from the Centers for Disease Control and Prevention, the DEA said that 66% of the 107,735 overdose deaths for the year ending August 2022 involved synthetic opioids such as fentanyl. The DEA said that the Sinaloa Cartel and Jalisco Cartel in Mexico, using chemicals sourced from China, are primarily responsible for trafficking fentanyl in the United States.
A version of this article originally appeared on Medscape.com.
The DEA warning comes on the heels of a Food and Drug Administration announcement that it would begin more closely monitoring imports of the raw materials and bulk shipments of xylazine, also known as “tranq” and “zombie drug.”
Xylazine was first approved by the FDA in 1972 as a sedative and analgesic for use only in animals, but is increasingly being detected in illicit street drugs, and is often mixed with fentanyl, cocaine, and methamphetamine.
The FDA warned in November that naloxone (Narcan) would not reverse xylazine-related overdoses because the tranquilizer is not an opioid. It does suppress respiration and repeated exposures may lead to dependence and withdrawal, said the agency. Users are also experiencing severe necrosis at injection sites.
“Xylazine is making the deadliest drug threat our country has ever faced, fentanyl, even deadlier,” said DEA Administrator Anne Milgram in a statement. “The DEA Laboratory System is reporting that in 2022 approximately 23% of fentanyl powder and 7% of fentanyl pills seized by the DEA contained xylazine.”
Xylazine use has spread quickly, from its start in the Philadelphia area to the Northeast, the South, and most recently the West.
Citing data from the Centers for Disease Control and Prevention, the DEA said that 66% of the 107,735 overdose deaths for the year ending August 2022 involved synthetic opioids such as fentanyl. The DEA said that the Sinaloa Cartel and Jalisco Cartel in Mexico, using chemicals sourced from China, are primarily responsible for trafficking fentanyl in the United States.
A version of this article originally appeared on Medscape.com.
The DEA warning comes on the heels of a Food and Drug Administration announcement that it would begin more closely monitoring imports of the raw materials and bulk shipments of xylazine, also known as “tranq” and “zombie drug.”
Xylazine was first approved by the FDA in 1972 as a sedative and analgesic for use only in animals, but is increasingly being detected in illicit street drugs, and is often mixed with fentanyl, cocaine, and methamphetamine.
The FDA warned in November that naloxone (Narcan) would not reverse xylazine-related overdoses because the tranquilizer is not an opioid. It does suppress respiration and repeated exposures may lead to dependence and withdrawal, said the agency. Users are also experiencing severe necrosis at injection sites.
“Xylazine is making the deadliest drug threat our country has ever faced, fentanyl, even deadlier,” said DEA Administrator Anne Milgram in a statement. “The DEA Laboratory System is reporting that in 2022 approximately 23% of fentanyl powder and 7% of fentanyl pills seized by the DEA contained xylazine.”
Xylazine use has spread quickly, from its start in the Philadelphia area to the Northeast, the South, and most recently the West.
Citing data from the Centers for Disease Control and Prevention, the DEA said that 66% of the 107,735 overdose deaths for the year ending August 2022 involved synthetic opioids such as fentanyl. The DEA said that the Sinaloa Cartel and Jalisco Cartel in Mexico, using chemicals sourced from China, are primarily responsible for trafficking fentanyl in the United States.
A version of this article originally appeared on Medscape.com.
FDA panels vote to modify isotretinoin iPLEDGE REMS
At a joint meeting of a drug for severe, nodular acne that is highly teratogenic.
The first vote was on whether to continue the 19-day lockout period for patients who can become pregnant and do not pick up their first prescription of isotretinoin within the 7-day prescription window. Those patients currently have to wait 19 days to get their second pregnancy test and receive the medication.
Most (17) of the 22 voting members voted not to continue the 19-day period; 4 voted to keep it; and 1 abstained. But there was no consensus on when the second pregnancy test should occur if the 19-day lockout is changed.
Ken Katz, MD, MSc, a dermatologist at Kaiser Permanente in San Francisco, was among those voting not to continue the 19-day lockout.
“I think this places an unduly high burden physically and psychologically on our patients. It seems arbitrary,” he said. “Likely we will miss some pregnancies; we are missing some already. But the burden is not matched by the benefit.”
The second question concerned patients who cannot become pregnant, and it asked when REMS should require that the prescriber document counseling the patient in the iPLEDGE system. The current requirement is monthly.
Listed options and the number of votes for each were:
- Only with the first prescription as part of patient enrollment (10)
- Monthly (1)
- Every 120 days (6)
- Some other frequency (5)
For this question too, while the members largely agreed the current monthly requirement is too burdensome, there was little agreement on what the most appropriate interval should be.
Lack of data
On both questions, several advisory committee members cited a lack of data on which they could base their decision.
On the documentation question, Megha Tollefson, MD, professor of dermatology at the Mayo Clinic, Rochester, Minn., said she voted for the fourth option (some other frequency) with the thought of yearly attestation.
“As a part of this, providers have to provide monthly counseling,” Dr. Tollefson said. “This is just a documentation requirement in the iPLEDGE system. I think most prescribers do document their monthly counseling in their own medical records. I would say it would be okay not to redocument that in iPLEDGE.”
The two votes came at the end of the second day of a joint meeting of the FDA’s Drug Safety and Risk Management Advisory Committee and Dermatologic and Ophthalmic Drugs Advisory Committee in which experts addressed ways to improve the iPLEDGE REMS for isotretinoin. A transition to a new platform for the iPLEDGE program caused chaos after its rollout at the end of 2021, resulting in extensive delays and denial of prescriptions.
The committees sought to balance reducing burden with maintaining safety and preventing fetal exposures to isotretinoin.
They were also tasked with discussing other REMS requirements without taking a vote on each topic.
Among those topics was whether home pregnancy tests, allowed during the COVID-19 public health emergency, should continue to be allowed. Most who spoke to the issue agreed that home tests should continue in an effort to increase access and decrease burden. Members suggested safeguards against falsified results that have been documented, including assigning names and barcodes to the test results and uploading the verification to the iPLEDGE website.
The advisory committees also discussed recommendations to encourage more participation in the iPLEDGE Pregnancy Registry.
The advisory committees’ recommendations to the FDA are nonbinding, but the FDA generally follows the recommendations of advisory panels.
A version of this article first appeared on Medscape.com.
At a joint meeting of a drug for severe, nodular acne that is highly teratogenic.
The first vote was on whether to continue the 19-day lockout period for patients who can become pregnant and do not pick up their first prescription of isotretinoin within the 7-day prescription window. Those patients currently have to wait 19 days to get their second pregnancy test and receive the medication.
Most (17) of the 22 voting members voted not to continue the 19-day period; 4 voted to keep it; and 1 abstained. But there was no consensus on when the second pregnancy test should occur if the 19-day lockout is changed.
Ken Katz, MD, MSc, a dermatologist at Kaiser Permanente in San Francisco, was among those voting not to continue the 19-day lockout.
“I think this places an unduly high burden physically and psychologically on our patients. It seems arbitrary,” he said. “Likely we will miss some pregnancies; we are missing some already. But the burden is not matched by the benefit.”
The second question concerned patients who cannot become pregnant, and it asked when REMS should require that the prescriber document counseling the patient in the iPLEDGE system. The current requirement is monthly.
Listed options and the number of votes for each were:
- Only with the first prescription as part of patient enrollment (10)
- Monthly (1)
- Every 120 days (6)
- Some other frequency (5)
For this question too, while the members largely agreed the current monthly requirement is too burdensome, there was little agreement on what the most appropriate interval should be.
Lack of data
On both questions, several advisory committee members cited a lack of data on which they could base their decision.
On the documentation question, Megha Tollefson, MD, professor of dermatology at the Mayo Clinic, Rochester, Minn., said she voted for the fourth option (some other frequency) with the thought of yearly attestation.
“As a part of this, providers have to provide monthly counseling,” Dr. Tollefson said. “This is just a documentation requirement in the iPLEDGE system. I think most prescribers do document their monthly counseling in their own medical records. I would say it would be okay not to redocument that in iPLEDGE.”
The two votes came at the end of the second day of a joint meeting of the FDA’s Drug Safety and Risk Management Advisory Committee and Dermatologic and Ophthalmic Drugs Advisory Committee in which experts addressed ways to improve the iPLEDGE REMS for isotretinoin. A transition to a new platform for the iPLEDGE program caused chaos after its rollout at the end of 2021, resulting in extensive delays and denial of prescriptions.
The committees sought to balance reducing burden with maintaining safety and preventing fetal exposures to isotretinoin.
They were also tasked with discussing other REMS requirements without taking a vote on each topic.
Among those topics was whether home pregnancy tests, allowed during the COVID-19 public health emergency, should continue to be allowed. Most who spoke to the issue agreed that home tests should continue in an effort to increase access and decrease burden. Members suggested safeguards against falsified results that have been documented, including assigning names and barcodes to the test results and uploading the verification to the iPLEDGE website.
The advisory committees also discussed recommendations to encourage more participation in the iPLEDGE Pregnancy Registry.
The advisory committees’ recommendations to the FDA are nonbinding, but the FDA generally follows the recommendations of advisory panels.
A version of this article first appeared on Medscape.com.
At a joint meeting of a drug for severe, nodular acne that is highly teratogenic.
The first vote was on whether to continue the 19-day lockout period for patients who can become pregnant and do not pick up their first prescription of isotretinoin within the 7-day prescription window. Those patients currently have to wait 19 days to get their second pregnancy test and receive the medication.
Most (17) of the 22 voting members voted not to continue the 19-day period; 4 voted to keep it; and 1 abstained. But there was no consensus on when the second pregnancy test should occur if the 19-day lockout is changed.
Ken Katz, MD, MSc, a dermatologist at Kaiser Permanente in San Francisco, was among those voting not to continue the 19-day lockout.
“I think this places an unduly high burden physically and psychologically on our patients. It seems arbitrary,” he said. “Likely we will miss some pregnancies; we are missing some already. But the burden is not matched by the benefit.”
The second question concerned patients who cannot become pregnant, and it asked when REMS should require that the prescriber document counseling the patient in the iPLEDGE system. The current requirement is monthly.
Listed options and the number of votes for each were:
- Only with the first prescription as part of patient enrollment (10)
- Monthly (1)
- Every 120 days (6)
- Some other frequency (5)
For this question too, while the members largely agreed the current monthly requirement is too burdensome, there was little agreement on what the most appropriate interval should be.
Lack of data
On both questions, several advisory committee members cited a lack of data on which they could base their decision.
On the documentation question, Megha Tollefson, MD, professor of dermatology at the Mayo Clinic, Rochester, Minn., said she voted for the fourth option (some other frequency) with the thought of yearly attestation.
“As a part of this, providers have to provide monthly counseling,” Dr. Tollefson said. “This is just a documentation requirement in the iPLEDGE system. I think most prescribers do document their monthly counseling in their own medical records. I would say it would be okay not to redocument that in iPLEDGE.”
The two votes came at the end of the second day of a joint meeting of the FDA’s Drug Safety and Risk Management Advisory Committee and Dermatologic and Ophthalmic Drugs Advisory Committee in which experts addressed ways to improve the iPLEDGE REMS for isotretinoin. A transition to a new platform for the iPLEDGE program caused chaos after its rollout at the end of 2021, resulting in extensive delays and denial of prescriptions.
The committees sought to balance reducing burden with maintaining safety and preventing fetal exposures to isotretinoin.
They were also tasked with discussing other REMS requirements without taking a vote on each topic.
Among those topics was whether home pregnancy tests, allowed during the COVID-19 public health emergency, should continue to be allowed. Most who spoke to the issue agreed that home tests should continue in an effort to increase access and decrease burden. Members suggested safeguards against falsified results that have been documented, including assigning names and barcodes to the test results and uploading the verification to the iPLEDGE website.
The advisory committees also discussed recommendations to encourage more participation in the iPLEDGE Pregnancy Registry.
The advisory committees’ recommendations to the FDA are nonbinding, but the FDA generally follows the recommendations of advisory panels.
A version of this article first appeared on Medscape.com.
Sweaty treatment for social anxiety could pass the sniff test
Getting sweet on sweat
Are you the sort of person who struggles in social situations? Have the past 3 years been a secret respite from the terror and exhaustion of meeting new people? We understand your plight. People kind of suck. And you don’t have to look far to be reminded of it.
Unfortunately, on occasion we all have to interact with other human beings. If you suffer from social anxiety, this is not a fun thing to do. But new research indicates that there may be a way to alleviate the stress for those with social anxiety: armpits.
Specifically, sweat from the armpits of other people. Yes, this means a group of scientists gathered up some volunteers and collected their armpit sweat while the volunteers watched a variety of movies (horror, comedy, romance, etc.). Our condolences to the poor unpaid interns tasked with gathering the sweat.
Once they had their precious new medicine, the researchers took a group of women and administered a round of mindfulness therapy. Some of the participants then received the various sweats, while the rest were forced to smell only clean air. (The horror!) Lo and behold, the sweat groups had their anxiety scores reduced by about 40% after their therapy, compared with just 17% in the control group.
The researchers also found that the source of the sweat didn’t matter. Their study subjects responded the same to sweat excreted during a scary movie as they did to sweat from a comedy, a result that surprised the researchers. They suggested chemosignals in the sweat may affect the treatment response and advised further research. Which means more sweat collection! They plan on testing emotionally neutral movies next time, and if we can make a humble suggestion, they also should try the sweatiest movies.
Before the Food and Drug Administration can approve armpit sweat as a treatment for social anxiety, we have some advice for those shut-in introverts out there. Next time you have to interact with rabid extroverts, instead of shaking their hands, walk up to them and take a deep whiff of their armpits. Establish dominance. Someone will feel awkward, and science has proved it won’t be you.
The puff that vaccinates
Ever been shot with a Nerf gun or hit with a foam pool tube? More annoying than painful, right? If we asked if you’d rather get pelted with one of those than receive a traditional vaccine injection, you would choose the former. Maybe someday you actually will.
During the boredom of the early pandemic lockdown, Jeremiah Gassensmith, PhD, of the department of chemistry and biochemistry at the University of Texas, Dallas, ordered a compressed gas–powered jet injection system to fool around with at home. Hey, who didn’t? Anyway, when it was time to go back to the lab he handed it over to one of his grad students, Yalini Wijesundara, and asked her to see what could be done with it.
In her tinkering she found that the jet injector could deliver metal-organic frameworks (MOFs) that can hold a bunch of different materials, like proteins and nucleic acids, through the skin.
Thus the “MOF-Jet” was born!
Jet injectors are nothing new, but they hurt. The MOF-Jet, however, is practically painless and cheaper than the gene guns that veterinarians use to inject biological cargo attached to the surface of a metal microparticle.
Changing the carrier gas also changes the time needed to break down the MOF and thus alters delivery of the drug inside. “If you shoot it with carbon dioxide, it will release its cargo faster within cells; if you use regular air, it will take 4 or 5 days,” Ms. Wijesundara explained in a written statement. That means the same drug could be released over different timescales without changing its formulation.
While testing on onion cells and mice, Ms. Wijesundara noted that it was as easy as “pointing and shooting” to distribute the puff of gas into the cells. A saving grace to those with needle anxiety. Not that we would know anything about needle anxiety.
More testing needs to be done before bringing this technology to human use, obviously, but we’re looking forward to saying goodbye to that dreaded prick and hello to a puff.
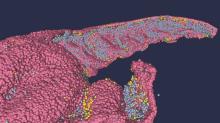
Your hippocampus is showing
Brain anatomy is one of the many, many things that’s not really our thing, but we do know a cool picture when we see one. Case in point: The image just below, which happens to be a full-scale, single-cell resolution model of the CA1 region of the hippocampus that “replicates the structure and architecture of the area, along with the position and relative connectivity of the neurons,” according to a statement from the Human Brain Project.
“We have performed a data mining operation on high resolution images of the human hippocampus, obtained from the BigBrain database. The position of individual neurons has been derived from a detailed analysis of these images,” said senior author Michele Migliore, PhD, of the Italian National Research Council’s Institute of Biophysics in Palermo.
Yes, he did say BigBrain database. BigBrain is – we checked and it’s definitely not this – a 3D model of a brain that was sectioned into 7,404 slices just 20 micrometers thick and then scanned by MRI. Digital reconstruction of those slices was done by supercomputer and the results are now available for analysis.
Dr. Migliore and his associates developed an image-processing algorithm to obtain neuronal positioning distribution and an algorithm to generate neuronal connectivity by approximating the shapes of dendrites and axons. (Our brains are starting to hurt just trying to write this.) “Some fit into narrow cones, others have a broad complex extension that can be approximated by dedicated geometrical volumes, and the connectivity to nearby neurons changes accordingly,” explained lead author Daniela Gandolfi of the University of Modena (Italy) and Reggio Emilia.
The investigators have made their dataset and the extraction methodology available on the EBRAINS platform and through the Human Brain Project and are moving on to other brain regions. And then, once everyone can find their way in and around the old gray matter, it should bring an end to conversations like this, which no doubt occur between male and female neuroscientists every day:
“Arnold, I think we’re lost.”
“Don’t worry, Bev, I know where I’m going.”
“Stop and ask this lady for directions.”
“I said I can find it.”
“Just ask her.”
“Fine. Excuse me, ma’am, can you tell us how to get to the corpora quadrigemina from here?
Getting sweet on sweat
Are you the sort of person who struggles in social situations? Have the past 3 years been a secret respite from the terror and exhaustion of meeting new people? We understand your plight. People kind of suck. And you don’t have to look far to be reminded of it.
Unfortunately, on occasion we all have to interact with other human beings. If you suffer from social anxiety, this is not a fun thing to do. But new research indicates that there may be a way to alleviate the stress for those with social anxiety: armpits.
Specifically, sweat from the armpits of other people. Yes, this means a group of scientists gathered up some volunteers and collected their armpit sweat while the volunteers watched a variety of movies (horror, comedy, romance, etc.). Our condolences to the poor unpaid interns tasked with gathering the sweat.
Once they had their precious new medicine, the researchers took a group of women and administered a round of mindfulness therapy. Some of the participants then received the various sweats, while the rest were forced to smell only clean air. (The horror!) Lo and behold, the sweat groups had their anxiety scores reduced by about 40% after their therapy, compared with just 17% in the control group.
The researchers also found that the source of the sweat didn’t matter. Their study subjects responded the same to sweat excreted during a scary movie as they did to sweat from a comedy, a result that surprised the researchers. They suggested chemosignals in the sweat may affect the treatment response and advised further research. Which means more sweat collection! They plan on testing emotionally neutral movies next time, and if we can make a humble suggestion, they also should try the sweatiest movies.
Before the Food and Drug Administration can approve armpit sweat as a treatment for social anxiety, we have some advice for those shut-in introverts out there. Next time you have to interact with rabid extroverts, instead of shaking their hands, walk up to them and take a deep whiff of their armpits. Establish dominance. Someone will feel awkward, and science has proved it won’t be you.
The puff that vaccinates
Ever been shot with a Nerf gun or hit with a foam pool tube? More annoying than painful, right? If we asked if you’d rather get pelted with one of those than receive a traditional vaccine injection, you would choose the former. Maybe someday you actually will.
During the boredom of the early pandemic lockdown, Jeremiah Gassensmith, PhD, of the department of chemistry and biochemistry at the University of Texas, Dallas, ordered a compressed gas–powered jet injection system to fool around with at home. Hey, who didn’t? Anyway, when it was time to go back to the lab he handed it over to one of his grad students, Yalini Wijesundara, and asked her to see what could be done with it.
In her tinkering she found that the jet injector could deliver metal-organic frameworks (MOFs) that can hold a bunch of different materials, like proteins and nucleic acids, through the skin.
Thus the “MOF-Jet” was born!
Jet injectors are nothing new, but they hurt. The MOF-Jet, however, is practically painless and cheaper than the gene guns that veterinarians use to inject biological cargo attached to the surface of a metal microparticle.
Changing the carrier gas also changes the time needed to break down the MOF and thus alters delivery of the drug inside. “If you shoot it with carbon dioxide, it will release its cargo faster within cells; if you use regular air, it will take 4 or 5 days,” Ms. Wijesundara explained in a written statement. That means the same drug could be released over different timescales without changing its formulation.
While testing on onion cells and mice, Ms. Wijesundara noted that it was as easy as “pointing and shooting” to distribute the puff of gas into the cells. A saving grace to those with needle anxiety. Not that we would know anything about needle anxiety.
More testing needs to be done before bringing this technology to human use, obviously, but we’re looking forward to saying goodbye to that dreaded prick and hello to a puff.
Your hippocampus is showing
Brain anatomy is one of the many, many things that’s not really our thing, but we do know a cool picture when we see one. Case in point: The image just below, which happens to be a full-scale, single-cell resolution model of the CA1 region of the hippocampus that “replicates the structure and architecture of the area, along with the position and relative connectivity of the neurons,” according to a statement from the Human Brain Project.
“We have performed a data mining operation on high resolution images of the human hippocampus, obtained from the BigBrain database. The position of individual neurons has been derived from a detailed analysis of these images,” said senior author Michele Migliore, PhD, of the Italian National Research Council’s Institute of Biophysics in Palermo.
Yes, he did say BigBrain database. BigBrain is – we checked and it’s definitely not this – a 3D model of a brain that was sectioned into 7,404 slices just 20 micrometers thick and then scanned by MRI. Digital reconstruction of those slices was done by supercomputer and the results are now available for analysis.
Dr. Migliore and his associates developed an image-processing algorithm to obtain neuronal positioning distribution and an algorithm to generate neuronal connectivity by approximating the shapes of dendrites and axons. (Our brains are starting to hurt just trying to write this.) “Some fit into narrow cones, others have a broad complex extension that can be approximated by dedicated geometrical volumes, and the connectivity to nearby neurons changes accordingly,” explained lead author Daniela Gandolfi of the University of Modena (Italy) and Reggio Emilia.
The investigators have made their dataset and the extraction methodology available on the EBRAINS platform and through the Human Brain Project and are moving on to other brain regions. And then, once everyone can find their way in and around the old gray matter, it should bring an end to conversations like this, which no doubt occur between male and female neuroscientists every day:
“Arnold, I think we’re lost.”
“Don’t worry, Bev, I know where I’m going.”
“Stop and ask this lady for directions.”
“I said I can find it.”
“Just ask her.”
“Fine. Excuse me, ma’am, can you tell us how to get to the corpora quadrigemina from here?
Getting sweet on sweat
Are you the sort of person who struggles in social situations? Have the past 3 years been a secret respite from the terror and exhaustion of meeting new people? We understand your plight. People kind of suck. And you don’t have to look far to be reminded of it.
Unfortunately, on occasion we all have to interact with other human beings. If you suffer from social anxiety, this is not a fun thing to do. But new research indicates that there may be a way to alleviate the stress for those with social anxiety: armpits.
Specifically, sweat from the armpits of other people. Yes, this means a group of scientists gathered up some volunteers and collected their armpit sweat while the volunteers watched a variety of movies (horror, comedy, romance, etc.). Our condolences to the poor unpaid interns tasked with gathering the sweat.
Once they had their precious new medicine, the researchers took a group of women and administered a round of mindfulness therapy. Some of the participants then received the various sweats, while the rest were forced to smell only clean air. (The horror!) Lo and behold, the sweat groups had their anxiety scores reduced by about 40% after their therapy, compared with just 17% in the control group.
The researchers also found that the source of the sweat didn’t matter. Their study subjects responded the same to sweat excreted during a scary movie as they did to sweat from a comedy, a result that surprised the researchers. They suggested chemosignals in the sweat may affect the treatment response and advised further research. Which means more sweat collection! They plan on testing emotionally neutral movies next time, and if we can make a humble suggestion, they also should try the sweatiest movies.
Before the Food and Drug Administration can approve armpit sweat as a treatment for social anxiety, we have some advice for those shut-in introverts out there. Next time you have to interact with rabid extroverts, instead of shaking their hands, walk up to them and take a deep whiff of their armpits. Establish dominance. Someone will feel awkward, and science has proved it won’t be you.
The puff that vaccinates
Ever been shot with a Nerf gun or hit with a foam pool tube? More annoying than painful, right? If we asked if you’d rather get pelted with one of those than receive a traditional vaccine injection, you would choose the former. Maybe someday you actually will.
During the boredom of the early pandemic lockdown, Jeremiah Gassensmith, PhD, of the department of chemistry and biochemistry at the University of Texas, Dallas, ordered a compressed gas–powered jet injection system to fool around with at home. Hey, who didn’t? Anyway, when it was time to go back to the lab he handed it over to one of his grad students, Yalini Wijesundara, and asked her to see what could be done with it.
In her tinkering she found that the jet injector could deliver metal-organic frameworks (MOFs) that can hold a bunch of different materials, like proteins and nucleic acids, through the skin.
Thus the “MOF-Jet” was born!
Jet injectors are nothing new, but they hurt. The MOF-Jet, however, is practically painless and cheaper than the gene guns that veterinarians use to inject biological cargo attached to the surface of a metal microparticle.
Changing the carrier gas also changes the time needed to break down the MOF and thus alters delivery of the drug inside. “If you shoot it with carbon dioxide, it will release its cargo faster within cells; if you use regular air, it will take 4 or 5 days,” Ms. Wijesundara explained in a written statement. That means the same drug could be released over different timescales without changing its formulation.
While testing on onion cells and mice, Ms. Wijesundara noted that it was as easy as “pointing and shooting” to distribute the puff of gas into the cells. A saving grace to those with needle anxiety. Not that we would know anything about needle anxiety.
More testing needs to be done before bringing this technology to human use, obviously, but we’re looking forward to saying goodbye to that dreaded prick and hello to a puff.
Your hippocampus is showing
Brain anatomy is one of the many, many things that’s not really our thing, but we do know a cool picture when we see one. Case in point: The image just below, which happens to be a full-scale, single-cell resolution model of the CA1 region of the hippocampus that “replicates the structure and architecture of the area, along with the position and relative connectivity of the neurons,” according to a statement from the Human Brain Project.
“We have performed a data mining operation on high resolution images of the human hippocampus, obtained from the BigBrain database. The position of individual neurons has been derived from a detailed analysis of these images,” said senior author Michele Migliore, PhD, of the Italian National Research Council’s Institute of Biophysics in Palermo.
Yes, he did say BigBrain database. BigBrain is – we checked and it’s definitely not this – a 3D model of a brain that was sectioned into 7,404 slices just 20 micrometers thick and then scanned by MRI. Digital reconstruction of those slices was done by supercomputer and the results are now available for analysis.
Dr. Migliore and his associates developed an image-processing algorithm to obtain neuronal positioning distribution and an algorithm to generate neuronal connectivity by approximating the shapes of dendrites and axons. (Our brains are starting to hurt just trying to write this.) “Some fit into narrow cones, others have a broad complex extension that can be approximated by dedicated geometrical volumes, and the connectivity to nearby neurons changes accordingly,” explained lead author Daniela Gandolfi of the University of Modena (Italy) and Reggio Emilia.
The investigators have made their dataset and the extraction methodology available on the EBRAINS platform and through the Human Brain Project and are moving on to other brain regions. And then, once everyone can find their way in and around the old gray matter, it should bring an end to conversations like this, which no doubt occur between male and female neuroscientists every day:
“Arnold, I think we’re lost.”
“Don’t worry, Bev, I know where I’m going.”
“Stop and ask this lady for directions.”
“I said I can find it.”
“Just ask her.”
“Fine. Excuse me, ma’am, can you tell us how to get to the corpora quadrigemina from here?
Mucus plugging phenotype associated with adverse features
In a real-life clinic setting study aimed at determining phenotypic associations of mucus plugging
Rory Chan, MBChB, of the University of Dundee (Scotland) and colleagues found conversely that the presence of these features was associated with an increased likelihood of mucus plugging.
Important pathophysiological characteristics of persistent asthma include mucus plugging, goblet cell hyperplasia, smooth muscle hypertrophy, and eosinophilic infiltration. Mucus plugging contributes significantly to airway obstruction and death in acute asthma, the investigators stated, noting further that the understanding of mucus plugging’s role in chronic asthma is increasing.
Their retrospective cohort study included 126 patients with respiratory physician-diagnosed moderate to severe asthma who had attended their clinic (January 2016–March 2022) and were receiving daily doses of inhaled corticosteroid (ICS) (≥ 800 mcg) and a second-line controller. All had prior high-resolution CT (HRCT) scans with mucus plugs identified by an experienced thoracic radiologist. Prior to the start of biologic therapy, a mucus plug score (MPS) signifying the number of affected lung segments (0-20) was calculated subsequently and considered along with pulmonary function testing, T2 inflammatory markers, asthma control data, and measures of peripheral blood eosinophils (PBE), as well as total IgG and IgE antibodies to Apergillus fumigatus.
The analysis showed that reduced forced expiratory volume in 1 second (FEV1)/forced vital capacity (FVC) ratio (OR, 3.01; 95% confidence interval, 1.14-7.97), two or more exacerbations per year (OR, 5.00; 95% CI, 1.55-16.11), raised PBE (OR, 3.23; 95% CI, 1.16-8.96), raised total IgE (OR, 3.20; 95% CI, 1.09-9.37), and Aspergillus fumigatus IgE titers (OR, 9.37; 95% CI, 1.82-48.20) all conferred significantly higher likelihood of the presence of mucus plugging. Highest prevalence of mucus plugs was in the right and left lower lung lobes (about 26% vs. about 10% and 14% in the middle and upper lobes).
Adjusted ORs in patients with impaired FEV1/FVC, showed the likelihood of mucus plugging to be 67% higher. In those with frequent exacerbations, they were 80% higher, and in those with raised PBE and IgE, 69% higher. Patients without mucus plugging had preserved FEV1 and FEV1/FVC.
Asthma patients with mucus plugging in the study exhibited higher levels of routinely measured T2 biomarkers, including blood eosinophils, FeNO, and total IgE, with median values all exceeding traditionally accepted cut points. Although patients with mucus plugging were receiving significantly higher ICS doses, and despite the suppressive effect of ICS on FeNO, they still had higher FeNO levels. “We therefore postulate that asthma patients with the MP phenotype might potentially experience greater treatment response to biologics targeting the underlying inflammatory endotype,” the investigators stated, adding that “the presence of mucus plugging should be recognized as a treatable trait for patients with severe asthma in terms of targeting therapy with biologics.”
They wrote that, “in a real-life clinic setting, the presence of mucus plugging detected on HRCT was associated with more severe exacerbations, more severe airflow obstruction, and greater T2 inflammation. This, in turn, suggests that imaging should be part of the routine workup of patients with poorly controlled severe asthma.”
In an accompanying editorial, Jorge Cedano, MD, Jiwoong Choi, PhD, and Mario Castro, MD, MPH, of the University of Kansas, Kansas City, cited that the prevalence and contribution of mucus plugging in the pathophysiology and morbidity of uncontrolled asthma is much greater than has been appreciated. They focused particularly on the suggestion that, even after adjusting for confounders, molds such as Apergillus may play a causal role, along with blood eosinophils, fractional exhaled nitric oxide, and total IgE, in T2 inflammation.
While current biologic therapies targeting the T2 phenotype have not yet been shown to reverse the progressive loss of lung function or lung remodeling process, the editorialists referenced a recent post hoc analysis of the CASCADE study showing mucus plugging reduction with the biologic tezepelumab versus placebo correlated with lung function improvement. “At least 20% of patients with moderate to severe asthma will experience progressive decline in lung function, more exacerbations, and worse asthma control despite the use of controller therapies. If physicians could identify the MP phenotype using computed tomography, then potentially earlier treatment with biologic therapy may improve asthma control and prevent future decline in lung function,” they said.
The study limitations listed included its retrospective observational nature and the fact that the study had only one senior thoracic radiologist interpreting the lung scans.
The study authors cited numerous conflicts of interest with pharmaceutical companies and medical societies. Dr. Castro reported affiliation or involvement in multiple organizations or entities with a financial or nonfinancial interest in the subject matter or materials discussed in the article.
In a real-life clinic setting study aimed at determining phenotypic associations of mucus plugging
Rory Chan, MBChB, of the University of Dundee (Scotland) and colleagues found conversely that the presence of these features was associated with an increased likelihood of mucus plugging.
Important pathophysiological characteristics of persistent asthma include mucus plugging, goblet cell hyperplasia, smooth muscle hypertrophy, and eosinophilic infiltration. Mucus plugging contributes significantly to airway obstruction and death in acute asthma, the investigators stated, noting further that the understanding of mucus plugging’s role in chronic asthma is increasing.
Their retrospective cohort study included 126 patients with respiratory physician-diagnosed moderate to severe asthma who had attended their clinic (January 2016–March 2022) and were receiving daily doses of inhaled corticosteroid (ICS) (≥ 800 mcg) and a second-line controller. All had prior high-resolution CT (HRCT) scans with mucus plugs identified by an experienced thoracic radiologist. Prior to the start of biologic therapy, a mucus plug score (MPS) signifying the number of affected lung segments (0-20) was calculated subsequently and considered along with pulmonary function testing, T2 inflammatory markers, asthma control data, and measures of peripheral blood eosinophils (PBE), as well as total IgG and IgE antibodies to Apergillus fumigatus.
The analysis showed that reduced forced expiratory volume in 1 second (FEV1)/forced vital capacity (FVC) ratio (OR, 3.01; 95% confidence interval, 1.14-7.97), two or more exacerbations per year (OR, 5.00; 95% CI, 1.55-16.11), raised PBE (OR, 3.23; 95% CI, 1.16-8.96), raised total IgE (OR, 3.20; 95% CI, 1.09-9.37), and Aspergillus fumigatus IgE titers (OR, 9.37; 95% CI, 1.82-48.20) all conferred significantly higher likelihood of the presence of mucus plugging. Highest prevalence of mucus plugs was in the right and left lower lung lobes (about 26% vs. about 10% and 14% in the middle and upper lobes).
Adjusted ORs in patients with impaired FEV1/FVC, showed the likelihood of mucus plugging to be 67% higher. In those with frequent exacerbations, they were 80% higher, and in those with raised PBE and IgE, 69% higher. Patients without mucus plugging had preserved FEV1 and FEV1/FVC.
Asthma patients with mucus plugging in the study exhibited higher levels of routinely measured T2 biomarkers, including blood eosinophils, FeNO, and total IgE, with median values all exceeding traditionally accepted cut points. Although patients with mucus plugging were receiving significantly higher ICS doses, and despite the suppressive effect of ICS on FeNO, they still had higher FeNO levels. “We therefore postulate that asthma patients with the MP phenotype might potentially experience greater treatment response to biologics targeting the underlying inflammatory endotype,” the investigators stated, adding that “the presence of mucus plugging should be recognized as a treatable trait for patients with severe asthma in terms of targeting therapy with biologics.”
They wrote that, “in a real-life clinic setting, the presence of mucus plugging detected on HRCT was associated with more severe exacerbations, more severe airflow obstruction, and greater T2 inflammation. This, in turn, suggests that imaging should be part of the routine workup of patients with poorly controlled severe asthma.”
In an accompanying editorial, Jorge Cedano, MD, Jiwoong Choi, PhD, and Mario Castro, MD, MPH, of the University of Kansas, Kansas City, cited that the prevalence and contribution of mucus plugging in the pathophysiology and morbidity of uncontrolled asthma is much greater than has been appreciated. They focused particularly on the suggestion that, even after adjusting for confounders, molds such as Apergillus may play a causal role, along with blood eosinophils, fractional exhaled nitric oxide, and total IgE, in T2 inflammation.
While current biologic therapies targeting the T2 phenotype have not yet been shown to reverse the progressive loss of lung function or lung remodeling process, the editorialists referenced a recent post hoc analysis of the CASCADE study showing mucus plugging reduction with the biologic tezepelumab versus placebo correlated with lung function improvement. “At least 20% of patients with moderate to severe asthma will experience progressive decline in lung function, more exacerbations, and worse asthma control despite the use of controller therapies. If physicians could identify the MP phenotype using computed tomography, then potentially earlier treatment with biologic therapy may improve asthma control and prevent future decline in lung function,” they said.
The study limitations listed included its retrospective observational nature and the fact that the study had only one senior thoracic radiologist interpreting the lung scans.
The study authors cited numerous conflicts of interest with pharmaceutical companies and medical societies. Dr. Castro reported affiliation or involvement in multiple organizations or entities with a financial or nonfinancial interest in the subject matter or materials discussed in the article.
In a real-life clinic setting study aimed at determining phenotypic associations of mucus plugging
Rory Chan, MBChB, of the University of Dundee (Scotland) and colleagues found conversely that the presence of these features was associated with an increased likelihood of mucus plugging.
Important pathophysiological characteristics of persistent asthma include mucus plugging, goblet cell hyperplasia, smooth muscle hypertrophy, and eosinophilic infiltration. Mucus plugging contributes significantly to airway obstruction and death in acute asthma, the investigators stated, noting further that the understanding of mucus plugging’s role in chronic asthma is increasing.
Their retrospective cohort study included 126 patients with respiratory physician-diagnosed moderate to severe asthma who had attended their clinic (January 2016–March 2022) and were receiving daily doses of inhaled corticosteroid (ICS) (≥ 800 mcg) and a second-line controller. All had prior high-resolution CT (HRCT) scans with mucus plugs identified by an experienced thoracic radiologist. Prior to the start of biologic therapy, a mucus plug score (MPS) signifying the number of affected lung segments (0-20) was calculated subsequently and considered along with pulmonary function testing, T2 inflammatory markers, asthma control data, and measures of peripheral blood eosinophils (PBE), as well as total IgG and IgE antibodies to Apergillus fumigatus.
The analysis showed that reduced forced expiratory volume in 1 second (FEV1)/forced vital capacity (FVC) ratio (OR, 3.01; 95% confidence interval, 1.14-7.97), two or more exacerbations per year (OR, 5.00; 95% CI, 1.55-16.11), raised PBE (OR, 3.23; 95% CI, 1.16-8.96), raised total IgE (OR, 3.20; 95% CI, 1.09-9.37), and Aspergillus fumigatus IgE titers (OR, 9.37; 95% CI, 1.82-48.20) all conferred significantly higher likelihood of the presence of mucus plugging. Highest prevalence of mucus plugs was in the right and left lower lung lobes (about 26% vs. about 10% and 14% in the middle and upper lobes).
Adjusted ORs in patients with impaired FEV1/FVC, showed the likelihood of mucus plugging to be 67% higher. In those with frequent exacerbations, they were 80% higher, and in those with raised PBE and IgE, 69% higher. Patients without mucus plugging had preserved FEV1 and FEV1/FVC.
Asthma patients with mucus plugging in the study exhibited higher levels of routinely measured T2 biomarkers, including blood eosinophils, FeNO, and total IgE, with median values all exceeding traditionally accepted cut points. Although patients with mucus plugging were receiving significantly higher ICS doses, and despite the suppressive effect of ICS on FeNO, they still had higher FeNO levels. “We therefore postulate that asthma patients with the MP phenotype might potentially experience greater treatment response to biologics targeting the underlying inflammatory endotype,” the investigators stated, adding that “the presence of mucus plugging should be recognized as a treatable trait for patients with severe asthma in terms of targeting therapy with biologics.”
They wrote that, “in a real-life clinic setting, the presence of mucus plugging detected on HRCT was associated with more severe exacerbations, more severe airflow obstruction, and greater T2 inflammation. This, in turn, suggests that imaging should be part of the routine workup of patients with poorly controlled severe asthma.”
In an accompanying editorial, Jorge Cedano, MD, Jiwoong Choi, PhD, and Mario Castro, MD, MPH, of the University of Kansas, Kansas City, cited that the prevalence and contribution of mucus plugging in the pathophysiology and morbidity of uncontrolled asthma is much greater than has been appreciated. They focused particularly on the suggestion that, even after adjusting for confounders, molds such as Apergillus may play a causal role, along with blood eosinophils, fractional exhaled nitric oxide, and total IgE, in T2 inflammation.
While current biologic therapies targeting the T2 phenotype have not yet been shown to reverse the progressive loss of lung function or lung remodeling process, the editorialists referenced a recent post hoc analysis of the CASCADE study showing mucus plugging reduction with the biologic tezepelumab versus placebo correlated with lung function improvement. “At least 20% of patients with moderate to severe asthma will experience progressive decline in lung function, more exacerbations, and worse asthma control despite the use of controller therapies. If physicians could identify the MP phenotype using computed tomography, then potentially earlier treatment with biologic therapy may improve asthma control and prevent future decline in lung function,” they said.
The study limitations listed included its retrospective observational nature and the fact that the study had only one senior thoracic radiologist interpreting the lung scans.
The study authors cited numerous conflicts of interest with pharmaceutical companies and medical societies. Dr. Castro reported affiliation or involvement in multiple organizations or entities with a financial or nonfinancial interest in the subject matter or materials discussed in the article.
FROM THE JOURNAL OF ALLERGY AND CLINICAL IMMUNOLOGY: IN PRACTICE
Early treatment considerations in RA, April 2023
In evaluating the importance of early aggressive treatment of rheumatoid arthritis (RA), we often look at prognostic factors for severe disease, such as seropositivity, elevated inflammatory markers, and erosions. Eberhard and colleagues looked at the relationship between damage as seen on radiography (including erosions and joint space narrowing) and pain and disability in early RA using an inception cohort with <12 months of symptoms. Over 200 patients in Sweden were followed for 5 years with clinical, laboratory, and radiographic evaluations. Of interest, pain was associated with female sex, tender joint count, and inflammatory markers at various time points but not with radiographic damage. This may reflect that pain is related to current inflammation rather than past joint damage or that pain is related to other factors, such as central sensitization. Radiographic damage was, however, associated with disability and thus remains an important target and outcome measure for assessing treatment effectiveness.
Leon and colleagues also looked at early RA but instead, at the category of difficult-to-treat RA (D2T RA), meaning persistent RA symptoms after a trial of at least two biologic or targeted synthetic disease-modifying antirheumatic drugs. In order to gain better insight in preventing D2T RA, the authors examined its association with potentially modifiable risk factors early in the course of disease. Of the over 600 patients followed in this inception cohort, only about 6% were classified as having D2T RA. The study found that patients who had D2T RA tended to be younger, with a higher tender joint count, higher pain scores, and a higher initial level of disability. The Disease Activity Score (DAS28) itself was higher in patients with D2T RA, but the difference did not reach statistical significance. The small number of patients (35) in the D2T RA group may have affected the findings as well as their wider applicability. However, it is interesting to consider whether the associations may also reflect the impact of noninflammatory factors, as in the previous study, on the classification of D2T RA.
Park and colleagues evaluated the difference in clinical outcomes in postmenopausal patients with RA who underwent menopause at younger than 45 years or 45 years or older. Among over 2800 patients in Korea, those who underwent early menopause were more likely to be seronegative and have high disease activity and worse patient-reported outcome scores in fatigue, sleep, and health-related quality of life despite comparable treatments and prevalence of erosions. The authors suggest this may be related to lower cumulative estrogen exposure; whether this correlates to inflammatory cytokine signatures is not yet known. However, as with the prior studies, central sensitization and noninflammatory pain may also contribute and should be considered in interpreting response to therapy.
Finally, addressing the potential risk for cancer in patients with RA before or during treatment with immunosuppressive medications, Miyata and colleagues reported a study that screened nearly 2200 patients who underwent CT (from neck to pelvis) and compared them with those who underwent routine screening with physical exam plus radiography. The study found that CT screening enhanced cancer detection, with a large number of cancers detected at an earlier stage with CT screening compared with routine screening. The overall number of cancers detected was low (33), and thus routine screening with neck-to-pelvis CT for all patients with RA may not be a cost-effective practice. However, it bears further examination for potentially higher-risk populations or specific cancers.
In evaluating the importance of early aggressive treatment of rheumatoid arthritis (RA), we often look at prognostic factors for severe disease, such as seropositivity, elevated inflammatory markers, and erosions. Eberhard and colleagues looked at the relationship between damage as seen on radiography (including erosions and joint space narrowing) and pain and disability in early RA using an inception cohort with <12 months of symptoms. Over 200 patients in Sweden were followed for 5 years with clinical, laboratory, and radiographic evaluations. Of interest, pain was associated with female sex, tender joint count, and inflammatory markers at various time points but not with radiographic damage. This may reflect that pain is related to current inflammation rather than past joint damage or that pain is related to other factors, such as central sensitization. Radiographic damage was, however, associated with disability and thus remains an important target and outcome measure for assessing treatment effectiveness.
Leon and colleagues also looked at early RA but instead, at the category of difficult-to-treat RA (D2T RA), meaning persistent RA symptoms after a trial of at least two biologic or targeted synthetic disease-modifying antirheumatic drugs. In order to gain better insight in preventing D2T RA, the authors examined its association with potentially modifiable risk factors early in the course of disease. Of the over 600 patients followed in this inception cohort, only about 6% were classified as having D2T RA. The study found that patients who had D2T RA tended to be younger, with a higher tender joint count, higher pain scores, and a higher initial level of disability. The Disease Activity Score (DAS28) itself was higher in patients with D2T RA, but the difference did not reach statistical significance. The small number of patients (35) in the D2T RA group may have affected the findings as well as their wider applicability. However, it is interesting to consider whether the associations may also reflect the impact of noninflammatory factors, as in the previous study, on the classification of D2T RA.
Park and colleagues evaluated the difference in clinical outcomes in postmenopausal patients with RA who underwent menopause at younger than 45 years or 45 years or older. Among over 2800 patients in Korea, those who underwent early menopause were more likely to be seronegative and have high disease activity and worse patient-reported outcome scores in fatigue, sleep, and health-related quality of life despite comparable treatments and prevalence of erosions. The authors suggest this may be related to lower cumulative estrogen exposure; whether this correlates to inflammatory cytokine signatures is not yet known. However, as with the prior studies, central sensitization and noninflammatory pain may also contribute and should be considered in interpreting response to therapy.
Finally, addressing the potential risk for cancer in patients with RA before or during treatment with immunosuppressive medications, Miyata and colleagues reported a study that screened nearly 2200 patients who underwent CT (from neck to pelvis) and compared them with those who underwent routine screening with physical exam plus radiography. The study found that CT screening enhanced cancer detection, with a large number of cancers detected at an earlier stage with CT screening compared with routine screening. The overall number of cancers detected was low (33), and thus routine screening with neck-to-pelvis CT for all patients with RA may not be a cost-effective practice. However, it bears further examination for potentially higher-risk populations or specific cancers.
In evaluating the importance of early aggressive treatment of rheumatoid arthritis (RA), we often look at prognostic factors for severe disease, such as seropositivity, elevated inflammatory markers, and erosions. Eberhard and colleagues looked at the relationship between damage as seen on radiography (including erosions and joint space narrowing) and pain and disability in early RA using an inception cohort with <12 months of symptoms. Over 200 patients in Sweden were followed for 5 years with clinical, laboratory, and radiographic evaluations. Of interest, pain was associated with female sex, tender joint count, and inflammatory markers at various time points but not with radiographic damage. This may reflect that pain is related to current inflammation rather than past joint damage or that pain is related to other factors, such as central sensitization. Radiographic damage was, however, associated with disability and thus remains an important target and outcome measure for assessing treatment effectiveness.
Leon and colleagues also looked at early RA but instead, at the category of difficult-to-treat RA (D2T RA), meaning persistent RA symptoms after a trial of at least two biologic or targeted synthetic disease-modifying antirheumatic drugs. In order to gain better insight in preventing D2T RA, the authors examined its association with potentially modifiable risk factors early in the course of disease. Of the over 600 patients followed in this inception cohort, only about 6% were classified as having D2T RA. The study found that patients who had D2T RA tended to be younger, with a higher tender joint count, higher pain scores, and a higher initial level of disability. The Disease Activity Score (DAS28) itself was higher in patients with D2T RA, but the difference did not reach statistical significance. The small number of patients (35) in the D2T RA group may have affected the findings as well as their wider applicability. However, it is interesting to consider whether the associations may also reflect the impact of noninflammatory factors, as in the previous study, on the classification of D2T RA.
Park and colleagues evaluated the difference in clinical outcomes in postmenopausal patients with RA who underwent menopause at younger than 45 years or 45 years or older. Among over 2800 patients in Korea, those who underwent early menopause were more likely to be seronegative and have high disease activity and worse patient-reported outcome scores in fatigue, sleep, and health-related quality of life despite comparable treatments and prevalence of erosions. The authors suggest this may be related to lower cumulative estrogen exposure; whether this correlates to inflammatory cytokine signatures is not yet known. However, as with the prior studies, central sensitization and noninflammatory pain may also contribute and should be considered in interpreting response to therapy.
Finally, addressing the potential risk for cancer in patients with RA before or during treatment with immunosuppressive medications, Miyata and colleagues reported a study that screened nearly 2200 patients who underwent CT (from neck to pelvis) and compared them with those who underwent routine screening with physical exam plus radiography. The study found that CT screening enhanced cancer detection, with a large number of cancers detected at an earlier stage with CT screening compared with routine screening. The overall number of cancers detected was low (33), and thus routine screening with neck-to-pelvis CT for all patients with RA may not be a cost-effective practice. However, it bears further examination for potentially higher-risk populations or specific cancers.
Commentary: Updates on the Treatment of Mantle Cell Lymphoma, April 2023
Mantle cell lymphoma (MCL) is an uncommon subtype of non-Hodgkin lymphoma (NHL) that is clinically heterogeneous, ranging from indolent to aggressive in nature. As with other subtypes of NHL, the treatment landscape is rapidly evolving.
Chemoimmunotherapy remains the standard first-line therapy for younger, fit patients. Although multiple induction regimens are used in this setting, it is typical to use a cytarabine-containing approach. Recently, the long-term analysis of the MCL Younger trial continued to demonstrate improved outcomes with this strategy.1 This phase 3 study included 497 patients aged ≥ 18 to < 66 years with previously untreated MCL who were randomly assigned to R-CHOP (cyclophosphamide, doxorubicin, prednisone, rituximab, and vincristine; n = 249) or R-DHAP (rituximab, dexamethasone, cytarabine, cisplatin; n = 248). After a median follow-up of 10.6 years, the R-DHAP vs R-CHOP arm continued to have a significantly longer time to treatment failure (hazard ratio [HR] 0.59; P = .038) and overall survival (Mantle Cell Lymphoma International Prognostic Index + Ki-67–adjusted HR 0.60; P = .0066).
Following chemoimmunotherapy, treatment for this patient population typically consists of consolidation with autologous stem cell transplantation (ASCT) and maintenance rituximab.2 Recently, the role of ASCT has been called into question.3 Preliminary data from the phase 3 TRIANGLE study demonstrated improvement in outcomes when the Bruton tyrosine kinase (BTK) inhibitor ibrutinib was added to chemoimmunotherapy, regardless of whether patients received ASCT.4 Additional studies evaluating the role of transplantation, particularly among patients who are minimal residual disease negative after chemoimmunotherapy, are ongoing (NCT03267433).
Options continue to expand in the relapsed/refractory setting. The chimeric antigen receptor (CAR) T-cell therapy, brexucabtagene autoleucel (brexu-cel), was approved by the US Food and Drug Administration for relapsed/refractory MCL on the basis of the results of the ZUMA-2 study.5 Recently, a multicenter, retrospective study demonstrated promising efficacy in the real world as well (Wang et al). This study was performed across 16 medical centers and included 189 patients with relapsed/refractory MCL who underwent leukapheresis for commercial manufacturing of brexu-cel, of which 168 received brexu-cel infusion. Of all patients receiving leukapheresis, 149 (79%) would not have met the eligibility criteria for ZUMA-2. At a median follow-up of 14.3 months after infusion, the best overall and complete response rates were 90% and 82%, respectively. The 6- and 12-month progression-free survival (PFS) rates were 69% (95% CI 61%-75%) and 59% (95% CI 51%-66%), respectively. This approach, however, was associated with significant toxicity, with a nonrelapse mortality rate of 9.1% at 1 year, primarily because of infections. The grade ≥ 3 cytokine release syndrome and neurotoxicity rates were 8% and 32%, respectively. Despite risks, this study confirms the role of CAR T-cell therapy for patients with relapsed/refractory MCL.
Other options in the relapsed setting include BTK and anti-apoptotic protein B-cell lymphoma (BCL-2) inhibitors. Although venetoclax, a BCL-2 inhibitor, has demonstrated activity in MCL in early-phase clinical trials, the role of this drug in clinical practice remains unclear.6,7 A recent multicenter, retrospective study evaluated the use of venetoclax in 81 adult patients with relapsed/refractory MCL, most of whom were heavily pretreated (median of three prior treatments) and had high-risk features, including high Ki-67 and TP53 alterations, who received venetoclax without (n = 50) or with (n = 31) other agents (Sawalha et al). In this study, venetoclax resulted in a good overall response rate (ORR) but short PFS. At a median follow-up of 16.4 months, patients had a median PFS and overall survival of 3.7 months (95% CI 2.3-5.6) and 12.5 months (95% CI 6.2-28.2), respectively, and an ORR of 40%. Studies of venetoclax in earlier lines of therapy and in combination with other agents are ongoing. There may also be a role for this treatment as a bridge to more definitive therapies, including CAR T-cell therapy or allogeneic stem cell transplantation. Other studies that are evaluating the role of bispecific antibodies and antibody drug conjugates are also underway, suggesting the potential for additional options in this patient population.
Additional References
1. Hermine O, Jiang L, Walewski J, et al. High-dose cytarabine and autologous stem-cell transplantation in mantle cell lymphoma: Long-term follow-up of the randomized Mantle Cell Lymphoma Younger Trial of the European Mantle Cell Lymphoma Network. J Clin Oncol. 2023;41:479-484. doi: 10.1200/JCO.22.01780
2. Le Gouill S, Thieblemont C, Oberic L, et al. Rituximab after autologous stem-cell transplantation in mantle-cell lymphoma. N Engl J Med. 2017;377:1250-1260. doi: 10.1056/NEJMoa1701769
3. Martin P, Cohen JB, Wang M, et al. Treatment outcomes and roles of transplantation and maintenance rituximab in patients with previously untreated mantle cell lymphoma: Results from large real-world cohorts. J Clin Oncol. 2023;41:541-554. doi: 10.1200/JCO.21.02698
4. Dreyling M, Doorduijn JK, Gine E, et al. Efficacy and safety of ibrutinib combined with standard first-line treatment or as substitute for autologous stem cell transplantation in younger patients with mantle cell lymphoma: Results from the randomized Triangle Trial by the European MCL Network. Blood. 2022;140(Suppl 1):1-3. doi: 10.1182/blood-2022-163018
5. Wang M, Munoz J, Goy A, et al. KTE-X19 CAR T-Cell therapy in relapsed or refractory mantle-cell lymphoma. N Engl J Med. 2020;382:1331-1342. doi: 10.1056/NEJMoa1914347
6. Davids MS, Roberts AW, Seymour JF, et al. Phase I first-in-human study of venetoclax in patients with relapsed or refractory non-Hodgkin lymphoma. J Clin Oncol. 2017;35:826-833. doi: 10.1200/JCO.2016.70.4320
7. Tam CS, Anderson MA, Pott C, et al. Ibrutinib plus venetoclax for the treatment of mantle-cell lymphoma. N Engl J Med. 2018;378:1211-1223. doi: 10.1056/NEJMoa1715519
Mantle cell lymphoma (MCL) is an uncommon subtype of non-Hodgkin lymphoma (NHL) that is clinically heterogeneous, ranging from indolent to aggressive in nature. As with other subtypes of NHL, the treatment landscape is rapidly evolving.
Chemoimmunotherapy remains the standard first-line therapy for younger, fit patients. Although multiple induction regimens are used in this setting, it is typical to use a cytarabine-containing approach. Recently, the long-term analysis of the MCL Younger trial continued to demonstrate improved outcomes with this strategy.1 This phase 3 study included 497 patients aged ≥ 18 to < 66 years with previously untreated MCL who were randomly assigned to R-CHOP (cyclophosphamide, doxorubicin, prednisone, rituximab, and vincristine; n = 249) or R-DHAP (rituximab, dexamethasone, cytarabine, cisplatin; n = 248). After a median follow-up of 10.6 years, the R-DHAP vs R-CHOP arm continued to have a significantly longer time to treatment failure (hazard ratio [HR] 0.59; P = .038) and overall survival (Mantle Cell Lymphoma International Prognostic Index + Ki-67–adjusted HR 0.60; P = .0066).
Following chemoimmunotherapy, treatment for this patient population typically consists of consolidation with autologous stem cell transplantation (ASCT) and maintenance rituximab.2 Recently, the role of ASCT has been called into question.3 Preliminary data from the phase 3 TRIANGLE study demonstrated improvement in outcomes when the Bruton tyrosine kinase (BTK) inhibitor ibrutinib was added to chemoimmunotherapy, regardless of whether patients received ASCT.4 Additional studies evaluating the role of transplantation, particularly among patients who are minimal residual disease negative after chemoimmunotherapy, are ongoing (NCT03267433).
Options continue to expand in the relapsed/refractory setting. The chimeric antigen receptor (CAR) T-cell therapy, brexucabtagene autoleucel (brexu-cel), was approved by the US Food and Drug Administration for relapsed/refractory MCL on the basis of the results of the ZUMA-2 study.5 Recently, a multicenter, retrospective study demonstrated promising efficacy in the real world as well (Wang et al). This study was performed across 16 medical centers and included 189 patients with relapsed/refractory MCL who underwent leukapheresis for commercial manufacturing of brexu-cel, of which 168 received brexu-cel infusion. Of all patients receiving leukapheresis, 149 (79%) would not have met the eligibility criteria for ZUMA-2. At a median follow-up of 14.3 months after infusion, the best overall and complete response rates were 90% and 82%, respectively. The 6- and 12-month progression-free survival (PFS) rates were 69% (95% CI 61%-75%) and 59% (95% CI 51%-66%), respectively. This approach, however, was associated with significant toxicity, with a nonrelapse mortality rate of 9.1% at 1 year, primarily because of infections. The grade ≥ 3 cytokine release syndrome and neurotoxicity rates were 8% and 32%, respectively. Despite risks, this study confirms the role of CAR T-cell therapy for patients with relapsed/refractory MCL.
Other options in the relapsed setting include BTK and anti-apoptotic protein B-cell lymphoma (BCL-2) inhibitors. Although venetoclax, a BCL-2 inhibitor, has demonstrated activity in MCL in early-phase clinical trials, the role of this drug in clinical practice remains unclear.6,7 A recent multicenter, retrospective study evaluated the use of venetoclax in 81 adult patients with relapsed/refractory MCL, most of whom were heavily pretreated (median of three prior treatments) and had high-risk features, including high Ki-67 and TP53 alterations, who received venetoclax without (n = 50) or with (n = 31) other agents (Sawalha et al). In this study, venetoclax resulted in a good overall response rate (ORR) but short PFS. At a median follow-up of 16.4 months, patients had a median PFS and overall survival of 3.7 months (95% CI 2.3-5.6) and 12.5 months (95% CI 6.2-28.2), respectively, and an ORR of 40%. Studies of venetoclax in earlier lines of therapy and in combination with other agents are ongoing. There may also be a role for this treatment as a bridge to more definitive therapies, including CAR T-cell therapy or allogeneic stem cell transplantation. Other studies that are evaluating the role of bispecific antibodies and antibody drug conjugates are also underway, suggesting the potential for additional options in this patient population.
Additional References
1. Hermine O, Jiang L, Walewski J, et al. High-dose cytarabine and autologous stem-cell transplantation in mantle cell lymphoma: Long-term follow-up of the randomized Mantle Cell Lymphoma Younger Trial of the European Mantle Cell Lymphoma Network. J Clin Oncol. 2023;41:479-484. doi: 10.1200/JCO.22.01780
2. Le Gouill S, Thieblemont C, Oberic L, et al. Rituximab after autologous stem-cell transplantation in mantle-cell lymphoma. N Engl J Med. 2017;377:1250-1260. doi: 10.1056/NEJMoa1701769
3. Martin P, Cohen JB, Wang M, et al. Treatment outcomes and roles of transplantation and maintenance rituximab in patients with previously untreated mantle cell lymphoma: Results from large real-world cohorts. J Clin Oncol. 2023;41:541-554. doi: 10.1200/JCO.21.02698
4. Dreyling M, Doorduijn JK, Gine E, et al. Efficacy and safety of ibrutinib combined with standard first-line treatment or as substitute for autologous stem cell transplantation in younger patients with mantle cell lymphoma: Results from the randomized Triangle Trial by the European MCL Network. Blood. 2022;140(Suppl 1):1-3. doi: 10.1182/blood-2022-163018
5. Wang M, Munoz J, Goy A, et al. KTE-X19 CAR T-Cell therapy in relapsed or refractory mantle-cell lymphoma. N Engl J Med. 2020;382:1331-1342. doi: 10.1056/NEJMoa1914347
6. Davids MS, Roberts AW, Seymour JF, et al. Phase I first-in-human study of venetoclax in patients with relapsed or refractory non-Hodgkin lymphoma. J Clin Oncol. 2017;35:826-833. doi: 10.1200/JCO.2016.70.4320
7. Tam CS, Anderson MA, Pott C, et al. Ibrutinib plus venetoclax for the treatment of mantle-cell lymphoma. N Engl J Med. 2018;378:1211-1223. doi: 10.1056/NEJMoa1715519
Mantle cell lymphoma (MCL) is an uncommon subtype of non-Hodgkin lymphoma (NHL) that is clinically heterogeneous, ranging from indolent to aggressive in nature. As with other subtypes of NHL, the treatment landscape is rapidly evolving.
Chemoimmunotherapy remains the standard first-line therapy for younger, fit patients. Although multiple induction regimens are used in this setting, it is typical to use a cytarabine-containing approach. Recently, the long-term analysis of the MCL Younger trial continued to demonstrate improved outcomes with this strategy.1 This phase 3 study included 497 patients aged ≥ 18 to < 66 years with previously untreated MCL who were randomly assigned to R-CHOP (cyclophosphamide, doxorubicin, prednisone, rituximab, and vincristine; n = 249) or R-DHAP (rituximab, dexamethasone, cytarabine, cisplatin; n = 248). After a median follow-up of 10.6 years, the R-DHAP vs R-CHOP arm continued to have a significantly longer time to treatment failure (hazard ratio [HR] 0.59; P = .038) and overall survival (Mantle Cell Lymphoma International Prognostic Index + Ki-67–adjusted HR 0.60; P = .0066).
Following chemoimmunotherapy, treatment for this patient population typically consists of consolidation with autologous stem cell transplantation (ASCT) and maintenance rituximab.2 Recently, the role of ASCT has been called into question.3 Preliminary data from the phase 3 TRIANGLE study demonstrated improvement in outcomes when the Bruton tyrosine kinase (BTK) inhibitor ibrutinib was added to chemoimmunotherapy, regardless of whether patients received ASCT.4 Additional studies evaluating the role of transplantation, particularly among patients who are minimal residual disease negative after chemoimmunotherapy, are ongoing (NCT03267433).
Options continue to expand in the relapsed/refractory setting. The chimeric antigen receptor (CAR) T-cell therapy, brexucabtagene autoleucel (brexu-cel), was approved by the US Food and Drug Administration for relapsed/refractory MCL on the basis of the results of the ZUMA-2 study.5 Recently, a multicenter, retrospective study demonstrated promising efficacy in the real world as well (Wang et al). This study was performed across 16 medical centers and included 189 patients with relapsed/refractory MCL who underwent leukapheresis for commercial manufacturing of brexu-cel, of which 168 received brexu-cel infusion. Of all patients receiving leukapheresis, 149 (79%) would not have met the eligibility criteria for ZUMA-2. At a median follow-up of 14.3 months after infusion, the best overall and complete response rates were 90% and 82%, respectively. The 6- and 12-month progression-free survival (PFS) rates were 69% (95% CI 61%-75%) and 59% (95% CI 51%-66%), respectively. This approach, however, was associated with significant toxicity, with a nonrelapse mortality rate of 9.1% at 1 year, primarily because of infections. The grade ≥ 3 cytokine release syndrome and neurotoxicity rates were 8% and 32%, respectively. Despite risks, this study confirms the role of CAR T-cell therapy for patients with relapsed/refractory MCL.
Other options in the relapsed setting include BTK and anti-apoptotic protein B-cell lymphoma (BCL-2) inhibitors. Although venetoclax, a BCL-2 inhibitor, has demonstrated activity in MCL in early-phase clinical trials, the role of this drug in clinical practice remains unclear.6,7 A recent multicenter, retrospective study evaluated the use of venetoclax in 81 adult patients with relapsed/refractory MCL, most of whom were heavily pretreated (median of three prior treatments) and had high-risk features, including high Ki-67 and TP53 alterations, who received venetoclax without (n = 50) or with (n = 31) other agents (Sawalha et al). In this study, venetoclax resulted in a good overall response rate (ORR) but short PFS. At a median follow-up of 16.4 months, patients had a median PFS and overall survival of 3.7 months (95% CI 2.3-5.6) and 12.5 months (95% CI 6.2-28.2), respectively, and an ORR of 40%. Studies of venetoclax in earlier lines of therapy and in combination with other agents are ongoing. There may also be a role for this treatment as a bridge to more definitive therapies, including CAR T-cell therapy or allogeneic stem cell transplantation. Other studies that are evaluating the role of bispecific antibodies and antibody drug conjugates are also underway, suggesting the potential for additional options in this patient population.
Additional References
1. Hermine O, Jiang L, Walewski J, et al. High-dose cytarabine and autologous stem-cell transplantation in mantle cell lymphoma: Long-term follow-up of the randomized Mantle Cell Lymphoma Younger Trial of the European Mantle Cell Lymphoma Network. J Clin Oncol. 2023;41:479-484. doi: 10.1200/JCO.22.01780
2. Le Gouill S, Thieblemont C, Oberic L, et al. Rituximab after autologous stem-cell transplantation in mantle-cell lymphoma. N Engl J Med. 2017;377:1250-1260. doi: 10.1056/NEJMoa1701769
3. Martin P, Cohen JB, Wang M, et al. Treatment outcomes and roles of transplantation and maintenance rituximab in patients with previously untreated mantle cell lymphoma: Results from large real-world cohorts. J Clin Oncol. 2023;41:541-554. doi: 10.1200/JCO.21.02698
4. Dreyling M, Doorduijn JK, Gine E, et al. Efficacy and safety of ibrutinib combined with standard first-line treatment or as substitute for autologous stem cell transplantation in younger patients with mantle cell lymphoma: Results from the randomized Triangle Trial by the European MCL Network. Blood. 2022;140(Suppl 1):1-3. doi: 10.1182/blood-2022-163018
5. Wang M, Munoz J, Goy A, et al. KTE-X19 CAR T-Cell therapy in relapsed or refractory mantle-cell lymphoma. N Engl J Med. 2020;382:1331-1342. doi: 10.1056/NEJMoa1914347
6. Davids MS, Roberts AW, Seymour JF, et al. Phase I first-in-human study of venetoclax in patients with relapsed or refractory non-Hodgkin lymphoma. J Clin Oncol. 2017;35:826-833. doi: 10.1200/JCO.2016.70.4320
7. Tam CS, Anderson MA, Pott C, et al. Ibrutinib plus venetoclax for the treatment of mantle-cell lymphoma. N Engl J Med. 2018;378:1211-1223. doi: 10.1056/NEJMoa1715519
Stutz: The psychiatrist as movie star
For as long as I can remember, psychiatrists have talked about what the appropriate boundaries are for self-disclosure about personal issues with patients. There is obviously no exact answer as to what is acceptable to disclose; this depends on the doctor, the patient, the “brand” of psychotherapy, the patient’s issues, the nature of what is being disclosed, and maybe the alignment of the stars on that particular day. “Stutz,” the Netflix documentary that Oscar-nominated actor/director Jonah Hill has made about his psychiatrist, Phil Stutz, MD, adds a whole new chapter to the discussion.
“Okay, entertain me,” Dr. Stutz says as his patient takes a seat. The therapeutic relationship and the paradigm Dr. Stutz has created to help his patients has been healing for Jonah Hill. The very serious and intimate dialogue that follows unfolds with moments of humor, warmth, and open affection. Hill candidly tells us why he is making this documentary – to share what he has learned and to honor his therapist – but we don’t know why Dr. Stutz has agreed to the endeavor and we’re left to our own inferences.
Dr. Stutz is the coauthor, with Barry Michels, of a best-selling self-help book, “The Tools: 5 Tools to Help You Find Courage, Creativity, and Willpower – and Inspire You to Live Life in Forward Motion.” He talks about his restlessness with the psychodynamic method during his training as a resident in New York – he wanted to offer his patients more immediate relief and a supervisor told him, “Don’t you dare!”
In the film, he talks about giving patients hope and direction. And Hill makes the comment, “In traditional therapy, you’re paying this person and you save all your problems for them, and they just listen, and your friends – who are idiots – give you advice, unsolicited, and you want your friends just to listen, and you want your therapist to give you advice!” Dr. Stutz gives advice and he is like no other therapist Jonah has ever had.
The premise of the film is that we are watching a single therapy session and Dr. Stutz will discuss the use of his tools and techniques that Hill has found helpful. Jonah is the interviewer, and when the doctor suggests it would be helpful if Jonah talked about his life, the patient/director rebuffs him; this documentary is about the psychiatrist.
Early in the film an alarm goes off, Dr. Stutz does not hear it, and Jonah has to remind him that it’s time for him to take his pills. The psychiatrist has Parkinson’s disease and how it has affected him becomes one focal point for the film. We later learn that he lost a younger brother as a child (something Hill did not know before they started filming) and grew up in the shadow of that loss. His extroverted father made it clear that medicine was the only acceptable career path for his son, and his introverted and depressed mother spent her days proclaiming that all men were as awful as her own abusive father.
About a third of the way through the film, the focus shifts. Jonah suddenly confesses that he is feeling stuck with regard to the movie, that he is troubled by the fact that he has not been able to share his distress with Dr. Stutz during their real-life, unfilmed therapy sessions, and the viewers learn that the single-session concept was disingenuous – they have been filming this documentary for two years, against a green screen and not in an office, always wearing the same clothes, and Jonah pulls off a wig that he wears to disguise the fact that he changed his hairstyle months earlier.
It’s a bit unnerving as they throw the wig around, and Jonah agrees to be more open about the issues he has struggled with. He acknowledges that this has been difficult, and he says, “I just keep asking myself, like, was this a f***ing horrible idea for a patient to make a movie about his therapist?” From my perspective as a psychiatrist-viewer, it’s a good question to ask!
Dr. Stutz reassures Jonah that it is okay to be vulnerable. “Failure, weakness, vulnerability – it’s like a connector, it connects you to the rest of the world.” A super-sized cardboard cutout of an obese 14-year-old Jonah now joins the room, and we learn that he continues to struggle with his self-image. Things get more real.
Peppered throughout the film, there are lessons from Dr. Stutz about his “tools,” constructs he uses to help people restructure their worlds and take action to move forward. One such construct he calls “the maze,” which occurs when one person in an interpersonal relationship is waiting for fairness and becomes preoccupied with feeling injured.
Jonah inquires about Dr. Stutz’s romantic life and the therapist replies with a transparency that overrides our usual professional boundaries. We all learn that Dr. Stutz is not in a relationship, he’s never been married, but there is a woman he has had some involvement with on and off for 40 years. Jonah’s line of questioning rivals that of any therapist. “How do you think it affects you, having your mom hate men and you being a man?” Dr. Stutz admits that he can never feel safe with women. “Did you ever override that wall you built with your mom and get close to a woman?” When Jonah professes, “I don’t feel anything but love for you and I just want you to be happy,” my own feeling was that the tables had turned too far, that the therapist’s failed romantic life risked being a burden to the patient.
Still, there is something about the relationship between the two men that is touching and beautiful. Dr. Stutz as a therapist is charismatic, caring, self-assured, and optimistic, and he radiates hope and certainty. He mixes an intense intimacy with humor in a way that is both authentic and entertaining. The interspersed jokes break the intensity, but they don’t diminish his wisdom and the healing he imparts.
Dr. Stutz is a psychiatrist, and his strength is clearly as a psychotherapist, yet there is not a single mention of psychotropic medications – there is a banter about recreational drugs and medications for Parkinson’s disease. If Hill is taking medication for depression or anxiety, and if prescribing is part of Dr. Stutz’s arsenal, the viewer is not made aware of this.
Dr. Stutz eschews the slow, detached, and “neutral” pace of psychodynamic therapy and the whole concept of the therapist as a blank wall for the transference to play out on, but here the transference screams: Jonah loves him, he respect and honors him, he wants him to be happy, and he is afraid of losing him.
“Stutz” is a movie about a larger-than-life psychiatrist, one whose warmth and inspiration are healing. I imagine his tools are helpful, but his personality is what carries the load. If a viewer has not had experience with psychiatry, and this film inspires him to begin therapy, there may be a good deal of disappointment. In this case, the patient is a successful actor, and one might wonder if that, together with the entire years-long project of filming, has altered the relationship well beyond the usual therapeutic hour.
Dr. Miller is a coauthor of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University Press, 2016). She has a private practice and is an assistant professor of psychiatry and behavioral sciences at Johns Hopkins University in Baltimore.
For as long as I can remember, psychiatrists have talked about what the appropriate boundaries are for self-disclosure about personal issues with patients. There is obviously no exact answer as to what is acceptable to disclose; this depends on the doctor, the patient, the “brand” of psychotherapy, the patient’s issues, the nature of what is being disclosed, and maybe the alignment of the stars on that particular day. “Stutz,” the Netflix documentary that Oscar-nominated actor/director Jonah Hill has made about his psychiatrist, Phil Stutz, MD, adds a whole new chapter to the discussion.
“Okay, entertain me,” Dr. Stutz says as his patient takes a seat. The therapeutic relationship and the paradigm Dr. Stutz has created to help his patients has been healing for Jonah Hill. The very serious and intimate dialogue that follows unfolds with moments of humor, warmth, and open affection. Hill candidly tells us why he is making this documentary – to share what he has learned and to honor his therapist – but we don’t know why Dr. Stutz has agreed to the endeavor and we’re left to our own inferences.
Dr. Stutz is the coauthor, with Barry Michels, of a best-selling self-help book, “The Tools: 5 Tools to Help You Find Courage, Creativity, and Willpower – and Inspire You to Live Life in Forward Motion.” He talks about his restlessness with the psychodynamic method during his training as a resident in New York – he wanted to offer his patients more immediate relief and a supervisor told him, “Don’t you dare!”
In the film, he talks about giving patients hope and direction. And Hill makes the comment, “In traditional therapy, you’re paying this person and you save all your problems for them, and they just listen, and your friends – who are idiots – give you advice, unsolicited, and you want your friends just to listen, and you want your therapist to give you advice!” Dr. Stutz gives advice and he is like no other therapist Jonah has ever had.
The premise of the film is that we are watching a single therapy session and Dr. Stutz will discuss the use of his tools and techniques that Hill has found helpful. Jonah is the interviewer, and when the doctor suggests it would be helpful if Jonah talked about his life, the patient/director rebuffs him; this documentary is about the psychiatrist.
Early in the film an alarm goes off, Dr. Stutz does not hear it, and Jonah has to remind him that it’s time for him to take his pills. The psychiatrist has Parkinson’s disease and how it has affected him becomes one focal point for the film. We later learn that he lost a younger brother as a child (something Hill did not know before they started filming) and grew up in the shadow of that loss. His extroverted father made it clear that medicine was the only acceptable career path for his son, and his introverted and depressed mother spent her days proclaiming that all men were as awful as her own abusive father.
About a third of the way through the film, the focus shifts. Jonah suddenly confesses that he is feeling stuck with regard to the movie, that he is troubled by the fact that he has not been able to share his distress with Dr. Stutz during their real-life, unfilmed therapy sessions, and the viewers learn that the single-session concept was disingenuous – they have been filming this documentary for two years, against a green screen and not in an office, always wearing the same clothes, and Jonah pulls off a wig that he wears to disguise the fact that he changed his hairstyle months earlier.
It’s a bit unnerving as they throw the wig around, and Jonah agrees to be more open about the issues he has struggled with. He acknowledges that this has been difficult, and he says, “I just keep asking myself, like, was this a f***ing horrible idea for a patient to make a movie about his therapist?” From my perspective as a psychiatrist-viewer, it’s a good question to ask!
Dr. Stutz reassures Jonah that it is okay to be vulnerable. “Failure, weakness, vulnerability – it’s like a connector, it connects you to the rest of the world.” A super-sized cardboard cutout of an obese 14-year-old Jonah now joins the room, and we learn that he continues to struggle with his self-image. Things get more real.
Peppered throughout the film, there are lessons from Dr. Stutz about his “tools,” constructs he uses to help people restructure their worlds and take action to move forward. One such construct he calls “the maze,” which occurs when one person in an interpersonal relationship is waiting for fairness and becomes preoccupied with feeling injured.
Jonah inquires about Dr. Stutz’s romantic life and the therapist replies with a transparency that overrides our usual professional boundaries. We all learn that Dr. Stutz is not in a relationship, he’s never been married, but there is a woman he has had some involvement with on and off for 40 years. Jonah’s line of questioning rivals that of any therapist. “How do you think it affects you, having your mom hate men and you being a man?” Dr. Stutz admits that he can never feel safe with women. “Did you ever override that wall you built with your mom and get close to a woman?” When Jonah professes, “I don’t feel anything but love for you and I just want you to be happy,” my own feeling was that the tables had turned too far, that the therapist’s failed romantic life risked being a burden to the patient.
Still, there is something about the relationship between the two men that is touching and beautiful. Dr. Stutz as a therapist is charismatic, caring, self-assured, and optimistic, and he radiates hope and certainty. He mixes an intense intimacy with humor in a way that is both authentic and entertaining. The interspersed jokes break the intensity, but they don’t diminish his wisdom and the healing he imparts.
Dr. Stutz is a psychiatrist, and his strength is clearly as a psychotherapist, yet there is not a single mention of psychotropic medications – there is a banter about recreational drugs and medications for Parkinson’s disease. If Hill is taking medication for depression or anxiety, and if prescribing is part of Dr. Stutz’s arsenal, the viewer is not made aware of this.
Dr. Stutz eschews the slow, detached, and “neutral” pace of psychodynamic therapy and the whole concept of the therapist as a blank wall for the transference to play out on, but here the transference screams: Jonah loves him, he respect and honors him, he wants him to be happy, and he is afraid of losing him.
“Stutz” is a movie about a larger-than-life psychiatrist, one whose warmth and inspiration are healing. I imagine his tools are helpful, but his personality is what carries the load. If a viewer has not had experience with psychiatry, and this film inspires him to begin therapy, there may be a good deal of disappointment. In this case, the patient is a successful actor, and one might wonder if that, together with the entire years-long project of filming, has altered the relationship well beyond the usual therapeutic hour.
Dr. Miller is a coauthor of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University Press, 2016). She has a private practice and is an assistant professor of psychiatry and behavioral sciences at Johns Hopkins University in Baltimore.
For as long as I can remember, psychiatrists have talked about what the appropriate boundaries are for self-disclosure about personal issues with patients. There is obviously no exact answer as to what is acceptable to disclose; this depends on the doctor, the patient, the “brand” of psychotherapy, the patient’s issues, the nature of what is being disclosed, and maybe the alignment of the stars on that particular day. “Stutz,” the Netflix documentary that Oscar-nominated actor/director Jonah Hill has made about his psychiatrist, Phil Stutz, MD, adds a whole new chapter to the discussion.
“Okay, entertain me,” Dr. Stutz says as his patient takes a seat. The therapeutic relationship and the paradigm Dr. Stutz has created to help his patients has been healing for Jonah Hill. The very serious and intimate dialogue that follows unfolds with moments of humor, warmth, and open affection. Hill candidly tells us why he is making this documentary – to share what he has learned and to honor his therapist – but we don’t know why Dr. Stutz has agreed to the endeavor and we’re left to our own inferences.
Dr. Stutz is the coauthor, with Barry Michels, of a best-selling self-help book, “The Tools: 5 Tools to Help You Find Courage, Creativity, and Willpower – and Inspire You to Live Life in Forward Motion.” He talks about his restlessness with the psychodynamic method during his training as a resident in New York – he wanted to offer his patients more immediate relief and a supervisor told him, “Don’t you dare!”
In the film, he talks about giving patients hope and direction. And Hill makes the comment, “In traditional therapy, you’re paying this person and you save all your problems for them, and they just listen, and your friends – who are idiots – give you advice, unsolicited, and you want your friends just to listen, and you want your therapist to give you advice!” Dr. Stutz gives advice and he is like no other therapist Jonah has ever had.
The premise of the film is that we are watching a single therapy session and Dr. Stutz will discuss the use of his tools and techniques that Hill has found helpful. Jonah is the interviewer, and when the doctor suggests it would be helpful if Jonah talked about his life, the patient/director rebuffs him; this documentary is about the psychiatrist.
Early in the film an alarm goes off, Dr. Stutz does not hear it, and Jonah has to remind him that it’s time for him to take his pills. The psychiatrist has Parkinson’s disease and how it has affected him becomes one focal point for the film. We later learn that he lost a younger brother as a child (something Hill did not know before they started filming) and grew up in the shadow of that loss. His extroverted father made it clear that medicine was the only acceptable career path for his son, and his introverted and depressed mother spent her days proclaiming that all men were as awful as her own abusive father.
About a third of the way through the film, the focus shifts. Jonah suddenly confesses that he is feeling stuck with regard to the movie, that he is troubled by the fact that he has not been able to share his distress with Dr. Stutz during their real-life, unfilmed therapy sessions, and the viewers learn that the single-session concept was disingenuous – they have been filming this documentary for two years, against a green screen and not in an office, always wearing the same clothes, and Jonah pulls off a wig that he wears to disguise the fact that he changed his hairstyle months earlier.
It’s a bit unnerving as they throw the wig around, and Jonah agrees to be more open about the issues he has struggled with. He acknowledges that this has been difficult, and he says, “I just keep asking myself, like, was this a f***ing horrible idea for a patient to make a movie about his therapist?” From my perspective as a psychiatrist-viewer, it’s a good question to ask!
Dr. Stutz reassures Jonah that it is okay to be vulnerable. “Failure, weakness, vulnerability – it’s like a connector, it connects you to the rest of the world.” A super-sized cardboard cutout of an obese 14-year-old Jonah now joins the room, and we learn that he continues to struggle with his self-image. Things get more real.
Peppered throughout the film, there are lessons from Dr. Stutz about his “tools,” constructs he uses to help people restructure their worlds and take action to move forward. One such construct he calls “the maze,” which occurs when one person in an interpersonal relationship is waiting for fairness and becomes preoccupied with feeling injured.
Jonah inquires about Dr. Stutz’s romantic life and the therapist replies with a transparency that overrides our usual professional boundaries. We all learn that Dr. Stutz is not in a relationship, he’s never been married, but there is a woman he has had some involvement with on and off for 40 years. Jonah’s line of questioning rivals that of any therapist. “How do you think it affects you, having your mom hate men and you being a man?” Dr. Stutz admits that he can never feel safe with women. “Did you ever override that wall you built with your mom and get close to a woman?” When Jonah professes, “I don’t feel anything but love for you and I just want you to be happy,” my own feeling was that the tables had turned too far, that the therapist’s failed romantic life risked being a burden to the patient.
Still, there is something about the relationship between the two men that is touching and beautiful. Dr. Stutz as a therapist is charismatic, caring, self-assured, and optimistic, and he radiates hope and certainty. He mixes an intense intimacy with humor in a way that is both authentic and entertaining. The interspersed jokes break the intensity, but they don’t diminish his wisdom and the healing he imparts.
Dr. Stutz is a psychiatrist, and his strength is clearly as a psychotherapist, yet there is not a single mention of psychotropic medications – there is a banter about recreational drugs and medications for Parkinson’s disease. If Hill is taking medication for depression or anxiety, and if prescribing is part of Dr. Stutz’s arsenal, the viewer is not made aware of this.
Dr. Stutz eschews the slow, detached, and “neutral” pace of psychodynamic therapy and the whole concept of the therapist as a blank wall for the transference to play out on, but here the transference screams: Jonah loves him, he respect and honors him, he wants him to be happy, and he is afraid of losing him.
“Stutz” is a movie about a larger-than-life psychiatrist, one whose warmth and inspiration are healing. I imagine his tools are helpful, but his personality is what carries the load. If a viewer has not had experience with psychiatry, and this film inspires him to begin therapy, there may be a good deal of disappointment. In this case, the patient is a successful actor, and one might wonder if that, together with the entire years-long project of filming, has altered the relationship well beyond the usual therapeutic hour.
Dr. Miller is a coauthor of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University Press, 2016). She has a private practice and is an assistant professor of psychiatry and behavioral sciences at Johns Hopkins University in Baltimore.