User login
Antidepressants benefit some patients with osteoarthritis pain
DENVER – Using antidepressants to treat osteoarthritis pain can benefit some individuals but appears to have a clinically unimportant reduction in pain when looking at all patients who have tried them, according to a study presented at the OARSI 2023 World Congress. The review was also published in the Cochrane Database of Systematic Reviews in October 2022.
In terms of implications for clinical practice, the findings “seem to suggest there is a subgroup that is more likely to respond to antidepressants,” Anita Wluka, PhD, MBBS, a professor in the School of Public Health and Preventive Medicine at Monash University in Melbourne, told attendees. The findings also raise an important research question: “How can we identify the patient phenotype likely to benefit so we can [minimize the] risk of those adverse events and effects?”
Osteoarthritis pain is heterogeneous, and an estimated 30% of the pain is neuropathic-like, likely including central and peripheral sensitization, Dr. Wluka said. Given that antidepressants affect multiple sites along these pathways, multiple organizations have issued a conditional recommendation for duloxetine in their osteoarthritis guidelines, including OARSI, the European Alliance of Associations for Rheumatology, and the American College of Rheumatology.
The Cochrane Collaboration therefore conducted a systematic review and meta-analysis of research on the benefits and harms of using antidepressants to treat symptomatic knee and hip osteoarthritis. The review included studies through January 2021 whose participants had knee and/or hip osteoarthritis and which compared antidepressant therapy with placebo or another intervention for at least 6 weeks. The authors looked at seven outcomes: overall pain on a 0-10 scale, clinical response (at least a 50% reduction in 24‐hour mean pain), physical function using the Western Ontario and McMaster Universities Arthritis Index (WOMAC), quality of life using the EQ-5D, the proportion of participants withdrawing because of adverse events, the proportion who experienced any adverse events, and the proportion who experienced serious adverse events.
The researchers considered a change on the pain scale of 0.5-1 points to be “slight to small,” a difference above 1 up to 2 to be “moderate,” and a difference greater than 2 points to be “large.” In assessing quality of life function on a scale of 0-100, a slight to small difference was 5-10, a moderate difference was 11-20, and a large difference was above 20.
Of the 18 articles the researchers identified for qualitative synthesis, 9 met the criteria for qualitative synthesis in the meta-analysis, including 7 studies only on the knee and 2 that included the knees and hips. All nine studies compared antidepressants with placebo, with or without NSAIDs. Most focused on serotonin and norepinephrine reuptake inhibitors (SNRIs) – six studies on duloxetine and one on milnacipran – while one included fluvoxamine and one included nortriptyline.
The trials included a combined 2,122 participants who were predominantly female with an average age range of 54-66. Trials ranged from 8 to 16 weeks. Five of the trials carried risk of attrition and reporting bias, and only one trial had low risk of bias across all domains.
In five trials with SNRIs and one trial with tricyclics (nortriptyline) totaling 1,904 participants, 45% of those receiving antidepressants had a clinical response, compared with 29% of patients who received placebo (risk ratio, 1.55; 95% CI, 1.31-1.92). This absolute improvement in pain occurred in 16% more participants taking antidepressants, giving a number needed to treat (NNT) of 6. Average improvement in WOMAC physical function was 10.5 points with placebo and 16.2 points with antidepressants, indicating a “small, clinically unimportant response,” the researchers concluded.
Withdrawals because of adverse events included 11% of the antidepressant group and 5% of the placebo group (RR, 2.15; 95% CI, 1.56-2.87), putting the NNT for a harmful outcome at 17.
For all nine trials together, however, the mean reduction in pain from antidepressants was 2.3 points, compared with 1.7 points with placebo, a statistically significant but ”clinically unimportant improvement,” the researchers concluded. Adverse events occurred in 64% of the antidepressant group, compared with 49% of the placebo group (RR, 1.27; 95% CI, 1.15-1.41), which put the NNT for a harmful outcome at 7. No significant difference in serious adverse events occurred between the groups.
The analysis was limited by the low number of trials, most of which were sponsored by industry and most of which used duloxetine. Further, few of the studies enrolled patients with osteoarthritis of the hip, none assessed medium- or long-term effects, and none stratified the participants for different types of pain (neuropathic-like or central or peripheral pain sensitization).
“My general impression is that there was a statistically significant difference found in favor of duloxetine and the antidepressants,” David J. Hunter, MBBS, PhD, MSc, of the University of Sydney, said after the presentation. “There is a real risk of harm, which I think is important to take into consideration, but at least for me as a clinician and in advising other clinicians, it’s one tool in our armamentarium. I think it’s really important to allow patients to make an informed decision about the potential benefit, the real risk of harm, and the fact that it is quite useful in some patients, and I use it in my clinical practice.”
Jeffrey N. Katz, MD, MS, of Brigham and Women’s Hospital in Boston, said he uses antidepressants in the same way in his practice and that other types of medications, such as TNF inhibitors, also carry risk of harm that may exceed that of antidepressants.
“I’ve had lots of people start duloxetine, and if they stop it, it’s usually because they just don’t tolerate it very well,” Dr. Katz said.
“We don’t want to throw too many things away,” Dr. Hunter added. “Our patients don’t necessarily have a lot of choices here from a pharmacologic perspective, so I think it’s one of those options that I want to keep in my tool kit, and that’s not necessarily going to change.”
The research did not involve outside funding, and Dr. Wluka reported having no industry disclosures. Disclosure information was unavailable for Dr. Katz and Dr. Hunter. The Congress was sponsored by the Osteoarthritis Research Society International.
DENVER – Using antidepressants to treat osteoarthritis pain can benefit some individuals but appears to have a clinically unimportant reduction in pain when looking at all patients who have tried them, according to a study presented at the OARSI 2023 World Congress. The review was also published in the Cochrane Database of Systematic Reviews in October 2022.
In terms of implications for clinical practice, the findings “seem to suggest there is a subgroup that is more likely to respond to antidepressants,” Anita Wluka, PhD, MBBS, a professor in the School of Public Health and Preventive Medicine at Monash University in Melbourne, told attendees. The findings also raise an important research question: “How can we identify the patient phenotype likely to benefit so we can [minimize the] risk of those adverse events and effects?”
Osteoarthritis pain is heterogeneous, and an estimated 30% of the pain is neuropathic-like, likely including central and peripheral sensitization, Dr. Wluka said. Given that antidepressants affect multiple sites along these pathways, multiple organizations have issued a conditional recommendation for duloxetine in their osteoarthritis guidelines, including OARSI, the European Alliance of Associations for Rheumatology, and the American College of Rheumatology.
The Cochrane Collaboration therefore conducted a systematic review and meta-analysis of research on the benefits and harms of using antidepressants to treat symptomatic knee and hip osteoarthritis. The review included studies through January 2021 whose participants had knee and/or hip osteoarthritis and which compared antidepressant therapy with placebo or another intervention for at least 6 weeks. The authors looked at seven outcomes: overall pain on a 0-10 scale, clinical response (at least a 50% reduction in 24‐hour mean pain), physical function using the Western Ontario and McMaster Universities Arthritis Index (WOMAC), quality of life using the EQ-5D, the proportion of participants withdrawing because of adverse events, the proportion who experienced any adverse events, and the proportion who experienced serious adverse events.
The researchers considered a change on the pain scale of 0.5-1 points to be “slight to small,” a difference above 1 up to 2 to be “moderate,” and a difference greater than 2 points to be “large.” In assessing quality of life function on a scale of 0-100, a slight to small difference was 5-10, a moderate difference was 11-20, and a large difference was above 20.
Of the 18 articles the researchers identified for qualitative synthesis, 9 met the criteria for qualitative synthesis in the meta-analysis, including 7 studies only on the knee and 2 that included the knees and hips. All nine studies compared antidepressants with placebo, with or without NSAIDs. Most focused on serotonin and norepinephrine reuptake inhibitors (SNRIs) – six studies on duloxetine and one on milnacipran – while one included fluvoxamine and one included nortriptyline.
The trials included a combined 2,122 participants who were predominantly female with an average age range of 54-66. Trials ranged from 8 to 16 weeks. Five of the trials carried risk of attrition and reporting bias, and only one trial had low risk of bias across all domains.
In five trials with SNRIs and one trial with tricyclics (nortriptyline) totaling 1,904 participants, 45% of those receiving antidepressants had a clinical response, compared with 29% of patients who received placebo (risk ratio, 1.55; 95% CI, 1.31-1.92). This absolute improvement in pain occurred in 16% more participants taking antidepressants, giving a number needed to treat (NNT) of 6. Average improvement in WOMAC physical function was 10.5 points with placebo and 16.2 points with antidepressants, indicating a “small, clinically unimportant response,” the researchers concluded.
Withdrawals because of adverse events included 11% of the antidepressant group and 5% of the placebo group (RR, 2.15; 95% CI, 1.56-2.87), putting the NNT for a harmful outcome at 17.
For all nine trials together, however, the mean reduction in pain from antidepressants was 2.3 points, compared with 1.7 points with placebo, a statistically significant but ”clinically unimportant improvement,” the researchers concluded. Adverse events occurred in 64% of the antidepressant group, compared with 49% of the placebo group (RR, 1.27; 95% CI, 1.15-1.41), which put the NNT for a harmful outcome at 7. No significant difference in serious adverse events occurred between the groups.
The analysis was limited by the low number of trials, most of which were sponsored by industry and most of which used duloxetine. Further, few of the studies enrolled patients with osteoarthritis of the hip, none assessed medium- or long-term effects, and none stratified the participants for different types of pain (neuropathic-like or central or peripheral pain sensitization).
“My general impression is that there was a statistically significant difference found in favor of duloxetine and the antidepressants,” David J. Hunter, MBBS, PhD, MSc, of the University of Sydney, said after the presentation. “There is a real risk of harm, which I think is important to take into consideration, but at least for me as a clinician and in advising other clinicians, it’s one tool in our armamentarium. I think it’s really important to allow patients to make an informed decision about the potential benefit, the real risk of harm, and the fact that it is quite useful in some patients, and I use it in my clinical practice.”
Jeffrey N. Katz, MD, MS, of Brigham and Women’s Hospital in Boston, said he uses antidepressants in the same way in his practice and that other types of medications, such as TNF inhibitors, also carry risk of harm that may exceed that of antidepressants.
“I’ve had lots of people start duloxetine, and if they stop it, it’s usually because they just don’t tolerate it very well,” Dr. Katz said.
“We don’t want to throw too many things away,” Dr. Hunter added. “Our patients don’t necessarily have a lot of choices here from a pharmacologic perspective, so I think it’s one of those options that I want to keep in my tool kit, and that’s not necessarily going to change.”
The research did not involve outside funding, and Dr. Wluka reported having no industry disclosures. Disclosure information was unavailable for Dr. Katz and Dr. Hunter. The Congress was sponsored by the Osteoarthritis Research Society International.
DENVER – Using antidepressants to treat osteoarthritis pain can benefit some individuals but appears to have a clinically unimportant reduction in pain when looking at all patients who have tried them, according to a study presented at the OARSI 2023 World Congress. The review was also published in the Cochrane Database of Systematic Reviews in October 2022.
In terms of implications for clinical practice, the findings “seem to suggest there is a subgroup that is more likely to respond to antidepressants,” Anita Wluka, PhD, MBBS, a professor in the School of Public Health and Preventive Medicine at Monash University in Melbourne, told attendees. The findings also raise an important research question: “How can we identify the patient phenotype likely to benefit so we can [minimize the] risk of those adverse events and effects?”
Osteoarthritis pain is heterogeneous, and an estimated 30% of the pain is neuropathic-like, likely including central and peripheral sensitization, Dr. Wluka said. Given that antidepressants affect multiple sites along these pathways, multiple organizations have issued a conditional recommendation for duloxetine in their osteoarthritis guidelines, including OARSI, the European Alliance of Associations for Rheumatology, and the American College of Rheumatology.
The Cochrane Collaboration therefore conducted a systematic review and meta-analysis of research on the benefits and harms of using antidepressants to treat symptomatic knee and hip osteoarthritis. The review included studies through January 2021 whose participants had knee and/or hip osteoarthritis and which compared antidepressant therapy with placebo or another intervention for at least 6 weeks. The authors looked at seven outcomes: overall pain on a 0-10 scale, clinical response (at least a 50% reduction in 24‐hour mean pain), physical function using the Western Ontario and McMaster Universities Arthritis Index (WOMAC), quality of life using the EQ-5D, the proportion of participants withdrawing because of adverse events, the proportion who experienced any adverse events, and the proportion who experienced serious adverse events.
The researchers considered a change on the pain scale of 0.5-1 points to be “slight to small,” a difference above 1 up to 2 to be “moderate,” and a difference greater than 2 points to be “large.” In assessing quality of life function on a scale of 0-100, a slight to small difference was 5-10, a moderate difference was 11-20, and a large difference was above 20.
Of the 18 articles the researchers identified for qualitative synthesis, 9 met the criteria for qualitative synthesis in the meta-analysis, including 7 studies only on the knee and 2 that included the knees and hips. All nine studies compared antidepressants with placebo, with or without NSAIDs. Most focused on serotonin and norepinephrine reuptake inhibitors (SNRIs) – six studies on duloxetine and one on milnacipran – while one included fluvoxamine and one included nortriptyline.
The trials included a combined 2,122 participants who were predominantly female with an average age range of 54-66. Trials ranged from 8 to 16 weeks. Five of the trials carried risk of attrition and reporting bias, and only one trial had low risk of bias across all domains.
In five trials with SNRIs and one trial with tricyclics (nortriptyline) totaling 1,904 participants, 45% of those receiving antidepressants had a clinical response, compared with 29% of patients who received placebo (risk ratio, 1.55; 95% CI, 1.31-1.92). This absolute improvement in pain occurred in 16% more participants taking antidepressants, giving a number needed to treat (NNT) of 6. Average improvement in WOMAC physical function was 10.5 points with placebo and 16.2 points with antidepressants, indicating a “small, clinically unimportant response,” the researchers concluded.
Withdrawals because of adverse events included 11% of the antidepressant group and 5% of the placebo group (RR, 2.15; 95% CI, 1.56-2.87), putting the NNT for a harmful outcome at 17.
For all nine trials together, however, the mean reduction in pain from antidepressants was 2.3 points, compared with 1.7 points with placebo, a statistically significant but ”clinically unimportant improvement,” the researchers concluded. Adverse events occurred in 64% of the antidepressant group, compared with 49% of the placebo group (RR, 1.27; 95% CI, 1.15-1.41), which put the NNT for a harmful outcome at 7. No significant difference in serious adverse events occurred between the groups.
The analysis was limited by the low number of trials, most of which were sponsored by industry and most of which used duloxetine. Further, few of the studies enrolled patients with osteoarthritis of the hip, none assessed medium- or long-term effects, and none stratified the participants for different types of pain (neuropathic-like or central or peripheral pain sensitization).
“My general impression is that there was a statistically significant difference found in favor of duloxetine and the antidepressants,” David J. Hunter, MBBS, PhD, MSc, of the University of Sydney, said after the presentation. “There is a real risk of harm, which I think is important to take into consideration, but at least for me as a clinician and in advising other clinicians, it’s one tool in our armamentarium. I think it’s really important to allow patients to make an informed decision about the potential benefit, the real risk of harm, and the fact that it is quite useful in some patients, and I use it in my clinical practice.”
Jeffrey N. Katz, MD, MS, of Brigham and Women’s Hospital in Boston, said he uses antidepressants in the same way in his practice and that other types of medications, such as TNF inhibitors, also carry risk of harm that may exceed that of antidepressants.
“I’ve had lots of people start duloxetine, and if they stop it, it’s usually because they just don’t tolerate it very well,” Dr. Katz said.
“We don’t want to throw too many things away,” Dr. Hunter added. “Our patients don’t necessarily have a lot of choices here from a pharmacologic perspective, so I think it’s one of those options that I want to keep in my tool kit, and that’s not necessarily going to change.”
The research did not involve outside funding, and Dr. Wluka reported having no industry disclosures. Disclosure information was unavailable for Dr. Katz and Dr. Hunter. The Congress was sponsored by the Osteoarthritis Research Society International.
FROM OARSI 2023
Limited treatment options exist for brittle nail syndrome
NEW ORLEANS – .
“The mainstay of treatment is irritant avoidance and moisturization,” Shari R. Lipner, MD, PhD, associate professor of clinical dermatology and director of the nail division at Weill Cornell Medicine, New York, said at the annual meeting of the American Academy of Dermatology. “This works well if patients are religious about doing it.”
Brittle nail syndrome affects about 20% of adults, she said, and is more common in females, particularly those older than age 50. Most cases are idiopathic, but some are secondary to dermatologic diseases including nail psoriasis and nail lichen planus, and systemic diseases such as hyperthyroidism and hypothyroidism. They are more common in patients in certain occupations such as carpentry. “The pathogenesis is poorly understood but is thought to be due to weakened intercellular keratinocyte bridges, decreased cholesterol sulphate in the nail plate, and reduced water content in the nail plate,” Dr. Lipner said.
Key clinical findings include onychoschizia (peeling of the nail plate), onychorrhexis (an increase in the longitudinal ridges and furrows, sometimes leading to splitting), and superficial granulation of keratin. Treatment involves general measures. “You want to treat the underlying cause and recommend that the patient avoid water and irritant exposure,” she said. Her general instructions for affected patients are to wear latex gloves for wet work and cotton gloves for dry work, avoid triclosan-based hand sanitizers, avoid nail cosmetics, minimize nail trauma, and foster moisturization.“It’s important to give these instructions verbally and in written form,” she said. “In our practice, we designed a QR code that links to our patient handout.”
According to Dr. Lipner, the promotion of vitamins and supplements such as biotin, vitamin D, amino acids, and chromium for treating brittle nail syndrome is rampant on the Internet and on social media, but no rigorously designed clinical trials have shown efficacy for any of them. “Very few people are deficient in biotin, except for those with inherited enzyme deficiencies,” and most people “can get all the biotin they need from a regular diet,” she said.
The initial rationale for using biotin for nails comes from the veterinary literature, she continued. In the 1940s, chickens with biotin deficiency developed fissures in their feet and parrot-like beaks. In the 1970s, pigs with biotin deficiency developed friable hooves, which was corrected with biotin supplementation. “By the 1980s it was standard practice to supplement the feet of pigs with biotin,” she said.
In a human trial from 1989, German researchers enrolled 71 patients with brittle nail syndrome who took oral biotin, 2.5 mg daily. Of the 45 patients evaluated, 41 (91%) showed improvement in firmness and hardness of the fingernails over the course of 5.5 months, but there was no good control group, Dr. Lipner said. In a follow-up study, the same German researchers used scanning electron microscopy to evaluate 22 patients with brittle nails who took oral biotin 2.5 mg daily and compared them with 10 patients with normal nails who did not take biotin. They found a 25% increase in nail plate thickness in the biotin group and onychoschizia resolved in 50% of patients who received biotin. “But again, there was no good control group,” Dr. Lipner said.
In a third study on the topic, researchers surveyed 46 patients who presented with onychorrhexis and/or onychoschizia on clinical exam and took 2.5 mg of biotin daily. Of the 35 survey respondents, 63% subjectively reported improvement in their nails at a mean of 2 months. “This is where we are today: There have been studies of only 80 patients that were done 25 years ago,” Dr. Lipner said. “That’s all of our evidence for biotin for the treatment of brittle nail syndrome.”
FDA warning about biotin
Additional cause for concern, she continued, is the safety communication issued by the FDA in 2017, stating that the use of biotin may interfere with certain lab tests such as thyroid tests and cardiac enzymes, in some cases leading to death. The safety communication was updated in 2019.
In 2018, Dr. Lipner and colleagues administered an anonymous survey to 447 patients at their clinic asking about their use of biotin supplements. Of the 447 patients, 34% reported current use of biotin. Among biotin users, 7% were aware of the FDA warning, 29% of respondents reported that it was recommended by either a primary care physician or a dermatologist, and 56% underwent laboratory testing while taking biotin. “It’s our duty to warn our patients about the evidence for biotin for treating brittle nails, and about this interference on laboratory tests,” Dr. Lipner said.
Other treatment options for brittle nail syndrome include two lacquers that are available by prescription. One contains hydroxypropyl chitosan, Equisetum arvense, and methylsulphonylmethane; the other contains 16% poly-ureaurethane, but has not been well studied. “These products can be very expensive if not covered by insurance,” Dr. Lipner said.
As an alternative, she recommends Nail Tek CITRA 2 Nail Strengthener, which is available for less than $10 from Walmart and other retailers.
Cyclosporine emulsion also has been studied for brittle nail syndrome, but results to date have been underwhelming. Dr. Lipner and colleagues are exploring the effect of platelet rich plasma for treating brittle nails on the premise that it will improve nail growth and promote healing, in a 16-week trial that has enrolled 10 patients and includes both a Physician Global Improvement Assessment (PGIA) and a Physician Global Assessment (PGA) score. “Our data is being analyzed by three independent nail experts, and we hope to report the findings next year,” she said.
Dr. Lipner reported having no disclosures relevant to her presentation.
NEW ORLEANS – .
“The mainstay of treatment is irritant avoidance and moisturization,” Shari R. Lipner, MD, PhD, associate professor of clinical dermatology and director of the nail division at Weill Cornell Medicine, New York, said at the annual meeting of the American Academy of Dermatology. “This works well if patients are religious about doing it.”
Brittle nail syndrome affects about 20% of adults, she said, and is more common in females, particularly those older than age 50. Most cases are idiopathic, but some are secondary to dermatologic diseases including nail psoriasis and nail lichen planus, and systemic diseases such as hyperthyroidism and hypothyroidism. They are more common in patients in certain occupations such as carpentry. “The pathogenesis is poorly understood but is thought to be due to weakened intercellular keratinocyte bridges, decreased cholesterol sulphate in the nail plate, and reduced water content in the nail plate,” Dr. Lipner said.
Key clinical findings include onychoschizia (peeling of the nail plate), onychorrhexis (an increase in the longitudinal ridges and furrows, sometimes leading to splitting), and superficial granulation of keratin. Treatment involves general measures. “You want to treat the underlying cause and recommend that the patient avoid water and irritant exposure,” she said. Her general instructions for affected patients are to wear latex gloves for wet work and cotton gloves for dry work, avoid triclosan-based hand sanitizers, avoid nail cosmetics, minimize nail trauma, and foster moisturization.“It’s important to give these instructions verbally and in written form,” she said. “In our practice, we designed a QR code that links to our patient handout.”
According to Dr. Lipner, the promotion of vitamins and supplements such as biotin, vitamin D, amino acids, and chromium for treating brittle nail syndrome is rampant on the Internet and on social media, but no rigorously designed clinical trials have shown efficacy for any of them. “Very few people are deficient in biotin, except for those with inherited enzyme deficiencies,” and most people “can get all the biotin they need from a regular diet,” she said.
The initial rationale for using biotin for nails comes from the veterinary literature, she continued. In the 1940s, chickens with biotin deficiency developed fissures in their feet and parrot-like beaks. In the 1970s, pigs with biotin deficiency developed friable hooves, which was corrected with biotin supplementation. “By the 1980s it was standard practice to supplement the feet of pigs with biotin,” she said.
In a human trial from 1989, German researchers enrolled 71 patients with brittle nail syndrome who took oral biotin, 2.5 mg daily. Of the 45 patients evaluated, 41 (91%) showed improvement in firmness and hardness of the fingernails over the course of 5.5 months, but there was no good control group, Dr. Lipner said. In a follow-up study, the same German researchers used scanning electron microscopy to evaluate 22 patients with brittle nails who took oral biotin 2.5 mg daily and compared them with 10 patients with normal nails who did not take biotin. They found a 25% increase in nail plate thickness in the biotin group and onychoschizia resolved in 50% of patients who received biotin. “But again, there was no good control group,” Dr. Lipner said.
In a third study on the topic, researchers surveyed 46 patients who presented with onychorrhexis and/or onychoschizia on clinical exam and took 2.5 mg of biotin daily. Of the 35 survey respondents, 63% subjectively reported improvement in their nails at a mean of 2 months. “This is where we are today: There have been studies of only 80 patients that were done 25 years ago,” Dr. Lipner said. “That’s all of our evidence for biotin for the treatment of brittle nail syndrome.”
FDA warning about biotin
Additional cause for concern, she continued, is the safety communication issued by the FDA in 2017, stating that the use of biotin may interfere with certain lab tests such as thyroid tests and cardiac enzymes, in some cases leading to death. The safety communication was updated in 2019.
In 2018, Dr. Lipner and colleagues administered an anonymous survey to 447 patients at their clinic asking about their use of biotin supplements. Of the 447 patients, 34% reported current use of biotin. Among biotin users, 7% were aware of the FDA warning, 29% of respondents reported that it was recommended by either a primary care physician or a dermatologist, and 56% underwent laboratory testing while taking biotin. “It’s our duty to warn our patients about the evidence for biotin for treating brittle nails, and about this interference on laboratory tests,” Dr. Lipner said.
Other treatment options for brittle nail syndrome include two lacquers that are available by prescription. One contains hydroxypropyl chitosan, Equisetum arvense, and methylsulphonylmethane; the other contains 16% poly-ureaurethane, but has not been well studied. “These products can be very expensive if not covered by insurance,” Dr. Lipner said.
As an alternative, she recommends Nail Tek CITRA 2 Nail Strengthener, which is available for less than $10 from Walmart and other retailers.
Cyclosporine emulsion also has been studied for brittle nail syndrome, but results to date have been underwhelming. Dr. Lipner and colleagues are exploring the effect of platelet rich plasma for treating brittle nails on the premise that it will improve nail growth and promote healing, in a 16-week trial that has enrolled 10 patients and includes both a Physician Global Improvement Assessment (PGIA) and a Physician Global Assessment (PGA) score. “Our data is being analyzed by three independent nail experts, and we hope to report the findings next year,” she said.
Dr. Lipner reported having no disclosures relevant to her presentation.
NEW ORLEANS – .
“The mainstay of treatment is irritant avoidance and moisturization,” Shari R. Lipner, MD, PhD, associate professor of clinical dermatology and director of the nail division at Weill Cornell Medicine, New York, said at the annual meeting of the American Academy of Dermatology. “This works well if patients are religious about doing it.”
Brittle nail syndrome affects about 20% of adults, she said, and is more common in females, particularly those older than age 50. Most cases are idiopathic, but some are secondary to dermatologic diseases including nail psoriasis and nail lichen planus, and systemic diseases such as hyperthyroidism and hypothyroidism. They are more common in patients in certain occupations such as carpentry. “The pathogenesis is poorly understood but is thought to be due to weakened intercellular keratinocyte bridges, decreased cholesterol sulphate in the nail plate, and reduced water content in the nail plate,” Dr. Lipner said.
Key clinical findings include onychoschizia (peeling of the nail plate), onychorrhexis (an increase in the longitudinal ridges and furrows, sometimes leading to splitting), and superficial granulation of keratin. Treatment involves general measures. “You want to treat the underlying cause and recommend that the patient avoid water and irritant exposure,” she said. Her general instructions for affected patients are to wear latex gloves for wet work and cotton gloves for dry work, avoid triclosan-based hand sanitizers, avoid nail cosmetics, minimize nail trauma, and foster moisturization.“It’s important to give these instructions verbally and in written form,” she said. “In our practice, we designed a QR code that links to our patient handout.”
According to Dr. Lipner, the promotion of vitamins and supplements such as biotin, vitamin D, amino acids, and chromium for treating brittle nail syndrome is rampant on the Internet and on social media, but no rigorously designed clinical trials have shown efficacy for any of them. “Very few people are deficient in biotin, except for those with inherited enzyme deficiencies,” and most people “can get all the biotin they need from a regular diet,” she said.
The initial rationale for using biotin for nails comes from the veterinary literature, she continued. In the 1940s, chickens with biotin deficiency developed fissures in their feet and parrot-like beaks. In the 1970s, pigs with biotin deficiency developed friable hooves, which was corrected with biotin supplementation. “By the 1980s it was standard practice to supplement the feet of pigs with biotin,” she said.
In a human trial from 1989, German researchers enrolled 71 patients with brittle nail syndrome who took oral biotin, 2.5 mg daily. Of the 45 patients evaluated, 41 (91%) showed improvement in firmness and hardness of the fingernails over the course of 5.5 months, but there was no good control group, Dr. Lipner said. In a follow-up study, the same German researchers used scanning electron microscopy to evaluate 22 patients with brittle nails who took oral biotin 2.5 mg daily and compared them with 10 patients with normal nails who did not take biotin. They found a 25% increase in nail plate thickness in the biotin group and onychoschizia resolved in 50% of patients who received biotin. “But again, there was no good control group,” Dr. Lipner said.
In a third study on the topic, researchers surveyed 46 patients who presented with onychorrhexis and/or onychoschizia on clinical exam and took 2.5 mg of biotin daily. Of the 35 survey respondents, 63% subjectively reported improvement in their nails at a mean of 2 months. “This is where we are today: There have been studies of only 80 patients that were done 25 years ago,” Dr. Lipner said. “That’s all of our evidence for biotin for the treatment of brittle nail syndrome.”
FDA warning about biotin
Additional cause for concern, she continued, is the safety communication issued by the FDA in 2017, stating that the use of biotin may interfere with certain lab tests such as thyroid tests and cardiac enzymes, in some cases leading to death. The safety communication was updated in 2019.
In 2018, Dr. Lipner and colleagues administered an anonymous survey to 447 patients at their clinic asking about their use of biotin supplements. Of the 447 patients, 34% reported current use of biotin. Among biotin users, 7% were aware of the FDA warning, 29% of respondents reported that it was recommended by either a primary care physician or a dermatologist, and 56% underwent laboratory testing while taking biotin. “It’s our duty to warn our patients about the evidence for biotin for treating brittle nails, and about this interference on laboratory tests,” Dr. Lipner said.
Other treatment options for brittle nail syndrome include two lacquers that are available by prescription. One contains hydroxypropyl chitosan, Equisetum arvense, and methylsulphonylmethane; the other contains 16% poly-ureaurethane, but has not been well studied. “These products can be very expensive if not covered by insurance,” Dr. Lipner said.
As an alternative, she recommends Nail Tek CITRA 2 Nail Strengthener, which is available for less than $10 from Walmart and other retailers.
Cyclosporine emulsion also has been studied for brittle nail syndrome, but results to date have been underwhelming. Dr. Lipner and colleagues are exploring the effect of platelet rich plasma for treating brittle nails on the premise that it will improve nail growth and promote healing, in a 16-week trial that has enrolled 10 patients and includes both a Physician Global Improvement Assessment (PGIA) and a Physician Global Assessment (PGA) score. “Our data is being analyzed by three independent nail experts, and we hope to report the findings next year,” she said.
Dr. Lipner reported having no disclosures relevant to her presentation.
AT AAD 2023
Commentary: Alisertib, trastuzumab, and treatment timing, April 2023
HER2-positive (HER2+) BC was associated with poor outcomes compared with other BC subtypes. However, the introduction of trastuzumab has drastically changed the treatment paradigm for these patients afflicted with HER2+ BC. The pivotal trials with trastuzumab included only a few patients with lower-risk HER2+ tumors; therefore, it was not clear whether these lower-risk patients can benefit from a de-escalated adjuvant regimen. The phase 2 APT trial prospectively investigated the safety and efficacy of 12 weeks of paclitaxel with trastuzumab, followed by 9 months of trastuzumab monotherapy, in patients with small (≤ 3 cm), node-negative, HER2+ BC. After a median follow-up of 10.8 years, the 10-year invasive disease-free survival was 91.3%, the recurrence-free interval was 96.3%, the overall survival rate was 94.3%, and the BC-specific survival rate was 98.8%.
The researchers also conducted an exploratory analysis in 284 patients using the HER2DX genomic test. This is a single 27-gene expression and clinical feature-based classifier developed for early-stage, HER2+ BC. The tool identified a subset of patients with a high HER2DX score (HERDX score ≥ 32) who might harbor an increased risk for long-term recurrence.
These excellent long-term outcomes from the APT trial support the use of the currently endorsed adjuvant regimen of paclitaxel and trastuzumab in patients with stage I HER2+ BC. Furthermore, the HER2DX risk score, if validated, may provide a promising genomic tool to identify a subset of these patients who are at increased risk for recurrence and therefore may benefit from additional therapy.
Prior studies have noted worse survival outcomes with longer times from BC diagnosis to surgical treatment; however, the specific time interval that is acceptable to wait between diagnosis and surgery is still unclear. A case series study by Weiner and colleagues looked at the association between time from BC diagnosis to primary breast surgery and overall survival. The study looked at 373,334 female patients from the National Cancer Database with stage I-III BC who underwent primary breast surgery. Results showed worse overall survival outcomes when time to surgery was 9 or more weeks compared with surgery between 0 and 4 weeks (hazard ratio 1.15; P < .001). Factors associated with longer times to surgery included younger age, uninsured or Medicaid status, and lower neighborhood household income. On the basis of these findings, surgery before 8 weeks from BC diagnosis appears to be an acceptable time frame to avoid unfavorable survival outcomes and allow for appropriate multidisciplinary care. Furthermore, it is critical to identify potential barriers in a timely manner to prevent prolonged delays in care.
In hormone receptor-positive (HR+) BC, adjuvant endocrine therapy (ET) is usually delayed until after adjuvant radiotherapy, although, the optimal sequence of both therapies is still unknown. The aim of the study by Sutton and colleagues was to assess the association between time from surgery to ET initiation and cancer outcomes in high-risk HR+ patients, particularly those with residual disease after neoadjuvant chemotherapy. The study analysed 179 patients with HR+ BC from a multi-institutional database who received adjuvant radiotherapy, of which 68 patients received adjuvant ET before or during radiotherapy and 111 patients received ET after cessation of radiotherapy. Results showed that an interval of >14 weeks between surgery and the receipt of ET was independently associated with worse recurrence-free survival compared with an interval of 14 or less weeks (hazard ratio 3.20; P = .02). Of interest, the study also showed that patients receiving ET before or during radiation were more likely to experience skin and soft tissue late radiation morbidity, and this was nonsignificantly associated with worse radiation-associated complication-free survival (hazard ratio 1.87; P = .06). Although prior studies have reported that the interval from surgery to ET does not affect cancer outcomes, this was not studied in a high-risk cohort who have received neoadjuvant chemotherapy. Further studies in larger prospective cohorts are needed to validate these findings. At this time, the risks and benefits of concurrent ET with radiation need to be assessed prior to making any treatment recommendations.
HER2-positive (HER2+) BC was associated with poor outcomes compared with other BC subtypes. However, the introduction of trastuzumab has drastically changed the treatment paradigm for these patients afflicted with HER2+ BC. The pivotal trials with trastuzumab included only a few patients with lower-risk HER2+ tumors; therefore, it was not clear whether these lower-risk patients can benefit from a de-escalated adjuvant regimen. The phase 2 APT trial prospectively investigated the safety and efficacy of 12 weeks of paclitaxel with trastuzumab, followed by 9 months of trastuzumab monotherapy, in patients with small (≤ 3 cm), node-negative, HER2+ BC. After a median follow-up of 10.8 years, the 10-year invasive disease-free survival was 91.3%, the recurrence-free interval was 96.3%, the overall survival rate was 94.3%, and the BC-specific survival rate was 98.8%.
The researchers also conducted an exploratory analysis in 284 patients using the HER2DX genomic test. This is a single 27-gene expression and clinical feature-based classifier developed for early-stage, HER2+ BC. The tool identified a subset of patients with a high HER2DX score (HERDX score ≥ 32) who might harbor an increased risk for long-term recurrence.
These excellent long-term outcomes from the APT trial support the use of the currently endorsed adjuvant regimen of paclitaxel and trastuzumab in patients with stage I HER2+ BC. Furthermore, the HER2DX risk score, if validated, may provide a promising genomic tool to identify a subset of these patients who are at increased risk for recurrence and therefore may benefit from additional therapy.
Prior studies have noted worse survival outcomes with longer times from BC diagnosis to surgical treatment; however, the specific time interval that is acceptable to wait between diagnosis and surgery is still unclear. A case series study by Weiner and colleagues looked at the association between time from BC diagnosis to primary breast surgery and overall survival. The study looked at 373,334 female patients from the National Cancer Database with stage I-III BC who underwent primary breast surgery. Results showed worse overall survival outcomes when time to surgery was 9 or more weeks compared with surgery between 0 and 4 weeks (hazard ratio 1.15; P < .001). Factors associated with longer times to surgery included younger age, uninsured or Medicaid status, and lower neighborhood household income. On the basis of these findings, surgery before 8 weeks from BC diagnosis appears to be an acceptable time frame to avoid unfavorable survival outcomes and allow for appropriate multidisciplinary care. Furthermore, it is critical to identify potential barriers in a timely manner to prevent prolonged delays in care.
In hormone receptor-positive (HR+) BC, adjuvant endocrine therapy (ET) is usually delayed until after adjuvant radiotherapy, although, the optimal sequence of both therapies is still unknown. The aim of the study by Sutton and colleagues was to assess the association between time from surgery to ET initiation and cancer outcomes in high-risk HR+ patients, particularly those with residual disease after neoadjuvant chemotherapy. The study analysed 179 patients with HR+ BC from a multi-institutional database who received adjuvant radiotherapy, of which 68 patients received adjuvant ET before or during radiotherapy and 111 patients received ET after cessation of radiotherapy. Results showed that an interval of >14 weeks between surgery and the receipt of ET was independently associated with worse recurrence-free survival compared with an interval of 14 or less weeks (hazard ratio 3.20; P = .02). Of interest, the study also showed that patients receiving ET before or during radiation were more likely to experience skin and soft tissue late radiation morbidity, and this was nonsignificantly associated with worse radiation-associated complication-free survival (hazard ratio 1.87; P = .06). Although prior studies have reported that the interval from surgery to ET does not affect cancer outcomes, this was not studied in a high-risk cohort who have received neoadjuvant chemotherapy. Further studies in larger prospective cohorts are needed to validate these findings. At this time, the risks and benefits of concurrent ET with radiation need to be assessed prior to making any treatment recommendations.
HER2-positive (HER2+) BC was associated with poor outcomes compared with other BC subtypes. However, the introduction of trastuzumab has drastically changed the treatment paradigm for these patients afflicted with HER2+ BC. The pivotal trials with trastuzumab included only a few patients with lower-risk HER2+ tumors; therefore, it was not clear whether these lower-risk patients can benefit from a de-escalated adjuvant regimen. The phase 2 APT trial prospectively investigated the safety and efficacy of 12 weeks of paclitaxel with trastuzumab, followed by 9 months of trastuzumab monotherapy, in patients with small (≤ 3 cm), node-negative, HER2+ BC. After a median follow-up of 10.8 years, the 10-year invasive disease-free survival was 91.3%, the recurrence-free interval was 96.3%, the overall survival rate was 94.3%, and the BC-specific survival rate was 98.8%.
The researchers also conducted an exploratory analysis in 284 patients using the HER2DX genomic test. This is a single 27-gene expression and clinical feature-based classifier developed for early-stage, HER2+ BC. The tool identified a subset of patients with a high HER2DX score (HERDX score ≥ 32) who might harbor an increased risk for long-term recurrence.
These excellent long-term outcomes from the APT trial support the use of the currently endorsed adjuvant regimen of paclitaxel and trastuzumab in patients with stage I HER2+ BC. Furthermore, the HER2DX risk score, if validated, may provide a promising genomic tool to identify a subset of these patients who are at increased risk for recurrence and therefore may benefit from additional therapy.
Prior studies have noted worse survival outcomes with longer times from BC diagnosis to surgical treatment; however, the specific time interval that is acceptable to wait between diagnosis and surgery is still unclear. A case series study by Weiner and colleagues looked at the association between time from BC diagnosis to primary breast surgery and overall survival. The study looked at 373,334 female patients from the National Cancer Database with stage I-III BC who underwent primary breast surgery. Results showed worse overall survival outcomes when time to surgery was 9 or more weeks compared with surgery between 0 and 4 weeks (hazard ratio 1.15; P < .001). Factors associated with longer times to surgery included younger age, uninsured or Medicaid status, and lower neighborhood household income. On the basis of these findings, surgery before 8 weeks from BC diagnosis appears to be an acceptable time frame to avoid unfavorable survival outcomes and allow for appropriate multidisciplinary care. Furthermore, it is critical to identify potential barriers in a timely manner to prevent prolonged delays in care.
In hormone receptor-positive (HR+) BC, adjuvant endocrine therapy (ET) is usually delayed until after adjuvant radiotherapy, although, the optimal sequence of both therapies is still unknown. The aim of the study by Sutton and colleagues was to assess the association between time from surgery to ET initiation and cancer outcomes in high-risk HR+ patients, particularly those with residual disease after neoadjuvant chemotherapy. The study analysed 179 patients with HR+ BC from a multi-institutional database who received adjuvant radiotherapy, of which 68 patients received adjuvant ET before or during radiotherapy and 111 patients received ET after cessation of radiotherapy. Results showed that an interval of >14 weeks between surgery and the receipt of ET was independently associated with worse recurrence-free survival compared with an interval of 14 or less weeks (hazard ratio 3.20; P = .02). Of interest, the study also showed that patients receiving ET before or during radiation were more likely to experience skin and soft tissue late radiation morbidity, and this was nonsignificantly associated with worse radiation-associated complication-free survival (hazard ratio 1.87; P = .06). Although prior studies have reported that the interval from surgery to ET does not affect cancer outcomes, this was not studied in a high-risk cohort who have received neoadjuvant chemotherapy. Further studies in larger prospective cohorts are needed to validate these findings. At this time, the risks and benefits of concurrent ET with radiation need to be assessed prior to making any treatment recommendations.
FDA Advisory panels consider easing isotretinoin requirements
for patients, pharmacies, and prescribers.
Isotretinoin, previously called Accutane, is marketed as Absorica, Absorica LD, Claravis, Amnesteem, Myorisan, and Zenatane.
In a joint meeting of the FDA’s Drug Safety and Risk Management Advisory Committee and Dermatologic and Ophthalmic Drugs Advisory Committee, experts addressed ways to improve the modified iPLEDGE Risk Evaluation and Mitigation Strategy (iPLEDGE REMS) for isotretinoin that caused chaos after its rollout at the end of 2021.
In January 2022, problems were multiplying with the program for clinicians, pharmacists, and patients, causing extensive delays and prescription denials. In response, the FDA said it would continue to meet with the Isotretinoin Products Manufacturers Group (IPMG) to resolve problems.
March 28 was the first day of a 2-day meeting addressing what can be done to reduce burden with the iPLEDGE REMS while maintaining safety and preventing fetal exposure to the drug.
Key areas of concern
The meeting focused on several key areas.
The 19-day lockout period
The lockout is a current restriction for patients who can become pregnant and do not pick up their first prescription of isotretinoin within the specified 7-day prescription window. Currently, those who miss the window must wait 19 days from the date of the first pregnancy test to take an additional pregnancy test to be eligible to receive the drug.
Lindsey Crist, PharmD, a risk management analyst for the FDA, who presented the FDA review committee’s analysis, acknowledged that the lockout period causes delays in treatment and adds frustration and costs.
She said it’s important to remember that the lockout applies only to the first prescription. “It’s intended as an additional layer of screening to detect pregnancy,” she said.
“At least 12 pregnancies have been identified during the 19-day lockout from March 2017–September of 2022,” she noted.
The FDA is looking to the advisory committee to provide recommendations on whether the lockout period should be changed.
Home testing
During the pandemic, iPLEDGE rules have been relaxed from having a pregnancy test done only at a Clinical Laboratory Improvement Amendments–certified laboratory and home pregnancy tests have been allowed. The question now is whether home tests should continue to be allowed.
Ms. Crist said that the FDA’s review committee recommends ending the allowance of home tests, citing insufficient data on use and the discovery of instances of falsification of pregnancy tests.
“One study at an academic medical center reviewed the medical records of 89 patients who used home pregnancy tests while taking isotretinoin during the public health emergency. It found that 15.7% submitted falsified pregnancy test results,” she said.
Ms. Crist added, however, that the review committee recommends allowing the tests to be done in a provider’s office as an alternative.
Documenting counseling patients who cannot get pregnant
Currently, this documentation must be done monthly, primarily to counsel patients against drug sharing or giving blood. Proposed changes include extending the intervals for attestation or eliminating it to reduce burden on clinicians.
IPMG representative Gregory Wedin, PharmD, pharmacovigilance and risk management director for Upsher-Smith Laboratories, said, “while we cannot support eliminating or extending the confirmation interval to a year, the [iPLEDGE] sponsors are agreeable [to] a 120-day confirmation interval.”
He said that while extending to 120 days would reduce burden on prescribers, it comes with risk in reducing oversight by a certified iPLEDGE prescriber and potentially increasing the risk for drug sharing.
“A patient may be more likely to share their drug with another person the further along with therapy they get as their condition improves,” Mr. Wedin said.
On March 29, the panel will hear more recommendations for and against modifications to iPLEDGE REMS and will vote on select modifications at the end of the meeting.
A version of this article first appeared on Medscape.com.
for patients, pharmacies, and prescribers.
Isotretinoin, previously called Accutane, is marketed as Absorica, Absorica LD, Claravis, Amnesteem, Myorisan, and Zenatane.
In a joint meeting of the FDA’s Drug Safety and Risk Management Advisory Committee and Dermatologic and Ophthalmic Drugs Advisory Committee, experts addressed ways to improve the modified iPLEDGE Risk Evaluation and Mitigation Strategy (iPLEDGE REMS) for isotretinoin that caused chaos after its rollout at the end of 2021.
In January 2022, problems were multiplying with the program for clinicians, pharmacists, and patients, causing extensive delays and prescription denials. In response, the FDA said it would continue to meet with the Isotretinoin Products Manufacturers Group (IPMG) to resolve problems.
March 28 was the first day of a 2-day meeting addressing what can be done to reduce burden with the iPLEDGE REMS while maintaining safety and preventing fetal exposure to the drug.
Key areas of concern
The meeting focused on several key areas.
The 19-day lockout period
The lockout is a current restriction for patients who can become pregnant and do not pick up their first prescription of isotretinoin within the specified 7-day prescription window. Currently, those who miss the window must wait 19 days from the date of the first pregnancy test to take an additional pregnancy test to be eligible to receive the drug.
Lindsey Crist, PharmD, a risk management analyst for the FDA, who presented the FDA review committee’s analysis, acknowledged that the lockout period causes delays in treatment and adds frustration and costs.
She said it’s important to remember that the lockout applies only to the first prescription. “It’s intended as an additional layer of screening to detect pregnancy,” she said.
“At least 12 pregnancies have been identified during the 19-day lockout from March 2017–September of 2022,” she noted.
The FDA is looking to the advisory committee to provide recommendations on whether the lockout period should be changed.
Home testing
During the pandemic, iPLEDGE rules have been relaxed from having a pregnancy test done only at a Clinical Laboratory Improvement Amendments–certified laboratory and home pregnancy tests have been allowed. The question now is whether home tests should continue to be allowed.
Ms. Crist said that the FDA’s review committee recommends ending the allowance of home tests, citing insufficient data on use and the discovery of instances of falsification of pregnancy tests.
“One study at an academic medical center reviewed the medical records of 89 patients who used home pregnancy tests while taking isotretinoin during the public health emergency. It found that 15.7% submitted falsified pregnancy test results,” she said.
Ms. Crist added, however, that the review committee recommends allowing the tests to be done in a provider’s office as an alternative.
Documenting counseling patients who cannot get pregnant
Currently, this documentation must be done monthly, primarily to counsel patients against drug sharing or giving blood. Proposed changes include extending the intervals for attestation or eliminating it to reduce burden on clinicians.
IPMG representative Gregory Wedin, PharmD, pharmacovigilance and risk management director for Upsher-Smith Laboratories, said, “while we cannot support eliminating or extending the confirmation interval to a year, the [iPLEDGE] sponsors are agreeable [to] a 120-day confirmation interval.”
He said that while extending to 120 days would reduce burden on prescribers, it comes with risk in reducing oversight by a certified iPLEDGE prescriber and potentially increasing the risk for drug sharing.
“A patient may be more likely to share their drug with another person the further along with therapy they get as their condition improves,” Mr. Wedin said.
On March 29, the panel will hear more recommendations for and against modifications to iPLEDGE REMS and will vote on select modifications at the end of the meeting.
A version of this article first appeared on Medscape.com.
for patients, pharmacies, and prescribers.
Isotretinoin, previously called Accutane, is marketed as Absorica, Absorica LD, Claravis, Amnesteem, Myorisan, and Zenatane.
In a joint meeting of the FDA’s Drug Safety and Risk Management Advisory Committee and Dermatologic and Ophthalmic Drugs Advisory Committee, experts addressed ways to improve the modified iPLEDGE Risk Evaluation and Mitigation Strategy (iPLEDGE REMS) for isotretinoin that caused chaos after its rollout at the end of 2021.
In January 2022, problems were multiplying with the program for clinicians, pharmacists, and patients, causing extensive delays and prescription denials. In response, the FDA said it would continue to meet with the Isotretinoin Products Manufacturers Group (IPMG) to resolve problems.
March 28 was the first day of a 2-day meeting addressing what can be done to reduce burden with the iPLEDGE REMS while maintaining safety and preventing fetal exposure to the drug.
Key areas of concern
The meeting focused on several key areas.
The 19-day lockout period
The lockout is a current restriction for patients who can become pregnant and do not pick up their first prescription of isotretinoin within the specified 7-day prescription window. Currently, those who miss the window must wait 19 days from the date of the first pregnancy test to take an additional pregnancy test to be eligible to receive the drug.
Lindsey Crist, PharmD, a risk management analyst for the FDA, who presented the FDA review committee’s analysis, acknowledged that the lockout period causes delays in treatment and adds frustration and costs.
She said it’s important to remember that the lockout applies only to the first prescription. “It’s intended as an additional layer of screening to detect pregnancy,” she said.
“At least 12 pregnancies have been identified during the 19-day lockout from March 2017–September of 2022,” she noted.
The FDA is looking to the advisory committee to provide recommendations on whether the lockout period should be changed.
Home testing
During the pandemic, iPLEDGE rules have been relaxed from having a pregnancy test done only at a Clinical Laboratory Improvement Amendments–certified laboratory and home pregnancy tests have been allowed. The question now is whether home tests should continue to be allowed.
Ms. Crist said that the FDA’s review committee recommends ending the allowance of home tests, citing insufficient data on use and the discovery of instances of falsification of pregnancy tests.
“One study at an academic medical center reviewed the medical records of 89 patients who used home pregnancy tests while taking isotretinoin during the public health emergency. It found that 15.7% submitted falsified pregnancy test results,” she said.
Ms. Crist added, however, that the review committee recommends allowing the tests to be done in a provider’s office as an alternative.
Documenting counseling patients who cannot get pregnant
Currently, this documentation must be done monthly, primarily to counsel patients against drug sharing or giving blood. Proposed changes include extending the intervals for attestation or eliminating it to reduce burden on clinicians.
IPMG representative Gregory Wedin, PharmD, pharmacovigilance and risk management director for Upsher-Smith Laboratories, said, “while we cannot support eliminating or extending the confirmation interval to a year, the [iPLEDGE] sponsors are agreeable [to] a 120-day confirmation interval.”
He said that while extending to 120 days would reduce burden on prescribers, it comes with risk in reducing oversight by a certified iPLEDGE prescriber and potentially increasing the risk for drug sharing.
“A patient may be more likely to share their drug with another person the further along with therapy they get as their condition improves,” Mr. Wedin said.
On March 29, the panel will hear more recommendations for and against modifications to iPLEDGE REMS and will vote on select modifications at the end of the meeting.
A version of this article first appeared on Medscape.com.
Commentary: Chemotherapies and gynecologic surgeries relative to breast cancer, April 2023
However, a combined analysis of two other trials (PlanB and SUCCESS C) did not show a benefit with the addition of anthracycline for most patients with human epidermal growth factor receptor 2 (HER2)–negative early breast cancer.2 Roy and colleagues performed a retrospective study using the Surveillance, Epidemiology, and End Results (SEER)-Medicare database, including 1106 women ≥ 66 years of age with node-positive TNBC, of whom 69.3% received adjuvant chemotherapy (N = 767). The use of chemotherapy led to a statistically significant improvement in survival outcomes (3-year cancer-specific survival [CSS] 81.8% vs 71.4%; overall survival 70.7% vs 51.3%). Although the anthracycline/taxane–based therapy did not improve CSS in the overall population vs taxane-based (hazard ratio [HR] 0.94; P = .79), among patients aged ≥ 76 years with four or more positive nodes, there was improvement in CSS with anthracycline/taxane therapy (HR 0.09; P = .02). These data further support the benefits of adjuvant chemotherapy in older patients when indicated; stimulate consideration of nonanthracycline combinations, particularly now with the use of immunotherapy for early TNBC; and highlight the need for inclusion of older individuals in clinical trials.
Treatment strategies to improve efficacy and minimize toxicity are highly desired for patients with early breast cancer (EBC). As an example, for small, node-negative, HER2-positive tumors, adjuvant systemic therapy with 12 weeks of paclitaxel/trastuzumab followed by continuation of trastuzumab to complete 1 full year has demonstrated excellent survival outcomes at over 10 years of follow-up.3 In the WSG-ADAPT-TP phase 2 trial, 375 patients with hormone receptor–positive , HER2-positive EBC were randomized to receive neoadjuvant T-DM1 (trastuzumab emtansine) with or without endocrine therapy or trastuzumab plus endocrine therapy. Similar 5-year invasive disease-free and overall survival rates were seen between the three arms. Patients who achieved a pathologic complete response (pCR) vs non-pCR had improved 5-year invasive disease-free survival (iDFS) rates (92.7% vs 82.7%; unadjusted HR 0.40). Furthermore, among the 117 patients who achieved pCR, the omission of adjuvant chemotherapy did not compromise survival outcomes (5-year iDFS 93% vs 92.1% for those who had vs those who did not have chemotherapy, respectively; unadjusted HR 1.15) (Harbeck et al). De-escalation approaches should ideally focus on the identification of biomarkers of response and resistance, as well as tools that can help predict patient outcomes and allow modification of therapy in real time. An example of this latter concept is the use of 18F-FDG-PET to identify patients with HER2-positive EBC who were likely to benefit from a chemotherapy-free dual HER2 blockade (trastuzumab/pertuzumab) treatment approach.4
Gynecologic surgery has been shown to reduce the risk for breast cancer,5 although the specific type of surgery and the impact of hormone replacement therapy add complexity to understanding the risks and outcomes for women. A prospective cohort, the Sister Study, included 50,701 women without a prior diagnosis of breast cancer but with a biological sister who had breast cancer; of these, 13.8% reported having hysterectomy only and 18.1% reported having bilateral oophorectomy with or without hysterectomy. Bilateral oophorectomy was inversely associated with breast cancer incidence (HR 0.91; 95% CI 0.83-1.00), with comparable results for women receiving estrogen only or combination estrogen plus progestin hormone replacement therapy. Contrary to these findings, having a hysterectomy only showed a positive association with breast cancer incidence (HR 1.12; 95% CI 1.02-1.23), with the strongest association among women who used combination estrogen and progestin therapy (HR 1.25; 95% CI 1.01-1.55) (Lovett et al). The impact of other gynecologic surgeries (such as salpingectomy)6 and the timing of the initiation of hormone replacement therapy, as well as the duration, should be investigated in future research.
Additional References
1. Blum JL, Flynn PJ, Yothers G, et al. Anthracyclines in early breast cancer: The ABC trials-USOR 06-090, NSABP B-46-I/USOR 07132, and NSABP B-49 (NRG oncology). J Clin Oncol. 2017;35:2647e55. doi: 10.1200/JCO.2016.71.4147
2. de Gregorio A, Janni W, Friedl TW, et al. The impact of anthracyclines in intermediate and high-risk HER2-negative early breast cancer-a pooled analysis of the randomised clinical trials PlanB and SUCCESS C. Br J Cancer. 2022;126:1715-1724. doi: 10.1038/s41416-021-01690-6
3. Tolaney SM, Tarantino P, Graham N, et al. Adjuvant paclitaxel and trastuzumab for node-negative, HER2-positive breast cancer: Final 10-year analysis of the open-label, single-arm, phase 2 APT trial. Lancet Oncol. 2023;24:273-285. doi: 10.1016/S1470-2045(23)00051-7
4. Pérez-García JM, Gebhart G, Ruiz Borrego M, et al; on behalf of PHERGain steering committee and trial investigators. Chemotherapy de-escalation using an 18F-FDG-PET-based pathological response-adapted strategy in patients with HER2-positive early breast cancer (PHERGain): A multicentre, randomised, open-label, non-comparative, phase 2 trial. Lancet Oncol. 2021;22:858-871. doi: 10.1016/S1470-2045(21)00122-4
5. Chow S, Raine-Bennett T, Samant ND, Postlethwaite DA, Holzapfel M. Breast cancer risk after hysterectomy with and without salpingo-oophorectomy for benign indications. Am J Obstet Gynecol. 2020;223:900.e1-900.e7. doi: 10.1016/j.ajog.2020.06.040
6. ACOG Committee Opinion No. 774: Opportunistic salpingectomy as a strategy for epithelial ovarian cancer prevention. Obstet Gynecol. 2019;133:e279-e284. doi: 10.1097/AOG.0000000000003164
However, a combined analysis of two other trials (PlanB and SUCCESS C) did not show a benefit with the addition of anthracycline for most patients with human epidermal growth factor receptor 2 (HER2)–negative early breast cancer.2 Roy and colleagues performed a retrospective study using the Surveillance, Epidemiology, and End Results (SEER)-Medicare database, including 1106 women ≥ 66 years of age with node-positive TNBC, of whom 69.3% received adjuvant chemotherapy (N = 767). The use of chemotherapy led to a statistically significant improvement in survival outcomes (3-year cancer-specific survival [CSS] 81.8% vs 71.4%; overall survival 70.7% vs 51.3%). Although the anthracycline/taxane–based therapy did not improve CSS in the overall population vs taxane-based (hazard ratio [HR] 0.94; P = .79), among patients aged ≥ 76 years with four or more positive nodes, there was improvement in CSS with anthracycline/taxane therapy (HR 0.09; P = .02). These data further support the benefits of adjuvant chemotherapy in older patients when indicated; stimulate consideration of nonanthracycline combinations, particularly now with the use of immunotherapy for early TNBC; and highlight the need for inclusion of older individuals in clinical trials.
Treatment strategies to improve efficacy and minimize toxicity are highly desired for patients with early breast cancer (EBC). As an example, for small, node-negative, HER2-positive tumors, adjuvant systemic therapy with 12 weeks of paclitaxel/trastuzumab followed by continuation of trastuzumab to complete 1 full year has demonstrated excellent survival outcomes at over 10 years of follow-up.3 In the WSG-ADAPT-TP phase 2 trial, 375 patients with hormone receptor–positive , HER2-positive EBC were randomized to receive neoadjuvant T-DM1 (trastuzumab emtansine) with or without endocrine therapy or trastuzumab plus endocrine therapy. Similar 5-year invasive disease-free and overall survival rates were seen between the three arms. Patients who achieved a pathologic complete response (pCR) vs non-pCR had improved 5-year invasive disease-free survival (iDFS) rates (92.7% vs 82.7%; unadjusted HR 0.40). Furthermore, among the 117 patients who achieved pCR, the omission of adjuvant chemotherapy did not compromise survival outcomes (5-year iDFS 93% vs 92.1% for those who had vs those who did not have chemotherapy, respectively; unadjusted HR 1.15) (Harbeck et al). De-escalation approaches should ideally focus on the identification of biomarkers of response and resistance, as well as tools that can help predict patient outcomes and allow modification of therapy in real time. An example of this latter concept is the use of 18F-FDG-PET to identify patients with HER2-positive EBC who were likely to benefit from a chemotherapy-free dual HER2 blockade (trastuzumab/pertuzumab) treatment approach.4
Gynecologic surgery has been shown to reduce the risk for breast cancer,5 although the specific type of surgery and the impact of hormone replacement therapy add complexity to understanding the risks and outcomes for women. A prospective cohort, the Sister Study, included 50,701 women without a prior diagnosis of breast cancer but with a biological sister who had breast cancer; of these, 13.8% reported having hysterectomy only and 18.1% reported having bilateral oophorectomy with or without hysterectomy. Bilateral oophorectomy was inversely associated with breast cancer incidence (HR 0.91; 95% CI 0.83-1.00), with comparable results for women receiving estrogen only or combination estrogen plus progestin hormone replacement therapy. Contrary to these findings, having a hysterectomy only showed a positive association with breast cancer incidence (HR 1.12; 95% CI 1.02-1.23), with the strongest association among women who used combination estrogen and progestin therapy (HR 1.25; 95% CI 1.01-1.55) (Lovett et al). The impact of other gynecologic surgeries (such as salpingectomy)6 and the timing of the initiation of hormone replacement therapy, as well as the duration, should be investigated in future research.
Additional References
1. Blum JL, Flynn PJ, Yothers G, et al. Anthracyclines in early breast cancer: The ABC trials-USOR 06-090, NSABP B-46-I/USOR 07132, and NSABP B-49 (NRG oncology). J Clin Oncol. 2017;35:2647e55. doi: 10.1200/JCO.2016.71.4147
2. de Gregorio A, Janni W, Friedl TW, et al. The impact of anthracyclines in intermediate and high-risk HER2-negative early breast cancer-a pooled analysis of the randomised clinical trials PlanB and SUCCESS C. Br J Cancer. 2022;126:1715-1724. doi: 10.1038/s41416-021-01690-6
3. Tolaney SM, Tarantino P, Graham N, et al. Adjuvant paclitaxel and trastuzumab for node-negative, HER2-positive breast cancer: Final 10-year analysis of the open-label, single-arm, phase 2 APT trial. Lancet Oncol. 2023;24:273-285. doi: 10.1016/S1470-2045(23)00051-7
4. Pérez-García JM, Gebhart G, Ruiz Borrego M, et al; on behalf of PHERGain steering committee and trial investigators. Chemotherapy de-escalation using an 18F-FDG-PET-based pathological response-adapted strategy in patients with HER2-positive early breast cancer (PHERGain): A multicentre, randomised, open-label, non-comparative, phase 2 trial. Lancet Oncol. 2021;22:858-871. doi: 10.1016/S1470-2045(21)00122-4
5. Chow S, Raine-Bennett T, Samant ND, Postlethwaite DA, Holzapfel M. Breast cancer risk after hysterectomy with and without salpingo-oophorectomy for benign indications. Am J Obstet Gynecol. 2020;223:900.e1-900.e7. doi: 10.1016/j.ajog.2020.06.040
6. ACOG Committee Opinion No. 774: Opportunistic salpingectomy as a strategy for epithelial ovarian cancer prevention. Obstet Gynecol. 2019;133:e279-e284. doi: 10.1097/AOG.0000000000003164
However, a combined analysis of two other trials (PlanB and SUCCESS C) did not show a benefit with the addition of anthracycline for most patients with human epidermal growth factor receptor 2 (HER2)–negative early breast cancer.2 Roy and colleagues performed a retrospective study using the Surveillance, Epidemiology, and End Results (SEER)-Medicare database, including 1106 women ≥ 66 years of age with node-positive TNBC, of whom 69.3% received adjuvant chemotherapy (N = 767). The use of chemotherapy led to a statistically significant improvement in survival outcomes (3-year cancer-specific survival [CSS] 81.8% vs 71.4%; overall survival 70.7% vs 51.3%). Although the anthracycline/taxane–based therapy did not improve CSS in the overall population vs taxane-based (hazard ratio [HR] 0.94; P = .79), among patients aged ≥ 76 years with four or more positive nodes, there was improvement in CSS with anthracycline/taxane therapy (HR 0.09; P = .02). These data further support the benefits of adjuvant chemotherapy in older patients when indicated; stimulate consideration of nonanthracycline combinations, particularly now with the use of immunotherapy for early TNBC; and highlight the need for inclusion of older individuals in clinical trials.
Treatment strategies to improve efficacy and minimize toxicity are highly desired for patients with early breast cancer (EBC). As an example, for small, node-negative, HER2-positive tumors, adjuvant systemic therapy with 12 weeks of paclitaxel/trastuzumab followed by continuation of trastuzumab to complete 1 full year has demonstrated excellent survival outcomes at over 10 years of follow-up.3 In the WSG-ADAPT-TP phase 2 trial, 375 patients with hormone receptor–positive , HER2-positive EBC were randomized to receive neoadjuvant T-DM1 (trastuzumab emtansine) with or without endocrine therapy or trastuzumab plus endocrine therapy. Similar 5-year invasive disease-free and overall survival rates were seen between the three arms. Patients who achieved a pathologic complete response (pCR) vs non-pCR had improved 5-year invasive disease-free survival (iDFS) rates (92.7% vs 82.7%; unadjusted HR 0.40). Furthermore, among the 117 patients who achieved pCR, the omission of adjuvant chemotherapy did not compromise survival outcomes (5-year iDFS 93% vs 92.1% for those who had vs those who did not have chemotherapy, respectively; unadjusted HR 1.15) (Harbeck et al). De-escalation approaches should ideally focus on the identification of biomarkers of response and resistance, as well as tools that can help predict patient outcomes and allow modification of therapy in real time. An example of this latter concept is the use of 18F-FDG-PET to identify patients with HER2-positive EBC who were likely to benefit from a chemotherapy-free dual HER2 blockade (trastuzumab/pertuzumab) treatment approach.4
Gynecologic surgery has been shown to reduce the risk for breast cancer,5 although the specific type of surgery and the impact of hormone replacement therapy add complexity to understanding the risks and outcomes for women. A prospective cohort, the Sister Study, included 50,701 women without a prior diagnosis of breast cancer but with a biological sister who had breast cancer; of these, 13.8% reported having hysterectomy only and 18.1% reported having bilateral oophorectomy with or without hysterectomy. Bilateral oophorectomy was inversely associated with breast cancer incidence (HR 0.91; 95% CI 0.83-1.00), with comparable results for women receiving estrogen only or combination estrogen plus progestin hormone replacement therapy. Contrary to these findings, having a hysterectomy only showed a positive association with breast cancer incidence (HR 1.12; 95% CI 1.02-1.23), with the strongest association among women who used combination estrogen and progestin therapy (HR 1.25; 95% CI 1.01-1.55) (Lovett et al). The impact of other gynecologic surgeries (such as salpingectomy)6 and the timing of the initiation of hormone replacement therapy, as well as the duration, should be investigated in future research.
Additional References
1. Blum JL, Flynn PJ, Yothers G, et al. Anthracyclines in early breast cancer: The ABC trials-USOR 06-090, NSABP B-46-I/USOR 07132, and NSABP B-49 (NRG oncology). J Clin Oncol. 2017;35:2647e55. doi: 10.1200/JCO.2016.71.4147
2. de Gregorio A, Janni W, Friedl TW, et al. The impact of anthracyclines in intermediate and high-risk HER2-negative early breast cancer-a pooled analysis of the randomised clinical trials PlanB and SUCCESS C. Br J Cancer. 2022;126:1715-1724. doi: 10.1038/s41416-021-01690-6
3. Tolaney SM, Tarantino P, Graham N, et al. Adjuvant paclitaxel and trastuzumab for node-negative, HER2-positive breast cancer: Final 10-year analysis of the open-label, single-arm, phase 2 APT trial. Lancet Oncol. 2023;24:273-285. doi: 10.1016/S1470-2045(23)00051-7
4. Pérez-García JM, Gebhart G, Ruiz Borrego M, et al; on behalf of PHERGain steering committee and trial investigators. Chemotherapy de-escalation using an 18F-FDG-PET-based pathological response-adapted strategy in patients with HER2-positive early breast cancer (PHERGain): A multicentre, randomised, open-label, non-comparative, phase 2 trial. Lancet Oncol. 2021;22:858-871. doi: 10.1016/S1470-2045(21)00122-4
5. Chow S, Raine-Bennett T, Samant ND, Postlethwaite DA, Holzapfel M. Breast cancer risk after hysterectomy with and without salpingo-oophorectomy for benign indications. Am J Obstet Gynecol. 2020;223:900.e1-900.e7. doi: 10.1016/j.ajog.2020.06.040
6. ACOG Committee Opinion No. 774: Opportunistic salpingectomy as a strategy for epithelial ovarian cancer prevention. Obstet Gynecol. 2019;133:e279-e284. doi: 10.1097/AOG.0000000000003164
Commentary: IL-31 inhibitor, e-cigarettes, and upadacitinib in AD, April 2023
Good news! There's not a lot to say about this. Dupilumab is so easy. No blood work, no immunosuppression. Dupilumab is highly effective and very safe. It's safe enough for children as young as 6 months! It's so effective that if it is not working, I question my diagnosis (Could it be contact dermatitis or mycosis fungoides instead?) and whether the patient is taking the medication properly.
Boesjes and colleagues describe in Acta Dermato-Venereologica the Dutch experience with upadacitinib in patients who have not been successfully treated with dupilumab or baricitinib. Presumably, such patients, because treatment with dupilumab or baricitinib or both was unsuccessful, have very resistant atopic dermatitis (either due to strong genetic propensity or perhaps because they don't take their medications). Despite having such refractory disease, most patients did well on the treatment with rapid disease improvement. Upadacitinib didn't work for everyone, though. About 30% of the patients discontinued upadacitinib treatment due to ineffectiveness, adverse events, or both (8.5%, 14.9%, and 6.4%, respectively).
How much of that ineffectiveness was due to poor adherence to taking the treatment was not assessed. Upadacitinib is extraordinarily effective for atopic dermatitis. I didn't think I would ever see a drug more effective than dupilumab for atopic dermatitis, but a low dose of upadacitinib (15 mg/day) seems about twice as effective as dupilumab for complete clearing of atopic dermatitis. The higher dose of 30 mg may be 3.5 times as effective as dupiliumab at getting atopic dermatitis completely clear.1
I dislike the word significant. Significant is ambiguous. It could mean that an observed association would not be likely to occur by chance, or it could mean that an observed association is clinically meaningful. Smith and colleagues in "Association between electronic cigarette use and atopic dermatitis among United States adults" reported finding a "significant" association between e-cigarette use and atopic dermatitis. A total of 23% of 2119 e-cigarette users had atopic dermatitis vs 17.1% of 26,444 nonusers. Clearly, the observed association was statistically significant (the 6% difference was not likely to occur due to chance alone). Is the finding clinically meaningful? I don't think it would affect our practice in any way.
The authors made the point that the study doesn't tell us whether e-cigarette use causes atopic dermatitis or if atopic dermatitis causes people to smoke. I wonder if just being younger (or some other factor) might make people more likely to use e-cigarettes and more likely to have atopic dermatitis (assuming atopic dermatitis gradually subsides over time, a dogma that may not be true).
Kabashima and colleagues report on the efficacy of the interleukin (IL)–31 antagonist nemolizumab. IL-31 mediates itch and having a new drug to block IL-31 may be a great treatment for our itchy patients. In this study, patients who had greater itch reduction had greater improvement in eczema and in quality of life. I'm quite sure that reducing itch improves patients' quality of life. But when it comes to the itch and the inflammation, I'm not sure which comes first. Does controlling the itch make the inflammation better? Maybe. Does controlling inflammation make itch better? Certainly.
For atopic patients with inflammation, controlling that inflammation seems to me to be the best approach, and we don't need more new treatments to accomplish that. For those patients who have a lot of itch and little inflammation, an IL-31 antagonist may be a revolutionary addition to our treatment options.
Additional References
1. Blauvelt A, Teixeira HD, Simpson EL, et al. Efficacy and safety of upadacitinib vs dupilumab in adults with moderate-to-severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2021;157:1047-1055. doi: 10.1001/jamadermatol.2021.3023. Erratum in: JAMA Dermatol. 2022;158:219. doi: 10.1001/jamadermatol.2021.5451
Good news! There's not a lot to say about this. Dupilumab is so easy. No blood work, no immunosuppression. Dupilumab is highly effective and very safe. It's safe enough for children as young as 6 months! It's so effective that if it is not working, I question my diagnosis (Could it be contact dermatitis or mycosis fungoides instead?) and whether the patient is taking the medication properly.
Boesjes and colleagues describe in Acta Dermato-Venereologica the Dutch experience with upadacitinib in patients who have not been successfully treated with dupilumab or baricitinib. Presumably, such patients, because treatment with dupilumab or baricitinib or both was unsuccessful, have very resistant atopic dermatitis (either due to strong genetic propensity or perhaps because they don't take their medications). Despite having such refractory disease, most patients did well on the treatment with rapid disease improvement. Upadacitinib didn't work for everyone, though. About 30% of the patients discontinued upadacitinib treatment due to ineffectiveness, adverse events, or both (8.5%, 14.9%, and 6.4%, respectively).
How much of that ineffectiveness was due to poor adherence to taking the treatment was not assessed. Upadacitinib is extraordinarily effective for atopic dermatitis. I didn't think I would ever see a drug more effective than dupilumab for atopic dermatitis, but a low dose of upadacitinib (15 mg/day) seems about twice as effective as dupilumab for complete clearing of atopic dermatitis. The higher dose of 30 mg may be 3.5 times as effective as dupiliumab at getting atopic dermatitis completely clear.1
I dislike the word significant. Significant is ambiguous. It could mean that an observed association would not be likely to occur by chance, or it could mean that an observed association is clinically meaningful. Smith and colleagues in "Association between electronic cigarette use and atopic dermatitis among United States adults" reported finding a "significant" association between e-cigarette use and atopic dermatitis. A total of 23% of 2119 e-cigarette users had atopic dermatitis vs 17.1% of 26,444 nonusers. Clearly, the observed association was statistically significant (the 6% difference was not likely to occur due to chance alone). Is the finding clinically meaningful? I don't think it would affect our practice in any way.
The authors made the point that the study doesn't tell us whether e-cigarette use causes atopic dermatitis or if atopic dermatitis causes people to smoke. I wonder if just being younger (or some other factor) might make people more likely to use e-cigarettes and more likely to have atopic dermatitis (assuming atopic dermatitis gradually subsides over time, a dogma that may not be true).
Kabashima and colleagues report on the efficacy of the interleukin (IL)–31 antagonist nemolizumab. IL-31 mediates itch and having a new drug to block IL-31 may be a great treatment for our itchy patients. In this study, patients who had greater itch reduction had greater improvement in eczema and in quality of life. I'm quite sure that reducing itch improves patients' quality of life. But when it comes to the itch and the inflammation, I'm not sure which comes first. Does controlling the itch make the inflammation better? Maybe. Does controlling inflammation make itch better? Certainly.
For atopic patients with inflammation, controlling that inflammation seems to me to be the best approach, and we don't need more new treatments to accomplish that. For those patients who have a lot of itch and little inflammation, an IL-31 antagonist may be a revolutionary addition to our treatment options.
Additional References
1. Blauvelt A, Teixeira HD, Simpson EL, et al. Efficacy and safety of upadacitinib vs dupilumab in adults with moderate-to-severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2021;157:1047-1055. doi: 10.1001/jamadermatol.2021.3023. Erratum in: JAMA Dermatol. 2022;158:219. doi: 10.1001/jamadermatol.2021.5451
Good news! There's not a lot to say about this. Dupilumab is so easy. No blood work, no immunosuppression. Dupilumab is highly effective and very safe. It's safe enough for children as young as 6 months! It's so effective that if it is not working, I question my diagnosis (Could it be contact dermatitis or mycosis fungoides instead?) and whether the patient is taking the medication properly.
Boesjes and colleagues describe in Acta Dermato-Venereologica the Dutch experience with upadacitinib in patients who have not been successfully treated with dupilumab or baricitinib. Presumably, such patients, because treatment with dupilumab or baricitinib or both was unsuccessful, have very resistant atopic dermatitis (either due to strong genetic propensity or perhaps because they don't take their medications). Despite having such refractory disease, most patients did well on the treatment with rapid disease improvement. Upadacitinib didn't work for everyone, though. About 30% of the patients discontinued upadacitinib treatment due to ineffectiveness, adverse events, or both (8.5%, 14.9%, and 6.4%, respectively).
How much of that ineffectiveness was due to poor adherence to taking the treatment was not assessed. Upadacitinib is extraordinarily effective for atopic dermatitis. I didn't think I would ever see a drug more effective than dupilumab for atopic dermatitis, but a low dose of upadacitinib (15 mg/day) seems about twice as effective as dupilumab for complete clearing of atopic dermatitis. The higher dose of 30 mg may be 3.5 times as effective as dupiliumab at getting atopic dermatitis completely clear.1
I dislike the word significant. Significant is ambiguous. It could mean that an observed association would not be likely to occur by chance, or it could mean that an observed association is clinically meaningful. Smith and colleagues in "Association between electronic cigarette use and atopic dermatitis among United States adults" reported finding a "significant" association between e-cigarette use and atopic dermatitis. A total of 23% of 2119 e-cigarette users had atopic dermatitis vs 17.1% of 26,444 nonusers. Clearly, the observed association was statistically significant (the 6% difference was not likely to occur due to chance alone). Is the finding clinically meaningful? I don't think it would affect our practice in any way.
The authors made the point that the study doesn't tell us whether e-cigarette use causes atopic dermatitis or if atopic dermatitis causes people to smoke. I wonder if just being younger (or some other factor) might make people more likely to use e-cigarettes and more likely to have atopic dermatitis (assuming atopic dermatitis gradually subsides over time, a dogma that may not be true).
Kabashima and colleagues report on the efficacy of the interleukin (IL)–31 antagonist nemolizumab. IL-31 mediates itch and having a new drug to block IL-31 may be a great treatment for our itchy patients. In this study, patients who had greater itch reduction had greater improvement in eczema and in quality of life. I'm quite sure that reducing itch improves patients' quality of life. But when it comes to the itch and the inflammation, I'm not sure which comes first. Does controlling the itch make the inflammation better? Maybe. Does controlling inflammation make itch better? Certainly.
For atopic patients with inflammation, controlling that inflammation seems to me to be the best approach, and we don't need more new treatments to accomplish that. For those patients who have a lot of itch and little inflammation, an IL-31 antagonist may be a revolutionary addition to our treatment options.
Additional References
1. Blauvelt A, Teixeira HD, Simpson EL, et al. Efficacy and safety of upadacitinib vs dupilumab in adults with moderate-to-severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2021;157:1047-1055. doi: 10.1001/jamadermatol.2021.3023. Erratum in: JAMA Dermatol. 2022;158:219. doi: 10.1001/jamadermatol.2021.5451
JAK inhibitor ivarmacitinib shows efficacy for atopic dermatitis in a pivotal trial
NEW ORLEANS – The presented as a late-breaker at the annual meeting of the American Academy of Dermatology.
Two doses were studied in the placebo-controlled trial and both demonstrated “a favorable benefit-to-risk profile in patients with moderate to severe AD,” reported Yan Zhao, MD, a clinician and researcher in the department of dermatology, Peking University People’s Hospital, Beijing.
In the study, called QUARTZ3, 336 patients aged 12 and older at 51 sites in China and Canada were randomized to 4 mg once-daily ivarmacitinib, 8 mg once-daily QD ivarmacitinib, or placebo. The mean age of the population was 32 years and approximately one-third were female.
The mean duration of AD for participants was 10 years. The mean baseline Eczema Area and Severity Index (EASI) score was near 30. On the Investigator Global Assessment (IGA) tool, approximately 40% had a score of 4, which is the highest score on the scale and indicates severe disease. The remaining patients had an IGA score of 3.
The co-primary endpoints were change in IGA and EASI scores at 16 weeks, and both improved rapidly, showing statistical significance relative to placebo by 4 weeks with no plateauing effect at the end of the 16-week trial. By week 16, the proportion of patients with an EASI score of 75, signifying a 75% improvement, was 66%, 54%, and 22% for the 8-mg dose of ivarmacitinib, 4-mg dose of ivarmacitinib, and placebo groups (P < .001 versus placebo for both doses of active therapy), respectively.
The pattern of the IGA response was similar. By week 16, the proportion of patients achieving an IGA score of 0 (clear) or 1 (almost clear) was 42%, 36%, and 9% for the 8-mg dose of ivarmacitinib, 4-mg dose of ivarmacitinib, and placebo groups, respectively. The advantage of either dose over placebo was highly significant (P < .001) at 8, 12, and 16 weeks.
For the WI-NRS (Worst Itch – Numeric Rating Scale), the advantage of the 8-mg dose relative to placebo was significant (P < .001) at the 1-week evaluation. By 2 weeks, the 4-mg dose had gained the same degree of statistical significance relative to placebo. After week 4, when the maximum proportion of patients with a WI-NRS score ≤ 4 was reached (50%, 35%, and 10% in the 8-mg, 4-mg, and placebo groups), and the relative advantage of active treatment persisted until the end of the 16-week study.
Two scales were used to evaluate change in quality of life. On the DLQI (Dermatology Life Quality Index) and POEM (Patient-Oriented Eczema Measure), improvements were again rapid and sustained. By week 4, improvement with the 8-mg dose was about fourfold greater (P < .001) than improvement with placebo for DLQI and about sixfold greater (P < .001) for POEM. For the 4-mg dose, the relative differences were approximately threefold and fourfold greater, and both were significant (P <.001).
There was no further gain in these quality-of-life scales from week 4 to week 16, but the advantages relative to placebo were generally sustained, Dr. Zhao reported.
Ivarmacitinib was safe and well-tolerated, according to Dr. Zhao. The proportion of patients with a treatment-emergent adverse event that led to drug discontinuation was numerically higher (5.4%) in the placebo group than in the 8-mg (3.6%) or 4-mg group (2.7%). Rates of infection in the three groups were similar, and there were no major adverse cardiovascular events (MACE) or thromboembolism observed in any group.
Ivarmacitinib, which has about a 10-fold greater selectivity for JAK1 than JAK2 and a more than 70-fold greater selectivity for JAK1 than JAK3, is being tested for rheumatoid arthritis, inflammatory bowel disease, and alopecia areata in addition to AD, Dr. Zhao said. She also reported that an application for new drug approval has been submitted in China. Efforts to pursue regulatory approval elsewhere are anticipated.
Currently, there are three JAK inhibitors licensed for the treatment of AD in the United States. Upadacitinib (Rinvoq) and abrocitinib (Cibinqo) are also once-daily oral JAK1-selective inhibitors. Regulatory approval for AD by the Food and Drug Administration was granted to both in early 2022 and both now have an indication for moderate to severe disease in patients ages 12 years and older.
In September 2021, the first U.S. approval of a drug in this class for AD was granted for a topical formulation of ruxolitinib (Opzelura), which has selectivity for both JAK1 and JAK2. The indication is for mild to moderate AD in patients aged 12 years and older.
In the phase 3 clinical trial that led to approval of abrocitinib for AD, the comparator groups included placebo and active treatment with 300 mg dupilumab administered subcutaneously every other week. The higher of two doses of abrocitinib (100 mg) was numerically superior to dupilumab in terms of EASI 75 response at week 12 and was statistically superior for relief of itch at week 2.
Relative to the first-generation JAK inhibitor tofacitinib (Xeljanz), both of the approved oral JAK inhibitors for AD, abrocitinib and upadacitinib, have greater JAK1-selectivity. However, selectivity for all JAK inhibitors is relative rather than absolute, according to a recent review article on oral JAK inhibitors for AD. Efficacy and safety are likely determined by relative inhibition of each of the four JAK enzymes (JAK1, JAK2, JAK3, and TYK2). Although JAK1 appears to be an important target for AD treatment, the clinical significance of the degree of selectivity among oral JAK inhibitors is not yet clear.
In an interview, the senior author of that review article, Emma Guttman-Yassky, MD, PhD, emphasized this point. She said there is no evidence and no basis on which to speculate that any one drug in this class is better than another for AD. Dr. Guttman-Yassky is a professor and system chair of dermatology and immunology at the Icahn School of Medicine at Mount Sinai, New York.
“The efficacy [of ivarmacitinib] seems, in general, to be in line with other JAK inhibitors,” said Dr. Guttman-Yassky, who attended the late-breaker session during which these data were presented. Although she acknowledged that rapid control of pruritus is important clinically, she said the speed of itch relief as reported in the phase 3 ivarmacitinib trial does not distinguish it from other oral drugs in the class.
Shawn Kwatra, MD, director of the Johns Hopkins Itch Center, Johns Hopkins University, Baltimore, agreed.
“The rapid effects on itch of ivarmacitinib are consistent with those observed by the already approved JAK1-selective inhibitors abrocitinib and upadacitinib,” he said in an interview.
This suggests that head-to-head trials will be needed to draw any conclusions about the relative efficacy and safety of existing and emerging oral JAK inhibitors for AD.
Dr. Zhao has reported a financial relationship with Reistone Biopharma, which is developing ivarmacitinib and provided funding for the trial. Dr. Guttman-Yassky has reported financial relationships with more than 20 pharmaceutical companies, including companies that make JAK inhibitors. Dr. Kwatra has reported financial relationships with AbbVie, Aslan, Arcutis Biotherapeutics, Castle Biosciences, Celldex, Galderma, Genzada, Incyte, Johnson & Johnson, Leo Pharma, Novartis, Pfizer, Regeneron, and Sanofi.
A version of this article first appeared on Medscape.com.
NEW ORLEANS – The presented as a late-breaker at the annual meeting of the American Academy of Dermatology.
Two doses were studied in the placebo-controlled trial and both demonstrated “a favorable benefit-to-risk profile in patients with moderate to severe AD,” reported Yan Zhao, MD, a clinician and researcher in the department of dermatology, Peking University People’s Hospital, Beijing.
In the study, called QUARTZ3, 336 patients aged 12 and older at 51 sites in China and Canada were randomized to 4 mg once-daily ivarmacitinib, 8 mg once-daily QD ivarmacitinib, or placebo. The mean age of the population was 32 years and approximately one-third were female.
The mean duration of AD for participants was 10 years. The mean baseline Eczema Area and Severity Index (EASI) score was near 30. On the Investigator Global Assessment (IGA) tool, approximately 40% had a score of 4, which is the highest score on the scale and indicates severe disease. The remaining patients had an IGA score of 3.
The co-primary endpoints were change in IGA and EASI scores at 16 weeks, and both improved rapidly, showing statistical significance relative to placebo by 4 weeks with no plateauing effect at the end of the 16-week trial. By week 16, the proportion of patients with an EASI score of 75, signifying a 75% improvement, was 66%, 54%, and 22% for the 8-mg dose of ivarmacitinib, 4-mg dose of ivarmacitinib, and placebo groups (P < .001 versus placebo for both doses of active therapy), respectively.
The pattern of the IGA response was similar. By week 16, the proportion of patients achieving an IGA score of 0 (clear) or 1 (almost clear) was 42%, 36%, and 9% for the 8-mg dose of ivarmacitinib, 4-mg dose of ivarmacitinib, and placebo groups, respectively. The advantage of either dose over placebo was highly significant (P < .001) at 8, 12, and 16 weeks.
For the WI-NRS (Worst Itch – Numeric Rating Scale), the advantage of the 8-mg dose relative to placebo was significant (P < .001) at the 1-week evaluation. By 2 weeks, the 4-mg dose had gained the same degree of statistical significance relative to placebo. After week 4, when the maximum proportion of patients with a WI-NRS score ≤ 4 was reached (50%, 35%, and 10% in the 8-mg, 4-mg, and placebo groups), and the relative advantage of active treatment persisted until the end of the 16-week study.
Two scales were used to evaluate change in quality of life. On the DLQI (Dermatology Life Quality Index) and POEM (Patient-Oriented Eczema Measure), improvements were again rapid and sustained. By week 4, improvement with the 8-mg dose was about fourfold greater (P < .001) than improvement with placebo for DLQI and about sixfold greater (P < .001) for POEM. For the 4-mg dose, the relative differences were approximately threefold and fourfold greater, and both were significant (P <.001).
There was no further gain in these quality-of-life scales from week 4 to week 16, but the advantages relative to placebo were generally sustained, Dr. Zhao reported.
Ivarmacitinib was safe and well-tolerated, according to Dr. Zhao. The proportion of patients with a treatment-emergent adverse event that led to drug discontinuation was numerically higher (5.4%) in the placebo group than in the 8-mg (3.6%) or 4-mg group (2.7%). Rates of infection in the three groups were similar, and there were no major adverse cardiovascular events (MACE) or thromboembolism observed in any group.
Ivarmacitinib, which has about a 10-fold greater selectivity for JAK1 than JAK2 and a more than 70-fold greater selectivity for JAK1 than JAK3, is being tested for rheumatoid arthritis, inflammatory bowel disease, and alopecia areata in addition to AD, Dr. Zhao said. She also reported that an application for new drug approval has been submitted in China. Efforts to pursue regulatory approval elsewhere are anticipated.
Currently, there are three JAK inhibitors licensed for the treatment of AD in the United States. Upadacitinib (Rinvoq) and abrocitinib (Cibinqo) are also once-daily oral JAK1-selective inhibitors. Regulatory approval for AD by the Food and Drug Administration was granted to both in early 2022 and both now have an indication for moderate to severe disease in patients ages 12 years and older.
In September 2021, the first U.S. approval of a drug in this class for AD was granted for a topical formulation of ruxolitinib (Opzelura), which has selectivity for both JAK1 and JAK2. The indication is for mild to moderate AD in patients aged 12 years and older.
In the phase 3 clinical trial that led to approval of abrocitinib for AD, the comparator groups included placebo and active treatment with 300 mg dupilumab administered subcutaneously every other week. The higher of two doses of abrocitinib (100 mg) was numerically superior to dupilumab in terms of EASI 75 response at week 12 and was statistically superior for relief of itch at week 2.
Relative to the first-generation JAK inhibitor tofacitinib (Xeljanz), both of the approved oral JAK inhibitors for AD, abrocitinib and upadacitinib, have greater JAK1-selectivity. However, selectivity for all JAK inhibitors is relative rather than absolute, according to a recent review article on oral JAK inhibitors for AD. Efficacy and safety are likely determined by relative inhibition of each of the four JAK enzymes (JAK1, JAK2, JAK3, and TYK2). Although JAK1 appears to be an important target for AD treatment, the clinical significance of the degree of selectivity among oral JAK inhibitors is not yet clear.
In an interview, the senior author of that review article, Emma Guttman-Yassky, MD, PhD, emphasized this point. She said there is no evidence and no basis on which to speculate that any one drug in this class is better than another for AD. Dr. Guttman-Yassky is a professor and system chair of dermatology and immunology at the Icahn School of Medicine at Mount Sinai, New York.
“The efficacy [of ivarmacitinib] seems, in general, to be in line with other JAK inhibitors,” said Dr. Guttman-Yassky, who attended the late-breaker session during which these data were presented. Although she acknowledged that rapid control of pruritus is important clinically, she said the speed of itch relief as reported in the phase 3 ivarmacitinib trial does not distinguish it from other oral drugs in the class.
Shawn Kwatra, MD, director of the Johns Hopkins Itch Center, Johns Hopkins University, Baltimore, agreed.
“The rapid effects on itch of ivarmacitinib are consistent with those observed by the already approved JAK1-selective inhibitors abrocitinib and upadacitinib,” he said in an interview.
This suggests that head-to-head trials will be needed to draw any conclusions about the relative efficacy and safety of existing and emerging oral JAK inhibitors for AD.
Dr. Zhao has reported a financial relationship with Reistone Biopharma, which is developing ivarmacitinib and provided funding for the trial. Dr. Guttman-Yassky has reported financial relationships with more than 20 pharmaceutical companies, including companies that make JAK inhibitors. Dr. Kwatra has reported financial relationships with AbbVie, Aslan, Arcutis Biotherapeutics, Castle Biosciences, Celldex, Galderma, Genzada, Incyte, Johnson & Johnson, Leo Pharma, Novartis, Pfizer, Regeneron, and Sanofi.
A version of this article first appeared on Medscape.com.
NEW ORLEANS – The presented as a late-breaker at the annual meeting of the American Academy of Dermatology.
Two doses were studied in the placebo-controlled trial and both demonstrated “a favorable benefit-to-risk profile in patients with moderate to severe AD,” reported Yan Zhao, MD, a clinician and researcher in the department of dermatology, Peking University People’s Hospital, Beijing.
In the study, called QUARTZ3, 336 patients aged 12 and older at 51 sites in China and Canada were randomized to 4 mg once-daily ivarmacitinib, 8 mg once-daily QD ivarmacitinib, or placebo. The mean age of the population was 32 years and approximately one-third were female.
The mean duration of AD for participants was 10 years. The mean baseline Eczema Area and Severity Index (EASI) score was near 30. On the Investigator Global Assessment (IGA) tool, approximately 40% had a score of 4, which is the highest score on the scale and indicates severe disease. The remaining patients had an IGA score of 3.
The co-primary endpoints were change in IGA and EASI scores at 16 weeks, and both improved rapidly, showing statistical significance relative to placebo by 4 weeks with no plateauing effect at the end of the 16-week trial. By week 16, the proportion of patients with an EASI score of 75, signifying a 75% improvement, was 66%, 54%, and 22% for the 8-mg dose of ivarmacitinib, 4-mg dose of ivarmacitinib, and placebo groups (P < .001 versus placebo for both doses of active therapy), respectively.
The pattern of the IGA response was similar. By week 16, the proportion of patients achieving an IGA score of 0 (clear) or 1 (almost clear) was 42%, 36%, and 9% for the 8-mg dose of ivarmacitinib, 4-mg dose of ivarmacitinib, and placebo groups, respectively. The advantage of either dose over placebo was highly significant (P < .001) at 8, 12, and 16 weeks.
For the WI-NRS (Worst Itch – Numeric Rating Scale), the advantage of the 8-mg dose relative to placebo was significant (P < .001) at the 1-week evaluation. By 2 weeks, the 4-mg dose had gained the same degree of statistical significance relative to placebo. After week 4, when the maximum proportion of patients with a WI-NRS score ≤ 4 was reached (50%, 35%, and 10% in the 8-mg, 4-mg, and placebo groups), and the relative advantage of active treatment persisted until the end of the 16-week study.
Two scales were used to evaluate change in quality of life. On the DLQI (Dermatology Life Quality Index) and POEM (Patient-Oriented Eczema Measure), improvements were again rapid and sustained. By week 4, improvement with the 8-mg dose was about fourfold greater (P < .001) than improvement with placebo for DLQI and about sixfold greater (P < .001) for POEM. For the 4-mg dose, the relative differences were approximately threefold and fourfold greater, and both were significant (P <.001).
There was no further gain in these quality-of-life scales from week 4 to week 16, but the advantages relative to placebo were generally sustained, Dr. Zhao reported.
Ivarmacitinib was safe and well-tolerated, according to Dr. Zhao. The proportion of patients with a treatment-emergent adverse event that led to drug discontinuation was numerically higher (5.4%) in the placebo group than in the 8-mg (3.6%) or 4-mg group (2.7%). Rates of infection in the three groups were similar, and there were no major adverse cardiovascular events (MACE) or thromboembolism observed in any group.
Ivarmacitinib, which has about a 10-fold greater selectivity for JAK1 than JAK2 and a more than 70-fold greater selectivity for JAK1 than JAK3, is being tested for rheumatoid arthritis, inflammatory bowel disease, and alopecia areata in addition to AD, Dr. Zhao said. She also reported that an application for new drug approval has been submitted in China. Efforts to pursue regulatory approval elsewhere are anticipated.
Currently, there are three JAK inhibitors licensed for the treatment of AD in the United States. Upadacitinib (Rinvoq) and abrocitinib (Cibinqo) are also once-daily oral JAK1-selective inhibitors. Regulatory approval for AD by the Food and Drug Administration was granted to both in early 2022 and both now have an indication for moderate to severe disease in patients ages 12 years and older.
In September 2021, the first U.S. approval of a drug in this class for AD was granted for a topical formulation of ruxolitinib (Opzelura), which has selectivity for both JAK1 and JAK2. The indication is for mild to moderate AD in patients aged 12 years and older.
In the phase 3 clinical trial that led to approval of abrocitinib for AD, the comparator groups included placebo and active treatment with 300 mg dupilumab administered subcutaneously every other week. The higher of two doses of abrocitinib (100 mg) was numerically superior to dupilumab in terms of EASI 75 response at week 12 and was statistically superior for relief of itch at week 2.
Relative to the first-generation JAK inhibitor tofacitinib (Xeljanz), both of the approved oral JAK inhibitors for AD, abrocitinib and upadacitinib, have greater JAK1-selectivity. However, selectivity for all JAK inhibitors is relative rather than absolute, according to a recent review article on oral JAK inhibitors for AD. Efficacy and safety are likely determined by relative inhibition of each of the four JAK enzymes (JAK1, JAK2, JAK3, and TYK2). Although JAK1 appears to be an important target for AD treatment, the clinical significance of the degree of selectivity among oral JAK inhibitors is not yet clear.
In an interview, the senior author of that review article, Emma Guttman-Yassky, MD, PhD, emphasized this point. She said there is no evidence and no basis on which to speculate that any one drug in this class is better than another for AD. Dr. Guttman-Yassky is a professor and system chair of dermatology and immunology at the Icahn School of Medicine at Mount Sinai, New York.
“The efficacy [of ivarmacitinib] seems, in general, to be in line with other JAK inhibitors,” said Dr. Guttman-Yassky, who attended the late-breaker session during which these data were presented. Although she acknowledged that rapid control of pruritus is important clinically, she said the speed of itch relief as reported in the phase 3 ivarmacitinib trial does not distinguish it from other oral drugs in the class.
Shawn Kwatra, MD, director of the Johns Hopkins Itch Center, Johns Hopkins University, Baltimore, agreed.
“The rapid effects on itch of ivarmacitinib are consistent with those observed by the already approved JAK1-selective inhibitors abrocitinib and upadacitinib,” he said in an interview.
This suggests that head-to-head trials will be needed to draw any conclusions about the relative efficacy and safety of existing and emerging oral JAK inhibitors for AD.
Dr. Zhao has reported a financial relationship with Reistone Biopharma, which is developing ivarmacitinib and provided funding for the trial. Dr. Guttman-Yassky has reported financial relationships with more than 20 pharmaceutical companies, including companies that make JAK inhibitors. Dr. Kwatra has reported financial relationships with AbbVie, Aslan, Arcutis Biotherapeutics, Castle Biosciences, Celldex, Galderma, Genzada, Incyte, Johnson & Johnson, Leo Pharma, Novartis, Pfizer, Regeneron, and Sanofi.
A version of this article first appeared on Medscape.com.
AT AAD 2023
COVID-19 potentially induced adult-onset IgA vasculitis
Plasma exchange successfully improved symptoms of immunoglobulin A vasculitis in an adult female patient who developed the condition after infection with COVID-19, according to a case report published in Cureus.
Immunoglobulin A (IgA) vasculitis can affect all ages, but is relatively rare in adults, and the etiology remains unclear, wrote Hassan Alwafi, MD, of Umm Al-Qura University, Makkah, Saudi Arabia, and colleagues.
COVID-19 has been associated with pulmonary and extrapulmonary complications, but , the authors wrote.
The authors described a case of a 41-year-old otherwise healthy Saudi Arabian woman who presented with an ascending rash on both lower extremities, along with arthralgia. Blood tests showed high blood urea nitrogen, creatinine, and inflammatory markers, and a negative immune panel. The patient had been infected with COVID-19 approximately 2 weeks before the onset of symptoms, but she was treated with supportive care and required no antiviral therapy of dexamethasone.
In addition, the patient’s urinalysis showed proteinuria and hematuria. After a kidney biopsy revealed additional abnormalities, the patient was started on intravenous methylprednisolone pulse therapy.
A few days after the initiation of therapy, the patient experienced nosebleeds and coughing up blood. After a chest x-ray showed bilateral pleural effusion, the patient was transferred to the ICU. The patient was started on intravenous piperacillin-tazobactam, and received two doses of intravenous immunoglobulin and plasma exchange after consultation with a nephrologist. Ultimately, the initial rash and other clinical symptoms improved, and the patient was discharged with a tapering schedule of oral prednisolone.
In this case, COVID-19 may have played a role in the development of IgA vasculitis, the authors said.
The authors also listed 21 cases of IgA vasculitis following COVID-19 infection, including 14 children and 7 adults. Of these, three cases had combined kidney and lung involvement, the two pediatric cases died from respiratory failure, while the adult case was successfully treated with steroid monotherapy.
“As COVID-19 is a novel disease and its pathogenic mechanism of causing IgA vasculitis is not well understood, every patient who is infected with or recently recovered from COVID-19 and presents with a skin rash or arthralgia should have baseline blood and urine tests done and should be treated promptly to avoid the emergence of irreversible consequences,” the authors wrote in their discussion.
Although case reports cannot prove a cause-and-effect link, the data from the cases in the current review suggest that COVID-19 infection may be an indirect trigger for IgA vasculitis, including cases associated with pulmonary renal syndrome, they said. However, more research is needed, especially on the efficacy of treatments in adults, they concluded.
The study received no outside funding. The researchers had no financial conflicts to disclose.
Plasma exchange successfully improved symptoms of immunoglobulin A vasculitis in an adult female patient who developed the condition after infection with COVID-19, according to a case report published in Cureus.
Immunoglobulin A (IgA) vasculitis can affect all ages, but is relatively rare in adults, and the etiology remains unclear, wrote Hassan Alwafi, MD, of Umm Al-Qura University, Makkah, Saudi Arabia, and colleagues.
COVID-19 has been associated with pulmonary and extrapulmonary complications, but , the authors wrote.
The authors described a case of a 41-year-old otherwise healthy Saudi Arabian woman who presented with an ascending rash on both lower extremities, along with arthralgia. Blood tests showed high blood urea nitrogen, creatinine, and inflammatory markers, and a negative immune panel. The patient had been infected with COVID-19 approximately 2 weeks before the onset of symptoms, but she was treated with supportive care and required no antiviral therapy of dexamethasone.
In addition, the patient’s urinalysis showed proteinuria and hematuria. After a kidney biopsy revealed additional abnormalities, the patient was started on intravenous methylprednisolone pulse therapy.
A few days after the initiation of therapy, the patient experienced nosebleeds and coughing up blood. After a chest x-ray showed bilateral pleural effusion, the patient was transferred to the ICU. The patient was started on intravenous piperacillin-tazobactam, and received two doses of intravenous immunoglobulin and plasma exchange after consultation with a nephrologist. Ultimately, the initial rash and other clinical symptoms improved, and the patient was discharged with a tapering schedule of oral prednisolone.
In this case, COVID-19 may have played a role in the development of IgA vasculitis, the authors said.
The authors also listed 21 cases of IgA vasculitis following COVID-19 infection, including 14 children and 7 adults. Of these, three cases had combined kidney and lung involvement, the two pediatric cases died from respiratory failure, while the adult case was successfully treated with steroid monotherapy.
“As COVID-19 is a novel disease and its pathogenic mechanism of causing IgA vasculitis is not well understood, every patient who is infected with or recently recovered from COVID-19 and presents with a skin rash or arthralgia should have baseline blood and urine tests done and should be treated promptly to avoid the emergence of irreversible consequences,” the authors wrote in their discussion.
Although case reports cannot prove a cause-and-effect link, the data from the cases in the current review suggest that COVID-19 infection may be an indirect trigger for IgA vasculitis, including cases associated with pulmonary renal syndrome, they said. However, more research is needed, especially on the efficacy of treatments in adults, they concluded.
The study received no outside funding. The researchers had no financial conflicts to disclose.
Plasma exchange successfully improved symptoms of immunoglobulin A vasculitis in an adult female patient who developed the condition after infection with COVID-19, according to a case report published in Cureus.
Immunoglobulin A (IgA) vasculitis can affect all ages, but is relatively rare in adults, and the etiology remains unclear, wrote Hassan Alwafi, MD, of Umm Al-Qura University, Makkah, Saudi Arabia, and colleagues.
COVID-19 has been associated with pulmonary and extrapulmonary complications, but , the authors wrote.
The authors described a case of a 41-year-old otherwise healthy Saudi Arabian woman who presented with an ascending rash on both lower extremities, along with arthralgia. Blood tests showed high blood urea nitrogen, creatinine, and inflammatory markers, and a negative immune panel. The patient had been infected with COVID-19 approximately 2 weeks before the onset of symptoms, but she was treated with supportive care and required no antiviral therapy of dexamethasone.
In addition, the patient’s urinalysis showed proteinuria and hematuria. After a kidney biopsy revealed additional abnormalities, the patient was started on intravenous methylprednisolone pulse therapy.
A few days after the initiation of therapy, the patient experienced nosebleeds and coughing up blood. After a chest x-ray showed bilateral pleural effusion, the patient was transferred to the ICU. The patient was started on intravenous piperacillin-tazobactam, and received two doses of intravenous immunoglobulin and plasma exchange after consultation with a nephrologist. Ultimately, the initial rash and other clinical symptoms improved, and the patient was discharged with a tapering schedule of oral prednisolone.
In this case, COVID-19 may have played a role in the development of IgA vasculitis, the authors said.
The authors also listed 21 cases of IgA vasculitis following COVID-19 infection, including 14 children and 7 adults. Of these, three cases had combined kidney and lung involvement, the two pediatric cases died from respiratory failure, while the adult case was successfully treated with steroid monotherapy.
“As COVID-19 is a novel disease and its pathogenic mechanism of causing IgA vasculitis is not well understood, every patient who is infected with or recently recovered from COVID-19 and presents with a skin rash or arthralgia should have baseline blood and urine tests done and should be treated promptly to avoid the emergence of irreversible consequences,” the authors wrote in their discussion.
Although case reports cannot prove a cause-and-effect link, the data from the cases in the current review suggest that COVID-19 infection may be an indirect trigger for IgA vasculitis, including cases associated with pulmonary renal syndrome, they said. However, more research is needed, especially on the efficacy of treatments in adults, they concluded.
The study received no outside funding. The researchers had no financial conflicts to disclose.
FROM CUREUS
Folic acid: A recommendation worth making
The US Preventive Services Task Force (USPSTF) recently published a draft recommendation on the use of folic acid before and during pregnancy to prevent fetal neural tube defects.1 This reaffirmation of the 2017 recommendation states that all persons planning to or who could become pregnant should take a daily supplement of folic acid.1,2 This is an “A” recommendation.
Neural tube defects are caused by a failure of the embryonic neural tube to close completely, which should occur in the first 28 days following fertilization. This is why folic acid is most effective if started at least 1 month before conception and continued for the first 2 to 3 months of pregnancy.
An estimated 3000 neural tube defects occur each year in the United States. Spina bifida, anencephaly, and encephalocele occur at respective rates of 3.9, 2.5, and 1.0 in 10,000 live births in the United States, which totals 7.4/10,000.3
Folic acid, if taken before and during pregnancy, can prevent about half of neural tube defects; if taken only during pregnancy, it prevents about one-third. If 50% of neural tube defects could be prevented with folic acid supplements, the number needed to treat (NNT) to prevent 1 case is about 3000.4
The case for supplementation. The recommended daily dose of folic acid is between 0.4 mg (400 μg) and 0.8 mg (800 μg), which is contained in many multivitamin products. Certain enriched cereal grain products in the United States have been fortified with folic acid for more than 2 decades, but it is unknown whether women in the United States are ingesting enough of these fortified foods to provide maximum prevention of neural tube defects. There are no known harms to mother or fetus from folic acid supplementation at recommended levels.
Room for improvement. Only 20% to 40% of people who are pregnant or trying to get pregnant, and 5% to 10% of people with an unplanned pregnancy, take folic acid supplements. Half of all pregnancies in the United States are unplanned.4 This leaves a lot of room for improvement in the prevention of neural tube defects.
An important recommendation, even if you don’t see the results. The NNT to prevent a case of neural tube defect is high; most family physicians providing perinatal care will not prevent a case during their career. And, as with most preventive interventions, we do not see the cases prevented. However, on a population-wide basis, if all women took folic acid as recommended, the number of severe birth defects prevented would be significant—making this simple recommendation worth mentioning to those of reproductive age.
1. USPSTF. Folic acid supplementation to prevent neural tube defects. Published February 21, 2023. Accessed March 22, 2023. https://uspreventiveservicestaskforce.org/home/getfilebytoken/sX6CTKHncTJT2nzmu7yLHh
2. USPSTF. Folic acid for the prevention of neural tube defects: preventive medication. Published January 10, 2017. Accessed March 22, 2023. www.uspreventiveservicestaskforce.org/uspstf/recommendation/folic-acid-for-the-prevention-of-neural-tube-defects-preventive-medication
3. Mai CT, Isenburg JL, Canfield MA, et al. National population-based estimates for major birth defects, 2010-2014. Birth Defects Res. 2019;111:1420-1435. doi: 10.1002/bdr2.1589
4. Viswanathan M, Urrutia RP, Hudson KN, et al. Folic acid supplementation to prevent neural tube defects: a limited systematic review update for the US Preventive Services Task Force. Evidence Synthesis No. 230. Published February 2023. Accessed March 22, 2023. www.uspreventiveservicestaskforce.org/home/getfilebytoken/AjUYoBvpfUBDAFjHeCcfPz
The US Preventive Services Task Force (USPSTF) recently published a draft recommendation on the use of folic acid before and during pregnancy to prevent fetal neural tube defects.1 This reaffirmation of the 2017 recommendation states that all persons planning to or who could become pregnant should take a daily supplement of folic acid.1,2 This is an “A” recommendation.
Neural tube defects are caused by a failure of the embryonic neural tube to close completely, which should occur in the first 28 days following fertilization. This is why folic acid is most effective if started at least 1 month before conception and continued for the first 2 to 3 months of pregnancy.
An estimated 3000 neural tube defects occur each year in the United States. Spina bifida, anencephaly, and encephalocele occur at respective rates of 3.9, 2.5, and 1.0 in 10,000 live births in the United States, which totals 7.4/10,000.3
Folic acid, if taken before and during pregnancy, can prevent about half of neural tube defects; if taken only during pregnancy, it prevents about one-third. If 50% of neural tube defects could be prevented with folic acid supplements, the number needed to treat (NNT) to prevent 1 case is about 3000.4
The case for supplementation. The recommended daily dose of folic acid is between 0.4 mg (400 μg) and 0.8 mg (800 μg), which is contained in many multivitamin products. Certain enriched cereal grain products in the United States have been fortified with folic acid for more than 2 decades, but it is unknown whether women in the United States are ingesting enough of these fortified foods to provide maximum prevention of neural tube defects. There are no known harms to mother or fetus from folic acid supplementation at recommended levels.
Room for improvement. Only 20% to 40% of people who are pregnant or trying to get pregnant, and 5% to 10% of people with an unplanned pregnancy, take folic acid supplements. Half of all pregnancies in the United States are unplanned.4 This leaves a lot of room for improvement in the prevention of neural tube defects.
An important recommendation, even if you don’t see the results. The NNT to prevent a case of neural tube defect is high; most family physicians providing perinatal care will not prevent a case during their career. And, as with most preventive interventions, we do not see the cases prevented. However, on a population-wide basis, if all women took folic acid as recommended, the number of severe birth defects prevented would be significant—making this simple recommendation worth mentioning to those of reproductive age.
The US Preventive Services Task Force (USPSTF) recently published a draft recommendation on the use of folic acid before and during pregnancy to prevent fetal neural tube defects.1 This reaffirmation of the 2017 recommendation states that all persons planning to or who could become pregnant should take a daily supplement of folic acid.1,2 This is an “A” recommendation.
Neural tube defects are caused by a failure of the embryonic neural tube to close completely, which should occur in the first 28 days following fertilization. This is why folic acid is most effective if started at least 1 month before conception and continued for the first 2 to 3 months of pregnancy.
An estimated 3000 neural tube defects occur each year in the United States. Spina bifida, anencephaly, and encephalocele occur at respective rates of 3.9, 2.5, and 1.0 in 10,000 live births in the United States, which totals 7.4/10,000.3
Folic acid, if taken before and during pregnancy, can prevent about half of neural tube defects; if taken only during pregnancy, it prevents about one-third. If 50% of neural tube defects could be prevented with folic acid supplements, the number needed to treat (NNT) to prevent 1 case is about 3000.4
The case for supplementation. The recommended daily dose of folic acid is between 0.4 mg (400 μg) and 0.8 mg (800 μg), which is contained in many multivitamin products. Certain enriched cereal grain products in the United States have been fortified with folic acid for more than 2 decades, but it is unknown whether women in the United States are ingesting enough of these fortified foods to provide maximum prevention of neural tube defects. There are no known harms to mother or fetus from folic acid supplementation at recommended levels.
Room for improvement. Only 20% to 40% of people who are pregnant or trying to get pregnant, and 5% to 10% of people with an unplanned pregnancy, take folic acid supplements. Half of all pregnancies in the United States are unplanned.4 This leaves a lot of room for improvement in the prevention of neural tube defects.
An important recommendation, even if you don’t see the results. The NNT to prevent a case of neural tube defect is high; most family physicians providing perinatal care will not prevent a case during their career. And, as with most preventive interventions, we do not see the cases prevented. However, on a population-wide basis, if all women took folic acid as recommended, the number of severe birth defects prevented would be significant—making this simple recommendation worth mentioning to those of reproductive age.
1. USPSTF. Folic acid supplementation to prevent neural tube defects. Published February 21, 2023. Accessed March 22, 2023. https://uspreventiveservicestaskforce.org/home/getfilebytoken/sX6CTKHncTJT2nzmu7yLHh
2. USPSTF. Folic acid for the prevention of neural tube defects: preventive medication. Published January 10, 2017. Accessed March 22, 2023. www.uspreventiveservicestaskforce.org/uspstf/recommendation/folic-acid-for-the-prevention-of-neural-tube-defects-preventive-medication
3. Mai CT, Isenburg JL, Canfield MA, et al. National population-based estimates for major birth defects, 2010-2014. Birth Defects Res. 2019;111:1420-1435. doi: 10.1002/bdr2.1589
4. Viswanathan M, Urrutia RP, Hudson KN, et al. Folic acid supplementation to prevent neural tube defects: a limited systematic review update for the US Preventive Services Task Force. Evidence Synthesis No. 230. Published February 2023. Accessed March 22, 2023. www.uspreventiveservicestaskforce.org/home/getfilebytoken/AjUYoBvpfUBDAFjHeCcfPz
1. USPSTF. Folic acid supplementation to prevent neural tube defects. Published February 21, 2023. Accessed March 22, 2023. https://uspreventiveservicestaskforce.org/home/getfilebytoken/sX6CTKHncTJT2nzmu7yLHh
2. USPSTF. Folic acid for the prevention of neural tube defects: preventive medication. Published January 10, 2017. Accessed March 22, 2023. www.uspreventiveservicestaskforce.org/uspstf/recommendation/folic-acid-for-the-prevention-of-neural-tube-defects-preventive-medication
3. Mai CT, Isenburg JL, Canfield MA, et al. National population-based estimates for major birth defects, 2010-2014. Birth Defects Res. 2019;111:1420-1435. doi: 10.1002/bdr2.1589
4. Viswanathan M, Urrutia RP, Hudson KN, et al. Folic acid supplementation to prevent neural tube defects: a limited systematic review update for the US Preventive Services Task Force. Evidence Synthesis No. 230. Published February 2023. Accessed March 22, 2023. www.uspreventiveservicestaskforce.org/home/getfilebytoken/AjUYoBvpfUBDAFjHeCcfPz
Advances in the treatment of fetal demise in the second and third trimester
Clinical care for fetal demise is complex and multidimensional, including empathic emotional support for the patient and family members who are experiencing a tragedy, investigation of the cause of the demise, and a plan for emptying the uterus. This editorial narrowly focuses on the options for treatment of fetal demise with the goal of emptying the uterus while minimizing complications.
When planning treatment of fetal demise, focus on fetal size and gestational age
Most guidelines for the treatment of fetal demise use gestational age to guide selection of a treatment.1,2 I believe that fetal size is as important as gestational age for selecting a treatment plan. When considering treatment, there are 2 reasons why fetal size is as important as gestational age:
- The physiologic processes that caused fetal demise may have caused fetal growth restriction, resulting in a fetal size that is 2 or more weeks below expected fetal size for gestational age.
- Fetal demise may have occurred weeks before the diagnosis was made, resulting in gestational age being greater than fetal size. This editorial will use ultrasonography estimate of fetal size in gestational weeks to guide treatment recommendations. When discussing fetal size, we will use the convention of weeks-days (w-d). Twenty-five weeks and zero days gestation is represented as 25w0d.
Treatment in the second and third trimester is a 2-step process
Step 1: Cervical preparation
In most cases of first trimester fetal demise, no cervical preparation is necessary. Cervical dilation with metal dilators followed by uterine evacuation with an appropriately sized vacuum catheter is a highly successful treatment.3 However for second and third trimester fetal demise, it is best to use a 2-step process, beginning with cervical preparation followed by emptying the uterus. For example, at a fetal size of 13w0d to 16w0d, cervical preparation can be achieved by administering a single buccal dose of misoprostol 400 µg 3 to 4 hours prior to uterine evacuation or by inserting a Dilapan-S (Medicem Inc) osmotic cervical dilator 3 to 6 hours prior to uterine evacuation.4-7 At a fetal size of 16w0d to 19w6d, cervical preparation can be achieved by placing osmotic cervical dilators 4 to 6 hours before surgical evacuation and administering buccal misoprostol 400 µg 3 hours before surgical evacuation.8
Alternatively, from 16w0d to 25w0d osmotic cervical dilators can be placed on day 1 of a 2-day process, and the patient can return on day 2 to have the cervical dilators removed followed by surgical evacuation of the uterus. Mifepristone 200 mg oral dose can be administered on day 1 to facilitate cervical preparation. In my practice, I use mifepristone 200 mg on day 1 when the fetal size is ≥20w0d gestation. Options for cervical preparation include use of osmotic dilators, cervical balloons, misoprostol, and/or mifepristone. These options are discussed below. With fetal demise, natural physiologic processes often have caused sufficient cervical softening and dilation that no cervical preparation is necessary and immediate uterine surgical evacuation or induction of labor can be initiated.
Step 2: Emptying the uterus
In the second and third trimesters, the approach to uterine evacuation is based on fetal size. At fetal sizes <25w0d, options for emptying the uterus include surgical evacuation with a vacuum catheter and grasping forceps or induction of labor with misoprostol followed by vaginal birth and expulsion of the placenta. At fetal sizes ˃25w0d gestation, following completion of cervical preparation, the most common approaches to uterine evacuation are induction of labor with misoprostol or oxytocin. Rarely, with a stillbirth at term, some clinicians will select hysterotomy to empty the uterus, avoiding uterine rupture during labor induction for patients at the highest risk, including those with a prior classical cesarean birth or more than 2 prior cesarean births with a low-transverse uterine incision.
Osmotic cervical dilators
The 2 most used cervical dilators are Dilapan-S, a polyacrylate-based hydrogel rod, and laminaria, dried compressed seaweed stipe (stalk) from Laminaria japonica or Laminaria digitata. Dilapan-S rods are available in diameters of 3 mm and 4 mm and rod lengths of 55 mm and 65 mm. Laminaria dilators are available in diameters of 2, 3, 4, 5, 6, 8 and 10 mm and rod length of 60 and 70 mm. Dilapan-S dilators reach near-maximal dilation in approximately 4 to 6 hours but continue to expand over the following 18 hours to achieve a maximum dilation of 3.3 to 3.6 times their dry diameter.9 Laminaria dilators expand to 2.7 to 2.9 times their dry diameter over 24 hours.9
A general rule is that as many dilators as possible should be placed until significant resistance to the placement of additional dilators is encountered.10 In my practice, for fetal size ≥20 weeks’ gestation, I place 2 Dilapan-S rods, 4 mm in diameter, 55 mm in length, and then encircle the Dilapan-S with laminaria rods that are 4 mm in diameter and 60 mm in length. Once cervical resistance to the placement of the 4 mm laminaria rods is observed, I encircle those laminaria with laminaria 2 mm in diameter, filling in the interstices between the 4 mm laminaria. The next day, cervical dilation is routinely ≥3 cm.
In a retrospective study of 491 patients undergoing pregnancy termination after 14 weeks’ gestation, with a mean gestational age of 24 weeks, compared with no osmotic cervical dilators, inserting osmotic cervical dilators the day before initiating misoprostol for induction of labor resulted in a decrease in time to delivery (428 min vs 640 min; P<.001) and a decrease in total misoprostol dose (990 µg vs 1,449 µg; P<.0001).11
Cervical balloons
All clinicians know that a Foley catheter or a Cook cervical ripening balloon can be used for cervical preparation in the third trimester.12,13 The Foley catheter also has been reported to be useful for cervical preparation in the second trimester. In one study of 43 patients 17 to 24 weeks’ gestation scheduled for a second-trimester dilation and evacuation, an intracervical Foley catheter was placed the evening before evacuation, and the balloon was inflated with 30 mL to 50 mL of saline. At the same time, mifepristone 200 mg was administered to the patients.14 The following day, dilation and evacuation was performed. In 72% of cases no additional cervical dilation was required on the day of evacuation. The investigators concluded that if osmotic cervical dilators are not available, the placement of an intracervical Foley catheter plus administration of mifepristone facilitates performance of an evacuation on the following day. If the patient prefers a 1-day procedure, the Foley can be inserted in the morning to facilitate cervical preparation, and the uterus can be evacuated in the afternoon.
Continue to: Misoprostol...
Misoprostol
Misoprostol, a derivative of prostaglandin E1, is useful for both cervical preparation and induction of labor. The dose of misoprostol and the route of administration are major determinants of uterine response.15-19 When administered by an oral route, misoprostol has fast onset and offset of action and often does not cause sustained uterine contractions. Hence, oral misoprostol, at a low dose is useful for cervical ripening, but not as useful for stimulation of sustained uterine contractions for induction of labor. When administered by a buccal or vaginal route, misoprostol has prolonged activity and often results in sustained uterine contractions. At any given dose of misoprostol, buccal and vaginal misoprostol administration are more effective than oral administration in inducing sustained uterine contractions sufficient to empty the uterus.15-19
Mifepristone
Mifepristone, an anti-progestin, is useful for cervical preparation and sensitizing myocytes to the action of uterotonics. Progesterone reduces cell-to-cell communication among uterine myocytes, facilitating uterine quiescence by suppressing connexin 43 and other proteins. Mifepristone blocks the effect of progesterone, inducing the production of myocyte connexin 43, enhancing efficient cell-to-cell communication, permitting uterine myoctes to contract in unison, creating the potential for powerful and sustained contractions.20-23 Randomized clinical trials report that administration of mifepristone 200 mg prior to misoprostol induced labor results in more rapid emptying of the uterus.24-27
It takes time for mifepristone to have its full effect on uterine myocytes. Hence, most protocols recommend waiting 24 hours following mifepristone administration before initiating treatment with an agent to stimulate uterine contractions such as misoprostol or oxytocin. However, preliminary data suggest that partial benefit of mifepristone can be obtained when initiating misoprostol 3 to 5 hours after mifepristone administration.28 In a study of 481 patients undergoing induction of labor in the second or third trimester, the time from initiation of misoprostol to vaginal birth was 15 hours with no mifepristone pretreatment, 13.2 hours if mifepristone was administered 3 to 5 hours before initiating misoprostol, 9.3 hours if mifepristone was administered 24 hours before initiating misoprostol, and 10.5 hours if mifepristone was administered 48 hours before initiating misoprostol.28
Fetal size <25w0d gestation: Cervical preparation and surgical evacuation
For fetal demise at a fetal size less than 25w0d, if clinical experts are available, the best treatment option is cervical preparation followed by surgical evacuation of the uterus using a vacuum catheter and grasping forceps to empty the uterus.29,30 A disadvantage of surgical evacuation of the uterus is that an intact fetus is not available for the patient to hold and mourn, and pathologic examination of an intact fetus is not possible. An alternative approach is cervical preparation followed by induction of labor using misoprostol with the goal of delivering an intact fetus. Although no prospective clinical trials are available comparing these 2 options, retrospective studies have reported that, at fetal size <25w0d gestation, compared with induction of labor, surgical evacuation of the uterus results in fewer complications,30 including fewer cases of retained placenta requiring an unplanned procedure and fewer presumed uterine infections.29
For surgical evacuation of fetal demise with a fetal size of <25w0d gestation, the first step on day 1 is placement of osmotic cervical dilators. In addition to osmotic cervical dilators, if the gestational age or fetal size is ≥19 weeks’ gestation an oral dose of mifepristone 200 mg to facilitate cervical preparation may be considered. On day 2, the osmotic dilators are removed and surgical evacuation is performed. In one randomized study, for pregnancies at 19 to 24 weeks’ gestation, compared with osmotic dilators alone, administration of mifepristone 200 mg at the time of placement of osmotic dilators resulted in fewer procedures that were difficult to complete.31 In some cases, 2 consecutive days of cervical preparation with osmotic dilators may be needed to properly prepare the cervix for uterine evacuation. For example, the cervix of a nulliparous teenage patient may require 2 days of cervical preparation with osmotic dilators to facilitate uterine evacuation. In some cases of fetal demise, the cervix is already dilated to ≥3 cm and surgical evacuation of the uterus or induction of labor can be initiated without the need for cervical preparation.
Continue to: Fetal size 14w0d to 28w6d gestation: Cervical preparation and induction of labor...
Fetal size 14w0d to 28w6d gestation: Cervical preparation and induction of labor
Treatment of fetal demise at 14w0d to 28w6d gestation with the goal of the vaginal birth of an intact fetus is optimized by the administration of mifepristone for cervical preparation followed by induction of labor with misoprostol.26,27
In one clinical trial, 66 patients with fetal demise between 14w0d and 28w6d gestation were randomly assigned to receive mifepristone 200 mg or placebo followed 24 to 48 hours later with initiation of misoprostol induction of labor.26 Among the patients from 14w0d to 24 weeks’ gestation, the misoprostol dose was 400 µg vaginally every 6 hours. For patients from 24w0d to 28 weeks’ gestation, the misoprostol dose was 200 µg vaginally every 4 hours. At 24 hours, a consultant obstetrician determined if additional misoprostol should be given. The median time from initiation of misoprostol to birth for the patients in the mifepristone and placebo groups was 6.8 hours and 10.5 hours (P=.002).
Compared with the patients in the placebo-misoprostol group, the patients in the mifepristone-misoprostol group required fewer doses of misoprostol (2.1 vs 3.4; P= .002) and a lower total dose of misoprostol (768 µg vs 1,182 µg; P=.003). All patients in the mifepristone group delivered within 24 hours. By contrast, 13% of the patients in the placebo group delivered more than 24 hours after the initiation of misoprostol treatment. Five patients were readmitted with retained products of conception needing suction curettage, 4 in the placebo group and 1 in the mifepristone group.26
In a second clinical trial, 105 patients with fetal demise after 20 weeks of gestation were randomly assigned to receive mifepristone 200 mg or placebo.27 In this study, 86% of the patients were ≥26w0d gestation, with a mean gestational age of approximately 32w2d. Thirty-six to 48 hours later, misoprostol induction of labor was initiated. Among the patients from 20 to 25 completed weeks of gestation, the misoprostoldose was 100 µg vaginally every 6 hours for a maximum of 4 doses. For patients from ≥26 weeks’ gestation, the misoprostol dose was 50 µg vaginally every 4 hours for a maximum of 6 doses. The median times from initiation of misoprostol to birth for the patients in the mifepristone and placebo groups were 9.8 hours and 16.3 hours, respectively (P=.001). Compared with the patients in the placebo-misoprostol group, the patients in the mifepristone-misoprostol group required a lower total dose of misoprostol (110 µg vs 198 µg; P<.001). Delivery within 24 hours following initiation of misoprostol occurred in 93% and 73% of the patients in the mifepristone and placebo groups, respectively (P<.001). Compared with patients in the mifepristone group, shivering occurred more frequently among patients in the placebo group (7.5% vs 19.2%; P=.09), likely because they received greater doses of misoprostol.27
Fetal size ≥29w0d gestation
At a fetal size ≥29w0d gestation, if the cervix is ripe with a Bishop score of ≥7, oxytocin induction of labor is often used as a first-line treatment. If the cervix is not ripe, misoprostol induction of labor may be considered at doses less than those used in the second trimester of pregnancy.32 TABLES 1,1, 26, 33–36 2,37 and 337 summarize regimens proposed for fetal size ≥29w0d. One regimen begins with an initial misoprostol dose of 50 µg. If adequate uterine contractions occur, the 50 µg dose is repeated every 4 hours up to 6 total doses. If contractions are inadequate, the dose can be increased to 100 µg every 4 hours for 5 additional doses.
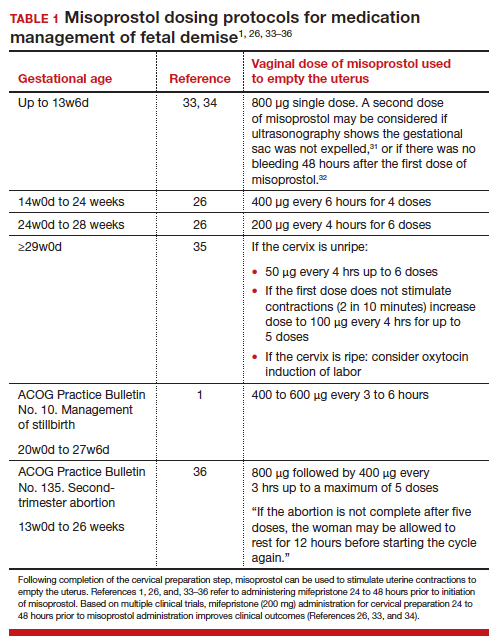
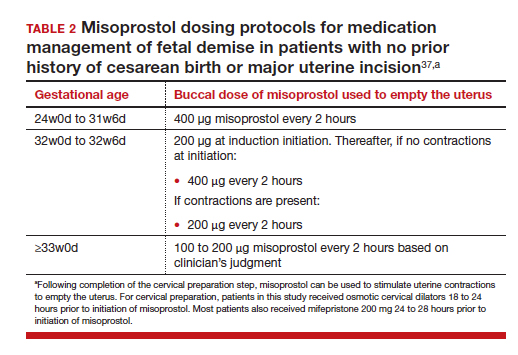

For fetal demise after 28w0d gestation, the
A multidisciplinary approach can optimize compassionate care
There are many gaps in the holistic care of patients and partners experiencing fetal demise. Patients with fetal demise often report that they did not receive sufficient information about the cause of the demise and wanted more opportunity to be involved in decision making about their care.39 The patient’s partner often reports feeling unacknowledged as a grieving parent.40 Fetal demise is experienced by many patients as a tragedy, triggering feelings of grief, anger, denial, anxiety and depression, sometimes resulting in isolation and substance misuse.
Using a 5-round Delphi process, experts identified 8 core goals in the care of patients with fetal demise:
- reduce stigma
- provide respectful care
- involve patients in care planning
- attempt to provide an explanation for the demise1
- acknowledge the depth of the grief response and provide emotional support
- offer information about ongoing psychological support
- provide information about future pregnancy planning
- provide opportunities for specialized training and support for care providers.41
Management of stillbirth is optimized by a multidisciplinary approach that includes the expert care of obstetrician-gynecologists, obstetric nurses, anesthesiologists, and expert consultation from social work, chaplaincy, and pathology. A heart-to-heart connection between clinician and patient is a key component of stillbirth care. ●
- American College of Obstetricians and Gynecologists. Management of stillbirth. ACOG Obstetric Care Consensus. No. 10. Obstet Gynecol. 2020;135:e110-132.
- Tsakiridis I, Giouleka S, Mamopoulos A, et al. Investigation and management of stillbirths: a descriptive review of major guidelines. J Perinat Med. 2022;50:796-813.
- Spingler T, Sonek J, Hoopman M, et al. Complication rate after termination of pregnancy due to fetal defects. Ultrasound Obstet Gynecol. 2023;Epub January 7.
- Goldberg AB, Drey EA, Whitaker AK, et al. Misoprostol compared with laminaria before early second-trimester surgical abortion: a randomized trial. Obstet Gynecol. 2005;106:234-241.
- Meirik O, My Huong NT, Piaggio G, et al. WHOR-GoP-MoF Regulation. Complications of first trimester abortion by vacuum aspiration after cervical preparation with and without misoprostol: a multicentre randomised trial. Lancet. 2012;379(9829):1817-1824.
- Bartz D, Maurer R, Allen RH, et al. Buccal misoprostol compared with synthetic osmotic cervical dilator before surgical abortion: a randomized controlled trial. Obstet Gynecol. 2013;122:57-63.
- Ngo LL, Mokashi M, Janiak E, et al. Acute complications with same-day versus overnight cervical preparation before dilation and evacuation at 14 to 16 weeks. Contraception. 2023;117:61-66.
- Kim CS, Dragoman M, Prosch L, et al. Same-day compared with overnight cervical preparation before dilation and evacuation between 16 and 19 6/7 weeks of gestation: a randomized controlled trial. Obstet Gynecol. 2022;139:1141-1144.
- Drunecky T, Reidingerova M, Plisova M, et al. Experimental comparison of properties of natural and synthetic osmotic dilators. Arch Gynecol Obstet. 2015;292:349-354.
- Hern WM. Laminaria versus Dilapan osmotic cervical dilators for outpatient dilation and evacuation abortion: randomized cohort comparison of 1001 patients. Am J Obstet Gynecol. 1994;171:1324-1328.
- Berthold C, Gomes David M, Gabriel P, et al. Effect of the addition of osmotic dilators to medical induction of labor abortion: a before-and-after study. Eur J Obstet Gynecol. 2020;244:185-189.
- Kemper JI, Li W, Goni S, et al. Foley catheter vs oral misoprostol for induction of labor: individual participant data meta-analysis. Ultrasound Obstet Gynecol. 2021;57:215-223.
- Attalli E, Kern Guy, Fouks Y, et al. Labor induction in third trimester non-viable fetus. J Matern Fetal Neonatal Med. 2022;Epub October 1.
- Fessehaye Sium A, Prager S, Wolderufael M, et al. Foley catheter for cervical preparation prior to second trimester dilation and evacuation: a supply-based alternative for surgical abortion: a case series. Contracept X. 2022;4:100085.
- Zieman M, Fong SK, Benowitz NL, et al. Absorption kinetics of misoprostol with oral or vaginal administration. Obstet Gynecol. 1997;90:88-92.
- Gemzell-Danilesson K, Marions L, Rodriguez A, et al. Comparison between oral and vaginal administration of misoprostol on uterine contractility. Obstet Gynecol. 1999;93:275-280.
- Aronsson A, Bygdeman M, Gemzell-Danielsson K. Effects of misoprostol on uterine contractility following different routes of administration. Hum Reprod. 2004;19:81-84.
- Meckstroth KR, Whitaker AK, Bertisch S, et al. Misoprostol administered by epithelial routes. Drug absorption and uterine response. Obstet Gynecol. 2006;108:582-590.
- Barbieri RL. Misoprostol: clinical pharmacology in obstetrics and gynecology. OBG Manag. 2022;34:8-10, 12.
- Andersen J, Grine E, Eng L, et al. Expression of connexin-43 in human myometrium and leiomyoma. Am J Obstet Gynecol. 1993;169:1266-1276.
- Ou CW, Orsino A, Lye SJ. Expression of connexin-43 and connexin-26 in the rat myometrium during pregnancy and labor is differentially regulated by mechanical and hormonal signals. Endocrinology. 1997;138:5398-5407.
- Petrocelli T, Lye SJ. Regulation of transcripts encoding the myometrial gap junction protein, connexin-43, by estrogen and progesterone. Endocrinology. 1993;133:284-290.
- Barbieri RL. Mifepristone for the treatment of miscarriage and fetal demise. OBG Manag. 2022;34:811, 15.
- Kapp N, Borgatta L, Stubblefield P, et al. Mifepristone in second-trimester medical abortion. Obstet Gynecol. 2007;110:1304-1310.
- Ngoc NTN, Shochet T, Raghavan S, et al. Mifepristone and misoprostol compared with misoprostol alone for second trimester abortion: a randomized controlled trial. Obstet Gynecol. 2011;118:601608.
- Allanson ER, Copson S, Spilsbury K, et al. Pretreatment with mifepristone compared with misoprostol alone for delivery after fetal death between 14 and 28 weeks of gestation. Obstet Gynecol. 2021;137:801-809.
- Chaudhuri P, Datta S. Mifepristone and misoprostol compared with misoprostol alone for induction of labor in intrauterine fetal death: a randomized trial. J Obstet Gynaecol Res. 2015;41:1884-1890.
- Prodan N, Breisch J, Hoopman M, et al. Dosing interval between mifepristone and misoprostol in second and third trimester termination. Arch Gynecol Obstet. 2019;299:675-679.
- Edlow AG, Hou MY, Maurer R, et al. Uterine evacuation for second trimester fetal death and maternal morbidity. Obstet Gynecol. 2011;117:1-10.
- Bryan AG, Grimes DA, Garrett JM, et al. Second-trimester abortion for fetal anomalies or fetal death. Obstet Gynecol. 2011;117:788-792.
- Goldberg AB, Fortin JA, Drey EA, et al. Cervical preparation before dilation and evacuation using adjunctive misoprostol or mifepristone compared with overnight osmotic dilators alone. Obstet Gynecol. 2015;126:599-609.
- Gomez-Ponce de Leon R, Wing D, Fiala C. Misoprostol for intrauterine fetal death. Int J Gynaecol Obstet. 2007;99(suppl 2):S190-S193.
- Schreiber C, Creinin MD, Atrio J, et al. Mifepristone pretreatment for the medical management of early pregnancy loss. N Engl J Med. 2018;378:2161-2170.
- Chu JJ, Devall AJ, Beeson LE, et al. Mifepristone and misoprostol versus misoprostol alone for the management of missed miscarriage (MifeMiso): a randomised, double-blind, placebo-controlled trial. Lancet. 2020;396:770-778.
- Gomez-Ponce de Leon R, Wing D, Fiala C. Misoprostol for intrauterine fetal death. Int J Gynaecol Obstet. 2007;99(suppl 2):S190-S193.
- American College of Obstetricians and Gynecologists. Second-trimester abortion. Practice Bulletin No. 135. Obstet Gynecol. 2013;121:1394-1406.
- Wingo E, Raifman S, Landau C, et al. Mifepristone-misoprostol versus misoprostol-alone regimen for medication abortion at ≥ 24 weeks gestation. Contraception. Appendix 1. 2020;102:99-103.
- American College of Obstetricians and Gynecologists. Vaginal birth after cesarean delivery. ACOG Practice Bulletin No. 205. Obstet Gynecol. 2019;133:e110-e127.
- Atkins B, Blencowe H, Boyle FM, et al. Is care of stillborn babies and their parents respectful? Results from an international online survey. BJOG. 2022;129:1731-1739.
- Haezell AEP, Siassakos D, Blencowe H, et al. Stillbirths: economic and psychosocial consequences. Lancet. 2016;387(10018):604-616.
- Shakespeare C, Merriel A, Bakhbakhi D, et al. The RESPECT Study for consensus on global bereavement care after stillbirth. Int J Gynaecol Obstet. 2020;149:137-147.
Clinical care for fetal demise is complex and multidimensional, including empathic emotional support for the patient and family members who are experiencing a tragedy, investigation of the cause of the demise, and a plan for emptying the uterus. This editorial narrowly focuses on the options for treatment of fetal demise with the goal of emptying the uterus while minimizing complications.
When planning treatment of fetal demise, focus on fetal size and gestational age
Most guidelines for the treatment of fetal demise use gestational age to guide selection of a treatment.1,2 I believe that fetal size is as important as gestational age for selecting a treatment plan. When considering treatment, there are 2 reasons why fetal size is as important as gestational age:
- The physiologic processes that caused fetal demise may have caused fetal growth restriction, resulting in a fetal size that is 2 or more weeks below expected fetal size for gestational age.
- Fetal demise may have occurred weeks before the diagnosis was made, resulting in gestational age being greater than fetal size. This editorial will use ultrasonography estimate of fetal size in gestational weeks to guide treatment recommendations. When discussing fetal size, we will use the convention of weeks-days (w-d). Twenty-five weeks and zero days gestation is represented as 25w0d.
Treatment in the second and third trimester is a 2-step process
Step 1: Cervical preparation
In most cases of first trimester fetal demise, no cervical preparation is necessary. Cervical dilation with metal dilators followed by uterine evacuation with an appropriately sized vacuum catheter is a highly successful treatment.3 However for second and third trimester fetal demise, it is best to use a 2-step process, beginning with cervical preparation followed by emptying the uterus. For example, at a fetal size of 13w0d to 16w0d, cervical preparation can be achieved by administering a single buccal dose of misoprostol 400 µg 3 to 4 hours prior to uterine evacuation or by inserting a Dilapan-S (Medicem Inc) osmotic cervical dilator 3 to 6 hours prior to uterine evacuation.4-7 At a fetal size of 16w0d to 19w6d, cervical preparation can be achieved by placing osmotic cervical dilators 4 to 6 hours before surgical evacuation and administering buccal misoprostol 400 µg 3 hours before surgical evacuation.8
Alternatively, from 16w0d to 25w0d osmotic cervical dilators can be placed on day 1 of a 2-day process, and the patient can return on day 2 to have the cervical dilators removed followed by surgical evacuation of the uterus. Mifepristone 200 mg oral dose can be administered on day 1 to facilitate cervical preparation. In my practice, I use mifepristone 200 mg on day 1 when the fetal size is ≥20w0d gestation. Options for cervical preparation include use of osmotic dilators, cervical balloons, misoprostol, and/or mifepristone. These options are discussed below. With fetal demise, natural physiologic processes often have caused sufficient cervical softening and dilation that no cervical preparation is necessary and immediate uterine surgical evacuation or induction of labor can be initiated.
Step 2: Emptying the uterus
In the second and third trimesters, the approach to uterine evacuation is based on fetal size. At fetal sizes <25w0d, options for emptying the uterus include surgical evacuation with a vacuum catheter and grasping forceps or induction of labor with misoprostol followed by vaginal birth and expulsion of the placenta. At fetal sizes ˃25w0d gestation, following completion of cervical preparation, the most common approaches to uterine evacuation are induction of labor with misoprostol or oxytocin. Rarely, with a stillbirth at term, some clinicians will select hysterotomy to empty the uterus, avoiding uterine rupture during labor induction for patients at the highest risk, including those with a prior classical cesarean birth or more than 2 prior cesarean births with a low-transverse uterine incision.
Osmotic cervical dilators
The 2 most used cervical dilators are Dilapan-S, a polyacrylate-based hydrogel rod, and laminaria, dried compressed seaweed stipe (stalk) from Laminaria japonica or Laminaria digitata. Dilapan-S rods are available in diameters of 3 mm and 4 mm and rod lengths of 55 mm and 65 mm. Laminaria dilators are available in diameters of 2, 3, 4, 5, 6, 8 and 10 mm and rod length of 60 and 70 mm. Dilapan-S dilators reach near-maximal dilation in approximately 4 to 6 hours but continue to expand over the following 18 hours to achieve a maximum dilation of 3.3 to 3.6 times their dry diameter.9 Laminaria dilators expand to 2.7 to 2.9 times their dry diameter over 24 hours.9
A general rule is that as many dilators as possible should be placed until significant resistance to the placement of additional dilators is encountered.10 In my practice, for fetal size ≥20 weeks’ gestation, I place 2 Dilapan-S rods, 4 mm in diameter, 55 mm in length, and then encircle the Dilapan-S with laminaria rods that are 4 mm in diameter and 60 mm in length. Once cervical resistance to the placement of the 4 mm laminaria rods is observed, I encircle those laminaria with laminaria 2 mm in diameter, filling in the interstices between the 4 mm laminaria. The next day, cervical dilation is routinely ≥3 cm.
In a retrospective study of 491 patients undergoing pregnancy termination after 14 weeks’ gestation, with a mean gestational age of 24 weeks, compared with no osmotic cervical dilators, inserting osmotic cervical dilators the day before initiating misoprostol for induction of labor resulted in a decrease in time to delivery (428 min vs 640 min; P<.001) and a decrease in total misoprostol dose (990 µg vs 1,449 µg; P<.0001).11
Cervical balloons
All clinicians know that a Foley catheter or a Cook cervical ripening balloon can be used for cervical preparation in the third trimester.12,13 The Foley catheter also has been reported to be useful for cervical preparation in the second trimester. In one study of 43 patients 17 to 24 weeks’ gestation scheduled for a second-trimester dilation and evacuation, an intracervical Foley catheter was placed the evening before evacuation, and the balloon was inflated with 30 mL to 50 mL of saline. At the same time, mifepristone 200 mg was administered to the patients.14 The following day, dilation and evacuation was performed. In 72% of cases no additional cervical dilation was required on the day of evacuation. The investigators concluded that if osmotic cervical dilators are not available, the placement of an intracervical Foley catheter plus administration of mifepristone facilitates performance of an evacuation on the following day. If the patient prefers a 1-day procedure, the Foley can be inserted in the morning to facilitate cervical preparation, and the uterus can be evacuated in the afternoon.
Continue to: Misoprostol...
Misoprostol
Misoprostol, a derivative of prostaglandin E1, is useful for both cervical preparation and induction of labor. The dose of misoprostol and the route of administration are major determinants of uterine response.15-19 When administered by an oral route, misoprostol has fast onset and offset of action and often does not cause sustained uterine contractions. Hence, oral misoprostol, at a low dose is useful for cervical ripening, but not as useful for stimulation of sustained uterine contractions for induction of labor. When administered by a buccal or vaginal route, misoprostol has prolonged activity and often results in sustained uterine contractions. At any given dose of misoprostol, buccal and vaginal misoprostol administration are more effective than oral administration in inducing sustained uterine contractions sufficient to empty the uterus.15-19
Mifepristone
Mifepristone, an anti-progestin, is useful for cervical preparation and sensitizing myocytes to the action of uterotonics. Progesterone reduces cell-to-cell communication among uterine myocytes, facilitating uterine quiescence by suppressing connexin 43 and other proteins. Mifepristone blocks the effect of progesterone, inducing the production of myocyte connexin 43, enhancing efficient cell-to-cell communication, permitting uterine myoctes to contract in unison, creating the potential for powerful and sustained contractions.20-23 Randomized clinical trials report that administration of mifepristone 200 mg prior to misoprostol induced labor results in more rapid emptying of the uterus.24-27
It takes time for mifepristone to have its full effect on uterine myocytes. Hence, most protocols recommend waiting 24 hours following mifepristone administration before initiating treatment with an agent to stimulate uterine contractions such as misoprostol or oxytocin. However, preliminary data suggest that partial benefit of mifepristone can be obtained when initiating misoprostol 3 to 5 hours after mifepristone administration.28 In a study of 481 patients undergoing induction of labor in the second or third trimester, the time from initiation of misoprostol to vaginal birth was 15 hours with no mifepristone pretreatment, 13.2 hours if mifepristone was administered 3 to 5 hours before initiating misoprostol, 9.3 hours if mifepristone was administered 24 hours before initiating misoprostol, and 10.5 hours if mifepristone was administered 48 hours before initiating misoprostol.28
Fetal size <25w0d gestation: Cervical preparation and surgical evacuation
For fetal demise at a fetal size less than 25w0d, if clinical experts are available, the best treatment option is cervical preparation followed by surgical evacuation of the uterus using a vacuum catheter and grasping forceps to empty the uterus.29,30 A disadvantage of surgical evacuation of the uterus is that an intact fetus is not available for the patient to hold and mourn, and pathologic examination of an intact fetus is not possible. An alternative approach is cervical preparation followed by induction of labor using misoprostol with the goal of delivering an intact fetus. Although no prospective clinical trials are available comparing these 2 options, retrospective studies have reported that, at fetal size <25w0d gestation, compared with induction of labor, surgical evacuation of the uterus results in fewer complications,30 including fewer cases of retained placenta requiring an unplanned procedure and fewer presumed uterine infections.29
For surgical evacuation of fetal demise with a fetal size of <25w0d gestation, the first step on day 1 is placement of osmotic cervical dilators. In addition to osmotic cervical dilators, if the gestational age or fetal size is ≥19 weeks’ gestation an oral dose of mifepristone 200 mg to facilitate cervical preparation may be considered. On day 2, the osmotic dilators are removed and surgical evacuation is performed. In one randomized study, for pregnancies at 19 to 24 weeks’ gestation, compared with osmotic dilators alone, administration of mifepristone 200 mg at the time of placement of osmotic dilators resulted in fewer procedures that were difficult to complete.31 In some cases, 2 consecutive days of cervical preparation with osmotic dilators may be needed to properly prepare the cervix for uterine evacuation. For example, the cervix of a nulliparous teenage patient may require 2 days of cervical preparation with osmotic dilators to facilitate uterine evacuation. In some cases of fetal demise, the cervix is already dilated to ≥3 cm and surgical evacuation of the uterus or induction of labor can be initiated without the need for cervical preparation.
Continue to: Fetal size 14w0d to 28w6d gestation: Cervical preparation and induction of labor...
Fetal size 14w0d to 28w6d gestation: Cervical preparation and induction of labor
Treatment of fetal demise at 14w0d to 28w6d gestation with the goal of the vaginal birth of an intact fetus is optimized by the administration of mifepristone for cervical preparation followed by induction of labor with misoprostol.26,27
In one clinical trial, 66 patients with fetal demise between 14w0d and 28w6d gestation were randomly assigned to receive mifepristone 200 mg or placebo followed 24 to 48 hours later with initiation of misoprostol induction of labor.26 Among the patients from 14w0d to 24 weeks’ gestation, the misoprostol dose was 400 µg vaginally every 6 hours. For patients from 24w0d to 28 weeks’ gestation, the misoprostol dose was 200 µg vaginally every 4 hours. At 24 hours, a consultant obstetrician determined if additional misoprostol should be given. The median time from initiation of misoprostol to birth for the patients in the mifepristone and placebo groups was 6.8 hours and 10.5 hours (P=.002).
Compared with the patients in the placebo-misoprostol group, the patients in the mifepristone-misoprostol group required fewer doses of misoprostol (2.1 vs 3.4; P= .002) and a lower total dose of misoprostol (768 µg vs 1,182 µg; P=.003). All patients in the mifepristone group delivered within 24 hours. By contrast, 13% of the patients in the placebo group delivered more than 24 hours after the initiation of misoprostol treatment. Five patients were readmitted with retained products of conception needing suction curettage, 4 in the placebo group and 1 in the mifepristone group.26
In a second clinical trial, 105 patients with fetal demise after 20 weeks of gestation were randomly assigned to receive mifepristone 200 mg or placebo.27 In this study, 86% of the patients were ≥26w0d gestation, with a mean gestational age of approximately 32w2d. Thirty-six to 48 hours later, misoprostol induction of labor was initiated. Among the patients from 20 to 25 completed weeks of gestation, the misoprostoldose was 100 µg vaginally every 6 hours for a maximum of 4 doses. For patients from ≥26 weeks’ gestation, the misoprostol dose was 50 µg vaginally every 4 hours for a maximum of 6 doses. The median times from initiation of misoprostol to birth for the patients in the mifepristone and placebo groups were 9.8 hours and 16.3 hours, respectively (P=.001). Compared with the patients in the placebo-misoprostol group, the patients in the mifepristone-misoprostol group required a lower total dose of misoprostol (110 µg vs 198 µg; P<.001). Delivery within 24 hours following initiation of misoprostol occurred in 93% and 73% of the patients in the mifepristone and placebo groups, respectively (P<.001). Compared with patients in the mifepristone group, shivering occurred more frequently among patients in the placebo group (7.5% vs 19.2%; P=.09), likely because they received greater doses of misoprostol.27
Fetal size ≥29w0d gestation
At a fetal size ≥29w0d gestation, if the cervix is ripe with a Bishop score of ≥7, oxytocin induction of labor is often used as a first-line treatment. If the cervix is not ripe, misoprostol induction of labor may be considered at doses less than those used in the second trimester of pregnancy.32 TABLES 1,1, 26, 33–36 2,37 and 337 summarize regimens proposed for fetal size ≥29w0d. One regimen begins with an initial misoprostol dose of 50 µg. If adequate uterine contractions occur, the 50 µg dose is repeated every 4 hours up to 6 total doses. If contractions are inadequate, the dose can be increased to 100 µg every 4 hours for 5 additional doses.



For fetal demise after 28w0d gestation, the
A multidisciplinary approach can optimize compassionate care
There are many gaps in the holistic care of patients and partners experiencing fetal demise. Patients with fetal demise often report that they did not receive sufficient information about the cause of the demise and wanted more opportunity to be involved in decision making about their care.39 The patient’s partner often reports feeling unacknowledged as a grieving parent.40 Fetal demise is experienced by many patients as a tragedy, triggering feelings of grief, anger, denial, anxiety and depression, sometimes resulting in isolation and substance misuse.
Using a 5-round Delphi process, experts identified 8 core goals in the care of patients with fetal demise:
- reduce stigma
- provide respectful care
- involve patients in care planning
- attempt to provide an explanation for the demise1
- acknowledge the depth of the grief response and provide emotional support
- offer information about ongoing psychological support
- provide information about future pregnancy planning
- provide opportunities for specialized training and support for care providers.41
Management of stillbirth is optimized by a multidisciplinary approach that includes the expert care of obstetrician-gynecologists, obstetric nurses, anesthesiologists, and expert consultation from social work, chaplaincy, and pathology. A heart-to-heart connection between clinician and patient is a key component of stillbirth care. ●
Clinical care for fetal demise is complex and multidimensional, including empathic emotional support for the patient and family members who are experiencing a tragedy, investigation of the cause of the demise, and a plan for emptying the uterus. This editorial narrowly focuses on the options for treatment of fetal demise with the goal of emptying the uterus while minimizing complications.
When planning treatment of fetal demise, focus on fetal size and gestational age
Most guidelines for the treatment of fetal demise use gestational age to guide selection of a treatment.1,2 I believe that fetal size is as important as gestational age for selecting a treatment plan. When considering treatment, there are 2 reasons why fetal size is as important as gestational age:
- The physiologic processes that caused fetal demise may have caused fetal growth restriction, resulting in a fetal size that is 2 or more weeks below expected fetal size for gestational age.
- Fetal demise may have occurred weeks before the diagnosis was made, resulting in gestational age being greater than fetal size. This editorial will use ultrasonography estimate of fetal size in gestational weeks to guide treatment recommendations. When discussing fetal size, we will use the convention of weeks-days (w-d). Twenty-five weeks and zero days gestation is represented as 25w0d.
Treatment in the second and third trimester is a 2-step process
Step 1: Cervical preparation
In most cases of first trimester fetal demise, no cervical preparation is necessary. Cervical dilation with metal dilators followed by uterine evacuation with an appropriately sized vacuum catheter is a highly successful treatment.3 However for second and third trimester fetal demise, it is best to use a 2-step process, beginning with cervical preparation followed by emptying the uterus. For example, at a fetal size of 13w0d to 16w0d, cervical preparation can be achieved by administering a single buccal dose of misoprostol 400 µg 3 to 4 hours prior to uterine evacuation or by inserting a Dilapan-S (Medicem Inc) osmotic cervical dilator 3 to 6 hours prior to uterine evacuation.4-7 At a fetal size of 16w0d to 19w6d, cervical preparation can be achieved by placing osmotic cervical dilators 4 to 6 hours before surgical evacuation and administering buccal misoprostol 400 µg 3 hours before surgical evacuation.8
Alternatively, from 16w0d to 25w0d osmotic cervical dilators can be placed on day 1 of a 2-day process, and the patient can return on day 2 to have the cervical dilators removed followed by surgical evacuation of the uterus. Mifepristone 200 mg oral dose can be administered on day 1 to facilitate cervical preparation. In my practice, I use mifepristone 200 mg on day 1 when the fetal size is ≥20w0d gestation. Options for cervical preparation include use of osmotic dilators, cervical balloons, misoprostol, and/or mifepristone. These options are discussed below. With fetal demise, natural physiologic processes often have caused sufficient cervical softening and dilation that no cervical preparation is necessary and immediate uterine surgical evacuation or induction of labor can be initiated.
Step 2: Emptying the uterus
In the second and third trimesters, the approach to uterine evacuation is based on fetal size. At fetal sizes <25w0d, options for emptying the uterus include surgical evacuation with a vacuum catheter and grasping forceps or induction of labor with misoprostol followed by vaginal birth and expulsion of the placenta. At fetal sizes ˃25w0d gestation, following completion of cervical preparation, the most common approaches to uterine evacuation are induction of labor with misoprostol or oxytocin. Rarely, with a stillbirth at term, some clinicians will select hysterotomy to empty the uterus, avoiding uterine rupture during labor induction for patients at the highest risk, including those with a prior classical cesarean birth or more than 2 prior cesarean births with a low-transverse uterine incision.
Osmotic cervical dilators
The 2 most used cervical dilators are Dilapan-S, a polyacrylate-based hydrogel rod, and laminaria, dried compressed seaweed stipe (stalk) from Laminaria japonica or Laminaria digitata. Dilapan-S rods are available in diameters of 3 mm and 4 mm and rod lengths of 55 mm and 65 mm. Laminaria dilators are available in diameters of 2, 3, 4, 5, 6, 8 and 10 mm and rod length of 60 and 70 mm. Dilapan-S dilators reach near-maximal dilation in approximately 4 to 6 hours but continue to expand over the following 18 hours to achieve a maximum dilation of 3.3 to 3.6 times their dry diameter.9 Laminaria dilators expand to 2.7 to 2.9 times their dry diameter over 24 hours.9
A general rule is that as many dilators as possible should be placed until significant resistance to the placement of additional dilators is encountered.10 In my practice, for fetal size ≥20 weeks’ gestation, I place 2 Dilapan-S rods, 4 mm in diameter, 55 mm in length, and then encircle the Dilapan-S with laminaria rods that are 4 mm in diameter and 60 mm in length. Once cervical resistance to the placement of the 4 mm laminaria rods is observed, I encircle those laminaria with laminaria 2 mm in diameter, filling in the interstices between the 4 mm laminaria. The next day, cervical dilation is routinely ≥3 cm.
In a retrospective study of 491 patients undergoing pregnancy termination after 14 weeks’ gestation, with a mean gestational age of 24 weeks, compared with no osmotic cervical dilators, inserting osmotic cervical dilators the day before initiating misoprostol for induction of labor resulted in a decrease in time to delivery (428 min vs 640 min; P<.001) and a decrease in total misoprostol dose (990 µg vs 1,449 µg; P<.0001).11
Cervical balloons
All clinicians know that a Foley catheter or a Cook cervical ripening balloon can be used for cervical preparation in the third trimester.12,13 The Foley catheter also has been reported to be useful for cervical preparation in the second trimester. In one study of 43 patients 17 to 24 weeks’ gestation scheduled for a second-trimester dilation and evacuation, an intracervical Foley catheter was placed the evening before evacuation, and the balloon was inflated with 30 mL to 50 mL of saline. At the same time, mifepristone 200 mg was administered to the patients.14 The following day, dilation and evacuation was performed. In 72% of cases no additional cervical dilation was required on the day of evacuation. The investigators concluded that if osmotic cervical dilators are not available, the placement of an intracervical Foley catheter plus administration of mifepristone facilitates performance of an evacuation on the following day. If the patient prefers a 1-day procedure, the Foley can be inserted in the morning to facilitate cervical preparation, and the uterus can be evacuated in the afternoon.
Continue to: Misoprostol...
Misoprostol
Misoprostol, a derivative of prostaglandin E1, is useful for both cervical preparation and induction of labor. The dose of misoprostol and the route of administration are major determinants of uterine response.15-19 When administered by an oral route, misoprostol has fast onset and offset of action and often does not cause sustained uterine contractions. Hence, oral misoprostol, at a low dose is useful for cervical ripening, but not as useful for stimulation of sustained uterine contractions for induction of labor. When administered by a buccal or vaginal route, misoprostol has prolonged activity and often results in sustained uterine contractions. At any given dose of misoprostol, buccal and vaginal misoprostol administration are more effective than oral administration in inducing sustained uterine contractions sufficient to empty the uterus.15-19
Mifepristone
Mifepristone, an anti-progestin, is useful for cervical preparation and sensitizing myocytes to the action of uterotonics. Progesterone reduces cell-to-cell communication among uterine myocytes, facilitating uterine quiescence by suppressing connexin 43 and other proteins. Mifepristone blocks the effect of progesterone, inducing the production of myocyte connexin 43, enhancing efficient cell-to-cell communication, permitting uterine myoctes to contract in unison, creating the potential for powerful and sustained contractions.20-23 Randomized clinical trials report that administration of mifepristone 200 mg prior to misoprostol induced labor results in more rapid emptying of the uterus.24-27
It takes time for mifepristone to have its full effect on uterine myocytes. Hence, most protocols recommend waiting 24 hours following mifepristone administration before initiating treatment with an agent to stimulate uterine contractions such as misoprostol or oxytocin. However, preliminary data suggest that partial benefit of mifepristone can be obtained when initiating misoprostol 3 to 5 hours after mifepristone administration.28 In a study of 481 patients undergoing induction of labor in the second or third trimester, the time from initiation of misoprostol to vaginal birth was 15 hours with no mifepristone pretreatment, 13.2 hours if mifepristone was administered 3 to 5 hours before initiating misoprostol, 9.3 hours if mifepristone was administered 24 hours before initiating misoprostol, and 10.5 hours if mifepristone was administered 48 hours before initiating misoprostol.28
Fetal size <25w0d gestation: Cervical preparation and surgical evacuation
For fetal demise at a fetal size less than 25w0d, if clinical experts are available, the best treatment option is cervical preparation followed by surgical evacuation of the uterus using a vacuum catheter and grasping forceps to empty the uterus.29,30 A disadvantage of surgical evacuation of the uterus is that an intact fetus is not available for the patient to hold and mourn, and pathologic examination of an intact fetus is not possible. An alternative approach is cervical preparation followed by induction of labor using misoprostol with the goal of delivering an intact fetus. Although no prospective clinical trials are available comparing these 2 options, retrospective studies have reported that, at fetal size <25w0d gestation, compared with induction of labor, surgical evacuation of the uterus results in fewer complications,30 including fewer cases of retained placenta requiring an unplanned procedure and fewer presumed uterine infections.29
For surgical evacuation of fetal demise with a fetal size of <25w0d gestation, the first step on day 1 is placement of osmotic cervical dilators. In addition to osmotic cervical dilators, if the gestational age or fetal size is ≥19 weeks’ gestation an oral dose of mifepristone 200 mg to facilitate cervical preparation may be considered. On day 2, the osmotic dilators are removed and surgical evacuation is performed. In one randomized study, for pregnancies at 19 to 24 weeks’ gestation, compared with osmotic dilators alone, administration of mifepristone 200 mg at the time of placement of osmotic dilators resulted in fewer procedures that were difficult to complete.31 In some cases, 2 consecutive days of cervical preparation with osmotic dilators may be needed to properly prepare the cervix for uterine evacuation. For example, the cervix of a nulliparous teenage patient may require 2 days of cervical preparation with osmotic dilators to facilitate uterine evacuation. In some cases of fetal demise, the cervix is already dilated to ≥3 cm and surgical evacuation of the uterus or induction of labor can be initiated without the need for cervical preparation.
Continue to: Fetal size 14w0d to 28w6d gestation: Cervical preparation and induction of labor...
Fetal size 14w0d to 28w6d gestation: Cervical preparation and induction of labor
Treatment of fetal demise at 14w0d to 28w6d gestation with the goal of the vaginal birth of an intact fetus is optimized by the administration of mifepristone for cervical preparation followed by induction of labor with misoprostol.26,27
In one clinical trial, 66 patients with fetal demise between 14w0d and 28w6d gestation were randomly assigned to receive mifepristone 200 mg or placebo followed 24 to 48 hours later with initiation of misoprostol induction of labor.26 Among the patients from 14w0d to 24 weeks’ gestation, the misoprostol dose was 400 µg vaginally every 6 hours. For patients from 24w0d to 28 weeks’ gestation, the misoprostol dose was 200 µg vaginally every 4 hours. At 24 hours, a consultant obstetrician determined if additional misoprostol should be given. The median time from initiation of misoprostol to birth for the patients in the mifepristone and placebo groups was 6.8 hours and 10.5 hours (P=.002).
Compared with the patients in the placebo-misoprostol group, the patients in the mifepristone-misoprostol group required fewer doses of misoprostol (2.1 vs 3.4; P= .002) and a lower total dose of misoprostol (768 µg vs 1,182 µg; P=.003). All patients in the mifepristone group delivered within 24 hours. By contrast, 13% of the patients in the placebo group delivered more than 24 hours after the initiation of misoprostol treatment. Five patients were readmitted with retained products of conception needing suction curettage, 4 in the placebo group and 1 in the mifepristone group.26
In a second clinical trial, 105 patients with fetal demise after 20 weeks of gestation were randomly assigned to receive mifepristone 200 mg or placebo.27 In this study, 86% of the patients were ≥26w0d gestation, with a mean gestational age of approximately 32w2d. Thirty-six to 48 hours later, misoprostol induction of labor was initiated. Among the patients from 20 to 25 completed weeks of gestation, the misoprostoldose was 100 µg vaginally every 6 hours for a maximum of 4 doses. For patients from ≥26 weeks’ gestation, the misoprostol dose was 50 µg vaginally every 4 hours for a maximum of 6 doses. The median times from initiation of misoprostol to birth for the patients in the mifepristone and placebo groups were 9.8 hours and 16.3 hours, respectively (P=.001). Compared with the patients in the placebo-misoprostol group, the patients in the mifepristone-misoprostol group required a lower total dose of misoprostol (110 µg vs 198 µg; P<.001). Delivery within 24 hours following initiation of misoprostol occurred in 93% and 73% of the patients in the mifepristone and placebo groups, respectively (P<.001). Compared with patients in the mifepristone group, shivering occurred more frequently among patients in the placebo group (7.5% vs 19.2%; P=.09), likely because they received greater doses of misoprostol.27
Fetal size ≥29w0d gestation
At a fetal size ≥29w0d gestation, if the cervix is ripe with a Bishop score of ≥7, oxytocin induction of labor is often used as a first-line treatment. If the cervix is not ripe, misoprostol induction of labor may be considered at doses less than those used in the second trimester of pregnancy.32 TABLES 1,1, 26, 33–36 2,37 and 337 summarize regimens proposed for fetal size ≥29w0d. One regimen begins with an initial misoprostol dose of 50 µg. If adequate uterine contractions occur, the 50 µg dose is repeated every 4 hours up to 6 total doses. If contractions are inadequate, the dose can be increased to 100 µg every 4 hours for 5 additional doses.



For fetal demise after 28w0d gestation, the
A multidisciplinary approach can optimize compassionate care
There are many gaps in the holistic care of patients and partners experiencing fetal demise. Patients with fetal demise often report that they did not receive sufficient information about the cause of the demise and wanted more opportunity to be involved in decision making about their care.39 The patient’s partner often reports feeling unacknowledged as a grieving parent.40 Fetal demise is experienced by many patients as a tragedy, triggering feelings of grief, anger, denial, anxiety and depression, sometimes resulting in isolation and substance misuse.
Using a 5-round Delphi process, experts identified 8 core goals in the care of patients with fetal demise:
- reduce stigma
- provide respectful care
- involve patients in care planning
- attempt to provide an explanation for the demise1
- acknowledge the depth of the grief response and provide emotional support
- offer information about ongoing psychological support
- provide information about future pregnancy planning
- provide opportunities for specialized training and support for care providers.41
Management of stillbirth is optimized by a multidisciplinary approach that includes the expert care of obstetrician-gynecologists, obstetric nurses, anesthesiologists, and expert consultation from social work, chaplaincy, and pathology. A heart-to-heart connection between clinician and patient is a key component of stillbirth care. ●
- American College of Obstetricians and Gynecologists. Management of stillbirth. ACOG Obstetric Care Consensus. No. 10. Obstet Gynecol. 2020;135:e110-132.
- Tsakiridis I, Giouleka S, Mamopoulos A, et al. Investigation and management of stillbirths: a descriptive review of major guidelines. J Perinat Med. 2022;50:796-813.
- Spingler T, Sonek J, Hoopman M, et al. Complication rate after termination of pregnancy due to fetal defects. Ultrasound Obstet Gynecol. 2023;Epub January 7.
- Goldberg AB, Drey EA, Whitaker AK, et al. Misoprostol compared with laminaria before early second-trimester surgical abortion: a randomized trial. Obstet Gynecol. 2005;106:234-241.
- Meirik O, My Huong NT, Piaggio G, et al. WHOR-GoP-MoF Regulation. Complications of first trimester abortion by vacuum aspiration after cervical preparation with and without misoprostol: a multicentre randomised trial. Lancet. 2012;379(9829):1817-1824.
- Bartz D, Maurer R, Allen RH, et al. Buccal misoprostol compared with synthetic osmotic cervical dilator before surgical abortion: a randomized controlled trial. Obstet Gynecol. 2013;122:57-63.
- Ngo LL, Mokashi M, Janiak E, et al. Acute complications with same-day versus overnight cervical preparation before dilation and evacuation at 14 to 16 weeks. Contraception. 2023;117:61-66.
- Kim CS, Dragoman M, Prosch L, et al. Same-day compared with overnight cervical preparation before dilation and evacuation between 16 and 19 6/7 weeks of gestation: a randomized controlled trial. Obstet Gynecol. 2022;139:1141-1144.
- Drunecky T, Reidingerova M, Plisova M, et al. Experimental comparison of properties of natural and synthetic osmotic dilators. Arch Gynecol Obstet. 2015;292:349-354.
- Hern WM. Laminaria versus Dilapan osmotic cervical dilators for outpatient dilation and evacuation abortion: randomized cohort comparison of 1001 patients. Am J Obstet Gynecol. 1994;171:1324-1328.
- Berthold C, Gomes David M, Gabriel P, et al. Effect of the addition of osmotic dilators to medical induction of labor abortion: a before-and-after study. Eur J Obstet Gynecol. 2020;244:185-189.
- Kemper JI, Li W, Goni S, et al. Foley catheter vs oral misoprostol for induction of labor: individual participant data meta-analysis. Ultrasound Obstet Gynecol. 2021;57:215-223.
- Attalli E, Kern Guy, Fouks Y, et al. Labor induction in third trimester non-viable fetus. J Matern Fetal Neonatal Med. 2022;Epub October 1.
- Fessehaye Sium A, Prager S, Wolderufael M, et al. Foley catheter for cervical preparation prior to second trimester dilation and evacuation: a supply-based alternative for surgical abortion: a case series. Contracept X. 2022;4:100085.
- Zieman M, Fong SK, Benowitz NL, et al. Absorption kinetics of misoprostol with oral or vaginal administration. Obstet Gynecol. 1997;90:88-92.
- Gemzell-Danilesson K, Marions L, Rodriguez A, et al. Comparison between oral and vaginal administration of misoprostol on uterine contractility. Obstet Gynecol. 1999;93:275-280.
- Aronsson A, Bygdeman M, Gemzell-Danielsson K. Effects of misoprostol on uterine contractility following different routes of administration. Hum Reprod. 2004;19:81-84.
- Meckstroth KR, Whitaker AK, Bertisch S, et al. Misoprostol administered by epithelial routes. Drug absorption and uterine response. Obstet Gynecol. 2006;108:582-590.
- Barbieri RL. Misoprostol: clinical pharmacology in obstetrics and gynecology. OBG Manag. 2022;34:8-10, 12.
- Andersen J, Grine E, Eng L, et al. Expression of connexin-43 in human myometrium and leiomyoma. Am J Obstet Gynecol. 1993;169:1266-1276.
- Ou CW, Orsino A, Lye SJ. Expression of connexin-43 and connexin-26 in the rat myometrium during pregnancy and labor is differentially regulated by mechanical and hormonal signals. Endocrinology. 1997;138:5398-5407.
- Petrocelli T, Lye SJ. Regulation of transcripts encoding the myometrial gap junction protein, connexin-43, by estrogen and progesterone. Endocrinology. 1993;133:284-290.
- Barbieri RL. Mifepristone for the treatment of miscarriage and fetal demise. OBG Manag. 2022;34:811, 15.
- Kapp N, Borgatta L, Stubblefield P, et al. Mifepristone in second-trimester medical abortion. Obstet Gynecol. 2007;110:1304-1310.
- Ngoc NTN, Shochet T, Raghavan S, et al. Mifepristone and misoprostol compared with misoprostol alone for second trimester abortion: a randomized controlled trial. Obstet Gynecol. 2011;118:601608.
- Allanson ER, Copson S, Spilsbury K, et al. Pretreatment with mifepristone compared with misoprostol alone for delivery after fetal death between 14 and 28 weeks of gestation. Obstet Gynecol. 2021;137:801-809.
- Chaudhuri P, Datta S. Mifepristone and misoprostol compared with misoprostol alone for induction of labor in intrauterine fetal death: a randomized trial. J Obstet Gynaecol Res. 2015;41:1884-1890.
- Prodan N, Breisch J, Hoopman M, et al. Dosing interval between mifepristone and misoprostol in second and third trimester termination. Arch Gynecol Obstet. 2019;299:675-679.
- Edlow AG, Hou MY, Maurer R, et al. Uterine evacuation for second trimester fetal death and maternal morbidity. Obstet Gynecol. 2011;117:1-10.
- Bryan AG, Grimes DA, Garrett JM, et al. Second-trimester abortion for fetal anomalies or fetal death. Obstet Gynecol. 2011;117:788-792.
- Goldberg AB, Fortin JA, Drey EA, et al. Cervical preparation before dilation and evacuation using adjunctive misoprostol or mifepristone compared with overnight osmotic dilators alone. Obstet Gynecol. 2015;126:599-609.
- Gomez-Ponce de Leon R, Wing D, Fiala C. Misoprostol for intrauterine fetal death. Int J Gynaecol Obstet. 2007;99(suppl 2):S190-S193.
- Schreiber C, Creinin MD, Atrio J, et al. Mifepristone pretreatment for the medical management of early pregnancy loss. N Engl J Med. 2018;378:2161-2170.
- Chu JJ, Devall AJ, Beeson LE, et al. Mifepristone and misoprostol versus misoprostol alone for the management of missed miscarriage (MifeMiso): a randomised, double-blind, placebo-controlled trial. Lancet. 2020;396:770-778.
- Gomez-Ponce de Leon R, Wing D, Fiala C. Misoprostol for intrauterine fetal death. Int J Gynaecol Obstet. 2007;99(suppl 2):S190-S193.
- American College of Obstetricians and Gynecologists. Second-trimester abortion. Practice Bulletin No. 135. Obstet Gynecol. 2013;121:1394-1406.
- Wingo E, Raifman S, Landau C, et al. Mifepristone-misoprostol versus misoprostol-alone regimen for medication abortion at ≥ 24 weeks gestation. Contraception. Appendix 1. 2020;102:99-103.
- American College of Obstetricians and Gynecologists. Vaginal birth after cesarean delivery. ACOG Practice Bulletin No. 205. Obstet Gynecol. 2019;133:e110-e127.
- Atkins B, Blencowe H, Boyle FM, et al. Is care of stillborn babies and their parents respectful? Results from an international online survey. BJOG. 2022;129:1731-1739.
- Haezell AEP, Siassakos D, Blencowe H, et al. Stillbirths: economic and psychosocial consequences. Lancet. 2016;387(10018):604-616.
- Shakespeare C, Merriel A, Bakhbakhi D, et al. The RESPECT Study for consensus on global bereavement care after stillbirth. Int J Gynaecol Obstet. 2020;149:137-147.
- American College of Obstetricians and Gynecologists. Management of stillbirth. ACOG Obstetric Care Consensus. No. 10. Obstet Gynecol. 2020;135:e110-132.
- Tsakiridis I, Giouleka S, Mamopoulos A, et al. Investigation and management of stillbirths: a descriptive review of major guidelines. J Perinat Med. 2022;50:796-813.
- Spingler T, Sonek J, Hoopman M, et al. Complication rate after termination of pregnancy due to fetal defects. Ultrasound Obstet Gynecol. 2023;Epub January 7.
- Goldberg AB, Drey EA, Whitaker AK, et al. Misoprostol compared with laminaria before early second-trimester surgical abortion: a randomized trial. Obstet Gynecol. 2005;106:234-241.
- Meirik O, My Huong NT, Piaggio G, et al. WHOR-GoP-MoF Regulation. Complications of first trimester abortion by vacuum aspiration after cervical preparation with and without misoprostol: a multicentre randomised trial. Lancet. 2012;379(9829):1817-1824.
- Bartz D, Maurer R, Allen RH, et al. Buccal misoprostol compared with synthetic osmotic cervical dilator before surgical abortion: a randomized controlled trial. Obstet Gynecol. 2013;122:57-63.
- Ngo LL, Mokashi M, Janiak E, et al. Acute complications with same-day versus overnight cervical preparation before dilation and evacuation at 14 to 16 weeks. Contraception. 2023;117:61-66.
- Kim CS, Dragoman M, Prosch L, et al. Same-day compared with overnight cervical preparation before dilation and evacuation between 16 and 19 6/7 weeks of gestation: a randomized controlled trial. Obstet Gynecol. 2022;139:1141-1144.
- Drunecky T, Reidingerova M, Plisova M, et al. Experimental comparison of properties of natural and synthetic osmotic dilators. Arch Gynecol Obstet. 2015;292:349-354.
- Hern WM. Laminaria versus Dilapan osmotic cervical dilators for outpatient dilation and evacuation abortion: randomized cohort comparison of 1001 patients. Am J Obstet Gynecol. 1994;171:1324-1328.
- Berthold C, Gomes David M, Gabriel P, et al. Effect of the addition of osmotic dilators to medical induction of labor abortion: a before-and-after study. Eur J Obstet Gynecol. 2020;244:185-189.
- Kemper JI, Li W, Goni S, et al. Foley catheter vs oral misoprostol for induction of labor: individual participant data meta-analysis. Ultrasound Obstet Gynecol. 2021;57:215-223.
- Attalli E, Kern Guy, Fouks Y, et al. Labor induction in third trimester non-viable fetus. J Matern Fetal Neonatal Med. 2022;Epub October 1.
- Fessehaye Sium A, Prager S, Wolderufael M, et al. Foley catheter for cervical preparation prior to second trimester dilation and evacuation: a supply-based alternative for surgical abortion: a case series. Contracept X. 2022;4:100085.
- Zieman M, Fong SK, Benowitz NL, et al. Absorption kinetics of misoprostol with oral or vaginal administration. Obstet Gynecol. 1997;90:88-92.
- Gemzell-Danilesson K, Marions L, Rodriguez A, et al. Comparison between oral and vaginal administration of misoprostol on uterine contractility. Obstet Gynecol. 1999;93:275-280.
- Aronsson A, Bygdeman M, Gemzell-Danielsson K. Effects of misoprostol on uterine contractility following different routes of administration. Hum Reprod. 2004;19:81-84.
- Meckstroth KR, Whitaker AK, Bertisch S, et al. Misoprostol administered by epithelial routes. Drug absorption and uterine response. Obstet Gynecol. 2006;108:582-590.
- Barbieri RL. Misoprostol: clinical pharmacology in obstetrics and gynecology. OBG Manag. 2022;34:8-10, 12.
- Andersen J, Grine E, Eng L, et al. Expression of connexin-43 in human myometrium and leiomyoma. Am J Obstet Gynecol. 1993;169:1266-1276.
- Ou CW, Orsino A, Lye SJ. Expression of connexin-43 and connexin-26 in the rat myometrium during pregnancy and labor is differentially regulated by mechanical and hormonal signals. Endocrinology. 1997;138:5398-5407.
- Petrocelli T, Lye SJ. Regulation of transcripts encoding the myometrial gap junction protein, connexin-43, by estrogen and progesterone. Endocrinology. 1993;133:284-290.
- Barbieri RL. Mifepristone for the treatment of miscarriage and fetal demise. OBG Manag. 2022;34:811, 15.
- Kapp N, Borgatta L, Stubblefield P, et al. Mifepristone in second-trimester medical abortion. Obstet Gynecol. 2007;110:1304-1310.
- Ngoc NTN, Shochet T, Raghavan S, et al. Mifepristone and misoprostol compared with misoprostol alone for second trimester abortion: a randomized controlled trial. Obstet Gynecol. 2011;118:601608.
- Allanson ER, Copson S, Spilsbury K, et al. Pretreatment with mifepristone compared with misoprostol alone for delivery after fetal death between 14 and 28 weeks of gestation. Obstet Gynecol. 2021;137:801-809.
- Chaudhuri P, Datta S. Mifepristone and misoprostol compared with misoprostol alone for induction of labor in intrauterine fetal death: a randomized trial. J Obstet Gynaecol Res. 2015;41:1884-1890.
- Prodan N, Breisch J, Hoopman M, et al. Dosing interval between mifepristone and misoprostol in second and third trimester termination. Arch Gynecol Obstet. 2019;299:675-679.
- Edlow AG, Hou MY, Maurer R, et al. Uterine evacuation for second trimester fetal death and maternal morbidity. Obstet Gynecol. 2011;117:1-10.
- Bryan AG, Grimes DA, Garrett JM, et al. Second-trimester abortion for fetal anomalies or fetal death. Obstet Gynecol. 2011;117:788-792.
- Goldberg AB, Fortin JA, Drey EA, et al. Cervical preparation before dilation and evacuation using adjunctive misoprostol or mifepristone compared with overnight osmotic dilators alone. Obstet Gynecol. 2015;126:599-609.
- Gomez-Ponce de Leon R, Wing D, Fiala C. Misoprostol for intrauterine fetal death. Int J Gynaecol Obstet. 2007;99(suppl 2):S190-S193.
- Schreiber C, Creinin MD, Atrio J, et al. Mifepristone pretreatment for the medical management of early pregnancy loss. N Engl J Med. 2018;378:2161-2170.
- Chu JJ, Devall AJ, Beeson LE, et al. Mifepristone and misoprostol versus misoprostol alone for the management of missed miscarriage (MifeMiso): a randomised, double-blind, placebo-controlled trial. Lancet. 2020;396:770-778.
- Gomez-Ponce de Leon R, Wing D, Fiala C. Misoprostol for intrauterine fetal death. Int J Gynaecol Obstet. 2007;99(suppl 2):S190-S193.
- American College of Obstetricians and Gynecologists. Second-trimester abortion. Practice Bulletin No. 135. Obstet Gynecol. 2013;121:1394-1406.
- Wingo E, Raifman S, Landau C, et al. Mifepristone-misoprostol versus misoprostol-alone regimen for medication abortion at ≥ 24 weeks gestation. Contraception. Appendix 1. 2020;102:99-103.
- American College of Obstetricians and Gynecologists. Vaginal birth after cesarean delivery. ACOG Practice Bulletin No. 205. Obstet Gynecol. 2019;133:e110-e127.
- Atkins B, Blencowe H, Boyle FM, et al. Is care of stillborn babies and their parents respectful? Results from an international online survey. BJOG. 2022;129:1731-1739.
- Haezell AEP, Siassakos D, Blencowe H, et al. Stillbirths: economic and psychosocial consequences. Lancet. 2016;387(10018):604-616.
- Shakespeare C, Merriel A, Bakhbakhi D, et al. The RESPECT Study for consensus on global bereavement care after stillbirth. Int J Gynaecol Obstet. 2020;149:137-147.