User login
Higher disability at early stages raises risk for progression to difficult-to-treat RA
Key clinical point: Younger patients and patients with higher disability scores at early stages were more likely to develop difficult-to-treat rheumatoid arthritis (RA). Thus focusing on severe disability during initial stages may alter disease course.
Major finding: Elevated initial disability score (odds ratio [OR] 1.89; P = .01) and a younger age at baseline (OR 0.95; P = .01) were associated with an increased risk for difficult-to-treat RA, whereas initial disease activity failed to show any influence.
Study details: The data come from a longitudinal, prospective cohort study including 631 patients with newly diagnosed RA, of which 35 patients developed difficult-to-treat RA.
Disclosures: This study was supported by the Instituto de Salud Carlos III, Spain, and other sources. The authors did not declare any conflicts of interest.
Source: Leon L et al. Difficult-to-treat rheumatoid arthritis (D2T RA): Clinical issues at early stages of disease. RMD Open. 2023;9:e002842 (Mar 8). Doi: 10.1136/rmdopen-2022-002842
Key clinical point: Younger patients and patients with higher disability scores at early stages were more likely to develop difficult-to-treat rheumatoid arthritis (RA). Thus focusing on severe disability during initial stages may alter disease course.
Major finding: Elevated initial disability score (odds ratio [OR] 1.89; P = .01) and a younger age at baseline (OR 0.95; P = .01) were associated with an increased risk for difficult-to-treat RA, whereas initial disease activity failed to show any influence.
Study details: The data come from a longitudinal, prospective cohort study including 631 patients with newly diagnosed RA, of which 35 patients developed difficult-to-treat RA.
Disclosures: This study was supported by the Instituto de Salud Carlos III, Spain, and other sources. The authors did not declare any conflicts of interest.
Source: Leon L et al. Difficult-to-treat rheumatoid arthritis (D2T RA): Clinical issues at early stages of disease. RMD Open. 2023;9:e002842 (Mar 8). Doi: 10.1136/rmdopen-2022-002842
Key clinical point: Younger patients and patients with higher disability scores at early stages were more likely to develop difficult-to-treat rheumatoid arthritis (RA). Thus focusing on severe disability during initial stages may alter disease course.
Major finding: Elevated initial disability score (odds ratio [OR] 1.89; P = .01) and a younger age at baseline (OR 0.95; P = .01) were associated with an increased risk for difficult-to-treat RA, whereas initial disease activity failed to show any influence.
Study details: The data come from a longitudinal, prospective cohort study including 631 patients with newly diagnosed RA, of which 35 patients developed difficult-to-treat RA.
Disclosures: This study was supported by the Instituto de Salud Carlos III, Spain, and other sources. The authors did not declare any conflicts of interest.
Source: Leon L et al. Difficult-to-treat rheumatoid arthritis (D2T RA): Clinical issues at early stages of disease. RMD Open. 2023;9:e002842 (Mar 8). Doi: 10.1136/rmdopen-2022-002842
Administering concomitant methotrexate at a half vs usual dose while initiating TNFi is feasible
Key clinical point: A nearly 50% reduction in methotrexate dose at the time of initiating a tumor necrosis factor inhibitor (TNFi) is possible in patients with rheumatoid arthritis (RA) who had an inadequate response to the initial maximum tolerated dose of methotrexate.
Major finding: Reduced-dose methotrexate+adalimumab was noninferior to maximal-dose methotrexate+adalimumab in achieving Simplified Disease Activity Index-based remission at week 48 (adjusted risk difference 6.4%; 90% CI −7.0% to 19.8%) and less frequently caused adverse events after 24 weeks (20% vs 35%). No deaths were reported.
Study details: Findings are from the MIRACLE trial including 291 methotrexate-naive patients with RA and inadequate response to the maximum tolerated methotrexate dose who were randomly assigned to initiate adalimumab in combination with the maximum tolerated dose or a reduced dose of methotrexate.
Disclosures: This study was funded by Eisai. Three authors declared being employees and shareholders of Eisai. Several authors declared receiving speaker honoraria, grants, research support, consulting fees, or royalties from Eisai and other sources.
Source: Tamai H et al and MIRACLE study collaborators. Reduced versus maximum tolerated methotrexate dose concomitant with adalimumab in patients with rheumatoid arthritis (MIRACLE): A randomised, open-label, non-inferiority trial. Lancet Rheumatol. 2023;5(4):E215-E224 (Apr). Doi: 10.1016/S2665-9913(23)00070-X
Key clinical point: A nearly 50% reduction in methotrexate dose at the time of initiating a tumor necrosis factor inhibitor (TNFi) is possible in patients with rheumatoid arthritis (RA) who had an inadequate response to the initial maximum tolerated dose of methotrexate.
Major finding: Reduced-dose methotrexate+adalimumab was noninferior to maximal-dose methotrexate+adalimumab in achieving Simplified Disease Activity Index-based remission at week 48 (adjusted risk difference 6.4%; 90% CI −7.0% to 19.8%) and less frequently caused adverse events after 24 weeks (20% vs 35%). No deaths were reported.
Study details: Findings are from the MIRACLE trial including 291 methotrexate-naive patients with RA and inadequate response to the maximum tolerated methotrexate dose who were randomly assigned to initiate adalimumab in combination with the maximum tolerated dose or a reduced dose of methotrexate.
Disclosures: This study was funded by Eisai. Three authors declared being employees and shareholders of Eisai. Several authors declared receiving speaker honoraria, grants, research support, consulting fees, or royalties from Eisai and other sources.
Source: Tamai H et al and MIRACLE study collaborators. Reduced versus maximum tolerated methotrexate dose concomitant with adalimumab in patients with rheumatoid arthritis (MIRACLE): A randomised, open-label, non-inferiority trial. Lancet Rheumatol. 2023;5(4):E215-E224 (Apr). Doi: 10.1016/S2665-9913(23)00070-X
Key clinical point: A nearly 50% reduction in methotrexate dose at the time of initiating a tumor necrosis factor inhibitor (TNFi) is possible in patients with rheumatoid arthritis (RA) who had an inadequate response to the initial maximum tolerated dose of methotrexate.
Major finding: Reduced-dose methotrexate+adalimumab was noninferior to maximal-dose methotrexate+adalimumab in achieving Simplified Disease Activity Index-based remission at week 48 (adjusted risk difference 6.4%; 90% CI −7.0% to 19.8%) and less frequently caused adverse events after 24 weeks (20% vs 35%). No deaths were reported.
Study details: Findings are from the MIRACLE trial including 291 methotrexate-naive patients with RA and inadequate response to the maximum tolerated methotrexate dose who were randomly assigned to initiate adalimumab in combination with the maximum tolerated dose or a reduced dose of methotrexate.
Disclosures: This study was funded by Eisai. Three authors declared being employees and shareholders of Eisai. Several authors declared receiving speaker honoraria, grants, research support, consulting fees, or royalties from Eisai and other sources.
Source: Tamai H et al and MIRACLE study collaborators. Reduced versus maximum tolerated methotrexate dose concomitant with adalimumab in patients with rheumatoid arthritis (MIRACLE): A randomised, open-label, non-inferiority trial. Lancet Rheumatol. 2023;5(4):E215-E224 (Apr). Doi: 10.1016/S2665-9913(23)00070-X
Anifrolumab shows promise in refractory discoid lupus erythematosus
a small retrospective study reports.
DLE, the most common form of chronic cutaneous lupus erythematosus, can permanently scar and disfigure patients, and traditional treatments such as antimalarials, steroid-sparing immunosuppressive agents, thalidomide, retinoids, and lenalidomide don’t consistently improve refractory DLE, the authors noted.
“All patients demonstrated significant improvement in symptomatology and disease activity within 2 months of initiating anifrolumab,” lead study author Katharina Shaw, MD, of the department of dermatology of Brigham and Women’s Hospital and Harvard Medical School, both in Boston, and colleagues wrote in a research letter published in JAMA Dermatology. “These early results highlight the potential for anifrolumab to be a viable therapeutic option for patients with DLE, particularly those with severe or recalcitrant disease.”
The Food and Drug Administration approved anifrolumab (Saphnelo), a human monoclonal antibody targeting type 1 interferon receptor subunit 1, in 2021 for adults with moderate to severe systemic lupus erythematosus, but it has not been approved for the treatment of DLE.
Dr. Shaw and colleagues queried the medical records from Brigham and Women’s Hospital and Massachusetts General Hospital, Boston, to find all cases of DLE based on biopsy, expert opinion, or both from January 2000 to October 2022.
The researchers identified eight female patients who had received anifrolumab for at least 8 weeks. The women were aged between 19 and 75 years (median, 42.5 years), and all had DLE recalcitrant to standard therapies and had been treated with hydroxychloroquine and between 1 and 10 other drugs, most commonly methotrexate and mycophenolate mofetil (MMF).
The authors looked for improvements in patient-reported symptoms and Cutaneous Lupus Erythematosus Disease Area and Severity Index scores, including CLASI A (activity) score 0-70, and CLASI-D (damage) score 0-56.
All patients showed significantly improved symptoms and disease activity within 2 months of their first infusion of the treatment. The mean decrease and mean percentage decrease in CLASI-A scores were 17.1 and 65.1%, respectively. The mean decrease and mean percentage decrease in CLASI-D scores were 0.5 and 2.9%, respectively.
The rapid clinical improvements with anifrolumab, compared with improvements with traditional medications, were striking, the authors wrote. “Given the risk for permanent scarring, dyspigmentation, and alopecia with poorly controlled DLE, the importance of rapidly mitigating disease activity cannot be overemphasized.”
They acknowledged that the results are limited by the study’s small sample size and retrospective design, and they recommend larger related prospective studies.
Asked to comment on the results, Kaveh Ardalan, MD, MS, assistant professor of pediatrics in the division of pediatric rheumatology at Duke University, Durham, N.C., said that finding new DLE therapeutics is important because of the huge impact of uncontrolled DLE on patients’ quality of life, body image, and social roles.
Dr. Ardalan noted that he sees DLE in his pediatric patients, “either as an isolated finding or in the context of systemic lupus erythematosus. Anifrolumab is not approved by the FDA to treat DLE or children.
“Randomized controlled trials, including the TULIP-1 and TULIP-2 studies of anifrolumab in systemic lupus, have indicated that lupus skin manifestations can improve in patients who receive anifrolumab,” said Dr. Ardalan, who was not involved in the study. “And we know that type I interferons are major drivers of cutaneous disease activity in patients with lupus, so targeting that mechanism with anifrolumab makes biological sense.”
The authors’ use of the validated CLASI classification system to quantify disease activity and damage over time, and their determination of the length of time for the drug to take effect are strengths of the study, he added.
Funding information was not provided. Two authors reported financial relationships with Pfizer, which does not manufacture anifrolumab. Dr. Ardalan reported no conflicts of interest with the study.
a small retrospective study reports.
DLE, the most common form of chronic cutaneous lupus erythematosus, can permanently scar and disfigure patients, and traditional treatments such as antimalarials, steroid-sparing immunosuppressive agents, thalidomide, retinoids, and lenalidomide don’t consistently improve refractory DLE, the authors noted.
“All patients demonstrated significant improvement in symptomatology and disease activity within 2 months of initiating anifrolumab,” lead study author Katharina Shaw, MD, of the department of dermatology of Brigham and Women’s Hospital and Harvard Medical School, both in Boston, and colleagues wrote in a research letter published in JAMA Dermatology. “These early results highlight the potential for anifrolumab to be a viable therapeutic option for patients with DLE, particularly those with severe or recalcitrant disease.”
The Food and Drug Administration approved anifrolumab (Saphnelo), a human monoclonal antibody targeting type 1 interferon receptor subunit 1, in 2021 for adults with moderate to severe systemic lupus erythematosus, but it has not been approved for the treatment of DLE.
Dr. Shaw and colleagues queried the medical records from Brigham and Women’s Hospital and Massachusetts General Hospital, Boston, to find all cases of DLE based on biopsy, expert opinion, or both from January 2000 to October 2022.
The researchers identified eight female patients who had received anifrolumab for at least 8 weeks. The women were aged between 19 and 75 years (median, 42.5 years), and all had DLE recalcitrant to standard therapies and had been treated with hydroxychloroquine and between 1 and 10 other drugs, most commonly methotrexate and mycophenolate mofetil (MMF).
The authors looked for improvements in patient-reported symptoms and Cutaneous Lupus Erythematosus Disease Area and Severity Index scores, including CLASI A (activity) score 0-70, and CLASI-D (damage) score 0-56.
All patients showed significantly improved symptoms and disease activity within 2 months of their first infusion of the treatment. The mean decrease and mean percentage decrease in CLASI-A scores were 17.1 and 65.1%, respectively. The mean decrease and mean percentage decrease in CLASI-D scores were 0.5 and 2.9%, respectively.
The rapid clinical improvements with anifrolumab, compared with improvements with traditional medications, were striking, the authors wrote. “Given the risk for permanent scarring, dyspigmentation, and alopecia with poorly controlled DLE, the importance of rapidly mitigating disease activity cannot be overemphasized.”
They acknowledged that the results are limited by the study’s small sample size and retrospective design, and they recommend larger related prospective studies.
Asked to comment on the results, Kaveh Ardalan, MD, MS, assistant professor of pediatrics in the division of pediatric rheumatology at Duke University, Durham, N.C., said that finding new DLE therapeutics is important because of the huge impact of uncontrolled DLE on patients’ quality of life, body image, and social roles.
Dr. Ardalan noted that he sees DLE in his pediatric patients, “either as an isolated finding or in the context of systemic lupus erythematosus. Anifrolumab is not approved by the FDA to treat DLE or children.
“Randomized controlled trials, including the TULIP-1 and TULIP-2 studies of anifrolumab in systemic lupus, have indicated that lupus skin manifestations can improve in patients who receive anifrolumab,” said Dr. Ardalan, who was not involved in the study. “And we know that type I interferons are major drivers of cutaneous disease activity in patients with lupus, so targeting that mechanism with anifrolumab makes biological sense.”
The authors’ use of the validated CLASI classification system to quantify disease activity and damage over time, and their determination of the length of time for the drug to take effect are strengths of the study, he added.
Funding information was not provided. Two authors reported financial relationships with Pfizer, which does not manufacture anifrolumab. Dr. Ardalan reported no conflicts of interest with the study.
a small retrospective study reports.
DLE, the most common form of chronic cutaneous lupus erythematosus, can permanently scar and disfigure patients, and traditional treatments such as antimalarials, steroid-sparing immunosuppressive agents, thalidomide, retinoids, and lenalidomide don’t consistently improve refractory DLE, the authors noted.
“All patients demonstrated significant improvement in symptomatology and disease activity within 2 months of initiating anifrolumab,” lead study author Katharina Shaw, MD, of the department of dermatology of Brigham and Women’s Hospital and Harvard Medical School, both in Boston, and colleagues wrote in a research letter published in JAMA Dermatology. “These early results highlight the potential for anifrolumab to be a viable therapeutic option for patients with DLE, particularly those with severe or recalcitrant disease.”
The Food and Drug Administration approved anifrolumab (Saphnelo), a human monoclonal antibody targeting type 1 interferon receptor subunit 1, in 2021 for adults with moderate to severe systemic lupus erythematosus, but it has not been approved for the treatment of DLE.
Dr. Shaw and colleagues queried the medical records from Brigham and Women’s Hospital and Massachusetts General Hospital, Boston, to find all cases of DLE based on biopsy, expert opinion, or both from January 2000 to October 2022.
The researchers identified eight female patients who had received anifrolumab for at least 8 weeks. The women were aged between 19 and 75 years (median, 42.5 years), and all had DLE recalcitrant to standard therapies and had been treated with hydroxychloroquine and between 1 and 10 other drugs, most commonly methotrexate and mycophenolate mofetil (MMF).
The authors looked for improvements in patient-reported symptoms and Cutaneous Lupus Erythematosus Disease Area and Severity Index scores, including CLASI A (activity) score 0-70, and CLASI-D (damage) score 0-56.
All patients showed significantly improved symptoms and disease activity within 2 months of their first infusion of the treatment. The mean decrease and mean percentage decrease in CLASI-A scores were 17.1 and 65.1%, respectively. The mean decrease and mean percentage decrease in CLASI-D scores were 0.5 and 2.9%, respectively.
The rapid clinical improvements with anifrolumab, compared with improvements with traditional medications, were striking, the authors wrote. “Given the risk for permanent scarring, dyspigmentation, and alopecia with poorly controlled DLE, the importance of rapidly mitigating disease activity cannot be overemphasized.”
They acknowledged that the results are limited by the study’s small sample size and retrospective design, and they recommend larger related prospective studies.
Asked to comment on the results, Kaveh Ardalan, MD, MS, assistant professor of pediatrics in the division of pediatric rheumatology at Duke University, Durham, N.C., said that finding new DLE therapeutics is important because of the huge impact of uncontrolled DLE on patients’ quality of life, body image, and social roles.
Dr. Ardalan noted that he sees DLE in his pediatric patients, “either as an isolated finding or in the context of systemic lupus erythematosus. Anifrolumab is not approved by the FDA to treat DLE or children.
“Randomized controlled trials, including the TULIP-1 and TULIP-2 studies of anifrolumab in systemic lupus, have indicated that lupus skin manifestations can improve in patients who receive anifrolumab,” said Dr. Ardalan, who was not involved in the study. “And we know that type I interferons are major drivers of cutaneous disease activity in patients with lupus, so targeting that mechanism with anifrolumab makes biological sense.”
The authors’ use of the validated CLASI classification system to quantify disease activity and damage over time, and their determination of the length of time for the drug to take effect are strengths of the study, he added.
Funding information was not provided. Two authors reported financial relationships with Pfizer, which does not manufacture anifrolumab. Dr. Ardalan reported no conflicts of interest with the study.
FROM JAMA DERMATOLOGY
New data forecast more oral PDE4 inhibitors for psoriasis
NEW ORLEANS – according to results of a phase 2 clinical trial presented as a late-breaker at the annual meeting of the American Academy of Dermatology.
The phase 2b data, which are prompting a phase 3 trial, suggest that the drug, called orismilast, “is a potential new addition to the psoriasis armamentarium,” reported Lars E. French, MD, professor and chair, department of dermatology, Ludwig Maximilian University of Munich (Germany).
At the same session, findings from another study supported off-label use of oral roflumilast (Daliresp and generic), a PDE4 inhibitor approved for severe chronic obstructive pulmonary disease (COPD). The only PDE4 inhibitors with an indication for psoriasis are roflumilast, approved as a cream (Zoryve), and apremilast (Otezla), approved as an oral therapy.
Phase 2 study of orismilast
In the orismilast trial, Dr. French attributed the efficacy observed to the potency of orismilast on the B and D subtypes of PDE4 associated with inflammation. One clue is that these specific subtypes are overly expressed in the skin of patients with either psoriasis or atopic dermatitis.
“When compared to apremilast, orismilast is at least two to fivefold more potent on all PDE4 isoforms and up to 39 times more potent on some of the PDE4 B and D isoforms,” said Dr. French, referring to preclinical findings in human whole blood and blood cells and in a mouse model of chronic inflammation.
The efficacy of orismilast in an immediate-release oral formulation was previously demonstrated in a recently published phase 2a trial, but the newest study tested a modified-release formulation of orismilast to test its potential to improve tolerability.
In the study, 202 adult patients with moderate to severe psoriasis (Psoriasis Area Severity Index [PASI] score ≥ 12) were randomly assigned to one of three doses of orismilast or to placebo. Each of the three doses – 20 mg, 30 mg, or 40 mg – were administered twice daily. The primary endpoint was change in PASI score at 16 weeks. Secondary endpoints included PASI 75 responses (signifying 75% clearance) and safety.
Relative to placebo, which was associated with a PASI improvement of 17%, all three of the tested orismilast doses were superior in a dose-dependent manner. The rates of response were 53%, 61%, and 64% for the 20-mg, 30-mg, and 40-mg twice-daily doses, respectively.
The PASI improvements were rapid, Dr. French said. At 4 weeks, PASI scores climbed from baseline by nearly 40% for those on all orismilast doses, which was more than double the improvement in the placebo group.
In the intention-to-treat analysis with missing data counted as nonresponders, the proportion of patients reaching PASI-75 scores at 16 weeks were 39%, 49%, 45%, and 17%, in the 20-mg, 30-mg, 40-mg, and placebo groups, respectively. The proportion of patients experiencing complete or near-complete skin clearance defined by a PASI 90 were 24%, 22%, 28%, and 8%, respectively.
The side-effect profile was consistent with other PDE4 inhibitors. The most common adverse events included gastrointestinal complaints, such as diarrhea and nausea, as well as headache and dizziness. But the majority of these events were of low grade, and they were largely confined to the first 4 weeks of treatment, which is a pattern reported with other PDE4 inhibitors in psoriasis and other chronic inflammatory diseases, such as COPD, according to Dr. French.
“There were no discontinuations for a treatment-related adverse event in the arms receiving either the 20-mg or the 30-mg doses,” Dr. French reported. There were only two serious adverse events, and neither were considered by trial investigators to be related to orismilast.
Based on the limited therapeutic gain but greater risk for adverse events on the 40-mg twice-daily dose, “the question is now whether to move forward with the 20-mg or the 30-mg dose,” said Dr. French, who said planning of a phase 3 trial is underway.
Phase 2 study of roflumilast
However, this was not the only set of data on an oral PDE4 inhibitor presented as a late-breaker at the AAD meeting. For clinicians looking for a more immediate and less expensive alternative to apremilast, another study indicated that off-label use of oral roflumilast is an option.
In an investigator-initiated, multicenter, double-blind, placebo-controlled trial conducted in Denmark, the rate of response to oral roflumilast at 24 weeks, including the clear or almost clear response, was on the same general order of magnitude as that seen in the orismilast study, reported Alexander Egeberg, MD, PhD, professor of dermatology, University of Copenhagen.
“At 24 weeks, 21.7% had achieved a PASI 90, and 8.7% achieved a PASI 100,” Dr. Egeberg said.
Oral roflumilast has been available for the treatment of COPD for more than 10 years and is now available in a generic formulation. This study was conducted independent of any pharmaceutical company involvement, and the high rate of response and low risk of adverse events suggests that patients can benefit from a PDE4 inhibitor in a very low-cost form.
“Generic oral roflumilast is cheaper than a Starbucks coffee,” Dr. Egeberg said.
In this trial, 46 patients were randomly assigned to placebo or to the COPD-approved roflumilast dose of 500 mcg once daily. The primary endpoint was change in PASI scores from baseline to week 12, which Dr. Egeberg pointed out is a shorter time frame than the 16 weeks more typical of psoriasis treatment studies.
At week 12, the median improvement in PASI was 34.8% in the roflumilast group versus 0% in the placebo group. Patients were then followed for an additional 12 weeks, but those randomized to placebo were switched to the active treatment. By week 24, the switch patients had largely caught up to those initiated on roflumilast for median PASI improvement (39.1% vs. 43.5%).
Similar to orismilast, roflumilast “was generally well tolerated,” Dr. Egeberg said. The adverse events were consistent with those associated with PDE4 inhibitors in previous trials, whether in psoriasis or COPD. There was only one serious adverse event, and it was not considered treatment related. Discontinuations for adverse events “were very low.”
In a population with a relatively high rate of smoking, Dr. Egeberg further reported, lung function was improved, a remark initially interpreted as a joke by some attending the presentation. However, Dr. Egeberg confirmed that lung function was monitored, and objective improvements were recorded.
By Danish law, the investigators were required to inform the manufacturers of roflumilast. Despite the results of this study, he is not aware of any plans to seek an indication for roflumilast in psoriasis, but he noted that the drug is readily available at a low price.
For those willing to offer this therapy off label, “you can start using it tomorrow if you’d like,” he said.
Dr. French reports financial relationships with Almirall, Amgen, Biotest, Galderma, Janssen Cilag, Leo Pharma, Pincell, Regeneron, UCB, and UNION Therapeutics, which provided funding for this trial. Dr. Egeberg reports financial relationships with Eli Lilly, Galderma, Janssen-Cilag, Novartis, and Pfizer.
A version of this article first appeared on Medscape.com.
NEW ORLEANS – according to results of a phase 2 clinical trial presented as a late-breaker at the annual meeting of the American Academy of Dermatology.
The phase 2b data, which are prompting a phase 3 trial, suggest that the drug, called orismilast, “is a potential new addition to the psoriasis armamentarium,” reported Lars E. French, MD, professor and chair, department of dermatology, Ludwig Maximilian University of Munich (Germany).
At the same session, findings from another study supported off-label use of oral roflumilast (Daliresp and generic), a PDE4 inhibitor approved for severe chronic obstructive pulmonary disease (COPD). The only PDE4 inhibitors with an indication for psoriasis are roflumilast, approved as a cream (Zoryve), and apremilast (Otezla), approved as an oral therapy.
Phase 2 study of orismilast
In the orismilast trial, Dr. French attributed the efficacy observed to the potency of orismilast on the B and D subtypes of PDE4 associated with inflammation. One clue is that these specific subtypes are overly expressed in the skin of patients with either psoriasis or atopic dermatitis.
“When compared to apremilast, orismilast is at least two to fivefold more potent on all PDE4 isoforms and up to 39 times more potent on some of the PDE4 B and D isoforms,” said Dr. French, referring to preclinical findings in human whole blood and blood cells and in a mouse model of chronic inflammation.
The efficacy of orismilast in an immediate-release oral formulation was previously demonstrated in a recently published phase 2a trial, but the newest study tested a modified-release formulation of orismilast to test its potential to improve tolerability.
In the study, 202 adult patients with moderate to severe psoriasis (Psoriasis Area Severity Index [PASI] score ≥ 12) were randomly assigned to one of three doses of orismilast or to placebo. Each of the three doses – 20 mg, 30 mg, or 40 mg – were administered twice daily. The primary endpoint was change in PASI score at 16 weeks. Secondary endpoints included PASI 75 responses (signifying 75% clearance) and safety.
Relative to placebo, which was associated with a PASI improvement of 17%, all three of the tested orismilast doses were superior in a dose-dependent manner. The rates of response were 53%, 61%, and 64% for the 20-mg, 30-mg, and 40-mg twice-daily doses, respectively.
The PASI improvements were rapid, Dr. French said. At 4 weeks, PASI scores climbed from baseline by nearly 40% for those on all orismilast doses, which was more than double the improvement in the placebo group.
In the intention-to-treat analysis with missing data counted as nonresponders, the proportion of patients reaching PASI-75 scores at 16 weeks were 39%, 49%, 45%, and 17%, in the 20-mg, 30-mg, 40-mg, and placebo groups, respectively. The proportion of patients experiencing complete or near-complete skin clearance defined by a PASI 90 were 24%, 22%, 28%, and 8%, respectively.
The side-effect profile was consistent with other PDE4 inhibitors. The most common adverse events included gastrointestinal complaints, such as diarrhea and nausea, as well as headache and dizziness. But the majority of these events were of low grade, and they were largely confined to the first 4 weeks of treatment, which is a pattern reported with other PDE4 inhibitors in psoriasis and other chronic inflammatory diseases, such as COPD, according to Dr. French.
“There were no discontinuations for a treatment-related adverse event in the arms receiving either the 20-mg or the 30-mg doses,” Dr. French reported. There were only two serious adverse events, and neither were considered by trial investigators to be related to orismilast.
Based on the limited therapeutic gain but greater risk for adverse events on the 40-mg twice-daily dose, “the question is now whether to move forward with the 20-mg or the 30-mg dose,” said Dr. French, who said planning of a phase 3 trial is underway.
Phase 2 study of roflumilast
However, this was not the only set of data on an oral PDE4 inhibitor presented as a late-breaker at the AAD meeting. For clinicians looking for a more immediate and less expensive alternative to apremilast, another study indicated that off-label use of oral roflumilast is an option.
In an investigator-initiated, multicenter, double-blind, placebo-controlled trial conducted in Denmark, the rate of response to oral roflumilast at 24 weeks, including the clear or almost clear response, was on the same general order of magnitude as that seen in the orismilast study, reported Alexander Egeberg, MD, PhD, professor of dermatology, University of Copenhagen.
“At 24 weeks, 21.7% had achieved a PASI 90, and 8.7% achieved a PASI 100,” Dr. Egeberg said.
Oral roflumilast has been available for the treatment of COPD for more than 10 years and is now available in a generic formulation. This study was conducted independent of any pharmaceutical company involvement, and the high rate of response and low risk of adverse events suggests that patients can benefit from a PDE4 inhibitor in a very low-cost form.
“Generic oral roflumilast is cheaper than a Starbucks coffee,” Dr. Egeberg said.
In this trial, 46 patients were randomly assigned to placebo or to the COPD-approved roflumilast dose of 500 mcg once daily. The primary endpoint was change in PASI scores from baseline to week 12, which Dr. Egeberg pointed out is a shorter time frame than the 16 weeks more typical of psoriasis treatment studies.
At week 12, the median improvement in PASI was 34.8% in the roflumilast group versus 0% in the placebo group. Patients were then followed for an additional 12 weeks, but those randomized to placebo were switched to the active treatment. By week 24, the switch patients had largely caught up to those initiated on roflumilast for median PASI improvement (39.1% vs. 43.5%).
Similar to orismilast, roflumilast “was generally well tolerated,” Dr. Egeberg said. The adverse events were consistent with those associated with PDE4 inhibitors in previous trials, whether in psoriasis or COPD. There was only one serious adverse event, and it was not considered treatment related. Discontinuations for adverse events “were very low.”
In a population with a relatively high rate of smoking, Dr. Egeberg further reported, lung function was improved, a remark initially interpreted as a joke by some attending the presentation. However, Dr. Egeberg confirmed that lung function was monitored, and objective improvements were recorded.
By Danish law, the investigators were required to inform the manufacturers of roflumilast. Despite the results of this study, he is not aware of any plans to seek an indication for roflumilast in psoriasis, but he noted that the drug is readily available at a low price.
For those willing to offer this therapy off label, “you can start using it tomorrow if you’d like,” he said.
Dr. French reports financial relationships with Almirall, Amgen, Biotest, Galderma, Janssen Cilag, Leo Pharma, Pincell, Regeneron, UCB, and UNION Therapeutics, which provided funding for this trial. Dr. Egeberg reports financial relationships with Eli Lilly, Galderma, Janssen-Cilag, Novartis, and Pfizer.
A version of this article first appeared on Medscape.com.
NEW ORLEANS – according to results of a phase 2 clinical trial presented as a late-breaker at the annual meeting of the American Academy of Dermatology.
The phase 2b data, which are prompting a phase 3 trial, suggest that the drug, called orismilast, “is a potential new addition to the psoriasis armamentarium,” reported Lars E. French, MD, professor and chair, department of dermatology, Ludwig Maximilian University of Munich (Germany).
At the same session, findings from another study supported off-label use of oral roflumilast (Daliresp and generic), a PDE4 inhibitor approved for severe chronic obstructive pulmonary disease (COPD). The only PDE4 inhibitors with an indication for psoriasis are roflumilast, approved as a cream (Zoryve), and apremilast (Otezla), approved as an oral therapy.
Phase 2 study of orismilast
In the orismilast trial, Dr. French attributed the efficacy observed to the potency of orismilast on the B and D subtypes of PDE4 associated with inflammation. One clue is that these specific subtypes are overly expressed in the skin of patients with either psoriasis or atopic dermatitis.
“When compared to apremilast, orismilast is at least two to fivefold more potent on all PDE4 isoforms and up to 39 times more potent on some of the PDE4 B and D isoforms,” said Dr. French, referring to preclinical findings in human whole blood and blood cells and in a mouse model of chronic inflammation.
The efficacy of orismilast in an immediate-release oral formulation was previously demonstrated in a recently published phase 2a trial, but the newest study tested a modified-release formulation of orismilast to test its potential to improve tolerability.
In the study, 202 adult patients with moderate to severe psoriasis (Psoriasis Area Severity Index [PASI] score ≥ 12) were randomly assigned to one of three doses of orismilast or to placebo. Each of the three doses – 20 mg, 30 mg, or 40 mg – were administered twice daily. The primary endpoint was change in PASI score at 16 weeks. Secondary endpoints included PASI 75 responses (signifying 75% clearance) and safety.
Relative to placebo, which was associated with a PASI improvement of 17%, all three of the tested orismilast doses were superior in a dose-dependent manner. The rates of response were 53%, 61%, and 64% for the 20-mg, 30-mg, and 40-mg twice-daily doses, respectively.
The PASI improvements were rapid, Dr. French said. At 4 weeks, PASI scores climbed from baseline by nearly 40% for those on all orismilast doses, which was more than double the improvement in the placebo group.
In the intention-to-treat analysis with missing data counted as nonresponders, the proportion of patients reaching PASI-75 scores at 16 weeks were 39%, 49%, 45%, and 17%, in the 20-mg, 30-mg, 40-mg, and placebo groups, respectively. The proportion of patients experiencing complete or near-complete skin clearance defined by a PASI 90 were 24%, 22%, 28%, and 8%, respectively.
The side-effect profile was consistent with other PDE4 inhibitors. The most common adverse events included gastrointestinal complaints, such as diarrhea and nausea, as well as headache and dizziness. But the majority of these events were of low grade, and they were largely confined to the first 4 weeks of treatment, which is a pattern reported with other PDE4 inhibitors in psoriasis and other chronic inflammatory diseases, such as COPD, according to Dr. French.
“There were no discontinuations for a treatment-related adverse event in the arms receiving either the 20-mg or the 30-mg doses,” Dr. French reported. There were only two serious adverse events, and neither were considered by trial investigators to be related to orismilast.
Based on the limited therapeutic gain but greater risk for adverse events on the 40-mg twice-daily dose, “the question is now whether to move forward with the 20-mg or the 30-mg dose,” said Dr. French, who said planning of a phase 3 trial is underway.
Phase 2 study of roflumilast
However, this was not the only set of data on an oral PDE4 inhibitor presented as a late-breaker at the AAD meeting. For clinicians looking for a more immediate and less expensive alternative to apremilast, another study indicated that off-label use of oral roflumilast is an option.
In an investigator-initiated, multicenter, double-blind, placebo-controlled trial conducted in Denmark, the rate of response to oral roflumilast at 24 weeks, including the clear or almost clear response, was on the same general order of magnitude as that seen in the orismilast study, reported Alexander Egeberg, MD, PhD, professor of dermatology, University of Copenhagen.
“At 24 weeks, 21.7% had achieved a PASI 90, and 8.7% achieved a PASI 100,” Dr. Egeberg said.
Oral roflumilast has been available for the treatment of COPD for more than 10 years and is now available in a generic formulation. This study was conducted independent of any pharmaceutical company involvement, and the high rate of response and low risk of adverse events suggests that patients can benefit from a PDE4 inhibitor in a very low-cost form.
“Generic oral roflumilast is cheaper than a Starbucks coffee,” Dr. Egeberg said.
In this trial, 46 patients were randomly assigned to placebo or to the COPD-approved roflumilast dose of 500 mcg once daily. The primary endpoint was change in PASI scores from baseline to week 12, which Dr. Egeberg pointed out is a shorter time frame than the 16 weeks more typical of psoriasis treatment studies.
At week 12, the median improvement in PASI was 34.8% in the roflumilast group versus 0% in the placebo group. Patients were then followed for an additional 12 weeks, but those randomized to placebo were switched to the active treatment. By week 24, the switch patients had largely caught up to those initiated on roflumilast for median PASI improvement (39.1% vs. 43.5%).
Similar to orismilast, roflumilast “was generally well tolerated,” Dr. Egeberg said. The adverse events were consistent with those associated with PDE4 inhibitors in previous trials, whether in psoriasis or COPD. There was only one serious adverse event, and it was not considered treatment related. Discontinuations for adverse events “were very low.”
In a population with a relatively high rate of smoking, Dr. Egeberg further reported, lung function was improved, a remark initially interpreted as a joke by some attending the presentation. However, Dr. Egeberg confirmed that lung function was monitored, and objective improvements were recorded.
By Danish law, the investigators were required to inform the manufacturers of roflumilast. Despite the results of this study, he is not aware of any plans to seek an indication for roflumilast in psoriasis, but he noted that the drug is readily available at a low price.
For those willing to offer this therapy off label, “you can start using it tomorrow if you’d like,” he said.
Dr. French reports financial relationships with Almirall, Amgen, Biotest, Galderma, Janssen Cilag, Leo Pharma, Pincell, Regeneron, UCB, and UNION Therapeutics, which provided funding for this trial. Dr. Egeberg reports financial relationships with Eli Lilly, Galderma, Janssen-Cilag, Novartis, and Pfizer.
A version of this article first appeared on Medscape.com.
AT AAD 2023
Poor bone health is a ‘robust’ dementia risk factor
After adjusting for relevant factors, adults with the lowest versus highest BMD at the femoral neck were 42% more likely to develop dementia over roughly 10 years.
“Our research has found a link between bone loss and dementia, but further studies are needed to better understand this connection between bone density and memory loss,” study investigator Mohammad Arfan Ikram, MD, PhD, with Erasmus University Medical Center in Rotterdam, the Netherlands, said in a statement.
“It’s possible that bone loss may occur already in the earliest phases of dementia, years before any clinical symptoms manifest themselves. If that were the case, bone loss could be an indicator of risk for dementia and people with bone loss could be targeted for screening and improved care,” Dr. Ikram added.
The study was published online in Neurology.
Common bedfellows
Low BMD and dementia commonly co-occur in the older population, with bone loss accelerating in dementia patients because of physical inactivity and poor nutrition. However, the extent to which bone loss already exists prior to the onset of dementia remains unclear.
The new findings are based on 3,651 adults (mean age 72 years, 58% women) in the Rotterdam Study who were free of dementia between 2002 and 2005. At that time, BMD at the femoral neck, lumbar spine, and total body were obtained using dual-energy radiography absorptiometry (DXA) and the trabecular bone score, which offers further details such as bone microarchitecture, was calculated. Participants were followed up until Jan. 1, 2020.
Analyses were adjusted for age, sex, education, physical activity, smoking status, body mass index, blood pressure, cholesterol, history of comorbidities (stroke and diabetes), and apolipoprotein E genotype.
During follow-up, 688 (19%) participants developed dementia, mostly Alzheimer’s disease (77%).
Throughout the entire follow-up period, lower BMD at the femoral neck (per standard deviation), but not at other bone sites, correlated with a higher risk for all-cause dementia (hazard ratio, 1.12; 95% confidence interval, 1.02-1.23) and Alzheimer’s disease (HR, 1.14; 95% CI, 1.02-1.28).
Within the first 10 years after baseline, the risk for dementia was greatest in individuals with the lowest BMD at the femoral neck (HR, 2.03; 95% CI, 1.39-2.96) and total body (HR, 1.42; 95% CI, 1.01-2.02) and lowest trabecular bone score (HR, 1.59; 95% CI, 1.11-2.28).
Only BMD at the femoral neck was related to incident all-cause dementia in the first 5 years of follow-up (HR, 2.13; 95% CI, 1.28-3.57).
These findings add “extra knowledge to previous findings that associations change with time, with the strength of the effect decreasing with increasing follow-up time,” the investigators noted.
They suggest that total BMD and trabecular bone score might occur as “prodromal features instead of causes of dementia and related toxic protein accumulation in the brain. In other words, persons with subclinical, incipient dementia may have poor bone health due to the dementia process instead of vice versa.”
The investigators noted that further research focusing on the predictive ability of BMD for dementia is necessary. “As an indicator of dementia risk, intervening in BMD may improve clinical care of these persons, especially considering the multicomorbidities and polypharmacy that are highly preventive in this group,” they concluded.
Little known bone-brain axis to blame?
In a comment, Shaheen Lakhan, MD, a neurologist and researcher in Boston, noted that “bone health is increasingly becoming front of mind in older adults. This study confirms an association between poor bone health – low bone mineral density and bone scores – and poor brain health.”
However, it’s unclear whether the link is causal – that is, whether poor bone health actually leads to poor brain health, and whether that can be staved off by directly supporting bone density,” Dr. Lakhan said.
“The link may very well be the little known ‘brain-bone axis’ – where our bones actually regulate our brain,” he added.
“Take for example the bone-generated hormone osteocalcin that crosses the blood-brain barrier and regulates brain functions like memory and cognition. Mice who don’t express the osteocalcin gene or are injected with antibodies that block osteocalcin actually have poor memory and worse anxiety,” Dr. Lakhan said.
“In any event, good bone health begins with healthy habits: a diet with plenty of calcium, vitamin D, and protein; a regimen of not just cardio, but also weight-bearing exercises; and staying clear of smoking and heavy alcohol intake,” he concluded.
The study was funded by Erasmus Medical Center and Erasmus University Rotterdam, the Netherlands Organization for Scientific Research, the Netherlands Organization for Health Research and Development, the Research Institute for Diseases in the Elderly, the Netherlands Genomics Initiative, the Ministry of Education, Culture and Science, the Ministry of Health, Welfare and Sports, the European Commission, and the Municipality of Rotterdam. Dr. Ikram and Dr. Lakhan report no relevant disclosures.
A version of this article first appeared on Medscape.com.
After adjusting for relevant factors, adults with the lowest versus highest BMD at the femoral neck were 42% more likely to develop dementia over roughly 10 years.
“Our research has found a link between bone loss and dementia, but further studies are needed to better understand this connection between bone density and memory loss,” study investigator Mohammad Arfan Ikram, MD, PhD, with Erasmus University Medical Center in Rotterdam, the Netherlands, said in a statement.
“It’s possible that bone loss may occur already in the earliest phases of dementia, years before any clinical symptoms manifest themselves. If that were the case, bone loss could be an indicator of risk for dementia and people with bone loss could be targeted for screening and improved care,” Dr. Ikram added.
The study was published online in Neurology.
Common bedfellows
Low BMD and dementia commonly co-occur in the older population, with bone loss accelerating in dementia patients because of physical inactivity and poor nutrition. However, the extent to which bone loss already exists prior to the onset of dementia remains unclear.
The new findings are based on 3,651 adults (mean age 72 years, 58% women) in the Rotterdam Study who were free of dementia between 2002 and 2005. At that time, BMD at the femoral neck, lumbar spine, and total body were obtained using dual-energy radiography absorptiometry (DXA) and the trabecular bone score, which offers further details such as bone microarchitecture, was calculated. Participants were followed up until Jan. 1, 2020.
Analyses were adjusted for age, sex, education, physical activity, smoking status, body mass index, blood pressure, cholesterol, history of comorbidities (stroke and diabetes), and apolipoprotein E genotype.
During follow-up, 688 (19%) participants developed dementia, mostly Alzheimer’s disease (77%).
Throughout the entire follow-up period, lower BMD at the femoral neck (per standard deviation), but not at other bone sites, correlated with a higher risk for all-cause dementia (hazard ratio, 1.12; 95% confidence interval, 1.02-1.23) and Alzheimer’s disease (HR, 1.14; 95% CI, 1.02-1.28).
Within the first 10 years after baseline, the risk for dementia was greatest in individuals with the lowest BMD at the femoral neck (HR, 2.03; 95% CI, 1.39-2.96) and total body (HR, 1.42; 95% CI, 1.01-2.02) and lowest trabecular bone score (HR, 1.59; 95% CI, 1.11-2.28).
Only BMD at the femoral neck was related to incident all-cause dementia in the first 5 years of follow-up (HR, 2.13; 95% CI, 1.28-3.57).
These findings add “extra knowledge to previous findings that associations change with time, with the strength of the effect decreasing with increasing follow-up time,” the investigators noted.
They suggest that total BMD and trabecular bone score might occur as “prodromal features instead of causes of dementia and related toxic protein accumulation in the brain. In other words, persons with subclinical, incipient dementia may have poor bone health due to the dementia process instead of vice versa.”
The investigators noted that further research focusing on the predictive ability of BMD for dementia is necessary. “As an indicator of dementia risk, intervening in BMD may improve clinical care of these persons, especially considering the multicomorbidities and polypharmacy that are highly preventive in this group,” they concluded.
Little known bone-brain axis to blame?
In a comment, Shaheen Lakhan, MD, a neurologist and researcher in Boston, noted that “bone health is increasingly becoming front of mind in older adults. This study confirms an association between poor bone health – low bone mineral density and bone scores – and poor brain health.”
However, it’s unclear whether the link is causal – that is, whether poor bone health actually leads to poor brain health, and whether that can be staved off by directly supporting bone density,” Dr. Lakhan said.
“The link may very well be the little known ‘brain-bone axis’ – where our bones actually regulate our brain,” he added.
“Take for example the bone-generated hormone osteocalcin that crosses the blood-brain barrier and regulates brain functions like memory and cognition. Mice who don’t express the osteocalcin gene or are injected with antibodies that block osteocalcin actually have poor memory and worse anxiety,” Dr. Lakhan said.
“In any event, good bone health begins with healthy habits: a diet with plenty of calcium, vitamin D, and protein; a regimen of not just cardio, but also weight-bearing exercises; and staying clear of smoking and heavy alcohol intake,” he concluded.
The study was funded by Erasmus Medical Center and Erasmus University Rotterdam, the Netherlands Organization for Scientific Research, the Netherlands Organization for Health Research and Development, the Research Institute for Diseases in the Elderly, the Netherlands Genomics Initiative, the Ministry of Education, Culture and Science, the Ministry of Health, Welfare and Sports, the European Commission, and the Municipality of Rotterdam. Dr. Ikram and Dr. Lakhan report no relevant disclosures.
A version of this article first appeared on Medscape.com.
After adjusting for relevant factors, adults with the lowest versus highest BMD at the femoral neck were 42% more likely to develop dementia over roughly 10 years.
“Our research has found a link between bone loss and dementia, but further studies are needed to better understand this connection between bone density and memory loss,” study investigator Mohammad Arfan Ikram, MD, PhD, with Erasmus University Medical Center in Rotterdam, the Netherlands, said in a statement.
“It’s possible that bone loss may occur already in the earliest phases of dementia, years before any clinical symptoms manifest themselves. If that were the case, bone loss could be an indicator of risk for dementia and people with bone loss could be targeted for screening and improved care,” Dr. Ikram added.
The study was published online in Neurology.
Common bedfellows
Low BMD and dementia commonly co-occur in the older population, with bone loss accelerating in dementia patients because of physical inactivity and poor nutrition. However, the extent to which bone loss already exists prior to the onset of dementia remains unclear.
The new findings are based on 3,651 adults (mean age 72 years, 58% women) in the Rotterdam Study who were free of dementia between 2002 and 2005. At that time, BMD at the femoral neck, lumbar spine, and total body were obtained using dual-energy radiography absorptiometry (DXA) and the trabecular bone score, which offers further details such as bone microarchitecture, was calculated. Participants were followed up until Jan. 1, 2020.
Analyses were adjusted for age, sex, education, physical activity, smoking status, body mass index, blood pressure, cholesterol, history of comorbidities (stroke and diabetes), and apolipoprotein E genotype.
During follow-up, 688 (19%) participants developed dementia, mostly Alzheimer’s disease (77%).
Throughout the entire follow-up period, lower BMD at the femoral neck (per standard deviation), but not at other bone sites, correlated with a higher risk for all-cause dementia (hazard ratio, 1.12; 95% confidence interval, 1.02-1.23) and Alzheimer’s disease (HR, 1.14; 95% CI, 1.02-1.28).
Within the first 10 years after baseline, the risk for dementia was greatest in individuals with the lowest BMD at the femoral neck (HR, 2.03; 95% CI, 1.39-2.96) and total body (HR, 1.42; 95% CI, 1.01-2.02) and lowest trabecular bone score (HR, 1.59; 95% CI, 1.11-2.28).
Only BMD at the femoral neck was related to incident all-cause dementia in the first 5 years of follow-up (HR, 2.13; 95% CI, 1.28-3.57).
These findings add “extra knowledge to previous findings that associations change with time, with the strength of the effect decreasing with increasing follow-up time,” the investigators noted.
They suggest that total BMD and trabecular bone score might occur as “prodromal features instead of causes of dementia and related toxic protein accumulation in the brain. In other words, persons with subclinical, incipient dementia may have poor bone health due to the dementia process instead of vice versa.”
The investigators noted that further research focusing on the predictive ability of BMD for dementia is necessary. “As an indicator of dementia risk, intervening in BMD may improve clinical care of these persons, especially considering the multicomorbidities and polypharmacy that are highly preventive in this group,” they concluded.
Little known bone-brain axis to blame?
In a comment, Shaheen Lakhan, MD, a neurologist and researcher in Boston, noted that “bone health is increasingly becoming front of mind in older adults. This study confirms an association between poor bone health – low bone mineral density and bone scores – and poor brain health.”
However, it’s unclear whether the link is causal – that is, whether poor bone health actually leads to poor brain health, and whether that can be staved off by directly supporting bone density,” Dr. Lakhan said.
“The link may very well be the little known ‘brain-bone axis’ – where our bones actually regulate our brain,” he added.
“Take for example the bone-generated hormone osteocalcin that crosses the blood-brain barrier and regulates brain functions like memory and cognition. Mice who don’t express the osteocalcin gene or are injected with antibodies that block osteocalcin actually have poor memory and worse anxiety,” Dr. Lakhan said.
“In any event, good bone health begins with healthy habits: a diet with plenty of calcium, vitamin D, and protein; a regimen of not just cardio, but also weight-bearing exercises; and staying clear of smoking and heavy alcohol intake,” he concluded.
The study was funded by Erasmus Medical Center and Erasmus University Rotterdam, the Netherlands Organization for Scientific Research, the Netherlands Organization for Health Research and Development, the Research Institute for Diseases in the Elderly, the Netherlands Genomics Initiative, the Ministry of Education, Culture and Science, the Ministry of Health, Welfare and Sports, the European Commission, and the Municipality of Rotterdam. Dr. Ikram and Dr. Lakhan report no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM NEUROLOGY
Longer telomeres tied to better brain health
, new research suggests.
“This is the largest and most systematic investigation of telomere length and brain structure and function,” said Anya Topiwala, of the University of Oxford (England). “We found that longer telomeres associated with protection against dementia. The links with brain structure, we think, offer a possible mechanism for this protection. The hope is, by understanding the mechanism, new treatment targets could be uncovered,” Dr. Topiwala said.
The study was published online in PLOS ONE.
UK Biobank cohort
Telomeres form protective caps at the ends of chromosomes, and they progressively shorten with age, which may increase susceptibility to age-related diseases including Alzheimer’s disease. The mechanism underlying this risk is unclear and may involve changes in brain structure and function. However, the relationship between telomere length and neuroimaging markers is poorly characterized.
Dr. Topiwala and colleagues compared telomere length in white blood cells to brain MRI and health record data in 31,661 middle-aged and older adults in UK Biobank. They found that longer leucocyte telomere length (LTL) was associated with a larger volume of global and subcortical grey matter and a larger hippocampus – both of which shrink in patients with Alzheimer’s disease. Longer telomeres were also associated with a thicker cerebral cortex, which thins as Alzheimer’s disease progresses.
Longer LTL was also associated with reduced incidence of dementia during follow-up (hazard ratio, 0.93; 95% confidence interval, 0.91-0.96).
Dr. Topiwala noted that many of the factors related to telomere shortening, such as age, genetics, and sex, can’t be changed. However, in a previous study, her team found that drinking alcohol may shorten telomere length. “So by this logic, reducing your alcohol intake could curb the shortening,” Dr. Topiwala said.
She said that a limitation of the study is that telomere length was measured in blood rather than brain and that it’s not clear at present how closely the two relate. Also, UK Biobank participants are generally more healthy than is the general population. Also, though telomere length and brain measures were associated, “we cannot from this study prove one is causing the other,” she added.
Need for more research
Commenting on the research, Percy Griffin, PhD, Alzheimer’s Association director of scientific engagement, said that it’s been “known for some time that shortened telomeres – the caps at the end of DNA – are associated with increased aging.”
This new study is “interesting,” said Dr. Percy, in that it shows an association between longer telomere length in white blood cells and healthier brain structures in the areas associated with Alzheimer’s disease. The longer telomeres were also associated with lower incidence of all-cause dementia.
But echoing Dr. Topiwala, “association does not mean causation,” Dr. Griffin said. “More research is needed to understand how diverse mechanisms contributing to Alzheimer’s and other dementia can be targeted.”
“The Alzheimer’s Association is accelerating the discovery of novel therapies through its Part the Cloud funding program, which has invested more than $65 million to accelerate the development of 65 drug development programs,” Dr. Griffin said.
The study had no specific funding. Dr. Topiwala and Dr. Griffin report no relevant disclosures.
A version of this article first appeared on Medscape.com.
, new research suggests.
“This is the largest and most systematic investigation of telomere length and brain structure and function,” said Anya Topiwala, of the University of Oxford (England). “We found that longer telomeres associated with protection against dementia. The links with brain structure, we think, offer a possible mechanism for this protection. The hope is, by understanding the mechanism, new treatment targets could be uncovered,” Dr. Topiwala said.
The study was published online in PLOS ONE.
UK Biobank cohort
Telomeres form protective caps at the ends of chromosomes, and they progressively shorten with age, which may increase susceptibility to age-related diseases including Alzheimer’s disease. The mechanism underlying this risk is unclear and may involve changes in brain structure and function. However, the relationship between telomere length and neuroimaging markers is poorly characterized.
Dr. Topiwala and colleagues compared telomere length in white blood cells to brain MRI and health record data in 31,661 middle-aged and older adults in UK Biobank. They found that longer leucocyte telomere length (LTL) was associated with a larger volume of global and subcortical grey matter and a larger hippocampus – both of which shrink in patients with Alzheimer’s disease. Longer telomeres were also associated with a thicker cerebral cortex, which thins as Alzheimer’s disease progresses.
Longer LTL was also associated with reduced incidence of dementia during follow-up (hazard ratio, 0.93; 95% confidence interval, 0.91-0.96).
Dr. Topiwala noted that many of the factors related to telomere shortening, such as age, genetics, and sex, can’t be changed. However, in a previous study, her team found that drinking alcohol may shorten telomere length. “So by this logic, reducing your alcohol intake could curb the shortening,” Dr. Topiwala said.
She said that a limitation of the study is that telomere length was measured in blood rather than brain and that it’s not clear at present how closely the two relate. Also, UK Biobank participants are generally more healthy than is the general population. Also, though telomere length and brain measures were associated, “we cannot from this study prove one is causing the other,” she added.
Need for more research
Commenting on the research, Percy Griffin, PhD, Alzheimer’s Association director of scientific engagement, said that it’s been “known for some time that shortened telomeres – the caps at the end of DNA – are associated with increased aging.”
This new study is “interesting,” said Dr. Percy, in that it shows an association between longer telomere length in white blood cells and healthier brain structures in the areas associated with Alzheimer’s disease. The longer telomeres were also associated with lower incidence of all-cause dementia.
But echoing Dr. Topiwala, “association does not mean causation,” Dr. Griffin said. “More research is needed to understand how diverse mechanisms contributing to Alzheimer’s and other dementia can be targeted.”
“The Alzheimer’s Association is accelerating the discovery of novel therapies through its Part the Cloud funding program, which has invested more than $65 million to accelerate the development of 65 drug development programs,” Dr. Griffin said.
The study had no specific funding. Dr. Topiwala and Dr. Griffin report no relevant disclosures.
A version of this article first appeared on Medscape.com.
, new research suggests.
“This is the largest and most systematic investigation of telomere length and brain structure and function,” said Anya Topiwala, of the University of Oxford (England). “We found that longer telomeres associated with protection against dementia. The links with brain structure, we think, offer a possible mechanism for this protection. The hope is, by understanding the mechanism, new treatment targets could be uncovered,” Dr. Topiwala said.
The study was published online in PLOS ONE.
UK Biobank cohort
Telomeres form protective caps at the ends of chromosomes, and they progressively shorten with age, which may increase susceptibility to age-related diseases including Alzheimer’s disease. The mechanism underlying this risk is unclear and may involve changes in brain structure and function. However, the relationship between telomere length and neuroimaging markers is poorly characterized.
Dr. Topiwala and colleagues compared telomere length in white blood cells to brain MRI and health record data in 31,661 middle-aged and older adults in UK Biobank. They found that longer leucocyte telomere length (LTL) was associated with a larger volume of global and subcortical grey matter and a larger hippocampus – both of which shrink in patients with Alzheimer’s disease. Longer telomeres were also associated with a thicker cerebral cortex, which thins as Alzheimer’s disease progresses.
Longer LTL was also associated with reduced incidence of dementia during follow-up (hazard ratio, 0.93; 95% confidence interval, 0.91-0.96).
Dr. Topiwala noted that many of the factors related to telomere shortening, such as age, genetics, and sex, can’t be changed. However, in a previous study, her team found that drinking alcohol may shorten telomere length. “So by this logic, reducing your alcohol intake could curb the shortening,” Dr. Topiwala said.
She said that a limitation of the study is that telomere length was measured in blood rather than brain and that it’s not clear at present how closely the two relate. Also, UK Biobank participants are generally more healthy than is the general population. Also, though telomere length and brain measures were associated, “we cannot from this study prove one is causing the other,” she added.
Need for more research
Commenting on the research, Percy Griffin, PhD, Alzheimer’s Association director of scientific engagement, said that it’s been “known for some time that shortened telomeres – the caps at the end of DNA – are associated with increased aging.”
This new study is “interesting,” said Dr. Percy, in that it shows an association between longer telomere length in white blood cells and healthier brain structures in the areas associated with Alzheimer’s disease. The longer telomeres were also associated with lower incidence of all-cause dementia.
But echoing Dr. Topiwala, “association does not mean causation,” Dr. Griffin said. “More research is needed to understand how diverse mechanisms contributing to Alzheimer’s and other dementia can be targeted.”
“The Alzheimer’s Association is accelerating the discovery of novel therapies through its Part the Cloud funding program, which has invested more than $65 million to accelerate the development of 65 drug development programs,” Dr. Griffin said.
The study had no specific funding. Dr. Topiwala and Dr. Griffin report no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM PLOS ONE
The desk
Recently, Dr. Jeffrey Benabio (I don’t believe we’ve ever met), wrote an enjoyable commentary mourning the loss of letters – the wonderful paper-and-pen documents that were, for the vast majority of human history, the main method of long distance communication. Even today, he notes, there’s something special about a letter, with the time and human effort required to sit down and put pen to paper, seal it into an envelope, and entrust it to the post office.
In his piece, Dr. Benabio describes his work desk as “a small surface, perhaps just enough for the monitor and a mug ... it has no drawers. It is lean and immaculate, but it has no soul.”
With all due respect, I can’t do that. I need a desk to function. A REAL one.
I was 9 when I got my first desk, far more than a 4th-grader needed. My dad was an attorney and had an extra desk from a partner who’d retired. It was big and heavy and made of wood. It had three drawers on each side, one in the middle, and pull-outs on each side in case you needed even more writing space. I loved it. As the years went by I did homework, wrote short stories, and built models on it. I covered the pull-outs with stickers for starship controls, so on a whim I could jump to hyperspace. In 1984 a brand-new Apple Macintosh, with 128K of RAM showed up on it. I began using the computer to write college papers, but most of my work at the desk still involved books and handwriting.
My current home desk has been with me through college, medical school, residency, and fellowship, and it continues with me today.
At my office, though, is my main desk. Before 2013 I was in a small back office, with only room for a tiny three-drawer college desk.
But in 2013 I moved into my own office, for the first time in my career. Now it was time to bring in my real desk, waiting in storage since my Dad had retired.
This is my desk now. It’s huge. It’s heavy. My dad bought it when he started his law practice in 1968. It has eight drawers, and my Dad’s original leather blotter is on top. It came with his chrome and brass letter opener in the top drawer. It has space for my computer, writing pads, exam tools (for people who can’t get on the exam table across the hall), business cards, a few baubles from my kids, stapler, tape dispenser, pen cup, phone, coffee mug, and a million other things.
It takes up a lot of space, but I don’t mind. There’s a human comfort to it and the organized disorder on top of it.
Everyone practices medicine differently. What works for me isn’t going to work for another doctor, and definitely not for another specialty.
But here, the big desk is part of my personal style. Sitting there gets me into “doctor mode” each day. I hope the more casual surroundings make it comfortable for patients, too.
It’s part of the soul of my practice, and I wouldn’t have it any other way.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Recently, Dr. Jeffrey Benabio (I don’t believe we’ve ever met), wrote an enjoyable commentary mourning the loss of letters – the wonderful paper-and-pen documents that were, for the vast majority of human history, the main method of long distance communication. Even today, he notes, there’s something special about a letter, with the time and human effort required to sit down and put pen to paper, seal it into an envelope, and entrust it to the post office.
In his piece, Dr. Benabio describes his work desk as “a small surface, perhaps just enough for the monitor and a mug ... it has no drawers. It is lean and immaculate, but it has no soul.”
With all due respect, I can’t do that. I need a desk to function. A REAL one.
I was 9 when I got my first desk, far more than a 4th-grader needed. My dad was an attorney and had an extra desk from a partner who’d retired. It was big and heavy and made of wood. It had three drawers on each side, one in the middle, and pull-outs on each side in case you needed even more writing space. I loved it. As the years went by I did homework, wrote short stories, and built models on it. I covered the pull-outs with stickers for starship controls, so on a whim I could jump to hyperspace. In 1984 a brand-new Apple Macintosh, with 128K of RAM showed up on it. I began using the computer to write college papers, but most of my work at the desk still involved books and handwriting.
My current home desk has been with me through college, medical school, residency, and fellowship, and it continues with me today.
At my office, though, is my main desk. Before 2013 I was in a small back office, with only room for a tiny three-drawer college desk.
But in 2013 I moved into my own office, for the first time in my career. Now it was time to bring in my real desk, waiting in storage since my Dad had retired.
This is my desk now. It’s huge. It’s heavy. My dad bought it when he started his law practice in 1968. It has eight drawers, and my Dad’s original leather blotter is on top. It came with his chrome and brass letter opener in the top drawer. It has space for my computer, writing pads, exam tools (for people who can’t get on the exam table across the hall), business cards, a few baubles from my kids, stapler, tape dispenser, pen cup, phone, coffee mug, and a million other things.
It takes up a lot of space, but I don’t mind. There’s a human comfort to it and the organized disorder on top of it.
Everyone practices medicine differently. What works for me isn’t going to work for another doctor, and definitely not for another specialty.
But here, the big desk is part of my personal style. Sitting there gets me into “doctor mode” each day. I hope the more casual surroundings make it comfortable for patients, too.
It’s part of the soul of my practice, and I wouldn’t have it any other way.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Recently, Dr. Jeffrey Benabio (I don’t believe we’ve ever met), wrote an enjoyable commentary mourning the loss of letters – the wonderful paper-and-pen documents that were, for the vast majority of human history, the main method of long distance communication. Even today, he notes, there’s something special about a letter, with the time and human effort required to sit down and put pen to paper, seal it into an envelope, and entrust it to the post office.
In his piece, Dr. Benabio describes his work desk as “a small surface, perhaps just enough for the monitor and a mug ... it has no drawers. It is lean and immaculate, but it has no soul.”
With all due respect, I can’t do that. I need a desk to function. A REAL one.
I was 9 when I got my first desk, far more than a 4th-grader needed. My dad was an attorney and had an extra desk from a partner who’d retired. It was big and heavy and made of wood. It had three drawers on each side, one in the middle, and pull-outs on each side in case you needed even more writing space. I loved it. As the years went by I did homework, wrote short stories, and built models on it. I covered the pull-outs with stickers for starship controls, so on a whim I could jump to hyperspace. In 1984 a brand-new Apple Macintosh, with 128K of RAM showed up on it. I began using the computer to write college papers, but most of my work at the desk still involved books and handwriting.
My current home desk has been with me through college, medical school, residency, and fellowship, and it continues with me today.
At my office, though, is my main desk. Before 2013 I was in a small back office, with only room for a tiny three-drawer college desk.
But in 2013 I moved into my own office, for the first time in my career. Now it was time to bring in my real desk, waiting in storage since my Dad had retired.
This is my desk now. It’s huge. It’s heavy. My dad bought it when he started his law practice in 1968. It has eight drawers, and my Dad’s original leather blotter is on top. It came with his chrome and brass letter opener in the top drawer. It has space for my computer, writing pads, exam tools (for people who can’t get on the exam table across the hall), business cards, a few baubles from my kids, stapler, tape dispenser, pen cup, phone, coffee mug, and a million other things.
It takes up a lot of space, but I don’t mind. There’s a human comfort to it and the organized disorder on top of it.
Everyone practices medicine differently. What works for me isn’t going to work for another doctor, and definitely not for another specialty.
But here, the big desk is part of my personal style. Sitting there gets me into “doctor mode” each day. I hope the more casual surroundings make it comfortable for patients, too.
It’s part of the soul of my practice, and I wouldn’t have it any other way.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
COVID in pregnancy may affect boys’ neurodevelopment: Study
Boys born to mothers infected with SARS‐CoV‐2 during pregnancy may be more likely to receive a diagnosis of a neurodevelopmental disorder by age 12 months, according to new research.
Andrea G. Edlow, MD, MSc, with Massachusetts General Hospital and Harvard Medical School in Boston, and colleagues examined data from 18,355 births between March 1, 2020, and May 31, 2021, at eight hospitals across two health systems in Massachusetts.
Of these births, 883 (4.8%) were to individuals who tested positive for SARS‐CoV‐2 during pregnancy. Among the children exposed to SARS‐CoV‐2 in the womb, 26 (3%) received a neurodevelopmental diagnosis, including disorders of motor function, speech and language, and psychological development, by age 1 year. In the group unexposed to the virus, 1.8% received such a diagnosis.
After adjusting for factors such as race, insurance, maternal age, and preterm birth, Dr. Edlow’s group found that a positive test for SARS-CoV-2 during pregnancy was associated with an increased risk for neurodevelopmental diagnoses at 12 months among boys (adjusted odds ratio, 1.94; 95% confidence interval, 1.12-3.17; P = .01), but not among girls.
In a subset of children with data available at 18 months, the correlation among boys at that age was less pronounced and not statistically significant (aOR, 1.42; 95% CI, 0.92-2.11; P = .10).
The findings were published online in JAMA Network Open
Prior epidemiological research has suggested that maternal infection during pregnancy is associated with heightened risk for a range of neurodevelopmental disorders, including autism and schizophrenia, in offspring, the authors wrote.
“The neurodevelopmental risk associated with maternal SARS-CoV-2 infection was disproportionately high in male infants, consistent with the known increased vulnerability of males in the face of prenatal adverse exposures,” Dr. Edlow said in a news release about the findings.
Larger studies and longer follow‐up are needed to confirm and reliably estimate the risk, the researchers said.
“It is not clear that the changes we can detect at 12 and 18 months will be indicative of persistent risks for disorders such as autism spectrum disorder, intellectual disability, or schizophrenia,” they write.
New data published online by the Centers for Disease Control and Prevention show that in 11 communities in 2020, 1 in 36 (2.8%) 8-year-old children had been identified with autism spectrum disorder, an increase from 2.3% in 2018. The data also show that the early months of the pandemic may have disrupted autism detection efforts among 4-year-olds.
The investigators were supported by grants from the National Institutes of Health and the Simons Foundation Autism Research Initiative. Coauthors disclosed consulting for or receiving personal fees from biotechnology and pharmaceutical companies.
A version of this article first appeared on Medscape.com.
Boys born to mothers infected with SARS‐CoV‐2 during pregnancy may be more likely to receive a diagnosis of a neurodevelopmental disorder by age 12 months, according to new research.
Andrea G. Edlow, MD, MSc, with Massachusetts General Hospital and Harvard Medical School in Boston, and colleagues examined data from 18,355 births between March 1, 2020, and May 31, 2021, at eight hospitals across two health systems in Massachusetts.
Of these births, 883 (4.8%) were to individuals who tested positive for SARS‐CoV‐2 during pregnancy. Among the children exposed to SARS‐CoV‐2 in the womb, 26 (3%) received a neurodevelopmental diagnosis, including disorders of motor function, speech and language, and psychological development, by age 1 year. In the group unexposed to the virus, 1.8% received such a diagnosis.
After adjusting for factors such as race, insurance, maternal age, and preterm birth, Dr. Edlow’s group found that a positive test for SARS-CoV-2 during pregnancy was associated with an increased risk for neurodevelopmental diagnoses at 12 months among boys (adjusted odds ratio, 1.94; 95% confidence interval, 1.12-3.17; P = .01), but not among girls.
In a subset of children with data available at 18 months, the correlation among boys at that age was less pronounced and not statistically significant (aOR, 1.42; 95% CI, 0.92-2.11; P = .10).
The findings were published online in JAMA Network Open
Prior epidemiological research has suggested that maternal infection during pregnancy is associated with heightened risk for a range of neurodevelopmental disorders, including autism and schizophrenia, in offspring, the authors wrote.
“The neurodevelopmental risk associated with maternal SARS-CoV-2 infection was disproportionately high in male infants, consistent with the known increased vulnerability of males in the face of prenatal adverse exposures,” Dr. Edlow said in a news release about the findings.
Larger studies and longer follow‐up are needed to confirm and reliably estimate the risk, the researchers said.
“It is not clear that the changes we can detect at 12 and 18 months will be indicative of persistent risks for disorders such as autism spectrum disorder, intellectual disability, or schizophrenia,” they write.
New data published online by the Centers for Disease Control and Prevention show that in 11 communities in 2020, 1 in 36 (2.8%) 8-year-old children had been identified with autism spectrum disorder, an increase from 2.3% in 2018. The data also show that the early months of the pandemic may have disrupted autism detection efforts among 4-year-olds.
The investigators were supported by grants from the National Institutes of Health and the Simons Foundation Autism Research Initiative. Coauthors disclosed consulting for or receiving personal fees from biotechnology and pharmaceutical companies.
A version of this article first appeared on Medscape.com.
Boys born to mothers infected with SARS‐CoV‐2 during pregnancy may be more likely to receive a diagnosis of a neurodevelopmental disorder by age 12 months, according to new research.
Andrea G. Edlow, MD, MSc, with Massachusetts General Hospital and Harvard Medical School in Boston, and colleagues examined data from 18,355 births between March 1, 2020, and May 31, 2021, at eight hospitals across two health systems in Massachusetts.
Of these births, 883 (4.8%) were to individuals who tested positive for SARS‐CoV‐2 during pregnancy. Among the children exposed to SARS‐CoV‐2 in the womb, 26 (3%) received a neurodevelopmental diagnosis, including disorders of motor function, speech and language, and psychological development, by age 1 year. In the group unexposed to the virus, 1.8% received such a diagnosis.
After adjusting for factors such as race, insurance, maternal age, and preterm birth, Dr. Edlow’s group found that a positive test for SARS-CoV-2 during pregnancy was associated with an increased risk for neurodevelopmental diagnoses at 12 months among boys (adjusted odds ratio, 1.94; 95% confidence interval, 1.12-3.17; P = .01), but not among girls.
In a subset of children with data available at 18 months, the correlation among boys at that age was less pronounced and not statistically significant (aOR, 1.42; 95% CI, 0.92-2.11; P = .10).
The findings were published online in JAMA Network Open
Prior epidemiological research has suggested that maternal infection during pregnancy is associated with heightened risk for a range of neurodevelopmental disorders, including autism and schizophrenia, in offspring, the authors wrote.
“The neurodevelopmental risk associated with maternal SARS-CoV-2 infection was disproportionately high in male infants, consistent with the known increased vulnerability of males in the face of prenatal adverse exposures,” Dr. Edlow said in a news release about the findings.
Larger studies and longer follow‐up are needed to confirm and reliably estimate the risk, the researchers said.
“It is not clear that the changes we can detect at 12 and 18 months will be indicative of persistent risks for disorders such as autism spectrum disorder, intellectual disability, or schizophrenia,” they write.
New data published online by the Centers for Disease Control and Prevention show that in 11 communities in 2020, 1 in 36 (2.8%) 8-year-old children had been identified with autism spectrum disorder, an increase from 2.3% in 2018. The data also show that the early months of the pandemic may have disrupted autism detection efforts among 4-year-olds.
The investigators were supported by grants from the National Institutes of Health and the Simons Foundation Autism Research Initiative. Coauthors disclosed consulting for or receiving personal fees from biotechnology and pharmaceutical companies.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
Consider life expectancy in surveillance colonoscopy advice
Among nearly 10,000 Medicare beneficiaries, the likelihood of finding advanced polyps or colorectal cancer (CRC) on surveillance colonoscopy was low. Yet, among patients for whom any follow-up recommendation – either for or against colonoscopy – was available, the vast majority (87%) were advised to return for the procedure in the future, even if their life expectancy was limited or there were no significant findings on their surveillance colonoscopy.
“These findings suggest that recommending against future surveillance colonoscopy in older adults with low-risk colonoscopy findings and/or limited life expectancy should be considered more frequently than is currently practiced,” say Audrey Calderwood, MD, with Dartmouth-Hitchcock Medical Center in Lebanon, N.H., and colleagues.
Because of the lack of clear guidance about when to stop recommending colonoscopies to older patients, it is not surprising that physicians recommend surveillance even for patients with low life expectancy, Ziad Gellad, MD, with Duke University Medical Center, Durham, N.C., said in an interview.
“As someone who performs these procedures, I can tell you that it is not easy to tell patients that they are too old to get preventive care, especially patients in whom your only interaction is the procedure itself,” said Dr. Gellad, who wasn’t involved in the study.
The study was published online in JAMA Internal Medicine.
Key findings
For older adults, surveillance after prior findings of colon polyps is the most frequent indication for colonoscopy. Data suggest that an estimated 5.6 million adults older than 75 will undergo follow-up colonoscopy annually by 2024.
For older adults with polyps, current guidelines recommend individualized decision-making about surveillance colonoscopy. That includes weighing the potential benefits (identifying and removing meaningful lesions to prevent CRC) against the burdens and potential harms (such as bleeding or perforation).
While most colon polyps are not harmful, a subset of polyps, if allowed to grow, can develop into cancer over 10-15 years. This long biological time line highlights the importance of considering life expectancy in deciding for whom surveillance colonoscopy should be recommended, Dr. Calderwood and colleagues note.
Using data from the New Hampshire Colonoscopy Registry, which is linked with the Medicare claims database, they evaluated surveillance colonoscopy findings and follow-up advice according to severity of findings and patients’ estimated life expectancy for 9,831 adults (mean age, 73; 54% men).
Life expectancy was 10+ years for 57.5% of patients, 5 to less than 10 years for 35%, and less than 5 years for 7.5%.
Overall, 791 patients (8%) were found to have advanced polyps (7.8%) or CRC (0.2%) on surveillance colonoscopy.
Recommendations to stop or continue future colonoscopy were available for 5,281 patients (53.7%). Among them, 4,588 (86.9%) were recommended to return for future colonoscopy, even when there were no significant colonoscopy findings or the patient’s life expectancy was limited.
Compared with life expectancy of less than 5 years, longer life expectancy was associated with advice to return for future colonoscopy regardless of clinical findings, with adjusted odds ratios of 21.5 and 2.7, respectively, for life expectancy of 10 or more years and of 5 to less than 10 years.
Among patients with no significant findings, 95% of those with life expectancy of 10 or more years were recommended to undergo repeat colonoscopy down the road, compared with 58% of those with estimated life expectancy of less than 5 years.
Among patients expected to live 5 to less than 10 years, future repeat colonoscopy was recommended for 75% who had no significant findings, 82% with one or two small polyps, and 88% with multiple polyps, advanced polyps, or CRC.
The recommended time to repeat colonoscopy was greater than life expectancy for 6.6% of patients with less than 5 years of life expectancy and 6% with 5 to less than 10 years of life expectancy.
Nuanced decisions
The findings “may help refine decision-making” about the potential benefits and harms of pursuing or stopping surveillance colonoscopy for older adults who have a history of polyps, Dr. Calderwood and colleagues say.
The risk for a colonoscopy complication has been estimated at 26 per 1,000 people, they note. That’s nearly 10 times greater than the potential benefits seen in their study (that is, identification of CRC in 23 of 9,831 people, or about 2.3 per 1,000).
In the study cohort, 10% of patients had comorbid conditions that have been associated with a higher risk for colonoscopy complications. Those with life expectancy of less than 5 years had higher rates of inadequate bowel preparation, which also is associated with increased risk for colonoscopy complications, including perforation.
Dr. Calderwood and colleagues suggest that clinicians use evidence regarding life expectancy and neoplasia progression to modify their recommendations for surveillance colonoscopy for older adults in the following ways:
- If life expectancy is less than 5 years, recommend against surveillance.
- If life expectancy is 5 to less than 10 years and the patient has only low-risk polyps, recommend against surveillance.
- If the patient is healthy with a life expectancy of 10+ years and has recently been found to have advanced polyps, recommend future surveillance colonoscopy, with a caveat that the ultimate decision is dependent on health and priorities at the time the colonoscopy is due to be performed.
- If future health is unknown or unclear, avoid giving definitive recommendations for future surveillance to allow the flexibility of deciding on the basis of risk and benefit when the time comes.
In comments to this news organization, Dr. Gellad noted that an assessment of patient life expectancy “is not readily accessible at the point of care. These are nuanced decisions that require shared decision-making. Sometimes that is best handled outside the procedure setting.”
Support for the study was provided by the National Cancer Institute. The authors have disclosed no relevant financial relationships. Dr. Gellad is a consultant for Merck and Novo Nordisk and is a cofounder of Higgs Boson.
A version of this article originally appeared on Medscape.com.
Among nearly 10,000 Medicare beneficiaries, the likelihood of finding advanced polyps or colorectal cancer (CRC) on surveillance colonoscopy was low. Yet, among patients for whom any follow-up recommendation – either for or against colonoscopy – was available, the vast majority (87%) were advised to return for the procedure in the future, even if their life expectancy was limited or there were no significant findings on their surveillance colonoscopy.
“These findings suggest that recommending against future surveillance colonoscopy in older adults with low-risk colonoscopy findings and/or limited life expectancy should be considered more frequently than is currently practiced,” say Audrey Calderwood, MD, with Dartmouth-Hitchcock Medical Center in Lebanon, N.H., and colleagues.
Because of the lack of clear guidance about when to stop recommending colonoscopies to older patients, it is not surprising that physicians recommend surveillance even for patients with low life expectancy, Ziad Gellad, MD, with Duke University Medical Center, Durham, N.C., said in an interview.
“As someone who performs these procedures, I can tell you that it is not easy to tell patients that they are too old to get preventive care, especially patients in whom your only interaction is the procedure itself,” said Dr. Gellad, who wasn’t involved in the study.
The study was published online in JAMA Internal Medicine.
Key findings
For older adults, surveillance after prior findings of colon polyps is the most frequent indication for colonoscopy. Data suggest that an estimated 5.6 million adults older than 75 will undergo follow-up colonoscopy annually by 2024.
For older adults with polyps, current guidelines recommend individualized decision-making about surveillance colonoscopy. That includes weighing the potential benefits (identifying and removing meaningful lesions to prevent CRC) against the burdens and potential harms (such as bleeding or perforation).
While most colon polyps are not harmful, a subset of polyps, if allowed to grow, can develop into cancer over 10-15 years. This long biological time line highlights the importance of considering life expectancy in deciding for whom surveillance colonoscopy should be recommended, Dr. Calderwood and colleagues note.
Using data from the New Hampshire Colonoscopy Registry, which is linked with the Medicare claims database, they evaluated surveillance colonoscopy findings and follow-up advice according to severity of findings and patients’ estimated life expectancy for 9,831 adults (mean age, 73; 54% men).
Life expectancy was 10+ years for 57.5% of patients, 5 to less than 10 years for 35%, and less than 5 years for 7.5%.
Overall, 791 patients (8%) were found to have advanced polyps (7.8%) or CRC (0.2%) on surveillance colonoscopy.
Recommendations to stop or continue future colonoscopy were available for 5,281 patients (53.7%). Among them, 4,588 (86.9%) were recommended to return for future colonoscopy, even when there were no significant colonoscopy findings or the patient’s life expectancy was limited.
Compared with life expectancy of less than 5 years, longer life expectancy was associated with advice to return for future colonoscopy regardless of clinical findings, with adjusted odds ratios of 21.5 and 2.7, respectively, for life expectancy of 10 or more years and of 5 to less than 10 years.
Among patients with no significant findings, 95% of those with life expectancy of 10 or more years were recommended to undergo repeat colonoscopy down the road, compared with 58% of those with estimated life expectancy of less than 5 years.
Among patients expected to live 5 to less than 10 years, future repeat colonoscopy was recommended for 75% who had no significant findings, 82% with one or two small polyps, and 88% with multiple polyps, advanced polyps, or CRC.
The recommended time to repeat colonoscopy was greater than life expectancy for 6.6% of patients with less than 5 years of life expectancy and 6% with 5 to less than 10 years of life expectancy.
Nuanced decisions
The findings “may help refine decision-making” about the potential benefits and harms of pursuing or stopping surveillance colonoscopy for older adults who have a history of polyps, Dr. Calderwood and colleagues say.
The risk for a colonoscopy complication has been estimated at 26 per 1,000 people, they note. That’s nearly 10 times greater than the potential benefits seen in their study (that is, identification of CRC in 23 of 9,831 people, or about 2.3 per 1,000).
In the study cohort, 10% of patients had comorbid conditions that have been associated with a higher risk for colonoscopy complications. Those with life expectancy of less than 5 years had higher rates of inadequate bowel preparation, which also is associated with increased risk for colonoscopy complications, including perforation.
Dr. Calderwood and colleagues suggest that clinicians use evidence regarding life expectancy and neoplasia progression to modify their recommendations for surveillance colonoscopy for older adults in the following ways:
- If life expectancy is less than 5 years, recommend against surveillance.
- If life expectancy is 5 to less than 10 years and the patient has only low-risk polyps, recommend against surveillance.
- If the patient is healthy with a life expectancy of 10+ years and has recently been found to have advanced polyps, recommend future surveillance colonoscopy, with a caveat that the ultimate decision is dependent on health and priorities at the time the colonoscopy is due to be performed.
- If future health is unknown or unclear, avoid giving definitive recommendations for future surveillance to allow the flexibility of deciding on the basis of risk and benefit when the time comes.
In comments to this news organization, Dr. Gellad noted that an assessment of patient life expectancy “is not readily accessible at the point of care. These are nuanced decisions that require shared decision-making. Sometimes that is best handled outside the procedure setting.”
Support for the study was provided by the National Cancer Institute. The authors have disclosed no relevant financial relationships. Dr. Gellad is a consultant for Merck and Novo Nordisk and is a cofounder of Higgs Boson.
A version of this article originally appeared on Medscape.com.
Among nearly 10,000 Medicare beneficiaries, the likelihood of finding advanced polyps or colorectal cancer (CRC) on surveillance colonoscopy was low. Yet, among patients for whom any follow-up recommendation – either for or against colonoscopy – was available, the vast majority (87%) were advised to return for the procedure in the future, even if their life expectancy was limited or there were no significant findings on their surveillance colonoscopy.
“These findings suggest that recommending against future surveillance colonoscopy in older adults with low-risk colonoscopy findings and/or limited life expectancy should be considered more frequently than is currently practiced,” say Audrey Calderwood, MD, with Dartmouth-Hitchcock Medical Center in Lebanon, N.H., and colleagues.
Because of the lack of clear guidance about when to stop recommending colonoscopies to older patients, it is not surprising that physicians recommend surveillance even for patients with low life expectancy, Ziad Gellad, MD, with Duke University Medical Center, Durham, N.C., said in an interview.
“As someone who performs these procedures, I can tell you that it is not easy to tell patients that they are too old to get preventive care, especially patients in whom your only interaction is the procedure itself,” said Dr. Gellad, who wasn’t involved in the study.
The study was published online in JAMA Internal Medicine.
Key findings
For older adults, surveillance after prior findings of colon polyps is the most frequent indication for colonoscopy. Data suggest that an estimated 5.6 million adults older than 75 will undergo follow-up colonoscopy annually by 2024.
For older adults with polyps, current guidelines recommend individualized decision-making about surveillance colonoscopy. That includes weighing the potential benefits (identifying and removing meaningful lesions to prevent CRC) against the burdens and potential harms (such as bleeding or perforation).
While most colon polyps are not harmful, a subset of polyps, if allowed to grow, can develop into cancer over 10-15 years. This long biological time line highlights the importance of considering life expectancy in deciding for whom surveillance colonoscopy should be recommended, Dr. Calderwood and colleagues note.
Using data from the New Hampshire Colonoscopy Registry, which is linked with the Medicare claims database, they evaluated surveillance colonoscopy findings and follow-up advice according to severity of findings and patients’ estimated life expectancy for 9,831 adults (mean age, 73; 54% men).
Life expectancy was 10+ years for 57.5% of patients, 5 to less than 10 years for 35%, and less than 5 years for 7.5%.
Overall, 791 patients (8%) were found to have advanced polyps (7.8%) or CRC (0.2%) on surveillance colonoscopy.
Recommendations to stop or continue future colonoscopy were available for 5,281 patients (53.7%). Among them, 4,588 (86.9%) were recommended to return for future colonoscopy, even when there were no significant colonoscopy findings or the patient’s life expectancy was limited.
Compared with life expectancy of less than 5 years, longer life expectancy was associated with advice to return for future colonoscopy regardless of clinical findings, with adjusted odds ratios of 21.5 and 2.7, respectively, for life expectancy of 10 or more years and of 5 to less than 10 years.
Among patients with no significant findings, 95% of those with life expectancy of 10 or more years were recommended to undergo repeat colonoscopy down the road, compared with 58% of those with estimated life expectancy of less than 5 years.
Among patients expected to live 5 to less than 10 years, future repeat colonoscopy was recommended for 75% who had no significant findings, 82% with one or two small polyps, and 88% with multiple polyps, advanced polyps, or CRC.
The recommended time to repeat colonoscopy was greater than life expectancy for 6.6% of patients with less than 5 years of life expectancy and 6% with 5 to less than 10 years of life expectancy.
Nuanced decisions
The findings “may help refine decision-making” about the potential benefits and harms of pursuing or stopping surveillance colonoscopy for older adults who have a history of polyps, Dr. Calderwood and colleagues say.
The risk for a colonoscopy complication has been estimated at 26 per 1,000 people, they note. That’s nearly 10 times greater than the potential benefits seen in their study (that is, identification of CRC in 23 of 9,831 people, or about 2.3 per 1,000).
In the study cohort, 10% of patients had comorbid conditions that have been associated with a higher risk for colonoscopy complications. Those with life expectancy of less than 5 years had higher rates of inadequate bowel preparation, which also is associated with increased risk for colonoscopy complications, including perforation.
Dr. Calderwood and colleagues suggest that clinicians use evidence regarding life expectancy and neoplasia progression to modify their recommendations for surveillance colonoscopy for older adults in the following ways:
- If life expectancy is less than 5 years, recommend against surveillance.
- If life expectancy is 5 to less than 10 years and the patient has only low-risk polyps, recommend against surveillance.
- If the patient is healthy with a life expectancy of 10+ years and has recently been found to have advanced polyps, recommend future surveillance colonoscopy, with a caveat that the ultimate decision is dependent on health and priorities at the time the colonoscopy is due to be performed.
- If future health is unknown or unclear, avoid giving definitive recommendations for future surveillance to allow the flexibility of deciding on the basis of risk and benefit when the time comes.
In comments to this news organization, Dr. Gellad noted that an assessment of patient life expectancy “is not readily accessible at the point of care. These are nuanced decisions that require shared decision-making. Sometimes that is best handled outside the procedure setting.”
Support for the study was provided by the National Cancer Institute. The authors have disclosed no relevant financial relationships. Dr. Gellad is a consultant for Merck and Novo Nordisk and is a cofounder of Higgs Boson.
A version of this article originally appeared on Medscape.com.
FROM JAMA INTERNAL MEDICINE
Multi-cancer early detection liquid biopsy testing: A predictive genetic test not quite ready for prime time
CASE Patient inquires about new technology to detect cancer
A 51-year-old woman (para 2) presents to your clinic for a routine gynecology exam. She is up to date on her screening mammogram and Pap testing. She has her first colonoscopy scheduled for next month. She has a 10-year remote smoking history, but she stopped smoking in her late twenties. Her cousin was recently diagnosed with skin cancer, her father had prostate cancer and is now in remission, and her paternal grandmother died of ovarian cancer. She knows ovarian cancer does not have an effective screening test, and she recently heard on the news about a new blood test that can detect cancer before symptoms start. She would like to know more about this test. Could it replace her next Pap, mammogram, and future colonoscopies? She also wants to know—How can a simple blood test detect cancer?
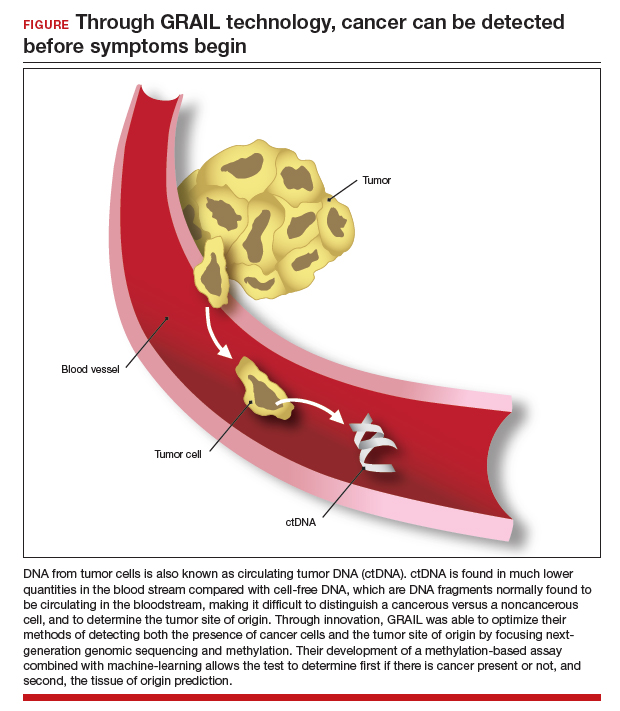
The power of genomics in cancer care
Since the first human genome was sequenced in 2000, the power of genomics has been evident across many aspects of medicine, including cancer care.1 Whereas the first human genome to be sequenced took more than 10 years to sequence and cost over $1 billion, sequencing of your entire genome can now be obtained for less than $400—with results in a week.2
Genomics is now an integral part of cancer care, with results having implications for both cancer risk and prevention as well as more individualized treatment. For example, a healthy 42-year-old patient with a strong family history of breast cancer may undergo genetic testing and discover she has a mutation in the tumor suppression gene BRCA1, which carries a 39% to 58% lifetime risk of ovarian cancer.3 By undergoing a risk-reducing bilateral salpingooophorectomy she will lower her ovarian cancer risk by up to 96%.4,5 A 67-year-old with a new diagnosis of stage III ovarian cancer and a BRCA2 mutation may be in remission for 5+ years due to her BRCA2 mutation, which makes her eligible for the use of the poly(ADPribose) polymerase (PARP) inhibitor olaparib.6 Genetic testing as illustrated above has led to decreased cancer-related mortality and prolonged survival.7 However, many women with such germline mutations are faced with difficult choices about surgical risk reduction, with the potential harms of early menopause and quality of life concerns. Having a test that does not just predict cancer risk but in fact quantifies that risk for the individual would greatly help in these decisions. Furthermore, more than 75% of ovarian cancers occur without a germline mutation.
Advances in genetic testing technology also have led to the ability to obtain genetic information from a simple blood test. For example, cell-free DNA (cfDNA), which is DNA fragments that are normally found to be circulating in the bloodstream, is routinely used as a screening tool for prenatal genetic testing to detect chromosomal abnormalities in the fetus.8 This technology relies on analyzing fetal free (non-cellular) DNA that is naturally found circulating in maternal blood. More recently, similar technology using cfDNA has been applied for the screening and characterization of certain cancers.9 This powerful technology can detect cancer before symptoms begin—all from a simple blood test, often referred to as a “liquid biopsy.” However, understanding the utility, supporting data, and target population for these tests is important before employing them as part of routine clinical practice.
Continue to: Current methods of cancer screening are limited...
Current methods of cancer screening are limited
Cancer is a leading cause of death worldwide, with nearly 10 million cancer-related deaths annually, and it may surpass cardiovascular disease as the leading cause over the course of the century.10,11 Many cancer deaths are in part due to late-stage diagnosis, when the cancer has already metastasized.12 Early detection of cancer improves outcomes and survival rates, but it is often difficult to detect early due to the lack of early symptoms with many cancers, which can limit cancer screening and issues with access to care.13
Currently, there are only 5 cancers: cervical, prostate, breast, colon, and lung (for high-risk adults) that are screened for in the general population (see "Cancer screening has helped save countless lives" at the end of this article).14 The Pap test to screen for cervical cancer, developed in the 1940s, has saved millions of women’s lives and reduced the mortality of cervical cancer by 70%.15 Coupled with the availability and implementation of the human papillomavirus (HPV) vaccine, cervical cancer rates are decreasing at substantial rates.16 However, there are no validated screening tests for uterine cancer, the most common gynecologic malignancy in the United States, or ovarian cancer, the most lethal.
Screening tests for cervical, prostate, breast, colon, and lung cancer have helped save millions of lives; however, these tests also come with high false-positive rates and the potential for overdiagnosis and overtreatment. For example, half of women undergoing mammograms will receive a false-positive result over a 10-year time period,17 and up to 50% of men undergoing prostate cancer screening have a positive prostate-specific antigen (PSA) test result when they do not actually have prostate cancer.18 Additionally, the positive predictive value of the current standard-of-care screening tests can be as low as <5%. Most diagnoses of cancer are made from a surgical biopsy, but these types of procedures can be difficult depending on the location or size of the tumor.19
The liquid biopsy. Given the limitations of current cancer screening and diagnostic tests, there is a great need for a more sensitive test that also can detect cancer from multiple organ sites. Liquid biopsy-based biomarkers can include circulating tumor cells, exosomes, microRNAs, and circulating tumor DNA (ctDNA). With advances in next-generation sequencing, ctDNA techniques remain the most promising.20
Methylation-based MCED testing: A new way of cancer screening
Multi-cancer early detection (MCED) technology was developed to address the need for better cancer screening and has the potential to detect up to 50 cancers with a simple blood test. This new technology opens the possibility for early detection of multiple cancers before symptoms even begin. MCED testing is sometimes referred to as “GRAIL” testing, after the American biotechnology company that developed the first commercially available MCED test, called the Galleri test (Galleri, Menlo Park, California). Although other biotechnology companies are developing similar technology (Exact Sciences, Madison, Wisconsin, and Freenome, South San Francisco, California, for example), this is the first test of its kind available to the public.21
The MCED test works by detecting the cfDNA fragments that are released into the blood passively by necrotic or apoptotic cells or secreted actively from tumor cells. The DNA from tumor cells is also known as circulating tumor DNA (ctDNA). CtDNA is found in much lower quantities in the blood stream compared with cfDNA from cells, making it difficult to distinguish a cancer versus a noncancer cell and to determine the tumor site of origin.22
Through innovation, the first example of detecting cancer through this method in fact came as a surprise result from an abnormal cfDNA test. A pregnant 37-yearold woman had a cfDNA result suggestive of aneuploidy for chromosomes 18 and 13; however, she gave birth to a normal male fetus. Shortly thereafter, a vaginal biopsy confirmed small-cell carcinoma with alterations in chromosomes 18 and 13.23 GRAIL testing for this patient was subsequently able to optimize their methods of detecting both the presence of cancer cells and the tumor site of origin by utilizing next-generation genomic sequencing and methylation. Their development of a methylation-based assay combined with 46 machine-learning allowed the test to determine, first, if there is cancer present or not, and second, the tissue of origin prediction. It is important to note that these tests are meant to be used in addition to standard-of-care screening tests, not as an alternative, and this is emphasized throughout the company’s website and the medical literature.24
Continue to: The process to develop and validate GRAIL’s blood-based cancer screening test...
The process to develop and validate GRAIL’s blood-based cancer screening test includes 4 large clinical trials of more than 180,000 participants, including those with cancer and those without. The Circulating Cell-Free Genome Atlas (CCGA) Study, was a prospective, case-controlled, observational study enrolling approximately 15,000 participants with 3 prespecified sub-studies. The first sub-study developed the machine-learning classifier for both early detection and tumor of origin detection.25,26
The highest performing assay from the first sub-study then went on to be further validated in the 2nd and 3rd sub-studies. The 3rd sub-study, published in the Annals of Oncology in 2021 looked at a cohort of 4,077 participants with and without cancer, and found the specificity of cancer signal detection to be 99.5% and the overall sensitivity to be 51.5%, with increasing sensitivity by cancer stage (stage I - 17%, stage II - 40%, stage III - 77%, and stage IV - 90.1%).24 The false-positive rate was low, at 0.7%, and the true positive rate was 88.7%. Notably, the test was able to correctly identify the tumor of origin for 93% of samples.24 The study overall demonstrated high specificity and accuracy of tumor site of origin and supported the use of this blood-based MCED assay.
The PATHFINDER study was another prospective, multicenter clinical trial that enrolled more than 6,000 participants in the United States. The participants were aged >50 years with or without additional cancer risk factors. The goal of this study was to determine the extent of testing required to achieve diagnosis after a “cancer signal detected” result. The study results found that, when MCED testing was added to the standard-of-care screening, the number of cancers detected doubled when compared with standard cancer screening alone.27,28 Of the 92 participants with positive cancer signals, 35 were diagnosed with cancer, and 71% of these cancer types did not have standard-ofcare screening. The tumor site of origin was correctly detected in 97% of cases, and there were less than 1% of false positives. Overall, the test led to diagnostic evaluation of 1.4% of patients and a cancer diagnosis in 0.5%.
Currently, there are 2 ongoing clinical trials to further evaluate the Galleri MCED test. The STRIVE trial that aims to prospectively validate the MCED test in a population of nearly 100,000 women undergoing mammography,29 and the SUMMIT trial,30 which is similarly aiming to validate the test in a group of individuals, half of whom have a significantly elevated risk of lung cancer.
With the promising results described above, the Galleri test became the first MCED test available for commercial use starting in 2022. It is only available for use in people who are aged 50 and older, have a family history of cancer, or are at an increased risk for cancer (although GRAIL does not elaborate on what constitutes increased risk). However, the Galleri test is only available through prescription—therefore, if interested, patients must ask their health care provider to register with GRAIL and order the test (https://www .galleri.com/hcp/the-galleri-test/ordering). Additionally, the test will cost the patient $949 and is not yet covered by insurances. Currently, several large health care groups such as the United States Department of Veterans Affairs, Cleveland Clinic, and Mercy hospitals have partnered with GRAIL to offer their test to certain patients for use as part of clinical trials. Currently, no MCED test, including the Galleri, is approved by the US Food and Drug Administration.
Incorporating MCED testing into clinical practice
The Galleri MCED test has promising potential to make multi-cancer screening feasible and obtainable, which could ultimately reduce late-stage cancer diagnosis and decrease mortality from all cancers. The compelling data from large cohorts and numerous clinical trials demonstrate its accuracy, reliability, reproducibility, and specificity. It can detect up to 50 different types of cancers, including cancers that affect our gynecologic patients, including breast, cervical, ovarian, and uterine. Additionally, its novel methylation-based assay accurately identifies the tumor site of origin in 97% of cases.28 Ongoing and future clinical trials will continue to validate and refine these methods and improve the sensitivity and positive-predictive value of this assay. As mentioned, although it has been incorporated into various large health care systems, it is not FDA approved and has not been validated in the general population. Additionally, it should not be used as a replacement for recommended screening.
CASE Resolved
The patient is eligible for the Galleri MCED test if ordered by her physician. However, she will need to pay for the test out-of-pocket. Due to her family history, she should consider germline genetic testing (either for herself, or if possible, for her father, who should meet criteria based on his prostate cancer).3 Panel testing for germline mutations has become much more accessible, and until MCED testing is ready for prime time, it remains one of the best ways to predict and prevent cancers. Additionally, she should continue to undergo routine screening for cervical, breast, and colon cancer as indicated. ●
- Mammography has helped reduce breast cancer mortality in the United States by nearly 40% since 19901
- Increases in screening for lung cancer with computed tomography in the United States are estimated to have saved more than 10,000 lives between 2014 and 20182
- Routine prostate specific antigen screening is no longer recommended for men at average risk for prostate cancer, and patients are advised to discuss risks and benefits of screening with their clinicians3
- Where screening programs have long been established, cervical cancer rates have decreased by as much as 65% over the past 40 years4
- 68% of colorectal cancer deaths could be prevented with increased screening, and one of the most effective ways to get screened is colonoscopy5
References
1. American College of Radiology website. https://www.acr.org/Practice-Management-Quality-Informatics/Practice-Toolkit/PatientResources/Mammography-Saves-Lives. Accessed March 1, 2023.
2. US lung cancer screening linked to earlier diagnosis and better survival. BMJ.com. https://www.bmj.com/company/newsroom/ us-lung-cancer-screening-linked-to-earlier-diagnosis-and-better-survival/. Accessed March 1, 2023.
3. Draisma G, Etzioni R, Tsodikov A, et al. Lead time and overdiagnosis in prostate-specific antigen screening: importance of methods and context. J Natl Cancer Inst. 2009;101:374-383.
4. Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA: Can J Clinicians. 2015;65:87-108.
5. Colon cancer coalition website. Fact check: Do colonoscopies save lives? https://coloncancercoalition.org/2022/10/11/fact-checkdo-colonoscopies-save-lives/#:~:text=According%20to%20the%20Centers%20for,get%20screened%20is%20a%20colonoscopy. Accessed March 1, 2023.
- Stratton MR, Campbell PJ, Futreal PA. The cancer genome. Nature. 2009;458:719-724.
- Davies K. The era of genomic medicine. Clin Med (Lond). 2013;13:594-601.
- National Comprehensive Cancer Network. Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic. Version 3.2023. February 13, 2023.
- Finch APM, Lubinski J, Møller P, et al. Impact of oophorectomy on cancer incidence and mortality in women with a BRCA1 or BRCA2 mutation. J Clin Oncol. 2014;32:1547-1553.
- Xiao Y-L, Wang K, Liu Q, et al. Risk reduction and survival benefit of risk-reducing salpingo-oophorectomy in hereditary breast cancer: meta-analysis and systematic review. Clin Breast Cancer. 2019;19:e48-e65.
- Moore K, Colombo N, Scambia G, et al. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2018;379:2495-2505.
- Pritchard D, Goodman C, Nadauld LD. Clinical utility of genomic testing in cancer care. JCO Precis Oncol. 2022;6:e2100349.
- Screening for fetal chromosomal abnormalities: ACOG Practice Bulletin summary, number 226. Obstet Gynecol. 2020;136:859-867.
- Yan Y-y, Guo Q-r, Wang F-h, et al. Cell-free DNA: hope and potential application in cancer. Front Cell Dev Biol. 2021;9.
- Bray F, Laversanne M, Weiderpass E, et al. The ever-increasing importance of cancer as a leading cause of premature death worldwide. Cancer. 2021;127:3029-3030.
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: a cancer journal for clinicians. 2021;71:209-249.
- Hawkes N. Cancer survival data emphasize importance of early diagnosis. BMJ. 2019;364:408.
- Neal RD, Tharmanathan P, France B, et al. Is increased time to diagnosis and treatment in symptomatic cancer associated with poorer outcomes? Systematic review. Br J Cancer. 2015;112:S92-S107.
- Centers for Disease Control and Prevention. Screening tests. https://www.cdc.gov/cancer/dcpc/prevention/screening. htm#print. Reviewed May 19, 2022. Accessed March 1, 2023.
- Wingo PA, Cardinez CJ, Landis SH, et al. Long-term trends in cancer mortality in the United States, 1930–1998. Cancer. 2003;97:3133-3275.
- Liao CI, Franceur AA, Kapp DS, et al. Trends in Human Papillomavirus–Associated Cancers, Demographic Characteristics, and Vaccinations in the US, 2001-2017. JAMA Netw Open. 2022;5:e222530. doi:10.1001/ jamanetworkopen.2022.2530.
- Ho T-QH, Bissell MCS, Kerlikowske K, et al. Cumulative probability of false-positive results after 10 years of screening with digital breast tomosynthesis vs digital mammography. JAMA Network Open. 2022;5:e222440.
- Martin RM, Donovan JL, Turner EL, et al. Effect of a low-intensity PSA-based screening intervention on prostate cancer mortality: the CAP randomized clinical trial. JAMA. 2018;319:883-895.
- Heitzer E, Ulz P, Geigl JB. Circulating tumor DNA as a liquid biopsy for cancer. Clin Chem. 2015;61:112-123.
- Dominguez-Vigil IG, Moreno-Martinez AK, Wang JY, et al. The dawn of the liquid biopsy in the fight against cancer. Oncotarget. 2018; 9:2912–2922. doi: 10.18632/ oncotarget.23131.
- GRAIL. https://grail.com/. Accessed March 1, 2023.
- Siravegna G, Marsoni S, Siena S, et al. Integrating liquid biopsies into the management of cancer. Nat Rev Clin Oncol. 2017;14:531-548.
- Osborne CM, Hardisty E, Devers P, et al. Discordant noninvasive prenatal testing results in a patient subsequently diagnosed with metastatic disease. Prenat Diagn. 2013;33:609-611.
- Klein EA, Richards D, Cohn A, et al. Clinical validation of a targeted methylation-based multi-cancer early detection test using an independent validation set. Ann Oncology. 2021;32:1167-1177.
- Li B, Wang C, Xu J, et al. Abstract A06: multiplatform analysis of early-stage cancer signatures in blood. Clin Cancer Res. 2020;26(11 supplement):A06-A.
- Shen SY, Singhania R, Fehringer G, et al. Sensitive tumour detection and classification using plasma cell-free DNA methylomes. Nature. 2018;563:579-583.
- Nadauld LD, McDonnell CH 3rd, Beer TM, et al. The PATHFINDER Study: assessment of the implementation of an investigational multi-cancer early detection test into clinical practice. Cancers (Basel). 2021;13.
- Klein EA. A prospective study of a multi-cancer early detection blood test in a clinical practice setting. Abstract presented at ESMO conference; Portland, OR. October 18, 2022.
- The STRIVE Study: development of a blood test for early detection of multiple cancer types. https://clinicaltrials.gov /ct2/show/NCT03085888. Accessed March 2, 2023.
- The SUMMIT Study: a cancer screening study (SUMMIT). https://clinicaltrials.gov/ct2/show/NCT03934866. Accessed March 2, 2023.
CASE Patient inquires about new technology to detect cancer
A 51-year-old woman (para 2) presents to your clinic for a routine gynecology exam. She is up to date on her screening mammogram and Pap testing. She has her first colonoscopy scheduled for next month. She has a 10-year remote smoking history, but she stopped smoking in her late twenties. Her cousin was recently diagnosed with skin cancer, her father had prostate cancer and is now in remission, and her paternal grandmother died of ovarian cancer. She knows ovarian cancer does not have an effective screening test, and she recently heard on the news about a new blood test that can detect cancer before symptoms start. She would like to know more about this test. Could it replace her next Pap, mammogram, and future colonoscopies? She also wants to know—How can a simple blood test detect cancer?

The power of genomics in cancer care
Since the first human genome was sequenced in 2000, the power of genomics has been evident across many aspects of medicine, including cancer care.1 Whereas the first human genome to be sequenced took more than 10 years to sequence and cost over $1 billion, sequencing of your entire genome can now be obtained for less than $400—with results in a week.2
Genomics is now an integral part of cancer care, with results having implications for both cancer risk and prevention as well as more individualized treatment. For example, a healthy 42-year-old patient with a strong family history of breast cancer may undergo genetic testing and discover she has a mutation in the tumor suppression gene BRCA1, which carries a 39% to 58% lifetime risk of ovarian cancer.3 By undergoing a risk-reducing bilateral salpingooophorectomy she will lower her ovarian cancer risk by up to 96%.4,5 A 67-year-old with a new diagnosis of stage III ovarian cancer and a BRCA2 mutation may be in remission for 5+ years due to her BRCA2 mutation, which makes her eligible for the use of the poly(ADPribose) polymerase (PARP) inhibitor olaparib.6 Genetic testing as illustrated above has led to decreased cancer-related mortality and prolonged survival.7 However, many women with such germline mutations are faced with difficult choices about surgical risk reduction, with the potential harms of early menopause and quality of life concerns. Having a test that does not just predict cancer risk but in fact quantifies that risk for the individual would greatly help in these decisions. Furthermore, more than 75% of ovarian cancers occur without a germline mutation.
Advances in genetic testing technology also have led to the ability to obtain genetic information from a simple blood test. For example, cell-free DNA (cfDNA), which is DNA fragments that are normally found to be circulating in the bloodstream, is routinely used as a screening tool for prenatal genetic testing to detect chromosomal abnormalities in the fetus.8 This technology relies on analyzing fetal free (non-cellular) DNA that is naturally found circulating in maternal blood. More recently, similar technology using cfDNA has been applied for the screening and characterization of certain cancers.9 This powerful technology can detect cancer before symptoms begin—all from a simple blood test, often referred to as a “liquid biopsy.” However, understanding the utility, supporting data, and target population for these tests is important before employing them as part of routine clinical practice.
Continue to: Current methods of cancer screening are limited...
Current methods of cancer screening are limited
Cancer is a leading cause of death worldwide, with nearly 10 million cancer-related deaths annually, and it may surpass cardiovascular disease as the leading cause over the course of the century.10,11 Many cancer deaths are in part due to late-stage diagnosis, when the cancer has already metastasized.12 Early detection of cancer improves outcomes and survival rates, but it is often difficult to detect early due to the lack of early symptoms with many cancers, which can limit cancer screening and issues with access to care.13
Currently, there are only 5 cancers: cervical, prostate, breast, colon, and lung (for high-risk adults) that are screened for in the general population (see "Cancer screening has helped save countless lives" at the end of this article).14 The Pap test to screen for cervical cancer, developed in the 1940s, has saved millions of women’s lives and reduced the mortality of cervical cancer by 70%.15 Coupled with the availability and implementation of the human papillomavirus (HPV) vaccine, cervical cancer rates are decreasing at substantial rates.16 However, there are no validated screening tests for uterine cancer, the most common gynecologic malignancy in the United States, or ovarian cancer, the most lethal.
Screening tests for cervical, prostate, breast, colon, and lung cancer have helped save millions of lives; however, these tests also come with high false-positive rates and the potential for overdiagnosis and overtreatment. For example, half of women undergoing mammograms will receive a false-positive result over a 10-year time period,17 and up to 50% of men undergoing prostate cancer screening have a positive prostate-specific antigen (PSA) test result when they do not actually have prostate cancer.18 Additionally, the positive predictive value of the current standard-of-care screening tests can be as low as <5%. Most diagnoses of cancer are made from a surgical biopsy, but these types of procedures can be difficult depending on the location or size of the tumor.19
The liquid biopsy. Given the limitations of current cancer screening and diagnostic tests, there is a great need for a more sensitive test that also can detect cancer from multiple organ sites. Liquid biopsy-based biomarkers can include circulating tumor cells, exosomes, microRNAs, and circulating tumor DNA (ctDNA). With advances in next-generation sequencing, ctDNA techniques remain the most promising.20
Methylation-based MCED testing: A new way of cancer screening
Multi-cancer early detection (MCED) technology was developed to address the need for better cancer screening and has the potential to detect up to 50 cancers with a simple blood test. This new technology opens the possibility for early detection of multiple cancers before symptoms even begin. MCED testing is sometimes referred to as “GRAIL” testing, after the American biotechnology company that developed the first commercially available MCED test, called the Galleri test (Galleri, Menlo Park, California). Although other biotechnology companies are developing similar technology (Exact Sciences, Madison, Wisconsin, and Freenome, South San Francisco, California, for example), this is the first test of its kind available to the public.21
The MCED test works by detecting the cfDNA fragments that are released into the blood passively by necrotic or apoptotic cells or secreted actively from tumor cells. The DNA from tumor cells is also known as circulating tumor DNA (ctDNA). CtDNA is found in much lower quantities in the blood stream compared with cfDNA from cells, making it difficult to distinguish a cancer versus a noncancer cell and to determine the tumor site of origin.22
Through innovation, the first example of detecting cancer through this method in fact came as a surprise result from an abnormal cfDNA test. A pregnant 37-yearold woman had a cfDNA result suggestive of aneuploidy for chromosomes 18 and 13; however, she gave birth to a normal male fetus. Shortly thereafter, a vaginal biopsy confirmed small-cell carcinoma with alterations in chromosomes 18 and 13.23 GRAIL testing for this patient was subsequently able to optimize their methods of detecting both the presence of cancer cells and the tumor site of origin by utilizing next-generation genomic sequencing and methylation. Their development of a methylation-based assay combined with 46 machine-learning allowed the test to determine, first, if there is cancer present or not, and second, the tissue of origin prediction. It is important to note that these tests are meant to be used in addition to standard-of-care screening tests, not as an alternative, and this is emphasized throughout the company’s website and the medical literature.24
Continue to: The process to develop and validate GRAIL’s blood-based cancer screening test...
The process to develop and validate GRAIL’s blood-based cancer screening test includes 4 large clinical trials of more than 180,000 participants, including those with cancer and those without. The Circulating Cell-Free Genome Atlas (CCGA) Study, was a prospective, case-controlled, observational study enrolling approximately 15,000 participants with 3 prespecified sub-studies. The first sub-study developed the machine-learning classifier for both early detection and tumor of origin detection.25,26
The highest performing assay from the first sub-study then went on to be further validated in the 2nd and 3rd sub-studies. The 3rd sub-study, published in the Annals of Oncology in 2021 looked at a cohort of 4,077 participants with and without cancer, and found the specificity of cancer signal detection to be 99.5% and the overall sensitivity to be 51.5%, with increasing sensitivity by cancer stage (stage I - 17%, stage II - 40%, stage III - 77%, and stage IV - 90.1%).24 The false-positive rate was low, at 0.7%, and the true positive rate was 88.7%. Notably, the test was able to correctly identify the tumor of origin for 93% of samples.24 The study overall demonstrated high specificity and accuracy of tumor site of origin and supported the use of this blood-based MCED assay.
The PATHFINDER study was another prospective, multicenter clinical trial that enrolled more than 6,000 participants in the United States. The participants were aged >50 years with or without additional cancer risk factors. The goal of this study was to determine the extent of testing required to achieve diagnosis after a “cancer signal detected” result. The study results found that, when MCED testing was added to the standard-of-care screening, the number of cancers detected doubled when compared with standard cancer screening alone.27,28 Of the 92 participants with positive cancer signals, 35 were diagnosed with cancer, and 71% of these cancer types did not have standard-ofcare screening. The tumor site of origin was correctly detected in 97% of cases, and there were less than 1% of false positives. Overall, the test led to diagnostic evaluation of 1.4% of patients and a cancer diagnosis in 0.5%.
Currently, there are 2 ongoing clinical trials to further evaluate the Galleri MCED test. The STRIVE trial that aims to prospectively validate the MCED test in a population of nearly 100,000 women undergoing mammography,29 and the SUMMIT trial,30 which is similarly aiming to validate the test in a group of individuals, half of whom have a significantly elevated risk of lung cancer.
With the promising results described above, the Galleri test became the first MCED test available for commercial use starting in 2022. It is only available for use in people who are aged 50 and older, have a family history of cancer, or are at an increased risk for cancer (although GRAIL does not elaborate on what constitutes increased risk). However, the Galleri test is only available through prescription—therefore, if interested, patients must ask their health care provider to register with GRAIL and order the test (https://www .galleri.com/hcp/the-galleri-test/ordering). Additionally, the test will cost the patient $949 and is not yet covered by insurances. Currently, several large health care groups such as the United States Department of Veterans Affairs, Cleveland Clinic, and Mercy hospitals have partnered with GRAIL to offer their test to certain patients for use as part of clinical trials. Currently, no MCED test, including the Galleri, is approved by the US Food and Drug Administration.
Incorporating MCED testing into clinical practice
The Galleri MCED test has promising potential to make multi-cancer screening feasible and obtainable, which could ultimately reduce late-stage cancer diagnosis and decrease mortality from all cancers. The compelling data from large cohorts and numerous clinical trials demonstrate its accuracy, reliability, reproducibility, and specificity. It can detect up to 50 different types of cancers, including cancers that affect our gynecologic patients, including breast, cervical, ovarian, and uterine. Additionally, its novel methylation-based assay accurately identifies the tumor site of origin in 97% of cases.28 Ongoing and future clinical trials will continue to validate and refine these methods and improve the sensitivity and positive-predictive value of this assay. As mentioned, although it has been incorporated into various large health care systems, it is not FDA approved and has not been validated in the general population. Additionally, it should not be used as a replacement for recommended screening.
CASE Resolved
The patient is eligible for the Galleri MCED test if ordered by her physician. However, she will need to pay for the test out-of-pocket. Due to her family history, she should consider germline genetic testing (either for herself, or if possible, for her father, who should meet criteria based on his prostate cancer).3 Panel testing for germline mutations has become much more accessible, and until MCED testing is ready for prime time, it remains one of the best ways to predict and prevent cancers. Additionally, she should continue to undergo routine screening for cervical, breast, and colon cancer as indicated. ●
- Mammography has helped reduce breast cancer mortality in the United States by nearly 40% since 19901
- Increases in screening for lung cancer with computed tomography in the United States are estimated to have saved more than 10,000 lives between 2014 and 20182
- Routine prostate specific antigen screening is no longer recommended for men at average risk for prostate cancer, and patients are advised to discuss risks and benefits of screening with their clinicians3
- Where screening programs have long been established, cervical cancer rates have decreased by as much as 65% over the past 40 years4
- 68% of colorectal cancer deaths could be prevented with increased screening, and one of the most effective ways to get screened is colonoscopy5
References
1. American College of Radiology website. https://www.acr.org/Practice-Management-Quality-Informatics/Practice-Toolkit/PatientResources/Mammography-Saves-Lives. Accessed March 1, 2023.
2. US lung cancer screening linked to earlier diagnosis and better survival. BMJ.com. https://www.bmj.com/company/newsroom/ us-lung-cancer-screening-linked-to-earlier-diagnosis-and-better-survival/. Accessed March 1, 2023.
3. Draisma G, Etzioni R, Tsodikov A, et al. Lead time and overdiagnosis in prostate-specific antigen screening: importance of methods and context. J Natl Cancer Inst. 2009;101:374-383.
4. Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA: Can J Clinicians. 2015;65:87-108.
5. Colon cancer coalition website. Fact check: Do colonoscopies save lives? https://coloncancercoalition.org/2022/10/11/fact-checkdo-colonoscopies-save-lives/#:~:text=According%20to%20the%20Centers%20for,get%20screened%20is%20a%20colonoscopy. Accessed March 1, 2023.
CASE Patient inquires about new technology to detect cancer
A 51-year-old woman (para 2) presents to your clinic for a routine gynecology exam. She is up to date on her screening mammogram and Pap testing. She has her first colonoscopy scheduled for next month. She has a 10-year remote smoking history, but she stopped smoking in her late twenties. Her cousin was recently diagnosed with skin cancer, her father had prostate cancer and is now in remission, and her paternal grandmother died of ovarian cancer. She knows ovarian cancer does not have an effective screening test, and she recently heard on the news about a new blood test that can detect cancer before symptoms start. She would like to know more about this test. Could it replace her next Pap, mammogram, and future colonoscopies? She also wants to know—How can a simple blood test detect cancer?

The power of genomics in cancer care
Since the first human genome was sequenced in 2000, the power of genomics has been evident across many aspects of medicine, including cancer care.1 Whereas the first human genome to be sequenced took more than 10 years to sequence and cost over $1 billion, sequencing of your entire genome can now be obtained for less than $400—with results in a week.2
Genomics is now an integral part of cancer care, with results having implications for both cancer risk and prevention as well as more individualized treatment. For example, a healthy 42-year-old patient with a strong family history of breast cancer may undergo genetic testing and discover she has a mutation in the tumor suppression gene BRCA1, which carries a 39% to 58% lifetime risk of ovarian cancer.3 By undergoing a risk-reducing bilateral salpingooophorectomy she will lower her ovarian cancer risk by up to 96%.4,5 A 67-year-old with a new diagnosis of stage III ovarian cancer and a BRCA2 mutation may be in remission for 5+ years due to her BRCA2 mutation, which makes her eligible for the use of the poly(ADPribose) polymerase (PARP) inhibitor olaparib.6 Genetic testing as illustrated above has led to decreased cancer-related mortality and prolonged survival.7 However, many women with such germline mutations are faced with difficult choices about surgical risk reduction, with the potential harms of early menopause and quality of life concerns. Having a test that does not just predict cancer risk but in fact quantifies that risk for the individual would greatly help in these decisions. Furthermore, more than 75% of ovarian cancers occur without a germline mutation.
Advances in genetic testing technology also have led to the ability to obtain genetic information from a simple blood test. For example, cell-free DNA (cfDNA), which is DNA fragments that are normally found to be circulating in the bloodstream, is routinely used as a screening tool for prenatal genetic testing to detect chromosomal abnormalities in the fetus.8 This technology relies on analyzing fetal free (non-cellular) DNA that is naturally found circulating in maternal blood. More recently, similar technology using cfDNA has been applied for the screening and characterization of certain cancers.9 This powerful technology can detect cancer before symptoms begin—all from a simple blood test, often referred to as a “liquid biopsy.” However, understanding the utility, supporting data, and target population for these tests is important before employing them as part of routine clinical practice.
Continue to: Current methods of cancer screening are limited...
Current methods of cancer screening are limited
Cancer is a leading cause of death worldwide, with nearly 10 million cancer-related deaths annually, and it may surpass cardiovascular disease as the leading cause over the course of the century.10,11 Many cancer deaths are in part due to late-stage diagnosis, when the cancer has already metastasized.12 Early detection of cancer improves outcomes and survival rates, but it is often difficult to detect early due to the lack of early symptoms with many cancers, which can limit cancer screening and issues with access to care.13
Currently, there are only 5 cancers: cervical, prostate, breast, colon, and lung (for high-risk adults) that are screened for in the general population (see "Cancer screening has helped save countless lives" at the end of this article).14 The Pap test to screen for cervical cancer, developed in the 1940s, has saved millions of women’s lives and reduced the mortality of cervical cancer by 70%.15 Coupled with the availability and implementation of the human papillomavirus (HPV) vaccine, cervical cancer rates are decreasing at substantial rates.16 However, there are no validated screening tests for uterine cancer, the most common gynecologic malignancy in the United States, or ovarian cancer, the most lethal.
Screening tests for cervical, prostate, breast, colon, and lung cancer have helped save millions of lives; however, these tests also come with high false-positive rates and the potential for overdiagnosis and overtreatment. For example, half of women undergoing mammograms will receive a false-positive result over a 10-year time period,17 and up to 50% of men undergoing prostate cancer screening have a positive prostate-specific antigen (PSA) test result when they do not actually have prostate cancer.18 Additionally, the positive predictive value of the current standard-of-care screening tests can be as low as <5%. Most diagnoses of cancer are made from a surgical biopsy, but these types of procedures can be difficult depending on the location or size of the tumor.19
The liquid biopsy. Given the limitations of current cancer screening and diagnostic tests, there is a great need for a more sensitive test that also can detect cancer from multiple organ sites. Liquid biopsy-based biomarkers can include circulating tumor cells, exosomes, microRNAs, and circulating tumor DNA (ctDNA). With advances in next-generation sequencing, ctDNA techniques remain the most promising.20
Methylation-based MCED testing: A new way of cancer screening
Multi-cancer early detection (MCED) technology was developed to address the need for better cancer screening and has the potential to detect up to 50 cancers with a simple blood test. This new technology opens the possibility for early detection of multiple cancers before symptoms even begin. MCED testing is sometimes referred to as “GRAIL” testing, after the American biotechnology company that developed the first commercially available MCED test, called the Galleri test (Galleri, Menlo Park, California). Although other biotechnology companies are developing similar technology (Exact Sciences, Madison, Wisconsin, and Freenome, South San Francisco, California, for example), this is the first test of its kind available to the public.21
The MCED test works by detecting the cfDNA fragments that are released into the blood passively by necrotic or apoptotic cells or secreted actively from tumor cells. The DNA from tumor cells is also known as circulating tumor DNA (ctDNA). CtDNA is found in much lower quantities in the blood stream compared with cfDNA from cells, making it difficult to distinguish a cancer versus a noncancer cell and to determine the tumor site of origin.22
Through innovation, the first example of detecting cancer through this method in fact came as a surprise result from an abnormal cfDNA test. A pregnant 37-yearold woman had a cfDNA result suggestive of aneuploidy for chromosomes 18 and 13; however, she gave birth to a normal male fetus. Shortly thereafter, a vaginal biopsy confirmed small-cell carcinoma with alterations in chromosomes 18 and 13.23 GRAIL testing for this patient was subsequently able to optimize their methods of detecting both the presence of cancer cells and the tumor site of origin by utilizing next-generation genomic sequencing and methylation. Their development of a methylation-based assay combined with 46 machine-learning allowed the test to determine, first, if there is cancer present or not, and second, the tissue of origin prediction. It is important to note that these tests are meant to be used in addition to standard-of-care screening tests, not as an alternative, and this is emphasized throughout the company’s website and the medical literature.24
Continue to: The process to develop and validate GRAIL’s blood-based cancer screening test...
The process to develop and validate GRAIL’s blood-based cancer screening test includes 4 large clinical trials of more than 180,000 participants, including those with cancer and those without. The Circulating Cell-Free Genome Atlas (CCGA) Study, was a prospective, case-controlled, observational study enrolling approximately 15,000 participants with 3 prespecified sub-studies. The first sub-study developed the machine-learning classifier for both early detection and tumor of origin detection.25,26
The highest performing assay from the first sub-study then went on to be further validated in the 2nd and 3rd sub-studies. The 3rd sub-study, published in the Annals of Oncology in 2021 looked at a cohort of 4,077 participants with and without cancer, and found the specificity of cancer signal detection to be 99.5% and the overall sensitivity to be 51.5%, with increasing sensitivity by cancer stage (stage I - 17%, stage II - 40%, stage III - 77%, and stage IV - 90.1%).24 The false-positive rate was low, at 0.7%, and the true positive rate was 88.7%. Notably, the test was able to correctly identify the tumor of origin for 93% of samples.24 The study overall demonstrated high specificity and accuracy of tumor site of origin and supported the use of this blood-based MCED assay.
The PATHFINDER study was another prospective, multicenter clinical trial that enrolled more than 6,000 participants in the United States. The participants were aged >50 years with or without additional cancer risk factors. The goal of this study was to determine the extent of testing required to achieve diagnosis after a “cancer signal detected” result. The study results found that, when MCED testing was added to the standard-of-care screening, the number of cancers detected doubled when compared with standard cancer screening alone.27,28 Of the 92 participants with positive cancer signals, 35 were diagnosed with cancer, and 71% of these cancer types did not have standard-ofcare screening. The tumor site of origin was correctly detected in 97% of cases, and there were less than 1% of false positives. Overall, the test led to diagnostic evaluation of 1.4% of patients and a cancer diagnosis in 0.5%.
Currently, there are 2 ongoing clinical trials to further evaluate the Galleri MCED test. The STRIVE trial that aims to prospectively validate the MCED test in a population of nearly 100,000 women undergoing mammography,29 and the SUMMIT trial,30 which is similarly aiming to validate the test in a group of individuals, half of whom have a significantly elevated risk of lung cancer.
With the promising results described above, the Galleri test became the first MCED test available for commercial use starting in 2022. It is only available for use in people who are aged 50 and older, have a family history of cancer, or are at an increased risk for cancer (although GRAIL does not elaborate on what constitutes increased risk). However, the Galleri test is only available through prescription—therefore, if interested, patients must ask their health care provider to register with GRAIL and order the test (https://www .galleri.com/hcp/the-galleri-test/ordering). Additionally, the test will cost the patient $949 and is not yet covered by insurances. Currently, several large health care groups such as the United States Department of Veterans Affairs, Cleveland Clinic, and Mercy hospitals have partnered with GRAIL to offer their test to certain patients for use as part of clinical trials. Currently, no MCED test, including the Galleri, is approved by the US Food and Drug Administration.
Incorporating MCED testing into clinical practice
The Galleri MCED test has promising potential to make multi-cancer screening feasible and obtainable, which could ultimately reduce late-stage cancer diagnosis and decrease mortality from all cancers. The compelling data from large cohorts and numerous clinical trials demonstrate its accuracy, reliability, reproducibility, and specificity. It can detect up to 50 different types of cancers, including cancers that affect our gynecologic patients, including breast, cervical, ovarian, and uterine. Additionally, its novel methylation-based assay accurately identifies the tumor site of origin in 97% of cases.28 Ongoing and future clinical trials will continue to validate and refine these methods and improve the sensitivity and positive-predictive value of this assay. As mentioned, although it has been incorporated into various large health care systems, it is not FDA approved and has not been validated in the general population. Additionally, it should not be used as a replacement for recommended screening.
CASE Resolved
The patient is eligible for the Galleri MCED test if ordered by her physician. However, she will need to pay for the test out-of-pocket. Due to her family history, she should consider germline genetic testing (either for herself, or if possible, for her father, who should meet criteria based on his prostate cancer).3 Panel testing for germline mutations has become much more accessible, and until MCED testing is ready for prime time, it remains one of the best ways to predict and prevent cancers. Additionally, she should continue to undergo routine screening for cervical, breast, and colon cancer as indicated. ●
- Mammography has helped reduce breast cancer mortality in the United States by nearly 40% since 19901
- Increases in screening for lung cancer with computed tomography in the United States are estimated to have saved more than 10,000 lives between 2014 and 20182
- Routine prostate specific antigen screening is no longer recommended for men at average risk for prostate cancer, and patients are advised to discuss risks and benefits of screening with their clinicians3
- Where screening programs have long been established, cervical cancer rates have decreased by as much as 65% over the past 40 years4
- 68% of colorectal cancer deaths could be prevented with increased screening, and one of the most effective ways to get screened is colonoscopy5
References
1. American College of Radiology website. https://www.acr.org/Practice-Management-Quality-Informatics/Practice-Toolkit/PatientResources/Mammography-Saves-Lives. Accessed March 1, 2023.
2. US lung cancer screening linked to earlier diagnosis and better survival. BMJ.com. https://www.bmj.com/company/newsroom/ us-lung-cancer-screening-linked-to-earlier-diagnosis-and-better-survival/. Accessed March 1, 2023.
3. Draisma G, Etzioni R, Tsodikov A, et al. Lead time and overdiagnosis in prostate-specific antigen screening: importance of methods and context. J Natl Cancer Inst. 2009;101:374-383.
4. Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA: Can J Clinicians. 2015;65:87-108.
5. Colon cancer coalition website. Fact check: Do colonoscopies save lives? https://coloncancercoalition.org/2022/10/11/fact-checkdo-colonoscopies-save-lives/#:~:text=According%20to%20the%20Centers%20for,get%20screened%20is%20a%20colonoscopy. Accessed March 1, 2023.
- Stratton MR, Campbell PJ, Futreal PA. The cancer genome. Nature. 2009;458:719-724.
- Davies K. The era of genomic medicine. Clin Med (Lond). 2013;13:594-601.
- National Comprehensive Cancer Network. Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic. Version 3.2023. February 13, 2023.
- Finch APM, Lubinski J, Møller P, et al. Impact of oophorectomy on cancer incidence and mortality in women with a BRCA1 or BRCA2 mutation. J Clin Oncol. 2014;32:1547-1553.
- Xiao Y-L, Wang K, Liu Q, et al. Risk reduction and survival benefit of risk-reducing salpingo-oophorectomy in hereditary breast cancer: meta-analysis and systematic review. Clin Breast Cancer. 2019;19:e48-e65.
- Moore K, Colombo N, Scambia G, et al. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2018;379:2495-2505.
- Pritchard D, Goodman C, Nadauld LD. Clinical utility of genomic testing in cancer care. JCO Precis Oncol. 2022;6:e2100349.
- Screening for fetal chromosomal abnormalities: ACOG Practice Bulletin summary, number 226. Obstet Gynecol. 2020;136:859-867.
- Yan Y-y, Guo Q-r, Wang F-h, et al. Cell-free DNA: hope and potential application in cancer. Front Cell Dev Biol. 2021;9.
- Bray F, Laversanne M, Weiderpass E, et al. The ever-increasing importance of cancer as a leading cause of premature death worldwide. Cancer. 2021;127:3029-3030.
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: a cancer journal for clinicians. 2021;71:209-249.
- Hawkes N. Cancer survival data emphasize importance of early diagnosis. BMJ. 2019;364:408.
- Neal RD, Tharmanathan P, France B, et al. Is increased time to diagnosis and treatment in symptomatic cancer associated with poorer outcomes? Systematic review. Br J Cancer. 2015;112:S92-S107.
- Centers for Disease Control and Prevention. Screening tests. https://www.cdc.gov/cancer/dcpc/prevention/screening. htm#print. Reviewed May 19, 2022. Accessed March 1, 2023.
- Wingo PA, Cardinez CJ, Landis SH, et al. Long-term trends in cancer mortality in the United States, 1930–1998. Cancer. 2003;97:3133-3275.
- Liao CI, Franceur AA, Kapp DS, et al. Trends in Human Papillomavirus–Associated Cancers, Demographic Characteristics, and Vaccinations in the US, 2001-2017. JAMA Netw Open. 2022;5:e222530. doi:10.1001/ jamanetworkopen.2022.2530.
- Ho T-QH, Bissell MCS, Kerlikowske K, et al. Cumulative probability of false-positive results after 10 years of screening with digital breast tomosynthesis vs digital mammography. JAMA Network Open. 2022;5:e222440.
- Martin RM, Donovan JL, Turner EL, et al. Effect of a low-intensity PSA-based screening intervention on prostate cancer mortality: the CAP randomized clinical trial. JAMA. 2018;319:883-895.
- Heitzer E, Ulz P, Geigl JB. Circulating tumor DNA as a liquid biopsy for cancer. Clin Chem. 2015;61:112-123.
- Dominguez-Vigil IG, Moreno-Martinez AK, Wang JY, et al. The dawn of the liquid biopsy in the fight against cancer. Oncotarget. 2018; 9:2912–2922. doi: 10.18632/ oncotarget.23131.
- GRAIL. https://grail.com/. Accessed March 1, 2023.
- Siravegna G, Marsoni S, Siena S, et al. Integrating liquid biopsies into the management of cancer. Nat Rev Clin Oncol. 2017;14:531-548.
- Osborne CM, Hardisty E, Devers P, et al. Discordant noninvasive prenatal testing results in a patient subsequently diagnosed with metastatic disease. Prenat Diagn. 2013;33:609-611.
- Klein EA, Richards D, Cohn A, et al. Clinical validation of a targeted methylation-based multi-cancer early detection test using an independent validation set. Ann Oncology. 2021;32:1167-1177.
- Li B, Wang C, Xu J, et al. Abstract A06: multiplatform analysis of early-stage cancer signatures in blood. Clin Cancer Res. 2020;26(11 supplement):A06-A.
- Shen SY, Singhania R, Fehringer G, et al. Sensitive tumour detection and classification using plasma cell-free DNA methylomes. Nature. 2018;563:579-583.
- Nadauld LD, McDonnell CH 3rd, Beer TM, et al. The PATHFINDER Study: assessment of the implementation of an investigational multi-cancer early detection test into clinical practice. Cancers (Basel). 2021;13.
- Klein EA. A prospective study of a multi-cancer early detection blood test in a clinical practice setting. Abstract presented at ESMO conference; Portland, OR. October 18, 2022.
- The STRIVE Study: development of a blood test for early detection of multiple cancer types. https://clinicaltrials.gov /ct2/show/NCT03085888. Accessed March 2, 2023.
- The SUMMIT Study: a cancer screening study (SUMMIT). https://clinicaltrials.gov/ct2/show/NCT03934866. Accessed March 2, 2023.
- Stratton MR, Campbell PJ, Futreal PA. The cancer genome. Nature. 2009;458:719-724.
- Davies K. The era of genomic medicine. Clin Med (Lond). 2013;13:594-601.
- National Comprehensive Cancer Network. Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic. Version 3.2023. February 13, 2023.
- Finch APM, Lubinski J, Møller P, et al. Impact of oophorectomy on cancer incidence and mortality in women with a BRCA1 or BRCA2 mutation. J Clin Oncol. 2014;32:1547-1553.
- Xiao Y-L, Wang K, Liu Q, et al. Risk reduction and survival benefit of risk-reducing salpingo-oophorectomy in hereditary breast cancer: meta-analysis and systematic review. Clin Breast Cancer. 2019;19:e48-e65.
- Moore K, Colombo N, Scambia G, et al. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2018;379:2495-2505.
- Pritchard D, Goodman C, Nadauld LD. Clinical utility of genomic testing in cancer care. JCO Precis Oncol. 2022;6:e2100349.
- Screening for fetal chromosomal abnormalities: ACOG Practice Bulletin summary, number 226. Obstet Gynecol. 2020;136:859-867.
- Yan Y-y, Guo Q-r, Wang F-h, et al. Cell-free DNA: hope and potential application in cancer. Front Cell Dev Biol. 2021;9.
- Bray F, Laversanne M, Weiderpass E, et al. The ever-increasing importance of cancer as a leading cause of premature death worldwide. Cancer. 2021;127:3029-3030.
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: a cancer journal for clinicians. 2021;71:209-249.
- Hawkes N. Cancer survival data emphasize importance of early diagnosis. BMJ. 2019;364:408.
- Neal RD, Tharmanathan P, France B, et al. Is increased time to diagnosis and treatment in symptomatic cancer associated with poorer outcomes? Systematic review. Br J Cancer. 2015;112:S92-S107.
- Centers for Disease Control and Prevention. Screening tests. https://www.cdc.gov/cancer/dcpc/prevention/screening. htm#print. Reviewed May 19, 2022. Accessed March 1, 2023.
- Wingo PA, Cardinez CJ, Landis SH, et al. Long-term trends in cancer mortality in the United States, 1930–1998. Cancer. 2003;97:3133-3275.
- Liao CI, Franceur AA, Kapp DS, et al. Trends in Human Papillomavirus–Associated Cancers, Demographic Characteristics, and Vaccinations in the US, 2001-2017. JAMA Netw Open. 2022;5:e222530. doi:10.1001/ jamanetworkopen.2022.2530.
- Ho T-QH, Bissell MCS, Kerlikowske K, et al. Cumulative probability of false-positive results after 10 years of screening with digital breast tomosynthesis vs digital mammography. JAMA Network Open. 2022;5:e222440.
- Martin RM, Donovan JL, Turner EL, et al. Effect of a low-intensity PSA-based screening intervention on prostate cancer mortality: the CAP randomized clinical trial. JAMA. 2018;319:883-895.
- Heitzer E, Ulz P, Geigl JB. Circulating tumor DNA as a liquid biopsy for cancer. Clin Chem. 2015;61:112-123.
- Dominguez-Vigil IG, Moreno-Martinez AK, Wang JY, et al. The dawn of the liquid biopsy in the fight against cancer. Oncotarget. 2018; 9:2912–2922. doi: 10.18632/ oncotarget.23131.
- GRAIL. https://grail.com/. Accessed March 1, 2023.
- Siravegna G, Marsoni S, Siena S, et al. Integrating liquid biopsies into the management of cancer. Nat Rev Clin Oncol. 2017;14:531-548.
- Osborne CM, Hardisty E, Devers P, et al. Discordant noninvasive prenatal testing results in a patient subsequently diagnosed with metastatic disease. Prenat Diagn. 2013;33:609-611.
- Klein EA, Richards D, Cohn A, et al. Clinical validation of a targeted methylation-based multi-cancer early detection test using an independent validation set. Ann Oncology. 2021;32:1167-1177.
- Li B, Wang C, Xu J, et al. Abstract A06: multiplatform analysis of early-stage cancer signatures in blood. Clin Cancer Res. 2020;26(11 supplement):A06-A.
- Shen SY, Singhania R, Fehringer G, et al. Sensitive tumour detection and classification using plasma cell-free DNA methylomes. Nature. 2018;563:579-583.
- Nadauld LD, McDonnell CH 3rd, Beer TM, et al. The PATHFINDER Study: assessment of the implementation of an investigational multi-cancer early detection test into clinical practice. Cancers (Basel). 2021;13.
- Klein EA. A prospective study of a multi-cancer early detection blood test in a clinical practice setting. Abstract presented at ESMO conference; Portland, OR. October 18, 2022.
- The STRIVE Study: development of a blood test for early detection of multiple cancer types. https://clinicaltrials.gov /ct2/show/NCT03085888. Accessed March 2, 2023.
- The SUMMIT Study: a cancer screening study (SUMMIT). https://clinicaltrials.gov/ct2/show/NCT03934866. Accessed March 2, 2023.









