User login
Thirty years of epilepsy therapy: ‘Plus ça change, plus c’est la même chose’?
Although the past 30 years have stirred up a whirlwind of neurological research that has dramatically expanded therapeutic options for patients with epilepsy, historical pioneers in the field might be disappointed at the fact that treatment response has remained stubbornly stagnant. “Plus ça change, plus c’est la même chose,” they might say: The more things change, the more they stay the same. In fact, since 1993, , with roughly two-thirds of patients achieving seizure freedom and a third still struggling with treatment resistance.
But if you widen the lens and look towards the horizon, things are “on the cusp and going like a rocket,” said Jacqueline A. French, MD, professor of neurology in the Comprehensive Epilepsy Center at NYU Langone Health, New York. While treatment response rates may be stuck, adverse effects of those treatments have plummeted, and even treatment-resistant patients dealing with residual seizures live a much freer life with far fewer and less serious episodes.
Simpler times
In the late 1980s, just as Dr. French was finishing her second epilepsy fellowship at Yale, it was “almost laughable that things were so simple,” she recalls. “There were a few major centers that were doing epilepsy surgery … and in the world of medication, there were just five major drugs: phenobarbital, primidone, carbamazepine, phenytoin, and valproate.” That all changed as she was settling in to her first academic position at the University of Pennsylvania, with the “explosive” introduction of felbamate, a new antiseizure drug whose precipitous rise and fall from favor cast a sobering shadow which set the course for future drug development in the field.
“The felbamate story has a lot to do with what came after, but it was a drug that was much more advantageous in regards to a lot of the things that we didn’t like about antiseizure medicines or antiepileptic drugs as we called them at that time,” she said. The older drugs affected the cerebellum, making people sleepy and unable to concentrate. They also came with the risk of serious adverse effects such as hepatic enzyme induction and teratogenicity. Not only was felbamate nonsedating, “it actually was a little bit alerting,” said Dr. French. “People felt so different and so great on it, and it was effective for some seizure types that we didn’t really have good drugs for.” Very quickly, felbamate became a first-line therapy. Within its first year on the market, 150,000 newly diagnosed patients were started on it, “which is unthinkable now,” she said.
Sure enough, it all came crashing down a year later, on Aug. 1, 1994, when the drug was urgently withdrawn by the U.S. Food and Drug Administration after being linked to the development of aplastic anemia. “There was a day that anybody who was there at the time will remember when we all got the news, that everybody had to be taken off the drug,” Dr. French recalled. “We spent the weekend in the chart room, looking chart by chart by chart, for who was on felbamate.”
Until then, Dr. French had been straddling the line between her interests in pharmacologic versus surgical treatments for epilepsy. In fact, during her second epilepsy fellowship, which was dedicated to surgery, she published “Characteristics of medial temporal lobe epilepsy” in Annals of Neurology, one of the most-cited papers of her career. “Epilepsy from the temporal lobe is the biggest and best shot on goal when you’re talking about sending somebody to epilepsy surgery and rendering them completely seizure free,” she said. “Early in my career at the University of Pennsylvania, it was all about identifying those patients. And you know, there is nothing more gratifying than taking somebody whose life has been devastated by frequent seizures, who is injuring themselves and not able to be independent, and doing a surgery, which is very safe, and then all the seizures are gone – which is probably why I was so excited by surgery at the time.”
For a while, in the early 1990s, temporal lobectomy eclipsed many of the other avenues in epilepsy treatment, but it too has given way to a much wider variety of more complex techniques, which may be less curative but more palliative.
More drug options
Meanwhile, the felbamate story had ignited debate in the field about safer drug development – pushing Dr. French into establishing what was then known as the Antiepileptic Drug Trials conference, later renamed the Epilepsy Therapies & Diagnostics Development Symposium – a forum that encouraged safer, but also swifter movement of drugs through the pipeline and onto the market. “After felbamate, came gabapentin, and then came to topiramate and lamotrigine, and very quickly there were many, many, many choices,” she explained. “But once stung, twice shy. Felbamate really gave us a new perspective on which patients we put on the new drugs. Now we have a process of starting them in people with treatment-resistant epilepsy first. The risk-benefit equation is more reasonable because they have lots of risks. And then we work our way back to people with newly diagnosed epilepsy.”
Disease-modifying therapies
Today, the medications used to treat epilepsy are referred to as antiseizure rather than antiepileptic drugs because they simply suppress seizure symptoms and do not address the cause. But the rocket that Dr. French is watching gain speed and momentum is the disease-modifying gene therapies – true antiepileptics that may significantly move the needle on the number and type of patients who can reach seizure freedom. “We spent the last 25 years not even thinking we would ever have antiepileptic therapies, and now in the last 5 years or so, we were pretty sure we will,” she said. “We have gene therapies that can intervene now – none yet that have actually reached approval, these are all currently in trials – but we certainly have high expectations that they will very soon be available.”
Improving patients’ lives
While gene therapy rockets ahead, new device developments are already improving life for patients, even despite ongoing seizures. A drug-delivering pump is still in trials, but could make a big difference to daily medication adherence, and wearable or implantable devices are being developed to track seizures. More accurate tracking has also revealed that many people’s seizures are actually quite predictable, with regular cycles allowing for the possibility of prophylactic medication when increased seizure activity is expected.
Despite 30 years of no change in the proportion of epilepsy patients experiencing treatment resistance, Dr. French said that drugs, devices, and surgeries have improved the lives of all patients – both treatment resistant and treatment sensitive. “The difference between almost seizure free and completely seizure free is a big one because it means you can’t drive, you may have difficulty with your employment, but being able to take a pill every day and feel otherwise completely normal? We’ve come a long way.”
Although the past 30 years have stirred up a whirlwind of neurological research that has dramatically expanded therapeutic options for patients with epilepsy, historical pioneers in the field might be disappointed at the fact that treatment response has remained stubbornly stagnant. “Plus ça change, plus c’est la même chose,” they might say: The more things change, the more they stay the same. In fact, since 1993, , with roughly two-thirds of patients achieving seizure freedom and a third still struggling with treatment resistance.
But if you widen the lens and look towards the horizon, things are “on the cusp and going like a rocket,” said Jacqueline A. French, MD, professor of neurology in the Comprehensive Epilepsy Center at NYU Langone Health, New York. While treatment response rates may be stuck, adverse effects of those treatments have plummeted, and even treatment-resistant patients dealing with residual seizures live a much freer life with far fewer and less serious episodes.
Simpler times
In the late 1980s, just as Dr. French was finishing her second epilepsy fellowship at Yale, it was “almost laughable that things were so simple,” she recalls. “There were a few major centers that were doing epilepsy surgery … and in the world of medication, there were just five major drugs: phenobarbital, primidone, carbamazepine, phenytoin, and valproate.” That all changed as she was settling in to her first academic position at the University of Pennsylvania, with the “explosive” introduction of felbamate, a new antiseizure drug whose precipitous rise and fall from favor cast a sobering shadow which set the course for future drug development in the field.
“The felbamate story has a lot to do with what came after, but it was a drug that was much more advantageous in regards to a lot of the things that we didn’t like about antiseizure medicines or antiepileptic drugs as we called them at that time,” she said. The older drugs affected the cerebellum, making people sleepy and unable to concentrate. They also came with the risk of serious adverse effects such as hepatic enzyme induction and teratogenicity. Not only was felbamate nonsedating, “it actually was a little bit alerting,” said Dr. French. “People felt so different and so great on it, and it was effective for some seizure types that we didn’t really have good drugs for.” Very quickly, felbamate became a first-line therapy. Within its first year on the market, 150,000 newly diagnosed patients were started on it, “which is unthinkable now,” she said.
Sure enough, it all came crashing down a year later, on Aug. 1, 1994, when the drug was urgently withdrawn by the U.S. Food and Drug Administration after being linked to the development of aplastic anemia. “There was a day that anybody who was there at the time will remember when we all got the news, that everybody had to be taken off the drug,” Dr. French recalled. “We spent the weekend in the chart room, looking chart by chart by chart, for who was on felbamate.”
Until then, Dr. French had been straddling the line between her interests in pharmacologic versus surgical treatments for epilepsy. In fact, during her second epilepsy fellowship, which was dedicated to surgery, she published “Characteristics of medial temporal lobe epilepsy” in Annals of Neurology, one of the most-cited papers of her career. “Epilepsy from the temporal lobe is the biggest and best shot on goal when you’re talking about sending somebody to epilepsy surgery and rendering them completely seizure free,” she said. “Early in my career at the University of Pennsylvania, it was all about identifying those patients. And you know, there is nothing more gratifying than taking somebody whose life has been devastated by frequent seizures, who is injuring themselves and not able to be independent, and doing a surgery, which is very safe, and then all the seizures are gone – which is probably why I was so excited by surgery at the time.”
For a while, in the early 1990s, temporal lobectomy eclipsed many of the other avenues in epilepsy treatment, but it too has given way to a much wider variety of more complex techniques, which may be less curative but more palliative.
More drug options
Meanwhile, the felbamate story had ignited debate in the field about safer drug development – pushing Dr. French into establishing what was then known as the Antiepileptic Drug Trials conference, later renamed the Epilepsy Therapies & Diagnostics Development Symposium – a forum that encouraged safer, but also swifter movement of drugs through the pipeline and onto the market. “After felbamate, came gabapentin, and then came to topiramate and lamotrigine, and very quickly there were many, many, many choices,” she explained. “But once stung, twice shy. Felbamate really gave us a new perspective on which patients we put on the new drugs. Now we have a process of starting them in people with treatment-resistant epilepsy first. The risk-benefit equation is more reasonable because they have lots of risks. And then we work our way back to people with newly diagnosed epilepsy.”
Disease-modifying therapies
Today, the medications used to treat epilepsy are referred to as antiseizure rather than antiepileptic drugs because they simply suppress seizure symptoms and do not address the cause. But the rocket that Dr. French is watching gain speed and momentum is the disease-modifying gene therapies – true antiepileptics that may significantly move the needle on the number and type of patients who can reach seizure freedom. “We spent the last 25 years not even thinking we would ever have antiepileptic therapies, and now in the last 5 years or so, we were pretty sure we will,” she said. “We have gene therapies that can intervene now – none yet that have actually reached approval, these are all currently in trials – but we certainly have high expectations that they will very soon be available.”
Improving patients’ lives
While gene therapy rockets ahead, new device developments are already improving life for patients, even despite ongoing seizures. A drug-delivering pump is still in trials, but could make a big difference to daily medication adherence, and wearable or implantable devices are being developed to track seizures. More accurate tracking has also revealed that many people’s seizures are actually quite predictable, with regular cycles allowing for the possibility of prophylactic medication when increased seizure activity is expected.
Despite 30 years of no change in the proportion of epilepsy patients experiencing treatment resistance, Dr. French said that drugs, devices, and surgeries have improved the lives of all patients – both treatment resistant and treatment sensitive. “The difference between almost seizure free and completely seizure free is a big one because it means you can’t drive, you may have difficulty with your employment, but being able to take a pill every day and feel otherwise completely normal? We’ve come a long way.”
Although the past 30 years have stirred up a whirlwind of neurological research that has dramatically expanded therapeutic options for patients with epilepsy, historical pioneers in the field might be disappointed at the fact that treatment response has remained stubbornly stagnant. “Plus ça change, plus c’est la même chose,” they might say: The more things change, the more they stay the same. In fact, since 1993, , with roughly two-thirds of patients achieving seizure freedom and a third still struggling with treatment resistance.
But if you widen the lens and look towards the horizon, things are “on the cusp and going like a rocket,” said Jacqueline A. French, MD, professor of neurology in the Comprehensive Epilepsy Center at NYU Langone Health, New York. While treatment response rates may be stuck, adverse effects of those treatments have plummeted, and even treatment-resistant patients dealing with residual seizures live a much freer life with far fewer and less serious episodes.
Simpler times
In the late 1980s, just as Dr. French was finishing her second epilepsy fellowship at Yale, it was “almost laughable that things were so simple,” she recalls. “There were a few major centers that were doing epilepsy surgery … and in the world of medication, there were just five major drugs: phenobarbital, primidone, carbamazepine, phenytoin, and valproate.” That all changed as she was settling in to her first academic position at the University of Pennsylvania, with the “explosive” introduction of felbamate, a new antiseizure drug whose precipitous rise and fall from favor cast a sobering shadow which set the course for future drug development in the field.
“The felbamate story has a lot to do with what came after, but it was a drug that was much more advantageous in regards to a lot of the things that we didn’t like about antiseizure medicines or antiepileptic drugs as we called them at that time,” she said. The older drugs affected the cerebellum, making people sleepy and unable to concentrate. They also came with the risk of serious adverse effects such as hepatic enzyme induction and teratogenicity. Not only was felbamate nonsedating, “it actually was a little bit alerting,” said Dr. French. “People felt so different and so great on it, and it was effective for some seizure types that we didn’t really have good drugs for.” Very quickly, felbamate became a first-line therapy. Within its first year on the market, 150,000 newly diagnosed patients were started on it, “which is unthinkable now,” she said.
Sure enough, it all came crashing down a year later, on Aug. 1, 1994, when the drug was urgently withdrawn by the U.S. Food and Drug Administration after being linked to the development of aplastic anemia. “There was a day that anybody who was there at the time will remember when we all got the news, that everybody had to be taken off the drug,” Dr. French recalled. “We spent the weekend in the chart room, looking chart by chart by chart, for who was on felbamate.”
Until then, Dr. French had been straddling the line between her interests in pharmacologic versus surgical treatments for epilepsy. In fact, during her second epilepsy fellowship, which was dedicated to surgery, she published “Characteristics of medial temporal lobe epilepsy” in Annals of Neurology, one of the most-cited papers of her career. “Epilepsy from the temporal lobe is the biggest and best shot on goal when you’re talking about sending somebody to epilepsy surgery and rendering them completely seizure free,” she said. “Early in my career at the University of Pennsylvania, it was all about identifying those patients. And you know, there is nothing more gratifying than taking somebody whose life has been devastated by frequent seizures, who is injuring themselves and not able to be independent, and doing a surgery, which is very safe, and then all the seizures are gone – which is probably why I was so excited by surgery at the time.”
For a while, in the early 1990s, temporal lobectomy eclipsed many of the other avenues in epilepsy treatment, but it too has given way to a much wider variety of more complex techniques, which may be less curative but more palliative.
More drug options
Meanwhile, the felbamate story had ignited debate in the field about safer drug development – pushing Dr. French into establishing what was then known as the Antiepileptic Drug Trials conference, later renamed the Epilepsy Therapies & Diagnostics Development Symposium – a forum that encouraged safer, but also swifter movement of drugs through the pipeline and onto the market. “After felbamate, came gabapentin, and then came to topiramate and lamotrigine, and very quickly there were many, many, many choices,” she explained. “But once stung, twice shy. Felbamate really gave us a new perspective on which patients we put on the new drugs. Now we have a process of starting them in people with treatment-resistant epilepsy first. The risk-benefit equation is more reasonable because they have lots of risks. And then we work our way back to people with newly diagnosed epilepsy.”
Disease-modifying therapies
Today, the medications used to treat epilepsy are referred to as antiseizure rather than antiepileptic drugs because they simply suppress seizure symptoms and do not address the cause. But the rocket that Dr. French is watching gain speed and momentum is the disease-modifying gene therapies – true antiepileptics that may significantly move the needle on the number and type of patients who can reach seizure freedom. “We spent the last 25 years not even thinking we would ever have antiepileptic therapies, and now in the last 5 years or so, we were pretty sure we will,” she said. “We have gene therapies that can intervene now – none yet that have actually reached approval, these are all currently in trials – but we certainly have high expectations that they will very soon be available.”
Improving patients’ lives
While gene therapy rockets ahead, new device developments are already improving life for patients, even despite ongoing seizures. A drug-delivering pump is still in trials, but could make a big difference to daily medication adherence, and wearable or implantable devices are being developed to track seizures. More accurate tracking has also revealed that many people’s seizures are actually quite predictable, with regular cycles allowing for the possibility of prophylactic medication when increased seizure activity is expected.
Despite 30 years of no change in the proportion of epilepsy patients experiencing treatment resistance, Dr. French said that drugs, devices, and surgeries have improved the lives of all patients – both treatment resistant and treatment sensitive. “The difference between almost seizure free and completely seizure free is a big one because it means you can’t drive, you may have difficulty with your employment, but being able to take a pill every day and feel otherwise completely normal? We’ve come a long way.”
Get action! – Teddy Roosevelt
“Papa! Where donut?” asks my 2½ year-old sitting with her legs dangling and hands folded in a bustling Starbucks. We’ve been waiting for 8 minutes and we’ve reached her limit of tolerance. She’s unimpressed by the queued customers who compliment her curly blonde hair, many of whom have come and gone since we’ve been waiting. I agree – how long does it take to pour a kiddie milk and grab a donut? We can both see it in the case right there!
No one likes to wait. Truly, one of the great benefits of the modern world is that wait times are now incredibly short. Many Starbucks customers, unlike my daughter, ordered their drink ahead and waited exactly 0 minutes to get their drink. What about Amazon? I ordered a bird feeder this morning and it’s already hanging in the yard. It’s still daylight. Feel like Himalayan Momo Dumplings tonight? Your food could arrive in 37 minutes. The modern wait standard has been set impossibly high for us.
Yes, for some. We created a whole room just for waiting. Airlines call theirs “The Platinum Executive Lounge.” Ours is “The waiting room.”
Excess waiting is a significant reason why health care gets beat up in reviews. We’re unable to keep up with the new expectations. Waiting is also a significant cause of distress. Many patients report the most difficult part of their cancer diagnosis was the waiting for results, not the treatment. It’s because when under stress, we are hardwired to take action. Binding patients into inaction while they wait is very uncomfortable.
Fortunately, the psychology of waiting is well understood and there are best practices that can help. First, anxiety makes waiting much worse. Conveying confidence and reassuring patients they are in the right place and that everything will be OK makes the wait time feel shorter for them. Uncertainty also compounds their apprehension. If you believe the diagnosis will be melanoma, tell them that at the time of the biopsy and tell them what you expect next. This is better than saying, “Well, that could be cancer. We’ll see.”
Knowing a wait time is also much better than not. Have your staff advise patients on how much longer they can expect before seeing you (telling them they’re next isn’t as effective). Advise that test results should be back by the end of next week. Of course, under promise and over deliver. When the results are back on Tuesday, you’ve got a pleased patient.
Explaining that you had to add in an urgent patient helps. Even if it’s not your fault, it’s still better to apologize. For example, the 78 highway, the left anterior descending artery to our office, has been closed because of a sinkhole this month (not kidding). I’ve been apologizing to a lot of patients saying that all our patients are arriving late, which is putting us behind. As they can envision the linear parking lot that used to be a highway, it helps.
Lastly, as any child can tell you, waiting has to not only be, but to also appear, fair. The only thing worse than waiting for an appointment, or donut, is seeing someone who came in after you get their donut before you do. If you’re pulling both Mohs and cosmetics patients from the same waiting area, then your surgery patients will see a lot of patients come and go while they are sitting. Demarcating one sitting area for Mohs and one for clinics might help. So does ordering ahead. I’d show my daughter how to use the app so we don’t have to wait so long next week, but she’s 2 and I’m quite sure she already knows.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
“Papa! Where donut?” asks my 2½ year-old sitting with her legs dangling and hands folded in a bustling Starbucks. We’ve been waiting for 8 minutes and we’ve reached her limit of tolerance. She’s unimpressed by the queued customers who compliment her curly blonde hair, many of whom have come and gone since we’ve been waiting. I agree – how long does it take to pour a kiddie milk and grab a donut? We can both see it in the case right there!
No one likes to wait. Truly, one of the great benefits of the modern world is that wait times are now incredibly short. Many Starbucks customers, unlike my daughter, ordered their drink ahead and waited exactly 0 minutes to get their drink. What about Amazon? I ordered a bird feeder this morning and it’s already hanging in the yard. It’s still daylight. Feel like Himalayan Momo Dumplings tonight? Your food could arrive in 37 minutes. The modern wait standard has been set impossibly high for us.
Yes, for some. We created a whole room just for waiting. Airlines call theirs “The Platinum Executive Lounge.” Ours is “The waiting room.”
Excess waiting is a significant reason why health care gets beat up in reviews. We’re unable to keep up with the new expectations. Waiting is also a significant cause of distress. Many patients report the most difficult part of their cancer diagnosis was the waiting for results, not the treatment. It’s because when under stress, we are hardwired to take action. Binding patients into inaction while they wait is very uncomfortable.
Fortunately, the psychology of waiting is well understood and there are best practices that can help. First, anxiety makes waiting much worse. Conveying confidence and reassuring patients they are in the right place and that everything will be OK makes the wait time feel shorter for them. Uncertainty also compounds their apprehension. If you believe the diagnosis will be melanoma, tell them that at the time of the biopsy and tell them what you expect next. This is better than saying, “Well, that could be cancer. We’ll see.”
Knowing a wait time is also much better than not. Have your staff advise patients on how much longer they can expect before seeing you (telling them they’re next isn’t as effective). Advise that test results should be back by the end of next week. Of course, under promise and over deliver. When the results are back on Tuesday, you’ve got a pleased patient.
Explaining that you had to add in an urgent patient helps. Even if it’s not your fault, it’s still better to apologize. For example, the 78 highway, the left anterior descending artery to our office, has been closed because of a sinkhole this month (not kidding). I’ve been apologizing to a lot of patients saying that all our patients are arriving late, which is putting us behind. As they can envision the linear parking lot that used to be a highway, it helps.
Lastly, as any child can tell you, waiting has to not only be, but to also appear, fair. The only thing worse than waiting for an appointment, or donut, is seeing someone who came in after you get their donut before you do. If you’re pulling both Mohs and cosmetics patients from the same waiting area, then your surgery patients will see a lot of patients come and go while they are sitting. Demarcating one sitting area for Mohs and one for clinics might help. So does ordering ahead. I’d show my daughter how to use the app so we don’t have to wait so long next week, but she’s 2 and I’m quite sure she already knows.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
“Papa! Where donut?” asks my 2½ year-old sitting with her legs dangling and hands folded in a bustling Starbucks. We’ve been waiting for 8 minutes and we’ve reached her limit of tolerance. She’s unimpressed by the queued customers who compliment her curly blonde hair, many of whom have come and gone since we’ve been waiting. I agree – how long does it take to pour a kiddie milk and grab a donut? We can both see it in the case right there!
No one likes to wait. Truly, one of the great benefits of the modern world is that wait times are now incredibly short. Many Starbucks customers, unlike my daughter, ordered their drink ahead and waited exactly 0 minutes to get their drink. What about Amazon? I ordered a bird feeder this morning and it’s already hanging in the yard. It’s still daylight. Feel like Himalayan Momo Dumplings tonight? Your food could arrive in 37 minutes. The modern wait standard has been set impossibly high for us.
Yes, for some. We created a whole room just for waiting. Airlines call theirs “The Platinum Executive Lounge.” Ours is “The waiting room.”
Excess waiting is a significant reason why health care gets beat up in reviews. We’re unable to keep up with the new expectations. Waiting is also a significant cause of distress. Many patients report the most difficult part of their cancer diagnosis was the waiting for results, not the treatment. It’s because when under stress, we are hardwired to take action. Binding patients into inaction while they wait is very uncomfortable.
Fortunately, the psychology of waiting is well understood and there are best practices that can help. First, anxiety makes waiting much worse. Conveying confidence and reassuring patients they are in the right place and that everything will be OK makes the wait time feel shorter for them. Uncertainty also compounds their apprehension. If you believe the diagnosis will be melanoma, tell them that at the time of the biopsy and tell them what you expect next. This is better than saying, “Well, that could be cancer. We’ll see.”
Knowing a wait time is also much better than not. Have your staff advise patients on how much longer they can expect before seeing you (telling them they’re next isn’t as effective). Advise that test results should be back by the end of next week. Of course, under promise and over deliver. When the results are back on Tuesday, you’ve got a pleased patient.
Explaining that you had to add in an urgent patient helps. Even if it’s not your fault, it’s still better to apologize. For example, the 78 highway, the left anterior descending artery to our office, has been closed because of a sinkhole this month (not kidding). I’ve been apologizing to a lot of patients saying that all our patients are arriving late, which is putting us behind. As they can envision the linear parking lot that used to be a highway, it helps.
Lastly, as any child can tell you, waiting has to not only be, but to also appear, fair. The only thing worse than waiting for an appointment, or donut, is seeing someone who came in after you get their donut before you do. If you’re pulling both Mohs and cosmetics patients from the same waiting area, then your surgery patients will see a lot of patients come and go while they are sitting. Demarcating one sitting area for Mohs and one for clinics might help. So does ordering ahead. I’d show my daughter how to use the app so we don’t have to wait so long next week, but she’s 2 and I’m quite sure she already knows.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
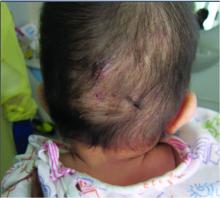
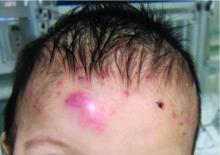
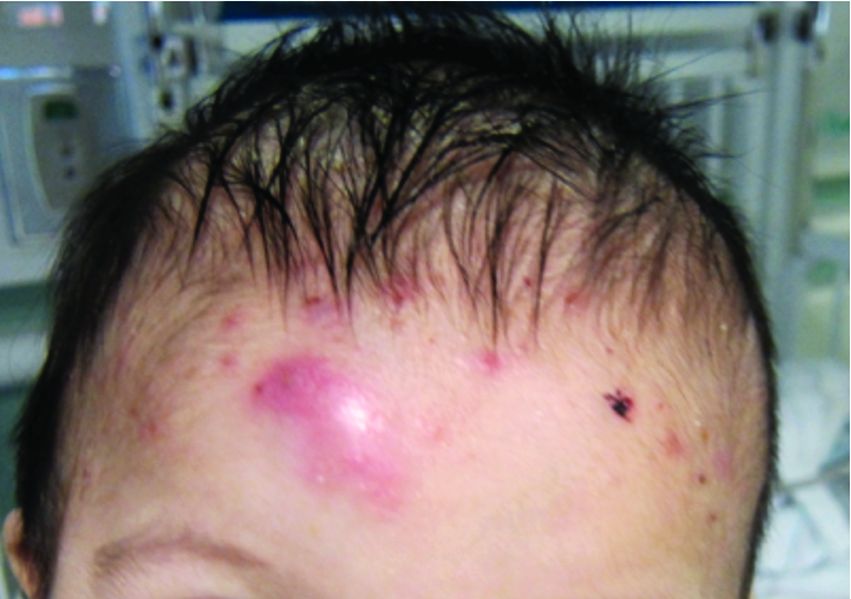
A 7-month-old male presents with pustules and inflamed papules on the scalp and extremities
The bacterial, fungal, and atypical mycobacterial cultures from the lesions performed at the emergency department were all negative.
Pediatric dermatology was consulted and a punch biopsy of one of the lesions was done. Histopathologic examination showed a mixed perifollicular infiltrate of predominantly eosinophils with some neutrophils and associated microabscesses. Periodic acid Schiff and Fite stains failed to reveal any organisms. CD1 immunostain was negative. Fresh tissue cultures for bacteria, fungi, and atypical mycobacteria were negative.
Given the clinical presentation of chronic recurrent sterile pustules on an infant with associated eosinophilia and the reported histopathologic findings, the patient was diagnosed with eosinophilic pustular folliculitis of infancy (EPFI).
EPFI is a rare and idiopathic cutaneous disorder present in children. About 70% of the cases reported occur in the first 6 month of life and rarely present past 3 years of age. EPF encompasses a group of conditions including the classic adult form, or Ofuji disease. EPF is seen in immunosuppressed patients, mainly HIV positive, and EPF is also seen in infants and children.
In EPFI, males are most commonly affected. The condition presents, as it did in our patient, with recurrent crops of sterile papules and pustules mainly on the scalp, but they can occur in other parts of the body. The lesions go away within a few weeks to months without leaving any scars but it can take months to years to resolve. Histopathologic analysis of the lesions show an eosinophilic infiltrate which can be follicular, perifollicular, or periadnexal with associated flame figures in about 26% of cases.
Aggressive treatment is usually not needed as lesions are self-limited. Lesions can be treated with topical corticosteroids and oral antihistamine medications like cetirizine if symptomatic.
If the lesions start to present during the neonatal period, one may consider in the differential diagnosis, neonatal rashes like transient neonatal pustular melanosis and erythema toxicum neonatorum. Both of these neonatal conditions tend to resolve in the first month of life, compared with EPFI where lesions can come and go for months to years. EPFI lesions can be described as pustules and inflammatory papules, as well as furuncles and vesicles. All of the lesions may be seen in one patient at one time, which will not be typical for transient neonatal pustular melanosis or erythema toxicum. Eosinophils can be seen in erythema toxicum but folliculitis is not present. The inflammatory infiltrate seen in transient neonatal pustular melanosis is polymorphonuclear, not eosinophilic.
Early in the presentation, infectious conditions like staphylococcal or streptococcal folliculitis, cellulitis and furunculosis, tinea capitis, atypical mycobacterial infections, herpes simplex, and parasitic infections like scabies should be considered. In young infants, empiric antibiotic treatment may be started until cultures are finalized. If there is a family history of pruritic papules and pustules, scabies should be considered. A scabies prep can be done to rule out this entity.
Langerhans cell histiocytosis can also present with pustules and papules in early infancy and also has a predilection for the scalp. When this condition is in question, a skin biopsy should be performed which shows a CD1 positive histiocytic infiltrate.
In conclusion, EPFI is a benign rare condition that can present in infants as recurrent pustules and papules, mainly on the scalp, which are self-limited and if symptomatic can be treated with topical corticosteroids and antihistamines.
References
Alonso-Castro L et al. Dermatol Online J. 2012 Oct 15;18(10):6.
Frølunde AS et al. Clin Case Rep. 2021 May 11;9(5):e04167.
Hernández-Martín Á et al. J Am Acad Dermatol. 2013 Jan;68(1):150-5.
The bacterial, fungal, and atypical mycobacterial cultures from the lesions performed at the emergency department were all negative.
Pediatric dermatology was consulted and a punch biopsy of one of the lesions was done. Histopathologic examination showed a mixed perifollicular infiltrate of predominantly eosinophils with some neutrophils and associated microabscesses. Periodic acid Schiff and Fite stains failed to reveal any organisms. CD1 immunostain was negative. Fresh tissue cultures for bacteria, fungi, and atypical mycobacteria were negative.
Given the clinical presentation of chronic recurrent sterile pustules on an infant with associated eosinophilia and the reported histopathologic findings, the patient was diagnosed with eosinophilic pustular folliculitis of infancy (EPFI).
EPFI is a rare and idiopathic cutaneous disorder present in children. About 70% of the cases reported occur in the first 6 month of life and rarely present past 3 years of age. EPF encompasses a group of conditions including the classic adult form, or Ofuji disease. EPF is seen in immunosuppressed patients, mainly HIV positive, and EPF is also seen in infants and children.
In EPFI, males are most commonly affected. The condition presents, as it did in our patient, with recurrent crops of sterile papules and pustules mainly on the scalp, but they can occur in other parts of the body. The lesions go away within a few weeks to months without leaving any scars but it can take months to years to resolve. Histopathologic analysis of the lesions show an eosinophilic infiltrate which can be follicular, perifollicular, or periadnexal with associated flame figures in about 26% of cases.
Aggressive treatment is usually not needed as lesions are self-limited. Lesions can be treated with topical corticosteroids and oral antihistamine medications like cetirizine if symptomatic.
If the lesions start to present during the neonatal period, one may consider in the differential diagnosis, neonatal rashes like transient neonatal pustular melanosis and erythema toxicum neonatorum. Both of these neonatal conditions tend to resolve in the first month of life, compared with EPFI where lesions can come and go for months to years. EPFI lesions can be described as pustules and inflammatory papules, as well as furuncles and vesicles. All of the lesions may be seen in one patient at one time, which will not be typical for transient neonatal pustular melanosis or erythema toxicum. Eosinophils can be seen in erythema toxicum but folliculitis is not present. The inflammatory infiltrate seen in transient neonatal pustular melanosis is polymorphonuclear, not eosinophilic.
Early in the presentation, infectious conditions like staphylococcal or streptococcal folliculitis, cellulitis and furunculosis, tinea capitis, atypical mycobacterial infections, herpes simplex, and parasitic infections like scabies should be considered. In young infants, empiric antibiotic treatment may be started until cultures are finalized. If there is a family history of pruritic papules and pustules, scabies should be considered. A scabies prep can be done to rule out this entity.
Langerhans cell histiocytosis can also present with pustules and papules in early infancy and also has a predilection for the scalp. When this condition is in question, a skin biopsy should be performed which shows a CD1 positive histiocytic infiltrate.
In conclusion, EPFI is a benign rare condition that can present in infants as recurrent pustules and papules, mainly on the scalp, which are self-limited and if symptomatic can be treated with topical corticosteroids and antihistamines.
References
Alonso-Castro L et al. Dermatol Online J. 2012 Oct 15;18(10):6.
Frølunde AS et al. Clin Case Rep. 2021 May 11;9(5):e04167.
Hernández-Martín Á et al. J Am Acad Dermatol. 2013 Jan;68(1):150-5.
The bacterial, fungal, and atypical mycobacterial cultures from the lesions performed at the emergency department were all negative.
Pediatric dermatology was consulted and a punch biopsy of one of the lesions was done. Histopathologic examination showed a mixed perifollicular infiltrate of predominantly eosinophils with some neutrophils and associated microabscesses. Periodic acid Schiff and Fite stains failed to reveal any organisms. CD1 immunostain was negative. Fresh tissue cultures for bacteria, fungi, and atypical mycobacteria were negative.
Given the clinical presentation of chronic recurrent sterile pustules on an infant with associated eosinophilia and the reported histopathologic findings, the patient was diagnosed with eosinophilic pustular folliculitis of infancy (EPFI).
EPFI is a rare and idiopathic cutaneous disorder present in children. About 70% of the cases reported occur in the first 6 month of life and rarely present past 3 years of age. EPF encompasses a group of conditions including the classic adult form, or Ofuji disease. EPF is seen in immunosuppressed patients, mainly HIV positive, and EPF is also seen in infants and children.
In EPFI, males are most commonly affected. The condition presents, as it did in our patient, with recurrent crops of sterile papules and pustules mainly on the scalp, but they can occur in other parts of the body. The lesions go away within a few weeks to months without leaving any scars but it can take months to years to resolve. Histopathologic analysis of the lesions show an eosinophilic infiltrate which can be follicular, perifollicular, or periadnexal with associated flame figures in about 26% of cases.
Aggressive treatment is usually not needed as lesions are self-limited. Lesions can be treated with topical corticosteroids and oral antihistamine medications like cetirizine if symptomatic.
If the lesions start to present during the neonatal period, one may consider in the differential diagnosis, neonatal rashes like transient neonatal pustular melanosis and erythema toxicum neonatorum. Both of these neonatal conditions tend to resolve in the first month of life, compared with EPFI where lesions can come and go for months to years. EPFI lesions can be described as pustules and inflammatory papules, as well as furuncles and vesicles. All of the lesions may be seen in one patient at one time, which will not be typical for transient neonatal pustular melanosis or erythema toxicum. Eosinophils can be seen in erythema toxicum but folliculitis is not present. The inflammatory infiltrate seen in transient neonatal pustular melanosis is polymorphonuclear, not eosinophilic.
Early in the presentation, infectious conditions like staphylococcal or streptococcal folliculitis, cellulitis and furunculosis, tinea capitis, atypical mycobacterial infections, herpes simplex, and parasitic infections like scabies should be considered. In young infants, empiric antibiotic treatment may be started until cultures are finalized. If there is a family history of pruritic papules and pustules, scabies should be considered. A scabies prep can be done to rule out this entity.
Langerhans cell histiocytosis can also present with pustules and papules in early infancy and also has a predilection for the scalp. When this condition is in question, a skin biopsy should be performed which shows a CD1 positive histiocytic infiltrate.
In conclusion, EPFI is a benign rare condition that can present in infants as recurrent pustules and papules, mainly on the scalp, which are self-limited and if symptomatic can be treated with topical corticosteroids and antihistamines.
References
Alonso-Castro L et al. Dermatol Online J. 2012 Oct 15;18(10):6.
Frølunde AS et al. Clin Case Rep. 2021 May 11;9(5):e04167.
Hernández-Martín Á et al. J Am Acad Dermatol. 2013 Jan;68(1):150-5.
A 7-month-old male is brought to the emergency department for evaluation of pustules and inflamed papules on the scalp and extremities for several weeks of duration. The parents report the lesions started about a month prior and he has already been treated with cephalexin, clindamycin, and sulfamethoxazole without any improvement. Cultures sent prior by the child's pediatrician did not reveal any fungus or bacteria. The parents report a low-grade fever for about 3 days.
He was born via natural vaginal delivery with no instrumentation or external monitoring. Mom had prenatal care. Besides the skin lesions, the baby has been healthy and growing well. He has no history of eczema or severe infections. He has not been hospitalized before.
On physical examination the baby was not febrile. On the scalp and forehead, he had diffusely distributed pustules, erythematous papules, and nodules. He also presented with scattered, fine, small, crusted 1-2-mm pink papules on the trunk and extremities. He had no adenopathy or hepatosplenomegaly.
At the emergency department, samples from one of the pustules were sent for bacterial, fungal, and atypical mycobacteria cultures. Laboratory test showed a normal blood count with associated eosinophilia (2.8 x 109 L), and normal liver and kidney function. A head ultrasound showed three ill-defined hypoechoic foci within the scalp.
The patient was admitted for treatment with broad-spectrum antibiotics and dermatology was consulted.
Medicare expands CGM coverage to more with type 2 diabetes
Medicare is now covering continuous glucose monitoring (CGM) for all beneficiaries with diabetes who use insulin, as well as those with a “history of problematic hypoglycemia.”
The new policy decision, announced earlier this year by the Centers for Medicare and Medicaid Services, means that coverage is expanded to those who take even just a single dose of basal insulin daily or who don’t take insulin but who for other reasons experience “problematic” hypoglycemia, defined as a history of more than one level 2 event (glucose < 54 mg/dL) or at least one level 3 event (< 54 mg/dL requiring assistance).
Previously, coverage was limited to those taking frequent daily insulin doses.
The additional number of people covered, most with type 2 diabetes, is estimated to be at least 1.5 million. That number could more than double if private insurers follow suit, reported an industry analyst.
Chuck Henderson, chief executive officer of the American Diabetes Association, said in a statement: “We applaud CMS’ decision allowing for all insulin-dependent people as well as others who have a history of problematic hypoglycemia to have access to a continuous glucose monitor, a potentially life-saving tool for diabetes management.”
According to Dexcom, which manufacturers the G6 and the recently approved G7 CGMs, the decision was based in part on their MOBILE study. The trial demonstrated the benefit of CGM in people with type 2 diabetes who use only basal insulin or have a history of problematic hypoglycemic events.
On April 14, Abbott, which manufactures the Freestyle Libre 2 and the recently approved Libre 3, received clearance from the U.S. Food and Drug Administration for the Libre 3’s stand-alone reader device. Previously, the Libre 3 had been approved for use only with a smartphone app. The small handheld reader is considered durable medical equipment, making it eligible for Medicare coverage. Abbott is “working on having the FreeStyle Libre 3 system available to Medicare beneficiaries,” the company said in a statement.
A version of this article first appeared on Medscape.com.
Medicare is now covering continuous glucose monitoring (CGM) for all beneficiaries with diabetes who use insulin, as well as those with a “history of problematic hypoglycemia.”
The new policy decision, announced earlier this year by the Centers for Medicare and Medicaid Services, means that coverage is expanded to those who take even just a single dose of basal insulin daily or who don’t take insulin but who for other reasons experience “problematic” hypoglycemia, defined as a history of more than one level 2 event (glucose < 54 mg/dL) or at least one level 3 event (< 54 mg/dL requiring assistance).
Previously, coverage was limited to those taking frequent daily insulin doses.
The additional number of people covered, most with type 2 diabetes, is estimated to be at least 1.5 million. That number could more than double if private insurers follow suit, reported an industry analyst.
Chuck Henderson, chief executive officer of the American Diabetes Association, said in a statement: “We applaud CMS’ decision allowing for all insulin-dependent people as well as others who have a history of problematic hypoglycemia to have access to a continuous glucose monitor, a potentially life-saving tool for diabetes management.”
According to Dexcom, which manufacturers the G6 and the recently approved G7 CGMs, the decision was based in part on their MOBILE study. The trial demonstrated the benefit of CGM in people with type 2 diabetes who use only basal insulin or have a history of problematic hypoglycemic events.
On April 14, Abbott, which manufactures the Freestyle Libre 2 and the recently approved Libre 3, received clearance from the U.S. Food and Drug Administration for the Libre 3’s stand-alone reader device. Previously, the Libre 3 had been approved for use only with a smartphone app. The small handheld reader is considered durable medical equipment, making it eligible for Medicare coverage. Abbott is “working on having the FreeStyle Libre 3 system available to Medicare beneficiaries,” the company said in a statement.
A version of this article first appeared on Medscape.com.
Medicare is now covering continuous glucose monitoring (CGM) for all beneficiaries with diabetes who use insulin, as well as those with a “history of problematic hypoglycemia.”
The new policy decision, announced earlier this year by the Centers for Medicare and Medicaid Services, means that coverage is expanded to those who take even just a single dose of basal insulin daily or who don’t take insulin but who for other reasons experience “problematic” hypoglycemia, defined as a history of more than one level 2 event (glucose < 54 mg/dL) or at least one level 3 event (< 54 mg/dL requiring assistance).
Previously, coverage was limited to those taking frequent daily insulin doses.
The additional number of people covered, most with type 2 diabetes, is estimated to be at least 1.5 million. That number could more than double if private insurers follow suit, reported an industry analyst.
Chuck Henderson, chief executive officer of the American Diabetes Association, said in a statement: “We applaud CMS’ decision allowing for all insulin-dependent people as well as others who have a history of problematic hypoglycemia to have access to a continuous glucose monitor, a potentially life-saving tool for diabetes management.”
According to Dexcom, which manufacturers the G6 and the recently approved G7 CGMs, the decision was based in part on their MOBILE study. The trial demonstrated the benefit of CGM in people with type 2 diabetes who use only basal insulin or have a history of problematic hypoglycemic events.
On April 14, Abbott, which manufactures the Freestyle Libre 2 and the recently approved Libre 3, received clearance from the U.S. Food and Drug Administration for the Libre 3’s stand-alone reader device. Previously, the Libre 3 had been approved for use only with a smartphone app. The small handheld reader is considered durable medical equipment, making it eligible for Medicare coverage. Abbott is “working on having the FreeStyle Libre 3 system available to Medicare beneficiaries,” the company said in a statement.
A version of this article first appeared on Medscape.com.
Closer to home: Melioidosis in the United States
Chest Infections & Disaster Response Network
Disaster Response & Global Health Section
caused by the gram-negative bacillus Burkholderia pseudomallei, does not usually appear on the differential diagnosis of patients in the United States. Historically endemic to South and Southeast Asia, Australia, Puerto Rico, and Central America, B. pseudomallei infects humans via direct inoculation of the skin, through inhalation, or by the ingestion of contaminated soil or water. Importation of melioidosis to the United States from civilian travelers, global commerce, or military personnel is becoming more common (Gee JE, et al. N Engl J Med. 2022;386[9]:861).
A case series of four patients across four states occurred in 2021. Contaminated aromatherapy sprays sold from a retailer whose supplier originated from India were identified as the source (Gee JE, et al). Two additional cases were reported in Mississippi spanning 2 years (CDC Health Alert Network. July 27, 2022). A case in Texas describes the zoonotic detection of the organism in a raccoon carcass (Petras JK, et al. MMWR. 2022;71:1597). Now, cases of U.S. domestic melioidosis have been described, with the CDC identifying areas of the Mississippi Gulf Coast as an endemic region.
The gold standard of diagnosis is the isolation of B. pseudomallei in culture. Serologic tests may also be useful. Automated bacterial identification systems may provide initially inaccurate results, delaying diagnosis and increasing mortality. Presenting symptoms are nonspecific and may resemble typical sepsis syndromes, as well as cavitary lung disease, mimicking TB. The diagnosis requires a high index of suspicion with targeted interviewing.
Clinicians should reevaluate patients with isolates identified as Burkholderia species, especially those who are unresponsive to standard empiric therapies. Treatment for melioidosis involves initial antibiotic therapy with ceftazidime, meropenem, or imipenem, followed by eradication therapy with trimethoprim-sulfamethoxazole or amoxicillin-clavulanate for up to 6 months (Wiersinga WJ, et al. N Engl J Med. 2012;367[11]:1035).
Zein Kattih, MD
Chest Infections & Disaster Response Network
Disaster Response & Global Health Section
caused by the gram-negative bacillus Burkholderia pseudomallei, does not usually appear on the differential diagnosis of patients in the United States. Historically endemic to South and Southeast Asia, Australia, Puerto Rico, and Central America, B. pseudomallei infects humans via direct inoculation of the skin, through inhalation, or by the ingestion of contaminated soil or water. Importation of melioidosis to the United States from civilian travelers, global commerce, or military personnel is becoming more common (Gee JE, et al. N Engl J Med. 2022;386[9]:861).
A case series of four patients across four states occurred in 2021. Contaminated aromatherapy sprays sold from a retailer whose supplier originated from India were identified as the source (Gee JE, et al). Two additional cases were reported in Mississippi spanning 2 years (CDC Health Alert Network. July 27, 2022). A case in Texas describes the zoonotic detection of the organism in a raccoon carcass (Petras JK, et al. MMWR. 2022;71:1597). Now, cases of U.S. domestic melioidosis have been described, with the CDC identifying areas of the Mississippi Gulf Coast as an endemic region.
The gold standard of diagnosis is the isolation of B. pseudomallei in culture. Serologic tests may also be useful. Automated bacterial identification systems may provide initially inaccurate results, delaying diagnosis and increasing mortality. Presenting symptoms are nonspecific and may resemble typical sepsis syndromes, as well as cavitary lung disease, mimicking TB. The diagnosis requires a high index of suspicion with targeted interviewing.
Clinicians should reevaluate patients with isolates identified as Burkholderia species, especially those who are unresponsive to standard empiric therapies. Treatment for melioidosis involves initial antibiotic therapy with ceftazidime, meropenem, or imipenem, followed by eradication therapy with trimethoprim-sulfamethoxazole or amoxicillin-clavulanate for up to 6 months (Wiersinga WJ, et al. N Engl J Med. 2012;367[11]:1035).
Zein Kattih, MD
Chest Infections & Disaster Response Network
Disaster Response & Global Health Section
caused by the gram-negative bacillus Burkholderia pseudomallei, does not usually appear on the differential diagnosis of patients in the United States. Historically endemic to South and Southeast Asia, Australia, Puerto Rico, and Central America, B. pseudomallei infects humans via direct inoculation of the skin, through inhalation, or by the ingestion of contaminated soil or water. Importation of melioidosis to the United States from civilian travelers, global commerce, or military personnel is becoming more common (Gee JE, et al. N Engl J Med. 2022;386[9]:861).
A case series of four patients across four states occurred in 2021. Contaminated aromatherapy sprays sold from a retailer whose supplier originated from India were identified as the source (Gee JE, et al). Two additional cases were reported in Mississippi spanning 2 years (CDC Health Alert Network. July 27, 2022). A case in Texas describes the zoonotic detection of the organism in a raccoon carcass (Petras JK, et al. MMWR. 2022;71:1597). Now, cases of U.S. domestic melioidosis have been described, with the CDC identifying areas of the Mississippi Gulf Coast as an endemic region.
The gold standard of diagnosis is the isolation of B. pseudomallei in culture. Serologic tests may also be useful. Automated bacterial identification systems may provide initially inaccurate results, delaying diagnosis and increasing mortality. Presenting symptoms are nonspecific and may resemble typical sepsis syndromes, as well as cavitary lung disease, mimicking TB. The diagnosis requires a high index of suspicion with targeted interviewing.
Clinicians should reevaluate patients with isolates identified as Burkholderia species, especially those who are unresponsive to standard empiric therapies. Treatment for melioidosis involves initial antibiotic therapy with ceftazidime, meropenem, or imipenem, followed by eradication therapy with trimethoprim-sulfamethoxazole or amoxicillin-clavulanate for up to 6 months (Wiersinga WJ, et al. N Engl J Med. 2012;367[11]:1035).
Zein Kattih, MD
Forgotten but not gone: EVALI epidemic continues
Rashelle Bernal vaped and ended up in an induced coma for a week. She was one of almost 3,000 people who were hospitalized during 2019 and early 2020 with severe lung damage from vaping and became part of what is now known as the epidemic of e-cigarette, or vaping, product use–associated lung injury (EVALI).
For many, the EVALI epidemic is a distant, pre-COVID memory.
But the vaping-related injuries are still happening. And for Ms. Bernal, the aftermath is her reality. Her pulmonologist from that time described the harm from the vape ingredients as an oil spill in her lungs. Eventually, the toxins would probably clear. But she will likely wrestle with the injuries for a very long time.
More than 3 years later, she frequently finds herself in the emergency department.
“If I get sick, if there’s anything that irritates my lungs – it could be something as simple as pollen in the air – it will cause me to get like a bacterial infection or other issues, and I can’t breathe,” Ms. Ms. Bernal, now 30, said in a recent interview. “I get really winded, to the point where I’ll walk up the stairs and I feel like I just ran a mile.”
In 2019 and 2020, a media firestorm erupted as hospitals notified the public of outbreaks of vaping-related lung injuries. News headlines reported e-cigarettes were killing teens from Texas to the Bronx. Investigators at the U.S. Centers for Disease Control and Prevention tracked most of the cases to vitamin E acetate, an additive in illicit cannabis vaping products intended to promote the metabolism of tetrahydrocannabinol (THC). The agency stopped tracking EVALI in February 2020.
But 2 months later, in April 2020, the agency’s National Center for Health Statistics implemented a diagnostic code, U07.0, for health care professionals in the United States to diagnose EVALI for the first time. The code is also used for lung damage related to use of electronic cigarettes and “dabbing” – a method of inhaling cannabis. Damage could include inflammation of the lungs, pulmonary hemorrhage, and eosinophilic pneumonia.
The incidence of these diagnoses appears to have risen sharply since 2020. In the last three months of 2020, a total of 11,300 medical claims included the U07.0 code. That figure rose to 22,000 in 2021 and hit 31,600 in 2022, according to data compiled for and provided to Medscape by Komodo Health, a health care technology company that holds a database of more than 330 million U.S. patients from Medicare, Medicaid, and commercial insurers’ medical, pharmacy, and laboratory claims.
Harm from vaping, including EVALI, has continued.
said Usha Periyanayagam, MD, MPH, head of clinic product and real-world evidence for Komodo and a former emergency medicine physician.
Where it started
Devika Rao, MD, a pediatric pulmonology specialist at UT Southwestern Medical Center, Dallas, has cared for most of her EVALI patients in the hospital, with the most recent case in early 2023. But in January, for the first time, she saw an EVALI patient in an outpatient clinic. The person had not been admitted to the hospital – like most were pre-pandemic. And like most who were seen during the pandemic, this patient had milder symptoms, not requiring intubation or take-home oxygen.
In 2019 and the beginning of 2020, many EVALI patients who were eventually hospitalized first sought help at urgent care centers or with primary care doctors and were presumed to have pneumonia or gastroenteritis and sent home.
“But they got worse, and they would present to our emergency room; their chest X-rays and CT scans showed extensive lung disease,” Dr. Rao said, adding that the damage was striking among patients all under age 18. “They were short of breath. Their oxygen levels were low. They had diminished lung function. And they had a lot of GI issues like abdominal pain and weight loss from nausea and vomiting.”
“These overwhelming inflammatory reactions that we see with EVALI,” said Karen M. Wilson, MD, MPH, a pediatric hospitalist at the University of Rochester (N.Y.) Medical Center and a tobacco use researcher. “You might find some microvascular changes with normal inhaling of smoke or aerosol, but you’re not going to find macro changes like we see with the EVALI.”
In late 2019, images of the CT scans of patients with EVALI were published, grabbing the attention of Arun Kannappan, MD, an assistant professor of pulmonary sciences and critical care at the University of Colorado Anschutz School of Medicine, Aurora. Dr. Kannappan knew a patient with such severe lung damage could develop acute respiratory distress syndrome, which means a patient would be put on a ventilator because their inflamed lungs could not oxygenate blood.
“That confers within somewhere between 30% to 50% chance of dying; it made all of the pulmonary specialists really turn their heads to make sure that we keep a lookout for it,” said Dr. Kannappan.
CT scans of lungs proved to be a critical diagnostic tool for doctors. Most of the images from patients showed acute inflammation and diffuse lung damage. Ehab Ali, MD, a critical care and pulmonary disease medicine specialist in Louisville, Ky., said the damage was often spread across both lungs in many areas and appeared opaque and hazy, known as “ground glass.” COVID-19, meanwhile, appeared differently in lung scans, often with damage that was more isolated.
But many diseases carry a “ground glass” appearance, with many potential causes, like infections, cigarette smoke, or an autoimmune condition.
“Before you even talk to the patient, you can immediately put it in your mind that ‘I’m going to ask this patient if they vape,’ when I see the distribution of ground glass appearance,” Dr. Ali said.
Dr. Ali said other factors, like the age of the patient – about three-quarters of EVALI patients are under age 34, according to the CDC – would spur him to ask about vaping. But because so many patients were young, discerning vape usage wasn’t always easy.
“When you’re talking to teenagers, if you ask them upon admission, with the parents in the room, they’re going say ‘no,’ ” said Rachel Boykan, MD, a pediatric hospitalist at Stony Brook (N.Y.) Children’s. She added that her hospital is still seeing cases.
Dr. Rao said it often takes two to three people asking a patient about any vape usage before they confess.
Ms. Bernal, who was 27 at the time of her hospital admission for EVALI, said she bought vapes with THC at a retail shop in California. She’d been a traditional marijuana smoker, using the leaf product, but switched when someone told her it was healthier to vape THC than inhale smoke from burned marijuana leaves into her lungs. “I thought this was safe.”
Dr. Rao and her colleagues recently published a study of 41 teenage patients with EVALI who were seen at Children’s Medical Center Dallas between December 2018 and July 2021. All but one reported using e-cigarettes containing THC, and the CDC in its most recent report from February 2020 said about 80% of patients had used vapes containing THC.
The CDC also found that vitamin E acetate, an oily substance that allows THC to travel from the lungs to the brain quickly and an ingredient used in the food and cosmetics industries, was found in many of the lungs of EVALI patients, though not all.
The aftermath
The outcomes of the thousands of patients who had EVALI – and those who may still be developing it – are largely untracked.
Bonnie Halpern-Felsher, PhD, director at the Stanford (Calif.) Reach Lab that bears her name and a researcher on tobacco in youth, said she and many of her colleagues are frustrated that the CDC is not continuing to collect data on EVALI.
“I know a lot of colleagues who’ve said that they’re still seeing EVALI, but because of COVID-19 they stopped collecting the data. And that’s been very frustrating because it’s hard to say whether the kinds of lung issues you’re having are related to e-cigarettes, generally, or EVALI,” Dr. Halpern-Felsher said.
Researchers and doctors affiliated with the American Thoracic Society published a report with solutions on how to better track EVALI. They recommended that a national case registry and biorepository be created.
Doctors also worry that many cases were missed. Dr. Boykan said that while protocol dictated nurses and other clinicians ask about a history of vaping – a key part of EVALI diagnosis – many did not. Dr. Ali, the Louisville critical care physician, said EVALI symptoms of nausea, cough, and fever are associated with viral infections.
“I’m sure that some of these cases might be discharged from the emergency room as a virus,” Dr. Ali said. “Most of the time patients would get prescribed steroids for viral infections, which may help EVALI patients even though it’s never been studied.”
Dr. Rao also said the treatment regimen at Children’s MC Dallas, which included high doses of intravenous steroids, seemed to help. But the best management approach for treatment, or long-term follow up care, has not been studied.
The report in the Annals of the American Thoracic Society said prospective studies are showing that a significant portion of patients with EVALI experience prolonged respiratory issues and cognitive and mood impairment. Dr. Rao said a common thread for many of her EVALI patients has been significant stress in their lives with school or family, which led them to vape in an attempt to reduce stress.
That was certainly the case for Ms. Bernal before her hospital admission. She had recently moved across the country for her husband’s job, was trying to buy a house, and had spent months in a hotel with three children. She vaped to cope.
But she said her mental and cognitive health has worsened. Back in Louisville, she saw a neurologist, who told her that her brain had shrunk, she said. She hasn’t found a new neurologist in Portland, Ore., where her family moved a year after the EVALI episode.
But she often finds herself overwhelmed and overstimulated with tasks that she said she never had problems with before. She tears up while talking about the newfound limitations. She struggled to find a primary care physician who could medically manage her mental health and a counselor who can understand what she’s been through with EVALI.
But, “a lot of doctors aren’t educated in it, and they don’t know how to respond or they don’t know what to do,” Ms. Bernal said. “And that makes me feel like, I guess, what I had wasn’t important.”
Ms. Bernal does have a new pulmonologist and is going in for a round of pulmonary tests soon because she often finds herself unable to breathe while completing simple tasks. She is tired of rushing to the ER. She wants answers, or some kind of treatment to help her feel normal again.
“I feel like this is my fault,” Ms. Bernal said. “Had I not smoked, I would be fine, and that’s hard to live with. Every day. Telling yourself, ‘It’s your fault.’ It’s been how many years now? And I still haven’t found peace yet. I don’t know if ever will.”
A version of this article first appeared on Medscape.com.
Rashelle Bernal vaped and ended up in an induced coma for a week. She was one of almost 3,000 people who were hospitalized during 2019 and early 2020 with severe lung damage from vaping and became part of what is now known as the epidemic of e-cigarette, or vaping, product use–associated lung injury (EVALI).
For many, the EVALI epidemic is a distant, pre-COVID memory.
But the vaping-related injuries are still happening. And for Ms. Bernal, the aftermath is her reality. Her pulmonologist from that time described the harm from the vape ingredients as an oil spill in her lungs. Eventually, the toxins would probably clear. But she will likely wrestle with the injuries for a very long time.
More than 3 years later, she frequently finds herself in the emergency department.
“If I get sick, if there’s anything that irritates my lungs – it could be something as simple as pollen in the air – it will cause me to get like a bacterial infection or other issues, and I can’t breathe,” Ms. Ms. Bernal, now 30, said in a recent interview. “I get really winded, to the point where I’ll walk up the stairs and I feel like I just ran a mile.”
In 2019 and 2020, a media firestorm erupted as hospitals notified the public of outbreaks of vaping-related lung injuries. News headlines reported e-cigarettes were killing teens from Texas to the Bronx. Investigators at the U.S. Centers for Disease Control and Prevention tracked most of the cases to vitamin E acetate, an additive in illicit cannabis vaping products intended to promote the metabolism of tetrahydrocannabinol (THC). The agency stopped tracking EVALI in February 2020.
But 2 months later, in April 2020, the agency’s National Center for Health Statistics implemented a diagnostic code, U07.0, for health care professionals in the United States to diagnose EVALI for the first time. The code is also used for lung damage related to use of electronic cigarettes and “dabbing” – a method of inhaling cannabis. Damage could include inflammation of the lungs, pulmonary hemorrhage, and eosinophilic pneumonia.
The incidence of these diagnoses appears to have risen sharply since 2020. In the last three months of 2020, a total of 11,300 medical claims included the U07.0 code. That figure rose to 22,000 in 2021 and hit 31,600 in 2022, according to data compiled for and provided to Medscape by Komodo Health, a health care technology company that holds a database of more than 330 million U.S. patients from Medicare, Medicaid, and commercial insurers’ medical, pharmacy, and laboratory claims.
Harm from vaping, including EVALI, has continued.
said Usha Periyanayagam, MD, MPH, head of clinic product and real-world evidence for Komodo and a former emergency medicine physician.
Where it started
Devika Rao, MD, a pediatric pulmonology specialist at UT Southwestern Medical Center, Dallas, has cared for most of her EVALI patients in the hospital, with the most recent case in early 2023. But in January, for the first time, she saw an EVALI patient in an outpatient clinic. The person had not been admitted to the hospital – like most were pre-pandemic. And like most who were seen during the pandemic, this patient had milder symptoms, not requiring intubation or take-home oxygen.
In 2019 and the beginning of 2020, many EVALI patients who were eventually hospitalized first sought help at urgent care centers or with primary care doctors and were presumed to have pneumonia or gastroenteritis and sent home.
“But they got worse, and they would present to our emergency room; their chest X-rays and CT scans showed extensive lung disease,” Dr. Rao said, adding that the damage was striking among patients all under age 18. “They were short of breath. Their oxygen levels were low. They had diminished lung function. And they had a lot of GI issues like abdominal pain and weight loss from nausea and vomiting.”
“These overwhelming inflammatory reactions that we see with EVALI,” said Karen M. Wilson, MD, MPH, a pediatric hospitalist at the University of Rochester (N.Y.) Medical Center and a tobacco use researcher. “You might find some microvascular changes with normal inhaling of smoke or aerosol, but you’re not going to find macro changes like we see with the EVALI.”
In late 2019, images of the CT scans of patients with EVALI were published, grabbing the attention of Arun Kannappan, MD, an assistant professor of pulmonary sciences and critical care at the University of Colorado Anschutz School of Medicine, Aurora. Dr. Kannappan knew a patient with such severe lung damage could develop acute respiratory distress syndrome, which means a patient would be put on a ventilator because their inflamed lungs could not oxygenate blood.
“That confers within somewhere between 30% to 50% chance of dying; it made all of the pulmonary specialists really turn their heads to make sure that we keep a lookout for it,” said Dr. Kannappan.
CT scans of lungs proved to be a critical diagnostic tool for doctors. Most of the images from patients showed acute inflammation and diffuse lung damage. Ehab Ali, MD, a critical care and pulmonary disease medicine specialist in Louisville, Ky., said the damage was often spread across both lungs in many areas and appeared opaque and hazy, known as “ground glass.” COVID-19, meanwhile, appeared differently in lung scans, often with damage that was more isolated.
But many diseases carry a “ground glass” appearance, with many potential causes, like infections, cigarette smoke, or an autoimmune condition.
“Before you even talk to the patient, you can immediately put it in your mind that ‘I’m going to ask this patient if they vape,’ when I see the distribution of ground glass appearance,” Dr. Ali said.
Dr. Ali said other factors, like the age of the patient – about three-quarters of EVALI patients are under age 34, according to the CDC – would spur him to ask about vaping. But because so many patients were young, discerning vape usage wasn’t always easy.
“When you’re talking to teenagers, if you ask them upon admission, with the parents in the room, they’re going say ‘no,’ ” said Rachel Boykan, MD, a pediatric hospitalist at Stony Brook (N.Y.) Children’s. She added that her hospital is still seeing cases.
Dr. Rao said it often takes two to three people asking a patient about any vape usage before they confess.
Ms. Bernal, who was 27 at the time of her hospital admission for EVALI, said she bought vapes with THC at a retail shop in California. She’d been a traditional marijuana smoker, using the leaf product, but switched when someone told her it was healthier to vape THC than inhale smoke from burned marijuana leaves into her lungs. “I thought this was safe.”
Dr. Rao and her colleagues recently published a study of 41 teenage patients with EVALI who were seen at Children’s Medical Center Dallas between December 2018 and July 2021. All but one reported using e-cigarettes containing THC, and the CDC in its most recent report from February 2020 said about 80% of patients had used vapes containing THC.
The CDC also found that vitamin E acetate, an oily substance that allows THC to travel from the lungs to the brain quickly and an ingredient used in the food and cosmetics industries, was found in many of the lungs of EVALI patients, though not all.
The aftermath
The outcomes of the thousands of patients who had EVALI – and those who may still be developing it – are largely untracked.
Bonnie Halpern-Felsher, PhD, director at the Stanford (Calif.) Reach Lab that bears her name and a researcher on tobacco in youth, said she and many of her colleagues are frustrated that the CDC is not continuing to collect data on EVALI.
“I know a lot of colleagues who’ve said that they’re still seeing EVALI, but because of COVID-19 they stopped collecting the data. And that’s been very frustrating because it’s hard to say whether the kinds of lung issues you’re having are related to e-cigarettes, generally, or EVALI,” Dr. Halpern-Felsher said.
Researchers and doctors affiliated with the American Thoracic Society published a report with solutions on how to better track EVALI. They recommended that a national case registry and biorepository be created.
Doctors also worry that many cases were missed. Dr. Boykan said that while protocol dictated nurses and other clinicians ask about a history of vaping – a key part of EVALI diagnosis – many did not. Dr. Ali, the Louisville critical care physician, said EVALI symptoms of nausea, cough, and fever are associated with viral infections.
“I’m sure that some of these cases might be discharged from the emergency room as a virus,” Dr. Ali said. “Most of the time patients would get prescribed steroids for viral infections, which may help EVALI patients even though it’s never been studied.”
Dr. Rao also said the treatment regimen at Children’s MC Dallas, which included high doses of intravenous steroids, seemed to help. But the best management approach for treatment, or long-term follow up care, has not been studied.
The report in the Annals of the American Thoracic Society said prospective studies are showing that a significant portion of patients with EVALI experience prolonged respiratory issues and cognitive and mood impairment. Dr. Rao said a common thread for many of her EVALI patients has been significant stress in their lives with school or family, which led them to vape in an attempt to reduce stress.
That was certainly the case for Ms. Bernal before her hospital admission. She had recently moved across the country for her husband’s job, was trying to buy a house, and had spent months in a hotel with three children. She vaped to cope.
But she said her mental and cognitive health has worsened. Back in Louisville, she saw a neurologist, who told her that her brain had shrunk, she said. She hasn’t found a new neurologist in Portland, Ore., where her family moved a year after the EVALI episode.
But she often finds herself overwhelmed and overstimulated with tasks that she said she never had problems with before. She tears up while talking about the newfound limitations. She struggled to find a primary care physician who could medically manage her mental health and a counselor who can understand what she’s been through with EVALI.
But, “a lot of doctors aren’t educated in it, and they don’t know how to respond or they don’t know what to do,” Ms. Bernal said. “And that makes me feel like, I guess, what I had wasn’t important.”
Ms. Bernal does have a new pulmonologist and is going in for a round of pulmonary tests soon because she often finds herself unable to breathe while completing simple tasks. She is tired of rushing to the ER. She wants answers, or some kind of treatment to help her feel normal again.
“I feel like this is my fault,” Ms. Bernal said. “Had I not smoked, I would be fine, and that’s hard to live with. Every day. Telling yourself, ‘It’s your fault.’ It’s been how many years now? And I still haven’t found peace yet. I don’t know if ever will.”
A version of this article first appeared on Medscape.com.
Rashelle Bernal vaped and ended up in an induced coma for a week. She was one of almost 3,000 people who were hospitalized during 2019 and early 2020 with severe lung damage from vaping and became part of what is now known as the epidemic of e-cigarette, or vaping, product use–associated lung injury (EVALI).
For many, the EVALI epidemic is a distant, pre-COVID memory.
But the vaping-related injuries are still happening. And for Ms. Bernal, the aftermath is her reality. Her pulmonologist from that time described the harm from the vape ingredients as an oil spill in her lungs. Eventually, the toxins would probably clear. But she will likely wrestle with the injuries for a very long time.
More than 3 years later, she frequently finds herself in the emergency department.
“If I get sick, if there’s anything that irritates my lungs – it could be something as simple as pollen in the air – it will cause me to get like a bacterial infection or other issues, and I can’t breathe,” Ms. Ms. Bernal, now 30, said in a recent interview. “I get really winded, to the point where I’ll walk up the stairs and I feel like I just ran a mile.”
In 2019 and 2020, a media firestorm erupted as hospitals notified the public of outbreaks of vaping-related lung injuries. News headlines reported e-cigarettes were killing teens from Texas to the Bronx. Investigators at the U.S. Centers for Disease Control and Prevention tracked most of the cases to vitamin E acetate, an additive in illicit cannabis vaping products intended to promote the metabolism of tetrahydrocannabinol (THC). The agency stopped tracking EVALI in February 2020.
But 2 months later, in April 2020, the agency’s National Center for Health Statistics implemented a diagnostic code, U07.0, for health care professionals in the United States to diagnose EVALI for the first time. The code is also used for lung damage related to use of electronic cigarettes and “dabbing” – a method of inhaling cannabis. Damage could include inflammation of the lungs, pulmonary hemorrhage, and eosinophilic pneumonia.
The incidence of these diagnoses appears to have risen sharply since 2020. In the last three months of 2020, a total of 11,300 medical claims included the U07.0 code. That figure rose to 22,000 in 2021 and hit 31,600 in 2022, according to data compiled for and provided to Medscape by Komodo Health, a health care technology company that holds a database of more than 330 million U.S. patients from Medicare, Medicaid, and commercial insurers’ medical, pharmacy, and laboratory claims.
Harm from vaping, including EVALI, has continued.
said Usha Periyanayagam, MD, MPH, head of clinic product and real-world evidence for Komodo and a former emergency medicine physician.
Where it started
Devika Rao, MD, a pediatric pulmonology specialist at UT Southwestern Medical Center, Dallas, has cared for most of her EVALI patients in the hospital, with the most recent case in early 2023. But in January, for the first time, she saw an EVALI patient in an outpatient clinic. The person had not been admitted to the hospital – like most were pre-pandemic. And like most who were seen during the pandemic, this patient had milder symptoms, not requiring intubation or take-home oxygen.
In 2019 and the beginning of 2020, many EVALI patients who were eventually hospitalized first sought help at urgent care centers or with primary care doctors and were presumed to have pneumonia or gastroenteritis and sent home.
“But they got worse, and they would present to our emergency room; their chest X-rays and CT scans showed extensive lung disease,” Dr. Rao said, adding that the damage was striking among patients all under age 18. “They were short of breath. Their oxygen levels were low. They had diminished lung function. And they had a lot of GI issues like abdominal pain and weight loss from nausea and vomiting.”
“These overwhelming inflammatory reactions that we see with EVALI,” said Karen M. Wilson, MD, MPH, a pediatric hospitalist at the University of Rochester (N.Y.) Medical Center and a tobacco use researcher. “You might find some microvascular changes with normal inhaling of smoke or aerosol, but you’re not going to find macro changes like we see with the EVALI.”
In late 2019, images of the CT scans of patients with EVALI were published, grabbing the attention of Arun Kannappan, MD, an assistant professor of pulmonary sciences and critical care at the University of Colorado Anschutz School of Medicine, Aurora. Dr. Kannappan knew a patient with such severe lung damage could develop acute respiratory distress syndrome, which means a patient would be put on a ventilator because their inflamed lungs could not oxygenate blood.
“That confers within somewhere between 30% to 50% chance of dying; it made all of the pulmonary specialists really turn their heads to make sure that we keep a lookout for it,” said Dr. Kannappan.
CT scans of lungs proved to be a critical diagnostic tool for doctors. Most of the images from patients showed acute inflammation and diffuse lung damage. Ehab Ali, MD, a critical care and pulmonary disease medicine specialist in Louisville, Ky., said the damage was often spread across both lungs in many areas and appeared opaque and hazy, known as “ground glass.” COVID-19, meanwhile, appeared differently in lung scans, often with damage that was more isolated.
But many diseases carry a “ground glass” appearance, with many potential causes, like infections, cigarette smoke, or an autoimmune condition.
“Before you even talk to the patient, you can immediately put it in your mind that ‘I’m going to ask this patient if they vape,’ when I see the distribution of ground glass appearance,” Dr. Ali said.
Dr. Ali said other factors, like the age of the patient – about three-quarters of EVALI patients are under age 34, according to the CDC – would spur him to ask about vaping. But because so many patients were young, discerning vape usage wasn’t always easy.
“When you’re talking to teenagers, if you ask them upon admission, with the parents in the room, they’re going say ‘no,’ ” said Rachel Boykan, MD, a pediatric hospitalist at Stony Brook (N.Y.) Children’s. She added that her hospital is still seeing cases.
Dr. Rao said it often takes two to three people asking a patient about any vape usage before they confess.
Ms. Bernal, who was 27 at the time of her hospital admission for EVALI, said she bought vapes with THC at a retail shop in California. She’d been a traditional marijuana smoker, using the leaf product, but switched when someone told her it was healthier to vape THC than inhale smoke from burned marijuana leaves into her lungs. “I thought this was safe.”
Dr. Rao and her colleagues recently published a study of 41 teenage patients with EVALI who were seen at Children’s Medical Center Dallas between December 2018 and July 2021. All but one reported using e-cigarettes containing THC, and the CDC in its most recent report from February 2020 said about 80% of patients had used vapes containing THC.
The CDC also found that vitamin E acetate, an oily substance that allows THC to travel from the lungs to the brain quickly and an ingredient used in the food and cosmetics industries, was found in many of the lungs of EVALI patients, though not all.
The aftermath
The outcomes of the thousands of patients who had EVALI – and those who may still be developing it – are largely untracked.
Bonnie Halpern-Felsher, PhD, director at the Stanford (Calif.) Reach Lab that bears her name and a researcher on tobacco in youth, said she and many of her colleagues are frustrated that the CDC is not continuing to collect data on EVALI.
“I know a lot of colleagues who’ve said that they’re still seeing EVALI, but because of COVID-19 they stopped collecting the data. And that’s been very frustrating because it’s hard to say whether the kinds of lung issues you’re having are related to e-cigarettes, generally, or EVALI,” Dr. Halpern-Felsher said.
Researchers and doctors affiliated with the American Thoracic Society published a report with solutions on how to better track EVALI. They recommended that a national case registry and biorepository be created.
Doctors also worry that many cases were missed. Dr. Boykan said that while protocol dictated nurses and other clinicians ask about a history of vaping – a key part of EVALI diagnosis – many did not. Dr. Ali, the Louisville critical care physician, said EVALI symptoms of nausea, cough, and fever are associated with viral infections.
“I’m sure that some of these cases might be discharged from the emergency room as a virus,” Dr. Ali said. “Most of the time patients would get prescribed steroids for viral infections, which may help EVALI patients even though it’s never been studied.”
Dr. Rao also said the treatment regimen at Children’s MC Dallas, which included high doses of intravenous steroids, seemed to help. But the best management approach for treatment, or long-term follow up care, has not been studied.
The report in the Annals of the American Thoracic Society said prospective studies are showing that a significant portion of patients with EVALI experience prolonged respiratory issues and cognitive and mood impairment. Dr. Rao said a common thread for many of her EVALI patients has been significant stress in their lives with school or family, which led them to vape in an attempt to reduce stress.
That was certainly the case for Ms. Bernal before her hospital admission. She had recently moved across the country for her husband’s job, was trying to buy a house, and had spent months in a hotel with three children. She vaped to cope.
But she said her mental and cognitive health has worsened. Back in Louisville, she saw a neurologist, who told her that her brain had shrunk, she said. She hasn’t found a new neurologist in Portland, Ore., where her family moved a year after the EVALI episode.
But she often finds herself overwhelmed and overstimulated with tasks that she said she never had problems with before. She tears up while talking about the newfound limitations. She struggled to find a primary care physician who could medically manage her mental health and a counselor who can understand what she’s been through with EVALI.
But, “a lot of doctors aren’t educated in it, and they don’t know how to respond or they don’t know what to do,” Ms. Bernal said. “And that makes me feel like, I guess, what I had wasn’t important.”
Ms. Bernal does have a new pulmonologist and is going in for a round of pulmonary tests soon because she often finds herself unable to breathe while completing simple tasks. She is tired of rushing to the ER. She wants answers, or some kind of treatment to help her feel normal again.
“I feel like this is my fault,” Ms. Bernal said. “Had I not smoked, I would be fine, and that’s hard to live with. Every day. Telling yourself, ‘It’s your fault.’ It’s been how many years now? And I still haven’t found peace yet. I don’t know if ever will.”
A version of this article first appeared on Medscape.com.
Sleep disturbances linked to post-COVID dyspnea
according to data from the U.K.’s CircCOVID study.
The researchers, led by John Blaikley, MRCP, PhD, respiratory physician and clinical scientist from the University of Manchester (England), found that sleep disturbance is a common problem after hospital admission for COVID-19 and may last for at least 1 year.
The study also showed that sleep disturbance after COVID hospitalization was associated with dyspnea and lower lung function. Further in-depth analysis revealed that the effects of sleep disturbance on dyspnea were partially mediated through both anxiety and muscle weakness; however, “this does not fully explain the association, suggesting other pathways are involved,” said Dr. Blaikley.
The study was jointly conducted by researchers from the University of Leicester (England), as well as 20 other U.K. institutes and the University of Helsinki. It was presented at the European Congress of Clinical Microbiology & Infectious Diseases and was simultaneously published in The Lancet Respiratory Medicine.
“Sleep disturbance is a common problem after hospitalization for COVID-19 and is associated with several symptoms in the post-COVID syndrome,” said Dr. Blaikley. “Clinicians should be aware of this association in their post-COVID syndrome clinics.”
He added that further work needs to be done to define the mechanism and to see whether the links are causal. “However, if they are, then treating sleep disturbance could have beneficial effects beyond improving sleep quality,” he said in an interview.
A large study recently showed that 4 in 10 people with post-COVID syndrome had moderate to severe sleep problems. Black people were at least three times more likely than White people to experience sleep problems. A total of 59% of all participants with long COVID reported having normal sleep or mild sleep disturbances, and 41% reported having moderate to severe sleep disturbances.
Unlike prior studies that evaluated sleep quality after COVID-19, which used either objective or subjective measures of sleep disturbance, the current study used both. “Using both measures revealed previously poorly described associations between sleep disturbance, breathlessness, reduced lung function, anxiety, and muscle weakness,” Dr. Blaikley pointed out.
Subjective and objective measures of sleep
The multicenter CircCOVID cohort study aimed to shed light on the prevalence and nature of sleep disturbance after patients are discharged from hospital for COVID-19 and to assess whether this was associated with dyspnea.
The study recruited a total of 2,320 participants who were part of a larger parent PHOSP-COVID study. After attending an early follow-up visit (at a median of 5 months after discharge from 83 U.K. hospitals for COVID-19), 638 participants provided data for analysis as measured by the Pittsburgh Sleep Quality Index (a subjective measure of sleep quality); 729 participants provided data for analysis as measured by actigraphy (an objective, wrist-worn, device-based measure of sleep quality) at a median of 7 months.
Breathlessness, the primary outcome, was assessed using the Dyspnea-12 validated questionnaire.
Actigraphy measurements were compared with an age-matched, sex-matched, body mass index (BMI)–matched, and time from discharge–matched cohort from the UK Biobank (a prepandemic comparator longitudinal cohort of 502,540 individuals, one-fifth of whom wore actigraphy devices). Sleep regularity was found to be 19% less in previously hospitalized patients with post-COVID syndrome, compared with matched controls who had been hospitalized for other reasons.
This “revealed that the actigraphy changes may be, in part, due to COVID-19 rather than hospitalization alone,” said Dr. Blaikley.
Data were collected at two time points after hospital discharge: 2-7 months (early), and 10-14 months (late). At the early time point, participants were clinically assessed with respect to anxiety, muscle function, and dyspnea, and lung function.
After discharge from hospital, the majority (62%) of post–COVID-19 participants reported poor sleep quality on the Pittsburgh Sleep Quality Index questionnaire. A “comparable” proportion (53%) felt that their quality of sleep had deteriorated following hospital discharge according to the numerical rating scale (subjective measure).
Also, sleep disturbance was found likely to persist for at least 12 months, since subjective sleep quality hardly changed between the early and late time points after hospital discharge.
Both subjective metrics (sleep quality and sleep quality deterioration after hospital discharge) and objective, device-based metrics (sleep regularity) were found to be associated with dyspnea and reduced lung function in patients with post-COVID syndrome.
“One of the striking findings in our study is the consistency with breathlessness and reduced lung function across different methods used to evaluate sleep,” highlighted Dr. Blaikley.
“The other striking finding was that participants following COVID-19 hospitalization actually slept longer [65 min; 95% confidence interval, 59-71 min] than participants hospitalized for non-COVID; however, their bedtimes were irregular, and it was this irregularity that was associated with breathlessness,” he added.
In comparison with nonhospitalized controls, also from the UK Biobank, study participants with lower sleep regularity had higher Dyspnea-12 scores (unadjusted effect estimate, 4.38; 95%: CI, 2.10-6.65). Those with poor sleep quality overall also had higher Dyspnea-12 scores (unadjusted effect estimate, 3.94; 95% CI, 2.78-5.10), and those who reported sleep quality deterioration had higher Dyspnea-12 scores (unadjusted effect estimate, 3,00; 95% CI, 1.82-4.28).
In comparison with hospitalized controls, CircCOVID participants had lower sleep regularity index (–19%; 95% CI, –20 to –16) and lower sleep efficiency (3.83 percentage points; 95% CI, 3.40-4.26).
Sleep disturbance after COVID hospitalization was also associated with lower lung function, from a 7% to a 14% reduction in predicted forced vital capacity, depending on which sleep measure used.
In an analysis of mediating factors active in the relationship between sleep disturbance and dyspnea/decreased lung function, the researchers found that reduced muscle function and anxiety, which are both recognized causes of dyspnea, could partially contribute to the association.
Regarding anxiety, and depending on the sleep metric, anxiety mediated 18%-39% of the effect of sleep disturbance on dyspnea, while muscle weakness mediated 27%-41% of this effect, reported Dr. Blaikley. Those with poor sleep quality were more likely to have mild, moderate, or severe anxiety, compared with participants who reported good-quality sleep.
A similar association was observed between anxiety and sleep quality deterioration.
“Two key questions are raised by our study: Do sleep interventions have a beneficial effect in post–COVID-19 syndrome, and are the associations causal?” asked Dr. Blaikley. “We hope to do a sleep intervention trial to answer these questions to explore if this is an effective treatment for post–COVID-19 syndrome.”
‘Underlying mechanisms remain unclear’
Amitava Banerjee, MD, professor of clinical data science and honorary consultant cardiologist, Institute of Health Informatics, UCL, London, welcomed the study but noted that it did not include nonhospitalized post-COVID patients.
“The majority of people with long COVID were not hospitalized for COVID, so the results may not be generalizable to this larger group,” she said in an interview. “Good-quality sleep is important for health and reduces risk of chronic diseases; quality of sleep is therefore likely to be important for those with long COVID in reducing their risk of chronic disease, but the role of sleep in the mechanism of long COVID needs further research.”
In a commentary also published in The Lancet Respiratory Medicine, W. Cameron McGuire, MD, pulmonary and critical care specialist from San Diego, California, and colleagues wrote: “These findings suggest that sleep disturbance, dyspnea, and anxiety are common after COVID-19 and are associated with one another, although the underlying mechanisms remain unclear.”
The commentators “applauded” the work overall but noted that the findings represent correlation rather than causation. “It is unclear whether sleep disturbance is causing anxiety or whether anxiety is contributing to poor sleep. ... For the sleep disturbances, increased BMI in the cohort reporting poor sleep, compared with those reporting good sleep might suggest underlying obstructive sleep apnea,” they wrote.
Dr. McGuire and colleagues added that many questions remain for researchers and clinicians, including “whether anxiety and dyspnoea are contributing to a low arousal threshold [disrupting sleep] ... whether the observed abnormalities (e.g., in dyspnea score) are clinically significant,” and “whether therapies such as glucocorticoids, anticoagulants, or previous vaccinations mitigate the observed abnormalities during COVID-19 recovery.”
Dr. Blaikley has received support to his institute from an MRC Transition Fellowship, Asthma + Lung UK, NIHR Manchester BRC, and UKRI; grants to his institution from the Small Business Research Initiative Home Spirometer and the National Institute of Academic Anaesthesia; and support from TEVA and Therakos for attending meetings. He is a committee member of the Royal Society of Medicine. A coauthor received funding from the National Institutes of Health and income for medical education from Zoll, Livanova, Jazz, and Eli Lilly. Dr. Banerjee is the chief investigator of STIMULATE-ICP (an NIHR-funded study) and has received research funding from AstraZeneca.
A version of this article first appeared on Medscape.com.
according to data from the U.K.’s CircCOVID study.
The researchers, led by John Blaikley, MRCP, PhD, respiratory physician and clinical scientist from the University of Manchester (England), found that sleep disturbance is a common problem after hospital admission for COVID-19 and may last for at least 1 year.
The study also showed that sleep disturbance after COVID hospitalization was associated with dyspnea and lower lung function. Further in-depth analysis revealed that the effects of sleep disturbance on dyspnea were partially mediated through both anxiety and muscle weakness; however, “this does not fully explain the association, suggesting other pathways are involved,” said Dr. Blaikley.
The study was jointly conducted by researchers from the University of Leicester (England), as well as 20 other U.K. institutes and the University of Helsinki. It was presented at the European Congress of Clinical Microbiology & Infectious Diseases and was simultaneously published in The Lancet Respiratory Medicine.
“Sleep disturbance is a common problem after hospitalization for COVID-19 and is associated with several symptoms in the post-COVID syndrome,” said Dr. Blaikley. “Clinicians should be aware of this association in their post-COVID syndrome clinics.”
He added that further work needs to be done to define the mechanism and to see whether the links are causal. “However, if they are, then treating sleep disturbance could have beneficial effects beyond improving sleep quality,” he said in an interview.
A large study recently showed that 4 in 10 people with post-COVID syndrome had moderate to severe sleep problems. Black people were at least three times more likely than White people to experience sleep problems. A total of 59% of all participants with long COVID reported having normal sleep or mild sleep disturbances, and 41% reported having moderate to severe sleep disturbances.
Unlike prior studies that evaluated sleep quality after COVID-19, which used either objective or subjective measures of sleep disturbance, the current study used both. “Using both measures revealed previously poorly described associations between sleep disturbance, breathlessness, reduced lung function, anxiety, and muscle weakness,” Dr. Blaikley pointed out.
Subjective and objective measures of sleep
The multicenter CircCOVID cohort study aimed to shed light on the prevalence and nature of sleep disturbance after patients are discharged from hospital for COVID-19 and to assess whether this was associated with dyspnea.
The study recruited a total of 2,320 participants who were part of a larger parent PHOSP-COVID study. After attending an early follow-up visit (at a median of 5 months after discharge from 83 U.K. hospitals for COVID-19), 638 participants provided data for analysis as measured by the Pittsburgh Sleep Quality Index (a subjective measure of sleep quality); 729 participants provided data for analysis as measured by actigraphy (an objective, wrist-worn, device-based measure of sleep quality) at a median of 7 months.
Breathlessness, the primary outcome, was assessed using the Dyspnea-12 validated questionnaire.
Actigraphy measurements were compared with an age-matched, sex-matched, body mass index (BMI)–matched, and time from discharge–matched cohort from the UK Biobank (a prepandemic comparator longitudinal cohort of 502,540 individuals, one-fifth of whom wore actigraphy devices). Sleep regularity was found to be 19% less in previously hospitalized patients with post-COVID syndrome, compared with matched controls who had been hospitalized for other reasons.
This “revealed that the actigraphy changes may be, in part, due to COVID-19 rather than hospitalization alone,” said Dr. Blaikley.
Data were collected at two time points after hospital discharge: 2-7 months (early), and 10-14 months (late). At the early time point, participants were clinically assessed with respect to anxiety, muscle function, and dyspnea, and lung function.
After discharge from hospital, the majority (62%) of post–COVID-19 participants reported poor sleep quality on the Pittsburgh Sleep Quality Index questionnaire. A “comparable” proportion (53%) felt that their quality of sleep had deteriorated following hospital discharge according to the numerical rating scale (subjective measure).
Also, sleep disturbance was found likely to persist for at least 12 months, since subjective sleep quality hardly changed between the early and late time points after hospital discharge.
Both subjective metrics (sleep quality and sleep quality deterioration after hospital discharge) and objective, device-based metrics (sleep regularity) were found to be associated with dyspnea and reduced lung function in patients with post-COVID syndrome.
“One of the striking findings in our study is the consistency with breathlessness and reduced lung function across different methods used to evaluate sleep,” highlighted Dr. Blaikley.
“The other striking finding was that participants following COVID-19 hospitalization actually slept longer [65 min; 95% confidence interval, 59-71 min] than participants hospitalized for non-COVID; however, their bedtimes were irregular, and it was this irregularity that was associated with breathlessness,” he added.
In comparison with nonhospitalized controls, also from the UK Biobank, study participants with lower sleep regularity had higher Dyspnea-12 scores (unadjusted effect estimate, 4.38; 95%: CI, 2.10-6.65). Those with poor sleep quality overall also had higher Dyspnea-12 scores (unadjusted effect estimate, 3.94; 95% CI, 2.78-5.10), and those who reported sleep quality deterioration had higher Dyspnea-12 scores (unadjusted effect estimate, 3,00; 95% CI, 1.82-4.28).
In comparison with hospitalized controls, CircCOVID participants had lower sleep regularity index (–19%; 95% CI, –20 to –16) and lower sleep efficiency (3.83 percentage points; 95% CI, 3.40-4.26).
Sleep disturbance after COVID hospitalization was also associated with lower lung function, from a 7% to a 14% reduction in predicted forced vital capacity, depending on which sleep measure used.
In an analysis of mediating factors active in the relationship between sleep disturbance and dyspnea/decreased lung function, the researchers found that reduced muscle function and anxiety, which are both recognized causes of dyspnea, could partially contribute to the association.
Regarding anxiety, and depending on the sleep metric, anxiety mediated 18%-39% of the effect of sleep disturbance on dyspnea, while muscle weakness mediated 27%-41% of this effect, reported Dr. Blaikley. Those with poor sleep quality were more likely to have mild, moderate, or severe anxiety, compared with participants who reported good-quality sleep.
A similar association was observed between anxiety and sleep quality deterioration.
“Two key questions are raised by our study: Do sleep interventions have a beneficial effect in post–COVID-19 syndrome, and are the associations causal?” asked Dr. Blaikley. “We hope to do a sleep intervention trial to answer these questions to explore if this is an effective treatment for post–COVID-19 syndrome.”
‘Underlying mechanisms remain unclear’
Amitava Banerjee, MD, professor of clinical data science and honorary consultant cardiologist, Institute of Health Informatics, UCL, London, welcomed the study but noted that it did not include nonhospitalized post-COVID patients.
“The majority of people with long COVID were not hospitalized for COVID, so the results may not be generalizable to this larger group,” she said in an interview. “Good-quality sleep is important for health and reduces risk of chronic diseases; quality of sleep is therefore likely to be important for those with long COVID in reducing their risk of chronic disease, but the role of sleep in the mechanism of long COVID needs further research.”
In a commentary also published in The Lancet Respiratory Medicine, W. Cameron McGuire, MD, pulmonary and critical care specialist from San Diego, California, and colleagues wrote: “These findings suggest that sleep disturbance, dyspnea, and anxiety are common after COVID-19 and are associated with one another, although the underlying mechanisms remain unclear.”
The commentators “applauded” the work overall but noted that the findings represent correlation rather than causation. “It is unclear whether sleep disturbance is causing anxiety or whether anxiety is contributing to poor sleep. ... For the sleep disturbances, increased BMI in the cohort reporting poor sleep, compared with those reporting good sleep might suggest underlying obstructive sleep apnea,” they wrote.
Dr. McGuire and colleagues added that many questions remain for researchers and clinicians, including “whether anxiety and dyspnoea are contributing to a low arousal threshold [disrupting sleep] ... whether the observed abnormalities (e.g., in dyspnea score) are clinically significant,” and “whether therapies such as glucocorticoids, anticoagulants, or previous vaccinations mitigate the observed abnormalities during COVID-19 recovery.”
Dr. Blaikley has received support to his institute from an MRC Transition Fellowship, Asthma + Lung UK, NIHR Manchester BRC, and UKRI; grants to his institution from the Small Business Research Initiative Home Spirometer and the National Institute of Academic Anaesthesia; and support from TEVA and Therakos for attending meetings. He is a committee member of the Royal Society of Medicine. A coauthor received funding from the National Institutes of Health and income for medical education from Zoll, Livanova, Jazz, and Eli Lilly. Dr. Banerjee is the chief investigator of STIMULATE-ICP (an NIHR-funded study) and has received research funding from AstraZeneca.
A version of this article first appeared on Medscape.com.
according to data from the U.K.’s CircCOVID study.
The researchers, led by John Blaikley, MRCP, PhD, respiratory physician and clinical scientist from the University of Manchester (England), found that sleep disturbance is a common problem after hospital admission for COVID-19 and may last for at least 1 year.
The study also showed that sleep disturbance after COVID hospitalization was associated with dyspnea and lower lung function. Further in-depth analysis revealed that the effects of sleep disturbance on dyspnea were partially mediated through both anxiety and muscle weakness; however, “this does not fully explain the association, suggesting other pathways are involved,” said Dr. Blaikley.
The study was jointly conducted by researchers from the University of Leicester (England), as well as 20 other U.K. institutes and the University of Helsinki. It was presented at the European Congress of Clinical Microbiology & Infectious Diseases and was simultaneously published in The Lancet Respiratory Medicine.
“Sleep disturbance is a common problem after hospitalization for COVID-19 and is associated with several symptoms in the post-COVID syndrome,” said Dr. Blaikley. “Clinicians should be aware of this association in their post-COVID syndrome clinics.”
He added that further work needs to be done to define the mechanism and to see whether the links are causal. “However, if they are, then treating sleep disturbance could have beneficial effects beyond improving sleep quality,” he said in an interview.
A large study recently showed that 4 in 10 people with post-COVID syndrome had moderate to severe sleep problems. Black people were at least three times more likely than White people to experience sleep problems. A total of 59% of all participants with long COVID reported having normal sleep or mild sleep disturbances, and 41% reported having moderate to severe sleep disturbances.
Unlike prior studies that evaluated sleep quality after COVID-19, which used either objective or subjective measures of sleep disturbance, the current study used both. “Using both measures revealed previously poorly described associations between sleep disturbance, breathlessness, reduced lung function, anxiety, and muscle weakness,” Dr. Blaikley pointed out.
Subjective and objective measures of sleep
The multicenter CircCOVID cohort study aimed to shed light on the prevalence and nature of sleep disturbance after patients are discharged from hospital for COVID-19 and to assess whether this was associated with dyspnea.
The study recruited a total of 2,320 participants who were part of a larger parent PHOSP-COVID study. After attending an early follow-up visit (at a median of 5 months after discharge from 83 U.K. hospitals for COVID-19), 638 participants provided data for analysis as measured by the Pittsburgh Sleep Quality Index (a subjective measure of sleep quality); 729 participants provided data for analysis as measured by actigraphy (an objective, wrist-worn, device-based measure of sleep quality) at a median of 7 months.
Breathlessness, the primary outcome, was assessed using the Dyspnea-12 validated questionnaire.
Actigraphy measurements were compared with an age-matched, sex-matched, body mass index (BMI)–matched, and time from discharge–matched cohort from the UK Biobank (a prepandemic comparator longitudinal cohort of 502,540 individuals, one-fifth of whom wore actigraphy devices). Sleep regularity was found to be 19% less in previously hospitalized patients with post-COVID syndrome, compared with matched controls who had been hospitalized for other reasons.
This “revealed that the actigraphy changes may be, in part, due to COVID-19 rather than hospitalization alone,” said Dr. Blaikley.
Data were collected at two time points after hospital discharge: 2-7 months (early), and 10-14 months (late). At the early time point, participants were clinically assessed with respect to anxiety, muscle function, and dyspnea, and lung function.
After discharge from hospital, the majority (62%) of post–COVID-19 participants reported poor sleep quality on the Pittsburgh Sleep Quality Index questionnaire. A “comparable” proportion (53%) felt that their quality of sleep had deteriorated following hospital discharge according to the numerical rating scale (subjective measure).
Also, sleep disturbance was found likely to persist for at least 12 months, since subjective sleep quality hardly changed between the early and late time points after hospital discharge.
Both subjective metrics (sleep quality and sleep quality deterioration after hospital discharge) and objective, device-based metrics (sleep regularity) were found to be associated with dyspnea and reduced lung function in patients with post-COVID syndrome.
“One of the striking findings in our study is the consistency with breathlessness and reduced lung function across different methods used to evaluate sleep,” highlighted Dr. Blaikley.
“The other striking finding was that participants following COVID-19 hospitalization actually slept longer [65 min; 95% confidence interval, 59-71 min] than participants hospitalized for non-COVID; however, their bedtimes were irregular, and it was this irregularity that was associated with breathlessness,” he added.
In comparison with nonhospitalized controls, also from the UK Biobank, study participants with lower sleep regularity had higher Dyspnea-12 scores (unadjusted effect estimate, 4.38; 95%: CI, 2.10-6.65). Those with poor sleep quality overall also had higher Dyspnea-12 scores (unadjusted effect estimate, 3.94; 95% CI, 2.78-5.10), and those who reported sleep quality deterioration had higher Dyspnea-12 scores (unadjusted effect estimate, 3,00; 95% CI, 1.82-4.28).
In comparison with hospitalized controls, CircCOVID participants had lower sleep regularity index (–19%; 95% CI, –20 to –16) and lower sleep efficiency (3.83 percentage points; 95% CI, 3.40-4.26).
Sleep disturbance after COVID hospitalization was also associated with lower lung function, from a 7% to a 14% reduction in predicted forced vital capacity, depending on which sleep measure used.
In an analysis of mediating factors active in the relationship between sleep disturbance and dyspnea/decreased lung function, the researchers found that reduced muscle function and anxiety, which are both recognized causes of dyspnea, could partially contribute to the association.
Regarding anxiety, and depending on the sleep metric, anxiety mediated 18%-39% of the effect of sleep disturbance on dyspnea, while muscle weakness mediated 27%-41% of this effect, reported Dr. Blaikley. Those with poor sleep quality were more likely to have mild, moderate, or severe anxiety, compared with participants who reported good-quality sleep.
A similar association was observed between anxiety and sleep quality deterioration.
“Two key questions are raised by our study: Do sleep interventions have a beneficial effect in post–COVID-19 syndrome, and are the associations causal?” asked Dr. Blaikley. “We hope to do a sleep intervention trial to answer these questions to explore if this is an effective treatment for post–COVID-19 syndrome.”
‘Underlying mechanisms remain unclear’
Amitava Banerjee, MD, professor of clinical data science and honorary consultant cardiologist, Institute of Health Informatics, UCL, London, welcomed the study but noted that it did not include nonhospitalized post-COVID patients.
“The majority of people with long COVID were not hospitalized for COVID, so the results may not be generalizable to this larger group,” she said in an interview. “Good-quality sleep is important for health and reduces risk of chronic diseases; quality of sleep is therefore likely to be important for those with long COVID in reducing their risk of chronic disease, but the role of sleep in the mechanism of long COVID needs further research.”
In a commentary also published in The Lancet Respiratory Medicine, W. Cameron McGuire, MD, pulmonary and critical care specialist from San Diego, California, and colleagues wrote: “These findings suggest that sleep disturbance, dyspnea, and anxiety are common after COVID-19 and are associated with one another, although the underlying mechanisms remain unclear.”
The commentators “applauded” the work overall but noted that the findings represent correlation rather than causation. “It is unclear whether sleep disturbance is causing anxiety or whether anxiety is contributing to poor sleep. ... For the sleep disturbances, increased BMI in the cohort reporting poor sleep, compared with those reporting good sleep might suggest underlying obstructive sleep apnea,” they wrote.
Dr. McGuire and colleagues added that many questions remain for researchers and clinicians, including “whether anxiety and dyspnoea are contributing to a low arousal threshold [disrupting sleep] ... whether the observed abnormalities (e.g., in dyspnea score) are clinically significant,” and “whether therapies such as glucocorticoids, anticoagulants, or previous vaccinations mitigate the observed abnormalities during COVID-19 recovery.”
Dr. Blaikley has received support to his institute from an MRC Transition Fellowship, Asthma + Lung UK, NIHR Manchester BRC, and UKRI; grants to his institution from the Small Business Research Initiative Home Spirometer and the National Institute of Academic Anaesthesia; and support from TEVA and Therakos for attending meetings. He is a committee member of the Royal Society of Medicine. A coauthor received funding from the National Institutes of Health and income for medical education from Zoll, Livanova, Jazz, and Eli Lilly. Dr. Banerjee is the chief investigator of STIMULATE-ICP (an NIHR-funded study) and has received research funding from AstraZeneca.
A version of this article first appeared on Medscape.com.
FROM ECCMID 2023
Metformin linked to reduced osteoarthritis risk
Patients taking metformin for type 2 diabetes had a lower risk of developing osteoarthritis than did patients taking a sulfonylurea, according to a cohort study published in JAMA Network Open. The findings jibe with those seen in a 2022 systematic review of preclinical and observational human studies finding potentially protective effects of metformin on osteoarthritis.
“Our study provides further, robust epidemiological evidence that metformin may be associated with protection in the development and progression of osteoarthritis in individuals with type 2 diabetes,” wrote Matthew C. Baker, MD, MS, an assistant professor of medicine in immunology and rheumatology at Stanford (Calif.) University, and his colleagues.
The findings also fit with the results of a poster presented at the Osteoarthritis Research Society International 2023 World Congress, although that abstract’s findings did not reach statistical significance.
In the published study, the researchers analyzed deidentified claims data from Optum’s Clinformatics Data Mart Database between December 2003 and December 2019. The database includes more than 15 million people with private insurance or Medicare Advantage Part D but does not include people with Medicaid, thereby excluding people from lower socioeconomic groups.
The researchers included all patients who were at least 40 years old, had type 2 diabetes, were taking metformin, and had been enrolled in the database for at least 1 uninterrupted year. They excluded anyone with type 1 diabetes or a prior diagnosis of osteoarthritis, inflammatory arthritis, or joint replacement. The authors then compared the incidence of osteoarthritis and joint replacement in these 20,937 participants to 20,937 control participants who were taking a sulfonylurea, matched to those taking metformin on the basis of age, sex, race, a comorbidity score, and duration of treatment. More than half the overall population (58%) was male with an average age of 62.
Patients needed to be on either drug for at least 3 months, but those who were initially treated with metformin before later taking a sulfonylurea could also be included and contribute to both groups. Those who first took a sulfonylurea and later switched to metformin were included only for the sulfonylurea group and censored after their switch to ensure the sulfonylurea group had enough participants. The comparison was further adjusted for age, sex, race, ethnicity, geographic region, education, comorbidities, and outpatient visit frequency.
The results revealed that those who were taking metformin were 24% less likely to develop osteoarthritis at least 3 months after starting the medication than were those taking a sulfonylurea (P < .001). The rate of joint replacements was not significantly different between those taking metformin and those taking a sulfonylurea. These two results did not change in a sensitivity analysis that compared patients who only ever took metformin or a sulfonylurea (as opposed to those who took one drug before switching to the other).
“When stratified by prior exposure to metformin within the sulfonylurea group, the observed benefit associated with metformin ... was attenuated in the people treated with a sulfonylurea with prior exposure to metformin, compared with those treated with a sulfonylurea with no prior exposure to metformin,” the authors further reported. A possible reason for this finding is that those taking a sulfonylurea after having previously taken metformin gained some protection from the earlier metformin exposure, the authors hypothesized.
This observational study could not show a causative effect from the metformin, but the researchers speculated on potential mechanisms if a causative effect were present, based on past research.
”Several preclinical studies have suggested a protective association of metformin in osteoarthritis through activating AMP-activated protein kinase signaling, decreasing the level of matrix metalloproteinase, increasing autophagy and reducing chondrocyte apoptosis, and augmenting chondroprotective and anti-inflammatory properties of mesenchymal stem cells,” the authors wrote.
Among this study’s limitations, however, was the lack of data on body mass index, which is associated with osteoarthritis in the literature and may differ between patients taking metformin versus a sulfonylurea. The researchers also did not have data on physical activity or a history of trauma to the joints, though there’s no reason to think these rates might differ between those taking one or the other medication.
Another substantial limitation is that all patients had type 2 diabetes, making it impossible to determine whether a similar protective effect from metformin might exist in people without diabetes.
Nonsignificant lower risk for posttraumatic knee osteoarthritis
Similar to the published study, the OARSI poster compared 5-year odds of incident osteoarthritis or total knee replacement surgery between patients taking metformin and those taking sulfonylureas, but it focused on younger patients, aged 18-40 years, who underwent anterior cruciate ligament or meniscus surgery.
Using data from MarketScan commercial insurance claims databases between 2006 and 2020, the authors identified 2,376 participants who were taking metformin or a sulfonylurea when they underwent their surgery or began taking it in the 6 months after their surgery. More than half the participants were female (57%) with an average age of 35.
Within 5 years, 10.8% of those taking metformin developed osteoarthritis, compared with 17.9% of those taking a sulfonylurea. In addition, 3% of those taking metformin underwent a total knee replacement, compared with 5.3% of those taking a sulfonylurea. After adjustment for age, sex, obesity, and a history of chronic kidney disease, liver disease, and depression, however, both risk difference and odds ratios were not statistically significant.
Risk of osteoarthritis was 17% lower in patients taking metformin (95% confidence interval, –0.18 to 0.09), whose odds of osteoarthritis were approximately half the odds of those taking a sulfonylurea (OR, 0.5; 95% CI, 0.21-1.67). Risk of a total knee replacement was 10% lower in metformin users (95% CI, –0.28 to 0.08) with a similar reduction in odds, compared with those taking a sulfonylurea (OR, 0.53; 95% CI, 0.2-1.44).
In this study, the researchers did not specifically determine whether the participants were diagnosed with diabetes, but they assumed all, or at least most, were, according to S. Reza Jafarzadeh, PhD, DVM, an assistant professor of medicine at Boston University.
“The goal was not to only focus on the diabetes population, but on people who received that exposure [of metformin or sulfonylureas],” Dr. Jafarzadeh said in an interview. Dr. Jafarzadeh noted that a larger randomized controlled trial is underway to look at whether metformin reduces the risk of osteoarthritis independent of whether a patient has diabetes.
The published study was funded by grants from the National Institutes of Health, the Department of Veterans Affairs, and Stanford University, and the authors reported no disclosures. The poster at OARSI was funded by NIH and the Arthritis Foundation, and the authors reported no disclosures.
Patients taking metformin for type 2 diabetes had a lower risk of developing osteoarthritis than did patients taking a sulfonylurea, according to a cohort study published in JAMA Network Open. The findings jibe with those seen in a 2022 systematic review of preclinical and observational human studies finding potentially protective effects of metformin on osteoarthritis.
“Our study provides further, robust epidemiological evidence that metformin may be associated with protection in the development and progression of osteoarthritis in individuals with type 2 diabetes,” wrote Matthew C. Baker, MD, MS, an assistant professor of medicine in immunology and rheumatology at Stanford (Calif.) University, and his colleagues.
The findings also fit with the results of a poster presented at the Osteoarthritis Research Society International 2023 World Congress, although that abstract’s findings did not reach statistical significance.
In the published study, the researchers analyzed deidentified claims data from Optum’s Clinformatics Data Mart Database between December 2003 and December 2019. The database includes more than 15 million people with private insurance or Medicare Advantage Part D but does not include people with Medicaid, thereby excluding people from lower socioeconomic groups.
The researchers included all patients who were at least 40 years old, had type 2 diabetes, were taking metformin, and had been enrolled in the database for at least 1 uninterrupted year. They excluded anyone with type 1 diabetes or a prior diagnosis of osteoarthritis, inflammatory arthritis, or joint replacement. The authors then compared the incidence of osteoarthritis and joint replacement in these 20,937 participants to 20,937 control participants who were taking a sulfonylurea, matched to those taking metformin on the basis of age, sex, race, a comorbidity score, and duration of treatment. More than half the overall population (58%) was male with an average age of 62.
Patients needed to be on either drug for at least 3 months, but those who were initially treated with metformin before later taking a sulfonylurea could also be included and contribute to both groups. Those who first took a sulfonylurea and later switched to metformin were included only for the sulfonylurea group and censored after their switch to ensure the sulfonylurea group had enough participants. The comparison was further adjusted for age, sex, race, ethnicity, geographic region, education, comorbidities, and outpatient visit frequency.
The results revealed that those who were taking metformin were 24% less likely to develop osteoarthritis at least 3 months after starting the medication than were those taking a sulfonylurea (P < .001). The rate of joint replacements was not significantly different between those taking metformin and those taking a sulfonylurea. These two results did not change in a sensitivity analysis that compared patients who only ever took metformin or a sulfonylurea (as opposed to those who took one drug before switching to the other).
“When stratified by prior exposure to metformin within the sulfonylurea group, the observed benefit associated with metformin ... was attenuated in the people treated with a sulfonylurea with prior exposure to metformin, compared with those treated with a sulfonylurea with no prior exposure to metformin,” the authors further reported. A possible reason for this finding is that those taking a sulfonylurea after having previously taken metformin gained some protection from the earlier metformin exposure, the authors hypothesized.
This observational study could not show a causative effect from the metformin, but the researchers speculated on potential mechanisms if a causative effect were present, based on past research.
”Several preclinical studies have suggested a protective association of metformin in osteoarthritis through activating AMP-activated protein kinase signaling, decreasing the level of matrix metalloproteinase, increasing autophagy and reducing chondrocyte apoptosis, and augmenting chondroprotective and anti-inflammatory properties of mesenchymal stem cells,” the authors wrote.
Among this study’s limitations, however, was the lack of data on body mass index, which is associated with osteoarthritis in the literature and may differ between patients taking metformin versus a sulfonylurea. The researchers also did not have data on physical activity or a history of trauma to the joints, though there’s no reason to think these rates might differ between those taking one or the other medication.
Another substantial limitation is that all patients had type 2 diabetes, making it impossible to determine whether a similar protective effect from metformin might exist in people without diabetes.
Nonsignificant lower risk for posttraumatic knee osteoarthritis
Similar to the published study, the OARSI poster compared 5-year odds of incident osteoarthritis or total knee replacement surgery between patients taking metformin and those taking sulfonylureas, but it focused on younger patients, aged 18-40 years, who underwent anterior cruciate ligament or meniscus surgery.
Using data from MarketScan commercial insurance claims databases between 2006 and 2020, the authors identified 2,376 participants who were taking metformin or a sulfonylurea when they underwent their surgery or began taking it in the 6 months after their surgery. More than half the participants were female (57%) with an average age of 35.
Within 5 years, 10.8% of those taking metformin developed osteoarthritis, compared with 17.9% of those taking a sulfonylurea. In addition, 3% of those taking metformin underwent a total knee replacement, compared with 5.3% of those taking a sulfonylurea. After adjustment for age, sex, obesity, and a history of chronic kidney disease, liver disease, and depression, however, both risk difference and odds ratios were not statistically significant.
Risk of osteoarthritis was 17% lower in patients taking metformin (95% confidence interval, –0.18 to 0.09), whose odds of osteoarthritis were approximately half the odds of those taking a sulfonylurea (OR, 0.5; 95% CI, 0.21-1.67). Risk of a total knee replacement was 10% lower in metformin users (95% CI, –0.28 to 0.08) with a similar reduction in odds, compared with those taking a sulfonylurea (OR, 0.53; 95% CI, 0.2-1.44).
In this study, the researchers did not specifically determine whether the participants were diagnosed with diabetes, but they assumed all, or at least most, were, according to S. Reza Jafarzadeh, PhD, DVM, an assistant professor of medicine at Boston University.
“The goal was not to only focus on the diabetes population, but on people who received that exposure [of metformin or sulfonylureas],” Dr. Jafarzadeh said in an interview. Dr. Jafarzadeh noted that a larger randomized controlled trial is underway to look at whether metformin reduces the risk of osteoarthritis independent of whether a patient has diabetes.
The published study was funded by grants from the National Institutes of Health, the Department of Veterans Affairs, and Stanford University, and the authors reported no disclosures. The poster at OARSI was funded by NIH and the Arthritis Foundation, and the authors reported no disclosures.
Patients taking metformin for type 2 diabetes had a lower risk of developing osteoarthritis than did patients taking a sulfonylurea, according to a cohort study published in JAMA Network Open. The findings jibe with those seen in a 2022 systematic review of preclinical and observational human studies finding potentially protective effects of metformin on osteoarthritis.
“Our study provides further, robust epidemiological evidence that metformin may be associated with protection in the development and progression of osteoarthritis in individuals with type 2 diabetes,” wrote Matthew C. Baker, MD, MS, an assistant professor of medicine in immunology and rheumatology at Stanford (Calif.) University, and his colleagues.
The findings also fit with the results of a poster presented at the Osteoarthritis Research Society International 2023 World Congress, although that abstract’s findings did not reach statistical significance.
In the published study, the researchers analyzed deidentified claims data from Optum’s Clinformatics Data Mart Database between December 2003 and December 2019. The database includes more than 15 million people with private insurance or Medicare Advantage Part D but does not include people with Medicaid, thereby excluding people from lower socioeconomic groups.
The researchers included all patients who were at least 40 years old, had type 2 diabetes, were taking metformin, and had been enrolled in the database for at least 1 uninterrupted year. They excluded anyone with type 1 diabetes or a prior diagnosis of osteoarthritis, inflammatory arthritis, or joint replacement. The authors then compared the incidence of osteoarthritis and joint replacement in these 20,937 participants to 20,937 control participants who were taking a sulfonylurea, matched to those taking metformin on the basis of age, sex, race, a comorbidity score, and duration of treatment. More than half the overall population (58%) was male with an average age of 62.
Patients needed to be on either drug for at least 3 months, but those who were initially treated with metformin before later taking a sulfonylurea could also be included and contribute to both groups. Those who first took a sulfonylurea and later switched to metformin were included only for the sulfonylurea group and censored after their switch to ensure the sulfonylurea group had enough participants. The comparison was further adjusted for age, sex, race, ethnicity, geographic region, education, comorbidities, and outpatient visit frequency.
The results revealed that those who were taking metformin were 24% less likely to develop osteoarthritis at least 3 months after starting the medication than were those taking a sulfonylurea (P < .001). The rate of joint replacements was not significantly different between those taking metformin and those taking a sulfonylurea. These two results did not change in a sensitivity analysis that compared patients who only ever took metformin or a sulfonylurea (as opposed to those who took one drug before switching to the other).
“When stratified by prior exposure to metformin within the sulfonylurea group, the observed benefit associated with metformin ... was attenuated in the people treated with a sulfonylurea with prior exposure to metformin, compared with those treated with a sulfonylurea with no prior exposure to metformin,” the authors further reported. A possible reason for this finding is that those taking a sulfonylurea after having previously taken metformin gained some protection from the earlier metformin exposure, the authors hypothesized.
This observational study could not show a causative effect from the metformin, but the researchers speculated on potential mechanisms if a causative effect were present, based on past research.
”Several preclinical studies have suggested a protective association of metformin in osteoarthritis through activating AMP-activated protein kinase signaling, decreasing the level of matrix metalloproteinase, increasing autophagy and reducing chondrocyte apoptosis, and augmenting chondroprotective and anti-inflammatory properties of mesenchymal stem cells,” the authors wrote.
Among this study’s limitations, however, was the lack of data on body mass index, which is associated with osteoarthritis in the literature and may differ between patients taking metformin versus a sulfonylurea. The researchers also did not have data on physical activity or a history of trauma to the joints, though there’s no reason to think these rates might differ between those taking one or the other medication.
Another substantial limitation is that all patients had type 2 diabetes, making it impossible to determine whether a similar protective effect from metformin might exist in people without diabetes.
Nonsignificant lower risk for posttraumatic knee osteoarthritis
Similar to the published study, the OARSI poster compared 5-year odds of incident osteoarthritis or total knee replacement surgery between patients taking metformin and those taking sulfonylureas, but it focused on younger patients, aged 18-40 years, who underwent anterior cruciate ligament or meniscus surgery.
Using data from MarketScan commercial insurance claims databases between 2006 and 2020, the authors identified 2,376 participants who were taking metformin or a sulfonylurea when they underwent their surgery or began taking it in the 6 months after their surgery. More than half the participants were female (57%) with an average age of 35.
Within 5 years, 10.8% of those taking metformin developed osteoarthritis, compared with 17.9% of those taking a sulfonylurea. In addition, 3% of those taking metformin underwent a total knee replacement, compared with 5.3% of those taking a sulfonylurea. After adjustment for age, sex, obesity, and a history of chronic kidney disease, liver disease, and depression, however, both risk difference and odds ratios were not statistically significant.
Risk of osteoarthritis was 17% lower in patients taking metformin (95% confidence interval, –0.18 to 0.09), whose odds of osteoarthritis were approximately half the odds of those taking a sulfonylurea (OR, 0.5; 95% CI, 0.21-1.67). Risk of a total knee replacement was 10% lower in metformin users (95% CI, –0.28 to 0.08) with a similar reduction in odds, compared with those taking a sulfonylurea (OR, 0.53; 95% CI, 0.2-1.44).
In this study, the researchers did not specifically determine whether the participants were diagnosed with diabetes, but they assumed all, or at least most, were, according to S. Reza Jafarzadeh, PhD, DVM, an assistant professor of medicine at Boston University.
“The goal was not to only focus on the diabetes population, but on people who received that exposure [of metformin or sulfonylureas],” Dr. Jafarzadeh said in an interview. Dr. Jafarzadeh noted that a larger randomized controlled trial is underway to look at whether metformin reduces the risk of osteoarthritis independent of whether a patient has diabetes.
The published study was funded by grants from the National Institutes of Health, the Department of Veterans Affairs, and Stanford University, and the authors reported no disclosures. The poster at OARSI was funded by NIH and the Arthritis Foundation, and the authors reported no disclosures.
FROM JAMA NETWORK OPEN AND OARSI 2023
Neuropsychiatric side effects of hormonal contraceptives: More common than you think!
Since its introduction in 1950, the combined oral contraceptive pill has been used by countless women as a method for birth control (Liao P. Can Fam Physician. 2012 Dec; 58[12]:e757-e760).
Hormonal contraception (HC) provides women with both contraceptive and noncontraceptive benefits, most notably a method for avoiding unintended pregnancy. In addition to being an effective method of contraception, oral contraceptive pills (OCPs) are well established for treating conditions such as hirsutism, pain symptoms associated with endometriosis and adenomyosis, and pelvic inflammatory disease, among others (Schindler A. Int J Endocrinol Metab. 2013 Winter;11[1]:41-7).
Combined hormonal contraceptives are also first-line treatment for women with menstrual disorders, and in women with polycystic ovary syndrome, can offer an effective long-term method to regulate their menstrual cycle, decrease androgens, clear up oily skin and acne, and reduce facial hair while also providing them with effective contraception (de Melo et al. Open Access J Contracept. 2017;8:13-23).
Associations between ‘the pill’ and mood effects remain controversial
More than 100 million women worldwide use hormonal contraceptives today, yet despite this, the data are mixed regarding the prevalence and extent of neuropsychiatric symptoms and mood changes associated with use of “the pill.” Some studies show combined oral contraceptives are associated with a decrease in general well-being, but had no effect on depression, in women compared with placebo (Zethraeus N et al. Fertil Steril. 2017 May;107[5]:1238-45).
However, a large Danish study published in JAMA Psychiatry of more than 1 million women found a significant association between use of hormonal contraception and antidepressant use or first diagnosis of depression, with adolescents having a higher rate of first depression diagnosis and antidepressant use compared with women 20–30 years old (Skovlund C et al. JAMA Psychiatry. 2016 Nov 1;73[11]:1154-62).
Studies have also shown long-term exposure to levonorgestrel is significantly associated with anxiety and sleep problems in women without a history of these issues (Slattery J et al. Drug Saf. 2018 Oct;41[10]:951-8). A recent small nationwide cohort study in France suggests this may also be true of levonorgestrel delivered by intrauterine devices (IUD) and the association may be dose-dependent (Roland N et al. JAMA. 2023;329[3]:257-9).
Of note, a study published in the American Journal of Psychiatry found a nearly twofold risk of suicide attempt and over threefold risk of suicide among women taking hormonal contraception compared with women who had never used hormonal contraceptives (Skovlund et al. Am J Psychiatry. 2017 Nov 17:appiajp201717060616).
Knowledge gaps make drawing conclusions difficult
The latest information on use of antidepressant and antianxiety medications in women of reproductive age (18-44 years) is sparse and, in some cases, outdated. According to data from the National Health and Nutrition Examination Survey, 18.6% of adult women 18 years or older reported using antidepressant medications within the last 30 days in 2017-2018, an increase from 13.8% in 2009-2010. Among women aged 15-44 year with private employer–sponsored insurance surveyed during 2008-2013, the results showed 15.4% of women filled a prescription for an antidepressant. We must look back further to find data on antianxiety medication use among women aged 18-44 years where use of antianxiety drugs (anxiolytics, sedatives, and hypnotics) was 4.3% between 2005 and 2008.
A lack of literature in this area is likely due to significant underreporting, and an inability to select patients who are sensitive to or at risk of developing neuropsychiatric symptoms resulting from hormonal contraception use because the true pathophysiology is unknown. Existing studies tend to use varying methods to assess mood changes, and do not usually specify hormonal contraceptive use type in their analyses (Schaffir J et al. Eur J Contracept Reprod Health Care. 2016 Oct;21[5]:347-55).
Studies of this nature also require large sample sizes, but the percentage of women who develop neuropsychiatric symptoms from hormonal contraceptive use has historically been relatively small. In the late 1990s, Rosenberg and colleagues found 46% of 1,657 women discontinued oral contraceptives due to side effects within 6 months of starting a new prescription; of these women, 5% reported mood changes as their reason for discontinuing oral contraceptives (Rosenberg M et al. Am J Obstet Gynecol. 1998 Sep;179[3 Pt 1]:577-82).
One might expect that, as lower dosage combined hormonal contraceptives were developed in the 1980s, that the rate of reporting psychological side effects would continue to decrease as well. Yet greater awareness of the potential for mood changes while on “the pill” as outlined by the lay press and social media may be leading to increased reporting of neuropsychiatric effects in women. In a recent cross-sectional survey of 188 women in New York, 43.6% said they experienced mood changes while on hormonal contraceptives, and 61.2% of women with histories of psychiatric illness reported mood changes they attributed to hormonal contraceptives (Martell S et al. Contracept Reprod Med. 2023;8:9).
Martell and colleagues found 48.3% of women cited side effects as a reason for discontinuing hormonal contraception, and 43 participants mentioned psychological side effects unprompted, including 2 patients with suicidal thoughts. The authors said this suggests “psychological side effects, at least in part, may have impacted” HC users’ decisions to switch from OCPs to an alternative method of contraception.
It is also not clear what risk factors exist for women who develop neuropsychiatric symptoms from hormonal contraceptive use. First, it is important to note that both progestin-only contraceptives and combined hormonal contraceptives are classified by the Centers for Disease Control and Prevention’s US Medical Eligibility Criteria for Contraceptive Use, 2016 as having no restrictions for use, including among patients with depression. While women in a smaller subgroup have significant neuropsychiatric symptoms related to their hormonal contraceptives, the underlying mechanism is unknown, and is thought to be largely related to the progestogen component of combined hormonal contraceptives or progestogen-only contraceptives (Mu E. Aust Prescr. 2022 Jun; 45[3]:75-9). We know that some women are hormone sensitive, while others are less so, and some not at all. Progestogens could affect mood as a direct action of the progestogen, because progestogens can be neurosteroids, or the progestogen effect could be mediated secondarily through a change in that woman’s own production of or bioavailability of androgens or naturally occurring estrogens (Giatti S. J Mol Endocrinol. 2016 Aug;57[2]:R109-26).
Here, we also find that currently available evidence limits our ability to draw firm conclusions. A study by Berry-Bibee and colleagues found a “low concern for clinically significant interactions” between hormonal contraception and psychotropic drugs, but was limited by quality/quantity of evidence (Berry-Bibee E et al. Contraception. 2016 Dec;94[6]:650-67). Interestingly, a study by Robinson and colleagues from the mid-2000s posited based on low evidence that “psychological response to the practice of contraception” was a potential explanation for the side effect profile of hormonal contraception (Robinson S et al. Med Hypotheses. 2004;63[2]:268-73).
Further, it may be that women with premenstrual dysphoric disorder (PMDD) might be selected for oral contraceptives, and they are predisposed to other neuropsychiatric problems. Estimates have placed the prevalence of comorbid psychiatric disorders such as anxiety, major depression, bipolar disorder, and posttraumatic stress disorder as high as 70% for women with PMDD (Sepede G et al. Neuropsychiatr Dis Treat. 2020;16:415-26). This phenomenon is not new, having been characterized in the lay literature nearly 20 years ago, by endocrinologist Geoffrey P. Redmond, MD (Redmond GP. The Hormonally Vulnerable Woman. New York: HarperCollins; 2005).
While the cause is not exactly idiosyncratic, They tend to have an entire spectrum of responses to the progestogens in combined or progestin-only contraceptives, ranging from just a flattened affect – which could easily be explained by their flattened level of endogenous hormones – to frank depression. Their frank depression, in turn, can be demonstrated to include suicidal ideation and actual suicide.
Compounding this issue is a woman’s perception of her sexuality. Some women with low sexual desire or sexual problems who are younger may have more distress about their problems compared with women of older reproductive age. While the reason for that is not clear, it may be that in the sexual arena, it is more important for some younger women to be a sexual person than in perimenopausal women, or that women who are younger are more likely to be partnered than women of older reproductive age. While the European Society of Sexual Medicine concluded in a 2019 position statement that there is inconclusive evidence whether hormonal contraception may be contributing to changes in sexual desire and sexual dysfunction, it appears that “a minority of women” experience “better or worse sexual functioning” from taking combined oral contraceptives (Both S et al. J Sex Med. 2019 Nov;16[11]:1681-95), suggesting that the majority of women report no significant changes.
Practitioners should discuss mood effects during consultation
An ob.gyn., primary care physicians, or others with prescriptive authority (i.e. nurse practitioners and physician assistants) in clinical practice may encounter a patient who seems to have mood side effects owing to progestogen-containing contraceptives that they prescribe. However, many ob.gyns. are likely unaware of the prevalence, or that some of those same patients can have such significant mood effects that they would become or are suicidal.
I believe questioning patients about mood effects during consultation and particularly during follow-up following the initiation of any hormonal contraceptive is worth a passing comment for every patient, which should include mood effects in broader discussion for anyone currently using an antidepressant, patients with a history of antidepressant use, and patients who have considered suicide. As we do with other drugs, these questions can be posed in the form of a questionnaire followed up by the practitioner in counseling.
Practitioners who encounter a patient with mood changes as a result of hormonal contraceptive use can consider changing to a nonhormonal method of birth control, or recommending the patient use a barrier method during sexual activity, as none of these options have neuropsychiatric side effects.
Ultimately, practitioners of all types need to engage in shared decision-making to identify the key benefits and risks of hormonal contraceptive use for each patient, which may involve trial and error to determine the ideal treatment. It is critical that practitioners of all types strike a balance between alleviating patient concerns about potential mood changes, monitoring patients with an appreciable risk of mood changes, and continuing patients on hormonal contraception for whom the benefits outweigh the risks.
Dr. Simon is a clinical professor at George Washington University and the medical director and founder of IntimMedicine Specialists in Washington, which provides patient-focused care for women across the reproductive life cycle. He is a past president of the International Society for the Study of Women’s Sexual Health and the North American Menopause Society. Dr. Simon has been a consultant to, received grant and research support from, and served on the speakers bureau for various pharmaceutical companies that develop combination hormonal contraceptives. Email Dr. Simon at [email protected].
Since its introduction in 1950, the combined oral contraceptive pill has been used by countless women as a method for birth control (Liao P. Can Fam Physician. 2012 Dec; 58[12]:e757-e760).
Hormonal contraception (HC) provides women with both contraceptive and noncontraceptive benefits, most notably a method for avoiding unintended pregnancy. In addition to being an effective method of contraception, oral contraceptive pills (OCPs) are well established for treating conditions such as hirsutism, pain symptoms associated with endometriosis and adenomyosis, and pelvic inflammatory disease, among others (Schindler A. Int J Endocrinol Metab. 2013 Winter;11[1]:41-7).
Combined hormonal contraceptives are also first-line treatment for women with menstrual disorders, and in women with polycystic ovary syndrome, can offer an effective long-term method to regulate their menstrual cycle, decrease androgens, clear up oily skin and acne, and reduce facial hair while also providing them with effective contraception (de Melo et al. Open Access J Contracept. 2017;8:13-23).
Associations between ‘the pill’ and mood effects remain controversial
More than 100 million women worldwide use hormonal contraceptives today, yet despite this, the data are mixed regarding the prevalence and extent of neuropsychiatric symptoms and mood changes associated with use of “the pill.” Some studies show combined oral contraceptives are associated with a decrease in general well-being, but had no effect on depression, in women compared with placebo (Zethraeus N et al. Fertil Steril. 2017 May;107[5]:1238-45).
However, a large Danish study published in JAMA Psychiatry of more than 1 million women found a significant association between use of hormonal contraception and antidepressant use or first diagnosis of depression, with adolescents having a higher rate of first depression diagnosis and antidepressant use compared with women 20–30 years old (Skovlund C et al. JAMA Psychiatry. 2016 Nov 1;73[11]:1154-62).
Studies have also shown long-term exposure to levonorgestrel is significantly associated with anxiety and sleep problems in women without a history of these issues (Slattery J et al. Drug Saf. 2018 Oct;41[10]:951-8). A recent small nationwide cohort study in France suggests this may also be true of levonorgestrel delivered by intrauterine devices (IUD) and the association may be dose-dependent (Roland N et al. JAMA. 2023;329[3]:257-9).
Of note, a study published in the American Journal of Psychiatry found a nearly twofold risk of suicide attempt and over threefold risk of suicide among women taking hormonal contraception compared with women who had never used hormonal contraceptives (Skovlund et al. Am J Psychiatry. 2017 Nov 17:appiajp201717060616).
Knowledge gaps make drawing conclusions difficult
The latest information on use of antidepressant and antianxiety medications in women of reproductive age (18-44 years) is sparse and, in some cases, outdated. According to data from the National Health and Nutrition Examination Survey, 18.6% of adult women 18 years or older reported using antidepressant medications within the last 30 days in 2017-2018, an increase from 13.8% in 2009-2010. Among women aged 15-44 year with private employer–sponsored insurance surveyed during 2008-2013, the results showed 15.4% of women filled a prescription for an antidepressant. We must look back further to find data on antianxiety medication use among women aged 18-44 years where use of antianxiety drugs (anxiolytics, sedatives, and hypnotics) was 4.3% between 2005 and 2008.
A lack of literature in this area is likely due to significant underreporting, and an inability to select patients who are sensitive to or at risk of developing neuropsychiatric symptoms resulting from hormonal contraception use because the true pathophysiology is unknown. Existing studies tend to use varying methods to assess mood changes, and do not usually specify hormonal contraceptive use type in their analyses (Schaffir J et al. Eur J Contracept Reprod Health Care. 2016 Oct;21[5]:347-55).
Studies of this nature also require large sample sizes, but the percentage of women who develop neuropsychiatric symptoms from hormonal contraceptive use has historically been relatively small. In the late 1990s, Rosenberg and colleagues found 46% of 1,657 women discontinued oral contraceptives due to side effects within 6 months of starting a new prescription; of these women, 5% reported mood changes as their reason for discontinuing oral contraceptives (Rosenberg M et al. Am J Obstet Gynecol. 1998 Sep;179[3 Pt 1]:577-82).
One might expect that, as lower dosage combined hormonal contraceptives were developed in the 1980s, that the rate of reporting psychological side effects would continue to decrease as well. Yet greater awareness of the potential for mood changes while on “the pill” as outlined by the lay press and social media may be leading to increased reporting of neuropsychiatric effects in women. In a recent cross-sectional survey of 188 women in New York, 43.6% said they experienced mood changes while on hormonal contraceptives, and 61.2% of women with histories of psychiatric illness reported mood changes they attributed to hormonal contraceptives (Martell S et al. Contracept Reprod Med. 2023;8:9).
Martell and colleagues found 48.3% of women cited side effects as a reason for discontinuing hormonal contraception, and 43 participants mentioned psychological side effects unprompted, including 2 patients with suicidal thoughts. The authors said this suggests “psychological side effects, at least in part, may have impacted” HC users’ decisions to switch from OCPs to an alternative method of contraception.
It is also not clear what risk factors exist for women who develop neuropsychiatric symptoms from hormonal contraceptive use. First, it is important to note that both progestin-only contraceptives and combined hormonal contraceptives are classified by the Centers for Disease Control and Prevention’s US Medical Eligibility Criteria for Contraceptive Use, 2016 as having no restrictions for use, including among patients with depression. While women in a smaller subgroup have significant neuropsychiatric symptoms related to their hormonal contraceptives, the underlying mechanism is unknown, and is thought to be largely related to the progestogen component of combined hormonal contraceptives or progestogen-only contraceptives (Mu E. Aust Prescr. 2022 Jun; 45[3]:75-9). We know that some women are hormone sensitive, while others are less so, and some not at all. Progestogens could affect mood as a direct action of the progestogen, because progestogens can be neurosteroids, or the progestogen effect could be mediated secondarily through a change in that woman’s own production of or bioavailability of androgens or naturally occurring estrogens (Giatti S. J Mol Endocrinol. 2016 Aug;57[2]:R109-26).
Here, we also find that currently available evidence limits our ability to draw firm conclusions. A study by Berry-Bibee and colleagues found a “low concern for clinically significant interactions” between hormonal contraception and psychotropic drugs, but was limited by quality/quantity of evidence (Berry-Bibee E et al. Contraception. 2016 Dec;94[6]:650-67). Interestingly, a study by Robinson and colleagues from the mid-2000s posited based on low evidence that “psychological response to the practice of contraception” was a potential explanation for the side effect profile of hormonal contraception (Robinson S et al. Med Hypotheses. 2004;63[2]:268-73).
Further, it may be that women with premenstrual dysphoric disorder (PMDD) might be selected for oral contraceptives, and they are predisposed to other neuropsychiatric problems. Estimates have placed the prevalence of comorbid psychiatric disorders such as anxiety, major depression, bipolar disorder, and posttraumatic stress disorder as high as 70% for women with PMDD (Sepede G et al. Neuropsychiatr Dis Treat. 2020;16:415-26). This phenomenon is not new, having been characterized in the lay literature nearly 20 years ago, by endocrinologist Geoffrey P. Redmond, MD (Redmond GP. The Hormonally Vulnerable Woman. New York: HarperCollins; 2005).
While the cause is not exactly idiosyncratic, They tend to have an entire spectrum of responses to the progestogens in combined or progestin-only contraceptives, ranging from just a flattened affect – which could easily be explained by their flattened level of endogenous hormones – to frank depression. Their frank depression, in turn, can be demonstrated to include suicidal ideation and actual suicide.
Compounding this issue is a woman’s perception of her sexuality. Some women with low sexual desire or sexual problems who are younger may have more distress about their problems compared with women of older reproductive age. While the reason for that is not clear, it may be that in the sexual arena, it is more important for some younger women to be a sexual person than in perimenopausal women, or that women who are younger are more likely to be partnered than women of older reproductive age. While the European Society of Sexual Medicine concluded in a 2019 position statement that there is inconclusive evidence whether hormonal contraception may be contributing to changes in sexual desire and sexual dysfunction, it appears that “a minority of women” experience “better or worse sexual functioning” from taking combined oral contraceptives (Both S et al. J Sex Med. 2019 Nov;16[11]:1681-95), suggesting that the majority of women report no significant changes.
Practitioners should discuss mood effects during consultation
An ob.gyn., primary care physicians, or others with prescriptive authority (i.e. nurse practitioners and physician assistants) in clinical practice may encounter a patient who seems to have mood side effects owing to progestogen-containing contraceptives that they prescribe. However, many ob.gyns. are likely unaware of the prevalence, or that some of those same patients can have such significant mood effects that they would become or are suicidal.
I believe questioning patients about mood effects during consultation and particularly during follow-up following the initiation of any hormonal contraceptive is worth a passing comment for every patient, which should include mood effects in broader discussion for anyone currently using an antidepressant, patients with a history of antidepressant use, and patients who have considered suicide. As we do with other drugs, these questions can be posed in the form of a questionnaire followed up by the practitioner in counseling.
Practitioners who encounter a patient with mood changes as a result of hormonal contraceptive use can consider changing to a nonhormonal method of birth control, or recommending the patient use a barrier method during sexual activity, as none of these options have neuropsychiatric side effects.
Ultimately, practitioners of all types need to engage in shared decision-making to identify the key benefits and risks of hormonal contraceptive use for each patient, which may involve trial and error to determine the ideal treatment. It is critical that practitioners of all types strike a balance between alleviating patient concerns about potential mood changes, monitoring patients with an appreciable risk of mood changes, and continuing patients on hormonal contraception for whom the benefits outweigh the risks.
Dr. Simon is a clinical professor at George Washington University and the medical director and founder of IntimMedicine Specialists in Washington, which provides patient-focused care for women across the reproductive life cycle. He is a past president of the International Society for the Study of Women’s Sexual Health and the North American Menopause Society. Dr. Simon has been a consultant to, received grant and research support from, and served on the speakers bureau for various pharmaceutical companies that develop combination hormonal contraceptives. Email Dr. Simon at [email protected].
Since its introduction in 1950, the combined oral contraceptive pill has been used by countless women as a method for birth control (Liao P. Can Fam Physician. 2012 Dec; 58[12]:e757-e760).
Hormonal contraception (HC) provides women with both contraceptive and noncontraceptive benefits, most notably a method for avoiding unintended pregnancy. In addition to being an effective method of contraception, oral contraceptive pills (OCPs) are well established for treating conditions such as hirsutism, pain symptoms associated with endometriosis and adenomyosis, and pelvic inflammatory disease, among others (Schindler A. Int J Endocrinol Metab. 2013 Winter;11[1]:41-7).
Combined hormonal contraceptives are also first-line treatment for women with menstrual disorders, and in women with polycystic ovary syndrome, can offer an effective long-term method to regulate their menstrual cycle, decrease androgens, clear up oily skin and acne, and reduce facial hair while also providing them with effective contraception (de Melo et al. Open Access J Contracept. 2017;8:13-23).
Associations between ‘the pill’ and mood effects remain controversial
More than 100 million women worldwide use hormonal contraceptives today, yet despite this, the data are mixed regarding the prevalence and extent of neuropsychiatric symptoms and mood changes associated with use of “the pill.” Some studies show combined oral contraceptives are associated with a decrease in general well-being, but had no effect on depression, in women compared with placebo (Zethraeus N et al. Fertil Steril. 2017 May;107[5]:1238-45).
However, a large Danish study published in JAMA Psychiatry of more than 1 million women found a significant association between use of hormonal contraception and antidepressant use or first diagnosis of depression, with adolescents having a higher rate of first depression diagnosis and antidepressant use compared with women 20–30 years old (Skovlund C et al. JAMA Psychiatry. 2016 Nov 1;73[11]:1154-62).
Studies have also shown long-term exposure to levonorgestrel is significantly associated with anxiety and sleep problems in women without a history of these issues (Slattery J et al. Drug Saf. 2018 Oct;41[10]:951-8). A recent small nationwide cohort study in France suggests this may also be true of levonorgestrel delivered by intrauterine devices (IUD) and the association may be dose-dependent (Roland N et al. JAMA. 2023;329[3]:257-9).
Of note, a study published in the American Journal of Psychiatry found a nearly twofold risk of suicide attempt and over threefold risk of suicide among women taking hormonal contraception compared with women who had never used hormonal contraceptives (Skovlund et al. Am J Psychiatry. 2017 Nov 17:appiajp201717060616).
Knowledge gaps make drawing conclusions difficult
The latest information on use of antidepressant and antianxiety medications in women of reproductive age (18-44 years) is sparse and, in some cases, outdated. According to data from the National Health and Nutrition Examination Survey, 18.6% of adult women 18 years or older reported using antidepressant medications within the last 30 days in 2017-2018, an increase from 13.8% in 2009-2010. Among women aged 15-44 year with private employer–sponsored insurance surveyed during 2008-2013, the results showed 15.4% of women filled a prescription for an antidepressant. We must look back further to find data on antianxiety medication use among women aged 18-44 years where use of antianxiety drugs (anxiolytics, sedatives, and hypnotics) was 4.3% between 2005 and 2008.
A lack of literature in this area is likely due to significant underreporting, and an inability to select patients who are sensitive to or at risk of developing neuropsychiatric symptoms resulting from hormonal contraception use because the true pathophysiology is unknown. Existing studies tend to use varying methods to assess mood changes, and do not usually specify hormonal contraceptive use type in their analyses (Schaffir J et al. Eur J Contracept Reprod Health Care. 2016 Oct;21[5]:347-55).
Studies of this nature also require large sample sizes, but the percentage of women who develop neuropsychiatric symptoms from hormonal contraceptive use has historically been relatively small. In the late 1990s, Rosenberg and colleagues found 46% of 1,657 women discontinued oral contraceptives due to side effects within 6 months of starting a new prescription; of these women, 5% reported mood changes as their reason for discontinuing oral contraceptives (Rosenberg M et al. Am J Obstet Gynecol. 1998 Sep;179[3 Pt 1]:577-82).
One might expect that, as lower dosage combined hormonal contraceptives were developed in the 1980s, that the rate of reporting psychological side effects would continue to decrease as well. Yet greater awareness of the potential for mood changes while on “the pill” as outlined by the lay press and social media may be leading to increased reporting of neuropsychiatric effects in women. In a recent cross-sectional survey of 188 women in New York, 43.6% said they experienced mood changes while on hormonal contraceptives, and 61.2% of women with histories of psychiatric illness reported mood changes they attributed to hormonal contraceptives (Martell S et al. Contracept Reprod Med. 2023;8:9).
Martell and colleagues found 48.3% of women cited side effects as a reason for discontinuing hormonal contraception, and 43 participants mentioned psychological side effects unprompted, including 2 patients with suicidal thoughts. The authors said this suggests “psychological side effects, at least in part, may have impacted” HC users’ decisions to switch from OCPs to an alternative method of contraception.
It is also not clear what risk factors exist for women who develop neuropsychiatric symptoms from hormonal contraceptive use. First, it is important to note that both progestin-only contraceptives and combined hormonal contraceptives are classified by the Centers for Disease Control and Prevention’s US Medical Eligibility Criteria for Contraceptive Use, 2016 as having no restrictions for use, including among patients with depression. While women in a smaller subgroup have significant neuropsychiatric symptoms related to their hormonal contraceptives, the underlying mechanism is unknown, and is thought to be largely related to the progestogen component of combined hormonal contraceptives or progestogen-only contraceptives (Mu E. Aust Prescr. 2022 Jun; 45[3]:75-9). We know that some women are hormone sensitive, while others are less so, and some not at all. Progestogens could affect mood as a direct action of the progestogen, because progestogens can be neurosteroids, or the progestogen effect could be mediated secondarily through a change in that woman’s own production of or bioavailability of androgens or naturally occurring estrogens (Giatti S. J Mol Endocrinol. 2016 Aug;57[2]:R109-26).
Here, we also find that currently available evidence limits our ability to draw firm conclusions. A study by Berry-Bibee and colleagues found a “low concern for clinically significant interactions” between hormonal contraception and psychotropic drugs, but was limited by quality/quantity of evidence (Berry-Bibee E et al. Contraception. 2016 Dec;94[6]:650-67). Interestingly, a study by Robinson and colleagues from the mid-2000s posited based on low evidence that “psychological response to the practice of contraception” was a potential explanation for the side effect profile of hormonal contraception (Robinson S et al. Med Hypotheses. 2004;63[2]:268-73).
Further, it may be that women with premenstrual dysphoric disorder (PMDD) might be selected for oral contraceptives, and they are predisposed to other neuropsychiatric problems. Estimates have placed the prevalence of comorbid psychiatric disorders such as anxiety, major depression, bipolar disorder, and posttraumatic stress disorder as high as 70% for women with PMDD (Sepede G et al. Neuropsychiatr Dis Treat. 2020;16:415-26). This phenomenon is not new, having been characterized in the lay literature nearly 20 years ago, by endocrinologist Geoffrey P. Redmond, MD (Redmond GP. The Hormonally Vulnerable Woman. New York: HarperCollins; 2005).
While the cause is not exactly idiosyncratic, They tend to have an entire spectrum of responses to the progestogens in combined or progestin-only contraceptives, ranging from just a flattened affect – which could easily be explained by their flattened level of endogenous hormones – to frank depression. Their frank depression, in turn, can be demonstrated to include suicidal ideation and actual suicide.
Compounding this issue is a woman’s perception of her sexuality. Some women with low sexual desire or sexual problems who are younger may have more distress about their problems compared with women of older reproductive age. While the reason for that is not clear, it may be that in the sexual arena, it is more important for some younger women to be a sexual person than in perimenopausal women, or that women who are younger are more likely to be partnered than women of older reproductive age. While the European Society of Sexual Medicine concluded in a 2019 position statement that there is inconclusive evidence whether hormonal contraception may be contributing to changes in sexual desire and sexual dysfunction, it appears that “a minority of women” experience “better or worse sexual functioning” from taking combined oral contraceptives (Both S et al. J Sex Med. 2019 Nov;16[11]:1681-95), suggesting that the majority of women report no significant changes.
Practitioners should discuss mood effects during consultation
An ob.gyn., primary care physicians, or others with prescriptive authority (i.e. nurse practitioners and physician assistants) in clinical practice may encounter a patient who seems to have mood side effects owing to progestogen-containing contraceptives that they prescribe. However, many ob.gyns. are likely unaware of the prevalence, or that some of those same patients can have such significant mood effects that they would become or are suicidal.
I believe questioning patients about mood effects during consultation and particularly during follow-up following the initiation of any hormonal contraceptive is worth a passing comment for every patient, which should include mood effects in broader discussion for anyone currently using an antidepressant, patients with a history of antidepressant use, and patients who have considered suicide. As we do with other drugs, these questions can be posed in the form of a questionnaire followed up by the practitioner in counseling.
Practitioners who encounter a patient with mood changes as a result of hormonal contraceptive use can consider changing to a nonhormonal method of birth control, or recommending the patient use a barrier method during sexual activity, as none of these options have neuropsychiatric side effects.
Ultimately, practitioners of all types need to engage in shared decision-making to identify the key benefits and risks of hormonal contraceptive use for each patient, which may involve trial and error to determine the ideal treatment. It is critical that practitioners of all types strike a balance between alleviating patient concerns about potential mood changes, monitoring patients with an appreciable risk of mood changes, and continuing patients on hormonal contraception for whom the benefits outweigh the risks.
Dr. Simon is a clinical professor at George Washington University and the medical director and founder of IntimMedicine Specialists in Washington, which provides patient-focused care for women across the reproductive life cycle. He is a past president of the International Society for the Study of Women’s Sexual Health and the North American Menopause Society. Dr. Simon has been a consultant to, received grant and research support from, and served on the speakers bureau for various pharmaceutical companies that develop combination hormonal contraceptives. Email Dr. Simon at [email protected].
ChatGPT as a tool in the ob.gyn. office
Artificial intelligence (AI) has recently gained significant public attention, primarily driven by the launch of a noteworthy program by OpenAI called Chat Generative Pre-trained Transformer (ChatGPT). This large language model is an AI system that enables users to interact with it using plain language. In just the first 2 months since its release, over 100 million subscribers have registered to use ChatGPT.
AI is now deeply integrated into our daily lives, pervading a wide array of smart devices such as phones, tablets, and numerous other gadgets that we rely on every day. These sophisticated technologies operate seamlessly in the background, often without us being consciously aware of their presence. Nevertheless, we greatly appreciate the way they enhance our lives by simplifying tasks and streamlining our routines.
A key factor contributing to ChatGPT’s popularity is its ability to accept input in the form of prompts in plain English. Our team published a comprehensive journal article showcasing examples of how this technology can be utilized by general ob.gyn. practitioners. ChatGPT has the potential to streamline work flow, generate letters to insurance companies, draft clinical plans, and assist with various other routine tasks in any ob.gyn. practice environment.
As with any new technology, it is essential for users to familiarize themselves with its capabilities and understand its limitations. A critical aspect to consider with the current version of ChatGPT is that it was developed using information primarily sourced from the Internet up until September 2021. This limitation is especially significant in the field of medicine, where practitioners consistently seek the most up-to-date and current information to inform their decisions.
However, it is expected that, in the near future, these programs will gain access to real-time information from a diverse range of sources, including but not limited to the Internet. This development will enhance the program’s utility and relevance in medical contexts.
Another limitation of ChatGPT is its propensity to “hallucinate.” Large language models are trained to respond to the best of their abilities, occasionally attempting to fill knowledge gaps with fabricated information. Being aware of these limitations enables users to recognize the program’s potential while also understanding the need for careful monitoring.
Our expertise and clinical knowledge are crucial in guiding and refining this technology, ensuring that it serves as a valuable tool in the ever-evolving landscape of medicine.
Another crucial factor to consider is that users must adhere to all guidelines and policies established within their work environment. This entails complying with HIPAA regulations and ensuring that any information generated by these programs is thoroughly examined before being integrated into practice work flows. As an increasing number of organizations develop specific guidelines for such technologies, the aim is to safeguard patients, users, and the organization itself. The individuals utilizing these technologies must exercise utmost caution and refrain from storing or sharing any sensitive or proprietary information.
The aim of this article is not to provide an exhaustive overview, but rather to offer a selection of examples and inspire readers to consider adopting these programs. By doing so, we hope to facilitate the exploration of innovative approaches that can improve the practice of ob.gyn.
The first example illustrates a common scenario that we frequently encounter – the situation in which an insurance company denies coverage for specific care or treatment for a patient. In fact, this was one of the first examples that was demonstrated by others on social media as a possible benefit for this technology. It effectively demonstrated the ability to save time and surmount the challenges of writer’s block that we occasionally encounter.
Prompt input:
“Write a letter to the insurance company who is denying cervical length surveillance for a patient with a short cervix and add references.”
ChatGPT response:
[Your Name]
[Your Address]
[City, State, Zip Code]
[Date]
[Insurance Company Name]
[Claims Department]
[Insurance Company Address]
[City, State, Zip Code]
Re: Request for Reconsideration – Cervical Length Surveillance
Policy Number: [Policy Number]
Insured: [Patient’s Name]
Date of Birth: [Patient’s Date of Birth]
Claim Number: [Claim Number]
Date of Service: [Date of Service]
Dear Claims Reviewer,
I am writing to request a reconsideration of the denial for cervical length surveillance for my patient, [Patient’s Name], who has been diagnosed with a short cervix. As a healthcare provider, I strongly believe that this is a medically necessary service for the patient in order to prevent preterm birth and its associated complications.
A short cervix is a well-established risk factor for preterm birth, and numerous studies have shown the efficacy of cervical length surveillance in the prevention and management of preterm birth. I kindly request that you reconsider the coverage for this essential service and refer to the following authoritative sources:
Berghella, V., & Saccone, G. (2019). Fetal fibronectin testing for reducing the risk of preterm birth. Cochrane Database of Systematic Reviews, 2019(7). doi:10.1002/14651858.CD004843.pub3
American College of Obstetricians and Gynecologists (2016). Practice Bulletin No. 171: Management of Preterm Labor. Obstetrics & Gynecology, 128(4), e155-e164. doi:10.1097/AOG.0000000000001711
Society for Maternal-Fetal Medicine (SMFM), McIntosh, J., Feltovich, H., Berghella, V., & Manuck, T. (2016). The role of routine cervical length screening in selected high- and low-risk women for preterm birth prevention. American Journal of Obstetrics & Gynecology, 215(3), B2-B7. doi:10.1016/j.ajog.2016.03.015
By denying coverage for cervical length surveillance, the insurance company is putting [Patient’s Name] at an increased risk for preterm birth and the numerous health complications that can accompany it, both for the mother and the infant. It is crucial to provide this service in order to improve health outcomes and reduce the risk of costly neonatal intensive care unit (NICU) admissions and long-term medical issues.
I kindly request that you review this case with the provided references and reconsider the coverage for cervical length surveillance for [Patient’s Name]. Thank you for your attention to this matter. Please feel free to contact me if you require any further information or clarification.
Sincerely,
[Your Name]
[Your Title]
[Your Phone Number]
[Your Email Address]
The document took less than 15 seconds to generate, it is well written and concise, and can also be utilized as a template for future use. But as mentioned above, it is important to confirm all the information within the document. For example, references 1 and 3 in the ChatGPT-generated letter above include document objective identifiers that do not correctly correspond to the reference.
Other uses that the reader might consider: patient education, helping to answer a patient question utilizing appropriate grade-level language, dietary plans for a patient - the potential list is endless. Another key aspect of utilizing these types of programs is understanding how to effectively ask a question. This involves providing ample detail about the inquiry you wish to have answered by the program. With some practice and review of guidance on how to do this from the Internet, one can become highly proficient at crafting questions and generating relevant responses. But as mentioned above, it is important to keep in mind that all information generated by this program needs to be vetted prior to utilization.
This groundbreaking technology is not only here to stay but will continue to become increasingly pervasive. It is already being integrated into conventional search engines such as Google and Bing. Microsoft has ambitious plans to incorporate this innovation into its entire suite of Office products. Just imagine working on a document and seeking assistance for editing or rephrasing, effortlessly searching your inbox for all emails containing a specific phrase or topic, or even crafting a PowerPoint presentation for a lecture while receiving help with both content and formatting. These scenarios offer just a glimpse of how AI programs can significantly assist and enhance our workflow.
I also anticipate that our patients will increasingly adopt this type of technology to generate customized lists of questions tailored to their specific medical conditions, which they can then ask their health care providers. Often, our patients express uncertainty about the appropriate questions to ask during a particular visit. Now envision a scenario in which they can effortlessly obtain a comprehensive list of relevant questions, specifically designed for their office consultation. This would empower them to engage more actively in their health care and enhance communication with their clinical team.
I highly recommend that readers explore and experiment with these programs. By doing so, we can provide valuable assistance and guidance not only within our specific medical specialties but also for our patients. In this way, we can effectively harness the power of technology to improve patient care and optimize our office work flow, ultimately benefiting both our patients and our practices.
Dr. Chavez is professor, department of obstetrics and gynecology, at NYU Long Island School of Medicine and director of maternal and fetal medicine at NYU Langone Hospital–Long Island, both in Mineola, N.Y. He has no disclosures.
Artificial intelligence (AI) has recently gained significant public attention, primarily driven by the launch of a noteworthy program by OpenAI called Chat Generative Pre-trained Transformer (ChatGPT). This large language model is an AI system that enables users to interact with it using plain language. In just the first 2 months since its release, over 100 million subscribers have registered to use ChatGPT.
AI is now deeply integrated into our daily lives, pervading a wide array of smart devices such as phones, tablets, and numerous other gadgets that we rely on every day. These sophisticated technologies operate seamlessly in the background, often without us being consciously aware of their presence. Nevertheless, we greatly appreciate the way they enhance our lives by simplifying tasks and streamlining our routines.
A key factor contributing to ChatGPT’s popularity is its ability to accept input in the form of prompts in plain English. Our team published a comprehensive journal article showcasing examples of how this technology can be utilized by general ob.gyn. practitioners. ChatGPT has the potential to streamline work flow, generate letters to insurance companies, draft clinical plans, and assist with various other routine tasks in any ob.gyn. practice environment.
As with any new technology, it is essential for users to familiarize themselves with its capabilities and understand its limitations. A critical aspect to consider with the current version of ChatGPT is that it was developed using information primarily sourced from the Internet up until September 2021. This limitation is especially significant in the field of medicine, where practitioners consistently seek the most up-to-date and current information to inform their decisions.
However, it is expected that, in the near future, these programs will gain access to real-time information from a diverse range of sources, including but not limited to the Internet. This development will enhance the program’s utility and relevance in medical contexts.
Another limitation of ChatGPT is its propensity to “hallucinate.” Large language models are trained to respond to the best of their abilities, occasionally attempting to fill knowledge gaps with fabricated information. Being aware of these limitations enables users to recognize the program’s potential while also understanding the need for careful monitoring.
Our expertise and clinical knowledge are crucial in guiding and refining this technology, ensuring that it serves as a valuable tool in the ever-evolving landscape of medicine.
Another crucial factor to consider is that users must adhere to all guidelines and policies established within their work environment. This entails complying with HIPAA regulations and ensuring that any information generated by these programs is thoroughly examined before being integrated into practice work flows. As an increasing number of organizations develop specific guidelines for such technologies, the aim is to safeguard patients, users, and the organization itself. The individuals utilizing these technologies must exercise utmost caution and refrain from storing or sharing any sensitive or proprietary information.
The aim of this article is not to provide an exhaustive overview, but rather to offer a selection of examples and inspire readers to consider adopting these programs. By doing so, we hope to facilitate the exploration of innovative approaches that can improve the practice of ob.gyn.
The first example illustrates a common scenario that we frequently encounter – the situation in which an insurance company denies coverage for specific care or treatment for a patient. In fact, this was one of the first examples that was demonstrated by others on social media as a possible benefit for this technology. It effectively demonstrated the ability to save time and surmount the challenges of writer’s block that we occasionally encounter.
Prompt input:
“Write a letter to the insurance company who is denying cervical length surveillance for a patient with a short cervix and add references.”
ChatGPT response:
[Your Name]
[Your Address]
[City, State, Zip Code]
[Date]
[Insurance Company Name]
[Claims Department]
[Insurance Company Address]
[City, State, Zip Code]
Re: Request for Reconsideration – Cervical Length Surveillance
Policy Number: [Policy Number]
Insured: [Patient’s Name]
Date of Birth: [Patient’s Date of Birth]
Claim Number: [Claim Number]
Date of Service: [Date of Service]
Dear Claims Reviewer,
I am writing to request a reconsideration of the denial for cervical length surveillance for my patient, [Patient’s Name], who has been diagnosed with a short cervix. As a healthcare provider, I strongly believe that this is a medically necessary service for the patient in order to prevent preterm birth and its associated complications.
A short cervix is a well-established risk factor for preterm birth, and numerous studies have shown the efficacy of cervical length surveillance in the prevention and management of preterm birth. I kindly request that you reconsider the coverage for this essential service and refer to the following authoritative sources:
Berghella, V., & Saccone, G. (2019). Fetal fibronectin testing for reducing the risk of preterm birth. Cochrane Database of Systematic Reviews, 2019(7). doi:10.1002/14651858.CD004843.pub3
American College of Obstetricians and Gynecologists (2016). Practice Bulletin No. 171: Management of Preterm Labor. Obstetrics & Gynecology, 128(4), e155-e164. doi:10.1097/AOG.0000000000001711
Society for Maternal-Fetal Medicine (SMFM), McIntosh, J., Feltovich, H., Berghella, V., & Manuck, T. (2016). The role of routine cervical length screening in selected high- and low-risk women for preterm birth prevention. American Journal of Obstetrics & Gynecology, 215(3), B2-B7. doi:10.1016/j.ajog.2016.03.015
By denying coverage for cervical length surveillance, the insurance company is putting [Patient’s Name] at an increased risk for preterm birth and the numerous health complications that can accompany it, both for the mother and the infant. It is crucial to provide this service in order to improve health outcomes and reduce the risk of costly neonatal intensive care unit (NICU) admissions and long-term medical issues.
I kindly request that you review this case with the provided references and reconsider the coverage for cervical length surveillance for [Patient’s Name]. Thank you for your attention to this matter. Please feel free to contact me if you require any further information or clarification.
Sincerely,
[Your Name]
[Your Title]
[Your Phone Number]
[Your Email Address]
The document took less than 15 seconds to generate, it is well written and concise, and can also be utilized as a template for future use. But as mentioned above, it is important to confirm all the information within the document. For example, references 1 and 3 in the ChatGPT-generated letter above include document objective identifiers that do not correctly correspond to the reference.
Other uses that the reader might consider: patient education, helping to answer a patient question utilizing appropriate grade-level language, dietary plans for a patient - the potential list is endless. Another key aspect of utilizing these types of programs is understanding how to effectively ask a question. This involves providing ample detail about the inquiry you wish to have answered by the program. With some practice and review of guidance on how to do this from the Internet, one can become highly proficient at crafting questions and generating relevant responses. But as mentioned above, it is important to keep in mind that all information generated by this program needs to be vetted prior to utilization.
This groundbreaking technology is not only here to stay but will continue to become increasingly pervasive. It is already being integrated into conventional search engines such as Google and Bing. Microsoft has ambitious plans to incorporate this innovation into its entire suite of Office products. Just imagine working on a document and seeking assistance for editing or rephrasing, effortlessly searching your inbox for all emails containing a specific phrase or topic, or even crafting a PowerPoint presentation for a lecture while receiving help with both content and formatting. These scenarios offer just a glimpse of how AI programs can significantly assist and enhance our workflow.
I also anticipate that our patients will increasingly adopt this type of technology to generate customized lists of questions tailored to their specific medical conditions, which they can then ask their health care providers. Often, our patients express uncertainty about the appropriate questions to ask during a particular visit. Now envision a scenario in which they can effortlessly obtain a comprehensive list of relevant questions, specifically designed for their office consultation. This would empower them to engage more actively in their health care and enhance communication with their clinical team.
I highly recommend that readers explore and experiment with these programs. By doing so, we can provide valuable assistance and guidance not only within our specific medical specialties but also for our patients. In this way, we can effectively harness the power of technology to improve patient care and optimize our office work flow, ultimately benefiting both our patients and our practices.
Dr. Chavez is professor, department of obstetrics and gynecology, at NYU Long Island School of Medicine and director of maternal and fetal medicine at NYU Langone Hospital–Long Island, both in Mineola, N.Y. He has no disclosures.
Artificial intelligence (AI) has recently gained significant public attention, primarily driven by the launch of a noteworthy program by OpenAI called Chat Generative Pre-trained Transformer (ChatGPT). This large language model is an AI system that enables users to interact with it using plain language. In just the first 2 months since its release, over 100 million subscribers have registered to use ChatGPT.
AI is now deeply integrated into our daily lives, pervading a wide array of smart devices such as phones, tablets, and numerous other gadgets that we rely on every day. These sophisticated technologies operate seamlessly in the background, often without us being consciously aware of their presence. Nevertheless, we greatly appreciate the way they enhance our lives by simplifying tasks and streamlining our routines.
A key factor contributing to ChatGPT’s popularity is its ability to accept input in the form of prompts in plain English. Our team published a comprehensive journal article showcasing examples of how this technology can be utilized by general ob.gyn. practitioners. ChatGPT has the potential to streamline work flow, generate letters to insurance companies, draft clinical plans, and assist with various other routine tasks in any ob.gyn. practice environment.
As with any new technology, it is essential for users to familiarize themselves with its capabilities and understand its limitations. A critical aspect to consider with the current version of ChatGPT is that it was developed using information primarily sourced from the Internet up until September 2021. This limitation is especially significant in the field of medicine, where practitioners consistently seek the most up-to-date and current information to inform their decisions.
However, it is expected that, in the near future, these programs will gain access to real-time information from a diverse range of sources, including but not limited to the Internet. This development will enhance the program’s utility and relevance in medical contexts.
Another limitation of ChatGPT is its propensity to “hallucinate.” Large language models are trained to respond to the best of their abilities, occasionally attempting to fill knowledge gaps with fabricated information. Being aware of these limitations enables users to recognize the program’s potential while also understanding the need for careful monitoring.
Our expertise and clinical knowledge are crucial in guiding and refining this technology, ensuring that it serves as a valuable tool in the ever-evolving landscape of medicine.
Another crucial factor to consider is that users must adhere to all guidelines and policies established within their work environment. This entails complying with HIPAA regulations and ensuring that any information generated by these programs is thoroughly examined before being integrated into practice work flows. As an increasing number of organizations develop specific guidelines for such technologies, the aim is to safeguard patients, users, and the organization itself. The individuals utilizing these technologies must exercise utmost caution and refrain from storing or sharing any sensitive or proprietary information.
The aim of this article is not to provide an exhaustive overview, but rather to offer a selection of examples and inspire readers to consider adopting these programs. By doing so, we hope to facilitate the exploration of innovative approaches that can improve the practice of ob.gyn.
The first example illustrates a common scenario that we frequently encounter – the situation in which an insurance company denies coverage for specific care or treatment for a patient. In fact, this was one of the first examples that was demonstrated by others on social media as a possible benefit for this technology. It effectively demonstrated the ability to save time and surmount the challenges of writer’s block that we occasionally encounter.
Prompt input:
“Write a letter to the insurance company who is denying cervical length surveillance for a patient with a short cervix and add references.”
ChatGPT response:
[Your Name]
[Your Address]
[City, State, Zip Code]
[Date]
[Insurance Company Name]
[Claims Department]
[Insurance Company Address]
[City, State, Zip Code]
Re: Request for Reconsideration – Cervical Length Surveillance
Policy Number: [Policy Number]
Insured: [Patient’s Name]
Date of Birth: [Patient’s Date of Birth]
Claim Number: [Claim Number]
Date of Service: [Date of Service]
Dear Claims Reviewer,
I am writing to request a reconsideration of the denial for cervical length surveillance for my patient, [Patient’s Name], who has been diagnosed with a short cervix. As a healthcare provider, I strongly believe that this is a medically necessary service for the patient in order to prevent preterm birth and its associated complications.
A short cervix is a well-established risk factor for preterm birth, and numerous studies have shown the efficacy of cervical length surveillance in the prevention and management of preterm birth. I kindly request that you reconsider the coverage for this essential service and refer to the following authoritative sources:
Berghella, V., & Saccone, G. (2019). Fetal fibronectin testing for reducing the risk of preterm birth. Cochrane Database of Systematic Reviews, 2019(7). doi:10.1002/14651858.CD004843.pub3
American College of Obstetricians and Gynecologists (2016). Practice Bulletin No. 171: Management of Preterm Labor. Obstetrics & Gynecology, 128(4), e155-e164. doi:10.1097/AOG.0000000000001711
Society for Maternal-Fetal Medicine (SMFM), McIntosh, J., Feltovich, H., Berghella, V., & Manuck, T. (2016). The role of routine cervical length screening in selected high- and low-risk women for preterm birth prevention. American Journal of Obstetrics & Gynecology, 215(3), B2-B7. doi:10.1016/j.ajog.2016.03.015
By denying coverage for cervical length surveillance, the insurance company is putting [Patient’s Name] at an increased risk for preterm birth and the numerous health complications that can accompany it, both for the mother and the infant. It is crucial to provide this service in order to improve health outcomes and reduce the risk of costly neonatal intensive care unit (NICU) admissions and long-term medical issues.
I kindly request that you review this case with the provided references and reconsider the coverage for cervical length surveillance for [Patient’s Name]. Thank you for your attention to this matter. Please feel free to contact me if you require any further information or clarification.
Sincerely,
[Your Name]
[Your Title]
[Your Phone Number]
[Your Email Address]
The document took less than 15 seconds to generate, it is well written and concise, and can also be utilized as a template for future use. But as mentioned above, it is important to confirm all the information within the document. For example, references 1 and 3 in the ChatGPT-generated letter above include document objective identifiers that do not correctly correspond to the reference.
Other uses that the reader might consider: patient education, helping to answer a patient question utilizing appropriate grade-level language, dietary plans for a patient - the potential list is endless. Another key aspect of utilizing these types of programs is understanding how to effectively ask a question. This involves providing ample detail about the inquiry you wish to have answered by the program. With some practice and review of guidance on how to do this from the Internet, one can become highly proficient at crafting questions and generating relevant responses. But as mentioned above, it is important to keep in mind that all information generated by this program needs to be vetted prior to utilization.
This groundbreaking technology is not only here to stay but will continue to become increasingly pervasive. It is already being integrated into conventional search engines such as Google and Bing. Microsoft has ambitious plans to incorporate this innovation into its entire suite of Office products. Just imagine working on a document and seeking assistance for editing or rephrasing, effortlessly searching your inbox for all emails containing a specific phrase or topic, or even crafting a PowerPoint presentation for a lecture while receiving help with both content and formatting. These scenarios offer just a glimpse of how AI programs can significantly assist and enhance our workflow.
I also anticipate that our patients will increasingly adopt this type of technology to generate customized lists of questions tailored to their specific medical conditions, which they can then ask their health care providers. Often, our patients express uncertainty about the appropriate questions to ask during a particular visit. Now envision a scenario in which they can effortlessly obtain a comprehensive list of relevant questions, specifically designed for their office consultation. This would empower them to engage more actively in their health care and enhance communication with their clinical team.
I highly recommend that readers explore and experiment with these programs. By doing so, we can provide valuable assistance and guidance not only within our specific medical specialties but also for our patients. In this way, we can effectively harness the power of technology to improve patient care and optimize our office work flow, ultimately benefiting both our patients and our practices.
Dr. Chavez is professor, department of obstetrics and gynecology, at NYU Long Island School of Medicine and director of maternal and fetal medicine at NYU Langone Hospital–Long Island, both in Mineola, N.Y. He has no disclosures.