User login
Endometriosis: What it is and how it is treated
A 62-year-old man with hypotension and an abnormal chest radiograph
Asymptomatic hyperuricemia: To treat or not to treat
Weight control and antipsychotics: How to tip the scales away from diabetes and heart disease
Weight gain is a potential problem for all patients who require treatment with antipsychotics. Those with schizophrenia face double jeopardy. Both the disorder and the use of virtually any available antipsychotic drug may be associated with weight gain, new-onset glucose intolerance, and type 2 diabetes mellitus.
Because of the cardiovascular risks and other morbidity associated with weight gain and glucose dysregulation,1 the psychiatrist must remain vigilant and manage these complications aggressively. In this article, we offer insights into the prevention and management of metabolic complications associated with the use of antipsychotic agents in patients with schizophrenia.
Weight gain and antipsychotics
Weight change was recognized as a feature of schizophrenia even before antipsychotic drugs were introduced in the 1950s.2 Schizophrenia—independent of drug treatment—also is a risk factor for the development of type 2 diabetes. In persons with schizophrenia, serum glucose levels increase more slowly, decline more gradually, and represent higher-than-normal reference values.3
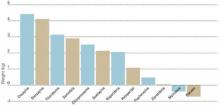
Figure 1 WEIGHT GAIN ASSOCIATED WITH ANTIPSYCHOTIC DRUG ADMINISTRATION
Values represent estimates of drug-induced weight gain after 10 weeks of drug administration.
Source: Allison et al. Am J Psychiatry 1999;156:1686-96; Brecher et al. Int J Psychiatry Clin Pract 2000;4:287-92.In 1999, Allison et al assessed the effects of conventional and atypical antipsychotics on body weight. Using 81 published articles, they estimated and compared weight changes associated with 10 antipsychotic agents and a placebo when given at standard dosages for 10 weeks.4 Comparative data on quetiapine, which were insufficient in 1999, have since been added (Figure 1).5
Patients who received a placebo lost 0.74 kg across 10 weeks. Weight changes with the conventional agents ranged from a reduction of 0.39 kg with molindone to an increase of 3.19 kg with thioridazine. Weight gains also were seen with all of the newer atypical agents, including clozapine (+4.45 kg), olanzapine (+4.15 kg), risperidone (+2.10 kg), and ziprasidone (+0.04 kg).
Fontaine et al have estimated that weight gain in patients with schizophrenia has its greatest impact on mortality in two scenarios:
- when patients are overweight before they start antipsychotic medication
- with greater degrees of weight gain across 10 years (Figure 2).
Whatever a patient’s starting weight, substantial weight gain with antipsychotic therapy increases the risk of impaired glucose tolerance and hypertension (Figure 3).6
Schizophrenia and diabetes
The prevalence of type 2 diabetes in patients with schizophrenia increased from 4.2% in 1956 to 17.2% in 1968, related in part to the introduction of phenothiazines.7 A recent study of data collected by the Schizophrenia Patient Outcomes Research Team (PORT)2 found higher rates of diabetes in persons with schizophrenia (lifetime prevalence, 14.9%) than in the general population (approximately 7.3%).1 Most patients in the PORT study were taking older antipsychotics, the use of which has occasionally been associated with carbohydrate dysregulation.
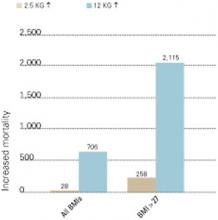
Figure 2 INCREASED MORTALITY ASSOCIATED WITH WEIGHT GAIN
Number of deaths associated with weight gains of 2.5 and 12 kg over 10 years, as related to all body mass index measurements (BMIs) and BMIs >27 (per 100,000 persons in U.S. population).
Source: Fontaine et al. Psychiatry Res 2001;101:277-88.The prevalence of new-onset diabetes with use of specific antipsychotics is unknown. Most information is contained in case reports, and proper epidemiologic studies await publication.
The most detailed report—a pooled study of published cases related to clozapine use—comes from the FDA’s Center for Drug Evaluation and Research.8 In this study, the authors identified 384 reports of diabetes that developed (in 242 patients) or was exacerbated (in 54 patients) in association with clozapine. Patient mean age was 40, and diabetes occurred more commonly in women than in men.
Diabetes developed most commonly within 6 months of starting treatment with clozapine, and one patient developed diabetes after a single 500-mg dose. Metabolic acidosis or ketosis occurred in 80 cases, and 25 subjects died during hyperglycemic episodes. Stopping clozapine or reducing the dosage improved glycemic control in 46 patients.8
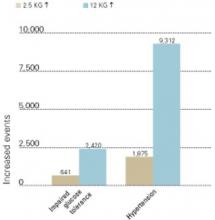
Figure 3 INCREASED MORBIDITY ASSOCIATED WITH WEIGHT GAIN
New cases of impaired glucose tolerance and hypertension that developed with weight gains of 2.5 and 12 kg over 10 years (per 100,000 persons in U.S. population).
Source: Fontaine et al. Psychiatry Res 2001;101:277-88.During antipsychotic therapy, it is important to measure patients’ fasting plasma glucose at least annually—and more often for high-risk patients (Table 1). The American Diabetes Association defines diabetes as a fasting serum or plasma glucose 126 mg/dl or a 2-hour postprandial serum or plasma glucose 200 mg/dl. In all patients, these tests should be repeated to confirm the diagnosis. Oral glucose tolerance testing is less convenient than fasting plasma glucose testing but more sensitive in identifying changes in carbohydrate metabolism.
As with weight gain, it is easier to prevent diabetes than to treat it. The psychiatrist can best help the patient with emerging carbohydrate dysregulation by collaborating with an internist, family physician, or endocrinologist.
Table 1
FACTORS RELATED TO HIGH RISK OF DEVELOPING TYPE 2 DIABETES
|
| Source: American Diabetes Association |
Weight gain with diabetes drugs Weight gain is associated not only with the use of antipsychotics but also with four classes of oral agents used to treat type 2 diabetes: sulfonylureas, meglitinides, phenylalanine derivatives, and thiazolidinediones. One class—biguanides—contributes to weight reduction, and one—alpha-glucosidase inhibitors—has a variable effect on body weight. These drugs also vary in their effects on serum lipids, including total cholesterol, LDL and HDL cholesterol, and triglycerides (Table 2).9
Many patients with type 2 diabetes require more than one agent to control plasma glucose. With time, insulin deficiency becomes more marked, and insulin therapy is frequently added to the regimen. Hypertension and hyperlipidemia are also very common in patients with type 2 diabetes and require medication to reduce the risk of cardiovascular events.10 As a result, the diabetic patient requiring antipsychotic drugs will likely need polypharmacy, and many of the drugs that might be used may lead to weight gain.
Assessing, managing weight gain
During each visit for the patient with schizophrenia, it is important to routinely weigh those receiving antipsychotics and ask about polydipsia and polyuria, which are early signs of incipient diabetes. A patient who is gaining significant weight (7% of baseline) while taking an antipsychotic and has risk factors for cardiovascular events (e.g., smoking, hypertension, hypertriglyceridemia) is a candidate for a change in antipsychotics.
Try to weigh patients at approximately the same time of day at each visit to compensate for possible diurnal weight changes related to polydipsia-hyponatremia syndrome.11 Patients with this syndrome can gain 5 to 10 lbs (or more) per day and excrete the retained fluid at night. It occurs in 5 to 10% of chronically psychotic patients requiring institutional care and in 1 to 2% of outpatients. Patients with schizophrenia complicated by this syndrome may manifest polydipsia and polyuria secondary to psychosis rather than emerging diabetes. Thus, the clinician must be alert to both diabetes and the polydipsia-hyponatremia syndrome in this setting.
Weight-control approaches
Patients who are taking sedating antipsychotics (e.g., clozapine, olanzapine, or low-potency phenothiazines) may gain up to 30 lbs per year if they become physically inactive and do not reduce their food consumption. Thus, it is important to work with such patients to decrease their caloric intake.
A weight-loss program that produces a loss of 0.5 to 1% of body weight per week is considered safe and acceptable.12 Mild to moderate obesity may be managed by reducing food intake by 500 calories and exercising 30 minutes each day.
CBT Cognitive-behavioral therapy (CBT) may help stem weight gain associated with antipsychotic use. Umbricht et al provided CBT to six patients with chronic psychosis who were receiving clozapine or olanzapine. Therapists in group and individual sessions focused on the causes of weight gain, lowcalorie nutrition, weight-loss guidelines, exercise programs, and relaxation strategies. Across 8 weeks, patients’ mean BMI decreased from 29.6 to 25.1 kg/m2
Table 2
METABOLIC EFFECTS OF ORAL ANTIHYPERGLYCEMIC DRUGS
| Class | Body weight | Total cholesterol | LDL | HDL | Triglycerides |
|---|---|---|---|---|---|
| Sulfonylureas Glipizide Glyburide Glimepiride | ▲ | ◄► | ◄► | ◄► | ◄► |
| Meglitinides Repaglinide | ▲ | ◄► | ◄► | ◄► | ◄► |
| Phenylalanine derivatives Nateglinide | ▲ | ◄► | ◄► | ◄► | ◄► |
| Biguanides Metformin | ▼ | ▼ | ▼ | ▲ | ▼ |
| Thiazolidinediones Pioglitazone Rosiglitazone | ▲ | ▲ | ▲ | ▲ | ▼ |
| Alpha-glucosidase inhibitors Acarbose Miglitol | ◄► | ◄► | ◄► | ◄► | ◄► |
| ▲ Increase ▼ Decrease ◄► Neutral effect/no change | |||||
Weight management program The Weight Watchers weight management program has shown mild success when offered to men and women with schizophrenia or schizoaffective disorder. Twenty-one patients who had gained an average of 32 lbs while taking olanzapine were enrolled in a Weight Watchers program at a psychiatric center.14 Mean starting BMI was 32 kg/m2 among the 11 patients who completed the 10-week program. Those 11 lost an average of 5 lbs.
All seven men lost weight. Three of the four women gained weight, and one woman lost 13 lbs. Study subjects remained clinically stable during the 10-week study. Two of the three women who did not lose weight had disabling psychiatric symptoms. Participation rates were similar to those of typical Weight Watchers clientele, suggesting that patients requiring antipsychotics might benefit from treatments used for other obese patients.
Patient education Educating patients about nutrition and exercise may help them control their rate of weight gain during antipsychotic therapy.
Littrell et al provided such an educational program for 1 hour per week for 4 months to six men and six women taking olanzapine for schizophrenia or schizoaffective disorder.15 Patients in the behavioral group gained 0.5 kg, compared with a control group that gained 2.9 kg. Mean increase in BMI was less for the behavioral group (0.3 kg/m2) than for the control patients (0.9 kg/m2). Men in both groups gained more weight than did women.
Pharmacologic approaches
Antiobesity medications are generally reserved for patients with a BMI 30 kg/m2 (threshold for obesity) or for those with a BMI 27 kg/m2 (threshold for overweight is 25 kg/m2) who have additional risk factors for cardiovascular disease, stroke, or diabetes.16
For patients with schizophrenia, who typically have a BMI 27 kg/m2, the presence of these risk factors alone may be enough to warrant consideration of an antiobesity agent. Adding any new drug to a patient’s regimen, however, increases the risk of an adverse interaction.
Antiobesity drugs work by a variety of mechanisms, including decreasing appetite, decreasing fat absorption, and increasing energy expenditure. Drugs may reduce caloric intake by decreasing appetite (anorectic drugs) or increasing satiety (appetite suppressants). Centrally-acting sympathomimetics or serotonergic drugs may suppress appetite.
In studies up to 2 years, the appetite suppressant sibutramine, with mixed serotonergic and noradrenergic reuptake inhibition properties, has been shown to cause more weight loss than a placebo in populations without schizophrenia.17 According to one case report, sibutramine use was associated with new-onset psychosis.18
Common side effects of sibutramine include headache, dry mouth, anorexia, constipation, and insomnia. Regular monitoring of blood pressure is required. Do not prescribe this drug for patients with cardiovascular disease, and avoid co-prescribing with MAO inhibitors and serotonergics.
Orlistat reduces fat absorption from the GI tract.19 Common side effects are largely confined to the GI tract and include oily spotting, flatulence, fecal urgency, fatty/oily stool, and oily evacuation.
Combination therapies
Researchers are studying whether adding adjunctive agents to antipsychotics reduces weight gain.
Clozapine plus quetiapine A group of 65 patients who experienced a mean body weight increase of 6.5 kg while taking clozapine for 6 months were then given clozapine plus quetiapine at chlorpromazine-equivalent dosing during the next 10 months. The patients lost a mean of 4.2 kg, and their glycemic control improved. Elevated glycosylated hemoglobin (HbA1c) became normal in those subjects (20% of participants) who had developed type 2 diabetes while taking clozapine alone. The authors theorized that the weight loss diminished insulin resistance, leading to better control of serum glucose levels.20
Olanzapine plus amantadine A group of 12 outpatients with axis I or II diagnoses had responded well clinically to olanzapine but had gained an average 7.3 kg over 1 to 11 months. In an open-label study, they continued their dosages of olanzapine and also were given amantadine, 100 to 300 mg/d. Amantadine was chosen for this trial because of its possible release of dopamine.
No dietary changes were made, but subjects gained no additional weight after amantadine was added. Over the next 3 to 6 months, they lost a mean 3.5 kg, which was 50% of the weight gain associated with olanzapine administration.21
Clozapine plus topiramate In clinical trials, the anticonvulsant topiramate has been associated with significant weight loss for up to 12 months in patients with seizure disorders.22 This agent, which also has mood-stabilizing effects, may be useful both for mood stabilization and weight loss in tandem with antipsychotic therapy.
In a case study,23 a 29-year-old man with schizophrenia who failed several trials of antipsychotic drugs experienced significant improvement with clozapine, 800 mg/d. Over 2 years, however, he developed myoclonic jerks and gained 45.5 kg (a 49% increase over baseline). When topiramate was added, starting with 25 mg/d and increasing to 125 mg/d, his mood improved and the myoclonic jerks stopped. During 5 months of combination therapy, the patient lost 21 kg without changing his eating habits.
Olanzapine and nizatidine Agents that block histamine (H 2) receptors in the digestive tract may be associated with weight loss when given at high doses, although the mechanism by which they contribute to weight loss is unclear. In a double-blind, placebo-controlled study,24 the H 2 blocker nizatidine was given to patients with schizophrenia who were taking olanzapine, 5 to 20 mg/d. In a 16-week trial, 132 patients were randomized to receive adjunctive treatment with low-dose nizatidine (150 mg bid), high-dose nizatidine (300 mg bid), or a placebo.
After 16 weeks, nizatidine demonstrated a dose-response effect when combined with olanzapine. Average weight gain was:
- 5.51 kg with a placebo
- 4.41 kg with low-dose nizatidine
- 2.76 kg with high-dose nizatidine (p =0.02 compared with a placebo).
In the high-dose nizatidine group, only 6% of patients gained more than 10 kg, and weight gain leveled off by week eight. Adverse events and clinical improvements were similar in the three groups.
Related resources
- Weight gain: A growing problem in schizophrenia management. J Clin Psychiatry 2001;62(suppl 7).
- Weight gain associated with the use of psychotropic medications. J Clin Psychiatry 1999;60(suppl 2).
- Effects of atypical antipsychotics on body weight and glucose regulation. J Clin Psychiatry 2001;62(suppl 23).
- National Heart, Lung, and Blood Institute. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults. www.nhlbi.nih.gov/guidelines/obesity/ob_home.htm
Drug brand names
- Amantadine • Symmetrel
- Clozapine • Clozaril
- Nizatidine • Axid
- Olanzapine • Zyprexa
- Orlistat • Xenical
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Sibutramine • Meridia
- Topiramate • Topamax
- Ziprasidone • Geodon
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article.
1. Mokdad AH, Bowman BA, Ford ES, Vinicor F, Marks JS, Koplan JP. The continuing epidemics of obesity and diabetes in the United States. JAMA 2001;286:1195-1200.
2. Dixon L, Weiden P, Delahanty J, et al. Prevalence and correlates of diabetes in national schizophrenia samples. Schizophr Bull 2000;26:903-12.
3. Braceland FJ, Meduna LJ, Vaichulis JA. Delayed action of insulin in schizophrenia. Am J Psychiatry 1945;102:108-10.
4. Allison DB, Mentore JL, Heo M, et al. Antipsychotic-induced weight gain: a comprehensive research synthesis. Am J Psychiatry 1999;156:1686-96.
5. Brecher M, Rak IW, Westhead EK. The long-term effect of quetiapine (“Seroquel’) monotherapy on weight in patients with schizophrenia. Int J Psychiatry Clin Pract 2000;4:287-92.
6. Fontaine KR, Heo M, Harrigan EP, Shear CL, Lakshiminarayanan M. Estimating the consequences of anti-psychotic induced weight gain on health and mortality rate. Psychiatry Res 2001;101:277-88.
7. Theonnard-Neumann E. Phenothiazines and diabetes in hospitalized women. Am J Psychiatry 1968;124:978-82.
8. Koller E, Schneider B, Bennett K, Dubitsky G. Clozapine-associated diabetes. Am J Med 2001;111:716-23.
9. Pendergrass ML. Pathophysiology and management of type 2 diabetes. In: Giles TD, Sowers JR, Weber MA (eds). Diabetes & cardiovascular disease: a practical primer. New Orleans: Institute of Professional Education, 2000;15-40.
10. Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults. Findings from the Third National Health and Nutrition Examination Survey. JAMA 2002;287:356-9.
11. Vieweg WVR, Leadbetter RA. The polydipsia-hyponatremia syndrome. Epidemiology, clinical features, and treatment. CNS Drugs 1997;7:121-38.
12. Thomas PR. Weighing the options: criteria for evaluating weight management programs. Washington, DC: National Academy Press, 1995.
13. Umbricht D, Flury H, Bridler R. Cognitive behavioral therapy for weight gain. Am J Psychiatry 2001;158:971.-
14. Ball M, Coons V, Buchanan R. A program for treating olanzapine-related weight gain. Psychiatric Services 2001;52:967-9.
15. Littrell KH, Petty RG, Hilligoss NM, Peabody CD, Johnson CG. Educational interventions for the management of antipsychotic-related weight gain. 41st annual meeting of the New Clinical Drug Evaluation Unit, Phoenix, AZ, May 28-31, 2001.
16. Greenberg I, Chan S, Blackburn GL. Nonpharmacologic and pharmacologic management of weight gain. J Clin Psychiatry 1999;60(suppl 21):31-6.
17. Wirth A, Krause J. Long-term weight loss with sibutramine: a randomized controlled trial. JAMA 2001;286:1331-9.
18. Taflinski T, Chojnacka J. Sibutramine-associated psychotic episode. Am J Psychiatry 2001;157:2057-8.
19. Glazer G. Long-term pharmacotherapy of obesity 2000. Arch Intern Med 2001;161:1814-24.
20. Reinstein M, Sirotovskaya L, Jones L. Effect of clozapine-quetiapine combination therapy on weight and glycaemic control. Clin Drug Invest 1999;18:99-104.
21. Floris M, Lejeune J, Deberdt W. Effect of amantadine on weight gain during olanzapine treatment. Eur Neuropsychopharmacol 2001;11:181-2.
22. Norton J, Potter D, Edwards K. Sustained weight loss associated with topiramate [abstract]. Epilepsia 1997;38(suppl 3):60.-
23. Dursun SM, Devarajan S. Clozapine weight gain, plus topiramate weight loss. Can J Psychiatry 2000;45:198.-
24. Breier A, Tanaka Y, Roychowdhury S, Clark WS. Nizatidine for the prevention of olanzapine-associated weight gain in schizophrenia and related disorders. A randomized controlled double blind study. 41st annual meeting of the New Clinical Drug Evaluation Unit, Phoenix, AZ, May 28-31 2001.
Weight gain is a potential problem for all patients who require treatment with antipsychotics. Those with schizophrenia face double jeopardy. Both the disorder and the use of virtually any available antipsychotic drug may be associated with weight gain, new-onset glucose intolerance, and type 2 diabetes mellitus.
Because of the cardiovascular risks and other morbidity associated with weight gain and glucose dysregulation,1 the psychiatrist must remain vigilant and manage these complications aggressively. In this article, we offer insights into the prevention and management of metabolic complications associated with the use of antipsychotic agents in patients with schizophrenia.
Weight gain and antipsychotics
Weight change was recognized as a feature of schizophrenia even before antipsychotic drugs were introduced in the 1950s.2 Schizophrenia—independent of drug treatment—also is a risk factor for the development of type 2 diabetes. In persons with schizophrenia, serum glucose levels increase more slowly, decline more gradually, and represent higher-than-normal reference values.3
Figure 1 WEIGHT GAIN ASSOCIATED WITH ANTIPSYCHOTIC DRUG ADMINISTRATION
Values represent estimates of drug-induced weight gain after 10 weeks of drug administration.
Source: Allison et al. Am J Psychiatry 1999;156:1686-96; Brecher et al. Int J Psychiatry Clin Pract 2000;4:287-92.In 1999, Allison et al assessed the effects of conventional and atypical antipsychotics on body weight. Using 81 published articles, they estimated and compared weight changes associated with 10 antipsychotic agents and a placebo when given at standard dosages for 10 weeks.4 Comparative data on quetiapine, which were insufficient in 1999, have since been added (Figure 1).5
Patients who received a placebo lost 0.74 kg across 10 weeks. Weight changes with the conventional agents ranged from a reduction of 0.39 kg with molindone to an increase of 3.19 kg with thioridazine. Weight gains also were seen with all of the newer atypical agents, including clozapine (+4.45 kg), olanzapine (+4.15 kg), risperidone (+2.10 kg), and ziprasidone (+0.04 kg).
Fontaine et al have estimated that weight gain in patients with schizophrenia has its greatest impact on mortality in two scenarios:
- when patients are overweight before they start antipsychotic medication
- with greater degrees of weight gain across 10 years (Figure 2).
Whatever a patient’s starting weight, substantial weight gain with antipsychotic therapy increases the risk of impaired glucose tolerance and hypertension (Figure 3).6
Schizophrenia and diabetes
The prevalence of type 2 diabetes in patients with schizophrenia increased from 4.2% in 1956 to 17.2% in 1968, related in part to the introduction of phenothiazines.7 A recent study of data collected by the Schizophrenia Patient Outcomes Research Team (PORT)2 found higher rates of diabetes in persons with schizophrenia (lifetime prevalence, 14.9%) than in the general population (approximately 7.3%).1 Most patients in the PORT study were taking older antipsychotics, the use of which has occasionally been associated with carbohydrate dysregulation.
Figure 2 INCREASED MORTALITY ASSOCIATED WITH WEIGHT GAIN
Number of deaths associated with weight gains of 2.5 and 12 kg over 10 years, as related to all body mass index measurements (BMIs) and BMIs >27 (per 100,000 persons in U.S. population).
Source: Fontaine et al. Psychiatry Res 2001;101:277-88.The prevalence of new-onset diabetes with use of specific antipsychotics is unknown. Most information is contained in case reports, and proper epidemiologic studies await publication.
The most detailed report—a pooled study of published cases related to clozapine use—comes from the FDA’s Center for Drug Evaluation and Research.8 In this study, the authors identified 384 reports of diabetes that developed (in 242 patients) or was exacerbated (in 54 patients) in association with clozapine. Patient mean age was 40, and diabetes occurred more commonly in women than in men.
Diabetes developed most commonly within 6 months of starting treatment with clozapine, and one patient developed diabetes after a single 500-mg dose. Metabolic acidosis or ketosis occurred in 80 cases, and 25 subjects died during hyperglycemic episodes. Stopping clozapine or reducing the dosage improved glycemic control in 46 patients.8
Figure 3 INCREASED MORBIDITY ASSOCIATED WITH WEIGHT GAIN
New cases of impaired glucose tolerance and hypertension that developed with weight gains of 2.5 and 12 kg over 10 years (per 100,000 persons in U.S. population).
Source: Fontaine et al. Psychiatry Res 2001;101:277-88.During antipsychotic therapy, it is important to measure patients’ fasting plasma glucose at least annually—and more often for high-risk patients (Table 1). The American Diabetes Association defines diabetes as a fasting serum or plasma glucose 126 mg/dl or a 2-hour postprandial serum or plasma glucose 200 mg/dl. In all patients, these tests should be repeated to confirm the diagnosis. Oral glucose tolerance testing is less convenient than fasting plasma glucose testing but more sensitive in identifying changes in carbohydrate metabolism.
As with weight gain, it is easier to prevent diabetes than to treat it. The psychiatrist can best help the patient with emerging carbohydrate dysregulation by collaborating with an internist, family physician, or endocrinologist.
Table 1
FACTORS RELATED TO HIGH RISK OF DEVELOPING TYPE 2 DIABETES
|
| Source: American Diabetes Association |
Weight gain with diabetes drugs Weight gain is associated not only with the use of antipsychotics but also with four classes of oral agents used to treat type 2 diabetes: sulfonylureas, meglitinides, phenylalanine derivatives, and thiazolidinediones. One class—biguanides—contributes to weight reduction, and one—alpha-glucosidase inhibitors—has a variable effect on body weight. These drugs also vary in their effects on serum lipids, including total cholesterol, LDL and HDL cholesterol, and triglycerides (Table 2).9
Many patients with type 2 diabetes require more than one agent to control plasma glucose. With time, insulin deficiency becomes more marked, and insulin therapy is frequently added to the regimen. Hypertension and hyperlipidemia are also very common in patients with type 2 diabetes and require medication to reduce the risk of cardiovascular events.10 As a result, the diabetic patient requiring antipsychotic drugs will likely need polypharmacy, and many of the drugs that might be used may lead to weight gain.
Assessing, managing weight gain
During each visit for the patient with schizophrenia, it is important to routinely weigh those receiving antipsychotics and ask about polydipsia and polyuria, which are early signs of incipient diabetes. A patient who is gaining significant weight (7% of baseline) while taking an antipsychotic and has risk factors for cardiovascular events (e.g., smoking, hypertension, hypertriglyceridemia) is a candidate for a change in antipsychotics.
Try to weigh patients at approximately the same time of day at each visit to compensate for possible diurnal weight changes related to polydipsia-hyponatremia syndrome.11 Patients with this syndrome can gain 5 to 10 lbs (or more) per day and excrete the retained fluid at night. It occurs in 5 to 10% of chronically psychotic patients requiring institutional care and in 1 to 2% of outpatients. Patients with schizophrenia complicated by this syndrome may manifest polydipsia and polyuria secondary to psychosis rather than emerging diabetes. Thus, the clinician must be alert to both diabetes and the polydipsia-hyponatremia syndrome in this setting.
Weight-control approaches
Patients who are taking sedating antipsychotics (e.g., clozapine, olanzapine, or low-potency phenothiazines) may gain up to 30 lbs per year if they become physically inactive and do not reduce their food consumption. Thus, it is important to work with such patients to decrease their caloric intake.
A weight-loss program that produces a loss of 0.5 to 1% of body weight per week is considered safe and acceptable.12 Mild to moderate obesity may be managed by reducing food intake by 500 calories and exercising 30 minutes each day.
CBT Cognitive-behavioral therapy (CBT) may help stem weight gain associated with antipsychotic use. Umbricht et al provided CBT to six patients with chronic psychosis who were receiving clozapine or olanzapine. Therapists in group and individual sessions focused on the causes of weight gain, lowcalorie nutrition, weight-loss guidelines, exercise programs, and relaxation strategies. Across 8 weeks, patients’ mean BMI decreased from 29.6 to 25.1 kg/m2
Table 2
METABOLIC EFFECTS OF ORAL ANTIHYPERGLYCEMIC DRUGS
| Class | Body weight | Total cholesterol | LDL | HDL | Triglycerides |
|---|---|---|---|---|---|
| Sulfonylureas Glipizide Glyburide Glimepiride | ▲ | ◄► | ◄► | ◄► | ◄► |
| Meglitinides Repaglinide | ▲ | ◄► | ◄► | ◄► | ◄► |
| Phenylalanine derivatives Nateglinide | ▲ | ◄► | ◄► | ◄► | ◄► |
| Biguanides Metformin | ▼ | ▼ | ▼ | ▲ | ▼ |
| Thiazolidinediones Pioglitazone Rosiglitazone | ▲ | ▲ | ▲ | ▲ | ▼ |
| Alpha-glucosidase inhibitors Acarbose Miglitol | ◄► | ◄► | ◄► | ◄► | ◄► |
| ▲ Increase ▼ Decrease ◄► Neutral effect/no change | |||||
Weight management program The Weight Watchers weight management program has shown mild success when offered to men and women with schizophrenia or schizoaffective disorder. Twenty-one patients who had gained an average of 32 lbs while taking olanzapine were enrolled in a Weight Watchers program at a psychiatric center.14 Mean starting BMI was 32 kg/m2 among the 11 patients who completed the 10-week program. Those 11 lost an average of 5 lbs.
All seven men lost weight. Three of the four women gained weight, and one woman lost 13 lbs. Study subjects remained clinically stable during the 10-week study. Two of the three women who did not lose weight had disabling psychiatric symptoms. Participation rates were similar to those of typical Weight Watchers clientele, suggesting that patients requiring antipsychotics might benefit from treatments used for other obese patients.
Patient education Educating patients about nutrition and exercise may help them control their rate of weight gain during antipsychotic therapy.
Littrell et al provided such an educational program for 1 hour per week for 4 months to six men and six women taking olanzapine for schizophrenia or schizoaffective disorder.15 Patients in the behavioral group gained 0.5 kg, compared with a control group that gained 2.9 kg. Mean increase in BMI was less for the behavioral group (0.3 kg/m2) than for the control patients (0.9 kg/m2). Men in both groups gained more weight than did women.
Pharmacologic approaches
Antiobesity medications are generally reserved for patients with a BMI 30 kg/m2 (threshold for obesity) or for those with a BMI 27 kg/m2 (threshold for overweight is 25 kg/m2) who have additional risk factors for cardiovascular disease, stroke, or diabetes.16
For patients with schizophrenia, who typically have a BMI 27 kg/m2, the presence of these risk factors alone may be enough to warrant consideration of an antiobesity agent. Adding any new drug to a patient’s regimen, however, increases the risk of an adverse interaction.
Antiobesity drugs work by a variety of mechanisms, including decreasing appetite, decreasing fat absorption, and increasing energy expenditure. Drugs may reduce caloric intake by decreasing appetite (anorectic drugs) or increasing satiety (appetite suppressants). Centrally-acting sympathomimetics or serotonergic drugs may suppress appetite.
In studies up to 2 years, the appetite suppressant sibutramine, with mixed serotonergic and noradrenergic reuptake inhibition properties, has been shown to cause more weight loss than a placebo in populations without schizophrenia.17 According to one case report, sibutramine use was associated with new-onset psychosis.18
Common side effects of sibutramine include headache, dry mouth, anorexia, constipation, and insomnia. Regular monitoring of blood pressure is required. Do not prescribe this drug for patients with cardiovascular disease, and avoid co-prescribing with MAO inhibitors and serotonergics.
Orlistat reduces fat absorption from the GI tract.19 Common side effects are largely confined to the GI tract and include oily spotting, flatulence, fecal urgency, fatty/oily stool, and oily evacuation.
Combination therapies
Researchers are studying whether adding adjunctive agents to antipsychotics reduces weight gain.
Clozapine plus quetiapine A group of 65 patients who experienced a mean body weight increase of 6.5 kg while taking clozapine for 6 months were then given clozapine plus quetiapine at chlorpromazine-equivalent dosing during the next 10 months. The patients lost a mean of 4.2 kg, and their glycemic control improved. Elevated glycosylated hemoglobin (HbA1c) became normal in those subjects (20% of participants) who had developed type 2 diabetes while taking clozapine alone. The authors theorized that the weight loss diminished insulin resistance, leading to better control of serum glucose levels.20
Olanzapine plus amantadine A group of 12 outpatients with axis I or II diagnoses had responded well clinically to olanzapine but had gained an average 7.3 kg over 1 to 11 months. In an open-label study, they continued their dosages of olanzapine and also were given amantadine, 100 to 300 mg/d. Amantadine was chosen for this trial because of its possible release of dopamine.
No dietary changes were made, but subjects gained no additional weight after amantadine was added. Over the next 3 to 6 months, they lost a mean 3.5 kg, which was 50% of the weight gain associated with olanzapine administration.21
Clozapine plus topiramate In clinical trials, the anticonvulsant topiramate has been associated with significant weight loss for up to 12 months in patients with seizure disorders.22 This agent, which also has mood-stabilizing effects, may be useful both for mood stabilization and weight loss in tandem with antipsychotic therapy.
In a case study,23 a 29-year-old man with schizophrenia who failed several trials of antipsychotic drugs experienced significant improvement with clozapine, 800 mg/d. Over 2 years, however, he developed myoclonic jerks and gained 45.5 kg (a 49% increase over baseline). When topiramate was added, starting with 25 mg/d and increasing to 125 mg/d, his mood improved and the myoclonic jerks stopped. During 5 months of combination therapy, the patient lost 21 kg without changing his eating habits.
Olanzapine and nizatidine Agents that block histamine (H 2) receptors in the digestive tract may be associated with weight loss when given at high doses, although the mechanism by which they contribute to weight loss is unclear. In a double-blind, placebo-controlled study,24 the H 2 blocker nizatidine was given to patients with schizophrenia who were taking olanzapine, 5 to 20 mg/d. In a 16-week trial, 132 patients were randomized to receive adjunctive treatment with low-dose nizatidine (150 mg bid), high-dose nizatidine (300 mg bid), or a placebo.
After 16 weeks, nizatidine demonstrated a dose-response effect when combined with olanzapine. Average weight gain was:
- 5.51 kg with a placebo
- 4.41 kg with low-dose nizatidine
- 2.76 kg with high-dose nizatidine (p =0.02 compared with a placebo).
In the high-dose nizatidine group, only 6% of patients gained more than 10 kg, and weight gain leveled off by week eight. Adverse events and clinical improvements were similar in the three groups.
Related resources
- Weight gain: A growing problem in schizophrenia management. J Clin Psychiatry 2001;62(suppl 7).
- Weight gain associated with the use of psychotropic medications. J Clin Psychiatry 1999;60(suppl 2).
- Effects of atypical antipsychotics on body weight and glucose regulation. J Clin Psychiatry 2001;62(suppl 23).
- National Heart, Lung, and Blood Institute. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults. www.nhlbi.nih.gov/guidelines/obesity/ob_home.htm
Drug brand names
- Amantadine • Symmetrel
- Clozapine • Clozaril
- Nizatidine • Axid
- Olanzapine • Zyprexa
- Orlistat • Xenical
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Sibutramine • Meridia
- Topiramate • Topamax
- Ziprasidone • Geodon
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article.
Weight gain is a potential problem for all patients who require treatment with antipsychotics. Those with schizophrenia face double jeopardy. Both the disorder and the use of virtually any available antipsychotic drug may be associated with weight gain, new-onset glucose intolerance, and type 2 diabetes mellitus.
Because of the cardiovascular risks and other morbidity associated with weight gain and glucose dysregulation,1 the psychiatrist must remain vigilant and manage these complications aggressively. In this article, we offer insights into the prevention and management of metabolic complications associated with the use of antipsychotic agents in patients with schizophrenia.
Weight gain and antipsychotics
Weight change was recognized as a feature of schizophrenia even before antipsychotic drugs were introduced in the 1950s.2 Schizophrenia—independent of drug treatment—also is a risk factor for the development of type 2 diabetes. In persons with schizophrenia, serum glucose levels increase more slowly, decline more gradually, and represent higher-than-normal reference values.3
Figure 1 WEIGHT GAIN ASSOCIATED WITH ANTIPSYCHOTIC DRUG ADMINISTRATION
Values represent estimates of drug-induced weight gain after 10 weeks of drug administration.
Source: Allison et al. Am J Psychiatry 1999;156:1686-96; Brecher et al. Int J Psychiatry Clin Pract 2000;4:287-92.In 1999, Allison et al assessed the effects of conventional and atypical antipsychotics on body weight. Using 81 published articles, they estimated and compared weight changes associated with 10 antipsychotic agents and a placebo when given at standard dosages for 10 weeks.4 Comparative data on quetiapine, which were insufficient in 1999, have since been added (Figure 1).5
Patients who received a placebo lost 0.74 kg across 10 weeks. Weight changes with the conventional agents ranged from a reduction of 0.39 kg with molindone to an increase of 3.19 kg with thioridazine. Weight gains also were seen with all of the newer atypical agents, including clozapine (+4.45 kg), olanzapine (+4.15 kg), risperidone (+2.10 kg), and ziprasidone (+0.04 kg).
Fontaine et al have estimated that weight gain in patients with schizophrenia has its greatest impact on mortality in two scenarios:
- when patients are overweight before they start antipsychotic medication
- with greater degrees of weight gain across 10 years (Figure 2).
Whatever a patient’s starting weight, substantial weight gain with antipsychotic therapy increases the risk of impaired glucose tolerance and hypertension (Figure 3).6
Schizophrenia and diabetes
The prevalence of type 2 diabetes in patients with schizophrenia increased from 4.2% in 1956 to 17.2% in 1968, related in part to the introduction of phenothiazines.7 A recent study of data collected by the Schizophrenia Patient Outcomes Research Team (PORT)2 found higher rates of diabetes in persons with schizophrenia (lifetime prevalence, 14.9%) than in the general population (approximately 7.3%).1 Most patients in the PORT study were taking older antipsychotics, the use of which has occasionally been associated with carbohydrate dysregulation.
Figure 2 INCREASED MORTALITY ASSOCIATED WITH WEIGHT GAIN
Number of deaths associated with weight gains of 2.5 and 12 kg over 10 years, as related to all body mass index measurements (BMIs) and BMIs >27 (per 100,000 persons in U.S. population).
Source: Fontaine et al. Psychiatry Res 2001;101:277-88.The prevalence of new-onset diabetes with use of specific antipsychotics is unknown. Most information is contained in case reports, and proper epidemiologic studies await publication.
The most detailed report—a pooled study of published cases related to clozapine use—comes from the FDA’s Center for Drug Evaluation and Research.8 In this study, the authors identified 384 reports of diabetes that developed (in 242 patients) or was exacerbated (in 54 patients) in association with clozapine. Patient mean age was 40, and diabetes occurred more commonly in women than in men.
Diabetes developed most commonly within 6 months of starting treatment with clozapine, and one patient developed diabetes after a single 500-mg dose. Metabolic acidosis or ketosis occurred in 80 cases, and 25 subjects died during hyperglycemic episodes. Stopping clozapine or reducing the dosage improved glycemic control in 46 patients.8
Figure 3 INCREASED MORBIDITY ASSOCIATED WITH WEIGHT GAIN
New cases of impaired glucose tolerance and hypertension that developed with weight gains of 2.5 and 12 kg over 10 years (per 100,000 persons in U.S. population).
Source: Fontaine et al. Psychiatry Res 2001;101:277-88.During antipsychotic therapy, it is important to measure patients’ fasting plasma glucose at least annually—and more often for high-risk patients (Table 1). The American Diabetes Association defines diabetes as a fasting serum or plasma glucose 126 mg/dl or a 2-hour postprandial serum or plasma glucose 200 mg/dl. In all patients, these tests should be repeated to confirm the diagnosis. Oral glucose tolerance testing is less convenient than fasting plasma glucose testing but more sensitive in identifying changes in carbohydrate metabolism.
As with weight gain, it is easier to prevent diabetes than to treat it. The psychiatrist can best help the patient with emerging carbohydrate dysregulation by collaborating with an internist, family physician, or endocrinologist.
Table 1
FACTORS RELATED TO HIGH RISK OF DEVELOPING TYPE 2 DIABETES
|
| Source: American Diabetes Association |
Weight gain with diabetes drugs Weight gain is associated not only with the use of antipsychotics but also with four classes of oral agents used to treat type 2 diabetes: sulfonylureas, meglitinides, phenylalanine derivatives, and thiazolidinediones. One class—biguanides—contributes to weight reduction, and one—alpha-glucosidase inhibitors—has a variable effect on body weight. These drugs also vary in their effects on serum lipids, including total cholesterol, LDL and HDL cholesterol, and triglycerides (Table 2).9
Many patients with type 2 diabetes require more than one agent to control plasma glucose. With time, insulin deficiency becomes more marked, and insulin therapy is frequently added to the regimen. Hypertension and hyperlipidemia are also very common in patients with type 2 diabetes and require medication to reduce the risk of cardiovascular events.10 As a result, the diabetic patient requiring antipsychotic drugs will likely need polypharmacy, and many of the drugs that might be used may lead to weight gain.
Assessing, managing weight gain
During each visit for the patient with schizophrenia, it is important to routinely weigh those receiving antipsychotics and ask about polydipsia and polyuria, which are early signs of incipient diabetes. A patient who is gaining significant weight (7% of baseline) while taking an antipsychotic and has risk factors for cardiovascular events (e.g., smoking, hypertension, hypertriglyceridemia) is a candidate for a change in antipsychotics.
Try to weigh patients at approximately the same time of day at each visit to compensate for possible diurnal weight changes related to polydipsia-hyponatremia syndrome.11 Patients with this syndrome can gain 5 to 10 lbs (or more) per day and excrete the retained fluid at night. It occurs in 5 to 10% of chronically psychotic patients requiring institutional care and in 1 to 2% of outpatients. Patients with schizophrenia complicated by this syndrome may manifest polydipsia and polyuria secondary to psychosis rather than emerging diabetes. Thus, the clinician must be alert to both diabetes and the polydipsia-hyponatremia syndrome in this setting.
Weight-control approaches
Patients who are taking sedating antipsychotics (e.g., clozapine, olanzapine, or low-potency phenothiazines) may gain up to 30 lbs per year if they become physically inactive and do not reduce their food consumption. Thus, it is important to work with such patients to decrease their caloric intake.
A weight-loss program that produces a loss of 0.5 to 1% of body weight per week is considered safe and acceptable.12 Mild to moderate obesity may be managed by reducing food intake by 500 calories and exercising 30 minutes each day.
CBT Cognitive-behavioral therapy (CBT) may help stem weight gain associated with antipsychotic use. Umbricht et al provided CBT to six patients with chronic psychosis who were receiving clozapine or olanzapine. Therapists in group and individual sessions focused on the causes of weight gain, lowcalorie nutrition, weight-loss guidelines, exercise programs, and relaxation strategies. Across 8 weeks, patients’ mean BMI decreased from 29.6 to 25.1 kg/m2
Table 2
METABOLIC EFFECTS OF ORAL ANTIHYPERGLYCEMIC DRUGS
| Class | Body weight | Total cholesterol | LDL | HDL | Triglycerides |
|---|---|---|---|---|---|
| Sulfonylureas Glipizide Glyburide Glimepiride | ▲ | ◄► | ◄► | ◄► | ◄► |
| Meglitinides Repaglinide | ▲ | ◄► | ◄► | ◄► | ◄► |
| Phenylalanine derivatives Nateglinide | ▲ | ◄► | ◄► | ◄► | ◄► |
| Biguanides Metformin | ▼ | ▼ | ▼ | ▲ | ▼ |
| Thiazolidinediones Pioglitazone Rosiglitazone | ▲ | ▲ | ▲ | ▲ | ▼ |
| Alpha-glucosidase inhibitors Acarbose Miglitol | ◄► | ◄► | ◄► | ◄► | ◄► |
| ▲ Increase ▼ Decrease ◄► Neutral effect/no change | |||||
Weight management program The Weight Watchers weight management program has shown mild success when offered to men and women with schizophrenia or schizoaffective disorder. Twenty-one patients who had gained an average of 32 lbs while taking olanzapine were enrolled in a Weight Watchers program at a psychiatric center.14 Mean starting BMI was 32 kg/m2 among the 11 patients who completed the 10-week program. Those 11 lost an average of 5 lbs.
All seven men lost weight. Three of the four women gained weight, and one woman lost 13 lbs. Study subjects remained clinically stable during the 10-week study. Two of the three women who did not lose weight had disabling psychiatric symptoms. Participation rates were similar to those of typical Weight Watchers clientele, suggesting that patients requiring antipsychotics might benefit from treatments used for other obese patients.
Patient education Educating patients about nutrition and exercise may help them control their rate of weight gain during antipsychotic therapy.
Littrell et al provided such an educational program for 1 hour per week for 4 months to six men and six women taking olanzapine for schizophrenia or schizoaffective disorder.15 Patients in the behavioral group gained 0.5 kg, compared with a control group that gained 2.9 kg. Mean increase in BMI was less for the behavioral group (0.3 kg/m2) than for the control patients (0.9 kg/m2). Men in both groups gained more weight than did women.
Pharmacologic approaches
Antiobesity medications are generally reserved for patients with a BMI 30 kg/m2 (threshold for obesity) or for those with a BMI 27 kg/m2 (threshold for overweight is 25 kg/m2) who have additional risk factors for cardiovascular disease, stroke, or diabetes.16
For patients with schizophrenia, who typically have a BMI 27 kg/m2, the presence of these risk factors alone may be enough to warrant consideration of an antiobesity agent. Adding any new drug to a patient’s regimen, however, increases the risk of an adverse interaction.
Antiobesity drugs work by a variety of mechanisms, including decreasing appetite, decreasing fat absorption, and increasing energy expenditure. Drugs may reduce caloric intake by decreasing appetite (anorectic drugs) or increasing satiety (appetite suppressants). Centrally-acting sympathomimetics or serotonergic drugs may suppress appetite.
In studies up to 2 years, the appetite suppressant sibutramine, with mixed serotonergic and noradrenergic reuptake inhibition properties, has been shown to cause more weight loss than a placebo in populations without schizophrenia.17 According to one case report, sibutramine use was associated with new-onset psychosis.18
Common side effects of sibutramine include headache, dry mouth, anorexia, constipation, and insomnia. Regular monitoring of blood pressure is required. Do not prescribe this drug for patients with cardiovascular disease, and avoid co-prescribing with MAO inhibitors and serotonergics.
Orlistat reduces fat absorption from the GI tract.19 Common side effects are largely confined to the GI tract and include oily spotting, flatulence, fecal urgency, fatty/oily stool, and oily evacuation.
Combination therapies
Researchers are studying whether adding adjunctive agents to antipsychotics reduces weight gain.
Clozapine plus quetiapine A group of 65 patients who experienced a mean body weight increase of 6.5 kg while taking clozapine for 6 months were then given clozapine plus quetiapine at chlorpromazine-equivalent dosing during the next 10 months. The patients lost a mean of 4.2 kg, and their glycemic control improved. Elevated glycosylated hemoglobin (HbA1c) became normal in those subjects (20% of participants) who had developed type 2 diabetes while taking clozapine alone. The authors theorized that the weight loss diminished insulin resistance, leading to better control of serum glucose levels.20
Olanzapine plus amantadine A group of 12 outpatients with axis I or II diagnoses had responded well clinically to olanzapine but had gained an average 7.3 kg over 1 to 11 months. In an open-label study, they continued their dosages of olanzapine and also were given amantadine, 100 to 300 mg/d. Amantadine was chosen for this trial because of its possible release of dopamine.
No dietary changes were made, but subjects gained no additional weight after amantadine was added. Over the next 3 to 6 months, they lost a mean 3.5 kg, which was 50% of the weight gain associated with olanzapine administration.21
Clozapine plus topiramate In clinical trials, the anticonvulsant topiramate has been associated with significant weight loss for up to 12 months in patients with seizure disorders.22 This agent, which also has mood-stabilizing effects, may be useful both for mood stabilization and weight loss in tandem with antipsychotic therapy.
In a case study,23 a 29-year-old man with schizophrenia who failed several trials of antipsychotic drugs experienced significant improvement with clozapine, 800 mg/d. Over 2 years, however, he developed myoclonic jerks and gained 45.5 kg (a 49% increase over baseline). When topiramate was added, starting with 25 mg/d and increasing to 125 mg/d, his mood improved and the myoclonic jerks stopped. During 5 months of combination therapy, the patient lost 21 kg without changing his eating habits.
Olanzapine and nizatidine Agents that block histamine (H 2) receptors in the digestive tract may be associated with weight loss when given at high doses, although the mechanism by which they contribute to weight loss is unclear. In a double-blind, placebo-controlled study,24 the H 2 blocker nizatidine was given to patients with schizophrenia who were taking olanzapine, 5 to 20 mg/d. In a 16-week trial, 132 patients were randomized to receive adjunctive treatment with low-dose nizatidine (150 mg bid), high-dose nizatidine (300 mg bid), or a placebo.
After 16 weeks, nizatidine demonstrated a dose-response effect when combined with olanzapine. Average weight gain was:
- 5.51 kg with a placebo
- 4.41 kg with low-dose nizatidine
- 2.76 kg with high-dose nizatidine (p =0.02 compared with a placebo).
In the high-dose nizatidine group, only 6% of patients gained more than 10 kg, and weight gain leveled off by week eight. Adverse events and clinical improvements were similar in the three groups.
Related resources
- Weight gain: A growing problem in schizophrenia management. J Clin Psychiatry 2001;62(suppl 7).
- Weight gain associated with the use of psychotropic medications. J Clin Psychiatry 1999;60(suppl 2).
- Effects of atypical antipsychotics on body weight and glucose regulation. J Clin Psychiatry 2001;62(suppl 23).
- National Heart, Lung, and Blood Institute. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults. www.nhlbi.nih.gov/guidelines/obesity/ob_home.htm
Drug brand names
- Amantadine • Symmetrel
- Clozapine • Clozaril
- Nizatidine • Axid
- Olanzapine • Zyprexa
- Orlistat • Xenical
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Sibutramine • Meridia
- Topiramate • Topamax
- Ziprasidone • Geodon
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article.
1. Mokdad AH, Bowman BA, Ford ES, Vinicor F, Marks JS, Koplan JP. The continuing epidemics of obesity and diabetes in the United States. JAMA 2001;286:1195-1200.
2. Dixon L, Weiden P, Delahanty J, et al. Prevalence and correlates of diabetes in national schizophrenia samples. Schizophr Bull 2000;26:903-12.
3. Braceland FJ, Meduna LJ, Vaichulis JA. Delayed action of insulin in schizophrenia. Am J Psychiatry 1945;102:108-10.
4. Allison DB, Mentore JL, Heo M, et al. Antipsychotic-induced weight gain: a comprehensive research synthesis. Am J Psychiatry 1999;156:1686-96.
5. Brecher M, Rak IW, Westhead EK. The long-term effect of quetiapine (“Seroquel’) monotherapy on weight in patients with schizophrenia. Int J Psychiatry Clin Pract 2000;4:287-92.
6. Fontaine KR, Heo M, Harrigan EP, Shear CL, Lakshiminarayanan M. Estimating the consequences of anti-psychotic induced weight gain on health and mortality rate. Psychiatry Res 2001;101:277-88.
7. Theonnard-Neumann E. Phenothiazines and diabetes in hospitalized women. Am J Psychiatry 1968;124:978-82.
8. Koller E, Schneider B, Bennett K, Dubitsky G. Clozapine-associated diabetes. Am J Med 2001;111:716-23.
9. Pendergrass ML. Pathophysiology and management of type 2 diabetes. In: Giles TD, Sowers JR, Weber MA (eds). Diabetes & cardiovascular disease: a practical primer. New Orleans: Institute of Professional Education, 2000;15-40.
10. Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults. Findings from the Third National Health and Nutrition Examination Survey. JAMA 2002;287:356-9.
11. Vieweg WVR, Leadbetter RA. The polydipsia-hyponatremia syndrome. Epidemiology, clinical features, and treatment. CNS Drugs 1997;7:121-38.
12. Thomas PR. Weighing the options: criteria for evaluating weight management programs. Washington, DC: National Academy Press, 1995.
13. Umbricht D, Flury H, Bridler R. Cognitive behavioral therapy for weight gain. Am J Psychiatry 2001;158:971.-
14. Ball M, Coons V, Buchanan R. A program for treating olanzapine-related weight gain. Psychiatric Services 2001;52:967-9.
15. Littrell KH, Petty RG, Hilligoss NM, Peabody CD, Johnson CG. Educational interventions for the management of antipsychotic-related weight gain. 41st annual meeting of the New Clinical Drug Evaluation Unit, Phoenix, AZ, May 28-31, 2001.
16. Greenberg I, Chan S, Blackburn GL. Nonpharmacologic and pharmacologic management of weight gain. J Clin Psychiatry 1999;60(suppl 21):31-6.
17. Wirth A, Krause J. Long-term weight loss with sibutramine: a randomized controlled trial. JAMA 2001;286:1331-9.
18. Taflinski T, Chojnacka J. Sibutramine-associated psychotic episode. Am J Psychiatry 2001;157:2057-8.
19. Glazer G. Long-term pharmacotherapy of obesity 2000. Arch Intern Med 2001;161:1814-24.
20. Reinstein M, Sirotovskaya L, Jones L. Effect of clozapine-quetiapine combination therapy on weight and glycaemic control. Clin Drug Invest 1999;18:99-104.
21. Floris M, Lejeune J, Deberdt W. Effect of amantadine on weight gain during olanzapine treatment. Eur Neuropsychopharmacol 2001;11:181-2.
22. Norton J, Potter D, Edwards K. Sustained weight loss associated with topiramate [abstract]. Epilepsia 1997;38(suppl 3):60.-
23. Dursun SM, Devarajan S. Clozapine weight gain, plus topiramate weight loss. Can J Psychiatry 2000;45:198.-
24. Breier A, Tanaka Y, Roychowdhury S, Clark WS. Nizatidine for the prevention of olanzapine-associated weight gain in schizophrenia and related disorders. A randomized controlled double blind study. 41st annual meeting of the New Clinical Drug Evaluation Unit, Phoenix, AZ, May 28-31 2001.
1. Mokdad AH, Bowman BA, Ford ES, Vinicor F, Marks JS, Koplan JP. The continuing epidemics of obesity and diabetes in the United States. JAMA 2001;286:1195-1200.
2. Dixon L, Weiden P, Delahanty J, et al. Prevalence and correlates of diabetes in national schizophrenia samples. Schizophr Bull 2000;26:903-12.
3. Braceland FJ, Meduna LJ, Vaichulis JA. Delayed action of insulin in schizophrenia. Am J Psychiatry 1945;102:108-10.
4. Allison DB, Mentore JL, Heo M, et al. Antipsychotic-induced weight gain: a comprehensive research synthesis. Am J Psychiatry 1999;156:1686-96.
5. Brecher M, Rak IW, Westhead EK. The long-term effect of quetiapine (“Seroquel’) monotherapy on weight in patients with schizophrenia. Int J Psychiatry Clin Pract 2000;4:287-92.
6. Fontaine KR, Heo M, Harrigan EP, Shear CL, Lakshiminarayanan M. Estimating the consequences of anti-psychotic induced weight gain on health and mortality rate. Psychiatry Res 2001;101:277-88.
7. Theonnard-Neumann E. Phenothiazines and diabetes in hospitalized women. Am J Psychiatry 1968;124:978-82.
8. Koller E, Schneider B, Bennett K, Dubitsky G. Clozapine-associated diabetes. Am J Med 2001;111:716-23.
9. Pendergrass ML. Pathophysiology and management of type 2 diabetes. In: Giles TD, Sowers JR, Weber MA (eds). Diabetes & cardiovascular disease: a practical primer. New Orleans: Institute of Professional Education, 2000;15-40.
10. Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults. Findings from the Third National Health and Nutrition Examination Survey. JAMA 2002;287:356-9.
11. Vieweg WVR, Leadbetter RA. The polydipsia-hyponatremia syndrome. Epidemiology, clinical features, and treatment. CNS Drugs 1997;7:121-38.
12. Thomas PR. Weighing the options: criteria for evaluating weight management programs. Washington, DC: National Academy Press, 1995.
13. Umbricht D, Flury H, Bridler R. Cognitive behavioral therapy for weight gain. Am J Psychiatry 2001;158:971.-
14. Ball M, Coons V, Buchanan R. A program for treating olanzapine-related weight gain. Psychiatric Services 2001;52:967-9.
15. Littrell KH, Petty RG, Hilligoss NM, Peabody CD, Johnson CG. Educational interventions for the management of antipsychotic-related weight gain. 41st annual meeting of the New Clinical Drug Evaluation Unit, Phoenix, AZ, May 28-31, 2001.
16. Greenberg I, Chan S, Blackburn GL. Nonpharmacologic and pharmacologic management of weight gain. J Clin Psychiatry 1999;60(suppl 21):31-6.
17. Wirth A, Krause J. Long-term weight loss with sibutramine: a randomized controlled trial. JAMA 2001;286:1331-9.
18. Taflinski T, Chojnacka J. Sibutramine-associated psychotic episode. Am J Psychiatry 2001;157:2057-8.
19. Glazer G. Long-term pharmacotherapy of obesity 2000. Arch Intern Med 2001;161:1814-24.
20. Reinstein M, Sirotovskaya L, Jones L. Effect of clozapine-quetiapine combination therapy on weight and glycaemic control. Clin Drug Invest 1999;18:99-104.
21. Floris M, Lejeune J, Deberdt W. Effect of amantadine on weight gain during olanzapine treatment. Eur Neuropsychopharmacol 2001;11:181-2.
22. Norton J, Potter D, Edwards K. Sustained weight loss associated with topiramate [abstract]. Epilepsia 1997;38(suppl 3):60.-
23. Dursun SM, Devarajan S. Clozapine weight gain, plus topiramate weight loss. Can J Psychiatry 2000;45:198.-
24. Breier A, Tanaka Y, Roychowdhury S, Clark WS. Nizatidine for the prevention of olanzapine-associated weight gain in schizophrenia and related disorders. A randomized controlled double blind study. 41st annual meeting of the New Clinical Drug Evaluation Unit, Phoenix, AZ, May 28-31 2001.
Weight control and antipsychotics: How to tip the scales away from diabetes and heart disease
Weight gain is a potential problem for all patients who require treatment with antipsychotics. Those with schizophrenia face double jeopardy. Both the disorder and the use of virtually any available antipsychotic drug may be associated with weight gain, new-onset glucose intolerance, and type 2 diabetes mellitus.
Because of the cardiovascular risks and other morbidity associated with weight gain and glucose dysregulation,1 the psychiatrist must remain vigilant and manage these complications aggressively. In this article, we offer insights into the prevention and management of metabolic complications associated with the use of antipsychotic agents in patients with schizophrenia.
Weight gain and antipsychotics
Weight change was recognized as a feature of schizophrenia even before antipsychotic drugs were introduced in the 1950s.2 Schizophrenia—independent of drug treatment—also is a risk factor for the development of type 2 diabetes. In persons with schizophrenia, serum glucose levels increase more slowly, decline more gradually, and represent higher-than-normal reference values.3
Figure 1 WEIGHT GAIN ASSOCIATED WITH ANTIPSYCHOTIC DRUG ADMINISTRATION
Values represent estimates of drug-induced weight gain after 10 weeks of drug administration.
Source: Allison et al. Am J Psychiatry 1999;156:1686-96; Brecher et al. Int J Psychiatry Clin Pract 2000;4:287-92.In 1999, Allison et al assessed the effects of conventional and atypical antipsychotics on body weight. Using 81 published articles, they estimated and compared weight changes associated with 10 antipsychotic agents and a placebo when given at standard dosages for 10 weeks.4 Comparative data on quetiapine, which were insufficient in 1999, have since been added (Figure 1).5
Patients who received a placebo lost 0.74 kg across 10 weeks. Weight changes with the conventional agents ranged from a reduction of 0.39 kg with molindone to an increase of 3.19 kg with thioridazine. Weight gains also were seen with all of the newer atypical agents, including clozapine (+4.45 kg), olanzapine (+4.15 kg), risperidone (+2.10 kg), and ziprasidone (+0.04 kg).
Fontaine et al have estimated that weight gain in patients with schizophrenia has its greatest impact on mortality in two scenarios:
- when patients are overweight before they start antipsychotic medication
- with greater degrees of weight gain across 10 years (Figure 2).
Whatever a patient’s starting weight, substantial weight gain with antipsychotic therapy increases the risk of impaired glucose tolerance and hypertension (Figure 3).6
Schizophrenia and diabetes
The prevalence of type 2 diabetes in patients with schizophrenia increased from 4.2% in 1956 to 17.2% in 1968, related in part to the introduction of phenothiazines.7 A recent study of data collected by the Schizophrenia Patient Outcomes Research Team (PORT)2 found higher rates of diabetes in persons with schizophrenia (lifetime prevalence, 14.9%) than in the general population (approximately 7.3%).1 Most patients in the PORT study were taking older antipsychotics, the use of which has occasionally been associated with carbohydrate dysregulation.
Figure 2 INCREASED MORTALITY ASSOCIATED WITH WEIGHT GAIN
Number of deaths associated with weight gains of 2.5 and 12 kg over 10 years, as related to all body mass index measurements (BMIs) and BMIs >27 (per 100,000 persons in U.S. population).
Source: Fontaine et al. Psychiatry Res 2001;101:277-88.The prevalence of new-onset diabetes with use of specific antipsychotics is unknown. Most information is contained in case reports, and proper epidemiologic studies await publication.
The most detailed report—a pooled study of published cases related to clozapine use—comes from the FDA’s Center for Drug Evaluation and Research.8 In this study, the authors identified 384 reports of diabetes that developed (in 242 patients) or was exacerbated (in 54 patients) in association with clozapine. Patient mean age was 40, and diabetes occurred more commonly in women than in men.
Diabetes developed most commonly within 6 months of starting treatment with clozapine, and one patient developed diabetes after a single 500-mg dose. Metabolic acidosis or ketosis occurred in 80 cases, and 25 subjects died during hyperglycemic episodes. Stopping clozapine or reducing the dosage improved glycemic control in 46 patients.8
Figure 3 INCREASED MORBIDITY ASSOCIATED WITH WEIGHT GAIN
New cases of impaired glucose tolerance and hypertension that developed with weight gains of 2.5 and 12 kg over 10 years (per 100,000 persons in U.S. population).
Source: Fontaine et al. Psychiatry Res 2001;101:277-88.During antipsychotic therapy, it is important to measure patients’ fasting plasma glucose at least annually—and more often for high-risk patients (Table 1). The American Diabetes Association defines diabetes as a fasting serum or plasma glucose 126 mg/dl or a 2-hour postprandial serum or plasma glucose 200 mg/dl. In all patients, these tests should be repeated to confirm the diagnosis. Oral glucose tolerance testing is less convenient than fasting plasma glucose testing but more sensitive in identifying changes in carbohydrate metabolism.
As with weight gain, it is easier to prevent diabetes than to treat it. The psychiatrist can best help the patient with emerging carbohydrate dysregulation by collaborating with an internist, family physician, or endocrinologist.
Table 1
FACTORS RELATED TO HIGH RISK OF DEVELOPING TYPE 2 DIABETES
|
| Source: American Diabetes Association |
Weight gain with diabetes drugs Weight gain is associated not only with the use of antipsychotics but also with four classes of oral agents used to treat type 2 diabetes: sulfonylureas, meglitinides, phenylalanine derivatives, and thiazolidinediones. One class—biguanides—contributes to weight reduction, and one—alpha-glucosidase inhibitors—has a variable effect on body weight. These drugs also vary in their effects on serum lipids, including total cholesterol, LDL and HDL cholesterol, and triglycerides (Table 2).9
Many patients with type 2 diabetes require more than one agent to control plasma glucose. With time, insulin deficiency becomes more marked, and insulin therapy is frequently added to the regimen. Hypertension and hyperlipidemia are also very common in patients with type 2 diabetes and require medication to reduce the risk of cardiovascular events.10 As a result, the diabetic patient requiring antipsychotic drugs will likely need polypharmacy, and many of the drugs that might be used may lead to weight gain.
Assessing, managing weight gain
During each visit for the patient with schizophrenia, it is important to routinely weigh those receiving antipsychotics and ask about polydipsia and polyuria, which are early signs of incipient diabetes. A patient who is gaining significant weight (7% of baseline) while taking an antipsychotic and has risk factors for cardiovascular events (e.g., smoking, hypertension, hypertriglyceridemia) is a candidate for a change in antipsychotics.
Try to weigh patients at approximately the same time of day at each visit to compensate for possible diurnal weight changes related to polydipsia-hyponatremia syndrome.11 Patients with this syndrome can gain 5 to 10 lbs (or more) per day and excrete the retained fluid at night. It occurs in 5 to 10% of chronically psychotic patients requiring institutional care and in 1 to 2% of outpatients. Patients with schizophrenia complicated by this syndrome may manifest polydipsia and polyuria secondary to psychosis rather than emerging diabetes. Thus, the clinician must be alert to both diabetes and the polydipsia-hyponatremia syndrome in this setting.
Weight-control approaches
Patients who are taking sedating antipsychotics (e.g., clozapine, olanzapine, or low-potency phenothiazines) may gain up to 30 lbs per year if they become physically inactive and do not reduce their food consumption. Thus, it is important to work with such patients to decrease their caloric intake.
A weight-loss program that produces a loss of 0.5 to 1% of body weight per week is considered safe and acceptable.12 Mild to moderate obesity may be managed by reducing food intake by 500 calories and exercising 30 minutes each day.
CBT Cognitive-behavioral therapy (CBT) may help stem weight gain associated with antipsychotic use. Umbricht et al provided CBT to six patients with chronic psychosis who were receiving clozapine or olanzapine. Therapists in group and individual sessions focused on the causes of weight gain, lowcalorie nutrition, weight-loss guidelines, exercise programs, and relaxation strategies. Across 8 weeks, patients’ mean BMI decreased from 29.6 to 25.1 kg/m2
Table 2
METABOLIC EFFECTS OF ORAL ANTIHYPERGLYCEMIC DRUGS
| Class | Body weight | Total cholesterol | LDL | HDL | Triglycerides |
|---|---|---|---|---|---|
| Sulfonylureas Glipizide Glyburide Glimepiride | ▲ | ◄► | ◄► | ◄► | ◄► |
| Meglitinides Repaglinide | ▲ | ◄► | ◄► | ◄► | ◄► |
| Phenylalanine derivatives Nateglinide | ▲ | ◄► | ◄► | ◄► | ◄► |
| Biguanides Metformin | ▼ | ▼ | ▼ | ▲ | ▼ |
| Thiazolidinediones Pioglitazone Rosiglitazone | ▲ | ▲ | ▲ | ▲ | ▼ |
| Alpha-glucosidase inhibitors Acarbose Miglitol | ◄► | ◄► | ◄► | ◄► | ◄► |
| ▲ Increase ▼ Decrease ◄► Neutral effect/no change | |||||
Weight management program The Weight Watchers weight management program has shown mild success when offered to men and women with schizophrenia or schizoaffective disorder. Twenty-one patients who had gained an average of 32 lbs while taking olanzapine were enrolled in a Weight Watchers program at a psychiatric center.14 Mean starting BMI was 32 kg/m2 among the 11 patients who completed the 10-week program. Those 11 lost an average of 5 lbs.
All seven men lost weight. Three of the four women gained weight, and one woman lost 13 lbs. Study subjects remained clinically stable during the 10-week study. Two of the three women who did not lose weight had disabling psychiatric symptoms. Participation rates were similar to those of typical Weight Watchers clientele, suggesting that patients requiring antipsychotics might benefit from treatments used for other obese patients.
Patient education Educating patients about nutrition and exercise may help them control their rate of weight gain during antipsychotic therapy.
Littrell et al provided such an educational program for 1 hour per week for 4 months to six men and six women taking olanzapine for schizophrenia or schizoaffective disorder.15 Patients in the behavioral group gained 0.5 kg, compared with a control group that gained 2.9 kg. Mean increase in BMI was less for the behavioral group (0.3 kg/m2) than for the control patients (0.9 kg/m2). Men in both groups gained more weight than did women.
Pharmacologic approaches
Antiobesity medications are generally reserved for patients with a BMI 30 kg/m2 (threshold for obesity) or for those with a BMI 27 kg/m2 (threshold for overweight is 25 kg/m2) who have additional risk factors for cardiovascular disease, stroke, or diabetes.16
For patients with schizophrenia, who typically have a BMI 27 kg/m2, the presence of these risk factors alone may be enough to warrant consideration of an antiobesity agent. Adding any new drug to a patient’s regimen, however, increases the risk of an adverse interaction.
Antiobesity drugs work by a variety of mechanisms, including decreasing appetite, decreasing fat absorption, and increasing energy expenditure. Drugs may reduce caloric intake by decreasing appetite (anorectic drugs) or increasing satiety (appetite suppressants). Centrally-acting sympathomimetics or serotonergic drugs may suppress appetite.
In studies up to 2 years, the appetite suppressant sibutramine, with mixed serotonergic and noradrenergic reuptake inhibition properties, has been shown to cause more weight loss than a placebo in populations without schizophrenia.17 According to one case report, sibutramine use was associated with new-onset psychosis.18
Common side effects of sibutramine include headache, dry mouth, anorexia, constipation, and insomnia. Regular monitoring of blood pressure is required. Do not prescribe this drug for patients with cardiovascular disease, and avoid co-prescribing with MAO inhibitors and serotonergics.
Orlistat reduces fat absorption from the GI tract.19 Common side effects are largely confined to the GI tract and include oily spotting, flatulence, fecal urgency, fatty/oily stool, and oily evacuation.
Combination therapies
Researchers are studying whether adding adjunctive agents to antipsychotics reduces weight gain.
Clozapine plus quetiapine A group of 65 patients who experienced a mean body weight increase of 6.5 kg while taking clozapine for 6 months were then given clozapine plus quetiapine at chlorpromazine-equivalent dosing during the next 10 months. The patients lost a mean of 4.2 kg, and their glycemic control improved. Elevated glycosylated hemoglobin (HbA1c) became normal in those subjects (20% of participants) who had developed type 2 diabetes while taking clozapine alone. The authors theorized that the weight loss diminished insulin resistance, leading to better control of serum glucose levels.20
Olanzapine plus amantadine A group of 12 outpatients with axis I or II diagnoses had responded well clinically to olanzapine but had gained an average 7.3 kg over 1 to 11 months. In an open-label study, they continued their dosages of olanzapine and also were given amantadine, 100 to 300 mg/d. Amantadine was chosen for this trial because of its possible release of dopamine.
No dietary changes were made, but subjects gained no additional weight after amantadine was added. Over the next 3 to 6 months, they lost a mean 3.5 kg, which was 50% of the weight gain associated with olanzapine administration.21
Clozapine plus topiramate In clinical trials, the anticonvulsant topiramate has been associated with significant weight loss for up to 12 months in patients with seizure disorders.22 This agent, which also has mood-stabilizing effects, may be useful both for mood stabilization and weight loss in tandem with antipsychotic therapy.
In a case study,23 a 29-year-old man with schizophrenia who failed several trials of antipsychotic drugs experienced significant improvement with clozapine, 800 mg/d. Over 2 years, however, he developed myoclonic jerks and gained 45.5 kg (a 49% increase over baseline). When topiramate was added, starting with 25 mg/d and increasing to 125 mg/d, his mood improved and the myoclonic jerks stopped. During 5 months of combination therapy, the patient lost 21 kg without changing his eating habits.
Olanzapine and nizatidine Agents that block histamine (H 2) receptors in the digestive tract may be associated with weight loss when given at high doses, although the mechanism by which they contribute to weight loss is unclear. In a double-blind, placebo-controlled study,24 the H 2 blocker nizatidine was given to patients with schizophrenia who were taking olanzapine, 5 to 20 mg/d. In a 16-week trial, 132 patients were randomized to receive adjunctive treatment with low-dose nizatidine (150 mg bid), high-dose nizatidine (300 mg bid), or a placebo.
After 16 weeks, nizatidine demonstrated a dose-response effect when combined with olanzapine. Average weight gain was:
- 5.51 kg with a placebo
- 4.41 kg with low-dose nizatidine
- 2.76 kg with high-dose nizatidine (p =0.02 compared with a placebo).
In the high-dose nizatidine group, only 6% of patients gained more than 10 kg, and weight gain leveled off by week eight. Adverse events and clinical improvements were similar in the three groups.
Related resources
- Weight gain: A growing problem in schizophrenia management. J Clin Psychiatry 2001;62(suppl 7).
- Weight gain associated with the use of psychotropic medications. J Clin Psychiatry 1999;60(suppl 2).
- Effects of atypical antipsychotics on body weight and glucose regulation. J Clin Psychiatry 2001;62(suppl 23).
- National Heart, Lung, and Blood Institute. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults. www.nhlbi.nih.gov/guidelines/obesity/ob_home.htm
Drug brand names
- Amantadine • Symmetrel
- Clozapine • Clozaril
- Nizatidine • Axid
- Olanzapine • Zyprexa
- Orlistat • Xenical
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Sibutramine • Meridia
- Topiramate • Topamax
- Ziprasidone • Geodon
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article.
1. Mokdad AH, Bowman BA, Ford ES, Vinicor F, Marks JS, Koplan JP. The continuing epidemics of obesity and diabetes in the United States. JAMA 2001;286:1195-1200.
2. Dixon L, Weiden P, Delahanty J, et al. Prevalence and correlates of diabetes in national schizophrenia samples. Schizophr Bull 2000;26:903-12.
3. Braceland FJ, Meduna LJ, Vaichulis JA. Delayed action of insulin in schizophrenia. Am J Psychiatry 1945;102:108-10.
4. Allison DB, Mentore JL, Heo M, et al. Antipsychotic-induced weight gain: a comprehensive research synthesis. Am J Psychiatry 1999;156:1686-96.
5. Brecher M, Rak IW, Westhead EK. The long-term effect of quetiapine (“Seroquel’) monotherapy on weight in patients with schizophrenia. Int J Psychiatry Clin Pract 2000;4:287-92.
6. Fontaine KR, Heo M, Harrigan EP, Shear CL, Lakshiminarayanan M. Estimating the consequences of anti-psychotic induced weight gain on health and mortality rate. Psychiatry Res 2001;101:277-88.
7. Theonnard-Neumann E. Phenothiazines and diabetes in hospitalized women. Am J Psychiatry 1968;124:978-82.
8. Koller E, Schneider B, Bennett K, Dubitsky G. Clozapine-associated diabetes. Am J Med 2001;111:716-23.
9. Pendergrass ML. Pathophysiology and management of type 2 diabetes. In: Giles TD, Sowers JR, Weber MA (eds). Diabetes & cardiovascular disease: a practical primer. New Orleans: Institute of Professional Education, 2000;15-40.
10. Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults. Findings from the Third National Health and Nutrition Examination Survey. JAMA 2002;287:356-9.
11. Vieweg WVR, Leadbetter RA. The polydipsia-hyponatremia syndrome. Epidemiology, clinical features, and treatment. CNS Drugs 1997;7:121-38.
12. Thomas PR. Weighing the options: criteria for evaluating weight management programs. Washington, DC: National Academy Press, 1995.
13. Umbricht D, Flury H, Bridler R. Cognitive behavioral therapy for weight gain. Am J Psychiatry 2001;158:971.-
14. Ball M, Coons V, Buchanan R. A program for treating olanzapine-related weight gain. Psychiatric Services 2001;52:967-9.
15. Littrell KH, Petty RG, Hilligoss NM, Peabody CD, Johnson CG. Educational interventions for the management of antipsychotic-related weight gain. 41st annual meeting of the New Clinical Drug Evaluation Unit, Phoenix, AZ, May 28-31, 2001.
16. Greenberg I, Chan S, Blackburn GL. Nonpharmacologic and pharmacologic management of weight gain. J Clin Psychiatry 1999;60(suppl 21):31-6.
17. Wirth A, Krause J. Long-term weight loss with sibutramine: a randomized controlled trial. JAMA 2001;286:1331-9.
18. Taflinski T, Chojnacka J. Sibutramine-associated psychotic episode. Am J Psychiatry 2001;157:2057-8.
19. Glazer G. Long-term pharmacotherapy of obesity 2000. Arch Intern Med 2001;161:1814-24.
20. Reinstein M, Sirotovskaya L, Jones L. Effect of clozapine-quetiapine combination therapy on weight and glycaemic control. Clin Drug Invest 1999;18:99-104.
21. Floris M, Lejeune J, Deberdt W. Effect of amantadine on weight gain during olanzapine treatment. Eur Neuropsychopharmacol 2001;11:181-2.
22. Norton J, Potter D, Edwards K. Sustained weight loss associated with topiramate [abstract]. Epilepsia 1997;38(suppl 3):60.-
23. Dursun SM, Devarajan S. Clozapine weight gain, plus topiramate weight loss. Can J Psychiatry 2000;45:198.-
24. Breier A, Tanaka Y, Roychowdhury S, Clark WS. Nizatidine for the prevention of olanzapine-associated weight gain in schizophrenia and related disorders. A randomized controlled double blind study. 41st annual meeting of the New Clinical Drug Evaluation Unit, Phoenix, AZ, May 28-31 2001.
Weight gain is a potential problem for all patients who require treatment with antipsychotics. Those with schizophrenia face double jeopardy. Both the disorder and the use of virtually any available antipsychotic drug may be associated with weight gain, new-onset glucose intolerance, and type 2 diabetes mellitus.
Because of the cardiovascular risks and other morbidity associated with weight gain and glucose dysregulation,1 the psychiatrist must remain vigilant and manage these complications aggressively. In this article, we offer insights into the prevention and management of metabolic complications associated with the use of antipsychotic agents in patients with schizophrenia.
Weight gain and antipsychotics
Weight change was recognized as a feature of schizophrenia even before antipsychotic drugs were introduced in the 1950s.2 Schizophrenia—independent of drug treatment—also is a risk factor for the development of type 2 diabetes. In persons with schizophrenia, serum glucose levels increase more slowly, decline more gradually, and represent higher-than-normal reference values.3
Figure 1 WEIGHT GAIN ASSOCIATED WITH ANTIPSYCHOTIC DRUG ADMINISTRATION
Values represent estimates of drug-induced weight gain after 10 weeks of drug administration.
Source: Allison et al. Am J Psychiatry 1999;156:1686-96; Brecher et al. Int J Psychiatry Clin Pract 2000;4:287-92.In 1999, Allison et al assessed the effects of conventional and atypical antipsychotics on body weight. Using 81 published articles, they estimated and compared weight changes associated with 10 antipsychotic agents and a placebo when given at standard dosages for 10 weeks.4 Comparative data on quetiapine, which were insufficient in 1999, have since been added (Figure 1).5
Patients who received a placebo lost 0.74 kg across 10 weeks. Weight changes with the conventional agents ranged from a reduction of 0.39 kg with molindone to an increase of 3.19 kg with thioridazine. Weight gains also were seen with all of the newer atypical agents, including clozapine (+4.45 kg), olanzapine (+4.15 kg), risperidone (+2.10 kg), and ziprasidone (+0.04 kg).
Fontaine et al have estimated that weight gain in patients with schizophrenia has its greatest impact on mortality in two scenarios:
- when patients are overweight before they start antipsychotic medication
- with greater degrees of weight gain across 10 years (Figure 2).
Whatever a patient’s starting weight, substantial weight gain with antipsychotic therapy increases the risk of impaired glucose tolerance and hypertension (Figure 3).6
Schizophrenia and diabetes
The prevalence of type 2 diabetes in patients with schizophrenia increased from 4.2% in 1956 to 17.2% in 1968, related in part to the introduction of phenothiazines.7 A recent study of data collected by the Schizophrenia Patient Outcomes Research Team (PORT)2 found higher rates of diabetes in persons with schizophrenia (lifetime prevalence, 14.9%) than in the general population (approximately 7.3%).1 Most patients in the PORT study were taking older antipsychotics, the use of which has occasionally been associated with carbohydrate dysregulation.
Figure 2 INCREASED MORTALITY ASSOCIATED WITH WEIGHT GAIN
Number of deaths associated with weight gains of 2.5 and 12 kg over 10 years, as related to all body mass index measurements (BMIs) and BMIs >27 (per 100,000 persons in U.S. population).
Source: Fontaine et al. Psychiatry Res 2001;101:277-88.The prevalence of new-onset diabetes with use of specific antipsychotics is unknown. Most information is contained in case reports, and proper epidemiologic studies await publication.
The most detailed report—a pooled study of published cases related to clozapine use—comes from the FDA’s Center for Drug Evaluation and Research.8 In this study, the authors identified 384 reports of diabetes that developed (in 242 patients) or was exacerbated (in 54 patients) in association with clozapine. Patient mean age was 40, and diabetes occurred more commonly in women than in men.
Diabetes developed most commonly within 6 months of starting treatment with clozapine, and one patient developed diabetes after a single 500-mg dose. Metabolic acidosis or ketosis occurred in 80 cases, and 25 subjects died during hyperglycemic episodes. Stopping clozapine or reducing the dosage improved glycemic control in 46 patients.8
Figure 3 INCREASED MORBIDITY ASSOCIATED WITH WEIGHT GAIN
New cases of impaired glucose tolerance and hypertension that developed with weight gains of 2.5 and 12 kg over 10 years (per 100,000 persons in U.S. population).
Source: Fontaine et al. Psychiatry Res 2001;101:277-88.During antipsychotic therapy, it is important to measure patients’ fasting plasma glucose at least annually—and more often for high-risk patients (Table 1). The American Diabetes Association defines diabetes as a fasting serum or plasma glucose 126 mg/dl or a 2-hour postprandial serum or plasma glucose 200 mg/dl. In all patients, these tests should be repeated to confirm the diagnosis. Oral glucose tolerance testing is less convenient than fasting plasma glucose testing but more sensitive in identifying changes in carbohydrate metabolism.
As with weight gain, it is easier to prevent diabetes than to treat it. The psychiatrist can best help the patient with emerging carbohydrate dysregulation by collaborating with an internist, family physician, or endocrinologist.
Table 1
FACTORS RELATED TO HIGH RISK OF DEVELOPING TYPE 2 DIABETES
|
| Source: American Diabetes Association |
Weight gain with diabetes drugs Weight gain is associated not only with the use of antipsychotics but also with four classes of oral agents used to treat type 2 diabetes: sulfonylureas, meglitinides, phenylalanine derivatives, and thiazolidinediones. One class—biguanides—contributes to weight reduction, and one—alpha-glucosidase inhibitors—has a variable effect on body weight. These drugs also vary in their effects on serum lipids, including total cholesterol, LDL and HDL cholesterol, and triglycerides (Table 2).9
Many patients with type 2 diabetes require more than one agent to control plasma glucose. With time, insulin deficiency becomes more marked, and insulin therapy is frequently added to the regimen. Hypertension and hyperlipidemia are also very common in patients with type 2 diabetes and require medication to reduce the risk of cardiovascular events.10 As a result, the diabetic patient requiring antipsychotic drugs will likely need polypharmacy, and many of the drugs that might be used may lead to weight gain.
Assessing, managing weight gain
During each visit for the patient with schizophrenia, it is important to routinely weigh those receiving antipsychotics and ask about polydipsia and polyuria, which are early signs of incipient diabetes. A patient who is gaining significant weight (7% of baseline) while taking an antipsychotic and has risk factors for cardiovascular events (e.g., smoking, hypertension, hypertriglyceridemia) is a candidate for a change in antipsychotics.
Try to weigh patients at approximately the same time of day at each visit to compensate for possible diurnal weight changes related to polydipsia-hyponatremia syndrome.11 Patients with this syndrome can gain 5 to 10 lbs (or more) per day and excrete the retained fluid at night. It occurs in 5 to 10% of chronically psychotic patients requiring institutional care and in 1 to 2% of outpatients. Patients with schizophrenia complicated by this syndrome may manifest polydipsia and polyuria secondary to psychosis rather than emerging diabetes. Thus, the clinician must be alert to both diabetes and the polydipsia-hyponatremia syndrome in this setting.
Weight-control approaches
Patients who are taking sedating antipsychotics (e.g., clozapine, olanzapine, or low-potency phenothiazines) may gain up to 30 lbs per year if they become physically inactive and do not reduce their food consumption. Thus, it is important to work with such patients to decrease their caloric intake.
A weight-loss program that produces a loss of 0.5 to 1% of body weight per week is considered safe and acceptable.12 Mild to moderate obesity may be managed by reducing food intake by 500 calories and exercising 30 minutes each day.
CBT Cognitive-behavioral therapy (CBT) may help stem weight gain associated with antipsychotic use. Umbricht et al provided CBT to six patients with chronic psychosis who were receiving clozapine or olanzapine. Therapists in group and individual sessions focused on the causes of weight gain, lowcalorie nutrition, weight-loss guidelines, exercise programs, and relaxation strategies. Across 8 weeks, patients’ mean BMI decreased from 29.6 to 25.1 kg/m2
Table 2
METABOLIC EFFECTS OF ORAL ANTIHYPERGLYCEMIC DRUGS
| Class | Body weight | Total cholesterol | LDL | HDL | Triglycerides |
|---|---|---|---|---|---|
| Sulfonylureas Glipizide Glyburide Glimepiride | ▲ | ◄► | ◄► | ◄► | ◄► |
| Meglitinides Repaglinide | ▲ | ◄► | ◄► | ◄► | ◄► |
| Phenylalanine derivatives Nateglinide | ▲ | ◄► | ◄► | ◄► | ◄► |
| Biguanides Metformin | ▼ | ▼ | ▼ | ▲ | ▼ |
| Thiazolidinediones Pioglitazone Rosiglitazone | ▲ | ▲ | ▲ | ▲ | ▼ |
| Alpha-glucosidase inhibitors Acarbose Miglitol | ◄► | ◄► | ◄► | ◄► | ◄► |
| ▲ Increase ▼ Decrease ◄► Neutral effect/no change | |||||
Weight management program The Weight Watchers weight management program has shown mild success when offered to men and women with schizophrenia or schizoaffective disorder. Twenty-one patients who had gained an average of 32 lbs while taking olanzapine were enrolled in a Weight Watchers program at a psychiatric center.14 Mean starting BMI was 32 kg/m2 among the 11 patients who completed the 10-week program. Those 11 lost an average of 5 lbs.
All seven men lost weight. Three of the four women gained weight, and one woman lost 13 lbs. Study subjects remained clinically stable during the 10-week study. Two of the three women who did not lose weight had disabling psychiatric symptoms. Participation rates were similar to those of typical Weight Watchers clientele, suggesting that patients requiring antipsychotics might benefit from treatments used for other obese patients.
Patient education Educating patients about nutrition and exercise may help them control their rate of weight gain during antipsychotic therapy.
Littrell et al provided such an educational program for 1 hour per week for 4 months to six men and six women taking olanzapine for schizophrenia or schizoaffective disorder.15 Patients in the behavioral group gained 0.5 kg, compared with a control group that gained 2.9 kg. Mean increase in BMI was less for the behavioral group (0.3 kg/m2) than for the control patients (0.9 kg/m2). Men in both groups gained more weight than did women.
Pharmacologic approaches
Antiobesity medications are generally reserved for patients with a BMI 30 kg/m2 (threshold for obesity) or for those with a BMI 27 kg/m2 (threshold for overweight is 25 kg/m2) who have additional risk factors for cardiovascular disease, stroke, or diabetes.16
For patients with schizophrenia, who typically have a BMI 27 kg/m2, the presence of these risk factors alone may be enough to warrant consideration of an antiobesity agent. Adding any new drug to a patient’s regimen, however, increases the risk of an adverse interaction.
Antiobesity drugs work by a variety of mechanisms, including decreasing appetite, decreasing fat absorption, and increasing energy expenditure. Drugs may reduce caloric intake by decreasing appetite (anorectic drugs) or increasing satiety (appetite suppressants). Centrally-acting sympathomimetics or serotonergic drugs may suppress appetite.
In studies up to 2 years, the appetite suppressant sibutramine, with mixed serotonergic and noradrenergic reuptake inhibition properties, has been shown to cause more weight loss than a placebo in populations without schizophrenia.17 According to one case report, sibutramine use was associated with new-onset psychosis.18
Common side effects of sibutramine include headache, dry mouth, anorexia, constipation, and insomnia. Regular monitoring of blood pressure is required. Do not prescribe this drug for patients with cardiovascular disease, and avoid co-prescribing with MAO inhibitors and serotonergics.
Orlistat reduces fat absorption from the GI tract.19 Common side effects are largely confined to the GI tract and include oily spotting, flatulence, fecal urgency, fatty/oily stool, and oily evacuation.
Combination therapies
Researchers are studying whether adding adjunctive agents to antipsychotics reduces weight gain.
Clozapine plus quetiapine A group of 65 patients who experienced a mean body weight increase of 6.5 kg while taking clozapine for 6 months were then given clozapine plus quetiapine at chlorpromazine-equivalent dosing during the next 10 months. The patients lost a mean of 4.2 kg, and their glycemic control improved. Elevated glycosylated hemoglobin (HbA1c) became normal in those subjects (20% of participants) who had developed type 2 diabetes while taking clozapine alone. The authors theorized that the weight loss diminished insulin resistance, leading to better control of serum glucose levels.20
Olanzapine plus amantadine A group of 12 outpatients with axis I or II diagnoses had responded well clinically to olanzapine but had gained an average 7.3 kg over 1 to 11 months. In an open-label study, they continued their dosages of olanzapine and also were given amantadine, 100 to 300 mg/d. Amantadine was chosen for this trial because of its possible release of dopamine.
No dietary changes were made, but subjects gained no additional weight after amantadine was added. Over the next 3 to 6 months, they lost a mean 3.5 kg, which was 50% of the weight gain associated with olanzapine administration.21
Clozapine plus topiramate In clinical trials, the anticonvulsant topiramate has been associated with significant weight loss for up to 12 months in patients with seizure disorders.22 This agent, which also has mood-stabilizing effects, may be useful both for mood stabilization and weight loss in tandem with antipsychotic therapy.
In a case study,23 a 29-year-old man with schizophrenia who failed several trials of antipsychotic drugs experienced significant improvement with clozapine, 800 mg/d. Over 2 years, however, he developed myoclonic jerks and gained 45.5 kg (a 49% increase over baseline). When topiramate was added, starting with 25 mg/d and increasing to 125 mg/d, his mood improved and the myoclonic jerks stopped. During 5 months of combination therapy, the patient lost 21 kg without changing his eating habits.
Olanzapine and nizatidine Agents that block histamine (H 2) receptors in the digestive tract may be associated with weight loss when given at high doses, although the mechanism by which they contribute to weight loss is unclear. In a double-blind, placebo-controlled study,24 the H 2 blocker nizatidine was given to patients with schizophrenia who were taking olanzapine, 5 to 20 mg/d. In a 16-week trial, 132 patients were randomized to receive adjunctive treatment with low-dose nizatidine (150 mg bid), high-dose nizatidine (300 mg bid), or a placebo.
After 16 weeks, nizatidine demonstrated a dose-response effect when combined with olanzapine. Average weight gain was:
- 5.51 kg with a placebo
- 4.41 kg with low-dose nizatidine
- 2.76 kg with high-dose nizatidine (p =0.02 compared with a placebo).
In the high-dose nizatidine group, only 6% of patients gained more than 10 kg, and weight gain leveled off by week eight. Adverse events and clinical improvements were similar in the three groups.
Related resources
- Weight gain: A growing problem in schizophrenia management. J Clin Psychiatry 2001;62(suppl 7).
- Weight gain associated with the use of psychotropic medications. J Clin Psychiatry 1999;60(suppl 2).
- Effects of atypical antipsychotics on body weight and glucose regulation. J Clin Psychiatry 2001;62(suppl 23).
- National Heart, Lung, and Blood Institute. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults. www.nhlbi.nih.gov/guidelines/obesity/ob_home.htm
Drug brand names
- Amantadine • Symmetrel
- Clozapine • Clozaril
- Nizatidine • Axid
- Olanzapine • Zyprexa
- Orlistat • Xenical
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Sibutramine • Meridia
- Topiramate • Topamax
- Ziprasidone • Geodon
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article.
Weight gain is a potential problem for all patients who require treatment with antipsychotics. Those with schizophrenia face double jeopardy. Both the disorder and the use of virtually any available antipsychotic drug may be associated with weight gain, new-onset glucose intolerance, and type 2 diabetes mellitus.
Because of the cardiovascular risks and other morbidity associated with weight gain and glucose dysregulation,1 the psychiatrist must remain vigilant and manage these complications aggressively. In this article, we offer insights into the prevention and management of metabolic complications associated with the use of antipsychotic agents in patients with schizophrenia.
Weight gain and antipsychotics
Weight change was recognized as a feature of schizophrenia even before antipsychotic drugs were introduced in the 1950s.2 Schizophrenia—independent of drug treatment—also is a risk factor for the development of type 2 diabetes. In persons with schizophrenia, serum glucose levels increase more slowly, decline more gradually, and represent higher-than-normal reference values.3
Figure 1 WEIGHT GAIN ASSOCIATED WITH ANTIPSYCHOTIC DRUG ADMINISTRATION
Values represent estimates of drug-induced weight gain after 10 weeks of drug administration.
Source: Allison et al. Am J Psychiatry 1999;156:1686-96; Brecher et al. Int J Psychiatry Clin Pract 2000;4:287-92.In 1999, Allison et al assessed the effects of conventional and atypical antipsychotics on body weight. Using 81 published articles, they estimated and compared weight changes associated with 10 antipsychotic agents and a placebo when given at standard dosages for 10 weeks.4 Comparative data on quetiapine, which were insufficient in 1999, have since been added (Figure 1).5
Patients who received a placebo lost 0.74 kg across 10 weeks. Weight changes with the conventional agents ranged from a reduction of 0.39 kg with molindone to an increase of 3.19 kg with thioridazine. Weight gains also were seen with all of the newer atypical agents, including clozapine (+4.45 kg), olanzapine (+4.15 kg), risperidone (+2.10 kg), and ziprasidone (+0.04 kg).
Fontaine et al have estimated that weight gain in patients with schizophrenia has its greatest impact on mortality in two scenarios:
- when patients are overweight before they start antipsychotic medication
- with greater degrees of weight gain across 10 years (Figure 2).
Whatever a patient’s starting weight, substantial weight gain with antipsychotic therapy increases the risk of impaired glucose tolerance and hypertension (Figure 3).6
Schizophrenia and diabetes
The prevalence of type 2 diabetes in patients with schizophrenia increased from 4.2% in 1956 to 17.2% in 1968, related in part to the introduction of phenothiazines.7 A recent study of data collected by the Schizophrenia Patient Outcomes Research Team (PORT)2 found higher rates of diabetes in persons with schizophrenia (lifetime prevalence, 14.9%) than in the general population (approximately 7.3%).1 Most patients in the PORT study were taking older antipsychotics, the use of which has occasionally been associated with carbohydrate dysregulation.
Figure 2 INCREASED MORTALITY ASSOCIATED WITH WEIGHT GAIN
Number of deaths associated with weight gains of 2.5 and 12 kg over 10 years, as related to all body mass index measurements (BMIs) and BMIs >27 (per 100,000 persons in U.S. population).
Source: Fontaine et al. Psychiatry Res 2001;101:277-88.The prevalence of new-onset diabetes with use of specific antipsychotics is unknown. Most information is contained in case reports, and proper epidemiologic studies await publication.
The most detailed report—a pooled study of published cases related to clozapine use—comes from the FDA’s Center for Drug Evaluation and Research.8 In this study, the authors identified 384 reports of diabetes that developed (in 242 patients) or was exacerbated (in 54 patients) in association with clozapine. Patient mean age was 40, and diabetes occurred more commonly in women than in men.
Diabetes developed most commonly within 6 months of starting treatment with clozapine, and one patient developed diabetes after a single 500-mg dose. Metabolic acidosis or ketosis occurred in 80 cases, and 25 subjects died during hyperglycemic episodes. Stopping clozapine or reducing the dosage improved glycemic control in 46 patients.8
Figure 3 INCREASED MORBIDITY ASSOCIATED WITH WEIGHT GAIN
New cases of impaired glucose tolerance and hypertension that developed with weight gains of 2.5 and 12 kg over 10 years (per 100,000 persons in U.S. population).
Source: Fontaine et al. Psychiatry Res 2001;101:277-88.During antipsychotic therapy, it is important to measure patients’ fasting plasma glucose at least annually—and more often for high-risk patients (Table 1). The American Diabetes Association defines diabetes as a fasting serum or plasma glucose 126 mg/dl or a 2-hour postprandial serum or plasma glucose 200 mg/dl. In all patients, these tests should be repeated to confirm the diagnosis. Oral glucose tolerance testing is less convenient than fasting plasma glucose testing but more sensitive in identifying changes in carbohydrate metabolism.
As with weight gain, it is easier to prevent diabetes than to treat it. The psychiatrist can best help the patient with emerging carbohydrate dysregulation by collaborating with an internist, family physician, or endocrinologist.
Table 1
FACTORS RELATED TO HIGH RISK OF DEVELOPING TYPE 2 DIABETES
|
| Source: American Diabetes Association |
Weight gain with diabetes drugs Weight gain is associated not only with the use of antipsychotics but also with four classes of oral agents used to treat type 2 diabetes: sulfonylureas, meglitinides, phenylalanine derivatives, and thiazolidinediones. One class—biguanides—contributes to weight reduction, and one—alpha-glucosidase inhibitors—has a variable effect on body weight. These drugs also vary in their effects on serum lipids, including total cholesterol, LDL and HDL cholesterol, and triglycerides (Table 2).9
Many patients with type 2 diabetes require more than one agent to control plasma glucose. With time, insulin deficiency becomes more marked, and insulin therapy is frequently added to the regimen. Hypertension and hyperlipidemia are also very common in patients with type 2 diabetes and require medication to reduce the risk of cardiovascular events.10 As a result, the diabetic patient requiring antipsychotic drugs will likely need polypharmacy, and many of the drugs that might be used may lead to weight gain.
Assessing, managing weight gain
During each visit for the patient with schizophrenia, it is important to routinely weigh those receiving antipsychotics and ask about polydipsia and polyuria, which are early signs of incipient diabetes. A patient who is gaining significant weight (7% of baseline) while taking an antipsychotic and has risk factors for cardiovascular events (e.g., smoking, hypertension, hypertriglyceridemia) is a candidate for a change in antipsychotics.
Try to weigh patients at approximately the same time of day at each visit to compensate for possible diurnal weight changes related to polydipsia-hyponatremia syndrome.11 Patients with this syndrome can gain 5 to 10 lbs (or more) per day and excrete the retained fluid at night. It occurs in 5 to 10% of chronically psychotic patients requiring institutional care and in 1 to 2% of outpatients. Patients with schizophrenia complicated by this syndrome may manifest polydipsia and polyuria secondary to psychosis rather than emerging diabetes. Thus, the clinician must be alert to both diabetes and the polydipsia-hyponatremia syndrome in this setting.
Weight-control approaches
Patients who are taking sedating antipsychotics (e.g., clozapine, olanzapine, or low-potency phenothiazines) may gain up to 30 lbs per year if they become physically inactive and do not reduce their food consumption. Thus, it is important to work with such patients to decrease their caloric intake.
A weight-loss program that produces a loss of 0.5 to 1% of body weight per week is considered safe and acceptable.12 Mild to moderate obesity may be managed by reducing food intake by 500 calories and exercising 30 minutes each day.
CBT Cognitive-behavioral therapy (CBT) may help stem weight gain associated with antipsychotic use. Umbricht et al provided CBT to six patients with chronic psychosis who were receiving clozapine or olanzapine. Therapists in group and individual sessions focused on the causes of weight gain, lowcalorie nutrition, weight-loss guidelines, exercise programs, and relaxation strategies. Across 8 weeks, patients’ mean BMI decreased from 29.6 to 25.1 kg/m2
Table 2
METABOLIC EFFECTS OF ORAL ANTIHYPERGLYCEMIC DRUGS
| Class | Body weight | Total cholesterol | LDL | HDL | Triglycerides |
|---|---|---|---|---|---|
| Sulfonylureas Glipizide Glyburide Glimepiride | ▲ | ◄► | ◄► | ◄► | ◄► |
| Meglitinides Repaglinide | ▲ | ◄► | ◄► | ◄► | ◄► |
| Phenylalanine derivatives Nateglinide | ▲ | ◄► | ◄► | ◄► | ◄► |
| Biguanides Metformin | ▼ | ▼ | ▼ | ▲ | ▼ |
| Thiazolidinediones Pioglitazone Rosiglitazone | ▲ | ▲ | ▲ | ▲ | ▼ |
| Alpha-glucosidase inhibitors Acarbose Miglitol | ◄► | ◄► | ◄► | ◄► | ◄► |
| ▲ Increase ▼ Decrease ◄► Neutral effect/no change | |||||
Weight management program The Weight Watchers weight management program has shown mild success when offered to men and women with schizophrenia or schizoaffective disorder. Twenty-one patients who had gained an average of 32 lbs while taking olanzapine were enrolled in a Weight Watchers program at a psychiatric center.14 Mean starting BMI was 32 kg/m2 among the 11 patients who completed the 10-week program. Those 11 lost an average of 5 lbs.
All seven men lost weight. Three of the four women gained weight, and one woman lost 13 lbs. Study subjects remained clinically stable during the 10-week study. Two of the three women who did not lose weight had disabling psychiatric symptoms. Participation rates were similar to those of typical Weight Watchers clientele, suggesting that patients requiring antipsychotics might benefit from treatments used for other obese patients.
Patient education Educating patients about nutrition and exercise may help them control their rate of weight gain during antipsychotic therapy.
Littrell et al provided such an educational program for 1 hour per week for 4 months to six men and six women taking olanzapine for schizophrenia or schizoaffective disorder.15 Patients in the behavioral group gained 0.5 kg, compared with a control group that gained 2.9 kg. Mean increase in BMI was less for the behavioral group (0.3 kg/m2) than for the control patients (0.9 kg/m2). Men in both groups gained more weight than did women.
Pharmacologic approaches
Antiobesity medications are generally reserved for patients with a BMI 30 kg/m2 (threshold for obesity) or for those with a BMI 27 kg/m2 (threshold for overweight is 25 kg/m2) who have additional risk factors for cardiovascular disease, stroke, or diabetes.16
For patients with schizophrenia, who typically have a BMI 27 kg/m2, the presence of these risk factors alone may be enough to warrant consideration of an antiobesity agent. Adding any new drug to a patient’s regimen, however, increases the risk of an adverse interaction.
Antiobesity drugs work by a variety of mechanisms, including decreasing appetite, decreasing fat absorption, and increasing energy expenditure. Drugs may reduce caloric intake by decreasing appetite (anorectic drugs) or increasing satiety (appetite suppressants). Centrally-acting sympathomimetics or serotonergic drugs may suppress appetite.
In studies up to 2 years, the appetite suppressant sibutramine, with mixed serotonergic and noradrenergic reuptake inhibition properties, has been shown to cause more weight loss than a placebo in populations without schizophrenia.17 According to one case report, sibutramine use was associated with new-onset psychosis.18
Common side effects of sibutramine include headache, dry mouth, anorexia, constipation, and insomnia. Regular monitoring of blood pressure is required. Do not prescribe this drug for patients with cardiovascular disease, and avoid co-prescribing with MAO inhibitors and serotonergics.
Orlistat reduces fat absorption from the GI tract.19 Common side effects are largely confined to the GI tract and include oily spotting, flatulence, fecal urgency, fatty/oily stool, and oily evacuation.
Combination therapies
Researchers are studying whether adding adjunctive agents to antipsychotics reduces weight gain.
Clozapine plus quetiapine A group of 65 patients who experienced a mean body weight increase of 6.5 kg while taking clozapine for 6 months were then given clozapine plus quetiapine at chlorpromazine-equivalent dosing during the next 10 months. The patients lost a mean of 4.2 kg, and their glycemic control improved. Elevated glycosylated hemoglobin (HbA1c) became normal in those subjects (20% of participants) who had developed type 2 diabetes while taking clozapine alone. The authors theorized that the weight loss diminished insulin resistance, leading to better control of serum glucose levels.20
Olanzapine plus amantadine A group of 12 outpatients with axis I or II diagnoses had responded well clinically to olanzapine but had gained an average 7.3 kg over 1 to 11 months. In an open-label study, they continued their dosages of olanzapine and also were given amantadine, 100 to 300 mg/d. Amantadine was chosen for this trial because of its possible release of dopamine.
No dietary changes were made, but subjects gained no additional weight after amantadine was added. Over the next 3 to 6 months, they lost a mean 3.5 kg, which was 50% of the weight gain associated with olanzapine administration.21
Clozapine plus topiramate In clinical trials, the anticonvulsant topiramate has been associated with significant weight loss for up to 12 months in patients with seizure disorders.22 This agent, which also has mood-stabilizing effects, may be useful both for mood stabilization and weight loss in tandem with antipsychotic therapy.
In a case study,23 a 29-year-old man with schizophrenia who failed several trials of antipsychotic drugs experienced significant improvement with clozapine, 800 mg/d. Over 2 years, however, he developed myoclonic jerks and gained 45.5 kg (a 49% increase over baseline). When topiramate was added, starting with 25 mg/d and increasing to 125 mg/d, his mood improved and the myoclonic jerks stopped. During 5 months of combination therapy, the patient lost 21 kg without changing his eating habits.
Olanzapine and nizatidine Agents that block histamine (H 2) receptors in the digestive tract may be associated with weight loss when given at high doses, although the mechanism by which they contribute to weight loss is unclear. In a double-blind, placebo-controlled study,24 the H 2 blocker nizatidine was given to patients with schizophrenia who were taking olanzapine, 5 to 20 mg/d. In a 16-week trial, 132 patients were randomized to receive adjunctive treatment with low-dose nizatidine (150 mg bid), high-dose nizatidine (300 mg bid), or a placebo.
After 16 weeks, nizatidine demonstrated a dose-response effect when combined with olanzapine. Average weight gain was:
- 5.51 kg with a placebo
- 4.41 kg with low-dose nizatidine
- 2.76 kg with high-dose nizatidine (p =0.02 compared with a placebo).
In the high-dose nizatidine group, only 6% of patients gained more than 10 kg, and weight gain leveled off by week eight. Adverse events and clinical improvements were similar in the three groups.
Related resources
- Weight gain: A growing problem in schizophrenia management. J Clin Psychiatry 2001;62(suppl 7).
- Weight gain associated with the use of psychotropic medications. J Clin Psychiatry 1999;60(suppl 2).
- Effects of atypical antipsychotics on body weight and glucose regulation. J Clin Psychiatry 2001;62(suppl 23).
- National Heart, Lung, and Blood Institute. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults. www.nhlbi.nih.gov/guidelines/obesity/ob_home.htm
Drug brand names
- Amantadine • Symmetrel
- Clozapine • Clozaril
- Nizatidine • Axid
- Olanzapine • Zyprexa
- Orlistat • Xenical
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Sibutramine • Meridia
- Topiramate • Topamax
- Ziprasidone • Geodon
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article.
1. Mokdad AH, Bowman BA, Ford ES, Vinicor F, Marks JS, Koplan JP. The continuing epidemics of obesity and diabetes in the United States. JAMA 2001;286:1195-1200.
2. Dixon L, Weiden P, Delahanty J, et al. Prevalence and correlates of diabetes in national schizophrenia samples. Schizophr Bull 2000;26:903-12.
3. Braceland FJ, Meduna LJ, Vaichulis JA. Delayed action of insulin in schizophrenia. Am J Psychiatry 1945;102:108-10.
4. Allison DB, Mentore JL, Heo M, et al. Antipsychotic-induced weight gain: a comprehensive research synthesis. Am J Psychiatry 1999;156:1686-96.
5. Brecher M, Rak IW, Westhead EK. The long-term effect of quetiapine (“Seroquel’) monotherapy on weight in patients with schizophrenia. Int J Psychiatry Clin Pract 2000;4:287-92.
6. Fontaine KR, Heo M, Harrigan EP, Shear CL, Lakshiminarayanan M. Estimating the consequences of anti-psychotic induced weight gain on health and mortality rate. Psychiatry Res 2001;101:277-88.
7. Theonnard-Neumann E. Phenothiazines and diabetes in hospitalized women. Am J Psychiatry 1968;124:978-82.
8. Koller E, Schneider B, Bennett K, Dubitsky G. Clozapine-associated diabetes. Am J Med 2001;111:716-23.
9. Pendergrass ML. Pathophysiology and management of type 2 diabetes. In: Giles TD, Sowers JR, Weber MA (eds). Diabetes & cardiovascular disease: a practical primer. New Orleans: Institute of Professional Education, 2000;15-40.
10. Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults. Findings from the Third National Health and Nutrition Examination Survey. JAMA 2002;287:356-9.
11. Vieweg WVR, Leadbetter RA. The polydipsia-hyponatremia syndrome. Epidemiology, clinical features, and treatment. CNS Drugs 1997;7:121-38.
12. Thomas PR. Weighing the options: criteria for evaluating weight management programs. Washington, DC: National Academy Press, 1995.
13. Umbricht D, Flury H, Bridler R. Cognitive behavioral therapy for weight gain. Am J Psychiatry 2001;158:971.-
14. Ball M, Coons V, Buchanan R. A program for treating olanzapine-related weight gain. Psychiatric Services 2001;52:967-9.
15. Littrell KH, Petty RG, Hilligoss NM, Peabody CD, Johnson CG. Educational interventions for the management of antipsychotic-related weight gain. 41st annual meeting of the New Clinical Drug Evaluation Unit, Phoenix, AZ, May 28-31, 2001.
16. Greenberg I, Chan S, Blackburn GL. Nonpharmacologic and pharmacologic management of weight gain. J Clin Psychiatry 1999;60(suppl 21):31-6.
17. Wirth A, Krause J. Long-term weight loss with sibutramine: a randomized controlled trial. JAMA 2001;286:1331-9.
18. Taflinski T, Chojnacka J. Sibutramine-associated psychotic episode. Am J Psychiatry 2001;157:2057-8.
19. Glazer G. Long-term pharmacotherapy of obesity 2000. Arch Intern Med 2001;161:1814-24.
20. Reinstein M, Sirotovskaya L, Jones L. Effect of clozapine-quetiapine combination therapy on weight and glycaemic control. Clin Drug Invest 1999;18:99-104.
21. Floris M, Lejeune J, Deberdt W. Effect of amantadine on weight gain during olanzapine treatment. Eur Neuropsychopharmacol 2001;11:181-2.
22. Norton J, Potter D, Edwards K. Sustained weight loss associated with topiramate [abstract]. Epilepsia 1997;38(suppl 3):60.-
23. Dursun SM, Devarajan S. Clozapine weight gain, plus topiramate weight loss. Can J Psychiatry 2000;45:198.-
24. Breier A, Tanaka Y, Roychowdhury S, Clark WS. Nizatidine for the prevention of olanzapine-associated weight gain in schizophrenia and related disorders. A randomized controlled double blind study. 41st annual meeting of the New Clinical Drug Evaluation Unit, Phoenix, AZ, May 28-31 2001.
1. Mokdad AH, Bowman BA, Ford ES, Vinicor F, Marks JS, Koplan JP. The continuing epidemics of obesity and diabetes in the United States. JAMA 2001;286:1195-1200.
2. Dixon L, Weiden P, Delahanty J, et al. Prevalence and correlates of diabetes in national schizophrenia samples. Schizophr Bull 2000;26:903-12.
3. Braceland FJ, Meduna LJ, Vaichulis JA. Delayed action of insulin in schizophrenia. Am J Psychiatry 1945;102:108-10.
4. Allison DB, Mentore JL, Heo M, et al. Antipsychotic-induced weight gain: a comprehensive research synthesis. Am J Psychiatry 1999;156:1686-96.
5. Brecher M, Rak IW, Westhead EK. The long-term effect of quetiapine (“Seroquel’) monotherapy on weight in patients with schizophrenia. Int J Psychiatry Clin Pract 2000;4:287-92.
6. Fontaine KR, Heo M, Harrigan EP, Shear CL, Lakshiminarayanan M. Estimating the consequences of anti-psychotic induced weight gain on health and mortality rate. Psychiatry Res 2001;101:277-88.
7. Theonnard-Neumann E. Phenothiazines and diabetes in hospitalized women. Am J Psychiatry 1968;124:978-82.
8. Koller E, Schneider B, Bennett K, Dubitsky G. Clozapine-associated diabetes. Am J Med 2001;111:716-23.
9. Pendergrass ML. Pathophysiology and management of type 2 diabetes. In: Giles TD, Sowers JR, Weber MA (eds). Diabetes & cardiovascular disease: a practical primer. New Orleans: Institute of Professional Education, 2000;15-40.
10. Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults. Findings from the Third National Health and Nutrition Examination Survey. JAMA 2002;287:356-9.
11. Vieweg WVR, Leadbetter RA. The polydipsia-hyponatremia syndrome. Epidemiology, clinical features, and treatment. CNS Drugs 1997;7:121-38.
12. Thomas PR. Weighing the options: criteria for evaluating weight management programs. Washington, DC: National Academy Press, 1995.
13. Umbricht D, Flury H, Bridler R. Cognitive behavioral therapy for weight gain. Am J Psychiatry 2001;158:971.-
14. Ball M, Coons V, Buchanan R. A program for treating olanzapine-related weight gain. Psychiatric Services 2001;52:967-9.
15. Littrell KH, Petty RG, Hilligoss NM, Peabody CD, Johnson CG. Educational interventions for the management of antipsychotic-related weight gain. 41st annual meeting of the New Clinical Drug Evaluation Unit, Phoenix, AZ, May 28-31, 2001.
16. Greenberg I, Chan S, Blackburn GL. Nonpharmacologic and pharmacologic management of weight gain. J Clin Psychiatry 1999;60(suppl 21):31-6.
17. Wirth A, Krause J. Long-term weight loss with sibutramine: a randomized controlled trial. JAMA 2001;286:1331-9.
18. Taflinski T, Chojnacka J. Sibutramine-associated psychotic episode. Am J Psychiatry 2001;157:2057-8.
19. Glazer G. Long-term pharmacotherapy of obesity 2000. Arch Intern Med 2001;161:1814-24.
20. Reinstein M, Sirotovskaya L, Jones L. Effect of clozapine-quetiapine combination therapy on weight and glycaemic control. Clin Drug Invest 1999;18:99-104.
21. Floris M, Lejeune J, Deberdt W. Effect of amantadine on weight gain during olanzapine treatment. Eur Neuropsychopharmacol 2001;11:181-2.
22. Norton J, Potter D, Edwards K. Sustained weight loss associated with topiramate [abstract]. Epilepsia 1997;38(suppl 3):60.-
23. Dursun SM, Devarajan S. Clozapine weight gain, plus topiramate weight loss. Can J Psychiatry 2000;45:198.-
24. Breier A, Tanaka Y, Roychowdhury S, Clark WS. Nizatidine for the prevention of olanzapine-associated weight gain in schizophrenia and related disorders. A randomized controlled double blind study. 41st annual meeting of the New Clinical Drug Evaluation Unit, Phoenix, AZ, May 28-31 2001.
Kleptomania: Emerging therapies target mood, impulsive behavior
What is kleptomania? An independent illness, a symptom of other psychiatric disorders, or merely criminal behavior? Kleptomania—a disorder defined by an inability to resist the impulse to steal—is one of psychiatry’s most poorly understood diagnoses, even though it has been recognized in the literature for almost 200 years.
Kleptomania causes notable distress and impaired functioning.1 People with kleptomania often suffer from comorbid mood, anxiety, substance use, and other impulse-control disorders.14 They experience the humiliation of repeated arrests, which leads to guilt, depression, and even suicide.1,5 Yet kleptomania usually goes undiagnosed and untreated, despite a lifetime prevalence as high as 0.6%.6
Case report: ‘I’m a thief’
“I’m a thief,” began Susan, age 39. “I steal something four or five times every week. I steal from grocery stores and clothing stores. Sometimes I might steal something like vanilla extract; other times an expensive men’s tie. I probably steal $200 worth of items every week.
“You probably won’t believe this, but I don’t want or need the stuff I take. I have plenty of money. I have no idea why I take the things I do. That’s why I’m so depressed. What kind of person does something like this?
The urge to steal “I was probably 14 when I started stealing. I would go to stores with my mother. When I saw certain objects, I would get urges to steal them. The odd thing was that the items I stole were so ridiculous. I remember stealing key chains for several months, maybe three or four times a week. When I got older, things got worse. I was having urges more often, and so I needed to steal more often.
| Myth | Fact |
|---|---|
| Only little old ladies are kleptomaniacs. | Men and women of all ages suffer from kleptomania. Most patients report that the disorder began in adolescence. |
| It’s just a phase kids go through. | Parents of adolescents might see stealing as a phase. In many cases this might be true, but stealing may also suggest an underlying psychopathology. |
| People who steal are “bad.” | People with kleptomania steal because of urges to steal, not because of moral weakness. Treatment, not judgment, is the appropriate response. |
“My entire life has been torment. Each day I worry about having the urges, and then I worry about being caught stealing. I can’t relax. I’ve been married for 17 years, and I haven’t told my husband. My secrecy is tearing our marriage apart. My husband thinks I’m having an affair because I’ve distanced myself emotionally from him.”
Table 1
SCREENING TEST FOR KLEPTOMANIA
| Yes | No | |
|---|---|---|
| 1. Do you steal or have urges to steal? | ○ | ○ |
| 2. Do thoughts of stealing or urges to steal preoccupy you? That is, do you often think about stealing or have urges to steal and wish the thoughts or urges occurred less often? | ○ | ○ |
| 3. Do you feel tense or anxious before you steal or when you have urges to steal? | ○ | ○ |
| 4. Do you feel pleasure or a sense of calm when you steal something? | ○ | ○ |
| 5. Has the stealing or urges to steal caused you much distress? | ○ | ○ |
| 6. Has the stealing or urges to steal significantly interfered with your life in some way? | ○ | ○ |
| A patient who answers “yes” to questions 1 through 4 and to question 5 or 6 is likely to have kleptomania. | ||
| Adapted from DSM-IV criteria, American Psychiatric Association, 2000 | ||
Susan described urges to steal almost every day. When the urges were mild, she could resist them. Other days they were severe, and Susan felt unable to control her behavior. At work, her urges distracted her from completing projects, and her performance suffered. The urge to steal would often compel Susan to leave work early so she could get to a store.
Calm, then guilt “Every time I steal something I feel both a thrill and a great sense of calm,” she said. “It feels good. The problem is that almost immediately after each theft, I feel guilty and ashamed. After I steal, I usually donate the items to the Salvation Army, throw them away, or give them away as gifts.”
Drug trials We started treating Susan with the selective serotonin reuptake inhibitor (SSRI), citalopram. She reported notable improvement in her mood after 3 weeks on a dosage of 60 mg/d and she had been attending weekly psychotherapy, although her stealing continued unchanged. The addition of naltrexone, 200 mg/d for 2 weeks, decreased the frequency of Susan’s stealing and reduced her urges to steal, but her symptoms continued to interfere significantly with her overall functioning.
We then added the atypical antipsychotic quetiapine, 100 mg bid, and Susan’s urges to steal and stealing behavior went into remission within 3 weeks. She has refrained from stealing for the last 9 months.
Making the diagnosis
In our clinic, we have treated more than 50 patients with kleptomania. Rather than coming to us through the criminal justice system, they are usually self-referred. Often they contact us after discovering on the Internet that we specialize in treating persons with this disorder.
In our experience, kleptomania typically goes undiagnosed in clinical settings, in part because patients are ashamed and embarrassed to discuss their symptoms with physicians unless specifically asked.1 If left untreated, however, kleptomania frequently becomes chronic.4 If persons with kleptomania are to seek treatment, it is important that family, friends, and mental health professionals understand the myths and facts about this disorder (Box).
To make the diagnosis, we use the simple screening instrument shown in Table 1.7 In general, because of high comorbidity with certain disorders, we screen every patient presenting to our clinic with a mood, substance use, anxiety, or eating disorder, or who has a problem with impulse control. Kleptomania is likely if the patient answers “yes” to questions 1 through 4 and to question 5 or 6. Stealing may be a symptom of several other psychiatric disorders, however, and misdiagnosis is fairly common (Table 2).6-8
Data suggest that the female-to-male ratio in kleptomania is approximately 2:1, with onset in adolescence. Typical individuals with kleptomania steal because they have urges to steal, often triggered by specific stimuli such as the sights and sounds of stores or feelings of loneliness or stress.1 Most patients with kleptomania are fairly specific about the types of stores from which they steal and the items they steal, and most hoard stolen items.1
Although many patients with kleptomania function quite well, others are severely debilitated in social and occupational realms. In a series of 22 patients with kleptomania:
- 64% had been apprehended
- 23% had served jail time
- 27% had been hospitalized because of their kleptomania symptoms
- 18% had considered or attempted suicide because of the distress associated with their kleptomania.1
Treatment recommendations
Patient history With patients who steal, we begin by identifying the motivation behind the stealing. Most patients with kleptomania report urges to steal. Some of these patients may have comorbid depression; for them, stealing makes them feel less depressed. Anger or irritability may point to borderline personality disorder. Stealing for the enjoyment of risk may suggest bipolar disorder.
Table 2
DIFFERENTIAL DIAGNOSIS: DISORDERS THAT MAY INVOLVE STEALING
| Misdiagnosis | How to distinguish from kleptomania |
|---|---|
| Bipolar disorder | Patients with bipolar disorder may steal as a result of the impulsivity of mania. In fact, the diagnostic criteria for kleptomania require the exclusion of mania as the cause of stealing.7 Patients with bipolar disorder report an elevated, expansive, or irritable mood while stealing. Patients with kleptomania tend to report a depressed mood when not stealing |
| Borderline personality disorder | Unlike patients with borderline personality disorder, patients with kleptomania do not report long histories of unstable relationships or negative self-image; inappropriate anger and “psychotic-like” symptoms are rare in patients with kleptomania |
| Antisocial personality disorder (ASPD, or conduct disorder) | Patients with kleptomania suffer intense shame and guilt, unlike those with ASPD. Also, most patients with kleptomania do not report other illegal or antisocial behavior. |
| Eating disorders | Data suggests that about one-third of patients with an eating disorder also steal.6,8 Patients with kleptomania, however, do not have disordered eating patterns or distorted body images common to patients with eating disorders. |
Many patients with kleptomania have comorbid mood, substance, or anxiety disorders. Treating these other symptoms while ignoring the symptoms of kleptomania may be unsuccessful. Comorbidity also may influence the choice of medication.
Medical assessment Case reports have associated the onset of kleptomania with a variety of medical conditions, including presenile cortical atrophy in a 25-year-old, a parietal tumor that caused blackouts and obliterated any memory of stealing episodes, narcolepsy, and an insulinoma that caused severe hypoglycemia.9 The relationship of these conditions with the onset of kleptomania is unclear, but the reports suggest that medical causes—although unlikely—should be ruled out before you consider kleptomania as a psychiatric illness.
Patient education Persons with kleptomania often feel that no one else has the same problem. They do not think of their behavior as being an illness. It is helpful to explain that kleptomania is treatable and to connect patients with educational books, self-help groups, and Web sites providing information and support (see “Related resources”).
Cognitive-behavioral therapy (CBT) Although the evidence is quite limited, covert sensitization, exposure and response prevention, and imaginal desensitization have all been shown effective in case reports.10
What medications are effective?
Only case reports, a case series of five subjects, and a single open-label treatment study involving 10 subjects with kleptomania have been done.
So far, uses of tricyclic antidepressants (imipramine, nortriptyline), SSRIs (fluoxetine, fluvoxamine, paroxetine), the opioid antagonist naltrexone, and mood stabilizers (lithium, valproate) have met with varying degrees of success. Strategies targeting urge and behavior reduction and mechanisms for coping with urges and behavior (e.g., cognitive-behavioral therapies) may represent important adjunctive components.2,11-17
No medications are FDA-approved for treating kleptomania. Therefore, it is important to inform patients of any off-label use of medications for this disorder, as well as the empirical basis for considering pharmacologic treatment.
SSRIs Only case reports exist on the use of SSRIs in treating kleptomania. The disorder may share a common pathology with pathologic gambling, and in our clinical experience appears to respond to similar treatments.18 We draw on research of pathologic gambling as well as our clinical experience in choosing SSRIs as first-line treatment, especially for patients with significant mood symptoms.19
We suggest titrating SSRIs to the maximum recommended dosage. As in the treatment of pathologic gambling, dosages of SSRIs required to treat kleptomania symptoms appear to be higher than average dosages required to treat depressive disorders. An SSRI should not be considered ineffective unless it has been tried for at least 10 to 12 weeks and the highest dosage tolerated or recommended by the manufacturer has been reached.
Response to SSRIs usually is characterized by decreased thoughts about stealing, decreased stealing behavior, and improvement in social and occupational functioning. If an SSRI is only partially effective, we consider augmentation with naltrexone, buspirone, or a mood stabilizer.
Naltrexone Patients taking naltrexone often report less-intense urges to steal. The urges may not disappear but are often sufficiently reduced so that the patient can resist them more easily. Patients also report that the thrill associated with stealing is reduced or eliminated.
Naltrexone was used in the first medication study of kleptomania and showed a significant decline in the intensity of urges to steal, stealing thoughts, and stealing behavior. Average dosage was 150 mg/d;11 a reduced dosage (e.g., 50 mg/d) may work in adolescents with kleptomania.20
Nausea as a side effect can be reduced by starting patients on 25 mg/d for the first 3 or 4 days and possibly adding ondansetron, 4 to 8 mg/d. Nausea and diarrhea are usually mild and resolve within the first week. Clinically, most patients respond to naltrexone within 2 weeks. After that, the dosage usually needs to be adjusted.
In patients with comorbid depression, augmentation with an SSRI may prevent worsening of untreated depressive symptoms. It is prudent to obtain liver function tests prior to naltrexone administration and again 3 to 4 weeks after starting the drug.21 Repeat testing should be performed at 2-to 4-week intervals for the next 2 months, then once a month for the following 3 months. After 6 months, testing three to four times a year is usually sufficient.
Nonsteroidal analgesics should not be used with high dosages of naltrexone (>50 mg/d), as concurrent use may increase the risk of hepatic transaminase elevation.21
Mood stabilizers Responses to lithium and valproate have been described in two case reports of patients with kleptomania.14,15 In the case of valproate, the effective dosage was 2,000 mg/d, whereas lithium reduced stealing urges at a serum level of 0.5 mEq/L.
Although it would be premature to recommend the use of mood stabilizers, their possible benefit may be related to their efficacy in bipolar disorder treatment and the existence of features (e.g., impulsivity) shared by kleptomania and bipolar disorder.
Atypical antipsychotics Although there is no evidence that atypical antipsychotics are useful in kleptomania, augmenting an SSRI with an atypical neuroleptic may be beneficial. Atypical antipsychotics have been explored as augmenting agents in the treatment of nonpsychotic disorders and behaviors, including pathologic gambling and obsessive-compulsive disorder.
The role of psychotherapy
Cognitive-behavioral therapy Based on the evidence of its effectiveness in treating pathologic gambling, CBT may hold promise as monotherapy for mild cases of kleptomania.
Combination therapy Combined pharmacologic and behavioral therapy may be the optimal treatment strategy for kleptomania. In our experience, patients who respond only partially or fail to respond to pharmacotherapy alone are more likely to find relief with a combination of drug and cognitive-behavioral therapies.
- Goldman MJ. Kleptomania: the compulsion to steal—what can be done? Far Hills, N New Horizon Press, 1998.
- Shoplifters Alternative http://www.shoplifters.org
- Impulse Control Disorders Clinic, University of Minnesota http://www.med.umn.edu/psychiatry/research/impulse.htm
Drug brand names
- Citalopram • Celexa
- Fluvoxamine • Luvox
- Imipramine • Tofranil
- Naltrexone • Revia
- Nortriptyline • Aventyl, Pamelor
- Paroxetine • Paxil
- Quetiapine • Seroquel
- Valproic acid • Depakote
Disclosure
The authors report no affiliation or financial arrangement with any of the companies whose products are mentioned in this article.
1. Grant JE, Kim SW. Clinical characteristics and associated psychopathology in 22 cases of kleptomania. Comp Psychiatry (in press).
2. McElroy SL, Pope HG, Hudson JI, Keck PE, White KL. Kleptomania: a report of 20 cases. Am J Psychiatry 1991;148:652-7.
3. Presta S, Marazziti D, Dell’Osso L, et al. Kleptomania: clinical features and comorbidity in an Italian sample. Comp Psychiatry 2002;43:7-12.
4. McElroy SL, Keck PE, Phillips KA. Kleptomania, compulsive buying, and binge-eating disorder. J Clin Psychiatry 1995;56:14-26.
5. McElroy SL, Hudson JI, Pope HG, Keck PE. Kleptomania: clinical characteristics and associated psychopathology. Psychol Med 1991;21:93-108.
6. Goldman MJ. Kleptomania: an overview. Psychiatric Ann 1992;22:68-71.
7. American Psychiatric Association Committee on Nomenclature and Statistics Diagnostic and statistical manual of mental disorders (4th ed, text rev). Washington, DC: American Psychiatric Association, 2000.
8. Krahn DD, Nairn K, Gosnell BA, Drewnowski A. Stealing in eating disordered patients. J Clin Psychiatry 1991;52:112-5.
9. Goldman MJ. Kleptomania: making sense of the nonsensical. Am J Psychiatry 1991;148:986-96.
10. Goldman MJ. Kleptomania: the compulsion to steal—what can be done? Far Hills, NJ: New Horizon Press, 1998.
11. Grant JE, Kim SW. An open-label study of naltrexone in the treatment of kleptomania. J Clin Psychiatry 2002;63:349-56.
12. Chong SA, Low BL. Treatment of kleptomania with fluvoxamine. Acta Psychiatr Scand 1996;93:314-5.
13. Kraus JE. Treatment of kleptomania with paroxetine. J Clin Psychiatry 1999;60:793.-
14. Burstein A. Fluoxetine lithium treatment for kleptomania. J Clin Psychiatry 1992;53:28-9.
15. Kmetz GF, McElroy SL, Collins DJ. Response of kleptomania and mixed mania to valproate. Am J Psychiatry 1997;154:580-1.
16. Lepkifker E, Dannon PN, Ziv R, Iancu I, Horesh N, Kotler M. The treatment of kleptomania with serotonin reuptake inhibitors. Clin Neuropharmacol 1999;22:40-3.
17. Durst R, Katz G, Knobler HY. Buspirone augmentation of fluvoxamine in the treatment of kleptomania. J Nerv Ment Dis 1997;185:586-8.
18. Kim SW. Opioid antagonists in the treatment of impulse-control disorders. J Clin Psychiatry 1998;59:159-64.
19. Grant JE, Kim SW. Pharmacotherapy of pathological gambling. Psychiatric Ann 2002;32:186-91.
20. Grant JE, Kim SW. Adolescent kleptomania treated with naltrexone: a case report. Eur Child Adolescent Psychiatry 2002;11:92-5.
21. Kim SW, Grant JE, et al. A preliminary report on a possible naltrexone and nonsteroidal analgesics interaction. J Clin Psychopharmacol 2001;21:632-4.
What is kleptomania? An independent illness, a symptom of other psychiatric disorders, or merely criminal behavior? Kleptomania—a disorder defined by an inability to resist the impulse to steal—is one of psychiatry’s most poorly understood diagnoses, even though it has been recognized in the literature for almost 200 years.
Kleptomania causes notable distress and impaired functioning.1 People with kleptomania often suffer from comorbid mood, anxiety, substance use, and other impulse-control disorders.14 They experience the humiliation of repeated arrests, which leads to guilt, depression, and even suicide.1,5 Yet kleptomania usually goes undiagnosed and untreated, despite a lifetime prevalence as high as 0.6%.6
Case report: ‘I’m a thief’
“I’m a thief,” began Susan, age 39. “I steal something four or five times every week. I steal from grocery stores and clothing stores. Sometimes I might steal something like vanilla extract; other times an expensive men’s tie. I probably steal $200 worth of items every week.
“You probably won’t believe this, but I don’t want or need the stuff I take. I have plenty of money. I have no idea why I take the things I do. That’s why I’m so depressed. What kind of person does something like this?
The urge to steal “I was probably 14 when I started stealing. I would go to stores with my mother. When I saw certain objects, I would get urges to steal them. The odd thing was that the items I stole were so ridiculous. I remember stealing key chains for several months, maybe three or four times a week. When I got older, things got worse. I was having urges more often, and so I needed to steal more often.
| Myth | Fact |
|---|---|
| Only little old ladies are kleptomaniacs. | Men and women of all ages suffer from kleptomania. Most patients report that the disorder began in adolescence. |
| It’s just a phase kids go through. | Parents of adolescents might see stealing as a phase. In many cases this might be true, but stealing may also suggest an underlying psychopathology. |
| People who steal are “bad.” | People with kleptomania steal because of urges to steal, not because of moral weakness. Treatment, not judgment, is the appropriate response. |
“My entire life has been torment. Each day I worry about having the urges, and then I worry about being caught stealing. I can’t relax. I’ve been married for 17 years, and I haven’t told my husband. My secrecy is tearing our marriage apart. My husband thinks I’m having an affair because I’ve distanced myself emotionally from him.”
Table 1
SCREENING TEST FOR KLEPTOMANIA
| Yes | No | |
|---|---|---|
| 1. Do you steal or have urges to steal? | ○ | ○ |
| 2. Do thoughts of stealing or urges to steal preoccupy you? That is, do you often think about stealing or have urges to steal and wish the thoughts or urges occurred less often? | ○ | ○ |
| 3. Do you feel tense or anxious before you steal or when you have urges to steal? | ○ | ○ |
| 4. Do you feel pleasure or a sense of calm when you steal something? | ○ | ○ |
| 5. Has the stealing or urges to steal caused you much distress? | ○ | ○ |
| 6. Has the stealing or urges to steal significantly interfered with your life in some way? | ○ | ○ |
| A patient who answers “yes” to questions 1 through 4 and to question 5 or 6 is likely to have kleptomania. | ||
| Adapted from DSM-IV criteria, American Psychiatric Association, 2000 | ||
Susan described urges to steal almost every day. When the urges were mild, she could resist them. Other days they were severe, and Susan felt unable to control her behavior. At work, her urges distracted her from completing projects, and her performance suffered. The urge to steal would often compel Susan to leave work early so she could get to a store.
Calm, then guilt “Every time I steal something I feel both a thrill and a great sense of calm,” she said. “It feels good. The problem is that almost immediately after each theft, I feel guilty and ashamed. After I steal, I usually donate the items to the Salvation Army, throw them away, or give them away as gifts.”
Drug trials We started treating Susan with the selective serotonin reuptake inhibitor (SSRI), citalopram. She reported notable improvement in her mood after 3 weeks on a dosage of 60 mg/d and she had been attending weekly psychotherapy, although her stealing continued unchanged. The addition of naltrexone, 200 mg/d for 2 weeks, decreased the frequency of Susan’s stealing and reduced her urges to steal, but her symptoms continued to interfere significantly with her overall functioning.
We then added the atypical antipsychotic quetiapine, 100 mg bid, and Susan’s urges to steal and stealing behavior went into remission within 3 weeks. She has refrained from stealing for the last 9 months.
Making the diagnosis
In our clinic, we have treated more than 50 patients with kleptomania. Rather than coming to us through the criminal justice system, they are usually self-referred. Often they contact us after discovering on the Internet that we specialize in treating persons with this disorder.
In our experience, kleptomania typically goes undiagnosed in clinical settings, in part because patients are ashamed and embarrassed to discuss their symptoms with physicians unless specifically asked.1 If left untreated, however, kleptomania frequently becomes chronic.4 If persons with kleptomania are to seek treatment, it is important that family, friends, and mental health professionals understand the myths and facts about this disorder (Box).
To make the diagnosis, we use the simple screening instrument shown in Table 1.7 In general, because of high comorbidity with certain disorders, we screen every patient presenting to our clinic with a mood, substance use, anxiety, or eating disorder, or who has a problem with impulse control. Kleptomania is likely if the patient answers “yes” to questions 1 through 4 and to question 5 or 6. Stealing may be a symptom of several other psychiatric disorders, however, and misdiagnosis is fairly common (Table 2).6-8
Data suggest that the female-to-male ratio in kleptomania is approximately 2:1, with onset in adolescence. Typical individuals with kleptomania steal because they have urges to steal, often triggered by specific stimuli such as the sights and sounds of stores or feelings of loneliness or stress.1 Most patients with kleptomania are fairly specific about the types of stores from which they steal and the items they steal, and most hoard stolen items.1
Although many patients with kleptomania function quite well, others are severely debilitated in social and occupational realms. In a series of 22 patients with kleptomania:
- 64% had been apprehended
- 23% had served jail time
- 27% had been hospitalized because of their kleptomania symptoms
- 18% had considered or attempted suicide because of the distress associated with their kleptomania.1
Treatment recommendations
Patient history With patients who steal, we begin by identifying the motivation behind the stealing. Most patients with kleptomania report urges to steal. Some of these patients may have comorbid depression; for them, stealing makes them feel less depressed. Anger or irritability may point to borderline personality disorder. Stealing for the enjoyment of risk may suggest bipolar disorder.
Table 2
DIFFERENTIAL DIAGNOSIS: DISORDERS THAT MAY INVOLVE STEALING
| Misdiagnosis | How to distinguish from kleptomania |
|---|---|
| Bipolar disorder | Patients with bipolar disorder may steal as a result of the impulsivity of mania. In fact, the diagnostic criteria for kleptomania require the exclusion of mania as the cause of stealing.7 Patients with bipolar disorder report an elevated, expansive, or irritable mood while stealing. Patients with kleptomania tend to report a depressed mood when not stealing |
| Borderline personality disorder | Unlike patients with borderline personality disorder, patients with kleptomania do not report long histories of unstable relationships or negative self-image; inappropriate anger and “psychotic-like” symptoms are rare in patients with kleptomania |
| Antisocial personality disorder (ASPD, or conduct disorder) | Patients with kleptomania suffer intense shame and guilt, unlike those with ASPD. Also, most patients with kleptomania do not report other illegal or antisocial behavior. |
| Eating disorders | Data suggests that about one-third of patients with an eating disorder also steal.6,8 Patients with kleptomania, however, do not have disordered eating patterns or distorted body images common to patients with eating disorders. |
Many patients with kleptomania have comorbid mood, substance, or anxiety disorders. Treating these other symptoms while ignoring the symptoms of kleptomania may be unsuccessful. Comorbidity also may influence the choice of medication.
Medical assessment Case reports have associated the onset of kleptomania with a variety of medical conditions, including presenile cortical atrophy in a 25-year-old, a parietal tumor that caused blackouts and obliterated any memory of stealing episodes, narcolepsy, and an insulinoma that caused severe hypoglycemia.9 The relationship of these conditions with the onset of kleptomania is unclear, but the reports suggest that medical causes—although unlikely—should be ruled out before you consider kleptomania as a psychiatric illness.
Patient education Persons with kleptomania often feel that no one else has the same problem. They do not think of their behavior as being an illness. It is helpful to explain that kleptomania is treatable and to connect patients with educational books, self-help groups, and Web sites providing information and support (see “Related resources”).
Cognitive-behavioral therapy (CBT) Although the evidence is quite limited, covert sensitization, exposure and response prevention, and imaginal desensitization have all been shown effective in case reports.10
What medications are effective?
Only case reports, a case series of five subjects, and a single open-label treatment study involving 10 subjects with kleptomania have been done.
So far, uses of tricyclic antidepressants (imipramine, nortriptyline), SSRIs (fluoxetine, fluvoxamine, paroxetine), the opioid antagonist naltrexone, and mood stabilizers (lithium, valproate) have met with varying degrees of success. Strategies targeting urge and behavior reduction and mechanisms for coping with urges and behavior (e.g., cognitive-behavioral therapies) may represent important adjunctive components.2,11-17
No medications are FDA-approved for treating kleptomania. Therefore, it is important to inform patients of any off-label use of medications for this disorder, as well as the empirical basis for considering pharmacologic treatment.
SSRIs Only case reports exist on the use of SSRIs in treating kleptomania. The disorder may share a common pathology with pathologic gambling, and in our clinical experience appears to respond to similar treatments.18 We draw on research of pathologic gambling as well as our clinical experience in choosing SSRIs as first-line treatment, especially for patients with significant mood symptoms.19
We suggest titrating SSRIs to the maximum recommended dosage. As in the treatment of pathologic gambling, dosages of SSRIs required to treat kleptomania symptoms appear to be higher than average dosages required to treat depressive disorders. An SSRI should not be considered ineffective unless it has been tried for at least 10 to 12 weeks and the highest dosage tolerated or recommended by the manufacturer has been reached.
Response to SSRIs usually is characterized by decreased thoughts about stealing, decreased stealing behavior, and improvement in social and occupational functioning. If an SSRI is only partially effective, we consider augmentation with naltrexone, buspirone, or a mood stabilizer.
Naltrexone Patients taking naltrexone often report less-intense urges to steal. The urges may not disappear but are often sufficiently reduced so that the patient can resist them more easily. Patients also report that the thrill associated with stealing is reduced or eliminated.
Naltrexone was used in the first medication study of kleptomania and showed a significant decline in the intensity of urges to steal, stealing thoughts, and stealing behavior. Average dosage was 150 mg/d;11 a reduced dosage (e.g., 50 mg/d) may work in adolescents with kleptomania.20
Nausea as a side effect can be reduced by starting patients on 25 mg/d for the first 3 or 4 days and possibly adding ondansetron, 4 to 8 mg/d. Nausea and diarrhea are usually mild and resolve within the first week. Clinically, most patients respond to naltrexone within 2 weeks. After that, the dosage usually needs to be adjusted.
In patients with comorbid depression, augmentation with an SSRI may prevent worsening of untreated depressive symptoms. It is prudent to obtain liver function tests prior to naltrexone administration and again 3 to 4 weeks after starting the drug.21 Repeat testing should be performed at 2-to 4-week intervals for the next 2 months, then once a month for the following 3 months. After 6 months, testing three to four times a year is usually sufficient.
Nonsteroidal analgesics should not be used with high dosages of naltrexone (>50 mg/d), as concurrent use may increase the risk of hepatic transaminase elevation.21
Mood stabilizers Responses to lithium and valproate have been described in two case reports of patients with kleptomania.14,15 In the case of valproate, the effective dosage was 2,000 mg/d, whereas lithium reduced stealing urges at a serum level of 0.5 mEq/L.
Although it would be premature to recommend the use of mood stabilizers, their possible benefit may be related to their efficacy in bipolar disorder treatment and the existence of features (e.g., impulsivity) shared by kleptomania and bipolar disorder.
Atypical antipsychotics Although there is no evidence that atypical antipsychotics are useful in kleptomania, augmenting an SSRI with an atypical neuroleptic may be beneficial. Atypical antipsychotics have been explored as augmenting agents in the treatment of nonpsychotic disorders and behaviors, including pathologic gambling and obsessive-compulsive disorder.
The role of psychotherapy
Cognitive-behavioral therapy Based on the evidence of its effectiveness in treating pathologic gambling, CBT may hold promise as monotherapy for mild cases of kleptomania.
Combination therapy Combined pharmacologic and behavioral therapy may be the optimal treatment strategy for kleptomania. In our experience, patients who respond only partially or fail to respond to pharmacotherapy alone are more likely to find relief with a combination of drug and cognitive-behavioral therapies.
- Goldman MJ. Kleptomania: the compulsion to steal—what can be done? Far Hills, N New Horizon Press, 1998.
- Shoplifters Alternative http://www.shoplifters.org
- Impulse Control Disorders Clinic, University of Minnesota http://www.med.umn.edu/psychiatry/research/impulse.htm
Drug brand names
- Citalopram • Celexa
- Fluvoxamine • Luvox
- Imipramine • Tofranil
- Naltrexone • Revia
- Nortriptyline • Aventyl, Pamelor
- Paroxetine • Paxil
- Quetiapine • Seroquel
- Valproic acid • Depakote
Disclosure
The authors report no affiliation or financial arrangement with any of the companies whose products are mentioned in this article.
What is kleptomania? An independent illness, a symptom of other psychiatric disorders, or merely criminal behavior? Kleptomania—a disorder defined by an inability to resist the impulse to steal—is one of psychiatry’s most poorly understood diagnoses, even though it has been recognized in the literature for almost 200 years.
Kleptomania causes notable distress and impaired functioning.1 People with kleptomania often suffer from comorbid mood, anxiety, substance use, and other impulse-control disorders.14 They experience the humiliation of repeated arrests, which leads to guilt, depression, and even suicide.1,5 Yet kleptomania usually goes undiagnosed and untreated, despite a lifetime prevalence as high as 0.6%.6
Case report: ‘I’m a thief’
“I’m a thief,” began Susan, age 39. “I steal something four or five times every week. I steal from grocery stores and clothing stores. Sometimes I might steal something like vanilla extract; other times an expensive men’s tie. I probably steal $200 worth of items every week.
“You probably won’t believe this, but I don’t want or need the stuff I take. I have plenty of money. I have no idea why I take the things I do. That’s why I’m so depressed. What kind of person does something like this?
The urge to steal “I was probably 14 when I started stealing. I would go to stores with my mother. When I saw certain objects, I would get urges to steal them. The odd thing was that the items I stole were so ridiculous. I remember stealing key chains for several months, maybe three or four times a week. When I got older, things got worse. I was having urges more often, and so I needed to steal more often.
| Myth | Fact |
|---|---|
| Only little old ladies are kleptomaniacs. | Men and women of all ages suffer from kleptomania. Most patients report that the disorder began in adolescence. |
| It’s just a phase kids go through. | Parents of adolescents might see stealing as a phase. In many cases this might be true, but stealing may also suggest an underlying psychopathology. |
| People who steal are “bad.” | People with kleptomania steal because of urges to steal, not because of moral weakness. Treatment, not judgment, is the appropriate response. |
“My entire life has been torment. Each day I worry about having the urges, and then I worry about being caught stealing. I can’t relax. I’ve been married for 17 years, and I haven’t told my husband. My secrecy is tearing our marriage apart. My husband thinks I’m having an affair because I’ve distanced myself emotionally from him.”
Table 1
SCREENING TEST FOR KLEPTOMANIA
| Yes | No | |
|---|---|---|
| 1. Do you steal or have urges to steal? | ○ | ○ |
| 2. Do thoughts of stealing or urges to steal preoccupy you? That is, do you often think about stealing or have urges to steal and wish the thoughts or urges occurred less often? | ○ | ○ |
| 3. Do you feel tense or anxious before you steal or when you have urges to steal? | ○ | ○ |
| 4. Do you feel pleasure or a sense of calm when you steal something? | ○ | ○ |
| 5. Has the stealing or urges to steal caused you much distress? | ○ | ○ |
| 6. Has the stealing or urges to steal significantly interfered with your life in some way? | ○ | ○ |
| A patient who answers “yes” to questions 1 through 4 and to question 5 or 6 is likely to have kleptomania. | ||
| Adapted from DSM-IV criteria, American Psychiatric Association, 2000 | ||
Susan described urges to steal almost every day. When the urges were mild, she could resist them. Other days they were severe, and Susan felt unable to control her behavior. At work, her urges distracted her from completing projects, and her performance suffered. The urge to steal would often compel Susan to leave work early so she could get to a store.
Calm, then guilt “Every time I steal something I feel both a thrill and a great sense of calm,” she said. “It feels good. The problem is that almost immediately after each theft, I feel guilty and ashamed. After I steal, I usually donate the items to the Salvation Army, throw them away, or give them away as gifts.”
Drug trials We started treating Susan with the selective serotonin reuptake inhibitor (SSRI), citalopram. She reported notable improvement in her mood after 3 weeks on a dosage of 60 mg/d and she had been attending weekly psychotherapy, although her stealing continued unchanged. The addition of naltrexone, 200 mg/d for 2 weeks, decreased the frequency of Susan’s stealing and reduced her urges to steal, but her symptoms continued to interfere significantly with her overall functioning.
We then added the atypical antipsychotic quetiapine, 100 mg bid, and Susan’s urges to steal and stealing behavior went into remission within 3 weeks. She has refrained from stealing for the last 9 months.
Making the diagnosis
In our clinic, we have treated more than 50 patients with kleptomania. Rather than coming to us through the criminal justice system, they are usually self-referred. Often they contact us after discovering on the Internet that we specialize in treating persons with this disorder.
In our experience, kleptomania typically goes undiagnosed in clinical settings, in part because patients are ashamed and embarrassed to discuss their symptoms with physicians unless specifically asked.1 If left untreated, however, kleptomania frequently becomes chronic.4 If persons with kleptomania are to seek treatment, it is important that family, friends, and mental health professionals understand the myths and facts about this disorder (Box).
To make the diagnosis, we use the simple screening instrument shown in Table 1.7 In general, because of high comorbidity with certain disorders, we screen every patient presenting to our clinic with a mood, substance use, anxiety, or eating disorder, or who has a problem with impulse control. Kleptomania is likely if the patient answers “yes” to questions 1 through 4 and to question 5 or 6. Stealing may be a symptom of several other psychiatric disorders, however, and misdiagnosis is fairly common (Table 2).6-8
Data suggest that the female-to-male ratio in kleptomania is approximately 2:1, with onset in adolescence. Typical individuals with kleptomania steal because they have urges to steal, often triggered by specific stimuli such as the sights and sounds of stores or feelings of loneliness or stress.1 Most patients with kleptomania are fairly specific about the types of stores from which they steal and the items they steal, and most hoard stolen items.1
Although many patients with kleptomania function quite well, others are severely debilitated in social and occupational realms. In a series of 22 patients with kleptomania:
- 64% had been apprehended
- 23% had served jail time
- 27% had been hospitalized because of their kleptomania symptoms
- 18% had considered or attempted suicide because of the distress associated with their kleptomania.1
Treatment recommendations
Patient history With patients who steal, we begin by identifying the motivation behind the stealing. Most patients with kleptomania report urges to steal. Some of these patients may have comorbid depression; for them, stealing makes them feel less depressed. Anger or irritability may point to borderline personality disorder. Stealing for the enjoyment of risk may suggest bipolar disorder.
Table 2
DIFFERENTIAL DIAGNOSIS: DISORDERS THAT MAY INVOLVE STEALING
| Misdiagnosis | How to distinguish from kleptomania |
|---|---|
| Bipolar disorder | Patients with bipolar disorder may steal as a result of the impulsivity of mania. In fact, the diagnostic criteria for kleptomania require the exclusion of mania as the cause of stealing.7 Patients with bipolar disorder report an elevated, expansive, or irritable mood while stealing. Patients with kleptomania tend to report a depressed mood when not stealing |
| Borderline personality disorder | Unlike patients with borderline personality disorder, patients with kleptomania do not report long histories of unstable relationships or negative self-image; inappropriate anger and “psychotic-like” symptoms are rare in patients with kleptomania |
| Antisocial personality disorder (ASPD, or conduct disorder) | Patients with kleptomania suffer intense shame and guilt, unlike those with ASPD. Also, most patients with kleptomania do not report other illegal or antisocial behavior. |
| Eating disorders | Data suggests that about one-third of patients with an eating disorder also steal.6,8 Patients with kleptomania, however, do not have disordered eating patterns or distorted body images common to patients with eating disorders. |
Many patients with kleptomania have comorbid mood, substance, or anxiety disorders. Treating these other symptoms while ignoring the symptoms of kleptomania may be unsuccessful. Comorbidity also may influence the choice of medication.
Medical assessment Case reports have associated the onset of kleptomania with a variety of medical conditions, including presenile cortical atrophy in a 25-year-old, a parietal tumor that caused blackouts and obliterated any memory of stealing episodes, narcolepsy, and an insulinoma that caused severe hypoglycemia.9 The relationship of these conditions with the onset of kleptomania is unclear, but the reports suggest that medical causes—although unlikely—should be ruled out before you consider kleptomania as a psychiatric illness.
Patient education Persons with kleptomania often feel that no one else has the same problem. They do not think of their behavior as being an illness. It is helpful to explain that kleptomania is treatable and to connect patients with educational books, self-help groups, and Web sites providing information and support (see “Related resources”).
Cognitive-behavioral therapy (CBT) Although the evidence is quite limited, covert sensitization, exposure and response prevention, and imaginal desensitization have all been shown effective in case reports.10
What medications are effective?
Only case reports, a case series of five subjects, and a single open-label treatment study involving 10 subjects with kleptomania have been done.
So far, uses of tricyclic antidepressants (imipramine, nortriptyline), SSRIs (fluoxetine, fluvoxamine, paroxetine), the opioid antagonist naltrexone, and mood stabilizers (lithium, valproate) have met with varying degrees of success. Strategies targeting urge and behavior reduction and mechanisms for coping with urges and behavior (e.g., cognitive-behavioral therapies) may represent important adjunctive components.2,11-17
No medications are FDA-approved for treating kleptomania. Therefore, it is important to inform patients of any off-label use of medications for this disorder, as well as the empirical basis for considering pharmacologic treatment.
SSRIs Only case reports exist on the use of SSRIs in treating kleptomania. The disorder may share a common pathology with pathologic gambling, and in our clinical experience appears to respond to similar treatments.18 We draw on research of pathologic gambling as well as our clinical experience in choosing SSRIs as first-line treatment, especially for patients with significant mood symptoms.19
We suggest titrating SSRIs to the maximum recommended dosage. As in the treatment of pathologic gambling, dosages of SSRIs required to treat kleptomania symptoms appear to be higher than average dosages required to treat depressive disorders. An SSRI should not be considered ineffective unless it has been tried for at least 10 to 12 weeks and the highest dosage tolerated or recommended by the manufacturer has been reached.
Response to SSRIs usually is characterized by decreased thoughts about stealing, decreased stealing behavior, and improvement in social and occupational functioning. If an SSRI is only partially effective, we consider augmentation with naltrexone, buspirone, or a mood stabilizer.
Naltrexone Patients taking naltrexone often report less-intense urges to steal. The urges may not disappear but are often sufficiently reduced so that the patient can resist them more easily. Patients also report that the thrill associated with stealing is reduced or eliminated.
Naltrexone was used in the first medication study of kleptomania and showed a significant decline in the intensity of urges to steal, stealing thoughts, and stealing behavior. Average dosage was 150 mg/d;11 a reduced dosage (e.g., 50 mg/d) may work in adolescents with kleptomania.20
Nausea as a side effect can be reduced by starting patients on 25 mg/d for the first 3 or 4 days and possibly adding ondansetron, 4 to 8 mg/d. Nausea and diarrhea are usually mild and resolve within the first week. Clinically, most patients respond to naltrexone within 2 weeks. After that, the dosage usually needs to be adjusted.
In patients with comorbid depression, augmentation with an SSRI may prevent worsening of untreated depressive symptoms. It is prudent to obtain liver function tests prior to naltrexone administration and again 3 to 4 weeks after starting the drug.21 Repeat testing should be performed at 2-to 4-week intervals for the next 2 months, then once a month for the following 3 months. After 6 months, testing three to four times a year is usually sufficient.
Nonsteroidal analgesics should not be used with high dosages of naltrexone (>50 mg/d), as concurrent use may increase the risk of hepatic transaminase elevation.21
Mood stabilizers Responses to lithium and valproate have been described in two case reports of patients with kleptomania.14,15 In the case of valproate, the effective dosage was 2,000 mg/d, whereas lithium reduced stealing urges at a serum level of 0.5 mEq/L.
Although it would be premature to recommend the use of mood stabilizers, their possible benefit may be related to their efficacy in bipolar disorder treatment and the existence of features (e.g., impulsivity) shared by kleptomania and bipolar disorder.
Atypical antipsychotics Although there is no evidence that atypical antipsychotics are useful in kleptomania, augmenting an SSRI with an atypical neuroleptic may be beneficial. Atypical antipsychotics have been explored as augmenting agents in the treatment of nonpsychotic disorders and behaviors, including pathologic gambling and obsessive-compulsive disorder.
The role of psychotherapy
Cognitive-behavioral therapy Based on the evidence of its effectiveness in treating pathologic gambling, CBT may hold promise as monotherapy for mild cases of kleptomania.
Combination therapy Combined pharmacologic and behavioral therapy may be the optimal treatment strategy for kleptomania. In our experience, patients who respond only partially or fail to respond to pharmacotherapy alone are more likely to find relief with a combination of drug and cognitive-behavioral therapies.
- Goldman MJ. Kleptomania: the compulsion to steal—what can be done? Far Hills, N New Horizon Press, 1998.
- Shoplifters Alternative http://www.shoplifters.org
- Impulse Control Disorders Clinic, University of Minnesota http://www.med.umn.edu/psychiatry/research/impulse.htm
Drug brand names
- Citalopram • Celexa
- Fluvoxamine • Luvox
- Imipramine • Tofranil
- Naltrexone • Revia
- Nortriptyline • Aventyl, Pamelor
- Paroxetine • Paxil
- Quetiapine • Seroquel
- Valproic acid • Depakote
Disclosure
The authors report no affiliation or financial arrangement with any of the companies whose products are mentioned in this article.
1. Grant JE, Kim SW. Clinical characteristics and associated psychopathology in 22 cases of kleptomania. Comp Psychiatry (in press).
2. McElroy SL, Pope HG, Hudson JI, Keck PE, White KL. Kleptomania: a report of 20 cases. Am J Psychiatry 1991;148:652-7.
3. Presta S, Marazziti D, Dell’Osso L, et al. Kleptomania: clinical features and comorbidity in an Italian sample. Comp Psychiatry 2002;43:7-12.
4. McElroy SL, Keck PE, Phillips KA. Kleptomania, compulsive buying, and binge-eating disorder. J Clin Psychiatry 1995;56:14-26.
5. McElroy SL, Hudson JI, Pope HG, Keck PE. Kleptomania: clinical characteristics and associated psychopathology. Psychol Med 1991;21:93-108.
6. Goldman MJ. Kleptomania: an overview. Psychiatric Ann 1992;22:68-71.
7. American Psychiatric Association Committee on Nomenclature and Statistics Diagnostic and statistical manual of mental disorders (4th ed, text rev). Washington, DC: American Psychiatric Association, 2000.
8. Krahn DD, Nairn K, Gosnell BA, Drewnowski A. Stealing in eating disordered patients. J Clin Psychiatry 1991;52:112-5.
9. Goldman MJ. Kleptomania: making sense of the nonsensical. Am J Psychiatry 1991;148:986-96.
10. Goldman MJ. Kleptomania: the compulsion to steal—what can be done? Far Hills, NJ: New Horizon Press, 1998.
11. Grant JE, Kim SW. An open-label study of naltrexone in the treatment of kleptomania. J Clin Psychiatry 2002;63:349-56.
12. Chong SA, Low BL. Treatment of kleptomania with fluvoxamine. Acta Psychiatr Scand 1996;93:314-5.
13. Kraus JE. Treatment of kleptomania with paroxetine. J Clin Psychiatry 1999;60:793.-
14. Burstein A. Fluoxetine lithium treatment for kleptomania. J Clin Psychiatry 1992;53:28-9.
15. Kmetz GF, McElroy SL, Collins DJ. Response of kleptomania and mixed mania to valproate. Am J Psychiatry 1997;154:580-1.
16. Lepkifker E, Dannon PN, Ziv R, Iancu I, Horesh N, Kotler M. The treatment of kleptomania with serotonin reuptake inhibitors. Clin Neuropharmacol 1999;22:40-3.
17. Durst R, Katz G, Knobler HY. Buspirone augmentation of fluvoxamine in the treatment of kleptomania. J Nerv Ment Dis 1997;185:586-8.
18. Kim SW. Opioid antagonists in the treatment of impulse-control disorders. J Clin Psychiatry 1998;59:159-64.
19. Grant JE, Kim SW. Pharmacotherapy of pathological gambling. Psychiatric Ann 2002;32:186-91.
20. Grant JE, Kim SW. Adolescent kleptomania treated with naltrexone: a case report. Eur Child Adolescent Psychiatry 2002;11:92-5.
21. Kim SW, Grant JE, et al. A preliminary report on a possible naltrexone and nonsteroidal analgesics interaction. J Clin Psychopharmacol 2001;21:632-4.
1. Grant JE, Kim SW. Clinical characteristics and associated psychopathology in 22 cases of kleptomania. Comp Psychiatry (in press).
2. McElroy SL, Pope HG, Hudson JI, Keck PE, White KL. Kleptomania: a report of 20 cases. Am J Psychiatry 1991;148:652-7.
3. Presta S, Marazziti D, Dell’Osso L, et al. Kleptomania: clinical features and comorbidity in an Italian sample. Comp Psychiatry 2002;43:7-12.
4. McElroy SL, Keck PE, Phillips KA. Kleptomania, compulsive buying, and binge-eating disorder. J Clin Psychiatry 1995;56:14-26.
5. McElroy SL, Hudson JI, Pope HG, Keck PE. Kleptomania: clinical characteristics and associated psychopathology. Psychol Med 1991;21:93-108.
6. Goldman MJ. Kleptomania: an overview. Psychiatric Ann 1992;22:68-71.
7. American Psychiatric Association Committee on Nomenclature and Statistics Diagnostic and statistical manual of mental disorders (4th ed, text rev). Washington, DC: American Psychiatric Association, 2000.
8. Krahn DD, Nairn K, Gosnell BA, Drewnowski A. Stealing in eating disordered patients. J Clin Psychiatry 1991;52:112-5.
9. Goldman MJ. Kleptomania: making sense of the nonsensical. Am J Psychiatry 1991;148:986-96.
10. Goldman MJ. Kleptomania: the compulsion to steal—what can be done? Far Hills, NJ: New Horizon Press, 1998.
11. Grant JE, Kim SW. An open-label study of naltrexone in the treatment of kleptomania. J Clin Psychiatry 2002;63:349-56.
12. Chong SA, Low BL. Treatment of kleptomania with fluvoxamine. Acta Psychiatr Scand 1996;93:314-5.
13. Kraus JE. Treatment of kleptomania with paroxetine. J Clin Psychiatry 1999;60:793.-
14. Burstein A. Fluoxetine lithium treatment for kleptomania. J Clin Psychiatry 1992;53:28-9.
15. Kmetz GF, McElroy SL, Collins DJ. Response of kleptomania and mixed mania to valproate. Am J Psychiatry 1997;154:580-1.
16. Lepkifker E, Dannon PN, Ziv R, Iancu I, Horesh N, Kotler M. The treatment of kleptomania with serotonin reuptake inhibitors. Clin Neuropharmacol 1999;22:40-3.
17. Durst R, Katz G, Knobler HY. Buspirone augmentation of fluvoxamine in the treatment of kleptomania. J Nerv Ment Dis 1997;185:586-8.
18. Kim SW. Opioid antagonists in the treatment of impulse-control disorders. J Clin Psychiatry 1998;59:159-64.
19. Grant JE, Kim SW. Pharmacotherapy of pathological gambling. Psychiatric Ann 2002;32:186-91.
20. Grant JE, Kim SW. Adolescent kleptomania treated with naltrexone: a case report. Eur Child Adolescent Psychiatry 2002;11:92-5.
21. Kim SW, Grant JE, et al. A preliminary report on a possible naltrexone and nonsteroidal analgesics interaction. J Clin Psychopharmacol 2001;21:632-4.
Kleptomania: Emerging therapies target mood, impulsive behavior
What is kleptomania? An independent illness, a symptom of other psychiatric disorders, or merely criminal behavior? Kleptomania—a disorder defined by an inability to resist the impulse to steal—is one of psychiatry’s most poorly understood diagnoses, even though it has been recognized in the literature for almost 200 years.
Kleptomania causes notable distress and impaired functioning.1 People with kleptomania often suffer from comorbid mood, anxiety, substance use, and other impulse-control disorders.14 They experience the humiliation of repeated arrests, which leads to guilt, depression, and even suicide.1,5 Yet kleptomania usually goes undiagnosed and untreated, despite a lifetime prevalence as high as 0.6%.6
Case report: ‘I’m a thief’
“I’m a thief,” began Susan, age 39. “I steal something four or five times every week. I steal from grocery stores and clothing stores. Sometimes I might steal something like vanilla extract; other times an expensive men’s tie. I probably steal $200 worth of items every week.
“You probably won’t believe this, but I don’t want or need the stuff I take. I have plenty of money. I have no idea why I take the things I do. That’s why I’m so depressed. What kind of person does something like this?
The urge to steal “I was probably 14 when I started stealing. I would go to stores with my mother. When I saw certain objects, I would get urges to steal them. The odd thing was that the items I stole were so ridiculous. I remember stealing key chains for several months, maybe three or four times a week. When I got older, things got worse. I was having urges more often, and so I needed to steal more often.
| Myth | Fact |
|---|---|
| Only little old ladies are kleptomaniacs. | Men and women of all ages suffer from kleptomania. Most patients report that the disorder began in adolescence. |
| It’s just a phase kids go through. | Parents of adolescents might see stealing as a phase. In many cases this might be true, but stealing may also suggest an underlying psychopathology. |
| People who steal are “bad.” | People with kleptomania steal because of urges to steal, not because of moral weakness. Treatment, not judgment, is the appropriate response. |
“My entire life has been torment. Each day I worry about having the urges, and then I worry about being caught stealing. I can’t relax. I’ve been married for 17 years, and I haven’t told my husband. My secrecy is tearing our marriage apart. My husband thinks I’m having an affair because I’ve distanced myself emotionally from him.”
Table 1
SCREENING TEST FOR KLEPTOMANIA
| Yes | No | |
|---|---|---|
| 1. Do you steal or have urges to steal? | ○ | ○ |
| 2. Do thoughts of stealing or urges to steal preoccupy you? That is, do you often think about stealing or have urges to steal and wish the thoughts or urges occurred less often? | ○ | ○ |
| 3. Do you feel tense or anxious before you steal or when you have urges to steal? | ○ | ○ |
| 4. Do you feel pleasure or a sense of calm when you steal something? | ○ | ○ |
| 5. Has the stealing or urges to steal caused you much distress? | ○ | ○ |
| 6. Has the stealing or urges to steal significantly interfered with your life in some way? | ○ | ○ |
| A patient who answers “yes” to questions 1 through 4 and to question 5 or 6 is likely to have kleptomania. | ||
| Adapted from DSM-IV criteria, American Psychiatric Association, 2000 | ||
Susan described urges to steal almost every day. When the urges were mild, she could resist them. Other days they were severe, and Susan felt unable to control her behavior. At work, her urges distracted her from completing projects, and her performance suffered. The urge to steal would often compel Susan to leave work early so she could get to a store.
Calm, then guilt “Every time I steal something I feel both a thrill and a great sense of calm,” she said. “It feels good. The problem is that almost immediately after each theft, I feel guilty and ashamed. After I steal, I usually donate the items to the Salvation Army, throw them away, or give them away as gifts.”
Drug trials We started treating Susan with the selective serotonin reuptake inhibitor (SSRI), citalopram. She reported notable improvement in her mood after 3 weeks on a dosage of 60 mg/d and she had been attending weekly psychotherapy, although her stealing continued unchanged. The addition of naltrexone, 200 mg/d for 2 weeks, decreased the frequency of Susan’s stealing and reduced her urges to steal, but her symptoms continued to interfere significantly with her overall functioning.
We then added the atypical antipsychotic quetiapine, 100 mg bid, and Susan’s urges to steal and stealing behavior went into remission within 3 weeks. She has refrained from stealing for the last 9 months.
Making the diagnosis
In our clinic, we have treated more than 50 patients with kleptomania. Rather than coming to us through the criminal justice system, they are usually self-referred. Often they contact us after discovering on the Internet that we specialize in treating persons with this disorder.
In our experience, kleptomania typically goes undiagnosed in clinical settings, in part because patients are ashamed and embarrassed to discuss their symptoms with physicians unless specifically asked.1 If left untreated, however, kleptomania frequently becomes chronic.4 If persons with kleptomania are to seek treatment, it is important that family, friends, and mental health professionals understand the myths and facts about this disorder (Box).
To make the diagnosis, we use the simple screening instrument shown in Table 1.7 In general, because of high comorbidity with certain disorders, we screen every patient presenting to our clinic with a mood, substance use, anxiety, or eating disorder, or who has a problem with impulse control. Kleptomania is likely if the patient answers “yes” to questions 1 through 4 and to question 5 or 6. Stealing may be a symptom of several other psychiatric disorders, however, and misdiagnosis is fairly common (Table 2).6-8
Data suggest that the female-to-male ratio in kleptomania is approximately 2:1, with onset in adolescence. Typical individuals with kleptomania steal because they have urges to steal, often triggered by specific stimuli such as the sights and sounds of stores or feelings of loneliness or stress.1 Most patients with kleptomania are fairly specific about the types of stores from which they steal and the items they steal, and most hoard stolen items.1
Although many patients with kleptomania function quite well, others are severely debilitated in social and occupational realms. In a series of 22 patients with kleptomania:
- 64% had been apprehended
- 23% had served jail time
- 27% had been hospitalized because of their kleptomania symptoms
- 18% had considered or attempted suicide because of the distress associated with their kleptomania.1
Treatment recommendations
Patient history With patients who steal, we begin by identifying the motivation behind the stealing. Most patients with kleptomania report urges to steal. Some of these patients may have comorbid depression; for them, stealing makes them feel less depressed. Anger or irritability may point to borderline personality disorder. Stealing for the enjoyment of risk may suggest bipolar disorder.
Table 2
DIFFERENTIAL DIAGNOSIS: DISORDERS THAT MAY INVOLVE STEALING
| Misdiagnosis | How to distinguish from kleptomania |
|---|---|
| Bipolar disorder | Patients with bipolar disorder may steal as a result of the impulsivity of mania. In fact, the diagnostic criteria for kleptomania require the exclusion of mania as the cause of stealing.7 Patients with bipolar disorder report an elevated, expansive, or irritable mood while stealing. Patients with kleptomania tend to report a depressed mood when not stealing |
| Borderline personality disorder | Unlike patients with borderline personality disorder, patients with kleptomania do not report long histories of unstable relationships or negative self-image; inappropriate anger and “psychotic-like” symptoms are rare in patients with kleptomania |
| Antisocial personality disorder (ASPD, or conduct disorder) | Patients with kleptomania suffer intense shame and guilt, unlike those with ASPD. Also, most patients with kleptomania do not report other illegal or antisocial behavior. |
| Eating disorders | Data suggests that about one-third of patients with an eating disorder also steal.6,8 Patients with kleptomania, however, do not have disordered eating patterns or distorted body images common to patients with eating disorders. |
Many patients with kleptomania have comorbid mood, substance, or anxiety disorders. Treating these other symptoms while ignoring the symptoms of kleptomania may be unsuccessful. Comorbidity also may influence the choice of medication.
Medical assessment Case reports have associated the onset of kleptomania with a variety of medical conditions, including presenile cortical atrophy in a 25-year-old, a parietal tumor that caused blackouts and obliterated any memory of stealing episodes, narcolepsy, and an insulinoma that caused severe hypoglycemia.9 The relationship of these conditions with the onset of kleptomania is unclear, but the reports suggest that medical causes—although unlikely—should be ruled out before you consider kleptomania as a psychiatric illness.
Patient education Persons with kleptomania often feel that no one else has the same problem. They do not think of their behavior as being an illness. It is helpful to explain that kleptomania is treatable and to connect patients with educational books, self-help groups, and Web sites providing information and support (see “Related resources”).
Cognitive-behavioral therapy (CBT) Although the evidence is quite limited, covert sensitization, exposure and response prevention, and imaginal desensitization have all been shown effective in case reports.10
What medications are effective?
Only case reports, a case series of five subjects, and a single open-label treatment study involving 10 subjects with kleptomania have been done.
So far, uses of tricyclic antidepressants (imipramine, nortriptyline), SSRIs (fluoxetine, fluvoxamine, paroxetine), the opioid antagonist naltrexone, and mood stabilizers (lithium, valproate) have met with varying degrees of success. Strategies targeting urge and behavior reduction and mechanisms for coping with urges and behavior (e.g., cognitive-behavioral therapies) may represent important adjunctive components.2,11-17
No medications are FDA-approved for treating kleptomania. Therefore, it is important to inform patients of any off-label use of medications for this disorder, as well as the empirical basis for considering pharmacologic treatment.
SSRIs Only case reports exist on the use of SSRIs in treating kleptomania. The disorder may share a common pathology with pathologic gambling, and in our clinical experience appears to respond to similar treatments.18 We draw on research of pathologic gambling as well as our clinical experience in choosing SSRIs as first-line treatment, especially for patients with significant mood symptoms.19
We suggest titrating SSRIs to the maximum recommended dosage. As in the treatment of pathologic gambling, dosages of SSRIs required to treat kleptomania symptoms appear to be higher than average dosages required to treat depressive disorders. An SSRI should not be considered ineffective unless it has been tried for at least 10 to 12 weeks and the highest dosage tolerated or recommended by the manufacturer has been reached.
Response to SSRIs usually is characterized by decreased thoughts about stealing, decreased stealing behavior, and improvement in social and occupational functioning. If an SSRI is only partially effective, we consider augmentation with naltrexone, buspirone, or a mood stabilizer.
Naltrexone Patients taking naltrexone often report less-intense urges to steal. The urges may not disappear but are often sufficiently reduced so that the patient can resist them more easily. Patients also report that the thrill associated with stealing is reduced or eliminated.
Naltrexone was used in the first medication study of kleptomania and showed a significant decline in the intensity of urges to steal, stealing thoughts, and stealing behavior. Average dosage was 150 mg/d;11 a reduced dosage (e.g., 50 mg/d) may work in adolescents with kleptomania.20
Nausea as a side effect can be reduced by starting patients on 25 mg/d for the first 3 or 4 days and possibly adding ondansetron, 4 to 8 mg/d. Nausea and diarrhea are usually mild and resolve within the first week. Clinically, most patients respond to naltrexone within 2 weeks. After that, the dosage usually needs to be adjusted.
In patients with comorbid depression, augmentation with an SSRI may prevent worsening of untreated depressive symptoms. It is prudent to obtain liver function tests prior to naltrexone administration and again 3 to 4 weeks after starting the drug.21 Repeat testing should be performed at 2-to 4-week intervals for the next 2 months, then once a month for the following 3 months. After 6 months, testing three to four times a year is usually sufficient.
Nonsteroidal analgesics should not be used with high dosages of naltrexone (>50 mg/d), as concurrent use may increase the risk of hepatic transaminase elevation.21
Mood stabilizers Responses to lithium and valproate have been described in two case reports of patients with kleptomania.14,15 In the case of valproate, the effective dosage was 2,000 mg/d, whereas lithium reduced stealing urges at a serum level of 0.5 mEq/L.
Although it would be premature to recommend the use of mood stabilizers, their possible benefit may be related to their efficacy in bipolar disorder treatment and the existence of features (e.g., impulsivity) shared by kleptomania and bipolar disorder.
Atypical antipsychotics Although there is no evidence that atypical antipsychotics are useful in kleptomania, augmenting an SSRI with an atypical neuroleptic may be beneficial. Atypical antipsychotics have been explored as augmenting agents in the treatment of nonpsychotic disorders and behaviors, including pathologic gambling and obsessive-compulsive disorder.
The role of psychotherapy
Cognitive-behavioral therapy Based on the evidence of its effectiveness in treating pathologic gambling, CBT may hold promise as monotherapy for mild cases of kleptomania.
Combination therapy Combined pharmacologic and behavioral therapy may be the optimal treatment strategy for kleptomania. In our experience, patients who respond only partially or fail to respond to pharmacotherapy alone are more likely to find relief with a combination of drug and cognitive-behavioral therapies.
- Goldman MJ. Kleptomania: the compulsion to steal—what can be done? Far Hills, N New Horizon Press, 1998.
- Shoplifters Alternative http://www.shoplifters.org
- Impulse Control Disorders Clinic, University of Minnesota http://www.med.umn.edu/psychiatry/research/impulse.htm
Drug brand names
- Citalopram • Celexa
- Fluvoxamine • Luvox
- Imipramine • Tofranil
- Naltrexone • Revia
- Nortriptyline • Aventyl, Pamelor
- Paroxetine • Paxil
- Quetiapine • Seroquel
- Valproic acid • Depakote
Disclosure
The authors report no affiliation or financial arrangement with any of the companies whose products are mentioned in this article.
1. Grant JE, Kim SW. Clinical characteristics and associated psychopathology in 22 cases of kleptomania. Comp Psychiatry (in press).
2. McElroy SL, Pope HG, Hudson JI, Keck PE, White KL. Kleptomania: a report of 20 cases. Am J Psychiatry 1991;148:652-7.
3. Presta S, Marazziti D, Dell’Osso L, et al. Kleptomania: clinical features and comorbidity in an Italian sample. Comp Psychiatry 2002;43:7-12.
4. McElroy SL, Keck PE, Phillips KA. Kleptomania, compulsive buying, and binge-eating disorder. J Clin Psychiatry 1995;56:14-26.
5. McElroy SL, Hudson JI, Pope HG, Keck PE. Kleptomania: clinical characteristics and associated psychopathology. Psychol Med 1991;21:93-108.
6. Goldman MJ. Kleptomania: an overview. Psychiatric Ann 1992;22:68-71.
7. American Psychiatric Association Committee on Nomenclature and Statistics Diagnostic and statistical manual of mental disorders (4th ed, text rev). Washington, DC: American Psychiatric Association, 2000.
8. Krahn DD, Nairn K, Gosnell BA, Drewnowski A. Stealing in eating disordered patients. J Clin Psychiatry 1991;52:112-5.
9. Goldman MJ. Kleptomania: making sense of the nonsensical. Am J Psychiatry 1991;148:986-96.
10. Goldman MJ. Kleptomania: the compulsion to steal—what can be done? Far Hills, NJ: New Horizon Press, 1998.
11. Grant JE, Kim SW. An open-label study of naltrexone in the treatment of kleptomania. J Clin Psychiatry 2002;63:349-56.
12. Chong SA, Low BL. Treatment of kleptomania with fluvoxamine. Acta Psychiatr Scand 1996;93:314-5.
13. Kraus JE. Treatment of kleptomania with paroxetine. J Clin Psychiatry 1999;60:793.-
14. Burstein A. Fluoxetine lithium treatment for kleptomania. J Clin Psychiatry 1992;53:28-9.
15. Kmetz GF, McElroy SL, Collins DJ. Response of kleptomania and mixed mania to valproate. Am J Psychiatry 1997;154:580-1.
16. Lepkifker E, Dannon PN, Ziv R, Iancu I, Horesh N, Kotler M. The treatment of kleptomania with serotonin reuptake inhibitors. Clin Neuropharmacol 1999;22:40-3.
17. Durst R, Katz G, Knobler HY. Buspirone augmentation of fluvoxamine in the treatment of kleptomania. J Nerv Ment Dis 1997;185:586-8.
18. Kim SW. Opioid antagonists in the treatment of impulse-control disorders. J Clin Psychiatry 1998;59:159-64.
19. Grant JE, Kim SW. Pharmacotherapy of pathological gambling. Psychiatric Ann 2002;32:186-91.
20. Grant JE, Kim SW. Adolescent kleptomania treated with naltrexone: a case report. Eur Child Adolescent Psychiatry 2002;11:92-5.
21. Kim SW, Grant JE, et al. A preliminary report on a possible naltrexone and nonsteroidal analgesics interaction. J Clin Psychopharmacol 2001;21:632-4.
What is kleptomania? An independent illness, a symptom of other psychiatric disorders, or merely criminal behavior? Kleptomania—a disorder defined by an inability to resist the impulse to steal—is one of psychiatry’s most poorly understood diagnoses, even though it has been recognized in the literature for almost 200 years.
Kleptomania causes notable distress and impaired functioning.1 People with kleptomania often suffer from comorbid mood, anxiety, substance use, and other impulse-control disorders.14 They experience the humiliation of repeated arrests, which leads to guilt, depression, and even suicide.1,5 Yet kleptomania usually goes undiagnosed and untreated, despite a lifetime prevalence as high as 0.6%.6
Case report: ‘I’m a thief’
“I’m a thief,” began Susan, age 39. “I steal something four or five times every week. I steal from grocery stores and clothing stores. Sometimes I might steal something like vanilla extract; other times an expensive men’s tie. I probably steal $200 worth of items every week.
“You probably won’t believe this, but I don’t want or need the stuff I take. I have plenty of money. I have no idea why I take the things I do. That’s why I’m so depressed. What kind of person does something like this?
The urge to steal “I was probably 14 when I started stealing. I would go to stores with my mother. When I saw certain objects, I would get urges to steal them. The odd thing was that the items I stole were so ridiculous. I remember stealing key chains for several months, maybe three or four times a week. When I got older, things got worse. I was having urges more often, and so I needed to steal more often.
| Myth | Fact |
|---|---|
| Only little old ladies are kleptomaniacs. | Men and women of all ages suffer from kleptomania. Most patients report that the disorder began in adolescence. |
| It’s just a phase kids go through. | Parents of adolescents might see stealing as a phase. In many cases this might be true, but stealing may also suggest an underlying psychopathology. |
| People who steal are “bad.” | People with kleptomania steal because of urges to steal, not because of moral weakness. Treatment, not judgment, is the appropriate response. |
“My entire life has been torment. Each day I worry about having the urges, and then I worry about being caught stealing. I can’t relax. I’ve been married for 17 years, and I haven’t told my husband. My secrecy is tearing our marriage apart. My husband thinks I’m having an affair because I’ve distanced myself emotionally from him.”
Table 1
SCREENING TEST FOR KLEPTOMANIA
| Yes | No | |
|---|---|---|
| 1. Do you steal or have urges to steal? | ○ | ○ |
| 2. Do thoughts of stealing or urges to steal preoccupy you? That is, do you often think about stealing or have urges to steal and wish the thoughts or urges occurred less often? | ○ | ○ |
| 3. Do you feel tense or anxious before you steal or when you have urges to steal? | ○ | ○ |
| 4. Do you feel pleasure or a sense of calm when you steal something? | ○ | ○ |
| 5. Has the stealing or urges to steal caused you much distress? | ○ | ○ |
| 6. Has the stealing or urges to steal significantly interfered with your life in some way? | ○ | ○ |
| A patient who answers “yes” to questions 1 through 4 and to question 5 or 6 is likely to have kleptomania. | ||
| Adapted from DSM-IV criteria, American Psychiatric Association, 2000 | ||
Susan described urges to steal almost every day. When the urges were mild, she could resist them. Other days they were severe, and Susan felt unable to control her behavior. At work, her urges distracted her from completing projects, and her performance suffered. The urge to steal would often compel Susan to leave work early so she could get to a store.
Calm, then guilt “Every time I steal something I feel both a thrill and a great sense of calm,” she said. “It feels good. The problem is that almost immediately after each theft, I feel guilty and ashamed. After I steal, I usually donate the items to the Salvation Army, throw them away, or give them away as gifts.”
Drug trials We started treating Susan with the selective serotonin reuptake inhibitor (SSRI), citalopram. She reported notable improvement in her mood after 3 weeks on a dosage of 60 mg/d and she had been attending weekly psychotherapy, although her stealing continued unchanged. The addition of naltrexone, 200 mg/d for 2 weeks, decreased the frequency of Susan’s stealing and reduced her urges to steal, but her symptoms continued to interfere significantly with her overall functioning.
We then added the atypical antipsychotic quetiapine, 100 mg bid, and Susan’s urges to steal and stealing behavior went into remission within 3 weeks. She has refrained from stealing for the last 9 months.
Making the diagnosis
In our clinic, we have treated more than 50 patients with kleptomania. Rather than coming to us through the criminal justice system, they are usually self-referred. Often they contact us after discovering on the Internet that we specialize in treating persons with this disorder.
In our experience, kleptomania typically goes undiagnosed in clinical settings, in part because patients are ashamed and embarrassed to discuss their symptoms with physicians unless specifically asked.1 If left untreated, however, kleptomania frequently becomes chronic.4 If persons with kleptomania are to seek treatment, it is important that family, friends, and mental health professionals understand the myths and facts about this disorder (Box).
To make the diagnosis, we use the simple screening instrument shown in Table 1.7 In general, because of high comorbidity with certain disorders, we screen every patient presenting to our clinic with a mood, substance use, anxiety, or eating disorder, or who has a problem with impulse control. Kleptomania is likely if the patient answers “yes” to questions 1 through 4 and to question 5 or 6. Stealing may be a symptom of several other psychiatric disorders, however, and misdiagnosis is fairly common (Table 2).6-8
Data suggest that the female-to-male ratio in kleptomania is approximately 2:1, with onset in adolescence. Typical individuals with kleptomania steal because they have urges to steal, often triggered by specific stimuli such as the sights and sounds of stores or feelings of loneliness or stress.1 Most patients with kleptomania are fairly specific about the types of stores from which they steal and the items they steal, and most hoard stolen items.1
Although many patients with kleptomania function quite well, others are severely debilitated in social and occupational realms. In a series of 22 patients with kleptomania:
- 64% had been apprehended
- 23% had served jail time
- 27% had been hospitalized because of their kleptomania symptoms
- 18% had considered or attempted suicide because of the distress associated with their kleptomania.1
Treatment recommendations
Patient history With patients who steal, we begin by identifying the motivation behind the stealing. Most patients with kleptomania report urges to steal. Some of these patients may have comorbid depression; for them, stealing makes them feel less depressed. Anger or irritability may point to borderline personality disorder. Stealing for the enjoyment of risk may suggest bipolar disorder.
Table 2
DIFFERENTIAL DIAGNOSIS: DISORDERS THAT MAY INVOLVE STEALING
| Misdiagnosis | How to distinguish from kleptomania |
|---|---|
| Bipolar disorder | Patients with bipolar disorder may steal as a result of the impulsivity of mania. In fact, the diagnostic criteria for kleptomania require the exclusion of mania as the cause of stealing.7 Patients with bipolar disorder report an elevated, expansive, or irritable mood while stealing. Patients with kleptomania tend to report a depressed mood when not stealing |
| Borderline personality disorder | Unlike patients with borderline personality disorder, patients with kleptomania do not report long histories of unstable relationships or negative self-image; inappropriate anger and “psychotic-like” symptoms are rare in patients with kleptomania |
| Antisocial personality disorder (ASPD, or conduct disorder) | Patients with kleptomania suffer intense shame and guilt, unlike those with ASPD. Also, most patients with kleptomania do not report other illegal or antisocial behavior. |
| Eating disorders | Data suggests that about one-third of patients with an eating disorder also steal.6,8 Patients with kleptomania, however, do not have disordered eating patterns or distorted body images common to patients with eating disorders. |
Many patients with kleptomania have comorbid mood, substance, or anxiety disorders. Treating these other symptoms while ignoring the symptoms of kleptomania may be unsuccessful. Comorbidity also may influence the choice of medication.
Medical assessment Case reports have associated the onset of kleptomania with a variety of medical conditions, including presenile cortical atrophy in a 25-year-old, a parietal tumor that caused blackouts and obliterated any memory of stealing episodes, narcolepsy, and an insulinoma that caused severe hypoglycemia.9 The relationship of these conditions with the onset of kleptomania is unclear, but the reports suggest that medical causes—although unlikely—should be ruled out before you consider kleptomania as a psychiatric illness.
Patient education Persons with kleptomania often feel that no one else has the same problem. They do not think of their behavior as being an illness. It is helpful to explain that kleptomania is treatable and to connect patients with educational books, self-help groups, and Web sites providing information and support (see “Related resources”).
Cognitive-behavioral therapy (CBT) Although the evidence is quite limited, covert sensitization, exposure and response prevention, and imaginal desensitization have all been shown effective in case reports.10
What medications are effective?
Only case reports, a case series of five subjects, and a single open-label treatment study involving 10 subjects with kleptomania have been done.
So far, uses of tricyclic antidepressants (imipramine, nortriptyline), SSRIs (fluoxetine, fluvoxamine, paroxetine), the opioid antagonist naltrexone, and mood stabilizers (lithium, valproate) have met with varying degrees of success. Strategies targeting urge and behavior reduction and mechanisms for coping with urges and behavior (e.g., cognitive-behavioral therapies) may represent important adjunctive components.2,11-17
No medications are FDA-approved for treating kleptomania. Therefore, it is important to inform patients of any off-label use of medications for this disorder, as well as the empirical basis for considering pharmacologic treatment.
SSRIs Only case reports exist on the use of SSRIs in treating kleptomania. The disorder may share a common pathology with pathologic gambling, and in our clinical experience appears to respond to similar treatments.18 We draw on research of pathologic gambling as well as our clinical experience in choosing SSRIs as first-line treatment, especially for patients with significant mood symptoms.19
We suggest titrating SSRIs to the maximum recommended dosage. As in the treatment of pathologic gambling, dosages of SSRIs required to treat kleptomania symptoms appear to be higher than average dosages required to treat depressive disorders. An SSRI should not be considered ineffective unless it has been tried for at least 10 to 12 weeks and the highest dosage tolerated or recommended by the manufacturer has been reached.
Response to SSRIs usually is characterized by decreased thoughts about stealing, decreased stealing behavior, and improvement in social and occupational functioning. If an SSRI is only partially effective, we consider augmentation with naltrexone, buspirone, or a mood stabilizer.
Naltrexone Patients taking naltrexone often report less-intense urges to steal. The urges may not disappear but are often sufficiently reduced so that the patient can resist them more easily. Patients also report that the thrill associated with stealing is reduced or eliminated.
Naltrexone was used in the first medication study of kleptomania and showed a significant decline in the intensity of urges to steal, stealing thoughts, and stealing behavior. Average dosage was 150 mg/d;11 a reduced dosage (e.g., 50 mg/d) may work in adolescents with kleptomania.20
Nausea as a side effect can be reduced by starting patients on 25 mg/d for the first 3 or 4 days and possibly adding ondansetron, 4 to 8 mg/d. Nausea and diarrhea are usually mild and resolve within the first week. Clinically, most patients respond to naltrexone within 2 weeks. After that, the dosage usually needs to be adjusted.
In patients with comorbid depression, augmentation with an SSRI may prevent worsening of untreated depressive symptoms. It is prudent to obtain liver function tests prior to naltrexone administration and again 3 to 4 weeks after starting the drug.21 Repeat testing should be performed at 2-to 4-week intervals for the next 2 months, then once a month for the following 3 months. After 6 months, testing three to four times a year is usually sufficient.
Nonsteroidal analgesics should not be used with high dosages of naltrexone (>50 mg/d), as concurrent use may increase the risk of hepatic transaminase elevation.21
Mood stabilizers Responses to lithium and valproate have been described in two case reports of patients with kleptomania.14,15 In the case of valproate, the effective dosage was 2,000 mg/d, whereas lithium reduced stealing urges at a serum level of 0.5 mEq/L.
Although it would be premature to recommend the use of mood stabilizers, their possible benefit may be related to their efficacy in bipolar disorder treatment and the existence of features (e.g., impulsivity) shared by kleptomania and bipolar disorder.
Atypical antipsychotics Although there is no evidence that atypical antipsychotics are useful in kleptomania, augmenting an SSRI with an atypical neuroleptic may be beneficial. Atypical antipsychotics have been explored as augmenting agents in the treatment of nonpsychotic disorders and behaviors, including pathologic gambling and obsessive-compulsive disorder.
The role of psychotherapy
Cognitive-behavioral therapy Based on the evidence of its effectiveness in treating pathologic gambling, CBT may hold promise as monotherapy for mild cases of kleptomania.
Combination therapy Combined pharmacologic and behavioral therapy may be the optimal treatment strategy for kleptomania. In our experience, patients who respond only partially or fail to respond to pharmacotherapy alone are more likely to find relief with a combination of drug and cognitive-behavioral therapies.
- Goldman MJ. Kleptomania: the compulsion to steal—what can be done? Far Hills, N New Horizon Press, 1998.
- Shoplifters Alternative http://www.shoplifters.org
- Impulse Control Disorders Clinic, University of Minnesota http://www.med.umn.edu/psychiatry/research/impulse.htm
Drug brand names
- Citalopram • Celexa
- Fluvoxamine • Luvox
- Imipramine • Tofranil
- Naltrexone • Revia
- Nortriptyline • Aventyl, Pamelor
- Paroxetine • Paxil
- Quetiapine • Seroquel
- Valproic acid • Depakote
Disclosure
The authors report no affiliation or financial arrangement with any of the companies whose products are mentioned in this article.
What is kleptomania? An independent illness, a symptom of other psychiatric disorders, or merely criminal behavior? Kleptomania—a disorder defined by an inability to resist the impulse to steal—is one of psychiatry’s most poorly understood diagnoses, even though it has been recognized in the literature for almost 200 years.
Kleptomania causes notable distress and impaired functioning.1 People with kleptomania often suffer from comorbid mood, anxiety, substance use, and other impulse-control disorders.14 They experience the humiliation of repeated arrests, which leads to guilt, depression, and even suicide.1,5 Yet kleptomania usually goes undiagnosed and untreated, despite a lifetime prevalence as high as 0.6%.6
Case report: ‘I’m a thief’
“I’m a thief,” began Susan, age 39. “I steal something four or five times every week. I steal from grocery stores and clothing stores. Sometimes I might steal something like vanilla extract; other times an expensive men’s tie. I probably steal $200 worth of items every week.
“You probably won’t believe this, but I don’t want or need the stuff I take. I have plenty of money. I have no idea why I take the things I do. That’s why I’m so depressed. What kind of person does something like this?
The urge to steal “I was probably 14 when I started stealing. I would go to stores with my mother. When I saw certain objects, I would get urges to steal them. The odd thing was that the items I stole were so ridiculous. I remember stealing key chains for several months, maybe three or four times a week. When I got older, things got worse. I was having urges more often, and so I needed to steal more often.
| Myth | Fact |
|---|---|
| Only little old ladies are kleptomaniacs. | Men and women of all ages suffer from kleptomania. Most patients report that the disorder began in adolescence. |
| It’s just a phase kids go through. | Parents of adolescents might see stealing as a phase. In many cases this might be true, but stealing may also suggest an underlying psychopathology. |
| People who steal are “bad.” | People with kleptomania steal because of urges to steal, not because of moral weakness. Treatment, not judgment, is the appropriate response. |
“My entire life has been torment. Each day I worry about having the urges, and then I worry about being caught stealing. I can’t relax. I’ve been married for 17 years, and I haven’t told my husband. My secrecy is tearing our marriage apart. My husband thinks I’m having an affair because I’ve distanced myself emotionally from him.”
Table 1
SCREENING TEST FOR KLEPTOMANIA
| Yes | No | |
|---|---|---|
| 1. Do you steal or have urges to steal? | ○ | ○ |
| 2. Do thoughts of stealing or urges to steal preoccupy you? That is, do you often think about stealing or have urges to steal and wish the thoughts or urges occurred less often? | ○ | ○ |
| 3. Do you feel tense or anxious before you steal or when you have urges to steal? | ○ | ○ |
| 4. Do you feel pleasure or a sense of calm when you steal something? | ○ | ○ |
| 5. Has the stealing or urges to steal caused you much distress? | ○ | ○ |
| 6. Has the stealing or urges to steal significantly interfered with your life in some way? | ○ | ○ |
| A patient who answers “yes” to questions 1 through 4 and to question 5 or 6 is likely to have kleptomania. | ||
| Adapted from DSM-IV criteria, American Psychiatric Association, 2000 | ||
Susan described urges to steal almost every day. When the urges were mild, she could resist them. Other days they were severe, and Susan felt unable to control her behavior. At work, her urges distracted her from completing projects, and her performance suffered. The urge to steal would often compel Susan to leave work early so she could get to a store.
Calm, then guilt “Every time I steal something I feel both a thrill and a great sense of calm,” she said. “It feels good. The problem is that almost immediately after each theft, I feel guilty and ashamed. After I steal, I usually donate the items to the Salvation Army, throw them away, or give them away as gifts.”
Drug trials We started treating Susan with the selective serotonin reuptake inhibitor (SSRI), citalopram. She reported notable improvement in her mood after 3 weeks on a dosage of 60 mg/d and she had been attending weekly psychotherapy, although her stealing continued unchanged. The addition of naltrexone, 200 mg/d for 2 weeks, decreased the frequency of Susan’s stealing and reduced her urges to steal, but her symptoms continued to interfere significantly with her overall functioning.
We then added the atypical antipsychotic quetiapine, 100 mg bid, and Susan’s urges to steal and stealing behavior went into remission within 3 weeks. She has refrained from stealing for the last 9 months.
Making the diagnosis
In our clinic, we have treated more than 50 patients with kleptomania. Rather than coming to us through the criminal justice system, they are usually self-referred. Often they contact us after discovering on the Internet that we specialize in treating persons with this disorder.
In our experience, kleptomania typically goes undiagnosed in clinical settings, in part because patients are ashamed and embarrassed to discuss their symptoms with physicians unless specifically asked.1 If left untreated, however, kleptomania frequently becomes chronic.4 If persons with kleptomania are to seek treatment, it is important that family, friends, and mental health professionals understand the myths and facts about this disorder (Box).
To make the diagnosis, we use the simple screening instrument shown in Table 1.7 In general, because of high comorbidity with certain disorders, we screen every patient presenting to our clinic with a mood, substance use, anxiety, or eating disorder, or who has a problem with impulse control. Kleptomania is likely if the patient answers “yes” to questions 1 through 4 and to question 5 or 6. Stealing may be a symptom of several other psychiatric disorders, however, and misdiagnosis is fairly common (Table 2).6-8
Data suggest that the female-to-male ratio in kleptomania is approximately 2:1, with onset in adolescence. Typical individuals with kleptomania steal because they have urges to steal, often triggered by specific stimuli such as the sights and sounds of stores or feelings of loneliness or stress.1 Most patients with kleptomania are fairly specific about the types of stores from which they steal and the items they steal, and most hoard stolen items.1
Although many patients with kleptomania function quite well, others are severely debilitated in social and occupational realms. In a series of 22 patients with kleptomania:
- 64% had been apprehended
- 23% had served jail time
- 27% had been hospitalized because of their kleptomania symptoms
- 18% had considered or attempted suicide because of the distress associated with their kleptomania.1
Treatment recommendations
Patient history With patients who steal, we begin by identifying the motivation behind the stealing. Most patients with kleptomania report urges to steal. Some of these patients may have comorbid depression; for them, stealing makes them feel less depressed. Anger or irritability may point to borderline personality disorder. Stealing for the enjoyment of risk may suggest bipolar disorder.
Table 2
DIFFERENTIAL DIAGNOSIS: DISORDERS THAT MAY INVOLVE STEALING
| Misdiagnosis | How to distinguish from kleptomania |
|---|---|
| Bipolar disorder | Patients with bipolar disorder may steal as a result of the impulsivity of mania. In fact, the diagnostic criteria for kleptomania require the exclusion of mania as the cause of stealing.7 Patients with bipolar disorder report an elevated, expansive, or irritable mood while stealing. Patients with kleptomania tend to report a depressed mood when not stealing |
| Borderline personality disorder | Unlike patients with borderline personality disorder, patients with kleptomania do not report long histories of unstable relationships or negative self-image; inappropriate anger and “psychotic-like” symptoms are rare in patients with kleptomania |
| Antisocial personality disorder (ASPD, or conduct disorder) | Patients with kleptomania suffer intense shame and guilt, unlike those with ASPD. Also, most patients with kleptomania do not report other illegal or antisocial behavior. |
| Eating disorders | Data suggests that about one-third of patients with an eating disorder also steal.6,8 Patients with kleptomania, however, do not have disordered eating patterns or distorted body images common to patients with eating disorders. |
Many patients with kleptomania have comorbid mood, substance, or anxiety disorders. Treating these other symptoms while ignoring the symptoms of kleptomania may be unsuccessful. Comorbidity also may influence the choice of medication.
Medical assessment Case reports have associated the onset of kleptomania with a variety of medical conditions, including presenile cortical atrophy in a 25-year-old, a parietal tumor that caused blackouts and obliterated any memory of stealing episodes, narcolepsy, and an insulinoma that caused severe hypoglycemia.9 The relationship of these conditions with the onset of kleptomania is unclear, but the reports suggest that medical causes—although unlikely—should be ruled out before you consider kleptomania as a psychiatric illness.
Patient education Persons with kleptomania often feel that no one else has the same problem. They do not think of their behavior as being an illness. It is helpful to explain that kleptomania is treatable and to connect patients with educational books, self-help groups, and Web sites providing information and support (see “Related resources”).
Cognitive-behavioral therapy (CBT) Although the evidence is quite limited, covert sensitization, exposure and response prevention, and imaginal desensitization have all been shown effective in case reports.10
What medications are effective?
Only case reports, a case series of five subjects, and a single open-label treatment study involving 10 subjects with kleptomania have been done.
So far, uses of tricyclic antidepressants (imipramine, nortriptyline), SSRIs (fluoxetine, fluvoxamine, paroxetine), the opioid antagonist naltrexone, and mood stabilizers (lithium, valproate) have met with varying degrees of success. Strategies targeting urge and behavior reduction and mechanisms for coping with urges and behavior (e.g., cognitive-behavioral therapies) may represent important adjunctive components.2,11-17
No medications are FDA-approved for treating kleptomania. Therefore, it is important to inform patients of any off-label use of medications for this disorder, as well as the empirical basis for considering pharmacologic treatment.
SSRIs Only case reports exist on the use of SSRIs in treating kleptomania. The disorder may share a common pathology with pathologic gambling, and in our clinical experience appears to respond to similar treatments.18 We draw on research of pathologic gambling as well as our clinical experience in choosing SSRIs as first-line treatment, especially for patients with significant mood symptoms.19
We suggest titrating SSRIs to the maximum recommended dosage. As in the treatment of pathologic gambling, dosages of SSRIs required to treat kleptomania symptoms appear to be higher than average dosages required to treat depressive disorders. An SSRI should not be considered ineffective unless it has been tried for at least 10 to 12 weeks and the highest dosage tolerated or recommended by the manufacturer has been reached.
Response to SSRIs usually is characterized by decreased thoughts about stealing, decreased stealing behavior, and improvement in social and occupational functioning. If an SSRI is only partially effective, we consider augmentation with naltrexone, buspirone, or a mood stabilizer.
Naltrexone Patients taking naltrexone often report less-intense urges to steal. The urges may not disappear but are often sufficiently reduced so that the patient can resist them more easily. Patients also report that the thrill associated with stealing is reduced or eliminated.
Naltrexone was used in the first medication study of kleptomania and showed a significant decline in the intensity of urges to steal, stealing thoughts, and stealing behavior. Average dosage was 150 mg/d;11 a reduced dosage (e.g., 50 mg/d) may work in adolescents with kleptomania.20
Nausea as a side effect can be reduced by starting patients on 25 mg/d for the first 3 or 4 days and possibly adding ondansetron, 4 to 8 mg/d. Nausea and diarrhea are usually mild and resolve within the first week. Clinically, most patients respond to naltrexone within 2 weeks. After that, the dosage usually needs to be adjusted.
In patients with comorbid depression, augmentation with an SSRI may prevent worsening of untreated depressive symptoms. It is prudent to obtain liver function tests prior to naltrexone administration and again 3 to 4 weeks after starting the drug.21 Repeat testing should be performed at 2-to 4-week intervals for the next 2 months, then once a month for the following 3 months. After 6 months, testing three to four times a year is usually sufficient.
Nonsteroidal analgesics should not be used with high dosages of naltrexone (>50 mg/d), as concurrent use may increase the risk of hepatic transaminase elevation.21
Mood stabilizers Responses to lithium and valproate have been described in two case reports of patients with kleptomania.14,15 In the case of valproate, the effective dosage was 2,000 mg/d, whereas lithium reduced stealing urges at a serum level of 0.5 mEq/L.
Although it would be premature to recommend the use of mood stabilizers, their possible benefit may be related to their efficacy in bipolar disorder treatment and the existence of features (e.g., impulsivity) shared by kleptomania and bipolar disorder.
Atypical antipsychotics Although there is no evidence that atypical antipsychotics are useful in kleptomania, augmenting an SSRI with an atypical neuroleptic may be beneficial. Atypical antipsychotics have been explored as augmenting agents in the treatment of nonpsychotic disorders and behaviors, including pathologic gambling and obsessive-compulsive disorder.
The role of psychotherapy
Cognitive-behavioral therapy Based on the evidence of its effectiveness in treating pathologic gambling, CBT may hold promise as monotherapy for mild cases of kleptomania.
Combination therapy Combined pharmacologic and behavioral therapy may be the optimal treatment strategy for kleptomania. In our experience, patients who respond only partially or fail to respond to pharmacotherapy alone are more likely to find relief with a combination of drug and cognitive-behavioral therapies.
- Goldman MJ. Kleptomania: the compulsion to steal—what can be done? Far Hills, N New Horizon Press, 1998.
- Shoplifters Alternative http://www.shoplifters.org
- Impulse Control Disorders Clinic, University of Minnesota http://www.med.umn.edu/psychiatry/research/impulse.htm
Drug brand names
- Citalopram • Celexa
- Fluvoxamine • Luvox
- Imipramine • Tofranil
- Naltrexone • Revia
- Nortriptyline • Aventyl, Pamelor
- Paroxetine • Paxil
- Quetiapine • Seroquel
- Valproic acid • Depakote
Disclosure
The authors report no affiliation or financial arrangement with any of the companies whose products are mentioned in this article.
1. Grant JE, Kim SW. Clinical characteristics and associated psychopathology in 22 cases of kleptomania. Comp Psychiatry (in press).
2. McElroy SL, Pope HG, Hudson JI, Keck PE, White KL. Kleptomania: a report of 20 cases. Am J Psychiatry 1991;148:652-7.
3. Presta S, Marazziti D, Dell’Osso L, et al. Kleptomania: clinical features and comorbidity in an Italian sample. Comp Psychiatry 2002;43:7-12.
4. McElroy SL, Keck PE, Phillips KA. Kleptomania, compulsive buying, and binge-eating disorder. J Clin Psychiatry 1995;56:14-26.
5. McElroy SL, Hudson JI, Pope HG, Keck PE. Kleptomania: clinical characteristics and associated psychopathology. Psychol Med 1991;21:93-108.
6. Goldman MJ. Kleptomania: an overview. Psychiatric Ann 1992;22:68-71.
7. American Psychiatric Association Committee on Nomenclature and Statistics Diagnostic and statistical manual of mental disorders (4th ed, text rev). Washington, DC: American Psychiatric Association, 2000.
8. Krahn DD, Nairn K, Gosnell BA, Drewnowski A. Stealing in eating disordered patients. J Clin Psychiatry 1991;52:112-5.
9. Goldman MJ. Kleptomania: making sense of the nonsensical. Am J Psychiatry 1991;148:986-96.
10. Goldman MJ. Kleptomania: the compulsion to steal—what can be done? Far Hills, NJ: New Horizon Press, 1998.
11. Grant JE, Kim SW. An open-label study of naltrexone in the treatment of kleptomania. J Clin Psychiatry 2002;63:349-56.
12. Chong SA, Low BL. Treatment of kleptomania with fluvoxamine. Acta Psychiatr Scand 1996;93:314-5.
13. Kraus JE. Treatment of kleptomania with paroxetine. J Clin Psychiatry 1999;60:793.-
14. Burstein A. Fluoxetine lithium treatment for kleptomania. J Clin Psychiatry 1992;53:28-9.
15. Kmetz GF, McElroy SL, Collins DJ. Response of kleptomania and mixed mania to valproate. Am J Psychiatry 1997;154:580-1.
16. Lepkifker E, Dannon PN, Ziv R, Iancu I, Horesh N, Kotler M. The treatment of kleptomania with serotonin reuptake inhibitors. Clin Neuropharmacol 1999;22:40-3.
17. Durst R, Katz G, Knobler HY. Buspirone augmentation of fluvoxamine in the treatment of kleptomania. J Nerv Ment Dis 1997;185:586-8.
18. Kim SW. Opioid antagonists in the treatment of impulse-control disorders. J Clin Psychiatry 1998;59:159-64.
19. Grant JE, Kim SW. Pharmacotherapy of pathological gambling. Psychiatric Ann 2002;32:186-91.
20. Grant JE, Kim SW. Adolescent kleptomania treated with naltrexone: a case report. Eur Child Adolescent Psychiatry 2002;11:92-5.
21. Kim SW, Grant JE, et al. A preliminary report on a possible naltrexone and nonsteroidal analgesics interaction. J Clin Psychopharmacol 2001;21:632-4.
1. Grant JE, Kim SW. Clinical characteristics and associated psychopathology in 22 cases of kleptomania. Comp Psychiatry (in press).
2. McElroy SL, Pope HG, Hudson JI, Keck PE, White KL. Kleptomania: a report of 20 cases. Am J Psychiatry 1991;148:652-7.
3. Presta S, Marazziti D, Dell’Osso L, et al. Kleptomania: clinical features and comorbidity in an Italian sample. Comp Psychiatry 2002;43:7-12.
4. McElroy SL, Keck PE, Phillips KA. Kleptomania, compulsive buying, and binge-eating disorder. J Clin Psychiatry 1995;56:14-26.
5. McElroy SL, Hudson JI, Pope HG, Keck PE. Kleptomania: clinical characteristics and associated psychopathology. Psychol Med 1991;21:93-108.
6. Goldman MJ. Kleptomania: an overview. Psychiatric Ann 1992;22:68-71.
7. American Psychiatric Association Committee on Nomenclature and Statistics Diagnostic and statistical manual of mental disorders (4th ed, text rev). Washington, DC: American Psychiatric Association, 2000.
8. Krahn DD, Nairn K, Gosnell BA, Drewnowski A. Stealing in eating disordered patients. J Clin Psychiatry 1991;52:112-5.
9. Goldman MJ. Kleptomania: making sense of the nonsensical. Am J Psychiatry 1991;148:986-96.
10. Goldman MJ. Kleptomania: the compulsion to steal—what can be done? Far Hills, NJ: New Horizon Press, 1998.
11. Grant JE, Kim SW. An open-label study of naltrexone in the treatment of kleptomania. J Clin Psychiatry 2002;63:349-56.
12. Chong SA, Low BL. Treatment of kleptomania with fluvoxamine. Acta Psychiatr Scand 1996;93:314-5.
13. Kraus JE. Treatment of kleptomania with paroxetine. J Clin Psychiatry 1999;60:793.-
14. Burstein A. Fluoxetine lithium treatment for kleptomania. J Clin Psychiatry 1992;53:28-9.
15. Kmetz GF, McElroy SL, Collins DJ. Response of kleptomania and mixed mania to valproate. Am J Psychiatry 1997;154:580-1.
16. Lepkifker E, Dannon PN, Ziv R, Iancu I, Horesh N, Kotler M. The treatment of kleptomania with serotonin reuptake inhibitors. Clin Neuropharmacol 1999;22:40-3.
17. Durst R, Katz G, Knobler HY. Buspirone augmentation of fluvoxamine in the treatment of kleptomania. J Nerv Ment Dis 1997;185:586-8.
18. Kim SW. Opioid antagonists in the treatment of impulse-control disorders. J Clin Psychiatry 1998;59:159-64.
19. Grant JE, Kim SW. Pharmacotherapy of pathological gambling. Psychiatric Ann 2002;32:186-91.
20. Grant JE, Kim SW. Adolescent kleptomania treated with naltrexone: a case report. Eur Child Adolescent Psychiatry 2002;11:92-5.
21. Kim SW, Grant JE, et al. A preliminary report on a possible naltrexone and nonsteroidal analgesics interaction. J Clin Psychopharmacol 2001;21:632-4.
From the Stanley Foundation Bipolar Network: New findings on suicide attempts, substance abuse, obesity, and more
Even with psychiatry’s most effective and best studied agents, many patients with bipolar disorder are inadequately treated and respond insufficiently to available psychopharmacologic agents. To address this problem, the Stanley Foundation Bipolar Network (SFBN) was created to explore acute and long-term response to treatment of bipolar disorder.
For the past 6 years, our group (Box) has followed an international cohort of patients with bipolar disorder and offered them opportunities to participate in clinical trials at seven field centers. We have made considerable progress as we focused on the longitudinal course of bipolar illness and evaluated various combinations of psychopharmacologic therapies. The SFBN also has explored factors that increase the risk of suicide, quantified the incidence of anxiety disorder and substance abuse, and looked at other variables that may affect treatment outcome.
This report for the readers of Current Psychiatry provides a brief state-of-the-art overview of what we have learned about the course and management of bipolar disorder. It brings together and summarizes the findings from studies published, in press, or under review. Our goal is to update current findings, which may help you provide optimum care for these at-risk patients.
Since Robert Prien’s pioneering studies in the 1970s, the National Institute of Mental Health (NIMH) had funded little work on prophylaxis of bipolar disorders or acute bipolar depression. Following two NIMH conferences on bipolar illness in 1989 and 1994, the Stanley Foundation Bipolar Network (SFBN) was established to help overcome these research deficiencies, with funding provided by the Theodore and Vada Stanley Foundation.
Patients were recruited into the network from the general community and with the use of newspaper and newsletter advertisements. In contrast to many other clinical populations, few who wished to enter the network were excluded. It was clear, however, that filling out demographic, clinical, and rating forms would require considerable effort from each patient. All patients gave written informed consent to participate in the naturalistic part of the study, and were reconsented for any open or blind randomized clinical trial.
Patients provided daily self-ratings on the National Institute of Mental Health Life Chart Method (NIMH-LCM), a unique longitudinal instrument. Clinicians then completed their own separate LCMs, as more severely ill patients often could not complete their self-ratings, and patients often underestimated the severity and functional disability associated with the hypomanic and manic phases of bipolar illness. With the use of the NIMH-LCM, network investigators were able, for the first time, to assess the details of mood fluctuations associated with bipolar illness and to more precisely delineate the qualitative and quantitative aspects of response to psychopharmacologic interventions.
The SFBN includes seven research field centers in the United States, the Netherlands, and Germany. The leaders of those centers collaborated with Robert M. Post, MD, to prepare this report of the network findings. The collaborators are:
| Gabriele S. Leverich, MSW, and Kirk D. Denicoff, MD Biological Psychiatry Branch National Institute of Mental Health Bethesda, MD | Paul E. Keck, Jr., MD, and Susan L. McElroy, MD Biological Psychiatry Program University of Cincinnati College of Medicine Cincinnati, OH |
| Lori L. Altshuler, MD, and Mark A. Frye, MD UCLA Mood Disorders Research Program VA Medical Center Los Angeles, CA | Willem A. Nolen, MD, and Ralph Kupka, MD Altrecht Institute for Mental Health Care University Medical Center Utrecht, The Netherlands |
| John Rush, MD, and Trisha Suppes, MD Department of Psychiatry Southwest Medical Center University of Texas, Dallas | Joerg Walden, MD Department of Psychiatry University of Freiburg Freiburg, Germany |
| Keith Kramlinger, MD Mayo Clinic, Rochester, MN (Administrative center) | Heinz Grunze, MD Psychiatrische Klinik der LMU Munich, Germany |
In part one, we discuss the demographics of our cohort of 676 community-based bipolar patients, including risk factors for suicide attempts, frequency of comorbid psychiatric diagnoses, problems of overweight and obesity, and preliminary findings about thyroiditis.
In part two, we describe our clinical trials assessing mood stabilizers and adjunctive agents such as atypical antipsychotics, second-generation antidepressants, and anticonvulsants. We also will address a soon-to-be analyzed study of omega-3 fatty acids.
Table 1
CHARACTERISTICS OF THE 676 BIPOLAR OUTPATIENTS
| Mean age | 41 | |
| % Female | 43 | |
| Diagnosis (%) | ||
| Bipolar I | 79 | |
| Bipolar II | 16 | |
| Bipolar NOS | 4 | |
| Marital status (%) | ||
| Married/cohabitating | 44 | |
| Divorced/separated | 22 | |
| Income (%) | ||
| <$20,000 | 37 | |
| $20,000-40,000 | 22 | |
| $40,000-100,000 | 32 | |
| >$100,000 | 9 | |
| Education (completed, %) | ||
| High school | 34 | |
| Jr. college/technical | 13 | |
| College, 4-year | 27 | |
| Graduate degree | 16 | |
| Positive family history (1st-degree relatives, %) | ||
| Bipolar disorder | 56 | |
| Unipolar disorder | 64 | |
| Schizophrenia | 24 | |
| Drug dependence | 44 | |
| Other psychiatric disorder | 52 | |
| Alcohol abuse/dependence | 52 | |
| Suicide (complete or serious attempt) | 38 | |
| Environmental adversity (%): | Child/adolescent | Adult |
| Physical abuse | 29 | 8 |
| Sexual abuse | 30 | 8 |
| Illness course and treatment (age in yrs) | ||
| Age of onset (symptoms) | 19 | |
| Early onset (age <14) | 34 | |
| Age at first treatment | 29 | |
| Age at first hospitalization | 30 | |
| Cycling pattern (n = 643, %) | ||
| Rapid (or faster cycling) | 47 | |
| Ultrarapid (or faster cycling) | 30 | |
| Rapid-cycling only | 17 | |
| Ultrarapid cycling only | 10 | |
| Ultradian cycling | 20 | |
Study population
The SFBN recruited outpatients with bipolar disorders I, II, or not otherwise specified (NOS). We did not exclude patients with comorbid illnesses, except those with active substance abuse disorder requiring treatment in another setting.1 Patients were recruited almost exclusively from the community and rarely from inpatient hospitalization. The average age was 41, and 43% were college graduates. Most were employed but indicated that their illness limited their employment level or ability to function at work (Table 1).
One striking finding was a decadelong lag between the onset of symptoms reaching thresholds for diagnosis and the onset of first treatment.2 This long delay could have adverse consequences for illness outcome and represents a major public health target. Age of onset appeared to have both genetic/familial and environmental determinants.3 Subjects with a history of both extreme early life adversities (i.e., physical or sexual abuse) and a positive family history of mood disorder had the earliest onset of bipolar illness. Those who had neither of these factors had late-onset illness.
Suicide attempts
Prior to network entry, 25 to 50% of our study population had made a serious suicide attempt (defined as requiring medical attention and/or hospitalization), pointing to bipolar disorder’s potential lethality.
History of abuse A history of physical or sexual abuse in childhood or adolescence was a prominent risk factor for attempted suicide.4 With either type of abuse there was an approximate 15% increase in incidence of suicide attempts over the baseline rate of about 25%. More than 55% of patients with a history of both physical and sexual abuse had made a serious suicide attempt. The frequency of physical and sexual abuse was also positively related to increased incidence of suicide attempts. Taken together, these data suggest a “stressor dose-response” relationship between adverse childhood or adolescent experiences and an increased incidence of suicide attempts.
Table 2
FACTORS THAT APPEAR TO INCREASE SUICIDE RISK IN BIPOLAR DISORDER*
| Course-of-illness characteristics |
| Early onset |
| More time ill |
| More time depressed |
| More-severe depression |
| Social and medical support |
| Loss of significant other |
| Lack of a confidant |
| Single motherhood |
| Decreased access to health care |
| Lack of medical insurance |
| Demographics |
| Less education |
| Lower income |
| Being single |
| Comorbidities |
| Axis I |
| Eating disorders |
| Anxiety disorders |
| Axis II |
| Cluster A, B, C |
| Medical |
| Traumatic life events |
| Sexual abuse |
| Physical abuse |
| Genetics |
| Family history of suicide and drug abuse |
| * Identified in the SFBN cohort |
Comorbidities Other factors that appear to be associated with bipolar disorder and increased risk of attempted suicide include anxiety and eating disorders, early onset of symptoms, at least four previous hospitalizations for depression, and medical comorbidity (Table 2). Limited access to psychiatric and medical services and inadequate health insurance also were correlated with having made a serious suicide attempt. Thus, lack of medical care might exacerbate demoralization and increase the risk of suicide.
Family history of suicide or serious suicide attempts and drug abuse were associated with an increase in suicide attempts, but family history of unipolar or bipolar illness were not. These data are consistent with other studies suggesting that vulnerability to suicide may have a separate genetic component from that of mood disorders per se.
Severity of illness Increased severity of bipolar illness also was associated with prior psychosocial stressors3 and serious suicide attempts. We confirmed self-reported history, using daily mood charting, the clinician-rated Inventory of Depressive Symptomatology (IDS-C), and the Young Mania Rating Scale (YMRS) to rate increase in percentage of time ill, percentage of time depressed, and severity of depression.
Clinical implications We are uncertain how directly these data regarding suicide attempts relate to completed suicide. However, patients with bipolar disorder are among those at highest risk for completed suicide, and a prior serious attempt is often a precursor to completed suicide.5,6
Factors that appear particularly related to increased risk of suicide in patients with bipolar disorder are family history of suicide, history of early psychosocial stressors, comorbid personality disorders, substance abuse, and a relatively severe course of bipolar illness. Patients with these risk factors need support for two types of psychosocial stressors:
- poor access to health care
- stressors related to concurrent events (e.g., occurring with new affective episodes).
It may also be important for clinicians to consider the prophylactic use of lithium because of its demonstrated ability to reduce mortality in bipolar illness.7 Additionally, we need data on the efficacy of new drugs in preventing affective episodes and reducing suicidal ideation and acts.
Common comorbidities
We found considerable axis I lifetime comorbidities in the SFBN cohort (Table 3), including a 42% incidence of anxiety disorder and a similarly high rate of substance abuse. In addition to bipolar disorder, patients averaged approximately two lifetime comorbid diagnoses:
- 35% had none
- 65% had one or more
- 42% had two or more
- 24% had three or more.
Table 3
PSYCHIATRIC COMORBIDITY IN BIPOLAR OUTPATIENTS (%)*.
| Any substance abuse disorder | 42% |
| Alcohol | 33 |
| Marijuana | 16 |
| Stimulant | 9 |
| Sedative | 8 |
| Opiate | 7 |
| Hallucinogens | 6 |
| Any anxiety disorder | 42% |
| Panic disorder | 20 |
| Social phobia | 16 |
| Simple phobia | 10 |
| OCD | 9 |
| PTSD | 7 |
| GAD | 3 |
| Other | 3 |
| Any eating disorder | 11% |
| Bulimia nervosa | 8 |
| Anorexia nervosa | 4 |
| * Lifetime comorbid axis I disorders based on Structured Clinical Interview for DSM-IV (SCID) interviews with 288 SFBN patients. | |
Men with bipolar disorder had a higher absolute prevalence of alcoholism, but the relative risk compared with the general population was much higher in women (odds ratio 7.35) compared with men (odds ratio 2.77). In women, alcohol use and abuse were related to history of social phobia, greater number of depressive episodes prior to network entry, and history of abusing more than one substance. In men, alcohol comorbidity was related to history of alcohol abuse in first-degree relatives and history of early physical abuse.
These data suggest that female patients, in particular, were self-medicating their affective episodes and comorbid anxiety disorders with alcohol. They suggest the importance of asking patients directly about alcohol use to identify the need for primary and secondary prevention. In an adolescent or young adult, affective illness—and bipolar disorder in particular—should be considered a major risk factor for subsequent problems with alcohol and drug abuse. When taking a careful history, the treating physician should include questions regarding any substance abuse.
Overweight and obesity
One-half of the women and two-thirds of the men in the SFBN were overweight at network entry.8 Although this rate of overweight sounds alarmingly high, it was not significantly different from rates in the general U.S. population. A greater percentage of women than men in the network had morbid obesity, however.
In our cohort, being overweight or obese was associated with an increased incidence of cardiovascular disease, diabetes, arthritis, hypertension, hypothyroidism, and cancer. The degree of obesity also correlated significantly with past use of the numerous psychotropic drugs that have been associated with weight gain. Also, as might be expected, increased weight was associated with physical inactivity.
Many of the psychotropic drugs routinely used to treat bipolar disorder are associated with weight gain. Among these are the mood stabilizers lithium and valproate and the atypical antipsychotics, with the exception of ziprasidone (and, to follow, aripiprazole). Lithium’s liability to cause weight gain may be counterbalanced by its antisuicide effects and its ability to reverse excess medical mortality associated with affective illness. Thus, lithium should continue to play a major therapeutic role in bipolar disorder.
It may be more effective and easier to prevent weight gain than to attempt to reduce weight that has been gained. Recommending exercise and other weight-reduction measures may be helpful, as may co-administering drugs associated with weight loss, such as topiramate.9
Thyroiditis
Antithyroid antibodies were detected in 28% of network patients, compared with rates of 3% in the general population and 18% in psychiatric controls.10 The increased rate was associated with the need to augment thyroid function in 17% of network patients, but it was not related to age, mood state, rapid cycling status, or lithium treatment.
These data indicate that bipolar patients are prone to autoimmune thyroid changes. Further analysis is required to ascertain whether this finding has therapeutic implications, especially related to adjunctive T3 and T4 treatment.11
1. Leverich GS, Nolen WA, Rush AJ, et al. The Stanley Foundation Bipolar Treatment Outcome Network. I. Longitudinal methodology. J Affect Disord 2001;67:33-44.
2. Suppes T, Leverich GS, Keck PE, et al. The Stanley Foundation Bipolar Treatment Outcome Network. II. Demographics and illness characteristics of the first 261 patients. J Affect Disord 2001b;67:45-59.
3. Leverich GS, McElroy SL, Suppes T, et al. Early physical and sexual abuse associated with an adverse course of bipolar illness. Biological Psychiatry 2002;51:288-97.
4. Leverich GS, Altshuler LL, Frye MA, et al. Factors associated with suicide attempts in 648 patients with bipolar disorder in the Stanley Foundation Bipolar Network. Manuscript submitted for publication, 2002.
5. Chen Y-W, Dilsaver SC. Lifetime rates of suicide attempts among subjects with bipolar and unipolar disorders relative to subjects with other axis I disorders. Biological Psychiatry 1996;39:896-9.
6. Kessler RC, Borges G, Walters EE. Prevalence of and risk factors for lifetime suicide attempts in the National Comorbidity Survey. Arch.Gen.Psychiatry 1999;56:617-26.
7. Ahrens B, Muller-Oerlinghausen B, Schou M, et al. Excess cardiovascular and suicide mortality of affective disorders may be reduced by lithium prophylaxis. J Affect Disord 1995;33:67-75.
8. McElroy SL, Frye MA, Suppes T, et al. Correlates of overweight and obesity in 644 patients with bipolar disorder. J Clin Psychiatry 2002;63:207-13.
9. McElroy SL, Suppes T, Keck PE, et al. Open-label adjunctive topiramate in the treatment of bipolar disorders. Biological Psychiatry 2000;47:1025-33.
10. Kupka RW, Nolen WA, Post RM, et al. High rate of autoimmune thyroiditis in bipolar disorder: lack of association with lithium exposure. Biological Psychiatry 2002;51:305-11.
11. Bauer M, Priebe S, Berghofer A, et al. Subjective response to and tolerability of long-term supraphysiological doses of levothyroxine in refractory mood disorders. J Affect Disord 2001;64:35-42.
Even with psychiatry’s most effective and best studied agents, many patients with bipolar disorder are inadequately treated and respond insufficiently to available psychopharmacologic agents. To address this problem, the Stanley Foundation Bipolar Network (SFBN) was created to explore acute and long-term response to treatment of bipolar disorder.
For the past 6 years, our group (Box) has followed an international cohort of patients with bipolar disorder and offered them opportunities to participate in clinical trials at seven field centers. We have made considerable progress as we focused on the longitudinal course of bipolar illness and evaluated various combinations of psychopharmacologic therapies. The SFBN also has explored factors that increase the risk of suicide, quantified the incidence of anxiety disorder and substance abuse, and looked at other variables that may affect treatment outcome.
This report for the readers of Current Psychiatry provides a brief state-of-the-art overview of what we have learned about the course and management of bipolar disorder. It brings together and summarizes the findings from studies published, in press, or under review. Our goal is to update current findings, which may help you provide optimum care for these at-risk patients.
Since Robert Prien’s pioneering studies in the 1970s, the National Institute of Mental Health (NIMH) had funded little work on prophylaxis of bipolar disorders or acute bipolar depression. Following two NIMH conferences on bipolar illness in 1989 and 1994, the Stanley Foundation Bipolar Network (SFBN) was established to help overcome these research deficiencies, with funding provided by the Theodore and Vada Stanley Foundation.
Patients were recruited into the network from the general community and with the use of newspaper and newsletter advertisements. In contrast to many other clinical populations, few who wished to enter the network were excluded. It was clear, however, that filling out demographic, clinical, and rating forms would require considerable effort from each patient. All patients gave written informed consent to participate in the naturalistic part of the study, and were reconsented for any open or blind randomized clinical trial.
Patients provided daily self-ratings on the National Institute of Mental Health Life Chart Method (NIMH-LCM), a unique longitudinal instrument. Clinicians then completed their own separate LCMs, as more severely ill patients often could not complete their self-ratings, and patients often underestimated the severity and functional disability associated with the hypomanic and manic phases of bipolar illness. With the use of the NIMH-LCM, network investigators were able, for the first time, to assess the details of mood fluctuations associated with bipolar illness and to more precisely delineate the qualitative and quantitative aspects of response to psychopharmacologic interventions.
The SFBN includes seven research field centers in the United States, the Netherlands, and Germany. The leaders of those centers collaborated with Robert M. Post, MD, to prepare this report of the network findings. The collaborators are:
| Gabriele S. Leverich, MSW, and Kirk D. Denicoff, MD Biological Psychiatry Branch National Institute of Mental Health Bethesda, MD | Paul E. Keck, Jr., MD, and Susan L. McElroy, MD Biological Psychiatry Program University of Cincinnati College of Medicine Cincinnati, OH |
| Lori L. Altshuler, MD, and Mark A. Frye, MD UCLA Mood Disorders Research Program VA Medical Center Los Angeles, CA | Willem A. Nolen, MD, and Ralph Kupka, MD Altrecht Institute for Mental Health Care University Medical Center Utrecht, The Netherlands |
| John Rush, MD, and Trisha Suppes, MD Department of Psychiatry Southwest Medical Center University of Texas, Dallas | Joerg Walden, MD Department of Psychiatry University of Freiburg Freiburg, Germany |
| Keith Kramlinger, MD Mayo Clinic, Rochester, MN (Administrative center) | Heinz Grunze, MD Psychiatrische Klinik der LMU Munich, Germany |
In part one, we discuss the demographics of our cohort of 676 community-based bipolar patients, including risk factors for suicide attempts, frequency of comorbid psychiatric diagnoses, problems of overweight and obesity, and preliminary findings about thyroiditis.
In part two, we describe our clinical trials assessing mood stabilizers and adjunctive agents such as atypical antipsychotics, second-generation antidepressants, and anticonvulsants. We also will address a soon-to-be analyzed study of omega-3 fatty acids.
Table 1
CHARACTERISTICS OF THE 676 BIPOLAR OUTPATIENTS
| Mean age | 41 | |
| % Female | 43 | |
| Diagnosis (%) | ||
| Bipolar I | 79 | |
| Bipolar II | 16 | |
| Bipolar NOS | 4 | |
| Marital status (%) | ||
| Married/cohabitating | 44 | |
| Divorced/separated | 22 | |
| Income (%) | ||
| <$20,000 | 37 | |
| $20,000-40,000 | 22 | |
| $40,000-100,000 | 32 | |
| >$100,000 | 9 | |
| Education (completed, %) | ||
| High school | 34 | |
| Jr. college/technical | 13 | |
| College, 4-year | 27 | |
| Graduate degree | 16 | |
| Positive family history (1st-degree relatives, %) | ||
| Bipolar disorder | 56 | |
| Unipolar disorder | 64 | |
| Schizophrenia | 24 | |
| Drug dependence | 44 | |
| Other psychiatric disorder | 52 | |
| Alcohol abuse/dependence | 52 | |
| Suicide (complete or serious attempt) | 38 | |
| Environmental adversity (%): | Child/adolescent | Adult |
| Physical abuse | 29 | 8 |
| Sexual abuse | 30 | 8 |
| Illness course and treatment (age in yrs) | ||
| Age of onset (symptoms) | 19 | |
| Early onset (age <14) | 34 | |
| Age at first treatment | 29 | |
| Age at first hospitalization | 30 | |
| Cycling pattern (n = 643, %) | ||
| Rapid (or faster cycling) | 47 | |
| Ultrarapid (or faster cycling) | 30 | |
| Rapid-cycling only | 17 | |
| Ultrarapid cycling only | 10 | |
| Ultradian cycling | 20 | |
Study population
The SFBN recruited outpatients with bipolar disorders I, II, or not otherwise specified (NOS). We did not exclude patients with comorbid illnesses, except those with active substance abuse disorder requiring treatment in another setting.1 Patients were recruited almost exclusively from the community and rarely from inpatient hospitalization. The average age was 41, and 43% were college graduates. Most were employed but indicated that their illness limited their employment level or ability to function at work (Table 1).
One striking finding was a decadelong lag between the onset of symptoms reaching thresholds for diagnosis and the onset of first treatment.2 This long delay could have adverse consequences for illness outcome and represents a major public health target. Age of onset appeared to have both genetic/familial and environmental determinants.3 Subjects with a history of both extreme early life adversities (i.e., physical or sexual abuse) and a positive family history of mood disorder had the earliest onset of bipolar illness. Those who had neither of these factors had late-onset illness.
Suicide attempts
Prior to network entry, 25 to 50% of our study population had made a serious suicide attempt (defined as requiring medical attention and/or hospitalization), pointing to bipolar disorder’s potential lethality.
History of abuse A history of physical or sexual abuse in childhood or adolescence was a prominent risk factor for attempted suicide.4 With either type of abuse there was an approximate 15% increase in incidence of suicide attempts over the baseline rate of about 25%. More than 55% of patients with a history of both physical and sexual abuse had made a serious suicide attempt. The frequency of physical and sexual abuse was also positively related to increased incidence of suicide attempts. Taken together, these data suggest a “stressor dose-response” relationship between adverse childhood or adolescent experiences and an increased incidence of suicide attempts.
Table 2
FACTORS THAT APPEAR TO INCREASE SUICIDE RISK IN BIPOLAR DISORDER*
| Course-of-illness characteristics |
| Early onset |
| More time ill |
| More time depressed |
| More-severe depression |
| Social and medical support |
| Loss of significant other |
| Lack of a confidant |
| Single motherhood |
| Decreased access to health care |
| Lack of medical insurance |
| Demographics |
| Less education |
| Lower income |
| Being single |
| Comorbidities |
| Axis I |
| Eating disorders |
| Anxiety disorders |
| Axis II |
| Cluster A, B, C |
| Medical |
| Traumatic life events |
| Sexual abuse |
| Physical abuse |
| Genetics |
| Family history of suicide and drug abuse |
| * Identified in the SFBN cohort |
Comorbidities Other factors that appear to be associated with bipolar disorder and increased risk of attempted suicide include anxiety and eating disorders, early onset of symptoms, at least four previous hospitalizations for depression, and medical comorbidity (Table 2). Limited access to psychiatric and medical services and inadequate health insurance also were correlated with having made a serious suicide attempt. Thus, lack of medical care might exacerbate demoralization and increase the risk of suicide.
Family history of suicide or serious suicide attempts and drug abuse were associated with an increase in suicide attempts, but family history of unipolar or bipolar illness were not. These data are consistent with other studies suggesting that vulnerability to suicide may have a separate genetic component from that of mood disorders per se.
Severity of illness Increased severity of bipolar illness also was associated with prior psychosocial stressors3 and serious suicide attempts. We confirmed self-reported history, using daily mood charting, the clinician-rated Inventory of Depressive Symptomatology (IDS-C), and the Young Mania Rating Scale (YMRS) to rate increase in percentage of time ill, percentage of time depressed, and severity of depression.
Clinical implications We are uncertain how directly these data regarding suicide attempts relate to completed suicide. However, patients with bipolar disorder are among those at highest risk for completed suicide, and a prior serious attempt is often a precursor to completed suicide.5,6
Factors that appear particularly related to increased risk of suicide in patients with bipolar disorder are family history of suicide, history of early psychosocial stressors, comorbid personality disorders, substance abuse, and a relatively severe course of bipolar illness. Patients with these risk factors need support for two types of psychosocial stressors:
- poor access to health care
- stressors related to concurrent events (e.g., occurring with new affective episodes).
It may also be important for clinicians to consider the prophylactic use of lithium because of its demonstrated ability to reduce mortality in bipolar illness.7 Additionally, we need data on the efficacy of new drugs in preventing affective episodes and reducing suicidal ideation and acts.
Common comorbidities
We found considerable axis I lifetime comorbidities in the SFBN cohort (Table 3), including a 42% incidence of anxiety disorder and a similarly high rate of substance abuse. In addition to bipolar disorder, patients averaged approximately two lifetime comorbid diagnoses:
- 35% had none
- 65% had one or more
- 42% had two or more
- 24% had three or more.
Table 3
PSYCHIATRIC COMORBIDITY IN BIPOLAR OUTPATIENTS (%)*.
| Any substance abuse disorder | 42% |
| Alcohol | 33 |
| Marijuana | 16 |
| Stimulant | 9 |
| Sedative | 8 |
| Opiate | 7 |
| Hallucinogens | 6 |
| Any anxiety disorder | 42% |
| Panic disorder | 20 |
| Social phobia | 16 |
| Simple phobia | 10 |
| OCD | 9 |
| PTSD | 7 |
| GAD | 3 |
| Other | 3 |
| Any eating disorder | 11% |
| Bulimia nervosa | 8 |
| Anorexia nervosa | 4 |
| * Lifetime comorbid axis I disorders based on Structured Clinical Interview for DSM-IV (SCID) interviews with 288 SFBN patients. | |
Men with bipolar disorder had a higher absolute prevalence of alcoholism, but the relative risk compared with the general population was much higher in women (odds ratio 7.35) compared with men (odds ratio 2.77). In women, alcohol use and abuse were related to history of social phobia, greater number of depressive episodes prior to network entry, and history of abusing more than one substance. In men, alcohol comorbidity was related to history of alcohol abuse in first-degree relatives and history of early physical abuse.
These data suggest that female patients, in particular, were self-medicating their affective episodes and comorbid anxiety disorders with alcohol. They suggest the importance of asking patients directly about alcohol use to identify the need for primary and secondary prevention. In an adolescent or young adult, affective illness—and bipolar disorder in particular—should be considered a major risk factor for subsequent problems with alcohol and drug abuse. When taking a careful history, the treating physician should include questions regarding any substance abuse.
Overweight and obesity
One-half of the women and two-thirds of the men in the SFBN were overweight at network entry.8 Although this rate of overweight sounds alarmingly high, it was not significantly different from rates in the general U.S. population. A greater percentage of women than men in the network had morbid obesity, however.
In our cohort, being overweight or obese was associated with an increased incidence of cardiovascular disease, diabetes, arthritis, hypertension, hypothyroidism, and cancer. The degree of obesity also correlated significantly with past use of the numerous psychotropic drugs that have been associated with weight gain. Also, as might be expected, increased weight was associated with physical inactivity.
Many of the psychotropic drugs routinely used to treat bipolar disorder are associated with weight gain. Among these are the mood stabilizers lithium and valproate and the atypical antipsychotics, with the exception of ziprasidone (and, to follow, aripiprazole). Lithium’s liability to cause weight gain may be counterbalanced by its antisuicide effects and its ability to reverse excess medical mortality associated with affective illness. Thus, lithium should continue to play a major therapeutic role in bipolar disorder.
It may be more effective and easier to prevent weight gain than to attempt to reduce weight that has been gained. Recommending exercise and other weight-reduction measures may be helpful, as may co-administering drugs associated with weight loss, such as topiramate.9
Thyroiditis
Antithyroid antibodies were detected in 28% of network patients, compared with rates of 3% in the general population and 18% in psychiatric controls.10 The increased rate was associated with the need to augment thyroid function in 17% of network patients, but it was not related to age, mood state, rapid cycling status, or lithium treatment.
These data indicate that bipolar patients are prone to autoimmune thyroid changes. Further analysis is required to ascertain whether this finding has therapeutic implications, especially related to adjunctive T3 and T4 treatment.11
Even with psychiatry’s most effective and best studied agents, many patients with bipolar disorder are inadequately treated and respond insufficiently to available psychopharmacologic agents. To address this problem, the Stanley Foundation Bipolar Network (SFBN) was created to explore acute and long-term response to treatment of bipolar disorder.
For the past 6 years, our group (Box) has followed an international cohort of patients with bipolar disorder and offered them opportunities to participate in clinical trials at seven field centers. We have made considerable progress as we focused on the longitudinal course of bipolar illness and evaluated various combinations of psychopharmacologic therapies. The SFBN also has explored factors that increase the risk of suicide, quantified the incidence of anxiety disorder and substance abuse, and looked at other variables that may affect treatment outcome.
This report for the readers of Current Psychiatry provides a brief state-of-the-art overview of what we have learned about the course and management of bipolar disorder. It brings together and summarizes the findings from studies published, in press, or under review. Our goal is to update current findings, which may help you provide optimum care for these at-risk patients.
Since Robert Prien’s pioneering studies in the 1970s, the National Institute of Mental Health (NIMH) had funded little work on prophylaxis of bipolar disorders or acute bipolar depression. Following two NIMH conferences on bipolar illness in 1989 and 1994, the Stanley Foundation Bipolar Network (SFBN) was established to help overcome these research deficiencies, with funding provided by the Theodore and Vada Stanley Foundation.
Patients were recruited into the network from the general community and with the use of newspaper and newsletter advertisements. In contrast to many other clinical populations, few who wished to enter the network were excluded. It was clear, however, that filling out demographic, clinical, and rating forms would require considerable effort from each patient. All patients gave written informed consent to participate in the naturalistic part of the study, and were reconsented for any open or blind randomized clinical trial.
Patients provided daily self-ratings on the National Institute of Mental Health Life Chart Method (NIMH-LCM), a unique longitudinal instrument. Clinicians then completed their own separate LCMs, as more severely ill patients often could not complete their self-ratings, and patients often underestimated the severity and functional disability associated with the hypomanic and manic phases of bipolar illness. With the use of the NIMH-LCM, network investigators were able, for the first time, to assess the details of mood fluctuations associated with bipolar illness and to more precisely delineate the qualitative and quantitative aspects of response to psychopharmacologic interventions.
The SFBN includes seven research field centers in the United States, the Netherlands, and Germany. The leaders of those centers collaborated with Robert M. Post, MD, to prepare this report of the network findings. The collaborators are:
| Gabriele S. Leverich, MSW, and Kirk D. Denicoff, MD Biological Psychiatry Branch National Institute of Mental Health Bethesda, MD | Paul E. Keck, Jr., MD, and Susan L. McElroy, MD Biological Psychiatry Program University of Cincinnati College of Medicine Cincinnati, OH |
| Lori L. Altshuler, MD, and Mark A. Frye, MD UCLA Mood Disorders Research Program VA Medical Center Los Angeles, CA | Willem A. Nolen, MD, and Ralph Kupka, MD Altrecht Institute for Mental Health Care University Medical Center Utrecht, The Netherlands |
| John Rush, MD, and Trisha Suppes, MD Department of Psychiatry Southwest Medical Center University of Texas, Dallas | Joerg Walden, MD Department of Psychiatry University of Freiburg Freiburg, Germany |
| Keith Kramlinger, MD Mayo Clinic, Rochester, MN (Administrative center) | Heinz Grunze, MD Psychiatrische Klinik der LMU Munich, Germany |
In part one, we discuss the demographics of our cohort of 676 community-based bipolar patients, including risk factors for suicide attempts, frequency of comorbid psychiatric diagnoses, problems of overweight and obesity, and preliminary findings about thyroiditis.
In part two, we describe our clinical trials assessing mood stabilizers and adjunctive agents such as atypical antipsychotics, second-generation antidepressants, and anticonvulsants. We also will address a soon-to-be analyzed study of omega-3 fatty acids.
Table 1
CHARACTERISTICS OF THE 676 BIPOLAR OUTPATIENTS
| Mean age | 41 | |
| % Female | 43 | |
| Diagnosis (%) | ||
| Bipolar I | 79 | |
| Bipolar II | 16 | |
| Bipolar NOS | 4 | |
| Marital status (%) | ||
| Married/cohabitating | 44 | |
| Divorced/separated | 22 | |
| Income (%) | ||
| <$20,000 | 37 | |
| $20,000-40,000 | 22 | |
| $40,000-100,000 | 32 | |
| >$100,000 | 9 | |
| Education (completed, %) | ||
| High school | 34 | |
| Jr. college/technical | 13 | |
| College, 4-year | 27 | |
| Graduate degree | 16 | |
| Positive family history (1st-degree relatives, %) | ||
| Bipolar disorder | 56 | |
| Unipolar disorder | 64 | |
| Schizophrenia | 24 | |
| Drug dependence | 44 | |
| Other psychiatric disorder | 52 | |
| Alcohol abuse/dependence | 52 | |
| Suicide (complete or serious attempt) | 38 | |
| Environmental adversity (%): | Child/adolescent | Adult |
| Physical abuse | 29 | 8 |
| Sexual abuse | 30 | 8 |
| Illness course and treatment (age in yrs) | ||
| Age of onset (symptoms) | 19 | |
| Early onset (age <14) | 34 | |
| Age at first treatment | 29 | |
| Age at first hospitalization | 30 | |
| Cycling pattern (n = 643, %) | ||
| Rapid (or faster cycling) | 47 | |
| Ultrarapid (or faster cycling) | 30 | |
| Rapid-cycling only | 17 | |
| Ultrarapid cycling only | 10 | |
| Ultradian cycling | 20 | |
Study population
The SFBN recruited outpatients with bipolar disorders I, II, or not otherwise specified (NOS). We did not exclude patients with comorbid illnesses, except those with active substance abuse disorder requiring treatment in another setting.1 Patients were recruited almost exclusively from the community and rarely from inpatient hospitalization. The average age was 41, and 43% were college graduates. Most were employed but indicated that their illness limited their employment level or ability to function at work (Table 1).
One striking finding was a decadelong lag between the onset of symptoms reaching thresholds for diagnosis and the onset of first treatment.2 This long delay could have adverse consequences for illness outcome and represents a major public health target. Age of onset appeared to have both genetic/familial and environmental determinants.3 Subjects with a history of both extreme early life adversities (i.e., physical or sexual abuse) and a positive family history of mood disorder had the earliest onset of bipolar illness. Those who had neither of these factors had late-onset illness.
Suicide attempts
Prior to network entry, 25 to 50% of our study population had made a serious suicide attempt (defined as requiring medical attention and/or hospitalization), pointing to bipolar disorder’s potential lethality.
History of abuse A history of physical or sexual abuse in childhood or adolescence was a prominent risk factor for attempted suicide.4 With either type of abuse there was an approximate 15% increase in incidence of suicide attempts over the baseline rate of about 25%. More than 55% of patients with a history of both physical and sexual abuse had made a serious suicide attempt. The frequency of physical and sexual abuse was also positively related to increased incidence of suicide attempts. Taken together, these data suggest a “stressor dose-response” relationship between adverse childhood or adolescent experiences and an increased incidence of suicide attempts.
Table 2
FACTORS THAT APPEAR TO INCREASE SUICIDE RISK IN BIPOLAR DISORDER*
| Course-of-illness characteristics |
| Early onset |
| More time ill |
| More time depressed |
| More-severe depression |
| Social and medical support |
| Loss of significant other |
| Lack of a confidant |
| Single motherhood |
| Decreased access to health care |
| Lack of medical insurance |
| Demographics |
| Less education |
| Lower income |
| Being single |
| Comorbidities |
| Axis I |
| Eating disorders |
| Anxiety disorders |
| Axis II |
| Cluster A, B, C |
| Medical |
| Traumatic life events |
| Sexual abuse |
| Physical abuse |
| Genetics |
| Family history of suicide and drug abuse |
| * Identified in the SFBN cohort |
Comorbidities Other factors that appear to be associated with bipolar disorder and increased risk of attempted suicide include anxiety and eating disorders, early onset of symptoms, at least four previous hospitalizations for depression, and medical comorbidity (Table 2). Limited access to psychiatric and medical services and inadequate health insurance also were correlated with having made a serious suicide attempt. Thus, lack of medical care might exacerbate demoralization and increase the risk of suicide.
Family history of suicide or serious suicide attempts and drug abuse were associated with an increase in suicide attempts, but family history of unipolar or bipolar illness were not. These data are consistent with other studies suggesting that vulnerability to suicide may have a separate genetic component from that of mood disorders per se.
Severity of illness Increased severity of bipolar illness also was associated with prior psychosocial stressors3 and serious suicide attempts. We confirmed self-reported history, using daily mood charting, the clinician-rated Inventory of Depressive Symptomatology (IDS-C), and the Young Mania Rating Scale (YMRS) to rate increase in percentage of time ill, percentage of time depressed, and severity of depression.
Clinical implications We are uncertain how directly these data regarding suicide attempts relate to completed suicide. However, patients with bipolar disorder are among those at highest risk for completed suicide, and a prior serious attempt is often a precursor to completed suicide.5,6
Factors that appear particularly related to increased risk of suicide in patients with bipolar disorder are family history of suicide, history of early psychosocial stressors, comorbid personality disorders, substance abuse, and a relatively severe course of bipolar illness. Patients with these risk factors need support for two types of psychosocial stressors:
- poor access to health care
- stressors related to concurrent events (e.g., occurring with new affective episodes).
It may also be important for clinicians to consider the prophylactic use of lithium because of its demonstrated ability to reduce mortality in bipolar illness.7 Additionally, we need data on the efficacy of new drugs in preventing affective episodes and reducing suicidal ideation and acts.
Common comorbidities
We found considerable axis I lifetime comorbidities in the SFBN cohort (Table 3), including a 42% incidence of anxiety disorder and a similarly high rate of substance abuse. In addition to bipolar disorder, patients averaged approximately two lifetime comorbid diagnoses:
- 35% had none
- 65% had one or more
- 42% had two or more
- 24% had three or more.
Table 3
PSYCHIATRIC COMORBIDITY IN BIPOLAR OUTPATIENTS (%)*.
| Any substance abuse disorder | 42% |
| Alcohol | 33 |
| Marijuana | 16 |
| Stimulant | 9 |
| Sedative | 8 |
| Opiate | 7 |
| Hallucinogens | 6 |
| Any anxiety disorder | 42% |
| Panic disorder | 20 |
| Social phobia | 16 |
| Simple phobia | 10 |
| OCD | 9 |
| PTSD | 7 |
| GAD | 3 |
| Other | 3 |
| Any eating disorder | 11% |
| Bulimia nervosa | 8 |
| Anorexia nervosa | 4 |
| * Lifetime comorbid axis I disorders based on Structured Clinical Interview for DSM-IV (SCID) interviews with 288 SFBN patients. | |
Men with bipolar disorder had a higher absolute prevalence of alcoholism, but the relative risk compared with the general population was much higher in women (odds ratio 7.35) compared with men (odds ratio 2.77). In women, alcohol use and abuse were related to history of social phobia, greater number of depressive episodes prior to network entry, and history of abusing more than one substance. In men, alcohol comorbidity was related to history of alcohol abuse in first-degree relatives and history of early physical abuse.
These data suggest that female patients, in particular, were self-medicating their affective episodes and comorbid anxiety disorders with alcohol. They suggest the importance of asking patients directly about alcohol use to identify the need for primary and secondary prevention. In an adolescent or young adult, affective illness—and bipolar disorder in particular—should be considered a major risk factor for subsequent problems with alcohol and drug abuse. When taking a careful history, the treating physician should include questions regarding any substance abuse.
Overweight and obesity
One-half of the women and two-thirds of the men in the SFBN were overweight at network entry.8 Although this rate of overweight sounds alarmingly high, it was not significantly different from rates in the general U.S. population. A greater percentage of women than men in the network had morbid obesity, however.
In our cohort, being overweight or obese was associated with an increased incidence of cardiovascular disease, diabetes, arthritis, hypertension, hypothyroidism, and cancer. The degree of obesity also correlated significantly with past use of the numerous psychotropic drugs that have been associated with weight gain. Also, as might be expected, increased weight was associated with physical inactivity.
Many of the psychotropic drugs routinely used to treat bipolar disorder are associated with weight gain. Among these are the mood stabilizers lithium and valproate and the atypical antipsychotics, with the exception of ziprasidone (and, to follow, aripiprazole). Lithium’s liability to cause weight gain may be counterbalanced by its antisuicide effects and its ability to reverse excess medical mortality associated with affective illness. Thus, lithium should continue to play a major therapeutic role in bipolar disorder.
It may be more effective and easier to prevent weight gain than to attempt to reduce weight that has been gained. Recommending exercise and other weight-reduction measures may be helpful, as may co-administering drugs associated with weight loss, such as topiramate.9
Thyroiditis
Antithyroid antibodies were detected in 28% of network patients, compared with rates of 3% in the general population and 18% in psychiatric controls.10 The increased rate was associated with the need to augment thyroid function in 17% of network patients, but it was not related to age, mood state, rapid cycling status, or lithium treatment.
These data indicate that bipolar patients are prone to autoimmune thyroid changes. Further analysis is required to ascertain whether this finding has therapeutic implications, especially related to adjunctive T3 and T4 treatment.11
1. Leverich GS, Nolen WA, Rush AJ, et al. The Stanley Foundation Bipolar Treatment Outcome Network. I. Longitudinal methodology. J Affect Disord 2001;67:33-44.
2. Suppes T, Leverich GS, Keck PE, et al. The Stanley Foundation Bipolar Treatment Outcome Network. II. Demographics and illness characteristics of the first 261 patients. J Affect Disord 2001b;67:45-59.
3. Leverich GS, McElroy SL, Suppes T, et al. Early physical and sexual abuse associated with an adverse course of bipolar illness. Biological Psychiatry 2002;51:288-97.
4. Leverich GS, Altshuler LL, Frye MA, et al. Factors associated with suicide attempts in 648 patients with bipolar disorder in the Stanley Foundation Bipolar Network. Manuscript submitted for publication, 2002.
5. Chen Y-W, Dilsaver SC. Lifetime rates of suicide attempts among subjects with bipolar and unipolar disorders relative to subjects with other axis I disorders. Biological Psychiatry 1996;39:896-9.
6. Kessler RC, Borges G, Walters EE. Prevalence of and risk factors for lifetime suicide attempts in the National Comorbidity Survey. Arch.Gen.Psychiatry 1999;56:617-26.
7. Ahrens B, Muller-Oerlinghausen B, Schou M, et al. Excess cardiovascular and suicide mortality of affective disorders may be reduced by lithium prophylaxis. J Affect Disord 1995;33:67-75.
8. McElroy SL, Frye MA, Suppes T, et al. Correlates of overweight and obesity in 644 patients with bipolar disorder. J Clin Psychiatry 2002;63:207-13.
9. McElroy SL, Suppes T, Keck PE, et al. Open-label adjunctive topiramate in the treatment of bipolar disorders. Biological Psychiatry 2000;47:1025-33.
10. Kupka RW, Nolen WA, Post RM, et al. High rate of autoimmune thyroiditis in bipolar disorder: lack of association with lithium exposure. Biological Psychiatry 2002;51:305-11.
11. Bauer M, Priebe S, Berghofer A, et al. Subjective response to and tolerability of long-term supraphysiological doses of levothyroxine in refractory mood disorders. J Affect Disord 2001;64:35-42.
1. Leverich GS, Nolen WA, Rush AJ, et al. The Stanley Foundation Bipolar Treatment Outcome Network. I. Longitudinal methodology. J Affect Disord 2001;67:33-44.
2. Suppes T, Leverich GS, Keck PE, et al. The Stanley Foundation Bipolar Treatment Outcome Network. II. Demographics and illness characteristics of the first 261 patients. J Affect Disord 2001b;67:45-59.
3. Leverich GS, McElroy SL, Suppes T, et al. Early physical and sexual abuse associated with an adverse course of bipolar illness. Biological Psychiatry 2002;51:288-97.
4. Leverich GS, Altshuler LL, Frye MA, et al. Factors associated with suicide attempts in 648 patients with bipolar disorder in the Stanley Foundation Bipolar Network. Manuscript submitted for publication, 2002.
5. Chen Y-W, Dilsaver SC. Lifetime rates of suicide attempts among subjects with bipolar and unipolar disorders relative to subjects with other axis I disorders. Biological Psychiatry 1996;39:896-9.
6. Kessler RC, Borges G, Walters EE. Prevalence of and risk factors for lifetime suicide attempts in the National Comorbidity Survey. Arch.Gen.Psychiatry 1999;56:617-26.
7. Ahrens B, Muller-Oerlinghausen B, Schou M, et al. Excess cardiovascular and suicide mortality of affective disorders may be reduced by lithium prophylaxis. J Affect Disord 1995;33:67-75.
8. McElroy SL, Frye MA, Suppes T, et al. Correlates of overweight and obesity in 644 patients with bipolar disorder. J Clin Psychiatry 2002;63:207-13.
9. McElroy SL, Suppes T, Keck PE, et al. Open-label adjunctive topiramate in the treatment of bipolar disorders. Biological Psychiatry 2000;47:1025-33.
10. Kupka RW, Nolen WA, Post RM, et al. High rate of autoimmune thyroiditis in bipolar disorder: lack of association with lithium exposure. Biological Psychiatry 2002;51:305-11.
11. Bauer M, Priebe S, Berghofer A, et al. Subjective response to and tolerability of long-term supraphysiological doses of levothyroxine in refractory mood disorders. J Affect Disord 2001;64:35-42.