User login
The DASH diet for high blood pressure: From clinical trial to dinner table
Changes in recommended treatments for mild and moderate asthma
- Every patient with persistent asthma, regardless of disease severity, should use a daily controller medication.
- Consider an inhaled corticosteroid (ICS) first when choosing controller medications for long-term treatment of mild, moderate, and severe persistent asthma in adults and children. Leukotriene modifiers, cromolyn, and nedocromil may be considered as alternative, not preferred, controller medications for patients with persistent asthma.
- Long-acting β2-adrenergic agonists should not be used as monotherapy.
- Long-term use of ICSs within labeled doses is safe for children in terms of growth, bone mineral density, and adrenal function; nonetheless, asthma should be monitored and ICS therapy stepped down to the lowest effective dose.
- Low-to medium-dose ICSs are not associated with the development of cataracts or glaucoma in children, but high cumulative lifetime doses may slightly increase the prevalence of cataracts in adults and elderly patients.
- ICSs are recommended for use in pregnant women with asthma; budesonide is the only ICS rated Pregnancy Category B.
Consider an adult with the following characteristics. To which disease severity would you assign this patient’s asthma?
- Forced expiratory volume in 1 second (FEV1) or peak expiratory flow (PEF) ≥80%
- PEF variability 20%–30%
- Daytime symptoms less than once a day
- Nighttime symptoms more than 1 night a week.
This patient is said to have moderate persistent asthma based on nighttime symptoms. An accurate classification of a patient’s asthma is the foundation for selecting an appropriate treatment strategy.
In 2002 the National Asthma Education and Prevention Program (NAEPP) updated select topics1from its 1997 Guidelines for the Diagnosis and Management of Asthma.2 These evidence-based revisions to the stepwise approach to asthma management were made following a systematic review of the literature (see Search function).
A comprehensive search of Medline and EMBASE databases was performed to identify controlled clinical studies relevant to each topic that were published (in English or foreign languages with English abstracts) from 1980 through August 2000. The search included studies published before 1980 if referenced in the post-1980 literature. Studies that did not include control groups were excluded, except for those reporting adverse effects of ICSs. Studies that met the study selection criteria established for each topic were included in a systematic review of the evidence. An expert panel reviewed the evidence, along with additional literature published since August 2000, and reached a consensus on whether the evidence supported 1997 guideline recommendations or indicated a need for revision. Writing committees were then assigned to developed position statements for each topic. The level of evidence for included studies was rated based on the system of Jadad and colleagues,3 where A = randomized controlled trials, rich body of data; B = randomized controlled trials, limited data; C = nonrandomized trials and observational studies; D = panel consensus judgment.
This article reviews the 2002 NAEPP recommendations for the use of controller medications for asthma, including:
- Relative effectiveness of inhaled corticosteroids (ICSs) versus other controller medications
- Safety of long-term ICS use in children
- Potential benefits of early ICS treatment.
We emphasize mild and moderate persistent asthma because the recommended treatments for these levels of severity have been most affected by the recent guideline changes. We also discuss a recent change by the US Food and Drug Administration (FDA) in its pregnancy category rating for an ICS.
2002 Stepwise approach to asthma management
New criteria for classifying asthma severity
The NAEPP classifies asthma severity according to symptoms and lung function in adults and children older than 5 years, and symptoms in children 5 years and younger.1 Persistent asthma is classified as mild, moderate, or severe according to the feature of greatest severity.
Asthma severity should be assigned according to symptoms before treatment.1 Because it is difficult to predict which infants and young children who wheeze with acute viral upper respiratory infection will go on to develop persistent asthma, new criteria have been detailed to help distinguish these children from those with transient wheeze (Table 1).1,4
TABLE 1
Criteria for children with intermittent wheeze
Infants and young children meeting these criteria should receive controller therapy for asthma:
|
AND presence of risk factors for development of persistent asthma:
|
Choosing pharmacologic treatment according to asthma classification
Quick-relief medications, which include the short-acting β2-agonists (SABAs), are taken as needed to promptly reverse acute airflow obstruction and relieve accompanying symptoms.2
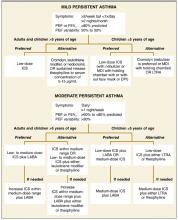
Asthma controller medications (ie, ICSs, cromolyn sodium, long-acting β2-adrenergic-agonists [LABAs], leukotriene modifiers, nedocromil, and theophylline) are used daily to achieve and maintain long-term control of persistent asthma. All patients with persistent asthma, regardless of disease severity, should use a daily controller. Criteria for determining asthma severity and updated recommendations for the use of controller treatment in mild and moderate persistent asthma are presented in the Figure.3,5 Levels of evidence justifying NAEPP treatment recommendations are shown in Table 2.
For use in children. Asthma controller medications approved for use in children younger than 5 years include the fluticasone dry-powder inhalers (Flovent, Rotadisk, and Flovent Diskus), which are approved for children as young as 4 years (Flovent Diskus is not yet commercially available), and nebulized budesonide inhalation suspension (Pulmicort Respules), which is approved for children as young as 12 months.
The LABAs formoterol (Foradil) and salmeterol (Serevent Diskus) are approved for children as young as 5 and 4 years, respectively. Cromolyn sodium nebulizer solution is approved for children as young as 2 years, and theophylline is available for use at any age.
Based on safety and extrapolation of efficacy data in older patients, the oral granule formulation of the leukotriene receptor antagonist (LTRA) montelukast (Singulair) is approved for children as young as 1 year, and the chewable tablets are approved for children 2 to 5 years of age. Zafirlukast (Accolate) is approved for use in children 5 years and older.
New recommendations for mild persistent asthma. Recommendations for the treatment of mild and moderate persistent asthma have changed considerably from the 1997 guidelines. ICSs are now the preferred controller medications, based on greater efficacy. The updated guidelines no longer recommend an initial trial of cromolyn or nedocromil for the treatment of mild persistent asthma; these agents, along with the leukotriene modifiers and slow-release theophylline, are now considered alternatives to low-dose ICSs for adults and children older than 5 years with mild persistent disease (Figure).
According to the NAEPP update, daily low-dose ICS treatment also is preferred for the control of mild persistent asthma in preschool children. As in older children, cromolyn and nedocromil are no longer considered appropriate initial treatments for infants and children 5 years and younger. Cromolyn is considered an alternative controller, whereas nedocromil is no longer recommended for use.
New recommendations for moderate persistent asthma. For adults and children older than 5 years with moderate persistent asthma, revision to the guidelines involved recommendation of a low- to medium-dose ICS plus a LABA as the preferred controller treatment (Figure). Comparative low, medium, and high daily doses for ICSs are shown in Table 3 .1
For preschool children, preferred controller treatments for moderate persistent asthma include low-dose ICSs plus a LABA, or increasing ICSs within the medium-dose range (Figure). Recommendations for the use of LABAs as add-on therapy in this age group are based on extrapolation of data from older patients, since therapy with an ICS/LABA combination has not been adequately studied in children younger than 5 years. Four studies included in the NAEPP evaluation showed clear benefit of medium-dose ICSs in this age group, supporting the use of medium-dose ICSs as a preferred option.6-9 LABAs are not recommended for use without an ICS, and the only ICS/LABA combination product currently available has been FDA approved only for patients aged 12 years and older.
TABLE 2
Levels of evidence for NAEPP assessments*
| Medication | NAEPP assessment | SOR* |
|---|---|---|
| ICS | Preferred treatment for children of all ages with persistent asthma | A (A) |
| SABA | ICSs improve asthma control compared with as-needed SABAs | A (A) |
| Cromolyn/nedocromil | For use as alternative, not preferred, treatment of mild persistent asthma in children of all ages (cromolyn) or children >5 years of age (nedocromil) | A (A) |
| LABA | For use with ICSs as the preferred combination treatment for moderate and severe persistent asthma in children >5 years of age | A (A) |
| For use as a preferred option for combination treatment in children 5 years of age | B (B) | |
| Leukotriene modifier | For use as alternative, not preferred, treatment of mild persistent asthma and as ICS adjunct in moderate persistent asthma | B (B) |
| Theophylline | For use as an alternative ICS add-on in moderate or severe persistent asthma if serum concentrations are monitored | D (D) |
| Not considered an alternative controller for young children with mild persistent asthma due to potential adverse effects in infants with frequent febrile illnesses | ||
| *Highest level of evidence available is reported. Strengths of recommendation are based on the method of Jadad et al.3 Strength of evidence based on the Oxford Center for Evidence-Based Medicine5 is in parentheses. SOR, strength of recommendation; NAEPP, National Asthma Education and Prevention Program; ICS, inhaled corticosteroid; SABA, short-acting β2-adrenergic agonist; LABA, long-acting β2-adrenergic agonist. | ||
TABLE 3
Estimated comparative daily doses for inhaled corticosteroids*
| Drug | Low daily dose | Medium daily dose | High daily dose | |||
|---|---|---|---|---|---|---|
| Adult | Child† | Adult | Child† | Adult | Child† | |
| Beclomethasone CFC 42 or 84 μg/puff | 168–504 μg | 84–336 μg | 504–840 μg | 336–672 μg | >840 μg | >672 μg |
| Beclomethasone HFA 40 or 80 μg/puff | 80–240 μg | 80–160 μg | 240–480 μg | 160–320 μg | >480 μg | >320 μg |
| Budesonide DPI 200 μg/inhalation | 200–600 μg | 200–400 μg | 600–1200 μg | 400–800 μg | >1200 μg | >800 μg |
| Budesonide inhalation suspension for nebulization (child dose) | 0.5mg | 1.0 mg | 2.0 mg | |||
| Fluticasone MDI 44, 110, or 220 μg/puff | 88–264 μg | 88–176 μg | 264–660 μg | 176–440 μg | >660 μg | >440 μg |
| Fluticasone DPI 50, 100, or 250 μg/inhalation | 100–300 μg | 100–200 μg | 300–600 μg | 200–400 μg | >600 μg | >400 μg |
| Triamcinolone acetonide 100 μg/puff | 400–1000 μg | 400–800 μg | 1000–2000 μg | 800–1200 μg | >2000 μg | >1200 μg |
| *The most important determinant of appropriate dosing is the clinician’s judgment of the patient’s response to therapy. This updated comparative dose chart is based on review of recently published clinical trials involving more than 5000 patients and published reviews. Some doses may be outside package labeling, especially in the high-dose range. | ||||||
| †Children 12 years of age. | ||||||
| CFC, chlorofluorocarbon; HFA, hydrofluoroalkane; DPI, dry-powder inhaler; MDI, metered-dose inhaler. | ||||||
FIGURE
Updated National Asthma Education and Prevention Program recommendations for long-term controller treatment in mild and moderate persistent asthma
Topics in the management of asthma in children
Recognizing the need for continual appraisal of the benefits and risks of asthma medications in children, the NAEPP Expert Panel considered new studies comparing the effectiveness of ICS monotherapy with that of as-needed SABAs and other controllers used as monotherapy in children with mild or moderate persistent asthma. In addition, the safety of long-term ICS use in children was evaluated based on vertical growth, bone mineral density, ocular toxicity, and adrenal suppression.
Effectiveness of ICSs compared with other asthma medications
Short-acting β2-adrenergic agonists. Eight studies met the eligibility criteria for evaluating the effectiveness of ICSs versus as-needed SABAs.6,10-16 Six studies (4 involving budesonide) in children 5 years and older showed that ICSs improve lung function and symptoms and reduce the need for emergency intervention compared with as-needed SABAs.1 Among all studies included in the NAEPP update, the Childhood Asthma Management Program (CAMP) Research Group Study,9 a placebo-controlled study of inhaled budesonide and nedocromil, contributed the most evidence. Studies with children 5 years and younger are limited to 2 small studies enrolling a total of 69 children.6,15 Consistent with studies of older children, these studies indicate that ICSs improve asthma control compared with as-needed SABAs.1
Cromolyn and nedocromil. Despite well-established safety profiles, cromolyn and nedocromil are no longer recommended as first-line therapy for children, even those with mild disease. New recommendations reflect the greater effectiveness of inhaled budesonide compared with nedocromil demonstrated in the CAMP study,10 and the lack of apparent benefit of cromolyn as maintenance treatment in childhood asthma reported by Tasche and colleagues in a systematic review of the literature.17
In the CAMP study, children 5 to 12 years of age receiving inhaled budesonide showed greater reductions in symptoms and albuterol use, lower rates of hospitalization and urgent care visits, and less need for additional asthma therapy and oral prednisone compared with placebo over 4 to 6 years of treatment.10 The marginal effectiveness of nedocromil demonstrated in the CAMP study mirrored that of cromolyn reported in the review of 24 randomized placebo-controlled studies by Tasche and colleagues.1,17
For children 5 years and younger, the NAEPP Expert Panel took into account 1 randomized placebo-controlled study conducted with children 2 to 5 years of age; it showed improvements in lung function, symptoms, and bronchial hyperre-activity with inhaled budesonide.9 Support for the new NAEPP recommendations preferring ICSs for preschool children is found in a more recent open-label study18 that showed greater symptom improvement and significantly lower rates of asthma exacerbations, urgent care visits, and oral prednisone use with budesonide inhalation suspension, compared with cromolyn sodium nebulizer solution (Intal Nebulizer Solution) in children 2 to 6 years of age with persistent asthma.
Leukotriene modifiers. The LTRAs zafir-lukast and montelukast are approved for use in children. According to the NAEPP Expert Panel, studies have shown only modest improvements in lung function and other asthma control outcomes with LTRA monotherapy in children as young as 6 and 2 years, respectively.1 Because studies comparing ICSs with LTRAs in children are lacking, findings of greater overall efficacy of ICSs in adults with persistent asthma have been extrapolated for use with children; clear superiority of ICSs versus LTRAs in most outcomes has resulted in the recommendation for ICSs as the preferred treatment for mild persistent asthma in children.
Long-acting β2-adrenergic agonists. There is no role for LABAs as monotherapy in asthma. No studies have compared the effectiveness of ICS versus LABA monotherapy in children younger than 5 years, and studies in older children have shown greater effectiveness of inhaled beclomethasone versus salmeterol.14,19 In the study by Verberne and colleagues, salmeterol monotherapy was associated with deterioration in FEV1.19 In a more recent study that included patients as young as 16 years, a switch from ICS to LABA treatment was associated with a significant increase in treatment failures and exacerbations.20
Theophylline. Only 1 study has compared outcomes with low-dose ICSs versus theophylline in adults and children.21 Although limited, the data support greater effectiveness of ICSs based on symptoms, bronchial hyperresponsiveness, and the need for β2-adrenergic agonists and oral corticosteroids.1
Safety of long-term ICS use in children
Systemic corticosteroids have the potential to suppress growth over the long term.2 Short-term growth studies with ICSs show an average reduction in growth velocity of 1 cm per year during the first year of treatment, but the CAMP study showed that initial reductions in growth velocity with inhaled budesonide were not maintained over a 4- to 6-year treatment period.1,10
Although catch-up growth was not observed in the CAMP study, Agertoft and Pedersen reported no effect of long-term treatment with inhaled budesonide (mean 9.2 years) on final adult height.22 Based on these long-term prospective studies of budesonide, showing only a transient reduction in growth velocity and attainment of expected final adult height, and retrospective studies including inhaled beclomethasone, the Expert Panel concluded that the ICS class is safe regarding growth effects.
According to the NAEPP Expert Panel, clinical study data for children monitored for up to 6 years strongly suggest that ICSs are safe when used at recommended doses (strength of recommendation: A).1 The panel could not rule out a potential cumulative effect of ICS use on some conditions, (eg, osteoporosis, cataracts, glaucoma) in adulthood, as sufficient long-term data are not available.
The panel did conclude that low- to medium-dose ICSs (Table 3) appear to have no serious adverse effects on bone mineral density in children.
Likewise, low- to medium-dose ICS use was not associated with the development of cataracts or glaucoma in children, although the potential for high cumulative lifetime doses of ICSs to slightly increase the prevalence of cataracts in adults and elderly patients was noted.
Strong evidence also indicates that ICS effects on adrenal function are usually clinically insignificant at low to medium doses; however, certain individuals may be at higher risk for hypothalam-ic pituitary adrenal axis effects while using conventional ICS doses.1
Although ICSs are safe when used within labeled dosing, it is still preferable to maintain doses at the lowest effective dose. In general, treatment should be reviewed every 1 to 6 months and doses reduced in a stepwise fashion when possible.1 For children showing a favorable response to treatment, a step down in dose should be considered, but not more frequently than every 3 months. If children show no clear response to treatment within 4 to 6 weeks, consider an alternative treatment or diagnosis.1
Safety of long-term ICS use in pregnant women
Uncontrolled asthma during pregnancy is associated with an increased risk of perinatal complications. 23 Since the consequences of not using asthma controllers during pregnancy can be worse than those with using them, daily controller treatment is recommended for all pregnant women with persistent asthma. 23
The American College of Obstetricians and Gynecologists and the American College of Allergy, Asthma and Immunology previously recommended cromolyn as the treatment of choice for pregnant women with mild persistent asthma. ICSs were recommended for patients whose asthma was inadequately controlled with cromolyn. 24 Beclomethasone and budesonide were the ICSs of choice for pregnant women and those who might become pregnant, with a preference for budesonide when high-dose therapy was indicated.24
These recommendations predate the 2002 NAEPP recommendations for ICSs as preferred therapy in mild persistent asthma and the 2004 NAEPP recommendations for ICSs as the first-choice controller therapy for mild persistent asthma during pregnancy. 25 Among ICSs, one (inhaled budesonide) has an FDA Pregnancy Category B rating based on studies showing no risk in pregnant women. 26,27 All other ICSs are rated Pregnancy Category C.
Based on current evidence, it seems reasonable to consider whether budesonide should now be the preferred therapy for mild persistent asthma during pregnancy.
Effects of early treatment on asthma progression
The potential for early ICS intervention to prevent progression of mild or moderate persistent asthma was evaluated solely with data from children enrolled in the CAMP study. 10 The NAEPP Expert Panel concluded that CAMP study data do not support a progressive decline in lung function in children aged 5 to 12 years with mild or moderate persistent asthma, but do suggest that lung function decline is influenced by age of asthma onset.
According to the panel, CAMP data suggest that most deficits in lung function growth due to childhood asthma occur during the first 3 years of life. Preliminary results of the recent START study (Inhaled Steroid Treatment As Regular Therapy in Early Asthma), 28 conducted with 7165 corticosteroidnaïve patients 5 to 66 years of age with recent onset mild persistent asthma, did show a decline in lung function in patients with mild persistent disease.
Although improvements in prebronchodilator and postbronchodilator FEV1 were significant after 3 years of treatment with inhaled budes-onide, differences from placebo in both outcomes were greatest after the first year. When patients with mild persistent disease inhaled budesonide once daily in addition to normal treatment within 2 years of asthma onset,28 they enjoyed considerable protection from severe and life-threatening asthma exacerbations and overall greater asthma control.
- Budesonide • Pulmicort
- Rhinocort Cromolyn • Intal
- Fluticasone • Flovent
- Formoterol • Foradil
- Montelukast • Singulair
- Nedocromil • Tilade
- Salmeterol • Servent
- Triamcinolone acetonide • Azmacort
- Zafirlukast • Accolate
Corresponding author
Gregory J. Redding, MD, Children’s Hospital and Regional Medical Center, 4800 Sand Point Way, NE, Seattle, WA 98105-0371. E-mail: [email protected].
1. National Asthma Education and Prevention Program. Expert panel report: guidelines for the diagnosis and management of asthma. Update on selected topics–2002. J Allergy Clin Immunol 2002;110(5 suppl):S141-S219.
2. National Asthma Education and Prevention Program Expert Panel Report 2: Guidelines for the Diagnosis and Management of Asthma. Bethesda, Md: National Heart, Lung, and Blood Institute; National Institutes of Health; 1997. Publication 97;4051.-
3. Jadad AR, Moher M, Browman GP, Booker L, Sigouin C, Fuentes M, et al. Systematic reviews and meta-analyses on treatment of asthma: critical evaluation. BMJ 2000;320:537-540.
4. Castro-Rodríguez JA, Holberg CJ, Wright AL, Martinez FD. A clinical index to define risk of asthma in young children with recurrent wheezing. Am J Respir Crit Care Med 2000;162:1403-1406.
5. Oxford Centre for Evidence-based Medicine Levels of Evidence Available atwww.cebm.net/levels_faq.asp . Accessed January 8, 2004.
6. Connett GJ, Warde C, Wooler E, Lenney W. Use of budes-onide in severe asthmatics aged 1–3 years. Arch Dis Child 1993;69:351-355.
7. de Blic J, Delacourt C, Le Bourgeois M, Mahut B, Ostinelli J, Caswell C, et al. Efficacy of nebulized budesonide in treatment of severe infantile asthma: a double-blind study. J Allergy Clin Immunol 1996;98:14-20.
8. Bisgaard H, Gillies J, Groenewald M, Maden C, . for an International Study Group The effect of inhaled fluticas-one propionate in the treatment of young asthmatic children: a dose comparison study. Am J Respir Crit Care Med 1999;160:126-131.
9. Nielsen KG, Bisgaard H. The effect of inhaled budesonide on symptoms, lung function, and cold air and metha-choline responsiveness in 2- to 5-year–old asthmatic children. Am J Respir Crit Care Med 2000;162:1500-1506.
10. Childhood Asthma Management Program Research Group. Long-term effects of budesonide or nedocromil in children with asthma. N Engl J Med 2000;343:1054-1063.
11. Agertoft L, Pedersen S. Effects of long-term treatment with an inhaled corticosteroid on growth and pulmonary function in asthmatic children. Respir Med 1994;88:373-381.
12. Hoekstra MO, Grol MH, Bouman K, Stijnen T, Koëter GH, Kauffman HF, et al. Fluticasone propionate in children with moderate asthma. Am J Respir Crit Care Med 1996;154:1039-1044.
13. Jónasson G, Carlsen K-H, Blomqvist P. Clinical efficacy of low-dose inhaled budesonide once or twice daily in children with mild asthma not previously treated with steroids. Eur Respir J 1998;12:1099-1104.
14. Simons FER and the Canadian Beclomethasone Dipropionate-Salmeterol Xinafoate Study Group. A comparison of beclomethasone, salmeterol, and placebo in children with asthma. N Engl J Med 1997;337:1659-1665.
15. Storr J, Lenney CA, Lenney W. Nebulized beclomethasone dipropionate in preschool asthma. Arch Dis Child 1986;61:270-273.
16. Van Essen-Zandvliet EE, Hughes MD, Waalkens HJ, Duiverman EJ, Pocock SJ, Kerrebijn KF. and the Dutch Chronic Non-Specific Lung Disease Study Group Effects of 22 months of treatment with inhaled corticosteroids and/or beta-2-agonists on lung function, airway responsiveness, and symptoms in children with asthma. Am Rev Respir Dis 1992;146:547-554.
17. Tasche MJA, Uijen JHJM, Bernsen RMD, de Jongste JC, van der Wouden JC. Inhaled disodium cromoglycate (DSCG) as maintenance therapy in children with asthma: a systematic review. Thorax 2000;55:913-920.
18. Leflein JG, Szefler SJ, Murphy KR, Fitzpatrick S, Cruz-Rivera M, Miller CJ, et al. Nebulized budesonide inhalation suspension compared with cromolyn sodium nebulizer solution for asthma in young children: results of a randomized outcomes trial. Pediatrics 2002;109:866-872.
19. Verberne AAPH, Frost C, Duiverman EJ, Grol MH, Kerrebijn KF. and the Dutch Paediatric Asthma Study Group Addition of salmeterol versus doubling the dose of beclomethasone in children with asthma. Am J Respir Crit Care Med 1998;158:213-219.
20. Lazarus SC, Boushey HA, Fahy JV, Chinchilli VM, Lemanske RF Jr, Sorkness CA, , et al. for the Asthma Clinical Research Network of the National Heart Lung and Blood Institute. Long-acting 2-agonist monotherapy vs continued therapy with inhaled corticosteroids in patients with persistent asthma: a randomized controlled trial. JAMA 2001;285:2583-2593.
21. Reed CE, Offord KP, Nelson HS, Li JT, Tinkelman DG. and the American Academy of Allergy, Asthma and Immunology Beclomethasone Dipropionate-Theophylline Study Group. Aerosol beclomethasone dipropionate spray compared with theophylline as primary treatment for chronic mild or moderate persistent asthma. J Allergy Clin Immunol 1998;101:14-23.
22. Agertoft L, Pedersen S. Effect of long-term treatment with inhaled budesonide on adult height in children with asthma. N Engl J Med 2000;343:1064-1069.
23. National Asthma Education Program (NAEP). Report of the Working Group on Asthma and Pregnancy: Management of Asthma during Pregnancy. Bethesda, Md: National Heart, Lung, and Blood Institute; National Institutes of Health, 1993. NIH Publication No. 96-141593.
24. American College of Obstetricians and Gynecologists (ACOG) and the American College of Allergy, Asthmaand Immunology (ACAAI). The use of newer asthma and allergy medications during pregnancy. Ann Allergy Asthma Immunol 2000;84:475-480.
25. National Asthma Education and Prevention Program. NAEPP Expert Panel Report. Managing Asthma During Pregnancy: Recommendations for Pharmacologic Treatment—Update 2004. Bethesda, Md: National Heart, Lung, and Blood Institute; National Institutes of Health. NIH Publication No. 04-5246. March 2004.
26. Källén B, Rydhstroem H, Äberg A. Congenital malformations after the use of inhaled budesonide in early pregnancy. Obstet Gynecol 1999;93:392-395.
27. Ericson A, Källén B. Use of drugs during pregnancy—unique Swedish registration method that can be improved. Information From the Swedish Medical Products Agency 1999;1:8-11.
28. Pauwels RA, Pedersen S, Busse WW, Tan WC, Chen Y-Z, Ohlsson SV, et al. for the START Investigators Group. Early intervention with budesonide in mild persistent asthma. Lancet 2003;361:1071-1076.
- Every patient with persistent asthma, regardless of disease severity, should use a daily controller medication.
- Consider an inhaled corticosteroid (ICS) first when choosing controller medications for long-term treatment of mild, moderate, and severe persistent asthma in adults and children. Leukotriene modifiers, cromolyn, and nedocromil may be considered as alternative, not preferred, controller medications for patients with persistent asthma.
- Long-acting β2-adrenergic agonists should not be used as monotherapy.
- Long-term use of ICSs within labeled doses is safe for children in terms of growth, bone mineral density, and adrenal function; nonetheless, asthma should be monitored and ICS therapy stepped down to the lowest effective dose.
- Low-to medium-dose ICSs are not associated with the development of cataracts or glaucoma in children, but high cumulative lifetime doses may slightly increase the prevalence of cataracts in adults and elderly patients.
- ICSs are recommended for use in pregnant women with asthma; budesonide is the only ICS rated Pregnancy Category B.
Consider an adult with the following characteristics. To which disease severity would you assign this patient’s asthma?
- Forced expiratory volume in 1 second (FEV1) or peak expiratory flow (PEF) ≥80%
- PEF variability 20%–30%
- Daytime symptoms less than once a day
- Nighttime symptoms more than 1 night a week.
This patient is said to have moderate persistent asthma based on nighttime symptoms. An accurate classification of a patient’s asthma is the foundation for selecting an appropriate treatment strategy.
In 2002 the National Asthma Education and Prevention Program (NAEPP) updated select topics1from its 1997 Guidelines for the Diagnosis and Management of Asthma.2 These evidence-based revisions to the stepwise approach to asthma management were made following a systematic review of the literature (see Search function).
A comprehensive search of Medline and EMBASE databases was performed to identify controlled clinical studies relevant to each topic that were published (in English or foreign languages with English abstracts) from 1980 through August 2000. The search included studies published before 1980 if referenced in the post-1980 literature. Studies that did not include control groups were excluded, except for those reporting adverse effects of ICSs. Studies that met the study selection criteria established for each topic were included in a systematic review of the evidence. An expert panel reviewed the evidence, along with additional literature published since August 2000, and reached a consensus on whether the evidence supported 1997 guideline recommendations or indicated a need for revision. Writing committees were then assigned to developed position statements for each topic. The level of evidence for included studies was rated based on the system of Jadad and colleagues,3 where A = randomized controlled trials, rich body of data; B = randomized controlled trials, limited data; C = nonrandomized trials and observational studies; D = panel consensus judgment.
This article reviews the 2002 NAEPP recommendations for the use of controller medications for asthma, including:
- Relative effectiveness of inhaled corticosteroids (ICSs) versus other controller medications
- Safety of long-term ICS use in children
- Potential benefits of early ICS treatment.
We emphasize mild and moderate persistent asthma because the recommended treatments for these levels of severity have been most affected by the recent guideline changes. We also discuss a recent change by the US Food and Drug Administration (FDA) in its pregnancy category rating for an ICS.
2002 Stepwise approach to asthma management
New criteria for classifying asthma severity
The NAEPP classifies asthma severity according to symptoms and lung function in adults and children older than 5 years, and symptoms in children 5 years and younger.1 Persistent asthma is classified as mild, moderate, or severe according to the feature of greatest severity.
Asthma severity should be assigned according to symptoms before treatment.1 Because it is difficult to predict which infants and young children who wheeze with acute viral upper respiratory infection will go on to develop persistent asthma, new criteria have been detailed to help distinguish these children from those with transient wheeze (Table 1).1,4
TABLE 1
Criteria for children with intermittent wheeze
Infants and young children meeting these criteria should receive controller therapy for asthma:
|
AND presence of risk factors for development of persistent asthma:
|
Choosing pharmacologic treatment according to asthma classification
Quick-relief medications, which include the short-acting β2-agonists (SABAs), are taken as needed to promptly reverse acute airflow obstruction and relieve accompanying symptoms.2
Asthma controller medications (ie, ICSs, cromolyn sodium, long-acting β2-adrenergic-agonists [LABAs], leukotriene modifiers, nedocromil, and theophylline) are used daily to achieve and maintain long-term control of persistent asthma. All patients with persistent asthma, regardless of disease severity, should use a daily controller. Criteria for determining asthma severity and updated recommendations for the use of controller treatment in mild and moderate persistent asthma are presented in the Figure.3,5 Levels of evidence justifying NAEPP treatment recommendations are shown in Table 2.
For use in children. Asthma controller medications approved for use in children younger than 5 years include the fluticasone dry-powder inhalers (Flovent, Rotadisk, and Flovent Diskus), which are approved for children as young as 4 years (Flovent Diskus is not yet commercially available), and nebulized budesonide inhalation suspension (Pulmicort Respules), which is approved for children as young as 12 months.
The LABAs formoterol (Foradil) and salmeterol (Serevent Diskus) are approved for children as young as 5 and 4 years, respectively. Cromolyn sodium nebulizer solution is approved for children as young as 2 years, and theophylline is available for use at any age.
Based on safety and extrapolation of efficacy data in older patients, the oral granule formulation of the leukotriene receptor antagonist (LTRA) montelukast (Singulair) is approved for children as young as 1 year, and the chewable tablets are approved for children 2 to 5 years of age. Zafirlukast (Accolate) is approved for use in children 5 years and older.
New recommendations for mild persistent asthma. Recommendations for the treatment of mild and moderate persistent asthma have changed considerably from the 1997 guidelines. ICSs are now the preferred controller medications, based on greater efficacy. The updated guidelines no longer recommend an initial trial of cromolyn or nedocromil for the treatment of mild persistent asthma; these agents, along with the leukotriene modifiers and slow-release theophylline, are now considered alternatives to low-dose ICSs for adults and children older than 5 years with mild persistent disease (Figure).
According to the NAEPP update, daily low-dose ICS treatment also is preferred for the control of mild persistent asthma in preschool children. As in older children, cromolyn and nedocromil are no longer considered appropriate initial treatments for infants and children 5 years and younger. Cromolyn is considered an alternative controller, whereas nedocromil is no longer recommended for use.
New recommendations for moderate persistent asthma. For adults and children older than 5 years with moderate persistent asthma, revision to the guidelines involved recommendation of a low- to medium-dose ICS plus a LABA as the preferred controller treatment (Figure). Comparative low, medium, and high daily doses for ICSs are shown in Table 3 .1
For preschool children, preferred controller treatments for moderate persistent asthma include low-dose ICSs plus a LABA, or increasing ICSs within the medium-dose range (Figure). Recommendations for the use of LABAs as add-on therapy in this age group are based on extrapolation of data from older patients, since therapy with an ICS/LABA combination has not been adequately studied in children younger than 5 years. Four studies included in the NAEPP evaluation showed clear benefit of medium-dose ICSs in this age group, supporting the use of medium-dose ICSs as a preferred option.6-9 LABAs are not recommended for use without an ICS, and the only ICS/LABA combination product currently available has been FDA approved only for patients aged 12 years and older.
TABLE 2
Levels of evidence for NAEPP assessments*
| Medication | NAEPP assessment | SOR* |
|---|---|---|
| ICS | Preferred treatment for children of all ages with persistent asthma | A (A) |
| SABA | ICSs improve asthma control compared with as-needed SABAs | A (A) |
| Cromolyn/nedocromil | For use as alternative, not preferred, treatment of mild persistent asthma in children of all ages (cromolyn) or children >5 years of age (nedocromil) | A (A) |
| LABA | For use with ICSs as the preferred combination treatment for moderate and severe persistent asthma in children >5 years of age | A (A) |
| For use as a preferred option for combination treatment in children 5 years of age | B (B) | |
| Leukotriene modifier | For use as alternative, not preferred, treatment of mild persistent asthma and as ICS adjunct in moderate persistent asthma | B (B) |
| Theophylline | For use as an alternative ICS add-on in moderate or severe persistent asthma if serum concentrations are monitored | D (D) |
| Not considered an alternative controller for young children with mild persistent asthma due to potential adverse effects in infants with frequent febrile illnesses | ||
| *Highest level of evidence available is reported. Strengths of recommendation are based on the method of Jadad et al.3 Strength of evidence based on the Oxford Center for Evidence-Based Medicine5 is in parentheses. SOR, strength of recommendation; NAEPP, National Asthma Education and Prevention Program; ICS, inhaled corticosteroid; SABA, short-acting β2-adrenergic agonist; LABA, long-acting β2-adrenergic agonist. | ||
TABLE 3
Estimated comparative daily doses for inhaled corticosteroids*
| Drug | Low daily dose | Medium daily dose | High daily dose | |||
|---|---|---|---|---|---|---|
| Adult | Child† | Adult | Child† | Adult | Child† | |
| Beclomethasone CFC 42 or 84 μg/puff | 168–504 μg | 84–336 μg | 504–840 μg | 336–672 μg | >840 μg | >672 μg |
| Beclomethasone HFA 40 or 80 μg/puff | 80–240 μg | 80–160 μg | 240–480 μg | 160–320 μg | >480 μg | >320 μg |
| Budesonide DPI 200 μg/inhalation | 200–600 μg | 200–400 μg | 600–1200 μg | 400–800 μg | >1200 μg | >800 μg |
| Budesonide inhalation suspension for nebulization (child dose) | 0.5mg | 1.0 mg | 2.0 mg | |||
| Fluticasone MDI 44, 110, or 220 μg/puff | 88–264 μg | 88–176 μg | 264–660 μg | 176–440 μg | >660 μg | >440 μg |
| Fluticasone DPI 50, 100, or 250 μg/inhalation | 100–300 μg | 100–200 μg | 300–600 μg | 200–400 μg | >600 μg | >400 μg |
| Triamcinolone acetonide 100 μg/puff | 400–1000 μg | 400–800 μg | 1000–2000 μg | 800–1200 μg | >2000 μg | >1200 μg |
| *The most important determinant of appropriate dosing is the clinician’s judgment of the patient’s response to therapy. This updated comparative dose chart is based on review of recently published clinical trials involving more than 5000 patients and published reviews. Some doses may be outside package labeling, especially in the high-dose range. | ||||||
| †Children 12 years of age. | ||||||
| CFC, chlorofluorocarbon; HFA, hydrofluoroalkane; DPI, dry-powder inhaler; MDI, metered-dose inhaler. | ||||||
FIGURE
Updated National Asthma Education and Prevention Program recommendations for long-term controller treatment in mild and moderate persistent asthma
Topics in the management of asthma in children
Recognizing the need for continual appraisal of the benefits and risks of asthma medications in children, the NAEPP Expert Panel considered new studies comparing the effectiveness of ICS monotherapy with that of as-needed SABAs and other controllers used as monotherapy in children with mild or moderate persistent asthma. In addition, the safety of long-term ICS use in children was evaluated based on vertical growth, bone mineral density, ocular toxicity, and adrenal suppression.
Effectiveness of ICSs compared with other asthma medications
Short-acting β2-adrenergic agonists. Eight studies met the eligibility criteria for evaluating the effectiveness of ICSs versus as-needed SABAs.6,10-16 Six studies (4 involving budesonide) in children 5 years and older showed that ICSs improve lung function and symptoms and reduce the need for emergency intervention compared with as-needed SABAs.1 Among all studies included in the NAEPP update, the Childhood Asthma Management Program (CAMP) Research Group Study,9 a placebo-controlled study of inhaled budesonide and nedocromil, contributed the most evidence. Studies with children 5 years and younger are limited to 2 small studies enrolling a total of 69 children.6,15 Consistent with studies of older children, these studies indicate that ICSs improve asthma control compared with as-needed SABAs.1
Cromolyn and nedocromil. Despite well-established safety profiles, cromolyn and nedocromil are no longer recommended as first-line therapy for children, even those with mild disease. New recommendations reflect the greater effectiveness of inhaled budesonide compared with nedocromil demonstrated in the CAMP study,10 and the lack of apparent benefit of cromolyn as maintenance treatment in childhood asthma reported by Tasche and colleagues in a systematic review of the literature.17
In the CAMP study, children 5 to 12 years of age receiving inhaled budesonide showed greater reductions in symptoms and albuterol use, lower rates of hospitalization and urgent care visits, and less need for additional asthma therapy and oral prednisone compared with placebo over 4 to 6 years of treatment.10 The marginal effectiveness of nedocromil demonstrated in the CAMP study mirrored that of cromolyn reported in the review of 24 randomized placebo-controlled studies by Tasche and colleagues.1,17
For children 5 years and younger, the NAEPP Expert Panel took into account 1 randomized placebo-controlled study conducted with children 2 to 5 years of age; it showed improvements in lung function, symptoms, and bronchial hyperre-activity with inhaled budesonide.9 Support for the new NAEPP recommendations preferring ICSs for preschool children is found in a more recent open-label study18 that showed greater symptom improvement and significantly lower rates of asthma exacerbations, urgent care visits, and oral prednisone use with budesonide inhalation suspension, compared with cromolyn sodium nebulizer solution (Intal Nebulizer Solution) in children 2 to 6 years of age with persistent asthma.
Leukotriene modifiers. The LTRAs zafir-lukast and montelukast are approved for use in children. According to the NAEPP Expert Panel, studies have shown only modest improvements in lung function and other asthma control outcomes with LTRA monotherapy in children as young as 6 and 2 years, respectively.1 Because studies comparing ICSs with LTRAs in children are lacking, findings of greater overall efficacy of ICSs in adults with persistent asthma have been extrapolated for use with children; clear superiority of ICSs versus LTRAs in most outcomes has resulted in the recommendation for ICSs as the preferred treatment for mild persistent asthma in children.
Long-acting β2-adrenergic agonists. There is no role for LABAs as monotherapy in asthma. No studies have compared the effectiveness of ICS versus LABA monotherapy in children younger than 5 years, and studies in older children have shown greater effectiveness of inhaled beclomethasone versus salmeterol.14,19 In the study by Verberne and colleagues, salmeterol monotherapy was associated with deterioration in FEV1.19 In a more recent study that included patients as young as 16 years, a switch from ICS to LABA treatment was associated with a significant increase in treatment failures and exacerbations.20
Theophylline. Only 1 study has compared outcomes with low-dose ICSs versus theophylline in adults and children.21 Although limited, the data support greater effectiveness of ICSs based on symptoms, bronchial hyperresponsiveness, and the need for β2-adrenergic agonists and oral corticosteroids.1
Safety of long-term ICS use in children
Systemic corticosteroids have the potential to suppress growth over the long term.2 Short-term growth studies with ICSs show an average reduction in growth velocity of 1 cm per year during the first year of treatment, but the CAMP study showed that initial reductions in growth velocity with inhaled budesonide were not maintained over a 4- to 6-year treatment period.1,10
Although catch-up growth was not observed in the CAMP study, Agertoft and Pedersen reported no effect of long-term treatment with inhaled budesonide (mean 9.2 years) on final adult height.22 Based on these long-term prospective studies of budesonide, showing only a transient reduction in growth velocity and attainment of expected final adult height, and retrospective studies including inhaled beclomethasone, the Expert Panel concluded that the ICS class is safe regarding growth effects.
According to the NAEPP Expert Panel, clinical study data for children monitored for up to 6 years strongly suggest that ICSs are safe when used at recommended doses (strength of recommendation: A).1 The panel could not rule out a potential cumulative effect of ICS use on some conditions, (eg, osteoporosis, cataracts, glaucoma) in adulthood, as sufficient long-term data are not available.
The panel did conclude that low- to medium-dose ICSs (Table 3) appear to have no serious adverse effects on bone mineral density in children.
Likewise, low- to medium-dose ICS use was not associated with the development of cataracts or glaucoma in children, although the potential for high cumulative lifetime doses of ICSs to slightly increase the prevalence of cataracts in adults and elderly patients was noted.
Strong evidence also indicates that ICS effects on adrenal function are usually clinically insignificant at low to medium doses; however, certain individuals may be at higher risk for hypothalam-ic pituitary adrenal axis effects while using conventional ICS doses.1
Although ICSs are safe when used within labeled dosing, it is still preferable to maintain doses at the lowest effective dose. In general, treatment should be reviewed every 1 to 6 months and doses reduced in a stepwise fashion when possible.1 For children showing a favorable response to treatment, a step down in dose should be considered, but not more frequently than every 3 months. If children show no clear response to treatment within 4 to 6 weeks, consider an alternative treatment or diagnosis.1
Safety of long-term ICS use in pregnant women
Uncontrolled asthma during pregnancy is associated with an increased risk of perinatal complications. 23 Since the consequences of not using asthma controllers during pregnancy can be worse than those with using them, daily controller treatment is recommended for all pregnant women with persistent asthma. 23
The American College of Obstetricians and Gynecologists and the American College of Allergy, Asthma and Immunology previously recommended cromolyn as the treatment of choice for pregnant women with mild persistent asthma. ICSs were recommended for patients whose asthma was inadequately controlled with cromolyn. 24 Beclomethasone and budesonide were the ICSs of choice for pregnant women and those who might become pregnant, with a preference for budesonide when high-dose therapy was indicated.24
These recommendations predate the 2002 NAEPP recommendations for ICSs as preferred therapy in mild persistent asthma and the 2004 NAEPP recommendations for ICSs as the first-choice controller therapy for mild persistent asthma during pregnancy. 25 Among ICSs, one (inhaled budesonide) has an FDA Pregnancy Category B rating based on studies showing no risk in pregnant women. 26,27 All other ICSs are rated Pregnancy Category C.
Based on current evidence, it seems reasonable to consider whether budesonide should now be the preferred therapy for mild persistent asthma during pregnancy.
Effects of early treatment on asthma progression
The potential for early ICS intervention to prevent progression of mild or moderate persistent asthma was evaluated solely with data from children enrolled in the CAMP study. 10 The NAEPP Expert Panel concluded that CAMP study data do not support a progressive decline in lung function in children aged 5 to 12 years with mild or moderate persistent asthma, but do suggest that lung function decline is influenced by age of asthma onset.
According to the panel, CAMP data suggest that most deficits in lung function growth due to childhood asthma occur during the first 3 years of life. Preliminary results of the recent START study (Inhaled Steroid Treatment As Regular Therapy in Early Asthma), 28 conducted with 7165 corticosteroidnaïve patients 5 to 66 years of age with recent onset mild persistent asthma, did show a decline in lung function in patients with mild persistent disease.
Although improvements in prebronchodilator and postbronchodilator FEV1 were significant after 3 years of treatment with inhaled budes-onide, differences from placebo in both outcomes were greatest after the first year. When patients with mild persistent disease inhaled budesonide once daily in addition to normal treatment within 2 years of asthma onset,28 they enjoyed considerable protection from severe and life-threatening asthma exacerbations and overall greater asthma control.
- Budesonide • Pulmicort
- Rhinocort Cromolyn • Intal
- Fluticasone • Flovent
- Formoterol • Foradil
- Montelukast • Singulair
- Nedocromil • Tilade
- Salmeterol • Servent
- Triamcinolone acetonide • Azmacort
- Zafirlukast • Accolate
Corresponding author
Gregory J. Redding, MD, Children’s Hospital and Regional Medical Center, 4800 Sand Point Way, NE, Seattle, WA 98105-0371. E-mail: [email protected].
- Every patient with persistent asthma, regardless of disease severity, should use a daily controller medication.
- Consider an inhaled corticosteroid (ICS) first when choosing controller medications for long-term treatment of mild, moderate, and severe persistent asthma in adults and children. Leukotriene modifiers, cromolyn, and nedocromil may be considered as alternative, not preferred, controller medications for patients with persistent asthma.
- Long-acting β2-adrenergic agonists should not be used as monotherapy.
- Long-term use of ICSs within labeled doses is safe for children in terms of growth, bone mineral density, and adrenal function; nonetheless, asthma should be monitored and ICS therapy stepped down to the lowest effective dose.
- Low-to medium-dose ICSs are not associated with the development of cataracts or glaucoma in children, but high cumulative lifetime doses may slightly increase the prevalence of cataracts in adults and elderly patients.
- ICSs are recommended for use in pregnant women with asthma; budesonide is the only ICS rated Pregnancy Category B.
Consider an adult with the following characteristics. To which disease severity would you assign this patient’s asthma?
- Forced expiratory volume in 1 second (FEV1) or peak expiratory flow (PEF) ≥80%
- PEF variability 20%–30%
- Daytime symptoms less than once a day
- Nighttime symptoms more than 1 night a week.
This patient is said to have moderate persistent asthma based on nighttime symptoms. An accurate classification of a patient’s asthma is the foundation for selecting an appropriate treatment strategy.
In 2002 the National Asthma Education and Prevention Program (NAEPP) updated select topics1from its 1997 Guidelines for the Diagnosis and Management of Asthma.2 These evidence-based revisions to the stepwise approach to asthma management were made following a systematic review of the literature (see Search function).
A comprehensive search of Medline and EMBASE databases was performed to identify controlled clinical studies relevant to each topic that were published (in English or foreign languages with English abstracts) from 1980 through August 2000. The search included studies published before 1980 if referenced in the post-1980 literature. Studies that did not include control groups were excluded, except for those reporting adverse effects of ICSs. Studies that met the study selection criteria established for each topic were included in a systematic review of the evidence. An expert panel reviewed the evidence, along with additional literature published since August 2000, and reached a consensus on whether the evidence supported 1997 guideline recommendations or indicated a need for revision. Writing committees were then assigned to developed position statements for each topic. The level of evidence for included studies was rated based on the system of Jadad and colleagues,3 where A = randomized controlled trials, rich body of data; B = randomized controlled trials, limited data; C = nonrandomized trials and observational studies; D = panel consensus judgment.
This article reviews the 2002 NAEPP recommendations for the use of controller medications for asthma, including:
- Relative effectiveness of inhaled corticosteroids (ICSs) versus other controller medications
- Safety of long-term ICS use in children
- Potential benefits of early ICS treatment.
We emphasize mild and moderate persistent asthma because the recommended treatments for these levels of severity have been most affected by the recent guideline changes. We also discuss a recent change by the US Food and Drug Administration (FDA) in its pregnancy category rating for an ICS.
2002 Stepwise approach to asthma management
New criteria for classifying asthma severity
The NAEPP classifies asthma severity according to symptoms and lung function in adults and children older than 5 years, and symptoms in children 5 years and younger.1 Persistent asthma is classified as mild, moderate, or severe according to the feature of greatest severity.
Asthma severity should be assigned according to symptoms before treatment.1 Because it is difficult to predict which infants and young children who wheeze with acute viral upper respiratory infection will go on to develop persistent asthma, new criteria have been detailed to help distinguish these children from those with transient wheeze (Table 1).1,4
TABLE 1
Criteria for children with intermittent wheeze
Infants and young children meeting these criteria should receive controller therapy for asthma:
|
AND presence of risk factors for development of persistent asthma:
|
Choosing pharmacologic treatment according to asthma classification
Quick-relief medications, which include the short-acting β2-agonists (SABAs), are taken as needed to promptly reverse acute airflow obstruction and relieve accompanying symptoms.2
Asthma controller medications (ie, ICSs, cromolyn sodium, long-acting β2-adrenergic-agonists [LABAs], leukotriene modifiers, nedocromil, and theophylline) are used daily to achieve and maintain long-term control of persistent asthma. All patients with persistent asthma, regardless of disease severity, should use a daily controller. Criteria for determining asthma severity and updated recommendations for the use of controller treatment in mild and moderate persistent asthma are presented in the Figure.3,5 Levels of evidence justifying NAEPP treatment recommendations are shown in Table 2.
For use in children. Asthma controller medications approved for use in children younger than 5 years include the fluticasone dry-powder inhalers (Flovent, Rotadisk, and Flovent Diskus), which are approved for children as young as 4 years (Flovent Diskus is not yet commercially available), and nebulized budesonide inhalation suspension (Pulmicort Respules), which is approved for children as young as 12 months.
The LABAs formoterol (Foradil) and salmeterol (Serevent Diskus) are approved for children as young as 5 and 4 years, respectively. Cromolyn sodium nebulizer solution is approved for children as young as 2 years, and theophylline is available for use at any age.
Based on safety and extrapolation of efficacy data in older patients, the oral granule formulation of the leukotriene receptor antagonist (LTRA) montelukast (Singulair) is approved for children as young as 1 year, and the chewable tablets are approved for children 2 to 5 years of age. Zafirlukast (Accolate) is approved for use in children 5 years and older.
New recommendations for mild persistent asthma. Recommendations for the treatment of mild and moderate persistent asthma have changed considerably from the 1997 guidelines. ICSs are now the preferred controller medications, based on greater efficacy. The updated guidelines no longer recommend an initial trial of cromolyn or nedocromil for the treatment of mild persistent asthma; these agents, along with the leukotriene modifiers and slow-release theophylline, are now considered alternatives to low-dose ICSs for adults and children older than 5 years with mild persistent disease (Figure).
According to the NAEPP update, daily low-dose ICS treatment also is preferred for the control of mild persistent asthma in preschool children. As in older children, cromolyn and nedocromil are no longer considered appropriate initial treatments for infants and children 5 years and younger. Cromolyn is considered an alternative controller, whereas nedocromil is no longer recommended for use.
New recommendations for moderate persistent asthma. For adults and children older than 5 years with moderate persistent asthma, revision to the guidelines involved recommendation of a low- to medium-dose ICS plus a LABA as the preferred controller treatment (Figure). Comparative low, medium, and high daily doses for ICSs are shown in Table 3 .1
For preschool children, preferred controller treatments for moderate persistent asthma include low-dose ICSs plus a LABA, or increasing ICSs within the medium-dose range (Figure). Recommendations for the use of LABAs as add-on therapy in this age group are based on extrapolation of data from older patients, since therapy with an ICS/LABA combination has not been adequately studied in children younger than 5 years. Four studies included in the NAEPP evaluation showed clear benefit of medium-dose ICSs in this age group, supporting the use of medium-dose ICSs as a preferred option.6-9 LABAs are not recommended for use without an ICS, and the only ICS/LABA combination product currently available has been FDA approved only for patients aged 12 years and older.
TABLE 2
Levels of evidence for NAEPP assessments*
| Medication | NAEPP assessment | SOR* |
|---|---|---|
| ICS | Preferred treatment for children of all ages with persistent asthma | A (A) |
| SABA | ICSs improve asthma control compared with as-needed SABAs | A (A) |
| Cromolyn/nedocromil | For use as alternative, not preferred, treatment of mild persistent asthma in children of all ages (cromolyn) or children >5 years of age (nedocromil) | A (A) |
| LABA | For use with ICSs as the preferred combination treatment for moderate and severe persistent asthma in children >5 years of age | A (A) |
| For use as a preferred option for combination treatment in children 5 years of age | B (B) | |
| Leukotriene modifier | For use as alternative, not preferred, treatment of mild persistent asthma and as ICS adjunct in moderate persistent asthma | B (B) |
| Theophylline | For use as an alternative ICS add-on in moderate or severe persistent asthma if serum concentrations are monitored | D (D) |
| Not considered an alternative controller for young children with mild persistent asthma due to potential adverse effects in infants with frequent febrile illnesses | ||
| *Highest level of evidence available is reported. Strengths of recommendation are based on the method of Jadad et al.3 Strength of evidence based on the Oxford Center for Evidence-Based Medicine5 is in parentheses. SOR, strength of recommendation; NAEPP, National Asthma Education and Prevention Program; ICS, inhaled corticosteroid; SABA, short-acting β2-adrenergic agonist; LABA, long-acting β2-adrenergic agonist. | ||
TABLE 3
Estimated comparative daily doses for inhaled corticosteroids*
| Drug | Low daily dose | Medium daily dose | High daily dose | |||
|---|---|---|---|---|---|---|
| Adult | Child† | Adult | Child† | Adult | Child† | |
| Beclomethasone CFC 42 or 84 μg/puff | 168–504 μg | 84–336 μg | 504–840 μg | 336–672 μg | >840 μg | >672 μg |
| Beclomethasone HFA 40 or 80 μg/puff | 80–240 μg | 80–160 μg | 240–480 μg | 160–320 μg | >480 μg | >320 μg |
| Budesonide DPI 200 μg/inhalation | 200–600 μg | 200–400 μg | 600–1200 μg | 400–800 μg | >1200 μg | >800 μg |
| Budesonide inhalation suspension for nebulization (child dose) | 0.5mg | 1.0 mg | 2.0 mg | |||
| Fluticasone MDI 44, 110, or 220 μg/puff | 88–264 μg | 88–176 μg | 264–660 μg | 176–440 μg | >660 μg | >440 μg |
| Fluticasone DPI 50, 100, or 250 μg/inhalation | 100–300 μg | 100–200 μg | 300–600 μg | 200–400 μg | >600 μg | >400 μg |
| Triamcinolone acetonide 100 μg/puff | 400–1000 μg | 400–800 μg | 1000–2000 μg | 800–1200 μg | >2000 μg | >1200 μg |
| *The most important determinant of appropriate dosing is the clinician’s judgment of the patient’s response to therapy. This updated comparative dose chart is based on review of recently published clinical trials involving more than 5000 patients and published reviews. Some doses may be outside package labeling, especially in the high-dose range. | ||||||
| †Children 12 years of age. | ||||||
| CFC, chlorofluorocarbon; HFA, hydrofluoroalkane; DPI, dry-powder inhaler; MDI, metered-dose inhaler. | ||||||
FIGURE
Updated National Asthma Education and Prevention Program recommendations for long-term controller treatment in mild and moderate persistent asthma
Topics in the management of asthma in children
Recognizing the need for continual appraisal of the benefits and risks of asthma medications in children, the NAEPP Expert Panel considered new studies comparing the effectiveness of ICS monotherapy with that of as-needed SABAs and other controllers used as monotherapy in children with mild or moderate persistent asthma. In addition, the safety of long-term ICS use in children was evaluated based on vertical growth, bone mineral density, ocular toxicity, and adrenal suppression.
Effectiveness of ICSs compared with other asthma medications
Short-acting β2-adrenergic agonists. Eight studies met the eligibility criteria for evaluating the effectiveness of ICSs versus as-needed SABAs.6,10-16 Six studies (4 involving budesonide) in children 5 years and older showed that ICSs improve lung function and symptoms and reduce the need for emergency intervention compared with as-needed SABAs.1 Among all studies included in the NAEPP update, the Childhood Asthma Management Program (CAMP) Research Group Study,9 a placebo-controlled study of inhaled budesonide and nedocromil, contributed the most evidence. Studies with children 5 years and younger are limited to 2 small studies enrolling a total of 69 children.6,15 Consistent with studies of older children, these studies indicate that ICSs improve asthma control compared with as-needed SABAs.1
Cromolyn and nedocromil. Despite well-established safety profiles, cromolyn and nedocromil are no longer recommended as first-line therapy for children, even those with mild disease. New recommendations reflect the greater effectiveness of inhaled budesonide compared with nedocromil demonstrated in the CAMP study,10 and the lack of apparent benefit of cromolyn as maintenance treatment in childhood asthma reported by Tasche and colleagues in a systematic review of the literature.17
In the CAMP study, children 5 to 12 years of age receiving inhaled budesonide showed greater reductions in symptoms and albuterol use, lower rates of hospitalization and urgent care visits, and less need for additional asthma therapy and oral prednisone compared with placebo over 4 to 6 years of treatment.10 The marginal effectiveness of nedocromil demonstrated in the CAMP study mirrored that of cromolyn reported in the review of 24 randomized placebo-controlled studies by Tasche and colleagues.1,17
For children 5 years and younger, the NAEPP Expert Panel took into account 1 randomized placebo-controlled study conducted with children 2 to 5 years of age; it showed improvements in lung function, symptoms, and bronchial hyperre-activity with inhaled budesonide.9 Support for the new NAEPP recommendations preferring ICSs for preschool children is found in a more recent open-label study18 that showed greater symptom improvement and significantly lower rates of asthma exacerbations, urgent care visits, and oral prednisone use with budesonide inhalation suspension, compared with cromolyn sodium nebulizer solution (Intal Nebulizer Solution) in children 2 to 6 years of age with persistent asthma.
Leukotriene modifiers. The LTRAs zafir-lukast and montelukast are approved for use in children. According to the NAEPP Expert Panel, studies have shown only modest improvements in lung function and other asthma control outcomes with LTRA monotherapy in children as young as 6 and 2 years, respectively.1 Because studies comparing ICSs with LTRAs in children are lacking, findings of greater overall efficacy of ICSs in adults with persistent asthma have been extrapolated for use with children; clear superiority of ICSs versus LTRAs in most outcomes has resulted in the recommendation for ICSs as the preferred treatment for mild persistent asthma in children.
Long-acting β2-adrenergic agonists. There is no role for LABAs as monotherapy in asthma. No studies have compared the effectiveness of ICS versus LABA monotherapy in children younger than 5 years, and studies in older children have shown greater effectiveness of inhaled beclomethasone versus salmeterol.14,19 In the study by Verberne and colleagues, salmeterol monotherapy was associated with deterioration in FEV1.19 In a more recent study that included patients as young as 16 years, a switch from ICS to LABA treatment was associated with a significant increase in treatment failures and exacerbations.20
Theophylline. Only 1 study has compared outcomes with low-dose ICSs versus theophylline in adults and children.21 Although limited, the data support greater effectiveness of ICSs based on symptoms, bronchial hyperresponsiveness, and the need for β2-adrenergic agonists and oral corticosteroids.1
Safety of long-term ICS use in children
Systemic corticosteroids have the potential to suppress growth over the long term.2 Short-term growth studies with ICSs show an average reduction in growth velocity of 1 cm per year during the first year of treatment, but the CAMP study showed that initial reductions in growth velocity with inhaled budesonide were not maintained over a 4- to 6-year treatment period.1,10
Although catch-up growth was not observed in the CAMP study, Agertoft and Pedersen reported no effect of long-term treatment with inhaled budesonide (mean 9.2 years) on final adult height.22 Based on these long-term prospective studies of budesonide, showing only a transient reduction in growth velocity and attainment of expected final adult height, and retrospective studies including inhaled beclomethasone, the Expert Panel concluded that the ICS class is safe regarding growth effects.
According to the NAEPP Expert Panel, clinical study data for children monitored for up to 6 years strongly suggest that ICSs are safe when used at recommended doses (strength of recommendation: A).1 The panel could not rule out a potential cumulative effect of ICS use on some conditions, (eg, osteoporosis, cataracts, glaucoma) in adulthood, as sufficient long-term data are not available.
The panel did conclude that low- to medium-dose ICSs (Table 3) appear to have no serious adverse effects on bone mineral density in children.
Likewise, low- to medium-dose ICS use was not associated with the development of cataracts or glaucoma in children, although the potential for high cumulative lifetime doses of ICSs to slightly increase the prevalence of cataracts in adults and elderly patients was noted.
Strong evidence also indicates that ICS effects on adrenal function are usually clinically insignificant at low to medium doses; however, certain individuals may be at higher risk for hypothalam-ic pituitary adrenal axis effects while using conventional ICS doses.1
Although ICSs are safe when used within labeled dosing, it is still preferable to maintain doses at the lowest effective dose. In general, treatment should be reviewed every 1 to 6 months and doses reduced in a stepwise fashion when possible.1 For children showing a favorable response to treatment, a step down in dose should be considered, but not more frequently than every 3 months. If children show no clear response to treatment within 4 to 6 weeks, consider an alternative treatment or diagnosis.1
Safety of long-term ICS use in pregnant women
Uncontrolled asthma during pregnancy is associated with an increased risk of perinatal complications. 23 Since the consequences of not using asthma controllers during pregnancy can be worse than those with using them, daily controller treatment is recommended for all pregnant women with persistent asthma. 23
The American College of Obstetricians and Gynecologists and the American College of Allergy, Asthma and Immunology previously recommended cromolyn as the treatment of choice for pregnant women with mild persistent asthma. ICSs were recommended for patients whose asthma was inadequately controlled with cromolyn. 24 Beclomethasone and budesonide were the ICSs of choice for pregnant women and those who might become pregnant, with a preference for budesonide when high-dose therapy was indicated.24
These recommendations predate the 2002 NAEPP recommendations for ICSs as preferred therapy in mild persistent asthma and the 2004 NAEPP recommendations for ICSs as the first-choice controller therapy for mild persistent asthma during pregnancy. 25 Among ICSs, one (inhaled budesonide) has an FDA Pregnancy Category B rating based on studies showing no risk in pregnant women. 26,27 All other ICSs are rated Pregnancy Category C.
Based on current evidence, it seems reasonable to consider whether budesonide should now be the preferred therapy for mild persistent asthma during pregnancy.
Effects of early treatment on asthma progression
The potential for early ICS intervention to prevent progression of mild or moderate persistent asthma was evaluated solely with data from children enrolled in the CAMP study. 10 The NAEPP Expert Panel concluded that CAMP study data do not support a progressive decline in lung function in children aged 5 to 12 years with mild or moderate persistent asthma, but do suggest that lung function decline is influenced by age of asthma onset.
According to the panel, CAMP data suggest that most deficits in lung function growth due to childhood asthma occur during the first 3 years of life. Preliminary results of the recent START study (Inhaled Steroid Treatment As Regular Therapy in Early Asthma), 28 conducted with 7165 corticosteroidnaïve patients 5 to 66 years of age with recent onset mild persistent asthma, did show a decline in lung function in patients with mild persistent disease.
Although improvements in prebronchodilator and postbronchodilator FEV1 were significant after 3 years of treatment with inhaled budes-onide, differences from placebo in both outcomes were greatest after the first year. When patients with mild persistent disease inhaled budesonide once daily in addition to normal treatment within 2 years of asthma onset,28 they enjoyed considerable protection from severe and life-threatening asthma exacerbations and overall greater asthma control.
- Budesonide • Pulmicort
- Rhinocort Cromolyn • Intal
- Fluticasone • Flovent
- Formoterol • Foradil
- Montelukast • Singulair
- Nedocromil • Tilade
- Salmeterol • Servent
- Triamcinolone acetonide • Azmacort
- Zafirlukast • Accolate
Corresponding author
Gregory J. Redding, MD, Children’s Hospital and Regional Medical Center, 4800 Sand Point Way, NE, Seattle, WA 98105-0371. E-mail: [email protected].
1. National Asthma Education and Prevention Program. Expert panel report: guidelines for the diagnosis and management of asthma. Update on selected topics–2002. J Allergy Clin Immunol 2002;110(5 suppl):S141-S219.
2. National Asthma Education and Prevention Program Expert Panel Report 2: Guidelines for the Diagnosis and Management of Asthma. Bethesda, Md: National Heart, Lung, and Blood Institute; National Institutes of Health; 1997. Publication 97;4051.-
3. Jadad AR, Moher M, Browman GP, Booker L, Sigouin C, Fuentes M, et al. Systematic reviews and meta-analyses on treatment of asthma: critical evaluation. BMJ 2000;320:537-540.
4. Castro-Rodríguez JA, Holberg CJ, Wright AL, Martinez FD. A clinical index to define risk of asthma in young children with recurrent wheezing. Am J Respir Crit Care Med 2000;162:1403-1406.
5. Oxford Centre for Evidence-based Medicine Levels of Evidence Available atwww.cebm.net/levels_faq.asp . Accessed January 8, 2004.
6. Connett GJ, Warde C, Wooler E, Lenney W. Use of budes-onide in severe asthmatics aged 1–3 years. Arch Dis Child 1993;69:351-355.
7. de Blic J, Delacourt C, Le Bourgeois M, Mahut B, Ostinelli J, Caswell C, et al. Efficacy of nebulized budesonide in treatment of severe infantile asthma: a double-blind study. J Allergy Clin Immunol 1996;98:14-20.
8. Bisgaard H, Gillies J, Groenewald M, Maden C, . for an International Study Group The effect of inhaled fluticas-one propionate in the treatment of young asthmatic children: a dose comparison study. Am J Respir Crit Care Med 1999;160:126-131.
9. Nielsen KG, Bisgaard H. The effect of inhaled budesonide on symptoms, lung function, and cold air and metha-choline responsiveness in 2- to 5-year–old asthmatic children. Am J Respir Crit Care Med 2000;162:1500-1506.
10. Childhood Asthma Management Program Research Group. Long-term effects of budesonide or nedocromil in children with asthma. N Engl J Med 2000;343:1054-1063.
11. Agertoft L, Pedersen S. Effects of long-term treatment with an inhaled corticosteroid on growth and pulmonary function in asthmatic children. Respir Med 1994;88:373-381.
12. Hoekstra MO, Grol MH, Bouman K, Stijnen T, Koëter GH, Kauffman HF, et al. Fluticasone propionate in children with moderate asthma. Am J Respir Crit Care Med 1996;154:1039-1044.
13. Jónasson G, Carlsen K-H, Blomqvist P. Clinical efficacy of low-dose inhaled budesonide once or twice daily in children with mild asthma not previously treated with steroids. Eur Respir J 1998;12:1099-1104.
14. Simons FER and the Canadian Beclomethasone Dipropionate-Salmeterol Xinafoate Study Group. A comparison of beclomethasone, salmeterol, and placebo in children with asthma. N Engl J Med 1997;337:1659-1665.
15. Storr J, Lenney CA, Lenney W. Nebulized beclomethasone dipropionate in preschool asthma. Arch Dis Child 1986;61:270-273.
16. Van Essen-Zandvliet EE, Hughes MD, Waalkens HJ, Duiverman EJ, Pocock SJ, Kerrebijn KF. and the Dutch Chronic Non-Specific Lung Disease Study Group Effects of 22 months of treatment with inhaled corticosteroids and/or beta-2-agonists on lung function, airway responsiveness, and symptoms in children with asthma. Am Rev Respir Dis 1992;146:547-554.
17. Tasche MJA, Uijen JHJM, Bernsen RMD, de Jongste JC, van der Wouden JC. Inhaled disodium cromoglycate (DSCG) as maintenance therapy in children with asthma: a systematic review. Thorax 2000;55:913-920.
18. Leflein JG, Szefler SJ, Murphy KR, Fitzpatrick S, Cruz-Rivera M, Miller CJ, et al. Nebulized budesonide inhalation suspension compared with cromolyn sodium nebulizer solution for asthma in young children: results of a randomized outcomes trial. Pediatrics 2002;109:866-872.
19. Verberne AAPH, Frost C, Duiverman EJ, Grol MH, Kerrebijn KF. and the Dutch Paediatric Asthma Study Group Addition of salmeterol versus doubling the dose of beclomethasone in children with asthma. Am J Respir Crit Care Med 1998;158:213-219.
20. Lazarus SC, Boushey HA, Fahy JV, Chinchilli VM, Lemanske RF Jr, Sorkness CA, , et al. for the Asthma Clinical Research Network of the National Heart Lung and Blood Institute. Long-acting 2-agonist monotherapy vs continued therapy with inhaled corticosteroids in patients with persistent asthma: a randomized controlled trial. JAMA 2001;285:2583-2593.
21. Reed CE, Offord KP, Nelson HS, Li JT, Tinkelman DG. and the American Academy of Allergy, Asthma and Immunology Beclomethasone Dipropionate-Theophylline Study Group. Aerosol beclomethasone dipropionate spray compared with theophylline as primary treatment for chronic mild or moderate persistent asthma. J Allergy Clin Immunol 1998;101:14-23.
22. Agertoft L, Pedersen S. Effect of long-term treatment with inhaled budesonide on adult height in children with asthma. N Engl J Med 2000;343:1064-1069.
23. National Asthma Education Program (NAEP). Report of the Working Group on Asthma and Pregnancy: Management of Asthma during Pregnancy. Bethesda, Md: National Heart, Lung, and Blood Institute; National Institutes of Health, 1993. NIH Publication No. 96-141593.
24. American College of Obstetricians and Gynecologists (ACOG) and the American College of Allergy, Asthmaand Immunology (ACAAI). The use of newer asthma and allergy medications during pregnancy. Ann Allergy Asthma Immunol 2000;84:475-480.
25. National Asthma Education and Prevention Program. NAEPP Expert Panel Report. Managing Asthma During Pregnancy: Recommendations for Pharmacologic Treatment—Update 2004. Bethesda, Md: National Heart, Lung, and Blood Institute; National Institutes of Health. NIH Publication No. 04-5246. March 2004.
26. Källén B, Rydhstroem H, Äberg A. Congenital malformations after the use of inhaled budesonide in early pregnancy. Obstet Gynecol 1999;93:392-395.
27. Ericson A, Källén B. Use of drugs during pregnancy—unique Swedish registration method that can be improved. Information From the Swedish Medical Products Agency 1999;1:8-11.
28. Pauwels RA, Pedersen S, Busse WW, Tan WC, Chen Y-Z, Ohlsson SV, et al. for the START Investigators Group. Early intervention with budesonide in mild persistent asthma. Lancet 2003;361:1071-1076.
1. National Asthma Education and Prevention Program. Expert panel report: guidelines for the diagnosis and management of asthma. Update on selected topics–2002. J Allergy Clin Immunol 2002;110(5 suppl):S141-S219.
2. National Asthma Education and Prevention Program Expert Panel Report 2: Guidelines for the Diagnosis and Management of Asthma. Bethesda, Md: National Heart, Lung, and Blood Institute; National Institutes of Health; 1997. Publication 97;4051.-
3. Jadad AR, Moher M, Browman GP, Booker L, Sigouin C, Fuentes M, et al. Systematic reviews and meta-analyses on treatment of asthma: critical evaluation. BMJ 2000;320:537-540.
4. Castro-Rodríguez JA, Holberg CJ, Wright AL, Martinez FD. A clinical index to define risk of asthma in young children with recurrent wheezing. Am J Respir Crit Care Med 2000;162:1403-1406.
5. Oxford Centre for Evidence-based Medicine Levels of Evidence Available atwww.cebm.net/levels_faq.asp . Accessed January 8, 2004.
6. Connett GJ, Warde C, Wooler E, Lenney W. Use of budes-onide in severe asthmatics aged 1–3 years. Arch Dis Child 1993;69:351-355.
7. de Blic J, Delacourt C, Le Bourgeois M, Mahut B, Ostinelli J, Caswell C, et al. Efficacy of nebulized budesonide in treatment of severe infantile asthma: a double-blind study. J Allergy Clin Immunol 1996;98:14-20.
8. Bisgaard H, Gillies J, Groenewald M, Maden C, . for an International Study Group The effect of inhaled fluticas-one propionate in the treatment of young asthmatic children: a dose comparison study. Am J Respir Crit Care Med 1999;160:126-131.
9. Nielsen KG, Bisgaard H. The effect of inhaled budesonide on symptoms, lung function, and cold air and metha-choline responsiveness in 2- to 5-year–old asthmatic children. Am J Respir Crit Care Med 2000;162:1500-1506.
10. Childhood Asthma Management Program Research Group. Long-term effects of budesonide or nedocromil in children with asthma. N Engl J Med 2000;343:1054-1063.
11. Agertoft L, Pedersen S. Effects of long-term treatment with an inhaled corticosteroid on growth and pulmonary function in asthmatic children. Respir Med 1994;88:373-381.
12. Hoekstra MO, Grol MH, Bouman K, Stijnen T, Koëter GH, Kauffman HF, et al. Fluticasone propionate in children with moderate asthma. Am J Respir Crit Care Med 1996;154:1039-1044.
13. Jónasson G, Carlsen K-H, Blomqvist P. Clinical efficacy of low-dose inhaled budesonide once or twice daily in children with mild asthma not previously treated with steroids. Eur Respir J 1998;12:1099-1104.
14. Simons FER and the Canadian Beclomethasone Dipropionate-Salmeterol Xinafoate Study Group. A comparison of beclomethasone, salmeterol, and placebo in children with asthma. N Engl J Med 1997;337:1659-1665.
15. Storr J, Lenney CA, Lenney W. Nebulized beclomethasone dipropionate in preschool asthma. Arch Dis Child 1986;61:270-273.
16. Van Essen-Zandvliet EE, Hughes MD, Waalkens HJ, Duiverman EJ, Pocock SJ, Kerrebijn KF. and the Dutch Chronic Non-Specific Lung Disease Study Group Effects of 22 months of treatment with inhaled corticosteroids and/or beta-2-agonists on lung function, airway responsiveness, and symptoms in children with asthma. Am Rev Respir Dis 1992;146:547-554.
17. Tasche MJA, Uijen JHJM, Bernsen RMD, de Jongste JC, van der Wouden JC. Inhaled disodium cromoglycate (DSCG) as maintenance therapy in children with asthma: a systematic review. Thorax 2000;55:913-920.
18. Leflein JG, Szefler SJ, Murphy KR, Fitzpatrick S, Cruz-Rivera M, Miller CJ, et al. Nebulized budesonide inhalation suspension compared with cromolyn sodium nebulizer solution for asthma in young children: results of a randomized outcomes trial. Pediatrics 2002;109:866-872.
19. Verberne AAPH, Frost C, Duiverman EJ, Grol MH, Kerrebijn KF. and the Dutch Paediatric Asthma Study Group Addition of salmeterol versus doubling the dose of beclomethasone in children with asthma. Am J Respir Crit Care Med 1998;158:213-219.
20. Lazarus SC, Boushey HA, Fahy JV, Chinchilli VM, Lemanske RF Jr, Sorkness CA, , et al. for the Asthma Clinical Research Network of the National Heart Lung and Blood Institute. Long-acting 2-agonist monotherapy vs continued therapy with inhaled corticosteroids in patients with persistent asthma: a randomized controlled trial. JAMA 2001;285:2583-2593.
21. Reed CE, Offord KP, Nelson HS, Li JT, Tinkelman DG. and the American Academy of Allergy, Asthma and Immunology Beclomethasone Dipropionate-Theophylline Study Group. Aerosol beclomethasone dipropionate spray compared with theophylline as primary treatment for chronic mild or moderate persistent asthma. J Allergy Clin Immunol 1998;101:14-23.
22. Agertoft L, Pedersen S. Effect of long-term treatment with inhaled budesonide on adult height in children with asthma. N Engl J Med 2000;343:1064-1069.
23. National Asthma Education Program (NAEP). Report of the Working Group on Asthma and Pregnancy: Management of Asthma during Pregnancy. Bethesda, Md: National Heart, Lung, and Blood Institute; National Institutes of Health, 1993. NIH Publication No. 96-141593.
24. American College of Obstetricians and Gynecologists (ACOG) and the American College of Allergy, Asthmaand Immunology (ACAAI). The use of newer asthma and allergy medications during pregnancy. Ann Allergy Asthma Immunol 2000;84:475-480.
25. National Asthma Education and Prevention Program. NAEPP Expert Panel Report. Managing Asthma During Pregnancy: Recommendations for Pharmacologic Treatment—Update 2004. Bethesda, Md: National Heart, Lung, and Blood Institute; National Institutes of Health. NIH Publication No. 04-5246. March 2004.
26. Källén B, Rydhstroem H, Äberg A. Congenital malformations after the use of inhaled budesonide in early pregnancy. Obstet Gynecol 1999;93:392-395.
27. Ericson A, Källén B. Use of drugs during pregnancy—unique Swedish registration method that can be improved. Information From the Swedish Medical Products Agency 1999;1:8-11.
28. Pauwels RA, Pedersen S, Busse WW, Tan WC, Chen Y-Z, Ohlsson SV, et al. for the START Investigators Group. Early intervention with budesonide in mild persistent asthma. Lancet 2003;361:1071-1076.
Suicidal patient jumps from building after protesting hospital discharge
Hudson County (NJ) superior court
A 44-year-old man with bipolar disorder and a history of suicidal behavior was hospitalized after telling the treating psychiatrist he had suicidal thoughts. Approximately 3 weeks later, the psychiatrist informed the patient he would be discharged.
Despite his objections, the patient was discharged 4 days later with the psychiatrist’s approval. This occurred even though the patient was found to be suicidal when he was evaluated that day by an in-hospital social services agency.
Two days later, the patient jumped from a 4-story building and sustained permanent partial paralysis. Subsequent treatments included spinal rod insertion, laminectomy, skin grafts, and 3 months’ rehabilitation.
The patient sued the psychiatrist for negligence for both the discharge and for inadequate medication management. The suit claimed that the prescribed mood stabilizer was dosed below therapeutic serum levels and that the psychiatrist switched the patient’s adjunctive antidepressant too close to his discharge date to determine its efficacy.
- The case was settled for $1 million.
Inappropriate drug therapy blamed for inducing fatal heart failure
Kings County (CA) superior court
A patient with severe mental illness died of congestive heart failure (CHF), and his three minor children argued that their father’s medications caused his death.
The patient’s surgeon and family practitioner observed that he had a history of acute psychosis and was taking haloperidol. He was also being treated for schizophrenia and bipolar disorder and was living in a group home. The patient was diagnosed with diabetes in 1998.
In 2000, a group home staff member observed ankle swelling and foam around the patient’s mouth. The consulting psychiatrist reduced the mood stabilizer dosage. The family physician subsequently saw the patient for complaints of cough, wheeze, dizziness, and foam on the mouth. Neither physician acknowledged the foam.
The patient was found dead the next day. Autopsy showed that he died of drug intoxication; toxicology studies showed that serum levels of two psychotropic medications were elevated.
The plaintiffs argued that the psychiatrist’s mismanagement of the psychotropics led to the patient’s death, and that the family practitioner failed to test for CHF. The psychiatrist argued that the drug levels were necessary to control the patient’s mental illness. The family practitioner questioned the autopsy conclusion and stated that relevant diagnostic studies were not ordered because the patient’s presentation was atypical for CHF.
- The jury settled in the defense’s favor.
Unattended patient sustains brain injury in attempted suicide
Unnamed county (MN) district court
A patient was hospitalized in the psychiatric unit with a diagnosis of major depressive disorder, suicidal ideation, and a defined suicide plan. The hospital psychiatrist ordered a suicide watch.
Three days after admission, hospital staff allowed the patient to use the psychiatric unit’s exercise equipment. When left unsupervised in the exercise room, the patient attempted suicide by hanging. Staff discovered and resuscitated the patient, but the hanging attempt resulted in severe anoxic brain injury, which caused permanent and total disability.
- The case was settled out of court for $2.75 million.
Bipolar teen attacks mother with knife; family blames misdiagnosis
Tarrant County (TX) district court
A 14-year-old boy was being treated in early 2000 by a psychiatric group for depression and hyperactivity, for which he was prescribed methylphenidate and paroxetine. Later that year, he became agitated and attacked his mother with a knife. He was arrested and charged with assault with a deadly weapon.
The plaintiff sued the psychiatrists for failure to diagnose his bipolar condition and showed that prescribing paroxetine without a mood stabilizer is contraindicated in bipolar patients. The defendants argued that the patient did not have bipolar disorder when the medications were prescribed.
- The jury found no negligence. Claims by the plaintiff’s mother were dismissed.
Hudson County (NJ) superior court
A 44-year-old man with bipolar disorder and a history of suicidal behavior was hospitalized after telling the treating psychiatrist he had suicidal thoughts. Approximately 3 weeks later, the psychiatrist informed the patient he would be discharged.
Despite his objections, the patient was discharged 4 days later with the psychiatrist’s approval. This occurred even though the patient was found to be suicidal when he was evaluated that day by an in-hospital social services agency.
Two days later, the patient jumped from a 4-story building and sustained permanent partial paralysis. Subsequent treatments included spinal rod insertion, laminectomy, skin grafts, and 3 months’ rehabilitation.
The patient sued the psychiatrist for negligence for both the discharge and for inadequate medication management. The suit claimed that the prescribed mood stabilizer was dosed below therapeutic serum levels and that the psychiatrist switched the patient’s adjunctive antidepressant too close to his discharge date to determine its efficacy.
- The case was settled for $1 million.
Inappropriate drug therapy blamed for inducing fatal heart failure
Kings County (CA) superior court
A patient with severe mental illness died of congestive heart failure (CHF), and his three minor children argued that their father’s medications caused his death.
The patient’s surgeon and family practitioner observed that he had a history of acute psychosis and was taking haloperidol. He was also being treated for schizophrenia and bipolar disorder and was living in a group home. The patient was diagnosed with diabetes in 1998.
In 2000, a group home staff member observed ankle swelling and foam around the patient’s mouth. The consulting psychiatrist reduced the mood stabilizer dosage. The family physician subsequently saw the patient for complaints of cough, wheeze, dizziness, and foam on the mouth. Neither physician acknowledged the foam.
The patient was found dead the next day. Autopsy showed that he died of drug intoxication; toxicology studies showed that serum levels of two psychotropic medications were elevated.
The plaintiffs argued that the psychiatrist’s mismanagement of the psychotropics led to the patient’s death, and that the family practitioner failed to test for CHF. The psychiatrist argued that the drug levels were necessary to control the patient’s mental illness. The family practitioner questioned the autopsy conclusion and stated that relevant diagnostic studies were not ordered because the patient’s presentation was atypical for CHF.
- The jury settled in the defense’s favor.
Unattended patient sustains brain injury in attempted suicide
Unnamed county (MN) district court
A patient was hospitalized in the psychiatric unit with a diagnosis of major depressive disorder, suicidal ideation, and a defined suicide plan. The hospital psychiatrist ordered a suicide watch.
Three days after admission, hospital staff allowed the patient to use the psychiatric unit’s exercise equipment. When left unsupervised in the exercise room, the patient attempted suicide by hanging. Staff discovered and resuscitated the patient, but the hanging attempt resulted in severe anoxic brain injury, which caused permanent and total disability.
- The case was settled out of court for $2.75 million.
Bipolar teen attacks mother with knife; family blames misdiagnosis
Tarrant County (TX) district court
A 14-year-old boy was being treated in early 2000 by a psychiatric group for depression and hyperactivity, for which he was prescribed methylphenidate and paroxetine. Later that year, he became agitated and attacked his mother with a knife. He was arrested and charged with assault with a deadly weapon.
The plaintiff sued the psychiatrists for failure to diagnose his bipolar condition and showed that prescribing paroxetine without a mood stabilizer is contraindicated in bipolar patients. The defendants argued that the patient did not have bipolar disorder when the medications were prescribed.
- The jury found no negligence. Claims by the plaintiff’s mother were dismissed.
Hudson County (NJ) superior court
A 44-year-old man with bipolar disorder and a history of suicidal behavior was hospitalized after telling the treating psychiatrist he had suicidal thoughts. Approximately 3 weeks later, the psychiatrist informed the patient he would be discharged.
Despite his objections, the patient was discharged 4 days later with the psychiatrist’s approval. This occurred even though the patient was found to be suicidal when he was evaluated that day by an in-hospital social services agency.
Two days later, the patient jumped from a 4-story building and sustained permanent partial paralysis. Subsequent treatments included spinal rod insertion, laminectomy, skin grafts, and 3 months’ rehabilitation.
The patient sued the psychiatrist for negligence for both the discharge and for inadequate medication management. The suit claimed that the prescribed mood stabilizer was dosed below therapeutic serum levels and that the psychiatrist switched the patient’s adjunctive antidepressant too close to his discharge date to determine its efficacy.
- The case was settled for $1 million.
Inappropriate drug therapy blamed for inducing fatal heart failure
Kings County (CA) superior court
A patient with severe mental illness died of congestive heart failure (CHF), and his three minor children argued that their father’s medications caused his death.
The patient’s surgeon and family practitioner observed that he had a history of acute psychosis and was taking haloperidol. He was also being treated for schizophrenia and bipolar disorder and was living in a group home. The patient was diagnosed with diabetes in 1998.
In 2000, a group home staff member observed ankle swelling and foam around the patient’s mouth. The consulting psychiatrist reduced the mood stabilizer dosage. The family physician subsequently saw the patient for complaints of cough, wheeze, dizziness, and foam on the mouth. Neither physician acknowledged the foam.
The patient was found dead the next day. Autopsy showed that he died of drug intoxication; toxicology studies showed that serum levels of two psychotropic medications were elevated.
The plaintiffs argued that the psychiatrist’s mismanagement of the psychotropics led to the patient’s death, and that the family practitioner failed to test for CHF. The psychiatrist argued that the drug levels were necessary to control the patient’s mental illness. The family practitioner questioned the autopsy conclusion and stated that relevant diagnostic studies were not ordered because the patient’s presentation was atypical for CHF.
- The jury settled in the defense’s favor.
Unattended patient sustains brain injury in attempted suicide
Unnamed county (MN) district court
A patient was hospitalized in the psychiatric unit with a diagnosis of major depressive disorder, suicidal ideation, and a defined suicide plan. The hospital psychiatrist ordered a suicide watch.
Three days after admission, hospital staff allowed the patient to use the psychiatric unit’s exercise equipment. When left unsupervised in the exercise room, the patient attempted suicide by hanging. Staff discovered and resuscitated the patient, but the hanging attempt resulted in severe anoxic brain injury, which caused permanent and total disability.
- The case was settled out of court for $2.75 million.
Bipolar teen attacks mother with knife; family blames misdiagnosis
Tarrant County (TX) district court
A 14-year-old boy was being treated in early 2000 by a psychiatric group for depression and hyperactivity, for which he was prescribed methylphenidate and paroxetine. Later that year, he became agitated and attacked his mother with a knife. He was arrested and charged with assault with a deadly weapon.
The plaintiff sued the psychiatrists for failure to diagnose his bipolar condition and showed that prescribing paroxetine without a mood stabilizer is contraindicated in bipolar patients. The defendants argued that the patient did not have bipolar disorder when the medications were prescribed.
- The jury found no negligence. Claims by the plaintiff’s mother were dismissed.
Angiotensin-receptor blockers in heart failure: Evidence from the CHARM trial
Nonalcoholic fatty liver disease and the epidemic of obesity
Cancer pain: How to measure the fifth vital sign
Lessons from the PROVE-IT trial. Higher dose of potent statin better for high-risk patients
Is conduct disorder real?
I am rebutting “How to reduce aggression in patients with conduct disorder” (Current Psychiatry, April 2004).
A 15-year-old ended his first two visits with me under police custody and was committed both times. After the first commitment, his grandmother filed a petition alleging unruly/delinquent behavior, and a judge ordered the boy to take his prescribed mood stabilizers. That was necessary because the hospital psychiatrist had determined that the boy was not mentally ill and that his grandmother needed parenting classes. The youth’s original diagnosis—conduct disorder and oppositional-defiant disorder (ODD)—contradicted my diagnosis: bipolar disorder, mixed.
During the second hospitalization, a psychiatrist diagnosed the youth as having attentiondeficit/hyperactivity disorder (ADHD). The doctor prescribed methylphenidate and oxcarbazepine, but the patient’s guardian did not consent to the medications.
Facing a sentence at the county juvenile detention center, the youth started taking olanzapine, 10 mg at bedtime, and lamotrigine, 25 mg bid titrated to 50 mg bid, as I had prescribed. His grandmother says that he no longer exhibits defiant behavior. At his third visit, he shook my hand and said, “Thank you for finding the right medications for me.”
I have seen hundreds of similar cases over 10 years. To paraphrase a colleague, diagnosing somebody with conduct disorder or ODD is like diagnosing a patient with a runny nose after a thorough emergency room examination.
I applaud the American Association of Community Psychiatry’s efforts to urge the American Psychiatric Association (APA) to abolish the conduct disorder diagnosis. I also support the many researchers who are requesting elimination of conduct disorder and ODD. These are not real and specific diagnoses but are alleged syndromes that express several conditions.
Manuel Mota-Castillo MD
Orlando, FL
Dr. Malone responds
It is hard to assess Dr. Mota-Castillo’s case based on the information he provided. Still, one would not refute any psychiatric syndrome by citing a single case.
Most psychiatric disorders are syndromes and affect heterogeneous groups. This is true for disorders that are more prevalent in adults—such as schizophrenia and mania—and for those that present in childhood and adolescence—such as conduct disorder, ODD, and ADHD. Heterogeneity within disorders is no doubt related to underlying individual differences in genetics and environment and contributes to differences in symptom expression and treatment response.
Dr. Mota-Castillo did not present symptoms listed under DSM-IV-TR, so it is unclear how the patient was diagnosed. Diagnoses:
- are one clinician’s impression or the consensus of several clinicians
- are based on one patient encounter or ongoing treatment
- occur with or without input from other sources, such as parents and school
- are made with or without validated structured interviews.
Conduct disorder and ODDare part of DSM diagnostic nomenclature,1 and the APA and American Academy of Child and Adolescent Psychiatry recognize both disorders. Reducing aggression associated with either disorder has long been the most common reason for psychiatric consultation in children.2
Also, Dr. Mota-Castillo prescribed olanzapine and lamotrigine, apparently for simultaneous use. The main point of our case was to discourage polypharmacy—something most experts agree should be avoided3 —by carefully starting one drug before adding a second. When a child receives two drugs at once, we cannot know the effect of either.
In the 15-year-old’s case, as often happens, the prescribed treatment might not have changed the symptoms; some symptoms remit spontaneously.
Nor does drug response clarify diagnosis. For example, both bipolar disorder and aggression in conduct disorder (and in many other conditions) may respond to an antipsychotic.4 Lithium and other treatments for mania have been shown to reduce severe aggression in nonmanic children and adolescents with conduct disorder.5,6
Richard P. Malone, MD
Associate professor
Eastern Pennsylvania Psychiatric Institute
Drexel University College of Medicine
Philadelphia, PA
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (4th ed-rev). Washington, DC: American Psychiatric Association, 2000.
- Kazdin AE. Conduct disorders in childhood and adolescence, vol. 9: developmental clinical psychology and psychiatry series. Newbury Park, CA: Sage Publications, 1987.
- Pappadopulos E, Macintyre JC II, Crismon ML, et al. Treatment recommendations for the use of antipsychotics for aggressive youth (TRAAY): Part II. J Am Acad Child Adolesc Psychiatry 2003;42(2):145–61.
- Malone RP, Delaney MA. Psychopharmacologic interventions in children with aggression: neuroleptics, lithium, and anticonvulsants. In: Coccaro EF (ed). Aggression: assessment and treatment.New York: Marcel Dekker, 2003:331–49.
- Malone RP, Delaney MA, Luebbert JF, et al. A double-blind placebo-controlled study of lithium in hospitalized aggressive children and adolescents with conduct disorder. Arch Gen Psychiatry 2000;57(7):649–54.
- Campbell M, Adams PB, Small AM, et al. Lithium in hospitalized aggressive children with conduct disorder: a double-blind and placebo-controlled study. J Am Acad Child Adolesc Psychiatry 1995;34(4):445–53.
I am rebutting “How to reduce aggression in patients with conduct disorder” (Current Psychiatry, April 2004).
A 15-year-old ended his first two visits with me under police custody and was committed both times. After the first commitment, his grandmother filed a petition alleging unruly/delinquent behavior, and a judge ordered the boy to take his prescribed mood stabilizers. That was necessary because the hospital psychiatrist had determined that the boy was not mentally ill and that his grandmother needed parenting classes. The youth’s original diagnosis—conduct disorder and oppositional-defiant disorder (ODD)—contradicted my diagnosis: bipolar disorder, mixed.
During the second hospitalization, a psychiatrist diagnosed the youth as having attentiondeficit/hyperactivity disorder (ADHD). The doctor prescribed methylphenidate and oxcarbazepine, but the patient’s guardian did not consent to the medications.
Facing a sentence at the county juvenile detention center, the youth started taking olanzapine, 10 mg at bedtime, and lamotrigine, 25 mg bid titrated to 50 mg bid, as I had prescribed. His grandmother says that he no longer exhibits defiant behavior. At his third visit, he shook my hand and said, “Thank you for finding the right medications for me.”
I have seen hundreds of similar cases over 10 years. To paraphrase a colleague, diagnosing somebody with conduct disorder or ODD is like diagnosing a patient with a runny nose after a thorough emergency room examination.
I applaud the American Association of Community Psychiatry’s efforts to urge the American Psychiatric Association (APA) to abolish the conduct disorder diagnosis. I also support the many researchers who are requesting elimination of conduct disorder and ODD. These are not real and specific diagnoses but are alleged syndromes that express several conditions.
Manuel Mota-Castillo MD
Orlando, FL
Dr. Malone responds
It is hard to assess Dr. Mota-Castillo’s case based on the information he provided. Still, one would not refute any psychiatric syndrome by citing a single case.
Most psychiatric disorders are syndromes and affect heterogeneous groups. This is true for disorders that are more prevalent in adults—such as schizophrenia and mania—and for those that present in childhood and adolescence—such as conduct disorder, ODD, and ADHD. Heterogeneity within disorders is no doubt related to underlying individual differences in genetics and environment and contributes to differences in symptom expression and treatment response.
Dr. Mota-Castillo did not present symptoms listed under DSM-IV-TR, so it is unclear how the patient was diagnosed. Diagnoses:
- are one clinician’s impression or the consensus of several clinicians
- are based on one patient encounter or ongoing treatment
- occur with or without input from other sources, such as parents and school
- are made with or without validated structured interviews.
Conduct disorder and ODDare part of DSM diagnostic nomenclature,1 and the APA and American Academy of Child and Adolescent Psychiatry recognize both disorders. Reducing aggression associated with either disorder has long been the most common reason for psychiatric consultation in children.2
Also, Dr. Mota-Castillo prescribed olanzapine and lamotrigine, apparently for simultaneous use. The main point of our case was to discourage polypharmacy—something most experts agree should be avoided3 —by carefully starting one drug before adding a second. When a child receives two drugs at once, we cannot know the effect of either.
In the 15-year-old’s case, as often happens, the prescribed treatment might not have changed the symptoms; some symptoms remit spontaneously.
Nor does drug response clarify diagnosis. For example, both bipolar disorder and aggression in conduct disorder (and in many other conditions) may respond to an antipsychotic.4 Lithium and other treatments for mania have been shown to reduce severe aggression in nonmanic children and adolescents with conduct disorder.5,6
Richard P. Malone, MD
Associate professor
Eastern Pennsylvania Psychiatric Institute
Drexel University College of Medicine
Philadelphia, PA
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (4th ed-rev). Washington, DC: American Psychiatric Association, 2000.
- Kazdin AE. Conduct disorders in childhood and adolescence, vol. 9: developmental clinical psychology and psychiatry series. Newbury Park, CA: Sage Publications, 1987.
- Pappadopulos E, Macintyre JC II, Crismon ML, et al. Treatment recommendations for the use of antipsychotics for aggressive youth (TRAAY): Part II. J Am Acad Child Adolesc Psychiatry 2003;42(2):145–61.
- Malone RP, Delaney MA. Psychopharmacologic interventions in children with aggression: neuroleptics, lithium, and anticonvulsants. In: Coccaro EF (ed). Aggression: assessment and treatment.New York: Marcel Dekker, 2003:331–49.
- Malone RP, Delaney MA, Luebbert JF, et al. A double-blind placebo-controlled study of lithium in hospitalized aggressive children and adolescents with conduct disorder. Arch Gen Psychiatry 2000;57(7):649–54.
- Campbell M, Adams PB, Small AM, et al. Lithium in hospitalized aggressive children with conduct disorder: a double-blind and placebo-controlled study. J Am Acad Child Adolesc Psychiatry 1995;34(4):445–53.
I am rebutting “How to reduce aggression in patients with conduct disorder” (Current Psychiatry, April 2004).
A 15-year-old ended his first two visits with me under police custody and was committed both times. After the first commitment, his grandmother filed a petition alleging unruly/delinquent behavior, and a judge ordered the boy to take his prescribed mood stabilizers. That was necessary because the hospital psychiatrist had determined that the boy was not mentally ill and that his grandmother needed parenting classes. The youth’s original diagnosis—conduct disorder and oppositional-defiant disorder (ODD)—contradicted my diagnosis: bipolar disorder, mixed.
During the second hospitalization, a psychiatrist diagnosed the youth as having attentiondeficit/hyperactivity disorder (ADHD). The doctor prescribed methylphenidate and oxcarbazepine, but the patient’s guardian did not consent to the medications.
Facing a sentence at the county juvenile detention center, the youth started taking olanzapine, 10 mg at bedtime, and lamotrigine, 25 mg bid titrated to 50 mg bid, as I had prescribed. His grandmother says that he no longer exhibits defiant behavior. At his third visit, he shook my hand and said, “Thank you for finding the right medications for me.”
I have seen hundreds of similar cases over 10 years. To paraphrase a colleague, diagnosing somebody with conduct disorder or ODD is like diagnosing a patient with a runny nose after a thorough emergency room examination.
I applaud the American Association of Community Psychiatry’s efforts to urge the American Psychiatric Association (APA) to abolish the conduct disorder diagnosis. I also support the many researchers who are requesting elimination of conduct disorder and ODD. These are not real and specific diagnoses but are alleged syndromes that express several conditions.
Manuel Mota-Castillo MD
Orlando, FL
Dr. Malone responds
It is hard to assess Dr. Mota-Castillo’s case based on the information he provided. Still, one would not refute any psychiatric syndrome by citing a single case.
Most psychiatric disorders are syndromes and affect heterogeneous groups. This is true for disorders that are more prevalent in adults—such as schizophrenia and mania—and for those that present in childhood and adolescence—such as conduct disorder, ODD, and ADHD. Heterogeneity within disorders is no doubt related to underlying individual differences in genetics and environment and contributes to differences in symptom expression and treatment response.
Dr. Mota-Castillo did not present symptoms listed under DSM-IV-TR, so it is unclear how the patient was diagnosed. Diagnoses:
- are one clinician’s impression or the consensus of several clinicians
- are based on one patient encounter or ongoing treatment
- occur with or without input from other sources, such as parents and school
- are made with or without validated structured interviews.
Conduct disorder and ODDare part of DSM diagnostic nomenclature,1 and the APA and American Academy of Child and Adolescent Psychiatry recognize both disorders. Reducing aggression associated with either disorder has long been the most common reason for psychiatric consultation in children.2
Also, Dr. Mota-Castillo prescribed olanzapine and lamotrigine, apparently for simultaneous use. The main point of our case was to discourage polypharmacy—something most experts agree should be avoided3 —by carefully starting one drug before adding a second. When a child receives two drugs at once, we cannot know the effect of either.
In the 15-year-old’s case, as often happens, the prescribed treatment might not have changed the symptoms; some symptoms remit spontaneously.
Nor does drug response clarify diagnosis. For example, both bipolar disorder and aggression in conduct disorder (and in many other conditions) may respond to an antipsychotic.4 Lithium and other treatments for mania have been shown to reduce severe aggression in nonmanic children and adolescents with conduct disorder.5,6
Richard P. Malone, MD
Associate professor
Eastern Pennsylvania Psychiatric Institute
Drexel University College of Medicine
Philadelphia, PA
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (4th ed-rev). Washington, DC: American Psychiatric Association, 2000.
- Kazdin AE. Conduct disorders in childhood and adolescence, vol. 9: developmental clinical psychology and psychiatry series. Newbury Park, CA: Sage Publications, 1987.
- Pappadopulos E, Macintyre JC II, Crismon ML, et al. Treatment recommendations for the use of antipsychotics for aggressive youth (TRAAY): Part II. J Am Acad Child Adolesc Psychiatry 2003;42(2):145–61.
- Malone RP, Delaney MA. Psychopharmacologic interventions in children with aggression: neuroleptics, lithium, and anticonvulsants. In: Coccaro EF (ed). Aggression: assessment and treatment.New York: Marcel Dekker, 2003:331–49.
- Malone RP, Delaney MA, Luebbert JF, et al. A double-blind placebo-controlled study of lithium in hospitalized aggressive children and adolescents with conduct disorder. Arch Gen Psychiatry 2000;57(7):649–54.
- Campbell M, Adams PB, Small AM, et al. Lithium in hospitalized aggressive children with conduct disorder: a double-blind and placebo-controlled study. J Am Acad Child Adolesc Psychiatry 1995;34(4):445–53.