User login
Editorial
In 10 short years, the explosive growth in the number of hospitalists has made hospital medicine programs the cornerstone of many innovations that support the institutions they serve: expanded inpatient care, developing consultative and comanagement services, hospital capacity management, improved patient quality and safety practices, and more. Hospitalist teams have demonstrated a genuine commitment to improving the hospital system, with literature supporting that hospitalists can positively affect cost, length of stay, quality of care, and, at academic institutions, education.1 To the casual observer, these hospitalist groups and the solutions they bring may seem fairly uniform; however, to the discerning eye, nothing could be farther from the truth. Hospital medicine programs, and their innovations, are as varied as the hospitals they serve.
Although the challenges encountered in hospital systems have clear, institution‐specific elements, common themes are often encountered by clinicians that parallel those seen at other facilities. Unfortunately, widely disseminated articles from peer‐reviewed journals on hospital‐based innovations have not been available for other hospitalists to use and glean ideas from for use at their home institutionsuntil now. The Journal of Hospital Medicine is pleased to announce the creation of that opportunity.
This year, the Journal of Hospital Medicine will publish articles on and later a supplement dedicated to innovations in hospital medicine. We invite authors to submit manuscripts related to any successful innovation they initiated in their hospital. We will consider any original work that pertains to hospital medicine, including but not limited to clinical innovations, educational programs, quality and safety initiatives, and administrative or academic issues. When available and appropriate, we encourage outcomes to be reported.
To be able to publish articles on a significant number of innovations, we request manuscripts be a maximum of 1500 words with no more than 2 tables or figures and fewer than 15 references. The deadline for submissions is August 1, 2007. All submitted manuscripts will undergo both editorial review by JHM staff and peer review. Authors should consult JHM's instructions for authors2 for guidelines on manuscript submission and preparation.
- How hospitalists add value: a special supplement to the Hospitalist.The Hospitalist.2005;9 (suppl 1).
- Journal of Hospital Medicine information for authors available at: www3.interscience.wiley.com/cgi‐bin/jabout/111081937/ForAuthors.html.
In 10 short years, the explosive growth in the number of hospitalists has made hospital medicine programs the cornerstone of many innovations that support the institutions they serve: expanded inpatient care, developing consultative and comanagement services, hospital capacity management, improved patient quality and safety practices, and more. Hospitalist teams have demonstrated a genuine commitment to improving the hospital system, with literature supporting that hospitalists can positively affect cost, length of stay, quality of care, and, at academic institutions, education.1 To the casual observer, these hospitalist groups and the solutions they bring may seem fairly uniform; however, to the discerning eye, nothing could be farther from the truth. Hospital medicine programs, and their innovations, are as varied as the hospitals they serve.
Although the challenges encountered in hospital systems have clear, institution‐specific elements, common themes are often encountered by clinicians that parallel those seen at other facilities. Unfortunately, widely disseminated articles from peer‐reviewed journals on hospital‐based innovations have not been available for other hospitalists to use and glean ideas from for use at their home institutionsuntil now. The Journal of Hospital Medicine is pleased to announce the creation of that opportunity.
This year, the Journal of Hospital Medicine will publish articles on and later a supplement dedicated to innovations in hospital medicine. We invite authors to submit manuscripts related to any successful innovation they initiated in their hospital. We will consider any original work that pertains to hospital medicine, including but not limited to clinical innovations, educational programs, quality and safety initiatives, and administrative or academic issues. When available and appropriate, we encourage outcomes to be reported.
To be able to publish articles on a significant number of innovations, we request manuscripts be a maximum of 1500 words with no more than 2 tables or figures and fewer than 15 references. The deadline for submissions is August 1, 2007. All submitted manuscripts will undergo both editorial review by JHM staff and peer review. Authors should consult JHM's instructions for authors2 for guidelines on manuscript submission and preparation.
In 10 short years, the explosive growth in the number of hospitalists has made hospital medicine programs the cornerstone of many innovations that support the institutions they serve: expanded inpatient care, developing consultative and comanagement services, hospital capacity management, improved patient quality and safety practices, and more. Hospitalist teams have demonstrated a genuine commitment to improving the hospital system, with literature supporting that hospitalists can positively affect cost, length of stay, quality of care, and, at academic institutions, education.1 To the casual observer, these hospitalist groups and the solutions they bring may seem fairly uniform; however, to the discerning eye, nothing could be farther from the truth. Hospital medicine programs, and their innovations, are as varied as the hospitals they serve.
Although the challenges encountered in hospital systems have clear, institution‐specific elements, common themes are often encountered by clinicians that parallel those seen at other facilities. Unfortunately, widely disseminated articles from peer‐reviewed journals on hospital‐based innovations have not been available for other hospitalists to use and glean ideas from for use at their home institutionsuntil now. The Journal of Hospital Medicine is pleased to announce the creation of that opportunity.
This year, the Journal of Hospital Medicine will publish articles on and later a supplement dedicated to innovations in hospital medicine. We invite authors to submit manuscripts related to any successful innovation they initiated in their hospital. We will consider any original work that pertains to hospital medicine, including but not limited to clinical innovations, educational programs, quality and safety initiatives, and administrative or academic issues. When available and appropriate, we encourage outcomes to be reported.
To be able to publish articles on a significant number of innovations, we request manuscripts be a maximum of 1500 words with no more than 2 tables or figures and fewer than 15 references. The deadline for submissions is August 1, 2007. All submitted manuscripts will undergo both editorial review by JHM staff and peer review. Authors should consult JHM's instructions for authors2 for guidelines on manuscript submission and preparation.
- How hospitalists add value: a special supplement to the Hospitalist.The Hospitalist.2005;9 (suppl 1).
- Journal of Hospital Medicine information for authors available at: www3.interscience.wiley.com/cgi‐bin/jabout/111081937/ForAuthors.html.
- How hospitalists add value: a special supplement to the Hospitalist.The Hospitalist.2005;9 (suppl 1).
- Journal of Hospital Medicine information for authors available at: www3.interscience.wiley.com/cgi‐bin/jabout/111081937/ForAuthors.html.
Expanding Hospitalist Roles to Public Health
The field of hospital medicine came into being in response to numerous factors involving physicians, patients, and hospitals themselves1 Now, years later, hospital medicine is a specialty that is growing, both in size and sophistication such that the role of the hospitalist is constantly evolving.2 A compelling function that has not yet been clearly articulated is the opportunity for hospitalists to serve as public health practitioners in their unique clinical environment. There is precedence for the power of collaboration between medicine and public health as has been seen with emergency medicine's willingness to embrace opportunities to advance public health.35
In public health, the programs, services, and institutions involved emphasize the prevention of disease and the health needs of the population as a whole. Public health activities vary with changing technology and social values, but the goals remain the same: to reduce the amount of disease, premature death, and disease‐associated discomfort and disability in the population.6 The authors of a leading textbook of public health, Scutchfield and Keck, contend that the most important skill for public health practice is the capacity to visualize the potential for health that exists in a community.6
Hospitalists care for a distinct subset of the general populationinpatients, only a small percentage of society in a given year. Yet over time hospitalists affect a substantial subset of the larger population that uses considerable health care resources.79 Furthermore, hospitalization can be a sentinel event with public health implications (eg, newly diagnosed HIV infection or acute myocardial infarction in a patient with an extended family of cigarette smokers). This presents an opportunity to educate and counsel both the patient and the patient's social network. One model of public health practice by hospitalists is to influence the patient, his or her family, and the community by touching and inspiring the hospitalized patient.
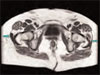
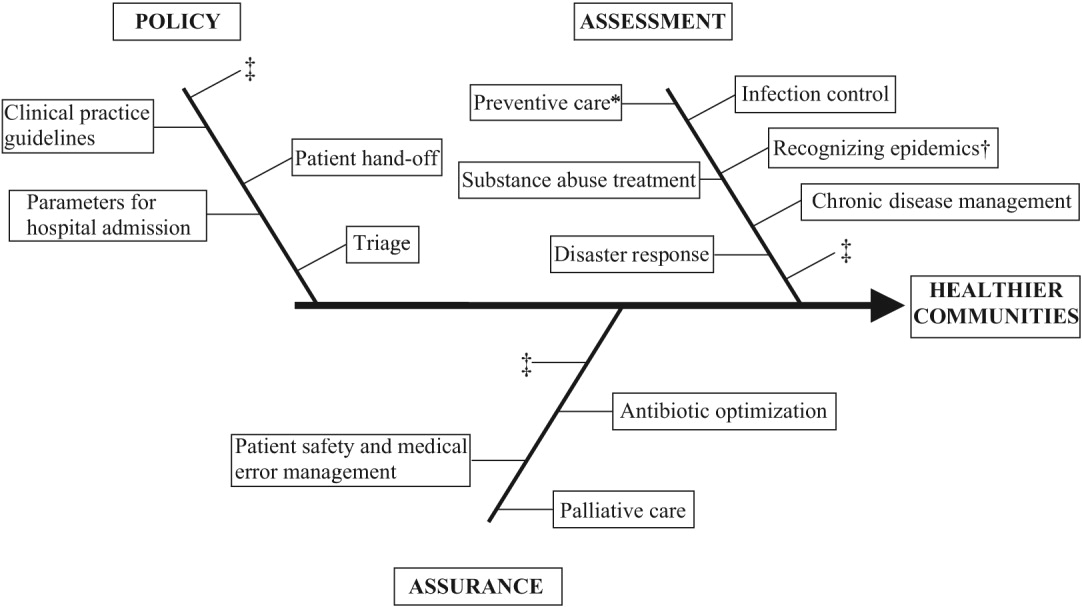
Hospitalists are already involved in many of the core functions of public health (assessment, assurance, and policy development; Fig. 1).10 Achieving ongoing success in this arena means developing hospitalists who are consciously in tune with their roles as public health practitioners.
In this article we define the specific public health contributions that hospitalists have made and describe the possibilities for further innovative advances. To this end, we outline specific public health roles under the broad categories of assessment, assurance, and policy. We point to advances in public health accomplished by hospitalists as well as those being performed by nonhospitalists in the hospital setting. We conclude by describing some of the barriers to and implications of hospitalists taking on public health roles.
ASSESSMENT
Assessment is the systematic collection, analysis, and dissemination of health status information.10 These activities include disease surveillance and investigation of acute outbreaks or changes in the epidemiology of chronic diseases. Assessment also involves understanding the health of a population and the key determinants of a population's health from a variety of perspectives: physical, biological, behavioral, social, cultural, and spiritual.6 Human health has been defined as a state characterized by anatomic integrity; ability to perform personally valued family work and community roles; ability to deal with physical, biologic, and social stress; a feeling of well‐being; and freedom from the risk of disease and untimely death.6 Hospitalists interact with individuals at times of stress and acute illness and thus have a unique opportunity to assess the strength, viability, and resources available to individuals. Key roles that may fall within the auspices of assessment in hospital medicine are infection control, epidemic recognition, disaster response, preventive care, substance abuse treatment, and chronic disease management.
Infection Control
Physicians caring for inpatients have a crucial stake in controlling hospital infection as exemplified by the work of Flanders et al. on preventing nosocomial infections, especially nosocomial pneumonia.11 They describe specific strategies to prevent iatrogenic spread such as washing hands before and after patient contact, establishing guidelines against the use of artificial fingernails, using indwelling devices such as catheters only when absolutely necessary, and using sterile barriers.11 Hospitalists such as Sanjay Saint have led the way in studying methods to reduce bladder catheterization, which has been associated with urinary tract infections12; others have collaborated on work to prevent infections in nursing homes.13 Given the importance of this field, there is room for further hospitalist involvement. Novel methods for infection control in hospitals have been studied by nonhospitalists such as Wisnivesky, who prospectively validated a clinical decision rule to predict the need for respiratory isolation of inpatients with suspected tuberculosis (TB). This prediction rule, which is based on clinical and chest radiographic findings, was able to accurately identify patients at low risk for TB from among inpatients with suspected active pulmonary TB isolated on admission to the hospital.14 Retrospective application of the prediction rule showed respiratory precautions were inappropriately implemented for a third of patients.14 These studies are examples of empiric public health research performed in the inpatient setting. In the infection control domain, candidate issues for further study could include interventions aimed at reducing rates of Clostridium difficile, developing programs for standardized surveillance of hospital infection, validating electronic markers for nosocomial infection, and taking innovative approaches to improving hand‐washing practices in the hospital.15, 16
Recognizing Epidemics
An excellent example of the importance of hospitalists embracing public health and remembering their patients are part of a community was the severe acute respiratory syndrome (SARS) outbreak in Toronto, Ontario, Canada. The outbreak is thought to have begun with a single traveler. With the transfer of patients and the movement of visitors and health care workers among facilities, SARS quickly spread through Toronto, making it the largest SARS‐affected area outside Asia.17 Approximately a month after the outbreak was recognized in Toronto, it was thought to be over, and the World Health Organization (WHO) removed Toronto from its SARS‐affected list.17 Unfortunately, patients with unrecognized SARS remained in health care institutions, including a patient transferred to a rehabilitation center. Infection quickly spread again, resulting in a second phase of the outbreak.17
The SARS outbreak served as a reminder that a global public health system is essential and taught many lessons17 germane to pandemics that recur annually (eg, influenza viruses) as well those that episodically threaten the health of the population (eg, avian flu). Proposed actions to prevent a repeat of the scenario that occurred with SARS in Toronto include assessing the current facilities (eg, isolation rooms and respiratory masks) at each institution, identifying health care workers willing to serve as an outbreak team, and the hiring staff to train hospital personnel in personal protective equipment (PPE) and infection control policies.18 The Centers for Disease Control and Prevention (CDC) contends that planning for the possibility of a virulent pandemic at the local, national, and global levels is critical to limiting the mortality and morbidity should such occur.19, 20 In a previous article, Pile and Gordon declared hospitalists are key players in institutional efforts to prepare for a viral pandemic such as influenza and should be aware of lessons that may be applied from responses to pandemics such as SARS.19 Well placed to recognize clinical trends that may herald epidemics, hospitalists can fulfill some of the necessary public health responsibilities delineated above.
Disaster Response
Natural disasters and terrorism are in the forefront of the popular press and are also high priorities in health care and public health.21 Terrorism and natural disasters cause significant injury, illness, and death.22 Hospital‐based health care providers fulfill a variety of roles when terrorist acts and disasters occur, including reporting, diagnosing, and managing illness, providing preventive measures (eg, vaccines and preparedness kits), preventing the secondary spread of disease, assisting in the investigation of the causes of disease outbreaks, participating in preparedness planning, and evaluating preparedness policies and programs.22 The experience gained in the aftermaths of Hurricanes Katrina and Rita with their unprecedented death, injury, destruction, and displacement should help to guide future response and recovery activities.23 Hospitalists were at the forefront of delivering care, living in their hospitals for days after Hurricane Katrina. Without question, hospitalists will be called on again to serve those affected by disasters.
Preventive Care
For many patients admitted to the hospital, meeting a hospitalist is their first encounter with a physician in years.24, 25 In these instances, hospitalists must ensure that patients' immunizations are up‐to‐date and arrange appropriate follow‐up care with primary care providers. Greenwald described an important role that hospitalists could play in HIV prevention by promoting HIV testing in the hospital.26 The CDC recently confirmed the wisdom of this approach and estimates that the 250,000 to 1.2 million people in the United States with HIV infection who do not know their serostatus play a significant role in HIV transmission.26, 27 In an effort to promote testing, the CDC has initiated a program aimed at incorporating HIV testing into routine medical care, as recommended by others.28 More than a quarter of patients with HIV in the United States are diagnosed in the hospital, and for many other patients, hospitalization is their only real opportunity to be tested.26, 29 Similarly, when hospitalists find elevated cholesterol or triglycerides in routine evaluations of patients who present with chest pain, they have to decide whether to initiate lipid‐lowering medications.30 The hospitalist is sometimes the only physician that patients repeatedly admitted, may see over prolonged periods. It follows that if hospitalists are remiss in delivering preventive care to such patients, they lose the opportunity to positively affect their long‐term health. In practice, hospitalists perform myriad preventive‐care functions, although there is scant literature supporting this role. Hospitalists have an opportunity to collaborate in research projects of hospital‐initiated preventive care that measure outcomes at the community level.
Substance Abuse
In the Unites States, 25%‐40% of hospital admissions are related to substance abuse and its sequelae.31 These patients frequently are admitted to general medicine services for detoxification or treatment of substance‐abuse‐related morbidity, although some American hospitals have specialized treatment and detoxification centers. There is a pressing need for more models of comprehensive care that address the complex issues of addiction, including the biological, social, cultural, spiritual, and developmental needs of patients.32
Hospitalists routinely counsel their patients with substance abuse problems and often consult a chemical dependency counselor, who provides patients with additional information about outpatient or inpatient facilities that may help them after their hospitalization. Unfortunately, because of the natural history of substance abuse, many of these patients are rehospitalized with the same problems even after going through rehabilitation. The adoption of a public health philosophy and approach by hospitalists may assist patients who have addictions through innovative multidisciplinary interventions while these patient are being detoxified. Traditionally, these responsibilities have fallen to primary care providers and psychologists in substance abuse medicine; but, as mentioned previously, many such patients are rehospitalized before they make it to their follow‐up appointments.
In a study examining smoking cessation practices among Norwegian hospital physicians, 98% of the doctors stated they ask their patients about their smoking habits, but fewer than 7% of these physicians regularly offer smoking‐cessation counseling, hand out materials, or give patients other advice about smoking cessation.33 That study illustrates that hospital doctors often ask about problems but can certainly improve in terms of intervention and follow‐up. Other works by nonhospitalist physicians have examined the real potential of inpatient interventions for smoking cessation. Most of this work involves a multidisciplinary approach that relies heavily on nurses. For example, Davies et al. evaluated the effectiveness of a hospital‐based intervention for smoking cessation among low‐income smokers using public health methodologies. The intervention was effective and promising as a way to affect smokers in underserved communities.34
Chronic Disease Management
Public health roles involving chronic disease management include surveillance, intervention design, and implementation of control programs.6 Given their access to data on hospitalized patients, hospitalists can carry out surveillance and empirical population‐based research about hospitalized patients with chronic illnesses. Thoughtfully designed protocols can measure the success of interventions initiated in patients while hospitalized, with further data collection and follow‐up after patients have returned to the community.35 Such endeavors can improve the likelihood that patients with chronic conditions are effectively referred to programs that will maintain their health and functional status.36 If hospitalists consider themselves public health providers, encounters with these hospitalized patients will go beyond noting that their chronic conditions are stable and instead will lay the groundwork to prospectively control these conditions. This approach would have the potential to reduce the number of future hospitalizations and lead to healthier communities.37 To truly carry this out effectively, coordinated collaboration between primary care providers and hospitalists will be necessary.
ASSURANCE
Assurance is the provision of access to necessary health services. It entails efforts to solve problems that threaten the health of populations and empowers individuals to maintain their own health. This is accomplished by either encouraging action, delegating to other entities (private or public sector), mandating specific requirements through regulation, or providing services directly.10 Hospitalist teams aim to ensure that the high‐quality services needed to protect the health of their community (hospitalized patients) are available and that this population receives proper consideration in the allocation of resources. The few studies to date that have directly examined the quality of care that hospitalists provide38 have done so using evidence‐based measures believed to correlate with improved health care outcomes.38 The ambiguities in assessing quality may in part limit such studies.39 Specific hospitalist roles that fall under the assurance umbrella include antibiotic optimization, palliative care, patient safety, and medical error management.
Antibiotic Optimization
Inappropriate use of antimicrobial treatment for infectious diseases has cost and public health implications.40 These inappropriate uses include giving antibiotics when not indicated, overusing broad‐spectrum antibiotics, making mismatches between microbes and medicines when cultures and information on test sensitivity are available, and using intravenous formulations when oral therapy would suffice.41 The public health impact goes way beyond increasing selective pressure for antimicrobial resistance to include safety, adverse events, and increased costs to both patient and hospitals.40 At our institution, the hospital medicine service and infectious disease division have jointly developed and implemented an intervention to reduce inappropriate antibiotic use. At other institutions, hospitalist teams have developed protocols for treating infectious diseases commonly encountered in the hospitalized patient.42 The recommendations of both Amin and Reddy for management of community‐ and hospital‐acquired pneumonia acknowledged that through establishment of clinical care pathways, variation in prescribing patterns among hospitalists can be decreased while optimizing outcomes.42 The work of Williams and colleagues is another example of advances by hospitalists. They reviewed the literature to determine that the use of combination antibiotics as empiric therapy for community‐acquired pneumonia is superior to the use of a single effective antibiotic in treating bacteremic patients with pneumococcal community‐acquired pneumonia.43
Palliative Care
Mortality is a vital outcome measure of public health research and interventions. Not surprisingly, many people are hospitalized in the final months of their life and often die in a hospital. Pantilat showed that hospitalists can respond to these circumstances and have the opportunity to improve care of the dying.4446 Muir et al. evaluated the convergence of the fields of palliative care medicine and hospital medicine and reviewed the opportunities for mutual education and improved patient care.47 They described how the confluence of the changing nature and site of death in the United States coupled with the reorganization of hospital care provides a strategic opportunity to improve end‐of‐life care.47 Hospitalists can ensure that care of the dying is delivered with skill, compassion, and expertise. And so it is imperative they be trained to accomplish this objective.47, 49
Fortunately, hospitalists already appear to enhance patientphysician communication. Auerbach looked at communication, care patterns, and outcomes of dying patients, comparing patients being cared for by hospitalists with those being care for by community‐based physicians. Hospitalists had discussions with patients or their families about care more often than did nonhospitalist physicians (91% versus 73%, respectively, P = .006).49 Because the delivery of high‐quality palliative care is time consuming and complex, alternative models for billing or the use of physician extenders or consultants may be necessary at some institutions.
Patient Safety and Medical Error Management
Hospitalists have been in the forefront of promoting a culture of patient safety.50 Their continuous presence in the hospital and their interactions with members of health care teams from multiple disciplines who share this goal make them important facilitators. Hospitalists have increasing involvement in systems‐based efforts aimed at reducing medical errors.50 Hospitalists are being asked to lead committees that adopt multidisciplinary approaches to reduce adverse events, morbidity, and mortality.50 These committees often have representation from pharmacy, nursing, and other key hospital stakeholders including from the administration.51 Quality assurance activities assess locally collected data and compare results with local and national benchmarks. There are several published examples of hospitalists engaged in patient safety and medical error management. For example, Shojania et al compiled evidence based safety practices in an effort to promote patient safety.52, 53 Schnipper studied the role of pharmacist counseling in preventing adverse drug events (ADEs) after hospitalization and found that pharmacist medication review, patient counseling, and telephone follow‐up were associated with a lower rate of preventable ADEs 30 days after hospital discharge.54 Moreover, Syed paired hospitalists and pharmacists to collaboratively prescribe medications appropriately. In one study there were fewer medication errors and adverse drug reactions in patients treated by a team led by hospitalists than in those treated by the control group, made up of nonhospitalist attendings.55
POLICY
Policy development defines health control goals and objectives and develops implementation plans for those goals.10 By necessity, it operates at the intersection of legislative, political, and regulatory processes.10 At many institutions, hospitalists have been involved in the development of policies ensuring that the core functions of assessment and assurance are addressed and maintained. In fact, hospitalists report that development of quality assurance and practice guidelines accounts for most of their nonclinical time.56 This role of hospitalists is supported by anecdotal reports rather than published empiric evidence.57 For example, at Johns Hopkins Bayview Medical Center, hospitalist‐led teams have developed triage and patient handoff policies designed to improve patient safety. Parameters for admission to the general medicine ward have been elaborated and are periodically refined by the hospitalist team.
Another area that falls within the genre of policy is development of clinical practice guidelines. Guidelines for the treatment of pneumonia, congestive heart failure, deep‐vein thrombosis prophylaxis, alcohol and drug withdrawal, pain management, delirium, and chronic obstructive pulmonary disease have been developed by nonhospitalists.58, 59 These areas are considered core competencies in hospital medicine, and as such, hospitalists have an obligation to review and refine these guidelines to ensure the best provision of care to our patients.59
Hospitalists have been engaged in upholding guidelines that affect community practice. For example, in a study comparing treatment of patients admitted with congestive heart failure by hospitalists compared with that by nonhospitalists, hospitalists were found to be more likely to document left ventricular function, a core measure of quality as defined by JCAHO.39, 60 Knowledge about cardiac function can direct future care for patients when they return to the community and into the care of their primary care providers. In another example, Rifkin found that patients with community‐acquired pneumonia treated by hospitalists were more rapidly converted to oral antibiotics from intravenous antibiotics, facilitating a shorter length of stay,61 which reduced the opportunity for nosocomial infections to propagate. Because hospitalists are skilled at following guidelines,59 it follows that they should seize the opportunity to develop more of them.
As the hospitalist movement continues to grow, hospitalists will likely be engaged in implementing citywide, statewide, and even national policies that ensure optimal care of the hospitalized patient.
BARRIERS TO HOSPITALISTS FOCUSING ON PUBLIC HEALTH
Hospitalists are involved in public health activities even though they may not recognize the extent of this involvement. However, there may be some drawbacks to hospitalists viewing each patient encounter as an opportunity for a public health intervention. First, in viewing a patient as part of a cohort, the individual needs of the patient may be overlooked. There is inherent tension between population‐based and individual‐based care, which is a challenge. Second, hospitalists are busy clinicians who may be most highly valued because of their focus on efficiency and cost savings in the acute care setting. This factor alone may prevent substantive involvement by hospitalists in public health practice. Moving beyond the management of an acute illness may interfere with this efficiency and cost effectiveness from the hospital's perspective. However, interventions that promote health and prevent or reduce rehospitalizations may be cost effective to society in the long run. Third, current billing systems do not adequately reward or reimburse providers for the extra time that may be necessary to engage in public health practice. Fourth, hospitalists may not have the awareness, interest, training, or commitment to engage in public health practice. Finally, there may not be effective collaboration and communication systems between primary care providers and hospitalists. This barrier limits or hinders many possibilities for the effective execution of several public health initiatives.
CONCLUSIONS AND IMPLICATIONS
Hospitalists and the specialty of hospital medicine materialized because of myriad economic forces and the need to provide safe, high‐quality care to hospitalized patients. In this article we have described the ways in which hospitalists can be explicitly involved in public health practice. Traditionally, physicians caring for hospitalized patients have collected information through histories and physical examinations, interpreted laboratory data and tests, and formulated assessments and plans of care. To become public health practitioners, hospitalists have to go beyond these tasks and consider public health thought processes, such as problem‐solving paradigms and theories of behavior change. In adopting this public health perspective, hospitalists may begin to think of a patient in the context of the larger community in order to define the problems facing the community, not just the patient, determine the magnitude of such problems, identify key stakeholders, create intervention/prevention strategies, set priorities and recommend interventions, and implement and evaluate those interventions. This approach forces providers to move beyond the physicianpatient model and draw on public health models to invoke change. Hopefully, future research will further convince hospitalists of the benefits of this approach. Although it may be easier to defer care and management decisions to an outpatient physician, data suggest that intervening when patients are in the hospital may be most effective.62, 63 For example, is it possible that patients are more likely to quit smoking when they are sick in the hospital than when they are in their usual state of health on a routine visit at their primary care provider's office?64 Further, although deferring care to a primary care provider (PCP) may be easier, it is not always possible given these barriers: (1) some patients are routinely rehospitalized, precluding primary care visits, (2) some recommendations may not be received by PCPs, and (3) PCPpatient encounters are brief and the agendas full, and there are limited resources to address recommendations from the hospital.
As hospitalists become more involved in public health practice, their collaboration with physicians and researchers in other fields, nurses, policymakers, and administrators will expand. Succeeding in this arena requires integrity, motivation, capacity, understanding, knowledge, and experience.65 It is hoped that hospitalists will embrace the opportunity and master the requisite skill set necessary to practice in and advance this field. As hospitalist fellowship programs are developed, public health practice skills could be incorporated into the curriculum. Currently 6 of 16 fellowship programs offer either a master of public health degree or public health courses.66 Public health skills can also be taught at Society of Hospital Medicine meetings and other continuing medical education events.
With the evolution of hospital medicine, hospitalists have to be malleable in order to optimally meet the needs of the population they serve. The possibilities are endless.
- ,.The Hospitalist movement 5 years later.JAMA.2002;287:487–494.
- Hospitals and Health Networks. Hospitalists: a specialty coming into its own. Available at: http://www.hhmag.com. Accessed February 27,2006.
- ,,.Emergency medicine and public health: new steps in old directions.Ann Emerg Med.2001;38:675–683.
- ,, et al.A public health approach to emergency medicine: preparing for the twenty‐first century.Acad Emerg Med.1994;1:277–286.
- ,.Emergency medicine in population‐based systems of care.Ann Emerg Med.1997;30:800–803.
- ,.Principles of Public Health Practice.Albany, NY:Delmar Publishing;1997.
- Centers for Medicare and Medicaid. Health care spending and growth rate continue to decline in 2004. Available at: http://www.cms.hhs.gov. Accessed October 31,2006.
- ,,, et al.National health expenditures, 2002.Health Care Financ Rev. Summer2004;25:4.
- ,,, et al.Health spending Projections Through 2015: Changes on the Horizon.Health Affairs2006;25:w61–w73.
- Institute of Medicine.Recommendations from the Future of Public Health. InThe Future of the Public's Health.Washington, DC:National Academic Press;2003:411–420.
- ,,.Nosocomial pneumonia: state of the science.Am J Infect Control.2006;34:84–93.
- ,,,,.A reminder reduces urinary catheterization in hospitalized patients.Jt Comm J Qual Patient Saf.2005;31:455–462.
- ,,,.Preventing infections in nursing homes: A survey of infection control practices in southeast Michigan.Am J Infect Control.2005;33:489–492.
- ,,,.Prospective validation of a prediction model for isolating inpatients with suspected pulmonary tuberculosis.Arch Intern Med.2005;165:453–457.
- ,.The Hospital Infection Standardised Surveillance (HISS) programme: analysis of a two‐year pilot.J Hosp Infect.2003;53:259–267.
- ,,,.A Laboratory‐Based, Hospital‐Wide, Electronic Marker for Nosocomial Infection.Am J Clin Pathol.2006;125:34–39.
- ,,.Severe acute respiratory syndrome.Arch Pathol Lab Med.2004;128:1346–1350.
- ,,.Severe acute respiratory syndrome: responses of the healthcare system to a global epidemic.Curr Opin Otolaryngol Head Neck Surg.2005;13:161–164.
- ,.Pandemic influenza and the hospitalist: apocalypse when?J Hosp Med.2006;1:118–123.
- Center for Disease Control and Prevention. Pandemic Influenza information for Health Professionals. Available at: http://www.cdc.gov/flu/pandemic/. Accessed October 31,2006.
- .US health policy in the aftermath of Hurricane Katrina.JAMA.2006;295:437–40
- Levy B,Sidel V, eds.Terrorism and Public Health.New York:Oxford University Press;2003.
- Centers for Disease Control and Prevention (CDC).Public health response to Hurricanes Katrina and Rita—United States 2005.MMWR Morb Mortal Wkly Rep.2006;55:229–231.
- ,,,,,.Racial and ethnic disparities in health: a view from the South Bronx.J Health Care Poor Underserved.2006;17:116–127.
- ,.Williams E. Health Seeking behaviors of African Americans: implications for health administration.J Health Hum Serv Adm.2005;28(1):68–95.
- .Routine rapid HIV testing in hospitals: another opportunity for hospitalists to improve care.J Hosp Med.2006;1:106–112.
- Centers for Disease Control and Prevention.Advancing HIV prevention: new strategies for a changing epidemic—United States, 2003.MMWR Morb Mortal Wkly Rep.2003;52:329–332.
- ,,, et al.Expanded screening for HIV in the United States—an analysis of cost‐effectiveness.N Engl J Med.2005;352:586–595.
- ,,,.Identifying undiagnosed human immunodeficiency virus: the yield for routine, voluntary, inpatient testing.Arch Intern Med.2002;162:887–892.
- ,,,,.Insufficient treatment of hypercholestrolemia among patients hospitalized with chest pain.Clin Cardiol.2006;29:259–262.
- .Medical management of alcoholic patients. In:Kissen B,Besleiter H, eds.Treatment and Rehabilitation of the Chronic Alcoholic.New York:Plenum Publishing Co.;1997.
- ,,.An integral approach to substance abuse.J Psychoactive Drugs.2005;37:363–371.
- ,,, et al.Smoking cessation practice among Norwegian hospital physicians.Tiddskr Nor laegeforen.2000;120:1629–1632.
- ,, et al.Evaluation of an intervention for hospitalized African American smokers.Am J Health Behav.2005;29:228–239.
- .Falling through the cracks: challenges and opportunities for improving transitional care for persons with continuous complex care needs [review].J Am Geriatr Soc.2003;51:549–555.
- ,,,,.Advances in hospital medicine: a review of key articles from the literature.Med Clin N Am.2002;86:797–823.
- ,,,,,.Comprehensive discharge planning with post discharge support for older patients with congestive heart failure.JAMA.2004;291:1358–1367.
- ,.The impact of hospitalists on the cost and quality of inpatient care in the United States: a research synthesis.Med Care Res Rev.2005;62:379–406.
- ,,,,,Quality of care for patients hospitalized with heart failure: assessing the impact of hospitalists.Arch Intern Med.2002;162:1251–1256.
- ,,,.Educational interventions to improve antibiotic use in the community: report from the International Forum on Antibiotic Resistance (IFAR) colloquim, 2002.Lancet Infect Dis.2004;4:44–53.
- ,,, et al.Systematic review of antimicrobial drug prescribing in hospitals.Emerg Infect Dis.2006;12:211–216.
- ,,,,,,.Recommendations for management of community and hospital acquired pneumonia‐the hospitalist perspective.Curr Opin Pulm Med.2004;10(suppl 1):S23–S27.
- ,,,,.Advances in hospital medicine: a review of key articles from the literature.Med Clin N Am.2002;86:797–823.
- .End‐of‐life care for the hospitalized patient.Med Clin N Am.2002;86:749–770.
- ,.Palliative care for patients with heart failure.JAMA.2004;291:2476–2482.
- ,.Prevalence and structure of palliative care services in California hospitals.Arch Intern Med.2003;163:1084–1088.
- ,.Palliative care and the hospitalist: an opportunity for cross‐fertilization.J Med.2001;111:10S–14S.
- .Palliative care in hospitals.J Hosp Med.2006;1:21–28.
- ,.End‐of‐life care in a voluntary hospitalist model: effects on communication, process of care, and patient symptoms.Am J Med.2004;116:669–675.
- ,,,Understanding medical error and improving patient safety in the inpatient setting,Med Clin N Am2002;86:847–867.
- , The hospitalist movement: ten issues to consider, hospital practice. Available at: http://www.hosppract.com/issues/1999/02/wachter.htm. Accessed March 14,2006.
- Shojania KG,Duncan BW,McDonald KM,Wachter RM, eds.Making health care safer: a critical analysis of patient safety practices. Evidence Report/Technology Assessment No. 43 from the Agency for Healthcare Research and Quality: AHRQ Publication No. 01‐E058;2001. Available at: http://www.ahrq.gov/clinic/ptsafety/.
- ,,,.Safe but sound: patient safety meets evidence‐based medicine.JAMA.2002;288:508–513.
- ,,, et al.Role of pharmacist counseling in preventing adverse drug events after hospitalization.Arch Intern Med.2006;166:565–571.
- Hospitalists, pharmacists partner to cut errors: shorter lengths of stay, lower med costs result. HealthCare Benchmarks and Quality Improvement.American Health Consultants, Inc.,2005.
- ,,,.Hospitalists and the practice of inpatient medicine: results of a survey of the National Association of Inpatient Physicians.Ann Intern Med.1999;130:343–349.
- ,,,,.Core competencies in hospital medicine: Development and methodology.J Hosp Med.2006;1:48–56.
- National guideline clearing house. Available at: http://www.guideline.gov. Accessed June 26,2006.
- ,,,,, eds.The core competencies in hospital medicine.J Hosp Med.2006;1(suppl 1).
- Joint Commission on Accreditation of Healthcare Organizations. Core Measures overview. Available at: http://www.jcaho.org/perfeas/coremeas/cm.ovrvw.html. Accessed February 1,2006.
- ,,,.,Comparison of processes and outcomes of pneumonia care between hospitalists and community‐based primary care physicians.Mayo Clin Proc.2002;77:1053–1058.
- ,.The effectiveness of a nursing inpatient smoking cessation program in individuals with cardiovascular disease.Nurs Res.2005;54:243–254.
- ,,,,,.Evaluation of an intervention for hospitalized African American smokers.Am J Health Behav.2005;29:228–239.
- .Smoking cessation: the case for hospital‐based interventions.Prof Nurse.2003;19(3):145–148..
- . Dee Hock's management principles, in his own words. Fast Company.1996;5:79. Available at: http://www.fastcompany.com/magazine/05/dee2.html.
- ,,,.Hospital Medicine Fellowships: Works in progress.Am J Med.2006;119(1):72.e1–e7.
The field of hospital medicine came into being in response to numerous factors involving physicians, patients, and hospitals themselves1 Now, years later, hospital medicine is a specialty that is growing, both in size and sophistication such that the role of the hospitalist is constantly evolving.2 A compelling function that has not yet been clearly articulated is the opportunity for hospitalists to serve as public health practitioners in their unique clinical environment. There is precedence for the power of collaboration between medicine and public health as has been seen with emergency medicine's willingness to embrace opportunities to advance public health.35
In public health, the programs, services, and institutions involved emphasize the prevention of disease and the health needs of the population as a whole. Public health activities vary with changing technology and social values, but the goals remain the same: to reduce the amount of disease, premature death, and disease‐associated discomfort and disability in the population.6 The authors of a leading textbook of public health, Scutchfield and Keck, contend that the most important skill for public health practice is the capacity to visualize the potential for health that exists in a community.6
Hospitalists care for a distinct subset of the general populationinpatients, only a small percentage of society in a given year. Yet over time hospitalists affect a substantial subset of the larger population that uses considerable health care resources.79 Furthermore, hospitalization can be a sentinel event with public health implications (eg, newly diagnosed HIV infection or acute myocardial infarction in a patient with an extended family of cigarette smokers). This presents an opportunity to educate and counsel both the patient and the patient's social network. One model of public health practice by hospitalists is to influence the patient, his or her family, and the community by touching and inspiring the hospitalized patient.
Hospitalists are already involved in many of the core functions of public health (assessment, assurance, and policy development; Fig. 1).10 Achieving ongoing success in this arena means developing hospitalists who are consciously in tune with their roles as public health practitioners.

In this article we define the specific public health contributions that hospitalists have made and describe the possibilities for further innovative advances. To this end, we outline specific public health roles under the broad categories of assessment, assurance, and policy. We point to advances in public health accomplished by hospitalists as well as those being performed by nonhospitalists in the hospital setting. We conclude by describing some of the barriers to and implications of hospitalists taking on public health roles.
ASSESSMENT
Assessment is the systematic collection, analysis, and dissemination of health status information.10 These activities include disease surveillance and investigation of acute outbreaks or changes in the epidemiology of chronic diseases. Assessment also involves understanding the health of a population and the key determinants of a population's health from a variety of perspectives: physical, biological, behavioral, social, cultural, and spiritual.6 Human health has been defined as a state characterized by anatomic integrity; ability to perform personally valued family work and community roles; ability to deal with physical, biologic, and social stress; a feeling of well‐being; and freedom from the risk of disease and untimely death.6 Hospitalists interact with individuals at times of stress and acute illness and thus have a unique opportunity to assess the strength, viability, and resources available to individuals. Key roles that may fall within the auspices of assessment in hospital medicine are infection control, epidemic recognition, disaster response, preventive care, substance abuse treatment, and chronic disease management.
Infection Control
Physicians caring for inpatients have a crucial stake in controlling hospital infection as exemplified by the work of Flanders et al. on preventing nosocomial infections, especially nosocomial pneumonia.11 They describe specific strategies to prevent iatrogenic spread such as washing hands before and after patient contact, establishing guidelines against the use of artificial fingernails, using indwelling devices such as catheters only when absolutely necessary, and using sterile barriers.11 Hospitalists such as Sanjay Saint have led the way in studying methods to reduce bladder catheterization, which has been associated with urinary tract infections12; others have collaborated on work to prevent infections in nursing homes.13 Given the importance of this field, there is room for further hospitalist involvement. Novel methods for infection control in hospitals have been studied by nonhospitalists such as Wisnivesky, who prospectively validated a clinical decision rule to predict the need for respiratory isolation of inpatients with suspected tuberculosis (TB). This prediction rule, which is based on clinical and chest radiographic findings, was able to accurately identify patients at low risk for TB from among inpatients with suspected active pulmonary TB isolated on admission to the hospital.14 Retrospective application of the prediction rule showed respiratory precautions were inappropriately implemented for a third of patients.14 These studies are examples of empiric public health research performed in the inpatient setting. In the infection control domain, candidate issues for further study could include interventions aimed at reducing rates of Clostridium difficile, developing programs for standardized surveillance of hospital infection, validating electronic markers for nosocomial infection, and taking innovative approaches to improving hand‐washing practices in the hospital.15, 16
Recognizing Epidemics
An excellent example of the importance of hospitalists embracing public health and remembering their patients are part of a community was the severe acute respiratory syndrome (SARS) outbreak in Toronto, Ontario, Canada. The outbreak is thought to have begun with a single traveler. With the transfer of patients and the movement of visitors and health care workers among facilities, SARS quickly spread through Toronto, making it the largest SARS‐affected area outside Asia.17 Approximately a month after the outbreak was recognized in Toronto, it was thought to be over, and the World Health Organization (WHO) removed Toronto from its SARS‐affected list.17 Unfortunately, patients with unrecognized SARS remained in health care institutions, including a patient transferred to a rehabilitation center. Infection quickly spread again, resulting in a second phase of the outbreak.17
The SARS outbreak served as a reminder that a global public health system is essential and taught many lessons17 germane to pandemics that recur annually (eg, influenza viruses) as well those that episodically threaten the health of the population (eg, avian flu). Proposed actions to prevent a repeat of the scenario that occurred with SARS in Toronto include assessing the current facilities (eg, isolation rooms and respiratory masks) at each institution, identifying health care workers willing to serve as an outbreak team, and the hiring staff to train hospital personnel in personal protective equipment (PPE) and infection control policies.18 The Centers for Disease Control and Prevention (CDC) contends that planning for the possibility of a virulent pandemic at the local, national, and global levels is critical to limiting the mortality and morbidity should such occur.19, 20 In a previous article, Pile and Gordon declared hospitalists are key players in institutional efforts to prepare for a viral pandemic such as influenza and should be aware of lessons that may be applied from responses to pandemics such as SARS.19 Well placed to recognize clinical trends that may herald epidemics, hospitalists can fulfill some of the necessary public health responsibilities delineated above.
Disaster Response
Natural disasters and terrorism are in the forefront of the popular press and are also high priorities in health care and public health.21 Terrorism and natural disasters cause significant injury, illness, and death.22 Hospital‐based health care providers fulfill a variety of roles when terrorist acts and disasters occur, including reporting, diagnosing, and managing illness, providing preventive measures (eg, vaccines and preparedness kits), preventing the secondary spread of disease, assisting in the investigation of the causes of disease outbreaks, participating in preparedness planning, and evaluating preparedness policies and programs.22 The experience gained in the aftermaths of Hurricanes Katrina and Rita with their unprecedented death, injury, destruction, and displacement should help to guide future response and recovery activities.23 Hospitalists were at the forefront of delivering care, living in their hospitals for days after Hurricane Katrina. Without question, hospitalists will be called on again to serve those affected by disasters.
Preventive Care
For many patients admitted to the hospital, meeting a hospitalist is their first encounter with a physician in years.24, 25 In these instances, hospitalists must ensure that patients' immunizations are up‐to‐date and arrange appropriate follow‐up care with primary care providers. Greenwald described an important role that hospitalists could play in HIV prevention by promoting HIV testing in the hospital.26 The CDC recently confirmed the wisdom of this approach and estimates that the 250,000 to 1.2 million people in the United States with HIV infection who do not know their serostatus play a significant role in HIV transmission.26, 27 In an effort to promote testing, the CDC has initiated a program aimed at incorporating HIV testing into routine medical care, as recommended by others.28 More than a quarter of patients with HIV in the United States are diagnosed in the hospital, and for many other patients, hospitalization is their only real opportunity to be tested.26, 29 Similarly, when hospitalists find elevated cholesterol or triglycerides in routine evaluations of patients who present with chest pain, they have to decide whether to initiate lipid‐lowering medications.30 The hospitalist is sometimes the only physician that patients repeatedly admitted, may see over prolonged periods. It follows that if hospitalists are remiss in delivering preventive care to such patients, they lose the opportunity to positively affect their long‐term health. In practice, hospitalists perform myriad preventive‐care functions, although there is scant literature supporting this role. Hospitalists have an opportunity to collaborate in research projects of hospital‐initiated preventive care that measure outcomes at the community level.
Substance Abuse
In the Unites States, 25%‐40% of hospital admissions are related to substance abuse and its sequelae.31 These patients frequently are admitted to general medicine services for detoxification or treatment of substance‐abuse‐related morbidity, although some American hospitals have specialized treatment and detoxification centers. There is a pressing need for more models of comprehensive care that address the complex issues of addiction, including the biological, social, cultural, spiritual, and developmental needs of patients.32
Hospitalists routinely counsel their patients with substance abuse problems and often consult a chemical dependency counselor, who provides patients with additional information about outpatient or inpatient facilities that may help them after their hospitalization. Unfortunately, because of the natural history of substance abuse, many of these patients are rehospitalized with the same problems even after going through rehabilitation. The adoption of a public health philosophy and approach by hospitalists may assist patients who have addictions through innovative multidisciplinary interventions while these patient are being detoxified. Traditionally, these responsibilities have fallen to primary care providers and psychologists in substance abuse medicine; but, as mentioned previously, many such patients are rehospitalized before they make it to their follow‐up appointments.
In a study examining smoking cessation practices among Norwegian hospital physicians, 98% of the doctors stated they ask their patients about their smoking habits, but fewer than 7% of these physicians regularly offer smoking‐cessation counseling, hand out materials, or give patients other advice about smoking cessation.33 That study illustrates that hospital doctors often ask about problems but can certainly improve in terms of intervention and follow‐up. Other works by nonhospitalist physicians have examined the real potential of inpatient interventions for smoking cessation. Most of this work involves a multidisciplinary approach that relies heavily on nurses. For example, Davies et al. evaluated the effectiveness of a hospital‐based intervention for smoking cessation among low‐income smokers using public health methodologies. The intervention was effective and promising as a way to affect smokers in underserved communities.34
Chronic Disease Management
Public health roles involving chronic disease management include surveillance, intervention design, and implementation of control programs.6 Given their access to data on hospitalized patients, hospitalists can carry out surveillance and empirical population‐based research about hospitalized patients with chronic illnesses. Thoughtfully designed protocols can measure the success of interventions initiated in patients while hospitalized, with further data collection and follow‐up after patients have returned to the community.35 Such endeavors can improve the likelihood that patients with chronic conditions are effectively referred to programs that will maintain their health and functional status.36 If hospitalists consider themselves public health providers, encounters with these hospitalized patients will go beyond noting that their chronic conditions are stable and instead will lay the groundwork to prospectively control these conditions. This approach would have the potential to reduce the number of future hospitalizations and lead to healthier communities.37 To truly carry this out effectively, coordinated collaboration between primary care providers and hospitalists will be necessary.
ASSURANCE
Assurance is the provision of access to necessary health services. It entails efforts to solve problems that threaten the health of populations and empowers individuals to maintain their own health. This is accomplished by either encouraging action, delegating to other entities (private or public sector), mandating specific requirements through regulation, or providing services directly.10 Hospitalist teams aim to ensure that the high‐quality services needed to protect the health of their community (hospitalized patients) are available and that this population receives proper consideration in the allocation of resources. The few studies to date that have directly examined the quality of care that hospitalists provide38 have done so using evidence‐based measures believed to correlate with improved health care outcomes.38 The ambiguities in assessing quality may in part limit such studies.39 Specific hospitalist roles that fall under the assurance umbrella include antibiotic optimization, palliative care, patient safety, and medical error management.
Antibiotic Optimization
Inappropriate use of antimicrobial treatment for infectious diseases has cost and public health implications.40 These inappropriate uses include giving antibiotics when not indicated, overusing broad‐spectrum antibiotics, making mismatches between microbes and medicines when cultures and information on test sensitivity are available, and using intravenous formulations when oral therapy would suffice.41 The public health impact goes way beyond increasing selective pressure for antimicrobial resistance to include safety, adverse events, and increased costs to both patient and hospitals.40 At our institution, the hospital medicine service and infectious disease division have jointly developed and implemented an intervention to reduce inappropriate antibiotic use. At other institutions, hospitalist teams have developed protocols for treating infectious diseases commonly encountered in the hospitalized patient.42 The recommendations of both Amin and Reddy for management of community‐ and hospital‐acquired pneumonia acknowledged that through establishment of clinical care pathways, variation in prescribing patterns among hospitalists can be decreased while optimizing outcomes.42 The work of Williams and colleagues is another example of advances by hospitalists. They reviewed the literature to determine that the use of combination antibiotics as empiric therapy for community‐acquired pneumonia is superior to the use of a single effective antibiotic in treating bacteremic patients with pneumococcal community‐acquired pneumonia.43
Palliative Care
Mortality is a vital outcome measure of public health research and interventions. Not surprisingly, many people are hospitalized in the final months of their life and often die in a hospital. Pantilat showed that hospitalists can respond to these circumstances and have the opportunity to improve care of the dying.4446 Muir et al. evaluated the convergence of the fields of palliative care medicine and hospital medicine and reviewed the opportunities for mutual education and improved patient care.47 They described how the confluence of the changing nature and site of death in the United States coupled with the reorganization of hospital care provides a strategic opportunity to improve end‐of‐life care.47 Hospitalists can ensure that care of the dying is delivered with skill, compassion, and expertise. And so it is imperative they be trained to accomplish this objective.47, 49
Fortunately, hospitalists already appear to enhance patientphysician communication. Auerbach looked at communication, care patterns, and outcomes of dying patients, comparing patients being cared for by hospitalists with those being care for by community‐based physicians. Hospitalists had discussions with patients or their families about care more often than did nonhospitalist physicians (91% versus 73%, respectively, P = .006).49 Because the delivery of high‐quality palliative care is time consuming and complex, alternative models for billing or the use of physician extenders or consultants may be necessary at some institutions.
Patient Safety and Medical Error Management
Hospitalists have been in the forefront of promoting a culture of patient safety.50 Their continuous presence in the hospital and their interactions with members of health care teams from multiple disciplines who share this goal make them important facilitators. Hospitalists have increasing involvement in systems‐based efforts aimed at reducing medical errors.50 Hospitalists are being asked to lead committees that adopt multidisciplinary approaches to reduce adverse events, morbidity, and mortality.50 These committees often have representation from pharmacy, nursing, and other key hospital stakeholders including from the administration.51 Quality assurance activities assess locally collected data and compare results with local and national benchmarks. There are several published examples of hospitalists engaged in patient safety and medical error management. For example, Shojania et al compiled evidence based safety practices in an effort to promote patient safety.52, 53 Schnipper studied the role of pharmacist counseling in preventing adverse drug events (ADEs) after hospitalization and found that pharmacist medication review, patient counseling, and telephone follow‐up were associated with a lower rate of preventable ADEs 30 days after hospital discharge.54 Moreover, Syed paired hospitalists and pharmacists to collaboratively prescribe medications appropriately. In one study there were fewer medication errors and adverse drug reactions in patients treated by a team led by hospitalists than in those treated by the control group, made up of nonhospitalist attendings.55
POLICY
Policy development defines health control goals and objectives and develops implementation plans for those goals.10 By necessity, it operates at the intersection of legislative, political, and regulatory processes.10 At many institutions, hospitalists have been involved in the development of policies ensuring that the core functions of assessment and assurance are addressed and maintained. In fact, hospitalists report that development of quality assurance and practice guidelines accounts for most of their nonclinical time.56 This role of hospitalists is supported by anecdotal reports rather than published empiric evidence.57 For example, at Johns Hopkins Bayview Medical Center, hospitalist‐led teams have developed triage and patient handoff policies designed to improve patient safety. Parameters for admission to the general medicine ward have been elaborated and are periodically refined by the hospitalist team.
Another area that falls within the genre of policy is development of clinical practice guidelines. Guidelines for the treatment of pneumonia, congestive heart failure, deep‐vein thrombosis prophylaxis, alcohol and drug withdrawal, pain management, delirium, and chronic obstructive pulmonary disease have been developed by nonhospitalists.58, 59 These areas are considered core competencies in hospital medicine, and as such, hospitalists have an obligation to review and refine these guidelines to ensure the best provision of care to our patients.59
Hospitalists have been engaged in upholding guidelines that affect community practice. For example, in a study comparing treatment of patients admitted with congestive heart failure by hospitalists compared with that by nonhospitalists, hospitalists were found to be more likely to document left ventricular function, a core measure of quality as defined by JCAHO.39, 60 Knowledge about cardiac function can direct future care for patients when they return to the community and into the care of their primary care providers. In another example, Rifkin found that patients with community‐acquired pneumonia treated by hospitalists were more rapidly converted to oral antibiotics from intravenous antibiotics, facilitating a shorter length of stay,61 which reduced the opportunity for nosocomial infections to propagate. Because hospitalists are skilled at following guidelines,59 it follows that they should seize the opportunity to develop more of them.
As the hospitalist movement continues to grow, hospitalists will likely be engaged in implementing citywide, statewide, and even national policies that ensure optimal care of the hospitalized patient.
BARRIERS TO HOSPITALISTS FOCUSING ON PUBLIC HEALTH
Hospitalists are involved in public health activities even though they may not recognize the extent of this involvement. However, there may be some drawbacks to hospitalists viewing each patient encounter as an opportunity for a public health intervention. First, in viewing a patient as part of a cohort, the individual needs of the patient may be overlooked. There is inherent tension between population‐based and individual‐based care, which is a challenge. Second, hospitalists are busy clinicians who may be most highly valued because of their focus on efficiency and cost savings in the acute care setting. This factor alone may prevent substantive involvement by hospitalists in public health practice. Moving beyond the management of an acute illness may interfere with this efficiency and cost effectiveness from the hospital's perspective. However, interventions that promote health and prevent or reduce rehospitalizations may be cost effective to society in the long run. Third, current billing systems do not adequately reward or reimburse providers for the extra time that may be necessary to engage in public health practice. Fourth, hospitalists may not have the awareness, interest, training, or commitment to engage in public health practice. Finally, there may not be effective collaboration and communication systems between primary care providers and hospitalists. This barrier limits or hinders many possibilities for the effective execution of several public health initiatives.
CONCLUSIONS AND IMPLICATIONS
Hospitalists and the specialty of hospital medicine materialized because of myriad economic forces and the need to provide safe, high‐quality care to hospitalized patients. In this article we have described the ways in which hospitalists can be explicitly involved in public health practice. Traditionally, physicians caring for hospitalized patients have collected information through histories and physical examinations, interpreted laboratory data and tests, and formulated assessments and plans of care. To become public health practitioners, hospitalists have to go beyond these tasks and consider public health thought processes, such as problem‐solving paradigms and theories of behavior change. In adopting this public health perspective, hospitalists may begin to think of a patient in the context of the larger community in order to define the problems facing the community, not just the patient, determine the magnitude of such problems, identify key stakeholders, create intervention/prevention strategies, set priorities and recommend interventions, and implement and evaluate those interventions. This approach forces providers to move beyond the physicianpatient model and draw on public health models to invoke change. Hopefully, future research will further convince hospitalists of the benefits of this approach. Although it may be easier to defer care and management decisions to an outpatient physician, data suggest that intervening when patients are in the hospital may be most effective.62, 63 For example, is it possible that patients are more likely to quit smoking when they are sick in the hospital than when they are in their usual state of health on a routine visit at their primary care provider's office?64 Further, although deferring care to a primary care provider (PCP) may be easier, it is not always possible given these barriers: (1) some patients are routinely rehospitalized, precluding primary care visits, (2) some recommendations may not be received by PCPs, and (3) PCPpatient encounters are brief and the agendas full, and there are limited resources to address recommendations from the hospital.
As hospitalists become more involved in public health practice, their collaboration with physicians and researchers in other fields, nurses, policymakers, and administrators will expand. Succeeding in this arena requires integrity, motivation, capacity, understanding, knowledge, and experience.65 It is hoped that hospitalists will embrace the opportunity and master the requisite skill set necessary to practice in and advance this field. As hospitalist fellowship programs are developed, public health practice skills could be incorporated into the curriculum. Currently 6 of 16 fellowship programs offer either a master of public health degree or public health courses.66 Public health skills can also be taught at Society of Hospital Medicine meetings and other continuing medical education events.
With the evolution of hospital medicine, hospitalists have to be malleable in order to optimally meet the needs of the population they serve. The possibilities are endless.
The field of hospital medicine came into being in response to numerous factors involving physicians, patients, and hospitals themselves1 Now, years later, hospital medicine is a specialty that is growing, both in size and sophistication such that the role of the hospitalist is constantly evolving.2 A compelling function that has not yet been clearly articulated is the opportunity for hospitalists to serve as public health practitioners in their unique clinical environment. There is precedence for the power of collaboration between medicine and public health as has been seen with emergency medicine's willingness to embrace opportunities to advance public health.35
In public health, the programs, services, and institutions involved emphasize the prevention of disease and the health needs of the population as a whole. Public health activities vary with changing technology and social values, but the goals remain the same: to reduce the amount of disease, premature death, and disease‐associated discomfort and disability in the population.6 The authors of a leading textbook of public health, Scutchfield and Keck, contend that the most important skill for public health practice is the capacity to visualize the potential for health that exists in a community.6
Hospitalists care for a distinct subset of the general populationinpatients, only a small percentage of society in a given year. Yet over time hospitalists affect a substantial subset of the larger population that uses considerable health care resources.79 Furthermore, hospitalization can be a sentinel event with public health implications (eg, newly diagnosed HIV infection or acute myocardial infarction in a patient with an extended family of cigarette smokers). This presents an opportunity to educate and counsel both the patient and the patient's social network. One model of public health practice by hospitalists is to influence the patient, his or her family, and the community by touching and inspiring the hospitalized patient.
Hospitalists are already involved in many of the core functions of public health (assessment, assurance, and policy development; Fig. 1).10 Achieving ongoing success in this arena means developing hospitalists who are consciously in tune with their roles as public health practitioners.

In this article we define the specific public health contributions that hospitalists have made and describe the possibilities for further innovative advances. To this end, we outline specific public health roles under the broad categories of assessment, assurance, and policy. We point to advances in public health accomplished by hospitalists as well as those being performed by nonhospitalists in the hospital setting. We conclude by describing some of the barriers to and implications of hospitalists taking on public health roles.
ASSESSMENT
Assessment is the systematic collection, analysis, and dissemination of health status information.10 These activities include disease surveillance and investigation of acute outbreaks or changes in the epidemiology of chronic diseases. Assessment also involves understanding the health of a population and the key determinants of a population's health from a variety of perspectives: physical, biological, behavioral, social, cultural, and spiritual.6 Human health has been defined as a state characterized by anatomic integrity; ability to perform personally valued family work and community roles; ability to deal with physical, biologic, and social stress; a feeling of well‐being; and freedom from the risk of disease and untimely death.6 Hospitalists interact with individuals at times of stress and acute illness and thus have a unique opportunity to assess the strength, viability, and resources available to individuals. Key roles that may fall within the auspices of assessment in hospital medicine are infection control, epidemic recognition, disaster response, preventive care, substance abuse treatment, and chronic disease management.
Infection Control
Physicians caring for inpatients have a crucial stake in controlling hospital infection as exemplified by the work of Flanders et al. on preventing nosocomial infections, especially nosocomial pneumonia.11 They describe specific strategies to prevent iatrogenic spread such as washing hands before and after patient contact, establishing guidelines against the use of artificial fingernails, using indwelling devices such as catheters only when absolutely necessary, and using sterile barriers.11 Hospitalists such as Sanjay Saint have led the way in studying methods to reduce bladder catheterization, which has been associated with urinary tract infections12; others have collaborated on work to prevent infections in nursing homes.13 Given the importance of this field, there is room for further hospitalist involvement. Novel methods for infection control in hospitals have been studied by nonhospitalists such as Wisnivesky, who prospectively validated a clinical decision rule to predict the need for respiratory isolation of inpatients with suspected tuberculosis (TB). This prediction rule, which is based on clinical and chest radiographic findings, was able to accurately identify patients at low risk for TB from among inpatients with suspected active pulmonary TB isolated on admission to the hospital.14 Retrospective application of the prediction rule showed respiratory precautions were inappropriately implemented for a third of patients.14 These studies are examples of empiric public health research performed in the inpatient setting. In the infection control domain, candidate issues for further study could include interventions aimed at reducing rates of Clostridium difficile, developing programs for standardized surveillance of hospital infection, validating electronic markers for nosocomial infection, and taking innovative approaches to improving hand‐washing practices in the hospital.15, 16
Recognizing Epidemics
An excellent example of the importance of hospitalists embracing public health and remembering their patients are part of a community was the severe acute respiratory syndrome (SARS) outbreak in Toronto, Ontario, Canada. The outbreak is thought to have begun with a single traveler. With the transfer of patients and the movement of visitors and health care workers among facilities, SARS quickly spread through Toronto, making it the largest SARS‐affected area outside Asia.17 Approximately a month after the outbreak was recognized in Toronto, it was thought to be over, and the World Health Organization (WHO) removed Toronto from its SARS‐affected list.17 Unfortunately, patients with unrecognized SARS remained in health care institutions, including a patient transferred to a rehabilitation center. Infection quickly spread again, resulting in a second phase of the outbreak.17
The SARS outbreak served as a reminder that a global public health system is essential and taught many lessons17 germane to pandemics that recur annually (eg, influenza viruses) as well those that episodically threaten the health of the population (eg, avian flu). Proposed actions to prevent a repeat of the scenario that occurred with SARS in Toronto include assessing the current facilities (eg, isolation rooms and respiratory masks) at each institution, identifying health care workers willing to serve as an outbreak team, and the hiring staff to train hospital personnel in personal protective equipment (PPE) and infection control policies.18 The Centers for Disease Control and Prevention (CDC) contends that planning for the possibility of a virulent pandemic at the local, national, and global levels is critical to limiting the mortality and morbidity should such occur.19, 20 In a previous article, Pile and Gordon declared hospitalists are key players in institutional efforts to prepare for a viral pandemic such as influenza and should be aware of lessons that may be applied from responses to pandemics such as SARS.19 Well placed to recognize clinical trends that may herald epidemics, hospitalists can fulfill some of the necessary public health responsibilities delineated above.
Disaster Response
Natural disasters and terrorism are in the forefront of the popular press and are also high priorities in health care and public health.21 Terrorism and natural disasters cause significant injury, illness, and death.22 Hospital‐based health care providers fulfill a variety of roles when terrorist acts and disasters occur, including reporting, diagnosing, and managing illness, providing preventive measures (eg, vaccines and preparedness kits), preventing the secondary spread of disease, assisting in the investigation of the causes of disease outbreaks, participating in preparedness planning, and evaluating preparedness policies and programs.22 The experience gained in the aftermaths of Hurricanes Katrina and Rita with their unprecedented death, injury, destruction, and displacement should help to guide future response and recovery activities.23 Hospitalists were at the forefront of delivering care, living in their hospitals for days after Hurricane Katrina. Without question, hospitalists will be called on again to serve those affected by disasters.
Preventive Care
For many patients admitted to the hospital, meeting a hospitalist is their first encounter with a physician in years.24, 25 In these instances, hospitalists must ensure that patients' immunizations are up‐to‐date and arrange appropriate follow‐up care with primary care providers. Greenwald described an important role that hospitalists could play in HIV prevention by promoting HIV testing in the hospital.26 The CDC recently confirmed the wisdom of this approach and estimates that the 250,000 to 1.2 million people in the United States with HIV infection who do not know their serostatus play a significant role in HIV transmission.26, 27 In an effort to promote testing, the CDC has initiated a program aimed at incorporating HIV testing into routine medical care, as recommended by others.28 More than a quarter of patients with HIV in the United States are diagnosed in the hospital, and for many other patients, hospitalization is their only real opportunity to be tested.26, 29 Similarly, when hospitalists find elevated cholesterol or triglycerides in routine evaluations of patients who present with chest pain, they have to decide whether to initiate lipid‐lowering medications.30 The hospitalist is sometimes the only physician that patients repeatedly admitted, may see over prolonged periods. It follows that if hospitalists are remiss in delivering preventive care to such patients, they lose the opportunity to positively affect their long‐term health. In practice, hospitalists perform myriad preventive‐care functions, although there is scant literature supporting this role. Hospitalists have an opportunity to collaborate in research projects of hospital‐initiated preventive care that measure outcomes at the community level.
Substance Abuse
In the Unites States, 25%‐40% of hospital admissions are related to substance abuse and its sequelae.31 These patients frequently are admitted to general medicine services for detoxification or treatment of substance‐abuse‐related morbidity, although some American hospitals have specialized treatment and detoxification centers. There is a pressing need for more models of comprehensive care that address the complex issues of addiction, including the biological, social, cultural, spiritual, and developmental needs of patients.32
Hospitalists routinely counsel their patients with substance abuse problems and often consult a chemical dependency counselor, who provides patients with additional information about outpatient or inpatient facilities that may help them after their hospitalization. Unfortunately, because of the natural history of substance abuse, many of these patients are rehospitalized with the same problems even after going through rehabilitation. The adoption of a public health philosophy and approach by hospitalists may assist patients who have addictions through innovative multidisciplinary interventions while these patient are being detoxified. Traditionally, these responsibilities have fallen to primary care providers and psychologists in substance abuse medicine; but, as mentioned previously, many such patients are rehospitalized before they make it to their follow‐up appointments.
In a study examining smoking cessation practices among Norwegian hospital physicians, 98% of the doctors stated they ask their patients about their smoking habits, but fewer than 7% of these physicians regularly offer smoking‐cessation counseling, hand out materials, or give patients other advice about smoking cessation.33 That study illustrates that hospital doctors often ask about problems but can certainly improve in terms of intervention and follow‐up. Other works by nonhospitalist physicians have examined the real potential of inpatient interventions for smoking cessation. Most of this work involves a multidisciplinary approach that relies heavily on nurses. For example, Davies et al. evaluated the effectiveness of a hospital‐based intervention for smoking cessation among low‐income smokers using public health methodologies. The intervention was effective and promising as a way to affect smokers in underserved communities.34
Chronic Disease Management
Public health roles involving chronic disease management include surveillance, intervention design, and implementation of control programs.6 Given their access to data on hospitalized patients, hospitalists can carry out surveillance and empirical population‐based research about hospitalized patients with chronic illnesses. Thoughtfully designed protocols can measure the success of interventions initiated in patients while hospitalized, with further data collection and follow‐up after patients have returned to the community.35 Such endeavors can improve the likelihood that patients with chronic conditions are effectively referred to programs that will maintain their health and functional status.36 If hospitalists consider themselves public health providers, encounters with these hospitalized patients will go beyond noting that their chronic conditions are stable and instead will lay the groundwork to prospectively control these conditions. This approach would have the potential to reduce the number of future hospitalizations and lead to healthier communities.37 To truly carry this out effectively, coordinated collaboration between primary care providers and hospitalists will be necessary.
ASSURANCE
Assurance is the provision of access to necessary health services. It entails efforts to solve problems that threaten the health of populations and empowers individuals to maintain their own health. This is accomplished by either encouraging action, delegating to other entities (private or public sector), mandating specific requirements through regulation, or providing services directly.10 Hospitalist teams aim to ensure that the high‐quality services needed to protect the health of their community (hospitalized patients) are available and that this population receives proper consideration in the allocation of resources. The few studies to date that have directly examined the quality of care that hospitalists provide38 have done so using evidence‐based measures believed to correlate with improved health care outcomes.38 The ambiguities in assessing quality may in part limit such studies.39 Specific hospitalist roles that fall under the assurance umbrella include antibiotic optimization, palliative care, patient safety, and medical error management.
Antibiotic Optimization
Inappropriate use of antimicrobial treatment for infectious diseases has cost and public health implications.40 These inappropriate uses include giving antibiotics when not indicated, overusing broad‐spectrum antibiotics, making mismatches between microbes and medicines when cultures and information on test sensitivity are available, and using intravenous formulations when oral therapy would suffice.41 The public health impact goes way beyond increasing selective pressure for antimicrobial resistance to include safety, adverse events, and increased costs to both patient and hospitals.40 At our institution, the hospital medicine service and infectious disease division have jointly developed and implemented an intervention to reduce inappropriate antibiotic use. At other institutions, hospitalist teams have developed protocols for treating infectious diseases commonly encountered in the hospitalized patient.42 The recommendations of both Amin and Reddy for management of community‐ and hospital‐acquired pneumonia acknowledged that through establishment of clinical care pathways, variation in prescribing patterns among hospitalists can be decreased while optimizing outcomes.42 The work of Williams and colleagues is another example of advances by hospitalists. They reviewed the literature to determine that the use of combination antibiotics as empiric therapy for community‐acquired pneumonia is superior to the use of a single effective antibiotic in treating bacteremic patients with pneumococcal community‐acquired pneumonia.43
Palliative Care
Mortality is a vital outcome measure of public health research and interventions. Not surprisingly, many people are hospitalized in the final months of their life and often die in a hospital. Pantilat showed that hospitalists can respond to these circumstances and have the opportunity to improve care of the dying.4446 Muir et al. evaluated the convergence of the fields of palliative care medicine and hospital medicine and reviewed the opportunities for mutual education and improved patient care.47 They described how the confluence of the changing nature and site of death in the United States coupled with the reorganization of hospital care provides a strategic opportunity to improve end‐of‐life care.47 Hospitalists can ensure that care of the dying is delivered with skill, compassion, and expertise. And so it is imperative they be trained to accomplish this objective.47, 49
Fortunately, hospitalists already appear to enhance patientphysician communication. Auerbach looked at communication, care patterns, and outcomes of dying patients, comparing patients being cared for by hospitalists with those being care for by community‐based physicians. Hospitalists had discussions with patients or their families about care more often than did nonhospitalist physicians (91% versus 73%, respectively, P = .006).49 Because the delivery of high‐quality palliative care is time consuming and complex, alternative models for billing or the use of physician extenders or consultants may be necessary at some institutions.
Patient Safety and Medical Error Management
Hospitalists have been in the forefront of promoting a culture of patient safety.50 Their continuous presence in the hospital and their interactions with members of health care teams from multiple disciplines who share this goal make them important facilitators. Hospitalists have increasing involvement in systems‐based efforts aimed at reducing medical errors.50 Hospitalists are being asked to lead committees that adopt multidisciplinary approaches to reduce adverse events, morbidity, and mortality.50 These committees often have representation from pharmacy, nursing, and other key hospital stakeholders including from the administration.51 Quality assurance activities assess locally collected data and compare results with local and national benchmarks. There are several published examples of hospitalists engaged in patient safety and medical error management. For example, Shojania et al compiled evidence based safety practices in an effort to promote patient safety.52, 53 Schnipper studied the role of pharmacist counseling in preventing adverse drug events (ADEs) after hospitalization and found that pharmacist medication review, patient counseling, and telephone follow‐up were associated with a lower rate of preventable ADEs 30 days after hospital discharge.54 Moreover, Syed paired hospitalists and pharmacists to collaboratively prescribe medications appropriately. In one study there were fewer medication errors and adverse drug reactions in patients treated by a team led by hospitalists than in those treated by the control group, made up of nonhospitalist attendings.55
POLICY
Policy development defines health control goals and objectives and develops implementation plans for those goals.10 By necessity, it operates at the intersection of legislative, political, and regulatory processes.10 At many institutions, hospitalists have been involved in the development of policies ensuring that the core functions of assessment and assurance are addressed and maintained. In fact, hospitalists report that development of quality assurance and practice guidelines accounts for most of their nonclinical time.56 This role of hospitalists is supported by anecdotal reports rather than published empiric evidence.57 For example, at Johns Hopkins Bayview Medical Center, hospitalist‐led teams have developed triage and patient handoff policies designed to improve patient safety. Parameters for admission to the general medicine ward have been elaborated and are periodically refined by the hospitalist team.
Another area that falls within the genre of policy is development of clinical practice guidelines. Guidelines for the treatment of pneumonia, congestive heart failure, deep‐vein thrombosis prophylaxis, alcohol and drug withdrawal, pain management, delirium, and chronic obstructive pulmonary disease have been developed by nonhospitalists.58, 59 These areas are considered core competencies in hospital medicine, and as such, hospitalists have an obligation to review and refine these guidelines to ensure the best provision of care to our patients.59
Hospitalists have been engaged in upholding guidelines that affect community practice. For example, in a study comparing treatment of patients admitted with congestive heart failure by hospitalists compared with that by nonhospitalists, hospitalists were found to be more likely to document left ventricular function, a core measure of quality as defined by JCAHO.39, 60 Knowledge about cardiac function can direct future care for patients when they return to the community and into the care of their primary care providers. In another example, Rifkin found that patients with community‐acquired pneumonia treated by hospitalists were more rapidly converted to oral antibiotics from intravenous antibiotics, facilitating a shorter length of stay,61 which reduced the opportunity for nosocomial infections to propagate. Because hospitalists are skilled at following guidelines,59 it follows that they should seize the opportunity to develop more of them.
As the hospitalist movement continues to grow, hospitalists will likely be engaged in implementing citywide, statewide, and even national policies that ensure optimal care of the hospitalized patient.
BARRIERS TO HOSPITALISTS FOCUSING ON PUBLIC HEALTH
Hospitalists are involved in public health activities even though they may not recognize the extent of this involvement. However, there may be some drawbacks to hospitalists viewing each patient encounter as an opportunity for a public health intervention. First, in viewing a patient as part of a cohort, the individual needs of the patient may be overlooked. There is inherent tension between population‐based and individual‐based care, which is a challenge. Second, hospitalists are busy clinicians who may be most highly valued because of their focus on efficiency and cost savings in the acute care setting. This factor alone may prevent substantive involvement by hospitalists in public health practice. Moving beyond the management of an acute illness may interfere with this efficiency and cost effectiveness from the hospital's perspective. However, interventions that promote health and prevent or reduce rehospitalizations may be cost effective to society in the long run. Third, current billing systems do not adequately reward or reimburse providers for the extra time that may be necessary to engage in public health practice. Fourth, hospitalists may not have the awareness, interest, training, or commitment to engage in public health practice. Finally, there may not be effective collaboration and communication systems between primary care providers and hospitalists. This barrier limits or hinders many possibilities for the effective execution of several public health initiatives.
CONCLUSIONS AND IMPLICATIONS
Hospitalists and the specialty of hospital medicine materialized because of myriad economic forces and the need to provide safe, high‐quality care to hospitalized patients. In this article we have described the ways in which hospitalists can be explicitly involved in public health practice. Traditionally, physicians caring for hospitalized patients have collected information through histories and physical examinations, interpreted laboratory data and tests, and formulated assessments and plans of care. To become public health practitioners, hospitalists have to go beyond these tasks and consider public health thought processes, such as problem‐solving paradigms and theories of behavior change. In adopting this public health perspective, hospitalists may begin to think of a patient in the context of the larger community in order to define the problems facing the community, not just the patient, determine the magnitude of such problems, identify key stakeholders, create intervention/prevention strategies, set priorities and recommend interventions, and implement and evaluate those interventions. This approach forces providers to move beyond the physicianpatient model and draw on public health models to invoke change. Hopefully, future research will further convince hospitalists of the benefits of this approach. Although it may be easier to defer care and management decisions to an outpatient physician, data suggest that intervening when patients are in the hospital may be most effective.62, 63 For example, is it possible that patients are more likely to quit smoking when they are sick in the hospital than when they are in their usual state of health on a routine visit at their primary care provider's office?64 Further, although deferring care to a primary care provider (PCP) may be easier, it is not always possible given these barriers: (1) some patients are routinely rehospitalized, precluding primary care visits, (2) some recommendations may not be received by PCPs, and (3) PCPpatient encounters are brief and the agendas full, and there are limited resources to address recommendations from the hospital.
As hospitalists become more involved in public health practice, their collaboration with physicians and researchers in other fields, nurses, policymakers, and administrators will expand. Succeeding in this arena requires integrity, motivation, capacity, understanding, knowledge, and experience.65 It is hoped that hospitalists will embrace the opportunity and master the requisite skill set necessary to practice in and advance this field. As hospitalist fellowship programs are developed, public health practice skills could be incorporated into the curriculum. Currently 6 of 16 fellowship programs offer either a master of public health degree or public health courses.66 Public health skills can also be taught at Society of Hospital Medicine meetings and other continuing medical education events.
With the evolution of hospital medicine, hospitalists have to be malleable in order to optimally meet the needs of the population they serve. The possibilities are endless.
- ,.The Hospitalist movement 5 years later.JAMA.2002;287:487–494.
- Hospitals and Health Networks. Hospitalists: a specialty coming into its own. Available at: http://www.hhmag.com. Accessed February 27,2006.
- ,,.Emergency medicine and public health: new steps in old directions.Ann Emerg Med.2001;38:675–683.
- ,, et al.A public health approach to emergency medicine: preparing for the twenty‐first century.Acad Emerg Med.1994;1:277–286.
- ,.Emergency medicine in population‐based systems of care.Ann Emerg Med.1997;30:800–803.
- ,.Principles of Public Health Practice.Albany, NY:Delmar Publishing;1997.
- Centers for Medicare and Medicaid. Health care spending and growth rate continue to decline in 2004. Available at: http://www.cms.hhs.gov. Accessed October 31,2006.
- ,,, et al.National health expenditures, 2002.Health Care Financ Rev. Summer2004;25:4.
- ,,, et al.Health spending Projections Through 2015: Changes on the Horizon.Health Affairs2006;25:w61–w73.
- Institute of Medicine.Recommendations from the Future of Public Health. InThe Future of the Public's Health.Washington, DC:National Academic Press;2003:411–420.
- ,,.Nosocomial pneumonia: state of the science.Am J Infect Control.2006;34:84–93.
- ,,,,.A reminder reduces urinary catheterization in hospitalized patients.Jt Comm J Qual Patient Saf.2005;31:455–462.
- ,,,.Preventing infections in nursing homes: A survey of infection control practices in southeast Michigan.Am J Infect Control.2005;33:489–492.
- ,,,.Prospective validation of a prediction model for isolating inpatients with suspected pulmonary tuberculosis.Arch Intern Med.2005;165:453–457.
- ,.The Hospital Infection Standardised Surveillance (HISS) programme: analysis of a two‐year pilot.J Hosp Infect.2003;53:259–267.
- ,,,.A Laboratory‐Based, Hospital‐Wide, Electronic Marker for Nosocomial Infection.Am J Clin Pathol.2006;125:34–39.
- ,,.Severe acute respiratory syndrome.Arch Pathol Lab Med.2004;128:1346–1350.
- ,,.Severe acute respiratory syndrome: responses of the healthcare system to a global epidemic.Curr Opin Otolaryngol Head Neck Surg.2005;13:161–164.
- ,.Pandemic influenza and the hospitalist: apocalypse when?J Hosp Med.2006;1:118–123.
- Center for Disease Control and Prevention. Pandemic Influenza information for Health Professionals. Available at: http://www.cdc.gov/flu/pandemic/. Accessed October 31,2006.
- .US health policy in the aftermath of Hurricane Katrina.JAMA.2006;295:437–40
- Levy B,Sidel V, eds.Terrorism and Public Health.New York:Oxford University Press;2003.
- Centers for Disease Control and Prevention (CDC).Public health response to Hurricanes Katrina and Rita—United States 2005.MMWR Morb Mortal Wkly Rep.2006;55:229–231.
- ,,,,,.Racial and ethnic disparities in health: a view from the South Bronx.J Health Care Poor Underserved.2006;17:116–127.
- ,.Williams E. Health Seeking behaviors of African Americans: implications for health administration.J Health Hum Serv Adm.2005;28(1):68–95.
- .Routine rapid HIV testing in hospitals: another opportunity for hospitalists to improve care.J Hosp Med.2006;1:106–112.
- Centers for Disease Control and Prevention.Advancing HIV prevention: new strategies for a changing epidemic—United States, 2003.MMWR Morb Mortal Wkly Rep.2003;52:329–332.
- ,,, et al.Expanded screening for HIV in the United States—an analysis of cost‐effectiveness.N Engl J Med.2005;352:586–595.
- ,,,.Identifying undiagnosed human immunodeficiency virus: the yield for routine, voluntary, inpatient testing.Arch Intern Med.2002;162:887–892.
- ,,,,.Insufficient treatment of hypercholestrolemia among patients hospitalized with chest pain.Clin Cardiol.2006;29:259–262.
- .Medical management of alcoholic patients. In:Kissen B,Besleiter H, eds.Treatment and Rehabilitation of the Chronic Alcoholic.New York:Plenum Publishing Co.;1997.
- ,,.An integral approach to substance abuse.J Psychoactive Drugs.2005;37:363–371.
- ,,, et al.Smoking cessation practice among Norwegian hospital physicians.Tiddskr Nor laegeforen.2000;120:1629–1632.
- ,, et al.Evaluation of an intervention for hospitalized African American smokers.Am J Health Behav.2005;29:228–239.
- .Falling through the cracks: challenges and opportunities for improving transitional care for persons with continuous complex care needs [review].J Am Geriatr Soc.2003;51:549–555.
- ,,,,.Advances in hospital medicine: a review of key articles from the literature.Med Clin N Am.2002;86:797–823.
- ,,,,,.Comprehensive discharge planning with post discharge support for older patients with congestive heart failure.JAMA.2004;291:1358–1367.
- ,.The impact of hospitalists on the cost and quality of inpatient care in the United States: a research synthesis.Med Care Res Rev.2005;62:379–406.
- ,,,,,Quality of care for patients hospitalized with heart failure: assessing the impact of hospitalists.Arch Intern Med.2002;162:1251–1256.
- ,,,.Educational interventions to improve antibiotic use in the community: report from the International Forum on Antibiotic Resistance (IFAR) colloquim, 2002.Lancet Infect Dis.2004;4:44–53.
- ,,, et al.Systematic review of antimicrobial drug prescribing in hospitals.Emerg Infect Dis.2006;12:211–216.
- ,,,,,,.Recommendations for management of community and hospital acquired pneumonia‐the hospitalist perspective.Curr Opin Pulm Med.2004;10(suppl 1):S23–S27.
- ,,,,.Advances in hospital medicine: a review of key articles from the literature.Med Clin N Am.2002;86:797–823.
- .End‐of‐life care for the hospitalized patient.Med Clin N Am.2002;86:749–770.
- ,.Palliative care for patients with heart failure.JAMA.2004;291:2476–2482.
- ,.Prevalence and structure of palliative care services in California hospitals.Arch Intern Med.2003;163:1084–1088.
- ,.Palliative care and the hospitalist: an opportunity for cross‐fertilization.J Med.2001;111:10S–14S.
- .Palliative care in hospitals.J Hosp Med.2006;1:21–28.
- ,.End‐of‐life care in a voluntary hospitalist model: effects on communication, process of care, and patient symptoms.Am J Med.2004;116:669–675.
- ,,,Understanding medical error and improving patient safety in the inpatient setting,Med Clin N Am2002;86:847–867.
- , The hospitalist movement: ten issues to consider, hospital practice. Available at: http://www.hosppract.com/issues/1999/02/wachter.htm. Accessed March 14,2006.
- Shojania KG,Duncan BW,McDonald KM,Wachter RM, eds.Making health care safer: a critical analysis of patient safety practices. Evidence Report/Technology Assessment No. 43 from the Agency for Healthcare Research and Quality: AHRQ Publication No. 01‐E058;2001. Available at: http://www.ahrq.gov/clinic/ptsafety/.
- ,,,.Safe but sound: patient safety meets evidence‐based medicine.JAMA.2002;288:508–513.
- ,,, et al.Role of pharmacist counseling in preventing adverse drug events after hospitalization.Arch Intern Med.2006;166:565–571.
- Hospitalists, pharmacists partner to cut errors: shorter lengths of stay, lower med costs result. HealthCare Benchmarks and Quality Improvement.American Health Consultants, Inc.,2005.
- ,,,.Hospitalists and the practice of inpatient medicine: results of a survey of the National Association of Inpatient Physicians.Ann Intern Med.1999;130:343–349.
- ,,,,.Core competencies in hospital medicine: Development and methodology.J Hosp Med.2006;1:48–56.
- National guideline clearing house. Available at: http://www.guideline.gov. Accessed June 26,2006.
- ,,,,, eds.The core competencies in hospital medicine.J Hosp Med.2006;1(suppl 1).
- Joint Commission on Accreditation of Healthcare Organizations. Core Measures overview. Available at: http://www.jcaho.org/perfeas/coremeas/cm.ovrvw.html. Accessed February 1,2006.
- ,,,.,Comparison of processes and outcomes of pneumonia care between hospitalists and community‐based primary care physicians.Mayo Clin Proc.2002;77:1053–1058.
- ,.The effectiveness of a nursing inpatient smoking cessation program in individuals with cardiovascular disease.Nurs Res.2005;54:243–254.
- ,,,,,.Evaluation of an intervention for hospitalized African American smokers.Am J Health Behav.2005;29:228–239.
- .Smoking cessation: the case for hospital‐based interventions.Prof Nurse.2003;19(3):145–148..
- . Dee Hock's management principles, in his own words. Fast Company.1996;5:79. Available at: http://www.fastcompany.com/magazine/05/dee2.html.
- ,,,.Hospital Medicine Fellowships: Works in progress.Am J Med.2006;119(1):72.e1–e7.
- ,.The Hospitalist movement 5 years later.JAMA.2002;287:487–494.
- Hospitals and Health Networks. Hospitalists: a specialty coming into its own. Available at: http://www.hhmag.com. Accessed February 27,2006.
- ,,.Emergency medicine and public health: new steps in old directions.Ann Emerg Med.2001;38:675–683.
- ,, et al.A public health approach to emergency medicine: preparing for the twenty‐first century.Acad Emerg Med.1994;1:277–286.
- ,.Emergency medicine in population‐based systems of care.Ann Emerg Med.1997;30:800–803.
- ,.Principles of Public Health Practice.Albany, NY:Delmar Publishing;1997.
- Centers for Medicare and Medicaid. Health care spending and growth rate continue to decline in 2004. Available at: http://www.cms.hhs.gov. Accessed October 31,2006.
- ,,, et al.National health expenditures, 2002.Health Care Financ Rev. Summer2004;25:4.
- ,,, et al.Health spending Projections Through 2015: Changes on the Horizon.Health Affairs2006;25:w61–w73.
- Institute of Medicine.Recommendations from the Future of Public Health. InThe Future of the Public's Health.Washington, DC:National Academic Press;2003:411–420.
- ,,.Nosocomial pneumonia: state of the science.Am J Infect Control.2006;34:84–93.
- ,,,,.A reminder reduces urinary catheterization in hospitalized patients.Jt Comm J Qual Patient Saf.2005;31:455–462.
- ,,,.Preventing infections in nursing homes: A survey of infection control practices in southeast Michigan.Am J Infect Control.2005;33:489–492.
- ,,,.Prospective validation of a prediction model for isolating inpatients with suspected pulmonary tuberculosis.Arch Intern Med.2005;165:453–457.
- ,.The Hospital Infection Standardised Surveillance (HISS) programme: analysis of a two‐year pilot.J Hosp Infect.2003;53:259–267.
- ,,,.A Laboratory‐Based, Hospital‐Wide, Electronic Marker for Nosocomial Infection.Am J Clin Pathol.2006;125:34–39.
- ,,.Severe acute respiratory syndrome.Arch Pathol Lab Med.2004;128:1346–1350.
- ,,.Severe acute respiratory syndrome: responses of the healthcare system to a global epidemic.Curr Opin Otolaryngol Head Neck Surg.2005;13:161–164.
- ,.Pandemic influenza and the hospitalist: apocalypse when?J Hosp Med.2006;1:118–123.
- Center for Disease Control and Prevention. Pandemic Influenza information for Health Professionals. Available at: http://www.cdc.gov/flu/pandemic/. Accessed October 31,2006.
- .US health policy in the aftermath of Hurricane Katrina.JAMA.2006;295:437–40
- Levy B,Sidel V, eds.Terrorism and Public Health.New York:Oxford University Press;2003.
- Centers for Disease Control and Prevention (CDC).Public health response to Hurricanes Katrina and Rita—United States 2005.MMWR Morb Mortal Wkly Rep.2006;55:229–231.
- ,,,,,.Racial and ethnic disparities in health: a view from the South Bronx.J Health Care Poor Underserved.2006;17:116–127.
- ,.Williams E. Health Seeking behaviors of African Americans: implications for health administration.J Health Hum Serv Adm.2005;28(1):68–95.
- .Routine rapid HIV testing in hospitals: another opportunity for hospitalists to improve care.J Hosp Med.2006;1:106–112.
- Centers for Disease Control and Prevention.Advancing HIV prevention: new strategies for a changing epidemic—United States, 2003.MMWR Morb Mortal Wkly Rep.2003;52:329–332.
- ,,, et al.Expanded screening for HIV in the United States—an analysis of cost‐effectiveness.N Engl J Med.2005;352:586–595.
- ,,,.Identifying undiagnosed human immunodeficiency virus: the yield for routine, voluntary, inpatient testing.Arch Intern Med.2002;162:887–892.
- ,,,,.Insufficient treatment of hypercholestrolemia among patients hospitalized with chest pain.Clin Cardiol.2006;29:259–262.
- .Medical management of alcoholic patients. In:Kissen B,Besleiter H, eds.Treatment and Rehabilitation of the Chronic Alcoholic.New York:Plenum Publishing Co.;1997.
- ,,.An integral approach to substance abuse.J Psychoactive Drugs.2005;37:363–371.
- ,,, et al.Smoking cessation practice among Norwegian hospital physicians.Tiddskr Nor laegeforen.2000;120:1629–1632.
- ,, et al.Evaluation of an intervention for hospitalized African American smokers.Am J Health Behav.2005;29:228–239.
- .Falling through the cracks: challenges and opportunities for improving transitional care for persons with continuous complex care needs [review].J Am Geriatr Soc.2003;51:549–555.
- ,,,,.Advances in hospital medicine: a review of key articles from the literature.Med Clin N Am.2002;86:797–823.
- ,,,,,.Comprehensive discharge planning with post discharge support for older patients with congestive heart failure.JAMA.2004;291:1358–1367.
- ,.The impact of hospitalists on the cost and quality of inpatient care in the United States: a research synthesis.Med Care Res Rev.2005;62:379–406.
- ,,,,,Quality of care for patients hospitalized with heart failure: assessing the impact of hospitalists.Arch Intern Med.2002;162:1251–1256.
- ,,,.Educational interventions to improve antibiotic use in the community: report from the International Forum on Antibiotic Resistance (IFAR) colloquim, 2002.Lancet Infect Dis.2004;4:44–53.
- ,,, et al.Systematic review of antimicrobial drug prescribing in hospitals.Emerg Infect Dis.2006;12:211–216.
- ,,,,,,.Recommendations for management of community and hospital acquired pneumonia‐the hospitalist perspective.Curr Opin Pulm Med.2004;10(suppl 1):S23–S27.
- ,,,,.Advances in hospital medicine: a review of key articles from the literature.Med Clin N Am.2002;86:797–823.
- .End‐of‐life care for the hospitalized patient.Med Clin N Am.2002;86:749–770.
- ,.Palliative care for patients with heart failure.JAMA.2004;291:2476–2482.
- ,.Prevalence and structure of palliative care services in California hospitals.Arch Intern Med.2003;163:1084–1088.
- ,.Palliative care and the hospitalist: an opportunity for cross‐fertilization.J Med.2001;111:10S–14S.
- .Palliative care in hospitals.J Hosp Med.2006;1:21–28.
- ,.End‐of‐life care in a voluntary hospitalist model: effects on communication, process of care, and patient symptoms.Am J Med.2004;116:669–675.
- ,,,Understanding medical error and improving patient safety in the inpatient setting,Med Clin N Am2002;86:847–867.
- , The hospitalist movement: ten issues to consider, hospital practice. Available at: http://www.hosppract.com/issues/1999/02/wachter.htm. Accessed March 14,2006.
- Shojania KG,Duncan BW,McDonald KM,Wachter RM, eds.Making health care safer: a critical analysis of patient safety practices. Evidence Report/Technology Assessment No. 43 from the Agency for Healthcare Research and Quality: AHRQ Publication No. 01‐E058;2001. Available at: http://www.ahrq.gov/clinic/ptsafety/.
- ,,,.Safe but sound: patient safety meets evidence‐based medicine.JAMA.2002;288:508–513.
- ,,, et al.Role of pharmacist counseling in preventing adverse drug events after hospitalization.Arch Intern Med.2006;166:565–571.
- Hospitalists, pharmacists partner to cut errors: shorter lengths of stay, lower med costs result. HealthCare Benchmarks and Quality Improvement.American Health Consultants, Inc.,2005.
- ,,,.Hospitalists and the practice of inpatient medicine: results of a survey of the National Association of Inpatient Physicians.Ann Intern Med.1999;130:343–349.
- ,,,,.Core competencies in hospital medicine: Development and methodology.J Hosp Med.2006;1:48–56.
- National guideline clearing house. Available at: http://www.guideline.gov. Accessed June 26,2006.
- ,,,,, eds.The core competencies in hospital medicine.J Hosp Med.2006;1(suppl 1).
- Joint Commission on Accreditation of Healthcare Organizations. Core Measures overview. Available at: http://www.jcaho.org/perfeas/coremeas/cm.ovrvw.html. Accessed February 1,2006.
- ,,,.,Comparison of processes and outcomes of pneumonia care between hospitalists and community‐based primary care physicians.Mayo Clin Proc.2002;77:1053–1058.
- ,.The effectiveness of a nursing inpatient smoking cessation program in individuals with cardiovascular disease.Nurs Res.2005;54:243–254.
- ,,,,,.Evaluation of an intervention for hospitalized African American smokers.Am J Health Behav.2005;29:228–239.
- .Smoking cessation: the case for hospital‐based interventions.Prof Nurse.2003;19(3):145–148..
- . Dee Hock's management principles, in his own words. Fast Company.1996;5:79. Available at: http://www.fastcompany.com/magazine/05/dee2.html.
- ,,,.Hospital Medicine Fellowships: Works in progress.Am J Med.2006;119(1):72.e1–e7.
Copyright © 2007 Society of Hospital Medicine
Hospital Reporting of Glomerular Filtration Rate
Chronic kidney disease is increasingly recognized as a significant public health issue, especially as our population ages. In the United States, it is estimated that 19.2 million individuals have chronic kidney disease (CKD), with an increasing prevalence in the elderly.1 CKD is associated with a higher mortality rate, as well as an increased risk of having several comorbidities, including anemia, coronary artery disease, and congestive heart failure.24 Early recognition, intervention, and management of patients with CKD by physicians has been shown to slow progression of disease and decrease complications.57 In the hospital setting, patients with CKD are at increased risk of medication dosing errors and acute renal failure (ARF).810
Serum creatinine is the most commonly used laboratory marker for assessing renal function. However, creatinine level is an imprecise measure of overall renal function, especially in older patients. The most recent National Kidney Foundation/ Kidney Disease Outcomes Quality Initiative (K/DOQI) guidelines recommend laboratory reporting of a calculated estimate of GFR.11 Equations used to calculate estimated GFR in adults, including the Cockcroft‐Gault (C‐G) equation, have been shown to provide an estimate of renal function, which can be used to clinically stratify varying levels of impaired renal function.11 Several studies have demonstrated that recognition of CKD by physicians is low in various clinical settings, especially in elderly patients.1215 Compliance with renal‐dose medication guidelines has also frequently been noted to be poor.16, 17
The investigators conducted a chart review study before and after reporting of estimated GFR to physicians in a hospital setting to assess the effect on physician recognition of CKD, the primary outcome. Secondary outcomes included the effect of reporting GFR on physician prescribing behaviors at the time of hospital discharge, including dosing of renal‐dosed antibiotics and use of nonsteroidal anti‐inflammatory (NSAID) and cyclooxygenase type 2 inhibitor (COX‐2) medications.
METHODS
This study was a retrospective chart review, with a prospective chart review as a comparison. Patients selected were admitted to a general medical floor in a 900‐bed academic medical center over the 2 years from 2002 to 2004. Computerized databases of laboratory values and weights obtained during hospitalization were used to select patients who fulfilled the following criteria: age > 65 years, all creatinine values during hospitalization < 1.6 mg/dL, and calculated estimated creatinine clearance (CrCl) < 60 mL/min using the Cockcroft‐Gault (C‐G) formula. The C‐G equation was developed for estimating CrCl and has also been extensively tested as a predictor of GFR. K/DOQI guidelines identify the C‐G equation as the most frequently used equation to estimate GFR in adults.11 To ensure steady‐state renal function, patients were excluded if creatinine varied by more than 0.4 mg/dL during their hospitalization. Based on an anticipated CKD recognition rate of 24%,13 our study sample size was selected to detect a 13% difference in the primary end point between the pre‐ and postintervention groups with 80% power. The study was approved by the institutional review board of the medical school.
Patient charts were reviewed with data obtained from the medical record, including physician notes, discharge summaries, orders, medication lists, and discharge prescriptions. Physician recognition was defined by documentation of CKD, calculated CrCl, or GFR in the physician notes or discharge summary. Charts were reviewed for diagnosis of hypertension (HTN) or diabetes (DM), and discharge medications including NSAID and COX‐2 medications and use and correct dosing of antibiotics requiring dose adjustment in patients with decreased GFR. Aspirin was not included as an NSAID.
For the prospective chart review portion, patients were selected at the time of admission on the basis of the same criteria. A notification was placed in the chart prominently listing the patient's estimated GFR calculated using the C‐G equation. Also included was a list of the stages of chronic renal disease based on the most recent K/DOQI guidelines11 and recommendations on dosing of select renal‐dosed antibiotics. Patients were again excluded if creatinine varied more than 0.4 mg/dL during their hospitalization.
Data Analysis
For statistical analysis, the association between recognition of CKD and the chart intervention, unadjusted for covariates, was evaluated using a contingency table. Additionally, the associations between recognition of CKD and other patient covariatessex, diabetes, hypertension, estimated GFRwere analyzed both individually and jointly. For individual covariate analysis, Fisher's exact test was used in all tests for association. For joint analysis, a set of relevant covariates was determined by stepwise logistic regression. The association of CKD recognition and the intervention was again analyzed using logistic regression while adjusting for this set of relevant covariates.
Finally, an analysis of appropriate medication prescribing at the time of hospital discharge was carried out to assess the effect of reporting estimated GFR. Prescription of NSAID or COX‐2 medications and correct dosing of renal‐dosed antibiotics at discharge were analyzed separately. As in the exploratory covariate analysis, Fisher's exact test for association was used.
RESULTS
Study Population
Characteristics of the study cohort are summarized in Table 1. The pre‐ and postintervention groups had 260 and 198 patients, respectively. Most were female. Average age, serum creatinine, and estimated GFR were similar in both groups.
| Characteristics | Preintervention | Postintervention |
|---|---|---|
| ||
| Total number | 260 | 198 |
| Age (years) | 81.1 6.6 | 82 6.8 |
| Sex (female) | 199 (76.5) | 168 (84.8) |
| Serum creatinine (mg/dL) | 0.98 0.2 | 0.9 0.2 |
| C‐G CrCl (mL/min) | 41.5 10.2 | 41.4 9.3 |
| DM | 58 (22.3) | 63 (31.8) |
| HTN | 190 (73.1) | 152 (76.7) |
| Physician recognition of CKD | 10 (3.9) | 25 (12.6) |
| NSAID or COX‐2 prescribed at discharge | 35 (13.5) | 21 (10.6) |
| Antibiotic requiring renal‐dose adjustment prescribed at discharge | 50 (19.2) | 29 (14.2) |
| Correct dosing of renal‐dosed antibiotic at discharge* | 28 (56.0) | 18 (62.1) |
Effect of Intervention on Recognition of CKD
Table 1 shows the number of patients recognized by physicians as having CKD in both groups. Prior to the study intervention, CKD was recognized in only 10 of 260 patients (3.9%), and following the intervention, rates increased to 25 of 198 patients (12.6%; P .001).
The results of the stepwise logistic regression of the covariates on CKD recognition showed that CKD recognition was modeled best with diabetes and lower estimated GFR. This corresponded well with the results of the individual covariate analyses. Thus, the primary outcome was again modeled by the intervention and the covariates diabetes and lower estimated GFR. With the addition of the covariates, the intervention was still a significant predictor of CKD recognition (P = .001), with an odds ratio of 4.07 (95% CI = (1.83,9.01)).
Effect of Intervention on Medication Prescribed at Hospital Discharge
Table 1 shows the number of patients discharged on NSAID/COX‐2 medications and renal‐dosed antibiotics in both the pre‐ and postintervention groups. Physicians prescribed NSAID/COX‐2 medications in 13.5% of patients preintervention and in 10.6% postintervention (P = .10). Overall, 12% of patients were discharged on a NSAID/COX‐2 medication. Reporting of estimated GFR did not have a significant effect on correct dosing of antibiotics at discharge (P = .81). Overall, 40% of renal‐dosed antibiotics were dosed incorrectly at the time of discharge.
DISCUSSION
This study has confirmed the findings of other investigators that significant CKD is underdiagnosed by physicians, especially in elderly patients with creatinine values within the normal laboratory range.13, 14 Investigators have demonstrated improved documentation of CKD with reporting of creatinine clearance and other simple educational interventions in an outpatient setting.13 In this study, reporting of estimated GFR did result in a significantly higher rate of recognition, but the overall rate was still very low in both groups (3.9%‐12.6%).
Although physician recognition of CKD did increase with the reporting of estimated GFR, this study found no significant impact on prescribing behaviors. Previous studies have shown an association between documentation of specific diagnoses and appropriate physician management.15, 18 However, the current data suggest that simply reporting GFR and increasing physician recognition of CKD may not lead to a significant decrease in medication dosing errors and that more extensive educational measures may be required.
Hospitalist physicians are increasingly serving as the primary caregivers for an aging population of hospitalized patients, and it is imperative that physicians recognize decreased GFR in elderly patients. Clearly, medication dosing errors are occurring in these patients, increasing the risk of adverse drug reactions.19 Elderly patients with renal impairment are also at increased risk of ARF while hospitalized.9, 10 Recognition of CKD by inpatient physicians identifies those patients who require preventive measures including maintenance of adequate hydration and avoidance of hypotension and nephrotoxic agents. Prevention of ARF in these patients has important clinical implications, as the mortality of patients is higher for elderly patients who develop hospital‐acquired ARF than for those presenting with community‐acquired ARF.20 Development of ARF has also been shown to increase length of hospitalization.21 Hospitalist physicians can also use the period of hospitalization as an opportunity to identify patients at risk of progressive CKD and in need of close follow‐up and possible referral to a nephrologist.
This study had several limitations. It was performed at a single institution, and therefore results may not be generalizable to all medical centers. The primary outcome of CKD documentation is an imperfect measure of recognition. The fact that chart documentation of CKD increased following the intervention suggests that documentation is associated with recognition, although it may be an underestimate. The effects of reporting estimated GFR on other secondary outcomes, including dosing of other medications, prevention of ARF, and length of hospital stay were not examined and deserve further investigation. The C‐G equation was chosen to calculate estimated GFR. There may be some advantage to using the Modification of Diet in Renal Disease equation as an alternative, but it is unclear if this is true in elderly female patients, who made up most of our study population.2225 Although using a prediction equation is clearly superior to using creatinine measurement solely to assess renal function in patients, further study is needed to identify the most accurate and effective formula for calculating estimated GFR in elderly patients.
The low rate of recognition of CKD by physicians found in this and other studies demonstrates the strong need for improvement in this area. Low recognition of CKD and a high rate of medication dosing errors despite reporting of the estimated GFR suggest that simply reporting GFR in addition to creatinine level is not sufficient. Further research is indicated to identify pragmatic educational tools and feedback mechanisms that effectively improve inpatient physician recognition of CKD and decrease medication dosing errors in elderly hospitalized patients.
Acknowledgements
The authors thank Christina Bennett for her assistance with data collection and Brian Waterman, MPH, of Waterman Research LLC, St. Louis, Missouri, for his assistance with statistical analyses.
- ,,,,.Prevalence of chronic kidney disease and decreased kidney function in the adult US population: Third National Health and Nutrition Examination Survey.Am J Kidney Dis.2003;41:1–12.
- ,,, et al.Anemia: an early complication of chronic renal insufficiencyAm J Kidney Dis.2001;38:803–812.
- ,,, et al.Mild renal insufficiency is associated with increased cardiovascular mortality: The Hoorn StudyKidney Int.2002;62:1402–1407.
- ,,,,.Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization.N Engl J Med.2004;351:1296–1305.
- ,,, et al.The timing of specialist evaluation in chronic kidney disease and mortalityAnn Intern Med.2002;137:479‐486.
- The effect of intensive treatment of diabetes on the development and progression of long‐term complications in insulin‐dependent diabetes mellitus.The Diabetes Control and Complications Trial Research Group.N Engl J Med.1993;329:977–986.
- ,,,,,.A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group.Ann Intern Med.1999;130:461–470.
- ,,.Calculation of the estimated creatinine clearance in avoiding drug dosing errors in the older patientAm J Med Sci.2001;322:133–136.
- ,.Acute renal failure in hospitalized patients: part I.Ann Pharmacother.2002;36:1261–1267.
- ,.Acute renal failure in hospitalized patients: part II.Ann Pharmacother.2002;36:1430–1442.
- .K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification.Am J Kid Dis.2002;39:S1–266.
- ,.An analysis of discharge drug prescribing amongst elderly patients with renal impairmentPostgrad Med J.1998;74:420–422.
- ,,, et al.Detection of chronic kidney disease with laboratory reporting of estimated glomerular filtration rate and an educational programArch Intern Med.2004;164:1788–1792.
- ,,,.Screening for renal disease using serum creatinine: who are we missing?Nephrol Dial Transplant.2001;16:1042–1046.
- ,,,.Early detection and treatment of renal disease in hospitalized diabetic and hypertensive patients: important differences between practice and published guidelines.Am J Kidney Dis.1997;29:368–375.
- ,,,.Compliance with dosing guidelines in patients with chronic kidney diseaseAnn Pharmacother.2004;38:853–858.
- ,,,,.Evaluation of dosage adjustment in patients with renal impairmentIntern Med J.2003;33:10–13.
- ,,.Current physician screening and treatment of hypercholesterolemic patients.Am J Med Sci.1993;306:124–128.
- ,,,,,.Concealed renal insufficiency and adverse drug reactions in elderly hospitalized patients.Arch Intern Med.2005;165:790–795.
- ,,,.Prognosis of ARF in hospitalized elderly patients.Am J Kidney Dis2004;44:410–409.
- ,.Epidemiology of acute renal failure: a prospective, multicenter, community‐based study. Madrid Acute Renal Failure Study Group.Kidney Int.1996;50:811–818.
- ,,,,.Performance of the modification of diet in renal disease and Cockcroft‐Gault equations in the estimation of GFR in health and in chronic kidney disease.J Am Soc Nephrol.2005;16:459–466.
- ,,,,.Predictive performance of the modification of diet in renal disease and Cockcroft‐Gault equations for estimating renal function.J Am Soc Nephrol.2005;16:763–773.
- ,,,,,.Can creatinine clearance be accurately predicted by formulae in octogenarian in‐patients?QJM.2004;97:281–287.
- ,,,,,.Estimation of glomerular filtration rate in older patients with chronic renal insufficiency: is the modification of diet in renal disease formula an improvement?J Am Geriatr Soc.2003;51:1012–1017.
Chronic kidney disease is increasingly recognized as a significant public health issue, especially as our population ages. In the United States, it is estimated that 19.2 million individuals have chronic kidney disease (CKD), with an increasing prevalence in the elderly.1 CKD is associated with a higher mortality rate, as well as an increased risk of having several comorbidities, including anemia, coronary artery disease, and congestive heart failure.24 Early recognition, intervention, and management of patients with CKD by physicians has been shown to slow progression of disease and decrease complications.57 In the hospital setting, patients with CKD are at increased risk of medication dosing errors and acute renal failure (ARF).810
Serum creatinine is the most commonly used laboratory marker for assessing renal function. However, creatinine level is an imprecise measure of overall renal function, especially in older patients. The most recent National Kidney Foundation/ Kidney Disease Outcomes Quality Initiative (K/DOQI) guidelines recommend laboratory reporting of a calculated estimate of GFR.11 Equations used to calculate estimated GFR in adults, including the Cockcroft‐Gault (C‐G) equation, have been shown to provide an estimate of renal function, which can be used to clinically stratify varying levels of impaired renal function.11 Several studies have demonstrated that recognition of CKD by physicians is low in various clinical settings, especially in elderly patients.1215 Compliance with renal‐dose medication guidelines has also frequently been noted to be poor.16, 17
The investigators conducted a chart review study before and after reporting of estimated GFR to physicians in a hospital setting to assess the effect on physician recognition of CKD, the primary outcome. Secondary outcomes included the effect of reporting GFR on physician prescribing behaviors at the time of hospital discharge, including dosing of renal‐dosed antibiotics and use of nonsteroidal anti‐inflammatory (NSAID) and cyclooxygenase type 2 inhibitor (COX‐2) medications.
METHODS
This study was a retrospective chart review, with a prospective chart review as a comparison. Patients selected were admitted to a general medical floor in a 900‐bed academic medical center over the 2 years from 2002 to 2004. Computerized databases of laboratory values and weights obtained during hospitalization were used to select patients who fulfilled the following criteria: age > 65 years, all creatinine values during hospitalization < 1.6 mg/dL, and calculated estimated creatinine clearance (CrCl) < 60 mL/min using the Cockcroft‐Gault (C‐G) formula. The C‐G equation was developed for estimating CrCl and has also been extensively tested as a predictor of GFR. K/DOQI guidelines identify the C‐G equation as the most frequently used equation to estimate GFR in adults.11 To ensure steady‐state renal function, patients were excluded if creatinine varied by more than 0.4 mg/dL during their hospitalization. Based on an anticipated CKD recognition rate of 24%,13 our study sample size was selected to detect a 13% difference in the primary end point between the pre‐ and postintervention groups with 80% power. The study was approved by the institutional review board of the medical school.
Patient charts were reviewed with data obtained from the medical record, including physician notes, discharge summaries, orders, medication lists, and discharge prescriptions. Physician recognition was defined by documentation of CKD, calculated CrCl, or GFR in the physician notes or discharge summary. Charts were reviewed for diagnosis of hypertension (HTN) or diabetes (DM), and discharge medications including NSAID and COX‐2 medications and use and correct dosing of antibiotics requiring dose adjustment in patients with decreased GFR. Aspirin was not included as an NSAID.
For the prospective chart review portion, patients were selected at the time of admission on the basis of the same criteria. A notification was placed in the chart prominently listing the patient's estimated GFR calculated using the C‐G equation. Also included was a list of the stages of chronic renal disease based on the most recent K/DOQI guidelines11 and recommendations on dosing of select renal‐dosed antibiotics. Patients were again excluded if creatinine varied more than 0.4 mg/dL during their hospitalization.
Data Analysis
For statistical analysis, the association between recognition of CKD and the chart intervention, unadjusted for covariates, was evaluated using a contingency table. Additionally, the associations between recognition of CKD and other patient covariatessex, diabetes, hypertension, estimated GFRwere analyzed both individually and jointly. For individual covariate analysis, Fisher's exact test was used in all tests for association. For joint analysis, a set of relevant covariates was determined by stepwise logistic regression. The association of CKD recognition and the intervention was again analyzed using logistic regression while adjusting for this set of relevant covariates.
Finally, an analysis of appropriate medication prescribing at the time of hospital discharge was carried out to assess the effect of reporting estimated GFR. Prescription of NSAID or COX‐2 medications and correct dosing of renal‐dosed antibiotics at discharge were analyzed separately. As in the exploratory covariate analysis, Fisher's exact test for association was used.
RESULTS
Study Population
Characteristics of the study cohort are summarized in Table 1. The pre‐ and postintervention groups had 260 and 198 patients, respectively. Most were female. Average age, serum creatinine, and estimated GFR were similar in both groups.
| Characteristics | Preintervention | Postintervention |
|---|---|---|
| ||
| Total number | 260 | 198 |
| Age (years) | 81.1 6.6 | 82 6.8 |
| Sex (female) | 199 (76.5) | 168 (84.8) |
| Serum creatinine (mg/dL) | 0.98 0.2 | 0.9 0.2 |
| C‐G CrCl (mL/min) | 41.5 10.2 | 41.4 9.3 |
| DM | 58 (22.3) | 63 (31.8) |
| HTN | 190 (73.1) | 152 (76.7) |
| Physician recognition of CKD | 10 (3.9) | 25 (12.6) |
| NSAID or COX‐2 prescribed at discharge | 35 (13.5) | 21 (10.6) |
| Antibiotic requiring renal‐dose adjustment prescribed at discharge | 50 (19.2) | 29 (14.2) |
| Correct dosing of renal‐dosed antibiotic at discharge* | 28 (56.0) | 18 (62.1) |
Effect of Intervention on Recognition of CKD
Table 1 shows the number of patients recognized by physicians as having CKD in both groups. Prior to the study intervention, CKD was recognized in only 10 of 260 patients (3.9%), and following the intervention, rates increased to 25 of 198 patients (12.6%; P .001).
The results of the stepwise logistic regression of the covariates on CKD recognition showed that CKD recognition was modeled best with diabetes and lower estimated GFR. This corresponded well with the results of the individual covariate analyses. Thus, the primary outcome was again modeled by the intervention and the covariates diabetes and lower estimated GFR. With the addition of the covariates, the intervention was still a significant predictor of CKD recognition (P = .001), with an odds ratio of 4.07 (95% CI = (1.83,9.01)).
Effect of Intervention on Medication Prescribed at Hospital Discharge
Table 1 shows the number of patients discharged on NSAID/COX‐2 medications and renal‐dosed antibiotics in both the pre‐ and postintervention groups. Physicians prescribed NSAID/COX‐2 medications in 13.5% of patients preintervention and in 10.6% postintervention (P = .10). Overall, 12% of patients were discharged on a NSAID/COX‐2 medication. Reporting of estimated GFR did not have a significant effect on correct dosing of antibiotics at discharge (P = .81). Overall, 40% of renal‐dosed antibiotics were dosed incorrectly at the time of discharge.
DISCUSSION
This study has confirmed the findings of other investigators that significant CKD is underdiagnosed by physicians, especially in elderly patients with creatinine values within the normal laboratory range.13, 14 Investigators have demonstrated improved documentation of CKD with reporting of creatinine clearance and other simple educational interventions in an outpatient setting.13 In this study, reporting of estimated GFR did result in a significantly higher rate of recognition, but the overall rate was still very low in both groups (3.9%‐12.6%).
Although physician recognition of CKD did increase with the reporting of estimated GFR, this study found no significant impact on prescribing behaviors. Previous studies have shown an association between documentation of specific diagnoses and appropriate physician management.15, 18 However, the current data suggest that simply reporting GFR and increasing physician recognition of CKD may not lead to a significant decrease in medication dosing errors and that more extensive educational measures may be required.
Hospitalist physicians are increasingly serving as the primary caregivers for an aging population of hospitalized patients, and it is imperative that physicians recognize decreased GFR in elderly patients. Clearly, medication dosing errors are occurring in these patients, increasing the risk of adverse drug reactions.19 Elderly patients with renal impairment are also at increased risk of ARF while hospitalized.9, 10 Recognition of CKD by inpatient physicians identifies those patients who require preventive measures including maintenance of adequate hydration and avoidance of hypotension and nephrotoxic agents. Prevention of ARF in these patients has important clinical implications, as the mortality of patients is higher for elderly patients who develop hospital‐acquired ARF than for those presenting with community‐acquired ARF.20 Development of ARF has also been shown to increase length of hospitalization.21 Hospitalist physicians can also use the period of hospitalization as an opportunity to identify patients at risk of progressive CKD and in need of close follow‐up and possible referral to a nephrologist.
This study had several limitations. It was performed at a single institution, and therefore results may not be generalizable to all medical centers. The primary outcome of CKD documentation is an imperfect measure of recognition. The fact that chart documentation of CKD increased following the intervention suggests that documentation is associated with recognition, although it may be an underestimate. The effects of reporting estimated GFR on other secondary outcomes, including dosing of other medications, prevention of ARF, and length of hospital stay were not examined and deserve further investigation. The C‐G equation was chosen to calculate estimated GFR. There may be some advantage to using the Modification of Diet in Renal Disease equation as an alternative, but it is unclear if this is true in elderly female patients, who made up most of our study population.2225 Although using a prediction equation is clearly superior to using creatinine measurement solely to assess renal function in patients, further study is needed to identify the most accurate and effective formula for calculating estimated GFR in elderly patients.
The low rate of recognition of CKD by physicians found in this and other studies demonstrates the strong need for improvement in this area. Low recognition of CKD and a high rate of medication dosing errors despite reporting of the estimated GFR suggest that simply reporting GFR in addition to creatinine level is not sufficient. Further research is indicated to identify pragmatic educational tools and feedback mechanisms that effectively improve inpatient physician recognition of CKD and decrease medication dosing errors in elderly hospitalized patients.
Acknowledgements
The authors thank Christina Bennett for her assistance with data collection and Brian Waterman, MPH, of Waterman Research LLC, St. Louis, Missouri, for his assistance with statistical analyses.
Chronic kidney disease is increasingly recognized as a significant public health issue, especially as our population ages. In the United States, it is estimated that 19.2 million individuals have chronic kidney disease (CKD), with an increasing prevalence in the elderly.1 CKD is associated with a higher mortality rate, as well as an increased risk of having several comorbidities, including anemia, coronary artery disease, and congestive heart failure.24 Early recognition, intervention, and management of patients with CKD by physicians has been shown to slow progression of disease and decrease complications.57 In the hospital setting, patients with CKD are at increased risk of medication dosing errors and acute renal failure (ARF).810
Serum creatinine is the most commonly used laboratory marker for assessing renal function. However, creatinine level is an imprecise measure of overall renal function, especially in older patients. The most recent National Kidney Foundation/ Kidney Disease Outcomes Quality Initiative (K/DOQI) guidelines recommend laboratory reporting of a calculated estimate of GFR.11 Equations used to calculate estimated GFR in adults, including the Cockcroft‐Gault (C‐G) equation, have been shown to provide an estimate of renal function, which can be used to clinically stratify varying levels of impaired renal function.11 Several studies have demonstrated that recognition of CKD by physicians is low in various clinical settings, especially in elderly patients.1215 Compliance with renal‐dose medication guidelines has also frequently been noted to be poor.16, 17
The investigators conducted a chart review study before and after reporting of estimated GFR to physicians in a hospital setting to assess the effect on physician recognition of CKD, the primary outcome. Secondary outcomes included the effect of reporting GFR on physician prescribing behaviors at the time of hospital discharge, including dosing of renal‐dosed antibiotics and use of nonsteroidal anti‐inflammatory (NSAID) and cyclooxygenase type 2 inhibitor (COX‐2) medications.
METHODS
This study was a retrospective chart review, with a prospective chart review as a comparison. Patients selected were admitted to a general medical floor in a 900‐bed academic medical center over the 2 years from 2002 to 2004. Computerized databases of laboratory values and weights obtained during hospitalization were used to select patients who fulfilled the following criteria: age > 65 years, all creatinine values during hospitalization < 1.6 mg/dL, and calculated estimated creatinine clearance (CrCl) < 60 mL/min using the Cockcroft‐Gault (C‐G) formula. The C‐G equation was developed for estimating CrCl and has also been extensively tested as a predictor of GFR. K/DOQI guidelines identify the C‐G equation as the most frequently used equation to estimate GFR in adults.11 To ensure steady‐state renal function, patients were excluded if creatinine varied by more than 0.4 mg/dL during their hospitalization. Based on an anticipated CKD recognition rate of 24%,13 our study sample size was selected to detect a 13% difference in the primary end point between the pre‐ and postintervention groups with 80% power. The study was approved by the institutional review board of the medical school.
Patient charts were reviewed with data obtained from the medical record, including physician notes, discharge summaries, orders, medication lists, and discharge prescriptions. Physician recognition was defined by documentation of CKD, calculated CrCl, or GFR in the physician notes or discharge summary. Charts were reviewed for diagnosis of hypertension (HTN) or diabetes (DM), and discharge medications including NSAID and COX‐2 medications and use and correct dosing of antibiotics requiring dose adjustment in patients with decreased GFR. Aspirin was not included as an NSAID.
For the prospective chart review portion, patients were selected at the time of admission on the basis of the same criteria. A notification was placed in the chart prominently listing the patient's estimated GFR calculated using the C‐G equation. Also included was a list of the stages of chronic renal disease based on the most recent K/DOQI guidelines11 and recommendations on dosing of select renal‐dosed antibiotics. Patients were again excluded if creatinine varied more than 0.4 mg/dL during their hospitalization.
Data Analysis
For statistical analysis, the association between recognition of CKD and the chart intervention, unadjusted for covariates, was evaluated using a contingency table. Additionally, the associations between recognition of CKD and other patient covariatessex, diabetes, hypertension, estimated GFRwere analyzed both individually and jointly. For individual covariate analysis, Fisher's exact test was used in all tests for association. For joint analysis, a set of relevant covariates was determined by stepwise logistic regression. The association of CKD recognition and the intervention was again analyzed using logistic regression while adjusting for this set of relevant covariates.
Finally, an analysis of appropriate medication prescribing at the time of hospital discharge was carried out to assess the effect of reporting estimated GFR. Prescription of NSAID or COX‐2 medications and correct dosing of renal‐dosed antibiotics at discharge were analyzed separately. As in the exploratory covariate analysis, Fisher's exact test for association was used.
RESULTS
Study Population
Characteristics of the study cohort are summarized in Table 1. The pre‐ and postintervention groups had 260 and 198 patients, respectively. Most were female. Average age, serum creatinine, and estimated GFR were similar in both groups.
| Characteristics | Preintervention | Postintervention |
|---|---|---|
| ||
| Total number | 260 | 198 |
| Age (years) | 81.1 6.6 | 82 6.8 |
| Sex (female) | 199 (76.5) | 168 (84.8) |
| Serum creatinine (mg/dL) | 0.98 0.2 | 0.9 0.2 |
| C‐G CrCl (mL/min) | 41.5 10.2 | 41.4 9.3 |
| DM | 58 (22.3) | 63 (31.8) |
| HTN | 190 (73.1) | 152 (76.7) |
| Physician recognition of CKD | 10 (3.9) | 25 (12.6) |
| NSAID or COX‐2 prescribed at discharge | 35 (13.5) | 21 (10.6) |
| Antibiotic requiring renal‐dose adjustment prescribed at discharge | 50 (19.2) | 29 (14.2) |
| Correct dosing of renal‐dosed antibiotic at discharge* | 28 (56.0) | 18 (62.1) |
Effect of Intervention on Recognition of CKD
Table 1 shows the number of patients recognized by physicians as having CKD in both groups. Prior to the study intervention, CKD was recognized in only 10 of 260 patients (3.9%), and following the intervention, rates increased to 25 of 198 patients (12.6%; P .001).
The results of the stepwise logistic regression of the covariates on CKD recognition showed that CKD recognition was modeled best with diabetes and lower estimated GFR. This corresponded well with the results of the individual covariate analyses. Thus, the primary outcome was again modeled by the intervention and the covariates diabetes and lower estimated GFR. With the addition of the covariates, the intervention was still a significant predictor of CKD recognition (P = .001), with an odds ratio of 4.07 (95% CI = (1.83,9.01)).
Effect of Intervention on Medication Prescribed at Hospital Discharge
Table 1 shows the number of patients discharged on NSAID/COX‐2 medications and renal‐dosed antibiotics in both the pre‐ and postintervention groups. Physicians prescribed NSAID/COX‐2 medications in 13.5% of patients preintervention and in 10.6% postintervention (P = .10). Overall, 12% of patients were discharged on a NSAID/COX‐2 medication. Reporting of estimated GFR did not have a significant effect on correct dosing of antibiotics at discharge (P = .81). Overall, 40% of renal‐dosed antibiotics were dosed incorrectly at the time of discharge.
DISCUSSION
This study has confirmed the findings of other investigators that significant CKD is underdiagnosed by physicians, especially in elderly patients with creatinine values within the normal laboratory range.13, 14 Investigators have demonstrated improved documentation of CKD with reporting of creatinine clearance and other simple educational interventions in an outpatient setting.13 In this study, reporting of estimated GFR did result in a significantly higher rate of recognition, but the overall rate was still very low in both groups (3.9%‐12.6%).
Although physician recognition of CKD did increase with the reporting of estimated GFR, this study found no significant impact on prescribing behaviors. Previous studies have shown an association between documentation of specific diagnoses and appropriate physician management.15, 18 However, the current data suggest that simply reporting GFR and increasing physician recognition of CKD may not lead to a significant decrease in medication dosing errors and that more extensive educational measures may be required.
Hospitalist physicians are increasingly serving as the primary caregivers for an aging population of hospitalized patients, and it is imperative that physicians recognize decreased GFR in elderly patients. Clearly, medication dosing errors are occurring in these patients, increasing the risk of adverse drug reactions.19 Elderly patients with renal impairment are also at increased risk of ARF while hospitalized.9, 10 Recognition of CKD by inpatient physicians identifies those patients who require preventive measures including maintenance of adequate hydration and avoidance of hypotension and nephrotoxic agents. Prevention of ARF in these patients has important clinical implications, as the mortality of patients is higher for elderly patients who develop hospital‐acquired ARF than for those presenting with community‐acquired ARF.20 Development of ARF has also been shown to increase length of hospitalization.21 Hospitalist physicians can also use the period of hospitalization as an opportunity to identify patients at risk of progressive CKD and in need of close follow‐up and possible referral to a nephrologist.
This study had several limitations. It was performed at a single institution, and therefore results may not be generalizable to all medical centers. The primary outcome of CKD documentation is an imperfect measure of recognition. The fact that chart documentation of CKD increased following the intervention suggests that documentation is associated with recognition, although it may be an underestimate. The effects of reporting estimated GFR on other secondary outcomes, including dosing of other medications, prevention of ARF, and length of hospital stay were not examined and deserve further investigation. The C‐G equation was chosen to calculate estimated GFR. There may be some advantage to using the Modification of Diet in Renal Disease equation as an alternative, but it is unclear if this is true in elderly female patients, who made up most of our study population.2225 Although using a prediction equation is clearly superior to using creatinine measurement solely to assess renal function in patients, further study is needed to identify the most accurate and effective formula for calculating estimated GFR in elderly patients.
The low rate of recognition of CKD by physicians found in this and other studies demonstrates the strong need for improvement in this area. Low recognition of CKD and a high rate of medication dosing errors despite reporting of the estimated GFR suggest that simply reporting GFR in addition to creatinine level is not sufficient. Further research is indicated to identify pragmatic educational tools and feedback mechanisms that effectively improve inpatient physician recognition of CKD and decrease medication dosing errors in elderly hospitalized patients.
Acknowledgements
The authors thank Christina Bennett for her assistance with data collection and Brian Waterman, MPH, of Waterman Research LLC, St. Louis, Missouri, for his assistance with statistical analyses.
- ,,,,.Prevalence of chronic kidney disease and decreased kidney function in the adult US population: Third National Health and Nutrition Examination Survey.Am J Kidney Dis.2003;41:1–12.
- ,,, et al.Anemia: an early complication of chronic renal insufficiencyAm J Kidney Dis.2001;38:803–812.
- ,,, et al.Mild renal insufficiency is associated with increased cardiovascular mortality: The Hoorn StudyKidney Int.2002;62:1402–1407.
- ,,,,.Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization.N Engl J Med.2004;351:1296–1305.
- ,,, et al.The timing of specialist evaluation in chronic kidney disease and mortalityAnn Intern Med.2002;137:479‐486.
- The effect of intensive treatment of diabetes on the development and progression of long‐term complications in insulin‐dependent diabetes mellitus.The Diabetes Control and Complications Trial Research Group.N Engl J Med.1993;329:977–986.
- ,,,,,.A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group.Ann Intern Med.1999;130:461–470.
- ,,.Calculation of the estimated creatinine clearance in avoiding drug dosing errors in the older patientAm J Med Sci.2001;322:133–136.
- ,.Acute renal failure in hospitalized patients: part I.Ann Pharmacother.2002;36:1261–1267.
- ,.Acute renal failure in hospitalized patients: part II.Ann Pharmacother.2002;36:1430–1442.
- .K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification.Am J Kid Dis.2002;39:S1–266.
- ,.An analysis of discharge drug prescribing amongst elderly patients with renal impairmentPostgrad Med J.1998;74:420–422.
- ,,, et al.Detection of chronic kidney disease with laboratory reporting of estimated glomerular filtration rate and an educational programArch Intern Med.2004;164:1788–1792.
- ,,,.Screening for renal disease using serum creatinine: who are we missing?Nephrol Dial Transplant.2001;16:1042–1046.
- ,,,.Early detection and treatment of renal disease in hospitalized diabetic and hypertensive patients: important differences between practice and published guidelines.Am J Kidney Dis.1997;29:368–375.
- ,,,.Compliance with dosing guidelines in patients with chronic kidney diseaseAnn Pharmacother.2004;38:853–858.
- ,,,,.Evaluation of dosage adjustment in patients with renal impairmentIntern Med J.2003;33:10–13.
- ,,.Current physician screening and treatment of hypercholesterolemic patients.Am J Med Sci.1993;306:124–128.
- ,,,,,.Concealed renal insufficiency and adverse drug reactions in elderly hospitalized patients.Arch Intern Med.2005;165:790–795.
- ,,,.Prognosis of ARF in hospitalized elderly patients.Am J Kidney Dis2004;44:410–409.
- ,.Epidemiology of acute renal failure: a prospective, multicenter, community‐based study. Madrid Acute Renal Failure Study Group.Kidney Int.1996;50:811–818.
- ,,,,.Performance of the modification of diet in renal disease and Cockcroft‐Gault equations in the estimation of GFR in health and in chronic kidney disease.J Am Soc Nephrol.2005;16:459–466.
- ,,,,.Predictive performance of the modification of diet in renal disease and Cockcroft‐Gault equations for estimating renal function.J Am Soc Nephrol.2005;16:763–773.
- ,,,,,.Can creatinine clearance be accurately predicted by formulae in octogenarian in‐patients?QJM.2004;97:281–287.
- ,,,,,.Estimation of glomerular filtration rate in older patients with chronic renal insufficiency: is the modification of diet in renal disease formula an improvement?J Am Geriatr Soc.2003;51:1012–1017.
- ,,,,.Prevalence of chronic kidney disease and decreased kidney function in the adult US population: Third National Health and Nutrition Examination Survey.Am J Kidney Dis.2003;41:1–12.
- ,,, et al.Anemia: an early complication of chronic renal insufficiencyAm J Kidney Dis.2001;38:803–812.
- ,,, et al.Mild renal insufficiency is associated with increased cardiovascular mortality: The Hoorn StudyKidney Int.2002;62:1402–1407.
- ,,,,.Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization.N Engl J Med.2004;351:1296–1305.
- ,,, et al.The timing of specialist evaluation in chronic kidney disease and mortalityAnn Intern Med.2002;137:479‐486.
- The effect of intensive treatment of diabetes on the development and progression of long‐term complications in insulin‐dependent diabetes mellitus.The Diabetes Control and Complications Trial Research Group.N Engl J Med.1993;329:977–986.
- ,,,,,.A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group.Ann Intern Med.1999;130:461–470.
- ,,.Calculation of the estimated creatinine clearance in avoiding drug dosing errors in the older patientAm J Med Sci.2001;322:133–136.
- ,.Acute renal failure in hospitalized patients: part I.Ann Pharmacother.2002;36:1261–1267.
- ,.Acute renal failure in hospitalized patients: part II.Ann Pharmacother.2002;36:1430–1442.
- .K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification.Am J Kid Dis.2002;39:S1–266.
- ,.An analysis of discharge drug prescribing amongst elderly patients with renal impairmentPostgrad Med J.1998;74:420–422.
- ,,, et al.Detection of chronic kidney disease with laboratory reporting of estimated glomerular filtration rate and an educational programArch Intern Med.2004;164:1788–1792.
- ,,,.Screening for renal disease using serum creatinine: who are we missing?Nephrol Dial Transplant.2001;16:1042–1046.
- ,,,.Early detection and treatment of renal disease in hospitalized diabetic and hypertensive patients: important differences between practice and published guidelines.Am J Kidney Dis.1997;29:368–375.
- ,,,.Compliance with dosing guidelines in patients with chronic kidney diseaseAnn Pharmacother.2004;38:853–858.
- ,,,,.Evaluation of dosage adjustment in patients with renal impairmentIntern Med J.2003;33:10–13.
- ,,.Current physician screening and treatment of hypercholesterolemic patients.Am J Med Sci.1993;306:124–128.
- ,,,,,.Concealed renal insufficiency and adverse drug reactions in elderly hospitalized patients.Arch Intern Med.2005;165:790–795.
- ,,,.Prognosis of ARF in hospitalized elderly patients.Am J Kidney Dis2004;44:410–409.
- ,.Epidemiology of acute renal failure: a prospective, multicenter, community‐based study. Madrid Acute Renal Failure Study Group.Kidney Int.1996;50:811–818.
- ,,,,.Performance of the modification of diet in renal disease and Cockcroft‐Gault equations in the estimation of GFR in health and in chronic kidney disease.J Am Soc Nephrol.2005;16:459–466.
- ,,,,.Predictive performance of the modification of diet in renal disease and Cockcroft‐Gault equations for estimating renal function.J Am Soc Nephrol.2005;16:763–773.
- ,,,,,.Can creatinine clearance be accurately predicted by formulae in octogenarian in‐patients?QJM.2004;97:281–287.
- ,,,,,.Estimation of glomerular filtration rate in older patients with chronic renal insufficiency: is the modification of diet in renal disease formula an improvement?J Am Geriatr Soc.2003;51:1012–1017.
Copyright © 2007 Society of Hospital Medicine
Dr. Smith Goes to Washington
Representing hospital medicine on Capitol Hill is an opportunity for physicians to educate policy makers and have a hand in the legislative process. Hospitalists who have had such experiences say that it can also be nerve wracking, eye opening, surprising, and very satisfying.
Rifkin Answers a Call to Arms
William Rifkin, MD, assistant professor of medicine and associate director of the Yale Primary Care Residency Program at the Yale University School of Medicine, New Haven, Conn., started his road to Capitol Hill by answering an SHM call to arms. “SHM was looking for someone in Connecticut to appear before the state Health Committee regarding proposed legislation that sought to regulate communications between hospitalists and primary care physicians,” says Dr. Rifkin, who was also asked to address the issue of whether the use of hospitalists should be mandatory or voluntary.
The call originally came from the Connecticut Medical Society, which—along with SHM—helped prepare Dr. Rifkin for his testimony. These groups’ public policy staffs coached him about his audience, the hearing process, and the key issues. They agreed his approach would be educational and informative in nature. He would explain what “hospital medicine is, the advantages and disadvantages, what is happening now in the field, and the issues being addressed by SHM,” he recalls. “I gave the committee members lots of literature and background information.”
Dr. Rifkin had some challenges to overcome in getting his message across to the legislators. “There is no data showing that lack of communication between hospitalists and community physicians has caused serious problems,” he explains. “I had to explain the difference between factual data and anecdotal information.”
Additionally, he recalls, one legislator seemed skeptical about the hospitalist’s role and kept referring to a health system that mandates the use of hospitalists. “I had to sit back and explain how SHM supports a voluntary model,” he says. “She kept talking about a reported example of a hospital that forced patients to use hospitalists. It was awkward because I suspected that the story was untrue or at least was missing some facts.”
By calmly relating SHM’s position and its reasons for preferring a voluntary model, Dr. Rifkin believes he was able to diffuse some of the tension. After the hearing, he approached the legislator and handed her the literature and statements from SHM. “I offered to stay in touch and suggested we find out about this hospital supposedly mandating hospitalists,” he says “Ultimately, I discovered that this story wasn’t true.”
Making a good impression can help legislators see physicians as colleagues rather than adversaries. “After the hearing, the committee sent the communications bill to the Connecticut Department of Public Health Best Practices Committee. This body was charged with making recommendations on communications best practices,” says Dr. Rifkin, who was asked to talk before this group. In fact, he adds, “They’ve asked me back repeatedly. I’m sort of a regular on the committee now.”
He is honored to have input and to present the hospitalist’s point of view. “Their recommendations likely will be similar to what SHM says regarding inpatient/outpatient communication,” he explains.
In retrospect, Dr. Rifkin believes he made a difference. “I felt as if I brought them some new information and taught legislators some things about hospital medicine they didn’t know before,” he says. “I think I helped them see that it would be counterproductive to dictate the specifics of inpatient/outpatient physician communication.”
When it comes to presenting testimony, Dr. Rifkin suggests, “it is best to acknowledge where legislators are right and use this as an opportunity for education. You don’t want to come across as dogmatic.”
While Dr. Rifkin enjoyed his experience, it was not without some surprises. He explains, “I left shaking my head and marveling, ‘Is this really how laws are made?’ ” He was surprised “about the lack of knowledge about the issues and the willingness to act on anecdotal information.”
Reporting back to SHM, Dr. Rifkin says, “It was good that I was there because—absent that—we could have ended up with some onerous rule that we then would have to undo.”
Another surprise for Dr. Rifkin was how long and tedious the process could be. “I was one of the last speakers on the agenda, and I did lots of waiting,” he states, adding, “If I had been nervous, it would have been a torturous eight hours.” Once he was in front of the microphone, Dr. Rifkin had just a few minutes to get his points across. He then answered questions for several additional minutes. “I had to watch the clock, and it was a little nerve-wracking to try to say everything I wanted to in a short time. But for the most part, it was actually enjoyable,” he offers.
Being active in advocacy efforts is a valuable, satisfying experience, and Dr. Rifkin urges his colleagues to carry the gauntlet. “We need to watch for opportunities to have input on legislation nationally and statewide. Hopefully, we’ll be able to have the same impact we had in Connecticut in other states as well,” he says. “Physicians need to be willing to get involved.”
—William Rifkin, MD, assistant professor of medicine and associate director, Yale Primary Care Residency Program, Yale University School of Medicine, New Haven, Conn.
Feinbloom: Testimony on the Fly
David Feinbloom, MD, a hospitalist at Beth Israel Deaconess Medical Center in Boston, had only two hours to prepare for his testimony about computerized physician order entry before the Massachusetts State Joint Committees on Health Care Financing and Economic Development and Emerging Technologies. “They wanted a clinician to explain how this system would improve quality and result in cost-saving,” he says.
Despite his lack of preparation time, he was familiar enough with the subject to speak in detail and answer questions. “I was a little nervous,” he admits, adding, “I would have preferred to have time to prepare a formal presentation, especially since I ended up having to write something up afterward for the official records.”
Dr. Feinbloom was one of the last speakers, and this had some disadvantages. First of all, he had to wait for hours. Additionally, “Many of the points I wanted to make already had been addressed. I didn’t get a lot of questions because there wasn’t much left to ask,” he explains.
The biggest surprise for Dr. Feinbloom was that the legislative process “is a little more mundane than I expected. It’s not like when you watch the news, and they have rousing, heated discussions.”
Also surprising was how receptive the committee members were about the issue. “Because part of the funding was coming from Blue Cross/Blue Shield, there wasn’t even any real controversy or debate from a budgetary standpoint,” he says. “There also was a big study showing that the system will pay for itself.”
Like Dr. Rifkin, Dr. Feinbloom believes his testimony had a positive effect. “I think that I brought to bear a realistic, ground-level view. I also brought some clinical examples of where this system is powerful, and I don’t think people realized this,” he says. “One of the senators had diabetes and told me he was surprised about how messy drug delivery in the hospital can be and how computerized systems can help. My examples stuck in his head as something he could relate to.”
Seymann: Another Kind of Testimony
Hospitalists don’t have to present testimony before a governmental body to have a positive effect on legislation and make a strong impression on lawmakers. Ask Gregory Seymann, MD, associate clinical professor, Division of Hospital Medicine, Department of Medicine, University of California San Diego School of Medicine. While in Washington, D.C., for the 2006 SHM Annual Meeting, he visited his House and Senate representatives.
“Our goal was to educate lawmakers about hospitalists—who we are and what we do—not to ask for favors or handouts,” explains Dr. Seymann. “Several of us went as a group to our senator’s office, and it was a rather short visit. We met with a staff person, who listened briefly and took our materials but asked few questions.”
When he went alone to his House representative, Susan Davis’ (D-CA) office, Dr. Seymann had a much different experience. The representative’s staff was extremely welcoming. “They told me that she was still in session marking up a bill, but that she really wanted to meet me,” he recalls. “They asked me if I could wait; and eventually they took me over to another building to meet her.”
Dr. Seymann’s House representative met with him for half an hour. “She was very pleasant, and I felt comfortable talking with her. I just gave her the basics of who we [hospitalists] are and what we do. She admitted that she didn’t know much about hospitalists and seemed interested in what I had to say,” he says. Davis asked several questions, Dr. Seymann notes. “She mostly wanted to know about how our practice differs from general internists and the difference between hospital and outpatient-based medicine,” he recalls, adding, “I felt like she heard me. The meeting exceeded my expectations.”
The difference between the two visits was striking. Dr. Seymann explains that it is important to realize that “you never know when something you say will make a difference or have an impact. You have to try and, sometimes, keep trying.”
Follow-up is important for these visits. “I sent e-mails on returning home to thank them for their time and remind them that I would be happy to help on hospital medicine issues in the future,” he says.
While Dr. Seymann believes he helped educate legislators about hospital medicine and the hospitalist’s role, he also learned something himself. “I realized that one person can effectively engage in the legislative process and that Congress is interested in what we have to say,” he says. Additionally, he observes, “They take the input of their constituents pretty seriously, and we have a role to play in ensuring that our voices are heard on issues that affect our patients and our profession.” TH
Joanne Kaldy is based in Maryland.
Representing hospital medicine on Capitol Hill is an opportunity for physicians to educate policy makers and have a hand in the legislative process. Hospitalists who have had such experiences say that it can also be nerve wracking, eye opening, surprising, and very satisfying.
Rifkin Answers a Call to Arms
William Rifkin, MD, assistant professor of medicine and associate director of the Yale Primary Care Residency Program at the Yale University School of Medicine, New Haven, Conn., started his road to Capitol Hill by answering an SHM call to arms. “SHM was looking for someone in Connecticut to appear before the state Health Committee regarding proposed legislation that sought to regulate communications between hospitalists and primary care physicians,” says Dr. Rifkin, who was also asked to address the issue of whether the use of hospitalists should be mandatory or voluntary.
The call originally came from the Connecticut Medical Society, which—along with SHM—helped prepare Dr. Rifkin for his testimony. These groups’ public policy staffs coached him about his audience, the hearing process, and the key issues. They agreed his approach would be educational and informative in nature. He would explain what “hospital medicine is, the advantages and disadvantages, what is happening now in the field, and the issues being addressed by SHM,” he recalls. “I gave the committee members lots of literature and background information.”
Dr. Rifkin had some challenges to overcome in getting his message across to the legislators. “There is no data showing that lack of communication between hospitalists and community physicians has caused serious problems,” he explains. “I had to explain the difference between factual data and anecdotal information.”
Additionally, he recalls, one legislator seemed skeptical about the hospitalist’s role and kept referring to a health system that mandates the use of hospitalists. “I had to sit back and explain how SHM supports a voluntary model,” he says. “She kept talking about a reported example of a hospital that forced patients to use hospitalists. It was awkward because I suspected that the story was untrue or at least was missing some facts.”
By calmly relating SHM’s position and its reasons for preferring a voluntary model, Dr. Rifkin believes he was able to diffuse some of the tension. After the hearing, he approached the legislator and handed her the literature and statements from SHM. “I offered to stay in touch and suggested we find out about this hospital supposedly mandating hospitalists,” he says “Ultimately, I discovered that this story wasn’t true.”
Making a good impression can help legislators see physicians as colleagues rather than adversaries. “After the hearing, the committee sent the communications bill to the Connecticut Department of Public Health Best Practices Committee. This body was charged with making recommendations on communications best practices,” says Dr. Rifkin, who was asked to talk before this group. In fact, he adds, “They’ve asked me back repeatedly. I’m sort of a regular on the committee now.”
He is honored to have input and to present the hospitalist’s point of view. “Their recommendations likely will be similar to what SHM says regarding inpatient/outpatient communication,” he explains.
In retrospect, Dr. Rifkin believes he made a difference. “I felt as if I brought them some new information and taught legislators some things about hospital medicine they didn’t know before,” he says. “I think I helped them see that it would be counterproductive to dictate the specifics of inpatient/outpatient physician communication.”
When it comes to presenting testimony, Dr. Rifkin suggests, “it is best to acknowledge where legislators are right and use this as an opportunity for education. You don’t want to come across as dogmatic.”
While Dr. Rifkin enjoyed his experience, it was not without some surprises. He explains, “I left shaking my head and marveling, ‘Is this really how laws are made?’ ” He was surprised “about the lack of knowledge about the issues and the willingness to act on anecdotal information.”
Reporting back to SHM, Dr. Rifkin says, “It was good that I was there because—absent that—we could have ended up with some onerous rule that we then would have to undo.”
Another surprise for Dr. Rifkin was how long and tedious the process could be. “I was one of the last speakers on the agenda, and I did lots of waiting,” he states, adding, “If I had been nervous, it would have been a torturous eight hours.” Once he was in front of the microphone, Dr. Rifkin had just a few minutes to get his points across. He then answered questions for several additional minutes. “I had to watch the clock, and it was a little nerve-wracking to try to say everything I wanted to in a short time. But for the most part, it was actually enjoyable,” he offers.
Being active in advocacy efforts is a valuable, satisfying experience, and Dr. Rifkin urges his colleagues to carry the gauntlet. “We need to watch for opportunities to have input on legislation nationally and statewide. Hopefully, we’ll be able to have the same impact we had in Connecticut in other states as well,” he says. “Physicians need to be willing to get involved.”
—William Rifkin, MD, assistant professor of medicine and associate director, Yale Primary Care Residency Program, Yale University School of Medicine, New Haven, Conn.
Feinbloom: Testimony on the Fly
David Feinbloom, MD, a hospitalist at Beth Israel Deaconess Medical Center in Boston, had only two hours to prepare for his testimony about computerized physician order entry before the Massachusetts State Joint Committees on Health Care Financing and Economic Development and Emerging Technologies. “They wanted a clinician to explain how this system would improve quality and result in cost-saving,” he says.
Despite his lack of preparation time, he was familiar enough with the subject to speak in detail and answer questions. “I was a little nervous,” he admits, adding, “I would have preferred to have time to prepare a formal presentation, especially since I ended up having to write something up afterward for the official records.”
Dr. Feinbloom was one of the last speakers, and this had some disadvantages. First of all, he had to wait for hours. Additionally, “Many of the points I wanted to make already had been addressed. I didn’t get a lot of questions because there wasn’t much left to ask,” he explains.
The biggest surprise for Dr. Feinbloom was that the legislative process “is a little more mundane than I expected. It’s not like when you watch the news, and they have rousing, heated discussions.”
Also surprising was how receptive the committee members were about the issue. “Because part of the funding was coming from Blue Cross/Blue Shield, there wasn’t even any real controversy or debate from a budgetary standpoint,” he says. “There also was a big study showing that the system will pay for itself.”
Like Dr. Rifkin, Dr. Feinbloom believes his testimony had a positive effect. “I think that I brought to bear a realistic, ground-level view. I also brought some clinical examples of where this system is powerful, and I don’t think people realized this,” he says. “One of the senators had diabetes and told me he was surprised about how messy drug delivery in the hospital can be and how computerized systems can help. My examples stuck in his head as something he could relate to.”
Seymann: Another Kind of Testimony
Hospitalists don’t have to present testimony before a governmental body to have a positive effect on legislation and make a strong impression on lawmakers. Ask Gregory Seymann, MD, associate clinical professor, Division of Hospital Medicine, Department of Medicine, University of California San Diego School of Medicine. While in Washington, D.C., for the 2006 SHM Annual Meeting, he visited his House and Senate representatives.
“Our goal was to educate lawmakers about hospitalists—who we are and what we do—not to ask for favors or handouts,” explains Dr. Seymann. “Several of us went as a group to our senator’s office, and it was a rather short visit. We met with a staff person, who listened briefly and took our materials but asked few questions.”
When he went alone to his House representative, Susan Davis’ (D-CA) office, Dr. Seymann had a much different experience. The representative’s staff was extremely welcoming. “They told me that she was still in session marking up a bill, but that she really wanted to meet me,” he recalls. “They asked me if I could wait; and eventually they took me over to another building to meet her.”
Dr. Seymann’s House representative met with him for half an hour. “She was very pleasant, and I felt comfortable talking with her. I just gave her the basics of who we [hospitalists] are and what we do. She admitted that she didn’t know much about hospitalists and seemed interested in what I had to say,” he says. Davis asked several questions, Dr. Seymann notes. “She mostly wanted to know about how our practice differs from general internists and the difference between hospital and outpatient-based medicine,” he recalls, adding, “I felt like she heard me. The meeting exceeded my expectations.”
The difference between the two visits was striking. Dr. Seymann explains that it is important to realize that “you never know when something you say will make a difference or have an impact. You have to try and, sometimes, keep trying.”
Follow-up is important for these visits. “I sent e-mails on returning home to thank them for their time and remind them that I would be happy to help on hospital medicine issues in the future,” he says.
While Dr. Seymann believes he helped educate legislators about hospital medicine and the hospitalist’s role, he also learned something himself. “I realized that one person can effectively engage in the legislative process and that Congress is interested in what we have to say,” he says. Additionally, he observes, “They take the input of their constituents pretty seriously, and we have a role to play in ensuring that our voices are heard on issues that affect our patients and our profession.” TH
Joanne Kaldy is based in Maryland.
Representing hospital medicine on Capitol Hill is an opportunity for physicians to educate policy makers and have a hand in the legislative process. Hospitalists who have had such experiences say that it can also be nerve wracking, eye opening, surprising, and very satisfying.
Rifkin Answers a Call to Arms
William Rifkin, MD, assistant professor of medicine and associate director of the Yale Primary Care Residency Program at the Yale University School of Medicine, New Haven, Conn., started his road to Capitol Hill by answering an SHM call to arms. “SHM was looking for someone in Connecticut to appear before the state Health Committee regarding proposed legislation that sought to regulate communications between hospitalists and primary care physicians,” says Dr. Rifkin, who was also asked to address the issue of whether the use of hospitalists should be mandatory or voluntary.
The call originally came from the Connecticut Medical Society, which—along with SHM—helped prepare Dr. Rifkin for his testimony. These groups’ public policy staffs coached him about his audience, the hearing process, and the key issues. They agreed his approach would be educational and informative in nature. He would explain what “hospital medicine is, the advantages and disadvantages, what is happening now in the field, and the issues being addressed by SHM,” he recalls. “I gave the committee members lots of literature and background information.”
Dr. Rifkin had some challenges to overcome in getting his message across to the legislators. “There is no data showing that lack of communication between hospitalists and community physicians has caused serious problems,” he explains. “I had to explain the difference between factual data and anecdotal information.”
Additionally, he recalls, one legislator seemed skeptical about the hospitalist’s role and kept referring to a health system that mandates the use of hospitalists. “I had to sit back and explain how SHM supports a voluntary model,” he says. “She kept talking about a reported example of a hospital that forced patients to use hospitalists. It was awkward because I suspected that the story was untrue or at least was missing some facts.”
By calmly relating SHM’s position and its reasons for preferring a voluntary model, Dr. Rifkin believes he was able to diffuse some of the tension. After the hearing, he approached the legislator and handed her the literature and statements from SHM. “I offered to stay in touch and suggested we find out about this hospital supposedly mandating hospitalists,” he says “Ultimately, I discovered that this story wasn’t true.”
Making a good impression can help legislators see physicians as colleagues rather than adversaries. “After the hearing, the committee sent the communications bill to the Connecticut Department of Public Health Best Practices Committee. This body was charged with making recommendations on communications best practices,” says Dr. Rifkin, who was asked to talk before this group. In fact, he adds, “They’ve asked me back repeatedly. I’m sort of a regular on the committee now.”
He is honored to have input and to present the hospitalist’s point of view. “Their recommendations likely will be similar to what SHM says regarding inpatient/outpatient communication,” he explains.
In retrospect, Dr. Rifkin believes he made a difference. “I felt as if I brought them some new information and taught legislators some things about hospital medicine they didn’t know before,” he says. “I think I helped them see that it would be counterproductive to dictate the specifics of inpatient/outpatient physician communication.”
When it comes to presenting testimony, Dr. Rifkin suggests, “it is best to acknowledge where legislators are right and use this as an opportunity for education. You don’t want to come across as dogmatic.”
While Dr. Rifkin enjoyed his experience, it was not without some surprises. He explains, “I left shaking my head and marveling, ‘Is this really how laws are made?’ ” He was surprised “about the lack of knowledge about the issues and the willingness to act on anecdotal information.”
Reporting back to SHM, Dr. Rifkin says, “It was good that I was there because—absent that—we could have ended up with some onerous rule that we then would have to undo.”
Another surprise for Dr. Rifkin was how long and tedious the process could be. “I was one of the last speakers on the agenda, and I did lots of waiting,” he states, adding, “If I had been nervous, it would have been a torturous eight hours.” Once he was in front of the microphone, Dr. Rifkin had just a few minutes to get his points across. He then answered questions for several additional minutes. “I had to watch the clock, and it was a little nerve-wracking to try to say everything I wanted to in a short time. But for the most part, it was actually enjoyable,” he offers.
Being active in advocacy efforts is a valuable, satisfying experience, and Dr. Rifkin urges his colleagues to carry the gauntlet. “We need to watch for opportunities to have input on legislation nationally and statewide. Hopefully, we’ll be able to have the same impact we had in Connecticut in other states as well,” he says. “Physicians need to be willing to get involved.”
—William Rifkin, MD, assistant professor of medicine and associate director, Yale Primary Care Residency Program, Yale University School of Medicine, New Haven, Conn.
Feinbloom: Testimony on the Fly
David Feinbloom, MD, a hospitalist at Beth Israel Deaconess Medical Center in Boston, had only two hours to prepare for his testimony about computerized physician order entry before the Massachusetts State Joint Committees on Health Care Financing and Economic Development and Emerging Technologies. “They wanted a clinician to explain how this system would improve quality and result in cost-saving,” he says.
Despite his lack of preparation time, he was familiar enough with the subject to speak in detail and answer questions. “I was a little nervous,” he admits, adding, “I would have preferred to have time to prepare a formal presentation, especially since I ended up having to write something up afterward for the official records.”
Dr. Feinbloom was one of the last speakers, and this had some disadvantages. First of all, he had to wait for hours. Additionally, “Many of the points I wanted to make already had been addressed. I didn’t get a lot of questions because there wasn’t much left to ask,” he explains.
The biggest surprise for Dr. Feinbloom was that the legislative process “is a little more mundane than I expected. It’s not like when you watch the news, and they have rousing, heated discussions.”
Also surprising was how receptive the committee members were about the issue. “Because part of the funding was coming from Blue Cross/Blue Shield, there wasn’t even any real controversy or debate from a budgetary standpoint,” he says. “There also was a big study showing that the system will pay for itself.”
Like Dr. Rifkin, Dr. Feinbloom believes his testimony had a positive effect. “I think that I brought to bear a realistic, ground-level view. I also brought some clinical examples of where this system is powerful, and I don’t think people realized this,” he says. “One of the senators had diabetes and told me he was surprised about how messy drug delivery in the hospital can be and how computerized systems can help. My examples stuck in his head as something he could relate to.”
Seymann: Another Kind of Testimony
Hospitalists don’t have to present testimony before a governmental body to have a positive effect on legislation and make a strong impression on lawmakers. Ask Gregory Seymann, MD, associate clinical professor, Division of Hospital Medicine, Department of Medicine, University of California San Diego School of Medicine. While in Washington, D.C., for the 2006 SHM Annual Meeting, he visited his House and Senate representatives.
“Our goal was to educate lawmakers about hospitalists—who we are and what we do—not to ask for favors or handouts,” explains Dr. Seymann. “Several of us went as a group to our senator’s office, and it was a rather short visit. We met with a staff person, who listened briefly and took our materials but asked few questions.”
When he went alone to his House representative, Susan Davis’ (D-CA) office, Dr. Seymann had a much different experience. The representative’s staff was extremely welcoming. “They told me that she was still in session marking up a bill, but that she really wanted to meet me,” he recalls. “They asked me if I could wait; and eventually they took me over to another building to meet her.”
Dr. Seymann’s House representative met with him for half an hour. “She was very pleasant, and I felt comfortable talking with her. I just gave her the basics of who we [hospitalists] are and what we do. She admitted that she didn’t know much about hospitalists and seemed interested in what I had to say,” he says. Davis asked several questions, Dr. Seymann notes. “She mostly wanted to know about how our practice differs from general internists and the difference between hospital and outpatient-based medicine,” he recalls, adding, “I felt like she heard me. The meeting exceeded my expectations.”
The difference between the two visits was striking. Dr. Seymann explains that it is important to realize that “you never know when something you say will make a difference or have an impact. You have to try and, sometimes, keep trying.”
Follow-up is important for these visits. “I sent e-mails on returning home to thank them for their time and remind them that I would be happy to help on hospital medicine issues in the future,” he says.
While Dr. Seymann believes he helped educate legislators about hospital medicine and the hospitalist’s role, he also learned something himself. “I realized that one person can effectively engage in the legislative process and that Congress is interested in what we have to say,” he says. Additionally, he observes, “They take the input of their constituents pretty seriously, and we have a role to play in ensuring that our voices are heard on issues that affect our patients and our profession.” TH
Joanne Kaldy is based in Maryland.
Medicine and Movies
It’s snowing outside, and the logs are burning in the fireplace. It’s a February blizzard. By the time you read this I’ll be thawed. I guess you know I’m not in San Diego. Despite Rochester’s well-earned reputation as the sizzling hot spot of the Minnesota-Iowa border, it seems like a cool night to think about renting some movies. But what are the best movies for a hospitalist? What are the absolute clunkers? Here are some suggestions.
“The Hospital” (1971)
This is my number one choice, no doubt about it. If you trained in New York City, it’s a bonus. This is a strange, dark comedy starring George C. Scott. His most famous, Oscar-winning role was in “Patton,” but I loved him in this medically titled-but-distinctly-different-from-“Dr. Strangelove” movie. In this flick, he plays the suicidal, alcoholic chief of internal medicine in a dysfunctional, deteriorating New York teaching hospital. On the edge of self-destruction, he meets the alluring yet bizarre Diana Rigg.
If you are at least as old as me—over 25, that is—you might remember her as Mrs. Emma Peel of the Avengers, the proto-feminist kickboxing genius in a leather body suit. Together, they try to solve a mystery involving unexpected deaths, in an atmosphere of abuse, lack of professionalism, and general mayhem. The Joint Commission would have a field day in this facility. See this movie with your hospital’s safety officer!
“No Way Out” (1950)
Next on my list is a movie that glorifies the doctor and his oath but still explores the politics of hospitals and race relations. This is another of my absolute favorite medical movies. It is Sidney Poitier’s first film. He plays the intern taking care of Richard Widmark and his brother—both of whom are rabid racists. When the brother dies following a lumbar puncture, a chain of events is set off that plunges the city into a race riot. Can Dr. Brooks clear his name by getting an autopsy before Ray Biddle hunts him down? This is a great movie to watch with a group of students—a conversation starter.
“Panic in the Streets” (1950)
Yup, it’s 1950 and Richard Widmark again. This time, he’s a public health officer who uncovers a case of plague in a very noir film-noir New Orleans. He must catch a killer who has been exposed to plague. The villain turns out to be Walter “Jack” Palance in his first movie. Watch Dr. Clint Reed chase Blackie through some scenes of New Orleans you won’t forget. Then play poker.
“Mother, Jugs & Speed” (1976)
Oh my, what is this doing on my list? I must be slipping. Bill Cosby and Raquel Welch star (guess which one is Jugs). Any movie with Dick Butkus and Larry Hagman in it can’t be all bad, can it? Yes, it can. Crazed ambulance drivers tear up the streets of L.A. when a new law decrees that whoever gets to the accident first gets the transport. Who says competition in the medical world isn’t good? This is just a modified version of pay for performance; too bad that, in this case (again), the performances are terrible!
“Fantastic Voyage” (1966)
I can’t stop thinking about Raquel Welch. In this movie she is a decade younger, an earnest young medical researcher who gets attacked by leukocytes. Watching her ultra-tight dive suit get covered in giant plastic antibodies almost made me want to go into immunology. The crew gets shrunk and injected into a diplomat’s body to dissolve a clot in his brain. Too bad they didn’t have tissue plasminogen activator (TPA). “The Simpsons” did a cover on this one that’s worth checking out.
“The Island of Lost Souls” (1933)
Watch this one with your favorite geneticist. In remakes, it’s called “The Island of Dr. Moreau,” the name of the book this movie was adapted from. Charles Laughton—the quintessential Quasimodo—creates beings that are half man/half beast, with the help of Bela Lugosi (sans pointy teeth and bats) and “the panther woman.” Her name is Lola. I think Dr. Moreau may have met her in a club down in old Soho. I guess you’ll have to drink champagne that tastes just like Coca-Cola with this one. Sorry, I couldn’t help myself.
“Le Roi de Coeur” (King of Hearts) (1966)
Many people consider this their favorite movie. Most of them went to college on the East Coast in the early 1980s and didn’t go home alone the night they saw this one. It involves some kind of operant conditioning. I just saw this movie again last week for the first time in 25 years, and I wasn’t disappointed. A Scottish ornithologist is taken for a bomb expert, and the denizens of a psychiatric hospital take over a small French town. It’s a love story and an anti-war movie. Watch this one with someone you love—or want to.
“The Cabinet of Dr. Caligari” (1920)
My sister Roberta told me about this one, so I knew it would be freaky. The first horror movie ever made, it’s a silent film. One of my favorite things about this film is its expressionist sets. It’s a must see for film buffs, but not one to watch with the kids. If you want to see another of my sister’s horror picks, try “Dead Ringers” (1988)—it’s about twin homicidal gynecologists. Not for the faint of heart.
“Flatliners” (1990)
Kiefer Sutherland, Kevin Bacon, Julia Roberts—yeah, that sounds like my medical school class. Actually, my class was more John Cleese, Marty Feldman, and Ruth Buzzi. In this film, medical students put themselves into cardiac arrest and then resuscitate one another at the last minute.
“M*A*S*H” (1970)
Still one of my favorites, and I loved the book even more. Anti-war, anti-bureaucracy, hilarious. Donald Sutherland and Elliott Gould are excellent as Hawkeye and Trapper John, and Sally Kellerman is the best Hot Lips. This is somewhat different from the series and is worth watching.
There are so many other movies I have enjoyed. There are Gregory Peck in “Captain Newman, MD” and Robin Williams in “Awakenings.” I even like Patrick Swayze in “City of Joy.” Also worth mentioning: “And the Band Played On,” “Coma,” “The Cider House Rules,” “The Unbearable Lightness of Being,” and “The Elephant Man.”
There are dozens more; some are great depictions of medicine, and some are total trash. Got a favorite I didn’t list? Send the name and a paragraph about why you like it to me at [email protected].
OK, I need to get out more. TH
Dr. Newman is the physician editor of The Hospitalist. He’s also consultant, Hospital Internal Medicine, and assistant professor of internal medicine and medical history, Mayo Clinic College of Medicine, Rochester, Minn.
It’s snowing outside, and the logs are burning in the fireplace. It’s a February blizzard. By the time you read this I’ll be thawed. I guess you know I’m not in San Diego. Despite Rochester’s well-earned reputation as the sizzling hot spot of the Minnesota-Iowa border, it seems like a cool night to think about renting some movies. But what are the best movies for a hospitalist? What are the absolute clunkers? Here are some suggestions.
“The Hospital” (1971)
This is my number one choice, no doubt about it. If you trained in New York City, it’s a bonus. This is a strange, dark comedy starring George C. Scott. His most famous, Oscar-winning role was in “Patton,” but I loved him in this medically titled-but-distinctly-different-from-“Dr. Strangelove” movie. In this flick, he plays the suicidal, alcoholic chief of internal medicine in a dysfunctional, deteriorating New York teaching hospital. On the edge of self-destruction, he meets the alluring yet bizarre Diana Rigg.
If you are at least as old as me—over 25, that is—you might remember her as Mrs. Emma Peel of the Avengers, the proto-feminist kickboxing genius in a leather body suit. Together, they try to solve a mystery involving unexpected deaths, in an atmosphere of abuse, lack of professionalism, and general mayhem. The Joint Commission would have a field day in this facility. See this movie with your hospital’s safety officer!
“No Way Out” (1950)
Next on my list is a movie that glorifies the doctor and his oath but still explores the politics of hospitals and race relations. This is another of my absolute favorite medical movies. It is Sidney Poitier’s first film. He plays the intern taking care of Richard Widmark and his brother—both of whom are rabid racists. When the brother dies following a lumbar puncture, a chain of events is set off that plunges the city into a race riot. Can Dr. Brooks clear his name by getting an autopsy before Ray Biddle hunts him down? This is a great movie to watch with a group of students—a conversation starter.
“Panic in the Streets” (1950)
Yup, it’s 1950 and Richard Widmark again. This time, he’s a public health officer who uncovers a case of plague in a very noir film-noir New Orleans. He must catch a killer who has been exposed to plague. The villain turns out to be Walter “Jack” Palance in his first movie. Watch Dr. Clint Reed chase Blackie through some scenes of New Orleans you won’t forget. Then play poker.
“Mother, Jugs & Speed” (1976)
Oh my, what is this doing on my list? I must be slipping. Bill Cosby and Raquel Welch star (guess which one is Jugs). Any movie with Dick Butkus and Larry Hagman in it can’t be all bad, can it? Yes, it can. Crazed ambulance drivers tear up the streets of L.A. when a new law decrees that whoever gets to the accident first gets the transport. Who says competition in the medical world isn’t good? This is just a modified version of pay for performance; too bad that, in this case (again), the performances are terrible!
“Fantastic Voyage” (1966)
I can’t stop thinking about Raquel Welch. In this movie she is a decade younger, an earnest young medical researcher who gets attacked by leukocytes. Watching her ultra-tight dive suit get covered in giant plastic antibodies almost made me want to go into immunology. The crew gets shrunk and injected into a diplomat’s body to dissolve a clot in his brain. Too bad they didn’t have tissue plasminogen activator (TPA). “The Simpsons” did a cover on this one that’s worth checking out.
“The Island of Lost Souls” (1933)
Watch this one with your favorite geneticist. In remakes, it’s called “The Island of Dr. Moreau,” the name of the book this movie was adapted from. Charles Laughton—the quintessential Quasimodo—creates beings that are half man/half beast, with the help of Bela Lugosi (sans pointy teeth and bats) and “the panther woman.” Her name is Lola. I think Dr. Moreau may have met her in a club down in old Soho. I guess you’ll have to drink champagne that tastes just like Coca-Cola with this one. Sorry, I couldn’t help myself.
“Le Roi de Coeur” (King of Hearts) (1966)
Many people consider this their favorite movie. Most of them went to college on the East Coast in the early 1980s and didn’t go home alone the night they saw this one. It involves some kind of operant conditioning. I just saw this movie again last week for the first time in 25 years, and I wasn’t disappointed. A Scottish ornithologist is taken for a bomb expert, and the denizens of a psychiatric hospital take over a small French town. It’s a love story and an anti-war movie. Watch this one with someone you love—or want to.
“The Cabinet of Dr. Caligari” (1920)
My sister Roberta told me about this one, so I knew it would be freaky. The first horror movie ever made, it’s a silent film. One of my favorite things about this film is its expressionist sets. It’s a must see for film buffs, but not one to watch with the kids. If you want to see another of my sister’s horror picks, try “Dead Ringers” (1988)—it’s about twin homicidal gynecologists. Not for the faint of heart.
“Flatliners” (1990)
Kiefer Sutherland, Kevin Bacon, Julia Roberts—yeah, that sounds like my medical school class. Actually, my class was more John Cleese, Marty Feldman, and Ruth Buzzi. In this film, medical students put themselves into cardiac arrest and then resuscitate one another at the last minute.
“M*A*S*H” (1970)
Still one of my favorites, and I loved the book even more. Anti-war, anti-bureaucracy, hilarious. Donald Sutherland and Elliott Gould are excellent as Hawkeye and Trapper John, and Sally Kellerman is the best Hot Lips. This is somewhat different from the series and is worth watching.
There are so many other movies I have enjoyed. There are Gregory Peck in “Captain Newman, MD” and Robin Williams in “Awakenings.” I even like Patrick Swayze in “City of Joy.” Also worth mentioning: “And the Band Played On,” “Coma,” “The Cider House Rules,” “The Unbearable Lightness of Being,” and “The Elephant Man.”
There are dozens more; some are great depictions of medicine, and some are total trash. Got a favorite I didn’t list? Send the name and a paragraph about why you like it to me at [email protected].
OK, I need to get out more. TH
Dr. Newman is the physician editor of The Hospitalist. He’s also consultant, Hospital Internal Medicine, and assistant professor of internal medicine and medical history, Mayo Clinic College of Medicine, Rochester, Minn.
It’s snowing outside, and the logs are burning in the fireplace. It’s a February blizzard. By the time you read this I’ll be thawed. I guess you know I’m not in San Diego. Despite Rochester’s well-earned reputation as the sizzling hot spot of the Minnesota-Iowa border, it seems like a cool night to think about renting some movies. But what are the best movies for a hospitalist? What are the absolute clunkers? Here are some suggestions.
“The Hospital” (1971)
This is my number one choice, no doubt about it. If you trained in New York City, it’s a bonus. This is a strange, dark comedy starring George C. Scott. His most famous, Oscar-winning role was in “Patton,” but I loved him in this medically titled-but-distinctly-different-from-“Dr. Strangelove” movie. In this flick, he plays the suicidal, alcoholic chief of internal medicine in a dysfunctional, deteriorating New York teaching hospital. On the edge of self-destruction, he meets the alluring yet bizarre Diana Rigg.
If you are at least as old as me—over 25, that is—you might remember her as Mrs. Emma Peel of the Avengers, the proto-feminist kickboxing genius in a leather body suit. Together, they try to solve a mystery involving unexpected deaths, in an atmosphere of abuse, lack of professionalism, and general mayhem. The Joint Commission would have a field day in this facility. See this movie with your hospital’s safety officer!
“No Way Out” (1950)
Next on my list is a movie that glorifies the doctor and his oath but still explores the politics of hospitals and race relations. This is another of my absolute favorite medical movies. It is Sidney Poitier’s first film. He plays the intern taking care of Richard Widmark and his brother—both of whom are rabid racists. When the brother dies following a lumbar puncture, a chain of events is set off that plunges the city into a race riot. Can Dr. Brooks clear his name by getting an autopsy before Ray Biddle hunts him down? This is a great movie to watch with a group of students—a conversation starter.
“Panic in the Streets” (1950)
Yup, it’s 1950 and Richard Widmark again. This time, he’s a public health officer who uncovers a case of plague in a very noir film-noir New Orleans. He must catch a killer who has been exposed to plague. The villain turns out to be Walter “Jack” Palance in his first movie. Watch Dr. Clint Reed chase Blackie through some scenes of New Orleans you won’t forget. Then play poker.
“Mother, Jugs & Speed” (1976)
Oh my, what is this doing on my list? I must be slipping. Bill Cosby and Raquel Welch star (guess which one is Jugs). Any movie with Dick Butkus and Larry Hagman in it can’t be all bad, can it? Yes, it can. Crazed ambulance drivers tear up the streets of L.A. when a new law decrees that whoever gets to the accident first gets the transport. Who says competition in the medical world isn’t good? This is just a modified version of pay for performance; too bad that, in this case (again), the performances are terrible!
“Fantastic Voyage” (1966)
I can’t stop thinking about Raquel Welch. In this movie she is a decade younger, an earnest young medical researcher who gets attacked by leukocytes. Watching her ultra-tight dive suit get covered in giant plastic antibodies almost made me want to go into immunology. The crew gets shrunk and injected into a diplomat’s body to dissolve a clot in his brain. Too bad they didn’t have tissue plasminogen activator (TPA). “The Simpsons” did a cover on this one that’s worth checking out.
“The Island of Lost Souls” (1933)
Watch this one with your favorite geneticist. In remakes, it’s called “The Island of Dr. Moreau,” the name of the book this movie was adapted from. Charles Laughton—the quintessential Quasimodo—creates beings that are half man/half beast, with the help of Bela Lugosi (sans pointy teeth and bats) and “the panther woman.” Her name is Lola. I think Dr. Moreau may have met her in a club down in old Soho. I guess you’ll have to drink champagne that tastes just like Coca-Cola with this one. Sorry, I couldn’t help myself.
“Le Roi de Coeur” (King of Hearts) (1966)
Many people consider this their favorite movie. Most of them went to college on the East Coast in the early 1980s and didn’t go home alone the night they saw this one. It involves some kind of operant conditioning. I just saw this movie again last week for the first time in 25 years, and I wasn’t disappointed. A Scottish ornithologist is taken for a bomb expert, and the denizens of a psychiatric hospital take over a small French town. It’s a love story and an anti-war movie. Watch this one with someone you love—or want to.
“The Cabinet of Dr. Caligari” (1920)
My sister Roberta told me about this one, so I knew it would be freaky. The first horror movie ever made, it’s a silent film. One of my favorite things about this film is its expressionist sets. It’s a must see for film buffs, but not one to watch with the kids. If you want to see another of my sister’s horror picks, try “Dead Ringers” (1988)—it’s about twin homicidal gynecologists. Not for the faint of heart.
“Flatliners” (1990)
Kiefer Sutherland, Kevin Bacon, Julia Roberts—yeah, that sounds like my medical school class. Actually, my class was more John Cleese, Marty Feldman, and Ruth Buzzi. In this film, medical students put themselves into cardiac arrest and then resuscitate one another at the last minute.
“M*A*S*H” (1970)
Still one of my favorites, and I loved the book even more. Anti-war, anti-bureaucracy, hilarious. Donald Sutherland and Elliott Gould are excellent as Hawkeye and Trapper John, and Sally Kellerman is the best Hot Lips. This is somewhat different from the series and is worth watching.
There are so many other movies I have enjoyed. There are Gregory Peck in “Captain Newman, MD” and Robin Williams in “Awakenings.” I even like Patrick Swayze in “City of Joy.” Also worth mentioning: “And the Band Played On,” “Coma,” “The Cider House Rules,” “The Unbearable Lightness of Being,” and “The Elephant Man.”
There are dozens more; some are great depictions of medicine, and some are total trash. Got a favorite I didn’t list? Send the name and a paragraph about why you like it to me at [email protected].
OK, I need to get out more. TH
Dr. Newman is the physician editor of The Hospitalist. He’s also consultant, Hospital Internal Medicine, and assistant professor of internal medicine and medical history, Mayo Clinic College of Medicine, Rochester, Minn.
Contractual Caution
Several times a week, I hear from doctors or administrators who want to discuss solutions to the latest crises occurring in their practices. There are three contractual issues that come up regularly in these conversations. One is how to handle the contractual provision for vacation time, and I addressed that in last month’s column.
This month, I’ll discuss the other two issues: payment for malpractice “tail coverage” and the inclusion of a non-compete clause in hospitalist employment contracts.
Malpractice Tail Coverage
Not long ago, I got a call from the worried administrator of a growing and successful hospitalist practice. She described a crisis that had started when a doctor decided to leave the practice to pursue fellowship training. The doctor was happy with the practice and enjoyed the time he had spent as part of it. Yet his departure had set off a wave of threatened resignations that risked the collapse of the practice.
It turns out that the doctors’ employment contract specified that the employer would pay malpractice tail coverage for any doctor who left the practice before completing two years of employment. After the two-year anniversary, the doctor would have to pay for tail coverage. Most doctors in this young practice were nearing their two-year anniversary, and they began to worry about having to assume this responsibility.
I spoke with one such doctor. The physician was happy with the practice and had not spent time thinking about leaving until faced with the issue of tail coverage. Like many hospitalists, she tended to think of her commitment to the practice more in terms of dating than as a marriage. She wanted to keep her options open to pursue other work in the future and thought there might be some chance she would move if she experienced a major life change like marriage. So—like several of her colleagues—she thought about leaving the practice ahead of the two-year anniversary, thus avoiding committing to paying tail coverage that could be as much as $25,000 per year, depending on how long she stayed with the practice. To her, assuming the risk of paying the tail coverage felt like punishment for staying in the practice for longer than two years rather than a reward for her loyalty.
Ultimately, the hospitalists and the multispecialty group that they were part of negotiated for the practice to pay the tail coverage regardless of the duration of a departing doctor’s employment with the group. The group paid for this in part by paying beginning hospitalists a lower salary; in a sense, the doctors were still paying a portion out of their own pockets, but it seemed less painful this way.
It is reasonably common in any specialty for a group to assume the risk of paying tail coverage if one of its doctors leaves the practice within the first two or three years because a doctor who decides to leave that quickly often does so after concluding that the practice is not as it was described during the recruiting process. But a doctor who departs later than that is more likely to do so because she has simply decided to pursue other options, and it seems reasonable that she should pay the expenses related to her departure. This is a reasonable approach, but there are several issues that might cause a practice to approach the issue differently for hospitalists than for other doctors.
- Hospitalist practice is likely to have a somewhat higher turnover in staffing than other physician groups for several reasons that I won’t enumerate here. So, like the woman in the anecdote above, everyone should acknowledge that the fact that a hospitalist is willing to stay longer than two or three years does not mean he or she will stay for a career. With this in mind, payment of tail coverage may be a bigger issue for hospitalists and may require a different approach than for other specialties, though whether the practice or the hospitalist should pay for it is still up for debate.
- Nationally, about half of hospitalists are employed by the hospital in which they work, and—in this case—malpractice insurance is usually provided by the employing hospital. Many or most hospitals have decided it is in their interest to pay for tail coverage for a departing doctor regardless of his duration of service. If a doctor were to decide not to buy tail coverage himself, then the hospital might become the deep-pocket target of a malpractice suit, instead of the doctor. For this reason, many hospitals have decided to go ahead and pay for the coverage instead of facing the risk that the doctor won’t buy it.
- Some hospitalists (most commonly those employed by hospitals) have an occurrence malpractice policy that doesn’t require tail coverage. Claims-made policies, which do require tail coverage, are much more common overall, but it is worth thinking about whether an occurrence policy might be better in your situation. If you’re unfamiliar with the differences between these policies, a good discussion can be found at www.physiciansnews.com/business/405.html, or just put “claims made + occurrence” in a search engine and you will find some good explanations.
The right approach to this issue will vary from one place to the next. In the current environment, with more hospitalist positions than there are doctors to fill them, many practices may need to agree to pay tail coverage for departing doctors.
Non-Compete Clauses
Non-compete clauses are common in physician contracts. They generally specify that a doctor who leaves a practice may not practice the same specialty of medicine within a defined geographic region for a specified period of time. The rationale for their inclusion in any specialty of medicine is complex but can be illustrated by an example that I watched play out while I was a resident.
With much fanfare, the hospital where I did my residency training in the 1980s recruited its first cardiac transplant surgeon, then bought new equipment and hired new staff to support the program. After about two years, the surgeon decided to move his practice to a hospital about 30 miles away, and the teaching hospital had made a big investment in a transplant program that it could no longer operate. Even if the hospital could have found a new transplant surgeon quickly, the original surgeon had developed relationships and referral sources from around the state, and most of these referrals would follow him to his new hospital.
I was only a resident and don’t know anything about why the doctor left or whether his contract had a non-compete provision. But it was clear to me that the hospital had made a big investment building the program around him and would now need to start over, working to recapture the referral relationships the departing doctor had taken with him. The hospital would have been smart to have a non-compete clause in place that would prohibit the surgeon from practicing in its market. It wouldn’t be fair to prevent the surgeon from leaving or practicing transplant surgery elsewhere, but it seems reasonable for the hospital to require that he not practice in a place that would be geographically close enough to interfere with its referrals.
There are better sources for the overall rationale of non-competes than this column, but some of the principal reasons they’re written into contracts include:
- To prevent a doctor from developing referral relationships—with the help of the employer practice—and then taking them across town to a competing group;
- To prevent a departing doctor from taking trade secrets about the way business is conducted—or future business plans—and using that information to benefit a competing practice; and
- To provide a means to reduce the chance that a practice incurs the expense of recruiting and getting the doctor established in practice, only to have the doctor quickly “jump ship” to a competing practice.
In most, but not all, cases, it is hard to argue that hospitalists can redirect referral sources or steal trade secrets when they leave a practice. Accordingly, these issues are rarely a good reason to include a non-compete.
Including a non-compete simply to prevent a doctor from jumping ship to a new practice has always struck me as the least legitimate reason; your practice should keep doctors from leaving because they like it there rather than because of a contractual provision that makes it difficult to switch to a different practice in the area. And including a non-compete clause comes at a cost of potentially scaring off the people you are trying to recruit, which could mean that it is hurting the practice more than helping it.
I’m not suggesting that non-competes have no place in hospitalist practice; they may be important and appropriate in some situations. But each hospitalist practice should take the time to think critically about whether to include one or not. Simply including it because it is common practice in other physician contracts may do more harm than good.
If you are a hospitalist and are considering signing a contract that includes a non-compete, don’t let this column lead you to believe that the practice is trying to treat you unfairly. But it is reasonable for you to ask the group representative why they see it as necessary. You might get lucky and find that they’re willing to delete or shorten it.
Conclusion
When it comes to hospitalist contracts, no one formula can apply to all practices or to all physicians. Careful analysis of the contract by both parties, however, along with a few well-thought-out questions, might prevent future problems. TH
Dr. Nelson has been a practicing hospitalist since 1988 and is a co-founder and past-president of SHM. He is a principal in Nelson/Flores Associates, a national hospitalist practice management consulting firm. This column represents his views and is not intended to reflect an official position of SHM.
Several times a week, I hear from doctors or administrators who want to discuss solutions to the latest crises occurring in their practices. There are three contractual issues that come up regularly in these conversations. One is how to handle the contractual provision for vacation time, and I addressed that in last month’s column.
This month, I’ll discuss the other two issues: payment for malpractice “tail coverage” and the inclusion of a non-compete clause in hospitalist employment contracts.
Malpractice Tail Coverage
Not long ago, I got a call from the worried administrator of a growing and successful hospitalist practice. She described a crisis that had started when a doctor decided to leave the practice to pursue fellowship training. The doctor was happy with the practice and enjoyed the time he had spent as part of it. Yet his departure had set off a wave of threatened resignations that risked the collapse of the practice.
It turns out that the doctors’ employment contract specified that the employer would pay malpractice tail coverage for any doctor who left the practice before completing two years of employment. After the two-year anniversary, the doctor would have to pay for tail coverage. Most doctors in this young practice were nearing their two-year anniversary, and they began to worry about having to assume this responsibility.
I spoke with one such doctor. The physician was happy with the practice and had not spent time thinking about leaving until faced with the issue of tail coverage. Like many hospitalists, she tended to think of her commitment to the practice more in terms of dating than as a marriage. She wanted to keep her options open to pursue other work in the future and thought there might be some chance she would move if she experienced a major life change like marriage. So—like several of her colleagues—she thought about leaving the practice ahead of the two-year anniversary, thus avoiding committing to paying tail coverage that could be as much as $25,000 per year, depending on how long she stayed with the practice. To her, assuming the risk of paying the tail coverage felt like punishment for staying in the practice for longer than two years rather than a reward for her loyalty.
Ultimately, the hospitalists and the multispecialty group that they were part of negotiated for the practice to pay the tail coverage regardless of the duration of a departing doctor’s employment with the group. The group paid for this in part by paying beginning hospitalists a lower salary; in a sense, the doctors were still paying a portion out of their own pockets, but it seemed less painful this way.
It is reasonably common in any specialty for a group to assume the risk of paying tail coverage if one of its doctors leaves the practice within the first two or three years because a doctor who decides to leave that quickly often does so after concluding that the practice is not as it was described during the recruiting process. But a doctor who departs later than that is more likely to do so because she has simply decided to pursue other options, and it seems reasonable that she should pay the expenses related to her departure. This is a reasonable approach, but there are several issues that might cause a practice to approach the issue differently for hospitalists than for other doctors.
- Hospitalist practice is likely to have a somewhat higher turnover in staffing than other physician groups for several reasons that I won’t enumerate here. So, like the woman in the anecdote above, everyone should acknowledge that the fact that a hospitalist is willing to stay longer than two or three years does not mean he or she will stay for a career. With this in mind, payment of tail coverage may be a bigger issue for hospitalists and may require a different approach than for other specialties, though whether the practice or the hospitalist should pay for it is still up for debate.
- Nationally, about half of hospitalists are employed by the hospital in which they work, and—in this case—malpractice insurance is usually provided by the employing hospital. Many or most hospitals have decided it is in their interest to pay for tail coverage for a departing doctor regardless of his duration of service. If a doctor were to decide not to buy tail coverage himself, then the hospital might become the deep-pocket target of a malpractice suit, instead of the doctor. For this reason, many hospitals have decided to go ahead and pay for the coverage instead of facing the risk that the doctor won’t buy it.
- Some hospitalists (most commonly those employed by hospitals) have an occurrence malpractice policy that doesn’t require tail coverage. Claims-made policies, which do require tail coverage, are much more common overall, but it is worth thinking about whether an occurrence policy might be better in your situation. If you’re unfamiliar with the differences between these policies, a good discussion can be found at www.physiciansnews.com/business/405.html, or just put “claims made + occurrence” in a search engine and you will find some good explanations.
The right approach to this issue will vary from one place to the next. In the current environment, with more hospitalist positions than there are doctors to fill them, many practices may need to agree to pay tail coverage for departing doctors.
Non-Compete Clauses
Non-compete clauses are common in physician contracts. They generally specify that a doctor who leaves a practice may not practice the same specialty of medicine within a defined geographic region for a specified period of time. The rationale for their inclusion in any specialty of medicine is complex but can be illustrated by an example that I watched play out while I was a resident.
With much fanfare, the hospital where I did my residency training in the 1980s recruited its first cardiac transplant surgeon, then bought new equipment and hired new staff to support the program. After about two years, the surgeon decided to move his practice to a hospital about 30 miles away, and the teaching hospital had made a big investment in a transplant program that it could no longer operate. Even if the hospital could have found a new transplant surgeon quickly, the original surgeon had developed relationships and referral sources from around the state, and most of these referrals would follow him to his new hospital.
I was only a resident and don’t know anything about why the doctor left or whether his contract had a non-compete provision. But it was clear to me that the hospital had made a big investment building the program around him and would now need to start over, working to recapture the referral relationships the departing doctor had taken with him. The hospital would have been smart to have a non-compete clause in place that would prohibit the surgeon from practicing in its market. It wouldn’t be fair to prevent the surgeon from leaving or practicing transplant surgery elsewhere, but it seems reasonable for the hospital to require that he not practice in a place that would be geographically close enough to interfere with its referrals.
There are better sources for the overall rationale of non-competes than this column, but some of the principal reasons they’re written into contracts include:
- To prevent a doctor from developing referral relationships—with the help of the employer practice—and then taking them across town to a competing group;
- To prevent a departing doctor from taking trade secrets about the way business is conducted—or future business plans—and using that information to benefit a competing practice; and
- To provide a means to reduce the chance that a practice incurs the expense of recruiting and getting the doctor established in practice, only to have the doctor quickly “jump ship” to a competing practice.
In most, but not all, cases, it is hard to argue that hospitalists can redirect referral sources or steal trade secrets when they leave a practice. Accordingly, these issues are rarely a good reason to include a non-compete.
Including a non-compete simply to prevent a doctor from jumping ship to a new practice has always struck me as the least legitimate reason; your practice should keep doctors from leaving because they like it there rather than because of a contractual provision that makes it difficult to switch to a different practice in the area. And including a non-compete clause comes at a cost of potentially scaring off the people you are trying to recruit, which could mean that it is hurting the practice more than helping it.
I’m not suggesting that non-competes have no place in hospitalist practice; they may be important and appropriate in some situations. But each hospitalist practice should take the time to think critically about whether to include one or not. Simply including it because it is common practice in other physician contracts may do more harm than good.
If you are a hospitalist and are considering signing a contract that includes a non-compete, don’t let this column lead you to believe that the practice is trying to treat you unfairly. But it is reasonable for you to ask the group representative why they see it as necessary. You might get lucky and find that they’re willing to delete or shorten it.
Conclusion
When it comes to hospitalist contracts, no one formula can apply to all practices or to all physicians. Careful analysis of the contract by both parties, however, along with a few well-thought-out questions, might prevent future problems. TH
Dr. Nelson has been a practicing hospitalist since 1988 and is a co-founder and past-president of SHM. He is a principal in Nelson/Flores Associates, a national hospitalist practice management consulting firm. This column represents his views and is not intended to reflect an official position of SHM.
Several times a week, I hear from doctors or administrators who want to discuss solutions to the latest crises occurring in their practices. There are three contractual issues that come up regularly in these conversations. One is how to handle the contractual provision for vacation time, and I addressed that in last month’s column.
This month, I’ll discuss the other two issues: payment for malpractice “tail coverage” and the inclusion of a non-compete clause in hospitalist employment contracts.
Malpractice Tail Coverage
Not long ago, I got a call from the worried administrator of a growing and successful hospitalist practice. She described a crisis that had started when a doctor decided to leave the practice to pursue fellowship training. The doctor was happy with the practice and enjoyed the time he had spent as part of it. Yet his departure had set off a wave of threatened resignations that risked the collapse of the practice.
It turns out that the doctors’ employment contract specified that the employer would pay malpractice tail coverage for any doctor who left the practice before completing two years of employment. After the two-year anniversary, the doctor would have to pay for tail coverage. Most doctors in this young practice were nearing their two-year anniversary, and they began to worry about having to assume this responsibility.
I spoke with one such doctor. The physician was happy with the practice and had not spent time thinking about leaving until faced with the issue of tail coverage. Like many hospitalists, she tended to think of her commitment to the practice more in terms of dating than as a marriage. She wanted to keep her options open to pursue other work in the future and thought there might be some chance she would move if she experienced a major life change like marriage. So—like several of her colleagues—she thought about leaving the practice ahead of the two-year anniversary, thus avoiding committing to paying tail coverage that could be as much as $25,000 per year, depending on how long she stayed with the practice. To her, assuming the risk of paying the tail coverage felt like punishment for staying in the practice for longer than two years rather than a reward for her loyalty.
Ultimately, the hospitalists and the multispecialty group that they were part of negotiated for the practice to pay the tail coverage regardless of the duration of a departing doctor’s employment with the group. The group paid for this in part by paying beginning hospitalists a lower salary; in a sense, the doctors were still paying a portion out of their own pockets, but it seemed less painful this way.
It is reasonably common in any specialty for a group to assume the risk of paying tail coverage if one of its doctors leaves the practice within the first two or three years because a doctor who decides to leave that quickly often does so after concluding that the practice is not as it was described during the recruiting process. But a doctor who departs later than that is more likely to do so because she has simply decided to pursue other options, and it seems reasonable that she should pay the expenses related to her departure. This is a reasonable approach, but there are several issues that might cause a practice to approach the issue differently for hospitalists than for other doctors.
- Hospitalist practice is likely to have a somewhat higher turnover in staffing than other physician groups for several reasons that I won’t enumerate here. So, like the woman in the anecdote above, everyone should acknowledge that the fact that a hospitalist is willing to stay longer than two or three years does not mean he or she will stay for a career. With this in mind, payment of tail coverage may be a bigger issue for hospitalists and may require a different approach than for other specialties, though whether the practice or the hospitalist should pay for it is still up for debate.
- Nationally, about half of hospitalists are employed by the hospital in which they work, and—in this case—malpractice insurance is usually provided by the employing hospital. Many or most hospitals have decided it is in their interest to pay for tail coverage for a departing doctor regardless of his duration of service. If a doctor were to decide not to buy tail coverage himself, then the hospital might become the deep-pocket target of a malpractice suit, instead of the doctor. For this reason, many hospitals have decided to go ahead and pay for the coverage instead of facing the risk that the doctor won’t buy it.
- Some hospitalists (most commonly those employed by hospitals) have an occurrence malpractice policy that doesn’t require tail coverage. Claims-made policies, which do require tail coverage, are much more common overall, but it is worth thinking about whether an occurrence policy might be better in your situation. If you’re unfamiliar with the differences between these policies, a good discussion can be found at www.physiciansnews.com/business/405.html, or just put “claims made + occurrence” in a search engine and you will find some good explanations.
The right approach to this issue will vary from one place to the next. In the current environment, with more hospitalist positions than there are doctors to fill them, many practices may need to agree to pay tail coverage for departing doctors.
Non-Compete Clauses
Non-compete clauses are common in physician contracts. They generally specify that a doctor who leaves a practice may not practice the same specialty of medicine within a defined geographic region for a specified period of time. The rationale for their inclusion in any specialty of medicine is complex but can be illustrated by an example that I watched play out while I was a resident.
With much fanfare, the hospital where I did my residency training in the 1980s recruited its first cardiac transplant surgeon, then bought new equipment and hired new staff to support the program. After about two years, the surgeon decided to move his practice to a hospital about 30 miles away, and the teaching hospital had made a big investment in a transplant program that it could no longer operate. Even if the hospital could have found a new transplant surgeon quickly, the original surgeon had developed relationships and referral sources from around the state, and most of these referrals would follow him to his new hospital.
I was only a resident and don’t know anything about why the doctor left or whether his contract had a non-compete provision. But it was clear to me that the hospital had made a big investment building the program around him and would now need to start over, working to recapture the referral relationships the departing doctor had taken with him. The hospital would have been smart to have a non-compete clause in place that would prohibit the surgeon from practicing in its market. It wouldn’t be fair to prevent the surgeon from leaving or practicing transplant surgery elsewhere, but it seems reasonable for the hospital to require that he not practice in a place that would be geographically close enough to interfere with its referrals.
There are better sources for the overall rationale of non-competes than this column, but some of the principal reasons they’re written into contracts include:
- To prevent a doctor from developing referral relationships—with the help of the employer practice—and then taking them across town to a competing group;
- To prevent a departing doctor from taking trade secrets about the way business is conducted—or future business plans—and using that information to benefit a competing practice; and
- To provide a means to reduce the chance that a practice incurs the expense of recruiting and getting the doctor established in practice, only to have the doctor quickly “jump ship” to a competing practice.
In most, but not all, cases, it is hard to argue that hospitalists can redirect referral sources or steal trade secrets when they leave a practice. Accordingly, these issues are rarely a good reason to include a non-compete.
Including a non-compete simply to prevent a doctor from jumping ship to a new practice has always struck me as the least legitimate reason; your practice should keep doctors from leaving because they like it there rather than because of a contractual provision that makes it difficult to switch to a different practice in the area. And including a non-compete clause comes at a cost of potentially scaring off the people you are trying to recruit, which could mean that it is hurting the practice more than helping it.
I’m not suggesting that non-competes have no place in hospitalist practice; they may be important and appropriate in some situations. But each hospitalist practice should take the time to think critically about whether to include one or not. Simply including it because it is common practice in other physician contracts may do more harm than good.
If you are a hospitalist and are considering signing a contract that includes a non-compete, don’t let this column lead you to believe that the practice is trying to treat you unfairly. But it is reasonable for you to ask the group representative why they see it as necessary. You might get lucky and find that they’re willing to delete or shorten it.
Conclusion
When it comes to hospitalist contracts, no one formula can apply to all practices or to all physicians. Careful analysis of the contract by both parties, however, along with a few well-thought-out questions, might prevent future problems. TH
Dr. Nelson has been a practicing hospitalist since 1988 and is a co-founder and past-president of SHM. He is a principal in Nelson/Flores Associates, a national hospitalist practice management consulting firm. This column represents his views and is not intended to reflect an official position of SHM.
Bacterial Meningitis, Non-Specific Troponin Elevation, Antibiotics for ECOPD, VTE Update, and More
Treatment of Bacterial Meningitis with Vancomycin
Ricard JD, Wolff M, Lacherade JC, et al. Levels of vancomycin in cerebrospinal fluid of adult patients receiving adjunctive corticosteroids to treat pneumococcal meningitis: a prospective multicenter observational study. Clin Infect Dis. 2007 Jan 15;44(2):250-255. Epub 2006 Dec 15.
In 2002, van de Beek and de Gans published a study demonstrating that adjuvant dexamethasone decreased mortality and improved neurological disability when given to patients with bacterial meningitis. Their results changed our treatment paradigm for this disease but left us with several questions. At what point in the treatment course does giving corticosteroids become ineffective? Do their results apply to all bacterial pathogens? Can the results be applied to the use of vancomycin in treating penicillin-resistant strains of Streptococcus pneumoniae? This final question arises from the disturbing ability of vancomycin to penetrate the cerebrospinal fluid (CSF). Previous data support this concern; thus, bactericidal titers may be inadequate within the CSF. Because meningeal inflammation exerts a strong influence over whether or not vancomycin enters the CSF, administering steroids may decrease its ability to do so. This study brings some clarity to the issue.
In this observational open multicenter trial from France, 14 adults were admitted to intensive care units with suspected pneumococcal meningitis. They were treated with intravenous cefotaxime, vancomycin, and dexamethasone. The vancomycin was given as a loading dose of 15 mg per kg of body weight followed by administration of a continuous infusion of 60 mg per kg of body weight per day. The diagnosis of pneumococcal meningitis was made using a CSF pleocytosis as well as one or more of the following: a positive culture from either the blood or CSF, a Gram stain showing Gram-positive diplococci, or pneumococcal antigens in the CSF as demonstrated by latex agglutination. Patients had a second lumbar puncture on either day two or three to measure vancomycin levels—among other markers of disease activity—in the CSF. Serum levels of vancomycin were drawn simultaneously.
Thirteen of the 14 patients had pneumococcal meningitis; one patient was found to have meningitis from Neisseria meningitidis. Seven patients had pneumococcal strains resistant to penicillin. Ten of the 14 patients required mechanical intubation. The second lumbar puncture demonstrated marked improvements in leukocyte counts, protein levels, and glucose levels. All subsequent cultures from the CSF were negative. Three patients died, two had neurological sequelae, and the remainder were discharged from the hospital without complications. Vancomycin concentrations in the serum ranged from 14.2 to 39.0 mg/L, with a mean of 25.2 mg/L; concentrations in the CSF ranged from 3.1 to 22.3 mg/L, with a mean of 7.9 mg/L. There was a significant correlation between vancomycin levels in the serum and those in the CSF (r = 0.68; P = 0.01). The concentration of vancomycin in the CSF was between four and 10 times the mean inhibitory concentrations (MICs). A linear correlation exists between penetration of vancomycin into CSF and serum levels. No evidence of drug toxicities was observed.
The results demonstrate that a therapeutic concentration of vancomycin can be achieved in the CSF. The continuous infusion of vancomycin with a loading dose, which has not been standard practice, has previously been shown to achieve targeted serum levels more quickly than intermittent dosing. Levels of serum vancomycin were likely higher in this study than when troughs of 15-20 mg/L are the goal. This data strongly suggests, however, that this same treatment regimen can obtain adequate vancomycin levels in the CSF while treating pneumococcal meningitis with adjunctive steroids.
Nonspecific elevations in troponins
Alcalai R, Planer D, Culhaoqlu A, et al. Acute coronary syndrome vs nonspecific troponin elevation: clinical predictors and survival analysis. Arch Intern Med. 2007 Feb 12;167(3):276-281.
In 2000, the American College of Cardiology (ACC) and the European Society of Cardiology (ESC) jointly produced a recommendation for a new definition of myocardial infarction. This proposal based the diagnosis primarily on the elevation of biomarkers specific to cardiac tissue, troponin T and troponin I. Since that time, as use of these blood tests has escalated, it is apparent that elevations in these biomarkers do not always translate into thrombotic coronary artery occlusion. Instead, we have seen that they are positive in a variety of clinical settings. These include sepsis, renal failure, pulmonary embolism, and atrial fibrillation. This investigation attempts to characterize the differences among patients presenting with acute coronary syndrome (ACS) and nonthrombotic troponin elevation (NTTE), to report on outcomes for each, and to note the positive predictive values (PPV) for elevated troponins across clinical settings.
Two hospitals in Israel collected data on all adult patients who experienced an elevation in troponin T (defined as at least 0.1 ng/mL) at any time during their hospital stay. Six hundred and fifteen patients were evaluated by age, sex, cardiovascular risk factors, history of ischemic heart disease, left ventricular function (LVF) by echocardiogram, serum creatine phosphokinase (CPK), and creatinine levels, as well as by which hospital service each had been admitted under. The highest troponin T value was used in the analysis, along with the creatinine level taken on the same day. Two physicians, one a specialist in internal medicine and the other a specialist in cardiology, independently determined the principal diagnosis in accordance with the ACC/ESC guidelines for thrombotic ACS and used other diagnostic studies for alternative diagnosis for conditions known to cause NTTE.
Patients were followed up for causes of mortality for up to two-and-a-half years. Kappa (k) was calculated for physician agreement regarding the principal diagnosis. To assess independent odds ratios and their 95% confidence intervals (CIs) of predictor variables for ACS, an unconditional multiple logistic regression analysis was used. The PPV for troponin T in the diagnosis of ACS was calculated. In-house mortality rates were measured. Long-term risk of death was assessed using Cox proportional hazard models.
The diagnosis of ACS was made in only 53% (326) of the patients. Forty-one percent (254) had NTTE, and the diagnosis was not determined in 6% (35). The diagnoses comprising NTTE included—in order from most to least common—cardiac non-ischemic conditions, sepsis, pulmonary diseases, and neurologic diseases. Using the multivariate analysis, the diagnostic predictors for ACS were history of hypertension or ischemic heart disease, age between 40 and 70 years, higher troponin levels (greater than 1.0 ng/mL), and normal renal function. Extreme age and admission to a surgical team were negative predictors for ACS. Gender, presence of diabetes, and LVF did not appear to make a difference.
The PPV of an elevated troponin T for ACS among all patients was only 56% (95% CI, 52%-60%). It became lower (27%) in those older than 70 with abnormal renal function and higher (90%) in those with a troponin T greater than 1.0 ng/mL and normal renal function. In-house mortality for all patients was 8%; for those with ACS, it was 3%, while for those with NTTE, it was—at 21%—almost eight times higher than the ACS group (P<0.001). Patients were followed up for mortality for a median of 22 months. The long-term mortality was also significantly better (P<0.001) for those with a diagnosis of ACS than for those with NTTE.
Since the incorporation of the ACC/ESC guidelines, the diagnosis of ACS has substantially increased. It is critical to distinguish between ACS and NTTE when using these very sensitive biomarkers, because the underlying cause of NTTE usually requires a drastically different therapy than that of ACS; in addition, misdiagnosing a myocardial infarction may lead to potentially harmful diagnostic studies and therapies in the form of coronary angiography, antithrombotics, and antiplatelet agents. Hospitalists should look for ACS when troponin T levels exceed 1.0 ng/mL in the face of normal renal function. Based on their data, the authors present an algorithm for working up ACS and NTTE that takes into consideration the clinical presentation, age, renal function, electrocardiographic changes, and troponin T levels. Though this is a retrospective trial, it provides guidance for a very common clinical scenario. We should be concerned about a patient’s prognosis when we encounter an elevated troponin in a setting of NTTE.
Guiding Antibiotic Therapy for COPD Exacerbations
Stolz D, Christ-Crain M, Bingisser R, et al. Antibiotic treatment of exacerbations of COPD: a randomized, controlled trial comparing procalcitonin-guidance with standard therapy. Chest. 2007 Jan;131(1):9-19.
Chronic obstructive pulmonary disease (COPD) is a leading cause of morbidity and mortality in the United States. Exacerbations of COPD (ECOPD) that require hospitalization are both common and costly. Though recent literature suggests that antibiotic therapy during exacerbations reduces morbidity and mortality and lowers the lack of response to treatment, controversy persists concerning whether or not these results are applicable to all patients with this condition. Procalcitonin is a protein not typically measurable in plasma. Levels of this protein rise with bacterial infections, but appear to be unaffected by inflammation from other etiologies such as autoimmune processes or viral infections. Measuring procalcitonin levels has already been shown to safely decrease the use of antibiotics in lower respiratory infections.
This single-center trial from Switzerland evaluated consecutive patients admitted from the emergency department with ECOPD. For 226 enrolled patients, symptoms were quantified, sputum was collected, spirometry was measured, and procalcitonin levels were evaluated. Attending physicians chose antibiotics, using current guidelines, for patients randomized to the standard therapy group. In the group randomized to procalcitonin guidance, antibiotics were given according to serum levels. No antibiotics were administered for levels below 0.1 micrograms (mcg)/L; antibiotics were encouraged for levels greater than 0.25 mcg/L. For levels between 0.1 and .25 mcg/L, antibiotics were encouraged or discouraged based on the clinical condition of the patient. The primary outcomes evaluated were total antibiotics used during hospitalization and up to six months following hospitalization. Secondary endpoints included clinical and laboratory data and six-month follow-up for exacerbation rate and time to the next ECOPD.
Procalcitonin guidance significantly decreased antibiotic administration compared with the standard-therapy arm (40% versus 72% respectively; P<0.0001) and antibiotic exposure (RR, 0.56; 95% CI, 0.43 to 0.73; P<0.0001). The absolute risk reduction was 31.5% (95% CI, 18.7 to 44.3%; p<0.0001). No difference in the mean time to the next exacerbation was noticed between the two groups. Clinical and laboratory measures at baseline and through the six-month follow-up demonstrated no significant differences.
Using procalcitonin levels to guide antibiotic therapy for ECOPD is a practice that is exciting and full of promise. Not only could costs be cut by omitting antibiotics for this treatment regimen in select patients, but some pressure will be relieved in terms of decreasing emerging bacterial resistance. Because procalcitonin levels have a lab turn-around time of approximately one hour, this test becomes even more attractive: decisions for treatment can be made while patients are still in the emergency department. On a cautionary note, there is more than one method of testing for procalcitonin levels, and this trial was done at only one center. Before widespread use of this test is applied, these results should be validated in a multicenter trial. In addition, one test should be used consistently for measuring procalcitonin levels.
Community-Associated MRSA and MSSA: Clinical and Epidemiologic Characteristics
Miller LG, Perdreau-Remington F, Bayer AS, et al. Clinical and epidemiologic characteristics cannot distinguish community-associated methicillin-resistant Staphylococcus aureus infection from methicillin-susceptible S. aureus infection: a prospective investigation. Clin Infect Dis. 2007 Feb 15;44(4):471-482. Epub 2007 Jan 19.
Methicillin-susceptible Staphylococcus aureus (MSSA) was, until very recently, the predominant strain seen in community-associated (CA) S. aureus infections. Now methicillin-resistant S aureus (MRSA) is a concern around the world. Deciding whether or not to treat empirically for MRSA in those patients who do not have risk factors for healthcare-associated (HCA) infections is difficult.
Investigators at the University of California-Los Angeles Medical Center (Torrance) prospectively evaluated consecutive patients admitted to the county hospital with S. aureus infections. Daily cultures of wounds, urine, blood, and sputum were taken. An extensive questionnaire was completed by 280 patients who provided information on exposures, demographic characteristics, and clinical characteristics. CA infections were defined as those not having a positive culture from a surgical site in a patient who, in the past year, had not lived in an extended living facility, had any indwelling devices, visited an infusion center, or received dialysis.
Of those evaluated, 202 patients (78%) had CA S. aureus and 78 (28%) had HCA S. aureus. Of those with the CA infections, 108 (60%) had MRSA and 72 (40%) had MSSA. Sensitivity, specificity, predictive values, and likelihood ratios for the risk factors evaluated were unable to distinguish CA-MRSA from CA-MSSA. For example, the sensitivities for most MRSA risk factors were less than 30%, and all the positive likelihood ratios were lower than three.
This study has very important consequences. Given the data presented, there is currently no way to consistently distinguish between CA-MRSA and CA-MSSA prior to culture results. It would be very reasonable in this population to treat for MRSA empirically. One limitation is that the information comes from a single center in an area that has a very diverse patient population. Also, because this was done at a county hospital, the resources for treating patients who would be cared for in the outpatient arena at other centers might not otherwise be available, thus generalizing this data to potential outpatients. Because the morbidity and mortality from a delay in treatment of MRSA infections is significant, however, it appears sensible to treat CA S. aureus empirically in areas where CA-MRSA is common, regardless of patients’ risk factors.
Venous Thromboembolism Update
King CS, Holley AB, Jackson JL, et al. Twice vs three times daily heparin dosing for thromboembolism prophylaxis in the general medical population: a metaanalysis. Chest. 2007 Feb;131(2):507-516.
Nijkeuter M, Sohne M, Tick LW, et al. The natural course of hemodynamically stable pulmonary embolism: clinical outcome and risk factors in a large prospective cohort study. Chest. 2007 Feb;131(2):517-523.
Segal JB, Streiff MB, Hoffman LV, et al. Management of venous thromboembolism: a systematic review for a practice guideline. Ann Intern Med. 2007 Feb 6;146(3):211-222.
Snow V, Qaseem A, Barry P, et al. Management of venous thromboembolism: a clinical practice guideline from the American College of Physicians and the American Academy of Family Physicians. Ann Intern Med. 2007 Feb 6;146 (3):204-210. Epub 2007 Jan 29.
The prevention and treatment of venous thromboembolism (VTE) is a skill set required for all hospitalists given the prevalence of this condition in hospitalized patients as well as the significant morbidity and mortality associated with the condition. Several articles that help to guide our decisions in managing VTE have been published recently.
We have no randomized controlled trials (RCT) comparing twice-daily (bid) with three-times-daily (tid) dosing of unfractionated heparin (UFH) for the prevention of VTE in medically ill patient populations. It is unlikely that such a study, involving an adequate number of patients, will ever be conducted. Though low molecular weight heparins (LMWH) are used more frequently for VTE prevention, many hospitalists still use UFH to prevent VTE in patients who are morbidly obese or who have profound renal insufficiency. King and colleagues have done a meta-analysis to find out whether or not tid dosing is superior to bid dosing for VTE prevention. Twelve studies, including almost 8,000 patients from 1966 to 2004, were reviewed. All patients were hospitalized for medical rather than surgical conditions.
Tid heparin significantly decreased the incidence of the combined outcome of pulmonary embolism (PE) and proximal deep vein thrombosis (DVT). There was a trend toward significance in decreasing the incidence of PE. There was a significant increase in the number of major bleeds with tid dosing compared with bid dosing. There are many limitations to this study: It is retrospective, the population is extremely heterogeneous, and varying methods have been employed to diagnosis VTE across the many studies from which data were pooled. This is likely the best data we will have for UFH in VTE prevention, however. In summary, tid dosing is preferred for high-risk patients, but bid dosing should be considered for those at risk for bleeding complications.
Data are limited for the clinical course of PE. Outpatient treatment of PE with LMWH is not uncommon in select patients, but choosing who is safe to treat in this arena is uncertain. Nijkeuter and colleagues assessed the incidence of recurrent VTE, hemorrhagic complications from therapy, mortality, risk factors for recurrence, and the course of these events from the time of diagnosis through a three-month follow-up period.
Six hundred and seventy-three patients completed the three-month follow-up. Twenty of them (3%) had recurrent VTE; 14 of these had recurrent PE. Recurrence predominantly transpired in the first three weeks of therapy. Of those with recurrent PE, 11 (79%) were fatal, and most of these occurred within the first week of diagnosis. Major bleeding occurred in 1.5% of the patients. Immobilization for more than three days was a significant risk factor for recurrence. Inpatient status, a diagnosis of COPD, and malignancy were independent risk factors for bleeding complications. Fifty-five patients (8.2%) died over the three-month period. Twenty percent died of fatal recurrent PE, while 4% suffered fatal hemorrhage.
Multivariate analysis revealed four characteristics as independent risk factors for mortality in patients with PE. These include age, inpatient status, immobilization for more than three days, and malignancy. It appears that the majority of recurrent and fatal PE occurs during the first week of therapy. Physicians should not discharge patients to home with LMWH for PE without considering these risk factors for hemorrhage, recurrence, and mortality.
Annals of Internal Medicine has published a systematic review of management issues in VTE to provide the framework for the American College of Physicians practice guidelines. These guidelines pool data from more than 100 randomized controlled trials and comment on six areas in VTE management. The following are quotes from this document.
Recommendation #1: Use low molecular-weight heparin (LMWH) rather than unfractionated heparin whenever possible for the initial inpatient treatment of deep vein thrombosis (DVT). Either unfractionated heparin or LMWH is appropriate for the initial treatment of pulmonary embolism.
Recommendation #2: Outpatient treatment of DVT, and possibly pulmonary embolism, with LMWH is safe and cost-effective for carefully selected patients and should be considered if the required support services are in place.
Recommendation #3: Compression stockings should be used routinely to prevent post-thrombotic syndrome, beginning within one month of diagnosis of proximal DVT and continuing for a minimum of one year after diagnosis.
Recommendation #4: There is insufficient evidence to make specific recommendations for types of anticoagulation management of VTE in pregnant women.
Recommendation #5: Anticoagulation should be maintained for three to six months for VTE secondary to transient risk factors and for more than 12 months for recurrent VTE. While the appropriate duration of anticoagulation for idiopathic or recurrent VTE is not definitively known, there is evidence of substantial benefit for extended-duration therapy.
Recommendation #6: LMWH is safe and efficacious for the long-term treatment of VTE in selected patients (and may be preferable for patients with cancer).
All of these seem reasonable and appropriate with a possible exception in the second recommendation. Using LMWH to treat patients diagnosed with PE in the outpatient setting is not well supported by data. The vast majority of trials involving the treatment of VTE with LMWH have been conducted on those with DVT; the number of patients in the trials with PE has been very small. The Food and Drug Administration has not approved LMWH for outpatient treatment of PE; LMWH is FDA approved in the outpatient setting only for the treatment of DVT. We know that the hemodynamic changes that can accompany PE may not occur for at least 24 hours. In addition, we now have data from the Nijkeuter study that point to dangers that may result from treating PE outside the hospital setting. At this time, we should treat PE with LMWH in the outpatient setting only with patients whose risk factors, clinical characteristics, and outpatient resources have been carefully scrutinized. TH
Treatment of Bacterial Meningitis with Vancomycin
Ricard JD, Wolff M, Lacherade JC, et al. Levels of vancomycin in cerebrospinal fluid of adult patients receiving adjunctive corticosteroids to treat pneumococcal meningitis: a prospective multicenter observational study. Clin Infect Dis. 2007 Jan 15;44(2):250-255. Epub 2006 Dec 15.
In 2002, van de Beek and de Gans published a study demonstrating that adjuvant dexamethasone decreased mortality and improved neurological disability when given to patients with bacterial meningitis. Their results changed our treatment paradigm for this disease but left us with several questions. At what point in the treatment course does giving corticosteroids become ineffective? Do their results apply to all bacterial pathogens? Can the results be applied to the use of vancomycin in treating penicillin-resistant strains of Streptococcus pneumoniae? This final question arises from the disturbing ability of vancomycin to penetrate the cerebrospinal fluid (CSF). Previous data support this concern; thus, bactericidal titers may be inadequate within the CSF. Because meningeal inflammation exerts a strong influence over whether or not vancomycin enters the CSF, administering steroids may decrease its ability to do so. This study brings some clarity to the issue.
In this observational open multicenter trial from France, 14 adults were admitted to intensive care units with suspected pneumococcal meningitis. They were treated with intravenous cefotaxime, vancomycin, and dexamethasone. The vancomycin was given as a loading dose of 15 mg per kg of body weight followed by administration of a continuous infusion of 60 mg per kg of body weight per day. The diagnosis of pneumococcal meningitis was made using a CSF pleocytosis as well as one or more of the following: a positive culture from either the blood or CSF, a Gram stain showing Gram-positive diplococci, or pneumococcal antigens in the CSF as demonstrated by latex agglutination. Patients had a second lumbar puncture on either day two or three to measure vancomycin levels—among other markers of disease activity—in the CSF. Serum levels of vancomycin were drawn simultaneously.
Thirteen of the 14 patients had pneumococcal meningitis; one patient was found to have meningitis from Neisseria meningitidis. Seven patients had pneumococcal strains resistant to penicillin. Ten of the 14 patients required mechanical intubation. The second lumbar puncture demonstrated marked improvements in leukocyte counts, protein levels, and glucose levels. All subsequent cultures from the CSF were negative. Three patients died, two had neurological sequelae, and the remainder were discharged from the hospital without complications. Vancomycin concentrations in the serum ranged from 14.2 to 39.0 mg/L, with a mean of 25.2 mg/L; concentrations in the CSF ranged from 3.1 to 22.3 mg/L, with a mean of 7.9 mg/L. There was a significant correlation between vancomycin levels in the serum and those in the CSF (r = 0.68; P = 0.01). The concentration of vancomycin in the CSF was between four and 10 times the mean inhibitory concentrations (MICs). A linear correlation exists between penetration of vancomycin into CSF and serum levels. No evidence of drug toxicities was observed.
The results demonstrate that a therapeutic concentration of vancomycin can be achieved in the CSF. The continuous infusion of vancomycin with a loading dose, which has not been standard practice, has previously been shown to achieve targeted serum levels more quickly than intermittent dosing. Levels of serum vancomycin were likely higher in this study than when troughs of 15-20 mg/L are the goal. This data strongly suggests, however, that this same treatment regimen can obtain adequate vancomycin levels in the CSF while treating pneumococcal meningitis with adjunctive steroids.
Nonspecific elevations in troponins
Alcalai R, Planer D, Culhaoqlu A, et al. Acute coronary syndrome vs nonspecific troponin elevation: clinical predictors and survival analysis. Arch Intern Med. 2007 Feb 12;167(3):276-281.
In 2000, the American College of Cardiology (ACC) and the European Society of Cardiology (ESC) jointly produced a recommendation for a new definition of myocardial infarction. This proposal based the diagnosis primarily on the elevation of biomarkers specific to cardiac tissue, troponin T and troponin I. Since that time, as use of these blood tests has escalated, it is apparent that elevations in these biomarkers do not always translate into thrombotic coronary artery occlusion. Instead, we have seen that they are positive in a variety of clinical settings. These include sepsis, renal failure, pulmonary embolism, and atrial fibrillation. This investigation attempts to characterize the differences among patients presenting with acute coronary syndrome (ACS) and nonthrombotic troponin elevation (NTTE), to report on outcomes for each, and to note the positive predictive values (PPV) for elevated troponins across clinical settings.
Two hospitals in Israel collected data on all adult patients who experienced an elevation in troponin T (defined as at least 0.1 ng/mL) at any time during their hospital stay. Six hundred and fifteen patients were evaluated by age, sex, cardiovascular risk factors, history of ischemic heart disease, left ventricular function (LVF) by echocardiogram, serum creatine phosphokinase (CPK), and creatinine levels, as well as by which hospital service each had been admitted under. The highest troponin T value was used in the analysis, along with the creatinine level taken on the same day. Two physicians, one a specialist in internal medicine and the other a specialist in cardiology, independently determined the principal diagnosis in accordance with the ACC/ESC guidelines for thrombotic ACS and used other diagnostic studies for alternative diagnosis for conditions known to cause NTTE.
Patients were followed up for causes of mortality for up to two-and-a-half years. Kappa (k) was calculated for physician agreement regarding the principal diagnosis. To assess independent odds ratios and their 95% confidence intervals (CIs) of predictor variables for ACS, an unconditional multiple logistic regression analysis was used. The PPV for troponin T in the diagnosis of ACS was calculated. In-house mortality rates were measured. Long-term risk of death was assessed using Cox proportional hazard models.
The diagnosis of ACS was made in only 53% (326) of the patients. Forty-one percent (254) had NTTE, and the diagnosis was not determined in 6% (35). The diagnoses comprising NTTE included—in order from most to least common—cardiac non-ischemic conditions, sepsis, pulmonary diseases, and neurologic diseases. Using the multivariate analysis, the diagnostic predictors for ACS were history of hypertension or ischemic heart disease, age between 40 and 70 years, higher troponin levels (greater than 1.0 ng/mL), and normal renal function. Extreme age and admission to a surgical team were negative predictors for ACS. Gender, presence of diabetes, and LVF did not appear to make a difference.
The PPV of an elevated troponin T for ACS among all patients was only 56% (95% CI, 52%-60%). It became lower (27%) in those older than 70 with abnormal renal function and higher (90%) in those with a troponin T greater than 1.0 ng/mL and normal renal function. In-house mortality for all patients was 8%; for those with ACS, it was 3%, while for those with NTTE, it was—at 21%—almost eight times higher than the ACS group (P<0.001). Patients were followed up for mortality for a median of 22 months. The long-term mortality was also significantly better (P<0.001) for those with a diagnosis of ACS than for those with NTTE.
Since the incorporation of the ACC/ESC guidelines, the diagnosis of ACS has substantially increased. It is critical to distinguish between ACS and NTTE when using these very sensitive biomarkers, because the underlying cause of NTTE usually requires a drastically different therapy than that of ACS; in addition, misdiagnosing a myocardial infarction may lead to potentially harmful diagnostic studies and therapies in the form of coronary angiography, antithrombotics, and antiplatelet agents. Hospitalists should look for ACS when troponin T levels exceed 1.0 ng/mL in the face of normal renal function. Based on their data, the authors present an algorithm for working up ACS and NTTE that takes into consideration the clinical presentation, age, renal function, electrocardiographic changes, and troponin T levels. Though this is a retrospective trial, it provides guidance for a very common clinical scenario. We should be concerned about a patient’s prognosis when we encounter an elevated troponin in a setting of NTTE.
Guiding Antibiotic Therapy for COPD Exacerbations
Stolz D, Christ-Crain M, Bingisser R, et al. Antibiotic treatment of exacerbations of COPD: a randomized, controlled trial comparing procalcitonin-guidance with standard therapy. Chest. 2007 Jan;131(1):9-19.
Chronic obstructive pulmonary disease (COPD) is a leading cause of morbidity and mortality in the United States. Exacerbations of COPD (ECOPD) that require hospitalization are both common and costly. Though recent literature suggests that antibiotic therapy during exacerbations reduces morbidity and mortality and lowers the lack of response to treatment, controversy persists concerning whether or not these results are applicable to all patients with this condition. Procalcitonin is a protein not typically measurable in plasma. Levels of this protein rise with bacterial infections, but appear to be unaffected by inflammation from other etiologies such as autoimmune processes or viral infections. Measuring procalcitonin levels has already been shown to safely decrease the use of antibiotics in lower respiratory infections.
This single-center trial from Switzerland evaluated consecutive patients admitted from the emergency department with ECOPD. For 226 enrolled patients, symptoms were quantified, sputum was collected, spirometry was measured, and procalcitonin levels were evaluated. Attending physicians chose antibiotics, using current guidelines, for patients randomized to the standard therapy group. In the group randomized to procalcitonin guidance, antibiotics were given according to serum levels. No antibiotics were administered for levels below 0.1 micrograms (mcg)/L; antibiotics were encouraged for levels greater than 0.25 mcg/L. For levels between 0.1 and .25 mcg/L, antibiotics were encouraged or discouraged based on the clinical condition of the patient. The primary outcomes evaluated were total antibiotics used during hospitalization and up to six months following hospitalization. Secondary endpoints included clinical and laboratory data and six-month follow-up for exacerbation rate and time to the next ECOPD.
Procalcitonin guidance significantly decreased antibiotic administration compared with the standard-therapy arm (40% versus 72% respectively; P<0.0001) and antibiotic exposure (RR, 0.56; 95% CI, 0.43 to 0.73; P<0.0001). The absolute risk reduction was 31.5% (95% CI, 18.7 to 44.3%; p<0.0001). No difference in the mean time to the next exacerbation was noticed between the two groups. Clinical and laboratory measures at baseline and through the six-month follow-up demonstrated no significant differences.
Using procalcitonin levels to guide antibiotic therapy for ECOPD is a practice that is exciting and full of promise. Not only could costs be cut by omitting antibiotics for this treatment regimen in select patients, but some pressure will be relieved in terms of decreasing emerging bacterial resistance. Because procalcitonin levels have a lab turn-around time of approximately one hour, this test becomes even more attractive: decisions for treatment can be made while patients are still in the emergency department. On a cautionary note, there is more than one method of testing for procalcitonin levels, and this trial was done at only one center. Before widespread use of this test is applied, these results should be validated in a multicenter trial. In addition, one test should be used consistently for measuring procalcitonin levels.
Community-Associated MRSA and MSSA: Clinical and Epidemiologic Characteristics
Miller LG, Perdreau-Remington F, Bayer AS, et al. Clinical and epidemiologic characteristics cannot distinguish community-associated methicillin-resistant Staphylococcus aureus infection from methicillin-susceptible S. aureus infection: a prospective investigation. Clin Infect Dis. 2007 Feb 15;44(4):471-482. Epub 2007 Jan 19.
Methicillin-susceptible Staphylococcus aureus (MSSA) was, until very recently, the predominant strain seen in community-associated (CA) S. aureus infections. Now methicillin-resistant S aureus (MRSA) is a concern around the world. Deciding whether or not to treat empirically for MRSA in those patients who do not have risk factors for healthcare-associated (HCA) infections is difficult.
Investigators at the University of California-Los Angeles Medical Center (Torrance) prospectively evaluated consecutive patients admitted to the county hospital with S. aureus infections. Daily cultures of wounds, urine, blood, and sputum were taken. An extensive questionnaire was completed by 280 patients who provided information on exposures, demographic characteristics, and clinical characteristics. CA infections were defined as those not having a positive culture from a surgical site in a patient who, in the past year, had not lived in an extended living facility, had any indwelling devices, visited an infusion center, or received dialysis.
Of those evaluated, 202 patients (78%) had CA S. aureus and 78 (28%) had HCA S. aureus. Of those with the CA infections, 108 (60%) had MRSA and 72 (40%) had MSSA. Sensitivity, specificity, predictive values, and likelihood ratios for the risk factors evaluated were unable to distinguish CA-MRSA from CA-MSSA. For example, the sensitivities for most MRSA risk factors were less than 30%, and all the positive likelihood ratios were lower than three.
This study has very important consequences. Given the data presented, there is currently no way to consistently distinguish between CA-MRSA and CA-MSSA prior to culture results. It would be very reasonable in this population to treat for MRSA empirically. One limitation is that the information comes from a single center in an area that has a very diverse patient population. Also, because this was done at a county hospital, the resources for treating patients who would be cared for in the outpatient arena at other centers might not otherwise be available, thus generalizing this data to potential outpatients. Because the morbidity and mortality from a delay in treatment of MRSA infections is significant, however, it appears sensible to treat CA S. aureus empirically in areas where CA-MRSA is common, regardless of patients’ risk factors.
Venous Thromboembolism Update
King CS, Holley AB, Jackson JL, et al. Twice vs three times daily heparin dosing for thromboembolism prophylaxis in the general medical population: a metaanalysis. Chest. 2007 Feb;131(2):507-516.
Nijkeuter M, Sohne M, Tick LW, et al. The natural course of hemodynamically stable pulmonary embolism: clinical outcome and risk factors in a large prospective cohort study. Chest. 2007 Feb;131(2):517-523.
Segal JB, Streiff MB, Hoffman LV, et al. Management of venous thromboembolism: a systematic review for a practice guideline. Ann Intern Med. 2007 Feb 6;146(3):211-222.
Snow V, Qaseem A, Barry P, et al. Management of venous thromboembolism: a clinical practice guideline from the American College of Physicians and the American Academy of Family Physicians. Ann Intern Med. 2007 Feb 6;146 (3):204-210. Epub 2007 Jan 29.
The prevention and treatment of venous thromboembolism (VTE) is a skill set required for all hospitalists given the prevalence of this condition in hospitalized patients as well as the significant morbidity and mortality associated with the condition. Several articles that help to guide our decisions in managing VTE have been published recently.
We have no randomized controlled trials (RCT) comparing twice-daily (bid) with three-times-daily (tid) dosing of unfractionated heparin (UFH) for the prevention of VTE in medically ill patient populations. It is unlikely that such a study, involving an adequate number of patients, will ever be conducted. Though low molecular weight heparins (LMWH) are used more frequently for VTE prevention, many hospitalists still use UFH to prevent VTE in patients who are morbidly obese or who have profound renal insufficiency. King and colleagues have done a meta-analysis to find out whether or not tid dosing is superior to bid dosing for VTE prevention. Twelve studies, including almost 8,000 patients from 1966 to 2004, were reviewed. All patients were hospitalized for medical rather than surgical conditions.
Tid heparin significantly decreased the incidence of the combined outcome of pulmonary embolism (PE) and proximal deep vein thrombosis (DVT). There was a trend toward significance in decreasing the incidence of PE. There was a significant increase in the number of major bleeds with tid dosing compared with bid dosing. There are many limitations to this study: It is retrospective, the population is extremely heterogeneous, and varying methods have been employed to diagnosis VTE across the many studies from which data were pooled. This is likely the best data we will have for UFH in VTE prevention, however. In summary, tid dosing is preferred for high-risk patients, but bid dosing should be considered for those at risk for bleeding complications.
Data are limited for the clinical course of PE. Outpatient treatment of PE with LMWH is not uncommon in select patients, but choosing who is safe to treat in this arena is uncertain. Nijkeuter and colleagues assessed the incidence of recurrent VTE, hemorrhagic complications from therapy, mortality, risk factors for recurrence, and the course of these events from the time of diagnosis through a three-month follow-up period.
Six hundred and seventy-three patients completed the three-month follow-up. Twenty of them (3%) had recurrent VTE; 14 of these had recurrent PE. Recurrence predominantly transpired in the first three weeks of therapy. Of those with recurrent PE, 11 (79%) were fatal, and most of these occurred within the first week of diagnosis. Major bleeding occurred in 1.5% of the patients. Immobilization for more than three days was a significant risk factor for recurrence. Inpatient status, a diagnosis of COPD, and malignancy were independent risk factors for bleeding complications. Fifty-five patients (8.2%) died over the three-month period. Twenty percent died of fatal recurrent PE, while 4% suffered fatal hemorrhage.
Multivariate analysis revealed four characteristics as independent risk factors for mortality in patients with PE. These include age, inpatient status, immobilization for more than three days, and malignancy. It appears that the majority of recurrent and fatal PE occurs during the first week of therapy. Physicians should not discharge patients to home with LMWH for PE without considering these risk factors for hemorrhage, recurrence, and mortality.
Annals of Internal Medicine has published a systematic review of management issues in VTE to provide the framework for the American College of Physicians practice guidelines. These guidelines pool data from more than 100 randomized controlled trials and comment on six areas in VTE management. The following are quotes from this document.
Recommendation #1: Use low molecular-weight heparin (LMWH) rather than unfractionated heparin whenever possible for the initial inpatient treatment of deep vein thrombosis (DVT). Either unfractionated heparin or LMWH is appropriate for the initial treatment of pulmonary embolism.
Recommendation #2: Outpatient treatment of DVT, and possibly pulmonary embolism, with LMWH is safe and cost-effective for carefully selected patients and should be considered if the required support services are in place.
Recommendation #3: Compression stockings should be used routinely to prevent post-thrombotic syndrome, beginning within one month of diagnosis of proximal DVT and continuing for a minimum of one year after diagnosis.
Recommendation #4: There is insufficient evidence to make specific recommendations for types of anticoagulation management of VTE in pregnant women.
Recommendation #5: Anticoagulation should be maintained for three to six months for VTE secondary to transient risk factors and for more than 12 months for recurrent VTE. While the appropriate duration of anticoagulation for idiopathic or recurrent VTE is not definitively known, there is evidence of substantial benefit for extended-duration therapy.
Recommendation #6: LMWH is safe and efficacious for the long-term treatment of VTE in selected patients (and may be preferable for patients with cancer).
All of these seem reasonable and appropriate with a possible exception in the second recommendation. Using LMWH to treat patients diagnosed with PE in the outpatient setting is not well supported by data. The vast majority of trials involving the treatment of VTE with LMWH have been conducted on those with DVT; the number of patients in the trials with PE has been very small. The Food and Drug Administration has not approved LMWH for outpatient treatment of PE; LMWH is FDA approved in the outpatient setting only for the treatment of DVT. We know that the hemodynamic changes that can accompany PE may not occur for at least 24 hours. In addition, we now have data from the Nijkeuter study that point to dangers that may result from treating PE outside the hospital setting. At this time, we should treat PE with LMWH in the outpatient setting only with patients whose risk factors, clinical characteristics, and outpatient resources have been carefully scrutinized. TH
Treatment of Bacterial Meningitis with Vancomycin
Ricard JD, Wolff M, Lacherade JC, et al. Levels of vancomycin in cerebrospinal fluid of adult patients receiving adjunctive corticosteroids to treat pneumococcal meningitis: a prospective multicenter observational study. Clin Infect Dis. 2007 Jan 15;44(2):250-255. Epub 2006 Dec 15.
In 2002, van de Beek and de Gans published a study demonstrating that adjuvant dexamethasone decreased mortality and improved neurological disability when given to patients with bacterial meningitis. Their results changed our treatment paradigm for this disease but left us with several questions. At what point in the treatment course does giving corticosteroids become ineffective? Do their results apply to all bacterial pathogens? Can the results be applied to the use of vancomycin in treating penicillin-resistant strains of Streptococcus pneumoniae? This final question arises from the disturbing ability of vancomycin to penetrate the cerebrospinal fluid (CSF). Previous data support this concern; thus, bactericidal titers may be inadequate within the CSF. Because meningeal inflammation exerts a strong influence over whether or not vancomycin enters the CSF, administering steroids may decrease its ability to do so. This study brings some clarity to the issue.
In this observational open multicenter trial from France, 14 adults were admitted to intensive care units with suspected pneumococcal meningitis. They were treated with intravenous cefotaxime, vancomycin, and dexamethasone. The vancomycin was given as a loading dose of 15 mg per kg of body weight followed by administration of a continuous infusion of 60 mg per kg of body weight per day. The diagnosis of pneumococcal meningitis was made using a CSF pleocytosis as well as one or more of the following: a positive culture from either the blood or CSF, a Gram stain showing Gram-positive diplococci, or pneumococcal antigens in the CSF as demonstrated by latex agglutination. Patients had a second lumbar puncture on either day two or three to measure vancomycin levels—among other markers of disease activity—in the CSF. Serum levels of vancomycin were drawn simultaneously.
Thirteen of the 14 patients had pneumococcal meningitis; one patient was found to have meningitis from Neisseria meningitidis. Seven patients had pneumococcal strains resistant to penicillin. Ten of the 14 patients required mechanical intubation. The second lumbar puncture demonstrated marked improvements in leukocyte counts, protein levels, and glucose levels. All subsequent cultures from the CSF were negative. Three patients died, two had neurological sequelae, and the remainder were discharged from the hospital without complications. Vancomycin concentrations in the serum ranged from 14.2 to 39.0 mg/L, with a mean of 25.2 mg/L; concentrations in the CSF ranged from 3.1 to 22.3 mg/L, with a mean of 7.9 mg/L. There was a significant correlation between vancomycin levels in the serum and those in the CSF (r = 0.68; P = 0.01). The concentration of vancomycin in the CSF was between four and 10 times the mean inhibitory concentrations (MICs). A linear correlation exists between penetration of vancomycin into CSF and serum levels. No evidence of drug toxicities was observed.
The results demonstrate that a therapeutic concentration of vancomycin can be achieved in the CSF. The continuous infusion of vancomycin with a loading dose, which has not been standard practice, has previously been shown to achieve targeted serum levels more quickly than intermittent dosing. Levels of serum vancomycin were likely higher in this study than when troughs of 15-20 mg/L are the goal. This data strongly suggests, however, that this same treatment regimen can obtain adequate vancomycin levels in the CSF while treating pneumococcal meningitis with adjunctive steroids.
Nonspecific elevations in troponins
Alcalai R, Planer D, Culhaoqlu A, et al. Acute coronary syndrome vs nonspecific troponin elevation: clinical predictors and survival analysis. Arch Intern Med. 2007 Feb 12;167(3):276-281.
In 2000, the American College of Cardiology (ACC) and the European Society of Cardiology (ESC) jointly produced a recommendation for a new definition of myocardial infarction. This proposal based the diagnosis primarily on the elevation of biomarkers specific to cardiac tissue, troponin T and troponin I. Since that time, as use of these blood tests has escalated, it is apparent that elevations in these biomarkers do not always translate into thrombotic coronary artery occlusion. Instead, we have seen that they are positive in a variety of clinical settings. These include sepsis, renal failure, pulmonary embolism, and atrial fibrillation. This investigation attempts to characterize the differences among patients presenting with acute coronary syndrome (ACS) and nonthrombotic troponin elevation (NTTE), to report on outcomes for each, and to note the positive predictive values (PPV) for elevated troponins across clinical settings.
Two hospitals in Israel collected data on all adult patients who experienced an elevation in troponin T (defined as at least 0.1 ng/mL) at any time during their hospital stay. Six hundred and fifteen patients were evaluated by age, sex, cardiovascular risk factors, history of ischemic heart disease, left ventricular function (LVF) by echocardiogram, serum creatine phosphokinase (CPK), and creatinine levels, as well as by which hospital service each had been admitted under. The highest troponin T value was used in the analysis, along with the creatinine level taken on the same day. Two physicians, one a specialist in internal medicine and the other a specialist in cardiology, independently determined the principal diagnosis in accordance with the ACC/ESC guidelines for thrombotic ACS and used other diagnostic studies for alternative diagnosis for conditions known to cause NTTE.
Patients were followed up for causes of mortality for up to two-and-a-half years. Kappa (k) was calculated for physician agreement regarding the principal diagnosis. To assess independent odds ratios and their 95% confidence intervals (CIs) of predictor variables for ACS, an unconditional multiple logistic regression analysis was used. The PPV for troponin T in the diagnosis of ACS was calculated. In-house mortality rates were measured. Long-term risk of death was assessed using Cox proportional hazard models.
The diagnosis of ACS was made in only 53% (326) of the patients. Forty-one percent (254) had NTTE, and the diagnosis was not determined in 6% (35). The diagnoses comprising NTTE included—in order from most to least common—cardiac non-ischemic conditions, sepsis, pulmonary diseases, and neurologic diseases. Using the multivariate analysis, the diagnostic predictors for ACS were history of hypertension or ischemic heart disease, age between 40 and 70 years, higher troponin levels (greater than 1.0 ng/mL), and normal renal function. Extreme age and admission to a surgical team were negative predictors for ACS. Gender, presence of diabetes, and LVF did not appear to make a difference.
The PPV of an elevated troponin T for ACS among all patients was only 56% (95% CI, 52%-60%). It became lower (27%) in those older than 70 with abnormal renal function and higher (90%) in those with a troponin T greater than 1.0 ng/mL and normal renal function. In-house mortality for all patients was 8%; for those with ACS, it was 3%, while for those with NTTE, it was—at 21%—almost eight times higher than the ACS group (P<0.001). Patients were followed up for mortality for a median of 22 months. The long-term mortality was also significantly better (P<0.001) for those with a diagnosis of ACS than for those with NTTE.
Since the incorporation of the ACC/ESC guidelines, the diagnosis of ACS has substantially increased. It is critical to distinguish between ACS and NTTE when using these very sensitive biomarkers, because the underlying cause of NTTE usually requires a drastically different therapy than that of ACS; in addition, misdiagnosing a myocardial infarction may lead to potentially harmful diagnostic studies and therapies in the form of coronary angiography, antithrombotics, and antiplatelet agents. Hospitalists should look for ACS when troponin T levels exceed 1.0 ng/mL in the face of normal renal function. Based on their data, the authors present an algorithm for working up ACS and NTTE that takes into consideration the clinical presentation, age, renal function, electrocardiographic changes, and troponin T levels. Though this is a retrospective trial, it provides guidance for a very common clinical scenario. We should be concerned about a patient’s prognosis when we encounter an elevated troponin in a setting of NTTE.
Guiding Antibiotic Therapy for COPD Exacerbations
Stolz D, Christ-Crain M, Bingisser R, et al. Antibiotic treatment of exacerbations of COPD: a randomized, controlled trial comparing procalcitonin-guidance with standard therapy. Chest. 2007 Jan;131(1):9-19.
Chronic obstructive pulmonary disease (COPD) is a leading cause of morbidity and mortality in the United States. Exacerbations of COPD (ECOPD) that require hospitalization are both common and costly. Though recent literature suggests that antibiotic therapy during exacerbations reduces morbidity and mortality and lowers the lack of response to treatment, controversy persists concerning whether or not these results are applicable to all patients with this condition. Procalcitonin is a protein not typically measurable in plasma. Levels of this protein rise with bacterial infections, but appear to be unaffected by inflammation from other etiologies such as autoimmune processes or viral infections. Measuring procalcitonin levels has already been shown to safely decrease the use of antibiotics in lower respiratory infections.
This single-center trial from Switzerland evaluated consecutive patients admitted from the emergency department with ECOPD. For 226 enrolled patients, symptoms were quantified, sputum was collected, spirometry was measured, and procalcitonin levels were evaluated. Attending physicians chose antibiotics, using current guidelines, for patients randomized to the standard therapy group. In the group randomized to procalcitonin guidance, antibiotics were given according to serum levels. No antibiotics were administered for levels below 0.1 micrograms (mcg)/L; antibiotics were encouraged for levels greater than 0.25 mcg/L. For levels between 0.1 and .25 mcg/L, antibiotics were encouraged or discouraged based on the clinical condition of the patient. The primary outcomes evaluated were total antibiotics used during hospitalization and up to six months following hospitalization. Secondary endpoints included clinical and laboratory data and six-month follow-up for exacerbation rate and time to the next ECOPD.
Procalcitonin guidance significantly decreased antibiotic administration compared with the standard-therapy arm (40% versus 72% respectively; P<0.0001) and antibiotic exposure (RR, 0.56; 95% CI, 0.43 to 0.73; P<0.0001). The absolute risk reduction was 31.5% (95% CI, 18.7 to 44.3%; p<0.0001). No difference in the mean time to the next exacerbation was noticed between the two groups. Clinical and laboratory measures at baseline and through the six-month follow-up demonstrated no significant differences.
Using procalcitonin levels to guide antibiotic therapy for ECOPD is a practice that is exciting and full of promise. Not only could costs be cut by omitting antibiotics for this treatment regimen in select patients, but some pressure will be relieved in terms of decreasing emerging bacterial resistance. Because procalcitonin levels have a lab turn-around time of approximately one hour, this test becomes even more attractive: decisions for treatment can be made while patients are still in the emergency department. On a cautionary note, there is more than one method of testing for procalcitonin levels, and this trial was done at only one center. Before widespread use of this test is applied, these results should be validated in a multicenter trial. In addition, one test should be used consistently for measuring procalcitonin levels.
Community-Associated MRSA and MSSA: Clinical and Epidemiologic Characteristics
Miller LG, Perdreau-Remington F, Bayer AS, et al. Clinical and epidemiologic characteristics cannot distinguish community-associated methicillin-resistant Staphylococcus aureus infection from methicillin-susceptible S. aureus infection: a prospective investigation. Clin Infect Dis. 2007 Feb 15;44(4):471-482. Epub 2007 Jan 19.
Methicillin-susceptible Staphylococcus aureus (MSSA) was, until very recently, the predominant strain seen in community-associated (CA) S. aureus infections. Now methicillin-resistant S aureus (MRSA) is a concern around the world. Deciding whether or not to treat empirically for MRSA in those patients who do not have risk factors for healthcare-associated (HCA) infections is difficult.
Investigators at the University of California-Los Angeles Medical Center (Torrance) prospectively evaluated consecutive patients admitted to the county hospital with S. aureus infections. Daily cultures of wounds, urine, blood, and sputum were taken. An extensive questionnaire was completed by 280 patients who provided information on exposures, demographic characteristics, and clinical characteristics. CA infections were defined as those not having a positive culture from a surgical site in a patient who, in the past year, had not lived in an extended living facility, had any indwelling devices, visited an infusion center, or received dialysis.
Of those evaluated, 202 patients (78%) had CA S. aureus and 78 (28%) had HCA S. aureus. Of those with the CA infections, 108 (60%) had MRSA and 72 (40%) had MSSA. Sensitivity, specificity, predictive values, and likelihood ratios for the risk factors evaluated were unable to distinguish CA-MRSA from CA-MSSA. For example, the sensitivities for most MRSA risk factors were less than 30%, and all the positive likelihood ratios were lower than three.
This study has very important consequences. Given the data presented, there is currently no way to consistently distinguish between CA-MRSA and CA-MSSA prior to culture results. It would be very reasonable in this population to treat for MRSA empirically. One limitation is that the information comes from a single center in an area that has a very diverse patient population. Also, because this was done at a county hospital, the resources for treating patients who would be cared for in the outpatient arena at other centers might not otherwise be available, thus generalizing this data to potential outpatients. Because the morbidity and mortality from a delay in treatment of MRSA infections is significant, however, it appears sensible to treat CA S. aureus empirically in areas where CA-MRSA is common, regardless of patients’ risk factors.
Venous Thromboembolism Update
King CS, Holley AB, Jackson JL, et al. Twice vs three times daily heparin dosing for thromboembolism prophylaxis in the general medical population: a metaanalysis. Chest. 2007 Feb;131(2):507-516.
Nijkeuter M, Sohne M, Tick LW, et al. The natural course of hemodynamically stable pulmonary embolism: clinical outcome and risk factors in a large prospective cohort study. Chest. 2007 Feb;131(2):517-523.
Segal JB, Streiff MB, Hoffman LV, et al. Management of venous thromboembolism: a systematic review for a practice guideline. Ann Intern Med. 2007 Feb 6;146(3):211-222.
Snow V, Qaseem A, Barry P, et al. Management of venous thromboembolism: a clinical practice guideline from the American College of Physicians and the American Academy of Family Physicians. Ann Intern Med. 2007 Feb 6;146 (3):204-210. Epub 2007 Jan 29.
The prevention and treatment of venous thromboembolism (VTE) is a skill set required for all hospitalists given the prevalence of this condition in hospitalized patients as well as the significant morbidity and mortality associated with the condition. Several articles that help to guide our decisions in managing VTE have been published recently.
We have no randomized controlled trials (RCT) comparing twice-daily (bid) with three-times-daily (tid) dosing of unfractionated heparin (UFH) for the prevention of VTE in medically ill patient populations. It is unlikely that such a study, involving an adequate number of patients, will ever be conducted. Though low molecular weight heparins (LMWH) are used more frequently for VTE prevention, many hospitalists still use UFH to prevent VTE in patients who are morbidly obese or who have profound renal insufficiency. King and colleagues have done a meta-analysis to find out whether or not tid dosing is superior to bid dosing for VTE prevention. Twelve studies, including almost 8,000 patients from 1966 to 2004, were reviewed. All patients were hospitalized for medical rather than surgical conditions.
Tid heparin significantly decreased the incidence of the combined outcome of pulmonary embolism (PE) and proximal deep vein thrombosis (DVT). There was a trend toward significance in decreasing the incidence of PE. There was a significant increase in the number of major bleeds with tid dosing compared with bid dosing. There are many limitations to this study: It is retrospective, the population is extremely heterogeneous, and varying methods have been employed to diagnosis VTE across the many studies from which data were pooled. This is likely the best data we will have for UFH in VTE prevention, however. In summary, tid dosing is preferred for high-risk patients, but bid dosing should be considered for those at risk for bleeding complications.
Data are limited for the clinical course of PE. Outpatient treatment of PE with LMWH is not uncommon in select patients, but choosing who is safe to treat in this arena is uncertain. Nijkeuter and colleagues assessed the incidence of recurrent VTE, hemorrhagic complications from therapy, mortality, risk factors for recurrence, and the course of these events from the time of diagnosis through a three-month follow-up period.
Six hundred and seventy-three patients completed the three-month follow-up. Twenty of them (3%) had recurrent VTE; 14 of these had recurrent PE. Recurrence predominantly transpired in the first three weeks of therapy. Of those with recurrent PE, 11 (79%) were fatal, and most of these occurred within the first week of diagnosis. Major bleeding occurred in 1.5% of the patients. Immobilization for more than three days was a significant risk factor for recurrence. Inpatient status, a diagnosis of COPD, and malignancy were independent risk factors for bleeding complications. Fifty-five patients (8.2%) died over the three-month period. Twenty percent died of fatal recurrent PE, while 4% suffered fatal hemorrhage.
Multivariate analysis revealed four characteristics as independent risk factors for mortality in patients with PE. These include age, inpatient status, immobilization for more than three days, and malignancy. It appears that the majority of recurrent and fatal PE occurs during the first week of therapy. Physicians should not discharge patients to home with LMWH for PE without considering these risk factors for hemorrhage, recurrence, and mortality.
Annals of Internal Medicine has published a systematic review of management issues in VTE to provide the framework for the American College of Physicians practice guidelines. These guidelines pool data from more than 100 randomized controlled trials and comment on six areas in VTE management. The following are quotes from this document.
Recommendation #1: Use low molecular-weight heparin (LMWH) rather than unfractionated heparin whenever possible for the initial inpatient treatment of deep vein thrombosis (DVT). Either unfractionated heparin or LMWH is appropriate for the initial treatment of pulmonary embolism.
Recommendation #2: Outpatient treatment of DVT, and possibly pulmonary embolism, with LMWH is safe and cost-effective for carefully selected patients and should be considered if the required support services are in place.
Recommendation #3: Compression stockings should be used routinely to prevent post-thrombotic syndrome, beginning within one month of diagnosis of proximal DVT and continuing for a minimum of one year after diagnosis.
Recommendation #4: There is insufficient evidence to make specific recommendations for types of anticoagulation management of VTE in pregnant women.
Recommendation #5: Anticoagulation should be maintained for three to six months for VTE secondary to transient risk factors and for more than 12 months for recurrent VTE. While the appropriate duration of anticoagulation for idiopathic or recurrent VTE is not definitively known, there is evidence of substantial benefit for extended-duration therapy.
Recommendation #6: LMWH is safe and efficacious for the long-term treatment of VTE in selected patients (and may be preferable for patients with cancer).
All of these seem reasonable and appropriate with a possible exception in the second recommendation. Using LMWH to treat patients diagnosed with PE in the outpatient setting is not well supported by data. The vast majority of trials involving the treatment of VTE with LMWH have been conducted on those with DVT; the number of patients in the trials with PE has been very small. The Food and Drug Administration has not approved LMWH for outpatient treatment of PE; LMWH is FDA approved in the outpatient setting only for the treatment of DVT. We know that the hemodynamic changes that can accompany PE may not occur for at least 24 hours. In addition, we now have data from the Nijkeuter study that point to dangers that may result from treating PE outside the hospital setting. At this time, we should treat PE with LMWH in the outpatient setting only with patients whose risk factors, clinical characteristics, and outpatient resources have been carefully scrutinized. TH
Homegrown Health
Tucked away in the University of Florida (UF), which is—with 50,000 students—the state’s largest university and the nation’s fourth largest, is its School of Medicine’s Community Health and Family Medicine (CHFM) department, along with that department’s hospital medicine program. The department remains a touchstone for its graduates, past and present, and has created hospitalist leaders to be proud of. Much of that pride emanates from R. Whit Curry Jr., MD, CHFM’s department chairman, who is a friend and mentor to several generations of family medicine physicians.
In late 2001, after a private hospital medicine group headed by John Nelson, MD, a UF graduate, SHM co-founder and columnist for this publication, it was natural for Shands Alachua General Hospital (SAGH) to turn to Dr. Curry to start a hospitalist program. Dr. Curry, who says wryly, “If you’ve seen one hospitalist program, you’ve seen one hospitalist program,” knew he could build a unique team of family medicine physicians into a cohesive hospital medicine group.
UF’s CHFM department had staffed SAGH with residents for years before the hospital asked Dr. Curry to start the hospitalist program. The group’s coverage had been limited to inpatient care for about 20 primary care physicians, with a similar arrangement for a for-profit HCA community hospital across town. SAGH needed a more ambitious hospitalist program, one that would cover unassigned patients as well as inpatient care for local doctors. At that point, SAGH issued a request for proposals (RFP) for a hospital medicine group, accepting UF’s CHFM proposal to fill the vacuum left by the departing group. Initially, SAGH would handle the 20 private physicians’ inpatients covered by the existing group, the overflow of unassigned patients from residency workload restrictions, and admitting and co-management for specialists.
Dr. Curry started building his team with Elizabeth Chmelik, MD, recruiting her as the program’s director straight from CHFM residency. The program’s first year—2002—was whirlwind hectic. Fortunately for the group, Marcia Miller, MD, a 1988 graduate of UF’s CHFM department, had burned herself out running a local private practice with two partners. She turned to Dr. Curry, her mentor and confidant. “I called him, told him I needed a job, and he hired me as the hospitalist program’s co-director,” says Dr. Miller. Her community-based partners endorsed her new career path, and she joined Dr. Chmelik in working every day of the program’s first year. “It was challenging,” she says, “but, with inpatient medicine, I became the doctor I was trained to be.”
Sherri Swilley, the department’s coordinator of administrative affairs, recalls the patient census growing so fast that she struggled to keep up with getting doctors temporary privileges while pursuing credentialing for the permanent staff. “The hospitalists had a fierce work ethic that carried us through those early years,” says Swilley.
Gainesville’s Medical Community
In spring 2003, the program added a local physician eager to exit private practice and a family medicine graduate who had worked in SAGH’s emergency department (ED) for 12 years. In June, they snagged two stellar family medicine graduates and a nocturnist. The nocturnist represents the program’s only turnover; her nightly 12 to 20 admissions proved too much to handle.
UF’s homegrown bunch of family physicians shaped this distinctive hospital medicine program. Dr. Chmelik, assistant professor and co-director of the UF College of Medicine’s hospitalist program at SAGH, says that although family physicians are outnumbered by internal medicine hospitalist physicians they add something special to the role.
“We want to go beyond the stereotype of the internal-medicine trained physician drudging through the hospital as an eternal extension of residency,” says Dr. Chmelik. “Family physicians excel at end-of-life issues and the biopsychosocial model of healthcare, both of which are very important in a hospital.”
Another addition to Dr. Chmelik’s black bag is her expert consulting on obstetric and pediatric patients; she can easily handle issues such as hypertension in a postpartum patient or post-appendectomy asthma in a child.
The Financial Tightrope
The University of Florida’s CHFM is closely tied to SAGH, a 75-year-old, 367-bed community hospital that serves as its training ground for residents and hospitalist fellows. Although SAGH is a vital part of Gainesville’s medical scene, its financial status has been shaky for some time.
In 2002, Moody’s Investor Service gave SAGH a negative rating, which was upgraded to stable in 2003.The upgrade of SAGH’s $312 million of outstanding debt issued by Alachua County Health Facilities Authority kept the wolf from the door temporarily. Talk persists that SAGH is in trouble, however, and a Gainesville group, the Health Care Is a Human Right Coalition, asserted in July 2006 that the hospital lacked leadership and that “there are no published plans to keep SAGH open beyond 2010.” SAGH spokesman Ralph Ives says, “Nothing could be further from the truth.” He points to SAGH’s investment in an 82-bed pediatric hospital in a renovated wing with its own hospital medicine group as an affirmation of the institution’s future.
Like other hospitals struggling with public payers and lots of unassigned patients, SAGH’s hospitalist program improves the hospital’s financial health by serving large numbers of patients cost-effectively. Conversely, hospitalist programs can sink because of inattention to financial basics. Early in SAGH’s history, the hospitalists were overwhelmed with a burgeoning census, leaving coding and billing for clerical staff to figure out at day’s end. This delayed billing and often failed to capture the correct diagnostic codes and level of severity for each patient encounter. As the program matured, the hospitalists and hospital administration agreed that physicians would do the coding themselves as they rounded. That change saved on the back end of clerical work and got the charges in promptly.
Each month, Swilley and the hospitalists review individual and group coding patterns, payer mix, the amount billed to each carrier, the amount of time bills spend in accounts receivable, and the services that have been denied. Hospitalists also receive daily reports on their charges and tips on improving coding. For example, if they’ve reviewed documents, Swilley reminds them to code that activity, just as she does a hospitalist who was swamped in the ED at 10 p.m. and left coding his encounters until 1 a.m., thereby losing one day of service. “A hospital is a business. Our hospitalists work hard; I teach them to work smarter and to pay attention to the bottom line,” says Swilley.
Being diligent about financial productivity matters greatly when it’s time for the CHFM department to negotiate with the hospital for its annual subsidy, which Dr. Curry says is about $500,000.
“When we present our budget, we include billing expenses, overhead, salary, and fringe benefits,” says Swilley. “The more detail we have on the number of patient encounters, [our] productivity, and the revenues we generate, the better position we’re in to get support.”
Like most other hospitals, SAGH struggles with tight reimbursement versus the need for an attractive physical plant. To that end, in 1998 it became the first hospital in Florida to adopt the Planetree model, which advocates patient-driven healthcare, serving body, mind, and spirit. “We’ve always been a deeply compassionate hospital,” says Lynne Mercadante, RN, SAGH’s director of Medical Staff Services, “and Planetree is a natural extension of our looking into our hearts and minds about treating the whole person.”
The hospitalists are deeply connected to the community, knowing when to bring in pastoral care or family therapy, as well as joining in celebrations for weddings and anniversaries.
SAGH has made typical Planetree physical changes, including remodeling one floor into six-patient nursing pods versus one nursing station for 42 patients, along with adding meditation rooms, libraries, a piano in the lobby, and a fish tank. Physicians, patients, and visitors are treated to Brahms’ Lullaby over the loudspeaker system every time a baby is born and can experience pet visitations and aromatherapy.
The Schedule
UF’s hospitalists initially adopted a rotation of seven days on, seven days off. When the group’s nocturnist left because of the heavy burden of night admissions, the nine hospitalists decided to cover call themselves instead of hiring a replacement nocturnist. Every ninth week, a hospitalist takes call Monday through Thursday, with residents carrying the weekend. It isn’t ideal, but it distributes call evenly and ensures hospitalists two free weekends a month.
Like most hospital medicine groups, UF’s hospitalists are trying to tinker with the schedule to make it more flexible. They had some give for Scott Medley, MD, who retired from his large Gainesville private practice of 10 physicians, 65 employees, five offices, and 40,000 patients in 2002 at age 55.
“My private [practice] kept me busy seven days a week. I had been there, done that, got the T-shirt,” says Dr. Medley. “I had enough of private practice, but I didn’t want to leave medicine. On reflection, I realized that what I enjoyed most is taking care of really sick patients, so I contacted Whit Curry, and he hired me as a hospitalist Monday through Friday mornings.”
Dr. Medley loves being an employee, doesn’t mind covering for younger colleagues on holidays, and is now collaborating with specialists who have been his friends and colleagues for 25 years. “I also enjoy teaching younger docs both the art and the economics of medicine—and not having to worry where my next paycheck is coming from,” he says.
Looking ahead, Dr. Miller relishes being a hospitalist at age 47, but wonders what the pace will feel like another decade out. To be more flexible, the group is considering allowing physicians to work more days but fewer hours, initiating job sharing, and recruiting more retirees like Dr. Medley, who can work shorter days and are willing to pinch-hit when coverage is tight. They are working toward consensus on maximizing flexibility while maintaining coverage. Still, there’s no easy solution to fluctuations in census, and Dr. Curry notes that some days span 7:30 a.m. to 3:30 p.m., while others stretch into the night. “We haven’t found a better solution yet to providing care 24 hours a day, 365 days a year,” concludes Dr. Medley.
Creating Value
Looking back at her quarter century at SAGH, Mercadante analyzes how the hospitalist medicine program improved things. “Without hospitalists, whoever was on call covered everyone in the ED, so you had neurologists treating patients with pneumonia. There was little continuity of care, unlike with the hospitalists who meet every morning discussing cases.”
In addition, the medical staff feel they have their lives back because call is covered, and they can count on the hospitalists to co-manage complicated cases. “When specialty physicians are in high demand and short supply, knowing that someone’s covering their hospitalized patients for chronic conditions is so important,” concludes Mercadante.
Looking to the future, UF’s hospitalists plan to build on their cohesiveness, collegiality, and emphasis on family medicine. With alumni of their school at the ready, they know where to turn to continue to grow the program. TH
Marlene Piturro also writes about scheduling in this issue.
Tucked away in the University of Florida (UF), which is—with 50,000 students—the state’s largest university and the nation’s fourth largest, is its School of Medicine’s Community Health and Family Medicine (CHFM) department, along with that department’s hospital medicine program. The department remains a touchstone for its graduates, past and present, and has created hospitalist leaders to be proud of. Much of that pride emanates from R. Whit Curry Jr., MD, CHFM’s department chairman, who is a friend and mentor to several generations of family medicine physicians.
In late 2001, after a private hospital medicine group headed by John Nelson, MD, a UF graduate, SHM co-founder and columnist for this publication, it was natural for Shands Alachua General Hospital (SAGH) to turn to Dr. Curry to start a hospitalist program. Dr. Curry, who says wryly, “If you’ve seen one hospitalist program, you’ve seen one hospitalist program,” knew he could build a unique team of family medicine physicians into a cohesive hospital medicine group.
UF’s CHFM department had staffed SAGH with residents for years before the hospital asked Dr. Curry to start the hospitalist program. The group’s coverage had been limited to inpatient care for about 20 primary care physicians, with a similar arrangement for a for-profit HCA community hospital across town. SAGH needed a more ambitious hospitalist program, one that would cover unassigned patients as well as inpatient care for local doctors. At that point, SAGH issued a request for proposals (RFP) for a hospital medicine group, accepting UF’s CHFM proposal to fill the vacuum left by the departing group. Initially, SAGH would handle the 20 private physicians’ inpatients covered by the existing group, the overflow of unassigned patients from residency workload restrictions, and admitting and co-management for specialists.
Dr. Curry started building his team with Elizabeth Chmelik, MD, recruiting her as the program’s director straight from CHFM residency. The program’s first year—2002—was whirlwind hectic. Fortunately for the group, Marcia Miller, MD, a 1988 graduate of UF’s CHFM department, had burned herself out running a local private practice with two partners. She turned to Dr. Curry, her mentor and confidant. “I called him, told him I needed a job, and he hired me as the hospitalist program’s co-director,” says Dr. Miller. Her community-based partners endorsed her new career path, and she joined Dr. Chmelik in working every day of the program’s first year. “It was challenging,” she says, “but, with inpatient medicine, I became the doctor I was trained to be.”
Sherri Swilley, the department’s coordinator of administrative affairs, recalls the patient census growing so fast that she struggled to keep up with getting doctors temporary privileges while pursuing credentialing for the permanent staff. “The hospitalists had a fierce work ethic that carried us through those early years,” says Swilley.
Gainesville’s Medical Community
In spring 2003, the program added a local physician eager to exit private practice and a family medicine graduate who had worked in SAGH’s emergency department (ED) for 12 years. In June, they snagged two stellar family medicine graduates and a nocturnist. The nocturnist represents the program’s only turnover; her nightly 12 to 20 admissions proved too much to handle.
UF’s homegrown bunch of family physicians shaped this distinctive hospital medicine program. Dr. Chmelik, assistant professor and co-director of the UF College of Medicine’s hospitalist program at SAGH, says that although family physicians are outnumbered by internal medicine hospitalist physicians they add something special to the role.
“We want to go beyond the stereotype of the internal-medicine trained physician drudging through the hospital as an eternal extension of residency,” says Dr. Chmelik. “Family physicians excel at end-of-life issues and the biopsychosocial model of healthcare, both of which are very important in a hospital.”
Another addition to Dr. Chmelik’s black bag is her expert consulting on obstetric and pediatric patients; she can easily handle issues such as hypertension in a postpartum patient or post-appendectomy asthma in a child.
The Financial Tightrope
The University of Florida’s CHFM is closely tied to SAGH, a 75-year-old, 367-bed community hospital that serves as its training ground for residents and hospitalist fellows. Although SAGH is a vital part of Gainesville’s medical scene, its financial status has been shaky for some time.
In 2002, Moody’s Investor Service gave SAGH a negative rating, which was upgraded to stable in 2003.The upgrade of SAGH’s $312 million of outstanding debt issued by Alachua County Health Facilities Authority kept the wolf from the door temporarily. Talk persists that SAGH is in trouble, however, and a Gainesville group, the Health Care Is a Human Right Coalition, asserted in July 2006 that the hospital lacked leadership and that “there are no published plans to keep SAGH open beyond 2010.” SAGH spokesman Ralph Ives says, “Nothing could be further from the truth.” He points to SAGH’s investment in an 82-bed pediatric hospital in a renovated wing with its own hospital medicine group as an affirmation of the institution’s future.
Like other hospitals struggling with public payers and lots of unassigned patients, SAGH’s hospitalist program improves the hospital’s financial health by serving large numbers of patients cost-effectively. Conversely, hospitalist programs can sink because of inattention to financial basics. Early in SAGH’s history, the hospitalists were overwhelmed with a burgeoning census, leaving coding and billing for clerical staff to figure out at day’s end. This delayed billing and often failed to capture the correct diagnostic codes and level of severity for each patient encounter. As the program matured, the hospitalists and hospital administration agreed that physicians would do the coding themselves as they rounded. That change saved on the back end of clerical work and got the charges in promptly.
Each month, Swilley and the hospitalists review individual and group coding patterns, payer mix, the amount billed to each carrier, the amount of time bills spend in accounts receivable, and the services that have been denied. Hospitalists also receive daily reports on their charges and tips on improving coding. For example, if they’ve reviewed documents, Swilley reminds them to code that activity, just as she does a hospitalist who was swamped in the ED at 10 p.m. and left coding his encounters until 1 a.m., thereby losing one day of service. “A hospital is a business. Our hospitalists work hard; I teach them to work smarter and to pay attention to the bottom line,” says Swilley.
Being diligent about financial productivity matters greatly when it’s time for the CHFM department to negotiate with the hospital for its annual subsidy, which Dr. Curry says is about $500,000.
“When we present our budget, we include billing expenses, overhead, salary, and fringe benefits,” says Swilley. “The more detail we have on the number of patient encounters, [our] productivity, and the revenues we generate, the better position we’re in to get support.”
Like most other hospitals, SAGH struggles with tight reimbursement versus the need for an attractive physical plant. To that end, in 1998 it became the first hospital in Florida to adopt the Planetree model, which advocates patient-driven healthcare, serving body, mind, and spirit. “We’ve always been a deeply compassionate hospital,” says Lynne Mercadante, RN, SAGH’s director of Medical Staff Services, “and Planetree is a natural extension of our looking into our hearts and minds about treating the whole person.”
The hospitalists are deeply connected to the community, knowing when to bring in pastoral care or family therapy, as well as joining in celebrations for weddings and anniversaries.
SAGH has made typical Planetree physical changes, including remodeling one floor into six-patient nursing pods versus one nursing station for 42 patients, along with adding meditation rooms, libraries, a piano in the lobby, and a fish tank. Physicians, patients, and visitors are treated to Brahms’ Lullaby over the loudspeaker system every time a baby is born and can experience pet visitations and aromatherapy.
The Schedule
UF’s hospitalists initially adopted a rotation of seven days on, seven days off. When the group’s nocturnist left because of the heavy burden of night admissions, the nine hospitalists decided to cover call themselves instead of hiring a replacement nocturnist. Every ninth week, a hospitalist takes call Monday through Thursday, with residents carrying the weekend. It isn’t ideal, but it distributes call evenly and ensures hospitalists two free weekends a month.
Like most hospital medicine groups, UF’s hospitalists are trying to tinker with the schedule to make it more flexible. They had some give for Scott Medley, MD, who retired from his large Gainesville private practice of 10 physicians, 65 employees, five offices, and 40,000 patients in 2002 at age 55.
“My private [practice] kept me busy seven days a week. I had been there, done that, got the T-shirt,” says Dr. Medley. “I had enough of private practice, but I didn’t want to leave medicine. On reflection, I realized that what I enjoyed most is taking care of really sick patients, so I contacted Whit Curry, and he hired me as a hospitalist Monday through Friday mornings.”
Dr. Medley loves being an employee, doesn’t mind covering for younger colleagues on holidays, and is now collaborating with specialists who have been his friends and colleagues for 25 years. “I also enjoy teaching younger docs both the art and the economics of medicine—and not having to worry where my next paycheck is coming from,” he says.
Looking ahead, Dr. Miller relishes being a hospitalist at age 47, but wonders what the pace will feel like another decade out. To be more flexible, the group is considering allowing physicians to work more days but fewer hours, initiating job sharing, and recruiting more retirees like Dr. Medley, who can work shorter days and are willing to pinch-hit when coverage is tight. They are working toward consensus on maximizing flexibility while maintaining coverage. Still, there’s no easy solution to fluctuations in census, and Dr. Curry notes that some days span 7:30 a.m. to 3:30 p.m., while others stretch into the night. “We haven’t found a better solution yet to providing care 24 hours a day, 365 days a year,” concludes Dr. Medley.
Creating Value
Looking back at her quarter century at SAGH, Mercadante analyzes how the hospitalist medicine program improved things. “Without hospitalists, whoever was on call covered everyone in the ED, so you had neurologists treating patients with pneumonia. There was little continuity of care, unlike with the hospitalists who meet every morning discussing cases.”
In addition, the medical staff feel they have their lives back because call is covered, and they can count on the hospitalists to co-manage complicated cases. “When specialty physicians are in high demand and short supply, knowing that someone’s covering their hospitalized patients for chronic conditions is so important,” concludes Mercadante.
Looking to the future, UF’s hospitalists plan to build on their cohesiveness, collegiality, and emphasis on family medicine. With alumni of their school at the ready, they know where to turn to continue to grow the program. TH
Marlene Piturro also writes about scheduling in this issue.
Tucked away in the University of Florida (UF), which is—with 50,000 students—the state’s largest university and the nation’s fourth largest, is its School of Medicine’s Community Health and Family Medicine (CHFM) department, along with that department’s hospital medicine program. The department remains a touchstone for its graduates, past and present, and has created hospitalist leaders to be proud of. Much of that pride emanates from R. Whit Curry Jr., MD, CHFM’s department chairman, who is a friend and mentor to several generations of family medicine physicians.
In late 2001, after a private hospital medicine group headed by John Nelson, MD, a UF graduate, SHM co-founder and columnist for this publication, it was natural for Shands Alachua General Hospital (SAGH) to turn to Dr. Curry to start a hospitalist program. Dr. Curry, who says wryly, “If you’ve seen one hospitalist program, you’ve seen one hospitalist program,” knew he could build a unique team of family medicine physicians into a cohesive hospital medicine group.
UF’s CHFM department had staffed SAGH with residents for years before the hospital asked Dr. Curry to start the hospitalist program. The group’s coverage had been limited to inpatient care for about 20 primary care physicians, with a similar arrangement for a for-profit HCA community hospital across town. SAGH needed a more ambitious hospitalist program, one that would cover unassigned patients as well as inpatient care for local doctors. At that point, SAGH issued a request for proposals (RFP) for a hospital medicine group, accepting UF’s CHFM proposal to fill the vacuum left by the departing group. Initially, SAGH would handle the 20 private physicians’ inpatients covered by the existing group, the overflow of unassigned patients from residency workload restrictions, and admitting and co-management for specialists.
Dr. Curry started building his team with Elizabeth Chmelik, MD, recruiting her as the program’s director straight from CHFM residency. The program’s first year—2002—was whirlwind hectic. Fortunately for the group, Marcia Miller, MD, a 1988 graduate of UF’s CHFM department, had burned herself out running a local private practice with two partners. She turned to Dr. Curry, her mentor and confidant. “I called him, told him I needed a job, and he hired me as the hospitalist program’s co-director,” says Dr. Miller. Her community-based partners endorsed her new career path, and she joined Dr. Chmelik in working every day of the program’s first year. “It was challenging,” she says, “but, with inpatient medicine, I became the doctor I was trained to be.”
Sherri Swilley, the department’s coordinator of administrative affairs, recalls the patient census growing so fast that she struggled to keep up with getting doctors temporary privileges while pursuing credentialing for the permanent staff. “The hospitalists had a fierce work ethic that carried us through those early years,” says Swilley.
Gainesville’s Medical Community
In spring 2003, the program added a local physician eager to exit private practice and a family medicine graduate who had worked in SAGH’s emergency department (ED) for 12 years. In June, they snagged two stellar family medicine graduates and a nocturnist. The nocturnist represents the program’s only turnover; her nightly 12 to 20 admissions proved too much to handle.
UF’s homegrown bunch of family physicians shaped this distinctive hospital medicine program. Dr. Chmelik, assistant professor and co-director of the UF College of Medicine’s hospitalist program at SAGH, says that although family physicians are outnumbered by internal medicine hospitalist physicians they add something special to the role.
“We want to go beyond the stereotype of the internal-medicine trained physician drudging through the hospital as an eternal extension of residency,” says Dr. Chmelik. “Family physicians excel at end-of-life issues and the biopsychosocial model of healthcare, both of which are very important in a hospital.”
Another addition to Dr. Chmelik’s black bag is her expert consulting on obstetric and pediatric patients; she can easily handle issues such as hypertension in a postpartum patient or post-appendectomy asthma in a child.
The Financial Tightrope
The University of Florida’s CHFM is closely tied to SAGH, a 75-year-old, 367-bed community hospital that serves as its training ground for residents and hospitalist fellows. Although SAGH is a vital part of Gainesville’s medical scene, its financial status has been shaky for some time.
In 2002, Moody’s Investor Service gave SAGH a negative rating, which was upgraded to stable in 2003.The upgrade of SAGH’s $312 million of outstanding debt issued by Alachua County Health Facilities Authority kept the wolf from the door temporarily. Talk persists that SAGH is in trouble, however, and a Gainesville group, the Health Care Is a Human Right Coalition, asserted in July 2006 that the hospital lacked leadership and that “there are no published plans to keep SAGH open beyond 2010.” SAGH spokesman Ralph Ives says, “Nothing could be further from the truth.” He points to SAGH’s investment in an 82-bed pediatric hospital in a renovated wing with its own hospital medicine group as an affirmation of the institution’s future.
Like other hospitals struggling with public payers and lots of unassigned patients, SAGH’s hospitalist program improves the hospital’s financial health by serving large numbers of patients cost-effectively. Conversely, hospitalist programs can sink because of inattention to financial basics. Early in SAGH’s history, the hospitalists were overwhelmed with a burgeoning census, leaving coding and billing for clerical staff to figure out at day’s end. This delayed billing and often failed to capture the correct diagnostic codes and level of severity for each patient encounter. As the program matured, the hospitalists and hospital administration agreed that physicians would do the coding themselves as they rounded. That change saved on the back end of clerical work and got the charges in promptly.
Each month, Swilley and the hospitalists review individual and group coding patterns, payer mix, the amount billed to each carrier, the amount of time bills spend in accounts receivable, and the services that have been denied. Hospitalists also receive daily reports on their charges and tips on improving coding. For example, if they’ve reviewed documents, Swilley reminds them to code that activity, just as she does a hospitalist who was swamped in the ED at 10 p.m. and left coding his encounters until 1 a.m., thereby losing one day of service. “A hospital is a business. Our hospitalists work hard; I teach them to work smarter and to pay attention to the bottom line,” says Swilley.
Being diligent about financial productivity matters greatly when it’s time for the CHFM department to negotiate with the hospital for its annual subsidy, which Dr. Curry says is about $500,000.
“When we present our budget, we include billing expenses, overhead, salary, and fringe benefits,” says Swilley. “The more detail we have on the number of patient encounters, [our] productivity, and the revenues we generate, the better position we’re in to get support.”
Like most other hospitals, SAGH struggles with tight reimbursement versus the need for an attractive physical plant. To that end, in 1998 it became the first hospital in Florida to adopt the Planetree model, which advocates patient-driven healthcare, serving body, mind, and spirit. “We’ve always been a deeply compassionate hospital,” says Lynne Mercadante, RN, SAGH’s director of Medical Staff Services, “and Planetree is a natural extension of our looking into our hearts and minds about treating the whole person.”
The hospitalists are deeply connected to the community, knowing when to bring in pastoral care or family therapy, as well as joining in celebrations for weddings and anniversaries.
SAGH has made typical Planetree physical changes, including remodeling one floor into six-patient nursing pods versus one nursing station for 42 patients, along with adding meditation rooms, libraries, a piano in the lobby, and a fish tank. Physicians, patients, and visitors are treated to Brahms’ Lullaby over the loudspeaker system every time a baby is born and can experience pet visitations and aromatherapy.
The Schedule
UF’s hospitalists initially adopted a rotation of seven days on, seven days off. When the group’s nocturnist left because of the heavy burden of night admissions, the nine hospitalists decided to cover call themselves instead of hiring a replacement nocturnist. Every ninth week, a hospitalist takes call Monday through Thursday, with residents carrying the weekend. It isn’t ideal, but it distributes call evenly and ensures hospitalists two free weekends a month.
Like most hospital medicine groups, UF’s hospitalists are trying to tinker with the schedule to make it more flexible. They had some give for Scott Medley, MD, who retired from his large Gainesville private practice of 10 physicians, 65 employees, five offices, and 40,000 patients in 2002 at age 55.
“My private [practice] kept me busy seven days a week. I had been there, done that, got the T-shirt,” says Dr. Medley. “I had enough of private practice, but I didn’t want to leave medicine. On reflection, I realized that what I enjoyed most is taking care of really sick patients, so I contacted Whit Curry, and he hired me as a hospitalist Monday through Friday mornings.”
Dr. Medley loves being an employee, doesn’t mind covering for younger colleagues on holidays, and is now collaborating with specialists who have been his friends and colleagues for 25 years. “I also enjoy teaching younger docs both the art and the economics of medicine—and not having to worry where my next paycheck is coming from,” he says.
Looking ahead, Dr. Miller relishes being a hospitalist at age 47, but wonders what the pace will feel like another decade out. To be more flexible, the group is considering allowing physicians to work more days but fewer hours, initiating job sharing, and recruiting more retirees like Dr. Medley, who can work shorter days and are willing to pinch-hit when coverage is tight. They are working toward consensus on maximizing flexibility while maintaining coverage. Still, there’s no easy solution to fluctuations in census, and Dr. Curry notes that some days span 7:30 a.m. to 3:30 p.m., while others stretch into the night. “We haven’t found a better solution yet to providing care 24 hours a day, 365 days a year,” concludes Dr. Medley.
Creating Value
Looking back at her quarter century at SAGH, Mercadante analyzes how the hospitalist medicine program improved things. “Without hospitalists, whoever was on call covered everyone in the ED, so you had neurologists treating patients with pneumonia. There was little continuity of care, unlike with the hospitalists who meet every morning discussing cases.”
In addition, the medical staff feel they have their lives back because call is covered, and they can count on the hospitalists to co-manage complicated cases. “When specialty physicians are in high demand and short supply, knowing that someone’s covering their hospitalized patients for chronic conditions is so important,” concludes Mercadante.
Looking to the future, UF’s hospitalists plan to build on their cohesiveness, collegiality, and emphasis on family medicine. With alumni of their school at the ready, they know where to turn to continue to grow the program. TH
Marlene Piturro also writes about scheduling in this issue.
TH's Pain Primer
Managing the pain of hospitalized patients is a fundamental ethical responsibility of hospitalists, enshrined as a core competency by SHM and, according to the Joint Commission on Healthcare Accreditation Organizations (JCAHO), a right for hospitalized patients.
Following last month’s exploration of IV pain medications (“Perfect Pain Control,” p. 40), this month we begin a three-part series on pain management issues in the hospital setting, based on interviews with working hospitalists and other pain experts.
Part one (below) provides a context for pain management and emphasizes assessment as the cornerstone of pain control. Next month, we will explore common dilemmas and difficult cases in pain management that can take hospitalists out of their comfort zone, along with the myths and realities of hot button topics such as addiction. The following month, we will chart the continuum of pain management modalities used in the hospital and discuss how working hospitalists can best utilize them for patients with special needs.
—Steven Pantilat, MD, hospitalist and palliative care physician, UCSF Medical Center
Listen to Your Patient’s Pain
Assessment and follow-up remain key to managing hospitalized patients’ pain. Stephen J. Bekanich, MD, a hospitalist at the University of Utah Medical Center in Salt Lake City and consultant on the medical center’s palliative care service, remembers a hospitalized patient whose pain problem was not what it seemed—although a more careful assessment showed the way to a solution. A woman in her early 80s who resided in a long-term-care facility was admitted to the hospital with out-of-control back pain and mild dementia. House staff fitted her with a patient-controlled analgesia (PCA) pump to treat her pain, with instructions to press the control button whenever she experienced pain. Dr. Bekanich got a call 48 hours later because the patient was still voicing significant pain complaints, despite the PCA.
“I found that her pain scores were taken by the nurses every four hours, which is not often enough when pain is out of control,” he says. “I also looked at a printout of the PCA history, which indicated that she had only pressed the button 10 or 12 times in 48 hours. You would have expected a lot more attempts, given her reports of pain.”
Dr. Bekanich showed the patient the PCA button and asked her, “ ‘What’s this?’ She replied, ‘I can’t see it. I don’t have my glasses here in the hospital.’ When I put it in her hand, she said, ‘This is what I use to call the nurse.’ ”
A small tag on the PCA handle indicated that the patient should push for pain, but the patient was unable to read it. Once Dr. Bekanich understood her functional limitations, he wrote a new order for continuous infusion of an opioid analgesic, which brought the pain under control.
This case illustrates several principles of effective pain management. First is the importance of assessing the various factors that influence pain and the physician’s need to look more deeply if the pain doesn’t respond to initial measures. “That should be a warning flag to ask, ‘OK, what am I missing?’ ” Also, for moderate to severe pain, a component of around-the-clock dosing or continuous infusion to bring the pain under control is just as important as having the availability of a PRN analgesic for responding to breakthrough pain, such as starting the patient on a PCA.
Pain: The Hospitalist’s Responsibility
According to Health, United States, 2006, the federal government’s annual, comprehensive report on America’s health, issued last November by the Centers for Disease Control and Prevention (CDC) National Center for Health Statistics, one-quarter of U.S. adults say they suffered a bout of pain lasting 24 hours or more in the past month. One in 10 says the pain lasted a year or more.
The CDC chose to focus on pain in the latest annual report “because it is rarely discussed as a condition in and of itself; it is mostly viewed as a byproduct of another condition,” says lead study author Amy Bernstein, who also cites the medical costs of pain and pain disparities among different population groups. Other studies have identified physicians’ self-reported discomfort with their training in pain management and with their ability to manage their patients’ pain.
Pain is also the reason many patients end up in the hospital, and treating pain should be the expectation of every hospitalist, says Robert V. Brody, MD, chief of the pain service at San Francisco General Hospital and a frequent presenter on pain management topics at clinical workshops for hospitalists. Effective pain management begins with the pain assessment, but equally important is the follow-up to reassess how the pain responds to initial measures, Dr. Brody says. If initial approaches fail to manage the pain, try again with a new dose, drug, or combination. Then reassess and repeat as often as necessary—viewing the pain challenge as a puzzle to be solved.
Pain is defined by the International Association for the Study of Pain as “an unpleasant experience associated with actual or potential tissue damage to a person’s body.” Key to that definition, notes Dr. Brody, is the recognition that pain is ultimately a subjective phenomenon, reflecting the patient’s perception of and emotional reaction to the unpleasant sensation. Patients are thus the best source of information on how much pain they are experiencing.
In recent years, standardized pain scales (typically ranging from zero—no pain, to 10—the worst pain imaginable) have gained currency in U.S. hospitals and other healthcare settings, thanks to the growing emphasis on pain management by groups such as the Joint Commission. (See “Pain Assessment Scales,” p. 49.) Such pain scales make it possible to quantify, chart, and track over time the patient’s subjective, self-reported pain scores. But while nurses may be regularly charting patients’ pain scores, hospitalists need to review those scores.
“We used to say that treating pain is not rocket science, but clearly there are skills and knowledge that hospitalists should acquire, including how to handle difficult issues around substance abuse or mental health,” explains Dr. Brody. “Certain basic rules of pain management can go a long way if you’re open to the belief that learning those rules is important and if you have an expectation that you will bring the patient’s pain under control.
“Talk to the patient,” he advises. “Ask what are the patient’s goals for pain relief.” The goal is not necessarily zero pain but a balance between pain relief and side effects from analgesics, based on functional status, defined goals, and the patient’s expressed preferences.
With practice, hospitalists can gain comfort with prescribing short-acting and long-acting opioids plus adjuvant treatments sufficient to address the majority of pain cases. They can also learn to convert between oral and intravenous opioid administration. But they must recognize when to call for reinforcements, such as the hospital’s pain service or a palliative care consultant, for assistance with more challenging cases. Ultimately, effective pain management in the hospital is multi-disciplinary, drawing at different times on the complementary perspectives of other team members, including the nurse, pharmacist, social worker, and chaplain.
“The first step to improving pain management is to develop awareness of the problem,” says Steven Pantilat, MD, a hospitalist, associate professor of clinical medicine, Department of Medicine, University of California, San Francisco, and past president of SHM. “But you also have to be comfortable giving adequate doses of these medications. You get comfortable through experience.”
Dr. Pantilat recommends that hospitalists stick with a few familiar opioids, both short-acting and long-acting. “But 90% of pain can be managed by a hospitalist without need for consultation.” He is also the past-president of SHM and the Alan M. Kates and John M. Burnard Endowed Chair in Palliative Care at UCSF.
Start with the Assessment
Pain assessment identifies the location, cause, intensity, duration, and nature of the pain, recognizing that many chronically ill patients may have more than one source of pain. It is important to establish why the patient is in pain because different pain responds to different treatments. It may also be helpful to know how long the patient has experienced the pain, how it was treated prior to the hospitalization, how it responded to treatment in the past, what makes the pain better or worse, and how it affects sleep, appetite, or physical activity.
Have the patient describe what the pain is like—the quality of the pain—using his or her own words, suggests Carol Jessop, MD, a hospitalist and palliative care physician at Alta Bates Summit Medical Center in Berkeley, Calif. There may also be psychological or spiritual elements of the pain—other sources that are not physical but contribute to a pain experience that is very real to the patient. A thorough pain assessment also evaluates the patient’s psychological state, including depression and anxiety, as well as past history of alcohol or drug use. It covers the patient’s and the patient’s family’s attitudes toward the use of opioid analgesics, their cultural context, and the meaning that the patient ascribes to his or her pain.
It can take a long time to gather all of that information as part of a comprehensive pain history, however—time that busy hospitalists may not be able to spare, says Dr. Bekanich. Fortunately, not every hospitalized patient requires this level of detail. But if there is reason to expect complications or difficulties in bringing the pain under control, if the pain doesn’t respond to standard analgesic treatments, or if there are reasons for avoiding opioid analgesics, then it may be worth making the time—or recruiting someone who can take a detailed pain history that would provide a baseline for future assessments.
“The most important thing to remember is that pain is what the patient says it is,” says Dr. Pantilat. “We are challenged by wondering whether the patient is really in pain. The answer has to be yes. You have to trust the patient unless you have specific reasons not to.
“It seems to me the first assessment of the patient’s pain may need to be more complex: Is there something new going on with this patient?” he continues. “If someone comes into the hospital with a new fracture or a kidney stone, you don’t need to spend as much time figuring out the pain’s source. But if it is chronic pain that has been unmanaged for a significant amount of time, that’s when you sit down and say, ‘OK, tell me about your pain.’ There’s no one size fits all in pain assessment.”
Other Issues
A special focus in pain assessment is recognizing neuropathic pain—resulting from injury or damage to the nerves themselves, which is different in nature and treatment from nociceptive pain and is also generally less responsive to opiate analgesics. Roughly 15% of the pain hospitalists see may be neuropathic, which can be suggested by certain words, such as burning, numbing, tingling, or shooting, in the patient’s description of the pain. Certain syndromes also suggest the possibility of neuropathic pain, including diabetes, HIV, alcoholism, radiation or chemotherapy, and amputation and phantom limb pain. Neuropathic pain may be treated with tricyclic antidepressants such as desipramine (Norpramin, Petrofrane) and nortriptyline (Pamelor, Aventyl) as well as with the anticonvulsant gabapentin.1,2
Another key issue in pain management involves side effects. With opioids, constipation is such a common side effect that experts recommend prescribing a laxative and/or stool softener every time an opioid analgesic is initiated. The physician must then stay on top of the issue, prescribing additional laxatives if the desired effect is not achieved. Other side effects of opioids, which must be balanced with their analgesic properties, include nausea, sedation, mental status changes, and respiratory suppression. A number of these side effects will dissipate after a few days on opioids, but constipation remains problematic.
Other basic principles of pain management, gathered from physicians interviewed for this article and from other pain resources (see “Resources and Tools,” p. 49) include:
- There is no absolute maximum dose of opioids; adjust dose based on individual need and response. If initial doses are not effective, titrate up based on percentages of the dose: 25%-50% for mild to moderate pain, 50%-100% for moderate to severe pain.
- Use the right duration in prescribing; short-acting opioids may be more effective when given every four hours than every six hours. PRN prescriptions are not recommended except for breakthrough pain. The World Health Organization’s Pain Ladder suggests an overall approach to dosing based on severity.
- Tailor the pain regimen while the patient is still in front of you, if possible. The patient’s response to intravenous analgesics should start to become clear within 10 minutes of initiation.
- The earlier you treat pain, the easier it will be to bring it under control.
- Oral administration is generally preferable to intravenous unless there is a reason to avoid using the oral route.
- Pain experts do not generally recommend meperidine as an analgesic.3
- Opioids are not recommended for all kinds of pain. Opioids may be avoided for patients with neuropathic pain, for those with existing constipation or nausea problems, or for morbidly obese patients with bad sleep apnea.
Finally, work with primary care physicians to plan for pain needs post-discharge, as well as for potential problems or barriers that may arise, especially if high doses of opioids are involved.
“One of the most difficult issues is addressing what will happen after the patient leaves the hospital,” says Dr. Bekanich. “That’s where the ball often gets dropped.”
He makes a point of calling the patient’s primary physician at the time of discharge and then dictates a letter, including the pain protocol, which is transcribed and faxed to the primary physician. “We don’t let these patients walk out the door without an appointment date already scheduled with a physician,” he says.
Benefits of Pain Relief
Dr. Jessop encourages hospitalists to take advantage of SHM’s core competency in pain management as a guide to improving their skills in this area. Managing patients’ pain is a win/win for the physician, the patient, and the institution. “Nothing feels better than getting a patient out of pain,” she says.
Better outcomes in pain management can help bring down hospital lengths of stay while driving up patient and staff satisfaction, adds Dr. Bekanich. Conversely, unrelieved pain not only leads to unnecessary suffering but also to patients who are depressed, slower to get up and start walking or eating, and reluctant to take deep breaths. “It’s hard to discharge a hospitalized patient whose pain is still out of control,” he says.
Dr. Bekanich reports that his own interest in learning pain management techniques resulted from watching his grandmother experience severe pain while struggling with cancer. “That was the driving force for me to say, ‘We can do so much better at this,’ and then get the training I needed,” he says.
Dr. Bekanich attended conferences offered by the Center to Advance Palliative Care and the American Academy of Hospice and Palliative Medicine. “I started to read a lot more in the pain literature,” he explains. “Initially, I was somewhat self-conscious about putting the new techniques into effect. So I’d call a pharmacist or a mentor to double check. I’m glad I did that.” TH
Larry Beresford is a regular contributor to The Hospitalist.
References
- Smith TE, Chong MS. Neuropathic pain. Hosp Med. 2000;61(11):760-766.
- Saarto T, Wiffen PJ. Antidepressants for neuropathic pain (review). Cochrane Database Syst Rev. 2005 Jul 20;(3)3:CD005454.
- Weissman DE. Fast Fact and Concept #71: Meperidine for pain—what’s all the fuss? [The End of Life/Palliative Education Resource Center.] Available at: www.eperc.mcw.edu/fastFact/ff_71.htm. Last accessed February 19, 2007.
Managing the pain of hospitalized patients is a fundamental ethical responsibility of hospitalists, enshrined as a core competency by SHM and, according to the Joint Commission on Healthcare Accreditation Organizations (JCAHO), a right for hospitalized patients.
Following last month’s exploration of IV pain medications (“Perfect Pain Control,” p. 40), this month we begin a three-part series on pain management issues in the hospital setting, based on interviews with working hospitalists and other pain experts.
Part one (below) provides a context for pain management and emphasizes assessment as the cornerstone of pain control. Next month, we will explore common dilemmas and difficult cases in pain management that can take hospitalists out of their comfort zone, along with the myths and realities of hot button topics such as addiction. The following month, we will chart the continuum of pain management modalities used in the hospital and discuss how working hospitalists can best utilize them for patients with special needs.
—Steven Pantilat, MD, hospitalist and palliative care physician, UCSF Medical Center
Listen to Your Patient’s Pain
Assessment and follow-up remain key to managing hospitalized patients’ pain. Stephen J. Bekanich, MD, a hospitalist at the University of Utah Medical Center in Salt Lake City and consultant on the medical center’s palliative care service, remembers a hospitalized patient whose pain problem was not what it seemed—although a more careful assessment showed the way to a solution. A woman in her early 80s who resided in a long-term-care facility was admitted to the hospital with out-of-control back pain and mild dementia. House staff fitted her with a patient-controlled analgesia (PCA) pump to treat her pain, with instructions to press the control button whenever she experienced pain. Dr. Bekanich got a call 48 hours later because the patient was still voicing significant pain complaints, despite the PCA.
“I found that her pain scores were taken by the nurses every four hours, which is not often enough when pain is out of control,” he says. “I also looked at a printout of the PCA history, which indicated that she had only pressed the button 10 or 12 times in 48 hours. You would have expected a lot more attempts, given her reports of pain.”
Dr. Bekanich showed the patient the PCA button and asked her, “ ‘What’s this?’ She replied, ‘I can’t see it. I don’t have my glasses here in the hospital.’ When I put it in her hand, she said, ‘This is what I use to call the nurse.’ ”
A small tag on the PCA handle indicated that the patient should push for pain, but the patient was unable to read it. Once Dr. Bekanich understood her functional limitations, he wrote a new order for continuous infusion of an opioid analgesic, which brought the pain under control.
This case illustrates several principles of effective pain management. First is the importance of assessing the various factors that influence pain and the physician’s need to look more deeply if the pain doesn’t respond to initial measures. “That should be a warning flag to ask, ‘OK, what am I missing?’ ” Also, for moderate to severe pain, a component of around-the-clock dosing or continuous infusion to bring the pain under control is just as important as having the availability of a PRN analgesic for responding to breakthrough pain, such as starting the patient on a PCA.
Pain: The Hospitalist’s Responsibility
According to Health, United States, 2006, the federal government’s annual, comprehensive report on America’s health, issued last November by the Centers for Disease Control and Prevention (CDC) National Center for Health Statistics, one-quarter of U.S. adults say they suffered a bout of pain lasting 24 hours or more in the past month. One in 10 says the pain lasted a year or more.
The CDC chose to focus on pain in the latest annual report “because it is rarely discussed as a condition in and of itself; it is mostly viewed as a byproduct of another condition,” says lead study author Amy Bernstein, who also cites the medical costs of pain and pain disparities among different population groups. Other studies have identified physicians’ self-reported discomfort with their training in pain management and with their ability to manage their patients’ pain.
Pain is also the reason many patients end up in the hospital, and treating pain should be the expectation of every hospitalist, says Robert V. Brody, MD, chief of the pain service at San Francisco General Hospital and a frequent presenter on pain management topics at clinical workshops for hospitalists. Effective pain management begins with the pain assessment, but equally important is the follow-up to reassess how the pain responds to initial measures, Dr. Brody says. If initial approaches fail to manage the pain, try again with a new dose, drug, or combination. Then reassess and repeat as often as necessary—viewing the pain challenge as a puzzle to be solved.
Pain is defined by the International Association for the Study of Pain as “an unpleasant experience associated with actual or potential tissue damage to a person’s body.” Key to that definition, notes Dr. Brody, is the recognition that pain is ultimately a subjective phenomenon, reflecting the patient’s perception of and emotional reaction to the unpleasant sensation. Patients are thus the best source of information on how much pain they are experiencing.
In recent years, standardized pain scales (typically ranging from zero—no pain, to 10—the worst pain imaginable) have gained currency in U.S. hospitals and other healthcare settings, thanks to the growing emphasis on pain management by groups such as the Joint Commission. (See “Pain Assessment Scales,” p. 49.) Such pain scales make it possible to quantify, chart, and track over time the patient’s subjective, self-reported pain scores. But while nurses may be regularly charting patients’ pain scores, hospitalists need to review those scores.
“We used to say that treating pain is not rocket science, but clearly there are skills and knowledge that hospitalists should acquire, including how to handle difficult issues around substance abuse or mental health,” explains Dr. Brody. “Certain basic rules of pain management can go a long way if you’re open to the belief that learning those rules is important and if you have an expectation that you will bring the patient’s pain under control.
“Talk to the patient,” he advises. “Ask what are the patient’s goals for pain relief.” The goal is not necessarily zero pain but a balance between pain relief and side effects from analgesics, based on functional status, defined goals, and the patient’s expressed preferences.
With practice, hospitalists can gain comfort with prescribing short-acting and long-acting opioids plus adjuvant treatments sufficient to address the majority of pain cases. They can also learn to convert between oral and intravenous opioid administration. But they must recognize when to call for reinforcements, such as the hospital’s pain service or a palliative care consultant, for assistance with more challenging cases. Ultimately, effective pain management in the hospital is multi-disciplinary, drawing at different times on the complementary perspectives of other team members, including the nurse, pharmacist, social worker, and chaplain.
“The first step to improving pain management is to develop awareness of the problem,” says Steven Pantilat, MD, a hospitalist, associate professor of clinical medicine, Department of Medicine, University of California, San Francisco, and past president of SHM. “But you also have to be comfortable giving adequate doses of these medications. You get comfortable through experience.”
Dr. Pantilat recommends that hospitalists stick with a few familiar opioids, both short-acting and long-acting. “But 90% of pain can be managed by a hospitalist without need for consultation.” He is also the past-president of SHM and the Alan M. Kates and John M. Burnard Endowed Chair in Palliative Care at UCSF.
Start with the Assessment
Pain assessment identifies the location, cause, intensity, duration, and nature of the pain, recognizing that many chronically ill patients may have more than one source of pain. It is important to establish why the patient is in pain because different pain responds to different treatments. It may also be helpful to know how long the patient has experienced the pain, how it was treated prior to the hospitalization, how it responded to treatment in the past, what makes the pain better or worse, and how it affects sleep, appetite, or physical activity.
Have the patient describe what the pain is like—the quality of the pain—using his or her own words, suggests Carol Jessop, MD, a hospitalist and palliative care physician at Alta Bates Summit Medical Center in Berkeley, Calif. There may also be psychological or spiritual elements of the pain—other sources that are not physical but contribute to a pain experience that is very real to the patient. A thorough pain assessment also evaluates the patient’s psychological state, including depression and anxiety, as well as past history of alcohol or drug use. It covers the patient’s and the patient’s family’s attitudes toward the use of opioid analgesics, their cultural context, and the meaning that the patient ascribes to his or her pain.
It can take a long time to gather all of that information as part of a comprehensive pain history, however—time that busy hospitalists may not be able to spare, says Dr. Bekanich. Fortunately, not every hospitalized patient requires this level of detail. But if there is reason to expect complications or difficulties in bringing the pain under control, if the pain doesn’t respond to standard analgesic treatments, or if there are reasons for avoiding opioid analgesics, then it may be worth making the time—or recruiting someone who can take a detailed pain history that would provide a baseline for future assessments.
“The most important thing to remember is that pain is what the patient says it is,” says Dr. Pantilat. “We are challenged by wondering whether the patient is really in pain. The answer has to be yes. You have to trust the patient unless you have specific reasons not to.
“It seems to me the first assessment of the patient’s pain may need to be more complex: Is there something new going on with this patient?” he continues. “If someone comes into the hospital with a new fracture or a kidney stone, you don’t need to spend as much time figuring out the pain’s source. But if it is chronic pain that has been unmanaged for a significant amount of time, that’s when you sit down and say, ‘OK, tell me about your pain.’ There’s no one size fits all in pain assessment.”
Other Issues
A special focus in pain assessment is recognizing neuropathic pain—resulting from injury or damage to the nerves themselves, which is different in nature and treatment from nociceptive pain and is also generally less responsive to opiate analgesics. Roughly 15% of the pain hospitalists see may be neuropathic, which can be suggested by certain words, such as burning, numbing, tingling, or shooting, in the patient’s description of the pain. Certain syndromes also suggest the possibility of neuropathic pain, including diabetes, HIV, alcoholism, radiation or chemotherapy, and amputation and phantom limb pain. Neuropathic pain may be treated with tricyclic antidepressants such as desipramine (Norpramin, Petrofrane) and nortriptyline (Pamelor, Aventyl) as well as with the anticonvulsant gabapentin.1,2
Another key issue in pain management involves side effects. With opioids, constipation is such a common side effect that experts recommend prescribing a laxative and/or stool softener every time an opioid analgesic is initiated. The physician must then stay on top of the issue, prescribing additional laxatives if the desired effect is not achieved. Other side effects of opioids, which must be balanced with their analgesic properties, include nausea, sedation, mental status changes, and respiratory suppression. A number of these side effects will dissipate after a few days on opioids, but constipation remains problematic.
Other basic principles of pain management, gathered from physicians interviewed for this article and from other pain resources (see “Resources and Tools,” p. 49) include:
- There is no absolute maximum dose of opioids; adjust dose based on individual need and response. If initial doses are not effective, titrate up based on percentages of the dose: 25%-50% for mild to moderate pain, 50%-100% for moderate to severe pain.
- Use the right duration in prescribing; short-acting opioids may be more effective when given every four hours than every six hours. PRN prescriptions are not recommended except for breakthrough pain. The World Health Organization’s Pain Ladder suggests an overall approach to dosing based on severity.
- Tailor the pain regimen while the patient is still in front of you, if possible. The patient’s response to intravenous analgesics should start to become clear within 10 minutes of initiation.
- The earlier you treat pain, the easier it will be to bring it under control.
- Oral administration is generally preferable to intravenous unless there is a reason to avoid using the oral route.
- Pain experts do not generally recommend meperidine as an analgesic.3
- Opioids are not recommended for all kinds of pain. Opioids may be avoided for patients with neuropathic pain, for those with existing constipation or nausea problems, or for morbidly obese patients with bad sleep apnea.
Finally, work with primary care physicians to plan for pain needs post-discharge, as well as for potential problems or barriers that may arise, especially if high doses of opioids are involved.
“One of the most difficult issues is addressing what will happen after the patient leaves the hospital,” says Dr. Bekanich. “That’s where the ball often gets dropped.”
He makes a point of calling the patient’s primary physician at the time of discharge and then dictates a letter, including the pain protocol, which is transcribed and faxed to the primary physician. “We don’t let these patients walk out the door without an appointment date already scheduled with a physician,” he says.
Benefits of Pain Relief
Dr. Jessop encourages hospitalists to take advantage of SHM’s core competency in pain management as a guide to improving their skills in this area. Managing patients’ pain is a win/win for the physician, the patient, and the institution. “Nothing feels better than getting a patient out of pain,” she says.
Better outcomes in pain management can help bring down hospital lengths of stay while driving up patient and staff satisfaction, adds Dr. Bekanich. Conversely, unrelieved pain not only leads to unnecessary suffering but also to patients who are depressed, slower to get up and start walking or eating, and reluctant to take deep breaths. “It’s hard to discharge a hospitalized patient whose pain is still out of control,” he says.
Dr. Bekanich reports that his own interest in learning pain management techniques resulted from watching his grandmother experience severe pain while struggling with cancer. “That was the driving force for me to say, ‘We can do so much better at this,’ and then get the training I needed,” he says.
Dr. Bekanich attended conferences offered by the Center to Advance Palliative Care and the American Academy of Hospice and Palliative Medicine. “I started to read a lot more in the pain literature,” he explains. “Initially, I was somewhat self-conscious about putting the new techniques into effect. So I’d call a pharmacist or a mentor to double check. I’m glad I did that.” TH
Larry Beresford is a regular contributor to The Hospitalist.
References
- Smith TE, Chong MS. Neuropathic pain. Hosp Med. 2000;61(11):760-766.
- Saarto T, Wiffen PJ. Antidepressants for neuropathic pain (review). Cochrane Database Syst Rev. 2005 Jul 20;(3)3:CD005454.
- Weissman DE. Fast Fact and Concept #71: Meperidine for pain—what’s all the fuss? [The End of Life/Palliative Education Resource Center.] Available at: www.eperc.mcw.edu/fastFact/ff_71.htm. Last accessed February 19, 2007.
Managing the pain of hospitalized patients is a fundamental ethical responsibility of hospitalists, enshrined as a core competency by SHM and, according to the Joint Commission on Healthcare Accreditation Organizations (JCAHO), a right for hospitalized patients.
Following last month’s exploration of IV pain medications (“Perfect Pain Control,” p. 40), this month we begin a three-part series on pain management issues in the hospital setting, based on interviews with working hospitalists and other pain experts.
Part one (below) provides a context for pain management and emphasizes assessment as the cornerstone of pain control. Next month, we will explore common dilemmas and difficult cases in pain management that can take hospitalists out of their comfort zone, along with the myths and realities of hot button topics such as addiction. The following month, we will chart the continuum of pain management modalities used in the hospital and discuss how working hospitalists can best utilize them for patients with special needs.
—Steven Pantilat, MD, hospitalist and palliative care physician, UCSF Medical Center
Listen to Your Patient’s Pain
Assessment and follow-up remain key to managing hospitalized patients’ pain. Stephen J. Bekanich, MD, a hospitalist at the University of Utah Medical Center in Salt Lake City and consultant on the medical center’s palliative care service, remembers a hospitalized patient whose pain problem was not what it seemed—although a more careful assessment showed the way to a solution. A woman in her early 80s who resided in a long-term-care facility was admitted to the hospital with out-of-control back pain and mild dementia. House staff fitted her with a patient-controlled analgesia (PCA) pump to treat her pain, with instructions to press the control button whenever she experienced pain. Dr. Bekanich got a call 48 hours later because the patient was still voicing significant pain complaints, despite the PCA.
“I found that her pain scores were taken by the nurses every four hours, which is not often enough when pain is out of control,” he says. “I also looked at a printout of the PCA history, which indicated that she had only pressed the button 10 or 12 times in 48 hours. You would have expected a lot more attempts, given her reports of pain.”
Dr. Bekanich showed the patient the PCA button and asked her, “ ‘What’s this?’ She replied, ‘I can’t see it. I don’t have my glasses here in the hospital.’ When I put it in her hand, she said, ‘This is what I use to call the nurse.’ ”
A small tag on the PCA handle indicated that the patient should push for pain, but the patient was unable to read it. Once Dr. Bekanich understood her functional limitations, he wrote a new order for continuous infusion of an opioid analgesic, which brought the pain under control.
This case illustrates several principles of effective pain management. First is the importance of assessing the various factors that influence pain and the physician’s need to look more deeply if the pain doesn’t respond to initial measures. “That should be a warning flag to ask, ‘OK, what am I missing?’ ” Also, for moderate to severe pain, a component of around-the-clock dosing or continuous infusion to bring the pain under control is just as important as having the availability of a PRN analgesic for responding to breakthrough pain, such as starting the patient on a PCA.
Pain: The Hospitalist’s Responsibility
According to Health, United States, 2006, the federal government’s annual, comprehensive report on America’s health, issued last November by the Centers for Disease Control and Prevention (CDC) National Center for Health Statistics, one-quarter of U.S. adults say they suffered a bout of pain lasting 24 hours or more in the past month. One in 10 says the pain lasted a year or more.
The CDC chose to focus on pain in the latest annual report “because it is rarely discussed as a condition in and of itself; it is mostly viewed as a byproduct of another condition,” says lead study author Amy Bernstein, who also cites the medical costs of pain and pain disparities among different population groups. Other studies have identified physicians’ self-reported discomfort with their training in pain management and with their ability to manage their patients’ pain.
Pain is also the reason many patients end up in the hospital, and treating pain should be the expectation of every hospitalist, says Robert V. Brody, MD, chief of the pain service at San Francisco General Hospital and a frequent presenter on pain management topics at clinical workshops for hospitalists. Effective pain management begins with the pain assessment, but equally important is the follow-up to reassess how the pain responds to initial measures, Dr. Brody says. If initial approaches fail to manage the pain, try again with a new dose, drug, or combination. Then reassess and repeat as often as necessary—viewing the pain challenge as a puzzle to be solved.
Pain is defined by the International Association for the Study of Pain as “an unpleasant experience associated with actual or potential tissue damage to a person’s body.” Key to that definition, notes Dr. Brody, is the recognition that pain is ultimately a subjective phenomenon, reflecting the patient’s perception of and emotional reaction to the unpleasant sensation. Patients are thus the best source of information on how much pain they are experiencing.
In recent years, standardized pain scales (typically ranging from zero—no pain, to 10—the worst pain imaginable) have gained currency in U.S. hospitals and other healthcare settings, thanks to the growing emphasis on pain management by groups such as the Joint Commission. (See “Pain Assessment Scales,” p. 49.) Such pain scales make it possible to quantify, chart, and track over time the patient’s subjective, self-reported pain scores. But while nurses may be regularly charting patients’ pain scores, hospitalists need to review those scores.
“We used to say that treating pain is not rocket science, but clearly there are skills and knowledge that hospitalists should acquire, including how to handle difficult issues around substance abuse or mental health,” explains Dr. Brody. “Certain basic rules of pain management can go a long way if you’re open to the belief that learning those rules is important and if you have an expectation that you will bring the patient’s pain under control.
“Talk to the patient,” he advises. “Ask what are the patient’s goals for pain relief.” The goal is not necessarily zero pain but a balance between pain relief and side effects from analgesics, based on functional status, defined goals, and the patient’s expressed preferences.
With practice, hospitalists can gain comfort with prescribing short-acting and long-acting opioids plus adjuvant treatments sufficient to address the majority of pain cases. They can also learn to convert between oral and intravenous opioid administration. But they must recognize when to call for reinforcements, such as the hospital’s pain service or a palliative care consultant, for assistance with more challenging cases. Ultimately, effective pain management in the hospital is multi-disciplinary, drawing at different times on the complementary perspectives of other team members, including the nurse, pharmacist, social worker, and chaplain.
“The first step to improving pain management is to develop awareness of the problem,” says Steven Pantilat, MD, a hospitalist, associate professor of clinical medicine, Department of Medicine, University of California, San Francisco, and past president of SHM. “But you also have to be comfortable giving adequate doses of these medications. You get comfortable through experience.”
Dr. Pantilat recommends that hospitalists stick with a few familiar opioids, both short-acting and long-acting. “But 90% of pain can be managed by a hospitalist without need for consultation.” He is also the past-president of SHM and the Alan M. Kates and John M. Burnard Endowed Chair in Palliative Care at UCSF.
Start with the Assessment
Pain assessment identifies the location, cause, intensity, duration, and nature of the pain, recognizing that many chronically ill patients may have more than one source of pain. It is important to establish why the patient is in pain because different pain responds to different treatments. It may also be helpful to know how long the patient has experienced the pain, how it was treated prior to the hospitalization, how it responded to treatment in the past, what makes the pain better or worse, and how it affects sleep, appetite, or physical activity.
Have the patient describe what the pain is like—the quality of the pain—using his or her own words, suggests Carol Jessop, MD, a hospitalist and palliative care physician at Alta Bates Summit Medical Center in Berkeley, Calif. There may also be psychological or spiritual elements of the pain—other sources that are not physical but contribute to a pain experience that is very real to the patient. A thorough pain assessment also evaluates the patient’s psychological state, including depression and anxiety, as well as past history of alcohol or drug use. It covers the patient’s and the patient’s family’s attitudes toward the use of opioid analgesics, their cultural context, and the meaning that the patient ascribes to his or her pain.
It can take a long time to gather all of that information as part of a comprehensive pain history, however—time that busy hospitalists may not be able to spare, says Dr. Bekanich. Fortunately, not every hospitalized patient requires this level of detail. But if there is reason to expect complications or difficulties in bringing the pain under control, if the pain doesn’t respond to standard analgesic treatments, or if there are reasons for avoiding opioid analgesics, then it may be worth making the time—or recruiting someone who can take a detailed pain history that would provide a baseline for future assessments.
“The most important thing to remember is that pain is what the patient says it is,” says Dr. Pantilat. “We are challenged by wondering whether the patient is really in pain. The answer has to be yes. You have to trust the patient unless you have specific reasons not to.
“It seems to me the first assessment of the patient’s pain may need to be more complex: Is there something new going on with this patient?” he continues. “If someone comes into the hospital with a new fracture or a kidney stone, you don’t need to spend as much time figuring out the pain’s source. But if it is chronic pain that has been unmanaged for a significant amount of time, that’s when you sit down and say, ‘OK, tell me about your pain.’ There’s no one size fits all in pain assessment.”
Other Issues
A special focus in pain assessment is recognizing neuropathic pain—resulting from injury or damage to the nerves themselves, which is different in nature and treatment from nociceptive pain and is also generally less responsive to opiate analgesics. Roughly 15% of the pain hospitalists see may be neuropathic, which can be suggested by certain words, such as burning, numbing, tingling, or shooting, in the patient’s description of the pain. Certain syndromes also suggest the possibility of neuropathic pain, including diabetes, HIV, alcoholism, radiation or chemotherapy, and amputation and phantom limb pain. Neuropathic pain may be treated with tricyclic antidepressants such as desipramine (Norpramin, Petrofrane) and nortriptyline (Pamelor, Aventyl) as well as with the anticonvulsant gabapentin.1,2
Another key issue in pain management involves side effects. With opioids, constipation is such a common side effect that experts recommend prescribing a laxative and/or stool softener every time an opioid analgesic is initiated. The physician must then stay on top of the issue, prescribing additional laxatives if the desired effect is not achieved. Other side effects of opioids, which must be balanced with their analgesic properties, include nausea, sedation, mental status changes, and respiratory suppression. A number of these side effects will dissipate after a few days on opioids, but constipation remains problematic.
Other basic principles of pain management, gathered from physicians interviewed for this article and from other pain resources (see “Resources and Tools,” p. 49) include:
- There is no absolute maximum dose of opioids; adjust dose based on individual need and response. If initial doses are not effective, titrate up based on percentages of the dose: 25%-50% for mild to moderate pain, 50%-100% for moderate to severe pain.
- Use the right duration in prescribing; short-acting opioids may be more effective when given every four hours than every six hours. PRN prescriptions are not recommended except for breakthrough pain. The World Health Organization’s Pain Ladder suggests an overall approach to dosing based on severity.
- Tailor the pain regimen while the patient is still in front of you, if possible. The patient’s response to intravenous analgesics should start to become clear within 10 minutes of initiation.
- The earlier you treat pain, the easier it will be to bring it under control.
- Oral administration is generally preferable to intravenous unless there is a reason to avoid using the oral route.
- Pain experts do not generally recommend meperidine as an analgesic.3
- Opioids are not recommended for all kinds of pain. Opioids may be avoided for patients with neuropathic pain, for those with existing constipation or nausea problems, or for morbidly obese patients with bad sleep apnea.
Finally, work with primary care physicians to plan for pain needs post-discharge, as well as for potential problems or barriers that may arise, especially if high doses of opioids are involved.
“One of the most difficult issues is addressing what will happen after the patient leaves the hospital,” says Dr. Bekanich. “That’s where the ball often gets dropped.”
He makes a point of calling the patient’s primary physician at the time of discharge and then dictates a letter, including the pain protocol, which is transcribed and faxed to the primary physician. “We don’t let these patients walk out the door without an appointment date already scheduled with a physician,” he says.
Benefits of Pain Relief
Dr. Jessop encourages hospitalists to take advantage of SHM’s core competency in pain management as a guide to improving their skills in this area. Managing patients’ pain is a win/win for the physician, the patient, and the institution. “Nothing feels better than getting a patient out of pain,” she says.
Better outcomes in pain management can help bring down hospital lengths of stay while driving up patient and staff satisfaction, adds Dr. Bekanich. Conversely, unrelieved pain not only leads to unnecessary suffering but also to patients who are depressed, slower to get up and start walking or eating, and reluctant to take deep breaths. “It’s hard to discharge a hospitalized patient whose pain is still out of control,” he says.
Dr. Bekanich reports that his own interest in learning pain management techniques resulted from watching his grandmother experience severe pain while struggling with cancer. “That was the driving force for me to say, ‘We can do so much better at this,’ and then get the training I needed,” he says.
Dr. Bekanich attended conferences offered by the Center to Advance Palliative Care and the American Academy of Hospice and Palliative Medicine. “I started to read a lot more in the pain literature,” he explains. “Initially, I was somewhat self-conscious about putting the new techniques into effect. So I’d call a pharmacist or a mentor to double check. I’m glad I did that.” TH
Larry Beresford is a regular contributor to The Hospitalist.
References
- Smith TE, Chong MS. Neuropathic pain. Hosp Med. 2000;61(11):760-766.
- Saarto T, Wiffen PJ. Antidepressants for neuropathic pain (review). Cochrane Database Syst Rev. 2005 Jul 20;(3)3:CD005454.
- Weissman DE. Fast Fact and Concept #71: Meperidine for pain—what’s all the fuss? [The End of Life/Palliative Education Resource Center.] Available at: www.eperc.mcw.edu/fastFact/ff_71.htm. Last accessed February 19, 2007.
Lateral Thigh Pain
A 67-year-old, slightly obese female was referred for poorly controlled hypertension and complaints of bilateral hip pain. Her pain worsens at night: It wakes her up when she rolls over on her hips. She also feels more pain when she is going up stairs.
Recently, she had difficulty bearing weight on her left hip. She has used a cane purchased at a yard sale for the past two and a half years. Daily ibuprofen provides her with little relief.
Physical Exam
On exam, we found that the patient had lateral thigh tenderness over the greater trochanter bilaterally and slight groin pain on internal/external rotation bilaterally. (See photos 1 and 2.)
We ordered an MRI because of concern that there might be a hip fracture. The results of the MRI indicate that both trochanteric bursae were inflamed and filled with fluid. The left bursa had much more fluid than the right. (See photo 3.)
Trochanteric Bursitis
We see trochanteric bursitis in runners and also after local trauma. This condition is most commonly found, however, in individuals with gait disturbances, such as those caused by a post-stroke condition, a discrepancy in leg length, pregnancy, medically complicated obesity, or improperly adjusted canes and walkers.
The pain caused by trochanteric bursitis may be severe and may radiate into the buttock or anterior thigh, mimicking fracture or radiculopathy.
The Trochanteric Bursae
There are three trochanteric bursae around the greater trochanter: two major and one minor. The minor bursa is the subgluteus minimus bursa, which is located above and slightly anterior to the proximal superior surface of the greater trochanter.
There are two major bursae. The subgluteus medius bursa can be found beneath the gluteus medius muscle, posterior and superior to the proximal edge of the greater trochanter. The subgluteus maximus bursa is lateral to the greater trochanter but separated from the trochanter by the gluteus medius muscle beneath the converging fibers of the tensor fascia lata and the gluteus maximus muscle and fascia as they join to form the iliotibial tract. Almond-shaped, 4 to 6 cm in length, and 2 to 4 cm in width, this bursa functions as a gliding mechanism for the anterior portion of the gluteus maximus tendon as it passes over the greater trochanter to insert into the iliotibial band. (See photo 4.)
Treatment
Although non-steroidal anti-inflammatory drugs (NSAIDs) provide relief to some patients, the elderly, who are most affected by trochanteric bursitis, frequently have contraindications to NSAID use. Local steroid injections provide durable relief for most patients. To limit recurrences, correct gait abnormalities and strengthen postural and hip muscles.
Trochanteric Bursitis Injection
To administer a trochanteric bursitis injection, first locate the subgluteus maximus bursa by palpating the greater trochanter. The bursa is located directly above the periosteum.
Next, identify an area of point tenderness. Instill a mixture of steroid and local anesthetic. The correct location will be confirmed by immediate pain relief; the steroid effect, however, may take up to week to provide pain relief. (See photo 5.)
Three-Month Follow-Up
The patient returned three months after her trochanateric bursae were injected with a depo-steroid preparation Today she no longer requires a cane. She is sleeping well and climbing stairs easily. Her blood pressure is well controlled, and she is on minimal medications after withdrawal of NSAIDs. She enjoys a renewed interest in German folk dancing.
Prevention
Prevention hinges on normalizing the gait abnormality that caused the bursal inflammation. That usually means strengthening the quadriceps—with or without a properly adjusted gait assist device such as a cane or an orthotic. (See photo 6.) TH
Dr. Ficalora is an associate professor of medicine at the Mayo Clinic College of Medicine, and Gerhart is a third-year medical student at the Mayo Clinic College of Medicine, Rochester, Minn.
A 67-year-old, slightly obese female was referred for poorly controlled hypertension and complaints of bilateral hip pain. Her pain worsens at night: It wakes her up when she rolls over on her hips. She also feels more pain when she is going up stairs.
Recently, she had difficulty bearing weight on her left hip. She has used a cane purchased at a yard sale for the past two and a half years. Daily ibuprofen provides her with little relief.
Physical Exam
On exam, we found that the patient had lateral thigh tenderness over the greater trochanter bilaterally and slight groin pain on internal/external rotation bilaterally. (See photos 1 and 2.)
We ordered an MRI because of concern that there might be a hip fracture. The results of the MRI indicate that both trochanteric bursae were inflamed and filled with fluid. The left bursa had much more fluid than the right. (See photo 3.)
Trochanteric Bursitis
We see trochanteric bursitis in runners and also after local trauma. This condition is most commonly found, however, in individuals with gait disturbances, such as those caused by a post-stroke condition, a discrepancy in leg length, pregnancy, medically complicated obesity, or improperly adjusted canes and walkers.
The pain caused by trochanteric bursitis may be severe and may radiate into the buttock or anterior thigh, mimicking fracture or radiculopathy.
The Trochanteric Bursae
There are three trochanteric bursae around the greater trochanter: two major and one minor. The minor bursa is the subgluteus minimus bursa, which is located above and slightly anterior to the proximal superior surface of the greater trochanter.
There are two major bursae. The subgluteus medius bursa can be found beneath the gluteus medius muscle, posterior and superior to the proximal edge of the greater trochanter. The subgluteus maximus bursa is lateral to the greater trochanter but separated from the trochanter by the gluteus medius muscle beneath the converging fibers of the tensor fascia lata and the gluteus maximus muscle and fascia as they join to form the iliotibial tract. Almond-shaped, 4 to 6 cm in length, and 2 to 4 cm in width, this bursa functions as a gliding mechanism for the anterior portion of the gluteus maximus tendon as it passes over the greater trochanter to insert into the iliotibial band. (See photo 4.)
Treatment
Although non-steroidal anti-inflammatory drugs (NSAIDs) provide relief to some patients, the elderly, who are most affected by trochanteric bursitis, frequently have contraindications to NSAID use. Local steroid injections provide durable relief for most patients. To limit recurrences, correct gait abnormalities and strengthen postural and hip muscles.
Trochanteric Bursitis Injection
To administer a trochanteric bursitis injection, first locate the subgluteus maximus bursa by palpating the greater trochanter. The bursa is located directly above the periosteum.
Next, identify an area of point tenderness. Instill a mixture of steroid and local anesthetic. The correct location will be confirmed by immediate pain relief; the steroid effect, however, may take up to week to provide pain relief. (See photo 5.)
Three-Month Follow-Up
The patient returned three months after her trochanateric bursae were injected with a depo-steroid preparation Today she no longer requires a cane. She is sleeping well and climbing stairs easily. Her blood pressure is well controlled, and she is on minimal medications after withdrawal of NSAIDs. She enjoys a renewed interest in German folk dancing.
Prevention
Prevention hinges on normalizing the gait abnormality that caused the bursal inflammation. That usually means strengthening the quadriceps—with or without a properly adjusted gait assist device such as a cane or an orthotic. (See photo 6.) TH
Dr. Ficalora is an associate professor of medicine at the Mayo Clinic College of Medicine, and Gerhart is a third-year medical student at the Mayo Clinic College of Medicine, Rochester, Minn.
A 67-year-old, slightly obese female was referred for poorly controlled hypertension and complaints of bilateral hip pain. Her pain worsens at night: It wakes her up when she rolls over on her hips. She also feels more pain when she is going up stairs.
Recently, she had difficulty bearing weight on her left hip. She has used a cane purchased at a yard sale for the past two and a half years. Daily ibuprofen provides her with little relief.
Physical Exam
On exam, we found that the patient had lateral thigh tenderness over the greater trochanter bilaterally and slight groin pain on internal/external rotation bilaterally. (See photos 1 and 2.)
We ordered an MRI because of concern that there might be a hip fracture. The results of the MRI indicate that both trochanteric bursae were inflamed and filled with fluid. The left bursa had much more fluid than the right. (See photo 3.)
Trochanteric Bursitis
We see trochanteric bursitis in runners and also after local trauma. This condition is most commonly found, however, in individuals with gait disturbances, such as those caused by a post-stroke condition, a discrepancy in leg length, pregnancy, medically complicated obesity, or improperly adjusted canes and walkers.
The pain caused by trochanteric bursitis may be severe and may radiate into the buttock or anterior thigh, mimicking fracture or radiculopathy.
The Trochanteric Bursae
There are three trochanteric bursae around the greater trochanter: two major and one minor. The minor bursa is the subgluteus minimus bursa, which is located above and slightly anterior to the proximal superior surface of the greater trochanter.
There are two major bursae. The subgluteus medius bursa can be found beneath the gluteus medius muscle, posterior and superior to the proximal edge of the greater trochanter. The subgluteus maximus bursa is lateral to the greater trochanter but separated from the trochanter by the gluteus medius muscle beneath the converging fibers of the tensor fascia lata and the gluteus maximus muscle and fascia as they join to form the iliotibial tract. Almond-shaped, 4 to 6 cm in length, and 2 to 4 cm in width, this bursa functions as a gliding mechanism for the anterior portion of the gluteus maximus tendon as it passes over the greater trochanter to insert into the iliotibial band. (See photo 4.)
Treatment
Although non-steroidal anti-inflammatory drugs (NSAIDs) provide relief to some patients, the elderly, who are most affected by trochanteric bursitis, frequently have contraindications to NSAID use. Local steroid injections provide durable relief for most patients. To limit recurrences, correct gait abnormalities and strengthen postural and hip muscles.
Trochanteric Bursitis Injection
To administer a trochanteric bursitis injection, first locate the subgluteus maximus bursa by palpating the greater trochanter. The bursa is located directly above the periosteum.
Next, identify an area of point tenderness. Instill a mixture of steroid and local anesthetic. The correct location will be confirmed by immediate pain relief; the steroid effect, however, may take up to week to provide pain relief. (See photo 5.)
Three-Month Follow-Up
The patient returned three months after her trochanateric bursae were injected with a depo-steroid preparation Today she no longer requires a cane. She is sleeping well and climbing stairs easily. Her blood pressure is well controlled, and she is on minimal medications after withdrawal of NSAIDs. She enjoys a renewed interest in German folk dancing.
Prevention
Prevention hinges on normalizing the gait abnormality that caused the bursal inflammation. That usually means strengthening the quadriceps—with or without a properly adjusted gait assist device such as a cane or an orthotic. (See photo 6.) TH
Dr. Ficalora is an associate professor of medicine at the Mayo Clinic College of Medicine, and Gerhart is a third-year medical student at the Mayo Clinic College of Medicine, Rochester, Minn.