User login
Atypical Presentation of Infiltrating Mucinous Carcinoma of the Breast
Benzoyl Peroxide Cleansers for the Treatment of Acne Vulgaris: Status Report on Available Data
A Diagnostic Pearl in Allergic Contact Dermatitis to Fragrances: The Atomizer Sign (See Erratum. 2009;83:49)
Did PSA finding get lost in the shuffle?...Woman sent home from ER dies of aneurysm...more
Did PSA finding get lost in the shuffle?
A SCREENING PROSTATE-SPECIFIC ANTIGEN (PSA) TEST ordered for a 76-year-old man by his primary care physician was within normal limits at 3.1. Two years later, the patient saw a urologist, who diagnosed renal cysts and bladder trabeculation based on a CT scan. Five months after that, the primary care physician ordered a second screening PSA, which was elevated at 12.
About a week later, the primary care physician noted that the patient was scheduled to see the urologist the next day, but didn’t indicate that the urologist had been informed of the elevated PSA or that the patient had been told of its significance. A letter from the primary care physician to the urologist after the patient’s visit stated that the patient was being treated for microscopic hematuria but didn’t mention elevated PSA. A letter several weeks later from the urologist to the primary care physician discussed the patient’s elevated PSA. The primary care physician didn’t contact the urologist to follow up on the finding, however.
After a year of testing, the urologist concluded that the hematuria was probably related to the kidney, or perhaps the prostate, and started the patient on dutasteride, which helped the bleeding. Two months after the start of treatment, the urologist ordered a PSA test, which was extremely elevated. A subsequent biopsy revealed adenocarcinoma, and a bone scan showed metastatic bony disease, which hadn’t shown up on a bone scan done 6 months before. The patient died 2 years later. The cause of death was listed as cardiopulmonary arrest, cardiogenic shock, and myocardial infarction.
PLAINTIFF’S CLAIM The plaintiff’s claim focused on the handling of the PSA test, though the specifics of the claim were not detailed in the case summary.
DOCTOR’S DEFENSE The primary care physician claimed that his nurse told the patient after the second PSA test that the PSA was 12 and encouraged the patient to see the urologist to discuss the elevated level. The physician also claimed that he had faxed the elevated PSA test result to the urologist and that the patient was reminded of the elevated PSA during his visit to the urologist. No information about the urologist’s defense was available.
VERDICT $325,000 Massachusetts settlement.
COMMENT Coordination of care and documentation of communication are keys to good patient care—and avoiding lawsuits.
Woman sent home from ER dies of aneurysm
SEVERE HEADACHES prompted a 38-year-old woman to visit her family physician, who referred her to a neurologist; an appointment was scheduled for more than a month later. A month after seeing the family physician, the patient went to the emergency room complaining of a severe headache.
A CT scan ordered by the ER physician showed a large mass in the patient’s brain. The ER physician gave the patient the scan report, told her to see her family doctor, and sent her home without consulting a neurosurgeon. Later that day, the aneurysm ruptured; the patient’s family took her to the hospital, where she died the next morning.
PLAINTIFF’S CLAIM The family physician should have ordered a CT scan, which would have revealed the aneurysm. The ER physician should have ordered an immediate neurologic consult, which would have led to surgical repair of the leaking aneurysm. Either measure would have saved the patient’s life.
DOCTOR’S DEFENSE The family physician claimed that the patient’s complaints weren’t urgent and he made a proper referral. The ER physician claimed that the patient wouldn’t have lived even if he’d arranged an immediate consult.
VERDICT $1.5 million Michigan verdict against the ER physician.
COMMENT This case illustrates the value of clearly documenting referrals and suggesting follow-up if a change in symptoms occurs.
Jaundiced newborn dies after slip-ups
AN INFANT BORN AT 36 WEEKS and the baby’s 20-year-old mother were discharged from the hospital fewer than 48 hours after delivery, with an appointment with a visiting nurse for the following day and a pediatrician 3 days later. Hospital medical records reported infrequent breast feeding, significant decrease in weight, and a bruise on the back of the infant’s head.
The visiting nurse who examined the baby noted moderate facial jaundice, mild jaundice in the groin, and slight jaundice in the sclera of the eyes, as well as the bruise on the back of the head. The nurse didn’t notify the pediatrician of the jaundice. The mother said that when she voiced concern about the jaundice, the nurse told her to feed the infant more often and expose her to sunlight.
The day after the nurse’s visit, the parents noticed that the baby was more jaundiced and had started to arch her back, grunt, and whine. The mother called the pediatrician’s office that day and reported the symptoms; the nurse told her that the pediatrician felt that he didn’t need to see the baby before her appointment the following day. As the symptoms worsened, the mother called the pediatrician’s office 3 more times before 6 PM, speaking with 2 nurses, neither of whom took a medical history.
The mother called again after the office had closed. A nurse arranged for the infant to be seen at the hospital, where the baby was admitted with a critically low temperature, decreased muscle tone, arching of the back, and an elevated bilirubin level of 35.4 mg/dL. Despite phototherapy and intubation, the infant’s condition deteriorated, and she was airlifted to another medical facility for more advanced care. The baby was given cardiopulmonary resuscitation on arrival, but died 4 hours later of acute bilirubin encephalopathy.
PLAINTIFF’S CLAIM In light of her symptoms, the baby shouldn’t have been discharged from the hospital. The visiting nurse should have reported the baby’s symptoms to the pediatrician or recommended that the parents take the baby to the doctor right away. The nurses in the pediatrician’s office were negligent in not taking a full medical history. The pediatrician should have seen the baby immediately. He failed to recognize the symptoms of possible hyperbilirubinemia, a medical emergency.
DOCTOR’S DEFENSE No information about the doctor’s or nurses’ defense is available.
VERDICT $460,000 Massachusetts settlement.
COMMENT This case illustrates, once again, the importance of care coordination and sharing information on a timely basis.
Did PSA finding get lost in the shuffle?
A SCREENING PROSTATE-SPECIFIC ANTIGEN (PSA) TEST ordered for a 76-year-old man by his primary care physician was within normal limits at 3.1. Two years later, the patient saw a urologist, who diagnosed renal cysts and bladder trabeculation based on a CT scan. Five months after that, the primary care physician ordered a second screening PSA, which was elevated at 12.
About a week later, the primary care physician noted that the patient was scheduled to see the urologist the next day, but didn’t indicate that the urologist had been informed of the elevated PSA or that the patient had been told of its significance. A letter from the primary care physician to the urologist after the patient’s visit stated that the patient was being treated for microscopic hematuria but didn’t mention elevated PSA. A letter several weeks later from the urologist to the primary care physician discussed the patient’s elevated PSA. The primary care physician didn’t contact the urologist to follow up on the finding, however.
After a year of testing, the urologist concluded that the hematuria was probably related to the kidney, or perhaps the prostate, and started the patient on dutasteride, which helped the bleeding. Two months after the start of treatment, the urologist ordered a PSA test, which was extremely elevated. A subsequent biopsy revealed adenocarcinoma, and a bone scan showed metastatic bony disease, which hadn’t shown up on a bone scan done 6 months before. The patient died 2 years later. The cause of death was listed as cardiopulmonary arrest, cardiogenic shock, and myocardial infarction.
PLAINTIFF’S CLAIM The plaintiff’s claim focused on the handling of the PSA test, though the specifics of the claim were not detailed in the case summary.
DOCTOR’S DEFENSE The primary care physician claimed that his nurse told the patient after the second PSA test that the PSA was 12 and encouraged the patient to see the urologist to discuss the elevated level. The physician also claimed that he had faxed the elevated PSA test result to the urologist and that the patient was reminded of the elevated PSA during his visit to the urologist. No information about the urologist’s defense was available.
VERDICT $325,000 Massachusetts settlement.
COMMENT Coordination of care and documentation of communication are keys to good patient care—and avoiding lawsuits.
Woman sent home from ER dies of aneurysm
SEVERE HEADACHES prompted a 38-year-old woman to visit her family physician, who referred her to a neurologist; an appointment was scheduled for more than a month later. A month after seeing the family physician, the patient went to the emergency room complaining of a severe headache.
A CT scan ordered by the ER physician showed a large mass in the patient’s brain. The ER physician gave the patient the scan report, told her to see her family doctor, and sent her home without consulting a neurosurgeon. Later that day, the aneurysm ruptured; the patient’s family took her to the hospital, where she died the next morning.
PLAINTIFF’S CLAIM The family physician should have ordered a CT scan, which would have revealed the aneurysm. The ER physician should have ordered an immediate neurologic consult, which would have led to surgical repair of the leaking aneurysm. Either measure would have saved the patient’s life.
DOCTOR’S DEFENSE The family physician claimed that the patient’s complaints weren’t urgent and he made a proper referral. The ER physician claimed that the patient wouldn’t have lived even if he’d arranged an immediate consult.
VERDICT $1.5 million Michigan verdict against the ER physician.
COMMENT This case illustrates the value of clearly documenting referrals and suggesting follow-up if a change in symptoms occurs.
Jaundiced newborn dies after slip-ups
AN INFANT BORN AT 36 WEEKS and the baby’s 20-year-old mother were discharged from the hospital fewer than 48 hours after delivery, with an appointment with a visiting nurse for the following day and a pediatrician 3 days later. Hospital medical records reported infrequent breast feeding, significant decrease in weight, and a bruise on the back of the infant’s head.
The visiting nurse who examined the baby noted moderate facial jaundice, mild jaundice in the groin, and slight jaundice in the sclera of the eyes, as well as the bruise on the back of the head. The nurse didn’t notify the pediatrician of the jaundice. The mother said that when she voiced concern about the jaundice, the nurse told her to feed the infant more often and expose her to sunlight.
The day after the nurse’s visit, the parents noticed that the baby was more jaundiced and had started to arch her back, grunt, and whine. The mother called the pediatrician’s office that day and reported the symptoms; the nurse told her that the pediatrician felt that he didn’t need to see the baby before her appointment the following day. As the symptoms worsened, the mother called the pediatrician’s office 3 more times before 6 PM, speaking with 2 nurses, neither of whom took a medical history.
The mother called again after the office had closed. A nurse arranged for the infant to be seen at the hospital, where the baby was admitted with a critically low temperature, decreased muscle tone, arching of the back, and an elevated bilirubin level of 35.4 mg/dL. Despite phototherapy and intubation, the infant’s condition deteriorated, and she was airlifted to another medical facility for more advanced care. The baby was given cardiopulmonary resuscitation on arrival, but died 4 hours later of acute bilirubin encephalopathy.
PLAINTIFF’S CLAIM In light of her symptoms, the baby shouldn’t have been discharged from the hospital. The visiting nurse should have reported the baby’s symptoms to the pediatrician or recommended that the parents take the baby to the doctor right away. The nurses in the pediatrician’s office were negligent in not taking a full medical history. The pediatrician should have seen the baby immediately. He failed to recognize the symptoms of possible hyperbilirubinemia, a medical emergency.
DOCTOR’S DEFENSE No information about the doctor’s or nurses’ defense is available.
VERDICT $460,000 Massachusetts settlement.
COMMENT This case illustrates, once again, the importance of care coordination and sharing information on a timely basis.
Did PSA finding get lost in the shuffle?
A SCREENING PROSTATE-SPECIFIC ANTIGEN (PSA) TEST ordered for a 76-year-old man by his primary care physician was within normal limits at 3.1. Two years later, the patient saw a urologist, who diagnosed renal cysts and bladder trabeculation based on a CT scan. Five months after that, the primary care physician ordered a second screening PSA, which was elevated at 12.
About a week later, the primary care physician noted that the patient was scheduled to see the urologist the next day, but didn’t indicate that the urologist had been informed of the elevated PSA or that the patient had been told of its significance. A letter from the primary care physician to the urologist after the patient’s visit stated that the patient was being treated for microscopic hematuria but didn’t mention elevated PSA. A letter several weeks later from the urologist to the primary care physician discussed the patient’s elevated PSA. The primary care physician didn’t contact the urologist to follow up on the finding, however.
After a year of testing, the urologist concluded that the hematuria was probably related to the kidney, or perhaps the prostate, and started the patient on dutasteride, which helped the bleeding. Two months after the start of treatment, the urologist ordered a PSA test, which was extremely elevated. A subsequent biopsy revealed adenocarcinoma, and a bone scan showed metastatic bony disease, which hadn’t shown up on a bone scan done 6 months before. The patient died 2 years later. The cause of death was listed as cardiopulmonary arrest, cardiogenic shock, and myocardial infarction.
PLAINTIFF’S CLAIM The plaintiff’s claim focused on the handling of the PSA test, though the specifics of the claim were not detailed in the case summary.
DOCTOR’S DEFENSE The primary care physician claimed that his nurse told the patient after the second PSA test that the PSA was 12 and encouraged the patient to see the urologist to discuss the elevated level. The physician also claimed that he had faxed the elevated PSA test result to the urologist and that the patient was reminded of the elevated PSA during his visit to the urologist. No information about the urologist’s defense was available.
VERDICT $325,000 Massachusetts settlement.
COMMENT Coordination of care and documentation of communication are keys to good patient care—and avoiding lawsuits.
Woman sent home from ER dies of aneurysm
SEVERE HEADACHES prompted a 38-year-old woman to visit her family physician, who referred her to a neurologist; an appointment was scheduled for more than a month later. A month after seeing the family physician, the patient went to the emergency room complaining of a severe headache.
A CT scan ordered by the ER physician showed a large mass in the patient’s brain. The ER physician gave the patient the scan report, told her to see her family doctor, and sent her home without consulting a neurosurgeon. Later that day, the aneurysm ruptured; the patient’s family took her to the hospital, where she died the next morning.
PLAINTIFF’S CLAIM The family physician should have ordered a CT scan, which would have revealed the aneurysm. The ER physician should have ordered an immediate neurologic consult, which would have led to surgical repair of the leaking aneurysm. Either measure would have saved the patient’s life.
DOCTOR’S DEFENSE The family physician claimed that the patient’s complaints weren’t urgent and he made a proper referral. The ER physician claimed that the patient wouldn’t have lived even if he’d arranged an immediate consult.
VERDICT $1.5 million Michigan verdict against the ER physician.
COMMENT This case illustrates the value of clearly documenting referrals and suggesting follow-up if a change in symptoms occurs.
Jaundiced newborn dies after slip-ups
AN INFANT BORN AT 36 WEEKS and the baby’s 20-year-old mother were discharged from the hospital fewer than 48 hours after delivery, with an appointment with a visiting nurse for the following day and a pediatrician 3 days later. Hospital medical records reported infrequent breast feeding, significant decrease in weight, and a bruise on the back of the infant’s head.
The visiting nurse who examined the baby noted moderate facial jaundice, mild jaundice in the groin, and slight jaundice in the sclera of the eyes, as well as the bruise on the back of the head. The nurse didn’t notify the pediatrician of the jaundice. The mother said that when she voiced concern about the jaundice, the nurse told her to feed the infant more often and expose her to sunlight.
The day after the nurse’s visit, the parents noticed that the baby was more jaundiced and had started to arch her back, grunt, and whine. The mother called the pediatrician’s office that day and reported the symptoms; the nurse told her that the pediatrician felt that he didn’t need to see the baby before her appointment the following day. As the symptoms worsened, the mother called the pediatrician’s office 3 more times before 6 PM, speaking with 2 nurses, neither of whom took a medical history.
The mother called again after the office had closed. A nurse arranged for the infant to be seen at the hospital, where the baby was admitted with a critically low temperature, decreased muscle tone, arching of the back, and an elevated bilirubin level of 35.4 mg/dL. Despite phototherapy and intubation, the infant’s condition deteriorated, and she was airlifted to another medical facility for more advanced care. The baby was given cardiopulmonary resuscitation on arrival, but died 4 hours later of acute bilirubin encephalopathy.
PLAINTIFF’S CLAIM In light of her symptoms, the baby shouldn’t have been discharged from the hospital. The visiting nurse should have reported the baby’s symptoms to the pediatrician or recommended that the parents take the baby to the doctor right away. The nurses in the pediatrician’s office were negligent in not taking a full medical history. The pediatrician should have seen the baby immediately. He failed to recognize the symptoms of possible hyperbilirubinemia, a medical emergency.
DOCTOR’S DEFENSE No information about the doctor’s or nurses’ defense is available.
VERDICT $460,000 Massachusetts settlement.
COMMENT This case illustrates, once again, the importance of care coordination and sharing information on a timely basis.
USPSTF scales back approach to lipid screening for women
When patients reached a certain age (36 for men, 46 for women), it used to mean that it was time, in the eyes of the United States Preventive Services Task Force (USPSTF), to screen for lipid disorders. But that’s changed for female patients.
The USPSTF’s latest recommendations (TABLE 1) on screening for lipid disorders in adults1 call for screening women only when coronary heart disease (CHD) risk factors are present, regardless of their age. (See TABLE 2 for a list of CHD risk factors.) That’s a major shift from the 2001 recommendation, which stated that all women over age 45 should be screened and women ages 20 to 45 should be screened if they were at elevated risk.
The recommendations for men remain the same: All men older than 35 should be screened, as should men who are between the ages of 20 and 35 who have other CHD risks.
TABLE 1
USPSTF lipid disorder screening recommendations at a glance
| Screening men • The United States Preventive Services Task Force (USPSTF) strongly recommends screening men ages 35 and older for lipid disorders. Grade A recommendation |
| • The USPSTF recommends screening men ages 20 to 35 for lipid disorders if they are at increased risk for coronary heart disease (CHD). Grade B recommendation |
| Screening women at increased risk • The USPSTF strongly recommends screening women ages 45 and older for lipid disorders if they are at increased risk for CHD. Grade A recommendation |
| • The USPSTF recommends screening women ages 20 to 45 for lipid disorders if they are at increased risk for CHD. Grade B recommendation |
| Screening young men and all women not at increased risk • The USPSTF makes no recommendation for or against routine screening for lipid disorders in men between the ages of 20 and 35, or in women ages 20 and older who are not at increased risk for CHD. Grade C recommendation |
TABLE 2
Risk factors for CHD
| • Diabetes |
| • Personal history of coronary heart disease (CHD) or noncoronary atherosclerosis (eg, abdominal aortic aneurysm, peripheral artery disease, and carotid artery stenosis) |
| • A family history of cardiovascular disease before age 50 in male relatives or age 60 in female relatives |
| • Tobacco use |
| • Hypertension |
| • Obesity (body mass index ≥30) |
A different approach from NIH and AHA
The revised updated recommendation for women over age 45 was based on 2 systematic evidence reviews2,3 that concluded, while treatment clearly benefits women with other risk factors, benefit has not been proven for women who are otherwise CHD risk free.
The recommendation for women conflicts with those of the National Institutes of Health and the American Heart Association; both recommend screening all adults starting at age 20—regardless of risk.
Screening those without risk isn’t ruled out
It is important to note that the task force is not recommending against screening in women (or men between the ages of 20 and 35) who do not have other CHD risks. The task force makes a C recommendation with wording that states, “The USPSTF makes no recommendation for or against routine provision of [the service]. The USPSTF found at least fair evidence that [the service] can improve health outcomes but concludes that the balance of benefits and harms is too close to justify a general recommendation” (TABLE 3).
The task force chose not to use the new wording for a C recommendation, adopted in 2007, which reads, “The USPSTF recommends against routinely providing the service. There may be considerations that support providing the service in an individual patient. There is at least moderate certainty that the net benefit is small.”
It is also important to realize that a large proportion of women have another CHD risk and will not fall into the C category recommendation.
TABLE 3
USPSTF recommendation categories
| A—Strongly recommended: The United States Preventive Services Task Force (USPSTF) strongly recommends that clinicians provide the service to eligible patients. The USPSTF found good evidence that the service improves important health outcomes and concludes that benefits substantially outweigh harms. |
| B—Recommended: The USPSTF recommends that clinicians provide the service to eligible patients. The USPSTF found at least fair evidence that the service improves important health outcomes and concludes that benefits outweigh harms. |
| C—No recommendation: The USPSTF makes no recommendation for or against routine provision of the service. The USPSTF found at least fair evidence that the service can improve health outcomes but concludes that the balance of benefits and harms is too close to justify a general recommendation. |
| D—Not recommended: The USPSTF recommends against routinely providing the service to asymptomatic patients. The USPSTF found at least fair evidence that the service is ineffective or that harms outweigh benefits. |
| I—Insufficient evidence to make a recommendation: The USPSTF concludes that the evidence is insufficient to recommend for or against routinely providing the service. Evidence that the service is effective is lacking, of poor quality, or conflicting, and the balance of benefits and harms cannot be determined. |
No need to look at triglycerides initially
The task force recommends screening with a fasting or nonfasting serum sample for total cholesterol and high-density lipoprotein cholesterol. The task force does not recommend including a triglyceride level because there is mixed and inclusive evidence that triglyceride levels are independently associated with CHD risk and scant evidence that treating isolated elevated triglyceride levels reduces the occurrence of CHD events. This approach also conflicts with other organizations that recommend screening with fasting lipid profiles that include a triglyceride level.
The task force states that an abnormal initial screen should be confirmed by a repeat test and, if confirmed, a fasting lipid panel should be obtained. Wide adoption of the task force recommendations would result in considerable savings in cost and patient inconvenience by avoiding complete fasting lipid panels as the initial screen.
The optimal frequency of screening is not established and the task force states that every 5 years is reasonable, although more frequent testing might be considered for those with high normal values, and less frequent intervals for those with optimal cholesterol levels and healthy lifestyles.
Treatment: Look beyond lifestyle
The screening recommendations are accompanied by a discussion of clinical considerations and a description of an approach to treatment for those with lipid disorders. The main point the task force makes is that all CHD risks should be addressed, and that lifestyle changes alone rarely reduce elevated cholesterol to an optimal level. (For more on the treatment of hyperlipidemia, see the National Heart, Lung, and Blood Institute’s Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults [Adult Treatment Panel III] at http://www.nhlbi.nih.gov/guidelines/cholesterol/index.htm.)
Time to rethink conventional opinion
The updated task force recommendations are a reminder that many widely used guidelines, including those on the prevention of CHD, are based on a lack of high-level evidence. Thus, it is not surprising that a rigorously evidence-based analysis, as preformed by the USPSTF, will frequently result in recommendations that are at variance with common practice and conventional opinion.
1. U.S. Preventive Services Task Force (USPSTF). Screening for lipid disorders in adults: recommendation statement. June 2008. Available at: http://www.ahrq.gov/clinic/uspstf08/lipid/lipidrs.htm. Accessed September 26, 2008.
2. Grady D, Chaput L, Kristof M. Systematic Review of Lipid Lowering Treatment to Reduce Risk of Coronary Heart Disease in Women. Rockville, Md: Agency for Healthcare Research and Quality; 2003.
3. Helfand M, Carson S. Screening for lipid disorders in adults: selective update of 2001 U.S. Preventive Services Task Force Review. June 2008. AHRQ publication number 08-05114-EF-1. Available at: http://www.ahrq.gov/clinic/uspstf08/lipid/lipides.pdf. Accessed September 26, 2008.
When patients reached a certain age (36 for men, 46 for women), it used to mean that it was time, in the eyes of the United States Preventive Services Task Force (USPSTF), to screen for lipid disorders. But that’s changed for female patients.
The USPSTF’s latest recommendations (TABLE 1) on screening for lipid disorders in adults1 call for screening women only when coronary heart disease (CHD) risk factors are present, regardless of their age. (See TABLE 2 for a list of CHD risk factors.) That’s a major shift from the 2001 recommendation, which stated that all women over age 45 should be screened and women ages 20 to 45 should be screened if they were at elevated risk.
The recommendations for men remain the same: All men older than 35 should be screened, as should men who are between the ages of 20 and 35 who have other CHD risks.
TABLE 1
USPSTF lipid disorder screening recommendations at a glance
| Screening men • The United States Preventive Services Task Force (USPSTF) strongly recommends screening men ages 35 and older for lipid disorders. Grade A recommendation |
| • The USPSTF recommends screening men ages 20 to 35 for lipid disorders if they are at increased risk for coronary heart disease (CHD). Grade B recommendation |
| Screening women at increased risk • The USPSTF strongly recommends screening women ages 45 and older for lipid disorders if they are at increased risk for CHD. Grade A recommendation |
| • The USPSTF recommends screening women ages 20 to 45 for lipid disorders if they are at increased risk for CHD. Grade B recommendation |
| Screening young men and all women not at increased risk • The USPSTF makes no recommendation for or against routine screening for lipid disorders in men between the ages of 20 and 35, or in women ages 20 and older who are not at increased risk for CHD. Grade C recommendation |
TABLE 2
Risk factors for CHD
| • Diabetes |
| • Personal history of coronary heart disease (CHD) or noncoronary atherosclerosis (eg, abdominal aortic aneurysm, peripheral artery disease, and carotid artery stenosis) |
| • A family history of cardiovascular disease before age 50 in male relatives or age 60 in female relatives |
| • Tobacco use |
| • Hypertension |
| • Obesity (body mass index ≥30) |
A different approach from NIH and AHA
The revised updated recommendation for women over age 45 was based on 2 systematic evidence reviews2,3 that concluded, while treatment clearly benefits women with other risk factors, benefit has not been proven for women who are otherwise CHD risk free.
The recommendation for women conflicts with those of the National Institutes of Health and the American Heart Association; both recommend screening all adults starting at age 20—regardless of risk.
Screening those without risk isn’t ruled out
It is important to note that the task force is not recommending against screening in women (or men between the ages of 20 and 35) who do not have other CHD risks. The task force makes a C recommendation with wording that states, “The USPSTF makes no recommendation for or against routine provision of [the service]. The USPSTF found at least fair evidence that [the service] can improve health outcomes but concludes that the balance of benefits and harms is too close to justify a general recommendation” (TABLE 3).
The task force chose not to use the new wording for a C recommendation, adopted in 2007, which reads, “The USPSTF recommends against routinely providing the service. There may be considerations that support providing the service in an individual patient. There is at least moderate certainty that the net benefit is small.”
It is also important to realize that a large proportion of women have another CHD risk and will not fall into the C category recommendation.
TABLE 3
USPSTF recommendation categories
| A—Strongly recommended: The United States Preventive Services Task Force (USPSTF) strongly recommends that clinicians provide the service to eligible patients. The USPSTF found good evidence that the service improves important health outcomes and concludes that benefits substantially outweigh harms. |
| B—Recommended: The USPSTF recommends that clinicians provide the service to eligible patients. The USPSTF found at least fair evidence that the service improves important health outcomes and concludes that benefits outweigh harms. |
| C—No recommendation: The USPSTF makes no recommendation for or against routine provision of the service. The USPSTF found at least fair evidence that the service can improve health outcomes but concludes that the balance of benefits and harms is too close to justify a general recommendation. |
| D—Not recommended: The USPSTF recommends against routinely providing the service to asymptomatic patients. The USPSTF found at least fair evidence that the service is ineffective or that harms outweigh benefits. |
| I—Insufficient evidence to make a recommendation: The USPSTF concludes that the evidence is insufficient to recommend for or against routinely providing the service. Evidence that the service is effective is lacking, of poor quality, or conflicting, and the balance of benefits and harms cannot be determined. |
No need to look at triglycerides initially
The task force recommends screening with a fasting or nonfasting serum sample for total cholesterol and high-density lipoprotein cholesterol. The task force does not recommend including a triglyceride level because there is mixed and inclusive evidence that triglyceride levels are independently associated with CHD risk and scant evidence that treating isolated elevated triglyceride levels reduces the occurrence of CHD events. This approach also conflicts with other organizations that recommend screening with fasting lipid profiles that include a triglyceride level.
The task force states that an abnormal initial screen should be confirmed by a repeat test and, if confirmed, a fasting lipid panel should be obtained. Wide adoption of the task force recommendations would result in considerable savings in cost and patient inconvenience by avoiding complete fasting lipid panels as the initial screen.
The optimal frequency of screening is not established and the task force states that every 5 years is reasonable, although more frequent testing might be considered for those with high normal values, and less frequent intervals for those with optimal cholesterol levels and healthy lifestyles.
Treatment: Look beyond lifestyle
The screening recommendations are accompanied by a discussion of clinical considerations and a description of an approach to treatment for those with lipid disorders. The main point the task force makes is that all CHD risks should be addressed, and that lifestyle changes alone rarely reduce elevated cholesterol to an optimal level. (For more on the treatment of hyperlipidemia, see the National Heart, Lung, and Blood Institute’s Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults [Adult Treatment Panel III] at http://www.nhlbi.nih.gov/guidelines/cholesterol/index.htm.)
Time to rethink conventional opinion
The updated task force recommendations are a reminder that many widely used guidelines, including those on the prevention of CHD, are based on a lack of high-level evidence. Thus, it is not surprising that a rigorously evidence-based analysis, as preformed by the USPSTF, will frequently result in recommendations that are at variance with common practice and conventional opinion.
When patients reached a certain age (36 for men, 46 for women), it used to mean that it was time, in the eyes of the United States Preventive Services Task Force (USPSTF), to screen for lipid disorders. But that’s changed for female patients.
The USPSTF’s latest recommendations (TABLE 1) on screening for lipid disorders in adults1 call for screening women only when coronary heart disease (CHD) risk factors are present, regardless of their age. (See TABLE 2 for a list of CHD risk factors.) That’s a major shift from the 2001 recommendation, which stated that all women over age 45 should be screened and women ages 20 to 45 should be screened if they were at elevated risk.
The recommendations for men remain the same: All men older than 35 should be screened, as should men who are between the ages of 20 and 35 who have other CHD risks.
TABLE 1
USPSTF lipid disorder screening recommendations at a glance
| Screening men • The United States Preventive Services Task Force (USPSTF) strongly recommends screening men ages 35 and older for lipid disorders. Grade A recommendation |
| • The USPSTF recommends screening men ages 20 to 35 for lipid disorders if they are at increased risk for coronary heart disease (CHD). Grade B recommendation |
| Screening women at increased risk • The USPSTF strongly recommends screening women ages 45 and older for lipid disorders if they are at increased risk for CHD. Grade A recommendation |
| • The USPSTF recommends screening women ages 20 to 45 for lipid disorders if they are at increased risk for CHD. Grade B recommendation |
| Screening young men and all women not at increased risk • The USPSTF makes no recommendation for or against routine screening for lipid disorders in men between the ages of 20 and 35, or in women ages 20 and older who are not at increased risk for CHD. Grade C recommendation |
TABLE 2
Risk factors for CHD
| • Diabetes |
| • Personal history of coronary heart disease (CHD) or noncoronary atherosclerosis (eg, abdominal aortic aneurysm, peripheral artery disease, and carotid artery stenosis) |
| • A family history of cardiovascular disease before age 50 in male relatives or age 60 in female relatives |
| • Tobacco use |
| • Hypertension |
| • Obesity (body mass index ≥30) |
A different approach from NIH and AHA
The revised updated recommendation for women over age 45 was based on 2 systematic evidence reviews2,3 that concluded, while treatment clearly benefits women with other risk factors, benefit has not been proven for women who are otherwise CHD risk free.
The recommendation for women conflicts with those of the National Institutes of Health and the American Heart Association; both recommend screening all adults starting at age 20—regardless of risk.
Screening those without risk isn’t ruled out
It is important to note that the task force is not recommending against screening in women (or men between the ages of 20 and 35) who do not have other CHD risks. The task force makes a C recommendation with wording that states, “The USPSTF makes no recommendation for or against routine provision of [the service]. The USPSTF found at least fair evidence that [the service] can improve health outcomes but concludes that the balance of benefits and harms is too close to justify a general recommendation” (TABLE 3).
The task force chose not to use the new wording for a C recommendation, adopted in 2007, which reads, “The USPSTF recommends against routinely providing the service. There may be considerations that support providing the service in an individual patient. There is at least moderate certainty that the net benefit is small.”
It is also important to realize that a large proportion of women have another CHD risk and will not fall into the C category recommendation.
TABLE 3
USPSTF recommendation categories
| A—Strongly recommended: The United States Preventive Services Task Force (USPSTF) strongly recommends that clinicians provide the service to eligible patients. The USPSTF found good evidence that the service improves important health outcomes and concludes that benefits substantially outweigh harms. |
| B—Recommended: The USPSTF recommends that clinicians provide the service to eligible patients. The USPSTF found at least fair evidence that the service improves important health outcomes and concludes that benefits outweigh harms. |
| C—No recommendation: The USPSTF makes no recommendation for or against routine provision of the service. The USPSTF found at least fair evidence that the service can improve health outcomes but concludes that the balance of benefits and harms is too close to justify a general recommendation. |
| D—Not recommended: The USPSTF recommends against routinely providing the service to asymptomatic patients. The USPSTF found at least fair evidence that the service is ineffective or that harms outweigh benefits. |
| I—Insufficient evidence to make a recommendation: The USPSTF concludes that the evidence is insufficient to recommend for or against routinely providing the service. Evidence that the service is effective is lacking, of poor quality, or conflicting, and the balance of benefits and harms cannot be determined. |
No need to look at triglycerides initially
The task force recommends screening with a fasting or nonfasting serum sample for total cholesterol and high-density lipoprotein cholesterol. The task force does not recommend including a triglyceride level because there is mixed and inclusive evidence that triglyceride levels are independently associated with CHD risk and scant evidence that treating isolated elevated triglyceride levels reduces the occurrence of CHD events. This approach also conflicts with other organizations that recommend screening with fasting lipid profiles that include a triglyceride level.
The task force states that an abnormal initial screen should be confirmed by a repeat test and, if confirmed, a fasting lipid panel should be obtained. Wide adoption of the task force recommendations would result in considerable savings in cost and patient inconvenience by avoiding complete fasting lipid panels as the initial screen.
The optimal frequency of screening is not established and the task force states that every 5 years is reasonable, although more frequent testing might be considered for those with high normal values, and less frequent intervals for those with optimal cholesterol levels and healthy lifestyles.
Treatment: Look beyond lifestyle
The screening recommendations are accompanied by a discussion of clinical considerations and a description of an approach to treatment for those with lipid disorders. The main point the task force makes is that all CHD risks should be addressed, and that lifestyle changes alone rarely reduce elevated cholesterol to an optimal level. (For more on the treatment of hyperlipidemia, see the National Heart, Lung, and Blood Institute’s Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults [Adult Treatment Panel III] at http://www.nhlbi.nih.gov/guidelines/cholesterol/index.htm.)
Time to rethink conventional opinion
The updated task force recommendations are a reminder that many widely used guidelines, including those on the prevention of CHD, are based on a lack of high-level evidence. Thus, it is not surprising that a rigorously evidence-based analysis, as preformed by the USPSTF, will frequently result in recommendations that are at variance with common practice and conventional opinion.
1. U.S. Preventive Services Task Force (USPSTF). Screening for lipid disorders in adults: recommendation statement. June 2008. Available at: http://www.ahrq.gov/clinic/uspstf08/lipid/lipidrs.htm. Accessed September 26, 2008.
2. Grady D, Chaput L, Kristof M. Systematic Review of Lipid Lowering Treatment to Reduce Risk of Coronary Heart Disease in Women. Rockville, Md: Agency for Healthcare Research and Quality; 2003.
3. Helfand M, Carson S. Screening for lipid disorders in adults: selective update of 2001 U.S. Preventive Services Task Force Review. June 2008. AHRQ publication number 08-05114-EF-1. Available at: http://www.ahrq.gov/clinic/uspstf08/lipid/lipides.pdf. Accessed September 26, 2008.
1. U.S. Preventive Services Task Force (USPSTF). Screening for lipid disorders in adults: recommendation statement. June 2008. Available at: http://www.ahrq.gov/clinic/uspstf08/lipid/lipidrs.htm. Accessed September 26, 2008.
2. Grady D, Chaput L, Kristof M. Systematic Review of Lipid Lowering Treatment to Reduce Risk of Coronary Heart Disease in Women. Rockville, Md: Agency for Healthcare Research and Quality; 2003.
3. Helfand M, Carson S. Screening for lipid disorders in adults: selective update of 2001 U.S. Preventive Services Task Force Review. June 2008. AHRQ publication number 08-05114-EF-1. Available at: http://www.ahrq.gov/clinic/uspstf08/lipid/lipides.pdf. Accessed September 26, 2008.
6 office tests to assess ovarian reserve, and what they tell you
The University of Medicine and Dentistry of New Jersey (UMDNJ) owns a patent relating to the use of anti-Müllerian hormone/Müllerian inhibiting substance for predicting ovarian response in women with infertility. The patent is based in part on work that Dr. Seifer carried out while employed at UMDNJ. In accordance with UMDNJ policy, Dr. Seifer, a named inventor on this patent, assigned his interest in the invention to UMDNJ. UMDNJ has a licensing agreement with Diagnostic Systems Laboratory for the use of the claimed invention. Dr. Seifer receives a portion of the royalties, as determined by UMDNJ policy, that UMDNJ gains from this licensing agreement.
CASE: Borderline test result prompts referral
A 36-year-old nulliparous woman is seen in your office for evaluation after 6 months of infertility. She is ovulatory, and has been using an ovulation-prediction kit to time intercourse. You learn that she had Chlamydia trachomatis infection in the distant past, but elicit no other significant medical or surgical history. She reports that she smoked approximately one pack of cigarettes a day for 15 years but gave up smoking 5 years ago.
You order a hysterosalpingogram, followed by day 3 testing of follicle-stimulating hormone (FSH). The hysterosalpingogram is normal; the FSH level is 7.5 mIU/mL and the estradiol level is 30 pg/mL—both in the normal range.
The patient asks for testing of anti-Müllerian hormone (AMH; also known as Müllerian-inhibiting substance) because she has read that it is a new marker of fertility. The result is 0.5 ng/mL, a borderline value. After reviewing these results, you refer her to a reproductive endocrinologist for further management.
Was the test for AMH indicated? And is this referral appropriate?
The referral is entirely appropriate, even though the patient has not been trying to conceive for a full year. Why? The AMH value suggests that her ovarian reserve is in early decline. She would benefit from evaluation by a subspecialist who can review the entire spectrum of treatments, including aggressive options such as ovulation induction and in vitro fertilization (IVF), to optimize her reproductive success.
This article reviews the various biomarkers available to assess ovarian reserve in women who experience infertility:
- day 3 (basal) FSH
- clomiphene citrate challenge
- gonadotropin-releasing hormone (GnRH) agonist stimulation
- inhibin-B
- antral follicle count (AFC)
- AMH.
The AFC and AMH tend to detect the earliest changes in ovarian reserve, followed, sequentially, by inhibin-B, the clomiphene citrate challenge test (CCCT), and basal FSH.
The tests we describe are used primarily to assess treatment prognosis in infertile women. In time, however, appropriate population screening of ovarian reserve may be feasible to provide many more women with information about their reproductive potential and help them shape their life plan.
What makes a test valuable?
Ovarian reserve describes a woman’s reproductive potential—specifically, the number and quality of oocytes she possesses.1 Biochemical tests of ovarian reserve emerged during the rise of assisted reproductive technologies (ART) in the late 1980s to predict both responsiveness to superovulation drugs and the odds of pregnancy with treatment.
Ideally, a test that assesses ovarian reserve should be affordable, straightforward, rapidly interpretable, and minimally invasive. It also should be able to detect changes that begin early in reproductive life. To be applicable to large populations of reproductive-age women, it should be of use anytime in the menstrual cycle, and should provide reproducible and highly accurate assessment of the reproductive aging process.
Our ability to offer tests that accurately measure ovarian reserve has a significant impact on women at risk of infertility and early menopause and on those who choose to delay childbearing for personal (nonmedical) reasons. These tests have become increasingly relevant because women are choosing to have their first child at a later age than their counterparts did 20 years ago:
- In 1980, 40% of women having their first baby were younger than 25 years, and only 5% were older than 35
- In 2000, 25% of women were younger than 25 when their first child was born, and 15% were older than 35.
Who should be tested?
Ovarian reserve is a complex clinical phenomenon that is influenced by age, genetics, and environmental variables. The decline in a woman’s ovarian reserve over time is irreversible; the trajectory of this decline is fundamental to the odds of fertility with age and the timing of the menopausal transition. At present, the markers used most often in clinical practice have some utility but also suffer from several drawbacks ( TABLE ).
For the general practitioner performing an infertility evaluation, we recommend focusing on the following groups of women for ovarian reserve testing:
- women over 30 years of age
- women with a history of exposure to a confirmed gonadotoxin, i.e., tobacco smoke, chemotherapy, radiation therapy
- women with a strong family history of early menopause or premature ovarian failure
- women who have had extensive ovarian surgery, i.e., cystectomy and unilateral oophorectomy.
Testing tends to have the highest yield in these groups. Women who have abnormal results should be referred to a reproductive endocrinologist for further evaluation and treatment.
The six tests are described below.
TABLE
How six markers of ovarian reserve stack up
| Test (year described) | Timing | Intracycle and intercycle variability | Sensitivity (specificity) | Reflects changes in ovarian reserve | Normal levels | Confounders | Out-of-pocket cost |
|---|---|---|---|---|---|---|---|
| Basal follicle-stimulating hormone (FSH) (1988) | Day 3 of menstrual cycle | Clinically significant | 7%–8% (98%–99%) | Late | • Early follicular phase FSH level <10 mIU/mL • Estradiol level <80 pg/mL | • High estradiol level (decreases) • Oral contraceptive use (decreases) • Pregnancy (decreases) | $125–$150 |
| Clomiphene challenge test (1989) | Days 3 and 10 of menstrual cycle | Clinically significant | 25%–40% (98%–99%) | Late | • Day 3 FSH level <10 mIU/mL; day 3 estradiol level <80 pg/mL • Day 10 FSH level <10 mIU/mL | • High day 3 estradiol level (decreases day 3 FSH) • Low day 10 estradiol (increases day 10 FSH) • Oral contraceptive use (decreases) • Pregnancy (decreases) | $550–$600 |
| GnRH agonist (1988) | Early follicular phase of menstrual cycle | Clinically significant | 32%–89% (79%–97%) | Late | Variable | • Oral contraceptives (decrease estradiol levels) • Pregnancy (increases estrogens) | $300–$350 |
| Inhibin-B (1997) | Early follicular phase of menstrual cycle | Clinically significant | 33%–81% (29%–95%) | Early | Variable in the literature; normal cutoffs range from ≥45–80 pg/mL | • Obesity (decreases) • PCOS (increases) • Exogenous FSH administration (increases) • Oral contraceptive use (decreases) | $150–$200 |
| Antral follicle count (1997) | Early follicular phase of menstrual cycle | Clinically significant (includes interobserver variability) | 8%–60% (33%–96%) | Earliest | ≥5–10 total antral follicles | • Oral contraceptive use (decreases) • Polycystic ovary syndrome (PCOS) (increases) | $300–$500 |
| Anti–Müllerian hormone/Müllerian-inhibiting substance (2002) | At any time; not cycle-dependent | Minimal | 49%–76% (89%–94%) | Earliest | >0.7 ng/mL | • PCOS (increases) • Obesity (decreases) • Exogenous FSH administration (decreases) | $150–$400 |
1 | Basal FSH—widely used but only moderately informative
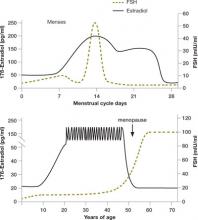
Day 3 FSH and the CCCT are the most widely used measures of ovarian reserve in ART practice. The use of early follicular-phase FSH as a marker of ovarian reserve and fertility was proposed 20 years ago with the emergence of IVF.2-4 The test is an indirect assessment of ovarian reserve in that it measures pituitary production of FSH in response to feedback from ovarian hormones. Estradiol and inhibin-B reach a nadir early in the menstrual cycle; measuring FSH on day 3 offers a glimpse of the functioning of the hypothalamic–pituitary–ovarian axis before ovarian hormone levels rise later in the cycle ( FIGURE 1 ).5,6
FIGURE 1 The HPO axis
The FSH level opens a window onto the function of the hypothalamic–pituitary–ovarian axis before ovarian hormone levels rise in the cycle. Women who have normal ovarian reserve have sufficient ovarian hormone production early in the menstrual cycle to maintain FSH levels within the normal range. Conversely, a “monotropic” elevation in FSH—one that is unaccompanied by a rise in luteinizing hormone (LH)—reflects poor hormone production from an aging pool of ovarian follicles and disinhibition of FSH production.5,6
FSH measurements are typically combined with estradiol to enhance the sensitivity of testing ( FIGURE 2, ). Premature elevations of estradiol early in the follicular phase are driven by rising FSH levels in women with declining ovarian reserve. Abnormally elevated estrogen levels then feed back negatively on pituitary production of FSH and mask an elevation that might otherwise reveal diminished ovarian reserve. Measurement of both FSH and estradiol on cycle day 3 may therefore help decrease the incidence of false-negative testing.
Commonly cited criteria for normal ovarian reserve are:
- early follicular phase FSH, <10 mIU/mL
- estradiol, <80 pg/mL1
It is extremely important to note, however, that these are general guidelines and that cutoffs are both laboratory- and practice-specific.
FIGURE 2 Monthly and lifetime variations in estradiol and FSH
How 17ß-estradiol and follicle-stimulating hormone levels vary over the menstrual cycle (top) and a woman’s lifetime (bottom).
2 | Clomiphene citrate—more sensitive than FSH testing
Like basal FSH testing, the CCCT is an indirect assessment of ovarian reserve. Unlike FSH testing, the CCCT is provocative. It involves administration of 100 mg of clomiphene citrate (Clomid) on days 5 through 9 of the menstrual cycle, with FSH and estradiol measured on days 3 and 10. Once clomiphene citrate is administered, FSH and LH levels rise, followed by an increase in estradiol and inhibin. Evidence suggests that the smaller follicular cohorts in women with diminished ovarian reserve produce less inhibin-B and estradiol and, therefore, less negative feedback on clomiphene-induced pituitary FSH release.6,7 The result: persistent elevation of the day 10 FSH value and a positive screen for diminished ovarian reserve.
In some women, day 10 FSH is elevated even after a normal day 3 value. This makes the CCCT more sensitive than basal FSH testing; it can identify women who might go unrecognized if evaluated by day 3 FSH and estradiol levels alone.
More expensive and labor-intensive than the alternatives
Interpretation of the CCCT requires that FSH and estradiol both be assessed on days 3 and 10. An elevated FSH (≥10 mIU/mL) on either day indicates diminished ovarian reserve. As with basal FSH testing, elevated estradiol (≥80 pg/mL) on day 3 is considered abnormal. The day 10 estradiol value of the CCCT reflects whether or not clomiphene citrate was administered appropriately, and should be elevated. However, the significance of the day 10 estradiol level has been debated with respect to its predictive value for pregnancy in infertile populations.8
The addition of day 10 FSH assessment improves the sensitivity of the CCCT over basal FSH measurement, but makes it a more expensive and labor-intensive test ( TABLE ).5,6 The CCCT involves administration of clomiphene citrate, a safe drug (though it can have side effects), and two blood draws instead of one. Nevertheless, both tests are relatively noninvasive, rapid measures of ovarian reserve.
Drawbacks of the tests
Both basal FSH testing and the CCCT are widely used, although support for their ability to predict ovarian reserve in the infertile population has been challenged recently. Newer data demonstrate that these tests are limited in their ability to predict outcome (pregnancy and response to superovulation drugs) in all but a narrow group of patients undergoing IVF. Performance is particularly limited in:
Additional drawbacks of basal FSH testing and the CCCT include:
- significant variability of test results from cycle to cycle (intercycle variability)
- limited time frame within which the tests can be performed (intracycle variability).
The basal FSH test and CCCT have high specificity (98% to 99% for each) as an assessment of reproductive performance in infertile women and generate few false-positive results.5,6 However, the high screen cutoffs that allow for such specificity come at a price: Few women will screen positive, and sensitivity of the tests is low (between 7% and 8% for basal FSH and between 25% and 40% for the CCCT). Such low sensitivity means that many women will not conceive after infertility treatment despite a normal test result.5,11 Overall, the tests are not highly informative for many women who get tested.
Once abnormal, normal results are meaningless
Once an FSH level or the CCCT has ever been abnormal, the patient has diminished ovarian reserve; normal values in subsequent menstrual cycles do not improve the odds of pregnancy with treatment.14 This fact can be a significant source of confusion and frustration for patients.
3 | GnRH agonist stimulation —no better than FSH testing
This test was developed in the search for a very sensitive assessment of ovarian reserve. It was designed to uncover subtle abnormalities in pituitary and ovarian dynamics. It involves administering a gonadotropin-releasing hormone (GnRH) agonist such as leuprolide acetate (Lupron) on day 2 or 3 of the menstrual cycle and measuring pituitary and ovarian hormone responses.5,15
One group of investigators demonstrated a correlation between stimulated estradiol levels and responsiveness during IVF,16 but other studies have shown that the test does not perform significantly better than day 3 FSH in predicting ovarian reserve.17,18
The sensitivity of GnRH agonist testing for pregnancy is moderate (32% to 89%); specificity ranges from 79% to 97%.19
4 | Inhibin-B—not helpful when used alone
This glycoprotein hormone produced by granulosa cells of developing follicles is a direct measure of ovarian reserve when assessed in the early follicular phase of the menstrual cycle.20 Women treated with IVF who have a low inhibin-B level—particularly when using cutoffs below the range of 45–80 pg/mL—have been shown to respond poorly to superovulation and have a lower pregnancy rate than women with high inhibin-B.21,22 One group of investigators demonstrated that women with clinical evidence of diminished ovarian reserve but a normal FSH level also had low inhibin-B production, suggesting that it may be a more sensitive marker than FSH.22
Inhibin-B testing involves a simple blood draw. However, the test has been incorporated into clinical assessment of ovarian reserve only to a limited degree, due to the lack of reliable assays and controversy concerning its prognostic value.23
Because of these limitations, routine testing of serum inhibin-B in isolation of other markers of ovarian reserve is not recommended.
5 | Antral follicle count—good predictor of IVF outcome
Transvaginal ultrasonographic determination of the number of ovarian follicles that measure between 2 mm and 10 mm in diameter in the early follicular phase of the cycle yields the AFC. As a direct marker of the cohort of growing follicles in the early menstrual cycle, the AFC is believed to correlate strongly with the number of primordial follicles present in the ovary and, therefore, ovarian reserve. Total AFCs of less than 5 to 10 are suggestive of diminished ovarian reserve.24,25
In IVF cycles, AFC has proven to be an accurate predictor of number of oocytes retrieved, risk of cycle cancellation, and odds of conception.24,25 Some investigators have even suggested that, compared with other markers of ovarian reserve, AFC is the best independent predictor of outcome in IVF cycles.7,26-27
In a group of normally cycling women with proven fertility, AFC also showed a strong correlation with age, declining slowly until age 37 and more rapidly thereafter.28,29
AFC sensitivity for pregnancy is moderate and varies widely in published reports (8% to 60%), whereas specificity tends to be higher (33% to 96%).19
Drawbacks of AFC
- Because of the need to perform transvaginal ultrasonography, AFC is a more invasive and often more expensive test than hormonal biomarkers
- Accurate assessment of AFC requires an experienced sonographer and can be limited in patients who have had pelvic surgery or uterine fibroids and in those who are obese
- Moderate interobserver and intercycle variability of AFC determinations limits its reproducibility29,30
- As with basal FSH measurement, the intercycle variability of AFC does not correlate well with IVF outcome in individual patients.30
6 | Anti-Müllerian hormone— many advantages
The drawbacks of the tests just described— e.g., intercycle variability, lack of uniform cutoffs, and limited ability to predict IVF outcomes—make the development of more reliable measures of ovarian reserve a priority in reproductive medicine. AMH is a highly promising marker that appears to have many advantages over other tests and may have the greatest power to predict ovarian aging in women of reproductive age.
How it works
AMH is a glycoprotein growth factor and a member of the transforming growth factor-ß superfamily.31 It is primarily produced by the pool of early-growing follicles, which are believed to serve as a proxy for the number of primordial follicles in the ovary. The number of primordial follicles at a given point in time represents the ovarian reserve. AMH levels above 0.7 ng/mL are considered normal; values between 0.3 ng/mL and 0.7 ng/mL are consistent with borderline ovarian reserve, according to 2007 data from Reprosource Corp.
AMH has been studied as a marker of ovarian reserve for 6 years, with multiple reports describing declines in levels with age and with diminishing oocyte numbers. It is undetectable at menopause.32
The age-related decline in AMH is gradual but measurable even in young women, consistently preceding changes in other markers of ovarian reserve such as FSH and inhibin-B.32-35 The longitudinal changes in AMH have been demonstrated in ovulatory premenopausal women and healthy volunteers with proven fertility.33,34 In one series of women followed over a mean of 4 years (ages 25 to 46), AMH testing was superior to day 3 FSH, inhibin-B, and AFC in its ability to predict the onset of cycle irregularity and the menopausal transition.33
Does it predict oocyte quality?
AMH has performed well as a biomarker, comparable in most series to AFC and superior to FSH. AMH levels are strongly correlated with the number of oocytes retrieved during IVF and the odds of cycle cancellation due to poor response35-41 —but does it accurately characterize oocyte quality, the other element of ovarian reserve?
Some reports have shown a strong association between AMH levels and surrogates of oocyte quality, including fertilization, oocyte morphology, embryo quality, and pregnancy and miscarriage rates,36-41 but others have not.42 Some reports demonstrate a relationship between AMH and some but not all surrogate markers of oocyte quality.40
Advantages of AMH
- It demonstrates minimal intracycle variability.32,43-45 Compared with other markers of ovarian reserve, which must be measured early in the follicular phase of the menstrual cycle, AMH can be assessed at random times, making it a more convenient method for patients and physicians
- It demonstrates minimal intercycle variability32,34
- AMH levels are not significantly affected by the hormonal changes of pregnancy, oral contraceptive use, or GnRH treatment, and can be measured in these settings.46,47
Utility of AMH is limited in PCOS and obesity
The ability to use AMH as a marker of ovarian aging in women who have polycystic ovary syndrome (PCOS) and in women who are obese may be limited by the ovulatory dysfunction in these populations. Circulating levels of AMH are higher in women with PCOS than in unaffected women, a finding thought to be indicative of oligo-ovulation and poor follicular development in polycystic ovaries.48-53
In a recent series investigating AMH levels in women with PCOS, AMH and the degree of insulin resistance were positively correlated, and the AMH level was negatively correlated with the number of menses in a year.49 The consistently positive correlation between AMH and PCOS may suggest a future role for this marker as a diagnostic tool.
In obese women who do not have PCOS, AMH production may be lower than in women of normal weight. In a recent series, normally cycling obese women in the later reproductive years were shown to have an AMH level 70% lower than those in women who were not obese.54 These differences have not been well studied in younger obese women.
Which test is best?
AMH may be preferable to the other tests to assess ovarian reserve because it can be measured any time during the menstrual cycle or between cycles. AMH measurement is also useful if a woman is taking oral contraceptives or leuprolide acetate because these medications may confound the results of the other test methods. In addition, AMH may be the earliest indicator of decline in ovarian reproductive function. As such, it may highlight cases that merit a search for other causes of infertility and make it possible to treat them in a timely manner.
Elevated AMH may reveal occult PCOS and warn of significant risk of ovarian hyperstimulation prior to ovulation induction with gonadotrophins, so that the clinician can plan smaller doses.
A normal female is born with 1 million to 2 million oocytes, a number that declines continuously, primarily through the process of follicular atresia. By the onset of puberty, the number of oocytes has declined to approximately 300,000. As a woman enters her late 30s, when the total number of oocytes is approximately 25,000, the pace of oocyte depletion begins to increase, as does the rate of spontaneous miscarriage.1,55,56
The effect of age on fertility is believed to arise from changes in both oocyte number and quality. Multiple investigators have found a greater frequency of cellular abnormalities in oocytes from older women.1,2,5,15,57
Although ovarian reserve declines with age in all women, women of similar ages can have very different degrees of ovarian reserve, and some women who have very poor ovarian reserve may never conceive, despite aggressive fertility treatment.
The biologic basis for differences in ovarian reserve among similar groups of women is not completely understood, but is probably rooted in genetic, lifestyle, and environmental factors that affect granulosa cell and oocyte function. Identifying sensitive biomarkers that can determine ovarian reserve independent of age is critical to predict fertility and age at menopause.5
1. Practice Committee of the American Society for Reproductive Medicine. Age and infertility in women. Fertil Steril. 2006;86:S248-S252.
2. Muasher SJ, Oehninger S, Simonetti S, Matta J, Ellis LM, Liu H-C. The value of basal and/or stimulated serum gonadotropin levels in prediction of stimulation response and in vitro fertilization outcome. Fertil Steril. 1988;50:298-307.
3. Scott RT, Toner JP, Muasher SJ, Oehninger S, Robinson S, Rosenwaks Z. Follicle stimulating hormone levels on cycle day 3 are predictive of in vitro fertilization outcome. Fertil Steril. 1989;51:651-654.
4. Toner JP, Philiput CB, Jones GS, Muasher SJ. Basal follicle stimulating hormone level is a better predictor of in vitro fertilization outcome than age. Fertil Steril. 1991;55:784-791.
5. Barnhart K, Osheroff J. Follicle stimulating hormone as a predictor of fertility. Curr Opin Obstet Gynecol. 1998;10:227-232.
6. Hofmann GE, Danforth DR, Seifer DB. Inhibin-B: the physiologic basis of the clomiphene citrate challenge test for ovarian reserve screening. Fertil Steril. 1998;69:474-477.
7. Yong PY, Baird DT, Thong KJ, McNeilly AS, Anderson RA. Prospective analysis of the relationships between the ovarian follicle cohort and basal FSH concentration, the inhibin response to exogenous FSH and ovarian follicle number at different stages of the normal menstrual cycle and after pituitary down-regulation. Hum Reprod. 2003;18:35-44.
8. Scott RT, Jr, Illions EH, Kost ER, Dellinger C, Hofmann GE, Navot D. Evaluation of the significance of the estradiol response during the clomiphene citrate challenge test. Fertil Steril. 1993;60:242-246.
9. Esposito MA, Coutifaris C, Barnhart KT. A moderately elevated day 3 FSH concentration has limited predictive value, especially in younger women. Hum Reprod. 2002;17:118-123.
10. Bancsi L, Broekmans FJM, Wol BWJ, Habbema DK, te Velde ER. Performance of basal follicle-stimulating hormone in the prediction of poor ovarian response and failure to become pregnant after in vitro fertilization: a meta-analysis. Fertil Steril. 2003;79:1091-1100.
11. Jain T, Soules MR, Collins JA. Comparison of basal follicle-stimulating hormone versus the clomiphene citrate challenge test for ovarian reserve screening. Fertil Steril. 2004;82:180-185.
12. Toner JP. Modest follicle-stimulating hormone elevations in younger women: warn but don’t disqualify. Fertil Steril. 2004;81:1493-1495.
13. Van Rooij IAJ, de Jong E, Broekmans FJM, Looman CWN, Habbeman DK, te Velde ER. High follicle-stimulating hormone levels should not necessarily lead to the exclusion of subfertile patients from treatment. Fertil Steril. 2004;81:1478-1485.
14. Scott RT, Hofmann GE, Oehninger S, Muasher SJ. Intercycle variability of day 3 follicle-stimulating hormone levels and its effect on stimulation quality in in vitro fertilization. Fertil Steril. 1990;54:297-302.
15. Bulkulmez O, Arici A. Assessment of ovarian reserve. Curr Opin Obstet Gynecol. 2004;16:231-237.
16. Ranieri DM, Quinn F, Makhlouf A, et al. Simultaneous evaluation of basal follicle-stimulating hormone and 17-beta-estradiol response to gonadotropin-releasing hormone analogue stimulation: an improved predictor of ovarian reserve. Fertil Steril. 1998;70:227-233.
17. Fujimoto VY, Klein NA, Battaglia DE, Bremmer WJ, Soules MR. The anterior pituitary response to a gonadotropin-releasing hormone challenge test in normal older reproductive age women. Fertil Steril. 1996;65:539-544.
18. Galtier-Dereure F, De Bouard V, Picto MC, et al. Ovarian reserve test with the gonadotrophin-releasing hormone agonist buserelin: correlation with in-vitro fertilization outcome. Hum Reprod. 1996;11:1393-1398.
19. Broekmans FJ, Fwee J, Hendricks DJ, Mol BW, Lambalk CB. A systematic review of tests predicting ovarian reserve and IVF outcome. Hum Reprod Update. 2006;12:685-718.
20. Klein NA, Illingworth PJ, Groome NP, NcNeilly AS, Battaglia DE, Soules MR. Decreased inhibin B secretion is associated with the monotropic FSH rise in older, ovulatory women: a study of serum and follicular fluid levels of dimeric inhibin A and B in spontaneous menstrual cycles. J Clin Endocrinol Metab. 1996;81:2742-2745.
21. Seifer DB, Lambert-Messerlian G, Hogan JW, et al. Day 3 serum inhibin-B is predictive of assisted reproductive technologies outcome. Fertil Steril. 1997;67:110-114.
22. Seifer DB, Scott RT, Jr, Bergh PA, et al. Women with declining ovarian reserve may demonstrate a decrease in day 3 serum inhibin B before a rise in day 3 follicle-stimulating hormone. Fertil Steril. 1999;72:63-65.
23. Corson SL, Gutmann J, Batzer FR, Wallace H, Klein N, Soules MR. Inhibin-B as a test of ovarian reserve for infertile women. Hum Reprod. 1999;14:2818-2821.
24. Tomas C, Nuojua-Huttunen S, Martikainen H. Pretreatment transvaginal ultrasound examination predicts ovarian responsiveness to gonadotrophins in in-vitro fertilization. Hum Reprod. 1997;12:220-223.
25. Chang MY, Chiang CH, Hsieh TT, Soong YK, Hsu KH. Use of the antral follicle count to predict the outcome of assisted reproductive technologies. Fertil Steril. 1998;69:505-510.
26. Hung E, Tang OS, Ho PC. The significance of the number of antral follicles prior to stimulation in predicting ovarian responses in an IVF programme. Hum Reprod. 2000;15:1937-1942.
27. Bancsi LFJMM, Broekmans FJM, Eijkemans MJC, de Jong FH, Habbema JDF, te Velde ER. Predictors of poor ovarian response in in vitro fertilization: a prospective study comparing basal markers of ovarian reserve. Fertil Steril. 2002;77:328-336.
28. Ng EH, Yeung WS, Fong DY, Ho PC. Effects of age on hormonal and ultrasound markers of ovarian reserve in Chinese women with proven fertility. Hum Reprod. 2003;18:2169-2174.
29. Scheffer GJ, Broekmans FJ, Dorland M, Habbema JD, Looman CW, te Velde ER. Antral follicle counts by transvaginal ultrasonography are related to age in women with proven natural fertility. Fertil Steril. 1999;72:845-851.
30. Hansen KR, Morris JL, Thyer AC, Soules MR. Reproductive aging and the variability in the ovarian antral follicle count: application in the clinical setting. Fertil Steril. 2003;80:577-583.
31. Cate RL, Mattaliano RJ, Hession C, et al. Isolation of the bovine and human genes for Müllerian inhibiting substance and expression of the human gene in animal cells. Cell. 1986;45:685-698.
32. de Vet A, Laven JSE, de Jong FH, Themmen APN, Fauser BCJM. Anti-Müllerian hormone serum levels: a putative marker for ovarian aging. Fertil Steril. 2002;77:357-362.
33. van Rooij IAJ, Broekmans FJM, Scheffer GJ, et al. Serum anti-Müllerian hormone levels best reflect the reproductive decline with age in normal women with proven fertility: a longitudinal study. Fertil Steril. 2005;83:979-987.
34. van Rooij IAJ, Tonkelaar I, Broekmans FJ, et al. Anti-Müllerian hormone is a promising predictor for the occurrence of the menopausal transition. Menopause. 2004;11:601-606.
35. Tremellen KP, Kolo M, Gilmore A, Lekamge DN. Anti-Müllerian hormone as a marker of ovarian reserve. Aust N Z J Obstet Gynaecol. 2005;45:20-24.
36. Silberstein T, MacLaughlin DT, Shai I, et al. Müllerian-inhibiting substance levels at the time of HCG administration in IVF cycles predict both ovarian reserve and embryo morphology. Hum Reprod. 2006;21:159-163.
37. Seifer DB, MacLaughlin DT, Christian BP, Feng B, Shelden RM. Early follicular serum Müllerian-inhibiting substance levels are associated with ovarian response during assisted reproductive technology cycles. Fertil Steril. 2002;77:468-471.
38. Ebner T, Sommergruber M, Moser M, Shebl O, Schreier-Lechner E, Tews G. Basal level anti-Müllerian hormone is associated with oocyte quality in stimulated cycles. Hum Reprod. 2006;21:2022-2026.
39. Hazout A, Bouchard P, Seifer DB, Aussage P, Junca AM, Cohen-Bacrie P. Serum anti-Müllerian hormone/Müllerian-inhibiting substance appears to be a more discriminatory marker of ART outcome than follicular stimulating hormone, inhibin B or estradiol. Fertil Steril. 2004;82:1323-1329.
40. Nelson SM, Yates RW, Fleming R. Serum anti-Müllerian hormone and FSH: prediction of live birth and extremes of response in stimulated cycles—implications for individualization of therapy. Hum Reprod. 2007;22:2414-2421.
41. Fanchin R, Mendez DH, Frydman N, et al. Anti-Müllerian hormone concentrations in the follicular fluid of the preovulatory follicle are predictive of the implantation potential of the ensuing embryo obtained by in vitro fertilization. J Clin Endocrinol Metab. 2007;92:1796-1802.
42. Smeenk JM, Sweep FC, Zielhuis GA, Kremer JA, Th omas CM, Braat DD. Anti-Müllerian hormone predicts ovarian responsiveness, but not embryo quality or pregnancy, after in vitro fertilization or intracyoplasmic sperm injection. Fertil Steril. 2007;87:223-226.
43. Hehenkamp WJ, Looman CW, Themmen AP, de Jong FM, Te Velde ER, Broekmans FJ. Anti-Müllerian hormone levels in the spontaneous menstrual cycle do not show substantial fluctuation. J Clin Endocrinol Metab. 2006;91:4057-4063.
44. La Marca A, Stabile G, Artenisio AC, Volpe A. Serum anti-Müllerian hormone throughout the menstrual cycle. Hum Reprod. 2006;21:3103-3107.
45. Tsepelidis S, Devreker F, Demeestere F, Flahaut I, Gervy A, Englert C. Stable serum levels of anti-Müllerian hormone during the menstrual cycle: a prospective study in normo-ovulatory women. Hum Reprod. 2007;22:1837-1840.
46. La Marca A, Giulini Orvieto R, De Leo V, Volpe A. Anti-Müllerian hormone concentrations in maternal serum during pregnancy. Hum Reprod. 2005;20:1569-1572.
47. Somunkiran A, Yavuz T, Yucel O, Ozdemir I. Anti-Müllerian hormone levels during hormonal contraception in women with polycystic ovary syndrome. Eur J Obstet Gynecol Reprod Biol. 2007;134:196-201.
48. Al-Qahtani A, Groome NP. Anti-Müllerian hormone: Cinderella finds new admirers. J Clin Endocrinol Metab. 2006;91:3760-3762.
49. La Marca A, Orvieto R, Giulini S, Jasonni VM, Volpe A, De Leo V. Müllerian-inhibiting substance in women with polycystic ovary syndrome: relationship with hormonal and metabolic characteristics. Fertil Steril. 2004;82:970-971.
50. Piltonen T, Morin-Papunen L, Koivunen R, Perheentupa A, Ruokonen A, Tapanainen JS. Serum anti-Müllerian hormone levels remain high until late reproductive age and decrease during metformin therapy in women with polycystic ovary syndrome. Hum Reprod. 2005;20:1820-1836.
51. Pigny P, Merlen E, Robert Y, et al. Elevated serum level of anti-Müllerian hormone in patients with polycystic ovary syndrome: relationship to the ovarian follicle excess and to the follicular arrest. J Clin Endocrinol Metab. 2003;88:5957-5962.
52. Cook CL, Siow Y, Brenner AG, Fallat ME. Relationship between serum anti-Müllerian substance and other reproductive hormones in untreated women with polycystic ovary syndrome and endometriosis. Fertil Steril. 1997;67:962-965.
53. Pellatt L, Hanna L, Brincat M, et al. Granulosa cell production of anti-Müllerian hormone is increased in polycystic ovaries. J Clin Endocrinol Metab. 2007;92:240-245.
54. Freeman EW, Gracia CG, Sammel MD, Lin H, Lim LC, Strauss JF, 3rd. Association of anti-Müllerian hormone levels with obesity in later reproductive-age women. Fertil Steril. 2007;87:101-106.
55. Scott RT, Opsahl MS, Leonardi MR, Neall GS, Illions EH, Navot D. Life table analysis of pregnancy rates in a general infertility population relative to ovarian reserve and patient age. Hum Reprod. 1995;10:1706-1710.
56. Speroff L. Fritz M. eds. Clinical Gynecologic Endocrinology and Infertility. 7th ed. Philadelphia: Lippincott Williams & Wilkins; 2004.
57. Lim AS, Tsakok MFH. Age-related decline in fertility: a link to degenerative oocytes? Fertil Steril. 1997;68:265-271.
The University of Medicine and Dentistry of New Jersey (UMDNJ) owns a patent relating to the use of anti-Müllerian hormone/Müllerian inhibiting substance for predicting ovarian response in women with infertility. The patent is based in part on work that Dr. Seifer carried out while employed at UMDNJ. In accordance with UMDNJ policy, Dr. Seifer, a named inventor on this patent, assigned his interest in the invention to UMDNJ. UMDNJ has a licensing agreement with Diagnostic Systems Laboratory for the use of the claimed invention. Dr. Seifer receives a portion of the royalties, as determined by UMDNJ policy, that UMDNJ gains from this licensing agreement.
CASE: Borderline test result prompts referral
A 36-year-old nulliparous woman is seen in your office for evaluation after 6 months of infertility. She is ovulatory, and has been using an ovulation-prediction kit to time intercourse. You learn that she had Chlamydia trachomatis infection in the distant past, but elicit no other significant medical or surgical history. She reports that she smoked approximately one pack of cigarettes a day for 15 years but gave up smoking 5 years ago.
You order a hysterosalpingogram, followed by day 3 testing of follicle-stimulating hormone (FSH). The hysterosalpingogram is normal; the FSH level is 7.5 mIU/mL and the estradiol level is 30 pg/mL—both in the normal range.
The patient asks for testing of anti-Müllerian hormone (AMH; also known as Müllerian-inhibiting substance) because she has read that it is a new marker of fertility. The result is 0.5 ng/mL, a borderline value. After reviewing these results, you refer her to a reproductive endocrinologist for further management.
Was the test for AMH indicated? And is this referral appropriate?
The referral is entirely appropriate, even though the patient has not been trying to conceive for a full year. Why? The AMH value suggests that her ovarian reserve is in early decline. She would benefit from evaluation by a subspecialist who can review the entire spectrum of treatments, including aggressive options such as ovulation induction and in vitro fertilization (IVF), to optimize her reproductive success.
This article reviews the various biomarkers available to assess ovarian reserve in women who experience infertility:
- day 3 (basal) FSH
- clomiphene citrate challenge
- gonadotropin-releasing hormone (GnRH) agonist stimulation
- inhibin-B
- antral follicle count (AFC)
- AMH.
The AFC and AMH tend to detect the earliest changes in ovarian reserve, followed, sequentially, by inhibin-B, the clomiphene citrate challenge test (CCCT), and basal FSH.
The tests we describe are used primarily to assess treatment prognosis in infertile women. In time, however, appropriate population screening of ovarian reserve may be feasible to provide many more women with information about their reproductive potential and help them shape their life plan.
What makes a test valuable?
Ovarian reserve describes a woman’s reproductive potential—specifically, the number and quality of oocytes she possesses.1 Biochemical tests of ovarian reserve emerged during the rise of assisted reproductive technologies (ART) in the late 1980s to predict both responsiveness to superovulation drugs and the odds of pregnancy with treatment.
Ideally, a test that assesses ovarian reserve should be affordable, straightforward, rapidly interpretable, and minimally invasive. It also should be able to detect changes that begin early in reproductive life. To be applicable to large populations of reproductive-age women, it should be of use anytime in the menstrual cycle, and should provide reproducible and highly accurate assessment of the reproductive aging process.
Our ability to offer tests that accurately measure ovarian reserve has a significant impact on women at risk of infertility and early menopause and on those who choose to delay childbearing for personal (nonmedical) reasons. These tests have become increasingly relevant because women are choosing to have their first child at a later age than their counterparts did 20 years ago:
- In 1980, 40% of women having their first baby were younger than 25 years, and only 5% were older than 35
- In 2000, 25% of women were younger than 25 when their first child was born, and 15% were older than 35.
Who should be tested?
Ovarian reserve is a complex clinical phenomenon that is influenced by age, genetics, and environmental variables. The decline in a woman’s ovarian reserve over time is irreversible; the trajectory of this decline is fundamental to the odds of fertility with age and the timing of the menopausal transition. At present, the markers used most often in clinical practice have some utility but also suffer from several drawbacks ( TABLE ).
For the general practitioner performing an infertility evaluation, we recommend focusing on the following groups of women for ovarian reserve testing:
- women over 30 years of age
- women with a history of exposure to a confirmed gonadotoxin, i.e., tobacco smoke, chemotherapy, radiation therapy
- women with a strong family history of early menopause or premature ovarian failure
- women who have had extensive ovarian surgery, i.e., cystectomy and unilateral oophorectomy.
Testing tends to have the highest yield in these groups. Women who have abnormal results should be referred to a reproductive endocrinologist for further evaluation and treatment.
The six tests are described below.
TABLE
How six markers of ovarian reserve stack up
| Test (year described) | Timing | Intracycle and intercycle variability | Sensitivity (specificity) | Reflects changes in ovarian reserve | Normal levels | Confounders | Out-of-pocket cost |
|---|---|---|---|---|---|---|---|
| Basal follicle-stimulating hormone (FSH) (1988) | Day 3 of menstrual cycle | Clinically significant | 7%–8% (98%–99%) | Late | • Early follicular phase FSH level <10 mIU/mL • Estradiol level <80 pg/mL | • High estradiol level (decreases) • Oral contraceptive use (decreases) • Pregnancy (decreases) | $125–$150 |
| Clomiphene challenge test (1989) | Days 3 and 10 of menstrual cycle | Clinically significant | 25%–40% (98%–99%) | Late | • Day 3 FSH level <10 mIU/mL; day 3 estradiol level <80 pg/mL • Day 10 FSH level <10 mIU/mL | • High day 3 estradiol level (decreases day 3 FSH) • Low day 10 estradiol (increases day 10 FSH) • Oral contraceptive use (decreases) • Pregnancy (decreases) | $550–$600 |
| GnRH agonist (1988) | Early follicular phase of menstrual cycle | Clinically significant | 32%–89% (79%–97%) | Late | Variable | • Oral contraceptives (decrease estradiol levels) • Pregnancy (increases estrogens) | $300–$350 |
| Inhibin-B (1997) | Early follicular phase of menstrual cycle | Clinically significant | 33%–81% (29%–95%) | Early | Variable in the literature; normal cutoffs range from ≥45–80 pg/mL | • Obesity (decreases) • PCOS (increases) • Exogenous FSH administration (increases) • Oral contraceptive use (decreases) | $150–$200 |
| Antral follicle count (1997) | Early follicular phase of menstrual cycle | Clinically significant (includes interobserver variability) | 8%–60% (33%–96%) | Earliest | ≥5–10 total antral follicles | • Oral contraceptive use (decreases) • Polycystic ovary syndrome (PCOS) (increases) | $300–$500 |
| Anti–Müllerian hormone/Müllerian-inhibiting substance (2002) | At any time; not cycle-dependent | Minimal | 49%–76% (89%–94%) | Earliest | >0.7 ng/mL | • PCOS (increases) • Obesity (decreases) • Exogenous FSH administration (decreases) | $150–$400 |
1 | Basal FSH—widely used but only moderately informative
Day 3 FSH and the CCCT are the most widely used measures of ovarian reserve in ART practice. The use of early follicular-phase FSH as a marker of ovarian reserve and fertility was proposed 20 years ago with the emergence of IVF.2-4 The test is an indirect assessment of ovarian reserve in that it measures pituitary production of FSH in response to feedback from ovarian hormones. Estradiol and inhibin-B reach a nadir early in the menstrual cycle; measuring FSH on day 3 offers a glimpse of the functioning of the hypothalamic–pituitary–ovarian axis before ovarian hormone levels rise later in the cycle ( FIGURE 1 ).5,6
FIGURE 1 The HPO axis
The FSH level opens a window onto the function of the hypothalamic–pituitary–ovarian axis before ovarian hormone levels rise in the cycle. Women who have normal ovarian reserve have sufficient ovarian hormone production early in the menstrual cycle to maintain FSH levels within the normal range. Conversely, a “monotropic” elevation in FSH—one that is unaccompanied by a rise in luteinizing hormone (LH)—reflects poor hormone production from an aging pool of ovarian follicles and disinhibition of FSH production.5,6
FSH measurements are typically combined with estradiol to enhance the sensitivity of testing ( FIGURE 2, ). Premature elevations of estradiol early in the follicular phase are driven by rising FSH levels in women with declining ovarian reserve. Abnormally elevated estrogen levels then feed back negatively on pituitary production of FSH and mask an elevation that might otherwise reveal diminished ovarian reserve. Measurement of both FSH and estradiol on cycle day 3 may therefore help decrease the incidence of false-negative testing.
Commonly cited criteria for normal ovarian reserve are:
- early follicular phase FSH, <10 mIU/mL
- estradiol, <80 pg/mL1
It is extremely important to note, however, that these are general guidelines and that cutoffs are both laboratory- and practice-specific.
FIGURE 2 Monthly and lifetime variations in estradiol and FSH
How 17ß-estradiol and follicle-stimulating hormone levels vary over the menstrual cycle (top) and a woman’s lifetime (bottom).
2 | Clomiphene citrate—more sensitive than FSH testing
Like basal FSH testing, the CCCT is an indirect assessment of ovarian reserve. Unlike FSH testing, the CCCT is provocative. It involves administration of 100 mg of clomiphene citrate (Clomid) on days 5 through 9 of the menstrual cycle, with FSH and estradiol measured on days 3 and 10. Once clomiphene citrate is administered, FSH and LH levels rise, followed by an increase in estradiol and inhibin. Evidence suggests that the smaller follicular cohorts in women with diminished ovarian reserve produce less inhibin-B and estradiol and, therefore, less negative feedback on clomiphene-induced pituitary FSH release.6,7 The result: persistent elevation of the day 10 FSH value and a positive screen for diminished ovarian reserve.
In some women, day 10 FSH is elevated even after a normal day 3 value. This makes the CCCT more sensitive than basal FSH testing; it can identify women who might go unrecognized if evaluated by day 3 FSH and estradiol levels alone.
More expensive and labor-intensive than the alternatives
Interpretation of the CCCT requires that FSH and estradiol both be assessed on days 3 and 10. An elevated FSH (≥10 mIU/mL) on either day indicates diminished ovarian reserve. As with basal FSH testing, elevated estradiol (≥80 pg/mL) on day 3 is considered abnormal. The day 10 estradiol value of the CCCT reflects whether or not clomiphene citrate was administered appropriately, and should be elevated. However, the significance of the day 10 estradiol level has been debated with respect to its predictive value for pregnancy in infertile populations.8
The addition of day 10 FSH assessment improves the sensitivity of the CCCT over basal FSH measurement, but makes it a more expensive and labor-intensive test ( TABLE ).5,6 The CCCT involves administration of clomiphene citrate, a safe drug (though it can have side effects), and two blood draws instead of one. Nevertheless, both tests are relatively noninvasive, rapid measures of ovarian reserve.
Drawbacks of the tests
Both basal FSH testing and the CCCT are widely used, although support for their ability to predict ovarian reserve in the infertile population has been challenged recently. Newer data demonstrate that these tests are limited in their ability to predict outcome (pregnancy and response to superovulation drugs) in all but a narrow group of patients undergoing IVF. Performance is particularly limited in:
Additional drawbacks of basal FSH testing and the CCCT include:
- significant variability of test results from cycle to cycle (intercycle variability)
- limited time frame within which the tests can be performed (intracycle variability).
The basal FSH test and CCCT have high specificity (98% to 99% for each) as an assessment of reproductive performance in infertile women and generate few false-positive results.5,6 However, the high screen cutoffs that allow for such specificity come at a price: Few women will screen positive, and sensitivity of the tests is low (between 7% and 8% for basal FSH and between 25% and 40% for the CCCT). Such low sensitivity means that many women will not conceive after infertility treatment despite a normal test result.5,11 Overall, the tests are not highly informative for many women who get tested.
Once abnormal, normal results are meaningless
Once an FSH level or the CCCT has ever been abnormal, the patient has diminished ovarian reserve; normal values in subsequent menstrual cycles do not improve the odds of pregnancy with treatment.14 This fact can be a significant source of confusion and frustration for patients.
3 | GnRH agonist stimulation —no better than FSH testing
This test was developed in the search for a very sensitive assessment of ovarian reserve. It was designed to uncover subtle abnormalities in pituitary and ovarian dynamics. It involves administering a gonadotropin-releasing hormone (GnRH) agonist such as leuprolide acetate (Lupron) on day 2 or 3 of the menstrual cycle and measuring pituitary and ovarian hormone responses.5,15
One group of investigators demonstrated a correlation between stimulated estradiol levels and responsiveness during IVF,16 but other studies have shown that the test does not perform significantly better than day 3 FSH in predicting ovarian reserve.17,18
The sensitivity of GnRH agonist testing for pregnancy is moderate (32% to 89%); specificity ranges from 79% to 97%.19
4 | Inhibin-B—not helpful when used alone
This glycoprotein hormone produced by granulosa cells of developing follicles is a direct measure of ovarian reserve when assessed in the early follicular phase of the menstrual cycle.20 Women treated with IVF who have a low inhibin-B level—particularly when using cutoffs below the range of 45–80 pg/mL—have been shown to respond poorly to superovulation and have a lower pregnancy rate than women with high inhibin-B.21,22 One group of investigators demonstrated that women with clinical evidence of diminished ovarian reserve but a normal FSH level also had low inhibin-B production, suggesting that it may be a more sensitive marker than FSH.22
Inhibin-B testing involves a simple blood draw. However, the test has been incorporated into clinical assessment of ovarian reserve only to a limited degree, due to the lack of reliable assays and controversy concerning its prognostic value.23
Because of these limitations, routine testing of serum inhibin-B in isolation of other markers of ovarian reserve is not recommended.
5 | Antral follicle count—good predictor of IVF outcome
Transvaginal ultrasonographic determination of the number of ovarian follicles that measure between 2 mm and 10 mm in diameter in the early follicular phase of the cycle yields the AFC. As a direct marker of the cohort of growing follicles in the early menstrual cycle, the AFC is believed to correlate strongly with the number of primordial follicles present in the ovary and, therefore, ovarian reserve. Total AFCs of less than 5 to 10 are suggestive of diminished ovarian reserve.24,25
In IVF cycles, AFC has proven to be an accurate predictor of number of oocytes retrieved, risk of cycle cancellation, and odds of conception.24,25 Some investigators have even suggested that, compared with other markers of ovarian reserve, AFC is the best independent predictor of outcome in IVF cycles.7,26-27
In a group of normally cycling women with proven fertility, AFC also showed a strong correlation with age, declining slowly until age 37 and more rapidly thereafter.28,29
AFC sensitivity for pregnancy is moderate and varies widely in published reports (8% to 60%), whereas specificity tends to be higher (33% to 96%).19
Drawbacks of AFC
- Because of the need to perform transvaginal ultrasonography, AFC is a more invasive and often more expensive test than hormonal biomarkers
- Accurate assessment of AFC requires an experienced sonographer and can be limited in patients who have had pelvic surgery or uterine fibroids and in those who are obese
- Moderate interobserver and intercycle variability of AFC determinations limits its reproducibility29,30
- As with basal FSH measurement, the intercycle variability of AFC does not correlate well with IVF outcome in individual patients.30
6 | Anti-Müllerian hormone— many advantages
The drawbacks of the tests just described— e.g., intercycle variability, lack of uniform cutoffs, and limited ability to predict IVF outcomes—make the development of more reliable measures of ovarian reserve a priority in reproductive medicine. AMH is a highly promising marker that appears to have many advantages over other tests and may have the greatest power to predict ovarian aging in women of reproductive age.
How it works
AMH is a glycoprotein growth factor and a member of the transforming growth factor-ß superfamily.31 It is primarily produced by the pool of early-growing follicles, which are believed to serve as a proxy for the number of primordial follicles in the ovary. The number of primordial follicles at a given point in time represents the ovarian reserve. AMH levels above 0.7 ng/mL are considered normal; values between 0.3 ng/mL and 0.7 ng/mL are consistent with borderline ovarian reserve, according to 2007 data from Reprosource Corp.
AMH has been studied as a marker of ovarian reserve for 6 years, with multiple reports describing declines in levels with age and with diminishing oocyte numbers. It is undetectable at menopause.32
The age-related decline in AMH is gradual but measurable even in young women, consistently preceding changes in other markers of ovarian reserve such as FSH and inhibin-B.32-35 The longitudinal changes in AMH have been demonstrated in ovulatory premenopausal women and healthy volunteers with proven fertility.33,34 In one series of women followed over a mean of 4 years (ages 25 to 46), AMH testing was superior to day 3 FSH, inhibin-B, and AFC in its ability to predict the onset of cycle irregularity and the menopausal transition.33
Does it predict oocyte quality?
AMH has performed well as a biomarker, comparable in most series to AFC and superior to FSH. AMH levels are strongly correlated with the number of oocytes retrieved during IVF and the odds of cycle cancellation due to poor response35-41 —but does it accurately characterize oocyte quality, the other element of ovarian reserve?
Some reports have shown a strong association between AMH levels and surrogates of oocyte quality, including fertilization, oocyte morphology, embryo quality, and pregnancy and miscarriage rates,36-41 but others have not.42 Some reports demonstrate a relationship between AMH and some but not all surrogate markers of oocyte quality.40
Advantages of AMH
- It demonstrates minimal intracycle variability.32,43-45 Compared with other markers of ovarian reserve, which must be measured early in the follicular phase of the menstrual cycle, AMH can be assessed at random times, making it a more convenient method for patients and physicians
- It demonstrates minimal intercycle variability32,34
- AMH levels are not significantly affected by the hormonal changes of pregnancy, oral contraceptive use, or GnRH treatment, and can be measured in these settings.46,47
Utility of AMH is limited in PCOS and obesity
The ability to use AMH as a marker of ovarian aging in women who have polycystic ovary syndrome (PCOS) and in women who are obese may be limited by the ovulatory dysfunction in these populations. Circulating levels of AMH are higher in women with PCOS than in unaffected women, a finding thought to be indicative of oligo-ovulation and poor follicular development in polycystic ovaries.48-53
In a recent series investigating AMH levels in women with PCOS, AMH and the degree of insulin resistance were positively correlated, and the AMH level was negatively correlated with the number of menses in a year.49 The consistently positive correlation between AMH and PCOS may suggest a future role for this marker as a diagnostic tool.
In obese women who do not have PCOS, AMH production may be lower than in women of normal weight. In a recent series, normally cycling obese women in the later reproductive years were shown to have an AMH level 70% lower than those in women who were not obese.54 These differences have not been well studied in younger obese women.
Which test is best?
AMH may be preferable to the other tests to assess ovarian reserve because it can be measured any time during the menstrual cycle or between cycles. AMH measurement is also useful if a woman is taking oral contraceptives or leuprolide acetate because these medications may confound the results of the other test methods. In addition, AMH may be the earliest indicator of decline in ovarian reproductive function. As such, it may highlight cases that merit a search for other causes of infertility and make it possible to treat them in a timely manner.
Elevated AMH may reveal occult PCOS and warn of significant risk of ovarian hyperstimulation prior to ovulation induction with gonadotrophins, so that the clinician can plan smaller doses.
A normal female is born with 1 million to 2 million oocytes, a number that declines continuously, primarily through the process of follicular atresia. By the onset of puberty, the number of oocytes has declined to approximately 300,000. As a woman enters her late 30s, when the total number of oocytes is approximately 25,000, the pace of oocyte depletion begins to increase, as does the rate of spontaneous miscarriage.1,55,56
The effect of age on fertility is believed to arise from changes in both oocyte number and quality. Multiple investigators have found a greater frequency of cellular abnormalities in oocytes from older women.1,2,5,15,57
Although ovarian reserve declines with age in all women, women of similar ages can have very different degrees of ovarian reserve, and some women who have very poor ovarian reserve may never conceive, despite aggressive fertility treatment.
The biologic basis for differences in ovarian reserve among similar groups of women is not completely understood, but is probably rooted in genetic, lifestyle, and environmental factors that affect granulosa cell and oocyte function. Identifying sensitive biomarkers that can determine ovarian reserve independent of age is critical to predict fertility and age at menopause.5
The University of Medicine and Dentistry of New Jersey (UMDNJ) owns a patent relating to the use of anti-Müllerian hormone/Müllerian inhibiting substance for predicting ovarian response in women with infertility. The patent is based in part on work that Dr. Seifer carried out while employed at UMDNJ. In accordance with UMDNJ policy, Dr. Seifer, a named inventor on this patent, assigned his interest in the invention to UMDNJ. UMDNJ has a licensing agreement with Diagnostic Systems Laboratory for the use of the claimed invention. Dr. Seifer receives a portion of the royalties, as determined by UMDNJ policy, that UMDNJ gains from this licensing agreement.
CASE: Borderline test result prompts referral
A 36-year-old nulliparous woman is seen in your office for evaluation after 6 months of infertility. She is ovulatory, and has been using an ovulation-prediction kit to time intercourse. You learn that she had Chlamydia trachomatis infection in the distant past, but elicit no other significant medical or surgical history. She reports that she smoked approximately one pack of cigarettes a day for 15 years but gave up smoking 5 years ago.
You order a hysterosalpingogram, followed by day 3 testing of follicle-stimulating hormone (FSH). The hysterosalpingogram is normal; the FSH level is 7.5 mIU/mL and the estradiol level is 30 pg/mL—both in the normal range.
The patient asks for testing of anti-Müllerian hormone (AMH; also known as Müllerian-inhibiting substance) because she has read that it is a new marker of fertility. The result is 0.5 ng/mL, a borderline value. After reviewing these results, you refer her to a reproductive endocrinologist for further management.
Was the test for AMH indicated? And is this referral appropriate?
The referral is entirely appropriate, even though the patient has not been trying to conceive for a full year. Why? The AMH value suggests that her ovarian reserve is in early decline. She would benefit from evaluation by a subspecialist who can review the entire spectrum of treatments, including aggressive options such as ovulation induction and in vitro fertilization (IVF), to optimize her reproductive success.
This article reviews the various biomarkers available to assess ovarian reserve in women who experience infertility:
- day 3 (basal) FSH
- clomiphene citrate challenge
- gonadotropin-releasing hormone (GnRH) agonist stimulation
- inhibin-B
- antral follicle count (AFC)
- AMH.
The AFC and AMH tend to detect the earliest changes in ovarian reserve, followed, sequentially, by inhibin-B, the clomiphene citrate challenge test (CCCT), and basal FSH.
The tests we describe are used primarily to assess treatment prognosis in infertile women. In time, however, appropriate population screening of ovarian reserve may be feasible to provide many more women with information about their reproductive potential and help them shape their life plan.
What makes a test valuable?
Ovarian reserve describes a woman’s reproductive potential—specifically, the number and quality of oocytes she possesses.1 Biochemical tests of ovarian reserve emerged during the rise of assisted reproductive technologies (ART) in the late 1980s to predict both responsiveness to superovulation drugs and the odds of pregnancy with treatment.
Ideally, a test that assesses ovarian reserve should be affordable, straightforward, rapidly interpretable, and minimally invasive. It also should be able to detect changes that begin early in reproductive life. To be applicable to large populations of reproductive-age women, it should be of use anytime in the menstrual cycle, and should provide reproducible and highly accurate assessment of the reproductive aging process.
Our ability to offer tests that accurately measure ovarian reserve has a significant impact on women at risk of infertility and early menopause and on those who choose to delay childbearing for personal (nonmedical) reasons. These tests have become increasingly relevant because women are choosing to have their first child at a later age than their counterparts did 20 years ago:
- In 1980, 40% of women having their first baby were younger than 25 years, and only 5% were older than 35
- In 2000, 25% of women were younger than 25 when their first child was born, and 15% were older than 35.
Who should be tested?
Ovarian reserve is a complex clinical phenomenon that is influenced by age, genetics, and environmental variables. The decline in a woman’s ovarian reserve over time is irreversible; the trajectory of this decline is fundamental to the odds of fertility with age and the timing of the menopausal transition. At present, the markers used most often in clinical practice have some utility but also suffer from several drawbacks ( TABLE ).
For the general practitioner performing an infertility evaluation, we recommend focusing on the following groups of women for ovarian reserve testing:
- women over 30 years of age
- women with a history of exposure to a confirmed gonadotoxin, i.e., tobacco smoke, chemotherapy, radiation therapy
- women with a strong family history of early menopause or premature ovarian failure
- women who have had extensive ovarian surgery, i.e., cystectomy and unilateral oophorectomy.
Testing tends to have the highest yield in these groups. Women who have abnormal results should be referred to a reproductive endocrinologist for further evaluation and treatment.
The six tests are described below.
TABLE
How six markers of ovarian reserve stack up
| Test (year described) | Timing | Intracycle and intercycle variability | Sensitivity (specificity) | Reflects changes in ovarian reserve | Normal levels | Confounders | Out-of-pocket cost |
|---|---|---|---|---|---|---|---|
| Basal follicle-stimulating hormone (FSH) (1988) | Day 3 of menstrual cycle | Clinically significant | 7%–8% (98%–99%) | Late | • Early follicular phase FSH level <10 mIU/mL • Estradiol level <80 pg/mL | • High estradiol level (decreases) • Oral contraceptive use (decreases) • Pregnancy (decreases) | $125–$150 |
| Clomiphene challenge test (1989) | Days 3 and 10 of menstrual cycle | Clinically significant | 25%–40% (98%–99%) | Late | • Day 3 FSH level <10 mIU/mL; day 3 estradiol level <80 pg/mL • Day 10 FSH level <10 mIU/mL | • High day 3 estradiol level (decreases day 3 FSH) • Low day 10 estradiol (increases day 10 FSH) • Oral contraceptive use (decreases) • Pregnancy (decreases) | $550–$600 |
| GnRH agonist (1988) | Early follicular phase of menstrual cycle | Clinically significant | 32%–89% (79%–97%) | Late | Variable | • Oral contraceptives (decrease estradiol levels) • Pregnancy (increases estrogens) | $300–$350 |
| Inhibin-B (1997) | Early follicular phase of menstrual cycle | Clinically significant | 33%–81% (29%–95%) | Early | Variable in the literature; normal cutoffs range from ≥45–80 pg/mL | • Obesity (decreases) • PCOS (increases) • Exogenous FSH administration (increases) • Oral contraceptive use (decreases) | $150–$200 |
| Antral follicle count (1997) | Early follicular phase of menstrual cycle | Clinically significant (includes interobserver variability) | 8%–60% (33%–96%) | Earliest | ≥5–10 total antral follicles | • Oral contraceptive use (decreases) • Polycystic ovary syndrome (PCOS) (increases) | $300–$500 |
| Anti–Müllerian hormone/Müllerian-inhibiting substance (2002) | At any time; not cycle-dependent | Minimal | 49%–76% (89%–94%) | Earliest | >0.7 ng/mL | • PCOS (increases) • Obesity (decreases) • Exogenous FSH administration (decreases) | $150–$400 |
1 | Basal FSH—widely used but only moderately informative
Day 3 FSH and the CCCT are the most widely used measures of ovarian reserve in ART practice. The use of early follicular-phase FSH as a marker of ovarian reserve and fertility was proposed 20 years ago with the emergence of IVF.2-4 The test is an indirect assessment of ovarian reserve in that it measures pituitary production of FSH in response to feedback from ovarian hormones. Estradiol and inhibin-B reach a nadir early in the menstrual cycle; measuring FSH on day 3 offers a glimpse of the functioning of the hypothalamic–pituitary–ovarian axis before ovarian hormone levels rise later in the cycle ( FIGURE 1 ).5,6
FIGURE 1 The HPO axis
The FSH level opens a window onto the function of the hypothalamic–pituitary–ovarian axis before ovarian hormone levels rise in the cycle. Women who have normal ovarian reserve have sufficient ovarian hormone production early in the menstrual cycle to maintain FSH levels within the normal range. Conversely, a “monotropic” elevation in FSH—one that is unaccompanied by a rise in luteinizing hormone (LH)—reflects poor hormone production from an aging pool of ovarian follicles and disinhibition of FSH production.5,6
FSH measurements are typically combined with estradiol to enhance the sensitivity of testing ( FIGURE 2, ). Premature elevations of estradiol early in the follicular phase are driven by rising FSH levels in women with declining ovarian reserve. Abnormally elevated estrogen levels then feed back negatively on pituitary production of FSH and mask an elevation that might otherwise reveal diminished ovarian reserve. Measurement of both FSH and estradiol on cycle day 3 may therefore help decrease the incidence of false-negative testing.
Commonly cited criteria for normal ovarian reserve are:
- early follicular phase FSH, <10 mIU/mL
- estradiol, <80 pg/mL1
It is extremely important to note, however, that these are general guidelines and that cutoffs are both laboratory- and practice-specific.
FIGURE 2 Monthly and lifetime variations in estradiol and FSH
How 17ß-estradiol and follicle-stimulating hormone levels vary over the menstrual cycle (top) and a woman’s lifetime (bottom).
2 | Clomiphene citrate—more sensitive than FSH testing
Like basal FSH testing, the CCCT is an indirect assessment of ovarian reserve. Unlike FSH testing, the CCCT is provocative. It involves administration of 100 mg of clomiphene citrate (Clomid) on days 5 through 9 of the menstrual cycle, with FSH and estradiol measured on days 3 and 10. Once clomiphene citrate is administered, FSH and LH levels rise, followed by an increase in estradiol and inhibin. Evidence suggests that the smaller follicular cohorts in women with diminished ovarian reserve produce less inhibin-B and estradiol and, therefore, less negative feedback on clomiphene-induced pituitary FSH release.6,7 The result: persistent elevation of the day 10 FSH value and a positive screen for diminished ovarian reserve.
In some women, day 10 FSH is elevated even after a normal day 3 value. This makes the CCCT more sensitive than basal FSH testing; it can identify women who might go unrecognized if evaluated by day 3 FSH and estradiol levels alone.
More expensive and labor-intensive than the alternatives
Interpretation of the CCCT requires that FSH and estradiol both be assessed on days 3 and 10. An elevated FSH (≥10 mIU/mL) on either day indicates diminished ovarian reserve. As with basal FSH testing, elevated estradiol (≥80 pg/mL) on day 3 is considered abnormal. The day 10 estradiol value of the CCCT reflects whether or not clomiphene citrate was administered appropriately, and should be elevated. However, the significance of the day 10 estradiol level has been debated with respect to its predictive value for pregnancy in infertile populations.8
The addition of day 10 FSH assessment improves the sensitivity of the CCCT over basal FSH measurement, but makes it a more expensive and labor-intensive test ( TABLE ).5,6 The CCCT involves administration of clomiphene citrate, a safe drug (though it can have side effects), and two blood draws instead of one. Nevertheless, both tests are relatively noninvasive, rapid measures of ovarian reserve.
Drawbacks of the tests
Both basal FSH testing and the CCCT are widely used, although support for their ability to predict ovarian reserve in the infertile population has been challenged recently. Newer data demonstrate that these tests are limited in their ability to predict outcome (pregnancy and response to superovulation drugs) in all but a narrow group of patients undergoing IVF. Performance is particularly limited in:
Additional drawbacks of basal FSH testing and the CCCT include:
- significant variability of test results from cycle to cycle (intercycle variability)
- limited time frame within which the tests can be performed (intracycle variability).
The basal FSH test and CCCT have high specificity (98% to 99% for each) as an assessment of reproductive performance in infertile women and generate few false-positive results.5,6 However, the high screen cutoffs that allow for such specificity come at a price: Few women will screen positive, and sensitivity of the tests is low (between 7% and 8% for basal FSH and between 25% and 40% for the CCCT). Such low sensitivity means that many women will not conceive after infertility treatment despite a normal test result.5,11 Overall, the tests are not highly informative for many women who get tested.
Once abnormal, normal results are meaningless
Once an FSH level or the CCCT has ever been abnormal, the patient has diminished ovarian reserve; normal values in subsequent menstrual cycles do not improve the odds of pregnancy with treatment.14 This fact can be a significant source of confusion and frustration for patients.
3 | GnRH agonist stimulation —no better than FSH testing
This test was developed in the search for a very sensitive assessment of ovarian reserve. It was designed to uncover subtle abnormalities in pituitary and ovarian dynamics. It involves administering a gonadotropin-releasing hormone (GnRH) agonist such as leuprolide acetate (Lupron) on day 2 or 3 of the menstrual cycle and measuring pituitary and ovarian hormone responses.5,15
One group of investigators demonstrated a correlation between stimulated estradiol levels and responsiveness during IVF,16 but other studies have shown that the test does not perform significantly better than day 3 FSH in predicting ovarian reserve.17,18
The sensitivity of GnRH agonist testing for pregnancy is moderate (32% to 89%); specificity ranges from 79% to 97%.19
4 | Inhibin-B—not helpful when used alone
This glycoprotein hormone produced by granulosa cells of developing follicles is a direct measure of ovarian reserve when assessed in the early follicular phase of the menstrual cycle.20 Women treated with IVF who have a low inhibin-B level—particularly when using cutoffs below the range of 45–80 pg/mL—have been shown to respond poorly to superovulation and have a lower pregnancy rate than women with high inhibin-B.21,22 One group of investigators demonstrated that women with clinical evidence of diminished ovarian reserve but a normal FSH level also had low inhibin-B production, suggesting that it may be a more sensitive marker than FSH.22
Inhibin-B testing involves a simple blood draw. However, the test has been incorporated into clinical assessment of ovarian reserve only to a limited degree, due to the lack of reliable assays and controversy concerning its prognostic value.23
Because of these limitations, routine testing of serum inhibin-B in isolation of other markers of ovarian reserve is not recommended.
5 | Antral follicle count—good predictor of IVF outcome
Transvaginal ultrasonographic determination of the number of ovarian follicles that measure between 2 mm and 10 mm in diameter in the early follicular phase of the cycle yields the AFC. As a direct marker of the cohort of growing follicles in the early menstrual cycle, the AFC is believed to correlate strongly with the number of primordial follicles present in the ovary and, therefore, ovarian reserve. Total AFCs of less than 5 to 10 are suggestive of diminished ovarian reserve.24,25
In IVF cycles, AFC has proven to be an accurate predictor of number of oocytes retrieved, risk of cycle cancellation, and odds of conception.24,25 Some investigators have even suggested that, compared with other markers of ovarian reserve, AFC is the best independent predictor of outcome in IVF cycles.7,26-27
In a group of normally cycling women with proven fertility, AFC also showed a strong correlation with age, declining slowly until age 37 and more rapidly thereafter.28,29
AFC sensitivity for pregnancy is moderate and varies widely in published reports (8% to 60%), whereas specificity tends to be higher (33% to 96%).19
Drawbacks of AFC
- Because of the need to perform transvaginal ultrasonography, AFC is a more invasive and often more expensive test than hormonal biomarkers
- Accurate assessment of AFC requires an experienced sonographer and can be limited in patients who have had pelvic surgery or uterine fibroids and in those who are obese
- Moderate interobserver and intercycle variability of AFC determinations limits its reproducibility29,30
- As with basal FSH measurement, the intercycle variability of AFC does not correlate well with IVF outcome in individual patients.30
6 | Anti-Müllerian hormone— many advantages
The drawbacks of the tests just described— e.g., intercycle variability, lack of uniform cutoffs, and limited ability to predict IVF outcomes—make the development of more reliable measures of ovarian reserve a priority in reproductive medicine. AMH is a highly promising marker that appears to have many advantages over other tests and may have the greatest power to predict ovarian aging in women of reproductive age.
How it works
AMH is a glycoprotein growth factor and a member of the transforming growth factor-ß superfamily.31 It is primarily produced by the pool of early-growing follicles, which are believed to serve as a proxy for the number of primordial follicles in the ovary. The number of primordial follicles at a given point in time represents the ovarian reserve. AMH levels above 0.7 ng/mL are considered normal; values between 0.3 ng/mL and 0.7 ng/mL are consistent with borderline ovarian reserve, according to 2007 data from Reprosource Corp.
AMH has been studied as a marker of ovarian reserve for 6 years, with multiple reports describing declines in levels with age and with diminishing oocyte numbers. It is undetectable at menopause.32
The age-related decline in AMH is gradual but measurable even in young women, consistently preceding changes in other markers of ovarian reserve such as FSH and inhibin-B.32-35 The longitudinal changes in AMH have been demonstrated in ovulatory premenopausal women and healthy volunteers with proven fertility.33,34 In one series of women followed over a mean of 4 years (ages 25 to 46), AMH testing was superior to day 3 FSH, inhibin-B, and AFC in its ability to predict the onset of cycle irregularity and the menopausal transition.33
Does it predict oocyte quality?
AMH has performed well as a biomarker, comparable in most series to AFC and superior to FSH. AMH levels are strongly correlated with the number of oocytes retrieved during IVF and the odds of cycle cancellation due to poor response35-41 —but does it accurately characterize oocyte quality, the other element of ovarian reserve?
Some reports have shown a strong association between AMH levels and surrogates of oocyte quality, including fertilization, oocyte morphology, embryo quality, and pregnancy and miscarriage rates,36-41 but others have not.42 Some reports demonstrate a relationship between AMH and some but not all surrogate markers of oocyte quality.40
Advantages of AMH
- It demonstrates minimal intracycle variability.32,43-45 Compared with other markers of ovarian reserve, which must be measured early in the follicular phase of the menstrual cycle, AMH can be assessed at random times, making it a more convenient method for patients and physicians
- It demonstrates minimal intercycle variability32,34
- AMH levels are not significantly affected by the hormonal changes of pregnancy, oral contraceptive use, or GnRH treatment, and can be measured in these settings.46,47
Utility of AMH is limited in PCOS and obesity
The ability to use AMH as a marker of ovarian aging in women who have polycystic ovary syndrome (PCOS) and in women who are obese may be limited by the ovulatory dysfunction in these populations. Circulating levels of AMH are higher in women with PCOS than in unaffected women, a finding thought to be indicative of oligo-ovulation and poor follicular development in polycystic ovaries.48-53
In a recent series investigating AMH levels in women with PCOS, AMH and the degree of insulin resistance were positively correlated, and the AMH level was negatively correlated with the number of menses in a year.49 The consistently positive correlation between AMH and PCOS may suggest a future role for this marker as a diagnostic tool.
In obese women who do not have PCOS, AMH production may be lower than in women of normal weight. In a recent series, normally cycling obese women in the later reproductive years were shown to have an AMH level 70% lower than those in women who were not obese.54 These differences have not been well studied in younger obese women.
Which test is best?
AMH may be preferable to the other tests to assess ovarian reserve because it can be measured any time during the menstrual cycle or between cycles. AMH measurement is also useful if a woman is taking oral contraceptives or leuprolide acetate because these medications may confound the results of the other test methods. In addition, AMH may be the earliest indicator of decline in ovarian reproductive function. As such, it may highlight cases that merit a search for other causes of infertility and make it possible to treat them in a timely manner.
Elevated AMH may reveal occult PCOS and warn of significant risk of ovarian hyperstimulation prior to ovulation induction with gonadotrophins, so that the clinician can plan smaller doses.
A normal female is born with 1 million to 2 million oocytes, a number that declines continuously, primarily through the process of follicular atresia. By the onset of puberty, the number of oocytes has declined to approximately 300,000. As a woman enters her late 30s, when the total number of oocytes is approximately 25,000, the pace of oocyte depletion begins to increase, as does the rate of spontaneous miscarriage.1,55,56
The effect of age on fertility is believed to arise from changes in both oocyte number and quality. Multiple investigators have found a greater frequency of cellular abnormalities in oocytes from older women.1,2,5,15,57
Although ovarian reserve declines with age in all women, women of similar ages can have very different degrees of ovarian reserve, and some women who have very poor ovarian reserve may never conceive, despite aggressive fertility treatment.
The biologic basis for differences in ovarian reserve among similar groups of women is not completely understood, but is probably rooted in genetic, lifestyle, and environmental factors that affect granulosa cell and oocyte function. Identifying sensitive biomarkers that can determine ovarian reserve independent of age is critical to predict fertility and age at menopause.5
1. Practice Committee of the American Society for Reproductive Medicine. Age and infertility in women. Fertil Steril. 2006;86:S248-S252.
2. Muasher SJ, Oehninger S, Simonetti S, Matta J, Ellis LM, Liu H-C. The value of basal and/or stimulated serum gonadotropin levels in prediction of stimulation response and in vitro fertilization outcome. Fertil Steril. 1988;50:298-307.
3. Scott RT, Toner JP, Muasher SJ, Oehninger S, Robinson S, Rosenwaks Z. Follicle stimulating hormone levels on cycle day 3 are predictive of in vitro fertilization outcome. Fertil Steril. 1989;51:651-654.
4. Toner JP, Philiput CB, Jones GS, Muasher SJ. Basal follicle stimulating hormone level is a better predictor of in vitro fertilization outcome than age. Fertil Steril. 1991;55:784-791.
5. Barnhart K, Osheroff J. Follicle stimulating hormone as a predictor of fertility. Curr Opin Obstet Gynecol. 1998;10:227-232.
6. Hofmann GE, Danforth DR, Seifer DB. Inhibin-B: the physiologic basis of the clomiphene citrate challenge test for ovarian reserve screening. Fertil Steril. 1998;69:474-477.
7. Yong PY, Baird DT, Thong KJ, McNeilly AS, Anderson RA. Prospective analysis of the relationships between the ovarian follicle cohort and basal FSH concentration, the inhibin response to exogenous FSH and ovarian follicle number at different stages of the normal menstrual cycle and after pituitary down-regulation. Hum Reprod. 2003;18:35-44.
8. Scott RT, Jr, Illions EH, Kost ER, Dellinger C, Hofmann GE, Navot D. Evaluation of the significance of the estradiol response during the clomiphene citrate challenge test. Fertil Steril. 1993;60:242-246.
9. Esposito MA, Coutifaris C, Barnhart KT. A moderately elevated day 3 FSH concentration has limited predictive value, especially in younger women. Hum Reprod. 2002;17:118-123.
10. Bancsi L, Broekmans FJM, Wol BWJ, Habbema DK, te Velde ER. Performance of basal follicle-stimulating hormone in the prediction of poor ovarian response and failure to become pregnant after in vitro fertilization: a meta-analysis. Fertil Steril. 2003;79:1091-1100.
11. Jain T, Soules MR, Collins JA. Comparison of basal follicle-stimulating hormone versus the clomiphene citrate challenge test for ovarian reserve screening. Fertil Steril. 2004;82:180-185.
12. Toner JP. Modest follicle-stimulating hormone elevations in younger women: warn but don’t disqualify. Fertil Steril. 2004;81:1493-1495.
13. Van Rooij IAJ, de Jong E, Broekmans FJM, Looman CWN, Habbeman DK, te Velde ER. High follicle-stimulating hormone levels should not necessarily lead to the exclusion of subfertile patients from treatment. Fertil Steril. 2004;81:1478-1485.
14. Scott RT, Hofmann GE, Oehninger S, Muasher SJ. Intercycle variability of day 3 follicle-stimulating hormone levels and its effect on stimulation quality in in vitro fertilization. Fertil Steril. 1990;54:297-302.
15. Bulkulmez O, Arici A. Assessment of ovarian reserve. Curr Opin Obstet Gynecol. 2004;16:231-237.
16. Ranieri DM, Quinn F, Makhlouf A, et al. Simultaneous evaluation of basal follicle-stimulating hormone and 17-beta-estradiol response to gonadotropin-releasing hormone analogue stimulation: an improved predictor of ovarian reserve. Fertil Steril. 1998;70:227-233.
17. Fujimoto VY, Klein NA, Battaglia DE, Bremmer WJ, Soules MR. The anterior pituitary response to a gonadotropin-releasing hormone challenge test in normal older reproductive age women. Fertil Steril. 1996;65:539-544.
18. Galtier-Dereure F, De Bouard V, Picto MC, et al. Ovarian reserve test with the gonadotrophin-releasing hormone agonist buserelin: correlation with in-vitro fertilization outcome. Hum Reprod. 1996;11:1393-1398.
19. Broekmans FJ, Fwee J, Hendricks DJ, Mol BW, Lambalk CB. A systematic review of tests predicting ovarian reserve and IVF outcome. Hum Reprod Update. 2006;12:685-718.
20. Klein NA, Illingworth PJ, Groome NP, NcNeilly AS, Battaglia DE, Soules MR. Decreased inhibin B secretion is associated with the monotropic FSH rise in older, ovulatory women: a study of serum and follicular fluid levels of dimeric inhibin A and B in spontaneous menstrual cycles. J Clin Endocrinol Metab. 1996;81:2742-2745.
21. Seifer DB, Lambert-Messerlian G, Hogan JW, et al. Day 3 serum inhibin-B is predictive of assisted reproductive technologies outcome. Fertil Steril. 1997;67:110-114.
22. Seifer DB, Scott RT, Jr, Bergh PA, et al. Women with declining ovarian reserve may demonstrate a decrease in day 3 serum inhibin B before a rise in day 3 follicle-stimulating hormone. Fertil Steril. 1999;72:63-65.
23. Corson SL, Gutmann J, Batzer FR, Wallace H, Klein N, Soules MR. Inhibin-B as a test of ovarian reserve for infertile women. Hum Reprod. 1999;14:2818-2821.
24. Tomas C, Nuojua-Huttunen S, Martikainen H. Pretreatment transvaginal ultrasound examination predicts ovarian responsiveness to gonadotrophins in in-vitro fertilization. Hum Reprod. 1997;12:220-223.
25. Chang MY, Chiang CH, Hsieh TT, Soong YK, Hsu KH. Use of the antral follicle count to predict the outcome of assisted reproductive technologies. Fertil Steril. 1998;69:505-510.
26. Hung E, Tang OS, Ho PC. The significance of the number of antral follicles prior to stimulation in predicting ovarian responses in an IVF programme. Hum Reprod. 2000;15:1937-1942.
27. Bancsi LFJMM, Broekmans FJM, Eijkemans MJC, de Jong FH, Habbema JDF, te Velde ER. Predictors of poor ovarian response in in vitro fertilization: a prospective study comparing basal markers of ovarian reserve. Fertil Steril. 2002;77:328-336.
28. Ng EH, Yeung WS, Fong DY, Ho PC. Effects of age on hormonal and ultrasound markers of ovarian reserve in Chinese women with proven fertility. Hum Reprod. 2003;18:2169-2174.
29. Scheffer GJ, Broekmans FJ, Dorland M, Habbema JD, Looman CW, te Velde ER. Antral follicle counts by transvaginal ultrasonography are related to age in women with proven natural fertility. Fertil Steril. 1999;72:845-851.
30. Hansen KR, Morris JL, Thyer AC, Soules MR. Reproductive aging and the variability in the ovarian antral follicle count: application in the clinical setting. Fertil Steril. 2003;80:577-583.
31. Cate RL, Mattaliano RJ, Hession C, et al. Isolation of the bovine and human genes for Müllerian inhibiting substance and expression of the human gene in animal cells. Cell. 1986;45:685-698.
32. de Vet A, Laven JSE, de Jong FH, Themmen APN, Fauser BCJM. Anti-Müllerian hormone serum levels: a putative marker for ovarian aging. Fertil Steril. 2002;77:357-362.
33. van Rooij IAJ, Broekmans FJM, Scheffer GJ, et al. Serum anti-Müllerian hormone levels best reflect the reproductive decline with age in normal women with proven fertility: a longitudinal study. Fertil Steril. 2005;83:979-987.
34. van Rooij IAJ, Tonkelaar I, Broekmans FJ, et al. Anti-Müllerian hormone is a promising predictor for the occurrence of the menopausal transition. Menopause. 2004;11:601-606.
35. Tremellen KP, Kolo M, Gilmore A, Lekamge DN. Anti-Müllerian hormone as a marker of ovarian reserve. Aust N Z J Obstet Gynaecol. 2005;45:20-24.
36. Silberstein T, MacLaughlin DT, Shai I, et al. Müllerian-inhibiting substance levels at the time of HCG administration in IVF cycles predict both ovarian reserve and embryo morphology. Hum Reprod. 2006;21:159-163.
37. Seifer DB, MacLaughlin DT, Christian BP, Feng B, Shelden RM. Early follicular serum Müllerian-inhibiting substance levels are associated with ovarian response during assisted reproductive technology cycles. Fertil Steril. 2002;77:468-471.
38. Ebner T, Sommergruber M, Moser M, Shebl O, Schreier-Lechner E, Tews G. Basal level anti-Müllerian hormone is associated with oocyte quality in stimulated cycles. Hum Reprod. 2006;21:2022-2026.
39. Hazout A, Bouchard P, Seifer DB, Aussage P, Junca AM, Cohen-Bacrie P. Serum anti-Müllerian hormone/Müllerian-inhibiting substance appears to be a more discriminatory marker of ART outcome than follicular stimulating hormone, inhibin B or estradiol. Fertil Steril. 2004;82:1323-1329.
40. Nelson SM, Yates RW, Fleming R. Serum anti-Müllerian hormone and FSH: prediction of live birth and extremes of response in stimulated cycles—implications for individualization of therapy. Hum Reprod. 2007;22:2414-2421.
41. Fanchin R, Mendez DH, Frydman N, et al. Anti-Müllerian hormone concentrations in the follicular fluid of the preovulatory follicle are predictive of the implantation potential of the ensuing embryo obtained by in vitro fertilization. J Clin Endocrinol Metab. 2007;92:1796-1802.
42. Smeenk JM, Sweep FC, Zielhuis GA, Kremer JA, Th omas CM, Braat DD. Anti-Müllerian hormone predicts ovarian responsiveness, but not embryo quality or pregnancy, after in vitro fertilization or intracyoplasmic sperm injection. Fertil Steril. 2007;87:223-226.
43. Hehenkamp WJ, Looman CW, Themmen AP, de Jong FM, Te Velde ER, Broekmans FJ. Anti-Müllerian hormone levels in the spontaneous menstrual cycle do not show substantial fluctuation. J Clin Endocrinol Metab. 2006;91:4057-4063.
44. La Marca A, Stabile G, Artenisio AC, Volpe A. Serum anti-Müllerian hormone throughout the menstrual cycle. Hum Reprod. 2006;21:3103-3107.
45. Tsepelidis S, Devreker F, Demeestere F, Flahaut I, Gervy A, Englert C. Stable serum levels of anti-Müllerian hormone during the menstrual cycle: a prospective study in normo-ovulatory women. Hum Reprod. 2007;22:1837-1840.
46. La Marca A, Giulini Orvieto R, De Leo V, Volpe A. Anti-Müllerian hormone concentrations in maternal serum during pregnancy. Hum Reprod. 2005;20:1569-1572.
47. Somunkiran A, Yavuz T, Yucel O, Ozdemir I. Anti-Müllerian hormone levels during hormonal contraception in women with polycystic ovary syndrome. Eur J Obstet Gynecol Reprod Biol. 2007;134:196-201.
48. Al-Qahtani A, Groome NP. Anti-Müllerian hormone: Cinderella finds new admirers. J Clin Endocrinol Metab. 2006;91:3760-3762.
49. La Marca A, Orvieto R, Giulini S, Jasonni VM, Volpe A, De Leo V. Müllerian-inhibiting substance in women with polycystic ovary syndrome: relationship with hormonal and metabolic characteristics. Fertil Steril. 2004;82:970-971.
50. Piltonen T, Morin-Papunen L, Koivunen R, Perheentupa A, Ruokonen A, Tapanainen JS. Serum anti-Müllerian hormone levels remain high until late reproductive age and decrease during metformin therapy in women with polycystic ovary syndrome. Hum Reprod. 2005;20:1820-1836.
51. Pigny P, Merlen E, Robert Y, et al. Elevated serum level of anti-Müllerian hormone in patients with polycystic ovary syndrome: relationship to the ovarian follicle excess and to the follicular arrest. J Clin Endocrinol Metab. 2003;88:5957-5962.
52. Cook CL, Siow Y, Brenner AG, Fallat ME. Relationship between serum anti-Müllerian substance and other reproductive hormones in untreated women with polycystic ovary syndrome and endometriosis. Fertil Steril. 1997;67:962-965.
53. Pellatt L, Hanna L, Brincat M, et al. Granulosa cell production of anti-Müllerian hormone is increased in polycystic ovaries. J Clin Endocrinol Metab. 2007;92:240-245.
54. Freeman EW, Gracia CG, Sammel MD, Lin H, Lim LC, Strauss JF, 3rd. Association of anti-Müllerian hormone levels with obesity in later reproductive-age women. Fertil Steril. 2007;87:101-106.
55. Scott RT, Opsahl MS, Leonardi MR, Neall GS, Illions EH, Navot D. Life table analysis of pregnancy rates in a general infertility population relative to ovarian reserve and patient age. Hum Reprod. 1995;10:1706-1710.
56. Speroff L. Fritz M. eds. Clinical Gynecologic Endocrinology and Infertility. 7th ed. Philadelphia: Lippincott Williams & Wilkins; 2004.
57. Lim AS, Tsakok MFH. Age-related decline in fertility: a link to degenerative oocytes? Fertil Steril. 1997;68:265-271.
1. Practice Committee of the American Society for Reproductive Medicine. Age and infertility in women. Fertil Steril. 2006;86:S248-S252.
2. Muasher SJ, Oehninger S, Simonetti S, Matta J, Ellis LM, Liu H-C. The value of basal and/or stimulated serum gonadotropin levels in prediction of stimulation response and in vitro fertilization outcome. Fertil Steril. 1988;50:298-307.
3. Scott RT, Toner JP, Muasher SJ, Oehninger S, Robinson S, Rosenwaks Z. Follicle stimulating hormone levels on cycle day 3 are predictive of in vitro fertilization outcome. Fertil Steril. 1989;51:651-654.
4. Toner JP, Philiput CB, Jones GS, Muasher SJ. Basal follicle stimulating hormone level is a better predictor of in vitro fertilization outcome than age. Fertil Steril. 1991;55:784-791.
5. Barnhart K, Osheroff J. Follicle stimulating hormone as a predictor of fertility. Curr Opin Obstet Gynecol. 1998;10:227-232.
6. Hofmann GE, Danforth DR, Seifer DB. Inhibin-B: the physiologic basis of the clomiphene citrate challenge test for ovarian reserve screening. Fertil Steril. 1998;69:474-477.
7. Yong PY, Baird DT, Thong KJ, McNeilly AS, Anderson RA. Prospective analysis of the relationships between the ovarian follicle cohort and basal FSH concentration, the inhibin response to exogenous FSH and ovarian follicle number at different stages of the normal menstrual cycle and after pituitary down-regulation. Hum Reprod. 2003;18:35-44.
8. Scott RT, Jr, Illions EH, Kost ER, Dellinger C, Hofmann GE, Navot D. Evaluation of the significance of the estradiol response during the clomiphene citrate challenge test. Fertil Steril. 1993;60:242-246.
9. Esposito MA, Coutifaris C, Barnhart KT. A moderately elevated day 3 FSH concentration has limited predictive value, especially in younger women. Hum Reprod. 2002;17:118-123.
10. Bancsi L, Broekmans FJM, Wol BWJ, Habbema DK, te Velde ER. Performance of basal follicle-stimulating hormone in the prediction of poor ovarian response and failure to become pregnant after in vitro fertilization: a meta-analysis. Fertil Steril. 2003;79:1091-1100.
11. Jain T, Soules MR, Collins JA. Comparison of basal follicle-stimulating hormone versus the clomiphene citrate challenge test for ovarian reserve screening. Fertil Steril. 2004;82:180-185.
12. Toner JP. Modest follicle-stimulating hormone elevations in younger women: warn but don’t disqualify. Fertil Steril. 2004;81:1493-1495.
13. Van Rooij IAJ, de Jong E, Broekmans FJM, Looman CWN, Habbeman DK, te Velde ER. High follicle-stimulating hormone levels should not necessarily lead to the exclusion of subfertile patients from treatment. Fertil Steril. 2004;81:1478-1485.
14. Scott RT, Hofmann GE, Oehninger S, Muasher SJ. Intercycle variability of day 3 follicle-stimulating hormone levels and its effect on stimulation quality in in vitro fertilization. Fertil Steril. 1990;54:297-302.
15. Bulkulmez O, Arici A. Assessment of ovarian reserve. Curr Opin Obstet Gynecol. 2004;16:231-237.
16. Ranieri DM, Quinn F, Makhlouf A, et al. Simultaneous evaluation of basal follicle-stimulating hormone and 17-beta-estradiol response to gonadotropin-releasing hormone analogue stimulation: an improved predictor of ovarian reserve. Fertil Steril. 1998;70:227-233.
17. Fujimoto VY, Klein NA, Battaglia DE, Bremmer WJ, Soules MR. The anterior pituitary response to a gonadotropin-releasing hormone challenge test in normal older reproductive age women. Fertil Steril. 1996;65:539-544.
18. Galtier-Dereure F, De Bouard V, Picto MC, et al. Ovarian reserve test with the gonadotrophin-releasing hormone agonist buserelin: correlation with in-vitro fertilization outcome. Hum Reprod. 1996;11:1393-1398.
19. Broekmans FJ, Fwee J, Hendricks DJ, Mol BW, Lambalk CB. A systematic review of tests predicting ovarian reserve and IVF outcome. Hum Reprod Update. 2006;12:685-718.
20. Klein NA, Illingworth PJ, Groome NP, NcNeilly AS, Battaglia DE, Soules MR. Decreased inhibin B secretion is associated with the monotropic FSH rise in older, ovulatory women: a study of serum and follicular fluid levels of dimeric inhibin A and B in spontaneous menstrual cycles. J Clin Endocrinol Metab. 1996;81:2742-2745.
21. Seifer DB, Lambert-Messerlian G, Hogan JW, et al. Day 3 serum inhibin-B is predictive of assisted reproductive technologies outcome. Fertil Steril. 1997;67:110-114.
22. Seifer DB, Scott RT, Jr, Bergh PA, et al. Women with declining ovarian reserve may demonstrate a decrease in day 3 serum inhibin B before a rise in day 3 follicle-stimulating hormone. Fertil Steril. 1999;72:63-65.
23. Corson SL, Gutmann J, Batzer FR, Wallace H, Klein N, Soules MR. Inhibin-B as a test of ovarian reserve for infertile women. Hum Reprod. 1999;14:2818-2821.
24. Tomas C, Nuojua-Huttunen S, Martikainen H. Pretreatment transvaginal ultrasound examination predicts ovarian responsiveness to gonadotrophins in in-vitro fertilization. Hum Reprod. 1997;12:220-223.
25. Chang MY, Chiang CH, Hsieh TT, Soong YK, Hsu KH. Use of the antral follicle count to predict the outcome of assisted reproductive technologies. Fertil Steril. 1998;69:505-510.
26. Hung E, Tang OS, Ho PC. The significance of the number of antral follicles prior to stimulation in predicting ovarian responses in an IVF programme. Hum Reprod. 2000;15:1937-1942.
27. Bancsi LFJMM, Broekmans FJM, Eijkemans MJC, de Jong FH, Habbema JDF, te Velde ER. Predictors of poor ovarian response in in vitro fertilization: a prospective study comparing basal markers of ovarian reserve. Fertil Steril. 2002;77:328-336.
28. Ng EH, Yeung WS, Fong DY, Ho PC. Effects of age on hormonal and ultrasound markers of ovarian reserve in Chinese women with proven fertility. Hum Reprod. 2003;18:2169-2174.
29. Scheffer GJ, Broekmans FJ, Dorland M, Habbema JD, Looman CW, te Velde ER. Antral follicle counts by transvaginal ultrasonography are related to age in women with proven natural fertility. Fertil Steril. 1999;72:845-851.
30. Hansen KR, Morris JL, Thyer AC, Soules MR. Reproductive aging and the variability in the ovarian antral follicle count: application in the clinical setting. Fertil Steril. 2003;80:577-583.
31. Cate RL, Mattaliano RJ, Hession C, et al. Isolation of the bovine and human genes for Müllerian inhibiting substance and expression of the human gene in animal cells. Cell. 1986;45:685-698.
32. de Vet A, Laven JSE, de Jong FH, Themmen APN, Fauser BCJM. Anti-Müllerian hormone serum levels: a putative marker for ovarian aging. Fertil Steril. 2002;77:357-362.
33. van Rooij IAJ, Broekmans FJM, Scheffer GJ, et al. Serum anti-Müllerian hormone levels best reflect the reproductive decline with age in normal women with proven fertility: a longitudinal study. Fertil Steril. 2005;83:979-987.
34. van Rooij IAJ, Tonkelaar I, Broekmans FJ, et al. Anti-Müllerian hormone is a promising predictor for the occurrence of the menopausal transition. Menopause. 2004;11:601-606.
35. Tremellen KP, Kolo M, Gilmore A, Lekamge DN. Anti-Müllerian hormone as a marker of ovarian reserve. Aust N Z J Obstet Gynaecol. 2005;45:20-24.
36. Silberstein T, MacLaughlin DT, Shai I, et al. Müllerian-inhibiting substance levels at the time of HCG administration in IVF cycles predict both ovarian reserve and embryo morphology. Hum Reprod. 2006;21:159-163.
37. Seifer DB, MacLaughlin DT, Christian BP, Feng B, Shelden RM. Early follicular serum Müllerian-inhibiting substance levels are associated with ovarian response during assisted reproductive technology cycles. Fertil Steril. 2002;77:468-471.
38. Ebner T, Sommergruber M, Moser M, Shebl O, Schreier-Lechner E, Tews G. Basal level anti-Müllerian hormone is associated with oocyte quality in stimulated cycles. Hum Reprod. 2006;21:2022-2026.
39. Hazout A, Bouchard P, Seifer DB, Aussage P, Junca AM, Cohen-Bacrie P. Serum anti-Müllerian hormone/Müllerian-inhibiting substance appears to be a more discriminatory marker of ART outcome than follicular stimulating hormone, inhibin B or estradiol. Fertil Steril. 2004;82:1323-1329.
40. Nelson SM, Yates RW, Fleming R. Serum anti-Müllerian hormone and FSH: prediction of live birth and extremes of response in stimulated cycles—implications for individualization of therapy. Hum Reprod. 2007;22:2414-2421.
41. Fanchin R, Mendez DH, Frydman N, et al. Anti-Müllerian hormone concentrations in the follicular fluid of the preovulatory follicle are predictive of the implantation potential of the ensuing embryo obtained by in vitro fertilization. J Clin Endocrinol Metab. 2007;92:1796-1802.
42. Smeenk JM, Sweep FC, Zielhuis GA, Kremer JA, Th omas CM, Braat DD. Anti-Müllerian hormone predicts ovarian responsiveness, but not embryo quality or pregnancy, after in vitro fertilization or intracyoplasmic sperm injection. Fertil Steril. 2007;87:223-226.
43. Hehenkamp WJ, Looman CW, Themmen AP, de Jong FM, Te Velde ER, Broekmans FJ. Anti-Müllerian hormone levels in the spontaneous menstrual cycle do not show substantial fluctuation. J Clin Endocrinol Metab. 2006;91:4057-4063.
44. La Marca A, Stabile G, Artenisio AC, Volpe A. Serum anti-Müllerian hormone throughout the menstrual cycle. Hum Reprod. 2006;21:3103-3107.
45. Tsepelidis S, Devreker F, Demeestere F, Flahaut I, Gervy A, Englert C. Stable serum levels of anti-Müllerian hormone during the menstrual cycle: a prospective study in normo-ovulatory women. Hum Reprod. 2007;22:1837-1840.
46. La Marca A, Giulini Orvieto R, De Leo V, Volpe A. Anti-Müllerian hormone concentrations in maternal serum during pregnancy. Hum Reprod. 2005;20:1569-1572.
47. Somunkiran A, Yavuz T, Yucel O, Ozdemir I. Anti-Müllerian hormone levels during hormonal contraception in women with polycystic ovary syndrome. Eur J Obstet Gynecol Reprod Biol. 2007;134:196-201.
48. Al-Qahtani A, Groome NP. Anti-Müllerian hormone: Cinderella finds new admirers. J Clin Endocrinol Metab. 2006;91:3760-3762.
49. La Marca A, Orvieto R, Giulini S, Jasonni VM, Volpe A, De Leo V. Müllerian-inhibiting substance in women with polycystic ovary syndrome: relationship with hormonal and metabolic characteristics. Fertil Steril. 2004;82:970-971.
50. Piltonen T, Morin-Papunen L, Koivunen R, Perheentupa A, Ruokonen A, Tapanainen JS. Serum anti-Müllerian hormone levels remain high until late reproductive age and decrease during metformin therapy in women with polycystic ovary syndrome. Hum Reprod. 2005;20:1820-1836.
51. Pigny P, Merlen E, Robert Y, et al. Elevated serum level of anti-Müllerian hormone in patients with polycystic ovary syndrome: relationship to the ovarian follicle excess and to the follicular arrest. J Clin Endocrinol Metab. 2003;88:5957-5962.
52. Cook CL, Siow Y, Brenner AG, Fallat ME. Relationship between serum anti-Müllerian substance and other reproductive hormones in untreated women with polycystic ovary syndrome and endometriosis. Fertil Steril. 1997;67:962-965.
53. Pellatt L, Hanna L, Brincat M, et al. Granulosa cell production of anti-Müllerian hormone is increased in polycystic ovaries. J Clin Endocrinol Metab. 2007;92:240-245.
54. Freeman EW, Gracia CG, Sammel MD, Lin H, Lim LC, Strauss JF, 3rd. Association of anti-Müllerian hormone levels with obesity in later reproductive-age women. Fertil Steril. 2007;87:101-106.
55. Scott RT, Opsahl MS, Leonardi MR, Neall GS, Illions EH, Navot D. Life table analysis of pregnancy rates in a general infertility population relative to ovarian reserve and patient age. Hum Reprod. 1995;10:1706-1710.
56. Speroff L. Fritz M. eds. Clinical Gynecologic Endocrinology and Infertility. 7th ed. Philadelphia: Lippincott Williams & Wilkins; 2004.
57. Lim AS, Tsakok MFH. Age-related decline in fertility: a link to degenerative oocytes? Fertil Steril. 1997;68:265-271.
How to safeguard the ureter and repair surgical injury
The author has no financial relationships relevant to this article.
CASE: Inadvertent ureteral transection
A gynecologic surgeon operates via Pfannenstiel incision to remove a 12-cm complex left adnexal mass from a 36-year-old obese woman. When she discovers that the mass is densely adherent to the pelvic peritoneum, the surgeon incises the peritoneum lateral to the mass and opens the retroperitoneal space. However, the size and relative immobility of the mass, coupled with the low transverse incision, impair visualization of retroperitoneal structures.
The surgeon clamps and divides the ovarian vessels above the mass but, afterward, suspects that the ureter has been transected and that its ends are included within the clamps. She separates the ovarian vessels above the clamp and ligates them, at which time transection of the ureter is confirmed.
How should she proceed?
The ureter is intimately associated with the female internal genitalia in a way that challenges the gynecologic surgeon to avoid it. In a small percentage of cases involving surgical extirpation in a woman who has severe pelvic pathology, ureteral injury may be inevitable.
Several variables predispose a patient to ureteral injury, including limited exposure, as in the opening case. Others include distorted anatomy of the urinary tract relative to internal genitalia and operations that require extensive resection of pelvic tissues.
This article describes:
- prevention and intraoperative recognition of ureteral injury during gynecologic surgery
- management of intraoperatively recognized ureteral injury.
Maintain a high index of suspicion
The surgeon in the opening case has already taken the first and most important step in ensuring a good outcome: She suspected ureteral injury. In high-risk situations, intraoperative recognition of ureteral injury is more likely when the operative field is inspected thoroughly during and at the conclusion of the surgical procedure.
In a high-risk case, the combined use of intravenous indigo carmine, careful inspection of the operative field, cystoscopy, and ureteral dissection is recommended and should be routine.
Common sites of injury
During gynecologic surgery, the ureter is susceptible to injury along its entire course through the pelvis (see “The ureter takes a course fraught with hazard,”).
During adnexectomy, the gonadal vessels are generally ligated 2 to 3 cm above the adnexa. The ureter lies in close proximity to these vessels and may inadvertently be included in the ligation.
During hysterectomy, the ureter is susceptible to injury as it passes through the parametrium a short distance from the uterus and vaginal fornix.
Sutures placed in the posterior lateral cul de sac during prolapse surgery lie near the midpelvic ureter, and sutures placed during vaginal cuff closure, anterior colporrhaphy, and retropubic urethropexy are in close proximity to the trigonal portion of the ureter.
The ureter extends from the renal pelvis to the bladder, with a length that ranges from 25 to 30 cm, depending on the patient’s height. It crosses the pelvic brim near the bifurcation of the common iliac artery, where it becomes the “pelvic” ureter. The abdominal and pelvic portions of the ureter are approximately equal in length.
ILLUSTRATIONS BY ROB FLEWELL FOR OBG MANAGEMENT
The blood supply of the ureter derives from branches of the major arterial system of the lower abdomen and pelvis. These branches reach the medial aspect of the abdominal ureter and the lateral side of the pelvic ureter to form an anastomotic vascular network protected by an adventitial layer surrounding the ureter.
The ureter is attached to the posterior lateral pelvic peritoneum running dorsal to ovarian vessels. At the midpelvis, it separates from the peritoneum to pierce the base of the broad ligament underneath the uterine artery. At this point, the ureter is about 1.5 to 2 cm lateral to the uterus and curves medially and ventrally, tunneling through the cardinal and vesicovaginal ligaments to enter the bladder trigone.
Risky procedures
In gynecologic surgery, ureteral injury occurs most often during abdominal hysterectomy—probably because of how frequently this operation is performed and the range of pathology managed. The incidence of ureteral injury is much higher during abdominal hysterectomy than vaginal hysterectomy.1-4
Laparoscopic hysterectomy also has been associated with a higher incidence of ureteral injury, especially in the early phase of training.5,6 Possible explanations include:
- greater difficulty identifying the ureter
- a steeper learning curve
- more frequent use of energy to hemostatically divide pedicles, with the potential for thermal injury
- less traction–countertraction, resulting in dissection closer to the ureter
- management of complex pathology.
Although the overall incidence of ureteral injury during adnexectomy is low, it is probably much higher in women undergoing this procedure after a previous hysterectomy or in the presence of complex adnexal pathology.
When injury is likely
Compromised exposure, distorted anatomy, and certain procedures can heighten the risk of ureteral injury. Large tumors may limit the ability of the surgeon to visualize or palpate the ureter (FIGURE 1). Extensive adhesions may cause similar difficulties, and a small incision or obesity may hinder identification of pelvic sidewall structures.
A number of pathologic conditions can distort the anatomy of the ureter, especially as it relates to the female genital tract:
- Malignancies such as ovarian cancer often encroach on and occasionally encase the ureter
- Pelvic inflammatory disease, endometriosis, and a history of surgery or pelvic radiotherapy can retract and encase the ureter toward the gynecologic tract
- Some masses expand against the lower ureter, such as cervical or broad-ligament leiomyomata or placenta previa with accreta
- During vaginal hysterectomy for complete uterine prolapse, the ureters frequently extend beyond the introitus well within the operative field
- Congenital anomalies of the ureter or hydroureter can also cause distortion.
Even in the presence of relatively normal anatomy, certain procedures predispose the ureter to injury. For example, radical hysterectomy involves the almost complete separation of the pelvic ureter from the gynecologic tract and its surrounding soft tissue. When pelvic pathology is significant, the plane of dissection will always be near the ureter.
FIGURE 1 Access to the ureter is obstructed, putting it in jeopardy
Large tumors may limit the ability of the surgeon to visualize or palpate the ureter.
Prevention is the best strategy
At least 50% of ureteral injuries reported during gynecologic surgery have occurred in the absence of a recognizable risk factor.2,7 Nevertheless, knowledge of anatomy and the ability to recognize situations in which there is an elevated risk for ureteral injury will best enable the surgeon to prevent such injury.
When a high-risk situation is encountered, critical preventive steps include:
- adequate exposure
- competent assistance
- exposure of the path of the ureter through the planned course of dissection. Dissecting the ureter beyond this area is usually unnecessary and may itself cause injury.
Skip preoperative IVP in most cases
The vast majority of women who undergo gynecologic surgery do not benefit from preoperative intravenous pyelography (IVP). This measure does not appear to reduce the likelihood of ureteral injury, even in the face of obvious gynecologic disease. However, preoperative identification of obvious ureteral involvement by the disease process is useful. In such cases, the plane of dissection will probably lie closer to the ureter. One of the goals of surgery will then be to clear the urinary tract from the affected area.
When there is a high index of suspicion of an abnormality such as obstruction, intrinsic ureteral endometriosis, or congenital anomaly, preoperative IVP is indicated.
A stent may be helpful in some cases
Ureteral stents are sometimes placed in order to aid in identification and dissection of the ureters during surgery. Some authors of reports on this topic, including Hoffman, believe that stents are useful in certain situations, such as excision of an ovarian remnant, radical vaginal hysterectomy, and when pelvic organs are encased by malignant ovarian tumors. However, stents do not clearly reduce the risk of injury and, in some cases, may increase the risk by providing a false sense of security and predisposing the ureter to adventitial injury during difficult dissection.
Anticipate the effects of disease
The surgeon must have a thorough knowledge of the gynecologic disease process as it relates to surgery involving the urinary tract. For example, an ovarian remnant will almost always be somewhat densely adherent to the pelvic ureter. When severe endometriosis involves the posterior leaf of the broad ligament, the ureter will often be fibrotically retracted toward the operative field.
Certain procedures have special challenges. During resection of adnexa, for example, it is important that the ureter be identified in the retroperitoneum before the ovarian vessels are ligated. During hysterectomy, soft tissues that contain the bladder and ureters should be mobilized caudally and laterally, respectively, creating a U-shaped region (“U” for urinary tract, FIGURE 2) to which the surgeon must limit dissection.
FIGURE 2 During hysterectomy, mobilize the bladder and ureter
Mobilize the soft tissues that contain the bladder and ureters caudally and laterally, respectively, creating a U-shaped region. During division of the paracervical tissues, the surgeon must remain within this region.
Intraoperative detection
Two main types of ureteral injury occur during gynecologic surgery: transection and destruction. The latter includes ligation, crushing, devascularization, and thermal injury.
Intraoperative detection of ureteral injury is more likely when the surgeon recognizes at the outset that the operation places the ureter at increased risk. When dissection has been difficult or complicated for any reason, be concerned about possible injury.
In general, ureteral injury is first recognized by careful inspection of the surgical field. Begin by instilling 5 ml of indigo carmine intravenously. Once the dye begins to appear in the Foley catheter, inspect the area of dissection under a small amount of irrigation fluid, looking for extravasation of dye that indicates partial or complete transection.
If no injury is identified, cystoscopy is the next step. I perform all major abdominal operations with the patient in the low lithotomy position, which provides easy access to the perineum. Cystoscopic identification of urine jetting from both ureteral orifices confirms patency. When only wisps of dye are observed, it is likely that the ureter in question has been partially occluded (e.g., by acute angulation). Failure of any urine to appear from one of the orifices highly suggests injury to that ureter.
During inspection of the operative field, attempt to pass a ureteral stent into the affected orifice. If the stent passes easily and dyed urine is seen to drip freely from it, look for possible angulation of the ureter. If you find none, remove the stent and inspect the orifice again for jetting urine.
If the ureteral stent will move only a few centimeters into the ureteral orifice, ligation (with or without transection) is likely. In this case, leave the stent in place. If the operative site is readily accessible, dissect the applicable area to identify the problem. Depending on the circumstances, you may wish to infuse dye through the stent to aid in operative identification or radiographic evaluation.
Intraoperative IVP may be useful, especially when cystoscopy is unavailable.
Fundamentals of repair
Repair of major injury to the pelvic ureter is generally best accomplished by ureteroneocystostomy or, in selected cases involving injury to the proximal pelvic ureter, by ureteroureterostomy.
When intraoperatively recognized injury to the pelvic ureter appears to be minor, it can be managed by placing a ureteral stent and a closed-suction pelvic drain. Also consider wrapping the injured area with vascularized tissue such as perivesical fat. Minor lacerations can be closed perpendicular to the axis of the ureter using interrupted 4-0 delayed absorbable suture.
Most injuries to the pelvic ureter are optimally managed by ureteroneocystostomy (FIGURE 3). When a significant portion of the pelvic ureter has been lost, ureteroneocystostomy usually requires a combination of:
- extensive mobilization of the bladder
- conservative mobilization of the ureter
- elongation of the bladder
- psoas hitch.
When necessary, mobilization of the kidney with suturing of the caudal perinephric fascia to the psoas muscle will bridge an additional 2- to 3-cm gap.
Major injury to the distal half of the pelvic ureter is repaired using straightforward ureteroneocystostomy.
When there is no significant pelvic disease and the distal ureter is healthy, injury to the proximal pelvic ureter during division of the ovarian vessels may be repaired via ureteroureterostomy. If the ureteral ends will be anastomosed on tension or there is any question about the integrity of the distal portion of the ureter, as when extensive distal ureterolysis has been necessary, consider ureteroneocystostomy.
FIGURE 3 When the distal ureter is injured
Most injuries to the pelvic ureter are managed optimally by ureteroneocystostomy.
Ureter injured during emergent hysterectomy
A 37-year-old woman, para 4, undergoes her fourth repeat cesarean section. When the OB attempts to manually extract the placenta, the patient begins to hemorrhage profusely. Conservative measures fail to stop the bleeding, and the patient becomes hypotensive. The physician performs emergent hysterectomy, taking large pedicles of tissue. Although the patient stabilizes, the doctor worries that the ureters may have been injured.
Resolution: Cystoscopy is performed to check for injury. Because indigo carmine does not spill from the left ureteral orifice, the physician passes a stent with the abdomen still open, and it stops within the most distal ligamentous pedicle. Upon deligation, indigo carmine begins to drain from the stent, which then passes easily.
The stent is withdrawn to below the site of injury, and dilute methylene blue is instilled through it while the ureter is observed under irrigation. No extravasation is noted. Because the ligature had been around a block of tissue that was thought to have acutely angulated rather than incorporated the ureter, the physician concludes that severe damage is unlikely. He places a 6 French double-J stent, wraps the damaged portion of the distal ureter in perivesical fat, and places a closed-suction pelvic drain. Healing is uneventful.
Obstruction is confirmed. Now the surgeon must find it
A 45-year-old woman, para 3, who has a symptomatic 14-weeks’ size myomatous uterus, undergoes vaginal hysterectomy. The surgeon ligates and divides the uterine vessel pedicles before beginning morcellation. At the completion of the procedure, during cystoscopy, indigo carmine fails to spill from the right ureteral orifice, suggesting injury to that ureter. The surgeon passes a stent into the ureter, and it stops approximately 6 cm from the orifice. A retrograde pyelogram confirms complete obstruction.
Resolution: With the stent left in place, the surgeon performs a midline laparotomy, tracing the ureter to the uterine artery pedicle in which it has been incorporated and transected. The distal ureter with the stent is found within soft tissue lateral to the cardinal ligament pedicle, and the transected end is securely ligated using 2–0 silk suture. After the bladder is mobilized, a ureteroneocystostomy is performed. The patient recovers fully.
Postoperative management
After repair of a ureteral injury, leave a closed-suction pelvic drain in place for 2 to 3 days so that any major urinary leak can be detected; it also enhances spontaneous closure and helps prevent potentially infected fluid from accumulating in the region of anastomosis.
The cystotomy performed during ureteroneocystostomy generally heals quickly with a low risk of complications.
Leave a large-bore (20 or 22 French) urethral Foley catheter in place for 2 weeks.
I recommend that a 6 French double-J ureteral stent be left in place for 6 weeks. Potential benefits of the stent include:
- prevention of stricture
- stabilization and immobilization of the ureter during healing
- reduced risk of extravasation of urine
- reduced risk of angulation of the ureter
- isolation of the repair from infection, retroperitoneal fibrosis, and cancer.
I perform IVP approximately 1 week after stent removal to ensure ureteral patency.
CASE RESOLVED
Exposure is improved by widening the incision and dividing the tendonous insertions of the rectus abdominus muscles. The surgeon then removes the mass, preserving the distal ureter, which is estimated to be 12 cm in length and to have intact adventitia.
The surgeon performs a double-spatulated end-to-end ureteroureterostomy over a 6 French double-J ureteral stent that has been passed proximally into the renal pelvis and distally into the bladder. The stent is removed 6 weeks postoperatively, and an IVP the following week demonstrates excellent patency.
The majority of payers consider ureterolysis integral to good surgical technique, but there can be exceptions when documentation supports existing codes. Three CPT codes describe this procedure:
50715 Ureterolysis, with or without repositioning of ureter for retroperitoneal fibrosis
50722 Ureterolysis for ovarian vein syndrome
50725 Ureterolysis for retrocaval ureter, with reanastomosis of upper urinary tract or vena cava
The key to getting paid will be to document the existence of the condition indicated by each of the codes.
The ICD-9 code for both retroperitoneal fibrosis and ovarian vein syndrome is the same, 593.4 (Other ureteric obstruction). If the patient requires ureterolysis for a retrocaval ureter, the code 753.4 (Other specified anomalies of ureter) would be reported instead. Note, however, that these procedure codes cannot be reported if the ureterolysis is performed laparoscopically. In that case, the most appropriate code is 50949 (Unlisted laparoscopy procedure, ureter).
When repair is necessary, you have several codes to choose from, but the supporting diagnosis code 998.2 (Accidental puncture or laceration during a procedure) must be indicated. If a Medicare patient is involved, the surgeon who created the injury would not be paid additionally for repair.
50780 Ureteroneocystostomy; anastomosis of single ureter to bladder
50782 Ureteroneocystostomy; anastomosis of duplicated ureter to bladder
50783 Ureteroneocystostomy; with extensive ureteral tailoring
50785 Ureteroneocystostomy; with vesico-psoas hitch or bladder flap
50760 Ureteroureterostomy; fusion of ureters
50770 Transureteroureterostomy, anastomosis of ureter to contralateral ureter—MELANIE WITT, RN, CPC-OBGYN, MA
1. St. Lezin MA, Stoller ML. Surgical ureteral injuries. Urology. 1991;38:497-506.
2. Liapis A, Bakas P, Giannopoulos V, Creatsas G. Ureteral injuries during gynecological surgery. Int Urogynecol J Pelvic Floor Dysfunct. 2001;12:391-394.
3. Vakili B, Chesson RR, Kyle BL, et al. The incidence of urinary tract injury during hysterectomy: a prospective analysis based on universal cystoscopy. Am J Obstet Gynecol. 2005;192:1599-1604.
4. Sakellariou P, Protopapas AG, Voulgaris Z, et al. Management of ureteric injuries during gynecological operations: 10 years experience. Eur J Obstet Gynecol Reprod Biol. 2002;101:179-184.
5. Assimos DG, Patterson LC, Taylor CL. Changing incidence and etiology of iatrogenic ureteral injuries. J Urol. 1994;152:2240-2246.
6. Härkki-Sirén P, Sjöberg J, Titinen A. Urinary tract injuries after hysterectomy. Obstet Gynecol. 1998;92:113-118.
7. Chan JK, Morrow J, Manetta A. Prevention of ureteral injuries in gynecologic surgery. Am J Obstet Gynecol. 2003;188:1273-1277.
The author has no financial relationships relevant to this article.
CASE: Inadvertent ureteral transection
A gynecologic surgeon operates via Pfannenstiel incision to remove a 12-cm complex left adnexal mass from a 36-year-old obese woman. When she discovers that the mass is densely adherent to the pelvic peritoneum, the surgeon incises the peritoneum lateral to the mass and opens the retroperitoneal space. However, the size and relative immobility of the mass, coupled with the low transverse incision, impair visualization of retroperitoneal structures.
The surgeon clamps and divides the ovarian vessels above the mass but, afterward, suspects that the ureter has been transected and that its ends are included within the clamps. She separates the ovarian vessels above the clamp and ligates them, at which time transection of the ureter is confirmed.
How should she proceed?
The ureter is intimately associated with the female internal genitalia in a way that challenges the gynecologic surgeon to avoid it. In a small percentage of cases involving surgical extirpation in a woman who has severe pelvic pathology, ureteral injury may be inevitable.
Several variables predispose a patient to ureteral injury, including limited exposure, as in the opening case. Others include distorted anatomy of the urinary tract relative to internal genitalia and operations that require extensive resection of pelvic tissues.
This article describes:
- prevention and intraoperative recognition of ureteral injury during gynecologic surgery
- management of intraoperatively recognized ureteral injury.
Maintain a high index of suspicion
The surgeon in the opening case has already taken the first and most important step in ensuring a good outcome: She suspected ureteral injury. In high-risk situations, intraoperative recognition of ureteral injury is more likely when the operative field is inspected thoroughly during and at the conclusion of the surgical procedure.
In a high-risk case, the combined use of intravenous indigo carmine, careful inspection of the operative field, cystoscopy, and ureteral dissection is recommended and should be routine.
Common sites of injury
During gynecologic surgery, the ureter is susceptible to injury along its entire course through the pelvis (see “The ureter takes a course fraught with hazard,”).
During adnexectomy, the gonadal vessels are generally ligated 2 to 3 cm above the adnexa. The ureter lies in close proximity to these vessels and may inadvertently be included in the ligation.
During hysterectomy, the ureter is susceptible to injury as it passes through the parametrium a short distance from the uterus and vaginal fornix.
Sutures placed in the posterior lateral cul de sac during prolapse surgery lie near the midpelvic ureter, and sutures placed during vaginal cuff closure, anterior colporrhaphy, and retropubic urethropexy are in close proximity to the trigonal portion of the ureter.
The ureter extends from the renal pelvis to the bladder, with a length that ranges from 25 to 30 cm, depending on the patient’s height. It crosses the pelvic brim near the bifurcation of the common iliac artery, where it becomes the “pelvic” ureter. The abdominal and pelvic portions of the ureter are approximately equal in length.
ILLUSTRATIONS BY ROB FLEWELL FOR OBG MANAGEMENT
The blood supply of the ureter derives from branches of the major arterial system of the lower abdomen and pelvis. These branches reach the medial aspect of the abdominal ureter and the lateral side of the pelvic ureter to form an anastomotic vascular network protected by an adventitial layer surrounding the ureter.
The ureter is attached to the posterior lateral pelvic peritoneum running dorsal to ovarian vessels. At the midpelvis, it separates from the peritoneum to pierce the base of the broad ligament underneath the uterine artery. At this point, the ureter is about 1.5 to 2 cm lateral to the uterus and curves medially and ventrally, tunneling through the cardinal and vesicovaginal ligaments to enter the bladder trigone.
Risky procedures
In gynecologic surgery, ureteral injury occurs most often during abdominal hysterectomy—probably because of how frequently this operation is performed and the range of pathology managed. The incidence of ureteral injury is much higher during abdominal hysterectomy than vaginal hysterectomy.1-4
Laparoscopic hysterectomy also has been associated with a higher incidence of ureteral injury, especially in the early phase of training.5,6 Possible explanations include:
- greater difficulty identifying the ureter
- a steeper learning curve
- more frequent use of energy to hemostatically divide pedicles, with the potential for thermal injury
- less traction–countertraction, resulting in dissection closer to the ureter
- management of complex pathology.
Although the overall incidence of ureteral injury during adnexectomy is low, it is probably much higher in women undergoing this procedure after a previous hysterectomy or in the presence of complex adnexal pathology.
When injury is likely
Compromised exposure, distorted anatomy, and certain procedures can heighten the risk of ureteral injury. Large tumors may limit the ability of the surgeon to visualize or palpate the ureter (FIGURE 1). Extensive adhesions may cause similar difficulties, and a small incision or obesity may hinder identification of pelvic sidewall structures.
A number of pathologic conditions can distort the anatomy of the ureter, especially as it relates to the female genital tract:
- Malignancies such as ovarian cancer often encroach on and occasionally encase the ureter
- Pelvic inflammatory disease, endometriosis, and a history of surgery or pelvic radiotherapy can retract and encase the ureter toward the gynecologic tract
- Some masses expand against the lower ureter, such as cervical or broad-ligament leiomyomata or placenta previa with accreta
- During vaginal hysterectomy for complete uterine prolapse, the ureters frequently extend beyond the introitus well within the operative field
- Congenital anomalies of the ureter or hydroureter can also cause distortion.
Even in the presence of relatively normal anatomy, certain procedures predispose the ureter to injury. For example, radical hysterectomy involves the almost complete separation of the pelvic ureter from the gynecologic tract and its surrounding soft tissue. When pelvic pathology is significant, the plane of dissection will always be near the ureter.
FIGURE 1 Access to the ureter is obstructed, putting it in jeopardy
Large tumors may limit the ability of the surgeon to visualize or palpate the ureter.
Prevention is the best strategy
At least 50% of ureteral injuries reported during gynecologic surgery have occurred in the absence of a recognizable risk factor.2,7 Nevertheless, knowledge of anatomy and the ability to recognize situations in which there is an elevated risk for ureteral injury will best enable the surgeon to prevent such injury.
When a high-risk situation is encountered, critical preventive steps include:
- adequate exposure
- competent assistance
- exposure of the path of the ureter through the planned course of dissection. Dissecting the ureter beyond this area is usually unnecessary and may itself cause injury.
Skip preoperative IVP in most cases
The vast majority of women who undergo gynecologic surgery do not benefit from preoperative intravenous pyelography (IVP). This measure does not appear to reduce the likelihood of ureteral injury, even in the face of obvious gynecologic disease. However, preoperative identification of obvious ureteral involvement by the disease process is useful. In such cases, the plane of dissection will probably lie closer to the ureter. One of the goals of surgery will then be to clear the urinary tract from the affected area.
When there is a high index of suspicion of an abnormality such as obstruction, intrinsic ureteral endometriosis, or congenital anomaly, preoperative IVP is indicated.
A stent may be helpful in some cases
Ureteral stents are sometimes placed in order to aid in identification and dissection of the ureters during surgery. Some authors of reports on this topic, including Hoffman, believe that stents are useful in certain situations, such as excision of an ovarian remnant, radical vaginal hysterectomy, and when pelvic organs are encased by malignant ovarian tumors. However, stents do not clearly reduce the risk of injury and, in some cases, may increase the risk by providing a false sense of security and predisposing the ureter to adventitial injury during difficult dissection.
Anticipate the effects of disease
The surgeon must have a thorough knowledge of the gynecologic disease process as it relates to surgery involving the urinary tract. For example, an ovarian remnant will almost always be somewhat densely adherent to the pelvic ureter. When severe endometriosis involves the posterior leaf of the broad ligament, the ureter will often be fibrotically retracted toward the operative field.
Certain procedures have special challenges. During resection of adnexa, for example, it is important that the ureter be identified in the retroperitoneum before the ovarian vessels are ligated. During hysterectomy, soft tissues that contain the bladder and ureters should be mobilized caudally and laterally, respectively, creating a U-shaped region (“U” for urinary tract, FIGURE 2) to which the surgeon must limit dissection.
FIGURE 2 During hysterectomy, mobilize the bladder and ureter
Mobilize the soft tissues that contain the bladder and ureters caudally and laterally, respectively, creating a U-shaped region. During division of the paracervical tissues, the surgeon must remain within this region.
Intraoperative detection
Two main types of ureteral injury occur during gynecologic surgery: transection and destruction. The latter includes ligation, crushing, devascularization, and thermal injury.
Intraoperative detection of ureteral injury is more likely when the surgeon recognizes at the outset that the operation places the ureter at increased risk. When dissection has been difficult or complicated for any reason, be concerned about possible injury.
In general, ureteral injury is first recognized by careful inspection of the surgical field. Begin by instilling 5 ml of indigo carmine intravenously. Once the dye begins to appear in the Foley catheter, inspect the area of dissection under a small amount of irrigation fluid, looking for extravasation of dye that indicates partial or complete transection.
If no injury is identified, cystoscopy is the next step. I perform all major abdominal operations with the patient in the low lithotomy position, which provides easy access to the perineum. Cystoscopic identification of urine jetting from both ureteral orifices confirms patency. When only wisps of dye are observed, it is likely that the ureter in question has been partially occluded (e.g., by acute angulation). Failure of any urine to appear from one of the orifices highly suggests injury to that ureter.
During inspection of the operative field, attempt to pass a ureteral stent into the affected orifice. If the stent passes easily and dyed urine is seen to drip freely from it, look for possible angulation of the ureter. If you find none, remove the stent and inspect the orifice again for jetting urine.
If the ureteral stent will move only a few centimeters into the ureteral orifice, ligation (with or without transection) is likely. In this case, leave the stent in place. If the operative site is readily accessible, dissect the applicable area to identify the problem. Depending on the circumstances, you may wish to infuse dye through the stent to aid in operative identification or radiographic evaluation.
Intraoperative IVP may be useful, especially when cystoscopy is unavailable.
Fundamentals of repair
Repair of major injury to the pelvic ureter is generally best accomplished by ureteroneocystostomy or, in selected cases involving injury to the proximal pelvic ureter, by ureteroureterostomy.
When intraoperatively recognized injury to the pelvic ureter appears to be minor, it can be managed by placing a ureteral stent and a closed-suction pelvic drain. Also consider wrapping the injured area with vascularized tissue such as perivesical fat. Minor lacerations can be closed perpendicular to the axis of the ureter using interrupted 4-0 delayed absorbable suture.
Most injuries to the pelvic ureter are optimally managed by ureteroneocystostomy (FIGURE 3). When a significant portion of the pelvic ureter has been lost, ureteroneocystostomy usually requires a combination of:
- extensive mobilization of the bladder
- conservative mobilization of the ureter
- elongation of the bladder
- psoas hitch.
When necessary, mobilization of the kidney with suturing of the caudal perinephric fascia to the psoas muscle will bridge an additional 2- to 3-cm gap.
Major injury to the distal half of the pelvic ureter is repaired using straightforward ureteroneocystostomy.
When there is no significant pelvic disease and the distal ureter is healthy, injury to the proximal pelvic ureter during division of the ovarian vessels may be repaired via ureteroureterostomy. If the ureteral ends will be anastomosed on tension or there is any question about the integrity of the distal portion of the ureter, as when extensive distal ureterolysis has been necessary, consider ureteroneocystostomy.
FIGURE 3 When the distal ureter is injured
Most injuries to the pelvic ureter are managed optimally by ureteroneocystostomy.
Ureter injured during emergent hysterectomy
A 37-year-old woman, para 4, undergoes her fourth repeat cesarean section. When the OB attempts to manually extract the placenta, the patient begins to hemorrhage profusely. Conservative measures fail to stop the bleeding, and the patient becomes hypotensive. The physician performs emergent hysterectomy, taking large pedicles of tissue. Although the patient stabilizes, the doctor worries that the ureters may have been injured.
Resolution: Cystoscopy is performed to check for injury. Because indigo carmine does not spill from the left ureteral orifice, the physician passes a stent with the abdomen still open, and it stops within the most distal ligamentous pedicle. Upon deligation, indigo carmine begins to drain from the stent, which then passes easily.
The stent is withdrawn to below the site of injury, and dilute methylene blue is instilled through it while the ureter is observed under irrigation. No extravasation is noted. Because the ligature had been around a block of tissue that was thought to have acutely angulated rather than incorporated the ureter, the physician concludes that severe damage is unlikely. He places a 6 French double-J stent, wraps the damaged portion of the distal ureter in perivesical fat, and places a closed-suction pelvic drain. Healing is uneventful.
Obstruction is confirmed. Now the surgeon must find it
A 45-year-old woman, para 3, who has a symptomatic 14-weeks’ size myomatous uterus, undergoes vaginal hysterectomy. The surgeon ligates and divides the uterine vessel pedicles before beginning morcellation. At the completion of the procedure, during cystoscopy, indigo carmine fails to spill from the right ureteral orifice, suggesting injury to that ureter. The surgeon passes a stent into the ureter, and it stops approximately 6 cm from the orifice. A retrograde pyelogram confirms complete obstruction.
Resolution: With the stent left in place, the surgeon performs a midline laparotomy, tracing the ureter to the uterine artery pedicle in which it has been incorporated and transected. The distal ureter with the stent is found within soft tissue lateral to the cardinal ligament pedicle, and the transected end is securely ligated using 2–0 silk suture. After the bladder is mobilized, a ureteroneocystostomy is performed. The patient recovers fully.
Postoperative management
After repair of a ureteral injury, leave a closed-suction pelvic drain in place for 2 to 3 days so that any major urinary leak can be detected; it also enhances spontaneous closure and helps prevent potentially infected fluid from accumulating in the region of anastomosis.
The cystotomy performed during ureteroneocystostomy generally heals quickly with a low risk of complications.
Leave a large-bore (20 or 22 French) urethral Foley catheter in place for 2 weeks.
I recommend that a 6 French double-J ureteral stent be left in place for 6 weeks. Potential benefits of the stent include:
- prevention of stricture
- stabilization and immobilization of the ureter during healing
- reduced risk of extravasation of urine
- reduced risk of angulation of the ureter
- isolation of the repair from infection, retroperitoneal fibrosis, and cancer.
I perform IVP approximately 1 week after stent removal to ensure ureteral patency.
CASE RESOLVED
Exposure is improved by widening the incision and dividing the tendonous insertions of the rectus abdominus muscles. The surgeon then removes the mass, preserving the distal ureter, which is estimated to be 12 cm in length and to have intact adventitia.
The surgeon performs a double-spatulated end-to-end ureteroureterostomy over a 6 French double-J ureteral stent that has been passed proximally into the renal pelvis and distally into the bladder. The stent is removed 6 weeks postoperatively, and an IVP the following week demonstrates excellent patency.
The majority of payers consider ureterolysis integral to good surgical technique, but there can be exceptions when documentation supports existing codes. Three CPT codes describe this procedure:
50715 Ureterolysis, with or without repositioning of ureter for retroperitoneal fibrosis
50722 Ureterolysis for ovarian vein syndrome
50725 Ureterolysis for retrocaval ureter, with reanastomosis of upper urinary tract or vena cava
The key to getting paid will be to document the existence of the condition indicated by each of the codes.
The ICD-9 code for both retroperitoneal fibrosis and ovarian vein syndrome is the same, 593.4 (Other ureteric obstruction). If the patient requires ureterolysis for a retrocaval ureter, the code 753.4 (Other specified anomalies of ureter) would be reported instead. Note, however, that these procedure codes cannot be reported if the ureterolysis is performed laparoscopically. In that case, the most appropriate code is 50949 (Unlisted laparoscopy procedure, ureter).
When repair is necessary, you have several codes to choose from, but the supporting diagnosis code 998.2 (Accidental puncture or laceration during a procedure) must be indicated. If a Medicare patient is involved, the surgeon who created the injury would not be paid additionally for repair.
50780 Ureteroneocystostomy; anastomosis of single ureter to bladder
50782 Ureteroneocystostomy; anastomosis of duplicated ureter to bladder
50783 Ureteroneocystostomy; with extensive ureteral tailoring
50785 Ureteroneocystostomy; with vesico-psoas hitch or bladder flap
50760 Ureteroureterostomy; fusion of ureters
50770 Transureteroureterostomy, anastomosis of ureter to contralateral ureter—MELANIE WITT, RN, CPC-OBGYN, MA
The author has no financial relationships relevant to this article.
CASE: Inadvertent ureteral transection
A gynecologic surgeon operates via Pfannenstiel incision to remove a 12-cm complex left adnexal mass from a 36-year-old obese woman. When she discovers that the mass is densely adherent to the pelvic peritoneum, the surgeon incises the peritoneum lateral to the mass and opens the retroperitoneal space. However, the size and relative immobility of the mass, coupled with the low transverse incision, impair visualization of retroperitoneal structures.
The surgeon clamps and divides the ovarian vessels above the mass but, afterward, suspects that the ureter has been transected and that its ends are included within the clamps. She separates the ovarian vessels above the clamp and ligates them, at which time transection of the ureter is confirmed.
How should she proceed?
The ureter is intimately associated with the female internal genitalia in a way that challenges the gynecologic surgeon to avoid it. In a small percentage of cases involving surgical extirpation in a woman who has severe pelvic pathology, ureteral injury may be inevitable.
Several variables predispose a patient to ureteral injury, including limited exposure, as in the opening case. Others include distorted anatomy of the urinary tract relative to internal genitalia and operations that require extensive resection of pelvic tissues.
This article describes:
- prevention and intraoperative recognition of ureteral injury during gynecologic surgery
- management of intraoperatively recognized ureteral injury.
Maintain a high index of suspicion
The surgeon in the opening case has already taken the first and most important step in ensuring a good outcome: She suspected ureteral injury. In high-risk situations, intraoperative recognition of ureteral injury is more likely when the operative field is inspected thoroughly during and at the conclusion of the surgical procedure.
In a high-risk case, the combined use of intravenous indigo carmine, careful inspection of the operative field, cystoscopy, and ureteral dissection is recommended and should be routine.
Common sites of injury
During gynecologic surgery, the ureter is susceptible to injury along its entire course through the pelvis (see “The ureter takes a course fraught with hazard,”).
During adnexectomy, the gonadal vessels are generally ligated 2 to 3 cm above the adnexa. The ureter lies in close proximity to these vessels and may inadvertently be included in the ligation.
During hysterectomy, the ureter is susceptible to injury as it passes through the parametrium a short distance from the uterus and vaginal fornix.
Sutures placed in the posterior lateral cul de sac during prolapse surgery lie near the midpelvic ureter, and sutures placed during vaginal cuff closure, anterior colporrhaphy, and retropubic urethropexy are in close proximity to the trigonal portion of the ureter.
The ureter extends from the renal pelvis to the bladder, with a length that ranges from 25 to 30 cm, depending on the patient’s height. It crosses the pelvic brim near the bifurcation of the common iliac artery, where it becomes the “pelvic” ureter. The abdominal and pelvic portions of the ureter are approximately equal in length.
ILLUSTRATIONS BY ROB FLEWELL FOR OBG MANAGEMENT
The blood supply of the ureter derives from branches of the major arterial system of the lower abdomen and pelvis. These branches reach the medial aspect of the abdominal ureter and the lateral side of the pelvic ureter to form an anastomotic vascular network protected by an adventitial layer surrounding the ureter.
The ureter is attached to the posterior lateral pelvic peritoneum running dorsal to ovarian vessels. At the midpelvis, it separates from the peritoneum to pierce the base of the broad ligament underneath the uterine artery. At this point, the ureter is about 1.5 to 2 cm lateral to the uterus and curves medially and ventrally, tunneling through the cardinal and vesicovaginal ligaments to enter the bladder trigone.
Risky procedures
In gynecologic surgery, ureteral injury occurs most often during abdominal hysterectomy—probably because of how frequently this operation is performed and the range of pathology managed. The incidence of ureteral injury is much higher during abdominal hysterectomy than vaginal hysterectomy.1-4
Laparoscopic hysterectomy also has been associated with a higher incidence of ureteral injury, especially in the early phase of training.5,6 Possible explanations include:
- greater difficulty identifying the ureter
- a steeper learning curve
- more frequent use of energy to hemostatically divide pedicles, with the potential for thermal injury
- less traction–countertraction, resulting in dissection closer to the ureter
- management of complex pathology.
Although the overall incidence of ureteral injury during adnexectomy is low, it is probably much higher in women undergoing this procedure after a previous hysterectomy or in the presence of complex adnexal pathology.
When injury is likely
Compromised exposure, distorted anatomy, and certain procedures can heighten the risk of ureteral injury. Large tumors may limit the ability of the surgeon to visualize or palpate the ureter (FIGURE 1). Extensive adhesions may cause similar difficulties, and a small incision or obesity may hinder identification of pelvic sidewall structures.
A number of pathologic conditions can distort the anatomy of the ureter, especially as it relates to the female genital tract:
- Malignancies such as ovarian cancer often encroach on and occasionally encase the ureter
- Pelvic inflammatory disease, endometriosis, and a history of surgery or pelvic radiotherapy can retract and encase the ureter toward the gynecologic tract
- Some masses expand against the lower ureter, such as cervical or broad-ligament leiomyomata or placenta previa with accreta
- During vaginal hysterectomy for complete uterine prolapse, the ureters frequently extend beyond the introitus well within the operative field
- Congenital anomalies of the ureter or hydroureter can also cause distortion.
Even in the presence of relatively normal anatomy, certain procedures predispose the ureter to injury. For example, radical hysterectomy involves the almost complete separation of the pelvic ureter from the gynecologic tract and its surrounding soft tissue. When pelvic pathology is significant, the plane of dissection will always be near the ureter.
FIGURE 1 Access to the ureter is obstructed, putting it in jeopardy
Large tumors may limit the ability of the surgeon to visualize or palpate the ureter.
Prevention is the best strategy
At least 50% of ureteral injuries reported during gynecologic surgery have occurred in the absence of a recognizable risk factor.2,7 Nevertheless, knowledge of anatomy and the ability to recognize situations in which there is an elevated risk for ureteral injury will best enable the surgeon to prevent such injury.
When a high-risk situation is encountered, critical preventive steps include:
- adequate exposure
- competent assistance
- exposure of the path of the ureter through the planned course of dissection. Dissecting the ureter beyond this area is usually unnecessary and may itself cause injury.
Skip preoperative IVP in most cases
The vast majority of women who undergo gynecologic surgery do not benefit from preoperative intravenous pyelography (IVP). This measure does not appear to reduce the likelihood of ureteral injury, even in the face of obvious gynecologic disease. However, preoperative identification of obvious ureteral involvement by the disease process is useful. In such cases, the plane of dissection will probably lie closer to the ureter. One of the goals of surgery will then be to clear the urinary tract from the affected area.
When there is a high index of suspicion of an abnormality such as obstruction, intrinsic ureteral endometriosis, or congenital anomaly, preoperative IVP is indicated.
A stent may be helpful in some cases
Ureteral stents are sometimes placed in order to aid in identification and dissection of the ureters during surgery. Some authors of reports on this topic, including Hoffman, believe that stents are useful in certain situations, such as excision of an ovarian remnant, radical vaginal hysterectomy, and when pelvic organs are encased by malignant ovarian tumors. However, stents do not clearly reduce the risk of injury and, in some cases, may increase the risk by providing a false sense of security and predisposing the ureter to adventitial injury during difficult dissection.
Anticipate the effects of disease
The surgeon must have a thorough knowledge of the gynecologic disease process as it relates to surgery involving the urinary tract. For example, an ovarian remnant will almost always be somewhat densely adherent to the pelvic ureter. When severe endometriosis involves the posterior leaf of the broad ligament, the ureter will often be fibrotically retracted toward the operative field.
Certain procedures have special challenges. During resection of adnexa, for example, it is important that the ureter be identified in the retroperitoneum before the ovarian vessels are ligated. During hysterectomy, soft tissues that contain the bladder and ureters should be mobilized caudally and laterally, respectively, creating a U-shaped region (“U” for urinary tract, FIGURE 2) to which the surgeon must limit dissection.
FIGURE 2 During hysterectomy, mobilize the bladder and ureter
Mobilize the soft tissues that contain the bladder and ureters caudally and laterally, respectively, creating a U-shaped region. During division of the paracervical tissues, the surgeon must remain within this region.
Intraoperative detection
Two main types of ureteral injury occur during gynecologic surgery: transection and destruction. The latter includes ligation, crushing, devascularization, and thermal injury.
Intraoperative detection of ureteral injury is more likely when the surgeon recognizes at the outset that the operation places the ureter at increased risk. When dissection has been difficult or complicated for any reason, be concerned about possible injury.
In general, ureteral injury is first recognized by careful inspection of the surgical field. Begin by instilling 5 ml of indigo carmine intravenously. Once the dye begins to appear in the Foley catheter, inspect the area of dissection under a small amount of irrigation fluid, looking for extravasation of dye that indicates partial or complete transection.
If no injury is identified, cystoscopy is the next step. I perform all major abdominal operations with the patient in the low lithotomy position, which provides easy access to the perineum. Cystoscopic identification of urine jetting from both ureteral orifices confirms patency. When only wisps of dye are observed, it is likely that the ureter in question has been partially occluded (e.g., by acute angulation). Failure of any urine to appear from one of the orifices highly suggests injury to that ureter.
During inspection of the operative field, attempt to pass a ureteral stent into the affected orifice. If the stent passes easily and dyed urine is seen to drip freely from it, look for possible angulation of the ureter. If you find none, remove the stent and inspect the orifice again for jetting urine.
If the ureteral stent will move only a few centimeters into the ureteral orifice, ligation (with or without transection) is likely. In this case, leave the stent in place. If the operative site is readily accessible, dissect the applicable area to identify the problem. Depending on the circumstances, you may wish to infuse dye through the stent to aid in operative identification or radiographic evaluation.
Intraoperative IVP may be useful, especially when cystoscopy is unavailable.
Fundamentals of repair
Repair of major injury to the pelvic ureter is generally best accomplished by ureteroneocystostomy or, in selected cases involving injury to the proximal pelvic ureter, by ureteroureterostomy.
When intraoperatively recognized injury to the pelvic ureter appears to be minor, it can be managed by placing a ureteral stent and a closed-suction pelvic drain. Also consider wrapping the injured area with vascularized tissue such as perivesical fat. Minor lacerations can be closed perpendicular to the axis of the ureter using interrupted 4-0 delayed absorbable suture.
Most injuries to the pelvic ureter are optimally managed by ureteroneocystostomy (FIGURE 3). When a significant portion of the pelvic ureter has been lost, ureteroneocystostomy usually requires a combination of:
- extensive mobilization of the bladder
- conservative mobilization of the ureter
- elongation of the bladder
- psoas hitch.
When necessary, mobilization of the kidney with suturing of the caudal perinephric fascia to the psoas muscle will bridge an additional 2- to 3-cm gap.
Major injury to the distal half of the pelvic ureter is repaired using straightforward ureteroneocystostomy.
When there is no significant pelvic disease and the distal ureter is healthy, injury to the proximal pelvic ureter during division of the ovarian vessels may be repaired via ureteroureterostomy. If the ureteral ends will be anastomosed on tension or there is any question about the integrity of the distal portion of the ureter, as when extensive distal ureterolysis has been necessary, consider ureteroneocystostomy.
FIGURE 3 When the distal ureter is injured
Most injuries to the pelvic ureter are managed optimally by ureteroneocystostomy.
Ureter injured during emergent hysterectomy
A 37-year-old woman, para 4, undergoes her fourth repeat cesarean section. When the OB attempts to manually extract the placenta, the patient begins to hemorrhage profusely. Conservative measures fail to stop the bleeding, and the patient becomes hypotensive. The physician performs emergent hysterectomy, taking large pedicles of tissue. Although the patient stabilizes, the doctor worries that the ureters may have been injured.
Resolution: Cystoscopy is performed to check for injury. Because indigo carmine does not spill from the left ureteral orifice, the physician passes a stent with the abdomen still open, and it stops within the most distal ligamentous pedicle. Upon deligation, indigo carmine begins to drain from the stent, which then passes easily.
The stent is withdrawn to below the site of injury, and dilute methylene blue is instilled through it while the ureter is observed under irrigation. No extravasation is noted. Because the ligature had been around a block of tissue that was thought to have acutely angulated rather than incorporated the ureter, the physician concludes that severe damage is unlikely. He places a 6 French double-J stent, wraps the damaged portion of the distal ureter in perivesical fat, and places a closed-suction pelvic drain. Healing is uneventful.
Obstruction is confirmed. Now the surgeon must find it
A 45-year-old woman, para 3, who has a symptomatic 14-weeks’ size myomatous uterus, undergoes vaginal hysterectomy. The surgeon ligates and divides the uterine vessel pedicles before beginning morcellation. At the completion of the procedure, during cystoscopy, indigo carmine fails to spill from the right ureteral orifice, suggesting injury to that ureter. The surgeon passes a stent into the ureter, and it stops approximately 6 cm from the orifice. A retrograde pyelogram confirms complete obstruction.
Resolution: With the stent left in place, the surgeon performs a midline laparotomy, tracing the ureter to the uterine artery pedicle in which it has been incorporated and transected. The distal ureter with the stent is found within soft tissue lateral to the cardinal ligament pedicle, and the transected end is securely ligated using 2–0 silk suture. After the bladder is mobilized, a ureteroneocystostomy is performed. The patient recovers fully.
Postoperative management
After repair of a ureteral injury, leave a closed-suction pelvic drain in place for 2 to 3 days so that any major urinary leak can be detected; it also enhances spontaneous closure and helps prevent potentially infected fluid from accumulating in the region of anastomosis.
The cystotomy performed during ureteroneocystostomy generally heals quickly with a low risk of complications.
Leave a large-bore (20 or 22 French) urethral Foley catheter in place for 2 weeks.
I recommend that a 6 French double-J ureteral stent be left in place for 6 weeks. Potential benefits of the stent include:
- prevention of stricture
- stabilization and immobilization of the ureter during healing
- reduced risk of extravasation of urine
- reduced risk of angulation of the ureter
- isolation of the repair from infection, retroperitoneal fibrosis, and cancer.
I perform IVP approximately 1 week after stent removal to ensure ureteral patency.
CASE RESOLVED
Exposure is improved by widening the incision and dividing the tendonous insertions of the rectus abdominus muscles. The surgeon then removes the mass, preserving the distal ureter, which is estimated to be 12 cm in length and to have intact adventitia.
The surgeon performs a double-spatulated end-to-end ureteroureterostomy over a 6 French double-J ureteral stent that has been passed proximally into the renal pelvis and distally into the bladder. The stent is removed 6 weeks postoperatively, and an IVP the following week demonstrates excellent patency.
The majority of payers consider ureterolysis integral to good surgical technique, but there can be exceptions when documentation supports existing codes. Three CPT codes describe this procedure:
50715 Ureterolysis, with or without repositioning of ureter for retroperitoneal fibrosis
50722 Ureterolysis for ovarian vein syndrome
50725 Ureterolysis for retrocaval ureter, with reanastomosis of upper urinary tract or vena cava
The key to getting paid will be to document the existence of the condition indicated by each of the codes.
The ICD-9 code for both retroperitoneal fibrosis and ovarian vein syndrome is the same, 593.4 (Other ureteric obstruction). If the patient requires ureterolysis for a retrocaval ureter, the code 753.4 (Other specified anomalies of ureter) would be reported instead. Note, however, that these procedure codes cannot be reported if the ureterolysis is performed laparoscopically. In that case, the most appropriate code is 50949 (Unlisted laparoscopy procedure, ureter).
When repair is necessary, you have several codes to choose from, but the supporting diagnosis code 998.2 (Accidental puncture or laceration during a procedure) must be indicated. If a Medicare patient is involved, the surgeon who created the injury would not be paid additionally for repair.
50780 Ureteroneocystostomy; anastomosis of single ureter to bladder
50782 Ureteroneocystostomy; anastomosis of duplicated ureter to bladder
50783 Ureteroneocystostomy; with extensive ureteral tailoring
50785 Ureteroneocystostomy; with vesico-psoas hitch or bladder flap
50760 Ureteroureterostomy; fusion of ureters
50770 Transureteroureterostomy, anastomosis of ureter to contralateral ureter—MELANIE WITT, RN, CPC-OBGYN, MA
1. St. Lezin MA, Stoller ML. Surgical ureteral injuries. Urology. 1991;38:497-506.
2. Liapis A, Bakas P, Giannopoulos V, Creatsas G. Ureteral injuries during gynecological surgery. Int Urogynecol J Pelvic Floor Dysfunct. 2001;12:391-394.
3. Vakili B, Chesson RR, Kyle BL, et al. The incidence of urinary tract injury during hysterectomy: a prospective analysis based on universal cystoscopy. Am J Obstet Gynecol. 2005;192:1599-1604.
4. Sakellariou P, Protopapas AG, Voulgaris Z, et al. Management of ureteric injuries during gynecological operations: 10 years experience. Eur J Obstet Gynecol Reprod Biol. 2002;101:179-184.
5. Assimos DG, Patterson LC, Taylor CL. Changing incidence and etiology of iatrogenic ureteral injuries. J Urol. 1994;152:2240-2246.
6. Härkki-Sirén P, Sjöberg J, Titinen A. Urinary tract injuries after hysterectomy. Obstet Gynecol. 1998;92:113-118.
7. Chan JK, Morrow J, Manetta A. Prevention of ureteral injuries in gynecologic surgery. Am J Obstet Gynecol. 2003;188:1273-1277.
1. St. Lezin MA, Stoller ML. Surgical ureteral injuries. Urology. 1991;38:497-506.
2. Liapis A, Bakas P, Giannopoulos V, Creatsas G. Ureteral injuries during gynecological surgery. Int Urogynecol J Pelvic Floor Dysfunct. 2001;12:391-394.
3. Vakili B, Chesson RR, Kyle BL, et al. The incidence of urinary tract injury during hysterectomy: a prospective analysis based on universal cystoscopy. Am J Obstet Gynecol. 2005;192:1599-1604.
4. Sakellariou P, Protopapas AG, Voulgaris Z, et al. Management of ureteric injuries during gynecological operations: 10 years experience. Eur J Obstet Gynecol Reprod Biol. 2002;101:179-184.
5. Assimos DG, Patterson LC, Taylor CL. Changing incidence and etiology of iatrogenic ureteral injuries. J Urol. 1994;152:2240-2246.
6. Härkki-Sirén P, Sjöberg J, Titinen A. Urinary tract injuries after hysterectomy. Obstet Gynecol. 1998;92:113-118.
7. Chan JK, Morrow J, Manetta A. Prevention of ureteral injuries in gynecologic surgery. Am J Obstet Gynecol. 2003;188:1273-1277.
Rabies Vaccine Supply Issue: Facts Are Key
The current limitation of the rabies vaccine supply presents an urgent, but not emergent, situation. In fact, nothing has changed regarding the indications for the vaccine's use. However, the supply issue does underscore the need for judicious use and careful attention to information gathering.
At this time, rabies vaccine is limited to postexposure prophylaxis and is not being given to travelers or individuals with occupational exposure risk. On Oct. 8, the Centers for Disease Control and Prevention announced that Novartis has collaborated with public health and government officials to provide additional supplies of RabAvert vaccine for postexposure prophylaxis without the need for a pass code or other restrictions. (Questions can be directed to Novartis customer service at 1-800-244-7668.)
A pass code is still required to receive Sanofi Pasteur Inc.'s IMOVAX. To obtain IMOVAX rabies vaccine, you must first contact your rabies state health official to conduct a risk assessment for the suspected exposure. (A list of those officials, along with the latest supply updates, is available at www.cdc.gov/rabies
The required form has specific information that should be collected so that an appropriate decision can be made for each patient. Basic information includes details regarding the animal species, the bite circumstances, and local rabies epidemiology. Even when the rabies vaccine supply is back to normal, practitioners will continue to be responsible for obtaining the relevant data that are necessary for making a decision about vaccine.
Children's Mercy Hospital has developed an easy-to-use form that practitioners can utilize now and in the future. Our infectious disease section data analyst, Josh Herigon, helped develop our current form, which can be accessed at http://www.childrensmercy.org/rabiesform
Parents of children who have had an animal bite are usually highly anxious and need to know that you are collecting all relevant information so that appropriate postexposure prophylaxis occurs in a timely fashion. In cases in which rabies postexposure prophylaxis is not recommended, parents need to understand the rationale for that decision.
Other key concepts include the following:
▸ Attempts should be made to recover the animal in all cases of exposure or possible exposure. If the animal is in a high-risk category, it should be immediately referred for rabies testing. Domestic animals that are acting normally should be observed, and referred for testing if they begin to exhibit abnormal behavior. Postexposure prophylaxis can be safely delayed for this period of time.
▸ If the animal can't be recovered, the next step depends upon the information you've gathered. If the animal is in the high-risk category and there was a bite wound, then postexposure prophylaxis—comprising both vaccine and rabies immune globulin—must be initiated.
▸ On the other hand, bites from low-risk animals that have escaped will rarely require vaccination. Indeed, the animal's ability to escape is a sign of noninfection, because a rabid animal is usually very sick and typically won't be able to make a quick getaway. Knowing whether the attack was provoked or not is also helpful, as a provoked animal is far less likely to be infected than is one that attacks for no apparent reason. Low-risk animals rarely carry rabies, and have never been documented to transmit it to a human in the United States.
▸ In an intermediate-risk situation, such as a dog bite in which the dog escapes, information such as the local rabies rates, the type and severity of the wound, and whether or not the attack was provoked will help you make the decision regarding whether or not to vaccinate. Again, consult with your local health officials or infectious disease specialist.
▸ Seeing a bat in the house commonly arouses concern about rabies. In the case of a preverbal child or an impaired (for example, drunk) adult who has no noticeable bite wound but who nevertheless may have been exposed, animal control should be called to capture the animal, and arrangements should be made with the local health department for rabies testing. If the bat cannot be captured, immunization plus rabies immune globulin is necessary. Approximately 5% of bats in the United States are rabid.
▸ Rabies vaccine is given intramuscularly on days 0, 3, 7, 14, and 28. The same dosage is used for both children and adults, but the injection is given in the deltoid in adults and in the anterolateral thigh in infants and children. Although primary care physicians don't typically administer rabies vaccine, it's important to educate patients about what's in store.
▸ Wound cleansing is extremely important. Irrigation (except in the case of puncture wounds), tetanus vaccination, antibiotic prophylaxis in appropriate cases, and wound closure when indicated are all essential. Animal studies suggest that wound cleansing reduces the chance of viral transmission.
▸ Officials at the CDC anticipate that the rabies vaccine supply will be fully restored in mid-2009, when Sanofi Pasteur's manufacturing facility in France is expected to be reopened. It was the scheduled closing of that facility in June 2007—combined with Novartis' inability to meet the remaining market demand—that resulted in the current supply problems. Hopefully, if we continue to practice judicious use of the vaccine even after the supply is restored, we can prevent a similar situation from reoccurring.
By the way, those of you practicing in Hawaii don't need to worry. Yours is the only U.S. state that has never had a documented case of rabies.
The current limitation of the rabies vaccine supply presents an urgent, but not emergent, situation. In fact, nothing has changed regarding the indications for the vaccine's use. However, the supply issue does underscore the need for judicious use and careful attention to information gathering.
At this time, rabies vaccine is limited to postexposure prophylaxis and is not being given to travelers or individuals with occupational exposure risk. On Oct. 8, the Centers for Disease Control and Prevention announced that Novartis has collaborated with public health and government officials to provide additional supplies of RabAvert vaccine for postexposure prophylaxis without the need for a pass code or other restrictions. (Questions can be directed to Novartis customer service at 1-800-244-7668.)
A pass code is still required to receive Sanofi Pasteur Inc.'s IMOVAX. To obtain IMOVAX rabies vaccine, you must first contact your rabies state health official to conduct a risk assessment for the suspected exposure. (A list of those officials, along with the latest supply updates, is available at www.cdc.gov/rabies
The required form has specific information that should be collected so that an appropriate decision can be made for each patient. Basic information includes details regarding the animal species, the bite circumstances, and local rabies epidemiology. Even when the rabies vaccine supply is back to normal, practitioners will continue to be responsible for obtaining the relevant data that are necessary for making a decision about vaccine.
Children's Mercy Hospital has developed an easy-to-use form that practitioners can utilize now and in the future. Our infectious disease section data analyst, Josh Herigon, helped develop our current form, which can be accessed at http://www.childrensmercy.org/rabiesform
Parents of children who have had an animal bite are usually highly anxious and need to know that you are collecting all relevant information so that appropriate postexposure prophylaxis occurs in a timely fashion. In cases in which rabies postexposure prophylaxis is not recommended, parents need to understand the rationale for that decision.
Other key concepts include the following:
▸ Attempts should be made to recover the animal in all cases of exposure or possible exposure. If the animal is in a high-risk category, it should be immediately referred for rabies testing. Domestic animals that are acting normally should be observed, and referred for testing if they begin to exhibit abnormal behavior. Postexposure prophylaxis can be safely delayed for this period of time.
▸ If the animal can't be recovered, the next step depends upon the information you've gathered. If the animal is in the high-risk category and there was a bite wound, then postexposure prophylaxis—comprising both vaccine and rabies immune globulin—must be initiated.
▸ On the other hand, bites from low-risk animals that have escaped will rarely require vaccination. Indeed, the animal's ability to escape is a sign of noninfection, because a rabid animal is usually very sick and typically won't be able to make a quick getaway. Knowing whether the attack was provoked or not is also helpful, as a provoked animal is far less likely to be infected than is one that attacks for no apparent reason. Low-risk animals rarely carry rabies, and have never been documented to transmit it to a human in the United States.
▸ In an intermediate-risk situation, such as a dog bite in which the dog escapes, information such as the local rabies rates, the type and severity of the wound, and whether or not the attack was provoked will help you make the decision regarding whether or not to vaccinate. Again, consult with your local health officials or infectious disease specialist.
▸ Seeing a bat in the house commonly arouses concern about rabies. In the case of a preverbal child or an impaired (for example, drunk) adult who has no noticeable bite wound but who nevertheless may have been exposed, animal control should be called to capture the animal, and arrangements should be made with the local health department for rabies testing. If the bat cannot be captured, immunization plus rabies immune globulin is necessary. Approximately 5% of bats in the United States are rabid.
▸ Rabies vaccine is given intramuscularly on days 0, 3, 7, 14, and 28. The same dosage is used for both children and adults, but the injection is given in the deltoid in adults and in the anterolateral thigh in infants and children. Although primary care physicians don't typically administer rabies vaccine, it's important to educate patients about what's in store.
▸ Wound cleansing is extremely important. Irrigation (except in the case of puncture wounds), tetanus vaccination, antibiotic prophylaxis in appropriate cases, and wound closure when indicated are all essential. Animal studies suggest that wound cleansing reduces the chance of viral transmission.
▸ Officials at the CDC anticipate that the rabies vaccine supply will be fully restored in mid-2009, when Sanofi Pasteur's manufacturing facility in France is expected to be reopened. It was the scheduled closing of that facility in June 2007—combined with Novartis' inability to meet the remaining market demand—that resulted in the current supply problems. Hopefully, if we continue to practice judicious use of the vaccine even after the supply is restored, we can prevent a similar situation from reoccurring.
By the way, those of you practicing in Hawaii don't need to worry. Yours is the only U.S. state that has never had a documented case of rabies.
The current limitation of the rabies vaccine supply presents an urgent, but not emergent, situation. In fact, nothing has changed regarding the indications for the vaccine's use. However, the supply issue does underscore the need for judicious use and careful attention to information gathering.
At this time, rabies vaccine is limited to postexposure prophylaxis and is not being given to travelers or individuals with occupational exposure risk. On Oct. 8, the Centers for Disease Control and Prevention announced that Novartis has collaborated with public health and government officials to provide additional supplies of RabAvert vaccine for postexposure prophylaxis without the need for a pass code or other restrictions. (Questions can be directed to Novartis customer service at 1-800-244-7668.)
A pass code is still required to receive Sanofi Pasteur Inc.'s IMOVAX. To obtain IMOVAX rabies vaccine, you must first contact your rabies state health official to conduct a risk assessment for the suspected exposure. (A list of those officials, along with the latest supply updates, is available at www.cdc.gov/rabies
The required form has specific information that should be collected so that an appropriate decision can be made for each patient. Basic information includes details regarding the animal species, the bite circumstances, and local rabies epidemiology. Even when the rabies vaccine supply is back to normal, practitioners will continue to be responsible for obtaining the relevant data that are necessary for making a decision about vaccine.
Children's Mercy Hospital has developed an easy-to-use form that practitioners can utilize now and in the future. Our infectious disease section data analyst, Josh Herigon, helped develop our current form, which can be accessed at http://www.childrensmercy.org/rabiesform
Parents of children who have had an animal bite are usually highly anxious and need to know that you are collecting all relevant information so that appropriate postexposure prophylaxis occurs in a timely fashion. In cases in which rabies postexposure prophylaxis is not recommended, parents need to understand the rationale for that decision.
Other key concepts include the following:
▸ Attempts should be made to recover the animal in all cases of exposure or possible exposure. If the animal is in a high-risk category, it should be immediately referred for rabies testing. Domestic animals that are acting normally should be observed, and referred for testing if they begin to exhibit abnormal behavior. Postexposure prophylaxis can be safely delayed for this period of time.
▸ If the animal can't be recovered, the next step depends upon the information you've gathered. If the animal is in the high-risk category and there was a bite wound, then postexposure prophylaxis—comprising both vaccine and rabies immune globulin—must be initiated.
▸ On the other hand, bites from low-risk animals that have escaped will rarely require vaccination. Indeed, the animal's ability to escape is a sign of noninfection, because a rabid animal is usually very sick and typically won't be able to make a quick getaway. Knowing whether the attack was provoked or not is also helpful, as a provoked animal is far less likely to be infected than is one that attacks for no apparent reason. Low-risk animals rarely carry rabies, and have never been documented to transmit it to a human in the United States.
▸ In an intermediate-risk situation, such as a dog bite in which the dog escapes, information such as the local rabies rates, the type and severity of the wound, and whether or not the attack was provoked will help you make the decision regarding whether or not to vaccinate. Again, consult with your local health officials or infectious disease specialist.
▸ Seeing a bat in the house commonly arouses concern about rabies. In the case of a preverbal child or an impaired (for example, drunk) adult who has no noticeable bite wound but who nevertheless may have been exposed, animal control should be called to capture the animal, and arrangements should be made with the local health department for rabies testing. If the bat cannot be captured, immunization plus rabies immune globulin is necessary. Approximately 5% of bats in the United States are rabid.
▸ Rabies vaccine is given intramuscularly on days 0, 3, 7, 14, and 28. The same dosage is used for both children and adults, but the injection is given in the deltoid in adults and in the anterolateral thigh in infants and children. Although primary care physicians don't typically administer rabies vaccine, it's important to educate patients about what's in store.
▸ Wound cleansing is extremely important. Irrigation (except in the case of puncture wounds), tetanus vaccination, antibiotic prophylaxis in appropriate cases, and wound closure when indicated are all essential. Animal studies suggest that wound cleansing reduces the chance of viral transmission.
▸ Officials at the CDC anticipate that the rabies vaccine supply will be fully restored in mid-2009, when Sanofi Pasteur's manufacturing facility in France is expected to be reopened. It was the scheduled closing of that facility in June 2007—combined with Novartis' inability to meet the remaining market demand—that resulted in the current supply problems. Hopefully, if we continue to practice judicious use of the vaccine even after the supply is restored, we can prevent a similar situation from reoccurring.
By the way, those of you practicing in Hawaii don't need to worry. Yours is the only U.S. state that has never had a documented case of rabies.
Glycemic Control Team Business Case
Implementation of targeted inpatient glycemic control by a multidisciplinary team in the hospital is a time‐intensive and labor‐intensive undertaking. A variety of business models may be applied including those in which the hospital provides financial support and those which will be self‐supporting through clinical billing revenues (Table 1). In practice, a combination of these strategies may be used.
| Model | Strategy | Target |
|---|---|---|
| A. Hospital‐Supported | 1. Improve documented patient acuity | Improve accuracy of documentation and coding of: |
| a. uncontrolled diabetes (DM); | ||
| b. unrecognized DM; | ||
| c. DM complications | ||
| 2. Increase capacity and denied payment for readmissions | Reduction in overall length of stay and readmission rates | |
| 3. Optimize resource utilization | Reduction in morbidity and mortality and reduction in intensive care unit length of stay | |
| B. Self‐Supported | 1. Allied health professional billings (NP, PA) | Salary offset through income generation |
| 2. Physician billings |
In order to make the case to hospital administration for investment of financial support for glycemic control programs, it is necessary to develop a business plan that will justify return on investment. The following discussion is a composite of information from the published literature, personal communication with hospital glycemic control champions, and the author's experience developing, implementing, and assessing outcomes for a glycemic control task force charged with improving glucose control in the 7 hospital MedStar Health system from which more than 45,000 patients are discharged annually with a diagnosis of diabetes.
The principles for making the business case for support of a glycemic control team are the same as those applied in other hospital‐based programs. While the clinical argument for a new process, resource, or staff member may be sound, the business plan will also be analyzed from nonclinical perspectives, with attention to its fiscal and operational feasibility. It is therefore important to involve hospital administration, operations, and finance representatives early, in order to obtain their support and address concerns early that would otherwise weaken the request. The information provided in this article is intended to provide practical guidance for developing a plan that is realistic and tailored to the needs of the individual institution.
A. HOSPITAL‐SUPPORTED MODELS
The business case for hospital support for the glycemic control team is based on opportunities to improve revenue through accurate documentation and coding, reduction in length of stay, and optimization of resource utilization through reduction in morbidity and mortality (cost aversion).
In order to understand opportunities for improved revenue, it is necessary to understand how hospitals are reimbursed for inpatient care. Medical record documentation is reviewed by hospital coding personnel who apply strict guidelines and ICD‐9‐CM nomenclature to establish the diagnoses and procedures for each case. The ICD‐9‐CM is a numerical coding scheme of more than 13,000 diagnoses and 5,000 procedures. The principal and secondary diagnoses and procedures are grouped into a Diagnosis Related Group (DRG) code. In most states, CMS (Medicare) reimburses hospitals a flat rate based on the DRG code assigned to each inpatient case. CMS calculates a specific DRG weight for each DRG code. The DRG weight is a number such as 0.895 or 1.24, which reflects the acuity of the patient assigned to a particular DRG code and expected resource utilization. The greater the weight, the greater the acuity and expected resource utilization. The overall Case Mix Index (CMI) is the simple average of the DRG weight for a population of inpatients. CMI indicates the relative severity of a patient population and is directly proportional to DRG payments. This index is valuable when making comparisons between hospitals or patient groups.
Each hospital is provided a single rate relative to a DRG weight of 1.0 which allows calculation of the expected revenue from CMS per case. The DRG payment for a Medicare patient is determined by multiplying the relative weight for the DRG by the hospital's blended rate: DRG PAYMENT = WEIGHT RATE. Each hospital's payment rate is defined by federal regulations and is updated annually to reflect inflation, technical adjustments, and budgetary constraints. There are separate rate calculations for large urban hospitals and other hospitals. There are also technical adjustments for local wage variations, teaching hospitals, and hospitals with a disproportionate share of indigent patients.1
Determination of allowable charges for inpatient care varies by location and payor. The State of Maryland, for example regulates hospital inpatient charges for all patients based on the CMI of each hospital. In this reimbursement system, the hospital must ensure that their total average charge per case is on par with their reported CMI, when compared to other hospitals. For non‐Medicare payors, reimbursement may also be based on a set DRG payment or a percentage of charges based on contractual stipulations. The business case will be based on the individual hospital's allowed charges.
Empirical studies in this area are lacking, perhaps in part because this is a sensitive data area. Publications tend to focus on potential rather than actual results. Profiling hospital improvement management performance for documentation, coding and reimbursement does allow hospitals to go beyond focused operational metrics to evaluate themselves in a broad industry context to determine whether they are in alignment with examples from other institutions.2
Given this background, specific strategies used to justify hospital support for the glycemic control will now be outlined.
1. Documentation Opportunities
The hospital glycemic control team can help optimize reimbursement through improved accuracy of physician documentation and of coding. Each opportunity should be determined in collaboration with the coding and finance departments. Education for providers and coders must be ongoing, especially in hospitals where staff turnover is common. It should also be made clear that coders cannot specify a diagnosis unless the provider has documented it in the chart. Some initiatives incorporate notifying providers when a documentation deficiency is identified in order to offer an opportunity to provide clarification.
Establishing a definition for each coding designation for diabetes will enable collection of specific data upon which the business case can be built.
a. Accuracy of Designating Diabetes as Controlled or Uncontrolled
The glycemic control team should come to a consensus on a definition of uncontrolled diabetes. In the absence of accepted criteria for this designation, the following descriptions are an attempt to provide some guidance. They are derived from the ICD‐9‐CM Professional 6th edition3 and the American Diabetes Association (ADA) article on management of diabetes and hyperglycemia in the hospital4
-
A nonspecific term indicating that the treatment regimen does not keep the blood sugar level of a patient within acceptable limits3
-
Admission BG over 180(200) mg/dL or 2 or more BG during the hospital stay over 180(200) mg/dL
-
Lesser persistent hyperglycemia outside guidelines set by AACE and ADA for hospital management, eg, fasting BG over 110 mg/dL and other BGs over 180 mg/dL on noncritical care units, could also be considered consistent with uncontrolled diabetes.
The modifier uncontrolled only applies when the patient has a known diagnosis of diabetes. CMS has recently revised reimbursement guidelines for uncontrolled diabetes. This designation no longer provides an incremental increase in reimbursement; however, it is still weighted in the determination of case mix index.
b. Unrecognized Diabetes
The key feature of this designation is that the diabetes is either unrecognized by the treating provider or is not clearly documented as diabetes in the medical record during the hospital stay. Beyond these key features, there are no clearly defined criteria for unrecognized diabetes. It is again contingent on the hospital team, including the coding specialists, to decide on blood glucose thresholds that will be applied to identify those cases which are to be designated unrecognized diabetes. Daily reports of patients with hyperglycemia could be generated in order to alert providers about its presence.
There is a paucity of data to guide us in defining a new diagnosis of diabetes in the hospital and multiple variables associated with the stress of illness and hospitalization are known to impact glucose tolerance. However, it seems reasonable to the authors to accept that a patient with a random BG greater than or equal to 200 mg/dL in the hospital has diabetes, particularly when symptoms of hyperglycemia are present, unless there are clearly extenuating circumstances that predispose to hyperglycemia, such as high‐dose glucocorticoid therapy. Less clear is the validity of designating a diabetes diagnosis if the fasting BG in the hospital is greater than or equal to 126 mg/dL, the standard cutoff in the outpatient setting. Hemoglobin A1C level may also help clarify underlying glucose tolerance. It is contingent upon the treating physician to confirm a diabetes diagnosis following discharge from the hospital when a lesser degree of hyperglycemia was present or the diabetes diagnosis was in question during the hospital stay.
c. Diabetes Complications
Accurate documentation of diabetes complications also provides opportunities for optimizing reimbursement. The ICD‐9‐CM1 classifies diabetes complications as follows:
-
Renal manifestations, eg, diabetic nephropathy
-
Ophthalmic manifestations, eg, diabetic retinopathy
-
Neurologic manifestations, eg, diabetic polyneuropathy, gastroparesis
-
Peripheral circulatory disorders, eg, peripheral angiopathy, gangrene
-
Other specified manifestations, eg, diabetic hypoglycemia; hypoglycemic shock; associated ulceration; diabetic bone changes; drug‐induced, eg, secondary to treatment with high‐dose glucocorticoids for acute medical condition
Within each of these opportunities, one must collect baseline data to accurately quantify potential for improvement or impact directly attributable to a glycemic control team initiative. Data will be gathered by chart review and/or by extraction from electronic data repositories and then correlated with known financial implications of improved accuracy of documentation for the given criteria, eg, impact on case‐mix ratio and or implications for direct billing and reimbursement to the hospital.
The steps necessary to quantify each of these opportunities include:
-
Defining the patient population to be assessed, eg, uncontrolled or unrecognized diabetes or diabetes with specific complications, as discussed above
-
Delineating the time period to be assessed, eg, baseline, or preimplementation and postimplementation of the intervention
-
Obtaining DRG (or other classification system) code and ICD‐9 principal and secondary code information
-
Review implications of improved coding on reimbursement rates for the hospital for the targeted area of opportunity, ie, if the selected opportunity, eg, uncontrolled diabetes is correctly documented and coded, what is the dollar amount/case that would potentially be recognized by the institution based on the new DRG codes assigned to these cases.
-
Extrapolate from the number of cases identified as having potential to be accurately coded, or the increased number of cases that are accurately coded as a result of the team intervention and the dollar amount of value per case to derive a projected total dollar amount that could or has been recognized for the hospital.
EXAMPLE: Potential for Improved Revenue Based on Allowed Charges for Uncontrolled Diabetes.
Assessment of potential for improved revenue based on allowed charges in a MedStar community teaching hospital with 344 beds was conducted using the case mix index (CMI) reimbursement system for the State of Maryland.5
Step 1. Define criteria for selection of specific population:
-
Hospital all discharges
-
Time period = Baseline FY 2006 Q3
-
Age 18 or greater, excluding cases with diabetic ketoacidosis or non‐ketotic hyperosmolar state (codes 250.1, 250.2, and 250.3)
Step 2. Obtain APR Diagnostic‐related group (DRG) and severity of illness (SOI) information for each case.
Step 3. List reviewed by rates and reimbursement specialist:
-
246 cases reviewed
-
Noted that all SOI levels 3 and 4 were not designated as having uncontrolled diabetes
-
49 of the 246 cases (19.9%) with potential for changes in allowed charge per case based on designation as uncontrolled diabetes.
| Item | Original (o) CMI | Improved (i) CMI |
|---|---|---|
| Case mix index (CMI) | 0.9269 | 0.9750 |
| Allowed charge/case | $8,531 | $8,973 |
| 246 cases (total allowed charge) | $2,098,522 (o) | $2,207,431 (i) |
| Q3 Potential for improved revenue (io) | $108,910 | |
| Annualized potential for improved revenue | $435,640 |
A similar process can be applied to demonstrate potential for improved revenue in systems where reimbursement is based on a combined case rate and percentage of charges based on contractual stipulation.
It is always advisable to use conservative, realistic assumptions when making such projections. Finally, one should note that in a hospital where successful efforts to optimize documentation and coding have been implemented such that the CMI, for example, has been maximized, it is less likely that financial benefit from incremental improvement in documentation will be recognized.
2. Reduction in Length of Stay and Readmissions
Financial benefit linked to reduction in length of stay (LOS) may be assessed in one of two ways. If reimbursement is predetermined based on DRG, shorter LOS means that fewer resources are spent caring for the patient. This model is known as cost aversion. It optimizes revenue recognized per case for the hospital. The second model focuses on throughput for hospital beds. If LOS is shortened there is increased availability of beds for additional billable patients to be admitted to the hospital. Newton and colleagues have applied the throughput model to successfully obtain hospital support for a nurse‐case manager diabetes management team, as shown in the example below.6 This model's success is contingent on high occupancy rates.
The concept that intervention by a glycemic control team can have a positive impact on LOS is not new. In a small study (N = 70) by Levetan and colleagues in 1995, the average LOS of patients cared for by a diabetes team was 3.6 1.7 days, which was 56% shorter than in diabetes patients who did not (8.2 6.2 days), P .001, and 35% shorter than that for patients who received a traditional individual Endocrine consult (5.5 3.4 days), P .05. Of note, LOS correlated significantly (P .0001) with time from admission to consultation, such that each 1‐day delay in consultation resulted in a 1‐day increase in length of stay.7 Admittedly, the magnitude of reduction in LOS that is currently feasible through implementation of glycemic control teams is likely less than was possible a decade ago.
EXAMPLE: Reduction in length of stay and resultant increase in patient throughput.
Newton and colleagues applied the throughput model to the results of an inpatient diabetes management program in Greenville, North Carolina, to calculate the return on investment for a multidisciplinary glycemic control team that uses endocrinologist supervised nurse case managers. A 0.26‐day reduction in LOS among 6,876 discharges for patients with diabetes was equated to 1,788 days saved per year, allowing an incremental annual inpatient volume of 350 patients with an average LOS of 5.11 days. Multiplying this incremental inpatient volume by the hospital's $6,357 revenue margin per patient is translated into a throughput value of $2,224,029 for the year. Based on salaries, consultant fees, data management and product services expended to implement their inpatient diabetes management program, these authors suggest that the throughput value allowed a 467% return on investment.6 The return would be even greater if averted expenditures were factored in.
3. Resource Utilization
Opportunities for cost savings through improved glycemic management may be assessed by analysis of geometric mean cost, expected cost for the selected practice and comparative cost deviation between patients with and without hyperglycemia. Many companies offer risk‐adjustment analysis software for hospitals. The Care Science software utilized by MedStar Health calculates a geometric mean cost for a given population of inpatient cases and compares this to the expected cost based on the population's clinical and demographic information. The cost of a specific inpatient case is calculated using the hospital's overall cost to charge ratio. The average cost for a given population is calculated using a geometric mean of these specific costs. Geometric mean is used to dampen any outlier effect of extremely high‐cost cases. A statistical model provided by the software company utilizes clinical and demographic information to calculate expected cost for an individual case or a population. In addition, analysis of the impact of glycemic control on morbidity and mortality will allow demonstration of cost savings attributable to the inpatient glycemic control initiative.
Relative to impact of glycemic control on morbidity, mortality and cost savings Furnary and colleagues have demonstrated the impact of targeted blood glucose control in diabetes patients undergoing open heart surgery (N = 4864) in an ongoing prospective, nonrandomized, interventional study. Continuous intravenous insulin infusion therapy (IIT) targeting BG 150 mg/dL was found to be associated independently with reduction in mortality risk and deep sternal wound infection by 57% and 66% respectively (P .0001 for both). Coronary artery bypass graft (CABG) surgery‐related mortality (2.5%) and deep sternal wound infection (DSWI) rates (0.8%) were normalized to that of the population without diabetes through implementation of targeted BG control using IIT for 3 days following cardiac surgery. Taking into account both direct and indirect costs of insulin therapy, additional costs and LOS attributable to DSWI this group estimates that intensive BG control realizes an overall cost savings of $680 per patient. The estimated cost saving was calculated based upon assumptions that the Portland protocol [reduced the incidence of DSWI by 1 case for every 83 patients in whom it was applied, off‐setting the cost of a single DSWI] + [reduction in LOS by 1 day accounted for by a 50 mg/dL reduction in BG] [the increased cost of implementing the protocol per patient]. The majority of savings are attributed to decreased costs for treatment of wound infections and to shorter length of hospital stay.8
Schmeltz and colleagues recently have reported reduction of surgical morbidity and mortality in diabetes patients undergoing cardiac surgery using IIT in the ICU followed by subcutaneous insulin outside the ICU. The authors hypothesize that the combination of IV and SQ insulin might be less costly and less nursing intensive than the 3 days of IV insulin therapy recommended by Furnary.9
EXAMPLE: Opportunity for cost savings through improved glycemic management.
Exploratory cost analysis for identification of potential resource utilization opportunities was carried out for a 33% sample of discharges from a 344‐bed community teaching hospital in the MedStar Health System for FY 2006, Quarter 3. Data were obtained from COMPAS (Clinical Outcomes Management and Process Analysis System), a database and software managed and licensed by Quovadx's CareScience division. The database warehouses patient characteristics, resource utilization, and most laboratory data for all inpatients. Analysis compared costs for cases with two or more BG > 180 mg/dL at some point during the hospital stay to those cases in which hyperglycemia was not present during the stay,3 as shown in Table 2. The data suggest a financial opportunity as evidenced by the delta in comparative cost deviation.
| Outcome | Cases with 2 or More BG at Some Point During Stay > 180 mg/dL | Cases with Controlled BG |
|---|---|---|
| Cases | 465 | 1,228 |
| Geometric mean cost | $10,312 | $5,272 |
| Expected cost (select practice) | $9,639 | $5,595 |
| Comparative cost deviation | $ 673 | ($ 323) |
| Comparative cost sig level | 90% sig | 90% sig |
Such analyses can serve as the basis for discussion with finance and operations to obtain an estimate of potential value of the glycemic control team to the hospital.
B. REVENUE GENERATION THROUGH BILLING FOR CLINICAL SERVICES
Implementation of targeted BG control in the hospital provides opportunities for an increase in the provision of clinical consultative services for diabetes management. Physicians and allied healthcare providers can bill when they provide such care, and the revenues offset costs of salary, fringe benefits and other expenses.
1. Nurse Practitioner (NP) Support Model
Northwestern University has successfully implemented a Glycemic Management Service (GMS) with the use of easy‐to‐follow insulin protocols guided by a formal management service. This model, implemented on inpatient surgical services utilizing Advanced NPs in conjunction with supervision by a board‐certified endocrinologist, has proven to be effective and financially viable. Revenue generated by GMS consultation has been able to provide salary support for the NPs and 25% of a supervising physician's salary.10
EXAMPLE: Justification of NP support through offset by billings for consultative diabetes management services.
Nurse Practitioners on the Northwestern glycemic management service did between 35 and 45 new patient plus follow‐up consults each per month in the first 7 months of 2006. Total monthly billings for each NP for new patient consults averaged $13,000 and for follow‐up consults averaged $12,600. This annualizes to billings of about $310,000 for each NP. If one assumes an annual salary of $80,000 for an NP plus 30% fringe benefits ($24,000), the total salary expenses incurred to support each NP is $104,000 (Mark Molitch, personal communication, 2008). Additional operating costs and contractual allowances must also be offset in the return on investment equation, as illustrated in the physician support model example below.
2. Physician Support Model
The case for return on investment (ROI) for physician consultation may be made in a similar fashion (Table 3). This model's success is contingent upon meeting the projected number of new consults and follow‐up visits.
| Physician‐Supported Model for Business Case | ||
|---|---|---|
| Item | Amount $ | Comments |
| A. Operating Revenue | ||
| ‐ Gross Patient Service Revenue | ||
| Professional Fees | 328,320 | Based on 4‐5 new level 4 consults/day generating $24,000/month and 2 level 2 follow‐up consults/day generating $5,760/month billings on average; balance in level 3 outpatient visits. |
| ‐ Deductions from Revenue | ||
| Contractual Allowances | (123,504) | |
| ‐ Net Patient Service Revenue | 204,816 | = 62% |
| Total operating revenue | 204,816 | |
| B. Operating Expenses | ||
| ‐ Personnel (salary) | (150,000) | 1.0 FTE endocrinologist |
| ‐ Benefits | (15,000) | |
| ‐ Purchased services | (18,443) | 9% billing fees |
| ‐ Risk Management | (11,000) | |
| ‐ Other operating expenses | (5,000) | Pager/phone/printed materials/CME |
| Total operating expenses | (199,433) | |
| C. Earnings from Operations | ||
| Net earnings | 5,383 | |
One should also note that reductions in length of stay attributed to the diabetes case management provided by the physician or NP/PA can potentially be factored in the resultant financial benefit equation.
Other: Diabetes Education in the Inpatient Setting
Finally, at this time, financial justification for direct support for inpatient diabetes education services is challenging as there is no mechanism whereby inpatient education services can be billed. The case is therefore supported by incorporating the role of the educator into the business plan for the diabetes case management team as a whole. Financial support is then justified indirectly via 1 or more mechanisms. Net positive collections for clinical services by the team physicians and/or allied healthcare providers who are NPs or PAs may be applied to defray the cost of educator salary. Reduction in length of stay and/or costs resulting from the team initiative may also be used in support of diabetes educator positions. The diabetes educator may also be incorporated as a member of the hospital education program in order to help meet the requirement that basic diabetes education be provided to enable safe discharge of the patient from the hospital into the primary care setting.
CONCLUSION
Financial justification for support of a hospital based glycemic control team is challenging but possible, as has been shown by Newton5 and by DeSantis and Molitch.10 Various models may be used individually or in combination to make the case to hospital administration for salary support for team members. The models that may be helpful in this regard include: improved documentation opportunities; reduction in length of stay; reimbursement for direct clinical diabetes case management consultative services by physicians and NPs or PAs, and demonstration of improved resource utilization for the hyperglycemic patient managed by the hospital glycemic control team.
- American Hospital Directory. Medicare Prospective Payment System. http://www.ahd.com/pps.html. Accessed September 5,2008.
- ,.How does your coding measure up?: analyzing performance data gives HIM a boost in managing revenue.J AHIMA.2005;76(7):26–31.
- Hart AC,Hopkins CA,Ford B, eds.ICD‐9‐CM Professional for Physicians.6th ed.Salt Lake City, UT:Ingenix;2006.
- ,,, et al.on behalf of the American Diabetes Association Writing Group.Management of diabetes and hyperglycemia in hospitals.Diabetes Care.2004;27:553–591.
- Analysis provided by MedStar Health Outcomes Department.
- ,.Financial implications of glycemic control: results of an inpatient diabetes management program.Endocr Pract.2006;12(Suppl 3):43–48.
- ,,,.Impact of endocrine and diabetes team consultation on hospital length of stay for patients with diabetes.Am J Med.1995;99:22–28.
- ,,.Effect of hyperglycemia and continuous intravenous insulin infusions on outcomes of cardiac surgical procedures: the Portland diabetes project.Endocr Pract.2004;10:21–33.
- ,,, et al.Reduction of surgical mortality and morbidity in diabetic patients undergoing cardiac surgery with a combined intravenous and subcutaneous insulin glucose management strategy.Diabetes Care.2007;30:823–828.
- ,,, et al.Inpatient management of hyperglycemia: the Northwestern experience.Endocr Pract.2006;12:491–505.
Implementation of targeted inpatient glycemic control by a multidisciplinary team in the hospital is a time‐intensive and labor‐intensive undertaking. A variety of business models may be applied including those in which the hospital provides financial support and those which will be self‐supporting through clinical billing revenues (Table 1). In practice, a combination of these strategies may be used.
| Model | Strategy | Target |
|---|---|---|
| A. Hospital‐Supported | 1. Improve documented patient acuity | Improve accuracy of documentation and coding of: |
| a. uncontrolled diabetes (DM); | ||
| b. unrecognized DM; | ||
| c. DM complications | ||
| 2. Increase capacity and denied payment for readmissions | Reduction in overall length of stay and readmission rates | |
| 3. Optimize resource utilization | Reduction in morbidity and mortality and reduction in intensive care unit length of stay | |
| B. Self‐Supported | 1. Allied health professional billings (NP, PA) | Salary offset through income generation |
| 2. Physician billings |
In order to make the case to hospital administration for investment of financial support for glycemic control programs, it is necessary to develop a business plan that will justify return on investment. The following discussion is a composite of information from the published literature, personal communication with hospital glycemic control champions, and the author's experience developing, implementing, and assessing outcomes for a glycemic control task force charged with improving glucose control in the 7 hospital MedStar Health system from which more than 45,000 patients are discharged annually with a diagnosis of diabetes.
The principles for making the business case for support of a glycemic control team are the same as those applied in other hospital‐based programs. While the clinical argument for a new process, resource, or staff member may be sound, the business plan will also be analyzed from nonclinical perspectives, with attention to its fiscal and operational feasibility. It is therefore important to involve hospital administration, operations, and finance representatives early, in order to obtain their support and address concerns early that would otherwise weaken the request. The information provided in this article is intended to provide practical guidance for developing a plan that is realistic and tailored to the needs of the individual institution.
A. HOSPITAL‐SUPPORTED MODELS
The business case for hospital support for the glycemic control team is based on opportunities to improve revenue through accurate documentation and coding, reduction in length of stay, and optimization of resource utilization through reduction in morbidity and mortality (cost aversion).
In order to understand opportunities for improved revenue, it is necessary to understand how hospitals are reimbursed for inpatient care. Medical record documentation is reviewed by hospital coding personnel who apply strict guidelines and ICD‐9‐CM nomenclature to establish the diagnoses and procedures for each case. The ICD‐9‐CM is a numerical coding scheme of more than 13,000 diagnoses and 5,000 procedures. The principal and secondary diagnoses and procedures are grouped into a Diagnosis Related Group (DRG) code. In most states, CMS (Medicare) reimburses hospitals a flat rate based on the DRG code assigned to each inpatient case. CMS calculates a specific DRG weight for each DRG code. The DRG weight is a number such as 0.895 or 1.24, which reflects the acuity of the patient assigned to a particular DRG code and expected resource utilization. The greater the weight, the greater the acuity and expected resource utilization. The overall Case Mix Index (CMI) is the simple average of the DRG weight for a population of inpatients. CMI indicates the relative severity of a patient population and is directly proportional to DRG payments. This index is valuable when making comparisons between hospitals or patient groups.
Each hospital is provided a single rate relative to a DRG weight of 1.0 which allows calculation of the expected revenue from CMS per case. The DRG payment for a Medicare patient is determined by multiplying the relative weight for the DRG by the hospital's blended rate: DRG PAYMENT = WEIGHT RATE. Each hospital's payment rate is defined by federal regulations and is updated annually to reflect inflation, technical adjustments, and budgetary constraints. There are separate rate calculations for large urban hospitals and other hospitals. There are also technical adjustments for local wage variations, teaching hospitals, and hospitals with a disproportionate share of indigent patients.1
Determination of allowable charges for inpatient care varies by location and payor. The State of Maryland, for example regulates hospital inpatient charges for all patients based on the CMI of each hospital. In this reimbursement system, the hospital must ensure that their total average charge per case is on par with their reported CMI, when compared to other hospitals. For non‐Medicare payors, reimbursement may also be based on a set DRG payment or a percentage of charges based on contractual stipulations. The business case will be based on the individual hospital's allowed charges.
Empirical studies in this area are lacking, perhaps in part because this is a sensitive data area. Publications tend to focus on potential rather than actual results. Profiling hospital improvement management performance for documentation, coding and reimbursement does allow hospitals to go beyond focused operational metrics to evaluate themselves in a broad industry context to determine whether they are in alignment with examples from other institutions.2
Given this background, specific strategies used to justify hospital support for the glycemic control will now be outlined.
1. Documentation Opportunities
The hospital glycemic control team can help optimize reimbursement through improved accuracy of physician documentation and of coding. Each opportunity should be determined in collaboration with the coding and finance departments. Education for providers and coders must be ongoing, especially in hospitals where staff turnover is common. It should also be made clear that coders cannot specify a diagnosis unless the provider has documented it in the chart. Some initiatives incorporate notifying providers when a documentation deficiency is identified in order to offer an opportunity to provide clarification.
Establishing a definition for each coding designation for diabetes will enable collection of specific data upon which the business case can be built.
a. Accuracy of Designating Diabetes as Controlled or Uncontrolled
The glycemic control team should come to a consensus on a definition of uncontrolled diabetes. In the absence of accepted criteria for this designation, the following descriptions are an attempt to provide some guidance. They are derived from the ICD‐9‐CM Professional 6th edition3 and the American Diabetes Association (ADA) article on management of diabetes and hyperglycemia in the hospital4
-
A nonspecific term indicating that the treatment regimen does not keep the blood sugar level of a patient within acceptable limits3
-
Admission BG over 180(200) mg/dL or 2 or more BG during the hospital stay over 180(200) mg/dL
-
Lesser persistent hyperglycemia outside guidelines set by AACE and ADA for hospital management, eg, fasting BG over 110 mg/dL and other BGs over 180 mg/dL on noncritical care units, could also be considered consistent with uncontrolled diabetes.
The modifier uncontrolled only applies when the patient has a known diagnosis of diabetes. CMS has recently revised reimbursement guidelines for uncontrolled diabetes. This designation no longer provides an incremental increase in reimbursement; however, it is still weighted in the determination of case mix index.
b. Unrecognized Diabetes
The key feature of this designation is that the diabetes is either unrecognized by the treating provider or is not clearly documented as diabetes in the medical record during the hospital stay. Beyond these key features, there are no clearly defined criteria for unrecognized diabetes. It is again contingent on the hospital team, including the coding specialists, to decide on blood glucose thresholds that will be applied to identify those cases which are to be designated unrecognized diabetes. Daily reports of patients with hyperglycemia could be generated in order to alert providers about its presence.
There is a paucity of data to guide us in defining a new diagnosis of diabetes in the hospital and multiple variables associated with the stress of illness and hospitalization are known to impact glucose tolerance. However, it seems reasonable to the authors to accept that a patient with a random BG greater than or equal to 200 mg/dL in the hospital has diabetes, particularly when symptoms of hyperglycemia are present, unless there are clearly extenuating circumstances that predispose to hyperglycemia, such as high‐dose glucocorticoid therapy. Less clear is the validity of designating a diabetes diagnosis if the fasting BG in the hospital is greater than or equal to 126 mg/dL, the standard cutoff in the outpatient setting. Hemoglobin A1C level may also help clarify underlying glucose tolerance. It is contingent upon the treating physician to confirm a diabetes diagnosis following discharge from the hospital when a lesser degree of hyperglycemia was present or the diabetes diagnosis was in question during the hospital stay.
c. Diabetes Complications
Accurate documentation of diabetes complications also provides opportunities for optimizing reimbursement. The ICD‐9‐CM1 classifies diabetes complications as follows:
-
Renal manifestations, eg, diabetic nephropathy
-
Ophthalmic manifestations, eg, diabetic retinopathy
-
Neurologic manifestations, eg, diabetic polyneuropathy, gastroparesis
-
Peripheral circulatory disorders, eg, peripheral angiopathy, gangrene
-
Other specified manifestations, eg, diabetic hypoglycemia; hypoglycemic shock; associated ulceration; diabetic bone changes; drug‐induced, eg, secondary to treatment with high‐dose glucocorticoids for acute medical condition
Within each of these opportunities, one must collect baseline data to accurately quantify potential for improvement or impact directly attributable to a glycemic control team initiative. Data will be gathered by chart review and/or by extraction from electronic data repositories and then correlated with known financial implications of improved accuracy of documentation for the given criteria, eg, impact on case‐mix ratio and or implications for direct billing and reimbursement to the hospital.
The steps necessary to quantify each of these opportunities include:
-
Defining the patient population to be assessed, eg, uncontrolled or unrecognized diabetes or diabetes with specific complications, as discussed above
-
Delineating the time period to be assessed, eg, baseline, or preimplementation and postimplementation of the intervention
-
Obtaining DRG (or other classification system) code and ICD‐9 principal and secondary code information
-
Review implications of improved coding on reimbursement rates for the hospital for the targeted area of opportunity, ie, if the selected opportunity, eg, uncontrolled diabetes is correctly documented and coded, what is the dollar amount/case that would potentially be recognized by the institution based on the new DRG codes assigned to these cases.
-
Extrapolate from the number of cases identified as having potential to be accurately coded, or the increased number of cases that are accurately coded as a result of the team intervention and the dollar amount of value per case to derive a projected total dollar amount that could or has been recognized for the hospital.
EXAMPLE: Potential for Improved Revenue Based on Allowed Charges for Uncontrolled Diabetes.
Assessment of potential for improved revenue based on allowed charges in a MedStar community teaching hospital with 344 beds was conducted using the case mix index (CMI) reimbursement system for the State of Maryland.5
Step 1. Define criteria for selection of specific population:
-
Hospital all discharges
-
Time period = Baseline FY 2006 Q3
-
Age 18 or greater, excluding cases with diabetic ketoacidosis or non‐ketotic hyperosmolar state (codes 250.1, 250.2, and 250.3)
Step 2. Obtain APR Diagnostic‐related group (DRG) and severity of illness (SOI) information for each case.
Step 3. List reviewed by rates and reimbursement specialist:
-
246 cases reviewed
-
Noted that all SOI levels 3 and 4 were not designated as having uncontrolled diabetes
-
49 of the 246 cases (19.9%) with potential for changes in allowed charge per case based on designation as uncontrolled diabetes.
| Item | Original (o) CMI | Improved (i) CMI |
|---|---|---|
| Case mix index (CMI) | 0.9269 | 0.9750 |
| Allowed charge/case | $8,531 | $8,973 |
| 246 cases (total allowed charge) | $2,098,522 (o) | $2,207,431 (i) |
| Q3 Potential for improved revenue (io) | $108,910 | |
| Annualized potential for improved revenue | $435,640 |
A similar process can be applied to demonstrate potential for improved revenue in systems where reimbursement is based on a combined case rate and percentage of charges based on contractual stipulation.
It is always advisable to use conservative, realistic assumptions when making such projections. Finally, one should note that in a hospital where successful efforts to optimize documentation and coding have been implemented such that the CMI, for example, has been maximized, it is less likely that financial benefit from incremental improvement in documentation will be recognized.
2. Reduction in Length of Stay and Readmissions
Financial benefit linked to reduction in length of stay (LOS) may be assessed in one of two ways. If reimbursement is predetermined based on DRG, shorter LOS means that fewer resources are spent caring for the patient. This model is known as cost aversion. It optimizes revenue recognized per case for the hospital. The second model focuses on throughput for hospital beds. If LOS is shortened there is increased availability of beds for additional billable patients to be admitted to the hospital. Newton and colleagues have applied the throughput model to successfully obtain hospital support for a nurse‐case manager diabetes management team, as shown in the example below.6 This model's success is contingent on high occupancy rates.
The concept that intervention by a glycemic control team can have a positive impact on LOS is not new. In a small study (N = 70) by Levetan and colleagues in 1995, the average LOS of patients cared for by a diabetes team was 3.6 1.7 days, which was 56% shorter than in diabetes patients who did not (8.2 6.2 days), P .001, and 35% shorter than that for patients who received a traditional individual Endocrine consult (5.5 3.4 days), P .05. Of note, LOS correlated significantly (P .0001) with time from admission to consultation, such that each 1‐day delay in consultation resulted in a 1‐day increase in length of stay.7 Admittedly, the magnitude of reduction in LOS that is currently feasible through implementation of glycemic control teams is likely less than was possible a decade ago.
EXAMPLE: Reduction in length of stay and resultant increase in patient throughput.
Newton and colleagues applied the throughput model to the results of an inpatient diabetes management program in Greenville, North Carolina, to calculate the return on investment for a multidisciplinary glycemic control team that uses endocrinologist supervised nurse case managers. A 0.26‐day reduction in LOS among 6,876 discharges for patients with diabetes was equated to 1,788 days saved per year, allowing an incremental annual inpatient volume of 350 patients with an average LOS of 5.11 days. Multiplying this incremental inpatient volume by the hospital's $6,357 revenue margin per patient is translated into a throughput value of $2,224,029 for the year. Based on salaries, consultant fees, data management and product services expended to implement their inpatient diabetes management program, these authors suggest that the throughput value allowed a 467% return on investment.6 The return would be even greater if averted expenditures were factored in.
3. Resource Utilization
Opportunities for cost savings through improved glycemic management may be assessed by analysis of geometric mean cost, expected cost for the selected practice and comparative cost deviation between patients with and without hyperglycemia. Many companies offer risk‐adjustment analysis software for hospitals. The Care Science software utilized by MedStar Health calculates a geometric mean cost for a given population of inpatient cases and compares this to the expected cost based on the population's clinical and demographic information. The cost of a specific inpatient case is calculated using the hospital's overall cost to charge ratio. The average cost for a given population is calculated using a geometric mean of these specific costs. Geometric mean is used to dampen any outlier effect of extremely high‐cost cases. A statistical model provided by the software company utilizes clinical and demographic information to calculate expected cost for an individual case or a population. In addition, analysis of the impact of glycemic control on morbidity and mortality will allow demonstration of cost savings attributable to the inpatient glycemic control initiative.
Relative to impact of glycemic control on morbidity, mortality and cost savings Furnary and colleagues have demonstrated the impact of targeted blood glucose control in diabetes patients undergoing open heart surgery (N = 4864) in an ongoing prospective, nonrandomized, interventional study. Continuous intravenous insulin infusion therapy (IIT) targeting BG 150 mg/dL was found to be associated independently with reduction in mortality risk and deep sternal wound infection by 57% and 66% respectively (P .0001 for both). Coronary artery bypass graft (CABG) surgery‐related mortality (2.5%) and deep sternal wound infection (DSWI) rates (0.8%) were normalized to that of the population without diabetes through implementation of targeted BG control using IIT for 3 days following cardiac surgery. Taking into account both direct and indirect costs of insulin therapy, additional costs and LOS attributable to DSWI this group estimates that intensive BG control realizes an overall cost savings of $680 per patient. The estimated cost saving was calculated based upon assumptions that the Portland protocol [reduced the incidence of DSWI by 1 case for every 83 patients in whom it was applied, off‐setting the cost of a single DSWI] + [reduction in LOS by 1 day accounted for by a 50 mg/dL reduction in BG] [the increased cost of implementing the protocol per patient]. The majority of savings are attributed to decreased costs for treatment of wound infections and to shorter length of hospital stay.8
Schmeltz and colleagues recently have reported reduction of surgical morbidity and mortality in diabetes patients undergoing cardiac surgery using IIT in the ICU followed by subcutaneous insulin outside the ICU. The authors hypothesize that the combination of IV and SQ insulin might be less costly and less nursing intensive than the 3 days of IV insulin therapy recommended by Furnary.9
EXAMPLE: Opportunity for cost savings through improved glycemic management.
Exploratory cost analysis for identification of potential resource utilization opportunities was carried out for a 33% sample of discharges from a 344‐bed community teaching hospital in the MedStar Health System for FY 2006, Quarter 3. Data were obtained from COMPAS (Clinical Outcomes Management and Process Analysis System), a database and software managed and licensed by Quovadx's CareScience division. The database warehouses patient characteristics, resource utilization, and most laboratory data for all inpatients. Analysis compared costs for cases with two or more BG > 180 mg/dL at some point during the hospital stay to those cases in which hyperglycemia was not present during the stay,3 as shown in Table 2. The data suggest a financial opportunity as evidenced by the delta in comparative cost deviation.
| Outcome | Cases with 2 or More BG at Some Point During Stay > 180 mg/dL | Cases with Controlled BG |
|---|---|---|
| Cases | 465 | 1,228 |
| Geometric mean cost | $10,312 | $5,272 |
| Expected cost (select practice) | $9,639 | $5,595 |
| Comparative cost deviation | $ 673 | ($ 323) |
| Comparative cost sig level | 90% sig | 90% sig |
Such analyses can serve as the basis for discussion with finance and operations to obtain an estimate of potential value of the glycemic control team to the hospital.
B. REVENUE GENERATION THROUGH BILLING FOR CLINICAL SERVICES
Implementation of targeted BG control in the hospital provides opportunities for an increase in the provision of clinical consultative services for diabetes management. Physicians and allied healthcare providers can bill when they provide such care, and the revenues offset costs of salary, fringe benefits and other expenses.
1. Nurse Practitioner (NP) Support Model
Northwestern University has successfully implemented a Glycemic Management Service (GMS) with the use of easy‐to‐follow insulin protocols guided by a formal management service. This model, implemented on inpatient surgical services utilizing Advanced NPs in conjunction with supervision by a board‐certified endocrinologist, has proven to be effective and financially viable. Revenue generated by GMS consultation has been able to provide salary support for the NPs and 25% of a supervising physician's salary.10
EXAMPLE: Justification of NP support through offset by billings for consultative diabetes management services.
Nurse Practitioners on the Northwestern glycemic management service did between 35 and 45 new patient plus follow‐up consults each per month in the first 7 months of 2006. Total monthly billings for each NP for new patient consults averaged $13,000 and for follow‐up consults averaged $12,600. This annualizes to billings of about $310,000 for each NP. If one assumes an annual salary of $80,000 for an NP plus 30% fringe benefits ($24,000), the total salary expenses incurred to support each NP is $104,000 (Mark Molitch, personal communication, 2008). Additional operating costs and contractual allowances must also be offset in the return on investment equation, as illustrated in the physician support model example below.
2. Physician Support Model
The case for return on investment (ROI) for physician consultation may be made in a similar fashion (Table 3). This model's success is contingent upon meeting the projected number of new consults and follow‐up visits.
| Physician‐Supported Model for Business Case | ||
|---|---|---|
| Item | Amount $ | Comments |
| A. Operating Revenue | ||
| ‐ Gross Patient Service Revenue | ||
| Professional Fees | 328,320 | Based on 4‐5 new level 4 consults/day generating $24,000/month and 2 level 2 follow‐up consults/day generating $5,760/month billings on average; balance in level 3 outpatient visits. |
| ‐ Deductions from Revenue | ||
| Contractual Allowances | (123,504) | |
| ‐ Net Patient Service Revenue | 204,816 | = 62% |
| Total operating revenue | 204,816 | |
| B. Operating Expenses | ||
| ‐ Personnel (salary) | (150,000) | 1.0 FTE endocrinologist |
| ‐ Benefits | (15,000) | |
| ‐ Purchased services | (18,443) | 9% billing fees |
| ‐ Risk Management | (11,000) | |
| ‐ Other operating expenses | (5,000) | Pager/phone/printed materials/CME |
| Total operating expenses | (199,433) | |
| C. Earnings from Operations | ||
| Net earnings | 5,383 | |
One should also note that reductions in length of stay attributed to the diabetes case management provided by the physician or NP/PA can potentially be factored in the resultant financial benefit equation.
Other: Diabetes Education in the Inpatient Setting
Finally, at this time, financial justification for direct support for inpatient diabetes education services is challenging as there is no mechanism whereby inpatient education services can be billed. The case is therefore supported by incorporating the role of the educator into the business plan for the diabetes case management team as a whole. Financial support is then justified indirectly via 1 or more mechanisms. Net positive collections for clinical services by the team physicians and/or allied healthcare providers who are NPs or PAs may be applied to defray the cost of educator salary. Reduction in length of stay and/or costs resulting from the team initiative may also be used in support of diabetes educator positions. The diabetes educator may also be incorporated as a member of the hospital education program in order to help meet the requirement that basic diabetes education be provided to enable safe discharge of the patient from the hospital into the primary care setting.
CONCLUSION
Financial justification for support of a hospital based glycemic control team is challenging but possible, as has been shown by Newton5 and by DeSantis and Molitch.10 Various models may be used individually or in combination to make the case to hospital administration for salary support for team members. The models that may be helpful in this regard include: improved documentation opportunities; reduction in length of stay; reimbursement for direct clinical diabetes case management consultative services by physicians and NPs or PAs, and demonstration of improved resource utilization for the hyperglycemic patient managed by the hospital glycemic control team.
Implementation of targeted inpatient glycemic control by a multidisciplinary team in the hospital is a time‐intensive and labor‐intensive undertaking. A variety of business models may be applied including those in which the hospital provides financial support and those which will be self‐supporting through clinical billing revenues (Table 1). In practice, a combination of these strategies may be used.
| Model | Strategy | Target |
|---|---|---|
| A. Hospital‐Supported | 1. Improve documented patient acuity | Improve accuracy of documentation and coding of: |
| a. uncontrolled diabetes (DM); | ||
| b. unrecognized DM; | ||
| c. DM complications | ||
| 2. Increase capacity and denied payment for readmissions | Reduction in overall length of stay and readmission rates | |
| 3. Optimize resource utilization | Reduction in morbidity and mortality and reduction in intensive care unit length of stay | |
| B. Self‐Supported | 1. Allied health professional billings (NP, PA) | Salary offset through income generation |
| 2. Physician billings |
In order to make the case to hospital administration for investment of financial support for glycemic control programs, it is necessary to develop a business plan that will justify return on investment. The following discussion is a composite of information from the published literature, personal communication with hospital glycemic control champions, and the author's experience developing, implementing, and assessing outcomes for a glycemic control task force charged with improving glucose control in the 7 hospital MedStar Health system from which more than 45,000 patients are discharged annually with a diagnosis of diabetes.
The principles for making the business case for support of a glycemic control team are the same as those applied in other hospital‐based programs. While the clinical argument for a new process, resource, or staff member may be sound, the business plan will also be analyzed from nonclinical perspectives, with attention to its fiscal and operational feasibility. It is therefore important to involve hospital administration, operations, and finance representatives early, in order to obtain their support and address concerns early that would otherwise weaken the request. The information provided in this article is intended to provide practical guidance for developing a plan that is realistic and tailored to the needs of the individual institution.
A. HOSPITAL‐SUPPORTED MODELS
The business case for hospital support for the glycemic control team is based on opportunities to improve revenue through accurate documentation and coding, reduction in length of stay, and optimization of resource utilization through reduction in morbidity and mortality (cost aversion).
In order to understand opportunities for improved revenue, it is necessary to understand how hospitals are reimbursed for inpatient care. Medical record documentation is reviewed by hospital coding personnel who apply strict guidelines and ICD‐9‐CM nomenclature to establish the diagnoses and procedures for each case. The ICD‐9‐CM is a numerical coding scheme of more than 13,000 diagnoses and 5,000 procedures. The principal and secondary diagnoses and procedures are grouped into a Diagnosis Related Group (DRG) code. In most states, CMS (Medicare) reimburses hospitals a flat rate based on the DRG code assigned to each inpatient case. CMS calculates a specific DRG weight for each DRG code. The DRG weight is a number such as 0.895 or 1.24, which reflects the acuity of the patient assigned to a particular DRG code and expected resource utilization. The greater the weight, the greater the acuity and expected resource utilization. The overall Case Mix Index (CMI) is the simple average of the DRG weight for a population of inpatients. CMI indicates the relative severity of a patient population and is directly proportional to DRG payments. This index is valuable when making comparisons between hospitals or patient groups.
Each hospital is provided a single rate relative to a DRG weight of 1.0 which allows calculation of the expected revenue from CMS per case. The DRG payment for a Medicare patient is determined by multiplying the relative weight for the DRG by the hospital's blended rate: DRG PAYMENT = WEIGHT RATE. Each hospital's payment rate is defined by federal regulations and is updated annually to reflect inflation, technical adjustments, and budgetary constraints. There are separate rate calculations for large urban hospitals and other hospitals. There are also technical adjustments for local wage variations, teaching hospitals, and hospitals with a disproportionate share of indigent patients.1
Determination of allowable charges for inpatient care varies by location and payor. The State of Maryland, for example regulates hospital inpatient charges for all patients based on the CMI of each hospital. In this reimbursement system, the hospital must ensure that their total average charge per case is on par with their reported CMI, when compared to other hospitals. For non‐Medicare payors, reimbursement may also be based on a set DRG payment or a percentage of charges based on contractual stipulations. The business case will be based on the individual hospital's allowed charges.
Empirical studies in this area are lacking, perhaps in part because this is a sensitive data area. Publications tend to focus on potential rather than actual results. Profiling hospital improvement management performance for documentation, coding and reimbursement does allow hospitals to go beyond focused operational metrics to evaluate themselves in a broad industry context to determine whether they are in alignment with examples from other institutions.2
Given this background, specific strategies used to justify hospital support for the glycemic control will now be outlined.
1. Documentation Opportunities
The hospital glycemic control team can help optimize reimbursement through improved accuracy of physician documentation and of coding. Each opportunity should be determined in collaboration with the coding and finance departments. Education for providers and coders must be ongoing, especially in hospitals where staff turnover is common. It should also be made clear that coders cannot specify a diagnosis unless the provider has documented it in the chart. Some initiatives incorporate notifying providers when a documentation deficiency is identified in order to offer an opportunity to provide clarification.
Establishing a definition for each coding designation for diabetes will enable collection of specific data upon which the business case can be built.
a. Accuracy of Designating Diabetes as Controlled or Uncontrolled
The glycemic control team should come to a consensus on a definition of uncontrolled diabetes. In the absence of accepted criteria for this designation, the following descriptions are an attempt to provide some guidance. They are derived from the ICD‐9‐CM Professional 6th edition3 and the American Diabetes Association (ADA) article on management of diabetes and hyperglycemia in the hospital4
-
A nonspecific term indicating that the treatment regimen does not keep the blood sugar level of a patient within acceptable limits3
-
Admission BG over 180(200) mg/dL or 2 or more BG during the hospital stay over 180(200) mg/dL
-
Lesser persistent hyperglycemia outside guidelines set by AACE and ADA for hospital management, eg, fasting BG over 110 mg/dL and other BGs over 180 mg/dL on noncritical care units, could also be considered consistent with uncontrolled diabetes.
The modifier uncontrolled only applies when the patient has a known diagnosis of diabetes. CMS has recently revised reimbursement guidelines for uncontrolled diabetes. This designation no longer provides an incremental increase in reimbursement; however, it is still weighted in the determination of case mix index.
b. Unrecognized Diabetes
The key feature of this designation is that the diabetes is either unrecognized by the treating provider or is not clearly documented as diabetes in the medical record during the hospital stay. Beyond these key features, there are no clearly defined criteria for unrecognized diabetes. It is again contingent on the hospital team, including the coding specialists, to decide on blood glucose thresholds that will be applied to identify those cases which are to be designated unrecognized diabetes. Daily reports of patients with hyperglycemia could be generated in order to alert providers about its presence.
There is a paucity of data to guide us in defining a new diagnosis of diabetes in the hospital and multiple variables associated with the stress of illness and hospitalization are known to impact glucose tolerance. However, it seems reasonable to the authors to accept that a patient with a random BG greater than or equal to 200 mg/dL in the hospital has diabetes, particularly when symptoms of hyperglycemia are present, unless there are clearly extenuating circumstances that predispose to hyperglycemia, such as high‐dose glucocorticoid therapy. Less clear is the validity of designating a diabetes diagnosis if the fasting BG in the hospital is greater than or equal to 126 mg/dL, the standard cutoff in the outpatient setting. Hemoglobin A1C level may also help clarify underlying glucose tolerance. It is contingent upon the treating physician to confirm a diabetes diagnosis following discharge from the hospital when a lesser degree of hyperglycemia was present or the diabetes diagnosis was in question during the hospital stay.
c. Diabetes Complications
Accurate documentation of diabetes complications also provides opportunities for optimizing reimbursement. The ICD‐9‐CM1 classifies diabetes complications as follows:
-
Renal manifestations, eg, diabetic nephropathy
-
Ophthalmic manifestations, eg, diabetic retinopathy
-
Neurologic manifestations, eg, diabetic polyneuropathy, gastroparesis
-
Peripheral circulatory disorders, eg, peripheral angiopathy, gangrene
-
Other specified manifestations, eg, diabetic hypoglycemia; hypoglycemic shock; associated ulceration; diabetic bone changes; drug‐induced, eg, secondary to treatment with high‐dose glucocorticoids for acute medical condition
Within each of these opportunities, one must collect baseline data to accurately quantify potential for improvement or impact directly attributable to a glycemic control team initiative. Data will be gathered by chart review and/or by extraction from electronic data repositories and then correlated with known financial implications of improved accuracy of documentation for the given criteria, eg, impact on case‐mix ratio and or implications for direct billing and reimbursement to the hospital.
The steps necessary to quantify each of these opportunities include:
-
Defining the patient population to be assessed, eg, uncontrolled or unrecognized diabetes or diabetes with specific complications, as discussed above
-
Delineating the time period to be assessed, eg, baseline, or preimplementation and postimplementation of the intervention
-
Obtaining DRG (or other classification system) code and ICD‐9 principal and secondary code information
-
Review implications of improved coding on reimbursement rates for the hospital for the targeted area of opportunity, ie, if the selected opportunity, eg, uncontrolled diabetes is correctly documented and coded, what is the dollar amount/case that would potentially be recognized by the institution based on the new DRG codes assigned to these cases.
-
Extrapolate from the number of cases identified as having potential to be accurately coded, or the increased number of cases that are accurately coded as a result of the team intervention and the dollar amount of value per case to derive a projected total dollar amount that could or has been recognized for the hospital.
EXAMPLE: Potential for Improved Revenue Based on Allowed Charges for Uncontrolled Diabetes.
Assessment of potential for improved revenue based on allowed charges in a MedStar community teaching hospital with 344 beds was conducted using the case mix index (CMI) reimbursement system for the State of Maryland.5
Step 1. Define criteria for selection of specific population:
-
Hospital all discharges
-
Time period = Baseline FY 2006 Q3
-
Age 18 or greater, excluding cases with diabetic ketoacidosis or non‐ketotic hyperosmolar state (codes 250.1, 250.2, and 250.3)
Step 2. Obtain APR Diagnostic‐related group (DRG) and severity of illness (SOI) information for each case.
Step 3. List reviewed by rates and reimbursement specialist:
-
246 cases reviewed
-
Noted that all SOI levels 3 and 4 were not designated as having uncontrolled diabetes
-
49 of the 246 cases (19.9%) with potential for changes in allowed charge per case based on designation as uncontrolled diabetes.
| Item | Original (o) CMI | Improved (i) CMI |
|---|---|---|
| Case mix index (CMI) | 0.9269 | 0.9750 |
| Allowed charge/case | $8,531 | $8,973 |
| 246 cases (total allowed charge) | $2,098,522 (o) | $2,207,431 (i) |
| Q3 Potential for improved revenue (io) | $108,910 | |
| Annualized potential for improved revenue | $435,640 |
A similar process can be applied to demonstrate potential for improved revenue in systems where reimbursement is based on a combined case rate and percentage of charges based on contractual stipulation.
It is always advisable to use conservative, realistic assumptions when making such projections. Finally, one should note that in a hospital where successful efforts to optimize documentation and coding have been implemented such that the CMI, for example, has been maximized, it is less likely that financial benefit from incremental improvement in documentation will be recognized.
2. Reduction in Length of Stay and Readmissions
Financial benefit linked to reduction in length of stay (LOS) may be assessed in one of two ways. If reimbursement is predetermined based on DRG, shorter LOS means that fewer resources are spent caring for the patient. This model is known as cost aversion. It optimizes revenue recognized per case for the hospital. The second model focuses on throughput for hospital beds. If LOS is shortened there is increased availability of beds for additional billable patients to be admitted to the hospital. Newton and colleagues have applied the throughput model to successfully obtain hospital support for a nurse‐case manager diabetes management team, as shown in the example below.6 This model's success is contingent on high occupancy rates.
The concept that intervention by a glycemic control team can have a positive impact on LOS is not new. In a small study (N = 70) by Levetan and colleagues in 1995, the average LOS of patients cared for by a diabetes team was 3.6 1.7 days, which was 56% shorter than in diabetes patients who did not (8.2 6.2 days), P .001, and 35% shorter than that for patients who received a traditional individual Endocrine consult (5.5 3.4 days), P .05. Of note, LOS correlated significantly (P .0001) with time from admission to consultation, such that each 1‐day delay in consultation resulted in a 1‐day increase in length of stay.7 Admittedly, the magnitude of reduction in LOS that is currently feasible through implementation of glycemic control teams is likely less than was possible a decade ago.
EXAMPLE: Reduction in length of stay and resultant increase in patient throughput.
Newton and colleagues applied the throughput model to the results of an inpatient diabetes management program in Greenville, North Carolina, to calculate the return on investment for a multidisciplinary glycemic control team that uses endocrinologist supervised nurse case managers. A 0.26‐day reduction in LOS among 6,876 discharges for patients with diabetes was equated to 1,788 days saved per year, allowing an incremental annual inpatient volume of 350 patients with an average LOS of 5.11 days. Multiplying this incremental inpatient volume by the hospital's $6,357 revenue margin per patient is translated into a throughput value of $2,224,029 for the year. Based on salaries, consultant fees, data management and product services expended to implement their inpatient diabetes management program, these authors suggest that the throughput value allowed a 467% return on investment.6 The return would be even greater if averted expenditures were factored in.
3. Resource Utilization
Opportunities for cost savings through improved glycemic management may be assessed by analysis of geometric mean cost, expected cost for the selected practice and comparative cost deviation between patients with and without hyperglycemia. Many companies offer risk‐adjustment analysis software for hospitals. The Care Science software utilized by MedStar Health calculates a geometric mean cost for a given population of inpatient cases and compares this to the expected cost based on the population's clinical and demographic information. The cost of a specific inpatient case is calculated using the hospital's overall cost to charge ratio. The average cost for a given population is calculated using a geometric mean of these specific costs. Geometric mean is used to dampen any outlier effect of extremely high‐cost cases. A statistical model provided by the software company utilizes clinical and demographic information to calculate expected cost for an individual case or a population. In addition, analysis of the impact of glycemic control on morbidity and mortality will allow demonstration of cost savings attributable to the inpatient glycemic control initiative.
Relative to impact of glycemic control on morbidity, mortality and cost savings Furnary and colleagues have demonstrated the impact of targeted blood glucose control in diabetes patients undergoing open heart surgery (N = 4864) in an ongoing prospective, nonrandomized, interventional study. Continuous intravenous insulin infusion therapy (IIT) targeting BG 150 mg/dL was found to be associated independently with reduction in mortality risk and deep sternal wound infection by 57% and 66% respectively (P .0001 for both). Coronary artery bypass graft (CABG) surgery‐related mortality (2.5%) and deep sternal wound infection (DSWI) rates (0.8%) were normalized to that of the population without diabetes through implementation of targeted BG control using IIT for 3 days following cardiac surgery. Taking into account both direct and indirect costs of insulin therapy, additional costs and LOS attributable to DSWI this group estimates that intensive BG control realizes an overall cost savings of $680 per patient. The estimated cost saving was calculated based upon assumptions that the Portland protocol [reduced the incidence of DSWI by 1 case for every 83 patients in whom it was applied, off‐setting the cost of a single DSWI] + [reduction in LOS by 1 day accounted for by a 50 mg/dL reduction in BG] [the increased cost of implementing the protocol per patient]. The majority of savings are attributed to decreased costs for treatment of wound infections and to shorter length of hospital stay.8
Schmeltz and colleagues recently have reported reduction of surgical morbidity and mortality in diabetes patients undergoing cardiac surgery using IIT in the ICU followed by subcutaneous insulin outside the ICU. The authors hypothesize that the combination of IV and SQ insulin might be less costly and less nursing intensive than the 3 days of IV insulin therapy recommended by Furnary.9
EXAMPLE: Opportunity for cost savings through improved glycemic management.
Exploratory cost analysis for identification of potential resource utilization opportunities was carried out for a 33% sample of discharges from a 344‐bed community teaching hospital in the MedStar Health System for FY 2006, Quarter 3. Data were obtained from COMPAS (Clinical Outcomes Management and Process Analysis System), a database and software managed and licensed by Quovadx's CareScience division. The database warehouses patient characteristics, resource utilization, and most laboratory data for all inpatients. Analysis compared costs for cases with two or more BG > 180 mg/dL at some point during the hospital stay to those cases in which hyperglycemia was not present during the stay,3 as shown in Table 2. The data suggest a financial opportunity as evidenced by the delta in comparative cost deviation.
| Outcome | Cases with 2 or More BG at Some Point During Stay > 180 mg/dL | Cases with Controlled BG |
|---|---|---|
| Cases | 465 | 1,228 |
| Geometric mean cost | $10,312 | $5,272 |
| Expected cost (select practice) | $9,639 | $5,595 |
| Comparative cost deviation | $ 673 | ($ 323) |
| Comparative cost sig level | 90% sig | 90% sig |
Such analyses can serve as the basis for discussion with finance and operations to obtain an estimate of potential value of the glycemic control team to the hospital.
B. REVENUE GENERATION THROUGH BILLING FOR CLINICAL SERVICES
Implementation of targeted BG control in the hospital provides opportunities for an increase in the provision of clinical consultative services for diabetes management. Physicians and allied healthcare providers can bill when they provide such care, and the revenues offset costs of salary, fringe benefits and other expenses.
1. Nurse Practitioner (NP) Support Model
Northwestern University has successfully implemented a Glycemic Management Service (GMS) with the use of easy‐to‐follow insulin protocols guided by a formal management service. This model, implemented on inpatient surgical services utilizing Advanced NPs in conjunction with supervision by a board‐certified endocrinologist, has proven to be effective and financially viable. Revenue generated by GMS consultation has been able to provide salary support for the NPs and 25% of a supervising physician's salary.10
EXAMPLE: Justification of NP support through offset by billings for consultative diabetes management services.
Nurse Practitioners on the Northwestern glycemic management service did between 35 and 45 new patient plus follow‐up consults each per month in the first 7 months of 2006. Total monthly billings for each NP for new patient consults averaged $13,000 and for follow‐up consults averaged $12,600. This annualizes to billings of about $310,000 for each NP. If one assumes an annual salary of $80,000 for an NP plus 30% fringe benefits ($24,000), the total salary expenses incurred to support each NP is $104,000 (Mark Molitch, personal communication, 2008). Additional operating costs and contractual allowances must also be offset in the return on investment equation, as illustrated in the physician support model example below.
2. Physician Support Model
The case for return on investment (ROI) for physician consultation may be made in a similar fashion (Table 3). This model's success is contingent upon meeting the projected number of new consults and follow‐up visits.
| Physician‐Supported Model for Business Case | ||
|---|---|---|
| Item | Amount $ | Comments |
| A. Operating Revenue | ||
| ‐ Gross Patient Service Revenue | ||
| Professional Fees | 328,320 | Based on 4‐5 new level 4 consults/day generating $24,000/month and 2 level 2 follow‐up consults/day generating $5,760/month billings on average; balance in level 3 outpatient visits. |
| ‐ Deductions from Revenue | ||
| Contractual Allowances | (123,504) | |
| ‐ Net Patient Service Revenue | 204,816 | = 62% |
| Total operating revenue | 204,816 | |
| B. Operating Expenses | ||
| ‐ Personnel (salary) | (150,000) | 1.0 FTE endocrinologist |
| ‐ Benefits | (15,000) | |
| ‐ Purchased services | (18,443) | 9% billing fees |
| ‐ Risk Management | (11,000) | |
| ‐ Other operating expenses | (5,000) | Pager/phone/printed materials/CME |
| Total operating expenses | (199,433) | |
| C. Earnings from Operations | ||
| Net earnings | 5,383 | |
One should also note that reductions in length of stay attributed to the diabetes case management provided by the physician or NP/PA can potentially be factored in the resultant financial benefit equation.
Other: Diabetes Education in the Inpatient Setting
Finally, at this time, financial justification for direct support for inpatient diabetes education services is challenging as there is no mechanism whereby inpatient education services can be billed. The case is therefore supported by incorporating the role of the educator into the business plan for the diabetes case management team as a whole. Financial support is then justified indirectly via 1 or more mechanisms. Net positive collections for clinical services by the team physicians and/or allied healthcare providers who are NPs or PAs may be applied to defray the cost of educator salary. Reduction in length of stay and/or costs resulting from the team initiative may also be used in support of diabetes educator positions. The diabetes educator may also be incorporated as a member of the hospital education program in order to help meet the requirement that basic diabetes education be provided to enable safe discharge of the patient from the hospital into the primary care setting.
CONCLUSION
Financial justification for support of a hospital based glycemic control team is challenging but possible, as has been shown by Newton5 and by DeSantis and Molitch.10 Various models may be used individually or in combination to make the case to hospital administration for salary support for team members. The models that may be helpful in this regard include: improved documentation opportunities; reduction in length of stay; reimbursement for direct clinical diabetes case management consultative services by physicians and NPs or PAs, and demonstration of improved resource utilization for the hyperglycemic patient managed by the hospital glycemic control team.
- American Hospital Directory. Medicare Prospective Payment System. http://www.ahd.com/pps.html. Accessed September 5,2008.
- ,.How does your coding measure up?: analyzing performance data gives HIM a boost in managing revenue.J AHIMA.2005;76(7):26–31.
- Hart AC,Hopkins CA,Ford B, eds.ICD‐9‐CM Professional for Physicians.6th ed.Salt Lake City, UT:Ingenix;2006.
- ,,, et al.on behalf of the American Diabetes Association Writing Group.Management of diabetes and hyperglycemia in hospitals.Diabetes Care.2004;27:553–591.
- Analysis provided by MedStar Health Outcomes Department.
- ,.Financial implications of glycemic control: results of an inpatient diabetes management program.Endocr Pract.2006;12(Suppl 3):43–48.
- ,,,.Impact of endocrine and diabetes team consultation on hospital length of stay for patients with diabetes.Am J Med.1995;99:22–28.
- ,,.Effect of hyperglycemia and continuous intravenous insulin infusions on outcomes of cardiac surgical procedures: the Portland diabetes project.Endocr Pract.2004;10:21–33.
- ,,, et al.Reduction of surgical mortality and morbidity in diabetic patients undergoing cardiac surgery with a combined intravenous and subcutaneous insulin glucose management strategy.Diabetes Care.2007;30:823–828.
- ,,, et al.Inpatient management of hyperglycemia: the Northwestern experience.Endocr Pract.2006;12:491–505.
- American Hospital Directory. Medicare Prospective Payment System. http://www.ahd.com/pps.html. Accessed September 5,2008.
- ,.How does your coding measure up?: analyzing performance data gives HIM a boost in managing revenue.J AHIMA.2005;76(7):26–31.
- Hart AC,Hopkins CA,Ford B, eds.ICD‐9‐CM Professional for Physicians.6th ed.Salt Lake City, UT:Ingenix;2006.
- ,,, et al.on behalf of the American Diabetes Association Writing Group.Management of diabetes and hyperglycemia in hospitals.Diabetes Care.2004;27:553–591.
- Analysis provided by MedStar Health Outcomes Department.
- ,.Financial implications of glycemic control: results of an inpatient diabetes management program.Endocr Pract.2006;12(Suppl 3):43–48.
- ,,,.Impact of endocrine and diabetes team consultation on hospital length of stay for patients with diabetes.Am J Med.1995;99:22–28.
- ,,.Effect of hyperglycemia and continuous intravenous insulin infusions on outcomes of cardiac surgical procedures: the Portland diabetes project.Endocr Pract.2004;10:21–33.
- ,,, et al.Reduction of surgical mortality and morbidity in diabetic patients undergoing cardiac surgery with a combined intravenous and subcutaneous insulin glucose management strategy.Diabetes Care.2007;30:823–828.
- ,,, et al.Inpatient management of hyperglycemia: the Northwestern experience.Endocr Pract.2006;12:491–505.
Metrics for Inpatient Glycemic Control
Data collection, analysis, and presentation are key to the success of any hospital glycemic control initiative. Such efforts enable the management team to track improvements in processes and outcomes, make necessary changes to their quality improvement efforts, justify the provision of necessary time and resources, and share their results with others. Reliable metrics for assessing glycemic control and frequency of hypoglycemia are essential to accomplish these tasks and to assess whether interventions result in more benefit than harm. Hypoglycemia metrics must be especially convincing because fear of hypoglycemia remains a major source of clinical inertia, impeding efforts to improve glucose control.
Currently, there are no official standards or guidelines for formulating metrics on the quality of inpatient glycemic control. This creates several problems. First, different metrics vary in their biases and in their responsiveness to change. Thus, use of a poor metric could lead to either a falsely positive or falsely negative impression that a quality improvement intervention is in fact improving glycemic control. Second, the proliferation of different measures and analytical plans in the research and quality improvement literature make it very difficult for hospitals to compare baseline performance, determine need for improvement, and understand which interventions may be most effective.
A related article in this supplement provides the rationale for improved inpatient glycemic control. That article argues that the current state of inpatient glycemic control, with the frequent occurrence of severe hyperglycemia and irrational insulin ordering, cannot be considered acceptable, especially given the large body of data (albeit largely observational) linking hyperglycemia to negative patient outcomes. However, regardless of whether one is an advocate or skeptic of tighter glucose control in the intensive care unit (ICU) and especially the non‐ICU setting, there is no question that standardized, valid, and reliable metrics are needed to compare efforts to improve glycemic control, better understand whether such control actually improves patient care, and closely monitor patient safety.
This article provides a summary of practical suggestions to assess glycemic control, insulin use patterns, and safety (hypoglycemia and severe hyperglycemia). In particular, we discuss the pros and cons of various measurement choices. We conclude with a tiered summary of recommendations for practical metrics that we hope will be useful to individual improvement teams. This article is not a consensus statement but rather a starting place that we hope will begin to standardize measurement across institutions and advance the dialogue on this subject. To more definitely address this problem, we call on the American Association of Clinical Endocrinologists (AACE), American Diabetes Association (ADA), Society of Hospital Medicine (SHM), and others to agree on consensus standards regarding metrics for the quality of inpatient glycemic control.
MEASURING GLYCEMIC CONTROL: GLUCOMETRICS
Glucometrics may be defined as the systematic analysis of blood glucose (BG) dataa phrase initially coined specifically for the inpatient setting. There are numerous ways to do these analyses, depending on which patients and glucose values are considered, the definitions used for hypoglycemia and hyperglycemia, the unit of measurement (eg, patient, patient‐day, individual glucose value), and the measure of control (eg, mean, median, percent of glucose readings within a certain range). We consider each of these dimensions in turn.
Defining the Target Patient Population
The first decision to be made is which patients to include in your analysis. Choices include the following:
-
Patients with a discharge diagnosis of diabetes: this group has face validity and intuitive appeal, is easy to identify retrospectively, and may capture some untested/untreated diabetics, but will miss patients with otherwise undiagnosed diabetes and stress hyperglycemia. It is also subject to the variable accuracy of billing codes.
-
Patients with a certain number of point‐of‐care (POC) glucose measurements: this group is also easy to identify, easy to measure, and will include patients with hyperglycemia without a previous diagnosis of diabetes, but will miss patients with untested/untreated hyperglycemia. Also, if glucose levels are checked on normoglycemic, nondiabetic patients, these values may dilute the overall assessment of glycemic control.
-
Patients treated with insulin in the hospital: this is a good choice if the purpose is mainly drug safety and avoidance of hypoglycemia, but by definition excludes most untreated patients.
-
Patients with 2 or more BG values (laboratory and/or POC) over a certain threshold (eg, >180 mg/dL). This will likely capture more patients with inpatient hyperglycemia, whether or not detected by the medical team, but is subject to wide variations in the frequency and timing of laboratory glucose testing, including whether or not the values are pre‐prandial (note that even preprandial POC glucose measurements are not always in fact fasting values).
Other considerations include the following:
-
Are there natural patient subgroups that should be measured and analyzed separately because of different guidelines? For example, there probably should be separate/emndependent inclusion criteria and analyses for critical care and noncritical care units because their glycemic targets and management considerations differ.
-
Which patients should be excluded? For example, if targeting subcutaneous insulin use in general hospitalized patients, one might eliminate those patients who are admitted specifically as the result of a diabetes emergency (eg, diabetic ketoacidosis [DKA] and hyperglycemic hyperosmolar state [HHS]), as their marked and prolonged hyperglycemia will skew BG data. Pregnant women should generally be excluded from broad‐based analyses or considered as a discrete category because they have very different targets for BG therapy. Patients with short lengths of stay may be less likely to benefit from tight glucose control and may also be considered for post hoc exclusion. One might also exclude patients with very few evaluable glucose readings (eg, fewer than 5) to ensure that measurement is meaningful for a given patient, keeping in mind that this may also exclude patients with undetected hyperglycemia, as mentioned above. Finally, patients receiving palliative care should also be considered for exclusion if feasible.
Recommendation: Do not limit analyses to only those patients with a diagnosis of diabetes or only those on insulin, which will lead to biased results.
-
For noncritical care patients, we recommend a combined approach: adult patients with a diagnosis of diabetes (e. g. using diagnosis‐related group [DRG] codes 294 or 295 or International Classification of Diseases 9th edition [ICD9] codes 250.xx) or with hyperglycemia (eg, 2 or more random laboratory and/or point of care (POC) BG values >180 mg/dL or 2 or more fasting BG values >130 mg/dL), excluding patients with DKA or HHS or who are pregnant.
-
For critical care units, we recommend either all patients, or patients with at least mild hyperglycemia (eg, 2 random glucose levels >140 mg/dL). Critical care patients with DKA, HHS, and pregnancy should be evaluated separately if possible.
Which Glucose Values to Include and Exclude
To answer this question, we first need to decide which method to use for BG measurement. There are several ways to measure BG, including the type of sample collected (capillary [fingerstick], arterial, and venous) and the technique used (central laboratory analyzing plasma, central laboratory analyzing whole blood [eg, from an arterial blood gas sample], glucose meter [usually calibrated to plasma], etc.). The POC (eg, capillary, glucose meter) glucose measurements alone are often preferred in the non‐ICU setting because laboratory plasma values generally provide little additional information and typically lower the mean glucose by including redundant fasting values.1 In critical care units, several different methods are often used together, and each merits inclusion. The inherent differences in calibration between the methods do not generally require separate analyses, especially given the frequency of testing in the ICU setting.
The next question is which values to include in analyses. In some situations, it may be most useful to focus on a certain period of hospitalization, such as the day of a procedure and the next 2 days in assessing the impact of the quality of perioperative care, or the first 14 days of a noncritical care stay to keep outliers for length of stay (LOS) from skewing the data. In the non‐ICU setting, it may be reasonable to exclude the first day of hospitalization, as early BG control is impacted by multiple variables beyond direct control of the clinician (eg, glucose control prior to admission, severity of presenting illness) and may not realistically reflect your interventions. (Keep in mind, however, that it may be useful to adjust for the admission glucose value in multivariable models given its importance to clinical outcomes and its strong relationship to subsequent inpatient glucose control.) However, in critical care units, it is reasonable to include the first day's readings in analyses given the high frequency of glucose measurements in this setting and the expectation that glucose control should be achieved within a few hours of starting an intravenous insulin infusion.
If feasible to do so with your institution's data capture methods, you may wish to select only the regularly scheduled (before each meal [qAC] and at bedtime [qHS], or every 6 hours [q6h]) glucose readings for inclusion in the summary data of glycemic control in the non‐ICU setting, thereby reducing bias caused by repeated measurements around extremes of glycemic excursions. An alternative in the non‐ICU setting is to censor glucose readings within 60 minutes of a previous reading.
Recommendation:
-
In the non‐ICU setting, we recommend first looking at all POC glucose values and if possible repeating the analyses excluding hospital day 1 and hospital day 15 and beyond, and also excluding glucose values measured within 60 minutes of a previous value.
-
In critical care units, we recommend evaluating all glucose readings used to guide care.
Units of Analysis
There are several different units of analysis, each with its own advantages and disadvantages:
-
Glucose value: this is the simplest measure and the one with the most statistical power. All glucose values for all patients of interest comprise the denominator. A report might say, for example, that 1% of the 1000 glucose values were 70 mg/dL during a certain period or that the mean of all glucose values collected for the month from patients in noncritical care areas was 160 mg/dL. The potential disadvantages of this approach are that these analyses are less clinically relevant than patient‐level analyses and that patients with many glucose readings and long hospitalizations may skew the data.
-
Patient (or the Patient Stay, [ie, the entire hospitalization]): all patients who are monitored make up the denominator. The numerator may be the percentage of patients with any hypoglycemia during their hospital stay or the percentage of patients achieving a certain mean glucose during their hospitalization, for example. This is inherently more clinically meaningful than using glucose value as a unit of analysis. A major disadvantage is not controlling for LOS effects. For example, a hospitalized patient with a long LOS is much more likely to be characterized as having at least 1 hypoglycemic value than is a patient with a shorter LOS. Another shortcoming is that this approach does not correct for uneven distribution of testing. A patient's mean glucose might be calculated on the basis of 8 glucose values on the first day of hospitalization, 4 on the second day, and 1 on the third day. Despite all these shortcomings, reporting by patient remains a popular and valid method of presenting glycemic control results, particularly when complemented by other views and refined to control for the number of readings per day.
-
Monitored Patient‐Day: The denominator in this setting is the total number of days a patient glucose level is monitored. The benefits of this method have been described and advocated in the literature.1 As with patient‐level analyses, this measure will be more rigorous and meaningful if the BG measures to be evaluated have been standardized. Typical reports might include percentage of monitored days with any hypoglycemia, or percentage of monitored days with all glucose values in the desired range. This unit of analysis may be considered more difficult to generate and to interpret. On the other hand, it is clinically relevant, less biased by LOS effects, and may be considered the most actionable metric by clinicians. This method provides a good balance when presented with data organized by patient.
The following example uses all 3 units of measurement, in this case to determine the rate of hypoglycemia, demonstrating the different but complementary information that each method provides:
-
In 1 month, 3900 POC glucose measurements were obtained from 286 patients representing 986 monitored patient‐days. With hypoglycemia defined as POC BG 60 mg/dL, the results showed the following:
-
50 of 3900 measurements (1.4%) were hypoglycemic 22 of 286 patients (7.7%) had 1 hypoglycemic episodes
-
40 of 986 monitored days (4.4%) had 1 hypoglycemic episodes.
The metric based on the number of glucose readings could be considered the least clinically relevant because it is unclear how many patients were affected; moreover, it may be based on variable testing patterns among patients, and could be influenced disproportionately by 1 patient with frequent hypoglycemia, many glucose readings, and/or a long LOS. One could argue that the patient‐stay metric is artificially elevated because a single hypoglycemic episode characterizes the entire stay as hypoglycemic. On the other hand, at least it acknowledges the number of patients affected by hypoglycemia. The patient‐day unit of analysis likely provides the most balanced view, one that is clinically relevant and measured over a standard period of time, and less biased by LOS and frequency of testing.
One way to express patient‐day glycemic control that deserves special mention is the patient‐day weighted mean. A mean glucose is calculated for each patient‐day, and then the mean is calculated across all patient‐days. The advantage of this approach is that it corrects for variation in the number of glucose readings each day; all hospital days are weighted equally.
Recommendation:
-
In noncritical care units, we recommend a combination of patient‐day and patient‐stay measures.
-
In critical care units, it is acceptable to also use glucose reading as the unit of measurement given more frequent and uniform data collection, but it should be complemented by more meaningful patient‐day and patient‐stay measures.
Measures of Control
In addition to deciding the unit(s) of analysis, another issue concerns which measures of control to use. These could include rates of hypoglycemia and hyperglycemia, percentage of glucose readings within various ranges (eg, 70, 70180, >180 mg/dL), mean glucose value, percentage of patient‐days during which the mean glucose is within various ranges, or the in control rate (ie, when all glucose values are within a certain range).
As with the various units of analysis, each of these measures of control has various advantages and disadvantages. For example, mean glucose is easy to report and understand, but masks extreme values. Percentage of glucose values within a certain range (eg, per patient, averaged across patients) presents a more complete picture but is a little harder to understand and will vary depending on the frequency of glucose monitoring. As mentioned above, this latter problem can be corrected in part by including only certain glucose values. Percent of glucose values within range may also be less sensitive to change than mean glucose (eg, a glucose that is lowered from 300 mg/dL to 200 mg/dL is still out of range). We recommend choosing a few, but not all, measures of control in order to get a complete picture of glycemic control. Over time one can then refine the measures being used to meet the needs of the glycemic control team and provide data that will drive the performance improvement process.
In critical care and perioperative settings, interest in glycemic control is often more intense around the time of a particular event such as major surgery or after admission to the ICU. Some measures commonly used in performing such analyses are:
-
All values outside a target range within a designated crucial period. For example, the University Healthcare Consortium and other organizations use a simple metric to gauge perioperative glycemic control. They collect the fasting glucose on postoperative days 1 and 2 and then calculate the percentage of postoperative days with any fasting glucose >200 mg/dL. Of course, this is a very liberal target, but it can always be lowered in a stepwise fashion once it is regularly being reached.
-
Three‐day blood glucose average. The Portland group uses the mean glucose of each patient for the period that includes the day of coronary artery bypass graft (CABG) surgery and the following 2 days. The 3‐day BG average (3‐BG) correlates very well with patient outcomes and can serve as a well‐defined target.2 It is likely that use of the 3‐BG would work well in other perioperative/trauma settings and could work in the medical ICU as well, with admission to the ICU as the starting point for calculation of the 3‐BG.
Hyperglycemic Index
Measuring the hyperglycemic index (HGI) is a validated method of summarizing glycemic control of ICU patients.3 It is designed to take into account the sometimes uneven distribution of patient testing. Time is plotted on the x‐axis and glucose values on the y‐axis. The HGI is calculated the area under the curve of glycemic values but above the upper limit of normal (ie, 110 mg/dL). Glucose values in the normal or hypoglycemic range are not included in the AUC. Mortality correlated well with this glycemic index. However, a recent observational study of glucometrics in patients hospitalized with acute myocardial infarction found that the simple mean of each patient's glucose values over the entire hospitalization was as predictive of in‐hospital mortality as the HGI or the time‐averaged glucose (AUC for all glucose values).4 In this study, metrics derived from glucose readings for the entire hospitalization were more predictive than those based on the first 24 or 48 hours or on the admission glucose.
Analyses Describing Change in Glycemic Control Over Time in the Hospital
In the critical care setting, this unit of analysis may be as simple as the mean time to reach the glycemic target on your insulin infusion protocol. On noncritical care wards, it is a bit more challenging to characterize the improvement (or clinical inertia) implied by failure of hyperglycemia to lessen as an inpatient stay progresses. One method is to calculate the mean glucose (or percentage of glucose values in a given range) for each patient on hospital day (HD) 1, and repeat for each HD (up to some reasonable limit, such as 5 or 7 days).
Recommendations:
-
In noncritical units, we recommend a limited set of complementary measures, such as the patient‐day weighted mean glucose, mean percent of glucose readings per patient that are within a certain range, and percentage of patients whose mean glucose is within a certain range on each hospital day.
-
In critical care units, it is often useful to focus measures around a certain critical event such as the 3‐day blood glucose average and to use measures such as the HGI that take advantage of more frequent blood glucose testing.
Definitions of Hyperglycemia and Hypoglycemia
Glucometrics outcomes will obviously depend on the thresholds established for hyperglycemia and hypoglycemia. Many centers define hypoglycemia as 60 mg/dL, whereas the ADA definition, based on physiologic changes that may take place, defines hypoglycemia (at least in the outpatient setting) as 70 mg/dL. Hypoglycemia may be further stratified by severity, with any glucose 40 mg/dL, for instance, defined as severe hypoglycemia.
Similarly, the definition of hyperglycemia (and therefore good control) must also be defined. Based on definitions developed by the ADA and AACE, the state of the medical literature, and current understanding of the pathophysiology of hyperglycemia, thresholds for critical care units include 110 mg/dL, 130 mg/dL, and 140 mg/dL, and options in noncritical care units include 130 mg/dL, 140 mg/dL, and 180 mg/dL. Because these thresholds implicitly assume adverse effects when glucose levels are above them, these levels are subject to revision as data become available confirming the benefits and safety of targeted glycemic control in various settings and patient populations.
Introducing optimal BG targets in a stepped fashion over time should also be considered. Furnary et al.2 have done this in the Portland Project, which tracks glycemic control in cardiac surgery patients receiving intravenous insulin therapy. The initial BG target for this project was 200 mg/dL; it was subsequently lowered stepwise over several years to 150 mg/dL, then to 120 mg/dL, and most recently to 110 mg/dL. This approach allows the safe introduction of targeted glycemic control and promotes acceptance of the concept by physicians and the allied nursing and medical staff.
Recommendations:
-
In noncritical care units, it is reasonable to use 40 mg/dL for severe hypoglycemia, 70 mg/dL for hypoglycemia, 130 mg/dL for fasting hyperglycemia, 180 mg/dL for random or postprandial hyperglycemia, and 300 mg/dL for severe hyperglycemia, keeping in mind that these thresholds are arbitrary. In critical care units, values from 110 mg/dL to 140 mg/dL might be better thresholds for hyperglycemia, but it may take time to safely and effectively move an organization toward these lower targets.
Other Considerations Relative to Glucometrics
Yale Glucometrics Website
The Yale Informatics group has put together a Web‐based resource (
Other Analytic Resources
Commercially available software, such as the RALS system (Medical Automation Systems, Inc., Charlottesville, VA) can gather POC glucose measurements directly from devices and provide real‐time reports of glycemic control, stratified by inpatient unit, using user‐defined targets for hypoglycemia and hyperglycemia. While they are no substitute for a dedicated, on‐site data analyst, such systems can be very useful for smaller hospitals with minimal data or information technology support staff.
APPROACHES TO ANALYSIS: RUN CHARTS
Most conventional clinical trials hold interventions fixed for a period of time and compare results with and without the intervention. For quality improvement studies, this is still a valid way to proceed, especially if studied as a randomized controlled trial. Such methods may be preferred when the clinical question is Does this type of intervention work in general? and the desired output is publication in peer‐reviewed journals so that others can learn about and adopt the intervention to their own institution. A before and after study with a similar analytic approach may also be valid, although concerns about temporal trends and cointerventions potentially compromise the validity of such studies. This approach again assumes that an intervention is held fixed over time such that it is clear what patients received during each time period.
If the desired result is improvement at a given institution (the question is Did we improve care?) then it may be preferable to present results over time using run‐charts. In a run chart, the x‐axis is time and the y‐axis the desired metric, such as patient‐day weighted mean glucose. Points in time when interventions were introduced or modified can be highlighted. Run charts have several advantages over before‐and‐after summaries: they do not require interventions remaining fixed and are more compatible with continuous quality improvement methods, it is easier to see the effect of different aspects of the interventions as they occur, one can get a quicker picture of whether something is working, and it is easier to separate out the impact of the intervention from secular trends. Finally, the use of run charts does not imply the absence of statistical rigor. Run charts with statistical process control (SPC) limits5 can easily convey when the observed time trend is unlikely to be due to chance using prespecified P values. (A full discussion of SPC and other methods to study quality improvement interventions is beyond the scope of this article.)
ASSESSING PATTERNS OF INSULIN USE AND ORDER SET UTILIZATION
Besides measuring the impact of quality improvement interventions on glucose control, it is important to measure processes such as proper insulin use. As mentioned in other articles in this supplement, processes are much more sensitive to change than outcomes. Failure to change processes should lead one to make changes to the intervention.
ICU and Perioperative Settings
For ICU and perioperative settings, the major process measure will likely be use of the insulin infusion order set. Designation of BG levels that trigger insulin infusion in these settings should be agreed upon in advance. The number of patients who meet the predefined glycemic criteria would make up the denominator, and the number of patients on the insulin infusion order set would make up the numerator.
NonCritical Care Units
On noncritical care units, measuring the percentage of subcutaneous insulin regimens that contain a basal insulin is a useful way to monitor the impact of an intervention. A more detailed analysis could examine the percentage of patients on simultaneous basal and nutritional insulin (if applicable). An important measure of clinical inertia is to track the percentage of patients who had changes in their insulin regimens on days after hypoglycemic or hyperglycemic excursions. Another important measure is the frequency with which the standardized order set is being used, analogous to the measure of insulin infusion use in the ICU. A final process measure, indirectly related to insulin use, is the frequency of use of oral diabetes agents, especially by patients for whom their use is contraindicated (eg, patients with congestive heart failure who are on thiazolidinediones and patients with renal insufficiency or receiving intravenous contrast continued on metformin).
OTHER CONSIDERATIONS AND METRICS
Examples of other metrics that can be used to track the success of quality improvement efforts include:
-
Glucose measurement within 8 hours of hospital admission.
-
Glycated hemoglobin (A1C) measurement obtained or available within 30 days of admission to help guide inpatient and especially discharge management.
-
Appropriate glucose testing in patients with diabetes or hyperglycemia (eg, 4 times per day in patients not on insulin infusion protocols, at least until 24 hours of euglycemia is documented).
-
The percentage of patients on insulin with on‐time tray delivery.
-
The timing of subcutaneous insulin administration in relation to glucose testing and nutrition delivery.
-
Documentation of carbohydrate intake among patients who are eating.
-
Satisfaction of physicians and nurses with order sets or protocols, using standard surveys.
-
Physician and nurse knowledge, attitudes, and beliefs about insulin administration, fear of hypoglycemia, treatment of hypoglycemia, and glycemic control in the hospital.
-
Patient satisfaction with their diabetes care in the hospital, including the education they received.
-
Nursing and physician education/certification in insulin prescribing, insulin administration, and other diabetes care issues.
-
Patient outcomes strongly associated with glycemic control, (eg, surgical wound infections, ICU LOS, catheter‐related bloodstream infections).
-
Appropriate treatment and documentation of hypoglycemia (eg, in accordance with hospital policy).
-
Documentation of severe hypoglycemic events through the hospital's adverse events reporting system (these may actually increase as change comes to the organization and as clinical personnel are more attuned to glycemic control).
-
Root causes of hypoglycemic events, which can be used to understand and prevent future events.
-
Appropriate transitions from IV to SC insulin regimens, (eg, starting basal insulin prior to discontinuing infusion in patients who have been on an insulin infusion of at least 2 units/hour or who have a known diagnosis of diabetes or A1C >7).
(Survey instruments and other measurement tools are available from the authors upon request.)
SHM GLYCEMIC CONTROL TASK FORCE SUMMARY RECOMMENDATIONS
The SHM Glycemic Control Task Force is working to develop standardized measures of inpatient glucose control and related indicators to track progress of hospital glycemic control initiatives (see the introduction to this supplement for a description of the charge and membership of this task force). The goals of the Task Force's metrics recommendations (Table 1) are several‐fold: (1) create a set of measurements that are complete but not overly burdensome; (2) create realistic measures that can be applied to institutions with different data management capabilities; and (3) allow for comparison across institutions for benchmarking purposes, evaluation of quality improvement projects, and reporting of results for formal research studies in this field.
| Measurement Issue | NonCritical Care Units | Critical Care Units | ||
|---|---|---|---|---|
| Tier 1 Recommendations | Tier 2 Recommendations | Tier 1 Recommendations | Tier 2 Recommendations | |
| ||||
| Patient inclusion and exclusion criteria | All adult patients with POC glucose testing (sampling acceptable). Exclude patients with DKA or HHS or who are pregnant. | All adult patients with diagnosis of diabetes by ICD‐9 code* or by glucose testing: random glucose (POC or laboratory) >180 mg/dL 2 or fasting glucose >130 mg/dL 2, excluding patients with DKA or HHS or who are pregnant. Additional analysis: exclude patients with 5 evaluable glucose readings, patients with LOS 2 days, or receiving palliative care. | All patients in every critical care unit (sampling acceptable). | Patients with DKA, HHS, or pregnancy in separate analyses. All patients in every critical care unit with random glucose (POC or laboratory) >140 mg/dL 2. |
| Glucose reading inclusion and exclusion criteria | All POC glucose values. | Additional analysis: exclude glucose values on hospital day 1 and on hospital day 15 and after. Additional analysis: exclude glucose values measured within 60 minutes of a previous value. | All POC and other glucose values used to guide care. | |
| Measures of safety | Analysis by patient‐day: Percentage of patient‐days with 1 or more values 40, 70, and >300 mg/dL. | Analysis by patient‐day: Percentage of patient‐days with 1 or more values 40, 70, and >300 mg/dL. | ||
| Measures of glucose control | Analysis by patient‐day: Percentage of patient‐days with mean 140, 180 mg/dL and/or Percentage of patient‐days with all values 180 mg/dL. | Analysis by patient‐day: Patient day‐weighted mean glucose. | Analysis by glucose reading: Percentage of readings 110, 140 mg/dL. | 3‐BG as above for all patients in critical care units.∥ Hyperglycemic index for all patients in critical care units (AUC of glucose values above target). |
| Analysis by patient stay: Percentage of patient stays with mean 140, 180 mg/dL. | Analysis by patient stay: Mean percentage of glucose readings of each patient 180 mg/dL. | Analysis by patient‐day: Percentage of patient‐days with mean 110, 140 mg/dL, and/or Percentage of patient‐days with all values 110, 140 mg/dL. | ||
| Analysis by hospital day: Percentage of patients with mean glucose readings 140, 180 mg/dL by hospital day (days 17). | Analysis by patient stay: 3‐day blood glucose average (3‐BG) for selected perioperative patients: Percentage of patients with 3‐BG 110, 140 mg/dL. Mean time (hours) to reach glycemic target (BG 110 or 140 mg/dL) on insulin infusion. | |||
| Measures of insulin use | Percentage of patients on any subcutaneous insulin that has a scheduled basal insulin component (glargine, NPH, or detemir). | Percentage of patients with at least 2 POC and/or laboratory glucose readings >180 mg/dL who have a scheduled basal insulin component. Percentage of eating patients with hyperglycemia as defined above with scheduled basal insulin and nutritional insulin. Percentage of patients and patient‐days with any changes in insulin orders the day after 2 or more episodes of hypoglycemia or hyperglycemia (ie, 70 or >180 mg/dL). | Percentage of patients with 2 POC or laboratory glucose readings >140 mg/dL placed on insulin infusion protocol. | |
| Other process measures | Glucose measured within 8 hours of hospital admission. | POC glucose testing at least 4 times a day for all patients with diabetes or hyperglycemia as defined above. | Glucose measured within 8 hours of hospital admission. | Appropriateness of hypoglycemia treatment and documentation. |
| A1C measurement obtained or available within 30 days of admission. | Measures of adherence to specific components of management protocol. | Frequency of BG testing (eg, per protocol if on insulin infusion; every 68 hours if not). | Clinical events of severe hypoglycemia reported through the organization's critical events reporting tool. | |
| Appropriateness of hypoglycemia treatment and documentation. | Root causes of hypoglycemia. | |||
| Clinical events of severe hypoglycemia reported through the organization's critical events reporting tool. | Appropriate use of IV‐to‐SC insulin transition protocol. | |||
| Root causes of hypoglycemia. | ||||
For each domain of glycemic management (glycemic control, safety, and insulin use), the task force chose a set of best measures. They are presented as two tiers of measurement standards, depending on the capabilities of the institution and the planned uses of the data. Tier 1 includes measures that, although they do take time and resources to collect, are feasible for most institutions. Tier 2 measures are recommended for hospitals with easy manipulation of electronic sources of data and for reporting quality‐of‐care measures for widespread publication, that is, in the context of a research study. It should be emphasized that these recommendations are only meant as a guide: the actual measures chosen should meet the needs and capabilities of each institution.
We recognize that few data support the recommendations made by this task force, that such data are needed, and that the field of data collection and analysis for hospital glycemic management is rapidly evolving. The hope is to begin the standardization process, promote dialogue in this field, and eventually reach a consensus in collaboration with the ADA, AACE, and other pertinent stakeholders.
CONCLUSIONS
Like the field of inpatient glycemic management itself, the field of devising metrics to measure the quality of inpatient glycemic control is also in its infancy and quickly evolving. One should not be paralyzed by the lack of consensus regarding measurementthe important point is to pick a few complementary metrics and begin the process. The table of recommendations can hopefully serve as a starting point for many institutions, with a focus on efficacy (glycemic control), safety (hypoglycemia), and process (insulin use patterns). As your institution gains experience with measurement and the field evolves, your metrics will likely change. We recommend keeping all process and outcome data in its raw form so that it can be summarized in different ways over time. It is also important not to wait for the perfect data collection tool before beginning to analyze data: sampling and paper processes are acceptable if automated data collection is not yet possible. Eventually, blood glucose meter readings should be downloaded into a central database that interfaces with hospital data repositories so data can be analyzed in conjunction with patient, service, and unit‐level information. Only with a rigorous measurement process can institutions hope to know whether their changes are resulting in improved care for patients.
- ,,, et al.“Glucometrics”—assessing the quality of inpatient glucose management.Diabetes Technol Ther.2006;8:560–569.
- ,,.Effect of hyperglycemia and continuous intravenous insulin infusions on outcomes of cardiac surgical procedures: the Portland Diabetic Project.Endocr Pract.2004;10(suppl 2):21–33.
- ,,.Hyperglycaemic index as a tool to assess glucose control: a retrospective study.Crit Care.2004;8:R122–R127.
- ,,, et al.Glucometrics in patients hospitalized with acute myocardial infarction: defining the optimal outcomes‐based measure of risk.Circulation.2008;117:1018–1027.
- ,,.Statistical process control as a tool for research and healthcare improvement.Qual Saf Health Care.2003;12:458–464.
Data collection, analysis, and presentation are key to the success of any hospital glycemic control initiative. Such efforts enable the management team to track improvements in processes and outcomes, make necessary changes to their quality improvement efforts, justify the provision of necessary time and resources, and share their results with others. Reliable metrics for assessing glycemic control and frequency of hypoglycemia are essential to accomplish these tasks and to assess whether interventions result in more benefit than harm. Hypoglycemia metrics must be especially convincing because fear of hypoglycemia remains a major source of clinical inertia, impeding efforts to improve glucose control.
Currently, there are no official standards or guidelines for formulating metrics on the quality of inpatient glycemic control. This creates several problems. First, different metrics vary in their biases and in their responsiveness to change. Thus, use of a poor metric could lead to either a falsely positive or falsely negative impression that a quality improvement intervention is in fact improving glycemic control. Second, the proliferation of different measures and analytical plans in the research and quality improvement literature make it very difficult for hospitals to compare baseline performance, determine need for improvement, and understand which interventions may be most effective.
A related article in this supplement provides the rationale for improved inpatient glycemic control. That article argues that the current state of inpatient glycemic control, with the frequent occurrence of severe hyperglycemia and irrational insulin ordering, cannot be considered acceptable, especially given the large body of data (albeit largely observational) linking hyperglycemia to negative patient outcomes. However, regardless of whether one is an advocate or skeptic of tighter glucose control in the intensive care unit (ICU) and especially the non‐ICU setting, there is no question that standardized, valid, and reliable metrics are needed to compare efforts to improve glycemic control, better understand whether such control actually improves patient care, and closely monitor patient safety.
This article provides a summary of practical suggestions to assess glycemic control, insulin use patterns, and safety (hypoglycemia and severe hyperglycemia). In particular, we discuss the pros and cons of various measurement choices. We conclude with a tiered summary of recommendations for practical metrics that we hope will be useful to individual improvement teams. This article is not a consensus statement but rather a starting place that we hope will begin to standardize measurement across institutions and advance the dialogue on this subject. To more definitely address this problem, we call on the American Association of Clinical Endocrinologists (AACE), American Diabetes Association (ADA), Society of Hospital Medicine (SHM), and others to agree on consensus standards regarding metrics for the quality of inpatient glycemic control.
MEASURING GLYCEMIC CONTROL: GLUCOMETRICS
Glucometrics may be defined as the systematic analysis of blood glucose (BG) dataa phrase initially coined specifically for the inpatient setting. There are numerous ways to do these analyses, depending on which patients and glucose values are considered, the definitions used for hypoglycemia and hyperglycemia, the unit of measurement (eg, patient, patient‐day, individual glucose value), and the measure of control (eg, mean, median, percent of glucose readings within a certain range). We consider each of these dimensions in turn.
Defining the Target Patient Population
The first decision to be made is which patients to include in your analysis. Choices include the following:
-
Patients with a discharge diagnosis of diabetes: this group has face validity and intuitive appeal, is easy to identify retrospectively, and may capture some untested/untreated diabetics, but will miss patients with otherwise undiagnosed diabetes and stress hyperglycemia. It is also subject to the variable accuracy of billing codes.
-
Patients with a certain number of point‐of‐care (POC) glucose measurements: this group is also easy to identify, easy to measure, and will include patients with hyperglycemia without a previous diagnosis of diabetes, but will miss patients with untested/untreated hyperglycemia. Also, if glucose levels are checked on normoglycemic, nondiabetic patients, these values may dilute the overall assessment of glycemic control.
-
Patients treated with insulin in the hospital: this is a good choice if the purpose is mainly drug safety and avoidance of hypoglycemia, but by definition excludes most untreated patients.
-
Patients with 2 or more BG values (laboratory and/or POC) over a certain threshold (eg, >180 mg/dL). This will likely capture more patients with inpatient hyperglycemia, whether or not detected by the medical team, but is subject to wide variations in the frequency and timing of laboratory glucose testing, including whether or not the values are pre‐prandial (note that even preprandial POC glucose measurements are not always in fact fasting values).
Other considerations include the following:
-
Are there natural patient subgroups that should be measured and analyzed separately because of different guidelines? For example, there probably should be separate/emndependent inclusion criteria and analyses for critical care and noncritical care units because their glycemic targets and management considerations differ.
-
Which patients should be excluded? For example, if targeting subcutaneous insulin use in general hospitalized patients, one might eliminate those patients who are admitted specifically as the result of a diabetes emergency (eg, diabetic ketoacidosis [DKA] and hyperglycemic hyperosmolar state [HHS]), as their marked and prolonged hyperglycemia will skew BG data. Pregnant women should generally be excluded from broad‐based analyses or considered as a discrete category because they have very different targets for BG therapy. Patients with short lengths of stay may be less likely to benefit from tight glucose control and may also be considered for post hoc exclusion. One might also exclude patients with very few evaluable glucose readings (eg, fewer than 5) to ensure that measurement is meaningful for a given patient, keeping in mind that this may also exclude patients with undetected hyperglycemia, as mentioned above. Finally, patients receiving palliative care should also be considered for exclusion if feasible.
Recommendation: Do not limit analyses to only those patients with a diagnosis of diabetes or only those on insulin, which will lead to biased results.
-
For noncritical care patients, we recommend a combined approach: adult patients with a diagnosis of diabetes (e. g. using diagnosis‐related group [DRG] codes 294 or 295 or International Classification of Diseases 9th edition [ICD9] codes 250.xx) or with hyperglycemia (eg, 2 or more random laboratory and/or point of care (POC) BG values >180 mg/dL or 2 or more fasting BG values >130 mg/dL), excluding patients with DKA or HHS or who are pregnant.
-
For critical care units, we recommend either all patients, or patients with at least mild hyperglycemia (eg, 2 random glucose levels >140 mg/dL). Critical care patients with DKA, HHS, and pregnancy should be evaluated separately if possible.
Which Glucose Values to Include and Exclude
To answer this question, we first need to decide which method to use for BG measurement. There are several ways to measure BG, including the type of sample collected (capillary [fingerstick], arterial, and venous) and the technique used (central laboratory analyzing plasma, central laboratory analyzing whole blood [eg, from an arterial blood gas sample], glucose meter [usually calibrated to plasma], etc.). The POC (eg, capillary, glucose meter) glucose measurements alone are often preferred in the non‐ICU setting because laboratory plasma values generally provide little additional information and typically lower the mean glucose by including redundant fasting values.1 In critical care units, several different methods are often used together, and each merits inclusion. The inherent differences in calibration between the methods do not generally require separate analyses, especially given the frequency of testing in the ICU setting.
The next question is which values to include in analyses. In some situations, it may be most useful to focus on a certain period of hospitalization, such as the day of a procedure and the next 2 days in assessing the impact of the quality of perioperative care, or the first 14 days of a noncritical care stay to keep outliers for length of stay (LOS) from skewing the data. In the non‐ICU setting, it may be reasonable to exclude the first day of hospitalization, as early BG control is impacted by multiple variables beyond direct control of the clinician (eg, glucose control prior to admission, severity of presenting illness) and may not realistically reflect your interventions. (Keep in mind, however, that it may be useful to adjust for the admission glucose value in multivariable models given its importance to clinical outcomes and its strong relationship to subsequent inpatient glucose control.) However, in critical care units, it is reasonable to include the first day's readings in analyses given the high frequency of glucose measurements in this setting and the expectation that glucose control should be achieved within a few hours of starting an intravenous insulin infusion.
If feasible to do so with your institution's data capture methods, you may wish to select only the regularly scheduled (before each meal [qAC] and at bedtime [qHS], or every 6 hours [q6h]) glucose readings for inclusion in the summary data of glycemic control in the non‐ICU setting, thereby reducing bias caused by repeated measurements around extremes of glycemic excursions. An alternative in the non‐ICU setting is to censor glucose readings within 60 minutes of a previous reading.
Recommendation:
-
In the non‐ICU setting, we recommend first looking at all POC glucose values and if possible repeating the analyses excluding hospital day 1 and hospital day 15 and beyond, and also excluding glucose values measured within 60 minutes of a previous value.
-
In critical care units, we recommend evaluating all glucose readings used to guide care.
Units of Analysis
There are several different units of analysis, each with its own advantages and disadvantages:
-
Glucose value: this is the simplest measure and the one with the most statistical power. All glucose values for all patients of interest comprise the denominator. A report might say, for example, that 1% of the 1000 glucose values were 70 mg/dL during a certain period or that the mean of all glucose values collected for the month from patients in noncritical care areas was 160 mg/dL. The potential disadvantages of this approach are that these analyses are less clinically relevant than patient‐level analyses and that patients with many glucose readings and long hospitalizations may skew the data.
-
Patient (or the Patient Stay, [ie, the entire hospitalization]): all patients who are monitored make up the denominator. The numerator may be the percentage of patients with any hypoglycemia during their hospital stay or the percentage of patients achieving a certain mean glucose during their hospitalization, for example. This is inherently more clinically meaningful than using glucose value as a unit of analysis. A major disadvantage is not controlling for LOS effects. For example, a hospitalized patient with a long LOS is much more likely to be characterized as having at least 1 hypoglycemic value than is a patient with a shorter LOS. Another shortcoming is that this approach does not correct for uneven distribution of testing. A patient's mean glucose might be calculated on the basis of 8 glucose values on the first day of hospitalization, 4 on the second day, and 1 on the third day. Despite all these shortcomings, reporting by patient remains a popular and valid method of presenting glycemic control results, particularly when complemented by other views and refined to control for the number of readings per day.
-
Monitored Patient‐Day: The denominator in this setting is the total number of days a patient glucose level is monitored. The benefits of this method have been described and advocated in the literature.1 As with patient‐level analyses, this measure will be more rigorous and meaningful if the BG measures to be evaluated have been standardized. Typical reports might include percentage of monitored days with any hypoglycemia, or percentage of monitored days with all glucose values in the desired range. This unit of analysis may be considered more difficult to generate and to interpret. On the other hand, it is clinically relevant, less biased by LOS effects, and may be considered the most actionable metric by clinicians. This method provides a good balance when presented with data organized by patient.
The following example uses all 3 units of measurement, in this case to determine the rate of hypoglycemia, demonstrating the different but complementary information that each method provides:
-
In 1 month, 3900 POC glucose measurements were obtained from 286 patients representing 986 monitored patient‐days. With hypoglycemia defined as POC BG 60 mg/dL, the results showed the following:
-
50 of 3900 measurements (1.4%) were hypoglycemic 22 of 286 patients (7.7%) had 1 hypoglycemic episodes
-
40 of 986 monitored days (4.4%) had 1 hypoglycemic episodes.
The metric based on the number of glucose readings could be considered the least clinically relevant because it is unclear how many patients were affected; moreover, it may be based on variable testing patterns among patients, and could be influenced disproportionately by 1 patient with frequent hypoglycemia, many glucose readings, and/or a long LOS. One could argue that the patient‐stay metric is artificially elevated because a single hypoglycemic episode characterizes the entire stay as hypoglycemic. On the other hand, at least it acknowledges the number of patients affected by hypoglycemia. The patient‐day unit of analysis likely provides the most balanced view, one that is clinically relevant and measured over a standard period of time, and less biased by LOS and frequency of testing.
One way to express patient‐day glycemic control that deserves special mention is the patient‐day weighted mean. A mean glucose is calculated for each patient‐day, and then the mean is calculated across all patient‐days. The advantage of this approach is that it corrects for variation in the number of glucose readings each day; all hospital days are weighted equally.
Recommendation:
-
In noncritical care units, we recommend a combination of patient‐day and patient‐stay measures.
-
In critical care units, it is acceptable to also use glucose reading as the unit of measurement given more frequent and uniform data collection, but it should be complemented by more meaningful patient‐day and patient‐stay measures.
Measures of Control
In addition to deciding the unit(s) of analysis, another issue concerns which measures of control to use. These could include rates of hypoglycemia and hyperglycemia, percentage of glucose readings within various ranges (eg, 70, 70180, >180 mg/dL), mean glucose value, percentage of patient‐days during which the mean glucose is within various ranges, or the in control rate (ie, when all glucose values are within a certain range).
As with the various units of analysis, each of these measures of control has various advantages and disadvantages. For example, mean glucose is easy to report and understand, but masks extreme values. Percentage of glucose values within a certain range (eg, per patient, averaged across patients) presents a more complete picture but is a little harder to understand and will vary depending on the frequency of glucose monitoring. As mentioned above, this latter problem can be corrected in part by including only certain glucose values. Percent of glucose values within range may also be less sensitive to change than mean glucose (eg, a glucose that is lowered from 300 mg/dL to 200 mg/dL is still out of range). We recommend choosing a few, but not all, measures of control in order to get a complete picture of glycemic control. Over time one can then refine the measures being used to meet the needs of the glycemic control team and provide data that will drive the performance improvement process.
In critical care and perioperative settings, interest in glycemic control is often more intense around the time of a particular event such as major surgery or after admission to the ICU. Some measures commonly used in performing such analyses are:
-
All values outside a target range within a designated crucial period. For example, the University Healthcare Consortium and other organizations use a simple metric to gauge perioperative glycemic control. They collect the fasting glucose on postoperative days 1 and 2 and then calculate the percentage of postoperative days with any fasting glucose >200 mg/dL. Of course, this is a very liberal target, but it can always be lowered in a stepwise fashion once it is regularly being reached.
-
Three‐day blood glucose average. The Portland group uses the mean glucose of each patient for the period that includes the day of coronary artery bypass graft (CABG) surgery and the following 2 days. The 3‐day BG average (3‐BG) correlates very well with patient outcomes and can serve as a well‐defined target.2 It is likely that use of the 3‐BG would work well in other perioperative/trauma settings and could work in the medical ICU as well, with admission to the ICU as the starting point for calculation of the 3‐BG.
Hyperglycemic Index
Measuring the hyperglycemic index (HGI) is a validated method of summarizing glycemic control of ICU patients.3 It is designed to take into account the sometimes uneven distribution of patient testing. Time is plotted on the x‐axis and glucose values on the y‐axis. The HGI is calculated the area under the curve of glycemic values but above the upper limit of normal (ie, 110 mg/dL). Glucose values in the normal or hypoglycemic range are not included in the AUC. Mortality correlated well with this glycemic index. However, a recent observational study of glucometrics in patients hospitalized with acute myocardial infarction found that the simple mean of each patient's glucose values over the entire hospitalization was as predictive of in‐hospital mortality as the HGI or the time‐averaged glucose (AUC for all glucose values).4 In this study, metrics derived from glucose readings for the entire hospitalization were more predictive than those based on the first 24 or 48 hours or on the admission glucose.
Analyses Describing Change in Glycemic Control Over Time in the Hospital
In the critical care setting, this unit of analysis may be as simple as the mean time to reach the glycemic target on your insulin infusion protocol. On noncritical care wards, it is a bit more challenging to characterize the improvement (or clinical inertia) implied by failure of hyperglycemia to lessen as an inpatient stay progresses. One method is to calculate the mean glucose (or percentage of glucose values in a given range) for each patient on hospital day (HD) 1, and repeat for each HD (up to some reasonable limit, such as 5 or 7 days).
Recommendations:
-
In noncritical units, we recommend a limited set of complementary measures, such as the patient‐day weighted mean glucose, mean percent of glucose readings per patient that are within a certain range, and percentage of patients whose mean glucose is within a certain range on each hospital day.
-
In critical care units, it is often useful to focus measures around a certain critical event such as the 3‐day blood glucose average and to use measures such as the HGI that take advantage of more frequent blood glucose testing.
Definitions of Hyperglycemia and Hypoglycemia
Glucometrics outcomes will obviously depend on the thresholds established for hyperglycemia and hypoglycemia. Many centers define hypoglycemia as 60 mg/dL, whereas the ADA definition, based on physiologic changes that may take place, defines hypoglycemia (at least in the outpatient setting) as 70 mg/dL. Hypoglycemia may be further stratified by severity, with any glucose 40 mg/dL, for instance, defined as severe hypoglycemia.
Similarly, the definition of hyperglycemia (and therefore good control) must also be defined. Based on definitions developed by the ADA and AACE, the state of the medical literature, and current understanding of the pathophysiology of hyperglycemia, thresholds for critical care units include 110 mg/dL, 130 mg/dL, and 140 mg/dL, and options in noncritical care units include 130 mg/dL, 140 mg/dL, and 180 mg/dL. Because these thresholds implicitly assume adverse effects when glucose levels are above them, these levels are subject to revision as data become available confirming the benefits and safety of targeted glycemic control in various settings and patient populations.
Introducing optimal BG targets in a stepped fashion over time should also be considered. Furnary et al.2 have done this in the Portland Project, which tracks glycemic control in cardiac surgery patients receiving intravenous insulin therapy. The initial BG target for this project was 200 mg/dL; it was subsequently lowered stepwise over several years to 150 mg/dL, then to 120 mg/dL, and most recently to 110 mg/dL. This approach allows the safe introduction of targeted glycemic control and promotes acceptance of the concept by physicians and the allied nursing and medical staff.
Recommendations:
-
In noncritical care units, it is reasonable to use 40 mg/dL for severe hypoglycemia, 70 mg/dL for hypoglycemia, 130 mg/dL for fasting hyperglycemia, 180 mg/dL for random or postprandial hyperglycemia, and 300 mg/dL for severe hyperglycemia, keeping in mind that these thresholds are arbitrary. In critical care units, values from 110 mg/dL to 140 mg/dL might be better thresholds for hyperglycemia, but it may take time to safely and effectively move an organization toward these lower targets.
Other Considerations Relative to Glucometrics
Yale Glucometrics Website
The Yale Informatics group has put together a Web‐based resource (
Other Analytic Resources
Commercially available software, such as the RALS system (Medical Automation Systems, Inc., Charlottesville, VA) can gather POC glucose measurements directly from devices and provide real‐time reports of glycemic control, stratified by inpatient unit, using user‐defined targets for hypoglycemia and hyperglycemia. While they are no substitute for a dedicated, on‐site data analyst, such systems can be very useful for smaller hospitals with minimal data or information technology support staff.
APPROACHES TO ANALYSIS: RUN CHARTS
Most conventional clinical trials hold interventions fixed for a period of time and compare results with and without the intervention. For quality improvement studies, this is still a valid way to proceed, especially if studied as a randomized controlled trial. Such methods may be preferred when the clinical question is Does this type of intervention work in general? and the desired output is publication in peer‐reviewed journals so that others can learn about and adopt the intervention to their own institution. A before and after study with a similar analytic approach may also be valid, although concerns about temporal trends and cointerventions potentially compromise the validity of such studies. This approach again assumes that an intervention is held fixed over time such that it is clear what patients received during each time period.
If the desired result is improvement at a given institution (the question is Did we improve care?) then it may be preferable to present results over time using run‐charts. In a run chart, the x‐axis is time and the y‐axis the desired metric, such as patient‐day weighted mean glucose. Points in time when interventions were introduced or modified can be highlighted. Run charts have several advantages over before‐and‐after summaries: they do not require interventions remaining fixed and are more compatible with continuous quality improvement methods, it is easier to see the effect of different aspects of the interventions as they occur, one can get a quicker picture of whether something is working, and it is easier to separate out the impact of the intervention from secular trends. Finally, the use of run charts does not imply the absence of statistical rigor. Run charts with statistical process control (SPC) limits5 can easily convey when the observed time trend is unlikely to be due to chance using prespecified P values. (A full discussion of SPC and other methods to study quality improvement interventions is beyond the scope of this article.)
ASSESSING PATTERNS OF INSULIN USE AND ORDER SET UTILIZATION
Besides measuring the impact of quality improvement interventions on glucose control, it is important to measure processes such as proper insulin use. As mentioned in other articles in this supplement, processes are much more sensitive to change than outcomes. Failure to change processes should lead one to make changes to the intervention.
ICU and Perioperative Settings
For ICU and perioperative settings, the major process measure will likely be use of the insulin infusion order set. Designation of BG levels that trigger insulin infusion in these settings should be agreed upon in advance. The number of patients who meet the predefined glycemic criteria would make up the denominator, and the number of patients on the insulin infusion order set would make up the numerator.
NonCritical Care Units
On noncritical care units, measuring the percentage of subcutaneous insulin regimens that contain a basal insulin is a useful way to monitor the impact of an intervention. A more detailed analysis could examine the percentage of patients on simultaneous basal and nutritional insulin (if applicable). An important measure of clinical inertia is to track the percentage of patients who had changes in their insulin regimens on days after hypoglycemic or hyperglycemic excursions. Another important measure is the frequency with which the standardized order set is being used, analogous to the measure of insulin infusion use in the ICU. A final process measure, indirectly related to insulin use, is the frequency of use of oral diabetes agents, especially by patients for whom their use is contraindicated (eg, patients with congestive heart failure who are on thiazolidinediones and patients with renal insufficiency or receiving intravenous contrast continued on metformin).
OTHER CONSIDERATIONS AND METRICS
Examples of other metrics that can be used to track the success of quality improvement efforts include:
-
Glucose measurement within 8 hours of hospital admission.
-
Glycated hemoglobin (A1C) measurement obtained or available within 30 days of admission to help guide inpatient and especially discharge management.
-
Appropriate glucose testing in patients with diabetes or hyperglycemia (eg, 4 times per day in patients not on insulin infusion protocols, at least until 24 hours of euglycemia is documented).
-
The percentage of patients on insulin with on‐time tray delivery.
-
The timing of subcutaneous insulin administration in relation to glucose testing and nutrition delivery.
-
Documentation of carbohydrate intake among patients who are eating.
-
Satisfaction of physicians and nurses with order sets or protocols, using standard surveys.
-
Physician and nurse knowledge, attitudes, and beliefs about insulin administration, fear of hypoglycemia, treatment of hypoglycemia, and glycemic control in the hospital.
-
Patient satisfaction with their diabetes care in the hospital, including the education they received.
-
Nursing and physician education/certification in insulin prescribing, insulin administration, and other diabetes care issues.
-
Patient outcomes strongly associated with glycemic control, (eg, surgical wound infections, ICU LOS, catheter‐related bloodstream infections).
-
Appropriate treatment and documentation of hypoglycemia (eg, in accordance with hospital policy).
-
Documentation of severe hypoglycemic events through the hospital's adverse events reporting system (these may actually increase as change comes to the organization and as clinical personnel are more attuned to glycemic control).
-
Root causes of hypoglycemic events, which can be used to understand and prevent future events.
-
Appropriate transitions from IV to SC insulin regimens, (eg, starting basal insulin prior to discontinuing infusion in patients who have been on an insulin infusion of at least 2 units/hour or who have a known diagnosis of diabetes or A1C >7).
(Survey instruments and other measurement tools are available from the authors upon request.)
SHM GLYCEMIC CONTROL TASK FORCE SUMMARY RECOMMENDATIONS
The SHM Glycemic Control Task Force is working to develop standardized measures of inpatient glucose control and related indicators to track progress of hospital glycemic control initiatives (see the introduction to this supplement for a description of the charge and membership of this task force). The goals of the Task Force's metrics recommendations (Table 1) are several‐fold: (1) create a set of measurements that are complete but not overly burdensome; (2) create realistic measures that can be applied to institutions with different data management capabilities; and (3) allow for comparison across institutions for benchmarking purposes, evaluation of quality improvement projects, and reporting of results for formal research studies in this field.
| Measurement Issue | NonCritical Care Units | Critical Care Units | ||
|---|---|---|---|---|
| Tier 1 Recommendations | Tier 2 Recommendations | Tier 1 Recommendations | Tier 2 Recommendations | |
| ||||
| Patient inclusion and exclusion criteria | All adult patients with POC glucose testing (sampling acceptable). Exclude patients with DKA or HHS or who are pregnant. | All adult patients with diagnosis of diabetes by ICD‐9 code* or by glucose testing: random glucose (POC or laboratory) >180 mg/dL 2 or fasting glucose >130 mg/dL 2, excluding patients with DKA or HHS or who are pregnant. Additional analysis: exclude patients with 5 evaluable glucose readings, patients with LOS 2 days, or receiving palliative care. | All patients in every critical care unit (sampling acceptable). | Patients with DKA, HHS, or pregnancy in separate analyses. All patients in every critical care unit with random glucose (POC or laboratory) >140 mg/dL 2. |
| Glucose reading inclusion and exclusion criteria | All POC glucose values. | Additional analysis: exclude glucose values on hospital day 1 and on hospital day 15 and after. Additional analysis: exclude glucose values measured within 60 minutes of a previous value. | All POC and other glucose values used to guide care. | |
| Measures of safety | Analysis by patient‐day: Percentage of patient‐days with 1 or more values 40, 70, and >300 mg/dL. | Analysis by patient‐day: Percentage of patient‐days with 1 or more values 40, 70, and >300 mg/dL. | ||
| Measures of glucose control | Analysis by patient‐day: Percentage of patient‐days with mean 140, 180 mg/dL and/or Percentage of patient‐days with all values 180 mg/dL. | Analysis by patient‐day: Patient day‐weighted mean glucose. | Analysis by glucose reading: Percentage of readings 110, 140 mg/dL. | 3‐BG as above for all patients in critical care units.∥ Hyperglycemic index for all patients in critical care units (AUC of glucose values above target). |
| Analysis by patient stay: Percentage of patient stays with mean 140, 180 mg/dL. | Analysis by patient stay: Mean percentage of glucose readings of each patient 180 mg/dL. | Analysis by patient‐day: Percentage of patient‐days with mean 110, 140 mg/dL, and/or Percentage of patient‐days with all values 110, 140 mg/dL. | ||
| Analysis by hospital day: Percentage of patients with mean glucose readings 140, 180 mg/dL by hospital day (days 17). | Analysis by patient stay: 3‐day blood glucose average (3‐BG) for selected perioperative patients: Percentage of patients with 3‐BG 110, 140 mg/dL. Mean time (hours) to reach glycemic target (BG 110 or 140 mg/dL) on insulin infusion. | |||
| Measures of insulin use | Percentage of patients on any subcutaneous insulin that has a scheduled basal insulin component (glargine, NPH, or detemir). | Percentage of patients with at least 2 POC and/or laboratory glucose readings >180 mg/dL who have a scheduled basal insulin component. Percentage of eating patients with hyperglycemia as defined above with scheduled basal insulin and nutritional insulin. Percentage of patients and patient‐days with any changes in insulin orders the day after 2 or more episodes of hypoglycemia or hyperglycemia (ie, 70 or >180 mg/dL). | Percentage of patients with 2 POC or laboratory glucose readings >140 mg/dL placed on insulin infusion protocol. | |
| Other process measures | Glucose measured within 8 hours of hospital admission. | POC glucose testing at least 4 times a day for all patients with diabetes or hyperglycemia as defined above. | Glucose measured within 8 hours of hospital admission. | Appropriateness of hypoglycemia treatment and documentation. |
| A1C measurement obtained or available within 30 days of admission. | Measures of adherence to specific components of management protocol. | Frequency of BG testing (eg, per protocol if on insulin infusion; every 68 hours if not). | Clinical events of severe hypoglycemia reported through the organization's critical events reporting tool. | |
| Appropriateness of hypoglycemia treatment and documentation. | Root causes of hypoglycemia. | |||
| Clinical events of severe hypoglycemia reported through the organization's critical events reporting tool. | Appropriate use of IV‐to‐SC insulin transition protocol. | |||
| Root causes of hypoglycemia. | ||||
For each domain of glycemic management (glycemic control, safety, and insulin use), the task force chose a set of best measures. They are presented as two tiers of measurement standards, depending on the capabilities of the institution and the planned uses of the data. Tier 1 includes measures that, although they do take time and resources to collect, are feasible for most institutions. Tier 2 measures are recommended for hospitals with easy manipulation of electronic sources of data and for reporting quality‐of‐care measures for widespread publication, that is, in the context of a research study. It should be emphasized that these recommendations are only meant as a guide: the actual measures chosen should meet the needs and capabilities of each institution.
We recognize that few data support the recommendations made by this task force, that such data are needed, and that the field of data collection and analysis for hospital glycemic management is rapidly evolving. The hope is to begin the standardization process, promote dialogue in this field, and eventually reach a consensus in collaboration with the ADA, AACE, and other pertinent stakeholders.
CONCLUSIONS
Like the field of inpatient glycemic management itself, the field of devising metrics to measure the quality of inpatient glycemic control is also in its infancy and quickly evolving. One should not be paralyzed by the lack of consensus regarding measurementthe important point is to pick a few complementary metrics and begin the process. The table of recommendations can hopefully serve as a starting point for many institutions, with a focus on efficacy (glycemic control), safety (hypoglycemia), and process (insulin use patterns). As your institution gains experience with measurement and the field evolves, your metrics will likely change. We recommend keeping all process and outcome data in its raw form so that it can be summarized in different ways over time. It is also important not to wait for the perfect data collection tool before beginning to analyze data: sampling and paper processes are acceptable if automated data collection is not yet possible. Eventually, blood glucose meter readings should be downloaded into a central database that interfaces with hospital data repositories so data can be analyzed in conjunction with patient, service, and unit‐level information. Only with a rigorous measurement process can institutions hope to know whether their changes are resulting in improved care for patients.
Data collection, analysis, and presentation are key to the success of any hospital glycemic control initiative. Such efforts enable the management team to track improvements in processes and outcomes, make necessary changes to their quality improvement efforts, justify the provision of necessary time and resources, and share their results with others. Reliable metrics for assessing glycemic control and frequency of hypoglycemia are essential to accomplish these tasks and to assess whether interventions result in more benefit than harm. Hypoglycemia metrics must be especially convincing because fear of hypoglycemia remains a major source of clinical inertia, impeding efforts to improve glucose control.
Currently, there are no official standards or guidelines for formulating metrics on the quality of inpatient glycemic control. This creates several problems. First, different metrics vary in their biases and in their responsiveness to change. Thus, use of a poor metric could lead to either a falsely positive or falsely negative impression that a quality improvement intervention is in fact improving glycemic control. Second, the proliferation of different measures and analytical plans in the research and quality improvement literature make it very difficult for hospitals to compare baseline performance, determine need for improvement, and understand which interventions may be most effective.
A related article in this supplement provides the rationale for improved inpatient glycemic control. That article argues that the current state of inpatient glycemic control, with the frequent occurrence of severe hyperglycemia and irrational insulin ordering, cannot be considered acceptable, especially given the large body of data (albeit largely observational) linking hyperglycemia to negative patient outcomes. However, regardless of whether one is an advocate or skeptic of tighter glucose control in the intensive care unit (ICU) and especially the non‐ICU setting, there is no question that standardized, valid, and reliable metrics are needed to compare efforts to improve glycemic control, better understand whether such control actually improves patient care, and closely monitor patient safety.
This article provides a summary of practical suggestions to assess glycemic control, insulin use patterns, and safety (hypoglycemia and severe hyperglycemia). In particular, we discuss the pros and cons of various measurement choices. We conclude with a tiered summary of recommendations for practical metrics that we hope will be useful to individual improvement teams. This article is not a consensus statement but rather a starting place that we hope will begin to standardize measurement across institutions and advance the dialogue on this subject. To more definitely address this problem, we call on the American Association of Clinical Endocrinologists (AACE), American Diabetes Association (ADA), Society of Hospital Medicine (SHM), and others to agree on consensus standards regarding metrics for the quality of inpatient glycemic control.
MEASURING GLYCEMIC CONTROL: GLUCOMETRICS
Glucometrics may be defined as the systematic analysis of blood glucose (BG) dataa phrase initially coined specifically for the inpatient setting. There are numerous ways to do these analyses, depending on which patients and glucose values are considered, the definitions used for hypoglycemia and hyperglycemia, the unit of measurement (eg, patient, patient‐day, individual glucose value), and the measure of control (eg, mean, median, percent of glucose readings within a certain range). We consider each of these dimensions in turn.
Defining the Target Patient Population
The first decision to be made is which patients to include in your analysis. Choices include the following:
-
Patients with a discharge diagnosis of diabetes: this group has face validity and intuitive appeal, is easy to identify retrospectively, and may capture some untested/untreated diabetics, but will miss patients with otherwise undiagnosed diabetes and stress hyperglycemia. It is also subject to the variable accuracy of billing codes.
-
Patients with a certain number of point‐of‐care (POC) glucose measurements: this group is also easy to identify, easy to measure, and will include patients with hyperglycemia without a previous diagnosis of diabetes, but will miss patients with untested/untreated hyperglycemia. Also, if glucose levels are checked on normoglycemic, nondiabetic patients, these values may dilute the overall assessment of glycemic control.
-
Patients treated with insulin in the hospital: this is a good choice if the purpose is mainly drug safety and avoidance of hypoglycemia, but by definition excludes most untreated patients.
-
Patients with 2 or more BG values (laboratory and/or POC) over a certain threshold (eg, >180 mg/dL). This will likely capture more patients with inpatient hyperglycemia, whether or not detected by the medical team, but is subject to wide variations in the frequency and timing of laboratory glucose testing, including whether or not the values are pre‐prandial (note that even preprandial POC glucose measurements are not always in fact fasting values).
Other considerations include the following:
-
Are there natural patient subgroups that should be measured and analyzed separately because of different guidelines? For example, there probably should be separate/emndependent inclusion criteria and analyses for critical care and noncritical care units because their glycemic targets and management considerations differ.
-
Which patients should be excluded? For example, if targeting subcutaneous insulin use in general hospitalized patients, one might eliminate those patients who are admitted specifically as the result of a diabetes emergency (eg, diabetic ketoacidosis [DKA] and hyperglycemic hyperosmolar state [HHS]), as their marked and prolonged hyperglycemia will skew BG data. Pregnant women should generally be excluded from broad‐based analyses or considered as a discrete category because they have very different targets for BG therapy. Patients with short lengths of stay may be less likely to benefit from tight glucose control and may also be considered for post hoc exclusion. One might also exclude patients with very few evaluable glucose readings (eg, fewer than 5) to ensure that measurement is meaningful for a given patient, keeping in mind that this may also exclude patients with undetected hyperglycemia, as mentioned above. Finally, patients receiving palliative care should also be considered for exclusion if feasible.
Recommendation: Do not limit analyses to only those patients with a diagnosis of diabetes or only those on insulin, which will lead to biased results.
-
For noncritical care patients, we recommend a combined approach: adult patients with a diagnosis of diabetes (e. g. using diagnosis‐related group [DRG] codes 294 or 295 or International Classification of Diseases 9th edition [ICD9] codes 250.xx) or with hyperglycemia (eg, 2 or more random laboratory and/or point of care (POC) BG values >180 mg/dL or 2 or more fasting BG values >130 mg/dL), excluding patients with DKA or HHS or who are pregnant.
-
For critical care units, we recommend either all patients, or patients with at least mild hyperglycemia (eg, 2 random glucose levels >140 mg/dL). Critical care patients with DKA, HHS, and pregnancy should be evaluated separately if possible.
Which Glucose Values to Include and Exclude
To answer this question, we first need to decide which method to use for BG measurement. There are several ways to measure BG, including the type of sample collected (capillary [fingerstick], arterial, and venous) and the technique used (central laboratory analyzing plasma, central laboratory analyzing whole blood [eg, from an arterial blood gas sample], glucose meter [usually calibrated to plasma], etc.). The POC (eg, capillary, glucose meter) glucose measurements alone are often preferred in the non‐ICU setting because laboratory plasma values generally provide little additional information and typically lower the mean glucose by including redundant fasting values.1 In critical care units, several different methods are often used together, and each merits inclusion. The inherent differences in calibration between the methods do not generally require separate analyses, especially given the frequency of testing in the ICU setting.
The next question is which values to include in analyses. In some situations, it may be most useful to focus on a certain period of hospitalization, such as the day of a procedure and the next 2 days in assessing the impact of the quality of perioperative care, or the first 14 days of a noncritical care stay to keep outliers for length of stay (LOS) from skewing the data. In the non‐ICU setting, it may be reasonable to exclude the first day of hospitalization, as early BG control is impacted by multiple variables beyond direct control of the clinician (eg, glucose control prior to admission, severity of presenting illness) and may not realistically reflect your interventions. (Keep in mind, however, that it may be useful to adjust for the admission glucose value in multivariable models given its importance to clinical outcomes and its strong relationship to subsequent inpatient glucose control.) However, in critical care units, it is reasonable to include the first day's readings in analyses given the high frequency of glucose measurements in this setting and the expectation that glucose control should be achieved within a few hours of starting an intravenous insulin infusion.
If feasible to do so with your institution's data capture methods, you may wish to select only the regularly scheduled (before each meal [qAC] and at bedtime [qHS], or every 6 hours [q6h]) glucose readings for inclusion in the summary data of glycemic control in the non‐ICU setting, thereby reducing bias caused by repeated measurements around extremes of glycemic excursions. An alternative in the non‐ICU setting is to censor glucose readings within 60 minutes of a previous reading.
Recommendation:
-
In the non‐ICU setting, we recommend first looking at all POC glucose values and if possible repeating the analyses excluding hospital day 1 and hospital day 15 and beyond, and also excluding glucose values measured within 60 minutes of a previous value.
-
In critical care units, we recommend evaluating all glucose readings used to guide care.
Units of Analysis
There are several different units of analysis, each with its own advantages and disadvantages:
-
Glucose value: this is the simplest measure and the one with the most statistical power. All glucose values for all patients of interest comprise the denominator. A report might say, for example, that 1% of the 1000 glucose values were 70 mg/dL during a certain period or that the mean of all glucose values collected for the month from patients in noncritical care areas was 160 mg/dL. The potential disadvantages of this approach are that these analyses are less clinically relevant than patient‐level analyses and that patients with many glucose readings and long hospitalizations may skew the data.
-
Patient (or the Patient Stay, [ie, the entire hospitalization]): all patients who are monitored make up the denominator. The numerator may be the percentage of patients with any hypoglycemia during their hospital stay or the percentage of patients achieving a certain mean glucose during their hospitalization, for example. This is inherently more clinically meaningful than using glucose value as a unit of analysis. A major disadvantage is not controlling for LOS effects. For example, a hospitalized patient with a long LOS is much more likely to be characterized as having at least 1 hypoglycemic value than is a patient with a shorter LOS. Another shortcoming is that this approach does not correct for uneven distribution of testing. A patient's mean glucose might be calculated on the basis of 8 glucose values on the first day of hospitalization, 4 on the second day, and 1 on the third day. Despite all these shortcomings, reporting by patient remains a popular and valid method of presenting glycemic control results, particularly when complemented by other views and refined to control for the number of readings per day.
-
Monitored Patient‐Day: The denominator in this setting is the total number of days a patient glucose level is monitored. The benefits of this method have been described and advocated in the literature.1 As with patient‐level analyses, this measure will be more rigorous and meaningful if the BG measures to be evaluated have been standardized. Typical reports might include percentage of monitored days with any hypoglycemia, or percentage of monitored days with all glucose values in the desired range. This unit of analysis may be considered more difficult to generate and to interpret. On the other hand, it is clinically relevant, less biased by LOS effects, and may be considered the most actionable metric by clinicians. This method provides a good balance when presented with data organized by patient.
The following example uses all 3 units of measurement, in this case to determine the rate of hypoglycemia, demonstrating the different but complementary information that each method provides:
-
In 1 month, 3900 POC glucose measurements were obtained from 286 patients representing 986 monitored patient‐days. With hypoglycemia defined as POC BG 60 mg/dL, the results showed the following:
-
50 of 3900 measurements (1.4%) were hypoglycemic 22 of 286 patients (7.7%) had 1 hypoglycemic episodes
-
40 of 986 monitored days (4.4%) had 1 hypoglycemic episodes.
The metric based on the number of glucose readings could be considered the least clinically relevant because it is unclear how many patients were affected; moreover, it may be based on variable testing patterns among patients, and could be influenced disproportionately by 1 patient with frequent hypoglycemia, many glucose readings, and/or a long LOS. One could argue that the patient‐stay metric is artificially elevated because a single hypoglycemic episode characterizes the entire stay as hypoglycemic. On the other hand, at least it acknowledges the number of patients affected by hypoglycemia. The patient‐day unit of analysis likely provides the most balanced view, one that is clinically relevant and measured over a standard period of time, and less biased by LOS and frequency of testing.
One way to express patient‐day glycemic control that deserves special mention is the patient‐day weighted mean. A mean glucose is calculated for each patient‐day, and then the mean is calculated across all patient‐days. The advantage of this approach is that it corrects for variation in the number of glucose readings each day; all hospital days are weighted equally.
Recommendation:
-
In noncritical care units, we recommend a combination of patient‐day and patient‐stay measures.
-
In critical care units, it is acceptable to also use glucose reading as the unit of measurement given more frequent and uniform data collection, but it should be complemented by more meaningful patient‐day and patient‐stay measures.
Measures of Control
In addition to deciding the unit(s) of analysis, another issue concerns which measures of control to use. These could include rates of hypoglycemia and hyperglycemia, percentage of glucose readings within various ranges (eg, 70, 70180, >180 mg/dL), mean glucose value, percentage of patient‐days during which the mean glucose is within various ranges, or the in control rate (ie, when all glucose values are within a certain range).
As with the various units of analysis, each of these measures of control has various advantages and disadvantages. For example, mean glucose is easy to report and understand, but masks extreme values. Percentage of glucose values within a certain range (eg, per patient, averaged across patients) presents a more complete picture but is a little harder to understand and will vary depending on the frequency of glucose monitoring. As mentioned above, this latter problem can be corrected in part by including only certain glucose values. Percent of glucose values within range may also be less sensitive to change than mean glucose (eg, a glucose that is lowered from 300 mg/dL to 200 mg/dL is still out of range). We recommend choosing a few, but not all, measures of control in order to get a complete picture of glycemic control. Over time one can then refine the measures being used to meet the needs of the glycemic control team and provide data that will drive the performance improvement process.
In critical care and perioperative settings, interest in glycemic control is often more intense around the time of a particular event such as major surgery or after admission to the ICU. Some measures commonly used in performing such analyses are:
-
All values outside a target range within a designated crucial period. For example, the University Healthcare Consortium and other organizations use a simple metric to gauge perioperative glycemic control. They collect the fasting glucose on postoperative days 1 and 2 and then calculate the percentage of postoperative days with any fasting glucose >200 mg/dL. Of course, this is a very liberal target, but it can always be lowered in a stepwise fashion once it is regularly being reached.
-
Three‐day blood glucose average. The Portland group uses the mean glucose of each patient for the period that includes the day of coronary artery bypass graft (CABG) surgery and the following 2 days. The 3‐day BG average (3‐BG) correlates very well with patient outcomes and can serve as a well‐defined target.2 It is likely that use of the 3‐BG would work well in other perioperative/trauma settings and could work in the medical ICU as well, with admission to the ICU as the starting point for calculation of the 3‐BG.
Hyperglycemic Index
Measuring the hyperglycemic index (HGI) is a validated method of summarizing glycemic control of ICU patients.3 It is designed to take into account the sometimes uneven distribution of patient testing. Time is plotted on the x‐axis and glucose values on the y‐axis. The HGI is calculated the area under the curve of glycemic values but above the upper limit of normal (ie, 110 mg/dL). Glucose values in the normal or hypoglycemic range are not included in the AUC. Mortality correlated well with this glycemic index. However, a recent observational study of glucometrics in patients hospitalized with acute myocardial infarction found that the simple mean of each patient's glucose values over the entire hospitalization was as predictive of in‐hospital mortality as the HGI or the time‐averaged glucose (AUC for all glucose values).4 In this study, metrics derived from glucose readings for the entire hospitalization were more predictive than those based on the first 24 or 48 hours or on the admission glucose.
Analyses Describing Change in Glycemic Control Over Time in the Hospital
In the critical care setting, this unit of analysis may be as simple as the mean time to reach the glycemic target on your insulin infusion protocol. On noncritical care wards, it is a bit more challenging to characterize the improvement (or clinical inertia) implied by failure of hyperglycemia to lessen as an inpatient stay progresses. One method is to calculate the mean glucose (or percentage of glucose values in a given range) for each patient on hospital day (HD) 1, and repeat for each HD (up to some reasonable limit, such as 5 or 7 days).
Recommendations:
-
In noncritical units, we recommend a limited set of complementary measures, such as the patient‐day weighted mean glucose, mean percent of glucose readings per patient that are within a certain range, and percentage of patients whose mean glucose is within a certain range on each hospital day.
-
In critical care units, it is often useful to focus measures around a certain critical event such as the 3‐day blood glucose average and to use measures such as the HGI that take advantage of more frequent blood glucose testing.
Definitions of Hyperglycemia and Hypoglycemia
Glucometrics outcomes will obviously depend on the thresholds established for hyperglycemia and hypoglycemia. Many centers define hypoglycemia as 60 mg/dL, whereas the ADA definition, based on physiologic changes that may take place, defines hypoglycemia (at least in the outpatient setting) as 70 mg/dL. Hypoglycemia may be further stratified by severity, with any glucose 40 mg/dL, for instance, defined as severe hypoglycemia.
Similarly, the definition of hyperglycemia (and therefore good control) must also be defined. Based on definitions developed by the ADA and AACE, the state of the medical literature, and current understanding of the pathophysiology of hyperglycemia, thresholds for critical care units include 110 mg/dL, 130 mg/dL, and 140 mg/dL, and options in noncritical care units include 130 mg/dL, 140 mg/dL, and 180 mg/dL. Because these thresholds implicitly assume adverse effects when glucose levels are above them, these levels are subject to revision as data become available confirming the benefits and safety of targeted glycemic control in various settings and patient populations.
Introducing optimal BG targets in a stepped fashion over time should also be considered. Furnary et al.2 have done this in the Portland Project, which tracks glycemic control in cardiac surgery patients receiving intravenous insulin therapy. The initial BG target for this project was 200 mg/dL; it was subsequently lowered stepwise over several years to 150 mg/dL, then to 120 mg/dL, and most recently to 110 mg/dL. This approach allows the safe introduction of targeted glycemic control and promotes acceptance of the concept by physicians and the allied nursing and medical staff.
Recommendations:
-
In noncritical care units, it is reasonable to use 40 mg/dL for severe hypoglycemia, 70 mg/dL for hypoglycemia, 130 mg/dL for fasting hyperglycemia, 180 mg/dL for random or postprandial hyperglycemia, and 300 mg/dL for severe hyperglycemia, keeping in mind that these thresholds are arbitrary. In critical care units, values from 110 mg/dL to 140 mg/dL might be better thresholds for hyperglycemia, but it may take time to safely and effectively move an organization toward these lower targets.
Other Considerations Relative to Glucometrics
Yale Glucometrics Website
The Yale Informatics group has put together a Web‐based resource (
Other Analytic Resources
Commercially available software, such as the RALS system (Medical Automation Systems, Inc., Charlottesville, VA) can gather POC glucose measurements directly from devices and provide real‐time reports of glycemic control, stratified by inpatient unit, using user‐defined targets for hypoglycemia and hyperglycemia. While they are no substitute for a dedicated, on‐site data analyst, such systems can be very useful for smaller hospitals with minimal data or information technology support staff.
APPROACHES TO ANALYSIS: RUN CHARTS
Most conventional clinical trials hold interventions fixed for a period of time and compare results with and without the intervention. For quality improvement studies, this is still a valid way to proceed, especially if studied as a randomized controlled trial. Such methods may be preferred when the clinical question is Does this type of intervention work in general? and the desired output is publication in peer‐reviewed journals so that others can learn about and adopt the intervention to their own institution. A before and after study with a similar analytic approach may also be valid, although concerns about temporal trends and cointerventions potentially compromise the validity of such studies. This approach again assumes that an intervention is held fixed over time such that it is clear what patients received during each time period.
If the desired result is improvement at a given institution (the question is Did we improve care?) then it may be preferable to present results over time using run‐charts. In a run chart, the x‐axis is time and the y‐axis the desired metric, such as patient‐day weighted mean glucose. Points in time when interventions were introduced or modified can be highlighted. Run charts have several advantages over before‐and‐after summaries: they do not require interventions remaining fixed and are more compatible with continuous quality improvement methods, it is easier to see the effect of different aspects of the interventions as they occur, one can get a quicker picture of whether something is working, and it is easier to separate out the impact of the intervention from secular trends. Finally, the use of run charts does not imply the absence of statistical rigor. Run charts with statistical process control (SPC) limits5 can easily convey when the observed time trend is unlikely to be due to chance using prespecified P values. (A full discussion of SPC and other methods to study quality improvement interventions is beyond the scope of this article.)
ASSESSING PATTERNS OF INSULIN USE AND ORDER SET UTILIZATION
Besides measuring the impact of quality improvement interventions on glucose control, it is important to measure processes such as proper insulin use. As mentioned in other articles in this supplement, processes are much more sensitive to change than outcomes. Failure to change processes should lead one to make changes to the intervention.
ICU and Perioperative Settings
For ICU and perioperative settings, the major process measure will likely be use of the insulin infusion order set. Designation of BG levels that trigger insulin infusion in these settings should be agreed upon in advance. The number of patients who meet the predefined glycemic criteria would make up the denominator, and the number of patients on the insulin infusion order set would make up the numerator.
NonCritical Care Units
On noncritical care units, measuring the percentage of subcutaneous insulin regimens that contain a basal insulin is a useful way to monitor the impact of an intervention. A more detailed analysis could examine the percentage of patients on simultaneous basal and nutritional insulin (if applicable). An important measure of clinical inertia is to track the percentage of patients who had changes in their insulin regimens on days after hypoglycemic or hyperglycemic excursions. Another important measure is the frequency with which the standardized order set is being used, analogous to the measure of insulin infusion use in the ICU. A final process measure, indirectly related to insulin use, is the frequency of use of oral diabetes agents, especially by patients for whom their use is contraindicated (eg, patients with congestive heart failure who are on thiazolidinediones and patients with renal insufficiency or receiving intravenous contrast continued on metformin).
OTHER CONSIDERATIONS AND METRICS
Examples of other metrics that can be used to track the success of quality improvement efforts include:
-
Glucose measurement within 8 hours of hospital admission.
-
Glycated hemoglobin (A1C) measurement obtained or available within 30 days of admission to help guide inpatient and especially discharge management.
-
Appropriate glucose testing in patients with diabetes or hyperglycemia (eg, 4 times per day in patients not on insulin infusion protocols, at least until 24 hours of euglycemia is documented).
-
The percentage of patients on insulin with on‐time tray delivery.
-
The timing of subcutaneous insulin administration in relation to glucose testing and nutrition delivery.
-
Documentation of carbohydrate intake among patients who are eating.
-
Satisfaction of physicians and nurses with order sets or protocols, using standard surveys.
-
Physician and nurse knowledge, attitudes, and beliefs about insulin administration, fear of hypoglycemia, treatment of hypoglycemia, and glycemic control in the hospital.
-
Patient satisfaction with their diabetes care in the hospital, including the education they received.
-
Nursing and physician education/certification in insulin prescribing, insulin administration, and other diabetes care issues.
-
Patient outcomes strongly associated with glycemic control, (eg, surgical wound infections, ICU LOS, catheter‐related bloodstream infections).
-
Appropriate treatment and documentation of hypoglycemia (eg, in accordance with hospital policy).
-
Documentation of severe hypoglycemic events through the hospital's adverse events reporting system (these may actually increase as change comes to the organization and as clinical personnel are more attuned to glycemic control).
-
Root causes of hypoglycemic events, which can be used to understand and prevent future events.
-
Appropriate transitions from IV to SC insulin regimens, (eg, starting basal insulin prior to discontinuing infusion in patients who have been on an insulin infusion of at least 2 units/hour or who have a known diagnosis of diabetes or A1C >7).
(Survey instruments and other measurement tools are available from the authors upon request.)
SHM GLYCEMIC CONTROL TASK FORCE SUMMARY RECOMMENDATIONS
The SHM Glycemic Control Task Force is working to develop standardized measures of inpatient glucose control and related indicators to track progress of hospital glycemic control initiatives (see the introduction to this supplement for a description of the charge and membership of this task force). The goals of the Task Force's metrics recommendations (Table 1) are several‐fold: (1) create a set of measurements that are complete but not overly burdensome; (2) create realistic measures that can be applied to institutions with different data management capabilities; and (3) allow for comparison across institutions for benchmarking purposes, evaluation of quality improvement projects, and reporting of results for formal research studies in this field.
| Measurement Issue | NonCritical Care Units | Critical Care Units | ||
|---|---|---|---|---|
| Tier 1 Recommendations | Tier 2 Recommendations | Tier 1 Recommendations | Tier 2 Recommendations | |
| ||||
| Patient inclusion and exclusion criteria | All adult patients with POC glucose testing (sampling acceptable). Exclude patients with DKA or HHS or who are pregnant. | All adult patients with diagnosis of diabetes by ICD‐9 code* or by glucose testing: random glucose (POC or laboratory) >180 mg/dL 2 or fasting glucose >130 mg/dL 2, excluding patients with DKA or HHS or who are pregnant. Additional analysis: exclude patients with 5 evaluable glucose readings, patients with LOS 2 days, or receiving palliative care. | All patients in every critical care unit (sampling acceptable). | Patients with DKA, HHS, or pregnancy in separate analyses. All patients in every critical care unit with random glucose (POC or laboratory) >140 mg/dL 2. |
| Glucose reading inclusion and exclusion criteria | All POC glucose values. | Additional analysis: exclude glucose values on hospital day 1 and on hospital day 15 and after. Additional analysis: exclude glucose values measured within 60 minutes of a previous value. | All POC and other glucose values used to guide care. | |
| Measures of safety | Analysis by patient‐day: Percentage of patient‐days with 1 or more values 40, 70, and >300 mg/dL. | Analysis by patient‐day: Percentage of patient‐days with 1 or more values 40, 70, and >300 mg/dL. | ||
| Measures of glucose control | Analysis by patient‐day: Percentage of patient‐days with mean 140, 180 mg/dL and/or Percentage of patient‐days with all values 180 mg/dL. | Analysis by patient‐day: Patient day‐weighted mean glucose. | Analysis by glucose reading: Percentage of readings 110, 140 mg/dL. | 3‐BG as above for all patients in critical care units.∥ Hyperglycemic index for all patients in critical care units (AUC of glucose values above target). |
| Analysis by patient stay: Percentage of patient stays with mean 140, 180 mg/dL. | Analysis by patient stay: Mean percentage of glucose readings of each patient 180 mg/dL. | Analysis by patient‐day: Percentage of patient‐days with mean 110, 140 mg/dL, and/or Percentage of patient‐days with all values 110, 140 mg/dL. | ||
| Analysis by hospital day: Percentage of patients with mean glucose readings 140, 180 mg/dL by hospital day (days 17). | Analysis by patient stay: 3‐day blood glucose average (3‐BG) for selected perioperative patients: Percentage of patients with 3‐BG 110, 140 mg/dL. Mean time (hours) to reach glycemic target (BG 110 or 140 mg/dL) on insulin infusion. | |||
| Measures of insulin use | Percentage of patients on any subcutaneous insulin that has a scheduled basal insulin component (glargine, NPH, or detemir). | Percentage of patients with at least 2 POC and/or laboratory glucose readings >180 mg/dL who have a scheduled basal insulin component. Percentage of eating patients with hyperglycemia as defined above with scheduled basal insulin and nutritional insulin. Percentage of patients and patient‐days with any changes in insulin orders the day after 2 or more episodes of hypoglycemia or hyperglycemia (ie, 70 or >180 mg/dL). | Percentage of patients with 2 POC or laboratory glucose readings >140 mg/dL placed on insulin infusion protocol. | |
| Other process measures | Glucose measured within 8 hours of hospital admission. | POC glucose testing at least 4 times a day for all patients with diabetes or hyperglycemia as defined above. | Glucose measured within 8 hours of hospital admission. | Appropriateness of hypoglycemia treatment and documentation. |
| A1C measurement obtained or available within 30 days of admission. | Measures of adherence to specific components of management protocol. | Frequency of BG testing (eg, per protocol if on insulin infusion; every 68 hours if not). | Clinical events of severe hypoglycemia reported through the organization's critical events reporting tool. | |
| Appropriateness of hypoglycemia treatment and documentation. | Root causes of hypoglycemia. | |||
| Clinical events of severe hypoglycemia reported through the organization's critical events reporting tool. | Appropriate use of IV‐to‐SC insulin transition protocol. | |||
| Root causes of hypoglycemia. | ||||
For each domain of glycemic management (glycemic control, safety, and insulin use), the task force chose a set of best measures. They are presented as two tiers of measurement standards, depending on the capabilities of the institution and the planned uses of the data. Tier 1 includes measures that, although they do take time and resources to collect, are feasible for most institutions. Tier 2 measures are recommended for hospitals with easy manipulation of electronic sources of data and for reporting quality‐of‐care measures for widespread publication, that is, in the context of a research study. It should be emphasized that these recommendations are only meant as a guide: the actual measures chosen should meet the needs and capabilities of each institution.
We recognize that few data support the recommendations made by this task force, that such data are needed, and that the field of data collection and analysis for hospital glycemic management is rapidly evolving. The hope is to begin the standardization process, promote dialogue in this field, and eventually reach a consensus in collaboration with the ADA, AACE, and other pertinent stakeholders.
CONCLUSIONS
Like the field of inpatient glycemic management itself, the field of devising metrics to measure the quality of inpatient glycemic control is also in its infancy and quickly evolving. One should not be paralyzed by the lack of consensus regarding measurementthe important point is to pick a few complementary metrics and begin the process. The table of recommendations can hopefully serve as a starting point for many institutions, with a focus on efficacy (glycemic control), safety (hypoglycemia), and process (insulin use patterns). As your institution gains experience with measurement and the field evolves, your metrics will likely change. We recommend keeping all process and outcome data in its raw form so that it can be summarized in different ways over time. It is also important not to wait for the perfect data collection tool before beginning to analyze data: sampling and paper processes are acceptable if automated data collection is not yet possible. Eventually, blood glucose meter readings should be downloaded into a central database that interfaces with hospital data repositories so data can be analyzed in conjunction with patient, service, and unit‐level information. Only with a rigorous measurement process can institutions hope to know whether their changes are resulting in improved care for patients.
- ,,, et al.“Glucometrics”—assessing the quality of inpatient glucose management.Diabetes Technol Ther.2006;8:560–569.
- ,,.Effect of hyperglycemia and continuous intravenous insulin infusions on outcomes of cardiac surgical procedures: the Portland Diabetic Project.Endocr Pract.2004;10(suppl 2):21–33.
- ,,.Hyperglycaemic index as a tool to assess glucose control: a retrospective study.Crit Care.2004;8:R122–R127.
- ,,, et al.Glucometrics in patients hospitalized with acute myocardial infarction: defining the optimal outcomes‐based measure of risk.Circulation.2008;117:1018–1027.
- ,,.Statistical process control as a tool for research and healthcare improvement.Qual Saf Health Care.2003;12:458–464.
- ,,, et al.“Glucometrics”—assessing the quality of inpatient glucose management.Diabetes Technol Ther.2006;8:560–569.
- ,,.Effect of hyperglycemia and continuous intravenous insulin infusions on outcomes of cardiac surgical procedures: the Portland Diabetic Project.Endocr Pract.2004;10(suppl 2):21–33.
- ,,.Hyperglycaemic index as a tool to assess glucose control: a retrospective study.Crit Care.2004;8:R122–R127.
- ,,, et al.Glucometrics in patients hospitalized with acute myocardial infarction: defining the optimal outcomes‐based measure of risk.Circulation.2008;117:1018–1027.
- ,,.Statistical process control as a tool for research and healthcare improvement.Qual Saf Health Care.2003;12:458–464.