User login
The Irritable Heart
The approach to clinical conundrums by an expert clinician is revealed through presentation of an actual patient's case in an approach typical of morning report. Similar to patient care, sequential pieces of information are provided to the clinician who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant.
A30‐year‐old woman was referred for evaluation of chest pain, palpitations, and exercise intolerance. She had been previously healthy, active, and physically fit. Five months prior to our evaluation, she had an elective C5C6 cervical spine discectomy with interbody allograft fusion for a chronic neck injury that occurred 11 years ago during gymnastics. Two weeks after spine surgery, the patient developed numbness and tingling of her left thumb and palm that occurred with exertion or exposure to cold and subsided with rest. These episodes increased in frequency and intensity and after 1 week became associated with sharp, occasionally stabbing chest pain that radiated to the left arm. On one occasion, the patient had an episode of exertional chest pain with prolonged left arm cyanosis. Emergent left upper extremity angiography revealed normal great vessel anatomy with spasm of the radial artery and collateral ulnar flow. The patient was diagnosed with Raynaud's phenomenon and was started on nifedipine. A subsequent rheumatologic evaluation was unrevealing, and the patient was empirically switched to amlodipine with no improvement in symptoms.
This otherwise very healthy 30‐year‐old developed a multitude of symptoms. The patient's chest pain is atypical and in a young woman is unlikely to signify atherosclerotic coronary disease, but it should not be entirely disregarded. Vasospasm triggered by exposure to cold does raise suspicion for Raynaud's phenomenon, which is not uncommon in this demographic. However, this presentation is quite unusual because the vasospasm was limited to one vascular distribution of one extremity. Associated coronary vasospasm could explain the other symptoms, although coronary spasm is generally not associated with Raynaud's phenomenon. Vasculitis may also affect the pulmonary vasculature, leading to pulmonary hypertension and exercise intolerance. The temporal association with her spine surgery is intriguing but of unclear significance.
The patient continued to have frequent exertional episodes of sharp precordial chest pain radiating to her left arm that were accompanied by dyspnea and left upper extremity symptoms despite amlodipine therapy. These now occurred with limited activity when she walked 1 to 2 blocks uphill. Over the previous 2 months, she had also noticed palpitations occurring reliably with exercise that were relieved with 15 to 20 min of rest. With prolonged episodes, she reported dizziness, nausea, and blurry vision that improved with lying down. She twice had syncope with these symptoms. She noted lower extremity edema while taking calcium channel blockers, but this had resolved after discontinuation of the drugs.
The patient's past medical history included several high‐school orthopedic injuries. She had 2 kidney stones at ages 18 and 23 and had an appendectomy at age 28. Her only medication was an oral contraceptive, and she had discontinued the amlodipine. She denied the use of tobacco, alcohol, herbal medications, or illicit substances. There was no family history of sudden death or heart disease.
Palpitations in a 30‐year‐old woman may signify a cardiac arrhythmia. Paroxysmal supraventricular arrhythmias, such as atrioventricular nodal reentrant tachycardia, atrial tachycardia, and atrial fibrillation, are well described in the young. Ventricular tachycardia (VT) is another possible cause and could be idiopathic or related to occult structural heart disease. Young patients typically tolerate lone arrhythmias quite well, and her failure to do so raises suspicion for concomitant structural heart disease. Her palpitations may be from appropriate sinus tachycardia, which could be compensatory because of inadequate cardiac output reserve, which in turn could be caused by valvular disease, congenital heart disease, or ventricular dysfunction. The exertional chest pain is worrisome for ischemia. Pulmonary hypertension, severe ventricular hypertrophy, or congenital anomalies of the coronary circulation could lead to subendocardial myocardial ischemia with exertion, resulting in angina, dyspnea, and arrhythmias. However, the patient also experiences exertional palpitations without chest pain, which may signify an exertional tachyarrhythmia possibly mediated by catecholamines. Based solely on the history, the differential diagnosis remains broad.
On physical examination, the patient was a fit, thin, healthy woman. Her blood pressure was 120/70 mm Hg supine in both arms and 115/75 mm Hg standing; her pulse was 85 supine and 110 standing, Oxygen saturation was 100% on room air. A cardiac exam revealed a normal jugular venous pressure, normal point of maximal impulse, regular rhythm with occasional ectopy, normal S1, and physiologically split S2 without extra heart sounds or murmurs. The right ventricular impulse was faintly palpable at the left sternal border. Head, neck, chest, abdominal, musculoskeletal, neurologic, extremity, and peripheral pulse examinations were normal.
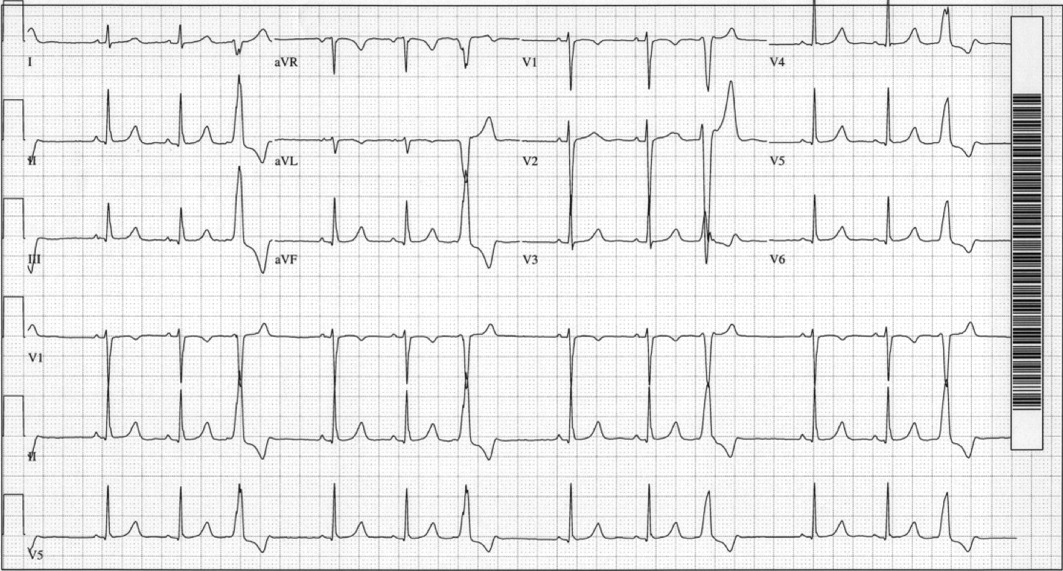
Laboratory data showed a normal complete blood count and normal chemistries. Serum tests for hepatitis C antibody, cardiolipin antibody, rheumatoid factor, cryoglobulins, and anti‐nuclear antibody were negative. The erythrocyte sedimentation rate and thyroid stimulating hormone levels were within normal limits. An electrocardiogram (ECG) demonstrated a normal sinus rhythm with frequent premature ventricular complexes (PVCs) and normal axis and intervals. (Figure 1). The PR segment was normal and without preexcitation. A prior ECG from 3 months ago was similar with ventricular trigeminy.
Her unremarkable cardiac examination does not favor structural or valvular heart disease, and there are no obvious stigmata of vasculitis. She did become mildly tachycardic upon standing, and this raises the possibility of orthostatic tachycardia. A comprehensive rheumatologic panel revealed no evidence of autoimmune disease or vasculitis, and the clinical constellation is not consistent with primary or secondary Raynaud's disease. The ECG demonstrates frequent monomorphic PVCs complexes with a left bundle branch block pattern and an inferior axis. This pattern suggests that the PVCs arise from the right ventricular outflow tract. Idiopathic right ventricular outflow tract VT and arrhythmogenic right ventricular dysplasia must be considered as a cause of exertional or catecholamine‐mediated tachycardia. The normal ECG argues against arrhythmogenic right ventricular dysplasia, in which patients typically have incomplete or complete right bundle branch block, right precordial T wave abnormalities, and occasionally epsilon waves. Her QT interval is normal, but excluding long‐QT syndrome with a single ECG has poor sensitivity. The next critical step is to document her cardiac rhythm during symptoms and to exclude malignant arrhythmias.
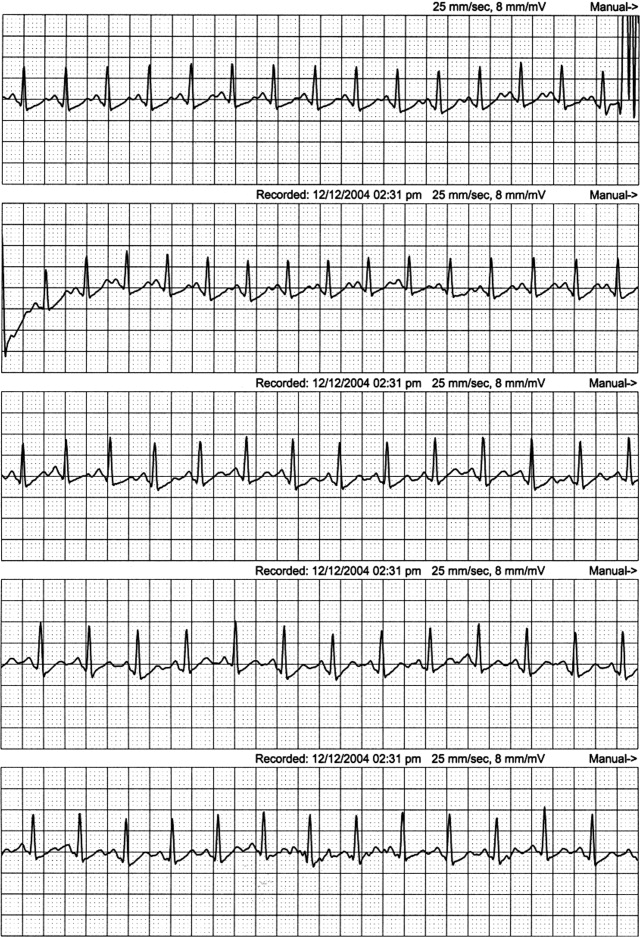
An event recorder and exercise echocardiogram were ordered. While the patient was wearing her event recorder, she had 4 episodes of exertional syncope while hiking and successfully triggered event recording before losing consciousness. She had chest pain and left arm pain after regaining consciousness. The patient came to the emergency room for evaluation. Her blood pressure was 116/80 mm Hg supine and 112/70 mm Hg seated. Her heart rate increased from 82 supine to 132 seated. The physical examination was unremarkable. ECG showed sinus rhythm with frequent PVCs. Troponin‐I measurements 10 hours apart were 0.7 and 0.3 g/L (normal < 1.1), with normal creatinine kinase and creatinine kinase MB fractions. Interrogation of the event recorder revealed multiple episodes of a narrow complex tachycardia with rates up to 180 bpm that correlated with symptoms (Figure 2). There were no episodes of wide complex tachycardia.
The patient was not hypotensive in the emergency room, but she had evidence of a marked orthostatic tachycardia. The minimal but significant troponin elevations are also troubling. Although her clinical picture is not consistent with an acute coronary syndrome, I am concerned about other mechanisms of myocardial ischemia or injury, such as a coronary anomaly or subendocardial ischemia from globally reduced myocardial perfusion. The presence of event recorder data from her syncopal events was fortuitous and revealed a supraventricular tachycardia. The arrhythmia was gradual in onset and resolution and had no triggers, such as premature atrial or ventricular complexes, which could suggest reentrant arrhythmias. The P wave morphology was also unchanged, and this argues against an atrial tachycardia. These findings are consistent with sinus tachycardia, which was notably out of proportion to her workload. This arrhythmia may be the primary cause of syncope, such as in inappropriate sinus tachycardia, or it may be a compensatory mechanism. Tachycardia from coronary vasospasm is often preceded by ST segment changes, which are not seen here. Although the event recorder had no episodes of VT, the patient's persistent frequent PVCs are still of concern. I would obtain an echocardiogram to exclude structural heart disease and an exercise test to exclude exertional VT. Finally, coronary angiography may be helpful in excluding congenital anomalies.
The patient was admitted for evaluation. An exercise treadmill test was performed, and the patient exercised 20 min on the standard Bruce protocol with a peak heart rate of 180 bpm. The test was notable for a premature rise in heart rate (in stage 1) without a rise in blood pressure. There were no symptoms or ST/T wave changes. Transthoracic echocardiogram showed normal left ventricular size and function with normal anatomy, valves, and hemodynamics. Coronary angiography showed a right dominant system with normal anatomy and no atherosclerotic disease.
Ventricular arrhythmias could not be elicited with exercise. Her high exercise tolerance virtually excluded hemodynamically significant structural or valvular disease, and this was confirmed by the echocardiogram. Coronary angiography excluded coronary anomalies and myocardial bridging. The most intriguing finding is the rise in the patient's heart rate out of proportion to the workload. This, along with her orthostatic tachycardia, raises the issue of inappropriate sinus tachycardia or postural orthostatic tachycardia syndrome (POTS). Carotid hypersensitivity is also a possibility. The patient was hiking when she fainted, and even light pressure on the patient's neck with head turning or from a camera strap, for example, could produce syncope. Although carotid hypersensitivity usually results in sinus bradycardia and AV block, it may be followed by reflex tachycardia, which was seen in this patient's event recordings. I would perform a tilt‐table test with carotid massage to make the diagnosis.
Tilt‐table testing was performed (Figure 3). Her supine blood pressure was 128/68 mm Hg, and her heart rate was 72 bpm with no change during the 10‐min supine period. Upon elevation to a 70‐degree tilt, the patient had an immediate increase in her heart rate to 160 bpm with a blood pressure nadir of 109/58 mm Hg and symptoms of palpitations, dizziness, dyspnea, chest pain, blurry vision, and nausea. Her peak heart rate was 172 bpm, and her peak blood pressure was 122/72 mm Hg. Vital signs did not change in response to carotid sinus massage in the supine or upright positions.
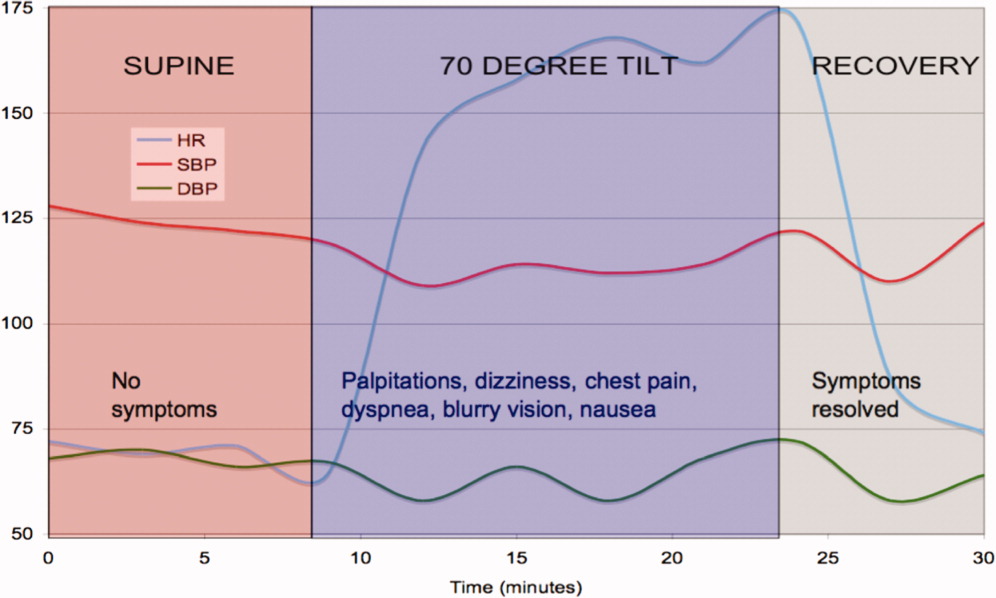
The tilt‐table test has 3 notable findings. First, her heart rate increased rapidly with tilt and decreased rapidly in supine recovery. Second, her usual symptoms started immediately after tilt and quickly resolved in recovery when vital signs returned to baseline. Finally, there was only a modest drop in blood pressure. These findings are classic for POTS. POTS is defined as symptomatic orthostasis with a heart rate increase of 30 bpm or a heart rate of 120 bpm. The physiologic lesions found in the syndrome are heterogeneous, but they all lead to a failure of orthostatic compensation. In POTS, the tachycardia is a reflex secondary to hypotension (baroreceptor reflex) or reduced preload (cardiac mechanoreceptors), in contrast to inappropriate sinus tachycardia. Interestingly, blood pressure is usually preserved until the final moments preceding syncope, when venous return further declines, tachycardia decreases the diastolic filling time and stroke volume, and mean arterial pressure sharply falls.
The patient was started on labetalol (200 mg 3 times daily), and her symptoms worsened. She also developed nausea and constipation. Midodrine and pindolol were also tried without success. She was then switched to fludrocortisone, salt supplementation, and leg support stockings with dramatic improvement.
COMMENTARY
In 1871, DeCosta1 published a report on the irritable heart, noting an affliction of extreme fatigue and exercise intolerance that occurred suddenly and without obvious cause. Subsequently, the terms vasoregulatory asthenia and neurocirculatory asthenia were used to link cardiovascular symptoms to impaired regulation of peripheral blood flow.2, 3 The term POTS was first used in 1982 to describe a single patient with postural tachycardia without hypotension and palpitations, weakness, abdominal pain, and presyncope.4
POTS is one of several disorders of autonomic control associated with orthostatic intolerance. The criteria for diagnosis are listed in Table 1. POTS typically occurs in women between the ages of 15 and 50 but tends to present during adolescence or young adulthood. The physiology has only recently been elucidated. When a person stands, 500 cc of the total blood volume is displaced to the dependent extremities and inferior mesenteric vessels.5 Normally, orthostatic stabilization occurs in less than 1 minute via 3 mechanisms: baroreceptor input, sympathetic reflex tachycardia and vasoconstriction, and enhanced venous return via the pumping action of skeletal muscles and venoconstriction. In POTS, there is a failure of at least one of these mechanisms, leading to decreased venous return, a 40% reduction in stroke volume, and cerebral hypoperfusion.6
| 1. Consistent symptoms of orthostatic intolerance [may include excessive fatigue, exercise intolerance, recurrent syncope or near syncope, dizziness, nausea, tachycardia, palpitations, visual disturbances, blurred vision, tunnel vision, tremulousness, weakness (most noticeable in the legs), chest discomfort, shortness of breath, mood swings, and gastrointestinal complaints] |
| 2. Heart rate increase 30 bpm or heart rate 120 bpm within 10 min of standing or head‐up tilt |
| 3. Absence of a known cause of autonomic neuropathy |
POTS is divided into 2 major subtypes on the basis of pathophysiology.5, 7 The partial dysautonomic form is the most common and the type that this patient most likely had. In this form, the development of an acquired peripheral autonomic neuropathy results in a failure of sympathetic venoconstriction, which leads to excessive venous pooling in the lower extremities and splanchnic circulation.8, 9 Failure to mobilize this venous reservoir upon standing leads to excessive orthostatic tachycardia secondary to a marked reduction in stroke volume. Peripheral arterial vasoconstriction is generally preserved, which is why midodrine, an arterial vasoconstrictor, did not improve symptoms. The labetalol may have further exacerbated peripheral pooling because of its alpha‐adrenergic blocking properties. Because total plasma volume is decreased and plasma renin activity is inappropriately low,10 volume expanders, including salt, low‐dose steroids, and fluids, can attenuate symptoms.11 The extrinsic venous compression from leg and abdominal support stockings may also dramatically reduce venous pooling.
In the less common hyperadrenergic form of POTS, patients may have orthostatic hypertension, tremulousness, cold, sweaty extremities, and anxiety due to an exaggerated response to beta‐adrenergic stimulation.7 The excessive sympathetic activity, which is poorly modulated by baroreflex activity, may be due to impaired mechanisms of norepinephrine reuptake by sympathetic ganglia.12 Consequently, serum norepinephrine levels are markedly elevated (>600 pg/mL).5
In adults, the presence of a POTS trigger is common and is usually an antecedent viral illness. Antibodies to the ganglionic acetylcholine receptor have been found in a subset of POTS patients,13 and this may suggest an idiopathic or postinflammatory autoimmune mechanism.14 This patient's presentation is unique because her symptoms developed after C5C6 spine surgery. The cervical spinal cord and sympathetic ganglia are dense with nerves involved in autonomic cardiovascular control, and damage to these fibers could explain the patient's physiology and symptoms. Among these, the descending vasomotor pathways traverse through the C5C8 area to innervate the splanchnic and leg venous circulation, receiving input from the heart along the way.15 The pattern of numbness and tingling fits the C5/C6 dermatomal distribution, as does the innervation of the radial artery. The frequent PVCs with a left bundle branch block pattern and inferior axis appear to arise from the right ventricular outflow tract and may be associated with regional sympathetic denervation, which has been described in idiopathic ventricular arrhythmias.16 POTS has been anecdotally reported after neck injury from motor vehicle accidents (whiplash), which is also thought to be related to cervical sympathetic nerve damage (B.P. Grubb, personal communication, 2005). Most cases of triggered POTS improve spontaneously after months to years, but this patient's prognosis remains uncertain because of the presumed mechanical disruption of the autonomic nerve fibers at the time of surgery.
This case demonstrates the complexities of arriving at a unifying diagnosis in the setting of a constellation of nonspecific symptoms and findings, some of which even suggest life‐threatening conditions. Because young women are primarily affected, symptoms of POTS can be mistakenly attributed to anxiety or other nonphysiological factors. A systematic approach excluded life‐threatening causes, including primary ventricular arrhythmias, coronary vasospasm, and coronary anomalies. The investigations narrowed the differential diagnosis, and the tilt‐table test confirmed POTS. Because the cardiac and circulatory dysautonomias encompass an array of distinct physiologic processes, understanding the patient's mechanism is critical to her management. The only effective therapies were those that counteracted venous pooling and improved venous return.
Teaching Points
-
The differential diagnosis of exertional syncope is extremely broad, ranging from benign to malignant conditions, and requires a systematic evaluation of the heart and circulatory system.
-
The diagnosis of POTS is elusive and frequently missed. Referral for tilt‐table testing is useful in identifying the mechanism of sinus tachycardia and syncope. Marked orthostatic tachycardia and symptoms of cerebral hypoperfusion out of proportion to the degree of hypotension strongly suggest POTS.
-
Cardiac and circulatory dysautonomias have distinct and varied mechanisms. Therapies, including beta‐blockers, vasoconstrictors, and volume expanders, must be directed at the underlying physiological defect.
- .An irritable heart.Am J Med Sci.1871;27:145–161.
- ,,,,,.Low physical working capacity in suspected heart cases due to inadequate adjustment of peripheral blood flow (vasoregulatory asthenia).Acta Med Scand.1957;158(6):413–436.
- ,,.Orthostatic tachycardia and orthostatic hypotension: defects in the return of venous blood to the heart.Am Heart J.1944;27:145–163.
- ,.Postural tachycardia syndrome. Reversal of sympathetic hyperresponsiveness and clinical improvement during sodium loading.Am J Med.1982;72(5):847–850.
- ,,.The postural orthostatic tachycardia syndrome: definitions, diagnosis, and management.Pacing Clin Electrophysiol.2003;26(8):1747– 1757.
- ,.Clinical disorders of the autonomic nervous system associated with orthostatic intolerance: an overview of classification, clinical evaluation, and management.Pacing Clin Electrophysiol.1999;22(5):798–810.
- ,.Idiopathic orthostatic intolerance and postural tachycardia syndromes.Am J Med Sci.1999;317(2):88–101.
- ,,, et al.Splanchnic‐mesenteric capacitance bed in the postural tachycardia syndrome (POTS).Auton Neurosci.2000;86(1–2):107–113.
- ,,,.Abnormal orthostatic changes in blood pressure and heart rate in subjects with intact sympathetic nervous function: evidence for excessive venous pooling.J Lab Clin Med.1988;111(3):326–335.
- ,,, et al.Renin‐aldosterone paradox and perturbed blood volume regulation underlying postural tachycardia syndrome.Circulation.2005;111(13):1574–1582.
- .Clinical practice. Neurocardiogenic syncope.N Engl J Med.2005;352(10):1004–1010.
- ,,, et al.Orthostatic intolerance and tachycardia associated with norepinephrine‐transporter deficiency.N Engl J Med.2000;342(8):541–549.
- ,,,,,.Autoantibodies to ganglionic acetylcholine receptors in autoimmune autonomic neuropathies.N Engl J Med.2000;343(12):847–855.
- ,,.The postural tachycardia syndrome: a concise guide to diagnosis and management.J Cardiovasc Electrophysiol.2006;17(1):108–112.
- ,,.Neurovegetative regulation of the vascular system. In:Lanzer P,Topol EJ, eds.Panvascular Medicine.Berlin, Germany:Springer‐Verlag;2002:175–187.
- ,,, et al.Regional cardiac sympathetic denervation in patients with ventricular tachycardia in the absence of coronary artery disease.J Am Coll Cardiol.1993;22(5):1344–1353.
The approach to clinical conundrums by an expert clinician is revealed through presentation of an actual patient's case in an approach typical of morning report. Similar to patient care, sequential pieces of information are provided to the clinician who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant.
A30‐year‐old woman was referred for evaluation of chest pain, palpitations, and exercise intolerance. She had been previously healthy, active, and physically fit. Five months prior to our evaluation, she had an elective C5C6 cervical spine discectomy with interbody allograft fusion for a chronic neck injury that occurred 11 years ago during gymnastics. Two weeks after spine surgery, the patient developed numbness and tingling of her left thumb and palm that occurred with exertion or exposure to cold and subsided with rest. These episodes increased in frequency and intensity and after 1 week became associated with sharp, occasionally stabbing chest pain that radiated to the left arm. On one occasion, the patient had an episode of exertional chest pain with prolonged left arm cyanosis. Emergent left upper extremity angiography revealed normal great vessel anatomy with spasm of the radial artery and collateral ulnar flow. The patient was diagnosed with Raynaud's phenomenon and was started on nifedipine. A subsequent rheumatologic evaluation was unrevealing, and the patient was empirically switched to amlodipine with no improvement in symptoms.
This otherwise very healthy 30‐year‐old developed a multitude of symptoms. The patient's chest pain is atypical and in a young woman is unlikely to signify atherosclerotic coronary disease, but it should not be entirely disregarded. Vasospasm triggered by exposure to cold does raise suspicion for Raynaud's phenomenon, which is not uncommon in this demographic. However, this presentation is quite unusual because the vasospasm was limited to one vascular distribution of one extremity. Associated coronary vasospasm could explain the other symptoms, although coronary spasm is generally not associated with Raynaud's phenomenon. Vasculitis may also affect the pulmonary vasculature, leading to pulmonary hypertension and exercise intolerance. The temporal association with her spine surgery is intriguing but of unclear significance.
The patient continued to have frequent exertional episodes of sharp precordial chest pain radiating to her left arm that were accompanied by dyspnea and left upper extremity symptoms despite amlodipine therapy. These now occurred with limited activity when she walked 1 to 2 blocks uphill. Over the previous 2 months, she had also noticed palpitations occurring reliably with exercise that were relieved with 15 to 20 min of rest. With prolonged episodes, she reported dizziness, nausea, and blurry vision that improved with lying down. She twice had syncope with these symptoms. She noted lower extremity edema while taking calcium channel blockers, but this had resolved after discontinuation of the drugs.
The patient's past medical history included several high‐school orthopedic injuries. She had 2 kidney stones at ages 18 and 23 and had an appendectomy at age 28. Her only medication was an oral contraceptive, and she had discontinued the amlodipine. She denied the use of tobacco, alcohol, herbal medications, or illicit substances. There was no family history of sudden death or heart disease.
Palpitations in a 30‐year‐old woman may signify a cardiac arrhythmia. Paroxysmal supraventricular arrhythmias, such as atrioventricular nodal reentrant tachycardia, atrial tachycardia, and atrial fibrillation, are well described in the young. Ventricular tachycardia (VT) is another possible cause and could be idiopathic or related to occult structural heart disease. Young patients typically tolerate lone arrhythmias quite well, and her failure to do so raises suspicion for concomitant structural heart disease. Her palpitations may be from appropriate sinus tachycardia, which could be compensatory because of inadequate cardiac output reserve, which in turn could be caused by valvular disease, congenital heart disease, or ventricular dysfunction. The exertional chest pain is worrisome for ischemia. Pulmonary hypertension, severe ventricular hypertrophy, or congenital anomalies of the coronary circulation could lead to subendocardial myocardial ischemia with exertion, resulting in angina, dyspnea, and arrhythmias. However, the patient also experiences exertional palpitations without chest pain, which may signify an exertional tachyarrhythmia possibly mediated by catecholamines. Based solely on the history, the differential diagnosis remains broad.
On physical examination, the patient was a fit, thin, healthy woman. Her blood pressure was 120/70 mm Hg supine in both arms and 115/75 mm Hg standing; her pulse was 85 supine and 110 standing, Oxygen saturation was 100% on room air. A cardiac exam revealed a normal jugular venous pressure, normal point of maximal impulse, regular rhythm with occasional ectopy, normal S1, and physiologically split S2 without extra heart sounds or murmurs. The right ventricular impulse was faintly palpable at the left sternal border. Head, neck, chest, abdominal, musculoskeletal, neurologic, extremity, and peripheral pulse examinations were normal.
Laboratory data showed a normal complete blood count and normal chemistries. Serum tests for hepatitis C antibody, cardiolipin antibody, rheumatoid factor, cryoglobulins, and anti‐nuclear antibody were negative. The erythrocyte sedimentation rate and thyroid stimulating hormone levels were within normal limits. An electrocardiogram (ECG) demonstrated a normal sinus rhythm with frequent premature ventricular complexes (PVCs) and normal axis and intervals. (Figure 1). The PR segment was normal and without preexcitation. A prior ECG from 3 months ago was similar with ventricular trigeminy.

Her unremarkable cardiac examination does not favor structural or valvular heart disease, and there are no obvious stigmata of vasculitis. She did become mildly tachycardic upon standing, and this raises the possibility of orthostatic tachycardia. A comprehensive rheumatologic panel revealed no evidence of autoimmune disease or vasculitis, and the clinical constellation is not consistent with primary or secondary Raynaud's disease. The ECG demonstrates frequent monomorphic PVCs complexes with a left bundle branch block pattern and an inferior axis. This pattern suggests that the PVCs arise from the right ventricular outflow tract. Idiopathic right ventricular outflow tract VT and arrhythmogenic right ventricular dysplasia must be considered as a cause of exertional or catecholamine‐mediated tachycardia. The normal ECG argues against arrhythmogenic right ventricular dysplasia, in which patients typically have incomplete or complete right bundle branch block, right precordial T wave abnormalities, and occasionally epsilon waves. Her QT interval is normal, but excluding long‐QT syndrome with a single ECG has poor sensitivity. The next critical step is to document her cardiac rhythm during symptoms and to exclude malignant arrhythmias.
An event recorder and exercise echocardiogram were ordered. While the patient was wearing her event recorder, she had 4 episodes of exertional syncope while hiking and successfully triggered event recording before losing consciousness. She had chest pain and left arm pain after regaining consciousness. The patient came to the emergency room for evaluation. Her blood pressure was 116/80 mm Hg supine and 112/70 mm Hg seated. Her heart rate increased from 82 supine to 132 seated. The physical examination was unremarkable. ECG showed sinus rhythm with frequent PVCs. Troponin‐I measurements 10 hours apart were 0.7 and 0.3 g/L (normal < 1.1), with normal creatinine kinase and creatinine kinase MB fractions. Interrogation of the event recorder revealed multiple episodes of a narrow complex tachycardia with rates up to 180 bpm that correlated with symptoms (Figure 2). There were no episodes of wide complex tachycardia.

The patient was not hypotensive in the emergency room, but she had evidence of a marked orthostatic tachycardia. The minimal but significant troponin elevations are also troubling. Although her clinical picture is not consistent with an acute coronary syndrome, I am concerned about other mechanisms of myocardial ischemia or injury, such as a coronary anomaly or subendocardial ischemia from globally reduced myocardial perfusion. The presence of event recorder data from her syncopal events was fortuitous and revealed a supraventricular tachycardia. The arrhythmia was gradual in onset and resolution and had no triggers, such as premature atrial or ventricular complexes, which could suggest reentrant arrhythmias. The P wave morphology was also unchanged, and this argues against an atrial tachycardia. These findings are consistent with sinus tachycardia, which was notably out of proportion to her workload. This arrhythmia may be the primary cause of syncope, such as in inappropriate sinus tachycardia, or it may be a compensatory mechanism. Tachycardia from coronary vasospasm is often preceded by ST segment changes, which are not seen here. Although the event recorder had no episodes of VT, the patient's persistent frequent PVCs are still of concern. I would obtain an echocardiogram to exclude structural heart disease and an exercise test to exclude exertional VT. Finally, coronary angiography may be helpful in excluding congenital anomalies.
The patient was admitted for evaluation. An exercise treadmill test was performed, and the patient exercised 20 min on the standard Bruce protocol with a peak heart rate of 180 bpm. The test was notable for a premature rise in heart rate (in stage 1) without a rise in blood pressure. There were no symptoms or ST/T wave changes. Transthoracic echocardiogram showed normal left ventricular size and function with normal anatomy, valves, and hemodynamics. Coronary angiography showed a right dominant system with normal anatomy and no atherosclerotic disease.
Ventricular arrhythmias could not be elicited with exercise. Her high exercise tolerance virtually excluded hemodynamically significant structural or valvular disease, and this was confirmed by the echocardiogram. Coronary angiography excluded coronary anomalies and myocardial bridging. The most intriguing finding is the rise in the patient's heart rate out of proportion to the workload. This, along with her orthostatic tachycardia, raises the issue of inappropriate sinus tachycardia or postural orthostatic tachycardia syndrome (POTS). Carotid hypersensitivity is also a possibility. The patient was hiking when she fainted, and even light pressure on the patient's neck with head turning or from a camera strap, for example, could produce syncope. Although carotid hypersensitivity usually results in sinus bradycardia and AV block, it may be followed by reflex tachycardia, which was seen in this patient's event recordings. I would perform a tilt‐table test with carotid massage to make the diagnosis.
Tilt‐table testing was performed (Figure 3). Her supine blood pressure was 128/68 mm Hg, and her heart rate was 72 bpm with no change during the 10‐min supine period. Upon elevation to a 70‐degree tilt, the patient had an immediate increase in her heart rate to 160 bpm with a blood pressure nadir of 109/58 mm Hg and symptoms of palpitations, dizziness, dyspnea, chest pain, blurry vision, and nausea. Her peak heart rate was 172 bpm, and her peak blood pressure was 122/72 mm Hg. Vital signs did not change in response to carotid sinus massage in the supine or upright positions.

The tilt‐table test has 3 notable findings. First, her heart rate increased rapidly with tilt and decreased rapidly in supine recovery. Second, her usual symptoms started immediately after tilt and quickly resolved in recovery when vital signs returned to baseline. Finally, there was only a modest drop in blood pressure. These findings are classic for POTS. POTS is defined as symptomatic orthostasis with a heart rate increase of 30 bpm or a heart rate of 120 bpm. The physiologic lesions found in the syndrome are heterogeneous, but they all lead to a failure of orthostatic compensation. In POTS, the tachycardia is a reflex secondary to hypotension (baroreceptor reflex) or reduced preload (cardiac mechanoreceptors), in contrast to inappropriate sinus tachycardia. Interestingly, blood pressure is usually preserved until the final moments preceding syncope, when venous return further declines, tachycardia decreases the diastolic filling time and stroke volume, and mean arterial pressure sharply falls.
The patient was started on labetalol (200 mg 3 times daily), and her symptoms worsened. She also developed nausea and constipation. Midodrine and pindolol were also tried without success. She was then switched to fludrocortisone, salt supplementation, and leg support stockings with dramatic improvement.
COMMENTARY
In 1871, DeCosta1 published a report on the irritable heart, noting an affliction of extreme fatigue and exercise intolerance that occurred suddenly and without obvious cause. Subsequently, the terms vasoregulatory asthenia and neurocirculatory asthenia were used to link cardiovascular symptoms to impaired regulation of peripheral blood flow.2, 3 The term POTS was first used in 1982 to describe a single patient with postural tachycardia without hypotension and palpitations, weakness, abdominal pain, and presyncope.4
POTS is one of several disorders of autonomic control associated with orthostatic intolerance. The criteria for diagnosis are listed in Table 1. POTS typically occurs in women between the ages of 15 and 50 but tends to present during adolescence or young adulthood. The physiology has only recently been elucidated. When a person stands, 500 cc of the total blood volume is displaced to the dependent extremities and inferior mesenteric vessels.5 Normally, orthostatic stabilization occurs in less than 1 minute via 3 mechanisms: baroreceptor input, sympathetic reflex tachycardia and vasoconstriction, and enhanced venous return via the pumping action of skeletal muscles and venoconstriction. In POTS, there is a failure of at least one of these mechanisms, leading to decreased venous return, a 40% reduction in stroke volume, and cerebral hypoperfusion.6
| 1. Consistent symptoms of orthostatic intolerance [may include excessive fatigue, exercise intolerance, recurrent syncope or near syncope, dizziness, nausea, tachycardia, palpitations, visual disturbances, blurred vision, tunnel vision, tremulousness, weakness (most noticeable in the legs), chest discomfort, shortness of breath, mood swings, and gastrointestinal complaints] |
| 2. Heart rate increase 30 bpm or heart rate 120 bpm within 10 min of standing or head‐up tilt |
| 3. Absence of a known cause of autonomic neuropathy |
POTS is divided into 2 major subtypes on the basis of pathophysiology.5, 7 The partial dysautonomic form is the most common and the type that this patient most likely had. In this form, the development of an acquired peripheral autonomic neuropathy results in a failure of sympathetic venoconstriction, which leads to excessive venous pooling in the lower extremities and splanchnic circulation.8, 9 Failure to mobilize this venous reservoir upon standing leads to excessive orthostatic tachycardia secondary to a marked reduction in stroke volume. Peripheral arterial vasoconstriction is generally preserved, which is why midodrine, an arterial vasoconstrictor, did not improve symptoms. The labetalol may have further exacerbated peripheral pooling because of its alpha‐adrenergic blocking properties. Because total plasma volume is decreased and plasma renin activity is inappropriately low,10 volume expanders, including salt, low‐dose steroids, and fluids, can attenuate symptoms.11 The extrinsic venous compression from leg and abdominal support stockings may also dramatically reduce venous pooling.
In the less common hyperadrenergic form of POTS, patients may have orthostatic hypertension, tremulousness, cold, sweaty extremities, and anxiety due to an exaggerated response to beta‐adrenergic stimulation.7 The excessive sympathetic activity, which is poorly modulated by baroreflex activity, may be due to impaired mechanisms of norepinephrine reuptake by sympathetic ganglia.12 Consequently, serum norepinephrine levels are markedly elevated (>600 pg/mL).5
In adults, the presence of a POTS trigger is common and is usually an antecedent viral illness. Antibodies to the ganglionic acetylcholine receptor have been found in a subset of POTS patients,13 and this may suggest an idiopathic or postinflammatory autoimmune mechanism.14 This patient's presentation is unique because her symptoms developed after C5C6 spine surgery. The cervical spinal cord and sympathetic ganglia are dense with nerves involved in autonomic cardiovascular control, and damage to these fibers could explain the patient's physiology and symptoms. Among these, the descending vasomotor pathways traverse through the C5C8 area to innervate the splanchnic and leg venous circulation, receiving input from the heart along the way.15 The pattern of numbness and tingling fits the C5/C6 dermatomal distribution, as does the innervation of the radial artery. The frequent PVCs with a left bundle branch block pattern and inferior axis appear to arise from the right ventricular outflow tract and may be associated with regional sympathetic denervation, which has been described in idiopathic ventricular arrhythmias.16 POTS has been anecdotally reported after neck injury from motor vehicle accidents (whiplash), which is also thought to be related to cervical sympathetic nerve damage (B.P. Grubb, personal communication, 2005). Most cases of triggered POTS improve spontaneously after months to years, but this patient's prognosis remains uncertain because of the presumed mechanical disruption of the autonomic nerve fibers at the time of surgery.
This case demonstrates the complexities of arriving at a unifying diagnosis in the setting of a constellation of nonspecific symptoms and findings, some of which even suggest life‐threatening conditions. Because young women are primarily affected, symptoms of POTS can be mistakenly attributed to anxiety or other nonphysiological factors. A systematic approach excluded life‐threatening causes, including primary ventricular arrhythmias, coronary vasospasm, and coronary anomalies. The investigations narrowed the differential diagnosis, and the tilt‐table test confirmed POTS. Because the cardiac and circulatory dysautonomias encompass an array of distinct physiologic processes, understanding the patient's mechanism is critical to her management. The only effective therapies were those that counteracted venous pooling and improved venous return.
Teaching Points
-
The differential diagnosis of exertional syncope is extremely broad, ranging from benign to malignant conditions, and requires a systematic evaluation of the heart and circulatory system.
-
The diagnosis of POTS is elusive and frequently missed. Referral for tilt‐table testing is useful in identifying the mechanism of sinus tachycardia and syncope. Marked orthostatic tachycardia and symptoms of cerebral hypoperfusion out of proportion to the degree of hypotension strongly suggest POTS.
-
Cardiac and circulatory dysautonomias have distinct and varied mechanisms. Therapies, including beta‐blockers, vasoconstrictors, and volume expanders, must be directed at the underlying physiological defect.
The approach to clinical conundrums by an expert clinician is revealed through presentation of an actual patient's case in an approach typical of morning report. Similar to patient care, sequential pieces of information are provided to the clinician who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant.
A30‐year‐old woman was referred for evaluation of chest pain, palpitations, and exercise intolerance. She had been previously healthy, active, and physically fit. Five months prior to our evaluation, she had an elective C5C6 cervical spine discectomy with interbody allograft fusion for a chronic neck injury that occurred 11 years ago during gymnastics. Two weeks after spine surgery, the patient developed numbness and tingling of her left thumb and palm that occurred with exertion or exposure to cold and subsided with rest. These episodes increased in frequency and intensity and after 1 week became associated with sharp, occasionally stabbing chest pain that radiated to the left arm. On one occasion, the patient had an episode of exertional chest pain with prolonged left arm cyanosis. Emergent left upper extremity angiography revealed normal great vessel anatomy with spasm of the radial artery and collateral ulnar flow. The patient was diagnosed with Raynaud's phenomenon and was started on nifedipine. A subsequent rheumatologic evaluation was unrevealing, and the patient was empirically switched to amlodipine with no improvement in symptoms.
This otherwise very healthy 30‐year‐old developed a multitude of symptoms. The patient's chest pain is atypical and in a young woman is unlikely to signify atherosclerotic coronary disease, but it should not be entirely disregarded. Vasospasm triggered by exposure to cold does raise suspicion for Raynaud's phenomenon, which is not uncommon in this demographic. However, this presentation is quite unusual because the vasospasm was limited to one vascular distribution of one extremity. Associated coronary vasospasm could explain the other symptoms, although coronary spasm is generally not associated with Raynaud's phenomenon. Vasculitis may also affect the pulmonary vasculature, leading to pulmonary hypertension and exercise intolerance. The temporal association with her spine surgery is intriguing but of unclear significance.
The patient continued to have frequent exertional episodes of sharp precordial chest pain radiating to her left arm that were accompanied by dyspnea and left upper extremity symptoms despite amlodipine therapy. These now occurred with limited activity when she walked 1 to 2 blocks uphill. Over the previous 2 months, she had also noticed palpitations occurring reliably with exercise that were relieved with 15 to 20 min of rest. With prolonged episodes, she reported dizziness, nausea, and blurry vision that improved with lying down. She twice had syncope with these symptoms. She noted lower extremity edema while taking calcium channel blockers, but this had resolved after discontinuation of the drugs.
The patient's past medical history included several high‐school orthopedic injuries. She had 2 kidney stones at ages 18 and 23 and had an appendectomy at age 28. Her only medication was an oral contraceptive, and she had discontinued the amlodipine. She denied the use of tobacco, alcohol, herbal medications, or illicit substances. There was no family history of sudden death or heart disease.
Palpitations in a 30‐year‐old woman may signify a cardiac arrhythmia. Paroxysmal supraventricular arrhythmias, such as atrioventricular nodal reentrant tachycardia, atrial tachycardia, and atrial fibrillation, are well described in the young. Ventricular tachycardia (VT) is another possible cause and could be idiopathic or related to occult structural heart disease. Young patients typically tolerate lone arrhythmias quite well, and her failure to do so raises suspicion for concomitant structural heart disease. Her palpitations may be from appropriate sinus tachycardia, which could be compensatory because of inadequate cardiac output reserve, which in turn could be caused by valvular disease, congenital heart disease, or ventricular dysfunction. The exertional chest pain is worrisome for ischemia. Pulmonary hypertension, severe ventricular hypertrophy, or congenital anomalies of the coronary circulation could lead to subendocardial myocardial ischemia with exertion, resulting in angina, dyspnea, and arrhythmias. However, the patient also experiences exertional palpitations without chest pain, which may signify an exertional tachyarrhythmia possibly mediated by catecholamines. Based solely on the history, the differential diagnosis remains broad.
On physical examination, the patient was a fit, thin, healthy woman. Her blood pressure was 120/70 mm Hg supine in both arms and 115/75 mm Hg standing; her pulse was 85 supine and 110 standing, Oxygen saturation was 100% on room air. A cardiac exam revealed a normal jugular venous pressure, normal point of maximal impulse, regular rhythm with occasional ectopy, normal S1, and physiologically split S2 without extra heart sounds or murmurs. The right ventricular impulse was faintly palpable at the left sternal border. Head, neck, chest, abdominal, musculoskeletal, neurologic, extremity, and peripheral pulse examinations were normal.
Laboratory data showed a normal complete blood count and normal chemistries. Serum tests for hepatitis C antibody, cardiolipin antibody, rheumatoid factor, cryoglobulins, and anti‐nuclear antibody were negative. The erythrocyte sedimentation rate and thyroid stimulating hormone levels were within normal limits. An electrocardiogram (ECG) demonstrated a normal sinus rhythm with frequent premature ventricular complexes (PVCs) and normal axis and intervals. (Figure 1). The PR segment was normal and without preexcitation. A prior ECG from 3 months ago was similar with ventricular trigeminy.

Her unremarkable cardiac examination does not favor structural or valvular heart disease, and there are no obvious stigmata of vasculitis. She did become mildly tachycardic upon standing, and this raises the possibility of orthostatic tachycardia. A comprehensive rheumatologic panel revealed no evidence of autoimmune disease or vasculitis, and the clinical constellation is not consistent with primary or secondary Raynaud's disease. The ECG demonstrates frequent monomorphic PVCs complexes with a left bundle branch block pattern and an inferior axis. This pattern suggests that the PVCs arise from the right ventricular outflow tract. Idiopathic right ventricular outflow tract VT and arrhythmogenic right ventricular dysplasia must be considered as a cause of exertional or catecholamine‐mediated tachycardia. The normal ECG argues against arrhythmogenic right ventricular dysplasia, in which patients typically have incomplete or complete right bundle branch block, right precordial T wave abnormalities, and occasionally epsilon waves. Her QT interval is normal, but excluding long‐QT syndrome with a single ECG has poor sensitivity. The next critical step is to document her cardiac rhythm during symptoms and to exclude malignant arrhythmias.
An event recorder and exercise echocardiogram were ordered. While the patient was wearing her event recorder, she had 4 episodes of exertional syncope while hiking and successfully triggered event recording before losing consciousness. She had chest pain and left arm pain after regaining consciousness. The patient came to the emergency room for evaluation. Her blood pressure was 116/80 mm Hg supine and 112/70 mm Hg seated. Her heart rate increased from 82 supine to 132 seated. The physical examination was unremarkable. ECG showed sinus rhythm with frequent PVCs. Troponin‐I measurements 10 hours apart were 0.7 and 0.3 g/L (normal < 1.1), with normal creatinine kinase and creatinine kinase MB fractions. Interrogation of the event recorder revealed multiple episodes of a narrow complex tachycardia with rates up to 180 bpm that correlated with symptoms (Figure 2). There were no episodes of wide complex tachycardia.

The patient was not hypotensive in the emergency room, but she had evidence of a marked orthostatic tachycardia. The minimal but significant troponin elevations are also troubling. Although her clinical picture is not consistent with an acute coronary syndrome, I am concerned about other mechanisms of myocardial ischemia or injury, such as a coronary anomaly or subendocardial ischemia from globally reduced myocardial perfusion. The presence of event recorder data from her syncopal events was fortuitous and revealed a supraventricular tachycardia. The arrhythmia was gradual in onset and resolution and had no triggers, such as premature atrial or ventricular complexes, which could suggest reentrant arrhythmias. The P wave morphology was also unchanged, and this argues against an atrial tachycardia. These findings are consistent with sinus tachycardia, which was notably out of proportion to her workload. This arrhythmia may be the primary cause of syncope, such as in inappropriate sinus tachycardia, or it may be a compensatory mechanism. Tachycardia from coronary vasospasm is often preceded by ST segment changes, which are not seen here. Although the event recorder had no episodes of VT, the patient's persistent frequent PVCs are still of concern. I would obtain an echocardiogram to exclude structural heart disease and an exercise test to exclude exertional VT. Finally, coronary angiography may be helpful in excluding congenital anomalies.
The patient was admitted for evaluation. An exercise treadmill test was performed, and the patient exercised 20 min on the standard Bruce protocol with a peak heart rate of 180 bpm. The test was notable for a premature rise in heart rate (in stage 1) without a rise in blood pressure. There were no symptoms or ST/T wave changes. Transthoracic echocardiogram showed normal left ventricular size and function with normal anatomy, valves, and hemodynamics. Coronary angiography showed a right dominant system with normal anatomy and no atherosclerotic disease.
Ventricular arrhythmias could not be elicited with exercise. Her high exercise tolerance virtually excluded hemodynamically significant structural or valvular disease, and this was confirmed by the echocardiogram. Coronary angiography excluded coronary anomalies and myocardial bridging. The most intriguing finding is the rise in the patient's heart rate out of proportion to the workload. This, along with her orthostatic tachycardia, raises the issue of inappropriate sinus tachycardia or postural orthostatic tachycardia syndrome (POTS). Carotid hypersensitivity is also a possibility. The patient was hiking when she fainted, and even light pressure on the patient's neck with head turning or from a camera strap, for example, could produce syncope. Although carotid hypersensitivity usually results in sinus bradycardia and AV block, it may be followed by reflex tachycardia, which was seen in this patient's event recordings. I would perform a tilt‐table test with carotid massage to make the diagnosis.
Tilt‐table testing was performed (Figure 3). Her supine blood pressure was 128/68 mm Hg, and her heart rate was 72 bpm with no change during the 10‐min supine period. Upon elevation to a 70‐degree tilt, the patient had an immediate increase in her heart rate to 160 bpm with a blood pressure nadir of 109/58 mm Hg and symptoms of palpitations, dizziness, dyspnea, chest pain, blurry vision, and nausea. Her peak heart rate was 172 bpm, and her peak blood pressure was 122/72 mm Hg. Vital signs did not change in response to carotid sinus massage in the supine or upright positions.

The tilt‐table test has 3 notable findings. First, her heart rate increased rapidly with tilt and decreased rapidly in supine recovery. Second, her usual symptoms started immediately after tilt and quickly resolved in recovery when vital signs returned to baseline. Finally, there was only a modest drop in blood pressure. These findings are classic for POTS. POTS is defined as symptomatic orthostasis with a heart rate increase of 30 bpm or a heart rate of 120 bpm. The physiologic lesions found in the syndrome are heterogeneous, but they all lead to a failure of orthostatic compensation. In POTS, the tachycardia is a reflex secondary to hypotension (baroreceptor reflex) or reduced preload (cardiac mechanoreceptors), in contrast to inappropriate sinus tachycardia. Interestingly, blood pressure is usually preserved until the final moments preceding syncope, when venous return further declines, tachycardia decreases the diastolic filling time and stroke volume, and mean arterial pressure sharply falls.
The patient was started on labetalol (200 mg 3 times daily), and her symptoms worsened. She also developed nausea and constipation. Midodrine and pindolol were also tried without success. She was then switched to fludrocortisone, salt supplementation, and leg support stockings with dramatic improvement.
COMMENTARY
In 1871, DeCosta1 published a report on the irritable heart, noting an affliction of extreme fatigue and exercise intolerance that occurred suddenly and without obvious cause. Subsequently, the terms vasoregulatory asthenia and neurocirculatory asthenia were used to link cardiovascular symptoms to impaired regulation of peripheral blood flow.2, 3 The term POTS was first used in 1982 to describe a single patient with postural tachycardia without hypotension and palpitations, weakness, abdominal pain, and presyncope.4
POTS is one of several disorders of autonomic control associated with orthostatic intolerance. The criteria for diagnosis are listed in Table 1. POTS typically occurs in women between the ages of 15 and 50 but tends to present during adolescence or young adulthood. The physiology has only recently been elucidated. When a person stands, 500 cc of the total blood volume is displaced to the dependent extremities and inferior mesenteric vessels.5 Normally, orthostatic stabilization occurs in less than 1 minute via 3 mechanisms: baroreceptor input, sympathetic reflex tachycardia and vasoconstriction, and enhanced venous return via the pumping action of skeletal muscles and venoconstriction. In POTS, there is a failure of at least one of these mechanisms, leading to decreased venous return, a 40% reduction in stroke volume, and cerebral hypoperfusion.6
| 1. Consistent symptoms of orthostatic intolerance [may include excessive fatigue, exercise intolerance, recurrent syncope or near syncope, dizziness, nausea, tachycardia, palpitations, visual disturbances, blurred vision, tunnel vision, tremulousness, weakness (most noticeable in the legs), chest discomfort, shortness of breath, mood swings, and gastrointestinal complaints] |
| 2. Heart rate increase 30 bpm or heart rate 120 bpm within 10 min of standing or head‐up tilt |
| 3. Absence of a known cause of autonomic neuropathy |
POTS is divided into 2 major subtypes on the basis of pathophysiology.5, 7 The partial dysautonomic form is the most common and the type that this patient most likely had. In this form, the development of an acquired peripheral autonomic neuropathy results in a failure of sympathetic venoconstriction, which leads to excessive venous pooling in the lower extremities and splanchnic circulation.8, 9 Failure to mobilize this venous reservoir upon standing leads to excessive orthostatic tachycardia secondary to a marked reduction in stroke volume. Peripheral arterial vasoconstriction is generally preserved, which is why midodrine, an arterial vasoconstrictor, did not improve symptoms. The labetalol may have further exacerbated peripheral pooling because of its alpha‐adrenergic blocking properties. Because total plasma volume is decreased and plasma renin activity is inappropriately low,10 volume expanders, including salt, low‐dose steroids, and fluids, can attenuate symptoms.11 The extrinsic venous compression from leg and abdominal support stockings may also dramatically reduce venous pooling.
In the less common hyperadrenergic form of POTS, patients may have orthostatic hypertension, tremulousness, cold, sweaty extremities, and anxiety due to an exaggerated response to beta‐adrenergic stimulation.7 The excessive sympathetic activity, which is poorly modulated by baroreflex activity, may be due to impaired mechanisms of norepinephrine reuptake by sympathetic ganglia.12 Consequently, serum norepinephrine levels are markedly elevated (>600 pg/mL).5
In adults, the presence of a POTS trigger is common and is usually an antecedent viral illness. Antibodies to the ganglionic acetylcholine receptor have been found in a subset of POTS patients,13 and this may suggest an idiopathic or postinflammatory autoimmune mechanism.14 This patient's presentation is unique because her symptoms developed after C5C6 spine surgery. The cervical spinal cord and sympathetic ganglia are dense with nerves involved in autonomic cardiovascular control, and damage to these fibers could explain the patient's physiology and symptoms. Among these, the descending vasomotor pathways traverse through the C5C8 area to innervate the splanchnic and leg venous circulation, receiving input from the heart along the way.15 The pattern of numbness and tingling fits the C5/C6 dermatomal distribution, as does the innervation of the radial artery. The frequent PVCs with a left bundle branch block pattern and inferior axis appear to arise from the right ventricular outflow tract and may be associated with regional sympathetic denervation, which has been described in idiopathic ventricular arrhythmias.16 POTS has been anecdotally reported after neck injury from motor vehicle accidents (whiplash), which is also thought to be related to cervical sympathetic nerve damage (B.P. Grubb, personal communication, 2005). Most cases of triggered POTS improve spontaneously after months to years, but this patient's prognosis remains uncertain because of the presumed mechanical disruption of the autonomic nerve fibers at the time of surgery.
This case demonstrates the complexities of arriving at a unifying diagnosis in the setting of a constellation of nonspecific symptoms and findings, some of which even suggest life‐threatening conditions. Because young women are primarily affected, symptoms of POTS can be mistakenly attributed to anxiety or other nonphysiological factors. A systematic approach excluded life‐threatening causes, including primary ventricular arrhythmias, coronary vasospasm, and coronary anomalies. The investigations narrowed the differential diagnosis, and the tilt‐table test confirmed POTS. Because the cardiac and circulatory dysautonomias encompass an array of distinct physiologic processes, understanding the patient's mechanism is critical to her management. The only effective therapies were those that counteracted venous pooling and improved venous return.
Teaching Points
-
The differential diagnosis of exertional syncope is extremely broad, ranging from benign to malignant conditions, and requires a systematic evaluation of the heart and circulatory system.
-
The diagnosis of POTS is elusive and frequently missed. Referral for tilt‐table testing is useful in identifying the mechanism of sinus tachycardia and syncope. Marked orthostatic tachycardia and symptoms of cerebral hypoperfusion out of proportion to the degree of hypotension strongly suggest POTS.
-
Cardiac and circulatory dysautonomias have distinct and varied mechanisms. Therapies, including beta‐blockers, vasoconstrictors, and volume expanders, must be directed at the underlying physiological defect.
- .An irritable heart.Am J Med Sci.1871;27:145–161.
- ,,,,,.Low physical working capacity in suspected heart cases due to inadequate adjustment of peripheral blood flow (vasoregulatory asthenia).Acta Med Scand.1957;158(6):413–436.
- ,,.Orthostatic tachycardia and orthostatic hypotension: defects in the return of venous blood to the heart.Am Heart J.1944;27:145–163.
- ,.Postural tachycardia syndrome. Reversal of sympathetic hyperresponsiveness and clinical improvement during sodium loading.Am J Med.1982;72(5):847–850.
- ,,.The postural orthostatic tachycardia syndrome: definitions, diagnosis, and management.Pacing Clin Electrophysiol.2003;26(8):1747– 1757.
- ,.Clinical disorders of the autonomic nervous system associated with orthostatic intolerance: an overview of classification, clinical evaluation, and management.Pacing Clin Electrophysiol.1999;22(5):798–810.
- ,.Idiopathic orthostatic intolerance and postural tachycardia syndromes.Am J Med Sci.1999;317(2):88–101.
- ,,, et al.Splanchnic‐mesenteric capacitance bed in the postural tachycardia syndrome (POTS).Auton Neurosci.2000;86(1–2):107–113.
- ,,,.Abnormal orthostatic changes in blood pressure and heart rate in subjects with intact sympathetic nervous function: evidence for excessive venous pooling.J Lab Clin Med.1988;111(3):326–335.
- ,,, et al.Renin‐aldosterone paradox and perturbed blood volume regulation underlying postural tachycardia syndrome.Circulation.2005;111(13):1574–1582.
- .Clinical practice. Neurocardiogenic syncope.N Engl J Med.2005;352(10):1004–1010.
- ,,, et al.Orthostatic intolerance and tachycardia associated with norepinephrine‐transporter deficiency.N Engl J Med.2000;342(8):541–549.
- ,,,,,.Autoantibodies to ganglionic acetylcholine receptors in autoimmune autonomic neuropathies.N Engl J Med.2000;343(12):847–855.
- ,,.The postural tachycardia syndrome: a concise guide to diagnosis and management.J Cardiovasc Electrophysiol.2006;17(1):108–112.
- ,,.Neurovegetative regulation of the vascular system. In:Lanzer P,Topol EJ, eds.Panvascular Medicine.Berlin, Germany:Springer‐Verlag;2002:175–187.
- ,,, et al.Regional cardiac sympathetic denervation in patients with ventricular tachycardia in the absence of coronary artery disease.J Am Coll Cardiol.1993;22(5):1344–1353.
- .An irritable heart.Am J Med Sci.1871;27:145–161.
- ,,,,,.Low physical working capacity in suspected heart cases due to inadequate adjustment of peripheral blood flow (vasoregulatory asthenia).Acta Med Scand.1957;158(6):413–436.
- ,,.Orthostatic tachycardia and orthostatic hypotension: defects in the return of venous blood to the heart.Am Heart J.1944;27:145–163.
- ,.Postural tachycardia syndrome. Reversal of sympathetic hyperresponsiveness and clinical improvement during sodium loading.Am J Med.1982;72(5):847–850.
- ,,.The postural orthostatic tachycardia syndrome: definitions, diagnosis, and management.Pacing Clin Electrophysiol.2003;26(8):1747– 1757.
- ,.Clinical disorders of the autonomic nervous system associated with orthostatic intolerance: an overview of classification, clinical evaluation, and management.Pacing Clin Electrophysiol.1999;22(5):798–810.
- ,.Idiopathic orthostatic intolerance and postural tachycardia syndromes.Am J Med Sci.1999;317(2):88–101.
- ,,, et al.Splanchnic‐mesenteric capacitance bed in the postural tachycardia syndrome (POTS).Auton Neurosci.2000;86(1–2):107–113.
- ,,,.Abnormal orthostatic changes in blood pressure and heart rate in subjects with intact sympathetic nervous function: evidence for excessive venous pooling.J Lab Clin Med.1988;111(3):326–335.
- ,,, et al.Renin‐aldosterone paradox and perturbed blood volume regulation underlying postural tachycardia syndrome.Circulation.2005;111(13):1574–1582.
- .Clinical practice. Neurocardiogenic syncope.N Engl J Med.2005;352(10):1004–1010.
- ,,, et al.Orthostatic intolerance and tachycardia associated with norepinephrine‐transporter deficiency.N Engl J Med.2000;342(8):541–549.
- ,,,,,.Autoantibodies to ganglionic acetylcholine receptors in autoimmune autonomic neuropathies.N Engl J Med.2000;343(12):847–855.
- ,,.The postural tachycardia syndrome: a concise guide to diagnosis and management.J Cardiovasc Electrophysiol.2006;17(1):108–112.
- ,,.Neurovegetative regulation of the vascular system. In:Lanzer P,Topol EJ, eds.Panvascular Medicine.Berlin, Germany:Springer‐Verlag;2002:175–187.
- ,,, et al.Regional cardiac sympathetic denervation in patients with ventricular tachycardia in the absence of coronary artery disease.J Am Coll Cardiol.1993;22(5):1344–1353.
Tobacco, Alcohol, and Drug Use Among Hospital Patients
Population‐based surveys of the adult US population estimate a prevalence of smoking of 25% and a prevalence of hazardous alcohol or illegal drug use of 23% and 8% respectively,1 with frequent concurrent use of these substances.2 The mortality associated with smoking and substance use is extremely high with tobacco first, alcohol third, and illicit drug use ninth as the leading causes of death in the US.3 Worldwide, the burden of disease from tobacco, alcohol, and illicit drugs accounts for almost 10% of all disability‐adjusted life years.4 Despite the availability of effective treatments,57 many patients do not receive professional intervention and few are offered comprehensive programs that address all of their harmful substance use.
Interventions have been successfully implemented for hospitalized smokers. Earlier work by Emmons8 and Orleans9 suggests that many smokers seek assistance to quit smoking during hospitalization. Over the past 15 years, hospital‐based smoking cessation interventions have been successfully implemented.10 Although mute on hospital‐based settings, the United States Preventive Service Task Force recommends screening and counseling interventions to reduce alcohol misuse among adults seen in primary care settings (B recommendation).6 Referral to specialized care is the accepted standard for most patients with substance dependence disorders7 regardless of the medical setting in which the diagnosis is made. Hospitalization provides a unique opportunity to initiate change in harmful substance use and smoking;11 however, interventions rarely are coordinated.
A high prevalence of smoking among substance users has been reported from population‐based surveys1215 and among patients in substance use treatment facilities.1618 Rates of concurrent smoking and substance use range from 35%44% in population‐based studies and may reach 80% in populations seeking substance use treatment.19 A recent hospital‐based study found at‐risk alcohol users were 3 times more likely to smoke.20 There are limited data describing concurrent smoking and substance use in the hospital population,15 and no reports describing the association between patients' willingness to quit smoking and readiness to change substance use behavior.
To better inform hospital‐based smoking and substance use intervention strategies, the epidemiology of smoking and substance use in the hospital population needs to be better described. Furthermore, there may be opportunities for synergy between these programs. In this study, we screened inpatients from multiple services at 2 hospitals for tobacco, alcohol, and illicit substance use. We report the prevalence and co‐occurrence of these behaviors and willingness to quit smoking among patients with and without at‐risk substance use.
METHODS
Data for this study were obtained for a 5‐year Substance Abuse and Mental Health Services Administration (SAMHSA) grant to the Illinois Office of the Governor. The grant was awarded to implement screening, brief intervention, brief treatment, and referral to treatment programs for patients of the Cook County Bureau of Health Services who had alcohol or other drug use disorders. We analyzed data collected from nonIntensive Care Unit patients who had been hospitalized on the internal medicine, family practice, HIV, or surgery services at John H. Stroger Jr Hospital of Cook County (formerly Cook County Hospital, a 464‐bed public, tertiary‐care hospital) or Provident Hospital of Cook County (a 100‐bed public community hospital), in Chicago, Illinois. Because internal medicine and family practice patients were similar in demographic characteristics and interview responses, we considered these as a single service. There is an HIV service at Stroger Hospital; all HIV‐infected patients are admitted or transferred to this service. For each patient, we used data collected from their initial hospitalization during a 9‐month study period (April 1, 2006 through December 31, 2006). Using hospital admission data, we estimated that 65% of patients were interviewed by a counselor; only 5% of patients could not be interviewed due to patient refusal or mental status changes.
Patients were screened for alcohol use, drug use, and smoking history by bedside interview. We defined at‐risk substance use as any illicit drug use within the previous 3 months or alcohol use that exceeded the National Institute for Alcohol Abuse and Alcoholism (NIAAA) guidelines for low‐risk drinking (no more than 5 drinks per day or 14 drinks per week for men up to age 65; no more than 3 drinks per day or 7 drinks per week for men over 65 and women). Based on their responses to questions about smoking history, patients were categorized into the following 4 groups: current smokers (ie, smoked within the previous 7 days), recent quitters (ie, quit within 8 days and 6 months), ex‐smokers (quit more than 6 months ago), or never smokers. Current smokers were also asked about their heaviness of smoking and willingness to quit. All smokers received a counseling session during hospitalization. All smokers who indicated a desire to quit were encouraged to call the Illinois Quitline after hospital discharge. Individuals who smoked between 10‐14 cigarettes per day and smoked their first cigarette within 30 minutes of waking or who smoked 15 or more cigarettes per day were classified as moderate or heavy smokers; all other smokers were classified as light smokers. We established these cut‐points by modifying the Public Health Service guideline and Heaviness of Smoking Index.5, 21, 22 The heaviness of smoking classification was used to guide recommendations to the primary service regarding the appropriateness of nicotine patch therapy during and after hospitalization. For moderate to heavy smokers who were willing to quit, the recommendation was to continue nicotine replacement after hospitalization.5
Patients were considered low health risk if their alcohol use did not exceed NIAAA guidelines and they reported no recent drug use. For all patients who reported alcohol use that exceeded the NIAAA guidelines or recent drug use, we administered the Texas Christian University Drug Screen II (TCU)23 to further characterize the severity of their use. Patients who had a TCU score of 3 were considered at‐risk substance users with substance dependence disorder; patients with scores of 2 or less were considered at‐risk substance users without dependence. Among all at‐risk substance users, we used a 10‐point visual analog scale to assess their readiness to change substance use. After evaluating the distribution and clustering of scores, we prespecified that a score 8 was indicative of a patient being ready to change their substance use behavior. This ruler has been successfully implemented as part of the Brief Negotiated Interview and Active Referral to Treatment Institute toolbox.24
Analysis
To facilitate comparison with other data sources, we used the same age categories as the National Survey on Drug Use and Health.1 Differences between proportions were evaluated by the chi‐squared test. We analyzed the trend in smoking behavior across the strata of substance use (ie, number of substances used and severity of use) using the Cochrane‐Armitage test for trend. To evaluate the association between substance use and smoking, multivariable models were constructed that included terms to adjust for age, race, gender, and hospital service; potential confounders (eg, age, race, gender, and service) were included in the final model if they significantly contributed to the outcome variable (P < 0.1). From these multivariable models, prevalence ratios were estimated using the binary log transformation in PROC GENMOD.25, 26 All data were analyzed using SAS version 9.0 (SAS Institute Inc., Cary, NC).
RESULTS
Patient Characteristics
Of the 7,714 unique patients interviewed at the 2 hospitals, we had data on smoking status for 7,391 (96%) (Table 1). The mean age was 50 years, most were male, cared for by the internal medicine or family practice service, and the most common racial/ethnic category was non‐Hispanic Black, followed by Hispanic, non‐Hispanic White, and Asian (Table 1). More than one‐quarter of patients reported at‐risk substance use other than tobacco; the most common substance used was alcohol followed by cocaine, marijuana, and then heroin (Table 1). Most patients who were at‐risk substance users (52%) met criteria for substance dependence disorder.23
| Characteristic | N | (%) | Smoking prevalence* (%) | Prevalence ratio (95% CI) | |
|---|---|---|---|---|---|
| |||||
| Age category | |||||
| 18‐25 | 479 | (6) | 35 | 2.6 | (2.1 to 3.1) |
| 26‐34 | 664 | (9) | 38 | 2.8 | (2.4 to 3.4) |
| 35‐44 | 1306 | (18) | 46 | 3.4 | (2.9 to 4.0) |
| 45‐54 | 2182 | (30) | 46 | 3.4 | (2.9 to 4.0) |
| 55‐64 | 1563 | (21) | 31 | 2.3 | (2.0 to 2.7) |
| 65 and older | 1185 | (16) | 13 | ref | |
| Race/Ethnicity | |||||
| Non Hispanic Black | 4990 | (68) | 45 | 3.0 | (2.2 to 4.0) |
| Non Hispanic White | 850 | (12) | 40 | 2.7 | (2.0 to 3.6) |
| Hispanic | 1222 | (17) | 19 | 1.3 | (0.9 to 1.7) |
| Asian | 253 | (3) | 15 | ref | |
| Other | 27 | (<1) | |||
| Gender | |||||
| Male | 4279 | (58) | 42 | 1.5 | (1.4 to 1.6) |
| Female | 3099 | (42) | 29 | ref | |
| Service | |||||
| HIV | 227 | (3) | 52 | 1.7 | (1.5 to 2.0) |
| Internal medicine or | 6278 | (85) | 36 | 1.2 | (1.1 to 1.3) |
| family practice | |||||
| Surgery | 886 | (12) | 31 | ref | |
Tobacco Use
Many hospitalized patients were current smokers (36%) and 35% of current smokers were moderate to heavy smokers. The prevalence of smoking varied significantly by age category, race, gender, and service. By age category, the prevalence of smoking peaked at 3554 years with lower rates of smoking at either extreme of age (Table 1). Non‐Hispanic Blacks and Whites had a prevalence of smoking 3‐fold higher than Asians; Hispanics were less likely to smoke than non‐Hispanic Whites or Blacks. Men were more likely to smoke than women, and patients on the HIV or internal medicine/family practice services had a higher prevalence of smoking compared to patients on the surgery service (Table 1).
The proportion of current smokers who were moderate to heavy smokers was similar between patients with no‐risk or low‐risk substance use and those who had at‐risk substance use without dependence (32% versus 34%, respectively); however, current smokers who were substance‐dependent were 40% more likely to be moderate to heavy smokers (48%) (prevalence ratio [PR]: 1.4, 95% confidence interval [CI]: 1.1 to 1.9).
Concurrent Tobacco and Substance Use
Compared to patients who reported low‐risk substance use, patients with at‐risk substance use had a dramatically higher prevalence of smoking (Table 2). In addition, there was a significant increase in the likelihood of smoking across the 3 levels of substance use and the number of substances used (Table 2).
| N | (%) | Smoking prevalence (%) | Adjusted prevalence ratio (95% CI)* | ||
|---|---|---|---|---|---|
| |||||
| Risk Index | |||||
| Low Health Risk | 5419 | (73) | 24 | ref | |
| At‐Risk, not dependent | 945 | (13) | 64 | 2.2 | (2.0 to 2.3) |
| At‐Risk, dependent | 1027 | (14) | 75 | 2.5 | (2.3 to 2.6) |
| Specific substance use | |||||
| Low Health Risk | 5419 | (73) | 24 | ref | |
| At‐Risk Alcohol Use | 1171 | (16) | 68 | 2.2 | (2.1 to 2.4) |
| At‐ Risk Marijuana Use | 688 | (9) | 70 | 2.1 | (2.0 to 2.3) |
| At‐Risk Cocaine Use | 503 | (7) | 79 | 2.4 | (2.2 to 2.6) |
| At‐Risk Heroin Use | 448 | (6) | 82 | 2.4 | (2.2 to 2.6) |
| Number of drugs | |||||
| None | 5419 | (73) | 24 | ref | |
| One | 1284 | (17) | 64 | 2.2 | (2.0 to 2.3) |
| Two or more | 688 | (9) | 81 | 2.6 | (2.5 to 2.8) |
Willingness to Quit
Most patients (61%) who smoked were willing to immediately quit smoking. After adjusting for other demographic confounders, non‐Hispanic Blacks and the elderly (age > 65) were more willing to quit (P < 0.05, data not shown). The substance use risk categories of low risk, at‐risk, and dependence were not associated with willingness to quit tobacco (Fig. 1, left panel).
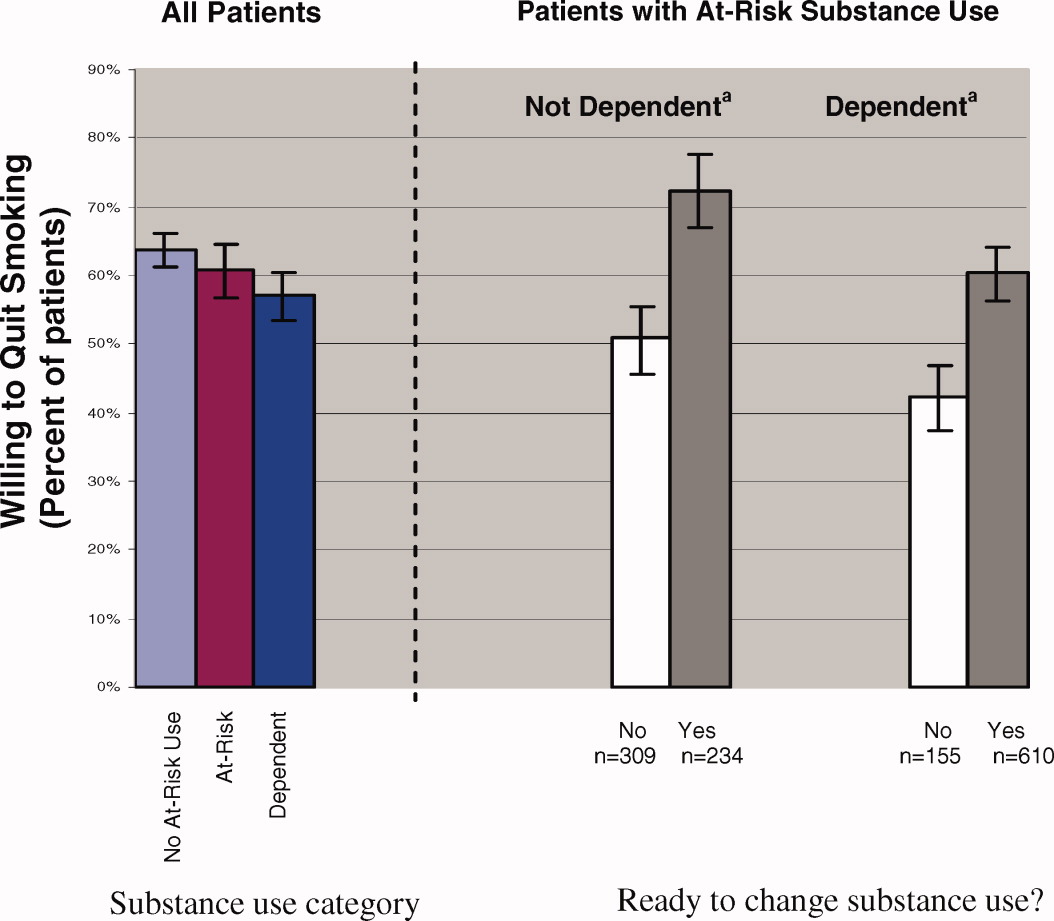
Regardless of substance use category, most patients were ready to change their substance use behavior (Fig. 1). Those patients who were ready to change their substance use behavior, regardless of whether they were substance‐dependent, were significantly more likely to report a willingness to quit smoking than those who were not ready to change (Fig. 1, right panel). In fact, at‐risk substance users without dependence who were ready to change their substance use were more willing to quit smoking than patients without at‐risk substance use (72% versus 64%; P < 0.05).
DISCUSSION
Among hospital patients, we found a 46% absolute increase in the prevalence of smoking among those who used illicit substances or alcohol above NIAAA guidelines compared to those who did not report such use. The prevalence of smoking increased across the spectrum of substance use, being highest for patients who met criteria for dependence. Also, patients who were substance dependent were more likely to be moderate to heavy smokers, suggesting an association between alcohol or other drug dependence disorders and nicotine dependence. Regardless of their patterns of substance use, most patients expressed a desire to immediately quit smoking and there was a strong association between willingness to quit smoking and readiness to reduce substance use.
In our hospital population, the prevalence of smoking among patients who use illicit drugs or at‐risk quantities of alcohol far exceeds estimates obtained from population‐based surveys. In addition to the relatively high prevalence of smoking, focusing attention on hospital patients who use substances is important for several other reasons. Individuals who use substances are less likely to receive health care from a primary care physician.28 Also, most patients who have substance use disorders do not enter treatment programs,1 even after hospitalization.29 Further, hospitals provide a setting that facilitates change; patients are temporarily required to stop smoking, and often they are available for relatively long counseling sessions. Finally, for patients without substance use disorders, hospital‐based smoking cessation intervention programs have been proven to be successful in several randomized controlled trials.10, 30
Because alcohol and drug use are so common among hospitalized smokers, it is unfortunate that there is little evidence from clinical trials to inform intervention strategies for patients with concurrent use. The clinical trials that form the evidence base for intervention among hospitalized smokers10 either have explicitly excluded patients who reported substance use,10, 15, 3133 did not assess baseline substance use,34, 35 or were underpowered to perform subgroup analyses on this population.36 Awaiting better evidence, we have chosen to routinely screen hospital patients for tobacco, alcohol, and drug use. For treatment strategies, we extrapolate the findings from successful interventions in the ambulatory setting37 or among hospital patients who do not use substances to our population. We offer smoking cessation interventions to patients regardless of other substance use.
Understanding the similarities and differences between smokers who use substances and those who do not is important in implementing successful strategies for smoking cessation. Rather than a step‐wise increase in heaviness of smoking across substance use categories (ie, no‐risk or low‐risk use, at‐risk use without dependence, and substance dependence), we found an increased heaviness of smoking only among substance‐dependent smokers; there was no difference in heaviness of smoking between those with at‐risk use without dependence and those with no‐risk or low‐risk use. Because interventions for patients who have nicotine dependence are more likely to succeed when pharmacotherapy is offered as an adjunct to behavior therapy,38 smokers who also are substance‐dependent likely will benefit from the addition of pharmacotherapy. One similarity is that all patients, regardless of substance use category, were willing to quit smoking. In fact, hospitalized smokers who were ready to change at‐risk substance use were more willing to quit smoking than patients who had no‐risk or low‐risk substance use. Previous investigators have found that smokers who use substances have fewer quit attempts,39 higher nicotine dependence,37, 39 and lower enrollment in smoking cessation interventions.38
Our study only includes data from patients at 2 public hospitals; therefore, our findings may not generalize to populations of higher socioeconomic status. Also, our smoking screening tool had relatively low sensitivity for categorizing current smokers as moderate to heavy smokers; therefore, we may have underestimated the number of moderate to heavy smokers.5, 22 Further, given our cross‐sectional study design, we were unable to evaluate whether patients who have at‐risk substance use remain willing to quit smoking after hospital discharge or to the effectiveness of our smoking cessation program. Finally, socially desirable responses may have caused patients to overstate their willingness to quit tobacco and readiness to change substance use. Additional research is needed to determine whether post‐hospitalization quit rates are similar between smokers with and without at‐risk substance use, and the optimal timing for smoking cessation interventions in relation to substance dependence treatment.40
Hospital patients who have substance use disorders are also highly likely to smoke, and these patients express a willingness to quit smoking. Given the frequency of concurrent smoking and other substance misuse and patients' desire to change both behaviors, there is a role for coordination of substance use and smoking cessation intervention programs.
- US Department of Health 24:201–208.
- ,,,.Actual causes of death in the United States, 2000.JAMA.2004;291:1238–1245.
- ,,.Global burden of disease from alcohol, illicit drugs and tobacco.Drug Alcohol Rev.2006;25:503–513.
- A clinical practice guideline for treating tobacco use and dependence,:A US Public Health Service report. The tobacco use and dependence clinical practice guideline panel, staff, and consortium representatives.JAMA.2000;283:3244–3254.
- ,,,,,U.S.Preventive Services Task Force. Behavioral counseling interventions in primary care to reduce risky/harmful alcohol use by adults: a summary of the evidence for the U.S. Preventive Services Task Force.Ann Intern Med.2004;140:557–568.
- Work Group on Substance Use Disorders,,, et al.Treatment of patients with substance use disorders, second edition. American Psychiatic Association.Am J Psych.2006;163(8 Suppl):75–82.
- ,.Smokers who are hospitalized: a window of opportunity for cessation interventions.Prev Med.1992;21;262–269.
- ,,.Helping hospitalized smokers quit: new directions for treatment and research.J Consult Clin Psychol.1993;61:778–89.
- ,,.Interventions for smoking cessation in hospitalised patients.Cochrane Database Sys Rev.2007;3(3);CD001837.
- ,,,.Expanding the roles of hospitalists physicians to include public health.J Hosp Med.2007;2:93–101.
- ,,,,.Smoking status as a clinical indicator for alcohol misuse in US adults.Arch Intern Med.2007;167:716–721.
- ,,,,.Alcohol high risk drinking, abuse and dependence among tobacco smoking medical care patients and the general population.Drug Alcohol Depend.2003;69:189–195.
- ,,,,.Nicotine dependence and psychiatric disorders in the United States: results from the national epidemiologic survey on alcohol and related conditions.Arch Gen Psychiatry.2004;61:1107–1115.
- ,,,,,.Smoking and mental illness: A population‐based prevalence study.JAMA.2000;284:2606–2610.
- .Clinical Implications of the association between smoking and alcoholism. In:Fertig JB,Allen JP, eds.Alcohol and Tobacco: From Basic Science to Clinical Practice.Bethesda, MD:NIAAA Research;1995:171–185.
- ,,,,,.Smoking and drinking among alcoholics in treatment: cross‐sectional and longitudinal relationships.J Stud Alcohol.2000;61:157–163.
- ,,, et al.Interrelationship of smoking and alcohol dependence, use and urges to use.J Stud Alcohol.1995;56:202–206.
- ,,.Tobacco cessation treatment for alcohol‐dependent smokers: when is the best time?Alcohol Res Health.2006;29:203–207.
- ,,, et al.Substance use in the general hospital.Addict Behav.2003;28:483–499.
- ,,,,.Measuring the heaviness of smoking: Using self‐reported time to the first cigarette of the day and number of cigarettes smoked per day.Br J Addict.1989;84:791–799.
- ,,,.The Heaviness of Smoking Index as a predictor of smoking cessation in Canada.Addict Behav.2007;32:1031–1042.
- ,,, et al.Effectiveness of screening instruments in detecting substance use disorders among prisoners.J Subst Abuse Treat.2000;18:349–358.
- Th BNI‐ART Institute, Readiness Ruler. http://www.ed.bmc.org/sbirt/techniques.php. Accessed August 20,2008.
- .A modified poisson regression approach to prospective studies with binary data.Am J Epidemiol.2004;159:702–706.
- ,,,.Estimating the relative risk in cohort studies and clinical trials of common outcomes.Am J Epidemiol.2003;157:940–943.
- ,.Do smokers with alcohol problems have more difficulty quitting?Drug Alcohol Depend.2006;82:91–102.
- .Emergency room and primary care services utilization and associated alcohol and drug use in the United States general population.Alcohol Alcohol.1999;34:581–589.
- ,,, et al.Brief intervention for medical inpatients with unhealthy alcohol use: a randomized, controlled trial.Ann Intern Med.2007;146:167–176.
- ,,.Smoking cessation interventions among hospitalized patients: what have we learned?Prev Med.2001;32:376–388.
- ,,, et al.Smoking cessation and severity of disease: the Coronary Artery Smoking Intervention Study.Health Psychol.1992;11:119–126.
- ,,,,.A randomized controlled trial of smoking cessation counseling after myocardial infarction.Prev Med.2000;30:261–268.
- ,.Comorbid cigarette and alcohol addiction: epidemiology and treatment.J Addict Dis.1998;17:55–66.
- ,,, et al.A case‐management system for coronary risk factor modification after acute myocardial infarction.Ann Intern Med.1994;120:721–729.
- ,,, et al.A nurse‐managed smoking cessation program for hospitalized smokers.Am J Public Health.1996;86:1557–1560.
- ,,,.Smoking cessation after surgery. A randomized trial.Arch Intern Med.1997;157:1371–1376.
- ,,,,.Efficacy of nicotine patch in smokers with a history of alcoholism.Alcohol Clin Exp Res.2003;27:946–954.
- ,,,.Predictors of tobacco quit attempts among recovering alcoholics.J Subst Abuse.1996;8:431–443.
- ,,.Is dependence on one drug associated with dependence on other drugs? The cases of alcohol, caffeine and nicotine.Am J Addict.2000;9:196–201.
- .Nicotine interventions with comorbid populations.Am J Prev Med.2007;33:S406–S413.
Population‐based surveys of the adult US population estimate a prevalence of smoking of 25% and a prevalence of hazardous alcohol or illegal drug use of 23% and 8% respectively,1 with frequent concurrent use of these substances.2 The mortality associated with smoking and substance use is extremely high with tobacco first, alcohol third, and illicit drug use ninth as the leading causes of death in the US.3 Worldwide, the burden of disease from tobacco, alcohol, and illicit drugs accounts for almost 10% of all disability‐adjusted life years.4 Despite the availability of effective treatments,57 many patients do not receive professional intervention and few are offered comprehensive programs that address all of their harmful substance use.
Interventions have been successfully implemented for hospitalized smokers. Earlier work by Emmons8 and Orleans9 suggests that many smokers seek assistance to quit smoking during hospitalization. Over the past 15 years, hospital‐based smoking cessation interventions have been successfully implemented.10 Although mute on hospital‐based settings, the United States Preventive Service Task Force recommends screening and counseling interventions to reduce alcohol misuse among adults seen in primary care settings (B recommendation).6 Referral to specialized care is the accepted standard for most patients with substance dependence disorders7 regardless of the medical setting in which the diagnosis is made. Hospitalization provides a unique opportunity to initiate change in harmful substance use and smoking;11 however, interventions rarely are coordinated.
A high prevalence of smoking among substance users has been reported from population‐based surveys1215 and among patients in substance use treatment facilities.1618 Rates of concurrent smoking and substance use range from 35%44% in population‐based studies and may reach 80% in populations seeking substance use treatment.19 A recent hospital‐based study found at‐risk alcohol users were 3 times more likely to smoke.20 There are limited data describing concurrent smoking and substance use in the hospital population,15 and no reports describing the association between patients' willingness to quit smoking and readiness to change substance use behavior.
To better inform hospital‐based smoking and substance use intervention strategies, the epidemiology of smoking and substance use in the hospital population needs to be better described. Furthermore, there may be opportunities for synergy between these programs. In this study, we screened inpatients from multiple services at 2 hospitals for tobacco, alcohol, and illicit substance use. We report the prevalence and co‐occurrence of these behaviors and willingness to quit smoking among patients with and without at‐risk substance use.
METHODS
Data for this study were obtained for a 5‐year Substance Abuse and Mental Health Services Administration (SAMHSA) grant to the Illinois Office of the Governor. The grant was awarded to implement screening, brief intervention, brief treatment, and referral to treatment programs for patients of the Cook County Bureau of Health Services who had alcohol or other drug use disorders. We analyzed data collected from nonIntensive Care Unit patients who had been hospitalized on the internal medicine, family practice, HIV, or surgery services at John H. Stroger Jr Hospital of Cook County (formerly Cook County Hospital, a 464‐bed public, tertiary‐care hospital) or Provident Hospital of Cook County (a 100‐bed public community hospital), in Chicago, Illinois. Because internal medicine and family practice patients were similar in demographic characteristics and interview responses, we considered these as a single service. There is an HIV service at Stroger Hospital; all HIV‐infected patients are admitted or transferred to this service. For each patient, we used data collected from their initial hospitalization during a 9‐month study period (April 1, 2006 through December 31, 2006). Using hospital admission data, we estimated that 65% of patients were interviewed by a counselor; only 5% of patients could not be interviewed due to patient refusal or mental status changes.
Patients were screened for alcohol use, drug use, and smoking history by bedside interview. We defined at‐risk substance use as any illicit drug use within the previous 3 months or alcohol use that exceeded the National Institute for Alcohol Abuse and Alcoholism (NIAAA) guidelines for low‐risk drinking (no more than 5 drinks per day or 14 drinks per week for men up to age 65; no more than 3 drinks per day or 7 drinks per week for men over 65 and women). Based on their responses to questions about smoking history, patients were categorized into the following 4 groups: current smokers (ie, smoked within the previous 7 days), recent quitters (ie, quit within 8 days and 6 months), ex‐smokers (quit more than 6 months ago), or never smokers. Current smokers were also asked about their heaviness of smoking and willingness to quit. All smokers received a counseling session during hospitalization. All smokers who indicated a desire to quit were encouraged to call the Illinois Quitline after hospital discharge. Individuals who smoked between 10‐14 cigarettes per day and smoked their first cigarette within 30 minutes of waking or who smoked 15 or more cigarettes per day were classified as moderate or heavy smokers; all other smokers were classified as light smokers. We established these cut‐points by modifying the Public Health Service guideline and Heaviness of Smoking Index.5, 21, 22 The heaviness of smoking classification was used to guide recommendations to the primary service regarding the appropriateness of nicotine patch therapy during and after hospitalization. For moderate to heavy smokers who were willing to quit, the recommendation was to continue nicotine replacement after hospitalization.5
Patients were considered low health risk if their alcohol use did not exceed NIAAA guidelines and they reported no recent drug use. For all patients who reported alcohol use that exceeded the NIAAA guidelines or recent drug use, we administered the Texas Christian University Drug Screen II (TCU)23 to further characterize the severity of their use. Patients who had a TCU score of 3 were considered at‐risk substance users with substance dependence disorder; patients with scores of 2 or less were considered at‐risk substance users without dependence. Among all at‐risk substance users, we used a 10‐point visual analog scale to assess their readiness to change substance use. After evaluating the distribution and clustering of scores, we prespecified that a score 8 was indicative of a patient being ready to change their substance use behavior. This ruler has been successfully implemented as part of the Brief Negotiated Interview and Active Referral to Treatment Institute toolbox.24
Analysis
To facilitate comparison with other data sources, we used the same age categories as the National Survey on Drug Use and Health.1 Differences between proportions were evaluated by the chi‐squared test. We analyzed the trend in smoking behavior across the strata of substance use (ie, number of substances used and severity of use) using the Cochrane‐Armitage test for trend. To evaluate the association between substance use and smoking, multivariable models were constructed that included terms to adjust for age, race, gender, and hospital service; potential confounders (eg, age, race, gender, and service) were included in the final model if they significantly contributed to the outcome variable (P < 0.1). From these multivariable models, prevalence ratios were estimated using the binary log transformation in PROC GENMOD.25, 26 All data were analyzed using SAS version 9.0 (SAS Institute Inc., Cary, NC).
RESULTS
Patient Characteristics
Of the 7,714 unique patients interviewed at the 2 hospitals, we had data on smoking status for 7,391 (96%) (Table 1). The mean age was 50 years, most were male, cared for by the internal medicine or family practice service, and the most common racial/ethnic category was non‐Hispanic Black, followed by Hispanic, non‐Hispanic White, and Asian (Table 1). More than one‐quarter of patients reported at‐risk substance use other than tobacco; the most common substance used was alcohol followed by cocaine, marijuana, and then heroin (Table 1). Most patients who were at‐risk substance users (52%) met criteria for substance dependence disorder.23
| Characteristic | N | (%) | Smoking prevalence* (%) | Prevalence ratio (95% CI) | |
|---|---|---|---|---|---|
| |||||
| Age category | |||||
| 18‐25 | 479 | (6) | 35 | 2.6 | (2.1 to 3.1) |
| 26‐34 | 664 | (9) | 38 | 2.8 | (2.4 to 3.4) |
| 35‐44 | 1306 | (18) | 46 | 3.4 | (2.9 to 4.0) |
| 45‐54 | 2182 | (30) | 46 | 3.4 | (2.9 to 4.0) |
| 55‐64 | 1563 | (21) | 31 | 2.3 | (2.0 to 2.7) |
| 65 and older | 1185 | (16) | 13 | ref | |
| Race/Ethnicity | |||||
| Non Hispanic Black | 4990 | (68) | 45 | 3.0 | (2.2 to 4.0) |
| Non Hispanic White | 850 | (12) | 40 | 2.7 | (2.0 to 3.6) |
| Hispanic | 1222 | (17) | 19 | 1.3 | (0.9 to 1.7) |
| Asian | 253 | (3) | 15 | ref | |
| Other | 27 | (<1) | |||
| Gender | |||||
| Male | 4279 | (58) | 42 | 1.5 | (1.4 to 1.6) |
| Female | 3099 | (42) | 29 | ref | |
| Service | |||||
| HIV | 227 | (3) | 52 | 1.7 | (1.5 to 2.0) |
| Internal medicine or | 6278 | (85) | 36 | 1.2 | (1.1 to 1.3) |
| family practice | |||||
| Surgery | 886 | (12) | 31 | ref | |
Tobacco Use
Many hospitalized patients were current smokers (36%) and 35% of current smokers were moderate to heavy smokers. The prevalence of smoking varied significantly by age category, race, gender, and service. By age category, the prevalence of smoking peaked at 3554 years with lower rates of smoking at either extreme of age (Table 1). Non‐Hispanic Blacks and Whites had a prevalence of smoking 3‐fold higher than Asians; Hispanics were less likely to smoke than non‐Hispanic Whites or Blacks. Men were more likely to smoke than women, and patients on the HIV or internal medicine/family practice services had a higher prevalence of smoking compared to patients on the surgery service (Table 1).
The proportion of current smokers who were moderate to heavy smokers was similar between patients with no‐risk or low‐risk substance use and those who had at‐risk substance use without dependence (32% versus 34%, respectively); however, current smokers who were substance‐dependent were 40% more likely to be moderate to heavy smokers (48%) (prevalence ratio [PR]: 1.4, 95% confidence interval [CI]: 1.1 to 1.9).
Concurrent Tobacco and Substance Use
Compared to patients who reported low‐risk substance use, patients with at‐risk substance use had a dramatically higher prevalence of smoking (Table 2). In addition, there was a significant increase in the likelihood of smoking across the 3 levels of substance use and the number of substances used (Table 2).
| N | (%) | Smoking prevalence (%) | Adjusted prevalence ratio (95% CI)* | ||
|---|---|---|---|---|---|
| |||||
| Risk Index | |||||
| Low Health Risk | 5419 | (73) | 24 | ref | |
| At‐Risk, not dependent | 945 | (13) | 64 | 2.2 | (2.0 to 2.3) |
| At‐Risk, dependent | 1027 | (14) | 75 | 2.5 | (2.3 to 2.6) |
| Specific substance use | |||||
| Low Health Risk | 5419 | (73) | 24 | ref | |
| At‐Risk Alcohol Use | 1171 | (16) | 68 | 2.2 | (2.1 to 2.4) |
| At‐ Risk Marijuana Use | 688 | (9) | 70 | 2.1 | (2.0 to 2.3) |
| At‐Risk Cocaine Use | 503 | (7) | 79 | 2.4 | (2.2 to 2.6) |
| At‐Risk Heroin Use | 448 | (6) | 82 | 2.4 | (2.2 to 2.6) |
| Number of drugs | |||||
| None | 5419 | (73) | 24 | ref | |
| One | 1284 | (17) | 64 | 2.2 | (2.0 to 2.3) |
| Two or more | 688 | (9) | 81 | 2.6 | (2.5 to 2.8) |
Willingness to Quit
Most patients (61%) who smoked were willing to immediately quit smoking. After adjusting for other demographic confounders, non‐Hispanic Blacks and the elderly (age > 65) were more willing to quit (P < 0.05, data not shown). The substance use risk categories of low risk, at‐risk, and dependence were not associated with willingness to quit tobacco (Fig. 1, left panel).

Regardless of substance use category, most patients were ready to change their substance use behavior (Fig. 1). Those patients who were ready to change their substance use behavior, regardless of whether they were substance‐dependent, were significantly more likely to report a willingness to quit smoking than those who were not ready to change (Fig. 1, right panel). In fact, at‐risk substance users without dependence who were ready to change their substance use were more willing to quit smoking than patients without at‐risk substance use (72% versus 64%; P < 0.05).
DISCUSSION
Among hospital patients, we found a 46% absolute increase in the prevalence of smoking among those who used illicit substances or alcohol above NIAAA guidelines compared to those who did not report such use. The prevalence of smoking increased across the spectrum of substance use, being highest for patients who met criteria for dependence. Also, patients who were substance dependent were more likely to be moderate to heavy smokers, suggesting an association between alcohol or other drug dependence disorders and nicotine dependence. Regardless of their patterns of substance use, most patients expressed a desire to immediately quit smoking and there was a strong association between willingness to quit smoking and readiness to reduce substance use.
In our hospital population, the prevalence of smoking among patients who use illicit drugs or at‐risk quantities of alcohol far exceeds estimates obtained from population‐based surveys. In addition to the relatively high prevalence of smoking, focusing attention on hospital patients who use substances is important for several other reasons. Individuals who use substances are less likely to receive health care from a primary care physician.28 Also, most patients who have substance use disorders do not enter treatment programs,1 even after hospitalization.29 Further, hospitals provide a setting that facilitates change; patients are temporarily required to stop smoking, and often they are available for relatively long counseling sessions. Finally, for patients without substance use disorders, hospital‐based smoking cessation intervention programs have been proven to be successful in several randomized controlled trials.10, 30
Because alcohol and drug use are so common among hospitalized smokers, it is unfortunate that there is little evidence from clinical trials to inform intervention strategies for patients with concurrent use. The clinical trials that form the evidence base for intervention among hospitalized smokers10 either have explicitly excluded patients who reported substance use,10, 15, 3133 did not assess baseline substance use,34, 35 or were underpowered to perform subgroup analyses on this population.36 Awaiting better evidence, we have chosen to routinely screen hospital patients for tobacco, alcohol, and drug use. For treatment strategies, we extrapolate the findings from successful interventions in the ambulatory setting37 or among hospital patients who do not use substances to our population. We offer smoking cessation interventions to patients regardless of other substance use.
Understanding the similarities and differences between smokers who use substances and those who do not is important in implementing successful strategies for smoking cessation. Rather than a step‐wise increase in heaviness of smoking across substance use categories (ie, no‐risk or low‐risk use, at‐risk use without dependence, and substance dependence), we found an increased heaviness of smoking only among substance‐dependent smokers; there was no difference in heaviness of smoking between those with at‐risk use without dependence and those with no‐risk or low‐risk use. Because interventions for patients who have nicotine dependence are more likely to succeed when pharmacotherapy is offered as an adjunct to behavior therapy,38 smokers who also are substance‐dependent likely will benefit from the addition of pharmacotherapy. One similarity is that all patients, regardless of substance use category, were willing to quit smoking. In fact, hospitalized smokers who were ready to change at‐risk substance use were more willing to quit smoking than patients who had no‐risk or low‐risk substance use. Previous investigators have found that smokers who use substances have fewer quit attempts,39 higher nicotine dependence,37, 39 and lower enrollment in smoking cessation interventions.38
Our study only includes data from patients at 2 public hospitals; therefore, our findings may not generalize to populations of higher socioeconomic status. Also, our smoking screening tool had relatively low sensitivity for categorizing current smokers as moderate to heavy smokers; therefore, we may have underestimated the number of moderate to heavy smokers.5, 22 Further, given our cross‐sectional study design, we were unable to evaluate whether patients who have at‐risk substance use remain willing to quit smoking after hospital discharge or to the effectiveness of our smoking cessation program. Finally, socially desirable responses may have caused patients to overstate their willingness to quit tobacco and readiness to change substance use. Additional research is needed to determine whether post‐hospitalization quit rates are similar between smokers with and without at‐risk substance use, and the optimal timing for smoking cessation interventions in relation to substance dependence treatment.40
Hospital patients who have substance use disorders are also highly likely to smoke, and these patients express a willingness to quit smoking. Given the frequency of concurrent smoking and other substance misuse and patients' desire to change both behaviors, there is a role for coordination of substance use and smoking cessation intervention programs.
Population‐based surveys of the adult US population estimate a prevalence of smoking of 25% and a prevalence of hazardous alcohol or illegal drug use of 23% and 8% respectively,1 with frequent concurrent use of these substances.2 The mortality associated with smoking and substance use is extremely high with tobacco first, alcohol third, and illicit drug use ninth as the leading causes of death in the US.3 Worldwide, the burden of disease from tobacco, alcohol, and illicit drugs accounts for almost 10% of all disability‐adjusted life years.4 Despite the availability of effective treatments,57 many patients do not receive professional intervention and few are offered comprehensive programs that address all of their harmful substance use.
Interventions have been successfully implemented for hospitalized smokers. Earlier work by Emmons8 and Orleans9 suggests that many smokers seek assistance to quit smoking during hospitalization. Over the past 15 years, hospital‐based smoking cessation interventions have been successfully implemented.10 Although mute on hospital‐based settings, the United States Preventive Service Task Force recommends screening and counseling interventions to reduce alcohol misuse among adults seen in primary care settings (B recommendation).6 Referral to specialized care is the accepted standard for most patients with substance dependence disorders7 regardless of the medical setting in which the diagnosis is made. Hospitalization provides a unique opportunity to initiate change in harmful substance use and smoking;11 however, interventions rarely are coordinated.
A high prevalence of smoking among substance users has been reported from population‐based surveys1215 and among patients in substance use treatment facilities.1618 Rates of concurrent smoking and substance use range from 35%44% in population‐based studies and may reach 80% in populations seeking substance use treatment.19 A recent hospital‐based study found at‐risk alcohol users were 3 times more likely to smoke.20 There are limited data describing concurrent smoking and substance use in the hospital population,15 and no reports describing the association between patients' willingness to quit smoking and readiness to change substance use behavior.
To better inform hospital‐based smoking and substance use intervention strategies, the epidemiology of smoking and substance use in the hospital population needs to be better described. Furthermore, there may be opportunities for synergy between these programs. In this study, we screened inpatients from multiple services at 2 hospitals for tobacco, alcohol, and illicit substance use. We report the prevalence and co‐occurrence of these behaviors and willingness to quit smoking among patients with and without at‐risk substance use.
METHODS
Data for this study were obtained for a 5‐year Substance Abuse and Mental Health Services Administration (SAMHSA) grant to the Illinois Office of the Governor. The grant was awarded to implement screening, brief intervention, brief treatment, and referral to treatment programs for patients of the Cook County Bureau of Health Services who had alcohol or other drug use disorders. We analyzed data collected from nonIntensive Care Unit patients who had been hospitalized on the internal medicine, family practice, HIV, or surgery services at John H. Stroger Jr Hospital of Cook County (formerly Cook County Hospital, a 464‐bed public, tertiary‐care hospital) or Provident Hospital of Cook County (a 100‐bed public community hospital), in Chicago, Illinois. Because internal medicine and family practice patients were similar in demographic characteristics and interview responses, we considered these as a single service. There is an HIV service at Stroger Hospital; all HIV‐infected patients are admitted or transferred to this service. For each patient, we used data collected from their initial hospitalization during a 9‐month study period (April 1, 2006 through December 31, 2006). Using hospital admission data, we estimated that 65% of patients were interviewed by a counselor; only 5% of patients could not be interviewed due to patient refusal or mental status changes.
Patients were screened for alcohol use, drug use, and smoking history by bedside interview. We defined at‐risk substance use as any illicit drug use within the previous 3 months or alcohol use that exceeded the National Institute for Alcohol Abuse and Alcoholism (NIAAA) guidelines for low‐risk drinking (no more than 5 drinks per day or 14 drinks per week for men up to age 65; no more than 3 drinks per day or 7 drinks per week for men over 65 and women). Based on their responses to questions about smoking history, patients were categorized into the following 4 groups: current smokers (ie, smoked within the previous 7 days), recent quitters (ie, quit within 8 days and 6 months), ex‐smokers (quit more than 6 months ago), or never smokers. Current smokers were also asked about their heaviness of smoking and willingness to quit. All smokers received a counseling session during hospitalization. All smokers who indicated a desire to quit were encouraged to call the Illinois Quitline after hospital discharge. Individuals who smoked between 10‐14 cigarettes per day and smoked their first cigarette within 30 minutes of waking or who smoked 15 or more cigarettes per day were classified as moderate or heavy smokers; all other smokers were classified as light smokers. We established these cut‐points by modifying the Public Health Service guideline and Heaviness of Smoking Index.5, 21, 22 The heaviness of smoking classification was used to guide recommendations to the primary service regarding the appropriateness of nicotine patch therapy during and after hospitalization. For moderate to heavy smokers who were willing to quit, the recommendation was to continue nicotine replacement after hospitalization.5
Patients were considered low health risk if their alcohol use did not exceed NIAAA guidelines and they reported no recent drug use. For all patients who reported alcohol use that exceeded the NIAAA guidelines or recent drug use, we administered the Texas Christian University Drug Screen II (TCU)23 to further characterize the severity of their use. Patients who had a TCU score of 3 were considered at‐risk substance users with substance dependence disorder; patients with scores of 2 or less were considered at‐risk substance users without dependence. Among all at‐risk substance users, we used a 10‐point visual analog scale to assess their readiness to change substance use. After evaluating the distribution and clustering of scores, we prespecified that a score 8 was indicative of a patient being ready to change their substance use behavior. This ruler has been successfully implemented as part of the Brief Negotiated Interview and Active Referral to Treatment Institute toolbox.24
Analysis
To facilitate comparison with other data sources, we used the same age categories as the National Survey on Drug Use and Health.1 Differences between proportions were evaluated by the chi‐squared test. We analyzed the trend in smoking behavior across the strata of substance use (ie, number of substances used and severity of use) using the Cochrane‐Armitage test for trend. To evaluate the association between substance use and smoking, multivariable models were constructed that included terms to adjust for age, race, gender, and hospital service; potential confounders (eg, age, race, gender, and service) were included in the final model if they significantly contributed to the outcome variable (P < 0.1). From these multivariable models, prevalence ratios were estimated using the binary log transformation in PROC GENMOD.25, 26 All data were analyzed using SAS version 9.0 (SAS Institute Inc., Cary, NC).
RESULTS
Patient Characteristics
Of the 7,714 unique patients interviewed at the 2 hospitals, we had data on smoking status for 7,391 (96%) (Table 1). The mean age was 50 years, most were male, cared for by the internal medicine or family practice service, and the most common racial/ethnic category was non‐Hispanic Black, followed by Hispanic, non‐Hispanic White, and Asian (Table 1). More than one‐quarter of patients reported at‐risk substance use other than tobacco; the most common substance used was alcohol followed by cocaine, marijuana, and then heroin (Table 1). Most patients who were at‐risk substance users (52%) met criteria for substance dependence disorder.23
| Characteristic | N | (%) | Smoking prevalence* (%) | Prevalence ratio (95% CI) | |
|---|---|---|---|---|---|
| |||||
| Age category | |||||
| 18‐25 | 479 | (6) | 35 | 2.6 | (2.1 to 3.1) |
| 26‐34 | 664 | (9) | 38 | 2.8 | (2.4 to 3.4) |
| 35‐44 | 1306 | (18) | 46 | 3.4 | (2.9 to 4.0) |
| 45‐54 | 2182 | (30) | 46 | 3.4 | (2.9 to 4.0) |
| 55‐64 | 1563 | (21) | 31 | 2.3 | (2.0 to 2.7) |
| 65 and older | 1185 | (16) | 13 | ref | |
| Race/Ethnicity | |||||
| Non Hispanic Black | 4990 | (68) | 45 | 3.0 | (2.2 to 4.0) |
| Non Hispanic White | 850 | (12) | 40 | 2.7 | (2.0 to 3.6) |
| Hispanic | 1222 | (17) | 19 | 1.3 | (0.9 to 1.7) |
| Asian | 253 | (3) | 15 | ref | |
| Other | 27 | (<1) | |||
| Gender | |||||
| Male | 4279 | (58) | 42 | 1.5 | (1.4 to 1.6) |
| Female | 3099 | (42) | 29 | ref | |
| Service | |||||
| HIV | 227 | (3) | 52 | 1.7 | (1.5 to 2.0) |
| Internal medicine or | 6278 | (85) | 36 | 1.2 | (1.1 to 1.3) |
| family practice | |||||
| Surgery | 886 | (12) | 31 | ref | |
Tobacco Use
Many hospitalized patients were current smokers (36%) and 35% of current smokers were moderate to heavy smokers. The prevalence of smoking varied significantly by age category, race, gender, and service. By age category, the prevalence of smoking peaked at 3554 years with lower rates of smoking at either extreme of age (Table 1). Non‐Hispanic Blacks and Whites had a prevalence of smoking 3‐fold higher than Asians; Hispanics were less likely to smoke than non‐Hispanic Whites or Blacks. Men were more likely to smoke than women, and patients on the HIV or internal medicine/family practice services had a higher prevalence of smoking compared to patients on the surgery service (Table 1).
The proportion of current smokers who were moderate to heavy smokers was similar between patients with no‐risk or low‐risk substance use and those who had at‐risk substance use without dependence (32% versus 34%, respectively); however, current smokers who were substance‐dependent were 40% more likely to be moderate to heavy smokers (48%) (prevalence ratio [PR]: 1.4, 95% confidence interval [CI]: 1.1 to 1.9).
Concurrent Tobacco and Substance Use
Compared to patients who reported low‐risk substance use, patients with at‐risk substance use had a dramatically higher prevalence of smoking (Table 2). In addition, there was a significant increase in the likelihood of smoking across the 3 levels of substance use and the number of substances used (Table 2).
| N | (%) | Smoking prevalence (%) | Adjusted prevalence ratio (95% CI)* | ||
|---|---|---|---|---|---|
| |||||
| Risk Index | |||||
| Low Health Risk | 5419 | (73) | 24 | ref | |
| At‐Risk, not dependent | 945 | (13) | 64 | 2.2 | (2.0 to 2.3) |
| At‐Risk, dependent | 1027 | (14) | 75 | 2.5 | (2.3 to 2.6) |
| Specific substance use | |||||
| Low Health Risk | 5419 | (73) | 24 | ref | |
| At‐Risk Alcohol Use | 1171 | (16) | 68 | 2.2 | (2.1 to 2.4) |
| At‐ Risk Marijuana Use | 688 | (9) | 70 | 2.1 | (2.0 to 2.3) |
| At‐Risk Cocaine Use | 503 | (7) | 79 | 2.4 | (2.2 to 2.6) |
| At‐Risk Heroin Use | 448 | (6) | 82 | 2.4 | (2.2 to 2.6) |
| Number of drugs | |||||
| None | 5419 | (73) | 24 | ref | |
| One | 1284 | (17) | 64 | 2.2 | (2.0 to 2.3) |
| Two or more | 688 | (9) | 81 | 2.6 | (2.5 to 2.8) |
Willingness to Quit
Most patients (61%) who smoked were willing to immediately quit smoking. After adjusting for other demographic confounders, non‐Hispanic Blacks and the elderly (age > 65) were more willing to quit (P < 0.05, data not shown). The substance use risk categories of low risk, at‐risk, and dependence were not associated with willingness to quit tobacco (Fig. 1, left panel).

Regardless of substance use category, most patients were ready to change their substance use behavior (Fig. 1). Those patients who were ready to change their substance use behavior, regardless of whether they were substance‐dependent, were significantly more likely to report a willingness to quit smoking than those who were not ready to change (Fig. 1, right panel). In fact, at‐risk substance users without dependence who were ready to change their substance use were more willing to quit smoking than patients without at‐risk substance use (72% versus 64%; P < 0.05).
DISCUSSION
Among hospital patients, we found a 46% absolute increase in the prevalence of smoking among those who used illicit substances or alcohol above NIAAA guidelines compared to those who did not report such use. The prevalence of smoking increased across the spectrum of substance use, being highest for patients who met criteria for dependence. Also, patients who were substance dependent were more likely to be moderate to heavy smokers, suggesting an association between alcohol or other drug dependence disorders and nicotine dependence. Regardless of their patterns of substance use, most patients expressed a desire to immediately quit smoking and there was a strong association between willingness to quit smoking and readiness to reduce substance use.
In our hospital population, the prevalence of smoking among patients who use illicit drugs or at‐risk quantities of alcohol far exceeds estimates obtained from population‐based surveys. In addition to the relatively high prevalence of smoking, focusing attention on hospital patients who use substances is important for several other reasons. Individuals who use substances are less likely to receive health care from a primary care physician.28 Also, most patients who have substance use disorders do not enter treatment programs,1 even after hospitalization.29 Further, hospitals provide a setting that facilitates change; patients are temporarily required to stop smoking, and often they are available for relatively long counseling sessions. Finally, for patients without substance use disorders, hospital‐based smoking cessation intervention programs have been proven to be successful in several randomized controlled trials.10, 30
Because alcohol and drug use are so common among hospitalized smokers, it is unfortunate that there is little evidence from clinical trials to inform intervention strategies for patients with concurrent use. The clinical trials that form the evidence base for intervention among hospitalized smokers10 either have explicitly excluded patients who reported substance use,10, 15, 3133 did not assess baseline substance use,34, 35 or were underpowered to perform subgroup analyses on this population.36 Awaiting better evidence, we have chosen to routinely screen hospital patients for tobacco, alcohol, and drug use. For treatment strategies, we extrapolate the findings from successful interventions in the ambulatory setting37 or among hospital patients who do not use substances to our population. We offer smoking cessation interventions to patients regardless of other substance use.
Understanding the similarities and differences between smokers who use substances and those who do not is important in implementing successful strategies for smoking cessation. Rather than a step‐wise increase in heaviness of smoking across substance use categories (ie, no‐risk or low‐risk use, at‐risk use without dependence, and substance dependence), we found an increased heaviness of smoking only among substance‐dependent smokers; there was no difference in heaviness of smoking between those with at‐risk use without dependence and those with no‐risk or low‐risk use. Because interventions for patients who have nicotine dependence are more likely to succeed when pharmacotherapy is offered as an adjunct to behavior therapy,38 smokers who also are substance‐dependent likely will benefit from the addition of pharmacotherapy. One similarity is that all patients, regardless of substance use category, were willing to quit smoking. In fact, hospitalized smokers who were ready to change at‐risk substance use were more willing to quit smoking than patients who had no‐risk or low‐risk substance use. Previous investigators have found that smokers who use substances have fewer quit attempts,39 higher nicotine dependence,37, 39 and lower enrollment in smoking cessation interventions.38
Our study only includes data from patients at 2 public hospitals; therefore, our findings may not generalize to populations of higher socioeconomic status. Also, our smoking screening tool had relatively low sensitivity for categorizing current smokers as moderate to heavy smokers; therefore, we may have underestimated the number of moderate to heavy smokers.5, 22 Further, given our cross‐sectional study design, we were unable to evaluate whether patients who have at‐risk substance use remain willing to quit smoking after hospital discharge or to the effectiveness of our smoking cessation program. Finally, socially desirable responses may have caused patients to overstate their willingness to quit tobacco and readiness to change substance use. Additional research is needed to determine whether post‐hospitalization quit rates are similar between smokers with and without at‐risk substance use, and the optimal timing for smoking cessation interventions in relation to substance dependence treatment.40
Hospital patients who have substance use disorders are also highly likely to smoke, and these patients express a willingness to quit smoking. Given the frequency of concurrent smoking and other substance misuse and patients' desire to change both behaviors, there is a role for coordination of substance use and smoking cessation intervention programs.
- US Department of Health 24:201–208.
- ,,,.Actual causes of death in the United States, 2000.JAMA.2004;291:1238–1245.
- ,,.Global burden of disease from alcohol, illicit drugs and tobacco.Drug Alcohol Rev.2006;25:503–513.
- A clinical practice guideline for treating tobacco use and dependence,:A US Public Health Service report. The tobacco use and dependence clinical practice guideline panel, staff, and consortium representatives.JAMA.2000;283:3244–3254.
- ,,,,,U.S.Preventive Services Task Force. Behavioral counseling interventions in primary care to reduce risky/harmful alcohol use by adults: a summary of the evidence for the U.S. Preventive Services Task Force.Ann Intern Med.2004;140:557–568.
- Work Group on Substance Use Disorders,,, et al.Treatment of patients with substance use disorders, second edition. American Psychiatic Association.Am J Psych.2006;163(8 Suppl):75–82.
- ,.Smokers who are hospitalized: a window of opportunity for cessation interventions.Prev Med.1992;21;262–269.
- ,,.Helping hospitalized smokers quit: new directions for treatment and research.J Consult Clin Psychol.1993;61:778–89.
- ,,.Interventions for smoking cessation in hospitalised patients.Cochrane Database Sys Rev.2007;3(3);CD001837.
- ,,,.Expanding the roles of hospitalists physicians to include public health.J Hosp Med.2007;2:93–101.
- ,,,,.Smoking status as a clinical indicator for alcohol misuse in US adults.Arch Intern Med.2007;167:716–721.
- ,,,,.Alcohol high risk drinking, abuse and dependence among tobacco smoking medical care patients and the general population.Drug Alcohol Depend.2003;69:189–195.
- ,,,,.Nicotine dependence and psychiatric disorders in the United States: results from the national epidemiologic survey on alcohol and related conditions.Arch Gen Psychiatry.2004;61:1107–1115.
- ,,,,,.Smoking and mental illness: A population‐based prevalence study.JAMA.2000;284:2606–2610.
- .Clinical Implications of the association between smoking and alcoholism. In:Fertig JB,Allen JP, eds.Alcohol and Tobacco: From Basic Science to Clinical Practice.Bethesda, MD:NIAAA Research;1995:171–185.
- ,,,,,.Smoking and drinking among alcoholics in treatment: cross‐sectional and longitudinal relationships.J Stud Alcohol.2000;61:157–163.
- ,,, et al.Interrelationship of smoking and alcohol dependence, use and urges to use.J Stud Alcohol.1995;56:202–206.
- ,,.Tobacco cessation treatment for alcohol‐dependent smokers: when is the best time?Alcohol Res Health.2006;29:203–207.
- ,,, et al.Substance use in the general hospital.Addict Behav.2003;28:483–499.
- ,,,,.Measuring the heaviness of smoking: Using self‐reported time to the first cigarette of the day and number of cigarettes smoked per day.Br J Addict.1989;84:791–799.
- ,,,.The Heaviness of Smoking Index as a predictor of smoking cessation in Canada.Addict Behav.2007;32:1031–1042.
- ,,, et al.Effectiveness of screening instruments in detecting substance use disorders among prisoners.J Subst Abuse Treat.2000;18:349–358.
- Th BNI‐ART Institute, Readiness Ruler. http://www.ed.bmc.org/sbirt/techniques.php. Accessed August 20,2008.
- .A modified poisson regression approach to prospective studies with binary data.Am J Epidemiol.2004;159:702–706.
- ,,,.Estimating the relative risk in cohort studies and clinical trials of common outcomes.Am J Epidemiol.2003;157:940–943.
- ,.Do smokers with alcohol problems have more difficulty quitting?Drug Alcohol Depend.2006;82:91–102.
- .Emergency room and primary care services utilization and associated alcohol and drug use in the United States general population.Alcohol Alcohol.1999;34:581–589.
- ,,, et al.Brief intervention for medical inpatients with unhealthy alcohol use: a randomized, controlled trial.Ann Intern Med.2007;146:167–176.
- ,,.Smoking cessation interventions among hospitalized patients: what have we learned?Prev Med.2001;32:376–388.
- ,,, et al.Smoking cessation and severity of disease: the Coronary Artery Smoking Intervention Study.Health Psychol.1992;11:119–126.
- ,,,,.A randomized controlled trial of smoking cessation counseling after myocardial infarction.Prev Med.2000;30:261–268.
- ,.Comorbid cigarette and alcohol addiction: epidemiology and treatment.J Addict Dis.1998;17:55–66.
- ,,, et al.A case‐management system for coronary risk factor modification after acute myocardial infarction.Ann Intern Med.1994;120:721–729.
- ,,, et al.A nurse‐managed smoking cessation program for hospitalized smokers.Am J Public Health.1996;86:1557–1560.
- ,,,.Smoking cessation after surgery. A randomized trial.Arch Intern Med.1997;157:1371–1376.
- ,,,,.Efficacy of nicotine patch in smokers with a history of alcoholism.Alcohol Clin Exp Res.2003;27:946–954.
- ,,,.Predictors of tobacco quit attempts among recovering alcoholics.J Subst Abuse.1996;8:431–443.
- ,,.Is dependence on one drug associated with dependence on other drugs? The cases of alcohol, caffeine and nicotine.Am J Addict.2000;9:196–201.
- .Nicotine interventions with comorbid populations.Am J Prev Med.2007;33:S406–S413.
- US Department of Health 24:201–208.
- ,,,.Actual causes of death in the United States, 2000.JAMA.2004;291:1238–1245.
- ,,.Global burden of disease from alcohol, illicit drugs and tobacco.Drug Alcohol Rev.2006;25:503–513.
- A clinical practice guideline for treating tobacco use and dependence,:A US Public Health Service report. The tobacco use and dependence clinical practice guideline panel, staff, and consortium representatives.JAMA.2000;283:3244–3254.
- ,,,,,U.S.Preventive Services Task Force. Behavioral counseling interventions in primary care to reduce risky/harmful alcohol use by adults: a summary of the evidence for the U.S. Preventive Services Task Force.Ann Intern Med.2004;140:557–568.
- Work Group on Substance Use Disorders,,, et al.Treatment of patients with substance use disorders, second edition. American Psychiatic Association.Am J Psych.2006;163(8 Suppl):75–82.
- ,.Smokers who are hospitalized: a window of opportunity for cessation interventions.Prev Med.1992;21;262–269.
- ,,.Helping hospitalized smokers quit: new directions for treatment and research.J Consult Clin Psychol.1993;61:778–89.
- ,,.Interventions for smoking cessation in hospitalised patients.Cochrane Database Sys Rev.2007;3(3);CD001837.
- ,,,.Expanding the roles of hospitalists physicians to include public health.J Hosp Med.2007;2:93–101.
- ,,,,.Smoking status as a clinical indicator for alcohol misuse in US adults.Arch Intern Med.2007;167:716–721.
- ,,,,.Alcohol high risk drinking, abuse and dependence among tobacco smoking medical care patients and the general population.Drug Alcohol Depend.2003;69:189–195.
- ,,,,.Nicotine dependence and psychiatric disorders in the United States: results from the national epidemiologic survey on alcohol and related conditions.Arch Gen Psychiatry.2004;61:1107–1115.
- ,,,,,.Smoking and mental illness: A population‐based prevalence study.JAMA.2000;284:2606–2610.
- .Clinical Implications of the association between smoking and alcoholism. In:Fertig JB,Allen JP, eds.Alcohol and Tobacco: From Basic Science to Clinical Practice.Bethesda, MD:NIAAA Research;1995:171–185.
- ,,,,,.Smoking and drinking among alcoholics in treatment: cross‐sectional and longitudinal relationships.J Stud Alcohol.2000;61:157–163.
- ,,, et al.Interrelationship of smoking and alcohol dependence, use and urges to use.J Stud Alcohol.1995;56:202–206.
- ,,.Tobacco cessation treatment for alcohol‐dependent smokers: when is the best time?Alcohol Res Health.2006;29:203–207.
- ,,, et al.Substance use in the general hospital.Addict Behav.2003;28:483–499.
- ,,,,.Measuring the heaviness of smoking: Using self‐reported time to the first cigarette of the day and number of cigarettes smoked per day.Br J Addict.1989;84:791–799.
- ,,,.The Heaviness of Smoking Index as a predictor of smoking cessation in Canada.Addict Behav.2007;32:1031–1042.
- ,,, et al.Effectiveness of screening instruments in detecting substance use disorders among prisoners.J Subst Abuse Treat.2000;18:349–358.
- Th BNI‐ART Institute, Readiness Ruler. http://www.ed.bmc.org/sbirt/techniques.php. Accessed August 20,2008.
- .A modified poisson regression approach to prospective studies with binary data.Am J Epidemiol.2004;159:702–706.
- ,,,.Estimating the relative risk in cohort studies and clinical trials of common outcomes.Am J Epidemiol.2003;157:940–943.
- ,.Do smokers with alcohol problems have more difficulty quitting?Drug Alcohol Depend.2006;82:91–102.
- .Emergency room and primary care services utilization and associated alcohol and drug use in the United States general population.Alcohol Alcohol.1999;34:581–589.
- ,,, et al.Brief intervention for medical inpatients with unhealthy alcohol use: a randomized, controlled trial.Ann Intern Med.2007;146:167–176.
- ,,.Smoking cessation interventions among hospitalized patients: what have we learned?Prev Med.2001;32:376–388.
- ,,, et al.Smoking cessation and severity of disease: the Coronary Artery Smoking Intervention Study.Health Psychol.1992;11:119–126.
- ,,,,.A randomized controlled trial of smoking cessation counseling after myocardial infarction.Prev Med.2000;30:261–268.
- ,.Comorbid cigarette and alcohol addiction: epidemiology and treatment.J Addict Dis.1998;17:55–66.
- ,,, et al.A case‐management system for coronary risk factor modification after acute myocardial infarction.Ann Intern Med.1994;120:721–729.
- ,,, et al.A nurse‐managed smoking cessation program for hospitalized smokers.Am J Public Health.1996;86:1557–1560.
- ,,,.Smoking cessation after surgery. A randomized trial.Arch Intern Med.1997;157:1371–1376.
- ,,,,.Efficacy of nicotine patch in smokers with a history of alcoholism.Alcohol Clin Exp Res.2003;27:946–954.
- ,,,.Predictors of tobacco quit attempts among recovering alcoholics.J Subst Abuse.1996;8:431–443.
- ,,.Is dependence on one drug associated with dependence on other drugs? The cases of alcohol, caffeine and nicotine.Am J Addict.2000;9:196–201.
- .Nicotine interventions with comorbid populations.Am J Prev Med.2007;33:S406–S413.
Copyright © 2008 Society of Hospital Medicine
Curriculum for the Hospitalized Aging Medical Patient
A crucial arena of innovative educational programs for the care of the elderly must include the hospital setting, a place of great cost, morbidity, and mortality for a population currently occupying approximately half of US hospital beds.1 With a marked acceleration in the number of persons living to an advanced age, there is a clear imperative to address the health‐care needs of the elderly, particularly the complex and frail.24 An educational grounding that steps beyond the traditional organ‐based models of disease to a much broader patient‐centered framework of care is necessary to aid physicians in advanced clinical decision‐making in the care of older patients. Organizing the medical care of the older patient within existing systems of care and a team care management network must also be improved.
Curricular materials and methods are widely available for teaching geriatric medicine,57 but most are geared toward outpatient care and management, with few addressing the care of the hospitalized, older medical patient.810 There is even less published on curricular materials, methods, and tools for such teaching outside of specialized hospital‐based geriatric units by nongeriatrics‐trained faculty.1113 Furthermore, the evaluation of geriatrics educational programs in the hospital setting has not been done with the ultimate assessment, the linking of educational programs to demonstrated changes in clinical practice and patient care outcomes.
To address these needs, we designed and implemented the Curriculum for the Hospitalized Aging Medical Patient (CHAMP) Faculty Development Program (FDP). CHAMP was funded by a grant from the Donald W. Reynolds Foundation Aging and Quality of Life Program with a matching commitment from the University of Chicago Department of Medicine. At the core of CHAMP are principles of care for the older patient in the hospital setting, with an emphasis on identifying and providing care for the complex and frail elderly with nongeriatrician inpatient medicine faculty as the primary teachers of these materials. The overall educational goals of the CHAMP FDP are the following: (1) to train hospitalists and general internists to recognize opportunities to teach geriatric medicine topics specific to the care of the hospitalized older patient; (2) to create teaching materials, tools, and methods that can be used in the busy medical inpatient setting at the bedside; (3) to create materials and tools that facilitate teaching the Accreditation Council for Graduate Medical Education (ACGME) core competencies14 during ward rounds; and (4) to increase the frequency and effectiveness with which this geriatrics content is taught in the hospital setting. This article describes the development and refinement of the CHAMP FDP and evaluation results to date.
METHODS
The CHAMP FDP was developed by a core group of geriatricians, hospitalists, general medicine faculty, and PhD educators from the Office of the Dean at the University of Chicago Pritzker School of Medicine. The core group piloted the FDP for themselves in spring 2004, and the FDP was offered to target learners annually from 2004 to 2006.
CHAMP Participants
The targeted faculty learners for the CHAMP FDP were hospitalists and general internists who attend on an inpatient medicine service for 1 to 4 months yearly. CHAMP Faculty Scholars were self‐selected from the eligible faculty of the University of Chicago. Approximately one‐third of the CHAMP Faculty Scholars held significant administrative and/or teaching positions in the Department of Medicine, residency program, or medical school. Overall, general internist and hospitalist faculty members of the University of Chicago are highly rated inpatient teachers with a 2004‐2007 average overall resident teaching rating of 3.79 (standard deviation = 0.53) on a scale of 1 to 4 (4 = outstanding). For each yearly cohort, we sought to train 8 to 10 Faculty Scholars. The Donald W. Reynolds Foundation grant funds supported the time of the Faculty Scholars to attend the CHAMP FDP 4 hours weekly for the 12 weeks of the course with release from a half‐day of outpatient clinical duties per week for the length of the FDP. Scholars also received continuing medical education credit for time spent in the FDP.
CHAMP Course Design, Structure, and Content
Design and Structure
The CHAMP FDP consists of twelve 4‐hour sessions given once weekly from September through November of each calendar year. Each session is composed of discrete teaching modules. During the first 2 hours of each session, 1 or 2 modules cover inpatient geriatric medicine content. The remaining 2 hours are devoted to modules consisting of the Stanford FDP for Medical Teachers: Improving Clinical Teaching (first 7 sessions)15, 16 and a course developed for the CHAMP FDP named Teaching on Today's Wards (remaining 5 sessions).
In addition to the overarching goals of the CHAMP FDP, each CHAMP module has specific learning objectives and an evaluation process based on the standard precepts of curriculum design.17 Further modifications of the CHAMP content and methods were strongly influenced by subsequent formal evaluative feedback on the course content, materials, and methods by the Faculty Scholars in each of the 4 FDP groups to date.
Geriatrics Content
The FDP geriatrics content and design model were developed as follows: reviewing existing published geriatrics curricular materials,5, 6, 8, 18 including high‐risk areas of geriatric hospital care;1922 drawing from the experience of the inpatient geriatric evaluation and treatment units;2325 and reviewing the Joint Commission mandates26 that have a particular impact on the care of the older hospitalized patients (eg, high‐risk medications, medication reconciliation, restraint use, and transitions of care). Final curricular materials were approved by consensus of the University of Chicago geriatrics/hospitalist core CHAMP faculty. A needs assessment surveying hospitalists at a regional Society of Hospital Medicine meeting showed a strong concordance between geriatrics topics that respondents thought they were least confident about in their knowledge, that they thought would be most useful to learn, and that we proposed for the core geriatrics topics for the CHAMP FDP, including pharmacy of aging, pressure ulcers, delirium, palliative care, decision‐making capacity, and dementia.27
Each geriatric topic is presented in 30‐ to 90‐minute teaching sessions with didactic lectures and case‐based discussions and is organized around 4 broad themes (Table 1). These lectures emphasize application of the content to bedside teaching during hospital medicine rounds. For example, the session on dementia focuses on assessing decision‐making capacity, the impact of dementia on the care of other medical illnesses and discharge decisions, dementia‐associated frailty with increased risk of hospitalization‐related adverse outcomes, and pain assessment in persons with dementia.
|
| Theme 1: Identify the frail/vulnerable elder |
| Identification and assessment of the vulnerable hospitalized older patient |
| Dementia in hospitalized older medical patients: Recognition of and screening for dementia, assessment of medical decision‐making capacity, implications for the treatment of nondementia illness, pain assessment, and improvement of the posthospitalization transition of care |
| Theme 2: Recognize and avoid hazards of hospitalization |
| Delirium: Diagnosis, treatment, risk stratification, and prevention |
| Falls: Assessment and prevention |
| Foley catheters: Scope of the problem, appropriate indications, and management |
| Deconditioning: Scope of the problem and prevention |
| Adverse drug reactions and medication errors: Principles of drug review |
| Pressure ulcers: Assessment, treatment, and prevention |
| Theme 3: Palliate and address end‐of‐life issues |
| Pain control: General principles and use of opiates |
| Symptom management in advanced disease: Nausea |
| Difficult conversations and advance directives |
| Hospice and palliative care and changing goals of care |
| Theme 4: Improve transitions of care |
| The ideal hospital discharge: Core components and determining destination |
| Destinations of posthospital care: Nursing homes for skilled rehabilitation and long‐term care |
The CHAMP materials created for teaching each topic at the bedside included topic‐specific teaching triggers, clinical teaching questions, and summary teaching points. The bedside teaching materials and other teaching tools, such as pocket cards with teaching triggers and clinical content (see the example in the appendix), commonly used geriatric measures (eg, the Confusion Assessment Method for delirium),28 and sample forms for teaching aspects of practice‐based learning and improvement and systems‐based practice, were available to Faculty Scholars electronically on the University of Chicago Course Management System (the CHALK E‐learning Web site). The CHAMP materials are now published at the University of Chicago Web site (
Teaching Content
The material referring to the process of teaching has been organized under 4 components in the CHAMP FDP.
The Stanford FDP for Medical Teachers15, 16
This established teaching skills course uses case scenarios and practice sessions to hone skills in key elements of teaching: learning climate, control of session, communication of goals, promotion of understanding and retention, evaluation, feedback, and promotion of self‐directed learning. This portion of the FDP was taught by a University of Chicago General Medicine faculty member trained and certified to teach the course at Stanford.
Teaching on Today's Wards
The Teaching on Today's Wards component was developed specifically for CHAMP to address the following: (1) to improve bedside teaching in the specific setting of the inpatient wards; (2) to increase the amount of geriatric medicine content taught by nongeriatrics faculty during bedside rounds; and (3) to teach the specific ACGME core competencies of professionalism, communication, practice‐based learning and improvement, and systems‐based practice during ward rounds (Table 2).
| ACGME Core Competency | Addressed in CHAMP Curriculum |
|---|---|
| |
| Knowledge/patient care | All geriatric lectures (see Table 1) |
| Professionalism | Geriatric lectures |
| 1. Advance directives and difficult conversations | |
| 2. Dementia: Decision‐making capacity | |
| Teaching on Today's Wards exercises and games | |
| 1. Process mapping | |
| 2. I Hope I Get a Good Team game | |
| 3. Deciding What To Teach/Missed Teaching Opportunities game | |
| Communication | Geriatric lectures |
| 1. Advance directives and difficult conversations | |
| 2. Dementia: Decision‐making capacity | |
| 3. Destinations for posthospital care: Nursing homes | |
| Teaching on Today's Wards exercises and games | |
| 1. Process mapping | |
| 2. Deciding What To Teach/Missed Teaching Opportunities game | |
| Systems‐based practice | Geriatric lectures |
| 1. Frailty: Screening | |
| 2. Delirium: Screening and prevention | |
| 3. Deconditioning: Prevention | |
| 4. Falls: Prevention | |
| 5. Pressure ulcers: Prevention | |
| 6. Drugs and aging: Drug review | |
| 7. Foley catheter: Indications for use | |
| 8. Ideal hospital discharge | |
| Teaching on Today's Wards exercises and games | |
| 1. Process mapping | |
| 2. Deciding What To Teach/Missed Teaching Opportunities game | |
| 3. Quality improvement projects | |
| Practice‐based learning and improvement | Teaching on Today's Wards exercises and games |
| 1. Case audit | |
| 2. Census audit | |
| 3. Process mapping | |
Session one of Teaching on Today's Wards takes the Faculty Scholars through an exploration of their teaching process on a postcall day using process mapping.29, 30 This technique, similar to constructing a flow chart, involves outlining the series of steps involved in one's actual (not ideal) process of postcall teaching. Faculty Scholars then explore how to recognize opportunities and add geriatric topics and the ACGME core competencies to their teaching on the basis of their own teaching process, skill sets, and clinical experience.
Session two explores goal setting, team dynamics, and the incorporation of more geriatrics teaching into the Faculty Scholar's teaching agenda through a series of interactive card game exercises facilitated in small group discussion. Card game 1, I Hope I Get a Good Team, allows learners to practice goal setting for their inpatient team using a hypothetical game card team based on the learning level, individuals' strengths and weaknesses, and individuals' roles in the team hierarchy. Card game 2, Deciding What To Teach/Missed Opportunities, helps learners develop a teaching agenda on any set of patients that incorporates the CHAMP geriatric topics and the ACGME core competencies.
Sessions three and four teach learners about the systems‐based practice and practice‐based learning and improvement competencies, including an introduction to quality improvement. These interactive sessions introduce Faculty Scholars to the plan‐do‐study‐act method,31 using the example of census and case audits32 to provide an objective and structured method of assessing care. These audits provide a structure for the medical team to review its actual care and management practices and for faculty to teach quality improvement. Examples of census audits developed by CHAMP faculty, including deep venous thrombosis prophylaxis, Foley catheter use, and use of proton pump inhibitors, provide models for the faculty learners to create their own audits.
The fifth session focuses on developing skills for life‐long learning. Based on previous work on medical education and evidence‐based medicine,33, 34 these sessions provide learners with a framework to identify and address knowledge gaps, obtain effective consultation, ask pertinent questions of learners, and self‐assess their teaching skills.
Observed Structured Teaching Exercises
Observed structured teaching exercises allow the deliberate practice of teaching new curricular materials and skills and have been shown to improve teaching skills for both faculty and resident teachers using standardized students in a simulated teaching environment.3537 The observed structured teaching exercises developed for CHAMP allow the Faculty Scholars to practice teaching geriatrics content using the one‐minute preceptor teaching method.38
Commitment to Change (CTC) Contracts
CTC contracts provide a method for sustaining CHAMP teaching. At the end of the FDP, we ask Faculty Scholars to sign a CTC contract,39, 40 selecting at least 1 geriatric topic and 1 topic from Teaching on Today's Wards to teach in future inpatient teaching attending months. Over the year(s) following the FDP, the CHAMP project director frequently contacts the Faculty Scholars via e‐mail and phone interviews before, during, and after each month of inpatient service. The CTC contract is formally reviewed and revised annually with each CHAMP Faculty Scholar by the CHAMP project director and a core CHAMP faculty member.
Evaluation
A comprehensive multilevel evaluation scheme was developed based on the work of Kirkpatrick,41 including participant experience and teaching and subsequent clinical outcomes. This article reports only on the knowledge, attitudes, and behavioral self‐report data collected from participants, and remaining data will be presented in future articles.
The evaluation of the FDP program includes many commonly used methods for evaluating faculty learners, including recollection and retention of course content and self‐reported behavioral changes regarding the incorporation of the material into clinical teaching and practice. The more proximal evaluation includes precourse and postcourse performance on a previously validated geriatric medicine knowledge test,4244 precourse and postcourse performance on a validated survey of attitudes regarding older persons and geriatric medicine,45 a self‐assessment survey measuring self‐reported importance of and confidence in practicing and teaching geriatric skills, and Faculty Scholars' reports of subsequent frequency of teaching on the geriatric medicine and Teaching on Today's Wards content.
Faculty Scholars' feedback regarding their reaction to and satisfaction with the CHAMP FDP includes immediate postsession evaluations of each individual CHAMP FDP session and its content.
Analyses
We calculated the overall satisfaction of the FDP by aggregating evaluations for all session modules across the 4 cohorts. Satisfaction was measured with 6 questions, which included an overall satisfaction question and were answered with 5‐point Likert scales.
Pre‐CHAMP and post‐CHAMP scores on the geriatrics knowledge test and geriatrics attitude scale were calculated for each participant and compared with paired‐sample t tests. Composite scores for the self‐reported behavior for importance of/confidence in practice and importance of/confidence in teaching were calculated for each set of responses from each participant. The average scores across all 14 geriatrics content items for importance of/confidence in practice and importance of/confidence in teaching were calculated pre‐CHAMP and post‐CHAMP and compared with a paired‐sample t test. Similarly, self‐reported behavior ratings of importance of/confidence in teaching were calculated by the averaging of responses across the 10 Teaching on Today's Wards items. Pre‐CHAMP and post‐CHAMP average scores were compared with paired‐sample t tests on SPSS version 14 (SPSS, Chicago, IL). Data from the pilot sessions were included in the analyses to provide adequate power.
RESULTS
We pilot‐tested the format, materials, methods, and evaluation components of the CHAMP FDP with the CHAMP core faculty in the spring of 2004. The revised CHAMP FDP was given in the fall of 2004 to the first group of 8 faculty learners. Similar annual CHAMP FDPs have occurred since 2004, with a total of 29 Faculty Scholars by 2006. This includes approximately half of the University of Chicago general medicine faculty and the majority of the hospitalist faculty. Geriatrics fellows, a medicine chief resident, and other internal medicine subspecialists have also taken the CHAMP FDP. The average evaluations of all CHAMP sessions by all participants are shown in Table 3.
| Rating Criteria* | Average (SD) | N |
|---|---|---|
| ||
| Teaching methods were appropriate for the content covered. | 4.5 0.8 | 571 |
| The module made an important contribution to my practice. | 4.4 0.9 | 566 |
| Supplemental materials were effectively used to enhance learning. | 4.0 1.6 | 433 |
| I feel prepared to teach the material covered in this module. | 4.1 1.0 | 567 |
| I feel prepared to incorporate this material into my practice. | 4.4 0.8 | 569 |
| Overall, this was a valuable educational experience. | 4.5 0.8 | 565 |
Faculty Scholars rated the FDP highly regarding preparation for teaching and incorporation of the material into their teaching and practice. Likewise, qualitative comments by the Faculty Scholars were strongly supportive of CHAMP:
Significantly more aware and confident in teaching around typical geriatric issues present in our patients.
Provided concrete, structured ideas about curriculum, learning goals, content materials and how to implement them.
The online teaching resources were something I used on an almost daily basis.
Wish we had this for outpatient.
CHAMP had a favorable impact on the Faculty Scholars across the domains of knowledge, attitudes, and perceived behavior change (Table 4). Significant differences on paired‐sample t tests found significant improvement on all but one measure (importance of teaching). After the CHAMP program, Faculty Scholars were more knowledgeable about geriatrics content (P = 0.023), had more positive attitudes to older patients (P = 0.049), and had greater confidence in their ability to care for older patients (P < 0.001) and teach geriatric medicine skills (P < 0.001) and Teaching on Today's Wards content (P < 0.001). There was a significant increase in the perceived importance of practicing the learned skills (P = 0.008) and Teaching on Today's Wards (P = 0.001). The increased importance of teaching geriatrics skills was marginally significant (P = 0.064).
| Domain | N | Average Response | SE | P Value* | ||
|---|---|---|---|---|---|---|
| Pre‐CHAMP | Post‐CHAMP | |||||
| ||||||
| Knowledge | Geriatric medicine knowledge test | 21 | 62.14 | 68.05 | 2.40 | 0.023 |
| Attitudes | Geriatrics attitude scale | 26 | 56.86 | 58.38 | 0.736 | 0.049 |
| Self‐report behavior change | Importance of practice | 28 | 4.40 | 4.62 | 0.078 | 0.008 |
| Confidence in practice | 28 | 3.59 | 4.33 | 0.096 | <0.001 | |
| Importance of teaching | 27 | 4.52 | 4.66 | 0.074 | 0.064 | |
| Confidence in teaching | 27 | 3.42 | 4.47 | 0.112 | <0.001 | |
| Importance of Teaching on Today's Wards∥ | 27 | 3.92 | 4.30 | 0.093 | 0.001 | |
| Confidence in Teaching on Today's Wards∥ | 27 | 2.81 | 4.05 | 0.136 | <0.001 | |
DISCUSSION
Central to CHAMP's design are (1) the creation of teaching materials and teaching resources that specifically address the challenges of teaching the care of the hospitalized older patient in busy hospital settings, (2) the provision of methods to reinforce the newly learned geriatrics teaching skills, and (3) a multidimensional evaluation scheme. The enthusiastic response to the CHAMP FDP and the evaluation results to date support the relevance and importance of CHAMP's focus, materials, and educational methods. The ideal outcome for our CHAMP FDP graduates is more informed, confident, and frequent teaching of geriatrics topics keyed to quality improvement and systems of care through a more streamlined but personalized bedside teaching process.13, 46 The CHAMP Faculty Scholar graduates' self‐report surveys of their performance and teaching of CHAMP course geriatrics skills did reveal a significant shift in clinical behavior, teaching, and confidence. Although the strongest indicator of perceived behavior change was in the enhanced self‐confidence in practicing and teaching, the significant changes in knowledge and attitude reinforce our observations of a shift in the mindset about teaching and caring for hospitalized elderly patients. This provides strong evidence for the efficacy of the CHAMP course in positively influencing participants.
Our biggest challenge with the CHAMP FDP was providing enough ongoing support to reinforce learning with an eye on the greater goal of changing teaching behaviors and clinical outcomes. After pilot testing, we added multiple types of support and follow‐up to the FDP: observed structured teaching exercises to practice CHAMP geriatrics content and teaching skills; modification of Teaching on Today's Wards through the addition of practice‐oriented exercises, games, and tutorials; frequent contact with our Faculty Scholar graduates post‐CHAMP FDP through CTC contracts; annual Faculty Scholars reunions; and continued access for the scholars to CHAMP materials on our Web site. Maintaining face‐to‐face contact between CHAMP core faculty and Faculty Scholars once the latter have finished the FDP has been challenging, largely because of clinical and teaching obligations over geographically separate sites. To overcome this, we are working to integrate CHAMP core faculty into hospitalist and general medicine section lecture series, increasing the frequency of CHAMP reunions, renewing CTC contracts with the Faculty Scholar graduates annually, and considering the concept of CHAMP core faculty guests attending during Faculty Scholars inpatient ward rounds.47
The CHAMP FDP and our evaluations to date have several limitations. First, FDP Scholars were volunteer participants who may have been more motivated to improve their geriatric care and teaching than nonparticipants. However, FDP Scholars had only moderate levels of geriatrics knowledge, attitudes, and confidence in their teaching on baseline testing and showed marked improvements in these domains after the FDP. In addition, Scholars' FDP participation was made possible by a reduction of other clinical obligations through direct reimbursement to their sections with CHAMP funds. Other incentives for CHAMP participation could include its focus on generalizable bedside teaching skills and provision of specific techniques for teaching the ACGME core competencies and quality improvement while using geriatrics content. Although the CHAMP FDP in its 48‐hour format is not sustainable or generalizable, the FDP modules and CHAMP materials were specifically designed to be usable in small pieces that could be incorporated into existing teaching structures, grand rounds, section meetings, teacher conferences, and continuing medical education workshops. CHAMP core group members have already presented and taught CHAMP components in many venues (see Dissemination on the CHAMP Web site). The excitement generated by CHAMP at national and specialty meetings, including multiple requests for materials, speaks to widespread interest in our CHAMP model. We are pursuing the creation of a mini‐CHAMP, an abbreviated FDP with an online component. These activities as well as feedback from users of CHAMP materials from the CHAMP Web site and the Portal of Geriatric Online Education will provide important opportunities for examining the use and acceptance of CHAMP outside our institution.
Another limitation of the CHAMP FDP is reliance on FDP Scholar self‐assessment in several of the evaluation components. Some studies have shown poor concordance between physicians' self‐assessment and external assessment over a range of domains.48 However, others have noted that despite these limitations, self‐assessment remains an essential tool for enabling physicians to discover the motivational discomfort of a performance gap, which may lead to changing concepts and mental models or changing work‐flow processes.49 Teaching on Today's Wards sessions in CHAMP emphasize self‐audit processes (such as process mapping and census audits) that can augment self‐assessment. We used such self‐audit processes in 1 small pilot study to date, providing summative and qualitative feedback to a group of FDP Scholars on their use of census audits.
However, the evaluation of the CHAMP FDP is enhanced by a yearly survey of all medical residents and medical students and by the linking of the teaching reported by residents and medical students to specific attendings. We have begun the analysis of resident perceptions of being taught CHAMP geriatrics topics by CHAMP faculty versus non‐CHAMP faculty. In addition, we are gathering data on patient‐level process of care and outcomes tied to the CHAMP FDP course session objectives by linking to the ongoing University of Chicago Hospitalist Project, a large clinical research project that enrolls general medicine inpatients in a study examining the quality of care and resource allocation for these patients.50 Because the ultimate goal of CHAMP is to improve the quality of care and outcomes for elderly hospitalized patients, the University of Chicago Hospitalist Project infrastructure was modified by the incorporation of the Vulnerable Elder Survey‐1351 and a process‐of‐care chart audit specifically based on the Assessing Care of the Vulnerable Elders Hospital Quality Indicators.52 Preliminary work included testing and validating these measures.53 Further evaluation of these clinical outcomes and CHAMP's efficacy and durability at the University of Chicago is ongoing and will be presented in future reports.
CONCLUSIONS
Through a collaboration of geriatricians, hospitalists, and general internists, the CHAMP FDP provides educational materials and methods keyed to bedside teaching in the fast‐paced world of the hospital. CHAMP improves faculty knowledge and attitudes and the frequency of teaching geriatrics topics and skills necessary to deliver quality care to the elderly hospitalized medical patient. Although the CHAMP FDP was developed and refined for use at a specific institution, the multitiered CHAMP FDP materials and methods have the potential for widespread use by multiple types of inpatient attendings for teaching the care of the older hospitalized medicine patient. Hospitalists in particular will require this expertise as both clinicians and teachers as their role, leadership, and influence continue to expand nationally.
Acknowledgements
The Curriculum for the Hospitalized Aging Medical Patient (CHAMP) Program was supported by funding from the Donald W. Reynolds Foundation with matching funds from the University of Chicago Department of Medicine, by the Hartford Foundation Geriatrics Center for Excellence, and by a Geriatric Academic Career Award to Don Scott. Presentations on CHAMP and its materials include a number of national and international meeting venues, including meetings of the Society of Hospital Medicine, the American Geriatrics Society, and the Association of Program Directors in Internal Medicine and the International Ottawa Conference.
APPENDIX
EXAMPLE OF A CHAMP POCKET CARD: FOLEY CATHETERS
| CHAMP: Foley Catheters | CHAMP: Inability to Void | |
|---|---|---|
| ||
| Catherine DuBeau, MD, Geriatrics, University of Chicago | Catherine DuBeau, MD, Geriatrics, University of Chicago | |
| 1. Does this patient have a catheter? Incorporate regular catheter checks on rounds as a practice‐based learning and improvement exercise. | 1. Is there a medical reason for this patient's inability to void? | |
| Two Basic Reasons | ||
| 2. Does this patient need a catheter? | Poor pump | |
| Only Four Indications | ▪ Meds: anticholinergics, Ca++ blockers, narcotics | |
| a. Inability to void | ▪ Sacral cord disease | |
| b. Urinary incontinence and | ▪ Neuropathy: DM, B12 | |
| ▪ Open sacral or perineal wound | ▪ Constipation/emmpaction | |
| ▪ Palliative care | Blocked outlet | |
| c. Urine output monitoring | ▪ Prostate disease | |
| ▪ Critical illnessfrequent/urgent monitoring needed | ▪ Suprasacral spinal cord disease (eg, MS) with detrusor‐sphincter dyssynergia | |
| ▪ Patient unable/unwilling to collect urine | ▪ Women: scarring, large cystocele | |
| d. After general or spinal anesthesia | ▪ Constipation/emmpaction | |
| 3. Why should catheter use be minimized? | Evaluation of Inability To Void | |
| a. Infection risk | ||
| ▪ Cause of 40% of nosocomial infections | Action Step | Possible Medical Reasons |
| b. Morbidity | ||
| ▪ Internal catheters | ||
| ○Associated with delirium | Review meds | ‐Cholinergics, narcotics, calcium channel blockers, ‐agonists |
| ○Urethral and meatal injury | ||
| ○Bladder and renal stones | ||
| ○Fever | Review med Hx | Diabetes with neuropathy, sacral/subsacral cord, B12, GU surgery or radiation |
| ○Polymicrobial bacteruria | ||
| ▪ External (condom) catheters | ||
| ○Penile cellulitus/necrosis | Physical exam | Womenpelvic for prolapse; all‐sacral root S2‐4anal wink and bulbocavernosus reflexes |
| ○Urinary retention | ||
| ○Bacteruria and infection | ||
| c. Foleys are uncomfortable/painful. | Postvoiding residual | This should have been done in the evaluation of the patient's inability to void and repeated after catheter removal with voiding trial. |
| d. Foleys are restrictive falls and delirium. | ||
| e. Cost | ||
- ,.2002 National Hospital Discharge Survey.Hyattsville, MD:National Center for Health Statistics;2002.Advance Data from Vital and Health Statistics 342.
- ,,, et al.The critical shortage of geriatrics faculty.J Am Geriatr Soc.1993;41:560–569.
- ,,, et al.Development of geriatrics‐oriented faculty in general internal medicine.Ann Intern Med.2003;139:615–620.
- ,,.General internal medicine and geriatrics: building a foundation to improve the training of general internists in the care of older adults.Ann Intern Med.2003;139:609–614.
- ,.Curriculum recommendations for resident training in nursing home care. A collaborative effort of the Society of General Internal Medicine Task Force on Geriatric Medicine, the Society of Teachers of Family Medicine Geriatrics Task Force, the American Medical Directors Association, and the American Geriatrics Society Education Committee.J Am Geriatr Soc.1994;42:1200–1201.
- ,,,,.Curriculum recommendations for resident training in geriatrics interdisciplinary team care.J Am Geriatr Soc.1999;47:1145–1148.
- ,,,,.A national survey on the current status of family practice residency education in geriatric medicine.Fam Med.2003;35:35–41.
- ,.ACGME requirements for geriatrics medicine curricula in medical specialties: progress made and progress needed.Acad Med.2005;80:279–285.
- ,,, et al.Improving geriatrics training in internal medicine residency programs: best practices and sustainable solutions.Ann Intern Med.2003;139:628–634.
- ,,,,.Core competencies in hospital medicine.J Hosp Med.2006;1(suppl 1):48–56.
- ,,,.A medical unit for the acute care of the elderly.J Am Geriatr Soc.1994;42:545–552.
- ,,, et al.Dissemination and characteristics of acute care of elders (ACE) units in the United States.Int J Technol Assess Health Care.2003;19:220–227.
- ,,,.Changing physician performance: a systematic review of the effect of continuing medical education strategies.J Am Med Assoc.1995;274:700–750.
- Accreditation Council for Graduate Medical Education. Outcome project: general competencies. Available at: http://www.acgme.org/outcome/comp/compfull.asp. Accessed October2005.
- ,,, et al.The Stanford faculty development program for medical teachers: a dissemination approach to faculty development for medical teachers.Teach Learn Med.1992;4:180–187.
- ,,.How do you get to teaching improvement? A longitudinal faculty development program for medical educators.Teach Learn Med.1998;11:52–57.
- .Curriculum Development for Medical Education: A Six‐Step Approach.Baltimore, MD:Johns Hopkins University Press;1998.
- .Acute hospital care. In:Cassel C,Cohen HJ,Larson EB, et al., eds.Geriatric Medicine,4th ed.New York:Springer‐Verlag;2003.
- ,,, et al.A multicomponent intervention to prevent delirium in hospitalized older patients.N Engl J Med.1999;340:669–676.
- ,,, et al.Importance of functional measures in predicting mortality among older hospitalized patients.JAMA.1998;279:1187–1193.
- ,,, et al.Cognitive screening predicts magnitude of functional recovery from admission to 3 months after discharge in hospitalized elders.J Gerontol Med Sci.2003;58:37–45.
- ,,, et al.Comprehensive discharge planning and home follow‐up of hospitalized elders: a randomized controlled trial.JAMA.1999;17:613–620.
- ,,, et al.A randomized trial of care in a hospital medical unit especially designed to improve the functional outcomes of acutely ill older patients.N Engl J Med.1995;332:1338–1344.
- ,,, et al.A controlled trial of inpatient and outpatient geriatric evaluation and management.N Engl J Med.2002;346:905–912.
- ,,, et al.Effects of a multicomponent intervention on functional outcomes and process of care in hospitalized older patients: a randomized controlled trial of acute care for the elders (ACE) in a community hospital.J Am Geriatr Soc.2000;48:1572–1581.
- The Joint Commission. Available at http://www.jcinc.com. Accessed April2008.
- ,,, et al.A learner's needs assessment in geriatric medicine for hospitalists. Paper to be presented at: American Geriatrics Society Annual Meeting; May2004; Las Vegas, NV.
- ,,, et al.Clarifying confusion: the confusion assessment method. A new method for detecting delirium.Ann Intern Med1990;113:941–948.
- ,.Meeting the JCAHO national patient safety goal: a model for building a standardized hand‐off protocol.Jt Comm J Qual Saf.2006;32:645–655.
- ,.Safety by design: understanding the dynamic complexity of redesigning care around the clinical microsystem.Qual Saf Health Care.2006;15(suppl 1):i10–i16.
- ,.The PDSA cycle at the core of learning in health professions education.Jt Comm J Qual Improv.1996;22:206–212.
- ,,.A case‐based approach to teaching practice‐based learning and improvement on the wards.Semin Med Pract.2005;8:64–74.
- ,,,.Does the structure of questions affect the outcome of curbside consultations with specialty colleagues?Arch Fam Med.2000;9:541–547.
- ,,.Enhancing medical student consultation request skills in an academic emergency department.J Emerg Med.1998;16:659–662.
- ,,,.Using an objective structured teaching evaluation for faculty development.Med Educ.2005;39:1160–1161.
- ,.Using standardised students in faculty development workshops to improve clinical teaching skills.Med Educ.2003;37:621.
- ,,,,,.Reliability and validity of an objective structured teaching examination for generalist resident teachers.Acad Med.2002;77:S29.
- ,,,.A five‐step “microskills” model of clinical teaching.J Am Board Fam Pract.1992;5:419–424.
- ,.Commitment to change: theoretical foundations, methods, and outcomes.J Cont Educ Health Prof.1999;19:200–207.
- .Commitment to change: a strategy for promoting educational effectiveness.J Cont Educ Health Prof.2000;20:156–163.
- .Evaluation of training. In:Craig R,Bittel I, eds.Training and Development Handbook.New York, NY:McGraw‐Hill;1967.
- ,,, et al.Development and evaluation of a geriatrics knowledge test for primary care residents.J Gen Intern Med.1997;12:450–452.
- ,.UNIPAC Three: Assessment and Treatment of Pain in the Terminally Ill.2nd ed.Glenview, IL:American Academy of Hospice and Palliative Care;2003.
- ,,, et al.Development and validation of a geriatric knowledge test for medical students.J Am Geriatr Soc.2004;52:983–988.
- ,,, et al.Development and validation of a geriatrics attitudes scale for primary care residents.J Am Geriatr Soc.1998;46:1425–1430.
- ,,,.No magic bullets: a systematic review of 102 trials of interventions to improve professional practice.Can Med Assoc J.1995;153:1423–1431.
- ,,,,.Faculty development in geriatrics for clinician educators: a unique model for skills acquisition and academic achievement.J Am Geriatr Soc.2005;53:516–521.
- ,,, et al.Accuracy of physician self‐assessment compared with observed measures of competence: a systematic review.JAMA.2006;296:1094–1102.
- ,.Self‐assessment in lifelong learning and improving performance in practice: physician know thyself.JAMA.2006;296:1137–1139.
- ,,, et al.Effects of physician experience on costs and outcomes on an academic general medicine service: results of a trial of hospitalists.Ann Intern Med.2002;137:866–874.
- ,,, et al.The vulnerable elders survey: a tool for identifying vulnerable older people in the community.J Am Geriatr Soc.2001;49:1691–1699.
- ,, and the ACOVE Investigators.Assessing care of vulnerable elders: ACOVE project overview.Ann Intern Med.2001;135:642–646.
- ,,, et al.Using assessing care of vulnerable elders quality indicators to measure quality of hospital care for vulnerable elders.J Am Geriatr Soc.2007;55:1705–1711.
A crucial arena of innovative educational programs for the care of the elderly must include the hospital setting, a place of great cost, morbidity, and mortality for a population currently occupying approximately half of US hospital beds.1 With a marked acceleration in the number of persons living to an advanced age, there is a clear imperative to address the health‐care needs of the elderly, particularly the complex and frail.24 An educational grounding that steps beyond the traditional organ‐based models of disease to a much broader patient‐centered framework of care is necessary to aid physicians in advanced clinical decision‐making in the care of older patients. Organizing the medical care of the older patient within existing systems of care and a team care management network must also be improved.
Curricular materials and methods are widely available for teaching geriatric medicine,57 but most are geared toward outpatient care and management, with few addressing the care of the hospitalized, older medical patient.810 There is even less published on curricular materials, methods, and tools for such teaching outside of specialized hospital‐based geriatric units by nongeriatrics‐trained faculty.1113 Furthermore, the evaluation of geriatrics educational programs in the hospital setting has not been done with the ultimate assessment, the linking of educational programs to demonstrated changes in clinical practice and patient care outcomes.
To address these needs, we designed and implemented the Curriculum for the Hospitalized Aging Medical Patient (CHAMP) Faculty Development Program (FDP). CHAMP was funded by a grant from the Donald W. Reynolds Foundation Aging and Quality of Life Program with a matching commitment from the University of Chicago Department of Medicine. At the core of CHAMP are principles of care for the older patient in the hospital setting, with an emphasis on identifying and providing care for the complex and frail elderly with nongeriatrician inpatient medicine faculty as the primary teachers of these materials. The overall educational goals of the CHAMP FDP are the following: (1) to train hospitalists and general internists to recognize opportunities to teach geriatric medicine topics specific to the care of the hospitalized older patient; (2) to create teaching materials, tools, and methods that can be used in the busy medical inpatient setting at the bedside; (3) to create materials and tools that facilitate teaching the Accreditation Council for Graduate Medical Education (ACGME) core competencies14 during ward rounds; and (4) to increase the frequency and effectiveness with which this geriatrics content is taught in the hospital setting. This article describes the development and refinement of the CHAMP FDP and evaluation results to date.
METHODS
The CHAMP FDP was developed by a core group of geriatricians, hospitalists, general medicine faculty, and PhD educators from the Office of the Dean at the University of Chicago Pritzker School of Medicine. The core group piloted the FDP for themselves in spring 2004, and the FDP was offered to target learners annually from 2004 to 2006.
CHAMP Participants
The targeted faculty learners for the CHAMP FDP were hospitalists and general internists who attend on an inpatient medicine service for 1 to 4 months yearly. CHAMP Faculty Scholars were self‐selected from the eligible faculty of the University of Chicago. Approximately one‐third of the CHAMP Faculty Scholars held significant administrative and/or teaching positions in the Department of Medicine, residency program, or medical school. Overall, general internist and hospitalist faculty members of the University of Chicago are highly rated inpatient teachers with a 2004‐2007 average overall resident teaching rating of 3.79 (standard deviation = 0.53) on a scale of 1 to 4 (4 = outstanding). For each yearly cohort, we sought to train 8 to 10 Faculty Scholars. The Donald W. Reynolds Foundation grant funds supported the time of the Faculty Scholars to attend the CHAMP FDP 4 hours weekly for the 12 weeks of the course with release from a half‐day of outpatient clinical duties per week for the length of the FDP. Scholars also received continuing medical education credit for time spent in the FDP.
CHAMP Course Design, Structure, and Content
Design and Structure
The CHAMP FDP consists of twelve 4‐hour sessions given once weekly from September through November of each calendar year. Each session is composed of discrete teaching modules. During the first 2 hours of each session, 1 or 2 modules cover inpatient geriatric medicine content. The remaining 2 hours are devoted to modules consisting of the Stanford FDP for Medical Teachers: Improving Clinical Teaching (first 7 sessions)15, 16 and a course developed for the CHAMP FDP named Teaching on Today's Wards (remaining 5 sessions).
In addition to the overarching goals of the CHAMP FDP, each CHAMP module has specific learning objectives and an evaluation process based on the standard precepts of curriculum design.17 Further modifications of the CHAMP content and methods were strongly influenced by subsequent formal evaluative feedback on the course content, materials, and methods by the Faculty Scholars in each of the 4 FDP groups to date.
Geriatrics Content
The FDP geriatrics content and design model were developed as follows: reviewing existing published geriatrics curricular materials,5, 6, 8, 18 including high‐risk areas of geriatric hospital care;1922 drawing from the experience of the inpatient geriatric evaluation and treatment units;2325 and reviewing the Joint Commission mandates26 that have a particular impact on the care of the older hospitalized patients (eg, high‐risk medications, medication reconciliation, restraint use, and transitions of care). Final curricular materials were approved by consensus of the University of Chicago geriatrics/hospitalist core CHAMP faculty. A needs assessment surveying hospitalists at a regional Society of Hospital Medicine meeting showed a strong concordance between geriatrics topics that respondents thought they were least confident about in their knowledge, that they thought would be most useful to learn, and that we proposed for the core geriatrics topics for the CHAMP FDP, including pharmacy of aging, pressure ulcers, delirium, palliative care, decision‐making capacity, and dementia.27
Each geriatric topic is presented in 30‐ to 90‐minute teaching sessions with didactic lectures and case‐based discussions and is organized around 4 broad themes (Table 1). These lectures emphasize application of the content to bedside teaching during hospital medicine rounds. For example, the session on dementia focuses on assessing decision‐making capacity, the impact of dementia on the care of other medical illnesses and discharge decisions, dementia‐associated frailty with increased risk of hospitalization‐related adverse outcomes, and pain assessment in persons with dementia.
|
| Theme 1: Identify the frail/vulnerable elder |
| Identification and assessment of the vulnerable hospitalized older patient |
| Dementia in hospitalized older medical patients: Recognition of and screening for dementia, assessment of medical decision‐making capacity, implications for the treatment of nondementia illness, pain assessment, and improvement of the posthospitalization transition of care |
| Theme 2: Recognize and avoid hazards of hospitalization |
| Delirium: Diagnosis, treatment, risk stratification, and prevention |
| Falls: Assessment and prevention |
| Foley catheters: Scope of the problem, appropriate indications, and management |
| Deconditioning: Scope of the problem and prevention |
| Adverse drug reactions and medication errors: Principles of drug review |
| Pressure ulcers: Assessment, treatment, and prevention |
| Theme 3: Palliate and address end‐of‐life issues |
| Pain control: General principles and use of opiates |
| Symptom management in advanced disease: Nausea |
| Difficult conversations and advance directives |
| Hospice and palliative care and changing goals of care |
| Theme 4: Improve transitions of care |
| The ideal hospital discharge: Core components and determining destination |
| Destinations of posthospital care: Nursing homes for skilled rehabilitation and long‐term care |
The CHAMP materials created for teaching each topic at the bedside included topic‐specific teaching triggers, clinical teaching questions, and summary teaching points. The bedside teaching materials and other teaching tools, such as pocket cards with teaching triggers and clinical content (see the example in the appendix), commonly used geriatric measures (eg, the Confusion Assessment Method for delirium),28 and sample forms for teaching aspects of practice‐based learning and improvement and systems‐based practice, were available to Faculty Scholars electronically on the University of Chicago Course Management System (the CHALK E‐learning Web site). The CHAMP materials are now published at the University of Chicago Web site (
Teaching Content
The material referring to the process of teaching has been organized under 4 components in the CHAMP FDP.
The Stanford FDP for Medical Teachers15, 16
This established teaching skills course uses case scenarios and practice sessions to hone skills in key elements of teaching: learning climate, control of session, communication of goals, promotion of understanding and retention, evaluation, feedback, and promotion of self‐directed learning. This portion of the FDP was taught by a University of Chicago General Medicine faculty member trained and certified to teach the course at Stanford.
Teaching on Today's Wards
The Teaching on Today's Wards component was developed specifically for CHAMP to address the following: (1) to improve bedside teaching in the specific setting of the inpatient wards; (2) to increase the amount of geriatric medicine content taught by nongeriatrics faculty during bedside rounds; and (3) to teach the specific ACGME core competencies of professionalism, communication, practice‐based learning and improvement, and systems‐based practice during ward rounds (Table 2).
| ACGME Core Competency | Addressed in CHAMP Curriculum |
|---|---|
| |
| Knowledge/patient care | All geriatric lectures (see Table 1) |
| Professionalism | Geriatric lectures |
| 1. Advance directives and difficult conversations | |
| 2. Dementia: Decision‐making capacity | |
| Teaching on Today's Wards exercises and games | |
| 1. Process mapping | |
| 2. I Hope I Get a Good Team game | |
| 3. Deciding What To Teach/Missed Teaching Opportunities game | |
| Communication | Geriatric lectures |
| 1. Advance directives and difficult conversations | |
| 2. Dementia: Decision‐making capacity | |
| 3. Destinations for posthospital care: Nursing homes | |
| Teaching on Today's Wards exercises and games | |
| 1. Process mapping | |
| 2. Deciding What To Teach/Missed Teaching Opportunities game | |
| Systems‐based practice | Geriatric lectures |
| 1. Frailty: Screening | |
| 2. Delirium: Screening and prevention | |
| 3. Deconditioning: Prevention | |
| 4. Falls: Prevention | |
| 5. Pressure ulcers: Prevention | |
| 6. Drugs and aging: Drug review | |
| 7. Foley catheter: Indications for use | |
| 8. Ideal hospital discharge | |
| Teaching on Today's Wards exercises and games | |
| 1. Process mapping | |
| 2. Deciding What To Teach/Missed Teaching Opportunities game | |
| 3. Quality improvement projects | |
| Practice‐based learning and improvement | Teaching on Today's Wards exercises and games |
| 1. Case audit | |
| 2. Census audit | |
| 3. Process mapping | |
Session one of Teaching on Today's Wards takes the Faculty Scholars through an exploration of their teaching process on a postcall day using process mapping.29, 30 This technique, similar to constructing a flow chart, involves outlining the series of steps involved in one's actual (not ideal) process of postcall teaching. Faculty Scholars then explore how to recognize opportunities and add geriatric topics and the ACGME core competencies to their teaching on the basis of their own teaching process, skill sets, and clinical experience.
Session two explores goal setting, team dynamics, and the incorporation of more geriatrics teaching into the Faculty Scholar's teaching agenda through a series of interactive card game exercises facilitated in small group discussion. Card game 1, I Hope I Get a Good Team, allows learners to practice goal setting for their inpatient team using a hypothetical game card team based on the learning level, individuals' strengths and weaknesses, and individuals' roles in the team hierarchy. Card game 2, Deciding What To Teach/Missed Opportunities, helps learners develop a teaching agenda on any set of patients that incorporates the CHAMP geriatric topics and the ACGME core competencies.
Sessions three and four teach learners about the systems‐based practice and practice‐based learning and improvement competencies, including an introduction to quality improvement. These interactive sessions introduce Faculty Scholars to the plan‐do‐study‐act method,31 using the example of census and case audits32 to provide an objective and structured method of assessing care. These audits provide a structure for the medical team to review its actual care and management practices and for faculty to teach quality improvement. Examples of census audits developed by CHAMP faculty, including deep venous thrombosis prophylaxis, Foley catheter use, and use of proton pump inhibitors, provide models for the faculty learners to create their own audits.
The fifth session focuses on developing skills for life‐long learning. Based on previous work on medical education and evidence‐based medicine,33, 34 these sessions provide learners with a framework to identify and address knowledge gaps, obtain effective consultation, ask pertinent questions of learners, and self‐assess their teaching skills.
Observed Structured Teaching Exercises
Observed structured teaching exercises allow the deliberate practice of teaching new curricular materials and skills and have been shown to improve teaching skills for both faculty and resident teachers using standardized students in a simulated teaching environment.3537 The observed structured teaching exercises developed for CHAMP allow the Faculty Scholars to practice teaching geriatrics content using the one‐minute preceptor teaching method.38
Commitment to Change (CTC) Contracts
CTC contracts provide a method for sustaining CHAMP teaching. At the end of the FDP, we ask Faculty Scholars to sign a CTC contract,39, 40 selecting at least 1 geriatric topic and 1 topic from Teaching on Today's Wards to teach in future inpatient teaching attending months. Over the year(s) following the FDP, the CHAMP project director frequently contacts the Faculty Scholars via e‐mail and phone interviews before, during, and after each month of inpatient service. The CTC contract is formally reviewed and revised annually with each CHAMP Faculty Scholar by the CHAMP project director and a core CHAMP faculty member.
Evaluation
A comprehensive multilevel evaluation scheme was developed based on the work of Kirkpatrick,41 including participant experience and teaching and subsequent clinical outcomes. This article reports only on the knowledge, attitudes, and behavioral self‐report data collected from participants, and remaining data will be presented in future articles.
The evaluation of the FDP program includes many commonly used methods for evaluating faculty learners, including recollection and retention of course content and self‐reported behavioral changes regarding the incorporation of the material into clinical teaching and practice. The more proximal evaluation includes precourse and postcourse performance on a previously validated geriatric medicine knowledge test,4244 precourse and postcourse performance on a validated survey of attitudes regarding older persons and geriatric medicine,45 a self‐assessment survey measuring self‐reported importance of and confidence in practicing and teaching geriatric skills, and Faculty Scholars' reports of subsequent frequency of teaching on the geriatric medicine and Teaching on Today's Wards content.
Faculty Scholars' feedback regarding their reaction to and satisfaction with the CHAMP FDP includes immediate postsession evaluations of each individual CHAMP FDP session and its content.
Analyses
We calculated the overall satisfaction of the FDP by aggregating evaluations for all session modules across the 4 cohorts. Satisfaction was measured with 6 questions, which included an overall satisfaction question and were answered with 5‐point Likert scales.
Pre‐CHAMP and post‐CHAMP scores on the geriatrics knowledge test and geriatrics attitude scale were calculated for each participant and compared with paired‐sample t tests. Composite scores for the self‐reported behavior for importance of/confidence in practice and importance of/confidence in teaching were calculated for each set of responses from each participant. The average scores across all 14 geriatrics content items for importance of/confidence in practice and importance of/confidence in teaching were calculated pre‐CHAMP and post‐CHAMP and compared with a paired‐sample t test. Similarly, self‐reported behavior ratings of importance of/confidence in teaching were calculated by the averaging of responses across the 10 Teaching on Today's Wards items. Pre‐CHAMP and post‐CHAMP average scores were compared with paired‐sample t tests on SPSS version 14 (SPSS, Chicago, IL). Data from the pilot sessions were included in the analyses to provide adequate power.
RESULTS
We pilot‐tested the format, materials, methods, and evaluation components of the CHAMP FDP with the CHAMP core faculty in the spring of 2004. The revised CHAMP FDP was given in the fall of 2004 to the first group of 8 faculty learners. Similar annual CHAMP FDPs have occurred since 2004, with a total of 29 Faculty Scholars by 2006. This includes approximately half of the University of Chicago general medicine faculty and the majority of the hospitalist faculty. Geriatrics fellows, a medicine chief resident, and other internal medicine subspecialists have also taken the CHAMP FDP. The average evaluations of all CHAMP sessions by all participants are shown in Table 3.
| Rating Criteria* | Average (SD) | N |
|---|---|---|
| ||
| Teaching methods were appropriate for the content covered. | 4.5 0.8 | 571 |
| The module made an important contribution to my practice. | 4.4 0.9 | 566 |
| Supplemental materials were effectively used to enhance learning. | 4.0 1.6 | 433 |
| I feel prepared to teach the material covered in this module. | 4.1 1.0 | 567 |
| I feel prepared to incorporate this material into my practice. | 4.4 0.8 | 569 |
| Overall, this was a valuable educational experience. | 4.5 0.8 | 565 |
Faculty Scholars rated the FDP highly regarding preparation for teaching and incorporation of the material into their teaching and practice. Likewise, qualitative comments by the Faculty Scholars were strongly supportive of CHAMP:
Significantly more aware and confident in teaching around typical geriatric issues present in our patients.
Provided concrete, structured ideas about curriculum, learning goals, content materials and how to implement them.
The online teaching resources were something I used on an almost daily basis.
Wish we had this for outpatient.
CHAMP had a favorable impact on the Faculty Scholars across the domains of knowledge, attitudes, and perceived behavior change (Table 4). Significant differences on paired‐sample t tests found significant improvement on all but one measure (importance of teaching). After the CHAMP program, Faculty Scholars were more knowledgeable about geriatrics content (P = 0.023), had more positive attitudes to older patients (P = 0.049), and had greater confidence in their ability to care for older patients (P < 0.001) and teach geriatric medicine skills (P < 0.001) and Teaching on Today's Wards content (P < 0.001). There was a significant increase in the perceived importance of practicing the learned skills (P = 0.008) and Teaching on Today's Wards (P = 0.001). The increased importance of teaching geriatrics skills was marginally significant (P = 0.064).
| Domain | N | Average Response | SE | P Value* | ||
|---|---|---|---|---|---|---|
| Pre‐CHAMP | Post‐CHAMP | |||||
| ||||||
| Knowledge | Geriatric medicine knowledge test | 21 | 62.14 | 68.05 | 2.40 | 0.023 |
| Attitudes | Geriatrics attitude scale | 26 | 56.86 | 58.38 | 0.736 | 0.049 |
| Self‐report behavior change | Importance of practice | 28 | 4.40 | 4.62 | 0.078 | 0.008 |
| Confidence in practice | 28 | 3.59 | 4.33 | 0.096 | <0.001 | |
| Importance of teaching | 27 | 4.52 | 4.66 | 0.074 | 0.064 | |
| Confidence in teaching | 27 | 3.42 | 4.47 | 0.112 | <0.001 | |
| Importance of Teaching on Today's Wards∥ | 27 | 3.92 | 4.30 | 0.093 | 0.001 | |
| Confidence in Teaching on Today's Wards∥ | 27 | 2.81 | 4.05 | 0.136 | <0.001 | |
DISCUSSION
Central to CHAMP's design are (1) the creation of teaching materials and teaching resources that specifically address the challenges of teaching the care of the hospitalized older patient in busy hospital settings, (2) the provision of methods to reinforce the newly learned geriatrics teaching skills, and (3) a multidimensional evaluation scheme. The enthusiastic response to the CHAMP FDP and the evaluation results to date support the relevance and importance of CHAMP's focus, materials, and educational methods. The ideal outcome for our CHAMP FDP graduates is more informed, confident, and frequent teaching of geriatrics topics keyed to quality improvement and systems of care through a more streamlined but personalized bedside teaching process.13, 46 The CHAMP Faculty Scholar graduates' self‐report surveys of their performance and teaching of CHAMP course geriatrics skills did reveal a significant shift in clinical behavior, teaching, and confidence. Although the strongest indicator of perceived behavior change was in the enhanced self‐confidence in practicing and teaching, the significant changes in knowledge and attitude reinforce our observations of a shift in the mindset about teaching and caring for hospitalized elderly patients. This provides strong evidence for the efficacy of the CHAMP course in positively influencing participants.
Our biggest challenge with the CHAMP FDP was providing enough ongoing support to reinforce learning with an eye on the greater goal of changing teaching behaviors and clinical outcomes. After pilot testing, we added multiple types of support and follow‐up to the FDP: observed structured teaching exercises to practice CHAMP geriatrics content and teaching skills; modification of Teaching on Today's Wards through the addition of practice‐oriented exercises, games, and tutorials; frequent contact with our Faculty Scholar graduates post‐CHAMP FDP through CTC contracts; annual Faculty Scholars reunions; and continued access for the scholars to CHAMP materials on our Web site. Maintaining face‐to‐face contact between CHAMP core faculty and Faculty Scholars once the latter have finished the FDP has been challenging, largely because of clinical and teaching obligations over geographically separate sites. To overcome this, we are working to integrate CHAMP core faculty into hospitalist and general medicine section lecture series, increasing the frequency of CHAMP reunions, renewing CTC contracts with the Faculty Scholar graduates annually, and considering the concept of CHAMP core faculty guests attending during Faculty Scholars inpatient ward rounds.47
The CHAMP FDP and our evaluations to date have several limitations. First, FDP Scholars were volunteer participants who may have been more motivated to improve their geriatric care and teaching than nonparticipants. However, FDP Scholars had only moderate levels of geriatrics knowledge, attitudes, and confidence in their teaching on baseline testing and showed marked improvements in these domains after the FDP. In addition, Scholars' FDP participation was made possible by a reduction of other clinical obligations through direct reimbursement to their sections with CHAMP funds. Other incentives for CHAMP participation could include its focus on generalizable bedside teaching skills and provision of specific techniques for teaching the ACGME core competencies and quality improvement while using geriatrics content. Although the CHAMP FDP in its 48‐hour format is not sustainable or generalizable, the FDP modules and CHAMP materials were specifically designed to be usable in small pieces that could be incorporated into existing teaching structures, grand rounds, section meetings, teacher conferences, and continuing medical education workshops. CHAMP core group members have already presented and taught CHAMP components in many venues (see Dissemination on the CHAMP Web site). The excitement generated by CHAMP at national and specialty meetings, including multiple requests for materials, speaks to widespread interest in our CHAMP model. We are pursuing the creation of a mini‐CHAMP, an abbreviated FDP with an online component. These activities as well as feedback from users of CHAMP materials from the CHAMP Web site and the Portal of Geriatric Online Education will provide important opportunities for examining the use and acceptance of CHAMP outside our institution.
Another limitation of the CHAMP FDP is reliance on FDP Scholar self‐assessment in several of the evaluation components. Some studies have shown poor concordance between physicians' self‐assessment and external assessment over a range of domains.48 However, others have noted that despite these limitations, self‐assessment remains an essential tool for enabling physicians to discover the motivational discomfort of a performance gap, which may lead to changing concepts and mental models or changing work‐flow processes.49 Teaching on Today's Wards sessions in CHAMP emphasize self‐audit processes (such as process mapping and census audits) that can augment self‐assessment. We used such self‐audit processes in 1 small pilot study to date, providing summative and qualitative feedback to a group of FDP Scholars on their use of census audits.
However, the evaluation of the CHAMP FDP is enhanced by a yearly survey of all medical residents and medical students and by the linking of the teaching reported by residents and medical students to specific attendings. We have begun the analysis of resident perceptions of being taught CHAMP geriatrics topics by CHAMP faculty versus non‐CHAMP faculty. In addition, we are gathering data on patient‐level process of care and outcomes tied to the CHAMP FDP course session objectives by linking to the ongoing University of Chicago Hospitalist Project, a large clinical research project that enrolls general medicine inpatients in a study examining the quality of care and resource allocation for these patients.50 Because the ultimate goal of CHAMP is to improve the quality of care and outcomes for elderly hospitalized patients, the University of Chicago Hospitalist Project infrastructure was modified by the incorporation of the Vulnerable Elder Survey‐1351 and a process‐of‐care chart audit specifically based on the Assessing Care of the Vulnerable Elders Hospital Quality Indicators.52 Preliminary work included testing and validating these measures.53 Further evaluation of these clinical outcomes and CHAMP's efficacy and durability at the University of Chicago is ongoing and will be presented in future reports.
CONCLUSIONS
Through a collaboration of geriatricians, hospitalists, and general internists, the CHAMP FDP provides educational materials and methods keyed to bedside teaching in the fast‐paced world of the hospital. CHAMP improves faculty knowledge and attitudes and the frequency of teaching geriatrics topics and skills necessary to deliver quality care to the elderly hospitalized medical patient. Although the CHAMP FDP was developed and refined for use at a specific institution, the multitiered CHAMP FDP materials and methods have the potential for widespread use by multiple types of inpatient attendings for teaching the care of the older hospitalized medicine patient. Hospitalists in particular will require this expertise as both clinicians and teachers as their role, leadership, and influence continue to expand nationally.
Acknowledgements
The Curriculum for the Hospitalized Aging Medical Patient (CHAMP) Program was supported by funding from the Donald W. Reynolds Foundation with matching funds from the University of Chicago Department of Medicine, by the Hartford Foundation Geriatrics Center for Excellence, and by a Geriatric Academic Career Award to Don Scott. Presentations on CHAMP and its materials include a number of national and international meeting venues, including meetings of the Society of Hospital Medicine, the American Geriatrics Society, and the Association of Program Directors in Internal Medicine and the International Ottawa Conference.
APPENDIX
EXAMPLE OF A CHAMP POCKET CARD: FOLEY CATHETERS
| CHAMP: Foley Catheters | CHAMP: Inability to Void | |
|---|---|---|
| ||
| Catherine DuBeau, MD, Geriatrics, University of Chicago | Catherine DuBeau, MD, Geriatrics, University of Chicago | |
| 1. Does this patient have a catheter? Incorporate regular catheter checks on rounds as a practice‐based learning and improvement exercise. | 1. Is there a medical reason for this patient's inability to void? | |
| Two Basic Reasons | ||
| 2. Does this patient need a catheter? | Poor pump | |
| Only Four Indications | ▪ Meds: anticholinergics, Ca++ blockers, narcotics | |
| a. Inability to void | ▪ Sacral cord disease | |
| b. Urinary incontinence and | ▪ Neuropathy: DM, B12 | |
| ▪ Open sacral or perineal wound | ▪ Constipation/emmpaction | |
| ▪ Palliative care | Blocked outlet | |
| c. Urine output monitoring | ▪ Prostate disease | |
| ▪ Critical illnessfrequent/urgent monitoring needed | ▪ Suprasacral spinal cord disease (eg, MS) with detrusor‐sphincter dyssynergia | |
| ▪ Patient unable/unwilling to collect urine | ▪ Women: scarring, large cystocele | |
| d. After general or spinal anesthesia | ▪ Constipation/emmpaction | |
| 3. Why should catheter use be minimized? | Evaluation of Inability To Void | |
| a. Infection risk | ||
| ▪ Cause of 40% of nosocomial infections | Action Step | Possible Medical Reasons |
| b. Morbidity | ||
| ▪ Internal catheters | ||
| ○Associated with delirium | Review meds | ‐Cholinergics, narcotics, calcium channel blockers, ‐agonists |
| ○Urethral and meatal injury | ||
| ○Bladder and renal stones | ||
| ○Fever | Review med Hx | Diabetes with neuropathy, sacral/subsacral cord, B12, GU surgery or radiation |
| ○Polymicrobial bacteruria | ||
| ▪ External (condom) catheters | ||
| ○Penile cellulitus/necrosis | Physical exam | Womenpelvic for prolapse; all‐sacral root S2‐4anal wink and bulbocavernosus reflexes |
| ○Urinary retention | ||
| ○Bacteruria and infection | ||
| c. Foleys are uncomfortable/painful. | Postvoiding residual | This should have been done in the evaluation of the patient's inability to void and repeated after catheter removal with voiding trial. |
| d. Foleys are restrictive falls and delirium. | ||
| e. Cost | ||
A crucial arena of innovative educational programs for the care of the elderly must include the hospital setting, a place of great cost, morbidity, and mortality for a population currently occupying approximately half of US hospital beds.1 With a marked acceleration in the number of persons living to an advanced age, there is a clear imperative to address the health‐care needs of the elderly, particularly the complex and frail.24 An educational grounding that steps beyond the traditional organ‐based models of disease to a much broader patient‐centered framework of care is necessary to aid physicians in advanced clinical decision‐making in the care of older patients. Organizing the medical care of the older patient within existing systems of care and a team care management network must also be improved.
Curricular materials and methods are widely available for teaching geriatric medicine,57 but most are geared toward outpatient care and management, with few addressing the care of the hospitalized, older medical patient.810 There is even less published on curricular materials, methods, and tools for such teaching outside of specialized hospital‐based geriatric units by nongeriatrics‐trained faculty.1113 Furthermore, the evaluation of geriatrics educational programs in the hospital setting has not been done with the ultimate assessment, the linking of educational programs to demonstrated changes in clinical practice and patient care outcomes.
To address these needs, we designed and implemented the Curriculum for the Hospitalized Aging Medical Patient (CHAMP) Faculty Development Program (FDP). CHAMP was funded by a grant from the Donald W. Reynolds Foundation Aging and Quality of Life Program with a matching commitment from the University of Chicago Department of Medicine. At the core of CHAMP are principles of care for the older patient in the hospital setting, with an emphasis on identifying and providing care for the complex and frail elderly with nongeriatrician inpatient medicine faculty as the primary teachers of these materials. The overall educational goals of the CHAMP FDP are the following: (1) to train hospitalists and general internists to recognize opportunities to teach geriatric medicine topics specific to the care of the hospitalized older patient; (2) to create teaching materials, tools, and methods that can be used in the busy medical inpatient setting at the bedside; (3) to create materials and tools that facilitate teaching the Accreditation Council for Graduate Medical Education (ACGME) core competencies14 during ward rounds; and (4) to increase the frequency and effectiveness with which this geriatrics content is taught in the hospital setting. This article describes the development and refinement of the CHAMP FDP and evaluation results to date.
METHODS
The CHAMP FDP was developed by a core group of geriatricians, hospitalists, general medicine faculty, and PhD educators from the Office of the Dean at the University of Chicago Pritzker School of Medicine. The core group piloted the FDP for themselves in spring 2004, and the FDP was offered to target learners annually from 2004 to 2006.
CHAMP Participants
The targeted faculty learners for the CHAMP FDP were hospitalists and general internists who attend on an inpatient medicine service for 1 to 4 months yearly. CHAMP Faculty Scholars were self‐selected from the eligible faculty of the University of Chicago. Approximately one‐third of the CHAMP Faculty Scholars held significant administrative and/or teaching positions in the Department of Medicine, residency program, or medical school. Overall, general internist and hospitalist faculty members of the University of Chicago are highly rated inpatient teachers with a 2004‐2007 average overall resident teaching rating of 3.79 (standard deviation = 0.53) on a scale of 1 to 4 (4 = outstanding). For each yearly cohort, we sought to train 8 to 10 Faculty Scholars. The Donald W. Reynolds Foundation grant funds supported the time of the Faculty Scholars to attend the CHAMP FDP 4 hours weekly for the 12 weeks of the course with release from a half‐day of outpatient clinical duties per week for the length of the FDP. Scholars also received continuing medical education credit for time spent in the FDP.
CHAMP Course Design, Structure, and Content
Design and Structure
The CHAMP FDP consists of twelve 4‐hour sessions given once weekly from September through November of each calendar year. Each session is composed of discrete teaching modules. During the first 2 hours of each session, 1 or 2 modules cover inpatient geriatric medicine content. The remaining 2 hours are devoted to modules consisting of the Stanford FDP for Medical Teachers: Improving Clinical Teaching (first 7 sessions)15, 16 and a course developed for the CHAMP FDP named Teaching on Today's Wards (remaining 5 sessions).
In addition to the overarching goals of the CHAMP FDP, each CHAMP module has specific learning objectives and an evaluation process based on the standard precepts of curriculum design.17 Further modifications of the CHAMP content and methods were strongly influenced by subsequent formal evaluative feedback on the course content, materials, and methods by the Faculty Scholars in each of the 4 FDP groups to date.
Geriatrics Content
The FDP geriatrics content and design model were developed as follows: reviewing existing published geriatrics curricular materials,5, 6, 8, 18 including high‐risk areas of geriatric hospital care;1922 drawing from the experience of the inpatient geriatric evaluation and treatment units;2325 and reviewing the Joint Commission mandates26 that have a particular impact on the care of the older hospitalized patients (eg, high‐risk medications, medication reconciliation, restraint use, and transitions of care). Final curricular materials were approved by consensus of the University of Chicago geriatrics/hospitalist core CHAMP faculty. A needs assessment surveying hospitalists at a regional Society of Hospital Medicine meeting showed a strong concordance between geriatrics topics that respondents thought they were least confident about in their knowledge, that they thought would be most useful to learn, and that we proposed for the core geriatrics topics for the CHAMP FDP, including pharmacy of aging, pressure ulcers, delirium, palliative care, decision‐making capacity, and dementia.27
Each geriatric topic is presented in 30‐ to 90‐minute teaching sessions with didactic lectures and case‐based discussions and is organized around 4 broad themes (Table 1). These lectures emphasize application of the content to bedside teaching during hospital medicine rounds. For example, the session on dementia focuses on assessing decision‐making capacity, the impact of dementia on the care of other medical illnesses and discharge decisions, dementia‐associated frailty with increased risk of hospitalization‐related adverse outcomes, and pain assessment in persons with dementia.
|
| Theme 1: Identify the frail/vulnerable elder |
| Identification and assessment of the vulnerable hospitalized older patient |
| Dementia in hospitalized older medical patients: Recognition of and screening for dementia, assessment of medical decision‐making capacity, implications for the treatment of nondementia illness, pain assessment, and improvement of the posthospitalization transition of care |
| Theme 2: Recognize and avoid hazards of hospitalization |
| Delirium: Diagnosis, treatment, risk stratification, and prevention |
| Falls: Assessment and prevention |
| Foley catheters: Scope of the problem, appropriate indications, and management |
| Deconditioning: Scope of the problem and prevention |
| Adverse drug reactions and medication errors: Principles of drug review |
| Pressure ulcers: Assessment, treatment, and prevention |
| Theme 3: Palliate and address end‐of‐life issues |
| Pain control: General principles and use of opiates |
| Symptom management in advanced disease: Nausea |
| Difficult conversations and advance directives |
| Hospice and palliative care and changing goals of care |
| Theme 4: Improve transitions of care |
| The ideal hospital discharge: Core components and determining destination |
| Destinations of posthospital care: Nursing homes for skilled rehabilitation and long‐term care |
The CHAMP materials created for teaching each topic at the bedside included topic‐specific teaching triggers, clinical teaching questions, and summary teaching points. The bedside teaching materials and other teaching tools, such as pocket cards with teaching triggers and clinical content (see the example in the appendix), commonly used geriatric measures (eg, the Confusion Assessment Method for delirium),28 and sample forms for teaching aspects of practice‐based learning and improvement and systems‐based practice, were available to Faculty Scholars electronically on the University of Chicago Course Management System (the CHALK E‐learning Web site). The CHAMP materials are now published at the University of Chicago Web site (
Teaching Content
The material referring to the process of teaching has been organized under 4 components in the CHAMP FDP.
The Stanford FDP for Medical Teachers15, 16
This established teaching skills course uses case scenarios and practice sessions to hone skills in key elements of teaching: learning climate, control of session, communication of goals, promotion of understanding and retention, evaluation, feedback, and promotion of self‐directed learning. This portion of the FDP was taught by a University of Chicago General Medicine faculty member trained and certified to teach the course at Stanford.
Teaching on Today's Wards
The Teaching on Today's Wards component was developed specifically for CHAMP to address the following: (1) to improve bedside teaching in the specific setting of the inpatient wards; (2) to increase the amount of geriatric medicine content taught by nongeriatrics faculty during bedside rounds; and (3) to teach the specific ACGME core competencies of professionalism, communication, practice‐based learning and improvement, and systems‐based practice during ward rounds (Table 2).
| ACGME Core Competency | Addressed in CHAMP Curriculum |
|---|---|
| |
| Knowledge/patient care | All geriatric lectures (see Table 1) |
| Professionalism | Geriatric lectures |
| 1. Advance directives and difficult conversations | |
| 2. Dementia: Decision‐making capacity | |
| Teaching on Today's Wards exercises and games | |
| 1. Process mapping | |
| 2. I Hope I Get a Good Team game | |
| 3. Deciding What To Teach/Missed Teaching Opportunities game | |
| Communication | Geriatric lectures |
| 1. Advance directives and difficult conversations | |
| 2. Dementia: Decision‐making capacity | |
| 3. Destinations for posthospital care: Nursing homes | |
| Teaching on Today's Wards exercises and games | |
| 1. Process mapping | |
| 2. Deciding What To Teach/Missed Teaching Opportunities game | |
| Systems‐based practice | Geriatric lectures |
| 1. Frailty: Screening | |
| 2. Delirium: Screening and prevention | |
| 3. Deconditioning: Prevention | |
| 4. Falls: Prevention | |
| 5. Pressure ulcers: Prevention | |
| 6. Drugs and aging: Drug review | |
| 7. Foley catheter: Indications for use | |
| 8. Ideal hospital discharge | |
| Teaching on Today's Wards exercises and games | |
| 1. Process mapping | |
| 2. Deciding What To Teach/Missed Teaching Opportunities game | |
| 3. Quality improvement projects | |
| Practice‐based learning and improvement | Teaching on Today's Wards exercises and games |
| 1. Case audit | |
| 2. Census audit | |
| 3. Process mapping | |
Session one of Teaching on Today's Wards takes the Faculty Scholars through an exploration of their teaching process on a postcall day using process mapping.29, 30 This technique, similar to constructing a flow chart, involves outlining the series of steps involved in one's actual (not ideal) process of postcall teaching. Faculty Scholars then explore how to recognize opportunities and add geriatric topics and the ACGME core competencies to their teaching on the basis of their own teaching process, skill sets, and clinical experience.
Session two explores goal setting, team dynamics, and the incorporation of more geriatrics teaching into the Faculty Scholar's teaching agenda through a series of interactive card game exercises facilitated in small group discussion. Card game 1, I Hope I Get a Good Team, allows learners to practice goal setting for their inpatient team using a hypothetical game card team based on the learning level, individuals' strengths and weaknesses, and individuals' roles in the team hierarchy. Card game 2, Deciding What To Teach/Missed Opportunities, helps learners develop a teaching agenda on any set of patients that incorporates the CHAMP geriatric topics and the ACGME core competencies.
Sessions three and four teach learners about the systems‐based practice and practice‐based learning and improvement competencies, including an introduction to quality improvement. These interactive sessions introduce Faculty Scholars to the plan‐do‐study‐act method,31 using the example of census and case audits32 to provide an objective and structured method of assessing care. These audits provide a structure for the medical team to review its actual care and management practices and for faculty to teach quality improvement. Examples of census audits developed by CHAMP faculty, including deep venous thrombosis prophylaxis, Foley catheter use, and use of proton pump inhibitors, provide models for the faculty learners to create their own audits.
The fifth session focuses on developing skills for life‐long learning. Based on previous work on medical education and evidence‐based medicine,33, 34 these sessions provide learners with a framework to identify and address knowledge gaps, obtain effective consultation, ask pertinent questions of learners, and self‐assess their teaching skills.
Observed Structured Teaching Exercises
Observed structured teaching exercises allow the deliberate practice of teaching new curricular materials and skills and have been shown to improve teaching skills for both faculty and resident teachers using standardized students in a simulated teaching environment.3537 The observed structured teaching exercises developed for CHAMP allow the Faculty Scholars to practice teaching geriatrics content using the one‐minute preceptor teaching method.38
Commitment to Change (CTC) Contracts
CTC contracts provide a method for sustaining CHAMP teaching. At the end of the FDP, we ask Faculty Scholars to sign a CTC contract,39, 40 selecting at least 1 geriatric topic and 1 topic from Teaching on Today's Wards to teach in future inpatient teaching attending months. Over the year(s) following the FDP, the CHAMP project director frequently contacts the Faculty Scholars via e‐mail and phone interviews before, during, and after each month of inpatient service. The CTC contract is formally reviewed and revised annually with each CHAMP Faculty Scholar by the CHAMP project director and a core CHAMP faculty member.
Evaluation
A comprehensive multilevel evaluation scheme was developed based on the work of Kirkpatrick,41 including participant experience and teaching and subsequent clinical outcomes. This article reports only on the knowledge, attitudes, and behavioral self‐report data collected from participants, and remaining data will be presented in future articles.
The evaluation of the FDP program includes many commonly used methods for evaluating faculty learners, including recollection and retention of course content and self‐reported behavioral changes regarding the incorporation of the material into clinical teaching and practice. The more proximal evaluation includes precourse and postcourse performance on a previously validated geriatric medicine knowledge test,4244 precourse and postcourse performance on a validated survey of attitudes regarding older persons and geriatric medicine,45 a self‐assessment survey measuring self‐reported importance of and confidence in practicing and teaching geriatric skills, and Faculty Scholars' reports of subsequent frequency of teaching on the geriatric medicine and Teaching on Today's Wards content.
Faculty Scholars' feedback regarding their reaction to and satisfaction with the CHAMP FDP includes immediate postsession evaluations of each individual CHAMP FDP session and its content.
Analyses
We calculated the overall satisfaction of the FDP by aggregating evaluations for all session modules across the 4 cohorts. Satisfaction was measured with 6 questions, which included an overall satisfaction question and were answered with 5‐point Likert scales.
Pre‐CHAMP and post‐CHAMP scores on the geriatrics knowledge test and geriatrics attitude scale were calculated for each participant and compared with paired‐sample t tests. Composite scores for the self‐reported behavior for importance of/confidence in practice and importance of/confidence in teaching were calculated for each set of responses from each participant. The average scores across all 14 geriatrics content items for importance of/confidence in practice and importance of/confidence in teaching were calculated pre‐CHAMP and post‐CHAMP and compared with a paired‐sample t test. Similarly, self‐reported behavior ratings of importance of/confidence in teaching were calculated by the averaging of responses across the 10 Teaching on Today's Wards items. Pre‐CHAMP and post‐CHAMP average scores were compared with paired‐sample t tests on SPSS version 14 (SPSS, Chicago, IL). Data from the pilot sessions were included in the analyses to provide adequate power.
RESULTS
We pilot‐tested the format, materials, methods, and evaluation components of the CHAMP FDP with the CHAMP core faculty in the spring of 2004. The revised CHAMP FDP was given in the fall of 2004 to the first group of 8 faculty learners. Similar annual CHAMP FDPs have occurred since 2004, with a total of 29 Faculty Scholars by 2006. This includes approximately half of the University of Chicago general medicine faculty and the majority of the hospitalist faculty. Geriatrics fellows, a medicine chief resident, and other internal medicine subspecialists have also taken the CHAMP FDP. The average evaluations of all CHAMP sessions by all participants are shown in Table 3.
| Rating Criteria* | Average (SD) | N |
|---|---|---|
| ||
| Teaching methods were appropriate for the content covered. | 4.5 0.8 | 571 |
| The module made an important contribution to my practice. | 4.4 0.9 | 566 |
| Supplemental materials were effectively used to enhance learning. | 4.0 1.6 | 433 |
| I feel prepared to teach the material covered in this module. | 4.1 1.0 | 567 |
| I feel prepared to incorporate this material into my practice. | 4.4 0.8 | 569 |
| Overall, this was a valuable educational experience. | 4.5 0.8 | 565 |
Faculty Scholars rated the FDP highly regarding preparation for teaching and incorporation of the material into their teaching and practice. Likewise, qualitative comments by the Faculty Scholars were strongly supportive of CHAMP:
Significantly more aware and confident in teaching around typical geriatric issues present in our patients.
Provided concrete, structured ideas about curriculum, learning goals, content materials and how to implement them.
The online teaching resources were something I used on an almost daily basis.
Wish we had this for outpatient.
CHAMP had a favorable impact on the Faculty Scholars across the domains of knowledge, attitudes, and perceived behavior change (Table 4). Significant differences on paired‐sample t tests found significant improvement on all but one measure (importance of teaching). After the CHAMP program, Faculty Scholars were more knowledgeable about geriatrics content (P = 0.023), had more positive attitudes to older patients (P = 0.049), and had greater confidence in their ability to care for older patients (P < 0.001) and teach geriatric medicine skills (P < 0.001) and Teaching on Today's Wards content (P < 0.001). There was a significant increase in the perceived importance of practicing the learned skills (P = 0.008) and Teaching on Today's Wards (P = 0.001). The increased importance of teaching geriatrics skills was marginally significant (P = 0.064).
| Domain | N | Average Response | SE | P Value* | ||
|---|---|---|---|---|---|---|
| Pre‐CHAMP | Post‐CHAMP | |||||
| ||||||
| Knowledge | Geriatric medicine knowledge test | 21 | 62.14 | 68.05 | 2.40 | 0.023 |
| Attitudes | Geriatrics attitude scale | 26 | 56.86 | 58.38 | 0.736 | 0.049 |
| Self‐report behavior change | Importance of practice | 28 | 4.40 | 4.62 | 0.078 | 0.008 |
| Confidence in practice | 28 | 3.59 | 4.33 | 0.096 | <0.001 | |
| Importance of teaching | 27 | 4.52 | 4.66 | 0.074 | 0.064 | |
| Confidence in teaching | 27 | 3.42 | 4.47 | 0.112 | <0.001 | |
| Importance of Teaching on Today's Wards∥ | 27 | 3.92 | 4.30 | 0.093 | 0.001 | |
| Confidence in Teaching on Today's Wards∥ | 27 | 2.81 | 4.05 | 0.136 | <0.001 | |
DISCUSSION
Central to CHAMP's design are (1) the creation of teaching materials and teaching resources that specifically address the challenges of teaching the care of the hospitalized older patient in busy hospital settings, (2) the provision of methods to reinforce the newly learned geriatrics teaching skills, and (3) a multidimensional evaluation scheme. The enthusiastic response to the CHAMP FDP and the evaluation results to date support the relevance and importance of CHAMP's focus, materials, and educational methods. The ideal outcome for our CHAMP FDP graduates is more informed, confident, and frequent teaching of geriatrics topics keyed to quality improvement and systems of care through a more streamlined but personalized bedside teaching process.13, 46 The CHAMP Faculty Scholar graduates' self‐report surveys of their performance and teaching of CHAMP course geriatrics skills did reveal a significant shift in clinical behavior, teaching, and confidence. Although the strongest indicator of perceived behavior change was in the enhanced self‐confidence in practicing and teaching, the significant changes in knowledge and attitude reinforce our observations of a shift in the mindset about teaching and caring for hospitalized elderly patients. This provides strong evidence for the efficacy of the CHAMP course in positively influencing participants.
Our biggest challenge with the CHAMP FDP was providing enough ongoing support to reinforce learning with an eye on the greater goal of changing teaching behaviors and clinical outcomes. After pilot testing, we added multiple types of support and follow‐up to the FDP: observed structured teaching exercises to practice CHAMP geriatrics content and teaching skills; modification of Teaching on Today's Wards through the addition of practice‐oriented exercises, games, and tutorials; frequent contact with our Faculty Scholar graduates post‐CHAMP FDP through CTC contracts; annual Faculty Scholars reunions; and continued access for the scholars to CHAMP materials on our Web site. Maintaining face‐to‐face contact between CHAMP core faculty and Faculty Scholars once the latter have finished the FDP has been challenging, largely because of clinical and teaching obligations over geographically separate sites. To overcome this, we are working to integrate CHAMP core faculty into hospitalist and general medicine section lecture series, increasing the frequency of CHAMP reunions, renewing CTC contracts with the Faculty Scholar graduates annually, and considering the concept of CHAMP core faculty guests attending during Faculty Scholars inpatient ward rounds.47
The CHAMP FDP and our evaluations to date have several limitations. First, FDP Scholars were volunteer participants who may have been more motivated to improve their geriatric care and teaching than nonparticipants. However, FDP Scholars had only moderate levels of geriatrics knowledge, attitudes, and confidence in their teaching on baseline testing and showed marked improvements in these domains after the FDP. In addition, Scholars' FDP participation was made possible by a reduction of other clinical obligations through direct reimbursement to their sections with CHAMP funds. Other incentives for CHAMP participation could include its focus on generalizable bedside teaching skills and provision of specific techniques for teaching the ACGME core competencies and quality improvement while using geriatrics content. Although the CHAMP FDP in its 48‐hour format is not sustainable or generalizable, the FDP modules and CHAMP materials were specifically designed to be usable in small pieces that could be incorporated into existing teaching structures, grand rounds, section meetings, teacher conferences, and continuing medical education workshops. CHAMP core group members have already presented and taught CHAMP components in many venues (see Dissemination on the CHAMP Web site). The excitement generated by CHAMP at national and specialty meetings, including multiple requests for materials, speaks to widespread interest in our CHAMP model. We are pursuing the creation of a mini‐CHAMP, an abbreviated FDP with an online component. These activities as well as feedback from users of CHAMP materials from the CHAMP Web site and the Portal of Geriatric Online Education will provide important opportunities for examining the use and acceptance of CHAMP outside our institution.
Another limitation of the CHAMP FDP is reliance on FDP Scholar self‐assessment in several of the evaluation components. Some studies have shown poor concordance between physicians' self‐assessment and external assessment over a range of domains.48 However, others have noted that despite these limitations, self‐assessment remains an essential tool for enabling physicians to discover the motivational discomfort of a performance gap, which may lead to changing concepts and mental models or changing work‐flow processes.49 Teaching on Today's Wards sessions in CHAMP emphasize self‐audit processes (such as process mapping and census audits) that can augment self‐assessment. We used such self‐audit processes in 1 small pilot study to date, providing summative and qualitative feedback to a group of FDP Scholars on their use of census audits.
However, the evaluation of the CHAMP FDP is enhanced by a yearly survey of all medical residents and medical students and by the linking of the teaching reported by residents and medical students to specific attendings. We have begun the analysis of resident perceptions of being taught CHAMP geriatrics topics by CHAMP faculty versus non‐CHAMP faculty. In addition, we are gathering data on patient‐level process of care and outcomes tied to the CHAMP FDP course session objectives by linking to the ongoing University of Chicago Hospitalist Project, a large clinical research project that enrolls general medicine inpatients in a study examining the quality of care and resource allocation for these patients.50 Because the ultimate goal of CHAMP is to improve the quality of care and outcomes for elderly hospitalized patients, the University of Chicago Hospitalist Project infrastructure was modified by the incorporation of the Vulnerable Elder Survey‐1351 and a process‐of‐care chart audit specifically based on the Assessing Care of the Vulnerable Elders Hospital Quality Indicators.52 Preliminary work included testing and validating these measures.53 Further evaluation of these clinical outcomes and CHAMP's efficacy and durability at the University of Chicago is ongoing and will be presented in future reports.
CONCLUSIONS
Through a collaboration of geriatricians, hospitalists, and general internists, the CHAMP FDP provides educational materials and methods keyed to bedside teaching in the fast‐paced world of the hospital. CHAMP improves faculty knowledge and attitudes and the frequency of teaching geriatrics topics and skills necessary to deliver quality care to the elderly hospitalized medical patient. Although the CHAMP FDP was developed and refined for use at a specific institution, the multitiered CHAMP FDP materials and methods have the potential for widespread use by multiple types of inpatient attendings for teaching the care of the older hospitalized medicine patient. Hospitalists in particular will require this expertise as both clinicians and teachers as their role, leadership, and influence continue to expand nationally.
Acknowledgements
The Curriculum for the Hospitalized Aging Medical Patient (CHAMP) Program was supported by funding from the Donald W. Reynolds Foundation with matching funds from the University of Chicago Department of Medicine, by the Hartford Foundation Geriatrics Center for Excellence, and by a Geriatric Academic Career Award to Don Scott. Presentations on CHAMP and its materials include a number of national and international meeting venues, including meetings of the Society of Hospital Medicine, the American Geriatrics Society, and the Association of Program Directors in Internal Medicine and the International Ottawa Conference.
APPENDIX
EXAMPLE OF A CHAMP POCKET CARD: FOLEY CATHETERS
| CHAMP: Foley Catheters | CHAMP: Inability to Void | |
|---|---|---|
| ||
| Catherine DuBeau, MD, Geriatrics, University of Chicago | Catherine DuBeau, MD, Geriatrics, University of Chicago | |
| 1. Does this patient have a catheter? Incorporate regular catheter checks on rounds as a practice‐based learning and improvement exercise. | 1. Is there a medical reason for this patient's inability to void? | |
| Two Basic Reasons | ||
| 2. Does this patient need a catheter? | Poor pump | |
| Only Four Indications | ▪ Meds: anticholinergics, Ca++ blockers, narcotics | |
| a. Inability to void | ▪ Sacral cord disease | |
| b. Urinary incontinence and | ▪ Neuropathy: DM, B12 | |
| ▪ Open sacral or perineal wound | ▪ Constipation/emmpaction | |
| ▪ Palliative care | Blocked outlet | |
| c. Urine output monitoring | ▪ Prostate disease | |
| ▪ Critical illnessfrequent/urgent monitoring needed | ▪ Suprasacral spinal cord disease (eg, MS) with detrusor‐sphincter dyssynergia | |
| ▪ Patient unable/unwilling to collect urine | ▪ Women: scarring, large cystocele | |
| d. After general or spinal anesthesia | ▪ Constipation/emmpaction | |
| 3. Why should catheter use be minimized? | Evaluation of Inability To Void | |
| a. Infection risk | ||
| ▪ Cause of 40% of nosocomial infections | Action Step | Possible Medical Reasons |
| b. Morbidity | ||
| ▪ Internal catheters | ||
| ○Associated with delirium | Review meds | ‐Cholinergics, narcotics, calcium channel blockers, ‐agonists |
| ○Urethral and meatal injury | ||
| ○Bladder and renal stones | ||
| ○Fever | Review med Hx | Diabetes with neuropathy, sacral/subsacral cord, B12, GU surgery or radiation |
| ○Polymicrobial bacteruria | ||
| ▪ External (condom) catheters | ||
| ○Penile cellulitus/necrosis | Physical exam | Womenpelvic for prolapse; all‐sacral root S2‐4anal wink and bulbocavernosus reflexes |
| ○Urinary retention | ||
| ○Bacteruria and infection | ||
| c. Foleys are uncomfortable/painful. | Postvoiding residual | This should have been done in the evaluation of the patient's inability to void and repeated after catheter removal with voiding trial. |
| d. Foleys are restrictive falls and delirium. | ||
| e. Cost | ||
- ,.2002 National Hospital Discharge Survey.Hyattsville, MD:National Center for Health Statistics;2002.Advance Data from Vital and Health Statistics 342.
- ,,, et al.The critical shortage of geriatrics faculty.J Am Geriatr Soc.1993;41:560–569.
- ,,, et al.Development of geriatrics‐oriented faculty in general internal medicine.Ann Intern Med.2003;139:615–620.
- ,,.General internal medicine and geriatrics: building a foundation to improve the training of general internists in the care of older adults.Ann Intern Med.2003;139:609–614.
- ,.Curriculum recommendations for resident training in nursing home care. A collaborative effort of the Society of General Internal Medicine Task Force on Geriatric Medicine, the Society of Teachers of Family Medicine Geriatrics Task Force, the American Medical Directors Association, and the American Geriatrics Society Education Committee.J Am Geriatr Soc.1994;42:1200–1201.
- ,,,,.Curriculum recommendations for resident training in geriatrics interdisciplinary team care.J Am Geriatr Soc.1999;47:1145–1148.
- ,,,,.A national survey on the current status of family practice residency education in geriatric medicine.Fam Med.2003;35:35–41.
- ,.ACGME requirements for geriatrics medicine curricula in medical specialties: progress made and progress needed.Acad Med.2005;80:279–285.
- ,,, et al.Improving geriatrics training in internal medicine residency programs: best practices and sustainable solutions.Ann Intern Med.2003;139:628–634.
- ,,,,.Core competencies in hospital medicine.J Hosp Med.2006;1(suppl 1):48–56.
- ,,,.A medical unit for the acute care of the elderly.J Am Geriatr Soc.1994;42:545–552.
- ,,, et al.Dissemination and characteristics of acute care of elders (ACE) units in the United States.Int J Technol Assess Health Care.2003;19:220–227.
- ,,,.Changing physician performance: a systematic review of the effect of continuing medical education strategies.J Am Med Assoc.1995;274:700–750.
- Accreditation Council for Graduate Medical Education. Outcome project: general competencies. Available at: http://www.acgme.org/outcome/comp/compfull.asp. Accessed October2005.
- ,,, et al.The Stanford faculty development program for medical teachers: a dissemination approach to faculty development for medical teachers.Teach Learn Med.1992;4:180–187.
- ,,.How do you get to teaching improvement? A longitudinal faculty development program for medical educators.Teach Learn Med.1998;11:52–57.
- .Curriculum Development for Medical Education: A Six‐Step Approach.Baltimore, MD:Johns Hopkins University Press;1998.
- .Acute hospital care. In:Cassel C,Cohen HJ,Larson EB, et al., eds.Geriatric Medicine,4th ed.New York:Springer‐Verlag;2003.
- ,,, et al.A multicomponent intervention to prevent delirium in hospitalized older patients.N Engl J Med.1999;340:669–676.
- ,,, et al.Importance of functional measures in predicting mortality among older hospitalized patients.JAMA.1998;279:1187–1193.
- ,,, et al.Cognitive screening predicts magnitude of functional recovery from admission to 3 months after discharge in hospitalized elders.J Gerontol Med Sci.2003;58:37–45.
- ,,, et al.Comprehensive discharge planning and home follow‐up of hospitalized elders: a randomized controlled trial.JAMA.1999;17:613–620.
- ,,, et al.A randomized trial of care in a hospital medical unit especially designed to improve the functional outcomes of acutely ill older patients.N Engl J Med.1995;332:1338–1344.
- ,,, et al.A controlled trial of inpatient and outpatient geriatric evaluation and management.N Engl J Med.2002;346:905–912.
- ,,, et al.Effects of a multicomponent intervention on functional outcomes and process of care in hospitalized older patients: a randomized controlled trial of acute care for the elders (ACE) in a community hospital.J Am Geriatr Soc.2000;48:1572–1581.
- The Joint Commission. Available at http://www.jcinc.com. Accessed April2008.
- ,,, et al.A learner's needs assessment in geriatric medicine for hospitalists. Paper to be presented at: American Geriatrics Society Annual Meeting; May2004; Las Vegas, NV.
- ,,, et al.Clarifying confusion: the confusion assessment method. A new method for detecting delirium.Ann Intern Med1990;113:941–948.
- ,.Meeting the JCAHO national patient safety goal: a model for building a standardized hand‐off protocol.Jt Comm J Qual Saf.2006;32:645–655.
- ,.Safety by design: understanding the dynamic complexity of redesigning care around the clinical microsystem.Qual Saf Health Care.2006;15(suppl 1):i10–i16.
- ,.The PDSA cycle at the core of learning in health professions education.Jt Comm J Qual Improv.1996;22:206–212.
- ,,.A case‐based approach to teaching practice‐based learning and improvement on the wards.Semin Med Pract.2005;8:64–74.
- ,,,.Does the structure of questions affect the outcome of curbside consultations with specialty colleagues?Arch Fam Med.2000;9:541–547.
- ,,.Enhancing medical student consultation request skills in an academic emergency department.J Emerg Med.1998;16:659–662.
- ,,,.Using an objective structured teaching evaluation for faculty development.Med Educ.2005;39:1160–1161.
- ,.Using standardised students in faculty development workshops to improve clinical teaching skills.Med Educ.2003;37:621.
- ,,,,,.Reliability and validity of an objective structured teaching examination for generalist resident teachers.Acad Med.2002;77:S29.
- ,,,.A five‐step “microskills” model of clinical teaching.J Am Board Fam Pract.1992;5:419–424.
- ,.Commitment to change: theoretical foundations, methods, and outcomes.J Cont Educ Health Prof.1999;19:200–207.
- .Commitment to change: a strategy for promoting educational effectiveness.J Cont Educ Health Prof.2000;20:156–163.
- .Evaluation of training. In:Craig R,Bittel I, eds.Training and Development Handbook.New York, NY:McGraw‐Hill;1967.
- ,,, et al.Development and evaluation of a geriatrics knowledge test for primary care residents.J Gen Intern Med.1997;12:450–452.
- ,.UNIPAC Three: Assessment and Treatment of Pain in the Terminally Ill.2nd ed.Glenview, IL:American Academy of Hospice and Palliative Care;2003.
- ,,, et al.Development and validation of a geriatric knowledge test for medical students.J Am Geriatr Soc.2004;52:983–988.
- ,,, et al.Development and validation of a geriatrics attitudes scale for primary care residents.J Am Geriatr Soc.1998;46:1425–1430.
- ,,,.No magic bullets: a systematic review of 102 trials of interventions to improve professional practice.Can Med Assoc J.1995;153:1423–1431.
- ,,,,.Faculty development in geriatrics for clinician educators: a unique model for skills acquisition and academic achievement.J Am Geriatr Soc.2005;53:516–521.
- ,,, et al.Accuracy of physician self‐assessment compared with observed measures of competence: a systematic review.JAMA.2006;296:1094–1102.
- ,.Self‐assessment in lifelong learning and improving performance in practice: physician know thyself.JAMA.2006;296:1137–1139.
- ,,, et al.Effects of physician experience on costs and outcomes on an academic general medicine service: results of a trial of hospitalists.Ann Intern Med.2002;137:866–874.
- ,,, et al.The vulnerable elders survey: a tool for identifying vulnerable older people in the community.J Am Geriatr Soc.2001;49:1691–1699.
- ,, and the ACOVE Investigators.Assessing care of vulnerable elders: ACOVE project overview.Ann Intern Med.2001;135:642–646.
- ,,, et al.Using assessing care of vulnerable elders quality indicators to measure quality of hospital care for vulnerable elders.J Am Geriatr Soc.2007;55:1705–1711.
- ,.2002 National Hospital Discharge Survey.Hyattsville, MD:National Center for Health Statistics;2002.Advance Data from Vital and Health Statistics 342.
- ,,, et al.The critical shortage of geriatrics faculty.J Am Geriatr Soc.1993;41:560–569.
- ,,, et al.Development of geriatrics‐oriented faculty in general internal medicine.Ann Intern Med.2003;139:615–620.
- ,,.General internal medicine and geriatrics: building a foundation to improve the training of general internists in the care of older adults.Ann Intern Med.2003;139:609–614.
- ,.Curriculum recommendations for resident training in nursing home care. A collaborative effort of the Society of General Internal Medicine Task Force on Geriatric Medicine, the Society of Teachers of Family Medicine Geriatrics Task Force, the American Medical Directors Association, and the American Geriatrics Society Education Committee.J Am Geriatr Soc.1994;42:1200–1201.
- ,,,,.Curriculum recommendations for resident training in geriatrics interdisciplinary team care.J Am Geriatr Soc.1999;47:1145–1148.
- ,,,,.A national survey on the current status of family practice residency education in geriatric medicine.Fam Med.2003;35:35–41.
- ,.ACGME requirements for geriatrics medicine curricula in medical specialties: progress made and progress needed.Acad Med.2005;80:279–285.
- ,,, et al.Improving geriatrics training in internal medicine residency programs: best practices and sustainable solutions.Ann Intern Med.2003;139:628–634.
- ,,,,.Core competencies in hospital medicine.J Hosp Med.2006;1(suppl 1):48–56.
- ,,,.A medical unit for the acute care of the elderly.J Am Geriatr Soc.1994;42:545–552.
- ,,, et al.Dissemination and characteristics of acute care of elders (ACE) units in the United States.Int J Technol Assess Health Care.2003;19:220–227.
- ,,,.Changing physician performance: a systematic review of the effect of continuing medical education strategies.J Am Med Assoc.1995;274:700–750.
- Accreditation Council for Graduate Medical Education. Outcome project: general competencies. Available at: http://www.acgme.org/outcome/comp/compfull.asp. Accessed October2005.
- ,,, et al.The Stanford faculty development program for medical teachers: a dissemination approach to faculty development for medical teachers.Teach Learn Med.1992;4:180–187.
- ,,.How do you get to teaching improvement? A longitudinal faculty development program for medical educators.Teach Learn Med.1998;11:52–57.
- .Curriculum Development for Medical Education: A Six‐Step Approach.Baltimore, MD:Johns Hopkins University Press;1998.
- .Acute hospital care. In:Cassel C,Cohen HJ,Larson EB, et al., eds.Geriatric Medicine,4th ed.New York:Springer‐Verlag;2003.
- ,,, et al.A multicomponent intervention to prevent delirium in hospitalized older patients.N Engl J Med.1999;340:669–676.
- ,,, et al.Importance of functional measures in predicting mortality among older hospitalized patients.JAMA.1998;279:1187–1193.
- ,,, et al.Cognitive screening predicts magnitude of functional recovery from admission to 3 months after discharge in hospitalized elders.J Gerontol Med Sci.2003;58:37–45.
- ,,, et al.Comprehensive discharge planning and home follow‐up of hospitalized elders: a randomized controlled trial.JAMA.1999;17:613–620.
- ,,, et al.A randomized trial of care in a hospital medical unit especially designed to improve the functional outcomes of acutely ill older patients.N Engl J Med.1995;332:1338–1344.
- ,,, et al.A controlled trial of inpatient and outpatient geriatric evaluation and management.N Engl J Med.2002;346:905–912.
- ,,, et al.Effects of a multicomponent intervention on functional outcomes and process of care in hospitalized older patients: a randomized controlled trial of acute care for the elders (ACE) in a community hospital.J Am Geriatr Soc.2000;48:1572–1581.
- The Joint Commission. Available at http://www.jcinc.com. Accessed April2008.
- ,,, et al.A learner's needs assessment in geriatric medicine for hospitalists. Paper to be presented at: American Geriatrics Society Annual Meeting; May2004; Las Vegas, NV.
- ,,, et al.Clarifying confusion: the confusion assessment method. A new method for detecting delirium.Ann Intern Med1990;113:941–948.
- ,.Meeting the JCAHO national patient safety goal: a model for building a standardized hand‐off protocol.Jt Comm J Qual Saf.2006;32:645–655.
- ,.Safety by design: understanding the dynamic complexity of redesigning care around the clinical microsystem.Qual Saf Health Care.2006;15(suppl 1):i10–i16.
- ,.The PDSA cycle at the core of learning in health professions education.Jt Comm J Qual Improv.1996;22:206–212.
- ,,.A case‐based approach to teaching practice‐based learning and improvement on the wards.Semin Med Pract.2005;8:64–74.
- ,,,.Does the structure of questions affect the outcome of curbside consultations with specialty colleagues?Arch Fam Med.2000;9:541–547.
- ,,.Enhancing medical student consultation request skills in an academic emergency department.J Emerg Med.1998;16:659–662.
- ,,,.Using an objective structured teaching evaluation for faculty development.Med Educ.2005;39:1160–1161.
- ,.Using standardised students in faculty development workshops to improve clinical teaching skills.Med Educ.2003;37:621.
- ,,,,,.Reliability and validity of an objective structured teaching examination for generalist resident teachers.Acad Med.2002;77:S29.
- ,,,.A five‐step “microskills” model of clinical teaching.J Am Board Fam Pract.1992;5:419–424.
- ,.Commitment to change: theoretical foundations, methods, and outcomes.J Cont Educ Health Prof.1999;19:200–207.
- .Commitment to change: a strategy for promoting educational effectiveness.J Cont Educ Health Prof.2000;20:156–163.
- .Evaluation of training. In:Craig R,Bittel I, eds.Training and Development Handbook.New York, NY:McGraw‐Hill;1967.
- ,,, et al.Development and evaluation of a geriatrics knowledge test for primary care residents.J Gen Intern Med.1997;12:450–452.
- ,.UNIPAC Three: Assessment and Treatment of Pain in the Terminally Ill.2nd ed.Glenview, IL:American Academy of Hospice and Palliative Care;2003.
- ,,, et al.Development and validation of a geriatric knowledge test for medical students.J Am Geriatr Soc.2004;52:983–988.
- ,,, et al.Development and validation of a geriatrics attitudes scale for primary care residents.J Am Geriatr Soc.1998;46:1425–1430.
- ,,,.No magic bullets: a systematic review of 102 trials of interventions to improve professional practice.Can Med Assoc J.1995;153:1423–1431.
- ,,,,.Faculty development in geriatrics for clinician educators: a unique model for skills acquisition and academic achievement.J Am Geriatr Soc.2005;53:516–521.
- ,,, et al.Accuracy of physician self‐assessment compared with observed measures of competence: a systematic review.JAMA.2006;296:1094–1102.
- ,.Self‐assessment in lifelong learning and improving performance in practice: physician know thyself.JAMA.2006;296:1137–1139.
- ,,, et al.Effects of physician experience on costs and outcomes on an academic general medicine service: results of a trial of hospitalists.Ann Intern Med.2002;137:866–874.
- ,,, et al.The vulnerable elders survey: a tool for identifying vulnerable older people in the community.J Am Geriatr Soc.2001;49:1691–1699.
- ,, and the ACOVE Investigators.Assessing care of vulnerable elders: ACOVE project overview.Ann Intern Med.2001;135:642–646.
- ,,, et al.Using assessing care of vulnerable elders quality indicators to measure quality of hospital care for vulnerable elders.J Am Geriatr Soc.2007;55:1705–1711.
Copyright © 2008 Society of Hospital Medicine
Admitting to a Readmit Problem
Admitting to a Readmit Problem
We have a friendly disagreement within our hospitalist group. Some of our physicians believe we should track readmission rates. They believe it is a marker of quality. Others do not. What do you think?
Richard Mackiewicz, MD, New York, NY
Dr. Hospitalist responds:
Policymakers certainly are thinking about hospital readmission rates these days. Hospital readmissions sometimes can indicate poor care or poor coordination of care. Most hospitalist programs do not track readmission rates…but maybe they should.
I have a feeling payers, such as Medicare, will implement policies in the future that will force hospitals and hospitalists to closely monitor readmission rates. Why do I think that? Because, aside from poor care, unnecessary readmissions cost the system money—lots of money. What if I told you 17% of your patients are readmitted to a hospital within 30 days? Not high enough? How about 31%?
I admit my hospitalist program doesn’t track readmission rates. I have no clue what percentage of our patients get readmitted within 30 days. But MedPAC does. A recent MedPAC analysis of 2005 Medicare Provider Analysis and Review data found 6.2% of patients discharged from hospitals are readmitted within seven days. This percentage grows to 11.3% at 15 days and 17.6% at 30 days. That 17.6% translates to roughly $15 billion in Medicare spending.
Data for patients with end-stage renal disease (ESRD) are even more staggering. Hospitalized ESRD patients are readmitted within seven days at a rate of 11.2%. Within 15 days, that becomes 20.4%. Within 30 days, 31.6% of patients with ESRD are readmitted to the hospital.
Surprised at the high numbers? I was. It’s not just patients of this type. Some of my patients get readmitted for reasons that have nothing to do with previous admissions. How can we prevent that? MedPAC ran numbers with only “potentially preventable hospital readmission rates.” The readmission rates for all comers were 5.2% at seven days, 8.8% at 15 days and 13.3% at 30 days. This translated to $5, $8, and $12 billion dollars, respectively, in potentially unnecessary spending of Medicare dollars.
If unnecessary hospital readmissions are so bad, why haven’t hospitals and hospitalists placed a bigger emphasis on preventing them? There are several reasons. One is a lack of awareness of the problem, but the main reason likely is lack of financial incentive to do so.
Most hospitals receive Medicare payment regardless of readmissions. In some states, CMS contractors and quality improvement organizations aggressively have denied payment for readmissions within 30 days, but these are the exceptions, not the rules. In many parts of the country, hospitals have no financial incentive to reduce readmissions unless they can fill the unused beds with more “profitable” patients.
Under the case-based DRG payment model, Medicare actually rewards hospitals for shorter lengths of stay. Hospitals have developed systems to encourage providers to discharge patients as quickly as possible. In fact, many hospitals even look at physicians’ inpatient length of stay as a measure of performance. From the physician perspective, why not discharge the patient as quickly as medically appropriate? The hospital commends you for doing so and if the patient is readmitted, you get to bill a higher admission code rather than a lower-paying subsequent day visit code. More admission and discharge billing means more money.
So how will policymakers address the issue of unnecessary hospital readmissions? Simple. They’ll restructure the compensation model. Medicare addressed the problem of hospital-acquired infections by not paying for them. Hospitals reacted by implementing measures to minimize and prevent the development of these complications. MedPAC has suggested Medicare disclose the risk-adjusted readmission rates for all hospitals and determine benchmark readmission rates for certain conditions (e.g., heart failure, COPD exacerbations, and CABG). Hospitals would receive payment based on how close they come to these benchmarks.
Depending on the approach, Medicare could take away dollars from low performers and/or pay more to high performers. Don’t expect Medicare to limit compensation incentives to acute care hospitals. Expect policy changes to also affect post-acute care facilities, home health providers and physicians.
One thing is certain: Hospitals and payers will expect and demand hospitalists to lead the effort to reduce unnecessary readmissions. No other group of physicians is better positioned to do so. How can hospitalists minimize the risk of hospital readmission? MedPAC has several suggestions:
- Provide better, safer care during the inpatient stay;
- Attend to patients’ medication needs at discharge;
- Improve communication with patients before and after; discharge;
- Improve communication with other providers; and
- Review practice patterns.
Do these suggestions sound familiar? They should. Most of them are signs of high functioning hospitalists and hospitalist groups, and you should already be doing them routinely. TH
Reference:
- MedPAC Report to the Congress: Promoting Greater Efficiency in Medicare, June 2007.
Admitting to a Readmit Problem
We have a friendly disagreement within our hospitalist group. Some of our physicians believe we should track readmission rates. They believe it is a marker of quality. Others do not. What do you think?
Richard Mackiewicz, MD, New York, NY
Dr. Hospitalist responds:
Policymakers certainly are thinking about hospital readmission rates these days. Hospital readmissions sometimes can indicate poor care or poor coordination of care. Most hospitalist programs do not track readmission rates…but maybe they should.
I have a feeling payers, such as Medicare, will implement policies in the future that will force hospitals and hospitalists to closely monitor readmission rates. Why do I think that? Because, aside from poor care, unnecessary readmissions cost the system money—lots of money. What if I told you 17% of your patients are readmitted to a hospital within 30 days? Not high enough? How about 31%?
I admit my hospitalist program doesn’t track readmission rates. I have no clue what percentage of our patients get readmitted within 30 days. But MedPAC does. A recent MedPAC analysis of 2005 Medicare Provider Analysis and Review data found 6.2% of patients discharged from hospitals are readmitted within seven days. This percentage grows to 11.3% at 15 days and 17.6% at 30 days. That 17.6% translates to roughly $15 billion in Medicare spending.
Data for patients with end-stage renal disease (ESRD) are even more staggering. Hospitalized ESRD patients are readmitted within seven days at a rate of 11.2%. Within 15 days, that becomes 20.4%. Within 30 days, 31.6% of patients with ESRD are readmitted to the hospital.
Surprised at the high numbers? I was. It’s not just patients of this type. Some of my patients get readmitted for reasons that have nothing to do with previous admissions. How can we prevent that? MedPAC ran numbers with only “potentially preventable hospital readmission rates.” The readmission rates for all comers were 5.2% at seven days, 8.8% at 15 days and 13.3% at 30 days. This translated to $5, $8, and $12 billion dollars, respectively, in potentially unnecessary spending of Medicare dollars.
If unnecessary hospital readmissions are so bad, why haven’t hospitals and hospitalists placed a bigger emphasis on preventing them? There are several reasons. One is a lack of awareness of the problem, but the main reason likely is lack of financial incentive to do so.
Most hospitals receive Medicare payment regardless of readmissions. In some states, CMS contractors and quality improvement organizations aggressively have denied payment for readmissions within 30 days, but these are the exceptions, not the rules. In many parts of the country, hospitals have no financial incentive to reduce readmissions unless they can fill the unused beds with more “profitable” patients.
Under the case-based DRG payment model, Medicare actually rewards hospitals for shorter lengths of stay. Hospitals have developed systems to encourage providers to discharge patients as quickly as possible. In fact, many hospitals even look at physicians’ inpatient length of stay as a measure of performance. From the physician perspective, why not discharge the patient as quickly as medically appropriate? The hospital commends you for doing so and if the patient is readmitted, you get to bill a higher admission code rather than a lower-paying subsequent day visit code. More admission and discharge billing means more money.
So how will policymakers address the issue of unnecessary hospital readmissions? Simple. They’ll restructure the compensation model. Medicare addressed the problem of hospital-acquired infections by not paying for them. Hospitals reacted by implementing measures to minimize and prevent the development of these complications. MedPAC has suggested Medicare disclose the risk-adjusted readmission rates for all hospitals and determine benchmark readmission rates for certain conditions (e.g., heart failure, COPD exacerbations, and CABG). Hospitals would receive payment based on how close they come to these benchmarks.
Depending on the approach, Medicare could take away dollars from low performers and/or pay more to high performers. Don’t expect Medicare to limit compensation incentives to acute care hospitals. Expect policy changes to also affect post-acute care facilities, home health providers and physicians.
One thing is certain: Hospitals and payers will expect and demand hospitalists to lead the effort to reduce unnecessary readmissions. No other group of physicians is better positioned to do so. How can hospitalists minimize the risk of hospital readmission? MedPAC has several suggestions:
- Provide better, safer care during the inpatient stay;
- Attend to patients’ medication needs at discharge;
- Improve communication with patients before and after; discharge;
- Improve communication with other providers; and
- Review practice patterns.
Do these suggestions sound familiar? They should. Most of them are signs of high functioning hospitalists and hospitalist groups, and you should already be doing them routinely. TH
Reference:
- MedPAC Report to the Congress: Promoting Greater Efficiency in Medicare, June 2007.
Admitting to a Readmit Problem
We have a friendly disagreement within our hospitalist group. Some of our physicians believe we should track readmission rates. They believe it is a marker of quality. Others do not. What do you think?
Richard Mackiewicz, MD, New York, NY
Dr. Hospitalist responds:
Policymakers certainly are thinking about hospital readmission rates these days. Hospital readmissions sometimes can indicate poor care or poor coordination of care. Most hospitalist programs do not track readmission rates…but maybe they should.
I have a feeling payers, such as Medicare, will implement policies in the future that will force hospitals and hospitalists to closely monitor readmission rates. Why do I think that? Because, aside from poor care, unnecessary readmissions cost the system money—lots of money. What if I told you 17% of your patients are readmitted to a hospital within 30 days? Not high enough? How about 31%?
I admit my hospitalist program doesn’t track readmission rates. I have no clue what percentage of our patients get readmitted within 30 days. But MedPAC does. A recent MedPAC analysis of 2005 Medicare Provider Analysis and Review data found 6.2% of patients discharged from hospitals are readmitted within seven days. This percentage grows to 11.3% at 15 days and 17.6% at 30 days. That 17.6% translates to roughly $15 billion in Medicare spending.
Data for patients with end-stage renal disease (ESRD) are even more staggering. Hospitalized ESRD patients are readmitted within seven days at a rate of 11.2%. Within 15 days, that becomes 20.4%. Within 30 days, 31.6% of patients with ESRD are readmitted to the hospital.
Surprised at the high numbers? I was. It’s not just patients of this type. Some of my patients get readmitted for reasons that have nothing to do with previous admissions. How can we prevent that? MedPAC ran numbers with only “potentially preventable hospital readmission rates.” The readmission rates for all comers were 5.2% at seven days, 8.8% at 15 days and 13.3% at 30 days. This translated to $5, $8, and $12 billion dollars, respectively, in potentially unnecessary spending of Medicare dollars.
If unnecessary hospital readmissions are so bad, why haven’t hospitals and hospitalists placed a bigger emphasis on preventing them? There are several reasons. One is a lack of awareness of the problem, but the main reason likely is lack of financial incentive to do so.
Most hospitals receive Medicare payment regardless of readmissions. In some states, CMS contractors and quality improvement organizations aggressively have denied payment for readmissions within 30 days, but these are the exceptions, not the rules. In many parts of the country, hospitals have no financial incentive to reduce readmissions unless they can fill the unused beds with more “profitable” patients.
Under the case-based DRG payment model, Medicare actually rewards hospitals for shorter lengths of stay. Hospitals have developed systems to encourage providers to discharge patients as quickly as possible. In fact, many hospitals even look at physicians’ inpatient length of stay as a measure of performance. From the physician perspective, why not discharge the patient as quickly as medically appropriate? The hospital commends you for doing so and if the patient is readmitted, you get to bill a higher admission code rather than a lower-paying subsequent day visit code. More admission and discharge billing means more money.
So how will policymakers address the issue of unnecessary hospital readmissions? Simple. They’ll restructure the compensation model. Medicare addressed the problem of hospital-acquired infections by not paying for them. Hospitals reacted by implementing measures to minimize and prevent the development of these complications. MedPAC has suggested Medicare disclose the risk-adjusted readmission rates for all hospitals and determine benchmark readmission rates for certain conditions (e.g., heart failure, COPD exacerbations, and CABG). Hospitals would receive payment based on how close they come to these benchmarks.
Depending on the approach, Medicare could take away dollars from low performers and/or pay more to high performers. Don’t expect Medicare to limit compensation incentives to acute care hospitals. Expect policy changes to also affect post-acute care facilities, home health providers and physicians.
One thing is certain: Hospitals and payers will expect and demand hospitalists to lead the effort to reduce unnecessary readmissions. No other group of physicians is better positioned to do so. How can hospitalists minimize the risk of hospital readmission? MedPAC has several suggestions:
- Provide better, safer care during the inpatient stay;
- Attend to patients’ medication needs at discharge;
- Improve communication with patients before and after; discharge;
- Improve communication with other providers; and
- Review practice patterns.
Do these suggestions sound familiar? They should. Most of them are signs of high functioning hospitalists and hospitalist groups, and you should already be doing them routinely. TH
Reference:
- MedPAC Report to the Congress: Promoting Greater Efficiency in Medicare, June 2007.
Maximizing NPPs in Hospitalist Practices
Last month, I recommended considering new and innovative roles for the non-physician providers (NPPs) (see The Hospitalist, September 2008, p. 61.). In this column I’ll discuss the economic and patient satisfaction issues related to NPPs in hospitalist practice.
Economics of NPPs
My experience suggests many practices follow a similar line of reasoning when adding NPPs: “We have six physician hospitalist FTEs and need to expand further, yet recruiting additional MDs is difficult. Perhaps we should add one or more NPPs instead. That should work out well economically since NPPs have lower salaries. After all, it seems to work for heart surgeons and orthopedists.”
This kind of reasoning has two flaws. The practice is, in essence, deciding to add NPPs because that process may be easier than finding additional MDs. The practice should instead consider what work needs to be done and decide whether there is a genuinely valuable role for an NPP.
Secondly, just because it makes financial sense for some specialties to add NPPs doesn’t mean it does for hospitalist groups. The salary gap between orthopedists or cardiac surgeons and NPPs is huge. The salary difference between a physician hospitalist and an NPP is much more modest.
From a strictly financial analysis, which ignores the many benefits of NPPs that don’t appear on financial statements, an NPP needs to increase the efficiency of an orthopedist or cardiac surgeon by only 10% to 20%. That same NPP would need to increase the efficiency of a hospitalist by more like 50%. (I estimated the percentages to illustrate the point. You should conduct a more-detailed analysis of your own situation to determine accurate percentages.)
I’ve worked with practices that have incorporated NPPs but failed to think carefully about their optimal roles. These staff end up functioning in a mostly clerical role, doing tasks such as faxing discharge information to PCPs, retrieving records from outside facilities, or handling billing functions for the doctors. Those practices should either change the NPPs’ roles or use the money to instead hire clerical help. That would leave money for other purposes, such as creating a more aggressive physician recruiting effort or hiring MDs to moonlight.
Local Factors Govern Economics, Practice
In addition to financial considerations surrounding NPPs, keep in mind licensure. Nurse practitioners are licensed as independent practitioners. Physician assistants are not. The laws governing scope of practice for both of these professionals vary from state to state. Additionally, hospital bylaws govern the boundaries of what NPPs can do without supervision. Two hospitals in the same community might have completely different rules. It is important to understand the state and individual hospital regulations that govern NPPs where you practice.
A PA’s work will nearly always require a physician being physically present during some portion of the patient visit and co-signing chart notes and orders. Nurse practitioners, on the other hand, may be able to perform certain patient-care activities independently. In the latter case, Medicare and other payers typically reimburse at 85% of the rate customarily paid to MDs for the same service.
Patient Perception of NPPs
Patients are increasingly more accepting of NPs and PAs. This seems especially true in settings with clear distinctions between the role of NPP and MD.
For example, my wife is perfectly happy to see a nurse practitioner for routine gynecological care, such as Pap smears. She knows the obstetrician handled the delivery of our children and is available anytime she’s needed.
My neighbor was pleased with his open-heart surgery experience and spoke glowingly of the NP who made rounds daily and assisted during the surgery. He knew the MD surgeon performed most of the operation but left the perioperative care up to the NP.
Patients on a hospitalist service may not see things the same way. My neighbor understood he was hospitalized for the purpose of open-heart surgery done by the MD. He looked at the perioperative care outside of the operation as a secondary issue.
Most medical admissions managed by hospitalists don’t have such clear marquee events in patients’ eyes. So it may be less natural for patients to feel OK about how the hospitalist and NPP divide up care responsibilities. Look at it this way: As hospitalists, we have limited face time with patients, and must make good use of it to establish trust and rapport. When we add an NPP to the care team, we ask patients to develop trust and rapport with two providers instead of just one.
Imagine a patient recently discharged from a hospitalist practice. Her friend asks how it went and which doctor she saw. The patient responds, “I couldn’t figure out who was really in charge of my care. Dr. Nelson’s name was on my armband, but I rarely saw him. Instead, I saw his assistant (the NPP) most of the time.” I suspect that patient will be much less likely to report high levels of satisfaction with her care than one who just saw a hospitalist.
Though I’m concerned that it might be more difficult to keep patients happy when NPPs are part of a hospitalist practice, most practices report this hasn’t been a problem. I’m not suggesting that concern about patient satisfaction means you shouldn’t use NPPs in your hospitalist practices. However, patient satisfaction is an issue to consider when organizing your practice—and an NPP’s role in it—to provide the greatest benefit to your patients. TH
Dr. Nelson has been a practicing hospitalist since 1988 and is co-founder and past president of SHM. He is a principal in Nelson/Flores Associates, a national hospitalist practice management consulting firm. He is also part of the faculty for SHM’s “Best Practices in Managing a Hospital Medicine Program” course. This column represents his views and is not intended to reflect an official position of SHM.
Last month, I recommended considering new and innovative roles for the non-physician providers (NPPs) (see The Hospitalist, September 2008, p. 61.). In this column I’ll discuss the economic and patient satisfaction issues related to NPPs in hospitalist practice.
Economics of NPPs
My experience suggests many practices follow a similar line of reasoning when adding NPPs: “We have six physician hospitalist FTEs and need to expand further, yet recruiting additional MDs is difficult. Perhaps we should add one or more NPPs instead. That should work out well economically since NPPs have lower salaries. After all, it seems to work for heart surgeons and orthopedists.”
This kind of reasoning has two flaws. The practice is, in essence, deciding to add NPPs because that process may be easier than finding additional MDs. The practice should instead consider what work needs to be done and decide whether there is a genuinely valuable role for an NPP.
Secondly, just because it makes financial sense for some specialties to add NPPs doesn’t mean it does for hospitalist groups. The salary gap between orthopedists or cardiac surgeons and NPPs is huge. The salary difference between a physician hospitalist and an NPP is much more modest.
From a strictly financial analysis, which ignores the many benefits of NPPs that don’t appear on financial statements, an NPP needs to increase the efficiency of an orthopedist or cardiac surgeon by only 10% to 20%. That same NPP would need to increase the efficiency of a hospitalist by more like 50%. (I estimated the percentages to illustrate the point. You should conduct a more-detailed analysis of your own situation to determine accurate percentages.)
I’ve worked with practices that have incorporated NPPs but failed to think carefully about their optimal roles. These staff end up functioning in a mostly clerical role, doing tasks such as faxing discharge information to PCPs, retrieving records from outside facilities, or handling billing functions for the doctors. Those practices should either change the NPPs’ roles or use the money to instead hire clerical help. That would leave money for other purposes, such as creating a more aggressive physician recruiting effort or hiring MDs to moonlight.
Local Factors Govern Economics, Practice
In addition to financial considerations surrounding NPPs, keep in mind licensure. Nurse practitioners are licensed as independent practitioners. Physician assistants are not. The laws governing scope of practice for both of these professionals vary from state to state. Additionally, hospital bylaws govern the boundaries of what NPPs can do without supervision. Two hospitals in the same community might have completely different rules. It is important to understand the state and individual hospital regulations that govern NPPs where you practice.
A PA’s work will nearly always require a physician being physically present during some portion of the patient visit and co-signing chart notes and orders. Nurse practitioners, on the other hand, may be able to perform certain patient-care activities independently. In the latter case, Medicare and other payers typically reimburse at 85% of the rate customarily paid to MDs for the same service.
Patient Perception of NPPs
Patients are increasingly more accepting of NPs and PAs. This seems especially true in settings with clear distinctions between the role of NPP and MD.
For example, my wife is perfectly happy to see a nurse practitioner for routine gynecological care, such as Pap smears. She knows the obstetrician handled the delivery of our children and is available anytime she’s needed.
My neighbor was pleased with his open-heart surgery experience and spoke glowingly of the NP who made rounds daily and assisted during the surgery. He knew the MD surgeon performed most of the operation but left the perioperative care up to the NP.
Patients on a hospitalist service may not see things the same way. My neighbor understood he was hospitalized for the purpose of open-heart surgery done by the MD. He looked at the perioperative care outside of the operation as a secondary issue.
Most medical admissions managed by hospitalists don’t have such clear marquee events in patients’ eyes. So it may be less natural for patients to feel OK about how the hospitalist and NPP divide up care responsibilities. Look at it this way: As hospitalists, we have limited face time with patients, and must make good use of it to establish trust and rapport. When we add an NPP to the care team, we ask patients to develop trust and rapport with two providers instead of just one.
Imagine a patient recently discharged from a hospitalist practice. Her friend asks how it went and which doctor she saw. The patient responds, “I couldn’t figure out who was really in charge of my care. Dr. Nelson’s name was on my armband, but I rarely saw him. Instead, I saw his assistant (the NPP) most of the time.” I suspect that patient will be much less likely to report high levels of satisfaction with her care than one who just saw a hospitalist.
Though I’m concerned that it might be more difficult to keep patients happy when NPPs are part of a hospitalist practice, most practices report this hasn’t been a problem. I’m not suggesting that concern about patient satisfaction means you shouldn’t use NPPs in your hospitalist practices. However, patient satisfaction is an issue to consider when organizing your practice—and an NPP’s role in it—to provide the greatest benefit to your patients. TH
Dr. Nelson has been a practicing hospitalist since 1988 and is co-founder and past president of SHM. He is a principal in Nelson/Flores Associates, a national hospitalist practice management consulting firm. He is also part of the faculty for SHM’s “Best Practices in Managing a Hospital Medicine Program” course. This column represents his views and is not intended to reflect an official position of SHM.
Last month, I recommended considering new and innovative roles for the non-physician providers (NPPs) (see The Hospitalist, September 2008, p. 61.). In this column I’ll discuss the economic and patient satisfaction issues related to NPPs in hospitalist practice.
Economics of NPPs
My experience suggests many practices follow a similar line of reasoning when adding NPPs: “We have six physician hospitalist FTEs and need to expand further, yet recruiting additional MDs is difficult. Perhaps we should add one or more NPPs instead. That should work out well economically since NPPs have lower salaries. After all, it seems to work for heart surgeons and orthopedists.”
This kind of reasoning has two flaws. The practice is, in essence, deciding to add NPPs because that process may be easier than finding additional MDs. The practice should instead consider what work needs to be done and decide whether there is a genuinely valuable role for an NPP.
Secondly, just because it makes financial sense for some specialties to add NPPs doesn’t mean it does for hospitalist groups. The salary gap between orthopedists or cardiac surgeons and NPPs is huge. The salary difference between a physician hospitalist and an NPP is much more modest.
From a strictly financial analysis, which ignores the many benefits of NPPs that don’t appear on financial statements, an NPP needs to increase the efficiency of an orthopedist or cardiac surgeon by only 10% to 20%. That same NPP would need to increase the efficiency of a hospitalist by more like 50%. (I estimated the percentages to illustrate the point. You should conduct a more-detailed analysis of your own situation to determine accurate percentages.)
I’ve worked with practices that have incorporated NPPs but failed to think carefully about their optimal roles. These staff end up functioning in a mostly clerical role, doing tasks such as faxing discharge information to PCPs, retrieving records from outside facilities, or handling billing functions for the doctors. Those practices should either change the NPPs’ roles or use the money to instead hire clerical help. That would leave money for other purposes, such as creating a more aggressive physician recruiting effort or hiring MDs to moonlight.
Local Factors Govern Economics, Practice
In addition to financial considerations surrounding NPPs, keep in mind licensure. Nurse practitioners are licensed as independent practitioners. Physician assistants are not. The laws governing scope of practice for both of these professionals vary from state to state. Additionally, hospital bylaws govern the boundaries of what NPPs can do without supervision. Two hospitals in the same community might have completely different rules. It is important to understand the state and individual hospital regulations that govern NPPs where you practice.
A PA’s work will nearly always require a physician being physically present during some portion of the patient visit and co-signing chart notes and orders. Nurse practitioners, on the other hand, may be able to perform certain patient-care activities independently. In the latter case, Medicare and other payers typically reimburse at 85% of the rate customarily paid to MDs for the same service.
Patient Perception of NPPs
Patients are increasingly more accepting of NPs and PAs. This seems especially true in settings with clear distinctions between the role of NPP and MD.
For example, my wife is perfectly happy to see a nurse practitioner for routine gynecological care, such as Pap smears. She knows the obstetrician handled the delivery of our children and is available anytime she’s needed.
My neighbor was pleased with his open-heart surgery experience and spoke glowingly of the NP who made rounds daily and assisted during the surgery. He knew the MD surgeon performed most of the operation but left the perioperative care up to the NP.
Patients on a hospitalist service may not see things the same way. My neighbor understood he was hospitalized for the purpose of open-heart surgery done by the MD. He looked at the perioperative care outside of the operation as a secondary issue.
Most medical admissions managed by hospitalists don’t have such clear marquee events in patients’ eyes. So it may be less natural for patients to feel OK about how the hospitalist and NPP divide up care responsibilities. Look at it this way: As hospitalists, we have limited face time with patients, and must make good use of it to establish trust and rapport. When we add an NPP to the care team, we ask patients to develop trust and rapport with two providers instead of just one.
Imagine a patient recently discharged from a hospitalist practice. Her friend asks how it went and which doctor she saw. The patient responds, “I couldn’t figure out who was really in charge of my care. Dr. Nelson’s name was on my armband, but I rarely saw him. Instead, I saw his assistant (the NPP) most of the time.” I suspect that patient will be much less likely to report high levels of satisfaction with her care than one who just saw a hospitalist.
Though I’m concerned that it might be more difficult to keep patients happy when NPPs are part of a hospitalist practice, most practices report this hasn’t been a problem. I’m not suggesting that concern about patient satisfaction means you shouldn’t use NPPs in your hospitalist practices. However, patient satisfaction is an issue to consider when organizing your practice—and an NPP’s role in it—to provide the greatest benefit to your patients. TH
Dr. Nelson has been a practicing hospitalist since 1988 and is co-founder and past president of SHM. He is a principal in Nelson/Flores Associates, a national hospitalist practice management consulting firm. He is also part of the faculty for SHM’s “Best Practices in Managing a Hospital Medicine Program” course. This column represents his views and is not intended to reflect an official position of SHM.
Something Interesting Happened
How did I get myself into this and, more importantly, how could I get out of it?
I could act like I had inadvertently shown up at the wrong room, “so sorry to barge in, I’ll be on my way now.” Or, I could fake an important page that would require me to attend to an “emergency.” Or, I could just tell the group, “look, as much as I’d love to meet with you all for two straight days, I really have more important things to do with my time.”
Problem was I had been part of the decision to call this meeting in the first place. What was I thinking?
For years I’ve sat on a capacity management committee that met frequently and tackled various projects, such as reducing length of stay, discharging patients earlier in the day, and improving the discharge process—all of which fell under the rubric of efficiently moving patients through the system so we could create space for more patients. This not only makes good business, sense but also is good for our patients who benefit from getting out of the hospital earlier and back to the recuperative comfort of their homes.
The committee had met for hours on end, discussing new methods to tackle old problems. What if…we developed a follow-up clinic that could see patients back shortly after discharge, had a discharge nurse whose only responsibility was to discharge patients, had a lounge that could hold discharged patients waiting for a ride, and so on.
Hour after hour, meeting after meeting, we searched for the elusive Rosetta stone that would unlock the mystery of the timely discharge. We often implemented a large intervention, then met again only to find that our glorious idea came up short. We’d scratch our heads, find someone to pin the blame on for these shortcomings and move on to the next doomed project. Ideas were waning, patience was frayed and morale was at an all-time low.
At our wits end we decided to get thinner, reduce waste, make cars.
Ok, not literally make cars but to use the methodology of the Toyota Production System (TPS) to remove waste, to get lean. Sounded like a good idea until I settled into my hardback chair for the meeting that first morning. I quickly was filled with the ominous dread that only results from mixing consultants, a trough full of meeting-issue scrambled eggs congealing over a Sterno flame, and a roomful of sleepy-eyed participants. Sprinkle in a two-day agenda and we had all the ingredients for a scalding caldron of tedium, bubbling over with boredom.
Then something interesting happened.
I became interested.
Our consultants initiated our journey by discussing the basis of lean Toyota production—the theory of Kaizen, or “change (Kai) for the good (Zen).” The essence of the process included multi-day continuous sessions (yikes) utilizing a cross-functional team consisting of leadership and front-line staff from all hospital disciplines—from doctors to nurses to transport to janitorial staff. It also focused on fast, continuous, experimental change.
Then something interesting happened.
We left the room.
A meeting that didn’t meet? What was this strange Japanese system? Well it turns out that another key tenet of the TPS is “gemba,” meaning “shop floor.” The idea is to spend as much time as possible observing the actual processes, out on the shop floor, not in the board room. So, rather than wallowing away in a meeting discussing what we thought the problem was, we actually went to see what the problem was.
We split into teams and were instructed to observe various parts of the discharge process. Specifically, we were charged with differentiating between processes that add value—things people would pay for—and processes that did not add value—things people wouldn’t pay for.
It is estimated that up to 40% of a nurse’s day is spent “nursing” an inefficient system. Any hospitalist who has spent time holding on the phone, chasing down a CT scan report, or scouring the documentation vortex that mysteriously confiscate charts only to just as mysteriously cough them back up 20 minutes later, knows how much time is wasted in a typical day.
Then something interesting happened.
We realized broken systems, not people, were to blame for most of our problems.
After several hours of observation the teams reconvened and discussed their findings. We discovered that efficiently discharging patients earlier in the day could not be accomplished simply by imploring the physicians to write the orders earlier in the day, an intervention that had been continuously failing since I was an intern 12 years earlier.
In fact, the committee discovered there wasn’t a single unifying solution to this problem. Rather, hundreds of gremlins were dwelling within the recesses of our hospital, together gumming up the system. In just one day of observation, our teams identified 70 different contused processes causing our system to hemorrhage inefficiency.
Then something interesting happened.
It was time to go home; our first day was complete.
The second day of Kaizen centered on “tests of change” that could be implemented immediately and then studied for effect. Each group proffered ideas to solve identified problems and then began implementing these changes, taking time to alter the intervention whenever a better method was uncovered.
For example, an inability to timely locate wheelchairs was slowing the transport of discharged patients out of the hospital. This problem was resolved by designating two wheelchairs for this activity alone; a lack of communication with the patient, family and nursing about the timing of discharge was addressed by placing a whiteboard in the room that physicians would use to catalogue the benchmarks for discharge as well as an anticipated discharge date and time; delays in social work planning were tackled by a five-minute “lightning round” between the doctors and the social workers at 8 a.m. every morning; redundant paperwork required to discharge a patient was consolidated.
On and on it went, every additional step exorcising another discharge gremlin.
Then something interesting happened.
We realized the key to efficiency lie not in changing one or two giant unruly processes rather in effecting multiple very small changes.
No one individual or system was to blame for delayed discharges. Years of patches, work-arounds and waste had accumulated in our system like the layers of paint covering the grime on the walls of an old house. We would need to slowly—but surely—chip away at these layers if we were going to achieve our goals. None of us were convinced these immediate changes would solve our problem, but for the first time we felt empowered to make the kind of changes that would lead us to real systems improvement.
Then something interesting happened.
The second day ended. We’d made a ton of progress and I didn’t even need to invoke that fake emergency page. TH
Dr. Glasheen is associate professor of medicine at the University of Colorado Denver, where he serves as director of the hospital mdicine program and the hospitalist Training program, and as associate program director of the Internal Medicine Residency Program.
How did I get myself into this and, more importantly, how could I get out of it?
I could act like I had inadvertently shown up at the wrong room, “so sorry to barge in, I’ll be on my way now.” Or, I could fake an important page that would require me to attend to an “emergency.” Or, I could just tell the group, “look, as much as I’d love to meet with you all for two straight days, I really have more important things to do with my time.”
Problem was I had been part of the decision to call this meeting in the first place. What was I thinking?
For years I’ve sat on a capacity management committee that met frequently and tackled various projects, such as reducing length of stay, discharging patients earlier in the day, and improving the discharge process—all of which fell under the rubric of efficiently moving patients through the system so we could create space for more patients. This not only makes good business, sense but also is good for our patients who benefit from getting out of the hospital earlier and back to the recuperative comfort of their homes.
The committee had met for hours on end, discussing new methods to tackle old problems. What if…we developed a follow-up clinic that could see patients back shortly after discharge, had a discharge nurse whose only responsibility was to discharge patients, had a lounge that could hold discharged patients waiting for a ride, and so on.
Hour after hour, meeting after meeting, we searched for the elusive Rosetta stone that would unlock the mystery of the timely discharge. We often implemented a large intervention, then met again only to find that our glorious idea came up short. We’d scratch our heads, find someone to pin the blame on for these shortcomings and move on to the next doomed project. Ideas were waning, patience was frayed and morale was at an all-time low.
At our wits end we decided to get thinner, reduce waste, make cars.
Ok, not literally make cars but to use the methodology of the Toyota Production System (TPS) to remove waste, to get lean. Sounded like a good idea until I settled into my hardback chair for the meeting that first morning. I quickly was filled with the ominous dread that only results from mixing consultants, a trough full of meeting-issue scrambled eggs congealing over a Sterno flame, and a roomful of sleepy-eyed participants. Sprinkle in a two-day agenda and we had all the ingredients for a scalding caldron of tedium, bubbling over with boredom.
Then something interesting happened.
I became interested.
Our consultants initiated our journey by discussing the basis of lean Toyota production—the theory of Kaizen, or “change (Kai) for the good (Zen).” The essence of the process included multi-day continuous sessions (yikes) utilizing a cross-functional team consisting of leadership and front-line staff from all hospital disciplines—from doctors to nurses to transport to janitorial staff. It also focused on fast, continuous, experimental change.
Then something interesting happened.
We left the room.
A meeting that didn’t meet? What was this strange Japanese system? Well it turns out that another key tenet of the TPS is “gemba,” meaning “shop floor.” The idea is to spend as much time as possible observing the actual processes, out on the shop floor, not in the board room. So, rather than wallowing away in a meeting discussing what we thought the problem was, we actually went to see what the problem was.
We split into teams and were instructed to observe various parts of the discharge process. Specifically, we were charged with differentiating between processes that add value—things people would pay for—and processes that did not add value—things people wouldn’t pay for.
It is estimated that up to 40% of a nurse’s day is spent “nursing” an inefficient system. Any hospitalist who has spent time holding on the phone, chasing down a CT scan report, or scouring the documentation vortex that mysteriously confiscate charts only to just as mysteriously cough them back up 20 minutes later, knows how much time is wasted in a typical day.
Then something interesting happened.
We realized broken systems, not people, were to blame for most of our problems.
After several hours of observation the teams reconvened and discussed their findings. We discovered that efficiently discharging patients earlier in the day could not be accomplished simply by imploring the physicians to write the orders earlier in the day, an intervention that had been continuously failing since I was an intern 12 years earlier.
In fact, the committee discovered there wasn’t a single unifying solution to this problem. Rather, hundreds of gremlins were dwelling within the recesses of our hospital, together gumming up the system. In just one day of observation, our teams identified 70 different contused processes causing our system to hemorrhage inefficiency.
Then something interesting happened.
It was time to go home; our first day was complete.
The second day of Kaizen centered on “tests of change” that could be implemented immediately and then studied for effect. Each group proffered ideas to solve identified problems and then began implementing these changes, taking time to alter the intervention whenever a better method was uncovered.
For example, an inability to timely locate wheelchairs was slowing the transport of discharged patients out of the hospital. This problem was resolved by designating two wheelchairs for this activity alone; a lack of communication with the patient, family and nursing about the timing of discharge was addressed by placing a whiteboard in the room that physicians would use to catalogue the benchmarks for discharge as well as an anticipated discharge date and time; delays in social work planning were tackled by a five-minute “lightning round” between the doctors and the social workers at 8 a.m. every morning; redundant paperwork required to discharge a patient was consolidated.
On and on it went, every additional step exorcising another discharge gremlin.
Then something interesting happened.
We realized the key to efficiency lie not in changing one or two giant unruly processes rather in effecting multiple very small changes.
No one individual or system was to blame for delayed discharges. Years of patches, work-arounds and waste had accumulated in our system like the layers of paint covering the grime on the walls of an old house. We would need to slowly—but surely—chip away at these layers if we were going to achieve our goals. None of us were convinced these immediate changes would solve our problem, but for the first time we felt empowered to make the kind of changes that would lead us to real systems improvement.
Then something interesting happened.
The second day ended. We’d made a ton of progress and I didn’t even need to invoke that fake emergency page. TH
Dr. Glasheen is associate professor of medicine at the University of Colorado Denver, where he serves as director of the hospital mdicine program and the hospitalist Training program, and as associate program director of the Internal Medicine Residency Program.
How did I get myself into this and, more importantly, how could I get out of it?
I could act like I had inadvertently shown up at the wrong room, “so sorry to barge in, I’ll be on my way now.” Or, I could fake an important page that would require me to attend to an “emergency.” Or, I could just tell the group, “look, as much as I’d love to meet with you all for two straight days, I really have more important things to do with my time.”
Problem was I had been part of the decision to call this meeting in the first place. What was I thinking?
For years I’ve sat on a capacity management committee that met frequently and tackled various projects, such as reducing length of stay, discharging patients earlier in the day, and improving the discharge process—all of which fell under the rubric of efficiently moving patients through the system so we could create space for more patients. This not only makes good business, sense but also is good for our patients who benefit from getting out of the hospital earlier and back to the recuperative comfort of their homes.
The committee had met for hours on end, discussing new methods to tackle old problems. What if…we developed a follow-up clinic that could see patients back shortly after discharge, had a discharge nurse whose only responsibility was to discharge patients, had a lounge that could hold discharged patients waiting for a ride, and so on.
Hour after hour, meeting after meeting, we searched for the elusive Rosetta stone that would unlock the mystery of the timely discharge. We often implemented a large intervention, then met again only to find that our glorious idea came up short. We’d scratch our heads, find someone to pin the blame on for these shortcomings and move on to the next doomed project. Ideas were waning, patience was frayed and morale was at an all-time low.
At our wits end we decided to get thinner, reduce waste, make cars.
Ok, not literally make cars but to use the methodology of the Toyota Production System (TPS) to remove waste, to get lean. Sounded like a good idea until I settled into my hardback chair for the meeting that first morning. I quickly was filled with the ominous dread that only results from mixing consultants, a trough full of meeting-issue scrambled eggs congealing over a Sterno flame, and a roomful of sleepy-eyed participants. Sprinkle in a two-day agenda and we had all the ingredients for a scalding caldron of tedium, bubbling over with boredom.
Then something interesting happened.
I became interested.
Our consultants initiated our journey by discussing the basis of lean Toyota production—the theory of Kaizen, or “change (Kai) for the good (Zen).” The essence of the process included multi-day continuous sessions (yikes) utilizing a cross-functional team consisting of leadership and front-line staff from all hospital disciplines—from doctors to nurses to transport to janitorial staff. It also focused on fast, continuous, experimental change.
Then something interesting happened.
We left the room.
A meeting that didn’t meet? What was this strange Japanese system? Well it turns out that another key tenet of the TPS is “gemba,” meaning “shop floor.” The idea is to spend as much time as possible observing the actual processes, out on the shop floor, not in the board room. So, rather than wallowing away in a meeting discussing what we thought the problem was, we actually went to see what the problem was.
We split into teams and were instructed to observe various parts of the discharge process. Specifically, we were charged with differentiating between processes that add value—things people would pay for—and processes that did not add value—things people wouldn’t pay for.
It is estimated that up to 40% of a nurse’s day is spent “nursing” an inefficient system. Any hospitalist who has spent time holding on the phone, chasing down a CT scan report, or scouring the documentation vortex that mysteriously confiscate charts only to just as mysteriously cough them back up 20 minutes later, knows how much time is wasted in a typical day.
Then something interesting happened.
We realized broken systems, not people, were to blame for most of our problems.
After several hours of observation the teams reconvened and discussed their findings. We discovered that efficiently discharging patients earlier in the day could not be accomplished simply by imploring the physicians to write the orders earlier in the day, an intervention that had been continuously failing since I was an intern 12 years earlier.
In fact, the committee discovered there wasn’t a single unifying solution to this problem. Rather, hundreds of gremlins were dwelling within the recesses of our hospital, together gumming up the system. In just one day of observation, our teams identified 70 different contused processes causing our system to hemorrhage inefficiency.
Then something interesting happened.
It was time to go home; our first day was complete.
The second day of Kaizen centered on “tests of change” that could be implemented immediately and then studied for effect. Each group proffered ideas to solve identified problems and then began implementing these changes, taking time to alter the intervention whenever a better method was uncovered.
For example, an inability to timely locate wheelchairs was slowing the transport of discharged patients out of the hospital. This problem was resolved by designating two wheelchairs for this activity alone; a lack of communication with the patient, family and nursing about the timing of discharge was addressed by placing a whiteboard in the room that physicians would use to catalogue the benchmarks for discharge as well as an anticipated discharge date and time; delays in social work planning were tackled by a five-minute “lightning round” between the doctors and the social workers at 8 a.m. every morning; redundant paperwork required to discharge a patient was consolidated.
On and on it went, every additional step exorcising another discharge gremlin.
Then something interesting happened.
We realized the key to efficiency lie not in changing one or two giant unruly processes rather in effecting multiple very small changes.
No one individual or system was to blame for delayed discharges. Years of patches, work-arounds and waste had accumulated in our system like the layers of paint covering the grime on the walls of an old house. We would need to slowly—but surely—chip away at these layers if we were going to achieve our goals. None of us were convinced these immediate changes would solve our problem, but for the first time we felt empowered to make the kind of changes that would lead us to real systems improvement.
Then something interesting happened.
The second day ended. We’d made a ton of progress and I didn’t even need to invoke that fake emergency page. TH
Dr. Glasheen is associate professor of medicine at the University of Colorado Denver, where he serves as director of the hospital mdicine program and the hospitalist Training program, and as associate program director of the Internal Medicine Residency Program.
A Gift Giving Guide for Hospitalists
As the holiday season fast approaches, our minds increasingly turn to gift giving. This season, I have given extensive thought to the needs of hospitalists, so if you are looking for that perfect gift, look no further.
I know that many of you have gift advice as well, so don’t hesitate to e-mail me your ideas. I will include those in a future column.
Without further delay, here are the Top 10 Gift Ideas for Hospitalists:
1. Improved Vital Sign Alert System: Nothing bothers a hospitalist more than a false alarm or, worse, no alert at all. Hospitalists need better knowledge of vital sign changes, particularly one that takes into effect rate of change.
2. Accurate respiratory rate: This is for the hospitalist who is particularly perturbed about the fact that 90 percent of one’s patients have a respiratory rate of 18. If you haven’t seen the new technology by Hoana Medical, check it out. If this technology pans out, my wish list would be for every hospitalist patient to be monitored and the respiratory rate tied into a sophisticated alert system.
3. An army of physical therapists: This will be hard to gift wrap, but given the fact that deconditioning sets in so fast in hospitalized patients, particularly in elderly patients, this gift is aimed at getting patients moving and preventing the deconditioning from starting.
4. Better nutrition: There is no greater cliché than hospital food. Any improvement would be appreciated. This is not only for the patients, but the physicians, as well. Too many hospitals have a plethora of fast food options, instead of a focus on wholesome choices.
5. Palliative Care: The ideal gift is fairly comprehensive, but it can be separated into several gifts over time. The first and easiest is teaching hospitalists palliative care skills. The second phase is having hospital personnel correctly identify--at admission--patients who need palliative care. The final phase, and the most elusive, is improving physician knowledge of which patients are good candidates for palliation and the correct action steps. This last gift is aimed at the hospitalist who has just admitted a severely demented bed bound patient with AIDs who is still on antiretroviral therapy but no hospice care. Or perhaps the patient with severe CHF admitted for the fifth time this month that is on the correct medications and does weigh himself daily, but also without hospice, or worse, no family understanding that he is dying.
6. More data to understand your practice: This is self explanatory, but many hospitalists are unable to obtain the clinical or financial data they need to understand their practice. Sometimes the data is present, but a better explanation of the data is necessary.
7. EMR that incorporates ALL data: If you can find this gift, please contact me immediately. I am willing to pay top dollar. The Information Technology (IT) department tells me it exists, but I have never actually put my hands on it. This is a single sign-on Electronic Medical Record that has all the clinical data that a hospitalist needs presented in an intuitive interface. And, it’s easily accessible from outside the hospital network.
8. Networked EMR: Link all those EMRs to hospitals across the country.
9. Comprehensive approach to delirium in hospitalized patients: This is for the hospitalist who has just spent the last two hours stopping all delirium provoking medications in a post-surgical elderly patient and talked at length to the family about the fact that this altered mentation is not permanent, not a stroke, and, yes, the anti-psychotic medications are the best medicine.
10. Maintenance of correct attending: This is for the hospitalist whose 15 patients all have a different attending assigned. This gift would ensure that the IT system has my name attached to all my patients at all times. Again, if you receive this gift, please contact me immediately.
In the spirit of being a tad more comprehensive, here are a few gift ideas for hospital medicine in general:
- More primary care physicians. Hospitalists know good primary care prevents hospitalization, but having a physician to refer a patient to after discharge is key.
- Hospital coverage for all U.S. citizens.
- A full understanding of hospital medicine by all hospital administrators.
- Uniform assessment of hospitals, so one can accurately grade/compare hospitals.
- Continued improvement in hospitalist leader skills.
- Continued better pay for hospitalists.
- More mid-level providers in hospital medicine.
- Continued improvement in teamwork amongst hospital personnel.
- Continued improvement in quality and patient safety.
And finally, the best gift of all…more hospitalists!
Happy Holidays! TH
Dr. Cawley is president of SHM.
As the holiday season fast approaches, our minds increasingly turn to gift giving. This season, I have given extensive thought to the needs of hospitalists, so if you are looking for that perfect gift, look no further.
I know that many of you have gift advice as well, so don’t hesitate to e-mail me your ideas. I will include those in a future column.
Without further delay, here are the Top 10 Gift Ideas for Hospitalists:
1. Improved Vital Sign Alert System: Nothing bothers a hospitalist more than a false alarm or, worse, no alert at all. Hospitalists need better knowledge of vital sign changes, particularly one that takes into effect rate of change.
2. Accurate respiratory rate: This is for the hospitalist who is particularly perturbed about the fact that 90 percent of one’s patients have a respiratory rate of 18. If you haven’t seen the new technology by Hoana Medical, check it out. If this technology pans out, my wish list would be for every hospitalist patient to be monitored and the respiratory rate tied into a sophisticated alert system.
3. An army of physical therapists: This will be hard to gift wrap, but given the fact that deconditioning sets in so fast in hospitalized patients, particularly in elderly patients, this gift is aimed at getting patients moving and preventing the deconditioning from starting.
4. Better nutrition: There is no greater cliché than hospital food. Any improvement would be appreciated. This is not only for the patients, but the physicians, as well. Too many hospitals have a plethora of fast food options, instead of a focus on wholesome choices.
5. Palliative Care: The ideal gift is fairly comprehensive, but it can be separated into several gifts over time. The first and easiest is teaching hospitalists palliative care skills. The second phase is having hospital personnel correctly identify--at admission--patients who need palliative care. The final phase, and the most elusive, is improving physician knowledge of which patients are good candidates for palliation and the correct action steps. This last gift is aimed at the hospitalist who has just admitted a severely demented bed bound patient with AIDs who is still on antiretroviral therapy but no hospice care. Or perhaps the patient with severe CHF admitted for the fifth time this month that is on the correct medications and does weigh himself daily, but also without hospice, or worse, no family understanding that he is dying.
6. More data to understand your practice: This is self explanatory, but many hospitalists are unable to obtain the clinical or financial data they need to understand their practice. Sometimes the data is present, but a better explanation of the data is necessary.
7. EMR that incorporates ALL data: If you can find this gift, please contact me immediately. I am willing to pay top dollar. The Information Technology (IT) department tells me it exists, but I have never actually put my hands on it. This is a single sign-on Electronic Medical Record that has all the clinical data that a hospitalist needs presented in an intuitive interface. And, it’s easily accessible from outside the hospital network.
8. Networked EMR: Link all those EMRs to hospitals across the country.
9. Comprehensive approach to delirium in hospitalized patients: This is for the hospitalist who has just spent the last two hours stopping all delirium provoking medications in a post-surgical elderly patient and talked at length to the family about the fact that this altered mentation is not permanent, not a stroke, and, yes, the anti-psychotic medications are the best medicine.
10. Maintenance of correct attending: This is for the hospitalist whose 15 patients all have a different attending assigned. This gift would ensure that the IT system has my name attached to all my patients at all times. Again, if you receive this gift, please contact me immediately.
In the spirit of being a tad more comprehensive, here are a few gift ideas for hospital medicine in general:
- More primary care physicians. Hospitalists know good primary care prevents hospitalization, but having a physician to refer a patient to after discharge is key.
- Hospital coverage for all U.S. citizens.
- A full understanding of hospital medicine by all hospital administrators.
- Uniform assessment of hospitals, so one can accurately grade/compare hospitals.
- Continued improvement in hospitalist leader skills.
- Continued better pay for hospitalists.
- More mid-level providers in hospital medicine.
- Continued improvement in teamwork amongst hospital personnel.
- Continued improvement in quality and patient safety.
And finally, the best gift of all…more hospitalists!
Happy Holidays! TH
Dr. Cawley is president of SHM.
As the holiday season fast approaches, our minds increasingly turn to gift giving. This season, I have given extensive thought to the needs of hospitalists, so if you are looking for that perfect gift, look no further.
I know that many of you have gift advice as well, so don’t hesitate to e-mail me your ideas. I will include those in a future column.
Without further delay, here are the Top 10 Gift Ideas for Hospitalists:
1. Improved Vital Sign Alert System: Nothing bothers a hospitalist more than a false alarm or, worse, no alert at all. Hospitalists need better knowledge of vital sign changes, particularly one that takes into effect rate of change.
2. Accurate respiratory rate: This is for the hospitalist who is particularly perturbed about the fact that 90 percent of one’s patients have a respiratory rate of 18. If you haven’t seen the new technology by Hoana Medical, check it out. If this technology pans out, my wish list would be for every hospitalist patient to be monitored and the respiratory rate tied into a sophisticated alert system.
3. An army of physical therapists: This will be hard to gift wrap, but given the fact that deconditioning sets in so fast in hospitalized patients, particularly in elderly patients, this gift is aimed at getting patients moving and preventing the deconditioning from starting.
4. Better nutrition: There is no greater cliché than hospital food. Any improvement would be appreciated. This is not only for the patients, but the physicians, as well. Too many hospitals have a plethora of fast food options, instead of a focus on wholesome choices.
5. Palliative Care: The ideal gift is fairly comprehensive, but it can be separated into several gifts over time. The first and easiest is teaching hospitalists palliative care skills. The second phase is having hospital personnel correctly identify--at admission--patients who need palliative care. The final phase, and the most elusive, is improving physician knowledge of which patients are good candidates for palliation and the correct action steps. This last gift is aimed at the hospitalist who has just admitted a severely demented bed bound patient with AIDs who is still on antiretroviral therapy but no hospice care. Or perhaps the patient with severe CHF admitted for the fifth time this month that is on the correct medications and does weigh himself daily, but also without hospice, or worse, no family understanding that he is dying.
6. More data to understand your practice: This is self explanatory, but many hospitalists are unable to obtain the clinical or financial data they need to understand their practice. Sometimes the data is present, but a better explanation of the data is necessary.
7. EMR that incorporates ALL data: If you can find this gift, please contact me immediately. I am willing to pay top dollar. The Information Technology (IT) department tells me it exists, but I have never actually put my hands on it. This is a single sign-on Electronic Medical Record that has all the clinical data that a hospitalist needs presented in an intuitive interface. And, it’s easily accessible from outside the hospital network.
8. Networked EMR: Link all those EMRs to hospitals across the country.
9. Comprehensive approach to delirium in hospitalized patients: This is for the hospitalist who has just spent the last two hours stopping all delirium provoking medications in a post-surgical elderly patient and talked at length to the family about the fact that this altered mentation is not permanent, not a stroke, and, yes, the anti-psychotic medications are the best medicine.
10. Maintenance of correct attending: This is for the hospitalist whose 15 patients all have a different attending assigned. This gift would ensure that the IT system has my name attached to all my patients at all times. Again, if you receive this gift, please contact me immediately.
In the spirit of being a tad more comprehensive, here are a few gift ideas for hospital medicine in general:
- More primary care physicians. Hospitalists know good primary care prevents hospitalization, but having a physician to refer a patient to after discharge is key.
- Hospital coverage for all U.S. citizens.
- A full understanding of hospital medicine by all hospital administrators.
- Uniform assessment of hospitals, so one can accurately grade/compare hospitals.
- Continued improvement in hospitalist leader skills.
- Continued better pay for hospitalists.
- More mid-level providers in hospital medicine.
- Continued improvement in teamwork amongst hospital personnel.
- Continued improvement in quality and patient safety.
And finally, the best gift of all…more hospitalists!
Happy Holidays! TH
Dr. Cawley is president of SHM.
Moving into the Future
The young specialty of hospital medicine has an even younger sibling—pediatric hospital medicine. “Just seven years ago, when I put on my pediatric hospitalist badge, people would ask me, ‘What is that?’” Douglas Carlson, MD, an associate professor at the Washington University School of Medicine in St. Louis, says. “They don’t do that anymore.”
Times certainly are a changing.
With an estimated 1,500 practitioners, pediatric hospitalists make up about 9% of the total hospitalist workforce in the United States. Growth in the pediatric field has been fueled by the need for expertise in treating hospitalized pediatric patients, the increasing complexity of hospitalized cases, mandates to reduce hospital costs and readmission rates, and the curtailment of resident hours.
“The biggest thing is the whole field is blossoming,” says SHM treasurer Jack Percelay, MD.
What Lies Ahead?
Pediatric hospital medicine may be young in years, but the primary focus is on the future. Such was the theme of the Pediatric Hospital Medicine Conference held by SHM, the American Academy of Pediatrics and the Academic Pediatrics Association in July in Denver.
“We are responsible for the future of hospital medicine,” keynote speaker Sanford Melzer, MD, of the University of Washington and Children’s Memorial Hospital and Regional Medical Center of Seattle says in an interview with The Hospitalist. “So what should that future look like?”
Pediatric hospitalists are in an ideal position to improve care, Dr. Melzer said. He outlined six crucial areas for action:
- Set standards of evidence-based patient care in areas not historically addressed, such as feeding tubes and severe reflux;
- Implement safety standards for issues such as medical errors, blood infections and hand-offs to other providers;
- Develop leaders who will work to bring about these changes;
- Stabilize the workforce by better defining pediatric hospital medicine as a career path;
- Create value for hospitals;
- Promote a holistic view of hospital care as a small part of the continuum of care for chronically ill children.
As Dr. Melzer succinctly puts it, “I am here to improve the whole system, not just to give kids meds and get them out.”
“I think our evolution in hospital medicine will follow that of ER physicians very closely,” says Dr. Carlson, who started out as an ER doctor. “Within 30 years, with fellowships and training, their specialty evolved.”
A similar progression is occurring with pediatric hospitalists. Carlson said he remembers when hospitalists would complain that their colleagues in other subspecialties would “treat them like glorified residents.” Not anymore. “We are now seen as equals,” he said.
Pediatric hospitalists bring a lot to the table, Dr. Carlson said, such as broad experience in treating acutely or chronically ill hospitalized children; the ability to coordinate care; knowledge in negotiating hospital routines and protocols; and the capacity to manage family fears.
But to survive and prosper, pediatric hospitalists must create value for their institutions, Dr. Melzer said. And value is exactly what evidence-based medicine can generate, he added. Establishing evidence-based guidelines for the treatment of the 10 most common conditions affecting 80% of patients would be a huge step forward in improving patient care, Melzer explained. It would create value for patients and, ultimately, the hospital.
Lending an Ear
Communication is another key, and can be particularly important in caring for children with life-threatening or terminal illnesses, said another speaker, Margaret Hood, MD, of Orlando Healthcare and Palliative Healthcare. Listening to patients and their families is a critical part of end-of-life care.
“The palliative care offered by pediatric hospitalists becomes a lifeline to patients and their families,” she explained. “Sensitive communications can foster hope, even when the news is bad.”
Dr. Hood told the poignant story of a baby born with a lethal heart problem. “I asked her parents, “What do you want?” she said. “They told me, ‘We want her heart to get better.’”
The doctor—and the family—knew that the baby would never get better. “What else would you like?” she asked. “To hold my little girl,” the mother answered. “I have only held her twice in two months.”
“We can do that.” Dr. Hood quickly replied.
Hope comes in many forms—this time in a mother’s arms, as she finally held her daughter before she died.
Frank Talk on Stress and Career Satisfaction
The 24/7 connection hospitalists have with their institutions is the basis for much of their expertise. Then again, that same 24/7 connection can be a source of extraordinary stress.
“It is variable work, with highs and lows in volume and in unscheduled care,” Dr. Carlson explained. “For hospitalized patients, we always need call coverage. That means odd hours—or being on-call in odd hours. It means night work or evening work. Stress carries risks of unplanned turnover, absenteeism, judgment and action errors, conflicts with colleagues, physical illness and mental fatigue.
“Hospitalists may be burning out even quicker than those in other specialties,” Dr. Carlson added. “Hospitalists love clinical care, they love what they do, but they are working in an environment where they must do more and more. We have to learn how to balance enthusiasm for taking care of patients with the demands of the job.”
One area of concern among hospitals and their pediatric hospitalists is workforce stability. Young women make up the majority of the workforce, and hospitals are “dealing continuously with women who are having families,” Dr. Melzer said.
“I have some people using it as a stepping stone to other specialties,” Dr. Carlson said. “They work as pediatric hospitalists while children are young, for flexibility.”
Both Carlson and Melzer believe a sharper definition of the pediatric hospital medicine career track would make a difference. “How do we get others in the hospital to make this job satisfactory?” Dr. Carlson asked. “Hospitalists enjoy the work, but want to balance it … and make a career out of this.”
Recognition from other medical colleagues is critical to job satisfaction. More and more, pediatric hospitalists are playing key leadership roles. “We are increasingly seen as the experts for hospitalized patients,” Dr. Carlson said. “I believe we can do things better than many specialists and many generalists, because we know how hospitals work—and we are there all the time.”
What’s Next?
Implementing plans for the future of pediatric hospital medicine will require collaboration among the many specialists and groups involved in the care of children. Dr. Melzer suggests convening a “leadership summit” for representatives from all of these associations.
Dr. Percelay agreed.
“It’s exciting,” he says. “The fact that the president of the American Board of Pediatrics came and spoke to our community, along with the presidents of SHM, the American Academy of Pediatrics, and the Academic Pediatric Association, is testimony to the role we are playing in the care of hospitalized children in the United States. We need to take a lot of care to make sure we maintain links between pediatric hospitalists and primary care pediatricians.”
That would be in the best interests of all children. TH
Carol Berczuk’s is a medical journalist based in New York.
The young specialty of hospital medicine has an even younger sibling—pediatric hospital medicine. “Just seven years ago, when I put on my pediatric hospitalist badge, people would ask me, ‘What is that?’” Douglas Carlson, MD, an associate professor at the Washington University School of Medicine in St. Louis, says. “They don’t do that anymore.”
Times certainly are a changing.
With an estimated 1,500 practitioners, pediatric hospitalists make up about 9% of the total hospitalist workforce in the United States. Growth in the pediatric field has been fueled by the need for expertise in treating hospitalized pediatric patients, the increasing complexity of hospitalized cases, mandates to reduce hospital costs and readmission rates, and the curtailment of resident hours.
“The biggest thing is the whole field is blossoming,” says SHM treasurer Jack Percelay, MD.
What Lies Ahead?
Pediatric hospital medicine may be young in years, but the primary focus is on the future. Such was the theme of the Pediatric Hospital Medicine Conference held by SHM, the American Academy of Pediatrics and the Academic Pediatrics Association in July in Denver.
“We are responsible for the future of hospital medicine,” keynote speaker Sanford Melzer, MD, of the University of Washington and Children’s Memorial Hospital and Regional Medical Center of Seattle says in an interview with The Hospitalist. “So what should that future look like?”
Pediatric hospitalists are in an ideal position to improve care, Dr. Melzer said. He outlined six crucial areas for action:
- Set standards of evidence-based patient care in areas not historically addressed, such as feeding tubes and severe reflux;
- Implement safety standards for issues such as medical errors, blood infections and hand-offs to other providers;
- Develop leaders who will work to bring about these changes;
- Stabilize the workforce by better defining pediatric hospital medicine as a career path;
- Create value for hospitals;
- Promote a holistic view of hospital care as a small part of the continuum of care for chronically ill children.
As Dr. Melzer succinctly puts it, “I am here to improve the whole system, not just to give kids meds and get them out.”
“I think our evolution in hospital medicine will follow that of ER physicians very closely,” says Dr. Carlson, who started out as an ER doctor. “Within 30 years, with fellowships and training, their specialty evolved.”
A similar progression is occurring with pediatric hospitalists. Carlson said he remembers when hospitalists would complain that their colleagues in other subspecialties would “treat them like glorified residents.” Not anymore. “We are now seen as equals,” he said.
Pediatric hospitalists bring a lot to the table, Dr. Carlson said, such as broad experience in treating acutely or chronically ill hospitalized children; the ability to coordinate care; knowledge in negotiating hospital routines and protocols; and the capacity to manage family fears.
But to survive and prosper, pediatric hospitalists must create value for their institutions, Dr. Melzer said. And value is exactly what evidence-based medicine can generate, he added. Establishing evidence-based guidelines for the treatment of the 10 most common conditions affecting 80% of patients would be a huge step forward in improving patient care, Melzer explained. It would create value for patients and, ultimately, the hospital.
Lending an Ear
Communication is another key, and can be particularly important in caring for children with life-threatening or terminal illnesses, said another speaker, Margaret Hood, MD, of Orlando Healthcare and Palliative Healthcare. Listening to patients and their families is a critical part of end-of-life care.
“The palliative care offered by pediatric hospitalists becomes a lifeline to patients and their families,” she explained. “Sensitive communications can foster hope, even when the news is bad.”
Dr. Hood told the poignant story of a baby born with a lethal heart problem. “I asked her parents, “What do you want?” she said. “They told me, ‘We want her heart to get better.’”
The doctor—and the family—knew that the baby would never get better. “What else would you like?” she asked. “To hold my little girl,” the mother answered. “I have only held her twice in two months.”
“We can do that.” Dr. Hood quickly replied.
Hope comes in many forms—this time in a mother’s arms, as she finally held her daughter before she died.
Frank Talk on Stress and Career Satisfaction
The 24/7 connection hospitalists have with their institutions is the basis for much of their expertise. Then again, that same 24/7 connection can be a source of extraordinary stress.
“It is variable work, with highs and lows in volume and in unscheduled care,” Dr. Carlson explained. “For hospitalized patients, we always need call coverage. That means odd hours—or being on-call in odd hours. It means night work or evening work. Stress carries risks of unplanned turnover, absenteeism, judgment and action errors, conflicts with colleagues, physical illness and mental fatigue.
“Hospitalists may be burning out even quicker than those in other specialties,” Dr. Carlson added. “Hospitalists love clinical care, they love what they do, but they are working in an environment where they must do more and more. We have to learn how to balance enthusiasm for taking care of patients with the demands of the job.”
One area of concern among hospitals and their pediatric hospitalists is workforce stability. Young women make up the majority of the workforce, and hospitals are “dealing continuously with women who are having families,” Dr. Melzer said.
“I have some people using it as a stepping stone to other specialties,” Dr. Carlson said. “They work as pediatric hospitalists while children are young, for flexibility.”
Both Carlson and Melzer believe a sharper definition of the pediatric hospital medicine career track would make a difference. “How do we get others in the hospital to make this job satisfactory?” Dr. Carlson asked. “Hospitalists enjoy the work, but want to balance it … and make a career out of this.”
Recognition from other medical colleagues is critical to job satisfaction. More and more, pediatric hospitalists are playing key leadership roles. “We are increasingly seen as the experts for hospitalized patients,” Dr. Carlson said. “I believe we can do things better than many specialists and many generalists, because we know how hospitals work—and we are there all the time.”
What’s Next?
Implementing plans for the future of pediatric hospital medicine will require collaboration among the many specialists and groups involved in the care of children. Dr. Melzer suggests convening a “leadership summit” for representatives from all of these associations.
Dr. Percelay agreed.
“It’s exciting,” he says. “The fact that the president of the American Board of Pediatrics came and spoke to our community, along with the presidents of SHM, the American Academy of Pediatrics, and the Academic Pediatric Association, is testimony to the role we are playing in the care of hospitalized children in the United States. We need to take a lot of care to make sure we maintain links between pediatric hospitalists and primary care pediatricians.”
That would be in the best interests of all children. TH
Carol Berczuk’s is a medical journalist based in New York.
The young specialty of hospital medicine has an even younger sibling—pediatric hospital medicine. “Just seven years ago, when I put on my pediatric hospitalist badge, people would ask me, ‘What is that?’” Douglas Carlson, MD, an associate professor at the Washington University School of Medicine in St. Louis, says. “They don’t do that anymore.”
Times certainly are a changing.
With an estimated 1,500 practitioners, pediatric hospitalists make up about 9% of the total hospitalist workforce in the United States. Growth in the pediatric field has been fueled by the need for expertise in treating hospitalized pediatric patients, the increasing complexity of hospitalized cases, mandates to reduce hospital costs and readmission rates, and the curtailment of resident hours.
“The biggest thing is the whole field is blossoming,” says SHM treasurer Jack Percelay, MD.
What Lies Ahead?
Pediatric hospital medicine may be young in years, but the primary focus is on the future. Such was the theme of the Pediatric Hospital Medicine Conference held by SHM, the American Academy of Pediatrics and the Academic Pediatrics Association in July in Denver.
“We are responsible for the future of hospital medicine,” keynote speaker Sanford Melzer, MD, of the University of Washington and Children’s Memorial Hospital and Regional Medical Center of Seattle says in an interview with The Hospitalist. “So what should that future look like?”
Pediatric hospitalists are in an ideal position to improve care, Dr. Melzer said. He outlined six crucial areas for action:
- Set standards of evidence-based patient care in areas not historically addressed, such as feeding tubes and severe reflux;
- Implement safety standards for issues such as medical errors, blood infections and hand-offs to other providers;
- Develop leaders who will work to bring about these changes;
- Stabilize the workforce by better defining pediatric hospital medicine as a career path;
- Create value for hospitals;
- Promote a holistic view of hospital care as a small part of the continuum of care for chronically ill children.
As Dr. Melzer succinctly puts it, “I am here to improve the whole system, not just to give kids meds and get them out.”
“I think our evolution in hospital medicine will follow that of ER physicians very closely,” says Dr. Carlson, who started out as an ER doctor. “Within 30 years, with fellowships and training, their specialty evolved.”
A similar progression is occurring with pediatric hospitalists. Carlson said he remembers when hospitalists would complain that their colleagues in other subspecialties would “treat them like glorified residents.” Not anymore. “We are now seen as equals,” he said.
Pediatric hospitalists bring a lot to the table, Dr. Carlson said, such as broad experience in treating acutely or chronically ill hospitalized children; the ability to coordinate care; knowledge in negotiating hospital routines and protocols; and the capacity to manage family fears.
But to survive and prosper, pediatric hospitalists must create value for their institutions, Dr. Melzer said. And value is exactly what evidence-based medicine can generate, he added. Establishing evidence-based guidelines for the treatment of the 10 most common conditions affecting 80% of patients would be a huge step forward in improving patient care, Melzer explained. It would create value for patients and, ultimately, the hospital.
Lending an Ear
Communication is another key, and can be particularly important in caring for children with life-threatening or terminal illnesses, said another speaker, Margaret Hood, MD, of Orlando Healthcare and Palliative Healthcare. Listening to patients and their families is a critical part of end-of-life care.
“The palliative care offered by pediatric hospitalists becomes a lifeline to patients and their families,” she explained. “Sensitive communications can foster hope, even when the news is bad.”
Dr. Hood told the poignant story of a baby born with a lethal heart problem. “I asked her parents, “What do you want?” she said. “They told me, ‘We want her heart to get better.’”
The doctor—and the family—knew that the baby would never get better. “What else would you like?” she asked. “To hold my little girl,” the mother answered. “I have only held her twice in two months.”
“We can do that.” Dr. Hood quickly replied.
Hope comes in many forms—this time in a mother’s arms, as she finally held her daughter before she died.
Frank Talk on Stress and Career Satisfaction
The 24/7 connection hospitalists have with their institutions is the basis for much of their expertise. Then again, that same 24/7 connection can be a source of extraordinary stress.
“It is variable work, with highs and lows in volume and in unscheduled care,” Dr. Carlson explained. “For hospitalized patients, we always need call coverage. That means odd hours—or being on-call in odd hours. It means night work or evening work. Stress carries risks of unplanned turnover, absenteeism, judgment and action errors, conflicts with colleagues, physical illness and mental fatigue.
“Hospitalists may be burning out even quicker than those in other specialties,” Dr. Carlson added. “Hospitalists love clinical care, they love what they do, but they are working in an environment where they must do more and more. We have to learn how to balance enthusiasm for taking care of patients with the demands of the job.”
One area of concern among hospitals and their pediatric hospitalists is workforce stability. Young women make up the majority of the workforce, and hospitals are “dealing continuously with women who are having families,” Dr. Melzer said.
“I have some people using it as a stepping stone to other specialties,” Dr. Carlson said. “They work as pediatric hospitalists while children are young, for flexibility.”
Both Carlson and Melzer believe a sharper definition of the pediatric hospital medicine career track would make a difference. “How do we get others in the hospital to make this job satisfactory?” Dr. Carlson asked. “Hospitalists enjoy the work, but want to balance it … and make a career out of this.”
Recognition from other medical colleagues is critical to job satisfaction. More and more, pediatric hospitalists are playing key leadership roles. “We are increasingly seen as the experts for hospitalized patients,” Dr. Carlson said. “I believe we can do things better than many specialists and many generalists, because we know how hospitals work—and we are there all the time.”
What’s Next?
Implementing plans for the future of pediatric hospital medicine will require collaboration among the many specialists and groups involved in the care of children. Dr. Melzer suggests convening a “leadership summit” for representatives from all of these associations.
Dr. Percelay agreed.
“It’s exciting,” he says. “The fact that the president of the American Board of Pediatrics came and spoke to our community, along with the presidents of SHM, the American Academy of Pediatrics, and the Academic Pediatric Association, is testimony to the role we are playing in the care of hospitalized children in the United States. We need to take a lot of care to make sure we maintain links between pediatric hospitalists and primary care pediatricians.”
That would be in the best interests of all children. TH
Carol Berczuk’s is a medical journalist based in New York.
The Religious Divide
Several years ago, a patient at Virtua West Jersey Hospital Marlton, in Marlton, N.J., was diagnosed with metastatic colon cancer with spinal metastases. The patient was septic, bleeding from a spinal wound, and was experiencing kidney failure. Hospitalists recommended stopping treatment and moving the patient to hospice care. The patient’s family refused, and told hospitalists that, according to their Christian faith, suffering was the only true path to heaven. Hospitalists kept the patient as comfortable as possible, but blood pressure problems and hypotension made it difficult for them to administer pain medication.
Hospitalists held numerous meetings with the family and medical and nursing staff to discuss the ethical implications of the situation. Two months later, the patient suffered cardiac arrest and died.
“The medical staff and family were continuously at odds because the patient was suffering so much,” says Marianne Holler, DO, a hospitalist at University of Medicine and Dentistry of New Jersey School of Osteopathic Medicine, who was part of the patient’s medical team. “We were never able to discontinue life support throughout [the patient’s] hospital stay.”
Whether planning a routine procedure or end-of-life care, hospitalists may be called into religious discussions with patients, their families, spiritual advisors, and hospital chaplains. While many hospitalists have received ethics and other professional training to prepare them for these conversations, some say the intersection of religion and medicine remains a challenging and multifaceted aspect of their practice.
—The Rev. Peter Yuichi Clark, PhD, Alta Bates Summit Medical Center, Berkeley, Calif.
A Hospitalist’s Belief
Hospitalists’ brief relationships with patients may influence the degree of knowledge they have about an individual’s religious beliefs, says Scott Enderby, DO, a hospitalist at Alta Bates Summit Medical Center in Berkeley, Calif. Over the years, primary care physicians may become less involved with a patient’s acute medical needs as they use hospitalist services to manage their inpatients, Dr. Enderby says. This means hospitalists must discuss patients’ wishes regarding code status and resuscitation, end-of-life care, and other necessary treatments.
When discussing religion and treatment, hospitalists must put aside their personal beliefs, and this may not always be easy, says Dr. Thomas McIlraith, MD, medical director of Hospital Medicine at Mercy Medical Group in Sacramento, Calif. Dr. McIlraith recalls a Jehovah’s Witness patient who cited religious beliefs when refusing a blood transfusion following a massive post-partum hemorrhage. The patient was severely anemic, and her hemoglobin levels plunged dangerously to 2 gm/dL. Leaders from the patient’s church asked Dr. McIlraith to try hemoglobin substitutes, but he was unable to do so because these substitutes still were experimental and associated with significant complications, he says.
Dr. McIlraith had to act fast. He instructed the obstetrician on the case to stop drawing hemoglobin levels; the patient needed every drop of blood she had to carry oxygen. He administered erythropoietin and iron to stimulate red blood cell production. He also put the patient on high flow oxygen to help saturate the plasma. The patient survived without a blood transfusion or significant complications.
“I didn’t think [the patient] was going to make it,” says Dr. McIlraith. “This was a very difficult situation because I knew they would have benefited from a blood transfusion. But, I presented them with their options and respected their wishes.”
Religious Diversity
Religious diversity can be another challenging aspect of patient care. In its 2008 U.S. Religious Landscapes Survey, the Pew Forum on Religion and Public Life interviewed 35,000 Americans age 18 and older and found “religious affiliation in the U.S. is both very diverse and extremely fluid.” The survey also found “people who are unaffiliated with any particular religion (16.1%) also exhibit remarkable internal diversity.”
Asking questions is the key to understanding a patient’s religious and spiritual needs, says the Rev. Peter Yuichi Clark, PhD, chaplain administrator at Alta Bates Summit Medical Center in Berkeley, Calif., who works closely with medical teams to assess and respond to these needs.
“I don’t assume I know what a patient’s religious needs are—even if I know what religion they profess to be,” Clark says. “Some patients may be very devout but do not practice certain aspects of their religion, while others follow a religion in name only but look for religious support during a time of crisis.”
Manish Patel, MD, a hospitalist and assistant professor with the division of General Internal Medicine at University of Medicine and Dentistry of New Jersey-Robert Wood Johnson Medical School, says it is impossible to predict an individual’s religious beliefs and how that may affect their hospital stay—even when the physician practices the same religion as the patient. For example, Dr. Patel knows that some, but not all, Hindus observe a strict vegetarian diet and that Vitamin B12 deficiencies are more prevalent in vegetarian populations. However, diet may not be the cause of this deficiency if the patient is not a vegetarian. Rather than assume, it’s important to ask Hindu patients if they observe a vegetarian diet, Dr. Patel says.
Some hospitalists find it difficult to engage patients in conversations about religion. In a study published in the June 2007 edition of the Journal of Palliative Medicine, researchers found physicians’ knowledge of factors relating to end-of-life care, which included patients’ religious and spiritual concerns and whether they affect decisions regarding end-of-life care, is poor.1
Hospitalists don’t have much time to get to know the person, so it’s even more important for them to have conversations about religion and end-of-life-care, says the study’s lead author Susan DesHarnais, PhD, of Pennsylvania State University’s Hershey Department of Public Health Sciences, Milton S. Hershey Medical Center College of Medicine. As important as these conversations are, Dr. DesHarnais learned hospitalists rarely have them.
When asked why she thinks these conversations rarely occur, Dr. DesHarnais said the research did not directly address that question, but she suspects the physicians don’t have a lot of time. Also, end-of-life decision-making is difficult, and some people are not comfortable talking about it, she says.
“Another factor may be that hospitalists are used to using technology for medical intervention more than they are used to working with people when not much more can be done,” she says.
Dr. Holler, who worked as a social worker before attending medical school, agrees that many physicians are uncomfortable with end-of-life decisions.
“Many physicians are 25 to 30 years old during their training,” says Dr. Holler. “They have been in school for many years. Some are discovering their own spiritual identity at the same time they are dealing, or learning to deal, with patients and families and where they are spiritually or religiously. Many haven’t dealt with these issues in their own personal lives yet.”
While Dr. Holler says she believes most doctors are caring and compassionate, end-of-life and religious discussions use different skill sets than those that preserve and extend life. “Often times we are not taught when enough is enough and how to convey that to patients and families,” says Dr. Holler. “Many doctors are afraid that they are conveying that they are giving up or that it isn’t worth it in the long run. So, many physicians find it easier to ‘keep going.’ ”
The Medical Community’s Response
The medical community is responding to shifting cultural and religious demographics, and more doctors are paying attention to religious diversity, Clark says. But a 2003 Joint Commision study of 60 public and private hospitals across the country, “Hospitals, Language and Culture: A Snapshot of the Nation,” found that hospitals still have work to do in this area.
“We found that hospitals are collecting data on patients’ religion, but it’s just not clear how they use it to improve services,” says Amy Wilson-Stronks, project director for health disparities with the Joint Commission and principal investigator of the study.
The current Joint Commission standards require hospitals to respect patients’ spiritual needs, beliefs, and values. Spiritual care issues first appeared in the 1969 accreditation manual and were adopted into standards in 1992, Wilson-Stronks says.
Accomodating Patients
Awareness and communication can benefit patients, hospitalists, and medical staff as a whole. For example, Alta Bates Summit’s intensive care unit staff in Berkely, Calif., turned to Chaplaincy Services about Muslim patients’ requests to continue their daily prayers, which include thorough washing of their hands, forearms, and other parts of their bodies (even when intravenous lines are attached). Chaplaincy Services reached out to an Islamic network group for advice and learned patients could rub a stone across their bodies to wash themselves. Chaplaincy Services now makes these stones available for staff and patients, Clark says.
Medical staff also works with Chaplaincy Services to accommodate Muslim patients’ wishes to face in the direction of Mecca during prayer, which can require maneuvering beds and other equipment, he says.
Some patients and their families may not understand how their religious tradition addresses code status, resuscitation, and when it is appropriate to withhold treatment, says Richard Rohr, MD, vice president of medical affairs at Cortland Regional Medical Center in Cortland, N.Y. While working as a hospitalist, Dr. Rohr suggested moving a terminal patient to palliative care and seeking a do not resuscitate (DNR) order. The patient’s family refused, and told Dr. Rohr they were Catholic and a DNR would violate their religious beliefs.
According to Dr. Rohr, DNR status and palliative care are described in the code of ethics adopted by the Catholic Health Association, and this type of care is generally provided at Catholic hospitals.
“I gently told them that this was within their religion, but they said no to palliative care and the DNR,” Dr. Rohr says. “The patient eventually died but it was much more difficult for them. They were subjected to active treatment that they couldn’t really benefit from.”
Families often seek the advice of spiritual advisors when making difficult decisions about code status and DNR orders. Barbara Egan, MD, a hospitalist at Memorial Sloan-Kettering Cancer Center in New York City, recalls treating an Orthodox Jewish patient who was suffering from end-stage disease. Death was imminent, and hospitalists recommended palliative care. The patient’s family members balked at the recommendation and insisted hospitalists “do everything possible” to treat their loved one. Soon after, the family’s rabbi arrived to counsel the family. After visiting the patient and speaking to medical staff about the prognosis, the rabbi urged the family not to pursue further treatment or artificial resuscitation. The patient was moved to a palliative care unit and passed away within a few days.
“The family’s rabbi told them exactly what I had: that there were no useful medical interventions for the patient,” Dr. Egan says. “But they really needed to hear it from him before they could come to an agreement on a DNR.”
Physicians’ reactions to religion at the bedside have evolved the past 25 years, says Kenneth Patrick, MD, ICU director at Chestnut Hill Hospital in Philadelphia. Physicians were more paternalistic then, and believed they knew what was best for their patients—and their families—regardless of their patient’s religious beliefs.
While serving as a fellow at Memorial Sloan-Kettering Cancer Center, Dr. Patrick worked with a terminally ill Buddhist patient in the intensive care unit. When death was imminent, the ICU director allowed Buddhist monks to light candles and pray in the room during the hours leading up to the patient’s death. At the time, this was not something that was normally done in a hospital, Dr. Patrick says. While the ritual may have kept medical staff from checking vital signs as often as they would have normally, he says this did not affect the patient’s treatment.
“I believe it is incumbent on the hospitalist to adjust his or her beliefs to be more accepting of our patients’ values,” Dr. Patrick says. “I can agree to any request I find to be reasonable and in the patient’s best interest, even if it is different than what I believe.” TH
Gina Gotsill is a journalist based in California.
Reference
- DesHarnais S, Carter RE, Hennessy W, Kurent JE, Carter C. Lack of concordance between physicians and patient: Reports on end-of-life care discussions. J Pall Med. 2007 June;10(3):728-740.
Several years ago, a patient at Virtua West Jersey Hospital Marlton, in Marlton, N.J., was diagnosed with metastatic colon cancer with spinal metastases. The patient was septic, bleeding from a spinal wound, and was experiencing kidney failure. Hospitalists recommended stopping treatment and moving the patient to hospice care. The patient’s family refused, and told hospitalists that, according to their Christian faith, suffering was the only true path to heaven. Hospitalists kept the patient as comfortable as possible, but blood pressure problems and hypotension made it difficult for them to administer pain medication.
Hospitalists held numerous meetings with the family and medical and nursing staff to discuss the ethical implications of the situation. Two months later, the patient suffered cardiac arrest and died.
“The medical staff and family were continuously at odds because the patient was suffering so much,” says Marianne Holler, DO, a hospitalist at University of Medicine and Dentistry of New Jersey School of Osteopathic Medicine, who was part of the patient’s medical team. “We were never able to discontinue life support throughout [the patient’s] hospital stay.”
Whether planning a routine procedure or end-of-life care, hospitalists may be called into religious discussions with patients, their families, spiritual advisors, and hospital chaplains. While many hospitalists have received ethics and other professional training to prepare them for these conversations, some say the intersection of religion and medicine remains a challenging and multifaceted aspect of their practice.
—The Rev. Peter Yuichi Clark, PhD, Alta Bates Summit Medical Center, Berkeley, Calif.
A Hospitalist’s Belief
Hospitalists’ brief relationships with patients may influence the degree of knowledge they have about an individual’s religious beliefs, says Scott Enderby, DO, a hospitalist at Alta Bates Summit Medical Center in Berkeley, Calif. Over the years, primary care physicians may become less involved with a patient’s acute medical needs as they use hospitalist services to manage their inpatients, Dr. Enderby says. This means hospitalists must discuss patients’ wishes regarding code status and resuscitation, end-of-life care, and other necessary treatments.
When discussing religion and treatment, hospitalists must put aside their personal beliefs, and this may not always be easy, says Dr. Thomas McIlraith, MD, medical director of Hospital Medicine at Mercy Medical Group in Sacramento, Calif. Dr. McIlraith recalls a Jehovah’s Witness patient who cited religious beliefs when refusing a blood transfusion following a massive post-partum hemorrhage. The patient was severely anemic, and her hemoglobin levels plunged dangerously to 2 gm/dL. Leaders from the patient’s church asked Dr. McIlraith to try hemoglobin substitutes, but he was unable to do so because these substitutes still were experimental and associated with significant complications, he says.
Dr. McIlraith had to act fast. He instructed the obstetrician on the case to stop drawing hemoglobin levels; the patient needed every drop of blood she had to carry oxygen. He administered erythropoietin and iron to stimulate red blood cell production. He also put the patient on high flow oxygen to help saturate the plasma. The patient survived without a blood transfusion or significant complications.
“I didn’t think [the patient] was going to make it,” says Dr. McIlraith. “This was a very difficult situation because I knew they would have benefited from a blood transfusion. But, I presented them with their options and respected their wishes.”
Religious Diversity
Religious diversity can be another challenging aspect of patient care. In its 2008 U.S. Religious Landscapes Survey, the Pew Forum on Religion and Public Life interviewed 35,000 Americans age 18 and older and found “religious affiliation in the U.S. is both very diverse and extremely fluid.” The survey also found “people who are unaffiliated with any particular religion (16.1%) also exhibit remarkable internal diversity.”
Asking questions is the key to understanding a patient’s religious and spiritual needs, says the Rev. Peter Yuichi Clark, PhD, chaplain administrator at Alta Bates Summit Medical Center in Berkeley, Calif., who works closely with medical teams to assess and respond to these needs.
“I don’t assume I know what a patient’s religious needs are—even if I know what religion they profess to be,” Clark says. “Some patients may be very devout but do not practice certain aspects of their religion, while others follow a religion in name only but look for religious support during a time of crisis.”
Manish Patel, MD, a hospitalist and assistant professor with the division of General Internal Medicine at University of Medicine and Dentistry of New Jersey-Robert Wood Johnson Medical School, says it is impossible to predict an individual’s religious beliefs and how that may affect their hospital stay—even when the physician practices the same religion as the patient. For example, Dr. Patel knows that some, but not all, Hindus observe a strict vegetarian diet and that Vitamin B12 deficiencies are more prevalent in vegetarian populations. However, diet may not be the cause of this deficiency if the patient is not a vegetarian. Rather than assume, it’s important to ask Hindu patients if they observe a vegetarian diet, Dr. Patel says.
Some hospitalists find it difficult to engage patients in conversations about religion. In a study published in the June 2007 edition of the Journal of Palliative Medicine, researchers found physicians’ knowledge of factors relating to end-of-life care, which included patients’ religious and spiritual concerns and whether they affect decisions regarding end-of-life care, is poor.1
Hospitalists don’t have much time to get to know the person, so it’s even more important for them to have conversations about religion and end-of-life-care, says the study’s lead author Susan DesHarnais, PhD, of Pennsylvania State University’s Hershey Department of Public Health Sciences, Milton S. Hershey Medical Center College of Medicine. As important as these conversations are, Dr. DesHarnais learned hospitalists rarely have them.
When asked why she thinks these conversations rarely occur, Dr. DesHarnais said the research did not directly address that question, but she suspects the physicians don’t have a lot of time. Also, end-of-life decision-making is difficult, and some people are not comfortable talking about it, she says.
“Another factor may be that hospitalists are used to using technology for medical intervention more than they are used to working with people when not much more can be done,” she says.
Dr. Holler, who worked as a social worker before attending medical school, agrees that many physicians are uncomfortable with end-of-life decisions.
“Many physicians are 25 to 30 years old during their training,” says Dr. Holler. “They have been in school for many years. Some are discovering their own spiritual identity at the same time they are dealing, or learning to deal, with patients and families and where they are spiritually or religiously. Many haven’t dealt with these issues in their own personal lives yet.”
While Dr. Holler says she believes most doctors are caring and compassionate, end-of-life and religious discussions use different skill sets than those that preserve and extend life. “Often times we are not taught when enough is enough and how to convey that to patients and families,” says Dr. Holler. “Many doctors are afraid that they are conveying that they are giving up or that it isn’t worth it in the long run. So, many physicians find it easier to ‘keep going.’ ”
The Medical Community’s Response
The medical community is responding to shifting cultural and religious demographics, and more doctors are paying attention to religious diversity, Clark says. But a 2003 Joint Commision study of 60 public and private hospitals across the country, “Hospitals, Language and Culture: A Snapshot of the Nation,” found that hospitals still have work to do in this area.
“We found that hospitals are collecting data on patients’ religion, but it’s just not clear how they use it to improve services,” says Amy Wilson-Stronks, project director for health disparities with the Joint Commission and principal investigator of the study.
The current Joint Commission standards require hospitals to respect patients’ spiritual needs, beliefs, and values. Spiritual care issues first appeared in the 1969 accreditation manual and were adopted into standards in 1992, Wilson-Stronks says.
Accomodating Patients
Awareness and communication can benefit patients, hospitalists, and medical staff as a whole. For example, Alta Bates Summit’s intensive care unit staff in Berkely, Calif., turned to Chaplaincy Services about Muslim patients’ requests to continue their daily prayers, which include thorough washing of their hands, forearms, and other parts of their bodies (even when intravenous lines are attached). Chaplaincy Services reached out to an Islamic network group for advice and learned patients could rub a stone across their bodies to wash themselves. Chaplaincy Services now makes these stones available for staff and patients, Clark says.
Medical staff also works with Chaplaincy Services to accommodate Muslim patients’ wishes to face in the direction of Mecca during prayer, which can require maneuvering beds and other equipment, he says.
Some patients and their families may not understand how their religious tradition addresses code status, resuscitation, and when it is appropriate to withhold treatment, says Richard Rohr, MD, vice president of medical affairs at Cortland Regional Medical Center in Cortland, N.Y. While working as a hospitalist, Dr. Rohr suggested moving a terminal patient to palliative care and seeking a do not resuscitate (DNR) order. The patient’s family refused, and told Dr. Rohr they were Catholic and a DNR would violate their religious beliefs.
According to Dr. Rohr, DNR status and palliative care are described in the code of ethics adopted by the Catholic Health Association, and this type of care is generally provided at Catholic hospitals.
“I gently told them that this was within their religion, but they said no to palliative care and the DNR,” Dr. Rohr says. “The patient eventually died but it was much more difficult for them. They were subjected to active treatment that they couldn’t really benefit from.”
Families often seek the advice of spiritual advisors when making difficult decisions about code status and DNR orders. Barbara Egan, MD, a hospitalist at Memorial Sloan-Kettering Cancer Center in New York City, recalls treating an Orthodox Jewish patient who was suffering from end-stage disease. Death was imminent, and hospitalists recommended palliative care. The patient’s family members balked at the recommendation and insisted hospitalists “do everything possible” to treat their loved one. Soon after, the family’s rabbi arrived to counsel the family. After visiting the patient and speaking to medical staff about the prognosis, the rabbi urged the family not to pursue further treatment or artificial resuscitation. The patient was moved to a palliative care unit and passed away within a few days.
“The family’s rabbi told them exactly what I had: that there were no useful medical interventions for the patient,” Dr. Egan says. “But they really needed to hear it from him before they could come to an agreement on a DNR.”
Physicians’ reactions to religion at the bedside have evolved the past 25 years, says Kenneth Patrick, MD, ICU director at Chestnut Hill Hospital in Philadelphia. Physicians were more paternalistic then, and believed they knew what was best for their patients—and their families—regardless of their patient’s religious beliefs.
While serving as a fellow at Memorial Sloan-Kettering Cancer Center, Dr. Patrick worked with a terminally ill Buddhist patient in the intensive care unit. When death was imminent, the ICU director allowed Buddhist monks to light candles and pray in the room during the hours leading up to the patient’s death. At the time, this was not something that was normally done in a hospital, Dr. Patrick says. While the ritual may have kept medical staff from checking vital signs as often as they would have normally, he says this did not affect the patient’s treatment.
“I believe it is incumbent on the hospitalist to adjust his or her beliefs to be more accepting of our patients’ values,” Dr. Patrick says. “I can agree to any request I find to be reasonable and in the patient’s best interest, even if it is different than what I believe.” TH
Gina Gotsill is a journalist based in California.
Reference
- DesHarnais S, Carter RE, Hennessy W, Kurent JE, Carter C. Lack of concordance between physicians and patient: Reports on end-of-life care discussions. J Pall Med. 2007 June;10(3):728-740.
Several years ago, a patient at Virtua West Jersey Hospital Marlton, in Marlton, N.J., was diagnosed with metastatic colon cancer with spinal metastases. The patient was septic, bleeding from a spinal wound, and was experiencing kidney failure. Hospitalists recommended stopping treatment and moving the patient to hospice care. The patient’s family refused, and told hospitalists that, according to their Christian faith, suffering was the only true path to heaven. Hospitalists kept the patient as comfortable as possible, but blood pressure problems and hypotension made it difficult for them to administer pain medication.
Hospitalists held numerous meetings with the family and medical and nursing staff to discuss the ethical implications of the situation. Two months later, the patient suffered cardiac arrest and died.
“The medical staff and family were continuously at odds because the patient was suffering so much,” says Marianne Holler, DO, a hospitalist at University of Medicine and Dentistry of New Jersey School of Osteopathic Medicine, who was part of the patient’s medical team. “We were never able to discontinue life support throughout [the patient’s] hospital stay.”
Whether planning a routine procedure or end-of-life care, hospitalists may be called into religious discussions with patients, their families, spiritual advisors, and hospital chaplains. While many hospitalists have received ethics and other professional training to prepare them for these conversations, some say the intersection of religion and medicine remains a challenging and multifaceted aspect of their practice.
—The Rev. Peter Yuichi Clark, PhD, Alta Bates Summit Medical Center, Berkeley, Calif.
A Hospitalist’s Belief
Hospitalists’ brief relationships with patients may influence the degree of knowledge they have about an individual’s religious beliefs, says Scott Enderby, DO, a hospitalist at Alta Bates Summit Medical Center in Berkeley, Calif. Over the years, primary care physicians may become less involved with a patient’s acute medical needs as they use hospitalist services to manage their inpatients, Dr. Enderby says. This means hospitalists must discuss patients’ wishes regarding code status and resuscitation, end-of-life care, and other necessary treatments.
When discussing religion and treatment, hospitalists must put aside their personal beliefs, and this may not always be easy, says Dr. Thomas McIlraith, MD, medical director of Hospital Medicine at Mercy Medical Group in Sacramento, Calif. Dr. McIlraith recalls a Jehovah’s Witness patient who cited religious beliefs when refusing a blood transfusion following a massive post-partum hemorrhage. The patient was severely anemic, and her hemoglobin levels plunged dangerously to 2 gm/dL. Leaders from the patient’s church asked Dr. McIlraith to try hemoglobin substitutes, but he was unable to do so because these substitutes still were experimental and associated with significant complications, he says.
Dr. McIlraith had to act fast. He instructed the obstetrician on the case to stop drawing hemoglobin levels; the patient needed every drop of blood she had to carry oxygen. He administered erythropoietin and iron to stimulate red blood cell production. He also put the patient on high flow oxygen to help saturate the plasma. The patient survived without a blood transfusion or significant complications.
“I didn’t think [the patient] was going to make it,” says Dr. McIlraith. “This was a very difficult situation because I knew they would have benefited from a blood transfusion. But, I presented them with their options and respected their wishes.”
Religious Diversity
Religious diversity can be another challenging aspect of patient care. In its 2008 U.S. Religious Landscapes Survey, the Pew Forum on Religion and Public Life interviewed 35,000 Americans age 18 and older and found “religious affiliation in the U.S. is both very diverse and extremely fluid.” The survey also found “people who are unaffiliated with any particular religion (16.1%) also exhibit remarkable internal diversity.”
Asking questions is the key to understanding a patient’s religious and spiritual needs, says the Rev. Peter Yuichi Clark, PhD, chaplain administrator at Alta Bates Summit Medical Center in Berkeley, Calif., who works closely with medical teams to assess and respond to these needs.
“I don’t assume I know what a patient’s religious needs are—even if I know what religion they profess to be,” Clark says. “Some patients may be very devout but do not practice certain aspects of their religion, while others follow a religion in name only but look for religious support during a time of crisis.”
Manish Patel, MD, a hospitalist and assistant professor with the division of General Internal Medicine at University of Medicine and Dentistry of New Jersey-Robert Wood Johnson Medical School, says it is impossible to predict an individual’s religious beliefs and how that may affect their hospital stay—even when the physician practices the same religion as the patient. For example, Dr. Patel knows that some, but not all, Hindus observe a strict vegetarian diet and that Vitamin B12 deficiencies are more prevalent in vegetarian populations. However, diet may not be the cause of this deficiency if the patient is not a vegetarian. Rather than assume, it’s important to ask Hindu patients if they observe a vegetarian diet, Dr. Patel says.
Some hospitalists find it difficult to engage patients in conversations about religion. In a study published in the June 2007 edition of the Journal of Palliative Medicine, researchers found physicians’ knowledge of factors relating to end-of-life care, which included patients’ religious and spiritual concerns and whether they affect decisions regarding end-of-life care, is poor.1
Hospitalists don’t have much time to get to know the person, so it’s even more important for them to have conversations about religion and end-of-life-care, says the study’s lead author Susan DesHarnais, PhD, of Pennsylvania State University’s Hershey Department of Public Health Sciences, Milton S. Hershey Medical Center College of Medicine. As important as these conversations are, Dr. DesHarnais learned hospitalists rarely have them.
When asked why she thinks these conversations rarely occur, Dr. DesHarnais said the research did not directly address that question, but she suspects the physicians don’t have a lot of time. Also, end-of-life decision-making is difficult, and some people are not comfortable talking about it, she says.
“Another factor may be that hospitalists are used to using technology for medical intervention more than they are used to working with people when not much more can be done,” she says.
Dr. Holler, who worked as a social worker before attending medical school, agrees that many physicians are uncomfortable with end-of-life decisions.
“Many physicians are 25 to 30 years old during their training,” says Dr. Holler. “They have been in school for many years. Some are discovering their own spiritual identity at the same time they are dealing, or learning to deal, with patients and families and where they are spiritually or religiously. Many haven’t dealt with these issues in their own personal lives yet.”
While Dr. Holler says she believes most doctors are caring and compassionate, end-of-life and religious discussions use different skill sets than those that preserve and extend life. “Often times we are not taught when enough is enough and how to convey that to patients and families,” says Dr. Holler. “Many doctors are afraid that they are conveying that they are giving up or that it isn’t worth it in the long run. So, many physicians find it easier to ‘keep going.’ ”
The Medical Community’s Response
The medical community is responding to shifting cultural and religious demographics, and more doctors are paying attention to religious diversity, Clark says. But a 2003 Joint Commision study of 60 public and private hospitals across the country, “Hospitals, Language and Culture: A Snapshot of the Nation,” found that hospitals still have work to do in this area.
“We found that hospitals are collecting data on patients’ religion, but it’s just not clear how they use it to improve services,” says Amy Wilson-Stronks, project director for health disparities with the Joint Commission and principal investigator of the study.
The current Joint Commission standards require hospitals to respect patients’ spiritual needs, beliefs, and values. Spiritual care issues first appeared in the 1969 accreditation manual and were adopted into standards in 1992, Wilson-Stronks says.
Accomodating Patients
Awareness and communication can benefit patients, hospitalists, and medical staff as a whole. For example, Alta Bates Summit’s intensive care unit staff in Berkely, Calif., turned to Chaplaincy Services about Muslim patients’ requests to continue their daily prayers, which include thorough washing of their hands, forearms, and other parts of their bodies (even when intravenous lines are attached). Chaplaincy Services reached out to an Islamic network group for advice and learned patients could rub a stone across their bodies to wash themselves. Chaplaincy Services now makes these stones available for staff and patients, Clark says.
Medical staff also works with Chaplaincy Services to accommodate Muslim patients’ wishes to face in the direction of Mecca during prayer, which can require maneuvering beds and other equipment, he says.
Some patients and their families may not understand how their religious tradition addresses code status, resuscitation, and when it is appropriate to withhold treatment, says Richard Rohr, MD, vice president of medical affairs at Cortland Regional Medical Center in Cortland, N.Y. While working as a hospitalist, Dr. Rohr suggested moving a terminal patient to palliative care and seeking a do not resuscitate (DNR) order. The patient’s family refused, and told Dr. Rohr they were Catholic and a DNR would violate their religious beliefs.
According to Dr. Rohr, DNR status and palliative care are described in the code of ethics adopted by the Catholic Health Association, and this type of care is generally provided at Catholic hospitals.
“I gently told them that this was within their religion, but they said no to palliative care and the DNR,” Dr. Rohr says. “The patient eventually died but it was much more difficult for them. They were subjected to active treatment that they couldn’t really benefit from.”
Families often seek the advice of spiritual advisors when making difficult decisions about code status and DNR orders. Barbara Egan, MD, a hospitalist at Memorial Sloan-Kettering Cancer Center in New York City, recalls treating an Orthodox Jewish patient who was suffering from end-stage disease. Death was imminent, and hospitalists recommended palliative care. The patient’s family members balked at the recommendation and insisted hospitalists “do everything possible” to treat their loved one. Soon after, the family’s rabbi arrived to counsel the family. After visiting the patient and speaking to medical staff about the prognosis, the rabbi urged the family not to pursue further treatment or artificial resuscitation. The patient was moved to a palliative care unit and passed away within a few days.
“The family’s rabbi told them exactly what I had: that there were no useful medical interventions for the patient,” Dr. Egan says. “But they really needed to hear it from him before they could come to an agreement on a DNR.”
Physicians’ reactions to religion at the bedside have evolved the past 25 years, says Kenneth Patrick, MD, ICU director at Chestnut Hill Hospital in Philadelphia. Physicians were more paternalistic then, and believed they knew what was best for their patients—and their families—regardless of their patient’s religious beliefs.
While serving as a fellow at Memorial Sloan-Kettering Cancer Center, Dr. Patrick worked with a terminally ill Buddhist patient in the intensive care unit. When death was imminent, the ICU director allowed Buddhist monks to light candles and pray in the room during the hours leading up to the patient’s death. At the time, this was not something that was normally done in a hospital, Dr. Patrick says. While the ritual may have kept medical staff from checking vital signs as often as they would have normally, he says this did not affect the patient’s treatment.
“I believe it is incumbent on the hospitalist to adjust his or her beliefs to be more accepting of our patients’ values,” Dr. Patrick says. “I can agree to any request I find to be reasonable and in the patient’s best interest, even if it is different than what I believe.” TH
Gina Gotsill is a journalist based in California.
Reference
- DesHarnais S, Carter RE, Hennessy W, Kurent JE, Carter C. Lack of concordance between physicians and patient: Reports on end-of-life care discussions. J Pall Med. 2007 June;10(3):728-740.
Patients’ Circumstances Count in Care Planning
Hospitals, insurers, and regulators today direct unparalleled time and resources toward battling medical errors. According to research from Saul Weiner, MD, a teacher and hospitalist at the University of Illinois, Chicago, one type of error is being ignored.
“I found that contextual errors are as least as common and probably even more serious in inpatient care,” Dr. Weiner says. Contextual errors occur when physicians neglect to recognize the crucial role a patient’s life plays in care planning, he says. “I would argue that there are contextual issues in virtually every admission,” he adds.
Dr. Weiner and his team are trying to prove the importance of systematizing how physicians learn to contextualize care. For their research, published in the September 2007 issue of Medical Decision Making, the doctors trained actors to present scenarios in which contextual information was essential to planning appropriate care. They then tested 54 internal medicine residents to see how many would provide contextually appropriate care. More than half made serious errors.1
Why Now?
From the time Dr. Weiner started precepting residents in 1997, he noticed many were quick to fit patients into categories where they could apply evidence-based approaches to care. “But I sometimes sensed that there was something not so straightforward about a particular patient’s situation, some unexplained issue for why the patient wasn’t taking his asthma meds properly, or was coming in today of all days,” he says. When he and the residents would re-question such patients, “key factors often arose that made everything change about the way we wanted to manage them.”
For him, the seminal case occurred in 2002 when a patient who came to the preoperative testing clinic for bariatric surgery. She qualified for surgery, although because of history of adhesions, the surgery was going to be open rather than laparoscopic. When she said she was looking forward to getting the surgery so she could better care for her son, a red flag went up for Dr. Weiner.
“Patients don’t typically offer such reflections,” he says. Further probing revealed that she had a son in his 20s with a fatal disease, she was the sole caregiver, and her husband was an alcoholic. “She really hadn’t processed the fact that she would not be able to do anything for 40 days,” Dr. Weiner says. “Suddenly, when she got that, she wound up canceling surgery.”
The importance of learning a patietn’s contextual factors depends on the setting and the availability of resources, such as case managers, social workers, and outpatient chronic disease teams. Even with those resources, the onus still is on the hospitalist to determine an effective plan of care using context, he says.
How Well Do Hospitalists Spot Red Flags?
Tuning up the radar to prevent these errors comes down to providers asking one question and listening to one answer: What is the best thing I can do for this patient at this time? Watch for red flags, statements patients make or actions they take that may signal something lying beneath the surface that could make a huge difference in outcomes, Dr. Weiner says.
Spotting red flags isn’t always easy for hospitalists, who don’t see the same patients on a regular basis, says William Stinnette, MD, hospitalist with Kaiser Permanente in San Rafael, Calif. “Unless patients are a part of that ‘revolving door,’ with frequent visits to the hospital, we have to work up most of our patients with limited background knowledge,” he says.
Dr. Stinnette, who has worked in hospital medicine groups in both small and large facilities, believes the ability to ferret out contextual factors depends on the setting and teh availability of resources, such as case managers, social workers, and outpatient chornic disease teams. Even with those resources, the onus is still on the hospitalist to determine an effective plan of care using context, he says.
Dr. Weiner agrees. “Clinical decision-making,” he writes in the Medical Decision Making article, “requires two distinct skills: the ability to classify patients’ conditions into diagnostic and management categories that permit the application of research evidence, and the ability to individualize (contextualize) care for patients whose circumstances and needs require variation from a standardized approach.”1
Though most hospitalists recognize the importance of doing this, according to Dr. Weiner, they lack a systematic and coherent way to do so.2
Standardized Training
Physicians are well trained in assessing biomedical information, but not in listening for contextual red flags. “When you need to know what’s unique about a particular patient that might be relevant to the case, you don’t really know what you’re searching for,” Dr. Weiner says. “You just need clarity, and that is theory-building or inductive reasoning, a very different skill.”
Since July, with support from the National Board of Medical Examiners, the University of Illinois, Chicago, team has been testing a contextual-care curriculum for fourth-year medical students to complete during their inpatient medical sub-internships. In the randomized controlled trial, half of the trainees receive the intervention, half do not. Those getting trained learn that when they intuitively feel there is more to a patient’s story, they should return to the patient to find out why.
They also learn to conduct a contextual history by asking simple, straightforward questions, keeping in mind potential areas of context, including the patient’s emotional state and cultural beliefs (see sidebar, “Cases of Context”). Through this process, the residents learn to identify ways to avoid contextual errors in their patients’ care.
At the end of the month-long curriculum, all trainees are tested in the patient laboratory. “The simulated patients are trained to present with complex histories,” Dr. Weiner says. If residents don’t probe properly, the patients don’t reveal the context. Both groups—those who’ve had the intervention and those who have not—interview the patients, and the study is tracking which physicians get the cases ‘correct.’ ”
The Cost of Errors
For a next phase of research, the university has enrolled about 100 physicians from multiple centers in the Chicago/Milwaukee area to participate in visits by undercover actors simulating real patients. The cases require uncovering contextual information to avoid making errors when planning their care.
After each visit, the team downloads and scores the physician’s note, identifying both unnecessary tests and missed opportunities. Using Medicare reimbursement data, the team assigns a dollar amount to these errors that would have occurred had the patients been real. Dr. Weiner predicts publicizing the financial costs will bring more attention to the problem.
Access to Care Issues
Actually, physicians should talk to their patients about the cost of their care, says David O. Meltzer, MD, PhD, associate professor and chief of the Section of Hospital Medicine at the University of Chicago Medical Center. In research published in the Journal of the American Medical Association in 2003, Dr. Meltzer and colleagues found patient costs may be associated with medication nonadherence and considerable economic burden.3
“You have to ask a patient the type of questions that will be most revealing, including the broader questions, such as ‘Are there any challenges in your life to getting the care that we’ve discussed after you leave the hospital?’ ” Dr. Stinnette, of Kaiser, says.
In their study, Dr. Meltzer and colleagues also showed that although physicians and patients agree it is important to talk about money issues, it doesn’t commonly happen.3
“In a public hospital where there are greater numbers of uninsured patients, I expect it is much more a frequent topic of conversation,” Dr. Meltzer says. “My suspicion is that the people at greatest risk are uninsured patients who are hospitalized in settings where most patients are insured, and physicians are not attuned to bringing up this issue.”
Although a great deal of literature now deals with physician-patient communication, the techniques seem too scripted to Dr. Weiner. “When you engage with the patient simply as one person who is concerned about another,” he says, “you much more naturally find out what you could do that might help them.”
References
- Weiner SJ, Schwartz A, Yudkowsky R, et al. Evaluating physician performance at individualizing care: A pilot study tracking contextual errors in medical decision-making. Med Decis Making 2007;27(6):726-34.
- Weiner SJ. Contextualizing medical decisions to individualize care: Lessons from the qualitative sciences. J Gen Intern Med 2004;19(3):281-5.
- Alexander GC, Casalino LP, Meltzer DO. Patient-physician communication about out-of-pocket costs. JAMA 2003;290(7):953-958.
Hospitals, insurers, and regulators today direct unparalleled time and resources toward battling medical errors. According to research from Saul Weiner, MD, a teacher and hospitalist at the University of Illinois, Chicago, one type of error is being ignored.
“I found that contextual errors are as least as common and probably even more serious in inpatient care,” Dr. Weiner says. Contextual errors occur when physicians neglect to recognize the crucial role a patient’s life plays in care planning, he says. “I would argue that there are contextual issues in virtually every admission,” he adds.
Dr. Weiner and his team are trying to prove the importance of systematizing how physicians learn to contextualize care. For their research, published in the September 2007 issue of Medical Decision Making, the doctors trained actors to present scenarios in which contextual information was essential to planning appropriate care. They then tested 54 internal medicine residents to see how many would provide contextually appropriate care. More than half made serious errors.1
Why Now?
From the time Dr. Weiner started precepting residents in 1997, he noticed many were quick to fit patients into categories where they could apply evidence-based approaches to care. “But I sometimes sensed that there was something not so straightforward about a particular patient’s situation, some unexplained issue for why the patient wasn’t taking his asthma meds properly, or was coming in today of all days,” he says. When he and the residents would re-question such patients, “key factors often arose that made everything change about the way we wanted to manage them.”
For him, the seminal case occurred in 2002 when a patient who came to the preoperative testing clinic for bariatric surgery. She qualified for surgery, although because of history of adhesions, the surgery was going to be open rather than laparoscopic. When she said she was looking forward to getting the surgery so she could better care for her son, a red flag went up for Dr. Weiner.
“Patients don’t typically offer such reflections,” he says. Further probing revealed that she had a son in his 20s with a fatal disease, she was the sole caregiver, and her husband was an alcoholic. “She really hadn’t processed the fact that she would not be able to do anything for 40 days,” Dr. Weiner says. “Suddenly, when she got that, she wound up canceling surgery.”
The importance of learning a patietn’s contextual factors depends on the setting and the availability of resources, such as case managers, social workers, and outpatient chronic disease teams. Even with those resources, the onus still is on the hospitalist to determine an effective plan of care using context, he says.
How Well Do Hospitalists Spot Red Flags?
Tuning up the radar to prevent these errors comes down to providers asking one question and listening to one answer: What is the best thing I can do for this patient at this time? Watch for red flags, statements patients make or actions they take that may signal something lying beneath the surface that could make a huge difference in outcomes, Dr. Weiner says.
Spotting red flags isn’t always easy for hospitalists, who don’t see the same patients on a regular basis, says William Stinnette, MD, hospitalist with Kaiser Permanente in San Rafael, Calif. “Unless patients are a part of that ‘revolving door,’ with frequent visits to the hospital, we have to work up most of our patients with limited background knowledge,” he says.
Dr. Stinnette, who has worked in hospital medicine groups in both small and large facilities, believes the ability to ferret out contextual factors depends on the setting and teh availability of resources, such as case managers, social workers, and outpatient chornic disease teams. Even with those resources, the onus is still on the hospitalist to determine an effective plan of care using context, he says.
Dr. Weiner agrees. “Clinical decision-making,” he writes in the Medical Decision Making article, “requires two distinct skills: the ability to classify patients’ conditions into diagnostic and management categories that permit the application of research evidence, and the ability to individualize (contextualize) care for patients whose circumstances and needs require variation from a standardized approach.”1
Though most hospitalists recognize the importance of doing this, according to Dr. Weiner, they lack a systematic and coherent way to do so.2
Standardized Training
Physicians are well trained in assessing biomedical information, but not in listening for contextual red flags. “When you need to know what’s unique about a particular patient that might be relevant to the case, you don’t really know what you’re searching for,” Dr. Weiner says. “You just need clarity, and that is theory-building or inductive reasoning, a very different skill.”
Since July, with support from the National Board of Medical Examiners, the University of Illinois, Chicago, team has been testing a contextual-care curriculum for fourth-year medical students to complete during their inpatient medical sub-internships. In the randomized controlled trial, half of the trainees receive the intervention, half do not. Those getting trained learn that when they intuitively feel there is more to a patient’s story, they should return to the patient to find out why.
They also learn to conduct a contextual history by asking simple, straightforward questions, keeping in mind potential areas of context, including the patient’s emotional state and cultural beliefs (see sidebar, “Cases of Context”). Through this process, the residents learn to identify ways to avoid contextual errors in their patients’ care.
At the end of the month-long curriculum, all trainees are tested in the patient laboratory. “The simulated patients are trained to present with complex histories,” Dr. Weiner says. If residents don’t probe properly, the patients don’t reveal the context. Both groups—those who’ve had the intervention and those who have not—interview the patients, and the study is tracking which physicians get the cases ‘correct.’ ”
The Cost of Errors
For a next phase of research, the university has enrolled about 100 physicians from multiple centers in the Chicago/Milwaukee area to participate in visits by undercover actors simulating real patients. The cases require uncovering contextual information to avoid making errors when planning their care.
After each visit, the team downloads and scores the physician’s note, identifying both unnecessary tests and missed opportunities. Using Medicare reimbursement data, the team assigns a dollar amount to these errors that would have occurred had the patients been real. Dr. Weiner predicts publicizing the financial costs will bring more attention to the problem.
Access to Care Issues
Actually, physicians should talk to their patients about the cost of their care, says David O. Meltzer, MD, PhD, associate professor and chief of the Section of Hospital Medicine at the University of Chicago Medical Center. In research published in the Journal of the American Medical Association in 2003, Dr. Meltzer and colleagues found patient costs may be associated with medication nonadherence and considerable economic burden.3
“You have to ask a patient the type of questions that will be most revealing, including the broader questions, such as ‘Are there any challenges in your life to getting the care that we’ve discussed after you leave the hospital?’ ” Dr. Stinnette, of Kaiser, says.
In their study, Dr. Meltzer and colleagues also showed that although physicians and patients agree it is important to talk about money issues, it doesn’t commonly happen.3
“In a public hospital where there are greater numbers of uninsured patients, I expect it is much more a frequent topic of conversation,” Dr. Meltzer says. “My suspicion is that the people at greatest risk are uninsured patients who are hospitalized in settings where most patients are insured, and physicians are not attuned to bringing up this issue.”
Although a great deal of literature now deals with physician-patient communication, the techniques seem too scripted to Dr. Weiner. “When you engage with the patient simply as one person who is concerned about another,” he says, “you much more naturally find out what you could do that might help them.”
References
- Weiner SJ, Schwartz A, Yudkowsky R, et al. Evaluating physician performance at individualizing care: A pilot study tracking contextual errors in medical decision-making. Med Decis Making 2007;27(6):726-34.
- Weiner SJ. Contextualizing medical decisions to individualize care: Lessons from the qualitative sciences. J Gen Intern Med 2004;19(3):281-5.
- Alexander GC, Casalino LP, Meltzer DO. Patient-physician communication about out-of-pocket costs. JAMA 2003;290(7):953-958.
Hospitals, insurers, and regulators today direct unparalleled time and resources toward battling medical errors. According to research from Saul Weiner, MD, a teacher and hospitalist at the University of Illinois, Chicago, one type of error is being ignored.
“I found that contextual errors are as least as common and probably even more serious in inpatient care,” Dr. Weiner says. Contextual errors occur when physicians neglect to recognize the crucial role a patient’s life plays in care planning, he says. “I would argue that there are contextual issues in virtually every admission,” he adds.
Dr. Weiner and his team are trying to prove the importance of systematizing how physicians learn to contextualize care. For their research, published in the September 2007 issue of Medical Decision Making, the doctors trained actors to present scenarios in which contextual information was essential to planning appropriate care. They then tested 54 internal medicine residents to see how many would provide contextually appropriate care. More than half made serious errors.1
Why Now?
From the time Dr. Weiner started precepting residents in 1997, he noticed many were quick to fit patients into categories where they could apply evidence-based approaches to care. “But I sometimes sensed that there was something not so straightforward about a particular patient’s situation, some unexplained issue for why the patient wasn’t taking his asthma meds properly, or was coming in today of all days,” he says. When he and the residents would re-question such patients, “key factors often arose that made everything change about the way we wanted to manage them.”
For him, the seminal case occurred in 2002 when a patient who came to the preoperative testing clinic for bariatric surgery. She qualified for surgery, although because of history of adhesions, the surgery was going to be open rather than laparoscopic. When she said she was looking forward to getting the surgery so she could better care for her son, a red flag went up for Dr. Weiner.
“Patients don’t typically offer such reflections,” he says. Further probing revealed that she had a son in his 20s with a fatal disease, she was the sole caregiver, and her husband was an alcoholic. “She really hadn’t processed the fact that she would not be able to do anything for 40 days,” Dr. Weiner says. “Suddenly, when she got that, she wound up canceling surgery.”
The importance of learning a patietn’s contextual factors depends on the setting and the availability of resources, such as case managers, social workers, and outpatient chronic disease teams. Even with those resources, the onus still is on the hospitalist to determine an effective plan of care using context, he says.
How Well Do Hospitalists Spot Red Flags?
Tuning up the radar to prevent these errors comes down to providers asking one question and listening to one answer: What is the best thing I can do for this patient at this time? Watch for red flags, statements patients make or actions they take that may signal something lying beneath the surface that could make a huge difference in outcomes, Dr. Weiner says.
Spotting red flags isn’t always easy for hospitalists, who don’t see the same patients on a regular basis, says William Stinnette, MD, hospitalist with Kaiser Permanente in San Rafael, Calif. “Unless patients are a part of that ‘revolving door,’ with frequent visits to the hospital, we have to work up most of our patients with limited background knowledge,” he says.
Dr. Stinnette, who has worked in hospital medicine groups in both small and large facilities, believes the ability to ferret out contextual factors depends on the setting and teh availability of resources, such as case managers, social workers, and outpatient chornic disease teams. Even with those resources, the onus is still on the hospitalist to determine an effective plan of care using context, he says.
Dr. Weiner agrees. “Clinical decision-making,” he writes in the Medical Decision Making article, “requires two distinct skills: the ability to classify patients’ conditions into diagnostic and management categories that permit the application of research evidence, and the ability to individualize (contextualize) care for patients whose circumstances and needs require variation from a standardized approach.”1
Though most hospitalists recognize the importance of doing this, according to Dr. Weiner, they lack a systematic and coherent way to do so.2
Standardized Training
Physicians are well trained in assessing biomedical information, but not in listening for contextual red flags. “When you need to know what’s unique about a particular patient that might be relevant to the case, you don’t really know what you’re searching for,” Dr. Weiner says. “You just need clarity, and that is theory-building or inductive reasoning, a very different skill.”
Since July, with support from the National Board of Medical Examiners, the University of Illinois, Chicago, team has been testing a contextual-care curriculum for fourth-year medical students to complete during their inpatient medical sub-internships. In the randomized controlled trial, half of the trainees receive the intervention, half do not. Those getting trained learn that when they intuitively feel there is more to a patient’s story, they should return to the patient to find out why.
They also learn to conduct a contextual history by asking simple, straightforward questions, keeping in mind potential areas of context, including the patient’s emotional state and cultural beliefs (see sidebar, “Cases of Context”). Through this process, the residents learn to identify ways to avoid contextual errors in their patients’ care.
At the end of the month-long curriculum, all trainees are tested in the patient laboratory. “The simulated patients are trained to present with complex histories,” Dr. Weiner says. If residents don’t probe properly, the patients don’t reveal the context. Both groups—those who’ve had the intervention and those who have not—interview the patients, and the study is tracking which physicians get the cases ‘correct.’ ”
The Cost of Errors
For a next phase of research, the university has enrolled about 100 physicians from multiple centers in the Chicago/Milwaukee area to participate in visits by undercover actors simulating real patients. The cases require uncovering contextual information to avoid making errors when planning their care.
After each visit, the team downloads and scores the physician’s note, identifying both unnecessary tests and missed opportunities. Using Medicare reimbursement data, the team assigns a dollar amount to these errors that would have occurred had the patients been real. Dr. Weiner predicts publicizing the financial costs will bring more attention to the problem.
Access to Care Issues
Actually, physicians should talk to their patients about the cost of their care, says David O. Meltzer, MD, PhD, associate professor and chief of the Section of Hospital Medicine at the University of Chicago Medical Center. In research published in the Journal of the American Medical Association in 2003, Dr. Meltzer and colleagues found patient costs may be associated with medication nonadherence and considerable economic burden.3
“You have to ask a patient the type of questions that will be most revealing, including the broader questions, such as ‘Are there any challenges in your life to getting the care that we’ve discussed after you leave the hospital?’ ” Dr. Stinnette, of Kaiser, says.
In their study, Dr. Meltzer and colleagues also showed that although physicians and patients agree it is important to talk about money issues, it doesn’t commonly happen.3
“In a public hospital where there are greater numbers of uninsured patients, I expect it is much more a frequent topic of conversation,” Dr. Meltzer says. “My suspicion is that the people at greatest risk are uninsured patients who are hospitalized in settings where most patients are insured, and physicians are not attuned to bringing up this issue.”
Although a great deal of literature now deals with physician-patient communication, the techniques seem too scripted to Dr. Weiner. “When you engage with the patient simply as one person who is concerned about another,” he says, “you much more naturally find out what you could do that might help them.”
References
- Weiner SJ, Schwartz A, Yudkowsky R, et al. Evaluating physician performance at individualizing care: A pilot study tracking contextual errors in medical decision-making. Med Decis Making 2007;27(6):726-34.
- Weiner SJ. Contextualizing medical decisions to individualize care: Lessons from the qualitative sciences. J Gen Intern Med 2004;19(3):281-5.
- Alexander GC, Casalino LP, Meltzer DO. Patient-physician communication about out-of-pocket costs. JAMA 2003;290(7):953-958.