User login
Shift-work disorder
The burden of shift-work disorder (SWD) is multifactorial—affecting relationships, health, and work performance. Lack of support, research, and treatment for SWD has economic and safety consequences for society in general. In this supplement, sleep experts suggest practical steps to relieve the adverse effects of SWD.
The burden of shift-work disorder (SWD) is multifactorial—affecting relationships, health, and work performance. Lack of support, research, and treatment for SWD has economic and safety consequences for society in general. In this supplement, sleep experts suggest practical steps to relieve the adverse effects of SWD.
The burden of shift-work disorder (SWD) is multifactorial—affecting relationships, health, and work performance. Lack of support, research, and treatment for SWD has economic and safety consequences for society in general. In this supplement, sleep experts suggest practical steps to relieve the adverse effects of SWD.
Support
Support for the publication of this supplement was provided by Cephalon, Inc. Editorial assistance was provided by Anthemis Consulting Ltd and supported by Cephalon, Inc.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily represent those of Cephalon, Inc., or the publishers. Any procedures, medications, or other courses of diagnosis or treatment discussed or suggested by the authors should not be used by clinicians without evaluation of their patients’ conditions and possible contraindications or dangers in use, review of any applicable manufacturer’s product information, and comparisons with the recommendations of other authorities. Content may include product information that is inconsistent with or outside the approved labeling for these products in the United States. Before prescribing any medication, you must familiarize yourself with the manufacturer’s product information.
This material was submitted by Anthemis Consulting Ltd on behalf of the authors. It has been edited and peer reviewed by The Journal of Family Practice.
Should we routinely screen for hypercapnia in sleep apnea patients before elective noncardiac surgery?
Yes. Obesity hypoventilation syndrome (OHS) is often undiagnosed and greatly increases perioperative risk. Therefore, we recommend trying to detect OHS in a timely manner. Treatment should begin without delay to avoid adverse perioperative outcomes, which can include acute-on-chronic respiratory failure requiring intensive-care monitoring and invasive mechanical ventilation, or death.
ALSO CALLED PICKWICKIAN SYNDROME
OHS is also known as Pickwickian syndrome, named for a character—a “fat boy” who is constantly falling asleep—in The Posthumous Papers of the Pickwick Club by Charles Dickens.
Salient features of OHS are:
- Obesity (body mass index ≥ 30 kg/m2)
- Sleep-disordered breathing (most patients with OHS are morbidly obese and have severe obstructive sleep apnea1)
- Chronic daytime alveolar hypoventilation: ie, Paco2 ≥ 45 mm Hg (normal range 35–45 mm Hg) and Pao2 < 70 mm Hg1 (normal range 85–95 mm Hg)
- No other identifiable cause of hypoventilation such as pulmonary disease (severe obstructive or restrictive), chest wall deformities, severe hypothyroidism, or neuromuscular disease.
WHY SCREEN FOR OHS?
Both obstructive sleep apnea and OHS worsen quality of life and increase the risk of serious disease and death.2–3 Patients with severe sleep apnea, particularly those with hypercapnia (ie, OHS) are at higher risk of cardiopulmonary complications in the perioperative period.
Compared with eucapnic patients with obstructive sleep apnea, patients with OHS have higher health care expenses, are at higher risk of developing serious cardiovascular diseases such as pulmonary hypertension and congestive heart failure, and are more likely to die sooner.4,5
Nowbar et al5 prospectively followed a group of severely obese patients after hospital discharge. At 18 months, 23% of those with OHS had died, compared with 9% of those without OHS. The groups were well matched for body mass index, age, and a number of comorbid conditions. Most of the deaths occurred in the first 3 months after hospital discharge. During the hospital stay, more patients with OHS were admitted to the intensive care unit and needed endotracheal intubation and mechanical ventilation, and more were discharged to a long-term facility.
A high level of suspicion can lead to early recognition and treatment, which may reduce the rate of adverse outcomes associated with undiagnosed and untreated OHS. Routine screening for hypercapnia in patients with sleep apnea might help to identify patients with OHS and allow for modifications in surgical approach, anesthetic technique, and postoperative monitoring, increasing patient safety.
HOW PREVALENT IS OHS?
Obstructive sleep apnea affects up to 20% of US adults and is undiagnosed and untreated in up to 90% of cases.6 Simple screening questionnaires have been shown to reliably identify patients at risk.7,8
To date, no population-based prevalence studies of OHS have been done.
The overall prevalence of OHS in patients with obstructive sleep apnea is better studied: multiple prospective and retrospective studies across various geographic regions with a variety of racial or ethnic populations have shown it to be between 10% and 20%.1,9 This range is very consistent among studies performed in Europe, the United States, and Japan, whether retrospective or prospective, and whether large or small.
The prevalence of OHS in the general adult population in the United States can, however, be estimated. If approximately 5% of the general US population has severe obesity (body mass index ≤ 40 kg/m2), if half of patients with severe obesity have obstructive sleep apnea,10 and if 15% of severely obese patients with sleep apnea have OHS, then a conservative estimated prevalence of OHS in the general adult US population is 0.37% (1 in 270 adults).
WHAT CAN BE DONE BEFORE ELECTIVE SURGERY?
Patients with OHS have an elevated serum bicarbonate level due to metabolic compensation for chronic respiratory acidosis. Moreover, they may have mild hypoxemia during wakefulness as measured by finger pulse oximetry.
The serum venous bicarbonate level is an easy and reasonable test to screen for hypercapnia in obese patients with obstructive sleep apnea because it is readily available, physiologically sensible, and less invasive than arterial puncture to measure blood gases.9
Arterial blood gas measurements, however, should be obtained to confirm the presence and severity of daytime hypercapnia in obese patients with hypoxemia during wakefulness or an elevated serum bicarbonate level.
Pulmonary function testing and chest imaging can exclude other causes of hypercapnia if hypercapnia is confirmed.
An overnight, attended polysomnographic study in a sleep laboratory is ultimately needed to establish the diagnosis and severity of obstructive sleep apnea and to titrate continuous positive airway pressure (CPAP) or bilevel positive airway pressure (BPAP) therapy. Since most patients with OHS have severe obstructive sleep apnea, in-laboratory attended polysomnography allows the clinician to both diagnose and intervene with PAP therapy (a “split-night” study). Home titration with an auto-CPAP device is not recommended because it does not have the ability to titrate PAP pressures in response to hypoxemia or hypoventilation. Patients with OHS require attended, laboratory-based PAP titration with or without supplemental oxygen.
CPAP or BPAP therapy should be started during the few days or weeks before surgery, and adherence should be emphasized. Anesthesiologists might reconsider the choice of anesthetic technique in favor of regional anesthesia and modify postoperative pain management to reduce opioid requirements. Reinstituting CPAP or BPAP therapy upon extubation or arrival in the postoperative recovery unit can further reduce the risk of respiratory complications. Additional monitoring such as continuous pulse oximetry when the patient is on the general ward should be considered.
- Mokhlesi B, Kryger MH, Grunstein RR. Assessment and management of patients with obesity hypoventilation syndrome. Proc Am Thorac Soc 2008; 5:218–225.
- Flegal KM, Graubard BI, Williamson DF, Gail MH. Excess deaths associated with underweight, overweight, and obesity. JAMA 2005; 293:1861–1867.
- Young T, Finn L, Peppard PE, et al. Sleep disordered breathing and mortality: eighteen-year follow-up of the Wisconsin sleep cohort. Sleep 2008; 31:1071–1078.
- Berg G, Delaive K, Manfreda J, Walld R, Kryger MH. The use of health-care resources in obesity-hypoventilation syndrome. Chest 2001; 120:377–383.
- Nowbar S, Burkart KM, Gonzales R, et al. Obesity-associated hypoventilation in hospitalized patients: prevalence, effects, and outcome. Am J Med 2004; 116:1–7.
- Kapur V, Strohl KP, Redline S, Iber C, O'Connor G, Nieto J. Underdiagnosis of sleep apnea syndrome in U.S. communities. Sleep Breath 2002; 6:49–54.
- Finkel KJ, Searleman AC, Tymkew H, et al. Prevalence of undiagnosed obstructive sleep apnea among adult surgical patients in an academic medical center. Sleep Med 2009; 10:753–758.
- Chung F, Yegneswaran B, Liao P, et al. STOP questionnaire: a tool to screen patients for obstructive sleep apnea. Anesthesiology 2008; 108:812–821.
- Mokhlesi B, Tulaimat A, Faibussowitsch I, Wang Y, Evans AT. Obesity hypoventilation syndrome: prevalence and predictors in patients with obstructive sleep apnea. Sleep Breath 2007; 11:117–124.
- Lee W, Nagubadi S, Kryger MH, Mokhlesi B. Epidemiology of obstructive sleep apnea: a population-based perspective. Expert Rev Respir Med 2008; 2:349–364.
Yes. Obesity hypoventilation syndrome (OHS) is often undiagnosed and greatly increases perioperative risk. Therefore, we recommend trying to detect OHS in a timely manner. Treatment should begin without delay to avoid adverse perioperative outcomes, which can include acute-on-chronic respiratory failure requiring intensive-care monitoring and invasive mechanical ventilation, or death.
ALSO CALLED PICKWICKIAN SYNDROME
OHS is also known as Pickwickian syndrome, named for a character—a “fat boy” who is constantly falling asleep—in The Posthumous Papers of the Pickwick Club by Charles Dickens.
Salient features of OHS are:
- Obesity (body mass index ≥ 30 kg/m2)
- Sleep-disordered breathing (most patients with OHS are morbidly obese and have severe obstructive sleep apnea1)
- Chronic daytime alveolar hypoventilation: ie, Paco2 ≥ 45 mm Hg (normal range 35–45 mm Hg) and Pao2 < 70 mm Hg1 (normal range 85–95 mm Hg)
- No other identifiable cause of hypoventilation such as pulmonary disease (severe obstructive or restrictive), chest wall deformities, severe hypothyroidism, or neuromuscular disease.
WHY SCREEN FOR OHS?
Both obstructive sleep apnea and OHS worsen quality of life and increase the risk of serious disease and death.2–3 Patients with severe sleep apnea, particularly those with hypercapnia (ie, OHS) are at higher risk of cardiopulmonary complications in the perioperative period.
Compared with eucapnic patients with obstructive sleep apnea, patients with OHS have higher health care expenses, are at higher risk of developing serious cardiovascular diseases such as pulmonary hypertension and congestive heart failure, and are more likely to die sooner.4,5
Nowbar et al5 prospectively followed a group of severely obese patients after hospital discharge. At 18 months, 23% of those with OHS had died, compared with 9% of those without OHS. The groups were well matched for body mass index, age, and a number of comorbid conditions. Most of the deaths occurred in the first 3 months after hospital discharge. During the hospital stay, more patients with OHS were admitted to the intensive care unit and needed endotracheal intubation and mechanical ventilation, and more were discharged to a long-term facility.
A high level of suspicion can lead to early recognition and treatment, which may reduce the rate of adverse outcomes associated with undiagnosed and untreated OHS. Routine screening for hypercapnia in patients with sleep apnea might help to identify patients with OHS and allow for modifications in surgical approach, anesthetic technique, and postoperative monitoring, increasing patient safety.
HOW PREVALENT IS OHS?
Obstructive sleep apnea affects up to 20% of US adults and is undiagnosed and untreated in up to 90% of cases.6 Simple screening questionnaires have been shown to reliably identify patients at risk.7,8
To date, no population-based prevalence studies of OHS have been done.
The overall prevalence of OHS in patients with obstructive sleep apnea is better studied: multiple prospective and retrospective studies across various geographic regions with a variety of racial or ethnic populations have shown it to be between 10% and 20%.1,9 This range is very consistent among studies performed in Europe, the United States, and Japan, whether retrospective or prospective, and whether large or small.
The prevalence of OHS in the general adult population in the United States can, however, be estimated. If approximately 5% of the general US population has severe obesity (body mass index ≤ 40 kg/m2), if half of patients with severe obesity have obstructive sleep apnea,10 and if 15% of severely obese patients with sleep apnea have OHS, then a conservative estimated prevalence of OHS in the general adult US population is 0.37% (1 in 270 adults).
WHAT CAN BE DONE BEFORE ELECTIVE SURGERY?
Patients with OHS have an elevated serum bicarbonate level due to metabolic compensation for chronic respiratory acidosis. Moreover, they may have mild hypoxemia during wakefulness as measured by finger pulse oximetry.
The serum venous bicarbonate level is an easy and reasonable test to screen for hypercapnia in obese patients with obstructive sleep apnea because it is readily available, physiologically sensible, and less invasive than arterial puncture to measure blood gases.9
Arterial blood gas measurements, however, should be obtained to confirm the presence and severity of daytime hypercapnia in obese patients with hypoxemia during wakefulness or an elevated serum bicarbonate level.
Pulmonary function testing and chest imaging can exclude other causes of hypercapnia if hypercapnia is confirmed.
An overnight, attended polysomnographic study in a sleep laboratory is ultimately needed to establish the diagnosis and severity of obstructive sleep apnea and to titrate continuous positive airway pressure (CPAP) or bilevel positive airway pressure (BPAP) therapy. Since most patients with OHS have severe obstructive sleep apnea, in-laboratory attended polysomnography allows the clinician to both diagnose and intervene with PAP therapy (a “split-night” study). Home titration with an auto-CPAP device is not recommended because it does not have the ability to titrate PAP pressures in response to hypoxemia or hypoventilation. Patients with OHS require attended, laboratory-based PAP titration with or without supplemental oxygen.
CPAP or BPAP therapy should be started during the few days or weeks before surgery, and adherence should be emphasized. Anesthesiologists might reconsider the choice of anesthetic technique in favor of regional anesthesia and modify postoperative pain management to reduce opioid requirements. Reinstituting CPAP or BPAP therapy upon extubation or arrival in the postoperative recovery unit can further reduce the risk of respiratory complications. Additional monitoring such as continuous pulse oximetry when the patient is on the general ward should be considered.
Yes. Obesity hypoventilation syndrome (OHS) is often undiagnosed and greatly increases perioperative risk. Therefore, we recommend trying to detect OHS in a timely manner. Treatment should begin without delay to avoid adverse perioperative outcomes, which can include acute-on-chronic respiratory failure requiring intensive-care monitoring and invasive mechanical ventilation, or death.
ALSO CALLED PICKWICKIAN SYNDROME
OHS is also known as Pickwickian syndrome, named for a character—a “fat boy” who is constantly falling asleep—in The Posthumous Papers of the Pickwick Club by Charles Dickens.
Salient features of OHS are:
- Obesity (body mass index ≥ 30 kg/m2)
- Sleep-disordered breathing (most patients with OHS are morbidly obese and have severe obstructive sleep apnea1)
- Chronic daytime alveolar hypoventilation: ie, Paco2 ≥ 45 mm Hg (normal range 35–45 mm Hg) and Pao2 < 70 mm Hg1 (normal range 85–95 mm Hg)
- No other identifiable cause of hypoventilation such as pulmonary disease (severe obstructive or restrictive), chest wall deformities, severe hypothyroidism, or neuromuscular disease.
WHY SCREEN FOR OHS?
Both obstructive sleep apnea and OHS worsen quality of life and increase the risk of serious disease and death.2–3 Patients with severe sleep apnea, particularly those with hypercapnia (ie, OHS) are at higher risk of cardiopulmonary complications in the perioperative period.
Compared with eucapnic patients with obstructive sleep apnea, patients with OHS have higher health care expenses, are at higher risk of developing serious cardiovascular diseases such as pulmonary hypertension and congestive heart failure, and are more likely to die sooner.4,5
Nowbar et al5 prospectively followed a group of severely obese patients after hospital discharge. At 18 months, 23% of those with OHS had died, compared with 9% of those without OHS. The groups were well matched for body mass index, age, and a number of comorbid conditions. Most of the deaths occurred in the first 3 months after hospital discharge. During the hospital stay, more patients with OHS were admitted to the intensive care unit and needed endotracheal intubation and mechanical ventilation, and more were discharged to a long-term facility.
A high level of suspicion can lead to early recognition and treatment, which may reduce the rate of adverse outcomes associated with undiagnosed and untreated OHS. Routine screening for hypercapnia in patients with sleep apnea might help to identify patients with OHS and allow for modifications in surgical approach, anesthetic technique, and postoperative monitoring, increasing patient safety.
HOW PREVALENT IS OHS?
Obstructive sleep apnea affects up to 20% of US adults and is undiagnosed and untreated in up to 90% of cases.6 Simple screening questionnaires have been shown to reliably identify patients at risk.7,8
To date, no population-based prevalence studies of OHS have been done.
The overall prevalence of OHS in patients with obstructive sleep apnea is better studied: multiple prospective and retrospective studies across various geographic regions with a variety of racial or ethnic populations have shown it to be between 10% and 20%.1,9 This range is very consistent among studies performed in Europe, the United States, and Japan, whether retrospective or prospective, and whether large or small.
The prevalence of OHS in the general adult population in the United States can, however, be estimated. If approximately 5% of the general US population has severe obesity (body mass index ≤ 40 kg/m2), if half of patients with severe obesity have obstructive sleep apnea,10 and if 15% of severely obese patients with sleep apnea have OHS, then a conservative estimated prevalence of OHS in the general adult US population is 0.37% (1 in 270 adults).
WHAT CAN BE DONE BEFORE ELECTIVE SURGERY?
Patients with OHS have an elevated serum bicarbonate level due to metabolic compensation for chronic respiratory acidosis. Moreover, they may have mild hypoxemia during wakefulness as measured by finger pulse oximetry.
The serum venous bicarbonate level is an easy and reasonable test to screen for hypercapnia in obese patients with obstructive sleep apnea because it is readily available, physiologically sensible, and less invasive than arterial puncture to measure blood gases.9
Arterial blood gas measurements, however, should be obtained to confirm the presence and severity of daytime hypercapnia in obese patients with hypoxemia during wakefulness or an elevated serum bicarbonate level.
Pulmonary function testing and chest imaging can exclude other causes of hypercapnia if hypercapnia is confirmed.
An overnight, attended polysomnographic study in a sleep laboratory is ultimately needed to establish the diagnosis and severity of obstructive sleep apnea and to titrate continuous positive airway pressure (CPAP) or bilevel positive airway pressure (BPAP) therapy. Since most patients with OHS have severe obstructive sleep apnea, in-laboratory attended polysomnography allows the clinician to both diagnose and intervene with PAP therapy (a “split-night” study). Home titration with an auto-CPAP device is not recommended because it does not have the ability to titrate PAP pressures in response to hypoxemia or hypoventilation. Patients with OHS require attended, laboratory-based PAP titration with or without supplemental oxygen.
CPAP or BPAP therapy should be started during the few days or weeks before surgery, and adherence should be emphasized. Anesthesiologists might reconsider the choice of anesthetic technique in favor of regional anesthesia and modify postoperative pain management to reduce opioid requirements. Reinstituting CPAP or BPAP therapy upon extubation or arrival in the postoperative recovery unit can further reduce the risk of respiratory complications. Additional monitoring such as continuous pulse oximetry when the patient is on the general ward should be considered.
- Mokhlesi B, Kryger MH, Grunstein RR. Assessment and management of patients with obesity hypoventilation syndrome. Proc Am Thorac Soc 2008; 5:218–225.
- Flegal KM, Graubard BI, Williamson DF, Gail MH. Excess deaths associated with underweight, overweight, and obesity. JAMA 2005; 293:1861–1867.
- Young T, Finn L, Peppard PE, et al. Sleep disordered breathing and mortality: eighteen-year follow-up of the Wisconsin sleep cohort. Sleep 2008; 31:1071–1078.
- Berg G, Delaive K, Manfreda J, Walld R, Kryger MH. The use of health-care resources in obesity-hypoventilation syndrome. Chest 2001; 120:377–383.
- Nowbar S, Burkart KM, Gonzales R, et al. Obesity-associated hypoventilation in hospitalized patients: prevalence, effects, and outcome. Am J Med 2004; 116:1–7.
- Kapur V, Strohl KP, Redline S, Iber C, O'Connor G, Nieto J. Underdiagnosis of sleep apnea syndrome in U.S. communities. Sleep Breath 2002; 6:49–54.
- Finkel KJ, Searleman AC, Tymkew H, et al. Prevalence of undiagnosed obstructive sleep apnea among adult surgical patients in an academic medical center. Sleep Med 2009; 10:753–758.
- Chung F, Yegneswaran B, Liao P, et al. STOP questionnaire: a tool to screen patients for obstructive sleep apnea. Anesthesiology 2008; 108:812–821.
- Mokhlesi B, Tulaimat A, Faibussowitsch I, Wang Y, Evans AT. Obesity hypoventilation syndrome: prevalence and predictors in patients with obstructive sleep apnea. Sleep Breath 2007; 11:117–124.
- Lee W, Nagubadi S, Kryger MH, Mokhlesi B. Epidemiology of obstructive sleep apnea: a population-based perspective. Expert Rev Respir Med 2008; 2:349–364.
- Mokhlesi B, Kryger MH, Grunstein RR. Assessment and management of patients with obesity hypoventilation syndrome. Proc Am Thorac Soc 2008; 5:218–225.
- Flegal KM, Graubard BI, Williamson DF, Gail MH. Excess deaths associated with underweight, overweight, and obesity. JAMA 2005; 293:1861–1867.
- Young T, Finn L, Peppard PE, et al. Sleep disordered breathing and mortality: eighteen-year follow-up of the Wisconsin sleep cohort. Sleep 2008; 31:1071–1078.
- Berg G, Delaive K, Manfreda J, Walld R, Kryger MH. The use of health-care resources in obesity-hypoventilation syndrome. Chest 2001; 120:377–383.
- Nowbar S, Burkart KM, Gonzales R, et al. Obesity-associated hypoventilation in hospitalized patients: prevalence, effects, and outcome. Am J Med 2004; 116:1–7.
- Kapur V, Strohl KP, Redline S, Iber C, O'Connor G, Nieto J. Underdiagnosis of sleep apnea syndrome in U.S. communities. Sleep Breath 2002; 6:49–54.
- Finkel KJ, Searleman AC, Tymkew H, et al. Prevalence of undiagnosed obstructive sleep apnea among adult surgical patients in an academic medical center. Sleep Med 2009; 10:753–758.
- Chung F, Yegneswaran B, Liao P, et al. STOP questionnaire: a tool to screen patients for obstructive sleep apnea. Anesthesiology 2008; 108:812–821.
- Mokhlesi B, Tulaimat A, Faibussowitsch I, Wang Y, Evans AT. Obesity hypoventilation syndrome: prevalence and predictors in patients with obstructive sleep apnea. Sleep Breath 2007; 11:117–124.
- Lee W, Nagubadi S, Kryger MH, Mokhlesi B. Epidemiology of obstructive sleep apnea: a population-based perspective. Expert Rev Respir Med 2008; 2:349–364.
Food allergy and eosinophilic esophagitis: Learning what to avoid
More children and even adults seem to be allergic to various foods these days than in the past. Also apparently on the rise is a linked condition, eosinophilic esophagitis.
The reason for these increases is not clear. This article confines itself to what we know about the mechanisms of food allergies and eosinophilic esophagitis, how to diagnose them, and how to treat them.
FOOD ALLERGIES ARE COMMON, AND MORE PREVALENT THAN EVER
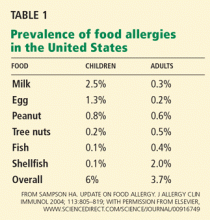
Food allergies—abnormal immune responses to food proteins1—affect an estimated 6% to 8% of young children and 3% to 4% of adults in the United States,2,3 and their prevalence appears to be rising in developed countries. Studies in US and British children indicate that peanut allergy has doubled in the past decade. 4
Any food can provoke a reaction, but only a few foods account for most of the significant allergic reactions: cow’s milk, soy, wheat, eggs, peanuts, tree nuts, fish, and shellfish.
Approximately 80% of allergies to milk, egg, wheat, and soy resolve by the time the patient reaches early adolescence.6 Fewer cases resolve in children with tree nut allergies (approximately 9%) or peanut allergy (20%),7,8 and allergies to fish and shellfish often develop or persist in adulthood.
A family history of an atopic disease such as asthma, allergic rhinitis, eczema, or food allergy is a risk factor for developing a food allergy. 3 Considering that the rate of peanut allergy has doubled in children over the past 10 years, environmental factors may also play a role.3
How we tolerate foods or become allergic to them
The gut, the largest mucosal organ in the body, is exposed to large quantities of foreign proteins daily. Most protein is broken down by stomach acid and digestive enzymes into lessantigenic peptides or is bound by secretory immunoglobulin A (IgA), which prevents it from being absorbed. Further, the epithelial cells lining the gut do not allow large molecules to pass easily, having tight intracellular junctions and being covered with mucus.
For these reasons, less than 2% of the protein in food is absorbed in an allergenic form.9 The reason food allergies are more prevalent in children is most likely that children have an immature gut barrier, lower IgA levels, a higher gastric pH, and lower proteolytic enzyme levels.
When dietary proteins do cross the gut barrier, the immune system normally suppresses the allergic response. Regulatory T cells, dendritic cells, and local immune responses play critical roles in the development of tolerance. Several types of regulatory T cells, such as Tr1 cells (which secrete interleukin 10), TH3 cells (which secrete transforming growth factor beta), CD4+CD25+ regulatory T cells, gamma-delta T cells, and CD8+ suppressor cells can all contribute to suppressing allergic responses.10 Dendritic cells also help induce tolerance by stimulating CD4+ T cells to secrete transforming growth factor beta, which leads to the production of interleukin 10 and additional transforming growth factor beta.11
Factors that contribute to food allergy
Many factors may contribute to whether a person becomes tolerant to or sensitized to a specific food protein.
The dose of antigen. Tolerance can develop after either high or low doses of antigens, but by different mechanisms.
The antigen structure. Soluble antigens are less sensitizing than particulate antigens.12,13
Processing of foods. Dry-roasted peanuts are more allergenic than raw or boiled peanuts, partly because they are less soluble.13
The route of initial exposure. Sensitization to food proteins can occur directly through the gut or the skin. Alternatively, it can occur indirectly via the respiratory tract. Skin exposure may be especially sensitizing in children with atopic dermatitis.14,15
The gut flora. When mice are raised in a germ-free environment, they fail to develop normal tolerance.16 They are also more likely to become sensitized if they are treated with antibiotics or if they lack toll-like receptors that recognize bacterial lipopolysaccharides.17 Furthermore, human studies suggest that probiotics promote tolerance, especially in preventing atopic dermatitis, although the studies have had conflicting results.18–21
The gastric pH. Murine and human studies reveal that antacid medications increase the risk of food allergy.22,23
Genetic susceptibility. A child with a sibling who is allergic to peanuts is approximately 10 times more likely to be allergic to peanuts than predicted by the rate in the general population. Although no risk-conferring gene has been identified, a study of twins showed concordance for peanut allergy in 64.3% of identical twins vs 6.8% of fraternal twins.24
Three types of immune responses to food
Immunologic reactions to foods can be divided into three categories: mediated by immunoglobulin E (IgE), non-IgE-mediated, and mixed. Therefore, these disorders can present as an acute, potentially life-threatening reaction or as a chronic disease such as eosinophilic gastoenteropathy.
IgE-mediated reactions are immediate hypersensitivity responses. In most patients, an IgE-mediated mechanism can be confirmed by a positive skin test or a test for food-specific IgE in the serum. In this article, the term “food allergy” refers to an IgE-mediated reaction to a food, unless otherwise indicated.
Non-IgE-mediated reactions have a delayed onset and chronic symptoms. Commonly, they are confined to the gastrointestinal tract; examples are food-protein-induced enterocolitis, proctitis, and proctocolitis and celiac disease.3,26,27 However, other diseases such as contact dermatitis, dermatitis herpetiformis, and food-induced pulmonary hemosiderosis (Heiner syndrome) are also considered non-IgE-mediated allergies.
Mixed-reaction disorders are chronic and include the eosinophilic gastroenteropathies, ie, eosinophilic proctocolitis, eosinophilic gastroenteritis, and eosinophilic esophagitis.28 The pathophysiology of these diseases is poorly understood. Many patients have evidence of allergic sensitivities to food or to environmental allergens, or both, but whether these sensitivities have a causal role in these disorders is not clear.
Atopic dermatitis, another complicated disease process, may be associated with mixedreaction food allergy, as approximately 35% of young children with moderate to severe atopic dermatitis have food allergies.29
Diagnosis of IgE-mediated food allergies
A thorough history and physical examination are key to diagnosing an IgE-mediated food allergy.
The history should include potential culprit foods, the quantity eaten, the timing of the onset of symptoms, and related factors such as exercise, alcohol intake, or medication use. Symptoms of an IgE-mediated reaction are generally rapid in onset but may be delayed up to a few hours, while non-IgE mediated symptoms may present several hours to days later.
Food challenge. A double-blind, placebocontrolled oral food challenge is the gold standard for the diagnosis of food allergies. (The food to be tested is hidden in other food or in capsules.) However, this test poses significant risks, and other diagnostic methods are more practical for screening.
Skin-prick tests with commercially available extracts are a rapid and sensitive method of screening for allergy to several foods.
Negative skin-prick tests have an estimated negative predictive value of more than 95% and can therefore exclude IgE-mediated food allergies.
A positive test indicates the presence of IgE against a specific food allergen and suggests a clinical food allergy, although the specificity of the test is only about 50%, making a positive result difficult to interpret. Although the size of the skin-test response does not necessarily correlate with the potential severity of a reaction, a response larger than 3 mm does indicate a greater likelihood of clinical reactivity. A positive test is most helpful in confirming the diagnosis of IgE-mediated food allergy when combined with a clear history of food-induced symptoms.
The proteins in commercially based extracts of most fruits and vegetables are often labile; therefore, skin testing with fresh fruits and vegetables may be indicated.30
Immunoassays. Radioallergosorbent tests (RASTs) and fluorescent enzyme immunoassays are used to identity food-specific IgE antibodies in the serum. The commercially available tests do not use radioactivity, but the term “RAST” is still commonly used.
Immunoassays are generally less sensitive and more costly than skin-prick tests, and their results are not immediately available, unlike those of skin-prick testing. However, these in vitro tests are not affected by antihistamine use and are useful in patients with severe dermatologic conditions or severe anaphylaxis, for whom skin-prick testing would not be appropriate.
However, unlike a negative skin-prick test, an undetectable serum food-specific IgE level has a low negative predictive value, and an undetectable level may be associated with symptoms of an allergic reaction for 10% to 25% of patients.29 Therefore, if one suspects an allergic reaction but no food-specific IgE can be detected in the serum, confirming the absence of a clinical allergy must be done with a skin-prick test or with a physician-supervised oral challenge, or both.
Managing food allergy by avoiding the allergen
Food allergies are managed by strictly avoiding food allergens and by taking medications such as self-injectable epinephrine for anaphylactic symptoms.
Patients and caregivers must be educated about reading food labels, avoiding high-risk situations such as eating at buffets and other restaurants with high risk of cross-contamination, wearing a medical-alert bracelet, recognizing and managing early symptoms of an allergic reaction, and calling for emergency services if they are having an allergic reaction. Since January 2006, the US Food and Drug Administration has required food manufacturers to list common food allergens on food labels (cow’s milk, soy, wheat, egg, peanut, tree nuts, fish, and shellfish), and the labeling must use simple, easily understood terms, such as “milk” instead of “whey.” However, it is still prudent to read all ingredients listed on the label.
Experimental treatments for food allergies
Humanized monoclonal anti-IgE antibodies such as talizumab (also known as TNX-901) and omalizumab (Xolair) have been developed, but their use in food allergy has been limited. In a study in patients with peanut allergy, injections of talizumab increased the threshold for sensitivity to peanuts in most patients, but 25% of the patients did not have any improvement.32 A study of omalizumab in patients with peanut allergy was stopped after adverse reactions developed during oral peanut challenges.33
Oral immunotherapy. Recent studies suggest it may be possible to induce oral tolerance in patients with IgE-mediated food allergy. Pilot studies have shown that frequent, increasing doses of food allergens (egg, milk, and peanut) may raise the threshold at which symptoms occur.34–36 Though these studies suggest that oral immunotherapy may protect some patients against a reaction if they accidentally ingest a food they are allergic to, some patients could not reach the goal doses because allergic symptoms were provoked.
At this early stage, these strategies must be considered investigational, and more randomized, placebo-controlled studies are needed. Further studies will also be needed to assess whether oral immunotherapy induces only short-term desensitization (in which case the allergen needs to be ingested daily to prevent reactions) or sustained tolerance (in which case the antigenic protein can be ingested without symptoms despite periods of abstinence).
THE ROLE OF FOOD ALLERGY IN EOSINOPHILIC ESOPHAGITIS
Eosinophilic esophagitis has been recognized with increasing frequency in both children and adults over the past several years. Symptoms can include difficulty feeding, failure to thrive, vomiting, epigastric or chest pain, dysphagia, and food impaction.
Diagnostic criteria for eosinophilic esophagitis are37:
- Clinical symptoms of esophageal dysfunction
- At least 15 eosinophils per high-power field in at least one esophageal biopsy specimen
- No response to a proton-pump inhibitor in high doses (up to 2 mg/kg/day) for 1 to 2 months, or normal results on pH probe monitoring of the esophagus (the reason for this criterion is that patients with gastroesophageal reflux disease can also have large numbers of eosinophils in the esophagus—more than 100 per highpower field38)
- Exclusion of other causes.
Though the cause of eosinophilic esophagitis is not completely understood, atopy has been strongly implicated as a factor. More than 50% of patients with eosinophilic esophagitis also have an atopic condition (eg, atopic dermatitis, allergic rhinitis, asthma), as well as positive results on skin-prick testing or measurement of antigen-specific IgE in the serum.39–41 Also, since most patients improve with either dietary restriction or elemental diets, food sensitization appears to play a considerable role.
As with atopic conditions such as asthma, atopic dermatitis, allergic rhinitis, and food allergy, eosinophilic esophagitis has been linked with immune responses involving helper T cell 2 (TH2). Adults and children with eosinophilic esophagitis have been found to have elevated eosinophil counts and total IgE levels in peripheral blood.37 In the esophagus, patients have elevated levels of the TH2 cytokines often seen in atopic patients (eg, interleukins 4, 5, and 13) and mast cells.42,43 In mice, eosinophilic esophagitis can be induced by allergen exposure and overexpression of TH2 cytokines.44,45 Expression of eotaxin-3, a potent eosinophil chemoattractant, was noted to be higher in children with eosinophilic esophagitis than in controls.46
Of interest, some patients with eosinophilic esophagitis say their symptoms vary with the seasons, correlating with seasonal changes in esophageal eosinophil levels.47,48
Studies linking eosinophilic esophagitis and food allergy in children
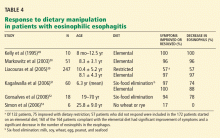
Kelly et al49 reported that 10 children with chronic symptomatic gastroesophageal reflux and eosinophilic esophagitis all had partial or complete resolution of symptoms on an elemental diet.
Markowitz et al50 found that symptoms of chronic reflux disease and eosinophilic esophagitis improved in 49 of 51 children on an elemental diet, and the number of eosinophils in the distal esophagus decreased significantly.
Liacouras et al39 reported similar findings in a 10-year experience. Of 132 children who had eosinophilic esophagitis, 75 improved with dietary restriction based on results of skin-prick and patch testing. The 57 patients who did not respond and 115 others were started on an elemental diet. Of the 164 patients who complied with the elemental diet, 160 had significant improvement of symptoms and a significant decrease in the number of eosinophils in the esophagus. Individual foods were reintroduced approximately every 5 days, and esophagogastroduodenoscopy with biopsies was performed 4 to 8 weeks after the last was reintroduced into the diet.
In a retrospective study, Kagalwalla et al51 reported that 60 children with eosinophilic esophagitis were treated with either an elemental diet or a six-food elimination diet (no milk, soy, wheat, egg, peanut, or seafood). The two groups showed similar clinical and histologic improvements.
Collectively, these studies in pediatric patients imply that food allergy is a significant factor in the pathogenesis of eosinophilic esophagitis.
Studies in adults
Fewer studies of the link between food allergy and eosinophilic esophagitis have been done in adults.
In a preliminary study, 18 adults followed the six-food elimination diet. Symptoms improved in 17 (94%), and histologic findings improved in 14 (78%).52
On the other hand, in six adult patients with eosinophilic esophagitis, Simon et al53 found that only one had improvement in symptoms after eliminating wheat and rye from the diet, and none had significant changes in the number of eosinophils in the esophagus.
In a 37-year-old man with eosinophilic esophagitis, symptoms improved after eliminating egg from his diet.54
Yamazaki et al55 measured expression of interleukin 5 and interleukin 13 in 15 adult patients with eosinophilic esophagitis. Food and aeroallergens that included milk, soy, dust mite, ragweed, and Aspergillus induced significantly more interleukin 5 production in these patients than in atopic controls, suggesting that both foods and aeroallergens may have a role in the pathogenesis of eosinophilic esophagitis in adults.
How to identify potential food triggers of eosinophilic esophagitis
Though elemental diets have been associated with a decrease in symptoms and esophageal eosinophilia, elemental formulas are expensive and unpalatable and pose a risk of nutritional deprivation. Identifying specific food allergens to eliminate from the diet in patients with eosinophilic esophagitis may be less expensive and more desirable than a very limited or elemental diet.
However, potential food triggers have been hard to identify in eosinophilic esophagitis. A recent consensus report did not recommend in vitro food allergy testing,37 owing to a lack of positive or negative predictive values for food-specific IgE level testing in eosinophilic esophagitis. Furthermore, the absence of IgE does not eliminate a food as a potential trigger, since non-IgE mechanisms may play a role.
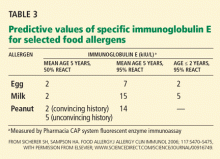
Skin-prick testing is one of the currently validated diagnostic methods. Several studies have used skin-prick testing of foods in patients with eosinophilic esophagitis. In these studies, approximately two-thirds of patients had positive test reactions to at least one food, most often to common food allergens such as cow’s milk, egg, soy, wheat, and peanut, but also to rye, beef, and bean.37 In a recent article,56 81% of adult patients with eosinophilic esophagitis had one or more allergens identified by skin-prick testing, and 50% of the patients tested positive for one or more food allergens.
Atopy patch testing. The combination of skin-prick testing and atopy patch testing may be more effective than skin-prick testing alone in identifying potential food triggers. Atopy patch testing has been used in the diagnosis of non-IgE cell-mediated (delayed) immune responses, in which T cells may play a significant role.
Atopy patch testing is similar to patch testing for contact dermatitis. It involves placing a small quantity of food on the skin and evaluating for a local delayed reaction after a set time.
In two studies,50,57 146 children with biopsy-proven eosinophilic esophagitis had foods eliminated from the diet on the basis of positive skin-prick tests and atopy patch tests. Approximately 77% of the children had significant reduction of esophageal eosinophils in biopsy specimens (from 20 per high-power field to 1.1). The foods most commonly implicated by skin-prick testing were cow’s milk, egg, wheat, peanut, shellfish, peas, beef, fish, rye, and tomato; those identified by atopy patch testing were cow’s milk, egg, wheat, corn, beef, milk, soy, rye, chicken, oats, and potato. The combination of both types of testing had a negative predictive value of 88% to 100% for all foods except milk, while the positive predictive value was greater than 74% for the most common foods causing eosinophilic esophagitis.58
Though atopy patch testing shows some usefulness in identifying foods that may elicit non-IgE-mediated reactions, currently these tests are not validated and have been evaluated in only a small number of studies. Currently, no standardized testing materials, methods of application, or interpretation of results exist, and no studies have included a control population to validate atopy patch testing. More studies are needed to validate atopy patch testing as a reliable diagnostic tool before it can be recommended as a component of routine diagnostic evaluation in patients with eosinophilic esophagitis.
- Bruijnzeel-Koomen C, Ortolani C, Aas K, et al. Adverse reactions to food. European Academy of Allergology and Clinical Immunology Subcommittee. Allergy 1995; 50:623–635.
- Sampson HA. Update on food allergy. J Allergy Clin Immunol 2004; 113:805–819.
- Sicherer SH, Sampson HA. 9. Food allergy. J Allergy Clin Immunol 2006; 117 (suppl 2):S470–S475.
- Sicherer SH, Munoz-Furlong A, Sampson HA. Prevalence of peanut and tree nut allergy in the United States determined by means of a random digit dial telephone survey: a 5-year follow-up study. J Allergy Clin Immunol 2003; 112:1203–1207.
- American College of Allergy, Asthma, & Immunology. Food allergy: a practice parameter. Ann Allergy Asthma Immunol 2006; 96( suppl 2):S1–S68.
- Wood RA. The natural history of food allergy. Pediatrics 2003; 111:1631–1637.
- Hourihane JO, Roberts SA, Warner JO. Resolution of peanut allergy: case-control study. BMJ 1998; 316:1271–1275.
- Fleischer DM, Conover-Walker MK, Matsui EC, Wood RA. The natural history of tree nut allergy. J Allergy Clin Immunol 2005; 116:1087–1093.
- Husby S, Foged N, Host A, Svehag SE. Passage of dietary antigens into the blood of children with coeliac disease. Quantification and size distribution of absorbed antigens. Gut 1987; 28:1062–1072.
- Mowat AM. Anatomical basis of tolerance and immunity to intestinal antigens. Nat Rev Immunol 2003; 3:331–341.
- Frossard CP, Tropia L, Hauser C, Eigenmann PA. Lymphocytes in Peyer patches regulate clinical tolerance in a murine model of food allergy. J Allergy Clin Immunol 2004; 113:958–964.
- Jain SL, Barone KS, Flanagan MP, Michael JG. Activation patterns of murine B cells after oral administration of an encapsulated soluble antigen. Vaccine 1996; 14:1291–1297.
- Kopper RA, Odum NJ, Sen M, Helm RM, Stanley JS, Burks AW. Peanut protein allergens: the effect of roasting on solubility and allergenicity. Int Arch Allergy Immunol 2005; 136:16–22.
- Lack G. Epidemiologic risks for food allergy. J Allergy Clin Immunol 2008; 121:1331–1336.
- Lack G, Fox D, Northstone K, Golding J; Avon Longitudinal Study of Parents and Children Study Team. Factors associated with the development of peanut allergy in childhood. N Engl J Med 2003; 348:977–985.
- Sudo N, Sawamura S, Tanaka K, Aiba Y, Kubo C, Koga Y. The requirement of intestinal bacterial flora for the development of an IgE production system fully susceptible to oral tolerance induction. J Immunol 1997; 159:1739–1745.
- Bashir ME, Louie S, Shi HN, Nagler-Anderson C. Toll-like receptor 4 signaling by intestinal microbes influences susceptibility to food allergy. J Immunol 2004; 172:6978–6987.
- Kopp MV, Hennemuth I, Heinzmann A, Urbanek R. Randomized, double-blind, placebo-controlled trial of probiotics for primary prevention: no clinical effects of lactobacillus GG supplementation. Pediatrics 2008; 121:e850–e856.
- Kukkonen K, Savilahti E, Haahtela T, et al. Probiotics and prebiotic galacto-oligosaccharides in the prevention of allergic diseases: a randomized, double-blind, placebo-controlled trial. J Allergy Clin Immunol 2007; 119:192–198.
- Osborn DA, Sinn JK. Probiotics in infants for prevention of allergic disease and food hypersensitivity. Cochrane Database Syst Rev 2007;CD006475.
- Prescott SL, Bjorksten B. Probiotics for the prevention or treatment of allergic diseases. J Allergy Clin Immunol 2007; 120:255–262.
- Untersmayr E, Jensen-Jarolim E. The role of protein digestibility and antacids on food allergy outcomes. J Allergy Clin Immunol 2008; 121:1301–1308.
- Untersmayr E, Scholl I, Swoboda I, et al. Antacid medication inhibits digestion of dietary proteins and causes food allergy: a fish allergy model in BALB/c mice. J Allergy Clin Immunol 2003; 112:616–623.
- Sicherer SH, Furlong TJ, Maes HH, Desnick RJ, Sampson HA, Gelb BD. Genetics of peanut allergy: a twin study. J Allergy Clin Immunol 2000; 106:53–56.
- Sicherer SH, Sampson HA. Food allergy: recent advances in pathophysiology and treatment. Annu Rev Med 2009; 60:261–277.
- Sampson HA, Anderson JA. Summary and recommendations: classification of gastrointestinal manifestations due to immunologic reactions to foods in infants and young children. J Pediatr Gastroenterol Nutr 2000; 30( suppl 1):S87–S94.
- Sampson HA, Sicherer SH, Birnbaum AH. AGA technical review on the evaluation of food allergy in gastrointestinal disorders. American Gastroenterological Association. Gastroenterology 2001; 120:1026–1040.
- Spergel JM, Pawlowski NA. Food allergy. Mechanisms, diagnosis, and management in children. Pediatr Clin North Am 2002; 49:73–96.
- Sampson HA. Utility of food-specific IgE concentrations in predicting symptomatic food allergy. J Allergy Clin Immunol 2001; 107:891–896.
- Ortolani C, Ispano M, Pastorello EA, Ansaloni R, Magri GC. Comparison of results of skin prick tests (with fresh foods and commercial food extracts) and RAST in 100 patients with oral allergy syndrome. J Allergy Clin Immunol 1989; 83:683–690.
- Perry TT, Matsui EC, Kay Conover-Walker M, Wood RA. The relationship of allergen-specific IgE levels and oral food challenge outcome. J Allergy Clin Immunol 2004; 114:144–149.
- Leung DY, Sampson HA, Yunginger JW, et al; Avon Longitudinal Study of Parents and Children Study Team. Effect of anti-IgE therapy in patients with peanut allergy. N Engl J Med 2003; 348:986–993.
- Sampson HA. A phase II, randomized double-blind, parallel-group, placebo-controlled, oral food challenge trial of Xolair (omalizumab) in peanut allergy (TOPS). J Allergy Clin Immunol 2007; 119 (suppl 1):S117.
- Buchanan AD, Green TD, Jones SM, et al Egg oral immunotherapy in nonanaphylactic children with egg allergy. J Allergy Clin Immunol 2007; 119:199–205.
- Burks AW, Jones SM. Egg oral immunotherapy in non-anaphylactic children with egg allergy: follow-up. J Allergy Clin Immunol 2008; 121:270–271.
- Skripak JM, Nash SD, Rowley H, et al. A randomized, double-blind, placebo-controlled study of milk oral immunotherapy for cow's milk allergy. J Allergy Clin Immunol 2008; 122:1154–1160.
- Furuta GT, Liacouras CA, Collins MH, et al; First International Gastrointestinal Eosinophil Research Symposium (FIGERS) Subcommittees. Eosinophilic esophagitis in children and adults: a systematic review and consensus recommendations for diagnosis and treatment. Gastroenterology 2007; 133:1342–1363.
- Rodrigo S, Abboud G, Oh D, et al. High intraepithelial eosinophil counts in esophageal squamous epithelium are not specific for eosinophilic esophagitis in adults. Am J Gastroenterol 2008; 103:435–442.
- Liacouras CA, Spergel JM, Ruchelli E, et al. Eosinophilic esophagitis: a 10-year experience in 381 children. Clin Gastroenterol Hepatol 2005; 3:1198–1206.
- Simon D, Marti H, Heer P, Simon HU, Braathen LR, Straumann A. Eosinophilic esophagitis is frequently associated with IgE-mediated allergic airway diseases. J Allergy Clin Immunol 2005; 115:1090–1092.
- Rothenberg ME, Mishra A, Collins MH, Putnam PE. Pathogenesis and clinical features of eosinophilic esophagitis. J Allergy Clin Immunol 2001; 108:891–894.
- Gupta SK, Fitzgerald JF, Kondratyuk T, HogenEsch H. Cytokine expression in normal and inflamed esophageal mucosa: a study into the pathogenesis of allergic eosinophilic esophagitis. J Pediatr Gastroenterol Nutr 2006; 42:22–26.
- Straumann A, Bauer M, Fischer B, Blaser K, Simon HU. Idiopathic eosinophilic esophagitis is associated with a T(H)2-type allergic inflammatory response. J Allergy Clin Immunol 2001; 108:954–961.
- Mishra A, Rothenberg ME. Intratracheal IL-13 induces eosinophilic esophagitis by an IL-5, eotaxin-1, and STAT6-dependent mechanism. Gastroenterology 2003; 125:1419–1427.
- Akei HS, Mishra A, Blanchard C, Rothenberg ME. Epicutaneous antigen exposure primes for experimental eosinophilic esophagitis in mice. Gastroenterology 2005; 129:985–994.
- Blanchard C, Wang N, Stringer KF, et al. Eotaxin-3 and a uniquely conserved gene-expression profile in eosinophilic esophagitis. J Clin Invest 2006; 116:536–547.
- Fogg MI, Ruchelli E, Spergel JM. Pollen and eosinophilic esophagitis. J Allergy Clin Immunol 2003; 112:796–797.
- Almansa C, Krishna M, Buchner AM, et al. Seasonal distribution in newly diagnosed cases of eosinophilic esophagitis in adults. Am J Gastroenterol 2009; 104:828–833.
- Kelly KJ, Lazenby AJ, Rowe PC, Yardley JH, Perman JA, Sampson HA. Eosinophilic esophagitis attributed to gastroesophageal reflux: improvement with an amino acid-based formula. Gastroenterology 1995; 109:1503–1512.
- Markowitz JE, Spergel JM, Ruchelli E, Liacouras CA. Elemental diet is an effective treatment for eosinophilic esophagitis in children and adolescents. Am J Gastroenterol 2003; 98:777–782.
- Kagalwalla AF, Sentongo TA, Ritz S, et al. Effect of six-food elimination diet on clinical and histologic outcomes in eosinophilic esophagitis. Clin Gastroenterol Hepatol 2006; 4:1097–1102.
- Gonsalves N, Yang GY, Doerfler B, et al. A prospective clinical trial of six food elimination diet and reintroduction of causative agents in adults with eosinophilic esophagitis [abstract]. Gastroenterology 2008; 134( suppl 1):A104–A105.
- Simon D, Straumann A, Wenk A, Spichtin H, Simon HU, Braathen LR. Eosinophilic esophagitis in adults—no clinical relevance of wheat and rye sensitizations. Allergy 2006; 61:1480–1483.
- Antón Remirez J, Escudero R, Caceres O, Fernandez-Benitez M. Eosinophilic esophagitis. Allergol Immunopathol (Madr) 2006; 34:79–81.
- Yamazaki K, Murray JA, Arora AS, et al. Allergen-specific in vitro cytokine production in adult patients with eosinophilic esophagitis. Dig Dis Sci 2006; 51:1934–1941.
- Penfield JD, Lang DM, Goldblum JR, Lopez R, Falk GW. The role of allergy evaluation in adults with eosinophilic esophagitis. J Clin Gastroenterol 2009(Epub ahead of print)
- Spergel JM, Andrews T, Brown-Whitehorn TF, Beausoleil JL, Liacouras CA. Treatment of eosinophilic esophagitis with specific food elimination diet directed by a combination of skin prick and patch tests. Ann Allergy Asthma Immunol 2005; 95:336–343.
- Spergel JM, Brown-Whitehorn T, Beausoleil JL, Shuker M, Liacouras CA. Predictive values for skin prick test and atopy patch test for eosinophilic esophagitis. J Allergy Clin Immunol 2007; 119:509–511.
More children and even adults seem to be allergic to various foods these days than in the past. Also apparently on the rise is a linked condition, eosinophilic esophagitis.
The reason for these increases is not clear. This article confines itself to what we know about the mechanisms of food allergies and eosinophilic esophagitis, how to diagnose them, and how to treat them.
FOOD ALLERGIES ARE COMMON, AND MORE PREVALENT THAN EVER
Food allergies—abnormal immune responses to food proteins1—affect an estimated 6% to 8% of young children and 3% to 4% of adults in the United States,2,3 and their prevalence appears to be rising in developed countries. Studies in US and British children indicate that peanut allergy has doubled in the past decade. 4
Any food can provoke a reaction, but only a few foods account for most of the significant allergic reactions: cow’s milk, soy, wheat, eggs, peanuts, tree nuts, fish, and shellfish.
Approximately 80% of allergies to milk, egg, wheat, and soy resolve by the time the patient reaches early adolescence.6 Fewer cases resolve in children with tree nut allergies (approximately 9%) or peanut allergy (20%),7,8 and allergies to fish and shellfish often develop or persist in adulthood.
A family history of an atopic disease such as asthma, allergic rhinitis, eczema, or food allergy is a risk factor for developing a food allergy. 3 Considering that the rate of peanut allergy has doubled in children over the past 10 years, environmental factors may also play a role.3
How we tolerate foods or become allergic to them
The gut, the largest mucosal organ in the body, is exposed to large quantities of foreign proteins daily. Most protein is broken down by stomach acid and digestive enzymes into lessantigenic peptides or is bound by secretory immunoglobulin A (IgA), which prevents it from being absorbed. Further, the epithelial cells lining the gut do not allow large molecules to pass easily, having tight intracellular junctions and being covered with mucus.
For these reasons, less than 2% of the protein in food is absorbed in an allergenic form.9 The reason food allergies are more prevalent in children is most likely that children have an immature gut barrier, lower IgA levels, a higher gastric pH, and lower proteolytic enzyme levels.
When dietary proteins do cross the gut barrier, the immune system normally suppresses the allergic response. Regulatory T cells, dendritic cells, and local immune responses play critical roles in the development of tolerance. Several types of regulatory T cells, such as Tr1 cells (which secrete interleukin 10), TH3 cells (which secrete transforming growth factor beta), CD4+CD25+ regulatory T cells, gamma-delta T cells, and CD8+ suppressor cells can all contribute to suppressing allergic responses.10 Dendritic cells also help induce tolerance by stimulating CD4+ T cells to secrete transforming growth factor beta, which leads to the production of interleukin 10 and additional transforming growth factor beta.11
Factors that contribute to food allergy
Many factors may contribute to whether a person becomes tolerant to or sensitized to a specific food protein.
The dose of antigen. Tolerance can develop after either high or low doses of antigens, but by different mechanisms.
The antigen structure. Soluble antigens are less sensitizing than particulate antigens.12,13
Processing of foods. Dry-roasted peanuts are more allergenic than raw or boiled peanuts, partly because they are less soluble.13
The route of initial exposure. Sensitization to food proteins can occur directly through the gut or the skin. Alternatively, it can occur indirectly via the respiratory tract. Skin exposure may be especially sensitizing in children with atopic dermatitis.14,15
The gut flora. When mice are raised in a germ-free environment, they fail to develop normal tolerance.16 They are also more likely to become sensitized if they are treated with antibiotics or if they lack toll-like receptors that recognize bacterial lipopolysaccharides.17 Furthermore, human studies suggest that probiotics promote tolerance, especially in preventing atopic dermatitis, although the studies have had conflicting results.18–21
The gastric pH. Murine and human studies reveal that antacid medications increase the risk of food allergy.22,23
Genetic susceptibility. A child with a sibling who is allergic to peanuts is approximately 10 times more likely to be allergic to peanuts than predicted by the rate in the general population. Although no risk-conferring gene has been identified, a study of twins showed concordance for peanut allergy in 64.3% of identical twins vs 6.8% of fraternal twins.24
Three types of immune responses to food
Immunologic reactions to foods can be divided into three categories: mediated by immunoglobulin E (IgE), non-IgE-mediated, and mixed. Therefore, these disorders can present as an acute, potentially life-threatening reaction or as a chronic disease such as eosinophilic gastoenteropathy.
IgE-mediated reactions are immediate hypersensitivity responses. In most patients, an IgE-mediated mechanism can be confirmed by a positive skin test or a test for food-specific IgE in the serum. In this article, the term “food allergy” refers to an IgE-mediated reaction to a food, unless otherwise indicated.
Non-IgE-mediated reactions have a delayed onset and chronic symptoms. Commonly, they are confined to the gastrointestinal tract; examples are food-protein-induced enterocolitis, proctitis, and proctocolitis and celiac disease.3,26,27 However, other diseases such as contact dermatitis, dermatitis herpetiformis, and food-induced pulmonary hemosiderosis (Heiner syndrome) are also considered non-IgE-mediated allergies.
Mixed-reaction disorders are chronic and include the eosinophilic gastroenteropathies, ie, eosinophilic proctocolitis, eosinophilic gastroenteritis, and eosinophilic esophagitis.28 The pathophysiology of these diseases is poorly understood. Many patients have evidence of allergic sensitivities to food or to environmental allergens, or both, but whether these sensitivities have a causal role in these disorders is not clear.
Atopic dermatitis, another complicated disease process, may be associated with mixedreaction food allergy, as approximately 35% of young children with moderate to severe atopic dermatitis have food allergies.29
Diagnosis of IgE-mediated food allergies
A thorough history and physical examination are key to diagnosing an IgE-mediated food allergy.
The history should include potential culprit foods, the quantity eaten, the timing of the onset of symptoms, and related factors such as exercise, alcohol intake, or medication use. Symptoms of an IgE-mediated reaction are generally rapid in onset but may be delayed up to a few hours, while non-IgE mediated symptoms may present several hours to days later.
Food challenge. A double-blind, placebocontrolled oral food challenge is the gold standard for the diagnosis of food allergies. (The food to be tested is hidden in other food or in capsules.) However, this test poses significant risks, and other diagnostic methods are more practical for screening.
Skin-prick tests with commercially available extracts are a rapid and sensitive method of screening for allergy to several foods.
Negative skin-prick tests have an estimated negative predictive value of more than 95% and can therefore exclude IgE-mediated food allergies.
A positive test indicates the presence of IgE against a specific food allergen and suggests a clinical food allergy, although the specificity of the test is only about 50%, making a positive result difficult to interpret. Although the size of the skin-test response does not necessarily correlate with the potential severity of a reaction, a response larger than 3 mm does indicate a greater likelihood of clinical reactivity. A positive test is most helpful in confirming the diagnosis of IgE-mediated food allergy when combined with a clear history of food-induced symptoms.
The proteins in commercially based extracts of most fruits and vegetables are often labile; therefore, skin testing with fresh fruits and vegetables may be indicated.30
Immunoassays. Radioallergosorbent tests (RASTs) and fluorescent enzyme immunoassays are used to identity food-specific IgE antibodies in the serum. The commercially available tests do not use radioactivity, but the term “RAST” is still commonly used.
Immunoassays are generally less sensitive and more costly than skin-prick tests, and their results are not immediately available, unlike those of skin-prick testing. However, these in vitro tests are not affected by antihistamine use and are useful in patients with severe dermatologic conditions or severe anaphylaxis, for whom skin-prick testing would not be appropriate.
However, unlike a negative skin-prick test, an undetectable serum food-specific IgE level has a low negative predictive value, and an undetectable level may be associated with symptoms of an allergic reaction for 10% to 25% of patients.29 Therefore, if one suspects an allergic reaction but no food-specific IgE can be detected in the serum, confirming the absence of a clinical allergy must be done with a skin-prick test or with a physician-supervised oral challenge, or both.
Managing food allergy by avoiding the allergen
Food allergies are managed by strictly avoiding food allergens and by taking medications such as self-injectable epinephrine for anaphylactic symptoms.
Patients and caregivers must be educated about reading food labels, avoiding high-risk situations such as eating at buffets and other restaurants with high risk of cross-contamination, wearing a medical-alert bracelet, recognizing and managing early symptoms of an allergic reaction, and calling for emergency services if they are having an allergic reaction. Since January 2006, the US Food and Drug Administration has required food manufacturers to list common food allergens on food labels (cow’s milk, soy, wheat, egg, peanut, tree nuts, fish, and shellfish), and the labeling must use simple, easily understood terms, such as “milk” instead of “whey.” However, it is still prudent to read all ingredients listed on the label.
Experimental treatments for food allergies
Humanized monoclonal anti-IgE antibodies such as talizumab (also known as TNX-901) and omalizumab (Xolair) have been developed, but their use in food allergy has been limited. In a study in patients with peanut allergy, injections of talizumab increased the threshold for sensitivity to peanuts in most patients, but 25% of the patients did not have any improvement.32 A study of omalizumab in patients with peanut allergy was stopped after adverse reactions developed during oral peanut challenges.33
Oral immunotherapy. Recent studies suggest it may be possible to induce oral tolerance in patients with IgE-mediated food allergy. Pilot studies have shown that frequent, increasing doses of food allergens (egg, milk, and peanut) may raise the threshold at which symptoms occur.34–36 Though these studies suggest that oral immunotherapy may protect some patients against a reaction if they accidentally ingest a food they are allergic to, some patients could not reach the goal doses because allergic symptoms were provoked.
At this early stage, these strategies must be considered investigational, and more randomized, placebo-controlled studies are needed. Further studies will also be needed to assess whether oral immunotherapy induces only short-term desensitization (in which case the allergen needs to be ingested daily to prevent reactions) or sustained tolerance (in which case the antigenic protein can be ingested without symptoms despite periods of abstinence).
THE ROLE OF FOOD ALLERGY IN EOSINOPHILIC ESOPHAGITIS
Eosinophilic esophagitis has been recognized with increasing frequency in both children and adults over the past several years. Symptoms can include difficulty feeding, failure to thrive, vomiting, epigastric or chest pain, dysphagia, and food impaction.
Diagnostic criteria for eosinophilic esophagitis are37:
- Clinical symptoms of esophageal dysfunction
- At least 15 eosinophils per high-power field in at least one esophageal biopsy specimen
- No response to a proton-pump inhibitor in high doses (up to 2 mg/kg/day) for 1 to 2 months, or normal results on pH probe monitoring of the esophagus (the reason for this criterion is that patients with gastroesophageal reflux disease can also have large numbers of eosinophils in the esophagus—more than 100 per highpower field38)
- Exclusion of other causes.
Though the cause of eosinophilic esophagitis is not completely understood, atopy has been strongly implicated as a factor. More than 50% of patients with eosinophilic esophagitis also have an atopic condition (eg, atopic dermatitis, allergic rhinitis, asthma), as well as positive results on skin-prick testing or measurement of antigen-specific IgE in the serum.39–41 Also, since most patients improve with either dietary restriction or elemental diets, food sensitization appears to play a considerable role.
As with atopic conditions such as asthma, atopic dermatitis, allergic rhinitis, and food allergy, eosinophilic esophagitis has been linked with immune responses involving helper T cell 2 (TH2). Adults and children with eosinophilic esophagitis have been found to have elevated eosinophil counts and total IgE levels in peripheral blood.37 In the esophagus, patients have elevated levels of the TH2 cytokines often seen in atopic patients (eg, interleukins 4, 5, and 13) and mast cells.42,43 In mice, eosinophilic esophagitis can be induced by allergen exposure and overexpression of TH2 cytokines.44,45 Expression of eotaxin-3, a potent eosinophil chemoattractant, was noted to be higher in children with eosinophilic esophagitis than in controls.46
Of interest, some patients with eosinophilic esophagitis say their symptoms vary with the seasons, correlating with seasonal changes in esophageal eosinophil levels.47,48
Studies linking eosinophilic esophagitis and food allergy in children
Kelly et al49 reported that 10 children with chronic symptomatic gastroesophageal reflux and eosinophilic esophagitis all had partial or complete resolution of symptoms on an elemental diet.
Markowitz et al50 found that symptoms of chronic reflux disease and eosinophilic esophagitis improved in 49 of 51 children on an elemental diet, and the number of eosinophils in the distal esophagus decreased significantly.
Liacouras et al39 reported similar findings in a 10-year experience. Of 132 children who had eosinophilic esophagitis, 75 improved with dietary restriction based on results of skin-prick and patch testing. The 57 patients who did not respond and 115 others were started on an elemental diet. Of the 164 patients who complied with the elemental diet, 160 had significant improvement of symptoms and a significant decrease in the number of eosinophils in the esophagus. Individual foods were reintroduced approximately every 5 days, and esophagogastroduodenoscopy with biopsies was performed 4 to 8 weeks after the last was reintroduced into the diet.
In a retrospective study, Kagalwalla et al51 reported that 60 children with eosinophilic esophagitis were treated with either an elemental diet or a six-food elimination diet (no milk, soy, wheat, egg, peanut, or seafood). The two groups showed similar clinical and histologic improvements.
Collectively, these studies in pediatric patients imply that food allergy is a significant factor in the pathogenesis of eosinophilic esophagitis.
Studies in adults
Fewer studies of the link between food allergy and eosinophilic esophagitis have been done in adults.
In a preliminary study, 18 adults followed the six-food elimination diet. Symptoms improved in 17 (94%), and histologic findings improved in 14 (78%).52
On the other hand, in six adult patients with eosinophilic esophagitis, Simon et al53 found that only one had improvement in symptoms after eliminating wheat and rye from the diet, and none had significant changes in the number of eosinophils in the esophagus.
In a 37-year-old man with eosinophilic esophagitis, symptoms improved after eliminating egg from his diet.54
Yamazaki et al55 measured expression of interleukin 5 and interleukin 13 in 15 adult patients with eosinophilic esophagitis. Food and aeroallergens that included milk, soy, dust mite, ragweed, and Aspergillus induced significantly more interleukin 5 production in these patients than in atopic controls, suggesting that both foods and aeroallergens may have a role in the pathogenesis of eosinophilic esophagitis in adults.
How to identify potential food triggers of eosinophilic esophagitis
Though elemental diets have been associated with a decrease in symptoms and esophageal eosinophilia, elemental formulas are expensive and unpalatable and pose a risk of nutritional deprivation. Identifying specific food allergens to eliminate from the diet in patients with eosinophilic esophagitis may be less expensive and more desirable than a very limited or elemental diet.
However, potential food triggers have been hard to identify in eosinophilic esophagitis. A recent consensus report did not recommend in vitro food allergy testing,37 owing to a lack of positive or negative predictive values for food-specific IgE level testing in eosinophilic esophagitis. Furthermore, the absence of IgE does not eliminate a food as a potential trigger, since non-IgE mechanisms may play a role.
Skin-prick testing is one of the currently validated diagnostic methods. Several studies have used skin-prick testing of foods in patients with eosinophilic esophagitis. In these studies, approximately two-thirds of patients had positive test reactions to at least one food, most often to common food allergens such as cow’s milk, egg, soy, wheat, and peanut, but also to rye, beef, and bean.37 In a recent article,56 81% of adult patients with eosinophilic esophagitis had one or more allergens identified by skin-prick testing, and 50% of the patients tested positive for one or more food allergens.
Atopy patch testing. The combination of skin-prick testing and atopy patch testing may be more effective than skin-prick testing alone in identifying potential food triggers. Atopy patch testing has been used in the diagnosis of non-IgE cell-mediated (delayed) immune responses, in which T cells may play a significant role.
Atopy patch testing is similar to patch testing for contact dermatitis. It involves placing a small quantity of food on the skin and evaluating for a local delayed reaction after a set time.
In two studies,50,57 146 children with biopsy-proven eosinophilic esophagitis had foods eliminated from the diet on the basis of positive skin-prick tests and atopy patch tests. Approximately 77% of the children had significant reduction of esophageal eosinophils in biopsy specimens (from 20 per high-power field to 1.1). The foods most commonly implicated by skin-prick testing were cow’s milk, egg, wheat, peanut, shellfish, peas, beef, fish, rye, and tomato; those identified by atopy patch testing were cow’s milk, egg, wheat, corn, beef, milk, soy, rye, chicken, oats, and potato. The combination of both types of testing had a negative predictive value of 88% to 100% for all foods except milk, while the positive predictive value was greater than 74% for the most common foods causing eosinophilic esophagitis.58
Though atopy patch testing shows some usefulness in identifying foods that may elicit non-IgE-mediated reactions, currently these tests are not validated and have been evaluated in only a small number of studies. Currently, no standardized testing materials, methods of application, or interpretation of results exist, and no studies have included a control population to validate atopy patch testing. More studies are needed to validate atopy patch testing as a reliable diagnostic tool before it can be recommended as a component of routine diagnostic evaluation in patients with eosinophilic esophagitis.
More children and even adults seem to be allergic to various foods these days than in the past. Also apparently on the rise is a linked condition, eosinophilic esophagitis.
The reason for these increases is not clear. This article confines itself to what we know about the mechanisms of food allergies and eosinophilic esophagitis, how to diagnose them, and how to treat them.
FOOD ALLERGIES ARE COMMON, AND MORE PREVALENT THAN EVER
Food allergies—abnormal immune responses to food proteins1—affect an estimated 6% to 8% of young children and 3% to 4% of adults in the United States,2,3 and their prevalence appears to be rising in developed countries. Studies in US and British children indicate that peanut allergy has doubled in the past decade. 4
Any food can provoke a reaction, but only a few foods account for most of the significant allergic reactions: cow’s milk, soy, wheat, eggs, peanuts, tree nuts, fish, and shellfish.
Approximately 80% of allergies to milk, egg, wheat, and soy resolve by the time the patient reaches early adolescence.6 Fewer cases resolve in children with tree nut allergies (approximately 9%) or peanut allergy (20%),7,8 and allergies to fish and shellfish often develop or persist in adulthood.
A family history of an atopic disease such as asthma, allergic rhinitis, eczema, or food allergy is a risk factor for developing a food allergy. 3 Considering that the rate of peanut allergy has doubled in children over the past 10 years, environmental factors may also play a role.3
How we tolerate foods or become allergic to them
The gut, the largest mucosal organ in the body, is exposed to large quantities of foreign proteins daily. Most protein is broken down by stomach acid and digestive enzymes into lessantigenic peptides or is bound by secretory immunoglobulin A (IgA), which prevents it from being absorbed. Further, the epithelial cells lining the gut do not allow large molecules to pass easily, having tight intracellular junctions and being covered with mucus.
For these reasons, less than 2% of the protein in food is absorbed in an allergenic form.9 The reason food allergies are more prevalent in children is most likely that children have an immature gut barrier, lower IgA levels, a higher gastric pH, and lower proteolytic enzyme levels.
When dietary proteins do cross the gut barrier, the immune system normally suppresses the allergic response. Regulatory T cells, dendritic cells, and local immune responses play critical roles in the development of tolerance. Several types of regulatory T cells, such as Tr1 cells (which secrete interleukin 10), TH3 cells (which secrete transforming growth factor beta), CD4+CD25+ regulatory T cells, gamma-delta T cells, and CD8+ suppressor cells can all contribute to suppressing allergic responses.10 Dendritic cells also help induce tolerance by stimulating CD4+ T cells to secrete transforming growth factor beta, which leads to the production of interleukin 10 and additional transforming growth factor beta.11
Factors that contribute to food allergy
Many factors may contribute to whether a person becomes tolerant to or sensitized to a specific food protein.
The dose of antigen. Tolerance can develop after either high or low doses of antigens, but by different mechanisms.
The antigen structure. Soluble antigens are less sensitizing than particulate antigens.12,13
Processing of foods. Dry-roasted peanuts are more allergenic than raw or boiled peanuts, partly because they are less soluble.13
The route of initial exposure. Sensitization to food proteins can occur directly through the gut or the skin. Alternatively, it can occur indirectly via the respiratory tract. Skin exposure may be especially sensitizing in children with atopic dermatitis.14,15
The gut flora. When mice are raised in a germ-free environment, they fail to develop normal tolerance.16 They are also more likely to become sensitized if they are treated with antibiotics or if they lack toll-like receptors that recognize bacterial lipopolysaccharides.17 Furthermore, human studies suggest that probiotics promote tolerance, especially in preventing atopic dermatitis, although the studies have had conflicting results.18–21
The gastric pH. Murine and human studies reveal that antacid medications increase the risk of food allergy.22,23
Genetic susceptibility. A child with a sibling who is allergic to peanuts is approximately 10 times more likely to be allergic to peanuts than predicted by the rate in the general population. Although no risk-conferring gene has been identified, a study of twins showed concordance for peanut allergy in 64.3% of identical twins vs 6.8% of fraternal twins.24
Three types of immune responses to food
Immunologic reactions to foods can be divided into three categories: mediated by immunoglobulin E (IgE), non-IgE-mediated, and mixed. Therefore, these disorders can present as an acute, potentially life-threatening reaction or as a chronic disease such as eosinophilic gastoenteropathy.
IgE-mediated reactions are immediate hypersensitivity responses. In most patients, an IgE-mediated mechanism can be confirmed by a positive skin test or a test for food-specific IgE in the serum. In this article, the term “food allergy” refers to an IgE-mediated reaction to a food, unless otherwise indicated.
Non-IgE-mediated reactions have a delayed onset and chronic symptoms. Commonly, they are confined to the gastrointestinal tract; examples are food-protein-induced enterocolitis, proctitis, and proctocolitis and celiac disease.3,26,27 However, other diseases such as contact dermatitis, dermatitis herpetiformis, and food-induced pulmonary hemosiderosis (Heiner syndrome) are also considered non-IgE-mediated allergies.
Mixed-reaction disorders are chronic and include the eosinophilic gastroenteropathies, ie, eosinophilic proctocolitis, eosinophilic gastroenteritis, and eosinophilic esophagitis.28 The pathophysiology of these diseases is poorly understood. Many patients have evidence of allergic sensitivities to food or to environmental allergens, or both, but whether these sensitivities have a causal role in these disorders is not clear.
Atopic dermatitis, another complicated disease process, may be associated with mixedreaction food allergy, as approximately 35% of young children with moderate to severe atopic dermatitis have food allergies.29
Diagnosis of IgE-mediated food allergies
A thorough history and physical examination are key to diagnosing an IgE-mediated food allergy.
The history should include potential culprit foods, the quantity eaten, the timing of the onset of symptoms, and related factors such as exercise, alcohol intake, or medication use. Symptoms of an IgE-mediated reaction are generally rapid in onset but may be delayed up to a few hours, while non-IgE mediated symptoms may present several hours to days later.
Food challenge. A double-blind, placebocontrolled oral food challenge is the gold standard for the diagnosis of food allergies. (The food to be tested is hidden in other food or in capsules.) However, this test poses significant risks, and other diagnostic methods are more practical for screening.
Skin-prick tests with commercially available extracts are a rapid and sensitive method of screening for allergy to several foods.
Negative skin-prick tests have an estimated negative predictive value of more than 95% and can therefore exclude IgE-mediated food allergies.
A positive test indicates the presence of IgE against a specific food allergen and suggests a clinical food allergy, although the specificity of the test is only about 50%, making a positive result difficult to interpret. Although the size of the skin-test response does not necessarily correlate with the potential severity of a reaction, a response larger than 3 mm does indicate a greater likelihood of clinical reactivity. A positive test is most helpful in confirming the diagnosis of IgE-mediated food allergy when combined with a clear history of food-induced symptoms.
The proteins in commercially based extracts of most fruits and vegetables are often labile; therefore, skin testing with fresh fruits and vegetables may be indicated.30
Immunoassays. Radioallergosorbent tests (RASTs) and fluorescent enzyme immunoassays are used to identity food-specific IgE antibodies in the serum. The commercially available tests do not use radioactivity, but the term “RAST” is still commonly used.
Immunoassays are generally less sensitive and more costly than skin-prick tests, and their results are not immediately available, unlike those of skin-prick testing. However, these in vitro tests are not affected by antihistamine use and are useful in patients with severe dermatologic conditions or severe anaphylaxis, for whom skin-prick testing would not be appropriate.
However, unlike a negative skin-prick test, an undetectable serum food-specific IgE level has a low negative predictive value, and an undetectable level may be associated with symptoms of an allergic reaction for 10% to 25% of patients.29 Therefore, if one suspects an allergic reaction but no food-specific IgE can be detected in the serum, confirming the absence of a clinical allergy must be done with a skin-prick test or with a physician-supervised oral challenge, or both.
Managing food allergy by avoiding the allergen
Food allergies are managed by strictly avoiding food allergens and by taking medications such as self-injectable epinephrine for anaphylactic symptoms.
Patients and caregivers must be educated about reading food labels, avoiding high-risk situations such as eating at buffets and other restaurants with high risk of cross-contamination, wearing a medical-alert bracelet, recognizing and managing early symptoms of an allergic reaction, and calling for emergency services if they are having an allergic reaction. Since January 2006, the US Food and Drug Administration has required food manufacturers to list common food allergens on food labels (cow’s milk, soy, wheat, egg, peanut, tree nuts, fish, and shellfish), and the labeling must use simple, easily understood terms, such as “milk” instead of “whey.” However, it is still prudent to read all ingredients listed on the label.
Experimental treatments for food allergies
Humanized monoclonal anti-IgE antibodies such as talizumab (also known as TNX-901) and omalizumab (Xolair) have been developed, but their use in food allergy has been limited. In a study in patients with peanut allergy, injections of talizumab increased the threshold for sensitivity to peanuts in most patients, but 25% of the patients did not have any improvement.32 A study of omalizumab in patients with peanut allergy was stopped after adverse reactions developed during oral peanut challenges.33
Oral immunotherapy. Recent studies suggest it may be possible to induce oral tolerance in patients with IgE-mediated food allergy. Pilot studies have shown that frequent, increasing doses of food allergens (egg, milk, and peanut) may raise the threshold at which symptoms occur.34–36 Though these studies suggest that oral immunotherapy may protect some patients against a reaction if they accidentally ingest a food they are allergic to, some patients could not reach the goal doses because allergic symptoms were provoked.
At this early stage, these strategies must be considered investigational, and more randomized, placebo-controlled studies are needed. Further studies will also be needed to assess whether oral immunotherapy induces only short-term desensitization (in which case the allergen needs to be ingested daily to prevent reactions) or sustained tolerance (in which case the antigenic protein can be ingested without symptoms despite periods of abstinence).
THE ROLE OF FOOD ALLERGY IN EOSINOPHILIC ESOPHAGITIS
Eosinophilic esophagitis has been recognized with increasing frequency in both children and adults over the past several years. Symptoms can include difficulty feeding, failure to thrive, vomiting, epigastric or chest pain, dysphagia, and food impaction.
Diagnostic criteria for eosinophilic esophagitis are37:
- Clinical symptoms of esophageal dysfunction
- At least 15 eosinophils per high-power field in at least one esophageal biopsy specimen
- No response to a proton-pump inhibitor in high doses (up to 2 mg/kg/day) for 1 to 2 months, or normal results on pH probe monitoring of the esophagus (the reason for this criterion is that patients with gastroesophageal reflux disease can also have large numbers of eosinophils in the esophagus—more than 100 per highpower field38)
- Exclusion of other causes.
Though the cause of eosinophilic esophagitis is not completely understood, atopy has been strongly implicated as a factor. More than 50% of patients with eosinophilic esophagitis also have an atopic condition (eg, atopic dermatitis, allergic rhinitis, asthma), as well as positive results on skin-prick testing or measurement of antigen-specific IgE in the serum.39–41 Also, since most patients improve with either dietary restriction or elemental diets, food sensitization appears to play a considerable role.
As with atopic conditions such as asthma, atopic dermatitis, allergic rhinitis, and food allergy, eosinophilic esophagitis has been linked with immune responses involving helper T cell 2 (TH2). Adults and children with eosinophilic esophagitis have been found to have elevated eosinophil counts and total IgE levels in peripheral blood.37 In the esophagus, patients have elevated levels of the TH2 cytokines often seen in atopic patients (eg, interleukins 4, 5, and 13) and mast cells.42,43 In mice, eosinophilic esophagitis can be induced by allergen exposure and overexpression of TH2 cytokines.44,45 Expression of eotaxin-3, a potent eosinophil chemoattractant, was noted to be higher in children with eosinophilic esophagitis than in controls.46
Of interest, some patients with eosinophilic esophagitis say their symptoms vary with the seasons, correlating with seasonal changes in esophageal eosinophil levels.47,48
Studies linking eosinophilic esophagitis and food allergy in children
Kelly et al49 reported that 10 children with chronic symptomatic gastroesophageal reflux and eosinophilic esophagitis all had partial or complete resolution of symptoms on an elemental diet.
Markowitz et al50 found that symptoms of chronic reflux disease and eosinophilic esophagitis improved in 49 of 51 children on an elemental diet, and the number of eosinophils in the distal esophagus decreased significantly.
Liacouras et al39 reported similar findings in a 10-year experience. Of 132 children who had eosinophilic esophagitis, 75 improved with dietary restriction based on results of skin-prick and patch testing. The 57 patients who did not respond and 115 others were started on an elemental diet. Of the 164 patients who complied with the elemental diet, 160 had significant improvement of symptoms and a significant decrease in the number of eosinophils in the esophagus. Individual foods were reintroduced approximately every 5 days, and esophagogastroduodenoscopy with biopsies was performed 4 to 8 weeks after the last was reintroduced into the diet.
In a retrospective study, Kagalwalla et al51 reported that 60 children with eosinophilic esophagitis were treated with either an elemental diet or a six-food elimination diet (no milk, soy, wheat, egg, peanut, or seafood). The two groups showed similar clinical and histologic improvements.
Collectively, these studies in pediatric patients imply that food allergy is a significant factor in the pathogenesis of eosinophilic esophagitis.
Studies in adults
Fewer studies of the link between food allergy and eosinophilic esophagitis have been done in adults.
In a preliminary study, 18 adults followed the six-food elimination diet. Symptoms improved in 17 (94%), and histologic findings improved in 14 (78%).52
On the other hand, in six adult patients with eosinophilic esophagitis, Simon et al53 found that only one had improvement in symptoms after eliminating wheat and rye from the diet, and none had significant changes in the number of eosinophils in the esophagus.
In a 37-year-old man with eosinophilic esophagitis, symptoms improved after eliminating egg from his diet.54
Yamazaki et al55 measured expression of interleukin 5 and interleukin 13 in 15 adult patients with eosinophilic esophagitis. Food and aeroallergens that included milk, soy, dust mite, ragweed, and Aspergillus induced significantly more interleukin 5 production in these patients than in atopic controls, suggesting that both foods and aeroallergens may have a role in the pathogenesis of eosinophilic esophagitis in adults.
How to identify potential food triggers of eosinophilic esophagitis
Though elemental diets have been associated with a decrease in symptoms and esophageal eosinophilia, elemental formulas are expensive and unpalatable and pose a risk of nutritional deprivation. Identifying specific food allergens to eliminate from the diet in patients with eosinophilic esophagitis may be less expensive and more desirable than a very limited or elemental diet.
However, potential food triggers have been hard to identify in eosinophilic esophagitis. A recent consensus report did not recommend in vitro food allergy testing,37 owing to a lack of positive or negative predictive values for food-specific IgE level testing in eosinophilic esophagitis. Furthermore, the absence of IgE does not eliminate a food as a potential trigger, since non-IgE mechanisms may play a role.
Skin-prick testing is one of the currently validated diagnostic methods. Several studies have used skin-prick testing of foods in patients with eosinophilic esophagitis. In these studies, approximately two-thirds of patients had positive test reactions to at least one food, most often to common food allergens such as cow’s milk, egg, soy, wheat, and peanut, but also to rye, beef, and bean.37 In a recent article,56 81% of adult patients with eosinophilic esophagitis had one or more allergens identified by skin-prick testing, and 50% of the patients tested positive for one or more food allergens.
Atopy patch testing. The combination of skin-prick testing and atopy patch testing may be more effective than skin-prick testing alone in identifying potential food triggers. Atopy patch testing has been used in the diagnosis of non-IgE cell-mediated (delayed) immune responses, in which T cells may play a significant role.
Atopy patch testing is similar to patch testing for contact dermatitis. It involves placing a small quantity of food on the skin and evaluating for a local delayed reaction after a set time.
In two studies,50,57 146 children with biopsy-proven eosinophilic esophagitis had foods eliminated from the diet on the basis of positive skin-prick tests and atopy patch tests. Approximately 77% of the children had significant reduction of esophageal eosinophils in biopsy specimens (from 20 per high-power field to 1.1). The foods most commonly implicated by skin-prick testing were cow’s milk, egg, wheat, peanut, shellfish, peas, beef, fish, rye, and tomato; those identified by atopy patch testing were cow’s milk, egg, wheat, corn, beef, milk, soy, rye, chicken, oats, and potato. The combination of both types of testing had a negative predictive value of 88% to 100% for all foods except milk, while the positive predictive value was greater than 74% for the most common foods causing eosinophilic esophagitis.58
Though atopy patch testing shows some usefulness in identifying foods that may elicit non-IgE-mediated reactions, currently these tests are not validated and have been evaluated in only a small number of studies. Currently, no standardized testing materials, methods of application, or interpretation of results exist, and no studies have included a control population to validate atopy patch testing. More studies are needed to validate atopy patch testing as a reliable diagnostic tool before it can be recommended as a component of routine diagnostic evaluation in patients with eosinophilic esophagitis.
- Bruijnzeel-Koomen C, Ortolani C, Aas K, et al. Adverse reactions to food. European Academy of Allergology and Clinical Immunology Subcommittee. Allergy 1995; 50:623–635.
- Sampson HA. Update on food allergy. J Allergy Clin Immunol 2004; 113:805–819.
- Sicherer SH, Sampson HA. 9. Food allergy. J Allergy Clin Immunol 2006; 117 (suppl 2):S470–S475.
- Sicherer SH, Munoz-Furlong A, Sampson HA. Prevalence of peanut and tree nut allergy in the United States determined by means of a random digit dial telephone survey: a 5-year follow-up study. J Allergy Clin Immunol 2003; 112:1203–1207.
- American College of Allergy, Asthma, & Immunology. Food allergy: a practice parameter. Ann Allergy Asthma Immunol 2006; 96( suppl 2):S1–S68.
- Wood RA. The natural history of food allergy. Pediatrics 2003; 111:1631–1637.
- Hourihane JO, Roberts SA, Warner JO. Resolution of peanut allergy: case-control study. BMJ 1998; 316:1271–1275.
- Fleischer DM, Conover-Walker MK, Matsui EC, Wood RA. The natural history of tree nut allergy. J Allergy Clin Immunol 2005; 116:1087–1093.
- Husby S, Foged N, Host A, Svehag SE. Passage of dietary antigens into the blood of children with coeliac disease. Quantification and size distribution of absorbed antigens. Gut 1987; 28:1062–1072.
- Mowat AM. Anatomical basis of tolerance and immunity to intestinal antigens. Nat Rev Immunol 2003; 3:331–341.
- Frossard CP, Tropia L, Hauser C, Eigenmann PA. Lymphocytes in Peyer patches regulate clinical tolerance in a murine model of food allergy. J Allergy Clin Immunol 2004; 113:958–964.
- Jain SL, Barone KS, Flanagan MP, Michael JG. Activation patterns of murine B cells after oral administration of an encapsulated soluble antigen. Vaccine 1996; 14:1291–1297.
- Kopper RA, Odum NJ, Sen M, Helm RM, Stanley JS, Burks AW. Peanut protein allergens: the effect of roasting on solubility and allergenicity. Int Arch Allergy Immunol 2005; 136:16–22.
- Lack G. Epidemiologic risks for food allergy. J Allergy Clin Immunol 2008; 121:1331–1336.
- Lack G, Fox D, Northstone K, Golding J; Avon Longitudinal Study of Parents and Children Study Team. Factors associated with the development of peanut allergy in childhood. N Engl J Med 2003; 348:977–985.
- Sudo N, Sawamura S, Tanaka K, Aiba Y, Kubo C, Koga Y. The requirement of intestinal bacterial flora for the development of an IgE production system fully susceptible to oral tolerance induction. J Immunol 1997; 159:1739–1745.
- Bashir ME, Louie S, Shi HN, Nagler-Anderson C. Toll-like receptor 4 signaling by intestinal microbes influences susceptibility to food allergy. J Immunol 2004; 172:6978–6987.
- Kopp MV, Hennemuth I, Heinzmann A, Urbanek R. Randomized, double-blind, placebo-controlled trial of probiotics for primary prevention: no clinical effects of lactobacillus GG supplementation. Pediatrics 2008; 121:e850–e856.
- Kukkonen K, Savilahti E, Haahtela T, et al. Probiotics and prebiotic galacto-oligosaccharides in the prevention of allergic diseases: a randomized, double-blind, placebo-controlled trial. J Allergy Clin Immunol 2007; 119:192–198.
- Osborn DA, Sinn JK. Probiotics in infants for prevention of allergic disease and food hypersensitivity. Cochrane Database Syst Rev 2007;CD006475.
- Prescott SL, Bjorksten B. Probiotics for the prevention or treatment of allergic diseases. J Allergy Clin Immunol 2007; 120:255–262.
- Untersmayr E, Jensen-Jarolim E. The role of protein digestibility and antacids on food allergy outcomes. J Allergy Clin Immunol 2008; 121:1301–1308.
- Untersmayr E, Scholl I, Swoboda I, et al. Antacid medication inhibits digestion of dietary proteins and causes food allergy: a fish allergy model in BALB/c mice. J Allergy Clin Immunol 2003; 112:616–623.
- Sicherer SH, Furlong TJ, Maes HH, Desnick RJ, Sampson HA, Gelb BD. Genetics of peanut allergy: a twin study. J Allergy Clin Immunol 2000; 106:53–56.
- Sicherer SH, Sampson HA. Food allergy: recent advances in pathophysiology and treatment. Annu Rev Med 2009; 60:261–277.
- Sampson HA, Anderson JA. Summary and recommendations: classification of gastrointestinal manifestations due to immunologic reactions to foods in infants and young children. J Pediatr Gastroenterol Nutr 2000; 30( suppl 1):S87–S94.
- Sampson HA, Sicherer SH, Birnbaum AH. AGA technical review on the evaluation of food allergy in gastrointestinal disorders. American Gastroenterological Association. Gastroenterology 2001; 120:1026–1040.
- Spergel JM, Pawlowski NA. Food allergy. Mechanisms, diagnosis, and management in children. Pediatr Clin North Am 2002; 49:73–96.
- Sampson HA. Utility of food-specific IgE concentrations in predicting symptomatic food allergy. J Allergy Clin Immunol 2001; 107:891–896.
- Ortolani C, Ispano M, Pastorello EA, Ansaloni R, Magri GC. Comparison of results of skin prick tests (with fresh foods and commercial food extracts) and RAST in 100 patients with oral allergy syndrome. J Allergy Clin Immunol 1989; 83:683–690.
- Perry TT, Matsui EC, Kay Conover-Walker M, Wood RA. The relationship of allergen-specific IgE levels and oral food challenge outcome. J Allergy Clin Immunol 2004; 114:144–149.
- Leung DY, Sampson HA, Yunginger JW, et al; Avon Longitudinal Study of Parents and Children Study Team. Effect of anti-IgE therapy in patients with peanut allergy. N Engl J Med 2003; 348:986–993.
- Sampson HA. A phase II, randomized double-blind, parallel-group, placebo-controlled, oral food challenge trial of Xolair (omalizumab) in peanut allergy (TOPS). J Allergy Clin Immunol 2007; 119 (suppl 1):S117.
- Buchanan AD, Green TD, Jones SM, et al Egg oral immunotherapy in nonanaphylactic children with egg allergy. J Allergy Clin Immunol 2007; 119:199–205.
- Burks AW, Jones SM. Egg oral immunotherapy in non-anaphylactic children with egg allergy: follow-up. J Allergy Clin Immunol 2008; 121:270–271.
- Skripak JM, Nash SD, Rowley H, et al. A randomized, double-blind, placebo-controlled study of milk oral immunotherapy for cow's milk allergy. J Allergy Clin Immunol 2008; 122:1154–1160.
- Furuta GT, Liacouras CA, Collins MH, et al; First International Gastrointestinal Eosinophil Research Symposium (FIGERS) Subcommittees. Eosinophilic esophagitis in children and adults: a systematic review and consensus recommendations for diagnosis and treatment. Gastroenterology 2007; 133:1342–1363.
- Rodrigo S, Abboud G, Oh D, et al. High intraepithelial eosinophil counts in esophageal squamous epithelium are not specific for eosinophilic esophagitis in adults. Am J Gastroenterol 2008; 103:435–442.
- Liacouras CA, Spergel JM, Ruchelli E, et al. Eosinophilic esophagitis: a 10-year experience in 381 children. Clin Gastroenterol Hepatol 2005; 3:1198–1206.
- Simon D, Marti H, Heer P, Simon HU, Braathen LR, Straumann A. Eosinophilic esophagitis is frequently associated with IgE-mediated allergic airway diseases. J Allergy Clin Immunol 2005; 115:1090–1092.
- Rothenberg ME, Mishra A, Collins MH, Putnam PE. Pathogenesis and clinical features of eosinophilic esophagitis. J Allergy Clin Immunol 2001; 108:891–894.
- Gupta SK, Fitzgerald JF, Kondratyuk T, HogenEsch H. Cytokine expression in normal and inflamed esophageal mucosa: a study into the pathogenesis of allergic eosinophilic esophagitis. J Pediatr Gastroenterol Nutr 2006; 42:22–26.
- Straumann A, Bauer M, Fischer B, Blaser K, Simon HU. Idiopathic eosinophilic esophagitis is associated with a T(H)2-type allergic inflammatory response. J Allergy Clin Immunol 2001; 108:954–961.
- Mishra A, Rothenberg ME. Intratracheal IL-13 induces eosinophilic esophagitis by an IL-5, eotaxin-1, and STAT6-dependent mechanism. Gastroenterology 2003; 125:1419–1427.
- Akei HS, Mishra A, Blanchard C, Rothenberg ME. Epicutaneous antigen exposure primes for experimental eosinophilic esophagitis in mice. Gastroenterology 2005; 129:985–994.
- Blanchard C, Wang N, Stringer KF, et al. Eotaxin-3 and a uniquely conserved gene-expression profile in eosinophilic esophagitis. J Clin Invest 2006; 116:536–547.
- Fogg MI, Ruchelli E, Spergel JM. Pollen and eosinophilic esophagitis. J Allergy Clin Immunol 2003; 112:796–797.
- Almansa C, Krishna M, Buchner AM, et al. Seasonal distribution in newly diagnosed cases of eosinophilic esophagitis in adults. Am J Gastroenterol 2009; 104:828–833.
- Kelly KJ, Lazenby AJ, Rowe PC, Yardley JH, Perman JA, Sampson HA. Eosinophilic esophagitis attributed to gastroesophageal reflux: improvement with an amino acid-based formula. Gastroenterology 1995; 109:1503–1512.
- Markowitz JE, Spergel JM, Ruchelli E, Liacouras CA. Elemental diet is an effective treatment for eosinophilic esophagitis in children and adolescents. Am J Gastroenterol 2003; 98:777–782.
- Kagalwalla AF, Sentongo TA, Ritz S, et al. Effect of six-food elimination diet on clinical and histologic outcomes in eosinophilic esophagitis. Clin Gastroenterol Hepatol 2006; 4:1097–1102.
- Gonsalves N, Yang GY, Doerfler B, et al. A prospective clinical trial of six food elimination diet and reintroduction of causative agents in adults with eosinophilic esophagitis [abstract]. Gastroenterology 2008; 134( suppl 1):A104–A105.
- Simon D, Straumann A, Wenk A, Spichtin H, Simon HU, Braathen LR. Eosinophilic esophagitis in adults—no clinical relevance of wheat and rye sensitizations. Allergy 2006; 61:1480–1483.
- Antón Remirez J, Escudero R, Caceres O, Fernandez-Benitez M. Eosinophilic esophagitis. Allergol Immunopathol (Madr) 2006; 34:79–81.
- Yamazaki K, Murray JA, Arora AS, et al. Allergen-specific in vitro cytokine production in adult patients with eosinophilic esophagitis. Dig Dis Sci 2006; 51:1934–1941.
- Penfield JD, Lang DM, Goldblum JR, Lopez R, Falk GW. The role of allergy evaluation in adults with eosinophilic esophagitis. J Clin Gastroenterol 2009(Epub ahead of print)
- Spergel JM, Andrews T, Brown-Whitehorn TF, Beausoleil JL, Liacouras CA. Treatment of eosinophilic esophagitis with specific food elimination diet directed by a combination of skin prick and patch tests. Ann Allergy Asthma Immunol 2005; 95:336–343.
- Spergel JM, Brown-Whitehorn T, Beausoleil JL, Shuker M, Liacouras CA. Predictive values for skin prick test and atopy patch test for eosinophilic esophagitis. J Allergy Clin Immunol 2007; 119:509–511.
- Bruijnzeel-Koomen C, Ortolani C, Aas K, et al. Adverse reactions to food. European Academy of Allergology and Clinical Immunology Subcommittee. Allergy 1995; 50:623–635.
- Sampson HA. Update on food allergy. J Allergy Clin Immunol 2004; 113:805–819.
- Sicherer SH, Sampson HA. 9. Food allergy. J Allergy Clin Immunol 2006; 117 (suppl 2):S470–S475.
- Sicherer SH, Munoz-Furlong A, Sampson HA. Prevalence of peanut and tree nut allergy in the United States determined by means of a random digit dial telephone survey: a 5-year follow-up study. J Allergy Clin Immunol 2003; 112:1203–1207.
- American College of Allergy, Asthma, & Immunology. Food allergy: a practice parameter. Ann Allergy Asthma Immunol 2006; 96( suppl 2):S1–S68.
- Wood RA. The natural history of food allergy. Pediatrics 2003; 111:1631–1637.
- Hourihane JO, Roberts SA, Warner JO. Resolution of peanut allergy: case-control study. BMJ 1998; 316:1271–1275.
- Fleischer DM, Conover-Walker MK, Matsui EC, Wood RA. The natural history of tree nut allergy. J Allergy Clin Immunol 2005; 116:1087–1093.
- Husby S, Foged N, Host A, Svehag SE. Passage of dietary antigens into the blood of children with coeliac disease. Quantification and size distribution of absorbed antigens. Gut 1987; 28:1062–1072.
- Mowat AM. Anatomical basis of tolerance and immunity to intestinal antigens. Nat Rev Immunol 2003; 3:331–341.
- Frossard CP, Tropia L, Hauser C, Eigenmann PA. Lymphocytes in Peyer patches regulate clinical tolerance in a murine model of food allergy. J Allergy Clin Immunol 2004; 113:958–964.
- Jain SL, Barone KS, Flanagan MP, Michael JG. Activation patterns of murine B cells after oral administration of an encapsulated soluble antigen. Vaccine 1996; 14:1291–1297.
- Kopper RA, Odum NJ, Sen M, Helm RM, Stanley JS, Burks AW. Peanut protein allergens: the effect of roasting on solubility and allergenicity. Int Arch Allergy Immunol 2005; 136:16–22.
- Lack G. Epidemiologic risks for food allergy. J Allergy Clin Immunol 2008; 121:1331–1336.
- Lack G, Fox D, Northstone K, Golding J; Avon Longitudinal Study of Parents and Children Study Team. Factors associated with the development of peanut allergy in childhood. N Engl J Med 2003; 348:977–985.
- Sudo N, Sawamura S, Tanaka K, Aiba Y, Kubo C, Koga Y. The requirement of intestinal bacterial flora for the development of an IgE production system fully susceptible to oral tolerance induction. J Immunol 1997; 159:1739–1745.
- Bashir ME, Louie S, Shi HN, Nagler-Anderson C. Toll-like receptor 4 signaling by intestinal microbes influences susceptibility to food allergy. J Immunol 2004; 172:6978–6987.
- Kopp MV, Hennemuth I, Heinzmann A, Urbanek R. Randomized, double-blind, placebo-controlled trial of probiotics for primary prevention: no clinical effects of lactobacillus GG supplementation. Pediatrics 2008; 121:e850–e856.
- Kukkonen K, Savilahti E, Haahtela T, et al. Probiotics and prebiotic galacto-oligosaccharides in the prevention of allergic diseases: a randomized, double-blind, placebo-controlled trial. J Allergy Clin Immunol 2007; 119:192–198.
- Osborn DA, Sinn JK. Probiotics in infants for prevention of allergic disease and food hypersensitivity. Cochrane Database Syst Rev 2007;CD006475.
- Prescott SL, Bjorksten B. Probiotics for the prevention or treatment of allergic diseases. J Allergy Clin Immunol 2007; 120:255–262.
- Untersmayr E, Jensen-Jarolim E. The role of protein digestibility and antacids on food allergy outcomes. J Allergy Clin Immunol 2008; 121:1301–1308.
- Untersmayr E, Scholl I, Swoboda I, et al. Antacid medication inhibits digestion of dietary proteins and causes food allergy: a fish allergy model in BALB/c mice. J Allergy Clin Immunol 2003; 112:616–623.
- Sicherer SH, Furlong TJ, Maes HH, Desnick RJ, Sampson HA, Gelb BD. Genetics of peanut allergy: a twin study. J Allergy Clin Immunol 2000; 106:53–56.
- Sicherer SH, Sampson HA. Food allergy: recent advances in pathophysiology and treatment. Annu Rev Med 2009; 60:261–277.
- Sampson HA, Anderson JA. Summary and recommendations: classification of gastrointestinal manifestations due to immunologic reactions to foods in infants and young children. J Pediatr Gastroenterol Nutr 2000; 30( suppl 1):S87–S94.
- Sampson HA, Sicherer SH, Birnbaum AH. AGA technical review on the evaluation of food allergy in gastrointestinal disorders. American Gastroenterological Association. Gastroenterology 2001; 120:1026–1040.
- Spergel JM, Pawlowski NA. Food allergy. Mechanisms, diagnosis, and management in children. Pediatr Clin North Am 2002; 49:73–96.
- Sampson HA. Utility of food-specific IgE concentrations in predicting symptomatic food allergy. J Allergy Clin Immunol 2001; 107:891–896.
- Ortolani C, Ispano M, Pastorello EA, Ansaloni R, Magri GC. Comparison of results of skin prick tests (with fresh foods and commercial food extracts) and RAST in 100 patients with oral allergy syndrome. J Allergy Clin Immunol 1989; 83:683–690.
- Perry TT, Matsui EC, Kay Conover-Walker M, Wood RA. The relationship of allergen-specific IgE levels and oral food challenge outcome. J Allergy Clin Immunol 2004; 114:144–149.
- Leung DY, Sampson HA, Yunginger JW, et al; Avon Longitudinal Study of Parents and Children Study Team. Effect of anti-IgE therapy in patients with peanut allergy. N Engl J Med 2003; 348:986–993.
- Sampson HA. A phase II, randomized double-blind, parallel-group, placebo-controlled, oral food challenge trial of Xolair (omalizumab) in peanut allergy (TOPS). J Allergy Clin Immunol 2007; 119 (suppl 1):S117.
- Buchanan AD, Green TD, Jones SM, et al Egg oral immunotherapy in nonanaphylactic children with egg allergy. J Allergy Clin Immunol 2007; 119:199–205.
- Burks AW, Jones SM. Egg oral immunotherapy in non-anaphylactic children with egg allergy: follow-up. J Allergy Clin Immunol 2008; 121:270–271.
- Skripak JM, Nash SD, Rowley H, et al. A randomized, double-blind, placebo-controlled study of milk oral immunotherapy for cow's milk allergy. J Allergy Clin Immunol 2008; 122:1154–1160.
- Furuta GT, Liacouras CA, Collins MH, et al; First International Gastrointestinal Eosinophil Research Symposium (FIGERS) Subcommittees. Eosinophilic esophagitis in children and adults: a systematic review and consensus recommendations for diagnosis and treatment. Gastroenterology 2007; 133:1342–1363.
- Rodrigo S, Abboud G, Oh D, et al. High intraepithelial eosinophil counts in esophageal squamous epithelium are not specific for eosinophilic esophagitis in adults. Am J Gastroenterol 2008; 103:435–442.
- Liacouras CA, Spergel JM, Ruchelli E, et al. Eosinophilic esophagitis: a 10-year experience in 381 children. Clin Gastroenterol Hepatol 2005; 3:1198–1206.
- Simon D, Marti H, Heer P, Simon HU, Braathen LR, Straumann A. Eosinophilic esophagitis is frequently associated with IgE-mediated allergic airway diseases. J Allergy Clin Immunol 2005; 115:1090–1092.
- Rothenberg ME, Mishra A, Collins MH, Putnam PE. Pathogenesis and clinical features of eosinophilic esophagitis. J Allergy Clin Immunol 2001; 108:891–894.
- Gupta SK, Fitzgerald JF, Kondratyuk T, HogenEsch H. Cytokine expression in normal and inflamed esophageal mucosa: a study into the pathogenesis of allergic eosinophilic esophagitis. J Pediatr Gastroenterol Nutr 2006; 42:22–26.
- Straumann A, Bauer M, Fischer B, Blaser K, Simon HU. Idiopathic eosinophilic esophagitis is associated with a T(H)2-type allergic inflammatory response. J Allergy Clin Immunol 2001; 108:954–961.
- Mishra A, Rothenberg ME. Intratracheal IL-13 induces eosinophilic esophagitis by an IL-5, eotaxin-1, and STAT6-dependent mechanism. Gastroenterology 2003; 125:1419–1427.
- Akei HS, Mishra A, Blanchard C, Rothenberg ME. Epicutaneous antigen exposure primes for experimental eosinophilic esophagitis in mice. Gastroenterology 2005; 129:985–994.
- Blanchard C, Wang N, Stringer KF, et al. Eotaxin-3 and a uniquely conserved gene-expression profile in eosinophilic esophagitis. J Clin Invest 2006; 116:536–547.
- Fogg MI, Ruchelli E, Spergel JM. Pollen and eosinophilic esophagitis. J Allergy Clin Immunol 2003; 112:796–797.
- Almansa C, Krishna M, Buchner AM, et al. Seasonal distribution in newly diagnosed cases of eosinophilic esophagitis in adults. Am J Gastroenterol 2009; 104:828–833.
- Kelly KJ, Lazenby AJ, Rowe PC, Yardley JH, Perman JA, Sampson HA. Eosinophilic esophagitis attributed to gastroesophageal reflux: improvement with an amino acid-based formula. Gastroenterology 1995; 109:1503–1512.
- Markowitz JE, Spergel JM, Ruchelli E, Liacouras CA. Elemental diet is an effective treatment for eosinophilic esophagitis in children and adolescents. Am J Gastroenterol 2003; 98:777–782.
- Kagalwalla AF, Sentongo TA, Ritz S, et al. Effect of six-food elimination diet on clinical and histologic outcomes in eosinophilic esophagitis. Clin Gastroenterol Hepatol 2006; 4:1097–1102.
- Gonsalves N, Yang GY, Doerfler B, et al. A prospective clinical trial of six food elimination diet and reintroduction of causative agents in adults with eosinophilic esophagitis [abstract]. Gastroenterology 2008; 134( suppl 1):A104–A105.
- Simon D, Straumann A, Wenk A, Spichtin H, Simon HU, Braathen LR. Eosinophilic esophagitis in adults—no clinical relevance of wheat and rye sensitizations. Allergy 2006; 61:1480–1483.
- Antón Remirez J, Escudero R, Caceres O, Fernandez-Benitez M. Eosinophilic esophagitis. Allergol Immunopathol (Madr) 2006; 34:79–81.
- Yamazaki K, Murray JA, Arora AS, et al. Allergen-specific in vitro cytokine production in adult patients with eosinophilic esophagitis. Dig Dis Sci 2006; 51:1934–1941.
- Penfield JD, Lang DM, Goldblum JR, Lopez R, Falk GW. The role of allergy evaluation in adults with eosinophilic esophagitis. J Clin Gastroenterol 2009(Epub ahead of print)
- Spergel JM, Andrews T, Brown-Whitehorn TF, Beausoleil JL, Liacouras CA. Treatment of eosinophilic esophagitis with specific food elimination diet directed by a combination of skin prick and patch tests. Ann Allergy Asthma Immunol 2005; 95:336–343.
- Spergel JM, Brown-Whitehorn T, Beausoleil JL, Shuker M, Liacouras CA. Predictive values for skin prick test and atopy patch test for eosinophilic esophagitis. J Allergy Clin Immunol 2007; 119:509–511.
KEY POINTS
- Food allergies can be classified as mediated by immunoglobulin E (IgE-mediated), non-IgE-mediated, or mixed. Their clinical presentation can vary from life-threatening anaphylaxis in IgE-mediated reactions to chronic, delayed symptoms as seen in eosinophilic esophagitis (a mixed reaction).
- The diagnosis of an IgE-mediated food allergy is made by taking a complete history and performing directed testing—skin-prick testing or measurement of foodspecific IgE levels in the serum, or both.
- Despite promising developments, food allergies continue to be treated primarily by telling patients to avoid allergens and to initiate therapy if ingestion occurs.
- Because most patients with eosinophilic esophagitis have a strong history of atopic disease and respond to allergen-free diets, a complete evaluation by a specialist in allergy and immunology is recommended.
Abdominal pain in a 20-year-old woman
A 20-year-old woman presents to the emergency department with postprandial epigastric and right-upper-quadrant pain, sometimes associated with nausea. She has been having six to eight loose bowel movements every day, with no blood or mucus, and she has lost about 20 lb despite a good appetite. The diarrhea did not improve when she tried omitting milk products and carbohydrates.
Her symptoms began several months ago, but she says that 3 days ago the pain worsened steadily, radiating to the middle of her back, with associated episodes of nonbloody, nonbilious emesis. She cannot keep down liquids or solids. She says she has never had such episodes in the past.
She reports no oral ulcers, urinary symptoms, skin rashes, musculoskeletal pain, or neurologic symptoms, and she denies being anxious or depressed.
She has no history of serious illness, surgery, or hospitalization. She smokes a half pack of cigarettes a day, drinks alcohol occasionally, and smokes marijuana occasionally. She is employed as a certified nursing assistant.
She is taking ethinyl estradiol-levonorgestrel pills for birth control and takes calcium carbonate as needed for abdominal discomfort. She is taking no other medications, including nonsteroidal anti-inflammatory drugs (NSAIDs).
Her maternal uncle died of colon cancer at age 32, and her mother had colon polyps on colonoscopy. There is no family history of inflammatory bowel disease or celiac sprue. Her father committed suicide.
Her laboratory values
- White blood cell count 10.2 × 109/L (normal range 4–11)
- Red blood cell count 4.71 × 1012/L (3.9–5.5)
- Hemoglobin 14.4 g/dL (12–16)
- Hematocrit 42.4% (37%–47%)
- Mean corpuscular volume 90 fL (83–99)
- Mean corpuscular hemoglobin 30.6 pg (27–33)
- Platelet count 230 × 109/L (150–400)
- Red cell distribution width 13.3% (11.5%–14.5%)
- Sodium 140 mmol/L (132–148)
- Potassium 3.3 mmol/L (3.5–5.0)
- Chloride 104 mmol/L (98–111)
- Bicarbonate 28 mmol/L (23–32)
- Blood urea nitrogen 9 mg/dL (8–25)
- Creatinine 0.8 mg/dL (0.7–1.4)
- Glucose 87 mg/dL (65–100)
- Alanine aminotransferase 26 U/L (0–45)
- Aspartate aminotransferase 21 U/L (7–40)
- Alkaline phosphatase 101 U/L (40–150)
- Total bilirubin 0.8 mg/dL (0–1.5)
- Albumin 3.5 g/dL (3.5–5)
- Pregnancy screen negative
- Urine toxicology screen negative.
Physical examination
The patient is very thin and appears quite uncomfortable. Her temperature is 99.7°F (37.6°C), pulse rate 101, respiratory rate 18, blood pressure 111/67 mm Hg, and oxygen saturation 96% on room air. Her skin is warm and dry. Her height is 66 inches, weight 116 lb, and body mass index 18.7.
Examination of the head and neck shows normal dentition, dry mucus membranes, and no oral exudates. The thyroid is normal, and no masses or lymphadenopathy are noted.
Heart sounds and rhythm are normal, and the lungs are clear with no crackles or rubs. The abdomen is scaphoid and soft, with no distention. She has epigastric tenderness but no rebound, guarding, rigidity, palpable mass, or costovertebral angle tenderness. Bowel sounds are normal. The neurologic examination is normal.
NARROWING THE DIAGNOSIS
1. Given the history and findings so far, which is the least likely cause of her symptoms?
- Lactose intolerance
- Celiac disease
- Crohn disease
- Duodenal ulcer
- Eating disorder
This young woman’s presentation has some features found in all of these conditions. However, the least likely is lactose intolerance.
Lactose intolerance results from a shortage of the enzyme lactase, which is normally produced by the cells that line the small intestine. Close to 50 million American adults have lactose intolerance. Common symptoms include nausea, cramps, bloating, gas, and diarrhea, which begin about 30 minutes to 2 hours after eating or drinking foods containing lactose.
Since the patient’s symptoms did not improve when she tried omitting milk products, and since lactose intolerance is rarely associated with pain radiating to the back and with severe vomiting, this is the least likely cause of her symptoms.
Celiac disease presents with a myriad of symptoms—sometimes without gastrointestinal (GI) symptoms. Anemia is the most common laboratory finding, due most often to iron deficiency, but also due to deficiencies of vitamin B12 and folate as a result of malabsorption.1
Our patient’s laboratory values—especially her red cell indices—do not confirm this finding. One must also remember, however, that hemoglobin tends to be falsely elevated in patients who are dehydrated.
Crohn disease often presents with occult blood loss, low-grade fever, weight loss, and anemia. Though the condition is most often ileocolic, it can affect any part of the gastrointestinal tract. Nevertheless, most patients with gastroduodenal involvement have previously been diagnosed with ileocolic disease, and gastroduodenal involvement manifests later. Nonradiating epigastric pain is very common. Obstructive symptoms due to gastroduodenal strictures (eg, postprandial vomiting, epigastric pain, weight loss, bloating) are also common. 2
Duodenal ulcer. The most important factors responsible for duodenal ulcers are NSAID use and Helicobacter pylori infection.3 Duodenal ulcers have a variety of clinical presentations, ranging from no symptoms to severe pain. Epigastric pain can be sharp, dull, burning, or penetrating. Many patients complain of a feeling of hunger and weight gain—as opposed to gastric ulcer, in which patients experience anorexia and weight loss. Abdominal pain generally occurs several hours after meals and often awakens the patient at night. Pain is often relieved by food, but this phenomenon is present in only 20% to 60% of patients and probably is not specific for duodenal ulcer.
Our patient does not use NSAIDs, but some of her symptoms, such as postprandial pain, epigastric pain radiating to the back, and nausea and vomiting are seen with duodenal ulcer.
Eating disorders. The two main types of eating disorders—anorexia nervosa and bulimia nervosa—have a significant diagnostic overlap,4 and a third type, binge-eating disorder, is currently being investigated and defined. Girls and women are 10 times as likely as boys and men to develop an eating disorder.
People with anorexia have a distorted view of their bodies. Even when they are extremely thin, they see themselves as too fat.
Bulimia is characterized by binge-eating, purging, and overexercising to compensate for the excess calories. Patients are often close to normal weight.
Binge-eating disorder involves the consumption of very large amounts of food in a short period of time. About 2% of all young adults in the United States struggle with bingeeating. They are either overweight or obese.
These disorders tend to be associated with other psychiatric disorders such as depression or obsessive-compulsive disorder. Our patient sought medical attention and was appropriately concerned about her weight loss, which make an eating disorder unlikely.
CASE CONTINUED: SHE UNDERGOES CT
2. Which of the following is the most likely diagnosis at this point?
- SMA syndrome
- Chronic mesenteric ischemia involving the SMA
- Megaduodenum due to a connective tissue disorder
SMA syndrome is the most likely diagnosis. Despite its name, this syndrome is not a vascular condition. It is an uncommon cause of proximal intestinal obstruction in which the duodenum is compressed between the SMA and the aorta. First described in 1861, it has also been known as cast syndrome, Wilkie syndrome, and arteriomesenteric duodenal obstruction.5
To date, more than 400 cases of this syndrome have been reported, twice as many in women as in men. Most patients are between 20 and 40 years of age at the time of diagnosis. Common presenting symptoms include postprandial abdominal pain, nausea, vomiting, and weight loss, which may further reduce the angle between the SMA and the aorta. Diarrhea is not generally associated with this syndrome, and in our patient’s case the diarrhea was thought to be unrelated to the SMA syndrome, since it subsided spontaneously.
Conditions and events that cause, contribute to, or worsen SMA syndrome include:
- Rapid weight loss (as in cancer or burns) or lean body habitus
- Prolonged bed rest
- Use of a body cast
- Malabsorption
- Spinal disease, deformity, or trauma
- Scoliosis surgery
- Rapid linear growth without compensatory weight gain
- Abnormally high and fixed position of the ligament of Treitz
- Abdominal surgery
- Cardiac cachexia
- Unusually low origin of the SMA.7
More common causes of mechanical smallbowel obstruction are adhesions, hernias, and tumors.8 Hyperactive, high-pitched peristalsis with rushes coinciding with cramps is typical. Abdominal cramps are centered around the umbilicus or in the epigastrium and are associated with vomiting; obstipation develops in patients with complete obstruction. Patients with partial obstruction may develop diarrhea. Paralytic ileus secondary to hypokalemia is an important consideration in partial obstruction. However, abdominal radiography and CT did not confirm an obstruction, and her symptoms persisted despite correction of the potassium level.
Chronic mesenteric ischemia can be caused by vasculitis, nonocclusive conditions that cause prolonged vasoconstriction (eg, cocaine ingestion), or reduced cardiac output.9 Symptoms are due to the gradual reduction in blood flow to the intestine that occurs during eating. Our patient’s toxicology report did not suggest cocaine abuse, and her history and the workup thus far do not suggest heart failure. A workup for vasculitis was negative.
Megaduodenum, SMA-like syndrome. In rare cases, dilation of the duodenum at the level of the SMA may be part of a generalized duodenal dilation caused by something other than obstruction due to mechanical compression. There are conditions, as described below, that cause an SMA-like syndrome.
A compression defect of the duodenum at the site where the SMA crossed the duodenum was found in a series of 11 cases of systemic sclerosis.10 These patients had definite dilation of the duodenum, but it was a result of atrophy of the muscle layers and replacement by collagenous tissue, changes that result in diminished peristalsis, loss of muscle tone, and dilation. The duodenum yields to pressure in its third portion under the SMA.
Several pathologic conditions, particularly connective tissue disorders, may predispose to the development of a megaduodenum that may result in an imprint on the duodenum at the level of the SMA. The most noteworthy of these conditions is scleroderma. Other conditions that can cause reduced duodenal peristalsis include diabetes, pancreatitis, dermatomyositis, lupus erythematosus, myxedema, and amyloidosis.11
It is important to distinguish SMA syndrome from SMA-like syndromes for several reasons.12 SMA-like syndromes result in loss of normal peristalsis. Further, the conditions have different outcomes, even though they are managed similarly initially, ie, with rehydration and parenteral nutrition. Surgery is to be avoided if possible in conditions that affect widespread areas of the intestine, such as scleroderma or diabetic neuropathy.
3. Which of the following is helpful in confirming SMA syndrome?
- CT of the abdomen
- Upper GI radiography series
- Upper GI endoscopy
All three can help confirm the diagnosis.
CT of the abdomen is a convenient, safe, rapid, readily available, and relatively noninvasive way to evaluate the aortomesenteric angle and to view retroperitoneal and mesenteric fat.13 Rehydration before injecting intravenous dye is important to avoid precipitating renal failure. In this patient, CT findings that helped make the diagnosis included a narrow aortomesenteric angle, compression of the duodenum, and a paucity of fat around the SMA.
An upper GI series can reveal dilation of the first and second portions of the duodenum and abrupt compression of the duodenal mucosal folds. Other findings can include a delay of 4 to 6 hours in gastroduodenal transit and relief of the obstruction when the patient is in the left lateral decubitus position. The Hayes maneuver refers to the disappearance of these radiologic features in the knee-chest position on cinefluoroscopy.14 The findings mentioned above are best noted in the supine position on both radiography and CT.
Endoscopy is necessary to rule out mechanical causes of duodenal obstruction. A pulsatile extrinsic compression suggests this condition but is found only occasionally.
Other imaging studies, such as ultrasonography, arteriography, and hypotonic duodenography, are used less often.
4. At this time, which of the following would be the most appropriate initial treatment in this patient?
- Conservative treatment
- Narcotics
- Duodenojejunostomy
Conservative treatment is indicated initially in all cases of SMA syndrome.15 This involves reversing precipitating factors and replacing fluid, electrolytes, and nutrition via total parenteral nutrition and nasogastric decompression.
To avoid keeping the patient on intravenous therapy for a prolonged time, it is important to start enteral feeding once the pain has subsided and the patient can tolerate it. A double-lumen nasojejunal tube is passed distal to the obstruction under fluoroscopic guidance. During feedings, the patient should be in the modified knee-chest, prone, or leftside-down position, all of which increase the aortomesenteric angle.
Delaying the treatment of SMA syndrome can increase the risk of morbidity and mortality by progressive malnutrition, dehydration, oliguria, electrolyte abnormalities (eg, hypokalemia), or intestinal perforation from prolonged ischemia.16,17
Narcotics and other drugs known to slow gut motility should be avoided.
Symptoms typically improve after restoration of normal body weight. If conservative treatment fails, or if the case is severe or chronic, surgery is required.18 Fortunately, this is not required often.
Duodenojejunostomy is the most common surgical treatment and involves creation of an alternate route between the duodenum and the jejunum, bypassing the compression between the aorta and the SMA. Other procedures include gastrojejunostomy, laparoscopic duodenojejunostomy, 19 a Roux-en-Y procedure, robotically assisted duodenojejunostomy, and anterior transposition of the third portion of the duodenum. Cleavage of the ligament of Treitz is another option, enabling the duodenum to drop away from the apex of the sharpened aortomesenteric angle.
WHEN TO CONSIDER SMA SYNDROME
The SMA syndrome is an uncommon cause of a very common presenting symptom, ie, abdominal pain. Nevertheless, it should be considered in the differential diagnosis of abdominal pain, especially in patients who have conditions that cause significant weight loss, such as anorexia nervosa, malabsorption, or hypercatabolic states such as burns, major surgery, severe injuries, or malignancies. The diagnosis is based on a thorough history and on supportive findings from CT and endoscopy.
In our patient, weight loss began with nonspecific diarrhea but perpetuated itself as SMA syndrome occurred.
Appropriate management consists of interrupting the cycle of weight loss and secondary upper gut obstruction. For patients in whom more definitive therapy is not feasible, a gastrostomy tube for decompression with a jejunal extension available for feeding appears to be a reasonable and safe treatment option. Duodenojejunostomy is considered the procedure of choice in severe cases.
CASE CONCLUDED
Fortunately, our patient responded well to conservative management. She was treated with intravenous hydration and correction of electrolytes and 10 days later was able to tolerate a soft diet. She was discharged in stable condition. At a follow-up visit 2 weeks later, she reported minimal abdominal discomfort, was able to tolerate meals, and had gained a few pounds. She continues to do well.
- Iovino P, Ciacci C, Sabbatini F, Acioli DM, D'Argenio G, Mazzacca G. Esophageal impairment in adult celiac disease with steatorrhea. Am J Gastroenterol 1998; 93:1243–1249.
- Loftus EV. Upper gastrointestinal tract Crohn’s disease. Clin Perspect Gastroenterol 2002; 5:188–191.
- Zapata-Colindres JC, Zepeda-Gómez S, Montaño-Loza A, Vázquez-Ballesteros E, de Jesús Villalobos J, Valdovinos-Andraca F. The association of Helicobacter pylori infection and nonsteroidal antiinflammatory drugs in peptic ulcer disease. Can J Gastroenterol 2006; 20:277–280.
- Milos G, Spindler A, Schnyder U, Fairburn CG. Instability of eating disorder diagnoses: prospective study. Br J Psychiatry 2005; 187:573–578.
- Wilkie DP. Chronic duodenal ileus. Br J Surg 1921; 9:204–214.
- Ozkurt H, Cenker MM, Bas N, Erturk SM, Basak M. Measurement of the distance and angle between the aorta and superior mesenteric artery: normal values in different BMI categories. Surg Radiol Anat 2007; 29:595–599.
- Lippl F, Hannig C, Weiss W, Allescher HD, Classen M, Kurjak M. Superior mesenteric artery syndrome: diagnosis and treatment from the gastroenterologist's view. J Gastroenterol 2002; 37:640–643.
- Balthazar EJ. George W. Holmes Lecture. CT of small-bowel obstruction. AJR Am J Roentgenol 1994; 162:255–261.
- Chang JB, Stein TA. Mesenteric ischemia: acute and chronic. Ann Vasc Surg 2003; 17:323–328.
- Gondos B. Duodenal compression defect and the “superior mesenteric artery syndrome” 1. Radiology 1977; 123:575–580.
- Cohen LB, Field SP, Sachar DB. The superior mesenteric artery syndrome. The disease that isn't, or is it? J Clin Gastroenterol 1985; 7:113–716.
- Ahmed AR, Taylor I. Superior mesenteric artery syndrome. Postgrad Med J 1997; 73:776–778.
- Santer R, Young C, Rossi T, Riddlesberger MM. Computed tomography in superior mesenteric artery syndrome. Pediatr Radiol 1991; 21:154–155.
- Lukes PJ, Rolny P, Nilson AE, Gamklou R, Darle N, Dotevall G. Diagnostic value of hypotonic duodenography in superior mesenteric artery syndrome. Acta Chir Scand 1978; 144:39–43.
- Dietz UA, Debus ES, Heuko-Valiati L, et al. Aorto-mesenteric artery compression syndrome. Chirurg 2000; 71:1345–1351.
- Lim JE, Duke GL, Eachempati SR. Superior mesenteric artery syndrome presenting with acute massive gastric dilatation, gastric wall pneumatosis, and portal venous gas. Surgery 2003; 134:840–843.
- Fuhrman MA, Felig DM, Tanchel ME. Superior mesenteric artery syndrome with obstructing duodenal bezoar. Gastrointest Endosc 2003; 57:387.
- Hines JR, Gore RM, Ballantyne GH. Superior mesenteric artery syndrome. Diagnostic criteria and therapeutic approaches. Am J Surg 1984; 148:630–632.
- Gersin KS, Heniford BT. Laparoscopic duodenojejunostomy for treatment of superior mesenteric artery syndrome. JSLS 1998; 2:281–284.
A 20-year-old woman presents to the emergency department with postprandial epigastric and right-upper-quadrant pain, sometimes associated with nausea. She has been having six to eight loose bowel movements every day, with no blood or mucus, and she has lost about 20 lb despite a good appetite. The diarrhea did not improve when she tried omitting milk products and carbohydrates.
Her symptoms began several months ago, but she says that 3 days ago the pain worsened steadily, radiating to the middle of her back, with associated episodes of nonbloody, nonbilious emesis. She cannot keep down liquids or solids. She says she has never had such episodes in the past.
She reports no oral ulcers, urinary symptoms, skin rashes, musculoskeletal pain, or neurologic symptoms, and she denies being anxious or depressed.
She has no history of serious illness, surgery, or hospitalization. She smokes a half pack of cigarettes a day, drinks alcohol occasionally, and smokes marijuana occasionally. She is employed as a certified nursing assistant.
She is taking ethinyl estradiol-levonorgestrel pills for birth control and takes calcium carbonate as needed for abdominal discomfort. She is taking no other medications, including nonsteroidal anti-inflammatory drugs (NSAIDs).
Her maternal uncle died of colon cancer at age 32, and her mother had colon polyps on colonoscopy. There is no family history of inflammatory bowel disease or celiac sprue. Her father committed suicide.
Her laboratory values
- White blood cell count 10.2 × 109/L (normal range 4–11)
- Red blood cell count 4.71 × 1012/L (3.9–5.5)
- Hemoglobin 14.4 g/dL (12–16)
- Hematocrit 42.4% (37%–47%)
- Mean corpuscular volume 90 fL (83–99)
- Mean corpuscular hemoglobin 30.6 pg (27–33)
- Platelet count 230 × 109/L (150–400)
- Red cell distribution width 13.3% (11.5%–14.5%)
- Sodium 140 mmol/L (132–148)
- Potassium 3.3 mmol/L (3.5–5.0)
- Chloride 104 mmol/L (98–111)
- Bicarbonate 28 mmol/L (23–32)
- Blood urea nitrogen 9 mg/dL (8–25)
- Creatinine 0.8 mg/dL (0.7–1.4)
- Glucose 87 mg/dL (65–100)
- Alanine aminotransferase 26 U/L (0–45)
- Aspartate aminotransferase 21 U/L (7–40)
- Alkaline phosphatase 101 U/L (40–150)
- Total bilirubin 0.8 mg/dL (0–1.5)
- Albumin 3.5 g/dL (3.5–5)
- Pregnancy screen negative
- Urine toxicology screen negative.
Physical examination
The patient is very thin and appears quite uncomfortable. Her temperature is 99.7°F (37.6°C), pulse rate 101, respiratory rate 18, blood pressure 111/67 mm Hg, and oxygen saturation 96% on room air. Her skin is warm and dry. Her height is 66 inches, weight 116 lb, and body mass index 18.7.
Examination of the head and neck shows normal dentition, dry mucus membranes, and no oral exudates. The thyroid is normal, and no masses or lymphadenopathy are noted.
Heart sounds and rhythm are normal, and the lungs are clear with no crackles or rubs. The abdomen is scaphoid and soft, with no distention. She has epigastric tenderness but no rebound, guarding, rigidity, palpable mass, or costovertebral angle tenderness. Bowel sounds are normal. The neurologic examination is normal.
NARROWING THE DIAGNOSIS
1. Given the history and findings so far, which is the least likely cause of her symptoms?
- Lactose intolerance
- Celiac disease
- Crohn disease
- Duodenal ulcer
- Eating disorder
This young woman’s presentation has some features found in all of these conditions. However, the least likely is lactose intolerance.
Lactose intolerance results from a shortage of the enzyme lactase, which is normally produced by the cells that line the small intestine. Close to 50 million American adults have lactose intolerance. Common symptoms include nausea, cramps, bloating, gas, and diarrhea, which begin about 30 minutes to 2 hours after eating or drinking foods containing lactose.
Since the patient’s symptoms did not improve when she tried omitting milk products, and since lactose intolerance is rarely associated with pain radiating to the back and with severe vomiting, this is the least likely cause of her symptoms.
Celiac disease presents with a myriad of symptoms—sometimes without gastrointestinal (GI) symptoms. Anemia is the most common laboratory finding, due most often to iron deficiency, but also due to deficiencies of vitamin B12 and folate as a result of malabsorption.1
Our patient’s laboratory values—especially her red cell indices—do not confirm this finding. One must also remember, however, that hemoglobin tends to be falsely elevated in patients who are dehydrated.
Crohn disease often presents with occult blood loss, low-grade fever, weight loss, and anemia. Though the condition is most often ileocolic, it can affect any part of the gastrointestinal tract. Nevertheless, most patients with gastroduodenal involvement have previously been diagnosed with ileocolic disease, and gastroduodenal involvement manifests later. Nonradiating epigastric pain is very common. Obstructive symptoms due to gastroduodenal strictures (eg, postprandial vomiting, epigastric pain, weight loss, bloating) are also common. 2
Duodenal ulcer. The most important factors responsible for duodenal ulcers are NSAID use and Helicobacter pylori infection.3 Duodenal ulcers have a variety of clinical presentations, ranging from no symptoms to severe pain. Epigastric pain can be sharp, dull, burning, or penetrating. Many patients complain of a feeling of hunger and weight gain—as opposed to gastric ulcer, in which patients experience anorexia and weight loss. Abdominal pain generally occurs several hours after meals and often awakens the patient at night. Pain is often relieved by food, but this phenomenon is present in only 20% to 60% of patients and probably is not specific for duodenal ulcer.
Our patient does not use NSAIDs, but some of her symptoms, such as postprandial pain, epigastric pain radiating to the back, and nausea and vomiting are seen with duodenal ulcer.
Eating disorders. The two main types of eating disorders—anorexia nervosa and bulimia nervosa—have a significant diagnostic overlap,4 and a third type, binge-eating disorder, is currently being investigated and defined. Girls and women are 10 times as likely as boys and men to develop an eating disorder.
People with anorexia have a distorted view of their bodies. Even when they are extremely thin, they see themselves as too fat.
Bulimia is characterized by binge-eating, purging, and overexercising to compensate for the excess calories. Patients are often close to normal weight.
Binge-eating disorder involves the consumption of very large amounts of food in a short period of time. About 2% of all young adults in the United States struggle with bingeeating. They are either overweight or obese.
These disorders tend to be associated with other psychiatric disorders such as depression or obsessive-compulsive disorder. Our patient sought medical attention and was appropriately concerned about her weight loss, which make an eating disorder unlikely.
CASE CONTINUED: SHE UNDERGOES CT
2. Which of the following is the most likely diagnosis at this point?
- SMA syndrome
- Chronic mesenteric ischemia involving the SMA
- Megaduodenum due to a connective tissue disorder
SMA syndrome is the most likely diagnosis. Despite its name, this syndrome is not a vascular condition. It is an uncommon cause of proximal intestinal obstruction in which the duodenum is compressed between the SMA and the aorta. First described in 1861, it has also been known as cast syndrome, Wilkie syndrome, and arteriomesenteric duodenal obstruction.5
To date, more than 400 cases of this syndrome have been reported, twice as many in women as in men. Most patients are between 20 and 40 years of age at the time of diagnosis. Common presenting symptoms include postprandial abdominal pain, nausea, vomiting, and weight loss, which may further reduce the angle between the SMA and the aorta. Diarrhea is not generally associated with this syndrome, and in our patient’s case the diarrhea was thought to be unrelated to the SMA syndrome, since it subsided spontaneously.
Conditions and events that cause, contribute to, or worsen SMA syndrome include:
- Rapid weight loss (as in cancer or burns) or lean body habitus
- Prolonged bed rest
- Use of a body cast
- Malabsorption
- Spinal disease, deformity, or trauma
- Scoliosis surgery
- Rapid linear growth without compensatory weight gain
- Abnormally high and fixed position of the ligament of Treitz
- Abdominal surgery
- Cardiac cachexia
- Unusually low origin of the SMA.7
More common causes of mechanical smallbowel obstruction are adhesions, hernias, and tumors.8 Hyperactive, high-pitched peristalsis with rushes coinciding with cramps is typical. Abdominal cramps are centered around the umbilicus or in the epigastrium and are associated with vomiting; obstipation develops in patients with complete obstruction. Patients with partial obstruction may develop diarrhea. Paralytic ileus secondary to hypokalemia is an important consideration in partial obstruction. However, abdominal radiography and CT did not confirm an obstruction, and her symptoms persisted despite correction of the potassium level.
Chronic mesenteric ischemia can be caused by vasculitis, nonocclusive conditions that cause prolonged vasoconstriction (eg, cocaine ingestion), or reduced cardiac output.9 Symptoms are due to the gradual reduction in blood flow to the intestine that occurs during eating. Our patient’s toxicology report did not suggest cocaine abuse, and her history and the workup thus far do not suggest heart failure. A workup for vasculitis was negative.
Megaduodenum, SMA-like syndrome. In rare cases, dilation of the duodenum at the level of the SMA may be part of a generalized duodenal dilation caused by something other than obstruction due to mechanical compression. There are conditions, as described below, that cause an SMA-like syndrome.
A compression defect of the duodenum at the site where the SMA crossed the duodenum was found in a series of 11 cases of systemic sclerosis.10 These patients had definite dilation of the duodenum, but it was a result of atrophy of the muscle layers and replacement by collagenous tissue, changes that result in diminished peristalsis, loss of muscle tone, and dilation. The duodenum yields to pressure in its third portion under the SMA.
Several pathologic conditions, particularly connective tissue disorders, may predispose to the development of a megaduodenum that may result in an imprint on the duodenum at the level of the SMA. The most noteworthy of these conditions is scleroderma. Other conditions that can cause reduced duodenal peristalsis include diabetes, pancreatitis, dermatomyositis, lupus erythematosus, myxedema, and amyloidosis.11
It is important to distinguish SMA syndrome from SMA-like syndromes for several reasons.12 SMA-like syndromes result in loss of normal peristalsis. Further, the conditions have different outcomes, even though they are managed similarly initially, ie, with rehydration and parenteral nutrition. Surgery is to be avoided if possible in conditions that affect widespread areas of the intestine, such as scleroderma or diabetic neuropathy.
3. Which of the following is helpful in confirming SMA syndrome?
- CT of the abdomen
- Upper GI radiography series
- Upper GI endoscopy
All three can help confirm the diagnosis.
CT of the abdomen is a convenient, safe, rapid, readily available, and relatively noninvasive way to evaluate the aortomesenteric angle and to view retroperitoneal and mesenteric fat.13 Rehydration before injecting intravenous dye is important to avoid precipitating renal failure. In this patient, CT findings that helped make the diagnosis included a narrow aortomesenteric angle, compression of the duodenum, and a paucity of fat around the SMA.
An upper GI series can reveal dilation of the first and second portions of the duodenum and abrupt compression of the duodenal mucosal folds. Other findings can include a delay of 4 to 6 hours in gastroduodenal transit and relief of the obstruction when the patient is in the left lateral decubitus position. The Hayes maneuver refers to the disappearance of these radiologic features in the knee-chest position on cinefluoroscopy.14 The findings mentioned above are best noted in the supine position on both radiography and CT.
Endoscopy is necessary to rule out mechanical causes of duodenal obstruction. A pulsatile extrinsic compression suggests this condition but is found only occasionally.
Other imaging studies, such as ultrasonography, arteriography, and hypotonic duodenography, are used less often.
4. At this time, which of the following would be the most appropriate initial treatment in this patient?
- Conservative treatment
- Narcotics
- Duodenojejunostomy
Conservative treatment is indicated initially in all cases of SMA syndrome.15 This involves reversing precipitating factors and replacing fluid, electrolytes, and nutrition via total parenteral nutrition and nasogastric decompression.
To avoid keeping the patient on intravenous therapy for a prolonged time, it is important to start enteral feeding once the pain has subsided and the patient can tolerate it. A double-lumen nasojejunal tube is passed distal to the obstruction under fluoroscopic guidance. During feedings, the patient should be in the modified knee-chest, prone, or leftside-down position, all of which increase the aortomesenteric angle.
Delaying the treatment of SMA syndrome can increase the risk of morbidity and mortality by progressive malnutrition, dehydration, oliguria, electrolyte abnormalities (eg, hypokalemia), or intestinal perforation from prolonged ischemia.16,17
Narcotics and other drugs known to slow gut motility should be avoided.
Symptoms typically improve after restoration of normal body weight. If conservative treatment fails, or if the case is severe or chronic, surgery is required.18 Fortunately, this is not required often.
Duodenojejunostomy is the most common surgical treatment and involves creation of an alternate route between the duodenum and the jejunum, bypassing the compression between the aorta and the SMA. Other procedures include gastrojejunostomy, laparoscopic duodenojejunostomy, 19 a Roux-en-Y procedure, robotically assisted duodenojejunostomy, and anterior transposition of the third portion of the duodenum. Cleavage of the ligament of Treitz is another option, enabling the duodenum to drop away from the apex of the sharpened aortomesenteric angle.
WHEN TO CONSIDER SMA SYNDROME
The SMA syndrome is an uncommon cause of a very common presenting symptom, ie, abdominal pain. Nevertheless, it should be considered in the differential diagnosis of abdominal pain, especially in patients who have conditions that cause significant weight loss, such as anorexia nervosa, malabsorption, or hypercatabolic states such as burns, major surgery, severe injuries, or malignancies. The diagnosis is based on a thorough history and on supportive findings from CT and endoscopy.
In our patient, weight loss began with nonspecific diarrhea but perpetuated itself as SMA syndrome occurred.
Appropriate management consists of interrupting the cycle of weight loss and secondary upper gut obstruction. For patients in whom more definitive therapy is not feasible, a gastrostomy tube for decompression with a jejunal extension available for feeding appears to be a reasonable and safe treatment option. Duodenojejunostomy is considered the procedure of choice in severe cases.
CASE CONCLUDED
Fortunately, our patient responded well to conservative management. She was treated with intravenous hydration and correction of electrolytes and 10 days later was able to tolerate a soft diet. She was discharged in stable condition. At a follow-up visit 2 weeks later, she reported minimal abdominal discomfort, was able to tolerate meals, and had gained a few pounds. She continues to do well.
A 20-year-old woman presents to the emergency department with postprandial epigastric and right-upper-quadrant pain, sometimes associated with nausea. She has been having six to eight loose bowel movements every day, with no blood or mucus, and she has lost about 20 lb despite a good appetite. The diarrhea did not improve when she tried omitting milk products and carbohydrates.
Her symptoms began several months ago, but she says that 3 days ago the pain worsened steadily, radiating to the middle of her back, with associated episodes of nonbloody, nonbilious emesis. She cannot keep down liquids or solids. She says she has never had such episodes in the past.
She reports no oral ulcers, urinary symptoms, skin rashes, musculoskeletal pain, or neurologic symptoms, and she denies being anxious or depressed.
She has no history of serious illness, surgery, or hospitalization. She smokes a half pack of cigarettes a day, drinks alcohol occasionally, and smokes marijuana occasionally. She is employed as a certified nursing assistant.
She is taking ethinyl estradiol-levonorgestrel pills for birth control and takes calcium carbonate as needed for abdominal discomfort. She is taking no other medications, including nonsteroidal anti-inflammatory drugs (NSAIDs).
Her maternal uncle died of colon cancer at age 32, and her mother had colon polyps on colonoscopy. There is no family history of inflammatory bowel disease or celiac sprue. Her father committed suicide.
Her laboratory values
- White blood cell count 10.2 × 109/L (normal range 4–11)
- Red blood cell count 4.71 × 1012/L (3.9–5.5)
- Hemoglobin 14.4 g/dL (12–16)
- Hematocrit 42.4% (37%–47%)
- Mean corpuscular volume 90 fL (83–99)
- Mean corpuscular hemoglobin 30.6 pg (27–33)
- Platelet count 230 × 109/L (150–400)
- Red cell distribution width 13.3% (11.5%–14.5%)
- Sodium 140 mmol/L (132–148)
- Potassium 3.3 mmol/L (3.5–5.0)
- Chloride 104 mmol/L (98–111)
- Bicarbonate 28 mmol/L (23–32)
- Blood urea nitrogen 9 mg/dL (8–25)
- Creatinine 0.8 mg/dL (0.7–1.4)
- Glucose 87 mg/dL (65–100)
- Alanine aminotransferase 26 U/L (0–45)
- Aspartate aminotransferase 21 U/L (7–40)
- Alkaline phosphatase 101 U/L (40–150)
- Total bilirubin 0.8 mg/dL (0–1.5)
- Albumin 3.5 g/dL (3.5–5)
- Pregnancy screen negative
- Urine toxicology screen negative.
Physical examination
The patient is very thin and appears quite uncomfortable. Her temperature is 99.7°F (37.6°C), pulse rate 101, respiratory rate 18, blood pressure 111/67 mm Hg, and oxygen saturation 96% on room air. Her skin is warm and dry. Her height is 66 inches, weight 116 lb, and body mass index 18.7.
Examination of the head and neck shows normal dentition, dry mucus membranes, and no oral exudates. The thyroid is normal, and no masses or lymphadenopathy are noted.
Heart sounds and rhythm are normal, and the lungs are clear with no crackles or rubs. The abdomen is scaphoid and soft, with no distention. She has epigastric tenderness but no rebound, guarding, rigidity, palpable mass, or costovertebral angle tenderness. Bowel sounds are normal. The neurologic examination is normal.
NARROWING THE DIAGNOSIS
1. Given the history and findings so far, which is the least likely cause of her symptoms?
- Lactose intolerance
- Celiac disease
- Crohn disease
- Duodenal ulcer
- Eating disorder
This young woman’s presentation has some features found in all of these conditions. However, the least likely is lactose intolerance.
Lactose intolerance results from a shortage of the enzyme lactase, which is normally produced by the cells that line the small intestine. Close to 50 million American adults have lactose intolerance. Common symptoms include nausea, cramps, bloating, gas, and diarrhea, which begin about 30 minutes to 2 hours after eating or drinking foods containing lactose.
Since the patient’s symptoms did not improve when she tried omitting milk products, and since lactose intolerance is rarely associated with pain radiating to the back and with severe vomiting, this is the least likely cause of her symptoms.
Celiac disease presents with a myriad of symptoms—sometimes without gastrointestinal (GI) symptoms. Anemia is the most common laboratory finding, due most often to iron deficiency, but also due to deficiencies of vitamin B12 and folate as a result of malabsorption.1
Our patient’s laboratory values—especially her red cell indices—do not confirm this finding. One must also remember, however, that hemoglobin tends to be falsely elevated in patients who are dehydrated.
Crohn disease often presents with occult blood loss, low-grade fever, weight loss, and anemia. Though the condition is most often ileocolic, it can affect any part of the gastrointestinal tract. Nevertheless, most patients with gastroduodenal involvement have previously been diagnosed with ileocolic disease, and gastroduodenal involvement manifests later. Nonradiating epigastric pain is very common. Obstructive symptoms due to gastroduodenal strictures (eg, postprandial vomiting, epigastric pain, weight loss, bloating) are also common. 2
Duodenal ulcer. The most important factors responsible for duodenal ulcers are NSAID use and Helicobacter pylori infection.3 Duodenal ulcers have a variety of clinical presentations, ranging from no symptoms to severe pain. Epigastric pain can be sharp, dull, burning, or penetrating. Many patients complain of a feeling of hunger and weight gain—as opposed to gastric ulcer, in which patients experience anorexia and weight loss. Abdominal pain generally occurs several hours after meals and often awakens the patient at night. Pain is often relieved by food, but this phenomenon is present in only 20% to 60% of patients and probably is not specific for duodenal ulcer.
Our patient does not use NSAIDs, but some of her symptoms, such as postprandial pain, epigastric pain radiating to the back, and nausea and vomiting are seen with duodenal ulcer.
Eating disorders. The two main types of eating disorders—anorexia nervosa and bulimia nervosa—have a significant diagnostic overlap,4 and a third type, binge-eating disorder, is currently being investigated and defined. Girls and women are 10 times as likely as boys and men to develop an eating disorder.
People with anorexia have a distorted view of their bodies. Even when they are extremely thin, they see themselves as too fat.
Bulimia is characterized by binge-eating, purging, and overexercising to compensate for the excess calories. Patients are often close to normal weight.
Binge-eating disorder involves the consumption of very large amounts of food in a short period of time. About 2% of all young adults in the United States struggle with bingeeating. They are either overweight or obese.
These disorders tend to be associated with other psychiatric disorders such as depression or obsessive-compulsive disorder. Our patient sought medical attention and was appropriately concerned about her weight loss, which make an eating disorder unlikely.
CASE CONTINUED: SHE UNDERGOES CT
2. Which of the following is the most likely diagnosis at this point?
- SMA syndrome
- Chronic mesenteric ischemia involving the SMA
- Megaduodenum due to a connective tissue disorder
SMA syndrome is the most likely diagnosis. Despite its name, this syndrome is not a vascular condition. It is an uncommon cause of proximal intestinal obstruction in which the duodenum is compressed between the SMA and the aorta. First described in 1861, it has also been known as cast syndrome, Wilkie syndrome, and arteriomesenteric duodenal obstruction.5
To date, more than 400 cases of this syndrome have been reported, twice as many in women as in men. Most patients are between 20 and 40 years of age at the time of diagnosis. Common presenting symptoms include postprandial abdominal pain, nausea, vomiting, and weight loss, which may further reduce the angle between the SMA and the aorta. Diarrhea is not generally associated with this syndrome, and in our patient’s case the diarrhea was thought to be unrelated to the SMA syndrome, since it subsided spontaneously.
Conditions and events that cause, contribute to, or worsen SMA syndrome include:
- Rapid weight loss (as in cancer or burns) or lean body habitus
- Prolonged bed rest
- Use of a body cast
- Malabsorption
- Spinal disease, deformity, or trauma
- Scoliosis surgery
- Rapid linear growth without compensatory weight gain
- Abnormally high and fixed position of the ligament of Treitz
- Abdominal surgery
- Cardiac cachexia
- Unusually low origin of the SMA.7
More common causes of mechanical smallbowel obstruction are adhesions, hernias, and tumors.8 Hyperactive, high-pitched peristalsis with rushes coinciding with cramps is typical. Abdominal cramps are centered around the umbilicus or in the epigastrium and are associated with vomiting; obstipation develops in patients with complete obstruction. Patients with partial obstruction may develop diarrhea. Paralytic ileus secondary to hypokalemia is an important consideration in partial obstruction. However, abdominal radiography and CT did not confirm an obstruction, and her symptoms persisted despite correction of the potassium level.
Chronic mesenteric ischemia can be caused by vasculitis, nonocclusive conditions that cause prolonged vasoconstriction (eg, cocaine ingestion), or reduced cardiac output.9 Symptoms are due to the gradual reduction in blood flow to the intestine that occurs during eating. Our patient’s toxicology report did not suggest cocaine abuse, and her history and the workup thus far do not suggest heart failure. A workup for vasculitis was negative.
Megaduodenum, SMA-like syndrome. In rare cases, dilation of the duodenum at the level of the SMA may be part of a generalized duodenal dilation caused by something other than obstruction due to mechanical compression. There are conditions, as described below, that cause an SMA-like syndrome.
A compression defect of the duodenum at the site where the SMA crossed the duodenum was found in a series of 11 cases of systemic sclerosis.10 These patients had definite dilation of the duodenum, but it was a result of atrophy of the muscle layers and replacement by collagenous tissue, changes that result in diminished peristalsis, loss of muscle tone, and dilation. The duodenum yields to pressure in its third portion under the SMA.
Several pathologic conditions, particularly connective tissue disorders, may predispose to the development of a megaduodenum that may result in an imprint on the duodenum at the level of the SMA. The most noteworthy of these conditions is scleroderma. Other conditions that can cause reduced duodenal peristalsis include diabetes, pancreatitis, dermatomyositis, lupus erythematosus, myxedema, and amyloidosis.11
It is important to distinguish SMA syndrome from SMA-like syndromes for several reasons.12 SMA-like syndromes result in loss of normal peristalsis. Further, the conditions have different outcomes, even though they are managed similarly initially, ie, with rehydration and parenteral nutrition. Surgery is to be avoided if possible in conditions that affect widespread areas of the intestine, such as scleroderma or diabetic neuropathy.
3. Which of the following is helpful in confirming SMA syndrome?
- CT of the abdomen
- Upper GI radiography series
- Upper GI endoscopy
All three can help confirm the diagnosis.
CT of the abdomen is a convenient, safe, rapid, readily available, and relatively noninvasive way to evaluate the aortomesenteric angle and to view retroperitoneal and mesenteric fat.13 Rehydration before injecting intravenous dye is important to avoid precipitating renal failure. In this patient, CT findings that helped make the diagnosis included a narrow aortomesenteric angle, compression of the duodenum, and a paucity of fat around the SMA.
An upper GI series can reveal dilation of the first and second portions of the duodenum and abrupt compression of the duodenal mucosal folds. Other findings can include a delay of 4 to 6 hours in gastroduodenal transit and relief of the obstruction when the patient is in the left lateral decubitus position. The Hayes maneuver refers to the disappearance of these radiologic features in the knee-chest position on cinefluoroscopy.14 The findings mentioned above are best noted in the supine position on both radiography and CT.
Endoscopy is necessary to rule out mechanical causes of duodenal obstruction. A pulsatile extrinsic compression suggests this condition but is found only occasionally.
Other imaging studies, such as ultrasonography, arteriography, and hypotonic duodenography, are used less often.
4. At this time, which of the following would be the most appropriate initial treatment in this patient?
- Conservative treatment
- Narcotics
- Duodenojejunostomy
Conservative treatment is indicated initially in all cases of SMA syndrome.15 This involves reversing precipitating factors and replacing fluid, electrolytes, and nutrition via total parenteral nutrition and nasogastric decompression.
To avoid keeping the patient on intravenous therapy for a prolonged time, it is important to start enteral feeding once the pain has subsided and the patient can tolerate it. A double-lumen nasojejunal tube is passed distal to the obstruction under fluoroscopic guidance. During feedings, the patient should be in the modified knee-chest, prone, or leftside-down position, all of which increase the aortomesenteric angle.
Delaying the treatment of SMA syndrome can increase the risk of morbidity and mortality by progressive malnutrition, dehydration, oliguria, electrolyte abnormalities (eg, hypokalemia), or intestinal perforation from prolonged ischemia.16,17
Narcotics and other drugs known to slow gut motility should be avoided.
Symptoms typically improve after restoration of normal body weight. If conservative treatment fails, or if the case is severe or chronic, surgery is required.18 Fortunately, this is not required often.
Duodenojejunostomy is the most common surgical treatment and involves creation of an alternate route between the duodenum and the jejunum, bypassing the compression between the aorta and the SMA. Other procedures include gastrojejunostomy, laparoscopic duodenojejunostomy, 19 a Roux-en-Y procedure, robotically assisted duodenojejunostomy, and anterior transposition of the third portion of the duodenum. Cleavage of the ligament of Treitz is another option, enabling the duodenum to drop away from the apex of the sharpened aortomesenteric angle.
WHEN TO CONSIDER SMA SYNDROME
The SMA syndrome is an uncommon cause of a very common presenting symptom, ie, abdominal pain. Nevertheless, it should be considered in the differential diagnosis of abdominal pain, especially in patients who have conditions that cause significant weight loss, such as anorexia nervosa, malabsorption, or hypercatabolic states such as burns, major surgery, severe injuries, or malignancies. The diagnosis is based on a thorough history and on supportive findings from CT and endoscopy.
In our patient, weight loss began with nonspecific diarrhea but perpetuated itself as SMA syndrome occurred.
Appropriate management consists of interrupting the cycle of weight loss and secondary upper gut obstruction. For patients in whom more definitive therapy is not feasible, a gastrostomy tube for decompression with a jejunal extension available for feeding appears to be a reasonable and safe treatment option. Duodenojejunostomy is considered the procedure of choice in severe cases.
CASE CONCLUDED
Fortunately, our patient responded well to conservative management. She was treated with intravenous hydration and correction of electrolytes and 10 days later was able to tolerate a soft diet. She was discharged in stable condition. At a follow-up visit 2 weeks later, she reported minimal abdominal discomfort, was able to tolerate meals, and had gained a few pounds. She continues to do well.
- Iovino P, Ciacci C, Sabbatini F, Acioli DM, D'Argenio G, Mazzacca G. Esophageal impairment in adult celiac disease with steatorrhea. Am J Gastroenterol 1998; 93:1243–1249.
- Loftus EV. Upper gastrointestinal tract Crohn’s disease. Clin Perspect Gastroenterol 2002; 5:188–191.
- Zapata-Colindres JC, Zepeda-Gómez S, Montaño-Loza A, Vázquez-Ballesteros E, de Jesús Villalobos J, Valdovinos-Andraca F. The association of Helicobacter pylori infection and nonsteroidal antiinflammatory drugs in peptic ulcer disease. Can J Gastroenterol 2006; 20:277–280.
- Milos G, Spindler A, Schnyder U, Fairburn CG. Instability of eating disorder diagnoses: prospective study. Br J Psychiatry 2005; 187:573–578.
- Wilkie DP. Chronic duodenal ileus. Br J Surg 1921; 9:204–214.
- Ozkurt H, Cenker MM, Bas N, Erturk SM, Basak M. Measurement of the distance and angle between the aorta and superior mesenteric artery: normal values in different BMI categories. Surg Radiol Anat 2007; 29:595–599.
- Lippl F, Hannig C, Weiss W, Allescher HD, Classen M, Kurjak M. Superior mesenteric artery syndrome: diagnosis and treatment from the gastroenterologist's view. J Gastroenterol 2002; 37:640–643.
- Balthazar EJ. George W. Holmes Lecture. CT of small-bowel obstruction. AJR Am J Roentgenol 1994; 162:255–261.
- Chang JB, Stein TA. Mesenteric ischemia: acute and chronic. Ann Vasc Surg 2003; 17:323–328.
- Gondos B. Duodenal compression defect and the “superior mesenteric artery syndrome” 1. Radiology 1977; 123:575–580.
- Cohen LB, Field SP, Sachar DB. The superior mesenteric artery syndrome. The disease that isn't, or is it? J Clin Gastroenterol 1985; 7:113–716.
- Ahmed AR, Taylor I. Superior mesenteric artery syndrome. Postgrad Med J 1997; 73:776–778.
- Santer R, Young C, Rossi T, Riddlesberger MM. Computed tomography in superior mesenteric artery syndrome. Pediatr Radiol 1991; 21:154–155.
- Lukes PJ, Rolny P, Nilson AE, Gamklou R, Darle N, Dotevall G. Diagnostic value of hypotonic duodenography in superior mesenteric artery syndrome. Acta Chir Scand 1978; 144:39–43.
- Dietz UA, Debus ES, Heuko-Valiati L, et al. Aorto-mesenteric artery compression syndrome. Chirurg 2000; 71:1345–1351.
- Lim JE, Duke GL, Eachempati SR. Superior mesenteric artery syndrome presenting with acute massive gastric dilatation, gastric wall pneumatosis, and portal venous gas. Surgery 2003; 134:840–843.
- Fuhrman MA, Felig DM, Tanchel ME. Superior mesenteric artery syndrome with obstructing duodenal bezoar. Gastrointest Endosc 2003; 57:387.
- Hines JR, Gore RM, Ballantyne GH. Superior mesenteric artery syndrome. Diagnostic criteria and therapeutic approaches. Am J Surg 1984; 148:630–632.
- Gersin KS, Heniford BT. Laparoscopic duodenojejunostomy for treatment of superior mesenteric artery syndrome. JSLS 1998; 2:281–284.
- Iovino P, Ciacci C, Sabbatini F, Acioli DM, D'Argenio G, Mazzacca G. Esophageal impairment in adult celiac disease with steatorrhea. Am J Gastroenterol 1998; 93:1243–1249.
- Loftus EV. Upper gastrointestinal tract Crohn’s disease. Clin Perspect Gastroenterol 2002; 5:188–191.
- Zapata-Colindres JC, Zepeda-Gómez S, Montaño-Loza A, Vázquez-Ballesteros E, de Jesús Villalobos J, Valdovinos-Andraca F. The association of Helicobacter pylori infection and nonsteroidal antiinflammatory drugs in peptic ulcer disease. Can J Gastroenterol 2006; 20:277–280.
- Milos G, Spindler A, Schnyder U, Fairburn CG. Instability of eating disorder diagnoses: prospective study. Br J Psychiatry 2005; 187:573–578.
- Wilkie DP. Chronic duodenal ileus. Br J Surg 1921; 9:204–214.
- Ozkurt H, Cenker MM, Bas N, Erturk SM, Basak M. Measurement of the distance and angle between the aorta and superior mesenteric artery: normal values in different BMI categories. Surg Radiol Anat 2007; 29:595–599.
- Lippl F, Hannig C, Weiss W, Allescher HD, Classen M, Kurjak M. Superior mesenteric artery syndrome: diagnosis and treatment from the gastroenterologist's view. J Gastroenterol 2002; 37:640–643.
- Balthazar EJ. George W. Holmes Lecture. CT of small-bowel obstruction. AJR Am J Roentgenol 1994; 162:255–261.
- Chang JB, Stein TA. Mesenteric ischemia: acute and chronic. Ann Vasc Surg 2003; 17:323–328.
- Gondos B. Duodenal compression defect and the “superior mesenteric artery syndrome” 1. Radiology 1977; 123:575–580.
- Cohen LB, Field SP, Sachar DB. The superior mesenteric artery syndrome. The disease that isn't, or is it? J Clin Gastroenterol 1985; 7:113–716.
- Ahmed AR, Taylor I. Superior mesenteric artery syndrome. Postgrad Med J 1997; 73:776–778.
- Santer R, Young C, Rossi T, Riddlesberger MM. Computed tomography in superior mesenteric artery syndrome. Pediatr Radiol 1991; 21:154–155.
- Lukes PJ, Rolny P, Nilson AE, Gamklou R, Darle N, Dotevall G. Diagnostic value of hypotonic duodenography in superior mesenteric artery syndrome. Acta Chir Scand 1978; 144:39–43.
- Dietz UA, Debus ES, Heuko-Valiati L, et al. Aorto-mesenteric artery compression syndrome. Chirurg 2000; 71:1345–1351.
- Lim JE, Duke GL, Eachempati SR. Superior mesenteric artery syndrome presenting with acute massive gastric dilatation, gastric wall pneumatosis, and portal venous gas. Surgery 2003; 134:840–843.
- Fuhrman MA, Felig DM, Tanchel ME. Superior mesenteric artery syndrome with obstructing duodenal bezoar. Gastrointest Endosc 2003; 57:387.
- Hines JR, Gore RM, Ballantyne GH. Superior mesenteric artery syndrome. Diagnostic criteria and therapeutic approaches. Am J Surg 1984; 148:630–632.
- Gersin KS, Heniford BT. Laparoscopic duodenojejunostomy for treatment of superior mesenteric artery syndrome. JSLS 1998; 2:281–284.
Myelodysplastic syndromes: A practical approach to diagnosis and treatment
Myelodysplastic syndromes (MDS) are a heterogeneous group of disorders of blood cell production in the bone marrow that can transform into acute myeloid leukemia (AML).1,2 They are diagnosed most often in the elderly.
Primary care physicians and geriatricians tend to be the first to identify the problem, as they recognize that cytopenias are not simply a normal consequence of aging.
MDS are considered to be cancers, akin to chronic leukemia or acute leukemia, with epidemiologic data tracked by national cancer registries and the US Centers for Disease Control and Prevention, under the auspices of the Surveillance, Epidemiology, and End Results (SEER) program.3
In this article, we briefly review the classification of MDS, current epidemiologic data, key diagnostic features, and current management options.
WHEN TO SUSPECT MDS
In many patients, MDS are asymptomatic and appear as an abnormality on a routine complete blood cell count (CBC) or as part of a workup for anemia. Symptoms develop as the bone marrow’s ability to produce normal-functioning blood cells is more and more compromised. The range of symptoms depends on the bone marrow cell type affected.
Patients with MDS typically have some degree of anemia, often detected incidentally on a routine CBC, or they have symptoms stemming from anemia or thrombocytopenia, or have recurrent infections.
Subtypes of MDS have different pathologic and clinical presentations and different prognoses. They are often categorized as lower-risk or higher-risk, depending on the likelihood of transforming to AML. Patients with lower-risk MDS survive a median of 3 to 7 years. Higher-risk types are pathobiologically similar to AML in older adults, and patients either develop AML or die of complications of MDS, on average within 1.5 years.
Several classification schemes and prognostic models guide the selection of the most appropriate therapy.
Older age and comorbidities such as coronary artery disease, chronic obstructive pulmonary disease, and chronic kidney disease make MDS more difficult to manage and worsen the prognosis.4
MOST PATIENTS ARE OLDER
Only since 2001, when MDS became reportable to SEER,3,5 has the epidemiology of MDS been reported in the United States.
MDS are currently diagnosed in an estimated 3.4 per 100,000 US citizens yearly.
The incidence rate increased from 3.28 per 100,000 per year in 2001 to 3.56 per 100,000 in 2004.5 The increase has been attributed to enhanced awareness of the disease and to the aging of the population, with the number of people age 65 or older in the United States expected to double from the year 2000 to 2030. Another factor is that effective therapies are now available, possibly making hematologists and oncologists more likely to pursue the diagnosis.
These numbers translate to 10,000 to 15,000 new cases annually, and given the life expectancy of patients affected by this disease (and the life-extending treatments for it), an estimated 30,000 to 60,000 Americans living with MDS.6,7
Even though MDS can occur at any age, most patients are older. The median age at diagnosis is 71 years,3,5,8 and 72% of patients are age 70 or older.3 The prevalence increases with age, to a rate of 36 per 100,000 in those age 80 and older.9 However, in areas of East Asia, it occurs at ages almost 2 decades younger than in the rest of the world.5
MDS are more common in men than in women and in whites than in blacks. Smoking appears to increase the risk, but alcohol consumption does not.10
About 10% of cases of MDS are secondary, most often due to radiation treatment or chemotherapy (particularly with alkylating agents and topoisomerase inhibitors) for cancer. The time from treatment of a primary malignancy (most often prostate, breast, bladder, lung, or non-Hodgkin lymphoma) to the development of MDS is about 5 years.5 A small number of cases are due to occupational exposure to radiation or benzene or other organic solvents, as might occur in the rubber industry (see below). Secondary MDS have a worse prognosis than primary (de novo) MDS.
GENETIC AND ENVIRONMENTAL FACTORS
The cause of de novo MDS is not known. Genetic and environmental factors probably both play a role. The lower median age at diagnosis in Eastern countries such as Japan than in the United States suggests that environmental factors11 such as smoking, ionizing radiation, and benzene exposure play a role.12,13 Some epidemiologic evidence suggests a higher incidence of MDS after exposure to solvents, hair dyes, and pesticides.13
Congenital conditions such as Down syndrome, Fanconi anemia, and Bloom syndrome are associated with MDS. Those affected usually present at an earlier age,13 suggesting a “multiple-hit” mechanism of cancer development with genetic and environmental factors. MDS rarely run in families.
SYMPTOMS ARE OFTEN NONSPECIFIC
Symptoms of MDS are often vague and nonspecific, and the diagnosis is often made during a workup for anemia, thrombocytopenia, or neutropenia discovered on a CBC. If present, signs and symptoms depend on the blood and bone marrow cell types that are affected.
When erythrocytes are affected (the most common situation), patients present with signs of anemia, including pallor, pale conjunctiva, tachycardia, hypotension, fatigue, headache, and exercise intolerance, or with signs and symptoms of a worsening underlying condition such as angina pectoris, heart failure, or emphysema.
When platelets or neutrophils are affected. Fewer than 20% of patients present with symptoms of isolated thrombocytopenia such as minor bleeding (eg, mucosal bleeding, petechiae, easy bruising, epistaxis) or major bleeding (eg, gastrointestinal bleeding, intracranial hemorrhage) or of isolated neutropenia (eg, fatigue, frequent bacterial infections of different organs systems).
Splenomegaly and lymphadenopathy are uncommon in MDS and, if detected, should raise suspicion of a myeloproliferative or lymphoproliferative neoplasm.
LABORATORY TESTS NEEDED
Complete blood cell count
Once the common causes of patient’s symptoms are evaluated, a CBC is needed to look for a hematologic cause. If a patient is ultimately determined to have MDS, anemia is the most common finding on the CBC: about 80% of patients with MDS are anemic at presentation. 6
Anemia associated with MDS can be microcytic, normocytic, or, most commonly, macrocytic. 14 Thrombocytopenia and neutropenia can be solitary or associated with anemia, and they are seen in about 40% of patients at the time of diagnosis.6 As the disease progresses, the degree of cytopenia worsens and, in most cases, preserved cell lineages are eventually affected.
Once cytopenia is discovered, a workup for the cause is needed. We emphasize a workup first for anemia, as it is the most common form of cytopenia in MDS. A workup for isolated thrombocytopenia or neutropenia usually requires a bone marrow examination earlier in the course, and we will discuss it only briefly here. Multilineage cytopenia almost always suggests abnormal bone marrow function and can be the basis for referral to a hematologist or oncologist.
Evaluation of anemia
If anemia is detected, it is reasonable to look for nonhematologic causes such as gastrointestinal bleeding, a cardiac cause, or a nutritional deficiency.
Anemia has a variety of possible hematologic causes, as shown in a study in the United States.15 When blood samples were collected from more than 2,000 people age 65 and older, 10.6% were found to have anemia, categorized as follows:
- Nutrient-deficiency anemia, related to low levels of vitamin B12, folate, or more commonly iron
- Anemia of chronic inflammation (formerly anemia of chronic disease, associated with a major medical disorder)
- Unexplained anemia (of those with unexplained anemia, 17.4% had blood findings compatible with MDS).15
- Tests for nutrient deficiencies such as iron, vitamin B12, and folate levels. Subsequent tests can include assessment for copper deficiencies. Vitamin B12 and copper deficiency can mimic MDS.
- Fecal occult blood testing, and, if positive, further evaluation for a source of gastrointestinal bleeding.
- Liver function tests, renal function tests, and tests for endocrine disorders, such as thyroid function tests.
- Review of drugs that can cause megaloblastoid erythropoiesis, such as methotrexate (Trexall), valproic acid (Depakote), phenytoin (Dilantin), phenobarbital (Luminal), sulfasalazine (Sulfazine), and zidovudine (Retrovir).
- Assesment of the responsiveness of the bone marrow to anemia, via a reticulocyte count or an erythropoietin level, or both, prior to any blood transfusion.
- Screening for relevant infections, including human immunodeficiency virus (HIV), hepatitis, or, in rare cases, parvovirus.
- Screening for lifestyle factors that may result in bone marrow suppression, such as excessive alcohol intake.
Evaluation of other cytopenias
In cases of isolated thrombocytopenia or combined bicytopenia (eg, anemia and thrombocytopenia), abdominal ultrasonography should be done to evaluate for splenomegaly.
Blood tests to evaluate for immune-mediated cytopenias, including idiopathic thrombocytopenic purpura and hemolytic anemia, include the direct and indirect Coombs antiglobulin tests, the lactate dehydrogenase level, the reticulocyte count, and the haptoglobin level. Other immune-mediated causes of cytopenia include connective tissue disorders and vasculitides, and an antinuclear antibody titer and rheumatoid factor level can also be considered.
Referral if tests are negative
If all these tests are negative, the next step is referral to a hematologist-oncologist for further workup, which may include a review of the peripheral blood smear; bone marrow aspiration and biopsy for evaluation of iron stores and bone marrow cellularity; and specialized tests such as assessment of antiplatelet antibodies, protein electrophoresis, or fluorescence in situ hybridization to evaluate for specific clonal disorders. The purpose of bone marrow aspiration and biopsy in MDS is to evaluate the morphology of the bone marrow and the patient’s cytogenetic profile. Each has its prognostic and therapeutic implications.
SCORING SYSTEMS FOR MDS, RATHER THAN STAGING SYSTEMS
The purpose of classification systems for any medical condition is to uniformly evaluate and group patients with a disease subtype to compare patient populations similarly throughout the world, to predict prognosis, and to dictate therapeutic directions.
MDS have two main classification systems, the FAB (French-American-British) and the WHO (World Health Organization). Revised in 2008,16 the WHO classification (Table 2, not available online)17 is widely accepted because it incorporates morphologic and cytogenetic factors and correlates with prognosis.18 The categories are distinguished by specific characteristics of peripheral blood and bone marrow.
Unlike many other cancers, MDS are not “staged.” Rather, prognostic systems have been devised to predict the risk of transformation to AML and to predict overall survival. These systems are based on:
- The number of myeloblasts in the bone marrow (the higher the count, the worse the prognosis)
- The number or degree of cytopenias
- Cytogenetic abnormalities (acquired genetic abnormalities in the neoplastic clone), found in about half of patients with MDS.19
The most widely used prognostic systems are the International Prognostic Scoring System (Table 3, not available online)2 and the WPSS (WHO Classification-based Prognostic Scoring System1). The latter system encorporates transfusion burden.
SUPPORTIVE CARE
Supportive care includes transfusion of blood products to minimize complications of cytopenias and to improve quality of life, as well as antibiotics to treat active infections.
Transfusions
Almost all patients with MDS need red cell transfusions at some point, while fewer need platelets. The frequency of transfusion depends on the extent of the disease and on comorbidities.
Red blood cells typically are given when the hemoglobin level falls below 8.5 g/dL, and platelets are given when the platelet count is below 100 × 109/L, in the absence of symptoms. Patients with symptomatic anemia should receive transfusion to relieve their symptoms. Some patients need transfusions occasionally, while others are transfusion-dependent.
Iron chelation
Blood product transfusions can lead to iron overload, particularly with a lifetime administration of more than 20 units, or with a year of continuous transfusions, and this is associated with diminished survival.20
However, considering the short survival of patients with MDS, the benefit of iron chelation is debatable. This intervention should be reserved for patients with lower-risk disease who are expected to survive more than 1 year and who have received more than 25 units of packed red blood cells.21
Antibiotics
Neutropenia is defined as an absolute neutrophil count less than 1.5 × 109/L. The risk of infection, particularly bacterial infection, is significantly increased when the neutrophil count is below 0.5 × 109/L. Fever (temperature > 100.4°F or 38.0°C) in neutropenic patients is an emergency, requiring hospitalization and immediate initiation of broad-spectrum antibiotics along with a workup for the cause of the fever.22 Prophylactic antibiotics have no proven role in MDS patients with neutropenia.
TREATMENT OF LOWER-RISK DISEASE
Erythropoiesis-stimulating agents
Once a patient starts to require red blood cell transfusions, an erythropoiesis-stimulating agent (EPA) can be considered.23,24 These include recombinant agents such as erythropoietin (Procrit) and darbepoetin alfa (Aranesp).
Response is measured as an improvement in hemoglobin or as independence from transfusions in those previously dependent on them. Patients most likely to respond are those whose pretransfusion erythropoietin level is below 100 IU/L and who have minimal transfusion needs.25,26 Addition of a colony-stimulating factor can be considered for patients with neutropenia. On average, about 40% of patients ultimately respond to an EPA, but those who respond eventually develop resistance to the agent. Retrospective data indicate that use of EPAs may improve survival in MDS.23,24
The recommended threshold hemoglobin level for starting an EPA is less than 10 g/dL. Patients need to be monitored with a CBC every time they receive treatment. The agent should be stopped once the hemoglobin level reaches 12 g/dL. A number of studies have shown lower survival rates when ESAs are used in nonhematologic malignancies, particularly if the malignancy is advanced and when the ESA is used to achieve a goal hemoglobin above 12 g/dL. There are no data to suggest a higher death rate in patients with hematologic malignancies who take ESAs. The use of ESAs in MDS patients should be judicious, however, and titrated to a goal hemoglobin level no higher than 12 g/dL.27
Other treatments
If ESA treatment is ineffective, other treatments may be considered, usually initiated by a hematologist or medical oncologist.
Immunosuppressive therapy with antithymocyte globulin (Thymoglobulin)28 is an option for patients with hypocellular or immune-mediated MDS. This treatment may decrease the need for transfusion and may improve the blood count.
Lenalidomide (Revlimid) for MDS with isolated chromosome 5q deletion29 can decrease the need for blood transfusion in approximately two-thirds of these patients.
Azacitidine (Vidaza) or decitabine (Dacogen), in patients with more advanced subtypes of MDS (eg, those with excess blasts) or with pancytopenia unresponsive to other therapies, can induce hematologic improvement and decrease transfusion dependence, as well as prolong survival.
Stem cell transplantation, for patients with more advanced subtypes of MDS and who have an appropriately matched donor, has the potential of being curative.
Experimental treatments are available in clinical trials.
TREATMENT OF HIGHER-RISK DISEASE
About 25% of patients with newly diagnosed MDS and 15% to 20% of patients with established MDS have higher-risk disease.30 These patients should almost always be followed by a hematologist or medical oncologist, with therapy initiated immediately, regardless of blood counts, given the high likelihood of transformation to AML or death within 1.5 years.
The treatment options for higher-risk disease include:
- Methyltransferase inhibitors such as azacitidine and decitabine31–34
- Cytotoxic chemotherapy (similar to treatment of acute myeloid leukemia)
- Bone marrow-hematopoeitic stem cell transplantation35,36
- Experimental treatments in clinical trials.
As mentioned earlier, outside of transplantation, only azacitidine has been shown to improve overall survival (with a doubling of survival at 2 years, to 50%), and no drug therapy is curative. Managing patient expectations for treatment outcome is thus crucial in higher-risk disease, and ongoing assessments of quality of life, both on or off therapy, should be considered obligatory.
Stem cell transplantation cures MDS
MDS are complex and heterogeneous, so treatment options range from supportive care to chemotherapy and allogeneic stem cell transplantation.6 The choice depends on the severity of disease, ie, lower-risk or higherrisk (Table 3, not available online), as well as on the prognosis, the availability of therapeutic options, and the patient’s expectations.
Hematopoietic stem cell transplantation is the only curative treatment for MDS. However, it is performed in fewer than 5% of patients,30 usually younger patients with few comorbidities, because the rate of transplantrelated death is high. Therefore, most treatments are palliative, aimed at improving the quality of life and prolonging survival.
The balance between risks and benefits of these treatments must be justifiable.30 Further, patients who have no symptoms or who have lower-risk disease need no treatment and may not for years. However, they do need close follow-up, because their symptoms will worsen and will eventually require treatment.
TAKE-HOME POINTS
- Myelodysplastic syndromes are more prevalent than previously realized. Mainly a disease of older adults, they should be suspected in any patient with unexplained cytopenia.
- Life expectancy at the time of diagnosis depends on the types of cells affected.
- Supportive and disease-altering options are available.
- Prompt referral to a hematologist or oncologist is important for confirmation of the diagnosis and initiation of an appropriate treatment plan. Patients with lower-risk disease can continue follow-up with their primary care provider once treatment goals and plans are established.
ACKNOWLEDGMENT
We thank Dr. Karl Theil of the Cleveland Clinic Department of Clinical Pathology for the photomicrographs used on the cover.
- Malcovati L, Nimer SD. Myelodysplastic syndromes: diagnosis and staging. Cancer Control 2008; 15 (suppl 4):4–13.
- Greenberg P, Cox C, LeBeau MM, et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood 1997; 89:2079–2088.
- Rollison DE, Howlader N, Smith MT, et al. Epidemiology of myelodysplastic syndromes and chronic myeloproliferative disorders in the United States, 2001–2004, using data from the NAACCR and SEER programs. Blood 2008; 112:45–52.
- Lichtman MA, Rowe JM. The relationship of patient age to the pathobiology of the clonal myeloid diseases. Semin Oncol 2004; 31:185–197.
- Ma X, Does M, Raza A, Mayne ST. Myelodysplastic syndromes: incidence and survival in the United States. Cancer 2007; 109:1536–1542.
- Steensma DP, Bennett JM. The myelodysplastic syndromes: diagnosis and treatment. Mayo Clin Proc 2006; 81:104–130.
- The MDS Foundation. http://www.mds-foundation.org/. Accessed August 27, 2009.
- Sekeres M, Cosgrove D, Falco A. Managing patients with low-risk MDS. Clin Adv Hematol Oncol 2006; 4( 7 suppl 16):1–10.
- Sandhu SK, Sekeres MA. Myelodysplastic syndromes: more prevalent than we know. Geriatrics 2008; 63:10–17.
- Strom SS, Gu Y, Gruschkus SK, Pierce SA, Estey EH. Risk factors of myelodysplastic syndromes: a case-control study. Leukemia 2005; 19:1912–1918.
- Kuendgen A, Matsuda A, Germing U. Differences in epidemiology of MDS between Western and Eastern countries: Ethnic differences or environmental influence? Leuk Res 2007; 31:103–104.
- Bjork J, Johansson B, Broberg K, Albin M. Smoking as a risk factor for myelodysplastic syndromes and acute myeloid leukemia and its relation to cytogenetic findings: a case-control study. Leuk Res 2009; 33:788–791.
- Germing U, Aul C, Niemeyer CM, Haas R, Bennett JM. Epidemiology, classification and prognosis of adults and children with myelodysplastic syndromes. Ann Hematol 2008; 87:691–699.
- Juneja SK, Imbert M, Jouault H, Scoazec JY, Sigaux F, Sultan C. Haematological features of primary myelodysplastic syndromes (PMDS) at initial presentation: a study of 118 cases. J Clin Pathol 1983; 36:1129–1135.
- Guralnik JM, Eisenstaedt RS, Ferrucci L, Klein HG, Woodman RC. Prevalence of anemia in persons 65 years and older in the United States: evidence for a high rate of unexplained anemia. Blood 2004; 104:2263–2268.
- Swerdlow SH, Campo E, Harris NL, et al. International Agency for Research on Cancer, World Health Organization. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues, 4th ed. International Agency for Research on Cancer: Lyon, France; 2008.
- Vardiman JW, Thiele J, Arber DA, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood 2009; 114:937–951.
- Bennett JM. A comparative review of classification systems in myelodysplastic syndromes (MDS). Semin Oncol 2005; 32( 4 suppl 5):S3–S10.
- Haase D. Cytogenetic features in myelodysplastic syndromes. Ann Hematol 2008; 87:515–526.
- Malcovati L, Della Porta MG, Cazzola M. Predicting survival and leukemic evolution in patients with myelodysplastic syndrome. Haematologica 2006; 91:1588–1590.
- Bowen D, Culligan D, Jowitt S, et al. Guidelines for the diagnosis and therapy of adult myelodysplastic syndromes. Br J Haematol 2003; 120:187–200.
- Segal BH, Freifeld AG, Baden LR, et al. Prevention and treatment of cancer-related infections. J Natl Compr Canc Netw 2008; 6:122–174.
- Golshayan AR, Jin T, Maciejewski J, et al. Efficacy of growth factors compared to other therapies for low-risk myelodysplastic syndromes. Br J Haematol 2007; 137:125–132.
- Jadersten M, Malcovati L, Dybedal I, et al. Erythropoietin and granulocyte-colony stimulating factor treatment associated with improved survival in myelodysplastic syndrome. J Clin Oncol 2008; 26:3607–3613.
- Hellstrom-Lindberg E, Gulbrandsen N, Lindberg G, et al; Scandinavian MDS Group. A validated decision model for treating the anaemia of myelodysplastic syndromes with erythropoietin + granulocyte colony-stimulating factor: significant effects on quality of life. Br J Haematol 2003; 120:1037–1046.
- Cheson BD, Greenberg PL, Bennett JM, et al. Clinical application and proposal for modification of the International Working Group (IWG) response criteria in myelodysplasia. Blood 2006; 108:419–425.
- ARANESP Prescribing Information. http://pi.amgen.com/united_states/aranesp/ckd/aranesp_pi_hcp_english.pdf. Accessed August 28, 2009.
- Molldrem JJ, Leifer E, Bahceci E, et al. Antithymocyte globulin for treatment of the bone marrow failure associated with myelodysplastic syndromes. Ann Intern Med 2002; 137:156–163.
- List A, Dewald G, Bennett J, et al; Myelodysplastic Syndrome-003 Study Investigators. Lenalidomide in the myelodysplastic syndrome with chromosome 5q deletion. N Engl J Med 2006; 355:1456–1465.
- Sekeres MA, Schoonen WM, Kantarjian H, et al. Characteristics of US patients with myelodysplastic syndromes: results of six crosssectional physician surveys. J Natl Cancer Inst 2008; 100:1542–1551.
- Stone R, Sekeres M, Garcia-Manero G, Lyons RM. Recent advances in low-and intermediate-1-risk myelodysplastic syndrome: developing a consensus for optimal therapy. Clin Adv Hematol Oncol 2008; 6:1–15.
- Kantarjian H, Issa JP, Rosenfeld CS, et al. Decitabine improves patient outcomes in myelodysplastic syndromes: results of a phase III randomized study. Cancer 2006; 106:1794–1803.
- Fenaux P, Mufti GJ, Hellstrom-Lindberg E, et al; International Vidaza High-Risk MDS Survival Study Group. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: a randomised, open-label, phase III study. Lancet Oncol 2009; 10:223–232.
- Silverman LR, Demakos EP, Peterson BL, et al. Randomized controlled trial of azacitidine in patients with the myelodysplastic syndrome: a study of the cancer and leukemia group B. J Clin Oncol 2002; 20:2429–2440.
- Giralt S. Bone marrow transplant in myelodysplastic syndromes: new technologies, same questions. Curr Hematol Rep 2005; 4:200–207.
- Cutler CS, Lee SJ, Greenberg P, et al. A decision analysis of allogeneic bone marrow transplantation for the myelodysplastic syndromes: delayed transplantation for low-risk myelodysplasia is associated with improved outcome. Blood 2004; 104:579–585.
Myelodysplastic syndromes (MDS) are a heterogeneous group of disorders of blood cell production in the bone marrow that can transform into acute myeloid leukemia (AML).1,2 They are diagnosed most often in the elderly.
Primary care physicians and geriatricians tend to be the first to identify the problem, as they recognize that cytopenias are not simply a normal consequence of aging.
MDS are considered to be cancers, akin to chronic leukemia or acute leukemia, with epidemiologic data tracked by national cancer registries and the US Centers for Disease Control and Prevention, under the auspices of the Surveillance, Epidemiology, and End Results (SEER) program.3
In this article, we briefly review the classification of MDS, current epidemiologic data, key diagnostic features, and current management options.
WHEN TO SUSPECT MDS
In many patients, MDS are asymptomatic and appear as an abnormality on a routine complete blood cell count (CBC) or as part of a workup for anemia. Symptoms develop as the bone marrow’s ability to produce normal-functioning blood cells is more and more compromised. The range of symptoms depends on the bone marrow cell type affected.
Patients with MDS typically have some degree of anemia, often detected incidentally on a routine CBC, or they have symptoms stemming from anemia or thrombocytopenia, or have recurrent infections.
Subtypes of MDS have different pathologic and clinical presentations and different prognoses. They are often categorized as lower-risk or higher-risk, depending on the likelihood of transforming to AML. Patients with lower-risk MDS survive a median of 3 to 7 years. Higher-risk types are pathobiologically similar to AML in older adults, and patients either develop AML or die of complications of MDS, on average within 1.5 years.
Several classification schemes and prognostic models guide the selection of the most appropriate therapy.
Older age and comorbidities such as coronary artery disease, chronic obstructive pulmonary disease, and chronic kidney disease make MDS more difficult to manage and worsen the prognosis.4
MOST PATIENTS ARE OLDER
Only since 2001, when MDS became reportable to SEER,3,5 has the epidemiology of MDS been reported in the United States.
MDS are currently diagnosed in an estimated 3.4 per 100,000 US citizens yearly.
The incidence rate increased from 3.28 per 100,000 per year in 2001 to 3.56 per 100,000 in 2004.5 The increase has been attributed to enhanced awareness of the disease and to the aging of the population, with the number of people age 65 or older in the United States expected to double from the year 2000 to 2030. Another factor is that effective therapies are now available, possibly making hematologists and oncologists more likely to pursue the diagnosis.
These numbers translate to 10,000 to 15,000 new cases annually, and given the life expectancy of patients affected by this disease (and the life-extending treatments for it), an estimated 30,000 to 60,000 Americans living with MDS.6,7
Even though MDS can occur at any age, most patients are older. The median age at diagnosis is 71 years,3,5,8 and 72% of patients are age 70 or older.3 The prevalence increases with age, to a rate of 36 per 100,000 in those age 80 and older.9 However, in areas of East Asia, it occurs at ages almost 2 decades younger than in the rest of the world.5
MDS are more common in men than in women and in whites than in blacks. Smoking appears to increase the risk, but alcohol consumption does not.10
About 10% of cases of MDS are secondary, most often due to radiation treatment or chemotherapy (particularly with alkylating agents and topoisomerase inhibitors) for cancer. The time from treatment of a primary malignancy (most often prostate, breast, bladder, lung, or non-Hodgkin lymphoma) to the development of MDS is about 5 years.5 A small number of cases are due to occupational exposure to radiation or benzene or other organic solvents, as might occur in the rubber industry (see below). Secondary MDS have a worse prognosis than primary (de novo) MDS.
GENETIC AND ENVIRONMENTAL FACTORS
The cause of de novo MDS is not known. Genetic and environmental factors probably both play a role. The lower median age at diagnosis in Eastern countries such as Japan than in the United States suggests that environmental factors11 such as smoking, ionizing radiation, and benzene exposure play a role.12,13 Some epidemiologic evidence suggests a higher incidence of MDS after exposure to solvents, hair dyes, and pesticides.13
Congenital conditions such as Down syndrome, Fanconi anemia, and Bloom syndrome are associated with MDS. Those affected usually present at an earlier age,13 suggesting a “multiple-hit” mechanism of cancer development with genetic and environmental factors. MDS rarely run in families.
SYMPTOMS ARE OFTEN NONSPECIFIC
Symptoms of MDS are often vague and nonspecific, and the diagnosis is often made during a workup for anemia, thrombocytopenia, or neutropenia discovered on a CBC. If present, signs and symptoms depend on the blood and bone marrow cell types that are affected.
When erythrocytes are affected (the most common situation), patients present with signs of anemia, including pallor, pale conjunctiva, tachycardia, hypotension, fatigue, headache, and exercise intolerance, or with signs and symptoms of a worsening underlying condition such as angina pectoris, heart failure, or emphysema.
When platelets or neutrophils are affected. Fewer than 20% of patients present with symptoms of isolated thrombocytopenia such as minor bleeding (eg, mucosal bleeding, petechiae, easy bruising, epistaxis) or major bleeding (eg, gastrointestinal bleeding, intracranial hemorrhage) or of isolated neutropenia (eg, fatigue, frequent bacterial infections of different organs systems).
Splenomegaly and lymphadenopathy are uncommon in MDS and, if detected, should raise suspicion of a myeloproliferative or lymphoproliferative neoplasm.
LABORATORY TESTS NEEDED
Complete blood cell count
Once the common causes of patient’s symptoms are evaluated, a CBC is needed to look for a hematologic cause. If a patient is ultimately determined to have MDS, anemia is the most common finding on the CBC: about 80% of patients with MDS are anemic at presentation. 6
Anemia associated with MDS can be microcytic, normocytic, or, most commonly, macrocytic. 14 Thrombocytopenia and neutropenia can be solitary or associated with anemia, and they are seen in about 40% of patients at the time of diagnosis.6 As the disease progresses, the degree of cytopenia worsens and, in most cases, preserved cell lineages are eventually affected.
Once cytopenia is discovered, a workup for the cause is needed. We emphasize a workup first for anemia, as it is the most common form of cytopenia in MDS. A workup for isolated thrombocytopenia or neutropenia usually requires a bone marrow examination earlier in the course, and we will discuss it only briefly here. Multilineage cytopenia almost always suggests abnormal bone marrow function and can be the basis for referral to a hematologist or oncologist.
Evaluation of anemia
If anemia is detected, it is reasonable to look for nonhematologic causes such as gastrointestinal bleeding, a cardiac cause, or a nutritional deficiency.
Anemia has a variety of possible hematologic causes, as shown in a study in the United States.15 When blood samples were collected from more than 2,000 people age 65 and older, 10.6% were found to have anemia, categorized as follows:
- Nutrient-deficiency anemia, related to low levels of vitamin B12, folate, or more commonly iron
- Anemia of chronic inflammation (formerly anemia of chronic disease, associated with a major medical disorder)
- Unexplained anemia (of those with unexplained anemia, 17.4% had blood findings compatible with MDS).15
- Tests for nutrient deficiencies such as iron, vitamin B12, and folate levels. Subsequent tests can include assessment for copper deficiencies. Vitamin B12 and copper deficiency can mimic MDS.
- Fecal occult blood testing, and, if positive, further evaluation for a source of gastrointestinal bleeding.
- Liver function tests, renal function tests, and tests for endocrine disorders, such as thyroid function tests.
- Review of drugs that can cause megaloblastoid erythropoiesis, such as methotrexate (Trexall), valproic acid (Depakote), phenytoin (Dilantin), phenobarbital (Luminal), sulfasalazine (Sulfazine), and zidovudine (Retrovir).
- Assesment of the responsiveness of the bone marrow to anemia, via a reticulocyte count or an erythropoietin level, or both, prior to any blood transfusion.
- Screening for relevant infections, including human immunodeficiency virus (HIV), hepatitis, or, in rare cases, parvovirus.
- Screening for lifestyle factors that may result in bone marrow suppression, such as excessive alcohol intake.
Evaluation of other cytopenias
In cases of isolated thrombocytopenia or combined bicytopenia (eg, anemia and thrombocytopenia), abdominal ultrasonography should be done to evaluate for splenomegaly.
Blood tests to evaluate for immune-mediated cytopenias, including idiopathic thrombocytopenic purpura and hemolytic anemia, include the direct and indirect Coombs antiglobulin tests, the lactate dehydrogenase level, the reticulocyte count, and the haptoglobin level. Other immune-mediated causes of cytopenia include connective tissue disorders and vasculitides, and an antinuclear antibody titer and rheumatoid factor level can also be considered.
Referral if tests are negative
If all these tests are negative, the next step is referral to a hematologist-oncologist for further workup, which may include a review of the peripheral blood smear; bone marrow aspiration and biopsy for evaluation of iron stores and bone marrow cellularity; and specialized tests such as assessment of antiplatelet antibodies, protein electrophoresis, or fluorescence in situ hybridization to evaluate for specific clonal disorders. The purpose of bone marrow aspiration and biopsy in MDS is to evaluate the morphology of the bone marrow and the patient’s cytogenetic profile. Each has its prognostic and therapeutic implications.
SCORING SYSTEMS FOR MDS, RATHER THAN STAGING SYSTEMS
The purpose of classification systems for any medical condition is to uniformly evaluate and group patients with a disease subtype to compare patient populations similarly throughout the world, to predict prognosis, and to dictate therapeutic directions.
MDS have two main classification systems, the FAB (French-American-British) and the WHO (World Health Organization). Revised in 2008,16 the WHO classification (Table 2, not available online)17 is widely accepted because it incorporates morphologic and cytogenetic factors and correlates with prognosis.18 The categories are distinguished by specific characteristics of peripheral blood and bone marrow.
Unlike many other cancers, MDS are not “staged.” Rather, prognostic systems have been devised to predict the risk of transformation to AML and to predict overall survival. These systems are based on:
- The number of myeloblasts in the bone marrow (the higher the count, the worse the prognosis)
- The number or degree of cytopenias
- Cytogenetic abnormalities (acquired genetic abnormalities in the neoplastic clone), found in about half of patients with MDS.19
The most widely used prognostic systems are the International Prognostic Scoring System (Table 3, not available online)2 and the WPSS (WHO Classification-based Prognostic Scoring System1). The latter system encorporates transfusion burden.
SUPPORTIVE CARE
Supportive care includes transfusion of blood products to minimize complications of cytopenias and to improve quality of life, as well as antibiotics to treat active infections.
Transfusions
Almost all patients with MDS need red cell transfusions at some point, while fewer need platelets. The frequency of transfusion depends on the extent of the disease and on comorbidities.
Red blood cells typically are given when the hemoglobin level falls below 8.5 g/dL, and platelets are given when the platelet count is below 100 × 109/L, in the absence of symptoms. Patients with symptomatic anemia should receive transfusion to relieve their symptoms. Some patients need transfusions occasionally, while others are transfusion-dependent.
Iron chelation
Blood product transfusions can lead to iron overload, particularly with a lifetime administration of more than 20 units, or with a year of continuous transfusions, and this is associated with diminished survival.20
However, considering the short survival of patients with MDS, the benefit of iron chelation is debatable. This intervention should be reserved for patients with lower-risk disease who are expected to survive more than 1 year and who have received more than 25 units of packed red blood cells.21
Antibiotics
Neutropenia is defined as an absolute neutrophil count less than 1.5 × 109/L. The risk of infection, particularly bacterial infection, is significantly increased when the neutrophil count is below 0.5 × 109/L. Fever (temperature > 100.4°F or 38.0°C) in neutropenic patients is an emergency, requiring hospitalization and immediate initiation of broad-spectrum antibiotics along with a workup for the cause of the fever.22 Prophylactic antibiotics have no proven role in MDS patients with neutropenia.
TREATMENT OF LOWER-RISK DISEASE
Erythropoiesis-stimulating agents
Once a patient starts to require red blood cell transfusions, an erythropoiesis-stimulating agent (EPA) can be considered.23,24 These include recombinant agents such as erythropoietin (Procrit) and darbepoetin alfa (Aranesp).
Response is measured as an improvement in hemoglobin or as independence from transfusions in those previously dependent on them. Patients most likely to respond are those whose pretransfusion erythropoietin level is below 100 IU/L and who have minimal transfusion needs.25,26 Addition of a colony-stimulating factor can be considered for patients with neutropenia. On average, about 40% of patients ultimately respond to an EPA, but those who respond eventually develop resistance to the agent. Retrospective data indicate that use of EPAs may improve survival in MDS.23,24
The recommended threshold hemoglobin level for starting an EPA is less than 10 g/dL. Patients need to be monitored with a CBC every time they receive treatment. The agent should be stopped once the hemoglobin level reaches 12 g/dL. A number of studies have shown lower survival rates when ESAs are used in nonhematologic malignancies, particularly if the malignancy is advanced and when the ESA is used to achieve a goal hemoglobin above 12 g/dL. There are no data to suggest a higher death rate in patients with hematologic malignancies who take ESAs. The use of ESAs in MDS patients should be judicious, however, and titrated to a goal hemoglobin level no higher than 12 g/dL.27
Other treatments
If ESA treatment is ineffective, other treatments may be considered, usually initiated by a hematologist or medical oncologist.
Immunosuppressive therapy with antithymocyte globulin (Thymoglobulin)28 is an option for patients with hypocellular or immune-mediated MDS. This treatment may decrease the need for transfusion and may improve the blood count.
Lenalidomide (Revlimid) for MDS with isolated chromosome 5q deletion29 can decrease the need for blood transfusion in approximately two-thirds of these patients.
Azacitidine (Vidaza) or decitabine (Dacogen), in patients with more advanced subtypes of MDS (eg, those with excess blasts) or with pancytopenia unresponsive to other therapies, can induce hematologic improvement and decrease transfusion dependence, as well as prolong survival.
Stem cell transplantation, for patients with more advanced subtypes of MDS and who have an appropriately matched donor, has the potential of being curative.
Experimental treatments are available in clinical trials.
TREATMENT OF HIGHER-RISK DISEASE
About 25% of patients with newly diagnosed MDS and 15% to 20% of patients with established MDS have higher-risk disease.30 These patients should almost always be followed by a hematologist or medical oncologist, with therapy initiated immediately, regardless of blood counts, given the high likelihood of transformation to AML or death within 1.5 years.
The treatment options for higher-risk disease include:
- Methyltransferase inhibitors such as azacitidine and decitabine31–34
- Cytotoxic chemotherapy (similar to treatment of acute myeloid leukemia)
- Bone marrow-hematopoeitic stem cell transplantation35,36
- Experimental treatments in clinical trials.
As mentioned earlier, outside of transplantation, only azacitidine has been shown to improve overall survival (with a doubling of survival at 2 years, to 50%), and no drug therapy is curative. Managing patient expectations for treatment outcome is thus crucial in higher-risk disease, and ongoing assessments of quality of life, both on or off therapy, should be considered obligatory.
Stem cell transplantation cures MDS
MDS are complex and heterogeneous, so treatment options range from supportive care to chemotherapy and allogeneic stem cell transplantation.6 The choice depends on the severity of disease, ie, lower-risk or higherrisk (Table 3, not available online), as well as on the prognosis, the availability of therapeutic options, and the patient’s expectations.
Hematopoietic stem cell transplantation is the only curative treatment for MDS. However, it is performed in fewer than 5% of patients,30 usually younger patients with few comorbidities, because the rate of transplantrelated death is high. Therefore, most treatments are palliative, aimed at improving the quality of life and prolonging survival.
The balance between risks and benefits of these treatments must be justifiable.30 Further, patients who have no symptoms or who have lower-risk disease need no treatment and may not for years. However, they do need close follow-up, because their symptoms will worsen and will eventually require treatment.
TAKE-HOME POINTS
- Myelodysplastic syndromes are more prevalent than previously realized. Mainly a disease of older adults, they should be suspected in any patient with unexplained cytopenia.
- Life expectancy at the time of diagnosis depends on the types of cells affected.
- Supportive and disease-altering options are available.
- Prompt referral to a hematologist or oncologist is important for confirmation of the diagnosis and initiation of an appropriate treatment plan. Patients with lower-risk disease can continue follow-up with their primary care provider once treatment goals and plans are established.
ACKNOWLEDGMENT
We thank Dr. Karl Theil of the Cleveland Clinic Department of Clinical Pathology for the photomicrographs used on the cover.
Myelodysplastic syndromes (MDS) are a heterogeneous group of disorders of blood cell production in the bone marrow that can transform into acute myeloid leukemia (AML).1,2 They are diagnosed most often in the elderly.
Primary care physicians and geriatricians tend to be the first to identify the problem, as they recognize that cytopenias are not simply a normal consequence of aging.
MDS are considered to be cancers, akin to chronic leukemia or acute leukemia, with epidemiologic data tracked by national cancer registries and the US Centers for Disease Control and Prevention, under the auspices of the Surveillance, Epidemiology, and End Results (SEER) program.3
In this article, we briefly review the classification of MDS, current epidemiologic data, key diagnostic features, and current management options.
WHEN TO SUSPECT MDS
In many patients, MDS are asymptomatic and appear as an abnormality on a routine complete blood cell count (CBC) or as part of a workup for anemia. Symptoms develop as the bone marrow’s ability to produce normal-functioning blood cells is more and more compromised. The range of symptoms depends on the bone marrow cell type affected.
Patients with MDS typically have some degree of anemia, often detected incidentally on a routine CBC, or they have symptoms stemming from anemia or thrombocytopenia, or have recurrent infections.
Subtypes of MDS have different pathologic and clinical presentations and different prognoses. They are often categorized as lower-risk or higher-risk, depending on the likelihood of transforming to AML. Patients with lower-risk MDS survive a median of 3 to 7 years. Higher-risk types are pathobiologically similar to AML in older adults, and patients either develop AML or die of complications of MDS, on average within 1.5 years.
Several classification schemes and prognostic models guide the selection of the most appropriate therapy.
Older age and comorbidities such as coronary artery disease, chronic obstructive pulmonary disease, and chronic kidney disease make MDS more difficult to manage and worsen the prognosis.4
MOST PATIENTS ARE OLDER
Only since 2001, when MDS became reportable to SEER,3,5 has the epidemiology of MDS been reported in the United States.
MDS are currently diagnosed in an estimated 3.4 per 100,000 US citizens yearly.
The incidence rate increased from 3.28 per 100,000 per year in 2001 to 3.56 per 100,000 in 2004.5 The increase has been attributed to enhanced awareness of the disease and to the aging of the population, with the number of people age 65 or older in the United States expected to double from the year 2000 to 2030. Another factor is that effective therapies are now available, possibly making hematologists and oncologists more likely to pursue the diagnosis.
These numbers translate to 10,000 to 15,000 new cases annually, and given the life expectancy of patients affected by this disease (and the life-extending treatments for it), an estimated 30,000 to 60,000 Americans living with MDS.6,7
Even though MDS can occur at any age, most patients are older. The median age at diagnosis is 71 years,3,5,8 and 72% of patients are age 70 or older.3 The prevalence increases with age, to a rate of 36 per 100,000 in those age 80 and older.9 However, in areas of East Asia, it occurs at ages almost 2 decades younger than in the rest of the world.5
MDS are more common in men than in women and in whites than in blacks. Smoking appears to increase the risk, but alcohol consumption does not.10
About 10% of cases of MDS are secondary, most often due to radiation treatment or chemotherapy (particularly with alkylating agents and topoisomerase inhibitors) for cancer. The time from treatment of a primary malignancy (most often prostate, breast, bladder, lung, or non-Hodgkin lymphoma) to the development of MDS is about 5 years.5 A small number of cases are due to occupational exposure to radiation or benzene or other organic solvents, as might occur in the rubber industry (see below). Secondary MDS have a worse prognosis than primary (de novo) MDS.
GENETIC AND ENVIRONMENTAL FACTORS
The cause of de novo MDS is not known. Genetic and environmental factors probably both play a role. The lower median age at diagnosis in Eastern countries such as Japan than in the United States suggests that environmental factors11 such as smoking, ionizing radiation, and benzene exposure play a role.12,13 Some epidemiologic evidence suggests a higher incidence of MDS after exposure to solvents, hair dyes, and pesticides.13
Congenital conditions such as Down syndrome, Fanconi anemia, and Bloom syndrome are associated with MDS. Those affected usually present at an earlier age,13 suggesting a “multiple-hit” mechanism of cancer development with genetic and environmental factors. MDS rarely run in families.
SYMPTOMS ARE OFTEN NONSPECIFIC
Symptoms of MDS are often vague and nonspecific, and the diagnosis is often made during a workup for anemia, thrombocytopenia, or neutropenia discovered on a CBC. If present, signs and symptoms depend on the blood and bone marrow cell types that are affected.
When erythrocytes are affected (the most common situation), patients present with signs of anemia, including pallor, pale conjunctiva, tachycardia, hypotension, fatigue, headache, and exercise intolerance, or with signs and symptoms of a worsening underlying condition such as angina pectoris, heart failure, or emphysema.
When platelets or neutrophils are affected. Fewer than 20% of patients present with symptoms of isolated thrombocytopenia such as minor bleeding (eg, mucosal bleeding, petechiae, easy bruising, epistaxis) or major bleeding (eg, gastrointestinal bleeding, intracranial hemorrhage) or of isolated neutropenia (eg, fatigue, frequent bacterial infections of different organs systems).
Splenomegaly and lymphadenopathy are uncommon in MDS and, if detected, should raise suspicion of a myeloproliferative or lymphoproliferative neoplasm.
LABORATORY TESTS NEEDED
Complete blood cell count
Once the common causes of patient’s symptoms are evaluated, a CBC is needed to look for a hematologic cause. If a patient is ultimately determined to have MDS, anemia is the most common finding on the CBC: about 80% of patients with MDS are anemic at presentation. 6
Anemia associated with MDS can be microcytic, normocytic, or, most commonly, macrocytic. 14 Thrombocytopenia and neutropenia can be solitary or associated with anemia, and they are seen in about 40% of patients at the time of diagnosis.6 As the disease progresses, the degree of cytopenia worsens and, in most cases, preserved cell lineages are eventually affected.
Once cytopenia is discovered, a workup for the cause is needed. We emphasize a workup first for anemia, as it is the most common form of cytopenia in MDS. A workup for isolated thrombocytopenia or neutropenia usually requires a bone marrow examination earlier in the course, and we will discuss it only briefly here. Multilineage cytopenia almost always suggests abnormal bone marrow function and can be the basis for referral to a hematologist or oncologist.
Evaluation of anemia
If anemia is detected, it is reasonable to look for nonhematologic causes such as gastrointestinal bleeding, a cardiac cause, or a nutritional deficiency.
Anemia has a variety of possible hematologic causes, as shown in a study in the United States.15 When blood samples were collected from more than 2,000 people age 65 and older, 10.6% were found to have anemia, categorized as follows:
- Nutrient-deficiency anemia, related to low levels of vitamin B12, folate, or more commonly iron
- Anemia of chronic inflammation (formerly anemia of chronic disease, associated with a major medical disorder)
- Unexplained anemia (of those with unexplained anemia, 17.4% had blood findings compatible with MDS).15
- Tests for nutrient deficiencies such as iron, vitamin B12, and folate levels. Subsequent tests can include assessment for copper deficiencies. Vitamin B12 and copper deficiency can mimic MDS.
- Fecal occult blood testing, and, if positive, further evaluation for a source of gastrointestinal bleeding.
- Liver function tests, renal function tests, and tests for endocrine disorders, such as thyroid function tests.
- Review of drugs that can cause megaloblastoid erythropoiesis, such as methotrexate (Trexall), valproic acid (Depakote), phenytoin (Dilantin), phenobarbital (Luminal), sulfasalazine (Sulfazine), and zidovudine (Retrovir).
- Assesment of the responsiveness of the bone marrow to anemia, via a reticulocyte count or an erythropoietin level, or both, prior to any blood transfusion.
- Screening for relevant infections, including human immunodeficiency virus (HIV), hepatitis, or, in rare cases, parvovirus.
- Screening for lifestyle factors that may result in bone marrow suppression, such as excessive alcohol intake.
Evaluation of other cytopenias
In cases of isolated thrombocytopenia or combined bicytopenia (eg, anemia and thrombocytopenia), abdominal ultrasonography should be done to evaluate for splenomegaly.
Blood tests to evaluate for immune-mediated cytopenias, including idiopathic thrombocytopenic purpura and hemolytic anemia, include the direct and indirect Coombs antiglobulin tests, the lactate dehydrogenase level, the reticulocyte count, and the haptoglobin level. Other immune-mediated causes of cytopenia include connective tissue disorders and vasculitides, and an antinuclear antibody titer and rheumatoid factor level can also be considered.
Referral if tests are negative
If all these tests are negative, the next step is referral to a hematologist-oncologist for further workup, which may include a review of the peripheral blood smear; bone marrow aspiration and biopsy for evaluation of iron stores and bone marrow cellularity; and specialized tests such as assessment of antiplatelet antibodies, protein electrophoresis, or fluorescence in situ hybridization to evaluate for specific clonal disorders. The purpose of bone marrow aspiration and biopsy in MDS is to evaluate the morphology of the bone marrow and the patient’s cytogenetic profile. Each has its prognostic and therapeutic implications.
SCORING SYSTEMS FOR MDS, RATHER THAN STAGING SYSTEMS
The purpose of classification systems for any medical condition is to uniformly evaluate and group patients with a disease subtype to compare patient populations similarly throughout the world, to predict prognosis, and to dictate therapeutic directions.
MDS have two main classification systems, the FAB (French-American-British) and the WHO (World Health Organization). Revised in 2008,16 the WHO classification (Table 2, not available online)17 is widely accepted because it incorporates morphologic and cytogenetic factors and correlates with prognosis.18 The categories are distinguished by specific characteristics of peripheral blood and bone marrow.
Unlike many other cancers, MDS are not “staged.” Rather, prognostic systems have been devised to predict the risk of transformation to AML and to predict overall survival. These systems are based on:
- The number of myeloblasts in the bone marrow (the higher the count, the worse the prognosis)
- The number or degree of cytopenias
- Cytogenetic abnormalities (acquired genetic abnormalities in the neoplastic clone), found in about half of patients with MDS.19
The most widely used prognostic systems are the International Prognostic Scoring System (Table 3, not available online)2 and the WPSS (WHO Classification-based Prognostic Scoring System1). The latter system encorporates transfusion burden.
SUPPORTIVE CARE
Supportive care includes transfusion of blood products to minimize complications of cytopenias and to improve quality of life, as well as antibiotics to treat active infections.
Transfusions
Almost all patients with MDS need red cell transfusions at some point, while fewer need platelets. The frequency of transfusion depends on the extent of the disease and on comorbidities.
Red blood cells typically are given when the hemoglobin level falls below 8.5 g/dL, and platelets are given when the platelet count is below 100 × 109/L, in the absence of symptoms. Patients with symptomatic anemia should receive transfusion to relieve their symptoms. Some patients need transfusions occasionally, while others are transfusion-dependent.
Iron chelation
Blood product transfusions can lead to iron overload, particularly with a lifetime administration of more than 20 units, or with a year of continuous transfusions, and this is associated with diminished survival.20
However, considering the short survival of patients with MDS, the benefit of iron chelation is debatable. This intervention should be reserved for patients with lower-risk disease who are expected to survive more than 1 year and who have received more than 25 units of packed red blood cells.21
Antibiotics
Neutropenia is defined as an absolute neutrophil count less than 1.5 × 109/L. The risk of infection, particularly bacterial infection, is significantly increased when the neutrophil count is below 0.5 × 109/L. Fever (temperature > 100.4°F or 38.0°C) in neutropenic patients is an emergency, requiring hospitalization and immediate initiation of broad-spectrum antibiotics along with a workup for the cause of the fever.22 Prophylactic antibiotics have no proven role in MDS patients with neutropenia.
TREATMENT OF LOWER-RISK DISEASE
Erythropoiesis-stimulating agents
Once a patient starts to require red blood cell transfusions, an erythropoiesis-stimulating agent (EPA) can be considered.23,24 These include recombinant agents such as erythropoietin (Procrit) and darbepoetin alfa (Aranesp).
Response is measured as an improvement in hemoglobin or as independence from transfusions in those previously dependent on them. Patients most likely to respond are those whose pretransfusion erythropoietin level is below 100 IU/L and who have minimal transfusion needs.25,26 Addition of a colony-stimulating factor can be considered for patients with neutropenia. On average, about 40% of patients ultimately respond to an EPA, but those who respond eventually develop resistance to the agent. Retrospective data indicate that use of EPAs may improve survival in MDS.23,24
The recommended threshold hemoglobin level for starting an EPA is less than 10 g/dL. Patients need to be monitored with a CBC every time they receive treatment. The agent should be stopped once the hemoglobin level reaches 12 g/dL. A number of studies have shown lower survival rates when ESAs are used in nonhematologic malignancies, particularly if the malignancy is advanced and when the ESA is used to achieve a goal hemoglobin above 12 g/dL. There are no data to suggest a higher death rate in patients with hematologic malignancies who take ESAs. The use of ESAs in MDS patients should be judicious, however, and titrated to a goal hemoglobin level no higher than 12 g/dL.27
Other treatments
If ESA treatment is ineffective, other treatments may be considered, usually initiated by a hematologist or medical oncologist.
Immunosuppressive therapy with antithymocyte globulin (Thymoglobulin)28 is an option for patients with hypocellular or immune-mediated MDS. This treatment may decrease the need for transfusion and may improve the blood count.
Lenalidomide (Revlimid) for MDS with isolated chromosome 5q deletion29 can decrease the need for blood transfusion in approximately two-thirds of these patients.
Azacitidine (Vidaza) or decitabine (Dacogen), in patients with more advanced subtypes of MDS (eg, those with excess blasts) or with pancytopenia unresponsive to other therapies, can induce hematologic improvement and decrease transfusion dependence, as well as prolong survival.
Stem cell transplantation, for patients with more advanced subtypes of MDS and who have an appropriately matched donor, has the potential of being curative.
Experimental treatments are available in clinical trials.
TREATMENT OF HIGHER-RISK DISEASE
About 25% of patients with newly diagnosed MDS and 15% to 20% of patients with established MDS have higher-risk disease.30 These patients should almost always be followed by a hematologist or medical oncologist, with therapy initiated immediately, regardless of blood counts, given the high likelihood of transformation to AML or death within 1.5 years.
The treatment options for higher-risk disease include:
- Methyltransferase inhibitors such as azacitidine and decitabine31–34
- Cytotoxic chemotherapy (similar to treatment of acute myeloid leukemia)
- Bone marrow-hematopoeitic stem cell transplantation35,36
- Experimental treatments in clinical trials.
As mentioned earlier, outside of transplantation, only azacitidine has been shown to improve overall survival (with a doubling of survival at 2 years, to 50%), and no drug therapy is curative. Managing patient expectations for treatment outcome is thus crucial in higher-risk disease, and ongoing assessments of quality of life, both on or off therapy, should be considered obligatory.
Stem cell transplantation cures MDS
MDS are complex and heterogeneous, so treatment options range from supportive care to chemotherapy and allogeneic stem cell transplantation.6 The choice depends on the severity of disease, ie, lower-risk or higherrisk (Table 3, not available online), as well as on the prognosis, the availability of therapeutic options, and the patient’s expectations.
Hematopoietic stem cell transplantation is the only curative treatment for MDS. However, it is performed in fewer than 5% of patients,30 usually younger patients with few comorbidities, because the rate of transplantrelated death is high. Therefore, most treatments are palliative, aimed at improving the quality of life and prolonging survival.
The balance between risks and benefits of these treatments must be justifiable.30 Further, patients who have no symptoms or who have lower-risk disease need no treatment and may not for years. However, they do need close follow-up, because their symptoms will worsen and will eventually require treatment.
TAKE-HOME POINTS
- Myelodysplastic syndromes are more prevalent than previously realized. Mainly a disease of older adults, they should be suspected in any patient with unexplained cytopenia.
- Life expectancy at the time of diagnosis depends on the types of cells affected.
- Supportive and disease-altering options are available.
- Prompt referral to a hematologist or oncologist is important for confirmation of the diagnosis and initiation of an appropriate treatment plan. Patients with lower-risk disease can continue follow-up with their primary care provider once treatment goals and plans are established.
ACKNOWLEDGMENT
We thank Dr. Karl Theil of the Cleveland Clinic Department of Clinical Pathology for the photomicrographs used on the cover.
- Malcovati L, Nimer SD. Myelodysplastic syndromes: diagnosis and staging. Cancer Control 2008; 15 (suppl 4):4–13.
- Greenberg P, Cox C, LeBeau MM, et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood 1997; 89:2079–2088.
- Rollison DE, Howlader N, Smith MT, et al. Epidemiology of myelodysplastic syndromes and chronic myeloproliferative disorders in the United States, 2001–2004, using data from the NAACCR and SEER programs. Blood 2008; 112:45–52.
- Lichtman MA, Rowe JM. The relationship of patient age to the pathobiology of the clonal myeloid diseases. Semin Oncol 2004; 31:185–197.
- Ma X, Does M, Raza A, Mayne ST. Myelodysplastic syndromes: incidence and survival in the United States. Cancer 2007; 109:1536–1542.
- Steensma DP, Bennett JM. The myelodysplastic syndromes: diagnosis and treatment. Mayo Clin Proc 2006; 81:104–130.
- The MDS Foundation. http://www.mds-foundation.org/. Accessed August 27, 2009.
- Sekeres M, Cosgrove D, Falco A. Managing patients with low-risk MDS. Clin Adv Hematol Oncol 2006; 4( 7 suppl 16):1–10.
- Sandhu SK, Sekeres MA. Myelodysplastic syndromes: more prevalent than we know. Geriatrics 2008; 63:10–17.
- Strom SS, Gu Y, Gruschkus SK, Pierce SA, Estey EH. Risk factors of myelodysplastic syndromes: a case-control study. Leukemia 2005; 19:1912–1918.
- Kuendgen A, Matsuda A, Germing U. Differences in epidemiology of MDS between Western and Eastern countries: Ethnic differences or environmental influence? Leuk Res 2007; 31:103–104.
- Bjork J, Johansson B, Broberg K, Albin M. Smoking as a risk factor for myelodysplastic syndromes and acute myeloid leukemia and its relation to cytogenetic findings: a case-control study. Leuk Res 2009; 33:788–791.
- Germing U, Aul C, Niemeyer CM, Haas R, Bennett JM. Epidemiology, classification and prognosis of adults and children with myelodysplastic syndromes. Ann Hematol 2008; 87:691–699.
- Juneja SK, Imbert M, Jouault H, Scoazec JY, Sigaux F, Sultan C. Haematological features of primary myelodysplastic syndromes (PMDS) at initial presentation: a study of 118 cases. J Clin Pathol 1983; 36:1129–1135.
- Guralnik JM, Eisenstaedt RS, Ferrucci L, Klein HG, Woodman RC. Prevalence of anemia in persons 65 years and older in the United States: evidence for a high rate of unexplained anemia. Blood 2004; 104:2263–2268.
- Swerdlow SH, Campo E, Harris NL, et al. International Agency for Research on Cancer, World Health Organization. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues, 4th ed. International Agency for Research on Cancer: Lyon, France; 2008.
- Vardiman JW, Thiele J, Arber DA, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood 2009; 114:937–951.
- Bennett JM. A comparative review of classification systems in myelodysplastic syndromes (MDS). Semin Oncol 2005; 32( 4 suppl 5):S3–S10.
- Haase D. Cytogenetic features in myelodysplastic syndromes. Ann Hematol 2008; 87:515–526.
- Malcovati L, Della Porta MG, Cazzola M. Predicting survival and leukemic evolution in patients with myelodysplastic syndrome. Haematologica 2006; 91:1588–1590.
- Bowen D, Culligan D, Jowitt S, et al. Guidelines for the diagnosis and therapy of adult myelodysplastic syndromes. Br J Haematol 2003; 120:187–200.
- Segal BH, Freifeld AG, Baden LR, et al. Prevention and treatment of cancer-related infections. J Natl Compr Canc Netw 2008; 6:122–174.
- Golshayan AR, Jin T, Maciejewski J, et al. Efficacy of growth factors compared to other therapies for low-risk myelodysplastic syndromes. Br J Haematol 2007; 137:125–132.
- Jadersten M, Malcovati L, Dybedal I, et al. Erythropoietin and granulocyte-colony stimulating factor treatment associated with improved survival in myelodysplastic syndrome. J Clin Oncol 2008; 26:3607–3613.
- Hellstrom-Lindberg E, Gulbrandsen N, Lindberg G, et al; Scandinavian MDS Group. A validated decision model for treating the anaemia of myelodysplastic syndromes with erythropoietin + granulocyte colony-stimulating factor: significant effects on quality of life. Br J Haematol 2003; 120:1037–1046.
- Cheson BD, Greenberg PL, Bennett JM, et al. Clinical application and proposal for modification of the International Working Group (IWG) response criteria in myelodysplasia. Blood 2006; 108:419–425.
- ARANESP Prescribing Information. http://pi.amgen.com/united_states/aranesp/ckd/aranesp_pi_hcp_english.pdf. Accessed August 28, 2009.
- Molldrem JJ, Leifer E, Bahceci E, et al. Antithymocyte globulin for treatment of the bone marrow failure associated with myelodysplastic syndromes. Ann Intern Med 2002; 137:156–163.
- List A, Dewald G, Bennett J, et al; Myelodysplastic Syndrome-003 Study Investigators. Lenalidomide in the myelodysplastic syndrome with chromosome 5q deletion. N Engl J Med 2006; 355:1456–1465.
- Sekeres MA, Schoonen WM, Kantarjian H, et al. Characteristics of US patients with myelodysplastic syndromes: results of six crosssectional physician surveys. J Natl Cancer Inst 2008; 100:1542–1551.
- Stone R, Sekeres M, Garcia-Manero G, Lyons RM. Recent advances in low-and intermediate-1-risk myelodysplastic syndrome: developing a consensus for optimal therapy. Clin Adv Hematol Oncol 2008; 6:1–15.
- Kantarjian H, Issa JP, Rosenfeld CS, et al. Decitabine improves patient outcomes in myelodysplastic syndromes: results of a phase III randomized study. Cancer 2006; 106:1794–1803.
- Fenaux P, Mufti GJ, Hellstrom-Lindberg E, et al; International Vidaza High-Risk MDS Survival Study Group. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: a randomised, open-label, phase III study. Lancet Oncol 2009; 10:223–232.
- Silverman LR, Demakos EP, Peterson BL, et al. Randomized controlled trial of azacitidine in patients with the myelodysplastic syndrome: a study of the cancer and leukemia group B. J Clin Oncol 2002; 20:2429–2440.
- Giralt S. Bone marrow transplant in myelodysplastic syndromes: new technologies, same questions. Curr Hematol Rep 2005; 4:200–207.
- Cutler CS, Lee SJ, Greenberg P, et al. A decision analysis of allogeneic bone marrow transplantation for the myelodysplastic syndromes: delayed transplantation for low-risk myelodysplasia is associated with improved outcome. Blood 2004; 104:579–585.
- Malcovati L, Nimer SD. Myelodysplastic syndromes: diagnosis and staging. Cancer Control 2008; 15 (suppl 4):4–13.
- Greenberg P, Cox C, LeBeau MM, et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood 1997; 89:2079–2088.
- Rollison DE, Howlader N, Smith MT, et al. Epidemiology of myelodysplastic syndromes and chronic myeloproliferative disorders in the United States, 2001–2004, using data from the NAACCR and SEER programs. Blood 2008; 112:45–52.
- Lichtman MA, Rowe JM. The relationship of patient age to the pathobiology of the clonal myeloid diseases. Semin Oncol 2004; 31:185–197.
- Ma X, Does M, Raza A, Mayne ST. Myelodysplastic syndromes: incidence and survival in the United States. Cancer 2007; 109:1536–1542.
- Steensma DP, Bennett JM. The myelodysplastic syndromes: diagnosis and treatment. Mayo Clin Proc 2006; 81:104–130.
- The MDS Foundation. http://www.mds-foundation.org/. Accessed August 27, 2009.
- Sekeres M, Cosgrove D, Falco A. Managing patients with low-risk MDS. Clin Adv Hematol Oncol 2006; 4( 7 suppl 16):1–10.
- Sandhu SK, Sekeres MA. Myelodysplastic syndromes: more prevalent than we know. Geriatrics 2008; 63:10–17.
- Strom SS, Gu Y, Gruschkus SK, Pierce SA, Estey EH. Risk factors of myelodysplastic syndromes: a case-control study. Leukemia 2005; 19:1912–1918.
- Kuendgen A, Matsuda A, Germing U. Differences in epidemiology of MDS between Western and Eastern countries: Ethnic differences or environmental influence? Leuk Res 2007; 31:103–104.
- Bjork J, Johansson B, Broberg K, Albin M. Smoking as a risk factor for myelodysplastic syndromes and acute myeloid leukemia and its relation to cytogenetic findings: a case-control study. Leuk Res 2009; 33:788–791.
- Germing U, Aul C, Niemeyer CM, Haas R, Bennett JM. Epidemiology, classification and prognosis of adults and children with myelodysplastic syndromes. Ann Hematol 2008; 87:691–699.
- Juneja SK, Imbert M, Jouault H, Scoazec JY, Sigaux F, Sultan C. Haematological features of primary myelodysplastic syndromes (PMDS) at initial presentation: a study of 118 cases. J Clin Pathol 1983; 36:1129–1135.
- Guralnik JM, Eisenstaedt RS, Ferrucci L, Klein HG, Woodman RC. Prevalence of anemia in persons 65 years and older in the United States: evidence for a high rate of unexplained anemia. Blood 2004; 104:2263–2268.
- Swerdlow SH, Campo E, Harris NL, et al. International Agency for Research on Cancer, World Health Organization. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues, 4th ed. International Agency for Research on Cancer: Lyon, France; 2008.
- Vardiman JW, Thiele J, Arber DA, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood 2009; 114:937–951.
- Bennett JM. A comparative review of classification systems in myelodysplastic syndromes (MDS). Semin Oncol 2005; 32( 4 suppl 5):S3–S10.
- Haase D. Cytogenetic features in myelodysplastic syndromes. Ann Hematol 2008; 87:515–526.
- Malcovati L, Della Porta MG, Cazzola M. Predicting survival and leukemic evolution in patients with myelodysplastic syndrome. Haematologica 2006; 91:1588–1590.
- Bowen D, Culligan D, Jowitt S, et al. Guidelines for the diagnosis and therapy of adult myelodysplastic syndromes. Br J Haematol 2003; 120:187–200.
- Segal BH, Freifeld AG, Baden LR, et al. Prevention and treatment of cancer-related infections. J Natl Compr Canc Netw 2008; 6:122–174.
- Golshayan AR, Jin T, Maciejewski J, et al. Efficacy of growth factors compared to other therapies for low-risk myelodysplastic syndromes. Br J Haematol 2007; 137:125–132.
- Jadersten M, Malcovati L, Dybedal I, et al. Erythropoietin and granulocyte-colony stimulating factor treatment associated with improved survival in myelodysplastic syndrome. J Clin Oncol 2008; 26:3607–3613.
- Hellstrom-Lindberg E, Gulbrandsen N, Lindberg G, et al; Scandinavian MDS Group. A validated decision model for treating the anaemia of myelodysplastic syndromes with erythropoietin + granulocyte colony-stimulating factor: significant effects on quality of life. Br J Haematol 2003; 120:1037–1046.
- Cheson BD, Greenberg PL, Bennett JM, et al. Clinical application and proposal for modification of the International Working Group (IWG) response criteria in myelodysplasia. Blood 2006; 108:419–425.
- ARANESP Prescribing Information. http://pi.amgen.com/united_states/aranesp/ckd/aranesp_pi_hcp_english.pdf. Accessed August 28, 2009.
- Molldrem JJ, Leifer E, Bahceci E, et al. Antithymocyte globulin for treatment of the bone marrow failure associated with myelodysplastic syndromes. Ann Intern Med 2002; 137:156–163.
- List A, Dewald G, Bennett J, et al; Myelodysplastic Syndrome-003 Study Investigators. Lenalidomide in the myelodysplastic syndrome with chromosome 5q deletion. N Engl J Med 2006; 355:1456–1465.
- Sekeres MA, Schoonen WM, Kantarjian H, et al. Characteristics of US patients with myelodysplastic syndromes: results of six crosssectional physician surveys. J Natl Cancer Inst 2008; 100:1542–1551.
- Stone R, Sekeres M, Garcia-Manero G, Lyons RM. Recent advances in low-and intermediate-1-risk myelodysplastic syndrome: developing a consensus for optimal therapy. Clin Adv Hematol Oncol 2008; 6:1–15.
- Kantarjian H, Issa JP, Rosenfeld CS, et al. Decitabine improves patient outcomes in myelodysplastic syndromes: results of a phase III randomized study. Cancer 2006; 106:1794–1803.
- Fenaux P, Mufti GJ, Hellstrom-Lindberg E, et al; International Vidaza High-Risk MDS Survival Study Group. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: a randomised, open-label, phase III study. Lancet Oncol 2009; 10:223–232.
- Silverman LR, Demakos EP, Peterson BL, et al. Randomized controlled trial of azacitidine in patients with the myelodysplastic syndrome: a study of the cancer and leukemia group B. J Clin Oncol 2002; 20:2429–2440.
- Giralt S. Bone marrow transplant in myelodysplastic syndromes: new technologies, same questions. Curr Hematol Rep 2005; 4:200–207.
- Cutler CS, Lee SJ, Greenberg P, et al. A decision analysis of allogeneic bone marrow transplantation for the myelodysplastic syndromes: delayed transplantation for low-risk myelodysplasia is associated with improved outcome. Blood 2004; 104:579–585.
KEY POINTS
- Multilineage cytopenia almost always suggests abnormal bone marrow function and can be the reason for referral to a hematologist-oncologist.
- Factors that make MDS more difficult to manage and that worsen the prognosis are older age at diagnosis and comorbidities such as coronary artery disease, chronic obstructive pulmonary disease, and chronic kidney disease.
- Patients with lower-risk disease can continue followup with their primary care provider once the treatment goals and plans are established.
Lesions on the hands, high aminotransferase levels
Q: Which is the most likely diagnosis?
- Addison disease
- Lupus erythematosus
- Polymorphous light eruption
- Porphyria cutanea tarda
- Bullous pemphigoid
A: Urine testing, including examination under ultraviolet light with a Wood lamp, indicates porphyria cutanea tarda. This is the most common porphyria, occurring mainly in men. Its true prevalence is not known but is estimated to be from 1:5,000 to 1:25,000.1
There are three types of porphyria cutanea tarda. About 80% of cases are type I, also referred to as “sporadic.” In type I, levels of uroporphyrinogen decarboxylase (UROD) in red blood cells are normal, but are low in the liver during episodes of the disease. In type II, UROD levels are about 50% below normal in all tissues. Type III is similar to type I, except that it occurs in more than one family member.
The genetic mutation that produces a deficiency of UROD leads to an excess of uroporphyrins and porphyrins that are partially decarboxylated and that irreversibly oxidize. When they are deposited in the skin and the skin is exposed to the sun, they cause the classic cutaneous manifestations.1
Risk factors2 for porphyria cutanea tarda can be extrinsic (eg, high iron blood levels,2,3 excessive ethanol intake, hepatitis C,2,4 human immunodeficiency virus, estrogen use,5 dialysis for end-stage renal disease) or intrinsic (altered iron metabolism or cytochrome P450 function2).
CLINICAL PRESENTATION
The cardinal symptom of porphyria cutanea tarda is photosensitivity, with the development of chronic blistering lesions on sun-exposed areas such as the hands, face, and forearms. Fluid-filled vesicles develop and rupture easily, and the denuded areas become crusted and heal slowly.5 Secondary infections can occur. Previous areas of blisters may appear atrophic, brownish, or violaceous. Small white plaques (milia) are also common and may precede or follow vesicle formation. These cutaneous lesions, however, are not specific to porphyria cutanea tarda and can appear in variegated porphyria and coproporphyria. Hypertrichosis5 and hyperpigmentation are usually present, mainly over the cheekbones and around the eyes. Patches of alopecia and hypopigmented sclerodermiform lesions may also be observed.
Porphyria cutanea tarda is usually accompanied by alterations in liver metabolism, affecting mainly aminotransferases and gammaglutamyltransferase. The absence of hepatitis C infection does not rule out porphyria cutanea tarda. About 50% of patients have pathologic structural changes in the liver such as lobular necrosis or fibrotic tracts, and 15% of patients have cirrhosis at presentation.6 The risk of hepatocellular carcinoma is clearly increased.6 Hepatitis C virus infection, iron overload, and excessive ethanol intake lead to a more severe liver disease.
DIAGNOSIS
The diagnosis of porphyria cutanea tarda is strongly suggested by the characteristic skin lesions in sun-exposed areas, but confirmation requires demonstration of high levels of uroporphyrins or coproporphyrins, or both.
Porphyrins accumulate in the liver, plasma, urine, and feces. Plasma porphyrin levels in porphyria cutanea tarda are usually above 10 μg/dL (normal < 1.4 μg/dL), and plasma fluorescence scanning usually shows a maximum fluorescence emission at an excitation wavelength of 619 nm. In this patient, however, the definitive diagnosis was made by chromatographic separation and the quantification of porphyrins in the urine and feces, which showed a predominance of uroporphyrins and heptacarboxyporphyrins in the urine and an excess of isocoproporphyrins in the feces.1
Analysis of UROD activity in erythrocytes can help determine the type of porphyria cutanea tarda. Type I and type III and porphyria cutanea tarda secondary to hepatotoxin exposure have normal levels, whereas type II and the hepatoerythropoietic form have abnormally low levels. Examination of the urine with a Wood lamp reveals coral pink fluorescence due to elimination of porphyrins, and this is another diagnostic clue.
Conditions that need to be ruled out include viral infection with hepatitis B or C or human immunodeficiency virus, iron overload, and hereditary hemochromatosis. Serum alpha fetoprotein level assessment, liver ultrasonography, or even biopsy may be indicated to exclude hepatocellular carcinoma.
TREATMENT
Once secondary causes of porphyria are excluded or treated (eg, advising the patient to avoid alcohol, discontinuing estrogens or iron intake), the next step in management is to reduce the patient’s porphyrin and iron loads. Phlebotomy is the standard way to reduce stores of iron throughout the body and particularly in the liver. It works by interrupting iron-mediated oxidative inhibition of hepatic UROD and the oxidation of hepatic porphyrinogens to porphyrinogens.
This adjustment must be gradual, with about 450 mL of blood removed at intervals of 1 to 2 weeks.7 This improves the cutaneous symptoms progressively, with resolution of vesicles in 2 to 3 months, improvement of skin fragility in 6 to 9 months, and normalization of porphyrin levels in 13 months. The scleroderma, atrophy, hyperpigmentation, and hypertrichosis respond more slowly and may take years to resolve.
Porphyria cutanea tarda can recur, usually with new exposure to risk factors. Treatment by phlebotomy may be stopped when the serum ferritin level has reached low-normal levels; the porphyrin levels may not yet be normal at that point but may continue to decline without additional phlebotomy sessions.
If phlebotomy is contraindicated, alternatives include iron chelation with deferoxamine (Desferal),7 or a low dose of chloroquine (Aralen) (125–250 mg orally twice a week) or hydroxychloroquine (Plaquenil) (100 mg orally twice a week) to avoid acute hepatic damage that may be caused by the release of large amounts of porphyrins that accompany standard dosing levels.
- Elder GH. Porphyria cutanea tarda. Semin Liver Dis 1998; 18:67–75.
- Mendez M, Rossetti MV, Del C, Batlle AM, Parera VE. The role of inherited and acquired factors in the development of porphyria cutanea tarda in the Argentinean population. J Am Acad Dermatol 2005; 52:417–424.
- Bonkovsky HL, Poh-Fitzpatrick M, Pimstone N, et al. Porphyria cutanea tarda, hepatitis C, and HFE gene mutations in North America. Hepatology 1998; 27:1661–1669.
- Ali A, Zein NN. Hepatitis C infection: a systemic disease with extrahepatic manifestations. Cleve Clin J Med 2005; 72:1005–1016.
- Grossman ME, Poh-Fitzpatrick MB. Porphyria cutanea tarda. diagnosis and management. Med Clin North Am 1980; 64:807–827.
- Cortés JM, Oliva H, Paradinas FJ, Hernandez-Guío C. The pathology of the liver in porphyria cutanea tarda. Histopathology 1980; 4:471–485.
- Rocchi E, Gibertini P, Cassanelli M, et al. Iron removal therapy in porphyria cutanea tarda: phlebotomy versus slow subcutaneous desferrioxamine infusion. Br J Dermatol 1986; 114:621–629.
Q: Which is the most likely diagnosis?
- Addison disease
- Lupus erythematosus
- Polymorphous light eruption
- Porphyria cutanea tarda
- Bullous pemphigoid
A: Urine testing, including examination under ultraviolet light with a Wood lamp, indicates porphyria cutanea tarda. This is the most common porphyria, occurring mainly in men. Its true prevalence is not known but is estimated to be from 1:5,000 to 1:25,000.1
There are three types of porphyria cutanea tarda. About 80% of cases are type I, also referred to as “sporadic.” In type I, levels of uroporphyrinogen decarboxylase (UROD) in red blood cells are normal, but are low in the liver during episodes of the disease. In type II, UROD levels are about 50% below normal in all tissues. Type III is similar to type I, except that it occurs in more than one family member.
The genetic mutation that produces a deficiency of UROD leads to an excess of uroporphyrins and porphyrins that are partially decarboxylated and that irreversibly oxidize. When they are deposited in the skin and the skin is exposed to the sun, they cause the classic cutaneous manifestations.1
Risk factors2 for porphyria cutanea tarda can be extrinsic (eg, high iron blood levels,2,3 excessive ethanol intake, hepatitis C,2,4 human immunodeficiency virus, estrogen use,5 dialysis for end-stage renal disease) or intrinsic (altered iron metabolism or cytochrome P450 function2).
CLINICAL PRESENTATION
The cardinal symptom of porphyria cutanea tarda is photosensitivity, with the development of chronic blistering lesions on sun-exposed areas such as the hands, face, and forearms. Fluid-filled vesicles develop and rupture easily, and the denuded areas become crusted and heal slowly.5 Secondary infections can occur. Previous areas of blisters may appear atrophic, brownish, or violaceous. Small white plaques (milia) are also common and may precede or follow vesicle formation. These cutaneous lesions, however, are not specific to porphyria cutanea tarda and can appear in variegated porphyria and coproporphyria. Hypertrichosis5 and hyperpigmentation are usually present, mainly over the cheekbones and around the eyes. Patches of alopecia and hypopigmented sclerodermiform lesions may also be observed.
Porphyria cutanea tarda is usually accompanied by alterations in liver metabolism, affecting mainly aminotransferases and gammaglutamyltransferase. The absence of hepatitis C infection does not rule out porphyria cutanea tarda. About 50% of patients have pathologic structural changes in the liver such as lobular necrosis or fibrotic tracts, and 15% of patients have cirrhosis at presentation.6 The risk of hepatocellular carcinoma is clearly increased.6 Hepatitis C virus infection, iron overload, and excessive ethanol intake lead to a more severe liver disease.
DIAGNOSIS
The diagnosis of porphyria cutanea tarda is strongly suggested by the characteristic skin lesions in sun-exposed areas, but confirmation requires demonstration of high levels of uroporphyrins or coproporphyrins, or both.
Porphyrins accumulate in the liver, plasma, urine, and feces. Plasma porphyrin levels in porphyria cutanea tarda are usually above 10 μg/dL (normal < 1.4 μg/dL), and plasma fluorescence scanning usually shows a maximum fluorescence emission at an excitation wavelength of 619 nm. In this patient, however, the definitive diagnosis was made by chromatographic separation and the quantification of porphyrins in the urine and feces, which showed a predominance of uroporphyrins and heptacarboxyporphyrins in the urine and an excess of isocoproporphyrins in the feces.1
Analysis of UROD activity in erythrocytes can help determine the type of porphyria cutanea tarda. Type I and type III and porphyria cutanea tarda secondary to hepatotoxin exposure have normal levels, whereas type II and the hepatoerythropoietic form have abnormally low levels. Examination of the urine with a Wood lamp reveals coral pink fluorescence due to elimination of porphyrins, and this is another diagnostic clue.
Conditions that need to be ruled out include viral infection with hepatitis B or C or human immunodeficiency virus, iron overload, and hereditary hemochromatosis. Serum alpha fetoprotein level assessment, liver ultrasonography, or even biopsy may be indicated to exclude hepatocellular carcinoma.
TREATMENT
Once secondary causes of porphyria are excluded or treated (eg, advising the patient to avoid alcohol, discontinuing estrogens or iron intake), the next step in management is to reduce the patient’s porphyrin and iron loads. Phlebotomy is the standard way to reduce stores of iron throughout the body and particularly in the liver. It works by interrupting iron-mediated oxidative inhibition of hepatic UROD and the oxidation of hepatic porphyrinogens to porphyrinogens.
This adjustment must be gradual, with about 450 mL of blood removed at intervals of 1 to 2 weeks.7 This improves the cutaneous symptoms progressively, with resolution of vesicles in 2 to 3 months, improvement of skin fragility in 6 to 9 months, and normalization of porphyrin levels in 13 months. The scleroderma, atrophy, hyperpigmentation, and hypertrichosis respond more slowly and may take years to resolve.
Porphyria cutanea tarda can recur, usually with new exposure to risk factors. Treatment by phlebotomy may be stopped when the serum ferritin level has reached low-normal levels; the porphyrin levels may not yet be normal at that point but may continue to decline without additional phlebotomy sessions.
If phlebotomy is contraindicated, alternatives include iron chelation with deferoxamine (Desferal),7 or a low dose of chloroquine (Aralen) (125–250 mg orally twice a week) or hydroxychloroquine (Plaquenil) (100 mg orally twice a week) to avoid acute hepatic damage that may be caused by the release of large amounts of porphyrins that accompany standard dosing levels.
Q: Which is the most likely diagnosis?
- Addison disease
- Lupus erythematosus
- Polymorphous light eruption
- Porphyria cutanea tarda
- Bullous pemphigoid
A: Urine testing, including examination under ultraviolet light with a Wood lamp, indicates porphyria cutanea tarda. This is the most common porphyria, occurring mainly in men. Its true prevalence is not known but is estimated to be from 1:5,000 to 1:25,000.1
There are three types of porphyria cutanea tarda. About 80% of cases are type I, also referred to as “sporadic.” In type I, levels of uroporphyrinogen decarboxylase (UROD) in red blood cells are normal, but are low in the liver during episodes of the disease. In type II, UROD levels are about 50% below normal in all tissues. Type III is similar to type I, except that it occurs in more than one family member.
The genetic mutation that produces a deficiency of UROD leads to an excess of uroporphyrins and porphyrins that are partially decarboxylated and that irreversibly oxidize. When they are deposited in the skin and the skin is exposed to the sun, they cause the classic cutaneous manifestations.1
Risk factors2 for porphyria cutanea tarda can be extrinsic (eg, high iron blood levels,2,3 excessive ethanol intake, hepatitis C,2,4 human immunodeficiency virus, estrogen use,5 dialysis for end-stage renal disease) or intrinsic (altered iron metabolism or cytochrome P450 function2).
CLINICAL PRESENTATION
The cardinal symptom of porphyria cutanea tarda is photosensitivity, with the development of chronic blistering lesions on sun-exposed areas such as the hands, face, and forearms. Fluid-filled vesicles develop and rupture easily, and the denuded areas become crusted and heal slowly.5 Secondary infections can occur. Previous areas of blisters may appear atrophic, brownish, or violaceous. Small white plaques (milia) are also common and may precede or follow vesicle formation. These cutaneous lesions, however, are not specific to porphyria cutanea tarda and can appear in variegated porphyria and coproporphyria. Hypertrichosis5 and hyperpigmentation are usually present, mainly over the cheekbones and around the eyes. Patches of alopecia and hypopigmented sclerodermiform lesions may also be observed.
Porphyria cutanea tarda is usually accompanied by alterations in liver metabolism, affecting mainly aminotransferases and gammaglutamyltransferase. The absence of hepatitis C infection does not rule out porphyria cutanea tarda. About 50% of patients have pathologic structural changes in the liver such as lobular necrosis or fibrotic tracts, and 15% of patients have cirrhosis at presentation.6 The risk of hepatocellular carcinoma is clearly increased.6 Hepatitis C virus infection, iron overload, and excessive ethanol intake lead to a more severe liver disease.
DIAGNOSIS
The diagnosis of porphyria cutanea tarda is strongly suggested by the characteristic skin lesions in sun-exposed areas, but confirmation requires demonstration of high levels of uroporphyrins or coproporphyrins, or both.
Porphyrins accumulate in the liver, plasma, urine, and feces. Plasma porphyrin levels in porphyria cutanea tarda are usually above 10 μg/dL (normal < 1.4 μg/dL), and plasma fluorescence scanning usually shows a maximum fluorescence emission at an excitation wavelength of 619 nm. In this patient, however, the definitive diagnosis was made by chromatographic separation and the quantification of porphyrins in the urine and feces, which showed a predominance of uroporphyrins and heptacarboxyporphyrins in the urine and an excess of isocoproporphyrins in the feces.1
Analysis of UROD activity in erythrocytes can help determine the type of porphyria cutanea tarda. Type I and type III and porphyria cutanea tarda secondary to hepatotoxin exposure have normal levels, whereas type II and the hepatoerythropoietic form have abnormally low levels. Examination of the urine with a Wood lamp reveals coral pink fluorescence due to elimination of porphyrins, and this is another diagnostic clue.
Conditions that need to be ruled out include viral infection with hepatitis B or C or human immunodeficiency virus, iron overload, and hereditary hemochromatosis. Serum alpha fetoprotein level assessment, liver ultrasonography, or even biopsy may be indicated to exclude hepatocellular carcinoma.
TREATMENT
Once secondary causes of porphyria are excluded or treated (eg, advising the patient to avoid alcohol, discontinuing estrogens or iron intake), the next step in management is to reduce the patient’s porphyrin and iron loads. Phlebotomy is the standard way to reduce stores of iron throughout the body and particularly in the liver. It works by interrupting iron-mediated oxidative inhibition of hepatic UROD and the oxidation of hepatic porphyrinogens to porphyrinogens.
This adjustment must be gradual, with about 450 mL of blood removed at intervals of 1 to 2 weeks.7 This improves the cutaneous symptoms progressively, with resolution of vesicles in 2 to 3 months, improvement of skin fragility in 6 to 9 months, and normalization of porphyrin levels in 13 months. The scleroderma, atrophy, hyperpigmentation, and hypertrichosis respond more slowly and may take years to resolve.
Porphyria cutanea tarda can recur, usually with new exposure to risk factors. Treatment by phlebotomy may be stopped when the serum ferritin level has reached low-normal levels; the porphyrin levels may not yet be normal at that point but may continue to decline without additional phlebotomy sessions.
If phlebotomy is contraindicated, alternatives include iron chelation with deferoxamine (Desferal),7 or a low dose of chloroquine (Aralen) (125–250 mg orally twice a week) or hydroxychloroquine (Plaquenil) (100 mg orally twice a week) to avoid acute hepatic damage that may be caused by the release of large amounts of porphyrins that accompany standard dosing levels.
- Elder GH. Porphyria cutanea tarda. Semin Liver Dis 1998; 18:67–75.
- Mendez M, Rossetti MV, Del C, Batlle AM, Parera VE. The role of inherited and acquired factors in the development of porphyria cutanea tarda in the Argentinean population. J Am Acad Dermatol 2005; 52:417–424.
- Bonkovsky HL, Poh-Fitzpatrick M, Pimstone N, et al. Porphyria cutanea tarda, hepatitis C, and HFE gene mutations in North America. Hepatology 1998; 27:1661–1669.
- Ali A, Zein NN. Hepatitis C infection: a systemic disease with extrahepatic manifestations. Cleve Clin J Med 2005; 72:1005–1016.
- Grossman ME, Poh-Fitzpatrick MB. Porphyria cutanea tarda. diagnosis and management. Med Clin North Am 1980; 64:807–827.
- Cortés JM, Oliva H, Paradinas FJ, Hernandez-Guío C. The pathology of the liver in porphyria cutanea tarda. Histopathology 1980; 4:471–485.
- Rocchi E, Gibertini P, Cassanelli M, et al. Iron removal therapy in porphyria cutanea tarda: phlebotomy versus slow subcutaneous desferrioxamine infusion. Br J Dermatol 1986; 114:621–629.
- Elder GH. Porphyria cutanea tarda. Semin Liver Dis 1998; 18:67–75.
- Mendez M, Rossetti MV, Del C, Batlle AM, Parera VE. The role of inherited and acquired factors in the development of porphyria cutanea tarda in the Argentinean population. J Am Acad Dermatol 2005; 52:417–424.
- Bonkovsky HL, Poh-Fitzpatrick M, Pimstone N, et al. Porphyria cutanea tarda, hepatitis C, and HFE gene mutations in North America. Hepatology 1998; 27:1661–1669.
- Ali A, Zein NN. Hepatitis C infection: a systemic disease with extrahepatic manifestations. Cleve Clin J Med 2005; 72:1005–1016.
- Grossman ME, Poh-Fitzpatrick MB. Porphyria cutanea tarda. diagnosis and management. Med Clin North Am 1980; 64:807–827.
- Cortés JM, Oliva H, Paradinas FJ, Hernandez-Guío C. The pathology of the liver in porphyria cutanea tarda. Histopathology 1980; 4:471–485.
- Rocchi E, Gibertini P, Cassanelli M, et al. Iron removal therapy in porphyria cutanea tarda: phlebotomy versus slow subcutaneous desferrioxamine infusion. Br J Dermatol 1986; 114:621–629.
Acetaminophen: Old drug, new warnings
Editor’s note: Portions of this article are based on an article previously published in an internal Cleveland Clinic publication, Pharmacotherapy Update. The version here has been revised, updated, and peer-reviewed.
This drug is generally considered safe, but high doses can be toxic. The number of overdoses is worrisome. In 2006 alone, the American Association of Poison Control Centers implicated acetaminophen in nearly 140,000 poisoning cases, in which more than 100 patients died.2 It is responsible for more emergency room visits than any other drug on the market.
According to a position statement from the American Association for the Study of Liver Diseases (AASLD),3 the incidence of acetaminophen-related liver toxicity has been steadily increasing over the past decade, and this drug is now the most common cause of acute liver failure.
MANY OVERDOSES ARE UNINTENTIONAL
Cases of acetaminophen-related liver toxicity can be categorized as either intentional (ie, due to a suicide attempt) or unintentional (ie, due to multiple therapeutic but excessive doses over a period of time, usually more than 3 days).
Up to 50% of cases are unintentional. Bower et al4 reviewed cases of acute liver failure that occurred in the Atlanta, GA, area between November 2000 and October 2004. Acetaminophen was the most common cause in adult patients. Of greater concern is that 61% of the acetaminophen-related cases were due to unintentional overdose. According to the Institute for Safe Medication Practices,5 one hospital (not named) reported that an average of one patient per day was given more than the recommended maximum daily acetaminophen dose of 4 g while in the hospital.
Many patients take more than one acetaminophen product
Unintentional overdoses or “therapeutic misadventures” are most often due to taking multiple products that contain acetaminophen, taking acetaminophen-narcotic combinations, and impulsive behavior involving a lack of understanding of possible injury in consuming multiple acetaminophen-containing products.3
In the US Food and Drug Administration (FDA) Medwatch Database, in 307 cases of unintentional acetaminophen overdose between 1998 and 2001, 25% of patients had been taking more than one acetaminophencontaining product.5
Larson et al6 found that one-third of patients who had had an unintentional acetaminophen overdose were taking an acetaminophen-narcotic combination in addition to another acetaminophen-containing product.
Many consumers don’t know they are taking acetaminophen
Many consumers don’t know that some of the drugs they take contain acetaminophen. This may be because many drug labels contain abbreviations for acetaminophen such as “APAP” or have inconsistent formatting that makes it difficult to determine if the product contains acetaminophen.
Others may not be aware of the total maximum recommended daily dose or may not be able to calculate the total daily intake from the information on the label. The problem is not only with over-the-counter products. For example, if a physician prescribes one or two tablets of hydrocodone/acetaminophen (Vicodin) 5 mg/500 mg every 4 to 6 hours, a patient could easily exceed the recommended maximum daily dose of 4 g of acetaminophen.
Toxicity can occur even at therapeutic doses
Acetaminophen hepatotoxicity can also occur even with therapeutic doses in certain conditions. Risk factors:
- Chronic alcohol use (ie, more than three drinks per day)
- Malnutrition
- Concurrent use of drugs that induce cytochrome P450 (CYP450) enzymes (more on this below).6
FIRST USED IN 1893
Acetaminophen was first used in medicine in 1893, and it became widely used after 1949, when it was found to be a less-toxic metabolite of two parent compounds, acetanilide and phenacetin.1
Acetaminophen is an effective antipyretic and analgesic, but its anti-inflammatory properties are minimal, especially compared with nonsteroidal anti-inflammatory drugs (NSAIDs). Nevertheless, acetaminophen is preferred over NSAIDs in some patients because it carries a lower risk of gastrointestinal toxicity (eg, ulceration, bleeding) and so may be better tolerated.1
INDICATIONS AND DOSAGE
Acetaminophen is indicated for mild to moderate pain or fever, including the pain of osteoarthritis. It is not recommended for chronic inflammatory conditions such as rheumatoid arthritis, since it lacks anti-inflammatory properties.
In adults and in children over age 12, the usual dosage is 325 to 650 mg orally or rectally every 4 to 6 hours, or 1,000 mg three to four times daily.
The current package label recommends that the total daily dose not exceed 4 g in most adults. Lower maximum daily doses (eg, 2 g) are recommended in patients who may be at higher risk of hepatotoxicity, such as those who drink heavily, are malnourished, or take enzyme-inducing drugs. Tylenol products currently include an alcohol warning, advising those who consume three or more alcoholic drinks a day to ask their doctor if they should take acetaminophen.
In children up to 12 years of age, the recommended dosage is 10 to 15 mg/kg orally or rectally every 4 to 6 hours. The maximum dosing for children in this age group should not exceed five doses (or 50 to 75 mg/kg) in 24 hours.7 In children under age 2 or weighing less than 11 kg, acetaminophen should only be used under the direction of a physician.
MOST ACETAMINOPHEN IS CONJUGATED AND THEN EXCRETED IN THE URINE
Acetaminophen usually has excellent bioavailability (up to 98%), but the exact amount absorbed varies, depending on the dosage form and concomitant use of other drugs.8
At therapeutic doses, the elimination halflife is about 2 hours. Peak plasma concentrations are reached 30 to 60 minutes after the dose is taken,1 but taking acetaminophen with opioids, anticholinergic drugs, or even food may delay the time to peak concentration by delaying gastric emptying.8
NAPQI is a toxic metabolite
Under normal circumstances, a small amount of acetaminophen undergoes hepatic metabolism by a different pathway, ie, by CYP450 enzymes, primarily CYP2E1 and to a lesser extent CYP1A2, CYP2A6, and CYP3A4, forming a toxic metabolite, N-acetyl-p-benzoquinone imine (NAPQI). Then, the sulfhydryl groups of glutathione convert NAPQI into harmless metabolites that are excreted in the urine.1,7
Well tolerated and relatively safe
At recommended doses, acetaminophen is well tolerated, and it is considered relatively safe when used according to labeling instructions.
Rarely, patients experience an erythematous or urticarial rash or other allergic complications.1
However, acetaminophen is a dose-dependent hepatotoxin, and excessive doses (intentional or unintentional) may lead to acute liver failure. In addition, even in therapeutic doses, acetaminophen may still cause transient liver enzyme elevations and possibly hepatotoxicity, particularly in people who are malnourished or alcoholic or are taking certain CYP450-inducing drugs.3,6,10
HOW ACETAMINOPHEN CAN INJURE THE LIVER
Glucuronidation and sulfation, the major metabolic pathways, become saturated after an acetaminophen overdose.7 When this happens, more of the toxic metabolite NAPQI is formed by CYP450-mediated N-hydroxylation. When glutathione is depleted after large doses of acetaminophen or in malnourished people, the toxic metabolite accumulates, resulting in liver damage (Figure 1).6
The liver is damaged by two mechanisms. In one, NAPQI binds to hepatic cell macromolecules, causing dysfunction of the enzymatic systems, structural and metabolic disarray, and eventually necrotic cell death. The other mechanism is oxidative stress due to depletion of glutathione.
In children, single acetaminophen doses of 120 to 150 mg/kg of body weight have been associated with hepatotoxicity,11 as have single doses of more than 150 mg/kg or a total dose of greater than 7.5 g in adults. However, the minimal dose associated with liver injury has ranged from 4 to 10 g, and in healthy volunteers even therapeutic doses of 1 g orally every 6 hours resulted in mild liver injury.12
Patients who are malnourished or fasting are thought to be at greater risk of acetaminophen hepatotoxicity because they may be deficient in glutathione at baseline. In addition, even at lower-than-therapeutic doses, induction of CYP450 enzymes by drugs or chronic alcohol consumption may lead to an increase in the formation of NAPQI, increasing the risk of hepatotoxicity. Examples of drugs that induce CYP450 enzymes to produce more NAPQI include the anticonvuslants phenytoin (Dilantin) and phenobarbital.
CLINICAL PRESENTATION OF ACETAMINOPHEN OVERDOSE
The diagnosis of acetaminophen overdose is often established by a thorough history. The pertinent information may be difficult to obtain, however, because the patient may be confused or stuporous at presentation, may not know that the overthe-counter products he or she has been taking contain acetaminophen, or may be embarrassed about taking too much acetaminophen.13
In acute intentional overdose, signs may not be apparent immediately
The symptoms of toxicity may not be apparent immediately after ingestion of an acute overdose of acetaminophen, but early recognition and treatment can prevent more severe liver damage, decreasing morbidity and the risk of death.9
Phase 1 of an acetaminophen overdose begins shortly after ingestion and can last for 12 to 24 hours. Patients may have signs of gastrointestinal upset, nausea, vomiting, anorexia, diaphoresis, and pallor. Although the signs and symptoms show a consistent pattern and are more pronounced after larger acute overdoses, they are not diagnostic or specific.
Phase 2 (up to 48 hours after ingestion). Patients may begin to feel better during this phase. However, the hepatic enzyme levels, the prothrombin time (PT), and the international normalized ratio (INR) may continue to rise, and right upper quadrant pain may develop. Additionally, other laboratory results may be abnormal, and renal insufficiency can occur due to acetaminophen-induced renal tubular necrosis.6 Most patients receive the antidote, acetylcysteine (Mycomyst, Acetadote) before or during this phase, and consequently, liver function gradually returns to normal.
Phase 3, if reached, may be marked by severe hepatic necrosis, typically 3 to 5 days after ingestion. Symptoms during this phase range from less severe (eg, nausea and general malaise) to more severe (eg, confusion and stupor). Also, at this time, liver enzyme levels can be as high as 10,000 IU/L or even higher, and lactic acidosis and coagulopathy may worsen. If death should occur, it is most likely from complications associated with fulminant hepatic failure, including cerebral edema, multiorgan-system failure, or sepsis.6
Phase 4. Patients who recover generally have complete recovery of liver function with no long-term sequelae..
In unintentional overdoses, patients may have low drug levels
Many patients who present with unintentional acetaminophen toxicity have been taking the drug or products that contain the drug over several days to treat an acute or chronic medical condition. They often have low or undetectable serum acetaminophen levels after 2 to 3 days of nonspecific symptoms.12
MANAGING ACETAMINOPHENHEN OVERDOSE
Unintentional overdoses occur over a more prolonged period, and therefore the nomogram is not useful in this situation.
Acetylcysteine is the antidote
Acetylcysteine is the antidote for acetaminophen toxicity and should be given within 8 hours of ingestion for maximal protection against hepatic injury in patients whose serum acetaminophen levels are above the “possible” toxicity line on the nomogram.15 If acetaminophen overdose is suspected but the time elapsed since ingestion cannot be determined, acetylcysteine should be given immediately regardless of the quantity of acetaminophen ingested. 16 In cases of unintentional overdose, it is often given at the discretion of the physician.
Acetylcysteine limits the toxicity of acetaminophen by increasing glutathione stores, binding with NAPQI as a substitute for glutathione, and enhancing sulfate conjugation.6 It may further limit acetaminophen toxicity by nonspecific mechanisms including anti-inflammatory, antioxidant, inotropic, and vasodilating effects.6 In addition, it may prevent further hepatic damage in any patient thought to have acetaminophen-related liver toxicity even beyond the first 12 hours of an overdose.1
Acetylcysteine is available in an oral (Mucomyst) and an intravenous (Acetadote) formulation, which are similar in efficacy. Because many patients find the taste of the oral solution unpleasant and difficult to tolerate, it should be diluted in a 1:3 ratio with cola, orange juice, or other drink to mask its flavor. This mixture should be used within 1 hour of preparation.
Anaphylactoid reactions have occurred in patients receiving intravenous acetylcysteine for acetaminophen overdose soon after the infusion was started, most commonly during the loading dose. The frequency of infusion-related reactions has been reported to be 0.2% to 20.8%.15
The recommended dosage for intravenous acetylcysteine is a loading dose of 150 mg/kg given over 60 minutes, followed by a second dose of 50 mg/kg given over 4 hours, and finally a third dose of 100 mg/kg given over 16 hours.15 For oral acetylcysteine, the loading dose is 140 mg/kg followed by 70 mg/kg every 4 hours for 17 additional doses.17
Other measures
Activated charcoal can be used if the patient presents within 1 to 2 hours after taking acetaminophen. However, the rapid gastrointestinal absorption of acetaminophen makes this treatment ineffective in most cases.9
FINDINGS FROM LARGE REGISTRIES
Findings in adults
Ostapowicz et al,19 as part of the Acute Liver Failure Study Group, in 2002 prospectively characterized the short-term outcomes of acute liver failure in a large number of patients at 17 tertiary care centers in the United States over approximately 41 months. All centers except one performed liver transplants. Eligible patients had to meet criteria for acute liver failure, including an INR higher than 1.5, evidence of hepatic encephalopathy, and presentation within 26 weeks of illness onset without apparent chronic liver disease.
Of the 308 cases of acute liver failure, 120 (39%) were from acetaminophen overdose, making this drug the most common cause of acute liver failure. Forty-four (37%) of the patients with acetaminophen-related acute liver failure were trying to commit suicide, 57% of cases were accidental, and the remaining reasons for acetaminophen overdose were unknown. The median amount ingested was 13.2 g/day (range 2.6–75 g), and 99 (83%) of the 120 patients took more than 4 g/day.
The patients with acetaminophen-related acute liver toxicity differed from those with other causes of acute liver failure such as idiosyncratic drug reactions or indeterminate causes. The acetaminophen group had a shorter duration of disease and higher serum levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), and serum creatinine than those with acute liver failure from other causes. They also had lower bilirubin levels and a lower arterial pH.
Rates of liver transplantation were 6% in the acetaminophen group, 53% in the group with other drug-induced liver toxicity, 51% in the indeterminate group, and 36% in the remaining groups. Of the acetaminophen group, 47% met the criteria for transplantation, but only 57% of those eligible were listed for transplantation. Those excluded from the transplant list had medical contraindications or were excluded for psychosocial reasons. The short-term transplant-free survival rate was 68% in the acetaminophen group.
Overall, 11% of the patients died, including 28% of the acetaminophen group. The authors concluded that most cases of liver injury in the United States are due to medications and may be preventable.
Larson et al,20 as part of the Acute Liver Failure Study Group, examined the incidence, risk factors, and outcomes of acetaminopheninduced acute liver failure at 22 tertiary care centers in the United States over a 6-year period. Of the 662 patients in the study, 302 had acetaminophen-related toxicity and 275 were included in the final analysis; some of them had been included in the study by Ostapowicz et al.19 During the study period, the number of cases of acute liver toxicity related to acetaminophen increased from 28% to 51%.
Of the patients enrolled in the study, 56% met the criteria for potentially toxic acetaminophen ingestion. Of those who met the criteria, 77% had detectable acetaminophen levels in their serum, and 91% had ALT levels higher than 1,000 IU/L. The time between ingestion and symptom onset ranged from 1 to 32 days, and the median dose was 24 g (range 1.2–180 g).
Of the overdose cases, 48% were unintentional, 44% were intentional, and the remaining 8% had no definable reason. Of the patients in the unintentional-overdose group, 38% were using more than one acetaminophen-containing product and 63% were taking combination products containing narcotics. Overeall, 44% of patients reported using narcotic-acetaminophen combination products. The unintentional-overdose group had lower serum acetaminophen levels than the intentional-overdose group, but they were more likely to present with severe hepatic encephalopathy.
The number of patients with an unintentional overdose was worrisome. Furthermore, one-third of the patients who were receiving an acetaminophen-narcotic combination product were taking an additional acetaminophencontaining product. The authors concluded that unintentional overdose is the leading cause of acetaminophen-related hepatotoxicity, and efforts to limit the over-the-counter package size and to restrict prescriptions of acetaminophen-narcotic combinations may be necessary to decrease the incidence of this preventable cause of acute liver failure.
Findings in children
Squires et al21 and the Pediatric Acute Liver Failure Study Group examined the pathogenesis, treatment, and outcome of acute liver failure in children (any age from birth to 18 years) with no previous evidence of chronic liver disease, evidence of acute liver injury, or hepatic-based coagulopathy. From December 1999 to December 2004, 348 patients were enrolled.
Fourteen percent of the cases were due to acetaminophen. The median dose of acetaminophen ingested was 183 mg/kg. Most of these patients were white and female, and 96% were over age 3. Hepatic encephalopathy was more common in the non-acetaminophen groups than in the acetaminophen group, although this is often difficult to assess in infants and children. Children with acetaminophen toxicity had the highest rate of spontaneous recovery: 45 (94%) of 48 recovered.
Although there are fewer acetaminophenrelated cases of acute liver failure in children than in adults, the use of acetaminophen in children is still worrisome. The authors concluded that if they do not have hepatic encephalopathy, children with acetaminopheninduced liver toxicity have an excellent prognosis.
THE FDA LOOKS AT THE PROBLEM
Why overdoses occur
A recent FDA report22 cited the following reasons for acetaminophen overdose:
- Some patients may experience liver injury at doses only slightly above the recommended 4-g daily limit.
- Some patients may be more prone to liver injury from acetaminophen.
- The symptoms associated with liver injury due to acetaminophen can be nonspecific and tend to evolve over several days.
- Many acetaminophen-containing products are available, including over-the-counter and prescription drugs with many different strengths and indications.
- Consumers are unaware of the risk of liver toxicity with acetaminophen.
- Prescription products are not always clearly labeled with acetaminophen as an ingredient.
- Pediatric dosage forms are available in many different concentrations.
New package labeling
Recommendations from an advisory panel
In addition, an FDA advisory panel recommended decreasing the maximum recommended daily dose (possibly to 3,250 mg/day in adults, although this is not final) to help prevent overdoses, and reducing the maximum amount in a single nonprescription dose of the drug to 650 mg.23 The panel also voted (by a narrow margin) to ban all acetaminophen-narcotic combination products.
As of this writing, the FDA has not adopted these recommendations. (Although the FDA is not obliged to follow the advice of its advisory panels, in most cases it does.) The recommendations about acetaminophen could take years to implement fully.
THE UNITED KINGDOM ACTED IN 1998
In response to a rising number of analgesicrelated deaths, the United Kingdom enacted legislation in 1998 to limit the package size available to consumers.24 Packages sold in general stores can contain no more than 16 capsules, while those sold in pharmacies can contain 24. Blister packs were also introduced to make it harder for people to impulsively take handfuls of tablets.25
Two studies since then both found that the number of deaths related to paracetamol (as acetaminophen is known in the United Kingdom) had fallen since the legislation was implemented.24,26 Greene et al27 found that patients who ingested potentially toxic doses of paracetamol had obtained the drug “in a manner contravening the 1998 legislation.”27 In other words, shops in London were not obeying the law.
SUMMARY
The new labeling and the proposed changes are sensible and draw needed attention to the problem of acetaminophen toxicity. To prevent unintentional acetaminophen overdoses, education of patients and health care professionals is urgently needed so that the dangers of consuming excess acetaminophen daily are understood.
- Burke A, Smyth EM, Fitzgerald GA. Analgesic-antipyretic agents: pharmacotherapy of gout. In:Brunton LL, Lazo JS, Parker K, editors. Goodman and Gilman’s the Pharmacological Basis of Therapeutics, 11th ed. New York: McGraw-Hill, 2006:671–716.
- Bronstein AC, Spyker DA, Cantilena LR, Green J, Rumack BH, Heard SE. 2006 Annual Report of the American Association of Poison Control Centers National Poison Data System (NPDS). Clin Toxicol (Phila) 2007; 45:815–917.
- Schwartz J, Stravitz T, Lee WM; American Association for the Study of Liver Disease Study Group. AASLD position on acetaminophen. www.aasld.org/about/publicpolicy/Documents/Public%2520Policy%2520Documents/AcetaminophenPosition.pdf.
- Bower WA, Johns M, Margolis HS, Williams IT, Bell BP. Populationbased surveillance for acute liver failure. Am J Gastroenterol 2007; 102:2459–2463.
- Institute for Safe Medication Practices. How are you preventing acetaminophen overdoses? www.ismp.org/newsletters/acutecare/articles/20030808.asp. Accessed 11/17/2009.
- Larson AM. Acetaminophen hepatotoxicity. Clin Liver Dis 2007; 11:525–548.
- Tylenol package insert. Fort Washington, PA: McNeil-PPC Inc.; 1999.
- Bizovi KE, Hendrickson RG. Chapter 34. Acetaminophen. In:Hoffman RS, Nelson LS, Howland MA, Lewin NA, Flomenbaum NE, Goldfrank LR, editors. Goldfrank’s Manual of Toxicologic Emergencies. 3rd ed. McGraw-Hill: New York, 2007. www.accessemergencymedicine.com/content.aspx?aID=88781. Accessed 11/7/2009.
- Hung OL, Nelson LS. Chapter 171. Acetaminophen. In:Tintinalli JE, Kelen GD, Stapcynski S, editors. Tintinalli's Emergency Medicine: A Comprehensive Study Guide. 6th ed. McGraw-Hill: New York, 2004. www.accessmedicine.com/content.aspx?aID=602606. Accessed 11/17/2009.
- Watkins PB, Kaplowitz N, Slattery JT, et al. Aminotransferase elevations in healthy adults receiving 4 grams of acetaminophen daily: a randomized controlled trial. JAMA 2006; 296:87–93.
- American Academy of Pediatrics Committee on Drugs. Acetaminophen toxicity in children. Pediatrics 2001; 108:1020–1024.
- Fontana RJ. Acute liver failure including acetaminophen overdose. Med Clin North Am 2008; 92:761–794.
- Heard KJ. Acetylcysteine for acetaminophen poisoning. N Engl J Med 2008; 359:285–292.
- Rumack BH, Matthew H. Acetaminophen poisoning and toxicity. Pediatrics 1975; 55:871–876.
- Acetadote package insert. Nashville, TN: Cumberland Pharmaceuticals; 2008Dec.
- Polson J, Lee WM; American Association for the Study of Liver Disease. AASLD position paper: the management of acute liver failure. Hepatology 2005; 41:1179–1197.
- Product Information: acetylcysteine inhalation solution, acetylcysteine inhalation solution. Hospira,Inc, Lake Forest, IL, 2004.
- O’Grady JG, Alexander GJ, Hayllar KM, Williams R. Early indicators of prognosis in fulminant hepatic failure. Gastroenterology 1989; 97:439–445.
- Ostapowicz G, Fontana RJ, Schiødt FV, et al. Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Ann Intern Med 2002; 137:947–954.
- Larson AM, Polson J, Fontana RJ, et al. Acetaminophen-induced acute liver failure: results of a United States multicenter, prospective study. Hepatology 2005; 42:1364–1372.
- Squires RH, Shneider BL, Bucuvalas J, et al. Acute liver failure in children: the first 348 patients in the Pediatric Acute Liver Failure Study Group. J Pediatr 2006; 148:652–658.
- US Food and Drug Administration. Questions and answers on final rule for labeling changes to over-the-counter pain relievers. www.fda.gov/Drugs/NewsEvents/ucm144068.htm. Accessed 10/30/2009.
- Perrone M. FDA panel recommends smaller doses of painkillers. Associated Press. Adelphi, MD. June 30, 2009.
- Hawton K, Simkin S, Deeks J, et al. UK legislation on analgesic packs: before and after study of long term effect on poisonings. BMJ 2004; 329:1076.
- Hughes B, Durran A, Langford NJ, Mutimer D. Paracetamol poisoning—impact of pack size restrictions. J Clin Pharmacol Ther 2003; 28:307–310.
- Wilkinson S, Taylor G, Templeton L, Mistral W, Salter E, Bennett P. Admissions to hospital for deliberate self-harm in England 1995–2000: an analysis of hospital episode statistics. J Public Health Med 2002; 24:179–183.
- Greene SL, Dargan PI, Leman P, Jones AL. Paracetamol availability and recent changes in paracetamol poisoning: is the 1998 legislation limiting availability of paracetamol being followed? Postgrad Med J 2006; 82:520–523.
Editor’s note: Portions of this article are based on an article previously published in an internal Cleveland Clinic publication, Pharmacotherapy Update. The version here has been revised, updated, and peer-reviewed.
This drug is generally considered safe, but high doses can be toxic. The number of overdoses is worrisome. In 2006 alone, the American Association of Poison Control Centers implicated acetaminophen in nearly 140,000 poisoning cases, in which more than 100 patients died.2 It is responsible for more emergency room visits than any other drug on the market.
According to a position statement from the American Association for the Study of Liver Diseases (AASLD),3 the incidence of acetaminophen-related liver toxicity has been steadily increasing over the past decade, and this drug is now the most common cause of acute liver failure.
MANY OVERDOSES ARE UNINTENTIONAL
Cases of acetaminophen-related liver toxicity can be categorized as either intentional (ie, due to a suicide attempt) or unintentional (ie, due to multiple therapeutic but excessive doses over a period of time, usually more than 3 days).
Up to 50% of cases are unintentional. Bower et al4 reviewed cases of acute liver failure that occurred in the Atlanta, GA, area between November 2000 and October 2004. Acetaminophen was the most common cause in adult patients. Of greater concern is that 61% of the acetaminophen-related cases were due to unintentional overdose. According to the Institute for Safe Medication Practices,5 one hospital (not named) reported that an average of one patient per day was given more than the recommended maximum daily acetaminophen dose of 4 g while in the hospital.
Many patients take more than one acetaminophen product
Unintentional overdoses or “therapeutic misadventures” are most often due to taking multiple products that contain acetaminophen, taking acetaminophen-narcotic combinations, and impulsive behavior involving a lack of understanding of possible injury in consuming multiple acetaminophen-containing products.3
In the US Food and Drug Administration (FDA) Medwatch Database, in 307 cases of unintentional acetaminophen overdose between 1998 and 2001, 25% of patients had been taking more than one acetaminophencontaining product.5
Larson et al6 found that one-third of patients who had had an unintentional acetaminophen overdose were taking an acetaminophen-narcotic combination in addition to another acetaminophen-containing product.
Many consumers don’t know they are taking acetaminophen
Many consumers don’t know that some of the drugs they take contain acetaminophen. This may be because many drug labels contain abbreviations for acetaminophen such as “APAP” or have inconsistent formatting that makes it difficult to determine if the product contains acetaminophen.
Others may not be aware of the total maximum recommended daily dose or may not be able to calculate the total daily intake from the information on the label. The problem is not only with over-the-counter products. For example, if a physician prescribes one or two tablets of hydrocodone/acetaminophen (Vicodin) 5 mg/500 mg every 4 to 6 hours, a patient could easily exceed the recommended maximum daily dose of 4 g of acetaminophen.
Toxicity can occur even at therapeutic doses
Acetaminophen hepatotoxicity can also occur even with therapeutic doses in certain conditions. Risk factors:
- Chronic alcohol use (ie, more than three drinks per day)
- Malnutrition
- Concurrent use of drugs that induce cytochrome P450 (CYP450) enzymes (more on this below).6
FIRST USED IN 1893
Acetaminophen was first used in medicine in 1893, and it became widely used after 1949, when it was found to be a less-toxic metabolite of two parent compounds, acetanilide and phenacetin.1
Acetaminophen is an effective antipyretic and analgesic, but its anti-inflammatory properties are minimal, especially compared with nonsteroidal anti-inflammatory drugs (NSAIDs). Nevertheless, acetaminophen is preferred over NSAIDs in some patients because it carries a lower risk of gastrointestinal toxicity (eg, ulceration, bleeding) and so may be better tolerated.1
INDICATIONS AND DOSAGE
Acetaminophen is indicated for mild to moderate pain or fever, including the pain of osteoarthritis. It is not recommended for chronic inflammatory conditions such as rheumatoid arthritis, since it lacks anti-inflammatory properties.
In adults and in children over age 12, the usual dosage is 325 to 650 mg orally or rectally every 4 to 6 hours, or 1,000 mg three to four times daily.
The current package label recommends that the total daily dose not exceed 4 g in most adults. Lower maximum daily doses (eg, 2 g) are recommended in patients who may be at higher risk of hepatotoxicity, such as those who drink heavily, are malnourished, or take enzyme-inducing drugs. Tylenol products currently include an alcohol warning, advising those who consume three or more alcoholic drinks a day to ask their doctor if they should take acetaminophen.
In children up to 12 years of age, the recommended dosage is 10 to 15 mg/kg orally or rectally every 4 to 6 hours. The maximum dosing for children in this age group should not exceed five doses (or 50 to 75 mg/kg) in 24 hours.7 In children under age 2 or weighing less than 11 kg, acetaminophen should only be used under the direction of a physician.
MOST ACETAMINOPHEN IS CONJUGATED AND THEN EXCRETED IN THE URINE
Acetaminophen usually has excellent bioavailability (up to 98%), but the exact amount absorbed varies, depending on the dosage form and concomitant use of other drugs.8
At therapeutic doses, the elimination halflife is about 2 hours. Peak plasma concentrations are reached 30 to 60 minutes after the dose is taken,1 but taking acetaminophen with opioids, anticholinergic drugs, or even food may delay the time to peak concentration by delaying gastric emptying.8
NAPQI is a toxic metabolite
Under normal circumstances, a small amount of acetaminophen undergoes hepatic metabolism by a different pathway, ie, by CYP450 enzymes, primarily CYP2E1 and to a lesser extent CYP1A2, CYP2A6, and CYP3A4, forming a toxic metabolite, N-acetyl-p-benzoquinone imine (NAPQI). Then, the sulfhydryl groups of glutathione convert NAPQI into harmless metabolites that are excreted in the urine.1,7
Well tolerated and relatively safe
At recommended doses, acetaminophen is well tolerated, and it is considered relatively safe when used according to labeling instructions.
Rarely, patients experience an erythematous or urticarial rash or other allergic complications.1
However, acetaminophen is a dose-dependent hepatotoxin, and excessive doses (intentional or unintentional) may lead to acute liver failure. In addition, even in therapeutic doses, acetaminophen may still cause transient liver enzyme elevations and possibly hepatotoxicity, particularly in people who are malnourished or alcoholic or are taking certain CYP450-inducing drugs.3,6,10
HOW ACETAMINOPHEN CAN INJURE THE LIVER
Glucuronidation and sulfation, the major metabolic pathways, become saturated after an acetaminophen overdose.7 When this happens, more of the toxic metabolite NAPQI is formed by CYP450-mediated N-hydroxylation. When glutathione is depleted after large doses of acetaminophen or in malnourished people, the toxic metabolite accumulates, resulting in liver damage (Figure 1).6
The liver is damaged by two mechanisms. In one, NAPQI binds to hepatic cell macromolecules, causing dysfunction of the enzymatic systems, structural and metabolic disarray, and eventually necrotic cell death. The other mechanism is oxidative stress due to depletion of glutathione.
In children, single acetaminophen doses of 120 to 150 mg/kg of body weight have been associated with hepatotoxicity,11 as have single doses of more than 150 mg/kg or a total dose of greater than 7.5 g in adults. However, the minimal dose associated with liver injury has ranged from 4 to 10 g, and in healthy volunteers even therapeutic doses of 1 g orally every 6 hours resulted in mild liver injury.12
Patients who are malnourished or fasting are thought to be at greater risk of acetaminophen hepatotoxicity because they may be deficient in glutathione at baseline. In addition, even at lower-than-therapeutic doses, induction of CYP450 enzymes by drugs or chronic alcohol consumption may lead to an increase in the formation of NAPQI, increasing the risk of hepatotoxicity. Examples of drugs that induce CYP450 enzymes to produce more NAPQI include the anticonvuslants phenytoin (Dilantin) and phenobarbital.
CLINICAL PRESENTATION OF ACETAMINOPHEN OVERDOSE
The diagnosis of acetaminophen overdose is often established by a thorough history. The pertinent information may be difficult to obtain, however, because the patient may be confused or stuporous at presentation, may not know that the overthe-counter products he or she has been taking contain acetaminophen, or may be embarrassed about taking too much acetaminophen.13
In acute intentional overdose, signs may not be apparent immediately
The symptoms of toxicity may not be apparent immediately after ingestion of an acute overdose of acetaminophen, but early recognition and treatment can prevent more severe liver damage, decreasing morbidity and the risk of death.9
Phase 1 of an acetaminophen overdose begins shortly after ingestion and can last for 12 to 24 hours. Patients may have signs of gastrointestinal upset, nausea, vomiting, anorexia, diaphoresis, and pallor. Although the signs and symptoms show a consistent pattern and are more pronounced after larger acute overdoses, they are not diagnostic or specific.
Phase 2 (up to 48 hours after ingestion). Patients may begin to feel better during this phase. However, the hepatic enzyme levels, the prothrombin time (PT), and the international normalized ratio (INR) may continue to rise, and right upper quadrant pain may develop. Additionally, other laboratory results may be abnormal, and renal insufficiency can occur due to acetaminophen-induced renal tubular necrosis.6 Most patients receive the antidote, acetylcysteine (Mycomyst, Acetadote) before or during this phase, and consequently, liver function gradually returns to normal.
Phase 3, if reached, may be marked by severe hepatic necrosis, typically 3 to 5 days after ingestion. Symptoms during this phase range from less severe (eg, nausea and general malaise) to more severe (eg, confusion and stupor). Also, at this time, liver enzyme levels can be as high as 10,000 IU/L or even higher, and lactic acidosis and coagulopathy may worsen. If death should occur, it is most likely from complications associated with fulminant hepatic failure, including cerebral edema, multiorgan-system failure, or sepsis.6
Phase 4. Patients who recover generally have complete recovery of liver function with no long-term sequelae..
In unintentional overdoses, patients may have low drug levels
Many patients who present with unintentional acetaminophen toxicity have been taking the drug or products that contain the drug over several days to treat an acute or chronic medical condition. They often have low or undetectable serum acetaminophen levels after 2 to 3 days of nonspecific symptoms.12
MANAGING ACETAMINOPHENHEN OVERDOSE
Unintentional overdoses occur over a more prolonged period, and therefore the nomogram is not useful in this situation.
Acetylcysteine is the antidote
Acetylcysteine is the antidote for acetaminophen toxicity and should be given within 8 hours of ingestion for maximal protection against hepatic injury in patients whose serum acetaminophen levels are above the “possible” toxicity line on the nomogram.15 If acetaminophen overdose is suspected but the time elapsed since ingestion cannot be determined, acetylcysteine should be given immediately regardless of the quantity of acetaminophen ingested. 16 In cases of unintentional overdose, it is often given at the discretion of the physician.
Acetylcysteine limits the toxicity of acetaminophen by increasing glutathione stores, binding with NAPQI as a substitute for glutathione, and enhancing sulfate conjugation.6 It may further limit acetaminophen toxicity by nonspecific mechanisms including anti-inflammatory, antioxidant, inotropic, and vasodilating effects.6 In addition, it may prevent further hepatic damage in any patient thought to have acetaminophen-related liver toxicity even beyond the first 12 hours of an overdose.1
Acetylcysteine is available in an oral (Mucomyst) and an intravenous (Acetadote) formulation, which are similar in efficacy. Because many patients find the taste of the oral solution unpleasant and difficult to tolerate, it should be diluted in a 1:3 ratio with cola, orange juice, or other drink to mask its flavor. This mixture should be used within 1 hour of preparation.
Anaphylactoid reactions have occurred in patients receiving intravenous acetylcysteine for acetaminophen overdose soon after the infusion was started, most commonly during the loading dose. The frequency of infusion-related reactions has been reported to be 0.2% to 20.8%.15
The recommended dosage for intravenous acetylcysteine is a loading dose of 150 mg/kg given over 60 minutes, followed by a second dose of 50 mg/kg given over 4 hours, and finally a third dose of 100 mg/kg given over 16 hours.15 For oral acetylcysteine, the loading dose is 140 mg/kg followed by 70 mg/kg every 4 hours for 17 additional doses.17
Other measures
Activated charcoal can be used if the patient presents within 1 to 2 hours after taking acetaminophen. However, the rapid gastrointestinal absorption of acetaminophen makes this treatment ineffective in most cases.9
FINDINGS FROM LARGE REGISTRIES
Findings in adults
Ostapowicz et al,19 as part of the Acute Liver Failure Study Group, in 2002 prospectively characterized the short-term outcomes of acute liver failure in a large number of patients at 17 tertiary care centers in the United States over approximately 41 months. All centers except one performed liver transplants. Eligible patients had to meet criteria for acute liver failure, including an INR higher than 1.5, evidence of hepatic encephalopathy, and presentation within 26 weeks of illness onset without apparent chronic liver disease.
Of the 308 cases of acute liver failure, 120 (39%) were from acetaminophen overdose, making this drug the most common cause of acute liver failure. Forty-four (37%) of the patients with acetaminophen-related acute liver failure were trying to commit suicide, 57% of cases were accidental, and the remaining reasons for acetaminophen overdose were unknown. The median amount ingested was 13.2 g/day (range 2.6–75 g), and 99 (83%) of the 120 patients took more than 4 g/day.
The patients with acetaminophen-related acute liver toxicity differed from those with other causes of acute liver failure such as idiosyncratic drug reactions or indeterminate causes. The acetaminophen group had a shorter duration of disease and higher serum levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), and serum creatinine than those with acute liver failure from other causes. They also had lower bilirubin levels and a lower arterial pH.
Rates of liver transplantation were 6% in the acetaminophen group, 53% in the group with other drug-induced liver toxicity, 51% in the indeterminate group, and 36% in the remaining groups. Of the acetaminophen group, 47% met the criteria for transplantation, but only 57% of those eligible were listed for transplantation. Those excluded from the transplant list had medical contraindications or were excluded for psychosocial reasons. The short-term transplant-free survival rate was 68% in the acetaminophen group.
Overall, 11% of the patients died, including 28% of the acetaminophen group. The authors concluded that most cases of liver injury in the United States are due to medications and may be preventable.
Larson et al,20 as part of the Acute Liver Failure Study Group, examined the incidence, risk factors, and outcomes of acetaminopheninduced acute liver failure at 22 tertiary care centers in the United States over a 6-year period. Of the 662 patients in the study, 302 had acetaminophen-related toxicity and 275 were included in the final analysis; some of them had been included in the study by Ostapowicz et al.19 During the study period, the number of cases of acute liver toxicity related to acetaminophen increased from 28% to 51%.
Of the patients enrolled in the study, 56% met the criteria for potentially toxic acetaminophen ingestion. Of those who met the criteria, 77% had detectable acetaminophen levels in their serum, and 91% had ALT levels higher than 1,000 IU/L. The time between ingestion and symptom onset ranged from 1 to 32 days, and the median dose was 24 g (range 1.2–180 g).
Of the overdose cases, 48% were unintentional, 44% were intentional, and the remaining 8% had no definable reason. Of the patients in the unintentional-overdose group, 38% were using more than one acetaminophen-containing product and 63% were taking combination products containing narcotics. Overeall, 44% of patients reported using narcotic-acetaminophen combination products. The unintentional-overdose group had lower serum acetaminophen levels than the intentional-overdose group, but they were more likely to present with severe hepatic encephalopathy.
The number of patients with an unintentional overdose was worrisome. Furthermore, one-third of the patients who were receiving an acetaminophen-narcotic combination product were taking an additional acetaminophencontaining product. The authors concluded that unintentional overdose is the leading cause of acetaminophen-related hepatotoxicity, and efforts to limit the over-the-counter package size and to restrict prescriptions of acetaminophen-narcotic combinations may be necessary to decrease the incidence of this preventable cause of acute liver failure.
Findings in children
Squires et al21 and the Pediatric Acute Liver Failure Study Group examined the pathogenesis, treatment, and outcome of acute liver failure in children (any age from birth to 18 years) with no previous evidence of chronic liver disease, evidence of acute liver injury, or hepatic-based coagulopathy. From December 1999 to December 2004, 348 patients were enrolled.
Fourteen percent of the cases were due to acetaminophen. The median dose of acetaminophen ingested was 183 mg/kg. Most of these patients were white and female, and 96% were over age 3. Hepatic encephalopathy was more common in the non-acetaminophen groups than in the acetaminophen group, although this is often difficult to assess in infants and children. Children with acetaminophen toxicity had the highest rate of spontaneous recovery: 45 (94%) of 48 recovered.
Although there are fewer acetaminophenrelated cases of acute liver failure in children than in adults, the use of acetaminophen in children is still worrisome. The authors concluded that if they do not have hepatic encephalopathy, children with acetaminopheninduced liver toxicity have an excellent prognosis.
THE FDA LOOKS AT THE PROBLEM
Why overdoses occur
A recent FDA report22 cited the following reasons for acetaminophen overdose:
- Some patients may experience liver injury at doses only slightly above the recommended 4-g daily limit.
- Some patients may be more prone to liver injury from acetaminophen.
- The symptoms associated with liver injury due to acetaminophen can be nonspecific and tend to evolve over several days.
- Many acetaminophen-containing products are available, including over-the-counter and prescription drugs with many different strengths and indications.
- Consumers are unaware of the risk of liver toxicity with acetaminophen.
- Prescription products are not always clearly labeled with acetaminophen as an ingredient.
- Pediatric dosage forms are available in many different concentrations.
New package labeling
Recommendations from an advisory panel
In addition, an FDA advisory panel recommended decreasing the maximum recommended daily dose (possibly to 3,250 mg/day in adults, although this is not final) to help prevent overdoses, and reducing the maximum amount in a single nonprescription dose of the drug to 650 mg.23 The panel also voted (by a narrow margin) to ban all acetaminophen-narcotic combination products.
As of this writing, the FDA has not adopted these recommendations. (Although the FDA is not obliged to follow the advice of its advisory panels, in most cases it does.) The recommendations about acetaminophen could take years to implement fully.
THE UNITED KINGDOM ACTED IN 1998
In response to a rising number of analgesicrelated deaths, the United Kingdom enacted legislation in 1998 to limit the package size available to consumers.24 Packages sold in general stores can contain no more than 16 capsules, while those sold in pharmacies can contain 24. Blister packs were also introduced to make it harder for people to impulsively take handfuls of tablets.25
Two studies since then both found that the number of deaths related to paracetamol (as acetaminophen is known in the United Kingdom) had fallen since the legislation was implemented.24,26 Greene et al27 found that patients who ingested potentially toxic doses of paracetamol had obtained the drug “in a manner contravening the 1998 legislation.”27 In other words, shops in London were not obeying the law.
SUMMARY
The new labeling and the proposed changes are sensible and draw needed attention to the problem of acetaminophen toxicity. To prevent unintentional acetaminophen overdoses, education of patients and health care professionals is urgently needed so that the dangers of consuming excess acetaminophen daily are understood.
Editor’s note: Portions of this article are based on an article previously published in an internal Cleveland Clinic publication, Pharmacotherapy Update. The version here has been revised, updated, and peer-reviewed.
This drug is generally considered safe, but high doses can be toxic. The number of overdoses is worrisome. In 2006 alone, the American Association of Poison Control Centers implicated acetaminophen in nearly 140,000 poisoning cases, in which more than 100 patients died.2 It is responsible for more emergency room visits than any other drug on the market.
According to a position statement from the American Association for the Study of Liver Diseases (AASLD),3 the incidence of acetaminophen-related liver toxicity has been steadily increasing over the past decade, and this drug is now the most common cause of acute liver failure.
MANY OVERDOSES ARE UNINTENTIONAL
Cases of acetaminophen-related liver toxicity can be categorized as either intentional (ie, due to a suicide attempt) or unintentional (ie, due to multiple therapeutic but excessive doses over a period of time, usually more than 3 days).
Up to 50% of cases are unintentional. Bower et al4 reviewed cases of acute liver failure that occurred in the Atlanta, GA, area between November 2000 and October 2004. Acetaminophen was the most common cause in adult patients. Of greater concern is that 61% of the acetaminophen-related cases were due to unintentional overdose. According to the Institute for Safe Medication Practices,5 one hospital (not named) reported that an average of one patient per day was given more than the recommended maximum daily acetaminophen dose of 4 g while in the hospital.
Many patients take more than one acetaminophen product
Unintentional overdoses or “therapeutic misadventures” are most often due to taking multiple products that contain acetaminophen, taking acetaminophen-narcotic combinations, and impulsive behavior involving a lack of understanding of possible injury in consuming multiple acetaminophen-containing products.3
In the US Food and Drug Administration (FDA) Medwatch Database, in 307 cases of unintentional acetaminophen overdose between 1998 and 2001, 25% of patients had been taking more than one acetaminophencontaining product.5
Larson et al6 found that one-third of patients who had had an unintentional acetaminophen overdose were taking an acetaminophen-narcotic combination in addition to another acetaminophen-containing product.
Many consumers don’t know they are taking acetaminophen
Many consumers don’t know that some of the drugs they take contain acetaminophen. This may be because many drug labels contain abbreviations for acetaminophen such as “APAP” or have inconsistent formatting that makes it difficult to determine if the product contains acetaminophen.
Others may not be aware of the total maximum recommended daily dose or may not be able to calculate the total daily intake from the information on the label. The problem is not only with over-the-counter products. For example, if a physician prescribes one or two tablets of hydrocodone/acetaminophen (Vicodin) 5 mg/500 mg every 4 to 6 hours, a patient could easily exceed the recommended maximum daily dose of 4 g of acetaminophen.
Toxicity can occur even at therapeutic doses
Acetaminophen hepatotoxicity can also occur even with therapeutic doses in certain conditions. Risk factors:
- Chronic alcohol use (ie, more than three drinks per day)
- Malnutrition
- Concurrent use of drugs that induce cytochrome P450 (CYP450) enzymes (more on this below).6
FIRST USED IN 1893
Acetaminophen was first used in medicine in 1893, and it became widely used after 1949, when it was found to be a less-toxic metabolite of two parent compounds, acetanilide and phenacetin.1
Acetaminophen is an effective antipyretic and analgesic, but its anti-inflammatory properties are minimal, especially compared with nonsteroidal anti-inflammatory drugs (NSAIDs). Nevertheless, acetaminophen is preferred over NSAIDs in some patients because it carries a lower risk of gastrointestinal toxicity (eg, ulceration, bleeding) and so may be better tolerated.1
INDICATIONS AND DOSAGE
Acetaminophen is indicated for mild to moderate pain or fever, including the pain of osteoarthritis. It is not recommended for chronic inflammatory conditions such as rheumatoid arthritis, since it lacks anti-inflammatory properties.
In adults and in children over age 12, the usual dosage is 325 to 650 mg orally or rectally every 4 to 6 hours, or 1,000 mg three to four times daily.
The current package label recommends that the total daily dose not exceed 4 g in most adults. Lower maximum daily doses (eg, 2 g) are recommended in patients who may be at higher risk of hepatotoxicity, such as those who drink heavily, are malnourished, or take enzyme-inducing drugs. Tylenol products currently include an alcohol warning, advising those who consume three or more alcoholic drinks a day to ask their doctor if they should take acetaminophen.
In children up to 12 years of age, the recommended dosage is 10 to 15 mg/kg orally or rectally every 4 to 6 hours. The maximum dosing for children in this age group should not exceed five doses (or 50 to 75 mg/kg) in 24 hours.7 In children under age 2 or weighing less than 11 kg, acetaminophen should only be used under the direction of a physician.
MOST ACETAMINOPHEN IS CONJUGATED AND THEN EXCRETED IN THE URINE
Acetaminophen usually has excellent bioavailability (up to 98%), but the exact amount absorbed varies, depending on the dosage form and concomitant use of other drugs.8
At therapeutic doses, the elimination halflife is about 2 hours. Peak plasma concentrations are reached 30 to 60 minutes after the dose is taken,1 but taking acetaminophen with opioids, anticholinergic drugs, or even food may delay the time to peak concentration by delaying gastric emptying.8
NAPQI is a toxic metabolite
Under normal circumstances, a small amount of acetaminophen undergoes hepatic metabolism by a different pathway, ie, by CYP450 enzymes, primarily CYP2E1 and to a lesser extent CYP1A2, CYP2A6, and CYP3A4, forming a toxic metabolite, N-acetyl-p-benzoquinone imine (NAPQI). Then, the sulfhydryl groups of glutathione convert NAPQI into harmless metabolites that are excreted in the urine.1,7
Well tolerated and relatively safe
At recommended doses, acetaminophen is well tolerated, and it is considered relatively safe when used according to labeling instructions.
Rarely, patients experience an erythematous or urticarial rash or other allergic complications.1
However, acetaminophen is a dose-dependent hepatotoxin, and excessive doses (intentional or unintentional) may lead to acute liver failure. In addition, even in therapeutic doses, acetaminophen may still cause transient liver enzyme elevations and possibly hepatotoxicity, particularly in people who are malnourished or alcoholic or are taking certain CYP450-inducing drugs.3,6,10
HOW ACETAMINOPHEN CAN INJURE THE LIVER
Glucuronidation and sulfation, the major metabolic pathways, become saturated after an acetaminophen overdose.7 When this happens, more of the toxic metabolite NAPQI is formed by CYP450-mediated N-hydroxylation. When glutathione is depleted after large doses of acetaminophen or in malnourished people, the toxic metabolite accumulates, resulting in liver damage (Figure 1).6
The liver is damaged by two mechanisms. In one, NAPQI binds to hepatic cell macromolecules, causing dysfunction of the enzymatic systems, structural and metabolic disarray, and eventually necrotic cell death. The other mechanism is oxidative stress due to depletion of glutathione.
In children, single acetaminophen doses of 120 to 150 mg/kg of body weight have been associated with hepatotoxicity,11 as have single doses of more than 150 mg/kg or a total dose of greater than 7.5 g in adults. However, the minimal dose associated with liver injury has ranged from 4 to 10 g, and in healthy volunteers even therapeutic doses of 1 g orally every 6 hours resulted in mild liver injury.12
Patients who are malnourished or fasting are thought to be at greater risk of acetaminophen hepatotoxicity because they may be deficient in glutathione at baseline. In addition, even at lower-than-therapeutic doses, induction of CYP450 enzymes by drugs or chronic alcohol consumption may lead to an increase in the formation of NAPQI, increasing the risk of hepatotoxicity. Examples of drugs that induce CYP450 enzymes to produce more NAPQI include the anticonvuslants phenytoin (Dilantin) and phenobarbital.
CLINICAL PRESENTATION OF ACETAMINOPHEN OVERDOSE
The diagnosis of acetaminophen overdose is often established by a thorough history. The pertinent information may be difficult to obtain, however, because the patient may be confused or stuporous at presentation, may not know that the overthe-counter products he or she has been taking contain acetaminophen, or may be embarrassed about taking too much acetaminophen.13
In acute intentional overdose, signs may not be apparent immediately
The symptoms of toxicity may not be apparent immediately after ingestion of an acute overdose of acetaminophen, but early recognition and treatment can prevent more severe liver damage, decreasing morbidity and the risk of death.9
Phase 1 of an acetaminophen overdose begins shortly after ingestion and can last for 12 to 24 hours. Patients may have signs of gastrointestinal upset, nausea, vomiting, anorexia, diaphoresis, and pallor. Although the signs and symptoms show a consistent pattern and are more pronounced after larger acute overdoses, they are not diagnostic or specific.
Phase 2 (up to 48 hours after ingestion). Patients may begin to feel better during this phase. However, the hepatic enzyme levels, the prothrombin time (PT), and the international normalized ratio (INR) may continue to rise, and right upper quadrant pain may develop. Additionally, other laboratory results may be abnormal, and renal insufficiency can occur due to acetaminophen-induced renal tubular necrosis.6 Most patients receive the antidote, acetylcysteine (Mycomyst, Acetadote) before or during this phase, and consequently, liver function gradually returns to normal.
Phase 3, if reached, may be marked by severe hepatic necrosis, typically 3 to 5 days after ingestion. Symptoms during this phase range from less severe (eg, nausea and general malaise) to more severe (eg, confusion and stupor). Also, at this time, liver enzyme levels can be as high as 10,000 IU/L or even higher, and lactic acidosis and coagulopathy may worsen. If death should occur, it is most likely from complications associated with fulminant hepatic failure, including cerebral edema, multiorgan-system failure, or sepsis.6
Phase 4. Patients who recover generally have complete recovery of liver function with no long-term sequelae..
In unintentional overdoses, patients may have low drug levels
Many patients who present with unintentional acetaminophen toxicity have been taking the drug or products that contain the drug over several days to treat an acute or chronic medical condition. They often have low or undetectable serum acetaminophen levels after 2 to 3 days of nonspecific symptoms.12
MANAGING ACETAMINOPHENHEN OVERDOSE
Unintentional overdoses occur over a more prolonged period, and therefore the nomogram is not useful in this situation.
Acetylcysteine is the antidote
Acetylcysteine is the antidote for acetaminophen toxicity and should be given within 8 hours of ingestion for maximal protection against hepatic injury in patients whose serum acetaminophen levels are above the “possible” toxicity line on the nomogram.15 If acetaminophen overdose is suspected but the time elapsed since ingestion cannot be determined, acetylcysteine should be given immediately regardless of the quantity of acetaminophen ingested. 16 In cases of unintentional overdose, it is often given at the discretion of the physician.
Acetylcysteine limits the toxicity of acetaminophen by increasing glutathione stores, binding with NAPQI as a substitute for glutathione, and enhancing sulfate conjugation.6 It may further limit acetaminophen toxicity by nonspecific mechanisms including anti-inflammatory, antioxidant, inotropic, and vasodilating effects.6 In addition, it may prevent further hepatic damage in any patient thought to have acetaminophen-related liver toxicity even beyond the first 12 hours of an overdose.1
Acetylcysteine is available in an oral (Mucomyst) and an intravenous (Acetadote) formulation, which are similar in efficacy. Because many patients find the taste of the oral solution unpleasant and difficult to tolerate, it should be diluted in a 1:3 ratio with cola, orange juice, or other drink to mask its flavor. This mixture should be used within 1 hour of preparation.
Anaphylactoid reactions have occurred in patients receiving intravenous acetylcysteine for acetaminophen overdose soon after the infusion was started, most commonly during the loading dose. The frequency of infusion-related reactions has been reported to be 0.2% to 20.8%.15
The recommended dosage for intravenous acetylcysteine is a loading dose of 150 mg/kg given over 60 minutes, followed by a second dose of 50 mg/kg given over 4 hours, and finally a third dose of 100 mg/kg given over 16 hours.15 For oral acetylcysteine, the loading dose is 140 mg/kg followed by 70 mg/kg every 4 hours for 17 additional doses.17
Other measures
Activated charcoal can be used if the patient presents within 1 to 2 hours after taking acetaminophen. However, the rapid gastrointestinal absorption of acetaminophen makes this treatment ineffective in most cases.9
FINDINGS FROM LARGE REGISTRIES
Findings in adults
Ostapowicz et al,19 as part of the Acute Liver Failure Study Group, in 2002 prospectively characterized the short-term outcomes of acute liver failure in a large number of patients at 17 tertiary care centers in the United States over approximately 41 months. All centers except one performed liver transplants. Eligible patients had to meet criteria for acute liver failure, including an INR higher than 1.5, evidence of hepatic encephalopathy, and presentation within 26 weeks of illness onset without apparent chronic liver disease.
Of the 308 cases of acute liver failure, 120 (39%) were from acetaminophen overdose, making this drug the most common cause of acute liver failure. Forty-four (37%) of the patients with acetaminophen-related acute liver failure were trying to commit suicide, 57% of cases were accidental, and the remaining reasons for acetaminophen overdose were unknown. The median amount ingested was 13.2 g/day (range 2.6–75 g), and 99 (83%) of the 120 patients took more than 4 g/day.
The patients with acetaminophen-related acute liver toxicity differed from those with other causes of acute liver failure such as idiosyncratic drug reactions or indeterminate causes. The acetaminophen group had a shorter duration of disease and higher serum levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), and serum creatinine than those with acute liver failure from other causes. They also had lower bilirubin levels and a lower arterial pH.
Rates of liver transplantation were 6% in the acetaminophen group, 53% in the group with other drug-induced liver toxicity, 51% in the indeterminate group, and 36% in the remaining groups. Of the acetaminophen group, 47% met the criteria for transplantation, but only 57% of those eligible were listed for transplantation. Those excluded from the transplant list had medical contraindications or were excluded for psychosocial reasons. The short-term transplant-free survival rate was 68% in the acetaminophen group.
Overall, 11% of the patients died, including 28% of the acetaminophen group. The authors concluded that most cases of liver injury in the United States are due to medications and may be preventable.
Larson et al,20 as part of the Acute Liver Failure Study Group, examined the incidence, risk factors, and outcomes of acetaminopheninduced acute liver failure at 22 tertiary care centers in the United States over a 6-year period. Of the 662 patients in the study, 302 had acetaminophen-related toxicity and 275 were included in the final analysis; some of them had been included in the study by Ostapowicz et al.19 During the study period, the number of cases of acute liver toxicity related to acetaminophen increased from 28% to 51%.
Of the patients enrolled in the study, 56% met the criteria for potentially toxic acetaminophen ingestion. Of those who met the criteria, 77% had detectable acetaminophen levels in their serum, and 91% had ALT levels higher than 1,000 IU/L. The time between ingestion and symptom onset ranged from 1 to 32 days, and the median dose was 24 g (range 1.2–180 g).
Of the overdose cases, 48% were unintentional, 44% were intentional, and the remaining 8% had no definable reason. Of the patients in the unintentional-overdose group, 38% were using more than one acetaminophen-containing product and 63% were taking combination products containing narcotics. Overeall, 44% of patients reported using narcotic-acetaminophen combination products. The unintentional-overdose group had lower serum acetaminophen levels than the intentional-overdose group, but they were more likely to present with severe hepatic encephalopathy.
The number of patients with an unintentional overdose was worrisome. Furthermore, one-third of the patients who were receiving an acetaminophen-narcotic combination product were taking an additional acetaminophencontaining product. The authors concluded that unintentional overdose is the leading cause of acetaminophen-related hepatotoxicity, and efforts to limit the over-the-counter package size and to restrict prescriptions of acetaminophen-narcotic combinations may be necessary to decrease the incidence of this preventable cause of acute liver failure.
Findings in children
Squires et al21 and the Pediatric Acute Liver Failure Study Group examined the pathogenesis, treatment, and outcome of acute liver failure in children (any age from birth to 18 years) with no previous evidence of chronic liver disease, evidence of acute liver injury, or hepatic-based coagulopathy. From December 1999 to December 2004, 348 patients were enrolled.
Fourteen percent of the cases were due to acetaminophen. The median dose of acetaminophen ingested was 183 mg/kg. Most of these patients were white and female, and 96% were over age 3. Hepatic encephalopathy was more common in the non-acetaminophen groups than in the acetaminophen group, although this is often difficult to assess in infants and children. Children with acetaminophen toxicity had the highest rate of spontaneous recovery: 45 (94%) of 48 recovered.
Although there are fewer acetaminophenrelated cases of acute liver failure in children than in adults, the use of acetaminophen in children is still worrisome. The authors concluded that if they do not have hepatic encephalopathy, children with acetaminopheninduced liver toxicity have an excellent prognosis.
THE FDA LOOKS AT THE PROBLEM
Why overdoses occur
A recent FDA report22 cited the following reasons for acetaminophen overdose:
- Some patients may experience liver injury at doses only slightly above the recommended 4-g daily limit.
- Some patients may be more prone to liver injury from acetaminophen.
- The symptoms associated with liver injury due to acetaminophen can be nonspecific and tend to evolve over several days.
- Many acetaminophen-containing products are available, including over-the-counter and prescription drugs with many different strengths and indications.
- Consumers are unaware of the risk of liver toxicity with acetaminophen.
- Prescription products are not always clearly labeled with acetaminophen as an ingredient.
- Pediatric dosage forms are available in many different concentrations.
New package labeling
Recommendations from an advisory panel
In addition, an FDA advisory panel recommended decreasing the maximum recommended daily dose (possibly to 3,250 mg/day in adults, although this is not final) to help prevent overdoses, and reducing the maximum amount in a single nonprescription dose of the drug to 650 mg.23 The panel also voted (by a narrow margin) to ban all acetaminophen-narcotic combination products.
As of this writing, the FDA has not adopted these recommendations. (Although the FDA is not obliged to follow the advice of its advisory panels, in most cases it does.) The recommendations about acetaminophen could take years to implement fully.
THE UNITED KINGDOM ACTED IN 1998
In response to a rising number of analgesicrelated deaths, the United Kingdom enacted legislation in 1998 to limit the package size available to consumers.24 Packages sold in general stores can contain no more than 16 capsules, while those sold in pharmacies can contain 24. Blister packs were also introduced to make it harder for people to impulsively take handfuls of tablets.25
Two studies since then both found that the number of deaths related to paracetamol (as acetaminophen is known in the United Kingdom) had fallen since the legislation was implemented.24,26 Greene et al27 found that patients who ingested potentially toxic doses of paracetamol had obtained the drug “in a manner contravening the 1998 legislation.”27 In other words, shops in London were not obeying the law.
SUMMARY
The new labeling and the proposed changes are sensible and draw needed attention to the problem of acetaminophen toxicity. To prevent unintentional acetaminophen overdoses, education of patients and health care professionals is urgently needed so that the dangers of consuming excess acetaminophen daily are understood.
- Burke A, Smyth EM, Fitzgerald GA. Analgesic-antipyretic agents: pharmacotherapy of gout. In:Brunton LL, Lazo JS, Parker K, editors. Goodman and Gilman’s the Pharmacological Basis of Therapeutics, 11th ed. New York: McGraw-Hill, 2006:671–716.
- Bronstein AC, Spyker DA, Cantilena LR, Green J, Rumack BH, Heard SE. 2006 Annual Report of the American Association of Poison Control Centers National Poison Data System (NPDS). Clin Toxicol (Phila) 2007; 45:815–917.
- Schwartz J, Stravitz T, Lee WM; American Association for the Study of Liver Disease Study Group. AASLD position on acetaminophen. www.aasld.org/about/publicpolicy/Documents/Public%2520Policy%2520Documents/AcetaminophenPosition.pdf.
- Bower WA, Johns M, Margolis HS, Williams IT, Bell BP. Populationbased surveillance for acute liver failure. Am J Gastroenterol 2007; 102:2459–2463.
- Institute for Safe Medication Practices. How are you preventing acetaminophen overdoses? www.ismp.org/newsletters/acutecare/articles/20030808.asp. Accessed 11/17/2009.
- Larson AM. Acetaminophen hepatotoxicity. Clin Liver Dis 2007; 11:525–548.
- Tylenol package insert. Fort Washington, PA: McNeil-PPC Inc.; 1999.
- Bizovi KE, Hendrickson RG. Chapter 34. Acetaminophen. In:Hoffman RS, Nelson LS, Howland MA, Lewin NA, Flomenbaum NE, Goldfrank LR, editors. Goldfrank’s Manual of Toxicologic Emergencies. 3rd ed. McGraw-Hill: New York, 2007. www.accessemergencymedicine.com/content.aspx?aID=88781. Accessed 11/7/2009.
- Hung OL, Nelson LS. Chapter 171. Acetaminophen. In:Tintinalli JE, Kelen GD, Stapcynski S, editors. Tintinalli's Emergency Medicine: A Comprehensive Study Guide. 6th ed. McGraw-Hill: New York, 2004. www.accessmedicine.com/content.aspx?aID=602606. Accessed 11/17/2009.
- Watkins PB, Kaplowitz N, Slattery JT, et al. Aminotransferase elevations in healthy adults receiving 4 grams of acetaminophen daily: a randomized controlled trial. JAMA 2006; 296:87–93.
- American Academy of Pediatrics Committee on Drugs. Acetaminophen toxicity in children. Pediatrics 2001; 108:1020–1024.
- Fontana RJ. Acute liver failure including acetaminophen overdose. Med Clin North Am 2008; 92:761–794.
- Heard KJ. Acetylcysteine for acetaminophen poisoning. N Engl J Med 2008; 359:285–292.
- Rumack BH, Matthew H. Acetaminophen poisoning and toxicity. Pediatrics 1975; 55:871–876.
- Acetadote package insert. Nashville, TN: Cumberland Pharmaceuticals; 2008Dec.
- Polson J, Lee WM; American Association for the Study of Liver Disease. AASLD position paper: the management of acute liver failure. Hepatology 2005; 41:1179–1197.
- Product Information: acetylcysteine inhalation solution, acetylcysteine inhalation solution. Hospira,Inc, Lake Forest, IL, 2004.
- O’Grady JG, Alexander GJ, Hayllar KM, Williams R. Early indicators of prognosis in fulminant hepatic failure. Gastroenterology 1989; 97:439–445.
- Ostapowicz G, Fontana RJ, Schiødt FV, et al. Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Ann Intern Med 2002; 137:947–954.
- Larson AM, Polson J, Fontana RJ, et al. Acetaminophen-induced acute liver failure: results of a United States multicenter, prospective study. Hepatology 2005; 42:1364–1372.
- Squires RH, Shneider BL, Bucuvalas J, et al. Acute liver failure in children: the first 348 patients in the Pediatric Acute Liver Failure Study Group. J Pediatr 2006; 148:652–658.
- US Food and Drug Administration. Questions and answers on final rule for labeling changes to over-the-counter pain relievers. www.fda.gov/Drugs/NewsEvents/ucm144068.htm. Accessed 10/30/2009.
- Perrone M. FDA panel recommends smaller doses of painkillers. Associated Press. Adelphi, MD. June 30, 2009.
- Hawton K, Simkin S, Deeks J, et al. UK legislation on analgesic packs: before and after study of long term effect on poisonings. BMJ 2004; 329:1076.
- Hughes B, Durran A, Langford NJ, Mutimer D. Paracetamol poisoning—impact of pack size restrictions. J Clin Pharmacol Ther 2003; 28:307–310.
- Wilkinson S, Taylor G, Templeton L, Mistral W, Salter E, Bennett P. Admissions to hospital for deliberate self-harm in England 1995–2000: an analysis of hospital episode statistics. J Public Health Med 2002; 24:179–183.
- Greene SL, Dargan PI, Leman P, Jones AL. Paracetamol availability and recent changes in paracetamol poisoning: is the 1998 legislation limiting availability of paracetamol being followed? Postgrad Med J 2006; 82:520–523.
- Burke A, Smyth EM, Fitzgerald GA. Analgesic-antipyretic agents: pharmacotherapy of gout. In:Brunton LL, Lazo JS, Parker K, editors. Goodman and Gilman’s the Pharmacological Basis of Therapeutics, 11th ed. New York: McGraw-Hill, 2006:671–716.
- Bronstein AC, Spyker DA, Cantilena LR, Green J, Rumack BH, Heard SE. 2006 Annual Report of the American Association of Poison Control Centers National Poison Data System (NPDS). Clin Toxicol (Phila) 2007; 45:815–917.
- Schwartz J, Stravitz T, Lee WM; American Association for the Study of Liver Disease Study Group. AASLD position on acetaminophen. www.aasld.org/about/publicpolicy/Documents/Public%2520Policy%2520Documents/AcetaminophenPosition.pdf.
- Bower WA, Johns M, Margolis HS, Williams IT, Bell BP. Populationbased surveillance for acute liver failure. Am J Gastroenterol 2007; 102:2459–2463.
- Institute for Safe Medication Practices. How are you preventing acetaminophen overdoses? www.ismp.org/newsletters/acutecare/articles/20030808.asp. Accessed 11/17/2009.
- Larson AM. Acetaminophen hepatotoxicity. Clin Liver Dis 2007; 11:525–548.
- Tylenol package insert. Fort Washington, PA: McNeil-PPC Inc.; 1999.
- Bizovi KE, Hendrickson RG. Chapter 34. Acetaminophen. In:Hoffman RS, Nelson LS, Howland MA, Lewin NA, Flomenbaum NE, Goldfrank LR, editors. Goldfrank’s Manual of Toxicologic Emergencies. 3rd ed. McGraw-Hill: New York, 2007. www.accessemergencymedicine.com/content.aspx?aID=88781. Accessed 11/7/2009.
- Hung OL, Nelson LS. Chapter 171. Acetaminophen. In:Tintinalli JE, Kelen GD, Stapcynski S, editors. Tintinalli's Emergency Medicine: A Comprehensive Study Guide. 6th ed. McGraw-Hill: New York, 2004. www.accessmedicine.com/content.aspx?aID=602606. Accessed 11/17/2009.
- Watkins PB, Kaplowitz N, Slattery JT, et al. Aminotransferase elevations in healthy adults receiving 4 grams of acetaminophen daily: a randomized controlled trial. JAMA 2006; 296:87–93.
- American Academy of Pediatrics Committee on Drugs. Acetaminophen toxicity in children. Pediatrics 2001; 108:1020–1024.
- Fontana RJ. Acute liver failure including acetaminophen overdose. Med Clin North Am 2008; 92:761–794.
- Heard KJ. Acetylcysteine for acetaminophen poisoning. N Engl J Med 2008; 359:285–292.
- Rumack BH, Matthew H. Acetaminophen poisoning and toxicity. Pediatrics 1975; 55:871–876.
- Acetadote package insert. Nashville, TN: Cumberland Pharmaceuticals; 2008Dec.
- Polson J, Lee WM; American Association for the Study of Liver Disease. AASLD position paper: the management of acute liver failure. Hepatology 2005; 41:1179–1197.
- Product Information: acetylcysteine inhalation solution, acetylcysteine inhalation solution. Hospira,Inc, Lake Forest, IL, 2004.
- O’Grady JG, Alexander GJ, Hayllar KM, Williams R. Early indicators of prognosis in fulminant hepatic failure. Gastroenterology 1989; 97:439–445.
- Ostapowicz G, Fontana RJ, Schiødt FV, et al. Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Ann Intern Med 2002; 137:947–954.
- Larson AM, Polson J, Fontana RJ, et al. Acetaminophen-induced acute liver failure: results of a United States multicenter, prospective study. Hepatology 2005; 42:1364–1372.
- Squires RH, Shneider BL, Bucuvalas J, et al. Acute liver failure in children: the first 348 patients in the Pediatric Acute Liver Failure Study Group. J Pediatr 2006; 148:652–658.
- US Food and Drug Administration. Questions and answers on final rule for labeling changes to over-the-counter pain relievers. www.fda.gov/Drugs/NewsEvents/ucm144068.htm. Accessed 10/30/2009.
- Perrone M. FDA panel recommends smaller doses of painkillers. Associated Press. Adelphi, MD. June 30, 2009.
- Hawton K, Simkin S, Deeks J, et al. UK legislation on analgesic packs: before and after study of long term effect on poisonings. BMJ 2004; 329:1076.
- Hughes B, Durran A, Langford NJ, Mutimer D. Paracetamol poisoning—impact of pack size restrictions. J Clin Pharmacol Ther 2003; 28:307–310.
- Wilkinson S, Taylor G, Templeton L, Mistral W, Salter E, Bennett P. Admissions to hospital for deliberate self-harm in England 1995–2000: an analysis of hospital episode statistics. J Public Health Med 2002; 24:179–183.
- Greene SL, Dargan PI, Leman P, Jones AL. Paracetamol availability and recent changes in paracetamol poisoning: is the 1998 legislation limiting availability of paracetamol being followed? Postgrad Med J 2006; 82:520–523.
KEY POINTS
- Acetaminophen is the leading cause of acute liver failure in the United States, and nearly half of acetaminophenassociated cases are due to unintentional overdose.
- In many cases of unintentional overdose, patients took more than one acetaminophen-containing product and did not know that both products contained this drug.
- Prescribers need to inform all patients, especially vulnerable ones (eg, those taking enzyme-inducing drugs, those who chronically use alcohol, and those who are malnourished) of the risks associated with acetaminophen.
- Although no consensus has been reached on what is a safe dose in patients with liver disease, 4 g/day is too much: a total daily dose of no more than 2 g is recommended to decrease the risk of toxicity in these patients.
Evidence, limes, and cement
In 1753, Lind2 described how he gave fresh fruit vs cider, vinegar, sulfuric acid, seawater, or barley water to 12 sailors aboard the HMS Salisbury in an effort to find a cure for scurvy. This landmark clinical trial has been hailed as an example of how clinical research can dramatically alter clinical practice. Yet practice did not change aboard British naval ships until almost 50 years after Lind’s treatise was published.3
For many reasons, randomized clinical trials may not immediately affect what physicians do. Sometimes, physicians believe that the trials were not well designed or well conducted, or that the results do not apply to their patients. I briefly discussed some limitations of evidence-based clinical decision-making in our September 2009 issue.4
Another reason is that the conclusions from some trials do not jibe with the experience of seasoned clinicians. That is why, this month, I have asked two physicians, a rheumatologist5 and a spine surgeon,6 to comment on how two studies7,8 have influenced their clinical practice. Both studies concluded that vertebroplasty (injecting cement to shore up osteoporotic vertebrae) was no more beneficial than a sham procedure in patients with vertebral compression fractures. Neither physician is ready to completely abandon vertebroplasty on the basis of these two studies. Thus, it seems that published evidence may provide us guidance and fruit for discussion, but does not give us certainty.
- Sackett DL, Rosenberg WM, Gray JA, Haynes RB, Richardson WS. Evidence based medicine: what it is and what it isn’t [editorial]. BMJ 1996; 312:71–72.
- Lind J. Treatise of the Scurvy in Three Parts. Containing an inquiry into the Nature, Causes and Cure of that Disease. Together with a Critical and Chronological View of what has been published on the subject. Edinburgh: Sands, Murray, and Cochran, 1753.
- Carpenter K. The History of Scurvy and Vitamin C. Cambridge, UK: Cambridge University Press, 1988.
- Mandell BF. Vertebroplasty, evidence, and health care reform: What is quality care? Cleve Clin J Med 2009; 76:497–502.
- Bolster MA. Consternation and questions about two vertebroplasty trials. Cleve Clin J Med 2010; 77:12–16.
- Orr RD. Vertebroplasty, cognitive dissonance, and evidence-based medicine: what do we do when the ‘evidence’ says we are wrong? Cleve Clin J Med 2010; 77:8–11.
- Kallmes DF, Comstock BA, Heagerty PJ, et al. A randomized trial of vertebroplasty for osteoporotic spinal fractures. N Engl J Med 2009; 361:569–579.
- Buchbinder R, Osborne RH, Ebeling PR, et al. A randomized trial of vertebroplasty for painful osteoporotic vertebral fractures. N Engl J Med 2009; 361:557–568.
In 1753, Lind2 described how he gave fresh fruit vs cider, vinegar, sulfuric acid, seawater, or barley water to 12 sailors aboard the HMS Salisbury in an effort to find a cure for scurvy. This landmark clinical trial has been hailed as an example of how clinical research can dramatically alter clinical practice. Yet practice did not change aboard British naval ships until almost 50 years after Lind’s treatise was published.3
For many reasons, randomized clinical trials may not immediately affect what physicians do. Sometimes, physicians believe that the trials were not well designed or well conducted, or that the results do not apply to their patients. I briefly discussed some limitations of evidence-based clinical decision-making in our September 2009 issue.4
Another reason is that the conclusions from some trials do not jibe with the experience of seasoned clinicians. That is why, this month, I have asked two physicians, a rheumatologist5 and a spine surgeon,6 to comment on how two studies7,8 have influenced their clinical practice. Both studies concluded that vertebroplasty (injecting cement to shore up osteoporotic vertebrae) was no more beneficial than a sham procedure in patients with vertebral compression fractures. Neither physician is ready to completely abandon vertebroplasty on the basis of these two studies. Thus, it seems that published evidence may provide us guidance and fruit for discussion, but does not give us certainty.
In 1753, Lind2 described how he gave fresh fruit vs cider, vinegar, sulfuric acid, seawater, or barley water to 12 sailors aboard the HMS Salisbury in an effort to find a cure for scurvy. This landmark clinical trial has been hailed as an example of how clinical research can dramatically alter clinical practice. Yet practice did not change aboard British naval ships until almost 50 years after Lind’s treatise was published.3
For many reasons, randomized clinical trials may not immediately affect what physicians do. Sometimes, physicians believe that the trials were not well designed or well conducted, or that the results do not apply to their patients. I briefly discussed some limitations of evidence-based clinical decision-making in our September 2009 issue.4
Another reason is that the conclusions from some trials do not jibe with the experience of seasoned clinicians. That is why, this month, I have asked two physicians, a rheumatologist5 and a spine surgeon,6 to comment on how two studies7,8 have influenced their clinical practice. Both studies concluded that vertebroplasty (injecting cement to shore up osteoporotic vertebrae) was no more beneficial than a sham procedure in patients with vertebral compression fractures. Neither physician is ready to completely abandon vertebroplasty on the basis of these two studies. Thus, it seems that published evidence may provide us guidance and fruit for discussion, but does not give us certainty.
- Sackett DL, Rosenberg WM, Gray JA, Haynes RB, Richardson WS. Evidence based medicine: what it is and what it isn’t [editorial]. BMJ 1996; 312:71–72.
- Lind J. Treatise of the Scurvy in Three Parts. Containing an inquiry into the Nature, Causes and Cure of that Disease. Together with a Critical and Chronological View of what has been published on the subject. Edinburgh: Sands, Murray, and Cochran, 1753.
- Carpenter K. The History of Scurvy and Vitamin C. Cambridge, UK: Cambridge University Press, 1988.
- Mandell BF. Vertebroplasty, evidence, and health care reform: What is quality care? Cleve Clin J Med 2009; 76:497–502.
- Bolster MA. Consternation and questions about two vertebroplasty trials. Cleve Clin J Med 2010; 77:12–16.
- Orr RD. Vertebroplasty, cognitive dissonance, and evidence-based medicine: what do we do when the ‘evidence’ says we are wrong? Cleve Clin J Med 2010; 77:8–11.
- Kallmes DF, Comstock BA, Heagerty PJ, et al. A randomized trial of vertebroplasty for osteoporotic spinal fractures. N Engl J Med 2009; 361:569–579.
- Buchbinder R, Osborne RH, Ebeling PR, et al. A randomized trial of vertebroplasty for painful osteoporotic vertebral fractures. N Engl J Med 2009; 361:557–568.
- Sackett DL, Rosenberg WM, Gray JA, Haynes RB, Richardson WS. Evidence based medicine: what it is and what it isn’t [editorial]. BMJ 1996; 312:71–72.
- Lind J. Treatise of the Scurvy in Three Parts. Containing an inquiry into the Nature, Causes and Cure of that Disease. Together with a Critical and Chronological View of what has been published on the subject. Edinburgh: Sands, Murray, and Cochran, 1753.
- Carpenter K. The History of Scurvy and Vitamin C. Cambridge, UK: Cambridge University Press, 1988.
- Mandell BF. Vertebroplasty, evidence, and health care reform: What is quality care? Cleve Clin J Med 2009; 76:497–502.
- Bolster MA. Consternation and questions about two vertebroplasty trials. Cleve Clin J Med 2010; 77:12–16.
- Orr RD. Vertebroplasty, cognitive dissonance, and evidence-based medicine: what do we do when the ‘evidence’ says we are wrong? Cleve Clin J Med 2010; 77:8–11.
- Kallmes DF, Comstock BA, Heagerty PJ, et al. A randomized trial of vertebroplasty for osteoporotic spinal fractures. N Engl J Med 2009; 361:569–579.
- Buchbinder R, Osborne RH, Ebeling PR, et al. A randomized trial of vertebroplasty for painful osteoporotic vertebral fractures. N Engl J Med 2009; 361:557–568.
Consternation and questions about two vertebroplasty trials
Confronted with the unexpected results of two trials of vertebroplasty,1,2 physicians are feeling some consternation, We had thought that percutaneous vertebroplasty helps patients with osteoporosis who sustain a painful vertebral insufficiency fracture. However, the trials found it to be no better than a sham procedure in terms of relieving pain.
How will these findings affect our practice? Should we abandon this popular procedure? Or are there other considerations that may mitigate these negative findings? And what should we tell our patients?
700,000 FRACTURES PER YEAR
Vertebral insufficiency fractures are the most common type of fracture in patients with osteoporosis. Every year in the United States, about 700,000 of them occur.
Nearly two-thirds are asymptomatic. The other one-third typically present with the acute onset of localized pain.
Vertebral insufficiency fractures often lead to chronic pain, impair the ability to walk and to perform daily activities, and accentuate thoracic kyphosis, which in turn can lead to pulmonary restrictive disease, and they raise the risk of death. Also, a patient who has a vertebral insufficiency fracture has a 20% risk of sustaining a new one within 1 year.3
Whether symptomatic or asymptomatic, finding a vertebral insufficiency fracture should prompt one to consider drug therapy for osteoporosis. In addition, until now, a patient who presented with the acute onset of back pain and whose evaluation revealed a vertebral insufficiency fracture would also be considered for a vertebral augmentation procedure, either vertebroplasty or kyphoplasty, to relieve the pain.
Vertebroplasty involves injecting polymethylmethacrylate cement percutaneously into the affected vertebral body. Kyphoplasty, a similar procedure, uses a balloon to create a cavity in the fractured vertebral body. After the balloon is withdrawn, the cavity is filled with cement.
TWO RANDOMIZED TRIALS OF SHAM VS REAL VERTEBROPLASTY
Two teams, Kallmes et al2 and Buchbinder et al,1 independently performed randomized controlled trials to see if vertebroplasty really relieves pain as well as has been reported in open studies, case series, and nonrandomized trials.4–7
In both trials, patients were randomized to undergo either sham vertebroplasty or real vertebroplasty. The sham procedure closely approximated the real procedure, including inserting a needle, infiltrating a local anesthetic, bupivacaine (Marcaine), into the periosteum of the posterior lamina1 or the pedicle of the target vertebrae,2 and opening a vial of polymethylmethacrylate so that the patient would smell the product.
Inclusion criteria
Patients in both trials had to have evidence of a recent (acute) or nonhealed vertebral insufficiency fracture.
Pain was the primary outcome measured
In both trials, the investigators assessed the patients’ pain at baseline and again at several specified intervals, using validated tools.
Kallmes et al assessed pain intensity and functional measures at 1 month (the primary outcome measured), and also at 3, 14, and 90 days and at 1 year.
Buchbinder et al assessed pain at 1 week and at 1, 3, and 6 months. The primary outcome measured was pain at 3 months. Secondary outcomes included quality-of-life measures, pain at rest, and pain at night.
Surprising results
In both trials, the mean pain scores were better than at baseline at all time points after the procedure in both the real-procedure and the sham-procedure groups. Moreover, the effect did not differ between the two treatment groups in either study.
QUESTIONS COMPLICATE THE ISSUE
These two trials should make us consider whether this intervention is warranted. We should, however, also consider some limitations of these studies that raise questions about how the conclusions should or should not alter practice.
Does local anesthetic continue to relieve pain?
In both the sham and the real procedure, the bupivacaine injection may have helped relieve pain to some extent afterward, as its anesthetic effect may last longer than we would expect from its 3-hour half-life. The effect could certainly have contributed to improvements in pain levels at the earlier time points after the procedure.
Was there selection bias?
Both studies were highly rigorous and were done at hospitals that had extensive experience with vertebroplasty. However, they may have harbored selection bias, as many more patients were screened than were randomized.
Buchbinder et al1 screened 468 patients. Of these, 30% declined to participate, and another 53% did not meet the eligibility criteria. In the end, only 78 patients were randomized.
Kallmes et al2 screened 1,813 patients, 300 of whom declined and 1,382 of whom were excluded, leaving 131 patients to be randomized. The reasons for exclusion were not specifically reported in many cases.
In both studies, it would be interesting to know how many of those who declined proceeded to undergo a vertebral augmentation procedure.
Did the trials have enough power?
In the study by Kallmes et al,2 recruitment got off to a slow start. Thus, after three patients were recruited, the inclusion requirements were liberalized. The study was originally designed to include 250 patients, which would have given it a power of greater than 80% to detect differences in primary and secondary outcomes. The design was revised to include 130 patients. The statistical power was still 80%, but this was to detect a greater difference in the outcomes than originally projected.
Had the window of opportunity already closed?
Vertebroplasty may have a window of opportunity within which it is most effective. Sooner is probably better than later, but it would be good to identify this time frame.
Kaufmann et al9 reported that patients with older fractures needed slightly more analgesic drugs after the procedure. It has been shown previously that patients who are the most likely to respond to a vertebral augmentation procedure are those with fractures that occurred between 1 and 12 months prior to the procedure and who have evidence that the fracture was recent, ie, edema on magnetic resonance imaging (MRI) or increased uptake on a bone scan.10
Other studies suggested that intervention works best in patients who have had uncontrolled pain lasting less than 6 weeks.8,11 (In the study by Buchbinder et al,1 only 32% of the patients in either group reported pain lasting less than 6 weeks.)
The study by Kallmes et al included patients whose pain had begun within 1 year previously. However, if the duration of pain (ie, the age of the fracture) was uncertain, MRI was done to look for edema, which would indicate the fracture was fresh. It is thus unclear whether all patients in this study truly had an acute or subacute fracture, since all did not undergo confirmatory MRI.
Why did so many patients cross over from sham to real treatment?
Patients in the Kallmes trial2 could cross over from one treatment group to the other as early as 1 month after the procedure. And, in fact, 43% of patients in the sham-treatment group did choose to cross over by 3 months. In contrast, after real vertebroplasty, significantly fewer—only 12% (P < .001)—crossed over to receive the sham procedure. The patients who crossed over from the sham-procedure group to receive vertebroplasty experienced an early improvement in pain, but this was not sustained at 1 or 3 months of follow-up.
The higher crossover rate in the shamprocedure group suggests they were dissatisfied with this intervention, although their outcomes were not significantly better after they got the real procedure. The patients who first received the sham treatment and elected to cross over to vertebroplasty had higher pain and disability scores at baseline. Thus, they may have had other, more chronic causes of pain or other factors affecting the likelihood of a response, particularly of a durable or sustained response.
How do the interventions compare with medical therapy?
Earlier studies showed that vertebroplasty relieves pain almost immediately.4–6 But the benefit does not last: at 6 weeks and up to 12 months later there is no difference in either pain or functional capacity reported in patients receiving vertebroplasty vs conservative treatment.4,6,7 It would thus appear that pain gradually diminishes over time after a vertebral insufficiency fracture, as the fracture heals.
The recent studies1,2 raise the possibility that the pain relief is due to the local anesthetic, not the vertebroplasty itself. We do not know, however, if either vertebroplasty or the sham procedure is superior to conservative medical management. Prospective multicenter trials are under way to address this question.11
Further complicating the issue, the two trials did not keep track of medical treatments patients were receiving concomitantly during the trial period. It is thus more difficult to compare the pain assessment outcomes following invasive procedures—real or sham.
Would kyphoplasty be better?
These studies addressed one procedure, vertebroplasty, and the results and conclusions should not be generalized to kyphoplasty. A prospective randomized trial of kyphoplasty is clearly warranted.
If kyphoplasty is found to be better than a sham procedure, then vertebroplasty should be re-examined in comparison with kyphoplasty. In any future studies, it will be important to select patients rigorously (eg, to include only patients with recent fractures), to match patients according to concomitant therapies, and to consider other potential superimposed causes of back pain in this elderly population, which has a high prevalence of back pain.
HOW SHOULD MY PRACTICE CHANGE? WHAT SHOULD I TELL PATIENTS?
Having considered the results, conclusions, and limitations of these two randomized trials, particularly in terms of recruitment, I cannot say that my practice has changed in terms of referring patients who have a vertebral compression fracture to an interventionalist. However, the education that I provide to patients has changed.
In my mind, the highest priority for a patient with a vertebral insufficiency fracture is to treat (or to reassess the current treatment of) the underlying systemic disease, ie, osteoporosis. This is especially true since most vertebral insufficiency fractures are asymptomatic.
On the other hand, a patient with a painful vertebral compression fracture needs prompt attention and consideration for interventional pain relief. Rapid pain relief is desirable. And in uncontrolled trials,4–7 vertebroplasty and kyphoplasty rapidly relieved vertebral pain. However, it may be that an anesthetic injection is equivalent to vertebroplasty and could accomplish the goal of immediate pain relief just as well.
The pain relief from sham or real vertebroplasty may not be durable, and 3 to 12 months later the pain benefit may be no greater than if more conservative therapy had been pursued.
It is essential to determine the most appropriate window for treatment as well as the most appropriate candidates on whom to perform a procedure. The recently published studies1,2 may have had significant patient selection bias and may not have optimized the window of opportunity for vertebral augmentation performance. There were many patients who declined the study, and some were excluded because of acute pain requiring hospitalization.
As a rheumatologist treating patients with osteoporosis, it is my responsibility to discuss with the patient and family the potential treatments available, to discuss the associated possible risks and benefits, to report on available evidence, and to refer patients to an appropriate interventional specialist if they desire. In light of the lack of superior pain reduction with vertebroplasty than with a sham procedure, many patients may opt for conservative therapy.
It is thus appropriate to determine the acuity of the fracture and to have a frank discussion with the patient about the options for pain management. Opiate drugs pose risks in elderly patients, particularly altered mentation, somnolence, interference with balance, and risk of falls. Vertebroplasty or anesthetic injection may rapidly relieve the pain and reduce the need for opiate therapy. Not yet subjected to the rigors of a randomized placebocontrolled trial, kyphoplasty may yet prove to be better than a sham intervention.
It is essential to determine if there is a role for vertebral augmentation in a select patient population—perhaps selected on the basis of the time that has elapsed since the fracture occurred (determined objectively), the severity of the fracture, and other factors. Perhaps a subset of patients would gain greater benefit from the procedure, whether it amounts solely to acute pain reduction or perhaps to a more durable response.
The recent studies by Kallmes et al2 and Buchbinder et al1 found vertebroplasty and sham vertebroplasty to be equally effective in reducing pain and improving function. However, given the limitations of each of these studies, particularly the low numbers of patients, it is difficult to establish that vertebral augmentation procedures should no longer be done. And vertebroplasty may still benefit correctly selected patients.
- Buchbinder R, Osborne RH, Ebeling PR, et a.l A randomized trial of vertebroplasty for painful osteoporotic vertebral fractures. N Engl J Med 2009; 361:557–568.
- Kallmes DF, Comstock BA, Heagerty PJ, et al. A randomized trial of vertebroplasty for osteoporotic spinal fractures. N Engl J Med 2009; 361:569–579.
- Francis RM, Aspray TJ, Hide G, Sutcliffe AM, Wilkinson P. Back pain in osteoporotic vertebral fractures. Osteoporos Int 2008; 19:895–903.
- Diamond TH, Champion B, Clark WA. Management of acute osteoporotic vertebral fractures: a nonrandomized trial comparing percutaneous vertebroplasty with conservative therapy. Am J Med 2003; 114:257–265.
- Voormolen MH, Mali WP, Lohle PN, et al. Percutaneous vertebroplasty compared with optimal pain medication treatment: short-term clinical outcome of patients with subacute or chronic painful osteoporotic vertebral compression fractures. The VERTOS study. AJNR Am J Neuroradiol 2007; 28:555–560.
- Alvarez L, Alcaraz M, Pérez-Higueras A, et al. Percutaneous vertebroplasty: functional improvement in patients with osteoporotic compression fractures. Spine (Phila PA 1976) 2006; 31:1113–1118.
- Rousing R, Andersen MO, Jespersen SM, Thomsen K, Lauritsen J. Percutaneous vertebroplasty compared to conservative treatment in patients with painful acute or subacute osteoporotic vertebral fractures: three-months follow-up in a clinical randomized study. Spine (Phila PA 1976) 2009; 34:1349–1354.
- Clark W, Lyon S, Burnes J. Trials of vertebroplasty for vertebral fractures. N Engl J Med 2009; 361:2097–2098.
- Kaufmann TJ, Jensen ME, Schweickert PA, Marx WF, Kallmes DF. Age of fracture and clinical outcomes of percutaneous vertebroplasty. AJNR Am J Neuroradiol 2001; 22:1860–1863.
- Maynard AS, Jensen ME, Schweickert PA, Marx WF, Short JG, Kallmes DF. Value of bone scan imaging in predicting pain relief from percutaneous vertebroplasty in osteoporotic vertebral fractures. AJNR Am J Neuroradiol 2000; 21:1807–1812.
- Klazen C, Verhaar H, Lampmann L, et al. VERTOS II: Percutaneous vertebroplasty versus conservative therapy in patients with painful osteoporotic vertebral compression fractures; rationale, objectives and design of a multicenter randomized controlled trial. Trials 2007; 8:33.
Confronted with the unexpected results of two trials of vertebroplasty,1,2 physicians are feeling some consternation, We had thought that percutaneous vertebroplasty helps patients with osteoporosis who sustain a painful vertebral insufficiency fracture. However, the trials found it to be no better than a sham procedure in terms of relieving pain.
How will these findings affect our practice? Should we abandon this popular procedure? Or are there other considerations that may mitigate these negative findings? And what should we tell our patients?
700,000 FRACTURES PER YEAR
Vertebral insufficiency fractures are the most common type of fracture in patients with osteoporosis. Every year in the United States, about 700,000 of them occur.
Nearly two-thirds are asymptomatic. The other one-third typically present with the acute onset of localized pain.
Vertebral insufficiency fractures often lead to chronic pain, impair the ability to walk and to perform daily activities, and accentuate thoracic kyphosis, which in turn can lead to pulmonary restrictive disease, and they raise the risk of death. Also, a patient who has a vertebral insufficiency fracture has a 20% risk of sustaining a new one within 1 year.3
Whether symptomatic or asymptomatic, finding a vertebral insufficiency fracture should prompt one to consider drug therapy for osteoporosis. In addition, until now, a patient who presented with the acute onset of back pain and whose evaluation revealed a vertebral insufficiency fracture would also be considered for a vertebral augmentation procedure, either vertebroplasty or kyphoplasty, to relieve the pain.
Vertebroplasty involves injecting polymethylmethacrylate cement percutaneously into the affected vertebral body. Kyphoplasty, a similar procedure, uses a balloon to create a cavity in the fractured vertebral body. After the balloon is withdrawn, the cavity is filled with cement.
TWO RANDOMIZED TRIALS OF SHAM VS REAL VERTEBROPLASTY
Two teams, Kallmes et al2 and Buchbinder et al,1 independently performed randomized controlled trials to see if vertebroplasty really relieves pain as well as has been reported in open studies, case series, and nonrandomized trials.4–7
In both trials, patients were randomized to undergo either sham vertebroplasty or real vertebroplasty. The sham procedure closely approximated the real procedure, including inserting a needle, infiltrating a local anesthetic, bupivacaine (Marcaine), into the periosteum of the posterior lamina1 or the pedicle of the target vertebrae,2 and opening a vial of polymethylmethacrylate so that the patient would smell the product.
Inclusion criteria
Patients in both trials had to have evidence of a recent (acute) or nonhealed vertebral insufficiency fracture.
Pain was the primary outcome measured
In both trials, the investigators assessed the patients’ pain at baseline and again at several specified intervals, using validated tools.
Kallmes et al assessed pain intensity and functional measures at 1 month (the primary outcome measured), and also at 3, 14, and 90 days and at 1 year.
Buchbinder et al assessed pain at 1 week and at 1, 3, and 6 months. The primary outcome measured was pain at 3 months. Secondary outcomes included quality-of-life measures, pain at rest, and pain at night.
Surprising results
In both trials, the mean pain scores were better than at baseline at all time points after the procedure in both the real-procedure and the sham-procedure groups. Moreover, the effect did not differ between the two treatment groups in either study.
QUESTIONS COMPLICATE THE ISSUE
These two trials should make us consider whether this intervention is warranted. We should, however, also consider some limitations of these studies that raise questions about how the conclusions should or should not alter practice.
Does local anesthetic continue to relieve pain?
In both the sham and the real procedure, the bupivacaine injection may have helped relieve pain to some extent afterward, as its anesthetic effect may last longer than we would expect from its 3-hour half-life. The effect could certainly have contributed to improvements in pain levels at the earlier time points after the procedure.
Was there selection bias?
Both studies were highly rigorous and were done at hospitals that had extensive experience with vertebroplasty. However, they may have harbored selection bias, as many more patients were screened than were randomized.
Buchbinder et al1 screened 468 patients. Of these, 30% declined to participate, and another 53% did not meet the eligibility criteria. In the end, only 78 patients were randomized.
Kallmes et al2 screened 1,813 patients, 300 of whom declined and 1,382 of whom were excluded, leaving 131 patients to be randomized. The reasons for exclusion were not specifically reported in many cases.
In both studies, it would be interesting to know how many of those who declined proceeded to undergo a vertebral augmentation procedure.
Did the trials have enough power?
In the study by Kallmes et al,2 recruitment got off to a slow start. Thus, after three patients were recruited, the inclusion requirements were liberalized. The study was originally designed to include 250 patients, which would have given it a power of greater than 80% to detect differences in primary and secondary outcomes. The design was revised to include 130 patients. The statistical power was still 80%, but this was to detect a greater difference in the outcomes than originally projected.
Had the window of opportunity already closed?
Vertebroplasty may have a window of opportunity within which it is most effective. Sooner is probably better than later, but it would be good to identify this time frame.
Kaufmann et al9 reported that patients with older fractures needed slightly more analgesic drugs after the procedure. It has been shown previously that patients who are the most likely to respond to a vertebral augmentation procedure are those with fractures that occurred between 1 and 12 months prior to the procedure and who have evidence that the fracture was recent, ie, edema on magnetic resonance imaging (MRI) or increased uptake on a bone scan.10
Other studies suggested that intervention works best in patients who have had uncontrolled pain lasting less than 6 weeks.8,11 (In the study by Buchbinder et al,1 only 32% of the patients in either group reported pain lasting less than 6 weeks.)
The study by Kallmes et al included patients whose pain had begun within 1 year previously. However, if the duration of pain (ie, the age of the fracture) was uncertain, MRI was done to look for edema, which would indicate the fracture was fresh. It is thus unclear whether all patients in this study truly had an acute or subacute fracture, since all did not undergo confirmatory MRI.
Why did so many patients cross over from sham to real treatment?
Patients in the Kallmes trial2 could cross over from one treatment group to the other as early as 1 month after the procedure. And, in fact, 43% of patients in the sham-treatment group did choose to cross over by 3 months. In contrast, after real vertebroplasty, significantly fewer—only 12% (P < .001)—crossed over to receive the sham procedure. The patients who crossed over from the sham-procedure group to receive vertebroplasty experienced an early improvement in pain, but this was not sustained at 1 or 3 months of follow-up.
The higher crossover rate in the shamprocedure group suggests they were dissatisfied with this intervention, although their outcomes were not significantly better after they got the real procedure. The patients who first received the sham treatment and elected to cross over to vertebroplasty had higher pain and disability scores at baseline. Thus, they may have had other, more chronic causes of pain or other factors affecting the likelihood of a response, particularly of a durable or sustained response.
How do the interventions compare with medical therapy?
Earlier studies showed that vertebroplasty relieves pain almost immediately.4–6 But the benefit does not last: at 6 weeks and up to 12 months later there is no difference in either pain or functional capacity reported in patients receiving vertebroplasty vs conservative treatment.4,6,7 It would thus appear that pain gradually diminishes over time after a vertebral insufficiency fracture, as the fracture heals.
The recent studies1,2 raise the possibility that the pain relief is due to the local anesthetic, not the vertebroplasty itself. We do not know, however, if either vertebroplasty or the sham procedure is superior to conservative medical management. Prospective multicenter trials are under way to address this question.11
Further complicating the issue, the two trials did not keep track of medical treatments patients were receiving concomitantly during the trial period. It is thus more difficult to compare the pain assessment outcomes following invasive procedures—real or sham.
Would kyphoplasty be better?
These studies addressed one procedure, vertebroplasty, and the results and conclusions should not be generalized to kyphoplasty. A prospective randomized trial of kyphoplasty is clearly warranted.
If kyphoplasty is found to be better than a sham procedure, then vertebroplasty should be re-examined in comparison with kyphoplasty. In any future studies, it will be important to select patients rigorously (eg, to include only patients with recent fractures), to match patients according to concomitant therapies, and to consider other potential superimposed causes of back pain in this elderly population, which has a high prevalence of back pain.
HOW SHOULD MY PRACTICE CHANGE? WHAT SHOULD I TELL PATIENTS?
Having considered the results, conclusions, and limitations of these two randomized trials, particularly in terms of recruitment, I cannot say that my practice has changed in terms of referring patients who have a vertebral compression fracture to an interventionalist. However, the education that I provide to patients has changed.
In my mind, the highest priority for a patient with a vertebral insufficiency fracture is to treat (or to reassess the current treatment of) the underlying systemic disease, ie, osteoporosis. This is especially true since most vertebral insufficiency fractures are asymptomatic.
On the other hand, a patient with a painful vertebral compression fracture needs prompt attention and consideration for interventional pain relief. Rapid pain relief is desirable. And in uncontrolled trials,4–7 vertebroplasty and kyphoplasty rapidly relieved vertebral pain. However, it may be that an anesthetic injection is equivalent to vertebroplasty and could accomplish the goal of immediate pain relief just as well.
The pain relief from sham or real vertebroplasty may not be durable, and 3 to 12 months later the pain benefit may be no greater than if more conservative therapy had been pursued.
It is essential to determine the most appropriate window for treatment as well as the most appropriate candidates on whom to perform a procedure. The recently published studies1,2 may have had significant patient selection bias and may not have optimized the window of opportunity for vertebral augmentation performance. There were many patients who declined the study, and some were excluded because of acute pain requiring hospitalization.
As a rheumatologist treating patients with osteoporosis, it is my responsibility to discuss with the patient and family the potential treatments available, to discuss the associated possible risks and benefits, to report on available evidence, and to refer patients to an appropriate interventional specialist if they desire. In light of the lack of superior pain reduction with vertebroplasty than with a sham procedure, many patients may opt for conservative therapy.
It is thus appropriate to determine the acuity of the fracture and to have a frank discussion with the patient about the options for pain management. Opiate drugs pose risks in elderly patients, particularly altered mentation, somnolence, interference with balance, and risk of falls. Vertebroplasty or anesthetic injection may rapidly relieve the pain and reduce the need for opiate therapy. Not yet subjected to the rigors of a randomized placebocontrolled trial, kyphoplasty may yet prove to be better than a sham intervention.
It is essential to determine if there is a role for vertebral augmentation in a select patient population—perhaps selected on the basis of the time that has elapsed since the fracture occurred (determined objectively), the severity of the fracture, and other factors. Perhaps a subset of patients would gain greater benefit from the procedure, whether it amounts solely to acute pain reduction or perhaps to a more durable response.
The recent studies by Kallmes et al2 and Buchbinder et al1 found vertebroplasty and sham vertebroplasty to be equally effective in reducing pain and improving function. However, given the limitations of each of these studies, particularly the low numbers of patients, it is difficult to establish that vertebral augmentation procedures should no longer be done. And vertebroplasty may still benefit correctly selected patients.
Confronted with the unexpected results of two trials of vertebroplasty,1,2 physicians are feeling some consternation, We had thought that percutaneous vertebroplasty helps patients with osteoporosis who sustain a painful vertebral insufficiency fracture. However, the trials found it to be no better than a sham procedure in terms of relieving pain.
How will these findings affect our practice? Should we abandon this popular procedure? Or are there other considerations that may mitigate these negative findings? And what should we tell our patients?
700,000 FRACTURES PER YEAR
Vertebral insufficiency fractures are the most common type of fracture in patients with osteoporosis. Every year in the United States, about 700,000 of them occur.
Nearly two-thirds are asymptomatic. The other one-third typically present with the acute onset of localized pain.
Vertebral insufficiency fractures often lead to chronic pain, impair the ability to walk and to perform daily activities, and accentuate thoracic kyphosis, which in turn can lead to pulmonary restrictive disease, and they raise the risk of death. Also, a patient who has a vertebral insufficiency fracture has a 20% risk of sustaining a new one within 1 year.3
Whether symptomatic or asymptomatic, finding a vertebral insufficiency fracture should prompt one to consider drug therapy for osteoporosis. In addition, until now, a patient who presented with the acute onset of back pain and whose evaluation revealed a vertebral insufficiency fracture would also be considered for a vertebral augmentation procedure, either vertebroplasty or kyphoplasty, to relieve the pain.
Vertebroplasty involves injecting polymethylmethacrylate cement percutaneously into the affected vertebral body. Kyphoplasty, a similar procedure, uses a balloon to create a cavity in the fractured vertebral body. After the balloon is withdrawn, the cavity is filled with cement.
TWO RANDOMIZED TRIALS OF SHAM VS REAL VERTEBROPLASTY
Two teams, Kallmes et al2 and Buchbinder et al,1 independently performed randomized controlled trials to see if vertebroplasty really relieves pain as well as has been reported in open studies, case series, and nonrandomized trials.4–7
In both trials, patients were randomized to undergo either sham vertebroplasty or real vertebroplasty. The sham procedure closely approximated the real procedure, including inserting a needle, infiltrating a local anesthetic, bupivacaine (Marcaine), into the periosteum of the posterior lamina1 or the pedicle of the target vertebrae,2 and opening a vial of polymethylmethacrylate so that the patient would smell the product.
Inclusion criteria
Patients in both trials had to have evidence of a recent (acute) or nonhealed vertebral insufficiency fracture.
Pain was the primary outcome measured
In both trials, the investigators assessed the patients’ pain at baseline and again at several specified intervals, using validated tools.
Kallmes et al assessed pain intensity and functional measures at 1 month (the primary outcome measured), and also at 3, 14, and 90 days and at 1 year.
Buchbinder et al assessed pain at 1 week and at 1, 3, and 6 months. The primary outcome measured was pain at 3 months. Secondary outcomes included quality-of-life measures, pain at rest, and pain at night.
Surprising results
In both trials, the mean pain scores were better than at baseline at all time points after the procedure in both the real-procedure and the sham-procedure groups. Moreover, the effect did not differ between the two treatment groups in either study.
QUESTIONS COMPLICATE THE ISSUE
These two trials should make us consider whether this intervention is warranted. We should, however, also consider some limitations of these studies that raise questions about how the conclusions should or should not alter practice.
Does local anesthetic continue to relieve pain?
In both the sham and the real procedure, the bupivacaine injection may have helped relieve pain to some extent afterward, as its anesthetic effect may last longer than we would expect from its 3-hour half-life. The effect could certainly have contributed to improvements in pain levels at the earlier time points after the procedure.
Was there selection bias?
Both studies were highly rigorous and were done at hospitals that had extensive experience with vertebroplasty. However, they may have harbored selection bias, as many more patients were screened than were randomized.
Buchbinder et al1 screened 468 patients. Of these, 30% declined to participate, and another 53% did not meet the eligibility criteria. In the end, only 78 patients were randomized.
Kallmes et al2 screened 1,813 patients, 300 of whom declined and 1,382 of whom were excluded, leaving 131 patients to be randomized. The reasons for exclusion were not specifically reported in many cases.
In both studies, it would be interesting to know how many of those who declined proceeded to undergo a vertebral augmentation procedure.
Did the trials have enough power?
In the study by Kallmes et al,2 recruitment got off to a slow start. Thus, after three patients were recruited, the inclusion requirements were liberalized. The study was originally designed to include 250 patients, which would have given it a power of greater than 80% to detect differences in primary and secondary outcomes. The design was revised to include 130 patients. The statistical power was still 80%, but this was to detect a greater difference in the outcomes than originally projected.
Had the window of opportunity already closed?
Vertebroplasty may have a window of opportunity within which it is most effective. Sooner is probably better than later, but it would be good to identify this time frame.
Kaufmann et al9 reported that patients with older fractures needed slightly more analgesic drugs after the procedure. It has been shown previously that patients who are the most likely to respond to a vertebral augmentation procedure are those with fractures that occurred between 1 and 12 months prior to the procedure and who have evidence that the fracture was recent, ie, edema on magnetic resonance imaging (MRI) or increased uptake on a bone scan.10
Other studies suggested that intervention works best in patients who have had uncontrolled pain lasting less than 6 weeks.8,11 (In the study by Buchbinder et al,1 only 32% of the patients in either group reported pain lasting less than 6 weeks.)
The study by Kallmes et al included patients whose pain had begun within 1 year previously. However, if the duration of pain (ie, the age of the fracture) was uncertain, MRI was done to look for edema, which would indicate the fracture was fresh. It is thus unclear whether all patients in this study truly had an acute or subacute fracture, since all did not undergo confirmatory MRI.
Why did so many patients cross over from sham to real treatment?
Patients in the Kallmes trial2 could cross over from one treatment group to the other as early as 1 month after the procedure. And, in fact, 43% of patients in the sham-treatment group did choose to cross over by 3 months. In contrast, after real vertebroplasty, significantly fewer—only 12% (P < .001)—crossed over to receive the sham procedure. The patients who crossed over from the sham-procedure group to receive vertebroplasty experienced an early improvement in pain, but this was not sustained at 1 or 3 months of follow-up.
The higher crossover rate in the shamprocedure group suggests they were dissatisfied with this intervention, although their outcomes were not significantly better after they got the real procedure. The patients who first received the sham treatment and elected to cross over to vertebroplasty had higher pain and disability scores at baseline. Thus, they may have had other, more chronic causes of pain or other factors affecting the likelihood of a response, particularly of a durable or sustained response.
How do the interventions compare with medical therapy?
Earlier studies showed that vertebroplasty relieves pain almost immediately.4–6 But the benefit does not last: at 6 weeks and up to 12 months later there is no difference in either pain or functional capacity reported in patients receiving vertebroplasty vs conservative treatment.4,6,7 It would thus appear that pain gradually diminishes over time after a vertebral insufficiency fracture, as the fracture heals.
The recent studies1,2 raise the possibility that the pain relief is due to the local anesthetic, not the vertebroplasty itself. We do not know, however, if either vertebroplasty or the sham procedure is superior to conservative medical management. Prospective multicenter trials are under way to address this question.11
Further complicating the issue, the two trials did not keep track of medical treatments patients were receiving concomitantly during the trial period. It is thus more difficult to compare the pain assessment outcomes following invasive procedures—real or sham.
Would kyphoplasty be better?
These studies addressed one procedure, vertebroplasty, and the results and conclusions should not be generalized to kyphoplasty. A prospective randomized trial of kyphoplasty is clearly warranted.
If kyphoplasty is found to be better than a sham procedure, then vertebroplasty should be re-examined in comparison with kyphoplasty. In any future studies, it will be important to select patients rigorously (eg, to include only patients with recent fractures), to match patients according to concomitant therapies, and to consider other potential superimposed causes of back pain in this elderly population, which has a high prevalence of back pain.
HOW SHOULD MY PRACTICE CHANGE? WHAT SHOULD I TELL PATIENTS?
Having considered the results, conclusions, and limitations of these two randomized trials, particularly in terms of recruitment, I cannot say that my practice has changed in terms of referring patients who have a vertebral compression fracture to an interventionalist. However, the education that I provide to patients has changed.
In my mind, the highest priority for a patient with a vertebral insufficiency fracture is to treat (or to reassess the current treatment of) the underlying systemic disease, ie, osteoporosis. This is especially true since most vertebral insufficiency fractures are asymptomatic.
On the other hand, a patient with a painful vertebral compression fracture needs prompt attention and consideration for interventional pain relief. Rapid pain relief is desirable. And in uncontrolled trials,4–7 vertebroplasty and kyphoplasty rapidly relieved vertebral pain. However, it may be that an anesthetic injection is equivalent to vertebroplasty and could accomplish the goal of immediate pain relief just as well.
The pain relief from sham or real vertebroplasty may not be durable, and 3 to 12 months later the pain benefit may be no greater than if more conservative therapy had been pursued.
It is essential to determine the most appropriate window for treatment as well as the most appropriate candidates on whom to perform a procedure. The recently published studies1,2 may have had significant patient selection bias and may not have optimized the window of opportunity for vertebral augmentation performance. There were many patients who declined the study, and some were excluded because of acute pain requiring hospitalization.
As a rheumatologist treating patients with osteoporosis, it is my responsibility to discuss with the patient and family the potential treatments available, to discuss the associated possible risks and benefits, to report on available evidence, and to refer patients to an appropriate interventional specialist if they desire. In light of the lack of superior pain reduction with vertebroplasty than with a sham procedure, many patients may opt for conservative therapy.
It is thus appropriate to determine the acuity of the fracture and to have a frank discussion with the patient about the options for pain management. Opiate drugs pose risks in elderly patients, particularly altered mentation, somnolence, interference with balance, and risk of falls. Vertebroplasty or anesthetic injection may rapidly relieve the pain and reduce the need for opiate therapy. Not yet subjected to the rigors of a randomized placebocontrolled trial, kyphoplasty may yet prove to be better than a sham intervention.
It is essential to determine if there is a role for vertebral augmentation in a select patient population—perhaps selected on the basis of the time that has elapsed since the fracture occurred (determined objectively), the severity of the fracture, and other factors. Perhaps a subset of patients would gain greater benefit from the procedure, whether it amounts solely to acute pain reduction or perhaps to a more durable response.
The recent studies by Kallmes et al2 and Buchbinder et al1 found vertebroplasty and sham vertebroplasty to be equally effective in reducing pain and improving function. However, given the limitations of each of these studies, particularly the low numbers of patients, it is difficult to establish that vertebral augmentation procedures should no longer be done. And vertebroplasty may still benefit correctly selected patients.
- Buchbinder R, Osborne RH, Ebeling PR, et a.l A randomized trial of vertebroplasty for painful osteoporotic vertebral fractures. N Engl J Med 2009; 361:557–568.
- Kallmes DF, Comstock BA, Heagerty PJ, et al. A randomized trial of vertebroplasty for osteoporotic spinal fractures. N Engl J Med 2009; 361:569–579.
- Francis RM, Aspray TJ, Hide G, Sutcliffe AM, Wilkinson P. Back pain in osteoporotic vertebral fractures. Osteoporos Int 2008; 19:895–903.
- Diamond TH, Champion B, Clark WA. Management of acute osteoporotic vertebral fractures: a nonrandomized trial comparing percutaneous vertebroplasty with conservative therapy. Am J Med 2003; 114:257–265.
- Voormolen MH, Mali WP, Lohle PN, et al. Percutaneous vertebroplasty compared with optimal pain medication treatment: short-term clinical outcome of patients with subacute or chronic painful osteoporotic vertebral compression fractures. The VERTOS study. AJNR Am J Neuroradiol 2007; 28:555–560.
- Alvarez L, Alcaraz M, Pérez-Higueras A, et al. Percutaneous vertebroplasty: functional improvement in patients with osteoporotic compression fractures. Spine (Phila PA 1976) 2006; 31:1113–1118.
- Rousing R, Andersen MO, Jespersen SM, Thomsen K, Lauritsen J. Percutaneous vertebroplasty compared to conservative treatment in patients with painful acute or subacute osteoporotic vertebral fractures: three-months follow-up in a clinical randomized study. Spine (Phila PA 1976) 2009; 34:1349–1354.
- Clark W, Lyon S, Burnes J. Trials of vertebroplasty for vertebral fractures. N Engl J Med 2009; 361:2097–2098.
- Kaufmann TJ, Jensen ME, Schweickert PA, Marx WF, Kallmes DF. Age of fracture and clinical outcomes of percutaneous vertebroplasty. AJNR Am J Neuroradiol 2001; 22:1860–1863.
- Maynard AS, Jensen ME, Schweickert PA, Marx WF, Short JG, Kallmes DF. Value of bone scan imaging in predicting pain relief from percutaneous vertebroplasty in osteoporotic vertebral fractures. AJNR Am J Neuroradiol 2000; 21:1807–1812.
- Klazen C, Verhaar H, Lampmann L, et al. VERTOS II: Percutaneous vertebroplasty versus conservative therapy in patients with painful osteoporotic vertebral compression fractures; rationale, objectives and design of a multicenter randomized controlled trial. Trials 2007; 8:33.
- Buchbinder R, Osborne RH, Ebeling PR, et a.l A randomized trial of vertebroplasty for painful osteoporotic vertebral fractures. N Engl J Med 2009; 361:557–568.
- Kallmes DF, Comstock BA, Heagerty PJ, et al. A randomized trial of vertebroplasty for osteoporotic spinal fractures. N Engl J Med 2009; 361:569–579.
- Francis RM, Aspray TJ, Hide G, Sutcliffe AM, Wilkinson P. Back pain in osteoporotic vertebral fractures. Osteoporos Int 2008; 19:895–903.
- Diamond TH, Champion B, Clark WA. Management of acute osteoporotic vertebral fractures: a nonrandomized trial comparing percutaneous vertebroplasty with conservative therapy. Am J Med 2003; 114:257–265.
- Voormolen MH, Mali WP, Lohle PN, et al. Percutaneous vertebroplasty compared with optimal pain medication treatment: short-term clinical outcome of patients with subacute or chronic painful osteoporotic vertebral compression fractures. The VERTOS study. AJNR Am J Neuroradiol 2007; 28:555–560.
- Alvarez L, Alcaraz M, Pérez-Higueras A, et al. Percutaneous vertebroplasty: functional improvement in patients with osteoporotic compression fractures. Spine (Phila PA 1976) 2006; 31:1113–1118.
- Rousing R, Andersen MO, Jespersen SM, Thomsen K, Lauritsen J. Percutaneous vertebroplasty compared to conservative treatment in patients with painful acute or subacute osteoporotic vertebral fractures: three-months follow-up in a clinical randomized study. Spine (Phila PA 1976) 2009; 34:1349–1354.
- Clark W, Lyon S, Burnes J. Trials of vertebroplasty for vertebral fractures. N Engl J Med 2009; 361:2097–2098.
- Kaufmann TJ, Jensen ME, Schweickert PA, Marx WF, Kallmes DF. Age of fracture and clinical outcomes of percutaneous vertebroplasty. AJNR Am J Neuroradiol 2001; 22:1860–1863.
- Maynard AS, Jensen ME, Schweickert PA, Marx WF, Short JG, Kallmes DF. Value of bone scan imaging in predicting pain relief from percutaneous vertebroplasty in osteoporotic vertebral fractures. AJNR Am J Neuroradiol 2000; 21:1807–1812.
- Klazen C, Verhaar H, Lampmann L, et al. VERTOS II: Percutaneous vertebroplasty versus conservative therapy in patients with painful osteoporotic vertebral compression fractures; rationale, objectives and design of a multicenter randomized controlled trial. Trials 2007; 8:33.
Vertebroplasty, cognitive dissonance, and evidence-based medicine: What do we do when the ‘evidence’ says we are wrong?
Cognitive dissonance describes how we respond to conflicting information that challenges our existing belief, the uncomfortable feeling we get when new evidence calls into question things that we “know” are true.
To the point: two recent clinical trials1,2 have called into question the efficacy of vertebroplasty for treating osteoporotic vertebral compression fractures and have led many of us to question many of our assumptions, not only about vertebroplasty but also about evidencebased medicine.
Osteoporotic vertebral compression fractures are very common: more than 700,000 are estimated to occur in the United States annually. 3 They are costly and are associated with a risk of death.4 Fortunately, most heal without problems over 4 to 6 weeks with conventional treatment, ie, activity modification, analgesics, and bracing.
However, some patients do not seem to do so well and are debilitated by the pain of the fracture. Conventional fracture surgery carries very high risk and poor outcomes,5 and so has been reserved mostly for patients with neurologic deficits.
VERTEBROPLASTY GOES MAINSTREAM
Given these facts, investigators began looking for alternative treatments. One that rose to the fore was polymethylmethacrylate cement to stabilize the fracture. This technique, called vertebroplasty, involves injecting liquid cement through a needle into the vertebral body, where it hardens and is thought to restore stability.
Since the first description of vertebroplasty for treating symptomatic hemangiomas,6 many papers have been published about the procedure and about similar ones, now grouped under the general heading of vertebral augmentation. This includes kyphoplasty and other newer proprietary techniques. These procedures have been widely accepted, and their use is growing. They have shown good results in several prospective case series, and nonrandomized and randomized controlled studies have shown them to be more effective than conventional medical treatment.7–25 For example, VERTOS, a small prospective randomized trial, showed that vertebroplasty was superior to conventional medical treatment.25 When Wardlaw et al24 showed that shortterm outcomes were better with kyphoplasty than with conventional medical therapy in a prospective randomized trial, many of us had moved past questioning whether vertebral augmentation is effective and were debating the relative merits of different methods and materials.
On a personal level, most of us became proponents of these procedures because we saw dramatic results—usually unequivocal. Most patients report significant improvement in pain immediately after the procedure, and many bedridden patients are able to leave the hospital within hours. In spine surgery, few procedures give such dramatic results with so few complications.
TWO NEW STUDIES UPSET ESTABLISHED BELIEF
This is why I am having such a hard time digesting the results of the trials by Buchbinder et al2 and Kallmes et al,1 published in the August 9, 2009, issue of the New England Journal of Medicine. Both were randomized controlled trials that used sham surgery rather than conventional medical treatment as the control. The sham procedure in each trial was the same as the intervention, with local anesthetic infiltration of the periosteum and mixing of the cement (so that the patients smelled its distinctive odor), but without placing the needle into the vertebra and injecting the cement.
In the study by Buchbinder et al,2 the real treatment had no benefit in any primary or secondary end point. This study did not allow crossovers.
In the study by Kallmes et al,1 more patients who received the real treatment reported clinically meaningful improvement in pain (a secondary end point), but the difference was not quite statistically significant (64% vs 48%, P = .06). In this trial, patients were allowed to cross over to the other study group after 1 month, and significantly more patients crossed over from the sham surgery group to the active treatment group than the other way around (43% vs 12%, P < .001).
My first instinct was to pick through the papers for flaws that would invalidate the results— and there were some problems. Both studies were initially planned to include more patients and therefore to have greater statistical power, but they were reassessed because of slow enrollment. In the study by Kallmes et al,1 the difference in clinically meaningful improvement might have reached statistical significance if the trial had been larger. The study by Buchbinder et al2 was a multicenter trial, but one center accounted for 53 (69%) of the 78 patients. Could this have biased the results?
The surgeon in me also seized for a while on the idea that since all of the interventions in both studies were done by interventional radiologists, the problem may have been in patient selection and that radiologists are not as astute as we are. However, even a surgeon’s ego cannot support this interpretation.
As I looked in more detail at the response I had written to these trials, I realized these criticisms were hardly fatal flaws, and the fact that two separate well-designed studies reached the same conclusion enhances their validity.
One concern that does bear some scrutiny is that the trials were too small to identify subgroups that may benefit from the procedure. In my experience, vertebral augmentation seems to have better results with certain types of fractures. Patients with a mobile pseudarthrotic cleft pattern of fracture seem to do much better than those with the more common nonmobile fracture.
THE POWERFUL PLACEBO EFFECT
Many commentaries on these two trials have discussed a famous study of a different procedure for a different condition. In this study, Moseley et al26 evaluated the use of arthroscopy to treat osteoarthritis of the knee and found that sham arthroscopy was as effective as real arthroscopy and that both were better than conventional treatment.
I was not long out of my orthopedic residency when this trial was published and was very aware of the debate that preceded it, as I once had to prepare a talk about it for resident rounds. I remember that there was a lively debate in the orthopedic community over the efficacy of the procedure before the results of this trial were released.
In contrast, the vertebral augmentation controversy had become a debate about the relative efficacy and the economics of specific techniques, not about the effectiveness of the entire concept. The mainstream had accepted the validity of the procedure, which was not the case in the knee arthroscopy trial.
In both vertebroplasty studies, the activetreatment groups and the sham-treatment groups all showed significant and rapid improvement in pain and disability, and these results were maintained over the study period. Though most vertebral compression fractures do heal, the clinical improvement is usually gradual over a period of weeks. This raises the possibility that the sham treatment was actualy an active placebo.
There is some evidence to support this possibility. In a randomized trial of the efficacy of selective nerve root blocks for lumbar radiculopathy, Riew et al27 showed that injection with a local anesthetic alone, although not as efficacious as a local anesthetic plus a corticosteroid at allowing patients to avoid surgery, showed an effect long after the expected duration of the anesthetic. The effect persisted even at 5 years of follow-up.28
Is it possible that the local anesthetic in this trial and the vertebroplasty trials acted as some sort of “reset button” for pain sensation? This is an area that may bear further investigation.
WHERE DOES THIS LEAVE US?
So where does this leave us? On one hand, randomized controlled trials comparing vertebral augmentation with conventional medical therapy24,25 showed augmentation to be beneficial. On the other hand, the studies by Kallmes et al1 and Buchbinder et al2 indicate vertebroplasty is no more effective than sham surgery.
It is very difficult for me to look at my own experience with vertebral augmentation and say that, on the basis of these trials, I am no longer going to offer it to my patients. I understand on an intellectual level that these trials call the efficacy of the procedure into question, but on a visceral level I cannot rationalize it. When faced with a patient who is barely ambulatory or in fact bed-bound due to pain, my experience tells me that vertebral augmentation has a very high chance of getting them ambulatory within hours. The trials of vertebroplasty would indicate this is a placebo effect or that local anesthetic alone is as effective, but I am not yet ready to make that leap.
Cognitive dissonance seems to rule.
- Kallmes DF, Comstock BA, Heagerty PJ, et al. A randomized trial of vertebroplasty for osteoporotic spinal fractures. N Engl J Med 2009; 361:569–579.
- Buchbinder R, Osborne RH, Ebeling PR, et al. A randomized trial of vertebroplasty for painful osteoporotic vertebral fractures. N Engl J Med 2009; 361:557–568.
- Carmona RH. Department of Health and Human Services. Office of the Surgeon General. Bone health and osteoporosis: A report of the Surgeon General (2004). www.surgeongeneral.gov/library/bonehealth/content.html. Accessed November 16, 2009.
- Kado DM, Browner WS, Palermo L, Nevitt MC, Genant HK, Cummings SR. Vertebral fractures and mortality in older women: a prospective study. Study of Osteoporotic Fractures Research Group. Arch Intern Med 1999; 159:1215–1220.
- Hu SS. Internal fixation in the osteoporotic spine. Spine (Phila PA 1976) 1997; 22( suppl 24):43S–48S.
- Galibert P, Deramond H, Rosat P, Le Gars D. Preliminary note on the treatment of vertebral angioma by percutaneous acrylic vertebroplasty. Neurochirurgie 1987; 33:166–168.
- Kasperk C, Hillmeier J, Noldge G, et al. Treatment of painful vertebral fractures by kyphoplasty in patients with primary osteoporosis: a prospective nonrandomized controlled study. J Bone Miner Res 2005; 20:604–612.
- Komp M, Ruetten S, Godolias G. Minimally invasive therapy for functionally unstable osteoporotic vertebral fracture by means of kyphoplasty: prospective comparative study of 19 surgically and 17 conservatively treated patients. J Miner Stoffwechs 2004; 11( suppl 1):13–15.
- Coumans JV, Reinhardt MK, Lieberman IH. Kyphoplasty for vertebral compression fractures: 1-year clinical outcomes from a prospective study. J Neurosurg 2003; 99 (suppl 1):44–50.
- Lieberman IH, Dudeney S, Reinhardt MK, Bell G. Initial outcome and efficacy of ‘kyphoplasty’ in the treatment of painful osteoporotic vertebral compression fractures. Spine (Phila PA 1976) 2001; 26:1631–1638.
- Garfin SR, Buckley RA, Ledlie J; Balloon Kyphoplasty Outcomes Group. Balloon kyphoplasty for symptomatic vertebral body compression fractures results in rapid, significant, and sustained improvements in back pain, function, and quality of life for elderly patients. Spine (Phila PA 1976) 2006; 31:2213–2220.
- Ledlie JT, Renfro MB. Kyphoplasty treatment of vertebral fractures: 2-year outcomes show sustained benefits. Spine (Phila PA 1976) 2006; 31:57–64.
- Majd ME, Farley S, Holt RT. Preliminary outcomes and efficacy of the first 360 consecutive kyphoplasties for the treatment of painful osteoporotic vertebral compression fractures. Spine J 2005; 5:244–255.
- Rhyne A, Banit D, Laxer E, Odum S, Nussman D. Kyphoplasty: report of eighty-two thoracolumbar osteoporotic vertebral fractures. J Orthop Trauma 2004; 18:294–299.
- Theodorou DJ, Theodorou SJ, Duncan TD, Garfin SR, Wong WH. Percutaneous balloon kyphoplasty for the correction of spinal deformity in painful vertebral body compression fractures. Clin Imaging 2002; 26:1–5.
- Berlemann U, Franz T, Orler R, Heini PF. Kyphoplasty for treatment of osteoporotic vertebral fractures: a prospective nonrandomized study. Eur Spine J 2004; 13:496–501.
- McGraw JK, Lippert JA, Minkus KD, Rami PM, Davis TM, Budzik RF. Prospective evaluation of pain relief in 100 patients undergoing percutaneous vertebroplasty: results and followup. J Vasc Interv Radiol 2002; 13:883–886.
- Zoarski GH, Snow P, Olan WJ, et al. Percutaneous vertebroplasty for osteoporotic compression fractures: quantitative prospective evaluation of long-term outcomes. J Vasc Interv Radiol 2002; 13:139–148.
- Evans AJ, Jensen ME, Kip KE, et al. Vertebral compression fractures: pain reduction and improvement in functional mobility after percutaneous polymethylmethacrylate vertebroplasty retrospective report of 245 cases. Radiology 2003; 226:366–372.
- Grohs JG, Matzner M, Trieb K, Krepler P. Minimal invasive stabilization of osteoporotic vertebral fractures: a prospective nonrandomized comparison of vertebroplasty and balloon kyphoplasty. J Spinal Disord Tech 2005; 18:238–242.
- Kallmes DF, Schweickert PA, Marx WF, Jensen ME. Vertebroplasty in the mid-and upper thoracic spine. AJNR Am J Neuroradiol 2002; 23:1117–1120.
- Grados F, Depriester C, Cayrolle G, Hardy N, Deramond H, Fardellone P. Long-term observations of vertebral osteoporotic fractures treated by percutaneous vertebroplasty. Rheumatology (Oxford) 2000; 39:1410–1414.
- Legroux-Gérot I, Lormeau C, Boutry N, Cotten A, Duquesnoy B, Cortet B. Long-term follow-up of vertebral osteoporotic fractures treated by percutaneous vertebroplasty. Clin Rheumatol 2004; 23:310–317.
- Wardlaw D, Cummings SR, Van Meirhaeghe J, et al. Efficacy and safety of balloon kyphoplasty compared with nonsurgical care for vertebral compression fracture (FREE): a randomised controlled trial. Lancet 2009; 373:1016–1024.
- Voormolen MH, Mali WP, Lohle PN, et al. Percutaneous vertebroplasty compared with optimal pain medication treatment: short-term outcomes of patients with subacute or chronic painful osteoporotic vertebral compression fractures. The VERTOS study. AJNR Am J Neuroradiol 2007: 28:555–560.
- Moseley JB, O'Malley K, Petersen NJ. A controlled trial of arthroscopic surgery for osteoarthritis of the knee. N Engl J Med 2002; 347:81–88.
- Riew KD, Yin Y, Gilula L, et al. The effect of nerve-root injections on the need for operative treatment of lumbar radicular pain. A prospective, randomized, controlled, double-blind study. J Bone Joint Surg Am 2000; 82–A:1589–1593.
- Riew KD, Park JB, Cho YS, et al. Nerve root blocks in the treatment of lumbar radicular pain. A minimum five-year follow-up. J Bone Joint Surg Am 2006; 88:1722–1725.
Cognitive dissonance describes how we respond to conflicting information that challenges our existing belief, the uncomfortable feeling we get when new evidence calls into question things that we “know” are true.
To the point: two recent clinical trials1,2 have called into question the efficacy of vertebroplasty for treating osteoporotic vertebral compression fractures and have led many of us to question many of our assumptions, not only about vertebroplasty but also about evidencebased medicine.
Osteoporotic vertebral compression fractures are very common: more than 700,000 are estimated to occur in the United States annually. 3 They are costly and are associated with a risk of death.4 Fortunately, most heal without problems over 4 to 6 weeks with conventional treatment, ie, activity modification, analgesics, and bracing.
However, some patients do not seem to do so well and are debilitated by the pain of the fracture. Conventional fracture surgery carries very high risk and poor outcomes,5 and so has been reserved mostly for patients with neurologic deficits.
VERTEBROPLASTY GOES MAINSTREAM
Given these facts, investigators began looking for alternative treatments. One that rose to the fore was polymethylmethacrylate cement to stabilize the fracture. This technique, called vertebroplasty, involves injecting liquid cement through a needle into the vertebral body, where it hardens and is thought to restore stability.
Since the first description of vertebroplasty for treating symptomatic hemangiomas,6 many papers have been published about the procedure and about similar ones, now grouped under the general heading of vertebral augmentation. This includes kyphoplasty and other newer proprietary techniques. These procedures have been widely accepted, and their use is growing. They have shown good results in several prospective case series, and nonrandomized and randomized controlled studies have shown them to be more effective than conventional medical treatment.7–25 For example, VERTOS, a small prospective randomized trial, showed that vertebroplasty was superior to conventional medical treatment.25 When Wardlaw et al24 showed that shortterm outcomes were better with kyphoplasty than with conventional medical therapy in a prospective randomized trial, many of us had moved past questioning whether vertebral augmentation is effective and were debating the relative merits of different methods and materials.
On a personal level, most of us became proponents of these procedures because we saw dramatic results—usually unequivocal. Most patients report significant improvement in pain immediately after the procedure, and many bedridden patients are able to leave the hospital within hours. In spine surgery, few procedures give such dramatic results with so few complications.
TWO NEW STUDIES UPSET ESTABLISHED BELIEF
This is why I am having such a hard time digesting the results of the trials by Buchbinder et al2 and Kallmes et al,1 published in the August 9, 2009, issue of the New England Journal of Medicine. Both were randomized controlled trials that used sham surgery rather than conventional medical treatment as the control. The sham procedure in each trial was the same as the intervention, with local anesthetic infiltration of the periosteum and mixing of the cement (so that the patients smelled its distinctive odor), but without placing the needle into the vertebra and injecting the cement.
In the study by Buchbinder et al,2 the real treatment had no benefit in any primary or secondary end point. This study did not allow crossovers.
In the study by Kallmes et al,1 more patients who received the real treatment reported clinically meaningful improvement in pain (a secondary end point), but the difference was not quite statistically significant (64% vs 48%, P = .06). In this trial, patients were allowed to cross over to the other study group after 1 month, and significantly more patients crossed over from the sham surgery group to the active treatment group than the other way around (43% vs 12%, P < .001).
My first instinct was to pick through the papers for flaws that would invalidate the results— and there were some problems. Both studies were initially planned to include more patients and therefore to have greater statistical power, but they were reassessed because of slow enrollment. In the study by Kallmes et al,1 the difference in clinically meaningful improvement might have reached statistical significance if the trial had been larger. The study by Buchbinder et al2 was a multicenter trial, but one center accounted for 53 (69%) of the 78 patients. Could this have biased the results?
The surgeon in me also seized for a while on the idea that since all of the interventions in both studies were done by interventional radiologists, the problem may have been in patient selection and that radiologists are not as astute as we are. However, even a surgeon’s ego cannot support this interpretation.
As I looked in more detail at the response I had written to these trials, I realized these criticisms were hardly fatal flaws, and the fact that two separate well-designed studies reached the same conclusion enhances their validity.
One concern that does bear some scrutiny is that the trials were too small to identify subgroups that may benefit from the procedure. In my experience, vertebral augmentation seems to have better results with certain types of fractures. Patients with a mobile pseudarthrotic cleft pattern of fracture seem to do much better than those with the more common nonmobile fracture.
THE POWERFUL PLACEBO EFFECT
Many commentaries on these two trials have discussed a famous study of a different procedure for a different condition. In this study, Moseley et al26 evaluated the use of arthroscopy to treat osteoarthritis of the knee and found that sham arthroscopy was as effective as real arthroscopy and that both were better than conventional treatment.
I was not long out of my orthopedic residency when this trial was published and was very aware of the debate that preceded it, as I once had to prepare a talk about it for resident rounds. I remember that there was a lively debate in the orthopedic community over the efficacy of the procedure before the results of this trial were released.
In contrast, the vertebral augmentation controversy had become a debate about the relative efficacy and the economics of specific techniques, not about the effectiveness of the entire concept. The mainstream had accepted the validity of the procedure, which was not the case in the knee arthroscopy trial.
In both vertebroplasty studies, the activetreatment groups and the sham-treatment groups all showed significant and rapid improvement in pain and disability, and these results were maintained over the study period. Though most vertebral compression fractures do heal, the clinical improvement is usually gradual over a period of weeks. This raises the possibility that the sham treatment was actualy an active placebo.
There is some evidence to support this possibility. In a randomized trial of the efficacy of selective nerve root blocks for lumbar radiculopathy, Riew et al27 showed that injection with a local anesthetic alone, although not as efficacious as a local anesthetic plus a corticosteroid at allowing patients to avoid surgery, showed an effect long after the expected duration of the anesthetic. The effect persisted even at 5 years of follow-up.28
Is it possible that the local anesthetic in this trial and the vertebroplasty trials acted as some sort of “reset button” for pain sensation? This is an area that may bear further investigation.
WHERE DOES THIS LEAVE US?
So where does this leave us? On one hand, randomized controlled trials comparing vertebral augmentation with conventional medical therapy24,25 showed augmentation to be beneficial. On the other hand, the studies by Kallmes et al1 and Buchbinder et al2 indicate vertebroplasty is no more effective than sham surgery.
It is very difficult for me to look at my own experience with vertebral augmentation and say that, on the basis of these trials, I am no longer going to offer it to my patients. I understand on an intellectual level that these trials call the efficacy of the procedure into question, but on a visceral level I cannot rationalize it. When faced with a patient who is barely ambulatory or in fact bed-bound due to pain, my experience tells me that vertebral augmentation has a very high chance of getting them ambulatory within hours. The trials of vertebroplasty would indicate this is a placebo effect or that local anesthetic alone is as effective, but I am not yet ready to make that leap.
Cognitive dissonance seems to rule.
Cognitive dissonance describes how we respond to conflicting information that challenges our existing belief, the uncomfortable feeling we get when new evidence calls into question things that we “know” are true.
To the point: two recent clinical trials1,2 have called into question the efficacy of vertebroplasty for treating osteoporotic vertebral compression fractures and have led many of us to question many of our assumptions, not only about vertebroplasty but also about evidencebased medicine.
Osteoporotic vertebral compression fractures are very common: more than 700,000 are estimated to occur in the United States annually. 3 They are costly and are associated with a risk of death.4 Fortunately, most heal without problems over 4 to 6 weeks with conventional treatment, ie, activity modification, analgesics, and bracing.
However, some patients do not seem to do so well and are debilitated by the pain of the fracture. Conventional fracture surgery carries very high risk and poor outcomes,5 and so has been reserved mostly for patients with neurologic deficits.
VERTEBROPLASTY GOES MAINSTREAM
Given these facts, investigators began looking for alternative treatments. One that rose to the fore was polymethylmethacrylate cement to stabilize the fracture. This technique, called vertebroplasty, involves injecting liquid cement through a needle into the vertebral body, where it hardens and is thought to restore stability.
Since the first description of vertebroplasty for treating symptomatic hemangiomas,6 many papers have been published about the procedure and about similar ones, now grouped under the general heading of vertebral augmentation. This includes kyphoplasty and other newer proprietary techniques. These procedures have been widely accepted, and their use is growing. They have shown good results in several prospective case series, and nonrandomized and randomized controlled studies have shown them to be more effective than conventional medical treatment.7–25 For example, VERTOS, a small prospective randomized trial, showed that vertebroplasty was superior to conventional medical treatment.25 When Wardlaw et al24 showed that shortterm outcomes were better with kyphoplasty than with conventional medical therapy in a prospective randomized trial, many of us had moved past questioning whether vertebral augmentation is effective and were debating the relative merits of different methods and materials.
On a personal level, most of us became proponents of these procedures because we saw dramatic results—usually unequivocal. Most patients report significant improvement in pain immediately after the procedure, and many bedridden patients are able to leave the hospital within hours. In spine surgery, few procedures give such dramatic results with so few complications.
TWO NEW STUDIES UPSET ESTABLISHED BELIEF
This is why I am having such a hard time digesting the results of the trials by Buchbinder et al2 and Kallmes et al,1 published in the August 9, 2009, issue of the New England Journal of Medicine. Both were randomized controlled trials that used sham surgery rather than conventional medical treatment as the control. The sham procedure in each trial was the same as the intervention, with local anesthetic infiltration of the periosteum and mixing of the cement (so that the patients smelled its distinctive odor), but without placing the needle into the vertebra and injecting the cement.
In the study by Buchbinder et al,2 the real treatment had no benefit in any primary or secondary end point. This study did not allow crossovers.
In the study by Kallmes et al,1 more patients who received the real treatment reported clinically meaningful improvement in pain (a secondary end point), but the difference was not quite statistically significant (64% vs 48%, P = .06). In this trial, patients were allowed to cross over to the other study group after 1 month, and significantly more patients crossed over from the sham surgery group to the active treatment group than the other way around (43% vs 12%, P < .001).
My first instinct was to pick through the papers for flaws that would invalidate the results— and there were some problems. Both studies were initially planned to include more patients and therefore to have greater statistical power, but they were reassessed because of slow enrollment. In the study by Kallmes et al,1 the difference in clinically meaningful improvement might have reached statistical significance if the trial had been larger. The study by Buchbinder et al2 was a multicenter trial, but one center accounted for 53 (69%) of the 78 patients. Could this have biased the results?
The surgeon in me also seized for a while on the idea that since all of the interventions in both studies were done by interventional radiologists, the problem may have been in patient selection and that radiologists are not as astute as we are. However, even a surgeon’s ego cannot support this interpretation.
As I looked in more detail at the response I had written to these trials, I realized these criticisms were hardly fatal flaws, and the fact that two separate well-designed studies reached the same conclusion enhances their validity.
One concern that does bear some scrutiny is that the trials were too small to identify subgroups that may benefit from the procedure. In my experience, vertebral augmentation seems to have better results with certain types of fractures. Patients with a mobile pseudarthrotic cleft pattern of fracture seem to do much better than those with the more common nonmobile fracture.
THE POWERFUL PLACEBO EFFECT
Many commentaries on these two trials have discussed a famous study of a different procedure for a different condition. In this study, Moseley et al26 evaluated the use of arthroscopy to treat osteoarthritis of the knee and found that sham arthroscopy was as effective as real arthroscopy and that both were better than conventional treatment.
I was not long out of my orthopedic residency when this trial was published and was very aware of the debate that preceded it, as I once had to prepare a talk about it for resident rounds. I remember that there was a lively debate in the orthopedic community over the efficacy of the procedure before the results of this trial were released.
In contrast, the vertebral augmentation controversy had become a debate about the relative efficacy and the economics of specific techniques, not about the effectiveness of the entire concept. The mainstream had accepted the validity of the procedure, which was not the case in the knee arthroscopy trial.
In both vertebroplasty studies, the activetreatment groups and the sham-treatment groups all showed significant and rapid improvement in pain and disability, and these results were maintained over the study period. Though most vertebral compression fractures do heal, the clinical improvement is usually gradual over a period of weeks. This raises the possibility that the sham treatment was actualy an active placebo.
There is some evidence to support this possibility. In a randomized trial of the efficacy of selective nerve root blocks for lumbar radiculopathy, Riew et al27 showed that injection with a local anesthetic alone, although not as efficacious as a local anesthetic plus a corticosteroid at allowing patients to avoid surgery, showed an effect long after the expected duration of the anesthetic. The effect persisted even at 5 years of follow-up.28
Is it possible that the local anesthetic in this trial and the vertebroplasty trials acted as some sort of “reset button” for pain sensation? This is an area that may bear further investigation.
WHERE DOES THIS LEAVE US?
So where does this leave us? On one hand, randomized controlled trials comparing vertebral augmentation with conventional medical therapy24,25 showed augmentation to be beneficial. On the other hand, the studies by Kallmes et al1 and Buchbinder et al2 indicate vertebroplasty is no more effective than sham surgery.
It is very difficult for me to look at my own experience with vertebral augmentation and say that, on the basis of these trials, I am no longer going to offer it to my patients. I understand on an intellectual level that these trials call the efficacy of the procedure into question, but on a visceral level I cannot rationalize it. When faced with a patient who is barely ambulatory or in fact bed-bound due to pain, my experience tells me that vertebral augmentation has a very high chance of getting them ambulatory within hours. The trials of vertebroplasty would indicate this is a placebo effect or that local anesthetic alone is as effective, but I am not yet ready to make that leap.
Cognitive dissonance seems to rule.
- Kallmes DF, Comstock BA, Heagerty PJ, et al. A randomized trial of vertebroplasty for osteoporotic spinal fractures. N Engl J Med 2009; 361:569–579.
- Buchbinder R, Osborne RH, Ebeling PR, et al. A randomized trial of vertebroplasty for painful osteoporotic vertebral fractures. N Engl J Med 2009; 361:557–568.
- Carmona RH. Department of Health and Human Services. Office of the Surgeon General. Bone health and osteoporosis: A report of the Surgeon General (2004). www.surgeongeneral.gov/library/bonehealth/content.html. Accessed November 16, 2009.
- Kado DM, Browner WS, Palermo L, Nevitt MC, Genant HK, Cummings SR. Vertebral fractures and mortality in older women: a prospective study. Study of Osteoporotic Fractures Research Group. Arch Intern Med 1999; 159:1215–1220.
- Hu SS. Internal fixation in the osteoporotic spine. Spine (Phila PA 1976) 1997; 22( suppl 24):43S–48S.
- Galibert P, Deramond H, Rosat P, Le Gars D. Preliminary note on the treatment of vertebral angioma by percutaneous acrylic vertebroplasty. Neurochirurgie 1987; 33:166–168.
- Kasperk C, Hillmeier J, Noldge G, et al. Treatment of painful vertebral fractures by kyphoplasty in patients with primary osteoporosis: a prospective nonrandomized controlled study. J Bone Miner Res 2005; 20:604–612.
- Komp M, Ruetten S, Godolias G. Minimally invasive therapy for functionally unstable osteoporotic vertebral fracture by means of kyphoplasty: prospective comparative study of 19 surgically and 17 conservatively treated patients. J Miner Stoffwechs 2004; 11( suppl 1):13–15.
- Coumans JV, Reinhardt MK, Lieberman IH. Kyphoplasty for vertebral compression fractures: 1-year clinical outcomes from a prospective study. J Neurosurg 2003; 99 (suppl 1):44–50.
- Lieberman IH, Dudeney S, Reinhardt MK, Bell G. Initial outcome and efficacy of ‘kyphoplasty’ in the treatment of painful osteoporotic vertebral compression fractures. Spine (Phila PA 1976) 2001; 26:1631–1638.
- Garfin SR, Buckley RA, Ledlie J; Balloon Kyphoplasty Outcomes Group. Balloon kyphoplasty for symptomatic vertebral body compression fractures results in rapid, significant, and sustained improvements in back pain, function, and quality of life for elderly patients. Spine (Phila PA 1976) 2006; 31:2213–2220.
- Ledlie JT, Renfro MB. Kyphoplasty treatment of vertebral fractures: 2-year outcomes show sustained benefits. Spine (Phila PA 1976) 2006; 31:57–64.
- Majd ME, Farley S, Holt RT. Preliminary outcomes and efficacy of the first 360 consecutive kyphoplasties for the treatment of painful osteoporotic vertebral compression fractures. Spine J 2005; 5:244–255.
- Rhyne A, Banit D, Laxer E, Odum S, Nussman D. Kyphoplasty: report of eighty-two thoracolumbar osteoporotic vertebral fractures. J Orthop Trauma 2004; 18:294–299.
- Theodorou DJ, Theodorou SJ, Duncan TD, Garfin SR, Wong WH. Percutaneous balloon kyphoplasty for the correction of spinal deformity in painful vertebral body compression fractures. Clin Imaging 2002; 26:1–5.
- Berlemann U, Franz T, Orler R, Heini PF. Kyphoplasty for treatment of osteoporotic vertebral fractures: a prospective nonrandomized study. Eur Spine J 2004; 13:496–501.
- McGraw JK, Lippert JA, Minkus KD, Rami PM, Davis TM, Budzik RF. Prospective evaluation of pain relief in 100 patients undergoing percutaneous vertebroplasty: results and followup. J Vasc Interv Radiol 2002; 13:883–886.
- Zoarski GH, Snow P, Olan WJ, et al. Percutaneous vertebroplasty for osteoporotic compression fractures: quantitative prospective evaluation of long-term outcomes. J Vasc Interv Radiol 2002; 13:139–148.
- Evans AJ, Jensen ME, Kip KE, et al. Vertebral compression fractures: pain reduction and improvement in functional mobility after percutaneous polymethylmethacrylate vertebroplasty retrospective report of 245 cases. Radiology 2003; 226:366–372.
- Grohs JG, Matzner M, Trieb K, Krepler P. Minimal invasive stabilization of osteoporotic vertebral fractures: a prospective nonrandomized comparison of vertebroplasty and balloon kyphoplasty. J Spinal Disord Tech 2005; 18:238–242.
- Kallmes DF, Schweickert PA, Marx WF, Jensen ME. Vertebroplasty in the mid-and upper thoracic spine. AJNR Am J Neuroradiol 2002; 23:1117–1120.
- Grados F, Depriester C, Cayrolle G, Hardy N, Deramond H, Fardellone P. Long-term observations of vertebral osteoporotic fractures treated by percutaneous vertebroplasty. Rheumatology (Oxford) 2000; 39:1410–1414.
- Legroux-Gérot I, Lormeau C, Boutry N, Cotten A, Duquesnoy B, Cortet B. Long-term follow-up of vertebral osteoporotic fractures treated by percutaneous vertebroplasty. Clin Rheumatol 2004; 23:310–317.
- Wardlaw D, Cummings SR, Van Meirhaeghe J, et al. Efficacy and safety of balloon kyphoplasty compared with nonsurgical care for vertebral compression fracture (FREE): a randomised controlled trial. Lancet 2009; 373:1016–1024.
- Voormolen MH, Mali WP, Lohle PN, et al. Percutaneous vertebroplasty compared with optimal pain medication treatment: short-term outcomes of patients with subacute or chronic painful osteoporotic vertebral compression fractures. The VERTOS study. AJNR Am J Neuroradiol 2007: 28:555–560.
- Moseley JB, O'Malley K, Petersen NJ. A controlled trial of arthroscopic surgery for osteoarthritis of the knee. N Engl J Med 2002; 347:81–88.
- Riew KD, Yin Y, Gilula L, et al. The effect of nerve-root injections on the need for operative treatment of lumbar radicular pain. A prospective, randomized, controlled, double-blind study. J Bone Joint Surg Am 2000; 82–A:1589–1593.
- Riew KD, Park JB, Cho YS, et al. Nerve root blocks in the treatment of lumbar radicular pain. A minimum five-year follow-up. J Bone Joint Surg Am 2006; 88:1722–1725.
- Kallmes DF, Comstock BA, Heagerty PJ, et al. A randomized trial of vertebroplasty for osteoporotic spinal fractures. N Engl J Med 2009; 361:569–579.
- Buchbinder R, Osborne RH, Ebeling PR, et al. A randomized trial of vertebroplasty for painful osteoporotic vertebral fractures. N Engl J Med 2009; 361:557–568.
- Carmona RH. Department of Health and Human Services. Office of the Surgeon General. Bone health and osteoporosis: A report of the Surgeon General (2004). www.surgeongeneral.gov/library/bonehealth/content.html. Accessed November 16, 2009.
- Kado DM, Browner WS, Palermo L, Nevitt MC, Genant HK, Cummings SR. Vertebral fractures and mortality in older women: a prospective study. Study of Osteoporotic Fractures Research Group. Arch Intern Med 1999; 159:1215–1220.
- Hu SS. Internal fixation in the osteoporotic spine. Spine (Phila PA 1976) 1997; 22( suppl 24):43S–48S.
- Galibert P, Deramond H, Rosat P, Le Gars D. Preliminary note on the treatment of vertebral angioma by percutaneous acrylic vertebroplasty. Neurochirurgie 1987; 33:166–168.
- Kasperk C, Hillmeier J, Noldge G, et al. Treatment of painful vertebral fractures by kyphoplasty in patients with primary osteoporosis: a prospective nonrandomized controlled study. J Bone Miner Res 2005; 20:604–612.
- Komp M, Ruetten S, Godolias G. Minimally invasive therapy for functionally unstable osteoporotic vertebral fracture by means of kyphoplasty: prospective comparative study of 19 surgically and 17 conservatively treated patients. J Miner Stoffwechs 2004; 11( suppl 1):13–15.
- Coumans JV, Reinhardt MK, Lieberman IH. Kyphoplasty for vertebral compression fractures: 1-year clinical outcomes from a prospective study. J Neurosurg 2003; 99 (suppl 1):44–50.
- Lieberman IH, Dudeney S, Reinhardt MK, Bell G. Initial outcome and efficacy of ‘kyphoplasty’ in the treatment of painful osteoporotic vertebral compression fractures. Spine (Phila PA 1976) 2001; 26:1631–1638.
- Garfin SR, Buckley RA, Ledlie J; Balloon Kyphoplasty Outcomes Group. Balloon kyphoplasty for symptomatic vertebral body compression fractures results in rapid, significant, and sustained improvements in back pain, function, and quality of life for elderly patients. Spine (Phila PA 1976) 2006; 31:2213–2220.
- Ledlie JT, Renfro MB. Kyphoplasty treatment of vertebral fractures: 2-year outcomes show sustained benefits. Spine (Phila PA 1976) 2006; 31:57–64.
- Majd ME, Farley S, Holt RT. Preliminary outcomes and efficacy of the first 360 consecutive kyphoplasties for the treatment of painful osteoporotic vertebral compression fractures. Spine J 2005; 5:244–255.
- Rhyne A, Banit D, Laxer E, Odum S, Nussman D. Kyphoplasty: report of eighty-two thoracolumbar osteoporotic vertebral fractures. J Orthop Trauma 2004; 18:294–299.
- Theodorou DJ, Theodorou SJ, Duncan TD, Garfin SR, Wong WH. Percutaneous balloon kyphoplasty for the correction of spinal deformity in painful vertebral body compression fractures. Clin Imaging 2002; 26:1–5.
- Berlemann U, Franz T, Orler R, Heini PF. Kyphoplasty for treatment of osteoporotic vertebral fractures: a prospective nonrandomized study. Eur Spine J 2004; 13:496–501.
- McGraw JK, Lippert JA, Minkus KD, Rami PM, Davis TM, Budzik RF. Prospective evaluation of pain relief in 100 patients undergoing percutaneous vertebroplasty: results and followup. J Vasc Interv Radiol 2002; 13:883–886.
- Zoarski GH, Snow P, Olan WJ, et al. Percutaneous vertebroplasty for osteoporotic compression fractures: quantitative prospective evaluation of long-term outcomes. J Vasc Interv Radiol 2002; 13:139–148.
- Evans AJ, Jensen ME, Kip KE, et al. Vertebral compression fractures: pain reduction and improvement in functional mobility after percutaneous polymethylmethacrylate vertebroplasty retrospective report of 245 cases. Radiology 2003; 226:366–372.
- Grohs JG, Matzner M, Trieb K, Krepler P. Minimal invasive stabilization of osteoporotic vertebral fractures: a prospective nonrandomized comparison of vertebroplasty and balloon kyphoplasty. J Spinal Disord Tech 2005; 18:238–242.
- Kallmes DF, Schweickert PA, Marx WF, Jensen ME. Vertebroplasty in the mid-and upper thoracic spine. AJNR Am J Neuroradiol 2002; 23:1117–1120.
- Grados F, Depriester C, Cayrolle G, Hardy N, Deramond H, Fardellone P. Long-term observations of vertebral osteoporotic fractures treated by percutaneous vertebroplasty. Rheumatology (Oxford) 2000; 39:1410–1414.
- Legroux-Gérot I, Lormeau C, Boutry N, Cotten A, Duquesnoy B, Cortet B. Long-term follow-up of vertebral osteoporotic fractures treated by percutaneous vertebroplasty. Clin Rheumatol 2004; 23:310–317.
- Wardlaw D, Cummings SR, Van Meirhaeghe J, et al. Efficacy and safety of balloon kyphoplasty compared with nonsurgical care for vertebral compression fracture (FREE): a randomised controlled trial. Lancet 2009; 373:1016–1024.
- Voormolen MH, Mali WP, Lohle PN, et al. Percutaneous vertebroplasty compared with optimal pain medication treatment: short-term outcomes of patients with subacute or chronic painful osteoporotic vertebral compression fractures. The VERTOS study. AJNR Am J Neuroradiol 2007: 28:555–560.
- Moseley JB, O'Malley K, Petersen NJ. A controlled trial of arthroscopic surgery for osteoarthritis of the knee. N Engl J Med 2002; 347:81–88.
- Riew KD, Yin Y, Gilula L, et al. The effect of nerve-root injections on the need for operative treatment of lumbar radicular pain. A prospective, randomized, controlled, double-blind study. J Bone Joint Surg Am 2000; 82–A:1589–1593.
- Riew KD, Park JB, Cho YS, et al. Nerve root blocks in the treatment of lumbar radicular pain. A minimum five-year follow-up. J Bone Joint Surg Am 2006; 88:1722–1725.