User login
SHM Boasts Diverse Membership, Leadership Lacks Non-Academic Presence
Who are you?
I am a 44-year-old Chinese-American male who works as a hospitalist at Beth Israel Deaconess Medical Center (BIDMC), an academic medical center in Boston. BIDMC is affiliated with Harvard Medical School, where I am an associate professor of medicine.
If you are about to join or renew your SHM membership, you can expect SHM to ask some questions it’s never asked before. What is your gender? What is your age? What is your ethnicity? I would not be surprised if you wondered why SHM is asking these questions. What does it have to do with my membership? Why are they asking now when they never asked before? I do not remember other professional medical societies asking these types of questions—should I be concerned? Is this an unnecessary invasion of privacy?
Call to Action
Nearly two years ago, when I had the good fortune of being elected SHM’s president-elect, I asked, What do we know about SHM members? As it turns out, it’s less than I thought we knew.
SHM has been in the survey business for years. The most visible survey is the annual productivity and compensation survey (see “Survey Insights,” p. TK). The data from this instrument have become very important, thanks to the many of you who have participated. In the early years of hospital medicine, everyone wanted to know how hard others were working and how much others were getting paid. If others had a better compensation package, it was the proof one needed to go marching into the C-suite asking for more support. If others were making less, it was the competitive advantage one needed to land the next hot-prospect hospitalist.
To be fair, I remember the surveys also asked about hospitalist age and employment model. But I don’t remember any questions about gender, race, or other personal information. The survey was the productivity and compensation survey, so maybe it had nothing to do with gender and race … but maybe it should.
Diverse, Yet Not So Much
Over the years, when I’ve walked the hallways at the SHM annual meeting, I got the sense that it was a reasonably diverse crowd. Take a look when you are at the San Diego Convention Center in April, and I expect you will agree. I grant you that it is generally a younger crowd than what one would find at most medical meetings, but I see people of many ethnic backgrounds, and there are equal parts women and men.
What was striking to me, however, was when I walked into some of the smaller conference rooms where the SHM committee meetings were being held and where the leaders sat. That crowd didn’t seem nearly as diverse as the crowd in the bigger rooms. I remember asking one of my colleagues whether he had the same perception. He told me he didn’t see it that way. Then again, it dawned on me that he is white and works at an academic medical center. What if he walked into leadership committee meetings filled with women from under-represented minority groups who work in community hospitals? My guess is that he would notice right away.
But my perception is biased, so when I became SHM president last spring, I asked that we assemble some facts about our members. SHM pulled together a task force, which developed a survey and took a snapshot of SHM membership. Some of you may have received this survey; it was sent out to thousands of SHM members.
The survey results, which were shared recently with SHM’s board of directors, confirmed my suspicions: SHM membership is a reasonably diverse crowd when it comes to gender and race. When it came to the SHM committee membership, I was right and wrong. The percentage of women and under-represented minorities on SHM committees reflected overall SHM membership reasonably well, but it was clear that fewer women and under-represented minorities held senior leadership positions, such as committee chairs and positions on the board. I suspect this is no different at other professional medical societies and more of a commentary on medicine than on SHM.
The most striking difference, however, did not have to do with race or gender, but instead had to do with employment model. Hospitalists who work at places other than academic medical centers are clearly under-represented in SHM leadership positions.
Action Item: New Knowledge, Better Understanding
So what do we do with this information? Am I suggesting that we set aside special seats at the board table for specific types of people or special-interest groups, some seats just for women, and some just for hospitalists who work in community hospitals? I am not advocating any such action.
I did ask SHM leadership to initiate action to help us continually understand the makeup of SHM membership and compare it to representation at the leadership level. SHM leadership overwhelmingly agreed. This is the reason you are being asked to volunteer personal information when you renew your membership.
It is my hope and belief that SHM will use this information appropriately when they organize committees and build leadership teams. This information, if used appropriately, will help SHM leadership understand its potential bias and guard against unintended consequences of their actions.
I recognize that some people will argue that the questions being asked are not sufficiently comprehensive. We should also be asking about other individual characteristics. You may or may not be right, but at this time, I think we are taking a step in the right direction. Further steps may be forthcoming in the future, but let’s not let perfection be the enemy of good.
If you have any comments about this article, please contact me at [email protected]. I’m also available on LinkedIn (JosephLi) and Twitter (@_JosephLi).
Dr. Li is president of SHM.
Who are you?
I am a 44-year-old Chinese-American male who works as a hospitalist at Beth Israel Deaconess Medical Center (BIDMC), an academic medical center in Boston. BIDMC is affiliated with Harvard Medical School, where I am an associate professor of medicine.
If you are about to join or renew your SHM membership, you can expect SHM to ask some questions it’s never asked before. What is your gender? What is your age? What is your ethnicity? I would not be surprised if you wondered why SHM is asking these questions. What does it have to do with my membership? Why are they asking now when they never asked before? I do not remember other professional medical societies asking these types of questions—should I be concerned? Is this an unnecessary invasion of privacy?
Call to Action
Nearly two years ago, when I had the good fortune of being elected SHM’s president-elect, I asked, What do we know about SHM members? As it turns out, it’s less than I thought we knew.
SHM has been in the survey business for years. The most visible survey is the annual productivity and compensation survey (see “Survey Insights,” p. TK). The data from this instrument have become very important, thanks to the many of you who have participated. In the early years of hospital medicine, everyone wanted to know how hard others were working and how much others were getting paid. If others had a better compensation package, it was the proof one needed to go marching into the C-suite asking for more support. If others were making less, it was the competitive advantage one needed to land the next hot-prospect hospitalist.
To be fair, I remember the surveys also asked about hospitalist age and employment model. But I don’t remember any questions about gender, race, or other personal information. The survey was the productivity and compensation survey, so maybe it had nothing to do with gender and race … but maybe it should.
Diverse, Yet Not So Much
Over the years, when I’ve walked the hallways at the SHM annual meeting, I got the sense that it was a reasonably diverse crowd. Take a look when you are at the San Diego Convention Center in April, and I expect you will agree. I grant you that it is generally a younger crowd than what one would find at most medical meetings, but I see people of many ethnic backgrounds, and there are equal parts women and men.
What was striking to me, however, was when I walked into some of the smaller conference rooms where the SHM committee meetings were being held and where the leaders sat. That crowd didn’t seem nearly as diverse as the crowd in the bigger rooms. I remember asking one of my colleagues whether he had the same perception. He told me he didn’t see it that way. Then again, it dawned on me that he is white and works at an academic medical center. What if he walked into leadership committee meetings filled with women from under-represented minority groups who work in community hospitals? My guess is that he would notice right away.
But my perception is biased, so when I became SHM president last spring, I asked that we assemble some facts about our members. SHM pulled together a task force, which developed a survey and took a snapshot of SHM membership. Some of you may have received this survey; it was sent out to thousands of SHM members.
The survey results, which were shared recently with SHM’s board of directors, confirmed my suspicions: SHM membership is a reasonably diverse crowd when it comes to gender and race. When it came to the SHM committee membership, I was right and wrong. The percentage of women and under-represented minorities on SHM committees reflected overall SHM membership reasonably well, but it was clear that fewer women and under-represented minorities held senior leadership positions, such as committee chairs and positions on the board. I suspect this is no different at other professional medical societies and more of a commentary on medicine than on SHM.
The most striking difference, however, did not have to do with race or gender, but instead had to do with employment model. Hospitalists who work at places other than academic medical centers are clearly under-represented in SHM leadership positions.
Action Item: New Knowledge, Better Understanding
So what do we do with this information? Am I suggesting that we set aside special seats at the board table for specific types of people or special-interest groups, some seats just for women, and some just for hospitalists who work in community hospitals? I am not advocating any such action.
I did ask SHM leadership to initiate action to help us continually understand the makeup of SHM membership and compare it to representation at the leadership level. SHM leadership overwhelmingly agreed. This is the reason you are being asked to volunteer personal information when you renew your membership.
It is my hope and belief that SHM will use this information appropriately when they organize committees and build leadership teams. This information, if used appropriately, will help SHM leadership understand its potential bias and guard against unintended consequences of their actions.
I recognize that some people will argue that the questions being asked are not sufficiently comprehensive. We should also be asking about other individual characteristics. You may or may not be right, but at this time, I think we are taking a step in the right direction. Further steps may be forthcoming in the future, but let’s not let perfection be the enemy of good.
If you have any comments about this article, please contact me at [email protected]. I’m also available on LinkedIn (JosephLi) and Twitter (@_JosephLi).
Dr. Li is president of SHM.
Who are you?
I am a 44-year-old Chinese-American male who works as a hospitalist at Beth Israel Deaconess Medical Center (BIDMC), an academic medical center in Boston. BIDMC is affiliated with Harvard Medical School, where I am an associate professor of medicine.
If you are about to join or renew your SHM membership, you can expect SHM to ask some questions it’s never asked before. What is your gender? What is your age? What is your ethnicity? I would not be surprised if you wondered why SHM is asking these questions. What does it have to do with my membership? Why are they asking now when they never asked before? I do not remember other professional medical societies asking these types of questions—should I be concerned? Is this an unnecessary invasion of privacy?
Call to Action
Nearly two years ago, when I had the good fortune of being elected SHM’s president-elect, I asked, What do we know about SHM members? As it turns out, it’s less than I thought we knew.
SHM has been in the survey business for years. The most visible survey is the annual productivity and compensation survey (see “Survey Insights,” p. TK). The data from this instrument have become very important, thanks to the many of you who have participated. In the early years of hospital medicine, everyone wanted to know how hard others were working and how much others were getting paid. If others had a better compensation package, it was the proof one needed to go marching into the C-suite asking for more support. If others were making less, it was the competitive advantage one needed to land the next hot-prospect hospitalist.
To be fair, I remember the surveys also asked about hospitalist age and employment model. But I don’t remember any questions about gender, race, or other personal information. The survey was the productivity and compensation survey, so maybe it had nothing to do with gender and race … but maybe it should.
Diverse, Yet Not So Much
Over the years, when I’ve walked the hallways at the SHM annual meeting, I got the sense that it was a reasonably diverse crowd. Take a look when you are at the San Diego Convention Center in April, and I expect you will agree. I grant you that it is generally a younger crowd than what one would find at most medical meetings, but I see people of many ethnic backgrounds, and there are equal parts women and men.
What was striking to me, however, was when I walked into some of the smaller conference rooms where the SHM committee meetings were being held and where the leaders sat. That crowd didn’t seem nearly as diverse as the crowd in the bigger rooms. I remember asking one of my colleagues whether he had the same perception. He told me he didn’t see it that way. Then again, it dawned on me that he is white and works at an academic medical center. What if he walked into leadership committee meetings filled with women from under-represented minority groups who work in community hospitals? My guess is that he would notice right away.
But my perception is biased, so when I became SHM president last spring, I asked that we assemble some facts about our members. SHM pulled together a task force, which developed a survey and took a snapshot of SHM membership. Some of you may have received this survey; it was sent out to thousands of SHM members.
The survey results, which were shared recently with SHM’s board of directors, confirmed my suspicions: SHM membership is a reasonably diverse crowd when it comes to gender and race. When it came to the SHM committee membership, I was right and wrong. The percentage of women and under-represented minorities on SHM committees reflected overall SHM membership reasonably well, but it was clear that fewer women and under-represented minorities held senior leadership positions, such as committee chairs and positions on the board. I suspect this is no different at other professional medical societies and more of a commentary on medicine than on SHM.
The most striking difference, however, did not have to do with race or gender, but instead had to do with employment model. Hospitalists who work at places other than academic medical centers are clearly under-represented in SHM leadership positions.
Action Item: New Knowledge, Better Understanding
So what do we do with this information? Am I suggesting that we set aside special seats at the board table for specific types of people or special-interest groups, some seats just for women, and some just for hospitalists who work in community hospitals? I am not advocating any such action.
I did ask SHM leadership to initiate action to help us continually understand the makeup of SHM membership and compare it to representation at the leadership level. SHM leadership overwhelmingly agreed. This is the reason you are being asked to volunteer personal information when you renew your membership.
It is my hope and belief that SHM will use this information appropriately when they organize committees and build leadership teams. This information, if used appropriately, will help SHM leadership understand its potential bias and guard against unintended consequences of their actions.
I recognize that some people will argue that the questions being asked are not sufficiently comprehensive. We should also be asking about other individual characteristics. You may or may not be right, but at this time, I think we are taking a step in the right direction. Further steps may be forthcoming in the future, but let’s not let perfection be the enemy of good.
If you have any comments about this article, please contact me at [email protected]. I’m also available on LinkedIn (JosephLi) and Twitter (@_JosephLi).
Dr. Li is president of SHM.
How to Get the Most Out of the HM12 Toolkit
I have a problem. OK, many problems. Marital discord, balky kids, bloated mortgage? No, fortunately, not those kinds of domestic problems—although I do struggle with reliably differentiating whites from darks. My biggest problem is work-related. And this isn’t new. Turns out, I have different problems at work every year. “Time to find a new job,” you say. Tell the boss to shove it? Produce an epic, Jerry Maguire-esque manifesto and ride off into the sunset with my goldfish and Renee Zellweger? Hmmm, Renee Zellweger…
No, no, that’s not it. Much more mundane, yet crucial, problems.
Problems like trying to sort out the implications of the impending value-based purchasing program—what does it mean for my group? How do I keep my hospitalist partners engaged, satisfied, and not burned out? How do I produce a schedule that emphasizes high-quality patient care, efficiency, and physician work-life balance? How can I reduce readmissions so my hospital administrator can go back to “administrating” someone other than me all day? What do I do with the perioperative beta blockade now that some of the original data have been called into question due to academic dishonesty? What does the Affordable Care Act really say, is it going stand up, and what does it mean for me, my patients, my salary, and my career?
These are all questions I am grappling with currently. They also are all questions that will be addressed at HM12, April 1-4 in San Diego. As such, I view the annual meeting as a kind of toolkit: Have a problem, reach into the HM12 toolkit, and pull out your solution. The beauty is its breadth. You might not care one iota about healthcare reform, scheduling, or group satisfaction. Fine: How about updates in new medications, management of hyponatremia, the unique challenges of women in medicine, managing acute ventilator issues, acute pain management, information technology, quality improvement, professionalism in the digital age, or listening to the latest in the management of Clostridium difficile from the world-renowned Dr. John Bartlett? All are tools in this year’s toolkit.
And this type of breadth means the annual meeting evolves with you. Early in my career, I reached for the clinical tools. Then it was practice development and management tools; now I tend to look for healthcare policy solutions. Suffice to say, whatever solution you are looking for, with nine tracks, eight pathways, seven pre-courses, three plenary sessions by healthcare luminaries, and two Research, Innovation, and Vignette sessions, HM12 has your tool.
How can you best access this trove of information? Here is some advice culled from my 10 years of attending SHM annual meetings.
They Won’t Leave A Light On For You Forever
Unless you’re Tom Bodett, I’d recommend you get a hotel room now. For my first annual meeting (which was also in San Diego), I registered late, found no hotel rooms in the city, and had to commute 30 minutes both ways. Not only is this inconvenient and costly (I had to rent a car), but it also takes you out of the action. You want to be on-site, especially after meeting hours, when a lot of the networking and fun happens.
Stay Out Late
OK, now that you have a room, don’t use it. Rooms are for sleeping. If you find yourself in your room not sleeping, then you are missing out on some of the richest aspects of the conference—meeting new people, catching up with colleagues you see only once a year, and bathing in the general excitement of being at a meeting with thousands of peers. This remains the most satisfying part of the annual meeting experience for me. It’s dinner with a colleague from another part of the country, coffee with a new acquaintance, or a drink with an old friend. It’s energizing, engaging, and reignites my passion for HM.
Sleep Is For Vacation
Staying with the hotel room theme, don’t sleep in. I realize San Diego in April can feel like a vacation, and truth be told, it should. However, you came to learn. It’s tempting to maximize pillow time instead of heading down to the first plenary session at 8 a.m.—after all, you stayed out late networking! Anyway, how interesting can it be? Very. Dr. Patrick Conway is going to lead off the meeting with a look at the implications of the Affordable Care Act for hospitalists. As a hospitalist and CMO of CMS, he should know. Come to this session, and so will you.
Declare a Major and a Minor
Remember college? Me, neither. But I do have a vague recollection of that kid-in-a-candy-store feeling of choice my freshman year. The rest is a blur of late nights, hungover Sundays, and weight gain. Just like the college course book, the HM12 agenda can be overwhelming. Choice is great, but how do you choose what to go to? Just like college, you need a plan. Spend time before the meeting charting your course. What do you want to learn? What knowledge gaps do you want to fill? Throw in something for fun. Peruse the website, print out or download the slide decks from the talks you are interested in, and have a plan to maximize your time in San Diego.
Divide and Conquer
Next, make a plan with your friends. Most attendees have at least one other group member attending the meeting. Don’t go to the same sessions. Why? You should share your findings with the rest of your group.
You’ll no doubt pick up a new method for patient handoffs, moving patients through the hospital more efficiently, creating an incentive plan, or developing a post-discharge clinic. Bring it home; share it; implement it.
Go to the RIV Sessions
“But wait,” you say, “I’m not a researcher.” Perhaps true, but you are a hospitalist. And this is the material that is coming down the pike. It’s the cool case you’ll encounter next month, the innovation that’ll help your patients avoid hospital infections, or the research that will inform the next VTE prophylaxis guideline.
Go Viral
Bring your business cards. And like a rhinovirus, give them to everyone. Entranced person next to you at the plenary? Card. New face at the Special Interest Forum for rural hospitalists? Card. Erudite-appearing character scanning the poster abstract on readmissions with you? Card. Bagel-versus-English-muffin-debating person in the breakfast line? Card.
The point is, don’t be shy. You are there to be part of the hospitalist movement—to learn, to share, to be part of the discussion, to help define our collective future. Do that. This isn’t the time to be a wallflower. Rather, say “hi” to the person next to you. Strike up a conversation; you never know where it may lead.
You Had Me At “Hello”
So tell your boss to “show me the money,” so that you, too, can utilize the HM12 toolkit. If he or she balks, tell them to “help me help you.” Because after attending the meeting, I’m confident that with a tear in your eye, you’ll sappily utter, “HM12, you complete me.”
Dr. Glasheen is physician editor of The Hospitalist.
I have a problem. OK, many problems. Marital discord, balky kids, bloated mortgage? No, fortunately, not those kinds of domestic problems—although I do struggle with reliably differentiating whites from darks. My biggest problem is work-related. And this isn’t new. Turns out, I have different problems at work every year. “Time to find a new job,” you say. Tell the boss to shove it? Produce an epic, Jerry Maguire-esque manifesto and ride off into the sunset with my goldfish and Renee Zellweger? Hmmm, Renee Zellweger…
No, no, that’s not it. Much more mundane, yet crucial, problems.
Problems like trying to sort out the implications of the impending value-based purchasing program—what does it mean for my group? How do I keep my hospitalist partners engaged, satisfied, and not burned out? How do I produce a schedule that emphasizes high-quality patient care, efficiency, and physician work-life balance? How can I reduce readmissions so my hospital administrator can go back to “administrating” someone other than me all day? What do I do with the perioperative beta blockade now that some of the original data have been called into question due to academic dishonesty? What does the Affordable Care Act really say, is it going stand up, and what does it mean for me, my patients, my salary, and my career?
These are all questions I am grappling with currently. They also are all questions that will be addressed at HM12, April 1-4 in San Diego. As such, I view the annual meeting as a kind of toolkit: Have a problem, reach into the HM12 toolkit, and pull out your solution. The beauty is its breadth. You might not care one iota about healthcare reform, scheduling, or group satisfaction. Fine: How about updates in new medications, management of hyponatremia, the unique challenges of women in medicine, managing acute ventilator issues, acute pain management, information technology, quality improvement, professionalism in the digital age, or listening to the latest in the management of Clostridium difficile from the world-renowned Dr. John Bartlett? All are tools in this year’s toolkit.
And this type of breadth means the annual meeting evolves with you. Early in my career, I reached for the clinical tools. Then it was practice development and management tools; now I tend to look for healthcare policy solutions. Suffice to say, whatever solution you are looking for, with nine tracks, eight pathways, seven pre-courses, three plenary sessions by healthcare luminaries, and two Research, Innovation, and Vignette sessions, HM12 has your tool.
How can you best access this trove of information? Here is some advice culled from my 10 years of attending SHM annual meetings.
They Won’t Leave A Light On For You Forever
Unless you’re Tom Bodett, I’d recommend you get a hotel room now. For my first annual meeting (which was also in San Diego), I registered late, found no hotel rooms in the city, and had to commute 30 minutes both ways. Not only is this inconvenient and costly (I had to rent a car), but it also takes you out of the action. You want to be on-site, especially after meeting hours, when a lot of the networking and fun happens.
Stay Out Late
OK, now that you have a room, don’t use it. Rooms are for sleeping. If you find yourself in your room not sleeping, then you are missing out on some of the richest aspects of the conference—meeting new people, catching up with colleagues you see only once a year, and bathing in the general excitement of being at a meeting with thousands of peers. This remains the most satisfying part of the annual meeting experience for me. It’s dinner with a colleague from another part of the country, coffee with a new acquaintance, or a drink with an old friend. It’s energizing, engaging, and reignites my passion for HM.
Sleep Is For Vacation
Staying with the hotel room theme, don’t sleep in. I realize San Diego in April can feel like a vacation, and truth be told, it should. However, you came to learn. It’s tempting to maximize pillow time instead of heading down to the first plenary session at 8 a.m.—after all, you stayed out late networking! Anyway, how interesting can it be? Very. Dr. Patrick Conway is going to lead off the meeting with a look at the implications of the Affordable Care Act for hospitalists. As a hospitalist and CMO of CMS, he should know. Come to this session, and so will you.
Declare a Major and a Minor
Remember college? Me, neither. But I do have a vague recollection of that kid-in-a-candy-store feeling of choice my freshman year. The rest is a blur of late nights, hungover Sundays, and weight gain. Just like the college course book, the HM12 agenda can be overwhelming. Choice is great, but how do you choose what to go to? Just like college, you need a plan. Spend time before the meeting charting your course. What do you want to learn? What knowledge gaps do you want to fill? Throw in something for fun. Peruse the website, print out or download the slide decks from the talks you are interested in, and have a plan to maximize your time in San Diego.
Divide and Conquer
Next, make a plan with your friends. Most attendees have at least one other group member attending the meeting. Don’t go to the same sessions. Why? You should share your findings with the rest of your group.
You’ll no doubt pick up a new method for patient handoffs, moving patients through the hospital more efficiently, creating an incentive plan, or developing a post-discharge clinic. Bring it home; share it; implement it.
Go to the RIV Sessions
“But wait,” you say, “I’m not a researcher.” Perhaps true, but you are a hospitalist. And this is the material that is coming down the pike. It’s the cool case you’ll encounter next month, the innovation that’ll help your patients avoid hospital infections, or the research that will inform the next VTE prophylaxis guideline.
Go Viral
Bring your business cards. And like a rhinovirus, give them to everyone. Entranced person next to you at the plenary? Card. New face at the Special Interest Forum for rural hospitalists? Card. Erudite-appearing character scanning the poster abstract on readmissions with you? Card. Bagel-versus-English-muffin-debating person in the breakfast line? Card.
The point is, don’t be shy. You are there to be part of the hospitalist movement—to learn, to share, to be part of the discussion, to help define our collective future. Do that. This isn’t the time to be a wallflower. Rather, say “hi” to the person next to you. Strike up a conversation; you never know where it may lead.
You Had Me At “Hello”
So tell your boss to “show me the money,” so that you, too, can utilize the HM12 toolkit. If he or she balks, tell them to “help me help you.” Because after attending the meeting, I’m confident that with a tear in your eye, you’ll sappily utter, “HM12, you complete me.”
Dr. Glasheen is physician editor of The Hospitalist.
I have a problem. OK, many problems. Marital discord, balky kids, bloated mortgage? No, fortunately, not those kinds of domestic problems—although I do struggle with reliably differentiating whites from darks. My biggest problem is work-related. And this isn’t new. Turns out, I have different problems at work every year. “Time to find a new job,” you say. Tell the boss to shove it? Produce an epic, Jerry Maguire-esque manifesto and ride off into the sunset with my goldfish and Renee Zellweger? Hmmm, Renee Zellweger…
No, no, that’s not it. Much more mundane, yet crucial, problems.
Problems like trying to sort out the implications of the impending value-based purchasing program—what does it mean for my group? How do I keep my hospitalist partners engaged, satisfied, and not burned out? How do I produce a schedule that emphasizes high-quality patient care, efficiency, and physician work-life balance? How can I reduce readmissions so my hospital administrator can go back to “administrating” someone other than me all day? What do I do with the perioperative beta blockade now that some of the original data have been called into question due to academic dishonesty? What does the Affordable Care Act really say, is it going stand up, and what does it mean for me, my patients, my salary, and my career?
These are all questions I am grappling with currently. They also are all questions that will be addressed at HM12, April 1-4 in San Diego. As such, I view the annual meeting as a kind of toolkit: Have a problem, reach into the HM12 toolkit, and pull out your solution. The beauty is its breadth. You might not care one iota about healthcare reform, scheduling, or group satisfaction. Fine: How about updates in new medications, management of hyponatremia, the unique challenges of women in medicine, managing acute ventilator issues, acute pain management, information technology, quality improvement, professionalism in the digital age, or listening to the latest in the management of Clostridium difficile from the world-renowned Dr. John Bartlett? All are tools in this year’s toolkit.
And this type of breadth means the annual meeting evolves with you. Early in my career, I reached for the clinical tools. Then it was practice development and management tools; now I tend to look for healthcare policy solutions. Suffice to say, whatever solution you are looking for, with nine tracks, eight pathways, seven pre-courses, three plenary sessions by healthcare luminaries, and two Research, Innovation, and Vignette sessions, HM12 has your tool.
How can you best access this trove of information? Here is some advice culled from my 10 years of attending SHM annual meetings.
They Won’t Leave A Light On For You Forever
Unless you’re Tom Bodett, I’d recommend you get a hotel room now. For my first annual meeting (which was also in San Diego), I registered late, found no hotel rooms in the city, and had to commute 30 minutes both ways. Not only is this inconvenient and costly (I had to rent a car), but it also takes you out of the action. You want to be on-site, especially after meeting hours, when a lot of the networking and fun happens.
Stay Out Late
OK, now that you have a room, don’t use it. Rooms are for sleeping. If you find yourself in your room not sleeping, then you are missing out on some of the richest aspects of the conference—meeting new people, catching up with colleagues you see only once a year, and bathing in the general excitement of being at a meeting with thousands of peers. This remains the most satisfying part of the annual meeting experience for me. It’s dinner with a colleague from another part of the country, coffee with a new acquaintance, or a drink with an old friend. It’s energizing, engaging, and reignites my passion for HM.
Sleep Is For Vacation
Staying with the hotel room theme, don’t sleep in. I realize San Diego in April can feel like a vacation, and truth be told, it should. However, you came to learn. It’s tempting to maximize pillow time instead of heading down to the first plenary session at 8 a.m.—after all, you stayed out late networking! Anyway, how interesting can it be? Very. Dr. Patrick Conway is going to lead off the meeting with a look at the implications of the Affordable Care Act for hospitalists. As a hospitalist and CMO of CMS, he should know. Come to this session, and so will you.
Declare a Major and a Minor
Remember college? Me, neither. But I do have a vague recollection of that kid-in-a-candy-store feeling of choice my freshman year. The rest is a blur of late nights, hungover Sundays, and weight gain. Just like the college course book, the HM12 agenda can be overwhelming. Choice is great, but how do you choose what to go to? Just like college, you need a plan. Spend time before the meeting charting your course. What do you want to learn? What knowledge gaps do you want to fill? Throw in something for fun. Peruse the website, print out or download the slide decks from the talks you are interested in, and have a plan to maximize your time in San Diego.
Divide and Conquer
Next, make a plan with your friends. Most attendees have at least one other group member attending the meeting. Don’t go to the same sessions. Why? You should share your findings with the rest of your group.
You’ll no doubt pick up a new method for patient handoffs, moving patients through the hospital more efficiently, creating an incentive plan, or developing a post-discharge clinic. Bring it home; share it; implement it.
Go to the RIV Sessions
“But wait,” you say, “I’m not a researcher.” Perhaps true, but you are a hospitalist. And this is the material that is coming down the pike. It’s the cool case you’ll encounter next month, the innovation that’ll help your patients avoid hospital infections, or the research that will inform the next VTE prophylaxis guideline.
Go Viral
Bring your business cards. And like a rhinovirus, give them to everyone. Entranced person next to you at the plenary? Card. New face at the Special Interest Forum for rural hospitalists? Card. Erudite-appearing character scanning the poster abstract on readmissions with you? Card. Bagel-versus-English-muffin-debating person in the breakfast line? Card.
The point is, don’t be shy. You are there to be part of the hospitalist movement—to learn, to share, to be part of the discussion, to help define our collective future. Do that. This isn’t the time to be a wallflower. Rather, say “hi” to the person next to you. Strike up a conversation; you never know where it may lead.
You Had Me At “Hello”
So tell your boss to “show me the money,” so that you, too, can utilize the HM12 toolkit. If he or she balks, tell them to “help me help you.” Because after attending the meeting, I’m confident that with a tear in your eye, you’ll sappily utter, “HM12, you complete me.”
Dr. Glasheen is physician editor of The Hospitalist.
John Nelson: ED Patient Throughput Is New Core Measure
To understand one reason why your hospital cares so much about patient throughput and discharging patients before noon, think of tables in a restaurant. A restaurant has a limited number of tables, and the more quickly they serve customers, the sooner they’re able to seat a new party. So by improving their throughput, they can serve more customers and increase profitability without having to add tables.
Of course, the more a restaurant pushes to increase throughput, the more it risks a decrease in the satisfaction of its customers. Too much focus on throughput could annoy customers so much that it puts them out of business (Seinfeld’s Soup Nazi is one notable exception).
Yet hospitals are likely to increase their customers’ satisfaction by improving “front-end” throughput from the ED to the inpatient unit. In fact, CMS added two new core measures (known as inpatient quality reporting, or IQR) that hospitals began reporting on Jan. 1:
- Median time from ED arrival to ED departure for admitted patients, and
- Admit decision time to ED departure for admitted patients.
You already knew about these, right? Although they don’t directly influence Medicare payments to your hospital now, there is a pretty good chance they could become part of the Hospital Value-Based Purchasing program in the future. So expect administrative leaders at your hospital to continue pestering you about ED throughput with even greater intensity.
I confess that improving throughput feels like one of the most difficult things for me to do, and I sweat when in meetings about it. Yet I know intellectually that we can do better. After all, it wasn’t that long ago that a patient could expect to wait hours to see an ED doctor, and shortening the wait seemed an impossible task. But EDs everywhere have done just that, and now lots of them post the current ED wait time (typically less than 10 or 15 minutes!) on the Internet or billboards. So if they can do it, then I’m sure hospitalists can, too.
I wrote about hospital throughput in my April 2009 and May 2010 columns, and included some strategies you might want to try. Here are a few more directed at ED-to-inpatient ward throughput.
The One-Admitter Approach
Most practices have no option other than a single admitter at night, but if your one admitter during daytime hours reliably gets behind, then you should have “rounders” help with admitting duties. While this is stressful for the rounder, and could sometimes delay the rounder’s ability to discharge patients early (more on that later), in most cases, the benefit will be worth the cost. In fact, I think the best arrangement is for all, or nearly all, daytime hospitalists to share admissions every day.
Remember, this approach will eliminate the admitter-to-rounder handoff from Day One to Day Two, resulting in a net increase in efficiency (and probably patient satisfaction). Plus, reduction handoff errors should decrease, because only one doctor, rather than two, will have to get to know the new patient.
Eliminate Impediments
Some hospitalists with inefficient work habits have an opportunity to become more efficient at taking a history and reviewing records, along with other steps in the admission process, without risking poor quality. But most are reasonably efficient at those tasks already and shouldn’t make them the focus of throughput efforts.
That said, most, or nearly all, hospitalists could shorten the time from when the ED pages on a new admission to when we appear in the ED to see the patient.
Here is one scenario that gives rise to the type of delay I’m talking about. The ED doctor pages a triage hospitalist, and the two finally speak directly 10 minutes later. After a five-minute conversation, the triage doctor pages the hospitalist who will actually admit the patient, waits 10 minutes for the return call, relays the information (possibly leaving out or misrepresenting some of what the ED doc said), and the admitting hospitalist heads to the ED 15 minutes later. Each person in this scenario acted reasonably promptly, but the clock starts with the first ED page. The hospitalist is already at 45 minutes when they arrive to see the patient!
You need to minimize “daisy chain” communication between the ED doctor and the hospitalist who will actually see the patient. In the scenario above, I think it would be best to eliminate the triage doctor entirely (see my December 2008 column for ideas about how to do this) so that the hospitalist who will actually admit the patient learns of the new referral quickly and speaks directly with the ED doctor.
Another reason for a poor “page from ED to hospitalist at patient’s bedside” time is that the admitting hospitalist just doesn’t make it a priority to get to the ED quickly. They might decide to round on two or three more patients before working on the new admission. Of course, we must triage and sometimes delay getting to the ED while attending to an unstable floor patient, but not too often, in my experience.
I’m not suggesting that others adopt my habit: I typically don’t call the ED back at all. Instead, I just head to the ED immediately after being paged, and usually arrive in less time than I would have spent on hold waiting for the ED doc to come to the phone.
When presented with several ED patients to admit at the same time or tied up with a critical patient on the floor, getting to the ED quickly isn’t possible.
Write Admission or “Holding” Orders and Move the Patient to His/Her Room
This is frustrating for the admitting hospitalist, because among other problems, families often leave when the patient moves to the inpatient ward, and additional tests likely will take longer to complete from the floor rather than ED. And while it is hard to quantify, I think this practice increases the risk of errors—though some studies have shown that long ED stays are associated with worse outcomes for the patient, too.
One of the marquee principles of ED medicine has historically been “ED doctors should never write admission orders.” Even so, I sense that this view is shifting, and many now see this as clinically reasonable, and in some cases necessary, to ensure good performance. Our ED docs have agreed to do this if there is concern that the hospitalist can’t finish writing orders within an hour of first being paged about the patient. Ask me in a year and I’ll let you know just how this is going.
And, of course, another option is for the admitting hospitalist to provide admission orders or “holding” orders by phone. I think this is safe for many patients, as long as it is followed by a bedside admission visit by the hospitalist before too long.
Next month: What about throughput at the discharge end of the hospital stay? And yes, improving throughput risks quality of care, yet we have to do it while maintaining or improving quality.
Dr. Nelson has been a practicing hospitalist since 1988 and is co-founder and past president of SHM. He is a principal in Nelson Flores Hospital Medicine Consultants, a national hospitalist practice management consulting firm.
To understand one reason why your hospital cares so much about patient throughput and discharging patients before noon, think of tables in a restaurant. A restaurant has a limited number of tables, and the more quickly they serve customers, the sooner they’re able to seat a new party. So by improving their throughput, they can serve more customers and increase profitability without having to add tables.
Of course, the more a restaurant pushes to increase throughput, the more it risks a decrease in the satisfaction of its customers. Too much focus on throughput could annoy customers so much that it puts them out of business (Seinfeld’s Soup Nazi is one notable exception).
Yet hospitals are likely to increase their customers’ satisfaction by improving “front-end” throughput from the ED to the inpatient unit. In fact, CMS added two new core measures (known as inpatient quality reporting, or IQR) that hospitals began reporting on Jan. 1:
- Median time from ED arrival to ED departure for admitted patients, and
- Admit decision time to ED departure for admitted patients.
You already knew about these, right? Although they don’t directly influence Medicare payments to your hospital now, there is a pretty good chance they could become part of the Hospital Value-Based Purchasing program in the future. So expect administrative leaders at your hospital to continue pestering you about ED throughput with even greater intensity.
I confess that improving throughput feels like one of the most difficult things for me to do, and I sweat when in meetings about it. Yet I know intellectually that we can do better. After all, it wasn’t that long ago that a patient could expect to wait hours to see an ED doctor, and shortening the wait seemed an impossible task. But EDs everywhere have done just that, and now lots of them post the current ED wait time (typically less than 10 or 15 minutes!) on the Internet or billboards. So if they can do it, then I’m sure hospitalists can, too.
I wrote about hospital throughput in my April 2009 and May 2010 columns, and included some strategies you might want to try. Here are a few more directed at ED-to-inpatient ward throughput.
The One-Admitter Approach
Most practices have no option other than a single admitter at night, but if your one admitter during daytime hours reliably gets behind, then you should have “rounders” help with admitting duties. While this is stressful for the rounder, and could sometimes delay the rounder’s ability to discharge patients early (more on that later), in most cases, the benefit will be worth the cost. In fact, I think the best arrangement is for all, or nearly all, daytime hospitalists to share admissions every day.
Remember, this approach will eliminate the admitter-to-rounder handoff from Day One to Day Two, resulting in a net increase in efficiency (and probably patient satisfaction). Plus, reduction handoff errors should decrease, because only one doctor, rather than two, will have to get to know the new patient.
Eliminate Impediments
Some hospitalists with inefficient work habits have an opportunity to become more efficient at taking a history and reviewing records, along with other steps in the admission process, without risking poor quality. But most are reasonably efficient at those tasks already and shouldn’t make them the focus of throughput efforts.
That said, most, or nearly all, hospitalists could shorten the time from when the ED pages on a new admission to when we appear in the ED to see the patient.
Here is one scenario that gives rise to the type of delay I’m talking about. The ED doctor pages a triage hospitalist, and the two finally speak directly 10 minutes later. After a five-minute conversation, the triage doctor pages the hospitalist who will actually admit the patient, waits 10 minutes for the return call, relays the information (possibly leaving out or misrepresenting some of what the ED doc said), and the admitting hospitalist heads to the ED 15 minutes later. Each person in this scenario acted reasonably promptly, but the clock starts with the first ED page. The hospitalist is already at 45 minutes when they arrive to see the patient!
You need to minimize “daisy chain” communication between the ED doctor and the hospitalist who will actually see the patient. In the scenario above, I think it would be best to eliminate the triage doctor entirely (see my December 2008 column for ideas about how to do this) so that the hospitalist who will actually admit the patient learns of the new referral quickly and speaks directly with the ED doctor.
Another reason for a poor “page from ED to hospitalist at patient’s bedside” time is that the admitting hospitalist just doesn’t make it a priority to get to the ED quickly. They might decide to round on two or three more patients before working on the new admission. Of course, we must triage and sometimes delay getting to the ED while attending to an unstable floor patient, but not too often, in my experience.
I’m not suggesting that others adopt my habit: I typically don’t call the ED back at all. Instead, I just head to the ED immediately after being paged, and usually arrive in less time than I would have spent on hold waiting for the ED doc to come to the phone.
When presented with several ED patients to admit at the same time or tied up with a critical patient on the floor, getting to the ED quickly isn’t possible.
Write Admission or “Holding” Orders and Move the Patient to His/Her Room
This is frustrating for the admitting hospitalist, because among other problems, families often leave when the patient moves to the inpatient ward, and additional tests likely will take longer to complete from the floor rather than ED. And while it is hard to quantify, I think this practice increases the risk of errors—though some studies have shown that long ED stays are associated with worse outcomes for the patient, too.
One of the marquee principles of ED medicine has historically been “ED doctors should never write admission orders.” Even so, I sense that this view is shifting, and many now see this as clinically reasonable, and in some cases necessary, to ensure good performance. Our ED docs have agreed to do this if there is concern that the hospitalist can’t finish writing orders within an hour of first being paged about the patient. Ask me in a year and I’ll let you know just how this is going.
And, of course, another option is for the admitting hospitalist to provide admission orders or “holding” orders by phone. I think this is safe for many patients, as long as it is followed by a bedside admission visit by the hospitalist before too long.
Next month: What about throughput at the discharge end of the hospital stay? And yes, improving throughput risks quality of care, yet we have to do it while maintaining or improving quality.
Dr. Nelson has been a practicing hospitalist since 1988 and is co-founder and past president of SHM. He is a principal in Nelson Flores Hospital Medicine Consultants, a national hospitalist practice management consulting firm.
To understand one reason why your hospital cares so much about patient throughput and discharging patients before noon, think of tables in a restaurant. A restaurant has a limited number of tables, and the more quickly they serve customers, the sooner they’re able to seat a new party. So by improving their throughput, they can serve more customers and increase profitability without having to add tables.
Of course, the more a restaurant pushes to increase throughput, the more it risks a decrease in the satisfaction of its customers. Too much focus on throughput could annoy customers so much that it puts them out of business (Seinfeld’s Soup Nazi is one notable exception).
Yet hospitals are likely to increase their customers’ satisfaction by improving “front-end” throughput from the ED to the inpatient unit. In fact, CMS added two new core measures (known as inpatient quality reporting, or IQR) that hospitals began reporting on Jan. 1:
- Median time from ED arrival to ED departure for admitted patients, and
- Admit decision time to ED departure for admitted patients.
You already knew about these, right? Although they don’t directly influence Medicare payments to your hospital now, there is a pretty good chance they could become part of the Hospital Value-Based Purchasing program in the future. So expect administrative leaders at your hospital to continue pestering you about ED throughput with even greater intensity.
I confess that improving throughput feels like one of the most difficult things for me to do, and I sweat when in meetings about it. Yet I know intellectually that we can do better. After all, it wasn’t that long ago that a patient could expect to wait hours to see an ED doctor, and shortening the wait seemed an impossible task. But EDs everywhere have done just that, and now lots of them post the current ED wait time (typically less than 10 or 15 minutes!) on the Internet or billboards. So if they can do it, then I’m sure hospitalists can, too.
I wrote about hospital throughput in my April 2009 and May 2010 columns, and included some strategies you might want to try. Here are a few more directed at ED-to-inpatient ward throughput.
The One-Admitter Approach
Most practices have no option other than a single admitter at night, but if your one admitter during daytime hours reliably gets behind, then you should have “rounders” help with admitting duties. While this is stressful for the rounder, and could sometimes delay the rounder’s ability to discharge patients early (more on that later), in most cases, the benefit will be worth the cost. In fact, I think the best arrangement is for all, or nearly all, daytime hospitalists to share admissions every day.
Remember, this approach will eliminate the admitter-to-rounder handoff from Day One to Day Two, resulting in a net increase in efficiency (and probably patient satisfaction). Plus, reduction handoff errors should decrease, because only one doctor, rather than two, will have to get to know the new patient.
Eliminate Impediments
Some hospitalists with inefficient work habits have an opportunity to become more efficient at taking a history and reviewing records, along with other steps in the admission process, without risking poor quality. But most are reasonably efficient at those tasks already and shouldn’t make them the focus of throughput efforts.
That said, most, or nearly all, hospitalists could shorten the time from when the ED pages on a new admission to when we appear in the ED to see the patient.
Here is one scenario that gives rise to the type of delay I’m talking about. The ED doctor pages a triage hospitalist, and the two finally speak directly 10 minutes later. After a five-minute conversation, the triage doctor pages the hospitalist who will actually admit the patient, waits 10 minutes for the return call, relays the information (possibly leaving out or misrepresenting some of what the ED doc said), and the admitting hospitalist heads to the ED 15 minutes later. Each person in this scenario acted reasonably promptly, but the clock starts with the first ED page. The hospitalist is already at 45 minutes when they arrive to see the patient!
You need to minimize “daisy chain” communication between the ED doctor and the hospitalist who will actually see the patient. In the scenario above, I think it would be best to eliminate the triage doctor entirely (see my December 2008 column for ideas about how to do this) so that the hospitalist who will actually admit the patient learns of the new referral quickly and speaks directly with the ED doctor.
Another reason for a poor “page from ED to hospitalist at patient’s bedside” time is that the admitting hospitalist just doesn’t make it a priority to get to the ED quickly. They might decide to round on two or three more patients before working on the new admission. Of course, we must triage and sometimes delay getting to the ED while attending to an unstable floor patient, but not too often, in my experience.
I’m not suggesting that others adopt my habit: I typically don’t call the ED back at all. Instead, I just head to the ED immediately after being paged, and usually arrive in less time than I would have spent on hold waiting for the ED doc to come to the phone.
When presented with several ED patients to admit at the same time or tied up with a critical patient on the floor, getting to the ED quickly isn’t possible.
Write Admission or “Holding” Orders and Move the Patient to His/Her Room
This is frustrating for the admitting hospitalist, because among other problems, families often leave when the patient moves to the inpatient ward, and additional tests likely will take longer to complete from the floor rather than ED. And while it is hard to quantify, I think this practice increases the risk of errors—though some studies have shown that long ED stays are associated with worse outcomes for the patient, too.
One of the marquee principles of ED medicine has historically been “ED doctors should never write admission orders.” Even so, I sense that this view is shifting, and many now see this as clinically reasonable, and in some cases necessary, to ensure good performance. Our ED docs have agreed to do this if there is concern that the hospitalist can’t finish writing orders within an hour of first being paged about the patient. Ask me in a year and I’ll let you know just how this is going.
And, of course, another option is for the admitting hospitalist to provide admission orders or “holding” orders by phone. I think this is safe for many patients, as long as it is followed by a bedside admission visit by the hospitalist before too long.
Next month: What about throughput at the discharge end of the hospital stay? And yes, improving throughput risks quality of care, yet we have to do it while maintaining or improving quality.
Dr. Nelson has been a practicing hospitalist since 1988 and is co-founder and past president of SHM. He is a principal in Nelson Flores Hospital Medicine Consultants, a national hospitalist practice management consulting firm.
Incentives to Improve Hospital Readmission Rates OK
Iam a practicing hospitalist as well as a consultant to hospitals and health systems. One of my clients has a question: Is it legal to incentivize hospitalists to reduce readmission rates?
Alexander Strachan Jr., MD, MBA, CEO and managing director, CrossWalk Consulting Group LLC, Mission Viejo, Calif.
Dr. Hospitalist responds:
If you recall, a few months ago we discussed gainsharing and its attendant implications for physicians. The other side of that coin would be something like readmission rates. How so? Well, Medicare prohibits gainsharing (when hospitals share with physicians in the savings from improved service utilization), with the exception of two small, ongoing demonstration projects. For readmission rates, there are proposed penalties for hospitals, but not for physicians, so again there is no direct linkage of incentives.
Let’s take a look at our subject again: readmissions. Definitely in the news, as starting Oct. 1, 2012, hospitals can be penalized for exceeding the target readmission rate for the diagnoses of acute myocardial infarction, heart failure, and pneumonia. No surprise, but these are the same diagnoses that are part of the value-based purchasing payment methodology. These are both offshoots from the controversial Affordable Care Act of 2010.
The penalty, which is set at 1% for fiscal-year 2013 and escalates to a maximum of 3% for fiscal-year 2015, is based not just on the diagnosis-related group (DRG) payments for those specific conditions, but also for all DRG payments the hospital receives in that fiscal year. Understandably, hospitals are paying attention to this. Physicians, on the other hand, are not directly connected to the penalty. However, as a hospitalist, I imagine that you are either employed by the hospital or your group has a contract with the hospital—and the hospital is paying part of your salary. We aren’t exactly “independent professionals” these days.
As for the question at hand, then can a hospital incentivize a physician directly to reduce the readmission rate? Sure! Why not? Sounds easy enough.
However, it bears taking a closer look at how this might happen. Remember, Medicare frowns upon the potential denial of care or reduction in services, which is why gainsharing still hasn’t made its way forward. For example, a hospital could not pay a physician to turn away a potential readmission at the door. If the hospital wants to pay a bonus for reducing the readmit rate to 5% from 10%, great. If the hospital wants to pay a physician $500 for each potential readmission that is sent home from the ED, bad idea.
Similarly, the readmission rate is based on inpatient admissions, and patients who are admitted as observation technically are outpatients. So, if the hospital (employer) encouraged the inappropriate use of observation status, it would be a big no-no.
So what are the potential solutions? Well, as above, the hospital could construct a bonus based on the improvement (or maintenance) of a specific readmission rate, but it can’t dictate a process that might be interpreted as a denial of care. Alternately, it could pay for a process that might be expected to have a positive impact on the readmission rate. The hospital could require notification of pending discharges for the three “targeted” diagnoses, which would then allow for more resources to be directed to the patient prior to discharge. From the other end, the hospital could promote seeing those specific patients at risk for readmission earlier in their presentation (in the ED) to engage the hospitalists in management of the disease.
There are many ways to creatively design a solution, but the most important point is to avoid the incentive for the denial of care. Yes, Medicare wants you to improve the hospital’s readmission rate, but the preferred approach is to provide more resources to this population, not fewer.
Iam a practicing hospitalist as well as a consultant to hospitals and health systems. One of my clients has a question: Is it legal to incentivize hospitalists to reduce readmission rates?
Alexander Strachan Jr., MD, MBA, CEO and managing director, CrossWalk Consulting Group LLC, Mission Viejo, Calif.
Dr. Hospitalist responds:
If you recall, a few months ago we discussed gainsharing and its attendant implications for physicians. The other side of that coin would be something like readmission rates. How so? Well, Medicare prohibits gainsharing (when hospitals share with physicians in the savings from improved service utilization), with the exception of two small, ongoing demonstration projects. For readmission rates, there are proposed penalties for hospitals, but not for physicians, so again there is no direct linkage of incentives.
Let’s take a look at our subject again: readmissions. Definitely in the news, as starting Oct. 1, 2012, hospitals can be penalized for exceeding the target readmission rate for the diagnoses of acute myocardial infarction, heart failure, and pneumonia. No surprise, but these are the same diagnoses that are part of the value-based purchasing payment methodology. These are both offshoots from the controversial Affordable Care Act of 2010.
The penalty, which is set at 1% for fiscal-year 2013 and escalates to a maximum of 3% for fiscal-year 2015, is based not just on the diagnosis-related group (DRG) payments for those specific conditions, but also for all DRG payments the hospital receives in that fiscal year. Understandably, hospitals are paying attention to this. Physicians, on the other hand, are not directly connected to the penalty. However, as a hospitalist, I imagine that you are either employed by the hospital or your group has a contract with the hospital—and the hospital is paying part of your salary. We aren’t exactly “independent professionals” these days.
As for the question at hand, then can a hospital incentivize a physician directly to reduce the readmission rate? Sure! Why not? Sounds easy enough.
However, it bears taking a closer look at how this might happen. Remember, Medicare frowns upon the potential denial of care or reduction in services, which is why gainsharing still hasn’t made its way forward. For example, a hospital could not pay a physician to turn away a potential readmission at the door. If the hospital wants to pay a bonus for reducing the readmit rate to 5% from 10%, great. If the hospital wants to pay a physician $500 for each potential readmission that is sent home from the ED, bad idea.
Similarly, the readmission rate is based on inpatient admissions, and patients who are admitted as observation technically are outpatients. So, if the hospital (employer) encouraged the inappropriate use of observation status, it would be a big no-no.
So what are the potential solutions? Well, as above, the hospital could construct a bonus based on the improvement (or maintenance) of a specific readmission rate, but it can’t dictate a process that might be interpreted as a denial of care. Alternately, it could pay for a process that might be expected to have a positive impact on the readmission rate. The hospital could require notification of pending discharges for the three “targeted” diagnoses, which would then allow for more resources to be directed to the patient prior to discharge. From the other end, the hospital could promote seeing those specific patients at risk for readmission earlier in their presentation (in the ED) to engage the hospitalists in management of the disease.
There are many ways to creatively design a solution, but the most important point is to avoid the incentive for the denial of care. Yes, Medicare wants you to improve the hospital’s readmission rate, but the preferred approach is to provide more resources to this population, not fewer.
Iam a practicing hospitalist as well as a consultant to hospitals and health systems. One of my clients has a question: Is it legal to incentivize hospitalists to reduce readmission rates?
Alexander Strachan Jr., MD, MBA, CEO and managing director, CrossWalk Consulting Group LLC, Mission Viejo, Calif.
Dr. Hospitalist responds:
If you recall, a few months ago we discussed gainsharing and its attendant implications for physicians. The other side of that coin would be something like readmission rates. How so? Well, Medicare prohibits gainsharing (when hospitals share with physicians in the savings from improved service utilization), with the exception of two small, ongoing demonstration projects. For readmission rates, there are proposed penalties for hospitals, but not for physicians, so again there is no direct linkage of incentives.
Let’s take a look at our subject again: readmissions. Definitely in the news, as starting Oct. 1, 2012, hospitals can be penalized for exceeding the target readmission rate for the diagnoses of acute myocardial infarction, heart failure, and pneumonia. No surprise, but these are the same diagnoses that are part of the value-based purchasing payment methodology. These are both offshoots from the controversial Affordable Care Act of 2010.
The penalty, which is set at 1% for fiscal-year 2013 and escalates to a maximum of 3% for fiscal-year 2015, is based not just on the diagnosis-related group (DRG) payments for those specific conditions, but also for all DRG payments the hospital receives in that fiscal year. Understandably, hospitals are paying attention to this. Physicians, on the other hand, are not directly connected to the penalty. However, as a hospitalist, I imagine that you are either employed by the hospital or your group has a contract with the hospital—and the hospital is paying part of your salary. We aren’t exactly “independent professionals” these days.
As for the question at hand, then can a hospital incentivize a physician directly to reduce the readmission rate? Sure! Why not? Sounds easy enough.
However, it bears taking a closer look at how this might happen. Remember, Medicare frowns upon the potential denial of care or reduction in services, which is why gainsharing still hasn’t made its way forward. For example, a hospital could not pay a physician to turn away a potential readmission at the door. If the hospital wants to pay a bonus for reducing the readmit rate to 5% from 10%, great. If the hospital wants to pay a physician $500 for each potential readmission that is sent home from the ED, bad idea.
Similarly, the readmission rate is based on inpatient admissions, and patients who are admitted as observation technically are outpatients. So, if the hospital (employer) encouraged the inappropriate use of observation status, it would be a big no-no.
So what are the potential solutions? Well, as above, the hospital could construct a bonus based on the improvement (or maintenance) of a specific readmission rate, but it can’t dictate a process that might be interpreted as a denial of care. Alternately, it could pay for a process that might be expected to have a positive impact on the readmission rate. The hospital could require notification of pending discharges for the three “targeted” diagnoses, which would then allow for more resources to be directed to the patient prior to discharge. From the other end, the hospital could promote seeing those specific patients at risk for readmission earlier in their presentation (in the ED) to engage the hospitalists in management of the disease.
There are many ways to creatively design a solution, but the most important point is to avoid the incentive for the denial of care. Yes, Medicare wants you to improve the hospital’s readmission rate, but the preferred approach is to provide more resources to this population, not fewer.
Pediatric Hospitalists Climb the Corporate Ladder
Pediatric hospitalists around the country have made inroads into hospital administration roles. Here are some of the movers and shakers:
Erin Stucky Fisher, MD, FAAP, MHM, medical director for quality improvement at Rady Children’s Hospital, San Diego
Paul Hain, MD, associate chief of staff and medical director for performance management and improvement, Monroe Carell Jr. Children’s Hospital at Vanderbilt, Nashville, Tenn.
Sanford Meltzer, MD, MBA, senior vice president for strategic planning at Seattle Children’s Hospital
Stephen Muething, MD, vice president of safety at Cincinnati Children’s Hospital
Steve Narang, MD, chief medical officer of Banner Health System’s Cardon Children’s Medical Center, Mesa, Ariz.
Brian M. Pate, MD, FHM, FAAP, vice chair of inpatient services for the department of pediatrics at Children’s Mercy Hospitals and Clinics, Kansas City
Shannon Connor Phillips, MD, MPH, FAAP, quality and patient safety officer at the Cleveland Clinic
Jeff Sperring, MD, CEO, Riley Hospital for Children at Indiana University Health, Indianapolis, Ind.
Editor’s note: We, of course, don’t know of all the hospitalists around the country that have risen to C-suite positions. Let us know if we missed one; send Editor Jason Carris an email ([email protected]) and we’ll add it to our monthly “Hospitalists on the Move” section.
Pediatric hospitalists around the country have made inroads into hospital administration roles. Here are some of the movers and shakers:
Erin Stucky Fisher, MD, FAAP, MHM, medical director for quality improvement at Rady Children’s Hospital, San Diego
Paul Hain, MD, associate chief of staff and medical director for performance management and improvement, Monroe Carell Jr. Children’s Hospital at Vanderbilt, Nashville, Tenn.
Sanford Meltzer, MD, MBA, senior vice president for strategic planning at Seattle Children’s Hospital
Stephen Muething, MD, vice president of safety at Cincinnati Children’s Hospital
Steve Narang, MD, chief medical officer of Banner Health System’s Cardon Children’s Medical Center, Mesa, Ariz.
Brian M. Pate, MD, FHM, FAAP, vice chair of inpatient services for the department of pediatrics at Children’s Mercy Hospitals and Clinics, Kansas City
Shannon Connor Phillips, MD, MPH, FAAP, quality and patient safety officer at the Cleveland Clinic
Jeff Sperring, MD, CEO, Riley Hospital for Children at Indiana University Health, Indianapolis, Ind.
Editor’s note: We, of course, don’t know of all the hospitalists around the country that have risen to C-suite positions. Let us know if we missed one; send Editor Jason Carris an email ([email protected]) and we’ll add it to our monthly “Hospitalists on the Move” section.
Pediatric hospitalists around the country have made inroads into hospital administration roles. Here are some of the movers and shakers:
Erin Stucky Fisher, MD, FAAP, MHM, medical director for quality improvement at Rady Children’s Hospital, San Diego
Paul Hain, MD, associate chief of staff and medical director for performance management and improvement, Monroe Carell Jr. Children’s Hospital at Vanderbilt, Nashville, Tenn.
Sanford Meltzer, MD, MBA, senior vice president for strategic planning at Seattle Children’s Hospital
Stephen Muething, MD, vice president of safety at Cincinnati Children’s Hospital
Steve Narang, MD, chief medical officer of Banner Health System’s Cardon Children’s Medical Center, Mesa, Ariz.
Brian M. Pate, MD, FHM, FAAP, vice chair of inpatient services for the department of pediatrics at Children’s Mercy Hospitals and Clinics, Kansas City
Shannon Connor Phillips, MD, MPH, FAAP, quality and patient safety officer at the Cleveland Clinic
Jeff Sperring, MD, CEO, Riley Hospital for Children at Indiana University Health, Indianapolis, Ind.
Editor’s note: We, of course, don’t know of all the hospitalists around the country that have risen to C-suite positions. Let us know if we missed one; send Editor Jason Carris an email ([email protected]) and we’ll add it to our monthly “Hospitalists on the Move” section.
Access Problems Persist Despite Health Insurance: Lessons from Massachusetts
A surprising lesson from Massachusetts is that expanding health insurance coverage does not automatically improve access to healthcare services. Here’s proof:
- More than half of primary-care physicians (PCPs) in Massachusetts are not accepting new patients.
- Wait times to see PCPs remain high: 48 days for internal medicine, 36 days for family medicine.
- The percentage of internal medicine physicians accepting Medicaid has decreased by double digits.
- Many physicians who accept Medicaid report that a lack of qualified specialists in their area is a major problem that limits their ability to provide high-quality care.
- Many physicians who accept a high proportion of Medicaid patients are in solo or two-physician practices, and have limited ability to expand hours of availability.
- ED use increased 10% from 2004 to 2008, and high levels of ED use have persisted since the reform law was enacted—a strong indicator of PCP shortages. Massachusetts has 491 ED visits per 1,000 residents, compared with a national average of 401 visits per 1,000 residents.
- Preventable hospitalization rates have not decreased, and are comparable to that of Medicaid patients and uninsured patients—remaining at about 10% from 2004 to 2008.
A surprising lesson from Massachusetts is that expanding health insurance coverage does not automatically improve access to healthcare services. Here’s proof:
- More than half of primary-care physicians (PCPs) in Massachusetts are not accepting new patients.
- Wait times to see PCPs remain high: 48 days for internal medicine, 36 days for family medicine.
- The percentage of internal medicine physicians accepting Medicaid has decreased by double digits.
- Many physicians who accept Medicaid report that a lack of qualified specialists in their area is a major problem that limits their ability to provide high-quality care.
- Many physicians who accept a high proportion of Medicaid patients are in solo or two-physician practices, and have limited ability to expand hours of availability.
- ED use increased 10% from 2004 to 2008, and high levels of ED use have persisted since the reform law was enacted—a strong indicator of PCP shortages. Massachusetts has 491 ED visits per 1,000 residents, compared with a national average of 401 visits per 1,000 residents.
- Preventable hospitalization rates have not decreased, and are comparable to that of Medicaid patients and uninsured patients—remaining at about 10% from 2004 to 2008.
A surprising lesson from Massachusetts is that expanding health insurance coverage does not automatically improve access to healthcare services. Here’s proof:
- More than half of primary-care physicians (PCPs) in Massachusetts are not accepting new patients.
- Wait times to see PCPs remain high: 48 days for internal medicine, 36 days for family medicine.
- The percentage of internal medicine physicians accepting Medicaid has decreased by double digits.
- Many physicians who accept Medicaid report that a lack of qualified specialists in their area is a major problem that limits their ability to provide high-quality care.
- Many physicians who accept a high proportion of Medicaid patients are in solo or two-physician practices, and have limited ability to expand hours of availability.
- ED use increased 10% from 2004 to 2008, and high levels of ED use have persisted since the reform law was enacted—a strong indicator of PCP shortages. Massachusetts has 491 ED visits per 1,000 residents, compared with a national average of 401 visits per 1,000 residents.
- Preventable hospitalization rates have not decreased, and are comparable to that of Medicaid patients and uninsured patients—remaining at about 10% from 2004 to 2008.
Negotiation Strategies for Better Compensation
The first step in negotiating is deciding to negotiate at all, Dr. Gebhard says. You must also recognize that many employers initially offer a lower compensation package because they expect negotiation to occur.
“You should have the mindset that everything is negotiable,” she says. “You have things to offer them and they have things to offer you, and it’s usually somewhere in between where you land.”
To prepare, a hospitalist should at minimum know what the local expectations are in pay, Dr. Fisher says. You might want to consider hiring a physician coach to learn effective negotiating strategies, Dr. Gebhard adds. Role-playing negotiation situations with a more experienced hospitalist can help, Dr. Reich says, as can attending negotiation skills workshops offered by SHM, the American Medical Women’s Association, and the American College of Physician Executives.
“It’s a matter of training people to feel negotiating is not self-serving or asking for more than what you’re valued at,” Dr. Fisher says. “It’s instead placing a value that’s appropriate and feeling confident that you’re asking for something that others in your same position would be asking for.”
Compensation isn’t the only negotiating point. “How much you’re worth is how many resources they’re going to invest in you so you can do the best job possible,” Dr. Brodsky says. “If you have adequate resources, then it’s much easier to bring yourself into a flexible situation because you’re getting what you need fairly. You can make the job look the way you want it to look while giving your employer fair value.”
Because people expect women to be communally interested rather than self-interested, a female hospitalist might want to approach negotiating from the standpoint of the common good of her family or the company, Dr. Gault says. “These sorts of requests aren’t met with surprise or negative judgment as much,” she says.
Nonetheless, women must be prepared for defeat.
“I think women should negotiate more. Not so much because it will be a successful strategy, but because in order to support one another, women have to get used to doing it,” Dr. Gault says. “We have to be willing to take the risk so that our perceptions and our ideas about what women should or shouldn’t do gradually shift over time.”
The first step in negotiating is deciding to negotiate at all, Dr. Gebhard says. You must also recognize that many employers initially offer a lower compensation package because they expect negotiation to occur.
“You should have the mindset that everything is negotiable,” she says. “You have things to offer them and they have things to offer you, and it’s usually somewhere in between where you land.”
To prepare, a hospitalist should at minimum know what the local expectations are in pay, Dr. Fisher says. You might want to consider hiring a physician coach to learn effective negotiating strategies, Dr. Gebhard adds. Role-playing negotiation situations with a more experienced hospitalist can help, Dr. Reich says, as can attending negotiation skills workshops offered by SHM, the American Medical Women’s Association, and the American College of Physician Executives.
“It’s a matter of training people to feel negotiating is not self-serving or asking for more than what you’re valued at,” Dr. Fisher says. “It’s instead placing a value that’s appropriate and feeling confident that you’re asking for something that others in your same position would be asking for.”
Compensation isn’t the only negotiating point. “How much you’re worth is how many resources they’re going to invest in you so you can do the best job possible,” Dr. Brodsky says. “If you have adequate resources, then it’s much easier to bring yourself into a flexible situation because you’re getting what you need fairly. You can make the job look the way you want it to look while giving your employer fair value.”
Because people expect women to be communally interested rather than self-interested, a female hospitalist might want to approach negotiating from the standpoint of the common good of her family or the company, Dr. Gault says. “These sorts of requests aren’t met with surprise or negative judgment as much,” she says.
Nonetheless, women must be prepared for defeat.
“I think women should negotiate more. Not so much because it will be a successful strategy, but because in order to support one another, women have to get used to doing it,” Dr. Gault says. “We have to be willing to take the risk so that our perceptions and our ideas about what women should or shouldn’t do gradually shift over time.”
The first step in negotiating is deciding to negotiate at all, Dr. Gebhard says. You must also recognize that many employers initially offer a lower compensation package because they expect negotiation to occur.
“You should have the mindset that everything is negotiable,” she says. “You have things to offer them and they have things to offer you, and it’s usually somewhere in between where you land.”
To prepare, a hospitalist should at minimum know what the local expectations are in pay, Dr. Fisher says. You might want to consider hiring a physician coach to learn effective negotiating strategies, Dr. Gebhard adds. Role-playing negotiation situations with a more experienced hospitalist can help, Dr. Reich says, as can attending negotiation skills workshops offered by SHM, the American Medical Women’s Association, and the American College of Physician Executives.
“It’s a matter of training people to feel negotiating is not self-serving or asking for more than what you’re valued at,” Dr. Fisher says. “It’s instead placing a value that’s appropriate and feeling confident that you’re asking for something that others in your same position would be asking for.”
Compensation isn’t the only negotiating point. “How much you’re worth is how many resources they’re going to invest in you so you can do the best job possible,” Dr. Brodsky says. “If you have adequate resources, then it’s much easier to bring yourself into a flexible situation because you’re getting what you need fairly. You can make the job look the way you want it to look while giving your employer fair value.”
Because people expect women to be communally interested rather than self-interested, a female hospitalist might want to approach negotiating from the standpoint of the common good of her family or the company, Dr. Gault says. “These sorts of requests aren’t met with surprise or negative judgment as much,” she says.
Nonetheless, women must be prepared for defeat.
“I think women should negotiate more. Not so much because it will be a successful strategy, but because in order to support one another, women have to get used to doing it,” Dr. Gault says. “We have to be willing to take the risk so that our perceptions and our ideas about what women should or shouldn’t do gradually shift over time.”
Online Exclusive: TK
Enter text here
Enter text here
Enter text here
Patient Experiences of Hospital Discharge
The transition from hospital to home is a complex event offering multiple provider‐identified opportunities to improve healthcare quality.18 Centering care delivery around patient needs and preferences is both inherently valuable and linked with better outcomes.9
The Care Transitions Measure (CTM) identifies 4 domains of patient experience related to hospital discharge: information transfer, patient and caregiver preparation, self‐management support, and empowerment to assert preferences.10 It discriminates between patients who do or do not experience a subsequent readmission or emergency room visit and between levels of care coordination.11 Quality indicators like the CTM are important tools for systematic healthcare improvement, but they provide a limited understanding of patient experiences, which can drive the transformation of systems.12, 13
With the exception of patients with a few specific clinical conditions, relatively little is known about how adult patients perceive the hospital‐to‐home transition.1417 They recall receiving discharge instructions but lack details about what to do if problems arise.18 They may lack important information despite receiving instruction.19 Caregivers report problems related to emotional support, discharge planning, and family participation,20 and patients and caregivers express anxiety, confusion, a sense of abandonment by the healthcare system, and the perception that their preferences are disregarded.21
As part of ongoing quality improvement activities, we sought to develop a richly detailed, patient‐centered view of the hospital‐to‐home transition. Our purpose was to understand patient and caregiver experiences during this pivotal healthcare experience.
METHODS
We used an applied ethnographic approach,22 conducting participant observation and video recording in‐depth, semi‐structured interviews in Kaiser Permanente Southern California, Colorado, and Hawaii. The United States' largest, private, not‐for‐profit integrated healthcare delivery system, Kaiser Permanente addresses all health needs for more than 8.9 million members.
To balance the pragmatic imperatives of quality improvement with obtaining enough information to understand patient experiences, we planned a sample of 24 patients across 3 settings with a mix of resource‐intensive and less‐intensive healthcare needs. We defined resource‐intensive needs as occurring among patients aged 65 or older with 3 or more chronic conditions. We asked hospital staff to identify patients by level of need and variety in diagnoses and illness severity, planned or unplanned hospitalizations, age, and ability to self manage. Reasons for admission included joint replacement, acute appendicitis, chronic illness exacerbation, complications of cancer chemotherapy, and others. We included patients who were inpatients or discharged no more than 3 weeks before interview. We excluded those under the age of 18 or discharged to non‐home settings. The project took place between September and November of 2008; 24 patients, half of whom were male, gave written informed consent for video recordings and authorization to distribute protected health information throughout and beyond Kaiser Permanente for quality improvement and educational purposes. Participants took part in interviews and observations lasting 1 to 3 hours; caregivers and family members participated in 9 instances.
Two or 3 observers attended each interview, which took place in the hospital on discharge day, at postdischarge appointments, or in patients' homes. Open‐ended questions prompted broad‐ranging inquiry into patients' lives, medical history, hospitalization experience, medications, care network, challenges, personal goals, and inner experience. Some questions were adapted and expanded from the CTM; others were prompts to demonstrate activities (eg, Can you show us how you organize your medications?). In addition to interviewing patients and caregivers, we observed interactions between patients, families, and hospital staff before discharge. We also observed patients and caregivers at home and when interacting with outpatient primary care providers. The purpose of observation was to understand the context of patient and caregiver experiences and to identify consistencies or discrepancies with their descriptions of experiences. (see Supporting Information In‐Home Interview Guide in the online version of this article)
Data included field notes and video recordings. In addition, observers summarized their strongest daily impressions as brief team stories that were shared with the observation team, local operations staff, and Kaiser Permanente national subject matter experts.23 Consistent with a grounded theory approach, interviews were professionally transcribed and qualitatively analyzed by multiple observers in iterative stages to develop broad domains of patient experiences.24 We clustered similar experiences and identified exemplar statements and behaviors. Team stories were analyzed separately, using a similar process. We reviewed recorded interviews to refine our emerging understanding of patient and caregiver experiences and discussed our observations and impressions about each domain. To maximize internal validity, an independent researcher who did not attend the interviews reviewed the transcripts and coding and participated in final qualitative analysis. Institutional review board approval was not required for this quality improvement project.
RESULTS
Patients and caregivers expressed or demonstrated 6 domains of experience as they transitioned from hospital to home (Table 1).
| Need | Key Observations |
|---|---|
| Translating knowledge into safe, health‐promoting actions at home | Even when patients and caregivers believe they have all needed information before discharge, they often find later that they are lacking knowledge or cannot translate it into contextually appropriate actions. |
| Patients and caregivers may inaccurately perceive that they have successfully translated knowledge into safe, health‐promoting actions. | |
| The day of discharge may not be the optimal time for learning. | |
| Inclusion of caregivers at every step of the transition process | Caregivers are integrally involved in the care for many patients. |
| Discharge teaching does not optimally include caregivers. | |
| Having readily available problem‐solving resources | Questions normally arise after the transition home as patients and caregivers engage in ongoing care activities. |
| Even patients and caregivers successfully providing care at home may need help interpreting experiences. | |
| Feeling connected to and trusting providers | Patients and caregivers highly value a feeling of being connected to providers, typically in the context of ongoing relationships. |
| Providers sometimes miss opportunities to connect with patients. | |
| Although investing in building connections with patients is time‐consuming for providers, patients may disregard communication unless it occurs. | |
| Transitioning from illness‐defined experience to normal life | Patients and caregivers want to return to a sense of normal life as quickly as possible. |
| This desire may interfere with the ability to absorb information and translate it, to prioritize healthcare needs, or to accurately assess the risk in a situation. | |
| Anticipating needs at home and making arrangements to meet them | Patients and caregivers require many types of help, but some may have trouble reconciling the need for assistance with the desire to return to a normal life. |
| Patients and caregivers find it stressful when needed arrangements have not been made. | |
| Some needed arrangements do not pertain strictly to healthcare (eg, help at home, meals). |
Translating Knowledge Into Safe, Health‐Promoting Actions at Home
A primary activity on discharge day was patient education provided by hospital staff. Topics included health conditions, medications, resources, activity, diet, equipment, supplies, and procedures. A nurse typically reviewed written instructions with the patient; the process ranged from thoughtful conversations to cursory recitation of printed information. Teaching was often sandwiched between other activities, and some staff members appeared pressured to complete it.
Patients and caregivers generally reported having all the information they needed; however, when we observed them at home, we noted that translating knowledge into safe, health‐promoting actions was a separate step. A common example was medication management. Patients or caregivers often rewrote the discharge medication list, grouping medications by purpose or creating charts of when to take each one. Patients and caregivers developed varying and somewhat complex systems for home medication management. For example, 1 patient taking 16 medications filled five 7‐day pillboxes each week; from these, he filled a tiny mug 5 times a day, placing it where it would remind him to take his medications. Patients interviewed about their medications at home often expressed uncertainty about their understanding of the medications and about how and why they were taking them.
When procedures were involved, such as dressing changes or administering intravenous (IV) solutions, in‐hospital teaching didn't always translate smoothly into safe action at home. A man who learned to administer total parenteral nutrition in the hospital found his first at‐home session unexpectedly challenging: I just got home and was behind schedule hooking up to the machine. I'm thinking, Which (tube) goes where? and getting real tired. I looked at the sheets. They have all the information you need, but it's too much for a tired person. I didn't want to read, and the pictures weren't clear, and I thought, I'll just try to remember what they said. (Patient #9)
We directly observed patients and caregivers failing to translate knowledge into safe, health‐promoting actions at home. Two days after discharge following a total knee replacement, a patient navigated a flight of stairs with a walker. In another instance, a caregiver hung an IV on a coat hanger hooked precariously to a mailbox as children raced around the room. An older man described strengthening and mobility exercises as instructed by his physical therapist but didn't perform them. Their reasoning was often unclear. For instance, after a nurse reviewed a list of discharge medications and left the room, despite verbal agreement with the instructions, the patient commented: Eight pills are too many. I'll take 3 today and 3 tomorrow and see how I feel. (Patient #27)
Inclusion of caregivers at Every Step of the Transition Process
After discharge, caregivers helped with or took responsibility for managing medications, wound care, administering intravenous antibiotics, adjusting diets, filling prescriptions, obtaining medical supplies and equipment, taking vital signs, interpreting signs and symptoms, monitoring health indicators, deciding who and when to call, and advocating for patients. When patients required hands‐on care tasks, such as dressing changes or intravenous medications, caregivers typically received instruction from hospital staff before discharge.
However, in many cases, including caregivers in discharge teaching appeared to be a low priority. In several instances, caregivers were unable to speak directly with a physician before the patient's discharge: I was hoping I could do that before she came home. I know it's hard to get hold of the doctors, but I wanted to know what to expect. (Caregiver #24)
Even when a caregiver was present, hospital staff frequently directed teaching exclusively toward the patient. For example, a nurse and patient sat side‐by‐side to review instructions; the highly motivated caregiver, seated across the room due to lack of space, was unable to see the written material. The integral role of caregivers in helping patients at home contrasted with their often peripheral role in in‐hospital transition processes.
Having Readily Available Problem‐Solving Resources
Patients and caregivers needed to know who and when to call for more information. They needed to discriminate between providers (eg, when to call a cardiologist vs a primary care provider), identify who to call in an urgent or emergent situation, and know how to access various resources. Some questions arose because patients lacked sufficient detail about what to expect. Even patients who successfully translated knowledge into safe, health‐promoting actions might need help interpreting observations: The wound is closed on top but not underneath, and the WoundVac is supposed to be working on the cells. I'm using the same amount of foam as when I started, so is it really healing? Shouldn't we be using less foam? We don't have anyone to answer the questions. (Patient #22)
Many patients with chronic conditions had direct numbers to their physicians' office; some had important numbers for a doctor or pharmacy on speed‐dial. Many patients and caregivers expressed a sense of pride at knowing how to navigate the healthcare system: I've learned how to get to him. I call downtown, and then they call out to his office. (Patient #8)
Other patients and caregivers gave conflicting messages; they said they knew who to call but provided few specifics: If he needed a nurse, I'd ask for the nurse assistant. I'll just do that or something. (Caregiver #20)
Feeling Connected to and Trusting Providers
For patients and caregivers, a critical aspect of communications with providers was a sense of connection, typically with a particular healthcare provider as part of an ongoing, trusting relationship. Patients expressed feeling respected, that their individual concerns and needs mattered, and that providers appreciated their emotional experiences, listened carefully without seeming rushed, and valued their knowledge. Successful experiences of connection were clearly meaningful to patients: The most important thing is how genuine the doctor is as a person. I pick up on that right away. It bothers me when they're not all there. It amazes me that they have the intellectual prowess to be a doctor, but there are other components that are not quite there yet. My doctor, he's got it all. (Patient #9)
This sense of connection often contrasted with what they may have experienced during short‐term relationships with providers in the hospital. In addition, providers sometimes overlooked opportunities to connect with patients. For instance, a clinic nurse, busy with intake, did not acknowledge a patient's repeated requests for help modifying his diet.
Transitioning From Illness‐Defined Experience to Normal Life
Patients and caregivers described or demonstrated a variety of ways of leavingor wanting to leavethe experience of illness behind, including feeling independent, useful, motivated, confident, and in control; helping others, including other patients in similar circumstances; feeling hopeful about recovery; and maintaining a sense of perspective.
This desire to get back to normal life affected the amount of information patients and caregivers absorbed on discharge day: I was so anxious to leave. I was like, Yeah, yeah, let's do this. I'm all packed. I've got one foot out the door. At home, I got ready to take my medication; the discharge instructions didn't jibe with what the doctor wrote. It was as much my fault as anyone's, because I was rushing to get home. (Patient #16)
Resuming usual activities, sleeping in one's own bed, eating familiar foods, being among friends and neighbors, and intentionally limiting the impact of a health condition on activities were all attempts to quickly restore a sense of normal life. Any milestone on the path to recovery seemed to help: I was so ecstatic in the car coming home. We were back on the road of real life. (Patient #22)
In some instances, the drive to feel a sense of normal life outweighed physical needs. For instance, a young woman with cancer delayed notifying her physician that she had cellulitis because she didn't want to interrupt her usual activities. After several days, she was taken to the emergency room by ambulance and admitted for IV antibiotics.
Anticipating Needs at Home and Making Arrangements to Meet Them
Patients and caregivers anticipated a variety of postdischarge needs. These included hands‐on healthcare tasks, grocery shopping, food preparation, and the like, as well as household maintenance, assistance with pets, and other daily activities that were unrelated to healthcare: I can't do it by myself. I can't just jump in the car and drive. So there are things that you need other people to help you with to get through the day. (Patient #9)
However many patients described a network of support including family members, neighbors, friends, clergy, and others. More than 1 helper was often required. However, patients sometimes found it difficult to reconcile the desire to return to normal life with needs for help. For example, an older woman refused a home health nursing visit for congestive heart failure because she felt it encroached on her independence. The same desire to return to normal life led patients to overestimate their ability to function independently. After a several‐day hospital stay for back surgery, a patient asked a friend to drop him off at home. He then used his walker to get to his car to retrieve a cart for his belongings. He pushed the walker with 1 hand and dragged the cart behind him up 2 floors to his apartment. Once inside, he went to bed, exhausted. In addition, it was sometimes difficult for patients to accurately anticipate needs. For example, a man who returned home alone after surgery suddenly realized his bed was much lower than the hospital bed; he wasn't sure he could get out of it without help.
Transportation home from the hospital and to outpatient appointments after discharge was a frequently identified need, leaving patients making hasty and suboptimal arrangements for a ride home, worried about keeping scheduled appointments, or both.
Patients and caregivers found it stressful when arrangements had not been made: First, we have to worry about getting home, and then I have to go to the medical supply store. What if she has to use the restroom? She has to wait until I get back. (Caregiver #8)
Patients and caregivers described experiences of making arrangements that were largely successful; however, they were also often time‐consuming.
DISCUSSION
Using an ethnographic approach, we identified 6 domains of patient and caregiver experience during the hospital‐to‐home transition. Many needs in these domains arose in the hours and days after patients returned home, and patients and caregivers often found it challenging to meet them. Our project adds a detailed, patient‐centered perspective on the transition from hospital to home.
The domains we identified share some conceptual territory with the dimensions of the Care Transition Measure and the Transitional Care Model,25 but generate a more detailed understanding of patient and caregiver experiences. Key findings include the fact that patients can find it challenging to translate knowledge into contextually appropriate action at home. This confirms some published results. For instance, estimates of outpatient adherence to complicated regimens range from 5% to 77%.2629 Significant opportunities exist to improve the reliability of translating medication instructions into systems that work at home,30 including aligning medication lists with physical aids (such as weekly pill boxes) and explaining medications in patient‐friendly terms. We also found that same‐day discharge teaching can be ineffective because patients are anxious to leave the hospital or staff members feel rushed. Emotion can interfere with cognition, and transferring information shortly before hospital discharge may overlook learning readiness, a fundamental principle of patient education.31, 32 In addition, the desire to return to normal life, coupled with uncertainty about who to call for clarification, can lead patients to simply do the best they can with whatever information they recall.
The literature refers to handoffs of patients from one provider to another as an episode of care is completed, but our findings suggest patients perceive hospitalization as an event occurring within ongoing relationships with the healthcare providers to whom they feel most connected.33, 34 Some patient and caregiver needs could be addressed by actively supporting these relationships during the hospital‐to‐home transition: explicitly acknowledging their importance to patients, ensuring that providers have discharge information, and framing discharge as a transition back to the care of trusted providers. Some of our findings require system‐level changes. Patients and caregivers with unmet transportation needs expressed anxiety about how or if help would materialize. Partnerships with community organizations could enable healthcare organizations to address needs like transportation that fall outside traditional discharge activities but significantly impact patient experiences. In addition, healthcare organizations are rarely designed for straightforward navigation; patient‐centered organizational designs could eliminate the need for patients and caregivers to learn how to navigate. For instance, a single point of contact for recently discharged patients might improve the process of finding help.
Strengths of our quality improvement project include the range of patients we interviewed and in‐depth observations and interviews across settings. Ethnography is ideal for generating a rich understanding of patient experiences, allowing us to observe needs patients did not mention, as well as the physical and emotional context of the transition. Weaknesses of our approach include the fact that the experiences reflected in each category were determined, to some extent, by the questions we asked. This may have constrained the variety of experiences patients reported. In addition, Kaiser Permanente's integrated nature may have affected our findings, although we believe patients and caregivers reported experiences that are likely universal.
Our project occurred in a healthcare system with an integrated electronic health record (EHR). Interventions to improve provider‐identified gaps in the discharge process often rely on information technology.3543 However, information technology does not eliminate continuity of care issues.44 Our EHR is widely used, but available information did not consistently ensure strong enough care coordination or good communication.
Including the patient's primary caregiver in discharge teaching appeared to be a relatively low priority for hospital staff, unless there was a hands‐on care task. Even when a primary caregiver was present, hospital staff frequently directed teaching exclusively toward the patient. The extent to which caregivers feel adequately prepared for their roles and responsibilities needs further exploration.
CONCLUSION
Our applied ethnographic approach reveals that patients experience several challenges while transitioning from hospital to home. Reducing readmissions is likely to remain challenging unless we broaden our understanding of the types of support and coaching required. We are translating our findings into quality improvement activities, conducting pilot projects focusing on risk stratification and tailoring of care, a specialized phone number for recently discharged patients, standardized same‐day discharge summaries to primary care providers, medication reconciliation, follow‐up phone calls, and scheduling appointments before discharge.
- ,,,.Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists.J Hosp Med.2007;2(5):314–323.
- ,,.Reconcilable differences: correcting medication errors at hospital admission and discharge.Qual Saf Health Care.2006;15(2):122–126.
- ,,,,,.Deficits in communication and information transfer between hospital‐based and primary care physicians: implications for patient safety and continuity of care.JAMA.2007;297(8):831–841.
- ,,,.Interventions to improve medication reconciliation in primary care.Ann Pharmacother.2009;43(10):1667–1675.
- ,,, et al.A quality improvement intervention to facilitate the transition of older adults from three hospitals back to their homes.J Am Geriatr Soc.2009;57(9):1540–1546.
- ,.The effects of a discharge planning and home follow‐up intervention on elders hospitalized with common medical and surgical cardiac conditions.J Cardiovasc Nurs.1999;14(1):44–54.
- ,,,,,.Improved quality in the hospital discharge summary reduces medication errors—LIMM: Landskrona Integrated Medicines Management.Eur J Clin Pharmacol.2009;65(10):1037–1046.
- ,,,,,.Omitted and unjustified medications in the discharge summary.Qual Saf Health Care.2009;18(3):205–208.
- ,,, et al.Evidence suggesting that a chronic disease self‐management program can improve health status while reducing hospitalization: a randomized trial.Med Care.1999;37(1):5–14.
- ,,,,,.Development and testing of a measure designed to assess the quality of care transitions.Int J Integr Care.2002;2:e02.
- ,,.Assessing the quality of preparation for posthospital care from the patient's perspective: the care transitions measure.Med Care.2005;43(3):246–255.
- ,.If you build it, will they come? Designing truly patient‐centered health care.Health Aff (Millwood).29(5):914–920.
- ,,,.Analysis 33:818–829.
- ,,, et al.Identifying factors associated with perceived success in the transition from hospital to home after brain injury.J Head Trauma Rehabil2011;April 25.
- ,,, et al.Perceived participation, experiences from persons with spinal cord injury in their transition period from hospital to home.Int J Rehabil Res.2010;July 31.
- ,,, et al.Reengagement in meaningful occupations during the transition from hospital to home for people with acquired brain injury and their family caregivers.Am J Occup Ther.2009;63:609–620.
- ,,.Hospital to home health care transition: patient, caregiver, and clinician perspectives.West J Nurs Res.2011;Mar 22.
- ,,.Survey on transition from inpatient to outpatient for patients on insulin: what really goes on at home?Endocr Pract.2010;16:785–791.
- ,,, et al.Carepartner experiences with hospital care.Med Care.1999;37:33–38.
- ,,, et al.Transitions of Care Consensus Policy Statement: American College of Physicians–Society of General Internal Medicine–Society of Hospital Medicine–American Geriatrics Society–American College of Emergency Physicians–Society of Academic Emergency Medicine.J Gen Intern Med.2009;24:971–976.
- .Case Study Research: Design and Methods.3rd ed.Thousand Oaks, CA:Sage Publications;2003.
- ,,.Writing Ethnographic Fieldnotes (Chicago Guides to Writing, Editing, and Publishing).Chicago, IL:University of Chicago Press;1995.
- .Learning From Strangers: The Art and Method of Qualitative Interview Studies.New York, NY:Free Press;1995.
- ,,, et al.Comprehensive discharge planning and home follow‐up of hospitalized elders: a randomized clinical trial.JAMA.1999;281(7):613–620.
- ,.Improving medication reconciliation in the 21st century.Curr Drug Saf.2008;3(3):227–229.
- ,,, et al.Trends in adherence to secondary prevention medications in elderly post‐myocardial infarction patients.Pharmacoepidemiol Drug Saf.2008;17:1189–1196.
- ,,, et al.Association between patient beliefs and medication adherence following hospitalization for acute coronary syndrome.Am Heart J.2011;161:855–863.
- ,,, et al.Adherence to statin therapy in elderly patients after hospitalization for coronary revascularization.Am J Cardiol.2011;107:1409–1414.
- ,.Medication use in the transition from hospital to home.Ann Acad Med Singapore.2008;37(2):136–141.
- .A model for assessing learning readiness for self‐direction of care in individuals with spinal cord injuries: a qualitative study.SCI Nurs.2004;21:69–74.
- .Motivational and emotional controls of cognition.Psychol Rev.1967;74(1):29–39.
- .Key legal principles for hospitalists.Dis Mon.2002;48(4):197–206.
- ,,, et al.Transition of care for hospitalized elderly patients—development of a discharge checklist for hospitalists.J Hosp Med.2006;1(6):354–360.
- ,,, et al.Electronic versus dictated hospital discharge summaries: a randomized controlled trial.J Gen Intern Med.2009;24(9):995–1001.
- ,,, et al.Creating a better discharge summary: improvement in quality and timeliness using an electronic discharge summary.J Hosp Med.2009;4(4):219–225.
- ,,,,.Electronic discharge summaries: the current state of play.HIM J.2007;36(3):30–36.
- ,,,.Patient and physician perceptions after software‐assisted hospital discharge: cluster randomized trial.J Hosp Med.2009;4(6):356–363.
- ,,, et al.Effect of an electronic medication reconciliation application and process redesign on potential adverse drug events: a cluster‐randomized trial.Arch Intern Med.2009;169(8):771–780.
- ,,,.Implementing online medication reconciliation at a large academic medical center.Jt Comm J Qual Patient Saf.2008;34(9):499–508.
- ,,,.Medication reconciliation: a necessity in promoting a safe hospital discharge.J Healthc Qual.2006;28(3):12–19.
- ,,,,.Effect of a computerized referral at hospital discharge on cardiac rehabilitation participation rates.J Cardiopulm Rehabil Prev.2009;29(6):365–369.
- ,,, et al.Implementation of an electronic system for medication reconciliation.Am J Health Syst Pharm.2007;64(4):404–422.
- ,,.Coordination of diabetes care in four delivery models using an electronic health record.Med Care.2009;47(9):993–999.
The transition from hospital to home is a complex event offering multiple provider‐identified opportunities to improve healthcare quality.18 Centering care delivery around patient needs and preferences is both inherently valuable and linked with better outcomes.9
The Care Transitions Measure (CTM) identifies 4 domains of patient experience related to hospital discharge: information transfer, patient and caregiver preparation, self‐management support, and empowerment to assert preferences.10 It discriminates between patients who do or do not experience a subsequent readmission or emergency room visit and between levels of care coordination.11 Quality indicators like the CTM are important tools for systematic healthcare improvement, but they provide a limited understanding of patient experiences, which can drive the transformation of systems.12, 13
With the exception of patients with a few specific clinical conditions, relatively little is known about how adult patients perceive the hospital‐to‐home transition.1417 They recall receiving discharge instructions but lack details about what to do if problems arise.18 They may lack important information despite receiving instruction.19 Caregivers report problems related to emotional support, discharge planning, and family participation,20 and patients and caregivers express anxiety, confusion, a sense of abandonment by the healthcare system, and the perception that their preferences are disregarded.21
As part of ongoing quality improvement activities, we sought to develop a richly detailed, patient‐centered view of the hospital‐to‐home transition. Our purpose was to understand patient and caregiver experiences during this pivotal healthcare experience.
METHODS
We used an applied ethnographic approach,22 conducting participant observation and video recording in‐depth, semi‐structured interviews in Kaiser Permanente Southern California, Colorado, and Hawaii. The United States' largest, private, not‐for‐profit integrated healthcare delivery system, Kaiser Permanente addresses all health needs for more than 8.9 million members.
To balance the pragmatic imperatives of quality improvement with obtaining enough information to understand patient experiences, we planned a sample of 24 patients across 3 settings with a mix of resource‐intensive and less‐intensive healthcare needs. We defined resource‐intensive needs as occurring among patients aged 65 or older with 3 or more chronic conditions. We asked hospital staff to identify patients by level of need and variety in diagnoses and illness severity, planned or unplanned hospitalizations, age, and ability to self manage. Reasons for admission included joint replacement, acute appendicitis, chronic illness exacerbation, complications of cancer chemotherapy, and others. We included patients who were inpatients or discharged no more than 3 weeks before interview. We excluded those under the age of 18 or discharged to non‐home settings. The project took place between September and November of 2008; 24 patients, half of whom were male, gave written informed consent for video recordings and authorization to distribute protected health information throughout and beyond Kaiser Permanente for quality improvement and educational purposes. Participants took part in interviews and observations lasting 1 to 3 hours; caregivers and family members participated in 9 instances.
Two or 3 observers attended each interview, which took place in the hospital on discharge day, at postdischarge appointments, or in patients' homes. Open‐ended questions prompted broad‐ranging inquiry into patients' lives, medical history, hospitalization experience, medications, care network, challenges, personal goals, and inner experience. Some questions were adapted and expanded from the CTM; others were prompts to demonstrate activities (eg, Can you show us how you organize your medications?). In addition to interviewing patients and caregivers, we observed interactions between patients, families, and hospital staff before discharge. We also observed patients and caregivers at home and when interacting with outpatient primary care providers. The purpose of observation was to understand the context of patient and caregiver experiences and to identify consistencies or discrepancies with their descriptions of experiences. (see Supporting Information In‐Home Interview Guide in the online version of this article)
Data included field notes and video recordings. In addition, observers summarized their strongest daily impressions as brief team stories that were shared with the observation team, local operations staff, and Kaiser Permanente national subject matter experts.23 Consistent with a grounded theory approach, interviews were professionally transcribed and qualitatively analyzed by multiple observers in iterative stages to develop broad domains of patient experiences.24 We clustered similar experiences and identified exemplar statements and behaviors. Team stories were analyzed separately, using a similar process. We reviewed recorded interviews to refine our emerging understanding of patient and caregiver experiences and discussed our observations and impressions about each domain. To maximize internal validity, an independent researcher who did not attend the interviews reviewed the transcripts and coding and participated in final qualitative analysis. Institutional review board approval was not required for this quality improvement project.
RESULTS
Patients and caregivers expressed or demonstrated 6 domains of experience as they transitioned from hospital to home (Table 1).
| Need | Key Observations |
|---|---|
| Translating knowledge into safe, health‐promoting actions at home | Even when patients and caregivers believe they have all needed information before discharge, they often find later that they are lacking knowledge or cannot translate it into contextually appropriate actions. |
| Patients and caregivers may inaccurately perceive that they have successfully translated knowledge into safe, health‐promoting actions. | |
| The day of discharge may not be the optimal time for learning. | |
| Inclusion of caregivers at every step of the transition process | Caregivers are integrally involved in the care for many patients. |
| Discharge teaching does not optimally include caregivers. | |
| Having readily available problem‐solving resources | Questions normally arise after the transition home as patients and caregivers engage in ongoing care activities. |
| Even patients and caregivers successfully providing care at home may need help interpreting experiences. | |
| Feeling connected to and trusting providers | Patients and caregivers highly value a feeling of being connected to providers, typically in the context of ongoing relationships. |
| Providers sometimes miss opportunities to connect with patients. | |
| Although investing in building connections with patients is time‐consuming for providers, patients may disregard communication unless it occurs. | |
| Transitioning from illness‐defined experience to normal life | Patients and caregivers want to return to a sense of normal life as quickly as possible. |
| This desire may interfere with the ability to absorb information and translate it, to prioritize healthcare needs, or to accurately assess the risk in a situation. | |
| Anticipating needs at home and making arrangements to meet them | Patients and caregivers require many types of help, but some may have trouble reconciling the need for assistance with the desire to return to a normal life. |
| Patients and caregivers find it stressful when needed arrangements have not been made. | |
| Some needed arrangements do not pertain strictly to healthcare (eg, help at home, meals). |
Translating Knowledge Into Safe, Health‐Promoting Actions at Home
A primary activity on discharge day was patient education provided by hospital staff. Topics included health conditions, medications, resources, activity, diet, equipment, supplies, and procedures. A nurse typically reviewed written instructions with the patient; the process ranged from thoughtful conversations to cursory recitation of printed information. Teaching was often sandwiched between other activities, and some staff members appeared pressured to complete it.
Patients and caregivers generally reported having all the information they needed; however, when we observed them at home, we noted that translating knowledge into safe, health‐promoting actions was a separate step. A common example was medication management. Patients or caregivers often rewrote the discharge medication list, grouping medications by purpose or creating charts of when to take each one. Patients and caregivers developed varying and somewhat complex systems for home medication management. For example, 1 patient taking 16 medications filled five 7‐day pillboxes each week; from these, he filled a tiny mug 5 times a day, placing it where it would remind him to take his medications. Patients interviewed about their medications at home often expressed uncertainty about their understanding of the medications and about how and why they were taking them.
When procedures were involved, such as dressing changes or administering intravenous (IV) solutions, in‐hospital teaching didn't always translate smoothly into safe action at home. A man who learned to administer total parenteral nutrition in the hospital found his first at‐home session unexpectedly challenging: I just got home and was behind schedule hooking up to the machine. I'm thinking, Which (tube) goes where? and getting real tired. I looked at the sheets. They have all the information you need, but it's too much for a tired person. I didn't want to read, and the pictures weren't clear, and I thought, I'll just try to remember what they said. (Patient #9)
We directly observed patients and caregivers failing to translate knowledge into safe, health‐promoting actions at home. Two days after discharge following a total knee replacement, a patient navigated a flight of stairs with a walker. In another instance, a caregiver hung an IV on a coat hanger hooked precariously to a mailbox as children raced around the room. An older man described strengthening and mobility exercises as instructed by his physical therapist but didn't perform them. Their reasoning was often unclear. For instance, after a nurse reviewed a list of discharge medications and left the room, despite verbal agreement with the instructions, the patient commented: Eight pills are too many. I'll take 3 today and 3 tomorrow and see how I feel. (Patient #27)
Inclusion of caregivers at Every Step of the Transition Process
After discharge, caregivers helped with or took responsibility for managing medications, wound care, administering intravenous antibiotics, adjusting diets, filling prescriptions, obtaining medical supplies and equipment, taking vital signs, interpreting signs and symptoms, monitoring health indicators, deciding who and when to call, and advocating for patients. When patients required hands‐on care tasks, such as dressing changes or intravenous medications, caregivers typically received instruction from hospital staff before discharge.
However, in many cases, including caregivers in discharge teaching appeared to be a low priority. In several instances, caregivers were unable to speak directly with a physician before the patient's discharge: I was hoping I could do that before she came home. I know it's hard to get hold of the doctors, but I wanted to know what to expect. (Caregiver #24)
Even when a caregiver was present, hospital staff frequently directed teaching exclusively toward the patient. For example, a nurse and patient sat side‐by‐side to review instructions; the highly motivated caregiver, seated across the room due to lack of space, was unable to see the written material. The integral role of caregivers in helping patients at home contrasted with their often peripheral role in in‐hospital transition processes.
Having Readily Available Problem‐Solving Resources
Patients and caregivers needed to know who and when to call for more information. They needed to discriminate between providers (eg, when to call a cardiologist vs a primary care provider), identify who to call in an urgent or emergent situation, and know how to access various resources. Some questions arose because patients lacked sufficient detail about what to expect. Even patients who successfully translated knowledge into safe, health‐promoting actions might need help interpreting observations: The wound is closed on top but not underneath, and the WoundVac is supposed to be working on the cells. I'm using the same amount of foam as when I started, so is it really healing? Shouldn't we be using less foam? We don't have anyone to answer the questions. (Patient #22)
Many patients with chronic conditions had direct numbers to their physicians' office; some had important numbers for a doctor or pharmacy on speed‐dial. Many patients and caregivers expressed a sense of pride at knowing how to navigate the healthcare system: I've learned how to get to him. I call downtown, and then they call out to his office. (Patient #8)
Other patients and caregivers gave conflicting messages; they said they knew who to call but provided few specifics: If he needed a nurse, I'd ask for the nurse assistant. I'll just do that or something. (Caregiver #20)
Feeling Connected to and Trusting Providers
For patients and caregivers, a critical aspect of communications with providers was a sense of connection, typically with a particular healthcare provider as part of an ongoing, trusting relationship. Patients expressed feeling respected, that their individual concerns and needs mattered, and that providers appreciated their emotional experiences, listened carefully without seeming rushed, and valued their knowledge. Successful experiences of connection were clearly meaningful to patients: The most important thing is how genuine the doctor is as a person. I pick up on that right away. It bothers me when they're not all there. It amazes me that they have the intellectual prowess to be a doctor, but there are other components that are not quite there yet. My doctor, he's got it all. (Patient #9)
This sense of connection often contrasted with what they may have experienced during short‐term relationships with providers in the hospital. In addition, providers sometimes overlooked opportunities to connect with patients. For instance, a clinic nurse, busy with intake, did not acknowledge a patient's repeated requests for help modifying his diet.
Transitioning From Illness‐Defined Experience to Normal Life
Patients and caregivers described or demonstrated a variety of ways of leavingor wanting to leavethe experience of illness behind, including feeling independent, useful, motivated, confident, and in control; helping others, including other patients in similar circumstances; feeling hopeful about recovery; and maintaining a sense of perspective.
This desire to get back to normal life affected the amount of information patients and caregivers absorbed on discharge day: I was so anxious to leave. I was like, Yeah, yeah, let's do this. I'm all packed. I've got one foot out the door. At home, I got ready to take my medication; the discharge instructions didn't jibe with what the doctor wrote. It was as much my fault as anyone's, because I was rushing to get home. (Patient #16)
Resuming usual activities, sleeping in one's own bed, eating familiar foods, being among friends and neighbors, and intentionally limiting the impact of a health condition on activities were all attempts to quickly restore a sense of normal life. Any milestone on the path to recovery seemed to help: I was so ecstatic in the car coming home. We were back on the road of real life. (Patient #22)
In some instances, the drive to feel a sense of normal life outweighed physical needs. For instance, a young woman with cancer delayed notifying her physician that she had cellulitis because she didn't want to interrupt her usual activities. After several days, she was taken to the emergency room by ambulance and admitted for IV antibiotics.
Anticipating Needs at Home and Making Arrangements to Meet Them
Patients and caregivers anticipated a variety of postdischarge needs. These included hands‐on healthcare tasks, grocery shopping, food preparation, and the like, as well as household maintenance, assistance with pets, and other daily activities that were unrelated to healthcare: I can't do it by myself. I can't just jump in the car and drive. So there are things that you need other people to help you with to get through the day. (Patient #9)
However many patients described a network of support including family members, neighbors, friends, clergy, and others. More than 1 helper was often required. However, patients sometimes found it difficult to reconcile the desire to return to normal life with needs for help. For example, an older woman refused a home health nursing visit for congestive heart failure because she felt it encroached on her independence. The same desire to return to normal life led patients to overestimate their ability to function independently. After a several‐day hospital stay for back surgery, a patient asked a friend to drop him off at home. He then used his walker to get to his car to retrieve a cart for his belongings. He pushed the walker with 1 hand and dragged the cart behind him up 2 floors to his apartment. Once inside, he went to bed, exhausted. In addition, it was sometimes difficult for patients to accurately anticipate needs. For example, a man who returned home alone after surgery suddenly realized his bed was much lower than the hospital bed; he wasn't sure he could get out of it without help.
Transportation home from the hospital and to outpatient appointments after discharge was a frequently identified need, leaving patients making hasty and suboptimal arrangements for a ride home, worried about keeping scheduled appointments, or both.
Patients and caregivers found it stressful when arrangements had not been made: First, we have to worry about getting home, and then I have to go to the medical supply store. What if she has to use the restroom? She has to wait until I get back. (Caregiver #8)
Patients and caregivers described experiences of making arrangements that were largely successful; however, they were also often time‐consuming.
DISCUSSION
Using an ethnographic approach, we identified 6 domains of patient and caregiver experience during the hospital‐to‐home transition. Many needs in these domains arose in the hours and days after patients returned home, and patients and caregivers often found it challenging to meet them. Our project adds a detailed, patient‐centered perspective on the transition from hospital to home.
The domains we identified share some conceptual territory with the dimensions of the Care Transition Measure and the Transitional Care Model,25 but generate a more detailed understanding of patient and caregiver experiences. Key findings include the fact that patients can find it challenging to translate knowledge into contextually appropriate action at home. This confirms some published results. For instance, estimates of outpatient adherence to complicated regimens range from 5% to 77%.2629 Significant opportunities exist to improve the reliability of translating medication instructions into systems that work at home,30 including aligning medication lists with physical aids (such as weekly pill boxes) and explaining medications in patient‐friendly terms. We also found that same‐day discharge teaching can be ineffective because patients are anxious to leave the hospital or staff members feel rushed. Emotion can interfere with cognition, and transferring information shortly before hospital discharge may overlook learning readiness, a fundamental principle of patient education.31, 32 In addition, the desire to return to normal life, coupled with uncertainty about who to call for clarification, can lead patients to simply do the best they can with whatever information they recall.
The literature refers to handoffs of patients from one provider to another as an episode of care is completed, but our findings suggest patients perceive hospitalization as an event occurring within ongoing relationships with the healthcare providers to whom they feel most connected.33, 34 Some patient and caregiver needs could be addressed by actively supporting these relationships during the hospital‐to‐home transition: explicitly acknowledging their importance to patients, ensuring that providers have discharge information, and framing discharge as a transition back to the care of trusted providers. Some of our findings require system‐level changes. Patients and caregivers with unmet transportation needs expressed anxiety about how or if help would materialize. Partnerships with community organizations could enable healthcare organizations to address needs like transportation that fall outside traditional discharge activities but significantly impact patient experiences. In addition, healthcare organizations are rarely designed for straightforward navigation; patient‐centered organizational designs could eliminate the need for patients and caregivers to learn how to navigate. For instance, a single point of contact for recently discharged patients might improve the process of finding help.
Strengths of our quality improvement project include the range of patients we interviewed and in‐depth observations and interviews across settings. Ethnography is ideal for generating a rich understanding of patient experiences, allowing us to observe needs patients did not mention, as well as the physical and emotional context of the transition. Weaknesses of our approach include the fact that the experiences reflected in each category were determined, to some extent, by the questions we asked. This may have constrained the variety of experiences patients reported. In addition, Kaiser Permanente's integrated nature may have affected our findings, although we believe patients and caregivers reported experiences that are likely universal.
Our project occurred in a healthcare system with an integrated electronic health record (EHR). Interventions to improve provider‐identified gaps in the discharge process often rely on information technology.3543 However, information technology does not eliminate continuity of care issues.44 Our EHR is widely used, but available information did not consistently ensure strong enough care coordination or good communication.
Including the patient's primary caregiver in discharge teaching appeared to be a relatively low priority for hospital staff, unless there was a hands‐on care task. Even when a primary caregiver was present, hospital staff frequently directed teaching exclusively toward the patient. The extent to which caregivers feel adequately prepared for their roles and responsibilities needs further exploration.
CONCLUSION
Our applied ethnographic approach reveals that patients experience several challenges while transitioning from hospital to home. Reducing readmissions is likely to remain challenging unless we broaden our understanding of the types of support and coaching required. We are translating our findings into quality improvement activities, conducting pilot projects focusing on risk stratification and tailoring of care, a specialized phone number for recently discharged patients, standardized same‐day discharge summaries to primary care providers, medication reconciliation, follow‐up phone calls, and scheduling appointments before discharge.
The transition from hospital to home is a complex event offering multiple provider‐identified opportunities to improve healthcare quality.18 Centering care delivery around patient needs and preferences is both inherently valuable and linked with better outcomes.9
The Care Transitions Measure (CTM) identifies 4 domains of patient experience related to hospital discharge: information transfer, patient and caregiver preparation, self‐management support, and empowerment to assert preferences.10 It discriminates between patients who do or do not experience a subsequent readmission or emergency room visit and between levels of care coordination.11 Quality indicators like the CTM are important tools for systematic healthcare improvement, but they provide a limited understanding of patient experiences, which can drive the transformation of systems.12, 13
With the exception of patients with a few specific clinical conditions, relatively little is known about how adult patients perceive the hospital‐to‐home transition.1417 They recall receiving discharge instructions but lack details about what to do if problems arise.18 They may lack important information despite receiving instruction.19 Caregivers report problems related to emotional support, discharge planning, and family participation,20 and patients and caregivers express anxiety, confusion, a sense of abandonment by the healthcare system, and the perception that their preferences are disregarded.21
As part of ongoing quality improvement activities, we sought to develop a richly detailed, patient‐centered view of the hospital‐to‐home transition. Our purpose was to understand patient and caregiver experiences during this pivotal healthcare experience.
METHODS
We used an applied ethnographic approach,22 conducting participant observation and video recording in‐depth, semi‐structured interviews in Kaiser Permanente Southern California, Colorado, and Hawaii. The United States' largest, private, not‐for‐profit integrated healthcare delivery system, Kaiser Permanente addresses all health needs for more than 8.9 million members.
To balance the pragmatic imperatives of quality improvement with obtaining enough information to understand patient experiences, we planned a sample of 24 patients across 3 settings with a mix of resource‐intensive and less‐intensive healthcare needs. We defined resource‐intensive needs as occurring among patients aged 65 or older with 3 or more chronic conditions. We asked hospital staff to identify patients by level of need and variety in diagnoses and illness severity, planned or unplanned hospitalizations, age, and ability to self manage. Reasons for admission included joint replacement, acute appendicitis, chronic illness exacerbation, complications of cancer chemotherapy, and others. We included patients who were inpatients or discharged no more than 3 weeks before interview. We excluded those under the age of 18 or discharged to non‐home settings. The project took place between September and November of 2008; 24 patients, half of whom were male, gave written informed consent for video recordings and authorization to distribute protected health information throughout and beyond Kaiser Permanente for quality improvement and educational purposes. Participants took part in interviews and observations lasting 1 to 3 hours; caregivers and family members participated in 9 instances.
Two or 3 observers attended each interview, which took place in the hospital on discharge day, at postdischarge appointments, or in patients' homes. Open‐ended questions prompted broad‐ranging inquiry into patients' lives, medical history, hospitalization experience, medications, care network, challenges, personal goals, and inner experience. Some questions were adapted and expanded from the CTM; others were prompts to demonstrate activities (eg, Can you show us how you organize your medications?). In addition to interviewing patients and caregivers, we observed interactions between patients, families, and hospital staff before discharge. We also observed patients and caregivers at home and when interacting with outpatient primary care providers. The purpose of observation was to understand the context of patient and caregiver experiences and to identify consistencies or discrepancies with their descriptions of experiences. (see Supporting Information In‐Home Interview Guide in the online version of this article)
Data included field notes and video recordings. In addition, observers summarized their strongest daily impressions as brief team stories that were shared with the observation team, local operations staff, and Kaiser Permanente national subject matter experts.23 Consistent with a grounded theory approach, interviews were professionally transcribed and qualitatively analyzed by multiple observers in iterative stages to develop broad domains of patient experiences.24 We clustered similar experiences and identified exemplar statements and behaviors. Team stories were analyzed separately, using a similar process. We reviewed recorded interviews to refine our emerging understanding of patient and caregiver experiences and discussed our observations and impressions about each domain. To maximize internal validity, an independent researcher who did not attend the interviews reviewed the transcripts and coding and participated in final qualitative analysis. Institutional review board approval was not required for this quality improvement project.
RESULTS
Patients and caregivers expressed or demonstrated 6 domains of experience as they transitioned from hospital to home (Table 1).
| Need | Key Observations |
|---|---|
| Translating knowledge into safe, health‐promoting actions at home | Even when patients and caregivers believe they have all needed information before discharge, they often find later that they are lacking knowledge or cannot translate it into contextually appropriate actions. |
| Patients and caregivers may inaccurately perceive that they have successfully translated knowledge into safe, health‐promoting actions. | |
| The day of discharge may not be the optimal time for learning. | |
| Inclusion of caregivers at every step of the transition process | Caregivers are integrally involved in the care for many patients. |
| Discharge teaching does not optimally include caregivers. | |
| Having readily available problem‐solving resources | Questions normally arise after the transition home as patients and caregivers engage in ongoing care activities. |
| Even patients and caregivers successfully providing care at home may need help interpreting experiences. | |
| Feeling connected to and trusting providers | Patients and caregivers highly value a feeling of being connected to providers, typically in the context of ongoing relationships. |
| Providers sometimes miss opportunities to connect with patients. | |
| Although investing in building connections with patients is time‐consuming for providers, patients may disregard communication unless it occurs. | |
| Transitioning from illness‐defined experience to normal life | Patients and caregivers want to return to a sense of normal life as quickly as possible. |
| This desire may interfere with the ability to absorb information and translate it, to prioritize healthcare needs, or to accurately assess the risk in a situation. | |
| Anticipating needs at home and making arrangements to meet them | Patients and caregivers require many types of help, but some may have trouble reconciling the need for assistance with the desire to return to a normal life. |
| Patients and caregivers find it stressful when needed arrangements have not been made. | |
| Some needed arrangements do not pertain strictly to healthcare (eg, help at home, meals). |
Translating Knowledge Into Safe, Health‐Promoting Actions at Home
A primary activity on discharge day was patient education provided by hospital staff. Topics included health conditions, medications, resources, activity, diet, equipment, supplies, and procedures. A nurse typically reviewed written instructions with the patient; the process ranged from thoughtful conversations to cursory recitation of printed information. Teaching was often sandwiched between other activities, and some staff members appeared pressured to complete it.
Patients and caregivers generally reported having all the information they needed; however, when we observed them at home, we noted that translating knowledge into safe, health‐promoting actions was a separate step. A common example was medication management. Patients or caregivers often rewrote the discharge medication list, grouping medications by purpose or creating charts of when to take each one. Patients and caregivers developed varying and somewhat complex systems for home medication management. For example, 1 patient taking 16 medications filled five 7‐day pillboxes each week; from these, he filled a tiny mug 5 times a day, placing it where it would remind him to take his medications. Patients interviewed about their medications at home often expressed uncertainty about their understanding of the medications and about how and why they were taking them.
When procedures were involved, such as dressing changes or administering intravenous (IV) solutions, in‐hospital teaching didn't always translate smoothly into safe action at home. A man who learned to administer total parenteral nutrition in the hospital found his first at‐home session unexpectedly challenging: I just got home and was behind schedule hooking up to the machine. I'm thinking, Which (tube) goes where? and getting real tired. I looked at the sheets. They have all the information you need, but it's too much for a tired person. I didn't want to read, and the pictures weren't clear, and I thought, I'll just try to remember what they said. (Patient #9)
We directly observed patients and caregivers failing to translate knowledge into safe, health‐promoting actions at home. Two days after discharge following a total knee replacement, a patient navigated a flight of stairs with a walker. In another instance, a caregiver hung an IV on a coat hanger hooked precariously to a mailbox as children raced around the room. An older man described strengthening and mobility exercises as instructed by his physical therapist but didn't perform them. Their reasoning was often unclear. For instance, after a nurse reviewed a list of discharge medications and left the room, despite verbal agreement with the instructions, the patient commented: Eight pills are too many. I'll take 3 today and 3 tomorrow and see how I feel. (Patient #27)
Inclusion of caregivers at Every Step of the Transition Process
After discharge, caregivers helped with or took responsibility for managing medications, wound care, administering intravenous antibiotics, adjusting diets, filling prescriptions, obtaining medical supplies and equipment, taking vital signs, interpreting signs and symptoms, monitoring health indicators, deciding who and when to call, and advocating for patients. When patients required hands‐on care tasks, such as dressing changes or intravenous medications, caregivers typically received instruction from hospital staff before discharge.
However, in many cases, including caregivers in discharge teaching appeared to be a low priority. In several instances, caregivers were unable to speak directly with a physician before the patient's discharge: I was hoping I could do that before she came home. I know it's hard to get hold of the doctors, but I wanted to know what to expect. (Caregiver #24)
Even when a caregiver was present, hospital staff frequently directed teaching exclusively toward the patient. For example, a nurse and patient sat side‐by‐side to review instructions; the highly motivated caregiver, seated across the room due to lack of space, was unable to see the written material. The integral role of caregivers in helping patients at home contrasted with their often peripheral role in in‐hospital transition processes.
Having Readily Available Problem‐Solving Resources
Patients and caregivers needed to know who and when to call for more information. They needed to discriminate between providers (eg, when to call a cardiologist vs a primary care provider), identify who to call in an urgent or emergent situation, and know how to access various resources. Some questions arose because patients lacked sufficient detail about what to expect. Even patients who successfully translated knowledge into safe, health‐promoting actions might need help interpreting observations: The wound is closed on top but not underneath, and the WoundVac is supposed to be working on the cells. I'm using the same amount of foam as when I started, so is it really healing? Shouldn't we be using less foam? We don't have anyone to answer the questions. (Patient #22)
Many patients with chronic conditions had direct numbers to their physicians' office; some had important numbers for a doctor or pharmacy on speed‐dial. Many patients and caregivers expressed a sense of pride at knowing how to navigate the healthcare system: I've learned how to get to him. I call downtown, and then they call out to his office. (Patient #8)
Other patients and caregivers gave conflicting messages; they said they knew who to call but provided few specifics: If he needed a nurse, I'd ask for the nurse assistant. I'll just do that or something. (Caregiver #20)
Feeling Connected to and Trusting Providers
For patients and caregivers, a critical aspect of communications with providers was a sense of connection, typically with a particular healthcare provider as part of an ongoing, trusting relationship. Patients expressed feeling respected, that their individual concerns and needs mattered, and that providers appreciated their emotional experiences, listened carefully without seeming rushed, and valued their knowledge. Successful experiences of connection were clearly meaningful to patients: The most important thing is how genuine the doctor is as a person. I pick up on that right away. It bothers me when they're not all there. It amazes me that they have the intellectual prowess to be a doctor, but there are other components that are not quite there yet. My doctor, he's got it all. (Patient #9)
This sense of connection often contrasted with what they may have experienced during short‐term relationships with providers in the hospital. In addition, providers sometimes overlooked opportunities to connect with patients. For instance, a clinic nurse, busy with intake, did not acknowledge a patient's repeated requests for help modifying his diet.
Transitioning From Illness‐Defined Experience to Normal Life
Patients and caregivers described or demonstrated a variety of ways of leavingor wanting to leavethe experience of illness behind, including feeling independent, useful, motivated, confident, and in control; helping others, including other patients in similar circumstances; feeling hopeful about recovery; and maintaining a sense of perspective.
This desire to get back to normal life affected the amount of information patients and caregivers absorbed on discharge day: I was so anxious to leave. I was like, Yeah, yeah, let's do this. I'm all packed. I've got one foot out the door. At home, I got ready to take my medication; the discharge instructions didn't jibe with what the doctor wrote. It was as much my fault as anyone's, because I was rushing to get home. (Patient #16)
Resuming usual activities, sleeping in one's own bed, eating familiar foods, being among friends and neighbors, and intentionally limiting the impact of a health condition on activities were all attempts to quickly restore a sense of normal life. Any milestone on the path to recovery seemed to help: I was so ecstatic in the car coming home. We were back on the road of real life. (Patient #22)
In some instances, the drive to feel a sense of normal life outweighed physical needs. For instance, a young woman with cancer delayed notifying her physician that she had cellulitis because she didn't want to interrupt her usual activities. After several days, she was taken to the emergency room by ambulance and admitted for IV antibiotics.
Anticipating Needs at Home and Making Arrangements to Meet Them
Patients and caregivers anticipated a variety of postdischarge needs. These included hands‐on healthcare tasks, grocery shopping, food preparation, and the like, as well as household maintenance, assistance with pets, and other daily activities that were unrelated to healthcare: I can't do it by myself. I can't just jump in the car and drive. So there are things that you need other people to help you with to get through the day. (Patient #9)
However many patients described a network of support including family members, neighbors, friends, clergy, and others. More than 1 helper was often required. However, patients sometimes found it difficult to reconcile the desire to return to normal life with needs for help. For example, an older woman refused a home health nursing visit for congestive heart failure because she felt it encroached on her independence. The same desire to return to normal life led patients to overestimate their ability to function independently. After a several‐day hospital stay for back surgery, a patient asked a friend to drop him off at home. He then used his walker to get to his car to retrieve a cart for his belongings. He pushed the walker with 1 hand and dragged the cart behind him up 2 floors to his apartment. Once inside, he went to bed, exhausted. In addition, it was sometimes difficult for patients to accurately anticipate needs. For example, a man who returned home alone after surgery suddenly realized his bed was much lower than the hospital bed; he wasn't sure he could get out of it without help.
Transportation home from the hospital and to outpatient appointments after discharge was a frequently identified need, leaving patients making hasty and suboptimal arrangements for a ride home, worried about keeping scheduled appointments, or both.
Patients and caregivers found it stressful when arrangements had not been made: First, we have to worry about getting home, and then I have to go to the medical supply store. What if she has to use the restroom? She has to wait until I get back. (Caregiver #8)
Patients and caregivers described experiences of making arrangements that were largely successful; however, they were also often time‐consuming.
DISCUSSION
Using an ethnographic approach, we identified 6 domains of patient and caregiver experience during the hospital‐to‐home transition. Many needs in these domains arose in the hours and days after patients returned home, and patients and caregivers often found it challenging to meet them. Our project adds a detailed, patient‐centered perspective on the transition from hospital to home.
The domains we identified share some conceptual territory with the dimensions of the Care Transition Measure and the Transitional Care Model,25 but generate a more detailed understanding of patient and caregiver experiences. Key findings include the fact that patients can find it challenging to translate knowledge into contextually appropriate action at home. This confirms some published results. For instance, estimates of outpatient adherence to complicated regimens range from 5% to 77%.2629 Significant opportunities exist to improve the reliability of translating medication instructions into systems that work at home,30 including aligning medication lists with physical aids (such as weekly pill boxes) and explaining medications in patient‐friendly terms. We also found that same‐day discharge teaching can be ineffective because patients are anxious to leave the hospital or staff members feel rushed. Emotion can interfere with cognition, and transferring information shortly before hospital discharge may overlook learning readiness, a fundamental principle of patient education.31, 32 In addition, the desire to return to normal life, coupled with uncertainty about who to call for clarification, can lead patients to simply do the best they can with whatever information they recall.
The literature refers to handoffs of patients from one provider to another as an episode of care is completed, but our findings suggest patients perceive hospitalization as an event occurring within ongoing relationships with the healthcare providers to whom they feel most connected.33, 34 Some patient and caregiver needs could be addressed by actively supporting these relationships during the hospital‐to‐home transition: explicitly acknowledging their importance to patients, ensuring that providers have discharge information, and framing discharge as a transition back to the care of trusted providers. Some of our findings require system‐level changes. Patients and caregivers with unmet transportation needs expressed anxiety about how or if help would materialize. Partnerships with community organizations could enable healthcare organizations to address needs like transportation that fall outside traditional discharge activities but significantly impact patient experiences. In addition, healthcare organizations are rarely designed for straightforward navigation; patient‐centered organizational designs could eliminate the need for patients and caregivers to learn how to navigate. For instance, a single point of contact for recently discharged patients might improve the process of finding help.
Strengths of our quality improvement project include the range of patients we interviewed and in‐depth observations and interviews across settings. Ethnography is ideal for generating a rich understanding of patient experiences, allowing us to observe needs patients did not mention, as well as the physical and emotional context of the transition. Weaknesses of our approach include the fact that the experiences reflected in each category were determined, to some extent, by the questions we asked. This may have constrained the variety of experiences patients reported. In addition, Kaiser Permanente's integrated nature may have affected our findings, although we believe patients and caregivers reported experiences that are likely universal.
Our project occurred in a healthcare system with an integrated electronic health record (EHR). Interventions to improve provider‐identified gaps in the discharge process often rely on information technology.3543 However, information technology does not eliminate continuity of care issues.44 Our EHR is widely used, but available information did not consistently ensure strong enough care coordination or good communication.
Including the patient's primary caregiver in discharge teaching appeared to be a relatively low priority for hospital staff, unless there was a hands‐on care task. Even when a primary caregiver was present, hospital staff frequently directed teaching exclusively toward the patient. The extent to which caregivers feel adequately prepared for their roles and responsibilities needs further exploration.
CONCLUSION
Our applied ethnographic approach reveals that patients experience several challenges while transitioning from hospital to home. Reducing readmissions is likely to remain challenging unless we broaden our understanding of the types of support and coaching required. We are translating our findings into quality improvement activities, conducting pilot projects focusing on risk stratification and tailoring of care, a specialized phone number for recently discharged patients, standardized same‐day discharge summaries to primary care providers, medication reconciliation, follow‐up phone calls, and scheduling appointments before discharge.
- ,,,.Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists.J Hosp Med.2007;2(5):314–323.
- ,,.Reconcilable differences: correcting medication errors at hospital admission and discharge.Qual Saf Health Care.2006;15(2):122–126.
- ,,,,,.Deficits in communication and information transfer between hospital‐based and primary care physicians: implications for patient safety and continuity of care.JAMA.2007;297(8):831–841.
- ,,,.Interventions to improve medication reconciliation in primary care.Ann Pharmacother.2009;43(10):1667–1675.
- ,,, et al.A quality improvement intervention to facilitate the transition of older adults from three hospitals back to their homes.J Am Geriatr Soc.2009;57(9):1540–1546.
- ,.The effects of a discharge planning and home follow‐up intervention on elders hospitalized with common medical and surgical cardiac conditions.J Cardiovasc Nurs.1999;14(1):44–54.
- ,,,,,.Improved quality in the hospital discharge summary reduces medication errors—LIMM: Landskrona Integrated Medicines Management.Eur J Clin Pharmacol.2009;65(10):1037–1046.
- ,,,,,.Omitted and unjustified medications in the discharge summary.Qual Saf Health Care.2009;18(3):205–208.
- ,,, et al.Evidence suggesting that a chronic disease self‐management program can improve health status while reducing hospitalization: a randomized trial.Med Care.1999;37(1):5–14.
- ,,,,,.Development and testing of a measure designed to assess the quality of care transitions.Int J Integr Care.2002;2:e02.
- ,,.Assessing the quality of preparation for posthospital care from the patient's perspective: the care transitions measure.Med Care.2005;43(3):246–255.
- ,.If you build it, will they come? Designing truly patient‐centered health care.Health Aff (Millwood).29(5):914–920.
- ,,,.Analysis 33:818–829.
- ,,, et al.Identifying factors associated with perceived success in the transition from hospital to home after brain injury.J Head Trauma Rehabil2011;April 25.
- ,,, et al.Perceived participation, experiences from persons with spinal cord injury in their transition period from hospital to home.Int J Rehabil Res.2010;July 31.
- ,,, et al.Reengagement in meaningful occupations during the transition from hospital to home for people with acquired brain injury and their family caregivers.Am J Occup Ther.2009;63:609–620.
- ,,.Hospital to home health care transition: patient, caregiver, and clinician perspectives.West J Nurs Res.2011;Mar 22.
- ,,.Survey on transition from inpatient to outpatient for patients on insulin: what really goes on at home?Endocr Pract.2010;16:785–791.
- ,,, et al.Carepartner experiences with hospital care.Med Care.1999;37:33–38.
- ,,, et al.Transitions of Care Consensus Policy Statement: American College of Physicians–Society of General Internal Medicine–Society of Hospital Medicine–American Geriatrics Society–American College of Emergency Physicians–Society of Academic Emergency Medicine.J Gen Intern Med.2009;24:971–976.
- .Case Study Research: Design and Methods.3rd ed.Thousand Oaks, CA:Sage Publications;2003.
- ,,.Writing Ethnographic Fieldnotes (Chicago Guides to Writing, Editing, and Publishing).Chicago, IL:University of Chicago Press;1995.
- .Learning From Strangers: The Art and Method of Qualitative Interview Studies.New York, NY:Free Press;1995.
- ,,, et al.Comprehensive discharge planning and home follow‐up of hospitalized elders: a randomized clinical trial.JAMA.1999;281(7):613–620.
- ,.Improving medication reconciliation in the 21st century.Curr Drug Saf.2008;3(3):227–229.
- ,,, et al.Trends in adherence to secondary prevention medications in elderly post‐myocardial infarction patients.Pharmacoepidemiol Drug Saf.2008;17:1189–1196.
- ,,, et al.Association between patient beliefs and medication adherence following hospitalization for acute coronary syndrome.Am Heart J.2011;161:855–863.
- ,,, et al.Adherence to statin therapy in elderly patients after hospitalization for coronary revascularization.Am J Cardiol.2011;107:1409–1414.
- ,.Medication use in the transition from hospital to home.Ann Acad Med Singapore.2008;37(2):136–141.
- .A model for assessing learning readiness for self‐direction of care in individuals with spinal cord injuries: a qualitative study.SCI Nurs.2004;21:69–74.
- .Motivational and emotional controls of cognition.Psychol Rev.1967;74(1):29–39.
- .Key legal principles for hospitalists.Dis Mon.2002;48(4):197–206.
- ,,, et al.Transition of care for hospitalized elderly patients—development of a discharge checklist for hospitalists.J Hosp Med.2006;1(6):354–360.
- ,,, et al.Electronic versus dictated hospital discharge summaries: a randomized controlled trial.J Gen Intern Med.2009;24(9):995–1001.
- ,,, et al.Creating a better discharge summary: improvement in quality and timeliness using an electronic discharge summary.J Hosp Med.2009;4(4):219–225.
- ,,,,.Electronic discharge summaries: the current state of play.HIM J.2007;36(3):30–36.
- ,,,.Patient and physician perceptions after software‐assisted hospital discharge: cluster randomized trial.J Hosp Med.2009;4(6):356–363.
- ,,, et al.Effect of an electronic medication reconciliation application and process redesign on potential adverse drug events: a cluster‐randomized trial.Arch Intern Med.2009;169(8):771–780.
- ,,,.Implementing online medication reconciliation at a large academic medical center.Jt Comm J Qual Patient Saf.2008;34(9):499–508.
- ,,,.Medication reconciliation: a necessity in promoting a safe hospital discharge.J Healthc Qual.2006;28(3):12–19.
- ,,,,.Effect of a computerized referral at hospital discharge on cardiac rehabilitation participation rates.J Cardiopulm Rehabil Prev.2009;29(6):365–369.
- ,,, et al.Implementation of an electronic system for medication reconciliation.Am J Health Syst Pharm.2007;64(4):404–422.
- ,,.Coordination of diabetes care in four delivery models using an electronic health record.Med Care.2009;47(9):993–999.
- ,,,.Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists.J Hosp Med.2007;2(5):314–323.
- ,,.Reconcilable differences: correcting medication errors at hospital admission and discharge.Qual Saf Health Care.2006;15(2):122–126.
- ,,,,,.Deficits in communication and information transfer between hospital‐based and primary care physicians: implications for patient safety and continuity of care.JAMA.2007;297(8):831–841.
- ,,,.Interventions to improve medication reconciliation in primary care.Ann Pharmacother.2009;43(10):1667–1675.
- ,,, et al.A quality improvement intervention to facilitate the transition of older adults from three hospitals back to their homes.J Am Geriatr Soc.2009;57(9):1540–1546.
- ,.The effects of a discharge planning and home follow‐up intervention on elders hospitalized with common medical and surgical cardiac conditions.J Cardiovasc Nurs.1999;14(1):44–54.
- ,,,,,.Improved quality in the hospital discharge summary reduces medication errors—LIMM: Landskrona Integrated Medicines Management.Eur J Clin Pharmacol.2009;65(10):1037–1046.
- ,,,,,.Omitted and unjustified medications in the discharge summary.Qual Saf Health Care.2009;18(3):205–208.
- ,,, et al.Evidence suggesting that a chronic disease self‐management program can improve health status while reducing hospitalization: a randomized trial.Med Care.1999;37(1):5–14.
- ,,,,,.Development and testing of a measure designed to assess the quality of care transitions.Int J Integr Care.2002;2:e02.
- ,,.Assessing the quality of preparation for posthospital care from the patient's perspective: the care transitions measure.Med Care.2005;43(3):246–255.
- ,.If you build it, will they come? Designing truly patient‐centered health care.Health Aff (Millwood).29(5):914–920.
- ,,,.Analysis 33:818–829.
- ,,, et al.Identifying factors associated with perceived success in the transition from hospital to home after brain injury.J Head Trauma Rehabil2011;April 25.
- ,,, et al.Perceived participation, experiences from persons with spinal cord injury in their transition period from hospital to home.Int J Rehabil Res.2010;July 31.
- ,,, et al.Reengagement in meaningful occupations during the transition from hospital to home for people with acquired brain injury and their family caregivers.Am J Occup Ther.2009;63:609–620.
- ,,.Hospital to home health care transition: patient, caregiver, and clinician perspectives.West J Nurs Res.2011;Mar 22.
- ,,.Survey on transition from inpatient to outpatient for patients on insulin: what really goes on at home?Endocr Pract.2010;16:785–791.
- ,,, et al.Carepartner experiences with hospital care.Med Care.1999;37:33–38.
- ,,, et al.Transitions of Care Consensus Policy Statement: American College of Physicians–Society of General Internal Medicine–Society of Hospital Medicine–American Geriatrics Society–American College of Emergency Physicians–Society of Academic Emergency Medicine.J Gen Intern Med.2009;24:971–976.
- .Case Study Research: Design and Methods.3rd ed.Thousand Oaks, CA:Sage Publications;2003.
- ,,.Writing Ethnographic Fieldnotes (Chicago Guides to Writing, Editing, and Publishing).Chicago, IL:University of Chicago Press;1995.
- .Learning From Strangers: The Art and Method of Qualitative Interview Studies.New York, NY:Free Press;1995.
- ,,, et al.Comprehensive discharge planning and home follow‐up of hospitalized elders: a randomized clinical trial.JAMA.1999;281(7):613–620.
- ,.Improving medication reconciliation in the 21st century.Curr Drug Saf.2008;3(3):227–229.
- ,,, et al.Trends in adherence to secondary prevention medications in elderly post‐myocardial infarction patients.Pharmacoepidemiol Drug Saf.2008;17:1189–1196.
- ,,, et al.Association between patient beliefs and medication adherence following hospitalization for acute coronary syndrome.Am Heart J.2011;161:855–863.
- ,,, et al.Adherence to statin therapy in elderly patients after hospitalization for coronary revascularization.Am J Cardiol.2011;107:1409–1414.
- ,.Medication use in the transition from hospital to home.Ann Acad Med Singapore.2008;37(2):136–141.
- .A model for assessing learning readiness for self‐direction of care in individuals with spinal cord injuries: a qualitative study.SCI Nurs.2004;21:69–74.
- .Motivational and emotional controls of cognition.Psychol Rev.1967;74(1):29–39.
- .Key legal principles for hospitalists.Dis Mon.2002;48(4):197–206.
- ,,, et al.Transition of care for hospitalized elderly patients—development of a discharge checklist for hospitalists.J Hosp Med.2006;1(6):354–360.
- ,,, et al.Electronic versus dictated hospital discharge summaries: a randomized controlled trial.J Gen Intern Med.2009;24(9):995–1001.
- ,,, et al.Creating a better discharge summary: improvement in quality and timeliness using an electronic discharge summary.J Hosp Med.2009;4(4):219–225.
- ,,,,.Electronic discharge summaries: the current state of play.HIM J.2007;36(3):30–36.
- ,,,.Patient and physician perceptions after software‐assisted hospital discharge: cluster randomized trial.J Hosp Med.2009;4(6):356–363.
- ,,, et al.Effect of an electronic medication reconciliation application and process redesign on potential adverse drug events: a cluster‐randomized trial.Arch Intern Med.2009;169(8):771–780.
- ,,,.Implementing online medication reconciliation at a large academic medical center.Jt Comm J Qual Patient Saf.2008;34(9):499–508.
- ,,,.Medication reconciliation: a necessity in promoting a safe hospital discharge.J Healthc Qual.2006;28(3):12–19.
- ,,,,.Effect of a computerized referral at hospital discharge on cardiac rehabilitation participation rates.J Cardiopulm Rehabil Prev.2009;29(6):365–369.
- ,,, et al.Implementation of an electronic system for medication reconciliation.Am J Health Syst Pharm.2007;64(4):404–422.
- ,,.Coordination of diabetes care in four delivery models using an electronic health record.Med Care.2009;47(9):993–999.
Copyright © 2012 Society of Hospital Medicine
PCP Referral
Over the past decade, research has demonstrated a value gap in US healthcare, characterized by rapidly rising costs and substandard quality.1, 2 Public reporting of hospital performance data is one of several strategies promoted to help address these deficiencies. To this end, a number of hospital rating services have created Web sites aimed at healthcare consumers.3 These services provide information about multiple aspects of healthcare quality, which in theory might be used by patients when deciding where to seek medical care.
Despite the increasing availability of publicly reported quality data comparing doctors and hospitals, a 2008 survey found that only 14% of Americans have seen and used such information in the past year, a decrease from 2006 (36%).4 A similar study in 2007 found that after seeking input from family and friends, patients generally rely on their primary care physician (PCP) to assist them to make decisions about where to have elective surgery.5 Surprisingly, almost nothing is known about how publicly reported data is used, if at all, by PCPs in the referral of patients to hospitals.
The physician is an important intermediary in the buying process for many healthcare services.6 Tertiary care hospitals depend on physician referrals for much of their patient volume.7 Until the emergence of the hospitalist model of care, most primary care physicians cared for their own hospitalized patients, and thus hospital referral decisions were largely driven by the PCP's admitting privileges. However, following the rapid expansion of the hospitalist movement,8, 9 there has been a sharp decrease in the number of PCPs who provide direct patient care for their hospitalized patients.8 As a result, PCPs may now have more choice in regards to hospital referrals for general medical conditions. Potential factors influencing a PCP's referral decisions might include familiarity with the hospital, care quality, patient convenience, satisfaction with the hospital, or hospital reputation.
Studies of cardiac surgery report cards in New York9 and Pennsylvania,10 conducted in the mid‐1990s, found that cardiologists did not use publicly reported mortality data in referral decisions, nor did they share it with patients. Over the past 2 decades, public reporting has grown exponentially, and now includes many measures of structure, processes, and outcomes for almost all US hospitals, available for free over the Internet. The growth of the patient safety movement and mandated public reporting might also have affected physicians' views about publicly reported quality data. We surveyed primary care physicians to determine the extent to which they use information about hospital quality in their referral decisions for community‐acquired pneumonia, and to identify other factors that might influence referral decisions.
METHODS
We obtained an e‐mail list of primary care physicians from the medical staff offices of all area hospitals within a 10‐mile radius of Springfield, MA (Baystate Medical Center, Holyoke Medical Center, and Mercy Medical Center). Baystate Medical Center is a 659‐bed academic medical center and Level 1 trauma center, while Holyoke and Mercy Medical Center are both 180‐bed acute care hospitals. Physicians were contacted via e‐mail from June through September of 2009, and asked to participate in an anonymous, 10‐minute, online survey accessible through an Internet link (SurveyMonkey.com) about factors influencing a primary care physician's hospital referral choice for a patient with pneumonia. To facilitate participation, we sent 2 follow‐up e‐mail reminders, and respondents who completed the entire survey received a $15 gift card. The study was approved by the institutional review board of Baystate Medical Center and closed to participation on September 23, 2009.
We created the online survey based on previous research7 and approximately 10 key informant interviews. The survey (see Supporting Information, Appendix, in the online version of this article) contained 13 demographic questions and 10 questions based on a case study of pneumonia (Figure 1). The instrument was pilot tested for clarity with a small group of primary care physicians at the author's institution and subsequently modified. We chose pneumonia because it is a common reason for a PCP to make an urgent hospital referral,11 and because there is a well‐established set of quality measures that are publicly reported.12 Unlike elective surgery, for which patients might research hospitals or surgeons on their own, patients with pneumonia would likely rely on their PCP to recommend a hospital for urgent referral. In contrast, PCPs know they will refer a number of pneumonia patients to hospitals each year and therefore might have an interest in comparing the publicly reported quality measures for local hospitals.

Respondents were shown the case study and asked to refer the hypothetical patient to 1 of 4 area hospitals. Respondents were asked to rate (on a 3‐point scale: not at all, somewhat, or very) the importance of the following factors in their referral decision: waiting time in the emergency room, distance traveled by the patient, experience of other patients, severity of patient's illness, patient's insurance, hospital's reputation among other physicians and partners, admitting privileges with a specific hospital, admitting arrangements with a hospitalist group, familiarity with the hospital, availability of subspecialists, quality of subspecialists, nursing quality, nursing staffing ratios, hospital's case volume for pneumonia, publicly available quality measures, patient preference, distance from your practice, shared electronic record system, and quality of hospital discharge summaries. Next, we measured provider's awareness of publicly reported hospital quality data and whether they used such data in referring patients or choosing their own medical care. Specifically, we asked about familiarity with the following 4 Web sites: Massachusetts Quality and Cost (a state‐specific Web site produced by the Massachusetts Executive Office of Health and Human Services)13; Hospital Compare (a Web site developed and maintained by Centers for Medicare and Medicaid Services [CMS] and the Department of Health and Human Services)14; Leapfrog Group (a private, nonprofit organization)15; and Health Grades (a private, for‐profit company).16
We then asked participants to rate the importance of the following performance measures when judging a hospital's performance: antibiotics within 6 hours of arrival to the hospital, appropriate initial antibiotic, blood culture drawn before antibiotics given, smoking cessation advice/counseling, oxygenation assessment, risk‐adjusted mortality, intensive care unit staffing, influenza vaccination, pneumococcal vaccination, Leapfrog's never events,15 volume, Leapfrog safe practices score, cost, computerized physician order entry system, Magnet status,17 and U.S. News & World Report's Best Hospitals designation.18 Lastly, we asked participants to state, using a 3‐point scale (agree, disagree, neutral), their level of agreement that the following factors, adapted from Schneider and Epstein,10 represented limitations of public reporting: 1) risk‐adjusted methods are inadequate to compare hospitals fairly; 2) mortality rates are an incomplete indication of the quality of a hospital's care; 3) hospitals can manipulate the data; and 4) ratings are inaccurate for hospitals with small caseloads.
Factors associated with physicians' knowledge of publicly reported data were analyzed with bivariate analysis. Since all factors are categorical, chi‐square analysis was used for bivariate analysis. No factor had a P value <0.2 on bivariate analysis, thus multiple logistic regression was not performed.
RESULTS
Of 194 primary care physicians who received invitations, 92 responded (response rate of 47%). See Table 1 for respondents' characteristics. All age groups were represented; most were male and between 3554 years of age. Respondents were evenly divided between those who owned their own practices (54%) and those working for a health system (46%). Ninety‐three percent of PCPs maintained admitting privileges (45% to more than 1 hospital), but only 20% continued to admit their own patients. When asked where they would send a hypothetical pneumonia patient, only 4% of PCPs chose a hospital to which they had never had admitting privileges.
| Variable | No. (%) of Respondents |
|---|---|
| Age | |
| 2534 | 5 (5) |
| 3544 | 27 (29) |
| 4554 | 24 (26) |
| >55 | 36 (39) |
| Gender | |
| Male | 65 (71) |
| Female | 27 (29) |
| Years out of medical school | |
| <6 | 6 (7) |
| 610 | 9 (10) |
| 1115 | 17 (18) |
| >15 | 60 (65) |
| % Patients seen who are covered by | |
| Medicaid: Mean (SD) | 28 (26) |
| Medicare: Mean (SD) | 31 (18) |
| Private: Mean (SD) | 40 (25) |
| Number of time doing patient care: Mean (SD) | 85 (23) |
| Number of patients admitted/sent to hospital/mo | |
| <6 | 40 (47) |
| 610 | 25 (29) |
| 1120 | 12 (14) |
| >20 | 8 (9) |
| Practice type | |
| Solo | 13 (15) |
| Single specialty group | 36 (42) |
| Multi‐specialty group | 36 (42) |
| Practice ownership | |
| Independent | 45 (54) |
| Health system | 38 (46) |
| Currently admits own patients | |
| Yes | 17 (20) |
| No | 66 (80) |
| Current hospital admitting privileges | |
| A | 63 (76) |
| B | 41 (49) |
| C | 3 (4) |
| D | 12 (14) |
| None | 6 (7) |
| Other | 2 (2) |
Physician's ratings of the importance of various factors in their referral decision are shown in Figure 2. The following factors were most often considered very important: familiarity with the hospital (70%), patient preference (62%), and admitting arrangements with a hospitalist group (62%). In contrast, only 18% of physicians viewed publicly available hospital quality measures as very important when making a referral decision. Factors most often rated not at all important to participants' decisions were patient insurance (48%), hospital's case volume for pneumonia (48%), and publicly available quality measures (42%).
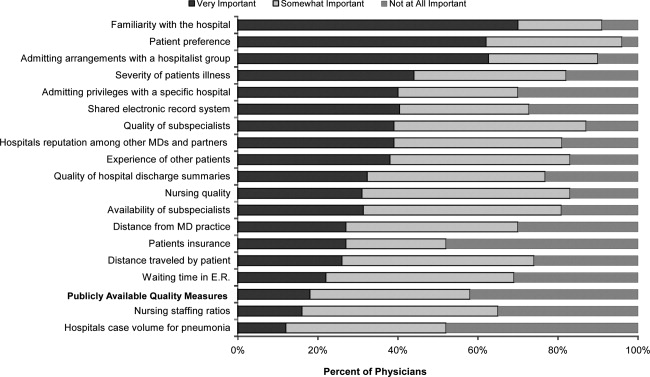
Of the 61% who were aware of Web sites that report hospital quality, most (52%) were familiar with Massachusetts Quality and Cost, while few (27%) were familiar with Hospital Compare. None of the physicians we surveyed reported having used publicly reported quality information when making a referral decision or having discussed such data with their patients. However, 49% stated that publicly reported performance data was somewhat and 10% very important to decisions regarding the medical care they receive. None of the demographic characteristics that we assessed (including age, gender, or years out of medical school) were associated with awareness of publicly reported data in bivariate analyses.
Respondents' ratings of specific quality measures appear in Figure 3. PCPs most often identified the following factors as being very important when judging hospital quality: percent of pneumonia patients given initial antibiotics within 6 hours after arrival (66%), percent of pneumonia patients given the most appropriate initial antibiotic (63%), and percent of pneumonia patients whose initial emergency room (ER) blood culture was performed prior to the administration of the first hospital dose of antibiotics (51%). The factors most often rated not at all important included: U.S. News & World Report's Best Hospitals designation (57%), Magnet Status (42%), and computer physician order entry system (40%).
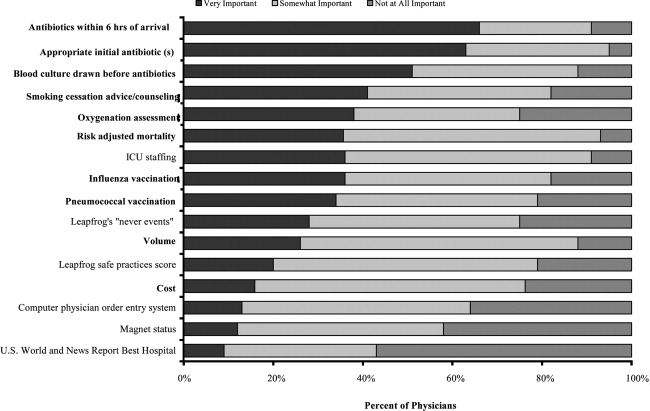
When asked about limitations of publicly reported performance data, 42% agreed that risk‐adjusted methods were inadequate to compare hospitals fairly, 76% agreed that mortality rates were an incomplete indication of the quality of hospitals care, 62% agreed that hospitals could manipulate the data, and 72% agreed that the ratings were inaccurate for hospitals with small caseloads.
DISCUSSION
In 2003, the Hospital Quality Alliance began a voluntary public reporting program of hospital performance measures, for pneumonia, acute myocardial infarction, and congestive heart failure, that was intended to encourage quality improvement activity by hospitals, and to provide patients and referring physicians with information to make better‐informed choices.19 These data are now easily available to the public through a free Web site (
Despite their lack of familiarity with Hospital Compare, it was the quality measures that are reported by Hospital Compare that they identified as the best indicators of hospital quality: appropriate initial antibiotic, antibiotics within 6 hours, and blood cultures performed prior to the administration of antibiotics. In fact, the 5 measures most often cited as very important to judging hospital quality were all measures reported on Hospital Compare.
As the US healthcare system becomes increasingly complex and costly, there is a growing interest in providing patients with physician and hospital performance data to help them select the provider.21 It is postulated that if patients took a more active role in choosing healthcare providers, and were forced to assume greater financial responsibility, then consumerism will force improvements in quality of care while maintaining or even lowering costs.21 However, studies demonstrate that most patients are unaware of performance data and, if they are aware, still value familiarity over quality ratings.4 Moreover, patients rely on the knowledge of their primary care physician to guide them.5
This is the first study we are aware of that examines how primary care physicians use publicly reported quality data in hospital referral decisions. Studies from more than a decade ago found that publicly reported data had minimal impact on referral decisions from cardiologists to cardiac surgeons. A survey of Pennsylvania's cardiologists and cardiac surgeons showed that although 82% were aware of risk‐adjusted mortality rates published for surgeons, only 10% of cardiologists reported these to be very important when evaluating the performance of a cardiothoracic surgeon. Furthermore, 87% of cardiologists stated that mortality and case volume information reported on cardiac surgeons had minimal or no influence on their referral practices.10 In 1997, a survey of cardiologists in New York found that only 38% of respondents reported that risk‐adjusted outcome data had affected their referrals to surgeons very much or somewhat.9 In addition, most authors conclude that public reporting has had little or no effect on market share.22 Despite growth in the number of measures and improved accessibility, our physicians were even less likely to be aware of, or use, publicly reported data than physicians a decade earlier.
Of course, even if public reporting does not influence referral patterns, it could still improve healthcare quality in several ways. First, feedback about performance may focus quality improvement activities in specific areas that represent gaps in care.10 This could take the form of an appeal to professionalism,23 or the desire to preserve one's reputation by not appearing on a list of poor performers.24 Second, hospitals' desire to appear on lists of high performers, such as U.S. News & World Report's hospital rankings, for marketing purposes, might stimulate improvement activities.10 Finally, publicly reported measures could form the basis for pay‐for‐performance incentives that further speed improvement.25
Our study has several limitations. First, our sample size was small and restricted to 1 region of 1 state, and may not be representative of either the state or nation as a whole. Still, our area has a high level of Internet use, and several local hospitals have been at the vanguard of the quality movement, generally scoring above both state and national averages on Hospital Compare. In addition, Massachusetts has made substantial efforts to promote its own public reporting program, and half the surveyed physicians reported being aware of the Massachusetts Quality and Cost Web site. The fact that not a single area physician surveyed used publicly reported data when making referral decisions is sobering. We believe it is unlikely that other areas of the country will have a substantially higher rate of use. Similarly, our response rate was under 50%. Physicians who did not take the survey may have differed in important ways from those who did. Nevertheless, our sample included a broad range of physician ages, practice types, and affiliations. It seems unlikely that those who did not respond would be more inclined to use publicly reported data than those who did. Second, we assessed decision‐making around a single medical condition. Physicians may have used publicly reported data for other decisions. However, the condition we chose was both urgent (as opposed to emergent) and possesses a robust set of publicly reported quality measures. If physicians do not use publicly reported data for this decision, it seems unlikely they would use it for conditions that have fewer reliable measures (eg, gall bladder surgery) or where the choice of hospital is generally made in an ambulance (eg, myocardial infarction). Finally, the low awareness of public reporting made it difficult for some physicians to answer some of the questions regarding publicly reported hospital quality data because they were unfamiliar with the language utilized by the Web sites (eg, magnet status, Leapfrog never events). It is possible that our results may have been altered slightly if a glossary had been provided.
Despite these limitations, our study suggests that more than 6 years after the launch of the Hospital Quality Alliance, primary care physicians do not appear to make use of these data when choosing a hospital for their patients suffering from pneumonia. Instead, they rely on familiarity with a hospital and past relationships. Even though a majority of the physicians surveyed no longer admitted their own patients, they continue to send patients to hospitals where they had privileges. This finding is not surprising, as physicians also cling to familiar therapies, and may be reluctant to prescribe a new medication or perform an unfamiliar procedure, even if it is indicated. Such reliance on familiarity may make physicians feel comfortable, but does not always result in the best care for patients. Acquiring familiarity, however, requires time and effort, something that physicians generally have in short supply; and while there are plenty of industry representatives to overcome physicians' hesitancy to prescribe new treatments, there are no analogous agents to educate physicians about public reporting or to help them overcome hesitancy about trying a new hospital.
Suspicion about the validity of public reporting may also play a role in the physicians' reported behavior. In past studies of cardiac report cards, cardiologists were most concerned that risk adjustment methods were inadequate (77%) and that mortality rates were an incomplete indicator of the quality of surgical care (74%). They were less concerned about manipulation of data (52%) or small caseloads (15%).10 Our physicians were also concerned that mortality rates were an incomplete measure of quality (76%) but less concerned about risk adjustment (42%), perhaps because many structure and process measures are not subject to risk adjustment. In contrast, they were somewhat more concerned that hospitals could manipulate the data (62%), which again may reflect process measures versus mortality statistics. Other reasons for not using the data may include a lack of awareness of the data or how to access it, or a belief that hospitals do not vary in quality.
Interestingly, even though most respondents were not aware of Hospital Compare, they found the information presented there to best reflect the overall hospital quality. Also, while respondents indicated that they did not use publicly reported data when referring patients, almost half of PCPs reported that publicly reported performance data was at least somewhat important in choosing their own medical care. Thus, although public reporting appears not to have reached its full potential, some publicly reported quality measures have clearly entered the consciousness of PCPs. In contrast, other highly touted measures such as computerized physician order entry systems were not appreciated, and popular designations such as U.S. News & World Report's Best Hospitals were least valued, even though 1 area hospital carries this designation. One conclusion might be that CMS should abandon Hospital Compare since neither patients4 nor providers use it. However, public reporting may improve quality in other ways. Moreover, physicians appear interested in the data even if they are not aware of it. Therefore, given the large investment by CMS and individual hospitals in collecting the data required for Hospital Compare, CMS might consider making greater efforts to increase primary care physician awareness of the Hospital Compare Web site. At the same time, high‐performing hospitals may want to communicate their performance scores to local PCPs as part of their marketing strategy. Future studies could assess whether such practices affect physician referral decisions and subsequent market share of high‐performing hospitals.
Acknowledgements
The authors of this study thank Jane Garb for her help with statistical analysis.
- Centers for Medicare and Medicaid Services. National Health Care Expenditures Data.2010. Available at: http://www.2.cms.gov/NationalHealthExpendData/25_NHE_Fact_Sheet.asp. Accessed April 22,year="2010"2010.
- ,,, et al.The quality of health care delivered to adults in the United States.N Engl J Med.2003;348(26):2635–2645.
- ,. The State‐of‐the‐Art of Online Hospital Public Reporting: a Review of Fifty‐One Websites. 2005. Available at: http://www.delmarvafoundation.org/newsAndPublications/reports/documents/WebSummariesFinal9.2.04.pdf. Accessed February 24,2012.
- . Kaiser Family Foundation. 2008 Update on Consumers' Views of Patient Safety and Quality Information. 2010. Available at: http://www.kff.org/kaiserpolls/upload/7819.pdf. Accessed April 20,2010.
- ,,.Choosing where to have major surgery: who makes the decision?Arch Surg.2007;142(3):242–246.
- ,,, et al.Resolving the gatekeeper conundrum: what patients value in primary care and referrals to specialists.JAMA.1999;282(3):261–266.
- ,,,.How physicians make referrals.J Health Care Mark.1993;13(2):6–17.
- ,,,.Growth in the care of older patients by hospitalists in the United States.N Engl J Med.2009;360(11):1102–1112.
- ,,,DeBuono BA. Public release of cardiac surgery outcomes data in New York: what do New York state cardiologists think of it?Am Heart J.1997;134(6):1120–1128.
- ,.Influence of cardiac‐surgery performance reports on referral practices and access to care. A survey of cardiovascular specialists.N Engl J Med.1996;335(4):251–256.
- ,,,,.Primary care summary of the British Thoracic Society Guidelines for the management of community acquired pneumonia in adults: 2009 update. Endorsed by the Royal College of General Practitioners and the Primary Care Respiratory Society UK.Prim Care Respir J.2010;19(1):21–27.
- Hospital Quality Alliance Quality Measures.2010. Available at: http://www.hospitalqualityalliance.org/hospitalqualityalliance/qualitymeasures/qualitymeasures.html. Accessed April 25,year="2010"2010.
- Massachusetts Executive Office of Health and Human Services. Massachusetts Executive Quality and Cost.2010. Available at: http://www.mass.gov/healthcareqc. Accessed February 24,year="2012"2012.
- Centers for Medicare and Medicaid Services. Hospital Compare.2010. Available at: http://www.hospitalcompare.hhs.gov. Accessed April 19,year="2010"2010.
- The Leapfrog Group for Patient Safety.2010. Available at: http://www.leapfroggroup.org/. Accessed April 23,year="2010"2010.
- Health Grades. 2010. Available at: http://www.healthgrades.com. Accessed April 19,2010.
- American Nurses Credentialing Center. Magnet Recognition Program. 2010. Available at: http://www.nursecredentialing.org/Magnet.aspx. Accessed April 15,2010.
- U.S. News 353(3):265–274.
- . US ads push patients to shop for hospitals. USA Today. May 20, 2008. Available at: http://www.usatoday.com/news/health/2008‐05‐20‐Hospitalads_N.htm. Accessed February 24, 2012.
- ,,.How do elderly patients decide where to go for major surgery? Telephone interview survey.BMJ.2005;331(7520):821.
- ,,, et al.Public reporting of cardiac surgery performance: part 1—history, rationale, consequences.Ann Thorac Surg.2011;92(3 suppl):S2–S11.
- ,,.Public reporting of hospital quality: recommendations to benefit patients and hospitals.J Hosp Med.2009;4(9):541–545.
- ,,, et al.When things go wrong: the impact of being a statistical outlier in publicly reported coronary artery bypass graft surgery mortality data.Am J Med Qual.2008;23(2):90–95.
- ,,, et al.Public reporting and pay for performance in hospital quality improvement.N Engl J Med.2007;356(5):486–496.
Over the past decade, research has demonstrated a value gap in US healthcare, characterized by rapidly rising costs and substandard quality.1, 2 Public reporting of hospital performance data is one of several strategies promoted to help address these deficiencies. To this end, a number of hospital rating services have created Web sites aimed at healthcare consumers.3 These services provide information about multiple aspects of healthcare quality, which in theory might be used by patients when deciding where to seek medical care.
Despite the increasing availability of publicly reported quality data comparing doctors and hospitals, a 2008 survey found that only 14% of Americans have seen and used such information in the past year, a decrease from 2006 (36%).4 A similar study in 2007 found that after seeking input from family and friends, patients generally rely on their primary care physician (PCP) to assist them to make decisions about where to have elective surgery.5 Surprisingly, almost nothing is known about how publicly reported data is used, if at all, by PCPs in the referral of patients to hospitals.
The physician is an important intermediary in the buying process for many healthcare services.6 Tertiary care hospitals depend on physician referrals for much of their patient volume.7 Until the emergence of the hospitalist model of care, most primary care physicians cared for their own hospitalized patients, and thus hospital referral decisions were largely driven by the PCP's admitting privileges. However, following the rapid expansion of the hospitalist movement,8, 9 there has been a sharp decrease in the number of PCPs who provide direct patient care for their hospitalized patients.8 As a result, PCPs may now have more choice in regards to hospital referrals for general medical conditions. Potential factors influencing a PCP's referral decisions might include familiarity with the hospital, care quality, patient convenience, satisfaction with the hospital, or hospital reputation.
Studies of cardiac surgery report cards in New York9 and Pennsylvania,10 conducted in the mid‐1990s, found that cardiologists did not use publicly reported mortality data in referral decisions, nor did they share it with patients. Over the past 2 decades, public reporting has grown exponentially, and now includes many measures of structure, processes, and outcomes for almost all US hospitals, available for free over the Internet. The growth of the patient safety movement and mandated public reporting might also have affected physicians' views about publicly reported quality data. We surveyed primary care physicians to determine the extent to which they use information about hospital quality in their referral decisions for community‐acquired pneumonia, and to identify other factors that might influence referral decisions.
METHODS
We obtained an e‐mail list of primary care physicians from the medical staff offices of all area hospitals within a 10‐mile radius of Springfield, MA (Baystate Medical Center, Holyoke Medical Center, and Mercy Medical Center). Baystate Medical Center is a 659‐bed academic medical center and Level 1 trauma center, while Holyoke and Mercy Medical Center are both 180‐bed acute care hospitals. Physicians were contacted via e‐mail from June through September of 2009, and asked to participate in an anonymous, 10‐minute, online survey accessible through an Internet link (SurveyMonkey.com) about factors influencing a primary care physician's hospital referral choice for a patient with pneumonia. To facilitate participation, we sent 2 follow‐up e‐mail reminders, and respondents who completed the entire survey received a $15 gift card. The study was approved by the institutional review board of Baystate Medical Center and closed to participation on September 23, 2009.
We created the online survey based on previous research7 and approximately 10 key informant interviews. The survey (see Supporting Information, Appendix, in the online version of this article) contained 13 demographic questions and 10 questions based on a case study of pneumonia (Figure 1). The instrument was pilot tested for clarity with a small group of primary care physicians at the author's institution and subsequently modified. We chose pneumonia because it is a common reason for a PCP to make an urgent hospital referral,11 and because there is a well‐established set of quality measures that are publicly reported.12 Unlike elective surgery, for which patients might research hospitals or surgeons on their own, patients with pneumonia would likely rely on their PCP to recommend a hospital for urgent referral. In contrast, PCPs know they will refer a number of pneumonia patients to hospitals each year and therefore might have an interest in comparing the publicly reported quality measures for local hospitals.

Respondents were shown the case study and asked to refer the hypothetical patient to 1 of 4 area hospitals. Respondents were asked to rate (on a 3‐point scale: not at all, somewhat, or very) the importance of the following factors in their referral decision: waiting time in the emergency room, distance traveled by the patient, experience of other patients, severity of patient's illness, patient's insurance, hospital's reputation among other physicians and partners, admitting privileges with a specific hospital, admitting arrangements with a hospitalist group, familiarity with the hospital, availability of subspecialists, quality of subspecialists, nursing quality, nursing staffing ratios, hospital's case volume for pneumonia, publicly available quality measures, patient preference, distance from your practice, shared electronic record system, and quality of hospital discharge summaries. Next, we measured provider's awareness of publicly reported hospital quality data and whether they used such data in referring patients or choosing their own medical care. Specifically, we asked about familiarity with the following 4 Web sites: Massachusetts Quality and Cost (a state‐specific Web site produced by the Massachusetts Executive Office of Health and Human Services)13; Hospital Compare (a Web site developed and maintained by Centers for Medicare and Medicaid Services [CMS] and the Department of Health and Human Services)14; Leapfrog Group (a private, nonprofit organization)15; and Health Grades (a private, for‐profit company).16
We then asked participants to rate the importance of the following performance measures when judging a hospital's performance: antibiotics within 6 hours of arrival to the hospital, appropriate initial antibiotic, blood culture drawn before antibiotics given, smoking cessation advice/counseling, oxygenation assessment, risk‐adjusted mortality, intensive care unit staffing, influenza vaccination, pneumococcal vaccination, Leapfrog's never events,15 volume, Leapfrog safe practices score, cost, computerized physician order entry system, Magnet status,17 and U.S. News & World Report's Best Hospitals designation.18 Lastly, we asked participants to state, using a 3‐point scale (agree, disagree, neutral), their level of agreement that the following factors, adapted from Schneider and Epstein,10 represented limitations of public reporting: 1) risk‐adjusted methods are inadequate to compare hospitals fairly; 2) mortality rates are an incomplete indication of the quality of a hospital's care; 3) hospitals can manipulate the data; and 4) ratings are inaccurate for hospitals with small caseloads.
Factors associated with physicians' knowledge of publicly reported data were analyzed with bivariate analysis. Since all factors are categorical, chi‐square analysis was used for bivariate analysis. No factor had a P value <0.2 on bivariate analysis, thus multiple logistic regression was not performed.
RESULTS
Of 194 primary care physicians who received invitations, 92 responded (response rate of 47%). See Table 1 for respondents' characteristics. All age groups were represented; most were male and between 3554 years of age. Respondents were evenly divided between those who owned their own practices (54%) and those working for a health system (46%). Ninety‐three percent of PCPs maintained admitting privileges (45% to more than 1 hospital), but only 20% continued to admit their own patients. When asked where they would send a hypothetical pneumonia patient, only 4% of PCPs chose a hospital to which they had never had admitting privileges.
| Variable | No. (%) of Respondents |
|---|---|
| Age | |
| 2534 | 5 (5) |
| 3544 | 27 (29) |
| 4554 | 24 (26) |
| >55 | 36 (39) |
| Gender | |
| Male | 65 (71) |
| Female | 27 (29) |
| Years out of medical school | |
| <6 | 6 (7) |
| 610 | 9 (10) |
| 1115 | 17 (18) |
| >15 | 60 (65) |
| % Patients seen who are covered by | |
| Medicaid: Mean (SD) | 28 (26) |
| Medicare: Mean (SD) | 31 (18) |
| Private: Mean (SD) | 40 (25) |
| Number of time doing patient care: Mean (SD) | 85 (23) |
| Number of patients admitted/sent to hospital/mo | |
| <6 | 40 (47) |
| 610 | 25 (29) |
| 1120 | 12 (14) |
| >20 | 8 (9) |
| Practice type | |
| Solo | 13 (15) |
| Single specialty group | 36 (42) |
| Multi‐specialty group | 36 (42) |
| Practice ownership | |
| Independent | 45 (54) |
| Health system | 38 (46) |
| Currently admits own patients | |
| Yes | 17 (20) |
| No | 66 (80) |
| Current hospital admitting privileges | |
| A | 63 (76) |
| B | 41 (49) |
| C | 3 (4) |
| D | 12 (14) |
| None | 6 (7) |
| Other | 2 (2) |
Physician's ratings of the importance of various factors in their referral decision are shown in Figure 2. The following factors were most often considered very important: familiarity with the hospital (70%), patient preference (62%), and admitting arrangements with a hospitalist group (62%). In contrast, only 18% of physicians viewed publicly available hospital quality measures as very important when making a referral decision. Factors most often rated not at all important to participants' decisions were patient insurance (48%), hospital's case volume for pneumonia (48%), and publicly available quality measures (42%).

Of the 61% who were aware of Web sites that report hospital quality, most (52%) were familiar with Massachusetts Quality and Cost, while few (27%) were familiar with Hospital Compare. None of the physicians we surveyed reported having used publicly reported quality information when making a referral decision or having discussed such data with their patients. However, 49% stated that publicly reported performance data was somewhat and 10% very important to decisions regarding the medical care they receive. None of the demographic characteristics that we assessed (including age, gender, or years out of medical school) were associated with awareness of publicly reported data in bivariate analyses.
Respondents' ratings of specific quality measures appear in Figure 3. PCPs most often identified the following factors as being very important when judging hospital quality: percent of pneumonia patients given initial antibiotics within 6 hours after arrival (66%), percent of pneumonia patients given the most appropriate initial antibiotic (63%), and percent of pneumonia patients whose initial emergency room (ER) blood culture was performed prior to the administration of the first hospital dose of antibiotics (51%). The factors most often rated not at all important included: U.S. News & World Report's Best Hospitals designation (57%), Magnet Status (42%), and computer physician order entry system (40%).

When asked about limitations of publicly reported performance data, 42% agreed that risk‐adjusted methods were inadequate to compare hospitals fairly, 76% agreed that mortality rates were an incomplete indication of the quality of hospitals care, 62% agreed that hospitals could manipulate the data, and 72% agreed that the ratings were inaccurate for hospitals with small caseloads.
DISCUSSION
In 2003, the Hospital Quality Alliance began a voluntary public reporting program of hospital performance measures, for pneumonia, acute myocardial infarction, and congestive heart failure, that was intended to encourage quality improvement activity by hospitals, and to provide patients and referring physicians with information to make better‐informed choices.19 These data are now easily available to the public through a free Web site (
Despite their lack of familiarity with Hospital Compare, it was the quality measures that are reported by Hospital Compare that they identified as the best indicators of hospital quality: appropriate initial antibiotic, antibiotics within 6 hours, and blood cultures performed prior to the administration of antibiotics. In fact, the 5 measures most often cited as very important to judging hospital quality were all measures reported on Hospital Compare.
As the US healthcare system becomes increasingly complex and costly, there is a growing interest in providing patients with physician and hospital performance data to help them select the provider.21 It is postulated that if patients took a more active role in choosing healthcare providers, and were forced to assume greater financial responsibility, then consumerism will force improvements in quality of care while maintaining or even lowering costs.21 However, studies demonstrate that most patients are unaware of performance data and, if they are aware, still value familiarity over quality ratings.4 Moreover, patients rely on the knowledge of their primary care physician to guide them.5
This is the first study we are aware of that examines how primary care physicians use publicly reported quality data in hospital referral decisions. Studies from more than a decade ago found that publicly reported data had minimal impact on referral decisions from cardiologists to cardiac surgeons. A survey of Pennsylvania's cardiologists and cardiac surgeons showed that although 82% were aware of risk‐adjusted mortality rates published for surgeons, only 10% of cardiologists reported these to be very important when evaluating the performance of a cardiothoracic surgeon. Furthermore, 87% of cardiologists stated that mortality and case volume information reported on cardiac surgeons had minimal or no influence on their referral practices.10 In 1997, a survey of cardiologists in New York found that only 38% of respondents reported that risk‐adjusted outcome data had affected their referrals to surgeons very much or somewhat.9 In addition, most authors conclude that public reporting has had little or no effect on market share.22 Despite growth in the number of measures and improved accessibility, our physicians were even less likely to be aware of, or use, publicly reported data than physicians a decade earlier.
Of course, even if public reporting does not influence referral patterns, it could still improve healthcare quality in several ways. First, feedback about performance may focus quality improvement activities in specific areas that represent gaps in care.10 This could take the form of an appeal to professionalism,23 or the desire to preserve one's reputation by not appearing on a list of poor performers.24 Second, hospitals' desire to appear on lists of high performers, such as U.S. News & World Report's hospital rankings, for marketing purposes, might stimulate improvement activities.10 Finally, publicly reported measures could form the basis for pay‐for‐performance incentives that further speed improvement.25
Our study has several limitations. First, our sample size was small and restricted to 1 region of 1 state, and may not be representative of either the state or nation as a whole. Still, our area has a high level of Internet use, and several local hospitals have been at the vanguard of the quality movement, generally scoring above both state and national averages on Hospital Compare. In addition, Massachusetts has made substantial efforts to promote its own public reporting program, and half the surveyed physicians reported being aware of the Massachusetts Quality and Cost Web site. The fact that not a single area physician surveyed used publicly reported data when making referral decisions is sobering. We believe it is unlikely that other areas of the country will have a substantially higher rate of use. Similarly, our response rate was under 50%. Physicians who did not take the survey may have differed in important ways from those who did. Nevertheless, our sample included a broad range of physician ages, practice types, and affiliations. It seems unlikely that those who did not respond would be more inclined to use publicly reported data than those who did. Second, we assessed decision‐making around a single medical condition. Physicians may have used publicly reported data for other decisions. However, the condition we chose was both urgent (as opposed to emergent) and possesses a robust set of publicly reported quality measures. If physicians do not use publicly reported data for this decision, it seems unlikely they would use it for conditions that have fewer reliable measures (eg, gall bladder surgery) or where the choice of hospital is generally made in an ambulance (eg, myocardial infarction). Finally, the low awareness of public reporting made it difficult for some physicians to answer some of the questions regarding publicly reported hospital quality data because they were unfamiliar with the language utilized by the Web sites (eg, magnet status, Leapfrog never events). It is possible that our results may have been altered slightly if a glossary had been provided.
Despite these limitations, our study suggests that more than 6 years after the launch of the Hospital Quality Alliance, primary care physicians do not appear to make use of these data when choosing a hospital for their patients suffering from pneumonia. Instead, they rely on familiarity with a hospital and past relationships. Even though a majority of the physicians surveyed no longer admitted their own patients, they continue to send patients to hospitals where they had privileges. This finding is not surprising, as physicians also cling to familiar therapies, and may be reluctant to prescribe a new medication or perform an unfamiliar procedure, even if it is indicated. Such reliance on familiarity may make physicians feel comfortable, but does not always result in the best care for patients. Acquiring familiarity, however, requires time and effort, something that physicians generally have in short supply; and while there are plenty of industry representatives to overcome physicians' hesitancy to prescribe new treatments, there are no analogous agents to educate physicians about public reporting or to help them overcome hesitancy about trying a new hospital.
Suspicion about the validity of public reporting may also play a role in the physicians' reported behavior. In past studies of cardiac report cards, cardiologists were most concerned that risk adjustment methods were inadequate (77%) and that mortality rates were an incomplete indicator of the quality of surgical care (74%). They were less concerned about manipulation of data (52%) or small caseloads (15%).10 Our physicians were also concerned that mortality rates were an incomplete measure of quality (76%) but less concerned about risk adjustment (42%), perhaps because many structure and process measures are not subject to risk adjustment. In contrast, they were somewhat more concerned that hospitals could manipulate the data (62%), which again may reflect process measures versus mortality statistics. Other reasons for not using the data may include a lack of awareness of the data or how to access it, or a belief that hospitals do not vary in quality.
Interestingly, even though most respondents were not aware of Hospital Compare, they found the information presented there to best reflect the overall hospital quality. Also, while respondents indicated that they did not use publicly reported data when referring patients, almost half of PCPs reported that publicly reported performance data was at least somewhat important in choosing their own medical care. Thus, although public reporting appears not to have reached its full potential, some publicly reported quality measures have clearly entered the consciousness of PCPs. In contrast, other highly touted measures such as computerized physician order entry systems were not appreciated, and popular designations such as U.S. News & World Report's Best Hospitals were least valued, even though 1 area hospital carries this designation. One conclusion might be that CMS should abandon Hospital Compare since neither patients4 nor providers use it. However, public reporting may improve quality in other ways. Moreover, physicians appear interested in the data even if they are not aware of it. Therefore, given the large investment by CMS and individual hospitals in collecting the data required for Hospital Compare, CMS might consider making greater efforts to increase primary care physician awareness of the Hospital Compare Web site. At the same time, high‐performing hospitals may want to communicate their performance scores to local PCPs as part of their marketing strategy. Future studies could assess whether such practices affect physician referral decisions and subsequent market share of high‐performing hospitals.
Acknowledgements
The authors of this study thank Jane Garb for her help with statistical analysis.
Over the past decade, research has demonstrated a value gap in US healthcare, characterized by rapidly rising costs and substandard quality.1, 2 Public reporting of hospital performance data is one of several strategies promoted to help address these deficiencies. To this end, a number of hospital rating services have created Web sites aimed at healthcare consumers.3 These services provide information about multiple aspects of healthcare quality, which in theory might be used by patients when deciding where to seek medical care.
Despite the increasing availability of publicly reported quality data comparing doctors and hospitals, a 2008 survey found that only 14% of Americans have seen and used such information in the past year, a decrease from 2006 (36%).4 A similar study in 2007 found that after seeking input from family and friends, patients generally rely on their primary care physician (PCP) to assist them to make decisions about where to have elective surgery.5 Surprisingly, almost nothing is known about how publicly reported data is used, if at all, by PCPs in the referral of patients to hospitals.
The physician is an important intermediary in the buying process for many healthcare services.6 Tertiary care hospitals depend on physician referrals for much of their patient volume.7 Until the emergence of the hospitalist model of care, most primary care physicians cared for their own hospitalized patients, and thus hospital referral decisions were largely driven by the PCP's admitting privileges. However, following the rapid expansion of the hospitalist movement,8, 9 there has been a sharp decrease in the number of PCPs who provide direct patient care for their hospitalized patients.8 As a result, PCPs may now have more choice in regards to hospital referrals for general medical conditions. Potential factors influencing a PCP's referral decisions might include familiarity with the hospital, care quality, patient convenience, satisfaction with the hospital, or hospital reputation.
Studies of cardiac surgery report cards in New York9 and Pennsylvania,10 conducted in the mid‐1990s, found that cardiologists did not use publicly reported mortality data in referral decisions, nor did they share it with patients. Over the past 2 decades, public reporting has grown exponentially, and now includes many measures of structure, processes, and outcomes for almost all US hospitals, available for free over the Internet. The growth of the patient safety movement and mandated public reporting might also have affected physicians' views about publicly reported quality data. We surveyed primary care physicians to determine the extent to which they use information about hospital quality in their referral decisions for community‐acquired pneumonia, and to identify other factors that might influence referral decisions.
METHODS
We obtained an e‐mail list of primary care physicians from the medical staff offices of all area hospitals within a 10‐mile radius of Springfield, MA (Baystate Medical Center, Holyoke Medical Center, and Mercy Medical Center). Baystate Medical Center is a 659‐bed academic medical center and Level 1 trauma center, while Holyoke and Mercy Medical Center are both 180‐bed acute care hospitals. Physicians were contacted via e‐mail from June through September of 2009, and asked to participate in an anonymous, 10‐minute, online survey accessible through an Internet link (SurveyMonkey.com) about factors influencing a primary care physician's hospital referral choice for a patient with pneumonia. To facilitate participation, we sent 2 follow‐up e‐mail reminders, and respondents who completed the entire survey received a $15 gift card. The study was approved by the institutional review board of Baystate Medical Center and closed to participation on September 23, 2009.
We created the online survey based on previous research7 and approximately 10 key informant interviews. The survey (see Supporting Information, Appendix, in the online version of this article) contained 13 demographic questions and 10 questions based on a case study of pneumonia (Figure 1). The instrument was pilot tested for clarity with a small group of primary care physicians at the author's institution and subsequently modified. We chose pneumonia because it is a common reason for a PCP to make an urgent hospital referral,11 and because there is a well‐established set of quality measures that are publicly reported.12 Unlike elective surgery, for which patients might research hospitals or surgeons on their own, patients with pneumonia would likely rely on their PCP to recommend a hospital for urgent referral. In contrast, PCPs know they will refer a number of pneumonia patients to hospitals each year and therefore might have an interest in comparing the publicly reported quality measures for local hospitals.

Respondents were shown the case study and asked to refer the hypothetical patient to 1 of 4 area hospitals. Respondents were asked to rate (on a 3‐point scale: not at all, somewhat, or very) the importance of the following factors in their referral decision: waiting time in the emergency room, distance traveled by the patient, experience of other patients, severity of patient's illness, patient's insurance, hospital's reputation among other physicians and partners, admitting privileges with a specific hospital, admitting arrangements with a hospitalist group, familiarity with the hospital, availability of subspecialists, quality of subspecialists, nursing quality, nursing staffing ratios, hospital's case volume for pneumonia, publicly available quality measures, patient preference, distance from your practice, shared electronic record system, and quality of hospital discharge summaries. Next, we measured provider's awareness of publicly reported hospital quality data and whether they used such data in referring patients or choosing their own medical care. Specifically, we asked about familiarity with the following 4 Web sites: Massachusetts Quality and Cost (a state‐specific Web site produced by the Massachusetts Executive Office of Health and Human Services)13; Hospital Compare (a Web site developed and maintained by Centers for Medicare and Medicaid Services [CMS] and the Department of Health and Human Services)14; Leapfrog Group (a private, nonprofit organization)15; and Health Grades (a private, for‐profit company).16
We then asked participants to rate the importance of the following performance measures when judging a hospital's performance: antibiotics within 6 hours of arrival to the hospital, appropriate initial antibiotic, blood culture drawn before antibiotics given, smoking cessation advice/counseling, oxygenation assessment, risk‐adjusted mortality, intensive care unit staffing, influenza vaccination, pneumococcal vaccination, Leapfrog's never events,15 volume, Leapfrog safe practices score, cost, computerized physician order entry system, Magnet status,17 and U.S. News & World Report's Best Hospitals designation.18 Lastly, we asked participants to state, using a 3‐point scale (agree, disagree, neutral), their level of agreement that the following factors, adapted from Schneider and Epstein,10 represented limitations of public reporting: 1) risk‐adjusted methods are inadequate to compare hospitals fairly; 2) mortality rates are an incomplete indication of the quality of a hospital's care; 3) hospitals can manipulate the data; and 4) ratings are inaccurate for hospitals with small caseloads.
Factors associated with physicians' knowledge of publicly reported data were analyzed with bivariate analysis. Since all factors are categorical, chi‐square analysis was used for bivariate analysis. No factor had a P value <0.2 on bivariate analysis, thus multiple logistic regression was not performed.
RESULTS
Of 194 primary care physicians who received invitations, 92 responded (response rate of 47%). See Table 1 for respondents' characteristics. All age groups were represented; most were male and between 3554 years of age. Respondents were evenly divided between those who owned their own practices (54%) and those working for a health system (46%). Ninety‐three percent of PCPs maintained admitting privileges (45% to more than 1 hospital), but only 20% continued to admit their own patients. When asked where they would send a hypothetical pneumonia patient, only 4% of PCPs chose a hospital to which they had never had admitting privileges.
| Variable | No. (%) of Respondents |
|---|---|
| Age | |
| 2534 | 5 (5) |
| 3544 | 27 (29) |
| 4554 | 24 (26) |
| >55 | 36 (39) |
| Gender | |
| Male | 65 (71) |
| Female | 27 (29) |
| Years out of medical school | |
| <6 | 6 (7) |
| 610 | 9 (10) |
| 1115 | 17 (18) |
| >15 | 60 (65) |
| % Patients seen who are covered by | |
| Medicaid: Mean (SD) | 28 (26) |
| Medicare: Mean (SD) | 31 (18) |
| Private: Mean (SD) | 40 (25) |
| Number of time doing patient care: Mean (SD) | 85 (23) |
| Number of patients admitted/sent to hospital/mo | |
| <6 | 40 (47) |
| 610 | 25 (29) |
| 1120 | 12 (14) |
| >20 | 8 (9) |
| Practice type | |
| Solo | 13 (15) |
| Single specialty group | 36 (42) |
| Multi‐specialty group | 36 (42) |
| Practice ownership | |
| Independent | 45 (54) |
| Health system | 38 (46) |
| Currently admits own patients | |
| Yes | 17 (20) |
| No | 66 (80) |
| Current hospital admitting privileges | |
| A | 63 (76) |
| B | 41 (49) |
| C | 3 (4) |
| D | 12 (14) |
| None | 6 (7) |
| Other | 2 (2) |
Physician's ratings of the importance of various factors in their referral decision are shown in Figure 2. The following factors were most often considered very important: familiarity with the hospital (70%), patient preference (62%), and admitting arrangements with a hospitalist group (62%). In contrast, only 18% of physicians viewed publicly available hospital quality measures as very important when making a referral decision. Factors most often rated not at all important to participants' decisions were patient insurance (48%), hospital's case volume for pneumonia (48%), and publicly available quality measures (42%).

Of the 61% who were aware of Web sites that report hospital quality, most (52%) were familiar with Massachusetts Quality and Cost, while few (27%) were familiar with Hospital Compare. None of the physicians we surveyed reported having used publicly reported quality information when making a referral decision or having discussed such data with their patients. However, 49% stated that publicly reported performance data was somewhat and 10% very important to decisions regarding the medical care they receive. None of the demographic characteristics that we assessed (including age, gender, or years out of medical school) were associated with awareness of publicly reported data in bivariate analyses.
Respondents' ratings of specific quality measures appear in Figure 3. PCPs most often identified the following factors as being very important when judging hospital quality: percent of pneumonia patients given initial antibiotics within 6 hours after arrival (66%), percent of pneumonia patients given the most appropriate initial antibiotic (63%), and percent of pneumonia patients whose initial emergency room (ER) blood culture was performed prior to the administration of the first hospital dose of antibiotics (51%). The factors most often rated not at all important included: U.S. News & World Report's Best Hospitals designation (57%), Magnet Status (42%), and computer physician order entry system (40%).

When asked about limitations of publicly reported performance data, 42% agreed that risk‐adjusted methods were inadequate to compare hospitals fairly, 76% agreed that mortality rates were an incomplete indication of the quality of hospitals care, 62% agreed that hospitals could manipulate the data, and 72% agreed that the ratings were inaccurate for hospitals with small caseloads.
DISCUSSION
In 2003, the Hospital Quality Alliance began a voluntary public reporting program of hospital performance measures, for pneumonia, acute myocardial infarction, and congestive heart failure, that was intended to encourage quality improvement activity by hospitals, and to provide patients and referring physicians with information to make better‐informed choices.19 These data are now easily available to the public through a free Web site (
Despite their lack of familiarity with Hospital Compare, it was the quality measures that are reported by Hospital Compare that they identified as the best indicators of hospital quality: appropriate initial antibiotic, antibiotics within 6 hours, and blood cultures performed prior to the administration of antibiotics. In fact, the 5 measures most often cited as very important to judging hospital quality were all measures reported on Hospital Compare.
As the US healthcare system becomes increasingly complex and costly, there is a growing interest in providing patients with physician and hospital performance data to help them select the provider.21 It is postulated that if patients took a more active role in choosing healthcare providers, and were forced to assume greater financial responsibility, then consumerism will force improvements in quality of care while maintaining or even lowering costs.21 However, studies demonstrate that most patients are unaware of performance data and, if they are aware, still value familiarity over quality ratings.4 Moreover, patients rely on the knowledge of their primary care physician to guide them.5
This is the first study we are aware of that examines how primary care physicians use publicly reported quality data in hospital referral decisions. Studies from more than a decade ago found that publicly reported data had minimal impact on referral decisions from cardiologists to cardiac surgeons. A survey of Pennsylvania's cardiologists and cardiac surgeons showed that although 82% were aware of risk‐adjusted mortality rates published for surgeons, only 10% of cardiologists reported these to be very important when evaluating the performance of a cardiothoracic surgeon. Furthermore, 87% of cardiologists stated that mortality and case volume information reported on cardiac surgeons had minimal or no influence on their referral practices.10 In 1997, a survey of cardiologists in New York found that only 38% of respondents reported that risk‐adjusted outcome data had affected their referrals to surgeons very much or somewhat.9 In addition, most authors conclude that public reporting has had little or no effect on market share.22 Despite growth in the number of measures and improved accessibility, our physicians were even less likely to be aware of, or use, publicly reported data than physicians a decade earlier.
Of course, even if public reporting does not influence referral patterns, it could still improve healthcare quality in several ways. First, feedback about performance may focus quality improvement activities in specific areas that represent gaps in care.10 This could take the form of an appeal to professionalism,23 or the desire to preserve one's reputation by not appearing on a list of poor performers.24 Second, hospitals' desire to appear on lists of high performers, such as U.S. News & World Report's hospital rankings, for marketing purposes, might stimulate improvement activities.10 Finally, publicly reported measures could form the basis for pay‐for‐performance incentives that further speed improvement.25
Our study has several limitations. First, our sample size was small and restricted to 1 region of 1 state, and may not be representative of either the state or nation as a whole. Still, our area has a high level of Internet use, and several local hospitals have been at the vanguard of the quality movement, generally scoring above both state and national averages on Hospital Compare. In addition, Massachusetts has made substantial efforts to promote its own public reporting program, and half the surveyed physicians reported being aware of the Massachusetts Quality and Cost Web site. The fact that not a single area physician surveyed used publicly reported data when making referral decisions is sobering. We believe it is unlikely that other areas of the country will have a substantially higher rate of use. Similarly, our response rate was under 50%. Physicians who did not take the survey may have differed in important ways from those who did. Nevertheless, our sample included a broad range of physician ages, practice types, and affiliations. It seems unlikely that those who did not respond would be more inclined to use publicly reported data than those who did. Second, we assessed decision‐making around a single medical condition. Physicians may have used publicly reported data for other decisions. However, the condition we chose was both urgent (as opposed to emergent) and possesses a robust set of publicly reported quality measures. If physicians do not use publicly reported data for this decision, it seems unlikely they would use it for conditions that have fewer reliable measures (eg, gall bladder surgery) or where the choice of hospital is generally made in an ambulance (eg, myocardial infarction). Finally, the low awareness of public reporting made it difficult for some physicians to answer some of the questions regarding publicly reported hospital quality data because they were unfamiliar with the language utilized by the Web sites (eg, magnet status, Leapfrog never events). It is possible that our results may have been altered slightly if a glossary had been provided.
Despite these limitations, our study suggests that more than 6 years after the launch of the Hospital Quality Alliance, primary care physicians do not appear to make use of these data when choosing a hospital for their patients suffering from pneumonia. Instead, they rely on familiarity with a hospital and past relationships. Even though a majority of the physicians surveyed no longer admitted their own patients, they continue to send patients to hospitals where they had privileges. This finding is not surprising, as physicians also cling to familiar therapies, and may be reluctant to prescribe a new medication or perform an unfamiliar procedure, even if it is indicated. Such reliance on familiarity may make physicians feel comfortable, but does not always result in the best care for patients. Acquiring familiarity, however, requires time and effort, something that physicians generally have in short supply; and while there are plenty of industry representatives to overcome physicians' hesitancy to prescribe new treatments, there are no analogous agents to educate physicians about public reporting or to help them overcome hesitancy about trying a new hospital.
Suspicion about the validity of public reporting may also play a role in the physicians' reported behavior. In past studies of cardiac report cards, cardiologists were most concerned that risk adjustment methods were inadequate (77%) and that mortality rates were an incomplete indicator of the quality of surgical care (74%). They were less concerned about manipulation of data (52%) or small caseloads (15%).10 Our physicians were also concerned that mortality rates were an incomplete measure of quality (76%) but less concerned about risk adjustment (42%), perhaps because many structure and process measures are not subject to risk adjustment. In contrast, they were somewhat more concerned that hospitals could manipulate the data (62%), which again may reflect process measures versus mortality statistics. Other reasons for not using the data may include a lack of awareness of the data or how to access it, or a belief that hospitals do not vary in quality.
Interestingly, even though most respondents were not aware of Hospital Compare, they found the information presented there to best reflect the overall hospital quality. Also, while respondents indicated that they did not use publicly reported data when referring patients, almost half of PCPs reported that publicly reported performance data was at least somewhat important in choosing their own medical care. Thus, although public reporting appears not to have reached its full potential, some publicly reported quality measures have clearly entered the consciousness of PCPs. In contrast, other highly touted measures such as computerized physician order entry systems were not appreciated, and popular designations such as U.S. News & World Report's Best Hospitals were least valued, even though 1 area hospital carries this designation. One conclusion might be that CMS should abandon Hospital Compare since neither patients4 nor providers use it. However, public reporting may improve quality in other ways. Moreover, physicians appear interested in the data even if they are not aware of it. Therefore, given the large investment by CMS and individual hospitals in collecting the data required for Hospital Compare, CMS might consider making greater efforts to increase primary care physician awareness of the Hospital Compare Web site. At the same time, high‐performing hospitals may want to communicate their performance scores to local PCPs as part of their marketing strategy. Future studies could assess whether such practices affect physician referral decisions and subsequent market share of high‐performing hospitals.
Acknowledgements
The authors of this study thank Jane Garb for her help with statistical analysis.
- Centers for Medicare and Medicaid Services. National Health Care Expenditures Data.2010. Available at: http://www.2.cms.gov/NationalHealthExpendData/25_NHE_Fact_Sheet.asp. Accessed April 22,year="2010"2010.
- ,,, et al.The quality of health care delivered to adults in the United States.N Engl J Med.2003;348(26):2635–2645.
- ,. The State‐of‐the‐Art of Online Hospital Public Reporting: a Review of Fifty‐One Websites. 2005. Available at: http://www.delmarvafoundation.org/newsAndPublications/reports/documents/WebSummariesFinal9.2.04.pdf. Accessed February 24,2012.
- . Kaiser Family Foundation. 2008 Update on Consumers' Views of Patient Safety and Quality Information. 2010. Available at: http://www.kff.org/kaiserpolls/upload/7819.pdf. Accessed April 20,2010.
- ,,.Choosing where to have major surgery: who makes the decision?Arch Surg.2007;142(3):242–246.
- ,,, et al.Resolving the gatekeeper conundrum: what patients value in primary care and referrals to specialists.JAMA.1999;282(3):261–266.
- ,,,.How physicians make referrals.J Health Care Mark.1993;13(2):6–17.
- ,,,.Growth in the care of older patients by hospitalists in the United States.N Engl J Med.2009;360(11):1102–1112.
- ,,,DeBuono BA. Public release of cardiac surgery outcomes data in New York: what do New York state cardiologists think of it?Am Heart J.1997;134(6):1120–1128.
- ,.Influence of cardiac‐surgery performance reports on referral practices and access to care. A survey of cardiovascular specialists.N Engl J Med.1996;335(4):251–256.
- ,,,,.Primary care summary of the British Thoracic Society Guidelines for the management of community acquired pneumonia in adults: 2009 update. Endorsed by the Royal College of General Practitioners and the Primary Care Respiratory Society UK.Prim Care Respir J.2010;19(1):21–27.
- Hospital Quality Alliance Quality Measures.2010. Available at: http://www.hospitalqualityalliance.org/hospitalqualityalliance/qualitymeasures/qualitymeasures.html. Accessed April 25,year="2010"2010.
- Massachusetts Executive Office of Health and Human Services. Massachusetts Executive Quality and Cost.2010. Available at: http://www.mass.gov/healthcareqc. Accessed February 24,year="2012"2012.
- Centers for Medicare and Medicaid Services. Hospital Compare.2010. Available at: http://www.hospitalcompare.hhs.gov. Accessed April 19,year="2010"2010.
- The Leapfrog Group for Patient Safety.2010. Available at: http://www.leapfroggroup.org/. Accessed April 23,year="2010"2010.
- Health Grades. 2010. Available at: http://www.healthgrades.com. Accessed April 19,2010.
- American Nurses Credentialing Center. Magnet Recognition Program. 2010. Available at: http://www.nursecredentialing.org/Magnet.aspx. Accessed April 15,2010.
- U.S. News 353(3):265–274.
- . US ads push patients to shop for hospitals. USA Today. May 20, 2008. Available at: http://www.usatoday.com/news/health/2008‐05‐20‐Hospitalads_N.htm. Accessed February 24, 2012.
- ,,.How do elderly patients decide where to go for major surgery? Telephone interview survey.BMJ.2005;331(7520):821.
- ,,, et al.Public reporting of cardiac surgery performance: part 1—history, rationale, consequences.Ann Thorac Surg.2011;92(3 suppl):S2–S11.
- ,,.Public reporting of hospital quality: recommendations to benefit patients and hospitals.J Hosp Med.2009;4(9):541–545.
- ,,, et al.When things go wrong: the impact of being a statistical outlier in publicly reported coronary artery bypass graft surgery mortality data.Am J Med Qual.2008;23(2):90–95.
- ,,, et al.Public reporting and pay for performance in hospital quality improvement.N Engl J Med.2007;356(5):486–496.
- Centers for Medicare and Medicaid Services. National Health Care Expenditures Data.2010. Available at: http://www.2.cms.gov/NationalHealthExpendData/25_NHE_Fact_Sheet.asp. Accessed April 22,year="2010"2010.
- ,,, et al.The quality of health care delivered to adults in the United States.N Engl J Med.2003;348(26):2635–2645.
- ,. The State‐of‐the‐Art of Online Hospital Public Reporting: a Review of Fifty‐One Websites. 2005. Available at: http://www.delmarvafoundation.org/newsAndPublications/reports/documents/WebSummariesFinal9.2.04.pdf. Accessed February 24,2012.
- . Kaiser Family Foundation. 2008 Update on Consumers' Views of Patient Safety and Quality Information. 2010. Available at: http://www.kff.org/kaiserpolls/upload/7819.pdf. Accessed April 20,2010.
- ,,.Choosing where to have major surgery: who makes the decision?Arch Surg.2007;142(3):242–246.
- ,,, et al.Resolving the gatekeeper conundrum: what patients value in primary care and referrals to specialists.JAMA.1999;282(3):261–266.
- ,,,.How physicians make referrals.J Health Care Mark.1993;13(2):6–17.
- ,,,.Growth in the care of older patients by hospitalists in the United States.N Engl J Med.2009;360(11):1102–1112.
- ,,,DeBuono BA. Public release of cardiac surgery outcomes data in New York: what do New York state cardiologists think of it?Am Heart J.1997;134(6):1120–1128.
- ,.Influence of cardiac‐surgery performance reports on referral practices and access to care. A survey of cardiovascular specialists.N Engl J Med.1996;335(4):251–256.
- ,,,,.Primary care summary of the British Thoracic Society Guidelines for the management of community acquired pneumonia in adults: 2009 update. Endorsed by the Royal College of General Practitioners and the Primary Care Respiratory Society UK.Prim Care Respir J.2010;19(1):21–27.
- Hospital Quality Alliance Quality Measures.2010. Available at: http://www.hospitalqualityalliance.org/hospitalqualityalliance/qualitymeasures/qualitymeasures.html. Accessed April 25,year="2010"2010.
- Massachusetts Executive Office of Health and Human Services. Massachusetts Executive Quality and Cost.2010. Available at: http://www.mass.gov/healthcareqc. Accessed February 24,year="2012"2012.
- Centers for Medicare and Medicaid Services. Hospital Compare.2010. Available at: http://www.hospitalcompare.hhs.gov. Accessed April 19,year="2010"2010.
- The Leapfrog Group for Patient Safety.2010. Available at: http://www.leapfroggroup.org/. Accessed April 23,year="2010"2010.
- Health Grades. 2010. Available at: http://www.healthgrades.com. Accessed April 19,2010.
- American Nurses Credentialing Center. Magnet Recognition Program. 2010. Available at: http://www.nursecredentialing.org/Magnet.aspx. Accessed April 15,2010.
- U.S. News 353(3):265–274.
- . US ads push patients to shop for hospitals. USA Today. May 20, 2008. Available at: http://www.usatoday.com/news/health/2008‐05‐20‐Hospitalads_N.htm. Accessed February 24, 2012.
- ,,.How do elderly patients decide where to go for major surgery? Telephone interview survey.BMJ.2005;331(7520):821.
- ,,, et al.Public reporting of cardiac surgery performance: part 1—history, rationale, consequences.Ann Thorac Surg.2011;92(3 suppl):S2–S11.
- ,,.Public reporting of hospital quality: recommendations to benefit patients and hospitals.J Hosp Med.2009;4(9):541–545.
- ,,, et al.When things go wrong: the impact of being a statistical outlier in publicly reported coronary artery bypass graft surgery mortality data.Am J Med Qual.2008;23(2):90–95.
- ,,, et al.Public reporting and pay for performance in hospital quality improvement.N Engl J Med.2007;356(5):486–496.
Copyright © 2012 Society of Hospital Medicine





