User login
Nasim Afsar, New SHM Board Member, Focuses on Improvement Initiatives
Nearly five years ago, when Nasim Afsar, MD, SFHM, was launching her career as a hospitalist at the University of California Los Angeles Medical Center, she began to appreciate the importance of quality issues for the field of hospital medicine.
Dr. Afsar, who recently was elected to the SHM board of directors, says, "I realized that evidence-based medicine should be the standard of care in hospitals, but we were nowhere near where we should be in addressing that at a systemic level."
Believing that her medical training had not fully prepared her for quality work, Dr. Afsar attended the Advanced Training Program at Intermountain Healthcare in Salt Lake City. "With that foundation, my focus ever since has been on improvement initiatives," she says.
In the years since, Dr. Afsar's quality responsibilities have grown steadily. She is now associate medical director of quality and safety at UCLA, executive director for quality and safety in its department of medicine, and director of quality for neurosurgery. That means about 80% of her work week is devoted to quality improvement (QI). And while her time seeing patients is less, she says the "clinical work is what inspires and motivates me, gives me my best ideas, and keeps me grounded."
Among the more than 40 quality and safety projects she has implemented at UCLA is "The ABCs of Hospitalized Patients," a multidisciplinary checklist designed to reduce the risk of eight common hospital-acquired conditions. "Within three weeks of implementing it, we started seeing significant improvement in every area, and we have been able to sustain that," she says. In 2009, she implemented a systemwide QI curriculum for the residents and fellows at UCLA.
Dr. Afsar, chair of SHM's Hospital Quality and Patient Safety Committee, says HM is challenged to make these kinds of quality approaches the standard of practice nationwide.
"Different institutions are doing different pieces of the quality movement very well," and SHM and its leaders on the board need to find a way to disseminate those best practices and integrate them into hospital practice, she says. "There are no simple ways to do that, but we know there are a lot of solutions out there."
Nearly five years ago, when Nasim Afsar, MD, SFHM, was launching her career as a hospitalist at the University of California Los Angeles Medical Center, she began to appreciate the importance of quality issues for the field of hospital medicine.
Dr. Afsar, who recently was elected to the SHM board of directors, says, "I realized that evidence-based medicine should be the standard of care in hospitals, but we were nowhere near where we should be in addressing that at a systemic level."
Believing that her medical training had not fully prepared her for quality work, Dr. Afsar attended the Advanced Training Program at Intermountain Healthcare in Salt Lake City. "With that foundation, my focus ever since has been on improvement initiatives," she says.
In the years since, Dr. Afsar's quality responsibilities have grown steadily. She is now associate medical director of quality and safety at UCLA, executive director for quality and safety in its department of medicine, and director of quality for neurosurgery. That means about 80% of her work week is devoted to quality improvement (QI). And while her time seeing patients is less, she says the "clinical work is what inspires and motivates me, gives me my best ideas, and keeps me grounded."
Among the more than 40 quality and safety projects she has implemented at UCLA is "The ABCs of Hospitalized Patients," a multidisciplinary checklist designed to reduce the risk of eight common hospital-acquired conditions. "Within three weeks of implementing it, we started seeing significant improvement in every area, and we have been able to sustain that," she says. In 2009, she implemented a systemwide QI curriculum for the residents and fellows at UCLA.
Dr. Afsar, chair of SHM's Hospital Quality and Patient Safety Committee, says HM is challenged to make these kinds of quality approaches the standard of practice nationwide.
"Different institutions are doing different pieces of the quality movement very well," and SHM and its leaders on the board need to find a way to disseminate those best practices and integrate them into hospital practice, she says. "There are no simple ways to do that, but we know there are a lot of solutions out there."
Nearly five years ago, when Nasim Afsar, MD, SFHM, was launching her career as a hospitalist at the University of California Los Angeles Medical Center, she began to appreciate the importance of quality issues for the field of hospital medicine.
Dr. Afsar, who recently was elected to the SHM board of directors, says, "I realized that evidence-based medicine should be the standard of care in hospitals, but we were nowhere near where we should be in addressing that at a systemic level."
Believing that her medical training had not fully prepared her for quality work, Dr. Afsar attended the Advanced Training Program at Intermountain Healthcare in Salt Lake City. "With that foundation, my focus ever since has been on improvement initiatives," she says.
In the years since, Dr. Afsar's quality responsibilities have grown steadily. She is now associate medical director of quality and safety at UCLA, executive director for quality and safety in its department of medicine, and director of quality for neurosurgery. That means about 80% of her work week is devoted to quality improvement (QI). And while her time seeing patients is less, she says the "clinical work is what inspires and motivates me, gives me my best ideas, and keeps me grounded."
Among the more than 40 quality and safety projects she has implemented at UCLA is "The ABCs of Hospitalized Patients," a multidisciplinary checklist designed to reduce the risk of eight common hospital-acquired conditions. "Within three weeks of implementing it, we started seeing significant improvement in every area, and we have been able to sustain that," she says. In 2009, she implemented a systemwide QI curriculum for the residents and fellows at UCLA.
Dr. Afsar, chair of SHM's Hospital Quality and Patient Safety Committee, says HM is challenged to make these kinds of quality approaches the standard of practice nationwide.
"Different institutions are doing different pieces of the quality movement very well," and SHM and its leaders on the board need to find a way to disseminate those best practices and integrate them into hospital practice, she says. "There are no simple ways to do that, but we know there are a lot of solutions out there."
Study: Medicare Pay for Performance Might Not Work as Currently Designed
Hospitalist Ashish Jha, MD, MPH, doesn't want people to take his research on the value of pay-for-performance models the wrong way. Although a new study he worked on found no evidence that the Medicare Premier Hospital Quality Incentive Demonstration (HQID) led to decreased rates of 30-day mortality, he believes the program's structure—not its concept—is at issue.
"It's not that pay for performance doesn't work,” says Dr. Jha, associate professor of health policy and management at Harvard School of Public Health in Boston. "What we had in the HQID was pretty small incentives and mostly focused on processes of care, some of which are important, many of which are not. When you have that as your structure, it's not shocking to see in retrospect that it didn't have a big impact on outcomes."
The report, "The Long-Term Effect of Premier Pay for Performance on Patient Outcomes," showed that the composite 30-day mortality rates for patients with acute myocardial infarction, congestive heart failure, pneumonia, and coronary-artery bypass grafts were similar for Premier and non-Premier hospitals (12.33% and 12.40%, respectively; 95% confidence interval, -0.40 to 0.26).
Dr. Jha says the results were surprising, but he believes that HQID, value-based purchasing, and any pay-for-performance model can only succeed if they more narrowly focus on outcomes. For example, he says, HQID should not have weighed reductions in 30-day mortality rates on par with providing smoking-cessation worksheets to patients at discharge.
"You need much stronger incentives," he says. If hospitals focus on outcomes—and, specifically, on the right outcomes—they will figure out what processes they need to engage in and refine, he says. "Hospitalists are going to be the key people there. If they know that their mortality rates are high, they're going to work on trying to figure out why."
Hospitalist Ashish Jha, MD, MPH, doesn't want people to take his research on the value of pay-for-performance models the wrong way. Although a new study he worked on found no evidence that the Medicare Premier Hospital Quality Incentive Demonstration (HQID) led to decreased rates of 30-day mortality, he believes the program's structure—not its concept—is at issue.
"It's not that pay for performance doesn't work,” says Dr. Jha, associate professor of health policy and management at Harvard School of Public Health in Boston. "What we had in the HQID was pretty small incentives and mostly focused on processes of care, some of which are important, many of which are not. When you have that as your structure, it's not shocking to see in retrospect that it didn't have a big impact on outcomes."
The report, "The Long-Term Effect of Premier Pay for Performance on Patient Outcomes," showed that the composite 30-day mortality rates for patients with acute myocardial infarction, congestive heart failure, pneumonia, and coronary-artery bypass grafts were similar for Premier and non-Premier hospitals (12.33% and 12.40%, respectively; 95% confidence interval, -0.40 to 0.26).
Dr. Jha says the results were surprising, but he believes that HQID, value-based purchasing, and any pay-for-performance model can only succeed if they more narrowly focus on outcomes. For example, he says, HQID should not have weighed reductions in 30-day mortality rates on par with providing smoking-cessation worksheets to patients at discharge.
"You need much stronger incentives," he says. If hospitals focus on outcomes—and, specifically, on the right outcomes—they will figure out what processes they need to engage in and refine, he says. "Hospitalists are going to be the key people there. If they know that their mortality rates are high, they're going to work on trying to figure out why."
Hospitalist Ashish Jha, MD, MPH, doesn't want people to take his research on the value of pay-for-performance models the wrong way. Although a new study he worked on found no evidence that the Medicare Premier Hospital Quality Incentive Demonstration (HQID) led to decreased rates of 30-day mortality, he believes the program's structure—not its concept—is at issue.
"It's not that pay for performance doesn't work,” says Dr. Jha, associate professor of health policy and management at Harvard School of Public Health in Boston. "What we had in the HQID was pretty small incentives and mostly focused on processes of care, some of which are important, many of which are not. When you have that as your structure, it's not shocking to see in retrospect that it didn't have a big impact on outcomes."
The report, "The Long-Term Effect of Premier Pay for Performance on Patient Outcomes," showed that the composite 30-day mortality rates for patients with acute myocardial infarction, congestive heart failure, pneumonia, and coronary-artery bypass grafts were similar for Premier and non-Premier hospitals (12.33% and 12.40%, respectively; 95% confidence interval, -0.40 to 0.26).
Dr. Jha says the results were surprising, but he believes that HQID, value-based purchasing, and any pay-for-performance model can only succeed if they more narrowly focus on outcomes. For example, he says, HQID should not have weighed reductions in 30-day mortality rates on par with providing smoking-cessation worksheets to patients at discharge.
"You need much stronger incentives," he says. If hospitals focus on outcomes—and, specifically, on the right outcomes—they will figure out what processes they need to engage in and refine, he says. "Hospitalists are going to be the key people there. If they know that their mortality rates are high, they're going to work on trying to figure out why."
Risk Evaluation and Mitigation Strategies (REMS): red tape, or a remedy for opioid abuse?
Are you aware that a significant change is coming to the way you prescribe opioid pain relievers for your patients? After 3 years of debate among the Food and Drug Administration (FDA), drug industry stakeholders, members of the pain and addiction communities, patient advocacy groups, and the public, the first large-scale, class-wide REMS is here. REMS is the acronym for Risk Evaluation and Mitigation Strategies. There is a good chance you are prescribing one or more of the affected medications, and adherence to the REMS requirements will be essential if you wish to continue prescribing them.
Before getting into the fine points of the opioid REMS, a little background about how it came into being is in order. On March 25, 2008, the Food and Drug Administration Amendments Act went into effect, granting the FDA authority to require a REMS for any product or product class it deemed to be a public health, safety, or welfare threat. Basically, REMS is an FDA-imposed “safety” program. The first medication to now have a single or class REMS is the class of extended-release (ER) and long-acting (LA) opioid analgesics.
Why opioid analgesics? In 2007, attempts to mitigate targeted risks associated with 30 drugs using RISKMaps were cited as inadequate by the FDA. RISKMaps are safety programs designed to minimize significant risks of certain medicines through FDA-approved labeling, reporting of adverse events, prescriber and patient education about risks, reminders, and performance-linked access systems that tie access to medications with documentation and laboratory testing.1 Passage of the FDA Amendments Act allowed the FDA to use its REMS authority to “improve” existing risk plans.
Forces for change
The FDA cites many good reasons for this change, primarily to ensure that the benefits of prescribing opioid analgesics outweigh the risks, and that patients in pain who need these drugs have access to them. Driving factors behind this move centered on the highly visible consequences associated with what FDA experts describe as misuse, abuse, and improper prescribing of 12 ER/LA opioid analgesics. According to FDA estimates, in 2007 more than 33 million Americans age 12 and older misused ER/LA opioids. Of the almost 28,000 Americans who died from unintended consequences of drug use, nearly 12,000 were associated with prescription analgesics.2
In my opinion, voluntary continuing medical education (CME) and professional organization guidelines added to the problem by failing to decrease overdoses and unintended deaths. This may come as no surprise, as such deaths often stem from diversion, and diverters typically are not subject to a CME requirement.
The ER/LA segment of the class was targeted for a variety of reasons. First, higher doses of ER/LA opiates packed into single units are believed to pose a greater threat than the millions of short-acting, immediate-release (IR) opioid analgesics units abused annually.3 Another reason for the move focused on the burden to the health system caused by more than 24 similar individual REMS existing in this class. That alone created a virtual paper, regulatory, and health system encumbrance that is expected to be alleviated by a class-wide REMS.
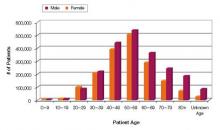
Increasing numbers of prescriptions were an additional consideration. The number of outpatient retail prescriptions dispensed for ER/LA and IR opiates rose dramatically between 2000 and 2009, from 9.3 million to 22.9 million ER/LA opioids and from 164.8 million to 234 million IR opioids [Figure 1].3 Who is prescribing them? You are. In 2009, primary care physicians were the top prescribers of ER/LA (43.8%) and IR (42.1%) opioid analgesics [Figure 2].3 Who are you prescribing them for? Not the elderly age group you might expect. The largest number of prescriptions were written for men and women between ages 50and 59 [Figure 3].3
| FIGURE 1: Total number of prescriptions dispensed for ER/LA and IR opioids from US outpatient retail pharmacies, 2000-2009. |
| ER, extended release; IR, immediate release; LA, long acting; TRx, total prescriptions. Source: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterial/Drug/AnestheticAndLifeSupportDrugsAdvisory Committee/UCM220950.pdf. |
| FIGURE 2: Total number of prescriptions dispensed in the United States by top 10 prescribing specialties for IR and ER/LA opioids, 2009 |
| ANES, anesthesiologists; DO, doctor of osteopathy; EM, emergency medicine; ER, extended release; FM, family medicine; GP, general practitioner; HEM, hematologists; IM, internal medicine; IR, immediate release; LA, long acting; NP, nurse practitioners; ORTH SURG, orthopedic surgeons; NEURO, neurologists; PA, physician assistants; PM&R, physical medicine and rehabilitation; TRx, total prescriptions. Source: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drug/AnestheticAndLifeSupportDrugsAdvisoryCommittee/UCM220950.pdf. |
| FIGURE 3: Total number of unique patients, stratified by age and sex, receiving a dispensed prescription for an ER/LA opioid product from US outpatient retail pharmacies, 2009 |
| ER, extended release; LA, long acting. Source: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/AnestheticAndLifeSupportDrugsAdvisoryCommittee/UCM220950.pdf. |
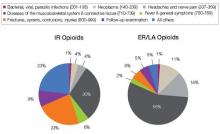
And what are you prescribing them for? Data from a 2009 survey of the prescribing habits of 3200 office-based physicians in 30 specialties showed that most prescriptions written for ER/LA and IR opioids are associated with diagnoses related to pain in the musculoskeletal system and connective tissue (56% [ER/LA] and 30% [IR]). For ER/LA
prescriptions the second most common diagnoses were headaches and nerve pain (14%), while for IR prescriptions they were fractures, sprains, and contusions (23%) [Figure 4].3
| FIGURE 4: Diagnoses associated with use (by grouped ICD-9 codes) for IR and ER/LA opioids as reported by office-based physicians in the United States, 2009 |
| ER, extended release; IR, immediate release; LA, long acting. Source: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/AnestheticAndLifeSupportDrugs AdvisoryCommittee/UCM220950.pdf. |
According to Janet Woodcock, MD, Director of the FDA’s Center for Drug Evaluation and Research, some physicians may not be clear about who should receive these drugs or how to manage patients in pain. As a result, some physicians may be reluctant to prescribe opioid analgesics, leaving patients without adequate pain relief. At the same time, other physicians overprescribe them, putting patients—and anyone with access to the family medicine cabinet—at risk.4
A REMS by any other name
And so REMS was conceived. On February 6, 2009, manufacturers of certain opioid drug products received a letter from the FDA informing them that their drugs would be required to have a risk management program, and inviting them to meet to discuss the design and development of such a REMS.5
Two years later, on April 19, 2011, an alarm in the form of an action plan was released by the Obama administration through the Office of National Drug Control Policy. The plan,
Epidemic: Responding to America’s Prescription Drug Abuse Crisis, outlined a set of measures to remedy the problem through education, monitoring, proper disposal of prescription drugs, and enforcement.6
REMS for opioids was the FDA’s response in support of the President’s plan. On the same day in April, 32 manufacturers of ER/LA opioids received a letter from the FDA informing them that they must meet new safety requirements concerning these medications under a single shared, standardized system [Table].
| TABLE: Long-acting and extended-release opioids requiring an opioid REMS |
| Brand Name Products |
| Trade Name | Generic Name | Sponsor | |
| 1 | Duragesic | Fentanyl transdermal system | Ortho-McNeil-Janssen |
| 2 | Dolophine | Methadone HCI tablets | Roxanne Laboratories |
| 3 | Avinza | Morphine sulfate extended-release capsules | King Pharmaceuticals/Pfizer |
| 4 | Kadian capsules | Morphine sulfate extended-release capsules | Actavis |
| 5 | MS Contin | Morphine sulfate controlled-release tablets | Purdue Pharma |
| 6 | Oramorph | Morphine sulfate sustained-release tablets | Xanodyne Pharmaceuticals |
| 7 | OxyContin | Oxycodone HCI controlled-release tablets | Purdue Pharma |
| 8 | Opana ER | Oxymorphone HCI extended-release tablets | Endo Pharmaceuticals |
| 9 | Exalgo | Hydromorphone HCI extendedrelease tablets | Mallinckrodt Inc/Covidien |
| 10 | Butrans | Buprenorphine transdermal system | Purdue Pharma |
| Generic Products |
| Drug Name | Generic Name | Sponsor | |
| 1 | Fentanyl | Fentanyl extended-release transdermal system | Actavis |
| 2 | Fentanyl | Fentanyl extended-release transdermal system | Lavipharm Labs |
| 3 | Fentanyl | Fentanyl extended-release transdermal system | Mallinckrodt Inc/Covidien |
| 4 | Fentanyl | Fentanyl extended-release transdermal system | Mylan Technologies |
| 5 | Fentanyl | Fentanyl extended-release transdermal system | Noven Pharmaceuticals |
| 6 | Fentanyl | Fentanyl extended-release transdermal system | Teva Pharmaceutical Industries |
| 7 | Fentanyl | Fentanyl extended-release transdermal system | Watson Pharmaceuticals |
| 8 | Methadone hydrochloride | Methadone HCl oral solution | The Pharmanetwork |
| 9 | Methadone hydrochloride | Methadone HCl oral solution | Mallinckrodt Inc/Covidien |
| 10 | Methadone hydrochloride | Methadone HCl oral solution | Sandoz |
| 11 | Methadone hydrochloride | Methadone HCl oral solution | Roxane Laboratories |
| 12 | Methadone hydrochloride | Methadone HCl oral solution | VistaPharm |
| 13 | Morphine sulfate | Morphine sulfate extendedrelease tablets | Endo Pharmaceuticals |
| 14 | Morphine sulfate | Morphine sulfate extendedrelease tablets | KV Pharmaceuticals |
| 15 | Morphine sulfate | Morphine sulfate extendedrelease tablets | Mallinckrodt Inc/Covidien |
| 16 | Morphine sulfate | Morphine sulfate extendedrelease tablets | Watson Pharmaceuticals |
| 17 | Morphine sulfate | Morphine sulfate extendedrelease tablets | Rhodes Pharmaceuticals |
| 18 | Oxycodone hydrochloride | *Oxycodone HCl extendedrelease tablets | Mallinckrodt Inc/Covidien |
| 19 | Oxycodone hydrochloride | *Oxycodone HCl extendedrelease tablets | Impax Laboratories |
| 20 | Oxycodone hydrochloride | *Oxycodone HCl extendedrelease tablets | Teva Pharmaceutical Industries |
| 21 | Oxycodone hydrochloride | *Oxycodone HCl extendedrelease tablets | Endo Pharmaceuticals |
| 22 | Oxycodone hydrochloride | Oxymorphone HCl extendedrelease tablets | Impax Laboratories |
| 23 | Oxycodone hydrochloride | Oxymorphone HCl extendedrelease tablets | Actavis |
| *Tentatively approved products. Source: U.S. Food & Drug Administration Web site. http://www.fda.gov/Drugs/DrugSafet/InformationbyDrugClass/ucm251735.htm. |
As outlined in this REMS, manufacturers must provide for the training of prescribers of opioid medications—training that covers proper patient selection, patient counseling in specific product use and risk, and assessment for addiction and tolerance. Manufacturers must also develop factual, nonpromotional patient information and medication guides that will be FDA regulated and approved. Finally, they will be asked to adhere to a timetable to assess whether REMS is meetings its goals.4,5
In May 2011, the FDA met with manufacturers to expand on how to coordinate and implement the REMS requirements.
Hope for a “new normal”
Will REMS for other large medication classes eventually reach beyond opioid analgesics, perhaps warranting practitioners to view REMS as being a good thing as opposed to a nuisance? Your decision to participate in REMS or pass and alter your care approach will need to be made soon. What will you do?
For you as an opioid prescriber, education is the focus, and you will soon be presented with voluntary prescriber education programs. The “hope” is that you will volunteer to take the opioid education program, fill out an electronic or fax form, and send it in to an administrator who will track all those who participate. Since “hope” will unlikely drive large-scale participation, when hope finally runs out the education will become mandatory. This will occur in a year or 2, and will likely become a Drug Enforcement Administration requirement for you to procure CII scheduling.
Unfortunately, there is no guarantee that deaths and overdoses will stop with the opioid REMS. The only guarantee is you will not be able to prescribe these medications at some point if you do not participate in the REMS.
So act now. To be notified when the opioid REMS training becomes available go to www.opioidREMS.com and register. It’s vital that you do ... and relatively painless.
REFERENCES
1. U.S. Department of Health and Human Services, Agency for Healthcare Research and Quality, Food and Drug Administration. Summary of public workshop. Implementation of risk minimization action plans (RiskMAPs) to support quality use of pharmaceuticals: opportunities and challenges. June 25-26, 2007.
2. US. Food and Drug Administration. FDA acts to reduce harm from opioid drugs. Consumer Updates. Available at: http://www.fda.gov/ForConsumers/ConsumerUpdates/ucm251830.htm.
3. Governale L. Outpatient Prescription Opioid Utilization in the U.S., Years 2000 – 2009. Food and Drug Administration, Division of Epidemiology. July 22, 2010. Avilable at: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/AnestheticAndLifeSupportDrugsAdvisoryCommittee/UCM220950.pdf
4. Marchand H, moderator. Opioid drugs and risk evaluation and mitigation strategies (REMS) Podcast/transcript. April 20, 2011. Available at: http://www.fda.gov/Drugs/DrugSafety/InformationbyDrugClass/ucm252649.htm.
5. U.S. Food and Drug Administration. Opioid drugs and risk mitigation strategies (REMS) Available at: http://www.fda.gov/Drugs/DrugSafety/InformationbyDrugClass/ucm163647.htm.
6. Epidemic: responding to America’s prescription drug abuse crisis. Available at: http://www.whitehousedrugpolicy.gov/publications/pdf/rx_abuse_plan.pdf.
Are you aware that a significant change is coming to the way you prescribe opioid pain relievers for your patients? After 3 years of debate among the Food and Drug Administration (FDA), drug industry stakeholders, members of the pain and addiction communities, patient advocacy groups, and the public, the first large-scale, class-wide REMS is here. REMS is the acronym for Risk Evaluation and Mitigation Strategies. There is a good chance you are prescribing one or more of the affected medications, and adherence to the REMS requirements will be essential if you wish to continue prescribing them.
Before getting into the fine points of the opioid REMS, a little background about how it came into being is in order. On March 25, 2008, the Food and Drug Administration Amendments Act went into effect, granting the FDA authority to require a REMS for any product or product class it deemed to be a public health, safety, or welfare threat. Basically, REMS is an FDA-imposed “safety” program. The first medication to now have a single or class REMS is the class of extended-release (ER) and long-acting (LA) opioid analgesics.
Why opioid analgesics? In 2007, attempts to mitigate targeted risks associated with 30 drugs using RISKMaps were cited as inadequate by the FDA. RISKMaps are safety programs designed to minimize significant risks of certain medicines through FDA-approved labeling, reporting of adverse events, prescriber and patient education about risks, reminders, and performance-linked access systems that tie access to medications with documentation and laboratory testing.1 Passage of the FDA Amendments Act allowed the FDA to use its REMS authority to “improve” existing risk plans.
Forces for change
The FDA cites many good reasons for this change, primarily to ensure that the benefits of prescribing opioid analgesics outweigh the risks, and that patients in pain who need these drugs have access to them. Driving factors behind this move centered on the highly visible consequences associated with what FDA experts describe as misuse, abuse, and improper prescribing of 12 ER/LA opioid analgesics. According to FDA estimates, in 2007 more than 33 million Americans age 12 and older misused ER/LA opioids. Of the almost 28,000 Americans who died from unintended consequences of drug use, nearly 12,000 were associated with prescription analgesics.2
In my opinion, voluntary continuing medical education (CME) and professional organization guidelines added to the problem by failing to decrease overdoses and unintended deaths. This may come as no surprise, as such deaths often stem from diversion, and diverters typically are not subject to a CME requirement.
The ER/LA segment of the class was targeted for a variety of reasons. First, higher doses of ER/LA opiates packed into single units are believed to pose a greater threat than the millions of short-acting, immediate-release (IR) opioid analgesics units abused annually.3 Another reason for the move focused on the burden to the health system caused by more than 24 similar individual REMS existing in this class. That alone created a virtual paper, regulatory, and health system encumbrance that is expected to be alleviated by a class-wide REMS.
Increasing numbers of prescriptions were an additional consideration. The number of outpatient retail prescriptions dispensed for ER/LA and IR opiates rose dramatically between 2000 and 2009, from 9.3 million to 22.9 million ER/LA opioids and from 164.8 million to 234 million IR opioids [Figure 1].3 Who is prescribing them? You are. In 2009, primary care physicians were the top prescribers of ER/LA (43.8%) and IR (42.1%) opioid analgesics [Figure 2].3 Who are you prescribing them for? Not the elderly age group you might expect. The largest number of prescriptions were written for men and women between ages 50and 59 [Figure 3].3
| FIGURE 1: Total number of prescriptions dispensed for ER/LA and IR opioids from US outpatient retail pharmacies, 2000-2009. |
| ER, extended release; IR, immediate release; LA, long acting; TRx, total prescriptions. Source: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterial/Drug/AnestheticAndLifeSupportDrugsAdvisory Committee/UCM220950.pdf. |
| FIGURE 2: Total number of prescriptions dispensed in the United States by top 10 prescribing specialties for IR and ER/LA opioids, 2009 |
| ANES, anesthesiologists; DO, doctor of osteopathy; EM, emergency medicine; ER, extended release; FM, family medicine; GP, general practitioner; HEM, hematologists; IM, internal medicine; IR, immediate release; LA, long acting; NP, nurse practitioners; ORTH SURG, orthopedic surgeons; NEURO, neurologists; PA, physician assistants; PM&R, physical medicine and rehabilitation; TRx, total prescriptions. Source: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drug/AnestheticAndLifeSupportDrugsAdvisoryCommittee/UCM220950.pdf. |
| FIGURE 3: Total number of unique patients, stratified by age and sex, receiving a dispensed prescription for an ER/LA opioid product from US outpatient retail pharmacies, 2009 |
| ER, extended release; LA, long acting. Source: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/AnestheticAndLifeSupportDrugsAdvisoryCommittee/UCM220950.pdf. |
And what are you prescribing them for? Data from a 2009 survey of the prescribing habits of 3200 office-based physicians in 30 specialties showed that most prescriptions written for ER/LA and IR opioids are associated with diagnoses related to pain in the musculoskeletal system and connective tissue (56% [ER/LA] and 30% [IR]). For ER/LA
prescriptions the second most common diagnoses were headaches and nerve pain (14%), while for IR prescriptions they were fractures, sprains, and contusions (23%) [Figure 4].3
| FIGURE 4: Diagnoses associated with use (by grouped ICD-9 codes) for IR and ER/LA opioids as reported by office-based physicians in the United States, 2009 |
| ER, extended release; IR, immediate release; LA, long acting. Source: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/AnestheticAndLifeSupportDrugs AdvisoryCommittee/UCM220950.pdf. |
According to Janet Woodcock, MD, Director of the FDA’s Center for Drug Evaluation and Research, some physicians may not be clear about who should receive these drugs or how to manage patients in pain. As a result, some physicians may be reluctant to prescribe opioid analgesics, leaving patients without adequate pain relief. At the same time, other physicians overprescribe them, putting patients—and anyone with access to the family medicine cabinet—at risk.4
A REMS by any other name
And so REMS was conceived. On February 6, 2009, manufacturers of certain opioid drug products received a letter from the FDA informing them that their drugs would be required to have a risk management program, and inviting them to meet to discuss the design and development of such a REMS.5
Two years later, on April 19, 2011, an alarm in the form of an action plan was released by the Obama administration through the Office of National Drug Control Policy. The plan,
Epidemic: Responding to America’s Prescription Drug Abuse Crisis, outlined a set of measures to remedy the problem through education, monitoring, proper disposal of prescription drugs, and enforcement.6
REMS for opioids was the FDA’s response in support of the President’s plan. On the same day in April, 32 manufacturers of ER/LA opioids received a letter from the FDA informing them that they must meet new safety requirements concerning these medications under a single shared, standardized system [Table].
| TABLE: Long-acting and extended-release opioids requiring an opioid REMS |
| Brand Name Products |
| Trade Name | Generic Name | Sponsor | |
| 1 | Duragesic | Fentanyl transdermal system | Ortho-McNeil-Janssen |
| 2 | Dolophine | Methadone HCI tablets | Roxanne Laboratories |
| 3 | Avinza | Morphine sulfate extended-release capsules | King Pharmaceuticals/Pfizer |
| 4 | Kadian capsules | Morphine sulfate extended-release capsules | Actavis |
| 5 | MS Contin | Morphine sulfate controlled-release tablets | Purdue Pharma |
| 6 | Oramorph | Morphine sulfate sustained-release tablets | Xanodyne Pharmaceuticals |
| 7 | OxyContin | Oxycodone HCI controlled-release tablets | Purdue Pharma |
| 8 | Opana ER | Oxymorphone HCI extended-release tablets | Endo Pharmaceuticals |
| 9 | Exalgo | Hydromorphone HCI extendedrelease tablets | Mallinckrodt Inc/Covidien |
| 10 | Butrans | Buprenorphine transdermal system | Purdue Pharma |
| Generic Products |
| Drug Name | Generic Name | Sponsor | |
| 1 | Fentanyl | Fentanyl extended-release transdermal system | Actavis |
| 2 | Fentanyl | Fentanyl extended-release transdermal system | Lavipharm Labs |
| 3 | Fentanyl | Fentanyl extended-release transdermal system | Mallinckrodt Inc/Covidien |
| 4 | Fentanyl | Fentanyl extended-release transdermal system | Mylan Technologies |
| 5 | Fentanyl | Fentanyl extended-release transdermal system | Noven Pharmaceuticals |
| 6 | Fentanyl | Fentanyl extended-release transdermal system | Teva Pharmaceutical Industries |
| 7 | Fentanyl | Fentanyl extended-release transdermal system | Watson Pharmaceuticals |
| 8 | Methadone hydrochloride | Methadone HCl oral solution | The Pharmanetwork |
| 9 | Methadone hydrochloride | Methadone HCl oral solution | Mallinckrodt Inc/Covidien |
| 10 | Methadone hydrochloride | Methadone HCl oral solution | Sandoz |
| 11 | Methadone hydrochloride | Methadone HCl oral solution | Roxane Laboratories |
| 12 | Methadone hydrochloride | Methadone HCl oral solution | VistaPharm |
| 13 | Morphine sulfate | Morphine sulfate extendedrelease tablets | Endo Pharmaceuticals |
| 14 | Morphine sulfate | Morphine sulfate extendedrelease tablets | KV Pharmaceuticals |
| 15 | Morphine sulfate | Morphine sulfate extendedrelease tablets | Mallinckrodt Inc/Covidien |
| 16 | Morphine sulfate | Morphine sulfate extendedrelease tablets | Watson Pharmaceuticals |
| 17 | Morphine sulfate | Morphine sulfate extendedrelease tablets | Rhodes Pharmaceuticals |
| 18 | Oxycodone hydrochloride | *Oxycodone HCl extendedrelease tablets | Mallinckrodt Inc/Covidien |
| 19 | Oxycodone hydrochloride | *Oxycodone HCl extendedrelease tablets | Impax Laboratories |
| 20 | Oxycodone hydrochloride | *Oxycodone HCl extendedrelease tablets | Teva Pharmaceutical Industries |
| 21 | Oxycodone hydrochloride | *Oxycodone HCl extendedrelease tablets | Endo Pharmaceuticals |
| 22 | Oxycodone hydrochloride | Oxymorphone HCl extendedrelease tablets | Impax Laboratories |
| 23 | Oxycodone hydrochloride | Oxymorphone HCl extendedrelease tablets | Actavis |
| *Tentatively approved products. Source: U.S. Food & Drug Administration Web site. http://www.fda.gov/Drugs/DrugSafet/InformationbyDrugClass/ucm251735.htm. |
As outlined in this REMS, manufacturers must provide for the training of prescribers of opioid medications—training that covers proper patient selection, patient counseling in specific product use and risk, and assessment for addiction and tolerance. Manufacturers must also develop factual, nonpromotional patient information and medication guides that will be FDA regulated and approved. Finally, they will be asked to adhere to a timetable to assess whether REMS is meetings its goals.4,5
In May 2011, the FDA met with manufacturers to expand on how to coordinate and implement the REMS requirements.
Hope for a “new normal”
Will REMS for other large medication classes eventually reach beyond opioid analgesics, perhaps warranting practitioners to view REMS as being a good thing as opposed to a nuisance? Your decision to participate in REMS or pass and alter your care approach will need to be made soon. What will you do?
For you as an opioid prescriber, education is the focus, and you will soon be presented with voluntary prescriber education programs. The “hope” is that you will volunteer to take the opioid education program, fill out an electronic or fax form, and send it in to an administrator who will track all those who participate. Since “hope” will unlikely drive large-scale participation, when hope finally runs out the education will become mandatory. This will occur in a year or 2, and will likely become a Drug Enforcement Administration requirement for you to procure CII scheduling.
Unfortunately, there is no guarantee that deaths and overdoses will stop with the opioid REMS. The only guarantee is you will not be able to prescribe these medications at some point if you do not participate in the REMS.
So act now. To be notified when the opioid REMS training becomes available go to www.opioidREMS.com and register. It’s vital that you do ... and relatively painless.
REFERENCES
1. U.S. Department of Health and Human Services, Agency for Healthcare Research and Quality, Food and Drug Administration. Summary of public workshop. Implementation of risk minimization action plans (RiskMAPs) to support quality use of pharmaceuticals: opportunities and challenges. June 25-26, 2007.
2. US. Food and Drug Administration. FDA acts to reduce harm from opioid drugs. Consumer Updates. Available at: http://www.fda.gov/ForConsumers/ConsumerUpdates/ucm251830.htm.
3. Governale L. Outpatient Prescription Opioid Utilization in the U.S., Years 2000 – 2009. Food and Drug Administration, Division of Epidemiology. July 22, 2010. Avilable at: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/AnestheticAndLifeSupportDrugsAdvisoryCommittee/UCM220950.pdf
4. Marchand H, moderator. Opioid drugs and risk evaluation and mitigation strategies (REMS) Podcast/transcript. April 20, 2011. Available at: http://www.fda.gov/Drugs/DrugSafety/InformationbyDrugClass/ucm252649.htm.
5. U.S. Food and Drug Administration. Opioid drugs and risk mitigation strategies (REMS) Available at: http://www.fda.gov/Drugs/DrugSafety/InformationbyDrugClass/ucm163647.htm.
6. Epidemic: responding to America’s prescription drug abuse crisis. Available at: http://www.whitehousedrugpolicy.gov/publications/pdf/rx_abuse_plan.pdf.
Are you aware that a significant change is coming to the way you prescribe opioid pain relievers for your patients? After 3 years of debate among the Food and Drug Administration (FDA), drug industry stakeholders, members of the pain and addiction communities, patient advocacy groups, and the public, the first large-scale, class-wide REMS is here. REMS is the acronym for Risk Evaluation and Mitigation Strategies. There is a good chance you are prescribing one or more of the affected medications, and adherence to the REMS requirements will be essential if you wish to continue prescribing them.
Before getting into the fine points of the opioid REMS, a little background about how it came into being is in order. On March 25, 2008, the Food and Drug Administration Amendments Act went into effect, granting the FDA authority to require a REMS for any product or product class it deemed to be a public health, safety, or welfare threat. Basically, REMS is an FDA-imposed “safety” program. The first medication to now have a single or class REMS is the class of extended-release (ER) and long-acting (LA) opioid analgesics.
Why opioid analgesics? In 2007, attempts to mitigate targeted risks associated with 30 drugs using RISKMaps were cited as inadequate by the FDA. RISKMaps are safety programs designed to minimize significant risks of certain medicines through FDA-approved labeling, reporting of adverse events, prescriber and patient education about risks, reminders, and performance-linked access systems that tie access to medications with documentation and laboratory testing.1 Passage of the FDA Amendments Act allowed the FDA to use its REMS authority to “improve” existing risk plans.
Forces for change
The FDA cites many good reasons for this change, primarily to ensure that the benefits of prescribing opioid analgesics outweigh the risks, and that patients in pain who need these drugs have access to them. Driving factors behind this move centered on the highly visible consequences associated with what FDA experts describe as misuse, abuse, and improper prescribing of 12 ER/LA opioid analgesics. According to FDA estimates, in 2007 more than 33 million Americans age 12 and older misused ER/LA opioids. Of the almost 28,000 Americans who died from unintended consequences of drug use, nearly 12,000 were associated with prescription analgesics.2
In my opinion, voluntary continuing medical education (CME) and professional organization guidelines added to the problem by failing to decrease overdoses and unintended deaths. This may come as no surprise, as such deaths often stem from diversion, and diverters typically are not subject to a CME requirement.
The ER/LA segment of the class was targeted for a variety of reasons. First, higher doses of ER/LA opiates packed into single units are believed to pose a greater threat than the millions of short-acting, immediate-release (IR) opioid analgesics units abused annually.3 Another reason for the move focused on the burden to the health system caused by more than 24 similar individual REMS existing in this class. That alone created a virtual paper, regulatory, and health system encumbrance that is expected to be alleviated by a class-wide REMS.
Increasing numbers of prescriptions were an additional consideration. The number of outpatient retail prescriptions dispensed for ER/LA and IR opiates rose dramatically between 2000 and 2009, from 9.3 million to 22.9 million ER/LA opioids and from 164.8 million to 234 million IR opioids [Figure 1].3 Who is prescribing them? You are. In 2009, primary care physicians were the top prescribers of ER/LA (43.8%) and IR (42.1%) opioid analgesics [Figure 2].3 Who are you prescribing them for? Not the elderly age group you might expect. The largest number of prescriptions were written for men and women between ages 50and 59 [Figure 3].3
| FIGURE 1: Total number of prescriptions dispensed for ER/LA and IR opioids from US outpatient retail pharmacies, 2000-2009. |
| ER, extended release; IR, immediate release; LA, long acting; TRx, total prescriptions. Source: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterial/Drug/AnestheticAndLifeSupportDrugsAdvisory Committee/UCM220950.pdf. |
| FIGURE 2: Total number of prescriptions dispensed in the United States by top 10 prescribing specialties for IR and ER/LA opioids, 2009 |
| ANES, anesthesiologists; DO, doctor of osteopathy; EM, emergency medicine; ER, extended release; FM, family medicine; GP, general practitioner; HEM, hematologists; IM, internal medicine; IR, immediate release; LA, long acting; NP, nurse practitioners; ORTH SURG, orthopedic surgeons; NEURO, neurologists; PA, physician assistants; PM&R, physical medicine and rehabilitation; TRx, total prescriptions. Source: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drug/AnestheticAndLifeSupportDrugsAdvisoryCommittee/UCM220950.pdf. |
| FIGURE 3: Total number of unique patients, stratified by age and sex, receiving a dispensed prescription for an ER/LA opioid product from US outpatient retail pharmacies, 2009 |
| ER, extended release; LA, long acting. Source: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/AnestheticAndLifeSupportDrugsAdvisoryCommittee/UCM220950.pdf. |
And what are you prescribing them for? Data from a 2009 survey of the prescribing habits of 3200 office-based physicians in 30 specialties showed that most prescriptions written for ER/LA and IR opioids are associated with diagnoses related to pain in the musculoskeletal system and connective tissue (56% [ER/LA] and 30% [IR]). For ER/LA
prescriptions the second most common diagnoses were headaches and nerve pain (14%), while for IR prescriptions they were fractures, sprains, and contusions (23%) [Figure 4].3
| FIGURE 4: Diagnoses associated with use (by grouped ICD-9 codes) for IR and ER/LA opioids as reported by office-based physicians in the United States, 2009 |
| ER, extended release; IR, immediate release; LA, long acting. Source: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/AnestheticAndLifeSupportDrugs AdvisoryCommittee/UCM220950.pdf. |
According to Janet Woodcock, MD, Director of the FDA’s Center for Drug Evaluation and Research, some physicians may not be clear about who should receive these drugs or how to manage patients in pain. As a result, some physicians may be reluctant to prescribe opioid analgesics, leaving patients without adequate pain relief. At the same time, other physicians overprescribe them, putting patients—and anyone with access to the family medicine cabinet—at risk.4
A REMS by any other name
And so REMS was conceived. On February 6, 2009, manufacturers of certain opioid drug products received a letter from the FDA informing them that their drugs would be required to have a risk management program, and inviting them to meet to discuss the design and development of such a REMS.5
Two years later, on April 19, 2011, an alarm in the form of an action plan was released by the Obama administration through the Office of National Drug Control Policy. The plan,
Epidemic: Responding to America’s Prescription Drug Abuse Crisis, outlined a set of measures to remedy the problem through education, monitoring, proper disposal of prescription drugs, and enforcement.6
REMS for opioids was the FDA’s response in support of the President’s plan. On the same day in April, 32 manufacturers of ER/LA opioids received a letter from the FDA informing them that they must meet new safety requirements concerning these medications under a single shared, standardized system [Table].
| TABLE: Long-acting and extended-release opioids requiring an opioid REMS |
| Brand Name Products |
| Trade Name | Generic Name | Sponsor | |
| 1 | Duragesic | Fentanyl transdermal system | Ortho-McNeil-Janssen |
| 2 | Dolophine | Methadone HCI tablets | Roxanne Laboratories |
| 3 | Avinza | Morphine sulfate extended-release capsules | King Pharmaceuticals/Pfizer |
| 4 | Kadian capsules | Morphine sulfate extended-release capsules | Actavis |
| 5 | MS Contin | Morphine sulfate controlled-release tablets | Purdue Pharma |
| 6 | Oramorph | Morphine sulfate sustained-release tablets | Xanodyne Pharmaceuticals |
| 7 | OxyContin | Oxycodone HCI controlled-release tablets | Purdue Pharma |
| 8 | Opana ER | Oxymorphone HCI extended-release tablets | Endo Pharmaceuticals |
| 9 | Exalgo | Hydromorphone HCI extendedrelease tablets | Mallinckrodt Inc/Covidien |
| 10 | Butrans | Buprenorphine transdermal system | Purdue Pharma |
| Generic Products |
| Drug Name | Generic Name | Sponsor | |
| 1 | Fentanyl | Fentanyl extended-release transdermal system | Actavis |
| 2 | Fentanyl | Fentanyl extended-release transdermal system | Lavipharm Labs |
| 3 | Fentanyl | Fentanyl extended-release transdermal system | Mallinckrodt Inc/Covidien |
| 4 | Fentanyl | Fentanyl extended-release transdermal system | Mylan Technologies |
| 5 | Fentanyl | Fentanyl extended-release transdermal system | Noven Pharmaceuticals |
| 6 | Fentanyl | Fentanyl extended-release transdermal system | Teva Pharmaceutical Industries |
| 7 | Fentanyl | Fentanyl extended-release transdermal system | Watson Pharmaceuticals |
| 8 | Methadone hydrochloride | Methadone HCl oral solution | The Pharmanetwork |
| 9 | Methadone hydrochloride | Methadone HCl oral solution | Mallinckrodt Inc/Covidien |
| 10 | Methadone hydrochloride | Methadone HCl oral solution | Sandoz |
| 11 | Methadone hydrochloride | Methadone HCl oral solution | Roxane Laboratories |
| 12 | Methadone hydrochloride | Methadone HCl oral solution | VistaPharm |
| 13 | Morphine sulfate | Morphine sulfate extendedrelease tablets | Endo Pharmaceuticals |
| 14 | Morphine sulfate | Morphine sulfate extendedrelease tablets | KV Pharmaceuticals |
| 15 | Morphine sulfate | Morphine sulfate extendedrelease tablets | Mallinckrodt Inc/Covidien |
| 16 | Morphine sulfate | Morphine sulfate extendedrelease tablets | Watson Pharmaceuticals |
| 17 | Morphine sulfate | Morphine sulfate extendedrelease tablets | Rhodes Pharmaceuticals |
| 18 | Oxycodone hydrochloride | *Oxycodone HCl extendedrelease tablets | Mallinckrodt Inc/Covidien |
| 19 | Oxycodone hydrochloride | *Oxycodone HCl extendedrelease tablets | Impax Laboratories |
| 20 | Oxycodone hydrochloride | *Oxycodone HCl extendedrelease tablets | Teva Pharmaceutical Industries |
| 21 | Oxycodone hydrochloride | *Oxycodone HCl extendedrelease tablets | Endo Pharmaceuticals |
| 22 | Oxycodone hydrochloride | Oxymorphone HCl extendedrelease tablets | Impax Laboratories |
| 23 | Oxycodone hydrochloride | Oxymorphone HCl extendedrelease tablets | Actavis |
| *Tentatively approved products. Source: U.S. Food & Drug Administration Web site. http://www.fda.gov/Drugs/DrugSafet/InformationbyDrugClass/ucm251735.htm. |
As outlined in this REMS, manufacturers must provide for the training of prescribers of opioid medications—training that covers proper patient selection, patient counseling in specific product use and risk, and assessment for addiction and tolerance. Manufacturers must also develop factual, nonpromotional patient information and medication guides that will be FDA regulated and approved. Finally, they will be asked to adhere to a timetable to assess whether REMS is meetings its goals.4,5
In May 2011, the FDA met with manufacturers to expand on how to coordinate and implement the REMS requirements.
Hope for a “new normal”
Will REMS for other large medication classes eventually reach beyond opioid analgesics, perhaps warranting practitioners to view REMS as being a good thing as opposed to a nuisance? Your decision to participate in REMS or pass and alter your care approach will need to be made soon. What will you do?
For you as an opioid prescriber, education is the focus, and you will soon be presented with voluntary prescriber education programs. The “hope” is that you will volunteer to take the opioid education program, fill out an electronic or fax form, and send it in to an administrator who will track all those who participate. Since “hope” will unlikely drive large-scale participation, when hope finally runs out the education will become mandatory. This will occur in a year or 2, and will likely become a Drug Enforcement Administration requirement for you to procure CII scheduling.
Unfortunately, there is no guarantee that deaths and overdoses will stop with the opioid REMS. The only guarantee is you will not be able to prescribe these medications at some point if you do not participate in the REMS.
So act now. To be notified when the opioid REMS training becomes available go to www.opioidREMS.com and register. It’s vital that you do ... and relatively painless.
REFERENCES
1. U.S. Department of Health and Human Services, Agency for Healthcare Research and Quality, Food and Drug Administration. Summary of public workshop. Implementation of risk minimization action plans (RiskMAPs) to support quality use of pharmaceuticals: opportunities and challenges. June 25-26, 2007.
2. US. Food and Drug Administration. FDA acts to reduce harm from opioid drugs. Consumer Updates. Available at: http://www.fda.gov/ForConsumers/ConsumerUpdates/ucm251830.htm.
3. Governale L. Outpatient Prescription Opioid Utilization in the U.S., Years 2000 – 2009. Food and Drug Administration, Division of Epidemiology. July 22, 2010. Avilable at: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/AnestheticAndLifeSupportDrugsAdvisoryCommittee/UCM220950.pdf
4. Marchand H, moderator. Opioid drugs and risk evaluation and mitigation strategies (REMS) Podcast/transcript. April 20, 2011. Available at: http://www.fda.gov/Drugs/DrugSafety/InformationbyDrugClass/ucm252649.htm.
5. U.S. Food and Drug Administration. Opioid drugs and risk mitigation strategies (REMS) Available at: http://www.fda.gov/Drugs/DrugSafety/InformationbyDrugClass/ucm163647.htm.
6. Epidemic: responding to America’s prescription drug abuse crisis. Available at: http://www.whitehousedrugpolicy.gov/publications/pdf/rx_abuse_plan.pdf.
The Hand That Feeds You
A 66‐year‐old man presented to the Emergency Department (ED) with rash and malaise in early April. He was in his usual state of good health until the morning of presentation, when he awoke feeling lethargic. Over the course of the day, his hands and feet grew cold and numb, his nose became dark red, and he developed a diffuse, net‐like red rash over his legs, hands, buttocks, and trunk. He had multiple maroon bowel movements. His wife noted that he became incoherent and brought him to the ED.
This apparently previously healthy man presented with an acute episode of fatigue and altered mental status accompanied by a prominent cutaneous eruption. The differential diagnosis will ultimately be guided by the morphology of the rash. At this stage, infectious diseases, drug or toxin exposure, and allergic processes including anaphylaxis must all be considered in this patient with rash and acute illness. The maroon bowel movements likely represent a gastrointestinal bleed that may be part of a unifying diagnosisa hematologic disorder, a vasculitis, or liver disease.
In the ED, the patient was reportedly febrile (exact temperature not recorded) with a blood pressure of 96/54 mmHg. He had pulse oximetry of 88% on room air and a diffuse purpuric rash. The patient was noted to have a leukocytosis, thrombocytopenia, coagulopathy, and an elevation of his creatinine and cardiac enzymes. He was given fluids, fresh frozen plasma, and broad‐spectrum antibiotics, and transferred directly to the intensive care unit of a tertiary medical center for further management.
Upon arrival to the intensive care unit, he complained of fatigue, progression of his nonpruritic, nonpainful rash, and worsening numbness and tingling of his extremities. He denied headache, nuchal rigidity, photophobia, vision or hearing changes, chest pain, cough, abdominal pain, myalgias, or arthralgias. While being interviewed, he had dark brown emesis and a bloody bowel movement.
The patient's past medical history included bacterial pericarditis as a teenager and remote hepatitis of unclear etiology. He rarely saw a physician, took no medications, and had no known medication allergies.
The patient worked as president of a software company and lived with his wife. He had smoked 1 to 2 packs of cigarettes a day for the past 30 years. He endorsed 2 of 4 CAGE criteria (need to Cut down, Annoyed when asked about alcohol, feel Guilty about drinking, need for an Eye opener), and his wife and had never been tested for human immunodeficiency virus (HIV). Family history was unremarkable.
The patient's presentation is concerning for a life‐threatening disease process with a rapid course. In the setting of the laboratory abnormalities demonstrating multi‐organ dysfunction, aggressive volume resuscitation and prompt initiation of broad‐spectrum antibiotics are indicated. The history does not reveal an obvious source of infection or exposure to a new drug, toxin, or allergen. His apparent gastrointestinal bleed could be explained by complications of liver disease from chronic alcohol use. For example, he could have variceal bleeding or gastropathy from portal hypertension. Alternatively, he may have bleeding secondary to a coagulopathy from decreased synthetic function of clotting factors. Other possibilities include a perforated viscus (eg, peptic ulcer) leading to bleeding and peritonitis or mesenteric ischemia, though the absence of abdominal pain makes these unlikely.
At this point, the overall presentation is most concerning for infection, especially given his chronic alcohol use and the vague history of hepatitis. The acute onset and severity of the illness are consistent with an aggressive, suppurative bacterial infection. The most likely causative organisms include gram‐negative bacteria, especially Neisseria meningitidis (with or without meningitis), as well as Staphylococcus aureus, Streptococcus pyogenes, and Rickettsia rickettsii (Rocky Mountain spotted fever).
Several months prior to presentation, he had traveled to Mexico. Two months prior to presentation, he made a trip to North Carolina and Ohio to visit his brother, who subsequently died of pneumonia. One month prior to presentation, he had traveled to urban China for work.
Because the presentation is so acute and the patient's travel took place over 1 month ago, this is unlikely to be a travel‐associated illness. Furthermore, the course is too acute to be consistent with endemic diseases of Central America and the midwestern United States, such as tuberculosis, brucellosis, and histoplasmosis.
He had a temperature of 38.7C. His heart rate was 110 beats per minute. His blood pressure was 115/78 mmHg, respiratory rate was 24 breaths per minute, and oxygen saturation was 99% on 6 liters via nasal cannula. The patient was a well‐nourished, middle‐aged man who appeared uncomfortable. He was in mild respiratory distress, though able to speak in full sentences. He was alert, coherent, and oriented to self, place, date, and time.
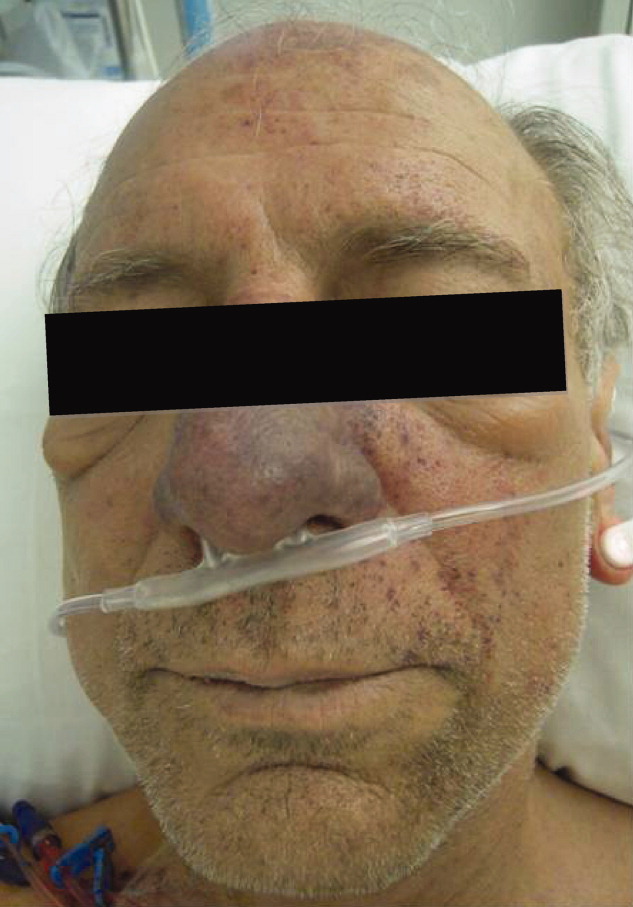
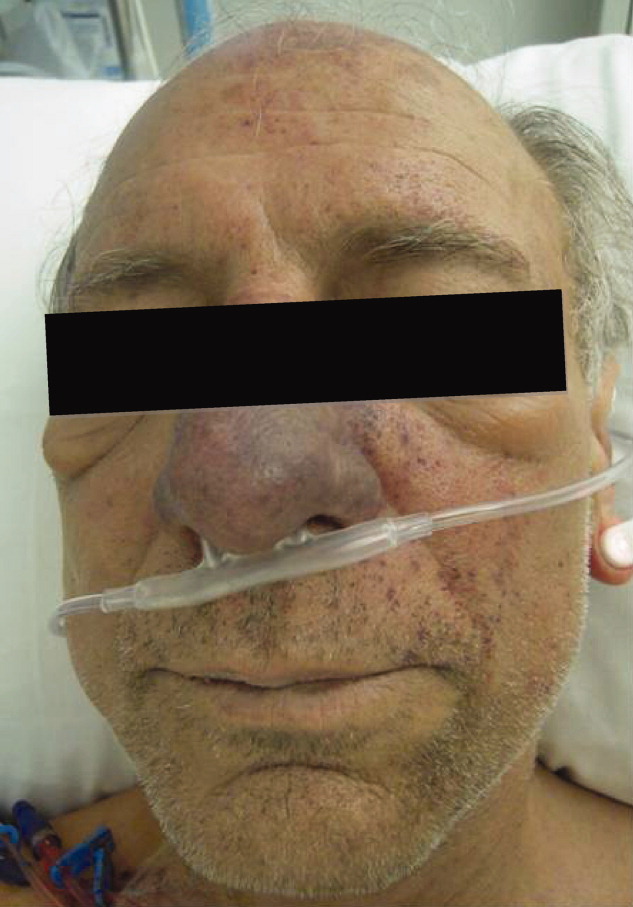
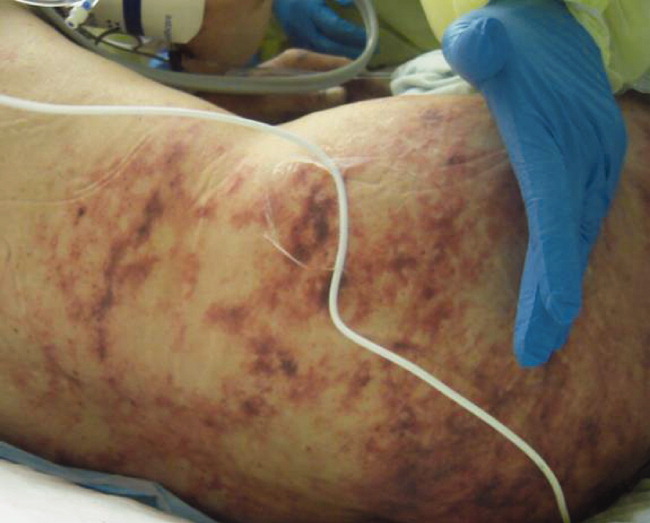
Skin examination revealed nonblanching purpuric papules coalescing into stellate plaques on his scalp, forehead, nose, cheeks, bilateral ears, hands, and feet (Figure 1). Acral surfaces, including hands and feet, were cyanotic without evidence of gangrene. He had nonblanching retiform purpuric plaques on his right flank, lower abdomen, low back, buttock, penis, scrotum, thighs, and legs (Figure 2). His right dorsal hand had 3 healing erosions of 3 to 10 mm in size without associated edema, erythema, or drainage.
Mucous membranes were dry without lesions. Cardiac examination demonstrated tachycardia without appreciable murmur. He was mildly tachypneic and his lungs were clear to auscultation without adventitious breath sounds. His abdominal examination was unremarkable. His hands and feet were cool with decreased sensation to touch. He had full range of motion and intact muscle strength, but mild bilateral dysmetria with finger‐nose‐finger testing. His radial and dorsalis pedis pulses were symmetric and brisk. Rectal exam revealed guaiac‐positive stool.
The patient's vital signs are compatible with the systemic inflammatory response syndrome. The presence of retiform purpura raises concerns for a systemic vasculitis with destruction of the vessel wall, or intravascular occlusion with thrombosis or emboli. Absence of murmur does not rule out endocarditis but makes it less likely. He has no risk factors for vasculitis, so the purpura, in conjunction with both bleeding and thrombosis, is much more suggestive of disseminated intravascular coagulation (DIC). This clotting disorder can result from a noninfectious trigger, such as acute pancreatitis or malignancy, but his presentation is more worrisome for a severe infection leading to DIC and complicated by purpura fulminans. He does not show signs of hepatic encephalopathy or cirrhosis, making decompensated liver disease a less likely inciting factor of his presentation.
Further exposure history was obtained: The patient often spent time outdoors near his rural home and used a weed‐whacker in his yard the day before admission. He owned 3 horses which he fed and often rode. He had 3 healthy dogs and had been bitten in attempts to break up fights among them, most recently 3 days prior to admission. He lived in mountain lion territory but had no direct exposure to lions. He had no known insect bites. He regularly drank well water, and consumed medium‐rare hamburgers 4 days prior to admission. One week prior to admission, a child with possible streptococcal pharyngitis visited his home.
With this history, the patient was treated with aggressive intravenous fluids and meningeal doses of ceftriaxone, vancomycin, and metronidazole.
In the summer, outdoor exposure to brush confers a risk of tick‐borne infections, including rickettsial diseases, ehrlichiosis, and spirochetal relapsing fever. However, this patient presented in the spring, and apart from rickettsial spotted fever, these illnesses tend to be indolent. It is conceivable, though unlikely, that the weed‐cutting device may have aerosolized fulminant zoonotic pathogens such as Francisella tularensis or plague that can be found in mountain lion territory.
Well water exposure suggests leptospirosis, which can present in a fulminant fashion with multi‐organ dysfunction, but is more often a subacute illness (developing over many days to a week or two). His ingestion of potentially undercooked meat raises the possibility of enterohemorrhagic infection complicated by the hemolytic uremic syndrome (HUS). However, while the purpuric rash and renal failure are compatible with HUS, the pace of illness and accompanying hypotension once again favor alternative infectious diagnoses.
The incubation period and presentation is concerning overwhelming bacterial infection related to the dog bite. Microbiological considerations include streptococcal species, Staphylococcus aureus, and gram‐negative organisms including Pasteurella species and Capnocytophaga canimorsus. The latter 2 organisms are of particular interest since they tend to cause severe sepsis in patients with alcoholism.
The antibiotic selection in this case is not straightforward. In general, empiric therapy for infections related to dog bites should include treatment for beta‐lactamaseproducing bacteria and anaerobes (eg, piperacillin/tazobactam). Yet, given the clinical presentation, severity of illness, and possible DIC, it is appropriate to be concerned about meningococcemia. Unfortunately, the tazobactam in piperacillin/tazobactam has poor central nervous system penetration so would be suboptimal treatment for meningitis. At this point, ceftriaxone, vancomycin, and metronidazole is a reasonable regimen.
Laboratory results were notable for blood urea nitrogen 50 mg/dL, creatinine 3.47 mg/dL, white cell count 21,800/L, with an absolute neutrophil count of 20,690/L, hematocrit 35.9%, platelet count 34,000/L, International Normalized Ratio 1.5, and partial thromboplastin time 44.0 seconds. His alanine aminotransferase was 356 U/L (1641 U/L), aspartate aminotransferase 959 U/L (1259 U/L), alkaline phosphatase 50 U/L (29111 U/L), and total bilirubin 1.7 mg/dL (0.31.3 mg/dL). Fibrinogen was 283 g/L (202430 g/L), lactate dehydrogenase was 1883 U/L (91185 IU/L), and uric acid was 10.5 mg/dL (3.77.7 mg/dL). His troponin I was 1.18 ng/mL (0.05 ng/ml), and his electrocardiogram showed sinus tachycardia but no evidence of myocardial ischemia. Chest x‐ray showed no infiltrate or evidence of volume overload. Lumbar puncture was deferred out of concern for ongoing disseminated intravascular coagulation.
Transthoracic echocardiogram revealed global hypokinesis and reduced left ventricular systolic function with ejection fraction of 35%. There was no evidence of vegetations or thrombus.
The patient's thrombocytopenia and prolonged coagulation parameters further support the presence of DIC. A peripheral blood smear should be examined. If microangiopathic changes are found, other diagnoses such as thrombotic thrombocytopenic purpura might be considered, although the rapid pace of illness and presence of hypotension still make sepsis with DIC more likely.
While septic shock often causes multi‐organ system failure secondary to hypoperfusion, the presumed rapid onset of hepatic and renal abnormalities suggests that microvascular thrombosis is playing a larger role in his organ system dysfunction. Microvascular thrombosis could also contribute to his myocardial injury, though globally depressed ejection fraction and elevated troponin might also be explained by infectious myocarditis. A third possibility is that his severe sepsis caused his myocardial dysfunction. Regardless of its etiology, the patient has no clinical evidence of congestive heart failure, so no specific therapy is required at this time. However, his cardiopulmonary exam should be monitored closely, and if he survives, he should have repeat echocardiography to monitor for resolution of the global hypokinesis.
Further evaluation revealed creatine kinase of 45,000 ng/ml (55380 ng/ml) and repeat troponin of >22 ng/ml. Protein C level was low at 30%. Testing for HIV was negative. Blood smear from time of transfer had few schistocytes. Urinalysis showed muddy brown casts but no dysmorphic red blood cells or red cell casts. The patient was placed on continuous veno‐venous hemofiltration (CVVH) for worsening renal failure and oliguria from presumed acute tubular necrosis in the setting of rhabdomyolysis and sepsis.
The patient has severe rhabdomyolysis that cannot fully be explained by his initial hypoperfusion and is more likely related to the overwhelming infection and microthrombosis. Rhabdomyolysis probably contributed to his acute tubular necrosis and renal failure.
Dermatology consultation identified the rash as likely purpura fulminans. They recommended a skin biopsy to rule out vasculitis. Three skin biopsies revealed micro‐vascular thrombosis; direct immunofluorescence test was negative for vasculitis; his skin tissue culture was negative for bacterial, mycobacterial, and fungal organisms.
Input from the dermatology service was key in identifying the rash. Purpura fulminans has a limited differential that includes severe infection from gram‐negative organisms and protein C and S deficiency. Since the biopsy results made vasculitis unlikely, the team was able to focus greater attention on potential pathogens such as Pasteurella species and C. canimorsus.
The biopsy also confirms the clinical suspicion that microvascular thrombosis is causing the patient's acute kidney injury, rhabdomyolysis, and myocardial ischemia. The presence of microvascular thrombosis prompts consideration of antithrombotic therapy such as heparin, but benefits of this therapy must be weighed against contraindications including bleeding and thrombocytopenia.
Ultimately out of concerns for recurrent gastrointestinal bleeding, the primary team decided not to treat with heparin or other antithrombotic therapy.
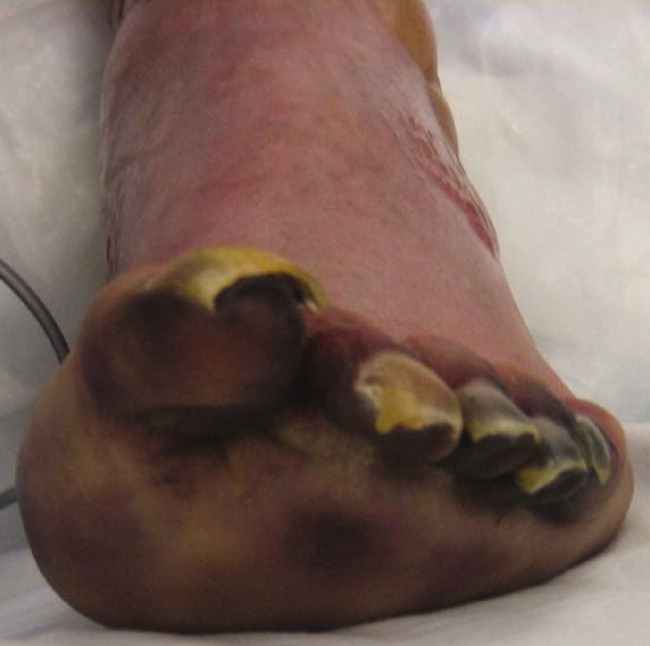
After several days of supportive care with antibiotics and renal replacement therapy, the patient showed gradual improvement of his retiform purpura, sensory neuropathy, laboratory data, and other markers of end‐organ dysfunction. Purpura of his fingertips, feet, and toes progressed to dry gangrene (Figure 3), which was monitored for potential need for amputation. He remained dependent on intermittent hemodialysis.
His initial antibiotic regimen was narrowed to ceftriaxone monotherapy. Five days after initial presentation, blood cultures drawn from the outside emergency department grew a gram‐negative rod in the anaerobic broth. Ten days later, this gram‐negative rod was identified as Capnocytophaga canimorsus. He was ultimately discharged to a skilled nursing facility.
Generally growth of an organism in broth only suggests either a very low inoculum or that the isolate is a contaminant. In this case, it was because the causative organism, C. canimorsus, is an obligate anaerobe and quite fastidious, so unlikely to grow easily. The identification of C. canimorsus from the initial blood culture is not surprising in this patient who presented with severe sepsis, DIC, and purpura fulminans after a recent dog bite. While the patient's chronic alcohol use may explain his fulminant infection from an atypical organism, one should always consider occult underlying malignancy as a predisposing factor, particularly in patients of this age group.
With the appropriate course of antibiotics, C. canimorsus infection should be completely cured. However, recovery of kidney and cardiac function could take weeks to months, and his dry gangrene may or may not resolve.
COMMENTARY
Capnocytophaga canimorsis sepsis is a rare and potentially deadly complication of dog bites that can present with rash, cellulitis, purpura fulminans, arthritis, meningitis, and endocarditis. The discussant considered a broad differential for the presentation of fever, rash, and acute illness. While the travel history was intriguing, the severity and pace of illness allowed him to focus attention on more recent infectious exposures. The ultimate key to the diagnosis was the patient's history of dog bite, an important but underrecognized source of serious infection in the United States.
According to the Centers for Disease Control and Prevention, there are approximately 4 million dog bites in the country each year. Of these, 300,000 bite victims seek care in the emergency department, resulting in 13,000 hospitalizations and 20 deaths annually.1 Infected dog bite wounds often grow polymicrobial flora. Pasteurella species are the most frequently found organisms in both dog and cat bite wounds. However, other aerobes such as streptococci, staphylococci, Moraxella, and Neisseria, as well as anaerobes including Fusobacterium and Bacteroides species, are also common.2
C. canimorsis is a facultative, fastidious gram‐negative bacillus found in the mouth flora of not only dogs but also cats and humans. It is often mistaken for other gram‐negative rod species.3 As with the patient described in this report, systemic infection from C. canimorsis can follow even superficial or well‐healed bite wounds.
Since this bacterium was first described in the literature 30 years ago, more than 100 cases of C. canimorsus infection have been described, with a mortality rate of nearly 30%.4 C. canimorsus occurs more frequently in males and in patients 50 to 70 years of age. Traditional risk factors include alcohol abuse, asplenia, immunosuppression, and corticosteroid treatment. However, in a case series of 56 isolates in California, only 10% of patients with Capnocytophaga sepsis were asplenic and none had alcohol abuse reported in their medical charts. In this series, median time from dog bite to the onset of symptoms was 3 days. Eighty‐five percent of patients presented with fever, while 32% had sepsis and 13% had DIC or septic shock.3
While C. canimorsus was once susceptible to a range of antibiotics, several reports from Canada and Europe document rising rates of beta‐lactamaseproducing strains that have caused clinically significant disease.5, 6 Individual susceptibility data take days to obtain, so it is important to start with empiric therapy. In general, empiric therapy for all serious dog bites should cover beta‐lactamaseproducing bacteria and anaerobes, for example, with amoxicillin/clavulanate, ampicillin/sulbactam, or piperacillin/tazobactam. If the patient is allergic to penicillin, clindamycin plus a fluoroquinolone can be used instead.
There are previous reports of purpura fulminans and symmetric peripheral gangrene following Capnocytophaga infection from dog bites.7, 8 Purpura fulminans is defined as rapidly progressive skin necrosis due to dermal vascular thrombosis, often in the setting of DIC. Early involvement occurs at acral sites, such as the nose, ears, fingers, and toes. Purpuric lesions often progress to skin necrosis or dry gangrene within 24 to 48 hours. In a review of 12 patients with purpura fulminans, only 9 survived. Eight of the 9 survivors required amputation of at least 1 limb, and 4 of them required 4‐limb amputation.7
In this patient who presented with fever and rash, the discussant recognized early on an underlying infectious etiology. Although the patient's exposure history led the discussant to consider a host of possibilities, the recognition of purpura fulminans allowed him to narrow his differential. Ultimately, the dog's bite clinched the diagnosis.
KEY TEACHING POINTS
-
Sepsis caused by C. canimorsus is often characterized by rash, cellulitis, arthritis, meningitis, and endocarditis. In some instances, infection can progress to purpura fulminans.
-
In cases where fastidious organisms are suspected as an infectious source, microbiology labs should be notified of suspected organisms so they can extend incubation periods or use special media to maximize culture yield and the likelihood of accurate identification.
-
Empiric therapy for serious dog bites should cover beta‐lactamaseproducing bacteria and anaerobes. Consider using amoxicillin/clavulanate, ampicillin/sulbactam, or piperacillin/tazobactam.
The approach to clinical conundrums by an expert clinician is revealed through the presentation of an actual patient's case in an approach typical of a morning report. Similarly to patient care, sequential pieces of information are provided to the clinician, who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant.
This icon represents the patient's case. Each paragraph that follows represents the discussant's thoughts.
Acknowledgements
The authors thank Snigdha Vallabhaneni, MD, from the UCSF Division of Infectious Diseases, for her contributions to the discussion on C. canimorsus. They also thank Kanade Shinkai, MD, PhD, from the UCSF Department of Dermatology, and Heather Nye, MD, PhD, from the UCSF Division of Hospital Medicine, for their review of the manuscript.
Disclosure: Nothing to report.
- ,,.Incidence of dog bite injuries treated in emergency departments.JAMA.1998;279:51–53.
- ,,,,.Bacteriologic analysis of infected dog and cat bites. Emergency Medicine Animal Bite Infection Study Group.N Engl J Med.1999;340:85–92.
- ,,,.Diagnosing Capnocytophaga canimorsus infections.Emerg Infect Dis.2006;12:340–342.
- ,,.Capnocytophaga canimorsus infections in human: review of the literature and cases report.Eur J Epidemiol.1996;12:521–533.
- ,,,,.Antimicrobial susceptibilities and beta‐lactamase characterization of Capnocytophaga species.Antimicrob Agents Chemother.1992;36:2197–2200.
- ,,,,,.Bacteremia due to Capnocytophaga species in patients with neutropenia: high frequency of beta‐lactamase‐producing strains.Clin Infect Dis.1999;28:1172–1174.
- ,,.Presentation and outcome of purpura fulminans associated with peripheral gangrene in 12 patients at Mayo Clinic.J Am Acad Dermatol.2007;57:944–956.
- ,,,.Capnocytophaga canimorsus sepsis with purpura fulminans and symmetrical gangrene following a dog bite in a shelter employee.Am J Med Sci.2004:327:369–372.
A 66‐year‐old man presented to the Emergency Department (ED) with rash and malaise in early April. He was in his usual state of good health until the morning of presentation, when he awoke feeling lethargic. Over the course of the day, his hands and feet grew cold and numb, his nose became dark red, and he developed a diffuse, net‐like red rash over his legs, hands, buttocks, and trunk. He had multiple maroon bowel movements. His wife noted that he became incoherent and brought him to the ED.
This apparently previously healthy man presented with an acute episode of fatigue and altered mental status accompanied by a prominent cutaneous eruption. The differential diagnosis will ultimately be guided by the morphology of the rash. At this stage, infectious diseases, drug or toxin exposure, and allergic processes including anaphylaxis must all be considered in this patient with rash and acute illness. The maroon bowel movements likely represent a gastrointestinal bleed that may be part of a unifying diagnosisa hematologic disorder, a vasculitis, or liver disease.
In the ED, the patient was reportedly febrile (exact temperature not recorded) with a blood pressure of 96/54 mmHg. He had pulse oximetry of 88% on room air and a diffuse purpuric rash. The patient was noted to have a leukocytosis, thrombocytopenia, coagulopathy, and an elevation of his creatinine and cardiac enzymes. He was given fluids, fresh frozen plasma, and broad‐spectrum antibiotics, and transferred directly to the intensive care unit of a tertiary medical center for further management.
Upon arrival to the intensive care unit, he complained of fatigue, progression of his nonpruritic, nonpainful rash, and worsening numbness and tingling of his extremities. He denied headache, nuchal rigidity, photophobia, vision or hearing changes, chest pain, cough, abdominal pain, myalgias, or arthralgias. While being interviewed, he had dark brown emesis and a bloody bowel movement.
The patient's past medical history included bacterial pericarditis as a teenager and remote hepatitis of unclear etiology. He rarely saw a physician, took no medications, and had no known medication allergies.
The patient worked as president of a software company and lived with his wife. He had smoked 1 to 2 packs of cigarettes a day for the past 30 years. He endorsed 2 of 4 CAGE criteria (need to Cut down, Annoyed when asked about alcohol, feel Guilty about drinking, need for an Eye opener), and his wife and had never been tested for human immunodeficiency virus (HIV). Family history was unremarkable.
The patient's presentation is concerning for a life‐threatening disease process with a rapid course. In the setting of the laboratory abnormalities demonstrating multi‐organ dysfunction, aggressive volume resuscitation and prompt initiation of broad‐spectrum antibiotics are indicated. The history does not reveal an obvious source of infection or exposure to a new drug, toxin, or allergen. His apparent gastrointestinal bleed could be explained by complications of liver disease from chronic alcohol use. For example, he could have variceal bleeding or gastropathy from portal hypertension. Alternatively, he may have bleeding secondary to a coagulopathy from decreased synthetic function of clotting factors. Other possibilities include a perforated viscus (eg, peptic ulcer) leading to bleeding and peritonitis or mesenteric ischemia, though the absence of abdominal pain makes these unlikely.
At this point, the overall presentation is most concerning for infection, especially given his chronic alcohol use and the vague history of hepatitis. The acute onset and severity of the illness are consistent with an aggressive, suppurative bacterial infection. The most likely causative organisms include gram‐negative bacteria, especially Neisseria meningitidis (with or without meningitis), as well as Staphylococcus aureus, Streptococcus pyogenes, and Rickettsia rickettsii (Rocky Mountain spotted fever).
Several months prior to presentation, he had traveled to Mexico. Two months prior to presentation, he made a trip to North Carolina and Ohio to visit his brother, who subsequently died of pneumonia. One month prior to presentation, he had traveled to urban China for work.
Because the presentation is so acute and the patient's travel took place over 1 month ago, this is unlikely to be a travel‐associated illness. Furthermore, the course is too acute to be consistent with endemic diseases of Central America and the midwestern United States, such as tuberculosis, brucellosis, and histoplasmosis.
He had a temperature of 38.7C. His heart rate was 110 beats per minute. His blood pressure was 115/78 mmHg, respiratory rate was 24 breaths per minute, and oxygen saturation was 99% on 6 liters via nasal cannula. The patient was a well‐nourished, middle‐aged man who appeared uncomfortable. He was in mild respiratory distress, though able to speak in full sentences. He was alert, coherent, and oriented to self, place, date, and time.
Skin examination revealed nonblanching purpuric papules coalescing into stellate plaques on his scalp, forehead, nose, cheeks, bilateral ears, hands, and feet (Figure 1). Acral surfaces, including hands and feet, were cyanotic without evidence of gangrene. He had nonblanching retiform purpuric plaques on his right flank, lower abdomen, low back, buttock, penis, scrotum, thighs, and legs (Figure 2). His right dorsal hand had 3 healing erosions of 3 to 10 mm in size without associated edema, erythema, or drainage.


Mucous membranes were dry without lesions. Cardiac examination demonstrated tachycardia without appreciable murmur. He was mildly tachypneic and his lungs were clear to auscultation without adventitious breath sounds. His abdominal examination was unremarkable. His hands and feet were cool with decreased sensation to touch. He had full range of motion and intact muscle strength, but mild bilateral dysmetria with finger‐nose‐finger testing. His radial and dorsalis pedis pulses were symmetric and brisk. Rectal exam revealed guaiac‐positive stool.
The patient's vital signs are compatible with the systemic inflammatory response syndrome. The presence of retiform purpura raises concerns for a systemic vasculitis with destruction of the vessel wall, or intravascular occlusion with thrombosis or emboli. Absence of murmur does not rule out endocarditis but makes it less likely. He has no risk factors for vasculitis, so the purpura, in conjunction with both bleeding and thrombosis, is much more suggestive of disseminated intravascular coagulation (DIC). This clotting disorder can result from a noninfectious trigger, such as acute pancreatitis or malignancy, but his presentation is more worrisome for a severe infection leading to DIC and complicated by purpura fulminans. He does not show signs of hepatic encephalopathy or cirrhosis, making decompensated liver disease a less likely inciting factor of his presentation.
Further exposure history was obtained: The patient often spent time outdoors near his rural home and used a weed‐whacker in his yard the day before admission. He owned 3 horses which he fed and often rode. He had 3 healthy dogs and had been bitten in attempts to break up fights among them, most recently 3 days prior to admission. He lived in mountain lion territory but had no direct exposure to lions. He had no known insect bites. He regularly drank well water, and consumed medium‐rare hamburgers 4 days prior to admission. One week prior to admission, a child with possible streptococcal pharyngitis visited his home.
With this history, the patient was treated with aggressive intravenous fluids and meningeal doses of ceftriaxone, vancomycin, and metronidazole.
In the summer, outdoor exposure to brush confers a risk of tick‐borne infections, including rickettsial diseases, ehrlichiosis, and spirochetal relapsing fever. However, this patient presented in the spring, and apart from rickettsial spotted fever, these illnesses tend to be indolent. It is conceivable, though unlikely, that the weed‐cutting device may have aerosolized fulminant zoonotic pathogens such as Francisella tularensis or plague that can be found in mountain lion territory.
Well water exposure suggests leptospirosis, which can present in a fulminant fashion with multi‐organ dysfunction, but is more often a subacute illness (developing over many days to a week or two). His ingestion of potentially undercooked meat raises the possibility of enterohemorrhagic infection complicated by the hemolytic uremic syndrome (HUS). However, while the purpuric rash and renal failure are compatible with HUS, the pace of illness and accompanying hypotension once again favor alternative infectious diagnoses.
The incubation period and presentation is concerning overwhelming bacterial infection related to the dog bite. Microbiological considerations include streptococcal species, Staphylococcus aureus, and gram‐negative organisms including Pasteurella species and Capnocytophaga canimorsus. The latter 2 organisms are of particular interest since they tend to cause severe sepsis in patients with alcoholism.
The antibiotic selection in this case is not straightforward. In general, empiric therapy for infections related to dog bites should include treatment for beta‐lactamaseproducing bacteria and anaerobes (eg, piperacillin/tazobactam). Yet, given the clinical presentation, severity of illness, and possible DIC, it is appropriate to be concerned about meningococcemia. Unfortunately, the tazobactam in piperacillin/tazobactam has poor central nervous system penetration so would be suboptimal treatment for meningitis. At this point, ceftriaxone, vancomycin, and metronidazole is a reasonable regimen.
Laboratory results were notable for blood urea nitrogen 50 mg/dL, creatinine 3.47 mg/dL, white cell count 21,800/L, with an absolute neutrophil count of 20,690/L, hematocrit 35.9%, platelet count 34,000/L, International Normalized Ratio 1.5, and partial thromboplastin time 44.0 seconds. His alanine aminotransferase was 356 U/L (1641 U/L), aspartate aminotransferase 959 U/L (1259 U/L), alkaline phosphatase 50 U/L (29111 U/L), and total bilirubin 1.7 mg/dL (0.31.3 mg/dL). Fibrinogen was 283 g/L (202430 g/L), lactate dehydrogenase was 1883 U/L (91185 IU/L), and uric acid was 10.5 mg/dL (3.77.7 mg/dL). His troponin I was 1.18 ng/mL (0.05 ng/ml), and his electrocardiogram showed sinus tachycardia but no evidence of myocardial ischemia. Chest x‐ray showed no infiltrate or evidence of volume overload. Lumbar puncture was deferred out of concern for ongoing disseminated intravascular coagulation.
Transthoracic echocardiogram revealed global hypokinesis and reduced left ventricular systolic function with ejection fraction of 35%. There was no evidence of vegetations or thrombus.
The patient's thrombocytopenia and prolonged coagulation parameters further support the presence of DIC. A peripheral blood smear should be examined. If microangiopathic changes are found, other diagnoses such as thrombotic thrombocytopenic purpura might be considered, although the rapid pace of illness and presence of hypotension still make sepsis with DIC more likely.
While septic shock often causes multi‐organ system failure secondary to hypoperfusion, the presumed rapid onset of hepatic and renal abnormalities suggests that microvascular thrombosis is playing a larger role in his organ system dysfunction. Microvascular thrombosis could also contribute to his myocardial injury, though globally depressed ejection fraction and elevated troponin might also be explained by infectious myocarditis. A third possibility is that his severe sepsis caused his myocardial dysfunction. Regardless of its etiology, the patient has no clinical evidence of congestive heart failure, so no specific therapy is required at this time. However, his cardiopulmonary exam should be monitored closely, and if he survives, he should have repeat echocardiography to monitor for resolution of the global hypokinesis.
Further evaluation revealed creatine kinase of 45,000 ng/ml (55380 ng/ml) and repeat troponin of >22 ng/ml. Protein C level was low at 30%. Testing for HIV was negative. Blood smear from time of transfer had few schistocytes. Urinalysis showed muddy brown casts but no dysmorphic red blood cells or red cell casts. The patient was placed on continuous veno‐venous hemofiltration (CVVH) for worsening renal failure and oliguria from presumed acute tubular necrosis in the setting of rhabdomyolysis and sepsis.
The patient has severe rhabdomyolysis that cannot fully be explained by his initial hypoperfusion and is more likely related to the overwhelming infection and microthrombosis. Rhabdomyolysis probably contributed to his acute tubular necrosis and renal failure.
Dermatology consultation identified the rash as likely purpura fulminans. They recommended a skin biopsy to rule out vasculitis. Three skin biopsies revealed micro‐vascular thrombosis; direct immunofluorescence test was negative for vasculitis; his skin tissue culture was negative for bacterial, mycobacterial, and fungal organisms.
Input from the dermatology service was key in identifying the rash. Purpura fulminans has a limited differential that includes severe infection from gram‐negative organisms and protein C and S deficiency. Since the biopsy results made vasculitis unlikely, the team was able to focus greater attention on potential pathogens such as Pasteurella species and C. canimorsus.
The biopsy also confirms the clinical suspicion that microvascular thrombosis is causing the patient's acute kidney injury, rhabdomyolysis, and myocardial ischemia. The presence of microvascular thrombosis prompts consideration of antithrombotic therapy such as heparin, but benefits of this therapy must be weighed against contraindications including bleeding and thrombocytopenia.
Ultimately out of concerns for recurrent gastrointestinal bleeding, the primary team decided not to treat with heparin or other antithrombotic therapy.
After several days of supportive care with antibiotics and renal replacement therapy, the patient showed gradual improvement of his retiform purpura, sensory neuropathy, laboratory data, and other markers of end‐organ dysfunction. Purpura of his fingertips, feet, and toes progressed to dry gangrene (Figure 3), which was monitored for potential need for amputation. He remained dependent on intermittent hemodialysis.

His initial antibiotic regimen was narrowed to ceftriaxone monotherapy. Five days after initial presentation, blood cultures drawn from the outside emergency department grew a gram‐negative rod in the anaerobic broth. Ten days later, this gram‐negative rod was identified as Capnocytophaga canimorsus. He was ultimately discharged to a skilled nursing facility.
Generally growth of an organism in broth only suggests either a very low inoculum or that the isolate is a contaminant. In this case, it was because the causative organism, C. canimorsus, is an obligate anaerobe and quite fastidious, so unlikely to grow easily. The identification of C. canimorsus from the initial blood culture is not surprising in this patient who presented with severe sepsis, DIC, and purpura fulminans after a recent dog bite. While the patient's chronic alcohol use may explain his fulminant infection from an atypical organism, one should always consider occult underlying malignancy as a predisposing factor, particularly in patients of this age group.
With the appropriate course of antibiotics, C. canimorsus infection should be completely cured. However, recovery of kidney and cardiac function could take weeks to months, and his dry gangrene may or may not resolve.
COMMENTARY
Capnocytophaga canimorsis sepsis is a rare and potentially deadly complication of dog bites that can present with rash, cellulitis, purpura fulminans, arthritis, meningitis, and endocarditis. The discussant considered a broad differential for the presentation of fever, rash, and acute illness. While the travel history was intriguing, the severity and pace of illness allowed him to focus attention on more recent infectious exposures. The ultimate key to the diagnosis was the patient's history of dog bite, an important but underrecognized source of serious infection in the United States.
According to the Centers for Disease Control and Prevention, there are approximately 4 million dog bites in the country each year. Of these, 300,000 bite victims seek care in the emergency department, resulting in 13,000 hospitalizations and 20 deaths annually.1 Infected dog bite wounds often grow polymicrobial flora. Pasteurella species are the most frequently found organisms in both dog and cat bite wounds. However, other aerobes such as streptococci, staphylococci, Moraxella, and Neisseria, as well as anaerobes including Fusobacterium and Bacteroides species, are also common.2
C. canimorsis is a facultative, fastidious gram‐negative bacillus found in the mouth flora of not only dogs but also cats and humans. It is often mistaken for other gram‐negative rod species.3 As with the patient described in this report, systemic infection from C. canimorsis can follow even superficial or well‐healed bite wounds.
Since this bacterium was first described in the literature 30 years ago, more than 100 cases of C. canimorsus infection have been described, with a mortality rate of nearly 30%.4 C. canimorsus occurs more frequently in males and in patients 50 to 70 years of age. Traditional risk factors include alcohol abuse, asplenia, immunosuppression, and corticosteroid treatment. However, in a case series of 56 isolates in California, only 10% of patients with Capnocytophaga sepsis were asplenic and none had alcohol abuse reported in their medical charts. In this series, median time from dog bite to the onset of symptoms was 3 days. Eighty‐five percent of patients presented with fever, while 32% had sepsis and 13% had DIC or septic shock.3
While C. canimorsus was once susceptible to a range of antibiotics, several reports from Canada and Europe document rising rates of beta‐lactamaseproducing strains that have caused clinically significant disease.5, 6 Individual susceptibility data take days to obtain, so it is important to start with empiric therapy. In general, empiric therapy for all serious dog bites should cover beta‐lactamaseproducing bacteria and anaerobes, for example, with amoxicillin/clavulanate, ampicillin/sulbactam, or piperacillin/tazobactam. If the patient is allergic to penicillin, clindamycin plus a fluoroquinolone can be used instead.
There are previous reports of purpura fulminans and symmetric peripheral gangrene following Capnocytophaga infection from dog bites.7, 8 Purpura fulminans is defined as rapidly progressive skin necrosis due to dermal vascular thrombosis, often in the setting of DIC. Early involvement occurs at acral sites, such as the nose, ears, fingers, and toes. Purpuric lesions often progress to skin necrosis or dry gangrene within 24 to 48 hours. In a review of 12 patients with purpura fulminans, only 9 survived. Eight of the 9 survivors required amputation of at least 1 limb, and 4 of them required 4‐limb amputation.7
In this patient who presented with fever and rash, the discussant recognized early on an underlying infectious etiology. Although the patient's exposure history led the discussant to consider a host of possibilities, the recognition of purpura fulminans allowed him to narrow his differential. Ultimately, the dog's bite clinched the diagnosis.
KEY TEACHING POINTS
-
Sepsis caused by C. canimorsus is often characterized by rash, cellulitis, arthritis, meningitis, and endocarditis. In some instances, infection can progress to purpura fulminans.
-
In cases where fastidious organisms are suspected as an infectious source, microbiology labs should be notified of suspected organisms so they can extend incubation periods or use special media to maximize culture yield and the likelihood of accurate identification.
-
Empiric therapy for serious dog bites should cover beta‐lactamaseproducing bacteria and anaerobes. Consider using amoxicillin/clavulanate, ampicillin/sulbactam, or piperacillin/tazobactam.
The approach to clinical conundrums by an expert clinician is revealed through the presentation of an actual patient's case in an approach typical of a morning report. Similarly to patient care, sequential pieces of information are provided to the clinician, who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant.
This icon represents the patient's case. Each paragraph that follows represents the discussant's thoughts.
Acknowledgements
The authors thank Snigdha Vallabhaneni, MD, from the UCSF Division of Infectious Diseases, for her contributions to the discussion on C. canimorsus. They also thank Kanade Shinkai, MD, PhD, from the UCSF Department of Dermatology, and Heather Nye, MD, PhD, from the UCSF Division of Hospital Medicine, for their review of the manuscript.
Disclosure: Nothing to report.
A 66‐year‐old man presented to the Emergency Department (ED) with rash and malaise in early April. He was in his usual state of good health until the morning of presentation, when he awoke feeling lethargic. Over the course of the day, his hands and feet grew cold and numb, his nose became dark red, and he developed a diffuse, net‐like red rash over his legs, hands, buttocks, and trunk. He had multiple maroon bowel movements. His wife noted that he became incoherent and brought him to the ED.
This apparently previously healthy man presented with an acute episode of fatigue and altered mental status accompanied by a prominent cutaneous eruption. The differential diagnosis will ultimately be guided by the morphology of the rash. At this stage, infectious diseases, drug or toxin exposure, and allergic processes including anaphylaxis must all be considered in this patient with rash and acute illness. The maroon bowel movements likely represent a gastrointestinal bleed that may be part of a unifying diagnosisa hematologic disorder, a vasculitis, or liver disease.
In the ED, the patient was reportedly febrile (exact temperature not recorded) with a blood pressure of 96/54 mmHg. He had pulse oximetry of 88% on room air and a diffuse purpuric rash. The patient was noted to have a leukocytosis, thrombocytopenia, coagulopathy, and an elevation of his creatinine and cardiac enzymes. He was given fluids, fresh frozen plasma, and broad‐spectrum antibiotics, and transferred directly to the intensive care unit of a tertiary medical center for further management.
Upon arrival to the intensive care unit, he complained of fatigue, progression of his nonpruritic, nonpainful rash, and worsening numbness and tingling of his extremities. He denied headache, nuchal rigidity, photophobia, vision or hearing changes, chest pain, cough, abdominal pain, myalgias, or arthralgias. While being interviewed, he had dark brown emesis and a bloody bowel movement.
The patient's past medical history included bacterial pericarditis as a teenager and remote hepatitis of unclear etiology. He rarely saw a physician, took no medications, and had no known medication allergies.
The patient worked as president of a software company and lived with his wife. He had smoked 1 to 2 packs of cigarettes a day for the past 30 years. He endorsed 2 of 4 CAGE criteria (need to Cut down, Annoyed when asked about alcohol, feel Guilty about drinking, need for an Eye opener), and his wife and had never been tested for human immunodeficiency virus (HIV). Family history was unremarkable.
The patient's presentation is concerning for a life‐threatening disease process with a rapid course. In the setting of the laboratory abnormalities demonstrating multi‐organ dysfunction, aggressive volume resuscitation and prompt initiation of broad‐spectrum antibiotics are indicated. The history does not reveal an obvious source of infection or exposure to a new drug, toxin, or allergen. His apparent gastrointestinal bleed could be explained by complications of liver disease from chronic alcohol use. For example, he could have variceal bleeding or gastropathy from portal hypertension. Alternatively, he may have bleeding secondary to a coagulopathy from decreased synthetic function of clotting factors. Other possibilities include a perforated viscus (eg, peptic ulcer) leading to bleeding and peritonitis or mesenteric ischemia, though the absence of abdominal pain makes these unlikely.
At this point, the overall presentation is most concerning for infection, especially given his chronic alcohol use and the vague history of hepatitis. The acute onset and severity of the illness are consistent with an aggressive, suppurative bacterial infection. The most likely causative organisms include gram‐negative bacteria, especially Neisseria meningitidis (with or without meningitis), as well as Staphylococcus aureus, Streptococcus pyogenes, and Rickettsia rickettsii (Rocky Mountain spotted fever).
Several months prior to presentation, he had traveled to Mexico. Two months prior to presentation, he made a trip to North Carolina and Ohio to visit his brother, who subsequently died of pneumonia. One month prior to presentation, he had traveled to urban China for work.
Because the presentation is so acute and the patient's travel took place over 1 month ago, this is unlikely to be a travel‐associated illness. Furthermore, the course is too acute to be consistent with endemic diseases of Central America and the midwestern United States, such as tuberculosis, brucellosis, and histoplasmosis.
He had a temperature of 38.7C. His heart rate was 110 beats per minute. His blood pressure was 115/78 mmHg, respiratory rate was 24 breaths per minute, and oxygen saturation was 99% on 6 liters via nasal cannula. The patient was a well‐nourished, middle‐aged man who appeared uncomfortable. He was in mild respiratory distress, though able to speak in full sentences. He was alert, coherent, and oriented to self, place, date, and time.
Skin examination revealed nonblanching purpuric papules coalescing into stellate plaques on his scalp, forehead, nose, cheeks, bilateral ears, hands, and feet (Figure 1). Acral surfaces, including hands and feet, were cyanotic without evidence of gangrene. He had nonblanching retiform purpuric plaques on his right flank, lower abdomen, low back, buttock, penis, scrotum, thighs, and legs (Figure 2). His right dorsal hand had 3 healing erosions of 3 to 10 mm in size without associated edema, erythema, or drainage.


Mucous membranes were dry without lesions. Cardiac examination demonstrated tachycardia without appreciable murmur. He was mildly tachypneic and his lungs were clear to auscultation without adventitious breath sounds. His abdominal examination was unremarkable. His hands and feet were cool with decreased sensation to touch. He had full range of motion and intact muscle strength, but mild bilateral dysmetria with finger‐nose‐finger testing. His radial and dorsalis pedis pulses were symmetric and brisk. Rectal exam revealed guaiac‐positive stool.
The patient's vital signs are compatible with the systemic inflammatory response syndrome. The presence of retiform purpura raises concerns for a systemic vasculitis with destruction of the vessel wall, or intravascular occlusion with thrombosis or emboli. Absence of murmur does not rule out endocarditis but makes it less likely. He has no risk factors for vasculitis, so the purpura, in conjunction with both bleeding and thrombosis, is much more suggestive of disseminated intravascular coagulation (DIC). This clotting disorder can result from a noninfectious trigger, such as acute pancreatitis or malignancy, but his presentation is more worrisome for a severe infection leading to DIC and complicated by purpura fulminans. He does not show signs of hepatic encephalopathy or cirrhosis, making decompensated liver disease a less likely inciting factor of his presentation.
Further exposure history was obtained: The patient often spent time outdoors near his rural home and used a weed‐whacker in his yard the day before admission. He owned 3 horses which he fed and often rode. He had 3 healthy dogs and had been bitten in attempts to break up fights among them, most recently 3 days prior to admission. He lived in mountain lion territory but had no direct exposure to lions. He had no known insect bites. He regularly drank well water, and consumed medium‐rare hamburgers 4 days prior to admission. One week prior to admission, a child with possible streptococcal pharyngitis visited his home.
With this history, the patient was treated with aggressive intravenous fluids and meningeal doses of ceftriaxone, vancomycin, and metronidazole.
In the summer, outdoor exposure to brush confers a risk of tick‐borne infections, including rickettsial diseases, ehrlichiosis, and spirochetal relapsing fever. However, this patient presented in the spring, and apart from rickettsial spotted fever, these illnesses tend to be indolent. It is conceivable, though unlikely, that the weed‐cutting device may have aerosolized fulminant zoonotic pathogens such as Francisella tularensis or plague that can be found in mountain lion territory.
Well water exposure suggests leptospirosis, which can present in a fulminant fashion with multi‐organ dysfunction, but is more often a subacute illness (developing over many days to a week or two). His ingestion of potentially undercooked meat raises the possibility of enterohemorrhagic infection complicated by the hemolytic uremic syndrome (HUS). However, while the purpuric rash and renal failure are compatible with HUS, the pace of illness and accompanying hypotension once again favor alternative infectious diagnoses.
The incubation period and presentation is concerning overwhelming bacterial infection related to the dog bite. Microbiological considerations include streptococcal species, Staphylococcus aureus, and gram‐negative organisms including Pasteurella species and Capnocytophaga canimorsus. The latter 2 organisms are of particular interest since they tend to cause severe sepsis in patients with alcoholism.
The antibiotic selection in this case is not straightforward. In general, empiric therapy for infections related to dog bites should include treatment for beta‐lactamaseproducing bacteria and anaerobes (eg, piperacillin/tazobactam). Yet, given the clinical presentation, severity of illness, and possible DIC, it is appropriate to be concerned about meningococcemia. Unfortunately, the tazobactam in piperacillin/tazobactam has poor central nervous system penetration so would be suboptimal treatment for meningitis. At this point, ceftriaxone, vancomycin, and metronidazole is a reasonable regimen.
Laboratory results were notable for blood urea nitrogen 50 mg/dL, creatinine 3.47 mg/dL, white cell count 21,800/L, with an absolute neutrophil count of 20,690/L, hematocrit 35.9%, platelet count 34,000/L, International Normalized Ratio 1.5, and partial thromboplastin time 44.0 seconds. His alanine aminotransferase was 356 U/L (1641 U/L), aspartate aminotransferase 959 U/L (1259 U/L), alkaline phosphatase 50 U/L (29111 U/L), and total bilirubin 1.7 mg/dL (0.31.3 mg/dL). Fibrinogen was 283 g/L (202430 g/L), lactate dehydrogenase was 1883 U/L (91185 IU/L), and uric acid was 10.5 mg/dL (3.77.7 mg/dL). His troponin I was 1.18 ng/mL (0.05 ng/ml), and his electrocardiogram showed sinus tachycardia but no evidence of myocardial ischemia. Chest x‐ray showed no infiltrate or evidence of volume overload. Lumbar puncture was deferred out of concern for ongoing disseminated intravascular coagulation.
Transthoracic echocardiogram revealed global hypokinesis and reduced left ventricular systolic function with ejection fraction of 35%. There was no evidence of vegetations or thrombus.
The patient's thrombocytopenia and prolonged coagulation parameters further support the presence of DIC. A peripheral blood smear should be examined. If microangiopathic changes are found, other diagnoses such as thrombotic thrombocytopenic purpura might be considered, although the rapid pace of illness and presence of hypotension still make sepsis with DIC more likely.
While septic shock often causes multi‐organ system failure secondary to hypoperfusion, the presumed rapid onset of hepatic and renal abnormalities suggests that microvascular thrombosis is playing a larger role in his organ system dysfunction. Microvascular thrombosis could also contribute to his myocardial injury, though globally depressed ejection fraction and elevated troponin might also be explained by infectious myocarditis. A third possibility is that his severe sepsis caused his myocardial dysfunction. Regardless of its etiology, the patient has no clinical evidence of congestive heart failure, so no specific therapy is required at this time. However, his cardiopulmonary exam should be monitored closely, and if he survives, he should have repeat echocardiography to monitor for resolution of the global hypokinesis.
Further evaluation revealed creatine kinase of 45,000 ng/ml (55380 ng/ml) and repeat troponin of >22 ng/ml. Protein C level was low at 30%. Testing for HIV was negative. Blood smear from time of transfer had few schistocytes. Urinalysis showed muddy brown casts but no dysmorphic red blood cells or red cell casts. The patient was placed on continuous veno‐venous hemofiltration (CVVH) for worsening renal failure and oliguria from presumed acute tubular necrosis in the setting of rhabdomyolysis and sepsis.
The patient has severe rhabdomyolysis that cannot fully be explained by his initial hypoperfusion and is more likely related to the overwhelming infection and microthrombosis. Rhabdomyolysis probably contributed to his acute tubular necrosis and renal failure.
Dermatology consultation identified the rash as likely purpura fulminans. They recommended a skin biopsy to rule out vasculitis. Three skin biopsies revealed micro‐vascular thrombosis; direct immunofluorescence test was negative for vasculitis; his skin tissue culture was negative for bacterial, mycobacterial, and fungal organisms.
Input from the dermatology service was key in identifying the rash. Purpura fulminans has a limited differential that includes severe infection from gram‐negative organisms and protein C and S deficiency. Since the biopsy results made vasculitis unlikely, the team was able to focus greater attention on potential pathogens such as Pasteurella species and C. canimorsus.
The biopsy also confirms the clinical suspicion that microvascular thrombosis is causing the patient's acute kidney injury, rhabdomyolysis, and myocardial ischemia. The presence of microvascular thrombosis prompts consideration of antithrombotic therapy such as heparin, but benefits of this therapy must be weighed against contraindications including bleeding and thrombocytopenia.
Ultimately out of concerns for recurrent gastrointestinal bleeding, the primary team decided not to treat with heparin or other antithrombotic therapy.
After several days of supportive care with antibiotics and renal replacement therapy, the patient showed gradual improvement of his retiform purpura, sensory neuropathy, laboratory data, and other markers of end‐organ dysfunction. Purpura of his fingertips, feet, and toes progressed to dry gangrene (Figure 3), which was monitored for potential need for amputation. He remained dependent on intermittent hemodialysis.

His initial antibiotic regimen was narrowed to ceftriaxone monotherapy. Five days after initial presentation, blood cultures drawn from the outside emergency department grew a gram‐negative rod in the anaerobic broth. Ten days later, this gram‐negative rod was identified as Capnocytophaga canimorsus. He was ultimately discharged to a skilled nursing facility.
Generally growth of an organism in broth only suggests either a very low inoculum or that the isolate is a contaminant. In this case, it was because the causative organism, C. canimorsus, is an obligate anaerobe and quite fastidious, so unlikely to grow easily. The identification of C. canimorsus from the initial blood culture is not surprising in this patient who presented with severe sepsis, DIC, and purpura fulminans after a recent dog bite. While the patient's chronic alcohol use may explain his fulminant infection from an atypical organism, one should always consider occult underlying malignancy as a predisposing factor, particularly in patients of this age group.
With the appropriate course of antibiotics, C. canimorsus infection should be completely cured. However, recovery of kidney and cardiac function could take weeks to months, and his dry gangrene may or may not resolve.
COMMENTARY
Capnocytophaga canimorsis sepsis is a rare and potentially deadly complication of dog bites that can present with rash, cellulitis, purpura fulminans, arthritis, meningitis, and endocarditis. The discussant considered a broad differential for the presentation of fever, rash, and acute illness. While the travel history was intriguing, the severity and pace of illness allowed him to focus attention on more recent infectious exposures. The ultimate key to the diagnosis was the patient's history of dog bite, an important but underrecognized source of serious infection in the United States.
According to the Centers for Disease Control and Prevention, there are approximately 4 million dog bites in the country each year. Of these, 300,000 bite victims seek care in the emergency department, resulting in 13,000 hospitalizations and 20 deaths annually.1 Infected dog bite wounds often grow polymicrobial flora. Pasteurella species are the most frequently found organisms in both dog and cat bite wounds. However, other aerobes such as streptococci, staphylococci, Moraxella, and Neisseria, as well as anaerobes including Fusobacterium and Bacteroides species, are also common.2
C. canimorsis is a facultative, fastidious gram‐negative bacillus found in the mouth flora of not only dogs but also cats and humans. It is often mistaken for other gram‐negative rod species.3 As with the patient described in this report, systemic infection from C. canimorsis can follow even superficial or well‐healed bite wounds.
Since this bacterium was first described in the literature 30 years ago, more than 100 cases of C. canimorsus infection have been described, with a mortality rate of nearly 30%.4 C. canimorsus occurs more frequently in males and in patients 50 to 70 years of age. Traditional risk factors include alcohol abuse, asplenia, immunosuppression, and corticosteroid treatment. However, in a case series of 56 isolates in California, only 10% of patients with Capnocytophaga sepsis were asplenic and none had alcohol abuse reported in their medical charts. In this series, median time from dog bite to the onset of symptoms was 3 days. Eighty‐five percent of patients presented with fever, while 32% had sepsis and 13% had DIC or septic shock.3
While C. canimorsus was once susceptible to a range of antibiotics, several reports from Canada and Europe document rising rates of beta‐lactamaseproducing strains that have caused clinically significant disease.5, 6 Individual susceptibility data take days to obtain, so it is important to start with empiric therapy. In general, empiric therapy for all serious dog bites should cover beta‐lactamaseproducing bacteria and anaerobes, for example, with amoxicillin/clavulanate, ampicillin/sulbactam, or piperacillin/tazobactam. If the patient is allergic to penicillin, clindamycin plus a fluoroquinolone can be used instead.
There are previous reports of purpura fulminans and symmetric peripheral gangrene following Capnocytophaga infection from dog bites.7, 8 Purpura fulminans is defined as rapidly progressive skin necrosis due to dermal vascular thrombosis, often in the setting of DIC. Early involvement occurs at acral sites, such as the nose, ears, fingers, and toes. Purpuric lesions often progress to skin necrosis or dry gangrene within 24 to 48 hours. In a review of 12 patients with purpura fulminans, only 9 survived. Eight of the 9 survivors required amputation of at least 1 limb, and 4 of them required 4‐limb amputation.7
In this patient who presented with fever and rash, the discussant recognized early on an underlying infectious etiology. Although the patient's exposure history led the discussant to consider a host of possibilities, the recognition of purpura fulminans allowed him to narrow his differential. Ultimately, the dog's bite clinched the diagnosis.
KEY TEACHING POINTS
-
Sepsis caused by C. canimorsus is often characterized by rash, cellulitis, arthritis, meningitis, and endocarditis. In some instances, infection can progress to purpura fulminans.
-
In cases where fastidious organisms are suspected as an infectious source, microbiology labs should be notified of suspected organisms so they can extend incubation periods or use special media to maximize culture yield and the likelihood of accurate identification.
-
Empiric therapy for serious dog bites should cover beta‐lactamaseproducing bacteria and anaerobes. Consider using amoxicillin/clavulanate, ampicillin/sulbactam, or piperacillin/tazobactam.
The approach to clinical conundrums by an expert clinician is revealed through the presentation of an actual patient's case in an approach typical of a morning report. Similarly to patient care, sequential pieces of information are provided to the clinician, who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant.
This icon represents the patient's case. Each paragraph that follows represents the discussant's thoughts.
Acknowledgements
The authors thank Snigdha Vallabhaneni, MD, from the UCSF Division of Infectious Diseases, for her contributions to the discussion on C. canimorsus. They also thank Kanade Shinkai, MD, PhD, from the UCSF Department of Dermatology, and Heather Nye, MD, PhD, from the UCSF Division of Hospital Medicine, for their review of the manuscript.
Disclosure: Nothing to report.
- ,,.Incidence of dog bite injuries treated in emergency departments.JAMA.1998;279:51–53.
- ,,,,.Bacteriologic analysis of infected dog and cat bites. Emergency Medicine Animal Bite Infection Study Group.N Engl J Med.1999;340:85–92.
- ,,,.Diagnosing Capnocytophaga canimorsus infections.Emerg Infect Dis.2006;12:340–342.
- ,,.Capnocytophaga canimorsus infections in human: review of the literature and cases report.Eur J Epidemiol.1996;12:521–533.
- ,,,,.Antimicrobial susceptibilities and beta‐lactamase characterization of Capnocytophaga species.Antimicrob Agents Chemother.1992;36:2197–2200.
- ,,,,,.Bacteremia due to Capnocytophaga species in patients with neutropenia: high frequency of beta‐lactamase‐producing strains.Clin Infect Dis.1999;28:1172–1174.
- ,,.Presentation and outcome of purpura fulminans associated with peripheral gangrene in 12 patients at Mayo Clinic.J Am Acad Dermatol.2007;57:944–956.
- ,,,.Capnocytophaga canimorsus sepsis with purpura fulminans and symmetrical gangrene following a dog bite in a shelter employee.Am J Med Sci.2004:327:369–372.
- ,,.Incidence of dog bite injuries treated in emergency departments.JAMA.1998;279:51–53.
- ,,,,.Bacteriologic analysis of infected dog and cat bites. Emergency Medicine Animal Bite Infection Study Group.N Engl J Med.1999;340:85–92.
- ,,,.Diagnosing Capnocytophaga canimorsus infections.Emerg Infect Dis.2006;12:340–342.
- ,,.Capnocytophaga canimorsus infections in human: review of the literature and cases report.Eur J Epidemiol.1996;12:521–533.
- ,,,,.Antimicrobial susceptibilities and beta‐lactamase characterization of Capnocytophaga species.Antimicrob Agents Chemother.1992;36:2197–2200.
- ,,,,,.Bacteremia due to Capnocytophaga species in patients with neutropenia: high frequency of beta‐lactamase‐producing strains.Clin Infect Dis.1999;28:1172–1174.
- ,,.Presentation and outcome of purpura fulminans associated with peripheral gangrene in 12 patients at Mayo Clinic.J Am Acad Dermatol.2007;57:944–956.
- ,,,.Capnocytophaga canimorsus sepsis with purpura fulminans and symmetrical gangrene following a dog bite in a shelter employee.Am J Med Sci.2004:327:369–372.
Adverse GERD Outcomes Rare After ALTE
Apparent life‐threatening events (ALTEs) are frightening for the parent/guardian and represent a challenge for the healthcare provider. ALTEs are defined as worrisome episodes of any combination of apnea, color change, change in muscle tone, choking or gagging.1 ALTEs account for 0.6% to 0.8% of emergency department (ED) visits for children <12 months old,2, 3 have an average length of stay (LOS) of 4.4 days and an average cost of $15,000 per hospitalization.4
Gastroesophageal reflux disease (GERD) is common in infancy11 and also is the most commonly (in 31%55% of ALTE cases) attributed cause of ALTE.2, 4, 5 It has been speculated that chemosensitivity to gastric acid results in laryngospasm, bronchospasm, and apnea. However, several small studies have failed to prove a causal link between reflux episodes and apnea.69 Furthermore, although consensus guidelines for GERD have been developed,14 the clinical use of testing for GERD remains highly variable. A study of infants discharged with an ALTE (n = 12,067) from 36 children's hospitals in the United States revealed extensive variability in the use of pH probes and upper gastrointestinal x‐ray series to diagnose GERD.4
The incidence of adverse outcomes associated with GERD after an ALTE remains unknown. It is also unknown whether an association exists between long‐term gastrointestinal (GI) outcomes and testing demonstrative of GERD or a diagnosis of GERD during hospitalization for ALTE. The primary objective of our study was to determine, in patients with an ALTE, the adverse outcomes associated with GERD (failure‐to‐thrive, aspiration pneumonia, and/or anti‐reflux surgery), the incidence of readmission for second ALTE, and death. Our secondary objective was to determine risk factors for adverse outcomes associated with GERD following an ALTE.
METHODS
Design
This was a retrospective cohort study. We reviewed electronic and paper medical charts of all infants <12 months of age admitted for ALTE between January 1, 1999 and December 31, 2003 to Primary Children's Medical Center in Salt Lake City, UT, which serves as the tertiary pediatric center for >1 million children and the primary facility for >270,000 children in Salt Lake County, UT.10 Primary Children's Medical Center is operated by a vertically integrated not‐for‐profit healthcare system (Intermountain Healthcare), which has 20 affiliated hospitals and EDs. The study was approved by the institutional review boards of the University of Utah and Intermountain Healthcare, and the privacy board of Intermountain Healthcare.
Participants
Patients were included if a computer search of ED chief complaint or hospital discharge diagnoses found one or more of the following keywords (or corresponding International Classification of Diseases, Ninth Revision [ICD‐9] codes if applicable): ALTE, altered mental status, apnea, breath‐holding spell, choking, GERD, hypotonia, lethargy, other convulsions, other neurologic diagnosis, other respiratory diagnosis, pallor, seizures, sleep apnea, stiff, syncope, and unresponsiveness. These diagnoses were chosen as potential proxy diagnoses or codes for possible ALTE, as ALTE did not have a corresponding ICD‐9 code at the time of this study.
Detailed review of the medical record included infants who were <12 months old at admission with a history consistent with ALTE, defined as an episode frightening to the observer with any combination of apnea, color change, change in muscle tone, choking, or gagging. Infants were excluded from the study if they had a previously documented underlying medical condition to explain the ALTE (such as a known seizure disorder) or had a clearly apparent diagnosis upon initial history and physical examination (such as bronchiolitis diagnosed in the emergency department) that would explain the event. Patients with unstable vital signs (eg, hypotension), trauma clearly apparent on admission, documented medication dosing error, or febrile seizure were also excluded. A complete list of exclusion criteria is found in Figure 1.
All hospital admissions, ED visits, and Pediatric GI clinic notes were reviewed for adverse outcomes associated with GERD, second ALTE admission, and death. The follow‐up time period included the original enrollment period (January 1, 1999 through December 31, 2003) through August 31, 2009.
Outcomes
Adverse outcomes associated with GERD were defined as aspiration pneumonia, failure‐to‐thrive (FTT; either admission or discharge diagnosis of FTT at another hospitalization, or follow‐up to gastroenterology clinic for FTT) and/or anti‐reflux surgery (Nissen fundoplication or gastrojejunal tube placement) as these are potential clinical consequences of having severe and uncontrolled GERD. We further collected readmission data for a second ALTE. Deaths and the attributed reasons were also collected.
Risk Factors
Potential risk factors for adverse outcomes associated with GERD (all during index hospitalization) included: age; prematurity; gender; previous event (as described by the parent, without previous ALTE hospitalization); primary discharge diagnosis of GERD; testing positive for reflux on index ALTE admission (upper GI x‐ray series, esophageal pH probe, swallow study, endoscopy, and/or consultation of pediatric gastroenterologist with results or assessments indicating gastroesophageal reflux); any anti‐reflux medication prescribed upon discharge; and LOS. We also considered diagnosis of neurologic impairment during follow‐up, which was defined as seizures or diagnosis of developmental delay from any etiology not recognized on index ALTE admission. We examined these risk factors as we postulated they might indicate higher risk for both ALTE and adverse outcomes associated with GERD, or might indicate a higher severity of initial event.
Analyses
Summary statistics were performed for adverse outcomes associated with GERD, readmission, and death. Univariate analyses were performed for risk factors using chi‐square tests for dichotomous predictors and Wilcoxon rank sum tests for nonparametric continuous predictors for any of the 3 adverse outcomes (FTT, aspiration pneumonia [AP], and/or anti‐reflux surgery) associated with GERD. All analyses were performed using SAS 9.13 (Carey, NC).
RESULTS
Eleven hundred forty‐eight infants with ALTE met inclusion criteria, from 187,903 patients meeting initial search criteria. Six hundred seventy‐one patients were excluded and 8 patients had missing charts. The study population of the 469‐patient cohort is shown in Figure 1.
Demographics are displayed in Table 1. The mean age was 65 days. One hundred three (22%) were premature. One hundred eighty‐nine patients (40%) had a primary discharge diagnosis of GERD; details of the diagnoses for the remaining patients are in Figure 1. Median length of follow‐up for the cohort was 7.8 years. The entire study period was 10.7 years.
| ALTE Cohort | |
|---|---|
| N = 469 | |
| |
| Female | 233 (49.7%) |
| Race | |
| Caucasian | 371 (79.1%) |
| Hispanic | 64 (13.6%) |
| Pacific Islander | 6 (1.3%) |
| Black | 4 (0.8%) |
| Other/unknown | 24 (5.1%) |
| Mean age in days (SD) | 65.2 (69.5) |
| Prematurity | 103 (22%) |
| Underwent testing for gastroesophageal reflux | 214 (45.6%) |
| Discharged on anti‐reflux medication | 238 (50.7%) |
| Previous event | 127 (27.1%) |
| Mean length of stay in days (SD) | 2.4 (2.4) |
| Later neurologic impairment (seizures or developmental delay) | 23 (4.9%) |
| Primary discharge diagnosis of GERD | 189 (40%) |
Eighteen patients (3.8%) had an adverse outcome associated with GERD. Four (0.9%) had aspiration pneumonia, 9 (1.9%) had failure‐to‐thrive, and 7 (1.5%) had a Nissen fundoplication (no patients had a gastrojejunal tube placed). Five patients had a gastrostomy tube placed at the time of fundoplication. Two patients had more than 1 adverse GI outcome; 1 patient had aspiration pneumonia and another had failure‐to‐thrive prior to their Nissen fundoplications.
Fifty‐six patients (11.9%) were readmitted for a second ALTE. Median time from index ALTE to second ALTE admission was 16.5 days (interquartile range: Q1, 8Q3, 32). Two (0.4%) patients died. Both (occurring at 18 months and 5.5 years after the initial ALTE hospitalization) were related to the children first developing seizure disorders and severe developmental delay. Neither of the patients who died had an index discharge diagnoses of GERD.
There was no significance of the following variables in predicting adverse outcomes associated with GERD: age, prematurity, gender, previous event, testing positive for reflux, primary discharge diagnosis of GERD, or discharge on anti‐reflux medications (see Table 2). Patients with adverse outcomes associated with GERD had longer mean LOS on the index ALTE hospitalization (4.3 days vs 2.4 days; P = 0.03) and a higher rate of neurologic impairment diagnosed in follow‐up (16.7% vs 4.4%; P = 0.02) than patients without long‐term adverse GI outcomes. Patients with neurological impairment diagnosed in follow‐up were more likely to eventually develop an adverse outcome associated with GERD, compared to patients without neurological impairment (odds ratio 8.4; 95% confidence interval 1.1516.1).
| AP, FTT, or Surgery | No Long‐Term Adverse GI Outcome | P Value | |
|---|---|---|---|
| N = 18 | N = 451 | ||
| |||
| Mean age in days (SD) | 51.9 (76.4) | 65.8 (69.3) | 0.27 |
| Prematurity | 1 (5.6%) | 102 (22.6%) | 0.08 |
| Male gender | 12 (66.7%) | 220 (48.8%) | 0.14 |
| Previous ALTE‐like event (no hospitalization) | 8 (44.4%) | 119 (26.4%) | 0.09 |
| Testing positive for reflux | 9 (50%) | 177 (39.3%) | 0.36 |
| Discharge diagnosis GERD | 9 (50%) | 180 (39.9%) | 0.39 |
| Discharged on anti‐reflux medication | 9 (50%) | 229 (50.7%) | 0.95 |
| Mean length of stay in days (SD) | 4.3 (4.7) | 2.4 (2.3) | 0.03 |
| Neurologic impairment diagnosed in follow‐up | 3 (16.7%) | 20 (4.4%) | 0.02 |
DISCUSSION
Our study had 2 main findings. First, infants admitted for an ALTE had a low percentage (3.8%) of adverse outcomes associated with GERD. Review of the literature provides little context to interpret this percentage. One study reports 9 per 100,000 of the general population <18 years of age having anti‐reflux surgery.24 The percentage of adverse outcomes associated with GERD converted to a rate in our study would likely reflect the bias of our center serving as a referral population for Utah and 5 surrounding states. Furthermore, there may be an additional bias of ALTE being a potential indication for anti‐reflux surgery for some clinicians, as well as confounding additional diagnoses (such as later neurologic impairment which might independently increase risk of study outcomes).
The second main finding of our study is that the development of neurologic impairment was predictive of developing adverse GI outcomes. As previous studies have shown that neurological impairment cannot be predicted during the initial ALTE hospitalization,14 adverse outcomes associated with GERD are similarly not predictable with current clinical approaches. Furthermore, the exact nature of the relationship between neurologic impairment and ALTEs remains unclear. While previous studies have described the increased prevalence of adverse neurological outcomes (such as seizures, developmental delay) in children who have had an ALTE, it is unclear what the precipitating reason for ALTE is in these infants (seizure, central apnea, GERD, etc).14
There is ongoing debate in the literature surrounding the optimal diagnosis of GERD in infants with ALTE. Recent guidelines state that investigations aimed to prove GERD causing an ALTE should include pH probe or impedance monitor testing, in combination with a sleep study, and discourages a GERD diagnosis based on upper GI‐series alone.14 Given the low use of pH probe and impedance monitoring at our institution during the study period (86% of the patients who had GI‐related testing had an upper GI‐series), we did not attempt to find the sensitivity or specificity of the different GI testing modalities for GERD in the setting of ALTE. The high use of upper‐GI series is not unique to our institutionone large study examining practice variation from 12,067 ALTE admissions in 36 children's hospitals, with 36.9% of infants (n = 4453) having a primary discharge diagnosis of gastroesophageal reflux, revealed that only 8.9% (SD 28%) received an esophageal pH probe, while 25.6% (SD 43.6%) had an upper GI‐series or swallow study.4
Given the difficulties in assigning a GERD diagnosis for ALTE infants, we focused on the long‐term adverse outcomes associated with GERD for the entire ALTE infant cohort. The 3 adverse outcomes we chose deserve some mention. Aspiration pneumonia is generally due to either primary aspiration (from dysfunctional swallowing) or secondary aspiration (from GERD). Failure‐to‐thrive can be due to ongoing GERD. Anti‐reflux surgery is often performed for severe GERD. While we believe that a prospective study with better diagnostic evaluations for GERD and apnea (such as pH probe or impedance monitor in combination with a sleep study) might help elucidate the unclear relationship between reflux episodes and ALTE, the low percentage of adverse outcomes associated with GERD after ALTE may suggest that such a study would be difficult both in terms of sample size and an unnecessary use of resources.
We also found that a high percentage (11.9%) of all patients had readmission for a second ALTE. This was substantially higher than the 2.5% readmission rate for second ALTE reported in a previous study of short‐term follow‐up (30 days).4 The number of readmissions in our study might be higher due to our comprehensive follow‐up, both in length of time and number of additional EDs and hospitals captured. Unfortunately, the retrospective nature of our study makes it difficult to determine if any interventions (prescription of anti‐reflux medication, education on reflux precautions) impacted the rate of readmission, as compliance was not measurable. Further studies should address why patients return with recurrent ALTE.
Interestingly, several potential risk factors did not predict long‐term adverse GI outcomes. For example, prematurity, a discharge diagnosis of GERD, or prescription of an anti‐reflux medication, were not associated with adverse GI outcomes. These findings support the concept that a diagnosis of GERD, at least as is commonly applied, is not meaningful in the setting of an ALTE. We did find associations with longer length of stay (LOS), and with eventual development of neurological impairment. Longer LOS might be a proxy for other subtle predictors that could influence adverse outcomes, such as requiring additional diagnostic tests prolonging hospitalization, or continued ALTEs while inpatient. The neurologic outcomes of patients with ALTE have been previously published, and the strong correlation between neurologic impairment and GERD has been well described.14, 25
There are several strengths of this study. This is the first study, to our knowledge, to look at adverse outcomes associated with GERD following ALTE, despite GERD being the most commonly attributed cause. The use of Intermountain Healthcare's electronic medical record system allowed for comprehensive tracking, over an extensive follow‐up period (median of 7.8 years), across 20 hospitals and EDs which care for the vast majority of pediatric patients in Utah. Finally, this large cohort of ALTE patients used clinical data from medical records and not only administrative data.
There are limitations of this study. This is a retrospective cohort study. Some of our study outcomes may be a result of pathophysiology other than GERD and, conversely, GERD may be a result of other issues (neurologic impairment). The small sample size and low percentage of the study outcomes make it possible that we did not detect true risk factors. Patients were lost to follow‐up if they moved or presented to a hospital not within the Intermountain Healthcare system. This study has slightly different patient numbers from 3 previously published studies for different outcomes on this cohort, as exclusion criteria for the different cohorts were different.14, 26, 27 Six patients had only their electronic medical record reviewed because the paper chart was missing.
IMPLICATIONS
The results of this study extend previous work of various outcomes regarding well‐appearing infants following an ALTE.14, 26, 27 In these studies, 3.9% and 3% were ultimately diagnosed with epilepsy and developmental delay, respectively; 1.4% were diagnosed with abusive head trauma; and 0.6% required otolaryngologic surgical intervention. In these previous studies, there were few predictors of these outcomes, with testing demonstrating largely normal results during the index ALTE admission.
Our study helps clinicians place the outcomes of aspiration pneumonia, failure‐to‐thrive, and anti‐reflux surgery into the context of these other studies when discharging infants from the hospital after an ALTE. Collectively, these studies provide clinicians with the information that, in the setting of a well‐appearing infant, few diagnostic tests in their ALTE patients will yield a definitive diagnosis. Ultimately, close follow‐up with further investigations if symptoms recur will be an important part of diagnosing the etiology of the ALTE in these infants.
We found that well‐appearing infants with ALTE, regardless of attributed cause, are at low risk for adverse outcomes associated with GERD. Only the eventual development of neurologic impairment or an increased length of stay during index ALTE hospitalization was found to be predictive of these outcomes.
Acknowledgements
The following individuals have made substantive intellectual contributions to this study: conception and design (G.Z., J.L.B., W.D.J., C.G.M., R.S.), acquisition of data (G.Z., J.L.B.), analysis (G.Z., RS) and interpretation of data (G.Z., J.L.B., W.D.J., C.G.M., R.S.). In addition, all listed authors have contributed to either drafting the article or revising it critically for important intellectual content. Finally, all listed authors have given final approval of this version submitted for publication. The authors also acknowledge Chelsea Welch for her assistance in data collection.
Disclosures: This study was presented in part at the national Pediatric Academic Societies meetings in Vancouver, Canada, May 2010 and in Denver, CO, May 2011. This study was supported by a National Institutes of Child Health and Human Development (NICHD) grant for Dr Srivastava (K23 HD052553), and a National Institute on Drug Abuse (NIDA) grant for Dr Bonkowsky (K08 DA24753). This research was supported in part by the Children's Health Research Center, University of Utah. There are no conflicts of interest.
- Infantile apnea and home monitoring.NIH Consensus Statement 1986 Sep 29‐Oct 1.Pediatrics.1987;79(2):292–299.
- ,.Causes of apparent life threatening events in infants: a systematic review.Arch Dis Child.2004;89:1043–1048.
- ,.Parental reported apnea, admissions to hospital and sudden infant death syndrome.Acta Paediatr.2001;90(4):417–422.
- ,,,.Variation in inpatient resource utilization and management of apparent life‐threatening events.J Pediatr.2008;152(5):629–635.
- ,,,,,.Discharge diagnoses in infants with apparent life‐threatening event.Pediatr Int.2003;45:560–563.
- ,,,,,.Lack of temporal relation between acid reflux in the proximal oesophagus and cardiorespiratory events in sleeping infants.Eur J Pediatr.1992;151(3):208–212.
- ,,, et al.Patterns of gastroesophageal reflux (GER) in patients with apparent life‐threatening events.J Pediatr Gastroenterol Nutr.1989;8(2):157–160.
- ,,, et al.Characteristics of continuous esophageal pH‐metering in infants with gastroesophageal reflux and apparent life‐threatening events.Eur J Pediatr Surg.1995;5(3);136–138.
- ,,,,.Apnea is not prolonged by acid gastroesophageal reflux in preterm infants.Pediatrics.2005;116:1059–1063.
- ,.Organizational responses to managed care: issues for academic health centers and implications for pediatric programs.Pediatrics.1998;101(4):805–811.
- ,,, et al.Pediatric gastroesophageal reflux clinical practice guidelines: joint recommendations of the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN) and the European Society for Pediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN).J Pediatr Gastroenterol Nutr.2009;49:498–547.
- ,,, et al.Prevalence and natural history of gastroesophageal reflux: pediatric prospective study.Pediatrics.2009;123(3):779–783.
- ,.Systematic review: the estra‐oesophageal symptoms of gastro‐oesophageal reflux disease in children.Aliment Pharmacol Ther.2009;29:258–272.
- ,,,.Death, child abuse, and adverse neurological outcomes of infants after an apparent life‐threatening event.Pediatrics.2008;122:125–131.
- ,,, et al.Abusive head injury as a cause of apparent life‐threatening events in infancy.Arch Pediatr Adolesc Med.2003;157:1011–1015.
- ,,.Accidental and nonaccidental poisonings as a cause of apparent life‐threatening events in infants.Pediatrics.2008;122:e359–e362.
- ,,,.Yield of diagnostic testing in infants who have had an apparent life‐threatening event.Pediatrics.2005;115:885–893.
- ,,, et al.Guidelines for evaluation and treatment of gastroesophageal reflux in infants and children: recommendations of the North American Society for Pediatric Gastroenterology and Nutrition.J Pediatr Gastroenterol Nutr.2001;32(suppl 2):S1–S31.
- ,,,.Comparison of barium radiology with esophageal pH monitoring in the diagnosis of gastroesophageal reflux disease.Am J Gastroenterol.1996;91(6):1181–1195.
- ,,, et al.Gastroesophageal reflux disease: correlation of esophageal pH testing and radiographic finding.Radiology.1992;185:483–486.
- ,,, et al.Gastro‐esophageal reflux demonstrated by radiography in infants less than 1 year of age. Comparison with pH monitoring.Acta Radiol.2003;44:136–138.
- ,,, et al.Double‐blind placebo controlled trial of omeprazole in irritable infants with gastroesophageal reflux.J Pediatr.2003;143:219–223.
- ,,, et al.Clinical predictors of pathological gastro‐oesophageal reflux in infants with persistant distress.J Paediatr Child Health.2006;42:134–139.
- ,,.National trends in the use of anti‐reflux procedures for children.Pediatrics.2006;118:1828–1835.
- ,,, et al.Prevalence and severity of feeding and nutritional problems in children with neurological impairment: Oxford feeding study.Dev Med Child Neurol.2000;42:674–680.
- ,,.Abusive head trauma in children presenting with an apparent life‐threatening event.J Pediatr.2010;157(5):821–825.
- ,,.Usefulness of airway evaluation in children initially seen with apparent life‐threatening event.Arch Otolaryngol Head Neck Surg.2011;137(4):359–362.
Apparent life‐threatening events (ALTEs) are frightening for the parent/guardian and represent a challenge for the healthcare provider. ALTEs are defined as worrisome episodes of any combination of apnea, color change, change in muscle tone, choking or gagging.1 ALTEs account for 0.6% to 0.8% of emergency department (ED) visits for children <12 months old,2, 3 have an average length of stay (LOS) of 4.4 days and an average cost of $15,000 per hospitalization.4
Gastroesophageal reflux disease (GERD) is common in infancy11 and also is the most commonly (in 31%55% of ALTE cases) attributed cause of ALTE.2, 4, 5 It has been speculated that chemosensitivity to gastric acid results in laryngospasm, bronchospasm, and apnea. However, several small studies have failed to prove a causal link between reflux episodes and apnea.69 Furthermore, although consensus guidelines for GERD have been developed,14 the clinical use of testing for GERD remains highly variable. A study of infants discharged with an ALTE (n = 12,067) from 36 children's hospitals in the United States revealed extensive variability in the use of pH probes and upper gastrointestinal x‐ray series to diagnose GERD.4
The incidence of adverse outcomes associated with GERD after an ALTE remains unknown. It is also unknown whether an association exists between long‐term gastrointestinal (GI) outcomes and testing demonstrative of GERD or a diagnosis of GERD during hospitalization for ALTE. The primary objective of our study was to determine, in patients with an ALTE, the adverse outcomes associated with GERD (failure‐to‐thrive, aspiration pneumonia, and/or anti‐reflux surgery), the incidence of readmission for second ALTE, and death. Our secondary objective was to determine risk factors for adverse outcomes associated with GERD following an ALTE.
METHODS
Design
This was a retrospective cohort study. We reviewed electronic and paper medical charts of all infants <12 months of age admitted for ALTE between January 1, 1999 and December 31, 2003 to Primary Children's Medical Center in Salt Lake City, UT, which serves as the tertiary pediatric center for >1 million children and the primary facility for >270,000 children in Salt Lake County, UT.10 Primary Children's Medical Center is operated by a vertically integrated not‐for‐profit healthcare system (Intermountain Healthcare), which has 20 affiliated hospitals and EDs. The study was approved by the institutional review boards of the University of Utah and Intermountain Healthcare, and the privacy board of Intermountain Healthcare.
Participants
Patients were included if a computer search of ED chief complaint or hospital discharge diagnoses found one or more of the following keywords (or corresponding International Classification of Diseases, Ninth Revision [ICD‐9] codes if applicable): ALTE, altered mental status, apnea, breath‐holding spell, choking, GERD, hypotonia, lethargy, other convulsions, other neurologic diagnosis, other respiratory diagnosis, pallor, seizures, sleep apnea, stiff, syncope, and unresponsiveness. These diagnoses were chosen as potential proxy diagnoses or codes for possible ALTE, as ALTE did not have a corresponding ICD‐9 code at the time of this study.
Detailed review of the medical record included infants who were <12 months old at admission with a history consistent with ALTE, defined as an episode frightening to the observer with any combination of apnea, color change, change in muscle tone, choking, or gagging. Infants were excluded from the study if they had a previously documented underlying medical condition to explain the ALTE (such as a known seizure disorder) or had a clearly apparent diagnosis upon initial history and physical examination (such as bronchiolitis diagnosed in the emergency department) that would explain the event. Patients with unstable vital signs (eg, hypotension), trauma clearly apparent on admission, documented medication dosing error, or febrile seizure were also excluded. A complete list of exclusion criteria is found in Figure 1.

All hospital admissions, ED visits, and Pediatric GI clinic notes were reviewed for adverse outcomes associated with GERD, second ALTE admission, and death. The follow‐up time period included the original enrollment period (January 1, 1999 through December 31, 2003) through August 31, 2009.
Outcomes
Adverse outcomes associated with GERD were defined as aspiration pneumonia, failure‐to‐thrive (FTT; either admission or discharge diagnosis of FTT at another hospitalization, or follow‐up to gastroenterology clinic for FTT) and/or anti‐reflux surgery (Nissen fundoplication or gastrojejunal tube placement) as these are potential clinical consequences of having severe and uncontrolled GERD. We further collected readmission data for a second ALTE. Deaths and the attributed reasons were also collected.
Risk Factors
Potential risk factors for adverse outcomes associated with GERD (all during index hospitalization) included: age; prematurity; gender; previous event (as described by the parent, without previous ALTE hospitalization); primary discharge diagnosis of GERD; testing positive for reflux on index ALTE admission (upper GI x‐ray series, esophageal pH probe, swallow study, endoscopy, and/or consultation of pediatric gastroenterologist with results or assessments indicating gastroesophageal reflux); any anti‐reflux medication prescribed upon discharge; and LOS. We also considered diagnosis of neurologic impairment during follow‐up, which was defined as seizures or diagnosis of developmental delay from any etiology not recognized on index ALTE admission. We examined these risk factors as we postulated they might indicate higher risk for both ALTE and adverse outcomes associated with GERD, or might indicate a higher severity of initial event.
Analyses
Summary statistics were performed for adverse outcomes associated with GERD, readmission, and death. Univariate analyses were performed for risk factors using chi‐square tests for dichotomous predictors and Wilcoxon rank sum tests for nonparametric continuous predictors for any of the 3 adverse outcomes (FTT, aspiration pneumonia [AP], and/or anti‐reflux surgery) associated with GERD. All analyses were performed using SAS 9.13 (Carey, NC).
RESULTS
Eleven hundred forty‐eight infants with ALTE met inclusion criteria, from 187,903 patients meeting initial search criteria. Six hundred seventy‐one patients were excluded and 8 patients had missing charts. The study population of the 469‐patient cohort is shown in Figure 1.
Demographics are displayed in Table 1. The mean age was 65 days. One hundred three (22%) were premature. One hundred eighty‐nine patients (40%) had a primary discharge diagnosis of GERD; details of the diagnoses for the remaining patients are in Figure 1. Median length of follow‐up for the cohort was 7.8 years. The entire study period was 10.7 years.
| ALTE Cohort | |
|---|---|
| N = 469 | |
| |
| Female | 233 (49.7%) |
| Race | |
| Caucasian | 371 (79.1%) |
| Hispanic | 64 (13.6%) |
| Pacific Islander | 6 (1.3%) |
| Black | 4 (0.8%) |
| Other/unknown | 24 (5.1%) |
| Mean age in days (SD) | 65.2 (69.5) |
| Prematurity | 103 (22%) |
| Underwent testing for gastroesophageal reflux | 214 (45.6%) |
| Discharged on anti‐reflux medication | 238 (50.7%) |
| Previous event | 127 (27.1%) |
| Mean length of stay in days (SD) | 2.4 (2.4) |
| Later neurologic impairment (seizures or developmental delay) | 23 (4.9%) |
| Primary discharge diagnosis of GERD | 189 (40%) |
Eighteen patients (3.8%) had an adverse outcome associated with GERD. Four (0.9%) had aspiration pneumonia, 9 (1.9%) had failure‐to‐thrive, and 7 (1.5%) had a Nissen fundoplication (no patients had a gastrojejunal tube placed). Five patients had a gastrostomy tube placed at the time of fundoplication. Two patients had more than 1 adverse GI outcome; 1 patient had aspiration pneumonia and another had failure‐to‐thrive prior to their Nissen fundoplications.
Fifty‐six patients (11.9%) were readmitted for a second ALTE. Median time from index ALTE to second ALTE admission was 16.5 days (interquartile range: Q1, 8Q3, 32). Two (0.4%) patients died. Both (occurring at 18 months and 5.5 years after the initial ALTE hospitalization) were related to the children first developing seizure disorders and severe developmental delay. Neither of the patients who died had an index discharge diagnoses of GERD.
There was no significance of the following variables in predicting adverse outcomes associated with GERD: age, prematurity, gender, previous event, testing positive for reflux, primary discharge diagnosis of GERD, or discharge on anti‐reflux medications (see Table 2). Patients with adverse outcomes associated with GERD had longer mean LOS on the index ALTE hospitalization (4.3 days vs 2.4 days; P = 0.03) and a higher rate of neurologic impairment diagnosed in follow‐up (16.7% vs 4.4%; P = 0.02) than patients without long‐term adverse GI outcomes. Patients with neurological impairment diagnosed in follow‐up were more likely to eventually develop an adverse outcome associated with GERD, compared to patients without neurological impairment (odds ratio 8.4; 95% confidence interval 1.1516.1).
| AP, FTT, or Surgery | No Long‐Term Adverse GI Outcome | P Value | |
|---|---|---|---|
| N = 18 | N = 451 | ||
| |||
| Mean age in days (SD) | 51.9 (76.4) | 65.8 (69.3) | 0.27 |
| Prematurity | 1 (5.6%) | 102 (22.6%) | 0.08 |
| Male gender | 12 (66.7%) | 220 (48.8%) | 0.14 |
| Previous ALTE‐like event (no hospitalization) | 8 (44.4%) | 119 (26.4%) | 0.09 |
| Testing positive for reflux | 9 (50%) | 177 (39.3%) | 0.36 |
| Discharge diagnosis GERD | 9 (50%) | 180 (39.9%) | 0.39 |
| Discharged on anti‐reflux medication | 9 (50%) | 229 (50.7%) | 0.95 |
| Mean length of stay in days (SD) | 4.3 (4.7) | 2.4 (2.3) | 0.03 |
| Neurologic impairment diagnosed in follow‐up | 3 (16.7%) | 20 (4.4%) | 0.02 |
DISCUSSION
Our study had 2 main findings. First, infants admitted for an ALTE had a low percentage (3.8%) of adverse outcomes associated with GERD. Review of the literature provides little context to interpret this percentage. One study reports 9 per 100,000 of the general population <18 years of age having anti‐reflux surgery.24 The percentage of adverse outcomes associated with GERD converted to a rate in our study would likely reflect the bias of our center serving as a referral population for Utah and 5 surrounding states. Furthermore, there may be an additional bias of ALTE being a potential indication for anti‐reflux surgery for some clinicians, as well as confounding additional diagnoses (such as later neurologic impairment which might independently increase risk of study outcomes).
The second main finding of our study is that the development of neurologic impairment was predictive of developing adverse GI outcomes. As previous studies have shown that neurological impairment cannot be predicted during the initial ALTE hospitalization,14 adverse outcomes associated with GERD are similarly not predictable with current clinical approaches. Furthermore, the exact nature of the relationship between neurologic impairment and ALTEs remains unclear. While previous studies have described the increased prevalence of adverse neurological outcomes (such as seizures, developmental delay) in children who have had an ALTE, it is unclear what the precipitating reason for ALTE is in these infants (seizure, central apnea, GERD, etc).14
There is ongoing debate in the literature surrounding the optimal diagnosis of GERD in infants with ALTE. Recent guidelines state that investigations aimed to prove GERD causing an ALTE should include pH probe or impedance monitor testing, in combination with a sleep study, and discourages a GERD diagnosis based on upper GI‐series alone.14 Given the low use of pH probe and impedance monitoring at our institution during the study period (86% of the patients who had GI‐related testing had an upper GI‐series), we did not attempt to find the sensitivity or specificity of the different GI testing modalities for GERD in the setting of ALTE. The high use of upper‐GI series is not unique to our institutionone large study examining practice variation from 12,067 ALTE admissions in 36 children's hospitals, with 36.9% of infants (n = 4453) having a primary discharge diagnosis of gastroesophageal reflux, revealed that only 8.9% (SD 28%) received an esophageal pH probe, while 25.6% (SD 43.6%) had an upper GI‐series or swallow study.4
Given the difficulties in assigning a GERD diagnosis for ALTE infants, we focused on the long‐term adverse outcomes associated with GERD for the entire ALTE infant cohort. The 3 adverse outcomes we chose deserve some mention. Aspiration pneumonia is generally due to either primary aspiration (from dysfunctional swallowing) or secondary aspiration (from GERD). Failure‐to‐thrive can be due to ongoing GERD. Anti‐reflux surgery is often performed for severe GERD. While we believe that a prospective study with better diagnostic evaluations for GERD and apnea (such as pH probe or impedance monitor in combination with a sleep study) might help elucidate the unclear relationship between reflux episodes and ALTE, the low percentage of adverse outcomes associated with GERD after ALTE may suggest that such a study would be difficult both in terms of sample size and an unnecessary use of resources.
We also found that a high percentage (11.9%) of all patients had readmission for a second ALTE. This was substantially higher than the 2.5% readmission rate for second ALTE reported in a previous study of short‐term follow‐up (30 days).4 The number of readmissions in our study might be higher due to our comprehensive follow‐up, both in length of time and number of additional EDs and hospitals captured. Unfortunately, the retrospective nature of our study makes it difficult to determine if any interventions (prescription of anti‐reflux medication, education on reflux precautions) impacted the rate of readmission, as compliance was not measurable. Further studies should address why patients return with recurrent ALTE.
Interestingly, several potential risk factors did not predict long‐term adverse GI outcomes. For example, prematurity, a discharge diagnosis of GERD, or prescription of an anti‐reflux medication, were not associated with adverse GI outcomes. These findings support the concept that a diagnosis of GERD, at least as is commonly applied, is not meaningful in the setting of an ALTE. We did find associations with longer length of stay (LOS), and with eventual development of neurological impairment. Longer LOS might be a proxy for other subtle predictors that could influence adverse outcomes, such as requiring additional diagnostic tests prolonging hospitalization, or continued ALTEs while inpatient. The neurologic outcomes of patients with ALTE have been previously published, and the strong correlation between neurologic impairment and GERD has been well described.14, 25
There are several strengths of this study. This is the first study, to our knowledge, to look at adverse outcomes associated with GERD following ALTE, despite GERD being the most commonly attributed cause. The use of Intermountain Healthcare's electronic medical record system allowed for comprehensive tracking, over an extensive follow‐up period (median of 7.8 years), across 20 hospitals and EDs which care for the vast majority of pediatric patients in Utah. Finally, this large cohort of ALTE patients used clinical data from medical records and not only administrative data.
There are limitations of this study. This is a retrospective cohort study. Some of our study outcomes may be a result of pathophysiology other than GERD and, conversely, GERD may be a result of other issues (neurologic impairment). The small sample size and low percentage of the study outcomes make it possible that we did not detect true risk factors. Patients were lost to follow‐up if they moved or presented to a hospital not within the Intermountain Healthcare system. This study has slightly different patient numbers from 3 previously published studies for different outcomes on this cohort, as exclusion criteria for the different cohorts were different.14, 26, 27 Six patients had only their electronic medical record reviewed because the paper chart was missing.
IMPLICATIONS
The results of this study extend previous work of various outcomes regarding well‐appearing infants following an ALTE.14, 26, 27 In these studies, 3.9% and 3% were ultimately diagnosed with epilepsy and developmental delay, respectively; 1.4% were diagnosed with abusive head trauma; and 0.6% required otolaryngologic surgical intervention. In these previous studies, there were few predictors of these outcomes, with testing demonstrating largely normal results during the index ALTE admission.
Our study helps clinicians place the outcomes of aspiration pneumonia, failure‐to‐thrive, and anti‐reflux surgery into the context of these other studies when discharging infants from the hospital after an ALTE. Collectively, these studies provide clinicians with the information that, in the setting of a well‐appearing infant, few diagnostic tests in their ALTE patients will yield a definitive diagnosis. Ultimately, close follow‐up with further investigations if symptoms recur will be an important part of diagnosing the etiology of the ALTE in these infants.
We found that well‐appearing infants with ALTE, regardless of attributed cause, are at low risk for adverse outcomes associated with GERD. Only the eventual development of neurologic impairment or an increased length of stay during index ALTE hospitalization was found to be predictive of these outcomes.
Acknowledgements
The following individuals have made substantive intellectual contributions to this study: conception and design (G.Z., J.L.B., W.D.J., C.G.M., R.S.), acquisition of data (G.Z., J.L.B.), analysis (G.Z., RS) and interpretation of data (G.Z., J.L.B., W.D.J., C.G.M., R.S.). In addition, all listed authors have contributed to either drafting the article or revising it critically for important intellectual content. Finally, all listed authors have given final approval of this version submitted for publication. The authors also acknowledge Chelsea Welch for her assistance in data collection.
Disclosures: This study was presented in part at the national Pediatric Academic Societies meetings in Vancouver, Canada, May 2010 and in Denver, CO, May 2011. This study was supported by a National Institutes of Child Health and Human Development (NICHD) grant for Dr Srivastava (K23 HD052553), and a National Institute on Drug Abuse (NIDA) grant for Dr Bonkowsky (K08 DA24753). This research was supported in part by the Children's Health Research Center, University of Utah. There are no conflicts of interest.
Apparent life‐threatening events (ALTEs) are frightening for the parent/guardian and represent a challenge for the healthcare provider. ALTEs are defined as worrisome episodes of any combination of apnea, color change, change in muscle tone, choking or gagging.1 ALTEs account for 0.6% to 0.8% of emergency department (ED) visits for children <12 months old,2, 3 have an average length of stay (LOS) of 4.4 days and an average cost of $15,000 per hospitalization.4
Gastroesophageal reflux disease (GERD) is common in infancy11 and also is the most commonly (in 31%55% of ALTE cases) attributed cause of ALTE.2, 4, 5 It has been speculated that chemosensitivity to gastric acid results in laryngospasm, bronchospasm, and apnea. However, several small studies have failed to prove a causal link between reflux episodes and apnea.69 Furthermore, although consensus guidelines for GERD have been developed,14 the clinical use of testing for GERD remains highly variable. A study of infants discharged with an ALTE (n = 12,067) from 36 children's hospitals in the United States revealed extensive variability in the use of pH probes and upper gastrointestinal x‐ray series to diagnose GERD.4
The incidence of adverse outcomes associated with GERD after an ALTE remains unknown. It is also unknown whether an association exists between long‐term gastrointestinal (GI) outcomes and testing demonstrative of GERD or a diagnosis of GERD during hospitalization for ALTE. The primary objective of our study was to determine, in patients with an ALTE, the adverse outcomes associated with GERD (failure‐to‐thrive, aspiration pneumonia, and/or anti‐reflux surgery), the incidence of readmission for second ALTE, and death. Our secondary objective was to determine risk factors for adverse outcomes associated with GERD following an ALTE.
METHODS
Design
This was a retrospective cohort study. We reviewed electronic and paper medical charts of all infants <12 months of age admitted for ALTE between January 1, 1999 and December 31, 2003 to Primary Children's Medical Center in Salt Lake City, UT, which serves as the tertiary pediatric center for >1 million children and the primary facility for >270,000 children in Salt Lake County, UT.10 Primary Children's Medical Center is operated by a vertically integrated not‐for‐profit healthcare system (Intermountain Healthcare), which has 20 affiliated hospitals and EDs. The study was approved by the institutional review boards of the University of Utah and Intermountain Healthcare, and the privacy board of Intermountain Healthcare.
Participants
Patients were included if a computer search of ED chief complaint or hospital discharge diagnoses found one or more of the following keywords (or corresponding International Classification of Diseases, Ninth Revision [ICD‐9] codes if applicable): ALTE, altered mental status, apnea, breath‐holding spell, choking, GERD, hypotonia, lethargy, other convulsions, other neurologic diagnosis, other respiratory diagnosis, pallor, seizures, sleep apnea, stiff, syncope, and unresponsiveness. These diagnoses were chosen as potential proxy diagnoses or codes for possible ALTE, as ALTE did not have a corresponding ICD‐9 code at the time of this study.
Detailed review of the medical record included infants who were <12 months old at admission with a history consistent with ALTE, defined as an episode frightening to the observer with any combination of apnea, color change, change in muscle tone, choking, or gagging. Infants were excluded from the study if they had a previously documented underlying medical condition to explain the ALTE (such as a known seizure disorder) or had a clearly apparent diagnosis upon initial history and physical examination (such as bronchiolitis diagnosed in the emergency department) that would explain the event. Patients with unstable vital signs (eg, hypotension), trauma clearly apparent on admission, documented medication dosing error, or febrile seizure were also excluded. A complete list of exclusion criteria is found in Figure 1.

All hospital admissions, ED visits, and Pediatric GI clinic notes were reviewed for adverse outcomes associated with GERD, second ALTE admission, and death. The follow‐up time period included the original enrollment period (January 1, 1999 through December 31, 2003) through August 31, 2009.
Outcomes
Adverse outcomes associated with GERD were defined as aspiration pneumonia, failure‐to‐thrive (FTT; either admission or discharge diagnosis of FTT at another hospitalization, or follow‐up to gastroenterology clinic for FTT) and/or anti‐reflux surgery (Nissen fundoplication or gastrojejunal tube placement) as these are potential clinical consequences of having severe and uncontrolled GERD. We further collected readmission data for a second ALTE. Deaths and the attributed reasons were also collected.
Risk Factors
Potential risk factors for adverse outcomes associated with GERD (all during index hospitalization) included: age; prematurity; gender; previous event (as described by the parent, without previous ALTE hospitalization); primary discharge diagnosis of GERD; testing positive for reflux on index ALTE admission (upper GI x‐ray series, esophageal pH probe, swallow study, endoscopy, and/or consultation of pediatric gastroenterologist with results or assessments indicating gastroesophageal reflux); any anti‐reflux medication prescribed upon discharge; and LOS. We also considered diagnosis of neurologic impairment during follow‐up, which was defined as seizures or diagnosis of developmental delay from any etiology not recognized on index ALTE admission. We examined these risk factors as we postulated they might indicate higher risk for both ALTE and adverse outcomes associated with GERD, or might indicate a higher severity of initial event.
Analyses
Summary statistics were performed for adverse outcomes associated with GERD, readmission, and death. Univariate analyses were performed for risk factors using chi‐square tests for dichotomous predictors and Wilcoxon rank sum tests for nonparametric continuous predictors for any of the 3 adverse outcomes (FTT, aspiration pneumonia [AP], and/or anti‐reflux surgery) associated with GERD. All analyses were performed using SAS 9.13 (Carey, NC).
RESULTS
Eleven hundred forty‐eight infants with ALTE met inclusion criteria, from 187,903 patients meeting initial search criteria. Six hundred seventy‐one patients were excluded and 8 patients had missing charts. The study population of the 469‐patient cohort is shown in Figure 1.
Demographics are displayed in Table 1. The mean age was 65 days. One hundred three (22%) were premature. One hundred eighty‐nine patients (40%) had a primary discharge diagnosis of GERD; details of the diagnoses for the remaining patients are in Figure 1. Median length of follow‐up for the cohort was 7.8 years. The entire study period was 10.7 years.
| ALTE Cohort | |
|---|---|
| N = 469 | |
| |
| Female | 233 (49.7%) |
| Race | |
| Caucasian | 371 (79.1%) |
| Hispanic | 64 (13.6%) |
| Pacific Islander | 6 (1.3%) |
| Black | 4 (0.8%) |
| Other/unknown | 24 (5.1%) |
| Mean age in days (SD) | 65.2 (69.5) |
| Prematurity | 103 (22%) |
| Underwent testing for gastroesophageal reflux | 214 (45.6%) |
| Discharged on anti‐reflux medication | 238 (50.7%) |
| Previous event | 127 (27.1%) |
| Mean length of stay in days (SD) | 2.4 (2.4) |
| Later neurologic impairment (seizures or developmental delay) | 23 (4.9%) |
| Primary discharge diagnosis of GERD | 189 (40%) |
Eighteen patients (3.8%) had an adverse outcome associated with GERD. Four (0.9%) had aspiration pneumonia, 9 (1.9%) had failure‐to‐thrive, and 7 (1.5%) had a Nissen fundoplication (no patients had a gastrojejunal tube placed). Five patients had a gastrostomy tube placed at the time of fundoplication. Two patients had more than 1 adverse GI outcome; 1 patient had aspiration pneumonia and another had failure‐to‐thrive prior to their Nissen fundoplications.
Fifty‐six patients (11.9%) were readmitted for a second ALTE. Median time from index ALTE to second ALTE admission was 16.5 days (interquartile range: Q1, 8Q3, 32). Two (0.4%) patients died. Both (occurring at 18 months and 5.5 years after the initial ALTE hospitalization) were related to the children first developing seizure disorders and severe developmental delay. Neither of the patients who died had an index discharge diagnoses of GERD.
There was no significance of the following variables in predicting adverse outcomes associated with GERD: age, prematurity, gender, previous event, testing positive for reflux, primary discharge diagnosis of GERD, or discharge on anti‐reflux medications (see Table 2). Patients with adverse outcomes associated with GERD had longer mean LOS on the index ALTE hospitalization (4.3 days vs 2.4 days; P = 0.03) and a higher rate of neurologic impairment diagnosed in follow‐up (16.7% vs 4.4%; P = 0.02) than patients without long‐term adverse GI outcomes. Patients with neurological impairment diagnosed in follow‐up were more likely to eventually develop an adverse outcome associated with GERD, compared to patients without neurological impairment (odds ratio 8.4; 95% confidence interval 1.1516.1).
| AP, FTT, or Surgery | No Long‐Term Adverse GI Outcome | P Value | |
|---|---|---|---|
| N = 18 | N = 451 | ||
| |||
| Mean age in days (SD) | 51.9 (76.4) | 65.8 (69.3) | 0.27 |
| Prematurity | 1 (5.6%) | 102 (22.6%) | 0.08 |
| Male gender | 12 (66.7%) | 220 (48.8%) | 0.14 |
| Previous ALTE‐like event (no hospitalization) | 8 (44.4%) | 119 (26.4%) | 0.09 |
| Testing positive for reflux | 9 (50%) | 177 (39.3%) | 0.36 |
| Discharge diagnosis GERD | 9 (50%) | 180 (39.9%) | 0.39 |
| Discharged on anti‐reflux medication | 9 (50%) | 229 (50.7%) | 0.95 |
| Mean length of stay in days (SD) | 4.3 (4.7) | 2.4 (2.3) | 0.03 |
| Neurologic impairment diagnosed in follow‐up | 3 (16.7%) | 20 (4.4%) | 0.02 |
DISCUSSION
Our study had 2 main findings. First, infants admitted for an ALTE had a low percentage (3.8%) of adverse outcomes associated with GERD. Review of the literature provides little context to interpret this percentage. One study reports 9 per 100,000 of the general population <18 years of age having anti‐reflux surgery.24 The percentage of adverse outcomes associated with GERD converted to a rate in our study would likely reflect the bias of our center serving as a referral population for Utah and 5 surrounding states. Furthermore, there may be an additional bias of ALTE being a potential indication for anti‐reflux surgery for some clinicians, as well as confounding additional diagnoses (such as later neurologic impairment which might independently increase risk of study outcomes).
The second main finding of our study is that the development of neurologic impairment was predictive of developing adverse GI outcomes. As previous studies have shown that neurological impairment cannot be predicted during the initial ALTE hospitalization,14 adverse outcomes associated with GERD are similarly not predictable with current clinical approaches. Furthermore, the exact nature of the relationship between neurologic impairment and ALTEs remains unclear. While previous studies have described the increased prevalence of adverse neurological outcomes (such as seizures, developmental delay) in children who have had an ALTE, it is unclear what the precipitating reason for ALTE is in these infants (seizure, central apnea, GERD, etc).14
There is ongoing debate in the literature surrounding the optimal diagnosis of GERD in infants with ALTE. Recent guidelines state that investigations aimed to prove GERD causing an ALTE should include pH probe or impedance monitor testing, in combination with a sleep study, and discourages a GERD diagnosis based on upper GI‐series alone.14 Given the low use of pH probe and impedance monitoring at our institution during the study period (86% of the patients who had GI‐related testing had an upper GI‐series), we did not attempt to find the sensitivity or specificity of the different GI testing modalities for GERD in the setting of ALTE. The high use of upper‐GI series is not unique to our institutionone large study examining practice variation from 12,067 ALTE admissions in 36 children's hospitals, with 36.9% of infants (n = 4453) having a primary discharge diagnosis of gastroesophageal reflux, revealed that only 8.9% (SD 28%) received an esophageal pH probe, while 25.6% (SD 43.6%) had an upper GI‐series or swallow study.4
Given the difficulties in assigning a GERD diagnosis for ALTE infants, we focused on the long‐term adverse outcomes associated with GERD for the entire ALTE infant cohort. The 3 adverse outcomes we chose deserve some mention. Aspiration pneumonia is generally due to either primary aspiration (from dysfunctional swallowing) or secondary aspiration (from GERD). Failure‐to‐thrive can be due to ongoing GERD. Anti‐reflux surgery is often performed for severe GERD. While we believe that a prospective study with better diagnostic evaluations for GERD and apnea (such as pH probe or impedance monitor in combination with a sleep study) might help elucidate the unclear relationship between reflux episodes and ALTE, the low percentage of adverse outcomes associated with GERD after ALTE may suggest that such a study would be difficult both in terms of sample size and an unnecessary use of resources.
We also found that a high percentage (11.9%) of all patients had readmission for a second ALTE. This was substantially higher than the 2.5% readmission rate for second ALTE reported in a previous study of short‐term follow‐up (30 days).4 The number of readmissions in our study might be higher due to our comprehensive follow‐up, both in length of time and number of additional EDs and hospitals captured. Unfortunately, the retrospective nature of our study makes it difficult to determine if any interventions (prescription of anti‐reflux medication, education on reflux precautions) impacted the rate of readmission, as compliance was not measurable. Further studies should address why patients return with recurrent ALTE.
Interestingly, several potential risk factors did not predict long‐term adverse GI outcomes. For example, prematurity, a discharge diagnosis of GERD, or prescription of an anti‐reflux medication, were not associated with adverse GI outcomes. These findings support the concept that a diagnosis of GERD, at least as is commonly applied, is not meaningful in the setting of an ALTE. We did find associations with longer length of stay (LOS), and with eventual development of neurological impairment. Longer LOS might be a proxy for other subtle predictors that could influence adverse outcomes, such as requiring additional diagnostic tests prolonging hospitalization, or continued ALTEs while inpatient. The neurologic outcomes of patients with ALTE have been previously published, and the strong correlation between neurologic impairment and GERD has been well described.14, 25
There are several strengths of this study. This is the first study, to our knowledge, to look at adverse outcomes associated with GERD following ALTE, despite GERD being the most commonly attributed cause. The use of Intermountain Healthcare's electronic medical record system allowed for comprehensive tracking, over an extensive follow‐up period (median of 7.8 years), across 20 hospitals and EDs which care for the vast majority of pediatric patients in Utah. Finally, this large cohort of ALTE patients used clinical data from medical records and not only administrative data.
There are limitations of this study. This is a retrospective cohort study. Some of our study outcomes may be a result of pathophysiology other than GERD and, conversely, GERD may be a result of other issues (neurologic impairment). The small sample size and low percentage of the study outcomes make it possible that we did not detect true risk factors. Patients were lost to follow‐up if they moved or presented to a hospital not within the Intermountain Healthcare system. This study has slightly different patient numbers from 3 previously published studies for different outcomes on this cohort, as exclusion criteria for the different cohorts were different.14, 26, 27 Six patients had only their electronic medical record reviewed because the paper chart was missing.
IMPLICATIONS
The results of this study extend previous work of various outcomes regarding well‐appearing infants following an ALTE.14, 26, 27 In these studies, 3.9% and 3% were ultimately diagnosed with epilepsy and developmental delay, respectively; 1.4% were diagnosed with abusive head trauma; and 0.6% required otolaryngologic surgical intervention. In these previous studies, there were few predictors of these outcomes, with testing demonstrating largely normal results during the index ALTE admission.
Our study helps clinicians place the outcomes of aspiration pneumonia, failure‐to‐thrive, and anti‐reflux surgery into the context of these other studies when discharging infants from the hospital after an ALTE. Collectively, these studies provide clinicians with the information that, in the setting of a well‐appearing infant, few diagnostic tests in their ALTE patients will yield a definitive diagnosis. Ultimately, close follow‐up with further investigations if symptoms recur will be an important part of diagnosing the etiology of the ALTE in these infants.
We found that well‐appearing infants with ALTE, regardless of attributed cause, are at low risk for adverse outcomes associated with GERD. Only the eventual development of neurologic impairment or an increased length of stay during index ALTE hospitalization was found to be predictive of these outcomes.
Acknowledgements
The following individuals have made substantive intellectual contributions to this study: conception and design (G.Z., J.L.B., W.D.J., C.G.M., R.S.), acquisition of data (G.Z., J.L.B.), analysis (G.Z., RS) and interpretation of data (G.Z., J.L.B., W.D.J., C.G.M., R.S.). In addition, all listed authors have contributed to either drafting the article or revising it critically for important intellectual content. Finally, all listed authors have given final approval of this version submitted for publication. The authors also acknowledge Chelsea Welch for her assistance in data collection.
Disclosures: This study was presented in part at the national Pediatric Academic Societies meetings in Vancouver, Canada, May 2010 and in Denver, CO, May 2011. This study was supported by a National Institutes of Child Health and Human Development (NICHD) grant for Dr Srivastava (K23 HD052553), and a National Institute on Drug Abuse (NIDA) grant for Dr Bonkowsky (K08 DA24753). This research was supported in part by the Children's Health Research Center, University of Utah. There are no conflicts of interest.
- Infantile apnea and home monitoring.NIH Consensus Statement 1986 Sep 29‐Oct 1.Pediatrics.1987;79(2):292–299.
- ,.Causes of apparent life threatening events in infants: a systematic review.Arch Dis Child.2004;89:1043–1048.
- ,.Parental reported apnea, admissions to hospital and sudden infant death syndrome.Acta Paediatr.2001;90(4):417–422.
- ,,,.Variation in inpatient resource utilization and management of apparent life‐threatening events.J Pediatr.2008;152(5):629–635.
- ,,,,,.Discharge diagnoses in infants with apparent life‐threatening event.Pediatr Int.2003;45:560–563.
- ,,,,,.Lack of temporal relation between acid reflux in the proximal oesophagus and cardiorespiratory events in sleeping infants.Eur J Pediatr.1992;151(3):208–212.
- ,,, et al.Patterns of gastroesophageal reflux (GER) in patients with apparent life‐threatening events.J Pediatr Gastroenterol Nutr.1989;8(2):157–160.
- ,,, et al.Characteristics of continuous esophageal pH‐metering in infants with gastroesophageal reflux and apparent life‐threatening events.Eur J Pediatr Surg.1995;5(3);136–138.
- ,,,,.Apnea is not prolonged by acid gastroesophageal reflux in preterm infants.Pediatrics.2005;116:1059–1063.
- ,.Organizational responses to managed care: issues for academic health centers and implications for pediatric programs.Pediatrics.1998;101(4):805–811.
- ,,, et al.Pediatric gastroesophageal reflux clinical practice guidelines: joint recommendations of the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN) and the European Society for Pediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN).J Pediatr Gastroenterol Nutr.2009;49:498–547.
- ,,, et al.Prevalence and natural history of gastroesophageal reflux: pediatric prospective study.Pediatrics.2009;123(3):779–783.
- ,.Systematic review: the estra‐oesophageal symptoms of gastro‐oesophageal reflux disease in children.Aliment Pharmacol Ther.2009;29:258–272.
- ,,,.Death, child abuse, and adverse neurological outcomes of infants after an apparent life‐threatening event.Pediatrics.2008;122:125–131.
- ,,, et al.Abusive head injury as a cause of apparent life‐threatening events in infancy.Arch Pediatr Adolesc Med.2003;157:1011–1015.
- ,,.Accidental and nonaccidental poisonings as a cause of apparent life‐threatening events in infants.Pediatrics.2008;122:e359–e362.
- ,,,.Yield of diagnostic testing in infants who have had an apparent life‐threatening event.Pediatrics.2005;115:885–893.
- ,,, et al.Guidelines for evaluation and treatment of gastroesophageal reflux in infants and children: recommendations of the North American Society for Pediatric Gastroenterology and Nutrition.J Pediatr Gastroenterol Nutr.2001;32(suppl 2):S1–S31.
- ,,,.Comparison of barium radiology with esophageal pH monitoring in the diagnosis of gastroesophageal reflux disease.Am J Gastroenterol.1996;91(6):1181–1195.
- ,,, et al.Gastroesophageal reflux disease: correlation of esophageal pH testing and radiographic finding.Radiology.1992;185:483–486.
- ,,, et al.Gastro‐esophageal reflux demonstrated by radiography in infants less than 1 year of age. Comparison with pH monitoring.Acta Radiol.2003;44:136–138.
- ,,, et al.Double‐blind placebo controlled trial of omeprazole in irritable infants with gastroesophageal reflux.J Pediatr.2003;143:219–223.
- ,,, et al.Clinical predictors of pathological gastro‐oesophageal reflux in infants with persistant distress.J Paediatr Child Health.2006;42:134–139.
- ,,.National trends in the use of anti‐reflux procedures for children.Pediatrics.2006;118:1828–1835.
- ,,, et al.Prevalence and severity of feeding and nutritional problems in children with neurological impairment: Oxford feeding study.Dev Med Child Neurol.2000;42:674–680.
- ,,.Abusive head trauma in children presenting with an apparent life‐threatening event.J Pediatr.2010;157(5):821–825.
- ,,.Usefulness of airway evaluation in children initially seen with apparent life‐threatening event.Arch Otolaryngol Head Neck Surg.2011;137(4):359–362.
- Infantile apnea and home monitoring.NIH Consensus Statement 1986 Sep 29‐Oct 1.Pediatrics.1987;79(2):292–299.
- ,.Causes of apparent life threatening events in infants: a systematic review.Arch Dis Child.2004;89:1043–1048.
- ,.Parental reported apnea, admissions to hospital and sudden infant death syndrome.Acta Paediatr.2001;90(4):417–422.
- ,,,.Variation in inpatient resource utilization and management of apparent life‐threatening events.J Pediatr.2008;152(5):629–635.
- ,,,,,.Discharge diagnoses in infants with apparent life‐threatening event.Pediatr Int.2003;45:560–563.
- ,,,,,.Lack of temporal relation between acid reflux in the proximal oesophagus and cardiorespiratory events in sleeping infants.Eur J Pediatr.1992;151(3):208–212.
- ,,, et al.Patterns of gastroesophageal reflux (GER) in patients with apparent life‐threatening events.J Pediatr Gastroenterol Nutr.1989;8(2):157–160.
- ,,, et al.Characteristics of continuous esophageal pH‐metering in infants with gastroesophageal reflux and apparent life‐threatening events.Eur J Pediatr Surg.1995;5(3);136–138.
- ,,,,.Apnea is not prolonged by acid gastroesophageal reflux in preterm infants.Pediatrics.2005;116:1059–1063.
- ,.Organizational responses to managed care: issues for academic health centers and implications for pediatric programs.Pediatrics.1998;101(4):805–811.
- ,,, et al.Pediatric gastroesophageal reflux clinical practice guidelines: joint recommendations of the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN) and the European Society for Pediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN).J Pediatr Gastroenterol Nutr.2009;49:498–547.
- ,,, et al.Prevalence and natural history of gastroesophageal reflux: pediatric prospective study.Pediatrics.2009;123(3):779–783.
- ,.Systematic review: the estra‐oesophageal symptoms of gastro‐oesophageal reflux disease in children.Aliment Pharmacol Ther.2009;29:258–272.
- ,,,.Death, child abuse, and adverse neurological outcomes of infants after an apparent life‐threatening event.Pediatrics.2008;122:125–131.
- ,,, et al.Abusive head injury as a cause of apparent life‐threatening events in infancy.Arch Pediatr Adolesc Med.2003;157:1011–1015.
- ,,.Accidental and nonaccidental poisonings as a cause of apparent life‐threatening events in infants.Pediatrics.2008;122:e359–e362.
- ,,,.Yield of diagnostic testing in infants who have had an apparent life‐threatening event.Pediatrics.2005;115:885–893.
- ,,, et al.Guidelines for evaluation and treatment of gastroesophageal reflux in infants and children: recommendations of the North American Society for Pediatric Gastroenterology and Nutrition.J Pediatr Gastroenterol Nutr.2001;32(suppl 2):S1–S31.
- ,,,.Comparison of barium radiology with esophageal pH monitoring in the diagnosis of gastroesophageal reflux disease.Am J Gastroenterol.1996;91(6):1181–1195.
- ,,, et al.Gastroesophageal reflux disease: correlation of esophageal pH testing and radiographic finding.Radiology.1992;185:483–486.
- ,,, et al.Gastro‐esophageal reflux demonstrated by radiography in infants less than 1 year of age. Comparison with pH monitoring.Acta Radiol.2003;44:136–138.
- ,,, et al.Double‐blind placebo controlled trial of omeprazole in irritable infants with gastroesophageal reflux.J Pediatr.2003;143:219–223.
- ,,, et al.Clinical predictors of pathological gastro‐oesophageal reflux in infants with persistant distress.J Paediatr Child Health.2006;42:134–139.
- ,,.National trends in the use of anti‐reflux procedures for children.Pediatrics.2006;118:1828–1835.
- ,,, et al.Prevalence and severity of feeding and nutritional problems in children with neurological impairment: Oxford feeding study.Dev Med Child Neurol.2000;42:674–680.
- ,,.Abusive head trauma in children presenting with an apparent life‐threatening event.J Pediatr.2010;157(5):821–825.
- ,,.Usefulness of airway evaluation in children initially seen with apparent life‐threatening event.Arch Otolaryngol Head Neck Surg.2011;137(4):359–362.
Copyright © 2012 Society of Hospital Medicine
Looks Aren’t Everything in Breast Reconstruction
Before-and-after photographs are the stock in trade of house painters, auto repair shops, and, yes, plastic and reconstructive surgeons. But a new study may make the last group pause, since it hints that far more is at play in breast cancer patients’ definition of “successful’’ breast reconstruction surgery than how their breasts appear.
The study from Liverpool, England (J Plast Reconstr Aesthet Surg (2012): doi:10.1016/j.bjps.2012.03.005) sidestepped traditional measures used to evaluate outcomes of aesthetic breast surgery and instead asked open-ended questions of survivors who had undergone reconstruction 1-8 years previously.
What the researchers discovered, not surprisingly, is that reconstruction patients are quite unlike cosmetic surgery patients in fundamental and important ways.
Of particular interest in the initial study cohort of 95 patients were 38 whose subjective evaluations of their surgical results completely contradicted objective ratings of cosmesis, the final appearance of the reconstructed breast(s) by surgeons and surgical nurses.
Incredibly, the association between women’s assessments and objective cosmesis ratings failed even to reach statistical significance.
In a structured data analysis of themes that arose in open-ended interviews with 27 of the survivors, the strongest link to women’s satisfaction with the procedure was the surgeon-patient relationship.
Next came the significance of reconstruction in what patients saw as the “completion of the cancer journey,” the authors wrote. “Patients who focused on this were positive about reconstruction that practitioners had rated negatively.”
A previous study asked patients about scarring, finding a correlation between scarring and dissatisfaction with reconstruction. But scarring wasn’t even a blip on the radar when, quoting from the Liverpool study, “we allowed patients to tell us what mattered to them rather than imposing our preconceptions.”
“It seems that surgeons and patients normally ‘talk different languages’; one technical and the other drawing more from relationships and patients’ sense of how normal they feel and appear and from their sense that reconstruction completes their cancer journey,” the investigators concluded. “In preoperative consultations, surgeons concentrate almost exclusively on the technical and cosmetic aspects of reconstruction: what can be achieved and what complications can occur.”
Of course, women who struggled with complications tended to factor that in to their assessments of their results, even if their final cosmetic outcome was considered by surgeons to be excellent.
Others were disappointed despite what seemed to surgeons to be excellent cosmetic results because, as one said, “I was expecting to feel feminine again, but I don’t, I don’t at all.”
What is perhaps even more interesting is to eavesdrop on the comments of women whose surgeons judged their cosmetic result to be poor.
Said one, “I had a really good relationship with (the surgeon) and I just found it so reassuring to see her. That was part of the whole thing really. She was just so positive, and so, well, just understanding I think … I was really glad that I had chosen that form of reconstruction because I had this regular contact with her.”
Said another, who felt “normal” despite what her surgeon considered to be a poor result: “If I didn’t have it done, I wouldn’t have felt normal at all. It would always remind me of what had happened.”
A highly complex patient-surgeon dance occurs when breast surgery is performed for more than cosmetic reasons, the study found.
One woman, disappointed with the way her reconstructed breast fit in a bra, could not bring herself to voice her concern with the surgeon she credited with saving her life.
“It’s very difficult to come face to face with somebody who says, ‘You’ve had cancer but we can get rid of it,’ and does their best… without seeming ungrateful,” she said, tearfully.
The study concludes with a fascinating discussion about the potential clinical implications of the findings.
Considering the profound influence of the patient-surgeon relationship on these particular patients, the investigators offer a cautionary suggestion to avoid being overly effusive about the cosmetic result they may see. Patients, they explain, may not necessarily share their enthusiasm, if they continue to struggle with the sense that cancer has marred their bodies, their sense of self, or their security in relationships.
“Both patient and surgeon have invested physically and emotionally in the procedure and it is difficult for either to admit to the other that it was “not worth it,” they note.
Women, on the other hand, who appear to be disproportionately pleased with the result of surgery that objectively achieved a poor result may simply be expressing relief and gratitude. “Their apparent satisfaction,” they wrote, “should not excuse poor surgical practice.” Rather, routine assessments of reconstructive practice should be made by objective sources, not simply patient report.
On the other hand, in individual patients, the objective in reconstructive surgery is patient satisfaction, they suggest. An unhappy patient might spur a conscientious surgeon to keep trying, perhaps through repeated procedures, to achieve a better result – a strategy that could be inappropriate and pointless considering that in some cases, “the reason for disappointment with reconstruction include many that the surgeon cannot influence surgically.”
It all suggests that communication between surgeons and reconstruction candidates and patients needs to be deep and candidly honest, informed by the emotional, sexual, and existential meaning the surgery holds.
In a word, it’s complicated.
Betsy Bates Freed, Psych.D., is a clinical psychologist in Santa Barbara, Calif., and a medical journalist.
Before-and-after photographs are the stock in trade of house painters, auto repair shops, and, yes, plastic and reconstructive surgeons. But a new study may make the last group pause, since it hints that far more is at play in breast cancer patients’ definition of “successful’’ breast reconstruction surgery than how their breasts appear.
The study from Liverpool, England (J Plast Reconstr Aesthet Surg (2012): doi:10.1016/j.bjps.2012.03.005) sidestepped traditional measures used to evaluate outcomes of aesthetic breast surgery and instead asked open-ended questions of survivors who had undergone reconstruction 1-8 years previously.
What the researchers discovered, not surprisingly, is that reconstruction patients are quite unlike cosmetic surgery patients in fundamental and important ways.
Of particular interest in the initial study cohort of 95 patients were 38 whose subjective evaluations of their surgical results completely contradicted objective ratings of cosmesis, the final appearance of the reconstructed breast(s) by surgeons and surgical nurses.
Incredibly, the association between women’s assessments and objective cosmesis ratings failed even to reach statistical significance.
In a structured data analysis of themes that arose in open-ended interviews with 27 of the survivors, the strongest link to women’s satisfaction with the procedure was the surgeon-patient relationship.
Next came the significance of reconstruction in what patients saw as the “completion of the cancer journey,” the authors wrote. “Patients who focused on this were positive about reconstruction that practitioners had rated negatively.”
A previous study asked patients about scarring, finding a correlation between scarring and dissatisfaction with reconstruction. But scarring wasn’t even a blip on the radar when, quoting from the Liverpool study, “we allowed patients to tell us what mattered to them rather than imposing our preconceptions.”
“It seems that surgeons and patients normally ‘talk different languages’; one technical and the other drawing more from relationships and patients’ sense of how normal they feel and appear and from their sense that reconstruction completes their cancer journey,” the investigators concluded. “In preoperative consultations, surgeons concentrate almost exclusively on the technical and cosmetic aspects of reconstruction: what can be achieved and what complications can occur.”
Of course, women who struggled with complications tended to factor that in to their assessments of their results, even if their final cosmetic outcome was considered by surgeons to be excellent.
Others were disappointed despite what seemed to surgeons to be excellent cosmetic results because, as one said, “I was expecting to feel feminine again, but I don’t, I don’t at all.”
What is perhaps even more interesting is to eavesdrop on the comments of women whose surgeons judged their cosmetic result to be poor.
Said one, “I had a really good relationship with (the surgeon) and I just found it so reassuring to see her. That was part of the whole thing really. She was just so positive, and so, well, just understanding I think … I was really glad that I had chosen that form of reconstruction because I had this regular contact with her.”
Said another, who felt “normal” despite what her surgeon considered to be a poor result: “If I didn’t have it done, I wouldn’t have felt normal at all. It would always remind me of what had happened.”
A highly complex patient-surgeon dance occurs when breast surgery is performed for more than cosmetic reasons, the study found.
One woman, disappointed with the way her reconstructed breast fit in a bra, could not bring herself to voice her concern with the surgeon she credited with saving her life.
“It’s very difficult to come face to face with somebody who says, ‘You’ve had cancer but we can get rid of it,’ and does their best… without seeming ungrateful,” she said, tearfully.
The study concludes with a fascinating discussion about the potential clinical implications of the findings.
Considering the profound influence of the patient-surgeon relationship on these particular patients, the investigators offer a cautionary suggestion to avoid being overly effusive about the cosmetic result they may see. Patients, they explain, may not necessarily share their enthusiasm, if they continue to struggle with the sense that cancer has marred their bodies, their sense of self, or their security in relationships.
“Both patient and surgeon have invested physically and emotionally in the procedure and it is difficult for either to admit to the other that it was “not worth it,” they note.
Women, on the other hand, who appear to be disproportionately pleased with the result of surgery that objectively achieved a poor result may simply be expressing relief and gratitude. “Their apparent satisfaction,” they wrote, “should not excuse poor surgical practice.” Rather, routine assessments of reconstructive practice should be made by objective sources, not simply patient report.
On the other hand, in individual patients, the objective in reconstructive surgery is patient satisfaction, they suggest. An unhappy patient might spur a conscientious surgeon to keep trying, perhaps through repeated procedures, to achieve a better result – a strategy that could be inappropriate and pointless considering that in some cases, “the reason for disappointment with reconstruction include many that the surgeon cannot influence surgically.”
It all suggests that communication between surgeons and reconstruction candidates and patients needs to be deep and candidly honest, informed by the emotional, sexual, and existential meaning the surgery holds.
In a word, it’s complicated.
Betsy Bates Freed, Psych.D., is a clinical psychologist in Santa Barbara, Calif., and a medical journalist.
Before-and-after photographs are the stock in trade of house painters, auto repair shops, and, yes, plastic and reconstructive surgeons. But a new study may make the last group pause, since it hints that far more is at play in breast cancer patients’ definition of “successful’’ breast reconstruction surgery than how their breasts appear.
The study from Liverpool, England (J Plast Reconstr Aesthet Surg (2012): doi:10.1016/j.bjps.2012.03.005) sidestepped traditional measures used to evaluate outcomes of aesthetic breast surgery and instead asked open-ended questions of survivors who had undergone reconstruction 1-8 years previously.
What the researchers discovered, not surprisingly, is that reconstruction patients are quite unlike cosmetic surgery patients in fundamental and important ways.
Of particular interest in the initial study cohort of 95 patients were 38 whose subjective evaluations of their surgical results completely contradicted objective ratings of cosmesis, the final appearance of the reconstructed breast(s) by surgeons and surgical nurses.
Incredibly, the association between women’s assessments and objective cosmesis ratings failed even to reach statistical significance.
In a structured data analysis of themes that arose in open-ended interviews with 27 of the survivors, the strongest link to women’s satisfaction with the procedure was the surgeon-patient relationship.
Next came the significance of reconstruction in what patients saw as the “completion of the cancer journey,” the authors wrote. “Patients who focused on this were positive about reconstruction that practitioners had rated negatively.”
A previous study asked patients about scarring, finding a correlation between scarring and dissatisfaction with reconstruction. But scarring wasn’t even a blip on the radar when, quoting from the Liverpool study, “we allowed patients to tell us what mattered to them rather than imposing our preconceptions.”
“It seems that surgeons and patients normally ‘talk different languages’; one technical and the other drawing more from relationships and patients’ sense of how normal they feel and appear and from their sense that reconstruction completes their cancer journey,” the investigators concluded. “In preoperative consultations, surgeons concentrate almost exclusively on the technical and cosmetic aspects of reconstruction: what can be achieved and what complications can occur.”
Of course, women who struggled with complications tended to factor that in to their assessments of their results, even if their final cosmetic outcome was considered by surgeons to be excellent.
Others were disappointed despite what seemed to surgeons to be excellent cosmetic results because, as one said, “I was expecting to feel feminine again, but I don’t, I don’t at all.”
What is perhaps even more interesting is to eavesdrop on the comments of women whose surgeons judged their cosmetic result to be poor.
Said one, “I had a really good relationship with (the surgeon) and I just found it so reassuring to see her. That was part of the whole thing really. She was just so positive, and so, well, just understanding I think … I was really glad that I had chosen that form of reconstruction because I had this regular contact with her.”
Said another, who felt “normal” despite what her surgeon considered to be a poor result: “If I didn’t have it done, I wouldn’t have felt normal at all. It would always remind me of what had happened.”
A highly complex patient-surgeon dance occurs when breast surgery is performed for more than cosmetic reasons, the study found.
One woman, disappointed with the way her reconstructed breast fit in a bra, could not bring herself to voice her concern with the surgeon she credited with saving her life.
“It’s very difficult to come face to face with somebody who says, ‘You’ve had cancer but we can get rid of it,’ and does their best… without seeming ungrateful,” she said, tearfully.
The study concludes with a fascinating discussion about the potential clinical implications of the findings.
Considering the profound influence of the patient-surgeon relationship on these particular patients, the investigators offer a cautionary suggestion to avoid being overly effusive about the cosmetic result they may see. Patients, they explain, may not necessarily share their enthusiasm, if they continue to struggle with the sense that cancer has marred their bodies, their sense of self, or their security in relationships.
“Both patient and surgeon have invested physically and emotionally in the procedure and it is difficult for either to admit to the other that it was “not worth it,” they note.
Women, on the other hand, who appear to be disproportionately pleased with the result of surgery that objectively achieved a poor result may simply be expressing relief and gratitude. “Their apparent satisfaction,” they wrote, “should not excuse poor surgical practice.” Rather, routine assessments of reconstructive practice should be made by objective sources, not simply patient report.
On the other hand, in individual patients, the objective in reconstructive surgery is patient satisfaction, they suggest. An unhappy patient might spur a conscientious surgeon to keep trying, perhaps through repeated procedures, to achieve a better result – a strategy that could be inappropriate and pointless considering that in some cases, “the reason for disappointment with reconstruction include many that the surgeon cannot influence surgically.”
It all suggests that communication between surgeons and reconstruction candidates and patients needs to be deep and candidly honest, informed by the emotional, sexual, and existential meaning the surgery holds.
In a word, it’s complicated.
Betsy Bates Freed, Psych.D., is a clinical psychologist in Santa Barbara, Calif., and a medical journalist.
Exploring the Role of Modified-Release Doxycycline in Rosacea

Topics
- Introduction - Exploring the Role of Modified-Release
- Doxycycline in Rosacea
- Comparing Antimicrobial and Anti-Inflammatory Doses of Oral Doxycycline in the Treatment of Rosacea
- Impact of Antibiotic Resistance on Dermatologic Practice
- The Effects of Modified-Release Doxycycline on Quality of Life
- Combining Anti-Inflammatory–Dose Doxycycline With Topical Metronidazole
To view the supplement, click the image above.
Faculty/Faculty Disclosure
Brian Berman, MD, PhD
Voluntary Professor of Dermatology and Cutaneous Surgery
University of Miami, Miller School of Medicine
Co-Director
Center for Clinical and Cosmetic Research, Skin & Cancer Associates, LLP,
Aventura, FL
Dr Berman has received funding for clinical grants from, is an investigator for, and is a consultant to, Galderma Laboratories, L.P.
Copyright © 2012 by Elsevier Inc.

Topics
- Introduction - Exploring the Role of Modified-Release
- Doxycycline in Rosacea
- Comparing Antimicrobial and Anti-Inflammatory Doses of Oral Doxycycline in the Treatment of Rosacea
- Impact of Antibiotic Resistance on Dermatologic Practice
- The Effects of Modified-Release Doxycycline on Quality of Life
- Combining Anti-Inflammatory–Dose Doxycycline With Topical Metronidazole
To view the supplement, click the image above.
Faculty/Faculty Disclosure
Brian Berman, MD, PhD
Voluntary Professor of Dermatology and Cutaneous Surgery
University of Miami, Miller School of Medicine
Co-Director
Center for Clinical and Cosmetic Research, Skin & Cancer Associates, LLP,
Aventura, FL
Dr Berman has received funding for clinical grants from, is an investigator for, and is a consultant to, Galderma Laboratories, L.P.
Copyright © 2012 by Elsevier Inc.

Topics
- Introduction - Exploring the Role of Modified-Release
- Doxycycline in Rosacea
- Comparing Antimicrobial and Anti-Inflammatory Doses of Oral Doxycycline in the Treatment of Rosacea
- Impact of Antibiotic Resistance on Dermatologic Practice
- The Effects of Modified-Release Doxycycline on Quality of Life
- Combining Anti-Inflammatory–Dose Doxycycline With Topical Metronidazole
To view the supplement, click the image above.
Faculty/Faculty Disclosure
Brian Berman, MD, PhD
Voluntary Professor of Dermatology and Cutaneous Surgery
University of Miami, Miller School of Medicine
Co-Director
Center for Clinical and Cosmetic Research, Skin & Cancer Associates, LLP,
Aventura, FL
Dr Berman has received funding for clinical grants from, is an investigator for, and is a consultant to, Galderma Laboratories, L.P.
Copyright © 2012 by Elsevier Inc.
Exploring the Role of Modified-Release Doxycycline in Rosacea
- Introduction - Exploring the Role of Modified-Release
- Doxycycline in Rosacea
- Comparing Antimicrobial and Anti-Inflammatory Doses of Oral Doxycycline in the Treatment of Rosacea
- Impact of Antibiotic Resistance on Dermatologic Practice
- The Effects of Modified-Release Doxycycline on Quality of Life
- Combining Anti-Inflammatory–Dose Doxycycline With Topical Metronidazole
To view the supplement, click the image above.
Faculty/Faculty Disclosure
Brian Berman, MD, PhD
Voluntary Professor of Dermatology and Cutaneous Surgery
University of Miami, Miller School of Medicine Co-Director
Center for Clinical and Cosmetic Research, Skin & Cancer Associates, LLP, Aventura, FL
Copyright (c) 2012 by Elsevier Inc.
- Introduction - Exploring the Role of Modified-Release
- Doxycycline in Rosacea
- Comparing Antimicrobial and Anti-Inflammatory Doses of Oral Doxycycline in the Treatment of Rosacea
- Impact of Antibiotic Resistance on Dermatologic Practice
- The Effects of Modified-Release Doxycycline on Quality of Life
- Combining Anti-Inflammatory–Dose Doxycycline With Topical Metronidazole
To view the supplement, click the image above.
Faculty/Faculty Disclosure
Brian Berman, MD, PhD
Voluntary Professor of Dermatology and Cutaneous Surgery
University of Miami, Miller School of Medicine Co-Director
Center for Clinical and Cosmetic Research, Skin & Cancer Associates, LLP, Aventura, FL
Copyright (c) 2012 by Elsevier Inc.
- Introduction - Exploring the Role of Modified-Release
- Doxycycline in Rosacea
- Comparing Antimicrobial and Anti-Inflammatory Doses of Oral Doxycycline in the Treatment of Rosacea
- Impact of Antibiotic Resistance on Dermatologic Practice
- The Effects of Modified-Release Doxycycline on Quality of Life
- Combining Anti-Inflammatory–Dose Doxycycline With Topical Metronidazole
To view the supplement, click the image above.
Faculty/Faculty Disclosure
Brian Berman, MD, PhD
Voluntary Professor of Dermatology and Cutaneous Surgery
University of Miami, Miller School of Medicine Co-Director
Center for Clinical and Cosmetic Research, Skin & Cancer Associates, LLP, Aventura, FL
Copyright (c) 2012 by Elsevier Inc.
Military Teens Face Unique Mental Health Challenges
"My Story: Blogs by Four Military Teens" is a book that gives voice to military teens by highlighting their feelings and experiences before, during, and after parental deployment. The four youths in "My Story" are fictional, but the stories are real in that the posts are a compilation of real life experiences of military kids.
"Adam" blogs, "My dad is one of the coolest, smartest, bravest men I’ve ever met, but sometimes I just can’t stand being around him. He’s a doctor – a surgeon – and is in the Air Force Reserves. He just returned from a second tour in Iraq, and he sure acts different. After his first tour, it took us some time to get caught up, but just when things got normal again, he got his orders to go back to Iraq. Now he’s finally home (for good?), but he just isn’t the same. He gets mad over the stupidest things and spends most of his time in his bedroom or in front of the computer.
"He’s still in ‘military mode,’ and orders us around way too much. He doesn’t joke around like before, and sometimes just hangs out in the garage by himself. We don’t talk much. I almost liked it better when he was gone. It was a lot quieter and less stressful around the house. Ashley, Lisa, and I just stay out of his way. Derrick is lucky – he’s leaving for college soon."
This book also provides support and education for military teens and preteens by honoring their unique joys and sacrifices, addressing their fears and hopes, and exploring how parental deployment affects their lives.
The book was written by Michelle D. Sherman, Ph.D., and her mother, DeAnne M. Sherman. Together, Dr. Sherman and her mother – a teacher – have written other books for teens, including "Finding My Way: A Teen’s Guide to Living with a Parent Who has Experienced Trauma" (Waco, Tex.: Seeds of Hope Publishers, 2005).
Dr. Sherman is a clinical psychologist at the Oklahoma City VA Medical Center, where she directs its Family Mental Health Program. She has dedicated her career to supporting families affected by mental illness and posttraumatic stress disorder, and has a special interest in the impact of parental mental illness and PTSD on youth.
"Think about the ... challenges facing our military teens," she writes in a guest post on the blog of an organization called Veterans Children. "Their parent(s) may be deployed once, twice, or even multiple times to a war zone. The parent may miss out on important events, such as prom, the school play, the state basketball tournament, and birthdays."
These young people are resilient, Dr. Sherman writes, but some are struggling with increased rates of anxiety, sleeping and behavioral problems, and the use of psychotropics.
"What does this tell us? ...They are affected by their parent’s deployment. We need to listen to them, provide resources, and make services available," writes Dr. Sherman, also is a clinical professor in the department of psychiatry and behavioral sciences at the University of Oklahoma Health Sciences Center and a research affiliate with the South Central Mental Illness Research, Education and Clinical Center (MIRECC).
In her work with veterans and families, Dr. Sherman has developed educational and support programs. Operation Enduring Families is a 5-session family education curriculum for Operation Enduring Freedom/Operation Iraqi Freedom (OEF/OIF) veterans/families, developed with Ursula B. Bowling, Psy.D., and Alan L. Doerman, Psy.D. This program is based on her S.A.F.E (Support and Family Education) program, an 18-session curriculum for those who care about someone with a mental illness/PTSD.
Dr. Sherman has developed an extensive resource list for OEF/OIF service members, and veterans and their families. (See box.) For a complete list, you can e-mail her at [email protected].
This column, "Families in Psychiatry," regularly appears in Clinical Psychiatry News, an Elsevier publication. Dr. Heru is an associate professor of psychiatry at the University of Colorado at Denver, Aurora. She has been a member of the Association of Family Psychiatrists since 2002 and currently serves as the organization’s treasurer. In addition, she is the coauthor of two books on working with families and is the author of numerous articles on this topic.
The following list of books and websites can be used to help military children of all ages cope with parental deployment:
Books
• "After the War Zone: A Practical Guide for Returning Troops and Their Families" (Cambridge, Mass.: Da Capo Press, 2008).
• "Back from the Front: Combat Trauma, Love, and the Family" (Brooklandville, Md.: Sidran Institute Press, 2007).
• "Courage After Fire: Coping Strategies for Returning Soldiers and Their Families" (Berkeley, Calif.: Ulysses Press, 2005).
• "I Miss You!: A Military Kid’s Book About Deployment" (Amherst, N.Y.: Prometheus Books, 2007).
• "Night Catch" (Jamestown, N.D.: Bubble Gum Press, 2005).
• "100 days and 99 nights" (New York: Little, Brown and Co. Books for Young Readers, 2008).
• "Sometimes We Were Brave" (Honesdale, Pa.: Boyds Mills Press, 2010).
• "The Fathers Are Coming Home" (New York: Margaret K. McElderry Books, 2010).
Websites
• "Courage to Care, Courage to Talk About War Injuries," developed by the Center for the Study of Traumatic Stress.
• "Military Child Bill of Rights."
• National Military Family Association.
• Song and video: "The Price of Peace."
• Video (58 minutes): "Returning From the War Zone: A Guide for Families of Military Members," created by the National Center for PTSD.
• SOAR (Student Online Achievement Resources).
• Students at the Center: An Education Resource for Families, the Military, and Schools.
• "Talk, Listen, Connect: Deployments, Homecomings, Changes."
• Veteran Parenting Toolkit, created by the Oklahoma City VA Family Mental Health Program.
• "Welcome Back Parenting: A Guide for Reconnecting Families After Military Deployment."
• DVD: "Young Children on the Homefront: Family Stories, Family, Strengths," developed by the nonprofit organization Zero to Three: National Center for Infants, Toddlers, and Families.
• "Young Heroes: Military Deployment Through the Eyes of Youth."
"My Story: Blogs by Four Military Teens" is a book that gives voice to military teens by highlighting their feelings and experiences before, during, and after parental deployment. The four youths in "My Story" are fictional, but the stories are real in that the posts are a compilation of real life experiences of military kids.
"Adam" blogs, "My dad is one of the coolest, smartest, bravest men I’ve ever met, but sometimes I just can’t stand being around him. He’s a doctor – a surgeon – and is in the Air Force Reserves. He just returned from a second tour in Iraq, and he sure acts different. After his first tour, it took us some time to get caught up, but just when things got normal again, he got his orders to go back to Iraq. Now he’s finally home (for good?), but he just isn’t the same. He gets mad over the stupidest things and spends most of his time in his bedroom or in front of the computer.
"He’s still in ‘military mode,’ and orders us around way too much. He doesn’t joke around like before, and sometimes just hangs out in the garage by himself. We don’t talk much. I almost liked it better when he was gone. It was a lot quieter and less stressful around the house. Ashley, Lisa, and I just stay out of his way. Derrick is lucky – he’s leaving for college soon."
This book also provides support and education for military teens and preteens by honoring their unique joys and sacrifices, addressing their fears and hopes, and exploring how parental deployment affects their lives.
The book was written by Michelle D. Sherman, Ph.D., and her mother, DeAnne M. Sherman. Together, Dr. Sherman and her mother – a teacher – have written other books for teens, including "Finding My Way: A Teen’s Guide to Living with a Parent Who has Experienced Trauma" (Waco, Tex.: Seeds of Hope Publishers, 2005).
Dr. Sherman is a clinical psychologist at the Oklahoma City VA Medical Center, where she directs its Family Mental Health Program. She has dedicated her career to supporting families affected by mental illness and posttraumatic stress disorder, and has a special interest in the impact of parental mental illness and PTSD on youth.
"Think about the ... challenges facing our military teens," she writes in a guest post on the blog of an organization called Veterans Children. "Their parent(s) may be deployed once, twice, or even multiple times to a war zone. The parent may miss out on important events, such as prom, the school play, the state basketball tournament, and birthdays."
These young people are resilient, Dr. Sherman writes, but some are struggling with increased rates of anxiety, sleeping and behavioral problems, and the use of psychotropics.
"What does this tell us? ...They are affected by their parent’s deployment. We need to listen to them, provide resources, and make services available," writes Dr. Sherman, also is a clinical professor in the department of psychiatry and behavioral sciences at the University of Oklahoma Health Sciences Center and a research affiliate with the South Central Mental Illness Research, Education and Clinical Center (MIRECC).
In her work with veterans and families, Dr. Sherman has developed educational and support programs. Operation Enduring Families is a 5-session family education curriculum for Operation Enduring Freedom/Operation Iraqi Freedom (OEF/OIF) veterans/families, developed with Ursula B. Bowling, Psy.D., and Alan L. Doerman, Psy.D. This program is based on her S.A.F.E (Support and Family Education) program, an 18-session curriculum for those who care about someone with a mental illness/PTSD.
Dr. Sherman has developed an extensive resource list for OEF/OIF service members, and veterans and their families. (See box.) For a complete list, you can e-mail her at [email protected].
This column, "Families in Psychiatry," regularly appears in Clinical Psychiatry News, an Elsevier publication. Dr. Heru is an associate professor of psychiatry at the University of Colorado at Denver, Aurora. She has been a member of the Association of Family Psychiatrists since 2002 and currently serves as the organization’s treasurer. In addition, she is the coauthor of two books on working with families and is the author of numerous articles on this topic.
The following list of books and websites can be used to help military children of all ages cope with parental deployment:
Books
• "After the War Zone: A Practical Guide for Returning Troops and Their Families" (Cambridge, Mass.: Da Capo Press, 2008).
• "Back from the Front: Combat Trauma, Love, and the Family" (Brooklandville, Md.: Sidran Institute Press, 2007).
• "Courage After Fire: Coping Strategies for Returning Soldiers and Their Families" (Berkeley, Calif.: Ulysses Press, 2005).
• "I Miss You!: A Military Kid’s Book About Deployment" (Amherst, N.Y.: Prometheus Books, 2007).
• "Night Catch" (Jamestown, N.D.: Bubble Gum Press, 2005).
• "100 days and 99 nights" (New York: Little, Brown and Co. Books for Young Readers, 2008).
• "Sometimes We Were Brave" (Honesdale, Pa.: Boyds Mills Press, 2010).
• "The Fathers Are Coming Home" (New York: Margaret K. McElderry Books, 2010).
Websites
• "Courage to Care, Courage to Talk About War Injuries," developed by the Center for the Study of Traumatic Stress.
• "Military Child Bill of Rights."
• National Military Family Association.
• Song and video: "The Price of Peace."
• Video (58 minutes): "Returning From the War Zone: A Guide for Families of Military Members," created by the National Center for PTSD.
• SOAR (Student Online Achievement Resources).
• Students at the Center: An Education Resource for Families, the Military, and Schools.
• "Talk, Listen, Connect: Deployments, Homecomings, Changes."
• Veteran Parenting Toolkit, created by the Oklahoma City VA Family Mental Health Program.
• "Welcome Back Parenting: A Guide for Reconnecting Families After Military Deployment."
• DVD: "Young Children on the Homefront: Family Stories, Family, Strengths," developed by the nonprofit organization Zero to Three: National Center for Infants, Toddlers, and Families.
• "Young Heroes: Military Deployment Through the Eyes of Youth."
"My Story: Blogs by Four Military Teens" is a book that gives voice to military teens by highlighting their feelings and experiences before, during, and after parental deployment. The four youths in "My Story" are fictional, but the stories are real in that the posts are a compilation of real life experiences of military kids.
"Adam" blogs, "My dad is one of the coolest, smartest, bravest men I’ve ever met, but sometimes I just can’t stand being around him. He’s a doctor – a surgeon – and is in the Air Force Reserves. He just returned from a second tour in Iraq, and he sure acts different. After his first tour, it took us some time to get caught up, but just when things got normal again, he got his orders to go back to Iraq. Now he’s finally home (for good?), but he just isn’t the same. He gets mad over the stupidest things and spends most of his time in his bedroom or in front of the computer.
"He’s still in ‘military mode,’ and orders us around way too much. He doesn’t joke around like before, and sometimes just hangs out in the garage by himself. We don’t talk much. I almost liked it better when he was gone. It was a lot quieter and less stressful around the house. Ashley, Lisa, and I just stay out of his way. Derrick is lucky – he’s leaving for college soon."
This book also provides support and education for military teens and preteens by honoring their unique joys and sacrifices, addressing their fears and hopes, and exploring how parental deployment affects their lives.
The book was written by Michelle D. Sherman, Ph.D., and her mother, DeAnne M. Sherman. Together, Dr. Sherman and her mother – a teacher – have written other books for teens, including "Finding My Way: A Teen’s Guide to Living with a Parent Who has Experienced Trauma" (Waco, Tex.: Seeds of Hope Publishers, 2005).
Dr. Sherman is a clinical psychologist at the Oklahoma City VA Medical Center, where she directs its Family Mental Health Program. She has dedicated her career to supporting families affected by mental illness and posttraumatic stress disorder, and has a special interest in the impact of parental mental illness and PTSD on youth.
"Think about the ... challenges facing our military teens," she writes in a guest post on the blog of an organization called Veterans Children. "Their parent(s) may be deployed once, twice, or even multiple times to a war zone. The parent may miss out on important events, such as prom, the school play, the state basketball tournament, and birthdays."
These young people are resilient, Dr. Sherman writes, but some are struggling with increased rates of anxiety, sleeping and behavioral problems, and the use of psychotropics.
"What does this tell us? ...They are affected by their parent’s deployment. We need to listen to them, provide resources, and make services available," writes Dr. Sherman, also is a clinical professor in the department of psychiatry and behavioral sciences at the University of Oklahoma Health Sciences Center and a research affiliate with the South Central Mental Illness Research, Education and Clinical Center (MIRECC).
In her work with veterans and families, Dr. Sherman has developed educational and support programs. Operation Enduring Families is a 5-session family education curriculum for Operation Enduring Freedom/Operation Iraqi Freedom (OEF/OIF) veterans/families, developed with Ursula B. Bowling, Psy.D., and Alan L. Doerman, Psy.D. This program is based on her S.A.F.E (Support and Family Education) program, an 18-session curriculum for those who care about someone with a mental illness/PTSD.
Dr. Sherman has developed an extensive resource list for OEF/OIF service members, and veterans and their families. (See box.) For a complete list, you can e-mail her at [email protected].
This column, "Families in Psychiatry," regularly appears in Clinical Psychiatry News, an Elsevier publication. Dr. Heru is an associate professor of psychiatry at the University of Colorado at Denver, Aurora. She has been a member of the Association of Family Psychiatrists since 2002 and currently serves as the organization’s treasurer. In addition, she is the coauthor of two books on working with families and is the author of numerous articles on this topic.
The following list of books and websites can be used to help military children of all ages cope with parental deployment:
Books
• "After the War Zone: A Practical Guide for Returning Troops and Their Families" (Cambridge, Mass.: Da Capo Press, 2008).
• "Back from the Front: Combat Trauma, Love, and the Family" (Brooklandville, Md.: Sidran Institute Press, 2007).
• "Courage After Fire: Coping Strategies for Returning Soldiers and Their Families" (Berkeley, Calif.: Ulysses Press, 2005).
• "I Miss You!: A Military Kid’s Book About Deployment" (Amherst, N.Y.: Prometheus Books, 2007).
• "Night Catch" (Jamestown, N.D.: Bubble Gum Press, 2005).
• "100 days and 99 nights" (New York: Little, Brown and Co. Books for Young Readers, 2008).
• "Sometimes We Were Brave" (Honesdale, Pa.: Boyds Mills Press, 2010).
• "The Fathers Are Coming Home" (New York: Margaret K. McElderry Books, 2010).
Websites
• "Courage to Care, Courage to Talk About War Injuries," developed by the Center for the Study of Traumatic Stress.
• "Military Child Bill of Rights."
• National Military Family Association.
• Song and video: "The Price of Peace."
• Video (58 minutes): "Returning From the War Zone: A Guide for Families of Military Members," created by the National Center for PTSD.
• SOAR (Student Online Achievement Resources).
• Students at the Center: An Education Resource for Families, the Military, and Schools.
• "Talk, Listen, Connect: Deployments, Homecomings, Changes."
• Veteran Parenting Toolkit, created by the Oklahoma City VA Family Mental Health Program.
• "Welcome Back Parenting: A Guide for Reconnecting Families After Military Deployment."
• DVD: "Young Children on the Homefront: Family Stories, Family, Strengths," developed by the nonprofit organization Zero to Three: National Center for Infants, Toddlers, and Families.
• "Young Heroes: Military Deployment Through the Eyes of Youth."
Urgent Discharge: What's the Rush?
Rehospitalization within 30 days of an initial acute coronary syndrome or heart failure event has now become a CMS quality measure that will affect overall hospital Medicare payments. It has been appreciated for some time that rehospitalization for these diagnoses has been unacceptably high.
A recent report indicates that a greater percentage of U.S. patients who experienced a STEMI are more likely to be rehospitalized within 30 days, compared with other Western countries (14.5% vs. 9.9%, respectively). That report (JAMA 2012;307:66-74) indicates that the increase is directly related to the shorter length of hospital stay in the United States. Among the 17 countries included in the report, the average duration was shortest in the United States (3 days) and longest in Germany (8 days). Predictors of readmission other than length of stay include the age of the patient and the presence of heart failure. The most interesting part of the story is how we arrived at this state of affairs.
For those of you who were not yet born or are too young to remember when Medicare was passed into law in 1965, I will give you a little history. And for those of you who were around at the time, I will provide a reminder.
As you undoubtedly know, Medicare, in addition to paying physicians’ fees also pays hospital costs. In the period between 1965 and 1983, using a payment system that was defined as "reasonable and allowable costs," Medicare payments to hospitals increased 10-fold, from $3 billion to $37 billion. In consequence, Congress passed a law in 1982 that created a prospective payment system for hospitals using diagnosis-related groups establishing a payment schedule for specific diagnoses, which included acute myocardial infarction and heart failure. With this schedule, hospitals were paid a fixed rate regardless of the number of procedures performed or duration of hospitalization. In order to minimize costs, hospitals accelerated discharges and shortened hospital length of stay. Emergency admissions resulted in urgent discharge.
In order to expedite the process of admission and discharge, hospitalists were hired to accelerate that process since practicing internists and cardiologists were not available to push the paperwork through fast enough to get the patients discharged quickly. Hospitals saw this additional layer of doctors caring for patients as financially profitable. As a result, hospital stays decreased markedly and payments to hospitals decreased by 52% from 1985 to 1990 and by an additional 37% between 1990 and 1995. Everyone seemed to be very happy with this, including the hospitals, Medicare, and doctors. As far as I know, patients were not consulted.
Cardiologists at that time were telling themselves how benign an acute MI is and began doing accelerated discharge after percutaneous coronary intervention. We prided ourselves on how patients could be discharged to home within 24-36 hours, but never actually reached the ultimate goal of a "drive-through PCI." The fact that patients with acute MI and heart failure were frequently readmitted was good business since each admission resulted in further Medicare payments both to the hospital and the doctors. Urging by some physicians to develop plans that could educate patients and develop discharge follow-up systems was met with incredulity by hospital administrators who saw readmission as a revenue source and discharge planning as costly.
It is important to emphasize that readmission not only reflects an important morbidity event, it also carries with it the potential for increased risk of mortality. In the report cited above, one-third of the deaths after hospitalization for a STEMI occurred within the same 30-day post-event period. The recent emphasis on decreasing door-to-balloon time, although effective in shortening that period, has had little effect on the mortality associated with an acute myocardial infarction. It is reasonable to assume that in placing a greater emphasis on insuring that patients are ready to leave the hospital, we can improve mortality and morbidity of both the ACS and heart failure patient. There really is no urgency to discharge patients other than improving the bottom line, and that imperative may no longer be relevant.
Dr. Goldstein, medical editor of Cardiology News, is professor of medicine at Wayne State University and division head emeritus of cardiovascular medicine at Henry Ford Hospital, both in Detroit. He is on data safety monitoring committees for the National Institutes of Health and several pharmaceutical companies.
Rehospitalization within 30 days of an initial acute coronary syndrome or heart failure event has now become a CMS quality measure that will affect overall hospital Medicare payments. It has been appreciated for some time that rehospitalization for these diagnoses has been unacceptably high.
A recent report indicates that a greater percentage of U.S. patients who experienced a STEMI are more likely to be rehospitalized within 30 days, compared with other Western countries (14.5% vs. 9.9%, respectively). That report (JAMA 2012;307:66-74) indicates that the increase is directly related to the shorter length of hospital stay in the United States. Among the 17 countries included in the report, the average duration was shortest in the United States (3 days) and longest in Germany (8 days). Predictors of readmission other than length of stay include the age of the patient and the presence of heart failure. The most interesting part of the story is how we arrived at this state of affairs.
For those of you who were not yet born or are too young to remember when Medicare was passed into law in 1965, I will give you a little history. And for those of you who were around at the time, I will provide a reminder.
As you undoubtedly know, Medicare, in addition to paying physicians’ fees also pays hospital costs. In the period between 1965 and 1983, using a payment system that was defined as "reasonable and allowable costs," Medicare payments to hospitals increased 10-fold, from $3 billion to $37 billion. In consequence, Congress passed a law in 1982 that created a prospective payment system for hospitals using diagnosis-related groups establishing a payment schedule for specific diagnoses, which included acute myocardial infarction and heart failure. With this schedule, hospitals were paid a fixed rate regardless of the number of procedures performed or duration of hospitalization. In order to minimize costs, hospitals accelerated discharges and shortened hospital length of stay. Emergency admissions resulted in urgent discharge.
In order to expedite the process of admission and discharge, hospitalists were hired to accelerate that process since practicing internists and cardiologists were not available to push the paperwork through fast enough to get the patients discharged quickly. Hospitals saw this additional layer of doctors caring for patients as financially profitable. As a result, hospital stays decreased markedly and payments to hospitals decreased by 52% from 1985 to 1990 and by an additional 37% between 1990 and 1995. Everyone seemed to be very happy with this, including the hospitals, Medicare, and doctors. As far as I know, patients were not consulted.
Cardiologists at that time were telling themselves how benign an acute MI is and began doing accelerated discharge after percutaneous coronary intervention. We prided ourselves on how patients could be discharged to home within 24-36 hours, but never actually reached the ultimate goal of a "drive-through PCI." The fact that patients with acute MI and heart failure were frequently readmitted was good business since each admission resulted in further Medicare payments both to the hospital and the doctors. Urging by some physicians to develop plans that could educate patients and develop discharge follow-up systems was met with incredulity by hospital administrators who saw readmission as a revenue source and discharge planning as costly.
It is important to emphasize that readmission not only reflects an important morbidity event, it also carries with it the potential for increased risk of mortality. In the report cited above, one-third of the deaths after hospitalization for a STEMI occurred within the same 30-day post-event period. The recent emphasis on decreasing door-to-balloon time, although effective in shortening that period, has had little effect on the mortality associated with an acute myocardial infarction. It is reasonable to assume that in placing a greater emphasis on insuring that patients are ready to leave the hospital, we can improve mortality and morbidity of both the ACS and heart failure patient. There really is no urgency to discharge patients other than improving the bottom line, and that imperative may no longer be relevant.
Dr. Goldstein, medical editor of Cardiology News, is professor of medicine at Wayne State University and division head emeritus of cardiovascular medicine at Henry Ford Hospital, both in Detroit. He is on data safety monitoring committees for the National Institutes of Health and several pharmaceutical companies.
Rehospitalization within 30 days of an initial acute coronary syndrome or heart failure event has now become a CMS quality measure that will affect overall hospital Medicare payments. It has been appreciated for some time that rehospitalization for these diagnoses has been unacceptably high.
A recent report indicates that a greater percentage of U.S. patients who experienced a STEMI are more likely to be rehospitalized within 30 days, compared with other Western countries (14.5% vs. 9.9%, respectively). That report (JAMA 2012;307:66-74) indicates that the increase is directly related to the shorter length of hospital stay in the United States. Among the 17 countries included in the report, the average duration was shortest in the United States (3 days) and longest in Germany (8 days). Predictors of readmission other than length of stay include the age of the patient and the presence of heart failure. The most interesting part of the story is how we arrived at this state of affairs.
For those of you who were not yet born or are too young to remember when Medicare was passed into law in 1965, I will give you a little history. And for those of you who were around at the time, I will provide a reminder.
As you undoubtedly know, Medicare, in addition to paying physicians’ fees also pays hospital costs. In the period between 1965 and 1983, using a payment system that was defined as "reasonable and allowable costs," Medicare payments to hospitals increased 10-fold, from $3 billion to $37 billion. In consequence, Congress passed a law in 1982 that created a prospective payment system for hospitals using diagnosis-related groups establishing a payment schedule for specific diagnoses, which included acute myocardial infarction and heart failure. With this schedule, hospitals were paid a fixed rate regardless of the number of procedures performed or duration of hospitalization. In order to minimize costs, hospitals accelerated discharges and shortened hospital length of stay. Emergency admissions resulted in urgent discharge.
In order to expedite the process of admission and discharge, hospitalists were hired to accelerate that process since practicing internists and cardiologists were not available to push the paperwork through fast enough to get the patients discharged quickly. Hospitals saw this additional layer of doctors caring for patients as financially profitable. As a result, hospital stays decreased markedly and payments to hospitals decreased by 52% from 1985 to 1990 and by an additional 37% between 1990 and 1995. Everyone seemed to be very happy with this, including the hospitals, Medicare, and doctors. As far as I know, patients were not consulted.
Cardiologists at that time were telling themselves how benign an acute MI is and began doing accelerated discharge after percutaneous coronary intervention. We prided ourselves on how patients could be discharged to home within 24-36 hours, but never actually reached the ultimate goal of a "drive-through PCI." The fact that patients with acute MI and heart failure were frequently readmitted was good business since each admission resulted in further Medicare payments both to the hospital and the doctors. Urging by some physicians to develop plans that could educate patients and develop discharge follow-up systems was met with incredulity by hospital administrators who saw readmission as a revenue source and discharge planning as costly.
It is important to emphasize that readmission not only reflects an important morbidity event, it also carries with it the potential for increased risk of mortality. In the report cited above, one-third of the deaths after hospitalization for a STEMI occurred within the same 30-day post-event period. The recent emphasis on decreasing door-to-balloon time, although effective in shortening that period, has had little effect on the mortality associated with an acute myocardial infarction. It is reasonable to assume that in placing a greater emphasis on insuring that patients are ready to leave the hospital, we can improve mortality and morbidity of both the ACS and heart failure patient. There really is no urgency to discharge patients other than improving the bottom line, and that imperative may no longer be relevant.
Dr. Goldstein, medical editor of Cardiology News, is professor of medicine at Wayne State University and division head emeritus of cardiovascular medicine at Henry Ford Hospital, both in Detroit. He is on data safety monitoring committees for the National Institutes of Health and several pharmaceutical companies.